
CARBOPHENOTHION JMPR 1972
IDENTITY
Chemical names
S-(4-chlorophenylthiomethyl) diethyl phosphorothiolothionate;
S-(4-chlorophenylthiomethyl) 0,0-diethyl phosphorodithioate
Synonyms
Trithion(R), Garrathion(R), R-1303, ENT 23708, nephocarp.
Empirical formula
C11H16O2Cl PS3
Structural formula
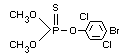 Physical and chemical properties
State: off-white to light amber coloured liquid
Boiling point: 82°C at 0.01 mm Hg
Vapour pressure: 3 × 10-7 mm Hg at 20°C
Specific gravity: 1.265 - 1.285 at 20°C
Molecular weight: 342.85
Refractive index: 1.590 - 1.597
Solubility: slightly soluble in water (<0.04 g/l at 20°C), Miscible
with most organic solvents (hydrocarbons, alcohols, ketones, esters)
Stability: stable to hydrolysis but oxidized to the phosphorothiolate
Purity: technical material about 95%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on absorption and distribution of carbophenothion in mammals
are not available. Following intraperitoneal administration to mice,
greater than 75% of the administered dose was excreted in the urine
within 24 hours. During the following 16 hours only 4-7% of the dose
was excreted. Carbophenothion is apparently rapidly eliminated from
the body (March et al., 1957).
Biotransformation
The metabolism of carbophenothion is qualitatively the same in mice,
insects and plants, the principal products being oxidative. Although
the transformation of carbophenothion to oxidative and hydrolytic
components has been postulated, little data are available on the
quantitative degradation in mammals. Oxidative metabolism of
carbophenothion has been shown to result in a substantial increase in
anticholinesterase activity except for the sulphone of the
phosphorodithioate. A quantitative difference in animal and plant
metabolites was the presence of an unidentified product on the surface
of plants which appeared to a minor degree in the internal parts and
was found as a minor component in mouse urine and in vitro tests
with mouse liver. The component is probably the phosphorodithioate
sulphoxide analogue (March et al., 1957). Five oxidative products of
carbophenothion have been identified (see figure 1).
S O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion oxygen analogue
(5 × 10-4M) (2 × 10-6M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion sulphoxide oxygen analogue sulphoxide
(8 × 10-6M) (2 × 10-7M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2O 6 4 2 2O 6 4
carbophenothion sulphone oxygen analogue sulphone
(1 × 10-4M) (1 × 10-8M)
Figure 1 - Oxidative products of carbophenothion
The number in parenthesis under the oxidative products in Figure 1 is
the I50 value for housefly head cholinesterase (March et al.,
1957).
Results of studies on the metabolism of carbophenothion in lettuce
have shown a typical pattern of oxidative metabolism resulting in the
sulphoxide and sulphone analogues of the phosphorothioate and
phosphorodithioate. Exposure of carbophenothion to UV light resulted
in the appearance of the phosphorodithioate sulphoxide analogue
(Menzies, 1969).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of White Leghorn hens (10 hens/group) were fed carbophenothion
in the diet at levels of 0, 10, 31.6 and 100 ppm for seven weeks. TOCP
was fed at 500 ppm as a positive control. Egg production was affected
at 100 ppm. Clinical signs of ataxia were evident in TOCP-fed hens but
were absent in animals fed all levels of carbophenothion. Histological
examination of nerve tissue showed slight evidence of demyelination in
TOCP treated hens but none with carbophenothion (Lobdell and Johnston,
1964).
Special studies on potentiation
Groups of dogs (1 male and 1 female/group) were fed carbophenothion (1
ppm) in the diet in combination with parathion (1 ppm),
methylparathion (5 ppm), EPN (20 ppm), demephion (2 ppm), or malathion
(100 ppm) for 45 days. No evidence of greater than additive depression
of cholinesterase activity was observed (Horn, 1957).
Carbophenothion in combination with five other organophosphates
(parathion, methylparathion, EPN, demephion and malathion) when
administered orally to rats did not show signs of potentiation of
acute toxicity (Horn, 1957; Shaffer and West, 1960).
Special studies on reproduction
Groups of rats (20 male and 20 female per group) were fed
carbophenothion in the diet and subjected to a standard
three-generation (2 litter per generation) reproduction study at
dosage levels of 0 and 20 ppm. At 20 ppm carbophenothion had an effect
on reproduction in all three generations. In the F0 generation a
reduced mean pup birthweight was observed and fewer young were alive
at weaning. In the F1b generation, an increased number of stillbirths
was observed in two litters and fewer young were alive at weaning in
the second (but not the first) litter. In the F2b generation, an
increase in stillbirths was again observed in two litters, a decreased
number of live births and again fewer pups were alive at weaning.
Foetal resorption was an apparent factor in three of 10 females
examined which had borne less than two litters. An examination of
stillborn pups for teratological effects of carbophenothion was
negative (Johnston and Scott, 1966).
Acute toxicity
Acute toxicity to carbophenothion in some animal species is summarized
in Table 1.
TABLE 1 Acute toxicity to carbophenothion in different animal species
Species Sex Route LD50 Reference
Rat M Oral 17-91 Gray, 1954; Elsea, 1955a,b; Horn, 1957;
Shaffer and West, 1960; Hagan et al.,
1961; Hayes, 1971
F Oral 10 Hayes, 1971
Oral 7-30 Edson et al., 1963
M Dermal 54 Hayes, 1971
F Dermal 27 Hayes, 1971
Mouse M Oral 106-218 Elsea, 1955b; Gray, 1954
Dog M and F IM Approx. Beliles, 1966
40
Rabbit Dermal 800 Edson et al., 1963
Duck M Oral 121 Tucker and Crabtree, 1970
A dose of 25 mg/kg administered to the eye of rabbits resulted in
death in one-third and signs of acute poisoning in the survivors
(Gray, 1954).
Signs of poisoning are typical of organophosphorus esters and include
salivation, lacrimation, diarrhea, miosis, tremors and other signs of
cholinergic stimulation.
Rats exposed to carbophenothion vapours at a dose of 1.04 mg/l for one
hour exhibited depression which subsided within one hour after
treatment (Bullock and Kamienski, 1971).
Atropine and PAM have been shown to be effective therapeutic agents
for acute poisoning by carbophenothion. Repeated administration with
both materials was necessary to insure complete survival of poisoned
animals (Elward and Meydins, 1964). Following acute IM administration
of carbophenothion, atropine and PAM administered IM were effective in
temporarily alleviating signs of poisoning. A return of toxic signs
was evident 2-4 hours after the administration of atropine and PAM
(Beliles, 1965).
Dogs were administered carbophenothion by IM injection at a dose
approximately five times the calculated LD50. This was followed by
atropine alone or in combination with PAM (atropine was administered
twice daily after signs of poisoning occurred and PAM administered
once). All dogs administered atropine alone died, although death was
delayed. One of six dogs receiving atropine and PAM survived.
Mortality in the dogs administered atropine and PAM was delayed.
Atropine plus PAM was a more effective therapeutic agent than atropine
alone. Signs of toxicity and mortality following lethal doses of
carbophenothion were alleviated with the two agents in combination
(Beliles, 1966).
Short-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for 90 days at dosages of 0, 5, 10, 22, 46
and 100 ppm. Clinical signs of toxicity, cholinergic stimulation, were
evident at 46 and 100 ppm. Growth of females was reduced at 100 ppm.
RBC cholinesterase inhibition was evident at levels of 10 ppm and
above, especially in females. Brain and plasma cholinesterase was
inhibited at 22 ppm and above. No effects were observed on survival,
food consumption and gross histological examination of tissues
(Fogleman, 1956; Lehman, 1965).
Dog
Groups of mongrel dogs (2 males and 2 females/group) were administered
carbophenothion orally by gelatin capsule, 6 days/week for 90 days, at
levels of 0.02, 0 04, 0.08, 0.25 and 0.80 mg/kg/day. Dogs at 0.25 and
0.80 mg/kg/day showed plasma cholinesterase depression and did not
receive the total dosage. At 0.02 mg/kg/day plasma cholinesterase was
depressed slightly, while at 0.04 mg/kg inhibition was more apparent.
RBC cholinesterase was normal at all treatment levels (Fogleman, 1956;
Lehman, 1965). Groups of purebred beagle dogs (3 males and 3 females/
group) were fed carbophenothion in the diet for two years at dosages
of 0, 5, 20 and 80 ppm. Erythrocyte and brain cholinesterase activity
were depressed at 80 ppm. Plasma cholinesterase activity was depressed
at all feeding levels at all examination intervals except the initial
one. At 20 and 80 ppm, the groups were distinguishable from controls
chiefly on the basis of decreased body-weight gain (in 80 ppm
females), greater degree of irritability and nervousness and increased
mean relative adrenal weight (in 80 ppm females). No effects were
noted on mortality, physical condition, food consumption, behaviour,
haemograms, clinical chemistry tests, urinalysis, neurological
examinations and gross or histological examination of tissues
(Johnston, 1967).
Cow
Groups of two lactating dairy cows were administered 0, 1, 3, 10 and
30 ppm of carbophenothion in the diet, 7 days/week, for 120 days. The
1 ppm group was changed to 20 ppm on day 23 of the study. A similar
group, receiving 15 ppm, was added to the study on day 70 and
continued for 50 days. Clinical signs of toxicity were evident at 30
ppm. Blood cholinesterase activity was significantly depressed at 20
and 30 ppm. Appearance and behaviour, food consumption and whole blood
cholinesterase activity were unaffected at 15 ppm and below (Weir,
1958).
Long-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for two years at dosage levels of 0, 5, 20
and 80 ppm. Although considerable mortality was observed in all groups
in this study, there was no relationship to the dietary intake. Rats
at 80 and 20 ppm feeding levels displayed tremors, loose stools and
hair loss as well as behaviour changes, including nervousness and
irritability. Females at 80 ppm showed reduced growth during the
second year of study. At 80 ppm, at 100 weeks of feeding, a gradual
lowering of hemoglobin became evident. RBC, plasma and brain
cholinesterase activity was inhibited at 80 and 20 ppm but not at 5
ppm. Cholinesterase inhibition in brain, RBC and plasma was more
evident in females than males. At 80 ppm females showed an increased
adrenal weight and ratio of organ to body-weight. Gross and
histological examinations showed no compound-related effect other than
the increased adrenal gland weight. Analyses of the incidence of
tissue mass showed no differences from control values. There were no
differences in respect to the frequency of neoplasm occurrence
(Johnston, 1967).
OBSERVATIONS IN MAN
The effects of carbophenothion noted on man are limited to several
acute poisoning incidents (Hearn, 1961) and a one-month subacute
administration to humans. Typical signs of cholinergic stimulation in
humans were obvious following ingestion of contaminated flour. This
poisoning was controlled with atropine and PAM. Five people were
administered carbophenothion at a dose of 0.8 mg/kg/day for 30 days.
There were no effects reported on plasma or RBC cholinesterase
activity (Rider et al., 1972).
COMMENT
Carbophenothion is rapidly excreted and is probably metabolized via
oxidative and hydrolytic pathways.
The compound does not induce demyelination and is not potentiated by
those organophosphates tested.
Cholinesterase depression was shown to occur in a rat reproduction
study, conducted at a dose level known to induce maternal
cholinesterase depression. Reduced pup weights, increased resorption
rate and postpartum mortality in pups was shown to occur in a
reproduction study in rats conducted at a dose level known to induce
maternal cholinesterase depression.
Plasma cholinesterase depression was the most sensitive criterion of
effect in both short- and long-term studies in rats and dogs.
A study in man did not result in plasma cholinesterase depression at
0.8 mg/kg/day. The meeting considered that the data available were
insufficient to enable an ADI to be based on this experiment. However,
the 0.8 mg/kg/day level was borne in mind when the study in dogs was
considered in the light of the apparent extreme difference in
sensitivity between man and dog. A no-effect level was not
demonstrated in dog at the lowest dose utilized (5 ppm or 0.125
mg/kg/day). In rat, 5 ppm (0.25 mg/kg/day) is a no-effect level with
regard to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous fruits, citrus
fruits, small fruits, vegetables and field crops, and for ectoparasite
control on cattle and sheep. It is also used as a cereal seed
dressing.
It is available as various strengths of emulsifiable concentrates,
wettable powders and dusts. Recommended treatment levels are as
follows:
1) Deciduous fruit - 1.25 lb to 2.0 lb a.i. per acre with pre-harvest
intervals of 30 days.
2) Citrus fruit - 2.5 lb to 5.0 lb a.i. per acre. Pre-harvest
intervals vary from 0 to 30 days depending on
amount of a.i. used per acre.
3) Vegetable crops - 1.0 lb a.i. per acre; 7 days pre-harvest
interval except for spinach (21 days).
4) Field crops - 1.0 lb a.i. per acre; 21 days pre-harvest
interval.
5) Cattle - 0.02% spray, no pre-slaughter interval.
6) Sheep - 0.026% tip spray; 0.062% saturation spray or
plunge dip - 14 day pre-slaughter interval.
Specific uses include the control of greenbug on sorghum (Ward et
al., 1970) and the control of castor white fly (Radha, 1971).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 2 gives typical results of analysis for residues of
carbophenothion following controlled application at stated rates to
various fruit and vegetable crops (Stauffer Chemical Co., 1972). The
data on citrus fruits refers to residues on whole fruits; evidence was
presented showing that practically all of the residue was retained on
the peel with only very small amounts (0.02 ppm or less) appearing in
the pulp or juice.
Tables 3 and 4 show residues of carbophenothion found in some tissues
of cattle and sheep at different intervals after treatment.
Table 5 gives results obtained from the analysis of milk from cows fed
carbophenothion at various concentrations to simulate feeding of
treated forage (Stauffer Chemical Co., 1972).
TABLE 2 Carbophenothion residues in various crops
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Oranges 0 0.03 0.80
7 0.03 0.67
14 0.03 0.55
21 0.03 0.47
28 0.03 0.38
Grapefruit 0 0.03 1.4
7 0.03 1.1
14 0.03 0.85
21 0.03 0.65
28 0.03 0.53
Lemons 0 0.12 4.0
7 0.12 3.3
14 0.12 2.5
21 0.12 2.2
28 0.12 1.9
Limes 0 0.03 0.95
7 0.03 0.79
14 0.03 0.65
21 0.03 0.53
28 0.03 0.43
Apples 0 0.03 0.73
2 0.03 0.30
12 0.03 0.46
14 0.03 0.88
19 0.03 0.36
26 0.03 0.29
29 0.03 0.37
Pears 33 0.06 0.41
Peaches 14 0.03 0.70
27 0.03 0.15
29 0.03 0.67
Prunes 28 0.04 0.24
29 0.04 0.47
29 0.04 0.42
Apricots 21 0.03 0.78
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Nectarines 14 0.03 0.52-0.66
37 0.03 0.33-0.42
Green alfalfa 0 0.25 70
10 0.25 20
20 0.25 5.5
Alfalfa hay 0 0.25 70
10 0.25 25
20 0.25 8
Soybean hay 0 0.25 70
10 0.25 25
20 0.25 8
Brussels 0 0.9 kg/ha 0.91
sprouts 7 0.9 kg/ha 0.28
Cauliflower 0 1.2 kg/ha 0.56
4 1.2 kg/ha 0.06
Broccoli 0 1.2 kg/ha 2.65
4 1.2 kg/ha 0.33
Sugar beet 7 1.2 3.9
(tops) 11 1.2 2.5
18 1.2 0.6
Sugar beet 0 0.25 0.01
(roots) 8 0.25 0.02
17 0.25 0.02
Spinach 2 4 oz/acre 26.4
5 11.2
10 3.8
15 0.70
2 8 oz/acre 63.4
5 25.8
10 12.5
15 1.7
0 12 oz/acre 36
4 14
22 1.56
29 0.16
2 16 oz/acre 95
5 32.2
10 15.1
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Spinach (cont'd) 15 2.4
Olives 66 0.06 0.08
88 0.06 0.04
119 0.06 0.02
Olive oil 66 0.06 0.16
88 0.06 0.06
119 0.06 0.02
Potatoes )
Rape seed ) Observed residues all below the
Walnuts (shelled) ) limit of determination (0.02 ppm)
Pecans (shelled) )
Information was also available (Stauffer Chemical Co., 1972) regarding
residues occurring in milk and cream from three cows spray-treated
with 0.02% carbophenothion; no residues above the limits of
determination were observed (<0.005 ppm for milk and <0.05 ppm for
cream).
FATE OF RESIDUES
In animals
Fat samples of cattle treated with carbophenothion have been examined
for the presence of carbophenothion, its sulphoxide and sulphone and
the corresponding oxygen-analogue compounds. The parent compound and
lesser amounts of its sulphoxide and sulphone were found (Table 6),
but the oxygen-analogues were not observed (Meyding and Browne, 1962).
In all cases the amounts of the oxygen-analogue, its sulphoxide and
sulphone were below 0.005 ppm.
In plants
Carbophenothion was applied to field-growing lettuce, and samples were
removed at various times after spraying and analysed for
carbophenothion and its oxidative metabolites. Carbophenothion
persisted in growing lettuce for 21 days. Five oxidation products
appeared within 4 hours of spraying and persisted for up to 21 days.
The results indicate that the principle route of oxidation involved
thioether oxidation to form the sulphoxide and then the sulphone,
followed by phosphorothionate oxidation to form the thiophosphate
sulphone (Coffin, 1964). (See also Figure 1). In these experiments the
amount of parent carbophenothion decreased rapidly from 7.0 ppm to 1.2
TABLE 3 Carbophenothion residues in cattle tissues1
Tissue Treatments Days since last treatment
(No.) 1 3 7/8 21 36 43
Perirenal fat 1 - - 0.13 0.09 - -
Subcutaneous fat 1 - - 0.09 0.10 - -
Omental fat 1 - - 0.17 0.15 - -
Perirenal fat 2 - - 0.63 0.20 - -
Subcutaneous fat 2 - - 0.19 0.14 - -
Omental fat 2 - - 0.41 0.24 - -
Perirenal fat 3 1.65 0.69 0.71 0.19 0.25 ND
Subcutaneous fat 3 0.62 0.61 0.37 0.10 0.14 0.04
Omental fat 3 1.12 0.74 0.47 0.25 0.31 0.02
Liver 3 0.019 <0.01 ND2 ND ND ND
Kidney 3 0.048 0.03 <0.01 ND ND ND
Skeletal muscle 3 0.036 0.01 ND ND ND ND
1 Residues found in the tissues of cattle following 1, 2 or 3 weekly spray exposures
to a 0.1% active ingredient suspension. Values represent net corrected ppm of
carbophenothion (Meyding and Browne, 1962).
2 ND = not detected
TABLE 4 Carbophenothion residues in sheep tissues1
Tissue Days after treatment
1 3 10 14
Fat unshorn 0.39 0.66 0.55 0.61
shorn 0.90 0.68 0.29 0.29
Muscle unshorn 0.054 0.072 0.047 0.042
shorn 0.049 0.086 0.032 0.026
TABLE 4 (continued)
Tissue Days after treatment
1 3 10 14
Kidney unshorn *2 0.036 0.045 *
shorn 0.014 0.020 0.020 *
Liver unshorn * 0.019 0.014 0.011
shorn 0.01 0.011 * 0.014
1 Residues found in various tissues of sheep resulting from plunge-dips
in a vat containing 0.05% carbophenothion (Meyding and Patchett, 1964).
Values given in ppm carbophenothion.
2 * = <0.01 ppm
TABLE 5 Carbophenothion residues in milk (ppm carbophenothion)
Duration of
treatment Treatment level (ppm in diet)
(days) 0 1 3 10 20 30
0 *1 * * * * 0.0014 *
7 * * * 0.0023, 0.0029 0.0101 0.0215, 0.0170
14 * * 0.0015, 0.0015 0.0028, 0.0024 0.0085, 0.0052 0.0210, 0.0149
28 * - 0.0015, 0.0024 0.0086, 0.0058 0.0103, 0.0104 0.0171
49 * - 0.0036, 0.0031 0.0058, 0.0047 0.0123, 0.0146 0.0141
70 * - 0.0033, 0.0011 0.0054, 0.0048 0.0115, 0.0088 0.0187
(mean) * * 0.0015 0.0045 0.0102 0.0178
1 * = less than 0.001 ppm.
TABLE 6 Carbophenothion and metabolites in cattle fat
Fat tissue Treatments Days since Carbophenothion -sulphoxide -sulphone
(no.) last treatment (ppm)
Perirenal 3 7 0.563 0.318 0.058
Omental 3 7 0.368 0.107 0.026
Subcutaneous 1 8 0.197 0.068 0.046
TABLE 7 Effect of storage and heat processing on carbophenothion residues
Heat Storage
Food Initial level processed Ambient 100°F
(ppm) temp.(1 yr)
Spinach 0.76 17% 71% 83%
Apricots 0.76 35% 84% 84%
ppm in the first three days and then more slowly to less than 0.1 ppm
at 21 days. The total residue declined more steadily from 8.3 ppm
to 0.1 ppm over the same period.
Evidence of residues in food in commerce or at consumption
Some limited information was available on residues of carbophenothion
occurring in food in commerce. Duggan et al. (1971) have presented
results of analyses from 1963 to 1969 of nearly 7 000 samples of U.S.
domestic and imported large fruits (apples, pears, oranges, peaches).
Of the few samples shown to contain residues of carbophenothion (less
than 1% of those examined), only 25 samples contained more than 0.1
ppm and one sample contained more than 1 ppm. No measurable amounts of
carbophenothion have been observed in various published total diet
studies.
Elkins et al. (1972) studied the effect of storage and heat
processing on the residue level of carbophenothion in canned spinach
and apricots. The results, shown in Table 7, are expressed in terms of
percentage reduction in the residue level.
METHODS OF RESIDUE ANALYSIS
Carbophenothion and its five oxidative metabolites are extracted from
non-fatty materials with aqueous acetonitrile, and from fat and oil
with hexane followed by partitioning in aqueous acetonitrile. The
residues are partially separated from crop extractives by an alumina
shakeout, a partition into chloroform, and an activated carbon
shakeout. The residues are oxidized to the sulphones with potassium
permanganate, further isolated and separated by liquid-solid
chromatography on silica gel H, and determined by gas-liquid
chromatography on OV-1 or QF-1 columns with flame photometric
detection in the phosphorus mode (Buxton, 1971 and Storherr et al.,
1971). These procedures should be suitable for regulatory purposes.
Bowman and Beroza (1970, 1971) published conditions for the gas
chromatography of organophosphorus pesticides, including
carbophenothion, using a flame photometric detector. A method based on
the extraction procedure of McLeod et al. (1967) in conjunction with
the T.L.C. enzymatic inhibition technique of Mendoza et al. (1968)
has been proposed by Mendoza et al. (1970). The presence of
carbophenothion in whole wheat flour could be detected down to 0.5
ppm. Carbophenothion and its five oxidative metabolites have been
determined by gas chromatography using a 5 ft column of 10% DC 200 and
the sulphur mode of the microcoulometric detector (Burke, 1965). A
method has been proposed for the detection and quantitative
determination of organothiophosphorus pesticides, including
carbophenothion, by in situ fluorimetry after separation on silica
gel layers. Yellowish-green fluorescent spots are obtained when the
plate is sprayed with a 3-hydroxyflavone, such as robinetin, after
bromination. Linear calibration curves up to 4 mg per spot of the
pesticide have been obtained and a relative standard deviation of
approximately 4% can be expected at the 1.0 µg level. Visual and
instrumental detection limits are around 0.04 µg per spot for certain
pesticides (Frei et al., 1971).
A thin-layer chromatographic procedure has been described for
separating and detecting carbophenothion and 16 other
cholinesterase-inhibiting pesticides on aluminum oxide and silica gel.
The detection of the anticholinesterase activity is based on the
hydrolysis of indophenyl acetate by bee brain cholinesterase. Limits
of detectable cholinesterase inhibitors are at the subnanogram level,
and the time required for detection is about 40 minutes (Winterlin
et al., 1968). A detection limit of 5 ng was obtained by Gardner
(1971) using a combined two-dimensional TLC - esterase method.
Patchett and Batchelder (1960) describe a two-phase hydrogen
peroxide-acetic acid-benzene system to oxidize carbophenothion to
strong cholinesterase inhibitors, which are subsequently determined by
measuring their inhibition of cholinesterase in human blood plasma.
The method is sensitive to 0.01 µg of carbophenothion and will detect
0.005 ppm residue in crops having low background values.
Villeneuve et al. (1970) investigated the separation and detection
of 13 organophosphorus pesticides, including carbophenothion, using a
combined TLC-GLC method. The separation of carbophenothion using a
silica gel microcolumn was investigated by Leoni (1971). A routine TLC
method for the determination of residues of organophosphorus
pesticides, including carbophenothion, has been proposed by Mees and
Antoine (1971). The procedure uses both silica gel and polyamide
coated plates.
Table 8 shows examples of national tolerances as reported to the Joint
Meeting.
TABLE 8
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
Australia Fruit and vegetables 1
Fat of meat of cattle and sheep 1
Belgium Fruit and vegetables 0.5
Canada Cherries, eggplants, peppers,
pimentos, plums, prunes, tomatoes 0.5
Apples, crabapples, onions,
pears, quinces 0.8
Federal
Republic
of Germany Rape, citrus fruit (without peel) 0.05
Netherlands Fruit and vegetables 0.05
TABLE 8 (cont'd)
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
U.S.A. Almond hulls 10
Alfalfa (green and hey),
clover (green and hay),
sugarbeet (root and tops) 5
Citrus fruit 2
Apples, apricots, snap beans,
lima beans, garden beets (root and
tops), melons 0.8
Cherries, cucumbers, eggplant, figs,
grapes, nectarines, olives, onions
(dry bulb and green), peaches,
pears, peas, peppers, plums,
quinces, pimentos, soybeans,
spinach, strawberries, summer
squash, tomatoes 0.8
Fat of meat of cattle, goats,
pigs and sheep 0.2
Dry beans, pecans, walnuts 0.1
APPRAISAL
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous, citrus and
small fruits, field crops, vegetables and for ectoparasite control on
cattle and sheep; it also has uses as a cereal seed dressing. The
pesticidal action is fairly persistent and residues are comprised
mostly of the parent compound together with its sulphoxide and, to a
lesser extent, its sulphone; the corresponding oxygen analogues are
rarely detected in animal tissue but have been observed as minor
components on treated vegetables. Following treatment of animals, the
bulk of the residue is contained in the fatty tissues. Residue data
was available following supervised trial treatments of fruit,
vegetables and animals, but no information was given regarding
foodstuffs moving in commerce. No residues have been observed in total
diet studies. Available multi-residue gas chromatographic procedures
should be suitable for application for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Temporary tolerances apply to the total residue, comprising
carbophenothion, its sulphoxide and sulphone together with their
corresponding oxygen analogues, if present, expressed as
carbophenothion.
ppm
Lemons 5
Spinach 2
Fat of meat of cattle and sheep 1
Grapefruit, limes, oranges, prunes,
apricots, nectarines, peaches 1
Broccoli, Brussels sprouts, cauliflower,
apples, pears 0.5
Olive oil 0.2
Olives, sugar beet 0.1
Milk and milk products (fat basis) 0.1
Potatoes, rape seed 0.02*
Walnuts and pecans (shelled) 0.02*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1976)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
2. An adequate reproduction study.
REFERENCES
Beliles, R.P. (1965) Trithion - safety evaluation by analysis of
cholinesterase reactivation with atropine and PAM after trithion
poisoning. Report Woodard Research Corporation. (unpublished)
Beliles, R.P. (1966) Trithion - safety evaluation by analyses of
cholinesterase reactivation with atropine and PAM after trithion
poisoning in the dog. Report Woodard Research Corporation.
(unpublished)
Bowman, M.C. and Beroza, M. (1970) G.L.C. retention time of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J. Ass. off. analyt. Chem., 53: 499-508.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on specially
washed Chromosorb W for multi-component residue determinations of
phosphorus and sulphur containing pesticides by flame photometric
G.L.C. J. Ass. off. analyt. Chem., 54: 1086-1092.
Bullock, C.H. and Kamienski, F.X. (1971) Acute inhalation screen, male
and female rats. Report Stauffer Chemical Company. (unpublished)
Burke, J.A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. off. Agric. Chem., 48: 1037-1058.
Buxton, R.W. (1971) Determination of residues of Trithion and its
oxygen analogues. Report Stauffer Chemical Co. (unpublished)
Coffin, D.E. (1964) Oxidative metabolism and persistence of Trithion
on field sprayed lettuce. J. Ass. off. Agric. Chem., 47: 662-667.
Duggan, R.E., Lipscomb, G.Q., Cox, E.L., Heatwole, R.E. and Kling,
R.C. (1971) Pesticide residue levels In food in the United States July
1, 1963 to June 30, 1969. Pestic. Monit. J., 5: 73-212.
Edson, E.F., Sanderson, D.M. and Noakes, D.N. (1963) Acute toxicity
data for pesticides. World Rev. Pest Control 2: 26-28.
Elsea, J.R. (1955a) Acute oral administration - rats. Report Hazleton
Laboratories. (unpublished)
Elsea, J.R. (1955b) Acute oral administration - mice. Report Hazleton
Laboratories. (unpublished)
Elkins, E.R., Farrow, R.P. and Kim, E.S. (1972) The effect of heat
processing and storage on pesticide residues in spinach and apricots.
J. Agr. Fd. Chem., 20: 286-291.
Elward, T.E. and Meyding, G.D. (1964) Carbophenothion. Reactivation
study - rats. Report No. 64-5 Stauffer Chemical Company. (unpublished)
Fogleman, R.W. (1956) Subacute feeding - rats. Report Hazleton
Laboratories. (unpublished)
Frei, R.W., Mallet, V. and Pothier, C. (1971) Flavones as fluorogenic
spray reagents for organothiophosphorus pesticides on silica gel
layers. J. Chromat., 59: 135-140.
Gardner, A.M. (1971) Confirmation of organophosphorus pesticide
residues at nanogram levels by two-dimensional T.L.C. J. Ass. off.
analyt. Chem., 54: 517-524.
Gray, E.H. (1954) Acute oral, dermal and eye application. Report
Hazleton Laboratories (unpublished)
Hagan, E.C., Jenner, P.M. and Fitzhugh, O.G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432.
Hayes, W.J., Jr. (1971) Clinical handbook on economic poisons. U.S.
EPA Pesticides Programs, Public Health Service Publication 476.
Hearn, C.E.D. (1961) Trithion poisoning. Bull. J. Ind. Med., 18:
231-233.
Horn, H.J. (1957) Trithion. Potentiation study, subacute feeding -
dogs, acute oral administration - rats. Report Hazleton Laboratories.
(unpublished)
Johnston, C.D. (1967) Trithion. Safety evaluation by two-year feeding
studies in the rat and the dog. Report Woodard Research Corporation.
(unpublished)
Johnston, C.D. and Scott, W.J. (1966) Trithion. Three generation
reproduction study in the rat. Report Woodard Research Corporation.
(unpublished)
Lehman, A.J. (1965) Summaries of pesticide toxicity. The Assoc. of
Food and Drug Officials of the U.S.
Leoni, V. (1971) The separation of fifty pesticides and related
compounds and polychlorobiphenyls into four groups by silica gel
microcolumn chromatography. J. Chromat., 62: 63-71.
Lobdell, B.J. and Johnston, C.D. (1964) Trithion-demyelination in
chickens. Report Woodard Research Corporation. (unpublished)
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1967) Metabolism of 32P
Trithion in the white mouse and American cockroach. Report Stauffer
Chemical Company. (unpublished)
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967)
Comparison of various carbon absorbents. Quantitative elution and
separation of 42 pesticides from a carbon-Solka Floc clean-up column.
J. Ass. off. analyt. Chem., 50: 1216-1228.
Mees, G. and Antoine, O. (1971) Routine method for the determination
of organophosphorus insecticides by thin-layer chromatography (in
French). J. Chromat., 58: 247-256.
Mendoza, C., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Enzymatic detection of 10 organophosphorus pesticides and carbaryl on
thin-layer chromatograms. Evaluation of indoxyl, substituted indoxyl
and 1-naphthyl acetates as substrates for esterases. Analyst, Lond.,
93: 34-38.
Mendoza, C., Wales, P.J. and McLeod, H.A. (1970) Simultaneous
detection of some organophosphorus pesticides in whole-wheat flour.
Bull. envir. Contam. Toxicol., 5: 276-278.
Menzies, C.M. (1969) Metabolism of pesticides. U.S. Dept. of the
Interior, Special scientific report No. 127.
Meyding, G.D. and Browne, H.L. (1962) Cattle spray program. Report
Hazelton-Nuclear Science Corporation. (unpublished)
Meyding, G.D. and Patchett, G.G. (1964) Garrathion 3.5E; Plunge-dip
toxicity/residue studies: sheep. Report Stauffer Chemical Co.
(unpublished)
Patchett, G.G. and Batchelder, G.H. (1960) Determination of Trithion
crop residues by cholinesterase inhibition measurement. J. Agr. Fd.
Chem., 8: 54-57.
Radha, N.V. (1971) Efficacy of some synthetic insecticides in the
control of the castor white fly, Trialeurodes ricini M. Madras Agr.
J., 58: 300-302.
Rider, J.A., Swader, J.I. and Puletti, E.J. (1972) Anticholinesterase
studies with Guthion, Phosdrin, Disyston and Trithion in human
subjects. Fed. Proc., 31: 520 (Abs # 1730).
Shaffer, B.C. and West, R. (1960) The acute and subacute toxicity of
technical tetram. Toxicology and applied pharmacology, 2:1-13.
Stauffer Chemical Co. (1972) Evaluation of residues in food.
(unpublished)
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticides in non-fatty foods. J. Ass. off. analyt.
Chem., 54: 513-516.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of Interior. Resource publication
No. 84.
Villeneuve, D.C., Butterfield, A.C., Grant, D.L. and McCully, K.A.
(1970) The detection, separation and quantitative recovery of 13
organophosphorus pesticides on silica gel GF254 thin-layer
chromatograms. J. Chromat., 48: 567-571.
Ward, C.R., Huddleston, E.W., Ashdown, D., Owens, J.C. and Polk, K.L.
(1970) Greenbug control on grain sorghum and the effects of tested
insecticides on other insects. J. Econ. Entomol., 63: 1929-1934.
Weir, R.J. (1958) Subacute feeding - dairy cows. Report Hazleton
Laboratories. (unpublished)
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Fd. Chem., 16: 808-812.
Physical and chemical properties
State: off-white to light amber coloured liquid
Boiling point: 82°C at 0.01 mm Hg
Vapour pressure: 3 × 10-7 mm Hg at 20°C
Specific gravity: 1.265 - 1.285 at 20°C
Molecular weight: 342.85
Refractive index: 1.590 - 1.597
Solubility: slightly soluble in water (<0.04 g/l at 20°C), Miscible
with most organic solvents (hydrocarbons, alcohols, ketones, esters)
Stability: stable to hydrolysis but oxidized to the phosphorothiolate
Purity: technical material about 95%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on absorption and distribution of carbophenothion in mammals
are not available. Following intraperitoneal administration to mice,
greater than 75% of the administered dose was excreted in the urine
within 24 hours. During the following 16 hours only 4-7% of the dose
was excreted. Carbophenothion is apparently rapidly eliminated from
the body (March et al., 1957).
Biotransformation
The metabolism of carbophenothion is qualitatively the same in mice,
insects and plants, the principal products being oxidative. Although
the transformation of carbophenothion to oxidative and hydrolytic
components has been postulated, little data are available on the
quantitative degradation in mammals. Oxidative metabolism of
carbophenothion has been shown to result in a substantial increase in
anticholinesterase activity except for the sulphone of the
phosphorodithioate. A quantitative difference in animal and plant
metabolites was the presence of an unidentified product on the surface
of plants which appeared to a minor degree in the internal parts and
was found as a minor component in mouse urine and in vitro tests
with mouse liver. The component is probably the phosphorodithioate
sulphoxide analogue (March et al., 1957). Five oxidative products of
carbophenothion have been identified (see figure 1).
S O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion oxygen analogue
(5 × 10-4M) (2 × 10-6M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion sulphoxide oxygen analogue sulphoxide
(8 × 10-6M) (2 × 10-7M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2O 6 4 2 2O 6 4
carbophenothion sulphone oxygen analogue sulphone
(1 × 10-4M) (1 × 10-8M)
Figure 1 - Oxidative products of carbophenothion
The number in parenthesis under the oxidative products in Figure 1 is
the I50 value for housefly head cholinesterase (March et al.,
1957).
Results of studies on the metabolism of carbophenothion in lettuce
have shown a typical pattern of oxidative metabolism resulting in the
sulphoxide and sulphone analogues of the phosphorothioate and
phosphorodithioate. Exposure of carbophenothion to UV light resulted
in the appearance of the phosphorodithioate sulphoxide analogue
(Menzies, 1969).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of White Leghorn hens (10 hens/group) were fed carbophenothion
in the diet at levels of 0, 10, 31.6 and 100 ppm for seven weeks. TOCP
was fed at 500 ppm as a positive control. Egg production was affected
at 100 ppm. Clinical signs of ataxia were evident in TOCP-fed hens but
were absent in animals fed all levels of carbophenothion. Histological
examination of nerve tissue showed slight evidence of demyelination in
TOCP treated hens but none with carbophenothion (Lobdell and Johnston,
1964).
Special studies on potentiation
Groups of dogs (1 male and 1 female/group) were fed carbophenothion (1
ppm) in the diet in combination with parathion (1 ppm),
methylparathion (5 ppm), EPN (20 ppm), demephion (2 ppm), or malathion
(100 ppm) for 45 days. No evidence of greater than additive depression
of cholinesterase activity was observed (Horn, 1957).
Carbophenothion in combination with five other organophosphates
(parathion, methylparathion, EPN, demephion and malathion) when
administered orally to rats did not show signs of potentiation of
acute toxicity (Horn, 1957; Shaffer and West, 1960).
Special studies on reproduction
Groups of rats (20 male and 20 female per group) were fed
carbophenothion in the diet and subjected to a standard
three-generation (2 litter per generation) reproduction study at
dosage levels of 0 and 20 ppm. At 20 ppm carbophenothion had an effect
on reproduction in all three generations. In the F0 generation a
reduced mean pup birthweight was observed and fewer young were alive
at weaning. In the F1b generation, an increased number of stillbirths
was observed in two litters and fewer young were alive at weaning in
the second (but not the first) litter. In the F2b generation, an
increase in stillbirths was again observed in two litters, a decreased
number of live births and again fewer pups were alive at weaning.
Foetal resorption was an apparent factor in three of 10 females
examined which had borne less than two litters. An examination of
stillborn pups for teratological effects of carbophenothion was
negative (Johnston and Scott, 1966).
Acute toxicity
Acute toxicity to carbophenothion in some animal species is summarized
in Table 1.
TABLE 1 Acute toxicity to carbophenothion in different animal species
Species Sex Route LD50 Reference
Rat M Oral 17-91 Gray, 1954; Elsea, 1955a,b; Horn, 1957;
Shaffer and West, 1960; Hagan et al.,
1961; Hayes, 1971
F Oral 10 Hayes, 1971
Oral 7-30 Edson et al., 1963
M Dermal 54 Hayes, 1971
F Dermal 27 Hayes, 1971
Mouse M Oral 106-218 Elsea, 1955b; Gray, 1954
Dog M and F IM Approx. Beliles, 1966
40
Rabbit Dermal 800 Edson et al., 1963
Duck M Oral 121 Tucker and Crabtree, 1970
A dose of 25 mg/kg administered to the eye of rabbits resulted in
death in one-third and signs of acute poisoning in the survivors
(Gray, 1954).
Signs of poisoning are typical of organophosphorus esters and include
salivation, lacrimation, diarrhea, miosis, tremors and other signs of
cholinergic stimulation.
Rats exposed to carbophenothion vapours at a dose of 1.04 mg/l for one
hour exhibited depression which subsided within one hour after
treatment (Bullock and Kamienski, 1971).
Atropine and PAM have been shown to be effective therapeutic agents
for acute poisoning by carbophenothion. Repeated administration with
both materials was necessary to insure complete survival of poisoned
animals (Elward and Meydins, 1964). Following acute IM administration
of carbophenothion, atropine and PAM administered IM were effective in
temporarily alleviating signs of poisoning. A return of toxic signs
was evident 2-4 hours after the administration of atropine and PAM
(Beliles, 1965).
Dogs were administered carbophenothion by IM injection at a dose
approximately five times the calculated LD50. This was followed by
atropine alone or in combination with PAM (atropine was administered
twice daily after signs of poisoning occurred and PAM administered
once). All dogs administered atropine alone died, although death was
delayed. One of six dogs receiving atropine and PAM survived.
Mortality in the dogs administered atropine and PAM was delayed.
Atropine plus PAM was a more effective therapeutic agent than atropine
alone. Signs of toxicity and mortality following lethal doses of
carbophenothion were alleviated with the two agents in combination
(Beliles, 1966).
Short-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for 90 days at dosages of 0, 5, 10, 22, 46
and 100 ppm. Clinical signs of toxicity, cholinergic stimulation, were
evident at 46 and 100 ppm. Growth of females was reduced at 100 ppm.
RBC cholinesterase inhibition was evident at levels of 10 ppm and
above, especially in females. Brain and plasma cholinesterase was
inhibited at 22 ppm and above. No effects were observed on survival,
food consumption and gross histological examination of tissues
(Fogleman, 1956; Lehman, 1965).
Dog
Groups of mongrel dogs (2 males and 2 females/group) were administered
carbophenothion orally by gelatin capsule, 6 days/week for 90 days, at
levels of 0.02, 0 04, 0.08, 0.25 and 0.80 mg/kg/day. Dogs at 0.25 and
0.80 mg/kg/day showed plasma cholinesterase depression and did not
receive the total dosage. At 0.02 mg/kg/day plasma cholinesterase was
depressed slightly, while at 0.04 mg/kg inhibition was more apparent.
RBC cholinesterase was normal at all treatment levels (Fogleman, 1956;
Lehman, 1965). Groups of purebred beagle dogs (3 males and 3 females/
group) were fed carbophenothion in the diet for two years at dosages
of 0, 5, 20 and 80 ppm. Erythrocyte and brain cholinesterase activity
were depressed at 80 ppm. Plasma cholinesterase activity was depressed
at all feeding levels at all examination intervals except the initial
one. At 20 and 80 ppm, the groups were distinguishable from controls
chiefly on the basis of decreased body-weight gain (in 80 ppm
females), greater degree of irritability and nervousness and increased
mean relative adrenal weight (in 80 ppm females). No effects were
noted on mortality, physical condition, food consumption, behaviour,
haemograms, clinical chemistry tests, urinalysis, neurological
examinations and gross or histological examination of tissues
(Johnston, 1967).
Cow
Groups of two lactating dairy cows were administered 0, 1, 3, 10 and
30 ppm of carbophenothion in the diet, 7 days/week, for 120 days. The
1 ppm group was changed to 20 ppm on day 23 of the study. A similar
group, receiving 15 ppm, was added to the study on day 70 and
continued for 50 days. Clinical signs of toxicity were evident at 30
ppm. Blood cholinesterase activity was significantly depressed at 20
and 30 ppm. Appearance and behaviour, food consumption and whole blood
cholinesterase activity were unaffected at 15 ppm and below (Weir,
1958).
Long-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for two years at dosage levels of 0, 5, 20
and 80 ppm. Although considerable mortality was observed in all groups
in this study, there was no relationship to the dietary intake. Rats
at 80 and 20 ppm feeding levels displayed tremors, loose stools and
hair loss as well as behaviour changes, including nervousness and
irritability. Females at 80 ppm showed reduced growth during the
second year of study. At 80 ppm, at 100 weeks of feeding, a gradual
lowering of hemoglobin became evident. RBC, plasma and brain
cholinesterase activity was inhibited at 80 and 20 ppm but not at 5
ppm. Cholinesterase inhibition in brain, RBC and plasma was more
evident in females than males. At 80 ppm females showed an increased
adrenal weight and ratio of organ to body-weight. Gross and
histological examinations showed no compound-related effect other than
the increased adrenal gland weight. Analyses of the incidence of
tissue mass showed no differences from control values. There were no
differences in respect to the frequency of neoplasm occurrence
(Johnston, 1967).
OBSERVATIONS IN MAN
The effects of carbophenothion noted on man are limited to several
acute poisoning incidents (Hearn, 1961) and a one-month subacute
administration to humans. Typical signs of cholinergic stimulation in
humans were obvious following ingestion of contaminated flour. This
poisoning was controlled with atropine and PAM. Five people were
administered carbophenothion at a dose of 0.8 mg/kg/day for 30 days.
There were no effects reported on plasma or RBC cholinesterase
activity (Rider et al., 1972).
COMMENT
Carbophenothion is rapidly excreted and is probably metabolized via
oxidative and hydrolytic pathways.
The compound does not induce demyelination and is not potentiated by
those organophosphates tested.
Cholinesterase depression was shown to occur in a rat reproduction
study, conducted at a dose level known to induce maternal
cholinesterase depression. Reduced pup weights, increased resorption
rate and postpartum mortality in pups was shown to occur in a
reproduction study in rats conducted at a dose level known to induce
maternal cholinesterase depression.
Plasma cholinesterase depression was the most sensitive criterion of
effect in both short- and long-term studies in rats and dogs.
A study in man did not result in plasma cholinesterase depression at
0.8 mg/kg/day. The meeting considered that the data available were
insufficient to enable an ADI to be based on this experiment. However,
the 0.8 mg/kg/day level was borne in mind when the study in dogs was
considered in the light of the apparent extreme difference in
sensitivity between man and dog. A no-effect level was not
demonstrated in dog at the lowest dose utilized (5 ppm or 0.125
mg/kg/day). In rat, 5 ppm (0.25 mg/kg/day) is a no-effect level with
regard to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous fruits, citrus
fruits, small fruits, vegetables and field crops, and for ectoparasite
control on cattle and sheep. It is also used as a cereal seed
dressing.
It is available as various strengths of emulsifiable concentrates,
wettable powders and dusts. Recommended treatment levels are as
follows:
1) Deciduous fruit - 1.25 lb to 2.0 lb a.i. per acre with pre-harvest
intervals of 30 days.
2) Citrus fruit - 2.5 lb to 5.0 lb a.i. per acre. Pre-harvest
intervals vary from 0 to 30 days depending on
amount of a.i. used per acre.
3) Vegetable crops - 1.0 lb a.i. per acre; 7 days pre-harvest
interval except for spinach (21 days).
4) Field crops - 1.0 lb a.i. per acre; 21 days pre-harvest
interval.
5) Cattle - 0.02% spray, no pre-slaughter interval.
6) Sheep - 0.026% tip spray; 0.062% saturation spray or
plunge dip - 14 day pre-slaughter interval.
Specific uses include the control of greenbug on sorghum (Ward et
al., 1970) and the control of castor white fly (Radha, 1971).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 2 gives typical results of analysis for residues of
carbophenothion following controlled application at stated rates to
various fruit and vegetable crops (Stauffer Chemical Co., 1972). The
data on citrus fruits refers to residues on whole fruits; evidence was
presented showing that practically all of the residue was retained on
the peel with only very small amounts (0.02 ppm or less) appearing in
the pulp or juice.
Tables 3 and 4 show residues of carbophenothion found in some tissues
of cattle and sheep at different intervals after treatment.
Table 5 gives results obtained from the analysis of milk from cows fed
carbophenothion at various concentrations to simulate feeding of
treated forage (Stauffer Chemical Co., 1972).
TABLE 2 Carbophenothion residues in various crops
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Oranges 0 0.03 0.80
7 0.03 0.67
14 0.03 0.55
21 0.03 0.47
28 0.03 0.38
Grapefruit 0 0.03 1.4
7 0.03 1.1
14 0.03 0.85
21 0.03 0.65
28 0.03 0.53
Lemons 0 0.12 4.0
7 0.12 3.3
14 0.12 2.5
21 0.12 2.2
28 0.12 1.9
Limes 0 0.03 0.95
7 0.03 0.79
14 0.03 0.65
21 0.03 0.53
28 0.03 0.43
Apples 0 0.03 0.73
2 0.03 0.30
12 0.03 0.46
14 0.03 0.88
19 0.03 0.36
26 0.03 0.29
29 0.03 0.37
Pears 33 0.06 0.41
Peaches 14 0.03 0.70
27 0.03 0.15
29 0.03 0.67
Prunes 28 0.04 0.24
29 0.04 0.47
29 0.04 0.42
Apricots 21 0.03 0.78
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Nectarines 14 0.03 0.52-0.66
37 0.03 0.33-0.42
Green alfalfa 0 0.25 70
10 0.25 20
20 0.25 5.5
Alfalfa hay 0 0.25 70
10 0.25 25
20 0.25 8
Soybean hay 0 0.25 70
10 0.25 25
20 0.25 8
Brussels 0 0.9 kg/ha 0.91
sprouts 7 0.9 kg/ha 0.28
Cauliflower 0 1.2 kg/ha 0.56
4 1.2 kg/ha 0.06
Broccoli 0 1.2 kg/ha 2.65
4 1.2 kg/ha 0.33
Sugar beet 7 1.2 3.9
(tops) 11 1.2 2.5
18 1.2 0.6
Sugar beet 0 0.25 0.01
(roots) 8 0.25 0.02
17 0.25 0.02
Spinach 2 4 oz/acre 26.4
5 11.2
10 3.8
15 0.70
2 8 oz/acre 63.4
5 25.8
10 12.5
15 1.7
0 12 oz/acre 36
4 14
22 1.56
29 0.16
2 16 oz/acre 95
5 32.2
10 15.1
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Spinach (cont'd) 15 2.4
Olives 66 0.06 0.08
88 0.06 0.04
119 0.06 0.02
Olive oil 66 0.06 0.16
88 0.06 0.06
119 0.06 0.02
Potatoes )
Rape seed ) Observed residues all below the
Walnuts (shelled) ) limit of determination (0.02 ppm)
Pecans (shelled) )
Information was also available (Stauffer Chemical Co., 1972) regarding
residues occurring in milk and cream from three cows spray-treated
with 0.02% carbophenothion; no residues above the limits of
determination were observed (<0.005 ppm for milk and <0.05 ppm for
cream).
FATE OF RESIDUES
In animals
Fat samples of cattle treated with carbophenothion have been examined
for the presence of carbophenothion, its sulphoxide and sulphone and
the corresponding oxygen-analogue compounds. The parent compound and
lesser amounts of its sulphoxide and sulphone were found (Table 6),
but the oxygen-analogues were not observed (Meyding and Browne, 1962).
In all cases the amounts of the oxygen-analogue, its sulphoxide and
sulphone were below 0.005 ppm.
In plants
Carbophenothion was applied to field-growing lettuce, and samples were
removed at various times after spraying and analysed for
carbophenothion and its oxidative metabolites. Carbophenothion
persisted in growing lettuce for 21 days. Five oxidation products
appeared within 4 hours of spraying and persisted for up to 21 days.
The results indicate that the principle route of oxidation involved
thioether oxidation to form the sulphoxide and then the sulphone,
followed by phosphorothionate oxidation to form the thiophosphate
sulphone (Coffin, 1964). (See also Figure 1). In these experiments the
amount of parent carbophenothion decreased rapidly from 7.0 ppm to 1.2
TABLE 3 Carbophenothion residues in cattle tissues1
Tissue Treatments Days since last treatment
(No.) 1 3 7/8 21 36 43
Perirenal fat 1 - - 0.13 0.09 - -
Subcutaneous fat 1 - - 0.09 0.10 - -
Omental fat 1 - - 0.17 0.15 - -
Perirenal fat 2 - - 0.63 0.20 - -
Subcutaneous fat 2 - - 0.19 0.14 - -
Omental fat 2 - - 0.41 0.24 - -
Perirenal fat 3 1.65 0.69 0.71 0.19 0.25 ND
Subcutaneous fat 3 0.62 0.61 0.37 0.10 0.14 0.04
Omental fat 3 1.12 0.74 0.47 0.25 0.31 0.02
Liver 3 0.019 <0.01 ND2 ND ND ND
Kidney 3 0.048 0.03 <0.01 ND ND ND
Skeletal muscle 3 0.036 0.01 ND ND ND ND
1 Residues found in the tissues of cattle following 1, 2 or 3 weekly spray exposures
to a 0.1% active ingredient suspension. Values represent net corrected ppm of
carbophenothion (Meyding and Browne, 1962).
2 ND = not detected
TABLE 4 Carbophenothion residues in sheep tissues1
Tissue Days after treatment
1 3 10 14
Fat unshorn 0.39 0.66 0.55 0.61
shorn 0.90 0.68 0.29 0.29
Muscle unshorn 0.054 0.072 0.047 0.042
shorn 0.049 0.086 0.032 0.026
TABLE 4 (continued)
Tissue Days after treatment
1 3 10 14
Kidney unshorn *2 0.036 0.045 *
shorn 0.014 0.020 0.020 *
Liver unshorn * 0.019 0.014 0.011
shorn 0.01 0.011 * 0.014
1 Residues found in various tissues of sheep resulting from plunge-dips
in a vat containing 0.05% carbophenothion (Meyding and Patchett, 1964).
Values given in ppm carbophenothion.
2 * = <0.01 ppm
TABLE 5 Carbophenothion residues in milk (ppm carbophenothion)
Duration of
treatment Treatment level (ppm in diet)
(days) 0 1 3 10 20 30
0 *1 * * * * 0.0014 *
7 * * * 0.0023, 0.0029 0.0101 0.0215, 0.0170
14 * * 0.0015, 0.0015 0.0028, 0.0024 0.0085, 0.0052 0.0210, 0.0149
28 * - 0.0015, 0.0024 0.0086, 0.0058 0.0103, 0.0104 0.0171
49 * - 0.0036, 0.0031 0.0058, 0.0047 0.0123, 0.0146 0.0141
70 * - 0.0033, 0.0011 0.0054, 0.0048 0.0115, 0.0088 0.0187
(mean) * * 0.0015 0.0045 0.0102 0.0178
1 * = less than 0.001 ppm.
TABLE 6 Carbophenothion and metabolites in cattle fat
Fat tissue Treatments Days since Carbophenothion -sulphoxide -sulphone
(no.) last treatment (ppm)
Perirenal 3 7 0.563 0.318 0.058
Omental 3 7 0.368 0.107 0.026
Subcutaneous 1 8 0.197 0.068 0.046
TABLE 7 Effect of storage and heat processing on carbophenothion residues
Heat Storage
Food Initial level processed Ambient 100°F
(ppm) temp.(1 yr)
Spinach 0.76 17% 71% 83%
Apricots 0.76 35% 84% 84%
ppm in the first three days and then more slowly to less than 0.1 ppm
at 21 days. The total residue declined more steadily from 8.3 ppm
to 0.1 ppm over the same period.
Evidence of residues in food in commerce or at consumption
Some limited information was available on residues of carbophenothion
occurring in food in commerce. Duggan et al. (1971) have presented
results of analyses from 1963 to 1969 of nearly 7 000 samples of U.S.
domestic and imported large fruits (apples, pears, oranges, peaches).
Of the few samples shown to contain residues of carbophenothion (less
than 1% of those examined), only 25 samples contained more than 0.1
ppm and one sample contained more than 1 ppm. No measurable amounts of
carbophenothion have been observed in various published total diet
studies.
Elkins et al. (1972) studied the effect of storage and heat
processing on the residue level of carbophenothion in canned spinach
and apricots. The results, shown in Table 7, are expressed in terms of
percentage reduction in the residue level.
METHODS OF RESIDUE ANALYSIS
Carbophenothion and its five oxidative metabolites are extracted from
non-fatty materials with aqueous acetonitrile, and from fat and oil
with hexane followed by partitioning in aqueous acetonitrile. The
residues are partially separated from crop extractives by an alumina
shakeout, a partition into chloroform, and an activated carbon
shakeout. The residues are oxidized to the sulphones with potassium
permanganate, further isolated and separated by liquid-solid
chromatography on silica gel H, and determined by gas-liquid
chromatography on OV-1 or QF-1 columns with flame photometric
detection in the phosphorus mode (Buxton, 1971 and Storherr et al.,
1971). These procedures should be suitable for regulatory purposes.
Bowman and Beroza (1970, 1971) published conditions for the gas
chromatography of organophosphorus pesticides, including
carbophenothion, using a flame photometric detector. A method based on
the extraction procedure of McLeod et al. (1967) in conjunction with
the T.L.C. enzymatic inhibition technique of Mendoza et al. (1968)
has been proposed by Mendoza et al. (1970). The presence of
carbophenothion in whole wheat flour could be detected down to 0.5
ppm. Carbophenothion and its five oxidative metabolites have been
determined by gas chromatography using a 5 ft column of 10% DC 200 and
the sulphur mode of the microcoulometric detector (Burke, 1965). A
method has been proposed for the detection and quantitative
determination of organothiophosphorus pesticides, including
carbophenothion, by in situ fluorimetry after separation on silica
gel layers. Yellowish-green fluorescent spots are obtained when the
plate is sprayed with a 3-hydroxyflavone, such as robinetin, after
bromination. Linear calibration curves up to 4 mg per spot of the
pesticide have been obtained and a relative standard deviation of
approximately 4% can be expected at the 1.0 µg level. Visual and
instrumental detection limits are around 0.04 µg per spot for certain
pesticides (Frei et al., 1971).
A thin-layer chromatographic procedure has been described for
separating and detecting carbophenothion and 16 other
cholinesterase-inhibiting pesticides on aluminum oxide and silica gel.
The detection of the anticholinesterase activity is based on the
hydrolysis of indophenyl acetate by bee brain cholinesterase. Limits
of detectable cholinesterase inhibitors are at the subnanogram level,
and the time required for detection is about 40 minutes (Winterlin
et al., 1968). A detection limit of 5 ng was obtained by Gardner
(1971) using a combined two-dimensional TLC - esterase method.
Patchett and Batchelder (1960) describe a two-phase hydrogen
peroxide-acetic acid-benzene system to oxidize carbophenothion to
strong cholinesterase inhibitors, which are subsequently determined by
measuring their inhibition of cholinesterase in human blood plasma.
The method is sensitive to 0.01 µg of carbophenothion and will detect
0.005 ppm residue in crops having low background values.
Villeneuve et al. (1970) investigated the separation and detection
of 13 organophosphorus pesticides, including carbophenothion, using a
combined TLC-GLC method. The separation of carbophenothion using a
silica gel microcolumn was investigated by Leoni (1971). A routine TLC
method for the determination of residues of organophosphorus
pesticides, including carbophenothion, has been proposed by Mees and
Antoine (1971). The procedure uses both silica gel and polyamide
coated plates.
Table 8 shows examples of national tolerances as reported to the Joint
Meeting.
TABLE 8
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
Australia Fruit and vegetables 1
Fat of meat of cattle and sheep 1
Belgium Fruit and vegetables 0.5
Canada Cherries, eggplants, peppers,
pimentos, plums, prunes, tomatoes 0.5
Apples, crabapples, onions,
pears, quinces 0.8
Federal
Republic
of Germany Rape, citrus fruit (without peel) 0.05
Netherlands Fruit and vegetables 0.05
TABLE 8 (cont'd)
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
U.S.A. Almond hulls 10
Alfalfa (green and hey),
clover (green and hay),
sugarbeet (root and tops) 5
Citrus fruit 2
Apples, apricots, snap beans,
lima beans, garden beets (root and
tops), melons 0.8
Cherries, cucumbers, eggplant, figs,
grapes, nectarines, olives, onions
(dry bulb and green), peaches,
pears, peas, peppers, plums,
quinces, pimentos, soybeans,
spinach, strawberries, summer
squash, tomatoes 0.8
Fat of meat of cattle, goats,
pigs and sheep 0.2
Dry beans, pecans, walnuts 0.1
APPRAISAL
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous, citrus and
small fruits, field crops, vegetables and for ectoparasite control on
cattle and sheep; it also has uses as a cereal seed dressing. The
pesticidal action is fairly persistent and residues are comprised
mostly of the parent compound together with its sulphoxide and, to a
lesser extent, its sulphone; the corresponding oxygen analogues are
rarely detected in animal tissue but have been observed as minor
components on treated vegetables. Following treatment of animals, the
bulk of the residue is contained in the fatty tissues. Residue data
was available following supervised trial treatments of fruit,
vegetables and animals, but no information was given regarding
foodstuffs moving in commerce. No residues have been observed in total
diet studies. Available multi-residue gas chromatographic procedures
should be suitable for application for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Temporary tolerances apply to the total residue, comprising
carbophenothion, its sulphoxide and sulphone together with their
corresponding oxygen analogues, if present, expressed as
carbophenothion.
ppm
Lemons 5
Spinach 2
Fat of meat of cattle and sheep 1
Grapefruit, limes, oranges, prunes,
apricots, nectarines, peaches 1
Broccoli, Brussels sprouts, cauliflower,
apples, pears 0.5
Olive oil 0.2
Olives, sugar beet 0.1
Milk and milk products (fat basis) 0.1
Potatoes, rape seed 0.02*
Walnuts and pecans (shelled) 0.02*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1976)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
2. An adequate reproduction study.
REFERENCES
Beliles, R.P. (1965) Trithion - safety evaluation by analysis of
cholinesterase reactivation with atropine and PAM after trithion
poisoning. Report Woodard Research Corporation. (unpublished)
Beliles, R.P. (1966) Trithion - safety evaluation by analyses of
cholinesterase reactivation with atropine and PAM after trithion
poisoning in the dog. Report Woodard Research Corporation.
(unpublished)
Bowman, M.C. and Beroza, M. (1970) G.L.C. retention time of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J. Ass. off. analyt. Chem., 53: 499-508.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on specially
washed Chromosorb W for multi-component residue determinations of
phosphorus and sulphur containing pesticides by flame photometric
G.L.C. J. Ass. off. analyt. Chem., 54: 1086-1092.
Bullock, C.H. and Kamienski, F.X. (1971) Acute inhalation screen, male
and female rats. Report Stauffer Chemical Company. (unpublished)
Burke, J.A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. off. Agric. Chem., 48: 1037-1058.
Buxton, R.W. (1971) Determination of residues of Trithion and its
oxygen analogues. Report Stauffer Chemical Co. (unpublished)
Coffin, D.E. (1964) Oxidative metabolism and persistence of Trithion
on field sprayed lettuce. J. Ass. off. Agric. Chem., 47: 662-667.
Duggan, R.E., Lipscomb, G.Q., Cox, E.L., Heatwole, R.E. and Kling,
R.C. (1971) Pesticide residue levels In food in the United States July
1, 1963 to June 30, 1969. Pestic. Monit. J., 5: 73-212.
Edson, E.F., Sanderson, D.M. and Noakes, D.N. (1963) Acute toxicity
data for pesticides. World Rev. Pest Control 2: 26-28.
Elsea, J.R. (1955a) Acute oral administration - rats. Report Hazleton
Laboratories. (unpublished)
Elsea, J.R. (1955b) Acute oral administration - mice. Report Hazleton
Laboratories. (unpublished)
Elkins, E.R., Farrow, R.P. and Kim, E.S. (1972) The effect of heat
processing and storage on pesticide residues in spinach and apricots.
J. Agr. Fd. Chem., 20: 286-291.
Elward, T.E. and Meyding, G.D. (1964) Carbophenothion. Reactivation
study - rats. Report No. 64-5 Stauffer Chemical Company. (unpublished)
Fogleman, R.W. (1956) Subacute feeding - rats. Report Hazleton
Laboratories. (unpublished)
Frei, R.W., Mallet, V. and Pothier, C. (1971) Flavones as fluorogenic
spray reagents for organothiophosphorus pesticides on silica gel
layers. J. Chromat., 59: 135-140.
Gardner, A.M. (1971) Confirmation of organophosphorus pesticide
residues at nanogram levels by two-dimensional T.L.C. J. Ass. off.
analyt. Chem., 54: 517-524.
Gray, E.H. (1954) Acute oral, dermal and eye application. Report
Hazleton Laboratories (unpublished)
Hagan, E.C., Jenner, P.M. and Fitzhugh, O.G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432.
Hayes, W.J., Jr. (1971) Clinical handbook on economic poisons. U.S.
EPA Pesticides Programs, Public Health Service Publication 476.
Hearn, C.E.D. (1961) Trithion poisoning. Bull. J. Ind. Med., 18:
231-233.
Horn, H.J. (1957) Trithion. Potentiation study, subacute feeding -
dogs, acute oral administration - rats. Report Hazleton Laboratories.
(unpublished)
Johnston, C.D. (1967) Trithion. Safety evaluation by two-year feeding
studies in the rat and the dog. Report Woodard Research Corporation.
(unpublished)
Johnston, C.D. and Scott, W.J. (1966) Trithion. Three generation
reproduction study in the rat. Report Woodard Research Corporation.
(unpublished)
Lehman, A.J. (1965) Summaries of pesticide toxicity. The Assoc. of
Food and Drug Officials of the U.S.
Leoni, V. (1971) The separation of fifty pesticides and related
compounds and polychlorobiphenyls into four groups by silica gel
microcolumn chromatography. J. Chromat., 62: 63-71.
Lobdell, B.J. and Johnston, C.D. (1964) Trithion-demyelination in
chickens. Report Woodard Research Corporation. (unpublished)
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1967) Metabolism of 32P
Trithion in the white mouse and American cockroach. Report Stauffer
Chemical Company. (unpublished)
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967)
Comparison of various carbon absorbents. Quantitative elution and
separation of 42 pesticides from a carbon-Solka Floc clean-up column.
J. Ass. off. analyt. Chem., 50: 1216-1228.
Mees, G. and Antoine, O. (1971) Routine method for the determination
of organophosphorus insecticides by thin-layer chromatography (in
French). J. Chromat., 58: 247-256.
Mendoza, C., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Enzymatic detection of 10 organophosphorus pesticides and carbaryl on
thin-layer chromatograms. Evaluation of indoxyl, substituted indoxyl
and 1-naphthyl acetates as substrates for esterases. Analyst, Lond.,
93: 34-38.
Mendoza, C., Wales, P.J. and McLeod, H.A. (1970) Simultaneous
detection of some organophosphorus pesticides in whole-wheat flour.
Bull. envir. Contam. Toxicol., 5: 276-278.
Menzies, C.M. (1969) Metabolism of pesticides. U.S. Dept. of the
Interior, Special scientific report No. 127.
Meyding, G.D. and Browne, H.L. (1962) Cattle spray program. Report
Hazelton-Nuclear Science Corporation. (unpublished)
Meyding, G.D. and Patchett, G.G. (1964) Garrathion 3.5E; Plunge-dip
toxicity/residue studies: sheep. Report Stauffer Chemical Co.
(unpublished)
Patchett, G.G. and Batchelder, G.H. (1960) Determination of Trithion
crop residues by cholinesterase inhibition measurement. J. Agr. Fd.
Chem., 8: 54-57.
Radha, N.V. (1971) Efficacy of some synthetic insecticides in the
control of the castor white fly, Trialeurodes ricini M. Madras Agr.
J., 58: 300-302.
Rider, J.A., Swader, J.I. and Puletti, E.J. (1972) Anticholinesterase
studies with Guthion, Phosdrin, Disyston and Trithion in human
subjects. Fed. Proc., 31: 520 (Abs # 1730).
Shaffer, B.C. and West, R. (1960) The acute and subacute toxicity of
technical tetram. Toxicology and applied pharmacology, 2:1-13.
Stauffer Chemical Co. (1972) Evaluation of residues in food.
(unpublished)
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticides in non-fatty foods. J. Ass. off. analyt.
Chem., 54: 513-516.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of Interior. Resource publication
No. 84.
Villeneuve, D.C., Butterfield, A.C., Grant, D.L. and McCully, K.A.
(1970) The detection, separation and quantitative recovery of 13
organophosphorus pesticides on silica gel GF254 thin-layer
chromatograms. J. Chromat., 48: 567-571.
Ward, C.R., Huddleston, E.W., Ashdown, D., Owens, J.C. and Polk, K.L.
(1970) Greenbug control on grain sorghum and the effects of tested
insecticides on other insects. J. Econ. Entomol., 63: 1929-1934.
Weir, R.J. (1958) Subacute feeding - dairy cows. Report Hazleton
Laboratories. (unpublished)
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Fd. Chem., 16: 808-812.
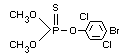 Physical and chemical properties
State: off-white to light amber coloured liquid
Boiling point: 82°C at 0.01 mm Hg
Vapour pressure: 3 × 10-7 mm Hg at 20°C
Specific gravity: 1.265 - 1.285 at 20°C
Molecular weight: 342.85
Refractive index: 1.590 - 1.597
Solubility: slightly soluble in water (<0.04 g/l at 20°C), Miscible
with most organic solvents (hydrocarbons, alcohols, ketones, esters)
Stability: stable to hydrolysis but oxidized to the phosphorothiolate
Purity: technical material about 95%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on absorption and distribution of carbophenothion in mammals
are not available. Following intraperitoneal administration to mice,
greater than 75% of the administered dose was excreted in the urine
within 24 hours. During the following 16 hours only 4-7% of the dose
was excreted. Carbophenothion is apparently rapidly eliminated from
the body (March et al., 1957).
Biotransformation
The metabolism of carbophenothion is qualitatively the same in mice,
insects and plants, the principal products being oxidative. Although
the transformation of carbophenothion to oxidative and hydrolytic
components has been postulated, little data are available on the
quantitative degradation in mammals. Oxidative metabolism of
carbophenothion has been shown to result in a substantial increase in
anticholinesterase activity except for the sulphone of the
phosphorodithioate. A quantitative difference in animal and plant
metabolites was the presence of an unidentified product on the surface
of plants which appeared to a minor degree in the internal parts and
was found as a minor component in mouse urine and in vitro tests
with mouse liver. The component is probably the phosphorodithioate
sulphoxide analogue (March et al., 1957). Five oxidative products of
carbophenothion have been identified (see figure 1).
S O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion oxygen analogue
(5 × 10-4M) (2 × 10-6M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion sulphoxide oxygen analogue sulphoxide
(8 × 10-6M) (2 × 10-7M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2O 6 4 2 2O 6 4
carbophenothion sulphone oxygen analogue sulphone
(1 × 10-4M) (1 × 10-8M)
Figure 1 - Oxidative products of carbophenothion
The number in parenthesis under the oxidative products in Figure 1 is
the I50 value for housefly head cholinesterase (March et al.,
1957).
Results of studies on the metabolism of carbophenothion in lettuce
have shown a typical pattern of oxidative metabolism resulting in the
sulphoxide and sulphone analogues of the phosphorothioate and
phosphorodithioate. Exposure of carbophenothion to UV light resulted
in the appearance of the phosphorodithioate sulphoxide analogue
(Menzies, 1969).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of White Leghorn hens (10 hens/group) were fed carbophenothion
in the diet at levels of 0, 10, 31.6 and 100 ppm for seven weeks. TOCP
was fed at 500 ppm as a positive control. Egg production was affected
at 100 ppm. Clinical signs of ataxia were evident in TOCP-fed hens but
were absent in animals fed all levels of carbophenothion. Histological
examination of nerve tissue showed slight evidence of demyelination in
TOCP treated hens but none with carbophenothion (Lobdell and Johnston,
1964).
Special studies on potentiation
Groups of dogs (1 male and 1 female/group) were fed carbophenothion (1
ppm) in the diet in combination with parathion (1 ppm),
methylparathion (5 ppm), EPN (20 ppm), demephion (2 ppm), or malathion
(100 ppm) for 45 days. No evidence of greater than additive depression
of cholinesterase activity was observed (Horn, 1957).
Carbophenothion in combination with five other organophosphates
(parathion, methylparathion, EPN, demephion and malathion) when
administered orally to rats did not show signs of potentiation of
acute toxicity (Horn, 1957; Shaffer and West, 1960).
Special studies on reproduction
Groups of rats (20 male and 20 female per group) were fed
carbophenothion in the diet and subjected to a standard
three-generation (2 litter per generation) reproduction study at
dosage levels of 0 and 20 ppm. At 20 ppm carbophenothion had an effect
on reproduction in all three generations. In the F0 generation a
reduced mean pup birthweight was observed and fewer young were alive
at weaning. In the F1b generation, an increased number of stillbirths
was observed in two litters and fewer young were alive at weaning in
the second (but not the first) litter. In the F2b generation, an
increase in stillbirths was again observed in two litters, a decreased
number of live births and again fewer pups were alive at weaning.
Foetal resorption was an apparent factor in three of 10 females
examined which had borne less than two litters. An examination of
stillborn pups for teratological effects of carbophenothion was
negative (Johnston and Scott, 1966).
Acute toxicity
Acute toxicity to carbophenothion in some animal species is summarized
in Table 1.
TABLE 1 Acute toxicity to carbophenothion in different animal species
Species Sex Route LD50 Reference
Rat M Oral 17-91 Gray, 1954; Elsea, 1955a,b; Horn, 1957;
Shaffer and West, 1960; Hagan et al.,
1961; Hayes, 1971
F Oral 10 Hayes, 1971
Oral 7-30 Edson et al., 1963
M Dermal 54 Hayes, 1971
F Dermal 27 Hayes, 1971
Mouse M Oral 106-218 Elsea, 1955b; Gray, 1954
Dog M and F IM Approx. Beliles, 1966
40
Rabbit Dermal 800 Edson et al., 1963
Duck M Oral 121 Tucker and Crabtree, 1970
A dose of 25 mg/kg administered to the eye of rabbits resulted in
death in one-third and signs of acute poisoning in the survivors
(Gray, 1954).
Signs of poisoning are typical of organophosphorus esters and include
salivation, lacrimation, diarrhea, miosis, tremors and other signs of
cholinergic stimulation.
Rats exposed to carbophenothion vapours at a dose of 1.04 mg/l for one
hour exhibited depression which subsided within one hour after
treatment (Bullock and Kamienski, 1971).
Atropine and PAM have been shown to be effective therapeutic agents
for acute poisoning by carbophenothion. Repeated administration with
both materials was necessary to insure complete survival of poisoned
animals (Elward and Meydins, 1964). Following acute IM administration
of carbophenothion, atropine and PAM administered IM were effective in
temporarily alleviating signs of poisoning. A return of toxic signs
was evident 2-4 hours after the administration of atropine and PAM
(Beliles, 1965).
Dogs were administered carbophenothion by IM injection at a dose
approximately five times the calculated LD50. This was followed by
atropine alone or in combination with PAM (atropine was administered
twice daily after signs of poisoning occurred and PAM administered
once). All dogs administered atropine alone died, although death was
delayed. One of six dogs receiving atropine and PAM survived.
Mortality in the dogs administered atropine and PAM was delayed.
Atropine plus PAM was a more effective therapeutic agent than atropine
alone. Signs of toxicity and mortality following lethal doses of
carbophenothion were alleviated with the two agents in combination
(Beliles, 1966).
Short-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for 90 days at dosages of 0, 5, 10, 22, 46
and 100 ppm. Clinical signs of toxicity, cholinergic stimulation, were
evident at 46 and 100 ppm. Growth of females was reduced at 100 ppm.
RBC cholinesterase inhibition was evident at levels of 10 ppm and
above, especially in females. Brain and plasma cholinesterase was
inhibited at 22 ppm and above. No effects were observed on survival,
food consumption and gross histological examination of tissues
(Fogleman, 1956; Lehman, 1965).
Dog
Groups of mongrel dogs (2 males and 2 females/group) were administered
carbophenothion orally by gelatin capsule, 6 days/week for 90 days, at
levels of 0.02, 0 04, 0.08, 0.25 and 0.80 mg/kg/day. Dogs at 0.25 and
0.80 mg/kg/day showed plasma cholinesterase depression and did not
receive the total dosage. At 0.02 mg/kg/day plasma cholinesterase was
depressed slightly, while at 0.04 mg/kg inhibition was more apparent.
RBC cholinesterase was normal at all treatment levels (Fogleman, 1956;
Lehman, 1965). Groups of purebred beagle dogs (3 males and 3 females/
group) were fed carbophenothion in the diet for two years at dosages
of 0, 5, 20 and 80 ppm. Erythrocyte and brain cholinesterase activity
were depressed at 80 ppm. Plasma cholinesterase activity was depressed
at all feeding levels at all examination intervals except the initial
one. At 20 and 80 ppm, the groups were distinguishable from controls
chiefly on the basis of decreased body-weight gain (in 80 ppm
females), greater degree of irritability and nervousness and increased
mean relative adrenal weight (in 80 ppm females). No effects were
noted on mortality, physical condition, food consumption, behaviour,
haemograms, clinical chemistry tests, urinalysis, neurological
examinations and gross or histological examination of tissues
(Johnston, 1967).
Cow
Groups of two lactating dairy cows were administered 0, 1, 3, 10 and
30 ppm of carbophenothion in the diet, 7 days/week, for 120 days. The
1 ppm group was changed to 20 ppm on day 23 of the study. A similar
group, receiving 15 ppm, was added to the study on day 70 and
continued for 50 days. Clinical signs of toxicity were evident at 30
ppm. Blood cholinesterase activity was significantly depressed at 20
and 30 ppm. Appearance and behaviour, food consumption and whole blood
cholinesterase activity were unaffected at 15 ppm and below (Weir,
1958).
Long-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for two years at dosage levels of 0, 5, 20
and 80 ppm. Although considerable mortality was observed in all groups
in this study, there was no relationship to the dietary intake. Rats
at 80 and 20 ppm feeding levels displayed tremors, loose stools and
hair loss as well as behaviour changes, including nervousness and
irritability. Females at 80 ppm showed reduced growth during the
second year of study. At 80 ppm, at 100 weeks of feeding, a gradual
lowering of hemoglobin became evident. RBC, plasma and brain
cholinesterase activity was inhibited at 80 and 20 ppm but not at 5
ppm. Cholinesterase inhibition in brain, RBC and plasma was more
evident in females than males. At 80 ppm females showed an increased
adrenal weight and ratio of organ to body-weight. Gross and
histological examinations showed no compound-related effect other than
the increased adrenal gland weight. Analyses of the incidence of
tissue mass showed no differences from control values. There were no
differences in respect to the frequency of neoplasm occurrence
(Johnston, 1967).
OBSERVATIONS IN MAN
The effects of carbophenothion noted on man are limited to several
acute poisoning incidents (Hearn, 1961) and a one-month subacute
administration to humans. Typical signs of cholinergic stimulation in
humans were obvious following ingestion of contaminated flour. This
poisoning was controlled with atropine and PAM. Five people were
administered carbophenothion at a dose of 0.8 mg/kg/day for 30 days.
There were no effects reported on plasma or RBC cholinesterase
activity (Rider et al., 1972).
COMMENT
Carbophenothion is rapidly excreted and is probably metabolized via
oxidative and hydrolytic pathways.
The compound does not induce demyelination and is not potentiated by
those organophosphates tested.
Cholinesterase depression was shown to occur in a rat reproduction
study, conducted at a dose level known to induce maternal
cholinesterase depression. Reduced pup weights, increased resorption
rate and postpartum mortality in pups was shown to occur in a
reproduction study in rats conducted at a dose level known to induce
maternal cholinesterase depression.
Plasma cholinesterase depression was the most sensitive criterion of
effect in both short- and long-term studies in rats and dogs.
A study in man did not result in plasma cholinesterase depression at
0.8 mg/kg/day. The meeting considered that the data available were
insufficient to enable an ADI to be based on this experiment. However,
the 0.8 mg/kg/day level was borne in mind when the study in dogs was
considered in the light of the apparent extreme difference in
sensitivity between man and dog. A no-effect level was not
demonstrated in dog at the lowest dose utilized (5 ppm or 0.125
mg/kg/day). In rat, 5 ppm (0.25 mg/kg/day) is a no-effect level with
regard to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous fruits, citrus
fruits, small fruits, vegetables and field crops, and for ectoparasite
control on cattle and sheep. It is also used as a cereal seed
dressing.
It is available as various strengths of emulsifiable concentrates,
wettable powders and dusts. Recommended treatment levels are as
follows:
1) Deciduous fruit - 1.25 lb to 2.0 lb a.i. per acre with pre-harvest
intervals of 30 days.
2) Citrus fruit - 2.5 lb to 5.0 lb a.i. per acre. Pre-harvest
intervals vary from 0 to 30 days depending on
amount of a.i. used per acre.
3) Vegetable crops - 1.0 lb a.i. per acre; 7 days pre-harvest
interval except for spinach (21 days).
4) Field crops - 1.0 lb a.i. per acre; 21 days pre-harvest
interval.
5) Cattle - 0.02% spray, no pre-slaughter interval.
6) Sheep - 0.026% tip spray; 0.062% saturation spray or
plunge dip - 14 day pre-slaughter interval.
Specific uses include the control of greenbug on sorghum (Ward et
al., 1970) and the control of castor white fly (Radha, 1971).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 2 gives typical results of analysis for residues of
carbophenothion following controlled application at stated rates to
various fruit and vegetable crops (Stauffer Chemical Co., 1972). The
data on citrus fruits refers to residues on whole fruits; evidence was
presented showing that practically all of the residue was retained on
the peel with only very small amounts (0.02 ppm or less) appearing in
the pulp or juice.
Tables 3 and 4 show residues of carbophenothion found in some tissues
of cattle and sheep at different intervals after treatment.
Table 5 gives results obtained from the analysis of milk from cows fed
carbophenothion at various concentrations to simulate feeding of
treated forage (Stauffer Chemical Co., 1972).
TABLE 2 Carbophenothion residues in various crops
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Oranges 0 0.03 0.80
7 0.03 0.67
14 0.03 0.55
21 0.03 0.47
28 0.03 0.38
Grapefruit 0 0.03 1.4
7 0.03 1.1
14 0.03 0.85
21 0.03 0.65
28 0.03 0.53
Lemons 0 0.12 4.0
7 0.12 3.3
14 0.12 2.5
21 0.12 2.2
28 0.12 1.9
Limes 0 0.03 0.95
7 0.03 0.79
14 0.03 0.65
21 0.03 0.53
28 0.03 0.43
Apples 0 0.03 0.73
2 0.03 0.30
12 0.03 0.46
14 0.03 0.88
19 0.03 0.36
26 0.03 0.29
29 0.03 0.37
Pears 33 0.06 0.41
Peaches 14 0.03 0.70
27 0.03 0.15
29 0.03 0.67
Prunes 28 0.04 0.24
29 0.04 0.47
29 0.04 0.42
Apricots 21 0.03 0.78
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Nectarines 14 0.03 0.52-0.66
37 0.03 0.33-0.42
Green alfalfa 0 0.25 70
10 0.25 20
20 0.25 5.5
Alfalfa hay 0 0.25 70
10 0.25 25
20 0.25 8
Soybean hay 0 0.25 70
10 0.25 25
20 0.25 8
Brussels 0 0.9 kg/ha 0.91
sprouts 7 0.9 kg/ha 0.28
Cauliflower 0 1.2 kg/ha 0.56
4 1.2 kg/ha 0.06
Broccoli 0 1.2 kg/ha 2.65
4 1.2 kg/ha 0.33
Sugar beet 7 1.2 3.9
(tops) 11 1.2 2.5
18 1.2 0.6
Sugar beet 0 0.25 0.01
(roots) 8 0.25 0.02
17 0.25 0.02
Spinach 2 4 oz/acre 26.4
5 11.2
10 3.8
15 0.70
2 8 oz/acre 63.4
5 25.8
10 12.5
15 1.7
0 12 oz/acre 36
4 14
22 1.56
29 0.16
2 16 oz/acre 95
5 32.2
10 15.1
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Spinach (cont'd) 15 2.4
Olives 66 0.06 0.08
88 0.06 0.04
119 0.06 0.02
Olive oil 66 0.06 0.16
88 0.06 0.06
119 0.06 0.02
Potatoes )
Rape seed ) Observed residues all below the
Walnuts (shelled) ) limit of determination (0.02 ppm)
Pecans (shelled) )
Information was also available (Stauffer Chemical Co., 1972) regarding
residues occurring in milk and cream from three cows spray-treated
with 0.02% carbophenothion; no residues above the limits of
determination were observed (<0.005 ppm for milk and <0.05 ppm for
cream).
FATE OF RESIDUES
In animals
Fat samples of cattle treated with carbophenothion have been examined
for the presence of carbophenothion, its sulphoxide and sulphone and
the corresponding oxygen-analogue compounds. The parent compound and
lesser amounts of its sulphoxide and sulphone were found (Table 6),
but the oxygen-analogues were not observed (Meyding and Browne, 1962).
In all cases the amounts of the oxygen-analogue, its sulphoxide and
sulphone were below 0.005 ppm.
In plants
Carbophenothion was applied to field-growing lettuce, and samples were
removed at various times after spraying and analysed for
carbophenothion and its oxidative metabolites. Carbophenothion
persisted in growing lettuce for 21 days. Five oxidation products
appeared within 4 hours of spraying and persisted for up to 21 days.
The results indicate that the principle route of oxidation involved
thioether oxidation to form the sulphoxide and then the sulphone,
followed by phosphorothionate oxidation to form the thiophosphate
sulphone (Coffin, 1964). (See also Figure 1). In these experiments the
amount of parent carbophenothion decreased rapidly from 7.0 ppm to 1.2
TABLE 3 Carbophenothion residues in cattle tissues1
Tissue Treatments Days since last treatment
(No.) 1 3 7/8 21 36 43
Perirenal fat 1 - - 0.13 0.09 - -
Subcutaneous fat 1 - - 0.09 0.10 - -
Omental fat 1 - - 0.17 0.15 - -
Perirenal fat 2 - - 0.63 0.20 - -
Subcutaneous fat 2 - - 0.19 0.14 - -
Omental fat 2 - - 0.41 0.24 - -
Perirenal fat 3 1.65 0.69 0.71 0.19 0.25 ND
Subcutaneous fat 3 0.62 0.61 0.37 0.10 0.14 0.04
Omental fat 3 1.12 0.74 0.47 0.25 0.31 0.02
Liver 3 0.019 <0.01 ND2 ND ND ND
Kidney 3 0.048 0.03 <0.01 ND ND ND
Skeletal muscle 3 0.036 0.01 ND ND ND ND
1 Residues found in the tissues of cattle following 1, 2 or 3 weekly spray exposures
to a 0.1% active ingredient suspension. Values represent net corrected ppm of
carbophenothion (Meyding and Browne, 1962).
2 ND = not detected
TABLE 4 Carbophenothion residues in sheep tissues1
Tissue Days after treatment
1 3 10 14
Fat unshorn 0.39 0.66 0.55 0.61
shorn 0.90 0.68 0.29 0.29
Muscle unshorn 0.054 0.072 0.047 0.042
shorn 0.049 0.086 0.032 0.026
TABLE 4 (continued)
Tissue Days after treatment
1 3 10 14
Kidney unshorn *2 0.036 0.045 *
shorn 0.014 0.020 0.020 *
Liver unshorn * 0.019 0.014 0.011
shorn 0.01 0.011 * 0.014
1 Residues found in various tissues of sheep resulting from plunge-dips
in a vat containing 0.05% carbophenothion (Meyding and Patchett, 1964).
Values given in ppm carbophenothion.
2 * = <0.01 ppm
TABLE 5 Carbophenothion residues in milk (ppm carbophenothion)
Duration of
treatment Treatment level (ppm in diet)
(days) 0 1 3 10 20 30
0 *1 * * * * 0.0014 *
7 * * * 0.0023, 0.0029 0.0101 0.0215, 0.0170
14 * * 0.0015, 0.0015 0.0028, 0.0024 0.0085, 0.0052 0.0210, 0.0149
28 * - 0.0015, 0.0024 0.0086, 0.0058 0.0103, 0.0104 0.0171
49 * - 0.0036, 0.0031 0.0058, 0.0047 0.0123, 0.0146 0.0141
70 * - 0.0033, 0.0011 0.0054, 0.0048 0.0115, 0.0088 0.0187
(mean) * * 0.0015 0.0045 0.0102 0.0178
1 * = less than 0.001 ppm.
TABLE 6 Carbophenothion and metabolites in cattle fat
Fat tissue Treatments Days since Carbophenothion -sulphoxide -sulphone
(no.) last treatment (ppm)
Perirenal 3 7 0.563 0.318 0.058
Omental 3 7 0.368 0.107 0.026
Subcutaneous 1 8 0.197 0.068 0.046
TABLE 7 Effect of storage and heat processing on carbophenothion residues
Heat Storage
Food Initial level processed Ambient 100°F
(ppm) temp.(1 yr)
Spinach 0.76 17% 71% 83%
Apricots 0.76 35% 84% 84%
ppm in the first three days and then more slowly to less than 0.1 ppm
at 21 days. The total residue declined more steadily from 8.3 ppm
to 0.1 ppm over the same period.
Evidence of residues in food in commerce or at consumption
Some limited information was available on residues of carbophenothion
occurring in food in commerce. Duggan et al. (1971) have presented
results of analyses from 1963 to 1969 of nearly 7 000 samples of U.S.
domestic and imported large fruits (apples, pears, oranges, peaches).
Of the few samples shown to contain residues of carbophenothion (less
than 1% of those examined), only 25 samples contained more than 0.1
ppm and one sample contained more than 1 ppm. No measurable amounts of
carbophenothion have been observed in various published total diet
studies.
Elkins et al. (1972) studied the effect of storage and heat
processing on the residue level of carbophenothion in canned spinach
and apricots. The results, shown in Table 7, are expressed in terms of
percentage reduction in the residue level.
METHODS OF RESIDUE ANALYSIS
Carbophenothion and its five oxidative metabolites are extracted from
non-fatty materials with aqueous acetonitrile, and from fat and oil
with hexane followed by partitioning in aqueous acetonitrile. The
residues are partially separated from crop extractives by an alumina
shakeout, a partition into chloroform, and an activated carbon
shakeout. The residues are oxidized to the sulphones with potassium
permanganate, further isolated and separated by liquid-solid
chromatography on silica gel H, and determined by gas-liquid
chromatography on OV-1 or QF-1 columns with flame photometric
detection in the phosphorus mode (Buxton, 1971 and Storherr et al.,
1971). These procedures should be suitable for regulatory purposes.
Bowman and Beroza (1970, 1971) published conditions for the gas
chromatography of organophosphorus pesticides, including
carbophenothion, using a flame photometric detector. A method based on
the extraction procedure of McLeod et al. (1967) in conjunction with
the T.L.C. enzymatic inhibition technique of Mendoza et al. (1968)
has been proposed by Mendoza et al. (1970). The presence of
carbophenothion in whole wheat flour could be detected down to 0.5
ppm. Carbophenothion and its five oxidative metabolites have been
determined by gas chromatography using a 5 ft column of 10% DC 200 and
the sulphur mode of the microcoulometric detector (Burke, 1965). A
method has been proposed for the detection and quantitative
determination of organothiophosphorus pesticides, including
carbophenothion, by in situ fluorimetry after separation on silica
gel layers. Yellowish-green fluorescent spots are obtained when the
plate is sprayed with a 3-hydroxyflavone, such as robinetin, after
bromination. Linear calibration curves up to 4 mg per spot of the
pesticide have been obtained and a relative standard deviation of
approximately 4% can be expected at the 1.0 µg level. Visual and
instrumental detection limits are around 0.04 µg per spot for certain
pesticides (Frei et al., 1971).
A thin-layer chromatographic procedure has been described for
separating and detecting carbophenothion and 16 other
cholinesterase-inhibiting pesticides on aluminum oxide and silica gel.
The detection of the anticholinesterase activity is based on the
hydrolysis of indophenyl acetate by bee brain cholinesterase. Limits
of detectable cholinesterase inhibitors are at the subnanogram level,
and the time required for detection is about 40 minutes (Winterlin
et al., 1968). A detection limit of 5 ng was obtained by Gardner
(1971) using a combined two-dimensional TLC - esterase method.
Patchett and Batchelder (1960) describe a two-phase hydrogen
peroxide-acetic acid-benzene system to oxidize carbophenothion to
strong cholinesterase inhibitors, which are subsequently determined by
measuring their inhibition of cholinesterase in human blood plasma.
The method is sensitive to 0.01 µg of carbophenothion and will detect
0.005 ppm residue in crops having low background values.
Villeneuve et al. (1970) investigated the separation and detection
of 13 organophosphorus pesticides, including carbophenothion, using a
combined TLC-GLC method. The separation of carbophenothion using a
silica gel microcolumn was investigated by Leoni (1971). A routine TLC
method for the determination of residues of organophosphorus
pesticides, including carbophenothion, has been proposed by Mees and
Antoine (1971). The procedure uses both silica gel and polyamide
coated plates.
Table 8 shows examples of national tolerances as reported to the Joint
Meeting.
TABLE 8
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
Australia Fruit and vegetables 1
Fat of meat of cattle and sheep 1
Belgium Fruit and vegetables 0.5
Canada Cherries, eggplants, peppers,
pimentos, plums, prunes, tomatoes 0.5
Apples, crabapples, onions,
pears, quinces 0.8
Federal
Republic
of Germany Rape, citrus fruit (without peel) 0.05
Netherlands Fruit and vegetables 0.05
TABLE 8 (cont'd)
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
U.S.A. Almond hulls 10
Alfalfa (green and hey),
clover (green and hay),
sugarbeet (root and tops) 5
Citrus fruit 2
Apples, apricots, snap beans,
lima beans, garden beets (root and
tops), melons 0.8
Cherries, cucumbers, eggplant, figs,
grapes, nectarines, olives, onions
(dry bulb and green), peaches,
pears, peas, peppers, plums,
quinces, pimentos, soybeans,
spinach, strawberries, summer
squash, tomatoes 0.8
Fat of meat of cattle, goats,
pigs and sheep 0.2
Dry beans, pecans, walnuts 0.1
APPRAISAL
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous, citrus and
small fruits, field crops, vegetables and for ectoparasite control on
cattle and sheep; it also has uses as a cereal seed dressing. The
pesticidal action is fairly persistent and residues are comprised
mostly of the parent compound together with its sulphoxide and, to a
lesser extent, its sulphone; the corresponding oxygen analogues are
rarely detected in animal tissue but have been observed as minor
components on treated vegetables. Following treatment of animals, the
bulk of the residue is contained in the fatty tissues. Residue data
was available following supervised trial treatments of fruit,
vegetables and animals, but no information was given regarding
foodstuffs moving in commerce. No residues have been observed in total
diet studies. Available multi-residue gas chromatographic procedures
should be suitable for application for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Temporary tolerances apply to the total residue, comprising
carbophenothion, its sulphoxide and sulphone together with their
corresponding oxygen analogues, if present, expressed as
carbophenothion.
ppm
Lemons 5
Spinach 2
Fat of meat of cattle and sheep 1
Grapefruit, limes, oranges, prunes,
apricots, nectarines, peaches 1
Broccoli, Brussels sprouts, cauliflower,
apples, pears 0.5
Olive oil 0.2
Olives, sugar beet 0.1
Milk and milk products (fat basis) 0.1
Potatoes, rape seed 0.02*
Walnuts and pecans (shelled) 0.02*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1976)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
2. An adequate reproduction study.
REFERENCES
Beliles, R.P. (1965) Trithion - safety evaluation by analysis of
cholinesterase reactivation with atropine and PAM after trithion
poisoning. Report Woodard Research Corporation. (unpublished)
Beliles, R.P. (1966) Trithion - safety evaluation by analyses of
cholinesterase reactivation with atropine and PAM after trithion
poisoning in the dog. Report Woodard Research Corporation.
(unpublished)
Bowman, M.C. and Beroza, M. (1970) G.L.C. retention time of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J. Ass. off. analyt. Chem., 53: 499-508.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on specially
washed Chromosorb W for multi-component residue determinations of
phosphorus and sulphur containing pesticides by flame photometric
G.L.C. J. Ass. off. analyt. Chem., 54: 1086-1092.
Bullock, C.H. and Kamienski, F.X. (1971) Acute inhalation screen, male
and female rats. Report Stauffer Chemical Company. (unpublished)
Burke, J.A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. off. Agric. Chem., 48: 1037-1058.
Buxton, R.W. (1971) Determination of residues of Trithion and its
oxygen analogues. Report Stauffer Chemical Co. (unpublished)
Coffin, D.E. (1964) Oxidative metabolism and persistence of Trithion
on field sprayed lettuce. J. Ass. off. Agric. Chem., 47: 662-667.
Duggan, R.E., Lipscomb, G.Q., Cox, E.L., Heatwole, R.E. and Kling,
R.C. (1971) Pesticide residue levels In food in the United States July
1, 1963 to June 30, 1969. Pestic. Monit. J., 5: 73-212.
Edson, E.F., Sanderson, D.M. and Noakes, D.N. (1963) Acute toxicity
data for pesticides. World Rev. Pest Control 2: 26-28.
Elsea, J.R. (1955a) Acute oral administration - rats. Report Hazleton
Laboratories. (unpublished)
Elsea, J.R. (1955b) Acute oral administration - mice. Report Hazleton
Laboratories. (unpublished)
Elkins, E.R., Farrow, R.P. and Kim, E.S. (1972) The effect of heat
processing and storage on pesticide residues in spinach and apricots.
J. Agr. Fd. Chem., 20: 286-291.
Elward, T.E. and Meyding, G.D. (1964) Carbophenothion. Reactivation
study - rats. Report No. 64-5 Stauffer Chemical Company. (unpublished)
Fogleman, R.W. (1956) Subacute feeding - rats. Report Hazleton
Laboratories. (unpublished)
Frei, R.W., Mallet, V. and Pothier, C. (1971) Flavones as fluorogenic
spray reagents for organothiophosphorus pesticides on silica gel
layers. J. Chromat., 59: 135-140.
Gardner, A.M. (1971) Confirmation of organophosphorus pesticide
residues at nanogram levels by two-dimensional T.L.C. J. Ass. off.
analyt. Chem., 54: 517-524.
Gray, E.H. (1954) Acute oral, dermal and eye application. Report
Hazleton Laboratories (unpublished)
Hagan, E.C., Jenner, P.M. and Fitzhugh, O.G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432.
Hayes, W.J., Jr. (1971) Clinical handbook on economic poisons. U.S.
EPA Pesticides Programs, Public Health Service Publication 476.
Hearn, C.E.D. (1961) Trithion poisoning. Bull. J. Ind. Med., 18:
231-233.
Horn, H.J. (1957) Trithion. Potentiation study, subacute feeding -
dogs, acute oral administration - rats. Report Hazleton Laboratories.
(unpublished)
Johnston, C.D. (1967) Trithion. Safety evaluation by two-year feeding
studies in the rat and the dog. Report Woodard Research Corporation.
(unpublished)
Johnston, C.D. and Scott, W.J. (1966) Trithion. Three generation
reproduction study in the rat. Report Woodard Research Corporation.
(unpublished)
Lehman, A.J. (1965) Summaries of pesticide toxicity. The Assoc. of
Food and Drug Officials of the U.S.
Leoni, V. (1971) The separation of fifty pesticides and related
compounds and polychlorobiphenyls into four groups by silica gel
microcolumn chromatography. J. Chromat., 62: 63-71.
Lobdell, B.J. and Johnston, C.D. (1964) Trithion-demyelination in
chickens. Report Woodard Research Corporation. (unpublished)
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1967) Metabolism of 32P
Trithion in the white mouse and American cockroach. Report Stauffer
Chemical Company. (unpublished)
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967)
Comparison of various carbon absorbents. Quantitative elution and
separation of 42 pesticides from a carbon-Solka Floc clean-up column.
J. Ass. off. analyt. Chem., 50: 1216-1228.
Mees, G. and Antoine, O. (1971) Routine method for the determination
of organophosphorus insecticides by thin-layer chromatography (in
French). J. Chromat., 58: 247-256.
Mendoza, C., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Enzymatic detection of 10 organophosphorus pesticides and carbaryl on
thin-layer chromatograms. Evaluation of indoxyl, substituted indoxyl
and 1-naphthyl acetates as substrates for esterases. Analyst, Lond.,
93: 34-38.
Mendoza, C., Wales, P.J. and McLeod, H.A. (1970) Simultaneous
detection of some organophosphorus pesticides in whole-wheat flour.
Bull. envir. Contam. Toxicol., 5: 276-278.
Menzies, C.M. (1969) Metabolism of pesticides. U.S. Dept. of the
Interior, Special scientific report No. 127.
Meyding, G.D. and Browne, H.L. (1962) Cattle spray program. Report
Hazelton-Nuclear Science Corporation. (unpublished)
Meyding, G.D. and Patchett, G.G. (1964) Garrathion 3.5E; Plunge-dip
toxicity/residue studies: sheep. Report Stauffer Chemical Co.
(unpublished)
Patchett, G.G. and Batchelder, G.H. (1960) Determination of Trithion
crop residues by cholinesterase inhibition measurement. J. Agr. Fd.
Chem., 8: 54-57.
Radha, N.V. (1971) Efficacy of some synthetic insecticides in the
control of the castor white fly, Trialeurodes ricini M. Madras Agr.
J., 58: 300-302.
Rider, J.A., Swader, J.I. and Puletti, E.J. (1972) Anticholinesterase
studies with Guthion, Phosdrin, Disyston and Trithion in human
subjects. Fed. Proc., 31: 520 (Abs # 1730).
Shaffer, B.C. and West, R. (1960) The acute and subacute toxicity of
technical tetram. Toxicology and applied pharmacology, 2:1-13.
Stauffer Chemical Co. (1972) Evaluation of residues in food.
(unpublished)
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticides in non-fatty foods. J. Ass. off. analyt.
Chem., 54: 513-516.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of Interior. Resource publication
No. 84.
Villeneuve, D.C., Butterfield, A.C., Grant, D.L. and McCully, K.A.
(1970) The detection, separation and quantitative recovery of 13
organophosphorus pesticides on silica gel GF254 thin-layer
chromatograms. J. Chromat., 48: 567-571.
Ward, C.R., Huddleston, E.W., Ashdown, D., Owens, J.C. and Polk, K.L.
(1970) Greenbug control on grain sorghum and the effects of tested
insecticides on other insects. J. Econ. Entomol., 63: 1929-1934.
Weir, R.J. (1958) Subacute feeding - dairy cows. Report Hazleton
Laboratories. (unpublished)
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Fd. Chem., 16: 808-812.
Physical and chemical properties
State: off-white to light amber coloured liquid
Boiling point: 82°C at 0.01 mm Hg
Vapour pressure: 3 × 10-7 mm Hg at 20°C
Specific gravity: 1.265 - 1.285 at 20°C
Molecular weight: 342.85
Refractive index: 1.590 - 1.597
Solubility: slightly soluble in water (<0.04 g/l at 20°C), Miscible
with most organic solvents (hydrocarbons, alcohols, ketones, esters)
Stability: stable to hydrolysis but oxidized to the phosphorothiolate
Purity: technical material about 95%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on absorption and distribution of carbophenothion in mammals
are not available. Following intraperitoneal administration to mice,
greater than 75% of the administered dose was excreted in the urine
within 24 hours. During the following 16 hours only 4-7% of the dose
was excreted. Carbophenothion is apparently rapidly eliminated from
the body (March et al., 1957).
Biotransformation
The metabolism of carbophenothion is qualitatively the same in mice,
insects and plants, the principal products being oxidative. Although
the transformation of carbophenothion to oxidative and hydrolytic
components has been postulated, little data are available on the
quantitative degradation in mammals. Oxidative metabolism of
carbophenothion has been shown to result in a substantial increase in
anticholinesterase activity except for the sulphone of the
phosphorodithioate. A quantitative difference in animal and plant
metabolites was the presence of an unidentified product on the surface
of plants which appeared to a minor degree in the internal parts and
was found as a minor component in mouse urine and in vitro tests
with mouse liver. The component is probably the phosphorodithioate
sulphoxide analogue (March et al., 1957). Five oxidative products of
carbophenothion have been identified (see figure 1).
S O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion oxygen analogue
(5 × 10-4M) (2 × 10-6M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2 6 4 2 2 6 4
carbophenothion sulphoxide oxygen analogue sulphoxide
(8 × 10-6M) (2 × 10-7M)
S O O O
(EtO) PSCH SC H pCl (EtO) PSCH SC H pCl
2 2O 6 4 2 2O 6 4
carbophenothion sulphone oxygen analogue sulphone
(1 × 10-4M) (1 × 10-8M)
Figure 1 - Oxidative products of carbophenothion
The number in parenthesis under the oxidative products in Figure 1 is
the I50 value for housefly head cholinesterase (March et al.,
1957).
Results of studies on the metabolism of carbophenothion in lettuce
have shown a typical pattern of oxidative metabolism resulting in the
sulphoxide and sulphone analogues of the phosphorothioate and
phosphorodithioate. Exposure of carbophenothion to UV light resulted
in the appearance of the phosphorodithioate sulphoxide analogue
(Menzies, 1969).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of White Leghorn hens (10 hens/group) were fed carbophenothion
in the diet at levels of 0, 10, 31.6 and 100 ppm for seven weeks. TOCP
was fed at 500 ppm as a positive control. Egg production was affected
at 100 ppm. Clinical signs of ataxia were evident in TOCP-fed hens but
were absent in animals fed all levels of carbophenothion. Histological
examination of nerve tissue showed slight evidence of demyelination in
TOCP treated hens but none with carbophenothion (Lobdell and Johnston,
1964).
Special studies on potentiation
Groups of dogs (1 male and 1 female/group) were fed carbophenothion (1
ppm) in the diet in combination with parathion (1 ppm),
methylparathion (5 ppm), EPN (20 ppm), demephion (2 ppm), or malathion
(100 ppm) for 45 days. No evidence of greater than additive depression
of cholinesterase activity was observed (Horn, 1957).
Carbophenothion in combination with five other organophosphates
(parathion, methylparathion, EPN, demephion and malathion) when
administered orally to rats did not show signs of potentiation of
acute toxicity (Horn, 1957; Shaffer and West, 1960).
Special studies on reproduction
Groups of rats (20 male and 20 female per group) were fed
carbophenothion in the diet and subjected to a standard
three-generation (2 litter per generation) reproduction study at
dosage levels of 0 and 20 ppm. At 20 ppm carbophenothion had an effect
on reproduction in all three generations. In the F0 generation a
reduced mean pup birthweight was observed and fewer young were alive
at weaning. In the F1b generation, an increased number of stillbirths
was observed in two litters and fewer young were alive at weaning in
the second (but not the first) litter. In the F2b generation, an
increase in stillbirths was again observed in two litters, a decreased
number of live births and again fewer pups were alive at weaning.
Foetal resorption was an apparent factor in three of 10 females
examined which had borne less than two litters. An examination of
stillborn pups for teratological effects of carbophenothion was
negative (Johnston and Scott, 1966).
Acute toxicity
Acute toxicity to carbophenothion in some animal species is summarized
in Table 1.
TABLE 1 Acute toxicity to carbophenothion in different animal species
Species Sex Route LD50 Reference
Rat M Oral 17-91 Gray, 1954; Elsea, 1955a,b; Horn, 1957;
Shaffer and West, 1960; Hagan et al.,
1961; Hayes, 1971
F Oral 10 Hayes, 1971
Oral 7-30 Edson et al., 1963
M Dermal 54 Hayes, 1971
F Dermal 27 Hayes, 1971
Mouse M Oral 106-218 Elsea, 1955b; Gray, 1954
Dog M and F IM Approx. Beliles, 1966
40
Rabbit Dermal 800 Edson et al., 1963
Duck M Oral 121 Tucker and Crabtree, 1970
A dose of 25 mg/kg administered to the eye of rabbits resulted in
death in one-third and signs of acute poisoning in the survivors
(Gray, 1954).
Signs of poisoning are typical of organophosphorus esters and include
salivation, lacrimation, diarrhea, miosis, tremors and other signs of
cholinergic stimulation.
Rats exposed to carbophenothion vapours at a dose of 1.04 mg/l for one
hour exhibited depression which subsided within one hour after
treatment (Bullock and Kamienski, 1971).
Atropine and PAM have been shown to be effective therapeutic agents
for acute poisoning by carbophenothion. Repeated administration with
both materials was necessary to insure complete survival of poisoned
animals (Elward and Meydins, 1964). Following acute IM administration
of carbophenothion, atropine and PAM administered IM were effective in
temporarily alleviating signs of poisoning. A return of toxic signs
was evident 2-4 hours after the administration of atropine and PAM
(Beliles, 1965).
Dogs were administered carbophenothion by IM injection at a dose
approximately five times the calculated LD50. This was followed by
atropine alone or in combination with PAM (atropine was administered
twice daily after signs of poisoning occurred and PAM administered
once). All dogs administered atropine alone died, although death was
delayed. One of six dogs receiving atropine and PAM survived.
Mortality in the dogs administered atropine and PAM was delayed.
Atropine plus PAM was a more effective therapeutic agent than atropine
alone. Signs of toxicity and mortality following lethal doses of
carbophenothion were alleviated with the two agents in combination
(Beliles, 1966).
Short-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for 90 days at dosages of 0, 5, 10, 22, 46
and 100 ppm. Clinical signs of toxicity, cholinergic stimulation, were
evident at 46 and 100 ppm. Growth of females was reduced at 100 ppm.
RBC cholinesterase inhibition was evident at levels of 10 ppm and
above, especially in females. Brain and plasma cholinesterase was
inhibited at 22 ppm and above. No effects were observed on survival,
food consumption and gross histological examination of tissues
(Fogleman, 1956; Lehman, 1965).
Dog
Groups of mongrel dogs (2 males and 2 females/group) were administered
carbophenothion orally by gelatin capsule, 6 days/week for 90 days, at
levels of 0.02, 0 04, 0.08, 0.25 and 0.80 mg/kg/day. Dogs at 0.25 and
0.80 mg/kg/day showed plasma cholinesterase depression and did not
receive the total dosage. At 0.02 mg/kg/day plasma cholinesterase was
depressed slightly, while at 0.04 mg/kg inhibition was more apparent.
RBC cholinesterase was normal at all treatment levels (Fogleman, 1956;
Lehman, 1965). Groups of purebred beagle dogs (3 males and 3 females/
group) were fed carbophenothion in the diet for two years at dosages
of 0, 5, 20 and 80 ppm. Erythrocyte and brain cholinesterase activity
were depressed at 80 ppm. Plasma cholinesterase activity was depressed
at all feeding levels at all examination intervals except the initial
one. At 20 and 80 ppm, the groups were distinguishable from controls
chiefly on the basis of decreased body-weight gain (in 80 ppm
females), greater degree of irritability and nervousness and increased
mean relative adrenal weight (in 80 ppm females). No effects were
noted on mortality, physical condition, food consumption, behaviour,
haemograms, clinical chemistry tests, urinalysis, neurological
examinations and gross or histological examination of tissues
(Johnston, 1967).
Cow
Groups of two lactating dairy cows were administered 0, 1, 3, 10 and
30 ppm of carbophenothion in the diet, 7 days/week, for 120 days. The
1 ppm group was changed to 20 ppm on day 23 of the study. A similar
group, receiving 15 ppm, was added to the study on day 70 and
continued for 50 days. Clinical signs of toxicity were evident at 30
ppm. Blood cholinesterase activity was significantly depressed at 20
and 30 ppm. Appearance and behaviour, food consumption and whole blood
cholinesterase activity were unaffected at 15 ppm and below (Weir,
1958).
Long-term studies
Rat
Groups of rats (25 males and 25 females/group) were fed
carbophenothion in the diet for two years at dosage levels of 0, 5, 20
and 80 ppm. Although considerable mortality was observed in all groups
in this study, there was no relationship to the dietary intake. Rats
at 80 and 20 ppm feeding levels displayed tremors, loose stools and
hair loss as well as behaviour changes, including nervousness and
irritability. Females at 80 ppm showed reduced growth during the
second year of study. At 80 ppm, at 100 weeks of feeding, a gradual
lowering of hemoglobin became evident. RBC, plasma and brain
cholinesterase activity was inhibited at 80 and 20 ppm but not at 5
ppm. Cholinesterase inhibition in brain, RBC and plasma was more
evident in females than males. At 80 ppm females showed an increased
adrenal weight and ratio of organ to body-weight. Gross and
histological examinations showed no compound-related effect other than
the increased adrenal gland weight. Analyses of the incidence of
tissue mass showed no differences from control values. There were no
differences in respect to the frequency of neoplasm occurrence
(Johnston, 1967).
OBSERVATIONS IN MAN
The effects of carbophenothion noted on man are limited to several
acute poisoning incidents (Hearn, 1961) and a one-month subacute
administration to humans. Typical signs of cholinergic stimulation in
humans were obvious following ingestion of contaminated flour. This
poisoning was controlled with atropine and PAM. Five people were
administered carbophenothion at a dose of 0.8 mg/kg/day for 30 days.
There were no effects reported on plasma or RBC cholinesterase
activity (Rider et al., 1972).
COMMENT
Carbophenothion is rapidly excreted and is probably metabolized via
oxidative and hydrolytic pathways.
The compound does not induce demyelination and is not potentiated by
those organophosphates tested.
Cholinesterase depression was shown to occur in a rat reproduction
study, conducted at a dose level known to induce maternal
cholinesterase depression. Reduced pup weights, increased resorption
rate and postpartum mortality in pups was shown to occur in a
reproduction study in rats conducted at a dose level known to induce
maternal cholinesterase depression.
Plasma cholinesterase depression was the most sensitive criterion of
effect in both short- and long-term studies in rats and dogs.
A study in man did not result in plasma cholinesterase depression at
0.8 mg/kg/day. The meeting considered that the data available were
insufficient to enable an ADI to be based on this experiment. However,
the 0.8 mg/kg/day level was borne in mind when the study in dogs was
considered in the light of the apparent extreme difference in
sensitivity between man and dog. A no-effect level was not
demonstrated in dog at the lowest dose utilized (5 ppm or 0.125
mg/kg/day). In rat, 5 ppm (0.25 mg/kg/day) is a no-effect level with
regard to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous fruits, citrus
fruits, small fruits, vegetables and field crops, and for ectoparasite
control on cattle and sheep. It is also used as a cereal seed
dressing.
It is available as various strengths of emulsifiable concentrates,
wettable powders and dusts. Recommended treatment levels are as
follows:
1) Deciduous fruit - 1.25 lb to 2.0 lb a.i. per acre with pre-harvest
intervals of 30 days.
2) Citrus fruit - 2.5 lb to 5.0 lb a.i. per acre. Pre-harvest
intervals vary from 0 to 30 days depending on
amount of a.i. used per acre.
3) Vegetable crops - 1.0 lb a.i. per acre; 7 days pre-harvest
interval except for spinach (21 days).
4) Field crops - 1.0 lb a.i. per acre; 21 days pre-harvest
interval.
5) Cattle - 0.02% spray, no pre-slaughter interval.
6) Sheep - 0.026% tip spray; 0.062% saturation spray or
plunge dip - 14 day pre-slaughter interval.
Specific uses include the control of greenbug on sorghum (Ward et
al., 1970) and the control of castor white fly (Radha, 1971).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 2 gives typical results of analysis for residues of
carbophenothion following controlled application at stated rates to
various fruit and vegetable crops (Stauffer Chemical Co., 1972). The
data on citrus fruits refers to residues on whole fruits; evidence was
presented showing that practically all of the residue was retained on
the peel with only very small amounts (0.02 ppm or less) appearing in
the pulp or juice.
Tables 3 and 4 show residues of carbophenothion found in some tissues
of cattle and sheep at different intervals after treatment.
Table 5 gives results obtained from the analysis of milk from cows fed
carbophenothion at various concentrations to simulate feeding of
treated forage (Stauffer Chemical Co., 1972).
TABLE 2 Carbophenothion residues in various crops
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Oranges 0 0.03 0.80
7 0.03 0.67
14 0.03 0.55
21 0.03 0.47
28 0.03 0.38
Grapefruit 0 0.03 1.4
7 0.03 1.1
14 0.03 0.85
21 0.03 0.65
28 0.03 0.53
Lemons 0 0.12 4.0
7 0.12 3.3
14 0.12 2.5
21 0.12 2.2
28 0.12 1.9
Limes 0 0.03 0.95
7 0.03 0.79
14 0.03 0.65
21 0.03 0.53
28 0.03 0.43
Apples 0 0.03 0.73
2 0.03 0.30
12 0.03 0.46
14 0.03 0.88
19 0.03 0.36
26 0.03 0.29
29 0.03 0.37
Pears 33 0.06 0.41
Peaches 14 0.03 0.70
27 0.03 0.15
29 0.03 0.67
Prunes 28 0.04 0.24
29 0.04 0.47
29 0.04 0.42
Apricots 21 0.03 0.78
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Nectarines 14 0.03 0.52-0.66
37 0.03 0.33-0.42
Green alfalfa 0 0.25 70
10 0.25 20
20 0.25 5.5
Alfalfa hay 0 0.25 70
10 0.25 25
20 0.25 8
Soybean hay 0 0.25 70
10 0.25 25
20 0.25 8
Brussels 0 0.9 kg/ha 0.91
sprouts 7 0.9 kg/ha 0.28
Cauliflower 0 1.2 kg/ha 0.56
4 1.2 kg/ha 0.06
Broccoli 0 1.2 kg/ha 2.65
4 1.2 kg/ha 0.33
Sugar beet 7 1.2 3.9
(tops) 11 1.2 2.5
18 1.2 0.6
Sugar beet 0 0.25 0.01
(roots) 8 0.25 0.02
17 0.25 0.02
Spinach 2 4 oz/acre 26.4
5 11.2
10 3.8
15 0.70
2 8 oz/acre 63.4
5 25.8
10 12.5
15 1.7
0 12 oz/acre 36
4 14
22 1.56
29 0.16
2 16 oz/acre 95
5 32.2
10 15.1
TABLE 2 (cont'd)
Crop Pre-harvest Rate Residue
interval (% a.i.) (ppm)
(days)
Spinach (cont'd) 15 2.4
Olives 66 0.06 0.08
88 0.06 0.04
119 0.06 0.02
Olive oil 66 0.06 0.16
88 0.06 0.06
119 0.06 0.02
Potatoes )
Rape seed ) Observed residues all below the
Walnuts (shelled) ) limit of determination (0.02 ppm)
Pecans (shelled) )
Information was also available (Stauffer Chemical Co., 1972) regarding
residues occurring in milk and cream from three cows spray-treated
with 0.02% carbophenothion; no residues above the limits of
determination were observed (<0.005 ppm for milk and <0.05 ppm for
cream).
FATE OF RESIDUES
In animals
Fat samples of cattle treated with carbophenothion have been examined
for the presence of carbophenothion, its sulphoxide and sulphone and
the corresponding oxygen-analogue compounds. The parent compound and
lesser amounts of its sulphoxide and sulphone were found (Table 6),
but the oxygen-analogues were not observed (Meyding and Browne, 1962).
In all cases the amounts of the oxygen-analogue, its sulphoxide and
sulphone were below 0.005 ppm.
In plants
Carbophenothion was applied to field-growing lettuce, and samples were
removed at various times after spraying and analysed for
carbophenothion and its oxidative metabolites. Carbophenothion
persisted in growing lettuce for 21 days. Five oxidation products
appeared within 4 hours of spraying and persisted for up to 21 days.
The results indicate that the principle route of oxidation involved
thioether oxidation to form the sulphoxide and then the sulphone,
followed by phosphorothionate oxidation to form the thiophosphate
sulphone (Coffin, 1964). (See also Figure 1). In these experiments the
amount of parent carbophenothion decreased rapidly from 7.0 ppm to 1.2
TABLE 3 Carbophenothion residues in cattle tissues1
Tissue Treatments Days since last treatment
(No.) 1 3 7/8 21 36 43
Perirenal fat 1 - - 0.13 0.09 - -
Subcutaneous fat 1 - - 0.09 0.10 - -
Omental fat 1 - - 0.17 0.15 - -
Perirenal fat 2 - - 0.63 0.20 - -
Subcutaneous fat 2 - - 0.19 0.14 - -
Omental fat 2 - - 0.41 0.24 - -
Perirenal fat 3 1.65 0.69 0.71 0.19 0.25 ND
Subcutaneous fat 3 0.62 0.61 0.37 0.10 0.14 0.04
Omental fat 3 1.12 0.74 0.47 0.25 0.31 0.02
Liver 3 0.019 <0.01 ND2 ND ND ND
Kidney 3 0.048 0.03 <0.01 ND ND ND
Skeletal muscle 3 0.036 0.01 ND ND ND ND
1 Residues found in the tissues of cattle following 1, 2 or 3 weekly spray exposures
to a 0.1% active ingredient suspension. Values represent net corrected ppm of
carbophenothion (Meyding and Browne, 1962).
2 ND = not detected
TABLE 4 Carbophenothion residues in sheep tissues1
Tissue Days after treatment
1 3 10 14
Fat unshorn 0.39 0.66 0.55 0.61
shorn 0.90 0.68 0.29 0.29
Muscle unshorn 0.054 0.072 0.047 0.042
shorn 0.049 0.086 0.032 0.026
TABLE 4 (continued)
Tissue Days after treatment
1 3 10 14
Kidney unshorn *2 0.036 0.045 *
shorn 0.014 0.020 0.020 *
Liver unshorn * 0.019 0.014 0.011
shorn 0.01 0.011 * 0.014
1 Residues found in various tissues of sheep resulting from plunge-dips
in a vat containing 0.05% carbophenothion (Meyding and Patchett, 1964).
Values given in ppm carbophenothion.
2 * = <0.01 ppm
TABLE 5 Carbophenothion residues in milk (ppm carbophenothion)
Duration of
treatment Treatment level (ppm in diet)
(days) 0 1 3 10 20 30
0 *1 * * * * 0.0014 *
7 * * * 0.0023, 0.0029 0.0101 0.0215, 0.0170
14 * * 0.0015, 0.0015 0.0028, 0.0024 0.0085, 0.0052 0.0210, 0.0149
28 * - 0.0015, 0.0024 0.0086, 0.0058 0.0103, 0.0104 0.0171
49 * - 0.0036, 0.0031 0.0058, 0.0047 0.0123, 0.0146 0.0141
70 * - 0.0033, 0.0011 0.0054, 0.0048 0.0115, 0.0088 0.0187
(mean) * * 0.0015 0.0045 0.0102 0.0178
1 * = less than 0.001 ppm.
TABLE 6 Carbophenothion and metabolites in cattle fat
Fat tissue Treatments Days since Carbophenothion -sulphoxide -sulphone
(no.) last treatment (ppm)
Perirenal 3 7 0.563 0.318 0.058
Omental 3 7 0.368 0.107 0.026
Subcutaneous 1 8 0.197 0.068 0.046
TABLE 7 Effect of storage and heat processing on carbophenothion residues
Heat Storage
Food Initial level processed Ambient 100°F
(ppm) temp.(1 yr)
Spinach 0.76 17% 71% 83%
Apricots 0.76 35% 84% 84%
ppm in the first three days and then more slowly to less than 0.1 ppm
at 21 days. The total residue declined more steadily from 8.3 ppm
to 0.1 ppm over the same period.
Evidence of residues in food in commerce or at consumption
Some limited information was available on residues of carbophenothion
occurring in food in commerce. Duggan et al. (1971) have presented
results of analyses from 1963 to 1969 of nearly 7 000 samples of U.S.
domestic and imported large fruits (apples, pears, oranges, peaches).
Of the few samples shown to contain residues of carbophenothion (less
than 1% of those examined), only 25 samples contained more than 0.1
ppm and one sample contained more than 1 ppm. No measurable amounts of
carbophenothion have been observed in various published total diet
studies.
Elkins et al. (1972) studied the effect of storage and heat
processing on the residue level of carbophenothion in canned spinach
and apricots. The results, shown in Table 7, are expressed in terms of
percentage reduction in the residue level.
METHODS OF RESIDUE ANALYSIS
Carbophenothion and its five oxidative metabolites are extracted from
non-fatty materials with aqueous acetonitrile, and from fat and oil
with hexane followed by partitioning in aqueous acetonitrile. The
residues are partially separated from crop extractives by an alumina
shakeout, a partition into chloroform, and an activated carbon
shakeout. The residues are oxidized to the sulphones with potassium
permanganate, further isolated and separated by liquid-solid
chromatography on silica gel H, and determined by gas-liquid
chromatography on OV-1 or QF-1 columns with flame photometric
detection in the phosphorus mode (Buxton, 1971 and Storherr et al.,
1971). These procedures should be suitable for regulatory purposes.
Bowman and Beroza (1970, 1971) published conditions for the gas
chromatography of organophosphorus pesticides, including
carbophenothion, using a flame photometric detector. A method based on
the extraction procedure of McLeod et al. (1967) in conjunction with
the T.L.C. enzymatic inhibition technique of Mendoza et al. (1968)
has been proposed by Mendoza et al. (1970). The presence of
carbophenothion in whole wheat flour could be detected down to 0.5
ppm. Carbophenothion and its five oxidative metabolites have been
determined by gas chromatography using a 5 ft column of 10% DC 200 and
the sulphur mode of the microcoulometric detector (Burke, 1965). A
method has been proposed for the detection and quantitative
determination of organothiophosphorus pesticides, including
carbophenothion, by in situ fluorimetry after separation on silica
gel layers. Yellowish-green fluorescent spots are obtained when the
plate is sprayed with a 3-hydroxyflavone, such as robinetin, after
bromination. Linear calibration curves up to 4 mg per spot of the
pesticide have been obtained and a relative standard deviation of
approximately 4% can be expected at the 1.0 µg level. Visual and
instrumental detection limits are around 0.04 µg per spot for certain
pesticides (Frei et al., 1971).
A thin-layer chromatographic procedure has been described for
separating and detecting carbophenothion and 16 other
cholinesterase-inhibiting pesticides on aluminum oxide and silica gel.
The detection of the anticholinesterase activity is based on the
hydrolysis of indophenyl acetate by bee brain cholinesterase. Limits
of detectable cholinesterase inhibitors are at the subnanogram level,
and the time required for detection is about 40 minutes (Winterlin
et al., 1968). A detection limit of 5 ng was obtained by Gardner
(1971) using a combined two-dimensional TLC - esterase method.
Patchett and Batchelder (1960) describe a two-phase hydrogen
peroxide-acetic acid-benzene system to oxidize carbophenothion to
strong cholinesterase inhibitors, which are subsequently determined by
measuring their inhibition of cholinesterase in human blood plasma.
The method is sensitive to 0.01 µg of carbophenothion and will detect
0.005 ppm residue in crops having low background values.
Villeneuve et al. (1970) investigated the separation and detection
of 13 organophosphorus pesticides, including carbophenothion, using a
combined TLC-GLC method. The separation of carbophenothion using a
silica gel microcolumn was investigated by Leoni (1971). A routine TLC
method for the determination of residues of organophosphorus
pesticides, including carbophenothion, has been proposed by Mees and
Antoine (1971). The procedure uses both silica gel and polyamide
coated plates.
Table 8 shows examples of national tolerances as reported to the Joint
Meeting.
TABLE 8
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
Australia Fruit and vegetables 1
Fat of meat of cattle and sheep 1
Belgium Fruit and vegetables 0.5
Canada Cherries, eggplants, peppers,
pimentos, plums, prunes, tomatoes 0.5
Apples, crabapples, onions,
pears, quinces 0.8
Federal
Republic
of Germany Rape, citrus fruit (without peel) 0.05
Netherlands Fruit and vegetables 0.05
TABLE 8 (cont'd)
Examples of national tolerances as reported to the meeting
Country Commodity Tolerance
ppm
U.S.A. Almond hulls 10
Alfalfa (green and hey),
clover (green and hay),
sugarbeet (root and tops) 5
Citrus fruit 2
Apples, apricots, snap beans,
lima beans, garden beets (root and
tops), melons 0.8
Cherries, cucumbers, eggplant, figs,
grapes, nectarines, olives, onions
(dry bulb and green), peaches,
pears, peas, peppers, plums,
quinces, pimentos, soybeans,
spinach, strawberries, summer
squash, tomatoes 0.8
Fat of meat of cattle, goats,
pigs and sheep 0.2
Dry beans, pecans, walnuts 0.1
APPRAISAL
Carbophenothion is a non-systemic organophosphorus insecticide and
acaricide used for pre-harvest treatments on deciduous, citrus and
small fruits, field crops, vegetables and for ectoparasite control on
cattle and sheep; it also has uses as a cereal seed dressing. The
pesticidal action is fairly persistent and residues are comprised
mostly of the parent compound together with its sulphoxide and, to a
lesser extent, its sulphone; the corresponding oxygen analogues are
rarely detected in animal tissue but have been observed as minor
components on treated vegetables. Following treatment of animals, the
bulk of the residue is contained in the fatty tissues. Residue data
was available following supervised trial treatments of fruit,
vegetables and animals, but no information was given regarding
foodstuffs moving in commerce. No residues have been observed in total
diet studies. Available multi-residue gas chromatographic procedures
should be suitable for application for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Temporary tolerances apply to the total residue, comprising
carbophenothion, its sulphoxide and sulphone together with their
corresponding oxygen analogues, if present, expressed as
carbophenothion.
ppm
Lemons 5
Spinach 2
Fat of meat of cattle and sheep 1
Grapefruit, limes, oranges, prunes,
apricots, nectarines, peaches 1
Broccoli, Brussels sprouts, cauliflower,
apples, pears 0.5
Olive oil 0.2
Olives, sugar beet 0.1
Milk and milk products (fat basis) 0.1
Potatoes, rape seed 0.02*
Walnuts and pecans (shelled) 0.02*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1976)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
2. An adequate reproduction study.
REFERENCES
Beliles, R.P. (1965) Trithion - safety evaluation by analysis of
cholinesterase reactivation with atropine and PAM after trithion
poisoning. Report Woodard Research Corporation. (unpublished)
Beliles, R.P. (1966) Trithion - safety evaluation by analyses of
cholinesterase reactivation with atropine and PAM after trithion
poisoning in the dog. Report Woodard Research Corporation.
(unpublished)
Bowman, M.C. and Beroza, M. (1970) G.L.C. retention time of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J. Ass. off. analyt. Chem., 53: 499-508.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on specially
washed Chromosorb W for multi-component residue determinations of
phosphorus and sulphur containing pesticides by flame photometric
G.L.C. J. Ass. off. analyt. Chem., 54: 1086-1092.
Bullock, C.H. and Kamienski, F.X. (1971) Acute inhalation screen, male
and female rats. Report Stauffer Chemical Company. (unpublished)
Burke, J.A. (1965) Gas chromatography for pesticide residue analysis;
some practical aspects. J. Ass. off. Agric. Chem., 48: 1037-1058.
Buxton, R.W. (1971) Determination of residues of Trithion and its
oxygen analogues. Report Stauffer Chemical Co. (unpublished)
Coffin, D.E. (1964) Oxidative metabolism and persistence of Trithion
on field sprayed lettuce. J. Ass. off. Agric. Chem., 47: 662-667.
Duggan, R.E., Lipscomb, G.Q., Cox, E.L., Heatwole, R.E. and Kling,
R.C. (1971) Pesticide residue levels In food in the United States July
1, 1963 to June 30, 1969. Pestic. Monit. J., 5: 73-212.
Edson, E.F., Sanderson, D.M. and Noakes, D.N. (1963) Acute toxicity
data for pesticides. World Rev. Pest Control 2: 26-28.
Elsea, J.R. (1955a) Acute oral administration - rats. Report Hazleton
Laboratories. (unpublished)
Elsea, J.R. (1955b) Acute oral administration - mice. Report Hazleton
Laboratories. (unpublished)
Elkins, E.R., Farrow, R.P. and Kim, E.S. (1972) The effect of heat
processing and storage on pesticide residues in spinach and apricots.
J. Agr. Fd. Chem., 20: 286-291.
Elward, T.E. and Meyding, G.D. (1964) Carbophenothion. Reactivation
study - rats. Report No. 64-5 Stauffer Chemical Company. (unpublished)
Fogleman, R.W. (1956) Subacute feeding - rats. Report Hazleton
Laboratories. (unpublished)
Frei, R.W., Mallet, V. and Pothier, C. (1971) Flavones as fluorogenic
spray reagents for organothiophosphorus pesticides on silica gel
layers. J. Chromat., 59: 135-140.
Gardner, A.M. (1971) Confirmation of organophosphorus pesticide
residues at nanogram levels by two-dimensional T.L.C. J. Ass. off.
analyt. Chem., 54: 517-524.
Gray, E.H. (1954) Acute oral, dermal and eye application. Report
Hazleton Laboratories (unpublished)
Hagan, E.C., Jenner, P.M. and Fitzhugh, O.G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432.
Hayes, W.J., Jr. (1971) Clinical handbook on economic poisons. U.S.
EPA Pesticides Programs, Public Health Service Publication 476.
Hearn, C.E.D. (1961) Trithion poisoning. Bull. J. Ind. Med., 18:
231-233.
Horn, H.J. (1957) Trithion. Potentiation study, subacute feeding -
dogs, acute oral administration - rats. Report Hazleton Laboratories.
(unpublished)
Johnston, C.D. (1967) Trithion. Safety evaluation by two-year feeding
studies in the rat and the dog. Report Woodard Research Corporation.
(unpublished)
Johnston, C.D. and Scott, W.J. (1966) Trithion. Three generation
reproduction study in the rat. Report Woodard Research Corporation.
(unpublished)
Lehman, A.J. (1965) Summaries of pesticide toxicity. The Assoc. of
Food and Drug Officials of the U.S.
Leoni, V. (1971) The separation of fifty pesticides and related
compounds and polychlorobiphenyls into four groups by silica gel
microcolumn chromatography. J. Chromat., 62: 63-71.
Lobdell, B.J. and Johnston, C.D. (1964) Trithion-demyelination in
chickens. Report Woodard Research Corporation. (unpublished)
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1967) Metabolism of 32P
Trithion in the white mouse and American cockroach. Report Stauffer
Chemical Company. (unpublished)
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967)
Comparison of various carbon absorbents. Quantitative elution and
separation of 42 pesticides from a carbon-Solka Floc clean-up column.
J. Ass. off. analyt. Chem., 50: 1216-1228.
Mees, G. and Antoine, O. (1971) Routine method for the determination
of organophosphorus insecticides by thin-layer chromatography (in
French). J. Chromat., 58: 247-256.
Mendoza, C., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Enzymatic detection of 10 organophosphorus pesticides and carbaryl on
thin-layer chromatograms. Evaluation of indoxyl, substituted indoxyl
and 1-naphthyl acetates as substrates for esterases. Analyst, Lond.,
93: 34-38.
Mendoza, C., Wales, P.J. and McLeod, H.A. (1970) Simultaneous
detection of some organophosphorus pesticides in whole-wheat flour.
Bull. envir. Contam. Toxicol., 5: 276-278.
Menzies, C.M. (1969) Metabolism of pesticides. U.S. Dept. of the
Interior, Special scientific report No. 127.
Meyding, G.D. and Browne, H.L. (1962) Cattle spray program. Report
Hazelton-Nuclear Science Corporation. (unpublished)
Meyding, G.D. and Patchett, G.G. (1964) Garrathion 3.5E; Plunge-dip
toxicity/residue studies: sheep. Report Stauffer Chemical Co.
(unpublished)
Patchett, G.G. and Batchelder, G.H. (1960) Determination of Trithion
crop residues by cholinesterase inhibition measurement. J. Agr. Fd.
Chem., 8: 54-57.
Radha, N.V. (1971) Efficacy of some synthetic insecticides in the
control of the castor white fly, Trialeurodes ricini M. Madras Agr.
J., 58: 300-302.
Rider, J.A., Swader, J.I. and Puletti, E.J. (1972) Anticholinesterase
studies with Guthion, Phosdrin, Disyston and Trithion in human
subjects. Fed. Proc., 31: 520 (Abs # 1730).
Shaffer, B.C. and West, R. (1960) The acute and subacute toxicity of
technical tetram. Toxicology and applied pharmacology, 2:1-13.
Stauffer Chemical Co. (1972) Evaluation of residues in food.
(unpublished)
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticides in non-fatty foods. J. Ass. off. analyt.
Chem., 54: 513-516.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of Interior. Resource publication
No. 84.
Villeneuve, D.C., Butterfield, A.C., Grant, D.L. and McCully, K.A.
(1970) The detection, separation and quantitative recovery of 13
organophosphorus pesticides on silica gel GF254 thin-layer
chromatograms. J. Chromat., 48: 567-571.
Ward, C.R., Huddleston, E.W., Ashdown, D., Owens, J.C. and Polk, K.L.
(1970) Greenbug control on grain sorghum and the effects of tested
insecticides on other insects. J. Econ. Entomol., 63: 1929-1934.
Weir, R.J. (1958) Subacute feeding - dairy cows. Report Hazleton
Laboratories. (unpublished)
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Fd. Chem., 16: 808-812.
