
PROPOXUR JMPR 1973
IDENTITY
Chemical name
2-isopropoxy-phenyl-N-methyl carbamate
Synonyms
PHC (common name in Japan)
Unden(R) (mainly for agricultural uses)
Baygon(R), Blattanex(R)
Bay 39007
OMS-33
Bö 58 12 315
Structural formula
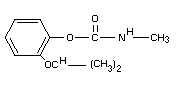 The technical material contains at least 95% propoxur.
Other information on identity and properties
(a) Composition of technical propoxur
Analysis of samples of technical propoxur gave the following
results:
Component %
propoxur min. 95%
O-isopropoxyphenol max. 3% )
)
N,N dimethyl-O-isopropoxy ) together
) max. 4.5%)
phenyl allophanate max. 2% )
1,2-diisopropoxybenzene max. 0.5%
(b) Physical and chemical properties
Physical state: white to cream coloured, crystalline powder
with mild phenolic odour
Molecule weight: 209.2
Melting point: techn. product 86-89°C;
pure propoxur 91.5°C
Vapour pressure: 6.5 x 10-6 mHg at 20°C;
1 x 10-2 mHg at 12°C
Specific gravity: D20 = 1.19
4
Solubility: in water of 20°C approx. 0.2%;
soluble in most polar organic solvents
Stability: stable under normal storage and use
conditions
Hydrolysis rate: propoxur is hydrolyzable in alkaline media;
half life values in aqueous solutions
at 20°C and pH 10.8 - 40 minutes
pH 11.8 - 11.5 minutes
pH 12.8 - 1 minute;
in a 1% aqueous solution at pH 7, it
hydrolyzes at a rate of 1.5% per day
Formulation used: wettable powder 50%;
liquid (EC) 20% w/w, gravity d204
approx. 1.09;
dust 1 and 2%;
fly and cockroach baits 1 and 2%;
balls against flies 50 mg propoxur/ball
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Radio-labelled propoxur was orally administered to rats at
dosages of 5-8 mg/kg. Rats eliminated 85% of the administered
radio-activity within 16 hours. Approximately 257 of the administered
dose was obtained as volatile compounds (CO2 : acetone; 85: 15) with
60% being found in the urine. Very small quantities of radio-activity
were observed in faeces, indicating that rapid absorption was
occurring. From this study, it is evident that propoxur is rapidly
absorbed and excreted in rats following acute administration (Everett
and Gronberg, 1970).
Biotransformation
The metabolic fate of propoxur has been studied in vivo in
mammals, plants and insects and in vitro using preparations from
various biological sources as well as using artificial systems to
examine the non-biological and environmental fate (Dorough and Casida,
1964; Oonnithan and Casida, 1966; Abdel-Wahab et al., 1966; Krishna
and Casida, 1966; Dorough et al., 1963; Kuhr and Casida, 1967;
Oonnithan and Casida, 1966, 1968; Tsukamoto and Casida, 1967a and b;
Casida et al., 1968; Balba and Casida, 1968; Shrivastava et al., 1969;
Kuhr, 1968, 1970; Everett and Gronberg, 1968; Gronberg, 1970; Metcalf
and Fukuto, 1965; Metcalf et al., 1967; Everett and Gronberg, 1971).
Incubation of propoxur with rat liver microsomes fortified with
various cofactors resulted in the formation of 2 hydroxyphenyl
methylcarbamate, 5 hydroxy propoxur, N-hydroxymethyl propoxur and
2-isopropoxyphenol. In vitro, in the presence of UDP-glucuronic
acid, these products would be conjugated. In vivo these same
metabolites were observed with an additional compound being
hydroxylated at the 2 carbon of the isopropoxy group. The major routes
of metabolism are depropylation to 2-hydroxyphenol-N-methylcarbamate
and hydrolysis to yield isopropoxy phenol. The minor routes of
metabolism are ring hydroxylation at the 5 or 6 position with
secondary hydroxylation at the 2 carbon of the allphatic sidechain and
n-methyl hydroxylation of the carbamate. The metabolites identified in
the rat appear to be the same as those found in plants, insects and
derived from in vitro systems. In vitro studies on the
photodecomposition of propoxur in solution resulted in the observation
that, of six N-methyl carbamates, propoxur was the only compound to be
completely stable under the conditions imposed (Crosby et al., 1965).
When exposed to light on a solid surface, propoxur was found to be
slightly degraded to two unidentified organic soluble materials
(Abdel-Wahab at al., 1966).
Effects on enzymes and other biochemical parameters
Propoxur is a biologically active material because of its
structural complimentarity to the active site of neptyl
cholinesterase. As a cholinesterase inhibitor, propoxur behaves as a
synthetic neurohormone that produces its toxic action by interrupting
the normal action of acetyl cholinesterase so that the substrata
acetylcholine accumulates at synaptic junctions. In vivo the signs
of poisoning are manifested by irritability. tremors, incoordination,
convulsions, paralysis and death. While several other carbamate
insecticides (carbaryl, Landrin, and others) produce a transient
anaesthetic effect following high dose administration, there is no
such effect noted with propoxur (Vandekar et al., 1971). In vivo and
in vitro studies have shown propoxur to be a potent inhibitor of
various types of cholinesterase. The I50 (inhibitor concentration
illiciting 50% inhibition) or the bimolecular rate constant
(LM-1min-1) can be seen in the following table.
The specificity of propoxur for true cholinesterase as evident by
its specificity towards bovine and RBC rather than for plasma or serum
cholinesterase is evident from the table. This selectivity was also
noted in studies on the effect of propoxur on man (see section,
Observations in man).
Vandekar et al. (1971) observed that depression of cholinesterase
activity in blood was determinable only during the short period of
time in which signs of poisoning occur. Following acute administration
of propoxur, it was observed that there was a correlation between the
severity of the signs of poisoning and the degree of depression of
erythrocyte cholinesterase. Signs of poisoning appeared initially when
the activity of the cholinesterase dropped below 50% of normal.
Following oral administration of propoxur to rats at a dose
approximately LD50, blood and brain cholinesterase activity was found
to be decreased by about 50% according to a spectrophotometric method
of analysis; however, the determination of cholinesterase activity by
an electrometric method or by a colorimetric method did not show this
inhibition.
As with other carbamate esters, propoxur does not appear to
exhibit any offset on other enzymes or other biochemical parameters.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female per group) were fed
propoxur in the diet at levels of 0, 250, 750, 2000 and 6000 ppm in a
standard three-generation, two-litter/generation reproduction study.
The two highest dietary levels affected the wellbeing of the parent
generation which resulted in a reduction in lactation and further
reduced the pup weight and the parental rate of growth. In addition,
there were effects at 6000 ppm which included smaller litter size. At
the conclusion of the study, examination of various tissues from the
pups of the F3B generation fed 2000 ppm and above showed that there
was a general reduction in the organ weights at three weeks of age.
However, the organ to body weight ratio of these animals corresponded
to that seen with the control, indicating as observed above that the
presence of 2000 ppm in the diet resulted in restricted growth in the
pups. Malformations were not observed in the histological examination
of selected tissues, including those that were noted under gross
examination to be small. Microscopic pathology showed no signs of
alterations attributable to the administration of propoxur. The
dietary concentration of 750 ppm and below did not effect fertility,
litter size and lactation (Loser, 1968a; Mawdesley-Thomas, 1969a).
CHOLINESTERASE INHIBITION
Enzyme I50 Ki Referencea
source (M) (LM-1min-1)
Bovine 7 x 10-7 Vandekar et al., 1971
0.5 - 1.0 x 105 (O'Brien, 1966;
(Reiner and Aldridge, 1967
Fly 0.1 - 8 x 10-8 (Weiden, 1971;
(Metcalf, 1971
1 x 105 Metcalf, 1971
Human serum 0.95 x 103 Metcalf, 1971
Haman plasma 2.3 x 10-5 Wilhelm, 1967
Human RBC 4.6 x 10-7 Wilhelm, 1967
a All values were referenced from various sources, either reviewed or presented as
original data in Bull. Wld Hlth Org., 1971, 44 (1,2,3), 1-470.
Special studies on mutagenicity
A "dominant-lethal study" was conducted on groups of 12 male mice
where the mice were administered a single i.p. dose of propoxur at
levels of 0, 2.5 or 5.0 mg/kg. For a period of six weeks, three virgin
females were exposed to each male at weekly intervals and reproduction
indices recorded. Males treated with 5 mg/kg had slightly lessened
physical activity which lasted one to two days following the treatment
and was reflected in the reduced number of implantations noted in the
treated groups with the females fertilized in the first week after
treatment. The acute administration of propoxur proved to have a
transient effect on the reproduction capability of the males, but
there was no evidence of early resorption which is indicative of an
absence of mutagenic defect (Arnold et al., 1971).
Special studies on neurotoxicity
Adult white leghorn hens wore orally administered proPoxur at
levels ranging from 100 to 1000 mg/kg in a single oral dose or as a
single i.p. dose at levels ranging from 25 to 100 mg/kg. In two of the
three trials, PAM (100 mg/kg) and atropine sulfate (50 mg/kg) were
injected intraperitoneally prior to treatment. No neurotoxic signs of
poisoning similar to that observed with TOCP were noted during periods
of up to six weeks of observation after treatment (Kimmerle, 1964,
1966a).
Groups of eight adult white leghorn hens were fed propoxur in the
diet at levels of 0, 300, 1500, 3000 and 4500 ppm for 30 days. No
evidence of delayed neurotoxic signs of poisoning was seen either
during the period of feeding or in a posttreatment observation period
of four weeks. Histological examination of sciatic nerve and spinal
cord showed no evidence of demyelination (Kimmerle, 1966a; Hobik,
1967).
Special studies on potentiation
Intraperitoneal administration of propoxur to adult female rats
in combination with 20 different anticholinesterase insecticides (19
organophosphates and one carbamate) administered at a dose level of
one-half of the LD50 did not result in an increase in the acute
toxicity (DuBois and Raymund, 1961b and c; Nelson, 1967). Propoxur
does not appear to potentiate antiesterase activity and, as it is a
weak inhibitor of pseudocholinesterase (serum cholinesterase Ki = 9.5
x 102 Lmol-1 min-1) it is doubtful that aliesterase inhibition, a
better indicator of potentiation, would be significantly reduced.
Special studies on teratogenicity
Groups of 10 pregnant rats were fed propoxur in the diet at
concentrations of 0, 1000, 3000 and 10 000 ppm during gestation.
Administration of 3000 ppm and above had an adverse effect on the
parents. At 10 000 ppm, there was a reduction in the number of fetuses
and an increase in the number of resorption sites. This was not
evident at 3000 ppm. There appeared to be a dose-dependent
relationship between the reduction in fetal weight (although only the
3000 and 10 000 ppm were statistically significant) accompanied by a
dose-dependent decrease in placental weight. The dietary concentration
of 1000 ppm was tolerated by the parents and the fetuses and, except
for a slight reduction in the average fetal weight, this level showed
no detrimental effects. Teratogenic abnormalities were not noted in
this study at any dosage level (Lorke, 1970).
Acute toxicity
(a) Original compound
Species Sex Route LD50 Reference
(Mg/kg)
Rat M & F oral 80-191 Ben Dyke et al., 1970;
DuBois and Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961, 1966b, 1971;
Klimmer, 1963
(cont'd)
Species Sex Route LD50 Reference
(Mg/kg)
Guinea-pig M oral 40 DuBois and Raymund, 1961a
Chicken F oral 150-750 DuBois, 1962;
Kimmerle, 1964
Rat M & F i.p. 10-30 DuBois and Raymund, 1961a;
Kimmerle, 1961;
Klimmer, 1963;
Nelson, 1967
Guinea-pig M i.p. 16 DuBois and Raymund, 1961a
Mouse M & F i.p. 14-20 DuBois and Raymund, 1961a
Rat M & F dermal 1000->2400 Ben Dyke et al., 1970;
DuBois and-Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961;
Klimmer, 1963
Rat i.v. 10.6 Vandekar, 1965
Rat i.m. 53 Vandekar, 1965
b) Metabolites
Species Sex Route LD50 Reference
(mg/kg)
2-bydroxyphenyl
N-methylcarbamate
Mice i.p. >167 Balba and Casida, 1968
5-hydroxy propoxur
Mice i.p. >56 Balba and Casida, 1968
4-hydroxy propoxur
Mice i.p. 52 Balba and Casida, 1968
Propoxur
Mice i.p. 12 Balba and Casida, 1968
O-isopropoxyphenol
Rat F oral >1000 DuBois, 1963
dermal
The signs of poisoning shown by propoxur are typical of those
induced by cholinesterase-inhibiting carbamate esters. Tremors, muscle
spasm, lacrimation, salivation and secretion of red tears were
observed in rats following acute dosing. The symptoms appeared rapidly
after administration and recovery was fast.
Following oral administration of propoxur to rats, i.p.
administration of atropine sulfate was found to be antidotal, while
treatment with oximes (PAM, 50 mg/kg and BH6, 20 mg/kg) afforded no
protection and were contraindicated (Kimmerle, 1961b).
Tetraethylammoniumchloride proved also to afford no protection from
the acute effects of propoxur (Kimmerle, 1971).
Subacute dermal toxicity
Groups of rabbits (five males and five females per group) were
treated dermally with propoxur at a level of 500 mg/kg for two weeks.
Residues of the material remaining on the skin prior to each
application were not washed off. Twenty-four hours after the final
application., the skin was washed with soap and water and the animals
observed for two further weeks. The treatment did not affect the
general behaviour and weight gain of the animal and clinical
examinations of blood, urine, liver and kidney function over the
two-week period were normal (Kimmerle and Solmeeke, 1971). Propoxur
was found to be nonirritating to the skin of rabbits when applied to
the inside of a rabbit's ear for 24 hours. No signs of irritation or
poisoning were observed when propoxur was applied to the shaved
abdominal skin of rats and allowed to remain for four hours (Kimmerle,
1961).
Short-term studies
Rat. Groups of rats (15 males and 15 females per group) were fed
propoxur in the diet at levels of 0, 1000, 2000, 4000 and 8000 ppm
(females were fed only dietary levels of 0 and 4000 ppm) for nine
weeks. There was an increase in mortality in the animals fed 4000 and
8000 ppm and a reduction in food consumption and weight gain was
observed in all animals (Löser, 1965).
Oral administration of propoxur to 25 male rats at a dose of 5
mg/kg/day, six days a week for six months (20 male rats served as
controls) resulted in no effects attributable to the administration of
the compound. Growth and food consumption were similar to the
controls, and gross and histological examination of tissues showed no
effects attributable to the administration of propoxur (Klimmer,
1963).
Groups of rats (12 males and 12 females per group) were fed
propoxur in the diet for 16 weeks at concentrations of 0, 5, 10, 50,
100 and 200 ppm (those animals fed 100 and 200 ppm were increased
after the first three weeks of feeding to 400 and 800 ppm respectively
for the remainder of the study). The administration of propoxur at
levels up to 800 ppm in the diet did not affect food consumption,
growth, mortality or gross and histological examination of tissues.
Cholinesterase activity, measured manometrically in the blood, brain
and submaxillary glands, was not affected (Root et al., 1963).
Rats were treated orally for four weeks at 0, 3, 10 and 30 mg/kg.
The highest level caused signs of poisoning and cholinesterase
depression was noted at 10 and 30 mg/kg, with no depression noted at
the low level (Eben and Kimmerle, 1973).
Groups of rats were fed propoxur in the diet at 0, 250, 750 and
2000 ppm for 15 weeks. Propoxur was tolerated with no signs of
poisoning and no consistent evidence of cholinesterase depression
(Eben and Kimmerle, 1973).
Groups of rats (10 males and 10 females per group) were fed
propoxur in the diet at concentrations of 0, 250, 500, 1000 and 2000
ppm for 16 weeks. Food consumption and body weight gain in the females
receiving concentrations of 1000 ppm and above were reduced. No
significant changes were noted with regard to organ weight data. The
1000 and 2000 ppm levels caused depression of cholinesterase activity
in whole blood after the twelfth week of testing and, on completion of
the feeding study, cholinesterase activity was depressed in plasma,
whole blood and brain. Although all clinical enzyme data were found to
be within the normal range, some histopathological changes were noted
in the livers of the animals which received 1000 and 2000 ppm. The
no-effect level in this study was judged to be 500 ppm (Syrowatka et
al., 1971).
Dog. Groups of beagle dogs (four males and four females per group)
were fed propoxur in the diet at concentrations of 0, 100, 250, 750
and 2000 ppm for two years. Mortality was evident at 2000 ppm. One of
the male dogs and none of the female dogs at this level survived to
the end of two years. Food consumption was reduced at this high level.
The animals receiving 2000 ppm at times exhibited signs of
cholinesterase depression, especially in the first six months of
testing. Dietary concentrations of 750 ppm and below did not affect
appearance, behaviour, food consumption or growth of the animals.
Haematological examinations and liver and kidney function tests showed
no effects of propoxur at any dosage level examined. Activity of
leucine-amino peptidase was slightly elevated at levels of 750 and
2000 ppm. Gross examination of tissues showed that there was a
slightly increased liver to body weight ratio in males at 2000 ppm.
There was no indication of cellular damage in any tissue as evidenced
by histological examination of tissues. Although there was a slight
increase in leucine-amino peptidase activity at 750 ppm, based upon
all other considerations, it was assumed that a level of 750 ppm is a
no-effect level. Based on food consumption data, the no-effect level
in dogs would be 50 mg/kg bw/day (Löser, 1968c; Mawdesley-Thomas,
1969c).
Long-term studies
Groups of SPF rats (25 males and 25 females per group, 50 males
and 50 females per control group) were fed propoxur in the diet at
levels of 0, 250, 750, 2000 and 6000 ppm for two years. Dietary
concentration of 6000 ppm caused a reduction in food consumption in
male rats, while 2000 ppm and above resulted in a similar effect in
females. This reduction in food intake was reflected in body weight
gains Of these two groups. On gross examination, at the end of two
years some slight effects were noted in some organs. especially liver
which was enlarged relative to the body weight at the highest feeding
level in male rats and at the highest three feeding levels in female
rats. This increased liver weight was not reflected in liver function
tests or in clinical chemistry examination. Cholinesterase examination
in whole blood (performed only at six months) showed no depression of
cholinesterase activity at 6000 ppm in males and females. Histological
examination of tissues showed no effects relating to the feeding of
propoxur. A no-effect level in this study, based upon increased
relative liver to body weight ratio, is 250 ppm (Loser, 1968b;
Mawdesley-Thomas, 1969b).
Observations in man
Because of the widespread experimental views of propoxur and
control through the auspices of WHO, some significant observations in
humans are available (Plestina, 1968; Dawson, 1964; Vandekar, 1969;
Vandekar et al., 1968, 1971). In a study undertaken to develop a
quantitative method for determining metabolites of propoxur. Dawson et
al. (1964) showed that oral administration of 110 and 116 mg/person
Produced no signs of illness. The level of urinary phenols reached 140
ppm in the absence of clinical signs of poisoning. In persons engaged
in spraying or other occupation exposure, urinary levels of 80 ppm are
uniformly associated with illness. In another study, Vandekar et al.
(1971) administered 135 mg/person to a male volunteer (1.5 mg/kg bw)
and within 20 minutes after ingestion described clinical signs of
poisoning due to the carbamate. Significant erythrocyte cholinesterase
depression was evident coinciding with clinical signs of poisoning,
while plasma cholinesterase depression was not observed. Two hours
after the ingestion of propoxur, there were no signs of poisoning and
the rapid disappearance of symptoms was consistent with the rapid
recovery of erythrocyte cholinesterase activity. Absorption and
excretion of propoxur was very rapid as evidenced by measurement of
urinary phenols which reached a maximum value within four hours of
almost 200 ppm. Of the total phenol content excreted, 81% was found
within five hours after administration. In another experiment, a
single dose of 0.36 mg/kg again produced a rapid fall of erythrocyte
cholinesterase to 57% of normal within 10 minutes. Cholinesterase
recovered to its normal value within three hours. Within 10 minutes of
the administration of propoxur, short-lasting stomach discomfort,
blurred vision, and moderate facial redness and sweating were evident
in the volunteer.
A number of experiments was carried out to study the effect of
storage or build-up of propoxur in the body. Human volunteers took
doses of either 0.15 or 0.2 mg/kg at half-hour intervals for a total
of five doses. In each subject a symptomless depression of erythrocyte
cholinesterase to about 60% of normal was observed. At the cessation
of dosing, enzyme recovery was rapid, being complete within two hours.
Similarly pronounced and as a rule symptomless daily depression and
reactivation of cholinesterase was observed in persons who are
occupationally exposed to propoxur. Studies in humans have shown that
depression of erythrocyte cholinesterase (rather than plasma
cholinesterase) is a significant indicator of exposure to propoxur.
This is consistent with the difference observed in the in vitro
affinity of propoxur for the two enzymes, the I50 values for
erythrocyte and plasma cholinesterase being 4.6 x 10-7 M and 2.3 x
10-5 M, respectively.
Vandekar et al. (1968) published the results of studies carried
out on spray operators and local inhabitants in Iran as part of a WHO
control programme. It was reported that following over-exposure some
spray operators and local inhabitants suffered mild temporary
cholinergic signs of poisoning (headache, nausea). In most of the
cases, the complaints were found to be due to heavy contamination of
propoxur on the skin.
It is evident from these studies that a single oral dose (between
0.2 and 0.4 mg/kg) of propoxur may produce symptoms in man of short
duration. Higher doses may be tolerated without evidence of poisoning
(although there is appreciable inhibition of erythrocyte
cholinesterase) if the higher doses are divided into portions and
administered over reasonably short periods of time. Within two hours
following exposure, cholinesterase depression would be expected to be
normal.
Comments
Propoxur, an anticholinesterase carbamate ester, induces typical
signs of cholinesterase inhibition in both laboratory animals and
humans. Reversible depression of cholinesterase activity is evident a
short time after exposure, although the sensitivity of various
cholinesterase sources differ in different animal species. Erythrocyte
cholinesterase is significantly more sensitive than plasma
cholinesterase in humans. The sensitivity of brain and plasma
cholinesterase appears to be of the same magnitude in rats. Although
0.36 mg/kg resulted in signs of acute poisoning in man, the repeated
administration of 0.2 mg/kg at half-hour intervals for 2.5 hours
resulted in no signs of poisoning, although cholinesterase activity
was depressed. This cholinesterase returned to normal within two hours
following exposure.
Propoxur is rapidly absorbed, metabolized and eliminated.
Teratogenicity and mutagenicity studies in the rat gave negative
results and reproduction was not affected by propoxur.
A long-term rat study provided no evidence of carcinogenic
activity. Liver weight was increased in both males and females at high
dosage levels. No changes were found in liver function tests, clinical
chemistry or on histological examination. In view of the histological
changes in the livers of rats exposed over a short period to 1000 ppm,
the increase in relative liver weight was considered a significant
effect. 250 ppm in the diet was accepted as the no-effect level in the
rat.
In a two-year dog study, a slight increase in leucine-amino
peptidase activity was not regarded as significant. The no-effect
level as evidenced by liver damage was 750 ppm in the diet which,
based on feed consumption data, was 50 mg/kg bw. Cholinesterase
depression was not observed in either the two-year rat or dog studies.
It was evident to the Meeting that the methodology used to determine
cholinesterase activity in these studies was not adequate to measure
depression caused by propoxur.
The no-effect level in the long-term study in the rat was used as
a basis for estimation of the ADI.
The rapid reversibility of acute signs of poisoning in man and
the fact that sensitivity to the toxic effects of propoxur decreased
during prolonged exposure was reassuring in estimating the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolic aspects
The metabolism of propoxur was studied in mammals by Dawson et
al. (1964), Everett and Gronberg (1970), Krishna and Casida (1966),
Waggoner and Olson (1971); in man by Dawson et al. (1964), Hayes
(1971); in insects by Metcalf et al. (1967), Shrivastava (1969); in
plants by Aba el Wahab (1966), Dorough and Casida (1964), Everett and
Gronberg (1968), Gronberg (1970), Kuhr and Casida (1967).
In vitro in animals studies were made by Crosby et al. (1965),
Dorough and Casida (1964) and Oonithan and Casida (1966, 1968).
In rats
The metabolites identified in the rat (see below) include those
found in plants, in insects and those derived from microsomes.
Whereas the oxidative pathways and hydrolytic degradation occur
in the same order of magnitude in mammals, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
Adsorption, distribution and excretion in mammals, including
biotransformation in rats: following oral administration in rats,
propoxur is rapidly ingested to the digestive tract, metabolized in
the body and excreted.
The rats eliminated 85% of the radio-activity after oral
administration of 14C carbonyl labelled, 14C isopropoxy labelled 3H
isopropyl labelled propoxur within 16 hours; 60% of this and amount
was excreted in the urine as conjugates and 20-25% as volatile
compounds in a C02/acetone ratio of 85:15. Only 1-5% of the activity
was found in the faeces in the same period (Everett and Gronberg,
1970).
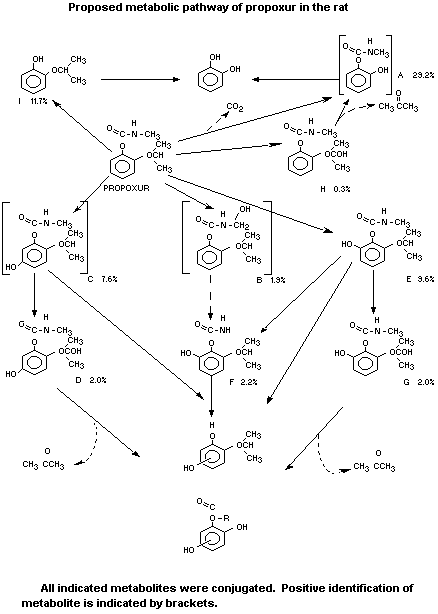
Evidence was obtained by the same authors that the major routes
of metabolism in rats are depropylation to
2-hydroxyphenyl-N-Methylcarbamate (further indicated as metabolite
A) and subsequent hydrolysis to isopropoxyphenol (metabolite I).
Minor metabolic pathways are ring hydroxylation at the 5 or 6
position (C and E), secondary hydroxylation of the 2-carbon atom of
the isopropoxy group (D and G) and hydroxylation of the N-methyl
group.
From the conjugated compounds in the urine, the following
metabolites were released by hydrolysis of the urine sample with
glucuronidase and/or acid. They were identified by their infra-red and
mass spectra as well as from the 3H/14C ratio yielded of double
labelled propoxur: 2-hydrophenyl-N-methylcarbamate (A),
2-isopropoxyphenyl-N-hydroxy methylearbamate (B) and
2-isopropoxy-5-hydroxyphenyl-N-methylearbamate (C).
The metabolic pathways of propoxur in rats as proposed by Everett
and Gronberg (1970) is shown in the diagram on the following page.
The technical material contains at least 95% propoxur.
Other information on identity and properties
(a) Composition of technical propoxur
Analysis of samples of technical propoxur gave the following
results:
Component %
propoxur min. 95%
O-isopropoxyphenol max. 3% )
)
N,N dimethyl-O-isopropoxy ) together
) max. 4.5%)
phenyl allophanate max. 2% )
1,2-diisopropoxybenzene max. 0.5%
(b) Physical and chemical properties
Physical state: white to cream coloured, crystalline powder
with mild phenolic odour
Molecule weight: 209.2
Melting point: techn. product 86-89°C;
pure propoxur 91.5°C
Vapour pressure: 6.5 x 10-6 mHg at 20°C;
1 x 10-2 mHg at 12°C
Specific gravity: D20 = 1.19
4
Solubility: in water of 20°C approx. 0.2%;
soluble in most polar organic solvents
Stability: stable under normal storage and use
conditions
Hydrolysis rate: propoxur is hydrolyzable in alkaline media;
half life values in aqueous solutions
at 20°C and pH 10.8 - 40 minutes
pH 11.8 - 11.5 minutes
pH 12.8 - 1 minute;
in a 1% aqueous solution at pH 7, it
hydrolyzes at a rate of 1.5% per day
Formulation used: wettable powder 50%;
liquid (EC) 20% w/w, gravity d204
approx. 1.09;
dust 1 and 2%;
fly and cockroach baits 1 and 2%;
balls against flies 50 mg propoxur/ball
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Radio-labelled propoxur was orally administered to rats at
dosages of 5-8 mg/kg. Rats eliminated 85% of the administered
radio-activity within 16 hours. Approximately 257 of the administered
dose was obtained as volatile compounds (CO2 : acetone; 85: 15) with
60% being found in the urine. Very small quantities of radio-activity
were observed in faeces, indicating that rapid absorption was
occurring. From this study, it is evident that propoxur is rapidly
absorbed and excreted in rats following acute administration (Everett
and Gronberg, 1970).
Biotransformation
The metabolic fate of propoxur has been studied in vivo in
mammals, plants and insects and in vitro using preparations from
various biological sources as well as using artificial systems to
examine the non-biological and environmental fate (Dorough and Casida,
1964; Oonnithan and Casida, 1966; Abdel-Wahab et al., 1966; Krishna
and Casida, 1966; Dorough et al., 1963; Kuhr and Casida, 1967;
Oonnithan and Casida, 1966, 1968; Tsukamoto and Casida, 1967a and b;
Casida et al., 1968; Balba and Casida, 1968; Shrivastava et al., 1969;
Kuhr, 1968, 1970; Everett and Gronberg, 1968; Gronberg, 1970; Metcalf
and Fukuto, 1965; Metcalf et al., 1967; Everett and Gronberg, 1971).
Incubation of propoxur with rat liver microsomes fortified with
various cofactors resulted in the formation of 2 hydroxyphenyl
methylcarbamate, 5 hydroxy propoxur, N-hydroxymethyl propoxur and
2-isopropoxyphenol. In vitro, in the presence of UDP-glucuronic
acid, these products would be conjugated. In vivo these same
metabolites were observed with an additional compound being
hydroxylated at the 2 carbon of the isopropoxy group. The major routes
of metabolism are depropylation to 2-hydroxyphenol-N-methylcarbamate
and hydrolysis to yield isopropoxy phenol. The minor routes of
metabolism are ring hydroxylation at the 5 or 6 position with
secondary hydroxylation at the 2 carbon of the allphatic sidechain and
n-methyl hydroxylation of the carbamate. The metabolites identified in
the rat appear to be the same as those found in plants, insects and
derived from in vitro systems. In vitro studies on the
photodecomposition of propoxur in solution resulted in the observation
that, of six N-methyl carbamates, propoxur was the only compound to be
completely stable under the conditions imposed (Crosby et al., 1965).
When exposed to light on a solid surface, propoxur was found to be
slightly degraded to two unidentified organic soluble materials
(Abdel-Wahab at al., 1966).
Effects on enzymes and other biochemical parameters
Propoxur is a biologically active material because of its
structural complimentarity to the active site of neptyl
cholinesterase. As a cholinesterase inhibitor, propoxur behaves as a
synthetic neurohormone that produces its toxic action by interrupting
the normal action of acetyl cholinesterase so that the substrata
acetylcholine accumulates at synaptic junctions. In vivo the signs
of poisoning are manifested by irritability. tremors, incoordination,
convulsions, paralysis and death. While several other carbamate
insecticides (carbaryl, Landrin, and others) produce a transient
anaesthetic effect following high dose administration, there is no
such effect noted with propoxur (Vandekar et al., 1971). In vivo and
in vitro studies have shown propoxur to be a potent inhibitor of
various types of cholinesterase. The I50 (inhibitor concentration
illiciting 50% inhibition) or the bimolecular rate constant
(LM-1min-1) can be seen in the following table.
The specificity of propoxur for true cholinesterase as evident by
its specificity towards bovine and RBC rather than for plasma or serum
cholinesterase is evident from the table. This selectivity was also
noted in studies on the effect of propoxur on man (see section,
Observations in man).
Vandekar et al. (1971) observed that depression of cholinesterase
activity in blood was determinable only during the short period of
time in which signs of poisoning occur. Following acute administration
of propoxur, it was observed that there was a correlation between the
severity of the signs of poisoning and the degree of depression of
erythrocyte cholinesterase. Signs of poisoning appeared initially when
the activity of the cholinesterase dropped below 50% of normal.
Following oral administration of propoxur to rats at a dose
approximately LD50, blood and brain cholinesterase activity was found
to be decreased by about 50% according to a spectrophotometric method
of analysis; however, the determination of cholinesterase activity by
an electrometric method or by a colorimetric method did not show this
inhibition.
As with other carbamate esters, propoxur does not appear to
exhibit any offset on other enzymes or other biochemical parameters.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female per group) were fed
propoxur in the diet at levels of 0, 250, 750, 2000 and 6000 ppm in a
standard three-generation, two-litter/generation reproduction study.
The two highest dietary levels affected the wellbeing of the parent
generation which resulted in a reduction in lactation and further
reduced the pup weight and the parental rate of growth. In addition,
there were effects at 6000 ppm which included smaller litter size. At
the conclusion of the study, examination of various tissues from the
pups of the F3B generation fed 2000 ppm and above showed that there
was a general reduction in the organ weights at three weeks of age.
However, the organ to body weight ratio of these animals corresponded
to that seen with the control, indicating as observed above that the
presence of 2000 ppm in the diet resulted in restricted growth in the
pups. Malformations were not observed in the histological examination
of selected tissues, including those that were noted under gross
examination to be small. Microscopic pathology showed no signs of
alterations attributable to the administration of propoxur. The
dietary concentration of 750 ppm and below did not effect fertility,
litter size and lactation (Loser, 1968a; Mawdesley-Thomas, 1969a).
CHOLINESTERASE INHIBITION
Enzyme I50 Ki Referencea
source (M) (LM-1min-1)
Bovine 7 x 10-7 Vandekar et al., 1971
0.5 - 1.0 x 105 (O'Brien, 1966;
(Reiner and Aldridge, 1967
Fly 0.1 - 8 x 10-8 (Weiden, 1971;
(Metcalf, 1971
1 x 105 Metcalf, 1971
Human serum 0.95 x 103 Metcalf, 1971
Haman plasma 2.3 x 10-5 Wilhelm, 1967
Human RBC 4.6 x 10-7 Wilhelm, 1967
a All values were referenced from various sources, either reviewed or presented as
original data in Bull. Wld Hlth Org., 1971, 44 (1,2,3), 1-470.
Special studies on mutagenicity
A "dominant-lethal study" was conducted on groups of 12 male mice
where the mice were administered a single i.p. dose of propoxur at
levels of 0, 2.5 or 5.0 mg/kg. For a period of six weeks, three virgin
females were exposed to each male at weekly intervals and reproduction
indices recorded. Males treated with 5 mg/kg had slightly lessened
physical activity which lasted one to two days following the treatment
and was reflected in the reduced number of implantations noted in the
treated groups with the females fertilized in the first week after
treatment. The acute administration of propoxur proved to have a
transient effect on the reproduction capability of the males, but
there was no evidence of early resorption which is indicative of an
absence of mutagenic defect (Arnold et al., 1971).
Special studies on neurotoxicity
Adult white leghorn hens wore orally administered proPoxur at
levels ranging from 100 to 1000 mg/kg in a single oral dose or as a
single i.p. dose at levels ranging from 25 to 100 mg/kg. In two of the
three trials, PAM (100 mg/kg) and atropine sulfate (50 mg/kg) were
injected intraperitoneally prior to treatment. No neurotoxic signs of
poisoning similar to that observed with TOCP were noted during periods
of up to six weeks of observation after treatment (Kimmerle, 1964,
1966a).
Groups of eight adult white leghorn hens were fed propoxur in the
diet at levels of 0, 300, 1500, 3000 and 4500 ppm for 30 days. No
evidence of delayed neurotoxic signs of poisoning was seen either
during the period of feeding or in a posttreatment observation period
of four weeks. Histological examination of sciatic nerve and spinal
cord showed no evidence of demyelination (Kimmerle, 1966a; Hobik,
1967).
Special studies on potentiation
Intraperitoneal administration of propoxur to adult female rats
in combination with 20 different anticholinesterase insecticides (19
organophosphates and one carbamate) administered at a dose level of
one-half of the LD50 did not result in an increase in the acute
toxicity (DuBois and Raymund, 1961b and c; Nelson, 1967). Propoxur
does not appear to potentiate antiesterase activity and, as it is a
weak inhibitor of pseudocholinesterase (serum cholinesterase Ki = 9.5
x 102 Lmol-1 min-1) it is doubtful that aliesterase inhibition, a
better indicator of potentiation, would be significantly reduced.
Special studies on teratogenicity
Groups of 10 pregnant rats were fed propoxur in the diet at
concentrations of 0, 1000, 3000 and 10 000 ppm during gestation.
Administration of 3000 ppm and above had an adverse effect on the
parents. At 10 000 ppm, there was a reduction in the number of fetuses
and an increase in the number of resorption sites. This was not
evident at 3000 ppm. There appeared to be a dose-dependent
relationship between the reduction in fetal weight (although only the
3000 and 10 000 ppm were statistically significant) accompanied by a
dose-dependent decrease in placental weight. The dietary concentration
of 1000 ppm was tolerated by the parents and the fetuses and, except
for a slight reduction in the average fetal weight, this level showed
no detrimental effects. Teratogenic abnormalities were not noted in
this study at any dosage level (Lorke, 1970).
Acute toxicity
(a) Original compound
Species Sex Route LD50 Reference
(Mg/kg)
Rat M & F oral 80-191 Ben Dyke et al., 1970;
DuBois and Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961, 1966b, 1971;
Klimmer, 1963
(cont'd)
Species Sex Route LD50 Reference
(Mg/kg)
Guinea-pig M oral 40 DuBois and Raymund, 1961a
Chicken F oral 150-750 DuBois, 1962;
Kimmerle, 1964
Rat M & F i.p. 10-30 DuBois and Raymund, 1961a;
Kimmerle, 1961;
Klimmer, 1963;
Nelson, 1967
Guinea-pig M i.p. 16 DuBois and Raymund, 1961a
Mouse M & F i.p. 14-20 DuBois and Raymund, 1961a
Rat M & F dermal 1000->2400 Ben Dyke et al., 1970;
DuBois and-Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961;
Klimmer, 1963
Rat i.v. 10.6 Vandekar, 1965
Rat i.m. 53 Vandekar, 1965
b) Metabolites
Species Sex Route LD50 Reference
(mg/kg)
2-bydroxyphenyl
N-methylcarbamate
Mice i.p. >167 Balba and Casida, 1968
5-hydroxy propoxur
Mice i.p. >56 Balba and Casida, 1968
4-hydroxy propoxur
Mice i.p. 52 Balba and Casida, 1968
Propoxur
Mice i.p. 12 Balba and Casida, 1968
O-isopropoxyphenol
Rat F oral >1000 DuBois, 1963
dermal
The signs of poisoning shown by propoxur are typical of those
induced by cholinesterase-inhibiting carbamate esters. Tremors, muscle
spasm, lacrimation, salivation and secretion of red tears were
observed in rats following acute dosing. The symptoms appeared rapidly
after administration and recovery was fast.
Following oral administration of propoxur to rats, i.p.
administration of atropine sulfate was found to be antidotal, while
treatment with oximes (PAM, 50 mg/kg and BH6, 20 mg/kg) afforded no
protection and were contraindicated (Kimmerle, 1961b).
Tetraethylammoniumchloride proved also to afford no protection from
the acute effects of propoxur (Kimmerle, 1971).
Subacute dermal toxicity
Groups of rabbits (five males and five females per group) were
treated dermally with propoxur at a level of 500 mg/kg for two weeks.
Residues of the material remaining on the skin prior to each
application were not washed off. Twenty-four hours after the final
application., the skin was washed with soap and water and the animals
observed for two further weeks. The treatment did not affect the
general behaviour and weight gain of the animal and clinical
examinations of blood, urine, liver and kidney function over the
two-week period were normal (Kimmerle and Solmeeke, 1971). Propoxur
was found to be nonirritating to the skin of rabbits when applied to
the inside of a rabbit's ear for 24 hours. No signs of irritation or
poisoning were observed when propoxur was applied to the shaved
abdominal skin of rats and allowed to remain for four hours (Kimmerle,
1961).
Short-term studies
Rat. Groups of rats (15 males and 15 females per group) were fed
propoxur in the diet at levels of 0, 1000, 2000, 4000 and 8000 ppm
(females were fed only dietary levels of 0 and 4000 ppm) for nine
weeks. There was an increase in mortality in the animals fed 4000 and
8000 ppm and a reduction in food consumption and weight gain was
observed in all animals (Löser, 1965).
Oral administration of propoxur to 25 male rats at a dose of 5
mg/kg/day, six days a week for six months (20 male rats served as
controls) resulted in no effects attributable to the administration of
the compound. Growth and food consumption were similar to the
controls, and gross and histological examination of tissues showed no
effects attributable to the administration of propoxur (Klimmer,
1963).
Groups of rats (12 males and 12 females per group) were fed
propoxur in the diet for 16 weeks at concentrations of 0, 5, 10, 50,
100 and 200 ppm (those animals fed 100 and 200 ppm were increased
after the first three weeks of feeding to 400 and 800 ppm respectively
for the remainder of the study). The administration of propoxur at
levels up to 800 ppm in the diet did not affect food consumption,
growth, mortality or gross and histological examination of tissues.
Cholinesterase activity, measured manometrically in the blood, brain
and submaxillary glands, was not affected (Root et al., 1963).
Rats were treated orally for four weeks at 0, 3, 10 and 30 mg/kg.
The highest level caused signs of poisoning and cholinesterase
depression was noted at 10 and 30 mg/kg, with no depression noted at
the low level (Eben and Kimmerle, 1973).
Groups of rats were fed propoxur in the diet at 0, 250, 750 and
2000 ppm for 15 weeks. Propoxur was tolerated with no signs of
poisoning and no consistent evidence of cholinesterase depression
(Eben and Kimmerle, 1973).
Groups of rats (10 males and 10 females per group) were fed
propoxur in the diet at concentrations of 0, 250, 500, 1000 and 2000
ppm for 16 weeks. Food consumption and body weight gain in the females
receiving concentrations of 1000 ppm and above were reduced. No
significant changes were noted with regard to organ weight data. The
1000 and 2000 ppm levels caused depression of cholinesterase activity
in whole blood after the twelfth week of testing and, on completion of
the feeding study, cholinesterase activity was depressed in plasma,
whole blood and brain. Although all clinical enzyme data were found to
be within the normal range, some histopathological changes were noted
in the livers of the animals which received 1000 and 2000 ppm. The
no-effect level in this study was judged to be 500 ppm (Syrowatka et
al., 1971).
Dog. Groups of beagle dogs (four males and four females per group)
were fed propoxur in the diet at concentrations of 0, 100, 250, 750
and 2000 ppm for two years. Mortality was evident at 2000 ppm. One of
the male dogs and none of the female dogs at this level survived to
the end of two years. Food consumption was reduced at this high level.
The animals receiving 2000 ppm at times exhibited signs of
cholinesterase depression, especially in the first six months of
testing. Dietary concentrations of 750 ppm and below did not affect
appearance, behaviour, food consumption or growth of the animals.
Haematological examinations and liver and kidney function tests showed
no effects of propoxur at any dosage level examined. Activity of
leucine-amino peptidase was slightly elevated at levels of 750 and
2000 ppm. Gross examination of tissues showed that there was a
slightly increased liver to body weight ratio in males at 2000 ppm.
There was no indication of cellular damage in any tissue as evidenced
by histological examination of tissues. Although there was a slight
increase in leucine-amino peptidase activity at 750 ppm, based upon
all other considerations, it was assumed that a level of 750 ppm is a
no-effect level. Based on food consumption data, the no-effect level
in dogs would be 50 mg/kg bw/day (Löser, 1968c; Mawdesley-Thomas,
1969c).
Long-term studies
Groups of SPF rats (25 males and 25 females per group, 50 males
and 50 females per control group) were fed propoxur in the diet at
levels of 0, 250, 750, 2000 and 6000 ppm for two years. Dietary
concentration of 6000 ppm caused a reduction in food consumption in
male rats, while 2000 ppm and above resulted in a similar effect in
females. This reduction in food intake was reflected in body weight
gains Of these two groups. On gross examination, at the end of two
years some slight effects were noted in some organs. especially liver
which was enlarged relative to the body weight at the highest feeding
level in male rats and at the highest three feeding levels in female
rats. This increased liver weight was not reflected in liver function
tests or in clinical chemistry examination. Cholinesterase examination
in whole blood (performed only at six months) showed no depression of
cholinesterase activity at 6000 ppm in males and females. Histological
examination of tissues showed no effects relating to the feeding of
propoxur. A no-effect level in this study, based upon increased
relative liver to body weight ratio, is 250 ppm (Loser, 1968b;
Mawdesley-Thomas, 1969b).
Observations in man
Because of the widespread experimental views of propoxur and
control through the auspices of WHO, some significant observations in
humans are available (Plestina, 1968; Dawson, 1964; Vandekar, 1969;
Vandekar et al., 1968, 1971). In a study undertaken to develop a
quantitative method for determining metabolites of propoxur. Dawson et
al. (1964) showed that oral administration of 110 and 116 mg/person
Produced no signs of illness. The level of urinary phenols reached 140
ppm in the absence of clinical signs of poisoning. In persons engaged
in spraying or other occupation exposure, urinary levels of 80 ppm are
uniformly associated with illness. In another study, Vandekar et al.
(1971) administered 135 mg/person to a male volunteer (1.5 mg/kg bw)
and within 20 minutes after ingestion described clinical signs of
poisoning due to the carbamate. Significant erythrocyte cholinesterase
depression was evident coinciding with clinical signs of poisoning,
while plasma cholinesterase depression was not observed. Two hours
after the ingestion of propoxur, there were no signs of poisoning and
the rapid disappearance of symptoms was consistent with the rapid
recovery of erythrocyte cholinesterase activity. Absorption and
excretion of propoxur was very rapid as evidenced by measurement of
urinary phenols which reached a maximum value within four hours of
almost 200 ppm. Of the total phenol content excreted, 81% was found
within five hours after administration. In another experiment, a
single dose of 0.36 mg/kg again produced a rapid fall of erythrocyte
cholinesterase to 57% of normal within 10 minutes. Cholinesterase
recovered to its normal value within three hours. Within 10 minutes of
the administration of propoxur, short-lasting stomach discomfort,
blurred vision, and moderate facial redness and sweating were evident
in the volunteer.
A number of experiments was carried out to study the effect of
storage or build-up of propoxur in the body. Human volunteers took
doses of either 0.15 or 0.2 mg/kg at half-hour intervals for a total
of five doses. In each subject a symptomless depression of erythrocyte
cholinesterase to about 60% of normal was observed. At the cessation
of dosing, enzyme recovery was rapid, being complete within two hours.
Similarly pronounced and as a rule symptomless daily depression and
reactivation of cholinesterase was observed in persons who are
occupationally exposed to propoxur. Studies in humans have shown that
depression of erythrocyte cholinesterase (rather than plasma
cholinesterase) is a significant indicator of exposure to propoxur.
This is consistent with the difference observed in the in vitro
affinity of propoxur for the two enzymes, the I50 values for
erythrocyte and plasma cholinesterase being 4.6 x 10-7 M and 2.3 x
10-5 M, respectively.
Vandekar et al. (1968) published the results of studies carried
out on spray operators and local inhabitants in Iran as part of a WHO
control programme. It was reported that following over-exposure some
spray operators and local inhabitants suffered mild temporary
cholinergic signs of poisoning (headache, nausea). In most of the
cases, the complaints were found to be due to heavy contamination of
propoxur on the skin.
It is evident from these studies that a single oral dose (between
0.2 and 0.4 mg/kg) of propoxur may produce symptoms in man of short
duration. Higher doses may be tolerated without evidence of poisoning
(although there is appreciable inhibition of erythrocyte
cholinesterase) if the higher doses are divided into portions and
administered over reasonably short periods of time. Within two hours
following exposure, cholinesterase depression would be expected to be
normal.
Comments
Propoxur, an anticholinesterase carbamate ester, induces typical
signs of cholinesterase inhibition in both laboratory animals and
humans. Reversible depression of cholinesterase activity is evident a
short time after exposure, although the sensitivity of various
cholinesterase sources differ in different animal species. Erythrocyte
cholinesterase is significantly more sensitive than plasma
cholinesterase in humans. The sensitivity of brain and plasma
cholinesterase appears to be of the same magnitude in rats. Although
0.36 mg/kg resulted in signs of acute poisoning in man, the repeated
administration of 0.2 mg/kg at half-hour intervals for 2.5 hours
resulted in no signs of poisoning, although cholinesterase activity
was depressed. This cholinesterase returned to normal within two hours
following exposure.
Propoxur is rapidly absorbed, metabolized and eliminated.
Teratogenicity and mutagenicity studies in the rat gave negative
results and reproduction was not affected by propoxur.
A long-term rat study provided no evidence of carcinogenic
activity. Liver weight was increased in both males and females at high
dosage levels. No changes were found in liver function tests, clinical
chemistry or on histological examination. In view of the histological
changes in the livers of rats exposed over a short period to 1000 ppm,
the increase in relative liver weight was considered a significant
effect. 250 ppm in the diet was accepted as the no-effect level in the
rat.
In a two-year dog study, a slight increase in leucine-amino
peptidase activity was not regarded as significant. The no-effect
level as evidenced by liver damage was 750 ppm in the diet which,
based on feed consumption data, was 50 mg/kg bw. Cholinesterase
depression was not observed in either the two-year rat or dog studies.
It was evident to the Meeting that the methodology used to determine
cholinesterase activity in these studies was not adequate to measure
depression caused by propoxur.
The no-effect level in the long-term study in the rat was used as
a basis for estimation of the ADI.
The rapid reversibility of acute signs of poisoning in man and
the fact that sensitivity to the toxic effects of propoxur decreased
during prolonged exposure was reassuring in estimating the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolic aspects
The metabolism of propoxur was studied in mammals by Dawson et
al. (1964), Everett and Gronberg (1970), Krishna and Casida (1966),
Waggoner and Olson (1971); in man by Dawson et al. (1964), Hayes
(1971); in insects by Metcalf et al. (1967), Shrivastava (1969); in
plants by Aba el Wahab (1966), Dorough and Casida (1964), Everett and
Gronberg (1968), Gronberg (1970), Kuhr and Casida (1967).
In vitro in animals studies were made by Crosby et al. (1965),
Dorough and Casida (1964) and Oonithan and Casida (1966, 1968).
In rats
The metabolites identified in the rat (see below) include those
found in plants, in insects and those derived from microsomes.
Whereas the oxidative pathways and hydrolytic degradation occur
in the same order of magnitude in mammals, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
Adsorption, distribution and excretion in mammals, including
biotransformation in rats: following oral administration in rats,
propoxur is rapidly ingested to the digestive tract, metabolized in
the body and excreted.
The rats eliminated 85% of the radio-activity after oral
administration of 14C carbonyl labelled, 14C isopropoxy labelled 3H
isopropyl labelled propoxur within 16 hours; 60% of this and amount
was excreted in the urine as conjugates and 20-25% as volatile
compounds in a C02/acetone ratio of 85:15. Only 1-5% of the activity
was found in the faeces in the same period (Everett and Gronberg,
1970).
Evidence was obtained by the same authors that the major routes
of metabolism in rats are depropylation to
2-hydroxyphenyl-N-Methylcarbamate (further indicated as metabolite
A) and subsequent hydrolysis to isopropoxyphenol (metabolite I).
Minor metabolic pathways are ring hydroxylation at the 5 or 6
position (C and E), secondary hydroxylation of the 2-carbon atom of
the isopropoxy group (D and G) and hydroxylation of the N-methyl
group.
From the conjugated compounds in the urine, the following
metabolites were released by hydrolysis of the urine sample with
glucuronidase and/or acid. They were identified by their infra-red and
mass spectra as well as from the 3H/14C ratio yielded of double
labelled propoxur: 2-hydrophenyl-N-methylcarbamate (A),
2-isopropoxyphenyl-N-hydroxy methylearbamate (B) and
2-isopropoxy-5-hydroxyphenyl-N-methylearbamate (C).
The metabolic pathways of propoxur in rats as proposed by Everett
and Gronberg (1970) is shown in the diagram on the following page.
 Krishna and Casida (1966) administered 14C carbonyl labelled
propoxur intraperitoneally to rats. After 48 hours only 2.1% of the
administered radio-activity remained in the body; 60% of the activity
was excreted in the urine in the first 29 hours, whereas only 1.2% was
excreted in the faeces. Within 48 hours 31.2% of the administered
radio-activity was expired as CO2.
From these data it may be concluded that the carbamate group was
cleaved from one-third of the injected propoxur dose.
In a similar experiment with 14C isopropyl labelled propoxur,
70-75% of the activity was excreted in the urine, whilst 30%. of the
administered activity was expired as 14CO2; 4% of the activity
remained in the body and only 0.7% was excreted in the faeces.
From this it may be concluded that the isopropyl group is cleaved
from about one-quarter of the injected dose.
Dorough and Casida (1964) incubated rat liver microsomes with
propoxur and obtained 30% conversion to a metabolite from which with
acid Isopropoxyphenol and formaldehyde were yielded. With
cochromatography and infra-red spectroscopy the metabolite was
confirmed with (B).
Oonithan and Casida (1968) studied the metabolic fate of propoxur
in a system containing rat liver microsomes and NADPH2. Two
metabolites were formed, probably (A) and (B).
In cattle
Waggoner and Olson (1971) determined residues in tissues and milk
of cattle after feeding on a diet containing propoxur for 28 days. In
those cases where residues could be detected the amounts of the
metabolite (A) was greater than the parent propoxur.
After feeding 7.5 mg/kg/day of propoxur, the residues of the
parent compound and the metabolite (A) were respectively:
ppm
propoxur (A)
kidney 0.04 0.13
milk 0.001 0.0027
In insects
Metcalf et al. (1967) treated flies with 14C isopropoxy labelled
propoxur and found metabolism to CO2. A pre-treatment with piperonyl
butoxide reduced the formation of CO2 to one-third.
Dorough et al. (1963) and Dorough and Casida (1964) found in
cockroaches the metabolite (B) after injection of propoxur.
Shrivastava et al. (1969) (see also Ruhr, 1968, 1970) studied
in vivo and in vitro the metabolism of propoxur in houseflies.
The following metabolites were isolated in order of decreasing amounts
(C), (A) and acetone, B and 2-isopropoxyphenyl carbamate. These
metabolites were all conjugated due to their hydroxyl groups and
volatilized in acetone (see also Casida et al., 1968).
Tsukamoto and Casida (1967a and b) investigated the metabolism of
propoxur in a system of house-fly microsomes and NADPH2; they found
the metabolites (A), (B) and (C) as major metabolites.
Propoxur is a non-systemic carbamate insecticide which is used
against a relatively broad spectrum of insects in field crops, fruit
and vegetables (aphids, including woolly aphid, lygus bugs,
leafhoppers, saw-flies, thrips, millipedes, etc.).
The product is registered and used in several countries in Europe
and in other parts of the world.
Propoxur is used extensively for hygienic purposes against
cockroaches, flies, etc. in homes, hotels, restaurants and warehouses.
Pre-harvest treatments
Major crops on which propoxur is used are rice, sugar cane, pome
and stone fruits, small fruits, vegetables and potatoes. The following
estimates can be given of the use in different areas:
rice about 30%
other field crops about 30%
cacao about 20%
other crops such as fruit,
vegetables, ornamentals about 20%.
The following table summarizes the recommendations in accordance
with good agricultural practice, including rates of application and
pre-harvest intervals.
Dosage rate Minimum pre-harvest
Crop g a.i./ha or interval recommended
g a.i./100 l days
Field crops
potatoes 250-600 g/ha 7
rice 400-750 g/ha 7
sugar cane 750-1000 g/ha 7
Cacao 250-600 g/ha 7
Fruits
Pome fruits
apple, pears 50-75 g/100 l 7
600-1200 g/ha
Stone fruits
peach, plums 50-75 g/100 l 7
900-1200 g/ha
Small fruits
blackberries, gooseberries,
red currants, raspberries,
strawberries 50-75 g/100 l 7
600-1200 g/ha
Vegetables (outdoors)
beans, cabbage, gherkins,
leek, lettuce, onionp
peas, spinach 400-750 g/ha 4-7
vegetables (glasshouses)
bell peppers, cucumbers,
gherkins, melons,
tomatoes 400-750 g/ha 4
Leafy vegetables such as
lettuce, spinach 400-750 g/ha 14-21
Post-harvest treatments
No treatments recommended.
Other uses
Propoxur is used on ornamentals and flower crops.
It is also extensively used in the hygienic sector in the form of
aerosols, thermal fog concentrates, baits, wettable powder and
emulsifiable liquids, against a number of household and domestic pests
such as bugs, cockroaches, flies, mosquitos, beetles, silverfish, etc.
Pre-harvest intervals officially established in
various countries in days
Austria
35 days, all fruit and vegetables
Belgium
21 days, lettuce and endive (glasshouse cultures) during
winter period
14 days, lettuce and endive (outdoors and glasshouse)
7 days, other vegetables except those mentioned below,
agricultural crops
3 days, gherkins, tomatoes (both under glass and outdoors),
bell peppers, cucumber, melons
Denmark
14 days, fruit, vegetables and field crops
Germany, Federal Republic of
21 days, cereals
14 days, potatoes
7 days, leafy vegetables (except lettuce), tomatoes,
gherkins, melons
4 days, pome and stone fruit, gooseberries, red and black
currants, raspberries, strawberries, cabbage, carrot,
celery, garden beet, leek, lettuce, onion, radish, horse
radish, sugar and fodder beet
Netherlands
21 days, lettuce and endive (glasshouse) between
November/March
14 days, lettuce and endive (outdoors.and glasshouse)
7 days, fruit, including berries, vegetables except those
mentioned below
4 days, gherkins (glasshouse and outdoors)
3 days, tomatoes, bell peppers, cucumbers, melons
New Zealand
21 days, all crops
Poland
7 days, potatoes
Spain
30 days, fruit, sugar beet, cotton
Sweden
14 days, vegetables
7 days, all other crops
United Kingdom
7 days, all outdoor crops
2 days, cucumbers, tomatoes (glasshouse)
Yugoslavia
21 days, fruit
Residue data from supervised trials
Residue data were obtained from trials on several fruits,
vegetables and field crops, such as apples. sour and sweet cherries,
peaches, plums, black and red currants, gooseberries, French beans,
bell peppers, red and white cabbage, savoy, carrots, cucumbers,
lettuce, leek, onions, peas, spinach, tomatoes, alfalfa, cereals,
rice, tobacco, cocoa, grassland. These data are summarized in Tables 1
and 2.
TABLE 1. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
Fruit
apples Belgium 1964 1 (75 g/100 l) w.p. 50% 0.3 n.d. n.d.
Germany, 1966 1 (75 g/100 l) w.p. 50% 1.6 0.96 0.48 0.43
Fed. Rep. 1966 1 (75 g/100 l) w.p. 50% 2.0 0.95 0.95 0.95 0.8
1964 1 (50 g/100 l) w.p. 50% 1.3 0.8 0.7 0.6 0.6
1964 1 (100 g/100 l) w.p. 50% 2.0 1.4 0.7 0.6 0.6
Netherlands 1964 2 (l.v.) w.p. 50% 1.0-1.4 0.5-0.7 0.4-0.5 0.2-0.4 0.1-0.2
1964 2 (h.v.) w.p. 50% 0.6-1.1 0.2 0.2 0.1-0.3 0.1-0.3
1965 2.5 (h.v.) w.p. 50% 0.33-1.6 0.38- 0.31-
0.80 0.44
1965 2.5 (h.v.) w.p. 50% 0.29- 0.28-
0.42 0.33
cherries, Germany, 1969 1 1.5 w.p. 50% 3.1 0.45 0.18
sour Fed. Rep. 1 1.5 w.p. 50% 5.0 0.3 0.24
cherries, Germany, 1968 1 (50 g/100 l) w.p. 50% 0.05
sweet Fed. Rep. 2 (50 g/100 l) w.p. 50% 0.06
peaches Germany, 1967 1 (50 g/100 l) w.p. 50% 1.55 0.5 0.25 0.2
Fed. Rep 1968 1 (75 g/100 l) w.p. 50% 3 2.0 1.25 0.65
1968 1 (75 g/100 l) w.p. 50% 8.7 2.36 1.65
1968 1 (75 g/100 l) w.p. 50% 2.9 1.8 0.9
1968 1 (75 g/100 l) w.p. 50% 3.9 1.5 0.5
plums Germany, 1967 1 (50 g/100 l) w.p. 50% 0.55 n.d. n.d. n.d.
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 2.16 0.52 0.2
1968 1 (75 g/100 l) w.p. 50% 3.71 1.75 1.5
1968 1 (75 g/100 l) w.p. 50% 3.05 1.5 0.7
1968 1 (75 g/100 l) w.p. 50% 1.6 0.7 0.15
1968 1 (75 g/100 l) w.p. 50% 2.75 1.45 0.7
1969 1 1.5 w.p. 50% 2.5 1.35 <0.05
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1969 1 1.5 w.p. 50% <0.05 <0.05 <0.05
red Netherlands 1965 1 0.75 (l.v.) w.p. 50% 1.31-2.11 <0.01- <0.01-
0.08 <0.01
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 7.25 0.75 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 14.1 0.64 0.61
1968 1 (75 g/100 l) w.p. 50% 8.2 1.1 0.64
black Netherlands 1964 1 (50 g/100 l) w.p. 50% 2.35 0.7
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 15 0.7 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 13.4 0.6 0.4
1 (75 g/100 l) w.p. 50% 16.7 2.45 1.35
1 (75 g/100 l) w.p. 50% 4.2 1.3 0.45 0.11
gooseberries Germany, 1968 1 (75 g/100 l) w.p. 50% 3.5 0.6 0.3
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 5.8 0.6 0.25
1968 1 (75 g/100 l) w.p. 50% 3.6 0.45
1968 1 (75 g/100 l) w.p. 50% 6.7 0.53 0.20
1968 1 (75 g/100 l) w.p. 50% 6.3 0.83 0.23
Vegetables
French Germany 1964 1 0.7 w.p. 50% 1.25 0.25 <0.1
bean Fed. Rep. 1969 1 0.45 w.p. 50% 1.65 0.55 0.20
1968 1 0.75 w.p. 50% 0.5 0.25
1968 1 0.75 w.p. 50% 1.6 1.1 1.0
1969 1 0.75 w.p. 50% 0.5 0.35 0.25
1969 1 0.75 w.p. 50% 0.6 0.4 0.25
1967 1 0.5 w.p. 50% 0.75 0.3 0.15
1968 1 0.45 w.p. 50% 0.9 0.2 0.1
1968 1 0.45 w.p. 50% 0.7 0.3 0.1
1969 1 0.45 w.p. 50% 1.6 0.5 0.08
UK 1964 1 0.7 w.p. 50% 0.25
-1.55
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
bell peppers Netherlands 1968 1 0.38 w.p. 50% 0.75 0.3 <0.1
(glasshouse)
red Germany, 1964 1 0.15 w.p. 50% 1.0 0.4 0.2 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 1.6 1.3 0.9 0.4
savoy Germany, 1964 1 0.15 w.p. 50% 3.9 0.9 0.2 <0.2
Fed. Rep. 1964 1 0.15 w.p. 50% 2.7 0.8 0.7 0.6
1968 1 0.6 w.p. 50% 5.3 2.1 0.7 1.2
1968 1 0.6 w.p. 50% 8.0 3.9 3.4 1.8
white Germany, 1964 1 0.15 w.p. 50% 2.2 1.3 0.6 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 5.8 2.1 1.2 0.6
carrots Germany, 1968 1 0.45 w.p. 50% 0.1 n.d n.d.
Fed. Rep. 1968 1 0.75 w.p. 50% n.d. 0.2 0.25
1968 1 0.75 w.p. 50% 0.1 0.15 0.25
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% 0.3 0.7 0 0.3
cucumbers Netherlands 1970 1 0.5 dust 2% 0.05 0.07 n.d.
(glasshouse) 1970 1 0.5 dust 2% 0.07 0.06 n.d.
leek Germany, 1968 1 0.45 w.p. 50% 0.5 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 0.6 0.1 n.d.
1968 1 0.45 w.p. 50% 10.9 1.1 1.0
1968 1 0.6 w.p. 50% 2.9 0.6 0.6
1968 1 0.75 w.p. 50% 2.3 0.25 0.1
1968 1 0.75 w.p. 50% 2.0 0.7 0.15
lettuce Germany, 1964 1 0.6 w.p. 50% 6.8 0.4 0.2 0.1
(outdoor) Fed. Rep. 1964 1 0.6 w.p. 50% 5.1 0.2 1.3 1.1
1 w.p. 50% 1.8 0.4 0.2 0.1
1 w.p. 50% 2.2 1.6 0.9 0.7
lettuce Netherlands 1963 1 0.66 w.p. 50% 17.2- 9.2- 5.4- 1.8-4.1 0.9-1.9 0.5-0.8
(glasshouse) 20.2 10.9 10.4
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1971 1 0.8-0.9 w.p. 50% 15.2 10.9 4.1
1971 1 0.6-0.9 w.p. 50% 10.0 7.45 3.1
1971 1 0.6-0.9 w.p. 50% 4.0 5.5 1.9
1971 1 0.6-0.9 w.p. 50% 8.5 7.0 2.7
1971 1 0.6-0.9 w.p. 50% 7.25 6.8 2.4
onions Germany, 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
1968 1 0.45 w.p. 50% 9.3 4.2 0.87
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50%
peas Germany, 1964 1 0.7 w.p. 50% 0.3 n.d. n.d.
pods Fed. Rep. 1964 0.4 0.1 <0.1
spinach Germany, 1968 1 0.45 w.p. 50% 5.7 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 6.8 n.d. n.d.
1969 1 0.45 w.p. 50% 32 0.6 0.06
1969 1 0.45 w.p. 50% 33.5 0.7 0.06
1969 1 0.75 w.p. 50% 27 7.3 0.6
tomatoes Netherlands 1971 1 0.5 dust 0.28 <0.05 n.d.
(glasshouse 1971 1 0.5 dust 0.33 <0.05 n.d.
1971 1 11 g/100 m3 smoke 0.07 n.d.
Field Crops
alfalfa USA 1970 1 1.0 w.p. 70% 59.9 12.2
green 1 1.0 w.p. 70% 11.6 2.17
1 1.0 w.p. 70% 15.5 2.06
1 1.0 w.p. 70% 65.8 7.14
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1 1.0 w.p. 70% 36.8 3.40 1.15 0.20 0.59(28)
1 1.0 w.p. 70% 65.8 7.14 3.17 0.66 <0.12(29)
hay 1 1.0 w.p. 70% 16.4 3.67
1 1.0 w.p. 70% 3.21 1.33
seed hulls 3 1.0 w.p. 70% 0.35(28)
3 1.0 w.p. 70% 0.18(29)
2 1.5 w.p. 70% 0.13(46)
2 1.5 w.p. 70% 0.13(92)
chaffs 3 1.0 w.p. 70% 0.63(28)
3 1.0 w.p. 70% 0.24(29)
0.13(46)
0.08(92)
barley USA 1970
grain 1 0.4a w.p. 50% <0.05
1 0.4a w.p. 50% <0.24
straw 1 0.4a w.p. 50% <0.03
1 0.4a w.p. 50% 0.32
oats USA 1970
grain 1 0.4a w.p. 50% 0.09
1 0.4a w.p. 50% 0.15
1 0.4a w.p. 50% 0.10
1 0.4a w.p. 50% <0.04(25)
straw 1 0.4a w.p. 50% 0.01
1 0.4a w.p. 50% 0.07
1 0.4a w.p. 50% <0.19
1 0.4a w.p. 50%
1 0.4a w.p. 50% 0.01(25)
rye USA 1970
grain 1 0.4a w.p. 70% <0.03
straw 1 0.4a w.p. 70% 0.04
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
wheat USA
grain 1 0.4 w.p. 50% 0.21
straw 1.41
pasture USA 1 0.25 w.p. 70% 11.82 6.99 1.02
grass U.L.V.
1 0.5 w.p. 70% 0.63 0.59 1.20 0.76
rangeland USA 1 0.25 w.p. 70% 29.8 1.49 0.76
grass
cacao
whole beans 2 0.5 E.C. 20% n.d.(24)
2 0.25 E.C. 20% n.d.(24)
shell 2 0.84 E.C. 20% 0.3 0.3 0.3(56)
3 0.84 E.C. 20% 0.3
nib 3 0.84 E.C. 20% <0.1
a seed treatment 500 g/100 kg seed and foliar application
h.v. = high volume; l.v. = low volume; U.L.V. = ultra-low volume
TABLE 2. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest interval in days
Crop Country Year No. rate Formulation
kg a.i./ha 1-10 11-20 21-30 31-40 41-50
Rice hulled Japan 1972 1 0.4 dust 1% 0.02 0.06-0.09
1 0.4 dust 1% 0.03 0.02-0.04
1 0.4 dust 1% 0.02 0.02
1 0.4 dust 1% 0.02 <0.02-0.19
1 0.4 dust 1% <0.02 0.02
1 0.4 dust 1% 0.02-0.11
1 0.4 dust 1% 0.21 0.02
1 0.4 dust 1% 0.02 0.06
Paddy rice Japan 1972 1 0.2-0.75 E.C. 25% 0.03 0.08-0.33
0.05-0.56
0.26-0.40 <0.02-0.05
0.47-0.54
Upland rice Japan 1972 1 0.2-0.75 E.C. 25% 0.18
1 0.2-0.75 E.C. 25% 0.19
1 0.2-0.75 E.C. 25% 0.11
1 0.2-0.75 E.C. 25% 0.13
Fate of residues
Influence of light
Propoxur exposed to light shows no or very slight
photo-decomposition. Crosby (1965) found that propoxur was the only
one out of six N-methylcarbamates which was not converted to other
cholinesterase inhibitors when exposed to ultra-violet light or
sunlight.
Propoxur on silica gel-coated plates exposed to long-wave length
ultra-violet light did not produce degradation products, whereas after
exposure to short-wave length ultra-violet light three metabolites,
which gave a colour reaction with ninhydrin (Abdel-Wahab et al.,
1966), were found.
The product did not show any changes when such plates were
exposed to sunlight, even when sensitizers were applied to the (Ivie
and Casida, 1971).
Inert surfaces
Propoxur disappears from inert surfaces more or less rapidly
mainly through volatilization. The rate of disappearance depends on
the nature of the surface. Fifty per cent. of a propoxur residue on
glass, kept under laboratory conditions, was still present after 1.8
hours (Abdel-Wahab et al., 1966).
The half-life of propoxur on porcelain saucers under field
conditions was about three days (Wright and Jackson, 1971), whereas on
polythene foils a half-life of two days was found (Marchart, 1970).
Propoxur sprayed as an aqueous emulsion concentrate on filter
papers, which were suspended vertically at 2.5 inch in a kitchen (mean
temperature 23°C (21-29°C) and mean relative humidity 52% (42-68%),
evaporated 50% in about six weeks (Links, 1965).
After three months, 60% of the active ingredient was still
present on Hessian bags, sprayed with a wettable powder and stored in
an unheated large storage shed (Linke, 1965).
When propoxur was applied to plywood panels, 50% of it evaporated
in 15 days irrespective of the formulation used (Dorough and Crouch,
1966).
Inconsistencies in the results of different authors may not only
be due to different temperatures but also to air movements (Marchart,
1970), the influence of which is large but has not yet been studied
quantitatively.
The evaporation of propoxur from animate or inanimate substrates
is of practical significance. A considerable proportion of residues on
plants is eliminated by volatilization (Abdel-Wahab at al., 1966;
Gronberg, 1970; Marchart, 1970, 1971). On the other hand, the activity
of propoxur in the vapour phase is utilized for the control of storage
pests and household and domestic pests (Gahan and Wilson, 1970; Wright
et al., 1969).
In water
Propoxur is relatively stable in water at pH levels of less than
7. The rate of hydrolysis, resulting in the formation of
isopropoxyphenol increases rapidly from pH 7 upwards. The half-life of
the parent compound in buffered solutions at 20°C and pH 8 was 16
days, at pH 9, 38 hours and at pH 10, three hours (Aly and El-Dib,
1971).
At temperatures of 30°C and pH 7, the half-life of the parent
propoxur was three days, at pH 9, 1.2 hours.
Flint and Shaw (1971) determined the half-life of propoxur in
field experiments in shallow open vessels filled with bottom silt and
lake water. In these experiments, 50% of the propoxur disappeared in
12.7 hours at pH 7 and 27-36°C.
In similar experiments, in which smaller nearly air-tight vessels
were used, and in a parallel experiment with a biologically sterile
system, the half-lives were 54.9 and 80.8 hours respectively;
temperature range was 5-22°C.
In soil
Propoxur evaporates from the soil; the amount which evaporates
increases with increasing moisture content of the soil.
The time required to decrease a soil residue to one-half of the
initial concentration ranges from six to eight weeks, depending on
soil type (Flint and Shaw, 1971).
Metabolism in soil
The metabolism of propoxur in different soil types was studied by
Church-and Flint (1971) with radio-labelled compounds. After a soil
application of 3H-isopropoxy, 14C-carbonyl labelled propoxur, the
3H activity remained organosoluble, whereas the 14C activity was
concentrated in the water-soluble fraction. The organosoluble activity
was composed of isopropoxyphenol and traces of propoxur. The 14C
activity was incorporated into unknown water-soluble materials which
no longer behaved as carbamates.
In sterile soils or under anaerobic soil conditions, the 14C
activity decreased whilst the 3H activity remained almost constant.
This indicates that in these conditions a simple chemical hydrolysis
to isopropoxyphenol was occurring (Flint and Shaw, 1971).
In biologically active soils, the 14C and 3H activity declined
sharply after nine days, which gives an indication of microbial
degradation. In this case, no conjugated compounds were found (Church
and Flint, 1971).
Flint and Shaw equilibrated aqueous solutions of propoxur with
different soil types. The adsorption of propoxur to soil particles was
poor under these conditions. The following adsorption co-efficients
were found: 0.63 for sandy loam, 0.49 for silty clay loam and 1.12 for
highly organic silty clay loam.
In freshly tilled soils propoxur can be moved laterally by water.
In soil leaching experiments, propoxur moved with the water front
passing through the packed soil columns. In view of the poor stability
of propoxur in aqueous systems, the normal use will give no risks of
contaminating ground or superficial water.
Propoxur at normal rates showed only a slight effect on soil
microorganisms (Church and Flint, 1971; Houseworth and Tweedy, 1972)
and on microorganisms in waste disposal lagoons.
In plants
A large proportion of propoxur applied to the leaf surface
evaporates (Everett and Gronberg, 1968; Marchart, 1970, 1971;
Abdel-Wahab et al., 1966).
With radio-labelled propoxur, it was demonstrated that only a
small amount penetrates from the leaf surface into the leaves. After
five days the parent propoxur comprised 69-98% of the total 14C
activity present. The material that penetrated was shown to be
primarily the parent propoxur and water-soluble metabolites, mainly
the ß glucoside of 2-hydroxyphenyl N-methylcarbamate.
No downward translocation of propoxur could be demonstrated
(Everett and Gronberg, 1968).
The uptake of propoxur by plant roots from an aqueous solution
was shown to be directly related to the water uptake. Propoxur and
metabolites were translocated from the aqueous solution to the surface
of the leaf from which there is some volatilization.
14C carbonyl labelled propoxur injected into the stems of beans
and cotton plants was found to be converted into watersoluble
metabolites which remained stable for a relatively long period
(Dorough and Casida, 1965). In subsequent experiments Abdel-Wahab at
al. (1966) found that the water-soluble metabolites still possessed a
carbamate structure. The half-life of the parent propoxur after
injection into bean plants was one day.
Kuhr and Casida (1967) identified with thin layer chromatography
the water-soluble metabolites. Following incubation with ß
glucosidase, ether-extractable aglycones of the water-soluble
metabolites were yielded to the extent of 76%. Tentative
identification by cochromatography showed that 91.3% of the mixture
consisted of 2-hydroxyphenyl-N-methylearbamate (metabolite "A"
according to the scheme of the metabolic pathway in the section "Fate
in animals" as proposed by Everett and Gronberg (1970)) and 4.9% of
metabolite B = 2-isopropoxyphenyl-N-hydroxymethylcarbamate.
In these studies the metabolites (A) and (B) accounted for 30.2%
and 1.5% respectively of the applied activity, six days after the
injection of propoxur in the bean plant, together comprising 96% of
the water-soluble metabolites.
Five days after foliar application of 14C carbonyl labelled and
isopropoxy labelled propoxur on bean and maize plants, the residue on
the leaves consisted practically only of the parent compound. A
negligible portion was metabolite (A) (<1%). Also in the plant the
largest proportion of the radio-active material extractable with
organic solvents consisted of propoxur. After 3, 5, 7, 9 and 14 days,
its share of the measurable activity was 58.7%, 45.0%, 51.3%, 50.1%
and 36.4% respectively.
A large proportion of the carbonyl labelled material was present
in the water phase, the proportion of isopropyl labelled being less,
thus indicating that the isopropoxy group was cleaved from a
considerable portion of the applied parent compound. This assumption
was supported by the detection of acetone in the air pulled through
the chamber (Everett and Gronberg, 1968).
In an experiment in which carbonyl labelled and isopropoxy
labelled propoxur was absorbed from water by the roots, the proportion
of 14C active compound increased continuously during the 14-day
study. The aglycones of the conjugated metabolites could be almost
completely released with ß glucosidase. Cochromatography showed
agreement with metabolites (A) and (B). The ratio of these compounds
was 9:1 (Everett and Gronberg, 1968).
In a later experiment, Gronberg (1970) found that 14 days after
uptake of labelled propoxur by maize roots 50% of the residue in the
plant was still the parent compound, 19.2% of the residue was
accounted for by (A) and 3.5% by (B). Both metabolites were released
from their conjugates by ß glucosidase and positively identified by
infra-red spectroscopy. It was shown that 2-isopropoxy-4
hydroxyphenyl-N-methylcarbate did not occur.
Methods of residue analysis
Bio-assay methods were developed for the detection of propoxur
residues in soil using house crickets (Burkhardt and Fairchild, 1967)
as test insects and on fruit crops using Daphnia magna (Parker et
al., 1970). Voss (1968) developed an automated procedure for residue
analysis of propoxur in aqueous extracts of fruits based on
cholinesterase inhibition.
The above-mentioned methods are not specific and therefore not
suitable for regulatory purposes. The methods have become obsolete.
Several colorimetric methods have been developed. The only
satisfactory methods involve hydrolysis to isopropoxyphenol, which is
converted to a dyestuff and measured photometrically (see following
table).
The limits of determination range from 0.05 to 0.1 ppm.
TLC methods
Several authors describe TLC methods for the analysis of
propoxur. The most suitable are those based on cholinesterase
inhibition, since they require the simplest clean-up procedure and can
be used for various crop types.
The limits of determination are usually about 0.1 ppm.
GLC methods
Since propoxur, in common with other carbamates, readily
decomposes at high temperatures, GLC methods had to be developed in
which propoxur is converted to a stable derivative, which permits
detection with an electron capture detector. Also a method is
described in which the flame photometric detector is used after
derivatization.
SPECTROMETRIC RESIDUE METHODS FOR PROPOXUR
Crop Detection Sensitivity Reference
Sugar beet, tops,
lettuce IR; N-H-stretching bond 0.2 ppm Niessen and Frehse (1963)
Grapes IR; N-H-stretching bond ? Broderick (1966)
Milk spectrophotofluorometry 1.4 ppm Bowman and Beroza (1967)
Fruits, vegetables,
potatoes, cereals,
hops Aminoantipyrinea 0.05-0.1 ppm Niessen and Frehse (1964)
Human urine Aminoantipyrinea 10-20 ppm Dawson et al. (1964)
Lettuce 2,6-dibromo-benzoquinone- 0.1 ppm van Gils (1970)
chloroimidea
Sugar beets, p-nitrobenzene
potatoes diazoniumfluoboratea 0.05 ppm George (1967)
a After saponification.
THIN LAYER RESIDUE METHODS FOR PROPOXUR
Crop Stationary Solvent Detection Sensitivity Reference
phase system
Water silica gel different dimethylamino-benzaldehyde; 0.1 ppm Abbot et al. (1967)
nitrobenzene-diazonium
fluoborate
Water silica gel different dimethylamino-benzaldehyde 0.1 ppm El-Dib (1970)
Peas, carrots silica gel acetone + cholinesterase 10 ng Mendoza and Shields
hexane inhibition (0.1 ppm) (1971)
20 + 80
Tobacco aluminiumoxide acetone + fast blue B; 0.5 ppm Nesemann and Seehofer
hexane dichloroquinone-chloroimide (1970)
10 + 90
Apples, beets, silica gel acetone + cholinesterase 1 µg Wales et al. (1968)
cabbage, carrots, hexane inhibition
lettuce,
raspberries, 20 + 80
poultry meat
GAS-CHROMATOGRAPHIC RESIDUE METHODS FOR PROPOXUR
Crop Column Derivativea Sensitivity Reference
Apples, cucumbers, Chromosorb W DMCS -chloroacetyl 0.04 ppm Argauer (1969)
tomatoes, milk XE - 60
Corn silage, milk Gaschrom Q -thiophosphoryl 0.02-0.04 ppm Bowman and Beroza (1967)
DC-200
Potatoes, sugar Gaschrom Q -trichloroacetyl 0.01-0.1 ppm Butler and MeDonough (1968)
beets, apples, DC-200
grass
Water, peas, Chromosorb GAWDMCS -2,4-dinitrophenyl 0.2 ppm Cohen et al. (1970)
lettuce, apples XE 60 + Epikote 1001
Spinach Anakrom A B S -2,4-dinitroanilineb 0.05-0.2 ppm Holden et al. (1969)
XE -60
Soil Gaschrom Q -trichloroacetyl 0.02 ppm Stanley (1971)
OV -1
Animal tissue, Gaschrom Q -trichloroacetyl 0.002-0.02 ppm Stanley and Thornton (1972)
milk OV -1
Alfalfa, corn, Gaschrom Q -trichloroacetyl 0.02-0.05 ppm Stanley et al. (1972)
grass, cereals
a Of isopropoxyphenol.
b From reaction with methylamine.
Although the above-mentioned GLC methods are relatively
time-consuming, they are nevertheless the methods of choice. They are
very sensitive and specific (limits 0.002-0.05 ppm).
TLC and GLC methods may be suitable or can be adapted for
regulatory purposes.
The determination of propoxur residues in the trials carried out
by Bayer and reported in this monograph is carried out by the
colorimetric method of Niessen and Frehse (1964). This method
determines only the parent compound and is not fully specific.
The method includes a step for precipitating plant constituents,
which is also used in the methods referred to above.
The analysis in the residue experiments carried out by Chemagro
mentioned in the monograph was by GLC as described by Stanley et al.
(1972). The method is rather complicated, but makes it possible to
determine the parent compound and the main metabolites ((A) and (B),
see page) separately with a high degree of sensitivity.
The conjugated metabolites (A) (2-hydroxyphenyl methylcarbamate)
and (B) (2-isopropoxyphenyl hydroxymethylcarbamate) are released by
enzyme hydrolysis before clean-up. The metabolite (A) is then
alkylated. Next, the compounds are saponified to the phenols and
converted to their trichloroacetyl derivatives, which are determined
by GLC with electron capture detection.
National tolerances
Belgium Fruits and vegetables, 3 ppm
except potatoes
Germany, Federal Fruit 3 ppm
Republic of Sugar Beets 3 ppm
Vegetables, except 3 ppm
Cabbage 4 ppm
Lettuce 4 ppm
Other plant products 0.5 ppm
France Fruit and Vegetables 3 ppm
Italy Fruit and Vegetables 2.25 ppm
Netherlands Fruit and Vegetables 3 ppm
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Propoxur is a non-systemic carbamate insecticide which is used on
a considerable scale in various countries against a relatively broad
spectrum of insects in field crops, fruits and vegetables, e.g.
aphids, lygus bugs, leafhoppers, thrips, sawflies, etc.
Propoxur is also used extensively for hygienic purposes against
cockroaches, flies, etc., and against insect pests on ornamentals and
flower crops.
The technical material contains minimal 95% of
2-isopropoxy-phenyl-N-methyl carbamate. The impurities in the
technical material are known.
Propoxur is marketed in the form of wettable powder, emulsifiable
concentrate, dust, fly and cockroach baits, and balls against flies.
The concentration/rates of application vary depending on pest,
crop and methods of application. Normal application rates are 250-1000
g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. Most of the
residue data obtained in Europe show only the parent compound. The
trials carried out in the United States of America and a limited
number of trials carried out elsewhere determined not only the parent
propoxur but also the two main plant metabolites, (A)
2-hydroxyphenyl-N-methylcarbamate and (B)
2-isopropoxyphenyl-N-hydroxymethylcarbamate.
Information is available on the fate of propoxur residues in
soil, in plants. in mammals and in other animals, e.g. flies.
Some data on products of animal origin after feeding the animals
on treated crops are available indicating that residues are very low.
It would be desirable to have the result of more critical studies
which have been carried out on cows, pigs and chickens, in order to
confirm that this is the true position.
The breakdown of propoxur in plants and animals follows similar
pathways. The same metabolites are identified in rats, plants and in
vitro with microsomes.
Whereas oxidative and hydrolytic degradation both occur and
proceed to the same degree in rats, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
The residues in foods of plant or animal origin, following
recommended directions for use and recommended pre-harvest
intervals, consist largely of the parent compound. In plant products,
the above-mentioned metabolites (A) and (B) occur in a ratio of about
9:1. These metabolites, however, normally represent less than
one-third of the total residue determined.
Little information is available on the rate of decrease in the
level of residue of propoxur and its metabolites during storage and
processing, including household cooking. Little information is
available on propoxur residues in food moving in commerce.
Thin layer chromatographic and gas chromatographic procedures,
specific for propoxur and its main metabolites occurring in plants
(i.e. the metabolites (A) and (B), see above) and/or in products of
animal origin, are available.
The above-mentioned GLC methods are rather time-consuming due to
the fact that propoxur has to be converted to derivatives which are
stable to the GLC conditions. These GLC methods and the TLC methods
may be suitable or can be adapted for regulatory purposes. The most
suitable TLC methods are those based on cholinesterase inhibition.
The limit of determination of the TLC methods is usually about
0.1 ppm. The GLC methods allow sensitive and specific analysis (limits
of detection depending on commodity 0.002-0.05 ppm) of residues in
most crops and products of animal origin.
RECOMMENDATIONS
The following tolerances are based on residues likely to be found
at harvest following currently used patterns. The residues are
determined as propoxur and the main metabolites and are expressed as
propoxur.
Interval on which
Tolerances recommendations
are based (days)
Fruit, including apples,
pears, cherries, peaches,
plums 3 4-7
Soft fruit, including red
currants, blackberries,
gooseberries, strawberries 3 4-7
Vegetables, except potatoes
and root vegetables 3 outdoor 4-7
glasshouse:
leafy vegetables 14,
other vegetables 3-7
Potatoes, root vegetables -
Raw cereals 0.5 14
Rice (hulled) 0.1 7
Cocoa beans 0.05a 7
Meat 0.05a -
Milk (whole) 0.05a -
Animal feedstuff 5 7-14
a At or about the limit of determination
The time interval between application and harvest which has been
used in determining the maximum residue limits is appropriate to the
agricultural practices in numerous countries.
FURTHER WORK OR INFORMATION
Desirable
1. Studies to elucidate the significance of the changes in relative
liver weight in the rat.
2. Studies, including pharmacokinetic studies, to elucidate the
relationships between toxicity and effects on cholinesterase levels in
various species.
3. A long-term study in an animal species other than the rat.
4. Continued epidemiological studies with emphasis on cholinesterase
activity.
5. Studies on behavioural responses especially with low-level
exposure.
6. Results of critical studies to determine the nature and level of
residues in meat (including poultry), milk, and eggs to confirm
recommendations for limits in animal products.
REFERENCES
Abbott, D.C., Blake, K.W., Tarrant, K.R. and Thomson, J. (1967)
Thin-layer chromatographic separation, identification and estimation
of residues of some carbamate and allied pesticides in soil and water.
J. Chromatography, 30: 136-142
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. (1966) Fate of
C14-carbonyl-labelled aryl methylearbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14: 290-299
Aly, O.M. and El-Dib, M.A. (1971) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Research, 5:
1191-1205
Argauer, R.J. (1969) Determination of residues of Banol and other
carbamate pesticides after hydrolysis and chloroacetylation. J. Agr.
Food Chem. 17: 888-892
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Baygon in albino mice. Unpublished report
submitted by Bayer AG
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of metbylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkyl-phenyl methylcarbamates. J. Agr. Food Chem. 16: 561-567
Barlow, F. and Hadaway, A. B. Interactions between insecticides,
cellulose and water, and their effects on insecticide toxicity and
persistence. Society of Chemical Industry Monograph No. 29 (London)
Bayer AG Rückstandsberichte 1964-1969
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) Acute toxicity
data for pesticides (1970). World Review of Pest Control, 9: 119-127
Bowman, M.C. and Beroza, M. (1967) Determination of Niagara NIA-10242
and its phenol degradation product in corn silage and milk and
determination of other carbamates by GLC of their thiophosphoryl
derivatives. J. Ass. off. analyt. Chem. 50: 926-933
Bowman, M.C. and Beroza, M. (1967) Spectrophotofluoreseent and
spectrophotophosphorescent data on insecticidal carbamates and the
analyses of five carbamates in milk by spectrophotofluorometry.
Residue Reviews, 17: 23-34
Broderick, E.J., Bourke, J.B., Mattik, L.R., Taschenberg, E.F. and
Avens, A.W. (1966) Determination of methylcarbamate pesticides in the
presence of methyl anthranilate in Concord grapes. J. Ass. off.
analyt. Chem. 49: 982-985
Brooks, G.D., Smith, E.A. and Schoof, H.F. (1967) Residual
effectiveness of eleven insecticides under weathering conditions
against Aedes aegypti. Mosquito News, 27: 93-99
Burkhardt, C.C. and Fairchild, M.L. (1967) Toxicity of insecticides to
house crickets and bioassay of treated soils in the laboratory. J.
Econ. Ent. 60: 1496-1503
Butler, L.I. and McDonough, L.M. Method for the determination of
residues of carbamate insecticides by electron-capture gas
chromatography. J. Agr. Food Chem. 16: 403-407
Casida, J.E., Shrivastava, S.P. and Esaae, E.G. (1968) Selective
recovery of volatile products from house flies treated with
radioactive insecticide chemicals and synergists, J. Econ. Ent. 61:
1339-1344
Chemagro Corporation (1971) Baygon, the effects on the environment.
Unpublished report submitted by Chemagro
Chemagro Corporation (1971) Unpublished report submitted by Chemagro
Church, D.D. and Flint, D.R. (1971) The fate of Baygon
(O-isopropoxy-phenyl-N-methylcarbamate) in soil. Unpublished report
submitted by Chemagro
Cohen, I.C., Norcup, J., Ruzicka, J.H.A. and Wheals, B.B. (1970) An
electron-capture gas chromatographic method for the determination of
some carbamate insecticides as 2,4-dinitrophenyl derivatives of their
phenol moieties. J. Chromatography, 49: 215-221
Crosby, D.G., Leitis, E. and Winterlin, W.L. (1965) Carbamate
insecticides - photodecomposition of carbamate insecticides. J. Agr.
Food Chem. 13: 204-207
Dawson. J.A., Heath, D.F., Rose, J.A., Thain, E.M. and Ward, J.B.
(1964) Excretion by humans of phenol derived in vivo from arprocarb.
I. Determination by gas chromatography. II. Colorimetric
determination. Bull. Wld Hlth Org. 30(1): 127-134
Dorough, H.W. and Casida, J.E. (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12: 294-304
Dorough, H.W. and Crouch, G.W. (1966) Persistence and residual
effectiveness of various formulations of Baygon (Bayer 39007) against
the house fly. J. Econ. Ent. 59: 1188-1190
Dorough, H.W., Leeling, N.C. and Casida, J.E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science,
140: 170-171
Douch, P.G.C. and Smith, J.N. (1971) Metabolism of
m-tert.-butylphenyl N-methylcarbamate in insects and mice. Biochem.
J. 125: 385-393
Douch, P.G.C. and Smith, J.N. (1971) The metabolism of
3,5-Di-tert.-butylphenyl N-methyl-carbamate in insects and by mouse
liver enzymes. Biochem. J. 125: 395-400
DuBois, K.P., (1962) University of Chicago The acute oral toxicity of
Bayer 39 007 to chickens. Unpublished report submitted by Bayer AG
DuBois, K.P. (1963) University of Chicago The acute toxicity of some
possible metabolites of Bayer 39 007, 44 646, 37 344 and Morestan.
Unpublished report submitted by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961a) University of Chicago The
acute toxicity of Bayer 39 007 to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961b) University of Chicago The
acute toxicity of Bayer 39 007 given in combination with other
anticholinesterase insecticides to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961c) University of Chicago The
acute toxicity of Bayer 39 007 and Bayer 37 344 in combination with
some other anticholinesterase insecticides to rats. Unpublished report
submitted by Bayer AG
Eben, A. and Kimmerle, G. (1973) Propoxur, effect of acute and
subacute oral doses on acetyl cholinesterase activity in plasma,
erythrocytes and brain of rats. Unpublished report submitted by Bayer
AG
Eichelberger, J.W. and Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Env. Sci and Techn. 5: 541-544
El-Dib, M.A. (1970) Thin layer chromatographic detection of carbamate
and phenylurea pesticide residues in natural waters. J. Ass. off.
analyt. Chem. 53: 756-760
Everett, L.J. and Gronberg, R.R. (1968) Plant metabolism of Baygon.
Unpublished report submitted by Chemagro
Everett, L.J. and Gronberg, R.R. (1970) The metabolic fate of Baygon
(2-isopropoxyphenyl-N-methyl-carbamate) in the rat. Unpublished
reported submitted by Chemagro
Flint, D.R. and Shaw, H.R., II (1971) The mobility and persistence
of Baygon in soil and water. Unpublished report submitted by Chemagro
Gahan, J.B. and Wilson, H.G. (1970) Seven insecticides as residual
sprays in buildings naturally infested with Anopheles
quadrimaculatus. Mosquito News, 30: 410-416
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharm. 14: 515-534
George, D.A., Rusk, H.W., Powell, D.M. and Landis, B.J. (1967) An
analytical method for o-isopropoxyphenyl methyl carbamate (Bayer
39007), its aphicidal value and persistence in potatoes and sugar
beets. J. Econ. Ent. 60: 82-84
van Gils, W.F. (1970) Spectrophotometric determination of propoxur
residues on vegetable matter. Analyst, 95: 88-90
Gronberg, R.R. (1970) Metabolism of Baygon in corn plants. Unpublished
report submitted by Chemagro
Hadaway, A.B. and Barlow, F.A (1964) A note on the sorption of
insecticides on tropical soils. Bull. Wld Hlth Org. 30: 146-148
Hayes, W.J., jr (1971) Studies on exposure during the use of
anti-cholinesterase pesticides. Bull. Wld Hlth Org. 44: 277-288
Hobik, H.P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitatsversuchen an Huhnern mit, BAYER
39007. Unpublished report submitted by Bayer AG
Holden, E.R., Jones, W.M. and Beroza, M. (1969) Determination of
residues of methyl-and dimethyl-carbamate insecticides by gas
chromatography of their 2,4-dinitroaniline derivatives. J. Agr. Food
Chem. 17: 56-59
Houseworth, L.D. and Tweedy, B.G. (1972) Effect of Baygon on microbial
populations. Unpublished report submitted by Chemagro
Ivie, G.W. and Casida, J.E. (1971) Sensitized photodecomposition and
photosensitizer activity of pesticide chemicals exposed to sunlight on
silica gel chromatoplates. Photosensitizers for the accelerated
degradation of chlorinated another insecticide chemicals exposed to
sunlight on bean leaves. J. Agr. Food Chem. 19: 405-416
Kimmerle, G. (1961) Preparation Dr. Bocker 58 12 315 (39 007).
Unpublished report submitted by Bayer AG
Kimmerle, G. (1964) E 39 007 (Bocker 58 12 315; Ht.-Nr. 3410)/
Neurotoxizitat. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966a) Neurotoxic studies on active ingredient Docker
58: 12 315 (Unden active ingredient). Unpublished report submitted by
Bayer AG
Kimmerle, G. (1966b) Unden-Wirkstoff (Bocker 58 12
315)/Antidotwirkung. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966c) Unden-Wirkstoff/Inhalationstoxizitat.
Unpublished report submitted by Bayer AG
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetra-ethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol. 27: 311-314
Kimmerle, G. and Solmecke, B. (1971) BAY 39 007/Subacute dermal
application to rabbits. Unpublished report submitted by Bayer AG
Klimmer, O.R., (1963) Pharmakologisches Institut der Universitat Bonn.
Toxikologische Prufung von BAYER 39 007. Unpublished report submitted
by Bayer AG
Krishna, J.G. and Casida, J.E. (1966) Insecticide metabolism - fate in
rats of the radiocarbon from ten variously labeled methyl-and
dimethylcarbamate-14C insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14: 98-105
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticide chemicals
in plants. J. Sci. Food Agr. pp. 44-49
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticides by
insects in vivo and in vitro. Mededel. Rijksfac.
Landbouwetenschappen, 33 (3): 647-657
Kuhr, R.J. (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J. Agr. Food Chem. 18: 1023-1030
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of
metabolites of methylcarbamate insecticide chemicals formed by
hydroxylation in bean plants. J. Agr. Food Chem. 15: 814-824
Linke, W. Trials to determine the persistence of Arprocarb when used
in food storage practice. Unpublished report submitted by Baywood
Chemicals Ltd., Techn. Dept. Technical Report No. TCR/130
Lorke, D. (1970) BAY 39 007/Untersuchungen auf embryotoxische
Wirkungen an der Ratte. Unpublished report submitted by Bayer AG
Löser, E. (1965) Fütterungsversuch Uber 2 Monate mit BAYER 39 007.
Unpublished report submitted by Bayer AG
Löser, E. (1968a) BAY 39 007/Generationsversuche an Ratten.
Unpublished report submitted by Bayer AG
Löser, E. (1968b) BAYER 39 007/Chronische toxikologische
Untersuchungen an Ratten. Unpublished report submitted by Bayer AG
Löser, E. (1968) BAY 39 007/Chronische toxikologische Untersuchungen
an Hunden. Unpublished report submitted by Bayer AG
Marchart, H. (1970) Insecticide residues in cocoa. Int. Atomic Energy
Agency, P-309/1: 1-10
Marchart, H. Evaluation of insecticides for the control of cocoa
capsids in Ghana. FAO Plant Protection Bull. 19: 97-109
Mawdesley-Thomas, L.E., (1969a) Huntingdon Research Centre, England.
Pathology report of the generation experiment in rats of the toxicity
of compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E. (1969b) Huntingdon Research Centre, England.
Pathology report of the two-year experiment in rats of the toxicity of
compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E., (1969c) Huntingdon Research Centre, England.
Pathology report of the two-year toxicity in dogs of compound BAY 39
007 by oral administration. Unpublished report submitted by Bayer AG
Mendoza, C.E. and Shields, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin layer chromatography. J. Ass. off.
analyt. Chem. 54: 507-512
Metcalf, R.L. (1971) Structure-activity relationships for insecticidal
carbamates. Bull. Wld Hlth Org. 44: 43-78
Metcalf, R.L. and Fukuto, T.R. (1965) Carbamate insecticides. Effects
of chemical structure on intoxication and detoxication of phenyl
N-methyl-carbamates in insects. J. Agr. Food Chem. 13: 220-231
Metcalf, R.L., Osman, M.F. and Fukuto, T.R. (1967) Metabolism of
14C-labeled carbamate insecticides to 14CO2 in the house fly. J.
Econ. Ent. 60: 445-450
Nelson, D.L. A study of the acute toxicity of BAYGON in 1967
combination with D.D.V.P. Unpublished report submitted by Bayer AG
Nesemann, E. and Seehofer, F. (1970) Screening procedures for
organophosphorus, organochlorine and carbamate pesticide residues on
tobacco. Beiträge zur Tabakforschung, 5: 207-214
Niessen, H. and Fretse, H. (1963) Eine infrarotspektroskopiscbe,
Methods zur Bestimmung von N-Methylcarbamat-Rückständen in Pflanzen.
Pflanzenschutz Nachrichten "Bayer", 16: 205-220
Niessen, H. and Frehae, H. (1964) Kolorimetrische Methods zur
Bestimung von Rücksthinden doe Insektizids Undon in pflanzlichem
Material. Pflanzenschutz-Nachrichten "Bayer", 17: 25-32
O'Brien, (1966) cited in Metcalf, 1971
Oonnithan, E.S. and Casida, J.E. (1966) Metabolites of methyl- and
dimethyl carbamate insecticide chemicals as formed by rat liver
microsomes. Bull. of Env. Cont. and Tox. 1 (2): 59-60
Oonnithan, E.S. and Casida, J.E. (1968) Oxidation of methyl- and
dimethyl carbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J. Agr. Food Chem.
16: 28-44
Pant, C.P. and Joshi, G.P. (1969) A field study of an airborne toxic
effect of Baygon residual spray. Mosquito News, 29: 674-677
Parker, B.L., Dewey, J.E. and Bache, C.A. (1970) Carbamate bio assay
using Daphnia magna. J. Econ. Ent. 63: 710-714
Plestina, Radovan (1968) Beitrag zur Erforschung der toxischen
Eigenschaften des o-Isopropoxyphenyömethylkarbamat. Magisterarbeit
Reiner, E. (1968) The inhibitory power of
2-isopropoxyphenyl-N-methyl-carbamate against serum cholinesterase of
various individuals. Arch. Toxikol. 23 (3): 237-239
Reiner and Aldrich (1967), cited in Metcalf, 1971
Reiner, E. and Simeon, V. (1968) The inhibitory power of 2-isopropoxy
1968 phenyl-N-methyl-carbamate against serum cholinesterase of various
individuals. Archiv f. Toxikologiet 23: 237-239
Root, M., Cowan, J. and Doull, J., University of Chicago. (1963)
Subacute oral toxicity of RAYER 39 007 to male and female rats.
Unpublished report submitted by Bayer AG
Shrivastava, S.P., Tsukamato, M. and Casida, J.E. (1969) Oxidative
metabolism of 14C-labeled Baygon by living house flies and by house
fly enzyme preparations. J. Econ. Ent. 62: 483-498
Stanley, C.W. (1971) A gas chromatographic method for the
determination of Baygon in soils. Unpublished report submitted by
Chemagro
Stanley, C.W. and Thornton, J.S. (1972) Gas chromatographic method for
residues of Baygon and its metabolites in animal tissues and milk. J.
Agr. Food Chem. 20: 1269-1273
Stanley, C.W., Thornton, J.S. and Katague, D.B. (1972) Gas
chromatographic method for residues of Baygon and metabolites in plant
tissues. J. Agr. Food Chem. 20: 1265-1269
Steelman, C.D., Colmerg A.R., Cabes, L., Barr, H.T. and Tower, B.A.
(1967) Relative toxicity of selected insecticides to bacterial
populations in waste disposal lagoons. J. Econ. Ent. 60: 467-468
Syrowatka, T., Jurek, A. and Nazarewiez, T. (1971) Short term chronic
toxicity study of o-isopropoxyphenyl-N-methyl carbamate (propoxur).
Rooz. Panstw. Zakl. Hig. 22: 579-589 (poln.)
Tsukamoto, M. and Casida, J.E. (1967) Albumin enhancement of oxidative
metabolism of methylearbamate insecticide chemicals by the house fly
microsome-NADPH2 system. J. Econ. Ent. 60: 617-619
Tsukamoto, M. and Casida, J.E. (1967) Metabolismus von Methylcarbamat
Insektiziden durch das NADPH2-benötiginde Enzymsystem der Hausfliege.
Nature (London), 213: 49-51
Vandekar, M. (1969) The effect on cholinesterase activity of storage
of undiluted whole blood sampled from men exposed to
C-isopropoxyphenylmethylcarbamate. Bull. Wld Hlth Org. 40: 91-96
Vandekar, M., Hedayat, E., Plestina, R. and Ahmady, G. (1968) A study
of the safety of o-isopropoxyphenylmetbyl-carbamate in an operational
field-trial in Iran. Bull. Wld Hlth Org. 38: 609-623
Vandekar, M., Plestina, R. and Wilhelm. K. (1971) Toxicity of
carbamates for mammals. Bull. Wld Hlth Org. 44: 241-249
Vandekar, M., Reiner, E., Svetlicic, B. and Fajdetic, T. (1965) Value
of ED50 testing in assessing hazards of acute poisoning by carbamates
and organophosphates. Brit. J. Industr. Med. 22: 317-320
Vandekar, M. and Wilford, K. (1969) The effect on cholinesterase
activity of storage of undiluted whole blood sampled from men exposed
to o-isopropoxyphenyl methylcarbamate (OMS-33). Bull. Wld Hlth Org.
40: 91-96
Voss, G. (1968) Peacock plasma, a useful cholinesterase source for
inhibition residue analysis of insecticidal carbamate. Bull. Env.
Cont. and Tox. 3: 339-347
Waggoner, T.B. and Olson, T.J. (1971) Effect of feeding
organophosphorus and carbamate pesticides to cattle. Paper No. 45,
Division of Pesticide Chemistry, 162nd National ACS Meeting, 12-17
September, Washington, D.C.
Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968) Pesticide residues
- TLC-enzyme inhibition procedure to detect some carbamate standards
and carbaryl in food extracts. J. Ass. off. analyt. Chem. 51:
1239-1242
Weiden, M.H.J. (1971) Toxicity of carbamate to insects. Bull. Wld
Hlth Org. 44: 203-213
Wilhelm (1967), cited in Vandekar et al., 1971
Wit, Sj. (1963) Residues of acaricides and aphicides on glasshouse
cultures (mevinphos, dichlorvos, naled, propoxur and nicotine).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1966) Residues of parathion and propoxur on red-currants.
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1969) Residues of propoxur on gherkins (glasshouse).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wright, J.W., Fritz, R.F., Hocking, K.S,, Babione, R., Gratz, N.G.,
Pal, R., Stiles, A.R. and Vandekar, M. (1969) Ortho-isopropoxyphenyl
methyl-carbamate (OMS-33) as a residual spray for control of
anopheline mosquitos. Bull. Wld Hlth Org. 40: 67-90
Wright, C.G. and Jackson, M.D. (1971) Propoxur, chlordane and diazinon
on porcelain china saucers after kitchen cabinet spraying. J. Econ.
Ent. 64: 457-459
Krishna and Casida (1966) administered 14C carbonyl labelled
propoxur intraperitoneally to rats. After 48 hours only 2.1% of the
administered radio-activity remained in the body; 60% of the activity
was excreted in the urine in the first 29 hours, whereas only 1.2% was
excreted in the faeces. Within 48 hours 31.2% of the administered
radio-activity was expired as CO2.
From these data it may be concluded that the carbamate group was
cleaved from one-third of the injected propoxur dose.
In a similar experiment with 14C isopropyl labelled propoxur,
70-75% of the activity was excreted in the urine, whilst 30%. of the
administered activity was expired as 14CO2; 4% of the activity
remained in the body and only 0.7% was excreted in the faeces.
From this it may be concluded that the isopropyl group is cleaved
from about one-quarter of the injected dose.
Dorough and Casida (1964) incubated rat liver microsomes with
propoxur and obtained 30% conversion to a metabolite from which with
acid Isopropoxyphenol and formaldehyde were yielded. With
cochromatography and infra-red spectroscopy the metabolite was
confirmed with (B).
Oonithan and Casida (1968) studied the metabolic fate of propoxur
in a system containing rat liver microsomes and NADPH2. Two
metabolites were formed, probably (A) and (B).
In cattle
Waggoner and Olson (1971) determined residues in tissues and milk
of cattle after feeding on a diet containing propoxur for 28 days. In
those cases where residues could be detected the amounts of the
metabolite (A) was greater than the parent propoxur.
After feeding 7.5 mg/kg/day of propoxur, the residues of the
parent compound and the metabolite (A) were respectively:
ppm
propoxur (A)
kidney 0.04 0.13
milk 0.001 0.0027
In insects
Metcalf et al. (1967) treated flies with 14C isopropoxy labelled
propoxur and found metabolism to CO2. A pre-treatment with piperonyl
butoxide reduced the formation of CO2 to one-third.
Dorough et al. (1963) and Dorough and Casida (1964) found in
cockroaches the metabolite (B) after injection of propoxur.
Shrivastava et al. (1969) (see also Ruhr, 1968, 1970) studied
in vivo and in vitro the metabolism of propoxur in houseflies.
The following metabolites were isolated in order of decreasing amounts
(C), (A) and acetone, B and 2-isopropoxyphenyl carbamate. These
metabolites were all conjugated due to their hydroxyl groups and
volatilized in acetone (see also Casida et al., 1968).
Tsukamoto and Casida (1967a and b) investigated the metabolism of
propoxur in a system of house-fly microsomes and NADPH2; they found
the metabolites (A), (B) and (C) as major metabolites.
Propoxur is a non-systemic carbamate insecticide which is used
against a relatively broad spectrum of insects in field crops, fruit
and vegetables (aphids, including woolly aphid, lygus bugs,
leafhoppers, saw-flies, thrips, millipedes, etc.).
The product is registered and used in several countries in Europe
and in other parts of the world.
Propoxur is used extensively for hygienic purposes against
cockroaches, flies, etc. in homes, hotels, restaurants and warehouses.
Pre-harvest treatments
Major crops on which propoxur is used are rice, sugar cane, pome
and stone fruits, small fruits, vegetables and potatoes. The following
estimates can be given of the use in different areas:
rice about 30%
other field crops about 30%
cacao about 20%
other crops such as fruit,
vegetables, ornamentals about 20%.
The following table summarizes the recommendations in accordance
with good agricultural practice, including rates of application and
pre-harvest intervals.
Dosage rate Minimum pre-harvest
Crop g a.i./ha or interval recommended
g a.i./100 l days
Field crops
potatoes 250-600 g/ha 7
rice 400-750 g/ha 7
sugar cane 750-1000 g/ha 7
Cacao 250-600 g/ha 7
Fruits
Pome fruits
apple, pears 50-75 g/100 l 7
600-1200 g/ha
Stone fruits
peach, plums 50-75 g/100 l 7
900-1200 g/ha
Small fruits
blackberries, gooseberries,
red currants, raspberries,
strawberries 50-75 g/100 l 7
600-1200 g/ha
Vegetables (outdoors)
beans, cabbage, gherkins,
leek, lettuce, onionp
peas, spinach 400-750 g/ha 4-7
vegetables (glasshouses)
bell peppers, cucumbers,
gherkins, melons,
tomatoes 400-750 g/ha 4
Leafy vegetables such as
lettuce, spinach 400-750 g/ha 14-21
Post-harvest treatments
No treatments recommended.
Other uses
Propoxur is used on ornamentals and flower crops.
It is also extensively used in the hygienic sector in the form of
aerosols, thermal fog concentrates, baits, wettable powder and
emulsifiable liquids, against a number of household and domestic pests
such as bugs, cockroaches, flies, mosquitos, beetles, silverfish, etc.
Pre-harvest intervals officially established in
various countries in days
Austria
35 days, all fruit and vegetables
Belgium
21 days, lettuce and endive (glasshouse cultures) during
winter period
14 days, lettuce and endive (outdoors and glasshouse)
7 days, other vegetables except those mentioned below,
agricultural crops
3 days, gherkins, tomatoes (both under glass and outdoors),
bell peppers, cucumber, melons
Denmark
14 days, fruit, vegetables and field crops
Germany, Federal Republic of
21 days, cereals
14 days, potatoes
7 days, leafy vegetables (except lettuce), tomatoes,
gherkins, melons
4 days, pome and stone fruit, gooseberries, red and black
currants, raspberries, strawberries, cabbage, carrot,
celery, garden beet, leek, lettuce, onion, radish, horse
radish, sugar and fodder beet
Netherlands
21 days, lettuce and endive (glasshouse) between
November/March
14 days, lettuce and endive (outdoors.and glasshouse)
7 days, fruit, including berries, vegetables except those
mentioned below
4 days, gherkins (glasshouse and outdoors)
3 days, tomatoes, bell peppers, cucumbers, melons
New Zealand
21 days, all crops
Poland
7 days, potatoes
Spain
30 days, fruit, sugar beet, cotton
Sweden
14 days, vegetables
7 days, all other crops
United Kingdom
7 days, all outdoor crops
2 days, cucumbers, tomatoes (glasshouse)
Yugoslavia
21 days, fruit
Residue data from supervised trials
Residue data were obtained from trials on several fruits,
vegetables and field crops, such as apples. sour and sweet cherries,
peaches, plums, black and red currants, gooseberries, French beans,
bell peppers, red and white cabbage, savoy, carrots, cucumbers,
lettuce, leek, onions, peas, spinach, tomatoes, alfalfa, cereals,
rice, tobacco, cocoa, grassland. These data are summarized in Tables 1
and 2.
TABLE 1. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
Fruit
apples Belgium 1964 1 (75 g/100 l) w.p. 50% 0.3 n.d. n.d.
Germany, 1966 1 (75 g/100 l) w.p. 50% 1.6 0.96 0.48 0.43
Fed. Rep. 1966 1 (75 g/100 l) w.p. 50% 2.0 0.95 0.95 0.95 0.8
1964 1 (50 g/100 l) w.p. 50% 1.3 0.8 0.7 0.6 0.6
1964 1 (100 g/100 l) w.p. 50% 2.0 1.4 0.7 0.6 0.6
Netherlands 1964 2 (l.v.) w.p. 50% 1.0-1.4 0.5-0.7 0.4-0.5 0.2-0.4 0.1-0.2
1964 2 (h.v.) w.p. 50% 0.6-1.1 0.2 0.2 0.1-0.3 0.1-0.3
1965 2.5 (h.v.) w.p. 50% 0.33-1.6 0.38- 0.31-
0.80 0.44
1965 2.5 (h.v.) w.p. 50% 0.29- 0.28-
0.42 0.33
cherries, Germany, 1969 1 1.5 w.p. 50% 3.1 0.45 0.18
sour Fed. Rep. 1 1.5 w.p. 50% 5.0 0.3 0.24
cherries, Germany, 1968 1 (50 g/100 l) w.p. 50% 0.05
sweet Fed. Rep. 2 (50 g/100 l) w.p. 50% 0.06
peaches Germany, 1967 1 (50 g/100 l) w.p. 50% 1.55 0.5 0.25 0.2
Fed. Rep 1968 1 (75 g/100 l) w.p. 50% 3 2.0 1.25 0.65
1968 1 (75 g/100 l) w.p. 50% 8.7 2.36 1.65
1968 1 (75 g/100 l) w.p. 50% 2.9 1.8 0.9
1968 1 (75 g/100 l) w.p. 50% 3.9 1.5 0.5
plums Germany, 1967 1 (50 g/100 l) w.p. 50% 0.55 n.d. n.d. n.d.
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 2.16 0.52 0.2
1968 1 (75 g/100 l) w.p. 50% 3.71 1.75 1.5
1968 1 (75 g/100 l) w.p. 50% 3.05 1.5 0.7
1968 1 (75 g/100 l) w.p. 50% 1.6 0.7 0.15
1968 1 (75 g/100 l) w.p. 50% 2.75 1.45 0.7
1969 1 1.5 w.p. 50% 2.5 1.35 <0.05
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1969 1 1.5 w.p. 50% <0.05 <0.05 <0.05
red Netherlands 1965 1 0.75 (l.v.) w.p. 50% 1.31-2.11 <0.01- <0.01-
0.08 <0.01
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 7.25 0.75 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 14.1 0.64 0.61
1968 1 (75 g/100 l) w.p. 50% 8.2 1.1 0.64
black Netherlands 1964 1 (50 g/100 l) w.p. 50% 2.35 0.7
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 15 0.7 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 13.4 0.6 0.4
1 (75 g/100 l) w.p. 50% 16.7 2.45 1.35
1 (75 g/100 l) w.p. 50% 4.2 1.3 0.45 0.11
gooseberries Germany, 1968 1 (75 g/100 l) w.p. 50% 3.5 0.6 0.3
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 5.8 0.6 0.25
1968 1 (75 g/100 l) w.p. 50% 3.6 0.45
1968 1 (75 g/100 l) w.p. 50% 6.7 0.53 0.20
1968 1 (75 g/100 l) w.p. 50% 6.3 0.83 0.23
Vegetables
French Germany 1964 1 0.7 w.p. 50% 1.25 0.25 <0.1
bean Fed. Rep. 1969 1 0.45 w.p. 50% 1.65 0.55 0.20
1968 1 0.75 w.p. 50% 0.5 0.25
1968 1 0.75 w.p. 50% 1.6 1.1 1.0
1969 1 0.75 w.p. 50% 0.5 0.35 0.25
1969 1 0.75 w.p. 50% 0.6 0.4 0.25
1967 1 0.5 w.p. 50% 0.75 0.3 0.15
1968 1 0.45 w.p. 50% 0.9 0.2 0.1
1968 1 0.45 w.p. 50% 0.7 0.3 0.1
1969 1 0.45 w.p. 50% 1.6 0.5 0.08
UK 1964 1 0.7 w.p. 50% 0.25
-1.55
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
bell peppers Netherlands 1968 1 0.38 w.p. 50% 0.75 0.3 <0.1
(glasshouse)
red Germany, 1964 1 0.15 w.p. 50% 1.0 0.4 0.2 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 1.6 1.3 0.9 0.4
savoy Germany, 1964 1 0.15 w.p. 50% 3.9 0.9 0.2 <0.2
Fed. Rep. 1964 1 0.15 w.p. 50% 2.7 0.8 0.7 0.6
1968 1 0.6 w.p. 50% 5.3 2.1 0.7 1.2
1968 1 0.6 w.p. 50% 8.0 3.9 3.4 1.8
white Germany, 1964 1 0.15 w.p. 50% 2.2 1.3 0.6 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 5.8 2.1 1.2 0.6
carrots Germany, 1968 1 0.45 w.p. 50% 0.1 n.d n.d.
Fed. Rep. 1968 1 0.75 w.p. 50% n.d. 0.2 0.25
1968 1 0.75 w.p. 50% 0.1 0.15 0.25
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% 0.3 0.7 0 0.3
cucumbers Netherlands 1970 1 0.5 dust 2% 0.05 0.07 n.d.
(glasshouse) 1970 1 0.5 dust 2% 0.07 0.06 n.d.
leek Germany, 1968 1 0.45 w.p. 50% 0.5 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 0.6 0.1 n.d.
1968 1 0.45 w.p. 50% 10.9 1.1 1.0
1968 1 0.6 w.p. 50% 2.9 0.6 0.6
1968 1 0.75 w.p. 50% 2.3 0.25 0.1
1968 1 0.75 w.p. 50% 2.0 0.7 0.15
lettuce Germany, 1964 1 0.6 w.p. 50% 6.8 0.4 0.2 0.1
(outdoor) Fed. Rep. 1964 1 0.6 w.p. 50% 5.1 0.2 1.3 1.1
1 w.p. 50% 1.8 0.4 0.2 0.1
1 w.p. 50% 2.2 1.6 0.9 0.7
lettuce Netherlands 1963 1 0.66 w.p. 50% 17.2- 9.2- 5.4- 1.8-4.1 0.9-1.9 0.5-0.8
(glasshouse) 20.2 10.9 10.4
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1971 1 0.8-0.9 w.p. 50% 15.2 10.9 4.1
1971 1 0.6-0.9 w.p. 50% 10.0 7.45 3.1
1971 1 0.6-0.9 w.p. 50% 4.0 5.5 1.9
1971 1 0.6-0.9 w.p. 50% 8.5 7.0 2.7
1971 1 0.6-0.9 w.p. 50% 7.25 6.8 2.4
onions Germany, 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
1968 1 0.45 w.p. 50% 9.3 4.2 0.87
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50%
peas Germany, 1964 1 0.7 w.p. 50% 0.3 n.d. n.d.
pods Fed. Rep. 1964 0.4 0.1 <0.1
spinach Germany, 1968 1 0.45 w.p. 50% 5.7 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 6.8 n.d. n.d.
1969 1 0.45 w.p. 50% 32 0.6 0.06
1969 1 0.45 w.p. 50% 33.5 0.7 0.06
1969 1 0.75 w.p. 50% 27 7.3 0.6
tomatoes Netherlands 1971 1 0.5 dust 0.28 <0.05 n.d.
(glasshouse 1971 1 0.5 dust 0.33 <0.05 n.d.
1971 1 11 g/100 m3 smoke 0.07 n.d.
Field Crops
alfalfa USA 1970 1 1.0 w.p. 70% 59.9 12.2
green 1 1.0 w.p. 70% 11.6 2.17
1 1.0 w.p. 70% 15.5 2.06
1 1.0 w.p. 70% 65.8 7.14
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1 1.0 w.p. 70% 36.8 3.40 1.15 0.20 0.59(28)
1 1.0 w.p. 70% 65.8 7.14 3.17 0.66 <0.12(29)
hay 1 1.0 w.p. 70% 16.4 3.67
1 1.0 w.p. 70% 3.21 1.33
seed hulls 3 1.0 w.p. 70% 0.35(28)
3 1.0 w.p. 70% 0.18(29)
2 1.5 w.p. 70% 0.13(46)
2 1.5 w.p. 70% 0.13(92)
chaffs 3 1.0 w.p. 70% 0.63(28)
3 1.0 w.p. 70% 0.24(29)
0.13(46)
0.08(92)
barley USA 1970
grain 1 0.4a w.p. 50% <0.05
1 0.4a w.p. 50% <0.24
straw 1 0.4a w.p. 50% <0.03
1 0.4a w.p. 50% 0.32
oats USA 1970
grain 1 0.4a w.p. 50% 0.09
1 0.4a w.p. 50% 0.15
1 0.4a w.p. 50% 0.10
1 0.4a w.p. 50% <0.04(25)
straw 1 0.4a w.p. 50% 0.01
1 0.4a w.p. 50% 0.07
1 0.4a w.p. 50% <0.19
1 0.4a w.p. 50%
1 0.4a w.p. 50% 0.01(25)
rye USA 1970
grain 1 0.4a w.p. 70% <0.03
straw 1 0.4a w.p. 70% 0.04
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
wheat USA
grain 1 0.4 w.p. 50% 0.21
straw 1.41
pasture USA 1 0.25 w.p. 70% 11.82 6.99 1.02
grass U.L.V.
1 0.5 w.p. 70% 0.63 0.59 1.20 0.76
rangeland USA 1 0.25 w.p. 70% 29.8 1.49 0.76
grass
cacao
whole beans 2 0.5 E.C. 20% n.d.(24)
2 0.25 E.C. 20% n.d.(24)
shell 2 0.84 E.C. 20% 0.3 0.3 0.3(56)
3 0.84 E.C. 20% 0.3
nib 3 0.84 E.C. 20% <0.1
a seed treatment 500 g/100 kg seed and foliar application
h.v. = high volume; l.v. = low volume; U.L.V. = ultra-low volume
TABLE 2. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest interval in days
Crop Country Year No. rate Formulation
kg a.i./ha 1-10 11-20 21-30 31-40 41-50
Rice hulled Japan 1972 1 0.4 dust 1% 0.02 0.06-0.09
1 0.4 dust 1% 0.03 0.02-0.04
1 0.4 dust 1% 0.02 0.02
1 0.4 dust 1% 0.02 <0.02-0.19
1 0.4 dust 1% <0.02 0.02
1 0.4 dust 1% 0.02-0.11
1 0.4 dust 1% 0.21 0.02
1 0.4 dust 1% 0.02 0.06
Paddy rice Japan 1972 1 0.2-0.75 E.C. 25% 0.03 0.08-0.33
0.05-0.56
0.26-0.40 <0.02-0.05
0.47-0.54
Upland rice Japan 1972 1 0.2-0.75 E.C. 25% 0.18
1 0.2-0.75 E.C. 25% 0.19
1 0.2-0.75 E.C. 25% 0.11
1 0.2-0.75 E.C. 25% 0.13
Fate of residues
Influence of light
Propoxur exposed to light shows no or very slight
photo-decomposition. Crosby (1965) found that propoxur was the only
one out of six N-methylcarbamates which was not converted to other
cholinesterase inhibitors when exposed to ultra-violet light or
sunlight.
Propoxur on silica gel-coated plates exposed to long-wave length
ultra-violet light did not produce degradation products, whereas after
exposure to short-wave length ultra-violet light three metabolites,
which gave a colour reaction with ninhydrin (Abdel-Wahab et al.,
1966), were found.
The product did not show any changes when such plates were
exposed to sunlight, even when sensitizers were applied to the (Ivie
and Casida, 1971).
Inert surfaces
Propoxur disappears from inert surfaces more or less rapidly
mainly through volatilization. The rate of disappearance depends on
the nature of the surface. Fifty per cent. of a propoxur residue on
glass, kept under laboratory conditions, was still present after 1.8
hours (Abdel-Wahab et al., 1966).
The half-life of propoxur on porcelain saucers under field
conditions was about three days (Wright and Jackson, 1971), whereas on
polythene foils a half-life of two days was found (Marchart, 1970).
Propoxur sprayed as an aqueous emulsion concentrate on filter
papers, which were suspended vertically at 2.5 inch in a kitchen (mean
temperature 23°C (21-29°C) and mean relative humidity 52% (42-68%),
evaporated 50% in about six weeks (Links, 1965).
After three months, 60% of the active ingredient was still
present on Hessian bags, sprayed with a wettable powder and stored in
an unheated large storage shed (Linke, 1965).
When propoxur was applied to plywood panels, 50% of it evaporated
in 15 days irrespective of the formulation used (Dorough and Crouch,
1966).
Inconsistencies in the results of different authors may not only
be due to different temperatures but also to air movements (Marchart,
1970), the influence of which is large but has not yet been studied
quantitatively.
The evaporation of propoxur from animate or inanimate substrates
is of practical significance. A considerable proportion of residues on
plants is eliminated by volatilization (Abdel-Wahab at al., 1966;
Gronberg, 1970; Marchart, 1970, 1971). On the other hand, the activity
of propoxur in the vapour phase is utilized for the control of storage
pests and household and domestic pests (Gahan and Wilson, 1970; Wright
et al., 1969).
In water
Propoxur is relatively stable in water at pH levels of less than
7. The rate of hydrolysis, resulting in the formation of
isopropoxyphenol increases rapidly from pH 7 upwards. The half-life of
the parent compound in buffered solutions at 20°C and pH 8 was 16
days, at pH 9, 38 hours and at pH 10, three hours (Aly and El-Dib,
1971).
At temperatures of 30°C and pH 7, the half-life of the parent
propoxur was three days, at pH 9, 1.2 hours.
Flint and Shaw (1971) determined the half-life of propoxur in
field experiments in shallow open vessels filled with bottom silt and
lake water. In these experiments, 50% of the propoxur disappeared in
12.7 hours at pH 7 and 27-36°C.
In similar experiments, in which smaller nearly air-tight vessels
were used, and in a parallel experiment with a biologically sterile
system, the half-lives were 54.9 and 80.8 hours respectively;
temperature range was 5-22°C.
In soil
Propoxur evaporates from the soil; the amount which evaporates
increases with increasing moisture content of the soil.
The time required to decrease a soil residue to one-half of the
initial concentration ranges from six to eight weeks, depending on
soil type (Flint and Shaw, 1971).
Metabolism in soil
The metabolism of propoxur in different soil types was studied by
Church-and Flint (1971) with radio-labelled compounds. After a soil
application of 3H-isopropoxy, 14C-carbonyl labelled propoxur, the
3H activity remained organosoluble, whereas the 14C activity was
concentrated in the water-soluble fraction. The organosoluble activity
was composed of isopropoxyphenol and traces of propoxur. The 14C
activity was incorporated into unknown water-soluble materials which
no longer behaved as carbamates.
In sterile soils or under anaerobic soil conditions, the 14C
activity decreased whilst the 3H activity remained almost constant.
This indicates that in these conditions a simple chemical hydrolysis
to isopropoxyphenol was occurring (Flint and Shaw, 1971).
In biologically active soils, the 14C and 3H activity declined
sharply after nine days, which gives an indication of microbial
degradation. In this case, no conjugated compounds were found (Church
and Flint, 1971).
Flint and Shaw equilibrated aqueous solutions of propoxur with
different soil types. The adsorption of propoxur to soil particles was
poor under these conditions. The following adsorption co-efficients
were found: 0.63 for sandy loam, 0.49 for silty clay loam and 1.12 for
highly organic silty clay loam.
In freshly tilled soils propoxur can be moved laterally by water.
In soil leaching experiments, propoxur moved with the water front
passing through the packed soil columns. In view of the poor stability
of propoxur in aqueous systems, the normal use will give no risks of
contaminating ground or superficial water.
Propoxur at normal rates showed only a slight effect on soil
microorganisms (Church and Flint, 1971; Houseworth and Tweedy, 1972)
and on microorganisms in waste disposal lagoons.
In plants
A large proportion of propoxur applied to the leaf surface
evaporates (Everett and Gronberg, 1968; Marchart, 1970, 1971;
Abdel-Wahab et al., 1966).
With radio-labelled propoxur, it was demonstrated that only a
small amount penetrates from the leaf surface into the leaves. After
five days the parent propoxur comprised 69-98% of the total 14C
activity present. The material that penetrated was shown to be
primarily the parent propoxur and water-soluble metabolites, mainly
the ß glucoside of 2-hydroxyphenyl N-methylcarbamate.
No downward translocation of propoxur could be demonstrated
(Everett and Gronberg, 1968).
The uptake of propoxur by plant roots from an aqueous solution
was shown to be directly related to the water uptake. Propoxur and
metabolites were translocated from the aqueous solution to the surface
of the leaf from which there is some volatilization.
14C carbonyl labelled propoxur injected into the stems of beans
and cotton plants was found to be converted into watersoluble
metabolites which remained stable for a relatively long period
(Dorough and Casida, 1965). In subsequent experiments Abdel-Wahab at
al. (1966) found that the water-soluble metabolites still possessed a
carbamate structure. The half-life of the parent propoxur after
injection into bean plants was one day.
Kuhr and Casida (1967) identified with thin layer chromatography
the water-soluble metabolites. Following incubation with ß
glucosidase, ether-extractable aglycones of the water-soluble
metabolites were yielded to the extent of 76%. Tentative
identification by cochromatography showed that 91.3% of the mixture
consisted of 2-hydroxyphenyl-N-methylearbamate (metabolite "A"
according to the scheme of the metabolic pathway in the section "Fate
in animals" as proposed by Everett and Gronberg (1970)) and 4.9% of
metabolite B = 2-isopropoxyphenyl-N-hydroxymethylcarbamate.
In these studies the metabolites (A) and (B) accounted for 30.2%
and 1.5% respectively of the applied activity, six days after the
injection of propoxur in the bean plant, together comprising 96% of
the water-soluble metabolites.
Five days after foliar application of 14C carbonyl labelled and
isopropoxy labelled propoxur on bean and maize plants, the residue on
the leaves consisted practically only of the parent compound. A
negligible portion was metabolite (A) (<1%). Also in the plant the
largest proportion of the radio-active material extractable with
organic solvents consisted of propoxur. After 3, 5, 7, 9 and 14 days,
its share of the measurable activity was 58.7%, 45.0%, 51.3%, 50.1%
and 36.4% respectively.
A large proportion of the carbonyl labelled material was present
in the water phase, the proportion of isopropyl labelled being less,
thus indicating that the isopropoxy group was cleaved from a
considerable portion of the applied parent compound. This assumption
was supported by the detection of acetone in the air pulled through
the chamber (Everett and Gronberg, 1968).
In an experiment in which carbonyl labelled and isopropoxy
labelled propoxur was absorbed from water by the roots, the proportion
of 14C active compound increased continuously during the 14-day
study. The aglycones of the conjugated metabolites could be almost
completely released with ß glucosidase. Cochromatography showed
agreement with metabolites (A) and (B). The ratio of these compounds
was 9:1 (Everett and Gronberg, 1968).
In a later experiment, Gronberg (1970) found that 14 days after
uptake of labelled propoxur by maize roots 50% of the residue in the
plant was still the parent compound, 19.2% of the residue was
accounted for by (A) and 3.5% by (B). Both metabolites were released
from their conjugates by ß glucosidase and positively identified by
infra-red spectroscopy. It was shown that 2-isopropoxy-4
hydroxyphenyl-N-methylcarbate did not occur.
Methods of residue analysis
Bio-assay methods were developed for the detection of propoxur
residues in soil using house crickets (Burkhardt and Fairchild, 1967)
as test insects and on fruit crops using Daphnia magna (Parker et
al., 1970). Voss (1968) developed an automated procedure for residue
analysis of propoxur in aqueous extracts of fruits based on
cholinesterase inhibition.
The above-mentioned methods are not specific and therefore not
suitable for regulatory purposes. The methods have become obsolete.
Several colorimetric methods have been developed. The only
satisfactory methods involve hydrolysis to isopropoxyphenol, which is
converted to a dyestuff and measured photometrically (see following
table).
The limits of determination range from 0.05 to 0.1 ppm.
TLC methods
Several authors describe TLC methods for the analysis of
propoxur. The most suitable are those based on cholinesterase
inhibition, since they require the simplest clean-up procedure and can
be used for various crop types.
The limits of determination are usually about 0.1 ppm.
GLC methods
Since propoxur, in common with other carbamates, readily
decomposes at high temperatures, GLC methods had to be developed in
which propoxur is converted to a stable derivative, which permits
detection with an electron capture detector. Also a method is
described in which the flame photometric detector is used after
derivatization.
SPECTROMETRIC RESIDUE METHODS FOR PROPOXUR
Crop Detection Sensitivity Reference
Sugar beet, tops,
lettuce IR; N-H-stretching bond 0.2 ppm Niessen and Frehse (1963)
Grapes IR; N-H-stretching bond ? Broderick (1966)
Milk spectrophotofluorometry 1.4 ppm Bowman and Beroza (1967)
Fruits, vegetables,
potatoes, cereals,
hops Aminoantipyrinea 0.05-0.1 ppm Niessen and Frehse (1964)
Human urine Aminoantipyrinea 10-20 ppm Dawson et al. (1964)
Lettuce 2,6-dibromo-benzoquinone- 0.1 ppm van Gils (1970)
chloroimidea
Sugar beets, p-nitrobenzene
potatoes diazoniumfluoboratea 0.05 ppm George (1967)
a After saponification.
THIN LAYER RESIDUE METHODS FOR PROPOXUR
Crop Stationary Solvent Detection Sensitivity Reference
phase system
Water silica gel different dimethylamino-benzaldehyde; 0.1 ppm Abbot et al. (1967)
nitrobenzene-diazonium
fluoborate
Water silica gel different dimethylamino-benzaldehyde 0.1 ppm El-Dib (1970)
Peas, carrots silica gel acetone + cholinesterase 10 ng Mendoza and Shields
hexane inhibition (0.1 ppm) (1971)
20 + 80
Tobacco aluminiumoxide acetone + fast blue B; 0.5 ppm Nesemann and Seehofer
hexane dichloroquinone-chloroimide (1970)
10 + 90
Apples, beets, silica gel acetone + cholinesterase 1 µg Wales et al. (1968)
cabbage, carrots, hexane inhibition
lettuce,
raspberries, 20 + 80
poultry meat
GAS-CHROMATOGRAPHIC RESIDUE METHODS FOR PROPOXUR
Crop Column Derivativea Sensitivity Reference
Apples, cucumbers, Chromosorb W DMCS -chloroacetyl 0.04 ppm Argauer (1969)
tomatoes, milk XE - 60
Corn silage, milk Gaschrom Q -thiophosphoryl 0.02-0.04 ppm Bowman and Beroza (1967)
DC-200
Potatoes, sugar Gaschrom Q -trichloroacetyl 0.01-0.1 ppm Butler and MeDonough (1968)
beets, apples, DC-200
grass
Water, peas, Chromosorb GAWDMCS -2,4-dinitrophenyl 0.2 ppm Cohen et al. (1970)
lettuce, apples XE 60 + Epikote 1001
Spinach Anakrom A B S -2,4-dinitroanilineb 0.05-0.2 ppm Holden et al. (1969)
XE -60
Soil Gaschrom Q -trichloroacetyl 0.02 ppm Stanley (1971)
OV -1
Animal tissue, Gaschrom Q -trichloroacetyl 0.002-0.02 ppm Stanley and Thornton (1972)
milk OV -1
Alfalfa, corn, Gaschrom Q -trichloroacetyl 0.02-0.05 ppm Stanley et al. (1972)
grass, cereals
a Of isopropoxyphenol.
b From reaction with methylamine.
Although the above-mentioned GLC methods are relatively
time-consuming, they are nevertheless the methods of choice. They are
very sensitive and specific (limits 0.002-0.05 ppm).
TLC and GLC methods may be suitable or can be adapted for
regulatory purposes.
The determination of propoxur residues in the trials carried out
by Bayer and reported in this monograph is carried out by the
colorimetric method of Niessen and Frehse (1964). This method
determines only the parent compound and is not fully specific.
The method includes a step for precipitating plant constituents,
which is also used in the methods referred to above.
The analysis in the residue experiments carried out by Chemagro
mentioned in the monograph was by GLC as described by Stanley et al.
(1972). The method is rather complicated, but makes it possible to
determine the parent compound and the main metabolites ((A) and (B),
see page) separately with a high degree of sensitivity.
The conjugated metabolites (A) (2-hydroxyphenyl methylcarbamate)
and (B) (2-isopropoxyphenyl hydroxymethylcarbamate) are released by
enzyme hydrolysis before clean-up. The metabolite (A) is then
alkylated. Next, the compounds are saponified to the phenols and
converted to their trichloroacetyl derivatives, which are determined
by GLC with electron capture detection.
National tolerances
Belgium Fruits and vegetables, 3 ppm
except potatoes
Germany, Federal Fruit 3 ppm
Republic of Sugar Beets 3 ppm
Vegetables, except 3 ppm
Cabbage 4 ppm
Lettuce 4 ppm
Other plant products 0.5 ppm
France Fruit and Vegetables 3 ppm
Italy Fruit and Vegetables 2.25 ppm
Netherlands Fruit and Vegetables 3 ppm
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Propoxur is a non-systemic carbamate insecticide which is used on
a considerable scale in various countries against a relatively broad
spectrum of insects in field crops, fruits and vegetables, e.g.
aphids, lygus bugs, leafhoppers, thrips, sawflies, etc.
Propoxur is also used extensively for hygienic purposes against
cockroaches, flies, etc., and against insect pests on ornamentals and
flower crops.
The technical material contains minimal 95% of
2-isopropoxy-phenyl-N-methyl carbamate. The impurities in the
technical material are known.
Propoxur is marketed in the form of wettable powder, emulsifiable
concentrate, dust, fly and cockroach baits, and balls against flies.
The concentration/rates of application vary depending on pest,
crop and methods of application. Normal application rates are 250-1000
g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. Most of the
residue data obtained in Europe show only the parent compound. The
trials carried out in the United States of America and a limited
number of trials carried out elsewhere determined not only the parent
propoxur but also the two main plant metabolites, (A)
2-hydroxyphenyl-N-methylcarbamate and (B)
2-isopropoxyphenyl-N-hydroxymethylcarbamate.
Information is available on the fate of propoxur residues in
soil, in plants. in mammals and in other animals, e.g. flies.
Some data on products of animal origin after feeding the animals
on treated crops are available indicating that residues are very low.
It would be desirable to have the result of more critical studies
which have been carried out on cows, pigs and chickens, in order to
confirm that this is the true position.
The breakdown of propoxur in plants and animals follows similar
pathways. The same metabolites are identified in rats, plants and in
vitro with microsomes.
Whereas oxidative and hydrolytic degradation both occur and
proceed to the same degree in rats, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
The residues in foods of plant or animal origin, following
recommended directions for use and recommended pre-harvest
intervals, consist largely of the parent compound. In plant products,
the above-mentioned metabolites (A) and (B) occur in a ratio of about
9:1. These metabolites, however, normally represent less than
one-third of the total residue determined.
Little information is available on the rate of decrease in the
level of residue of propoxur and its metabolites during storage and
processing, including household cooking. Little information is
available on propoxur residues in food moving in commerce.
Thin layer chromatographic and gas chromatographic procedures,
specific for propoxur and its main metabolites occurring in plants
(i.e. the metabolites (A) and (B), see above) and/or in products of
animal origin, are available.
The above-mentioned GLC methods are rather time-consuming due to
the fact that propoxur has to be converted to derivatives which are
stable to the GLC conditions. These GLC methods and the TLC methods
may be suitable or can be adapted for regulatory purposes. The most
suitable TLC methods are those based on cholinesterase inhibition.
The limit of determination of the TLC methods is usually about
0.1 ppm. The GLC methods allow sensitive and specific analysis (limits
of detection depending on commodity 0.002-0.05 ppm) of residues in
most crops and products of animal origin.
RECOMMENDATIONS
The following tolerances are based on residues likely to be found
at harvest following currently used patterns. The residues are
determined as propoxur and the main metabolites and are expressed as
propoxur.
Interval on which
Tolerances recommendations
are based (days)
Fruit, including apples,
pears, cherries, peaches,
plums 3 4-7
Soft fruit, including red
currants, blackberries,
gooseberries, strawberries 3 4-7
Vegetables, except potatoes
and root vegetables 3 outdoor 4-7
glasshouse:
leafy vegetables 14,
other vegetables 3-7
Potatoes, root vegetables -
Raw cereals 0.5 14
Rice (hulled) 0.1 7
Cocoa beans 0.05a 7
Meat 0.05a -
Milk (whole) 0.05a -
Animal feedstuff 5 7-14
a At or about the limit of determination
The time interval between application and harvest which has been
used in determining the maximum residue limits is appropriate to the
agricultural practices in numerous countries.
FURTHER WORK OR INFORMATION
Desirable
1. Studies to elucidate the significance of the changes in relative
liver weight in the rat.
2. Studies, including pharmacokinetic studies, to elucidate the
relationships between toxicity and effects on cholinesterase levels in
various species.
3. A long-term study in an animal species other than the rat.
4. Continued epidemiological studies with emphasis on cholinesterase
activity.
5. Studies on behavioural responses especially with low-level
exposure.
6. Results of critical studies to determine the nature and level of
residues in meat (including poultry), milk, and eggs to confirm
recommendations for limits in animal products.
REFERENCES
Abbott, D.C., Blake, K.W., Tarrant, K.R. and Thomson, J. (1967)
Thin-layer chromatographic separation, identification and estimation
of residues of some carbamate and allied pesticides in soil and water.
J. Chromatography, 30: 136-142
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. (1966) Fate of
C14-carbonyl-labelled aryl methylearbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14: 290-299
Aly, O.M. and El-Dib, M.A. (1971) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Research, 5:
1191-1205
Argauer, R.J. (1969) Determination of residues of Banol and other
carbamate pesticides after hydrolysis and chloroacetylation. J. Agr.
Food Chem. 17: 888-892
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Baygon in albino mice. Unpublished report
submitted by Bayer AG
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of metbylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkyl-phenyl methylcarbamates. J. Agr. Food Chem. 16: 561-567
Barlow, F. and Hadaway, A. B. Interactions between insecticides,
cellulose and water, and their effects on insecticide toxicity and
persistence. Society of Chemical Industry Monograph No. 29 (London)
Bayer AG Rückstandsberichte 1964-1969
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) Acute toxicity
data for pesticides (1970). World Review of Pest Control, 9: 119-127
Bowman, M.C. and Beroza, M. (1967) Determination of Niagara NIA-10242
and its phenol degradation product in corn silage and milk and
determination of other carbamates by GLC of their thiophosphoryl
derivatives. J. Ass. off. analyt. Chem. 50: 926-933
Bowman, M.C. and Beroza, M. (1967) Spectrophotofluoreseent and
spectrophotophosphorescent data on insecticidal carbamates and the
analyses of five carbamates in milk by spectrophotofluorometry.
Residue Reviews, 17: 23-34
Broderick, E.J., Bourke, J.B., Mattik, L.R., Taschenberg, E.F. and
Avens, A.W. (1966) Determination of methylcarbamate pesticides in the
presence of methyl anthranilate in Concord grapes. J. Ass. off.
analyt. Chem. 49: 982-985
Brooks, G.D., Smith, E.A. and Schoof, H.F. (1967) Residual
effectiveness of eleven insecticides under weathering conditions
against Aedes aegypti. Mosquito News, 27: 93-99
Burkhardt, C.C. and Fairchild, M.L. (1967) Toxicity of insecticides to
house crickets and bioassay of treated soils in the laboratory. J.
Econ. Ent. 60: 1496-1503
Butler, L.I. and McDonough, L.M. Method for the determination of
residues of carbamate insecticides by electron-capture gas
chromatography. J. Agr. Food Chem. 16: 403-407
Casida, J.E., Shrivastava, S.P. and Esaae, E.G. (1968) Selective
recovery of volatile products from house flies treated with
radioactive insecticide chemicals and synergists, J. Econ. Ent. 61:
1339-1344
Chemagro Corporation (1971) Baygon, the effects on the environment.
Unpublished report submitted by Chemagro
Chemagro Corporation (1971) Unpublished report submitted by Chemagro
Church, D.D. and Flint, D.R. (1971) The fate of Baygon
(O-isopropoxy-phenyl-N-methylcarbamate) in soil. Unpublished report
submitted by Chemagro
Cohen, I.C., Norcup, J., Ruzicka, J.H.A. and Wheals, B.B. (1970) An
electron-capture gas chromatographic method for the determination of
some carbamate insecticides as 2,4-dinitrophenyl derivatives of their
phenol moieties. J. Chromatography, 49: 215-221
Crosby, D.G., Leitis, E. and Winterlin, W.L. (1965) Carbamate
insecticides - photodecomposition of carbamate insecticides. J. Agr.
Food Chem. 13: 204-207
Dawson. J.A., Heath, D.F., Rose, J.A., Thain, E.M. and Ward, J.B.
(1964) Excretion by humans of phenol derived in vivo from arprocarb.
I. Determination by gas chromatography. II. Colorimetric
determination. Bull. Wld Hlth Org. 30(1): 127-134
Dorough, H.W. and Casida, J.E. (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12: 294-304
Dorough, H.W. and Crouch, G.W. (1966) Persistence and residual
effectiveness of various formulations of Baygon (Bayer 39007) against
the house fly. J. Econ. Ent. 59: 1188-1190
Dorough, H.W., Leeling, N.C. and Casida, J.E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science,
140: 170-171
Douch, P.G.C. and Smith, J.N. (1971) Metabolism of
m-tert.-butylphenyl N-methylcarbamate in insects and mice. Biochem.
J. 125: 385-393
Douch, P.G.C. and Smith, J.N. (1971) The metabolism of
3,5-Di-tert.-butylphenyl N-methyl-carbamate in insects and by mouse
liver enzymes. Biochem. J. 125: 395-400
DuBois, K.P., (1962) University of Chicago The acute oral toxicity of
Bayer 39 007 to chickens. Unpublished report submitted by Bayer AG
DuBois, K.P. (1963) University of Chicago The acute toxicity of some
possible metabolites of Bayer 39 007, 44 646, 37 344 and Morestan.
Unpublished report submitted by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961a) University of Chicago The
acute toxicity of Bayer 39 007 to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961b) University of Chicago The
acute toxicity of Bayer 39 007 given in combination with other
anticholinesterase insecticides to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961c) University of Chicago The
acute toxicity of Bayer 39 007 and Bayer 37 344 in combination with
some other anticholinesterase insecticides to rats. Unpublished report
submitted by Bayer AG
Eben, A. and Kimmerle, G. (1973) Propoxur, effect of acute and
subacute oral doses on acetyl cholinesterase activity in plasma,
erythrocytes and brain of rats. Unpublished report submitted by Bayer
AG
Eichelberger, J.W. and Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Env. Sci and Techn. 5: 541-544
El-Dib, M.A. (1970) Thin layer chromatographic detection of carbamate
and phenylurea pesticide residues in natural waters. J. Ass. off.
analyt. Chem. 53: 756-760
Everett, L.J. and Gronberg, R.R. (1968) Plant metabolism of Baygon.
Unpublished report submitted by Chemagro
Everett, L.J. and Gronberg, R.R. (1970) The metabolic fate of Baygon
(2-isopropoxyphenyl-N-methyl-carbamate) in the rat. Unpublished
reported submitted by Chemagro
Flint, D.R. and Shaw, H.R., II (1971) The mobility and persistence
of Baygon in soil and water. Unpublished report submitted by Chemagro
Gahan, J.B. and Wilson, H.G. (1970) Seven insecticides as residual
sprays in buildings naturally infested with Anopheles
quadrimaculatus. Mosquito News, 30: 410-416
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharm. 14: 515-534
George, D.A., Rusk, H.W., Powell, D.M. and Landis, B.J. (1967) An
analytical method for o-isopropoxyphenyl methyl carbamate (Bayer
39007), its aphicidal value and persistence in potatoes and sugar
beets. J. Econ. Ent. 60: 82-84
van Gils, W.F. (1970) Spectrophotometric determination of propoxur
residues on vegetable matter. Analyst, 95: 88-90
Gronberg, R.R. (1970) Metabolism of Baygon in corn plants. Unpublished
report submitted by Chemagro
Hadaway, A.B. and Barlow, F.A (1964) A note on the sorption of
insecticides on tropical soils. Bull. Wld Hlth Org. 30: 146-148
Hayes, W.J., jr (1971) Studies on exposure during the use of
anti-cholinesterase pesticides. Bull. Wld Hlth Org. 44: 277-288
Hobik, H.P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitatsversuchen an Huhnern mit, BAYER
39007. Unpublished report submitted by Bayer AG
Holden, E.R., Jones, W.M. and Beroza, M. (1969) Determination of
residues of methyl-and dimethyl-carbamate insecticides by gas
chromatography of their 2,4-dinitroaniline derivatives. J. Agr. Food
Chem. 17: 56-59
Houseworth, L.D. and Tweedy, B.G. (1972) Effect of Baygon on microbial
populations. Unpublished report submitted by Chemagro
Ivie, G.W. and Casida, J.E. (1971) Sensitized photodecomposition and
photosensitizer activity of pesticide chemicals exposed to sunlight on
silica gel chromatoplates. Photosensitizers for the accelerated
degradation of chlorinated another insecticide chemicals exposed to
sunlight on bean leaves. J. Agr. Food Chem. 19: 405-416
Kimmerle, G. (1961) Preparation Dr. Bocker 58 12 315 (39 007).
Unpublished report submitted by Bayer AG
Kimmerle, G. (1964) E 39 007 (Bocker 58 12 315; Ht.-Nr. 3410)/
Neurotoxizitat. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966a) Neurotoxic studies on active ingredient Docker
58: 12 315 (Unden active ingredient). Unpublished report submitted by
Bayer AG
Kimmerle, G. (1966b) Unden-Wirkstoff (Bocker 58 12
315)/Antidotwirkung. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966c) Unden-Wirkstoff/Inhalationstoxizitat.
Unpublished report submitted by Bayer AG
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetra-ethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol. 27: 311-314
Kimmerle, G. and Solmecke, B. (1971) BAY 39 007/Subacute dermal
application to rabbits. Unpublished report submitted by Bayer AG
Klimmer, O.R., (1963) Pharmakologisches Institut der Universitat Bonn.
Toxikologische Prufung von BAYER 39 007. Unpublished report submitted
by Bayer AG
Krishna, J.G. and Casida, J.E. (1966) Insecticide metabolism - fate in
rats of the radiocarbon from ten variously labeled methyl-and
dimethylcarbamate-14C insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14: 98-105
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticide chemicals
in plants. J. Sci. Food Agr. pp. 44-49
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticides by
insects in vivo and in vitro. Mededel. Rijksfac.
Landbouwetenschappen, 33 (3): 647-657
Kuhr, R.J. (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J. Agr. Food Chem. 18: 1023-1030
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of
metabolites of methylcarbamate insecticide chemicals formed by
hydroxylation in bean plants. J. Agr. Food Chem. 15: 814-824
Linke, W. Trials to determine the persistence of Arprocarb when used
in food storage practice. Unpublished report submitted by Baywood
Chemicals Ltd., Techn. Dept. Technical Report No. TCR/130
Lorke, D. (1970) BAY 39 007/Untersuchungen auf embryotoxische
Wirkungen an der Ratte. Unpublished report submitted by Bayer AG
Löser, E. (1965) Fütterungsversuch Uber 2 Monate mit BAYER 39 007.
Unpublished report submitted by Bayer AG
Löser, E. (1968a) BAY 39 007/Generationsversuche an Ratten.
Unpublished report submitted by Bayer AG
Löser, E. (1968b) BAYER 39 007/Chronische toxikologische
Untersuchungen an Ratten. Unpublished report submitted by Bayer AG
Löser, E. (1968) BAY 39 007/Chronische toxikologische Untersuchungen
an Hunden. Unpublished report submitted by Bayer AG
Marchart, H. (1970) Insecticide residues in cocoa. Int. Atomic Energy
Agency, P-309/1: 1-10
Marchart, H. Evaluation of insecticides for the control of cocoa
capsids in Ghana. FAO Plant Protection Bull. 19: 97-109
Mawdesley-Thomas, L.E., (1969a) Huntingdon Research Centre, England.
Pathology report of the generation experiment in rats of the toxicity
of compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E. (1969b) Huntingdon Research Centre, England.
Pathology report of the two-year experiment in rats of the toxicity of
compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E., (1969c) Huntingdon Research Centre, England.
Pathology report of the two-year toxicity in dogs of compound BAY 39
007 by oral administration. Unpublished report submitted by Bayer AG
Mendoza, C.E. and Shields, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin layer chromatography. J. Ass. off.
analyt. Chem. 54: 507-512
Metcalf, R.L. (1971) Structure-activity relationships for insecticidal
carbamates. Bull. Wld Hlth Org. 44: 43-78
Metcalf, R.L. and Fukuto, T.R. (1965) Carbamate insecticides. Effects
of chemical structure on intoxication and detoxication of phenyl
N-methyl-carbamates in insects. J. Agr. Food Chem. 13: 220-231
Metcalf, R.L., Osman, M.F. and Fukuto, T.R. (1967) Metabolism of
14C-labeled carbamate insecticides to 14CO2 in the house fly. J.
Econ. Ent. 60: 445-450
Nelson, D.L. A study of the acute toxicity of BAYGON in 1967
combination with D.D.V.P. Unpublished report submitted by Bayer AG
Nesemann, E. and Seehofer, F. (1970) Screening procedures for
organophosphorus, organochlorine and carbamate pesticide residues on
tobacco. Beiträge zur Tabakforschung, 5: 207-214
Niessen, H. and Fretse, H. (1963) Eine infrarotspektroskopiscbe,
Methods zur Bestimmung von N-Methylcarbamat-Rückständen in Pflanzen.
Pflanzenschutz Nachrichten "Bayer", 16: 205-220
Niessen, H. and Frehae, H. (1964) Kolorimetrische Methods zur
Bestimung von Rücksthinden doe Insektizids Undon in pflanzlichem
Material. Pflanzenschutz-Nachrichten "Bayer", 17: 25-32
O'Brien, (1966) cited in Metcalf, 1971
Oonnithan, E.S. and Casida, J.E. (1966) Metabolites of methyl- and
dimethyl carbamate insecticide chemicals as formed by rat liver
microsomes. Bull. of Env. Cont. and Tox. 1 (2): 59-60
Oonnithan, E.S. and Casida, J.E. (1968) Oxidation of methyl- and
dimethyl carbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J. Agr. Food Chem.
16: 28-44
Pant, C.P. and Joshi, G.P. (1969) A field study of an airborne toxic
effect of Baygon residual spray. Mosquito News, 29: 674-677
Parker, B.L., Dewey, J.E. and Bache, C.A. (1970) Carbamate bio assay
using Daphnia magna. J. Econ. Ent. 63: 710-714
Plestina, Radovan (1968) Beitrag zur Erforschung der toxischen
Eigenschaften des o-Isopropoxyphenyömethylkarbamat. Magisterarbeit
Reiner, E. (1968) The inhibitory power of
2-isopropoxyphenyl-N-methyl-carbamate against serum cholinesterase of
various individuals. Arch. Toxikol. 23 (3): 237-239
Reiner and Aldrich (1967), cited in Metcalf, 1971
Reiner, E. and Simeon, V. (1968) The inhibitory power of 2-isopropoxy
1968 phenyl-N-methyl-carbamate against serum cholinesterase of various
individuals. Archiv f. Toxikologiet 23: 237-239
Root, M., Cowan, J. and Doull, J., University of Chicago. (1963)
Subacute oral toxicity of RAYER 39 007 to male and female rats.
Unpublished report submitted by Bayer AG
Shrivastava, S.P., Tsukamato, M. and Casida, J.E. (1969) Oxidative
metabolism of 14C-labeled Baygon by living house flies and by house
fly enzyme preparations. J. Econ. Ent. 62: 483-498
Stanley, C.W. (1971) A gas chromatographic method for the
determination of Baygon in soils. Unpublished report submitted by
Chemagro
Stanley, C.W. and Thornton, J.S. (1972) Gas chromatographic method for
residues of Baygon and its metabolites in animal tissues and milk. J.
Agr. Food Chem. 20: 1269-1273
Stanley, C.W., Thornton, J.S. and Katague, D.B. (1972) Gas
chromatographic method for residues of Baygon and metabolites in plant
tissues. J. Agr. Food Chem. 20: 1265-1269
Steelman, C.D., Colmerg A.R., Cabes, L., Barr, H.T. and Tower, B.A.
(1967) Relative toxicity of selected insecticides to bacterial
populations in waste disposal lagoons. J. Econ. Ent. 60: 467-468
Syrowatka, T., Jurek, A. and Nazarewiez, T. (1971) Short term chronic
toxicity study of o-isopropoxyphenyl-N-methyl carbamate (propoxur).
Rooz. Panstw. Zakl. Hig. 22: 579-589 (poln.)
Tsukamoto, M. and Casida, J.E. (1967) Albumin enhancement of oxidative
metabolism of methylearbamate insecticide chemicals by the house fly
microsome-NADPH2 system. J. Econ. Ent. 60: 617-619
Tsukamoto, M. and Casida, J.E. (1967) Metabolismus von Methylcarbamat
Insektiziden durch das NADPH2-benötiginde Enzymsystem der Hausfliege.
Nature (London), 213: 49-51
Vandekar, M. (1969) The effect on cholinesterase activity of storage
of undiluted whole blood sampled from men exposed to
C-isopropoxyphenylmethylcarbamate. Bull. Wld Hlth Org. 40: 91-96
Vandekar, M., Hedayat, E., Plestina, R. and Ahmady, G. (1968) A study
of the safety of o-isopropoxyphenylmetbyl-carbamate in an operational
field-trial in Iran. Bull. Wld Hlth Org. 38: 609-623
Vandekar, M., Plestina, R. and Wilhelm. K. (1971) Toxicity of
carbamates for mammals. Bull. Wld Hlth Org. 44: 241-249
Vandekar, M., Reiner, E., Svetlicic, B. and Fajdetic, T. (1965) Value
of ED50 testing in assessing hazards of acute poisoning by carbamates
and organophosphates. Brit. J. Industr. Med. 22: 317-320
Vandekar, M. and Wilford, K. (1969) The effect on cholinesterase
activity of storage of undiluted whole blood sampled from men exposed
to o-isopropoxyphenyl methylcarbamate (OMS-33). Bull. Wld Hlth Org.
40: 91-96
Voss, G. (1968) Peacock plasma, a useful cholinesterase source for
inhibition residue analysis of insecticidal carbamate. Bull. Env.
Cont. and Tox. 3: 339-347
Waggoner, T.B. and Olson, T.J. (1971) Effect of feeding
organophosphorus and carbamate pesticides to cattle. Paper No. 45,
Division of Pesticide Chemistry, 162nd National ACS Meeting, 12-17
September, Washington, D.C.
Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968) Pesticide residues
- TLC-enzyme inhibition procedure to detect some carbamate standards
and carbaryl in food extracts. J. Ass. off. analyt. Chem. 51:
1239-1242
Weiden, M.H.J. (1971) Toxicity of carbamate to insects. Bull. Wld
Hlth Org. 44: 203-213
Wilhelm (1967), cited in Vandekar et al., 1971
Wit, Sj. (1963) Residues of acaricides and aphicides on glasshouse
cultures (mevinphos, dichlorvos, naled, propoxur and nicotine).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1966) Residues of parathion and propoxur on red-currants.
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1969) Residues of propoxur on gherkins (glasshouse).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wright, J.W., Fritz, R.F., Hocking, K.S,, Babione, R., Gratz, N.G.,
Pal, R., Stiles, A.R. and Vandekar, M. (1969) Ortho-isopropoxyphenyl
methyl-carbamate (OMS-33) as a residual spray for control of
anopheline mosquitos. Bull. Wld Hlth Org. 40: 67-90
Wright, C.G. and Jackson, M.D. (1971) Propoxur, chlordane and diazinon
on porcelain china saucers after kitchen cabinet spraying. J. Econ.
Ent. 64: 457-459
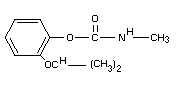 The technical material contains at least 95% propoxur.
Other information on identity and properties
(a) Composition of technical propoxur
Analysis of samples of technical propoxur gave the following
results:
Component %
propoxur min. 95%
O-isopropoxyphenol max. 3% )
)
N,N dimethyl-O-isopropoxy ) together
) max. 4.5%)
phenyl allophanate max. 2% )
1,2-diisopropoxybenzene max. 0.5%
(b) Physical and chemical properties
Physical state: white to cream coloured, crystalline powder
with mild phenolic odour
Molecule weight: 209.2
Melting point: techn. product 86-89°C;
pure propoxur 91.5°C
Vapour pressure: 6.5 x 10-6 mHg at 20°C;
1 x 10-2 mHg at 12°C
Specific gravity: D20 = 1.19
4
Solubility: in water of 20°C approx. 0.2%;
soluble in most polar organic solvents
Stability: stable under normal storage and use
conditions
Hydrolysis rate: propoxur is hydrolyzable in alkaline media;
half life values in aqueous solutions
at 20°C and pH 10.8 - 40 minutes
pH 11.8 - 11.5 minutes
pH 12.8 - 1 minute;
in a 1% aqueous solution at pH 7, it
hydrolyzes at a rate of 1.5% per day
Formulation used: wettable powder 50%;
liquid (EC) 20% w/w, gravity d204
approx. 1.09;
dust 1 and 2%;
fly and cockroach baits 1 and 2%;
balls against flies 50 mg propoxur/ball
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Radio-labelled propoxur was orally administered to rats at
dosages of 5-8 mg/kg. Rats eliminated 85% of the administered
radio-activity within 16 hours. Approximately 257 of the administered
dose was obtained as volatile compounds (CO2 : acetone; 85: 15) with
60% being found in the urine. Very small quantities of radio-activity
were observed in faeces, indicating that rapid absorption was
occurring. From this study, it is evident that propoxur is rapidly
absorbed and excreted in rats following acute administration (Everett
and Gronberg, 1970).
Biotransformation
The metabolic fate of propoxur has been studied in vivo in
mammals, plants and insects and in vitro using preparations from
various biological sources as well as using artificial systems to
examine the non-biological and environmental fate (Dorough and Casida,
1964; Oonnithan and Casida, 1966; Abdel-Wahab et al., 1966; Krishna
and Casida, 1966; Dorough et al., 1963; Kuhr and Casida, 1967;
Oonnithan and Casida, 1966, 1968; Tsukamoto and Casida, 1967a and b;
Casida et al., 1968; Balba and Casida, 1968; Shrivastava et al., 1969;
Kuhr, 1968, 1970; Everett and Gronberg, 1968; Gronberg, 1970; Metcalf
and Fukuto, 1965; Metcalf et al., 1967; Everett and Gronberg, 1971).
Incubation of propoxur with rat liver microsomes fortified with
various cofactors resulted in the formation of 2 hydroxyphenyl
methylcarbamate, 5 hydroxy propoxur, N-hydroxymethyl propoxur and
2-isopropoxyphenol. In vitro, in the presence of UDP-glucuronic
acid, these products would be conjugated. In vivo these same
metabolites were observed with an additional compound being
hydroxylated at the 2 carbon of the isopropoxy group. The major routes
of metabolism are depropylation to 2-hydroxyphenol-N-methylcarbamate
and hydrolysis to yield isopropoxy phenol. The minor routes of
metabolism are ring hydroxylation at the 5 or 6 position with
secondary hydroxylation at the 2 carbon of the allphatic sidechain and
n-methyl hydroxylation of the carbamate. The metabolites identified in
the rat appear to be the same as those found in plants, insects and
derived from in vitro systems. In vitro studies on the
photodecomposition of propoxur in solution resulted in the observation
that, of six N-methyl carbamates, propoxur was the only compound to be
completely stable under the conditions imposed (Crosby et al., 1965).
When exposed to light on a solid surface, propoxur was found to be
slightly degraded to two unidentified organic soluble materials
(Abdel-Wahab at al., 1966).
Effects on enzymes and other biochemical parameters
Propoxur is a biologically active material because of its
structural complimentarity to the active site of neptyl
cholinesterase. As a cholinesterase inhibitor, propoxur behaves as a
synthetic neurohormone that produces its toxic action by interrupting
the normal action of acetyl cholinesterase so that the substrata
acetylcholine accumulates at synaptic junctions. In vivo the signs
of poisoning are manifested by irritability. tremors, incoordination,
convulsions, paralysis and death. While several other carbamate
insecticides (carbaryl, Landrin, and others) produce a transient
anaesthetic effect following high dose administration, there is no
such effect noted with propoxur (Vandekar et al., 1971). In vivo and
in vitro studies have shown propoxur to be a potent inhibitor of
various types of cholinesterase. The I50 (inhibitor concentration
illiciting 50% inhibition) or the bimolecular rate constant
(LM-1min-1) can be seen in the following table.
The specificity of propoxur for true cholinesterase as evident by
its specificity towards bovine and RBC rather than for plasma or serum
cholinesterase is evident from the table. This selectivity was also
noted in studies on the effect of propoxur on man (see section,
Observations in man).
Vandekar et al. (1971) observed that depression of cholinesterase
activity in blood was determinable only during the short period of
time in which signs of poisoning occur. Following acute administration
of propoxur, it was observed that there was a correlation between the
severity of the signs of poisoning and the degree of depression of
erythrocyte cholinesterase. Signs of poisoning appeared initially when
the activity of the cholinesterase dropped below 50% of normal.
Following oral administration of propoxur to rats at a dose
approximately LD50, blood and brain cholinesterase activity was found
to be decreased by about 50% according to a spectrophotometric method
of analysis; however, the determination of cholinesterase activity by
an electrometric method or by a colorimetric method did not show this
inhibition.
As with other carbamate esters, propoxur does not appear to
exhibit any offset on other enzymes or other biochemical parameters.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female per group) were fed
propoxur in the diet at levels of 0, 250, 750, 2000 and 6000 ppm in a
standard three-generation, two-litter/generation reproduction study.
The two highest dietary levels affected the wellbeing of the parent
generation which resulted in a reduction in lactation and further
reduced the pup weight and the parental rate of growth. In addition,
there were effects at 6000 ppm which included smaller litter size. At
the conclusion of the study, examination of various tissues from the
pups of the F3B generation fed 2000 ppm and above showed that there
was a general reduction in the organ weights at three weeks of age.
However, the organ to body weight ratio of these animals corresponded
to that seen with the control, indicating as observed above that the
presence of 2000 ppm in the diet resulted in restricted growth in the
pups. Malformations were not observed in the histological examination
of selected tissues, including those that were noted under gross
examination to be small. Microscopic pathology showed no signs of
alterations attributable to the administration of propoxur. The
dietary concentration of 750 ppm and below did not effect fertility,
litter size and lactation (Loser, 1968a; Mawdesley-Thomas, 1969a).
CHOLINESTERASE INHIBITION
Enzyme I50 Ki Referencea
source (M) (LM-1min-1)
Bovine 7 x 10-7 Vandekar et al., 1971
0.5 - 1.0 x 105 (O'Brien, 1966;
(Reiner and Aldridge, 1967
Fly 0.1 - 8 x 10-8 (Weiden, 1971;
(Metcalf, 1971
1 x 105 Metcalf, 1971
Human serum 0.95 x 103 Metcalf, 1971
Haman plasma 2.3 x 10-5 Wilhelm, 1967
Human RBC 4.6 x 10-7 Wilhelm, 1967
a All values were referenced from various sources, either reviewed or presented as
original data in Bull. Wld Hlth Org., 1971, 44 (1,2,3), 1-470.
Special studies on mutagenicity
A "dominant-lethal study" was conducted on groups of 12 male mice
where the mice were administered a single i.p. dose of propoxur at
levels of 0, 2.5 or 5.0 mg/kg. For a period of six weeks, three virgin
females were exposed to each male at weekly intervals and reproduction
indices recorded. Males treated with 5 mg/kg had slightly lessened
physical activity which lasted one to two days following the treatment
and was reflected in the reduced number of implantations noted in the
treated groups with the females fertilized in the first week after
treatment. The acute administration of propoxur proved to have a
transient effect on the reproduction capability of the males, but
there was no evidence of early resorption which is indicative of an
absence of mutagenic defect (Arnold et al., 1971).
Special studies on neurotoxicity
Adult white leghorn hens wore orally administered proPoxur at
levels ranging from 100 to 1000 mg/kg in a single oral dose or as a
single i.p. dose at levels ranging from 25 to 100 mg/kg. In two of the
three trials, PAM (100 mg/kg) and atropine sulfate (50 mg/kg) were
injected intraperitoneally prior to treatment. No neurotoxic signs of
poisoning similar to that observed with TOCP were noted during periods
of up to six weeks of observation after treatment (Kimmerle, 1964,
1966a).
Groups of eight adult white leghorn hens were fed propoxur in the
diet at levels of 0, 300, 1500, 3000 and 4500 ppm for 30 days. No
evidence of delayed neurotoxic signs of poisoning was seen either
during the period of feeding or in a posttreatment observation period
of four weeks. Histological examination of sciatic nerve and spinal
cord showed no evidence of demyelination (Kimmerle, 1966a; Hobik,
1967).
Special studies on potentiation
Intraperitoneal administration of propoxur to adult female rats
in combination with 20 different anticholinesterase insecticides (19
organophosphates and one carbamate) administered at a dose level of
one-half of the LD50 did not result in an increase in the acute
toxicity (DuBois and Raymund, 1961b and c; Nelson, 1967). Propoxur
does not appear to potentiate antiesterase activity and, as it is a
weak inhibitor of pseudocholinesterase (serum cholinesterase Ki = 9.5
x 102 Lmol-1 min-1) it is doubtful that aliesterase inhibition, a
better indicator of potentiation, would be significantly reduced.
Special studies on teratogenicity
Groups of 10 pregnant rats were fed propoxur in the diet at
concentrations of 0, 1000, 3000 and 10 000 ppm during gestation.
Administration of 3000 ppm and above had an adverse effect on the
parents. At 10 000 ppm, there was a reduction in the number of fetuses
and an increase in the number of resorption sites. This was not
evident at 3000 ppm. There appeared to be a dose-dependent
relationship between the reduction in fetal weight (although only the
3000 and 10 000 ppm were statistically significant) accompanied by a
dose-dependent decrease in placental weight. The dietary concentration
of 1000 ppm was tolerated by the parents and the fetuses and, except
for a slight reduction in the average fetal weight, this level showed
no detrimental effects. Teratogenic abnormalities were not noted in
this study at any dosage level (Lorke, 1970).
Acute toxicity
(a) Original compound
Species Sex Route LD50 Reference
(Mg/kg)
Rat M & F oral 80-191 Ben Dyke et al., 1970;
DuBois and Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961, 1966b, 1971;
Klimmer, 1963
(cont'd)
Species Sex Route LD50 Reference
(Mg/kg)
Guinea-pig M oral 40 DuBois and Raymund, 1961a
Chicken F oral 150-750 DuBois, 1962;
Kimmerle, 1964
Rat M & F i.p. 10-30 DuBois and Raymund, 1961a;
Kimmerle, 1961;
Klimmer, 1963;
Nelson, 1967
Guinea-pig M i.p. 16 DuBois and Raymund, 1961a
Mouse M & F i.p. 14-20 DuBois and Raymund, 1961a
Rat M & F dermal 1000->2400 Ben Dyke et al., 1970;
DuBois and-Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961;
Klimmer, 1963
Rat i.v. 10.6 Vandekar, 1965
Rat i.m. 53 Vandekar, 1965
b) Metabolites
Species Sex Route LD50 Reference
(mg/kg)
2-bydroxyphenyl
N-methylcarbamate
Mice i.p. >167 Balba and Casida, 1968
5-hydroxy propoxur
Mice i.p. >56 Balba and Casida, 1968
4-hydroxy propoxur
Mice i.p. 52 Balba and Casida, 1968
Propoxur
Mice i.p. 12 Balba and Casida, 1968
O-isopropoxyphenol
Rat F oral >1000 DuBois, 1963
dermal
The signs of poisoning shown by propoxur are typical of those
induced by cholinesterase-inhibiting carbamate esters. Tremors, muscle
spasm, lacrimation, salivation and secretion of red tears were
observed in rats following acute dosing. The symptoms appeared rapidly
after administration and recovery was fast.
Following oral administration of propoxur to rats, i.p.
administration of atropine sulfate was found to be antidotal, while
treatment with oximes (PAM, 50 mg/kg and BH6, 20 mg/kg) afforded no
protection and were contraindicated (Kimmerle, 1961b).
Tetraethylammoniumchloride proved also to afford no protection from
the acute effects of propoxur (Kimmerle, 1971).
Subacute dermal toxicity
Groups of rabbits (five males and five females per group) were
treated dermally with propoxur at a level of 500 mg/kg for two weeks.
Residues of the material remaining on the skin prior to each
application were not washed off. Twenty-four hours after the final
application., the skin was washed with soap and water and the animals
observed for two further weeks. The treatment did not affect the
general behaviour and weight gain of the animal and clinical
examinations of blood, urine, liver and kidney function over the
two-week period were normal (Kimmerle and Solmeeke, 1971). Propoxur
was found to be nonirritating to the skin of rabbits when applied to
the inside of a rabbit's ear for 24 hours. No signs of irritation or
poisoning were observed when propoxur was applied to the shaved
abdominal skin of rats and allowed to remain for four hours (Kimmerle,
1961).
Short-term studies
Rat. Groups of rats (15 males and 15 females per group) were fed
propoxur in the diet at levels of 0, 1000, 2000, 4000 and 8000 ppm
(females were fed only dietary levels of 0 and 4000 ppm) for nine
weeks. There was an increase in mortality in the animals fed 4000 and
8000 ppm and a reduction in food consumption and weight gain was
observed in all animals (Löser, 1965).
Oral administration of propoxur to 25 male rats at a dose of 5
mg/kg/day, six days a week for six months (20 male rats served as
controls) resulted in no effects attributable to the administration of
the compound. Growth and food consumption were similar to the
controls, and gross and histological examination of tissues showed no
effects attributable to the administration of propoxur (Klimmer,
1963).
Groups of rats (12 males and 12 females per group) were fed
propoxur in the diet for 16 weeks at concentrations of 0, 5, 10, 50,
100 and 200 ppm (those animals fed 100 and 200 ppm were increased
after the first three weeks of feeding to 400 and 800 ppm respectively
for the remainder of the study). The administration of propoxur at
levels up to 800 ppm in the diet did not affect food consumption,
growth, mortality or gross and histological examination of tissues.
Cholinesterase activity, measured manometrically in the blood, brain
and submaxillary glands, was not affected (Root et al., 1963).
Rats were treated orally for four weeks at 0, 3, 10 and 30 mg/kg.
The highest level caused signs of poisoning and cholinesterase
depression was noted at 10 and 30 mg/kg, with no depression noted at
the low level (Eben and Kimmerle, 1973).
Groups of rats were fed propoxur in the diet at 0, 250, 750 and
2000 ppm for 15 weeks. Propoxur was tolerated with no signs of
poisoning and no consistent evidence of cholinesterase depression
(Eben and Kimmerle, 1973).
Groups of rats (10 males and 10 females per group) were fed
propoxur in the diet at concentrations of 0, 250, 500, 1000 and 2000
ppm for 16 weeks. Food consumption and body weight gain in the females
receiving concentrations of 1000 ppm and above were reduced. No
significant changes were noted with regard to organ weight data. The
1000 and 2000 ppm levels caused depression of cholinesterase activity
in whole blood after the twelfth week of testing and, on completion of
the feeding study, cholinesterase activity was depressed in plasma,
whole blood and brain. Although all clinical enzyme data were found to
be within the normal range, some histopathological changes were noted
in the livers of the animals which received 1000 and 2000 ppm. The
no-effect level in this study was judged to be 500 ppm (Syrowatka et
al., 1971).
Dog. Groups of beagle dogs (four males and four females per group)
were fed propoxur in the diet at concentrations of 0, 100, 250, 750
and 2000 ppm for two years. Mortality was evident at 2000 ppm. One of
the male dogs and none of the female dogs at this level survived to
the end of two years. Food consumption was reduced at this high level.
The animals receiving 2000 ppm at times exhibited signs of
cholinesterase depression, especially in the first six months of
testing. Dietary concentrations of 750 ppm and below did not affect
appearance, behaviour, food consumption or growth of the animals.
Haematological examinations and liver and kidney function tests showed
no effects of propoxur at any dosage level examined. Activity of
leucine-amino peptidase was slightly elevated at levels of 750 and
2000 ppm. Gross examination of tissues showed that there was a
slightly increased liver to body weight ratio in males at 2000 ppm.
There was no indication of cellular damage in any tissue as evidenced
by histological examination of tissues. Although there was a slight
increase in leucine-amino peptidase activity at 750 ppm, based upon
all other considerations, it was assumed that a level of 750 ppm is a
no-effect level. Based on food consumption data, the no-effect level
in dogs would be 50 mg/kg bw/day (Löser, 1968c; Mawdesley-Thomas,
1969c).
Long-term studies
Groups of SPF rats (25 males and 25 females per group, 50 males
and 50 females per control group) were fed propoxur in the diet at
levels of 0, 250, 750, 2000 and 6000 ppm for two years. Dietary
concentration of 6000 ppm caused a reduction in food consumption in
male rats, while 2000 ppm and above resulted in a similar effect in
females. This reduction in food intake was reflected in body weight
gains Of these two groups. On gross examination, at the end of two
years some slight effects were noted in some organs. especially liver
which was enlarged relative to the body weight at the highest feeding
level in male rats and at the highest three feeding levels in female
rats. This increased liver weight was not reflected in liver function
tests or in clinical chemistry examination. Cholinesterase examination
in whole blood (performed only at six months) showed no depression of
cholinesterase activity at 6000 ppm in males and females. Histological
examination of tissues showed no effects relating to the feeding of
propoxur. A no-effect level in this study, based upon increased
relative liver to body weight ratio, is 250 ppm (Loser, 1968b;
Mawdesley-Thomas, 1969b).
Observations in man
Because of the widespread experimental views of propoxur and
control through the auspices of WHO, some significant observations in
humans are available (Plestina, 1968; Dawson, 1964; Vandekar, 1969;
Vandekar et al., 1968, 1971). In a study undertaken to develop a
quantitative method for determining metabolites of propoxur. Dawson et
al. (1964) showed that oral administration of 110 and 116 mg/person
Produced no signs of illness. The level of urinary phenols reached 140
ppm in the absence of clinical signs of poisoning. In persons engaged
in spraying or other occupation exposure, urinary levels of 80 ppm are
uniformly associated with illness. In another study, Vandekar et al.
(1971) administered 135 mg/person to a male volunteer (1.5 mg/kg bw)
and within 20 minutes after ingestion described clinical signs of
poisoning due to the carbamate. Significant erythrocyte cholinesterase
depression was evident coinciding with clinical signs of poisoning,
while plasma cholinesterase depression was not observed. Two hours
after the ingestion of propoxur, there were no signs of poisoning and
the rapid disappearance of symptoms was consistent with the rapid
recovery of erythrocyte cholinesterase activity. Absorption and
excretion of propoxur was very rapid as evidenced by measurement of
urinary phenols which reached a maximum value within four hours of
almost 200 ppm. Of the total phenol content excreted, 81% was found
within five hours after administration. In another experiment, a
single dose of 0.36 mg/kg again produced a rapid fall of erythrocyte
cholinesterase to 57% of normal within 10 minutes. Cholinesterase
recovered to its normal value within three hours. Within 10 minutes of
the administration of propoxur, short-lasting stomach discomfort,
blurred vision, and moderate facial redness and sweating were evident
in the volunteer.
A number of experiments was carried out to study the effect of
storage or build-up of propoxur in the body. Human volunteers took
doses of either 0.15 or 0.2 mg/kg at half-hour intervals for a total
of five doses. In each subject a symptomless depression of erythrocyte
cholinesterase to about 60% of normal was observed. At the cessation
of dosing, enzyme recovery was rapid, being complete within two hours.
Similarly pronounced and as a rule symptomless daily depression and
reactivation of cholinesterase was observed in persons who are
occupationally exposed to propoxur. Studies in humans have shown that
depression of erythrocyte cholinesterase (rather than plasma
cholinesterase) is a significant indicator of exposure to propoxur.
This is consistent with the difference observed in the in vitro
affinity of propoxur for the two enzymes, the I50 values for
erythrocyte and plasma cholinesterase being 4.6 x 10-7 M and 2.3 x
10-5 M, respectively.
Vandekar et al. (1968) published the results of studies carried
out on spray operators and local inhabitants in Iran as part of a WHO
control programme. It was reported that following over-exposure some
spray operators and local inhabitants suffered mild temporary
cholinergic signs of poisoning (headache, nausea). In most of the
cases, the complaints were found to be due to heavy contamination of
propoxur on the skin.
It is evident from these studies that a single oral dose (between
0.2 and 0.4 mg/kg) of propoxur may produce symptoms in man of short
duration. Higher doses may be tolerated without evidence of poisoning
(although there is appreciable inhibition of erythrocyte
cholinesterase) if the higher doses are divided into portions and
administered over reasonably short periods of time. Within two hours
following exposure, cholinesterase depression would be expected to be
normal.
Comments
Propoxur, an anticholinesterase carbamate ester, induces typical
signs of cholinesterase inhibition in both laboratory animals and
humans. Reversible depression of cholinesterase activity is evident a
short time after exposure, although the sensitivity of various
cholinesterase sources differ in different animal species. Erythrocyte
cholinesterase is significantly more sensitive than plasma
cholinesterase in humans. The sensitivity of brain and plasma
cholinesterase appears to be of the same magnitude in rats. Although
0.36 mg/kg resulted in signs of acute poisoning in man, the repeated
administration of 0.2 mg/kg at half-hour intervals for 2.5 hours
resulted in no signs of poisoning, although cholinesterase activity
was depressed. This cholinesterase returned to normal within two hours
following exposure.
Propoxur is rapidly absorbed, metabolized and eliminated.
Teratogenicity and mutagenicity studies in the rat gave negative
results and reproduction was not affected by propoxur.
A long-term rat study provided no evidence of carcinogenic
activity. Liver weight was increased in both males and females at high
dosage levels. No changes were found in liver function tests, clinical
chemistry or on histological examination. In view of the histological
changes in the livers of rats exposed over a short period to 1000 ppm,
the increase in relative liver weight was considered a significant
effect. 250 ppm in the diet was accepted as the no-effect level in the
rat.
In a two-year dog study, a slight increase in leucine-amino
peptidase activity was not regarded as significant. The no-effect
level as evidenced by liver damage was 750 ppm in the diet which,
based on feed consumption data, was 50 mg/kg bw. Cholinesterase
depression was not observed in either the two-year rat or dog studies.
It was evident to the Meeting that the methodology used to determine
cholinesterase activity in these studies was not adequate to measure
depression caused by propoxur.
The no-effect level in the long-term study in the rat was used as
a basis for estimation of the ADI.
The rapid reversibility of acute signs of poisoning in man and
the fact that sensitivity to the toxic effects of propoxur decreased
during prolonged exposure was reassuring in estimating the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolic aspects
The metabolism of propoxur was studied in mammals by Dawson et
al. (1964), Everett and Gronberg (1970), Krishna and Casida (1966),
Waggoner and Olson (1971); in man by Dawson et al. (1964), Hayes
(1971); in insects by Metcalf et al. (1967), Shrivastava (1969); in
plants by Aba el Wahab (1966), Dorough and Casida (1964), Everett and
Gronberg (1968), Gronberg (1970), Kuhr and Casida (1967).
In vitro in animals studies were made by Crosby et al. (1965),
Dorough and Casida (1964) and Oonithan and Casida (1966, 1968).
In rats
The metabolites identified in the rat (see below) include those
found in plants, in insects and those derived from microsomes.
Whereas the oxidative pathways and hydrolytic degradation occur
in the same order of magnitude in mammals, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
Adsorption, distribution and excretion in mammals, including
biotransformation in rats: following oral administration in rats,
propoxur is rapidly ingested to the digestive tract, metabolized in
the body and excreted.
The rats eliminated 85% of the radio-activity after oral
administration of 14C carbonyl labelled, 14C isopropoxy labelled 3H
isopropyl labelled propoxur within 16 hours; 60% of this and amount
was excreted in the urine as conjugates and 20-25% as volatile
compounds in a C02/acetone ratio of 85:15. Only 1-5% of the activity
was found in the faeces in the same period (Everett and Gronberg,
1970).
Evidence was obtained by the same authors that the major routes
of metabolism in rats are depropylation to
2-hydroxyphenyl-N-Methylcarbamate (further indicated as metabolite
A) and subsequent hydrolysis to isopropoxyphenol (metabolite I).
Minor metabolic pathways are ring hydroxylation at the 5 or 6
position (C and E), secondary hydroxylation of the 2-carbon atom of
the isopropoxy group (D and G) and hydroxylation of the N-methyl
group.
From the conjugated compounds in the urine, the following
metabolites were released by hydrolysis of the urine sample with
glucuronidase and/or acid. They were identified by their infra-red and
mass spectra as well as from the 3H/14C ratio yielded of double
labelled propoxur: 2-hydrophenyl-N-methylcarbamate (A),
2-isopropoxyphenyl-N-hydroxy methylearbamate (B) and
2-isopropoxy-5-hydroxyphenyl-N-methylearbamate (C).
The metabolic pathways of propoxur in rats as proposed by Everett
and Gronberg (1970) is shown in the diagram on the following page.
The technical material contains at least 95% propoxur.
Other information on identity and properties
(a) Composition of technical propoxur
Analysis of samples of technical propoxur gave the following
results:
Component %
propoxur min. 95%
O-isopropoxyphenol max. 3% )
)
N,N dimethyl-O-isopropoxy ) together
) max. 4.5%)
phenyl allophanate max. 2% )
1,2-diisopropoxybenzene max. 0.5%
(b) Physical and chemical properties
Physical state: white to cream coloured, crystalline powder
with mild phenolic odour
Molecule weight: 209.2
Melting point: techn. product 86-89°C;
pure propoxur 91.5°C
Vapour pressure: 6.5 x 10-6 mHg at 20°C;
1 x 10-2 mHg at 12°C
Specific gravity: D20 = 1.19
4
Solubility: in water of 20°C approx. 0.2%;
soluble in most polar organic solvents
Stability: stable under normal storage and use
conditions
Hydrolysis rate: propoxur is hydrolyzable in alkaline media;
half life values in aqueous solutions
at 20°C and pH 10.8 - 40 minutes
pH 11.8 - 11.5 minutes
pH 12.8 - 1 minute;
in a 1% aqueous solution at pH 7, it
hydrolyzes at a rate of 1.5% per day
Formulation used: wettable powder 50%;
liquid (EC) 20% w/w, gravity d204
approx. 1.09;
dust 1 and 2%;
fly and cockroach baits 1 and 2%;
balls against flies 50 mg propoxur/ball
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
Radio-labelled propoxur was orally administered to rats at
dosages of 5-8 mg/kg. Rats eliminated 85% of the administered
radio-activity within 16 hours. Approximately 257 of the administered
dose was obtained as volatile compounds (CO2 : acetone; 85: 15) with
60% being found in the urine. Very small quantities of radio-activity
were observed in faeces, indicating that rapid absorption was
occurring. From this study, it is evident that propoxur is rapidly
absorbed and excreted in rats following acute administration (Everett
and Gronberg, 1970).
Biotransformation
The metabolic fate of propoxur has been studied in vivo in
mammals, plants and insects and in vitro using preparations from
various biological sources as well as using artificial systems to
examine the non-biological and environmental fate (Dorough and Casida,
1964; Oonnithan and Casida, 1966; Abdel-Wahab et al., 1966; Krishna
and Casida, 1966; Dorough et al., 1963; Kuhr and Casida, 1967;
Oonnithan and Casida, 1966, 1968; Tsukamoto and Casida, 1967a and b;
Casida et al., 1968; Balba and Casida, 1968; Shrivastava et al., 1969;
Kuhr, 1968, 1970; Everett and Gronberg, 1968; Gronberg, 1970; Metcalf
and Fukuto, 1965; Metcalf et al., 1967; Everett and Gronberg, 1971).
Incubation of propoxur with rat liver microsomes fortified with
various cofactors resulted in the formation of 2 hydroxyphenyl
methylcarbamate, 5 hydroxy propoxur, N-hydroxymethyl propoxur and
2-isopropoxyphenol. In vitro, in the presence of UDP-glucuronic
acid, these products would be conjugated. In vivo these same
metabolites were observed with an additional compound being
hydroxylated at the 2 carbon of the isopropoxy group. The major routes
of metabolism are depropylation to 2-hydroxyphenol-N-methylcarbamate
and hydrolysis to yield isopropoxy phenol. The minor routes of
metabolism are ring hydroxylation at the 5 or 6 position with
secondary hydroxylation at the 2 carbon of the allphatic sidechain and
n-methyl hydroxylation of the carbamate. The metabolites identified in
the rat appear to be the same as those found in plants, insects and
derived from in vitro systems. In vitro studies on the
photodecomposition of propoxur in solution resulted in the observation
that, of six N-methyl carbamates, propoxur was the only compound to be
completely stable under the conditions imposed (Crosby et al., 1965).
When exposed to light on a solid surface, propoxur was found to be
slightly degraded to two unidentified organic soluble materials
(Abdel-Wahab at al., 1966).
Effects on enzymes and other biochemical parameters
Propoxur is a biologically active material because of its
structural complimentarity to the active site of neptyl
cholinesterase. As a cholinesterase inhibitor, propoxur behaves as a
synthetic neurohormone that produces its toxic action by interrupting
the normal action of acetyl cholinesterase so that the substrata
acetylcholine accumulates at synaptic junctions. In vivo the signs
of poisoning are manifested by irritability. tremors, incoordination,
convulsions, paralysis and death. While several other carbamate
insecticides (carbaryl, Landrin, and others) produce a transient
anaesthetic effect following high dose administration, there is no
such effect noted with propoxur (Vandekar et al., 1971). In vivo and
in vitro studies have shown propoxur to be a potent inhibitor of
various types of cholinesterase. The I50 (inhibitor concentration
illiciting 50% inhibition) or the bimolecular rate constant
(LM-1min-1) can be seen in the following table.
The specificity of propoxur for true cholinesterase as evident by
its specificity towards bovine and RBC rather than for plasma or serum
cholinesterase is evident from the table. This selectivity was also
noted in studies on the effect of propoxur on man (see section,
Observations in man).
Vandekar et al. (1971) observed that depression of cholinesterase
activity in blood was determinable only during the short period of
time in which signs of poisoning occur. Following acute administration
of propoxur, it was observed that there was a correlation between the
severity of the signs of poisoning and the degree of depression of
erythrocyte cholinesterase. Signs of poisoning appeared initially when
the activity of the cholinesterase dropped below 50% of normal.
Following oral administration of propoxur to rats at a dose
approximately LD50, blood and brain cholinesterase activity was found
to be decreased by about 50% according to a spectrophotometric method
of analysis; however, the determination of cholinesterase activity by
an electrometric method or by a colorimetric method did not show this
inhibition.
As with other carbamate esters, propoxur does not appear to
exhibit any offset on other enzymes or other biochemical parameters.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female per group) were fed
propoxur in the diet at levels of 0, 250, 750, 2000 and 6000 ppm in a
standard three-generation, two-litter/generation reproduction study.
The two highest dietary levels affected the wellbeing of the parent
generation which resulted in a reduction in lactation and further
reduced the pup weight and the parental rate of growth. In addition,
there were effects at 6000 ppm which included smaller litter size. At
the conclusion of the study, examination of various tissues from the
pups of the F3B generation fed 2000 ppm and above showed that there
was a general reduction in the organ weights at three weeks of age.
However, the organ to body weight ratio of these animals corresponded
to that seen with the control, indicating as observed above that the
presence of 2000 ppm in the diet resulted in restricted growth in the
pups. Malformations were not observed in the histological examination
of selected tissues, including those that were noted under gross
examination to be small. Microscopic pathology showed no signs of
alterations attributable to the administration of propoxur. The
dietary concentration of 750 ppm and below did not effect fertility,
litter size and lactation (Loser, 1968a; Mawdesley-Thomas, 1969a).
CHOLINESTERASE INHIBITION
Enzyme I50 Ki Referencea
source (M) (LM-1min-1)
Bovine 7 x 10-7 Vandekar et al., 1971
0.5 - 1.0 x 105 (O'Brien, 1966;
(Reiner and Aldridge, 1967
Fly 0.1 - 8 x 10-8 (Weiden, 1971;
(Metcalf, 1971
1 x 105 Metcalf, 1971
Human serum 0.95 x 103 Metcalf, 1971
Haman plasma 2.3 x 10-5 Wilhelm, 1967
Human RBC 4.6 x 10-7 Wilhelm, 1967
a All values were referenced from various sources, either reviewed or presented as
original data in Bull. Wld Hlth Org., 1971, 44 (1,2,3), 1-470.
Special studies on mutagenicity
A "dominant-lethal study" was conducted on groups of 12 male mice
where the mice were administered a single i.p. dose of propoxur at
levels of 0, 2.5 or 5.0 mg/kg. For a period of six weeks, three virgin
females were exposed to each male at weekly intervals and reproduction
indices recorded. Males treated with 5 mg/kg had slightly lessened
physical activity which lasted one to two days following the treatment
and was reflected in the reduced number of implantations noted in the
treated groups with the females fertilized in the first week after
treatment. The acute administration of propoxur proved to have a
transient effect on the reproduction capability of the males, but
there was no evidence of early resorption which is indicative of an
absence of mutagenic defect (Arnold et al., 1971).
Special studies on neurotoxicity
Adult white leghorn hens wore orally administered proPoxur at
levels ranging from 100 to 1000 mg/kg in a single oral dose or as a
single i.p. dose at levels ranging from 25 to 100 mg/kg. In two of the
three trials, PAM (100 mg/kg) and atropine sulfate (50 mg/kg) were
injected intraperitoneally prior to treatment. No neurotoxic signs of
poisoning similar to that observed with TOCP were noted during periods
of up to six weeks of observation after treatment (Kimmerle, 1964,
1966a).
Groups of eight adult white leghorn hens were fed propoxur in the
diet at levels of 0, 300, 1500, 3000 and 4500 ppm for 30 days. No
evidence of delayed neurotoxic signs of poisoning was seen either
during the period of feeding or in a posttreatment observation period
of four weeks. Histological examination of sciatic nerve and spinal
cord showed no evidence of demyelination (Kimmerle, 1966a; Hobik,
1967).
Special studies on potentiation
Intraperitoneal administration of propoxur to adult female rats
in combination with 20 different anticholinesterase insecticides (19
organophosphates and one carbamate) administered at a dose level of
one-half of the LD50 did not result in an increase in the acute
toxicity (DuBois and Raymund, 1961b and c; Nelson, 1967). Propoxur
does not appear to potentiate antiesterase activity and, as it is a
weak inhibitor of pseudocholinesterase (serum cholinesterase Ki = 9.5
x 102 Lmol-1 min-1) it is doubtful that aliesterase inhibition, a
better indicator of potentiation, would be significantly reduced.
Special studies on teratogenicity
Groups of 10 pregnant rats were fed propoxur in the diet at
concentrations of 0, 1000, 3000 and 10 000 ppm during gestation.
Administration of 3000 ppm and above had an adverse effect on the
parents. At 10 000 ppm, there was a reduction in the number of fetuses
and an increase in the number of resorption sites. This was not
evident at 3000 ppm. There appeared to be a dose-dependent
relationship between the reduction in fetal weight (although only the
3000 and 10 000 ppm were statistically significant) accompanied by a
dose-dependent decrease in placental weight. The dietary concentration
of 1000 ppm was tolerated by the parents and the fetuses and, except
for a slight reduction in the average fetal weight, this level showed
no detrimental effects. Teratogenic abnormalities were not noted in
this study at any dosage level (Lorke, 1970).
Acute toxicity
(a) Original compound
Species Sex Route LD50 Reference
(Mg/kg)
Rat M & F oral 80-191 Ben Dyke et al., 1970;
DuBois and Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961, 1966b, 1971;
Klimmer, 1963
(cont'd)
Species Sex Route LD50 Reference
(Mg/kg)
Guinea-pig M oral 40 DuBois and Raymund, 1961a
Chicken F oral 150-750 DuBois, 1962;
Kimmerle, 1964
Rat M & F i.p. 10-30 DuBois and Raymund, 1961a;
Kimmerle, 1961;
Klimmer, 1963;
Nelson, 1967
Guinea-pig M i.p. 16 DuBois and Raymund, 1961a
Mouse M & F i.p. 14-20 DuBois and Raymund, 1961a
Rat M & F dermal 1000->2400 Ben Dyke et al., 1970;
DuBois and-Raymund, 1961a;
Gaines, 1969;
Kimmerle, 1961;
Klimmer, 1963
Rat i.v. 10.6 Vandekar, 1965
Rat i.m. 53 Vandekar, 1965
b) Metabolites
Species Sex Route LD50 Reference
(mg/kg)
2-bydroxyphenyl
N-methylcarbamate
Mice i.p. >167 Balba and Casida, 1968
5-hydroxy propoxur
Mice i.p. >56 Balba and Casida, 1968
4-hydroxy propoxur
Mice i.p. 52 Balba and Casida, 1968
Propoxur
Mice i.p. 12 Balba and Casida, 1968
O-isopropoxyphenol
Rat F oral >1000 DuBois, 1963
dermal
The signs of poisoning shown by propoxur are typical of those
induced by cholinesterase-inhibiting carbamate esters. Tremors, muscle
spasm, lacrimation, salivation and secretion of red tears were
observed in rats following acute dosing. The symptoms appeared rapidly
after administration and recovery was fast.
Following oral administration of propoxur to rats, i.p.
administration of atropine sulfate was found to be antidotal, while
treatment with oximes (PAM, 50 mg/kg and BH6, 20 mg/kg) afforded no
protection and were contraindicated (Kimmerle, 1961b).
Tetraethylammoniumchloride proved also to afford no protection from
the acute effects of propoxur (Kimmerle, 1971).
Subacute dermal toxicity
Groups of rabbits (five males and five females per group) were
treated dermally with propoxur at a level of 500 mg/kg for two weeks.
Residues of the material remaining on the skin prior to each
application were not washed off. Twenty-four hours after the final
application., the skin was washed with soap and water and the animals
observed for two further weeks. The treatment did not affect the
general behaviour and weight gain of the animal and clinical
examinations of blood, urine, liver and kidney function over the
two-week period were normal (Kimmerle and Solmeeke, 1971). Propoxur
was found to be nonirritating to the skin of rabbits when applied to
the inside of a rabbit's ear for 24 hours. No signs of irritation or
poisoning were observed when propoxur was applied to the shaved
abdominal skin of rats and allowed to remain for four hours (Kimmerle,
1961).
Short-term studies
Rat. Groups of rats (15 males and 15 females per group) were fed
propoxur in the diet at levels of 0, 1000, 2000, 4000 and 8000 ppm
(females were fed only dietary levels of 0 and 4000 ppm) for nine
weeks. There was an increase in mortality in the animals fed 4000 and
8000 ppm and a reduction in food consumption and weight gain was
observed in all animals (Löser, 1965).
Oral administration of propoxur to 25 male rats at a dose of 5
mg/kg/day, six days a week for six months (20 male rats served as
controls) resulted in no effects attributable to the administration of
the compound. Growth and food consumption were similar to the
controls, and gross and histological examination of tissues showed no
effects attributable to the administration of propoxur (Klimmer,
1963).
Groups of rats (12 males and 12 females per group) were fed
propoxur in the diet for 16 weeks at concentrations of 0, 5, 10, 50,
100 and 200 ppm (those animals fed 100 and 200 ppm were increased
after the first three weeks of feeding to 400 and 800 ppm respectively
for the remainder of the study). The administration of propoxur at
levels up to 800 ppm in the diet did not affect food consumption,
growth, mortality or gross and histological examination of tissues.
Cholinesterase activity, measured manometrically in the blood, brain
and submaxillary glands, was not affected (Root et al., 1963).
Rats were treated orally for four weeks at 0, 3, 10 and 30 mg/kg.
The highest level caused signs of poisoning and cholinesterase
depression was noted at 10 and 30 mg/kg, with no depression noted at
the low level (Eben and Kimmerle, 1973).
Groups of rats were fed propoxur in the diet at 0, 250, 750 and
2000 ppm for 15 weeks. Propoxur was tolerated with no signs of
poisoning and no consistent evidence of cholinesterase depression
(Eben and Kimmerle, 1973).
Groups of rats (10 males and 10 females per group) were fed
propoxur in the diet at concentrations of 0, 250, 500, 1000 and 2000
ppm for 16 weeks. Food consumption and body weight gain in the females
receiving concentrations of 1000 ppm and above were reduced. No
significant changes were noted with regard to organ weight data. The
1000 and 2000 ppm levels caused depression of cholinesterase activity
in whole blood after the twelfth week of testing and, on completion of
the feeding study, cholinesterase activity was depressed in plasma,
whole blood and brain. Although all clinical enzyme data were found to
be within the normal range, some histopathological changes were noted
in the livers of the animals which received 1000 and 2000 ppm. The
no-effect level in this study was judged to be 500 ppm (Syrowatka et
al., 1971).
Dog. Groups of beagle dogs (four males and four females per group)
were fed propoxur in the diet at concentrations of 0, 100, 250, 750
and 2000 ppm for two years. Mortality was evident at 2000 ppm. One of
the male dogs and none of the female dogs at this level survived to
the end of two years. Food consumption was reduced at this high level.
The animals receiving 2000 ppm at times exhibited signs of
cholinesterase depression, especially in the first six months of
testing. Dietary concentrations of 750 ppm and below did not affect
appearance, behaviour, food consumption or growth of the animals.
Haematological examinations and liver and kidney function tests showed
no effects of propoxur at any dosage level examined. Activity of
leucine-amino peptidase was slightly elevated at levels of 750 and
2000 ppm. Gross examination of tissues showed that there was a
slightly increased liver to body weight ratio in males at 2000 ppm.
There was no indication of cellular damage in any tissue as evidenced
by histological examination of tissues. Although there was a slight
increase in leucine-amino peptidase activity at 750 ppm, based upon
all other considerations, it was assumed that a level of 750 ppm is a
no-effect level. Based on food consumption data, the no-effect level
in dogs would be 50 mg/kg bw/day (Löser, 1968c; Mawdesley-Thomas,
1969c).
Long-term studies
Groups of SPF rats (25 males and 25 females per group, 50 males
and 50 females per control group) were fed propoxur in the diet at
levels of 0, 250, 750, 2000 and 6000 ppm for two years. Dietary
concentration of 6000 ppm caused a reduction in food consumption in
male rats, while 2000 ppm and above resulted in a similar effect in
females. This reduction in food intake was reflected in body weight
gains Of these two groups. On gross examination, at the end of two
years some slight effects were noted in some organs. especially liver
which was enlarged relative to the body weight at the highest feeding
level in male rats and at the highest three feeding levels in female
rats. This increased liver weight was not reflected in liver function
tests or in clinical chemistry examination. Cholinesterase examination
in whole blood (performed only at six months) showed no depression of
cholinesterase activity at 6000 ppm in males and females. Histological
examination of tissues showed no effects relating to the feeding of
propoxur. A no-effect level in this study, based upon increased
relative liver to body weight ratio, is 250 ppm (Loser, 1968b;
Mawdesley-Thomas, 1969b).
Observations in man
Because of the widespread experimental views of propoxur and
control through the auspices of WHO, some significant observations in
humans are available (Plestina, 1968; Dawson, 1964; Vandekar, 1969;
Vandekar et al., 1968, 1971). In a study undertaken to develop a
quantitative method for determining metabolites of propoxur. Dawson et
al. (1964) showed that oral administration of 110 and 116 mg/person
Produced no signs of illness. The level of urinary phenols reached 140
ppm in the absence of clinical signs of poisoning. In persons engaged
in spraying or other occupation exposure, urinary levels of 80 ppm are
uniformly associated with illness. In another study, Vandekar et al.
(1971) administered 135 mg/person to a male volunteer (1.5 mg/kg bw)
and within 20 minutes after ingestion described clinical signs of
poisoning due to the carbamate. Significant erythrocyte cholinesterase
depression was evident coinciding with clinical signs of poisoning,
while plasma cholinesterase depression was not observed. Two hours
after the ingestion of propoxur, there were no signs of poisoning and
the rapid disappearance of symptoms was consistent with the rapid
recovery of erythrocyte cholinesterase activity. Absorption and
excretion of propoxur was very rapid as evidenced by measurement of
urinary phenols which reached a maximum value within four hours of
almost 200 ppm. Of the total phenol content excreted, 81% was found
within five hours after administration. In another experiment, a
single dose of 0.36 mg/kg again produced a rapid fall of erythrocyte
cholinesterase to 57% of normal within 10 minutes. Cholinesterase
recovered to its normal value within three hours. Within 10 minutes of
the administration of propoxur, short-lasting stomach discomfort,
blurred vision, and moderate facial redness and sweating were evident
in the volunteer.
A number of experiments was carried out to study the effect of
storage or build-up of propoxur in the body. Human volunteers took
doses of either 0.15 or 0.2 mg/kg at half-hour intervals for a total
of five doses. In each subject a symptomless depression of erythrocyte
cholinesterase to about 60% of normal was observed. At the cessation
of dosing, enzyme recovery was rapid, being complete within two hours.
Similarly pronounced and as a rule symptomless daily depression and
reactivation of cholinesterase was observed in persons who are
occupationally exposed to propoxur. Studies in humans have shown that
depression of erythrocyte cholinesterase (rather than plasma
cholinesterase) is a significant indicator of exposure to propoxur.
This is consistent with the difference observed in the in vitro
affinity of propoxur for the two enzymes, the I50 values for
erythrocyte and plasma cholinesterase being 4.6 x 10-7 M and 2.3 x
10-5 M, respectively.
Vandekar et al. (1968) published the results of studies carried
out on spray operators and local inhabitants in Iran as part of a WHO
control programme. It was reported that following over-exposure some
spray operators and local inhabitants suffered mild temporary
cholinergic signs of poisoning (headache, nausea). In most of the
cases, the complaints were found to be due to heavy contamination of
propoxur on the skin.
It is evident from these studies that a single oral dose (between
0.2 and 0.4 mg/kg) of propoxur may produce symptoms in man of short
duration. Higher doses may be tolerated without evidence of poisoning
(although there is appreciable inhibition of erythrocyte
cholinesterase) if the higher doses are divided into portions and
administered over reasonably short periods of time. Within two hours
following exposure, cholinesterase depression would be expected to be
normal.
Comments
Propoxur, an anticholinesterase carbamate ester, induces typical
signs of cholinesterase inhibition in both laboratory animals and
humans. Reversible depression of cholinesterase activity is evident a
short time after exposure, although the sensitivity of various
cholinesterase sources differ in different animal species. Erythrocyte
cholinesterase is significantly more sensitive than plasma
cholinesterase in humans. The sensitivity of brain and plasma
cholinesterase appears to be of the same magnitude in rats. Although
0.36 mg/kg resulted in signs of acute poisoning in man, the repeated
administration of 0.2 mg/kg at half-hour intervals for 2.5 hours
resulted in no signs of poisoning, although cholinesterase activity
was depressed. This cholinesterase returned to normal within two hours
following exposure.
Propoxur is rapidly absorbed, metabolized and eliminated.
Teratogenicity and mutagenicity studies in the rat gave negative
results and reproduction was not affected by propoxur.
A long-term rat study provided no evidence of carcinogenic
activity. Liver weight was increased in both males and females at high
dosage levels. No changes were found in liver function tests, clinical
chemistry or on histological examination. In view of the histological
changes in the livers of rats exposed over a short period to 1000 ppm,
the increase in relative liver weight was considered a significant
effect. 250 ppm in the diet was accepted as the no-effect level in the
rat.
In a two-year dog study, a slight increase in leucine-amino
peptidase activity was not regarded as significant. The no-effect
level as evidenced by liver damage was 750 ppm in the diet which,
based on feed consumption data, was 50 mg/kg bw. Cholinesterase
depression was not observed in either the two-year rat or dog studies.
It was evident to the Meeting that the methodology used to determine
cholinesterase activity in these studies was not adequate to measure
depression caused by propoxur.
The no-effect level in the long-term study in the rat was used as
a basis for estimation of the ADI.
The rapid reversibility of acute signs of poisoning in man and
the fact that sensitivity to the toxic effects of propoxur decreased
during prolonged exposure was reassuring in estimating the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.02 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolic aspects
The metabolism of propoxur was studied in mammals by Dawson et
al. (1964), Everett and Gronberg (1970), Krishna and Casida (1966),
Waggoner and Olson (1971); in man by Dawson et al. (1964), Hayes
(1971); in insects by Metcalf et al. (1967), Shrivastava (1969); in
plants by Aba el Wahab (1966), Dorough and Casida (1964), Everett and
Gronberg (1968), Gronberg (1970), Kuhr and Casida (1967).
In vitro in animals studies were made by Crosby et al. (1965),
Dorough and Casida (1964) and Oonithan and Casida (1966, 1968).
In rats
The metabolites identified in the rat (see below) include those
found in plants, in insects and those derived from microsomes.
Whereas the oxidative pathways and hydrolytic degradation occur
in the same order of magnitude in mammals, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
Adsorption, distribution and excretion in mammals, including
biotransformation in rats: following oral administration in rats,
propoxur is rapidly ingested to the digestive tract, metabolized in
the body and excreted.
The rats eliminated 85% of the radio-activity after oral
administration of 14C carbonyl labelled, 14C isopropoxy labelled 3H
isopropyl labelled propoxur within 16 hours; 60% of this and amount
was excreted in the urine as conjugates and 20-25% as volatile
compounds in a C02/acetone ratio of 85:15. Only 1-5% of the activity
was found in the faeces in the same period (Everett and Gronberg,
1970).
Evidence was obtained by the same authors that the major routes
of metabolism in rats are depropylation to
2-hydroxyphenyl-N-Methylcarbamate (further indicated as metabolite
A) and subsequent hydrolysis to isopropoxyphenol (metabolite I).
Minor metabolic pathways are ring hydroxylation at the 5 or 6
position (C and E), secondary hydroxylation of the 2-carbon atom of
the isopropoxy group (D and G) and hydroxylation of the N-methyl
group.
From the conjugated compounds in the urine, the following
metabolites were released by hydrolysis of the urine sample with
glucuronidase and/or acid. They were identified by their infra-red and
mass spectra as well as from the 3H/14C ratio yielded of double
labelled propoxur: 2-hydrophenyl-N-methylcarbamate (A),
2-isopropoxyphenyl-N-hydroxy methylearbamate (B) and
2-isopropoxy-5-hydroxyphenyl-N-methylearbamate (C).
The metabolic pathways of propoxur in rats as proposed by Everett
and Gronberg (1970) is shown in the diagram on the following page.
 Krishna and Casida (1966) administered 14C carbonyl labelled
propoxur intraperitoneally to rats. After 48 hours only 2.1% of the
administered radio-activity remained in the body; 60% of the activity
was excreted in the urine in the first 29 hours, whereas only 1.2% was
excreted in the faeces. Within 48 hours 31.2% of the administered
radio-activity was expired as CO2.
From these data it may be concluded that the carbamate group was
cleaved from one-third of the injected propoxur dose.
In a similar experiment with 14C isopropyl labelled propoxur,
70-75% of the activity was excreted in the urine, whilst 30%. of the
administered activity was expired as 14CO2; 4% of the activity
remained in the body and only 0.7% was excreted in the faeces.
From this it may be concluded that the isopropyl group is cleaved
from about one-quarter of the injected dose.
Dorough and Casida (1964) incubated rat liver microsomes with
propoxur and obtained 30% conversion to a metabolite from which with
acid Isopropoxyphenol and formaldehyde were yielded. With
cochromatography and infra-red spectroscopy the metabolite was
confirmed with (B).
Oonithan and Casida (1968) studied the metabolic fate of propoxur
in a system containing rat liver microsomes and NADPH2. Two
metabolites were formed, probably (A) and (B).
In cattle
Waggoner and Olson (1971) determined residues in tissues and milk
of cattle after feeding on a diet containing propoxur for 28 days. In
those cases where residues could be detected the amounts of the
metabolite (A) was greater than the parent propoxur.
After feeding 7.5 mg/kg/day of propoxur, the residues of the
parent compound and the metabolite (A) were respectively:
ppm
propoxur (A)
kidney 0.04 0.13
milk 0.001 0.0027
In insects
Metcalf et al. (1967) treated flies with 14C isopropoxy labelled
propoxur and found metabolism to CO2. A pre-treatment with piperonyl
butoxide reduced the formation of CO2 to one-third.
Dorough et al. (1963) and Dorough and Casida (1964) found in
cockroaches the metabolite (B) after injection of propoxur.
Shrivastava et al. (1969) (see also Ruhr, 1968, 1970) studied
in vivo and in vitro the metabolism of propoxur in houseflies.
The following metabolites were isolated in order of decreasing amounts
(C), (A) and acetone, B and 2-isopropoxyphenyl carbamate. These
metabolites were all conjugated due to their hydroxyl groups and
volatilized in acetone (see also Casida et al., 1968).
Tsukamoto and Casida (1967a and b) investigated the metabolism of
propoxur in a system of house-fly microsomes and NADPH2; they found
the metabolites (A), (B) and (C) as major metabolites.
Propoxur is a non-systemic carbamate insecticide which is used
against a relatively broad spectrum of insects in field crops, fruit
and vegetables (aphids, including woolly aphid, lygus bugs,
leafhoppers, saw-flies, thrips, millipedes, etc.).
The product is registered and used in several countries in Europe
and in other parts of the world.
Propoxur is used extensively for hygienic purposes against
cockroaches, flies, etc. in homes, hotels, restaurants and warehouses.
Pre-harvest treatments
Major crops on which propoxur is used are rice, sugar cane, pome
and stone fruits, small fruits, vegetables and potatoes. The following
estimates can be given of the use in different areas:
rice about 30%
other field crops about 30%
cacao about 20%
other crops such as fruit,
vegetables, ornamentals about 20%.
The following table summarizes the recommendations in accordance
with good agricultural practice, including rates of application and
pre-harvest intervals.
Dosage rate Minimum pre-harvest
Crop g a.i./ha or interval recommended
g a.i./100 l days
Field crops
potatoes 250-600 g/ha 7
rice 400-750 g/ha 7
sugar cane 750-1000 g/ha 7
Cacao 250-600 g/ha 7
Fruits
Pome fruits
apple, pears 50-75 g/100 l 7
600-1200 g/ha
Stone fruits
peach, plums 50-75 g/100 l 7
900-1200 g/ha
Small fruits
blackberries, gooseberries,
red currants, raspberries,
strawberries 50-75 g/100 l 7
600-1200 g/ha
Vegetables (outdoors)
beans, cabbage, gherkins,
leek, lettuce, onionp
peas, spinach 400-750 g/ha 4-7
vegetables (glasshouses)
bell peppers, cucumbers,
gherkins, melons,
tomatoes 400-750 g/ha 4
Leafy vegetables such as
lettuce, spinach 400-750 g/ha 14-21
Post-harvest treatments
No treatments recommended.
Other uses
Propoxur is used on ornamentals and flower crops.
It is also extensively used in the hygienic sector in the form of
aerosols, thermal fog concentrates, baits, wettable powder and
emulsifiable liquids, against a number of household and domestic pests
such as bugs, cockroaches, flies, mosquitos, beetles, silverfish, etc.
Pre-harvest intervals officially established in
various countries in days
Austria
35 days, all fruit and vegetables
Belgium
21 days, lettuce and endive (glasshouse cultures) during
winter period
14 days, lettuce and endive (outdoors and glasshouse)
7 days, other vegetables except those mentioned below,
agricultural crops
3 days, gherkins, tomatoes (both under glass and outdoors),
bell peppers, cucumber, melons
Denmark
14 days, fruit, vegetables and field crops
Germany, Federal Republic of
21 days, cereals
14 days, potatoes
7 days, leafy vegetables (except lettuce), tomatoes,
gherkins, melons
4 days, pome and stone fruit, gooseberries, red and black
currants, raspberries, strawberries, cabbage, carrot,
celery, garden beet, leek, lettuce, onion, radish, horse
radish, sugar and fodder beet
Netherlands
21 days, lettuce and endive (glasshouse) between
November/March
14 days, lettuce and endive (outdoors.and glasshouse)
7 days, fruit, including berries, vegetables except those
mentioned below
4 days, gherkins (glasshouse and outdoors)
3 days, tomatoes, bell peppers, cucumbers, melons
New Zealand
21 days, all crops
Poland
7 days, potatoes
Spain
30 days, fruit, sugar beet, cotton
Sweden
14 days, vegetables
7 days, all other crops
United Kingdom
7 days, all outdoor crops
2 days, cucumbers, tomatoes (glasshouse)
Yugoslavia
21 days, fruit
Residue data from supervised trials
Residue data were obtained from trials on several fruits,
vegetables and field crops, such as apples. sour and sweet cherries,
peaches, plums, black and red currants, gooseberries, French beans,
bell peppers, red and white cabbage, savoy, carrots, cucumbers,
lettuce, leek, onions, peas, spinach, tomatoes, alfalfa, cereals,
rice, tobacco, cocoa, grassland. These data are summarized in Tables 1
and 2.
TABLE 1. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
Fruit
apples Belgium 1964 1 (75 g/100 l) w.p. 50% 0.3 n.d. n.d.
Germany, 1966 1 (75 g/100 l) w.p. 50% 1.6 0.96 0.48 0.43
Fed. Rep. 1966 1 (75 g/100 l) w.p. 50% 2.0 0.95 0.95 0.95 0.8
1964 1 (50 g/100 l) w.p. 50% 1.3 0.8 0.7 0.6 0.6
1964 1 (100 g/100 l) w.p. 50% 2.0 1.4 0.7 0.6 0.6
Netherlands 1964 2 (l.v.) w.p. 50% 1.0-1.4 0.5-0.7 0.4-0.5 0.2-0.4 0.1-0.2
1964 2 (h.v.) w.p. 50% 0.6-1.1 0.2 0.2 0.1-0.3 0.1-0.3
1965 2.5 (h.v.) w.p. 50% 0.33-1.6 0.38- 0.31-
0.80 0.44
1965 2.5 (h.v.) w.p. 50% 0.29- 0.28-
0.42 0.33
cherries, Germany, 1969 1 1.5 w.p. 50% 3.1 0.45 0.18
sour Fed. Rep. 1 1.5 w.p. 50% 5.0 0.3 0.24
cherries, Germany, 1968 1 (50 g/100 l) w.p. 50% 0.05
sweet Fed. Rep. 2 (50 g/100 l) w.p. 50% 0.06
peaches Germany, 1967 1 (50 g/100 l) w.p. 50% 1.55 0.5 0.25 0.2
Fed. Rep 1968 1 (75 g/100 l) w.p. 50% 3 2.0 1.25 0.65
1968 1 (75 g/100 l) w.p. 50% 8.7 2.36 1.65
1968 1 (75 g/100 l) w.p. 50% 2.9 1.8 0.9
1968 1 (75 g/100 l) w.p. 50% 3.9 1.5 0.5
plums Germany, 1967 1 (50 g/100 l) w.p. 50% 0.55 n.d. n.d. n.d.
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 2.16 0.52 0.2
1968 1 (75 g/100 l) w.p. 50% 3.71 1.75 1.5
1968 1 (75 g/100 l) w.p. 50% 3.05 1.5 0.7
1968 1 (75 g/100 l) w.p. 50% 1.6 0.7 0.15
1968 1 (75 g/100 l) w.p. 50% 2.75 1.45 0.7
1969 1 1.5 w.p. 50% 2.5 1.35 <0.05
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1969 1 1.5 w.p. 50% <0.05 <0.05 <0.05
red Netherlands 1965 1 0.75 (l.v.) w.p. 50% 1.31-2.11 <0.01- <0.01-
0.08 <0.01
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 7.25 0.75 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 14.1 0.64 0.61
1968 1 (75 g/100 l) w.p. 50% 8.2 1.1 0.64
black Netherlands 1964 1 (50 g/100 l) w.p. 50% 2.35 0.7
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 15 0.7 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 13.4 0.6 0.4
1 (75 g/100 l) w.p. 50% 16.7 2.45 1.35
1 (75 g/100 l) w.p. 50% 4.2 1.3 0.45 0.11
gooseberries Germany, 1968 1 (75 g/100 l) w.p. 50% 3.5 0.6 0.3
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 5.8 0.6 0.25
1968 1 (75 g/100 l) w.p. 50% 3.6 0.45
1968 1 (75 g/100 l) w.p. 50% 6.7 0.53 0.20
1968 1 (75 g/100 l) w.p. 50% 6.3 0.83 0.23
Vegetables
French Germany 1964 1 0.7 w.p. 50% 1.25 0.25 <0.1
bean Fed. Rep. 1969 1 0.45 w.p. 50% 1.65 0.55 0.20
1968 1 0.75 w.p. 50% 0.5 0.25
1968 1 0.75 w.p. 50% 1.6 1.1 1.0
1969 1 0.75 w.p. 50% 0.5 0.35 0.25
1969 1 0.75 w.p. 50% 0.6 0.4 0.25
1967 1 0.5 w.p. 50% 0.75 0.3 0.15
1968 1 0.45 w.p. 50% 0.9 0.2 0.1
1968 1 0.45 w.p. 50% 0.7 0.3 0.1
1969 1 0.45 w.p. 50% 1.6 0.5 0.08
UK 1964 1 0.7 w.p. 50% 0.25
-1.55
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
bell peppers Netherlands 1968 1 0.38 w.p. 50% 0.75 0.3 <0.1
(glasshouse)
red Germany, 1964 1 0.15 w.p. 50% 1.0 0.4 0.2 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 1.6 1.3 0.9 0.4
savoy Germany, 1964 1 0.15 w.p. 50% 3.9 0.9 0.2 <0.2
Fed. Rep. 1964 1 0.15 w.p. 50% 2.7 0.8 0.7 0.6
1968 1 0.6 w.p. 50% 5.3 2.1 0.7 1.2
1968 1 0.6 w.p. 50% 8.0 3.9 3.4 1.8
white Germany, 1964 1 0.15 w.p. 50% 2.2 1.3 0.6 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 5.8 2.1 1.2 0.6
carrots Germany, 1968 1 0.45 w.p. 50% 0.1 n.d n.d.
Fed. Rep. 1968 1 0.75 w.p. 50% n.d. 0.2 0.25
1968 1 0.75 w.p. 50% 0.1 0.15 0.25
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% 0.3 0.7 0 0.3
cucumbers Netherlands 1970 1 0.5 dust 2% 0.05 0.07 n.d.
(glasshouse) 1970 1 0.5 dust 2% 0.07 0.06 n.d.
leek Germany, 1968 1 0.45 w.p. 50% 0.5 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 0.6 0.1 n.d.
1968 1 0.45 w.p. 50% 10.9 1.1 1.0
1968 1 0.6 w.p. 50% 2.9 0.6 0.6
1968 1 0.75 w.p. 50% 2.3 0.25 0.1
1968 1 0.75 w.p. 50% 2.0 0.7 0.15
lettuce Germany, 1964 1 0.6 w.p. 50% 6.8 0.4 0.2 0.1
(outdoor) Fed. Rep. 1964 1 0.6 w.p. 50% 5.1 0.2 1.3 1.1
1 w.p. 50% 1.8 0.4 0.2 0.1
1 w.p. 50% 2.2 1.6 0.9 0.7
lettuce Netherlands 1963 1 0.66 w.p. 50% 17.2- 9.2- 5.4- 1.8-4.1 0.9-1.9 0.5-0.8
(glasshouse) 20.2 10.9 10.4
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1971 1 0.8-0.9 w.p. 50% 15.2 10.9 4.1
1971 1 0.6-0.9 w.p. 50% 10.0 7.45 3.1
1971 1 0.6-0.9 w.p. 50% 4.0 5.5 1.9
1971 1 0.6-0.9 w.p. 50% 8.5 7.0 2.7
1971 1 0.6-0.9 w.p. 50% 7.25 6.8 2.4
onions Germany, 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
1968 1 0.45 w.p. 50% 9.3 4.2 0.87
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50%
peas Germany, 1964 1 0.7 w.p. 50% 0.3 n.d. n.d.
pods Fed. Rep. 1964 0.4 0.1 <0.1
spinach Germany, 1968 1 0.45 w.p. 50% 5.7 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 6.8 n.d. n.d.
1969 1 0.45 w.p. 50% 32 0.6 0.06
1969 1 0.45 w.p. 50% 33.5 0.7 0.06
1969 1 0.75 w.p. 50% 27 7.3 0.6
tomatoes Netherlands 1971 1 0.5 dust 0.28 <0.05 n.d.
(glasshouse 1971 1 0.5 dust 0.33 <0.05 n.d.
1971 1 11 g/100 m3 smoke 0.07 n.d.
Field Crops
alfalfa USA 1970 1 1.0 w.p. 70% 59.9 12.2
green 1 1.0 w.p. 70% 11.6 2.17
1 1.0 w.p. 70% 15.5 2.06
1 1.0 w.p. 70% 65.8 7.14
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1 1.0 w.p. 70% 36.8 3.40 1.15 0.20 0.59(28)
1 1.0 w.p. 70% 65.8 7.14 3.17 0.66 <0.12(29)
hay 1 1.0 w.p. 70% 16.4 3.67
1 1.0 w.p. 70% 3.21 1.33
seed hulls 3 1.0 w.p. 70% 0.35(28)
3 1.0 w.p. 70% 0.18(29)
2 1.5 w.p. 70% 0.13(46)
2 1.5 w.p. 70% 0.13(92)
chaffs 3 1.0 w.p. 70% 0.63(28)
3 1.0 w.p. 70% 0.24(29)
0.13(46)
0.08(92)
barley USA 1970
grain 1 0.4a w.p. 50% <0.05
1 0.4a w.p. 50% <0.24
straw 1 0.4a w.p. 50% <0.03
1 0.4a w.p. 50% 0.32
oats USA 1970
grain 1 0.4a w.p. 50% 0.09
1 0.4a w.p. 50% 0.15
1 0.4a w.p. 50% 0.10
1 0.4a w.p. 50% <0.04(25)
straw 1 0.4a w.p. 50% 0.01
1 0.4a w.p. 50% 0.07
1 0.4a w.p. 50% <0.19
1 0.4a w.p. 50%
1 0.4a w.p. 50% 0.01(25)
rye USA 1970
grain 1 0.4a w.p. 70% <0.03
straw 1 0.4a w.p. 70% 0.04
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
wheat USA
grain 1 0.4 w.p. 50% 0.21
straw 1.41
pasture USA 1 0.25 w.p. 70% 11.82 6.99 1.02
grass U.L.V.
1 0.5 w.p. 70% 0.63 0.59 1.20 0.76
rangeland USA 1 0.25 w.p. 70% 29.8 1.49 0.76
grass
cacao
whole beans 2 0.5 E.C. 20% n.d.(24)
2 0.25 E.C. 20% n.d.(24)
shell 2 0.84 E.C. 20% 0.3 0.3 0.3(56)
3 0.84 E.C. 20% 0.3
nib 3 0.84 E.C. 20% <0.1
a seed treatment 500 g/100 kg seed and foliar application
h.v. = high volume; l.v. = low volume; U.L.V. = ultra-low volume
TABLE 2. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest interval in days
Crop Country Year No. rate Formulation
kg a.i./ha 1-10 11-20 21-30 31-40 41-50
Rice hulled Japan 1972 1 0.4 dust 1% 0.02 0.06-0.09
1 0.4 dust 1% 0.03 0.02-0.04
1 0.4 dust 1% 0.02 0.02
1 0.4 dust 1% 0.02 <0.02-0.19
1 0.4 dust 1% <0.02 0.02
1 0.4 dust 1% 0.02-0.11
1 0.4 dust 1% 0.21 0.02
1 0.4 dust 1% 0.02 0.06
Paddy rice Japan 1972 1 0.2-0.75 E.C. 25% 0.03 0.08-0.33
0.05-0.56
0.26-0.40 <0.02-0.05
0.47-0.54
Upland rice Japan 1972 1 0.2-0.75 E.C. 25% 0.18
1 0.2-0.75 E.C. 25% 0.19
1 0.2-0.75 E.C. 25% 0.11
1 0.2-0.75 E.C. 25% 0.13
Fate of residues
Influence of light
Propoxur exposed to light shows no or very slight
photo-decomposition. Crosby (1965) found that propoxur was the only
one out of six N-methylcarbamates which was not converted to other
cholinesterase inhibitors when exposed to ultra-violet light or
sunlight.
Propoxur on silica gel-coated plates exposed to long-wave length
ultra-violet light did not produce degradation products, whereas after
exposure to short-wave length ultra-violet light three metabolites,
which gave a colour reaction with ninhydrin (Abdel-Wahab et al.,
1966), were found.
The product did not show any changes when such plates were
exposed to sunlight, even when sensitizers were applied to the (Ivie
and Casida, 1971).
Inert surfaces
Propoxur disappears from inert surfaces more or less rapidly
mainly through volatilization. The rate of disappearance depends on
the nature of the surface. Fifty per cent. of a propoxur residue on
glass, kept under laboratory conditions, was still present after 1.8
hours (Abdel-Wahab et al., 1966).
The half-life of propoxur on porcelain saucers under field
conditions was about three days (Wright and Jackson, 1971), whereas on
polythene foils a half-life of two days was found (Marchart, 1970).
Propoxur sprayed as an aqueous emulsion concentrate on filter
papers, which were suspended vertically at 2.5 inch in a kitchen (mean
temperature 23°C (21-29°C) and mean relative humidity 52% (42-68%),
evaporated 50% in about six weeks (Links, 1965).
After three months, 60% of the active ingredient was still
present on Hessian bags, sprayed with a wettable powder and stored in
an unheated large storage shed (Linke, 1965).
When propoxur was applied to plywood panels, 50% of it evaporated
in 15 days irrespective of the formulation used (Dorough and Crouch,
1966).
Inconsistencies in the results of different authors may not only
be due to different temperatures but also to air movements (Marchart,
1970), the influence of which is large but has not yet been studied
quantitatively.
The evaporation of propoxur from animate or inanimate substrates
is of practical significance. A considerable proportion of residues on
plants is eliminated by volatilization (Abdel-Wahab at al., 1966;
Gronberg, 1970; Marchart, 1970, 1971). On the other hand, the activity
of propoxur in the vapour phase is utilized for the control of storage
pests and household and domestic pests (Gahan and Wilson, 1970; Wright
et al., 1969).
In water
Propoxur is relatively stable in water at pH levels of less than
7. The rate of hydrolysis, resulting in the formation of
isopropoxyphenol increases rapidly from pH 7 upwards. The half-life of
the parent compound in buffered solutions at 20°C and pH 8 was 16
days, at pH 9, 38 hours and at pH 10, three hours (Aly and El-Dib,
1971).
At temperatures of 30°C and pH 7, the half-life of the parent
propoxur was three days, at pH 9, 1.2 hours.
Flint and Shaw (1971) determined the half-life of propoxur in
field experiments in shallow open vessels filled with bottom silt and
lake water. In these experiments, 50% of the propoxur disappeared in
12.7 hours at pH 7 and 27-36°C.
In similar experiments, in which smaller nearly air-tight vessels
were used, and in a parallel experiment with a biologically sterile
system, the half-lives were 54.9 and 80.8 hours respectively;
temperature range was 5-22°C.
In soil
Propoxur evaporates from the soil; the amount which evaporates
increases with increasing moisture content of the soil.
The time required to decrease a soil residue to one-half of the
initial concentration ranges from six to eight weeks, depending on
soil type (Flint and Shaw, 1971).
Metabolism in soil
The metabolism of propoxur in different soil types was studied by
Church-and Flint (1971) with radio-labelled compounds. After a soil
application of 3H-isopropoxy, 14C-carbonyl labelled propoxur, the
3H activity remained organosoluble, whereas the 14C activity was
concentrated in the water-soluble fraction. The organosoluble activity
was composed of isopropoxyphenol and traces of propoxur. The 14C
activity was incorporated into unknown water-soluble materials which
no longer behaved as carbamates.
In sterile soils or under anaerobic soil conditions, the 14C
activity decreased whilst the 3H activity remained almost constant.
This indicates that in these conditions a simple chemical hydrolysis
to isopropoxyphenol was occurring (Flint and Shaw, 1971).
In biologically active soils, the 14C and 3H activity declined
sharply after nine days, which gives an indication of microbial
degradation. In this case, no conjugated compounds were found (Church
and Flint, 1971).
Flint and Shaw equilibrated aqueous solutions of propoxur with
different soil types. The adsorption of propoxur to soil particles was
poor under these conditions. The following adsorption co-efficients
were found: 0.63 for sandy loam, 0.49 for silty clay loam and 1.12 for
highly organic silty clay loam.
In freshly tilled soils propoxur can be moved laterally by water.
In soil leaching experiments, propoxur moved with the water front
passing through the packed soil columns. In view of the poor stability
of propoxur in aqueous systems, the normal use will give no risks of
contaminating ground or superficial water.
Propoxur at normal rates showed only a slight effect on soil
microorganisms (Church and Flint, 1971; Houseworth and Tweedy, 1972)
and on microorganisms in waste disposal lagoons.
In plants
A large proportion of propoxur applied to the leaf surface
evaporates (Everett and Gronberg, 1968; Marchart, 1970, 1971;
Abdel-Wahab et al., 1966).
With radio-labelled propoxur, it was demonstrated that only a
small amount penetrates from the leaf surface into the leaves. After
five days the parent propoxur comprised 69-98% of the total 14C
activity present. The material that penetrated was shown to be
primarily the parent propoxur and water-soluble metabolites, mainly
the ß glucoside of 2-hydroxyphenyl N-methylcarbamate.
No downward translocation of propoxur could be demonstrated
(Everett and Gronberg, 1968).
The uptake of propoxur by plant roots from an aqueous solution
was shown to be directly related to the water uptake. Propoxur and
metabolites were translocated from the aqueous solution to the surface
of the leaf from which there is some volatilization.
14C carbonyl labelled propoxur injected into the stems of beans
and cotton plants was found to be converted into watersoluble
metabolites which remained stable for a relatively long period
(Dorough and Casida, 1965). In subsequent experiments Abdel-Wahab at
al. (1966) found that the water-soluble metabolites still possessed a
carbamate structure. The half-life of the parent propoxur after
injection into bean plants was one day.
Kuhr and Casida (1967) identified with thin layer chromatography
the water-soluble metabolites. Following incubation with ß
glucosidase, ether-extractable aglycones of the water-soluble
metabolites were yielded to the extent of 76%. Tentative
identification by cochromatography showed that 91.3% of the mixture
consisted of 2-hydroxyphenyl-N-methylearbamate (metabolite "A"
according to the scheme of the metabolic pathway in the section "Fate
in animals" as proposed by Everett and Gronberg (1970)) and 4.9% of
metabolite B = 2-isopropoxyphenyl-N-hydroxymethylcarbamate.
In these studies the metabolites (A) and (B) accounted for 30.2%
and 1.5% respectively of the applied activity, six days after the
injection of propoxur in the bean plant, together comprising 96% of
the water-soluble metabolites.
Five days after foliar application of 14C carbonyl labelled and
isopropoxy labelled propoxur on bean and maize plants, the residue on
the leaves consisted practically only of the parent compound. A
negligible portion was metabolite (A) (<1%). Also in the plant the
largest proportion of the radio-active material extractable with
organic solvents consisted of propoxur. After 3, 5, 7, 9 and 14 days,
its share of the measurable activity was 58.7%, 45.0%, 51.3%, 50.1%
and 36.4% respectively.
A large proportion of the carbonyl labelled material was present
in the water phase, the proportion of isopropyl labelled being less,
thus indicating that the isopropoxy group was cleaved from a
considerable portion of the applied parent compound. This assumption
was supported by the detection of acetone in the air pulled through
the chamber (Everett and Gronberg, 1968).
In an experiment in which carbonyl labelled and isopropoxy
labelled propoxur was absorbed from water by the roots, the proportion
of 14C active compound increased continuously during the 14-day
study. The aglycones of the conjugated metabolites could be almost
completely released with ß glucosidase. Cochromatography showed
agreement with metabolites (A) and (B). The ratio of these compounds
was 9:1 (Everett and Gronberg, 1968).
In a later experiment, Gronberg (1970) found that 14 days after
uptake of labelled propoxur by maize roots 50% of the residue in the
plant was still the parent compound, 19.2% of the residue was
accounted for by (A) and 3.5% by (B). Both metabolites were released
from their conjugates by ß glucosidase and positively identified by
infra-red spectroscopy. It was shown that 2-isopropoxy-4
hydroxyphenyl-N-methylcarbate did not occur.
Methods of residue analysis
Bio-assay methods were developed for the detection of propoxur
residues in soil using house crickets (Burkhardt and Fairchild, 1967)
as test insects and on fruit crops using Daphnia magna (Parker et
al., 1970). Voss (1968) developed an automated procedure for residue
analysis of propoxur in aqueous extracts of fruits based on
cholinesterase inhibition.
The above-mentioned methods are not specific and therefore not
suitable for regulatory purposes. The methods have become obsolete.
Several colorimetric methods have been developed. The only
satisfactory methods involve hydrolysis to isopropoxyphenol, which is
converted to a dyestuff and measured photometrically (see following
table).
The limits of determination range from 0.05 to 0.1 ppm.
TLC methods
Several authors describe TLC methods for the analysis of
propoxur. The most suitable are those based on cholinesterase
inhibition, since they require the simplest clean-up procedure and can
be used for various crop types.
The limits of determination are usually about 0.1 ppm.
GLC methods
Since propoxur, in common with other carbamates, readily
decomposes at high temperatures, GLC methods had to be developed in
which propoxur is converted to a stable derivative, which permits
detection with an electron capture detector. Also a method is
described in which the flame photometric detector is used after
derivatization.
SPECTROMETRIC RESIDUE METHODS FOR PROPOXUR
Crop Detection Sensitivity Reference
Sugar beet, tops,
lettuce IR; N-H-stretching bond 0.2 ppm Niessen and Frehse (1963)
Grapes IR; N-H-stretching bond ? Broderick (1966)
Milk spectrophotofluorometry 1.4 ppm Bowman and Beroza (1967)
Fruits, vegetables,
potatoes, cereals,
hops Aminoantipyrinea 0.05-0.1 ppm Niessen and Frehse (1964)
Human urine Aminoantipyrinea 10-20 ppm Dawson et al. (1964)
Lettuce 2,6-dibromo-benzoquinone- 0.1 ppm van Gils (1970)
chloroimidea
Sugar beets, p-nitrobenzene
potatoes diazoniumfluoboratea 0.05 ppm George (1967)
a After saponification.
THIN LAYER RESIDUE METHODS FOR PROPOXUR
Crop Stationary Solvent Detection Sensitivity Reference
phase system
Water silica gel different dimethylamino-benzaldehyde; 0.1 ppm Abbot et al. (1967)
nitrobenzene-diazonium
fluoborate
Water silica gel different dimethylamino-benzaldehyde 0.1 ppm El-Dib (1970)
Peas, carrots silica gel acetone + cholinesterase 10 ng Mendoza and Shields
hexane inhibition (0.1 ppm) (1971)
20 + 80
Tobacco aluminiumoxide acetone + fast blue B; 0.5 ppm Nesemann and Seehofer
hexane dichloroquinone-chloroimide (1970)
10 + 90
Apples, beets, silica gel acetone + cholinesterase 1 µg Wales et al. (1968)
cabbage, carrots, hexane inhibition
lettuce,
raspberries, 20 + 80
poultry meat
GAS-CHROMATOGRAPHIC RESIDUE METHODS FOR PROPOXUR
Crop Column Derivativea Sensitivity Reference
Apples, cucumbers, Chromosorb W DMCS -chloroacetyl 0.04 ppm Argauer (1969)
tomatoes, milk XE - 60
Corn silage, milk Gaschrom Q -thiophosphoryl 0.02-0.04 ppm Bowman and Beroza (1967)
DC-200
Potatoes, sugar Gaschrom Q -trichloroacetyl 0.01-0.1 ppm Butler and MeDonough (1968)
beets, apples, DC-200
grass
Water, peas, Chromosorb GAWDMCS -2,4-dinitrophenyl 0.2 ppm Cohen et al. (1970)
lettuce, apples XE 60 + Epikote 1001
Spinach Anakrom A B S -2,4-dinitroanilineb 0.05-0.2 ppm Holden et al. (1969)
XE -60
Soil Gaschrom Q -trichloroacetyl 0.02 ppm Stanley (1971)
OV -1
Animal tissue, Gaschrom Q -trichloroacetyl 0.002-0.02 ppm Stanley and Thornton (1972)
milk OV -1
Alfalfa, corn, Gaschrom Q -trichloroacetyl 0.02-0.05 ppm Stanley et al. (1972)
grass, cereals
a Of isopropoxyphenol.
b From reaction with methylamine.
Although the above-mentioned GLC methods are relatively
time-consuming, they are nevertheless the methods of choice. They are
very sensitive and specific (limits 0.002-0.05 ppm).
TLC and GLC methods may be suitable or can be adapted for
regulatory purposes.
The determination of propoxur residues in the trials carried out
by Bayer and reported in this monograph is carried out by the
colorimetric method of Niessen and Frehse (1964). This method
determines only the parent compound and is not fully specific.
The method includes a step for precipitating plant constituents,
which is also used in the methods referred to above.
The analysis in the residue experiments carried out by Chemagro
mentioned in the monograph was by GLC as described by Stanley et al.
(1972). The method is rather complicated, but makes it possible to
determine the parent compound and the main metabolites ((A) and (B),
see page) separately with a high degree of sensitivity.
The conjugated metabolites (A) (2-hydroxyphenyl methylcarbamate)
and (B) (2-isopropoxyphenyl hydroxymethylcarbamate) are released by
enzyme hydrolysis before clean-up. The metabolite (A) is then
alkylated. Next, the compounds are saponified to the phenols and
converted to their trichloroacetyl derivatives, which are determined
by GLC with electron capture detection.
National tolerances
Belgium Fruits and vegetables, 3 ppm
except potatoes
Germany, Federal Fruit 3 ppm
Republic of Sugar Beets 3 ppm
Vegetables, except 3 ppm
Cabbage 4 ppm
Lettuce 4 ppm
Other plant products 0.5 ppm
France Fruit and Vegetables 3 ppm
Italy Fruit and Vegetables 2.25 ppm
Netherlands Fruit and Vegetables 3 ppm
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Propoxur is a non-systemic carbamate insecticide which is used on
a considerable scale in various countries against a relatively broad
spectrum of insects in field crops, fruits and vegetables, e.g.
aphids, lygus bugs, leafhoppers, thrips, sawflies, etc.
Propoxur is also used extensively for hygienic purposes against
cockroaches, flies, etc., and against insect pests on ornamentals and
flower crops.
The technical material contains minimal 95% of
2-isopropoxy-phenyl-N-methyl carbamate. The impurities in the
technical material are known.
Propoxur is marketed in the form of wettable powder, emulsifiable
concentrate, dust, fly and cockroach baits, and balls against flies.
The concentration/rates of application vary depending on pest,
crop and methods of application. Normal application rates are 250-1000
g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. Most of the
residue data obtained in Europe show only the parent compound. The
trials carried out in the United States of America and a limited
number of trials carried out elsewhere determined not only the parent
propoxur but also the two main plant metabolites, (A)
2-hydroxyphenyl-N-methylcarbamate and (B)
2-isopropoxyphenyl-N-hydroxymethylcarbamate.
Information is available on the fate of propoxur residues in
soil, in plants. in mammals and in other animals, e.g. flies.
Some data on products of animal origin after feeding the animals
on treated crops are available indicating that residues are very low.
It would be desirable to have the result of more critical studies
which have been carried out on cows, pigs and chickens, in order to
confirm that this is the true position.
The breakdown of propoxur in plants and animals follows similar
pathways. The same metabolites are identified in rats, plants and in
vitro with microsomes.
Whereas oxidative and hydrolytic degradation both occur and
proceed to the same degree in rats, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
The residues in foods of plant or animal origin, following
recommended directions for use and recommended pre-harvest
intervals, consist largely of the parent compound. In plant products,
the above-mentioned metabolites (A) and (B) occur in a ratio of about
9:1. These metabolites, however, normally represent less than
one-third of the total residue determined.
Little information is available on the rate of decrease in the
level of residue of propoxur and its metabolites during storage and
processing, including household cooking. Little information is
available on propoxur residues in food moving in commerce.
Thin layer chromatographic and gas chromatographic procedures,
specific for propoxur and its main metabolites occurring in plants
(i.e. the metabolites (A) and (B), see above) and/or in products of
animal origin, are available.
The above-mentioned GLC methods are rather time-consuming due to
the fact that propoxur has to be converted to derivatives which are
stable to the GLC conditions. These GLC methods and the TLC methods
may be suitable or can be adapted for regulatory purposes. The most
suitable TLC methods are those based on cholinesterase inhibition.
The limit of determination of the TLC methods is usually about
0.1 ppm. The GLC methods allow sensitive and specific analysis (limits
of detection depending on commodity 0.002-0.05 ppm) of residues in
most crops and products of animal origin.
RECOMMENDATIONS
The following tolerances are based on residues likely to be found
at harvest following currently used patterns. The residues are
determined as propoxur and the main metabolites and are expressed as
propoxur.
Interval on which
Tolerances recommendations
are based (days)
Fruit, including apples,
pears, cherries, peaches,
plums 3 4-7
Soft fruit, including red
currants, blackberries,
gooseberries, strawberries 3 4-7
Vegetables, except potatoes
and root vegetables 3 outdoor 4-7
glasshouse:
leafy vegetables 14,
other vegetables 3-7
Potatoes, root vegetables -
Raw cereals 0.5 14
Rice (hulled) 0.1 7
Cocoa beans 0.05a 7
Meat 0.05a -
Milk (whole) 0.05a -
Animal feedstuff 5 7-14
a At or about the limit of determination
The time interval between application and harvest which has been
used in determining the maximum residue limits is appropriate to the
agricultural practices in numerous countries.
FURTHER WORK OR INFORMATION
Desirable
1. Studies to elucidate the significance of the changes in relative
liver weight in the rat.
2. Studies, including pharmacokinetic studies, to elucidate the
relationships between toxicity and effects on cholinesterase levels in
various species.
3. A long-term study in an animal species other than the rat.
4. Continued epidemiological studies with emphasis on cholinesterase
activity.
5. Studies on behavioural responses especially with low-level
exposure.
6. Results of critical studies to determine the nature and level of
residues in meat (including poultry), milk, and eggs to confirm
recommendations for limits in animal products.
REFERENCES
Abbott, D.C., Blake, K.W., Tarrant, K.R. and Thomson, J. (1967)
Thin-layer chromatographic separation, identification and estimation
of residues of some carbamate and allied pesticides in soil and water.
J. Chromatography, 30: 136-142
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. (1966) Fate of
C14-carbonyl-labelled aryl methylearbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14: 290-299
Aly, O.M. and El-Dib, M.A. (1971) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Research, 5:
1191-1205
Argauer, R.J. (1969) Determination of residues of Banol and other
carbamate pesticides after hydrolysis and chloroacetylation. J. Agr.
Food Chem. 17: 888-892
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Baygon in albino mice. Unpublished report
submitted by Bayer AG
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of metbylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkyl-phenyl methylcarbamates. J. Agr. Food Chem. 16: 561-567
Barlow, F. and Hadaway, A. B. Interactions between insecticides,
cellulose and water, and their effects on insecticide toxicity and
persistence. Society of Chemical Industry Monograph No. 29 (London)
Bayer AG Rückstandsberichte 1964-1969
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) Acute toxicity
data for pesticides (1970). World Review of Pest Control, 9: 119-127
Bowman, M.C. and Beroza, M. (1967) Determination of Niagara NIA-10242
and its phenol degradation product in corn silage and milk and
determination of other carbamates by GLC of their thiophosphoryl
derivatives. J. Ass. off. analyt. Chem. 50: 926-933
Bowman, M.C. and Beroza, M. (1967) Spectrophotofluoreseent and
spectrophotophosphorescent data on insecticidal carbamates and the
analyses of five carbamates in milk by spectrophotofluorometry.
Residue Reviews, 17: 23-34
Broderick, E.J., Bourke, J.B., Mattik, L.R., Taschenberg, E.F. and
Avens, A.W. (1966) Determination of methylcarbamate pesticides in the
presence of methyl anthranilate in Concord grapes. J. Ass. off.
analyt. Chem. 49: 982-985
Brooks, G.D., Smith, E.A. and Schoof, H.F. (1967) Residual
effectiveness of eleven insecticides under weathering conditions
against Aedes aegypti. Mosquito News, 27: 93-99
Burkhardt, C.C. and Fairchild, M.L. (1967) Toxicity of insecticides to
house crickets and bioassay of treated soils in the laboratory. J.
Econ. Ent. 60: 1496-1503
Butler, L.I. and McDonough, L.M. Method for the determination of
residues of carbamate insecticides by electron-capture gas
chromatography. J. Agr. Food Chem. 16: 403-407
Casida, J.E., Shrivastava, S.P. and Esaae, E.G. (1968) Selective
recovery of volatile products from house flies treated with
radioactive insecticide chemicals and synergists, J. Econ. Ent. 61:
1339-1344
Chemagro Corporation (1971) Baygon, the effects on the environment.
Unpublished report submitted by Chemagro
Chemagro Corporation (1971) Unpublished report submitted by Chemagro
Church, D.D. and Flint, D.R. (1971) The fate of Baygon
(O-isopropoxy-phenyl-N-methylcarbamate) in soil. Unpublished report
submitted by Chemagro
Cohen, I.C., Norcup, J., Ruzicka, J.H.A. and Wheals, B.B. (1970) An
electron-capture gas chromatographic method for the determination of
some carbamate insecticides as 2,4-dinitrophenyl derivatives of their
phenol moieties. J. Chromatography, 49: 215-221
Crosby, D.G., Leitis, E. and Winterlin, W.L. (1965) Carbamate
insecticides - photodecomposition of carbamate insecticides. J. Agr.
Food Chem. 13: 204-207
Dawson. J.A., Heath, D.F., Rose, J.A., Thain, E.M. and Ward, J.B.
(1964) Excretion by humans of phenol derived in vivo from arprocarb.
I. Determination by gas chromatography. II. Colorimetric
determination. Bull. Wld Hlth Org. 30(1): 127-134
Dorough, H.W. and Casida, J.E. (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12: 294-304
Dorough, H.W. and Crouch, G.W. (1966) Persistence and residual
effectiveness of various formulations of Baygon (Bayer 39007) against
the house fly. J. Econ. Ent. 59: 1188-1190
Dorough, H.W., Leeling, N.C. and Casida, J.E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science,
140: 170-171
Douch, P.G.C. and Smith, J.N. (1971) Metabolism of
m-tert.-butylphenyl N-methylcarbamate in insects and mice. Biochem.
J. 125: 385-393
Douch, P.G.C. and Smith, J.N. (1971) The metabolism of
3,5-Di-tert.-butylphenyl N-methyl-carbamate in insects and by mouse
liver enzymes. Biochem. J. 125: 395-400
DuBois, K.P., (1962) University of Chicago The acute oral toxicity of
Bayer 39 007 to chickens. Unpublished report submitted by Bayer AG
DuBois, K.P. (1963) University of Chicago The acute toxicity of some
possible metabolites of Bayer 39 007, 44 646, 37 344 and Morestan.
Unpublished report submitted by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961a) University of Chicago The
acute toxicity of Bayer 39 007 to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961b) University of Chicago The
acute toxicity of Bayer 39 007 given in combination with other
anticholinesterase insecticides to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961c) University of Chicago The
acute toxicity of Bayer 39 007 and Bayer 37 344 in combination with
some other anticholinesterase insecticides to rats. Unpublished report
submitted by Bayer AG
Eben, A. and Kimmerle, G. (1973) Propoxur, effect of acute and
subacute oral doses on acetyl cholinesterase activity in plasma,
erythrocytes and brain of rats. Unpublished report submitted by Bayer
AG
Eichelberger, J.W. and Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Env. Sci and Techn. 5: 541-544
El-Dib, M.A. (1970) Thin layer chromatographic detection of carbamate
and phenylurea pesticide residues in natural waters. J. Ass. off.
analyt. Chem. 53: 756-760
Everett, L.J. and Gronberg, R.R. (1968) Plant metabolism of Baygon.
Unpublished report submitted by Chemagro
Everett, L.J. and Gronberg, R.R. (1970) The metabolic fate of Baygon
(2-isopropoxyphenyl-N-methyl-carbamate) in the rat. Unpublished
reported submitted by Chemagro
Flint, D.R. and Shaw, H.R., II (1971) The mobility and persistence
of Baygon in soil and water. Unpublished report submitted by Chemagro
Gahan, J.B. and Wilson, H.G. (1970) Seven insecticides as residual
sprays in buildings naturally infested with Anopheles
quadrimaculatus. Mosquito News, 30: 410-416
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharm. 14: 515-534
George, D.A., Rusk, H.W., Powell, D.M. and Landis, B.J. (1967) An
analytical method for o-isopropoxyphenyl methyl carbamate (Bayer
39007), its aphicidal value and persistence in potatoes and sugar
beets. J. Econ. Ent. 60: 82-84
van Gils, W.F. (1970) Spectrophotometric determination of propoxur
residues on vegetable matter. Analyst, 95: 88-90
Gronberg, R.R. (1970) Metabolism of Baygon in corn plants. Unpublished
report submitted by Chemagro
Hadaway, A.B. and Barlow, F.A (1964) A note on the sorption of
insecticides on tropical soils. Bull. Wld Hlth Org. 30: 146-148
Hayes, W.J., jr (1971) Studies on exposure during the use of
anti-cholinesterase pesticides. Bull. Wld Hlth Org. 44: 277-288
Hobik, H.P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitatsversuchen an Huhnern mit, BAYER
39007. Unpublished report submitted by Bayer AG
Holden, E.R., Jones, W.M. and Beroza, M. (1969) Determination of
residues of methyl-and dimethyl-carbamate insecticides by gas
chromatography of their 2,4-dinitroaniline derivatives. J. Agr. Food
Chem. 17: 56-59
Houseworth, L.D. and Tweedy, B.G. (1972) Effect of Baygon on microbial
populations. Unpublished report submitted by Chemagro
Ivie, G.W. and Casida, J.E. (1971) Sensitized photodecomposition and
photosensitizer activity of pesticide chemicals exposed to sunlight on
silica gel chromatoplates. Photosensitizers for the accelerated
degradation of chlorinated another insecticide chemicals exposed to
sunlight on bean leaves. J. Agr. Food Chem. 19: 405-416
Kimmerle, G. (1961) Preparation Dr. Bocker 58 12 315 (39 007).
Unpublished report submitted by Bayer AG
Kimmerle, G. (1964) E 39 007 (Bocker 58 12 315; Ht.-Nr. 3410)/
Neurotoxizitat. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966a) Neurotoxic studies on active ingredient Docker
58: 12 315 (Unden active ingredient). Unpublished report submitted by
Bayer AG
Kimmerle, G. (1966b) Unden-Wirkstoff (Bocker 58 12
315)/Antidotwirkung. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966c) Unden-Wirkstoff/Inhalationstoxizitat.
Unpublished report submitted by Bayer AG
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetra-ethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol. 27: 311-314
Kimmerle, G. and Solmecke, B. (1971) BAY 39 007/Subacute dermal
application to rabbits. Unpublished report submitted by Bayer AG
Klimmer, O.R., (1963) Pharmakologisches Institut der Universitat Bonn.
Toxikologische Prufung von BAYER 39 007. Unpublished report submitted
by Bayer AG
Krishna, J.G. and Casida, J.E. (1966) Insecticide metabolism - fate in
rats of the radiocarbon from ten variously labeled methyl-and
dimethylcarbamate-14C insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14: 98-105
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticide chemicals
in plants. J. Sci. Food Agr. pp. 44-49
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticides by
insects in vivo and in vitro. Mededel. Rijksfac.
Landbouwetenschappen, 33 (3): 647-657
Kuhr, R.J. (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J. Agr. Food Chem. 18: 1023-1030
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of
metabolites of methylcarbamate insecticide chemicals formed by
hydroxylation in bean plants. J. Agr. Food Chem. 15: 814-824
Linke, W. Trials to determine the persistence of Arprocarb when used
in food storage practice. Unpublished report submitted by Baywood
Chemicals Ltd., Techn. Dept. Technical Report No. TCR/130
Lorke, D. (1970) BAY 39 007/Untersuchungen auf embryotoxische
Wirkungen an der Ratte. Unpublished report submitted by Bayer AG
Löser, E. (1965) Fütterungsversuch Uber 2 Monate mit BAYER 39 007.
Unpublished report submitted by Bayer AG
Löser, E. (1968a) BAY 39 007/Generationsversuche an Ratten.
Unpublished report submitted by Bayer AG
Löser, E. (1968b) BAYER 39 007/Chronische toxikologische
Untersuchungen an Ratten. Unpublished report submitted by Bayer AG
Löser, E. (1968) BAY 39 007/Chronische toxikologische Untersuchungen
an Hunden. Unpublished report submitted by Bayer AG
Marchart, H. (1970) Insecticide residues in cocoa. Int. Atomic Energy
Agency, P-309/1: 1-10
Marchart, H. Evaluation of insecticides for the control of cocoa
capsids in Ghana. FAO Plant Protection Bull. 19: 97-109
Mawdesley-Thomas, L.E., (1969a) Huntingdon Research Centre, England.
Pathology report of the generation experiment in rats of the toxicity
of compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E. (1969b) Huntingdon Research Centre, England.
Pathology report of the two-year experiment in rats of the toxicity of
compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E., (1969c) Huntingdon Research Centre, England.
Pathology report of the two-year toxicity in dogs of compound BAY 39
007 by oral administration. Unpublished report submitted by Bayer AG
Mendoza, C.E. and Shields, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin layer chromatography. J. Ass. off.
analyt. Chem. 54: 507-512
Metcalf, R.L. (1971) Structure-activity relationships for insecticidal
carbamates. Bull. Wld Hlth Org. 44: 43-78
Metcalf, R.L. and Fukuto, T.R. (1965) Carbamate insecticides. Effects
of chemical structure on intoxication and detoxication of phenyl
N-methyl-carbamates in insects. J. Agr. Food Chem. 13: 220-231
Metcalf, R.L., Osman, M.F. and Fukuto, T.R. (1967) Metabolism of
14C-labeled carbamate insecticides to 14CO2 in the house fly. J.
Econ. Ent. 60: 445-450
Nelson, D.L. A study of the acute toxicity of BAYGON in 1967
combination with D.D.V.P. Unpublished report submitted by Bayer AG
Nesemann, E. and Seehofer, F. (1970) Screening procedures for
organophosphorus, organochlorine and carbamate pesticide residues on
tobacco. Beiträge zur Tabakforschung, 5: 207-214
Niessen, H. and Fretse, H. (1963) Eine infrarotspektroskopiscbe,
Methods zur Bestimmung von N-Methylcarbamat-Rückständen in Pflanzen.
Pflanzenschutz Nachrichten "Bayer", 16: 205-220
Niessen, H. and Frehae, H. (1964) Kolorimetrische Methods zur
Bestimung von Rücksthinden doe Insektizids Undon in pflanzlichem
Material. Pflanzenschutz-Nachrichten "Bayer", 17: 25-32
O'Brien, (1966) cited in Metcalf, 1971
Oonnithan, E.S. and Casida, J.E. (1966) Metabolites of methyl- and
dimethyl carbamate insecticide chemicals as formed by rat liver
microsomes. Bull. of Env. Cont. and Tox. 1 (2): 59-60
Oonnithan, E.S. and Casida, J.E. (1968) Oxidation of methyl- and
dimethyl carbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J. Agr. Food Chem.
16: 28-44
Pant, C.P. and Joshi, G.P. (1969) A field study of an airborne toxic
effect of Baygon residual spray. Mosquito News, 29: 674-677
Parker, B.L., Dewey, J.E. and Bache, C.A. (1970) Carbamate bio assay
using Daphnia magna. J. Econ. Ent. 63: 710-714
Plestina, Radovan (1968) Beitrag zur Erforschung der toxischen
Eigenschaften des o-Isopropoxyphenyömethylkarbamat. Magisterarbeit
Reiner, E. (1968) The inhibitory power of
2-isopropoxyphenyl-N-methyl-carbamate against serum cholinesterase of
various individuals. Arch. Toxikol. 23 (3): 237-239
Reiner and Aldrich (1967), cited in Metcalf, 1971
Reiner, E. and Simeon, V. (1968) The inhibitory power of 2-isopropoxy
1968 phenyl-N-methyl-carbamate against serum cholinesterase of various
individuals. Archiv f. Toxikologiet 23: 237-239
Root, M., Cowan, J. and Doull, J., University of Chicago. (1963)
Subacute oral toxicity of RAYER 39 007 to male and female rats.
Unpublished report submitted by Bayer AG
Shrivastava, S.P., Tsukamato, M. and Casida, J.E. (1969) Oxidative
metabolism of 14C-labeled Baygon by living house flies and by house
fly enzyme preparations. J. Econ. Ent. 62: 483-498
Stanley, C.W. (1971) A gas chromatographic method for the
determination of Baygon in soils. Unpublished report submitted by
Chemagro
Stanley, C.W. and Thornton, J.S. (1972) Gas chromatographic method for
residues of Baygon and its metabolites in animal tissues and milk. J.
Agr. Food Chem. 20: 1269-1273
Stanley, C.W., Thornton, J.S. and Katague, D.B. (1972) Gas
chromatographic method for residues of Baygon and metabolites in plant
tissues. J. Agr. Food Chem. 20: 1265-1269
Steelman, C.D., Colmerg A.R., Cabes, L., Barr, H.T. and Tower, B.A.
(1967) Relative toxicity of selected insecticides to bacterial
populations in waste disposal lagoons. J. Econ. Ent. 60: 467-468
Syrowatka, T., Jurek, A. and Nazarewiez, T. (1971) Short term chronic
toxicity study of o-isopropoxyphenyl-N-methyl carbamate (propoxur).
Rooz. Panstw. Zakl. Hig. 22: 579-589 (poln.)
Tsukamoto, M. and Casida, J.E. (1967) Albumin enhancement of oxidative
metabolism of methylearbamate insecticide chemicals by the house fly
microsome-NADPH2 system. J. Econ. Ent. 60: 617-619
Tsukamoto, M. and Casida, J.E. (1967) Metabolismus von Methylcarbamat
Insektiziden durch das NADPH2-benötiginde Enzymsystem der Hausfliege.
Nature (London), 213: 49-51
Vandekar, M. (1969) The effect on cholinesterase activity of storage
of undiluted whole blood sampled from men exposed to
C-isopropoxyphenylmethylcarbamate. Bull. Wld Hlth Org. 40: 91-96
Vandekar, M., Hedayat, E., Plestina, R. and Ahmady, G. (1968) A study
of the safety of o-isopropoxyphenylmetbyl-carbamate in an operational
field-trial in Iran. Bull. Wld Hlth Org. 38: 609-623
Vandekar, M., Plestina, R. and Wilhelm. K. (1971) Toxicity of
carbamates for mammals. Bull. Wld Hlth Org. 44: 241-249
Vandekar, M., Reiner, E., Svetlicic, B. and Fajdetic, T. (1965) Value
of ED50 testing in assessing hazards of acute poisoning by carbamates
and organophosphates. Brit. J. Industr. Med. 22: 317-320
Vandekar, M. and Wilford, K. (1969) The effect on cholinesterase
activity of storage of undiluted whole blood sampled from men exposed
to o-isopropoxyphenyl methylcarbamate (OMS-33). Bull. Wld Hlth Org.
40: 91-96
Voss, G. (1968) Peacock plasma, a useful cholinesterase source for
inhibition residue analysis of insecticidal carbamate. Bull. Env.
Cont. and Tox. 3: 339-347
Waggoner, T.B. and Olson, T.J. (1971) Effect of feeding
organophosphorus and carbamate pesticides to cattle. Paper No. 45,
Division of Pesticide Chemistry, 162nd National ACS Meeting, 12-17
September, Washington, D.C.
Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968) Pesticide residues
- TLC-enzyme inhibition procedure to detect some carbamate standards
and carbaryl in food extracts. J. Ass. off. analyt. Chem. 51:
1239-1242
Weiden, M.H.J. (1971) Toxicity of carbamate to insects. Bull. Wld
Hlth Org. 44: 203-213
Wilhelm (1967), cited in Vandekar et al., 1971
Wit, Sj. (1963) Residues of acaricides and aphicides on glasshouse
cultures (mevinphos, dichlorvos, naled, propoxur and nicotine).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1966) Residues of parathion and propoxur on red-currants.
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1969) Residues of propoxur on gherkins (glasshouse).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wright, J.W., Fritz, R.F., Hocking, K.S,, Babione, R., Gratz, N.G.,
Pal, R., Stiles, A.R. and Vandekar, M. (1969) Ortho-isopropoxyphenyl
methyl-carbamate (OMS-33) as a residual spray for control of
anopheline mosquitos. Bull. Wld Hlth Org. 40: 67-90
Wright, C.G. and Jackson, M.D. (1971) Propoxur, chlordane and diazinon
on porcelain china saucers after kitchen cabinet spraying. J. Econ.
Ent. 64: 457-459
Krishna and Casida (1966) administered 14C carbonyl labelled
propoxur intraperitoneally to rats. After 48 hours only 2.1% of the
administered radio-activity remained in the body; 60% of the activity
was excreted in the urine in the first 29 hours, whereas only 1.2% was
excreted in the faeces. Within 48 hours 31.2% of the administered
radio-activity was expired as CO2.
From these data it may be concluded that the carbamate group was
cleaved from one-third of the injected propoxur dose.
In a similar experiment with 14C isopropyl labelled propoxur,
70-75% of the activity was excreted in the urine, whilst 30%. of the
administered activity was expired as 14CO2; 4% of the activity
remained in the body and only 0.7% was excreted in the faeces.
From this it may be concluded that the isopropyl group is cleaved
from about one-quarter of the injected dose.
Dorough and Casida (1964) incubated rat liver microsomes with
propoxur and obtained 30% conversion to a metabolite from which with
acid Isopropoxyphenol and formaldehyde were yielded. With
cochromatography and infra-red spectroscopy the metabolite was
confirmed with (B).
Oonithan and Casida (1968) studied the metabolic fate of propoxur
in a system containing rat liver microsomes and NADPH2. Two
metabolites were formed, probably (A) and (B).
In cattle
Waggoner and Olson (1971) determined residues in tissues and milk
of cattle after feeding on a diet containing propoxur for 28 days. In
those cases where residues could be detected the amounts of the
metabolite (A) was greater than the parent propoxur.
After feeding 7.5 mg/kg/day of propoxur, the residues of the
parent compound and the metabolite (A) were respectively:
ppm
propoxur (A)
kidney 0.04 0.13
milk 0.001 0.0027
In insects
Metcalf et al. (1967) treated flies with 14C isopropoxy labelled
propoxur and found metabolism to CO2. A pre-treatment with piperonyl
butoxide reduced the formation of CO2 to one-third.
Dorough et al. (1963) and Dorough and Casida (1964) found in
cockroaches the metabolite (B) after injection of propoxur.
Shrivastava et al. (1969) (see also Ruhr, 1968, 1970) studied
in vivo and in vitro the metabolism of propoxur in houseflies.
The following metabolites were isolated in order of decreasing amounts
(C), (A) and acetone, B and 2-isopropoxyphenyl carbamate. These
metabolites were all conjugated due to their hydroxyl groups and
volatilized in acetone (see also Casida et al., 1968).
Tsukamoto and Casida (1967a and b) investigated the metabolism of
propoxur in a system of house-fly microsomes and NADPH2; they found
the metabolites (A), (B) and (C) as major metabolites.
Propoxur is a non-systemic carbamate insecticide which is used
against a relatively broad spectrum of insects in field crops, fruit
and vegetables (aphids, including woolly aphid, lygus bugs,
leafhoppers, saw-flies, thrips, millipedes, etc.).
The product is registered and used in several countries in Europe
and in other parts of the world.
Propoxur is used extensively for hygienic purposes against
cockroaches, flies, etc. in homes, hotels, restaurants and warehouses.
Pre-harvest treatments
Major crops on which propoxur is used are rice, sugar cane, pome
and stone fruits, small fruits, vegetables and potatoes. The following
estimates can be given of the use in different areas:
rice about 30%
other field crops about 30%
cacao about 20%
other crops such as fruit,
vegetables, ornamentals about 20%.
The following table summarizes the recommendations in accordance
with good agricultural practice, including rates of application and
pre-harvest intervals.
Dosage rate Minimum pre-harvest
Crop g a.i./ha or interval recommended
g a.i./100 l days
Field crops
potatoes 250-600 g/ha 7
rice 400-750 g/ha 7
sugar cane 750-1000 g/ha 7
Cacao 250-600 g/ha 7
Fruits
Pome fruits
apple, pears 50-75 g/100 l 7
600-1200 g/ha
Stone fruits
peach, plums 50-75 g/100 l 7
900-1200 g/ha
Small fruits
blackberries, gooseberries,
red currants, raspberries,
strawberries 50-75 g/100 l 7
600-1200 g/ha
Vegetables (outdoors)
beans, cabbage, gherkins,
leek, lettuce, onionp
peas, spinach 400-750 g/ha 4-7
vegetables (glasshouses)
bell peppers, cucumbers,
gherkins, melons,
tomatoes 400-750 g/ha 4
Leafy vegetables such as
lettuce, spinach 400-750 g/ha 14-21
Post-harvest treatments
No treatments recommended.
Other uses
Propoxur is used on ornamentals and flower crops.
It is also extensively used in the hygienic sector in the form of
aerosols, thermal fog concentrates, baits, wettable powder and
emulsifiable liquids, against a number of household and domestic pests
such as bugs, cockroaches, flies, mosquitos, beetles, silverfish, etc.
Pre-harvest intervals officially established in
various countries in days
Austria
35 days, all fruit and vegetables
Belgium
21 days, lettuce and endive (glasshouse cultures) during
winter period
14 days, lettuce and endive (outdoors and glasshouse)
7 days, other vegetables except those mentioned below,
agricultural crops
3 days, gherkins, tomatoes (both under glass and outdoors),
bell peppers, cucumber, melons
Denmark
14 days, fruit, vegetables and field crops
Germany, Federal Republic of
21 days, cereals
14 days, potatoes
7 days, leafy vegetables (except lettuce), tomatoes,
gherkins, melons
4 days, pome and stone fruit, gooseberries, red and black
currants, raspberries, strawberries, cabbage, carrot,
celery, garden beet, leek, lettuce, onion, radish, horse
radish, sugar and fodder beet
Netherlands
21 days, lettuce and endive (glasshouse) between
November/March
14 days, lettuce and endive (outdoors.and glasshouse)
7 days, fruit, including berries, vegetables except those
mentioned below
4 days, gherkins (glasshouse and outdoors)
3 days, tomatoes, bell peppers, cucumbers, melons
New Zealand
21 days, all crops
Poland
7 days, potatoes
Spain
30 days, fruit, sugar beet, cotton
Sweden
14 days, vegetables
7 days, all other crops
United Kingdom
7 days, all outdoor crops
2 days, cucumbers, tomatoes (glasshouse)
Yugoslavia
21 days, fruit
Residue data from supervised trials
Residue data were obtained from trials on several fruits,
vegetables and field crops, such as apples. sour and sweet cherries,
peaches, plums, black and red currants, gooseberries, French beans,
bell peppers, red and white cabbage, savoy, carrots, cucumbers,
lettuce, leek, onions, peas, spinach, tomatoes, alfalfa, cereals,
rice, tobacco, cocoa, grassland. These data are summarized in Tables 1
and 2.
TABLE 1. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
Fruit
apples Belgium 1964 1 (75 g/100 l) w.p. 50% 0.3 n.d. n.d.
Germany, 1966 1 (75 g/100 l) w.p. 50% 1.6 0.96 0.48 0.43
Fed. Rep. 1966 1 (75 g/100 l) w.p. 50% 2.0 0.95 0.95 0.95 0.8
1964 1 (50 g/100 l) w.p. 50% 1.3 0.8 0.7 0.6 0.6
1964 1 (100 g/100 l) w.p. 50% 2.0 1.4 0.7 0.6 0.6
Netherlands 1964 2 (l.v.) w.p. 50% 1.0-1.4 0.5-0.7 0.4-0.5 0.2-0.4 0.1-0.2
1964 2 (h.v.) w.p. 50% 0.6-1.1 0.2 0.2 0.1-0.3 0.1-0.3
1965 2.5 (h.v.) w.p. 50% 0.33-1.6 0.38- 0.31-
0.80 0.44
1965 2.5 (h.v.) w.p. 50% 0.29- 0.28-
0.42 0.33
cherries, Germany, 1969 1 1.5 w.p. 50% 3.1 0.45 0.18
sour Fed. Rep. 1 1.5 w.p. 50% 5.0 0.3 0.24
cherries, Germany, 1968 1 (50 g/100 l) w.p. 50% 0.05
sweet Fed. Rep. 2 (50 g/100 l) w.p. 50% 0.06
peaches Germany, 1967 1 (50 g/100 l) w.p. 50% 1.55 0.5 0.25 0.2
Fed. Rep 1968 1 (75 g/100 l) w.p. 50% 3 2.0 1.25 0.65
1968 1 (75 g/100 l) w.p. 50% 8.7 2.36 1.65
1968 1 (75 g/100 l) w.p. 50% 2.9 1.8 0.9
1968 1 (75 g/100 l) w.p. 50% 3.9 1.5 0.5
plums Germany, 1967 1 (50 g/100 l) w.p. 50% 0.55 n.d. n.d. n.d.
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 2.16 0.52 0.2
1968 1 (75 g/100 l) w.p. 50% 3.71 1.75 1.5
1968 1 (75 g/100 l) w.p. 50% 3.05 1.5 0.7
1968 1 (75 g/100 l) w.p. 50% 1.6 0.7 0.15
1968 1 (75 g/100 l) w.p. 50% 2.75 1.45 0.7
1969 1 1.5 w.p. 50% 2.5 1.35 <0.05
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1969 1 1.5 w.p. 50% <0.05 <0.05 <0.05
red Netherlands 1965 1 0.75 (l.v.) w.p. 50% 1.31-2.11 <0.01- <0.01-
0.08 <0.01
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 7.25 0.75 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 14.1 0.64 0.61
1968 1 (75 g/100 l) w.p. 50% 8.2 1.1 0.64
black Netherlands 1964 1 (50 g/100 l) w.p. 50% 2.35 0.7
currants Germany, 1968 1 (75 g/100 l) w.p. 50% 15 0.7 0.45
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 13.4 0.6 0.4
1 (75 g/100 l) w.p. 50% 16.7 2.45 1.35
1 (75 g/100 l) w.p. 50% 4.2 1.3 0.45 0.11
gooseberries Germany, 1968 1 (75 g/100 l) w.p. 50% 3.5 0.6 0.3
Fed. Rep. 1968 1 (75 g/100 l) w.p. 50% 5.8 0.6 0.25
1968 1 (75 g/100 l) w.p. 50% 3.6 0.45
1968 1 (75 g/100 l) w.p. 50% 6.7 0.53 0.20
1968 1 (75 g/100 l) w.p. 50% 6.3 0.83 0.23
Vegetables
French Germany 1964 1 0.7 w.p. 50% 1.25 0.25 <0.1
bean Fed. Rep. 1969 1 0.45 w.p. 50% 1.65 0.55 0.20
1968 1 0.75 w.p. 50% 0.5 0.25
1968 1 0.75 w.p. 50% 1.6 1.1 1.0
1969 1 0.75 w.p. 50% 0.5 0.35 0.25
1969 1 0.75 w.p. 50% 0.6 0.4 0.25
1967 1 0.5 w.p. 50% 0.75 0.3 0.15
1968 1 0.45 w.p. 50% 0.9 0.2 0.1
1968 1 0.45 w.p. 50% 0.7 0.3 0.1
1969 1 0.45 w.p. 50% 1.6 0.5 0.08
UK 1964 1 0.7 w.p. 50% 0.25
-1.55
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
bell peppers Netherlands 1968 1 0.38 w.p. 50% 0.75 0.3 <0.1
(glasshouse)
red Germany, 1964 1 0.15 w.p. 50% 1.0 0.4 0.2 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 1.6 1.3 0.9 0.4
savoy Germany, 1964 1 0.15 w.p. 50% 3.9 0.9 0.2 <0.2
Fed. Rep. 1964 1 0.15 w.p. 50% 2.7 0.8 0.7 0.6
1968 1 0.6 w.p. 50% 5.3 2.1 0.7 1.2
1968 1 0.6 w.p. 50% 8.0 3.9 3.4 1.8
white Germany, 1964 1 0.15 w.p. 50% 2.2 1.3 0.6 <0.2
cabbage Fed. Rep. 1964 1 0.6 w.p. 50% 5.8 2.1 1.2 0.6
carrots Germany, 1968 1 0.45 w.p. 50% 0.1 n.d n.d.
Fed. Rep. 1968 1 0.75 w.p. 50% n.d. 0.2 0.25
1968 1 0.75 w.p. 50% 0.1 0.15 0.25
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% 0.3 0.7 0 0.3
cucumbers Netherlands 1970 1 0.5 dust 2% 0.05 0.07 n.d.
(glasshouse) 1970 1 0.5 dust 2% 0.07 0.06 n.d.
leek Germany, 1968 1 0.45 w.p. 50% 0.5 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 0.6 0.1 n.d.
1968 1 0.45 w.p. 50% 10.9 1.1 1.0
1968 1 0.6 w.p. 50% 2.9 0.6 0.6
1968 1 0.75 w.p. 50% 2.3 0.25 0.1
1968 1 0.75 w.p. 50% 2.0 0.7 0.15
lettuce Germany, 1964 1 0.6 w.p. 50% 6.8 0.4 0.2 0.1
(outdoor) Fed. Rep. 1964 1 0.6 w.p. 50% 5.1 0.2 1.3 1.1
1 w.p. 50% 1.8 0.4 0.2 0.1
1 w.p. 50% 2.2 1.6 0.9 0.7
lettuce Netherlands 1963 1 0.66 w.p. 50% 17.2- 9.2- 5.4- 1.8-4.1 0.9-1.9 0.5-0.8
(glasshouse) 20.2 10.9 10.4
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1971 1 0.8-0.9 w.p. 50% 15.2 10.9 4.1
1971 1 0.6-0.9 w.p. 50% 10.0 7.45 3.1
1971 1 0.6-0.9 w.p. 50% 4.0 5.5 1.9
1971 1 0.6-0.9 w.p. 50% 8.5 7.0 2.7
1971 1 0.6-0.9 w.p. 50% 7.25 6.8 2.4
onions Germany, 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% n.d. n.d. n.d.
1968 1 0.45 w.p. 50% 9.3 4.2 0.87
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1968 1 0.45 w.p. 50% <0.05 <0.05 <0.05
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50% n.d. n.d. n.d.
1969 1 0.75 w.p. 50%
peas Germany, 1964 1 0.7 w.p. 50% 0.3 n.d. n.d.
pods Fed. Rep. 1964 0.4 0.1 <0.1
spinach Germany, 1968 1 0.45 w.p. 50% 5.7 n.d. n.d.
Fed. Rep. 1968 1 0.45 w.p. 50% 6.8 n.d. n.d.
1969 1 0.45 w.p. 50% 32 0.6 0.06
1969 1 0.45 w.p. 50% 33.5 0.7 0.06
1969 1 0.75 w.p. 50% 27 7.3 0.6
tomatoes Netherlands 1971 1 0.5 dust 0.28 <0.05 n.d.
(glasshouse 1971 1 0.5 dust 0.33 <0.05 n.d.
1971 1 11 g/100 m3 smoke 0.07 n.d.
Field Crops
alfalfa USA 1970 1 1.0 w.p. 70% 59.9 12.2
green 1 1.0 w.p. 70% 11.6 2.17
1 1.0 w.p. 70% 15.5 2.06
1 1.0 w.p. 70% 65.8 7.14
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
1 1.0 w.p. 70% 36.8 3.40 1.15 0.20 0.59(28)
1 1.0 w.p. 70% 65.8 7.14 3.17 0.66 <0.12(29)
hay 1 1.0 w.p. 70% 16.4 3.67
1 1.0 w.p. 70% 3.21 1.33
seed hulls 3 1.0 w.p. 70% 0.35(28)
3 1.0 w.p. 70% 0.18(29)
2 1.5 w.p. 70% 0.13(46)
2 1.5 w.p. 70% 0.13(92)
chaffs 3 1.0 w.p. 70% 0.63(28)
3 1.0 w.p. 70% 0.24(29)
0.13(46)
0.08(92)
barley USA 1970
grain 1 0.4a w.p. 50% <0.05
1 0.4a w.p. 50% <0.24
straw 1 0.4a w.p. 50% <0.03
1 0.4a w.p. 50% 0.32
oats USA 1970
grain 1 0.4a w.p. 50% 0.09
1 0.4a w.p. 50% 0.15
1 0.4a w.p. 50% 0.10
1 0.4a w.p. 50% <0.04(25)
straw 1 0.4a w.p. 50% 0.01
1 0.4a w.p. 50% 0.07
1 0.4a w.p. 50% <0.19
1 0.4a w.p. 50%
1 0.4a w.p. 50% 0.01(25)
rye USA 1970
grain 1 0.4a w.p. 70% <0.03
straw 1 0.4a w.p. 70% 0.04
TABLE 1. (Cont'd.)
Application Pre-harvest intervals
Crop Country Year
No. rate kg a.i./ha Formulation >21
(... g/100 l) 0 2/3 4/6 7/8 10/13 14/17 20/21 (days)
wheat USA
grain 1 0.4 w.p. 50% 0.21
straw 1.41
pasture USA 1 0.25 w.p. 70% 11.82 6.99 1.02
grass U.L.V.
1 0.5 w.p. 70% 0.63 0.59 1.20 0.76
rangeland USA 1 0.25 w.p. 70% 29.8 1.49 0.76
grass
cacao
whole beans 2 0.5 E.C. 20% n.d.(24)
2 0.25 E.C. 20% n.d.(24)
shell 2 0.84 E.C. 20% 0.3 0.3 0.3(56)
3 0.84 E.C. 20% 0.3
nib 3 0.84 E.C. 20% <0.1
a seed treatment 500 g/100 kg seed and foliar application
h.v. = high volume; l.v. = low volume; U.L.V. = ultra-low volume
TABLE 2. RESIDUES OF PROPOXUR IN ppm
Application Pre-harvest interval in days
Crop Country Year No. rate Formulation
kg a.i./ha 1-10 11-20 21-30 31-40 41-50
Rice hulled Japan 1972 1 0.4 dust 1% 0.02 0.06-0.09
1 0.4 dust 1% 0.03 0.02-0.04
1 0.4 dust 1% 0.02 0.02
1 0.4 dust 1% 0.02 <0.02-0.19
1 0.4 dust 1% <0.02 0.02
1 0.4 dust 1% 0.02-0.11
1 0.4 dust 1% 0.21 0.02
1 0.4 dust 1% 0.02 0.06
Paddy rice Japan 1972 1 0.2-0.75 E.C. 25% 0.03 0.08-0.33
0.05-0.56
0.26-0.40 <0.02-0.05
0.47-0.54
Upland rice Japan 1972 1 0.2-0.75 E.C. 25% 0.18
1 0.2-0.75 E.C. 25% 0.19
1 0.2-0.75 E.C. 25% 0.11
1 0.2-0.75 E.C. 25% 0.13
Fate of residues
Influence of light
Propoxur exposed to light shows no or very slight
photo-decomposition. Crosby (1965) found that propoxur was the only
one out of six N-methylcarbamates which was not converted to other
cholinesterase inhibitors when exposed to ultra-violet light or
sunlight.
Propoxur on silica gel-coated plates exposed to long-wave length
ultra-violet light did not produce degradation products, whereas after
exposure to short-wave length ultra-violet light three metabolites,
which gave a colour reaction with ninhydrin (Abdel-Wahab et al.,
1966), were found.
The product did not show any changes when such plates were
exposed to sunlight, even when sensitizers were applied to the (Ivie
and Casida, 1971).
Inert surfaces
Propoxur disappears from inert surfaces more or less rapidly
mainly through volatilization. The rate of disappearance depends on
the nature of the surface. Fifty per cent. of a propoxur residue on
glass, kept under laboratory conditions, was still present after 1.8
hours (Abdel-Wahab et al., 1966).
The half-life of propoxur on porcelain saucers under field
conditions was about three days (Wright and Jackson, 1971), whereas on
polythene foils a half-life of two days was found (Marchart, 1970).
Propoxur sprayed as an aqueous emulsion concentrate on filter
papers, which were suspended vertically at 2.5 inch in a kitchen (mean
temperature 23°C (21-29°C) and mean relative humidity 52% (42-68%),
evaporated 50% in about six weeks (Links, 1965).
After three months, 60% of the active ingredient was still
present on Hessian bags, sprayed with a wettable powder and stored in
an unheated large storage shed (Linke, 1965).
When propoxur was applied to plywood panels, 50% of it evaporated
in 15 days irrespective of the formulation used (Dorough and Crouch,
1966).
Inconsistencies in the results of different authors may not only
be due to different temperatures but also to air movements (Marchart,
1970), the influence of which is large but has not yet been studied
quantitatively.
The evaporation of propoxur from animate or inanimate substrates
is of practical significance. A considerable proportion of residues on
plants is eliminated by volatilization (Abdel-Wahab at al., 1966;
Gronberg, 1970; Marchart, 1970, 1971). On the other hand, the activity
of propoxur in the vapour phase is utilized for the control of storage
pests and household and domestic pests (Gahan and Wilson, 1970; Wright
et al., 1969).
In water
Propoxur is relatively stable in water at pH levels of less than
7. The rate of hydrolysis, resulting in the formation of
isopropoxyphenol increases rapidly from pH 7 upwards. The half-life of
the parent compound in buffered solutions at 20°C and pH 8 was 16
days, at pH 9, 38 hours and at pH 10, three hours (Aly and El-Dib,
1971).
At temperatures of 30°C and pH 7, the half-life of the parent
propoxur was three days, at pH 9, 1.2 hours.
Flint and Shaw (1971) determined the half-life of propoxur in
field experiments in shallow open vessels filled with bottom silt and
lake water. In these experiments, 50% of the propoxur disappeared in
12.7 hours at pH 7 and 27-36°C.
In similar experiments, in which smaller nearly air-tight vessels
were used, and in a parallel experiment with a biologically sterile
system, the half-lives were 54.9 and 80.8 hours respectively;
temperature range was 5-22°C.
In soil
Propoxur evaporates from the soil; the amount which evaporates
increases with increasing moisture content of the soil.
The time required to decrease a soil residue to one-half of the
initial concentration ranges from six to eight weeks, depending on
soil type (Flint and Shaw, 1971).
Metabolism in soil
The metabolism of propoxur in different soil types was studied by
Church-and Flint (1971) with radio-labelled compounds. After a soil
application of 3H-isopropoxy, 14C-carbonyl labelled propoxur, the
3H activity remained organosoluble, whereas the 14C activity was
concentrated in the water-soluble fraction. The organosoluble activity
was composed of isopropoxyphenol and traces of propoxur. The 14C
activity was incorporated into unknown water-soluble materials which
no longer behaved as carbamates.
In sterile soils or under anaerobic soil conditions, the 14C
activity decreased whilst the 3H activity remained almost constant.
This indicates that in these conditions a simple chemical hydrolysis
to isopropoxyphenol was occurring (Flint and Shaw, 1971).
In biologically active soils, the 14C and 3H activity declined
sharply after nine days, which gives an indication of microbial
degradation. In this case, no conjugated compounds were found (Church
and Flint, 1971).
Flint and Shaw equilibrated aqueous solutions of propoxur with
different soil types. The adsorption of propoxur to soil particles was
poor under these conditions. The following adsorption co-efficients
were found: 0.63 for sandy loam, 0.49 for silty clay loam and 1.12 for
highly organic silty clay loam.
In freshly tilled soils propoxur can be moved laterally by water.
In soil leaching experiments, propoxur moved with the water front
passing through the packed soil columns. In view of the poor stability
of propoxur in aqueous systems, the normal use will give no risks of
contaminating ground or superficial water.
Propoxur at normal rates showed only a slight effect on soil
microorganisms (Church and Flint, 1971; Houseworth and Tweedy, 1972)
and on microorganisms in waste disposal lagoons.
In plants
A large proportion of propoxur applied to the leaf surface
evaporates (Everett and Gronberg, 1968; Marchart, 1970, 1971;
Abdel-Wahab et al., 1966).
With radio-labelled propoxur, it was demonstrated that only a
small amount penetrates from the leaf surface into the leaves. After
five days the parent propoxur comprised 69-98% of the total 14C
activity present. The material that penetrated was shown to be
primarily the parent propoxur and water-soluble metabolites, mainly
the ß glucoside of 2-hydroxyphenyl N-methylcarbamate.
No downward translocation of propoxur could be demonstrated
(Everett and Gronberg, 1968).
The uptake of propoxur by plant roots from an aqueous solution
was shown to be directly related to the water uptake. Propoxur and
metabolites were translocated from the aqueous solution to the surface
of the leaf from which there is some volatilization.
14C carbonyl labelled propoxur injected into the stems of beans
and cotton plants was found to be converted into watersoluble
metabolites which remained stable for a relatively long period
(Dorough and Casida, 1965). In subsequent experiments Abdel-Wahab at
al. (1966) found that the water-soluble metabolites still possessed a
carbamate structure. The half-life of the parent propoxur after
injection into bean plants was one day.
Kuhr and Casida (1967) identified with thin layer chromatography
the water-soluble metabolites. Following incubation with ß
glucosidase, ether-extractable aglycones of the water-soluble
metabolites were yielded to the extent of 76%. Tentative
identification by cochromatography showed that 91.3% of the mixture
consisted of 2-hydroxyphenyl-N-methylearbamate (metabolite "A"
according to the scheme of the metabolic pathway in the section "Fate
in animals" as proposed by Everett and Gronberg (1970)) and 4.9% of
metabolite B = 2-isopropoxyphenyl-N-hydroxymethylcarbamate.
In these studies the metabolites (A) and (B) accounted for 30.2%
and 1.5% respectively of the applied activity, six days after the
injection of propoxur in the bean plant, together comprising 96% of
the water-soluble metabolites.
Five days after foliar application of 14C carbonyl labelled and
isopropoxy labelled propoxur on bean and maize plants, the residue on
the leaves consisted practically only of the parent compound. A
negligible portion was metabolite (A) (<1%). Also in the plant the
largest proportion of the radio-active material extractable with
organic solvents consisted of propoxur. After 3, 5, 7, 9 and 14 days,
its share of the measurable activity was 58.7%, 45.0%, 51.3%, 50.1%
and 36.4% respectively.
A large proportion of the carbonyl labelled material was present
in the water phase, the proportion of isopropyl labelled being less,
thus indicating that the isopropoxy group was cleaved from a
considerable portion of the applied parent compound. This assumption
was supported by the detection of acetone in the air pulled through
the chamber (Everett and Gronberg, 1968).
In an experiment in which carbonyl labelled and isopropoxy
labelled propoxur was absorbed from water by the roots, the proportion
of 14C active compound increased continuously during the 14-day
study. The aglycones of the conjugated metabolites could be almost
completely released with ß glucosidase. Cochromatography showed
agreement with metabolites (A) and (B). The ratio of these compounds
was 9:1 (Everett and Gronberg, 1968).
In a later experiment, Gronberg (1970) found that 14 days after
uptake of labelled propoxur by maize roots 50% of the residue in the
plant was still the parent compound, 19.2% of the residue was
accounted for by (A) and 3.5% by (B). Both metabolites were released
from their conjugates by ß glucosidase and positively identified by
infra-red spectroscopy. It was shown that 2-isopropoxy-4
hydroxyphenyl-N-methylcarbate did not occur.
Methods of residue analysis
Bio-assay methods were developed for the detection of propoxur
residues in soil using house crickets (Burkhardt and Fairchild, 1967)
as test insects and on fruit crops using Daphnia magna (Parker et
al., 1970). Voss (1968) developed an automated procedure for residue
analysis of propoxur in aqueous extracts of fruits based on
cholinesterase inhibition.
The above-mentioned methods are not specific and therefore not
suitable for regulatory purposes. The methods have become obsolete.
Several colorimetric methods have been developed. The only
satisfactory methods involve hydrolysis to isopropoxyphenol, which is
converted to a dyestuff and measured photometrically (see following
table).
The limits of determination range from 0.05 to 0.1 ppm.
TLC methods
Several authors describe TLC methods for the analysis of
propoxur. The most suitable are those based on cholinesterase
inhibition, since they require the simplest clean-up procedure and can
be used for various crop types.
The limits of determination are usually about 0.1 ppm.
GLC methods
Since propoxur, in common with other carbamates, readily
decomposes at high temperatures, GLC methods had to be developed in
which propoxur is converted to a stable derivative, which permits
detection with an electron capture detector. Also a method is
described in which the flame photometric detector is used after
derivatization.
SPECTROMETRIC RESIDUE METHODS FOR PROPOXUR
Crop Detection Sensitivity Reference
Sugar beet, tops,
lettuce IR; N-H-stretching bond 0.2 ppm Niessen and Frehse (1963)
Grapes IR; N-H-stretching bond ? Broderick (1966)
Milk spectrophotofluorometry 1.4 ppm Bowman and Beroza (1967)
Fruits, vegetables,
potatoes, cereals,
hops Aminoantipyrinea 0.05-0.1 ppm Niessen and Frehse (1964)
Human urine Aminoantipyrinea 10-20 ppm Dawson et al. (1964)
Lettuce 2,6-dibromo-benzoquinone- 0.1 ppm van Gils (1970)
chloroimidea
Sugar beets, p-nitrobenzene
potatoes diazoniumfluoboratea 0.05 ppm George (1967)
a After saponification.
THIN LAYER RESIDUE METHODS FOR PROPOXUR
Crop Stationary Solvent Detection Sensitivity Reference
phase system
Water silica gel different dimethylamino-benzaldehyde; 0.1 ppm Abbot et al. (1967)
nitrobenzene-diazonium
fluoborate
Water silica gel different dimethylamino-benzaldehyde 0.1 ppm El-Dib (1970)
Peas, carrots silica gel acetone + cholinesterase 10 ng Mendoza and Shields
hexane inhibition (0.1 ppm) (1971)
20 + 80
Tobacco aluminiumoxide acetone + fast blue B; 0.5 ppm Nesemann and Seehofer
hexane dichloroquinone-chloroimide (1970)
10 + 90
Apples, beets, silica gel acetone + cholinesterase 1 µg Wales et al. (1968)
cabbage, carrots, hexane inhibition
lettuce,
raspberries, 20 + 80
poultry meat
GAS-CHROMATOGRAPHIC RESIDUE METHODS FOR PROPOXUR
Crop Column Derivativea Sensitivity Reference
Apples, cucumbers, Chromosorb W DMCS -chloroacetyl 0.04 ppm Argauer (1969)
tomatoes, milk XE - 60
Corn silage, milk Gaschrom Q -thiophosphoryl 0.02-0.04 ppm Bowman and Beroza (1967)
DC-200
Potatoes, sugar Gaschrom Q -trichloroacetyl 0.01-0.1 ppm Butler and MeDonough (1968)
beets, apples, DC-200
grass
Water, peas, Chromosorb GAWDMCS -2,4-dinitrophenyl 0.2 ppm Cohen et al. (1970)
lettuce, apples XE 60 + Epikote 1001
Spinach Anakrom A B S -2,4-dinitroanilineb 0.05-0.2 ppm Holden et al. (1969)
XE -60
Soil Gaschrom Q -trichloroacetyl 0.02 ppm Stanley (1971)
OV -1
Animal tissue, Gaschrom Q -trichloroacetyl 0.002-0.02 ppm Stanley and Thornton (1972)
milk OV -1
Alfalfa, corn, Gaschrom Q -trichloroacetyl 0.02-0.05 ppm Stanley et al. (1972)
grass, cereals
a Of isopropoxyphenol.
b From reaction with methylamine.
Although the above-mentioned GLC methods are relatively
time-consuming, they are nevertheless the methods of choice. They are
very sensitive and specific (limits 0.002-0.05 ppm).
TLC and GLC methods may be suitable or can be adapted for
regulatory purposes.
The determination of propoxur residues in the trials carried out
by Bayer and reported in this monograph is carried out by the
colorimetric method of Niessen and Frehse (1964). This method
determines only the parent compound and is not fully specific.
The method includes a step for precipitating plant constituents,
which is also used in the methods referred to above.
The analysis in the residue experiments carried out by Chemagro
mentioned in the monograph was by GLC as described by Stanley et al.
(1972). The method is rather complicated, but makes it possible to
determine the parent compound and the main metabolites ((A) and (B),
see page) separately with a high degree of sensitivity.
The conjugated metabolites (A) (2-hydroxyphenyl methylcarbamate)
and (B) (2-isopropoxyphenyl hydroxymethylcarbamate) are released by
enzyme hydrolysis before clean-up. The metabolite (A) is then
alkylated. Next, the compounds are saponified to the phenols and
converted to their trichloroacetyl derivatives, which are determined
by GLC with electron capture detection.
National tolerances
Belgium Fruits and vegetables, 3 ppm
except potatoes
Germany, Federal Fruit 3 ppm
Republic of Sugar Beets 3 ppm
Vegetables, except 3 ppm
Cabbage 4 ppm
Lettuce 4 ppm
Other plant products 0.5 ppm
France Fruit and Vegetables 3 ppm
Italy Fruit and Vegetables 2.25 ppm
Netherlands Fruit and Vegetables 3 ppm
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Propoxur is a non-systemic carbamate insecticide which is used on
a considerable scale in various countries against a relatively broad
spectrum of insects in field crops, fruits and vegetables, e.g.
aphids, lygus bugs, leafhoppers, thrips, sawflies, etc.
Propoxur is also used extensively for hygienic purposes against
cockroaches, flies, etc., and against insect pests on ornamentals and
flower crops.
The technical material contains minimal 95% of
2-isopropoxy-phenyl-N-methyl carbamate. The impurities in the
technical material are known.
Propoxur is marketed in the form of wettable powder, emulsifiable
concentrate, dust, fly and cockroach baits, and balls against flies.
The concentration/rates of application vary depending on pest,
crop and methods of application. Normal application rates are 250-1000
g/ha.
The residue data available were obtained from different countries
and regions with different climatic and pest conditions. Most of the
residue data obtained in Europe show only the parent compound. The
trials carried out in the United States of America and a limited
number of trials carried out elsewhere determined not only the parent
propoxur but also the two main plant metabolites, (A)
2-hydroxyphenyl-N-methylcarbamate and (B)
2-isopropoxyphenyl-N-hydroxymethylcarbamate.
Information is available on the fate of propoxur residues in
soil, in plants. in mammals and in other animals, e.g. flies.
Some data on products of animal origin after feeding the animals
on treated crops are available indicating that residues are very low.
It would be desirable to have the result of more critical studies
which have been carried out on cows, pigs and chickens, in order to
confirm that this is the true position.
The breakdown of propoxur in plants and animals follows similar
pathways. The same metabolites are identified in rats, plants and in
vitro with microsomes.
Whereas oxidative and hydrolytic degradation both occur and
proceed to the same degree in rats, the formation of oxidation
products predominates in plants. In soil, however, hydrolytic
degradation predominates.
The residues in foods of plant or animal origin, following
recommended directions for use and recommended pre-harvest
intervals, consist largely of the parent compound. In plant products,
the above-mentioned metabolites (A) and (B) occur in a ratio of about
9:1. These metabolites, however, normally represent less than
one-third of the total residue determined.
Little information is available on the rate of decrease in the
level of residue of propoxur and its metabolites during storage and
processing, including household cooking. Little information is
available on propoxur residues in food moving in commerce.
Thin layer chromatographic and gas chromatographic procedures,
specific for propoxur and its main metabolites occurring in plants
(i.e. the metabolites (A) and (B), see above) and/or in products of
animal origin, are available.
The above-mentioned GLC methods are rather time-consuming due to
the fact that propoxur has to be converted to derivatives which are
stable to the GLC conditions. These GLC methods and the TLC methods
may be suitable or can be adapted for regulatory purposes. The most
suitable TLC methods are those based on cholinesterase inhibition.
The limit of determination of the TLC methods is usually about
0.1 ppm. The GLC methods allow sensitive and specific analysis (limits
of detection depending on commodity 0.002-0.05 ppm) of residues in
most crops and products of animal origin.
RECOMMENDATIONS
The following tolerances are based on residues likely to be found
at harvest following currently used patterns. The residues are
determined as propoxur and the main metabolites and are expressed as
propoxur.
Interval on which
Tolerances recommendations
are based (days)
Fruit, including apples,
pears, cherries, peaches,
plums 3 4-7
Soft fruit, including red
currants, blackberries,
gooseberries, strawberries 3 4-7
Vegetables, except potatoes
and root vegetables 3 outdoor 4-7
glasshouse:
leafy vegetables 14,
other vegetables 3-7
Potatoes, root vegetables -
Raw cereals 0.5 14
Rice (hulled) 0.1 7
Cocoa beans 0.05a 7
Meat 0.05a -
Milk (whole) 0.05a -
Animal feedstuff 5 7-14
a At or about the limit of determination
The time interval between application and harvest which has been
used in determining the maximum residue limits is appropriate to the
agricultural practices in numerous countries.
FURTHER WORK OR INFORMATION
Desirable
1. Studies to elucidate the significance of the changes in relative
liver weight in the rat.
2. Studies, including pharmacokinetic studies, to elucidate the
relationships between toxicity and effects on cholinesterase levels in
various species.
3. A long-term study in an animal species other than the rat.
4. Continued epidemiological studies with emphasis on cholinesterase
activity.
5. Studies on behavioural responses especially with low-level
exposure.
6. Results of critical studies to determine the nature and level of
residues in meat (including poultry), milk, and eggs to confirm
recommendations for limits in animal products.
REFERENCES
Abbott, D.C., Blake, K.W., Tarrant, K.R. and Thomson, J. (1967)
Thin-layer chromatographic separation, identification and estimation
of residues of some carbamate and allied pesticides in soil and water.
J. Chromatography, 30: 136-142
Abdel-Wahab, A.M., Kuhr, R.J. and Casida, J.E. (1966) Fate of
C14-carbonyl-labelled aryl methylearbamate insecticide chemicals in
and on bean plants. J. Agr. Food Chem. 14: 290-299
Aly, O.M. and El-Dib, M.A. (1971) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Research, 5:
1191-1205
Argauer, R.J. (1969) Determination of residues of Banol and other
carbamate pesticides after hydrolysis and chloroacetylation. J. Agr.
Food Chem. 17: 888-892
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Baygon in albino mice. Unpublished report
submitted by Bayer AG
Balba, M.H. and Casida, J.E. (1968) Synthesis of possible metabolites
of metbylcarbamate insecticide chemicals. Hydroxyaryl and
hydroxyalkyl-phenyl methylcarbamates. J. Agr. Food Chem. 16: 561-567
Barlow, F. and Hadaway, A. B. Interactions between insecticides,
cellulose and water, and their effects on insecticide toxicity and
persistence. Society of Chemical Industry Monograph No. 29 (London)
Bayer AG Rückstandsberichte 1964-1969
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) Acute toxicity
data for pesticides (1970). World Review of Pest Control, 9: 119-127
Bowman, M.C. and Beroza, M. (1967) Determination of Niagara NIA-10242
and its phenol degradation product in corn silage and milk and
determination of other carbamates by GLC of their thiophosphoryl
derivatives. J. Ass. off. analyt. Chem. 50: 926-933
Bowman, M.C. and Beroza, M. (1967) Spectrophotofluoreseent and
spectrophotophosphorescent data on insecticidal carbamates and the
analyses of five carbamates in milk by spectrophotofluorometry.
Residue Reviews, 17: 23-34
Broderick, E.J., Bourke, J.B., Mattik, L.R., Taschenberg, E.F. and
Avens, A.W. (1966) Determination of methylcarbamate pesticides in the
presence of methyl anthranilate in Concord grapes. J. Ass. off.
analyt. Chem. 49: 982-985
Brooks, G.D., Smith, E.A. and Schoof, H.F. (1967) Residual
effectiveness of eleven insecticides under weathering conditions
against Aedes aegypti. Mosquito News, 27: 93-99
Burkhardt, C.C. and Fairchild, M.L. (1967) Toxicity of insecticides to
house crickets and bioassay of treated soils in the laboratory. J.
Econ. Ent. 60: 1496-1503
Butler, L.I. and McDonough, L.M. Method for the determination of
residues of carbamate insecticides by electron-capture gas
chromatography. J. Agr. Food Chem. 16: 403-407
Casida, J.E., Shrivastava, S.P. and Esaae, E.G. (1968) Selective
recovery of volatile products from house flies treated with
radioactive insecticide chemicals and synergists, J. Econ. Ent. 61:
1339-1344
Chemagro Corporation (1971) Baygon, the effects on the environment.
Unpublished report submitted by Chemagro
Chemagro Corporation (1971) Unpublished report submitted by Chemagro
Church, D.D. and Flint, D.R. (1971) The fate of Baygon
(O-isopropoxy-phenyl-N-methylcarbamate) in soil. Unpublished report
submitted by Chemagro
Cohen, I.C., Norcup, J., Ruzicka, J.H.A. and Wheals, B.B. (1970) An
electron-capture gas chromatographic method for the determination of
some carbamate insecticides as 2,4-dinitrophenyl derivatives of their
phenol moieties. J. Chromatography, 49: 215-221
Crosby, D.G., Leitis, E. and Winterlin, W.L. (1965) Carbamate
insecticides - photodecomposition of carbamate insecticides. J. Agr.
Food Chem. 13: 204-207
Dawson. J.A., Heath, D.F., Rose, J.A., Thain, E.M. and Ward, J.B.
(1964) Excretion by humans of phenol derived in vivo from arprocarb.
I. Determination by gas chromatography. II. Colorimetric
determination. Bull. Wld Hlth Org. 30(1): 127-134
Dorough, H.W. and Casida, J.E. (1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J. Agr. Food Chem. 12: 294-304
Dorough, H.W. and Crouch, G.W. (1966) Persistence and residual
effectiveness of various formulations of Baygon (Bayer 39007) against
the house fly. J. Econ. Ent. 59: 1188-1190
Dorough, H.W., Leeling, N.C. and Casida, J.E. (1963) Nonhydrolytic
pathway in metabolism of N-methylcarbamate insecticides. Science,
140: 170-171
Douch, P.G.C. and Smith, J.N. (1971) Metabolism of
m-tert.-butylphenyl N-methylcarbamate in insects and mice. Biochem.
J. 125: 385-393
Douch, P.G.C. and Smith, J.N. (1971) The metabolism of
3,5-Di-tert.-butylphenyl N-methyl-carbamate in insects and by mouse
liver enzymes. Biochem. J. 125: 395-400
DuBois, K.P., (1962) University of Chicago The acute oral toxicity of
Bayer 39 007 to chickens. Unpublished report submitted by Bayer AG
DuBois, K.P. (1963) University of Chicago The acute toxicity of some
possible metabolites of Bayer 39 007, 44 646, 37 344 and Morestan.
Unpublished report submitted by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961a) University of Chicago The
acute toxicity of Bayer 39 007 to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961b) University of Chicago The
acute toxicity of Bayer 39 007 given in combination with other
anticholinesterase insecticides to rats. Unpublished report submitted
by Bayer AG
DuBois, K.P. and Raymund, A.B., (1961c) University of Chicago The
acute toxicity of Bayer 39 007 and Bayer 37 344 in combination with
some other anticholinesterase insecticides to rats. Unpublished report
submitted by Bayer AG
Eben, A. and Kimmerle, G. (1973) Propoxur, effect of acute and
subacute oral doses on acetyl cholinesterase activity in plasma,
erythrocytes and brain of rats. Unpublished report submitted by Bayer
AG
Eichelberger, J.W. and Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Env. Sci and Techn. 5: 541-544
El-Dib, M.A. (1970) Thin layer chromatographic detection of carbamate
and phenylurea pesticide residues in natural waters. J. Ass. off.
analyt. Chem. 53: 756-760
Everett, L.J. and Gronberg, R.R. (1968) Plant metabolism of Baygon.
Unpublished report submitted by Chemagro
Everett, L.J. and Gronberg, R.R. (1970) The metabolic fate of Baygon
(2-isopropoxyphenyl-N-methyl-carbamate) in the rat. Unpublished
reported submitted by Chemagro
Flint, D.R. and Shaw, H.R., II (1971) The mobility and persistence
of Baygon in soil and water. Unpublished report submitted by Chemagro
Gahan, J.B. and Wilson, H.G. (1970) Seven insecticides as residual
sprays in buildings naturally infested with Anopheles
quadrimaculatus. Mosquito News, 30: 410-416
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharm. 14: 515-534
George, D.A., Rusk, H.W., Powell, D.M. and Landis, B.J. (1967) An
analytical method for o-isopropoxyphenyl methyl carbamate (Bayer
39007), its aphicidal value and persistence in potatoes and sugar
beets. J. Econ. Ent. 60: 82-84
van Gils, W.F. (1970) Spectrophotometric determination of propoxur
residues on vegetable matter. Analyst, 95: 88-90
Gronberg, R.R. (1970) Metabolism of Baygon in corn plants. Unpublished
report submitted by Chemagro
Hadaway, A.B. and Barlow, F.A (1964) A note on the sorption of
insecticides on tropical soils. Bull. Wld Hlth Org. 30: 146-148
Hayes, W.J., jr (1971) Studies on exposure during the use of
anti-cholinesterase pesticides. Bull. Wld Hlth Org. 44: 277-288
Hobik, H.P. (1967) Histologische Untersuchungen von Ruckenmark und
Nervi ischiadici aus Neurotoxizitatsversuchen an Huhnern mit, BAYER
39007. Unpublished report submitted by Bayer AG
Holden, E.R., Jones, W.M. and Beroza, M. (1969) Determination of
residues of methyl-and dimethyl-carbamate insecticides by gas
chromatography of their 2,4-dinitroaniline derivatives. J. Agr. Food
Chem. 17: 56-59
Houseworth, L.D. and Tweedy, B.G. (1972) Effect of Baygon on microbial
populations. Unpublished report submitted by Chemagro
Ivie, G.W. and Casida, J.E. (1971) Sensitized photodecomposition and
photosensitizer activity of pesticide chemicals exposed to sunlight on
silica gel chromatoplates. Photosensitizers for the accelerated
degradation of chlorinated another insecticide chemicals exposed to
sunlight on bean leaves. J. Agr. Food Chem. 19: 405-416
Kimmerle, G. (1961) Preparation Dr. Bocker 58 12 315 (39 007).
Unpublished report submitted by Bayer AG
Kimmerle, G. (1964) E 39 007 (Bocker 58 12 315; Ht.-Nr. 3410)/
Neurotoxizitat. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966a) Neurotoxic studies on active ingredient Docker
58: 12 315 (Unden active ingredient). Unpublished report submitted by
Bayer AG
Kimmerle, G. (1966b) Unden-Wirkstoff (Bocker 58 12
315)/Antidotwirkung. Unpublished report submitted by Bayer AG
Kimmerle, G. (1966c) Unden-Wirkstoff/Inhalationstoxizitat.
Unpublished report submitted by Bayer AG
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetra-ethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol. 27: 311-314
Kimmerle, G. and Solmecke, B. (1971) BAY 39 007/Subacute dermal
application to rabbits. Unpublished report submitted by Bayer AG
Klimmer, O.R., (1963) Pharmakologisches Institut der Universitat Bonn.
Toxikologische Prufung von BAYER 39 007. Unpublished report submitted
by Bayer AG
Krishna, J.G. and Casida, J.E. (1966) Insecticide metabolism - fate in
rats of the radiocarbon from ten variously labeled methyl-and
dimethylcarbamate-14C insecticide chemicals and their hydrolysis
products. J. Agr. Food Chem. 14: 98-105
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticide chemicals
in plants. J. Sci. Food Agr. pp. 44-49
Kuhr, R.J. (1968) Metabolism of methylcarbamate insecticides by
insects in vivo and in vitro. Mededel. Rijksfac.
Landbouwetenschappen, 33 (3): 647-657
Kuhr, R.J. (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J. Agr. Food Chem. 18: 1023-1030
Kuhr, R.J. and Casida, J.E. (1967) Persistent glycosides of
metabolites of methylcarbamate insecticide chemicals formed by
hydroxylation in bean plants. J. Agr. Food Chem. 15: 814-824
Linke, W. Trials to determine the persistence of Arprocarb when used
in food storage practice. Unpublished report submitted by Baywood
Chemicals Ltd., Techn. Dept. Technical Report No. TCR/130
Lorke, D. (1970) BAY 39 007/Untersuchungen auf embryotoxische
Wirkungen an der Ratte. Unpublished report submitted by Bayer AG
Löser, E. (1965) Fütterungsversuch Uber 2 Monate mit BAYER 39 007.
Unpublished report submitted by Bayer AG
Löser, E. (1968a) BAY 39 007/Generationsversuche an Ratten.
Unpublished report submitted by Bayer AG
Löser, E. (1968b) BAYER 39 007/Chronische toxikologische
Untersuchungen an Ratten. Unpublished report submitted by Bayer AG
Löser, E. (1968) BAY 39 007/Chronische toxikologische Untersuchungen
an Hunden. Unpublished report submitted by Bayer AG
Marchart, H. (1970) Insecticide residues in cocoa. Int. Atomic Energy
Agency, P-309/1: 1-10
Marchart, H. Evaluation of insecticides for the control of cocoa
capsids in Ghana. FAO Plant Protection Bull. 19: 97-109
Mawdesley-Thomas, L.E., (1969a) Huntingdon Research Centre, England.
Pathology report of the generation experiment in rats of the toxicity
of compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E. (1969b) Huntingdon Research Centre, England.
Pathology report of the two-year experiment in rats of the toxicity of
compound BAY 39 007 by oral administration. Unpublished report
submitted by Bayer AG
Mawdesley-Thomas, L.E., (1969c) Huntingdon Research Centre, England.
Pathology report of the two-year toxicity in dogs of compound BAY 39
007 by oral administration. Unpublished report submitted by Bayer AG
Mendoza, C.E. and Shields, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin layer chromatography. J. Ass. off.
analyt. Chem. 54: 507-512
Metcalf, R.L. (1971) Structure-activity relationships for insecticidal
carbamates. Bull. Wld Hlth Org. 44: 43-78
Metcalf, R.L. and Fukuto, T.R. (1965) Carbamate insecticides. Effects
of chemical structure on intoxication and detoxication of phenyl
N-methyl-carbamates in insects. J. Agr. Food Chem. 13: 220-231
Metcalf, R.L., Osman, M.F. and Fukuto, T.R. (1967) Metabolism of
14C-labeled carbamate insecticides to 14CO2 in the house fly. J.
Econ. Ent. 60: 445-450
Nelson, D.L. A study of the acute toxicity of BAYGON in 1967
combination with D.D.V.P. Unpublished report submitted by Bayer AG
Nesemann, E. and Seehofer, F. (1970) Screening procedures for
organophosphorus, organochlorine and carbamate pesticide residues on
tobacco. Beiträge zur Tabakforschung, 5: 207-214
Niessen, H. and Fretse, H. (1963) Eine infrarotspektroskopiscbe,
Methods zur Bestimmung von N-Methylcarbamat-Rückständen in Pflanzen.
Pflanzenschutz Nachrichten "Bayer", 16: 205-220
Niessen, H. and Frehae, H. (1964) Kolorimetrische Methods zur
Bestimung von Rücksthinden doe Insektizids Undon in pflanzlichem
Material. Pflanzenschutz-Nachrichten "Bayer", 17: 25-32
O'Brien, (1966) cited in Metcalf, 1971
Oonnithan, E.S. and Casida, J.E. (1966) Metabolites of methyl- and
dimethyl carbamate insecticide chemicals as formed by rat liver
microsomes. Bull. of Env. Cont. and Tox. 1 (2): 59-60
Oonnithan, E.S. and Casida, J.E. (1968) Oxidation of methyl- and
dimethyl carbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J. Agr. Food Chem.
16: 28-44
Pant, C.P. and Joshi, G.P. (1969) A field study of an airborne toxic
effect of Baygon residual spray. Mosquito News, 29: 674-677
Parker, B.L., Dewey, J.E. and Bache, C.A. (1970) Carbamate bio assay
using Daphnia magna. J. Econ. Ent. 63: 710-714
Plestina, Radovan (1968) Beitrag zur Erforschung der toxischen
Eigenschaften des o-Isopropoxyphenyömethylkarbamat. Magisterarbeit
Reiner, E. (1968) The inhibitory power of
2-isopropoxyphenyl-N-methyl-carbamate against serum cholinesterase of
various individuals. Arch. Toxikol. 23 (3): 237-239
Reiner and Aldrich (1967), cited in Metcalf, 1971
Reiner, E. and Simeon, V. (1968) The inhibitory power of 2-isopropoxy
1968 phenyl-N-methyl-carbamate against serum cholinesterase of various
individuals. Archiv f. Toxikologiet 23: 237-239
Root, M., Cowan, J. and Doull, J., University of Chicago. (1963)
Subacute oral toxicity of RAYER 39 007 to male and female rats.
Unpublished report submitted by Bayer AG
Shrivastava, S.P., Tsukamato, M. and Casida, J.E. (1969) Oxidative
metabolism of 14C-labeled Baygon by living house flies and by house
fly enzyme preparations. J. Econ. Ent. 62: 483-498
Stanley, C.W. (1971) A gas chromatographic method for the
determination of Baygon in soils. Unpublished report submitted by
Chemagro
Stanley, C.W. and Thornton, J.S. (1972) Gas chromatographic method for
residues of Baygon and its metabolites in animal tissues and milk. J.
Agr. Food Chem. 20: 1269-1273
Stanley, C.W., Thornton, J.S. and Katague, D.B. (1972) Gas
chromatographic method for residues of Baygon and metabolites in plant
tissues. J. Agr. Food Chem. 20: 1265-1269
Steelman, C.D., Colmerg A.R., Cabes, L., Barr, H.T. and Tower, B.A.
(1967) Relative toxicity of selected insecticides to bacterial
populations in waste disposal lagoons. J. Econ. Ent. 60: 467-468
Syrowatka, T., Jurek, A. and Nazarewiez, T. (1971) Short term chronic
toxicity study of o-isopropoxyphenyl-N-methyl carbamate (propoxur).
Rooz. Panstw. Zakl. Hig. 22: 579-589 (poln.)
Tsukamoto, M. and Casida, J.E. (1967) Albumin enhancement of oxidative
metabolism of methylearbamate insecticide chemicals by the house fly
microsome-NADPH2 system. J. Econ. Ent. 60: 617-619
Tsukamoto, M. and Casida, J.E. (1967) Metabolismus von Methylcarbamat
Insektiziden durch das NADPH2-benötiginde Enzymsystem der Hausfliege.
Nature (London), 213: 49-51
Vandekar, M. (1969) The effect on cholinesterase activity of storage
of undiluted whole blood sampled from men exposed to
C-isopropoxyphenylmethylcarbamate. Bull. Wld Hlth Org. 40: 91-96
Vandekar, M., Hedayat, E., Plestina, R. and Ahmady, G. (1968) A study
of the safety of o-isopropoxyphenylmetbyl-carbamate in an operational
field-trial in Iran. Bull. Wld Hlth Org. 38: 609-623
Vandekar, M., Plestina, R. and Wilhelm. K. (1971) Toxicity of
carbamates for mammals. Bull. Wld Hlth Org. 44: 241-249
Vandekar, M., Reiner, E., Svetlicic, B. and Fajdetic, T. (1965) Value
of ED50 testing in assessing hazards of acute poisoning by carbamates
and organophosphates. Brit. J. Industr. Med. 22: 317-320
Vandekar, M. and Wilford, K. (1969) The effect on cholinesterase
activity of storage of undiluted whole blood sampled from men exposed
to o-isopropoxyphenyl methylcarbamate (OMS-33). Bull. Wld Hlth Org.
40: 91-96
Voss, G. (1968) Peacock plasma, a useful cholinesterase source for
inhibition residue analysis of insecticidal carbamate. Bull. Env.
Cont. and Tox. 3: 339-347
Waggoner, T.B. and Olson, T.J. (1971) Effect of feeding
organophosphorus and carbamate pesticides to cattle. Paper No. 45,
Division of Pesticide Chemistry, 162nd National ACS Meeting, 12-17
September, Washington, D.C.
Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968) Pesticide residues
- TLC-enzyme inhibition procedure to detect some carbamate standards
and carbaryl in food extracts. J. Ass. off. analyt. Chem. 51:
1239-1242
Weiden, M.H.J. (1971) Toxicity of carbamate to insects. Bull. Wld
Hlth Org. 44: 203-213
Wilhelm (1967), cited in Vandekar et al., 1971
Wit, Sj. (1963) Residues of acaricides and aphicides on glasshouse
cultures (mevinphos, dichlorvos, naled, propoxur and nicotine).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1966) Residues of parathion and propoxur on red-currants.
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wit, Sj. (1969) Residues of propoxur on gherkins (glasshouse).
Unpublished report submitted by the Nat. Inst. Public Health
Netherlands
Wright, J.W., Fritz, R.F., Hocking, K.S,, Babione, R., Gratz, N.G.,
Pal, R., Stiles, A.R. and Vandekar, M. (1969) Ortho-isopropoxyphenyl
methyl-carbamate (OMS-33) as a residual spray for control of
anopheline mosquitos. Bull. Wld Hlth Org. 40: 67-90
Wright, C.G. and Jackson, M.D. (1971) Propoxur, chlordane and diazinon
on porcelain china saucers after kitchen cabinet spraying. J. Econ.
Ent. 64: 457-459
