
CHLORMEQUAT JMPR 1972
Explanation
The data relative to identity and residues in food and their
evaluation were reviewed at the 1970 Joint FAO/WHO Meeting on
Pesticide Residues (FAO/WHO, 1971). At that time the original reports
of most of the biochemical and toxicological studies conducted with
chlormequat were not available. Many of these data are now reviewed.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
An oral dose of chlormequat (chlorocholine chloride) labelled with
14C was administered to male rats. The bulk (60.6%) was excreted in
the urine in four hours and 96% was eliminated within 46.5 hours.
Faecal excretion accounted for 2.3% and <1% was expired as 14CO2.
The remaining 0.5% was found in the tissues, the largest amount being
in the carcass (0.25%), intestines (0.11%) and liver (0.08%).
Chromatography indicated that the radioactivity was all in the form of
chlormequat (Blinn, 1967).
In an earlier study 330 mg were administered to male and female rats.
The compound was detected in the urine after 30 min. and reached a
peak at 1-8 hours. It could no longer be detected after 56 hours
(Shaffer, 1970).
In an analysis of the urine of rats that had received 200 mg/kg of
chlormequat orally, only chlormequat and two other compounds, which
may have been other salts of chlorocholine, were found. Choline itself
was not identified (Bronisz und Romanowski, 1968).
Rats were administered orally a single (60 mg) dose of 15N-labelled
chlormequat or they received 2 mg of the labelled compound daily for
100 days. After the single dose the amount of compound in the brain
decreased rather quickly, but there was considerable accumulation in
the kidneys over the 20 days of the investigation. After the
continuous administration, chlormequat was noted particularly in
active muscles such as the heart and diaphragm (Bier and Ackermann,
1970).
When a lactating cow received a single oral dose of 1 000 mg of
15N-labelled chlormequat, the compound was demonstrated in the milk
and urine three hours after administration. The main excretion of
chlormequat was 15-39 hours after administration, the total during
that time being 489 mg. Only 22 mg was excreted in the milk and the
concentration in the milk never exceeded 1 ppm, the peak
concentrations being 12-60 hours after administration (Lampeter and
Bier, 1970).
Effect on enzymes and other biochemical parameters
Suggestions that chlormequat may inhibit acetylcholinesterase and
microsomal oxidation are not based on substantial evidence. A study in
which rats were fed diets containing 500 - 3 000 ppm chlorocholine
and/or chlormequat, with or without cysteine, produced no evidence to
suggest that chlormequat has a lipotropic effect on the formation of
fatty liver produced by choline deficiency (Proll et al., 1970).
Groups of 4 male and 4 female rats were fed a diet containing 0 or 2
000 ppm of chlormequat for 21 days. The animals were then sacrificed
for cholinesterase determinations. There was no difference between
test animals and controls with respect to plasma, RBC or brain
cholinesterase (Levinskas, 1965).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of 20 male and 20 female rats received diets containing 0, 100,
300 or 900 ppm of chlormequat in a standard three-generation study.
Chlormequat produced no abnormalities in the appearance, behaviour,
food intake, weight gain, fertility, gestation, lactation or viability
of offspring. No foetal malformations or macro- or
micro-histopathological abnormalities could be attributed to
chlormequat (Leuschner et al., 1967).
In a long-term feeding study in which rats received a diet containing
10 to 1 000 ppm chlormequat, there was no evidence of effect on
fertility, development of young or of teratogenicity (Ackermann
et al., 1970).
Special studies on teratogenicity
Groups of pregnant mice received an i.p. injection (30 mg/kg) of
chlormequat either on days 14 and 15 or on days 11 to 15 inclusive, of
gestation. Another group of mice received 200 mg/kg by gavage daily on
days 11 to 15. On day 19 all mice were sacrificed. The mean number of
foetuses per mother, foetal size, frequency of resorption and
incidence of malformation did not vary between test and control
animals (Shaffer, 1970).
Pregnant mice were fed dietary levels of 0, 1 000 or 10 000 ppm of
chlormequat from days 1 to 15 of gestation or 25 000 ppm from days 11
to 15 inclusive. All animals were sacrificed on day 19. The average
number of foetuses per mother, size of foetuses and number of
resorption sites did not vary between test and control groups. The
number of malformations among foetuses of mothers fed 1.0% or 2.5% of
chlormequat was slightly higher than that of the controls (Shaffer,
1970).
Groups of mature male and female mice were fed dietary levels of 0,
1 000 or 5 000 ppm of chlormequat for ten weeks. Matings were
conducted after 1, 3, 4 and 10 weeks between the controls and animals
on the various dose levels. Upon sacrifice on day 19 of gestation, the
feeding of chlormequat was found to have no effect on the fertility of
the mice and produced no terata among the offspring (Shaffer, 1970).
Groups of rats were fed dietary levels of 0, 1 000 and 5 000 ppm of
chlormequat from days 1 to 21 of gestation. The animals were
sacrificed the day before parturition and no teratogenic effect was
observed (Shaffer, 1970).
Groups of pregnant golden hamsters (treatment groups of 8 animals and
15 controls) were given chlormequat by gavage at levels of 0, 25, 50,
100, 200, 300 or 400 mg/kg body-weight once on day 8 of gestation.
Another group received 100 mg/kg daily on days 7, 8 and 9 of
gestation. Signs of toxicity were evident in the higher dosed groups.
All surviving animals were sacrificed on day 14 of gestation. The
number of foetuses produced was less than in the controls and an
increase in foetal resorption was observed at 100 mg/kg and above.
Foetal size and weight was significantly reduced at 200 mg/kg and
above. No abnormalities were encountered in the foetuses from the
groups given 0, 25 or 50 mg/kg nor in the group given one dose of 100
mg/kg. Malformed or underdeveloped foetuses occurred in the groups
given three doses of 100 mg/kg and above. Malformations consisted of
anophthalamus, microphthalamus, encephaloceles, head deformation,
harelip, polydactylism, subcutaneous effusion of blood and body oedema
(Juszkiewicz et al., 1970).
Pregnant rabbits were fed 1 000 ppm chlormequat from day 1 to 28 of
pregnancy. Two days before parturition the animals were sacrificed. No
evidence of teratogenicity was observed (Shaffer, 1970).
Acute toxicity
Acute toxicity to chlormequat has been studied in several animal
species, and findings are summarized in Table 1.
Short-term studies
Rat
Rats exposed to chlormequat at doses equal to or greater than 18 mg/kg
showed slight traces of damage to the internal organs. At high doses
(162 mg/kg) changes in the liver and lungs appeared in the form of
diffuse fatty degeneration and hemorrhagic inflammation, respectively.
Necrotic changes in the testicles were observed in one animal being
treated with a dose of 18 mg/kg body-weight. No effects were noted at
6 mg/kg or below in this study, the results of which have not been
confirmed (Niepolomski et al., 1970).
TABLE 1 Acute toxicity of chlormequat in different animal species
LD50
Species Route mg/kg Reference
bodyweight
Mouse oral 215 - 1 020 Oettel, 1965; Levinskas
and Shaffer, 1966,
Ignatiev, 1967.
Mouse ip 60 Shaffer, 1970
Hamster oral 1 070 Levinskas and Shaffer,
1966
Rat oral 330-750 Oettel, 1965; Levinskas
and Shaffer, 1966; Ignatiev,
1967; Anonymous, 1969;
Stefaniak, 1969
Guinea oral 215 Oettel, 1965
pig
Guinea oral 620 Levinskas and Shaffer,
pig 1966
Rabbit oral 60-81 Oettel, 1965; Levinskas
and Shaffer, 1966;
Anonymous, 1969
Cat oral 7-50 Oettel, 1965; Levinskas
and Shaffer, 1966;
Anonymous, 1969
Dog oral 100 Anonymous, 1969
Dog oral <50 Levinskas and Shaffer, 1966
Monkey oral >800 Costs et al., 1967
Sheep oral >150-<200 Schulz et al., 1970
Groups of ten male rats were fed dietary levels of chlormequat of 0,
500, 1 000 or 2 000 ppm for 29 days. There were no deaths nor were
there any signs of abnormal behaviour. Mean body-weight gain and mean
food intake did not differ between test animals and controls. At
sacrifice, after completion of the study, no gross pathological
abnormalities were observed (Levinskas and Shaffer, 1962).
Rats fed the equivalent of 600 mg/kg body-weight of chlormequat in
their diet for three months showed no adverse effect other than
reduced weight gain. When the daily dietary dosage was increased to
1 200 mg/kg the only abnormality was a more pronounced reduction in
growth rate. No gross or histopathological abnormalities were observed
(Shaffer, 1970).
Groups each containing 20 male and 20 female rats were fed dietary
levels of 0, 200, 600 or 1 800 ppm of chlormequat for 90 days. There
were no deaths and there was no difference in behaviour, blood
chemistry or appearance between test and control. The mean weight gain
in the males (but not the females) was significantly less in the group
fed 1 800 ppm compared to the controls and other test groups. A trend
was observed toward increased kidney to body-weight ratios in females,
and liver to body-weight ratios in males with increasing doses. These
increases appeared to be significant only at the 1 800 ppm level.
Histological examination of all major organs from animals fed 1 800
ppm were normal (Levinskas, 1965).
Addition of 10 or 1 000 ppm of chlormequat to a suboptimal,
protein-deficient, diet in rats resulted in significant reduction in
relative liver weights (Ackermann et al., 1970).
Cat
Two castrated male and two female cats received 1 mg/kg body-weight
per day of chlormequat five days a week for six months without any
harmful effects as evidenced by behaviour, weight gain and absence of
toxic signs and abnormal results of blood analyses (Shaffer, 1970).
Dog
Groups of dogs (2 males and 2 females/group) received dietary levels
of 0, 20, 60 or 180 ppm of chlormequat for 106-108 days. Food intake,
weight gain, behaviour, appearance, clinical and chemical tests were
unaffected by chlormequat. At the end of the test period, gross
histopathological examination of all main organs revealed no lesions
that could be related to feeding chlormequat. Organ to body-weight
ratios showed no marked changes (Levinskas, 1965).
Groups of dogs (10 males and 10 females in the control and 3 males and
3 females in the test groups) were fed dietary levels of 0, 100, 300
or 1 000 ppm chlormequat for two years. Some animals on 1000 ppm
exhibited excessive salivation and weakness of the hindquarters. This
problem was reduced or overcome by feeding the dogs a daily ration in
two stages rather than all at once. One male and one female on 1 000
ppm died at 22 and 38 days. At 300 ppm and below there were no overt
signs of toxicity. Food intake was unaffected at all levels. Extensive
blood and urine analysis revealed no abnormalities except the presence
of chlormequat in the urine of the test animals. At sacrifice, organ
to body-weight ratios were not significantly different between test
and control groups. Extensive gross and histopathological examination
of all major organs revealed no changes attributable to feeding
chlormequat (BASF, 1967b).
Monkey
One of four rhesus monkeys receiving an oral dose of 500 mg/kg
body-weight of chlormequat died. At this high dose level heavy
salivation and emesis were noted, but the survivors appeared normal
after administration and remained so during the 7 day follow-up
period. Gross pathology revealed little change that could be related
to chlormequat (Costa et al., 1967).
Sheep
Sheep administered a single dose of 200 mg/kg body-weight of
chlormequat died in 5-20 hours. Serum cholinesterase, total bilirubin
and direct bilirubin were decreased. Acute liver damage was found in
three and massive excretion of protein in the proximal segment of the
renal tubules was described (Schulz et al., 1970).
Signs of intoxication after a single dose in all species studied
appear characteristic of cholinergic agents and most deaths occur
between 6 and 24 hours after administration of the dose. The variation
in species susceptibility is reported to be typical of ganglionic
blocking agents of the decamethonium type (Shaffer, 1970).
Daily administration of 1, 2, 5 or 10 mg/kg body-weight of chlormequat
to sheep was not lethal. There were no important changes in the
clinical status or in the results of laboratory tests and there was no
evidence of injury to the parenchyma of internal organs. The feeding
of meat from these sheep to cats and dogs had no adverse effect
(Schulz et al., 1970).
Long-term studies
Mouse
Groups of 52 male and 52 female CFLP mice were fed dietary levels of 0
or 1 000 ppm of chlormequat for up to 78 weeks. Survival was not
adversely affected. The rate of gain in body-weight began to be
reduced in the test group after 24 weeks and was about 6% lower in the
surviving animals during the final weeks of the study. No overt signs
due to treatment were seen. The incidence of benign lung tumours was
higher (20 out of 52) in the males fed 1 000 ppm of chlormequat than
in the controls (10 out of 51). It was considered that this incidence
in the treated mice fell within the normal range under the conditions
studied. Incidence of lung tumours in the males and incidence of
tumours in all other organs examined in both species was not
significantly higher in the test group than in the controls (Weldon
et al., 1971).
Two hybrid strains of mice (18 males and 18 females of each
strain/group) were given chlormequat by gavage at a dose of 21.5 mg/kg
from 7 to 28 days and then fed a diet containing 65 ppm for 18 months.
Based upon the number of hepatomas encountered in test and control
groups the authors considered that the evidence was inconclusive to
categorize chlormequat as being tumorigenic and stated that further
studies were required (Innes et al., 1969). Hepatomas were found in
five males of each strain (5 out of 18) compared to the control values
of 6 out of 257 for one strain and 7 out of 240 for the other strain
(Shaffer, 1970).
Rat
Groups each of 50 male and 50 female rats were fed diets containing 0,
500 or 1 000 ppm of chlormequat for two years. There were no signs of
abnormal behaviour or appearance or other signs of toxicity. Food
intake and body-weight gain were comparable in test and control
groups. Haematological and blood chemical determinations were normal,
as were urinalysis and microscopic examination of urine sediment.
Gross and histopathological examination of organs revealed no
abnormalities attributable to chlormequat (BASF, 1967a).
COMMENTS
Chlormequat is a stable quaternary compound that appears to act by
depolarizing the post-junctional membrane, and although the signs of
toxicity from high dosages in mammals resemble those of
anticholinesterase agents, it does not inhibit cholinesterase.
Chlormequat is rapidly absorbed and excreted mainly unchanged in the
urine of mammals.
A three-generation study in rats did not reveal adverse effects on
reproduction. Teratogenic studies in several species indicate effects
only at dose levels exceeding 50 mg/kg/day.
Long-term studies in mice and rats indicate no effects at 1 000 ppm.
However, in the rat, reproduction study levels of 300 ppm may have
caused delayed maturation of stages of spermatogenesis in 9-week-old
offspring whilst 100 ppm caused no effect. A two-year study in dogs
demonstrated a no-effect level at 300 ppm; at 1 000 ppm some signs of
cholinergic effect were noted.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 1 000 ppm in the diet equivalent to 150 mg/kg
body-weight/day.
Rat: 100 ppm in the diet equivalent to 5.0 mg/kg
body-weight/day.
Dog: 300 ppm in the diet equivalent to 7.5 mg/kg
body-weight/day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.05 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
In addition to those uses referred to in the 1970 evaluations
(FAO/WHO, 1971) information was received on the use of chlormequat on
pears for promoting fruit bud formation and reducing excessive
vegetative growth. The major use, however, remains on cereal crops,
especially wheat, to reduce straw length, strengthen straw and prevent
lodging.
Pears are treated about 14 days after full flowering with chlormequat
solutions at 0.1 - 0.2%. Treatment may be repeated once 14 days later.
Split applications at low concentrations are more effective than a
single treatment at higher concentrations.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Wheat
One application with 4.5 kg a.i./ha when wheat was 10-20 cm in height
and one spraying with 3 kg a.i./ha at a height of 30-35 cm gave
residues of 0.5-1 ppm in wheat grain (Jung and Henjes, 1964a).
In experiments on winter wheat with applications of from 1.2 - 6 kg
a.i./ha, the residues summarized in Table 2 were found in wheat grain
and straw.
Winter wheat treated with 1.25 to 10 kg a.i./ha 3-4 months before
harvest was found to have residues in grain ranging from 0.02 - 2.36
ppm. Spring varieties treated with 1.2 - 10 kg a.i./ha 2-3 months
before harvest had residues ranging from 0 - 2.5 ppm in the grain at
harvest. In a variety of soft wheat grown in Italy and treated at
various stages of growth with 2 and 4 kg a.i./ha the following
residues were detected in the grain: 1.36 - 2.18 ppm when treated 2
weeks after initiation of stem elongation; 2.24 - 6.78 ppm, when
treated at the boot stage (Cyanamid International 1966).
TABLE 2 Chlormequat residues in wheat
Residues (ppm)
Chlormequat Grain Straw
(kg a.i./ha) Average Range Average Range
1.2 0.5
2.4 0.8 N.D.1 - 2.0 15.8 N.D. - 40
3.0 - N.D. - 0.5 20 10 - 40
6.0 0.5
1 N.D. = not detectable (<0.5 ppm).
In dry years when the dose is high or the application is made late,
higher chlormequat residues can be found in the grain of treated wheat
plants. It seems that high rainfall can reduce or eliminate
chlormequat residues resulting from high or late application of the
growth regulator. After the wet summer of 1965 analysis of wheat grain
from a crop which had been treated with 12 kg a.i. chlormequat/ha,
showed no trace of any chlormequat (Jung and El-Fouly, 1966). It is
interesting to observe that residues decrease with increasing storage
time. This is obviously connected with the enzymatic transformation of
chlormequat in the plant. Jung and El-Fouly (1966) found only 0.5 ppm
or less in 12 samples of wheat grain, which originally contained
1.0 - 2.5 ppm chlormequat, after they had been stored for 12 months.
Rye
For short-straw winter ryes, chlormequat is applied at the rate of
1.5 kg a.i./ha at a growth height of 20-30 cm. Analysis of rye grain
from the 1966 harvest after 1.8 kg a.i./ha had been applied, showed
1.0-1.3 ppm chlormequat in the dry grain, average of four locations
(Jung, 1968a).
Oats
The question of chlormequat residues in oats is of special interest,
as the most effective time of chlormequat application is when growth
height is 40-50 cm. Harvest samples from field trials in various parts
of West Germany in 1967 showed a definite dependency on the time of
treatment (Jung, 1968b).
Early sprayed plants, when treated at a height of 20-30 cm with
1.5 - 3 kg a.i./ha, generally showed not more than 1.0 to 1.4 ppm
chlormequat in the dry grain. The values for late treated (at a growth
height of 40-50 cm) were 3.8 and 6.1 ppm, respectively. Straw residues
averaged 1.2 - 1.7 ppm (early) and 9.5 - 14.1 ppm (late) from the two
different times of application. Analysis of oat grain and straw of the
1968 harvest, based on an average of 11 field trials in all parts of
West Germany (Jung, 1969), are given in Table 3.
TABLE 3 Chlormequat residues in grain and straw of oats from
field trials1
Treatment2 Chlormequat in the dry grain
(l/ha Cycocel) (ppm)
Grain Straw
Untreated 0.4 0.7
2 1.7 5.9
4 2.3 9.9
6 3.5 16.2
1 Average of 11 field trials at each treatment rate.
2 At growth height of 40-50 cm.
Analysis of samples harvested from trials at the same location near
Cologne showed an average residue in grain of 0.9 - 1.5 ppm
chlormequat (dry basis), after treatment with 1 and 1.5 kg a.i./ha at
a growth height of 20 cm, and of 2.3 - 3.5 ppm chlormequat after
treatment with the same quantity at a growth height of 40 cm. The
straw of early treated plants was found to have an average of
0.7 - 1.1 and later treated plants 4.2 - 11.4 ppm (Jung, 1969a).
Results are shown in Table 4.
Apples and pears
In 1967 after treatment of eight varieties of apples with 0.3%
chlormequat solution, residue levels were found to range between 0.95
and 1.6 ppm in the fresh fruit. Repeated treatments led to residue
levels up to 3 ppm. The method used was capable of determining 0.2 ppm
chlormequat in apples (Jung, 1969b).
In 1968, five apple varieties were sprayed with 0.2% chlormequat
solution. The residue in the fresh fruit was between 0.2 and 1.4 ppm.
In the year following a single treatment with 0.2% chlormequat, no
residue was traceable in the apples. After two sprays, about 0.4 ppm
could be traced in the fruit the following year. Apples from trees
which had been treated with chlormequat in both 1967 and 1968
contained residues of 0.4 - 1.4 ppm when harvested in 1969.
The analyses of treated pears showed a distinct dependence on the date
of treatment. After a normal treatment with 0.2% solution, the average
of four varieties was 0.2 to 1 ppm chlormequat in the fresh fruit. An
extreme four-time spraying with 0.25% chlormequat solution led to
residue of 6 ppm. Fruit from the succeeding year did not contain any
residue (Jung, 1969b).
Vegetables
Analysis of several types of vegetables in 1967 and 1968 (Jung, 1969b)
led to the following results: cucumbers in the pot, which had been
treated with 0.25% solution, contained 1.6 ppm chlormequat. After
treating the soil with 0.15% and 0.5% solution and comparative spray
treatment with 0.25% solution, the residue fluctuated between 0.2 and
1.1 ppm chlormequat. Capsicum, after spraying with 0.15 - 1.0%
solution, contained 5.8 - 8.9 ppm chlormequat. In tomatoes which were
cultivated under greenhouse conditions no residue was found after soil
treatment with 10 and 30 mg chlormequat per pot, and 0.5 - 1.4 ppm
chlormequat was found after spraying with 0.1 - 0.25% solution.
Tomatoes raised in the field showed 0.6 ppm chlormequat in the fruit
after soil treatment with 50 cc 0.5% solution. No residue could be
traced in radish after soil and spray treatment with 0.15 - 0.25%
solutions.
TABLE 4 Chlormequat residues in grain and straw of oats
Treatment Nitrogen Growth Chlormequat, dry basis
(kg a.i./ha) fertilizer stage (ppm)
(kg/ha) Grain Straw
Untreated 60 0.37 0.34
Untreated 80 0.41 0.24
1.0 60 0.89 0.93
1.5 1.25 0.72
1.0 80 at 20 cm 1.16 1.11
1.5 1.47 0.77
1.0 60 2.42 4.16
1.5 3.23 6.61
1.0 80 at 40 cm 2.18 5.23
1.5 3.52 11.45
FATE OF RESIDUES
In animals
Orloski (1970) reported experiments designed to determine chlormequat
residues in the milk of dairy cows receiving 10 ppm chlormequat in the
ration for 14 consecutive days. Each animal received 128 mg
chlormequat daily via oral dose syringe. The results show trace
residues of chlormequat ranging from 0.024 to 0.034 ppm at each of
milk samplings 1, 4, 7 and 14 days during feeding. When the
chlormequat was removed from the ration no residues above the limit of
detection (0.01 ppm) were found in the milk.
In plants
The chlormequat level in vegetative parts of plants declines quickly
after treatment. Residue analyses based on the half-quantitative
method, in 1963 showed 2200 and 3600 ppm for winter wheat ("Werla")
and 1200 and 2400 ppm for summer wheat ("Koga") after treatment with 2
and 4 kg chlormequat/ha. After five weeks the residue in the complete
plant was reduced to 7 and 25 ppm, respectively, in winter wheat and
17 and 47 ppm, respectively, in summer wheat. No chlormequat could be
traced in the ears. The ripe wheat grains were also free of traceable
residue; 5 and 7 ppm chlormequat were found in the ripe straw of
winter wheat and 6 and 11 ppm chlormequat in the straw of summer wheat
(Jung and Henjes, 1964a).
Analysis of summer wheat in the year 1965, after treatment with up to
12 kg chlormequat/ha, showed the same tendency (Jung and El-Fouly,
1966). A similar rapid reduction of residues was also found by Mooney
and Pasarela (1967) in wheat plants of the "Redcoat" type. The residue
level was reduced from 218 ppm on the day of the treatment to 125 ppm
after 21 days, 20 ppm after 42 days and 5 ppm (straw) after 64 days.
After 91 days, the average residue content in six samples was 1.4 ppm
and in the straw 2.8 ppm. The untreated grain sample contained 0.15
ppm chlormequat. The plants had been treated with 4.5 kg a.i/ha at a
growth height of 20 cm, the precipitation during the vegetation period
being 344.5 mm. The biological half-life for chlormequat determined by
this study was 13 days.
High chlormequat applications frequently increase the content of
choline chloride (CC) shortly after treatment, and it therefore is
possible that chlormequat is transformed into CC and possibly betain.
Consequently, Jung and El-Fouly (1966) carried out tests in vivo and
in vitro to determine whether chlormequat is transformed into betain
and/or choline chloride. Their results are shown in Table 5.
It is not fully understood whether chlormequat, which is closely
related to the choline and betain contained in plants, is already
acting in the plant as a "natural" growth regulator. On the other
hand, the quarternary ammonium combinations choline and betain,
chemically closely related to chlormequat, can be traced in the plant
in relatively large quantities (Jung and El-Fouly, 1966; Jung and
Henjes, 1964b; Paxton and Mayr, 1962).
In the process of decomposition in vitro, transformation evidently
starts a few minutes after chlormequat solution has been added to the
plant extract. In each case, choline chloride could be traced as the
decomposition product.
It is noticeable that chlormequat decomposes differently in leaf
extracts of different plants. Plants sensitive to chlormequat seem to
decompose the growth regulator more slowly than plants which are less
sensitive. Perhaps the explanation for the different effect of
chlormequat on different plants and/or plant types can be found here.
If the speed of decomposition of chlormequat in wheat is 100, the
relative values in maize and rice would be 180, in beetroot and apples
130, in rapeseed 120, in poinsettia and cotton 100, in tomatoes 70, in
beans 40 and in grapes and chrysanthemums 20 (Jung and El-Fouly,
1966).
TABLE 5 Choline chloride and betain content in wheat plants after
treatment with high rates of chlormequat
Treatment 3 days after treatment 24 days after treatment
(kg a.i./
ha) Chlormequat CC betain Chlormequat CC betain
(ppm) (ppm) (ppm) (ppm) (ppm) (ppm)
Untreated - 3 100 3 500 - 900 1 000
6 1 400 3 900 4 300 100 900 1 200
12 2 800 3 800 4 200 200 1 000 1 300
These authors have established the following scheme (figure 1) for the
decomposition of chlorine choline chloride.
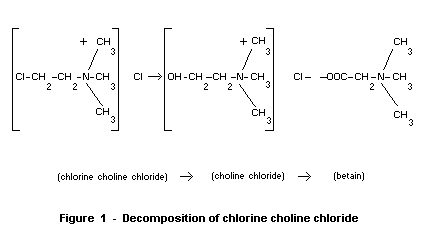 (chlorine choline chloride)-> (choline chloride)-> (betain)
Figure 1 - Decomposition of chlorine choline chloride
As choline occupies a central position in plant metabolism and can be
oxidized to betain, the increase of betain content after chlormequat
treatment could thus be explained. Paxton and Mayr (1962) have shown
the transformation of choline and betain in a further scheme.
According to the latest research, the enzymatic system controlling the
decomposition of chlormequat into CC, is pH-dependent and thermostable
(Jung and El-Fouly 1969). On the whole, the decomposition of
chlormequat increases with the increasing pH value of the plant
extract and reached a maximum at pH6. Above this value the rate of
decomposition decreases since chlormequat is decomposed in alkaline
medium. Heading (40-90°C) the plant extracts and subsequent cooling to
room temperature did not affect the rate of decomposition of
chlormequat which was added later. Only by cooking the extracts for
several hours could inactivation be obtained. Temperatures which
increase from 10-40°C, however, accelerate the decomposition of
chlormequat into CC, if the chlormequat is already present in the
extract. The enzymatic system only becomes active in the presence of
at least two components (one precipitated with ZnCl2 and one
dialysable). Schneider (1967) demonstrated chromatographically that
radio-labelled chlormequat was transformed into a substance identical
to choline in barley and chrysanthemum sprouts. He was able to isolate
radioactive choline or radioactive choline metabolites from plant
tissue, which had been standing in chlormequat-containing solutions
for some time. During decomposition the carbohydrate skeleton of
chlormequat seems, therefore to remain intact. The unknown metabolites
were identical to the metabolites isolated from plants after a choline
or choline plus chlormequat treatment. An additional combination,
which only appeared after chlormequat treatment, is taken as an
intermediate of the decomposition processes. On the whole, 50-80% of
the added radioactivity was found unchanged in the chlormequat
solution, 15-40% in the residue solutions and 35-40% in the methanol
extracts. At least 10-15% of the added radioactivity had been
transformed in metabolic products of chlormequat.
Schneider (1967) sees a contradiction in the relatively fast
decomposition of chlormequat and its long lasting effect on plant
growth. Since choline also, though at ten times higher concentrations
than chlormequat, delayed the growth of barley roots and sprouts in
such tests, this author believes that the effect normally assigned to
chlormequat alone could result from chlormequat choline and further
unknown metabolites of these combinations. The contradiction between
chlormequat decomposition and lasting effect could be explained if the
metabolism of chlormequat only takes place in one part of the plant
tissue, whilst other parts store unchanged chlormequat. This
explanation would comply with observations of Sachs and Kofranek
(1963), who have noted that chlormequat activities mainly take place
in the subapical meristem.
In soil
Chlormequat decomposition in soil takes place relatively fast. Cathey
and Stuart (1961) assess the persistency of choline chloride in soil
as only three weeks. The opinion that the soil does not provide the
plant with a continuous subsequent supply of chlormequat is also
shared by Schneider (1967). Trials were carried out by Jung (1965) in
which four different soils were treated with 5 mg chlormequat 0-6
weeks before sowing wheat seeds in all pots. After 4-6 weeks there was
almost complete inactivation. In further tests it was established that
chlormequat in quantities of 3, 30 and 300 kg/ha did not exert a
definable influence on the microbic activity of five different soils.
CO2 production and nitrification were taken as the basis for these
observations (Jung, 1964).
In storage and processing
Jung and El-Fouly (1966) found that chlormequat residues in wheat
decline significantly in storage. Twelve samples of wheat which
originally contained 1.0 - 2.5 ppm chlormequat were found to have less
than 0.5 ppm after being stored for 12 months.
Cyanamid International (1971) reports trials to determine the level of
chlormequat residues in milling products processed from wheat treated
with chlormequat at the rate of 1.2 kg per ha by aircraft and
harvested 95 days later. The wheat was milled five months after
harvest and the bran and flour collected. The flour was made into
bread. The treated grain was found to contain 0.26 ppm chlormequat.
The bran contained 0.8 ppm, the flour 0.32 ppm and the bread 0.06 ppm
chlormequat. It is apparent that milling does not substantially reduce
the level of chlormequat residues in wheat but that baking of the
flour in the production of bread largely eliminates the residue. The
analytical method used had a limit of determination of 0.05 ppm.
The work of Tafuri et al. (1970) indicates that wine made from
grapes grown under chlormequat treatment will contain residues
substantially similar to those in the fresh grapes (up to 1 ppm).
APPRAISAL
Chlormequat is used as a growth regulator in cereals, grapes and
pears.
Residues in wheat grain which mostly lie below 1 ppm, may range up to
2 ppm. When such wheat is milled, the flour will contain substantially
similar levels but these are largely destroyed in the baking of bread.
Cows fed on the straw of chlormequat-treated cereals excrete small
traces of chlormequat in milk during the period of feeding, but
residues disappear as soon as the treated feed is withdrawn.
The treatment of pear trees at flowering in order to prevent
vegetative growth and encourage fruit bud formation for the next
season gives rise to residues ranging up to 2.6 ppm in fruit at
harvest. Residues are not found in the succeeding crop.
The gas chromatographic method of Tafuri et al. (1970) is
recommended as suitable for regulatory purposes. It is suitable for
use with the commodities included in the list of recommended
tolerances having a limit of determination of 0.05 ppm.
RECOMMENDATIONS
TOLERANCES
The following tolerances are for residues likely to be found at
harvest. Due to the stability of chlormequat residues, it is not
anticipated that residue levels will change significantly in storage,
although they will be largely destroyed by cooking.
ppm
Oats and rye 5
Wheat 3
Pears 3
Grapes, raisins and other dried
vine fruits 1
Milk and milk products 0.1*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on other registered uses for chlormequat.
2. Further information on residues of chlormequat in raw
agricultural commodities from a number of different countries.
3. Information on chlormequat residues in commodities moving in
international trade.
REFERENCES
Ackermann, H. and Kretzschmann, F. (1970) Einfluss von
Chlorcholinchlorid auf die Toxizität von Cholinesterase-Inhibitoren.
Arch. exp. Vet.-Med., 24 (4): 1045-1047.
Ackermann, H., Proll, J. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid l. Mitteilung:
Einfluss von Chlorcholinchlorid auf Wachstum, Futterverwertung,
Organveränderungen und Nachkommenschaft. Arch. exp. Vet.-Med., 24 (4):
1049-1058.
Anonymous. (1969) Justification for consideration of chlormequat as
plant growth regulator at 4th session of the Codex Committee on
Pesticide Residues Arnhem, Netherlands.
BASF, (1967a) Zweijahre-Ratten-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
BASF, (1967b) Zweijahre-Hunde-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
Bier, H. and Ackermann, H. (1970) Lokalisiesung und Anreicherung von
Chlorcholinchlorid bei der Ratte nach peroraler Applikation. Arch.
exp. Vet.-Med., 24(4): 1023-1026.
Blinn, R.C. (1967) Plant growth regulant. Biochemical behaviour of
2-chloroethyl trimethylammonium chloride in wheat and in rats. J. Agr.
Fd. Chem., 15: 984-988.
Bronisz, H. and Romanowski, H. (1968) Chromatographic study of the
urine of the rat poisoned with chlorocholine chloride (CCC) (in
Polish) Acta. Pol. Pharm., 25(6): 611
Cathey, H.M. and Stuart, N.W. (1961) Comparative plant growth
retarding activity of Amo 1618, Phosphon and CCC. Botan. Gaz., 123: 51
Costa, D.G., Hansen, W.D. and Woodard, G. (1967) Cyclocel. Toxicity in
the monkey following single oral doses. Report Woodard Research
Corporation submitted to American Cyanamid Co. (unpublished)
Cyanamid International. (1966) "Cycocel" plant growth reguland.
Technical information published by American Cyanamid Co.
Cyanamid International. (1971) Cycocel residues in wheat grains, wheat
bran, wheat flour and bread. Technical report No. RLG 53.
(unpublished)
FAO/WHO. (1971) 1970 evaluations of some pesticide residues in food.
FAO/AGP/1970/M/12/1; WHO/Food Add./71.42.
Friess, S.L. and McCarville, W.J. (1954) Nature of the
acetylcholinesterase surface. II. The ring effect in enzymic
inhibitors of the substituted ethylenediamine type. J. Am. Chem. Soc.,
76: 2260-2261.
Ignatiev, A.D. (1967) Toxicological evaluation of chlorocholine
chloride and its agricultural use in controlling plant growth (in
Russian). Chemistry in Agriculture, Moscow, 5: 37-39 (cited in
Lampeter and Bier, 1970).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst., 42: 1101-1114.
Jung, J. (1964) "Uber den einfluss von Chlorcholinchlorid (CCC) auf
biologische Vorgänge im Boden" (on the influence of chlorine choline
chloride chlormequat on biological activity in soil); Zeitschr. f.
Pflanzenernähr., Düngg. u. Bodenkde., 107: 153-164.
Jung, J. (1965) "Das Verhalten von CCC in Pflanzen und Boden;
CCC-Symposium der BASF. (Behaviour of chlormequat in plants and soil.)
Chlormequat symposium, BASE.
Jung, J. (1968a) "CCC-Rückstände an Roggen nach den Versuchsplänen D
III und D III d; Interne BASF-Mitteilungen (Chlormequat residue in rye
according to trial plan D III and D III d, Internal BASF Information.)
Jung, J. (1968b) "Rückstandsuntersuchungen an CCC-behandelten
Haferbeständen; Interne BASF-Mitteilungen Nr. 581. (Residue trials
with chlormequat-treated oats. Internal BASF Information No. 581.)
Jung, J. (1969a) "Ergebnisse der Rückstandsuntersuchung an
CCC-behandelten Haferbeständen der Vegetationsperiode 1968; Interne
BASF-Mitteilung No. 617. (Results of the residue examination of
chlormequat-treated oats from vegetation period 1968. Internal BASF
Information No. 617.)
Jung, J. (1969b) "CCC-Rückstandsuntersuchungen an Apfeln, Birnen und
einigen Gemüsearten; Interne BASF Mitteilungen No. 616. (CCC
Chlormequat residue examinations with apples, pears and several
vegetables. Internal BASF Information No. 616.)
Jung, J. and El-Fouly, M.M. (1966) "Uber den Abbau von
Chlorcholinchlorid (CCC) in der Pflanze" (On the decomposition of
chlorine choline chloride (CCC) in the plant): "Zeitschr. f.
Pflanzenernährg., Düngg. u. Bodenkde., 114: 128.
Jung, J. and El-Fouly, M.M. (1969) Some factors affecting the
degradation of (2-chloroethyl)-trimethylammoniumchloride by wheat
plant extracts. Experientia, 25: 587.
Jung, J. and Henjes, G. (1964a) "Nachweis und halbquantitative
Bestimmung von Chlorcholinchlorid (CCC) in Weizenkörnern und-stroh"
(Proof and half quantitative determination of chlorcholinchloride
(CCC) in wheat grain and straw); "Zeitschr. f. Pflanzenernährg.,
Düngg. u. Bodenkde., 106: 108.
Jung. J. and Henjes, G. (1964b) "Analytische Untersuchungen an
Weizenproben aus CCC-Versuchen" (Analytical research on wheat samples
of CCC-trials), "Landw. Forschg., 17: 267.
Juszkiewicz, T., Rakalska, Z. and Dzierzawski, A. (1970) Effet
embryopathique du chlorure de chlorocholine (CCC) chez le hamster
doré. Europ. J. Toxicol., 3(5): 265-270.
Lampeter, W. and Bier, H. (1970) Ausscheidung von chlorcholinchlorid
über Milch und Harn nach oraler Applikation von 1g15N-markiertem
Chlorcholinchlorid an eine laktierende Kuh. Arch. exp. Vet. Med.,
24(4): 1027-1031.
Leuschner, F., Leuschner, A. and Schwerdtfeger, W. (1967) Uber die
chronische Verträglichkeit von CCC. Chronischer Reproduktionsversuch
uber 3 Generationen an Wistar-Ratten. Report from Laboratory for
Pharmacology and Toxicology, Hamburg, Germany submitted by Cyanamid
GMBH. (unpublished)
Levinskas, G.J. (1965) Cyclocel(R), growth regulator;
2-chlorethyl-trimethylammonium chloride. Report on 90-day feeding
trials on dogs and rats conducted at the Central Medical Department,
American Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1962)
2-chloroethyl-trimethylammonium chloride; CL 38,555; limited release
toxicity studies. Report from Central Medical Department, American
Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1966) Cyclocel(R) plant growth
regulant. Single oral dose toxicity. Report from Central Medical
Department, American Cyanamid Company. (unpublished)
Lüder, W., Ackermann, H. and Proll, J. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 2. Meittlung:
Einfluss von Chlorcholinchlorid auf Liberatmung, Esterase-und
Transaminaseak-tivetät. Arch. exp. Vet. Med., 24(4): 1059-1064.
Mooney, R.P. and Pasarela, N.R. (1967) Determination of
chlorcholinchloride residues in wheat grain, straw and green wheat
foliage. J. Agr. Fd. Chem., 15: 989.
Niepolomski, W., Szczurek, A., Knapek, R. and Kolodziejczyk, A. (1970)
Evaluation of the chronic toxicity of chlorocholine chloride (CCC)
based on morphological studies. (in Polish). Med. Pracy., 21(1): 1-6.
Oettel, H. (1965) Zur Toxizität von Chlorcholinchlorid (CCC).
CCC-Symposium der BASF 14 Dezember 1965 (cited in Schulz and Lampeter,
1970).
Orloski, E.J. (1970) Cycocel residues in milk. Report No. C225
American Cyanamid. (unpublished)
Paxton, R.C. and Mayr, H.H. (1962) "Untersuchungen über das natürliche
Vorkommen quaternärer Ammoniumbasen in Lycopersicum esculentum"
(Examinations on the natural presence of quaternary ammonium bases in
Lycopersicum esculentum). Planta, 59: 165.
Proll, J., Ackermann, H. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 3. Mitteilung: zur
lipotropen Wirkung von Chlorcholinchlorid. Arch. exp. Vet. Med.,
24(4): 1065-1068.
Sachs, R.M. and Kofranek, A.M. (1963) Comparative cytohistological
studies on inhibition and promotion of stem growth in Chrysanthemum
morifolium. Am. J. Botany, 50: 772.
Schneider, E.F. (1967) Conversion of the plant growth retardant
(2-chlor-ethyl)-trimethyl-ammoniumchloride to choline in shoots of
chrysanthemum and barley. Can. J. Biochem., 45: 395.
Schulz, J.A., Eichelberger, P., Schäppel, K.F. and Johannsen, U.
(1970) Untersuchungen zur Verträglichkeit das Chlorcholinchlorids nach
oraler Verabreichung an Schafe. Arch exp. Vet. Med., 24(4): 1033-1044.
Schulz, J.A. and Lampeter, W., (1970) Vorwortzuden Untersuchungen über
Chlorcholinchlorid-Rückstände im Pflanzenmaterial die Wirkung des
Chlorcholinchlorids auf Warmblüter und über den Verbleib des
Chlorcholinchlorids bei Verfütterung an Ratten und an die Kuh. Arch.
exp. Vet. Med., 24(4): 1013-1018.
Shaffer, C.B. (1970) Chlormequat. "Monograph" from Central Medical
Department, American Cyanamid Co. submitted to Joint FAO/WHO Meeting
on Pesticide Residues.
Stefaniak, B. (1969) Severe toxicity of chlorocholine chloride (CCC)
to rats (in Polish). Med. weteryn, 25(5): 285-286.
Tafari, F., Businelli, M., Scarponi, L. and Giusquiani, P.L. (1970)
Chlorocholine chloride residues in grapes and their fate in
winemaking. J. Agr. Fd. Chem., 18: 869-871.
Weldon, G.H., Hunter, B., Hague, P.H. and Spicer E.J.F. (1971)
Long-term feeding study of Cycocel in the mouse. Report No.
4059/71/217 from Huntingdon Research Centre submitted to American
Cyanamid Company and to Cyanamid of Great Britain Ltd. (unpublished)
(chlorine choline chloride)-> (choline chloride)-> (betain)
Figure 1 - Decomposition of chlorine choline chloride
As choline occupies a central position in plant metabolism and can be
oxidized to betain, the increase of betain content after chlormequat
treatment could thus be explained. Paxton and Mayr (1962) have shown
the transformation of choline and betain in a further scheme.
According to the latest research, the enzymatic system controlling the
decomposition of chlormequat into CC, is pH-dependent and thermostable
(Jung and El-Fouly 1969). On the whole, the decomposition of
chlormequat increases with the increasing pH value of the plant
extract and reached a maximum at pH6. Above this value the rate of
decomposition decreases since chlormequat is decomposed in alkaline
medium. Heading (40-90°C) the plant extracts and subsequent cooling to
room temperature did not affect the rate of decomposition of
chlormequat which was added later. Only by cooking the extracts for
several hours could inactivation be obtained. Temperatures which
increase from 10-40°C, however, accelerate the decomposition of
chlormequat into CC, if the chlormequat is already present in the
extract. The enzymatic system only becomes active in the presence of
at least two components (one precipitated with ZnCl2 and one
dialysable). Schneider (1967) demonstrated chromatographically that
radio-labelled chlormequat was transformed into a substance identical
to choline in barley and chrysanthemum sprouts. He was able to isolate
radioactive choline or radioactive choline metabolites from plant
tissue, which had been standing in chlormequat-containing solutions
for some time. During decomposition the carbohydrate skeleton of
chlormequat seems, therefore to remain intact. The unknown metabolites
were identical to the metabolites isolated from plants after a choline
or choline plus chlormequat treatment. An additional combination,
which only appeared after chlormequat treatment, is taken as an
intermediate of the decomposition processes. On the whole, 50-80% of
the added radioactivity was found unchanged in the chlormequat
solution, 15-40% in the residue solutions and 35-40% in the methanol
extracts. At least 10-15% of the added radioactivity had been
transformed in metabolic products of chlormequat.
Schneider (1967) sees a contradiction in the relatively fast
decomposition of chlormequat and its long lasting effect on plant
growth. Since choline also, though at ten times higher concentrations
than chlormequat, delayed the growth of barley roots and sprouts in
such tests, this author believes that the effect normally assigned to
chlormequat alone could result from chlormequat choline and further
unknown metabolites of these combinations. The contradiction between
chlormequat decomposition and lasting effect could be explained if the
metabolism of chlormequat only takes place in one part of the plant
tissue, whilst other parts store unchanged chlormequat. This
explanation would comply with observations of Sachs and Kofranek
(1963), who have noted that chlormequat activities mainly take place
in the subapical meristem.
In soil
Chlormequat decomposition in soil takes place relatively fast. Cathey
and Stuart (1961) assess the persistency of choline chloride in soil
as only three weeks. The opinion that the soil does not provide the
plant with a continuous subsequent supply of chlormequat is also
shared by Schneider (1967). Trials were carried out by Jung (1965) in
which four different soils were treated with 5 mg chlormequat 0-6
weeks before sowing wheat seeds in all pots. After 4-6 weeks there was
almost complete inactivation. In further tests it was established that
chlormequat in quantities of 3, 30 and 300 kg/ha did not exert a
definable influence on the microbic activity of five different soils.
CO2 production and nitrification were taken as the basis for these
observations (Jung, 1964).
In storage and processing
Jung and El-Fouly (1966) found that chlormequat residues in wheat
decline significantly in storage. Twelve samples of wheat which
originally contained 1.0 - 2.5 ppm chlormequat were found to have less
than 0.5 ppm after being stored for 12 months.
Cyanamid International (1971) reports trials to determine the level of
chlormequat residues in milling products processed from wheat treated
with chlormequat at the rate of 1.2 kg per ha by aircraft and
harvested 95 days later. The wheat was milled five months after
harvest and the bran and flour collected. The flour was made into
bread. The treated grain was found to contain 0.26 ppm chlormequat.
The bran contained 0.8 ppm, the flour 0.32 ppm and the bread 0.06 ppm
chlormequat. It is apparent that milling does not substantially reduce
the level of chlormequat residues in wheat but that baking of the
flour in the production of bread largely eliminates the residue. The
analytical method used had a limit of determination of 0.05 ppm.
The work of Tafuri et al. (1970) indicates that wine made from
grapes grown under chlormequat treatment will contain residues
substantially similar to those in the fresh grapes (up to 1 ppm).
APPRAISAL
Chlormequat is used as a growth regulator in cereals, grapes and
pears.
Residues in wheat grain which mostly lie below 1 ppm, may range up to
2 ppm. When such wheat is milled, the flour will contain substantially
similar levels but these are largely destroyed in the baking of bread.
Cows fed on the straw of chlormequat-treated cereals excrete small
traces of chlormequat in milk during the period of feeding, but
residues disappear as soon as the treated feed is withdrawn.
The treatment of pear trees at flowering in order to prevent
vegetative growth and encourage fruit bud formation for the next
season gives rise to residues ranging up to 2.6 ppm in fruit at
harvest. Residues are not found in the succeeding crop.
The gas chromatographic method of Tafuri et al. (1970) is
recommended as suitable for regulatory purposes. It is suitable for
use with the commodities included in the list of recommended
tolerances having a limit of determination of 0.05 ppm.
RECOMMENDATIONS
TOLERANCES
The following tolerances are for residues likely to be found at
harvest. Due to the stability of chlormequat residues, it is not
anticipated that residue levels will change significantly in storage,
although they will be largely destroyed by cooking.
ppm
Oats and rye 5
Wheat 3
Pears 3
Grapes, raisins and other dried
vine fruits 1
Milk and milk products 0.1*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on other registered uses for chlormequat.
2. Further information on residues of chlormequat in raw
agricultural commodities from a number of different countries.
3. Information on chlormequat residues in commodities moving in
international trade.
REFERENCES
Ackermann, H. and Kretzschmann, F. (1970) Einfluss von
Chlorcholinchlorid auf die Toxizität von Cholinesterase-Inhibitoren.
Arch. exp. Vet.-Med., 24 (4): 1045-1047.
Ackermann, H., Proll, J. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid l. Mitteilung:
Einfluss von Chlorcholinchlorid auf Wachstum, Futterverwertung,
Organveränderungen und Nachkommenschaft. Arch. exp. Vet.-Med., 24 (4):
1049-1058.
Anonymous. (1969) Justification for consideration of chlormequat as
plant growth regulator at 4th session of the Codex Committee on
Pesticide Residues Arnhem, Netherlands.
BASF, (1967a) Zweijahre-Ratten-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
BASF, (1967b) Zweijahre-Hunde-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
Bier, H. and Ackermann, H. (1970) Lokalisiesung und Anreicherung von
Chlorcholinchlorid bei der Ratte nach peroraler Applikation. Arch.
exp. Vet.-Med., 24(4): 1023-1026.
Blinn, R.C. (1967) Plant growth regulant. Biochemical behaviour of
2-chloroethyl trimethylammonium chloride in wheat and in rats. J. Agr.
Fd. Chem., 15: 984-988.
Bronisz, H. and Romanowski, H. (1968) Chromatographic study of the
urine of the rat poisoned with chlorocholine chloride (CCC) (in
Polish) Acta. Pol. Pharm., 25(6): 611
Cathey, H.M. and Stuart, N.W. (1961) Comparative plant growth
retarding activity of Amo 1618, Phosphon and CCC. Botan. Gaz., 123: 51
Costa, D.G., Hansen, W.D. and Woodard, G. (1967) Cyclocel. Toxicity in
the monkey following single oral doses. Report Woodard Research
Corporation submitted to American Cyanamid Co. (unpublished)
Cyanamid International. (1966) "Cycocel" plant growth reguland.
Technical information published by American Cyanamid Co.
Cyanamid International. (1971) Cycocel residues in wheat grains, wheat
bran, wheat flour and bread. Technical report No. RLG 53.
(unpublished)
FAO/WHO. (1971) 1970 evaluations of some pesticide residues in food.
FAO/AGP/1970/M/12/1; WHO/Food Add./71.42.
Friess, S.L. and McCarville, W.J. (1954) Nature of the
acetylcholinesterase surface. II. The ring effect in enzymic
inhibitors of the substituted ethylenediamine type. J. Am. Chem. Soc.,
76: 2260-2261.
Ignatiev, A.D. (1967) Toxicological evaluation of chlorocholine
chloride and its agricultural use in controlling plant growth (in
Russian). Chemistry in Agriculture, Moscow, 5: 37-39 (cited in
Lampeter and Bier, 1970).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst., 42: 1101-1114.
Jung, J. (1964) "Uber den einfluss von Chlorcholinchlorid (CCC) auf
biologische Vorgänge im Boden" (on the influence of chlorine choline
chloride chlormequat on biological activity in soil); Zeitschr. f.
Pflanzenernähr., Düngg. u. Bodenkde., 107: 153-164.
Jung, J. (1965) "Das Verhalten von CCC in Pflanzen und Boden;
CCC-Symposium der BASF. (Behaviour of chlormequat in plants and soil.)
Chlormequat symposium, BASE.
Jung, J. (1968a) "CCC-Rückstände an Roggen nach den Versuchsplänen D
III und D III d; Interne BASF-Mitteilungen (Chlormequat residue in rye
according to trial plan D III and D III d, Internal BASF Information.)
Jung, J. (1968b) "Rückstandsuntersuchungen an CCC-behandelten
Haferbeständen; Interne BASF-Mitteilungen Nr. 581. (Residue trials
with chlormequat-treated oats. Internal BASF Information No. 581.)
Jung, J. (1969a) "Ergebnisse der Rückstandsuntersuchung an
CCC-behandelten Haferbeständen der Vegetationsperiode 1968; Interne
BASF-Mitteilung No. 617. (Results of the residue examination of
chlormequat-treated oats from vegetation period 1968. Internal BASF
Information No. 617.)
Jung, J. (1969b) "CCC-Rückstandsuntersuchungen an Apfeln, Birnen und
einigen Gemüsearten; Interne BASF Mitteilungen No. 616. (CCC
Chlormequat residue examinations with apples, pears and several
vegetables. Internal BASF Information No. 616.)
Jung, J. and El-Fouly, M.M. (1966) "Uber den Abbau von
Chlorcholinchlorid (CCC) in der Pflanze" (On the decomposition of
chlorine choline chloride (CCC) in the plant): "Zeitschr. f.
Pflanzenernährg., Düngg. u. Bodenkde., 114: 128.
Jung, J. and El-Fouly, M.M. (1969) Some factors affecting the
degradation of (2-chloroethyl)-trimethylammoniumchloride by wheat
plant extracts. Experientia, 25: 587.
Jung, J. and Henjes, G. (1964a) "Nachweis und halbquantitative
Bestimmung von Chlorcholinchlorid (CCC) in Weizenkörnern und-stroh"
(Proof and half quantitative determination of chlorcholinchloride
(CCC) in wheat grain and straw); "Zeitschr. f. Pflanzenernährg.,
Düngg. u. Bodenkde., 106: 108.
Jung. J. and Henjes, G. (1964b) "Analytische Untersuchungen an
Weizenproben aus CCC-Versuchen" (Analytical research on wheat samples
of CCC-trials), "Landw. Forschg., 17: 267.
Juszkiewicz, T., Rakalska, Z. and Dzierzawski, A. (1970) Effet
embryopathique du chlorure de chlorocholine (CCC) chez le hamster
doré. Europ. J. Toxicol., 3(5): 265-270.
Lampeter, W. and Bier, H. (1970) Ausscheidung von chlorcholinchlorid
über Milch und Harn nach oraler Applikation von 1g15N-markiertem
Chlorcholinchlorid an eine laktierende Kuh. Arch. exp. Vet. Med.,
24(4): 1027-1031.
Leuschner, F., Leuschner, A. and Schwerdtfeger, W. (1967) Uber die
chronische Verträglichkeit von CCC. Chronischer Reproduktionsversuch
uber 3 Generationen an Wistar-Ratten. Report from Laboratory for
Pharmacology and Toxicology, Hamburg, Germany submitted by Cyanamid
GMBH. (unpublished)
Levinskas, G.J. (1965) Cyclocel(R), growth regulator;
2-chlorethyl-trimethylammonium chloride. Report on 90-day feeding
trials on dogs and rats conducted at the Central Medical Department,
American Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1962)
2-chloroethyl-trimethylammonium chloride; CL 38,555; limited release
toxicity studies. Report from Central Medical Department, American
Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1966) Cyclocel(R) plant growth
regulant. Single oral dose toxicity. Report from Central Medical
Department, American Cyanamid Company. (unpublished)
Lüder, W., Ackermann, H. and Proll, J. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 2. Meittlung:
Einfluss von Chlorcholinchlorid auf Liberatmung, Esterase-und
Transaminaseak-tivetät. Arch. exp. Vet. Med., 24(4): 1059-1064.
Mooney, R.P. and Pasarela, N.R. (1967) Determination of
chlorcholinchloride residues in wheat grain, straw and green wheat
foliage. J. Agr. Fd. Chem., 15: 989.
Niepolomski, W., Szczurek, A., Knapek, R. and Kolodziejczyk, A. (1970)
Evaluation of the chronic toxicity of chlorocholine chloride (CCC)
based on morphological studies. (in Polish). Med. Pracy., 21(1): 1-6.
Oettel, H. (1965) Zur Toxizität von Chlorcholinchlorid (CCC).
CCC-Symposium der BASF 14 Dezember 1965 (cited in Schulz and Lampeter,
1970).
Orloski, E.J. (1970) Cycocel residues in milk. Report No. C225
American Cyanamid. (unpublished)
Paxton, R.C. and Mayr, H.H. (1962) "Untersuchungen über das natürliche
Vorkommen quaternärer Ammoniumbasen in Lycopersicum esculentum"
(Examinations on the natural presence of quaternary ammonium bases in
Lycopersicum esculentum). Planta, 59: 165.
Proll, J., Ackermann, H. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 3. Mitteilung: zur
lipotropen Wirkung von Chlorcholinchlorid. Arch. exp. Vet. Med.,
24(4): 1065-1068.
Sachs, R.M. and Kofranek, A.M. (1963) Comparative cytohistological
studies on inhibition and promotion of stem growth in Chrysanthemum
morifolium. Am. J. Botany, 50: 772.
Schneider, E.F. (1967) Conversion of the plant growth retardant
(2-chlor-ethyl)-trimethyl-ammoniumchloride to choline in shoots of
chrysanthemum and barley. Can. J. Biochem., 45: 395.
Schulz, J.A., Eichelberger, P., Schäppel, K.F. and Johannsen, U.
(1970) Untersuchungen zur Verträglichkeit das Chlorcholinchlorids nach
oraler Verabreichung an Schafe. Arch exp. Vet. Med., 24(4): 1033-1044.
Schulz, J.A. and Lampeter, W., (1970) Vorwortzuden Untersuchungen über
Chlorcholinchlorid-Rückstände im Pflanzenmaterial die Wirkung des
Chlorcholinchlorids auf Warmblüter und über den Verbleib des
Chlorcholinchlorids bei Verfütterung an Ratten und an die Kuh. Arch.
exp. Vet. Med., 24(4): 1013-1018.
Shaffer, C.B. (1970) Chlormequat. "Monograph" from Central Medical
Department, American Cyanamid Co. submitted to Joint FAO/WHO Meeting
on Pesticide Residues.
Stefaniak, B. (1969) Severe toxicity of chlorocholine chloride (CCC)
to rats (in Polish). Med. weteryn, 25(5): 285-286.
Tafari, F., Businelli, M., Scarponi, L. and Giusquiani, P.L. (1970)
Chlorocholine chloride residues in grapes and their fate in
winemaking. J. Agr. Fd. Chem., 18: 869-871.
Weldon, G.H., Hunter, B., Hague, P.H. and Spicer E.J.F. (1971)
Long-term feeding study of Cycocel in the mouse. Report No.
4059/71/217 from Huntingdon Research Centre submitted to American
Cyanamid Company and to Cyanamid of Great Britain Ltd. (unpublished)
 (chlorine choline chloride)-> (choline chloride)-> (betain)
Figure 1 - Decomposition of chlorine choline chloride
As choline occupies a central position in plant metabolism and can be
oxidized to betain, the increase of betain content after chlormequat
treatment could thus be explained. Paxton and Mayr (1962) have shown
the transformation of choline and betain in a further scheme.
According to the latest research, the enzymatic system controlling the
decomposition of chlormequat into CC, is pH-dependent and thermostable
(Jung and El-Fouly 1969). On the whole, the decomposition of
chlormequat increases with the increasing pH value of the plant
extract and reached a maximum at pH6. Above this value the rate of
decomposition decreases since chlormequat is decomposed in alkaline
medium. Heading (40-90°C) the plant extracts and subsequent cooling to
room temperature did not affect the rate of decomposition of
chlormequat which was added later. Only by cooking the extracts for
several hours could inactivation be obtained. Temperatures which
increase from 10-40°C, however, accelerate the decomposition of
chlormequat into CC, if the chlormequat is already present in the
extract. The enzymatic system only becomes active in the presence of
at least two components (one precipitated with ZnCl2 and one
dialysable). Schneider (1967) demonstrated chromatographically that
radio-labelled chlormequat was transformed into a substance identical
to choline in barley and chrysanthemum sprouts. He was able to isolate
radioactive choline or radioactive choline metabolites from plant
tissue, which had been standing in chlormequat-containing solutions
for some time. During decomposition the carbohydrate skeleton of
chlormequat seems, therefore to remain intact. The unknown metabolites
were identical to the metabolites isolated from plants after a choline
or choline plus chlormequat treatment. An additional combination,
which only appeared after chlormequat treatment, is taken as an
intermediate of the decomposition processes. On the whole, 50-80% of
the added radioactivity was found unchanged in the chlormequat
solution, 15-40% in the residue solutions and 35-40% in the methanol
extracts. At least 10-15% of the added radioactivity had been
transformed in metabolic products of chlormequat.
Schneider (1967) sees a contradiction in the relatively fast
decomposition of chlormequat and its long lasting effect on plant
growth. Since choline also, though at ten times higher concentrations
than chlormequat, delayed the growth of barley roots and sprouts in
such tests, this author believes that the effect normally assigned to
chlormequat alone could result from chlormequat choline and further
unknown metabolites of these combinations. The contradiction between
chlormequat decomposition and lasting effect could be explained if the
metabolism of chlormequat only takes place in one part of the plant
tissue, whilst other parts store unchanged chlormequat. This
explanation would comply with observations of Sachs and Kofranek
(1963), who have noted that chlormequat activities mainly take place
in the subapical meristem.
In soil
Chlormequat decomposition in soil takes place relatively fast. Cathey
and Stuart (1961) assess the persistency of choline chloride in soil
as only three weeks. The opinion that the soil does not provide the
plant with a continuous subsequent supply of chlormequat is also
shared by Schneider (1967). Trials were carried out by Jung (1965) in
which four different soils were treated with 5 mg chlormequat 0-6
weeks before sowing wheat seeds in all pots. After 4-6 weeks there was
almost complete inactivation. In further tests it was established that
chlormequat in quantities of 3, 30 and 300 kg/ha did not exert a
definable influence on the microbic activity of five different soils.
CO2 production and nitrification were taken as the basis for these
observations (Jung, 1964).
In storage and processing
Jung and El-Fouly (1966) found that chlormequat residues in wheat
decline significantly in storage. Twelve samples of wheat which
originally contained 1.0 - 2.5 ppm chlormequat were found to have less
than 0.5 ppm after being stored for 12 months.
Cyanamid International (1971) reports trials to determine the level of
chlormequat residues in milling products processed from wheat treated
with chlormequat at the rate of 1.2 kg per ha by aircraft and
harvested 95 days later. The wheat was milled five months after
harvest and the bran and flour collected. The flour was made into
bread. The treated grain was found to contain 0.26 ppm chlormequat.
The bran contained 0.8 ppm, the flour 0.32 ppm and the bread 0.06 ppm
chlormequat. It is apparent that milling does not substantially reduce
the level of chlormequat residues in wheat but that baking of the
flour in the production of bread largely eliminates the residue. The
analytical method used had a limit of determination of 0.05 ppm.
The work of Tafuri et al. (1970) indicates that wine made from
grapes grown under chlormequat treatment will contain residues
substantially similar to those in the fresh grapes (up to 1 ppm).
APPRAISAL
Chlormequat is used as a growth regulator in cereals, grapes and
pears.
Residues in wheat grain which mostly lie below 1 ppm, may range up to
2 ppm. When such wheat is milled, the flour will contain substantially
similar levels but these are largely destroyed in the baking of bread.
Cows fed on the straw of chlormequat-treated cereals excrete small
traces of chlormequat in milk during the period of feeding, but
residues disappear as soon as the treated feed is withdrawn.
The treatment of pear trees at flowering in order to prevent
vegetative growth and encourage fruit bud formation for the next
season gives rise to residues ranging up to 2.6 ppm in fruit at
harvest. Residues are not found in the succeeding crop.
The gas chromatographic method of Tafuri et al. (1970) is
recommended as suitable for regulatory purposes. It is suitable for
use with the commodities included in the list of recommended
tolerances having a limit of determination of 0.05 ppm.
RECOMMENDATIONS
TOLERANCES
The following tolerances are for residues likely to be found at
harvest. Due to the stability of chlormequat residues, it is not
anticipated that residue levels will change significantly in storage,
although they will be largely destroyed by cooking.
ppm
Oats and rye 5
Wheat 3
Pears 3
Grapes, raisins and other dried
vine fruits 1
Milk and milk products 0.1*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on other registered uses for chlormequat.
2. Further information on residues of chlormequat in raw
agricultural commodities from a number of different countries.
3. Information on chlormequat residues in commodities moving in
international trade.
REFERENCES
Ackermann, H. and Kretzschmann, F. (1970) Einfluss von
Chlorcholinchlorid auf die Toxizität von Cholinesterase-Inhibitoren.
Arch. exp. Vet.-Med., 24 (4): 1045-1047.
Ackermann, H., Proll, J. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid l. Mitteilung:
Einfluss von Chlorcholinchlorid auf Wachstum, Futterverwertung,
Organveränderungen und Nachkommenschaft. Arch. exp. Vet.-Med., 24 (4):
1049-1058.
Anonymous. (1969) Justification for consideration of chlormequat as
plant growth regulator at 4th session of the Codex Committee on
Pesticide Residues Arnhem, Netherlands.
BASF, (1967a) Zweijahre-Ratten-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
BASF, (1967b) Zweijahre-Hunde-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
Bier, H. and Ackermann, H. (1970) Lokalisiesung und Anreicherung von
Chlorcholinchlorid bei der Ratte nach peroraler Applikation. Arch.
exp. Vet.-Med., 24(4): 1023-1026.
Blinn, R.C. (1967) Plant growth regulant. Biochemical behaviour of
2-chloroethyl trimethylammonium chloride in wheat and in rats. J. Agr.
Fd. Chem., 15: 984-988.
Bronisz, H. and Romanowski, H. (1968) Chromatographic study of the
urine of the rat poisoned with chlorocholine chloride (CCC) (in
Polish) Acta. Pol. Pharm., 25(6): 611
Cathey, H.M. and Stuart, N.W. (1961) Comparative plant growth
retarding activity of Amo 1618, Phosphon and CCC. Botan. Gaz., 123: 51
Costa, D.G., Hansen, W.D. and Woodard, G. (1967) Cyclocel. Toxicity in
the monkey following single oral doses. Report Woodard Research
Corporation submitted to American Cyanamid Co. (unpublished)
Cyanamid International. (1966) "Cycocel" plant growth reguland.
Technical information published by American Cyanamid Co.
Cyanamid International. (1971) Cycocel residues in wheat grains, wheat
bran, wheat flour and bread. Technical report No. RLG 53.
(unpublished)
FAO/WHO. (1971) 1970 evaluations of some pesticide residues in food.
FAO/AGP/1970/M/12/1; WHO/Food Add./71.42.
Friess, S.L. and McCarville, W.J. (1954) Nature of the
acetylcholinesterase surface. II. The ring effect in enzymic
inhibitors of the substituted ethylenediamine type. J. Am. Chem. Soc.,
76: 2260-2261.
Ignatiev, A.D. (1967) Toxicological evaluation of chlorocholine
chloride and its agricultural use in controlling plant growth (in
Russian). Chemistry in Agriculture, Moscow, 5: 37-39 (cited in
Lampeter and Bier, 1970).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst., 42: 1101-1114.
Jung, J. (1964) "Uber den einfluss von Chlorcholinchlorid (CCC) auf
biologische Vorgänge im Boden" (on the influence of chlorine choline
chloride chlormequat on biological activity in soil); Zeitschr. f.
Pflanzenernähr., Düngg. u. Bodenkde., 107: 153-164.
Jung, J. (1965) "Das Verhalten von CCC in Pflanzen und Boden;
CCC-Symposium der BASF. (Behaviour of chlormequat in plants and soil.)
Chlormequat symposium, BASE.
Jung, J. (1968a) "CCC-Rückstände an Roggen nach den Versuchsplänen D
III und D III d; Interne BASF-Mitteilungen (Chlormequat residue in rye
according to trial plan D III and D III d, Internal BASF Information.)
Jung, J. (1968b) "Rückstandsuntersuchungen an CCC-behandelten
Haferbeständen; Interne BASF-Mitteilungen Nr. 581. (Residue trials
with chlormequat-treated oats. Internal BASF Information No. 581.)
Jung, J. (1969a) "Ergebnisse der Rückstandsuntersuchung an
CCC-behandelten Haferbeständen der Vegetationsperiode 1968; Interne
BASF-Mitteilung No. 617. (Results of the residue examination of
chlormequat-treated oats from vegetation period 1968. Internal BASF
Information No. 617.)
Jung, J. (1969b) "CCC-Rückstandsuntersuchungen an Apfeln, Birnen und
einigen Gemüsearten; Interne BASF Mitteilungen No. 616. (CCC
Chlormequat residue examinations with apples, pears and several
vegetables. Internal BASF Information No. 616.)
Jung, J. and El-Fouly, M.M. (1966) "Uber den Abbau von
Chlorcholinchlorid (CCC) in der Pflanze" (On the decomposition of
chlorine choline chloride (CCC) in the plant): "Zeitschr. f.
Pflanzenernährg., Düngg. u. Bodenkde., 114: 128.
Jung, J. and El-Fouly, M.M. (1969) Some factors affecting the
degradation of (2-chloroethyl)-trimethylammoniumchloride by wheat
plant extracts. Experientia, 25: 587.
Jung, J. and Henjes, G. (1964a) "Nachweis und halbquantitative
Bestimmung von Chlorcholinchlorid (CCC) in Weizenkörnern und-stroh"
(Proof and half quantitative determination of chlorcholinchloride
(CCC) in wheat grain and straw); "Zeitschr. f. Pflanzenernährg.,
Düngg. u. Bodenkde., 106: 108.
Jung. J. and Henjes, G. (1964b) "Analytische Untersuchungen an
Weizenproben aus CCC-Versuchen" (Analytical research on wheat samples
of CCC-trials), "Landw. Forschg., 17: 267.
Juszkiewicz, T., Rakalska, Z. and Dzierzawski, A. (1970) Effet
embryopathique du chlorure de chlorocholine (CCC) chez le hamster
doré. Europ. J. Toxicol., 3(5): 265-270.
Lampeter, W. and Bier, H. (1970) Ausscheidung von chlorcholinchlorid
über Milch und Harn nach oraler Applikation von 1g15N-markiertem
Chlorcholinchlorid an eine laktierende Kuh. Arch. exp. Vet. Med.,
24(4): 1027-1031.
Leuschner, F., Leuschner, A. and Schwerdtfeger, W. (1967) Uber die
chronische Verträglichkeit von CCC. Chronischer Reproduktionsversuch
uber 3 Generationen an Wistar-Ratten. Report from Laboratory for
Pharmacology and Toxicology, Hamburg, Germany submitted by Cyanamid
GMBH. (unpublished)
Levinskas, G.J. (1965) Cyclocel(R), growth regulator;
2-chlorethyl-trimethylammonium chloride. Report on 90-day feeding
trials on dogs and rats conducted at the Central Medical Department,
American Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1962)
2-chloroethyl-trimethylammonium chloride; CL 38,555; limited release
toxicity studies. Report from Central Medical Department, American
Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1966) Cyclocel(R) plant growth
regulant. Single oral dose toxicity. Report from Central Medical
Department, American Cyanamid Company. (unpublished)
Lüder, W., Ackermann, H. and Proll, J. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 2. Meittlung:
Einfluss von Chlorcholinchlorid auf Liberatmung, Esterase-und
Transaminaseak-tivetät. Arch. exp. Vet. Med., 24(4): 1059-1064.
Mooney, R.P. and Pasarela, N.R. (1967) Determination of
chlorcholinchloride residues in wheat grain, straw and green wheat
foliage. J. Agr. Fd. Chem., 15: 989.
Niepolomski, W., Szczurek, A., Knapek, R. and Kolodziejczyk, A. (1970)
Evaluation of the chronic toxicity of chlorocholine chloride (CCC)
based on morphological studies. (in Polish). Med. Pracy., 21(1): 1-6.
Oettel, H. (1965) Zur Toxizität von Chlorcholinchlorid (CCC).
CCC-Symposium der BASF 14 Dezember 1965 (cited in Schulz and Lampeter,
1970).
Orloski, E.J. (1970) Cycocel residues in milk. Report No. C225
American Cyanamid. (unpublished)
Paxton, R.C. and Mayr, H.H. (1962) "Untersuchungen über das natürliche
Vorkommen quaternärer Ammoniumbasen in Lycopersicum esculentum"
(Examinations on the natural presence of quaternary ammonium bases in
Lycopersicum esculentum). Planta, 59: 165.
Proll, J., Ackermann, H. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 3. Mitteilung: zur
lipotropen Wirkung von Chlorcholinchlorid. Arch. exp. Vet. Med.,
24(4): 1065-1068.
Sachs, R.M. and Kofranek, A.M. (1963) Comparative cytohistological
studies on inhibition and promotion of stem growth in Chrysanthemum
morifolium. Am. J. Botany, 50: 772.
Schneider, E.F. (1967) Conversion of the plant growth retardant
(2-chlor-ethyl)-trimethyl-ammoniumchloride to choline in shoots of
chrysanthemum and barley. Can. J. Biochem., 45: 395.
Schulz, J.A., Eichelberger, P., Schäppel, K.F. and Johannsen, U.
(1970) Untersuchungen zur Verträglichkeit das Chlorcholinchlorids nach
oraler Verabreichung an Schafe. Arch exp. Vet. Med., 24(4): 1033-1044.
Schulz, J.A. and Lampeter, W., (1970) Vorwortzuden Untersuchungen über
Chlorcholinchlorid-Rückstände im Pflanzenmaterial die Wirkung des
Chlorcholinchlorids auf Warmblüter und über den Verbleib des
Chlorcholinchlorids bei Verfütterung an Ratten und an die Kuh. Arch.
exp. Vet. Med., 24(4): 1013-1018.
Shaffer, C.B. (1970) Chlormequat. "Monograph" from Central Medical
Department, American Cyanamid Co. submitted to Joint FAO/WHO Meeting
on Pesticide Residues.
Stefaniak, B. (1969) Severe toxicity of chlorocholine chloride (CCC)
to rats (in Polish). Med. weteryn, 25(5): 285-286.
Tafari, F., Businelli, M., Scarponi, L. and Giusquiani, P.L. (1970)
Chlorocholine chloride residues in grapes and their fate in
winemaking. J. Agr. Fd. Chem., 18: 869-871.
Weldon, G.H., Hunter, B., Hague, P.H. and Spicer E.J.F. (1971)
Long-term feeding study of Cycocel in the mouse. Report No.
4059/71/217 from Huntingdon Research Centre submitted to American
Cyanamid Company and to Cyanamid of Great Britain Ltd. (unpublished)
(chlorine choline chloride)-> (choline chloride)-> (betain)
Figure 1 - Decomposition of chlorine choline chloride
As choline occupies a central position in plant metabolism and can be
oxidized to betain, the increase of betain content after chlormequat
treatment could thus be explained. Paxton and Mayr (1962) have shown
the transformation of choline and betain in a further scheme.
According to the latest research, the enzymatic system controlling the
decomposition of chlormequat into CC, is pH-dependent and thermostable
(Jung and El-Fouly 1969). On the whole, the decomposition of
chlormequat increases with the increasing pH value of the plant
extract and reached a maximum at pH6. Above this value the rate of
decomposition decreases since chlormequat is decomposed in alkaline
medium. Heading (40-90°C) the plant extracts and subsequent cooling to
room temperature did not affect the rate of decomposition of
chlormequat which was added later. Only by cooking the extracts for
several hours could inactivation be obtained. Temperatures which
increase from 10-40°C, however, accelerate the decomposition of
chlormequat into CC, if the chlormequat is already present in the
extract. The enzymatic system only becomes active in the presence of
at least two components (one precipitated with ZnCl2 and one
dialysable). Schneider (1967) demonstrated chromatographically that
radio-labelled chlormequat was transformed into a substance identical
to choline in barley and chrysanthemum sprouts. He was able to isolate
radioactive choline or radioactive choline metabolites from plant
tissue, which had been standing in chlormequat-containing solutions
for some time. During decomposition the carbohydrate skeleton of
chlormequat seems, therefore to remain intact. The unknown metabolites
were identical to the metabolites isolated from plants after a choline
or choline plus chlormequat treatment. An additional combination,
which only appeared after chlormequat treatment, is taken as an
intermediate of the decomposition processes. On the whole, 50-80% of
the added radioactivity was found unchanged in the chlormequat
solution, 15-40% in the residue solutions and 35-40% in the methanol
extracts. At least 10-15% of the added radioactivity had been
transformed in metabolic products of chlormequat.
Schneider (1967) sees a contradiction in the relatively fast
decomposition of chlormequat and its long lasting effect on plant
growth. Since choline also, though at ten times higher concentrations
than chlormequat, delayed the growth of barley roots and sprouts in
such tests, this author believes that the effect normally assigned to
chlormequat alone could result from chlormequat choline and further
unknown metabolites of these combinations. The contradiction between
chlormequat decomposition and lasting effect could be explained if the
metabolism of chlormequat only takes place in one part of the plant
tissue, whilst other parts store unchanged chlormequat. This
explanation would comply with observations of Sachs and Kofranek
(1963), who have noted that chlormequat activities mainly take place
in the subapical meristem.
In soil
Chlormequat decomposition in soil takes place relatively fast. Cathey
and Stuart (1961) assess the persistency of choline chloride in soil
as only three weeks. The opinion that the soil does not provide the
plant with a continuous subsequent supply of chlormequat is also
shared by Schneider (1967). Trials were carried out by Jung (1965) in
which four different soils were treated with 5 mg chlormequat 0-6
weeks before sowing wheat seeds in all pots. After 4-6 weeks there was
almost complete inactivation. In further tests it was established that
chlormequat in quantities of 3, 30 and 300 kg/ha did not exert a
definable influence on the microbic activity of five different soils.
CO2 production and nitrification were taken as the basis for these
observations (Jung, 1964).
In storage and processing
Jung and El-Fouly (1966) found that chlormequat residues in wheat
decline significantly in storage. Twelve samples of wheat which
originally contained 1.0 - 2.5 ppm chlormequat were found to have less
than 0.5 ppm after being stored for 12 months.
Cyanamid International (1971) reports trials to determine the level of
chlormequat residues in milling products processed from wheat treated
with chlormequat at the rate of 1.2 kg per ha by aircraft and
harvested 95 days later. The wheat was milled five months after
harvest and the bran and flour collected. The flour was made into
bread. The treated grain was found to contain 0.26 ppm chlormequat.
The bran contained 0.8 ppm, the flour 0.32 ppm and the bread 0.06 ppm
chlormequat. It is apparent that milling does not substantially reduce
the level of chlormequat residues in wheat but that baking of the
flour in the production of bread largely eliminates the residue. The
analytical method used had a limit of determination of 0.05 ppm.
The work of Tafuri et al. (1970) indicates that wine made from
grapes grown under chlormequat treatment will contain residues
substantially similar to those in the fresh grapes (up to 1 ppm).
APPRAISAL
Chlormequat is used as a growth regulator in cereals, grapes and
pears.
Residues in wheat grain which mostly lie below 1 ppm, may range up to
2 ppm. When such wheat is milled, the flour will contain substantially
similar levels but these are largely destroyed in the baking of bread.
Cows fed on the straw of chlormequat-treated cereals excrete small
traces of chlormequat in milk during the period of feeding, but
residues disappear as soon as the treated feed is withdrawn.
The treatment of pear trees at flowering in order to prevent
vegetative growth and encourage fruit bud formation for the next
season gives rise to residues ranging up to 2.6 ppm in fruit at
harvest. Residues are not found in the succeeding crop.
The gas chromatographic method of Tafuri et al. (1970) is
recommended as suitable for regulatory purposes. It is suitable for
use with the commodities included in the list of recommended
tolerances having a limit of determination of 0.05 ppm.
RECOMMENDATIONS
TOLERANCES
The following tolerances are for residues likely to be found at
harvest. Due to the stability of chlormequat residues, it is not
anticipated that residue levels will change significantly in storage,
although they will be largely destroyed by cooking.
ppm
Oats and rye 5
Wheat 3
Pears 3
Grapes, raisins and other dried
vine fruits 1
Milk and milk products 0.1*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on other registered uses for chlormequat.
2. Further information on residues of chlormequat in raw
agricultural commodities from a number of different countries.
3. Information on chlormequat residues in commodities moving in
international trade.
REFERENCES
Ackermann, H. and Kretzschmann, F. (1970) Einfluss von
Chlorcholinchlorid auf die Toxizität von Cholinesterase-Inhibitoren.
Arch. exp. Vet.-Med., 24 (4): 1045-1047.
Ackermann, H., Proll, J. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid l. Mitteilung:
Einfluss von Chlorcholinchlorid auf Wachstum, Futterverwertung,
Organveränderungen und Nachkommenschaft. Arch. exp. Vet.-Med., 24 (4):
1049-1058.
Anonymous. (1969) Justification for consideration of chlormequat as
plant growth regulator at 4th session of the Codex Committee on
Pesticide Residues Arnhem, Netherlands.
BASF, (1967a) Zweijahre-Ratten-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
BASF, (1967b) Zweijahre-Hunde-Fütterungsversuch. Report from
Badische-Anilin-und Soda Fabrik submitted by Cyanamid GMBH.
(unpublished)
Bier, H. and Ackermann, H. (1970) Lokalisiesung und Anreicherung von
Chlorcholinchlorid bei der Ratte nach peroraler Applikation. Arch.
exp. Vet.-Med., 24(4): 1023-1026.
Blinn, R.C. (1967) Plant growth regulant. Biochemical behaviour of
2-chloroethyl trimethylammonium chloride in wheat and in rats. J. Agr.
Fd. Chem., 15: 984-988.
Bronisz, H. and Romanowski, H. (1968) Chromatographic study of the
urine of the rat poisoned with chlorocholine chloride (CCC) (in
Polish) Acta. Pol. Pharm., 25(6): 611
Cathey, H.M. and Stuart, N.W. (1961) Comparative plant growth
retarding activity of Amo 1618, Phosphon and CCC. Botan. Gaz., 123: 51
Costa, D.G., Hansen, W.D. and Woodard, G. (1967) Cyclocel. Toxicity in
the monkey following single oral doses. Report Woodard Research
Corporation submitted to American Cyanamid Co. (unpublished)
Cyanamid International. (1966) "Cycocel" plant growth reguland.
Technical information published by American Cyanamid Co.
Cyanamid International. (1971) Cycocel residues in wheat grains, wheat
bran, wheat flour and bread. Technical report No. RLG 53.
(unpublished)
FAO/WHO. (1971) 1970 evaluations of some pesticide residues in food.
FAO/AGP/1970/M/12/1; WHO/Food Add./71.42.
Friess, S.L. and McCarville, W.J. (1954) Nature of the
acetylcholinesterase surface. II. The ring effect in enzymic
inhibitors of the substituted ethylenediamine type. J. Am. Chem. Soc.,
76: 2260-2261.
Ignatiev, A.D. (1967) Toxicological evaluation of chlorocholine
chloride and its agricultural use in controlling plant growth (in
Russian). Chemistry in Agriculture, Moscow, 5: 37-39 (cited in
Lampeter and Bier, 1970).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst., 42: 1101-1114.
Jung, J. (1964) "Uber den einfluss von Chlorcholinchlorid (CCC) auf
biologische Vorgänge im Boden" (on the influence of chlorine choline
chloride chlormequat on biological activity in soil); Zeitschr. f.
Pflanzenernähr., Düngg. u. Bodenkde., 107: 153-164.
Jung, J. (1965) "Das Verhalten von CCC in Pflanzen und Boden;
CCC-Symposium der BASF. (Behaviour of chlormequat in plants and soil.)
Chlormequat symposium, BASE.
Jung, J. (1968a) "CCC-Rückstände an Roggen nach den Versuchsplänen D
III und D III d; Interne BASF-Mitteilungen (Chlormequat residue in rye
according to trial plan D III and D III d, Internal BASF Information.)
Jung, J. (1968b) "Rückstandsuntersuchungen an CCC-behandelten
Haferbeständen; Interne BASF-Mitteilungen Nr. 581. (Residue trials
with chlormequat-treated oats. Internal BASF Information No. 581.)
Jung, J. (1969a) "Ergebnisse der Rückstandsuntersuchung an
CCC-behandelten Haferbeständen der Vegetationsperiode 1968; Interne
BASF-Mitteilung No. 617. (Results of the residue examination of
chlormequat-treated oats from vegetation period 1968. Internal BASF
Information No. 617.)
Jung, J. (1969b) "CCC-Rückstandsuntersuchungen an Apfeln, Birnen und
einigen Gemüsearten; Interne BASF Mitteilungen No. 616. (CCC
Chlormequat residue examinations with apples, pears and several
vegetables. Internal BASF Information No. 616.)
Jung, J. and El-Fouly, M.M. (1966) "Uber den Abbau von
Chlorcholinchlorid (CCC) in der Pflanze" (On the decomposition of
chlorine choline chloride (CCC) in the plant): "Zeitschr. f.
Pflanzenernährg., Düngg. u. Bodenkde., 114: 128.
Jung, J. and El-Fouly, M.M. (1969) Some factors affecting the
degradation of (2-chloroethyl)-trimethylammoniumchloride by wheat
plant extracts. Experientia, 25: 587.
Jung, J. and Henjes, G. (1964a) "Nachweis und halbquantitative
Bestimmung von Chlorcholinchlorid (CCC) in Weizenkörnern und-stroh"
(Proof and half quantitative determination of chlorcholinchloride
(CCC) in wheat grain and straw); "Zeitschr. f. Pflanzenernährg.,
Düngg. u. Bodenkde., 106: 108.
Jung. J. and Henjes, G. (1964b) "Analytische Untersuchungen an
Weizenproben aus CCC-Versuchen" (Analytical research on wheat samples
of CCC-trials), "Landw. Forschg., 17: 267.
Juszkiewicz, T., Rakalska, Z. and Dzierzawski, A. (1970) Effet
embryopathique du chlorure de chlorocholine (CCC) chez le hamster
doré. Europ. J. Toxicol., 3(5): 265-270.
Lampeter, W. and Bier, H. (1970) Ausscheidung von chlorcholinchlorid
über Milch und Harn nach oraler Applikation von 1g15N-markiertem
Chlorcholinchlorid an eine laktierende Kuh. Arch. exp. Vet. Med.,
24(4): 1027-1031.
Leuschner, F., Leuschner, A. and Schwerdtfeger, W. (1967) Uber die
chronische Verträglichkeit von CCC. Chronischer Reproduktionsversuch
uber 3 Generationen an Wistar-Ratten. Report from Laboratory for
Pharmacology and Toxicology, Hamburg, Germany submitted by Cyanamid
GMBH. (unpublished)
Levinskas, G.J. (1965) Cyclocel(R), growth regulator;
2-chlorethyl-trimethylammonium chloride. Report on 90-day feeding
trials on dogs and rats conducted at the Central Medical Department,
American Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1962)
2-chloroethyl-trimethylammonium chloride; CL 38,555; limited release
toxicity studies. Report from Central Medical Department, American
Cyanamid Company. (unpublished)
Levinskas, G.J. and Shaffer, C.B. (1966) Cyclocel(R) plant growth
regulant. Single oral dose toxicity. Report from Central Medical
Department, American Cyanamid Company. (unpublished)
Lüder, W., Ackermann, H. and Proll, J. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 2. Meittlung:
Einfluss von Chlorcholinchlorid auf Liberatmung, Esterase-und
Transaminaseak-tivetät. Arch. exp. Vet. Med., 24(4): 1059-1064.
Mooney, R.P. and Pasarela, N.R. (1967) Determination of
chlorcholinchloride residues in wheat grain, straw and green wheat
foliage. J. Agr. Fd. Chem., 15: 989.
Niepolomski, W., Szczurek, A., Knapek, R. and Kolodziejczyk, A. (1970)
Evaluation of the chronic toxicity of chlorocholine chloride (CCC)
based on morphological studies. (in Polish). Med. Pracy., 21(1): 1-6.
Oettel, H. (1965) Zur Toxizität von Chlorcholinchlorid (CCC).
CCC-Symposium der BASF 14 Dezember 1965 (cited in Schulz and Lampeter,
1970).
Orloski, E.J. (1970) Cycocel residues in milk. Report No. C225
American Cyanamid. (unpublished)
Paxton, R.C. and Mayr, H.H. (1962) "Untersuchungen über das natürliche
Vorkommen quaternärer Ammoniumbasen in Lycopersicum esculentum"
(Examinations on the natural presence of quaternary ammonium bases in
Lycopersicum esculentum). Planta, 59: 165.
Proll, J., Ackermann, H. and Lüder, W. (1970) Untersuchungen zur
toxikologischen Beurteilung von Chlorcholinchlorid. 3. Mitteilung: zur
lipotropen Wirkung von Chlorcholinchlorid. Arch. exp. Vet. Med.,
24(4): 1065-1068.
Sachs, R.M. and Kofranek, A.M. (1963) Comparative cytohistological
studies on inhibition and promotion of stem growth in Chrysanthemum
morifolium. Am. J. Botany, 50: 772.
Schneider, E.F. (1967) Conversion of the plant growth retardant
(2-chlor-ethyl)-trimethyl-ammoniumchloride to choline in shoots of
chrysanthemum and barley. Can. J. Biochem., 45: 395.
Schulz, J.A., Eichelberger, P., Schäppel, K.F. and Johannsen, U.
(1970) Untersuchungen zur Verträglichkeit das Chlorcholinchlorids nach
oraler Verabreichung an Schafe. Arch exp. Vet. Med., 24(4): 1033-1044.
Schulz, J.A. and Lampeter, W., (1970) Vorwortzuden Untersuchungen über
Chlorcholinchlorid-Rückstände im Pflanzenmaterial die Wirkung des
Chlorcholinchlorids auf Warmblüter und über den Verbleib des
Chlorcholinchlorids bei Verfütterung an Ratten und an die Kuh. Arch.
exp. Vet. Med., 24(4): 1013-1018.
Shaffer, C.B. (1970) Chlormequat. "Monograph" from Central Medical
Department, American Cyanamid Co. submitted to Joint FAO/WHO Meeting
on Pesticide Residues.
Stefaniak, B. (1969) Severe toxicity of chlorocholine chloride (CCC)
to rats (in Polish). Med. weteryn, 25(5): 285-286.
Tafari, F., Businelli, M., Scarponi, L. and Giusquiani, P.L. (1970)
Chlorocholine chloride residues in grapes and their fate in
winemaking. J. Agr. Fd. Chem., 18: 869-871.
Weldon, G.H., Hunter, B., Hague, P.H. and Spicer E.J.F. (1971)
Long-term feeding study of Cycocel in the mouse. Report No.
4059/71/217 from Huntingdon Research Centre submitted to American
Cyanamid Company and to Cyanamid of Great Britain Ltd. (unpublished)
