
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
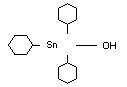
TRICYCLOHEXYLTIN HYDROXIDE
IDENTITY
Chemical name
Tricyclohexyltin hydroxide
Synonyms
DOWCO (R) 213, PLICTRAN (R)
Structural formula
 Other relevant chemical properties
The compound in a white, crystalline powder with an apparent melting
point of 245°C. The compound undergoes loss of water at 120-130°C and
In converted to the bis (tricyclohexyltin) oxide. The technical
product has the following solubilities at 25°C: acetone 0.13 g/100 ml,
benzene 1.6 g/100 ml, carbon tetrachloride 2.8 g/100 ml, chloroform
21.6 g/ml, methanol 3.7 g/100 ml and water < 0.0001 g/100 ml.
Purity
The technical product contains 95-96% tricyclohexyltin hydroxide. It
is formulated as a wettable powder.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
In the studies with rats and dogs, in addition to the parent compound,
dicyclohexyltin oxide and trace amounts of cyclohexylstannoic acid in
organic tin were identified (Smith and Fischer, 1970; Anon, 1970a).
The metabolic fate of the limited amounts of compound absorbed from
the gastrointestinal tract in animals appears to occur by the
sequence:
(C6H11)3 SnOH---> (C6H11)2 SnO---> C6H11SnO2H---> Sn+4
Absorption, distribution and excretion
Two rats received, each in a gelatine capsule, a single oral dose of 5
mg of 119Sn-labelled tricyclohexyltin hydroxide, corresponding to 25
mg/kg body-weight. Essentially quantitative recovery (99.9 and 100
percent) of the total radioactivity was obtained in the excreta
collected for ten days after dosing. Most (75-80 percent) was excreted
in the first four days after administration. Almost all of the
radioactivity occurred in the faeces (97.5 and 98.9 percent versus 1.8
to 2.5 percent in the urine). The practical lack of gastrointestinal
absorption was also confirmed by the absence of radioactivity in the
bile of two guinea pigs, which were administered the labelled compound
and sacrificed, respectively, 24 and 48 hours after dosing (Smith and
Fischer, 1970).
Groups of rats and dogs of both sexes were fed tricyclohexyltin
hydroxide in their diet at rates of 0.75, 3, 6 and 12 mg/kg
body-weight per day, for periods ranging from 45 days to two years.
Tin compounds, primarily the parent compound, were distributed through
the tissues only in trace amounts. As an example: in rats which were
fed 3 mg compound/kg/day, the levels were: fat (0.1 ppm tin) < muscle
< adrenals < heart < brain <liver kidney (0.8 ppm). The biological
half-life of the tin compounds was approximately 5 to 40 days after
compound withdrawal from the diet (Anon, 1970a). Dicyclohexyltin
oxide, trace amounts of cyclohexylstannoic acid, and inorganic tin
were identified as metabolites in rats and dogs. Generally, from 60 to
95 percent of the tin was present as organotin (Smith and Fischer,
1970; Anon, 1970a).
Rats were fed 100 ppm of 119Sn-labelled tricyclohexyltin hydroxide for
90 days, and several tissues were analysed for uptake of 119Sn.
Continuous uptake resulted in an equilibrium being reached between
40-60 days with the females accumulating slightly more than the males.
Tin was eliminated slowly from the tissues upon cessation of feeding,
with the brain being one of the slowest tissues. Traces of tin were
noted after 115 days, although the major quantity was removed in 20
days (Smith and Fischer, 1970).
Effect on enzymes and other biochemical parameters
In a series of pharmacological and biochemical studies, intravenous
injection to dogs and cats resulted in no effect on blood pressure,
pulse rate or cholinesterase levels, and in no potentiation of drugs
(epinphrine chloride, norepinephrine bitartrate, tyramine
monohydrochloride or nicotine sulfate). The primary effect noted on
intravenous injection was stimulation of respiration accompanied by an
increase in blood lactate and a decrease in blood pCO2, without
changes in either pH or pO2 in the blood (Hine at al., 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Japanese Quail
Investigations were started on four groups of birds, each containing
eight to ten females plus an equal number of males. Dietary levels of
0, 1, 10 or 100 ppm of tricyclohexyltin hydroxide were given to the
adults (ten weeks of age) of the first generation, and, afterwards,
through the entire life cycle of the second and the third generation.
Body and egg weights, food-consumption, survival of live chicks from
eggs, adult mortality, general physical condition and appearance and
gross observation of the ovaries and gonads were comparable to the
controls for all dietary levels. Similarly, no gross teratogenic or
lenticular effects were observed in any of the treated groups.
Definite effects on embryonic mortality, egg production, fertility and
hatchability were observed at the 100 ppm dietary levels. At 10 ppm,
there were effects of questionable significance on egg fertility and
hatchability. None of those criteria was affected at the level of 1
ppm (Stevenson and Kenaga, 1969).
Rabbit
Groups of pregnant New Zealand white rabbits, 28 in all, received oral
doses of respectively 0, 0.75 or 3 mg tricyclohexyltin hydroxide/kg
body-weight/day on the eighth to the 16th day inclusive of gestation.
There was no evidence of significant adverse effects due to the
compound as judged by indices of fertility, gestation, viability and
lactation or by examination of the foetuses for teratogenic effects
(Hine at al., 1969b).
Rat
A three generation (two litters per generation) reproduction,
fertility and teratology study was conducted in groups of 10 male and
20 female rats maintained continuously on diets containing 0, 12.5, 50
or 100 ppm of tricyclohexyltin hydroxide (the uppermost dietary level
corresponds to approximately 4 to 6 mg test compound/kg
body-weight/day on the basis of adult and female rats). There was no
evidence of significant adverse effects due to the compound under test
when judged by indices of fertility, gestation, viability and
lactation, or by examination of the foetuses for teratogenic effects
(Hine et al., 1969a).
Special studies on metabolites and photodecomposition products
In addition to having been identified as metabolites in animals (Smith
and Fischer, 1970), dicyclohexyltin oxide and cyclohexylstannoic acid
occur as a relatively minor proportion of the residues on fruit,
having been formed by sunlight degradation (Getzendaner, 1968) (see
'Fate of Residues, in plants').
LD50 values for dicyclohexyltin oxide, as determined in five studies
involving varying strains of rats, averaged 355 mg/kg body-weight
(Anon, 1970c). Cyclohexylstannoic acid exhibits low toxicity to rats
with an LD50 value of < 3 600 mg/kg body-weight (Hine, 1967).
Male and female rats were fed dicyclohexyltin oxide in their diet for
90 days at dose levels of 0, 1, 3 and 6 mg/kg body-weight/day. No
unusual alterations in behaviour and appearance occured during this
study nor were any pharmacodynamic or toxic signs exhibited which
could be related to the feeding of the test compound in the diet. No
compound related variations were found in haematological and
biochemical values, in gross and microscopic examination of tissues
and in organ-weights and ratios (Wazeter et al., 1968).
Acute toxicity
TABLE I
Summary of acute toxicities by various workers
LD50(mg/kg)
Animal Route body-weight Reference
Chick (M) Oral 650 Olson, 1964
Mouse,
Peromyscus Oral 7101/ Kenaga, 1968
Mouse,
Swiss White Oral 10701/ Kenaga, 1968
Rat Oral 5402/ Anon, 1970b
Rat ip 13 Hine et al., 1969a
Guinea pig (M) Oral 780 Olson, 1964
Guinea pig (M) ip 9 Norris, 1968
Rabbit Oral >500,<1000 Olson, 1964
Rabbit ip >126 Norris, 1968
Dog iv 14 Hine et al., 1969a
Cat iv 6 Hine et al., 1969a
1/ Approximate lethal dosage (100 percent deaths)
2/ Average LD50 value from 10 studies in both sexes of three strains
These data reveal that acute toxicity by the oral route is, because of
very poor gastrointestinal absorption, considerably lower than by the
parenteral route.
The compound appears to be principally a depressant of the central
nervous system, but not nearly to the same extent as the triethyltin
acetate in comparable doses. Neither interperitoneal, intravenous nor
oral administration resulted in cerebral oedema or significant
pathologic lesions (Hine at al., 1969).
Death usually occurred within two to seven days after administration.
Acute signs of poisoning in rats include anal wetting, piloerection,
wet nares and mouth, lethargy, hyporeflexia, arching, depressed
respiration, emaciation, diarrhoea and flexure of all four limbs when
picked up by the scruff of the neck. Gross examination of the dead
animals showed that the adrenal gland of rats was 2-3 times heavier
than the controls and the spleen was 0.3-0.4 time heavier than the
controls (Hine, 1966).
Short-term studies
Dog
A total of 96 beagle dogs, half male and half female, averaging eight
months of age, were fed tricyclohexyltin hydroxide in the diet, at
levels adjusted to give ingestion rates of 0, 0.75, 3 or 6 mg/kg
body-weight/day, for two years, or of 12 mg/kg/day for six months.
Animals were sacrificed in each group after 3, 6, 12 or 24 months. All
diets containing the compound under test were rejected by the animals
to some extent during the first stages of the treatment, particularly
at the highest levels. Diets designed to administer 12 mg/kg/day were
very unpalatable; many dogs completely refused diets at this level,
most progressively lost weight and some died of starvation. Ingestion
of 6 mg/kg/day for two years caused no direct toxic effect on tissues,
but did cause an apparent reduction in final body weight, which in
turn affected organs to body-weight ratios, but no pathological
changes were found. At the 3 mg/kg/day dietary level, mortality,
behaviour, food intake, growth rate, gross and microscopic pathology,
haematology and biochemical values were comparable to the controls.
The same conclusion applies to organ-weights and ratios, except for
statistically significant increased heart to body-weight ratio
averages, but not actual heart weights, in both sexes, and average
liver weight and ratio in the males only. These observations were not
associated with significant alterations in these two organs at either
the interim or final necropsy periods or upon microscopic examination.
Copper content in the urine was decreased at all dose levels examined.
A notable undefined effect in dogs was a tanning or brown
discoloration of the serosal surface of the small intestine in dogs
fed 3 and 6 mg/kg levels. This effect was not noted at 0.75 mg/kg.
Measurement of tin content indicated higher levels of tin in all
tissues examined. Levels in the males were lower than in the females
and were proportional in both sexes to the dietary intake. An
equilibrium level was apparently reached between six and 12 weeks of
feeding and remained constant for the remainder of the testing.
Tissues of rats and dogs contained similar concentrations of tin,
except for the liver of dogs, which contained five to 14 times the tin
content of rats and a higher proportion of inorganic tin. No signs of
toxic injury were observed at the level of 0.75 mg/kg (Hine, 1970).
Rabbit
Tricyclohexyltin hydroxide administered to the conjunctival sac
resulted in irritational corneal injury which subsided in one week
(Olson, 1964).
Groups of rabbits (ten male and ton female) were treated dermally at
doses of 0, 1.2, 6, 12 or 60 mg/kg/body-weight/day, five days per week
for three weeks. All animals developed erythema, oedema, atonia,
desquamation and fissuring of the skin. The signs of toxicity were
severe at all done levels examined. Other than dermal problems, no
other effects were noted (Wazeter, 1969).
Rat
Exploratory experiments in rats showed that an oral dose of 12.5 mg/kg
body-weight/day did not produce tissue damage when given daily by
gavage for 19 days. A dose of 25 mg/kg/day was toxic within two weeks,
and doses of 50 and 100 mg/kg/day were toxic within one week. Rats
that received 12 mg/kg body-weight/day for ten or 16 weeks showed no
direct toxic effects. Poor weight gain observed at this dietary level
was judged to be caused by unpalatability of diets containing
tricyclohexyltin hydroxide. Due to diet unpalatability, higher doses
had to be administered by gavage. Direct toxic effects were not
achieved until doses of 25 mg/kg bodyweight/day were administered.
This level resulted in clinical symptoms, such as severe diarrhoea and
weight loss, as well as morphological effects, such as
gastroenteritis, intrahepatic and extrahepatic cholangitis,
degenerative changes in adrenal glands and toxic nephrosis (Tucker,
1966).
Long-term studies
Rat
A total of 720 rats, half males and half females, aged four weeks,
were fed 0, 0.75, 3, 6 or 12 mg tricyclohexyltin hydroxide/kg
body-weight/day for up to two years. None of the dietary levels of
treatment caused any change in behaviour, mortality records,
haematologic and biochemical values, gross appearances and
histological characteristics of organs and tissues. Effects observed
at the 12 mg/kg level wore decreased weight gains in both sexes and
increased organ to body-weight ratios for spleen and liver in the
females. The pattern of tumour incidence appeared to be random and not
suggestive of a dose relationship. The occurrence of many cysts seen
throughout this study at all levels may be related to the particular
strain of animals, although in the females at 12 mg/kg, 17 liver cysts
and five pituitary cysts were noted as compared to one liver cyst and
five pituitary cysts in the controls. At six mg/kg, only a slight
decrease of food intake and a related mild depression of growth rate
were observed during the first three months on treatment. These
effects were absent at the level of 3 mg/kg (Hine, 1970a).
OBSERVATIONS IN MAN
A wettable powder formulation (50 percent tricyclohexyltin hydroxide)
was examined for its irritation and sensitization potential to human
subjects. No adverse reactions were observed in 53 females following
sensitization applications and a challenge application 20 days later
of 0.5 ml of a 0.1 percent emulsion. Tricyclohexyltin hydroxide was
not dermally irritating at a concentration of about 0.01 mg/kg
body-weight (Laker et al., 1966).
COMMENT
The Meeting expressed concern that the only toxicological data that
had been presented for consideration were unpublished, and had
therefore not already been subject to critical scrutiny by the general
scientific community.
A difficulty with the toxicological evaluation of this compound is
that its presence made laboratory diets unpalatable. A need for
carefully controlled paired-feeding experiments was therefore evident.
Concern was expressed that residual tin remained in the tissues of
exposed animals for prolonged periods and that brown discoloration of
the serosal surface of the small intestine occurred in dogs fed 3
mg/kg body-weight/day or higher dose levels of the compound. It was
noted that, in dogs fed 0.75 mg/kg body-weight/day or more, there was
a reduction in urinary copper output. In view of this observation, the
need for information on the copper content of the liver of exposed
animals was stressed. The observation of an increased incidence of
cysts of the liver and pituitary gland of female rats fed 12 mg/kg
body-weight/day was regarded with some concern. For these reasons the
Committee considered that only a temporary acceptable daily intake
could be established.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: level in the diet adjusted to give 0.75 mg/kg body-weight/day
Rat: level in the diet adjusted to give 3 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0075 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Tricyclohexyltin hydroxide is a non-systemic acaricide used or under
development for use in several countries to control phytophagous mites
on apple and pear trees. The compound exhibits little or no activity
against insects,including pollinating bees,and it is relatively
harmless to predatory mites. Thus it may be advantageously applied in
integrated control schemes (Gray, 1968; Zambelli et al., 1968; Jeppson
et al., 1968). Typically, tricyclohexyltin hydroxide is recommended
for use in dilute sprays at concentrations of 15 to 30 g/100 litres,
the total amount applied per unit area ranging from 0.42 to 1.68
kg/hectare.
In addition to use on apples and pears, tricyclohexyltin hydroxide is
expected to prove useful for control of mites on stone fruits, citrus,
grapes and certain other crops.
Post-harvest treatments
Post-harvest use on apples is under investigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following typical data (Table II) are extracted from unpublished
reports supplied to FAO/WHO by Dow Chemical Company (Getzendaner,
1968; Komblas, 1969; Getzendaner and Corbin, 1969; Anon, 1970b).
FATE OF RESIDUES
General comments
Tricyclohexyltin hydroxide is decomposed by sunlight as evidenced by
(1) a half-life of 8 "sunny days" for disappearance from glass slides
(Smith et al., 1970c) and (2) detection of inorganic tin as
approximately 15 to 30 or more percent of the total tin residues on
fruit exposed to 3 or 4 applications of the miticide (Getzendaner and
Corbin, 1969).
TABLE II
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 15 1 0 0.8 -
8 1.0 -
16 0.6 -
32 0.5 -
TABLE II (cont'd)
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 23 4 0 1.7 2.5
14 1.6 2.2
28 1.1 1.5
35 0.9 1.0
Netherlands 25 4 0 1.2 1.9
14 1.2 1.6
28 0.5 0.7
42 0.5 0.7
Italy 30 2 7 1.1 1.3
16 1.2 1.3
28 0.8 1.0
U.S.A. 30 3 17 1.9 -
Australia 30 3 0 - 1.94
France 30 1 0 0.7 1.8
15 0.5 0.5
42 0.4 0.4
Japan 33 3 0 2.0 -
14 1.5 -
30 1.1 -
PEARS
U.S.A. 23 3 0 1.4 2.1
15 1.1 1.4
29 0.7 1.0
42 0.6 0.6
61 0.4 0.6
Australia 23 1 31 - 1.0
Italy 30 2 7 1.0 1.5
16 0.8 1.0
28 0.4 0.6
Photodegradation occurs by the sequence, which is identical with that
owing in the animal body:
Other relevant chemical properties
The compound in a white, crystalline powder with an apparent melting
point of 245°C. The compound undergoes loss of water at 120-130°C and
In converted to the bis (tricyclohexyltin) oxide. The technical
product has the following solubilities at 25°C: acetone 0.13 g/100 ml,
benzene 1.6 g/100 ml, carbon tetrachloride 2.8 g/100 ml, chloroform
21.6 g/ml, methanol 3.7 g/100 ml and water < 0.0001 g/100 ml.
Purity
The technical product contains 95-96% tricyclohexyltin hydroxide. It
is formulated as a wettable powder.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
In the studies with rats and dogs, in addition to the parent compound,
dicyclohexyltin oxide and trace amounts of cyclohexylstannoic acid in
organic tin were identified (Smith and Fischer, 1970; Anon, 1970a).
The metabolic fate of the limited amounts of compound absorbed from
the gastrointestinal tract in animals appears to occur by the
sequence:
(C6H11)3 SnOH---> (C6H11)2 SnO---> C6H11SnO2H---> Sn+4
Absorption, distribution and excretion
Two rats received, each in a gelatine capsule, a single oral dose of 5
mg of 119Sn-labelled tricyclohexyltin hydroxide, corresponding to 25
mg/kg body-weight. Essentially quantitative recovery (99.9 and 100
percent) of the total radioactivity was obtained in the excreta
collected for ten days after dosing. Most (75-80 percent) was excreted
in the first four days after administration. Almost all of the
radioactivity occurred in the faeces (97.5 and 98.9 percent versus 1.8
to 2.5 percent in the urine). The practical lack of gastrointestinal
absorption was also confirmed by the absence of radioactivity in the
bile of two guinea pigs, which were administered the labelled compound
and sacrificed, respectively, 24 and 48 hours after dosing (Smith and
Fischer, 1970).
Groups of rats and dogs of both sexes were fed tricyclohexyltin
hydroxide in their diet at rates of 0.75, 3, 6 and 12 mg/kg
body-weight per day, for periods ranging from 45 days to two years.
Tin compounds, primarily the parent compound, were distributed through
the tissues only in trace amounts. As an example: in rats which were
fed 3 mg compound/kg/day, the levels were: fat (0.1 ppm tin) < muscle
< adrenals < heart < brain <liver kidney (0.8 ppm). The biological
half-life of the tin compounds was approximately 5 to 40 days after
compound withdrawal from the diet (Anon, 1970a). Dicyclohexyltin
oxide, trace amounts of cyclohexylstannoic acid, and inorganic tin
were identified as metabolites in rats and dogs. Generally, from 60 to
95 percent of the tin was present as organotin (Smith and Fischer,
1970; Anon, 1970a).
Rats were fed 100 ppm of 119Sn-labelled tricyclohexyltin hydroxide for
90 days, and several tissues were analysed for uptake of 119Sn.
Continuous uptake resulted in an equilibrium being reached between
40-60 days with the females accumulating slightly more than the males.
Tin was eliminated slowly from the tissues upon cessation of feeding,
with the brain being one of the slowest tissues. Traces of tin were
noted after 115 days, although the major quantity was removed in 20
days (Smith and Fischer, 1970).
Effect on enzymes and other biochemical parameters
In a series of pharmacological and biochemical studies, intravenous
injection to dogs and cats resulted in no effect on blood pressure,
pulse rate or cholinesterase levels, and in no potentiation of drugs
(epinphrine chloride, norepinephrine bitartrate, tyramine
monohydrochloride or nicotine sulfate). The primary effect noted on
intravenous injection was stimulation of respiration accompanied by an
increase in blood lactate and a decrease in blood pCO2, without
changes in either pH or pO2 in the blood (Hine at al., 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Japanese Quail
Investigations were started on four groups of birds, each containing
eight to ten females plus an equal number of males. Dietary levels of
0, 1, 10 or 100 ppm of tricyclohexyltin hydroxide were given to the
adults (ten weeks of age) of the first generation, and, afterwards,
through the entire life cycle of the second and the third generation.
Body and egg weights, food-consumption, survival of live chicks from
eggs, adult mortality, general physical condition and appearance and
gross observation of the ovaries and gonads were comparable to the
controls for all dietary levels. Similarly, no gross teratogenic or
lenticular effects were observed in any of the treated groups.
Definite effects on embryonic mortality, egg production, fertility and
hatchability were observed at the 100 ppm dietary levels. At 10 ppm,
there were effects of questionable significance on egg fertility and
hatchability. None of those criteria was affected at the level of 1
ppm (Stevenson and Kenaga, 1969).
Rabbit
Groups of pregnant New Zealand white rabbits, 28 in all, received oral
doses of respectively 0, 0.75 or 3 mg tricyclohexyltin hydroxide/kg
body-weight/day on the eighth to the 16th day inclusive of gestation.
There was no evidence of significant adverse effects due to the
compound as judged by indices of fertility, gestation, viability and
lactation or by examination of the foetuses for teratogenic effects
(Hine at al., 1969b).
Rat
A three generation (two litters per generation) reproduction,
fertility and teratology study was conducted in groups of 10 male and
20 female rats maintained continuously on diets containing 0, 12.5, 50
or 100 ppm of tricyclohexyltin hydroxide (the uppermost dietary level
corresponds to approximately 4 to 6 mg test compound/kg
body-weight/day on the basis of adult and female rats). There was no
evidence of significant adverse effects due to the compound under test
when judged by indices of fertility, gestation, viability and
lactation, or by examination of the foetuses for teratogenic effects
(Hine et al., 1969a).
Special studies on metabolites and photodecomposition products
In addition to having been identified as metabolites in animals (Smith
and Fischer, 1970), dicyclohexyltin oxide and cyclohexylstannoic acid
occur as a relatively minor proportion of the residues on fruit,
having been formed by sunlight degradation (Getzendaner, 1968) (see
'Fate of Residues, in plants').
LD50 values for dicyclohexyltin oxide, as determined in five studies
involving varying strains of rats, averaged 355 mg/kg body-weight
(Anon, 1970c). Cyclohexylstannoic acid exhibits low toxicity to rats
with an LD50 value of < 3 600 mg/kg body-weight (Hine, 1967).
Male and female rats were fed dicyclohexyltin oxide in their diet for
90 days at dose levels of 0, 1, 3 and 6 mg/kg body-weight/day. No
unusual alterations in behaviour and appearance occured during this
study nor were any pharmacodynamic or toxic signs exhibited which
could be related to the feeding of the test compound in the diet. No
compound related variations were found in haematological and
biochemical values, in gross and microscopic examination of tissues
and in organ-weights and ratios (Wazeter et al., 1968).
Acute toxicity
TABLE I
Summary of acute toxicities by various workers
LD50(mg/kg)
Animal Route body-weight Reference
Chick (M) Oral 650 Olson, 1964
Mouse,
Peromyscus Oral 7101/ Kenaga, 1968
Mouse,
Swiss White Oral 10701/ Kenaga, 1968
Rat Oral 5402/ Anon, 1970b
Rat ip 13 Hine et al., 1969a
Guinea pig (M) Oral 780 Olson, 1964
Guinea pig (M) ip 9 Norris, 1968
Rabbit Oral >500,<1000 Olson, 1964
Rabbit ip >126 Norris, 1968
Dog iv 14 Hine et al., 1969a
Cat iv 6 Hine et al., 1969a
1/ Approximate lethal dosage (100 percent deaths)
2/ Average LD50 value from 10 studies in both sexes of three strains
These data reveal that acute toxicity by the oral route is, because of
very poor gastrointestinal absorption, considerably lower than by the
parenteral route.
The compound appears to be principally a depressant of the central
nervous system, but not nearly to the same extent as the triethyltin
acetate in comparable doses. Neither interperitoneal, intravenous nor
oral administration resulted in cerebral oedema or significant
pathologic lesions (Hine at al., 1969).
Death usually occurred within two to seven days after administration.
Acute signs of poisoning in rats include anal wetting, piloerection,
wet nares and mouth, lethargy, hyporeflexia, arching, depressed
respiration, emaciation, diarrhoea and flexure of all four limbs when
picked up by the scruff of the neck. Gross examination of the dead
animals showed that the adrenal gland of rats was 2-3 times heavier
than the controls and the spleen was 0.3-0.4 time heavier than the
controls (Hine, 1966).
Short-term studies
Dog
A total of 96 beagle dogs, half male and half female, averaging eight
months of age, were fed tricyclohexyltin hydroxide in the diet, at
levels adjusted to give ingestion rates of 0, 0.75, 3 or 6 mg/kg
body-weight/day, for two years, or of 12 mg/kg/day for six months.
Animals were sacrificed in each group after 3, 6, 12 or 24 months. All
diets containing the compound under test were rejected by the animals
to some extent during the first stages of the treatment, particularly
at the highest levels. Diets designed to administer 12 mg/kg/day were
very unpalatable; many dogs completely refused diets at this level,
most progressively lost weight and some died of starvation. Ingestion
of 6 mg/kg/day for two years caused no direct toxic effect on tissues,
but did cause an apparent reduction in final body weight, which in
turn affected organs to body-weight ratios, but no pathological
changes were found. At the 3 mg/kg/day dietary level, mortality,
behaviour, food intake, growth rate, gross and microscopic pathology,
haematology and biochemical values were comparable to the controls.
The same conclusion applies to organ-weights and ratios, except for
statistically significant increased heart to body-weight ratio
averages, but not actual heart weights, in both sexes, and average
liver weight and ratio in the males only. These observations were not
associated with significant alterations in these two organs at either
the interim or final necropsy periods or upon microscopic examination.
Copper content in the urine was decreased at all dose levels examined.
A notable undefined effect in dogs was a tanning or brown
discoloration of the serosal surface of the small intestine in dogs
fed 3 and 6 mg/kg levels. This effect was not noted at 0.75 mg/kg.
Measurement of tin content indicated higher levels of tin in all
tissues examined. Levels in the males were lower than in the females
and were proportional in both sexes to the dietary intake. An
equilibrium level was apparently reached between six and 12 weeks of
feeding and remained constant for the remainder of the testing.
Tissues of rats and dogs contained similar concentrations of tin,
except for the liver of dogs, which contained five to 14 times the tin
content of rats and a higher proportion of inorganic tin. No signs of
toxic injury were observed at the level of 0.75 mg/kg (Hine, 1970).
Rabbit
Tricyclohexyltin hydroxide administered to the conjunctival sac
resulted in irritational corneal injury which subsided in one week
(Olson, 1964).
Groups of rabbits (ten male and ton female) were treated dermally at
doses of 0, 1.2, 6, 12 or 60 mg/kg/body-weight/day, five days per week
for three weeks. All animals developed erythema, oedema, atonia,
desquamation and fissuring of the skin. The signs of toxicity were
severe at all done levels examined. Other than dermal problems, no
other effects were noted (Wazeter, 1969).
Rat
Exploratory experiments in rats showed that an oral dose of 12.5 mg/kg
body-weight/day did not produce tissue damage when given daily by
gavage for 19 days. A dose of 25 mg/kg/day was toxic within two weeks,
and doses of 50 and 100 mg/kg/day were toxic within one week. Rats
that received 12 mg/kg body-weight/day for ten or 16 weeks showed no
direct toxic effects. Poor weight gain observed at this dietary level
was judged to be caused by unpalatability of diets containing
tricyclohexyltin hydroxide. Due to diet unpalatability, higher doses
had to be administered by gavage. Direct toxic effects were not
achieved until doses of 25 mg/kg bodyweight/day were administered.
This level resulted in clinical symptoms, such as severe diarrhoea and
weight loss, as well as morphological effects, such as
gastroenteritis, intrahepatic and extrahepatic cholangitis,
degenerative changes in adrenal glands and toxic nephrosis (Tucker,
1966).
Long-term studies
Rat
A total of 720 rats, half males and half females, aged four weeks,
were fed 0, 0.75, 3, 6 or 12 mg tricyclohexyltin hydroxide/kg
body-weight/day for up to two years. None of the dietary levels of
treatment caused any change in behaviour, mortality records,
haematologic and biochemical values, gross appearances and
histological characteristics of organs and tissues. Effects observed
at the 12 mg/kg level wore decreased weight gains in both sexes and
increased organ to body-weight ratios for spleen and liver in the
females. The pattern of tumour incidence appeared to be random and not
suggestive of a dose relationship. The occurrence of many cysts seen
throughout this study at all levels may be related to the particular
strain of animals, although in the females at 12 mg/kg, 17 liver cysts
and five pituitary cysts were noted as compared to one liver cyst and
five pituitary cysts in the controls. At six mg/kg, only a slight
decrease of food intake and a related mild depression of growth rate
were observed during the first three months on treatment. These
effects were absent at the level of 3 mg/kg (Hine, 1970a).
OBSERVATIONS IN MAN
A wettable powder formulation (50 percent tricyclohexyltin hydroxide)
was examined for its irritation and sensitization potential to human
subjects. No adverse reactions were observed in 53 females following
sensitization applications and a challenge application 20 days later
of 0.5 ml of a 0.1 percent emulsion. Tricyclohexyltin hydroxide was
not dermally irritating at a concentration of about 0.01 mg/kg
body-weight (Laker et al., 1966).
COMMENT
The Meeting expressed concern that the only toxicological data that
had been presented for consideration were unpublished, and had
therefore not already been subject to critical scrutiny by the general
scientific community.
A difficulty with the toxicological evaluation of this compound is
that its presence made laboratory diets unpalatable. A need for
carefully controlled paired-feeding experiments was therefore evident.
Concern was expressed that residual tin remained in the tissues of
exposed animals for prolonged periods and that brown discoloration of
the serosal surface of the small intestine occurred in dogs fed 3
mg/kg body-weight/day or higher dose levels of the compound. It was
noted that, in dogs fed 0.75 mg/kg body-weight/day or more, there was
a reduction in urinary copper output. In view of this observation, the
need for information on the copper content of the liver of exposed
animals was stressed. The observation of an increased incidence of
cysts of the liver and pituitary gland of female rats fed 12 mg/kg
body-weight/day was regarded with some concern. For these reasons the
Committee considered that only a temporary acceptable daily intake
could be established.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: level in the diet adjusted to give 0.75 mg/kg body-weight/day
Rat: level in the diet adjusted to give 3 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0075 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Tricyclohexyltin hydroxide is a non-systemic acaricide used or under
development for use in several countries to control phytophagous mites
on apple and pear trees. The compound exhibits little or no activity
against insects,including pollinating bees,and it is relatively
harmless to predatory mites. Thus it may be advantageously applied in
integrated control schemes (Gray, 1968; Zambelli et al., 1968; Jeppson
et al., 1968). Typically, tricyclohexyltin hydroxide is recommended
for use in dilute sprays at concentrations of 15 to 30 g/100 litres,
the total amount applied per unit area ranging from 0.42 to 1.68
kg/hectare.
In addition to use on apples and pears, tricyclohexyltin hydroxide is
expected to prove useful for control of mites on stone fruits, citrus,
grapes and certain other crops.
Post-harvest treatments
Post-harvest use on apples is under investigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following typical data (Table II) are extracted from unpublished
reports supplied to FAO/WHO by Dow Chemical Company (Getzendaner,
1968; Komblas, 1969; Getzendaner and Corbin, 1969; Anon, 1970b).
FATE OF RESIDUES
General comments
Tricyclohexyltin hydroxide is decomposed by sunlight as evidenced by
(1) a half-life of 8 "sunny days" for disappearance from glass slides
(Smith et al., 1970c) and (2) detection of inorganic tin as
approximately 15 to 30 or more percent of the total tin residues on
fruit exposed to 3 or 4 applications of the miticide (Getzendaner and
Corbin, 1969).
TABLE II
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 15 1 0 0.8 -
8 1.0 -
16 0.6 -
32 0.5 -
TABLE II (cont'd)
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 23 4 0 1.7 2.5
14 1.6 2.2
28 1.1 1.5
35 0.9 1.0
Netherlands 25 4 0 1.2 1.9
14 1.2 1.6
28 0.5 0.7
42 0.5 0.7
Italy 30 2 7 1.1 1.3
16 1.2 1.3
28 0.8 1.0
U.S.A. 30 3 17 1.9 -
Australia 30 3 0 - 1.94
France 30 1 0 0.7 1.8
15 0.5 0.5
42 0.4 0.4
Japan 33 3 0 2.0 -
14 1.5 -
30 1.1 -
PEARS
U.S.A. 23 3 0 1.4 2.1
15 1.1 1.4
29 0.7 1.0
42 0.6 0.6
61 0.4 0.6
Australia 23 1 31 - 1.0
Italy 30 2 7 1.0 1.5
16 0.8 1.0
28 0.4 0.6
Photodegradation occurs by the sequence, which is identical with that
owing in the animal body:
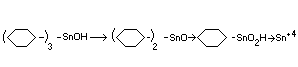 Tricyclohexyltin hydroxide is not volatile from dry surfaces, but
'co-distils', with water from moist surfaces at a slow but finite rate
(Smith et al., 1970). Field experiments show that the half-life of the
compound is about four days on grass and about three weeks on apples
and pears; the disappearance results from photodegradation,
'co-distillation' with water from moist surfaces and weathering
factors (Getzendaner and Gentry, 1970a; Getzendaner and Corbin, 1969).
In animals
As indicated under "BIOCHEMICAL ASPECTS", tricyclohexyltin hydroxide
is not readily absorbed from the gastrointestinal tract.
When the miticide is fed daily to rats, dogs or cattle, tin compounds,
primarily unchanged tricyclohexyltin hydroxide, accumulate in the
tissues to a limited extent, reach equilibrium values in a few months
to a year, and are slowly eliminated from the tissues after the
compound is withdrawn from the diet. It does not selectively partition
into fat, and the distribution pattern in tissues is similar for rats,
dogs and cattle (Smith and Fischer, 1970; Anon, 1970a; Getzendaner and
Gentry, 1970b). For example, tissues from calves fed 30 ppm of the
compound in the diet for 90 days showed 0.07 ppm total tin in fat,
0.09 ppm in muscle, 0.37 ppm in liver and 0.20 ppm in kidney
(Getzendaner and Gentry, 1970b).
Levels of tricyclohexyltin hydroxide equivalent were 0.05 ppm in milk
and 0.4 ppm in cream obtained from cows fed 100 ppm of the compound in
the diet for 53 days (Getzendaner and Gentry, 1970c).
In plants
Residues occurring on apples were identified by a series of extraction
techniques followed by analysis. Tricyclohexyltin hydroxide was the
main component of the tin residues, comprising 67% (0.66 ppm) of the
residue (eight days post-treatment). Small amounts of dicyclohexyltin
oxide and cyclohexylstannoic acid were present (5% of each), as well
as 23% inorganic tin (Getzendaner, 1968).
Tricyclohexyltin hydroxide and its sunlight decomposition products are
not translocated in plants to a significant extent. This has been
demonstrated by (1) the absence of significant residues of tin
compounds in the interior portions of fruit, even after periods of
several months between application of the first of up to 5 spray
applications and harvest (Getzendaner and Corbin, 1969), (2) the
absence of radioactive residues in bean and maize plants grown in soil
treated with exaggerated amounts (3 lb/acre) of 119Sn-labelled
miticide or its degradation (Smith at al. 1970), and (3) the essential
absence of translocation across the narrow thickness of the leaf of
cotton plants as determined by bioassay experiments (Allison, 1969).
In soil
Tricyclohexyltin hydroxide is markedly absorbed by soil and it is not
leached from soil, as demonstrated by soil-column leaching studies and
by study of the transfer from water to soil (Smith et al., 1970a,
Smith and Taylor, 1970; Whitney, 1966). No significant amounts of the
miticide will remain in or on the soil from one growing season to the
next.
In storage and processing
Results of several residue trials, involving separate analysis of peel
and pulp or surface extraction, indicate that at least 90% of the
residue in or on apples and pears is on the surface of the fruit
(Getzendaner, 1968; Komblas, 1969; Getzendaner and Corbin, 1969).
Typically, about 0.1 ppm tricyclohexyltin hydroxide equivalent may be
expected in fruit when the peeling is removed. Mild water washing
removed up to 50% of the residue from apples and pears with an average
of >20% removed (Getzendaner and Corbin, 1969).
Evidence of residues in food in commerce or at consumption
No information is available.
METHODS OF RESIDUE ANALYSIS
Corbin (1970) recently published a method suitable for determination
of the total organotin and inorganic tin present in fruit The sample
is subjected to a wet oxidation treatment with sulfuric and nitric
acids to destroy organic matter, remove volatile acids and halogens
and convert the tin to a soluble inorganic form. The tin is then
separated from elements which would interfere in the colorimetric
method (heavy metals) and from non-interfering salts. This separation
involves extracting tin as SnI4, with n-hexane from a strong sulfuric
solution of KI. Evaporation of hexane and conversion to sulfate leaves
the tin in a form measurable by a dithiol colorimetric method.
Sensitivity of the method is 0.01 ppm. Optional steps are included in
the method for removal of arsenic and antimony, if these elements are
suspected of being present in amounts which might cause interference
(> 0.15 mg and > 2 mg, respectively).
A complementary method has been developed for determining residues of
organotin in fruit by M & T Chemicals (Anon, 1969). Tri-, di-, and
monocyclohexyltin are solubilized by treatment of the fruit sample
with hydrochloric acid (9 molar) and then extracted with chloroform
from inorganic tin and heavy metals, which remain in the acidic
aqueous phase. The organotin compounds are then oxidized and
determined colorimetrically.
NATIONAL TOLERANCES
Tolerances have been established (provisional) or are proposed for
residues of tricyclohexyltin hydroxide in or on apples and pears in
various countries including the following:
Country Tolerance Withdrawal between
ppm treatment and harvest, days
Australia1 3 2
Belgium 12 28
Italy 1 20
Israel 1 30
Netherlands 1 28
U.S.A. 23 14
1 Stone fruits
2 Proposed
3 Tolerance proposed on 23 June 1970
Use of the miticide is registered for apples and pears in Chile,
France and Korea, but without provision for tolerance.
APPRAISAL
Tricyclohexyltin hydroxide is a non-systematic acaricide now used in
many countries and likely to be used worldwide on apples and pears,
with indications of future use on citrus, stone fruits and other
crops. Post-harvest use on apples is under investigation. The compound
is relatively harmless to beneficial insects and mites, and it is
usefully applied to integrated control schemes. Tricyclohexyltin
hydroxide is used as wettable powder at a rate of about 0.5 to 1.5 kg
per hectare.
Comprehensive residue data on tricyclohexyltin hydroxide on apples and
pears are available from the United States and from five other
countries, which are consistent with those from the U.S.A. Residue
data on other crops were not available to the Meeting. When apples and
pears are peeled, most of the tricyclohexyltin hydroxide residue is
removed; only 0.1 ppm may be expected in the fruit flesh. Washing is
found to remove 20-50% of the residues, more from apples than from
pears.
Field experiments show that the residues of tricyclohexyltin hydroxide
declined to half in about three weeks on apples and pears. The
disappearance is due to photodegradation, codistillation with water
and weathering factors. The residues are found to occur on the surface
of the treated plants. The degradation of tricyclohexyltin hydroxide
is found to produce dicyclohexyltin oxide and monocyclohexylstannoic
acid and finally inorganic tin salts.
The absorption of tricyclohexyltin hydroxide from the gastrointestinal
tract of animals is small. The distribution patterns of the compound
are determined in rats, dogs and cattle. When cows were fed residues
of 30 and 100 ppm, the residues found in milk were less than 0.1 ppm
and in various tissues less than 0.5 ppm. Limited feeding of apple and
pear pulp to cows is not expected to produce detectable residues in
meat or milk.
A colorimetric method with a detectability of 0.01 ppm tin is
available to determine tricyclohexyltin hydroxide and its organic
degradation products. The method is not able to distinguish between
the various compounds,but reflects the total amount of the compounds
in the sample analyzed.
RECOMMENDATIONS FOR TEMPORARY TOLERANCE
The tolerance is expressed in terms of tricyclohexyltin hydroxide. The
organic degradation products and the inorganic tin salts derived from
tricyclohexyltin hydroxide are not included in the tolerance. A
temporary tolerance of 2 ppm, based on an interval of two weeks from
the last treatment to the harvest, is recommended for apples and
pears.
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Further studies on the effects of exposure to tricyclohexyltin
hydroxide on copper balance and, in particular, on the copper
content of the liver.
2. Further information on the occurrence of liver and pituitary gland
cysts in female rate fed the compound.
3. Further information on the effect of the compound on the rate of
body-weight gain after allowing for the unpalatability of test
diets.
4. Further information on the significance of the brown discoloration
of the serosal surface of the intestine of treated animals.
5. Establishment of analytical procedures capable of distinguishing
qualitatively between tricyclohexyltin compounds and other
organotin compounds, particularly the fentin compounds, and, where
possible, of quantitative measurement of the separate compounds.
DESIRABLE
Data on residues of tricyclohexyltin hydroxide on apples and pears
moving in international commerce.
REFERENCES
Allison, W.E. (1969) An evaluation of the systemic (soil and foliar)
acaricidal activity of tricyclohexyltin hydroxide. Unpublished report
from the Dow Chemical Co.
Anon. (1969) Determination of small amounts of organotin in macerated
fruit. Method No. TA-2-2. Unpublished report from M & T Chemicals,
Inc.
Anon. (1970a) Tin content of tissues of rats and dogs fed varying
amounts of tricyclohexyltin hydroxide in the daily diet for two years.
Unpublished report from the Dow Chemical Co.
Anon. (1970b) Compilation of studies on residues of tricyclohexyltin
hydroxide in or on apples and pears: Australia, France, Netherlands,
and Japan. Unpublished report
Anon. (1970c) Dicyclohexyltin oxide: summary of acute oral toxicity
tests. Unpublished report from the Dow Chemical Company
Corbin, H.B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Offic. Anal.
Chemists, 53: 140-146
Getzendaner, M.E. (1968) A review of residue information on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Getzendaner, M.E. and Corbin, H.A. (1969) Residue study: inorganic
and organic tin compounds in or on apples and pears from field
applications of PLICTRAN(R) miticide. Unpublished report from the Dow
Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970a) Determination of tin
residues in orchard grass exposed to tricyclohexyltin hydroxide
miticide. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970b) Determination of tin
residues in beef calves fed tricyclohexyltin hydroxide for ninety
days. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970c) Determination of tin
residues in milk, cream, and body tissue of dairy cows fed
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Gray, H.E. (1968) PLICTRAN miticide - new approach to mite control.
Down to Earth, 23 (4): 3-5
Hine, C.H. (1966) Acute oral toxicity of tricyclohexyltin hydroxide in
Long Evans strain rats (17 March 1966). Unpublished report from the
Hine Laboratories, Inc. to Dow Chemical Co.
Hine, C.H. (1967) Acute oral toxicity: Cyclohexylstannoic-acid,
dicyclohexyltin oxide, tetracyclohexyltin, hexacyclohexylditin.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Brownlow, E.K., Cummins, J.T., Eisenlord, G.H. and Wong,
L.C.K. (1969) Studies on the mechanism of action of DOWCO 213.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969a) DOWCO 213
reproduction and teratology studies in rats. Unpublished report from
the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969b) DOWCO 213
reproduction and teratology studies in rabbits. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970a) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in dogs. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970b) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in rats. Unpublished report
from the Hine Laboratories, Inc.
Jeppson, L.R., Jesser, M.J. and Complin, J.O. (1968) Responses of the
pacific spider mite and the citrus red mite to laboratory and field
applications of tricyclohexyltin hydroxide. J. Econ. Entomology, 61:
1502-1505
Kenaga, E.E. (1968) The rodent toxicity and repellency of
tricyclohexyltin compounds to two species of mice. Unpublished report
from the Dow Chemical Co.
Komblas and Kostas N. (1969) Determination of residues of PLICTRAN
acaricide on apples and pears in Italy. (Translation of report of
Societá Italo-Americana Prodotti Antiparassitari, Balliera/Bologna,
Italy). Unpublished report from the Dow Chemical Co.
Laker, T.L., Elsea, J.R. and Ede, M. (1966) Repeated insult patch test
on M-2527 and cloth material TE98-SEU3501. Unpublished report from
Hill Top Research, Inc.
Norris, J.M. (1968) Toxicity of tricyclohexyltin hydroxide via i.p.
route in laboratory animals. Unpublished report from the Dow Chemical
Co.
Olson, K.J. (1964) Results of range finding toxicological tests on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Smith, G.N., Axelson, R.J. and Schiesser, L.H. (1970) The loss of
PLICTRAN(R) miticide from various soils. Unpublished report from the
Dow Chemical Co.
Smith, G.N. and Fischer, F.S. (1970) Metabolism of PLICTRAN(R)
miticide. Observations on the absorption, distribution, and excretion
of tricyclohexyltin-Sn119 hydroxide in white rats. Unpublished report
from the Dow Chemical Co.
Smith, G.N., Fischer, F.S. and Axelson, R.J. (1970) The volatilization
and photodecomposition of PLICTRAN(R) miticide. Unpublished report
from the Dow Chemical Co.
Smith, G.N. and Taylor, Y.S. (1970) The absorption and translocation
of PLICTRAN miticide and its decomposition products in bean and corn
plants. Unpublished report from the Dow Chemical Co.
Stevenson, G.T. and Kenaga, E.E. (1969) The effects of dietary feeding
of tricyclohexyltin hydroxide on the life stages of the Japanese
Quail. Unpublished report from the Dow Chemical Co.
Tucker, W.E. (1966) Oral toxicity studies of tricyclohexyltin
hydroxide in rats. Unpublished data from the Dow Chemical Co.
Wazeter, F.X., Buller, R.H., Geil, R.G. and Long, J.E. (1968)
Dicyclohexyltin oxide: 90-day feeding study in the Long-Evans rat.
Unpublished report from the International Research and Development
Corporation
Wazeter, F.X. (1969) 21-day dermal study in,albino rabbits-PLICTRAN
M-3180 miticide. Unpublished report from the International Research
and Development Corporation
Whitney, W.K. (1966) Leaching tests with DOWCO 213 in soil
Zambelli, N., Komblas, W. and Kovacs, A. (1968) PLICTRAN miticide for
the control of spider mites in Italy. Down to Earth, 24(3): 25-31
Tricyclohexyltin hydroxide is not volatile from dry surfaces, but
'co-distils', with water from moist surfaces at a slow but finite rate
(Smith et al., 1970). Field experiments show that the half-life of the
compound is about four days on grass and about three weeks on apples
and pears; the disappearance results from photodegradation,
'co-distillation' with water from moist surfaces and weathering
factors (Getzendaner and Gentry, 1970a; Getzendaner and Corbin, 1969).
In animals
As indicated under "BIOCHEMICAL ASPECTS", tricyclohexyltin hydroxide
is not readily absorbed from the gastrointestinal tract.
When the miticide is fed daily to rats, dogs or cattle, tin compounds,
primarily unchanged tricyclohexyltin hydroxide, accumulate in the
tissues to a limited extent, reach equilibrium values in a few months
to a year, and are slowly eliminated from the tissues after the
compound is withdrawn from the diet. It does not selectively partition
into fat, and the distribution pattern in tissues is similar for rats,
dogs and cattle (Smith and Fischer, 1970; Anon, 1970a; Getzendaner and
Gentry, 1970b). For example, tissues from calves fed 30 ppm of the
compound in the diet for 90 days showed 0.07 ppm total tin in fat,
0.09 ppm in muscle, 0.37 ppm in liver and 0.20 ppm in kidney
(Getzendaner and Gentry, 1970b).
Levels of tricyclohexyltin hydroxide equivalent were 0.05 ppm in milk
and 0.4 ppm in cream obtained from cows fed 100 ppm of the compound in
the diet for 53 days (Getzendaner and Gentry, 1970c).
In plants
Residues occurring on apples were identified by a series of extraction
techniques followed by analysis. Tricyclohexyltin hydroxide was the
main component of the tin residues, comprising 67% (0.66 ppm) of the
residue (eight days post-treatment). Small amounts of dicyclohexyltin
oxide and cyclohexylstannoic acid were present (5% of each), as well
as 23% inorganic tin (Getzendaner, 1968).
Tricyclohexyltin hydroxide and its sunlight decomposition products are
not translocated in plants to a significant extent. This has been
demonstrated by (1) the absence of significant residues of tin
compounds in the interior portions of fruit, even after periods of
several months between application of the first of up to 5 spray
applications and harvest (Getzendaner and Corbin, 1969), (2) the
absence of radioactive residues in bean and maize plants grown in soil
treated with exaggerated amounts (3 lb/acre) of 119Sn-labelled
miticide or its degradation (Smith at al. 1970), and (3) the essential
absence of translocation across the narrow thickness of the leaf of
cotton plants as determined by bioassay experiments (Allison, 1969).
In soil
Tricyclohexyltin hydroxide is markedly absorbed by soil and it is not
leached from soil, as demonstrated by soil-column leaching studies and
by study of the transfer from water to soil (Smith et al., 1970a,
Smith and Taylor, 1970; Whitney, 1966). No significant amounts of the
miticide will remain in or on the soil from one growing season to the
next.
In storage and processing
Results of several residue trials, involving separate analysis of peel
and pulp or surface extraction, indicate that at least 90% of the
residue in or on apples and pears is on the surface of the fruit
(Getzendaner, 1968; Komblas, 1969; Getzendaner and Corbin, 1969).
Typically, about 0.1 ppm tricyclohexyltin hydroxide equivalent may be
expected in fruit when the peeling is removed. Mild water washing
removed up to 50% of the residue from apples and pears with an average
of >20% removed (Getzendaner and Corbin, 1969).
Evidence of residues in food in commerce or at consumption
No information is available.
METHODS OF RESIDUE ANALYSIS
Corbin (1970) recently published a method suitable for determination
of the total organotin and inorganic tin present in fruit The sample
is subjected to a wet oxidation treatment with sulfuric and nitric
acids to destroy organic matter, remove volatile acids and halogens
and convert the tin to a soluble inorganic form. The tin is then
separated from elements which would interfere in the colorimetric
method (heavy metals) and from non-interfering salts. This separation
involves extracting tin as SnI4, with n-hexane from a strong sulfuric
solution of KI. Evaporation of hexane and conversion to sulfate leaves
the tin in a form measurable by a dithiol colorimetric method.
Sensitivity of the method is 0.01 ppm. Optional steps are included in
the method for removal of arsenic and antimony, if these elements are
suspected of being present in amounts which might cause interference
(> 0.15 mg and > 2 mg, respectively).
A complementary method has been developed for determining residues of
organotin in fruit by M & T Chemicals (Anon, 1969). Tri-, di-, and
monocyclohexyltin are solubilized by treatment of the fruit sample
with hydrochloric acid (9 molar) and then extracted with chloroform
from inorganic tin and heavy metals, which remain in the acidic
aqueous phase. The organotin compounds are then oxidized and
determined colorimetrically.
NATIONAL TOLERANCES
Tolerances have been established (provisional) or are proposed for
residues of tricyclohexyltin hydroxide in or on apples and pears in
various countries including the following:
Country Tolerance Withdrawal between
ppm treatment and harvest, days
Australia1 3 2
Belgium 12 28
Italy 1 20
Israel 1 30
Netherlands 1 28
U.S.A. 23 14
1 Stone fruits
2 Proposed
3 Tolerance proposed on 23 June 1970
Use of the miticide is registered for apples and pears in Chile,
France and Korea, but without provision for tolerance.
APPRAISAL
Tricyclohexyltin hydroxide is a non-systematic acaricide now used in
many countries and likely to be used worldwide on apples and pears,
with indications of future use on citrus, stone fruits and other
crops. Post-harvest use on apples is under investigation. The compound
is relatively harmless to beneficial insects and mites, and it is
usefully applied to integrated control schemes. Tricyclohexyltin
hydroxide is used as wettable powder at a rate of about 0.5 to 1.5 kg
per hectare.
Comprehensive residue data on tricyclohexyltin hydroxide on apples and
pears are available from the United States and from five other
countries, which are consistent with those from the U.S.A. Residue
data on other crops were not available to the Meeting. When apples and
pears are peeled, most of the tricyclohexyltin hydroxide residue is
removed; only 0.1 ppm may be expected in the fruit flesh. Washing is
found to remove 20-50% of the residues, more from apples than from
pears.
Field experiments show that the residues of tricyclohexyltin hydroxide
declined to half in about three weeks on apples and pears. The
disappearance is due to photodegradation, codistillation with water
and weathering factors. The residues are found to occur on the surface
of the treated plants. The degradation of tricyclohexyltin hydroxide
is found to produce dicyclohexyltin oxide and monocyclohexylstannoic
acid and finally inorganic tin salts.
The absorption of tricyclohexyltin hydroxide from the gastrointestinal
tract of animals is small. The distribution patterns of the compound
are determined in rats, dogs and cattle. When cows were fed residues
of 30 and 100 ppm, the residues found in milk were less than 0.1 ppm
and in various tissues less than 0.5 ppm. Limited feeding of apple and
pear pulp to cows is not expected to produce detectable residues in
meat or milk.
A colorimetric method with a detectability of 0.01 ppm tin is
available to determine tricyclohexyltin hydroxide and its organic
degradation products. The method is not able to distinguish between
the various compounds,but reflects the total amount of the compounds
in the sample analyzed.
RECOMMENDATIONS FOR TEMPORARY TOLERANCE
The tolerance is expressed in terms of tricyclohexyltin hydroxide. The
organic degradation products and the inorganic tin salts derived from
tricyclohexyltin hydroxide are not included in the tolerance. A
temporary tolerance of 2 ppm, based on an interval of two weeks from
the last treatment to the harvest, is recommended for apples and
pears.
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Further studies on the effects of exposure to tricyclohexyltin
hydroxide on copper balance and, in particular, on the copper
content of the liver.
2. Further information on the occurrence of liver and pituitary gland
cysts in female rate fed the compound.
3. Further information on the effect of the compound on the rate of
body-weight gain after allowing for the unpalatability of test
diets.
4. Further information on the significance of the brown discoloration
of the serosal surface of the intestine of treated animals.
5. Establishment of analytical procedures capable of distinguishing
qualitatively between tricyclohexyltin compounds and other
organotin compounds, particularly the fentin compounds, and, where
possible, of quantitative measurement of the separate compounds.
DESIRABLE
Data on residues of tricyclohexyltin hydroxide on apples and pears
moving in international commerce.
REFERENCES
Allison, W.E. (1969) An evaluation of the systemic (soil and foliar)
acaricidal activity of tricyclohexyltin hydroxide. Unpublished report
from the Dow Chemical Co.
Anon. (1969) Determination of small amounts of organotin in macerated
fruit. Method No. TA-2-2. Unpublished report from M & T Chemicals,
Inc.
Anon. (1970a) Tin content of tissues of rats and dogs fed varying
amounts of tricyclohexyltin hydroxide in the daily diet for two years.
Unpublished report from the Dow Chemical Co.
Anon. (1970b) Compilation of studies on residues of tricyclohexyltin
hydroxide in or on apples and pears: Australia, France, Netherlands,
and Japan. Unpublished report
Anon. (1970c) Dicyclohexyltin oxide: summary of acute oral toxicity
tests. Unpublished report from the Dow Chemical Company
Corbin, H.B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Offic. Anal.
Chemists, 53: 140-146
Getzendaner, M.E. (1968) A review of residue information on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Getzendaner, M.E. and Corbin, H.A. (1969) Residue study: inorganic
and organic tin compounds in or on apples and pears from field
applications of PLICTRAN(R) miticide. Unpublished report from the Dow
Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970a) Determination of tin
residues in orchard grass exposed to tricyclohexyltin hydroxide
miticide. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970b) Determination of tin
residues in beef calves fed tricyclohexyltin hydroxide for ninety
days. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970c) Determination of tin
residues in milk, cream, and body tissue of dairy cows fed
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Gray, H.E. (1968) PLICTRAN miticide - new approach to mite control.
Down to Earth, 23 (4): 3-5
Hine, C.H. (1966) Acute oral toxicity of tricyclohexyltin hydroxide in
Long Evans strain rats (17 March 1966). Unpublished report from the
Hine Laboratories, Inc. to Dow Chemical Co.
Hine, C.H. (1967) Acute oral toxicity: Cyclohexylstannoic-acid,
dicyclohexyltin oxide, tetracyclohexyltin, hexacyclohexylditin.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Brownlow, E.K., Cummins, J.T., Eisenlord, G.H. and Wong,
L.C.K. (1969) Studies on the mechanism of action of DOWCO 213.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969a) DOWCO 213
reproduction and teratology studies in rats. Unpublished report from
the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969b) DOWCO 213
reproduction and teratology studies in rabbits. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970a) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in dogs. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970b) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in rats. Unpublished report
from the Hine Laboratories, Inc.
Jeppson, L.R., Jesser, M.J. and Complin, J.O. (1968) Responses of the
pacific spider mite and the citrus red mite to laboratory and field
applications of tricyclohexyltin hydroxide. J. Econ. Entomology, 61:
1502-1505
Kenaga, E.E. (1968) The rodent toxicity and repellency of
tricyclohexyltin compounds to two species of mice. Unpublished report
from the Dow Chemical Co.
Komblas and Kostas N. (1969) Determination of residues of PLICTRAN
acaricide on apples and pears in Italy. (Translation of report of
Societá Italo-Americana Prodotti Antiparassitari, Balliera/Bologna,
Italy). Unpublished report from the Dow Chemical Co.
Laker, T.L., Elsea, J.R. and Ede, M. (1966) Repeated insult patch test
on M-2527 and cloth material TE98-SEU3501. Unpublished report from
Hill Top Research, Inc.
Norris, J.M. (1968) Toxicity of tricyclohexyltin hydroxide via i.p.
route in laboratory animals. Unpublished report from the Dow Chemical
Co.
Olson, K.J. (1964) Results of range finding toxicological tests on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Smith, G.N., Axelson, R.J. and Schiesser, L.H. (1970) The loss of
PLICTRAN(R) miticide from various soils. Unpublished report from the
Dow Chemical Co.
Smith, G.N. and Fischer, F.S. (1970) Metabolism of PLICTRAN(R)
miticide. Observations on the absorption, distribution, and excretion
of tricyclohexyltin-Sn119 hydroxide in white rats. Unpublished report
from the Dow Chemical Co.
Smith, G.N., Fischer, F.S. and Axelson, R.J. (1970) The volatilization
and photodecomposition of PLICTRAN(R) miticide. Unpublished report
from the Dow Chemical Co.
Smith, G.N. and Taylor, Y.S. (1970) The absorption and translocation
of PLICTRAN miticide and its decomposition products in bean and corn
plants. Unpublished report from the Dow Chemical Co.
Stevenson, G.T. and Kenaga, E.E. (1969) The effects of dietary feeding
of tricyclohexyltin hydroxide on the life stages of the Japanese
Quail. Unpublished report from the Dow Chemical Co.
Tucker, W.E. (1966) Oral toxicity studies of tricyclohexyltin
hydroxide in rats. Unpublished data from the Dow Chemical Co.
Wazeter, F.X., Buller, R.H., Geil, R.G. and Long, J.E. (1968)
Dicyclohexyltin oxide: 90-day feeding study in the Long-Evans rat.
Unpublished report from the International Research and Development
Corporation
Wazeter, F.X. (1969) 21-day dermal study in,albino rabbits-PLICTRAN
M-3180 miticide. Unpublished report from the International Research
and Development Corporation
Whitney, W.K. (1966) Leaching tests with DOWCO 213 in soil
Zambelli, N., Komblas, W. and Kovacs, A. (1968) PLICTRAN miticide for
the control of spider mites in Italy. Down to Earth, 24(3): 25-31
 Other relevant chemical properties
The compound in a white, crystalline powder with an apparent melting
point of 245°C. The compound undergoes loss of water at 120-130°C and
In converted to the bis (tricyclohexyltin) oxide. The technical
product has the following solubilities at 25°C: acetone 0.13 g/100 ml,
benzene 1.6 g/100 ml, carbon tetrachloride 2.8 g/100 ml, chloroform
21.6 g/ml, methanol 3.7 g/100 ml and water < 0.0001 g/100 ml.
Purity
The technical product contains 95-96% tricyclohexyltin hydroxide. It
is formulated as a wettable powder.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
In the studies with rats and dogs, in addition to the parent compound,
dicyclohexyltin oxide and trace amounts of cyclohexylstannoic acid in
organic tin were identified (Smith and Fischer, 1970; Anon, 1970a).
The metabolic fate of the limited amounts of compound absorbed from
the gastrointestinal tract in animals appears to occur by the
sequence:
(C6H11)3 SnOH---> (C6H11)2 SnO---> C6H11SnO2H---> Sn+4
Absorption, distribution and excretion
Two rats received, each in a gelatine capsule, a single oral dose of 5
mg of 119Sn-labelled tricyclohexyltin hydroxide, corresponding to 25
mg/kg body-weight. Essentially quantitative recovery (99.9 and 100
percent) of the total radioactivity was obtained in the excreta
collected for ten days after dosing. Most (75-80 percent) was excreted
in the first four days after administration. Almost all of the
radioactivity occurred in the faeces (97.5 and 98.9 percent versus 1.8
to 2.5 percent in the urine). The practical lack of gastrointestinal
absorption was also confirmed by the absence of radioactivity in the
bile of two guinea pigs, which were administered the labelled compound
and sacrificed, respectively, 24 and 48 hours after dosing (Smith and
Fischer, 1970).
Groups of rats and dogs of both sexes were fed tricyclohexyltin
hydroxide in their diet at rates of 0.75, 3, 6 and 12 mg/kg
body-weight per day, for periods ranging from 45 days to two years.
Tin compounds, primarily the parent compound, were distributed through
the tissues only in trace amounts. As an example: in rats which were
fed 3 mg compound/kg/day, the levels were: fat (0.1 ppm tin) < muscle
< adrenals < heart < brain <liver kidney (0.8 ppm). The biological
half-life of the tin compounds was approximately 5 to 40 days after
compound withdrawal from the diet (Anon, 1970a). Dicyclohexyltin
oxide, trace amounts of cyclohexylstannoic acid, and inorganic tin
were identified as metabolites in rats and dogs. Generally, from 60 to
95 percent of the tin was present as organotin (Smith and Fischer,
1970; Anon, 1970a).
Rats were fed 100 ppm of 119Sn-labelled tricyclohexyltin hydroxide for
90 days, and several tissues were analysed for uptake of 119Sn.
Continuous uptake resulted in an equilibrium being reached between
40-60 days with the females accumulating slightly more than the males.
Tin was eliminated slowly from the tissues upon cessation of feeding,
with the brain being one of the slowest tissues. Traces of tin were
noted after 115 days, although the major quantity was removed in 20
days (Smith and Fischer, 1970).
Effect on enzymes and other biochemical parameters
In a series of pharmacological and biochemical studies, intravenous
injection to dogs and cats resulted in no effect on blood pressure,
pulse rate or cholinesterase levels, and in no potentiation of drugs
(epinphrine chloride, norepinephrine bitartrate, tyramine
monohydrochloride or nicotine sulfate). The primary effect noted on
intravenous injection was stimulation of respiration accompanied by an
increase in blood lactate and a decrease in blood pCO2, without
changes in either pH or pO2 in the blood (Hine at al., 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Japanese Quail
Investigations were started on four groups of birds, each containing
eight to ten females plus an equal number of males. Dietary levels of
0, 1, 10 or 100 ppm of tricyclohexyltin hydroxide were given to the
adults (ten weeks of age) of the first generation, and, afterwards,
through the entire life cycle of the second and the third generation.
Body and egg weights, food-consumption, survival of live chicks from
eggs, adult mortality, general physical condition and appearance and
gross observation of the ovaries and gonads were comparable to the
controls for all dietary levels. Similarly, no gross teratogenic or
lenticular effects were observed in any of the treated groups.
Definite effects on embryonic mortality, egg production, fertility and
hatchability were observed at the 100 ppm dietary levels. At 10 ppm,
there were effects of questionable significance on egg fertility and
hatchability. None of those criteria was affected at the level of 1
ppm (Stevenson and Kenaga, 1969).
Rabbit
Groups of pregnant New Zealand white rabbits, 28 in all, received oral
doses of respectively 0, 0.75 or 3 mg tricyclohexyltin hydroxide/kg
body-weight/day on the eighth to the 16th day inclusive of gestation.
There was no evidence of significant adverse effects due to the
compound as judged by indices of fertility, gestation, viability and
lactation or by examination of the foetuses for teratogenic effects
(Hine at al., 1969b).
Rat
A three generation (two litters per generation) reproduction,
fertility and teratology study was conducted in groups of 10 male and
20 female rats maintained continuously on diets containing 0, 12.5, 50
or 100 ppm of tricyclohexyltin hydroxide (the uppermost dietary level
corresponds to approximately 4 to 6 mg test compound/kg
body-weight/day on the basis of adult and female rats). There was no
evidence of significant adverse effects due to the compound under test
when judged by indices of fertility, gestation, viability and
lactation, or by examination of the foetuses for teratogenic effects
(Hine et al., 1969a).
Special studies on metabolites and photodecomposition products
In addition to having been identified as metabolites in animals (Smith
and Fischer, 1970), dicyclohexyltin oxide and cyclohexylstannoic acid
occur as a relatively minor proportion of the residues on fruit,
having been formed by sunlight degradation (Getzendaner, 1968) (see
'Fate of Residues, in plants').
LD50 values for dicyclohexyltin oxide, as determined in five studies
involving varying strains of rats, averaged 355 mg/kg body-weight
(Anon, 1970c). Cyclohexylstannoic acid exhibits low toxicity to rats
with an LD50 value of < 3 600 mg/kg body-weight (Hine, 1967).
Male and female rats were fed dicyclohexyltin oxide in their diet for
90 days at dose levels of 0, 1, 3 and 6 mg/kg body-weight/day. No
unusual alterations in behaviour and appearance occured during this
study nor were any pharmacodynamic or toxic signs exhibited which
could be related to the feeding of the test compound in the diet. No
compound related variations were found in haematological and
biochemical values, in gross and microscopic examination of tissues
and in organ-weights and ratios (Wazeter et al., 1968).
Acute toxicity
TABLE I
Summary of acute toxicities by various workers
LD50(mg/kg)
Animal Route body-weight Reference
Chick (M) Oral 650 Olson, 1964
Mouse,
Peromyscus Oral 7101/ Kenaga, 1968
Mouse,
Swiss White Oral 10701/ Kenaga, 1968
Rat Oral 5402/ Anon, 1970b
Rat ip 13 Hine et al., 1969a
Guinea pig (M) Oral 780 Olson, 1964
Guinea pig (M) ip 9 Norris, 1968
Rabbit Oral >500,<1000 Olson, 1964
Rabbit ip >126 Norris, 1968
Dog iv 14 Hine et al., 1969a
Cat iv 6 Hine et al., 1969a
1/ Approximate lethal dosage (100 percent deaths)
2/ Average LD50 value from 10 studies in both sexes of three strains
These data reveal that acute toxicity by the oral route is, because of
very poor gastrointestinal absorption, considerably lower than by the
parenteral route.
The compound appears to be principally a depressant of the central
nervous system, but not nearly to the same extent as the triethyltin
acetate in comparable doses. Neither interperitoneal, intravenous nor
oral administration resulted in cerebral oedema or significant
pathologic lesions (Hine at al., 1969).
Death usually occurred within two to seven days after administration.
Acute signs of poisoning in rats include anal wetting, piloerection,
wet nares and mouth, lethargy, hyporeflexia, arching, depressed
respiration, emaciation, diarrhoea and flexure of all four limbs when
picked up by the scruff of the neck. Gross examination of the dead
animals showed that the adrenal gland of rats was 2-3 times heavier
than the controls and the spleen was 0.3-0.4 time heavier than the
controls (Hine, 1966).
Short-term studies
Dog
A total of 96 beagle dogs, half male and half female, averaging eight
months of age, were fed tricyclohexyltin hydroxide in the diet, at
levels adjusted to give ingestion rates of 0, 0.75, 3 or 6 mg/kg
body-weight/day, for two years, or of 12 mg/kg/day for six months.
Animals were sacrificed in each group after 3, 6, 12 or 24 months. All
diets containing the compound under test were rejected by the animals
to some extent during the first stages of the treatment, particularly
at the highest levels. Diets designed to administer 12 mg/kg/day were
very unpalatable; many dogs completely refused diets at this level,
most progressively lost weight and some died of starvation. Ingestion
of 6 mg/kg/day for two years caused no direct toxic effect on tissues,
but did cause an apparent reduction in final body weight, which in
turn affected organs to body-weight ratios, but no pathological
changes were found. At the 3 mg/kg/day dietary level, mortality,
behaviour, food intake, growth rate, gross and microscopic pathology,
haematology and biochemical values were comparable to the controls.
The same conclusion applies to organ-weights and ratios, except for
statistically significant increased heart to body-weight ratio
averages, but not actual heart weights, in both sexes, and average
liver weight and ratio in the males only. These observations were not
associated with significant alterations in these two organs at either
the interim or final necropsy periods or upon microscopic examination.
Copper content in the urine was decreased at all dose levels examined.
A notable undefined effect in dogs was a tanning or brown
discoloration of the serosal surface of the small intestine in dogs
fed 3 and 6 mg/kg levels. This effect was not noted at 0.75 mg/kg.
Measurement of tin content indicated higher levels of tin in all
tissues examined. Levels in the males were lower than in the females
and were proportional in both sexes to the dietary intake. An
equilibrium level was apparently reached between six and 12 weeks of
feeding and remained constant for the remainder of the testing.
Tissues of rats and dogs contained similar concentrations of tin,
except for the liver of dogs, which contained five to 14 times the tin
content of rats and a higher proportion of inorganic tin. No signs of
toxic injury were observed at the level of 0.75 mg/kg (Hine, 1970).
Rabbit
Tricyclohexyltin hydroxide administered to the conjunctival sac
resulted in irritational corneal injury which subsided in one week
(Olson, 1964).
Groups of rabbits (ten male and ton female) were treated dermally at
doses of 0, 1.2, 6, 12 or 60 mg/kg/body-weight/day, five days per week
for three weeks. All animals developed erythema, oedema, atonia,
desquamation and fissuring of the skin. The signs of toxicity were
severe at all done levels examined. Other than dermal problems, no
other effects were noted (Wazeter, 1969).
Rat
Exploratory experiments in rats showed that an oral dose of 12.5 mg/kg
body-weight/day did not produce tissue damage when given daily by
gavage for 19 days. A dose of 25 mg/kg/day was toxic within two weeks,
and doses of 50 and 100 mg/kg/day were toxic within one week. Rats
that received 12 mg/kg body-weight/day for ten or 16 weeks showed no
direct toxic effects. Poor weight gain observed at this dietary level
was judged to be caused by unpalatability of diets containing
tricyclohexyltin hydroxide. Due to diet unpalatability, higher doses
had to be administered by gavage. Direct toxic effects were not
achieved until doses of 25 mg/kg bodyweight/day were administered.
This level resulted in clinical symptoms, such as severe diarrhoea and
weight loss, as well as morphological effects, such as
gastroenteritis, intrahepatic and extrahepatic cholangitis,
degenerative changes in adrenal glands and toxic nephrosis (Tucker,
1966).
Long-term studies
Rat
A total of 720 rats, half males and half females, aged four weeks,
were fed 0, 0.75, 3, 6 or 12 mg tricyclohexyltin hydroxide/kg
body-weight/day for up to two years. None of the dietary levels of
treatment caused any change in behaviour, mortality records,
haematologic and biochemical values, gross appearances and
histological characteristics of organs and tissues. Effects observed
at the 12 mg/kg level wore decreased weight gains in both sexes and
increased organ to body-weight ratios for spleen and liver in the
females. The pattern of tumour incidence appeared to be random and not
suggestive of a dose relationship. The occurrence of many cysts seen
throughout this study at all levels may be related to the particular
strain of animals, although in the females at 12 mg/kg, 17 liver cysts
and five pituitary cysts were noted as compared to one liver cyst and
five pituitary cysts in the controls. At six mg/kg, only a slight
decrease of food intake and a related mild depression of growth rate
were observed during the first three months on treatment. These
effects were absent at the level of 3 mg/kg (Hine, 1970a).
OBSERVATIONS IN MAN
A wettable powder formulation (50 percent tricyclohexyltin hydroxide)
was examined for its irritation and sensitization potential to human
subjects. No adverse reactions were observed in 53 females following
sensitization applications and a challenge application 20 days later
of 0.5 ml of a 0.1 percent emulsion. Tricyclohexyltin hydroxide was
not dermally irritating at a concentration of about 0.01 mg/kg
body-weight (Laker et al., 1966).
COMMENT
The Meeting expressed concern that the only toxicological data that
had been presented for consideration were unpublished, and had
therefore not already been subject to critical scrutiny by the general
scientific community.
A difficulty with the toxicological evaluation of this compound is
that its presence made laboratory diets unpalatable. A need for
carefully controlled paired-feeding experiments was therefore evident.
Concern was expressed that residual tin remained in the tissues of
exposed animals for prolonged periods and that brown discoloration of
the serosal surface of the small intestine occurred in dogs fed 3
mg/kg body-weight/day or higher dose levels of the compound. It was
noted that, in dogs fed 0.75 mg/kg body-weight/day or more, there was
a reduction in urinary copper output. In view of this observation, the
need for information on the copper content of the liver of exposed
animals was stressed. The observation of an increased incidence of
cysts of the liver and pituitary gland of female rats fed 12 mg/kg
body-weight/day was regarded with some concern. For these reasons the
Committee considered that only a temporary acceptable daily intake
could be established.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: level in the diet adjusted to give 0.75 mg/kg body-weight/day
Rat: level in the diet adjusted to give 3 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0075 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Tricyclohexyltin hydroxide is a non-systemic acaricide used or under
development for use in several countries to control phytophagous mites
on apple and pear trees. The compound exhibits little or no activity
against insects,including pollinating bees,and it is relatively
harmless to predatory mites. Thus it may be advantageously applied in
integrated control schemes (Gray, 1968; Zambelli et al., 1968; Jeppson
et al., 1968). Typically, tricyclohexyltin hydroxide is recommended
for use in dilute sprays at concentrations of 15 to 30 g/100 litres,
the total amount applied per unit area ranging from 0.42 to 1.68
kg/hectare.
In addition to use on apples and pears, tricyclohexyltin hydroxide is
expected to prove useful for control of mites on stone fruits, citrus,
grapes and certain other crops.
Post-harvest treatments
Post-harvest use on apples is under investigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following typical data (Table II) are extracted from unpublished
reports supplied to FAO/WHO by Dow Chemical Company (Getzendaner,
1968; Komblas, 1969; Getzendaner and Corbin, 1969; Anon, 1970b).
FATE OF RESIDUES
General comments
Tricyclohexyltin hydroxide is decomposed by sunlight as evidenced by
(1) a half-life of 8 "sunny days" for disappearance from glass slides
(Smith et al., 1970c) and (2) detection of inorganic tin as
approximately 15 to 30 or more percent of the total tin residues on
fruit exposed to 3 or 4 applications of the miticide (Getzendaner and
Corbin, 1969).
TABLE II
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 15 1 0 0.8 -
8 1.0 -
16 0.6 -
32 0.5 -
TABLE II (cont'd)
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 23 4 0 1.7 2.5
14 1.6 2.2
28 1.1 1.5
35 0.9 1.0
Netherlands 25 4 0 1.2 1.9
14 1.2 1.6
28 0.5 0.7
42 0.5 0.7
Italy 30 2 7 1.1 1.3
16 1.2 1.3
28 0.8 1.0
U.S.A. 30 3 17 1.9 -
Australia 30 3 0 - 1.94
France 30 1 0 0.7 1.8
15 0.5 0.5
42 0.4 0.4
Japan 33 3 0 2.0 -
14 1.5 -
30 1.1 -
PEARS
U.S.A. 23 3 0 1.4 2.1
15 1.1 1.4
29 0.7 1.0
42 0.6 0.6
61 0.4 0.6
Australia 23 1 31 - 1.0
Italy 30 2 7 1.0 1.5
16 0.8 1.0
28 0.4 0.6
Photodegradation occurs by the sequence, which is identical with that
owing in the animal body:
Other relevant chemical properties
The compound in a white, crystalline powder with an apparent melting
point of 245°C. The compound undergoes loss of water at 120-130°C and
In converted to the bis (tricyclohexyltin) oxide. The technical
product has the following solubilities at 25°C: acetone 0.13 g/100 ml,
benzene 1.6 g/100 ml, carbon tetrachloride 2.8 g/100 ml, chloroform
21.6 g/ml, methanol 3.7 g/100 ml and water < 0.0001 g/100 ml.
Purity
The technical product contains 95-96% tricyclohexyltin hydroxide. It
is formulated as a wettable powder.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
In the studies with rats and dogs, in addition to the parent compound,
dicyclohexyltin oxide and trace amounts of cyclohexylstannoic acid in
organic tin were identified (Smith and Fischer, 1970; Anon, 1970a).
The metabolic fate of the limited amounts of compound absorbed from
the gastrointestinal tract in animals appears to occur by the
sequence:
(C6H11)3 SnOH---> (C6H11)2 SnO---> C6H11SnO2H---> Sn+4
Absorption, distribution and excretion
Two rats received, each in a gelatine capsule, a single oral dose of 5
mg of 119Sn-labelled tricyclohexyltin hydroxide, corresponding to 25
mg/kg body-weight. Essentially quantitative recovery (99.9 and 100
percent) of the total radioactivity was obtained in the excreta
collected for ten days after dosing. Most (75-80 percent) was excreted
in the first four days after administration. Almost all of the
radioactivity occurred in the faeces (97.5 and 98.9 percent versus 1.8
to 2.5 percent in the urine). The practical lack of gastrointestinal
absorption was also confirmed by the absence of radioactivity in the
bile of two guinea pigs, which were administered the labelled compound
and sacrificed, respectively, 24 and 48 hours after dosing (Smith and
Fischer, 1970).
Groups of rats and dogs of both sexes were fed tricyclohexyltin
hydroxide in their diet at rates of 0.75, 3, 6 and 12 mg/kg
body-weight per day, for periods ranging from 45 days to two years.
Tin compounds, primarily the parent compound, were distributed through
the tissues only in trace amounts. As an example: in rats which were
fed 3 mg compound/kg/day, the levels were: fat (0.1 ppm tin) < muscle
< adrenals < heart < brain <liver kidney (0.8 ppm). The biological
half-life of the tin compounds was approximately 5 to 40 days after
compound withdrawal from the diet (Anon, 1970a). Dicyclohexyltin
oxide, trace amounts of cyclohexylstannoic acid, and inorganic tin
were identified as metabolites in rats and dogs. Generally, from 60 to
95 percent of the tin was present as organotin (Smith and Fischer,
1970; Anon, 1970a).
Rats were fed 100 ppm of 119Sn-labelled tricyclohexyltin hydroxide for
90 days, and several tissues were analysed for uptake of 119Sn.
Continuous uptake resulted in an equilibrium being reached between
40-60 days with the females accumulating slightly more than the males.
Tin was eliminated slowly from the tissues upon cessation of feeding,
with the brain being one of the slowest tissues. Traces of tin were
noted after 115 days, although the major quantity was removed in 20
days (Smith and Fischer, 1970).
Effect on enzymes and other biochemical parameters
In a series of pharmacological and biochemical studies, intravenous
injection to dogs and cats resulted in no effect on blood pressure,
pulse rate or cholinesterase levels, and in no potentiation of drugs
(epinphrine chloride, norepinephrine bitartrate, tyramine
monohydrochloride or nicotine sulfate). The primary effect noted on
intravenous injection was stimulation of respiration accompanied by an
increase in blood lactate and a decrease in blood pCO2, without
changes in either pH or pO2 in the blood (Hine at al., 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Japanese Quail
Investigations were started on four groups of birds, each containing
eight to ten females plus an equal number of males. Dietary levels of
0, 1, 10 or 100 ppm of tricyclohexyltin hydroxide were given to the
adults (ten weeks of age) of the first generation, and, afterwards,
through the entire life cycle of the second and the third generation.
Body and egg weights, food-consumption, survival of live chicks from
eggs, adult mortality, general physical condition and appearance and
gross observation of the ovaries and gonads were comparable to the
controls for all dietary levels. Similarly, no gross teratogenic or
lenticular effects were observed in any of the treated groups.
Definite effects on embryonic mortality, egg production, fertility and
hatchability were observed at the 100 ppm dietary levels. At 10 ppm,
there were effects of questionable significance on egg fertility and
hatchability. None of those criteria was affected at the level of 1
ppm (Stevenson and Kenaga, 1969).
Rabbit
Groups of pregnant New Zealand white rabbits, 28 in all, received oral
doses of respectively 0, 0.75 or 3 mg tricyclohexyltin hydroxide/kg
body-weight/day on the eighth to the 16th day inclusive of gestation.
There was no evidence of significant adverse effects due to the
compound as judged by indices of fertility, gestation, viability and
lactation or by examination of the foetuses for teratogenic effects
(Hine at al., 1969b).
Rat
A three generation (two litters per generation) reproduction,
fertility and teratology study was conducted in groups of 10 male and
20 female rats maintained continuously on diets containing 0, 12.5, 50
or 100 ppm of tricyclohexyltin hydroxide (the uppermost dietary level
corresponds to approximately 4 to 6 mg test compound/kg
body-weight/day on the basis of adult and female rats). There was no
evidence of significant adverse effects due to the compound under test
when judged by indices of fertility, gestation, viability and
lactation, or by examination of the foetuses for teratogenic effects
(Hine et al., 1969a).
Special studies on metabolites and photodecomposition products
In addition to having been identified as metabolites in animals (Smith
and Fischer, 1970), dicyclohexyltin oxide and cyclohexylstannoic acid
occur as a relatively minor proportion of the residues on fruit,
having been formed by sunlight degradation (Getzendaner, 1968) (see
'Fate of Residues, in plants').
LD50 values for dicyclohexyltin oxide, as determined in five studies
involving varying strains of rats, averaged 355 mg/kg body-weight
(Anon, 1970c). Cyclohexylstannoic acid exhibits low toxicity to rats
with an LD50 value of < 3 600 mg/kg body-weight (Hine, 1967).
Male and female rats were fed dicyclohexyltin oxide in their diet for
90 days at dose levels of 0, 1, 3 and 6 mg/kg body-weight/day. No
unusual alterations in behaviour and appearance occured during this
study nor were any pharmacodynamic or toxic signs exhibited which
could be related to the feeding of the test compound in the diet. No
compound related variations were found in haematological and
biochemical values, in gross and microscopic examination of tissues
and in organ-weights and ratios (Wazeter et al., 1968).
Acute toxicity
TABLE I
Summary of acute toxicities by various workers
LD50(mg/kg)
Animal Route body-weight Reference
Chick (M) Oral 650 Olson, 1964
Mouse,
Peromyscus Oral 7101/ Kenaga, 1968
Mouse,
Swiss White Oral 10701/ Kenaga, 1968
Rat Oral 5402/ Anon, 1970b
Rat ip 13 Hine et al., 1969a
Guinea pig (M) Oral 780 Olson, 1964
Guinea pig (M) ip 9 Norris, 1968
Rabbit Oral >500,<1000 Olson, 1964
Rabbit ip >126 Norris, 1968
Dog iv 14 Hine et al., 1969a
Cat iv 6 Hine et al., 1969a
1/ Approximate lethal dosage (100 percent deaths)
2/ Average LD50 value from 10 studies in both sexes of three strains
These data reveal that acute toxicity by the oral route is, because of
very poor gastrointestinal absorption, considerably lower than by the
parenteral route.
The compound appears to be principally a depressant of the central
nervous system, but not nearly to the same extent as the triethyltin
acetate in comparable doses. Neither interperitoneal, intravenous nor
oral administration resulted in cerebral oedema or significant
pathologic lesions (Hine at al., 1969).
Death usually occurred within two to seven days after administration.
Acute signs of poisoning in rats include anal wetting, piloerection,
wet nares and mouth, lethargy, hyporeflexia, arching, depressed
respiration, emaciation, diarrhoea and flexure of all four limbs when
picked up by the scruff of the neck. Gross examination of the dead
animals showed that the adrenal gland of rats was 2-3 times heavier
than the controls and the spleen was 0.3-0.4 time heavier than the
controls (Hine, 1966).
Short-term studies
Dog
A total of 96 beagle dogs, half male and half female, averaging eight
months of age, were fed tricyclohexyltin hydroxide in the diet, at
levels adjusted to give ingestion rates of 0, 0.75, 3 or 6 mg/kg
body-weight/day, for two years, or of 12 mg/kg/day for six months.
Animals were sacrificed in each group after 3, 6, 12 or 24 months. All
diets containing the compound under test were rejected by the animals
to some extent during the first stages of the treatment, particularly
at the highest levels. Diets designed to administer 12 mg/kg/day were
very unpalatable; many dogs completely refused diets at this level,
most progressively lost weight and some died of starvation. Ingestion
of 6 mg/kg/day for two years caused no direct toxic effect on tissues,
but did cause an apparent reduction in final body weight, which in
turn affected organs to body-weight ratios, but no pathological
changes were found. At the 3 mg/kg/day dietary level, mortality,
behaviour, food intake, growth rate, gross and microscopic pathology,
haematology and biochemical values were comparable to the controls.
The same conclusion applies to organ-weights and ratios, except for
statistically significant increased heart to body-weight ratio
averages, but not actual heart weights, in both sexes, and average
liver weight and ratio in the males only. These observations were not
associated with significant alterations in these two organs at either
the interim or final necropsy periods or upon microscopic examination.
Copper content in the urine was decreased at all dose levels examined.
A notable undefined effect in dogs was a tanning or brown
discoloration of the serosal surface of the small intestine in dogs
fed 3 and 6 mg/kg levels. This effect was not noted at 0.75 mg/kg.
Measurement of tin content indicated higher levels of tin in all
tissues examined. Levels in the males were lower than in the females
and were proportional in both sexes to the dietary intake. An
equilibrium level was apparently reached between six and 12 weeks of
feeding and remained constant for the remainder of the testing.
Tissues of rats and dogs contained similar concentrations of tin,
except for the liver of dogs, which contained five to 14 times the tin
content of rats and a higher proportion of inorganic tin. No signs of
toxic injury were observed at the level of 0.75 mg/kg (Hine, 1970).
Rabbit
Tricyclohexyltin hydroxide administered to the conjunctival sac
resulted in irritational corneal injury which subsided in one week
(Olson, 1964).
Groups of rabbits (ten male and ton female) were treated dermally at
doses of 0, 1.2, 6, 12 or 60 mg/kg/body-weight/day, five days per week
for three weeks. All animals developed erythema, oedema, atonia,
desquamation and fissuring of the skin. The signs of toxicity were
severe at all done levels examined. Other than dermal problems, no
other effects were noted (Wazeter, 1969).
Rat
Exploratory experiments in rats showed that an oral dose of 12.5 mg/kg
body-weight/day did not produce tissue damage when given daily by
gavage for 19 days. A dose of 25 mg/kg/day was toxic within two weeks,
and doses of 50 and 100 mg/kg/day were toxic within one week. Rats
that received 12 mg/kg body-weight/day for ten or 16 weeks showed no
direct toxic effects. Poor weight gain observed at this dietary level
was judged to be caused by unpalatability of diets containing
tricyclohexyltin hydroxide. Due to diet unpalatability, higher doses
had to be administered by gavage. Direct toxic effects were not
achieved until doses of 25 mg/kg bodyweight/day were administered.
This level resulted in clinical symptoms, such as severe diarrhoea and
weight loss, as well as morphological effects, such as
gastroenteritis, intrahepatic and extrahepatic cholangitis,
degenerative changes in adrenal glands and toxic nephrosis (Tucker,
1966).
Long-term studies
Rat
A total of 720 rats, half males and half females, aged four weeks,
were fed 0, 0.75, 3, 6 or 12 mg tricyclohexyltin hydroxide/kg
body-weight/day for up to two years. None of the dietary levels of
treatment caused any change in behaviour, mortality records,
haematologic and biochemical values, gross appearances and
histological characteristics of organs and tissues. Effects observed
at the 12 mg/kg level wore decreased weight gains in both sexes and
increased organ to body-weight ratios for spleen and liver in the
females. The pattern of tumour incidence appeared to be random and not
suggestive of a dose relationship. The occurrence of many cysts seen
throughout this study at all levels may be related to the particular
strain of animals, although in the females at 12 mg/kg, 17 liver cysts
and five pituitary cysts were noted as compared to one liver cyst and
five pituitary cysts in the controls. At six mg/kg, only a slight
decrease of food intake and a related mild depression of growth rate
were observed during the first three months on treatment. These
effects were absent at the level of 3 mg/kg (Hine, 1970a).
OBSERVATIONS IN MAN
A wettable powder formulation (50 percent tricyclohexyltin hydroxide)
was examined for its irritation and sensitization potential to human
subjects. No adverse reactions were observed in 53 females following
sensitization applications and a challenge application 20 days later
of 0.5 ml of a 0.1 percent emulsion. Tricyclohexyltin hydroxide was
not dermally irritating at a concentration of about 0.01 mg/kg
body-weight (Laker et al., 1966).
COMMENT
The Meeting expressed concern that the only toxicological data that
had been presented for consideration were unpublished, and had
therefore not already been subject to critical scrutiny by the general
scientific community.
A difficulty with the toxicological evaluation of this compound is
that its presence made laboratory diets unpalatable. A need for
carefully controlled paired-feeding experiments was therefore evident.
Concern was expressed that residual tin remained in the tissues of
exposed animals for prolonged periods and that brown discoloration of
the serosal surface of the small intestine occurred in dogs fed 3
mg/kg body-weight/day or higher dose levels of the compound. It was
noted that, in dogs fed 0.75 mg/kg body-weight/day or more, there was
a reduction in urinary copper output. In view of this observation, the
need for information on the copper content of the liver of exposed
animals was stressed. The observation of an increased incidence of
cysts of the liver and pituitary gland of female rats fed 12 mg/kg
body-weight/day was regarded with some concern. For these reasons the
Committee considered that only a temporary acceptable daily intake
could be established.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: level in the diet adjusted to give 0.75 mg/kg body-weight/day
Rat: level in the diet adjusted to give 3 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0075 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Tricyclohexyltin hydroxide is a non-systemic acaricide used or under
development for use in several countries to control phytophagous mites
on apple and pear trees. The compound exhibits little or no activity
against insects,including pollinating bees,and it is relatively
harmless to predatory mites. Thus it may be advantageously applied in
integrated control schemes (Gray, 1968; Zambelli et al., 1968; Jeppson
et al., 1968). Typically, tricyclohexyltin hydroxide is recommended
for use in dilute sprays at concentrations of 15 to 30 g/100 litres,
the total amount applied per unit area ranging from 0.42 to 1.68
kg/hectare.
In addition to use on apples and pears, tricyclohexyltin hydroxide is
expected to prove useful for control of mites on stone fruits, citrus,
grapes and certain other crops.
Post-harvest treatments
Post-harvest use on apples is under investigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following typical data (Table II) are extracted from unpublished
reports supplied to FAO/WHO by Dow Chemical Company (Getzendaner,
1968; Komblas, 1969; Getzendaner and Corbin, 1969; Anon, 1970b).
FATE OF RESIDUES
General comments
Tricyclohexyltin hydroxide is decomposed by sunlight as evidenced by
(1) a half-life of 8 "sunny days" for disappearance from glass slides
(Smith et al., 1970c) and (2) detection of inorganic tin as
approximately 15 to 30 or more percent of the total tin residues on
fruit exposed to 3 or 4 applications of the miticide (Getzendaner and
Corbin, 1969).
TABLE II
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 15 1 0 0.8 -
8 1.0 -
16 0.6 -
32 0.5 -
TABLE II (cont'd)
Residues found in apples and pears in various countries
Rate of Number Post-treatment Residue, total tin,
Location application of interval as Cy3SnOH, ppm
g/100 l Treatments days
average maximum
APPLES
U.S.A. 23 4 0 1.7 2.5
14 1.6 2.2
28 1.1 1.5
35 0.9 1.0
Netherlands 25 4 0 1.2 1.9
14 1.2 1.6
28 0.5 0.7
42 0.5 0.7
Italy 30 2 7 1.1 1.3
16 1.2 1.3
28 0.8 1.0
U.S.A. 30 3 17 1.9 -
Australia 30 3 0 - 1.94
France 30 1 0 0.7 1.8
15 0.5 0.5
42 0.4 0.4
Japan 33 3 0 2.0 -
14 1.5 -
30 1.1 -
PEARS
U.S.A. 23 3 0 1.4 2.1
15 1.1 1.4
29 0.7 1.0
42 0.6 0.6
61 0.4 0.6
Australia 23 1 31 - 1.0
Italy 30 2 7 1.0 1.5
16 0.8 1.0
28 0.4 0.6
Photodegradation occurs by the sequence, which is identical with that
owing in the animal body:
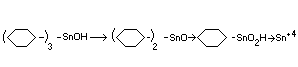 Tricyclohexyltin hydroxide is not volatile from dry surfaces, but
'co-distils', with water from moist surfaces at a slow but finite rate
(Smith et al., 1970). Field experiments show that the half-life of the
compound is about four days on grass and about three weeks on apples
and pears; the disappearance results from photodegradation,
'co-distillation' with water from moist surfaces and weathering
factors (Getzendaner and Gentry, 1970a; Getzendaner and Corbin, 1969).
In animals
As indicated under "BIOCHEMICAL ASPECTS", tricyclohexyltin hydroxide
is not readily absorbed from the gastrointestinal tract.
When the miticide is fed daily to rats, dogs or cattle, tin compounds,
primarily unchanged tricyclohexyltin hydroxide, accumulate in the
tissues to a limited extent, reach equilibrium values in a few months
to a year, and are slowly eliminated from the tissues after the
compound is withdrawn from the diet. It does not selectively partition
into fat, and the distribution pattern in tissues is similar for rats,
dogs and cattle (Smith and Fischer, 1970; Anon, 1970a; Getzendaner and
Gentry, 1970b). For example, tissues from calves fed 30 ppm of the
compound in the diet for 90 days showed 0.07 ppm total tin in fat,
0.09 ppm in muscle, 0.37 ppm in liver and 0.20 ppm in kidney
(Getzendaner and Gentry, 1970b).
Levels of tricyclohexyltin hydroxide equivalent were 0.05 ppm in milk
and 0.4 ppm in cream obtained from cows fed 100 ppm of the compound in
the diet for 53 days (Getzendaner and Gentry, 1970c).
In plants
Residues occurring on apples were identified by a series of extraction
techniques followed by analysis. Tricyclohexyltin hydroxide was the
main component of the tin residues, comprising 67% (0.66 ppm) of the
residue (eight days post-treatment). Small amounts of dicyclohexyltin
oxide and cyclohexylstannoic acid were present (5% of each), as well
as 23% inorganic tin (Getzendaner, 1968).
Tricyclohexyltin hydroxide and its sunlight decomposition products are
not translocated in plants to a significant extent. This has been
demonstrated by (1) the absence of significant residues of tin
compounds in the interior portions of fruit, even after periods of
several months between application of the first of up to 5 spray
applications and harvest (Getzendaner and Corbin, 1969), (2) the
absence of radioactive residues in bean and maize plants grown in soil
treated with exaggerated amounts (3 lb/acre) of 119Sn-labelled
miticide or its degradation (Smith at al. 1970), and (3) the essential
absence of translocation across the narrow thickness of the leaf of
cotton plants as determined by bioassay experiments (Allison, 1969).
In soil
Tricyclohexyltin hydroxide is markedly absorbed by soil and it is not
leached from soil, as demonstrated by soil-column leaching studies and
by study of the transfer from water to soil (Smith et al., 1970a,
Smith and Taylor, 1970; Whitney, 1966). No significant amounts of the
miticide will remain in or on the soil from one growing season to the
next.
In storage and processing
Results of several residue trials, involving separate analysis of peel
and pulp or surface extraction, indicate that at least 90% of the
residue in or on apples and pears is on the surface of the fruit
(Getzendaner, 1968; Komblas, 1969; Getzendaner and Corbin, 1969).
Typically, about 0.1 ppm tricyclohexyltin hydroxide equivalent may be
expected in fruit when the peeling is removed. Mild water washing
removed up to 50% of the residue from apples and pears with an average
of >20% removed (Getzendaner and Corbin, 1969).
Evidence of residues in food in commerce or at consumption
No information is available.
METHODS OF RESIDUE ANALYSIS
Corbin (1970) recently published a method suitable for determination
of the total organotin and inorganic tin present in fruit The sample
is subjected to a wet oxidation treatment with sulfuric and nitric
acids to destroy organic matter, remove volatile acids and halogens
and convert the tin to a soluble inorganic form. The tin is then
separated from elements which would interfere in the colorimetric
method (heavy metals) and from non-interfering salts. This separation
involves extracting tin as SnI4, with n-hexane from a strong sulfuric
solution of KI. Evaporation of hexane and conversion to sulfate leaves
the tin in a form measurable by a dithiol colorimetric method.
Sensitivity of the method is 0.01 ppm. Optional steps are included in
the method for removal of arsenic and antimony, if these elements are
suspected of being present in amounts which might cause interference
(> 0.15 mg and > 2 mg, respectively).
A complementary method has been developed for determining residues of
organotin in fruit by M & T Chemicals (Anon, 1969). Tri-, di-, and
monocyclohexyltin are solubilized by treatment of the fruit sample
with hydrochloric acid (9 molar) and then extracted with chloroform
from inorganic tin and heavy metals, which remain in the acidic
aqueous phase. The organotin compounds are then oxidized and
determined colorimetrically.
NATIONAL TOLERANCES
Tolerances have been established (provisional) or are proposed for
residues of tricyclohexyltin hydroxide in or on apples and pears in
various countries including the following:
Country Tolerance Withdrawal between
ppm treatment and harvest, days
Australia1 3 2
Belgium 12 28
Italy 1 20
Israel 1 30
Netherlands 1 28
U.S.A. 23 14
1 Stone fruits
2 Proposed
3 Tolerance proposed on 23 June 1970
Use of the miticide is registered for apples and pears in Chile,
France and Korea, but without provision for tolerance.
APPRAISAL
Tricyclohexyltin hydroxide is a non-systematic acaricide now used in
many countries and likely to be used worldwide on apples and pears,
with indications of future use on citrus, stone fruits and other
crops. Post-harvest use on apples is under investigation. The compound
is relatively harmless to beneficial insects and mites, and it is
usefully applied to integrated control schemes. Tricyclohexyltin
hydroxide is used as wettable powder at a rate of about 0.5 to 1.5 kg
per hectare.
Comprehensive residue data on tricyclohexyltin hydroxide on apples and
pears are available from the United States and from five other
countries, which are consistent with those from the U.S.A. Residue
data on other crops were not available to the Meeting. When apples and
pears are peeled, most of the tricyclohexyltin hydroxide residue is
removed; only 0.1 ppm may be expected in the fruit flesh. Washing is
found to remove 20-50% of the residues, more from apples than from
pears.
Field experiments show that the residues of tricyclohexyltin hydroxide
declined to half in about three weeks on apples and pears. The
disappearance is due to photodegradation, codistillation with water
and weathering factors. The residues are found to occur on the surface
of the treated plants. The degradation of tricyclohexyltin hydroxide
is found to produce dicyclohexyltin oxide and monocyclohexylstannoic
acid and finally inorganic tin salts.
The absorption of tricyclohexyltin hydroxide from the gastrointestinal
tract of animals is small. The distribution patterns of the compound
are determined in rats, dogs and cattle. When cows were fed residues
of 30 and 100 ppm, the residues found in milk were less than 0.1 ppm
and in various tissues less than 0.5 ppm. Limited feeding of apple and
pear pulp to cows is not expected to produce detectable residues in
meat or milk.
A colorimetric method with a detectability of 0.01 ppm tin is
available to determine tricyclohexyltin hydroxide and its organic
degradation products. The method is not able to distinguish between
the various compounds,but reflects the total amount of the compounds
in the sample analyzed.
RECOMMENDATIONS FOR TEMPORARY TOLERANCE
The tolerance is expressed in terms of tricyclohexyltin hydroxide. The
organic degradation products and the inorganic tin salts derived from
tricyclohexyltin hydroxide are not included in the tolerance. A
temporary tolerance of 2 ppm, based on an interval of two weeks from
the last treatment to the harvest, is recommended for apples and
pears.
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Further studies on the effects of exposure to tricyclohexyltin
hydroxide on copper balance and, in particular, on the copper
content of the liver.
2. Further information on the occurrence of liver and pituitary gland
cysts in female rate fed the compound.
3. Further information on the effect of the compound on the rate of
body-weight gain after allowing for the unpalatability of test
diets.
4. Further information on the significance of the brown discoloration
of the serosal surface of the intestine of treated animals.
5. Establishment of analytical procedures capable of distinguishing
qualitatively between tricyclohexyltin compounds and other
organotin compounds, particularly the fentin compounds, and, where
possible, of quantitative measurement of the separate compounds.
DESIRABLE
Data on residues of tricyclohexyltin hydroxide on apples and pears
moving in international commerce.
REFERENCES
Allison, W.E. (1969) An evaluation of the systemic (soil and foliar)
acaricidal activity of tricyclohexyltin hydroxide. Unpublished report
from the Dow Chemical Co.
Anon. (1969) Determination of small amounts of organotin in macerated
fruit. Method No. TA-2-2. Unpublished report from M & T Chemicals,
Inc.
Anon. (1970a) Tin content of tissues of rats and dogs fed varying
amounts of tricyclohexyltin hydroxide in the daily diet for two years.
Unpublished report from the Dow Chemical Co.
Anon. (1970b) Compilation of studies on residues of tricyclohexyltin
hydroxide in or on apples and pears: Australia, France, Netherlands,
and Japan. Unpublished report
Anon. (1970c) Dicyclohexyltin oxide: summary of acute oral toxicity
tests. Unpublished report from the Dow Chemical Company
Corbin, H.B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Offic. Anal.
Chemists, 53: 140-146
Getzendaner, M.E. (1968) A review of residue information on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Getzendaner, M.E. and Corbin, H.A. (1969) Residue study: inorganic
and organic tin compounds in or on apples and pears from field
applications of PLICTRAN(R) miticide. Unpublished report from the Dow
Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970a) Determination of tin
residues in orchard grass exposed to tricyclohexyltin hydroxide
miticide. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970b) Determination of tin
residues in beef calves fed tricyclohexyltin hydroxide for ninety
days. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970c) Determination of tin
residues in milk, cream, and body tissue of dairy cows fed
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Gray, H.E. (1968) PLICTRAN miticide - new approach to mite control.
Down to Earth, 23 (4): 3-5
Hine, C.H. (1966) Acute oral toxicity of tricyclohexyltin hydroxide in
Long Evans strain rats (17 March 1966). Unpublished report from the
Hine Laboratories, Inc. to Dow Chemical Co.
Hine, C.H. (1967) Acute oral toxicity: Cyclohexylstannoic-acid,
dicyclohexyltin oxide, tetracyclohexyltin, hexacyclohexylditin.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Brownlow, E.K., Cummins, J.T., Eisenlord, G.H. and Wong,
L.C.K. (1969) Studies on the mechanism of action of DOWCO 213.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969a) DOWCO 213
reproduction and teratology studies in rats. Unpublished report from
the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969b) DOWCO 213
reproduction and teratology studies in rabbits. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970a) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in dogs. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970b) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in rats. Unpublished report
from the Hine Laboratories, Inc.
Jeppson, L.R., Jesser, M.J. and Complin, J.O. (1968) Responses of the
pacific spider mite and the citrus red mite to laboratory and field
applications of tricyclohexyltin hydroxide. J. Econ. Entomology, 61:
1502-1505
Kenaga, E.E. (1968) The rodent toxicity and repellency of
tricyclohexyltin compounds to two species of mice. Unpublished report
from the Dow Chemical Co.
Komblas and Kostas N. (1969) Determination of residues of PLICTRAN
acaricide on apples and pears in Italy. (Translation of report of
Societá Italo-Americana Prodotti Antiparassitari, Balliera/Bologna,
Italy). Unpublished report from the Dow Chemical Co.
Laker, T.L., Elsea, J.R. and Ede, M. (1966) Repeated insult patch test
on M-2527 and cloth material TE98-SEU3501. Unpublished report from
Hill Top Research, Inc.
Norris, J.M. (1968) Toxicity of tricyclohexyltin hydroxide via i.p.
route in laboratory animals. Unpublished report from the Dow Chemical
Co.
Olson, K.J. (1964) Results of range finding toxicological tests on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Smith, G.N., Axelson, R.J. and Schiesser, L.H. (1970) The loss of
PLICTRAN(R) miticide from various soils. Unpublished report from the
Dow Chemical Co.
Smith, G.N. and Fischer, F.S. (1970) Metabolism of PLICTRAN(R)
miticide. Observations on the absorption, distribution, and excretion
of tricyclohexyltin-Sn119 hydroxide in white rats. Unpublished report
from the Dow Chemical Co.
Smith, G.N., Fischer, F.S. and Axelson, R.J. (1970) The volatilization
and photodecomposition of PLICTRAN(R) miticide. Unpublished report
from the Dow Chemical Co.
Smith, G.N. and Taylor, Y.S. (1970) The absorption and translocation
of PLICTRAN miticide and its decomposition products in bean and corn
plants. Unpublished report from the Dow Chemical Co.
Stevenson, G.T. and Kenaga, E.E. (1969) The effects of dietary feeding
of tricyclohexyltin hydroxide on the life stages of the Japanese
Quail. Unpublished report from the Dow Chemical Co.
Tucker, W.E. (1966) Oral toxicity studies of tricyclohexyltin
hydroxide in rats. Unpublished data from the Dow Chemical Co.
Wazeter, F.X., Buller, R.H., Geil, R.G. and Long, J.E. (1968)
Dicyclohexyltin oxide: 90-day feeding study in the Long-Evans rat.
Unpublished report from the International Research and Development
Corporation
Wazeter, F.X. (1969) 21-day dermal study in,albino rabbits-PLICTRAN
M-3180 miticide. Unpublished report from the International Research
and Development Corporation
Whitney, W.K. (1966) Leaching tests with DOWCO 213 in soil
Zambelli, N., Komblas, W. and Kovacs, A. (1968) PLICTRAN miticide for
the control of spider mites in Italy. Down to Earth, 24(3): 25-31
Tricyclohexyltin hydroxide is not volatile from dry surfaces, but
'co-distils', with water from moist surfaces at a slow but finite rate
(Smith et al., 1970). Field experiments show that the half-life of the
compound is about four days on grass and about three weeks on apples
and pears; the disappearance results from photodegradation,
'co-distillation' with water from moist surfaces and weathering
factors (Getzendaner and Gentry, 1970a; Getzendaner and Corbin, 1969).
In animals
As indicated under "BIOCHEMICAL ASPECTS", tricyclohexyltin hydroxide
is not readily absorbed from the gastrointestinal tract.
When the miticide is fed daily to rats, dogs or cattle, tin compounds,
primarily unchanged tricyclohexyltin hydroxide, accumulate in the
tissues to a limited extent, reach equilibrium values in a few months
to a year, and are slowly eliminated from the tissues after the
compound is withdrawn from the diet. It does not selectively partition
into fat, and the distribution pattern in tissues is similar for rats,
dogs and cattle (Smith and Fischer, 1970; Anon, 1970a; Getzendaner and
Gentry, 1970b). For example, tissues from calves fed 30 ppm of the
compound in the diet for 90 days showed 0.07 ppm total tin in fat,
0.09 ppm in muscle, 0.37 ppm in liver and 0.20 ppm in kidney
(Getzendaner and Gentry, 1970b).
Levels of tricyclohexyltin hydroxide equivalent were 0.05 ppm in milk
and 0.4 ppm in cream obtained from cows fed 100 ppm of the compound in
the diet for 53 days (Getzendaner and Gentry, 1970c).
In plants
Residues occurring on apples were identified by a series of extraction
techniques followed by analysis. Tricyclohexyltin hydroxide was the
main component of the tin residues, comprising 67% (0.66 ppm) of the
residue (eight days post-treatment). Small amounts of dicyclohexyltin
oxide and cyclohexylstannoic acid were present (5% of each), as well
as 23% inorganic tin (Getzendaner, 1968).
Tricyclohexyltin hydroxide and its sunlight decomposition products are
not translocated in plants to a significant extent. This has been
demonstrated by (1) the absence of significant residues of tin
compounds in the interior portions of fruit, even after periods of
several months between application of the first of up to 5 spray
applications and harvest (Getzendaner and Corbin, 1969), (2) the
absence of radioactive residues in bean and maize plants grown in soil
treated with exaggerated amounts (3 lb/acre) of 119Sn-labelled
miticide or its degradation (Smith at al. 1970), and (3) the essential
absence of translocation across the narrow thickness of the leaf of
cotton plants as determined by bioassay experiments (Allison, 1969).
In soil
Tricyclohexyltin hydroxide is markedly absorbed by soil and it is not
leached from soil, as demonstrated by soil-column leaching studies and
by study of the transfer from water to soil (Smith et al., 1970a,
Smith and Taylor, 1970; Whitney, 1966). No significant amounts of the
miticide will remain in or on the soil from one growing season to the
next.
In storage and processing
Results of several residue trials, involving separate analysis of peel
and pulp or surface extraction, indicate that at least 90% of the
residue in or on apples and pears is on the surface of the fruit
(Getzendaner, 1968; Komblas, 1969; Getzendaner and Corbin, 1969).
Typically, about 0.1 ppm tricyclohexyltin hydroxide equivalent may be
expected in fruit when the peeling is removed. Mild water washing
removed up to 50% of the residue from apples and pears with an average
of >20% removed (Getzendaner and Corbin, 1969).
Evidence of residues in food in commerce or at consumption
No information is available.
METHODS OF RESIDUE ANALYSIS
Corbin (1970) recently published a method suitable for determination
of the total organotin and inorganic tin present in fruit The sample
is subjected to a wet oxidation treatment with sulfuric and nitric
acids to destroy organic matter, remove volatile acids and halogens
and convert the tin to a soluble inorganic form. The tin is then
separated from elements which would interfere in the colorimetric
method (heavy metals) and from non-interfering salts. This separation
involves extracting tin as SnI4, with n-hexane from a strong sulfuric
solution of KI. Evaporation of hexane and conversion to sulfate leaves
the tin in a form measurable by a dithiol colorimetric method.
Sensitivity of the method is 0.01 ppm. Optional steps are included in
the method for removal of arsenic and antimony, if these elements are
suspected of being present in amounts which might cause interference
(> 0.15 mg and > 2 mg, respectively).
A complementary method has been developed for determining residues of
organotin in fruit by M & T Chemicals (Anon, 1969). Tri-, di-, and
monocyclohexyltin are solubilized by treatment of the fruit sample
with hydrochloric acid (9 molar) and then extracted with chloroform
from inorganic tin and heavy metals, which remain in the acidic
aqueous phase. The organotin compounds are then oxidized and
determined colorimetrically.
NATIONAL TOLERANCES
Tolerances have been established (provisional) or are proposed for
residues of tricyclohexyltin hydroxide in or on apples and pears in
various countries including the following:
Country Tolerance Withdrawal between
ppm treatment and harvest, days
Australia1 3 2
Belgium 12 28
Italy 1 20
Israel 1 30
Netherlands 1 28
U.S.A. 23 14
1 Stone fruits
2 Proposed
3 Tolerance proposed on 23 June 1970
Use of the miticide is registered for apples and pears in Chile,
France and Korea, but without provision for tolerance.
APPRAISAL
Tricyclohexyltin hydroxide is a non-systematic acaricide now used in
many countries and likely to be used worldwide on apples and pears,
with indications of future use on citrus, stone fruits and other
crops. Post-harvest use on apples is under investigation. The compound
is relatively harmless to beneficial insects and mites, and it is
usefully applied to integrated control schemes. Tricyclohexyltin
hydroxide is used as wettable powder at a rate of about 0.5 to 1.5 kg
per hectare.
Comprehensive residue data on tricyclohexyltin hydroxide on apples and
pears are available from the United States and from five other
countries, which are consistent with those from the U.S.A. Residue
data on other crops were not available to the Meeting. When apples and
pears are peeled, most of the tricyclohexyltin hydroxide residue is
removed; only 0.1 ppm may be expected in the fruit flesh. Washing is
found to remove 20-50% of the residues, more from apples than from
pears.
Field experiments show that the residues of tricyclohexyltin hydroxide
declined to half in about three weeks on apples and pears. The
disappearance is due to photodegradation, codistillation with water
and weathering factors. The residues are found to occur on the surface
of the treated plants. The degradation of tricyclohexyltin hydroxide
is found to produce dicyclohexyltin oxide and monocyclohexylstannoic
acid and finally inorganic tin salts.
The absorption of tricyclohexyltin hydroxide from the gastrointestinal
tract of animals is small. The distribution patterns of the compound
are determined in rats, dogs and cattle. When cows were fed residues
of 30 and 100 ppm, the residues found in milk were less than 0.1 ppm
and in various tissues less than 0.5 ppm. Limited feeding of apple and
pear pulp to cows is not expected to produce detectable residues in
meat or milk.
A colorimetric method with a detectability of 0.01 ppm tin is
available to determine tricyclohexyltin hydroxide and its organic
degradation products. The method is not able to distinguish between
the various compounds,but reflects the total amount of the compounds
in the sample analyzed.
RECOMMENDATIONS FOR TEMPORARY TOLERANCE
The tolerance is expressed in terms of tricyclohexyltin hydroxide. The
organic degradation products and the inorganic tin salts derived from
tricyclohexyltin hydroxide are not included in the tolerance. A
temporary tolerance of 2 ppm, based on an interval of two weeks from
the last treatment to the harvest, is recommended for apples and
pears.
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Further studies on the effects of exposure to tricyclohexyltin
hydroxide on copper balance and, in particular, on the copper
content of the liver.
2. Further information on the occurrence of liver and pituitary gland
cysts in female rate fed the compound.
3. Further information on the effect of the compound on the rate of
body-weight gain after allowing for the unpalatability of test
diets.
4. Further information on the significance of the brown discoloration
of the serosal surface of the intestine of treated animals.
5. Establishment of analytical procedures capable of distinguishing
qualitatively between tricyclohexyltin compounds and other
organotin compounds, particularly the fentin compounds, and, where
possible, of quantitative measurement of the separate compounds.
DESIRABLE
Data on residues of tricyclohexyltin hydroxide on apples and pears
moving in international commerce.
REFERENCES
Allison, W.E. (1969) An evaluation of the systemic (soil and foliar)
acaricidal activity of tricyclohexyltin hydroxide. Unpublished report
from the Dow Chemical Co.
Anon. (1969) Determination of small amounts of organotin in macerated
fruit. Method No. TA-2-2. Unpublished report from M & T Chemicals,
Inc.
Anon. (1970a) Tin content of tissues of rats and dogs fed varying
amounts of tricyclohexyltin hydroxide in the daily diet for two years.
Unpublished report from the Dow Chemical Co.
Anon. (1970b) Compilation of studies on residues of tricyclohexyltin
hydroxide in or on apples and pears: Australia, France, Netherlands,
and Japan. Unpublished report
Anon. (1970c) Dicyclohexyltin oxide: summary of acute oral toxicity
tests. Unpublished report from the Dow Chemical Company
Corbin, H.B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Offic. Anal.
Chemists, 53: 140-146
Getzendaner, M.E. (1968) A review of residue information on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Getzendaner, M.E. and Corbin, H.A. (1969) Residue study: inorganic
and organic tin compounds in or on apples and pears from field
applications of PLICTRAN(R) miticide. Unpublished report from the Dow
Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970a) Determination of tin
residues in orchard grass exposed to tricyclohexyltin hydroxide
miticide. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970b) Determination of tin
residues in beef calves fed tricyclohexyltin hydroxide for ninety
days. Unpublished report from the Dow Chemical Co.
Getzendaner, M.E. and Gentry, W.M. (1970c) Determination of tin
residues in milk, cream, and body tissue of dairy cows fed
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Gray, H.E. (1968) PLICTRAN miticide - new approach to mite control.
Down to Earth, 23 (4): 3-5
Hine, C.H. (1966) Acute oral toxicity of tricyclohexyltin hydroxide in
Long Evans strain rats (17 March 1966). Unpublished report from the
Hine Laboratories, Inc. to Dow Chemical Co.
Hine, C.H. (1967) Acute oral toxicity: Cyclohexylstannoic-acid,
dicyclohexyltin oxide, tetracyclohexyltin, hexacyclohexylditin.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Brownlow, E.K., Cummins, J.T., Eisenlord, G.H. and Wong,
L.C.K. (1969) Studies on the mechanism of action of DOWCO 213.
Unpublished report from the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969a) DOWCO 213
reproduction and teratology studies in rats. Unpublished report from
the Hine Laboratories, Inc.
Hine, C.H., Eisenlord, G.H. and Laudel, A. (1969b) DOWCO 213
reproduction and teratology studies in rabbits. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970a) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in dogs. Unpublished report
from the Hine Laboratories, Inc.
Hine, C.H. (1970b) Results of two-year dietary feeding study with
tricyclohexyltin hydroxide (DOWCO 213) in rats. Unpublished report
from the Hine Laboratories, Inc.
Jeppson, L.R., Jesser, M.J. and Complin, J.O. (1968) Responses of the
pacific spider mite and the citrus red mite to laboratory and field
applications of tricyclohexyltin hydroxide. J. Econ. Entomology, 61:
1502-1505
Kenaga, E.E. (1968) The rodent toxicity and repellency of
tricyclohexyltin compounds to two species of mice. Unpublished report
from the Dow Chemical Co.
Komblas and Kostas N. (1969) Determination of residues of PLICTRAN
acaricide on apples and pears in Italy. (Translation of report of
Societá Italo-Americana Prodotti Antiparassitari, Balliera/Bologna,
Italy). Unpublished report from the Dow Chemical Co.
Laker, T.L., Elsea, J.R. and Ede, M. (1966) Repeated insult patch test
on M-2527 and cloth material TE98-SEU3501. Unpublished report from
Hill Top Research, Inc.
Norris, J.M. (1968) Toxicity of tricyclohexyltin hydroxide via i.p.
route in laboratory animals. Unpublished report from the Dow Chemical
Co.
Olson, K.J. (1964) Results of range finding toxicological tests on
tricyclohexyltin hydroxide. Unpublished report from the Dow Chemical
Co.
Smith, G.N., Axelson, R.J. and Schiesser, L.H. (1970) The loss of
PLICTRAN(R) miticide from various soils. Unpublished report from the
Dow Chemical Co.
Smith, G.N. and Fischer, F.S. (1970) Metabolism of PLICTRAN(R)
miticide. Observations on the absorption, distribution, and excretion
of tricyclohexyltin-Sn119 hydroxide in white rats. Unpublished report
from the Dow Chemical Co.
Smith, G.N., Fischer, F.S. and Axelson, R.J. (1970) The volatilization
and photodecomposition of PLICTRAN(R) miticide. Unpublished report
from the Dow Chemical Co.
Smith, G.N. and Taylor, Y.S. (1970) The absorption and translocation
of PLICTRAN miticide and its decomposition products in bean and corn
plants. Unpublished report from the Dow Chemical Co.
Stevenson, G.T. and Kenaga, E.E. (1969) The effects of dietary feeding
of tricyclohexyltin hydroxide on the life stages of the Japanese
Quail. Unpublished report from the Dow Chemical Co.
Tucker, W.E. (1966) Oral toxicity studies of tricyclohexyltin
hydroxide in rats. Unpublished data from the Dow Chemical Co.
Wazeter, F.X., Buller, R.H., Geil, R.G. and Long, J.E. (1968)
Dicyclohexyltin oxide: 90-day feeding study in the Long-Evans rat.
Unpublished report from the International Research and Development
Corporation
Wazeter, F.X. (1969) 21-day dermal study in,albino rabbits-PLICTRAN
M-3180 miticide. Unpublished report from the International Research
and Development Corporation
Whitney, W.K. (1966) Leaching tests with DOWCO 213 in soil
Zambelli, N., Komblas, W. and Kovacs, A. (1968) PLICTRAN miticide for
the control of spider mites in Italy. Down to Earth, 24(3): 25-31
