
WHO Pesticide Residues Series, No. 1
1971 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The evaluations contained in these monographs were prepared by the
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues that met
in Geneva from 22 to 29 November 1971.1
World Health Organization
Geneva
1972
1 Pesticide Residues in Food: Report of the 1971 Joint Meeting of
the FAO Working Party of Experts on Pesticide Residues and the WHO
Expert Committee on Pesticide Residues, Wld Hlth Org. techn. Rep.
Ser., No. 502; FAO Agricultural Studies, 1972, No. 88.
These monographs are also issued by the Food and Agriculture
Organization of the United Nations, Rome, as document AGP-1971/M/9/1.
FAO and WHO 1972
TRICHLORONAT
IDENTITY
Chemical names
O-ethyl-O-(2,4,5-trichlorophenyl)-ethylmonothiophosphonate
ethyl 2,4,5-trichlorophenyl ethylphosphonothionate
Synonyms
(R) Agritox, (R) Phytosol, (R) Agrisil, BAY 37 289, S 4400
Structural formula
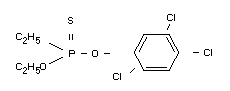 Other information on identity and properties
The active ingredient is a light brown liquid and has a boiling point
of 108°C at 0.01 mm Hg. It has a vapour pressure of 1.5 × 10-5 mm Hg
at 20°C and a volatility of 0.27 mg/m3 at 20°C. Its solubility in
water at 20°C is approximately 50 ppm, in kerosenes poor, but in
alcohol, acetone, chlorinated hydrocarbons and aromatic solvents good.
The stability of the active ingredient to hydrolysis is high in the
acid range, but in the alkaline range it decomposes quite readily
(Bayer, 1969).
Composition of the technical trichloronat is reported to be (Bayer,
1971):
active ingredient 93.0 - 95.0%
free 2,4,5-trichlorophenol 0.05 - 0.5%
O-ethyl-O-(2,4-dichlorophenyl)-ethylmonothiophosphonate 0.4 - 1.0%
O,O-diethyl ethylthiophosphonate 0.1 - 0.5%
sum of two unknown, low boiling compounds 1.0 - 1.5%
sum of three to four unknown, high
boiling compounds 3.0 - 5.0%
H2O 0.01 - 0.9%
Furthermore an analytical method for the determination of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the technical material has
been worked out. Samples analysed have contained less than 0.1 ppm of
"dioxin" which is the limit of detection. It is guaranteed by the
manufacturer that the "dioxin" content of the trichloronat
preparations is less than 0.1 ppm in terms of the active ingredient
(Bayer, 1971).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption and distribution
No information available.
Bio-transformation
No information available.
Excretion
No information available.
Effects on enzymes and other biochemical parameters
Three groups of three male rats orally dosed with 7.5, 3.8, or 1.9
mg/kg showed whole blood cholinesterase depression of 30, 15, and 0%
after three hours, 35, 25 and 15% after 24 hours, and 15, 0-10, and 0%
after 72 hours respectively (Kimmerle, G., 1962).
Trichloronat is ineffective as a cholinesterase inhibitor in vitro,
but is a strong inhibitor in vivo after i.p. administration of
7 mg/kg to rats. Maximum inhibition of brain, serum, and submaxillary
gland cholinesterase occurred in 1-6 hours. Reversal of inhibition was
slow, only returning to 60-75% of normal after 7 days (Root et al.,
1969).
Inhalation exposure of groups of 10 hens, for four hours five times
weekly for four weeks resulted in 60.4, and 76.7% whole blood
cholinesterase depression at air concentrations of 0.033, and 0.048
mg/l respectively (Kimmerle, G., 1968).
TOXICOLOGICAL STUDIES
Special studies
Reproduction
Four groups each comprising 10 male and 20 female rats were fed 0, 3,
10 and 30 ppm 95% pure trichloronat in the diet through three
generations. The second litter from each generation, F1b, and F2b,
was used as parents to establish the next generation.
No adverse effects were noted among the parental animals except for
depression of growth rate at 30 ppm. Reproductive performance was
normal at all dose levels, but pup growth was depressed at 30 ppm,
although litter size and birth weight were normal. In the third
generation, the survival of the pups was adversely affected by 30 ppm
trichloronat. No terata were observed (Löser, 1971).
Neurotoxicity
Exposure of groups of five hens to 0.055, 0.126, 0.248, or 0.585 mg/l
air for four hours resulted in 100% mortality at the two upper dose
levels. Examination of survivors 42 hours after exposure did not
reveal neurotoxic effects. Further groups pre-treated i.m. with 100 mg
PAM/kg and 50 mg atropine sulphate/kg exposed to 0.185, 0.4, 0.583 or
0.784 mg/l air resulted in three deaths out of five at the upper two
dose levels. All survivors exposed to 0.4 mg/l air and above showed
neurotoxic effects. Four-hour exposures repeated on five consecutive
days at 0.053, 0.126, 0.143 or 0.162 mg/l air did not result in
neurotoxic effects, although 1/5 and 5/5 hens died at the two top dose
levels. Birds similarly exposed after PAM and atropine treatment, to
levels of 0.222, or 0.376 mg/l air, all showed neurotoxic effects.
Neurotoxic effects were not observed following four-hour exposures
five times weekly for four weeks to air concentrations of 0.033 or
0.048 mg/l air (Kimmerle, 1968).
Neurotoxic effects were not observed in 1-2 year-old hens unprotected
against the cholinergic effects of trichloronat when single doses up
to those which proved lethal were given. However, when the hens were
protected with i.p. injections of atropine sulphate and 2-PAM, higher
doses of trichloronat could be given and 100 mg/kg i.p. or 150 mg/kg
orally produced neurotoxic effects. This effect of trichloronat was
not detected in a sub-acute experiment in which 0, 250, 500, 1000 and
2000 ppm trichloronat in the diet was fed to groups of eight hens for
30 days. Two hens per group were killed after the treatment period,
the rest were killed 30 days later; neurotoxic effects were not
observed (Kimmerle, 1965).
Potentiation
Trichloronat administered simultaneously with malathion resulted in a
two-fold potentiation of the malathion toxicity (Root et al., 1969).
Toxicity of contaminants
2,4,5-Trichlorophenol is known to occur as a contaminant of
trichloronat at a level of 1-2%.
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
2,3,7,8-Tetrachlorodibenzo-p-dioxin ("dioxin") is also a possible
contaminant of trichloronat. The sole manufacturer of trichloronat
states that the level of "dioxin" is below 0.1 ppm in technical
trichloronat. (See "Identity").
Acute toxicity
Species Route LD50 Reference
(mg/kg)
Mouse i.p. 33-35 DuBois and Kinoshita, 1963
Rat (M) oral 16-55 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1968; Kimmerle, 1967
Rat (F) oral 16-37.5 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Rat (M) i.p. 10-14 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Rat (F) i.p. 11-37.5 Dubois et al., 1966;
Kimmerle, 1962
Species Route LD50 Reference
(mg/kg)
Rat dermal 64-250 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Guinea-pig oral 40-100 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Guinea-pig i.p. 26 DuBois and Kinoshita, 1963
(M)
Rabbit oral 25-50 Kimmerle, 1962
Cat oral 10-25 Kimmerle, 1962; Kimmerle,
1968
Chicken oral 45 DuBois, 1963
Groups of five female rats were injected i.p., daily for 60 days, at
dose levels of 0, 2, 4, 6 and 8 mg/kg/day. Mortality resulted in 2 out
of 5, 4 out of 5, and 5 out of 5 at 4, 6 and 8 mg/kg respectively.
Body weight was reduced at all dose levels, although some recovery
occurred at 2 and 4 mg/kg after 20 days on the test. Survivors
autopsied at 60 days showed marked depression of serum, submaxillary
gland, and brain cholinesterase (DuBois et al., 1966).
Rabbit. A pair of rabbits dosed orally, five times weekly for two
weeks with 5 mg/kg/dose did not lose body weight or display symptoms
of poisoning (Kimmerle, 1962).
Liver function tests (BSP, SG.OT, SG-PT) were unaffected by 20 mg/kg
(one rabbit) or 10 mg/kg (two rabbits) administered as a single oral
dose (Kimmerle, 1962).
Dog. Groups of beagle dogs were fed 0, 2, 5, 10 (two males and two
females/group or 25 (one male and one female) ppm in dry diet for 12
weeks. At termination of the study, erythrocyte cholinesterase was
depressed at 25 ppm (40%) in the females. Plasma cholinesterase was
depressed in both sexes at 10 ppm (39% in males and 27% in females)
but in females only at 25 ppm (41%). Brain cholinesterase was
comparable to control values for all groups. Liver cholinesterase was
depressed in males at 5 ppm and above (depression exceeding 20%), and
in females at 10 ppm and above. Thyroid weight and weight ratio were
decreased at 25 ppm (Root at al., 1968b). Histopathological changes
including lymphocytic proliferation of the intestinal wall were not
dose-related (Hibbs and Nelson, 1968).
Groups of two male and two female beagle dogs were fed 0 or 5 ppm in
the dry diet for two years. A further group was fed 25 ppm for 18
months. The 25 ppm dose level was then increased to 50 ppm for the
next seven weeks, to 100 ppm for the subsequent 14 weeks and finally
to 200 ppm until the termination of the study. A final group was fed 2
ppm for two years, and then 1 ppm for a further 13 weeks, and then
control diet for the final eight weeks prior to autopsy. Body symptoms
in all cases reported were typical of cholinesterase depression.
Short-term studies
Rat. Groups of 10 male rats were incubated five times weekly for
eight weeks with 0.75, 1.5, 3, 6, or 12 mg/kg/dose followed by a four
week observation period. Symptoms of cholinesterase depression were
seen at 12 mg/kg after the first dose only (Kimmerle, 1968).
Groups of 20 male and 20 female rats were fed 0, 1, 3, 10 or 30 ppm of
95% pure trichloronat in the diet for three months. Final body weight
was comparable, but males fed 10 and 30 ppm showed a slight dose
related lag in weight gain during the second month. Signs of
cholinesterase depression were seen during the morning from the second
week in rats fed 30 ppm and in some rats fed 10 ppm. Plasma and
erythrocyte cholinesterase levels were depressed at 10 and 30 ppm,
erythrocyte cholinesterase being depressed slightly more than plasma
cholinesterase. Depression at 3 ppm in plasma and erythrocytes was
less than 20% (Löser, 1968).
Groups of 15 male and 15 female rats were fed 2.25, 4.5, 9 or 18 ppm
of technical trichloronat in the diet for four months. The control
group comprised 30 male and 30 female rats. Cholinesterase depression
in whole blood was apparent in females at 4.5 and 9 ppm at four weeks.
At eight weeks, male rats showed depression at 9 and 18 ppm, and
females at 18 ppm only. At 12 and 16 weeks, cholinesterase depression
was apparent in both sexes at 18 ppm only (Löser, 1966).
Histopathological examination revealed low-grade fatty changes in
isolated epithelial cells of the liver of five male rats fed 18 ppm
(Hobik, 1967).
Five groups of 10 male and 10 female rats were fed 0, 2, 5, 10 or 25
ppm in the diet for 16 weeks. Terminal cholinesterase determinations
indicated depression occurred in erythrocytes at 10 and 25 ppm, in
brain and submaxillary glands at 25 ppm and in plasma of female rats
at 25 ppm. Depression was less than 20% in all other groups, and less
than 10% except for plasma in females at 10 ppm and erythrocytes in
males at 5 ppm (Root at al., 1968a). The presence of dilated kidney
tubules with eosinophilic casts was observed in all groups, but the
incidence was considerably greater in the test groups (Grey at al.,
1968). Weight and food consumption were reduced at 200 ppm. Signs of
cholinesterase depression became apparent within three weeks of
feeding 200 ppm. One male dog died after nine weeks exposure at this
level. Erythrocyte cholinesterase was comparable to controls until the
dose was increased to 75 ppm, at which dose depression occurred. The
depression increased when the dose was increased to 200 ppm. Plasma
cholinesterase was depressed at 2 ppm and above, depression being
about 20% at 2 ppm. When the dose was reduced to 1 ppm, recovery to
99% of normal activity occurred. At autopsy, liver cholinesterase was
inhibited 45% at 5 ppm. No reduction was observed in the group reduced
from 2 to 1 to 0 ppm. Brain cholinesterase was reduced 10% at 5 ppm,
and 72% at the top dose level. No compound related histological
changes were apparent (Root et al., 1970).
Long-term studies
Rat. Four groups of 30 male and 30 female rats were fed 1, 3, 10, or
30 ppm of 95% pure trichloronat in the diet for two years. The control
group comprised 60 males and 60 females. Body weight of males at 30
ppm was reduced, female body weight at 30 ppm was reduced between 9
and 12 months. Food consumption at 30 ppm was marginally reduced.
Average absolute weight of male heart, lung, liver, kidney, and spleen
were depressed, but organ/body weight ratio was unaffected. Signs of
cholinesterase depression were apparent in the morning during the
first three months in the 30 ppm group. Plasma and erythrocyte
cholinesterase were depressed at 10 and 30 ppm, although at 10 ppm the
depression became marginal as the study progressed. Terminal brain
cholinesterase was depressed at 10 and 30 ppm in both sexes, and
marginally depressed (17%) in males at 3 ppm. Histopathological data
have not been submitted (Löser, 1970).
Observations in man
No information is available.
Comments
Trichloronat is a persistent organophosphorus insecticide used for
soil treatment. No information is available on the absorption,
distribution, excretion or general metabolism in animals.
Short-term studies in the rat and dog, one long-term (two-year) study
in the rat and a three-generation reproduction study in rats are
available. In the short-term study some histopathological changes were
observed, the nature of which could not be assessed. In the long-term
studies insufficient information was available both on gross and
histopathology of the organs.
For this reason and because there is no information on the metabolism
of this persistent insecticide the Meeting decided that no acceptable
daily intake for man could be established at this time.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichloronat is an insecticidal compound with a contract and stomach
action and with a residual activity of relatively long duration for a
phosphorus insecticide. It is especially used for the control of
soil-inhabiting developmental stages of different insect species and
vegetable fly larvae. The formulations currently on the market are as
follows (Bayer, 1969; 1971):
2.5% granular (Gr.)
7.5% granular (Gr.)
20.0% seed dressing powder (S.D.P.)
50.0% emulsifiable concentrate (E.C.)
Practically all trichloronat manufactured is applied to vegetable
crops.
Pre-harvest treatments
The recommended usages of trichloronat (Bayer, 1969) with regard to
crops, pests, formulations, and rates are given below:
Onions, leeks Hylemyia antiqua (Onion fly)
50% E.C. 10 litres per hectare, spray presowing and work
in just below the surface;
0.1% (250 cm3 per running metre), drench at
egg-laying;
7.5% Gr. 25 kg/hectare (or 7.5 g/10 metres), row treatment
at sowing;
2.5% Gr. 80-100 kg/hectare (or 2.5 g per running metre),
row treatment at sowing;
20% S.D.P. 75-100 g/kg seed, before treatment, moisten
seed using 50-75 cm3 of water; sow within 24
hours of treatment.
Brassica crops Hylemyia brassicae (Cabbage root fly)
Hylemyia floralis (Turnip fly)
50% E.C. 0.04% (1 litre/m2), treatment of nursery seedbed
or 10 litres per hectare, spray presowing, and
work in just below surface, or
0.1% (250 cm3 per running metre, or 80-100 cm3/
Plant), drench at egg-laying;
7.5% Gr. 4 g/m2, treatment of seedbed, or 25 kg/hectare,
row treatment at transplanting;
Onions, leeks Hylemyia antiqua (Onion fly)
2.5% Gr. 1-2 kg/m3 for treatment of nursery bed soil, or
800 g/100 metres, row treatment at planting or
100 kg/hectare, row treatment at transplanting,
or 1-2 g/plant applied to stem base at
transplanting (also as mixture with sand), or
3-5 g/plant hole at transplanting.
Carrots Psila rosae (Carrot fly)
50% E.C. 10 litres/hectare, spray presowing and work in
just below surface;
2.5% Gr. 100 kg/hectare, row treatment at sowing;
20% S.D.P. 100 g/kg seed.
Cereals Hylemyia coarctata (Wheat bulb fly)
2.5% Gr. 80-100 kg/hectare, broadcast treatment;
20% S.D.P. 250 g/100 kg seed.
If fungicides are additionally applied use only
non-mercurial products.
Bananas Cosmopolites sordidus (Banana weevil borer)
2.5% Gr. 40-60 g/banana plant, apply in a radius of up
to 40 cm around stem.
Grassland Costelytra zealandica, Heteronychus arator,
Oncopera intricata, Wiseana
2.5% Gr. 20-80 kg/hectare, broadcast treatment;
50% E.C. 2 litres/hectare, broadcast treatment.
Trichloronat is recommended for testing on some additional pests.
Other uses
The compound is also used for termite-proofing of polyethylene,
plasticized PVC and rubber.
Residues resulting from supervised trials
The residue data from supervised trials are given in Table I. They are
compiled from the documentation made available by Bayer (1971).
TABLE I
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Onions Germany 50% E.C. 10 l/ha 100-133 n.d.-0.02
Belgium " 8 l/ha 35 n.d.
Germany " 0.1% spray
250 ml/row-m 58; 71 5.5; 1.6
Germany 2.5% Gr. 200 kg/ha 163 n.d.
" " 1.5; 2.5 g/
row-m 100-133 n.d.-0.1
Finland 20% S.D.P. 100 g/kg seed 91-119 n.d.-0.3
Denmark " 3; 5 g/kg
transplant roots 82; 110 0.2; 1.2
Leeks Germany 50% E.C. 10 l/ha 121 n.d.
Cabbages Germany 50% E.C. 2% spray 2 ml/
plant 55 n.d.
Belgium " 0.2% spray
100 ml/plant 48 n.d.
Germany " 0.1% spray
250 ml/row-m 110 n.d.
USA E.C. 1 x 0.13 g a.i./
plant + 2 × 0.18 g/
a.i./row-m 23-44 n.d.
Germany 2.5% Gr. 2 g/plant 31; 62 n.d.
Cauliflower Germany 50% E.C. 6; 8; 10 l/ha 50-69 n.d.-0.01
" " 0.05% spray
100 ml/plant 55 n.d.
" " 0.1% spray
80 ml/plant 42; 52 n.d.
" " 2% spray 2 ml/
plant 69 n.d.
Cauliflower USA E.C. 1 × 0.13 g a.i./ 42-61 n.d.
plant + 2 × 0.18 g
a.i./row-m
Germany 2.5% Gr. 2 g/plant 35-60 n.d.-0.1
" 20% S.D.P. 100 g/kg seed 76 n.d.
Broccoli USA E.C. 1 × 0.13 g a.i./ 26-41 n.d.
plant + 2 × 0.18 g
a.i./row-m
Brussels USA E.C. 1 × 0.13 g a.i./ 40-58 n.d.
sprouts plant + 2 × 0.18 g
a.i./row-m
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Kohlrabi Germany 50% E.C. 6; 8; 10 kg/ha 30-50 0.01-0.08
" " 0.05% spray
100 ml/plant 19-45 0.01-0.1
" 2.5% Gr. 1 g/plant 19-45 0.04-0.4
Carrots Denmark 50% E.C. 4 l/ha 76-174 0.05-0.2
" " 8 l/ha " 0.04-0.2
Belgium " " 44; 79 3.6;* 5.0*
Germany " 10 l/ha 94; 97 0.2; 0.5
" " " 68-148 0.06-0.4
" 2.5% Gr. 100 kg/ha 64-92 0.05-0.3
Norway " " 62-139 0.3-0.6
France " " 135-172 0.01-0.02
Belgium " " 51; 81 n.d.
Italy " " 193 0.2
Denmark " 80 kg/ha 76 19.0*
" " " 90 11.0*
" " " 100-187 1.1-3.8*
Germany " 150 kg/ha 46-166 n.d.-2.0*
Holland " 2.5 g/row-m 111 0.6
Sweden " 3.5 g/row-m 156 0.8
" " 5.0 g/row-m " 1.4
" " 9.5 g/row-m " 2.3
Denmark 20% S.D.P. 100 g/kg 76-174 0.03-0.3
Finland " " 85-119 0.02-0.2
Barley Germany 2.5% Gr. 100 kg/ha 135 n.d.
Corn " 2.5% Gr. 80 kg/ha 169 n.d.
kernels USA Gr.; E.C. 5.6 kg a.i./ha 34-114 n.d.
cobs " " " " n.d.
forage " " " " n.d.
Oats Germany 2.5% Gr. 100; 150 kg/ha 97; 135 n.d.
Rye " 20% S.D.P. 250 g/kg seed 294 n.d.
Bananas Ecuador 2.5% Gr. 45; 67.5 g/plant 3-180 n.d.
Potatoes Germany 2.5% Gr. 150 kg/ha 150 0.02
Belgium " " 49; 62 0.3; n.d.
sweet USA Gr.; E.C. 5.6 kg a.i./ha 116-164 n.d.
Radish Germany 50% E.C 5; 10 kg/ha 21; 36 0.01; 0.1
" 2.5% Gr. 100 kg/ha 21; 36; 41 0.03; 0.04;
0.1
" 20% S.D.P. 100 g/kg seed 58 0.5-0.7
Finland " " 47 0.5
Rutabaga Finland 20% S.D.P. 100 g/kg seed 91; 135 n.d.; 0.05
Denmark " " 73; 153 n.d.
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Sugar-beets
roots Germany 2.5% Gr. 150 kg/ha 160 n.d.
tops " " " " n.d.
roots USA 10% Gr. 0.1-0.3 g a.i./
row-m 147-190 n.d.-0.1
tops " " " " n.d.-0.02
Turnips
roots Germany 2.5% Gr. 150 kg/ha 175 n.d.
tops " " " 163 0.01
1 E.C. = emulsifiable concentrate.
Gr. = granular.
S.D.P. = seed dressing powder.
2 Rate given in terms of formulation if not indicated
by a.i. = active ingredient.
3 n.d. = non-detectable (varies 0.01-0.1 ppm).
* Considered that the residue has not met the local requirements
of good agricultural practice.
It has been found (Brewerton et al., 1968) that the trichloronat
residues in pasture crops were at or below 5 mg/kg (dry matter basis)
after two weeks from treatment at 0.5 and 1 kg active ingredient per
hectare, after one month from treatment at 2 kg a.i. per ha, and after
two months from treatment at 4 kg a.i. per ha.
Fate of residues
General comments
Trichloronat is a thiophosphonate which is highly effective against
insects which live in or on soil (Homeyer, 1969). In crop protection,
it is used only as a soil insecticide. The compound itself causes only
slight depression of cholinesterase activity in vitro (Root et al.,
1969) whilst, on the other hand, the trichloronat-oxone, formed by
oxidation, has a strong anticholinesterase action. Trichloronat can be
metabolized into O-ethyl-O(2,4,5-trichlorophenyl)-ethylphosphonate,
2,4,5-trichlorophenol, O-ethyl-ethanephosphothioic acid,
O-ethyl-ethanephosphonic acid, and ethanephosphonic acid. Degradation
is effected mainly by splitting of the P-O-aryl bond with formation of
2,4,5-trichloro-phenol and O-ethyl-ethanethiophosphonic acid. On
account of its very low stability, the latter is further broken down
at a fast rate to O-ethyl-ethanephosphonic acid and finally to
ethanephosphonic acid.
In animals
No relevant data are available for the evaluation of trichloronat in
the feed (e.g. pasture grass) of the domestic animals.
In plants
It is known from biological experiments that trichloronat does not
have a systemic action. Chemical analysis showed (Möllhoff, 1968a)
that the compound is able, to a limited extent to penetrate into the
plant and to be translocated within it just as observed also for
parathion and similar compounds. Despite massive trichloronat
treatment, viz. application of 50 ppm to potted soil, the trichloronat
concentration in the aerial plant parts of China cabbage after 14 days
amounted to only 0.05 ppm. Later analyses of kohlrabi showed that
following application as a soil drench or granular treatment as
recommended, i.e. at 50 mg of active ingredient per plant, the maximum
residue amounted to 0.2 ppm after 30 to 40 days; the concentration in
the leaves was higher than in the edible root. In larger plants, e.g.
cabbages or bananas, the compound does not move upwards to a
sufficient extent to produce measurable concentrations (>0.01 ppm) in
the aerial plant parts. In root vegetables, the bulk of the absorbed
trichloronat is present in the peel. In an experiment in which lettuce
was sprayed with trichloronat (not done commercially), the
trichloronat residues decreased at the same rate as those of parathion
(Möllhoff, 1968b).
Plants can convert trichloronat to the oxone. Following massive
treatment, the concentrations may reach levels equivalent to 5-10% of
the respective trichloronat content. But no concentrations exceeding
0.05 ppm have been found in any instance (Bayer, 1971). The oxone
migrates at a fast rate in the plant. China cabbage grown in potted
soil containing an initial concentration of 19 ppm of oxone absorbed
so much of the oxone after two to seven days that the concentration in
the plants reached a level of 0.77 ppm (Möllhoff, 1968a). But in
comparison with trichloronat, the oxone is broken down in the plant at
a much faster rate (Möllhoff, 1968b).
When trichloronat is broken down in the plant, 2,4,5-trichlorophenol
is liberated. Very small concentrations of this metabolite were found
in tobacco and beets (Bayer, 1971). A further study of the metabolism
of trichloronat in plants is still in progress.
In soil
A study was undertaken by Tu (1970) to establish whether trichloronat
has any effect on microbial activities related to soil fertility.
Application of trichloronat to soil at rates of 10 and 100 ppm
affected the populations of bacteria and fungi for periods of one and
two weeks. The application did not have a permanently harmful effect
on nitrification, sulfur oxidation and phosphorus mineralization. On
the other hand, it significantly stimulated ammonification. There are
indications that trichloronat like other organo-phosphorus
insecticides undergo microbial degradation in soil. Harris (1969)
compared the persistence of biological activity of insecticides,
including trichloronat, in soil. The insecticides were divided into
three groups: (1) highly residual; (2) moderately residual; and (3)
slightly residual. In muck soil, trichloronat was classified into
group (3) and in sandy loam it was placed into group (2). According to
the results of experiments with granular formulations and emulsions in
the United States of America and in Germany (Bayer, 1971), the
concentration of trichloronat in soil decreases to a level of 50%
within 50 to 115 days. Bro-Rasmussen et al. (1970) studied the
persistence of organophosphorus insecticides in soil in Denmark, and
found that trichloronat has a half-life of 141 days.
The metabolites were found to reach their peak concentrations in soil
30 to 60 days after incorporation of the parent compound.
Trichloronat-oxone was not detectable (limit of determination of 0.01
ppm) in non-planted soil in any study. Oxone has been found in soil
only in one experiment on potted China cabbage (Möllhoff, 1968a) in
which there was a trichloronat concentration of 50 ppm in the soil.
When the experiment was terminated after 14 days, the oxone
concentration had reached a level of 0.05 ppm. In a parallel
experiment in which the oxone itself was applied into the soil, the
oxone concentration decreased from 19.3 ppm to a level of 0.4 ppm in
14 days. Therefore, it seems unlikely that the oxone is concentrated
in soil.
Splitting of the P-O-ethyl bond of trichloronat or its oxone was not
observed in soil samples (limit of determination of 0.01 ppm). The
2,4,5-trichlorophenol which is liberated by the splitting of the
P-O-aryl bond reached concentrations averaging 0.3 ppm in soil (Bayer,
1971; Möllhoff, 1971a; see Table II). The simultaneously liberated
O-ethyl-ethanethiophosphonic acid was detected in traces only under
favourable laboratory conditions and following addition of 40 ppm of
trichloronat to soil. In a field experiment with a starting value of
5.5 ppm of trichloronat, O-ethyl-ethanephosphonic acid and
ethanephosphonic acid reached maximum concentrations of 0.1-0.15 ppm
each (Table II). Ethane-thiophosphonic acid was not found in any
instance. In the above-mentioned laboratory experiment in which 40 ppm
of trichloronat was applied to the soil, the peak concentration of
O-ethyl-ethanephosphonic acid was 0.2 ppm and that of ethanephosphonic
acid was 1.2 ppm. Under laboratory conditions, the latter has in soil
a half-life of 15 to 20 days, as against a half-life of about 50 days
for trichloronat.
TABLE II. RESIDUES OF TRICHLORONAT AND ITS METABOLITES IN SOILS (µg/kg) (Mölhoff, 1971a)
EtO S EtO O HO S HO O EtO S EtO O HO S HO O
\ // \ // \ // \ // \ // \ // \ // \ //
Days P P P P HO-R P P P P
Soil after / \ / \ / \ / \ / \ / \ / \ / \ Total P
No. applic. Et O-R Et O-R Et O-R Et O-R Et OH Et OH Et OH Et OH (%)
1 0 5 510 n.d. n.d. n.d. 180 n.d. 60 n.d. 90 100
14 4 400 n.d. n.d. n.d. 200 n.d. 140 n.d. 110 78
Opladen 31 4 860 n.d. n.d. n.d. 250 n.d. 80 n.d. 50 75
60 2 340 n.d. trace n.d. 280 n.d. 80 n.d. 130 40
89 2 050 n.d. n.d. n.d. 50 n.d. <10 n.d. 100 36
119 1 120 n.d. n.d. n.d. 70 n.d. <10 n.d. 60 20
150 890 n.d. n.d. n.d. 40 n.d. <10 n.d. 30 15
180 990 n.d. n.d. n.d. nd n.d. nd n.d. nd 18
2 0 2 670 n.d. n.d. n.d. 90 n.d. 20 n.d. 30 100
14 2 700 n.d. n.d. n.d. 100 n.d. 20 n.d. 30 105
Höfchen 31 2 760 n.d. n.d. n.d. 30 n.d. 20 n.d. 40 109
60 2 350 n.d. n.d. n.d. 100 n.d. 30 n.d. 50 91
89 770 n.d.
119 790 n.d.
150 1 060 n.d.
180 900 n.d.
Other information on identity and properties
The active ingredient is a light brown liquid and has a boiling point
of 108°C at 0.01 mm Hg. It has a vapour pressure of 1.5 × 10-5 mm Hg
at 20°C and a volatility of 0.27 mg/m3 at 20°C. Its solubility in
water at 20°C is approximately 50 ppm, in kerosenes poor, but in
alcohol, acetone, chlorinated hydrocarbons and aromatic solvents good.
The stability of the active ingredient to hydrolysis is high in the
acid range, but in the alkaline range it decomposes quite readily
(Bayer, 1969).
Composition of the technical trichloronat is reported to be (Bayer,
1971):
active ingredient 93.0 - 95.0%
free 2,4,5-trichlorophenol 0.05 - 0.5%
O-ethyl-O-(2,4-dichlorophenyl)-ethylmonothiophosphonate 0.4 - 1.0%
O,O-diethyl ethylthiophosphonate 0.1 - 0.5%
sum of two unknown, low boiling compounds 1.0 - 1.5%
sum of three to four unknown, high
boiling compounds 3.0 - 5.0%
H2O 0.01 - 0.9%
Furthermore an analytical method for the determination of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the technical material has
been worked out. Samples analysed have contained less than 0.1 ppm of
"dioxin" which is the limit of detection. It is guaranteed by the
manufacturer that the "dioxin" content of the trichloronat
preparations is less than 0.1 ppm in terms of the active ingredient
(Bayer, 1971).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption and distribution
No information available.
Bio-transformation
No information available.
Excretion
No information available.
Effects on enzymes and other biochemical parameters
Three groups of three male rats orally dosed with 7.5, 3.8, or 1.9
mg/kg showed whole blood cholinesterase depression of 30, 15, and 0%
after three hours, 35, 25 and 15% after 24 hours, and 15, 0-10, and 0%
after 72 hours respectively (Kimmerle, G., 1962).
Trichloronat is ineffective as a cholinesterase inhibitor in vitro,
but is a strong inhibitor in vivo after i.p. administration of
7 mg/kg to rats. Maximum inhibition of brain, serum, and submaxillary
gland cholinesterase occurred in 1-6 hours. Reversal of inhibition was
slow, only returning to 60-75% of normal after 7 days (Root et al.,
1969).
Inhalation exposure of groups of 10 hens, for four hours five times
weekly for four weeks resulted in 60.4, and 76.7% whole blood
cholinesterase depression at air concentrations of 0.033, and 0.048
mg/l respectively (Kimmerle, G., 1968).
TOXICOLOGICAL STUDIES
Special studies
Reproduction
Four groups each comprising 10 male and 20 female rats were fed 0, 3,
10 and 30 ppm 95% pure trichloronat in the diet through three
generations. The second litter from each generation, F1b, and F2b,
was used as parents to establish the next generation.
No adverse effects were noted among the parental animals except for
depression of growth rate at 30 ppm. Reproductive performance was
normal at all dose levels, but pup growth was depressed at 30 ppm,
although litter size and birth weight were normal. In the third
generation, the survival of the pups was adversely affected by 30 ppm
trichloronat. No terata were observed (Löser, 1971).
Neurotoxicity
Exposure of groups of five hens to 0.055, 0.126, 0.248, or 0.585 mg/l
air for four hours resulted in 100% mortality at the two upper dose
levels. Examination of survivors 42 hours after exposure did not
reveal neurotoxic effects. Further groups pre-treated i.m. with 100 mg
PAM/kg and 50 mg atropine sulphate/kg exposed to 0.185, 0.4, 0.583 or
0.784 mg/l air resulted in three deaths out of five at the upper two
dose levels. All survivors exposed to 0.4 mg/l air and above showed
neurotoxic effects. Four-hour exposures repeated on five consecutive
days at 0.053, 0.126, 0.143 or 0.162 mg/l air did not result in
neurotoxic effects, although 1/5 and 5/5 hens died at the two top dose
levels. Birds similarly exposed after PAM and atropine treatment, to
levels of 0.222, or 0.376 mg/l air, all showed neurotoxic effects.
Neurotoxic effects were not observed following four-hour exposures
five times weekly for four weeks to air concentrations of 0.033 or
0.048 mg/l air (Kimmerle, 1968).
Neurotoxic effects were not observed in 1-2 year-old hens unprotected
against the cholinergic effects of trichloronat when single doses up
to those which proved lethal were given. However, when the hens were
protected with i.p. injections of atropine sulphate and 2-PAM, higher
doses of trichloronat could be given and 100 mg/kg i.p. or 150 mg/kg
orally produced neurotoxic effects. This effect of trichloronat was
not detected in a sub-acute experiment in which 0, 250, 500, 1000 and
2000 ppm trichloronat in the diet was fed to groups of eight hens for
30 days. Two hens per group were killed after the treatment period,
the rest were killed 30 days later; neurotoxic effects were not
observed (Kimmerle, 1965).
Potentiation
Trichloronat administered simultaneously with malathion resulted in a
two-fold potentiation of the malathion toxicity (Root et al., 1969).
Toxicity of contaminants
2,4,5-Trichlorophenol is known to occur as a contaminant of
trichloronat at a level of 1-2%.
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
2,3,7,8-Tetrachlorodibenzo-p-dioxin ("dioxin") is also a possible
contaminant of trichloronat. The sole manufacturer of trichloronat
states that the level of "dioxin" is below 0.1 ppm in technical
trichloronat. (See "Identity").
Acute toxicity
Species Route LD50 Reference
(mg/kg)
Mouse i.p. 33-35 DuBois and Kinoshita, 1963
Rat (M) oral 16-55 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1968; Kimmerle, 1967
Rat (F) oral 16-37.5 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Rat (M) i.p. 10-14 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Rat (F) i.p. 11-37.5 Dubois et al., 1966;
Kimmerle, 1962
Species Route LD50 Reference
(mg/kg)
Rat dermal 64-250 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Guinea-pig oral 40-100 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Guinea-pig i.p. 26 DuBois and Kinoshita, 1963
(M)
Rabbit oral 25-50 Kimmerle, 1962
Cat oral 10-25 Kimmerle, 1962; Kimmerle,
1968
Chicken oral 45 DuBois, 1963
Groups of five female rats were injected i.p., daily for 60 days, at
dose levels of 0, 2, 4, 6 and 8 mg/kg/day. Mortality resulted in 2 out
of 5, 4 out of 5, and 5 out of 5 at 4, 6 and 8 mg/kg respectively.
Body weight was reduced at all dose levels, although some recovery
occurred at 2 and 4 mg/kg after 20 days on the test. Survivors
autopsied at 60 days showed marked depression of serum, submaxillary
gland, and brain cholinesterase (DuBois et al., 1966).
Rabbit. A pair of rabbits dosed orally, five times weekly for two
weeks with 5 mg/kg/dose did not lose body weight or display symptoms
of poisoning (Kimmerle, 1962).
Liver function tests (BSP, SG.OT, SG-PT) were unaffected by 20 mg/kg
(one rabbit) or 10 mg/kg (two rabbits) administered as a single oral
dose (Kimmerle, 1962).
Dog. Groups of beagle dogs were fed 0, 2, 5, 10 (two males and two
females/group or 25 (one male and one female) ppm in dry diet for 12
weeks. At termination of the study, erythrocyte cholinesterase was
depressed at 25 ppm (40%) in the females. Plasma cholinesterase was
depressed in both sexes at 10 ppm (39% in males and 27% in females)
but in females only at 25 ppm (41%). Brain cholinesterase was
comparable to control values for all groups. Liver cholinesterase was
depressed in males at 5 ppm and above (depression exceeding 20%), and
in females at 10 ppm and above. Thyroid weight and weight ratio were
decreased at 25 ppm (Root at al., 1968b). Histopathological changes
including lymphocytic proliferation of the intestinal wall were not
dose-related (Hibbs and Nelson, 1968).
Groups of two male and two female beagle dogs were fed 0 or 5 ppm in
the dry diet for two years. A further group was fed 25 ppm for 18
months. The 25 ppm dose level was then increased to 50 ppm for the
next seven weeks, to 100 ppm for the subsequent 14 weeks and finally
to 200 ppm until the termination of the study. A final group was fed 2
ppm for two years, and then 1 ppm for a further 13 weeks, and then
control diet for the final eight weeks prior to autopsy. Body symptoms
in all cases reported were typical of cholinesterase depression.
Short-term studies
Rat. Groups of 10 male rats were incubated five times weekly for
eight weeks with 0.75, 1.5, 3, 6, or 12 mg/kg/dose followed by a four
week observation period. Symptoms of cholinesterase depression were
seen at 12 mg/kg after the first dose only (Kimmerle, 1968).
Groups of 20 male and 20 female rats were fed 0, 1, 3, 10 or 30 ppm of
95% pure trichloronat in the diet for three months. Final body weight
was comparable, but males fed 10 and 30 ppm showed a slight dose
related lag in weight gain during the second month. Signs of
cholinesterase depression were seen during the morning from the second
week in rats fed 30 ppm and in some rats fed 10 ppm. Plasma and
erythrocyte cholinesterase levels were depressed at 10 and 30 ppm,
erythrocyte cholinesterase being depressed slightly more than plasma
cholinesterase. Depression at 3 ppm in plasma and erythrocytes was
less than 20% (Löser, 1968).
Groups of 15 male and 15 female rats were fed 2.25, 4.5, 9 or 18 ppm
of technical trichloronat in the diet for four months. The control
group comprised 30 male and 30 female rats. Cholinesterase depression
in whole blood was apparent in females at 4.5 and 9 ppm at four weeks.
At eight weeks, male rats showed depression at 9 and 18 ppm, and
females at 18 ppm only. At 12 and 16 weeks, cholinesterase depression
was apparent in both sexes at 18 ppm only (Löser, 1966).
Histopathological examination revealed low-grade fatty changes in
isolated epithelial cells of the liver of five male rats fed 18 ppm
(Hobik, 1967).
Five groups of 10 male and 10 female rats were fed 0, 2, 5, 10 or 25
ppm in the diet for 16 weeks. Terminal cholinesterase determinations
indicated depression occurred in erythrocytes at 10 and 25 ppm, in
brain and submaxillary glands at 25 ppm and in plasma of female rats
at 25 ppm. Depression was less than 20% in all other groups, and less
than 10% except for plasma in females at 10 ppm and erythrocytes in
males at 5 ppm (Root at al., 1968a). The presence of dilated kidney
tubules with eosinophilic casts was observed in all groups, but the
incidence was considerably greater in the test groups (Grey at al.,
1968). Weight and food consumption were reduced at 200 ppm. Signs of
cholinesterase depression became apparent within three weeks of
feeding 200 ppm. One male dog died after nine weeks exposure at this
level. Erythrocyte cholinesterase was comparable to controls until the
dose was increased to 75 ppm, at which dose depression occurred. The
depression increased when the dose was increased to 200 ppm. Plasma
cholinesterase was depressed at 2 ppm and above, depression being
about 20% at 2 ppm. When the dose was reduced to 1 ppm, recovery to
99% of normal activity occurred. At autopsy, liver cholinesterase was
inhibited 45% at 5 ppm. No reduction was observed in the group reduced
from 2 to 1 to 0 ppm. Brain cholinesterase was reduced 10% at 5 ppm,
and 72% at the top dose level. No compound related histological
changes were apparent (Root et al., 1970).
Long-term studies
Rat. Four groups of 30 male and 30 female rats were fed 1, 3, 10, or
30 ppm of 95% pure trichloronat in the diet for two years. The control
group comprised 60 males and 60 females. Body weight of males at 30
ppm was reduced, female body weight at 30 ppm was reduced between 9
and 12 months. Food consumption at 30 ppm was marginally reduced.
Average absolute weight of male heart, lung, liver, kidney, and spleen
were depressed, but organ/body weight ratio was unaffected. Signs of
cholinesterase depression were apparent in the morning during the
first three months in the 30 ppm group. Plasma and erythrocyte
cholinesterase were depressed at 10 and 30 ppm, although at 10 ppm the
depression became marginal as the study progressed. Terminal brain
cholinesterase was depressed at 10 and 30 ppm in both sexes, and
marginally depressed (17%) in males at 3 ppm. Histopathological data
have not been submitted (Löser, 1970).
Observations in man
No information is available.
Comments
Trichloronat is a persistent organophosphorus insecticide used for
soil treatment. No information is available on the absorption,
distribution, excretion or general metabolism in animals.
Short-term studies in the rat and dog, one long-term (two-year) study
in the rat and a three-generation reproduction study in rats are
available. In the short-term study some histopathological changes were
observed, the nature of which could not be assessed. In the long-term
studies insufficient information was available both on gross and
histopathology of the organs.
For this reason and because there is no information on the metabolism
of this persistent insecticide the Meeting decided that no acceptable
daily intake for man could be established at this time.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichloronat is an insecticidal compound with a contract and stomach
action and with a residual activity of relatively long duration for a
phosphorus insecticide. It is especially used for the control of
soil-inhabiting developmental stages of different insect species and
vegetable fly larvae. The formulations currently on the market are as
follows (Bayer, 1969; 1971):
2.5% granular (Gr.)
7.5% granular (Gr.)
20.0% seed dressing powder (S.D.P.)
50.0% emulsifiable concentrate (E.C.)
Practically all trichloronat manufactured is applied to vegetable
crops.
Pre-harvest treatments
The recommended usages of trichloronat (Bayer, 1969) with regard to
crops, pests, formulations, and rates are given below:
Onions, leeks Hylemyia antiqua (Onion fly)
50% E.C. 10 litres per hectare, spray presowing and work
in just below the surface;
0.1% (250 cm3 per running metre), drench at
egg-laying;
7.5% Gr. 25 kg/hectare (or 7.5 g/10 metres), row treatment
at sowing;
2.5% Gr. 80-100 kg/hectare (or 2.5 g per running metre),
row treatment at sowing;
20% S.D.P. 75-100 g/kg seed, before treatment, moisten
seed using 50-75 cm3 of water; sow within 24
hours of treatment.
Brassica crops Hylemyia brassicae (Cabbage root fly)
Hylemyia floralis (Turnip fly)
50% E.C. 0.04% (1 litre/m2), treatment of nursery seedbed
or 10 litres per hectare, spray presowing, and
work in just below surface, or
0.1% (250 cm3 per running metre, or 80-100 cm3/
Plant), drench at egg-laying;
7.5% Gr. 4 g/m2, treatment of seedbed, or 25 kg/hectare,
row treatment at transplanting;
Onions, leeks Hylemyia antiqua (Onion fly)
2.5% Gr. 1-2 kg/m3 for treatment of nursery bed soil, or
800 g/100 metres, row treatment at planting or
100 kg/hectare, row treatment at transplanting,
or 1-2 g/plant applied to stem base at
transplanting (also as mixture with sand), or
3-5 g/plant hole at transplanting.
Carrots Psila rosae (Carrot fly)
50% E.C. 10 litres/hectare, spray presowing and work in
just below surface;
2.5% Gr. 100 kg/hectare, row treatment at sowing;
20% S.D.P. 100 g/kg seed.
Cereals Hylemyia coarctata (Wheat bulb fly)
2.5% Gr. 80-100 kg/hectare, broadcast treatment;
20% S.D.P. 250 g/100 kg seed.
If fungicides are additionally applied use only
non-mercurial products.
Bananas Cosmopolites sordidus (Banana weevil borer)
2.5% Gr. 40-60 g/banana plant, apply in a radius of up
to 40 cm around stem.
Grassland Costelytra zealandica, Heteronychus arator,
Oncopera intricata, Wiseana
2.5% Gr. 20-80 kg/hectare, broadcast treatment;
50% E.C. 2 litres/hectare, broadcast treatment.
Trichloronat is recommended for testing on some additional pests.
Other uses
The compound is also used for termite-proofing of polyethylene,
plasticized PVC and rubber.
Residues resulting from supervised trials
The residue data from supervised trials are given in Table I. They are
compiled from the documentation made available by Bayer (1971).
TABLE I
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Onions Germany 50% E.C. 10 l/ha 100-133 n.d.-0.02
Belgium " 8 l/ha 35 n.d.
Germany " 0.1% spray
250 ml/row-m 58; 71 5.5; 1.6
Germany 2.5% Gr. 200 kg/ha 163 n.d.
" " 1.5; 2.5 g/
row-m 100-133 n.d.-0.1
Finland 20% S.D.P. 100 g/kg seed 91-119 n.d.-0.3
Denmark " 3; 5 g/kg
transplant roots 82; 110 0.2; 1.2
Leeks Germany 50% E.C. 10 l/ha 121 n.d.
Cabbages Germany 50% E.C. 2% spray 2 ml/
plant 55 n.d.
Belgium " 0.2% spray
100 ml/plant 48 n.d.
Germany " 0.1% spray
250 ml/row-m 110 n.d.
USA E.C. 1 x 0.13 g a.i./
plant + 2 × 0.18 g/
a.i./row-m 23-44 n.d.
Germany 2.5% Gr. 2 g/plant 31; 62 n.d.
Cauliflower Germany 50% E.C. 6; 8; 10 l/ha 50-69 n.d.-0.01
" " 0.05% spray
100 ml/plant 55 n.d.
" " 0.1% spray
80 ml/plant 42; 52 n.d.
" " 2% spray 2 ml/
plant 69 n.d.
Cauliflower USA E.C. 1 × 0.13 g a.i./ 42-61 n.d.
plant + 2 × 0.18 g
a.i./row-m
Germany 2.5% Gr. 2 g/plant 35-60 n.d.-0.1
" 20% S.D.P. 100 g/kg seed 76 n.d.
Broccoli USA E.C. 1 × 0.13 g a.i./ 26-41 n.d.
plant + 2 × 0.18 g
a.i./row-m
Brussels USA E.C. 1 × 0.13 g a.i./ 40-58 n.d.
sprouts plant + 2 × 0.18 g
a.i./row-m
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Kohlrabi Germany 50% E.C. 6; 8; 10 kg/ha 30-50 0.01-0.08
" " 0.05% spray
100 ml/plant 19-45 0.01-0.1
" 2.5% Gr. 1 g/plant 19-45 0.04-0.4
Carrots Denmark 50% E.C. 4 l/ha 76-174 0.05-0.2
" " 8 l/ha " 0.04-0.2
Belgium " " 44; 79 3.6;* 5.0*
Germany " 10 l/ha 94; 97 0.2; 0.5
" " " 68-148 0.06-0.4
" 2.5% Gr. 100 kg/ha 64-92 0.05-0.3
Norway " " 62-139 0.3-0.6
France " " 135-172 0.01-0.02
Belgium " " 51; 81 n.d.
Italy " " 193 0.2
Denmark " 80 kg/ha 76 19.0*
" " " 90 11.0*
" " " 100-187 1.1-3.8*
Germany " 150 kg/ha 46-166 n.d.-2.0*
Holland " 2.5 g/row-m 111 0.6
Sweden " 3.5 g/row-m 156 0.8
" " 5.0 g/row-m " 1.4
" " 9.5 g/row-m " 2.3
Denmark 20% S.D.P. 100 g/kg 76-174 0.03-0.3
Finland " " 85-119 0.02-0.2
Barley Germany 2.5% Gr. 100 kg/ha 135 n.d.
Corn " 2.5% Gr. 80 kg/ha 169 n.d.
kernels USA Gr.; E.C. 5.6 kg a.i./ha 34-114 n.d.
cobs " " " " n.d.
forage " " " " n.d.
Oats Germany 2.5% Gr. 100; 150 kg/ha 97; 135 n.d.
Rye " 20% S.D.P. 250 g/kg seed 294 n.d.
Bananas Ecuador 2.5% Gr. 45; 67.5 g/plant 3-180 n.d.
Potatoes Germany 2.5% Gr. 150 kg/ha 150 0.02
Belgium " " 49; 62 0.3; n.d.
sweet USA Gr.; E.C. 5.6 kg a.i./ha 116-164 n.d.
Radish Germany 50% E.C 5; 10 kg/ha 21; 36 0.01; 0.1
" 2.5% Gr. 100 kg/ha 21; 36; 41 0.03; 0.04;
0.1
" 20% S.D.P. 100 g/kg seed 58 0.5-0.7
Finland " " 47 0.5
Rutabaga Finland 20% S.D.P. 100 g/kg seed 91; 135 n.d.; 0.05
Denmark " " 73; 153 n.d.
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Sugar-beets
roots Germany 2.5% Gr. 150 kg/ha 160 n.d.
tops " " " " n.d.
roots USA 10% Gr. 0.1-0.3 g a.i./
row-m 147-190 n.d.-0.1
tops " " " " n.d.-0.02
Turnips
roots Germany 2.5% Gr. 150 kg/ha 175 n.d.
tops " " " 163 0.01
1 E.C. = emulsifiable concentrate.
Gr. = granular.
S.D.P. = seed dressing powder.
2 Rate given in terms of formulation if not indicated
by a.i. = active ingredient.
3 n.d. = non-detectable (varies 0.01-0.1 ppm).
* Considered that the residue has not met the local requirements
of good agricultural practice.
It has been found (Brewerton et al., 1968) that the trichloronat
residues in pasture crops were at or below 5 mg/kg (dry matter basis)
after two weeks from treatment at 0.5 and 1 kg active ingredient per
hectare, after one month from treatment at 2 kg a.i. per ha, and after
two months from treatment at 4 kg a.i. per ha.
Fate of residues
General comments
Trichloronat is a thiophosphonate which is highly effective against
insects which live in or on soil (Homeyer, 1969). In crop protection,
it is used only as a soil insecticide. The compound itself causes only
slight depression of cholinesterase activity in vitro (Root et al.,
1969) whilst, on the other hand, the trichloronat-oxone, formed by
oxidation, has a strong anticholinesterase action. Trichloronat can be
metabolized into O-ethyl-O(2,4,5-trichlorophenyl)-ethylphosphonate,
2,4,5-trichlorophenol, O-ethyl-ethanephosphothioic acid,
O-ethyl-ethanephosphonic acid, and ethanephosphonic acid. Degradation
is effected mainly by splitting of the P-O-aryl bond with formation of
2,4,5-trichloro-phenol and O-ethyl-ethanethiophosphonic acid. On
account of its very low stability, the latter is further broken down
at a fast rate to O-ethyl-ethanephosphonic acid and finally to
ethanephosphonic acid.
In animals
No relevant data are available for the evaluation of trichloronat in
the feed (e.g. pasture grass) of the domestic animals.
In plants
It is known from biological experiments that trichloronat does not
have a systemic action. Chemical analysis showed (Möllhoff, 1968a)
that the compound is able, to a limited extent to penetrate into the
plant and to be translocated within it just as observed also for
parathion and similar compounds. Despite massive trichloronat
treatment, viz. application of 50 ppm to potted soil, the trichloronat
concentration in the aerial plant parts of China cabbage after 14 days
amounted to only 0.05 ppm. Later analyses of kohlrabi showed that
following application as a soil drench or granular treatment as
recommended, i.e. at 50 mg of active ingredient per plant, the maximum
residue amounted to 0.2 ppm after 30 to 40 days; the concentration in
the leaves was higher than in the edible root. In larger plants, e.g.
cabbages or bananas, the compound does not move upwards to a
sufficient extent to produce measurable concentrations (>0.01 ppm) in
the aerial plant parts. In root vegetables, the bulk of the absorbed
trichloronat is present in the peel. In an experiment in which lettuce
was sprayed with trichloronat (not done commercially), the
trichloronat residues decreased at the same rate as those of parathion
(Möllhoff, 1968b).
Plants can convert trichloronat to the oxone. Following massive
treatment, the concentrations may reach levels equivalent to 5-10% of
the respective trichloronat content. But no concentrations exceeding
0.05 ppm have been found in any instance (Bayer, 1971). The oxone
migrates at a fast rate in the plant. China cabbage grown in potted
soil containing an initial concentration of 19 ppm of oxone absorbed
so much of the oxone after two to seven days that the concentration in
the plants reached a level of 0.77 ppm (Möllhoff, 1968a). But in
comparison with trichloronat, the oxone is broken down in the plant at
a much faster rate (Möllhoff, 1968b).
When trichloronat is broken down in the plant, 2,4,5-trichlorophenol
is liberated. Very small concentrations of this metabolite were found
in tobacco and beets (Bayer, 1971). A further study of the metabolism
of trichloronat in plants is still in progress.
In soil
A study was undertaken by Tu (1970) to establish whether trichloronat
has any effect on microbial activities related to soil fertility.
Application of trichloronat to soil at rates of 10 and 100 ppm
affected the populations of bacteria and fungi for periods of one and
two weeks. The application did not have a permanently harmful effect
on nitrification, sulfur oxidation and phosphorus mineralization. On
the other hand, it significantly stimulated ammonification. There are
indications that trichloronat like other organo-phosphorus
insecticides undergo microbial degradation in soil. Harris (1969)
compared the persistence of biological activity of insecticides,
including trichloronat, in soil. The insecticides were divided into
three groups: (1) highly residual; (2) moderately residual; and (3)
slightly residual. In muck soil, trichloronat was classified into
group (3) and in sandy loam it was placed into group (2). According to
the results of experiments with granular formulations and emulsions in
the United States of America and in Germany (Bayer, 1971), the
concentration of trichloronat in soil decreases to a level of 50%
within 50 to 115 days. Bro-Rasmussen et al. (1970) studied the
persistence of organophosphorus insecticides in soil in Denmark, and
found that trichloronat has a half-life of 141 days.
The metabolites were found to reach their peak concentrations in soil
30 to 60 days after incorporation of the parent compound.
Trichloronat-oxone was not detectable (limit of determination of 0.01
ppm) in non-planted soil in any study. Oxone has been found in soil
only in one experiment on potted China cabbage (Möllhoff, 1968a) in
which there was a trichloronat concentration of 50 ppm in the soil.
When the experiment was terminated after 14 days, the oxone
concentration had reached a level of 0.05 ppm. In a parallel
experiment in which the oxone itself was applied into the soil, the
oxone concentration decreased from 19.3 ppm to a level of 0.4 ppm in
14 days. Therefore, it seems unlikely that the oxone is concentrated
in soil.
Splitting of the P-O-ethyl bond of trichloronat or its oxone was not
observed in soil samples (limit of determination of 0.01 ppm). The
2,4,5-trichlorophenol which is liberated by the splitting of the
P-O-aryl bond reached concentrations averaging 0.3 ppm in soil (Bayer,
1971; Möllhoff, 1971a; see Table II). The simultaneously liberated
O-ethyl-ethanethiophosphonic acid was detected in traces only under
favourable laboratory conditions and following addition of 40 ppm of
trichloronat to soil. In a field experiment with a starting value of
5.5 ppm of trichloronat, O-ethyl-ethanephosphonic acid and
ethanephosphonic acid reached maximum concentrations of 0.1-0.15 ppm
each (Table II). Ethane-thiophosphonic acid was not found in any
instance. In the above-mentioned laboratory experiment in which 40 ppm
of trichloronat was applied to the soil, the peak concentration of
O-ethyl-ethanephosphonic acid was 0.2 ppm and that of ethanephosphonic
acid was 1.2 ppm. Under laboratory conditions, the latter has in soil
a half-life of 15 to 20 days, as against a half-life of about 50 days
for trichloronat.
TABLE II. RESIDUES OF TRICHLORONAT AND ITS METABOLITES IN SOILS (µg/kg) (Mölhoff, 1971a)
EtO S EtO O HO S HO O EtO S EtO O HO S HO O
\ // \ // \ // \ // \ // \ // \ // \ //
Days P P P P HO-R P P P P
Soil after / \ / \ / \ / \ / \ / \ / \ / \ Total P
No. applic. Et O-R Et O-R Et O-R Et O-R Et OH Et OH Et OH Et OH (%)
1 0 5 510 n.d. n.d. n.d. 180 n.d. 60 n.d. 90 100
14 4 400 n.d. n.d. n.d. 200 n.d. 140 n.d. 110 78
Opladen 31 4 860 n.d. n.d. n.d. 250 n.d. 80 n.d. 50 75
60 2 340 n.d. trace n.d. 280 n.d. 80 n.d. 130 40
89 2 050 n.d. n.d. n.d. 50 n.d. <10 n.d. 100 36
119 1 120 n.d. n.d. n.d. 70 n.d. <10 n.d. 60 20
150 890 n.d. n.d. n.d. 40 n.d. <10 n.d. 30 15
180 990 n.d. n.d. n.d. nd n.d. nd n.d. nd 18
2 0 2 670 n.d. n.d. n.d. 90 n.d. 20 n.d. 30 100
14 2 700 n.d. n.d. n.d. 100 n.d. 20 n.d. 30 105
Höfchen 31 2 760 n.d. n.d. n.d. 30 n.d. 20 n.d. 40 109
60 2 350 n.d. n.d. n.d. 100 n.d. 30 n.d. 50 91
89 770 n.d.
119 790 n.d.
150 1 060 n.d.
180 900 n.d.
 n.d. non detectable
Assuming that trichloronat is applied, according to the
recommendations, only once a year to the same field and taking into
account the degradation rates presented before, there seems to be no
accumulation of trichloronat in the soils to be expected.
The behaviour of trichloronat in simulated field environment was
studied to determine its relative potential for contaminating water
stores. Residues in runoff water from field soil plots were less than
1% within a 14-day interval of application. Leaching studies in the
laboratory for high nitrogen, clay and sandy loam soils indicated that
rainfalls of 368, 447 and 103 inches, respectively, would
theoretically be required to leach the compound 12 inches into the
soil (Shaw et al., 1971). The half-life of trichloronat in neutral or
alkaline water is very short. It was found to be 51 hours in water
buffered to a pH of 7 at 30°C (Shaw et al., 1971). Measurements in
isopropanol/water 1:1 (v/v) at pH 11.5, equivalent to pH 10.5 in
water, produced a half-life of 6.2 hours for trichloronat at 37°C; the
half-life value found for parathion was 28.3 hours, and thus greater
by a factor of 4.5 (Hofer, 1969). A similar factor, viz. 5.5, was
obtained by Möllhoff (1971b) in measurements in distilled water at
26°C. The half-life values were 110 days for parathion and paraoxone,
50 days for parathion-methyl, 20 days for trichloronat and
paraoxone-methyl, and 60 days for trichloronat-oxone.
In storage and processing
Residues of trichloronat, trichloronat-oxone and 2,4,5-trichlorophenol
in cold-stored (approximately -20°C) cabbage and potatoes did not
decrease in 168 to 224 days (Chemagro Corp., 1968). Kohlrabi roots
containing 0.3 ppm of trichloronat were boiled unpeeled for 30 minutes
in twice the amount by weight of water in a closed vessel, and then
analysed together with the water. Twenty-seven per cent of the
compound was decomposed (Bayer, 1971). Washed carrots contained 0.35
ppm of residue; after they had been peeled, no more residue was
detectable in them (limit of determination of 0.07 ppm) (Bayer, 1971).
Washed turnips, carrots and onions contained no detectable residues
after being peeled (Anon., Finland, 1969). The peel of carrots
contained 0.66 ppm of residue as against only 0.02 ppm in the pulp.
After these carrots had been mechanically washed, the peel was found
still to contain 0.32 ppm residue and the pulp contained 0.04 ppm of
residue. After the carrots had been blanched for one minute at 100°C,
no more residues were detectable in them (<0.02 ppm). A similar
result was obtained for normal preservation (Martens, 1970). In a
study to investigate the effect of processing on trichloronat in sugar
beets, a laboratory procedure was employed which simulated the
industrial process; the results showed that trichloronat,
trichloronat-oxone, and 2,4,5-trichlorophenol were completely degraded
during the first initial boiling and liming step (Katague and
Anderson, 1968a). Cigarettes containing trichloronat,
trichloronat-oxone and 2,4,5-trichlorophenol were smoked and the smoke
was analysed. Approximately 15% of each of the three compounds was
found in the smoke (Olson and Anderson, 1968).
Evidence of residues in food in commerce or at consumption
From 1964 to 1968, Renvall and Åkerblom (1971) analysed 2396 samples
of domestic and imported fruit and vegetables obtained from the
Swedish market. Only about 0.01% of these samples, i.e. two or three,
contained trichloronat. The residue level was less than 0.1 ppm. The
limit of determination was 0.01 ppm. In 1968, Krause and Kirchhoff
(1969, 1970) carried out analyses of market samples of fruit and
vegetables produced in or imported into Germany. None of the 70
analysed samples contained detectable residues of trichloronat (limit
of determination of 0.01 ppm).
Methods of residue analysis
Studies of metabolites have shown that following customary application
of trichloronat, the oxone will occur, if at all, only in low
concentrations because its half-life is considerably shorter than that
of the parent compound (Möllhoff, 1968a, 1968b), 2,4,5-trichlorophenol
also occurs only in very low concentrations or not at all. Therefore,
determination of the parent compound itself is sufficient for
regulatory purposes.
Renvall and Åkerblom (1971) developed a thin-layer chromatographic
method for determining residues of organophosphorus insecticides,
including trichloronat, in fruit and vegetables, and which has a limit
of determination of 0.1 ppm.
Trichloronat can also always be co-determined by multiresidue methods
for gas-chromatographic determination of organophosphorus
insecticides, which are suitable for determining parathion (Beckman
and Garber, 1969; Krause and Kirchhoff, 1970; Möllhoff, 1967, 1968b;
Renvall and Åkerblom, 1971; Sans, 1967). Detectors that have been used
include the electron-capture detector (Brewerton at al., 1968;
Möllhoff, 1967), the thermionic detector and the flame photometric
detector (Bowman and Beroza, 1970).
There are two special methods for the determination of trichloronat
residues. One is based on the determination of the phenol group
(Katague and Anderson, 1966), and the other is based on the
determination of the parent compound itself (Möllhoff, 1967; 1968a,
1968b).
In the method based on the determination of the phenol, trichloronat
and its oxone are determined together and the free
2,4,5-trichlorophenol is determined separately. After separating the
free 2,4,5-trichlorophenol, trichloronat and its oxone are saponified.
The resultantly liberated phenol and the previously separated phenol
which is already free are separately acetylated and determined by
gas-chromatography using an electron-capture detector. The limit of
determination is generally 0.01 ppm. For confirmation of the results,
two columns (Katague and Anderson, 1968b) is used. In a study
conducted by Katague and Anderson (1967), 43 organophosphorus
pesticides were checked for possible interference with the
trichloronat residue analysis method. The trichloronat analysis is
interfered with only by fenchlorphos (Ronnel) and its oxygen analogue
(they were not included in the above study) because they contain the
same phenol as trichloronat.
The second special method of trichloronat residue analysis with
terminal gas-chromatographic determination permits trichloronat and
its oxygen analogue to be detected separately with the phosphorus or
the electron-capture detector. By using suitable columns, it was
possible to separate trichloronat from 35 organophosphorus
insecticides including fenchlorphos (Ronnel). The response of the
electron-capture detector to trichloronat is three times greater than
that of the thermionic phosphorus detector but interference peaks
occur on concentrating the extracts of some crops. Organochlorine
compounds may also interfere. Therefore, the phosphorus detector
should be preferably used for the determination.
The metabolites that cannot be assayed by either of these two methods,
can be determined in soil and plant samples by a gas-chromatographic
method described by Möllhoff (1970).
Examples of national tolerances
Tolerance Safety
Country Crop in ppm interval
in days
Belgium Cabbage 60
Carrots 90
Fruit, vegetables (incl.
cabbage, onions), excl.
potatoes 0.1
Canada Cole crops N.R.
Denmark Onion, cabbage and carrot
seed (as seed dressing) 0
Finland General (as soil drench) 84
Italy General 20
Netherlands Cabbage (soil treatment) 42
Beans 0
Cabbage, leeks, onions 0.1
Miscellaneous 0
Norway Edible root crops
(as granular) 90
Appraisal
Trichloronat is an organothiophosphorus insecticide which is used
against soil insects, especially vegetable fly larvae.
It is chiefly recommended for treatments of onions, leeks, brassica
crops, carrots, cereals, bananas and grassland. Types of application
are seed dressing, granular broadcast, soil spray, transplant and soil
drench. Amounts applied are up to 5 kg active ingredient per hectare.
Technical trichloronat is reported to contain 93-95% active
ingredient. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) may exist
as an impurity in the technical material. It is guaranteed by the sole
manufacturer that the dioxin content is below the limit of detection
of the analytical method (0.1 ppm).
Residue data are available from several countries.
Trichloronat is subject to degradation in soils, plants and animals.
Prom the degradation products trichloronat-oxone is of toxicological
importance, but it is existing only in insignificant amounts in the
terminal residues.
From the treated seeds or soil, trichloronat is sorbed to the surface
of the plant roots and is migrating only in minor amounts into the
aerial parts of the crops. From the root crops, carrots have shown
highest incidence and magnitude of residues. It has been demonstrated
that peeling, blanching, etc. processes decrease the residues of
carrots and other root crops to or below the levels of analytical
detection limits.
There is a TLC method with a detection limit of 0.1 ppm, GLC
multi-residue methods, and two specific methods with detection limits
of about 0.01 ppm for determining the residues of trichloronat in
various crops.
The evaluation of residue data is based on the assumptions that the
technical trichloronat contain less than 0.1 ppm dioxin and that it is
applied only into soils or to seeds and no treatments of the growing
crops are made. Any such treatments are not regarded as good
agricultural practice.
Since no residues have been detected (less than 0.01 ppm) in bananas
and cereals from recommended usages no tolerances are needed for those
crops.
Since an acceptable daily intake for trichloronat was not established
by the Meeting, no recommendations for tolerances are made.
As a result of recommended use of trichloronat, following residue
levels need not be exceeded:
Onions, leeks, kohlrabi, radish 0.5 ppm
Cabbage, cauliflower, broccoli,
brussels sprouts, rutabagas,
turnips, sugar beets, potatoes 0.1 ppm
Carrots 1 ppm
Further work or information
Required (before an acceptable daily intake can be established or
tolerances recommended):
1. Information on the absorption, distribution, excretion and
general metabolism of trichloronat in at least one mammalian
species.
2. Comprehensive information on the gross and histopathological
findings particularly after long-term administration of this
compound.
3. Relevant data for the evaluation of trichloronat in the feed of
domestic animals, e.g. fodder crops.
REFERENCES
Anon., Finland. (1969) State Institute of Agr. Chem., Helsinki,
Finland
Bayer AG, Farbenfabriken, Pflanzenschutz. (1969) (R)Phytosol,
(R)Agritox (Bayer 37289, S 4400). Leverkusen. E. 1-659/23536
Bayer AG, Farbenfabriken, Pflanzenschutz. (1971) Documentation on
trichloronat for FAO
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system.
J.A.O.A.C., 52, 286-293
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulfur on four thermally
stable columns. J.A.O.A.C., 53: 499-508
Brewerton, H.V., Gibbs, M. M. and Perrot, D. C. F. (1968)
Fensulgothion and "Bayer 37 289" residues in pasture. New Zealand
J. Agr. Res., 11: 303-312
Bro-Rasmussen, F., Noddegaard, E. and Voldum-Clausen, K. (1970)
Comparison of the disappearance of eight organophosphorus insecticides
from soil in laboratory and in outdoor experiments. Pesticide Sci.,
1: 179-199
Chemagro Corporation, Research Dept., Kansas City, U.S.A., Rep. No. 23
060. (1968) The effect of frozen storage at 0 to -10°F on Bay 37 289.
Residues in cabbage and potatoes
DuBois, K. P. (1963) The acute oral toxicity of Bayer 37289 to
chickens. Unpublished report of the Toxicity Lab., University of
Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P. and Kinoshita, F. (1963) The acute toxicity of Bayer
37289 to mammals. Unpublished report of the Toxicity Lab., University
of Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P., Kinoshita, F. and Flynn, M. The sub-acute parenteral
toxicity of Bayer 37289 to rats. Unpublished report of the Toxicity
Lab., University of Chicago, submitted by Farbenfabriken Bayer
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Grey, A. P., Hibbs, C. M. and Nelson, D. L. (1969) Histologic
evaluation of Bayer 37289 treated rats - addendum to report of Root et
al., 1968a. Unpublished report of Chemagro Corp. submitted by
Farbenfabriken Bayer
Harris, C. R. (1969) Laboratory studies on the persistence of
biological activity of some insecticides in soils. J. Econ. Entomol.,
62: 1437-1441
Hibbs, C. and Nelson, D. L. (1968) Microscopic findings in tissues of
male and female dogs fed Bay 37289 for twelve weeks - addendum to
report of Root et al., 1968b. Unpublished report of Chemagro Corp.
submitted by Farbenfabriken Bayer
Hobik, H. P. (1967) Histologische Untersuchungen. Unpublished report
submitted by Farbenfabriken Bayer
Hofer, W. (1969) Farbenfabriken Bayer A.G. Personal communication
Homeyer, B. (1969) Trichloronat, ein neues Mittel gegen Bodeninsekten.
Mededelingen Rijksfakulteit Landbouw-Wetenschappen Gent, 34: 598-606
Katague, D. B. and Anderson, C. A. (1966) A gas-chromatographic method
for the determination of Bay 37 289, its oxygen analogue, and
2,4,5-trichlorophenol in crops. J. Agr. Food Chem., 14: 505-508
Katague, D. B. and Anderson, C. A. (1967) An interference study for
the residue method for Bay 37 289, its oxygen analog, and
2,4,5-trichlorophenol. Chemagro Corp. Report No. 20 482. Unpublished
Katague, D. B. and Anderson, C. A. (1968a) Effect of processing on Bay
37 289 residues in sugar beets. Chemagro Corp. Report No. 23 418.
Unpublished
Katague, D. B. and Anderson, C. A. (1968b) Data for a confirmatory gas
chromatographic method for Bay 37 289 and its metabolites. Chemagro
Corp. Report No. 23 427. Unpublished
Kimmerle, G. (1962) Re 54400 (E37289). Unpublished report submitted by
Farbenfabriken Bayer
Kimmerle, G. (1965) Untersuchungen zur Neurotoxitat von Bayer 37289
bei Huhnen. Unpublished report submitted by Farbenfabriken Bayer
Kimmerle, G. (1968) Bayer 37289. Toxikologische Untersuchunger
No. 667, submitted by Farbenfabriken Bayer
Krause, C. and Kirchhoff, J. (1969) Organophosphatrückstände auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen.
Nachrichtenblatt Dtsch. Pflanzenschutz-dienst, Braunschweig,
21: 81-84
Krause, C. and Kirchhoff, J. (1970) Gaschromatographische Bestimmung
von Organophosphatrückständen auf Marktproben von Obst und Gemüse.
Dtsch. Lebensmittel-Rundschau, 66: 194-199
Löser, E. (1966) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1968) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1970) Chronisch toxikologische Untersuchungen an Ratten.
Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1971) Bay 37289 Generationsversuche an Ratten. Unpublished
report submitted by Farbenfabriken Bayer
Martens, P. H. (1970) Influence des traitements de conserverie sur la
degradation des residus de pesticides appliques sur carottes. Faculté
des Sciences Agronomiques de L'Etat, Gembloux, Belgique; Centre de
Recherches de Phytopharmacie, Rapport d'Activite 1970, No. 706
McCollister, D. D. Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol.,
3: 63-70
Möllhoff, E. (1967) Gaschromatographische Bestimmung von Rückständen
in Pflanzen und Bodenproben nach Anwendung von Präparaten der
(R)E 605-Reihe und von (R)Agritox. Pflanzenschutz-Nachrichten
"Bayer", 20: 557-574
Möllhoff, E. (1968a) Versuche zur Aufnahme von Agritox durch Pflanzen
aus dem Boden. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1968b) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzon nach Anwendung von Präparaten der (R)E
605-und (R)Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21:
331-358
Möllhoff, E. (1970) Methods zur Extraktion aus Pflanzen und Boden, zur
Trennung und zum gaschromatographischen Nachweis von
Organophosphorverbindungen und ihren Um- und Abbau-produkten.
Vorläufiger Bericht. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1971a) Untersuchung über den Metabolismus von
Trichloronat im Boden. Farbenfabriken Bayer A.C. Unpublished
Möllhoff, E. (1971b) Verhalten einiger Organophosphorverbindungen in
destilliertem Wasser. Farbenfabriken Bayer A.G. Unpublished
Olson, T. J. and Anderson, C. A. (1968) Determination of Bay 37 289
residues in cigarette smoke. Chemagro Corp. Report No. 23 066.
Unpublished
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin layer chromatography. J.A.O.A.C.,
50: 1088-1098
Renvall, S. and Åkerblom, M. (1971) Determination of organophosphorus
pesticide residues in fruits and vegetables on the Swedish market from
1964 to 1968. Residue Reviews, 34: 1-26
Root, M., Kinoshita, F. K., Flynn, M. and DuBois, K. P. (1969)
Toxicity and anticholinesterase action of O-ethyl
O-2,4,5-trichlorophenyl ethylphosphonothioate. Toxicol. appl.
Pharmacol., 14 (3): 620. Abstr. Papers 18th Ann. Meet. Soc.
Toxicol., Williamsburg, Virginia
Root, M., Meskauskas, J. Kinoshita, F. and Flynn, M. (1970) The
chronic toxicity of Bayer 37289 to male and female dogs. Unpublished
report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn, M. and Kempf, C.
(1968a) Sub-acute oral toxicity of Bayer 37289 to male and female
rats. Unpublished report of the Toxicity Lab., University of Chicago,
submitted by Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn M. and Groks, D.
(1968b) Sub-acute oral toxicity of Bay 37289 to male and female dogs.
Report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Sans, W. W. (1967) Multiple insecticide residue determination using
column chromatography, chemical conversion, and gas-liquid
chromatography. J. Agr. Food Chem., 15: 192-198
Shaw, H. R., Flint, D. R. and Waggoner, T. B. (1971) Bay 37 289
- Water stability and soil runoff, leaching and adsorption studies.
Chemagro Corp. Report No. 29 062. Unpublished
Tu, C. M. (1970) Effect of four organophosphorus insecticides on
microbial activities in soil. Appl. Microbiol., 19: 479-484
n.d. non detectable
Assuming that trichloronat is applied, according to the
recommendations, only once a year to the same field and taking into
account the degradation rates presented before, there seems to be no
accumulation of trichloronat in the soils to be expected.
The behaviour of trichloronat in simulated field environment was
studied to determine its relative potential for contaminating water
stores. Residues in runoff water from field soil plots were less than
1% within a 14-day interval of application. Leaching studies in the
laboratory for high nitrogen, clay and sandy loam soils indicated that
rainfalls of 368, 447 and 103 inches, respectively, would
theoretically be required to leach the compound 12 inches into the
soil (Shaw et al., 1971). The half-life of trichloronat in neutral or
alkaline water is very short. It was found to be 51 hours in water
buffered to a pH of 7 at 30°C (Shaw et al., 1971). Measurements in
isopropanol/water 1:1 (v/v) at pH 11.5, equivalent to pH 10.5 in
water, produced a half-life of 6.2 hours for trichloronat at 37°C; the
half-life value found for parathion was 28.3 hours, and thus greater
by a factor of 4.5 (Hofer, 1969). A similar factor, viz. 5.5, was
obtained by Möllhoff (1971b) in measurements in distilled water at
26°C. The half-life values were 110 days for parathion and paraoxone,
50 days for parathion-methyl, 20 days for trichloronat and
paraoxone-methyl, and 60 days for trichloronat-oxone.
In storage and processing
Residues of trichloronat, trichloronat-oxone and 2,4,5-trichlorophenol
in cold-stored (approximately -20°C) cabbage and potatoes did not
decrease in 168 to 224 days (Chemagro Corp., 1968). Kohlrabi roots
containing 0.3 ppm of trichloronat were boiled unpeeled for 30 minutes
in twice the amount by weight of water in a closed vessel, and then
analysed together with the water. Twenty-seven per cent of the
compound was decomposed (Bayer, 1971). Washed carrots contained 0.35
ppm of residue; after they had been peeled, no more residue was
detectable in them (limit of determination of 0.07 ppm) (Bayer, 1971).
Washed turnips, carrots and onions contained no detectable residues
after being peeled (Anon., Finland, 1969). The peel of carrots
contained 0.66 ppm of residue as against only 0.02 ppm in the pulp.
After these carrots had been mechanically washed, the peel was found
still to contain 0.32 ppm residue and the pulp contained 0.04 ppm of
residue. After the carrots had been blanched for one minute at 100°C,
no more residues were detectable in them (<0.02 ppm). A similar
result was obtained for normal preservation (Martens, 1970). In a
study to investigate the effect of processing on trichloronat in sugar
beets, a laboratory procedure was employed which simulated the
industrial process; the results showed that trichloronat,
trichloronat-oxone, and 2,4,5-trichlorophenol were completely degraded
during the first initial boiling and liming step (Katague and
Anderson, 1968a). Cigarettes containing trichloronat,
trichloronat-oxone and 2,4,5-trichlorophenol were smoked and the smoke
was analysed. Approximately 15% of each of the three compounds was
found in the smoke (Olson and Anderson, 1968).
Evidence of residues in food in commerce or at consumption
From 1964 to 1968, Renvall and Åkerblom (1971) analysed 2396 samples
of domestic and imported fruit and vegetables obtained from the
Swedish market. Only about 0.01% of these samples, i.e. two or three,
contained trichloronat. The residue level was less than 0.1 ppm. The
limit of determination was 0.01 ppm. In 1968, Krause and Kirchhoff
(1969, 1970) carried out analyses of market samples of fruit and
vegetables produced in or imported into Germany. None of the 70
analysed samples contained detectable residues of trichloronat (limit
of determination of 0.01 ppm).
Methods of residue analysis
Studies of metabolites have shown that following customary application
of trichloronat, the oxone will occur, if at all, only in low
concentrations because its half-life is considerably shorter than that
of the parent compound (Möllhoff, 1968a, 1968b), 2,4,5-trichlorophenol
also occurs only in very low concentrations or not at all. Therefore,
determination of the parent compound itself is sufficient for
regulatory purposes.
Renvall and Åkerblom (1971) developed a thin-layer chromatographic
method for determining residues of organophosphorus insecticides,
including trichloronat, in fruit and vegetables, and which has a limit
of determination of 0.1 ppm.
Trichloronat can also always be co-determined by multiresidue methods
for gas-chromatographic determination of organophosphorus
insecticides, which are suitable for determining parathion (Beckman
and Garber, 1969; Krause and Kirchhoff, 1970; Möllhoff, 1967, 1968b;
Renvall and Åkerblom, 1971; Sans, 1967). Detectors that have been used
include the electron-capture detector (Brewerton at al., 1968;
Möllhoff, 1967), the thermionic detector and the flame photometric
detector (Bowman and Beroza, 1970).
There are two special methods for the determination of trichloronat
residues. One is based on the determination of the phenol group
(Katague and Anderson, 1966), and the other is based on the
determination of the parent compound itself (Möllhoff, 1967; 1968a,
1968b).
In the method based on the determination of the phenol, trichloronat
and its oxone are determined together and the free
2,4,5-trichlorophenol is determined separately. After separating the
free 2,4,5-trichlorophenol, trichloronat and its oxone are saponified.
The resultantly liberated phenol and the previously separated phenol
which is already free are separately acetylated and determined by
gas-chromatography using an electron-capture detector. The limit of
determination is generally 0.01 ppm. For confirmation of the results,
two columns (Katague and Anderson, 1968b) is used. In a study
conducted by Katague and Anderson (1967), 43 organophosphorus
pesticides were checked for possible interference with the
trichloronat residue analysis method. The trichloronat analysis is
interfered with only by fenchlorphos (Ronnel) and its oxygen analogue
(they were not included in the above study) because they contain the
same phenol as trichloronat.
The second special method of trichloronat residue analysis with
terminal gas-chromatographic determination permits trichloronat and
its oxygen analogue to be detected separately with the phosphorus or
the electron-capture detector. By using suitable columns, it was
possible to separate trichloronat from 35 organophosphorus
insecticides including fenchlorphos (Ronnel). The response of the
electron-capture detector to trichloronat is three times greater than
that of the thermionic phosphorus detector but interference peaks
occur on concentrating the extracts of some crops. Organochlorine
compounds may also interfere. Therefore, the phosphorus detector
should be preferably used for the determination.
The metabolites that cannot be assayed by either of these two methods,
can be determined in soil and plant samples by a gas-chromatographic
method described by Möllhoff (1970).
Examples of national tolerances
Tolerance Safety
Country Crop in ppm interval
in days
Belgium Cabbage 60
Carrots 90
Fruit, vegetables (incl.
cabbage, onions), excl.
potatoes 0.1
Canada Cole crops N.R.
Denmark Onion, cabbage and carrot
seed (as seed dressing) 0
Finland General (as soil drench) 84
Italy General 20
Netherlands Cabbage (soil treatment) 42
Beans 0
Cabbage, leeks, onions 0.1
Miscellaneous 0
Norway Edible root crops
(as granular) 90
Appraisal
Trichloronat is an organothiophosphorus insecticide which is used
against soil insects, especially vegetable fly larvae.
It is chiefly recommended for treatments of onions, leeks, brassica
crops, carrots, cereals, bananas and grassland. Types of application
are seed dressing, granular broadcast, soil spray, transplant and soil
drench. Amounts applied are up to 5 kg active ingredient per hectare.
Technical trichloronat is reported to contain 93-95% active
ingredient. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) may exist
as an impurity in the technical material. It is guaranteed by the sole
manufacturer that the dioxin content is below the limit of detection
of the analytical method (0.1 ppm).
Residue data are available from several countries.
Trichloronat is subject to degradation in soils, plants and animals.
Prom the degradation products trichloronat-oxone is of toxicological
importance, but it is existing only in insignificant amounts in the
terminal residues.
From the treated seeds or soil, trichloronat is sorbed to the surface
of the plant roots and is migrating only in minor amounts into the
aerial parts of the crops. From the root crops, carrots have shown
highest incidence and magnitude of residues. It has been demonstrated
that peeling, blanching, etc. processes decrease the residues of
carrots and other root crops to or below the levels of analytical
detection limits.
There is a TLC method with a detection limit of 0.1 ppm, GLC
multi-residue methods, and two specific methods with detection limits
of about 0.01 ppm for determining the residues of trichloronat in
various crops.
The evaluation of residue data is based on the assumptions that the
technical trichloronat contain less than 0.1 ppm dioxin and that it is
applied only into soils or to seeds and no treatments of the growing
crops are made. Any such treatments are not regarded as good
agricultural practice.
Since no residues have been detected (less than 0.01 ppm) in bananas
and cereals from recommended usages no tolerances are needed for those
crops.
Since an acceptable daily intake for trichloronat was not established
by the Meeting, no recommendations for tolerances are made.
As a result of recommended use of trichloronat, following residue
levels need not be exceeded:
Onions, leeks, kohlrabi, radish 0.5 ppm
Cabbage, cauliflower, broccoli,
brussels sprouts, rutabagas,
turnips, sugar beets, potatoes 0.1 ppm
Carrots 1 ppm
Further work or information
Required (before an acceptable daily intake can be established or
tolerances recommended):
1. Information on the absorption, distribution, excretion and
general metabolism of trichloronat in at least one mammalian
species.
2. Comprehensive information on the gross and histopathological
findings particularly after long-term administration of this
compound.
3. Relevant data for the evaluation of trichloronat in the feed of
domestic animals, e.g. fodder crops.
REFERENCES
Anon., Finland. (1969) State Institute of Agr. Chem., Helsinki,
Finland
Bayer AG, Farbenfabriken, Pflanzenschutz. (1969) (R)Phytosol,
(R)Agritox (Bayer 37289, S 4400). Leverkusen. E. 1-659/23536
Bayer AG, Farbenfabriken, Pflanzenschutz. (1971) Documentation on
trichloronat for FAO
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system.
J.A.O.A.C., 52, 286-293
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulfur on four thermally
stable columns. J.A.O.A.C., 53: 499-508
Brewerton, H.V., Gibbs, M. M. and Perrot, D. C. F. (1968)
Fensulgothion and "Bayer 37 289" residues in pasture. New Zealand
J. Agr. Res., 11: 303-312
Bro-Rasmussen, F., Noddegaard, E. and Voldum-Clausen, K. (1970)
Comparison of the disappearance of eight organophosphorus insecticides
from soil in laboratory and in outdoor experiments. Pesticide Sci.,
1: 179-199
Chemagro Corporation, Research Dept., Kansas City, U.S.A., Rep. No. 23
060. (1968) The effect of frozen storage at 0 to -10°F on Bay 37 289.
Residues in cabbage and potatoes
DuBois, K. P. (1963) The acute oral toxicity of Bayer 37289 to
chickens. Unpublished report of the Toxicity Lab., University of
Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P. and Kinoshita, F. (1963) The acute toxicity of Bayer
37289 to mammals. Unpublished report of the Toxicity Lab., University
of Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P., Kinoshita, F. and Flynn, M. The sub-acute parenteral
toxicity of Bayer 37289 to rats. Unpublished report of the Toxicity
Lab., University of Chicago, submitted by Farbenfabriken Bayer
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Grey, A. P., Hibbs, C. M. and Nelson, D. L. (1969) Histologic
evaluation of Bayer 37289 treated rats - addendum to report of Root et
al., 1968a. Unpublished report of Chemagro Corp. submitted by
Farbenfabriken Bayer
Harris, C. R. (1969) Laboratory studies on the persistence of
biological activity of some insecticides in soils. J. Econ. Entomol.,
62: 1437-1441
Hibbs, C. and Nelson, D. L. (1968) Microscopic findings in tissues of
male and female dogs fed Bay 37289 for twelve weeks - addendum to
report of Root et al., 1968b. Unpublished report of Chemagro Corp.
submitted by Farbenfabriken Bayer
Hobik, H. P. (1967) Histologische Untersuchungen. Unpublished report
submitted by Farbenfabriken Bayer
Hofer, W. (1969) Farbenfabriken Bayer A.G. Personal communication
Homeyer, B. (1969) Trichloronat, ein neues Mittel gegen Bodeninsekten.
Mededelingen Rijksfakulteit Landbouw-Wetenschappen Gent, 34: 598-606
Katague, D. B. and Anderson, C. A. (1966) A gas-chromatographic method
for the determination of Bay 37 289, its oxygen analogue, and
2,4,5-trichlorophenol in crops. J. Agr. Food Chem., 14: 505-508
Katague, D. B. and Anderson, C. A. (1967) An interference study for
the residue method for Bay 37 289, its oxygen analog, and
2,4,5-trichlorophenol. Chemagro Corp. Report No. 20 482. Unpublished
Katague, D. B. and Anderson, C. A. (1968a) Effect of processing on Bay
37 289 residues in sugar beets. Chemagro Corp. Report No. 23 418.
Unpublished
Katague, D. B. and Anderson, C. A. (1968b) Data for a confirmatory gas
chromatographic method for Bay 37 289 and its metabolites. Chemagro
Corp. Report No. 23 427. Unpublished
Kimmerle, G. (1962) Re 54400 (E37289). Unpublished report submitted by
Farbenfabriken Bayer
Kimmerle, G. (1965) Untersuchungen zur Neurotoxitat von Bayer 37289
bei Huhnen. Unpublished report submitted by Farbenfabriken Bayer
Kimmerle, G. (1968) Bayer 37289. Toxikologische Untersuchunger
No. 667, submitted by Farbenfabriken Bayer
Krause, C. and Kirchhoff, J. (1969) Organophosphatrückstände auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen.
Nachrichtenblatt Dtsch. Pflanzenschutz-dienst, Braunschweig,
21: 81-84
Krause, C. and Kirchhoff, J. (1970) Gaschromatographische Bestimmung
von Organophosphatrückständen auf Marktproben von Obst und Gemüse.
Dtsch. Lebensmittel-Rundschau, 66: 194-199
Löser, E. (1966) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1968) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1970) Chronisch toxikologische Untersuchungen an Ratten.
Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1971) Bay 37289 Generationsversuche an Ratten. Unpublished
report submitted by Farbenfabriken Bayer
Martens, P. H. (1970) Influence des traitements de conserverie sur la
degradation des residus de pesticides appliques sur carottes. Faculté
des Sciences Agronomiques de L'Etat, Gembloux, Belgique; Centre de
Recherches de Phytopharmacie, Rapport d'Activite 1970, No. 706
McCollister, D. D. Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol.,
3: 63-70
Möllhoff, E. (1967) Gaschromatographische Bestimmung von Rückständen
in Pflanzen und Bodenproben nach Anwendung von Präparaten der
(R)E 605-Reihe und von (R)Agritox. Pflanzenschutz-Nachrichten
"Bayer", 20: 557-574
Möllhoff, E. (1968a) Versuche zur Aufnahme von Agritox durch Pflanzen
aus dem Boden. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1968b) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzon nach Anwendung von Präparaten der (R)E
605-und (R)Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21:
331-358
Möllhoff, E. (1970) Methods zur Extraktion aus Pflanzen und Boden, zur
Trennung und zum gaschromatographischen Nachweis von
Organophosphorverbindungen und ihren Um- und Abbau-produkten.
Vorläufiger Bericht. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1971a) Untersuchung über den Metabolismus von
Trichloronat im Boden. Farbenfabriken Bayer A.C. Unpublished
Möllhoff, E. (1971b) Verhalten einiger Organophosphorverbindungen in
destilliertem Wasser. Farbenfabriken Bayer A.G. Unpublished
Olson, T. J. and Anderson, C. A. (1968) Determination of Bay 37 289
residues in cigarette smoke. Chemagro Corp. Report No. 23 066.
Unpublished
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin layer chromatography. J.A.O.A.C.,
50: 1088-1098
Renvall, S. and Åkerblom, M. (1971) Determination of organophosphorus
pesticide residues in fruits and vegetables on the Swedish market from
1964 to 1968. Residue Reviews, 34: 1-26
Root, M., Kinoshita, F. K., Flynn, M. and DuBois, K. P. (1969)
Toxicity and anticholinesterase action of O-ethyl
O-2,4,5-trichlorophenyl ethylphosphonothioate. Toxicol. appl.
Pharmacol., 14 (3): 620. Abstr. Papers 18th Ann. Meet. Soc.
Toxicol., Williamsburg, Virginia
Root, M., Meskauskas, J. Kinoshita, F. and Flynn, M. (1970) The
chronic toxicity of Bayer 37289 to male and female dogs. Unpublished
report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn, M. and Kempf, C.
(1968a) Sub-acute oral toxicity of Bayer 37289 to male and female
rats. Unpublished report of the Toxicity Lab., University of Chicago,
submitted by Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn M. and Groks, D.
(1968b) Sub-acute oral toxicity of Bay 37289 to male and female dogs.
Report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Sans, W. W. (1967) Multiple insecticide residue determination using
column chromatography, chemical conversion, and gas-liquid
chromatography. J. Agr. Food Chem., 15: 192-198
Shaw, H. R., Flint, D. R. and Waggoner, T. B. (1971) Bay 37 289
- Water stability and soil runoff, leaching and adsorption studies.
Chemagro Corp. Report No. 29 062. Unpublished
Tu, C. M. (1970) Effect of four organophosphorus insecticides on
microbial activities in soil. Appl. Microbiol., 19: 479-484
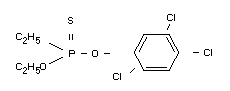 Other information on identity and properties
The active ingredient is a light brown liquid and has a boiling point
of 108°C at 0.01 mm Hg. It has a vapour pressure of 1.5 × 10-5 mm Hg
at 20°C and a volatility of 0.27 mg/m3 at 20°C. Its solubility in
water at 20°C is approximately 50 ppm, in kerosenes poor, but in
alcohol, acetone, chlorinated hydrocarbons and aromatic solvents good.
The stability of the active ingredient to hydrolysis is high in the
acid range, but in the alkaline range it decomposes quite readily
(Bayer, 1969).
Composition of the technical trichloronat is reported to be (Bayer,
1971):
active ingredient 93.0 - 95.0%
free 2,4,5-trichlorophenol 0.05 - 0.5%
O-ethyl-O-(2,4-dichlorophenyl)-ethylmonothiophosphonate 0.4 - 1.0%
O,O-diethyl ethylthiophosphonate 0.1 - 0.5%
sum of two unknown, low boiling compounds 1.0 - 1.5%
sum of three to four unknown, high
boiling compounds 3.0 - 5.0%
H2O 0.01 - 0.9%
Furthermore an analytical method for the determination of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the technical material has
been worked out. Samples analysed have contained less than 0.1 ppm of
"dioxin" which is the limit of detection. It is guaranteed by the
manufacturer that the "dioxin" content of the trichloronat
preparations is less than 0.1 ppm in terms of the active ingredient
(Bayer, 1971).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption and distribution
No information available.
Bio-transformation
No information available.
Excretion
No information available.
Effects on enzymes and other biochemical parameters
Three groups of three male rats orally dosed with 7.5, 3.8, or 1.9
mg/kg showed whole blood cholinesterase depression of 30, 15, and 0%
after three hours, 35, 25 and 15% after 24 hours, and 15, 0-10, and 0%
after 72 hours respectively (Kimmerle, G., 1962).
Trichloronat is ineffective as a cholinesterase inhibitor in vitro,
but is a strong inhibitor in vivo after i.p. administration of
7 mg/kg to rats. Maximum inhibition of brain, serum, and submaxillary
gland cholinesterase occurred in 1-6 hours. Reversal of inhibition was
slow, only returning to 60-75% of normal after 7 days (Root et al.,
1969).
Inhalation exposure of groups of 10 hens, for four hours five times
weekly for four weeks resulted in 60.4, and 76.7% whole blood
cholinesterase depression at air concentrations of 0.033, and 0.048
mg/l respectively (Kimmerle, G., 1968).
TOXICOLOGICAL STUDIES
Special studies
Reproduction
Four groups each comprising 10 male and 20 female rats were fed 0, 3,
10 and 30 ppm 95% pure trichloronat in the diet through three
generations. The second litter from each generation, F1b, and F2b,
was used as parents to establish the next generation.
No adverse effects were noted among the parental animals except for
depression of growth rate at 30 ppm. Reproductive performance was
normal at all dose levels, but pup growth was depressed at 30 ppm,
although litter size and birth weight were normal. In the third
generation, the survival of the pups was adversely affected by 30 ppm
trichloronat. No terata were observed (Löser, 1971).
Neurotoxicity
Exposure of groups of five hens to 0.055, 0.126, 0.248, or 0.585 mg/l
air for four hours resulted in 100% mortality at the two upper dose
levels. Examination of survivors 42 hours after exposure did not
reveal neurotoxic effects. Further groups pre-treated i.m. with 100 mg
PAM/kg and 50 mg atropine sulphate/kg exposed to 0.185, 0.4, 0.583 or
0.784 mg/l air resulted in three deaths out of five at the upper two
dose levels. All survivors exposed to 0.4 mg/l air and above showed
neurotoxic effects. Four-hour exposures repeated on five consecutive
days at 0.053, 0.126, 0.143 or 0.162 mg/l air did not result in
neurotoxic effects, although 1/5 and 5/5 hens died at the two top dose
levels. Birds similarly exposed after PAM and atropine treatment, to
levels of 0.222, or 0.376 mg/l air, all showed neurotoxic effects.
Neurotoxic effects were not observed following four-hour exposures
five times weekly for four weeks to air concentrations of 0.033 or
0.048 mg/l air (Kimmerle, 1968).
Neurotoxic effects were not observed in 1-2 year-old hens unprotected
against the cholinergic effects of trichloronat when single doses up
to those which proved lethal were given. However, when the hens were
protected with i.p. injections of atropine sulphate and 2-PAM, higher
doses of trichloronat could be given and 100 mg/kg i.p. or 150 mg/kg
orally produced neurotoxic effects. This effect of trichloronat was
not detected in a sub-acute experiment in which 0, 250, 500, 1000 and
2000 ppm trichloronat in the diet was fed to groups of eight hens for
30 days. Two hens per group were killed after the treatment period,
the rest were killed 30 days later; neurotoxic effects were not
observed (Kimmerle, 1965).
Potentiation
Trichloronat administered simultaneously with malathion resulted in a
two-fold potentiation of the malathion toxicity (Root et al., 1969).
Toxicity of contaminants
2,4,5-Trichlorophenol is known to occur as a contaminant of
trichloronat at a level of 1-2%.
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
2,3,7,8-Tetrachlorodibenzo-p-dioxin ("dioxin") is also a possible
contaminant of trichloronat. The sole manufacturer of trichloronat
states that the level of "dioxin" is below 0.1 ppm in technical
trichloronat. (See "Identity").
Acute toxicity
Species Route LD50 Reference
(mg/kg)
Mouse i.p. 33-35 DuBois and Kinoshita, 1963
Rat (M) oral 16-55 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1968; Kimmerle, 1967
Rat (F) oral 16-37.5 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Rat (M) i.p. 10-14 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Rat (F) i.p. 11-37.5 Dubois et al., 1966;
Kimmerle, 1962
Species Route LD50 Reference
(mg/kg)
Rat dermal 64-250 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Guinea-pig oral 40-100 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Guinea-pig i.p. 26 DuBois and Kinoshita, 1963
(M)
Rabbit oral 25-50 Kimmerle, 1962
Cat oral 10-25 Kimmerle, 1962; Kimmerle,
1968
Chicken oral 45 DuBois, 1963
Groups of five female rats were injected i.p., daily for 60 days, at
dose levels of 0, 2, 4, 6 and 8 mg/kg/day. Mortality resulted in 2 out
of 5, 4 out of 5, and 5 out of 5 at 4, 6 and 8 mg/kg respectively.
Body weight was reduced at all dose levels, although some recovery
occurred at 2 and 4 mg/kg after 20 days on the test. Survivors
autopsied at 60 days showed marked depression of serum, submaxillary
gland, and brain cholinesterase (DuBois et al., 1966).
Rabbit. A pair of rabbits dosed orally, five times weekly for two
weeks with 5 mg/kg/dose did not lose body weight or display symptoms
of poisoning (Kimmerle, 1962).
Liver function tests (BSP, SG.OT, SG-PT) were unaffected by 20 mg/kg
(one rabbit) or 10 mg/kg (two rabbits) administered as a single oral
dose (Kimmerle, 1962).
Dog. Groups of beagle dogs were fed 0, 2, 5, 10 (two males and two
females/group or 25 (one male and one female) ppm in dry diet for 12
weeks. At termination of the study, erythrocyte cholinesterase was
depressed at 25 ppm (40%) in the females. Plasma cholinesterase was
depressed in both sexes at 10 ppm (39% in males and 27% in females)
but in females only at 25 ppm (41%). Brain cholinesterase was
comparable to control values for all groups. Liver cholinesterase was
depressed in males at 5 ppm and above (depression exceeding 20%), and
in females at 10 ppm and above. Thyroid weight and weight ratio were
decreased at 25 ppm (Root at al., 1968b). Histopathological changes
including lymphocytic proliferation of the intestinal wall were not
dose-related (Hibbs and Nelson, 1968).
Groups of two male and two female beagle dogs were fed 0 or 5 ppm in
the dry diet for two years. A further group was fed 25 ppm for 18
months. The 25 ppm dose level was then increased to 50 ppm for the
next seven weeks, to 100 ppm for the subsequent 14 weeks and finally
to 200 ppm until the termination of the study. A final group was fed 2
ppm for two years, and then 1 ppm for a further 13 weeks, and then
control diet for the final eight weeks prior to autopsy. Body symptoms
in all cases reported were typical of cholinesterase depression.
Short-term studies
Rat. Groups of 10 male rats were incubated five times weekly for
eight weeks with 0.75, 1.5, 3, 6, or 12 mg/kg/dose followed by a four
week observation period. Symptoms of cholinesterase depression were
seen at 12 mg/kg after the first dose only (Kimmerle, 1968).
Groups of 20 male and 20 female rats were fed 0, 1, 3, 10 or 30 ppm of
95% pure trichloronat in the diet for three months. Final body weight
was comparable, but males fed 10 and 30 ppm showed a slight dose
related lag in weight gain during the second month. Signs of
cholinesterase depression were seen during the morning from the second
week in rats fed 30 ppm and in some rats fed 10 ppm. Plasma and
erythrocyte cholinesterase levels were depressed at 10 and 30 ppm,
erythrocyte cholinesterase being depressed slightly more than plasma
cholinesterase. Depression at 3 ppm in plasma and erythrocytes was
less than 20% (Löser, 1968).
Groups of 15 male and 15 female rats were fed 2.25, 4.5, 9 or 18 ppm
of technical trichloronat in the diet for four months. The control
group comprised 30 male and 30 female rats. Cholinesterase depression
in whole blood was apparent in females at 4.5 and 9 ppm at four weeks.
At eight weeks, male rats showed depression at 9 and 18 ppm, and
females at 18 ppm only. At 12 and 16 weeks, cholinesterase depression
was apparent in both sexes at 18 ppm only (Löser, 1966).
Histopathological examination revealed low-grade fatty changes in
isolated epithelial cells of the liver of five male rats fed 18 ppm
(Hobik, 1967).
Five groups of 10 male and 10 female rats were fed 0, 2, 5, 10 or 25
ppm in the diet for 16 weeks. Terminal cholinesterase determinations
indicated depression occurred in erythrocytes at 10 and 25 ppm, in
brain and submaxillary glands at 25 ppm and in plasma of female rats
at 25 ppm. Depression was less than 20% in all other groups, and less
than 10% except for plasma in females at 10 ppm and erythrocytes in
males at 5 ppm (Root at al., 1968a). The presence of dilated kidney
tubules with eosinophilic casts was observed in all groups, but the
incidence was considerably greater in the test groups (Grey at al.,
1968). Weight and food consumption were reduced at 200 ppm. Signs of
cholinesterase depression became apparent within three weeks of
feeding 200 ppm. One male dog died after nine weeks exposure at this
level. Erythrocyte cholinesterase was comparable to controls until the
dose was increased to 75 ppm, at which dose depression occurred. The
depression increased when the dose was increased to 200 ppm. Plasma
cholinesterase was depressed at 2 ppm and above, depression being
about 20% at 2 ppm. When the dose was reduced to 1 ppm, recovery to
99% of normal activity occurred. At autopsy, liver cholinesterase was
inhibited 45% at 5 ppm. No reduction was observed in the group reduced
from 2 to 1 to 0 ppm. Brain cholinesterase was reduced 10% at 5 ppm,
and 72% at the top dose level. No compound related histological
changes were apparent (Root et al., 1970).
Long-term studies
Rat. Four groups of 30 male and 30 female rats were fed 1, 3, 10, or
30 ppm of 95% pure trichloronat in the diet for two years. The control
group comprised 60 males and 60 females. Body weight of males at 30
ppm was reduced, female body weight at 30 ppm was reduced between 9
and 12 months. Food consumption at 30 ppm was marginally reduced.
Average absolute weight of male heart, lung, liver, kidney, and spleen
were depressed, but organ/body weight ratio was unaffected. Signs of
cholinesterase depression were apparent in the morning during the
first three months in the 30 ppm group. Plasma and erythrocyte
cholinesterase were depressed at 10 and 30 ppm, although at 10 ppm the
depression became marginal as the study progressed. Terminal brain
cholinesterase was depressed at 10 and 30 ppm in both sexes, and
marginally depressed (17%) in males at 3 ppm. Histopathological data
have not been submitted (Löser, 1970).
Observations in man
No information is available.
Comments
Trichloronat is a persistent organophosphorus insecticide used for
soil treatment. No information is available on the absorption,
distribution, excretion or general metabolism in animals.
Short-term studies in the rat and dog, one long-term (two-year) study
in the rat and a three-generation reproduction study in rats are
available. In the short-term study some histopathological changes were
observed, the nature of which could not be assessed. In the long-term
studies insufficient information was available both on gross and
histopathology of the organs.
For this reason and because there is no information on the metabolism
of this persistent insecticide the Meeting decided that no acceptable
daily intake for man could be established at this time.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichloronat is an insecticidal compound with a contract and stomach
action and with a residual activity of relatively long duration for a
phosphorus insecticide. It is especially used for the control of
soil-inhabiting developmental stages of different insect species and
vegetable fly larvae. The formulations currently on the market are as
follows (Bayer, 1969; 1971):
2.5% granular (Gr.)
7.5% granular (Gr.)
20.0% seed dressing powder (S.D.P.)
50.0% emulsifiable concentrate (E.C.)
Practically all trichloronat manufactured is applied to vegetable
crops.
Pre-harvest treatments
The recommended usages of trichloronat (Bayer, 1969) with regard to
crops, pests, formulations, and rates are given below:
Onions, leeks Hylemyia antiqua (Onion fly)
50% E.C. 10 litres per hectare, spray presowing and work
in just below the surface;
0.1% (250 cm3 per running metre), drench at
egg-laying;
7.5% Gr. 25 kg/hectare (or 7.5 g/10 metres), row treatment
at sowing;
2.5% Gr. 80-100 kg/hectare (or 2.5 g per running metre),
row treatment at sowing;
20% S.D.P. 75-100 g/kg seed, before treatment, moisten
seed using 50-75 cm3 of water; sow within 24
hours of treatment.
Brassica crops Hylemyia brassicae (Cabbage root fly)
Hylemyia floralis (Turnip fly)
50% E.C. 0.04% (1 litre/m2), treatment of nursery seedbed
or 10 litres per hectare, spray presowing, and
work in just below surface, or
0.1% (250 cm3 per running metre, or 80-100 cm3/
Plant), drench at egg-laying;
7.5% Gr. 4 g/m2, treatment of seedbed, or 25 kg/hectare,
row treatment at transplanting;
Onions, leeks Hylemyia antiqua (Onion fly)
2.5% Gr. 1-2 kg/m3 for treatment of nursery bed soil, or
800 g/100 metres, row treatment at planting or
100 kg/hectare, row treatment at transplanting,
or 1-2 g/plant applied to stem base at
transplanting (also as mixture with sand), or
3-5 g/plant hole at transplanting.
Carrots Psila rosae (Carrot fly)
50% E.C. 10 litres/hectare, spray presowing and work in
just below surface;
2.5% Gr. 100 kg/hectare, row treatment at sowing;
20% S.D.P. 100 g/kg seed.
Cereals Hylemyia coarctata (Wheat bulb fly)
2.5% Gr. 80-100 kg/hectare, broadcast treatment;
20% S.D.P. 250 g/100 kg seed.
If fungicides are additionally applied use only
non-mercurial products.
Bananas Cosmopolites sordidus (Banana weevil borer)
2.5% Gr. 40-60 g/banana plant, apply in a radius of up
to 40 cm around stem.
Grassland Costelytra zealandica, Heteronychus arator,
Oncopera intricata, Wiseana
2.5% Gr. 20-80 kg/hectare, broadcast treatment;
50% E.C. 2 litres/hectare, broadcast treatment.
Trichloronat is recommended for testing on some additional pests.
Other uses
The compound is also used for termite-proofing of polyethylene,
plasticized PVC and rubber.
Residues resulting from supervised trials
The residue data from supervised trials are given in Table I. They are
compiled from the documentation made available by Bayer (1971).
TABLE I
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Onions Germany 50% E.C. 10 l/ha 100-133 n.d.-0.02
Belgium " 8 l/ha 35 n.d.
Germany " 0.1% spray
250 ml/row-m 58; 71 5.5; 1.6
Germany 2.5% Gr. 200 kg/ha 163 n.d.
" " 1.5; 2.5 g/
row-m 100-133 n.d.-0.1
Finland 20% S.D.P. 100 g/kg seed 91-119 n.d.-0.3
Denmark " 3; 5 g/kg
transplant roots 82; 110 0.2; 1.2
Leeks Germany 50% E.C. 10 l/ha 121 n.d.
Cabbages Germany 50% E.C. 2% spray 2 ml/
plant 55 n.d.
Belgium " 0.2% spray
100 ml/plant 48 n.d.
Germany " 0.1% spray
250 ml/row-m 110 n.d.
USA E.C. 1 x 0.13 g a.i./
plant + 2 × 0.18 g/
a.i./row-m 23-44 n.d.
Germany 2.5% Gr. 2 g/plant 31; 62 n.d.
Cauliflower Germany 50% E.C. 6; 8; 10 l/ha 50-69 n.d.-0.01
" " 0.05% spray
100 ml/plant 55 n.d.
" " 0.1% spray
80 ml/plant 42; 52 n.d.
" " 2% spray 2 ml/
plant 69 n.d.
Cauliflower USA E.C. 1 × 0.13 g a.i./ 42-61 n.d.
plant + 2 × 0.18 g
a.i./row-m
Germany 2.5% Gr. 2 g/plant 35-60 n.d.-0.1
" 20% S.D.P. 100 g/kg seed 76 n.d.
Broccoli USA E.C. 1 × 0.13 g a.i./ 26-41 n.d.
plant + 2 × 0.18 g
a.i./row-m
Brussels USA E.C. 1 × 0.13 g a.i./ 40-58 n.d.
sprouts plant + 2 × 0.18 g
a.i./row-m
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Kohlrabi Germany 50% E.C. 6; 8; 10 kg/ha 30-50 0.01-0.08
" " 0.05% spray
100 ml/plant 19-45 0.01-0.1
" 2.5% Gr. 1 g/plant 19-45 0.04-0.4
Carrots Denmark 50% E.C. 4 l/ha 76-174 0.05-0.2
" " 8 l/ha " 0.04-0.2
Belgium " " 44; 79 3.6;* 5.0*
Germany " 10 l/ha 94; 97 0.2; 0.5
" " " 68-148 0.06-0.4
" 2.5% Gr. 100 kg/ha 64-92 0.05-0.3
Norway " " 62-139 0.3-0.6
France " " 135-172 0.01-0.02
Belgium " " 51; 81 n.d.
Italy " " 193 0.2
Denmark " 80 kg/ha 76 19.0*
" " " 90 11.0*
" " " 100-187 1.1-3.8*
Germany " 150 kg/ha 46-166 n.d.-2.0*
Holland " 2.5 g/row-m 111 0.6
Sweden " 3.5 g/row-m 156 0.8
" " 5.0 g/row-m " 1.4
" " 9.5 g/row-m " 2.3
Denmark 20% S.D.P. 100 g/kg 76-174 0.03-0.3
Finland " " 85-119 0.02-0.2
Barley Germany 2.5% Gr. 100 kg/ha 135 n.d.
Corn " 2.5% Gr. 80 kg/ha 169 n.d.
kernels USA Gr.; E.C. 5.6 kg a.i./ha 34-114 n.d.
cobs " " " " n.d.
forage " " " " n.d.
Oats Germany 2.5% Gr. 100; 150 kg/ha 97; 135 n.d.
Rye " 20% S.D.P. 250 g/kg seed 294 n.d.
Bananas Ecuador 2.5% Gr. 45; 67.5 g/plant 3-180 n.d.
Potatoes Germany 2.5% Gr. 150 kg/ha 150 0.02
Belgium " " 49; 62 0.3; n.d.
sweet USA Gr.; E.C. 5.6 kg a.i./ha 116-164 n.d.
Radish Germany 50% E.C 5; 10 kg/ha 21; 36 0.01; 0.1
" 2.5% Gr. 100 kg/ha 21; 36; 41 0.03; 0.04;
0.1
" 20% S.D.P. 100 g/kg seed 58 0.5-0.7
Finland " " 47 0.5
Rutabaga Finland 20% S.D.P. 100 g/kg seed 91; 135 n.d.; 0.05
Denmark " " 73; 153 n.d.
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Sugar-beets
roots Germany 2.5% Gr. 150 kg/ha 160 n.d.
tops " " " " n.d.
roots USA 10% Gr. 0.1-0.3 g a.i./
row-m 147-190 n.d.-0.1
tops " " " " n.d.-0.02
Turnips
roots Germany 2.5% Gr. 150 kg/ha 175 n.d.
tops " " " 163 0.01
1 E.C. = emulsifiable concentrate.
Gr. = granular.
S.D.P. = seed dressing powder.
2 Rate given in terms of formulation if not indicated
by a.i. = active ingredient.
3 n.d. = non-detectable (varies 0.01-0.1 ppm).
* Considered that the residue has not met the local requirements
of good agricultural practice.
It has been found (Brewerton et al., 1968) that the trichloronat
residues in pasture crops were at or below 5 mg/kg (dry matter basis)
after two weeks from treatment at 0.5 and 1 kg active ingredient per
hectare, after one month from treatment at 2 kg a.i. per ha, and after
two months from treatment at 4 kg a.i. per ha.
Fate of residues
General comments
Trichloronat is a thiophosphonate which is highly effective against
insects which live in or on soil (Homeyer, 1969). In crop protection,
it is used only as a soil insecticide. The compound itself causes only
slight depression of cholinesterase activity in vitro (Root et al.,
1969) whilst, on the other hand, the trichloronat-oxone, formed by
oxidation, has a strong anticholinesterase action. Trichloronat can be
metabolized into O-ethyl-O(2,4,5-trichlorophenyl)-ethylphosphonate,
2,4,5-trichlorophenol, O-ethyl-ethanephosphothioic acid,
O-ethyl-ethanephosphonic acid, and ethanephosphonic acid. Degradation
is effected mainly by splitting of the P-O-aryl bond with formation of
2,4,5-trichloro-phenol and O-ethyl-ethanethiophosphonic acid. On
account of its very low stability, the latter is further broken down
at a fast rate to O-ethyl-ethanephosphonic acid and finally to
ethanephosphonic acid.
In animals
No relevant data are available for the evaluation of trichloronat in
the feed (e.g. pasture grass) of the domestic animals.
In plants
It is known from biological experiments that trichloronat does not
have a systemic action. Chemical analysis showed (Möllhoff, 1968a)
that the compound is able, to a limited extent to penetrate into the
plant and to be translocated within it just as observed also for
parathion and similar compounds. Despite massive trichloronat
treatment, viz. application of 50 ppm to potted soil, the trichloronat
concentration in the aerial plant parts of China cabbage after 14 days
amounted to only 0.05 ppm. Later analyses of kohlrabi showed that
following application as a soil drench or granular treatment as
recommended, i.e. at 50 mg of active ingredient per plant, the maximum
residue amounted to 0.2 ppm after 30 to 40 days; the concentration in
the leaves was higher than in the edible root. In larger plants, e.g.
cabbages or bananas, the compound does not move upwards to a
sufficient extent to produce measurable concentrations (>0.01 ppm) in
the aerial plant parts. In root vegetables, the bulk of the absorbed
trichloronat is present in the peel. In an experiment in which lettuce
was sprayed with trichloronat (not done commercially), the
trichloronat residues decreased at the same rate as those of parathion
(Möllhoff, 1968b).
Plants can convert trichloronat to the oxone. Following massive
treatment, the concentrations may reach levels equivalent to 5-10% of
the respective trichloronat content. But no concentrations exceeding
0.05 ppm have been found in any instance (Bayer, 1971). The oxone
migrates at a fast rate in the plant. China cabbage grown in potted
soil containing an initial concentration of 19 ppm of oxone absorbed
so much of the oxone after two to seven days that the concentration in
the plants reached a level of 0.77 ppm (Möllhoff, 1968a). But in
comparison with trichloronat, the oxone is broken down in the plant at
a much faster rate (Möllhoff, 1968b).
When trichloronat is broken down in the plant, 2,4,5-trichlorophenol
is liberated. Very small concentrations of this metabolite were found
in tobacco and beets (Bayer, 1971). A further study of the metabolism
of trichloronat in plants is still in progress.
In soil
A study was undertaken by Tu (1970) to establish whether trichloronat
has any effect on microbial activities related to soil fertility.
Application of trichloronat to soil at rates of 10 and 100 ppm
affected the populations of bacteria and fungi for periods of one and
two weeks. The application did not have a permanently harmful effect
on nitrification, sulfur oxidation and phosphorus mineralization. On
the other hand, it significantly stimulated ammonification. There are
indications that trichloronat like other organo-phosphorus
insecticides undergo microbial degradation in soil. Harris (1969)
compared the persistence of biological activity of insecticides,
including trichloronat, in soil. The insecticides were divided into
three groups: (1) highly residual; (2) moderately residual; and (3)
slightly residual. In muck soil, trichloronat was classified into
group (3) and in sandy loam it was placed into group (2). According to
the results of experiments with granular formulations and emulsions in
the United States of America and in Germany (Bayer, 1971), the
concentration of trichloronat in soil decreases to a level of 50%
within 50 to 115 days. Bro-Rasmussen et al. (1970) studied the
persistence of organophosphorus insecticides in soil in Denmark, and
found that trichloronat has a half-life of 141 days.
The metabolites were found to reach their peak concentrations in soil
30 to 60 days after incorporation of the parent compound.
Trichloronat-oxone was not detectable (limit of determination of 0.01
ppm) in non-planted soil in any study. Oxone has been found in soil
only in one experiment on potted China cabbage (Möllhoff, 1968a) in
which there was a trichloronat concentration of 50 ppm in the soil.
When the experiment was terminated after 14 days, the oxone
concentration had reached a level of 0.05 ppm. In a parallel
experiment in which the oxone itself was applied into the soil, the
oxone concentration decreased from 19.3 ppm to a level of 0.4 ppm in
14 days. Therefore, it seems unlikely that the oxone is concentrated
in soil.
Splitting of the P-O-ethyl bond of trichloronat or its oxone was not
observed in soil samples (limit of determination of 0.01 ppm). The
2,4,5-trichlorophenol which is liberated by the splitting of the
P-O-aryl bond reached concentrations averaging 0.3 ppm in soil (Bayer,
1971; Möllhoff, 1971a; see Table II). The simultaneously liberated
O-ethyl-ethanethiophosphonic acid was detected in traces only under
favourable laboratory conditions and following addition of 40 ppm of
trichloronat to soil. In a field experiment with a starting value of
5.5 ppm of trichloronat, O-ethyl-ethanephosphonic acid and
ethanephosphonic acid reached maximum concentrations of 0.1-0.15 ppm
each (Table II). Ethane-thiophosphonic acid was not found in any
instance. In the above-mentioned laboratory experiment in which 40 ppm
of trichloronat was applied to the soil, the peak concentration of
O-ethyl-ethanephosphonic acid was 0.2 ppm and that of ethanephosphonic
acid was 1.2 ppm. Under laboratory conditions, the latter has in soil
a half-life of 15 to 20 days, as against a half-life of about 50 days
for trichloronat.
TABLE II. RESIDUES OF TRICHLORONAT AND ITS METABOLITES IN SOILS (µg/kg) (Mölhoff, 1971a)
EtO S EtO O HO S HO O EtO S EtO O HO S HO O
\ // \ // \ // \ // \ // \ // \ // \ //
Days P P P P HO-R P P P P
Soil after / \ / \ / \ / \ / \ / \ / \ / \ Total P
No. applic. Et O-R Et O-R Et O-R Et O-R Et OH Et OH Et OH Et OH (%)
1 0 5 510 n.d. n.d. n.d. 180 n.d. 60 n.d. 90 100
14 4 400 n.d. n.d. n.d. 200 n.d. 140 n.d. 110 78
Opladen 31 4 860 n.d. n.d. n.d. 250 n.d. 80 n.d. 50 75
60 2 340 n.d. trace n.d. 280 n.d. 80 n.d. 130 40
89 2 050 n.d. n.d. n.d. 50 n.d. <10 n.d. 100 36
119 1 120 n.d. n.d. n.d. 70 n.d. <10 n.d. 60 20
150 890 n.d. n.d. n.d. 40 n.d. <10 n.d. 30 15
180 990 n.d. n.d. n.d. nd n.d. nd n.d. nd 18
2 0 2 670 n.d. n.d. n.d. 90 n.d. 20 n.d. 30 100
14 2 700 n.d. n.d. n.d. 100 n.d. 20 n.d. 30 105
Höfchen 31 2 760 n.d. n.d. n.d. 30 n.d. 20 n.d. 40 109
60 2 350 n.d. n.d. n.d. 100 n.d. 30 n.d. 50 91
89 770 n.d.
119 790 n.d.
150 1 060 n.d.
180 900 n.d.
Other information on identity and properties
The active ingredient is a light brown liquid and has a boiling point
of 108°C at 0.01 mm Hg. It has a vapour pressure of 1.5 × 10-5 mm Hg
at 20°C and a volatility of 0.27 mg/m3 at 20°C. Its solubility in
water at 20°C is approximately 50 ppm, in kerosenes poor, but in
alcohol, acetone, chlorinated hydrocarbons and aromatic solvents good.
The stability of the active ingredient to hydrolysis is high in the
acid range, but in the alkaline range it decomposes quite readily
(Bayer, 1969).
Composition of the technical trichloronat is reported to be (Bayer,
1971):
active ingredient 93.0 - 95.0%
free 2,4,5-trichlorophenol 0.05 - 0.5%
O-ethyl-O-(2,4-dichlorophenyl)-ethylmonothiophosphonate 0.4 - 1.0%
O,O-diethyl ethylthiophosphonate 0.1 - 0.5%
sum of two unknown, low boiling compounds 1.0 - 1.5%
sum of three to four unknown, high
boiling compounds 3.0 - 5.0%
H2O 0.01 - 0.9%
Furthermore an analytical method for the determination of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the technical material has
been worked out. Samples analysed have contained less than 0.1 ppm of
"dioxin" which is the limit of detection. It is guaranteed by the
manufacturer that the "dioxin" content of the trichloronat
preparations is less than 0.1 ppm in terms of the active ingredient
(Bayer, 1971).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption and distribution
No information available.
Bio-transformation
No information available.
Excretion
No information available.
Effects on enzymes and other biochemical parameters
Three groups of three male rats orally dosed with 7.5, 3.8, or 1.9
mg/kg showed whole blood cholinesterase depression of 30, 15, and 0%
after three hours, 35, 25 and 15% after 24 hours, and 15, 0-10, and 0%
after 72 hours respectively (Kimmerle, G., 1962).
Trichloronat is ineffective as a cholinesterase inhibitor in vitro,
but is a strong inhibitor in vivo after i.p. administration of
7 mg/kg to rats. Maximum inhibition of brain, serum, and submaxillary
gland cholinesterase occurred in 1-6 hours. Reversal of inhibition was
slow, only returning to 60-75% of normal after 7 days (Root et al.,
1969).
Inhalation exposure of groups of 10 hens, for four hours five times
weekly for four weeks resulted in 60.4, and 76.7% whole blood
cholinesterase depression at air concentrations of 0.033, and 0.048
mg/l respectively (Kimmerle, G., 1968).
TOXICOLOGICAL STUDIES
Special studies
Reproduction
Four groups each comprising 10 male and 20 female rats were fed 0, 3,
10 and 30 ppm 95% pure trichloronat in the diet through three
generations. The second litter from each generation, F1b, and F2b,
was used as parents to establish the next generation.
No adverse effects were noted among the parental animals except for
depression of growth rate at 30 ppm. Reproductive performance was
normal at all dose levels, but pup growth was depressed at 30 ppm,
although litter size and birth weight were normal. In the third
generation, the survival of the pups was adversely affected by 30 ppm
trichloronat. No terata were observed (Löser, 1971).
Neurotoxicity
Exposure of groups of five hens to 0.055, 0.126, 0.248, or 0.585 mg/l
air for four hours resulted in 100% mortality at the two upper dose
levels. Examination of survivors 42 hours after exposure did not
reveal neurotoxic effects. Further groups pre-treated i.m. with 100 mg
PAM/kg and 50 mg atropine sulphate/kg exposed to 0.185, 0.4, 0.583 or
0.784 mg/l air resulted in three deaths out of five at the upper two
dose levels. All survivors exposed to 0.4 mg/l air and above showed
neurotoxic effects. Four-hour exposures repeated on five consecutive
days at 0.053, 0.126, 0.143 or 0.162 mg/l air did not result in
neurotoxic effects, although 1/5 and 5/5 hens died at the two top dose
levels. Birds similarly exposed after PAM and atropine treatment, to
levels of 0.222, or 0.376 mg/l air, all showed neurotoxic effects.
Neurotoxic effects were not observed following four-hour exposures
five times weekly for four weeks to air concentrations of 0.033 or
0.048 mg/l air (Kimmerle, 1968).
Neurotoxic effects were not observed in 1-2 year-old hens unprotected
against the cholinergic effects of trichloronat when single doses up
to those which proved lethal were given. However, when the hens were
protected with i.p. injections of atropine sulphate and 2-PAM, higher
doses of trichloronat could be given and 100 mg/kg i.p. or 150 mg/kg
orally produced neurotoxic effects. This effect of trichloronat was
not detected in a sub-acute experiment in which 0, 250, 500, 1000 and
2000 ppm trichloronat in the diet was fed to groups of eight hens for
30 days. Two hens per group were killed after the treatment period,
the rest were killed 30 days later; neurotoxic effects were not
observed (Kimmerle, 1965).
Potentiation
Trichloronat administered simultaneously with malathion resulted in a
two-fold potentiation of the malathion toxicity (Root et al., 1969).
Toxicity of contaminants
2,4,5-Trichlorophenol is known to occur as a contaminant of
trichloronat at a level of 1-2%.
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
2,3,7,8-Tetrachlorodibenzo-p-dioxin ("dioxin") is also a possible
contaminant of trichloronat. The sole manufacturer of trichloronat
states that the level of "dioxin" is below 0.1 ppm in technical
trichloronat. (See "Identity").
Acute toxicity
Species Route LD50 Reference
(mg/kg)
Mouse i.p. 33-35 DuBois and Kinoshita, 1963
Rat (M) oral 16-55 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1968; Kimmerle, 1967
Rat (F) oral 16-37.5 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Rat (M) i.p. 10-14 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Rat (F) i.p. 11-37.5 Dubois et al., 1966;
Kimmerle, 1962
Species Route LD50 Reference
(mg/kg)
Rat dermal 64-250 DuBois and Kinoshita, 1963;
Gaines, 1969; Kimmerle,
1962
Guinea-pig oral 40-100 DuBois and Kinoshita, 1963;
Kimmerle, 1962
Guinea-pig i.p. 26 DuBois and Kinoshita, 1963
(M)
Rabbit oral 25-50 Kimmerle, 1962
Cat oral 10-25 Kimmerle, 1962; Kimmerle,
1968
Chicken oral 45 DuBois, 1963
Groups of five female rats were injected i.p., daily for 60 days, at
dose levels of 0, 2, 4, 6 and 8 mg/kg/day. Mortality resulted in 2 out
of 5, 4 out of 5, and 5 out of 5 at 4, 6 and 8 mg/kg respectively.
Body weight was reduced at all dose levels, although some recovery
occurred at 2 and 4 mg/kg after 20 days on the test. Survivors
autopsied at 60 days showed marked depression of serum, submaxillary
gland, and brain cholinesterase (DuBois et al., 1966).
Rabbit. A pair of rabbits dosed orally, five times weekly for two
weeks with 5 mg/kg/dose did not lose body weight or display symptoms
of poisoning (Kimmerle, 1962).
Liver function tests (BSP, SG.OT, SG-PT) were unaffected by 20 mg/kg
(one rabbit) or 10 mg/kg (two rabbits) administered as a single oral
dose (Kimmerle, 1962).
Dog. Groups of beagle dogs were fed 0, 2, 5, 10 (two males and two
females/group or 25 (one male and one female) ppm in dry diet for 12
weeks. At termination of the study, erythrocyte cholinesterase was
depressed at 25 ppm (40%) in the females. Plasma cholinesterase was
depressed in both sexes at 10 ppm (39% in males and 27% in females)
but in females only at 25 ppm (41%). Brain cholinesterase was
comparable to control values for all groups. Liver cholinesterase was
depressed in males at 5 ppm and above (depression exceeding 20%), and
in females at 10 ppm and above. Thyroid weight and weight ratio were
decreased at 25 ppm (Root at al., 1968b). Histopathological changes
including lymphocytic proliferation of the intestinal wall were not
dose-related (Hibbs and Nelson, 1968).
Groups of two male and two female beagle dogs were fed 0 or 5 ppm in
the dry diet for two years. A further group was fed 25 ppm for 18
months. The 25 ppm dose level was then increased to 50 ppm for the
next seven weeks, to 100 ppm for the subsequent 14 weeks and finally
to 200 ppm until the termination of the study. A final group was fed 2
ppm for two years, and then 1 ppm for a further 13 weeks, and then
control diet for the final eight weeks prior to autopsy. Body symptoms
in all cases reported were typical of cholinesterase depression.
Short-term studies
Rat. Groups of 10 male rats were incubated five times weekly for
eight weeks with 0.75, 1.5, 3, 6, or 12 mg/kg/dose followed by a four
week observation period. Symptoms of cholinesterase depression were
seen at 12 mg/kg after the first dose only (Kimmerle, 1968).
Groups of 20 male and 20 female rats were fed 0, 1, 3, 10 or 30 ppm of
95% pure trichloronat in the diet for three months. Final body weight
was comparable, but males fed 10 and 30 ppm showed a slight dose
related lag in weight gain during the second month. Signs of
cholinesterase depression were seen during the morning from the second
week in rats fed 30 ppm and in some rats fed 10 ppm. Plasma and
erythrocyte cholinesterase levels were depressed at 10 and 30 ppm,
erythrocyte cholinesterase being depressed slightly more than plasma
cholinesterase. Depression at 3 ppm in plasma and erythrocytes was
less than 20% (Löser, 1968).
Groups of 15 male and 15 female rats were fed 2.25, 4.5, 9 or 18 ppm
of technical trichloronat in the diet for four months. The control
group comprised 30 male and 30 female rats. Cholinesterase depression
in whole blood was apparent in females at 4.5 and 9 ppm at four weeks.
At eight weeks, male rats showed depression at 9 and 18 ppm, and
females at 18 ppm only. At 12 and 16 weeks, cholinesterase depression
was apparent in both sexes at 18 ppm only (Löser, 1966).
Histopathological examination revealed low-grade fatty changes in
isolated epithelial cells of the liver of five male rats fed 18 ppm
(Hobik, 1967).
Five groups of 10 male and 10 female rats were fed 0, 2, 5, 10 or 25
ppm in the diet for 16 weeks. Terminal cholinesterase determinations
indicated depression occurred in erythrocytes at 10 and 25 ppm, in
brain and submaxillary glands at 25 ppm and in plasma of female rats
at 25 ppm. Depression was less than 20% in all other groups, and less
than 10% except for plasma in females at 10 ppm and erythrocytes in
males at 5 ppm (Root at al., 1968a). The presence of dilated kidney
tubules with eosinophilic casts was observed in all groups, but the
incidence was considerably greater in the test groups (Grey at al.,
1968). Weight and food consumption were reduced at 200 ppm. Signs of
cholinesterase depression became apparent within three weeks of
feeding 200 ppm. One male dog died after nine weeks exposure at this
level. Erythrocyte cholinesterase was comparable to controls until the
dose was increased to 75 ppm, at which dose depression occurred. The
depression increased when the dose was increased to 200 ppm. Plasma
cholinesterase was depressed at 2 ppm and above, depression being
about 20% at 2 ppm. When the dose was reduced to 1 ppm, recovery to
99% of normal activity occurred. At autopsy, liver cholinesterase was
inhibited 45% at 5 ppm. No reduction was observed in the group reduced
from 2 to 1 to 0 ppm. Brain cholinesterase was reduced 10% at 5 ppm,
and 72% at the top dose level. No compound related histological
changes were apparent (Root et al., 1970).
Long-term studies
Rat. Four groups of 30 male and 30 female rats were fed 1, 3, 10, or
30 ppm of 95% pure trichloronat in the diet for two years. The control
group comprised 60 males and 60 females. Body weight of males at 30
ppm was reduced, female body weight at 30 ppm was reduced between 9
and 12 months. Food consumption at 30 ppm was marginally reduced.
Average absolute weight of male heart, lung, liver, kidney, and spleen
were depressed, but organ/body weight ratio was unaffected. Signs of
cholinesterase depression were apparent in the morning during the
first three months in the 30 ppm group. Plasma and erythrocyte
cholinesterase were depressed at 10 and 30 ppm, although at 10 ppm the
depression became marginal as the study progressed. Terminal brain
cholinesterase was depressed at 10 and 30 ppm in both sexes, and
marginally depressed (17%) in males at 3 ppm. Histopathological data
have not been submitted (Löser, 1970).
Observations in man
No information is available.
Comments
Trichloronat is a persistent organophosphorus insecticide used for
soil treatment. No information is available on the absorption,
distribution, excretion or general metabolism in animals.
Short-term studies in the rat and dog, one long-term (two-year) study
in the rat and a three-generation reproduction study in rats are
available. In the short-term study some histopathological changes were
observed, the nature of which could not be assessed. In the long-term
studies insufficient information was available both on gross and
histopathology of the organs.
For this reason and because there is no information on the metabolism
of this persistent insecticide the Meeting decided that no acceptable
daily intake for man could be established at this time.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Trichloronat is an insecticidal compound with a contract and stomach
action and with a residual activity of relatively long duration for a
phosphorus insecticide. It is especially used for the control of
soil-inhabiting developmental stages of different insect species and
vegetable fly larvae. The formulations currently on the market are as
follows (Bayer, 1969; 1971):
2.5% granular (Gr.)
7.5% granular (Gr.)
20.0% seed dressing powder (S.D.P.)
50.0% emulsifiable concentrate (E.C.)
Practically all trichloronat manufactured is applied to vegetable
crops.
Pre-harvest treatments
The recommended usages of trichloronat (Bayer, 1969) with regard to
crops, pests, formulations, and rates are given below:
Onions, leeks Hylemyia antiqua (Onion fly)
50% E.C. 10 litres per hectare, spray presowing and work
in just below the surface;
0.1% (250 cm3 per running metre), drench at
egg-laying;
7.5% Gr. 25 kg/hectare (or 7.5 g/10 metres), row treatment
at sowing;
2.5% Gr. 80-100 kg/hectare (or 2.5 g per running metre),
row treatment at sowing;
20% S.D.P. 75-100 g/kg seed, before treatment, moisten
seed using 50-75 cm3 of water; sow within 24
hours of treatment.
Brassica crops Hylemyia brassicae (Cabbage root fly)
Hylemyia floralis (Turnip fly)
50% E.C. 0.04% (1 litre/m2), treatment of nursery seedbed
or 10 litres per hectare, spray presowing, and
work in just below surface, or
0.1% (250 cm3 per running metre, or 80-100 cm3/
Plant), drench at egg-laying;
7.5% Gr. 4 g/m2, treatment of seedbed, or 25 kg/hectare,
row treatment at transplanting;
Onions, leeks Hylemyia antiqua (Onion fly)
2.5% Gr. 1-2 kg/m3 for treatment of nursery bed soil, or
800 g/100 metres, row treatment at planting or
100 kg/hectare, row treatment at transplanting,
or 1-2 g/plant applied to stem base at
transplanting (also as mixture with sand), or
3-5 g/plant hole at transplanting.
Carrots Psila rosae (Carrot fly)
50% E.C. 10 litres/hectare, spray presowing and work in
just below surface;
2.5% Gr. 100 kg/hectare, row treatment at sowing;
20% S.D.P. 100 g/kg seed.
Cereals Hylemyia coarctata (Wheat bulb fly)
2.5% Gr. 80-100 kg/hectare, broadcast treatment;
20% S.D.P. 250 g/100 kg seed.
If fungicides are additionally applied use only
non-mercurial products.
Bananas Cosmopolites sordidus (Banana weevil borer)
2.5% Gr. 40-60 g/banana plant, apply in a radius of up
to 40 cm around stem.
Grassland Costelytra zealandica, Heteronychus arator,
Oncopera intricata, Wiseana
2.5% Gr. 20-80 kg/hectare, broadcast treatment;
50% E.C. 2 litres/hectare, broadcast treatment.
Trichloronat is recommended for testing on some additional pests.
Other uses
The compound is also used for termite-proofing of polyethylene,
plasticized PVC and rubber.
Residues resulting from supervised trials
The residue data from supervised trials are given in Table I. They are
compiled from the documentation made available by Bayer (1971).
TABLE I
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Onions Germany 50% E.C. 10 l/ha 100-133 n.d.-0.02
Belgium " 8 l/ha 35 n.d.
Germany " 0.1% spray
250 ml/row-m 58; 71 5.5; 1.6
Germany 2.5% Gr. 200 kg/ha 163 n.d.
" " 1.5; 2.5 g/
row-m 100-133 n.d.-0.1
Finland 20% S.D.P. 100 g/kg seed 91-119 n.d.-0.3
Denmark " 3; 5 g/kg
transplant roots 82; 110 0.2; 1.2
Leeks Germany 50% E.C. 10 l/ha 121 n.d.
Cabbages Germany 50% E.C. 2% spray 2 ml/
plant 55 n.d.
Belgium " 0.2% spray
100 ml/plant 48 n.d.
Germany " 0.1% spray
250 ml/row-m 110 n.d.
USA E.C. 1 x 0.13 g a.i./
plant + 2 × 0.18 g/
a.i./row-m 23-44 n.d.
Germany 2.5% Gr. 2 g/plant 31; 62 n.d.
Cauliflower Germany 50% E.C. 6; 8; 10 l/ha 50-69 n.d.-0.01
" " 0.05% spray
100 ml/plant 55 n.d.
" " 0.1% spray
80 ml/plant 42; 52 n.d.
" " 2% spray 2 ml/
plant 69 n.d.
Cauliflower USA E.C. 1 × 0.13 g a.i./ 42-61 n.d.
plant + 2 × 0.18 g
a.i./row-m
Germany 2.5% Gr. 2 g/plant 35-60 n.d.-0.1
" 20% S.D.P. 100 g/kg seed 76 n.d.
Broccoli USA E.C. 1 × 0.13 g a.i./ 26-41 n.d.
plant + 2 × 0.18 g
a.i./row-m
Brussels USA E.C. 1 × 0.13 g a.i./ 40-58 n.d.
sprouts plant + 2 × 0.18 g
a.i./row-m
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Kohlrabi Germany 50% E.C. 6; 8; 10 kg/ha 30-50 0.01-0.08
" " 0.05% spray
100 ml/plant 19-45 0.01-0.1
" 2.5% Gr. 1 g/plant 19-45 0.04-0.4
Carrots Denmark 50% E.C. 4 l/ha 76-174 0.05-0.2
" " 8 l/ha " 0.04-0.2
Belgium " " 44; 79 3.6;* 5.0*
Germany " 10 l/ha 94; 97 0.2; 0.5
" " " 68-148 0.06-0.4
" 2.5% Gr. 100 kg/ha 64-92 0.05-0.3
Norway " " 62-139 0.3-0.6
France " " 135-172 0.01-0.02
Belgium " " 51; 81 n.d.
Italy " " 193 0.2
Denmark " 80 kg/ha 76 19.0*
" " " 90 11.0*
" " " 100-187 1.1-3.8*
Germany " 150 kg/ha 46-166 n.d.-2.0*
Holland " 2.5 g/row-m 111 0.6
Sweden " 3.5 g/row-m 156 0.8
" " 5.0 g/row-m " 1.4
" " 9.5 g/row-m " 2.3
Denmark 20% S.D.P. 100 g/kg 76-174 0.03-0.3
Finland " " 85-119 0.02-0.2
Barley Germany 2.5% Gr. 100 kg/ha 135 n.d.
Corn " 2.5% Gr. 80 kg/ha 169 n.d.
kernels USA Gr.; E.C. 5.6 kg a.i./ha 34-114 n.d.
cobs " " " " n.d.
forage " " " " n.d.
Oats Germany 2.5% Gr. 100; 150 kg/ha 97; 135 n.d.
Rye " 20% S.D.P. 250 g/kg seed 294 n.d.
Bananas Ecuador 2.5% Gr. 45; 67.5 g/plant 3-180 n.d.
Potatoes Germany 2.5% Gr. 150 kg/ha 150 0.02
Belgium " " 49; 62 0.3; n.d.
sweet USA Gr.; E.C. 5.6 kg a.i./ha 116-164 n.d.
Radish Germany 50% E.C 5; 10 kg/ha 21; 36 0.01; 0.1
" 2.5% Gr. 100 kg/ha 21; 36; 41 0.03; 0.04;
0.1
" 20% S.D.P. 100 g/kg seed 58 0.5-0.7
Finland " " 47 0.5
Rutabaga Finland 20% S.D.P. 100 g/kg seed 91; 135 n.d.; 0.05
Denmark " " 73; 153 n.d.
TABLE I (Cont'd.)
Crop Country Formulation Application Pre-harvest Residue
used1 rate2 interval (ppm)3
(days)
Sugar-beets
roots Germany 2.5% Gr. 150 kg/ha 160 n.d.
tops " " " " n.d.
roots USA 10% Gr. 0.1-0.3 g a.i./
row-m 147-190 n.d.-0.1
tops " " " " n.d.-0.02
Turnips
roots Germany 2.5% Gr. 150 kg/ha 175 n.d.
tops " " " 163 0.01
1 E.C. = emulsifiable concentrate.
Gr. = granular.
S.D.P. = seed dressing powder.
2 Rate given in terms of formulation if not indicated
by a.i. = active ingredient.
3 n.d. = non-detectable (varies 0.01-0.1 ppm).
* Considered that the residue has not met the local requirements
of good agricultural practice.
It has been found (Brewerton et al., 1968) that the trichloronat
residues in pasture crops were at or below 5 mg/kg (dry matter basis)
after two weeks from treatment at 0.5 and 1 kg active ingredient per
hectare, after one month from treatment at 2 kg a.i. per ha, and after
two months from treatment at 4 kg a.i. per ha.
Fate of residues
General comments
Trichloronat is a thiophosphonate which is highly effective against
insects which live in or on soil (Homeyer, 1969). In crop protection,
it is used only as a soil insecticide. The compound itself causes only
slight depression of cholinesterase activity in vitro (Root et al.,
1969) whilst, on the other hand, the trichloronat-oxone, formed by
oxidation, has a strong anticholinesterase action. Trichloronat can be
metabolized into O-ethyl-O(2,4,5-trichlorophenyl)-ethylphosphonate,
2,4,5-trichlorophenol, O-ethyl-ethanephosphothioic acid,
O-ethyl-ethanephosphonic acid, and ethanephosphonic acid. Degradation
is effected mainly by splitting of the P-O-aryl bond with formation of
2,4,5-trichloro-phenol and O-ethyl-ethanethiophosphonic acid. On
account of its very low stability, the latter is further broken down
at a fast rate to O-ethyl-ethanephosphonic acid and finally to
ethanephosphonic acid.
In animals
No relevant data are available for the evaluation of trichloronat in
the feed (e.g. pasture grass) of the domestic animals.
In plants
It is known from biological experiments that trichloronat does not
have a systemic action. Chemical analysis showed (Möllhoff, 1968a)
that the compound is able, to a limited extent to penetrate into the
plant and to be translocated within it just as observed also for
parathion and similar compounds. Despite massive trichloronat
treatment, viz. application of 50 ppm to potted soil, the trichloronat
concentration in the aerial plant parts of China cabbage after 14 days
amounted to only 0.05 ppm. Later analyses of kohlrabi showed that
following application as a soil drench or granular treatment as
recommended, i.e. at 50 mg of active ingredient per plant, the maximum
residue amounted to 0.2 ppm after 30 to 40 days; the concentration in
the leaves was higher than in the edible root. In larger plants, e.g.
cabbages or bananas, the compound does not move upwards to a
sufficient extent to produce measurable concentrations (>0.01 ppm) in
the aerial plant parts. In root vegetables, the bulk of the absorbed
trichloronat is present in the peel. In an experiment in which lettuce
was sprayed with trichloronat (not done commercially), the
trichloronat residues decreased at the same rate as those of parathion
(Möllhoff, 1968b).
Plants can convert trichloronat to the oxone. Following massive
treatment, the concentrations may reach levels equivalent to 5-10% of
the respective trichloronat content. But no concentrations exceeding
0.05 ppm have been found in any instance (Bayer, 1971). The oxone
migrates at a fast rate in the plant. China cabbage grown in potted
soil containing an initial concentration of 19 ppm of oxone absorbed
so much of the oxone after two to seven days that the concentration in
the plants reached a level of 0.77 ppm (Möllhoff, 1968a). But in
comparison with trichloronat, the oxone is broken down in the plant at
a much faster rate (Möllhoff, 1968b).
When trichloronat is broken down in the plant, 2,4,5-trichlorophenol
is liberated. Very small concentrations of this metabolite were found
in tobacco and beets (Bayer, 1971). A further study of the metabolism
of trichloronat in plants is still in progress.
In soil
A study was undertaken by Tu (1970) to establish whether trichloronat
has any effect on microbial activities related to soil fertility.
Application of trichloronat to soil at rates of 10 and 100 ppm
affected the populations of bacteria and fungi for periods of one and
two weeks. The application did not have a permanently harmful effect
on nitrification, sulfur oxidation and phosphorus mineralization. On
the other hand, it significantly stimulated ammonification. There are
indications that trichloronat like other organo-phosphorus
insecticides undergo microbial degradation in soil. Harris (1969)
compared the persistence of biological activity of insecticides,
including trichloronat, in soil. The insecticides were divided into
three groups: (1) highly residual; (2) moderately residual; and (3)
slightly residual. In muck soil, trichloronat was classified into
group (3) and in sandy loam it was placed into group (2). According to
the results of experiments with granular formulations and emulsions in
the United States of America and in Germany (Bayer, 1971), the
concentration of trichloronat in soil decreases to a level of 50%
within 50 to 115 days. Bro-Rasmussen et al. (1970) studied the
persistence of organophosphorus insecticides in soil in Denmark, and
found that trichloronat has a half-life of 141 days.
The metabolites were found to reach their peak concentrations in soil
30 to 60 days after incorporation of the parent compound.
Trichloronat-oxone was not detectable (limit of determination of 0.01
ppm) in non-planted soil in any study. Oxone has been found in soil
only in one experiment on potted China cabbage (Möllhoff, 1968a) in
which there was a trichloronat concentration of 50 ppm in the soil.
When the experiment was terminated after 14 days, the oxone
concentration had reached a level of 0.05 ppm. In a parallel
experiment in which the oxone itself was applied into the soil, the
oxone concentration decreased from 19.3 ppm to a level of 0.4 ppm in
14 days. Therefore, it seems unlikely that the oxone is concentrated
in soil.
Splitting of the P-O-ethyl bond of trichloronat or its oxone was not
observed in soil samples (limit of determination of 0.01 ppm). The
2,4,5-trichlorophenol which is liberated by the splitting of the
P-O-aryl bond reached concentrations averaging 0.3 ppm in soil (Bayer,
1971; Möllhoff, 1971a; see Table II). The simultaneously liberated
O-ethyl-ethanethiophosphonic acid was detected in traces only under
favourable laboratory conditions and following addition of 40 ppm of
trichloronat to soil. In a field experiment with a starting value of
5.5 ppm of trichloronat, O-ethyl-ethanephosphonic acid and
ethanephosphonic acid reached maximum concentrations of 0.1-0.15 ppm
each (Table II). Ethane-thiophosphonic acid was not found in any
instance. In the above-mentioned laboratory experiment in which 40 ppm
of trichloronat was applied to the soil, the peak concentration of
O-ethyl-ethanephosphonic acid was 0.2 ppm and that of ethanephosphonic
acid was 1.2 ppm. Under laboratory conditions, the latter has in soil
a half-life of 15 to 20 days, as against a half-life of about 50 days
for trichloronat.
TABLE II. RESIDUES OF TRICHLORONAT AND ITS METABOLITES IN SOILS (µg/kg) (Mölhoff, 1971a)
EtO S EtO O HO S HO O EtO S EtO O HO S HO O
\ // \ // \ // \ // \ // \ // \ // \ //
Days P P P P HO-R P P P P
Soil after / \ / \ / \ / \ / \ / \ / \ / \ Total P
No. applic. Et O-R Et O-R Et O-R Et O-R Et OH Et OH Et OH Et OH (%)
1 0 5 510 n.d. n.d. n.d. 180 n.d. 60 n.d. 90 100
14 4 400 n.d. n.d. n.d. 200 n.d. 140 n.d. 110 78
Opladen 31 4 860 n.d. n.d. n.d. 250 n.d. 80 n.d. 50 75
60 2 340 n.d. trace n.d. 280 n.d. 80 n.d. 130 40
89 2 050 n.d. n.d. n.d. 50 n.d. <10 n.d. 100 36
119 1 120 n.d. n.d. n.d. 70 n.d. <10 n.d. 60 20
150 890 n.d. n.d. n.d. 40 n.d. <10 n.d. 30 15
180 990 n.d. n.d. n.d. nd n.d. nd n.d. nd 18
2 0 2 670 n.d. n.d. n.d. 90 n.d. 20 n.d. 30 100
14 2 700 n.d. n.d. n.d. 100 n.d. 20 n.d. 30 105
Höfchen 31 2 760 n.d. n.d. n.d. 30 n.d. 20 n.d. 40 109
60 2 350 n.d. n.d. n.d. 100 n.d. 30 n.d. 50 91
89 770 n.d.
119 790 n.d.
150 1 060 n.d.
180 900 n.d.
 n.d. non detectable
Assuming that trichloronat is applied, according to the
recommendations, only once a year to the same field and taking into
account the degradation rates presented before, there seems to be no
accumulation of trichloronat in the soils to be expected.
The behaviour of trichloronat in simulated field environment was
studied to determine its relative potential for contaminating water
stores. Residues in runoff water from field soil plots were less than
1% within a 14-day interval of application. Leaching studies in the
laboratory for high nitrogen, clay and sandy loam soils indicated that
rainfalls of 368, 447 and 103 inches, respectively, would
theoretically be required to leach the compound 12 inches into the
soil (Shaw et al., 1971). The half-life of trichloronat in neutral or
alkaline water is very short. It was found to be 51 hours in water
buffered to a pH of 7 at 30°C (Shaw et al., 1971). Measurements in
isopropanol/water 1:1 (v/v) at pH 11.5, equivalent to pH 10.5 in
water, produced a half-life of 6.2 hours for trichloronat at 37°C; the
half-life value found for parathion was 28.3 hours, and thus greater
by a factor of 4.5 (Hofer, 1969). A similar factor, viz. 5.5, was
obtained by Möllhoff (1971b) in measurements in distilled water at
26°C. The half-life values were 110 days for parathion and paraoxone,
50 days for parathion-methyl, 20 days for trichloronat and
paraoxone-methyl, and 60 days for trichloronat-oxone.
In storage and processing
Residues of trichloronat, trichloronat-oxone and 2,4,5-trichlorophenol
in cold-stored (approximately -20°C) cabbage and potatoes did not
decrease in 168 to 224 days (Chemagro Corp., 1968). Kohlrabi roots
containing 0.3 ppm of trichloronat were boiled unpeeled for 30 minutes
in twice the amount by weight of water in a closed vessel, and then
analysed together with the water. Twenty-seven per cent of the
compound was decomposed (Bayer, 1971). Washed carrots contained 0.35
ppm of residue; after they had been peeled, no more residue was
detectable in them (limit of determination of 0.07 ppm) (Bayer, 1971).
Washed turnips, carrots and onions contained no detectable residues
after being peeled (Anon., Finland, 1969). The peel of carrots
contained 0.66 ppm of residue as against only 0.02 ppm in the pulp.
After these carrots had been mechanically washed, the peel was found
still to contain 0.32 ppm residue and the pulp contained 0.04 ppm of
residue. After the carrots had been blanched for one minute at 100°C,
no more residues were detectable in them (<0.02 ppm). A similar
result was obtained for normal preservation (Martens, 1970). In a
study to investigate the effect of processing on trichloronat in sugar
beets, a laboratory procedure was employed which simulated the
industrial process; the results showed that trichloronat,
trichloronat-oxone, and 2,4,5-trichlorophenol were completely degraded
during the first initial boiling and liming step (Katague and
Anderson, 1968a). Cigarettes containing trichloronat,
trichloronat-oxone and 2,4,5-trichlorophenol were smoked and the smoke
was analysed. Approximately 15% of each of the three compounds was
found in the smoke (Olson and Anderson, 1968).
Evidence of residues in food in commerce or at consumption
From 1964 to 1968, Renvall and Åkerblom (1971) analysed 2396 samples
of domestic and imported fruit and vegetables obtained from the
Swedish market. Only about 0.01% of these samples, i.e. two or three,
contained trichloronat. The residue level was less than 0.1 ppm. The
limit of determination was 0.01 ppm. In 1968, Krause and Kirchhoff
(1969, 1970) carried out analyses of market samples of fruit and
vegetables produced in or imported into Germany. None of the 70
analysed samples contained detectable residues of trichloronat (limit
of determination of 0.01 ppm).
Methods of residue analysis
Studies of metabolites have shown that following customary application
of trichloronat, the oxone will occur, if at all, only in low
concentrations because its half-life is considerably shorter than that
of the parent compound (Möllhoff, 1968a, 1968b), 2,4,5-trichlorophenol
also occurs only in very low concentrations or not at all. Therefore,
determination of the parent compound itself is sufficient for
regulatory purposes.
Renvall and Åkerblom (1971) developed a thin-layer chromatographic
method for determining residues of organophosphorus insecticides,
including trichloronat, in fruit and vegetables, and which has a limit
of determination of 0.1 ppm.
Trichloronat can also always be co-determined by multiresidue methods
for gas-chromatographic determination of organophosphorus
insecticides, which are suitable for determining parathion (Beckman
and Garber, 1969; Krause and Kirchhoff, 1970; Möllhoff, 1967, 1968b;
Renvall and Åkerblom, 1971; Sans, 1967). Detectors that have been used
include the electron-capture detector (Brewerton at al., 1968;
Möllhoff, 1967), the thermionic detector and the flame photometric
detector (Bowman and Beroza, 1970).
There are two special methods for the determination of trichloronat
residues. One is based on the determination of the phenol group
(Katague and Anderson, 1966), and the other is based on the
determination of the parent compound itself (Möllhoff, 1967; 1968a,
1968b).
In the method based on the determination of the phenol, trichloronat
and its oxone are determined together and the free
2,4,5-trichlorophenol is determined separately. After separating the
free 2,4,5-trichlorophenol, trichloronat and its oxone are saponified.
The resultantly liberated phenol and the previously separated phenol
which is already free are separately acetylated and determined by
gas-chromatography using an electron-capture detector. The limit of
determination is generally 0.01 ppm. For confirmation of the results,
two columns (Katague and Anderson, 1968b) is used. In a study
conducted by Katague and Anderson (1967), 43 organophosphorus
pesticides were checked for possible interference with the
trichloronat residue analysis method. The trichloronat analysis is
interfered with only by fenchlorphos (Ronnel) and its oxygen analogue
(they were not included in the above study) because they contain the
same phenol as trichloronat.
The second special method of trichloronat residue analysis with
terminal gas-chromatographic determination permits trichloronat and
its oxygen analogue to be detected separately with the phosphorus or
the electron-capture detector. By using suitable columns, it was
possible to separate trichloronat from 35 organophosphorus
insecticides including fenchlorphos (Ronnel). The response of the
electron-capture detector to trichloronat is three times greater than
that of the thermionic phosphorus detector but interference peaks
occur on concentrating the extracts of some crops. Organochlorine
compounds may also interfere. Therefore, the phosphorus detector
should be preferably used for the determination.
The metabolites that cannot be assayed by either of these two methods,
can be determined in soil and plant samples by a gas-chromatographic
method described by Möllhoff (1970).
Examples of national tolerances
Tolerance Safety
Country Crop in ppm interval
in days
Belgium Cabbage 60
Carrots 90
Fruit, vegetables (incl.
cabbage, onions), excl.
potatoes 0.1
Canada Cole crops N.R.
Denmark Onion, cabbage and carrot
seed (as seed dressing) 0
Finland General (as soil drench) 84
Italy General 20
Netherlands Cabbage (soil treatment) 42
Beans 0
Cabbage, leeks, onions 0.1
Miscellaneous 0
Norway Edible root crops
(as granular) 90
Appraisal
Trichloronat is an organothiophosphorus insecticide which is used
against soil insects, especially vegetable fly larvae.
It is chiefly recommended for treatments of onions, leeks, brassica
crops, carrots, cereals, bananas and grassland. Types of application
are seed dressing, granular broadcast, soil spray, transplant and soil
drench. Amounts applied are up to 5 kg active ingredient per hectare.
Technical trichloronat is reported to contain 93-95% active
ingredient. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) may exist
as an impurity in the technical material. It is guaranteed by the sole
manufacturer that the dioxin content is below the limit of detection
of the analytical method (0.1 ppm).
Residue data are available from several countries.
Trichloronat is subject to degradation in soils, plants and animals.
Prom the degradation products trichloronat-oxone is of toxicological
importance, but it is existing only in insignificant amounts in the
terminal residues.
From the treated seeds or soil, trichloronat is sorbed to the surface
of the plant roots and is migrating only in minor amounts into the
aerial parts of the crops. From the root crops, carrots have shown
highest incidence and magnitude of residues. It has been demonstrated
that peeling, blanching, etc. processes decrease the residues of
carrots and other root crops to or below the levels of analytical
detection limits.
There is a TLC method with a detection limit of 0.1 ppm, GLC
multi-residue methods, and two specific methods with detection limits
of about 0.01 ppm for determining the residues of trichloronat in
various crops.
The evaluation of residue data is based on the assumptions that the
technical trichloronat contain less than 0.1 ppm dioxin and that it is
applied only into soils or to seeds and no treatments of the growing
crops are made. Any such treatments are not regarded as good
agricultural practice.
Since no residues have been detected (less than 0.01 ppm) in bananas
and cereals from recommended usages no tolerances are needed for those
crops.
Since an acceptable daily intake for trichloronat was not established
by the Meeting, no recommendations for tolerances are made.
As a result of recommended use of trichloronat, following residue
levels need not be exceeded:
Onions, leeks, kohlrabi, radish 0.5 ppm
Cabbage, cauliflower, broccoli,
brussels sprouts, rutabagas,
turnips, sugar beets, potatoes 0.1 ppm
Carrots 1 ppm
Further work or information
Required (before an acceptable daily intake can be established or
tolerances recommended):
1. Information on the absorption, distribution, excretion and
general metabolism of trichloronat in at least one mammalian
species.
2. Comprehensive information on the gross and histopathological
findings particularly after long-term administration of this
compound.
3. Relevant data for the evaluation of trichloronat in the feed of
domestic animals, e.g. fodder crops.
REFERENCES
Anon., Finland. (1969) State Institute of Agr. Chem., Helsinki,
Finland
Bayer AG, Farbenfabriken, Pflanzenschutz. (1969) (R)Phytosol,
(R)Agritox (Bayer 37289, S 4400). Leverkusen. E. 1-659/23536
Bayer AG, Farbenfabriken, Pflanzenschutz. (1971) Documentation on
trichloronat for FAO
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system.
J.A.O.A.C., 52, 286-293
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulfur on four thermally
stable columns. J.A.O.A.C., 53: 499-508
Brewerton, H.V., Gibbs, M. M. and Perrot, D. C. F. (1968)
Fensulgothion and "Bayer 37 289" residues in pasture. New Zealand
J. Agr. Res., 11: 303-312
Bro-Rasmussen, F., Noddegaard, E. and Voldum-Clausen, K. (1970)
Comparison of the disappearance of eight organophosphorus insecticides
from soil in laboratory and in outdoor experiments. Pesticide Sci.,
1: 179-199
Chemagro Corporation, Research Dept., Kansas City, U.S.A., Rep. No. 23
060. (1968) The effect of frozen storage at 0 to -10°F on Bay 37 289.
Residues in cabbage and potatoes
DuBois, K. P. (1963) The acute oral toxicity of Bayer 37289 to
chickens. Unpublished report of the Toxicity Lab., University of
Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P. and Kinoshita, F. (1963) The acute toxicity of Bayer
37289 to mammals. Unpublished report of the Toxicity Lab., University
of Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P., Kinoshita, F. and Flynn, M. The sub-acute parenteral
toxicity of Bayer 37289 to rats. Unpublished report of the Toxicity
Lab., University of Chicago, submitted by Farbenfabriken Bayer
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Grey, A. P., Hibbs, C. M. and Nelson, D. L. (1969) Histologic
evaluation of Bayer 37289 treated rats - addendum to report of Root et
al., 1968a. Unpublished report of Chemagro Corp. submitted by
Farbenfabriken Bayer
Harris, C. R. (1969) Laboratory studies on the persistence of
biological activity of some insecticides in soils. J. Econ. Entomol.,
62: 1437-1441
Hibbs, C. and Nelson, D. L. (1968) Microscopic findings in tissues of
male and female dogs fed Bay 37289 for twelve weeks - addendum to
report of Root et al., 1968b. Unpublished report of Chemagro Corp.
submitted by Farbenfabriken Bayer
Hobik, H. P. (1967) Histologische Untersuchungen. Unpublished report
submitted by Farbenfabriken Bayer
Hofer, W. (1969) Farbenfabriken Bayer A.G. Personal communication
Homeyer, B. (1969) Trichloronat, ein neues Mittel gegen Bodeninsekten.
Mededelingen Rijksfakulteit Landbouw-Wetenschappen Gent, 34: 598-606
Katague, D. B. and Anderson, C. A. (1966) A gas-chromatographic method
for the determination of Bay 37 289, its oxygen analogue, and
2,4,5-trichlorophenol in crops. J. Agr. Food Chem., 14: 505-508
Katague, D. B. and Anderson, C. A. (1967) An interference study for
the residue method for Bay 37 289, its oxygen analog, and
2,4,5-trichlorophenol. Chemagro Corp. Report No. 20 482. Unpublished
Katague, D. B. and Anderson, C. A. (1968a) Effect of processing on Bay
37 289 residues in sugar beets. Chemagro Corp. Report No. 23 418.
Unpublished
Katague, D. B. and Anderson, C. A. (1968b) Data for a confirmatory gas
chromatographic method for Bay 37 289 and its metabolites. Chemagro
Corp. Report No. 23 427. Unpublished
Kimmerle, G. (1962) Re 54400 (E37289). Unpublished report submitted by
Farbenfabriken Bayer
Kimmerle, G. (1965) Untersuchungen zur Neurotoxitat von Bayer 37289
bei Huhnen. Unpublished report submitted by Farbenfabriken Bayer
Kimmerle, G. (1968) Bayer 37289. Toxikologische Untersuchunger
No. 667, submitted by Farbenfabriken Bayer
Krause, C. and Kirchhoff, J. (1969) Organophosphatrückstände auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen.
Nachrichtenblatt Dtsch. Pflanzenschutz-dienst, Braunschweig,
21: 81-84
Krause, C. and Kirchhoff, J. (1970) Gaschromatographische Bestimmung
von Organophosphatrückständen auf Marktproben von Obst und Gemüse.
Dtsch. Lebensmittel-Rundschau, 66: 194-199
Löser, E. (1966) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1968) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1970) Chronisch toxikologische Untersuchungen an Ratten.
Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1971) Bay 37289 Generationsversuche an Ratten. Unpublished
report submitted by Farbenfabriken Bayer
Martens, P. H. (1970) Influence des traitements de conserverie sur la
degradation des residus de pesticides appliques sur carottes. Faculté
des Sciences Agronomiques de L'Etat, Gembloux, Belgique; Centre de
Recherches de Phytopharmacie, Rapport d'Activite 1970, No. 706
McCollister, D. D. Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol.,
3: 63-70
Möllhoff, E. (1967) Gaschromatographische Bestimmung von Rückständen
in Pflanzen und Bodenproben nach Anwendung von Präparaten der
(R)E 605-Reihe und von (R)Agritox. Pflanzenschutz-Nachrichten
"Bayer", 20: 557-574
Möllhoff, E. (1968a) Versuche zur Aufnahme von Agritox durch Pflanzen
aus dem Boden. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1968b) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzon nach Anwendung von Präparaten der (R)E
605-und (R)Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21:
331-358
Möllhoff, E. (1970) Methods zur Extraktion aus Pflanzen und Boden, zur
Trennung und zum gaschromatographischen Nachweis von
Organophosphorverbindungen und ihren Um- und Abbau-produkten.
Vorläufiger Bericht. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1971a) Untersuchung über den Metabolismus von
Trichloronat im Boden. Farbenfabriken Bayer A.C. Unpublished
Möllhoff, E. (1971b) Verhalten einiger Organophosphorverbindungen in
destilliertem Wasser. Farbenfabriken Bayer A.G. Unpublished
Olson, T. J. and Anderson, C. A. (1968) Determination of Bay 37 289
residues in cigarette smoke. Chemagro Corp. Report No. 23 066.
Unpublished
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin layer chromatography. J.A.O.A.C.,
50: 1088-1098
Renvall, S. and Åkerblom, M. (1971) Determination of organophosphorus
pesticide residues in fruits and vegetables on the Swedish market from
1964 to 1968. Residue Reviews, 34: 1-26
Root, M., Kinoshita, F. K., Flynn, M. and DuBois, K. P. (1969)
Toxicity and anticholinesterase action of O-ethyl
O-2,4,5-trichlorophenyl ethylphosphonothioate. Toxicol. appl.
Pharmacol., 14 (3): 620. Abstr. Papers 18th Ann. Meet. Soc.
Toxicol., Williamsburg, Virginia
Root, M., Meskauskas, J. Kinoshita, F. and Flynn, M. (1970) The
chronic toxicity of Bayer 37289 to male and female dogs. Unpublished
report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn, M. and Kempf, C.
(1968a) Sub-acute oral toxicity of Bayer 37289 to male and female
rats. Unpublished report of the Toxicity Lab., University of Chicago,
submitted by Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn M. and Groks, D.
(1968b) Sub-acute oral toxicity of Bay 37289 to male and female dogs.
Report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Sans, W. W. (1967) Multiple insecticide residue determination using
column chromatography, chemical conversion, and gas-liquid
chromatography. J. Agr. Food Chem., 15: 192-198
Shaw, H. R., Flint, D. R. and Waggoner, T. B. (1971) Bay 37 289
- Water stability and soil runoff, leaching and adsorption studies.
Chemagro Corp. Report No. 29 062. Unpublished
Tu, C. M. (1970) Effect of four organophosphorus insecticides on
microbial activities in soil. Appl. Microbiol., 19: 479-484
n.d. non detectable
Assuming that trichloronat is applied, according to the
recommendations, only once a year to the same field and taking into
account the degradation rates presented before, there seems to be no
accumulation of trichloronat in the soils to be expected.
The behaviour of trichloronat in simulated field environment was
studied to determine its relative potential for contaminating water
stores. Residues in runoff water from field soil plots were less than
1% within a 14-day interval of application. Leaching studies in the
laboratory for high nitrogen, clay and sandy loam soils indicated that
rainfalls of 368, 447 and 103 inches, respectively, would
theoretically be required to leach the compound 12 inches into the
soil (Shaw et al., 1971). The half-life of trichloronat in neutral or
alkaline water is very short. It was found to be 51 hours in water
buffered to a pH of 7 at 30°C (Shaw et al., 1971). Measurements in
isopropanol/water 1:1 (v/v) at pH 11.5, equivalent to pH 10.5 in
water, produced a half-life of 6.2 hours for trichloronat at 37°C; the
half-life value found for parathion was 28.3 hours, and thus greater
by a factor of 4.5 (Hofer, 1969). A similar factor, viz. 5.5, was
obtained by Möllhoff (1971b) in measurements in distilled water at
26°C. The half-life values were 110 days for parathion and paraoxone,
50 days for parathion-methyl, 20 days for trichloronat and
paraoxone-methyl, and 60 days for trichloronat-oxone.
In storage and processing
Residues of trichloronat, trichloronat-oxone and 2,4,5-trichlorophenol
in cold-stored (approximately -20°C) cabbage and potatoes did not
decrease in 168 to 224 days (Chemagro Corp., 1968). Kohlrabi roots
containing 0.3 ppm of trichloronat were boiled unpeeled for 30 minutes
in twice the amount by weight of water in a closed vessel, and then
analysed together with the water. Twenty-seven per cent of the
compound was decomposed (Bayer, 1971). Washed carrots contained 0.35
ppm of residue; after they had been peeled, no more residue was
detectable in them (limit of determination of 0.07 ppm) (Bayer, 1971).
Washed turnips, carrots and onions contained no detectable residues
after being peeled (Anon., Finland, 1969). The peel of carrots
contained 0.66 ppm of residue as against only 0.02 ppm in the pulp.
After these carrots had been mechanically washed, the peel was found
still to contain 0.32 ppm residue and the pulp contained 0.04 ppm of
residue. After the carrots had been blanched for one minute at 100°C,
no more residues were detectable in them (<0.02 ppm). A similar
result was obtained for normal preservation (Martens, 1970). In a
study to investigate the effect of processing on trichloronat in sugar
beets, a laboratory procedure was employed which simulated the
industrial process; the results showed that trichloronat,
trichloronat-oxone, and 2,4,5-trichlorophenol were completely degraded
during the first initial boiling and liming step (Katague and
Anderson, 1968a). Cigarettes containing trichloronat,
trichloronat-oxone and 2,4,5-trichlorophenol were smoked and the smoke
was analysed. Approximately 15% of each of the three compounds was
found in the smoke (Olson and Anderson, 1968).
Evidence of residues in food in commerce or at consumption
From 1964 to 1968, Renvall and Åkerblom (1971) analysed 2396 samples
of domestic and imported fruit and vegetables obtained from the
Swedish market. Only about 0.01% of these samples, i.e. two or three,
contained trichloronat. The residue level was less than 0.1 ppm. The
limit of determination was 0.01 ppm. In 1968, Krause and Kirchhoff
(1969, 1970) carried out analyses of market samples of fruit and
vegetables produced in or imported into Germany. None of the 70
analysed samples contained detectable residues of trichloronat (limit
of determination of 0.01 ppm).
Methods of residue analysis
Studies of metabolites have shown that following customary application
of trichloronat, the oxone will occur, if at all, only in low
concentrations because its half-life is considerably shorter than that
of the parent compound (Möllhoff, 1968a, 1968b), 2,4,5-trichlorophenol
also occurs only in very low concentrations or not at all. Therefore,
determination of the parent compound itself is sufficient for
regulatory purposes.
Renvall and Åkerblom (1971) developed a thin-layer chromatographic
method for determining residues of organophosphorus insecticides,
including trichloronat, in fruit and vegetables, and which has a limit
of determination of 0.1 ppm.
Trichloronat can also always be co-determined by multiresidue methods
for gas-chromatographic determination of organophosphorus
insecticides, which are suitable for determining parathion (Beckman
and Garber, 1969; Krause and Kirchhoff, 1970; Möllhoff, 1967, 1968b;
Renvall and Åkerblom, 1971; Sans, 1967). Detectors that have been used
include the electron-capture detector (Brewerton at al., 1968;
Möllhoff, 1967), the thermionic detector and the flame photometric
detector (Bowman and Beroza, 1970).
There are two special methods for the determination of trichloronat
residues. One is based on the determination of the phenol group
(Katague and Anderson, 1966), and the other is based on the
determination of the parent compound itself (Möllhoff, 1967; 1968a,
1968b).
In the method based on the determination of the phenol, trichloronat
and its oxone are determined together and the free
2,4,5-trichlorophenol is determined separately. After separating the
free 2,4,5-trichlorophenol, trichloronat and its oxone are saponified.
The resultantly liberated phenol and the previously separated phenol
which is already free are separately acetylated and determined by
gas-chromatography using an electron-capture detector. The limit of
determination is generally 0.01 ppm. For confirmation of the results,
two columns (Katague and Anderson, 1968b) is used. In a study
conducted by Katague and Anderson (1967), 43 organophosphorus
pesticides were checked for possible interference with the
trichloronat residue analysis method. The trichloronat analysis is
interfered with only by fenchlorphos (Ronnel) and its oxygen analogue
(they were not included in the above study) because they contain the
same phenol as trichloronat.
The second special method of trichloronat residue analysis with
terminal gas-chromatographic determination permits trichloronat and
its oxygen analogue to be detected separately with the phosphorus or
the electron-capture detector. By using suitable columns, it was
possible to separate trichloronat from 35 organophosphorus
insecticides including fenchlorphos (Ronnel). The response of the
electron-capture detector to trichloronat is three times greater than
that of the thermionic phosphorus detector but interference peaks
occur on concentrating the extracts of some crops. Organochlorine
compounds may also interfere. Therefore, the phosphorus detector
should be preferably used for the determination.
The metabolites that cannot be assayed by either of these two methods,
can be determined in soil and plant samples by a gas-chromatographic
method described by Möllhoff (1970).
Examples of national tolerances
Tolerance Safety
Country Crop in ppm interval
in days
Belgium Cabbage 60
Carrots 90
Fruit, vegetables (incl.
cabbage, onions), excl.
potatoes 0.1
Canada Cole crops N.R.
Denmark Onion, cabbage and carrot
seed (as seed dressing) 0
Finland General (as soil drench) 84
Italy General 20
Netherlands Cabbage (soil treatment) 42
Beans 0
Cabbage, leeks, onions 0.1
Miscellaneous 0
Norway Edible root crops
(as granular) 90
Appraisal
Trichloronat is an organothiophosphorus insecticide which is used
against soil insects, especially vegetable fly larvae.
It is chiefly recommended for treatments of onions, leeks, brassica
crops, carrots, cereals, bananas and grassland. Types of application
are seed dressing, granular broadcast, soil spray, transplant and soil
drench. Amounts applied are up to 5 kg active ingredient per hectare.
Technical trichloronat is reported to contain 93-95% active
ingredient. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) may exist
as an impurity in the technical material. It is guaranteed by the sole
manufacturer that the dioxin content is below the limit of detection
of the analytical method (0.1 ppm).
Residue data are available from several countries.
Trichloronat is subject to degradation in soils, plants and animals.
Prom the degradation products trichloronat-oxone is of toxicological
importance, but it is existing only in insignificant amounts in the
terminal residues.
From the treated seeds or soil, trichloronat is sorbed to the surface
of the plant roots and is migrating only in minor amounts into the
aerial parts of the crops. From the root crops, carrots have shown
highest incidence and magnitude of residues. It has been demonstrated
that peeling, blanching, etc. processes decrease the residues of
carrots and other root crops to or below the levels of analytical
detection limits.
There is a TLC method with a detection limit of 0.1 ppm, GLC
multi-residue methods, and two specific methods with detection limits
of about 0.01 ppm for determining the residues of trichloronat in
various crops.
The evaluation of residue data is based on the assumptions that the
technical trichloronat contain less than 0.1 ppm dioxin and that it is
applied only into soils or to seeds and no treatments of the growing
crops are made. Any such treatments are not regarded as good
agricultural practice.
Since no residues have been detected (less than 0.01 ppm) in bananas
and cereals from recommended usages no tolerances are needed for those
crops.
Since an acceptable daily intake for trichloronat was not established
by the Meeting, no recommendations for tolerances are made.
As a result of recommended use of trichloronat, following residue
levels need not be exceeded:
Onions, leeks, kohlrabi, radish 0.5 ppm
Cabbage, cauliflower, broccoli,
brussels sprouts, rutabagas,
turnips, sugar beets, potatoes 0.1 ppm
Carrots 1 ppm
Further work or information
Required (before an acceptable daily intake can be established or
tolerances recommended):
1. Information on the absorption, distribution, excretion and
general metabolism of trichloronat in at least one mammalian
species.
2. Comprehensive information on the gross and histopathological
findings particularly after long-term administration of this
compound.
3. Relevant data for the evaluation of trichloronat in the feed of
domestic animals, e.g. fodder crops.
REFERENCES
Anon., Finland. (1969) State Institute of Agr. Chem., Helsinki,
Finland
Bayer AG, Farbenfabriken, Pflanzenschutz. (1969) (R)Phytosol,
(R)Agritox (Bayer 37289, S 4400). Leverkusen. E. 1-659/23536
Bayer AG, Farbenfabriken, Pflanzenschutz. (1971) Documentation on
trichloronat for FAO
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system.
J.A.O.A.C., 52, 286-293
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulfur on four thermally
stable columns. J.A.O.A.C., 53: 499-508
Brewerton, H.V., Gibbs, M. M. and Perrot, D. C. F. (1968)
Fensulgothion and "Bayer 37 289" residues in pasture. New Zealand
J. Agr. Res., 11: 303-312
Bro-Rasmussen, F., Noddegaard, E. and Voldum-Clausen, K. (1970)
Comparison of the disappearance of eight organophosphorus insecticides
from soil in laboratory and in outdoor experiments. Pesticide Sci.,
1: 179-199
Chemagro Corporation, Research Dept., Kansas City, U.S.A., Rep. No. 23
060. (1968) The effect of frozen storage at 0 to -10°F on Bay 37 289.
Residues in cabbage and potatoes
DuBois, K. P. (1963) The acute oral toxicity of Bayer 37289 to
chickens. Unpublished report of the Toxicity Lab., University of
Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P. and Kinoshita, F. (1963) The acute toxicity of Bayer
37289 to mammals. Unpublished report of the Toxicity Lab., University
of Chicago, submitted by Farbenfabriken Bayer
DuBois, K. P., Kinoshita, F. and Flynn, M. The sub-acute parenteral
toxicity of Bayer 37289 to rats. Unpublished report of the Toxicity
Lab., University of Chicago, submitted by Farbenfabriken Bayer
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Grey, A. P., Hibbs, C. M. and Nelson, D. L. (1969) Histologic
evaluation of Bayer 37289 treated rats - addendum to report of Root et
al., 1968a. Unpublished report of Chemagro Corp. submitted by
Farbenfabriken Bayer
Harris, C. R. (1969) Laboratory studies on the persistence of
biological activity of some insecticides in soils. J. Econ. Entomol.,
62: 1437-1441
Hibbs, C. and Nelson, D. L. (1968) Microscopic findings in tissues of
male and female dogs fed Bay 37289 for twelve weeks - addendum to
report of Root et al., 1968b. Unpublished report of Chemagro Corp.
submitted by Farbenfabriken Bayer
Hobik, H. P. (1967) Histologische Untersuchungen. Unpublished report
submitted by Farbenfabriken Bayer
Hofer, W. (1969) Farbenfabriken Bayer A.G. Personal communication
Homeyer, B. (1969) Trichloronat, ein neues Mittel gegen Bodeninsekten.
Mededelingen Rijksfakulteit Landbouw-Wetenschappen Gent, 34: 598-606
Katague, D. B. and Anderson, C. A. (1966) A gas-chromatographic method
for the determination of Bay 37 289, its oxygen analogue, and
2,4,5-trichlorophenol in crops. J. Agr. Food Chem., 14: 505-508
Katague, D. B. and Anderson, C. A. (1967) An interference study for
the residue method for Bay 37 289, its oxygen analog, and
2,4,5-trichlorophenol. Chemagro Corp. Report No. 20 482. Unpublished
Katague, D. B. and Anderson, C. A. (1968a) Effect of processing on Bay
37 289 residues in sugar beets. Chemagro Corp. Report No. 23 418.
Unpublished
Katague, D. B. and Anderson, C. A. (1968b) Data for a confirmatory gas
chromatographic method for Bay 37 289 and its metabolites. Chemagro
Corp. Report No. 23 427. Unpublished
Kimmerle, G. (1962) Re 54400 (E37289). Unpublished report submitted by
Farbenfabriken Bayer
Kimmerle, G. (1965) Untersuchungen zur Neurotoxitat von Bayer 37289
bei Huhnen. Unpublished report submitted by Farbenfabriken Bayer
Kimmerle, G. (1968) Bayer 37289. Toxikologische Untersuchunger
No. 667, submitted by Farbenfabriken Bayer
Krause, C. and Kirchhoff, J. (1969) Organophosphatrückstände auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen.
Nachrichtenblatt Dtsch. Pflanzenschutz-dienst, Braunschweig,
21: 81-84
Krause, C. and Kirchhoff, J. (1970) Gaschromatographische Bestimmung
von Organophosphatrückständen auf Marktproben von Obst und Gemüse.
Dtsch. Lebensmittel-Rundschau, 66: 194-199
Löser, E. (1966) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1968) Subchronische toxikologische Untersuchungen an
Ratten. Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1970) Chronisch toxikologische Untersuchungen an Ratten.
Unpublished report submitted by Farbenfabriken Bayer
Löser, E. (1971) Bay 37289 Generationsversuche an Ratten. Unpublished
report submitted by Farbenfabriken Bayer
Martens, P. H. (1970) Influence des traitements de conserverie sur la
degradation des residus de pesticides appliques sur carottes. Faculté
des Sciences Agronomiques de L'Etat, Gembloux, Belgique; Centre de
Recherches de Phytopharmacie, Rapport d'Activite 1970, No. 706
McCollister, D. D. Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol.,
3: 63-70
Möllhoff, E. (1967) Gaschromatographische Bestimmung von Rückständen
in Pflanzen und Bodenproben nach Anwendung von Präparaten der
(R)E 605-Reihe und von (R)Agritox. Pflanzenschutz-Nachrichten
"Bayer", 20: 557-574
Möllhoff, E. (1968a) Versuche zur Aufnahme von Agritox durch Pflanzen
aus dem Boden. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1968b) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzon nach Anwendung von Präparaten der (R)E
605-und (R)Agritox-Reihe. Pflanzenschutz-Nachrichten "Bayer", 21:
331-358
Möllhoff, E. (1970) Methods zur Extraktion aus Pflanzen und Boden, zur
Trennung und zum gaschromatographischen Nachweis von
Organophosphorverbindungen und ihren Um- und Abbau-produkten.
Vorläufiger Bericht. Farbenfabriken Bayer A.G. Unpublished
Möllhoff, E. (1971a) Untersuchung über den Metabolismus von
Trichloronat im Boden. Farbenfabriken Bayer A.C. Unpublished
Möllhoff, E. (1971b) Verhalten einiger Organophosphorverbindungen in
destilliertem Wasser. Farbenfabriken Bayer A.G. Unpublished
Olson, T. J. and Anderson, C. A. (1968) Determination of Bay 37 289
residues in cigarette smoke. Chemagro Corp. Report No. 23 066.
Unpublished
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin layer chromatography. J.A.O.A.C.,
50: 1088-1098
Renvall, S. and Åkerblom, M. (1971) Determination of organophosphorus
pesticide residues in fruits and vegetables on the Swedish market from
1964 to 1968. Residue Reviews, 34: 1-26
Root, M., Kinoshita, F. K., Flynn, M. and DuBois, K. P. (1969)
Toxicity and anticholinesterase action of O-ethyl
O-2,4,5-trichlorophenyl ethylphosphonothioate. Toxicol. appl.
Pharmacol., 14 (3): 620. Abstr. Papers 18th Ann. Meet. Soc.
Toxicol., Williamsburg, Virginia
Root, M., Meskauskas, J. Kinoshita, F. and Flynn, M. (1970) The
chronic toxicity of Bayer 37289 to male and female dogs. Unpublished
report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn, M. and Kempf, C.
(1968a) Sub-acute oral toxicity of Bayer 37289 to male and female
rats. Unpublished report of the Toxicity Lab., University of Chicago,
submitted by Farbenfabriken Bayer
Root, M., Meskauskas, J., Kinoshita, F., Flynn M. and Groks, D.
(1968b) Sub-acute oral toxicity of Bay 37289 to male and female dogs.
Report of the Toxicity Lab., University of Chicago, submitted by
Farbenfabriken Bayer
Sans, W. W. (1967) Multiple insecticide residue determination using
column chromatography, chemical conversion, and gas-liquid
chromatography. J. Agr. Food Chem., 15: 192-198
Shaw, H. R., Flint, D. R. and Waggoner, T. B. (1971) Bay 37 289
- Water stability and soil runoff, leaching and adsorption studies.
Chemagro Corp. Report No. 29 062. Unpublished
Tu, C. M. (1970) Effect of four organophosphorus insecticides on
microbial activities in soil. Appl. Microbiol., 19: 479-484
