
AZINPHOS-ETHYL JMPR 1973
IDENTITY
Chemical name
S-(3,4-dihydro-4-oxobenzo(d)-1,2,3-triazin-3-ylmethyl)
O,O-diethyl phosphorodithioate.
Synonyms
O,O-diethyl
S-(3,4-dihydro-4-oxobenzo(d)-(1,2,3)-triazin-3-ylmethyl)
phosphorodithionate; triazotion (common name in USSR);
Ethyl-Gusathion(R) Ethyl-Guthion(R). (R)Gusathion A,
(R)Gusathion K-forte; (R)Gusathion H.
Bay 16259
R 1513
Structural formula
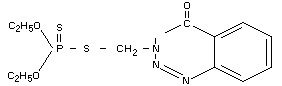 empirical formula: C12H16N3O3PS2
Other information on identity and properties
(a) Composition of technical azinphos-ethyl
The technical material contains a minimum of 92% azinphos-ethyl.
Impurities
triethyl thiophosphate and
triethyldithiophosphate together max. 2.0%
bis(3,4-dihydro-4-oxo-1,2,3-triazin-3-ylmethyl)-sulfide together
and -disulfide max. 1.5%
3-chloromethyl-3,4-dihydro-4-oxo-1,2,3
benzotriazin and (3,4-dihydro-4-oxo-1,2,3
benzotriazin-3 ylmethyl)-methylsulfide together max. 1.5%
ethylene-bis (O,O-diethyl-dithiophosphate) max. 1.9%
0,S-diethyl-S-(3,4-dihydro-4-oxo-1,2,3-
benzotriazin-3-yl(-methyl)-dithiophosphate max. 0.7%
bis (3,3-dihydro-4-oxo-benzotriazin-3-yl-methyl)-ether max. 0.6%
0-ethyl-S-(3,4, dihydro-4-oxo-1,2,3
benzotriazin 3-yl-methyl)-dithio-phosphoric
acid max. 0.5%
water max. 0.2%
(b) Physical and chemical properties of azinphos-ethyl
Physical state: colourless crystals.
Molecular weight: 345.4.
Melting point: 53°C (pure material).
Boiling point: 147°C at 0.01 mm Hg
111°C at 0.001 mm Hg
Volatility: 0.0012 mg/cu metre at 10°C
0.0042 mg/cu metre at 20°C
0.0128 mg/cu metre at 30°C
0.0372 mg/cu metre at 40°C
Vapour pressure: 6.3 x 10-8 mm Hg at 10°C
2.2 x 10-7 mm Hg at 20°C
7 x 10-7 mm Hg at 30°C
2.1 x 10-6 mm Hg at 40°C
Specific gravity: D 20 = 1.284
4
Refractive index: N 53 = 1.592
D
Solubility: practically insoluble in water,
soluble in most organic solvents
Formulations used: emulsifiable liquid 25% w/v, 40% w/v
wettable powder 25%, 33%, 40%
ULV formulation 500 g/l
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Following oral and I.V. administration of 14C-labelled
azinphos-ethyl to male rats in doses of 0.1 or 2 mg/kg bw, about 60%
of the activity was excreted in urine and 30% in faeces in 48 hours.
Approximately 30% of a 2 mg/kg dose was eliminated in bile within 24
hours; less than 0.1% was eliminated in expired air, indicating that
the benzotriazine ring does not undergo extensive degradation.
Approximately 6% of activity was present in the body 48 hours after
administration, the mean concentration in tissues being equivalent to
0.1 ppm. The highest equivalent concentration (0.2-0.3 ppm
(0.00002%-0.00003%)) was found in blood and 0.15-0.2 ppm
(0.000015%-0.00002%) was found in lungs, liver and kidneys. The
results of an exactly similar study using azinphos-methyl showed no
differences, indicating that mammals metabolize both compounds in a
similar manner (Anon., 1973).
Effects on enzymes and other biochemical parameters
Brain cholinesterase activity in vitro was reduced by 50% by
1.4 x 10-5 molar azinphos-ethyl; this was considered to be due to the
presence of impurities with anticholinesterase activity. A single I.P.
dose of 3.4 mg azinphos-ethyl/kg reduced serum, brain and submaxillary
gland cholinesterase activity to approximately the same extent.
Maximum inhibition occurred within an hour and activity returned to
normal gradually, reversal not being complete by 70 hours. On
incubation with liver preparation, azinphos-ethyl was transformed into
a potent anticholinesterase compound, probably the oxygen analogue
(Dubois et al., 1959).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of three hens received a single dose of 10 or 25 mg
azinphos-ethyl/kg and were observed for five weeks. One bird on the 25
mg/kg level died after 26 days but no signs of demyelinating disease
developed (Kimmerle, 1960).
Groups of four hens were fed on diet containing 0, 75, 150, 300
and 600 ppm (0, 0.0075, 0.015, 0.03 and 0.06%) azinphos-ethyl for 30
days. Loss of body weight occurred at the two highest dosage levels
and a significant depression of whole blood cholinesterase activity at
the 600 ppm (0.06%) level. No signs of neurological damage occurred
during the dosage period or during the subsequent four-week
observation period. Histological examination of femoral fixed brain,
spinal cord and femoral nerve failed to detect evidence of
demyelination or nerve damage (Kimmerle, 1968; Grasso, 1969).
Special studies on potentiation and antidotes
No significant potentiation of acute toxicity occurred with
azinphos-ethyl in combination with parathion, methyl parathion,
systox, EPN, malathion, trithion, phosdrin, sevin, delnav, OMPA,
diazinon, azinphos, di-syston, Co-Ral, Folex or Ronnel. A twofold
potentiation occurred with azinphos-ethyl and ethion (Dubois and
Raymond, 1960a; Dubois, 1963; Kimmerle, 1966a).
The effects of azinphos-ethyl are counteracted by atropine and by
reactivating compounds. The two drugs given in combination give more
pronounced effects (Kimmerle, 1966; Lorke and Kimmerle, 1968; Dubois
et al., 1959).
Acute toxicity
LD50
Species Route Sex mg/kg bw References
Mouse I.P. 3.8-4.0 Dubois et al., 1959
Rat Oral 12.0-20.5 Ben-Dyke et al., 1970
Kimmerle, 1959,
1966 a & b
F 7.0 Dubois et al., 1959
M 15.2
Rat I.P. F 4.4 Dubois et al., 1959
M 9.2
M 7.5 Kimmerle, 1959
Rat Dermal 75-280 Ben-Dyke et al., 1970
Dubois et al., 1959
Kimmerle, 1959
Guinea-pig Oral 17 Dubois et al., 1959
Guinea-pig I.P. 8.0 Dubois et al., 1959
Chicks Oral 34 Sherman et al., 1967
F = Female; M = Male.
Short-term studies
Rat. Twenty-four male rats received an oral dose of 1 mg
azinphos-ethyl/kg bw on each of 28 consecutive working days. The rate
of weight gain was normal and signs of poisoning did not occur.
Erythrocyte cholinesterase showed 50% depression after 2, 82% after 3
and 90% after 28 days' treatment. Activity had returned to normal 35
days after treatment stopped (Kimmerle, 1959).
Groups of five female rats received daily I.P. injections of
azinphos-ethyl at dosage levels of 0.5, 1, 2 and 3 mg/kg bw for 60
days. Only the two highest dosage levels caused a reduction in the
rate of weight gain and an increased mortality (Dubois and Raymond,
1960b).
Groups of 15 male and 15 female rats received diet containing 0,
1, 2, 4 and 8 ppm (0, 0.0001, 0.0002, 0.0004 and 0.0008%) for three
months. No effects were found on growth rate, food consumption,
mortality rate or on the results of haematological investigations and
analysis of blood. The 4 ppm (0.0004%) level had no significant effect
on plasma enzyme but depressed the erythrocyte cholinesterase activity
after one month. The 8 ppm (0.0008%) level depressed plasma enzyme
after only one week's exposure and prolonged exposure caused no
further depression; erythrocyte enzyme activity continued to fall
during the first month's exposure. In general, females appeared to be
more sensitive than males. Autopsy, determination of organ weights and
histological examination of organs and tissues detected no abnormality
in animals fed azinphos-ethyl (Löser, 1969; Mawdesley-Thomas and
Urwin, 1969).
Groups of 12 male and 12 female rats were fed on diets containing
0, 5, 10 and 50 ppm (0, 0.0005, 0.001 and 0.005%) azinphos-ethyl for
16 weeks. The growth rate of female animals was unaffected while male
rats receiving 50 ppm (0.005%) showed a slightly reduced rate of
growth compared with controls. No animals showed signs of
cholinesterase inhibition but determination of the activity of the
enzyme in serum, erythrocytes, submaxillary and brain tissue of five
male and five female rats of each group showed that it was markedly
inhibited in the 50 ppm (0.005%) group. Inhibition was most marked in
erythrocytes (87%) and serum (85%) and less marked In brain (24% in
males and 72% in females) and gland tissue (16% in males and 59% in
females). Serum and erythrocyte activity was depressed (52% and 80%
respectively) in rats fed 10 ppm (0.001%) azinphos-ethyl in their
diet. Only erythrocyte enzyme was significantly inhibited in rats on 5
ppm (0.0005%) diet; males and females did not differ in this respect.
The remaining seven animals of each sex of each group were autopsied
and the organs weighed and examined histologically but the results of
this examination are not reported (Doull and Root, 1960).
Dog. Groups of two male and two female dogs aged between 6 and 14
months were fed for 12 weeks on diets containing 0.25, 0.5, 1, 2, 3
and 10 ppm, (0.000025, 0.0005, 0.0001, 0.0002, 0.0003 and 0.001%)
azinphos-ethyl. The serum and erythrocyte cholinesterase activity was
determined periodically before, during and following the dosage
period. Dogs receiving 3 and 10 ppm (0.0003 and 0.001%) diets
exhibited signs of cholinesterase activity depression and were taken
off the diets after 1 and 6 weeks respectively. Over 50% inhibition
occurred in the serum and erythrocytes of dogs fed on 2 ppm (0.0002%)
diet. Erythrocyte enzyme activity was not inhibited in dogs receiving
the 0.25% diet but the serum enzyme was inhibited by about 20%. Since
the serum cholinesterase activity in control animals was inhibited by
10% the no-effect level was considered to be approximately 0.25 ppm
(0.000025%). In the animals affected, activity was reduced during the
first week of the test and then remained constant while the
erythrocyte enzyme tended to decrease gradually throughout the 12-week
test period. Cholinesterase activity returned to normal in 3-4 weeks
when dogs on the highest dosage levels were transferred to control
diet and in 2-3 weeks with the 0.5 and 1 ppm (0.00005 and 0.0001%)
groups (Doull et al., 1963).
Long-term studies
No data available.
Comments
Azinphos-ethyl is absorbed from the gastrointestinal tract and
excreted in urine and bile. It does not accumulate in tissues. The
absorption, distribution and excretion of radiolabel by rats are
similar in animals administered 14C-labelled azinphos-ethyl or
-methyl. However, insufficient data are available to determine the
metabolic pathway of azinphos-ethyl and to compare it with that of
azinphos-methyl, although there is evidence that in both the
benzotriazine moiety remains intact.
Azinphos-ethyl inhibits acetylcholinesterase activity in plasma,
erythrocyte, brain and submaxillary gland and a series of low doses
causes the plasma activity of rats and dogs to fall rapidly to a
stable level while erythrocyte acetylcholinesterase tends to fall more
gradually over a longer period. Female rats were more sensitive than
males as shown by the degree of enzyme inhibition and, in some tests,
by LD50 values. Recovery of enzyme activity took several weeks
following cessation of exposure of rats.
Short-term studies in rats show the no-effect level to be 2 ppm
(0.0002%) in the diet. The only toxic effects seen were attributed to
depression of cholinesterase activity. In an investigation using dogs,
in which only plasma and erythrocyte cholinesterase activity were
recorded, the no-effect level was 0.25 ppm (0.000025%) in the diet.
The Meeting was unable to estimate an acceptable daily intake for
this substance in the absence of sufficient information on the
identity and toxicity of its metabolites, on its possible effects on
reproduction, on its long-term toxicity and on its carcinogenic and
teratogenic and mutagenic potential.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-ethyl is a non-systemic organophosphorus insecticide,
which is used against a relatively broad spectrum of insects e.g.
Lepidopterous larvae, beetles and their larvae, aphids, jassids and
spider mites on various crops: cotton, rice, sugar and fodder beets;
fruits such as apples, pears, citrus-fruit, grapes; tobacco.
Azinphos-ethyl is used in about 35 countries in Europe, Africa,
Asia, Australia, New Zealand, North, Central and South America.
The amounts used on different crops vary greatly from year to
year depending on the pest situation. A rough estimate may be given:
cotton and other agricultural crops 60%
fruit crops (including grapes) 30%
vegetables (including potatoes) 10%
Pre-harvest applications
Azinphos-ethyl is generally used at dosages of 0.04-0.05% active
ingredient. The officially registered and/or recommended uses of
azinphos-ethyl are summarized below, with the application rates and
pre-harvest intervals.
Crop Dosage rate Minimum pre-harvest
g a.i./ha interval - days
Agricultural crops
cotton 250-1 000 5
rice 300-500 14
tobacco 300-600 14
sugar- and fodder beets 300-500 14
Fruit crops
apples, pears, peaches,
plums and cherries 800-1 000 14-21
citrus fruit 800-1 000 14-21
grapes 800-1 000 14-21
Vegetables
brassicas 300-500 7-14
melons 300-500 7-14
tomatoes 300-500 7-14
potatoes 300- 14-
Hops 400-800 14
Pre-harvest intervals officially recommended in different
countries, days
Australia general 21 days
Bulgaria sugar beets 14 days
Fed. Rep. of field crops as:
Germany cereals, rape, alfalfa,
sugar and fodder beets
potatoes 14 days
beans 21 days
cucumbers, tomatoes 14 days
United Kingdom apples, pears 21 days
cucumbers and tomatoes
(glasshouse cultures) 2 days
France general 15 days
Italy general 20 days
Morocco olives 30 days
other crops 15 days
New Zealand root vegetables 14 days
smooth-skinned fruit 14 days
other fruit and
vegetables 21 days
Netherlands potatoes 28 days
Portugal tomatoes for industrial
processing 5 days
all other crops 21 days
Switzerland fruit, grapes 21 days
potatoes 21 days
Spain cotton 21 days (2%
dust 15 days)
sugar beets 21 days
hazelnuts 21 days
South Africa potatoes 21 days
Pre-harvest intervals officially recommended in different
countries, days
South Africa cotton 5 days (not
(Cont'd.) to be used for
feeding purposes
until after 35 days)
Yugoslavia fruit 21 days
Residue data from supervised trials
Residue data are available from trials on various fruit,
vegetables and field crops: apple; kidney bean, soybean, cauliflower,
kohlrabi, savoy, potatoes; tobacco; the data obtained are summarized
in the following table.
Residues in food moving in commerce
In the Federal Republic of Germany in 1965 and 1968 a total of
228 samples of fruit and vegetables, home produced and imported,
analysed for organophosphorous compounds.
Only in one of these 220 samples azinphos-ethyl was detected, the
residue level being less than 0.1 ppm (Krause, 1969).
Azinphos-ethyl was not found in any of the 378 sub-samples of a
total diet study carried out in England and Wales in 1966-67 (Abbott
et al., 1970).
Fate of residues
In mammals
14C-labelled azinphos-ethyl and azinphos-methyl was administered
orally and intravenously to about 50 male rats (Sprague - Dawley
approx. 170 g). The compounds were applied in a solvent mixture
containing 5% Cremophor EL.
The radioactivity measured related to the sum of the unchanged
parent compound and its metabolites.
After oral as well as after intravenous administration about 65%
(57-68%) of the applied activity was eliminated in the urine within 48
hours, and about 30% (26-34%) in the faeces (see table).
TABLE 1. RESIDUES OF AZINPHOS-ETHYL + P = 0 ANALOGUE IN PPM, TOTAL RESIDUE
[TWO FIGURES ... (...), AZINPHOS-ETHYL AND P = 0 ANALOGUE SEPARATELY]
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Field crops
cottonseed USA 1968 14 0.56 E.C. 12 <0.01 <0.01
10 " <0.01 <0.01
13 " 0.01 0.01
10 " <0.01 0.01
gin trash USA 1968 14 0.56 E.C.12 2.71 1.96
10 " 1.28 0.75
13 " 0.22 0.12
10 " 5.07 3.07
foliage USA 1968 14 0.56 E.C.12 16.45 8.81
10 " 1.20 0.60
13 " 0.35 <0.07
10 " 11.41 11.47
rape seed Germany 1971 1 0.3 w.p. 33% n.d.(74)
Fed.Rep.
Fruit crops
Apple
Cox's and UK 1961 3 1.13 w.p. 25% 0.3 <0.3 <0.3 <0.3 n.d.(47)
Worcester
Cox's UK 1961 2 1.4 w.p. 25% 1.04 0.66 0.43 0.42
Golden
Delicious Belgium 1969 1 1.25 E.C. 50% 0.94 0.44 0.19 0.09 (42)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Vegetables
French Germany 1972 1 0.3 E.C.250g/l 0.6 0.05 n.d. n.d.
beans Fed.Rep. 1972 1 0.3 0.6 0.06 n.d. n.d.
1972 1 0.3 0.12 0.07 n.d. n.d.
soybean USA 1968 3 0.42 E.C. 12 0.55
(0.003)
green plant 1969 3 0.42 " 0.79
(0.003)
whole 1968 3 0.42 " 3.08 0.87
(0.04) (0.03)
1969 3 0.42 " 14.91 4.62 0.75 0.24
(<0.02) (<0.02) (<0.02) (<0.02)
1968 3 0.42 " 18.0 3.6 1.6 0.9
(<0.03) (<0.16) (<0.03) (0.39)
soybean USA 1968 3 0.42 E.C. 12 0.26
vines (dry) (<0.03)
(45 d)
1969 3 " " 0.25
(<0.06)
1968 3 " " 0.14
(<0.02)
(55 d)
1969 3 " " 0.01
(<0.03)
(48 d)
1968 3 " " 0.23
(<0.04)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
beans(dry) 1968 3 0.42 E.C. 12 0.10
(<0.03)
(45 d)
1969 3 " " 0.01
(<0.02)
1968 3 " " 0.01
(<0.01)
(55 d)
1969 3 " " 0.09
(<0.02)
(48 d)
1968 3 " " 0.01
(<0.01)
cauliflower Germany 1967 1 0.5 w.p.33% 2.95 0.15
Fed. Rep.
kohlrabi Germany 1968 1 0.5 w.p. 33% 0.25 0.07 0.04 n.d.
(without Fed.Rep. 1967 1 0.5 " 0.3 0.06 0.01
leaves) 1967 1 0.5 " 0.4 0.03 <0.01
1967 1 0.3 " 0.15 0.12 0.08
savoy Germany 1 0.3 w.p. 33% 0.32 0.27 0.15 0.12 0.14
Fed.Rep. (36 d)
white
cabbage Germany 1967 1 0.3 w.p. 33% 0.18 0.07 0.06 <0.02
Fed. Rep.
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
potatoes USA 1962 8 0.42 E.C. 12 n.d.
1962 8 " " n.d.
1962 8 " 1 " n.d.
1962 8 " " n.d.
1962 8 " " n.d.
Netherlands 1972 1 0.20 w.p.25% n.d.
1972 1 " " n.d.
tomatoes New
Zealand 1968 2 1.25 E.C. 44 1.03a 0.9a 0.78a 0.5la
1968 2 " 1.5a 0.98a 1.28a 0.80a
1968 2 11.25 w.p. 25 1.10a 1.15a 0.66a 0.62a
1968 2 " 1.97a 1.62a 0.94a 0.33a
tobacco
cured USA 1967 3 0.84b E.C. 12 227 119 63 22
1968 3 0.84b " 373 282 150 75
1967 3 0.56 E.C. 12 117 138 27 29
1967 3 " " 79.5 58.6 40.5 7.2
1967 3 " " 170 67 29 19
1967 3 " " 87 103 14 8.7
1967 3 " " 254 119 61 22
1967 3 " " 207 140 68 40
n.d. = not detectable.
a Only residues on the peel; residue in the pulp 0.03-0.09 ppm.
b Overdosage
Route of Dosage Number of % of activity applied
Compound administration mg/kg animals excreted in 48 hours
urine faeces
azinphos-ethyl i.v. 2 5 66 ± 2 26 ± 4
i.v. 0.1 5 59 ± 2 31 ± 4
p.o. 2 4 62 ± 2 30 ± 5
p.o. 0.1 5 57 ± 5 34 ± 6
azinphos-methyl i.v. 2 4 68 ± 3 26 ± 4
i.v. 0.1 5 63 ± 3 29 ± 4
p.o. 2 5 68 ± 4 26 ± 2
About 50% of the dosage administered was excreted in 8-9 hours.
Elimination in the bile
Following intravenous administration of 2 mg/kg azinphos-ethyl or
azinphos to each of three animals an average of approximately 30% of
the applied activity was eliminated in the bile within 24 hours.
Elimination of azinphos in the air
Following oral or intravenous administration of 14C-labelled
azinphos-ethyl less than 0.1% of the applied activity was eliminated
in the expired air within 24 hours. This indicates that the
benzotriazine ring in azinphos-ethyl does not undergo extensive
metabolic degradation.
Forty-eight hours after oral and intravenous administration of 2
mg/kg azinphos-ethyl or azinphos to each of four or five animals 6% of
the applied activity was still present in the animal; approximately 1%
was present in the gastrointestinal tract.
The mean concentration of azinphos-ethyl in the animal tissue
(except the gastrointestinal tract) was at that moment about 0.1 µg/g
tissue irrespective of the compound applied and the route of
administration. Autoradiography and quantitative determination in
single tissues showed that the activity was fairly evenly distributed.
The highest concentrations were in the blood (0.2-0.3 µg/g).
The equivalent concentrations in lung, liver and kidneys were
0.15-0.2 µg/g.
The concentrations measured in other organs were below the values
mentioned above.
Fate of residues in plants
The metabolism of azinphos-ethyl was studied in cotton and bean
plants under field conditions (Olson, 1969).
The residue following application of azinphos-ethyl consisted
partly of the P = 0 metabolite presumably owing to its higher chemical
stability. After 14 days approximately 10% of the residue could be
determined by TLC and GLC as the P = 0 analogue.
Methods of residue analysis
Several colorimetric, thin layer and gas chromatographic methods
for residue analysis of azinphos-ethyl and relevant metabolites are
described in the literature. Only those methods will be referred to
which may be suitable for regulatory purposes or which can be adapted
for these purposes.
Colorimetric methods
Miles (1964) developed a rapid colorimetric method for residue
analysis of azinphos-ethyl, azinphos and their P = 0 metabolites in
plant material.
The method is based on the hydrolysis of the pesticide or
metabolite molecule after separation in a medium of acetic and
hydrochloric acid and the simultaneous coupling of the presumably
formed intermediate benzazimide with N-(1-naphtyl)-ethylene diamine to
produce a violet solution with an absorption maximum at 556 nm.
The plant material is extracted with chloroform and the extract
is carried through a clean-up procedure using Attaclay-celite. The
oxygen analogues are separated in a Florisil column. Azinphos-ethyl
and azinphos-methyl are eluated with chloroform, the P = 0 analogues
with an acetonechloroform mixture.
The recoveries in plant material range from 78 to 97%.
The lower limit of detection is about 5 µg of azinphosethyl and
the P = 0 analogue.
The method, which is specific for the benzotriazinyl group, was
proved to be suitable for residue analysis on apples, tomatoes,
cabbage and tobacco. No interference occurs with milligram quantities
of carbaryl or the following organophosphorous pesticides: demeton,
diazinon, dimethoate, disulfoton, EPN, malathion, parathion, phorate,
schradan and trichlorphon.
A similar colorimetric method for residue analysis of
azinphos-ethyl in apples, potatoes, alfalfa and watermelons was
described by Möllhoff (1969).
The plant samples are treated with acetone; the parent compound
is extracted with chloroform and then chromatographed in isopropanolic
solution for clean-up. The alkaline hydrolysis leads to anthranilic
acid, which is diazotized and coupled with N-(1 naphtyl)-ethylene
diamine.
The P = 0 compound of azinphos-ethyl and azinphos are
co-determined with the method.
Parathion and 4-nitrophenol do not interfere with the
determination.
The limit of detection of the method is about 0.1-0.05 ppm.
The recoveries by the colorimetric method (Meagher et al., 1960)
were found to be similar to those obtained with GLC methods (Wagner,
1973).
COMPARISON OF RECOVERIES OF AZINPHOS-ETHYL BY COLORIMETRIC AND GLC
METHODS OF ANALYSIS
Recovery %
Crop Added ppm
GLC Colorimetry
potato 0.1 86 100
0.2 93 99
citrus 0.1 76 72
0.2 79 82
TLC method
Kirchhof (1970) developed a thin layer chromatographic separation
of azinphos-ethyl, azinphos-methyl and their P = 0 analogues on silica
gel coated plates combined with the colorimetric method of Miles
(1964).
The limit of determination of the method on apples, lettuce,
kohlrabi and onions is 0.1-0.2 µg for the parent compounds and their
P = 0 metabolites alone or in combination.
The method does not require a special clean-up procedure. There
is no interference from other insecticides, fungicides, herbicides or
plant constituents.
GLC method
Residues of azinphos-ethyl, azinphos and their P = 0 analogues
can be determined by gas chromatography with a high degree of
sensitivity and specificity.
Especially GLC methods utilizing a phosphorous-specific detector
e.g. a modified flame ionization detector (thermionic detector) proved
to be particularly suitable for regulatory purposes.
Simultaneous GLC determination of azinphos-ethyl and its P = 0
analogue in soybeans is described by Olson (1969). Azinphos is also
co-determined,
Following extraction of the plant samples (green plants, dry
beans and vines) with chloroform, interfering plant constituents are
precipitated. The resultant extract is directly injected into the
gas-chromatograph.
Under the given conditions azinphos, azinphos-ethyl
and the P = 0 analogue of azinphos-ethyl are identified by their
retention times which are 13.3, 17.3 and 15 minutes respectively.
The limit of determination in this rapid procedure is about 0.005
ppm.
A similar procedure for GLC analysis of azinphos-ethyl and
azinphos in cotton is described by Olson (1969a and b).
Other organophosphorous compounds which may be used in cotton,
such as diazinon, disulfoton and its sulfoxide and sulfone, demeton
and its sulfoxide and sulfone, malathion and malaoxon did not
interfere with the determination of azinphosethyl, azinphos and their
P = 0 metabolites.
A similar GLC method for determination of azinphos-ethyl,
azinphos and their P = 0 metabolites in cigarette smoke is described
by Olson (1969c).
A method for the gas chromatographic determination of residues of
azinphos-ethyl, azinphos-methyl and their P = 0 analogues in milk is
described by Hunt et al. (1970). The extraction procedure corresponds
to that of Adams et al. (1966). The sensitivity of this method for the
determination of azinphos-methyl and the -ethyl analogue in milk
samples is about 0.04 ppm and for the P = 0 analogue of
azinphos-methyl about 0.06 ppm.
New and generally applicable procedures for the extraction of
residues of carbaryl, malathion, phosphamidon, azinphos-methyl and
parathion from plant material are described by Watts (1971). The three
described procedures which consist of sample blending with
acetonitrile or ethyl acetate and an exhaustive Soxhlet extraction
procedure using 10% methanol in chloroform, were compared. They were
all found to give very good recoveries.
National tolerances
Australia fruit 2.0
vegetables 1.0
cereals 1.0
potatoes 0 (at or about limit of detection)
Belgium fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4
potatoes 0.05
Fed. Rep. of Germany fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4 (except root vegetables)
celeriac 0.4
other food
crops 0.05
Italy
azinphos-ethyl and
-methyl together 0.4
Netherlands
azinphos-ethyl and
-methyl together fruit crops 0.4
vegetables 0.4 (except potatoes)
potatoes 0.05
Switzerland
azinphos-ethyl and fruit crops
-methyl together incl.
grapes 0.4
vegetables 0.4
South Africa fruit,
vegetables 2
Appraisal
Azinphos-ethyl is a non-systemic organophosphorus insecticide
with contact action as well as stomach poison action used on a
considerable scale in many countries on a relatively wide range of
crops.
The main uses are foliar applications on field crops, such as
cotton, sugarbeet, fruit and vegetables against a wide range of
insects.
Technical azinphos-ethyl contains a minimum of 92% of the pure
compound. The impurities in the technical material are known.
Azinphos-ethyl is marketed in the form of wettable powders,
emulsifiable liquids and as a ULV formulation.
The concentration/rates of application vary, depending on pest,
crop and method of application; normal application rates are
250-1000 g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. The data
presented for azinphos-ethyl including the P = 0 analogue are, with
few exceptions, representative of those likely to result from good
agricultural practice.
Limited information is available on the fate of residues in
plants. The residues which may occur in food of plant origin,
following recommended directions for use and recommended pre-harvest
intervals consist largely of the parent chemical and to a small extent
of the P = 0 analogue. The residue may contain up to 10% of the P = 0
analogue.
Information considered provided a comparison between the nature
and fate of azinphos-ethyl and its methyl analogue. The only
noticeable difference is the greater persistence of the P = 0 analogue
of the azinphos-ethyl. The P = 0 analogue is not detected at harvest
following the use of azinphos-methyl.
Only limited information is available on the rate of decrease of
the residue of azinphos-ethyl and the P = 0 analogue in crops during
storage and processing, including household cooking. Information was
presented on the extent of carry over of residues in tobacco smoke
following application to tobacco.
Extensive data was available from supervised trials on
cauliflower, kohlrabi, savoy cabbage, white cabbage, french beans. It
was considered that a sufficient range of vegetable crops had been
studied to enable recommendations to be made for vegetables as a
group.
Little information is available on azinphos-ethyl in food in
commerce. A rapid and sensitive colorimetric method is described for
the determination of azinphos-ethyl and the P = 0 analogue together.
The method is specific for the benzotriazinol group and thus
azinphos-ethyl and azinphosmethyl cannot be determined separately.
Gas chromatographic procedures are available for specific
determination of azinphos-ethyl and the P = 0 analogue. Appropriate
extraction and clean-up procedures in food products of plant and
animal origin are available. These methods are suitable for regulatory
purposes.
RECOMMENDATIONS
The following tolerances are recommended for azinphosethyl and
its P = 0 metabolite expressed as azinphos-ethyl.
Commodity Tolerance Pre-harvest intervals on
(ppm) which recommendations are
based (days)
Tomatoes 1 14 (outdoors)
Apples, pears 0.5 21
Vegetables, except
tomatoes and potatoes 0.5 14-21
Soybeans (dry) 0.2 14-21
Potatoes 0.05a 14
Cotton seed, rape seed 0.05a 14
a At or about the limit of determination
N.B. In those cases where a mixture of residues of azinphos-ethyl
and azinphos-methyl occurs together on a commodity, except tomatoes,
the total residue of both compounds including their P = 0 analogues
may not exceed the levels recommended for azinphos-methyl. In case of
tomatoes the total residue should not exceed the level recommended
above for azinphos-ethyl, 1 ppm.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Studies to identify metabolites in plants and animals.
3. Studies to investigate the toxicity of metabolites.
4. Studies to detect effects on reproduction.
5. Studies to detect teratogenic activity.
Desirable
1. Additional information on the nature of terminal residues
in plants, animals and their products.
2. Data on disappearance of residues during storage and
cooking of vegetables and fruits.
REFERENCES
Abbot, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G.
1970 Pesticide residues in the total diet in England
and Wales, 1966-1967. III. Organophosphorous
pesticide residues in the total diet. Pestic. Sci
1: 10-13
Adams, J. M. and Anderson, C. A. Spectrophotofluormetric
1966 method for Guthion residues in milk and animal
tissues. J. Agric. Fd. Chem. 14: 53-55
Anderson, C. A. A Comparison of the Residue Decline Rates
1963 for Guthion and Ethyl Homolog on Alfalfa, Apples
and Lespedezza. Chemagro Report No. 10 394
(unpublished)
Anon. Pharmacokinetic studies with 14C-labelled Gusathion
1973 A and Gusathion M (provisional results)
Unpublished report from Isotopen-Institut,
submitted by Bayer A.G.
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. Acute
1970 toxicity data for pesticides. World Review of Pest
Control, 9: 119-127
Doull, J. and Root, M. The Effect of Diets containing Ethyl
1960 Guthion on Rats. Unpublished report from
University of Chicago
Doull, J., Root, M. and Cowns, J. Determination of the safe
1963 dietary level of Ethyl Guthion for dogs.
Unpublished report from University of Chicago
Dubois, K. P. The Acute Toxicity of Ethyl Guthion in
1963 Combination with Four Other Organic Phosphates.
Unpublished report from University of Chicago
Dubois, K. P. and Raymund, A. B. The Acute Toxicity of Ethyl
1960a Guthion in Combination with Other
Anticholinesterase Agents. Unpublished report from
University of Chicago
Dubois, K. P. and Raymund, A. B. The Subacute Toxicity of
1960b Ethyl Guthion to Rats. Unpublished report from
University of Chicago
Dubois, K. P., Schmalgemeier, D. and Leighton, L. B.
1959 Studies on the Toxicity and Anticholinesterase
Action of Ethyl Guthion. Unpublished report from
University of Chicago
Grasso, P. Results of histological examination of brain,
1969 spinal cord and a peripheral nerve from hens
treated with Ethyl-gusathion for 30 days.
Unpublished report from British Industrial
Research Association, submitted by Bayer A.G.
Hunt, L. M. and Gilbert, B. N. Simultaneous determinations of
1970 azinphos-ethyl, azinphos-methyl and its oxygen
analogue by gas liquid chromatography. Bull. of
Env. Cont. and Tox. 5: 42-46
Kimmerle, G. Active substance R1513 (Ethyl Gusathion;
1959 No. 2078). Unpublished report from Bayer A.G.
Kimmerle, G. Ethyl-Gusathion. Unpublished report from
1960 Bayer A.G.
Kimmerle, G. Gusathion A/Antidotwirkung und Potentzierung.
1966a Unpublished report from Bayer A.G.
Kimmerle, G. Gusathion A-Wirkstoff (R 1513; Ht. - Nr. 3651).
1966b Unpublished report from Bayer A.G.
Kimmerle, G. Äthyl-Gusation/Subchronische Neurotoxizitäts
1968 versuche bei Hühnern. Unpublished report from
Bayer A.G.
Kimmerle, G. and Lorke, D. Toxicology of insecticidal
1968 organophosphates. Pflanzenschutz-Nachrichten,
Bayer, 21: 111-142
Kirchhoff, J. Spezifischer dünnschichtchromatographischer
1970 Nachweis von Gusathion-Rückständen in Pflanzen
extrakten. Pflanzenschutznachrichten, Bayer, 23:
365-366
Krause, Ch. und Kirchhoff, J. Organophosphatrückstände auf
1969 Marktproben von Obst and Gemüse sowie auf
Getreideerzeugnissen. Deutsch. Pflanzenschutzd.
(Braunschweig), 21, 1969: 81-84
Lorke, D. and Kimmerle, G. The Action of Reactivators in
1969 Phosphoric - Acid-Ester Poisoning. Naunyn
Schmiedebergs Arch. Pharmak. exp. Path. 263: 237
Löser, E. BAY 16 259/Subchronische toxikologische
1969 Untersuchungen an Ratten. Unpublished report from
Bayer A.G.
Mawdesley-Thomas, L. E. and Urwin, C. Pathology Report of
1969 BAY 16 259. Sub-chronic Toxicity Study in Rats
Administered over Three Months. Unpublished report
from Huntingdon Research Centre, submitted by
Bayer A.G.
Meagher, W. R., Adams. J. M., Anderson, C. A. and Macdougall, D.
1960 Colorimetric determination of Guthion residues in
crops. J. Agric. Fd. Chem., 8: 282-286
Miles, J. R. W. A new colorimetric method for determination
1964 of residues of Guthion and Ethyl Guthion and their
oxygen analogs. J.A.O.A.C. 47: 882-885
Möllhoff, E. Kolorimetrische Bestimmung von Azinphos-äthyl. 1969
Rückstands analytik von Pflanzenschutmitteln.
(DFG), Verlag Chemie GmbH, Weinheim/Bergstr.
Olson, T. J. Investigation of the possible metabolism of
1969 Ethyl-Guthion on crops. Chemagro Report No. 25
151 (unpublished)
Olson, T. J. Determination of residues of Guthion M-E in
1969a soybeans by thermionic emission gas
chromatography. Chemagro Report No. 25 152
(unpublished)
Olson, T. J. A confirmatory gas chromatographic procedure for
1969b the Guthion M-E residue method. Chemagro Report
No. 25 911 (unpublished)
Olson, T. J. A study of the possible interferences or other
1969c pesticides with the analytical method for Guthion
M-E in cotton. Chemagro Report No. 25 912
(unpublished)
Olson, T. J. Determination of Guthion M-E residues in
1969d cigarette smoke. Chemagro Report No. 25 121
(unpublished)
Olthoff, P. D. A. and de Mey, R. C. H. Residues of azinphos
1973 ethyl on potatoes. Report No. 3954 Central
Institute for Nutrition and Food Research,
Netherlands
Schumann, S. A. and Anderson, R. J. Determination of Guthion
1965 M-E residues in clover by electron capture gas
chromatography. Chemagro Report No. 16 571
(unpublished)
Sherman, M., Herrick, R. B., Ross, E. and Chang, M. T. Y.
1967 Further Studies of the acute and subacute toxicity
of Insecticides to Chicks, Toxicol. and Appl.
Pharmacol. 11: 49-67
Wagner, Kl. Report of Bayer A.G., Leverkusen (Pflanzenschutz
1973 AT/Biologische Forschung, Institut für
Rückstandsanalytik) (unpublished)
Watts, R. R. Extraction efficiency study - Examination of three
procedures for extracting 14C-labeled and
unlabeled residues of organophosphorus pesticides
and carbaryl from bean leaves and kale. J.A.O.A.C.
54: 953-958
empirical formula: C12H16N3O3PS2
Other information on identity and properties
(a) Composition of technical azinphos-ethyl
The technical material contains a minimum of 92% azinphos-ethyl.
Impurities
triethyl thiophosphate and
triethyldithiophosphate together max. 2.0%
bis(3,4-dihydro-4-oxo-1,2,3-triazin-3-ylmethyl)-sulfide together
and -disulfide max. 1.5%
3-chloromethyl-3,4-dihydro-4-oxo-1,2,3
benzotriazin and (3,4-dihydro-4-oxo-1,2,3
benzotriazin-3 ylmethyl)-methylsulfide together max. 1.5%
ethylene-bis (O,O-diethyl-dithiophosphate) max. 1.9%
0,S-diethyl-S-(3,4-dihydro-4-oxo-1,2,3-
benzotriazin-3-yl(-methyl)-dithiophosphate max. 0.7%
bis (3,3-dihydro-4-oxo-benzotriazin-3-yl-methyl)-ether max. 0.6%
0-ethyl-S-(3,4, dihydro-4-oxo-1,2,3
benzotriazin 3-yl-methyl)-dithio-phosphoric
acid max. 0.5%
water max. 0.2%
(b) Physical and chemical properties of azinphos-ethyl
Physical state: colourless crystals.
Molecular weight: 345.4.
Melting point: 53°C (pure material).
Boiling point: 147°C at 0.01 mm Hg
111°C at 0.001 mm Hg
Volatility: 0.0012 mg/cu metre at 10°C
0.0042 mg/cu metre at 20°C
0.0128 mg/cu metre at 30°C
0.0372 mg/cu metre at 40°C
Vapour pressure: 6.3 x 10-8 mm Hg at 10°C
2.2 x 10-7 mm Hg at 20°C
7 x 10-7 mm Hg at 30°C
2.1 x 10-6 mm Hg at 40°C
Specific gravity: D 20 = 1.284
4
Refractive index: N 53 = 1.592
D
Solubility: practically insoluble in water,
soluble in most organic solvents
Formulations used: emulsifiable liquid 25% w/v, 40% w/v
wettable powder 25%, 33%, 40%
ULV formulation 500 g/l
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Following oral and I.V. administration of 14C-labelled
azinphos-ethyl to male rats in doses of 0.1 or 2 mg/kg bw, about 60%
of the activity was excreted in urine and 30% in faeces in 48 hours.
Approximately 30% of a 2 mg/kg dose was eliminated in bile within 24
hours; less than 0.1% was eliminated in expired air, indicating that
the benzotriazine ring does not undergo extensive degradation.
Approximately 6% of activity was present in the body 48 hours after
administration, the mean concentration in tissues being equivalent to
0.1 ppm. The highest equivalent concentration (0.2-0.3 ppm
(0.00002%-0.00003%)) was found in blood and 0.15-0.2 ppm
(0.000015%-0.00002%) was found in lungs, liver and kidneys. The
results of an exactly similar study using azinphos-methyl showed no
differences, indicating that mammals metabolize both compounds in a
similar manner (Anon., 1973).
Effects on enzymes and other biochemical parameters
Brain cholinesterase activity in vitro was reduced by 50% by
1.4 x 10-5 molar azinphos-ethyl; this was considered to be due to the
presence of impurities with anticholinesterase activity. A single I.P.
dose of 3.4 mg azinphos-ethyl/kg reduced serum, brain and submaxillary
gland cholinesterase activity to approximately the same extent.
Maximum inhibition occurred within an hour and activity returned to
normal gradually, reversal not being complete by 70 hours. On
incubation with liver preparation, azinphos-ethyl was transformed into
a potent anticholinesterase compound, probably the oxygen analogue
(Dubois et al., 1959).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of three hens received a single dose of 10 or 25 mg
azinphos-ethyl/kg and were observed for five weeks. One bird on the 25
mg/kg level died after 26 days but no signs of demyelinating disease
developed (Kimmerle, 1960).
Groups of four hens were fed on diet containing 0, 75, 150, 300
and 600 ppm (0, 0.0075, 0.015, 0.03 and 0.06%) azinphos-ethyl for 30
days. Loss of body weight occurred at the two highest dosage levels
and a significant depression of whole blood cholinesterase activity at
the 600 ppm (0.06%) level. No signs of neurological damage occurred
during the dosage period or during the subsequent four-week
observation period. Histological examination of femoral fixed brain,
spinal cord and femoral nerve failed to detect evidence of
demyelination or nerve damage (Kimmerle, 1968; Grasso, 1969).
Special studies on potentiation and antidotes
No significant potentiation of acute toxicity occurred with
azinphos-ethyl in combination with parathion, methyl parathion,
systox, EPN, malathion, trithion, phosdrin, sevin, delnav, OMPA,
diazinon, azinphos, di-syston, Co-Ral, Folex or Ronnel. A twofold
potentiation occurred with azinphos-ethyl and ethion (Dubois and
Raymond, 1960a; Dubois, 1963; Kimmerle, 1966a).
The effects of azinphos-ethyl are counteracted by atropine and by
reactivating compounds. The two drugs given in combination give more
pronounced effects (Kimmerle, 1966; Lorke and Kimmerle, 1968; Dubois
et al., 1959).
Acute toxicity
LD50
Species Route Sex mg/kg bw References
Mouse I.P. 3.8-4.0 Dubois et al., 1959
Rat Oral 12.0-20.5 Ben-Dyke et al., 1970
Kimmerle, 1959,
1966 a & b
F 7.0 Dubois et al., 1959
M 15.2
Rat I.P. F 4.4 Dubois et al., 1959
M 9.2
M 7.5 Kimmerle, 1959
Rat Dermal 75-280 Ben-Dyke et al., 1970
Dubois et al., 1959
Kimmerle, 1959
Guinea-pig Oral 17 Dubois et al., 1959
Guinea-pig I.P. 8.0 Dubois et al., 1959
Chicks Oral 34 Sherman et al., 1967
F = Female; M = Male.
Short-term studies
Rat. Twenty-four male rats received an oral dose of 1 mg
azinphos-ethyl/kg bw on each of 28 consecutive working days. The rate
of weight gain was normal and signs of poisoning did not occur.
Erythrocyte cholinesterase showed 50% depression after 2, 82% after 3
and 90% after 28 days' treatment. Activity had returned to normal 35
days after treatment stopped (Kimmerle, 1959).
Groups of five female rats received daily I.P. injections of
azinphos-ethyl at dosage levels of 0.5, 1, 2 and 3 mg/kg bw for 60
days. Only the two highest dosage levels caused a reduction in the
rate of weight gain and an increased mortality (Dubois and Raymond,
1960b).
Groups of 15 male and 15 female rats received diet containing 0,
1, 2, 4 and 8 ppm (0, 0.0001, 0.0002, 0.0004 and 0.0008%) for three
months. No effects were found on growth rate, food consumption,
mortality rate or on the results of haematological investigations and
analysis of blood. The 4 ppm (0.0004%) level had no significant effect
on plasma enzyme but depressed the erythrocyte cholinesterase activity
after one month. The 8 ppm (0.0008%) level depressed plasma enzyme
after only one week's exposure and prolonged exposure caused no
further depression; erythrocyte enzyme activity continued to fall
during the first month's exposure. In general, females appeared to be
more sensitive than males. Autopsy, determination of organ weights and
histological examination of organs and tissues detected no abnormality
in animals fed azinphos-ethyl (Löser, 1969; Mawdesley-Thomas and
Urwin, 1969).
Groups of 12 male and 12 female rats were fed on diets containing
0, 5, 10 and 50 ppm (0, 0.0005, 0.001 and 0.005%) azinphos-ethyl for
16 weeks. The growth rate of female animals was unaffected while male
rats receiving 50 ppm (0.005%) showed a slightly reduced rate of
growth compared with controls. No animals showed signs of
cholinesterase inhibition but determination of the activity of the
enzyme in serum, erythrocytes, submaxillary and brain tissue of five
male and five female rats of each group showed that it was markedly
inhibited in the 50 ppm (0.005%) group. Inhibition was most marked in
erythrocytes (87%) and serum (85%) and less marked In brain (24% in
males and 72% in females) and gland tissue (16% in males and 59% in
females). Serum and erythrocyte activity was depressed (52% and 80%
respectively) in rats fed 10 ppm (0.001%) azinphos-ethyl in their
diet. Only erythrocyte enzyme was significantly inhibited in rats on 5
ppm (0.0005%) diet; males and females did not differ in this respect.
The remaining seven animals of each sex of each group were autopsied
and the organs weighed and examined histologically but the results of
this examination are not reported (Doull and Root, 1960).
Dog. Groups of two male and two female dogs aged between 6 and 14
months were fed for 12 weeks on diets containing 0.25, 0.5, 1, 2, 3
and 10 ppm, (0.000025, 0.0005, 0.0001, 0.0002, 0.0003 and 0.001%)
azinphos-ethyl. The serum and erythrocyte cholinesterase activity was
determined periodically before, during and following the dosage
period. Dogs receiving 3 and 10 ppm (0.0003 and 0.001%) diets
exhibited signs of cholinesterase activity depression and were taken
off the diets after 1 and 6 weeks respectively. Over 50% inhibition
occurred in the serum and erythrocytes of dogs fed on 2 ppm (0.0002%)
diet. Erythrocyte enzyme activity was not inhibited in dogs receiving
the 0.25% diet but the serum enzyme was inhibited by about 20%. Since
the serum cholinesterase activity in control animals was inhibited by
10% the no-effect level was considered to be approximately 0.25 ppm
(0.000025%). In the animals affected, activity was reduced during the
first week of the test and then remained constant while the
erythrocyte enzyme tended to decrease gradually throughout the 12-week
test period. Cholinesterase activity returned to normal in 3-4 weeks
when dogs on the highest dosage levels were transferred to control
diet and in 2-3 weeks with the 0.5 and 1 ppm (0.00005 and 0.0001%)
groups (Doull et al., 1963).
Long-term studies
No data available.
Comments
Azinphos-ethyl is absorbed from the gastrointestinal tract and
excreted in urine and bile. It does not accumulate in tissues. The
absorption, distribution and excretion of radiolabel by rats are
similar in animals administered 14C-labelled azinphos-ethyl or
-methyl. However, insufficient data are available to determine the
metabolic pathway of azinphos-ethyl and to compare it with that of
azinphos-methyl, although there is evidence that in both the
benzotriazine moiety remains intact.
Azinphos-ethyl inhibits acetylcholinesterase activity in plasma,
erythrocyte, brain and submaxillary gland and a series of low doses
causes the plasma activity of rats and dogs to fall rapidly to a
stable level while erythrocyte acetylcholinesterase tends to fall more
gradually over a longer period. Female rats were more sensitive than
males as shown by the degree of enzyme inhibition and, in some tests,
by LD50 values. Recovery of enzyme activity took several weeks
following cessation of exposure of rats.
Short-term studies in rats show the no-effect level to be 2 ppm
(0.0002%) in the diet. The only toxic effects seen were attributed to
depression of cholinesterase activity. In an investigation using dogs,
in which only plasma and erythrocyte cholinesterase activity were
recorded, the no-effect level was 0.25 ppm (0.000025%) in the diet.
The Meeting was unable to estimate an acceptable daily intake for
this substance in the absence of sufficient information on the
identity and toxicity of its metabolites, on its possible effects on
reproduction, on its long-term toxicity and on its carcinogenic and
teratogenic and mutagenic potential.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-ethyl is a non-systemic organophosphorus insecticide,
which is used against a relatively broad spectrum of insects e.g.
Lepidopterous larvae, beetles and their larvae, aphids, jassids and
spider mites on various crops: cotton, rice, sugar and fodder beets;
fruits such as apples, pears, citrus-fruit, grapes; tobacco.
Azinphos-ethyl is used in about 35 countries in Europe, Africa,
Asia, Australia, New Zealand, North, Central and South America.
The amounts used on different crops vary greatly from year to
year depending on the pest situation. A rough estimate may be given:
cotton and other agricultural crops 60%
fruit crops (including grapes) 30%
vegetables (including potatoes) 10%
Pre-harvest applications
Azinphos-ethyl is generally used at dosages of 0.04-0.05% active
ingredient. The officially registered and/or recommended uses of
azinphos-ethyl are summarized below, with the application rates and
pre-harvest intervals.
Crop Dosage rate Minimum pre-harvest
g a.i./ha interval - days
Agricultural crops
cotton 250-1 000 5
rice 300-500 14
tobacco 300-600 14
sugar- and fodder beets 300-500 14
Fruit crops
apples, pears, peaches,
plums and cherries 800-1 000 14-21
citrus fruit 800-1 000 14-21
grapes 800-1 000 14-21
Vegetables
brassicas 300-500 7-14
melons 300-500 7-14
tomatoes 300-500 7-14
potatoes 300- 14-
Hops 400-800 14
Pre-harvest intervals officially recommended in different
countries, days
Australia general 21 days
Bulgaria sugar beets 14 days
Fed. Rep. of field crops as:
Germany cereals, rape, alfalfa,
sugar and fodder beets
potatoes 14 days
beans 21 days
cucumbers, tomatoes 14 days
United Kingdom apples, pears 21 days
cucumbers and tomatoes
(glasshouse cultures) 2 days
France general 15 days
Italy general 20 days
Morocco olives 30 days
other crops 15 days
New Zealand root vegetables 14 days
smooth-skinned fruit 14 days
other fruit and
vegetables 21 days
Netherlands potatoes 28 days
Portugal tomatoes for industrial
processing 5 days
all other crops 21 days
Switzerland fruit, grapes 21 days
potatoes 21 days
Spain cotton 21 days (2%
dust 15 days)
sugar beets 21 days
hazelnuts 21 days
South Africa potatoes 21 days
Pre-harvest intervals officially recommended in different
countries, days
South Africa cotton 5 days (not
(Cont'd.) to be used for
feeding purposes
until after 35 days)
Yugoslavia fruit 21 days
Residue data from supervised trials
Residue data are available from trials on various fruit,
vegetables and field crops: apple; kidney bean, soybean, cauliflower,
kohlrabi, savoy, potatoes; tobacco; the data obtained are summarized
in the following table.
Residues in food moving in commerce
In the Federal Republic of Germany in 1965 and 1968 a total of
228 samples of fruit and vegetables, home produced and imported,
analysed for organophosphorous compounds.
Only in one of these 220 samples azinphos-ethyl was detected, the
residue level being less than 0.1 ppm (Krause, 1969).
Azinphos-ethyl was not found in any of the 378 sub-samples of a
total diet study carried out in England and Wales in 1966-67 (Abbott
et al., 1970).
Fate of residues
In mammals
14C-labelled azinphos-ethyl and azinphos-methyl was administered
orally and intravenously to about 50 male rats (Sprague - Dawley
approx. 170 g). The compounds were applied in a solvent mixture
containing 5% Cremophor EL.
The radioactivity measured related to the sum of the unchanged
parent compound and its metabolites.
After oral as well as after intravenous administration about 65%
(57-68%) of the applied activity was eliminated in the urine within 48
hours, and about 30% (26-34%) in the faeces (see table).
TABLE 1. RESIDUES OF AZINPHOS-ETHYL + P = 0 ANALOGUE IN PPM, TOTAL RESIDUE
[TWO FIGURES ... (...), AZINPHOS-ETHYL AND P = 0 ANALOGUE SEPARATELY]
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Field crops
cottonseed USA 1968 14 0.56 E.C. 12 <0.01 <0.01
10 " <0.01 <0.01
13 " 0.01 0.01
10 " <0.01 0.01
gin trash USA 1968 14 0.56 E.C.12 2.71 1.96
10 " 1.28 0.75
13 " 0.22 0.12
10 " 5.07 3.07
foliage USA 1968 14 0.56 E.C.12 16.45 8.81
10 " 1.20 0.60
13 " 0.35 <0.07
10 " 11.41 11.47
rape seed Germany 1971 1 0.3 w.p. 33% n.d.(74)
Fed.Rep.
Fruit crops
Apple
Cox's and UK 1961 3 1.13 w.p. 25% 0.3 <0.3 <0.3 <0.3 n.d.(47)
Worcester
Cox's UK 1961 2 1.4 w.p. 25% 1.04 0.66 0.43 0.42
Golden
Delicious Belgium 1969 1 1.25 E.C. 50% 0.94 0.44 0.19 0.09 (42)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Vegetables
French Germany 1972 1 0.3 E.C.250g/l 0.6 0.05 n.d. n.d.
beans Fed.Rep. 1972 1 0.3 0.6 0.06 n.d. n.d.
1972 1 0.3 0.12 0.07 n.d. n.d.
soybean USA 1968 3 0.42 E.C. 12 0.55
(0.003)
green plant 1969 3 0.42 " 0.79
(0.003)
whole 1968 3 0.42 " 3.08 0.87
(0.04) (0.03)
1969 3 0.42 " 14.91 4.62 0.75 0.24
(<0.02) (<0.02) (<0.02) (<0.02)
1968 3 0.42 " 18.0 3.6 1.6 0.9
(<0.03) (<0.16) (<0.03) (0.39)
soybean USA 1968 3 0.42 E.C. 12 0.26
vines (dry) (<0.03)
(45 d)
1969 3 " " 0.25
(<0.06)
1968 3 " " 0.14
(<0.02)
(55 d)
1969 3 " " 0.01
(<0.03)
(48 d)
1968 3 " " 0.23
(<0.04)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
beans(dry) 1968 3 0.42 E.C. 12 0.10
(<0.03)
(45 d)
1969 3 " " 0.01
(<0.02)
1968 3 " " 0.01
(<0.01)
(55 d)
1969 3 " " 0.09
(<0.02)
(48 d)
1968 3 " " 0.01
(<0.01)
cauliflower Germany 1967 1 0.5 w.p.33% 2.95 0.15
Fed. Rep.
kohlrabi Germany 1968 1 0.5 w.p. 33% 0.25 0.07 0.04 n.d.
(without Fed.Rep. 1967 1 0.5 " 0.3 0.06 0.01
leaves) 1967 1 0.5 " 0.4 0.03 <0.01
1967 1 0.3 " 0.15 0.12 0.08
savoy Germany 1 0.3 w.p. 33% 0.32 0.27 0.15 0.12 0.14
Fed.Rep. (36 d)
white
cabbage Germany 1967 1 0.3 w.p. 33% 0.18 0.07 0.06 <0.02
Fed. Rep.
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
potatoes USA 1962 8 0.42 E.C. 12 n.d.
1962 8 " " n.d.
1962 8 " 1 " n.d.
1962 8 " " n.d.
1962 8 " " n.d.
Netherlands 1972 1 0.20 w.p.25% n.d.
1972 1 " " n.d.
tomatoes New
Zealand 1968 2 1.25 E.C. 44 1.03a 0.9a 0.78a 0.5la
1968 2 " 1.5a 0.98a 1.28a 0.80a
1968 2 11.25 w.p. 25 1.10a 1.15a 0.66a 0.62a
1968 2 " 1.97a 1.62a 0.94a 0.33a
tobacco
cured USA 1967 3 0.84b E.C. 12 227 119 63 22
1968 3 0.84b " 373 282 150 75
1967 3 0.56 E.C. 12 117 138 27 29
1967 3 " " 79.5 58.6 40.5 7.2
1967 3 " " 170 67 29 19
1967 3 " " 87 103 14 8.7
1967 3 " " 254 119 61 22
1967 3 " " 207 140 68 40
n.d. = not detectable.
a Only residues on the peel; residue in the pulp 0.03-0.09 ppm.
b Overdosage
Route of Dosage Number of % of activity applied
Compound administration mg/kg animals excreted in 48 hours
urine faeces
azinphos-ethyl i.v. 2 5 66 ± 2 26 ± 4
i.v. 0.1 5 59 ± 2 31 ± 4
p.o. 2 4 62 ± 2 30 ± 5
p.o. 0.1 5 57 ± 5 34 ± 6
azinphos-methyl i.v. 2 4 68 ± 3 26 ± 4
i.v. 0.1 5 63 ± 3 29 ± 4
p.o. 2 5 68 ± 4 26 ± 2
About 50% of the dosage administered was excreted in 8-9 hours.
Elimination in the bile
Following intravenous administration of 2 mg/kg azinphos-ethyl or
azinphos to each of three animals an average of approximately 30% of
the applied activity was eliminated in the bile within 24 hours.
Elimination of azinphos in the air
Following oral or intravenous administration of 14C-labelled
azinphos-ethyl less than 0.1% of the applied activity was eliminated
in the expired air within 24 hours. This indicates that the
benzotriazine ring in azinphos-ethyl does not undergo extensive
metabolic degradation.
Forty-eight hours after oral and intravenous administration of 2
mg/kg azinphos-ethyl or azinphos to each of four or five animals 6% of
the applied activity was still present in the animal; approximately 1%
was present in the gastrointestinal tract.
The mean concentration of azinphos-ethyl in the animal tissue
(except the gastrointestinal tract) was at that moment about 0.1 µg/g
tissue irrespective of the compound applied and the route of
administration. Autoradiography and quantitative determination in
single tissues showed that the activity was fairly evenly distributed.
The highest concentrations were in the blood (0.2-0.3 µg/g).
The equivalent concentrations in lung, liver and kidneys were
0.15-0.2 µg/g.
The concentrations measured in other organs were below the values
mentioned above.
Fate of residues in plants
The metabolism of azinphos-ethyl was studied in cotton and bean
plants under field conditions (Olson, 1969).
The residue following application of azinphos-ethyl consisted
partly of the P = 0 metabolite presumably owing to its higher chemical
stability. After 14 days approximately 10% of the residue could be
determined by TLC and GLC as the P = 0 analogue.
Methods of residue analysis
Several colorimetric, thin layer and gas chromatographic methods
for residue analysis of azinphos-ethyl and relevant metabolites are
described in the literature. Only those methods will be referred to
which may be suitable for regulatory purposes or which can be adapted
for these purposes.
Colorimetric methods
Miles (1964) developed a rapid colorimetric method for residue
analysis of azinphos-ethyl, azinphos and their P = 0 metabolites in
plant material.
The method is based on the hydrolysis of the pesticide or
metabolite molecule after separation in a medium of acetic and
hydrochloric acid and the simultaneous coupling of the presumably
formed intermediate benzazimide with N-(1-naphtyl)-ethylene diamine to
produce a violet solution with an absorption maximum at 556 nm.
The plant material is extracted with chloroform and the extract
is carried through a clean-up procedure using Attaclay-celite. The
oxygen analogues are separated in a Florisil column. Azinphos-ethyl
and azinphos-methyl are eluated with chloroform, the P = 0 analogues
with an acetonechloroform mixture.
The recoveries in plant material range from 78 to 97%.
The lower limit of detection is about 5 µg of azinphosethyl and
the P = 0 analogue.
The method, which is specific for the benzotriazinyl group, was
proved to be suitable for residue analysis on apples, tomatoes,
cabbage and tobacco. No interference occurs with milligram quantities
of carbaryl or the following organophosphorous pesticides: demeton,
diazinon, dimethoate, disulfoton, EPN, malathion, parathion, phorate,
schradan and trichlorphon.
A similar colorimetric method for residue analysis of
azinphos-ethyl in apples, potatoes, alfalfa and watermelons was
described by Möllhoff (1969).
The plant samples are treated with acetone; the parent compound
is extracted with chloroform and then chromatographed in isopropanolic
solution for clean-up. The alkaline hydrolysis leads to anthranilic
acid, which is diazotized and coupled with N-(1 naphtyl)-ethylene
diamine.
The P = 0 compound of azinphos-ethyl and azinphos are
co-determined with the method.
Parathion and 4-nitrophenol do not interfere with the
determination.
The limit of detection of the method is about 0.1-0.05 ppm.
The recoveries by the colorimetric method (Meagher et al., 1960)
were found to be similar to those obtained with GLC methods (Wagner,
1973).
COMPARISON OF RECOVERIES OF AZINPHOS-ETHYL BY COLORIMETRIC AND GLC
METHODS OF ANALYSIS
Recovery %
Crop Added ppm
GLC Colorimetry
potato 0.1 86 100
0.2 93 99
citrus 0.1 76 72
0.2 79 82
TLC method
Kirchhof (1970) developed a thin layer chromatographic separation
of azinphos-ethyl, azinphos-methyl and their P = 0 analogues on silica
gel coated plates combined with the colorimetric method of Miles
(1964).
The limit of determination of the method on apples, lettuce,
kohlrabi and onions is 0.1-0.2 µg for the parent compounds and their
P = 0 metabolites alone or in combination.
The method does not require a special clean-up procedure. There
is no interference from other insecticides, fungicides, herbicides or
plant constituents.
GLC method
Residues of azinphos-ethyl, azinphos and their P = 0 analogues
can be determined by gas chromatography with a high degree of
sensitivity and specificity.
Especially GLC methods utilizing a phosphorous-specific detector
e.g. a modified flame ionization detector (thermionic detector) proved
to be particularly suitable for regulatory purposes.
Simultaneous GLC determination of azinphos-ethyl and its P = 0
analogue in soybeans is described by Olson (1969). Azinphos is also
co-determined,
Following extraction of the plant samples (green plants, dry
beans and vines) with chloroform, interfering plant constituents are
precipitated. The resultant extract is directly injected into the
gas-chromatograph.
Under the given conditions azinphos, azinphos-ethyl
and the P = 0 analogue of azinphos-ethyl are identified by their
retention times which are 13.3, 17.3 and 15 minutes respectively.
The limit of determination in this rapid procedure is about 0.005
ppm.
A similar procedure for GLC analysis of azinphos-ethyl and
azinphos in cotton is described by Olson (1969a and b).
Other organophosphorous compounds which may be used in cotton,
such as diazinon, disulfoton and its sulfoxide and sulfone, demeton
and its sulfoxide and sulfone, malathion and malaoxon did not
interfere with the determination of azinphosethyl, azinphos and their
P = 0 metabolites.
A similar GLC method for determination of azinphos-ethyl,
azinphos and their P = 0 metabolites in cigarette smoke is described
by Olson (1969c).
A method for the gas chromatographic determination of residues of
azinphos-ethyl, azinphos-methyl and their P = 0 analogues in milk is
described by Hunt et al. (1970). The extraction procedure corresponds
to that of Adams et al. (1966). The sensitivity of this method for the
determination of azinphos-methyl and the -ethyl analogue in milk
samples is about 0.04 ppm and for the P = 0 analogue of
azinphos-methyl about 0.06 ppm.
New and generally applicable procedures for the extraction of
residues of carbaryl, malathion, phosphamidon, azinphos-methyl and
parathion from plant material are described by Watts (1971). The three
described procedures which consist of sample blending with
acetonitrile or ethyl acetate and an exhaustive Soxhlet extraction
procedure using 10% methanol in chloroform, were compared. They were
all found to give very good recoveries.
National tolerances
Australia fruit 2.0
vegetables 1.0
cereals 1.0
potatoes 0 (at or about limit of detection)
Belgium fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4
potatoes 0.05
Fed. Rep. of Germany fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4 (except root vegetables)
celeriac 0.4
other food
crops 0.05
Italy
azinphos-ethyl and
-methyl together 0.4
Netherlands
azinphos-ethyl and
-methyl together fruit crops 0.4
vegetables 0.4 (except potatoes)
potatoes 0.05
Switzerland
azinphos-ethyl and fruit crops
-methyl together incl.
grapes 0.4
vegetables 0.4
South Africa fruit,
vegetables 2
Appraisal
Azinphos-ethyl is a non-systemic organophosphorus insecticide
with contact action as well as stomach poison action used on a
considerable scale in many countries on a relatively wide range of
crops.
The main uses are foliar applications on field crops, such as
cotton, sugarbeet, fruit and vegetables against a wide range of
insects.
Technical azinphos-ethyl contains a minimum of 92% of the pure
compound. The impurities in the technical material are known.
Azinphos-ethyl is marketed in the form of wettable powders,
emulsifiable liquids and as a ULV formulation.
The concentration/rates of application vary, depending on pest,
crop and method of application; normal application rates are
250-1000 g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. The data
presented for azinphos-ethyl including the P = 0 analogue are, with
few exceptions, representative of those likely to result from good
agricultural practice.
Limited information is available on the fate of residues in
plants. The residues which may occur in food of plant origin,
following recommended directions for use and recommended pre-harvest
intervals consist largely of the parent chemical and to a small extent
of the P = 0 analogue. The residue may contain up to 10% of the P = 0
analogue.
Information considered provided a comparison between the nature
and fate of azinphos-ethyl and its methyl analogue. The only
noticeable difference is the greater persistence of the P = 0 analogue
of the azinphos-ethyl. The P = 0 analogue is not detected at harvest
following the use of azinphos-methyl.
Only limited information is available on the rate of decrease of
the residue of azinphos-ethyl and the P = 0 analogue in crops during
storage and processing, including household cooking. Information was
presented on the extent of carry over of residues in tobacco smoke
following application to tobacco.
Extensive data was available from supervised trials on
cauliflower, kohlrabi, savoy cabbage, white cabbage, french beans. It
was considered that a sufficient range of vegetable crops had been
studied to enable recommendations to be made for vegetables as a
group.
Little information is available on azinphos-ethyl in food in
commerce. A rapid and sensitive colorimetric method is described for
the determination of azinphos-ethyl and the P = 0 analogue together.
The method is specific for the benzotriazinol group and thus
azinphos-ethyl and azinphosmethyl cannot be determined separately.
Gas chromatographic procedures are available for specific
determination of azinphos-ethyl and the P = 0 analogue. Appropriate
extraction and clean-up procedures in food products of plant and
animal origin are available. These methods are suitable for regulatory
purposes.
RECOMMENDATIONS
The following tolerances are recommended for azinphosethyl and
its P = 0 metabolite expressed as azinphos-ethyl.
Commodity Tolerance Pre-harvest intervals on
(ppm) which recommendations are
based (days)
Tomatoes 1 14 (outdoors)
Apples, pears 0.5 21
Vegetables, except
tomatoes and potatoes 0.5 14-21
Soybeans (dry) 0.2 14-21
Potatoes 0.05a 14
Cotton seed, rape seed 0.05a 14
a At or about the limit of determination
N.B. In those cases where a mixture of residues of azinphos-ethyl
and azinphos-methyl occurs together on a commodity, except tomatoes,
the total residue of both compounds including their P = 0 analogues
may not exceed the levels recommended for azinphos-methyl. In case of
tomatoes the total residue should not exceed the level recommended
above for azinphos-ethyl, 1 ppm.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Studies to identify metabolites in plants and animals.
3. Studies to investigate the toxicity of metabolites.
4. Studies to detect effects on reproduction.
5. Studies to detect teratogenic activity.
Desirable
1. Additional information on the nature of terminal residues
in plants, animals and their products.
2. Data on disappearance of residues during storage and
cooking of vegetables and fruits.
REFERENCES
Abbot, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G.
1970 Pesticide residues in the total diet in England
and Wales, 1966-1967. III. Organophosphorous
pesticide residues in the total diet. Pestic. Sci
1: 10-13
Adams, J. M. and Anderson, C. A. Spectrophotofluormetric
1966 method for Guthion residues in milk and animal
tissues. J. Agric. Fd. Chem. 14: 53-55
Anderson, C. A. A Comparison of the Residue Decline Rates
1963 for Guthion and Ethyl Homolog on Alfalfa, Apples
and Lespedezza. Chemagro Report No. 10 394
(unpublished)
Anon. Pharmacokinetic studies with 14C-labelled Gusathion
1973 A and Gusathion M (provisional results)
Unpublished report from Isotopen-Institut,
submitted by Bayer A.G.
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. Acute
1970 toxicity data for pesticides. World Review of Pest
Control, 9: 119-127
Doull, J. and Root, M. The Effect of Diets containing Ethyl
1960 Guthion on Rats. Unpublished report from
University of Chicago
Doull, J., Root, M. and Cowns, J. Determination of the safe
1963 dietary level of Ethyl Guthion for dogs.
Unpublished report from University of Chicago
Dubois, K. P. The Acute Toxicity of Ethyl Guthion in
1963 Combination with Four Other Organic Phosphates.
Unpublished report from University of Chicago
Dubois, K. P. and Raymund, A. B. The Acute Toxicity of Ethyl
1960a Guthion in Combination with Other
Anticholinesterase Agents. Unpublished report from
University of Chicago
Dubois, K. P. and Raymund, A. B. The Subacute Toxicity of
1960b Ethyl Guthion to Rats. Unpublished report from
University of Chicago
Dubois, K. P., Schmalgemeier, D. and Leighton, L. B.
1959 Studies on the Toxicity and Anticholinesterase
Action of Ethyl Guthion. Unpublished report from
University of Chicago
Grasso, P. Results of histological examination of brain,
1969 spinal cord and a peripheral nerve from hens
treated with Ethyl-gusathion for 30 days.
Unpublished report from British Industrial
Research Association, submitted by Bayer A.G.
Hunt, L. M. and Gilbert, B. N. Simultaneous determinations of
1970 azinphos-ethyl, azinphos-methyl and its oxygen
analogue by gas liquid chromatography. Bull. of
Env. Cont. and Tox. 5: 42-46
Kimmerle, G. Active substance R1513 (Ethyl Gusathion;
1959 No. 2078). Unpublished report from Bayer A.G.
Kimmerle, G. Ethyl-Gusathion. Unpublished report from
1960 Bayer A.G.
Kimmerle, G. Gusathion A/Antidotwirkung und Potentzierung.
1966a Unpublished report from Bayer A.G.
Kimmerle, G. Gusathion A-Wirkstoff (R 1513; Ht. - Nr. 3651).
1966b Unpublished report from Bayer A.G.
Kimmerle, G. Äthyl-Gusation/Subchronische Neurotoxizitäts
1968 versuche bei Hühnern. Unpublished report from
Bayer A.G.
Kimmerle, G. and Lorke, D. Toxicology of insecticidal
1968 organophosphates. Pflanzenschutz-Nachrichten,
Bayer, 21: 111-142
Kirchhoff, J. Spezifischer dünnschichtchromatographischer
1970 Nachweis von Gusathion-Rückständen in Pflanzen
extrakten. Pflanzenschutznachrichten, Bayer, 23:
365-366
Krause, Ch. und Kirchhoff, J. Organophosphatrückstände auf
1969 Marktproben von Obst and Gemüse sowie auf
Getreideerzeugnissen. Deutsch. Pflanzenschutzd.
(Braunschweig), 21, 1969: 81-84
Lorke, D. and Kimmerle, G. The Action of Reactivators in
1969 Phosphoric - Acid-Ester Poisoning. Naunyn
Schmiedebergs Arch. Pharmak. exp. Path. 263: 237
Löser, E. BAY 16 259/Subchronische toxikologische
1969 Untersuchungen an Ratten. Unpublished report from
Bayer A.G.
Mawdesley-Thomas, L. E. and Urwin, C. Pathology Report of
1969 BAY 16 259. Sub-chronic Toxicity Study in Rats
Administered over Three Months. Unpublished report
from Huntingdon Research Centre, submitted by
Bayer A.G.
Meagher, W. R., Adams. J. M., Anderson, C. A. and Macdougall, D.
1960 Colorimetric determination of Guthion residues in
crops. J. Agric. Fd. Chem., 8: 282-286
Miles, J. R. W. A new colorimetric method for determination
1964 of residues of Guthion and Ethyl Guthion and their
oxygen analogs. J.A.O.A.C. 47: 882-885
Möllhoff, E. Kolorimetrische Bestimmung von Azinphos-äthyl. 1969
Rückstands analytik von Pflanzenschutmitteln.
(DFG), Verlag Chemie GmbH, Weinheim/Bergstr.
Olson, T. J. Investigation of the possible metabolism of
1969 Ethyl-Guthion on crops. Chemagro Report No. 25
151 (unpublished)
Olson, T. J. Determination of residues of Guthion M-E in
1969a soybeans by thermionic emission gas
chromatography. Chemagro Report No. 25 152
(unpublished)
Olson, T. J. A confirmatory gas chromatographic procedure for
1969b the Guthion M-E residue method. Chemagro Report
No. 25 911 (unpublished)
Olson, T. J. A study of the possible interferences or other
1969c pesticides with the analytical method for Guthion
M-E in cotton. Chemagro Report No. 25 912
(unpublished)
Olson, T. J. Determination of Guthion M-E residues in
1969d cigarette smoke. Chemagro Report No. 25 121
(unpublished)
Olthoff, P. D. A. and de Mey, R. C. H. Residues of azinphos
1973 ethyl on potatoes. Report No. 3954 Central
Institute for Nutrition and Food Research,
Netherlands
Schumann, S. A. and Anderson, R. J. Determination of Guthion
1965 M-E residues in clover by electron capture gas
chromatography. Chemagro Report No. 16 571
(unpublished)
Sherman, M., Herrick, R. B., Ross, E. and Chang, M. T. Y.
1967 Further Studies of the acute and subacute toxicity
of Insecticides to Chicks, Toxicol. and Appl.
Pharmacol. 11: 49-67
Wagner, Kl. Report of Bayer A.G., Leverkusen (Pflanzenschutz
1973 AT/Biologische Forschung, Institut für
Rückstandsanalytik) (unpublished)
Watts, R. R. Extraction efficiency study - Examination of three
procedures for extracting 14C-labeled and
unlabeled residues of organophosphorus pesticides
and carbaryl from bean leaves and kale. J.A.O.A.C.
54: 953-958
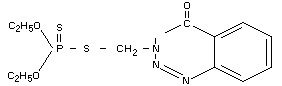 empirical formula: C12H16N3O3PS2
Other information on identity and properties
(a) Composition of technical azinphos-ethyl
The technical material contains a minimum of 92% azinphos-ethyl.
Impurities
triethyl thiophosphate and
triethyldithiophosphate together max. 2.0%
bis(3,4-dihydro-4-oxo-1,2,3-triazin-3-ylmethyl)-sulfide together
and -disulfide max. 1.5%
3-chloromethyl-3,4-dihydro-4-oxo-1,2,3
benzotriazin and (3,4-dihydro-4-oxo-1,2,3
benzotriazin-3 ylmethyl)-methylsulfide together max. 1.5%
ethylene-bis (O,O-diethyl-dithiophosphate) max. 1.9%
0,S-diethyl-S-(3,4-dihydro-4-oxo-1,2,3-
benzotriazin-3-yl(-methyl)-dithiophosphate max. 0.7%
bis (3,3-dihydro-4-oxo-benzotriazin-3-yl-methyl)-ether max. 0.6%
0-ethyl-S-(3,4, dihydro-4-oxo-1,2,3
benzotriazin 3-yl-methyl)-dithio-phosphoric
acid max. 0.5%
water max. 0.2%
(b) Physical and chemical properties of azinphos-ethyl
Physical state: colourless crystals.
Molecular weight: 345.4.
Melting point: 53°C (pure material).
Boiling point: 147°C at 0.01 mm Hg
111°C at 0.001 mm Hg
Volatility: 0.0012 mg/cu metre at 10°C
0.0042 mg/cu metre at 20°C
0.0128 mg/cu metre at 30°C
0.0372 mg/cu metre at 40°C
Vapour pressure: 6.3 x 10-8 mm Hg at 10°C
2.2 x 10-7 mm Hg at 20°C
7 x 10-7 mm Hg at 30°C
2.1 x 10-6 mm Hg at 40°C
Specific gravity: D 20 = 1.284
4
Refractive index: N 53 = 1.592
D
Solubility: practically insoluble in water,
soluble in most organic solvents
Formulations used: emulsifiable liquid 25% w/v, 40% w/v
wettable powder 25%, 33%, 40%
ULV formulation 500 g/l
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Following oral and I.V. administration of 14C-labelled
azinphos-ethyl to male rats in doses of 0.1 or 2 mg/kg bw, about 60%
of the activity was excreted in urine and 30% in faeces in 48 hours.
Approximately 30% of a 2 mg/kg dose was eliminated in bile within 24
hours; less than 0.1% was eliminated in expired air, indicating that
the benzotriazine ring does not undergo extensive degradation.
Approximately 6% of activity was present in the body 48 hours after
administration, the mean concentration in tissues being equivalent to
0.1 ppm. The highest equivalent concentration (0.2-0.3 ppm
(0.00002%-0.00003%)) was found in blood and 0.15-0.2 ppm
(0.000015%-0.00002%) was found in lungs, liver and kidneys. The
results of an exactly similar study using azinphos-methyl showed no
differences, indicating that mammals metabolize both compounds in a
similar manner (Anon., 1973).
Effects on enzymes and other biochemical parameters
Brain cholinesterase activity in vitro was reduced by 50% by
1.4 x 10-5 molar azinphos-ethyl; this was considered to be due to the
presence of impurities with anticholinesterase activity. A single I.P.
dose of 3.4 mg azinphos-ethyl/kg reduced serum, brain and submaxillary
gland cholinesterase activity to approximately the same extent.
Maximum inhibition occurred within an hour and activity returned to
normal gradually, reversal not being complete by 70 hours. On
incubation with liver preparation, azinphos-ethyl was transformed into
a potent anticholinesterase compound, probably the oxygen analogue
(Dubois et al., 1959).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of three hens received a single dose of 10 or 25 mg
azinphos-ethyl/kg and were observed for five weeks. One bird on the 25
mg/kg level died after 26 days but no signs of demyelinating disease
developed (Kimmerle, 1960).
Groups of four hens were fed on diet containing 0, 75, 150, 300
and 600 ppm (0, 0.0075, 0.015, 0.03 and 0.06%) azinphos-ethyl for 30
days. Loss of body weight occurred at the two highest dosage levels
and a significant depression of whole blood cholinesterase activity at
the 600 ppm (0.06%) level. No signs of neurological damage occurred
during the dosage period or during the subsequent four-week
observation period. Histological examination of femoral fixed brain,
spinal cord and femoral nerve failed to detect evidence of
demyelination or nerve damage (Kimmerle, 1968; Grasso, 1969).
Special studies on potentiation and antidotes
No significant potentiation of acute toxicity occurred with
azinphos-ethyl in combination with parathion, methyl parathion,
systox, EPN, malathion, trithion, phosdrin, sevin, delnav, OMPA,
diazinon, azinphos, di-syston, Co-Ral, Folex or Ronnel. A twofold
potentiation occurred with azinphos-ethyl and ethion (Dubois and
Raymond, 1960a; Dubois, 1963; Kimmerle, 1966a).
The effects of azinphos-ethyl are counteracted by atropine and by
reactivating compounds. The two drugs given in combination give more
pronounced effects (Kimmerle, 1966; Lorke and Kimmerle, 1968; Dubois
et al., 1959).
Acute toxicity
LD50
Species Route Sex mg/kg bw References
Mouse I.P. 3.8-4.0 Dubois et al., 1959
Rat Oral 12.0-20.5 Ben-Dyke et al., 1970
Kimmerle, 1959,
1966 a & b
F 7.0 Dubois et al., 1959
M 15.2
Rat I.P. F 4.4 Dubois et al., 1959
M 9.2
M 7.5 Kimmerle, 1959
Rat Dermal 75-280 Ben-Dyke et al., 1970
Dubois et al., 1959
Kimmerle, 1959
Guinea-pig Oral 17 Dubois et al., 1959
Guinea-pig I.P. 8.0 Dubois et al., 1959
Chicks Oral 34 Sherman et al., 1967
F = Female; M = Male.
Short-term studies
Rat. Twenty-four male rats received an oral dose of 1 mg
azinphos-ethyl/kg bw on each of 28 consecutive working days. The rate
of weight gain was normal and signs of poisoning did not occur.
Erythrocyte cholinesterase showed 50% depression after 2, 82% after 3
and 90% after 28 days' treatment. Activity had returned to normal 35
days after treatment stopped (Kimmerle, 1959).
Groups of five female rats received daily I.P. injections of
azinphos-ethyl at dosage levels of 0.5, 1, 2 and 3 mg/kg bw for 60
days. Only the two highest dosage levels caused a reduction in the
rate of weight gain and an increased mortality (Dubois and Raymond,
1960b).
Groups of 15 male and 15 female rats received diet containing 0,
1, 2, 4 and 8 ppm (0, 0.0001, 0.0002, 0.0004 and 0.0008%) for three
months. No effects were found on growth rate, food consumption,
mortality rate or on the results of haematological investigations and
analysis of blood. The 4 ppm (0.0004%) level had no significant effect
on plasma enzyme but depressed the erythrocyte cholinesterase activity
after one month. The 8 ppm (0.0008%) level depressed plasma enzyme
after only one week's exposure and prolonged exposure caused no
further depression; erythrocyte enzyme activity continued to fall
during the first month's exposure. In general, females appeared to be
more sensitive than males. Autopsy, determination of organ weights and
histological examination of organs and tissues detected no abnormality
in animals fed azinphos-ethyl (Löser, 1969; Mawdesley-Thomas and
Urwin, 1969).
Groups of 12 male and 12 female rats were fed on diets containing
0, 5, 10 and 50 ppm (0, 0.0005, 0.001 and 0.005%) azinphos-ethyl for
16 weeks. The growth rate of female animals was unaffected while male
rats receiving 50 ppm (0.005%) showed a slightly reduced rate of
growth compared with controls. No animals showed signs of
cholinesterase inhibition but determination of the activity of the
enzyme in serum, erythrocytes, submaxillary and brain tissue of five
male and five female rats of each group showed that it was markedly
inhibited in the 50 ppm (0.005%) group. Inhibition was most marked in
erythrocytes (87%) and serum (85%) and less marked In brain (24% in
males and 72% in females) and gland tissue (16% in males and 59% in
females). Serum and erythrocyte activity was depressed (52% and 80%
respectively) in rats fed 10 ppm (0.001%) azinphos-ethyl in their
diet. Only erythrocyte enzyme was significantly inhibited in rats on 5
ppm (0.0005%) diet; males and females did not differ in this respect.
The remaining seven animals of each sex of each group were autopsied
and the organs weighed and examined histologically but the results of
this examination are not reported (Doull and Root, 1960).
Dog. Groups of two male and two female dogs aged between 6 and 14
months were fed for 12 weeks on diets containing 0.25, 0.5, 1, 2, 3
and 10 ppm, (0.000025, 0.0005, 0.0001, 0.0002, 0.0003 and 0.001%)
azinphos-ethyl. The serum and erythrocyte cholinesterase activity was
determined periodically before, during and following the dosage
period. Dogs receiving 3 and 10 ppm (0.0003 and 0.001%) diets
exhibited signs of cholinesterase activity depression and were taken
off the diets after 1 and 6 weeks respectively. Over 50% inhibition
occurred in the serum and erythrocytes of dogs fed on 2 ppm (0.0002%)
diet. Erythrocyte enzyme activity was not inhibited in dogs receiving
the 0.25% diet but the serum enzyme was inhibited by about 20%. Since
the serum cholinesterase activity in control animals was inhibited by
10% the no-effect level was considered to be approximately 0.25 ppm
(0.000025%). In the animals affected, activity was reduced during the
first week of the test and then remained constant while the
erythrocyte enzyme tended to decrease gradually throughout the 12-week
test period. Cholinesterase activity returned to normal in 3-4 weeks
when dogs on the highest dosage levels were transferred to control
diet and in 2-3 weeks with the 0.5 and 1 ppm (0.00005 and 0.0001%)
groups (Doull et al., 1963).
Long-term studies
No data available.
Comments
Azinphos-ethyl is absorbed from the gastrointestinal tract and
excreted in urine and bile. It does not accumulate in tissues. The
absorption, distribution and excretion of radiolabel by rats are
similar in animals administered 14C-labelled azinphos-ethyl or
-methyl. However, insufficient data are available to determine the
metabolic pathway of azinphos-ethyl and to compare it with that of
azinphos-methyl, although there is evidence that in both the
benzotriazine moiety remains intact.
Azinphos-ethyl inhibits acetylcholinesterase activity in plasma,
erythrocyte, brain and submaxillary gland and a series of low doses
causes the plasma activity of rats and dogs to fall rapidly to a
stable level while erythrocyte acetylcholinesterase tends to fall more
gradually over a longer period. Female rats were more sensitive than
males as shown by the degree of enzyme inhibition and, in some tests,
by LD50 values. Recovery of enzyme activity took several weeks
following cessation of exposure of rats.
Short-term studies in rats show the no-effect level to be 2 ppm
(0.0002%) in the diet. The only toxic effects seen were attributed to
depression of cholinesterase activity. In an investigation using dogs,
in which only plasma and erythrocyte cholinesterase activity were
recorded, the no-effect level was 0.25 ppm (0.000025%) in the diet.
The Meeting was unable to estimate an acceptable daily intake for
this substance in the absence of sufficient information on the
identity and toxicity of its metabolites, on its possible effects on
reproduction, on its long-term toxicity and on its carcinogenic and
teratogenic and mutagenic potential.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-ethyl is a non-systemic organophosphorus insecticide,
which is used against a relatively broad spectrum of insects e.g.
Lepidopterous larvae, beetles and their larvae, aphids, jassids and
spider mites on various crops: cotton, rice, sugar and fodder beets;
fruits such as apples, pears, citrus-fruit, grapes; tobacco.
Azinphos-ethyl is used in about 35 countries in Europe, Africa,
Asia, Australia, New Zealand, North, Central and South America.
The amounts used on different crops vary greatly from year to
year depending on the pest situation. A rough estimate may be given:
cotton and other agricultural crops 60%
fruit crops (including grapes) 30%
vegetables (including potatoes) 10%
Pre-harvest applications
Azinphos-ethyl is generally used at dosages of 0.04-0.05% active
ingredient. The officially registered and/or recommended uses of
azinphos-ethyl are summarized below, with the application rates and
pre-harvest intervals.
Crop Dosage rate Minimum pre-harvest
g a.i./ha interval - days
Agricultural crops
cotton 250-1 000 5
rice 300-500 14
tobacco 300-600 14
sugar- and fodder beets 300-500 14
Fruit crops
apples, pears, peaches,
plums and cherries 800-1 000 14-21
citrus fruit 800-1 000 14-21
grapes 800-1 000 14-21
Vegetables
brassicas 300-500 7-14
melons 300-500 7-14
tomatoes 300-500 7-14
potatoes 300- 14-
Hops 400-800 14
Pre-harvest intervals officially recommended in different
countries, days
Australia general 21 days
Bulgaria sugar beets 14 days
Fed. Rep. of field crops as:
Germany cereals, rape, alfalfa,
sugar and fodder beets
potatoes 14 days
beans 21 days
cucumbers, tomatoes 14 days
United Kingdom apples, pears 21 days
cucumbers and tomatoes
(glasshouse cultures) 2 days
France general 15 days
Italy general 20 days
Morocco olives 30 days
other crops 15 days
New Zealand root vegetables 14 days
smooth-skinned fruit 14 days
other fruit and
vegetables 21 days
Netherlands potatoes 28 days
Portugal tomatoes for industrial
processing 5 days
all other crops 21 days
Switzerland fruit, grapes 21 days
potatoes 21 days
Spain cotton 21 days (2%
dust 15 days)
sugar beets 21 days
hazelnuts 21 days
South Africa potatoes 21 days
Pre-harvest intervals officially recommended in different
countries, days
South Africa cotton 5 days (not
(Cont'd.) to be used for
feeding purposes
until after 35 days)
Yugoslavia fruit 21 days
Residue data from supervised trials
Residue data are available from trials on various fruit,
vegetables and field crops: apple; kidney bean, soybean, cauliflower,
kohlrabi, savoy, potatoes; tobacco; the data obtained are summarized
in the following table.
Residues in food moving in commerce
In the Federal Republic of Germany in 1965 and 1968 a total of
228 samples of fruit and vegetables, home produced and imported,
analysed for organophosphorous compounds.
Only in one of these 220 samples azinphos-ethyl was detected, the
residue level being less than 0.1 ppm (Krause, 1969).
Azinphos-ethyl was not found in any of the 378 sub-samples of a
total diet study carried out in England and Wales in 1966-67 (Abbott
et al., 1970).
Fate of residues
In mammals
14C-labelled azinphos-ethyl and azinphos-methyl was administered
orally and intravenously to about 50 male rats (Sprague - Dawley
approx. 170 g). The compounds were applied in a solvent mixture
containing 5% Cremophor EL.
The radioactivity measured related to the sum of the unchanged
parent compound and its metabolites.
After oral as well as after intravenous administration about 65%
(57-68%) of the applied activity was eliminated in the urine within 48
hours, and about 30% (26-34%) in the faeces (see table).
TABLE 1. RESIDUES OF AZINPHOS-ETHYL + P = 0 ANALOGUE IN PPM, TOTAL RESIDUE
[TWO FIGURES ... (...), AZINPHOS-ETHYL AND P = 0 ANALOGUE SEPARATELY]
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Field crops
cottonseed USA 1968 14 0.56 E.C. 12 <0.01 <0.01
10 " <0.01 <0.01
13 " 0.01 0.01
10 " <0.01 0.01
gin trash USA 1968 14 0.56 E.C.12 2.71 1.96
10 " 1.28 0.75
13 " 0.22 0.12
10 " 5.07 3.07
foliage USA 1968 14 0.56 E.C.12 16.45 8.81
10 " 1.20 0.60
13 " 0.35 <0.07
10 " 11.41 11.47
rape seed Germany 1971 1 0.3 w.p. 33% n.d.(74)
Fed.Rep.
Fruit crops
Apple
Cox's and UK 1961 3 1.13 w.p. 25% 0.3 <0.3 <0.3 <0.3 n.d.(47)
Worcester
Cox's UK 1961 2 1.4 w.p. 25% 1.04 0.66 0.43 0.42
Golden
Delicious Belgium 1969 1 1.25 E.C. 50% 0.94 0.44 0.19 0.09 (42)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Vegetables
French Germany 1972 1 0.3 E.C.250g/l 0.6 0.05 n.d. n.d.
beans Fed.Rep. 1972 1 0.3 0.6 0.06 n.d. n.d.
1972 1 0.3 0.12 0.07 n.d. n.d.
soybean USA 1968 3 0.42 E.C. 12 0.55
(0.003)
green plant 1969 3 0.42 " 0.79
(0.003)
whole 1968 3 0.42 " 3.08 0.87
(0.04) (0.03)
1969 3 0.42 " 14.91 4.62 0.75 0.24
(<0.02) (<0.02) (<0.02) (<0.02)
1968 3 0.42 " 18.0 3.6 1.6 0.9
(<0.03) (<0.16) (<0.03) (0.39)
soybean USA 1968 3 0.42 E.C. 12 0.26
vines (dry) (<0.03)
(45 d)
1969 3 " " 0.25
(<0.06)
1968 3 " " 0.14
(<0.02)
(55 d)
1969 3 " " 0.01
(<0.03)
(48 d)
1968 3 " " 0.23
(<0.04)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
beans(dry) 1968 3 0.42 E.C. 12 0.10
(<0.03)
(45 d)
1969 3 " " 0.01
(<0.02)
1968 3 " " 0.01
(<0.01)
(55 d)
1969 3 " " 0.09
(<0.02)
(48 d)
1968 3 " " 0.01
(<0.01)
cauliflower Germany 1967 1 0.5 w.p.33% 2.95 0.15
Fed. Rep.
kohlrabi Germany 1968 1 0.5 w.p. 33% 0.25 0.07 0.04 n.d.
(without Fed.Rep. 1967 1 0.5 " 0.3 0.06 0.01
leaves) 1967 1 0.5 " 0.4 0.03 <0.01
1967 1 0.3 " 0.15 0.12 0.08
savoy Germany 1 0.3 w.p. 33% 0.32 0.27 0.15 0.12 0.14
Fed.Rep. (36 d)
white
cabbage Germany 1967 1 0.3 w.p. 33% 0.18 0.07 0.06 <0.02
Fed. Rep.
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
potatoes USA 1962 8 0.42 E.C. 12 n.d.
1962 8 " " n.d.
1962 8 " 1 " n.d.
1962 8 " " n.d.
1962 8 " " n.d.
Netherlands 1972 1 0.20 w.p.25% n.d.
1972 1 " " n.d.
tomatoes New
Zealand 1968 2 1.25 E.C. 44 1.03a 0.9a 0.78a 0.5la
1968 2 " 1.5a 0.98a 1.28a 0.80a
1968 2 11.25 w.p. 25 1.10a 1.15a 0.66a 0.62a
1968 2 " 1.97a 1.62a 0.94a 0.33a
tobacco
cured USA 1967 3 0.84b E.C. 12 227 119 63 22
1968 3 0.84b " 373 282 150 75
1967 3 0.56 E.C. 12 117 138 27 29
1967 3 " " 79.5 58.6 40.5 7.2
1967 3 " " 170 67 29 19
1967 3 " " 87 103 14 8.7
1967 3 " " 254 119 61 22
1967 3 " " 207 140 68 40
n.d. = not detectable.
a Only residues on the peel; residue in the pulp 0.03-0.09 ppm.
b Overdosage
Route of Dosage Number of % of activity applied
Compound administration mg/kg animals excreted in 48 hours
urine faeces
azinphos-ethyl i.v. 2 5 66 ± 2 26 ± 4
i.v. 0.1 5 59 ± 2 31 ± 4
p.o. 2 4 62 ± 2 30 ± 5
p.o. 0.1 5 57 ± 5 34 ± 6
azinphos-methyl i.v. 2 4 68 ± 3 26 ± 4
i.v. 0.1 5 63 ± 3 29 ± 4
p.o. 2 5 68 ± 4 26 ± 2
About 50% of the dosage administered was excreted in 8-9 hours.
Elimination in the bile
Following intravenous administration of 2 mg/kg azinphos-ethyl or
azinphos to each of three animals an average of approximately 30% of
the applied activity was eliminated in the bile within 24 hours.
Elimination of azinphos in the air
Following oral or intravenous administration of 14C-labelled
azinphos-ethyl less than 0.1% of the applied activity was eliminated
in the expired air within 24 hours. This indicates that the
benzotriazine ring in azinphos-ethyl does not undergo extensive
metabolic degradation.
Forty-eight hours after oral and intravenous administration of 2
mg/kg azinphos-ethyl or azinphos to each of four or five animals 6% of
the applied activity was still present in the animal; approximately 1%
was present in the gastrointestinal tract.
The mean concentration of azinphos-ethyl in the animal tissue
(except the gastrointestinal tract) was at that moment about 0.1 µg/g
tissue irrespective of the compound applied and the route of
administration. Autoradiography and quantitative determination in
single tissues showed that the activity was fairly evenly distributed.
The highest concentrations were in the blood (0.2-0.3 µg/g).
The equivalent concentrations in lung, liver and kidneys were
0.15-0.2 µg/g.
The concentrations measured in other organs were below the values
mentioned above.
Fate of residues in plants
The metabolism of azinphos-ethyl was studied in cotton and bean
plants under field conditions (Olson, 1969).
The residue following application of azinphos-ethyl consisted
partly of the P = 0 metabolite presumably owing to its higher chemical
stability. After 14 days approximately 10% of the residue could be
determined by TLC and GLC as the P = 0 analogue.
Methods of residue analysis
Several colorimetric, thin layer and gas chromatographic methods
for residue analysis of azinphos-ethyl and relevant metabolites are
described in the literature. Only those methods will be referred to
which may be suitable for regulatory purposes or which can be adapted
for these purposes.
Colorimetric methods
Miles (1964) developed a rapid colorimetric method for residue
analysis of azinphos-ethyl, azinphos and their P = 0 metabolites in
plant material.
The method is based on the hydrolysis of the pesticide or
metabolite molecule after separation in a medium of acetic and
hydrochloric acid and the simultaneous coupling of the presumably
formed intermediate benzazimide with N-(1-naphtyl)-ethylene diamine to
produce a violet solution with an absorption maximum at 556 nm.
The plant material is extracted with chloroform and the extract
is carried through a clean-up procedure using Attaclay-celite. The
oxygen analogues are separated in a Florisil column. Azinphos-ethyl
and azinphos-methyl are eluated with chloroform, the P = 0 analogues
with an acetonechloroform mixture.
The recoveries in plant material range from 78 to 97%.
The lower limit of detection is about 5 µg of azinphosethyl and
the P = 0 analogue.
The method, which is specific for the benzotriazinyl group, was
proved to be suitable for residue analysis on apples, tomatoes,
cabbage and tobacco. No interference occurs with milligram quantities
of carbaryl or the following organophosphorous pesticides: demeton,
diazinon, dimethoate, disulfoton, EPN, malathion, parathion, phorate,
schradan and trichlorphon.
A similar colorimetric method for residue analysis of
azinphos-ethyl in apples, potatoes, alfalfa and watermelons was
described by Möllhoff (1969).
The plant samples are treated with acetone; the parent compound
is extracted with chloroform and then chromatographed in isopropanolic
solution for clean-up. The alkaline hydrolysis leads to anthranilic
acid, which is diazotized and coupled with N-(1 naphtyl)-ethylene
diamine.
The P = 0 compound of azinphos-ethyl and azinphos are
co-determined with the method.
Parathion and 4-nitrophenol do not interfere with the
determination.
The limit of detection of the method is about 0.1-0.05 ppm.
The recoveries by the colorimetric method (Meagher et al., 1960)
were found to be similar to those obtained with GLC methods (Wagner,
1973).
COMPARISON OF RECOVERIES OF AZINPHOS-ETHYL BY COLORIMETRIC AND GLC
METHODS OF ANALYSIS
Recovery %
Crop Added ppm
GLC Colorimetry
potato 0.1 86 100
0.2 93 99
citrus 0.1 76 72
0.2 79 82
TLC method
Kirchhof (1970) developed a thin layer chromatographic separation
of azinphos-ethyl, azinphos-methyl and their P = 0 analogues on silica
gel coated plates combined with the colorimetric method of Miles
(1964).
The limit of determination of the method on apples, lettuce,
kohlrabi and onions is 0.1-0.2 µg for the parent compounds and their
P = 0 metabolites alone or in combination.
The method does not require a special clean-up procedure. There
is no interference from other insecticides, fungicides, herbicides or
plant constituents.
GLC method
Residues of azinphos-ethyl, azinphos and their P = 0 analogues
can be determined by gas chromatography with a high degree of
sensitivity and specificity.
Especially GLC methods utilizing a phosphorous-specific detector
e.g. a modified flame ionization detector (thermionic detector) proved
to be particularly suitable for regulatory purposes.
Simultaneous GLC determination of azinphos-ethyl and its P = 0
analogue in soybeans is described by Olson (1969). Azinphos is also
co-determined,
Following extraction of the plant samples (green plants, dry
beans and vines) with chloroform, interfering plant constituents are
precipitated. The resultant extract is directly injected into the
gas-chromatograph.
Under the given conditions azinphos, azinphos-ethyl
and the P = 0 analogue of azinphos-ethyl are identified by their
retention times which are 13.3, 17.3 and 15 minutes respectively.
The limit of determination in this rapid procedure is about 0.005
ppm.
A similar procedure for GLC analysis of azinphos-ethyl and
azinphos in cotton is described by Olson (1969a and b).
Other organophosphorous compounds which may be used in cotton,
such as diazinon, disulfoton and its sulfoxide and sulfone, demeton
and its sulfoxide and sulfone, malathion and malaoxon did not
interfere with the determination of azinphosethyl, azinphos and their
P = 0 metabolites.
A similar GLC method for determination of azinphos-ethyl,
azinphos and their P = 0 metabolites in cigarette smoke is described
by Olson (1969c).
A method for the gas chromatographic determination of residues of
azinphos-ethyl, azinphos-methyl and their P = 0 analogues in milk is
described by Hunt et al. (1970). The extraction procedure corresponds
to that of Adams et al. (1966). The sensitivity of this method for the
determination of azinphos-methyl and the -ethyl analogue in milk
samples is about 0.04 ppm and for the P = 0 analogue of
azinphos-methyl about 0.06 ppm.
New and generally applicable procedures for the extraction of
residues of carbaryl, malathion, phosphamidon, azinphos-methyl and
parathion from plant material are described by Watts (1971). The three
described procedures which consist of sample blending with
acetonitrile or ethyl acetate and an exhaustive Soxhlet extraction
procedure using 10% methanol in chloroform, were compared. They were
all found to give very good recoveries.
National tolerances
Australia fruit 2.0
vegetables 1.0
cereals 1.0
potatoes 0 (at or about limit of detection)
Belgium fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4
potatoes 0.05
Fed. Rep. of Germany fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4 (except root vegetables)
celeriac 0.4
other food
crops 0.05
Italy
azinphos-ethyl and
-methyl together 0.4
Netherlands
azinphos-ethyl and
-methyl together fruit crops 0.4
vegetables 0.4 (except potatoes)
potatoes 0.05
Switzerland
azinphos-ethyl and fruit crops
-methyl together incl.
grapes 0.4
vegetables 0.4
South Africa fruit,
vegetables 2
Appraisal
Azinphos-ethyl is a non-systemic organophosphorus insecticide
with contact action as well as stomach poison action used on a
considerable scale in many countries on a relatively wide range of
crops.
The main uses are foliar applications on field crops, such as
cotton, sugarbeet, fruit and vegetables against a wide range of
insects.
Technical azinphos-ethyl contains a minimum of 92% of the pure
compound. The impurities in the technical material are known.
Azinphos-ethyl is marketed in the form of wettable powders,
emulsifiable liquids and as a ULV formulation.
The concentration/rates of application vary, depending on pest,
crop and method of application; normal application rates are
250-1000 g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. The data
presented for azinphos-ethyl including the P = 0 analogue are, with
few exceptions, representative of those likely to result from good
agricultural practice.
Limited information is available on the fate of residues in
plants. The residues which may occur in food of plant origin,
following recommended directions for use and recommended pre-harvest
intervals consist largely of the parent chemical and to a small extent
of the P = 0 analogue. The residue may contain up to 10% of the P = 0
analogue.
Information considered provided a comparison between the nature
and fate of azinphos-ethyl and its methyl analogue. The only
noticeable difference is the greater persistence of the P = 0 analogue
of the azinphos-ethyl. The P = 0 analogue is not detected at harvest
following the use of azinphos-methyl.
Only limited information is available on the rate of decrease of
the residue of azinphos-ethyl and the P = 0 analogue in crops during
storage and processing, including household cooking. Information was
presented on the extent of carry over of residues in tobacco smoke
following application to tobacco.
Extensive data was available from supervised trials on
cauliflower, kohlrabi, savoy cabbage, white cabbage, french beans. It
was considered that a sufficient range of vegetable crops had been
studied to enable recommendations to be made for vegetables as a
group.
Little information is available on azinphos-ethyl in food in
commerce. A rapid and sensitive colorimetric method is described for
the determination of azinphos-ethyl and the P = 0 analogue together.
The method is specific for the benzotriazinol group and thus
azinphos-ethyl and azinphosmethyl cannot be determined separately.
Gas chromatographic procedures are available for specific
determination of azinphos-ethyl and the P = 0 analogue. Appropriate
extraction and clean-up procedures in food products of plant and
animal origin are available. These methods are suitable for regulatory
purposes.
RECOMMENDATIONS
The following tolerances are recommended for azinphosethyl and
its P = 0 metabolite expressed as azinphos-ethyl.
Commodity Tolerance Pre-harvest intervals on
(ppm) which recommendations are
based (days)
Tomatoes 1 14 (outdoors)
Apples, pears 0.5 21
Vegetables, except
tomatoes and potatoes 0.5 14-21
Soybeans (dry) 0.2 14-21
Potatoes 0.05a 14
Cotton seed, rape seed 0.05a 14
a At or about the limit of determination
N.B. In those cases where a mixture of residues of azinphos-ethyl
and azinphos-methyl occurs together on a commodity, except tomatoes,
the total residue of both compounds including their P = 0 analogues
may not exceed the levels recommended for azinphos-methyl. In case of
tomatoes the total residue should not exceed the level recommended
above for azinphos-ethyl, 1 ppm.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Studies to identify metabolites in plants and animals.
3. Studies to investigate the toxicity of metabolites.
4. Studies to detect effects on reproduction.
5. Studies to detect teratogenic activity.
Desirable
1. Additional information on the nature of terminal residues
in plants, animals and their products.
2. Data on disappearance of residues during storage and
cooking of vegetables and fruits.
REFERENCES
Abbot, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G.
1970 Pesticide residues in the total diet in England
and Wales, 1966-1967. III. Organophosphorous
pesticide residues in the total diet. Pestic. Sci
1: 10-13
Adams, J. M. and Anderson, C. A. Spectrophotofluormetric
1966 method for Guthion residues in milk and animal
tissues. J. Agric. Fd. Chem. 14: 53-55
Anderson, C. A. A Comparison of the Residue Decline Rates
1963 for Guthion and Ethyl Homolog on Alfalfa, Apples
and Lespedezza. Chemagro Report No. 10 394
(unpublished)
Anon. Pharmacokinetic studies with 14C-labelled Gusathion
1973 A and Gusathion M (provisional results)
Unpublished report from Isotopen-Institut,
submitted by Bayer A.G.
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. Acute
1970 toxicity data for pesticides. World Review of Pest
Control, 9: 119-127
Doull, J. and Root, M. The Effect of Diets containing Ethyl
1960 Guthion on Rats. Unpublished report from
University of Chicago
Doull, J., Root, M. and Cowns, J. Determination of the safe
1963 dietary level of Ethyl Guthion for dogs.
Unpublished report from University of Chicago
Dubois, K. P. The Acute Toxicity of Ethyl Guthion in
1963 Combination with Four Other Organic Phosphates.
Unpublished report from University of Chicago
Dubois, K. P. and Raymund, A. B. The Acute Toxicity of Ethyl
1960a Guthion in Combination with Other
Anticholinesterase Agents. Unpublished report from
University of Chicago
Dubois, K. P. and Raymund, A. B. The Subacute Toxicity of
1960b Ethyl Guthion to Rats. Unpublished report from
University of Chicago
Dubois, K. P., Schmalgemeier, D. and Leighton, L. B.
1959 Studies on the Toxicity and Anticholinesterase
Action of Ethyl Guthion. Unpublished report from
University of Chicago
Grasso, P. Results of histological examination of brain,
1969 spinal cord and a peripheral nerve from hens
treated with Ethyl-gusathion for 30 days.
Unpublished report from British Industrial
Research Association, submitted by Bayer A.G.
Hunt, L. M. and Gilbert, B. N. Simultaneous determinations of
1970 azinphos-ethyl, azinphos-methyl and its oxygen
analogue by gas liquid chromatography. Bull. of
Env. Cont. and Tox. 5: 42-46
Kimmerle, G. Active substance R1513 (Ethyl Gusathion;
1959 No. 2078). Unpublished report from Bayer A.G.
Kimmerle, G. Ethyl-Gusathion. Unpublished report from
1960 Bayer A.G.
Kimmerle, G. Gusathion A/Antidotwirkung und Potentzierung.
1966a Unpublished report from Bayer A.G.
Kimmerle, G. Gusathion A-Wirkstoff (R 1513; Ht. - Nr. 3651).
1966b Unpublished report from Bayer A.G.
Kimmerle, G. Äthyl-Gusation/Subchronische Neurotoxizitäts
1968 versuche bei Hühnern. Unpublished report from
Bayer A.G.
Kimmerle, G. and Lorke, D. Toxicology of insecticidal
1968 organophosphates. Pflanzenschutz-Nachrichten,
Bayer, 21: 111-142
Kirchhoff, J. Spezifischer dünnschichtchromatographischer
1970 Nachweis von Gusathion-Rückständen in Pflanzen
extrakten. Pflanzenschutznachrichten, Bayer, 23:
365-366
Krause, Ch. und Kirchhoff, J. Organophosphatrückstände auf
1969 Marktproben von Obst and Gemüse sowie auf
Getreideerzeugnissen. Deutsch. Pflanzenschutzd.
(Braunschweig), 21, 1969: 81-84
Lorke, D. and Kimmerle, G. The Action of Reactivators in
1969 Phosphoric - Acid-Ester Poisoning. Naunyn
Schmiedebergs Arch. Pharmak. exp. Path. 263: 237
Löser, E. BAY 16 259/Subchronische toxikologische
1969 Untersuchungen an Ratten. Unpublished report from
Bayer A.G.
Mawdesley-Thomas, L. E. and Urwin, C. Pathology Report of
1969 BAY 16 259. Sub-chronic Toxicity Study in Rats
Administered over Three Months. Unpublished report
from Huntingdon Research Centre, submitted by
Bayer A.G.
Meagher, W. R., Adams. J. M., Anderson, C. A. and Macdougall, D.
1960 Colorimetric determination of Guthion residues in
crops. J. Agric. Fd. Chem., 8: 282-286
Miles, J. R. W. A new colorimetric method for determination
1964 of residues of Guthion and Ethyl Guthion and their
oxygen analogs. J.A.O.A.C. 47: 882-885
Möllhoff, E. Kolorimetrische Bestimmung von Azinphos-äthyl. 1969
Rückstands analytik von Pflanzenschutmitteln.
(DFG), Verlag Chemie GmbH, Weinheim/Bergstr.
Olson, T. J. Investigation of the possible metabolism of
1969 Ethyl-Guthion on crops. Chemagro Report No. 25
151 (unpublished)
Olson, T. J. Determination of residues of Guthion M-E in
1969a soybeans by thermionic emission gas
chromatography. Chemagro Report No. 25 152
(unpublished)
Olson, T. J. A confirmatory gas chromatographic procedure for
1969b the Guthion M-E residue method. Chemagro Report
No. 25 911 (unpublished)
Olson, T. J. A study of the possible interferences or other
1969c pesticides with the analytical method for Guthion
M-E in cotton. Chemagro Report No. 25 912
(unpublished)
Olson, T. J. Determination of Guthion M-E residues in
1969d cigarette smoke. Chemagro Report No. 25 121
(unpublished)
Olthoff, P. D. A. and de Mey, R. C. H. Residues of azinphos
1973 ethyl on potatoes. Report No. 3954 Central
Institute for Nutrition and Food Research,
Netherlands
Schumann, S. A. and Anderson, R. J. Determination of Guthion
1965 M-E residues in clover by electron capture gas
chromatography. Chemagro Report No. 16 571
(unpublished)
Sherman, M., Herrick, R. B., Ross, E. and Chang, M. T. Y.
1967 Further Studies of the acute and subacute toxicity
of Insecticides to Chicks, Toxicol. and Appl.
Pharmacol. 11: 49-67
Wagner, Kl. Report of Bayer A.G., Leverkusen (Pflanzenschutz
1973 AT/Biologische Forschung, Institut für
Rückstandsanalytik) (unpublished)
Watts, R. R. Extraction efficiency study - Examination of three
procedures for extracting 14C-labeled and
unlabeled residues of organophosphorus pesticides
and carbaryl from bean leaves and kale. J.A.O.A.C.
54: 953-958
empirical formula: C12H16N3O3PS2
Other information on identity and properties
(a) Composition of technical azinphos-ethyl
The technical material contains a minimum of 92% azinphos-ethyl.
Impurities
triethyl thiophosphate and
triethyldithiophosphate together max. 2.0%
bis(3,4-dihydro-4-oxo-1,2,3-triazin-3-ylmethyl)-sulfide together
and -disulfide max. 1.5%
3-chloromethyl-3,4-dihydro-4-oxo-1,2,3
benzotriazin and (3,4-dihydro-4-oxo-1,2,3
benzotriazin-3 ylmethyl)-methylsulfide together max. 1.5%
ethylene-bis (O,O-diethyl-dithiophosphate) max. 1.9%
0,S-diethyl-S-(3,4-dihydro-4-oxo-1,2,3-
benzotriazin-3-yl(-methyl)-dithiophosphate max. 0.7%
bis (3,3-dihydro-4-oxo-benzotriazin-3-yl-methyl)-ether max. 0.6%
0-ethyl-S-(3,4, dihydro-4-oxo-1,2,3
benzotriazin 3-yl-methyl)-dithio-phosphoric
acid max. 0.5%
water max. 0.2%
(b) Physical and chemical properties of azinphos-ethyl
Physical state: colourless crystals.
Molecular weight: 345.4.
Melting point: 53°C (pure material).
Boiling point: 147°C at 0.01 mm Hg
111°C at 0.001 mm Hg
Volatility: 0.0012 mg/cu metre at 10°C
0.0042 mg/cu metre at 20°C
0.0128 mg/cu metre at 30°C
0.0372 mg/cu metre at 40°C
Vapour pressure: 6.3 x 10-8 mm Hg at 10°C
2.2 x 10-7 mm Hg at 20°C
7 x 10-7 mm Hg at 30°C
2.1 x 10-6 mm Hg at 40°C
Specific gravity: D 20 = 1.284
4
Refractive index: N 53 = 1.592
D
Solubility: practically insoluble in water,
soluble in most organic solvents
Formulations used: emulsifiable liquid 25% w/v, 40% w/v
wettable powder 25%, 33%, 40%
ULV formulation 500 g/l
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Following oral and I.V. administration of 14C-labelled
azinphos-ethyl to male rats in doses of 0.1 or 2 mg/kg bw, about 60%
of the activity was excreted in urine and 30% in faeces in 48 hours.
Approximately 30% of a 2 mg/kg dose was eliminated in bile within 24
hours; less than 0.1% was eliminated in expired air, indicating that
the benzotriazine ring does not undergo extensive degradation.
Approximately 6% of activity was present in the body 48 hours after
administration, the mean concentration in tissues being equivalent to
0.1 ppm. The highest equivalent concentration (0.2-0.3 ppm
(0.00002%-0.00003%)) was found in blood and 0.15-0.2 ppm
(0.000015%-0.00002%) was found in lungs, liver and kidneys. The
results of an exactly similar study using azinphos-methyl showed no
differences, indicating that mammals metabolize both compounds in a
similar manner (Anon., 1973).
Effects on enzymes and other biochemical parameters
Brain cholinesterase activity in vitro was reduced by 50% by
1.4 x 10-5 molar azinphos-ethyl; this was considered to be due to the
presence of impurities with anticholinesterase activity. A single I.P.
dose of 3.4 mg azinphos-ethyl/kg reduced serum, brain and submaxillary
gland cholinesterase activity to approximately the same extent.
Maximum inhibition occurred within an hour and activity returned to
normal gradually, reversal not being complete by 70 hours. On
incubation with liver preparation, azinphos-ethyl was transformed into
a potent anticholinesterase compound, probably the oxygen analogue
(Dubois et al., 1959).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Groups of three hens received a single dose of 10 or 25 mg
azinphos-ethyl/kg and were observed for five weeks. One bird on the 25
mg/kg level died after 26 days but no signs of demyelinating disease
developed (Kimmerle, 1960).
Groups of four hens were fed on diet containing 0, 75, 150, 300
and 600 ppm (0, 0.0075, 0.015, 0.03 and 0.06%) azinphos-ethyl for 30
days. Loss of body weight occurred at the two highest dosage levels
and a significant depression of whole blood cholinesterase activity at
the 600 ppm (0.06%) level. No signs of neurological damage occurred
during the dosage period or during the subsequent four-week
observation period. Histological examination of femoral fixed brain,
spinal cord and femoral nerve failed to detect evidence of
demyelination or nerve damage (Kimmerle, 1968; Grasso, 1969).
Special studies on potentiation and antidotes
No significant potentiation of acute toxicity occurred with
azinphos-ethyl in combination with parathion, methyl parathion,
systox, EPN, malathion, trithion, phosdrin, sevin, delnav, OMPA,
diazinon, azinphos, di-syston, Co-Ral, Folex or Ronnel. A twofold
potentiation occurred with azinphos-ethyl and ethion (Dubois and
Raymond, 1960a; Dubois, 1963; Kimmerle, 1966a).
The effects of azinphos-ethyl are counteracted by atropine and by
reactivating compounds. The two drugs given in combination give more
pronounced effects (Kimmerle, 1966; Lorke and Kimmerle, 1968; Dubois
et al., 1959).
Acute toxicity
LD50
Species Route Sex mg/kg bw References
Mouse I.P. 3.8-4.0 Dubois et al., 1959
Rat Oral 12.0-20.5 Ben-Dyke et al., 1970
Kimmerle, 1959,
1966 a & b
F 7.0 Dubois et al., 1959
M 15.2
Rat I.P. F 4.4 Dubois et al., 1959
M 9.2
M 7.5 Kimmerle, 1959
Rat Dermal 75-280 Ben-Dyke et al., 1970
Dubois et al., 1959
Kimmerle, 1959
Guinea-pig Oral 17 Dubois et al., 1959
Guinea-pig I.P. 8.0 Dubois et al., 1959
Chicks Oral 34 Sherman et al., 1967
F = Female; M = Male.
Short-term studies
Rat. Twenty-four male rats received an oral dose of 1 mg
azinphos-ethyl/kg bw on each of 28 consecutive working days. The rate
of weight gain was normal and signs of poisoning did not occur.
Erythrocyte cholinesterase showed 50% depression after 2, 82% after 3
and 90% after 28 days' treatment. Activity had returned to normal 35
days after treatment stopped (Kimmerle, 1959).
Groups of five female rats received daily I.P. injections of
azinphos-ethyl at dosage levels of 0.5, 1, 2 and 3 mg/kg bw for 60
days. Only the two highest dosage levels caused a reduction in the
rate of weight gain and an increased mortality (Dubois and Raymond,
1960b).
Groups of 15 male and 15 female rats received diet containing 0,
1, 2, 4 and 8 ppm (0, 0.0001, 0.0002, 0.0004 and 0.0008%) for three
months. No effects were found on growth rate, food consumption,
mortality rate or on the results of haematological investigations and
analysis of blood. The 4 ppm (0.0004%) level had no significant effect
on plasma enzyme but depressed the erythrocyte cholinesterase activity
after one month. The 8 ppm (0.0008%) level depressed plasma enzyme
after only one week's exposure and prolonged exposure caused no
further depression; erythrocyte enzyme activity continued to fall
during the first month's exposure. In general, females appeared to be
more sensitive than males. Autopsy, determination of organ weights and
histological examination of organs and tissues detected no abnormality
in animals fed azinphos-ethyl (Löser, 1969; Mawdesley-Thomas and
Urwin, 1969).
Groups of 12 male and 12 female rats were fed on diets containing
0, 5, 10 and 50 ppm (0, 0.0005, 0.001 and 0.005%) azinphos-ethyl for
16 weeks. The growth rate of female animals was unaffected while male
rats receiving 50 ppm (0.005%) showed a slightly reduced rate of
growth compared with controls. No animals showed signs of
cholinesterase inhibition but determination of the activity of the
enzyme in serum, erythrocytes, submaxillary and brain tissue of five
male and five female rats of each group showed that it was markedly
inhibited in the 50 ppm (0.005%) group. Inhibition was most marked in
erythrocytes (87%) and serum (85%) and less marked In brain (24% in
males and 72% in females) and gland tissue (16% in males and 59% in
females). Serum and erythrocyte activity was depressed (52% and 80%
respectively) in rats fed 10 ppm (0.001%) azinphos-ethyl in their
diet. Only erythrocyte enzyme was significantly inhibited in rats on 5
ppm (0.0005%) diet; males and females did not differ in this respect.
The remaining seven animals of each sex of each group were autopsied
and the organs weighed and examined histologically but the results of
this examination are not reported (Doull and Root, 1960).
Dog. Groups of two male and two female dogs aged between 6 and 14
months were fed for 12 weeks on diets containing 0.25, 0.5, 1, 2, 3
and 10 ppm, (0.000025, 0.0005, 0.0001, 0.0002, 0.0003 and 0.001%)
azinphos-ethyl. The serum and erythrocyte cholinesterase activity was
determined periodically before, during and following the dosage
period. Dogs receiving 3 and 10 ppm (0.0003 and 0.001%) diets
exhibited signs of cholinesterase activity depression and were taken
off the diets after 1 and 6 weeks respectively. Over 50% inhibition
occurred in the serum and erythrocytes of dogs fed on 2 ppm (0.0002%)
diet. Erythrocyte enzyme activity was not inhibited in dogs receiving
the 0.25% diet but the serum enzyme was inhibited by about 20%. Since
the serum cholinesterase activity in control animals was inhibited by
10% the no-effect level was considered to be approximately 0.25 ppm
(0.000025%). In the animals affected, activity was reduced during the
first week of the test and then remained constant while the
erythrocyte enzyme tended to decrease gradually throughout the 12-week
test period. Cholinesterase activity returned to normal in 3-4 weeks
when dogs on the highest dosage levels were transferred to control
diet and in 2-3 weeks with the 0.5 and 1 ppm (0.00005 and 0.0001%)
groups (Doull et al., 1963).
Long-term studies
No data available.
Comments
Azinphos-ethyl is absorbed from the gastrointestinal tract and
excreted in urine and bile. It does not accumulate in tissues. The
absorption, distribution and excretion of radiolabel by rats are
similar in animals administered 14C-labelled azinphos-ethyl or
-methyl. However, insufficient data are available to determine the
metabolic pathway of azinphos-ethyl and to compare it with that of
azinphos-methyl, although there is evidence that in both the
benzotriazine moiety remains intact.
Azinphos-ethyl inhibits acetylcholinesterase activity in plasma,
erythrocyte, brain and submaxillary gland and a series of low doses
causes the plasma activity of rats and dogs to fall rapidly to a
stable level while erythrocyte acetylcholinesterase tends to fall more
gradually over a longer period. Female rats were more sensitive than
males as shown by the degree of enzyme inhibition and, in some tests,
by LD50 values. Recovery of enzyme activity took several weeks
following cessation of exposure of rats.
Short-term studies in rats show the no-effect level to be 2 ppm
(0.0002%) in the diet. The only toxic effects seen were attributed to
depression of cholinesterase activity. In an investigation using dogs,
in which only plasma and erythrocyte cholinesterase activity were
recorded, the no-effect level was 0.25 ppm (0.000025%) in the diet.
The Meeting was unable to estimate an acceptable daily intake for
this substance in the absence of sufficient information on the
identity and toxicity of its metabolites, on its possible effects on
reproduction, on its long-term toxicity and on its carcinogenic and
teratogenic and mutagenic potential.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-ethyl is a non-systemic organophosphorus insecticide,
which is used against a relatively broad spectrum of insects e.g.
Lepidopterous larvae, beetles and their larvae, aphids, jassids and
spider mites on various crops: cotton, rice, sugar and fodder beets;
fruits such as apples, pears, citrus-fruit, grapes; tobacco.
Azinphos-ethyl is used in about 35 countries in Europe, Africa,
Asia, Australia, New Zealand, North, Central and South America.
The amounts used on different crops vary greatly from year to
year depending on the pest situation. A rough estimate may be given:
cotton and other agricultural crops 60%
fruit crops (including grapes) 30%
vegetables (including potatoes) 10%
Pre-harvest applications
Azinphos-ethyl is generally used at dosages of 0.04-0.05% active
ingredient. The officially registered and/or recommended uses of
azinphos-ethyl are summarized below, with the application rates and
pre-harvest intervals.
Crop Dosage rate Minimum pre-harvest
g a.i./ha interval - days
Agricultural crops
cotton 250-1 000 5
rice 300-500 14
tobacco 300-600 14
sugar- and fodder beets 300-500 14
Fruit crops
apples, pears, peaches,
plums and cherries 800-1 000 14-21
citrus fruit 800-1 000 14-21
grapes 800-1 000 14-21
Vegetables
brassicas 300-500 7-14
melons 300-500 7-14
tomatoes 300-500 7-14
potatoes 300- 14-
Hops 400-800 14
Pre-harvest intervals officially recommended in different
countries, days
Australia general 21 days
Bulgaria sugar beets 14 days
Fed. Rep. of field crops as:
Germany cereals, rape, alfalfa,
sugar and fodder beets
potatoes 14 days
beans 21 days
cucumbers, tomatoes 14 days
United Kingdom apples, pears 21 days
cucumbers and tomatoes
(glasshouse cultures) 2 days
France general 15 days
Italy general 20 days
Morocco olives 30 days
other crops 15 days
New Zealand root vegetables 14 days
smooth-skinned fruit 14 days
other fruit and
vegetables 21 days
Netherlands potatoes 28 days
Portugal tomatoes for industrial
processing 5 days
all other crops 21 days
Switzerland fruit, grapes 21 days
potatoes 21 days
Spain cotton 21 days (2%
dust 15 days)
sugar beets 21 days
hazelnuts 21 days
South Africa potatoes 21 days
Pre-harvest intervals officially recommended in different
countries, days
South Africa cotton 5 days (not
(Cont'd.) to be used for
feeding purposes
until after 35 days)
Yugoslavia fruit 21 days
Residue data from supervised trials
Residue data are available from trials on various fruit,
vegetables and field crops: apple; kidney bean, soybean, cauliflower,
kohlrabi, savoy, potatoes; tobacco; the data obtained are summarized
in the following table.
Residues in food moving in commerce
In the Federal Republic of Germany in 1965 and 1968 a total of
228 samples of fruit and vegetables, home produced and imported,
analysed for organophosphorous compounds.
Only in one of these 220 samples azinphos-ethyl was detected, the
residue level being less than 0.1 ppm (Krause, 1969).
Azinphos-ethyl was not found in any of the 378 sub-samples of a
total diet study carried out in England and Wales in 1966-67 (Abbott
et al., 1970).
Fate of residues
In mammals
14C-labelled azinphos-ethyl and azinphos-methyl was administered
orally and intravenously to about 50 male rats (Sprague - Dawley
approx. 170 g). The compounds were applied in a solvent mixture
containing 5% Cremophor EL.
The radioactivity measured related to the sum of the unchanged
parent compound and its metabolites.
After oral as well as after intravenous administration about 65%
(57-68%) of the applied activity was eliminated in the urine within 48
hours, and about 30% (26-34%) in the faeces (see table).
TABLE 1. RESIDUES OF AZINPHOS-ETHYL + P = 0 ANALOGUE IN PPM, TOTAL RESIDUE
[TWO FIGURES ... (...), AZINPHOS-ETHYL AND P = 0 ANALOGUE SEPARATELY]
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Field crops
cottonseed USA 1968 14 0.56 E.C. 12 <0.01 <0.01
10 " <0.01 <0.01
13 " 0.01 0.01
10 " <0.01 0.01
gin trash USA 1968 14 0.56 E.C.12 2.71 1.96
10 " 1.28 0.75
13 " 0.22 0.12
10 " 5.07 3.07
foliage USA 1968 14 0.56 E.C.12 16.45 8.81
10 " 1.20 0.60
13 " 0.35 <0.07
10 " 11.41 11.47
rape seed Germany 1971 1 0.3 w.p. 33% n.d.(74)
Fed.Rep.
Fruit crops
Apple
Cox's and UK 1961 3 1.13 w.p. 25% 0.3 <0.3 <0.3 <0.3 n.d.(47)
Worcester
Cox's UK 1961 2 1.4 w.p. 25% 1.04 0.66 0.43 0.42
Golden
Delicious Belgium 1969 1 1.25 E.C. 50% 0.94 0.44 0.19 0.09 (42)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
Vegetables
French Germany 1972 1 0.3 E.C.250g/l 0.6 0.05 n.d. n.d.
beans Fed.Rep. 1972 1 0.3 0.6 0.06 n.d. n.d.
1972 1 0.3 0.12 0.07 n.d. n.d.
soybean USA 1968 3 0.42 E.C. 12 0.55
(0.003)
green plant 1969 3 0.42 " 0.79
(0.003)
whole 1968 3 0.42 " 3.08 0.87
(0.04) (0.03)
1969 3 0.42 " 14.91 4.62 0.75 0.24
(<0.02) (<0.02) (<0.02) (<0.02)
1968 3 0.42 " 18.0 3.6 1.6 0.9
(<0.03) (<0.16) (<0.03) (0.39)
soybean USA 1968 3 0.42 E.C. 12 0.26
vines (dry) (<0.03)
(45 d)
1969 3 " " 0.25
(<0.06)
1968 3 " " 0.14
(<0.02)
(55 d)
1969 3 " " 0.01
(<0.03)
(48 d)
1968 3 " " 0.23
(<0.04)
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
beans(dry) 1968 3 0.42 E.C. 12 0.10
(<0.03)
(45 d)
1969 3 " " 0.01
(<0.02)
1968 3 " " 0.01
(<0.01)
(55 d)
1969 3 " " 0.09
(<0.02)
(48 d)
1968 3 " " 0.01
(<0.01)
cauliflower Germany 1967 1 0.5 w.p.33% 2.95 0.15
Fed. Rep.
kohlrabi Germany 1968 1 0.5 w.p. 33% 0.25 0.07 0.04 n.d.
(without Fed.Rep. 1967 1 0.5 " 0.3 0.06 0.01
leaves) 1967 1 0.5 " 0.4 0.03 <0.01
1967 1 0.3 " 0.15 0.12 0.08
savoy Germany 1 0.3 w.p. 33% 0.32 0.27 0.15 0.12 0.14
Fed.Rep. (36 d)
white
cabbage Germany 1967 1 0.3 w.p. 33% 0.18 0.07 0.06 <0.02
Fed. Rep.
TABLE 1. (Cont'd.)
Application Pre-harvest interval in days
Crop Country Year rate kg >35
No. a.i./ha formulation 0 2/3 6/8 10/13 14/15 21/23 28/31 (...days)
potatoes USA 1962 8 0.42 E.C. 12 n.d.
1962 8 " " n.d.
1962 8 " 1 " n.d.
1962 8 " " n.d.
1962 8 " " n.d.
Netherlands 1972 1 0.20 w.p.25% n.d.
1972 1 " " n.d.
tomatoes New
Zealand 1968 2 1.25 E.C. 44 1.03a 0.9a 0.78a 0.5la
1968 2 " 1.5a 0.98a 1.28a 0.80a
1968 2 11.25 w.p. 25 1.10a 1.15a 0.66a 0.62a
1968 2 " 1.97a 1.62a 0.94a 0.33a
tobacco
cured USA 1967 3 0.84b E.C. 12 227 119 63 22
1968 3 0.84b " 373 282 150 75
1967 3 0.56 E.C. 12 117 138 27 29
1967 3 " " 79.5 58.6 40.5 7.2
1967 3 " " 170 67 29 19
1967 3 " " 87 103 14 8.7
1967 3 " " 254 119 61 22
1967 3 " " 207 140 68 40
n.d. = not detectable.
a Only residues on the peel; residue in the pulp 0.03-0.09 ppm.
b Overdosage
Route of Dosage Number of % of activity applied
Compound administration mg/kg animals excreted in 48 hours
urine faeces
azinphos-ethyl i.v. 2 5 66 ± 2 26 ± 4
i.v. 0.1 5 59 ± 2 31 ± 4
p.o. 2 4 62 ± 2 30 ± 5
p.o. 0.1 5 57 ± 5 34 ± 6
azinphos-methyl i.v. 2 4 68 ± 3 26 ± 4
i.v. 0.1 5 63 ± 3 29 ± 4
p.o. 2 5 68 ± 4 26 ± 2
About 50% of the dosage administered was excreted in 8-9 hours.
Elimination in the bile
Following intravenous administration of 2 mg/kg azinphos-ethyl or
azinphos to each of three animals an average of approximately 30% of
the applied activity was eliminated in the bile within 24 hours.
Elimination of azinphos in the air
Following oral or intravenous administration of 14C-labelled
azinphos-ethyl less than 0.1% of the applied activity was eliminated
in the expired air within 24 hours. This indicates that the
benzotriazine ring in azinphos-ethyl does not undergo extensive
metabolic degradation.
Forty-eight hours after oral and intravenous administration of 2
mg/kg azinphos-ethyl or azinphos to each of four or five animals 6% of
the applied activity was still present in the animal; approximately 1%
was present in the gastrointestinal tract.
The mean concentration of azinphos-ethyl in the animal tissue
(except the gastrointestinal tract) was at that moment about 0.1 µg/g
tissue irrespective of the compound applied and the route of
administration. Autoradiography and quantitative determination in
single tissues showed that the activity was fairly evenly distributed.
The highest concentrations were in the blood (0.2-0.3 µg/g).
The equivalent concentrations in lung, liver and kidneys were
0.15-0.2 µg/g.
The concentrations measured in other organs were below the values
mentioned above.
Fate of residues in plants
The metabolism of azinphos-ethyl was studied in cotton and bean
plants under field conditions (Olson, 1969).
The residue following application of azinphos-ethyl consisted
partly of the P = 0 metabolite presumably owing to its higher chemical
stability. After 14 days approximately 10% of the residue could be
determined by TLC and GLC as the P = 0 analogue.
Methods of residue analysis
Several colorimetric, thin layer and gas chromatographic methods
for residue analysis of azinphos-ethyl and relevant metabolites are
described in the literature. Only those methods will be referred to
which may be suitable for regulatory purposes or which can be adapted
for these purposes.
Colorimetric methods
Miles (1964) developed a rapid colorimetric method for residue
analysis of azinphos-ethyl, azinphos and their P = 0 metabolites in
plant material.
The method is based on the hydrolysis of the pesticide or
metabolite molecule after separation in a medium of acetic and
hydrochloric acid and the simultaneous coupling of the presumably
formed intermediate benzazimide with N-(1-naphtyl)-ethylene diamine to
produce a violet solution with an absorption maximum at 556 nm.
The plant material is extracted with chloroform and the extract
is carried through a clean-up procedure using Attaclay-celite. The
oxygen analogues are separated in a Florisil column. Azinphos-ethyl
and azinphos-methyl are eluated with chloroform, the P = 0 analogues
with an acetonechloroform mixture.
The recoveries in plant material range from 78 to 97%.
The lower limit of detection is about 5 µg of azinphosethyl and
the P = 0 analogue.
The method, which is specific for the benzotriazinyl group, was
proved to be suitable for residue analysis on apples, tomatoes,
cabbage and tobacco. No interference occurs with milligram quantities
of carbaryl or the following organophosphorous pesticides: demeton,
diazinon, dimethoate, disulfoton, EPN, malathion, parathion, phorate,
schradan and trichlorphon.
A similar colorimetric method for residue analysis of
azinphos-ethyl in apples, potatoes, alfalfa and watermelons was
described by Möllhoff (1969).
The plant samples are treated with acetone; the parent compound
is extracted with chloroform and then chromatographed in isopropanolic
solution for clean-up. The alkaline hydrolysis leads to anthranilic
acid, which is diazotized and coupled with N-(1 naphtyl)-ethylene
diamine.
The P = 0 compound of azinphos-ethyl and azinphos are
co-determined with the method.
Parathion and 4-nitrophenol do not interfere with the
determination.
The limit of detection of the method is about 0.1-0.05 ppm.
The recoveries by the colorimetric method (Meagher et al., 1960)
were found to be similar to those obtained with GLC methods (Wagner,
1973).
COMPARISON OF RECOVERIES OF AZINPHOS-ETHYL BY COLORIMETRIC AND GLC
METHODS OF ANALYSIS
Recovery %
Crop Added ppm
GLC Colorimetry
potato 0.1 86 100
0.2 93 99
citrus 0.1 76 72
0.2 79 82
TLC method
Kirchhof (1970) developed a thin layer chromatographic separation
of azinphos-ethyl, azinphos-methyl and their P = 0 analogues on silica
gel coated plates combined with the colorimetric method of Miles
(1964).
The limit of determination of the method on apples, lettuce,
kohlrabi and onions is 0.1-0.2 µg for the parent compounds and their
P = 0 metabolites alone or in combination.
The method does not require a special clean-up procedure. There
is no interference from other insecticides, fungicides, herbicides or
plant constituents.
GLC method
Residues of azinphos-ethyl, azinphos and their P = 0 analogues
can be determined by gas chromatography with a high degree of
sensitivity and specificity.
Especially GLC methods utilizing a phosphorous-specific detector
e.g. a modified flame ionization detector (thermionic detector) proved
to be particularly suitable for regulatory purposes.
Simultaneous GLC determination of azinphos-ethyl and its P = 0
analogue in soybeans is described by Olson (1969). Azinphos is also
co-determined,
Following extraction of the plant samples (green plants, dry
beans and vines) with chloroform, interfering plant constituents are
precipitated. The resultant extract is directly injected into the
gas-chromatograph.
Under the given conditions azinphos, azinphos-ethyl
and the P = 0 analogue of azinphos-ethyl are identified by their
retention times which are 13.3, 17.3 and 15 minutes respectively.
The limit of determination in this rapid procedure is about 0.005
ppm.
A similar procedure for GLC analysis of azinphos-ethyl and
azinphos in cotton is described by Olson (1969a and b).
Other organophosphorous compounds which may be used in cotton,
such as diazinon, disulfoton and its sulfoxide and sulfone, demeton
and its sulfoxide and sulfone, malathion and malaoxon did not
interfere with the determination of azinphosethyl, azinphos and their
P = 0 metabolites.
A similar GLC method for determination of azinphos-ethyl,
azinphos and their P = 0 metabolites in cigarette smoke is described
by Olson (1969c).
A method for the gas chromatographic determination of residues of
azinphos-ethyl, azinphos-methyl and their P = 0 analogues in milk is
described by Hunt et al. (1970). The extraction procedure corresponds
to that of Adams et al. (1966). The sensitivity of this method for the
determination of azinphos-methyl and the -ethyl analogue in milk
samples is about 0.04 ppm and for the P = 0 analogue of
azinphos-methyl about 0.06 ppm.
New and generally applicable procedures for the extraction of
residues of carbaryl, malathion, phosphamidon, azinphos-methyl and
parathion from plant material are described by Watts (1971). The three
described procedures which consist of sample blending with
acetonitrile or ethyl acetate and an exhaustive Soxhlet extraction
procedure using 10% methanol in chloroform, were compared. They were
all found to give very good recoveries.
National tolerances
Australia fruit 2.0
vegetables 1.0
cereals 1.0
potatoes 0 (at or about limit of detection)
Belgium fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4
potatoes 0.05
Fed. Rep. of Germany fruit 0.4
azinphos-ethyl and
-methyl together vegetables 0.4 (except root vegetables)
celeriac 0.4
other food
crops 0.05
Italy
azinphos-ethyl and
-methyl together 0.4
Netherlands
azinphos-ethyl and
-methyl together fruit crops 0.4
vegetables 0.4 (except potatoes)
potatoes 0.05
Switzerland
azinphos-ethyl and fruit crops
-methyl together incl.
grapes 0.4
vegetables 0.4
South Africa fruit,
vegetables 2
Appraisal
Azinphos-ethyl is a non-systemic organophosphorus insecticide
with contact action as well as stomach poison action used on a
considerable scale in many countries on a relatively wide range of
crops.
The main uses are foliar applications on field crops, such as
cotton, sugarbeet, fruit and vegetables against a wide range of
insects.
Technical azinphos-ethyl contains a minimum of 92% of the pure
compound. The impurities in the technical material are known.
Azinphos-ethyl is marketed in the form of wettable powders,
emulsifiable liquids and as a ULV formulation.
The concentration/rates of application vary, depending on pest,
crop and method of application; normal application rates are
250-1000 g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. The data
presented for azinphos-ethyl including the P = 0 analogue are, with
few exceptions, representative of those likely to result from good
agricultural practice.
Limited information is available on the fate of residues in
plants. The residues which may occur in food of plant origin,
following recommended directions for use and recommended pre-harvest
intervals consist largely of the parent chemical and to a small extent
of the P = 0 analogue. The residue may contain up to 10% of the P = 0
analogue.
Information considered provided a comparison between the nature
and fate of azinphos-ethyl and its methyl analogue. The only
noticeable difference is the greater persistence of the P = 0 analogue
of the azinphos-ethyl. The P = 0 analogue is not detected at harvest
following the use of azinphos-methyl.
Only limited information is available on the rate of decrease of
the residue of azinphos-ethyl and the P = 0 analogue in crops during
storage and processing, including household cooking. Information was
presented on the extent of carry over of residues in tobacco smoke
following application to tobacco.
Extensive data was available from supervised trials on
cauliflower, kohlrabi, savoy cabbage, white cabbage, french beans. It
was considered that a sufficient range of vegetable crops had been
studied to enable recommendations to be made for vegetables as a
group.
Little information is available on azinphos-ethyl in food in
commerce. A rapid and sensitive colorimetric method is described for
the determination of azinphos-ethyl and the P = 0 analogue together.
The method is specific for the benzotriazinol group and thus
azinphos-ethyl and azinphosmethyl cannot be determined separately.
Gas chromatographic procedures are available for specific
determination of azinphos-ethyl and the P = 0 analogue. Appropriate
extraction and clean-up procedures in food products of plant and
animal origin are available. These methods are suitable for regulatory
purposes.
RECOMMENDATIONS
The following tolerances are recommended for azinphosethyl and
its P = 0 metabolite expressed as azinphos-ethyl.
Commodity Tolerance Pre-harvest intervals on
(ppm) which recommendations are
based (days)
Tomatoes 1 14 (outdoors)
Apples, pears 0.5 21
Vegetables, except
tomatoes and potatoes 0.5 14-21
Soybeans (dry) 0.2 14-21
Potatoes 0.05a 14
Cotton seed, rape seed 0.05a 14
a At or about the limit of determination
N.B. In those cases where a mixture of residues of azinphos-ethyl
and azinphos-methyl occurs together on a commodity, except tomatoes,
the total residue of both compounds including their P = 0 analogues
may not exceed the levels recommended for azinphos-methyl. In case of
tomatoes the total residue should not exceed the level recommended
above for azinphos-ethyl, 1 ppm.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Studies to identify metabolites in plants and animals.
3. Studies to investigate the toxicity of metabolites.
4. Studies to detect effects on reproduction.
5. Studies to detect teratogenic activity.
Desirable
1. Additional information on the nature of terminal residues
in plants, animals and their products.
2. Data on disappearance of residues during storage and
cooking of vegetables and fruits.
REFERENCES
Abbot, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G.
1970 Pesticide residues in the total diet in England
and Wales, 1966-1967. III. Organophosphorous
pesticide residues in the total diet. Pestic. Sci
1: 10-13
Adams, J. M. and Anderson, C. A. Spectrophotofluormetric
1966 method for Guthion residues in milk and animal
tissues. J. Agric. Fd. Chem. 14: 53-55
Anderson, C. A. A Comparison of the Residue Decline Rates
1963 for Guthion and Ethyl Homolog on Alfalfa, Apples
and Lespedezza. Chemagro Report No. 10 394
(unpublished)
Anon. Pharmacokinetic studies with 14C-labelled Gusathion
1973 A and Gusathion M (provisional results)
Unpublished report from Isotopen-Institut,
submitted by Bayer A.G.
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. Acute
1970 toxicity data for pesticides. World Review of Pest
Control, 9: 119-127
Doull, J. and Root, M. The Effect of Diets containing Ethyl
1960 Guthion on Rats. Unpublished report from
University of Chicago
Doull, J., Root, M. and Cowns, J. Determination of the safe
1963 dietary level of Ethyl Guthion for dogs.
Unpublished report from University of Chicago
Dubois, K. P. The Acute Toxicity of Ethyl Guthion in
1963 Combination with Four Other Organic Phosphates.
Unpublished report from University of Chicago
Dubois, K. P. and Raymund, A. B. The Acute Toxicity of Ethyl
1960a Guthion in Combination with Other
Anticholinesterase Agents. Unpublished report from
University of Chicago
Dubois, K. P. and Raymund, A. B. The Subacute Toxicity of
1960b Ethyl Guthion to Rats. Unpublished report from
University of Chicago
Dubois, K. P., Schmalgemeier, D. and Leighton, L. B.
1959 Studies on the Toxicity and Anticholinesterase
Action of Ethyl Guthion. Unpublished report from
University of Chicago
Grasso, P. Results of histological examination of brain,
1969 spinal cord and a peripheral nerve from hens
treated with Ethyl-gusathion for 30 days.
Unpublished report from British Industrial
Research Association, submitted by Bayer A.G.
Hunt, L. M. and Gilbert, B. N. Simultaneous determinations of
1970 azinphos-ethyl, azinphos-methyl and its oxygen
analogue by gas liquid chromatography. Bull. of
Env. Cont. and Tox. 5: 42-46
Kimmerle, G. Active substance R1513 (Ethyl Gusathion;
1959 No. 2078). Unpublished report from Bayer A.G.
Kimmerle, G. Ethyl-Gusathion. Unpublished report from
1960 Bayer A.G.
Kimmerle, G. Gusathion A/Antidotwirkung und Potentzierung.
1966a Unpublished report from Bayer A.G.
Kimmerle, G. Gusathion A-Wirkstoff (R 1513; Ht. - Nr. 3651).
1966b Unpublished report from Bayer A.G.
Kimmerle, G. Äthyl-Gusation/Subchronische Neurotoxizitäts
1968 versuche bei Hühnern. Unpublished report from
Bayer A.G.
Kimmerle, G. and Lorke, D. Toxicology of insecticidal
1968 organophosphates. Pflanzenschutz-Nachrichten,
Bayer, 21: 111-142
Kirchhoff, J. Spezifischer dünnschichtchromatographischer
1970 Nachweis von Gusathion-Rückständen in Pflanzen
extrakten. Pflanzenschutznachrichten, Bayer, 23:
365-366
Krause, Ch. und Kirchhoff, J. Organophosphatrückstände auf
1969 Marktproben von Obst and Gemüse sowie auf
Getreideerzeugnissen. Deutsch. Pflanzenschutzd.
(Braunschweig), 21, 1969: 81-84
Lorke, D. and Kimmerle, G. The Action of Reactivators in
1969 Phosphoric - Acid-Ester Poisoning. Naunyn
Schmiedebergs Arch. Pharmak. exp. Path. 263: 237
Löser, E. BAY 16 259/Subchronische toxikologische
1969 Untersuchungen an Ratten. Unpublished report from
Bayer A.G.
Mawdesley-Thomas, L. E. and Urwin, C. Pathology Report of
1969 BAY 16 259. Sub-chronic Toxicity Study in Rats
Administered over Three Months. Unpublished report
from Huntingdon Research Centre, submitted by
Bayer A.G.
Meagher, W. R., Adams. J. M., Anderson, C. A. and Macdougall, D.
1960 Colorimetric determination of Guthion residues in
crops. J. Agric. Fd. Chem., 8: 282-286
Miles, J. R. W. A new colorimetric method for determination
1964 of residues of Guthion and Ethyl Guthion and their
oxygen analogs. J.A.O.A.C. 47: 882-885
Möllhoff, E. Kolorimetrische Bestimmung von Azinphos-äthyl. 1969
Rückstands analytik von Pflanzenschutmitteln.
(DFG), Verlag Chemie GmbH, Weinheim/Bergstr.
Olson, T. J. Investigation of the possible metabolism of
1969 Ethyl-Guthion on crops. Chemagro Report No. 25
151 (unpublished)
Olson, T. J. Determination of residues of Guthion M-E in
1969a soybeans by thermionic emission gas
chromatography. Chemagro Report No. 25 152
(unpublished)
Olson, T. J. A confirmatory gas chromatographic procedure for
1969b the Guthion M-E residue method. Chemagro Report
No. 25 911 (unpublished)
Olson, T. J. A study of the possible interferences or other
1969c pesticides with the analytical method for Guthion
M-E in cotton. Chemagro Report No. 25 912
(unpublished)
Olson, T. J. Determination of Guthion M-E residues in
1969d cigarette smoke. Chemagro Report No. 25 121
(unpublished)
Olthoff, P. D. A. and de Mey, R. C. H. Residues of azinphos
1973 ethyl on potatoes. Report No. 3954 Central
Institute for Nutrition and Food Research,
Netherlands
Schumann, S. A. and Anderson, R. J. Determination of Guthion
1965 M-E residues in clover by electron capture gas
chromatography. Chemagro Report No. 16 571
(unpublished)
Sherman, M., Herrick, R. B., Ross, E. and Chang, M. T. Y.
1967 Further Studies of the acute and subacute toxicity
of Insecticides to Chicks, Toxicol. and Appl.
Pharmacol. 11: 49-67
Wagner, Kl. Report of Bayer A.G., Leverkusen (Pflanzenschutz
1973 AT/Biologische Forschung, Institut für
Rückstandsanalytik) (unpublished)
Watts, R. R. Extraction efficiency study - Examination of three
procedures for extracting 14C-labeled and
unlabeled residues of organophosphorus pesticides
and carbaryl from bean leaves and kale. J.A.O.A.C.
54: 953-958
