
BROMOPHOS-ETHYL JMPR 1972
IDENTITY
Chemical name
O,O-diethyl-O-(4-bromo-2,5-dichlorophenyl)
Synonyms
Nexagan(R), S-2225, SHG-2225, ethyl bromophos, bromophosethyl
Structural formula
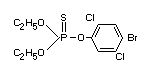 Other information on identity and properties
Molecular weight: 394.0
State: yellow fluid
Density: d20 = 1.53
20
Boiling point: 122-123°C at 10-3 torr.
Vapour pressure: 4.6 x 10-5 mm Hg at 30°C
Solubility: at room temperature miscible with most organic
solvents; practically insoluble in water.
Stability: stable in aqueous suspension. Saponification only
occurs in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-diethylphosphorothioate:
approx. 1.0% (chlorine position not defined)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies using bromophos-ethyl labelled with 3H in the benzene ring
demonstrated that, in the rat, the substance is absorbed following
oral administration but accumulation generally or in specific organs
does not occur. Apart from the G.I. tract, only the liver, kidney,
spleen and brain showed any substantial activity after 4 hours, and
this declined sharply in 24 hours. Almost total excretion of activity
occurred within 4 days following 5 daily oral doses of approximately 8
mg/kg. The distribution pattern was essentially the same after
intraperitoneal injection, but excretion was slower due to its slow
release from peritoneal and mesenteric fat.
Following a single 8 mg/kg oral dose of 3H-labelled bromophos-ethyl,
40% of the activity appeared in urine and 60% in faeces. When
administered intraperitoneally or subcutaneously, the major portion
also occurred in faeces. Radioactivity was detected in bile 40 minutes
after administration into the duodenum of 3H-labelled material and
40% was excreted by this route in 9 hours. The identity of the
substance in bile has not been established (Boehringer, 1967).
Biotransformation
No bromophos-ethyl, the oxidation product bromoxon-ethyl or
desethyl-bromophos-ethyl, which would be formed by cleavage of the
C2H5-O-P bond, were found in the urine or faeces of rats after oral
dosage. The metabolite dichloro-bromophenol and its conjugates were
identified in urine and faeces and accounted for 85-90% of
administered product, showing that the principal metabolic route
involved cleavage of the phenyl-O-P bond (Boehringer, 1967).
Effects on enzymes and other biochemical parameters
A single dose of 42 mg/kg of bromophos-ethyl to rats caused maximum
inhibition of cholinesterase in plasma and RBC after 8 and 2 hours
respectively. Values showing less than 20% inhibition occurred after
16 and 48 hours respectively (Muacevic, 1968). A single dose of 15
mg/kg to sheep inhibited RBC cholinesterase after 24 hours. A normal
level was found 24 hours later. A dose of 10 mg/kg was without effect
(Terblanche, 1966). In short-term (Muacevic, 1966; Leuschner, 1966,
1967) and long-term studies (Leuschner et al., 1969a, 1969b) in rats
and in short-term studies in dogs (Leuschner, 1966, 1967; Leuschner
et al., 1968) and cattle (Vuuren, 1964; Muacevic, 1966), inhibition
of plasma, RBC, brain and liver cholinesterase was noted. In general,
plasma cholinesterase was the most sensitive of the enzymes.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Bromophos-ethyl, 600 mg/kg (the assumed LD50) was administered orally
to 10 one-year-old hens; they were treated with atropine and
toxogonin. No neurological abnormalities were seen in the 6-week
observation period (Muacevic, 1968).
Three groups of 10 one-year-old hens were administered orally 0, 1.25,
or 2.5 mg/kg/day of bromophos-ethyl on 6 days a week for one month.
Only one bird at the top dosage level showed general weakness;
neuropathological examination revealed no changes in this or other
birds examined (Muacevic, 1968).
Special studies on pharmacology
Atropine sulphate and toxigonin were shown to protect rats and mice
from a lethal dose of bromophos-ethyl. Norscopolamine also protected,
but salicylaldoxime showed no positive effect (Muacevic, 1967; 1968).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0,
1.25, 3.0 and 7.2 mg/kg/day approximately of bromophos-ethyl. Three
generations were examined, the parent F1 and F2 generation each
producing two litters, and mating taking place between week 9 and 15
and between week 21 and 16. No abnormalities were seen in the
behaviour, appearance, body-weight, haematological indices, or in
organs during pathological examinations in adult animals. No effect on
reproduction was found in the two lower dosage groups, but in the 7.2
mg/kg group, which showed significant inhibition of liver, brain and
plasma cholinesterase, the fertility rate and litter size were
decreased and the number of stillbirths increased. The young also
tended to have a lower body-weight than controls. However no runts or
animals with congenital malformations were discovered (Leuschner
et al., 1969b).
Special studies on teratogenicity
Groups of 20 pregnant female rats were administered 0, 0.03, 1.7, 3.5,
7.0 or 14.0 mg/kg body-weight/day of bromophos-ethyl by gastric
intubation from day 6 to 15 of gestation. Animals were killed at day
20 and uteri and foetuses examined. No significant differences from
controls were found in the number of foetuses or reabsorption sites or
in foetus weights. No malformations, runts or dead foetuses were found
in any of the treated groups (Leuschner, 1967).
Acute toxicity
The acute toxicity of technical bromophos-ethyl has been studied in
various animal species. A summary of the results of these studies is
given in Table 1.
TABLE 1 Acute toxicity of technical bromophos-ethyl in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 210-550 Barnes, 1968; Muacevic, 1964,
1967
Rat oral 52-127 Barnes, 1968; Muacevic, 1964,
1966; Leuschner, 1966, 1967
Rabbit oral 28 Muacevic, 1970
Rabbit dermal 100-600 Bradford, 1967; Muacevic, 1967
Dog oral 335(approx.) Muacevic, 1970
Quail oral 200 Muacevic, 1970
Hen oral 600(approx.) Muacevic, 1969
Potentiation of acute toxicity
Potentiation of the acute toxicity of bromophos-ethyl has been
demonstrated to occur in the rat with bromophos, chlorfenvinphos,
coumaphos, malathion, mevinphos and parathion-methyl, and in the mouse
with chlorfenvinphos, diazinon, dimethoate, ethion and malathion.
Slight potentiation with parathion was found in both species
(Muacevic, 1966, 1967, 1968).
Short-term studies
Rat
Groups of 6 male and 6 female rats were fed for 4 weeks on diets
providing 0, 1 and 10 mg/kg/day of bromophos-ethyl. No significant
changes in behaviour, growth rate, food intake or macroscopic
appearance of organs could be observed (Leuschner, 1966).
Groups of 16 male rats were administered daily by gavage 0, 1, 5, 20
or 60 mg/kg body-weight of bromophos-ethyl suspension. The highest
dose was discontinued after 2 days since 9 animals had died. In the
remaining groups no abnormalities in behaviour, food consumption,
growth rate, haematological indices or liver function were found. No
pathological changes in organs were found at autopsy, and the livers,
kidneys and lungs were microscopically normal (Boehringer, 1964).
Groups of 42 male and 42 female rats were administered diets providing
0, 0.78, 1.25, 3.0 and 7.2 mg/kg body-weight of bromophos-ethyl/day.
During the 16-week period of administration no abnormalities in
behaviour, body-weight, food consumption or blood counts were
detected. Necropsy of 5 animals of each group revealed no macroscopic
abnormalities attributable to treatment. Dosage levels of 1.25 mg/kg
upwards caused a marked increase in the quantities of ascorbic and
dehydroascorbic acids excreted in the urine (Leuschner, 1967).
Dog
Three groups of 2 male and 2 female mongrel dogs were administered
daily in capsules 0, 1.6 or 12.5 mg/kg body-weight of bromophos-ethyl
for 2 weeks. The highest dose level was increased to 25 mg/kg for a
further week, and during this period these animals became quiescent,
lost weight and developed diarrhoea. The composition of urine and
blood counts were normal in all animals. The two lower dosage levels
were without untoward effect (Leuschner, 1966).
Four groups of 2 male and 2 female beagle dogs received daily capsules
containing 0, 0.52, 1.25 or 3.0 mg bromophos-ethyl for 16 weeks. No
abnormalities in behaviour, food consumption, body-weight,
haematological indices, the activity of serum enzymes, chemical
constituents of blood or urine constituents were found in treated
animals. No pathological changes were found in the eye, and organ
weight changes did not occur. The 1.25 mg/kg group excreted an
increased amount of ascorbic and dehydroascorbic acids in their urine
and three times the normal amount was excreted by the 3.0 mg/kg group.
The adrenal was the only organ in which changes occurred which could
be attributed to treatment. Narrowing of the fascicular zone and
dispersal of the glomerulose zone, the cells showing a high lipoid
content, and fibrous dispersal of the capsule were described, the
degree of change being most marked in the 3.0 mg/kg group (Leuschner,
1967).
Six groups of beagle dogs were administered each day, in capsules,
sugar (control), coumaphos or bromophos-ethyl as follows: sugar, 1 g;
coumaphos, 3.0 mg/kg; bromophos-ethyl, 0.26 mg/kg and 0.39 mg/kg (3
male and 3 female dogs), 1.25 mg/kg (5 males and 5 females), 3.0 mg/kg
(6 males and 6 females). The administration continued for 18 weeks
after which a number of animals of each group received no treatment
for four weeks. Depression of plasma cholinesterase activity occurred
at the 1.25 mg/kg dosage level, but no definite decrease in enzyme
activity occurred at any dosage level in the RBC, brain or adrenal
gland. Caumaphos, however, inhibited RBC and brain enzyme at the 3.0
mg/kg/day level. Four weeks after treatment ceased all cholinesterase
levels had returned to normal. Urinary ascorbic and dehydroascorbic
acids were increased slightly in the 0.39 mg/kg group and the increase
was marked with higher dosage levels of bromophos-ethyl and with
coumaphos. The values also returned to normal when administration
ceased. Histological examination showed that in bromophos-ethyl
treated animals only the 3.0 mg/kg group showed a moderate broadening
of the fasciculate zone of the adrenal and a relative increase in
eosinophilic staining in the frontal lobe of the pituitary. The
appearance of the adrenals and pituitary were normal after treatment
had ceased for four weeks (Leuschner et al., 1968).
Five groups of 4 male and 4 female beagle dogs were fed for 2 years on
diets containing 0, 10, 20, 30 and 120 ppm bromophos-ethyl. No
influence was seen on behaviour, food intake or growth of treated
animals. Analysis of blood for chemical constituents, including
cortisol, and of urine failed to demonstrate abnormalities. All serum
enzymes, except cholinesterase, were of normal activity. The weight
and macro- and microscopic appearance of organs, including the adrenal
and pituitary glands and bone marrow, were normal when animals were
killed during and at the end of the test. The dosage threshold at
which inhibition of cholinesterase occurred was considered to be
between 10 and 20 ppm for plasma enzyme and above 120 ppm for RBC and
brain enzymes. Urine ascorbic and dehydroascorbic acid levels were
increased in the 30 and 120 ppm groups. Excretion was maximum at
between 6 and 9 months, and then it decreased. No untoward effects
could be detected in the 10 ppm group (Leuschner et al., 1971).
Rabbit
Groups of 2 male and 2 female rabbits were administered 0, 2.5, 12.5
and 62.5 mg/kg/day of bromophos-ethyl on scarified or intact skin in
the form of an ointment for 6 hours each day for 21 days. They were
observed for a further 14 days before necropsy. One male of the
highest dosage group died and body-weight gain was retarded in the two
highest groups. Varying degrees of liver necrosis, myocardial scarring
and renal changes were found at the highest dosage level but not at
lower levels (Stötzer et al., 1970).
Long-term studies
Rat
Groups of 42 male and 42 female rats, each weighing from 166 to 214 g,
were fed diets providing 0.78, 1.25, 3.0 or 7.2 mg/kg body-weight/day
of bromophos-ethyl for 2 years. The threshold levels for a 20%
inhibition of activity lay between 0.78 and 1.25 mg/kg for plasma and
3.0 and 7.2 mg/kg for erythrocyte, brain and liver cholinesterase.
Urinary ascorbic and dehydroascorbic acid excretion was elevated at
all dosage levels; a 78% increase was seen at the 0.78 mg/kg level.
With increasing age, the amounts excreted became less marked but were
still significantly high at 2 years in the 3.0 and 7.2 mg/kg groups.
The mean body-weights of rats of both sexes on 3.0 and 7.2 mg/kg
bromophos-ethyl were slightly elevated above that of the controls
throughout much of the test. Urine analysis showed that the incidence
of occurrence of erythrocytes, protein, ketone bodies and white blood
cells was reduced in the 3.0 and 7.2 mg/kg groups compared with
controls. No significant differences from controls were seen with
regard to behaviour, survival, food intake, and the results of
haematological investigations or with regard to gross or microscopic
changes in organs or tumour incidence and type (Leuschner et al.,
1969a).
COMMENT
Bromophos-ethyl is absorbed from the gastro-intestinal tract and
excreted in urine and faeces principally as dichloro-bromophenol and
its conjugates. Accumulation does not occur following oral ingestion.
Bromophos-ethyl inhibits cholinesterase, the plasma enzyme being the
most sensitive. Acute potentiation was observed in combination with
several other organo-phosphates in rats and mice. In 2-year studies in
dogs and rats the no-effect levels for plasma cholinesterase
inhibition were 0.4 and 0.78 mg/kg body-weight/day, respectively.
Bromophos-ethyl does not cause delayed neurological injury. Studies in
rats did not indicate ill effects on reproduction or teratogenic
activity.
In studies in rats and dogs urinary excretion of ascorbic and
dehydroascorbic acids was increased. The no-effect level in dogs was
0.26 mg/kg/day. A no-effect level was not demonstrated for rats.
Investigations to find the cause of the increase were not reported.
The long-term study in rats showed that, at the dosage levels
employed, bromophos-ethyl had no carcinogenic activity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: 0.26 mg/kg body-weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos-ethyl is an organo-phosphorus insecticide and acaricide with
a broad spectrum of activity and moderate mammalian toxicity. It acts
as a contact and stomach poison. It is available as an emulsifiable
concentrate, ULV concentrate, wettable powder, granular or dust
formulation as well as dip formulations. Bromophos-ethyl is compatible
with insecticides and fungicides.
This substance is of moderate toxicity to bees and should not be
sprayed on flowering crops during the flight of bees.
Bromophos-ethyl has been used in Argentina, Australia, Austria,
Belgium, Brazil, Colombia, the Federal Republic of Germany, the
Netherlands, New Zealand, Nicaragua, Pakistan, South Africa and
Venezuela.
Pre-harvest treatments
Bromophos-ethyl is applied on various crops, mainly fruits, field
crops, vegetables, cereals, maize, rice, cotton and tobacco, and is
effective against a large number of chewing and sucking insect pests,
especially caterpillars, vegetable root maggots, fruit flies, bean
fly, aphids and beetles.
Depending on the different crops and the main pest species present the
recommended concentrations of spray wash vary between 0.02% and 0.1%
a.i. and the rates of application between 0.2 and 1.2 kg a.i./ha. The
majority of crops are tolerant to normal rates of the insecticide.
Occasionally slight damage was noticed, in particular in connection
with high dosages.
The withholding periods range from 14 to 28 days, depending on the
local conditions and crop.
Other uses
Bromophos-ethyl is used in the sector of animal health and tick
control. The compound is active against ticks, including strains of
the genus Boophilus which are resistant to chlorinated hydrocarbons
and other phosphorus preparations, as well as against blowflies, lice
and other ectoparasites. It also controls mosquitoes, mosquito larvae
and ants, revealing a remarkable residual effect. Bromophos-ethyl is
used particularly as a mosquito larvicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables and field crops (Boehringer, 1971-1972). Summaries
of much of this information have recently been published (Eichler
1971). The following tables 2 and 3 present a summary of available
data along with relevant information on rates of application, number
of applications and preharvest interval.
In trials in the Netherlands (Anonymous, 1970), bromophos-ethyl was
applied to carrots at 6 kg a.i./ha either by soil surface treatment
with granules prior to seeding or by foliar treatment with wettable
powder or emulsifiable concentrate 81 and 89 days after planting. The
residues obtained are shown in table 4.
Distribution of residues in 1-year-old beef cattle was determined in
Germany (Boehringer, 1966) after spraying to runoff 1 or 4 times with
bromophos-ethyl emulsion (0.05% a.i.). The results are summarized in
table 5.
In later trials (Boehringer, 1968), 6 cows were sprayed three times at
5-day intervals (14 days is more usual) with 0.1% a.i. emulsion (twice
the concentration generally used). Table 6 summarizes these results.
Two experimental trials on residues in beef cattle were conducted in
Australia. In the first of these, two groups of 9 cattle were sprayed
with bromophos-ethyl at a concentration of 0.05% (Snelson, 1968).
Group 1 was treated once and Group 2 had three treatments at 5-day
intervals. Residues were determined in heart, kidney, omental and
subcutaneous fat with the results shown in Table 7.
The highest residues were in samples of heart and kidney fat. In the
second trial, six steers were sprayed each with 2 gallons of 0.05%
bromophos-ethyl and samples were taken for analysis at 4, 7 and 21
days after exposure (Harvey, 1968). Residues were distributed as shown
in Table 8.
Dipping leads to higher uptake and significantly higher residues in
fat than does spraying with the same concentration of bromophos-ethyl.
In a series of dipping trials under the direction of the Board of Tick
Control in Australia, 12 cattle were dipped once in 0.05%
bromophos-ethyl emulsion and groups of four were slaughtered 1, 4 and
8 days post treatment and 12 cattle were dipped three times at
intervals of 5 days in the same bath and groups of four were
slaughtered, as before, 1, 4 and 8 days after the third dipping
(Snelson, 1972). Results are given in Table 9.
Further trials showed that the uptake varied considerably depending on
the condition of the animals (fat versus thin), length of hair and
time of year. It was demonstrated that the half-life of
bromophos-ethyl residues in internal fat of cattle is 10 to 12 days.
Table 2 Bromophos-ethyl residues in fruit1
Crop Application Days between last treatment and harvest
No. Rate a.i. 0 1 2 3 4 5 7 8 10 14 15 21 22 28 30
(g/plant)
Apple 1 1.8 2.20 1.40 1.95 1.35
1 0.9 1.25 0.7 1.03 1.20 0.76 0.55
1 0.9 0.58 0.36 0.33 0.30 0.28
1 1.8 1.17 1.13 0.81 1.23
5 1.2 3.45 2.65 2.05 1.95
6 1.5 4.90 3.70 2.50 2.30
Pear 1 0.36 0.59 0.32 0.32 0.26 0.15
Peach 1 0.5 0.38 0.39
Yellow plum 1 0.9 1.40 0.81
Plum 1 1.8 2.08 1.14 1.35 1.11
3 1.8 2.28 1.24 0.73 0.51
1 0.54 0.36 0.34 0.14 0.13 0.12
Sweet Cherry 1 1.8 0.87 0.45 0.27 0.07 0.03
1 1.8 0.21 0.17 0.09
1 1.8 0.89 0.27 0.16 0.14 0.03
Strawberry 1 2882 0.36 0.07
Gooseberry 1 0.36 0.33 0.52 0.26 0.31 0.30
Red-currant 1 3602 1.18 0.95
Black-currant 1 0.36 0.78 0.12
1 residue figures given in ppm
2 g/ha
Table 3a Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 0 1 3 4 5 7 14 21 28 30
(g/ha a.i.)
Garden
lettuce 1 108 0.31 0.28 0.18
1 216 2.35 1.09 1.03 0.37 0.16 0.10
1 108 1.05 0.45 0.09
1 108 0.31 0.28 0.18
Spinach 1 216 2.72 1.60 0.42 0.03
1 216 6.84 4.42 2.22
1 288 8.32 6.66 3.26 2.78 0.44
Carrot 2 0.182
1 0.103
2 0.182
1 0.183
Cauliflower 2 28.84 <0.02
Kohlrabi 2 324
White cabbage 1 360 <0.08 <0.08
1 216 0.20 0.16 0.07 0.04 0.02 <0.02
Brussels
sprouts 3 540 0.57 0.51
Sugar beet 1 144
Onion 2 0.23
2 0.23
2 0.23
Tubercelery
tuber 3 160 0.19-
0.36
leaf 3 160 2.67-
4.09
Rape seed 1 216
Rape oil 1 216
Horsebean 1 600 <0.02
Kidney bean 2 324 0.18 0.07 0.03 0.02 <0.02
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
Table 3b Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 31 35 42 49 54 56 62 64 66 71 107
(g/ha a.i.
Garden
lettuce 1 108
1 216
1 108
1 108
Spinach 1 216 0.02
1 216
1 288
Carrot 2 0.182 1.21
1 0.103 0.40
2 0.182 1.53
1 0.183 1.42
Cauliflower 2 28.84
Kohlrabi 2 324 0.05
White
cabbage 1 360
1 216
Brussels
sprouts 3 540
Sugar beet 1 144 <0.02
Onion 2 0.23 <0.02
2 0.23 <0.02
2 0.23 <0.02
Tubercelery
tuber 3 160
leaf 3 160
Rape seed 1 216 0.08
Rape oil 1 216 0.33
Horsebean 1 600
Kidney bean 2 324
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
TABLE 4 Bromophos-ethyl residues in carrots
Crop Method of Rate of Time between Residues
application application last treatment (ppm)
(kg/ha/a.i.) and harvest
(months)
Carrots soil surface 6 3 0.22 - 0.24
soil surface 6 3 0.17 - 0.77
foliar spray 6 2 0.49 - 1.5
foliar spray 6 2 0.84 - 1.6
TABLE 5 Distribution of bromophos-ethyl residues in cattle tissue, 1966
Treatment Sample interval Residues (ppm)1
Animal (0.05% (days after Back Leg
a.i.) last treatment) muscle muscle Brain Kidney Liver Fat
12 1 spray 1 0.77 0.90 0.76 <0.13 <0.14 n.d.
2 4 sprays 3 n.d. <0.18NS n.d. n.d. <0.15NS n.d.
3 4 sprays 1 <0.18NS n.d. n.d. n.d. n.d. 0.67*
4 4 sprays 11 n.d. n.d. n.d. n.d. n.d. <0.20NS
5 Control -- -- 0.056 -- 0.049 0.052 0.053 0.076
1 n.d. = not detected; NS = not statistically significant; * = statistically significant.
2 High probability that samples were contaminated during taking and handling; data not usable.
TABLE 6 Distribution of bromophos-ethyl residues in cattle tissue, 1968
Sample interval Residues (ppm)1,2
Animal (days after Fillet Roasting Kidney Liver Kidney Subcutaneous
last treatment) beef fat fat
924 1 0.015 0.015 0.010 <0.005 0.132 0.056
621 1 0.017 0.006 0.006 <0.005 0.193 0.065
110 3 0.010 0.008 0.007 <0.005 0.236 n.a.
102 3 0.007 0.007 0.010 <0.005 0.219 n.a.
605 7 <0.005 <0.005 <0.005 <0.005 0.218 n.a.
228 7 <0.005 0.010 <0.005 <0.005 0.183 n.a.
1 n.a. = not analyzed.
2 Determination by gas chromatography, mean of three determinations.
Bromophos-ethyl residues in milk from three dairy cows each sprayed
with two gallons of 0.05% active ingredient are shown in Table 10
(Snelson, 1968).
Dipping of dairy cows likewise gives rise to significant residues in
milk which are all transferred to the butterfat. The level of residues
in the milk of dipped cows was only slightly higher than those
reported in Table 10 (Snelson, 1972). The residue level reached a peak
on the first and second days after dipping and thereafter declined
rapidly, with a half-life calculated to be between 1-2 and 1-4 days.
TABLE 7 Bromophos-ethyl residues in fat of beef cattle, trial 1
Post-treatment day Group 1, Group 2,
1 treatment (ppm) 3 treatments (ppm)
1 0.08 - 2.9 0.3 - 2.6
4 0.1 - 2.0 0.1 - 1.6
8 Nil - 0.7 Nil - 0.6
TABLE 8 Bromophos-ethyl residues in fat of steers, trial 2
Interval between Residues (ppm)
treatment and Liver Kidney Muscle Omental Perirenal
sampling (days) fat fat
4 <0.05 <0.05 <0.05 0.82 0.63
<0.05 <0.05 <0.05 0.62 0.36
7 <0.05 <0.05 <0.05 0.70 0.63
<0.05 <0.05 <0.05 0.21 0.45
21 <0.05 <0.05 <0.05 0.24 0.31
<0.05 <0.05 <0.05 0.40 0.29
TABLE 9 Bromophos-ethyl residues in cattle after dipping
Interval
between Residues bromophos-ethyl (ppm)
last dipping dipped once dipped 3 times
and slaughter Internal Subcutaneous Internal Subcutaneous
(days) fat fat fat fat
1 0.15 - 0.23 0.13 - 0.26 1.47 - 3.36 1.2 - 2.32
4 0.21 - 0.37 0.15 - 0.27 1.38 - 1.83 0.5 - 2.05
8 0.36 - 0.87 0.23 - 0.50 2.04 - 2.48 0.78 - 2.56
TABLE 10 Bromophos-ethyl residues in milk
Time after Residues (ppm)
Treatment treatment in butterfat in milk
(h)
Nexagan 0.05% 5 0.03 0.002
(single
application) 21 1.01 0.072
29 0.71,0.67,0.42 0.038,0.026,0.017
45 1.17,0.48,0.60 0.060,0.028,0.026
53 0.79,0.60,0.47 0.042,0.028,0.021
69 0.47 0.017
77 0.63 0.038
93 0.25 0.016
10 days 0.02 0.001
In trials in Germany (Boehringer, 1968), three cows were each sprayed
with 5 liters of 0.05%. a.i. suspension and milk samples were taken
during the following four days. A second treatment was performed seven
days later without sampling. A third treatment followed 14 days after
the start of the trial, and subsequent milkings were analyzed for a
total time of 21 days. Maximum residues (0.098, 0.077, 0.089 ppm) were
found about 24 hours after treatment, decreasing within about four
days below the limit of determination (<0.02 ppm). There was no
accumulation of residues after the third treatment.
FATE OF RESIDUES
General comments
By analogy with bromophos, the major metabolites of bromophos-ethyl
would be expected to be 2,5-dichloro-4-bromophenol, bromoxon-ethyl,
and desethyl bromophos-ethyl (see Figure 1 of preceding bromophos
monograph). The major points of difference appear to be that no
desethyl-bromophos-ethyl is found after oral administration to rats
and that somewhat higher ratios of bromoxon-ethyl to bromophos-ethyl
is found in plants, especially lettuce (Eichler, 1971).
In animals
The metabolism and excretion of 3H-labelled bromophos-ethyl in the
rat was studied by Stiasni (1967). No specific organic accumulation
occurred following oral and parenteral administration. Quantitative
excretion occurred between 8 and 12 days. Neither unchanged
bromophos-ethyl nor bromoxon-ethyl could be found in the excrements
following oral administration. Desethyl-bromophos-ethyl was also
missing among the metabolites. The only metabolites identified were
dichloro-bromophenol and its conjugates.
In plants
No tests have been carried out with radioactively labelled
bromophos-ethyl in plants. However, in connection with supervised
trials for determining residues in fruits and vegetables,
bromoxon-ethyl residues were determined in apples, Brussels sprouts
and lettuce (Eichler, 1971). In apples, the bromoxon-ethyl residues
never exceeded 0.005 ppm (<1% of bromophos-ethyl residue). In
Brussels sprouts, the highest bromoxon-ethyl residue found was 0.026
ppm (5% of bromophos-ethyl residue). In lettuce, after several
sprayings, bromoxon-ethyl residues reached a maximum of 0.07 ppm (6.4%
of bromophos-ethyl residue) in three days. The highest ratio found was
8.8% (0.014 ppm) after 14 days.
In soil
Bromophos-ethyl E.C. was applied one time at 0.5 g a.i./m2 to high
moorland soil (acid, high organic content), Ingelheim sand and clay
soil. Zero to 20 cm-deep samples were taken periodically for 26 weeks
and analyzed with the results shown in Table 11 (Eichler, 1970).
In comparison with bromophos, bromophos-ethyl degrades somewhat more
slowly in soil; however, neither compound shows any significant
persistence.
In storage and processing
Leber and Deckers (1968) examined the effects on residues in beef
under storage at +4° and -18°C and after cooking (roasting). No
significant decrease in residues was observed in meat, kidney and
kidney fat after a 7-day storage period at +4°C or after a 30-day
storage period at -18°C. Neither 15 minutes of frying of 100 g steaks
nor one hour of boiling of 100 g of beef in 200 ml of water resulted
in any significant decrease in residues of bromophos-ethyl.
Evidence of residues in food in commerce or at consumption
In connection with a tick eradication program in Australia, perirenal
fat from 17 cattle known to have been dipped in 0.05% Nexagan on 27
occasions over a period of 14 months was analyzed for residues
(Harvey, 1968). Three samples had residues between 0.5 and 1.0 ppm,
five between 1.0 and 1.5 ppm, seven between 1.5 and 2.0 ppm and two
samples had 2.18 and 2.68 ppm, respectively.
TABLE 11 Bromophos-ethyl residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-
soil time (weeks) Bromophos-ethyl bromophenol
High moorland 0 13.302 <0.10
1 6.26 <0.10
3 4.95 0.54
6 3.92 <0.10
9 3.29 0.60
13 3.44 1.30
26 1.25 <0.10
Sandy 0 2.343 <0.10
1 1.40 <0.10
3 0.64 0.13
6 0.40 <0.10
9 0.16 <0.10
13 0.12 <0.10
26 <0.02 <0.10
Clay 0 2.764 <0.10
1 2.16 <0.10
3 0.44 0.17
6 0.32 <0.10
9 0.26 <0.10
13 0.14 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 0.844-0.032 log X; r = 0.9173
3 Y = 0.128-0.076 log X; r = 0.9721
4 Y = 0.175-0.076 log X; r = 0.9544
Samples of internal fat taken from animals, with unknown treatment
history, being slaughtered in the cattle tick zone in Australia during
1971/72 revealed some to contain bromophos-ethyl residues. Of 1 055
samples examined, 52 contained bromophos-ethyl, mostly below 0.5 ppm,
the highest being 0.8 ppm (Snelson, 1972). During the same period 589
samples of butter and cheese were examined for organo-phosphorus
residues. Only three samples were found to contain bromophos-ethyl
residues, all at 0.1 ppm.
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos-ethyl. These
have recently been reviewed and summarized by Eichler (1971). A method
has been developed by Leber and Deckers (1968) for all crops and
animal tissues. It utilizes gas chromatography with a phosphorus
specific detector and can estimate quantities down to the range of
0.001 to 0.01 ppm with a 100 g sample. The method includes procedures
for determining bromoxon-ethyl either by colorimetry or by gas
chromatography with a sensitivity of 0.03 to 0.05 ppm. Confirmation of
residues can be accomplished by the thin-layer chromatographic
techniques suggested in the method.
Bromophos-ethyl is among the pesticides listed as being detectable by
the multi-residue gas chromatographic procedure of Abbott et al.
(1970), and that method is the most suitable for regulatory use. The
method of Leber and Deckers would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Examples of national tolerances of bromophos-ethyl residues are
reported in Table 12.
TABLE 12 Examples of national tolerances as reported to meeting
Country Commodity Tolerance
Australia Fat of meat of cattle
and sheep 3 ppm
Milk and milk products
(fat basis) 1 ppm
The Netherlands Fruit and vegetables 0.4 ppm
APPRAISAL
Bromophos-ethyl is a non-systemic halogen-containing organo-phosphorus
insecticide and acaricide used on fruit, vegetables, field crops,
cereals, maize, rice, cotton and tobacco. It is also used extensively
in tick control, for other ectoparasites of domestic animals and as a
mosquito larvicide.
Supervised trials with foliar treatments on fruits and vegetables have
shown that bromophos-ethyl is somewhat more persistent than bromophos
and requires a pre-harvest interval about twice as long. The rate of
residue decline is highly dependent on many factors, especially
botanical species and morphological structure, which require that
tolerance recommendations be made on an individual commodity basis
rather than on broad crop categories.
Groups of beef cattle were sprayed at recommended rates one or more
times in trials in several countries. Highest residues were found in
omental, heart and perirenal fat, ranging in one trial from 0.1 to 2.0
ppm at four days post-treatment.
Spray treatment of dairy cows at recommended rates resulted in a
residue maximum of 1.17 ppm in butterfat at 45 hours post-treatment.
This fell to 0.02 ppm in butterfat after ten days. There was no
accumulation of milk residues from the multiple treatments.
The metabolites of bromophos-ethyl most likely to be found are
2,5-dichloro-4-bromophenol and bromoxon-ethyl. Only
dichloro-bromophenol and its conjugates were found in the excrement of
rats after oral administration of labelled bromophos-ethyl. The
dichloro-bromophenol also appears to be the only soil metabolite.
Although no studies have been conducted with the labelled compound in
plants, supervised field trials have shown small amounts of
bromoxon-ethyl residues in apples, Brussels sprouts and lettuce (up to
0.07 ppm in the latter case).
Available multi-residue gas chromatographic procedures are suitable
for use for regulatory purposes and are recommended.
Although bromophos-ethyl is recommended for cereals, maize, cotton,
rice and in animal health, there were no data available for these
commodities except fat of meat of cattle and milk. Therefore, no
recommendations could be made for tolerances on these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are recommended for
bromophos-ethyl.
ppm
Apples, carrots, fat of meat of cattle,
plums, pears, spinach 2
Brussels sprouts, redcurrants 1
Celeriac, gooseberries, peaches,
rape seed oil, cherries (sweet) 0.5
Blackcurrants, lettuce 0.2
Rape seed, strawberries, cabbage 0.1
Kohlrabi, French beans 0.05
Cauliflower, beans (without pods),
onions, sugarbeets, milk (whole) 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1975)
Further studies evaluating the significance of urinary excretion of
ascorbic and dehydroascorbic acids and its relevance to man.
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials on maize, rice and other cereals,
cotton, domestic animals, other than cattle, and milk products.
DESIRABLE
A study to determine dose levels causing no carboxyl-esterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1967.
III. Organo-phosphorus pesticide residues in the total diet. Pestic.
Sci. 1: 10-13.
Anonymous. (1970) Utrecht report, project CvF/PD4.2.(2.2.03).
Barnes, J.M. (1968) WHO insecticide evaluation and testing programme.
Stage I - mammalian toxicity report. Medical Research Council,
Carshalton. (unpublished).
Battelle Institute. (1964) Toxicity tests with the substance S2225 in
rats, guinea pigs and rabbits. (unpublished)
Boehringer. (1966) Residue trials and examination of cholinesterase
activity after spraying with bromophos-ethyl on cattle. Report C.H.
Boehringer Sohn.
Boehringer. (1967) Investigations concerning absorption, distribution,
excretion and metabolism of bromophos-ethyl-3H in rats. Report C.H.
Boehringer Sohn. (unpublished)
Boehringer. (1968) Determination of residues of bromophos-ethyl in
cattle. Report C.H. Boehringer Sohn.
Boehringer. (1971-1972) Residue investigation reports, C.H. Boehringer
Sohn.
Bradford, H.A. (1967) Acute dermal toxicity - bromophos-ethyl
(compound 70625). Report Eli Lilly and Company. (unpublished)
Eichler, D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
Eichler, D. (1970) Report C.H. Boehringer Sohn "Uber den Abban von
Bromophos und Bromophos-ethyl in verschiedenen Böden".
Harvey, J.M. (1968) Bromophos-ethyl residues in meat products.
Communication with C.H. Boehringer Sohn.
Leber G. and Deckers, W. (1968) Determination of residues of bromophos
and bromophos-ethyl. Proc. Brit. Insecticide Fungicide Conf.,
Brighton, Eng. 4: 570.
Leuschner, F. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Standinger, H.J. (1968) About the short-term toxicity studies on
bromophos-ethyl - charge 1487 - in Beagle dogs (with special attention
to the toxicology of the adrenal gland). Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Dontenwill, W. (1969a) About the chronic toxicity of bromophos-ethyl
in Wistar rats following oral application. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969b)
About the chronic tolerance of bromophos-ethyl in the reproduction
test over three generations of Wistar rats. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Standinger, H.J., Schwerdtfeger, W. and
Dontenwill, W. (1971) Two years oral toxicity study in Beagle dogs
with bromophos-ethyl. Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Report on Shg 2225. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1969) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1970) Reports C.H. Boehringer Sohn. (unpublished)
Snelson, J.T. (1968) Bromophos-ethyl residues in milk. Communication
with C.H. Boehringer Sohn.
Snelson, J.T. (1967 - 1968) Residue studies - bromophos-ethyl.
Communications with C.H. Boehringer Sohn.
Snelson, J.T. (1972) Results from experimental investigations and
residue surveys with bromophos-ethyl following dipping in Australia.
Report to Joint Meeting.
Stiasni, M. (1967) Data from C.H. Boehringer Sohn. (unpublished)
Stötzer, H., Herbst, M., Baumgartner, R. and Guęnard, J. (1970)
Subacute dermal toxicity of the substance bromophos-ethyl in rabbits
(New Zealand White). Report C.H. Boehringer Sohn. (unpublished)
Terblanche. (1966) Toxicity of bromophos-ethyl. Department of
Agricultural Technical Services, Onderstepoort, South Africa.
(unpublished)
Vuuren, P.J.J. (1964) Whale-blood cholinesterase levels in cattle
sprayed at weekly intervals with S2225 (CELA). Agricura Laboratoria
Ltd., Silverton, South Africa. (unpublished)
Other information on identity and properties
Molecular weight: 394.0
State: yellow fluid
Density: d20 = 1.53
20
Boiling point: 122-123°C at 10-3 torr.
Vapour pressure: 4.6 x 10-5 mm Hg at 30°C
Solubility: at room temperature miscible with most organic
solvents; practically insoluble in water.
Stability: stable in aqueous suspension. Saponification only
occurs in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-diethylphosphorothioate:
approx. 1.0% (chlorine position not defined)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies using bromophos-ethyl labelled with 3H in the benzene ring
demonstrated that, in the rat, the substance is absorbed following
oral administration but accumulation generally or in specific organs
does not occur. Apart from the G.I. tract, only the liver, kidney,
spleen and brain showed any substantial activity after 4 hours, and
this declined sharply in 24 hours. Almost total excretion of activity
occurred within 4 days following 5 daily oral doses of approximately 8
mg/kg. The distribution pattern was essentially the same after
intraperitoneal injection, but excretion was slower due to its slow
release from peritoneal and mesenteric fat.
Following a single 8 mg/kg oral dose of 3H-labelled bromophos-ethyl,
40% of the activity appeared in urine and 60% in faeces. When
administered intraperitoneally or subcutaneously, the major portion
also occurred in faeces. Radioactivity was detected in bile 40 minutes
after administration into the duodenum of 3H-labelled material and
40% was excreted by this route in 9 hours. The identity of the
substance in bile has not been established (Boehringer, 1967).
Biotransformation
No bromophos-ethyl, the oxidation product bromoxon-ethyl or
desethyl-bromophos-ethyl, which would be formed by cleavage of the
C2H5-O-P bond, were found in the urine or faeces of rats after oral
dosage. The metabolite dichloro-bromophenol and its conjugates were
identified in urine and faeces and accounted for 85-90% of
administered product, showing that the principal metabolic route
involved cleavage of the phenyl-O-P bond (Boehringer, 1967).
Effects on enzymes and other biochemical parameters
A single dose of 42 mg/kg of bromophos-ethyl to rats caused maximum
inhibition of cholinesterase in plasma and RBC after 8 and 2 hours
respectively. Values showing less than 20% inhibition occurred after
16 and 48 hours respectively (Muacevic, 1968). A single dose of 15
mg/kg to sheep inhibited RBC cholinesterase after 24 hours. A normal
level was found 24 hours later. A dose of 10 mg/kg was without effect
(Terblanche, 1966). In short-term (Muacevic, 1966; Leuschner, 1966,
1967) and long-term studies (Leuschner et al., 1969a, 1969b) in rats
and in short-term studies in dogs (Leuschner, 1966, 1967; Leuschner
et al., 1968) and cattle (Vuuren, 1964; Muacevic, 1966), inhibition
of plasma, RBC, brain and liver cholinesterase was noted. In general,
plasma cholinesterase was the most sensitive of the enzymes.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Bromophos-ethyl, 600 mg/kg (the assumed LD50) was administered orally
to 10 one-year-old hens; they were treated with atropine and
toxogonin. No neurological abnormalities were seen in the 6-week
observation period (Muacevic, 1968).
Three groups of 10 one-year-old hens were administered orally 0, 1.25,
or 2.5 mg/kg/day of bromophos-ethyl on 6 days a week for one month.
Only one bird at the top dosage level showed general weakness;
neuropathological examination revealed no changes in this or other
birds examined (Muacevic, 1968).
Special studies on pharmacology
Atropine sulphate and toxigonin were shown to protect rats and mice
from a lethal dose of bromophos-ethyl. Norscopolamine also protected,
but salicylaldoxime showed no positive effect (Muacevic, 1967; 1968).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0,
1.25, 3.0 and 7.2 mg/kg/day approximately of bromophos-ethyl. Three
generations were examined, the parent F1 and F2 generation each
producing two litters, and mating taking place between week 9 and 15
and between week 21 and 16. No abnormalities were seen in the
behaviour, appearance, body-weight, haematological indices, or in
organs during pathological examinations in adult animals. No effect on
reproduction was found in the two lower dosage groups, but in the 7.2
mg/kg group, which showed significant inhibition of liver, brain and
plasma cholinesterase, the fertility rate and litter size were
decreased and the number of stillbirths increased. The young also
tended to have a lower body-weight than controls. However no runts or
animals with congenital malformations were discovered (Leuschner
et al., 1969b).
Special studies on teratogenicity
Groups of 20 pregnant female rats were administered 0, 0.03, 1.7, 3.5,
7.0 or 14.0 mg/kg body-weight/day of bromophos-ethyl by gastric
intubation from day 6 to 15 of gestation. Animals were killed at day
20 and uteri and foetuses examined. No significant differences from
controls were found in the number of foetuses or reabsorption sites or
in foetus weights. No malformations, runts or dead foetuses were found
in any of the treated groups (Leuschner, 1967).
Acute toxicity
The acute toxicity of technical bromophos-ethyl has been studied in
various animal species. A summary of the results of these studies is
given in Table 1.
TABLE 1 Acute toxicity of technical bromophos-ethyl in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 210-550 Barnes, 1968; Muacevic, 1964,
1967
Rat oral 52-127 Barnes, 1968; Muacevic, 1964,
1966; Leuschner, 1966, 1967
Rabbit oral 28 Muacevic, 1970
Rabbit dermal 100-600 Bradford, 1967; Muacevic, 1967
Dog oral 335(approx.) Muacevic, 1970
Quail oral 200 Muacevic, 1970
Hen oral 600(approx.) Muacevic, 1969
Potentiation of acute toxicity
Potentiation of the acute toxicity of bromophos-ethyl has been
demonstrated to occur in the rat with bromophos, chlorfenvinphos,
coumaphos, malathion, mevinphos and parathion-methyl, and in the mouse
with chlorfenvinphos, diazinon, dimethoate, ethion and malathion.
Slight potentiation with parathion was found in both species
(Muacevic, 1966, 1967, 1968).
Short-term studies
Rat
Groups of 6 male and 6 female rats were fed for 4 weeks on diets
providing 0, 1 and 10 mg/kg/day of bromophos-ethyl. No significant
changes in behaviour, growth rate, food intake or macroscopic
appearance of organs could be observed (Leuschner, 1966).
Groups of 16 male rats were administered daily by gavage 0, 1, 5, 20
or 60 mg/kg body-weight of bromophos-ethyl suspension. The highest
dose was discontinued after 2 days since 9 animals had died. In the
remaining groups no abnormalities in behaviour, food consumption,
growth rate, haematological indices or liver function were found. No
pathological changes in organs were found at autopsy, and the livers,
kidneys and lungs were microscopically normal (Boehringer, 1964).
Groups of 42 male and 42 female rats were administered diets providing
0, 0.78, 1.25, 3.0 and 7.2 mg/kg body-weight of bromophos-ethyl/day.
During the 16-week period of administration no abnormalities in
behaviour, body-weight, food consumption or blood counts were
detected. Necropsy of 5 animals of each group revealed no macroscopic
abnormalities attributable to treatment. Dosage levels of 1.25 mg/kg
upwards caused a marked increase in the quantities of ascorbic and
dehydroascorbic acids excreted in the urine (Leuschner, 1967).
Dog
Three groups of 2 male and 2 female mongrel dogs were administered
daily in capsules 0, 1.6 or 12.5 mg/kg body-weight of bromophos-ethyl
for 2 weeks. The highest dose level was increased to 25 mg/kg for a
further week, and during this period these animals became quiescent,
lost weight and developed diarrhoea. The composition of urine and
blood counts were normal in all animals. The two lower dosage levels
were without untoward effect (Leuschner, 1966).
Four groups of 2 male and 2 female beagle dogs received daily capsules
containing 0, 0.52, 1.25 or 3.0 mg bromophos-ethyl for 16 weeks. No
abnormalities in behaviour, food consumption, body-weight,
haematological indices, the activity of serum enzymes, chemical
constituents of blood or urine constituents were found in treated
animals. No pathological changes were found in the eye, and organ
weight changes did not occur. The 1.25 mg/kg group excreted an
increased amount of ascorbic and dehydroascorbic acids in their urine
and three times the normal amount was excreted by the 3.0 mg/kg group.
The adrenal was the only organ in which changes occurred which could
be attributed to treatment. Narrowing of the fascicular zone and
dispersal of the glomerulose zone, the cells showing a high lipoid
content, and fibrous dispersal of the capsule were described, the
degree of change being most marked in the 3.0 mg/kg group (Leuschner,
1967).
Six groups of beagle dogs were administered each day, in capsules,
sugar (control), coumaphos or bromophos-ethyl as follows: sugar, 1 g;
coumaphos, 3.0 mg/kg; bromophos-ethyl, 0.26 mg/kg and 0.39 mg/kg (3
male and 3 female dogs), 1.25 mg/kg (5 males and 5 females), 3.0 mg/kg
(6 males and 6 females). The administration continued for 18 weeks
after which a number of animals of each group received no treatment
for four weeks. Depression of plasma cholinesterase activity occurred
at the 1.25 mg/kg dosage level, but no definite decrease in enzyme
activity occurred at any dosage level in the RBC, brain or adrenal
gland. Caumaphos, however, inhibited RBC and brain enzyme at the 3.0
mg/kg/day level. Four weeks after treatment ceased all cholinesterase
levels had returned to normal. Urinary ascorbic and dehydroascorbic
acids were increased slightly in the 0.39 mg/kg group and the increase
was marked with higher dosage levels of bromophos-ethyl and with
coumaphos. The values also returned to normal when administration
ceased. Histological examination showed that in bromophos-ethyl
treated animals only the 3.0 mg/kg group showed a moderate broadening
of the fasciculate zone of the adrenal and a relative increase in
eosinophilic staining in the frontal lobe of the pituitary. The
appearance of the adrenals and pituitary were normal after treatment
had ceased for four weeks (Leuschner et al., 1968).
Five groups of 4 male and 4 female beagle dogs were fed for 2 years on
diets containing 0, 10, 20, 30 and 120 ppm bromophos-ethyl. No
influence was seen on behaviour, food intake or growth of treated
animals. Analysis of blood for chemical constituents, including
cortisol, and of urine failed to demonstrate abnormalities. All serum
enzymes, except cholinesterase, were of normal activity. The weight
and macro- and microscopic appearance of organs, including the adrenal
and pituitary glands and bone marrow, were normal when animals were
killed during and at the end of the test. The dosage threshold at
which inhibition of cholinesterase occurred was considered to be
between 10 and 20 ppm for plasma enzyme and above 120 ppm for RBC and
brain enzymes. Urine ascorbic and dehydroascorbic acid levels were
increased in the 30 and 120 ppm groups. Excretion was maximum at
between 6 and 9 months, and then it decreased. No untoward effects
could be detected in the 10 ppm group (Leuschner et al., 1971).
Rabbit
Groups of 2 male and 2 female rabbits were administered 0, 2.5, 12.5
and 62.5 mg/kg/day of bromophos-ethyl on scarified or intact skin in
the form of an ointment for 6 hours each day for 21 days. They were
observed for a further 14 days before necropsy. One male of the
highest dosage group died and body-weight gain was retarded in the two
highest groups. Varying degrees of liver necrosis, myocardial scarring
and renal changes were found at the highest dosage level but not at
lower levels (Stötzer et al., 1970).
Long-term studies
Rat
Groups of 42 male and 42 female rats, each weighing from 166 to 214 g,
were fed diets providing 0.78, 1.25, 3.0 or 7.2 mg/kg body-weight/day
of bromophos-ethyl for 2 years. The threshold levels for a 20%
inhibition of activity lay between 0.78 and 1.25 mg/kg for plasma and
3.0 and 7.2 mg/kg for erythrocyte, brain and liver cholinesterase.
Urinary ascorbic and dehydroascorbic acid excretion was elevated at
all dosage levels; a 78% increase was seen at the 0.78 mg/kg level.
With increasing age, the amounts excreted became less marked but were
still significantly high at 2 years in the 3.0 and 7.2 mg/kg groups.
The mean body-weights of rats of both sexes on 3.0 and 7.2 mg/kg
bromophos-ethyl were slightly elevated above that of the controls
throughout much of the test. Urine analysis showed that the incidence
of occurrence of erythrocytes, protein, ketone bodies and white blood
cells was reduced in the 3.0 and 7.2 mg/kg groups compared with
controls. No significant differences from controls were seen with
regard to behaviour, survival, food intake, and the results of
haematological investigations or with regard to gross or microscopic
changes in organs or tumour incidence and type (Leuschner et al.,
1969a).
COMMENT
Bromophos-ethyl is absorbed from the gastro-intestinal tract and
excreted in urine and faeces principally as dichloro-bromophenol and
its conjugates. Accumulation does not occur following oral ingestion.
Bromophos-ethyl inhibits cholinesterase, the plasma enzyme being the
most sensitive. Acute potentiation was observed in combination with
several other organo-phosphates in rats and mice. In 2-year studies in
dogs and rats the no-effect levels for plasma cholinesterase
inhibition were 0.4 and 0.78 mg/kg body-weight/day, respectively.
Bromophos-ethyl does not cause delayed neurological injury. Studies in
rats did not indicate ill effects on reproduction or teratogenic
activity.
In studies in rats and dogs urinary excretion of ascorbic and
dehydroascorbic acids was increased. The no-effect level in dogs was
0.26 mg/kg/day. A no-effect level was not demonstrated for rats.
Investigations to find the cause of the increase were not reported.
The long-term study in rats showed that, at the dosage levels
employed, bromophos-ethyl had no carcinogenic activity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: 0.26 mg/kg body-weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos-ethyl is an organo-phosphorus insecticide and acaricide with
a broad spectrum of activity and moderate mammalian toxicity. It acts
as a contact and stomach poison. It is available as an emulsifiable
concentrate, ULV concentrate, wettable powder, granular or dust
formulation as well as dip formulations. Bromophos-ethyl is compatible
with insecticides and fungicides.
This substance is of moderate toxicity to bees and should not be
sprayed on flowering crops during the flight of bees.
Bromophos-ethyl has been used in Argentina, Australia, Austria,
Belgium, Brazil, Colombia, the Federal Republic of Germany, the
Netherlands, New Zealand, Nicaragua, Pakistan, South Africa and
Venezuela.
Pre-harvest treatments
Bromophos-ethyl is applied on various crops, mainly fruits, field
crops, vegetables, cereals, maize, rice, cotton and tobacco, and is
effective against a large number of chewing and sucking insect pests,
especially caterpillars, vegetable root maggots, fruit flies, bean
fly, aphids and beetles.
Depending on the different crops and the main pest species present the
recommended concentrations of spray wash vary between 0.02% and 0.1%
a.i. and the rates of application between 0.2 and 1.2 kg a.i./ha. The
majority of crops are tolerant to normal rates of the insecticide.
Occasionally slight damage was noticed, in particular in connection
with high dosages.
The withholding periods range from 14 to 28 days, depending on the
local conditions and crop.
Other uses
Bromophos-ethyl is used in the sector of animal health and tick
control. The compound is active against ticks, including strains of
the genus Boophilus which are resistant to chlorinated hydrocarbons
and other phosphorus preparations, as well as against blowflies, lice
and other ectoparasites. It also controls mosquitoes, mosquito larvae
and ants, revealing a remarkable residual effect. Bromophos-ethyl is
used particularly as a mosquito larvicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables and field crops (Boehringer, 1971-1972). Summaries
of much of this information have recently been published (Eichler
1971). The following tables 2 and 3 present a summary of available
data along with relevant information on rates of application, number
of applications and preharvest interval.
In trials in the Netherlands (Anonymous, 1970), bromophos-ethyl was
applied to carrots at 6 kg a.i./ha either by soil surface treatment
with granules prior to seeding or by foliar treatment with wettable
powder or emulsifiable concentrate 81 and 89 days after planting. The
residues obtained are shown in table 4.
Distribution of residues in 1-year-old beef cattle was determined in
Germany (Boehringer, 1966) after spraying to runoff 1 or 4 times with
bromophos-ethyl emulsion (0.05% a.i.). The results are summarized in
table 5.
In later trials (Boehringer, 1968), 6 cows were sprayed three times at
5-day intervals (14 days is more usual) with 0.1% a.i. emulsion (twice
the concentration generally used). Table 6 summarizes these results.
Two experimental trials on residues in beef cattle were conducted in
Australia. In the first of these, two groups of 9 cattle were sprayed
with bromophos-ethyl at a concentration of 0.05% (Snelson, 1968).
Group 1 was treated once and Group 2 had three treatments at 5-day
intervals. Residues were determined in heart, kidney, omental and
subcutaneous fat with the results shown in Table 7.
The highest residues were in samples of heart and kidney fat. In the
second trial, six steers were sprayed each with 2 gallons of 0.05%
bromophos-ethyl and samples were taken for analysis at 4, 7 and 21
days after exposure (Harvey, 1968). Residues were distributed as shown
in Table 8.
Dipping leads to higher uptake and significantly higher residues in
fat than does spraying with the same concentration of bromophos-ethyl.
In a series of dipping trials under the direction of the Board of Tick
Control in Australia, 12 cattle were dipped once in 0.05%
bromophos-ethyl emulsion and groups of four were slaughtered 1, 4 and
8 days post treatment and 12 cattle were dipped three times at
intervals of 5 days in the same bath and groups of four were
slaughtered, as before, 1, 4 and 8 days after the third dipping
(Snelson, 1972). Results are given in Table 9.
Further trials showed that the uptake varied considerably depending on
the condition of the animals (fat versus thin), length of hair and
time of year. It was demonstrated that the half-life of
bromophos-ethyl residues in internal fat of cattle is 10 to 12 days.
Table 2 Bromophos-ethyl residues in fruit1
Crop Application Days between last treatment and harvest
No. Rate a.i. 0 1 2 3 4 5 7 8 10 14 15 21 22 28 30
(g/plant)
Apple 1 1.8 2.20 1.40 1.95 1.35
1 0.9 1.25 0.7 1.03 1.20 0.76 0.55
1 0.9 0.58 0.36 0.33 0.30 0.28
1 1.8 1.17 1.13 0.81 1.23
5 1.2 3.45 2.65 2.05 1.95
6 1.5 4.90 3.70 2.50 2.30
Pear 1 0.36 0.59 0.32 0.32 0.26 0.15
Peach 1 0.5 0.38 0.39
Yellow plum 1 0.9 1.40 0.81
Plum 1 1.8 2.08 1.14 1.35 1.11
3 1.8 2.28 1.24 0.73 0.51
1 0.54 0.36 0.34 0.14 0.13 0.12
Sweet Cherry 1 1.8 0.87 0.45 0.27 0.07 0.03
1 1.8 0.21 0.17 0.09
1 1.8 0.89 0.27 0.16 0.14 0.03
Strawberry 1 2882 0.36 0.07
Gooseberry 1 0.36 0.33 0.52 0.26 0.31 0.30
Red-currant 1 3602 1.18 0.95
Black-currant 1 0.36 0.78 0.12
1 residue figures given in ppm
2 g/ha
Table 3a Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 0 1 3 4 5 7 14 21 28 30
(g/ha a.i.)
Garden
lettuce 1 108 0.31 0.28 0.18
1 216 2.35 1.09 1.03 0.37 0.16 0.10
1 108 1.05 0.45 0.09
1 108 0.31 0.28 0.18
Spinach 1 216 2.72 1.60 0.42 0.03
1 216 6.84 4.42 2.22
1 288 8.32 6.66 3.26 2.78 0.44
Carrot 2 0.182
1 0.103
2 0.182
1 0.183
Cauliflower 2 28.84 <0.02
Kohlrabi 2 324
White cabbage 1 360 <0.08 <0.08
1 216 0.20 0.16 0.07 0.04 0.02 <0.02
Brussels
sprouts 3 540 0.57 0.51
Sugar beet 1 144
Onion 2 0.23
2 0.23
2 0.23
Tubercelery
tuber 3 160 0.19-
0.36
leaf 3 160 2.67-
4.09
Rape seed 1 216
Rape oil 1 216
Horsebean 1 600 <0.02
Kidney bean 2 324 0.18 0.07 0.03 0.02 <0.02
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
Table 3b Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 31 35 42 49 54 56 62 64 66 71 107
(g/ha a.i.
Garden
lettuce 1 108
1 216
1 108
1 108
Spinach 1 216 0.02
1 216
1 288
Carrot 2 0.182 1.21
1 0.103 0.40
2 0.182 1.53
1 0.183 1.42
Cauliflower 2 28.84
Kohlrabi 2 324 0.05
White
cabbage 1 360
1 216
Brussels
sprouts 3 540
Sugar beet 1 144 <0.02
Onion 2 0.23 <0.02
2 0.23 <0.02
2 0.23 <0.02
Tubercelery
tuber 3 160
leaf 3 160
Rape seed 1 216 0.08
Rape oil 1 216 0.33
Horsebean 1 600
Kidney bean 2 324
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
TABLE 4 Bromophos-ethyl residues in carrots
Crop Method of Rate of Time between Residues
application application last treatment (ppm)
(kg/ha/a.i.) and harvest
(months)
Carrots soil surface 6 3 0.22 - 0.24
soil surface 6 3 0.17 - 0.77
foliar spray 6 2 0.49 - 1.5
foliar spray 6 2 0.84 - 1.6
TABLE 5 Distribution of bromophos-ethyl residues in cattle tissue, 1966
Treatment Sample interval Residues (ppm)1
Animal (0.05% (days after Back Leg
a.i.) last treatment) muscle muscle Brain Kidney Liver Fat
12 1 spray 1 0.77 0.90 0.76 <0.13 <0.14 n.d.
2 4 sprays 3 n.d. <0.18NS n.d. n.d. <0.15NS n.d.
3 4 sprays 1 <0.18NS n.d. n.d. n.d. n.d. 0.67*
4 4 sprays 11 n.d. n.d. n.d. n.d. n.d. <0.20NS
5 Control -- -- 0.056 -- 0.049 0.052 0.053 0.076
1 n.d. = not detected; NS = not statistically significant; * = statistically significant.
2 High probability that samples were contaminated during taking and handling; data not usable.
TABLE 6 Distribution of bromophos-ethyl residues in cattle tissue, 1968
Sample interval Residues (ppm)1,2
Animal (days after Fillet Roasting Kidney Liver Kidney Subcutaneous
last treatment) beef fat fat
924 1 0.015 0.015 0.010 <0.005 0.132 0.056
621 1 0.017 0.006 0.006 <0.005 0.193 0.065
110 3 0.010 0.008 0.007 <0.005 0.236 n.a.
102 3 0.007 0.007 0.010 <0.005 0.219 n.a.
605 7 <0.005 <0.005 <0.005 <0.005 0.218 n.a.
228 7 <0.005 0.010 <0.005 <0.005 0.183 n.a.
1 n.a. = not analyzed.
2 Determination by gas chromatography, mean of three determinations.
Bromophos-ethyl residues in milk from three dairy cows each sprayed
with two gallons of 0.05% active ingredient are shown in Table 10
(Snelson, 1968).
Dipping of dairy cows likewise gives rise to significant residues in
milk which are all transferred to the butterfat. The level of residues
in the milk of dipped cows was only slightly higher than those
reported in Table 10 (Snelson, 1972). The residue level reached a peak
on the first and second days after dipping and thereafter declined
rapidly, with a half-life calculated to be between 1-2 and 1-4 days.
TABLE 7 Bromophos-ethyl residues in fat of beef cattle, trial 1
Post-treatment day Group 1, Group 2,
1 treatment (ppm) 3 treatments (ppm)
1 0.08 - 2.9 0.3 - 2.6
4 0.1 - 2.0 0.1 - 1.6
8 Nil - 0.7 Nil - 0.6
TABLE 8 Bromophos-ethyl residues in fat of steers, trial 2
Interval between Residues (ppm)
treatment and Liver Kidney Muscle Omental Perirenal
sampling (days) fat fat
4 <0.05 <0.05 <0.05 0.82 0.63
<0.05 <0.05 <0.05 0.62 0.36
7 <0.05 <0.05 <0.05 0.70 0.63
<0.05 <0.05 <0.05 0.21 0.45
21 <0.05 <0.05 <0.05 0.24 0.31
<0.05 <0.05 <0.05 0.40 0.29
TABLE 9 Bromophos-ethyl residues in cattle after dipping
Interval
between Residues bromophos-ethyl (ppm)
last dipping dipped once dipped 3 times
and slaughter Internal Subcutaneous Internal Subcutaneous
(days) fat fat fat fat
1 0.15 - 0.23 0.13 - 0.26 1.47 - 3.36 1.2 - 2.32
4 0.21 - 0.37 0.15 - 0.27 1.38 - 1.83 0.5 - 2.05
8 0.36 - 0.87 0.23 - 0.50 2.04 - 2.48 0.78 - 2.56
TABLE 10 Bromophos-ethyl residues in milk
Time after Residues (ppm)
Treatment treatment in butterfat in milk
(h)
Nexagan 0.05% 5 0.03 0.002
(single
application) 21 1.01 0.072
29 0.71,0.67,0.42 0.038,0.026,0.017
45 1.17,0.48,0.60 0.060,0.028,0.026
53 0.79,0.60,0.47 0.042,0.028,0.021
69 0.47 0.017
77 0.63 0.038
93 0.25 0.016
10 days 0.02 0.001
In trials in Germany (Boehringer, 1968), three cows were each sprayed
with 5 liters of 0.05%. a.i. suspension and milk samples were taken
during the following four days. A second treatment was performed seven
days later without sampling. A third treatment followed 14 days after
the start of the trial, and subsequent milkings were analyzed for a
total time of 21 days. Maximum residues (0.098, 0.077, 0.089 ppm) were
found about 24 hours after treatment, decreasing within about four
days below the limit of determination (<0.02 ppm). There was no
accumulation of residues after the third treatment.
FATE OF RESIDUES
General comments
By analogy with bromophos, the major metabolites of bromophos-ethyl
would be expected to be 2,5-dichloro-4-bromophenol, bromoxon-ethyl,
and desethyl bromophos-ethyl (see Figure 1 of preceding bromophos
monograph). The major points of difference appear to be that no
desethyl-bromophos-ethyl is found after oral administration to rats
and that somewhat higher ratios of bromoxon-ethyl to bromophos-ethyl
is found in plants, especially lettuce (Eichler, 1971).
In animals
The metabolism and excretion of 3H-labelled bromophos-ethyl in the
rat was studied by Stiasni (1967). No specific organic accumulation
occurred following oral and parenteral administration. Quantitative
excretion occurred between 8 and 12 days. Neither unchanged
bromophos-ethyl nor bromoxon-ethyl could be found in the excrements
following oral administration. Desethyl-bromophos-ethyl was also
missing among the metabolites. The only metabolites identified were
dichloro-bromophenol and its conjugates.
In plants
No tests have been carried out with radioactively labelled
bromophos-ethyl in plants. However, in connection with supervised
trials for determining residues in fruits and vegetables,
bromoxon-ethyl residues were determined in apples, Brussels sprouts
and lettuce (Eichler, 1971). In apples, the bromoxon-ethyl residues
never exceeded 0.005 ppm (<1% of bromophos-ethyl residue). In
Brussels sprouts, the highest bromoxon-ethyl residue found was 0.026
ppm (5% of bromophos-ethyl residue). In lettuce, after several
sprayings, bromoxon-ethyl residues reached a maximum of 0.07 ppm (6.4%
of bromophos-ethyl residue) in three days. The highest ratio found was
8.8% (0.014 ppm) after 14 days.
In soil
Bromophos-ethyl E.C. was applied one time at 0.5 g a.i./m2 to high
moorland soil (acid, high organic content), Ingelheim sand and clay
soil. Zero to 20 cm-deep samples were taken periodically for 26 weeks
and analyzed with the results shown in Table 11 (Eichler, 1970).
In comparison with bromophos, bromophos-ethyl degrades somewhat more
slowly in soil; however, neither compound shows any significant
persistence.
In storage and processing
Leber and Deckers (1968) examined the effects on residues in beef
under storage at +4° and -18°C and after cooking (roasting). No
significant decrease in residues was observed in meat, kidney and
kidney fat after a 7-day storage period at +4°C or after a 30-day
storage period at -18°C. Neither 15 minutes of frying of 100 g steaks
nor one hour of boiling of 100 g of beef in 200 ml of water resulted
in any significant decrease in residues of bromophos-ethyl.
Evidence of residues in food in commerce or at consumption
In connection with a tick eradication program in Australia, perirenal
fat from 17 cattle known to have been dipped in 0.05% Nexagan on 27
occasions over a period of 14 months was analyzed for residues
(Harvey, 1968). Three samples had residues between 0.5 and 1.0 ppm,
five between 1.0 and 1.5 ppm, seven between 1.5 and 2.0 ppm and two
samples had 2.18 and 2.68 ppm, respectively.
TABLE 11 Bromophos-ethyl residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-
soil time (weeks) Bromophos-ethyl bromophenol
High moorland 0 13.302 <0.10
1 6.26 <0.10
3 4.95 0.54
6 3.92 <0.10
9 3.29 0.60
13 3.44 1.30
26 1.25 <0.10
Sandy 0 2.343 <0.10
1 1.40 <0.10
3 0.64 0.13
6 0.40 <0.10
9 0.16 <0.10
13 0.12 <0.10
26 <0.02 <0.10
Clay 0 2.764 <0.10
1 2.16 <0.10
3 0.44 0.17
6 0.32 <0.10
9 0.26 <0.10
13 0.14 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 0.844-0.032 log X; r = 0.9173
3 Y = 0.128-0.076 log X; r = 0.9721
4 Y = 0.175-0.076 log X; r = 0.9544
Samples of internal fat taken from animals, with unknown treatment
history, being slaughtered in the cattle tick zone in Australia during
1971/72 revealed some to contain bromophos-ethyl residues. Of 1 055
samples examined, 52 contained bromophos-ethyl, mostly below 0.5 ppm,
the highest being 0.8 ppm (Snelson, 1972). During the same period 589
samples of butter and cheese were examined for organo-phosphorus
residues. Only three samples were found to contain bromophos-ethyl
residues, all at 0.1 ppm.
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos-ethyl. These
have recently been reviewed and summarized by Eichler (1971). A method
has been developed by Leber and Deckers (1968) for all crops and
animal tissues. It utilizes gas chromatography with a phosphorus
specific detector and can estimate quantities down to the range of
0.001 to 0.01 ppm with a 100 g sample. The method includes procedures
for determining bromoxon-ethyl either by colorimetry or by gas
chromatography with a sensitivity of 0.03 to 0.05 ppm. Confirmation of
residues can be accomplished by the thin-layer chromatographic
techniques suggested in the method.
Bromophos-ethyl is among the pesticides listed as being detectable by
the multi-residue gas chromatographic procedure of Abbott et al.
(1970), and that method is the most suitable for regulatory use. The
method of Leber and Deckers would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Examples of national tolerances of bromophos-ethyl residues are
reported in Table 12.
TABLE 12 Examples of national tolerances as reported to meeting
Country Commodity Tolerance
Australia Fat of meat of cattle
and sheep 3 ppm
Milk and milk products
(fat basis) 1 ppm
The Netherlands Fruit and vegetables 0.4 ppm
APPRAISAL
Bromophos-ethyl is a non-systemic halogen-containing organo-phosphorus
insecticide and acaricide used on fruit, vegetables, field crops,
cereals, maize, rice, cotton and tobacco. It is also used extensively
in tick control, for other ectoparasites of domestic animals and as a
mosquito larvicide.
Supervised trials with foliar treatments on fruits and vegetables have
shown that bromophos-ethyl is somewhat more persistent than bromophos
and requires a pre-harvest interval about twice as long. The rate of
residue decline is highly dependent on many factors, especially
botanical species and morphological structure, which require that
tolerance recommendations be made on an individual commodity basis
rather than on broad crop categories.
Groups of beef cattle were sprayed at recommended rates one or more
times in trials in several countries. Highest residues were found in
omental, heart and perirenal fat, ranging in one trial from 0.1 to 2.0
ppm at four days post-treatment.
Spray treatment of dairy cows at recommended rates resulted in a
residue maximum of 1.17 ppm in butterfat at 45 hours post-treatment.
This fell to 0.02 ppm in butterfat after ten days. There was no
accumulation of milk residues from the multiple treatments.
The metabolites of bromophos-ethyl most likely to be found are
2,5-dichloro-4-bromophenol and bromoxon-ethyl. Only
dichloro-bromophenol and its conjugates were found in the excrement of
rats after oral administration of labelled bromophos-ethyl. The
dichloro-bromophenol also appears to be the only soil metabolite.
Although no studies have been conducted with the labelled compound in
plants, supervised field trials have shown small amounts of
bromoxon-ethyl residues in apples, Brussels sprouts and lettuce (up to
0.07 ppm in the latter case).
Available multi-residue gas chromatographic procedures are suitable
for use for regulatory purposes and are recommended.
Although bromophos-ethyl is recommended for cereals, maize, cotton,
rice and in animal health, there were no data available for these
commodities except fat of meat of cattle and milk. Therefore, no
recommendations could be made for tolerances on these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are recommended for
bromophos-ethyl.
ppm
Apples, carrots, fat of meat of cattle,
plums, pears, spinach 2
Brussels sprouts, redcurrants 1
Celeriac, gooseberries, peaches,
rape seed oil, cherries (sweet) 0.5
Blackcurrants, lettuce 0.2
Rape seed, strawberries, cabbage 0.1
Kohlrabi, French beans 0.05
Cauliflower, beans (without pods),
onions, sugarbeets, milk (whole) 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1975)
Further studies evaluating the significance of urinary excretion of
ascorbic and dehydroascorbic acids and its relevance to man.
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials on maize, rice and other cereals,
cotton, domestic animals, other than cattle, and milk products.
DESIRABLE
A study to determine dose levels causing no carboxyl-esterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1967.
III. Organo-phosphorus pesticide residues in the total diet. Pestic.
Sci. 1: 10-13.
Anonymous. (1970) Utrecht report, project CvF/PD4.2.(2.2.03).
Barnes, J.M. (1968) WHO insecticide evaluation and testing programme.
Stage I - mammalian toxicity report. Medical Research Council,
Carshalton. (unpublished).
Battelle Institute. (1964) Toxicity tests with the substance S2225 in
rats, guinea pigs and rabbits. (unpublished)
Boehringer. (1966) Residue trials and examination of cholinesterase
activity after spraying with bromophos-ethyl on cattle. Report C.H.
Boehringer Sohn.
Boehringer. (1967) Investigations concerning absorption, distribution,
excretion and metabolism of bromophos-ethyl-3H in rats. Report C.H.
Boehringer Sohn. (unpublished)
Boehringer. (1968) Determination of residues of bromophos-ethyl in
cattle. Report C.H. Boehringer Sohn.
Boehringer. (1971-1972) Residue investigation reports, C.H. Boehringer
Sohn.
Bradford, H.A. (1967) Acute dermal toxicity - bromophos-ethyl
(compound 70625). Report Eli Lilly and Company. (unpublished)
Eichler, D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
Eichler, D. (1970) Report C.H. Boehringer Sohn "Uber den Abban von
Bromophos und Bromophos-ethyl in verschiedenen Böden".
Harvey, J.M. (1968) Bromophos-ethyl residues in meat products.
Communication with C.H. Boehringer Sohn.
Leber G. and Deckers, W. (1968) Determination of residues of bromophos
and bromophos-ethyl. Proc. Brit. Insecticide Fungicide Conf.,
Brighton, Eng. 4: 570.
Leuschner, F. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Standinger, H.J. (1968) About the short-term toxicity studies on
bromophos-ethyl - charge 1487 - in Beagle dogs (with special attention
to the toxicology of the adrenal gland). Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Dontenwill, W. (1969a) About the chronic toxicity of bromophos-ethyl
in Wistar rats following oral application. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969b)
About the chronic tolerance of bromophos-ethyl in the reproduction
test over three generations of Wistar rats. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Standinger, H.J., Schwerdtfeger, W. and
Dontenwill, W. (1971) Two years oral toxicity study in Beagle dogs
with bromophos-ethyl. Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Report on Shg 2225. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1969) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1970) Reports C.H. Boehringer Sohn. (unpublished)
Snelson, J.T. (1968) Bromophos-ethyl residues in milk. Communication
with C.H. Boehringer Sohn.
Snelson, J.T. (1967 - 1968) Residue studies - bromophos-ethyl.
Communications with C.H. Boehringer Sohn.
Snelson, J.T. (1972) Results from experimental investigations and
residue surveys with bromophos-ethyl following dipping in Australia.
Report to Joint Meeting.
Stiasni, M. (1967) Data from C.H. Boehringer Sohn. (unpublished)
Stötzer, H., Herbst, M., Baumgartner, R. and Guęnard, J. (1970)
Subacute dermal toxicity of the substance bromophos-ethyl in rabbits
(New Zealand White). Report C.H. Boehringer Sohn. (unpublished)
Terblanche. (1966) Toxicity of bromophos-ethyl. Department of
Agricultural Technical Services, Onderstepoort, South Africa.
(unpublished)
Vuuren, P.J.J. (1964) Whale-blood cholinesterase levels in cattle
sprayed at weekly intervals with S2225 (CELA). Agricura Laboratoria
Ltd., Silverton, South Africa. (unpublished)
 Other information on identity and properties
Molecular weight: 394.0
State: yellow fluid
Density: d20 = 1.53
20
Boiling point: 122-123°C at 10-3 torr.
Vapour pressure: 4.6 x 10-5 mm Hg at 30°C
Solubility: at room temperature miscible with most organic
solvents; practically insoluble in water.
Stability: stable in aqueous suspension. Saponification only
occurs in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-diethylphosphorothioate:
approx. 1.0% (chlorine position not defined)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies using bromophos-ethyl labelled with 3H in the benzene ring
demonstrated that, in the rat, the substance is absorbed following
oral administration but accumulation generally or in specific organs
does not occur. Apart from the G.I. tract, only the liver, kidney,
spleen and brain showed any substantial activity after 4 hours, and
this declined sharply in 24 hours. Almost total excretion of activity
occurred within 4 days following 5 daily oral doses of approximately 8
mg/kg. The distribution pattern was essentially the same after
intraperitoneal injection, but excretion was slower due to its slow
release from peritoneal and mesenteric fat.
Following a single 8 mg/kg oral dose of 3H-labelled bromophos-ethyl,
40% of the activity appeared in urine and 60% in faeces. When
administered intraperitoneally or subcutaneously, the major portion
also occurred in faeces. Radioactivity was detected in bile 40 minutes
after administration into the duodenum of 3H-labelled material and
40% was excreted by this route in 9 hours. The identity of the
substance in bile has not been established (Boehringer, 1967).
Biotransformation
No bromophos-ethyl, the oxidation product bromoxon-ethyl or
desethyl-bromophos-ethyl, which would be formed by cleavage of the
C2H5-O-P bond, were found in the urine or faeces of rats after oral
dosage. The metabolite dichloro-bromophenol and its conjugates were
identified in urine and faeces and accounted for 85-90% of
administered product, showing that the principal metabolic route
involved cleavage of the phenyl-O-P bond (Boehringer, 1967).
Effects on enzymes and other biochemical parameters
A single dose of 42 mg/kg of bromophos-ethyl to rats caused maximum
inhibition of cholinesterase in plasma and RBC after 8 and 2 hours
respectively. Values showing less than 20% inhibition occurred after
16 and 48 hours respectively (Muacevic, 1968). A single dose of 15
mg/kg to sheep inhibited RBC cholinesterase after 24 hours. A normal
level was found 24 hours later. A dose of 10 mg/kg was without effect
(Terblanche, 1966). In short-term (Muacevic, 1966; Leuschner, 1966,
1967) and long-term studies (Leuschner et al., 1969a, 1969b) in rats
and in short-term studies in dogs (Leuschner, 1966, 1967; Leuschner
et al., 1968) and cattle (Vuuren, 1964; Muacevic, 1966), inhibition
of plasma, RBC, brain and liver cholinesterase was noted. In general,
plasma cholinesterase was the most sensitive of the enzymes.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Bromophos-ethyl, 600 mg/kg (the assumed LD50) was administered orally
to 10 one-year-old hens; they were treated with atropine and
toxogonin. No neurological abnormalities were seen in the 6-week
observation period (Muacevic, 1968).
Three groups of 10 one-year-old hens were administered orally 0, 1.25,
or 2.5 mg/kg/day of bromophos-ethyl on 6 days a week for one month.
Only one bird at the top dosage level showed general weakness;
neuropathological examination revealed no changes in this or other
birds examined (Muacevic, 1968).
Special studies on pharmacology
Atropine sulphate and toxigonin were shown to protect rats and mice
from a lethal dose of bromophos-ethyl. Norscopolamine also protected,
but salicylaldoxime showed no positive effect (Muacevic, 1967; 1968).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0,
1.25, 3.0 and 7.2 mg/kg/day approximately of bromophos-ethyl. Three
generations were examined, the parent F1 and F2 generation each
producing two litters, and mating taking place between week 9 and 15
and between week 21 and 16. No abnormalities were seen in the
behaviour, appearance, body-weight, haematological indices, or in
organs during pathological examinations in adult animals. No effect on
reproduction was found in the two lower dosage groups, but in the 7.2
mg/kg group, which showed significant inhibition of liver, brain and
plasma cholinesterase, the fertility rate and litter size were
decreased and the number of stillbirths increased. The young also
tended to have a lower body-weight than controls. However no runts or
animals with congenital malformations were discovered (Leuschner
et al., 1969b).
Special studies on teratogenicity
Groups of 20 pregnant female rats were administered 0, 0.03, 1.7, 3.5,
7.0 or 14.0 mg/kg body-weight/day of bromophos-ethyl by gastric
intubation from day 6 to 15 of gestation. Animals were killed at day
20 and uteri and foetuses examined. No significant differences from
controls were found in the number of foetuses or reabsorption sites or
in foetus weights. No malformations, runts or dead foetuses were found
in any of the treated groups (Leuschner, 1967).
Acute toxicity
The acute toxicity of technical bromophos-ethyl has been studied in
various animal species. A summary of the results of these studies is
given in Table 1.
TABLE 1 Acute toxicity of technical bromophos-ethyl in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 210-550 Barnes, 1968; Muacevic, 1964,
1967
Rat oral 52-127 Barnes, 1968; Muacevic, 1964,
1966; Leuschner, 1966, 1967
Rabbit oral 28 Muacevic, 1970
Rabbit dermal 100-600 Bradford, 1967; Muacevic, 1967
Dog oral 335(approx.) Muacevic, 1970
Quail oral 200 Muacevic, 1970
Hen oral 600(approx.) Muacevic, 1969
Potentiation of acute toxicity
Potentiation of the acute toxicity of bromophos-ethyl has been
demonstrated to occur in the rat with bromophos, chlorfenvinphos,
coumaphos, malathion, mevinphos and parathion-methyl, and in the mouse
with chlorfenvinphos, diazinon, dimethoate, ethion and malathion.
Slight potentiation with parathion was found in both species
(Muacevic, 1966, 1967, 1968).
Short-term studies
Rat
Groups of 6 male and 6 female rats were fed for 4 weeks on diets
providing 0, 1 and 10 mg/kg/day of bromophos-ethyl. No significant
changes in behaviour, growth rate, food intake or macroscopic
appearance of organs could be observed (Leuschner, 1966).
Groups of 16 male rats were administered daily by gavage 0, 1, 5, 20
or 60 mg/kg body-weight of bromophos-ethyl suspension. The highest
dose was discontinued after 2 days since 9 animals had died. In the
remaining groups no abnormalities in behaviour, food consumption,
growth rate, haematological indices or liver function were found. No
pathological changes in organs were found at autopsy, and the livers,
kidneys and lungs were microscopically normal (Boehringer, 1964).
Groups of 42 male and 42 female rats were administered diets providing
0, 0.78, 1.25, 3.0 and 7.2 mg/kg body-weight of bromophos-ethyl/day.
During the 16-week period of administration no abnormalities in
behaviour, body-weight, food consumption or blood counts were
detected. Necropsy of 5 animals of each group revealed no macroscopic
abnormalities attributable to treatment. Dosage levels of 1.25 mg/kg
upwards caused a marked increase in the quantities of ascorbic and
dehydroascorbic acids excreted in the urine (Leuschner, 1967).
Dog
Three groups of 2 male and 2 female mongrel dogs were administered
daily in capsules 0, 1.6 or 12.5 mg/kg body-weight of bromophos-ethyl
for 2 weeks. The highest dose level was increased to 25 mg/kg for a
further week, and during this period these animals became quiescent,
lost weight and developed diarrhoea. The composition of urine and
blood counts were normal in all animals. The two lower dosage levels
were without untoward effect (Leuschner, 1966).
Four groups of 2 male and 2 female beagle dogs received daily capsules
containing 0, 0.52, 1.25 or 3.0 mg bromophos-ethyl for 16 weeks. No
abnormalities in behaviour, food consumption, body-weight,
haematological indices, the activity of serum enzymes, chemical
constituents of blood or urine constituents were found in treated
animals. No pathological changes were found in the eye, and organ
weight changes did not occur. The 1.25 mg/kg group excreted an
increased amount of ascorbic and dehydroascorbic acids in their urine
and three times the normal amount was excreted by the 3.0 mg/kg group.
The adrenal was the only organ in which changes occurred which could
be attributed to treatment. Narrowing of the fascicular zone and
dispersal of the glomerulose zone, the cells showing a high lipoid
content, and fibrous dispersal of the capsule were described, the
degree of change being most marked in the 3.0 mg/kg group (Leuschner,
1967).
Six groups of beagle dogs were administered each day, in capsules,
sugar (control), coumaphos or bromophos-ethyl as follows: sugar, 1 g;
coumaphos, 3.0 mg/kg; bromophos-ethyl, 0.26 mg/kg and 0.39 mg/kg (3
male and 3 female dogs), 1.25 mg/kg (5 males and 5 females), 3.0 mg/kg
(6 males and 6 females). The administration continued for 18 weeks
after which a number of animals of each group received no treatment
for four weeks. Depression of plasma cholinesterase activity occurred
at the 1.25 mg/kg dosage level, but no definite decrease in enzyme
activity occurred at any dosage level in the RBC, brain or adrenal
gland. Caumaphos, however, inhibited RBC and brain enzyme at the 3.0
mg/kg/day level. Four weeks after treatment ceased all cholinesterase
levels had returned to normal. Urinary ascorbic and dehydroascorbic
acids were increased slightly in the 0.39 mg/kg group and the increase
was marked with higher dosage levels of bromophos-ethyl and with
coumaphos. The values also returned to normal when administration
ceased. Histological examination showed that in bromophos-ethyl
treated animals only the 3.0 mg/kg group showed a moderate broadening
of the fasciculate zone of the adrenal and a relative increase in
eosinophilic staining in the frontal lobe of the pituitary. The
appearance of the adrenals and pituitary were normal after treatment
had ceased for four weeks (Leuschner et al., 1968).
Five groups of 4 male and 4 female beagle dogs were fed for 2 years on
diets containing 0, 10, 20, 30 and 120 ppm bromophos-ethyl. No
influence was seen on behaviour, food intake or growth of treated
animals. Analysis of blood for chemical constituents, including
cortisol, and of urine failed to demonstrate abnormalities. All serum
enzymes, except cholinesterase, were of normal activity. The weight
and macro- and microscopic appearance of organs, including the adrenal
and pituitary glands and bone marrow, were normal when animals were
killed during and at the end of the test. The dosage threshold at
which inhibition of cholinesterase occurred was considered to be
between 10 and 20 ppm for plasma enzyme and above 120 ppm for RBC and
brain enzymes. Urine ascorbic and dehydroascorbic acid levels were
increased in the 30 and 120 ppm groups. Excretion was maximum at
between 6 and 9 months, and then it decreased. No untoward effects
could be detected in the 10 ppm group (Leuschner et al., 1971).
Rabbit
Groups of 2 male and 2 female rabbits were administered 0, 2.5, 12.5
and 62.5 mg/kg/day of bromophos-ethyl on scarified or intact skin in
the form of an ointment for 6 hours each day for 21 days. They were
observed for a further 14 days before necropsy. One male of the
highest dosage group died and body-weight gain was retarded in the two
highest groups. Varying degrees of liver necrosis, myocardial scarring
and renal changes were found at the highest dosage level but not at
lower levels (Stötzer et al., 1970).
Long-term studies
Rat
Groups of 42 male and 42 female rats, each weighing from 166 to 214 g,
were fed diets providing 0.78, 1.25, 3.0 or 7.2 mg/kg body-weight/day
of bromophos-ethyl for 2 years. The threshold levels for a 20%
inhibition of activity lay between 0.78 and 1.25 mg/kg for plasma and
3.0 and 7.2 mg/kg for erythrocyte, brain and liver cholinesterase.
Urinary ascorbic and dehydroascorbic acid excretion was elevated at
all dosage levels; a 78% increase was seen at the 0.78 mg/kg level.
With increasing age, the amounts excreted became less marked but were
still significantly high at 2 years in the 3.0 and 7.2 mg/kg groups.
The mean body-weights of rats of both sexes on 3.0 and 7.2 mg/kg
bromophos-ethyl were slightly elevated above that of the controls
throughout much of the test. Urine analysis showed that the incidence
of occurrence of erythrocytes, protein, ketone bodies and white blood
cells was reduced in the 3.0 and 7.2 mg/kg groups compared with
controls. No significant differences from controls were seen with
regard to behaviour, survival, food intake, and the results of
haematological investigations or with regard to gross or microscopic
changes in organs or tumour incidence and type (Leuschner et al.,
1969a).
COMMENT
Bromophos-ethyl is absorbed from the gastro-intestinal tract and
excreted in urine and faeces principally as dichloro-bromophenol and
its conjugates. Accumulation does not occur following oral ingestion.
Bromophos-ethyl inhibits cholinesterase, the plasma enzyme being the
most sensitive. Acute potentiation was observed in combination with
several other organo-phosphates in rats and mice. In 2-year studies in
dogs and rats the no-effect levels for plasma cholinesterase
inhibition were 0.4 and 0.78 mg/kg body-weight/day, respectively.
Bromophos-ethyl does not cause delayed neurological injury. Studies in
rats did not indicate ill effects on reproduction or teratogenic
activity.
In studies in rats and dogs urinary excretion of ascorbic and
dehydroascorbic acids was increased. The no-effect level in dogs was
0.26 mg/kg/day. A no-effect level was not demonstrated for rats.
Investigations to find the cause of the increase were not reported.
The long-term study in rats showed that, at the dosage levels
employed, bromophos-ethyl had no carcinogenic activity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: 0.26 mg/kg body-weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos-ethyl is an organo-phosphorus insecticide and acaricide with
a broad spectrum of activity and moderate mammalian toxicity. It acts
as a contact and stomach poison. It is available as an emulsifiable
concentrate, ULV concentrate, wettable powder, granular or dust
formulation as well as dip formulations. Bromophos-ethyl is compatible
with insecticides and fungicides.
This substance is of moderate toxicity to bees and should not be
sprayed on flowering crops during the flight of bees.
Bromophos-ethyl has been used in Argentina, Australia, Austria,
Belgium, Brazil, Colombia, the Federal Republic of Germany, the
Netherlands, New Zealand, Nicaragua, Pakistan, South Africa and
Venezuela.
Pre-harvest treatments
Bromophos-ethyl is applied on various crops, mainly fruits, field
crops, vegetables, cereals, maize, rice, cotton and tobacco, and is
effective against a large number of chewing and sucking insect pests,
especially caterpillars, vegetable root maggots, fruit flies, bean
fly, aphids and beetles.
Depending on the different crops and the main pest species present the
recommended concentrations of spray wash vary between 0.02% and 0.1%
a.i. and the rates of application between 0.2 and 1.2 kg a.i./ha. The
majority of crops are tolerant to normal rates of the insecticide.
Occasionally slight damage was noticed, in particular in connection
with high dosages.
The withholding periods range from 14 to 28 days, depending on the
local conditions and crop.
Other uses
Bromophos-ethyl is used in the sector of animal health and tick
control. The compound is active against ticks, including strains of
the genus Boophilus which are resistant to chlorinated hydrocarbons
and other phosphorus preparations, as well as against blowflies, lice
and other ectoparasites. It also controls mosquitoes, mosquito larvae
and ants, revealing a remarkable residual effect. Bromophos-ethyl is
used particularly as a mosquito larvicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables and field crops (Boehringer, 1971-1972). Summaries
of much of this information have recently been published (Eichler
1971). The following tables 2 and 3 present a summary of available
data along with relevant information on rates of application, number
of applications and preharvest interval.
In trials in the Netherlands (Anonymous, 1970), bromophos-ethyl was
applied to carrots at 6 kg a.i./ha either by soil surface treatment
with granules prior to seeding or by foliar treatment with wettable
powder or emulsifiable concentrate 81 and 89 days after planting. The
residues obtained are shown in table 4.
Distribution of residues in 1-year-old beef cattle was determined in
Germany (Boehringer, 1966) after spraying to runoff 1 or 4 times with
bromophos-ethyl emulsion (0.05% a.i.). The results are summarized in
table 5.
In later trials (Boehringer, 1968), 6 cows were sprayed three times at
5-day intervals (14 days is more usual) with 0.1% a.i. emulsion (twice
the concentration generally used). Table 6 summarizes these results.
Two experimental trials on residues in beef cattle were conducted in
Australia. In the first of these, two groups of 9 cattle were sprayed
with bromophos-ethyl at a concentration of 0.05% (Snelson, 1968).
Group 1 was treated once and Group 2 had three treatments at 5-day
intervals. Residues were determined in heart, kidney, omental and
subcutaneous fat with the results shown in Table 7.
The highest residues were in samples of heart and kidney fat. In the
second trial, six steers were sprayed each with 2 gallons of 0.05%
bromophos-ethyl and samples were taken for analysis at 4, 7 and 21
days after exposure (Harvey, 1968). Residues were distributed as shown
in Table 8.
Dipping leads to higher uptake and significantly higher residues in
fat than does spraying with the same concentration of bromophos-ethyl.
In a series of dipping trials under the direction of the Board of Tick
Control in Australia, 12 cattle were dipped once in 0.05%
bromophos-ethyl emulsion and groups of four were slaughtered 1, 4 and
8 days post treatment and 12 cattle were dipped three times at
intervals of 5 days in the same bath and groups of four were
slaughtered, as before, 1, 4 and 8 days after the third dipping
(Snelson, 1972). Results are given in Table 9.
Further trials showed that the uptake varied considerably depending on
the condition of the animals (fat versus thin), length of hair and
time of year. It was demonstrated that the half-life of
bromophos-ethyl residues in internal fat of cattle is 10 to 12 days.
Table 2 Bromophos-ethyl residues in fruit1
Crop Application Days between last treatment and harvest
No. Rate a.i. 0 1 2 3 4 5 7 8 10 14 15 21 22 28 30
(g/plant)
Apple 1 1.8 2.20 1.40 1.95 1.35
1 0.9 1.25 0.7 1.03 1.20 0.76 0.55
1 0.9 0.58 0.36 0.33 0.30 0.28
1 1.8 1.17 1.13 0.81 1.23
5 1.2 3.45 2.65 2.05 1.95
6 1.5 4.90 3.70 2.50 2.30
Pear 1 0.36 0.59 0.32 0.32 0.26 0.15
Peach 1 0.5 0.38 0.39
Yellow plum 1 0.9 1.40 0.81
Plum 1 1.8 2.08 1.14 1.35 1.11
3 1.8 2.28 1.24 0.73 0.51
1 0.54 0.36 0.34 0.14 0.13 0.12
Sweet Cherry 1 1.8 0.87 0.45 0.27 0.07 0.03
1 1.8 0.21 0.17 0.09
1 1.8 0.89 0.27 0.16 0.14 0.03
Strawberry 1 2882 0.36 0.07
Gooseberry 1 0.36 0.33 0.52 0.26 0.31 0.30
Red-currant 1 3602 1.18 0.95
Black-currant 1 0.36 0.78 0.12
1 residue figures given in ppm
2 g/ha
Table 3a Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 0 1 3 4 5 7 14 21 28 30
(g/ha a.i.)
Garden
lettuce 1 108 0.31 0.28 0.18
1 216 2.35 1.09 1.03 0.37 0.16 0.10
1 108 1.05 0.45 0.09
1 108 0.31 0.28 0.18
Spinach 1 216 2.72 1.60 0.42 0.03
1 216 6.84 4.42 2.22
1 288 8.32 6.66 3.26 2.78 0.44
Carrot 2 0.182
1 0.103
2 0.182
1 0.183
Cauliflower 2 28.84 <0.02
Kohlrabi 2 324
White cabbage 1 360 <0.08 <0.08
1 216 0.20 0.16 0.07 0.04 0.02 <0.02
Brussels
sprouts 3 540 0.57 0.51
Sugar beet 1 144
Onion 2 0.23
2 0.23
2 0.23
Tubercelery
tuber 3 160 0.19-
0.36
leaf 3 160 2.67-
4.09
Rape seed 1 216
Rape oil 1 216
Horsebean 1 600 <0.02
Kidney bean 2 324 0.18 0.07 0.03 0.02 <0.02
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
Table 3b Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 31 35 42 49 54 56 62 64 66 71 107
(g/ha a.i.
Garden
lettuce 1 108
1 216
1 108
1 108
Spinach 1 216 0.02
1 216
1 288
Carrot 2 0.182 1.21
1 0.103 0.40
2 0.182 1.53
1 0.183 1.42
Cauliflower 2 28.84
Kohlrabi 2 324 0.05
White
cabbage 1 360
1 216
Brussels
sprouts 3 540
Sugar beet 1 144 <0.02
Onion 2 0.23 <0.02
2 0.23 <0.02
2 0.23 <0.02
Tubercelery
tuber 3 160
leaf 3 160
Rape seed 1 216 0.08
Rape oil 1 216 0.33
Horsebean 1 600
Kidney bean 2 324
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
TABLE 4 Bromophos-ethyl residues in carrots
Crop Method of Rate of Time between Residues
application application last treatment (ppm)
(kg/ha/a.i.) and harvest
(months)
Carrots soil surface 6 3 0.22 - 0.24
soil surface 6 3 0.17 - 0.77
foliar spray 6 2 0.49 - 1.5
foliar spray 6 2 0.84 - 1.6
TABLE 5 Distribution of bromophos-ethyl residues in cattle tissue, 1966
Treatment Sample interval Residues (ppm)1
Animal (0.05% (days after Back Leg
a.i.) last treatment) muscle muscle Brain Kidney Liver Fat
12 1 spray 1 0.77 0.90 0.76 <0.13 <0.14 n.d.
2 4 sprays 3 n.d. <0.18NS n.d. n.d. <0.15NS n.d.
3 4 sprays 1 <0.18NS n.d. n.d. n.d. n.d. 0.67*
4 4 sprays 11 n.d. n.d. n.d. n.d. n.d. <0.20NS
5 Control -- -- 0.056 -- 0.049 0.052 0.053 0.076
1 n.d. = not detected; NS = not statistically significant; * = statistically significant.
2 High probability that samples were contaminated during taking and handling; data not usable.
TABLE 6 Distribution of bromophos-ethyl residues in cattle tissue, 1968
Sample interval Residues (ppm)1,2
Animal (days after Fillet Roasting Kidney Liver Kidney Subcutaneous
last treatment) beef fat fat
924 1 0.015 0.015 0.010 <0.005 0.132 0.056
621 1 0.017 0.006 0.006 <0.005 0.193 0.065
110 3 0.010 0.008 0.007 <0.005 0.236 n.a.
102 3 0.007 0.007 0.010 <0.005 0.219 n.a.
605 7 <0.005 <0.005 <0.005 <0.005 0.218 n.a.
228 7 <0.005 0.010 <0.005 <0.005 0.183 n.a.
1 n.a. = not analyzed.
2 Determination by gas chromatography, mean of three determinations.
Bromophos-ethyl residues in milk from three dairy cows each sprayed
with two gallons of 0.05% active ingredient are shown in Table 10
(Snelson, 1968).
Dipping of dairy cows likewise gives rise to significant residues in
milk which are all transferred to the butterfat. The level of residues
in the milk of dipped cows was only slightly higher than those
reported in Table 10 (Snelson, 1972). The residue level reached a peak
on the first and second days after dipping and thereafter declined
rapidly, with a half-life calculated to be between 1-2 and 1-4 days.
TABLE 7 Bromophos-ethyl residues in fat of beef cattle, trial 1
Post-treatment day Group 1, Group 2,
1 treatment (ppm) 3 treatments (ppm)
1 0.08 - 2.9 0.3 - 2.6
4 0.1 - 2.0 0.1 - 1.6
8 Nil - 0.7 Nil - 0.6
TABLE 8 Bromophos-ethyl residues in fat of steers, trial 2
Interval between Residues (ppm)
treatment and Liver Kidney Muscle Omental Perirenal
sampling (days) fat fat
4 <0.05 <0.05 <0.05 0.82 0.63
<0.05 <0.05 <0.05 0.62 0.36
7 <0.05 <0.05 <0.05 0.70 0.63
<0.05 <0.05 <0.05 0.21 0.45
21 <0.05 <0.05 <0.05 0.24 0.31
<0.05 <0.05 <0.05 0.40 0.29
TABLE 9 Bromophos-ethyl residues in cattle after dipping
Interval
between Residues bromophos-ethyl (ppm)
last dipping dipped once dipped 3 times
and slaughter Internal Subcutaneous Internal Subcutaneous
(days) fat fat fat fat
1 0.15 - 0.23 0.13 - 0.26 1.47 - 3.36 1.2 - 2.32
4 0.21 - 0.37 0.15 - 0.27 1.38 - 1.83 0.5 - 2.05
8 0.36 - 0.87 0.23 - 0.50 2.04 - 2.48 0.78 - 2.56
TABLE 10 Bromophos-ethyl residues in milk
Time after Residues (ppm)
Treatment treatment in butterfat in milk
(h)
Nexagan 0.05% 5 0.03 0.002
(single
application) 21 1.01 0.072
29 0.71,0.67,0.42 0.038,0.026,0.017
45 1.17,0.48,0.60 0.060,0.028,0.026
53 0.79,0.60,0.47 0.042,0.028,0.021
69 0.47 0.017
77 0.63 0.038
93 0.25 0.016
10 days 0.02 0.001
In trials in Germany (Boehringer, 1968), three cows were each sprayed
with 5 liters of 0.05%. a.i. suspension and milk samples were taken
during the following four days. A second treatment was performed seven
days later without sampling. A third treatment followed 14 days after
the start of the trial, and subsequent milkings were analyzed for a
total time of 21 days. Maximum residues (0.098, 0.077, 0.089 ppm) were
found about 24 hours after treatment, decreasing within about four
days below the limit of determination (<0.02 ppm). There was no
accumulation of residues after the third treatment.
FATE OF RESIDUES
General comments
By analogy with bromophos, the major metabolites of bromophos-ethyl
would be expected to be 2,5-dichloro-4-bromophenol, bromoxon-ethyl,
and desethyl bromophos-ethyl (see Figure 1 of preceding bromophos
monograph). The major points of difference appear to be that no
desethyl-bromophos-ethyl is found after oral administration to rats
and that somewhat higher ratios of bromoxon-ethyl to bromophos-ethyl
is found in plants, especially lettuce (Eichler, 1971).
In animals
The metabolism and excretion of 3H-labelled bromophos-ethyl in the
rat was studied by Stiasni (1967). No specific organic accumulation
occurred following oral and parenteral administration. Quantitative
excretion occurred between 8 and 12 days. Neither unchanged
bromophos-ethyl nor bromoxon-ethyl could be found in the excrements
following oral administration. Desethyl-bromophos-ethyl was also
missing among the metabolites. The only metabolites identified were
dichloro-bromophenol and its conjugates.
In plants
No tests have been carried out with radioactively labelled
bromophos-ethyl in plants. However, in connection with supervised
trials for determining residues in fruits and vegetables,
bromoxon-ethyl residues were determined in apples, Brussels sprouts
and lettuce (Eichler, 1971). In apples, the bromoxon-ethyl residues
never exceeded 0.005 ppm (<1% of bromophos-ethyl residue). In
Brussels sprouts, the highest bromoxon-ethyl residue found was 0.026
ppm (5% of bromophos-ethyl residue). In lettuce, after several
sprayings, bromoxon-ethyl residues reached a maximum of 0.07 ppm (6.4%
of bromophos-ethyl residue) in three days. The highest ratio found was
8.8% (0.014 ppm) after 14 days.
In soil
Bromophos-ethyl E.C. was applied one time at 0.5 g a.i./m2 to high
moorland soil (acid, high organic content), Ingelheim sand and clay
soil. Zero to 20 cm-deep samples were taken periodically for 26 weeks
and analyzed with the results shown in Table 11 (Eichler, 1970).
In comparison with bromophos, bromophos-ethyl degrades somewhat more
slowly in soil; however, neither compound shows any significant
persistence.
In storage and processing
Leber and Deckers (1968) examined the effects on residues in beef
under storage at +4° and -18°C and after cooking (roasting). No
significant decrease in residues was observed in meat, kidney and
kidney fat after a 7-day storage period at +4°C or after a 30-day
storage period at -18°C. Neither 15 minutes of frying of 100 g steaks
nor one hour of boiling of 100 g of beef in 200 ml of water resulted
in any significant decrease in residues of bromophos-ethyl.
Evidence of residues in food in commerce or at consumption
In connection with a tick eradication program in Australia, perirenal
fat from 17 cattle known to have been dipped in 0.05% Nexagan on 27
occasions over a period of 14 months was analyzed for residues
(Harvey, 1968). Three samples had residues between 0.5 and 1.0 ppm,
five between 1.0 and 1.5 ppm, seven between 1.5 and 2.0 ppm and two
samples had 2.18 and 2.68 ppm, respectively.
TABLE 11 Bromophos-ethyl residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-
soil time (weeks) Bromophos-ethyl bromophenol
High moorland 0 13.302 <0.10
1 6.26 <0.10
3 4.95 0.54
6 3.92 <0.10
9 3.29 0.60
13 3.44 1.30
26 1.25 <0.10
Sandy 0 2.343 <0.10
1 1.40 <0.10
3 0.64 0.13
6 0.40 <0.10
9 0.16 <0.10
13 0.12 <0.10
26 <0.02 <0.10
Clay 0 2.764 <0.10
1 2.16 <0.10
3 0.44 0.17
6 0.32 <0.10
9 0.26 <0.10
13 0.14 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 0.844-0.032 log X; r = 0.9173
3 Y = 0.128-0.076 log X; r = 0.9721
4 Y = 0.175-0.076 log X; r = 0.9544
Samples of internal fat taken from animals, with unknown treatment
history, being slaughtered in the cattle tick zone in Australia during
1971/72 revealed some to contain bromophos-ethyl residues. Of 1 055
samples examined, 52 contained bromophos-ethyl, mostly below 0.5 ppm,
the highest being 0.8 ppm (Snelson, 1972). During the same period 589
samples of butter and cheese were examined for organo-phosphorus
residues. Only three samples were found to contain bromophos-ethyl
residues, all at 0.1 ppm.
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos-ethyl. These
have recently been reviewed and summarized by Eichler (1971). A method
has been developed by Leber and Deckers (1968) for all crops and
animal tissues. It utilizes gas chromatography with a phosphorus
specific detector and can estimate quantities down to the range of
0.001 to 0.01 ppm with a 100 g sample. The method includes procedures
for determining bromoxon-ethyl either by colorimetry or by gas
chromatography with a sensitivity of 0.03 to 0.05 ppm. Confirmation of
residues can be accomplished by the thin-layer chromatographic
techniques suggested in the method.
Bromophos-ethyl is among the pesticides listed as being detectable by
the multi-residue gas chromatographic procedure of Abbott et al.
(1970), and that method is the most suitable for regulatory use. The
method of Leber and Deckers would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Examples of national tolerances of bromophos-ethyl residues are
reported in Table 12.
TABLE 12 Examples of national tolerances as reported to meeting
Country Commodity Tolerance
Australia Fat of meat of cattle
and sheep 3 ppm
Milk and milk products
(fat basis) 1 ppm
The Netherlands Fruit and vegetables 0.4 ppm
APPRAISAL
Bromophos-ethyl is a non-systemic halogen-containing organo-phosphorus
insecticide and acaricide used on fruit, vegetables, field crops,
cereals, maize, rice, cotton and tobacco. It is also used extensively
in tick control, for other ectoparasites of domestic animals and as a
mosquito larvicide.
Supervised trials with foliar treatments on fruits and vegetables have
shown that bromophos-ethyl is somewhat more persistent than bromophos
and requires a pre-harvest interval about twice as long. The rate of
residue decline is highly dependent on many factors, especially
botanical species and morphological structure, which require that
tolerance recommendations be made on an individual commodity basis
rather than on broad crop categories.
Groups of beef cattle were sprayed at recommended rates one or more
times in trials in several countries. Highest residues were found in
omental, heart and perirenal fat, ranging in one trial from 0.1 to 2.0
ppm at four days post-treatment.
Spray treatment of dairy cows at recommended rates resulted in a
residue maximum of 1.17 ppm in butterfat at 45 hours post-treatment.
This fell to 0.02 ppm in butterfat after ten days. There was no
accumulation of milk residues from the multiple treatments.
The metabolites of bromophos-ethyl most likely to be found are
2,5-dichloro-4-bromophenol and bromoxon-ethyl. Only
dichloro-bromophenol and its conjugates were found in the excrement of
rats after oral administration of labelled bromophos-ethyl. The
dichloro-bromophenol also appears to be the only soil metabolite.
Although no studies have been conducted with the labelled compound in
plants, supervised field trials have shown small amounts of
bromoxon-ethyl residues in apples, Brussels sprouts and lettuce (up to
0.07 ppm in the latter case).
Available multi-residue gas chromatographic procedures are suitable
for use for regulatory purposes and are recommended.
Although bromophos-ethyl is recommended for cereals, maize, cotton,
rice and in animal health, there were no data available for these
commodities except fat of meat of cattle and milk. Therefore, no
recommendations could be made for tolerances on these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are recommended for
bromophos-ethyl.
ppm
Apples, carrots, fat of meat of cattle,
plums, pears, spinach 2
Brussels sprouts, redcurrants 1
Celeriac, gooseberries, peaches,
rape seed oil, cherries (sweet) 0.5
Blackcurrants, lettuce 0.2
Rape seed, strawberries, cabbage 0.1
Kohlrabi, French beans 0.05
Cauliflower, beans (without pods),
onions, sugarbeets, milk (whole) 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1975)
Further studies evaluating the significance of urinary excretion of
ascorbic and dehydroascorbic acids and its relevance to man.
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials on maize, rice and other cereals,
cotton, domestic animals, other than cattle, and milk products.
DESIRABLE
A study to determine dose levels causing no carboxyl-esterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1967.
III. Organo-phosphorus pesticide residues in the total diet. Pestic.
Sci. 1: 10-13.
Anonymous. (1970) Utrecht report, project CvF/PD4.2.(2.2.03).
Barnes, J.M. (1968) WHO insecticide evaluation and testing programme.
Stage I - mammalian toxicity report. Medical Research Council,
Carshalton. (unpublished).
Battelle Institute. (1964) Toxicity tests with the substance S2225 in
rats, guinea pigs and rabbits. (unpublished)
Boehringer. (1966) Residue trials and examination of cholinesterase
activity after spraying with bromophos-ethyl on cattle. Report C.H.
Boehringer Sohn.
Boehringer. (1967) Investigations concerning absorption, distribution,
excretion and metabolism of bromophos-ethyl-3H in rats. Report C.H.
Boehringer Sohn. (unpublished)
Boehringer. (1968) Determination of residues of bromophos-ethyl in
cattle. Report C.H. Boehringer Sohn.
Boehringer. (1971-1972) Residue investigation reports, C.H. Boehringer
Sohn.
Bradford, H.A. (1967) Acute dermal toxicity - bromophos-ethyl
(compound 70625). Report Eli Lilly and Company. (unpublished)
Eichler, D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
Eichler, D. (1970) Report C.H. Boehringer Sohn "Uber den Abban von
Bromophos und Bromophos-ethyl in verschiedenen Böden".
Harvey, J.M. (1968) Bromophos-ethyl residues in meat products.
Communication with C.H. Boehringer Sohn.
Leber G. and Deckers, W. (1968) Determination of residues of bromophos
and bromophos-ethyl. Proc. Brit. Insecticide Fungicide Conf.,
Brighton, Eng. 4: 570.
Leuschner, F. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Standinger, H.J. (1968) About the short-term toxicity studies on
bromophos-ethyl - charge 1487 - in Beagle dogs (with special attention
to the toxicology of the adrenal gland). Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Dontenwill, W. (1969a) About the chronic toxicity of bromophos-ethyl
in Wistar rats following oral application. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969b)
About the chronic tolerance of bromophos-ethyl in the reproduction
test over three generations of Wistar rats. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Standinger, H.J., Schwerdtfeger, W. and
Dontenwill, W. (1971) Two years oral toxicity study in Beagle dogs
with bromophos-ethyl. Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Report on Shg 2225. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1969) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1970) Reports C.H. Boehringer Sohn. (unpublished)
Snelson, J.T. (1968) Bromophos-ethyl residues in milk. Communication
with C.H. Boehringer Sohn.
Snelson, J.T. (1967 - 1968) Residue studies - bromophos-ethyl.
Communications with C.H. Boehringer Sohn.
Snelson, J.T. (1972) Results from experimental investigations and
residue surveys with bromophos-ethyl following dipping in Australia.
Report to Joint Meeting.
Stiasni, M. (1967) Data from C.H. Boehringer Sohn. (unpublished)
Stötzer, H., Herbst, M., Baumgartner, R. and Guęnard, J. (1970)
Subacute dermal toxicity of the substance bromophos-ethyl in rabbits
(New Zealand White). Report C.H. Boehringer Sohn. (unpublished)
Terblanche. (1966) Toxicity of bromophos-ethyl. Department of
Agricultural Technical Services, Onderstepoort, South Africa.
(unpublished)
Vuuren, P.J.J. (1964) Whale-blood cholinesterase levels in cattle
sprayed at weekly intervals with S2225 (CELA). Agricura Laboratoria
Ltd., Silverton, South Africa. (unpublished)
Other information on identity and properties
Molecular weight: 394.0
State: yellow fluid
Density: d20 = 1.53
20
Boiling point: 122-123°C at 10-3 torr.
Vapour pressure: 4.6 x 10-5 mm Hg at 30°C
Solubility: at room temperature miscible with most organic
solvents; practically insoluble in water.
Stability: stable in aqueous suspension. Saponification only
occurs in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-diethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-diethylphosphorothioate:
approx. 1.0% (chlorine position not defined)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies using bromophos-ethyl labelled with 3H in the benzene ring
demonstrated that, in the rat, the substance is absorbed following
oral administration but accumulation generally or in specific organs
does not occur. Apart from the G.I. tract, only the liver, kidney,
spleen and brain showed any substantial activity after 4 hours, and
this declined sharply in 24 hours. Almost total excretion of activity
occurred within 4 days following 5 daily oral doses of approximately 8
mg/kg. The distribution pattern was essentially the same after
intraperitoneal injection, but excretion was slower due to its slow
release from peritoneal and mesenteric fat.
Following a single 8 mg/kg oral dose of 3H-labelled bromophos-ethyl,
40% of the activity appeared in urine and 60% in faeces. When
administered intraperitoneally or subcutaneously, the major portion
also occurred in faeces. Radioactivity was detected in bile 40 minutes
after administration into the duodenum of 3H-labelled material and
40% was excreted by this route in 9 hours. The identity of the
substance in bile has not been established (Boehringer, 1967).
Biotransformation
No bromophos-ethyl, the oxidation product bromoxon-ethyl or
desethyl-bromophos-ethyl, which would be formed by cleavage of the
C2H5-O-P bond, were found in the urine or faeces of rats after oral
dosage. The metabolite dichloro-bromophenol and its conjugates were
identified in urine and faeces and accounted for 85-90% of
administered product, showing that the principal metabolic route
involved cleavage of the phenyl-O-P bond (Boehringer, 1967).
Effects on enzymes and other biochemical parameters
A single dose of 42 mg/kg of bromophos-ethyl to rats caused maximum
inhibition of cholinesterase in plasma and RBC after 8 and 2 hours
respectively. Values showing less than 20% inhibition occurred after
16 and 48 hours respectively (Muacevic, 1968). A single dose of 15
mg/kg to sheep inhibited RBC cholinesterase after 24 hours. A normal
level was found 24 hours later. A dose of 10 mg/kg was without effect
(Terblanche, 1966). In short-term (Muacevic, 1966; Leuschner, 1966,
1967) and long-term studies (Leuschner et al., 1969a, 1969b) in rats
and in short-term studies in dogs (Leuschner, 1966, 1967; Leuschner
et al., 1968) and cattle (Vuuren, 1964; Muacevic, 1966), inhibition
of plasma, RBC, brain and liver cholinesterase was noted. In general,
plasma cholinesterase was the most sensitive of the enzymes.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Bromophos-ethyl, 600 mg/kg (the assumed LD50) was administered orally
to 10 one-year-old hens; they were treated with atropine and
toxogonin. No neurological abnormalities were seen in the 6-week
observation period (Muacevic, 1968).
Three groups of 10 one-year-old hens were administered orally 0, 1.25,
or 2.5 mg/kg/day of bromophos-ethyl on 6 days a week for one month.
Only one bird at the top dosage level showed general weakness;
neuropathological examination revealed no changes in this or other
birds examined (Muacevic, 1968).
Special studies on pharmacology
Atropine sulphate and toxigonin were shown to protect rats and mice
from a lethal dose of bromophos-ethyl. Norscopolamine also protected,
but salicylaldoxime showed no positive effect (Muacevic, 1967; 1968).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0,
1.25, 3.0 and 7.2 mg/kg/day approximately of bromophos-ethyl. Three
generations were examined, the parent F1 and F2 generation each
producing two litters, and mating taking place between week 9 and 15
and between week 21 and 16. No abnormalities were seen in the
behaviour, appearance, body-weight, haematological indices, or in
organs during pathological examinations in adult animals. No effect on
reproduction was found in the two lower dosage groups, but in the 7.2
mg/kg group, which showed significant inhibition of liver, brain and
plasma cholinesterase, the fertility rate and litter size were
decreased and the number of stillbirths increased. The young also
tended to have a lower body-weight than controls. However no runts or
animals with congenital malformations were discovered (Leuschner
et al., 1969b).
Special studies on teratogenicity
Groups of 20 pregnant female rats were administered 0, 0.03, 1.7, 3.5,
7.0 or 14.0 mg/kg body-weight/day of bromophos-ethyl by gastric
intubation from day 6 to 15 of gestation. Animals were killed at day
20 and uteri and foetuses examined. No significant differences from
controls were found in the number of foetuses or reabsorption sites or
in foetus weights. No malformations, runts or dead foetuses were found
in any of the treated groups (Leuschner, 1967).
Acute toxicity
The acute toxicity of technical bromophos-ethyl has been studied in
various animal species. A summary of the results of these studies is
given in Table 1.
TABLE 1 Acute toxicity of technical bromophos-ethyl in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 210-550 Barnes, 1968; Muacevic, 1964,
1967
Rat oral 52-127 Barnes, 1968; Muacevic, 1964,
1966; Leuschner, 1966, 1967
Rabbit oral 28 Muacevic, 1970
Rabbit dermal 100-600 Bradford, 1967; Muacevic, 1967
Dog oral 335(approx.) Muacevic, 1970
Quail oral 200 Muacevic, 1970
Hen oral 600(approx.) Muacevic, 1969
Potentiation of acute toxicity
Potentiation of the acute toxicity of bromophos-ethyl has been
demonstrated to occur in the rat with bromophos, chlorfenvinphos,
coumaphos, malathion, mevinphos and parathion-methyl, and in the mouse
with chlorfenvinphos, diazinon, dimethoate, ethion and malathion.
Slight potentiation with parathion was found in both species
(Muacevic, 1966, 1967, 1968).
Short-term studies
Rat
Groups of 6 male and 6 female rats were fed for 4 weeks on diets
providing 0, 1 and 10 mg/kg/day of bromophos-ethyl. No significant
changes in behaviour, growth rate, food intake or macroscopic
appearance of organs could be observed (Leuschner, 1966).
Groups of 16 male rats were administered daily by gavage 0, 1, 5, 20
or 60 mg/kg body-weight of bromophos-ethyl suspension. The highest
dose was discontinued after 2 days since 9 animals had died. In the
remaining groups no abnormalities in behaviour, food consumption,
growth rate, haematological indices or liver function were found. No
pathological changes in organs were found at autopsy, and the livers,
kidneys and lungs were microscopically normal (Boehringer, 1964).
Groups of 42 male and 42 female rats were administered diets providing
0, 0.78, 1.25, 3.0 and 7.2 mg/kg body-weight of bromophos-ethyl/day.
During the 16-week period of administration no abnormalities in
behaviour, body-weight, food consumption or blood counts were
detected. Necropsy of 5 animals of each group revealed no macroscopic
abnormalities attributable to treatment. Dosage levels of 1.25 mg/kg
upwards caused a marked increase in the quantities of ascorbic and
dehydroascorbic acids excreted in the urine (Leuschner, 1967).
Dog
Three groups of 2 male and 2 female mongrel dogs were administered
daily in capsules 0, 1.6 or 12.5 mg/kg body-weight of bromophos-ethyl
for 2 weeks. The highest dose level was increased to 25 mg/kg for a
further week, and during this period these animals became quiescent,
lost weight and developed diarrhoea. The composition of urine and
blood counts were normal in all animals. The two lower dosage levels
were without untoward effect (Leuschner, 1966).
Four groups of 2 male and 2 female beagle dogs received daily capsules
containing 0, 0.52, 1.25 or 3.0 mg bromophos-ethyl for 16 weeks. No
abnormalities in behaviour, food consumption, body-weight,
haematological indices, the activity of serum enzymes, chemical
constituents of blood or urine constituents were found in treated
animals. No pathological changes were found in the eye, and organ
weight changes did not occur. The 1.25 mg/kg group excreted an
increased amount of ascorbic and dehydroascorbic acids in their urine
and three times the normal amount was excreted by the 3.0 mg/kg group.
The adrenal was the only organ in which changes occurred which could
be attributed to treatment. Narrowing of the fascicular zone and
dispersal of the glomerulose zone, the cells showing a high lipoid
content, and fibrous dispersal of the capsule were described, the
degree of change being most marked in the 3.0 mg/kg group (Leuschner,
1967).
Six groups of beagle dogs were administered each day, in capsules,
sugar (control), coumaphos or bromophos-ethyl as follows: sugar, 1 g;
coumaphos, 3.0 mg/kg; bromophos-ethyl, 0.26 mg/kg and 0.39 mg/kg (3
male and 3 female dogs), 1.25 mg/kg (5 males and 5 females), 3.0 mg/kg
(6 males and 6 females). The administration continued for 18 weeks
after which a number of animals of each group received no treatment
for four weeks. Depression of plasma cholinesterase activity occurred
at the 1.25 mg/kg dosage level, but no definite decrease in enzyme
activity occurred at any dosage level in the RBC, brain or adrenal
gland. Caumaphos, however, inhibited RBC and brain enzyme at the 3.0
mg/kg/day level. Four weeks after treatment ceased all cholinesterase
levels had returned to normal. Urinary ascorbic and dehydroascorbic
acids were increased slightly in the 0.39 mg/kg group and the increase
was marked with higher dosage levels of bromophos-ethyl and with
coumaphos. The values also returned to normal when administration
ceased. Histological examination showed that in bromophos-ethyl
treated animals only the 3.0 mg/kg group showed a moderate broadening
of the fasciculate zone of the adrenal and a relative increase in
eosinophilic staining in the frontal lobe of the pituitary. The
appearance of the adrenals and pituitary were normal after treatment
had ceased for four weeks (Leuschner et al., 1968).
Five groups of 4 male and 4 female beagle dogs were fed for 2 years on
diets containing 0, 10, 20, 30 and 120 ppm bromophos-ethyl. No
influence was seen on behaviour, food intake or growth of treated
animals. Analysis of blood for chemical constituents, including
cortisol, and of urine failed to demonstrate abnormalities. All serum
enzymes, except cholinesterase, were of normal activity. The weight
and macro- and microscopic appearance of organs, including the adrenal
and pituitary glands and bone marrow, were normal when animals were
killed during and at the end of the test. The dosage threshold at
which inhibition of cholinesterase occurred was considered to be
between 10 and 20 ppm for plasma enzyme and above 120 ppm for RBC and
brain enzymes. Urine ascorbic and dehydroascorbic acid levels were
increased in the 30 and 120 ppm groups. Excretion was maximum at
between 6 and 9 months, and then it decreased. No untoward effects
could be detected in the 10 ppm group (Leuschner et al., 1971).
Rabbit
Groups of 2 male and 2 female rabbits were administered 0, 2.5, 12.5
and 62.5 mg/kg/day of bromophos-ethyl on scarified or intact skin in
the form of an ointment for 6 hours each day for 21 days. They were
observed for a further 14 days before necropsy. One male of the
highest dosage group died and body-weight gain was retarded in the two
highest groups. Varying degrees of liver necrosis, myocardial scarring
and renal changes were found at the highest dosage level but not at
lower levels (Stötzer et al., 1970).
Long-term studies
Rat
Groups of 42 male and 42 female rats, each weighing from 166 to 214 g,
were fed diets providing 0.78, 1.25, 3.0 or 7.2 mg/kg body-weight/day
of bromophos-ethyl for 2 years. The threshold levels for a 20%
inhibition of activity lay between 0.78 and 1.25 mg/kg for plasma and
3.0 and 7.2 mg/kg for erythrocyte, brain and liver cholinesterase.
Urinary ascorbic and dehydroascorbic acid excretion was elevated at
all dosage levels; a 78% increase was seen at the 0.78 mg/kg level.
With increasing age, the amounts excreted became less marked but were
still significantly high at 2 years in the 3.0 and 7.2 mg/kg groups.
The mean body-weights of rats of both sexes on 3.0 and 7.2 mg/kg
bromophos-ethyl were slightly elevated above that of the controls
throughout much of the test. Urine analysis showed that the incidence
of occurrence of erythrocytes, protein, ketone bodies and white blood
cells was reduced in the 3.0 and 7.2 mg/kg groups compared with
controls. No significant differences from controls were seen with
regard to behaviour, survival, food intake, and the results of
haematological investigations or with regard to gross or microscopic
changes in organs or tumour incidence and type (Leuschner et al.,
1969a).
COMMENT
Bromophos-ethyl is absorbed from the gastro-intestinal tract and
excreted in urine and faeces principally as dichloro-bromophenol and
its conjugates. Accumulation does not occur following oral ingestion.
Bromophos-ethyl inhibits cholinesterase, the plasma enzyme being the
most sensitive. Acute potentiation was observed in combination with
several other organo-phosphates in rats and mice. In 2-year studies in
dogs and rats the no-effect levels for plasma cholinesterase
inhibition were 0.4 and 0.78 mg/kg body-weight/day, respectively.
Bromophos-ethyl does not cause delayed neurological injury. Studies in
rats did not indicate ill effects on reproduction or teratogenic
activity.
In studies in rats and dogs urinary excretion of ascorbic and
dehydroascorbic acids was increased. The no-effect level in dogs was
0.26 mg/kg/day. A no-effect level was not demonstrated for rats.
Investigations to find the cause of the increase were not reported.
The long-term study in rats showed that, at the dosage levels
employed, bromophos-ethyl had no carcinogenic activity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Dog: 0.26 mg/kg body-weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos-ethyl is an organo-phosphorus insecticide and acaricide with
a broad spectrum of activity and moderate mammalian toxicity. It acts
as a contact and stomach poison. It is available as an emulsifiable
concentrate, ULV concentrate, wettable powder, granular or dust
formulation as well as dip formulations. Bromophos-ethyl is compatible
with insecticides and fungicides.
This substance is of moderate toxicity to bees and should not be
sprayed on flowering crops during the flight of bees.
Bromophos-ethyl has been used in Argentina, Australia, Austria,
Belgium, Brazil, Colombia, the Federal Republic of Germany, the
Netherlands, New Zealand, Nicaragua, Pakistan, South Africa and
Venezuela.
Pre-harvest treatments
Bromophos-ethyl is applied on various crops, mainly fruits, field
crops, vegetables, cereals, maize, rice, cotton and tobacco, and is
effective against a large number of chewing and sucking insect pests,
especially caterpillars, vegetable root maggots, fruit flies, bean
fly, aphids and beetles.
Depending on the different crops and the main pest species present the
recommended concentrations of spray wash vary between 0.02% and 0.1%
a.i. and the rates of application between 0.2 and 1.2 kg a.i./ha. The
majority of crops are tolerant to normal rates of the insecticide.
Occasionally slight damage was noticed, in particular in connection
with high dosages.
The withholding periods range from 14 to 28 days, depending on the
local conditions and crop.
Other uses
Bromophos-ethyl is used in the sector of animal health and tick
control. The compound is active against ticks, including strains of
the genus Boophilus which are resistant to chlorinated hydrocarbons
and other phosphorus preparations, as well as against blowflies, lice
and other ectoparasites. It also controls mosquitoes, mosquito larvae
and ants, revealing a remarkable residual effect. Bromophos-ethyl is
used particularly as a mosquito larvicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables and field crops (Boehringer, 1971-1972). Summaries
of much of this information have recently been published (Eichler
1971). The following tables 2 and 3 present a summary of available
data along with relevant information on rates of application, number
of applications and preharvest interval.
In trials in the Netherlands (Anonymous, 1970), bromophos-ethyl was
applied to carrots at 6 kg a.i./ha either by soil surface treatment
with granules prior to seeding or by foliar treatment with wettable
powder or emulsifiable concentrate 81 and 89 days after planting. The
residues obtained are shown in table 4.
Distribution of residues in 1-year-old beef cattle was determined in
Germany (Boehringer, 1966) after spraying to runoff 1 or 4 times with
bromophos-ethyl emulsion (0.05% a.i.). The results are summarized in
table 5.
In later trials (Boehringer, 1968), 6 cows were sprayed three times at
5-day intervals (14 days is more usual) with 0.1% a.i. emulsion (twice
the concentration generally used). Table 6 summarizes these results.
Two experimental trials on residues in beef cattle were conducted in
Australia. In the first of these, two groups of 9 cattle were sprayed
with bromophos-ethyl at a concentration of 0.05% (Snelson, 1968).
Group 1 was treated once and Group 2 had three treatments at 5-day
intervals. Residues were determined in heart, kidney, omental and
subcutaneous fat with the results shown in Table 7.
The highest residues were in samples of heart and kidney fat. In the
second trial, six steers were sprayed each with 2 gallons of 0.05%
bromophos-ethyl and samples were taken for analysis at 4, 7 and 21
days after exposure (Harvey, 1968). Residues were distributed as shown
in Table 8.
Dipping leads to higher uptake and significantly higher residues in
fat than does spraying with the same concentration of bromophos-ethyl.
In a series of dipping trials under the direction of the Board of Tick
Control in Australia, 12 cattle were dipped once in 0.05%
bromophos-ethyl emulsion and groups of four were slaughtered 1, 4 and
8 days post treatment and 12 cattle were dipped three times at
intervals of 5 days in the same bath and groups of four were
slaughtered, as before, 1, 4 and 8 days after the third dipping
(Snelson, 1972). Results are given in Table 9.
Further trials showed that the uptake varied considerably depending on
the condition of the animals (fat versus thin), length of hair and
time of year. It was demonstrated that the half-life of
bromophos-ethyl residues in internal fat of cattle is 10 to 12 days.
Table 2 Bromophos-ethyl residues in fruit1
Crop Application Days between last treatment and harvest
No. Rate a.i. 0 1 2 3 4 5 7 8 10 14 15 21 22 28 30
(g/plant)
Apple 1 1.8 2.20 1.40 1.95 1.35
1 0.9 1.25 0.7 1.03 1.20 0.76 0.55
1 0.9 0.58 0.36 0.33 0.30 0.28
1 1.8 1.17 1.13 0.81 1.23
5 1.2 3.45 2.65 2.05 1.95
6 1.5 4.90 3.70 2.50 2.30
Pear 1 0.36 0.59 0.32 0.32 0.26 0.15
Peach 1 0.5 0.38 0.39
Yellow plum 1 0.9 1.40 0.81
Plum 1 1.8 2.08 1.14 1.35 1.11
3 1.8 2.28 1.24 0.73 0.51
1 0.54 0.36 0.34 0.14 0.13 0.12
Sweet Cherry 1 1.8 0.87 0.45 0.27 0.07 0.03
1 1.8 0.21 0.17 0.09
1 1.8 0.89 0.27 0.16 0.14 0.03
Strawberry 1 2882 0.36 0.07
Gooseberry 1 0.36 0.33 0.52 0.26 0.31 0.30
Red-currant 1 3602 1.18 0.95
Black-currant 1 0.36 0.78 0.12
1 residue figures given in ppm
2 g/ha
Table 3a Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 0 1 3 4 5 7 14 21 28 30
(g/ha a.i.)
Garden
lettuce 1 108 0.31 0.28 0.18
1 216 2.35 1.09 1.03 0.37 0.16 0.10
1 108 1.05 0.45 0.09
1 108 0.31 0.28 0.18
Spinach 1 216 2.72 1.60 0.42 0.03
1 216 6.84 4.42 2.22
1 288 8.32 6.66 3.26 2.78 0.44
Carrot 2 0.182
1 0.103
2 0.182
1 0.183
Cauliflower 2 28.84 <0.02
Kohlrabi 2 324
White cabbage 1 360 <0.08 <0.08
1 216 0.20 0.16 0.07 0.04 0.02 <0.02
Brussels
sprouts 3 540 0.57 0.51
Sugar beet 1 144
Onion 2 0.23
2 0.23
2 0.23
Tubercelery
tuber 3 160 0.19-
0.36
leaf 3 160 2.67-
4.09
Rape seed 1 216
Rape oil 1 216
Horsebean 1 600 <0.02
Kidney bean 2 324 0.18 0.07 0.03 0.02 <0.02
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
Table 3b Bromophos-ethyl residues in vegetables and field crops1
Crop Application Days between last treatment and harvest
No. Rate 31 35 42 49 54 56 62 64 66 71 107
(g/ha a.i.
Garden
lettuce 1 108
1 216
1 108
1 108
Spinach 1 216 0.02
1 216
1 288
Carrot 2 0.182 1.21
1 0.103 0.40
2 0.182 1.53
1 0.183 1.42
Cauliflower 2 28.84
Kohlrabi 2 324 0.05
White
cabbage 1 360
1 216
Brussels
sprouts 3 540
Sugar beet 1 144 <0.02
Onion 2 0.23 <0.02
2 0.23 <0.02
2 0.23 <0.02
Tubercelery
tuber 3 160
leaf 3 160
Rape seed 1 216 0.08
Rape oil 1 216 0.33
Horsebean 1 600
Kidney bean 2 324
1 residue figures given in ppm. 2 g/m (2x). 3 g/m. 4 mg/plant.
TABLE 4 Bromophos-ethyl residues in carrots
Crop Method of Rate of Time between Residues
application application last treatment (ppm)
(kg/ha/a.i.) and harvest
(months)
Carrots soil surface 6 3 0.22 - 0.24
soil surface 6 3 0.17 - 0.77
foliar spray 6 2 0.49 - 1.5
foliar spray 6 2 0.84 - 1.6
TABLE 5 Distribution of bromophos-ethyl residues in cattle tissue, 1966
Treatment Sample interval Residues (ppm)1
Animal (0.05% (days after Back Leg
a.i.) last treatment) muscle muscle Brain Kidney Liver Fat
12 1 spray 1 0.77 0.90 0.76 <0.13 <0.14 n.d.
2 4 sprays 3 n.d. <0.18NS n.d. n.d. <0.15NS n.d.
3 4 sprays 1 <0.18NS n.d. n.d. n.d. n.d. 0.67*
4 4 sprays 11 n.d. n.d. n.d. n.d. n.d. <0.20NS
5 Control -- -- 0.056 -- 0.049 0.052 0.053 0.076
1 n.d. = not detected; NS = not statistically significant; * = statistically significant.
2 High probability that samples were contaminated during taking and handling; data not usable.
TABLE 6 Distribution of bromophos-ethyl residues in cattle tissue, 1968
Sample interval Residues (ppm)1,2
Animal (days after Fillet Roasting Kidney Liver Kidney Subcutaneous
last treatment) beef fat fat
924 1 0.015 0.015 0.010 <0.005 0.132 0.056
621 1 0.017 0.006 0.006 <0.005 0.193 0.065
110 3 0.010 0.008 0.007 <0.005 0.236 n.a.
102 3 0.007 0.007 0.010 <0.005 0.219 n.a.
605 7 <0.005 <0.005 <0.005 <0.005 0.218 n.a.
228 7 <0.005 0.010 <0.005 <0.005 0.183 n.a.
1 n.a. = not analyzed.
2 Determination by gas chromatography, mean of three determinations.
Bromophos-ethyl residues in milk from three dairy cows each sprayed
with two gallons of 0.05% active ingredient are shown in Table 10
(Snelson, 1968).
Dipping of dairy cows likewise gives rise to significant residues in
milk which are all transferred to the butterfat. The level of residues
in the milk of dipped cows was only slightly higher than those
reported in Table 10 (Snelson, 1972). The residue level reached a peak
on the first and second days after dipping and thereafter declined
rapidly, with a half-life calculated to be between 1-2 and 1-4 days.
TABLE 7 Bromophos-ethyl residues in fat of beef cattle, trial 1
Post-treatment day Group 1, Group 2,
1 treatment (ppm) 3 treatments (ppm)
1 0.08 - 2.9 0.3 - 2.6
4 0.1 - 2.0 0.1 - 1.6
8 Nil - 0.7 Nil - 0.6
TABLE 8 Bromophos-ethyl residues in fat of steers, trial 2
Interval between Residues (ppm)
treatment and Liver Kidney Muscle Omental Perirenal
sampling (days) fat fat
4 <0.05 <0.05 <0.05 0.82 0.63
<0.05 <0.05 <0.05 0.62 0.36
7 <0.05 <0.05 <0.05 0.70 0.63
<0.05 <0.05 <0.05 0.21 0.45
21 <0.05 <0.05 <0.05 0.24 0.31
<0.05 <0.05 <0.05 0.40 0.29
TABLE 9 Bromophos-ethyl residues in cattle after dipping
Interval
between Residues bromophos-ethyl (ppm)
last dipping dipped once dipped 3 times
and slaughter Internal Subcutaneous Internal Subcutaneous
(days) fat fat fat fat
1 0.15 - 0.23 0.13 - 0.26 1.47 - 3.36 1.2 - 2.32
4 0.21 - 0.37 0.15 - 0.27 1.38 - 1.83 0.5 - 2.05
8 0.36 - 0.87 0.23 - 0.50 2.04 - 2.48 0.78 - 2.56
TABLE 10 Bromophos-ethyl residues in milk
Time after Residues (ppm)
Treatment treatment in butterfat in milk
(h)
Nexagan 0.05% 5 0.03 0.002
(single
application) 21 1.01 0.072
29 0.71,0.67,0.42 0.038,0.026,0.017
45 1.17,0.48,0.60 0.060,0.028,0.026
53 0.79,0.60,0.47 0.042,0.028,0.021
69 0.47 0.017
77 0.63 0.038
93 0.25 0.016
10 days 0.02 0.001
In trials in Germany (Boehringer, 1968), three cows were each sprayed
with 5 liters of 0.05%. a.i. suspension and milk samples were taken
during the following four days. A second treatment was performed seven
days later without sampling. A third treatment followed 14 days after
the start of the trial, and subsequent milkings were analyzed for a
total time of 21 days. Maximum residues (0.098, 0.077, 0.089 ppm) were
found about 24 hours after treatment, decreasing within about four
days below the limit of determination (<0.02 ppm). There was no
accumulation of residues after the third treatment.
FATE OF RESIDUES
General comments
By analogy with bromophos, the major metabolites of bromophos-ethyl
would be expected to be 2,5-dichloro-4-bromophenol, bromoxon-ethyl,
and desethyl bromophos-ethyl (see Figure 1 of preceding bromophos
monograph). The major points of difference appear to be that no
desethyl-bromophos-ethyl is found after oral administration to rats
and that somewhat higher ratios of bromoxon-ethyl to bromophos-ethyl
is found in plants, especially lettuce (Eichler, 1971).
In animals
The metabolism and excretion of 3H-labelled bromophos-ethyl in the
rat was studied by Stiasni (1967). No specific organic accumulation
occurred following oral and parenteral administration. Quantitative
excretion occurred between 8 and 12 days. Neither unchanged
bromophos-ethyl nor bromoxon-ethyl could be found in the excrements
following oral administration. Desethyl-bromophos-ethyl was also
missing among the metabolites. The only metabolites identified were
dichloro-bromophenol and its conjugates.
In plants
No tests have been carried out with radioactively labelled
bromophos-ethyl in plants. However, in connection with supervised
trials for determining residues in fruits and vegetables,
bromoxon-ethyl residues were determined in apples, Brussels sprouts
and lettuce (Eichler, 1971). In apples, the bromoxon-ethyl residues
never exceeded 0.005 ppm (<1% of bromophos-ethyl residue). In
Brussels sprouts, the highest bromoxon-ethyl residue found was 0.026
ppm (5% of bromophos-ethyl residue). In lettuce, after several
sprayings, bromoxon-ethyl residues reached a maximum of 0.07 ppm (6.4%
of bromophos-ethyl residue) in three days. The highest ratio found was
8.8% (0.014 ppm) after 14 days.
In soil
Bromophos-ethyl E.C. was applied one time at 0.5 g a.i./m2 to high
moorland soil (acid, high organic content), Ingelheim sand and clay
soil. Zero to 20 cm-deep samples were taken periodically for 26 weeks
and analyzed with the results shown in Table 11 (Eichler, 1970).
In comparison with bromophos, bromophos-ethyl degrades somewhat more
slowly in soil; however, neither compound shows any significant
persistence.
In storage and processing
Leber and Deckers (1968) examined the effects on residues in beef
under storage at +4° and -18°C and after cooking (roasting). No
significant decrease in residues was observed in meat, kidney and
kidney fat after a 7-day storage period at +4°C or after a 30-day
storage period at -18°C. Neither 15 minutes of frying of 100 g steaks
nor one hour of boiling of 100 g of beef in 200 ml of water resulted
in any significant decrease in residues of bromophos-ethyl.
Evidence of residues in food in commerce or at consumption
In connection with a tick eradication program in Australia, perirenal
fat from 17 cattle known to have been dipped in 0.05% Nexagan on 27
occasions over a period of 14 months was analyzed for residues
(Harvey, 1968). Three samples had residues between 0.5 and 1.0 ppm,
five between 1.0 and 1.5 ppm, seven between 1.5 and 2.0 ppm and two
samples had 2.18 and 2.68 ppm, respectively.
TABLE 11 Bromophos-ethyl residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-
soil time (weeks) Bromophos-ethyl bromophenol
High moorland 0 13.302 <0.10
1 6.26 <0.10
3 4.95 0.54
6 3.92 <0.10
9 3.29 0.60
13 3.44 1.30
26 1.25 <0.10
Sandy 0 2.343 <0.10
1 1.40 <0.10
3 0.64 0.13
6 0.40 <0.10
9 0.16 <0.10
13 0.12 <0.10
26 <0.02 <0.10
Clay 0 2.764 <0.10
1 2.16 <0.10
3 0.44 0.17
6 0.32 <0.10
9 0.26 <0.10
13 0.14 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 0.844-0.032 log X; r = 0.9173
3 Y = 0.128-0.076 log X; r = 0.9721
4 Y = 0.175-0.076 log X; r = 0.9544
Samples of internal fat taken from animals, with unknown treatment
history, being slaughtered in the cattle tick zone in Australia during
1971/72 revealed some to contain bromophos-ethyl residues. Of 1 055
samples examined, 52 contained bromophos-ethyl, mostly below 0.5 ppm,
the highest being 0.8 ppm (Snelson, 1972). During the same period 589
samples of butter and cheese were examined for organo-phosphorus
residues. Only three samples were found to contain bromophos-ethyl
residues, all at 0.1 ppm.
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos-ethyl. These
have recently been reviewed and summarized by Eichler (1971). A method
has been developed by Leber and Deckers (1968) for all crops and
animal tissues. It utilizes gas chromatography with a phosphorus
specific detector and can estimate quantities down to the range of
0.001 to 0.01 ppm with a 100 g sample. The method includes procedures
for determining bromoxon-ethyl either by colorimetry or by gas
chromatography with a sensitivity of 0.03 to 0.05 ppm. Confirmation of
residues can be accomplished by the thin-layer chromatographic
techniques suggested in the method.
Bromophos-ethyl is among the pesticides listed as being detectable by
the multi-residue gas chromatographic procedure of Abbott et al.
(1970), and that method is the most suitable for regulatory use. The
method of Leber and Deckers would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Examples of national tolerances of bromophos-ethyl residues are
reported in Table 12.
TABLE 12 Examples of national tolerances as reported to meeting
Country Commodity Tolerance
Australia Fat of meat of cattle
and sheep 3 ppm
Milk and milk products
(fat basis) 1 ppm
The Netherlands Fruit and vegetables 0.4 ppm
APPRAISAL
Bromophos-ethyl is a non-systemic halogen-containing organo-phosphorus
insecticide and acaricide used on fruit, vegetables, field crops,
cereals, maize, rice, cotton and tobacco. It is also used extensively
in tick control, for other ectoparasites of domestic animals and as a
mosquito larvicide.
Supervised trials with foliar treatments on fruits and vegetables have
shown that bromophos-ethyl is somewhat more persistent than bromophos
and requires a pre-harvest interval about twice as long. The rate of
residue decline is highly dependent on many factors, especially
botanical species and morphological structure, which require that
tolerance recommendations be made on an individual commodity basis
rather than on broad crop categories.
Groups of beef cattle were sprayed at recommended rates one or more
times in trials in several countries. Highest residues were found in
omental, heart and perirenal fat, ranging in one trial from 0.1 to 2.0
ppm at four days post-treatment.
Spray treatment of dairy cows at recommended rates resulted in a
residue maximum of 1.17 ppm in butterfat at 45 hours post-treatment.
This fell to 0.02 ppm in butterfat after ten days. There was no
accumulation of milk residues from the multiple treatments.
The metabolites of bromophos-ethyl most likely to be found are
2,5-dichloro-4-bromophenol and bromoxon-ethyl. Only
dichloro-bromophenol and its conjugates were found in the excrement of
rats after oral administration of labelled bromophos-ethyl. The
dichloro-bromophenol also appears to be the only soil metabolite.
Although no studies have been conducted with the labelled compound in
plants, supervised field trials have shown small amounts of
bromoxon-ethyl residues in apples, Brussels sprouts and lettuce (up to
0.07 ppm in the latter case).
Available multi-residue gas chromatographic procedures are suitable
for use for regulatory purposes and are recommended.
Although bromophos-ethyl is recommended for cereals, maize, cotton,
rice and in animal health, there were no data available for these
commodities except fat of meat of cattle and milk. Therefore, no
recommendations could be made for tolerances on these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are recommended for
bromophos-ethyl.
ppm
Apples, carrots, fat of meat of cattle,
plums, pears, spinach 2
Brussels sprouts, redcurrants 1
Celeriac, gooseberries, peaches,
rape seed oil, cherries (sweet) 0.5
Blackcurrants, lettuce 0.2
Rape seed, strawberries, cabbage 0.1
Kohlrabi, French beans 0.05
Cauliflower, beans (without pods),
onions, sugarbeets, milk (whole) 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1975)
Further studies evaluating the significance of urinary excretion of
ascorbic and dehydroascorbic acids and its relevance to man.
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials on maize, rice and other cereals,
cotton, domestic animals, other than cattle, and milk products.
DESIRABLE
A study to determine dose levels causing no carboxyl-esterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1967.
III. Organo-phosphorus pesticide residues in the total diet. Pestic.
Sci. 1: 10-13.
Anonymous. (1970) Utrecht report, project CvF/PD4.2.(2.2.03).
Barnes, J.M. (1968) WHO insecticide evaluation and testing programme.
Stage I - mammalian toxicity report. Medical Research Council,
Carshalton. (unpublished).
Battelle Institute. (1964) Toxicity tests with the substance S2225 in
rats, guinea pigs and rabbits. (unpublished)
Boehringer. (1966) Residue trials and examination of cholinesterase
activity after spraying with bromophos-ethyl on cattle. Report C.H.
Boehringer Sohn.
Boehringer. (1967) Investigations concerning absorption, distribution,
excretion and metabolism of bromophos-ethyl-3H in rats. Report C.H.
Boehringer Sohn. (unpublished)
Boehringer. (1968) Determination of residues of bromophos-ethyl in
cattle. Report C.H. Boehringer Sohn.
Boehringer. (1971-1972) Residue investigation reports, C.H. Boehringer
Sohn.
Bradford, H.A. (1967) Acute dermal toxicity - bromophos-ethyl
(compound 70625). Report Eli Lilly and Company. (unpublished)
Eichler, D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
Eichler, D. (1970) Report C.H. Boehringer Sohn "Uber den Abban von
Bromophos und Bromophos-ethyl in verschiedenen Böden".
Harvey, J.M. (1968) Bromophos-ethyl residues in meat products.
Communication with C.H. Boehringer Sohn.
Leber G. and Deckers, W. (1968) Determination of residues of bromophos
and bromophos-ethyl. Proc. Brit. Insecticide Fungicide Conf.,
Brighton, Eng. 4: 570.
Leuschner, F. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Standinger, H.J. (1968) About the short-term toxicity studies on
bromophos-ethyl - charge 1487 - in Beagle dogs (with special attention
to the toxicology of the adrenal gland). Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Pliess, G. and
Dontenwill, W. (1969a) About the chronic toxicity of bromophos-ethyl
in Wistar rats following oral application. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969b)
About the chronic tolerance of bromophos-ethyl in the reproduction
test over three generations of Wistar rats. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Standinger, H.J., Schwerdtfeger, W. and
Dontenwill, W. (1971) Two years oral toxicity study in Beagle dogs
with bromophos-ethyl. Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Report on Shg 2225. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1969) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1970) Reports C.H. Boehringer Sohn. (unpublished)
Snelson, J.T. (1968) Bromophos-ethyl residues in milk. Communication
with C.H. Boehringer Sohn.
Snelson, J.T. (1967 - 1968) Residue studies - bromophos-ethyl.
Communications with C.H. Boehringer Sohn.
Snelson, J.T. (1972) Results from experimental investigations and
residue surveys with bromophos-ethyl following dipping in Australia.
Report to Joint Meeting.
Stiasni, M. (1967) Data from C.H. Boehringer Sohn. (unpublished)
Stötzer, H., Herbst, M., Baumgartner, R. and Guęnard, J. (1970)
Subacute dermal toxicity of the substance bromophos-ethyl in rabbits
(New Zealand White). Report C.H. Boehringer Sohn. (unpublished)
Terblanche. (1966) Toxicity of bromophos-ethyl. Department of
Agricultural Technical Services, Onderstepoort, South Africa.
(unpublished)
Vuuren, P.J.J. (1964) Whale-blood cholinesterase levels in cattle
sprayed at weekly intervals with S2225 (CELA). Agricura Laboratoria
Ltd., Silverton, South Africa. (unpublished)
