
CHLOROTHALONIL JMPR 1974
IDENTITY
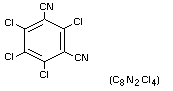
Chemical name
2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile
Synonyms
2,4,5,6-tetrachloroisophthalonitrile, DAC-2787(R), Daconil
2787(R).
Structural Formula
 Other Information on Identity and Properties
Molecular weight: 265.9
State: White crystalline solid
Melting point: 250-251°C
Boiling point: 350°C at 760 mm Hg
Vapour Pressures:
Vapour Pressures Temperatures
mm Hg °C
< 0.1 40
9.2 170
17.4 191
27.3 212
43.3 230
Solubility:
Solvent % by weight at 25°C
acetone 2
AR-60 6
AR-55 3
cyclohexanone 3
dimethyl sulfoxide 2
dimethyl formamide 3
kerosene <1
mineral seal oil <1
methyl ethyl ketone 2
xylene 8
water 0.6 ppm
Stability: Stable under normal temperatures of storage. Chemically
stable in alkaline or acidic aqueous media. Stable to
ultraviolet radiation.
Purity of technical material:
Ingredient Range (%) Average (%)
2,4,5,6-tetrachloro 95.6-98.5 97.6
-1,3-benzenedicarbonitrile
tetrachlorophthalonitrile - <0.1
tetrachloroterephtholonitrile <0.1-1.6 0.5
pentachlorobenzonitrile 0.5-2.5 1.2
partially chlorinated 0.2-1.0 0.4
dicyanobenzenes (all isomers)
unchlorinated dicyanobenzenes <0.1-1.6 0.3
(all isomers)
insolublein xylene <0.1-1.0 0.2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Following acute oral administration to dogs, chlorothalonil (500
mg/kg) was rapidly excreted, mainly unchanged, in the faeces.
Approximately 90% of the administered dose was found in faeces within
72 hours. No chlorothalonil or metabolites were found in blood or
urine when examined using methods having a sensitivity of 1 ppm for
blood and 0.1 ppm for urine (Skinner and Stallard, 1967). Urine and
faecal samples were collected from dogs and rats fed chlorothalonil in
the diet for approximately ten months. In both species excretion of
unchanged chlorothalonil in faeces was proportional to the
concentration in the diet. Rats fed a dose of 0.15% excreted 21% in
the faeces; those fed 1.5% excreted 67%; dogs fed 0.15% excreted
9-15%; those fed 1.5% excreted 67-71%; and those fed 3% excreted
86-86% unchanged chlorothalonil in the faeces. Assays based on total
chlorine content established that recovery in faeces of chlorothalonil
consumed in the diet was complete. The total chlorine assay and
incomplete recovery of chlorothalonil or metabolites from faeces
suggests a biotransformation product excreted with the faeces and not
identified.
Three male and three female weanling rats were fed for 22 days at
5000 ppm in the diet. They were orally administered 14C
chlorothalonil at a dose of 2.8 µ Ci/rat. The dose (1.5 mg) did not
significantly increase the daily input of chlorothalonil. After 264
hours, 95% of the material was recovered, predominantly in faeces
(88%) and urine (5%) with none detected in tissues or as CO2.
Chlorothalonil was not rapidly removed from the animals body (43% in
24 hours; 64% in 48 hours, and 76% in 72 hours) (Ryer and Sullivan,
1966).
Studies on the transformation of chlorothalonil in soil (Stallard
and Wolfe, 1967; Duane, 1970) have isolated and identified a
metabolite of chlorothalonil as
4-hydroxy-2,5,6-trichloroisophthalo-nitrile (DAC-3701).
Other Information on Identity and Properties
Molecular weight: 265.9
State: White crystalline solid
Melting point: 250-251°C
Boiling point: 350°C at 760 mm Hg
Vapour Pressures:
Vapour Pressures Temperatures
mm Hg °C
< 0.1 40
9.2 170
17.4 191
27.3 212
43.3 230
Solubility:
Solvent % by weight at 25°C
acetone 2
AR-60 6
AR-55 3
cyclohexanone 3
dimethyl sulfoxide 2
dimethyl formamide 3
kerosene <1
mineral seal oil <1
methyl ethyl ketone 2
xylene 8
water 0.6 ppm
Stability: Stable under normal temperatures of storage. Chemically
stable in alkaline or acidic aqueous media. Stable to
ultraviolet radiation.
Purity of technical material:
Ingredient Range (%) Average (%)
2,4,5,6-tetrachloro 95.6-98.5 97.6
-1,3-benzenedicarbonitrile
tetrachlorophthalonitrile - <0.1
tetrachloroterephtholonitrile <0.1-1.6 0.5
pentachlorobenzonitrile 0.5-2.5 1.2
partially chlorinated 0.2-1.0 0.4
dicyanobenzenes (all isomers)
unchlorinated dicyanobenzenes <0.1-1.6 0.3
(all isomers)
insolublein xylene <0.1-1.0 0.2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Following acute oral administration to dogs, chlorothalonil (500
mg/kg) was rapidly excreted, mainly unchanged, in the faeces.
Approximately 90% of the administered dose was found in faeces within
72 hours. No chlorothalonil or metabolites were found in blood or
urine when examined using methods having a sensitivity of 1 ppm for
blood and 0.1 ppm for urine (Skinner and Stallard, 1967). Urine and
faecal samples were collected from dogs and rats fed chlorothalonil in
the diet for approximately ten months. In both species excretion of
unchanged chlorothalonil in faeces was proportional to the
concentration in the diet. Rats fed a dose of 0.15% excreted 21% in
the faeces; those fed 1.5% excreted 67%; dogs fed 0.15% excreted
9-15%; those fed 1.5% excreted 67-71%; and those fed 3% excreted
86-86% unchanged chlorothalonil in the faeces. Assays based on total
chlorine content established that recovery in faeces of chlorothalonil
consumed in the diet was complete. The total chlorine assay and
incomplete recovery of chlorothalonil or metabolites from faeces
suggests a biotransformation product excreted with the faeces and not
identified.
Three male and three female weanling rats were fed for 22 days at
5000 ppm in the diet. They were orally administered 14C
chlorothalonil at a dose of 2.8 µ Ci/rat. The dose (1.5 mg) did not
significantly increase the daily input of chlorothalonil. After 264
hours, 95% of the material was recovered, predominantly in faeces
(88%) and urine (5%) with none detected in tissues or as CO2.
Chlorothalonil was not rapidly removed from the animals body (43% in
24 hours; 64% in 48 hours, and 76% in 72 hours) (Ryer and Sullivan,
1966).
Studies on the transformation of chlorothalonil in soil (Stallard
and Wolfe, 1967; Duane, 1970) have isolated and identified a
metabolite of chlorothalonil as
4-hydroxy-2,5,6-trichloroisophthalo-nitrile (DAC-3701).
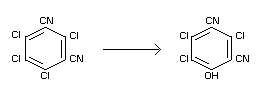 Residues and biotransformations of chlorothalonil in farm animals,
plants and soil are discussed in the sections "Residues resulting from
supervised trials" and "Fate of residues."
Pharmacological effects
Chlorothalonil has a cathartic effect which is apparently due to
irritation of the gastro-intestinal tract. This was evidenced by a
dose related decrease in urinary volume and increase in urine specific
gravity. Studies using rats subjected to levels of 0, 1500, and 15,000
ppm in the diet showed a dose related decrease in retention of an
orally administered dye indicative of a potential laxative effect.
Some experiments utilizing selected animals on feeding studies
suggested an increase in water intake, a decrease in urine volume, and
an increase in fecal moisture which was dose related especially at
high concentrations in the diet (Paynter, 1967c; Skinner and Stallard,
1967).
Chlorothalonil administered orally at one gm/kg to mice increased
intestinal mobility as measured by the percentage of the small
intestine traversed by a charcoal marker within a selected time
interval. The laxative action of chlorothalonil was significantly
reduced by pre-treatment with corn oil but not with atropine or
papaverine (Teeters, 1966).
Residue analyses of tissues and organs of animals fed
chlorothalonil in the diet indicated that there was no accumulation in
any body organ. Small quantities of the 4-hydroxy metabolite were
observed in liver and kidney with no parent compound noted (Wolfe and
Stallard, 1968a).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse
The potential genetic hazard of chlorothalonil was evaluated in
mice by the host mediated assay, in vivo cytogenic testing and the
dominant lethal test. The compound did not produce any measurable
mutagenic response when evaluated in vitro against eight indicator
organisms of the Salmonella typhimurium, histidine auxotroph tester
strains which can be reverted by both base substitution and frame
shift mutagens. In these tester strains, both repair deficient and
repair competent strains were used. When the tester strains were
inoculated into animals treated with chlorothalonil daily for five
days at an oral dose of 6.5 mg/kg (10 animals per treatment), no
increase in the number of mutations over the control were found. The
positive control, dimethylnitrosamine, increased mutation frequency in
mice (ip, 4 mg/kg).
Following oral administration of chlorothalonil daily for five
days at 6.5 mg/kg, an examination of bone marrow for the presence of
cells with micronuclei indicative of cytogenic abnormalities was
negative.
Treated orally for five days at 6.5 mg/kg, two male mice were
mated sequentially with untreated females in a standard dominant
lethal test. No adverse effect on fertility, implantation or foetal
mortality was observed. A positive control (triethylene melamine),
administered ip at 0.5 mg/kg, produced an increase in early death at
the post meiotic period. At the concentration tested in the three
procedures used, chlorothalonil did not produce mutagenic effects
(Legator, 1974).
Special studies on reproduction
Rat
Groups of rats (10 males and 20 females per group) were fed
chlorothalonil in the diet at levels of 0, 0.15, 1.5, and 3.0% in a
standard three generation, two litters per generation, reproduction
study. Because of food refusal and poor weight gain, the test using
the two higher levels was interrupted. After the first litter of pups
(F1a), the high level of treatment which had been reduced to 2% was
discontinued and a new dose level of 0.5% (with another control group)
was substituted. The full three generation reproduction test was
ultimately performed using dosage levels of 0, 0.15, 0.5 and 1.5% in
the diet. At the high discontinued dose level there was a significant
growth depression in nursing litters and an emaciated appearance in
the pups at weaning. There was also a reduction of fertility and
lactation at this maternally toxic level.
Growth suppression in pups was noted at all test intervals
through all three generations. It was considered that while smaller,
pups were still within a normal weight range, no malformations were
observed and reproduction indices were not affected. Necropsy
examination performed on parents and on the terminal F3b generation
revealed gross changes in the kidneys and in the G.I. tract. Gross
changes included enlargement and distention of the caecum and colon,
soft faecal matter, and occasional thickening of the stomach wall.
Microscopic changes were also evident in stomach and kidney.
Chlorothalonil examined at maternally toxic dietary levels did not
have an effect on reproduction in the rat (Paynter, 1967a).
Groups of rats (10 males and 20 females per group) were fed the
chlorothalonil metabolite, 4-hydroxy 2,5,6-trichloroisophthalonitrile,
in the diet at levels of 0, 10, 50, 100 and 200 ppm for 70 days prior
to mating in 3 generation, one litter per generation, reproduction
study. Growth was reduced, clinical chemistry changes (increased SGPT)
were noted and gross and microscopic changes observed at 200 mg/kg.
Reproduction was affected at 100 mg/kg as evidenced by reduced litter
size and weight as well as decreased survival. Milk analysis revealed
the 4-hydroxy metabolite in the stomach curd of 7 day pups in a
concentration comparable to that fed to the parents. No effects were
noted at 50 mg/kg on the reproduction parameters recorded (Hastings
and Jessup, 1974).
Special studies on teratogenicity
Rabbit
Groups of 8 pregnant rabbits were administered chlorothalonil
from day 8 to day 16 of gestation. The initial dose on days eight and
nine (0, 180, and 375 ml/kg/day) was reduced because of decreased food
consumption to 0, 62.5, and 31.25 mg/kg/day respectively for the
remainder of the study. Deaths were noted in the treated groups and
there was a severe weight loss with the treated dose. There was no
apparent effect on the embryo and while there was a considerable
effect on the adults, chlorothalonil is not considered to be a
teratogen (Paynter, 1966).
Acute toxicity
TABLE 1. Acute toxicity of chlorothalonil
Species Route LD50 mg/kg References
Rat Oral 10,000 Powers, 1965
Doyle and Elsea, 1963
Inhalation 4.7 mg/kg Beasley and Leong, 1965
Dog Oral > 5,000 Paynter, 1965a
Rabbit Dermal 10,000 Doyle and Elsea, 1963
Signs of poisoning include depression, diarrhoea and an unkempt
appearance. 3 mg crystalline technical product applied to the
conjunctival sac produced a mild irritation which was probably
mechanical (Doyle and Elsea, 1963).
Acute studies of the 4-hydroxy metabolite showed an oral LD50 in
male rats of 332 mg/kg. An LD50 could not be found in dogs because of
emesis at all dose levels (Wazeter, 1971; Wazeter and Goldenthal,
1972).
Short term studies
Rat (inhalation)
Groups of rats (15 males and 15 females per group) were exposed
to chlorothalonil by inhalation 6 hours/day, 5 days/week for three
weeks. This exposure was at a mean concentration of 12.2 mg/l at a
respirable particle size range of 1-5 microns (91%), 6-25 microns
(9%), and 26-40 microns (<1%). There were no deaths or untoward
behaviour among any of the animals. Growth was normal, and gross and
histological examinations did not reveal any compound-related effects
(Holliday et al., 1973).
Rat (feeding)
Groups of rats (10 males and 10 females per group) were fed diets
containing 5000 ppm chlorothalonil for 4 weeks. In an attempt to
determine the effect of chlorothalonil on absorption and utilization
of protein, fat, and amino acids, 10 amino acids varying in
concentration from 0.2 to 0.9% were added to the diet. On the basis of
a ten week feeding of dietary levels containing chlorothalonil plus
casein and corn oil or chlorothalonil plus amino acids, it was
concluded that chlorothalonil did not interfere directly with the
absorption and utilization of protein, fat, or amino acids. The
reduction in weight gain predominantly noted at this high feeding
level of chlorothalonil was presumably due to catharsis and not to
absorption difficulties (Paynter, 1967b).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of O, 250, 500, 750 and 1500 ppm
for 22 weeks. No mortality was observed and appearance and behaviour
were normal. Growth was slightly reduced at all feeding levels in
males and at 750 and 1500 ppm in the females. Food consumption was
comparable in all groups.
Haematological and urine analysis values were within normal
limits. An increase in liver-kidney weight was noted especially in
males at the two higher dosage levels. Kidney alterations,
characterized by irregular swelling of the tubular epithelium,
epithelial degeneration, and tubular dilation were seen in all test
groups with the males affected to a greater degree than females
(Blackmore and Shott, 1968).
Groups of rats (10 males and 10 females per group) were
administered chlorothalonil by oral gavage at dosage levels of 0, 0.5,
1.0, 2.0, 4.0 and 8.0 gm/kg five days a week for 13 weeks.
Administration of 4.0 gm/kg resulted in a slight, non-significant
reduction of growth, poor general condition, and reduced white blood
count. No significant findings were observed with regard to
elucidating the potential renal toxicity problem observed in longer
studies. Studies performed in this test indicated that chlorothalonil
did not result in a specific abnormality. It was suggested there might
be a decrease in resistance and defence mechanisms, making rats more
susceptible to naturally occurring infections (Sterner and Loveless,
1963).
Rabbit (dermal)
Groups of albino rabbits were daily administered a 75%
formulation of chlorothalonil to either intact or abraded skin, 5 days
per week, for three weeks. (Groups of two males and two females served
as controls; 5 males and 5 females were treated groups.) Animals were
administered chlorothalonil at dose levels of 0, 500, or 1000 mg/kg.
Repeated application of chlorothalonil to the intact or abraded
skin of rabbits resulted in dose-related dermal irritation consisting
of erythema, atonia, and desquamation. The degree of irritation was
more severe to the abraded skin. A number of animals at the high level
showed atypical haematological values in conjunction with diarrhoea
and/or dermal irritation as a result of application of chlorothalonil.
As might be expected, histological examination of the skin revealed
the presence of a moderate degree of acanthosis particularly in the
abraded skin areas, hyperkeratosis, rarely focal parakeratosis, and
slight to moderate leucocytic infiltration. No pathological
abnormalities were noted in other tissues (Paynter, 1965b).
Dog
Groups of dogs (4 males and 4 females per group) were fed
chlorothalonil in the diet at 0, 250, 500 and 750 ppm for sixteen
weeks. There was no mortality and no apparent effect on behaviour or
growth in any of the dogs tested. Haematological, clinical chemistry
and urine analysis values were normal with the exception of slightly
raised PBI values at high dose levels in females. Gross and
microscopic examination of organs and tissues did not reveal any
compound-related abnormalities (Paynter and Murphy, 1967).
Groups of beagle dogs (8 males and 8 females per group) were fed
chlorothalonil in the diet for two years at dosage levels of 0, 60 and
120 ppm. There were no effects noted on behaviour and growth over the
course of the study. Clinical chemistry values including haematology,
biochemistry and urine analysis were comparable to the controls at all
levels of feeding. Gross and microscopic examination of tissues and
organs performed on animals sacrificed at 12 months indicated a
compound-related change in the kidney. Further examination of tissues
and organs at 24 months did not show chlorothalonil-related
abnormalities. A slight degree renal tubule vacuolation in two of four
animals at 120 ppm after two years in the absence of other changes
(urinalyses values) was considered questionable especially as a slight
degree of vacuolation was noted in control as well as other treated
animals (Holsing and Voelker, 1970).
Long term studies
Rat
Groups of rats (35 males and 35 females per group; 70 males and
70 females were utilized for the control group) were fed
chlorothalonil in the diet for two years at levels of 0, 1500, 15,000,
and 30,000 ppm. Because of food refusal and poor weight gain at the
two highest dose levels, the compound was discontinued within one
week. The rats were fed basal diets for two weeks, after which
chlorothalonil administration was resumed and dietary levels increased
until the end of nine weeks when the intermediate group was fed 15,000
ppm. This intermediate group was then continued for the remainder of
the two year period. The high dose level was increased at biweekly
intervals until the sixteenth week when the group treatment was
terminated.
Growth suppression was observed at all levels and was
dose-related. This reduction in growth was reversible, as noted when
the high dose group recovered after being removed from the diet
containing chlorothalonil and fed a control diet. Haematological and
urine analysis values were within the normal range. PBI values were
generally reduced. Chlorothalonil had a cathartic action as evidenced
by increased water consumption and increased weight of faeces. Organ
weight and organ-to-body weight ratios increased for the liver and
kidney at the higher levels. Microscopic examination of the thyroid,
stomach, kidney, and liver revealed pathological changes.
A distinct alteration was produced in the squamous epithelium of
the cardiac portion of the stomach in most of the male and female
rats. The squamous epithelium was rather consistently thickened and
covered by a layer of keratin. In the kidneys of the higher dose
animals, the epithelium of the proximal convoluted tubules was paler
than usual and uniformly enlarged. At the termination of the study the
thyroid glands of the 15,000 ppm animals exhibited an increase in
epithelial pigmentation. Changes in the stomach at all levels included
acanthosis and hyperkeratosis.
At 13 weeks, the kidneys of both sexes fed 1500 ppm were similar
to those of the control. Gross and microscopic pathological
differences especially in males were, however, observed at the one and
two year intervals at this dose level. Liver changes, predominantly in
females, were characterized by an enlargement of the cells in the
nuclei, particularly in the central lobular area, and the formation of
large multinucleated cells in the pericentral area.
No increase in tumor formation was evident. Alterations in the
thyroid and the stomach appeared to be reversible; the alterations in
the liver were slight and confined to the females; the alterations in
the kidney were not reversible (Paynter, 1967c).
When samples of urine and faeces were taken for metabolism
studies (Skinner and Stallard, 1967) from rats from this long-term
study, data were reported for the volume of urine collected from the
rat over the period of sampling. The volume of urine was found to
decrease proportionately as the dietary dose increased. Conversely,
there appeared to be an increase in faecal excretion although the food
consumption remained constant.
Groups of rats (15 males and 15 females per group) were fed
chlorothalonil in the diet at levels of 0, 500, 1000, and 5000 ppm for
76 weeks. There were no apparent effects on behaviour and growth with
the exception of animals at the high level where food refusal was
noted. In a separate paired feeding study, there was no effect of
chlorothalonil on growth or behaviour. Increased liver weight, kidney
weight, and kidney to body weight ratio was apparent at the higher
test intervals. Microscopic examination indicated chiefly tubular
hypertrophy, epithelial irregularity and vacuolation. The degree of
kidney damage was dose-related and appeared to increase in severity in
males. No other adverse effects were noted in this study (Paynter and
Busey, 1967).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of 0 and 5000 ppm for two years.
Growth suppression in both males and females was evident throughout
the two year period. Survival was not affected and haematological,
biochemical and urine analysis values were within a normal range. A
cathartic effect of chlorothalonil was suggested as evidenced by
increased water consumption and increased weight of faecal excretion.
Organ weight and organ-to-body weight ratios, were increased for the
kidney and caecum. Histological examination of the kidneys at one year
again showed evidence of tubular hypertrophy and epithelial
alterations. No significant effects were noted with other tissues and
organs (Paynter and Crews, 1967).
Groups of rats (50 males and 50 females per group) were fed
chlorothalonil in the diet at levels of 0, 4, 10, 20, 30, 40 and 60
ppm for two years. No effects were seen on appearance, behaviour,
growth, food consumption or mortality. Haematological, clinical
chemistry and urine analysis values were normal. Animals sacrificed at
13, 52 and 104 weeks were examined for gross and microscopic defects.
A major lesion observed in studies at higher dose levels, necrosis of
the epithelial lining of the proximal tubules in the deep portion of
the cortex, was observed sporadically in this experiment at the one
and two year intervals. Vacuolation observed at 13 weeks predominantly
in females at 4 mg/kg and above, was not noted at later intervals.
There was no dose-related histological effect noted. A no-effect level
in this study would be 60 ppm (Holsing and Shott, 1970).
COMMENT
Chlorothalonil is rapidly excreted primarily unchanged. A
metabolite in plants and animals, the 4-hydroxy compound, is more
acutely toxic and more persistent than the parent molecule.
Studies performed at maternally toxic levels showed no effects of
chlorothalonil on reproduction. Growth retardation of pups was
believed to be the result of ingestion of treated diet. The results of
a reproduction study with the 4-hydroxy metabolite were negative at
low levels although secretion into the milk was observed. The results
of mutagenesis and teratogenesis tests were negative within the
parameters of the defined studies. Results of long and short term
chlorothalonil studies at high dietary levels to rats and dogs suggest
a toxicological problem associated with the kidney. Kidney changes
were characterized microscopically as hypertrophy, dilation,
cytoplasmic vacuolation, and hyperplasia of the epithelial cells of
proximal tubules and grossly as enlarged, greenish-brown granular
kidneys. No clinical effects were noted although some renal pathology
was suggested by a reduced urine volume. The significance of
histological changes in the kidney at lower dose levels needs
clarification.
No direct observations in man were reported.
Low-level feeding studies in rat and dog showed no effects in rat
at 60 ppm and in dog at 120 ppm forming the basis for allocating a
temporary ADI for man.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Rat: 60 ppm in the diet, equivalent to 3.0 mg/kg bw.
Dog: 120 ppm in the diet, equivalent to 3.0 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Chlorothalonil is a fungicide with broad spectrum activity. It is
effective against many fungus diseases which damage vegetables (rusts,
anthracnose, downy mildews, leaf spots, soft rot, leaf blight, scab,
early blight, late blight, pink rot, powdery mildew, etc.); agronomic
crops (downy mildew, leaf spots, leaf rust, brown spot, blue mold,
etc.), small fruits (anthracnose, gray mold powdery mildew, black rot,
downy mildew, ripe rot, leaf scorch, leaf blight, etc.); tree fruits
and nuts (scab, powdery mildew, black rot, white rot, fly speck, sooty
blotch, bitter rot, fire blight, blossom blight, leaf spot, melanose,
canker, greasy spot, leaf curl, etc.); tropical crops (signatoka,
black pod, coffee berry disease, leaf disease); turf and ornamental
crops. It has been tested on over 60 crops throughout the world and is
non-phytotoxic to most of these crop plants (Diamond Shamrock, 1974).
Literature available to the meeting further confirmed the fungicidal
effectiveness of chlorothalonil in both field and glasshouse tests
(DiDaris et al., 1965; Turner et al., 1964; Turner and Lamont, 1965).
Chlorothalonil is available as a wettable powder, dust and in
tablet form. The following formulations are produced.
Daconil 2787 W-75, a 75% wettable powder designed to be mixed
with water and applied as a spray. Registered for use in the
U.S.A. for turf and ornamentals and in 20 other countries for use
on a variety of crops (Diamond Shamrock, 1971).
Bravo W-75, a 75% wettable powder formulation registered for use
in the U.S.A. on agricultural crops.
Termil tollets - a tollet containing 8 g active ingredient
formulation to be vaporized by a suitable heat source at
temperatures of 315-425°C in a shallow pan.
Exotherm Termil - a powder containing 20% active ingredients
packaged in 100 g cans. Application is made by removing the lid
from the can and igniting the powder to generate smoke.
The recommended application rates for various crops using the
wettable powder formulation Daconil 2787 W-75 are as follows:
Application
Crop rate
Vegetable crops (asparagus, beans, 1000-3400 g/ha
broccoli, Brussels sprouts, cabbage,
cantaloupe, carrot, cauliflower, celery,
chinese cabbage, corn (sweet), pumpkin,
squash, tomato, turnip, watermelon)
Agronomic crops (hop, peanut, spearmint, 1000-2300 g/ha
sugar beet, tobacco)
Small fruits (blackberry, blueberry, 120-480 g/100 litres
currant, grape, raspberry, strawberry)
Tree fruits and nuts (apple, cherry, 120-360 g/100 litres
grapefruit, lemon, orange, peach,
pecan, pear, tangerine)
Tropical crops (banana, cacao, coffee, 240-480 g/100 litres
rubber)
Thermal dusting with Termil tollets or Exotherm Termil is used to
control a variety of diseases of ornamentals and vegetable crops
(asparagus, snap beans, cucumbers and tomatoes) grown in glasshouses,
at an application rate of 7-7.5 g a.i./100 cu.m. of space.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits and vegetables (Diamond Shamrock, 1974). A summary of much of
this information appears in Table 2 together with details of rates and
number of applications and pre-harvest intervals used. The majority of
these data are from trials in the U.S.A. with a few trials from
Canadian locations.
Chlorothalonil residues were detected on most aboveground crops
at the time of harvest. The higher residues occurred on leafy
vegetables, e.g. lettuce, escarole, chicory, spinach, celery and
crucifers including collards and kale, with generally lower levels on
melons, fruits and root crops. High residues were detected also in
lima bean plants, sugar beet tops and peanut hay which may be used for
animal feeds. The amount of fungicide applied, time interval between
last application and harvest, surface area, weight and surface
structure of the crop are factors that affect the level of the
residue. The residue level diminishes with time after application.
Glasshouse trials of thermal dusting were carried out on
cucumbers, leaf lettuce and tomatoes. The residue data are summarized
in Table 3. In general, the residue levels were low, <4 mg/kg,
following a pre-harvest interval of 1 day.
Only negligible residues of the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, DAC 3701 (see
"Biotransformation" and "Fate of Residues"), were detected in most
commodities. Only in the case of peaches were significant residues
(up to 0.5 mg/kg) detected routinely. Peanut hay and lima beans
(including pods) also sometimes showed residues of 0.5-1 mg/kg.
A series of experiments were performed to see if chlorothalonil
or the 4-hydroxy metabolite could be a residue in meat or milk from
feeding these products to cows. Groups of cows (4 cows/group) were fed
chlorothalonil plus the 4-hydroxy metabolite at dosage levels of 0, 25
ppm chlorothalonil plus 0.2 ppm metabolite, 75 ppm chlorothalonil plus
0.6 ppm metabolite, and 250 ppm chlorothalonil plus 2.0 ppm metabolite
for 30 days. Half the animals were sacrificed at the end of the test
and half after a 32 day recovery period. At a sensitivity of 0.02 ppm,
no chlorothalonil was found in milk (Wolfe and Stallard, 1969). A
small quantity of the metabolite was noted in milk: details are given
in Table 4. Residues in the milk reached maxima of 0.20, 0.26 and 0.78
respectively at the 3 feeding levels after about 18 days (Wolfe and
Stallard, 1970a). At the 30-day interval small residues of
chlorothalonil and the metabolite were seen in muscle, fat, liver and
kidney. Residues of the metabolite were respectively 0.51, 2.1, 0.93
and 3.7 mg/kg at the highest feeding level. No residues were seen
after the 32 days recovery period (Wolfe and Stallard, 1970b).
TABLE 2a. Residues of chlorothalonil from supervised trials.
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 12 13 1 9 0-0.7 0.15
(tops removed) 1.7 9 0-7 3 8-9 0.1-3.6 0.3-2.8
1.9 9 2 1 6 1-8-5.8 4.4
2.5 7-10 0-7 4 6-8 0-8.7 0.02-7.3
Broccoli 1.3 9 1-15 3 9 0.01-9.0 0.01-6.0
2.5 6-8 0-12 4 5-9 0-2.6 0-1.4
Brussels
sprouts 1.3 4-5 0-21 4 9-18 0.7-3.5 1.7-2.5
1.7 5 8 1 3 4.6-5.3 5.0
2.5 4-8 0-18 2 3,5 0.9-4.3 1.0-3.5
3.4 5 0-14 4 2-5 2.0-11 3.2-11
Cabbage 1.7 5-9 0 2 3,7 0.8-2.7 1.5-1.6
2.5 7-9 0-7 5 5-9 0-0.2 <0.01-0.4
Cauliflower 0.8 2 33 1 9 0.03-0.09 0.06
2.5 2-10 0-33 5 7-9 0-1.9 0.02-1.2
Cucumber 0.8 4-9 0-6 5 6-9 0-1.1 0.05-0.9
1.3 4-9 0-6 4 6-18 0-1.2 0.1-0.8
1.7 1-12 0-7 12 3-23 0-2.5 0.2-2.4
2.5 4-13 0-14 12 6-9 0-2.8 0.01-1.8
Summer squash 0.8 6-9 0-3 2 5,6 0.07-0.9 0-2-0.3
1.3 6-9 0-3 2 6 0.2-1.2 0.45-0.9
1.7 1-9 0-9 9 3-25 0-2.0 0-1-1.3
2.5 5 0-14 3 3 0.06-0.25 0.07-0.2
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Cantaloupe
(whole melons) 1.7 7-11 0-14 4 5-17 0-1.1 0.02-0.6
2.5 7 4 1 12 0.7-1.8 1.1
(pulp) 2.5 7 0 1 5 0.05-0.1 0.07
Muskmelon
(pulp) 1.3 11 0 1 3 0 0
(rind) 1.3 11 0 1 3 0.03-0.2 0.1
(pulp) 2.5 11 0 1 3 0 0
(rind) 2.5 11 0 1 3 1.4-1.7 1.5
Honeydew melon 1.7 10 0-14 3 5-8 0-1.6 0.03-0.7
2.5 7 0-14 3 6 0.07-0.4 0.1-0.25
Winter squash
(whole) 1.7 5-10 0 2 3 0.04-1.7 0.05-1.4
(pulp) 1.7 5-10 0 2 3,8 0 0
(whole) 2.5 7 7 1 3 0.8-2.3 1.4
Watermelon
(whole) 0.8 8-10 0-14 4 3 0-0.6 0.02-0.35
(rind) 1.3 7 14 1 3 0.2-0.4 0.3
(whole) 1.7 8-10 0-14 4 3 0.03-1.0 0.08-0.5
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(whole) 2.5 8-10 0-14 4 0.05-1.0 0.05-0.8
Pumpkin (whole) 1.7 10 0-7 3 3 0.8-1.8 0.8-1.4
(pulp) 1.7 10 0 1 2 0 0
Tomato 0.6 18 3 1 9 0-0.1 0.05
1.3 6-18 0-14 5 6-18 0.03-5.5 0.05-4.4
1.7 4-10 0-15 17 3-27 0.01-5.5 0.09-3.4
2.5 5-18 0-14 12 3-32 0.2-4.5 0.2-4.5
Peanuts (nuts) 0.8 6 78 1 3 0.03 0.3
1.3 4-12 4-78 11 4-24 0-0.3 0-0.09
(hulls) 1.3 4-12 4-55 9 3-12 0-0.8 0.09-0.5
(hay) 1.3 4-11 4-68 11 2-30 0.96 0.3-89
(nuts) 1.7 6 78 1 3 0.02-0.04 0.03
Potatoes
(whole) 0.8 10 12 2 9 0 0
(peeled) 0.8 13 15 1 6 0 0
(peelings) 0.8 13 15 1 3 0-0.01 0
Potatoes
(whole) 1.3 3-13 12-23 16 35-48 0-0.07 0-0.02
(peeled) 1.3 10-13 0-15 4 33 0-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(peelings) 1.3 13 15 1 4 0-0.05 0.01
(whole) 1.7 5-12 0-23 11 19-44 0-0.06 0-0.01
(peeled) 1.7 5-12 0-13 3 21 0 0
(peelings) 1.7 5-12 0-14 6 14 0-0.15 0.06
(whole) 2.5 10 14 1 9 0-0.01 0
Sugar beets
(roots) 1.3 4 61 1 10 0-0.02 0.01
1.7 4-6 14-41 9 9-27 0-0.5 0-0.3
(tops) 1.7 4-6 14-41 3 9 0-5-17 0.9-11
(roots) 2.5 3-6 14-59 5 5-12 0-1.2 0.02-0.5
(tops) 2.5 3-5 14-59 3 6-17 0.2-32 1.2-16
(roots) 3.4 5 23 1 6 0.03-0.09 0.05
Sweet corn
(kernels
and cob) 1.3 14 14 2 17 0 0
1.7 10 0 1 9 0-0.03 0
2.5 11 0-7 3 5-9 0.01-0.1 0.04
Snap beans 1.7 5-8 0-7 5 5-17 0.04-14 0.1-11
2.5 8 0-7 3 9 0.8-10 2-3-5.4
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Lima beans
(beans) 1.3 10 0 1 3 0-2-0.4 0.3
(plants) 1.3 10 0 1 9 220-535 400
(beans) 1.7 7 0-15 2 5-9 0-0.1 0-0.7
(pod + bean) 1.7 13 0 1 3 10-13 12
(plants) 1.7 4-13 0-15 3 2-9 22-310 47-117
Celery 0.8 5-24 0-14 6 8-9 0.2-21 0.4-18
1.3 9-24 0-14 5 6-9 0.1-52 0.4-29
1.4 8 7-14 2 6-8 0.06-1.7 0.1-1
1.7 9-24 0-14 7 6-9 0.1-53 0.7-34
1.8 8 7-14 2 6 0.1-5.4 0.3-3.4
1.9 26 7-14 2 6-8 0.2-17 0.6-12
2.5 5-14 0-14 5 8-9 0.6-17 1.4-10
3.4 10-18 1-14 4 6-9 2.8-26 4.5-18
Oranges (whole) 1.3 1-14 0-14 3 6 1.8-5.1 2.7-4.3
(peel) 1.3 1 173-397 5 20 0-0.7 0.1
Grapefruit
(whole) 0.4-0.8 1 309 2 6 0.01-0.02 0.01
1.3 1 200-309 3 11 0-0.05 0.02
(peel) 1.3 1-2 22-309 6 6-17 0-0.2 0.06-0.09
Limes (whole) 1.3 1 119-280 2 12 0-0.01 0
Lemons (whole) 1.3 1 280 1 4 0.01-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Tangerines
(whole) 1.3 1 280 1 6 0.01 0.01
Cherries 0.4 1-4 3-10 2 4-12 0-0.2 0.04-0.2
0.6 4 0-24 5 2-13 0.1-11 0.1-9.3
1.0 4 20 1 6 0.1-0.2 0.2
1.3 3-5 2-24 5 5-14 0.7-7.8 1.4-5.7
1.5 4-5 20-24 3 18 1-10 3.1-4.2
Peaches 0.6 4 8 1 3 6-8 6.7
0.8 4 8 1 6 3.3-6.3 5.0
1.0 7 0-14 3 3 6.4-19 8-15
1.3 4-11 6-14 3 3-12 2.8-20 4-16
1.5 6-7 0-25 11 3-18 1.3-45 4-31
1.7 4 8 1 3 27-28 28
2.5 4 8 1 3 32-54 45
Currants 1.0 4 3 1 6 16-20 18
1.3 4 3 1 6 16-20 18
1.5 4 3 1 6 20-23 23
Blackberries 1.3 2 4 1 12 4-11 7.3
1.7 2 23-48 2 12 0.5-2 1.2
1.9 2 4 1 12 3-16 9.6
2.5 2 0-16 3 9 0.3-8 2-4
3.0 2 0-16 4 7-12 0.5-43 1.6-21
3.4 2 0-16 3 9 1.3-9.3 2.5-4
Raspberries 0.4 2 4 1 6 0.05-1 0.3
0.8 2 4 1 6 0.2-2.4 1.2
1.3 2-3 0-8 3 6-12 0.4-6.5 0.7-4.1
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
1.5 2 0-8 2 6 0.6-2.7 0.7-1.7
1.7 3 0-7 3 3 5.6-20 5-7-15
Collards 2.5 3 0-14 4 9 3.3-88 5.7-69
Kale 2.5 3 0-14 4 6 1.5-71 2.8-62
Escarole 1.7 8 0-7 3 4-6 0.4-24 1.3-15
Endive 0.4 5 1 1 2 24-28 26
0.8 5 1 1 3 22-44 31
Chicory 1.7 8 0-7 3 3-5 0.04-30 0.3-24
Lettuce leaf 1.3 3 0 1 8 13-43 24
2.5 3-4 0-7 3 8-9 5-89 13-68
Lettuce head 1.7 4-7 0-14 5 2-12 0.1-100 1.3-86
Spinach 1.3 5 3-8 2 6 4-48 14-29
Turnip greens 1.7 2 2 1 7 5-10 7.2
Onions, green 1.7 3-4 0-7 3 6-8 0.6-11 1.1-7.6
2.5 3-4 0-7 3 6-7 1.3-24 2.0-18
Onions, mature 0.8-1.3 8-9 12-14 2 30 0-0.1 0-0.05
dry 1.7-2.5 5-9 7-9 2 105 0-0.3 0-0.1
Peppers 1.7 9-13 0-7 2 3-9 0.4-9.4 1.2-8.4
2.5 7 0-7 3 9 0.1-3.3 0.5-1.9
TABLE 2b. Residues of hydroxy-metabolite (DAC-3701) from supervised trials
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 8 0-7 2 3,5 0.01-0.06 0.01-0.04
(tops removed) 2.5 7 0 1 6 0.04 0.04
Broccoli 1.3 9 1-15 3 9 0 0
2.5 8 0 1 7 0-0.06 0.01
Brussels sprouts 2.5 8 0 1 5 0.01-0.02 0.02
Cabbage 2.5 9 0 1 6 0 0
Cucumber 0.8 7 0 1 6 0-0.03 < 0.01
1.7 7 0 1 6 0-0.03 < 0.01
2.5 6 0 1 3 0 0
Cantaloupe
(whole melons) 1.7 7 4 1 13 0.02-0.04 0.03
2.5 7 4 1 8 0.02-0.08 0.05
(pulp) 2.5 5 1 1 5 0.08-0.14 0.1
Watermelon
(rind) 1.3 7 14 1 3 0 0
Tomato 1.3 6 0 1 6 0-0.2 0.01
1.7 5 1 1 3 0.06-0.1 0.08
2.5 5-8 0-1 3 11 0.01-0.1 0.02-0.09
Peanuts (nuts) 1.3 4-12 4-55 6 10-20 0 0
(hulls) 1.3 4-12 6-55 5 3-9 0-0.05 0-0.02
TABLE 2b. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Peanuts
(hay) 1.3 4-11 4-55 6 9-33 0-0.6 0.03-0.4
Sugar beets
(roots) 2.5 3 25 2 9 0-0.01 0.01
(tops) 2.5 3 23 1 6 0 0
(roots) 3.4 5 23 1 6 0-0.01 0.01
Lima beans
(pod + bean) 1.7 4 3 1 3 0.8-1.1 1.0
Celery 0.8 20 0 1 4 0.05-0.1 0.05
1.4 8 7-14 2 2-5 0.05-0.2 0.09-0.15
Peaches 0.6 3 0-10 2 3 0.1-0.3 0.2
0.8 2-10 0-10 4 2-5 0-1-0.4 0.2-0.3
1.3 2-4 0-12 4 2-3 0.01-0.5 0.01-0.3
Lettuce head 1.3 4 1-7 2 4 0-0.04 0.01
1.7 4 21 1 6 0-0.04 0.02
Spinach 1.3 5 3 1 8 0.04-0.3 0.1
Turnip greens 1.7 2 2 1 5 0-0.02 0
Onions, mature 0.8-1.3 5-8 14 3-8 3-8 0.0.02 0-0.01
dry 1.7-2.5 8 - 3-5 3-5 0-0.2 0.1-0.2
TABLE 3. Chlorothalonil and DAC-3701 residues in glasshouse crops from supervised trials
Residues (ppm)
Days from No. of Range Means or
Crop and Rate No. of application No. of determinations individual range of
residue g/1000 ft3 applications to harvest trials per trial determinations) means/trial
Cucumbers
chlorothalonil 2 12-15 1 4 3 0.01-0.09 0.03-0.06
DAC-3701 2 12-15 1 4 3 0 0
Lettuce, leaf
chlorothalonil 2 5-12 1 7 3 0.05-3.6 0.05-3.0
Tomatoes
chlorothalonil 2 1-8 1 8 2-3 0.02-0.4 0.03-0.3
1.8 3-8 1 6 4 0.1-3.7 0.2-3.0
2 12-18 1 5 3 0.5-1.4 0.7-1.4
2 1-9 1 9 3 0-0.8 0-0.6
2 8-16 0.5 9 3 0.04-0.7 0.08-0.6
DAC-3701 2 1-8 1 8 2-3 0 0
TABLE 4. DAC-3701 residues (mg/kg) in cows milk during
feeding trial
Residue (mg/kg at 0.2*, 0.6** and 2.0*** ppm
DAC-3071 in diet
Days 0 0.2 0.6 2.0
0 < 0.03 < 0.03 < 0.03 < 0.03
2 < 0.03 < 0.03 < 0.03 0.06
4 0.04 0.04 0.06 0.16
8 0.04 0.08 0.10 0.51
14 < 0.03 0.13 0.16 0.57
18 < 0.03 0.20 0.26 0.74
22 < 0.03 0.11 0.17 0.78
26 < 0.03 0.11 0.13 0.59
30 < 0.03 0.16 0.26 0.74
37 < 0.03 0.06 0.12 0.55
44 < 0.03 0.04 0.16 0.16
51 < 0.03 < 0.03 < 0.03 0.06
60 < 0.03 < 0.03 < 0.03 < 0.03
* +25 ppm chlorothalonil
** +75 ppm chlorothalonil
*** +250 ppm chlorothalonil
Residues of chlorothalonil were not detected in the tissues of
dogs and rats fed levels in the diet of up to 30 000 mg/kg (Wolfe and
Stallard, 1968a) although small quantities of the hydroxy metabolite
were found in the liver and kidney.
No residues of chlorothalonil were detected in milk from a
Holstein cow fed the compound at the 5 ppm level in the ration for
four days (Gutenmann and Lisk, 1966). The method was sensitive to
about 0.03 ppm.
FATE OF RESIDUES
General comments
The disappearance and fate of chlorothalonil residues is
reasonably well documented. The only identified metabolite is the
4-hydroxy compound, DAC-3701, mentioned previously. Although
chlorothalonil residues decrease quite rapidly on plants, the actual
fate is unknown.
In animals
In the experiment by Gutenmann and Lisk (1966) mentioned above,
samples of milk, urine and faeces were collected throughout the
feeding period and for five days thereafter. No residues of
chlorothalonil, acid products of nitrile hydrolysis or conjugates of
acidic or phenolic derivatives were detected. In the same series of
experiments, chlorothalonil disappeared rapidly when incubated with
rumen juice at the 1 ppm level. Two unidentified metabolites were
formed. Feeding studies with dogs and rats showed that chlorothalonil
was rapidly eliminated in the faeces with negligible amounts in the
urine (Skinner and Stallard, 1967). The eliminated material was mostly
unchanged chlorothalonil. Analysis of tissue samples from rats and
dogs from chronic feeding studies of one and two years indicated no
detectable storage of chlorothalonil. A radio-tracer study with rats
confirmed that elimination of chlorothalonil was complete and there
was no significant tissue storage (Ryer and Sullivan, 1966; Skinner
and Stallard, 1967).
Dogs and rats were fed for 2 years on diets containing 1500,
15 000 and 30 000 mg/kg of chlorothalonil (Wolfe and Stallard, 1968a).
Residues were not detected in muscle tissue. Residues of
4-hydroxy-2,5,6-1,3-benzene-dicarbonitrile (DAC-3701) in kidney
tissues of dogs were <1.5 mg/kg and <3.5 mg/kg in the livers of
dogs and rats. Residue levels of the hydroxy metabolite were <0.25
mg/kg in the urine of the dogs and rats.
In the feeding experiment involving both chlorothalonil and
DAC-3701 (Wolfe and Stallard, 1969, 1970a, 1970b; see preceding
section and Table 4), the DAC-3701 residues in the milk accounted for
10-16% of the administered metabolite, but it was not possible to
determine whether part of the milk residue derived from the
chlorothalonil in the diet.
In plants
In studies with ring labelled 14C-chlorothalonil, Kunkel (1967a)
found no evidence of translocation from topical applications on
cucumber cotyledons, cucumber leaves, cucumber hypocotyls, bean
unifolilate leaves or tomato leaves. He also demonstrated that the
labelled compound was not translocated into the aerial parts of corn
or tomato plants when they were cultivated for 23 days in soil treated
with 14C-chlorothalonil. In another study, Kunkel (1967b) showed that
autoradiographic techniques did not demonstrate 14C-movement or
translocation within root systems of sweet corn, cucumber or tomato
grown in soil treated with ring-labelled chlorothalonil. Since
DAC-3701 is a soil metabolite of chlorothalonil and 14C activity was
not translocated from the soil, the experiments also showed that the
4-hydroxy metabolite is not translocated into the root system or
aerial parts of these plants. The residue data from supervised trials
(Table 2) showed that DAC-3701 is a residue following chlorothalonil
treatment. Of the crops tested for DAC-3701 residues, peaches
contained up to 0.5 mg/kg, spinach up to 0.3 mg/kg, onions up to 0.2
mg/kg, celery up to 0.2 mg/kg, cantaloupe up to 0.1 mg/kg and lima
bean plants up to 1 mg/kg. Negligible residues of DAC-3701 (<0.1 ppm)
were reported for carrots, tomatoes, peanuts (except peanut hay),
sugar beets, turnip greens, broccoli, Brussels sprouts, cabbage,
cucumber, watermelon and lettuce.
In soil
Tests conducted under both laboratory and field conditions
demonstrated that chlorothalonil is rapidly degraded in soil (Stallard
and Wolfe, 1967). In the laboratory experiments its half-life in the
types of soils tested ranged from 4 to over 40 days. The degradation
rate increased with increasing organic matter content, moisture
content and temperature but appeared to be independent of pH within
the range 6-8. Chlorothalonil was evidently not lost by
volatilization, since it disappeared rapidly from treated soil
incubated in tightly closed jars.
In the field trials, turf plots in 3 locations in the U.S.A. were
treated with chlorothalonil and the half-life of the chlorothalonil
residues ranged from 26-45 days. The data indicated a decreased
degradation rate during the winter months.
4-Hydroxy-2,5,6-trichloro-1,3-benzenecarbonitrile (DAC-3701) was
the major soil degradation product following treatment with 14C-ring
labelled chlorothalonil and incubation (Duane, 1970). This compound
accounted for over 80% of the radioactivity extracted from the soil.
(Approximately 20% of the radioactivity was not extracted.) A second
degradation product, more polar than DAC-3701 and without a hydroxyl
group, was detected but not identified. During these experiments it
was shown that 14C-chlorothalonil was not volatilized from the soil
and no volatile degradation products were formed.
Laboratory experiments with 5 representative soil types
demonstrated that the half-life values for DAC-3701 ranged from 36
days in a sandy loam type soil to 220 days in clay type soil (Wolfe
and Stallard, 1968b).
Duane (1970) demonstrated that bacteria isolated from soil were
capable of metabolizing chlorothalonil in culture media. Thus it may
be assumed that naturally occurring soil microorganisms are in part
responsible for the rapid loss of chlorothalonil under field
conditions.
In storage and processing
Some data are available on the effect of washing, field trimming
and peeling on the residue levels of chlorothalonil in some
commodities (Diamond Shamrock, 1974). Table 5 summarizes the data on
the effect of washing with water. Residues on carrots, cucumbers,
summer squash, tomatoes, currants and cranberries were reduced by
more than 50%. Washing was least effective with the leafy vegetables.
Significant reduction in chlorothalonil residues results from trimming
cabbage and head lettuce (Table 6). Chlorothalonil residue are
concentrated on the surface and as a result peeling removes
almost all the residues leaving the pulp of many fruits and
vegetables almost residue-free (Table 7). Following processing,
residues of chlorothalonil were not detected ( <0.1 mg/kg) in canned
spinach (Table 8). The fate of any DAC-3701 residues was not
determined.
TABLE 5. Effect of washing with water on residues of
chlorothalonil
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Carrots 4.39-7.34 1.05-3.53 52-76
Cucumbers 0.08-0.45 0.01-0.03 87-95
Squash, summer 0.21-1.33 0.01-0.12 76-97
Tomatoes 0.21-0.82 0.02-0.08 90
Celery 1.04-20.0 0.76-8.18 0-59
Peaches 7.6-15.0 4.9-8.6 35-45
Currants 18.3 2.6 86
Cranberries 0.91 0.25 73
TABLE 5. (Cont'd.)
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Collards 5.73-69.0 1.7-31.3 31-70
Kale 2.8-62.0 1.6-20.0 43-74
Escarole 1.32-4.1 0.63-2.6 36-44
Chicory 0.31-4.0 0.23-1.5 26-62
Lettuce 23.7-67.6 8.9-37.1 45-70
Cauliflower 1.23 0.85 30
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not available to the Meeting to indicate the level and
incidence of chlorothalonil residues in food moving in commerce or in
food at the time of consumption. Chlorothalonil residues were not
determined in the U.S.A. national food and feed monitoring program
(Duggan and Cook, 1971).
METHODS OF RESIDUE ANALYSIS
Residues of chlorothalonil and its metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile DAC-3701), can be
determined by gas-liquid chromotography (GLC) with either electron
capture or microcoulometric (halide) detectors (Wolfe and Stallard,
1970). The parent compound is chromatographed directly but the
metabolite is converted to the methyl ether by reacting with
diazomethane prior to chromatography.
Crop and soil samples are extracted with acidified acetone. The
acetone is removed by distillation and the residues are partitioned
into diethyl ether. The ether extract is cleaned-up on a Florisil
chromatographic column. Chlorothalonil is eluted with 5%
acetone-dichloromethane and DAC-3701 with 50% acetone-dichloromethane.
Following reaction with diazomethane, the methyl ether of DAC-3701 and
chlorothalonil are determined by GLC either by electron capture or
microcoulometric detection. The sensitivity of the method is
TABLE 6. Effect of trimming on residues of chlorothalonil in cabbage and lettuce
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop kg/ha applications to harvest determinations (mg/kg) (mg/kg)
Cabbage
(field trimmed) 1.7 9 0 7 0.77-2.67 1.60
1.7 5 0 3 1.25-1.72 1.47
(market trimmed) 2.5 7-8 0-7 7-9 0.00-0.18 <0.01-0.03
Lettuce
(whole untrimmed
heads) 1.7 4-7 0-7 2-12 11.5-100 33.7-85.5
1.7 4 14 11 0.10-3.0 1.33
(trimmed heads) 1.3 5 1-7 4 0.15-1.98 0.53-0.95
1.7 4 2-14 6 0.07-2.9 0.80-0.90
TABLE 7. Effect of peeling on chlorothalonil residues
Crop Residues (mg/kg)
Cucumbers Peelings - 1.26; Pulp - <0.01
Muskmelon Rind - 0.11-1.50; Pulp - 0.00
Winter squash Whole - 0.05-1.42; Pulp - 0.00
Pumpkin Whole - 1.42; Pulp - 0.00
Cantaloupe Whole - 1.09; Edible portion - 0.07
Peanut Hulls - 0.13-0.51; Meat - 0.00
Potatoes Peelings - 0.00-0.06; Pulp - 0.00
Lima beans Pod and beans - 11.9; Beans - 0.00
Grapefruit Peel - 0.09; Whole - 0.02
Oranges Whole - 2.70-4.29; Pulp - <0.01-0.08;
Juice - 0.00
TABLE 8. Effect of canning on chlorothalonil residues in spinach
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop % applications to harvest analysis (ppm) (ppm)
Spinach
(raw) control 0 - 6 <0.1 <0.1
0.11 5 3 6 16.1-47.8 29.1
0.11 5 8 6 3.7-30.9 13.6
(processed,
canned) control 0 - 3 <0.1 <0.1
0.11 4 16 3 <0.1 <0.1
0.02 mg/kg. Recovery values obtained from fortified crops were greater
than 72 and 76% for chlorothalonil and DAC-3701 respectively. Some
modifications to the method described above are required for animal
tissue (Wolfe and Stallard, 1970b) and milk samples (Wolfe and
Stallard, 1969, 1970c). Average, recoveries for chlorothalonil and
DAC-3701 respectively were:
muscle - 81, 91%
fat - 76, 91%
kidney - 82, 84%
liver - 93 83%
milk - 88, 83%
The sensitivity of the method for milk is 0.02 mg/kg for
chlorothalonil and 0.03 mg/kg for DAC 3701. Chlorothalonil is not
recovered by the Mills Florisil multi-residue procedure but is
recovered (McMahon et al., 1973) in the alternative Florisil elution
system (Mills et al., 1972). Recovery from deactivated Florisil
(Osadchuk et al., 1971) is achieved by elution with 10% ethyl acetate
in hexane (McLeod and Ritcey, 1973).
Chlorothalonil can be recovered through the carbon-cellulose
cleanup procedure of McLeod et al. (1967). Gutenmann and Lisk (1966)
determined chlorothalonil by electron capture GLC in milk, urine and
faeces after extracting with acetone-phosphoric acid and partitioning
into hexane. Possible acid metabolites were determined after
diazomethane esterification of the evaporated acetone extract.
With the exception of the Mills Florisil multiresidue procedure,
the multiresidue procedures discussed above appear to be suitable for
regulatory determination of the parent compound. When the total
residue, chlorothalonil and DAC-3701, is required the GLC method
proposed by Wolfe and Stallard (1970c) can be recommended for
regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Some examples of national tolerances were reported to the Meeting
and are listed in Table 9.
TABLE 9. Examples of national tolerances as reported to the Meeting
Tolerance
Count Commodity (mg/kg)
Australia Beets, carrots, corn, cucumbers,
onions, peppers, potatoes,
tomatoes 7
Peanuts 0.2
TABLE 9. (cont'd)
Tolerance
Count Commodity (mg/kg)
Canada Celery 15
Broccoli, Brussels sprouts,
cabbage, cauliflower, cucumbers,
melons, pumpkins, snap beans,
squash, tomatoes 5
Carrots 1
Peanuts 0.3
Netherlands Potatoes 0.05
Apples, apricots, beets, carrots,
citrus, corn, cucumbers, grapes,
melons, onions, peaches, pears,
peppers, plums, tomatoes 0.01
Switzerland Potatoes 0.05
U.S.A. Celery 15*
Broccoli, Brussels sprouts, cabbage,
cauliflower, cucumbers, melons,
pumpkins, snap beans, squash
(summer and winter), tomatoes 5*
Carrots, sweet corn (kernels plus
cob with husks removed) 1*
Peanuts 0.3*
Potatoes 0.1*
* Including metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzen-edicarbonitrile.
APPRAISAL
Chlorothalonil is a broad-spectrum fungicide with effective
action against many fungus diseases which damage vegetable, tree,
small fruit and other agricultural crops, turf and ornamentals. The
use pattern of chlorothalonil is such that residues remain on most
above-ground crops at the time of harvest. It is desirable to have
some residue of the fungicide on the mature crop to protect it from
disease organisms during shipment.
4-Hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile is a major
metabolite of chlorothalonil in soil and a metabolite in plants, but
only negligible residues were found on most crops investigated.
Chlorothalonil does not translocate into plants from the soil or from
topical application.
Extensive crop residue data indicated that chlorothalonil is a
relatively persistent fungicide. The level of the residue depends on
such factors as rate of the fungicide applied, time interval between
last application and harvest and the surface area, weight and surface
structure of the crop. The residue level diminishes with time after
application, and pre-harvest intervals are therefore recommended for
some crops. Some data are available on the effects of washing,
trimming and peeling on chlorothalonil residues.
Chlorothalonil residues do not occur in the tissues or milk of
cows, but residues of the 4-hydroxy metabolite are found when cows are
fed chlorothalonil and the 4-hydroxy compound together in their
ration. The residue levels depend on the levels fed and reach a steady
value in the milk after 18 days. Residues in milk declined to below
detection level within 21 days after withdrawal. It was not determined
whether residues of the 4-hydroxy metabolite would occur in the milk
and tissues of cows if only chlorothalonil were ingested.
Since no data were provided on residues in crops that may be fed
to animals, it was not possible to recommend maximum residue limits
for milk and meat.
Most available multi-residue GLC methods appear to be suitable
for the determination of the parent compound, but chlorothalonil is
not recovered by the original Mills Florisil multi-residue cleanup
procedure (McMahon et al., 1973). The GLC method proposed by Wolfe
and Stallard (1970) for chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile appears to be
suitable for regulatory purposes when the total residue is required.
National tolerances are in effect in a number of countries.
RECOMMENDATIONS
The following maximum residue limits are recommended for
chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, expressed as
chlorothalonil.
TEMPORARY TOLERANCES
Pre-harvest
interval on which
Limit recommendations
Commodity (mg/kg) are based (days)
Peaches 30 7
Currants (black, red and white) 25 3
Celery 15 7
Peppers 10 1
Blackberries, raspberries, 10 7
cherries, chicory sprouts
Collards, kale, endive, 10 14
lettuce (head)
Broccoli, Brussels sprouts, 5 7
cabbage, cauliflower, beans
(green including pod), oranges,
onions, cranberries
Cucumbers, melons, pumpkins, 5 1
squash, tomatoes
Carrots, sweet corn, sugar 1 1
beets
Lima beans, peanuts (whole) 0.5 0
Peanuts (kernel), potatoes 0.1 0
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Additional study to resolve lower limit of kidney effects in rat.
2. Define growth reduction in pups relative to dietary ingestion or
secretion into milk.
3. Data on residues of chlorothalonil and the 4-hydroxy metabolite
in crops that may be fed to animals.
4. The results of feeding studies on dairy cattle understood to be
in progress to determine the level and nature of residues in milk
and tissues.
DESIRABLE
1. Observations in man.
2. Residue data for food moving in commerce.
3. Further information on effects of processing, including household
cooking, on residues.
REFERENCES
Beasley, A. and Leong, K. (1965). Acute inhalation exposure - rats.
Report from Hazelton Laboratories Inc., submitted by The Diamond
Shamrock Chemical Co. (Unpublished)
Blackmore, R. and Shott, L. (1968). Final report four month feeding
study - rats. Report from Hazelton Laboratories, Inc. (Unpublished)
Diamond Shamrock (1971). Documentation on the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Diamond Shamrock (1974). Documentation concerning the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Di Dario, A., Curry, T.L., Thayer, P. and Turner, J.J. (1965). The
biological performance of tetrachloroisophthalonitrile as influenced
by particle size and crystalline form. Phytopathology, 55:1055.
Doyle, R. and Elsea, J. (1963). Acute oral, dermal and eye toxicity
and irritation studies on DAC-2787. Report from Hill Top Research
Institute Inc., submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Duane, W.C. (1970). Biodegradation of Daconil 2787(R). Report
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Duggan, R.E. and Cook, H.R. (1971). National food and feed monitoring
Program. Pestic. Monit. J., 5:37-43.
Gutenmann, W.H. and Lisk, D.J. (1966). Metabolism of Daconil and
Dacthal pesticides in lactating cows. J. Dairy Sci., 49:1272-1276.
Hastings, T.F. and Jessup, D.C. (1974). 3-Generation reproduction
study in albino rats using DAC-3701 in the diet. Report from BIO/TOX
Research Laboratories, Inc. Submitted to WHO by Diamond Shamrock
Chemical Co. (Unpublished)
Holliday, W., Schadeberg, K., Goode, J. and Keplinger, M. (1973).
Twenty-one day subacute aerosol inhalation toxicity study with Bravo
6F(R) in albino rats. Report from Industrial Bio Test Laboratories,
Inc. (Unpublished)
Holsing, G. and Shott, L. (1970). Two year dietary administration
- rats. Report from Hazelton Laboratories Inc. submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Holsing, G. and Voelker, R. (1970). Final report 104 week dietary
administration - dogs. Report from Hazelton Laboratories Inc.
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967a). Absence of 14C movement in crop plant organs
after topical application and soil amendment studies with isotopic
Daconil 2787. Report to Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967b). Movement of 14C in or on roots of crop species
grown in soil amended with isotopic Daconil 2787. Report to Diamond
Shamrock Chemical Co. (Unpublished)
Legator, M. (1974). Mutagenic Testing with DAC 2787. Report from
Division of Genetics, Roger Williams General Hospital and Brown
University, Division of Biological and Medical Sciences submitted by
Diamond Shamrock Chemical Co. (Unpublished
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967).
Comparison of various carbon adsorbents and quantitative elution and
separation of forty-two pesticides from a carbon-Solka Floc cleanup
column. J. Ass. off. analyt. Chem., 50:1216-1228.
McLeod, H.A. and Ritcey, W.R. (1973). Analytical methods for pesticide
residues in foods. Information Canada, Ottawa, Canada.
McMahon, B.M., Sawyer, L.D. and Corneliussen, P.E. (editors) (1973).
Pesticide analytical manual. Volume 1. Methods which detect multiple
residues. Food and Drug Administration, Washington, D.C.
Mills, P.A., Bong, B.A., Kamps, L.R. and Burke, J.A. (1972). Elution
solvent system for Florisil column cleanup in organochlorine pesticide
residue analyses. J. Ass. off. analyt. Chem., 55:39-43.
Osadchuk, M., Romach, M. and McCully, K.A. (1971). Cleanup and
separation procedures for multi-pesticide residue analysis in
monitoring and regulatory laboratories. Proc. Second Intern. IUPAC
Congress Pestic. Chem. Vol. IV, Methods in Residue Analysis, p.
357-383. A.S. Tahori (editor), Gordon and Breach Science Publishers,
New York.
Paynter, O. (1965a). Oral dose range - dogs - final report. Report of
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1965b). Repeated dermal application - rabbits. Report
from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1966). Reproduction - rabbit. Report from Hazelton
Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1967a). Three generation reproduction study - rats.
Report from Hazelton Laboratories Inc. submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1967b). Ten week amino acid feeding study - rats. Report
from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. (1967c). Final report - two year dietary feeding - rats.
Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Busey, W. (1967). Long term (76 weeks) feeding study,
rats, DAC 2787. Final Report from Hazelton Laboratories Inc.
(Unpublished)
Paynter, O. and Crews, L. (1967). Final report - two year dietary
feeding - rats. Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Murphy, J. (1967). Sixteen week dietary feeding -
dogs. Report from Hazelton Laboratories Inc., submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Powers, M. (1965). Acute oral administration - rats. Report from
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Ryer, F.H. and Sullivan, J.B. (1966). Radiotracer metabolism study.
Report from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Skinner, W.A. and Stallard, D.E. (1967). Daconil 2787(R) animal
metabolism studies. Report from and submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Stallard, D.E. and Wolfe, A.L. (1967). The fate of
2,4,5,6-tetrachloroisophthalonitrile (Daconil 2787(R)) in soil.
Report from Diamond Shamrock Chemical Co. (Unpublished)
Sterner, W. and Loveless, L. (1963). A study of the subacute toxicity
of tetrachloroisophthalonitrile to rats. Report from International
Bio-Research, Inc. (Unpublished)
Teeters, W. (1966). In vivo assay of spasmodic activity, mice, DAC
2787. Final report from Hazelton Laboratories Inc. (Unpublished)
Turner, N.J. and Lamont, D. (1965). Control of fungal diseases in the
greenhouse with thermally induced dusts of
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
23:51-54.
Turner, N.J., Limpel, L.E., Battershell, R.D., Bluestone, H., Lamont,
D. (1964). A new foliage protectant fungicide,
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
22:303-310.
Wazeter, F. (1971). Acute oral LD50 in male albino rats. Report from
International Research and Development Corporation. (Unpublished)
Wazeter, F. and Goldenthal, E. (1972). Acute oral toxicity in beagle
dogs. Report from International Research and Development Corporation.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968a). Analysis of tissues and organs
for storage of the Daconil metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968b). The fate of DAC-3701
(4-hydroxy-2,5,6-trichloroisophthalonitrile) in soil. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1969). Residues in milk from cows fed
2,4,5,6-tetra-chloroisophthalonitrile. Diamond Shamrock Chemical Co.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970a). Residues in milk from cows fed
2,5,6-trichloro-4-hydroxyisophthalonitrile. Diamond Shamrock Chemical
Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970b). Residues in tissues of dairy
cows fed Daconil 2787 and 2,5,6-trichloro-4-hydroxyisophthalonitrile.
Diamond Shamrock Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970c). Analytical method for
determination of Daconil 2787 and DAC-3701 residues. Diamond Shamrock
Chemical Co. (Unpublished)
Residues and biotransformations of chlorothalonil in farm animals,
plants and soil are discussed in the sections "Residues resulting from
supervised trials" and "Fate of residues."
Pharmacological effects
Chlorothalonil has a cathartic effect which is apparently due to
irritation of the gastro-intestinal tract. This was evidenced by a
dose related decrease in urinary volume and increase in urine specific
gravity. Studies using rats subjected to levels of 0, 1500, and 15,000
ppm in the diet showed a dose related decrease in retention of an
orally administered dye indicative of a potential laxative effect.
Some experiments utilizing selected animals on feeding studies
suggested an increase in water intake, a decrease in urine volume, and
an increase in fecal moisture which was dose related especially at
high concentrations in the diet (Paynter, 1967c; Skinner and Stallard,
1967).
Chlorothalonil administered orally at one gm/kg to mice increased
intestinal mobility as measured by the percentage of the small
intestine traversed by a charcoal marker within a selected time
interval. The laxative action of chlorothalonil was significantly
reduced by pre-treatment with corn oil but not with atropine or
papaverine (Teeters, 1966).
Residue analyses of tissues and organs of animals fed
chlorothalonil in the diet indicated that there was no accumulation in
any body organ. Small quantities of the 4-hydroxy metabolite were
observed in liver and kidney with no parent compound noted (Wolfe and
Stallard, 1968a).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse
The potential genetic hazard of chlorothalonil was evaluated in
mice by the host mediated assay, in vivo cytogenic testing and the
dominant lethal test. The compound did not produce any measurable
mutagenic response when evaluated in vitro against eight indicator
organisms of the Salmonella typhimurium, histidine auxotroph tester
strains which can be reverted by both base substitution and frame
shift mutagens. In these tester strains, both repair deficient and
repair competent strains were used. When the tester strains were
inoculated into animals treated with chlorothalonil daily for five
days at an oral dose of 6.5 mg/kg (10 animals per treatment), no
increase in the number of mutations over the control were found. The
positive control, dimethylnitrosamine, increased mutation frequency in
mice (ip, 4 mg/kg).
Following oral administration of chlorothalonil daily for five
days at 6.5 mg/kg, an examination of bone marrow for the presence of
cells with micronuclei indicative of cytogenic abnormalities was
negative.
Treated orally for five days at 6.5 mg/kg, two male mice were
mated sequentially with untreated females in a standard dominant
lethal test. No adverse effect on fertility, implantation or foetal
mortality was observed. A positive control (triethylene melamine),
administered ip at 0.5 mg/kg, produced an increase in early death at
the post meiotic period. At the concentration tested in the three
procedures used, chlorothalonil did not produce mutagenic effects
(Legator, 1974).
Special studies on reproduction
Rat
Groups of rats (10 males and 20 females per group) were fed
chlorothalonil in the diet at levels of 0, 0.15, 1.5, and 3.0% in a
standard three generation, two litters per generation, reproduction
study. Because of food refusal and poor weight gain, the test using
the two higher levels was interrupted. After the first litter of pups
(F1a), the high level of treatment which had been reduced to 2% was
discontinued and a new dose level of 0.5% (with another control group)
was substituted. The full three generation reproduction test was
ultimately performed using dosage levels of 0, 0.15, 0.5 and 1.5% in
the diet. At the high discontinued dose level there was a significant
growth depression in nursing litters and an emaciated appearance in
the pups at weaning. There was also a reduction of fertility and
lactation at this maternally toxic level.
Growth suppression in pups was noted at all test intervals
through all three generations. It was considered that while smaller,
pups were still within a normal weight range, no malformations were
observed and reproduction indices were not affected. Necropsy
examination performed on parents and on the terminal F3b generation
revealed gross changes in the kidneys and in the G.I. tract. Gross
changes included enlargement and distention of the caecum and colon,
soft faecal matter, and occasional thickening of the stomach wall.
Microscopic changes were also evident in stomach and kidney.
Chlorothalonil examined at maternally toxic dietary levels did not
have an effect on reproduction in the rat (Paynter, 1967a).
Groups of rats (10 males and 20 females per group) were fed the
chlorothalonil metabolite, 4-hydroxy 2,5,6-trichloroisophthalonitrile,
in the diet at levels of 0, 10, 50, 100 and 200 ppm for 70 days prior
to mating in 3 generation, one litter per generation, reproduction
study. Growth was reduced, clinical chemistry changes (increased SGPT)
were noted and gross and microscopic changes observed at 200 mg/kg.
Reproduction was affected at 100 mg/kg as evidenced by reduced litter
size and weight as well as decreased survival. Milk analysis revealed
the 4-hydroxy metabolite in the stomach curd of 7 day pups in a
concentration comparable to that fed to the parents. No effects were
noted at 50 mg/kg on the reproduction parameters recorded (Hastings
and Jessup, 1974).
Special studies on teratogenicity
Rabbit
Groups of 8 pregnant rabbits were administered chlorothalonil
from day 8 to day 16 of gestation. The initial dose on days eight and
nine (0, 180, and 375 ml/kg/day) was reduced because of decreased food
consumption to 0, 62.5, and 31.25 mg/kg/day respectively for the
remainder of the study. Deaths were noted in the treated groups and
there was a severe weight loss with the treated dose. There was no
apparent effect on the embryo and while there was a considerable
effect on the adults, chlorothalonil is not considered to be a
teratogen (Paynter, 1966).
Acute toxicity
TABLE 1. Acute toxicity of chlorothalonil
Species Route LD50 mg/kg References
Rat Oral 10,000 Powers, 1965
Doyle and Elsea, 1963
Inhalation 4.7 mg/kg Beasley and Leong, 1965
Dog Oral > 5,000 Paynter, 1965a
Rabbit Dermal 10,000 Doyle and Elsea, 1963
Signs of poisoning include depression, diarrhoea and an unkempt
appearance. 3 mg crystalline technical product applied to the
conjunctival sac produced a mild irritation which was probably
mechanical (Doyle and Elsea, 1963).
Acute studies of the 4-hydroxy metabolite showed an oral LD50 in
male rats of 332 mg/kg. An LD50 could not be found in dogs because of
emesis at all dose levels (Wazeter, 1971; Wazeter and Goldenthal,
1972).
Short term studies
Rat (inhalation)
Groups of rats (15 males and 15 females per group) were exposed
to chlorothalonil by inhalation 6 hours/day, 5 days/week for three
weeks. This exposure was at a mean concentration of 12.2 mg/l at a
respirable particle size range of 1-5 microns (91%), 6-25 microns
(9%), and 26-40 microns (<1%). There were no deaths or untoward
behaviour among any of the animals. Growth was normal, and gross and
histological examinations did not reveal any compound-related effects
(Holliday et al., 1973).
Rat (feeding)
Groups of rats (10 males and 10 females per group) were fed diets
containing 5000 ppm chlorothalonil for 4 weeks. In an attempt to
determine the effect of chlorothalonil on absorption and utilization
of protein, fat, and amino acids, 10 amino acids varying in
concentration from 0.2 to 0.9% were added to the diet. On the basis of
a ten week feeding of dietary levels containing chlorothalonil plus
casein and corn oil or chlorothalonil plus amino acids, it was
concluded that chlorothalonil did not interfere directly with the
absorption and utilization of protein, fat, or amino acids. The
reduction in weight gain predominantly noted at this high feeding
level of chlorothalonil was presumably due to catharsis and not to
absorption difficulties (Paynter, 1967b).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of O, 250, 500, 750 and 1500 ppm
for 22 weeks. No mortality was observed and appearance and behaviour
were normal. Growth was slightly reduced at all feeding levels in
males and at 750 and 1500 ppm in the females. Food consumption was
comparable in all groups.
Haematological and urine analysis values were within normal
limits. An increase in liver-kidney weight was noted especially in
males at the two higher dosage levels. Kidney alterations,
characterized by irregular swelling of the tubular epithelium,
epithelial degeneration, and tubular dilation were seen in all test
groups with the males affected to a greater degree than females
(Blackmore and Shott, 1968).
Groups of rats (10 males and 10 females per group) were
administered chlorothalonil by oral gavage at dosage levels of 0, 0.5,
1.0, 2.0, 4.0 and 8.0 gm/kg five days a week for 13 weeks.
Administration of 4.0 gm/kg resulted in a slight, non-significant
reduction of growth, poor general condition, and reduced white blood
count. No significant findings were observed with regard to
elucidating the potential renal toxicity problem observed in longer
studies. Studies performed in this test indicated that chlorothalonil
did not result in a specific abnormality. It was suggested there might
be a decrease in resistance and defence mechanisms, making rats more
susceptible to naturally occurring infections (Sterner and Loveless,
1963).
Rabbit (dermal)
Groups of albino rabbits were daily administered a 75%
formulation of chlorothalonil to either intact or abraded skin, 5 days
per week, for three weeks. (Groups of two males and two females served
as controls; 5 males and 5 females were treated groups.) Animals were
administered chlorothalonil at dose levels of 0, 500, or 1000 mg/kg.
Repeated application of chlorothalonil to the intact or abraded
skin of rabbits resulted in dose-related dermal irritation consisting
of erythema, atonia, and desquamation. The degree of irritation was
more severe to the abraded skin. A number of animals at the high level
showed atypical haematological values in conjunction with diarrhoea
and/or dermal irritation as a result of application of chlorothalonil.
As might be expected, histological examination of the skin revealed
the presence of a moderate degree of acanthosis particularly in the
abraded skin areas, hyperkeratosis, rarely focal parakeratosis, and
slight to moderate leucocytic infiltration. No pathological
abnormalities were noted in other tissues (Paynter, 1965b).
Dog
Groups of dogs (4 males and 4 females per group) were fed
chlorothalonil in the diet at 0, 250, 500 and 750 ppm for sixteen
weeks. There was no mortality and no apparent effect on behaviour or
growth in any of the dogs tested. Haematological, clinical chemistry
and urine analysis values were normal with the exception of slightly
raised PBI values at high dose levels in females. Gross and
microscopic examination of organs and tissues did not reveal any
compound-related abnormalities (Paynter and Murphy, 1967).
Groups of beagle dogs (8 males and 8 females per group) were fed
chlorothalonil in the diet for two years at dosage levels of 0, 60 and
120 ppm. There were no effects noted on behaviour and growth over the
course of the study. Clinical chemistry values including haematology,
biochemistry and urine analysis were comparable to the controls at all
levels of feeding. Gross and microscopic examination of tissues and
organs performed on animals sacrificed at 12 months indicated a
compound-related change in the kidney. Further examination of tissues
and organs at 24 months did not show chlorothalonil-related
abnormalities. A slight degree renal tubule vacuolation in two of four
animals at 120 ppm after two years in the absence of other changes
(urinalyses values) was considered questionable especially as a slight
degree of vacuolation was noted in control as well as other treated
animals (Holsing and Voelker, 1970).
Long term studies
Rat
Groups of rats (35 males and 35 females per group; 70 males and
70 females were utilized for the control group) were fed
chlorothalonil in the diet for two years at levels of 0, 1500, 15,000,
and 30,000 ppm. Because of food refusal and poor weight gain at the
two highest dose levels, the compound was discontinued within one
week. The rats were fed basal diets for two weeks, after which
chlorothalonil administration was resumed and dietary levels increased
until the end of nine weeks when the intermediate group was fed 15,000
ppm. This intermediate group was then continued for the remainder of
the two year period. The high dose level was increased at biweekly
intervals until the sixteenth week when the group treatment was
terminated.
Growth suppression was observed at all levels and was
dose-related. This reduction in growth was reversible, as noted when
the high dose group recovered after being removed from the diet
containing chlorothalonil and fed a control diet. Haematological and
urine analysis values were within the normal range. PBI values were
generally reduced. Chlorothalonil had a cathartic action as evidenced
by increased water consumption and increased weight of faeces. Organ
weight and organ-to-body weight ratios increased for the liver and
kidney at the higher levels. Microscopic examination of the thyroid,
stomach, kidney, and liver revealed pathological changes.
A distinct alteration was produced in the squamous epithelium of
the cardiac portion of the stomach in most of the male and female
rats. The squamous epithelium was rather consistently thickened and
covered by a layer of keratin. In the kidneys of the higher dose
animals, the epithelium of the proximal convoluted tubules was paler
than usual and uniformly enlarged. At the termination of the study the
thyroid glands of the 15,000 ppm animals exhibited an increase in
epithelial pigmentation. Changes in the stomach at all levels included
acanthosis and hyperkeratosis.
At 13 weeks, the kidneys of both sexes fed 1500 ppm were similar
to those of the control. Gross and microscopic pathological
differences especially in males were, however, observed at the one and
two year intervals at this dose level. Liver changes, predominantly in
females, were characterized by an enlargement of the cells in the
nuclei, particularly in the central lobular area, and the formation of
large multinucleated cells in the pericentral area.
No increase in tumor formation was evident. Alterations in the
thyroid and the stomach appeared to be reversible; the alterations in
the liver were slight and confined to the females; the alterations in
the kidney were not reversible (Paynter, 1967c).
When samples of urine and faeces were taken for metabolism
studies (Skinner and Stallard, 1967) from rats from this long-term
study, data were reported for the volume of urine collected from the
rat over the period of sampling. The volume of urine was found to
decrease proportionately as the dietary dose increased. Conversely,
there appeared to be an increase in faecal excretion although the food
consumption remained constant.
Groups of rats (15 males and 15 females per group) were fed
chlorothalonil in the diet at levels of 0, 500, 1000, and 5000 ppm for
76 weeks. There were no apparent effects on behaviour and growth with
the exception of animals at the high level where food refusal was
noted. In a separate paired feeding study, there was no effect of
chlorothalonil on growth or behaviour. Increased liver weight, kidney
weight, and kidney to body weight ratio was apparent at the higher
test intervals. Microscopic examination indicated chiefly tubular
hypertrophy, epithelial irregularity and vacuolation. The degree of
kidney damage was dose-related and appeared to increase in severity in
males. No other adverse effects were noted in this study (Paynter and
Busey, 1967).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of 0 and 5000 ppm for two years.
Growth suppression in both males and females was evident throughout
the two year period. Survival was not affected and haematological,
biochemical and urine analysis values were within a normal range. A
cathartic effect of chlorothalonil was suggested as evidenced by
increased water consumption and increased weight of faecal excretion.
Organ weight and organ-to-body weight ratios, were increased for the
kidney and caecum. Histological examination of the kidneys at one year
again showed evidence of tubular hypertrophy and epithelial
alterations. No significant effects were noted with other tissues and
organs (Paynter and Crews, 1967).
Groups of rats (50 males and 50 females per group) were fed
chlorothalonil in the diet at levels of 0, 4, 10, 20, 30, 40 and 60
ppm for two years. No effects were seen on appearance, behaviour,
growth, food consumption or mortality. Haematological, clinical
chemistry and urine analysis values were normal. Animals sacrificed at
13, 52 and 104 weeks were examined for gross and microscopic defects.
A major lesion observed in studies at higher dose levels, necrosis of
the epithelial lining of the proximal tubules in the deep portion of
the cortex, was observed sporadically in this experiment at the one
and two year intervals. Vacuolation observed at 13 weeks predominantly
in females at 4 mg/kg and above, was not noted at later intervals.
There was no dose-related histological effect noted. A no-effect level
in this study would be 60 ppm (Holsing and Shott, 1970).
COMMENT
Chlorothalonil is rapidly excreted primarily unchanged. A
metabolite in plants and animals, the 4-hydroxy compound, is more
acutely toxic and more persistent than the parent molecule.
Studies performed at maternally toxic levels showed no effects of
chlorothalonil on reproduction. Growth retardation of pups was
believed to be the result of ingestion of treated diet. The results of
a reproduction study with the 4-hydroxy metabolite were negative at
low levels although secretion into the milk was observed. The results
of mutagenesis and teratogenesis tests were negative within the
parameters of the defined studies. Results of long and short term
chlorothalonil studies at high dietary levels to rats and dogs suggest
a toxicological problem associated with the kidney. Kidney changes
were characterized microscopically as hypertrophy, dilation,
cytoplasmic vacuolation, and hyperplasia of the epithelial cells of
proximal tubules and grossly as enlarged, greenish-brown granular
kidneys. No clinical effects were noted although some renal pathology
was suggested by a reduced urine volume. The significance of
histological changes in the kidney at lower dose levels needs
clarification.
No direct observations in man were reported.
Low-level feeding studies in rat and dog showed no effects in rat
at 60 ppm and in dog at 120 ppm forming the basis for allocating a
temporary ADI for man.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Rat: 60 ppm in the diet, equivalent to 3.0 mg/kg bw.
Dog: 120 ppm in the diet, equivalent to 3.0 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Chlorothalonil is a fungicide with broad spectrum activity. It is
effective against many fungus diseases which damage vegetables (rusts,
anthracnose, downy mildews, leaf spots, soft rot, leaf blight, scab,
early blight, late blight, pink rot, powdery mildew, etc.); agronomic
crops (downy mildew, leaf spots, leaf rust, brown spot, blue mold,
etc.), small fruits (anthracnose, gray mold powdery mildew, black rot,
downy mildew, ripe rot, leaf scorch, leaf blight, etc.); tree fruits
and nuts (scab, powdery mildew, black rot, white rot, fly speck, sooty
blotch, bitter rot, fire blight, blossom blight, leaf spot, melanose,
canker, greasy spot, leaf curl, etc.); tropical crops (signatoka,
black pod, coffee berry disease, leaf disease); turf and ornamental
crops. It has been tested on over 60 crops throughout the world and is
non-phytotoxic to most of these crop plants (Diamond Shamrock, 1974).
Literature available to the meeting further confirmed the fungicidal
effectiveness of chlorothalonil in both field and glasshouse tests
(DiDaris et al., 1965; Turner et al., 1964; Turner and Lamont, 1965).
Chlorothalonil is available as a wettable powder, dust and in
tablet form. The following formulations are produced.
Daconil 2787 W-75, a 75% wettable powder designed to be mixed
with water and applied as a spray. Registered for use in the
U.S.A. for turf and ornamentals and in 20 other countries for use
on a variety of crops (Diamond Shamrock, 1971).
Bravo W-75, a 75% wettable powder formulation registered for use
in the U.S.A. on agricultural crops.
Termil tollets - a tollet containing 8 g active ingredient
formulation to be vaporized by a suitable heat source at
temperatures of 315-425°C in a shallow pan.
Exotherm Termil - a powder containing 20% active ingredients
packaged in 100 g cans. Application is made by removing the lid
from the can and igniting the powder to generate smoke.
The recommended application rates for various crops using the
wettable powder formulation Daconil 2787 W-75 are as follows:
Application
Crop rate
Vegetable crops (asparagus, beans, 1000-3400 g/ha
broccoli, Brussels sprouts, cabbage,
cantaloupe, carrot, cauliflower, celery,
chinese cabbage, corn (sweet), pumpkin,
squash, tomato, turnip, watermelon)
Agronomic crops (hop, peanut, spearmint, 1000-2300 g/ha
sugar beet, tobacco)
Small fruits (blackberry, blueberry, 120-480 g/100 litres
currant, grape, raspberry, strawberry)
Tree fruits and nuts (apple, cherry, 120-360 g/100 litres
grapefruit, lemon, orange, peach,
pecan, pear, tangerine)
Tropical crops (banana, cacao, coffee, 240-480 g/100 litres
rubber)
Thermal dusting with Termil tollets or Exotherm Termil is used to
control a variety of diseases of ornamentals and vegetable crops
(asparagus, snap beans, cucumbers and tomatoes) grown in glasshouses,
at an application rate of 7-7.5 g a.i./100 cu.m. of space.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits and vegetables (Diamond Shamrock, 1974). A summary of much of
this information appears in Table 2 together with details of rates and
number of applications and pre-harvest intervals used. The majority of
these data are from trials in the U.S.A. with a few trials from
Canadian locations.
Chlorothalonil residues were detected on most aboveground crops
at the time of harvest. The higher residues occurred on leafy
vegetables, e.g. lettuce, escarole, chicory, spinach, celery and
crucifers including collards and kale, with generally lower levels on
melons, fruits and root crops. High residues were detected also in
lima bean plants, sugar beet tops and peanut hay which may be used for
animal feeds. The amount of fungicide applied, time interval between
last application and harvest, surface area, weight and surface
structure of the crop are factors that affect the level of the
residue. The residue level diminishes with time after application.
Glasshouse trials of thermal dusting were carried out on
cucumbers, leaf lettuce and tomatoes. The residue data are summarized
in Table 3. In general, the residue levels were low, <4 mg/kg,
following a pre-harvest interval of 1 day.
Only negligible residues of the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, DAC 3701 (see
"Biotransformation" and "Fate of Residues"), were detected in most
commodities. Only in the case of peaches were significant residues
(up to 0.5 mg/kg) detected routinely. Peanut hay and lima beans
(including pods) also sometimes showed residues of 0.5-1 mg/kg.
A series of experiments were performed to see if chlorothalonil
or the 4-hydroxy metabolite could be a residue in meat or milk from
feeding these products to cows. Groups of cows (4 cows/group) were fed
chlorothalonil plus the 4-hydroxy metabolite at dosage levels of 0, 25
ppm chlorothalonil plus 0.2 ppm metabolite, 75 ppm chlorothalonil plus
0.6 ppm metabolite, and 250 ppm chlorothalonil plus 2.0 ppm metabolite
for 30 days. Half the animals were sacrificed at the end of the test
and half after a 32 day recovery period. At a sensitivity of 0.02 ppm,
no chlorothalonil was found in milk (Wolfe and Stallard, 1969). A
small quantity of the metabolite was noted in milk: details are given
in Table 4. Residues in the milk reached maxima of 0.20, 0.26 and 0.78
respectively at the 3 feeding levels after about 18 days (Wolfe and
Stallard, 1970a). At the 30-day interval small residues of
chlorothalonil and the metabolite were seen in muscle, fat, liver and
kidney. Residues of the metabolite were respectively 0.51, 2.1, 0.93
and 3.7 mg/kg at the highest feeding level. No residues were seen
after the 32 days recovery period (Wolfe and Stallard, 1970b).
TABLE 2a. Residues of chlorothalonil from supervised trials.
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 12 13 1 9 0-0.7 0.15
(tops removed) 1.7 9 0-7 3 8-9 0.1-3.6 0.3-2.8
1.9 9 2 1 6 1-8-5.8 4.4
2.5 7-10 0-7 4 6-8 0-8.7 0.02-7.3
Broccoli 1.3 9 1-15 3 9 0.01-9.0 0.01-6.0
2.5 6-8 0-12 4 5-9 0-2.6 0-1.4
Brussels
sprouts 1.3 4-5 0-21 4 9-18 0.7-3.5 1.7-2.5
1.7 5 8 1 3 4.6-5.3 5.0
2.5 4-8 0-18 2 3,5 0.9-4.3 1.0-3.5
3.4 5 0-14 4 2-5 2.0-11 3.2-11
Cabbage 1.7 5-9 0 2 3,7 0.8-2.7 1.5-1.6
2.5 7-9 0-7 5 5-9 0-0.2 <0.01-0.4
Cauliflower 0.8 2 33 1 9 0.03-0.09 0.06
2.5 2-10 0-33 5 7-9 0-1.9 0.02-1.2
Cucumber 0.8 4-9 0-6 5 6-9 0-1.1 0.05-0.9
1.3 4-9 0-6 4 6-18 0-1.2 0.1-0.8
1.7 1-12 0-7 12 3-23 0-2.5 0.2-2.4
2.5 4-13 0-14 12 6-9 0-2.8 0.01-1.8
Summer squash 0.8 6-9 0-3 2 5,6 0.07-0.9 0-2-0.3
1.3 6-9 0-3 2 6 0.2-1.2 0.45-0.9
1.7 1-9 0-9 9 3-25 0-2.0 0-1-1.3
2.5 5 0-14 3 3 0.06-0.25 0.07-0.2
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Cantaloupe
(whole melons) 1.7 7-11 0-14 4 5-17 0-1.1 0.02-0.6
2.5 7 4 1 12 0.7-1.8 1.1
(pulp) 2.5 7 0 1 5 0.05-0.1 0.07
Muskmelon
(pulp) 1.3 11 0 1 3 0 0
(rind) 1.3 11 0 1 3 0.03-0.2 0.1
(pulp) 2.5 11 0 1 3 0 0
(rind) 2.5 11 0 1 3 1.4-1.7 1.5
Honeydew melon 1.7 10 0-14 3 5-8 0-1.6 0.03-0.7
2.5 7 0-14 3 6 0.07-0.4 0.1-0.25
Winter squash
(whole) 1.7 5-10 0 2 3 0.04-1.7 0.05-1.4
(pulp) 1.7 5-10 0 2 3,8 0 0
(whole) 2.5 7 7 1 3 0.8-2.3 1.4
Watermelon
(whole) 0.8 8-10 0-14 4 3 0-0.6 0.02-0.35
(rind) 1.3 7 14 1 3 0.2-0.4 0.3
(whole) 1.7 8-10 0-14 4 3 0.03-1.0 0.08-0.5
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(whole) 2.5 8-10 0-14 4 0.05-1.0 0.05-0.8
Pumpkin (whole) 1.7 10 0-7 3 3 0.8-1.8 0.8-1.4
(pulp) 1.7 10 0 1 2 0 0
Tomato 0.6 18 3 1 9 0-0.1 0.05
1.3 6-18 0-14 5 6-18 0.03-5.5 0.05-4.4
1.7 4-10 0-15 17 3-27 0.01-5.5 0.09-3.4
2.5 5-18 0-14 12 3-32 0.2-4.5 0.2-4.5
Peanuts (nuts) 0.8 6 78 1 3 0.03 0.3
1.3 4-12 4-78 11 4-24 0-0.3 0-0.09
(hulls) 1.3 4-12 4-55 9 3-12 0-0.8 0.09-0.5
(hay) 1.3 4-11 4-68 11 2-30 0.96 0.3-89
(nuts) 1.7 6 78 1 3 0.02-0.04 0.03
Potatoes
(whole) 0.8 10 12 2 9 0 0
(peeled) 0.8 13 15 1 6 0 0
(peelings) 0.8 13 15 1 3 0-0.01 0
Potatoes
(whole) 1.3 3-13 12-23 16 35-48 0-0.07 0-0.02
(peeled) 1.3 10-13 0-15 4 33 0-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(peelings) 1.3 13 15 1 4 0-0.05 0.01
(whole) 1.7 5-12 0-23 11 19-44 0-0.06 0-0.01
(peeled) 1.7 5-12 0-13 3 21 0 0
(peelings) 1.7 5-12 0-14 6 14 0-0.15 0.06
(whole) 2.5 10 14 1 9 0-0.01 0
Sugar beets
(roots) 1.3 4 61 1 10 0-0.02 0.01
1.7 4-6 14-41 9 9-27 0-0.5 0-0.3
(tops) 1.7 4-6 14-41 3 9 0-5-17 0.9-11
(roots) 2.5 3-6 14-59 5 5-12 0-1.2 0.02-0.5
(tops) 2.5 3-5 14-59 3 6-17 0.2-32 1.2-16
(roots) 3.4 5 23 1 6 0.03-0.09 0.05
Sweet corn
(kernels
and cob) 1.3 14 14 2 17 0 0
1.7 10 0 1 9 0-0.03 0
2.5 11 0-7 3 5-9 0.01-0.1 0.04
Snap beans 1.7 5-8 0-7 5 5-17 0.04-14 0.1-11
2.5 8 0-7 3 9 0.8-10 2-3-5.4
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Lima beans
(beans) 1.3 10 0 1 3 0-2-0.4 0.3
(plants) 1.3 10 0 1 9 220-535 400
(beans) 1.7 7 0-15 2 5-9 0-0.1 0-0.7
(pod + bean) 1.7 13 0 1 3 10-13 12
(plants) 1.7 4-13 0-15 3 2-9 22-310 47-117
Celery 0.8 5-24 0-14 6 8-9 0.2-21 0.4-18
1.3 9-24 0-14 5 6-9 0.1-52 0.4-29
1.4 8 7-14 2 6-8 0.06-1.7 0.1-1
1.7 9-24 0-14 7 6-9 0.1-53 0.7-34
1.8 8 7-14 2 6 0.1-5.4 0.3-3.4
1.9 26 7-14 2 6-8 0.2-17 0.6-12
2.5 5-14 0-14 5 8-9 0.6-17 1.4-10
3.4 10-18 1-14 4 6-9 2.8-26 4.5-18
Oranges (whole) 1.3 1-14 0-14 3 6 1.8-5.1 2.7-4.3
(peel) 1.3 1 173-397 5 20 0-0.7 0.1
Grapefruit
(whole) 0.4-0.8 1 309 2 6 0.01-0.02 0.01
1.3 1 200-309 3 11 0-0.05 0.02
(peel) 1.3 1-2 22-309 6 6-17 0-0.2 0.06-0.09
Limes (whole) 1.3 1 119-280 2 12 0-0.01 0
Lemons (whole) 1.3 1 280 1 4 0.01-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Tangerines
(whole) 1.3 1 280 1 6 0.01 0.01
Cherries 0.4 1-4 3-10 2 4-12 0-0.2 0.04-0.2
0.6 4 0-24 5 2-13 0.1-11 0.1-9.3
1.0 4 20 1 6 0.1-0.2 0.2
1.3 3-5 2-24 5 5-14 0.7-7.8 1.4-5.7
1.5 4-5 20-24 3 18 1-10 3.1-4.2
Peaches 0.6 4 8 1 3 6-8 6.7
0.8 4 8 1 6 3.3-6.3 5.0
1.0 7 0-14 3 3 6.4-19 8-15
1.3 4-11 6-14 3 3-12 2.8-20 4-16
1.5 6-7 0-25 11 3-18 1.3-45 4-31
1.7 4 8 1 3 27-28 28
2.5 4 8 1 3 32-54 45
Currants 1.0 4 3 1 6 16-20 18
1.3 4 3 1 6 16-20 18
1.5 4 3 1 6 20-23 23
Blackberries 1.3 2 4 1 12 4-11 7.3
1.7 2 23-48 2 12 0.5-2 1.2
1.9 2 4 1 12 3-16 9.6
2.5 2 0-16 3 9 0.3-8 2-4
3.0 2 0-16 4 7-12 0.5-43 1.6-21
3.4 2 0-16 3 9 1.3-9.3 2.5-4
Raspberries 0.4 2 4 1 6 0.05-1 0.3
0.8 2 4 1 6 0.2-2.4 1.2
1.3 2-3 0-8 3 6-12 0.4-6.5 0.7-4.1
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
1.5 2 0-8 2 6 0.6-2.7 0.7-1.7
1.7 3 0-7 3 3 5.6-20 5-7-15
Collards 2.5 3 0-14 4 9 3.3-88 5.7-69
Kale 2.5 3 0-14 4 6 1.5-71 2.8-62
Escarole 1.7 8 0-7 3 4-6 0.4-24 1.3-15
Endive 0.4 5 1 1 2 24-28 26
0.8 5 1 1 3 22-44 31
Chicory 1.7 8 0-7 3 3-5 0.04-30 0.3-24
Lettuce leaf 1.3 3 0 1 8 13-43 24
2.5 3-4 0-7 3 8-9 5-89 13-68
Lettuce head 1.7 4-7 0-14 5 2-12 0.1-100 1.3-86
Spinach 1.3 5 3-8 2 6 4-48 14-29
Turnip greens 1.7 2 2 1 7 5-10 7.2
Onions, green 1.7 3-4 0-7 3 6-8 0.6-11 1.1-7.6
2.5 3-4 0-7 3 6-7 1.3-24 2.0-18
Onions, mature 0.8-1.3 8-9 12-14 2 30 0-0.1 0-0.05
dry 1.7-2.5 5-9 7-9 2 105 0-0.3 0-0.1
Peppers 1.7 9-13 0-7 2 3-9 0.4-9.4 1.2-8.4
2.5 7 0-7 3 9 0.1-3.3 0.5-1.9
TABLE 2b. Residues of hydroxy-metabolite (DAC-3701) from supervised trials
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 8 0-7 2 3,5 0.01-0.06 0.01-0.04
(tops removed) 2.5 7 0 1 6 0.04 0.04
Broccoli 1.3 9 1-15 3 9 0 0
2.5 8 0 1 7 0-0.06 0.01
Brussels sprouts 2.5 8 0 1 5 0.01-0.02 0.02
Cabbage 2.5 9 0 1 6 0 0
Cucumber 0.8 7 0 1 6 0-0.03 < 0.01
1.7 7 0 1 6 0-0.03 < 0.01
2.5 6 0 1 3 0 0
Cantaloupe
(whole melons) 1.7 7 4 1 13 0.02-0.04 0.03
2.5 7 4 1 8 0.02-0.08 0.05
(pulp) 2.5 5 1 1 5 0.08-0.14 0.1
Watermelon
(rind) 1.3 7 14 1 3 0 0
Tomato 1.3 6 0 1 6 0-0.2 0.01
1.7 5 1 1 3 0.06-0.1 0.08
2.5 5-8 0-1 3 11 0.01-0.1 0.02-0.09
Peanuts (nuts) 1.3 4-12 4-55 6 10-20 0 0
(hulls) 1.3 4-12 6-55 5 3-9 0-0.05 0-0.02
TABLE 2b. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Peanuts
(hay) 1.3 4-11 4-55 6 9-33 0-0.6 0.03-0.4
Sugar beets
(roots) 2.5 3 25 2 9 0-0.01 0.01
(tops) 2.5 3 23 1 6 0 0
(roots) 3.4 5 23 1 6 0-0.01 0.01
Lima beans
(pod + bean) 1.7 4 3 1 3 0.8-1.1 1.0
Celery 0.8 20 0 1 4 0.05-0.1 0.05
1.4 8 7-14 2 2-5 0.05-0.2 0.09-0.15
Peaches 0.6 3 0-10 2 3 0.1-0.3 0.2
0.8 2-10 0-10 4 2-5 0-1-0.4 0.2-0.3
1.3 2-4 0-12 4 2-3 0.01-0.5 0.01-0.3
Lettuce head 1.3 4 1-7 2 4 0-0.04 0.01
1.7 4 21 1 6 0-0.04 0.02
Spinach 1.3 5 3 1 8 0.04-0.3 0.1
Turnip greens 1.7 2 2 1 5 0-0.02 0
Onions, mature 0.8-1.3 5-8 14 3-8 3-8 0.0.02 0-0.01
dry 1.7-2.5 8 - 3-5 3-5 0-0.2 0.1-0.2
TABLE 3. Chlorothalonil and DAC-3701 residues in glasshouse crops from supervised trials
Residues (ppm)
Days from No. of Range Means or
Crop and Rate No. of application No. of determinations individual range of
residue g/1000 ft3 applications to harvest trials per trial determinations) means/trial
Cucumbers
chlorothalonil 2 12-15 1 4 3 0.01-0.09 0.03-0.06
DAC-3701 2 12-15 1 4 3 0 0
Lettuce, leaf
chlorothalonil 2 5-12 1 7 3 0.05-3.6 0.05-3.0
Tomatoes
chlorothalonil 2 1-8 1 8 2-3 0.02-0.4 0.03-0.3
1.8 3-8 1 6 4 0.1-3.7 0.2-3.0
2 12-18 1 5 3 0.5-1.4 0.7-1.4
2 1-9 1 9 3 0-0.8 0-0.6
2 8-16 0.5 9 3 0.04-0.7 0.08-0.6
DAC-3701 2 1-8 1 8 2-3 0 0
TABLE 4. DAC-3701 residues (mg/kg) in cows milk during
feeding trial
Residue (mg/kg at 0.2*, 0.6** and 2.0*** ppm
DAC-3071 in diet
Days 0 0.2 0.6 2.0
0 < 0.03 < 0.03 < 0.03 < 0.03
2 < 0.03 < 0.03 < 0.03 0.06
4 0.04 0.04 0.06 0.16
8 0.04 0.08 0.10 0.51
14 < 0.03 0.13 0.16 0.57
18 < 0.03 0.20 0.26 0.74
22 < 0.03 0.11 0.17 0.78
26 < 0.03 0.11 0.13 0.59
30 < 0.03 0.16 0.26 0.74
37 < 0.03 0.06 0.12 0.55
44 < 0.03 0.04 0.16 0.16
51 < 0.03 < 0.03 < 0.03 0.06
60 < 0.03 < 0.03 < 0.03 < 0.03
* +25 ppm chlorothalonil
** +75 ppm chlorothalonil
*** +250 ppm chlorothalonil
Residues of chlorothalonil were not detected in the tissues of
dogs and rats fed levels in the diet of up to 30 000 mg/kg (Wolfe and
Stallard, 1968a) although small quantities of the hydroxy metabolite
were found in the liver and kidney.
No residues of chlorothalonil were detected in milk from a
Holstein cow fed the compound at the 5 ppm level in the ration for
four days (Gutenmann and Lisk, 1966). The method was sensitive to
about 0.03 ppm.
FATE OF RESIDUES
General comments
The disappearance and fate of chlorothalonil residues is
reasonably well documented. The only identified metabolite is the
4-hydroxy compound, DAC-3701, mentioned previously. Although
chlorothalonil residues decrease quite rapidly on plants, the actual
fate is unknown.
In animals
In the experiment by Gutenmann and Lisk (1966) mentioned above,
samples of milk, urine and faeces were collected throughout the
feeding period and for five days thereafter. No residues of
chlorothalonil, acid products of nitrile hydrolysis or conjugates of
acidic or phenolic derivatives were detected. In the same series of
experiments, chlorothalonil disappeared rapidly when incubated with
rumen juice at the 1 ppm level. Two unidentified metabolites were
formed. Feeding studies with dogs and rats showed that chlorothalonil
was rapidly eliminated in the faeces with negligible amounts in the
urine (Skinner and Stallard, 1967). The eliminated material was mostly
unchanged chlorothalonil. Analysis of tissue samples from rats and
dogs from chronic feeding studies of one and two years indicated no
detectable storage of chlorothalonil. A radio-tracer study with rats
confirmed that elimination of chlorothalonil was complete and there
was no significant tissue storage (Ryer and Sullivan, 1966; Skinner
and Stallard, 1967).
Dogs and rats were fed for 2 years on diets containing 1500,
15 000 and 30 000 mg/kg of chlorothalonil (Wolfe and Stallard, 1968a).
Residues were not detected in muscle tissue. Residues of
4-hydroxy-2,5,6-1,3-benzene-dicarbonitrile (DAC-3701) in kidney
tissues of dogs were <1.5 mg/kg and <3.5 mg/kg in the livers of
dogs and rats. Residue levels of the hydroxy metabolite were <0.25
mg/kg in the urine of the dogs and rats.
In the feeding experiment involving both chlorothalonil and
DAC-3701 (Wolfe and Stallard, 1969, 1970a, 1970b; see preceding
section and Table 4), the DAC-3701 residues in the milk accounted for
10-16% of the administered metabolite, but it was not possible to
determine whether part of the milk residue derived from the
chlorothalonil in the diet.
In plants
In studies with ring labelled 14C-chlorothalonil, Kunkel (1967a)
found no evidence of translocation from topical applications on
cucumber cotyledons, cucumber leaves, cucumber hypocotyls, bean
unifolilate leaves or tomato leaves. He also demonstrated that the
labelled compound was not translocated into the aerial parts of corn
or tomato plants when they were cultivated for 23 days in soil treated
with 14C-chlorothalonil. In another study, Kunkel (1967b) showed that
autoradiographic techniques did not demonstrate 14C-movement or
translocation within root systems of sweet corn, cucumber or tomato
grown in soil treated with ring-labelled chlorothalonil. Since
DAC-3701 is a soil metabolite of chlorothalonil and 14C activity was
not translocated from the soil, the experiments also showed that the
4-hydroxy metabolite is not translocated into the root system or
aerial parts of these plants. The residue data from supervised trials
(Table 2) showed that DAC-3701 is a residue following chlorothalonil
treatment. Of the crops tested for DAC-3701 residues, peaches
contained up to 0.5 mg/kg, spinach up to 0.3 mg/kg, onions up to 0.2
mg/kg, celery up to 0.2 mg/kg, cantaloupe up to 0.1 mg/kg and lima
bean plants up to 1 mg/kg. Negligible residues of DAC-3701 (<0.1 ppm)
were reported for carrots, tomatoes, peanuts (except peanut hay),
sugar beets, turnip greens, broccoli, Brussels sprouts, cabbage,
cucumber, watermelon and lettuce.
In soil
Tests conducted under both laboratory and field conditions
demonstrated that chlorothalonil is rapidly degraded in soil (Stallard
and Wolfe, 1967). In the laboratory experiments its half-life in the
types of soils tested ranged from 4 to over 40 days. The degradation
rate increased with increasing organic matter content, moisture
content and temperature but appeared to be independent of pH within
the range 6-8. Chlorothalonil was evidently not lost by
volatilization, since it disappeared rapidly from treated soil
incubated in tightly closed jars.
In the field trials, turf plots in 3 locations in the U.S.A. were
treated with chlorothalonil and the half-life of the chlorothalonil
residues ranged from 26-45 days. The data indicated a decreased
degradation rate during the winter months.
4-Hydroxy-2,5,6-trichloro-1,3-benzenecarbonitrile (DAC-3701) was
the major soil degradation product following treatment with 14C-ring
labelled chlorothalonil and incubation (Duane, 1970). This compound
accounted for over 80% of the radioactivity extracted from the soil.
(Approximately 20% of the radioactivity was not extracted.) A second
degradation product, more polar than DAC-3701 and without a hydroxyl
group, was detected but not identified. During these experiments it
was shown that 14C-chlorothalonil was not volatilized from the soil
and no volatile degradation products were formed.
Laboratory experiments with 5 representative soil types
demonstrated that the half-life values for DAC-3701 ranged from 36
days in a sandy loam type soil to 220 days in clay type soil (Wolfe
and Stallard, 1968b).
Duane (1970) demonstrated that bacteria isolated from soil were
capable of metabolizing chlorothalonil in culture media. Thus it may
be assumed that naturally occurring soil microorganisms are in part
responsible for the rapid loss of chlorothalonil under field
conditions.
In storage and processing
Some data are available on the effect of washing, field trimming
and peeling on the residue levels of chlorothalonil in some
commodities (Diamond Shamrock, 1974). Table 5 summarizes the data on
the effect of washing with water. Residues on carrots, cucumbers,
summer squash, tomatoes, currants and cranberries were reduced by
more than 50%. Washing was least effective with the leafy vegetables.
Significant reduction in chlorothalonil residues results from trimming
cabbage and head lettuce (Table 6). Chlorothalonil residue are
concentrated on the surface and as a result peeling removes
almost all the residues leaving the pulp of many fruits and
vegetables almost residue-free (Table 7). Following processing,
residues of chlorothalonil were not detected ( <0.1 mg/kg) in canned
spinach (Table 8). The fate of any DAC-3701 residues was not
determined.
TABLE 5. Effect of washing with water on residues of
chlorothalonil
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Carrots 4.39-7.34 1.05-3.53 52-76
Cucumbers 0.08-0.45 0.01-0.03 87-95
Squash, summer 0.21-1.33 0.01-0.12 76-97
Tomatoes 0.21-0.82 0.02-0.08 90
Celery 1.04-20.0 0.76-8.18 0-59
Peaches 7.6-15.0 4.9-8.6 35-45
Currants 18.3 2.6 86
Cranberries 0.91 0.25 73
TABLE 5. (Cont'd.)
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Collards 5.73-69.0 1.7-31.3 31-70
Kale 2.8-62.0 1.6-20.0 43-74
Escarole 1.32-4.1 0.63-2.6 36-44
Chicory 0.31-4.0 0.23-1.5 26-62
Lettuce 23.7-67.6 8.9-37.1 45-70
Cauliflower 1.23 0.85 30
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not available to the Meeting to indicate the level and
incidence of chlorothalonil residues in food moving in commerce or in
food at the time of consumption. Chlorothalonil residues were not
determined in the U.S.A. national food and feed monitoring program
(Duggan and Cook, 1971).
METHODS OF RESIDUE ANALYSIS
Residues of chlorothalonil and its metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile DAC-3701), can be
determined by gas-liquid chromotography (GLC) with either electron
capture or microcoulometric (halide) detectors (Wolfe and Stallard,
1970). The parent compound is chromatographed directly but the
metabolite is converted to the methyl ether by reacting with
diazomethane prior to chromatography.
Crop and soil samples are extracted with acidified acetone. The
acetone is removed by distillation and the residues are partitioned
into diethyl ether. The ether extract is cleaned-up on a Florisil
chromatographic column. Chlorothalonil is eluted with 5%
acetone-dichloromethane and DAC-3701 with 50% acetone-dichloromethane.
Following reaction with diazomethane, the methyl ether of DAC-3701 and
chlorothalonil are determined by GLC either by electron capture or
microcoulometric detection. The sensitivity of the method is
TABLE 6. Effect of trimming on residues of chlorothalonil in cabbage and lettuce
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop kg/ha applications to harvest determinations (mg/kg) (mg/kg)
Cabbage
(field trimmed) 1.7 9 0 7 0.77-2.67 1.60
1.7 5 0 3 1.25-1.72 1.47
(market trimmed) 2.5 7-8 0-7 7-9 0.00-0.18 <0.01-0.03
Lettuce
(whole untrimmed
heads) 1.7 4-7 0-7 2-12 11.5-100 33.7-85.5
1.7 4 14 11 0.10-3.0 1.33
(trimmed heads) 1.3 5 1-7 4 0.15-1.98 0.53-0.95
1.7 4 2-14 6 0.07-2.9 0.80-0.90
TABLE 7. Effect of peeling on chlorothalonil residues
Crop Residues (mg/kg)
Cucumbers Peelings - 1.26; Pulp - <0.01
Muskmelon Rind - 0.11-1.50; Pulp - 0.00
Winter squash Whole - 0.05-1.42; Pulp - 0.00
Pumpkin Whole - 1.42; Pulp - 0.00
Cantaloupe Whole - 1.09; Edible portion - 0.07
Peanut Hulls - 0.13-0.51; Meat - 0.00
Potatoes Peelings - 0.00-0.06; Pulp - 0.00
Lima beans Pod and beans - 11.9; Beans - 0.00
Grapefruit Peel - 0.09; Whole - 0.02
Oranges Whole - 2.70-4.29; Pulp - <0.01-0.08;
Juice - 0.00
TABLE 8. Effect of canning on chlorothalonil residues in spinach
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop % applications to harvest analysis (ppm) (ppm)
Spinach
(raw) control 0 - 6 <0.1 <0.1
0.11 5 3 6 16.1-47.8 29.1
0.11 5 8 6 3.7-30.9 13.6
(processed,
canned) control 0 - 3 <0.1 <0.1
0.11 4 16 3 <0.1 <0.1
0.02 mg/kg. Recovery values obtained from fortified crops were greater
than 72 and 76% for chlorothalonil and DAC-3701 respectively. Some
modifications to the method described above are required for animal
tissue (Wolfe and Stallard, 1970b) and milk samples (Wolfe and
Stallard, 1969, 1970c). Average, recoveries for chlorothalonil and
DAC-3701 respectively were:
muscle - 81, 91%
fat - 76, 91%
kidney - 82, 84%
liver - 93 83%
milk - 88, 83%
The sensitivity of the method for milk is 0.02 mg/kg for
chlorothalonil and 0.03 mg/kg for DAC 3701. Chlorothalonil is not
recovered by the Mills Florisil multi-residue procedure but is
recovered (McMahon et al., 1973) in the alternative Florisil elution
system (Mills et al., 1972). Recovery from deactivated Florisil
(Osadchuk et al., 1971) is achieved by elution with 10% ethyl acetate
in hexane (McLeod and Ritcey, 1973).
Chlorothalonil can be recovered through the carbon-cellulose
cleanup procedure of McLeod et al. (1967). Gutenmann and Lisk (1966)
determined chlorothalonil by electron capture GLC in milk, urine and
faeces after extracting with acetone-phosphoric acid and partitioning
into hexane. Possible acid metabolites were determined after
diazomethane esterification of the evaporated acetone extract.
With the exception of the Mills Florisil multiresidue procedure,
the multiresidue procedures discussed above appear to be suitable for
regulatory determination of the parent compound. When the total
residue, chlorothalonil and DAC-3701, is required the GLC method
proposed by Wolfe and Stallard (1970c) can be recommended for
regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Some examples of national tolerances were reported to the Meeting
and are listed in Table 9.
TABLE 9. Examples of national tolerances as reported to the Meeting
Tolerance
Count Commodity (mg/kg)
Australia Beets, carrots, corn, cucumbers,
onions, peppers, potatoes,
tomatoes 7
Peanuts 0.2
TABLE 9. (cont'd)
Tolerance
Count Commodity (mg/kg)
Canada Celery 15
Broccoli, Brussels sprouts,
cabbage, cauliflower, cucumbers,
melons, pumpkins, snap beans,
squash, tomatoes 5
Carrots 1
Peanuts 0.3
Netherlands Potatoes 0.05
Apples, apricots, beets, carrots,
citrus, corn, cucumbers, grapes,
melons, onions, peaches, pears,
peppers, plums, tomatoes 0.01
Switzerland Potatoes 0.05
U.S.A. Celery 15*
Broccoli, Brussels sprouts, cabbage,
cauliflower, cucumbers, melons,
pumpkins, snap beans, squash
(summer and winter), tomatoes 5*
Carrots, sweet corn (kernels plus
cob with husks removed) 1*
Peanuts 0.3*
Potatoes 0.1*
* Including metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzen-edicarbonitrile.
APPRAISAL
Chlorothalonil is a broad-spectrum fungicide with effective
action against many fungus diseases which damage vegetable, tree,
small fruit and other agricultural crops, turf and ornamentals. The
use pattern of chlorothalonil is such that residues remain on most
above-ground crops at the time of harvest. It is desirable to have
some residue of the fungicide on the mature crop to protect it from
disease organisms during shipment.
4-Hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile is a major
metabolite of chlorothalonil in soil and a metabolite in plants, but
only negligible residues were found on most crops investigated.
Chlorothalonil does not translocate into plants from the soil or from
topical application.
Extensive crop residue data indicated that chlorothalonil is a
relatively persistent fungicide. The level of the residue depends on
such factors as rate of the fungicide applied, time interval between
last application and harvest and the surface area, weight and surface
structure of the crop. The residue level diminishes with time after
application, and pre-harvest intervals are therefore recommended for
some crops. Some data are available on the effects of washing,
trimming and peeling on chlorothalonil residues.
Chlorothalonil residues do not occur in the tissues or milk of
cows, but residues of the 4-hydroxy metabolite are found when cows are
fed chlorothalonil and the 4-hydroxy compound together in their
ration. The residue levels depend on the levels fed and reach a steady
value in the milk after 18 days. Residues in milk declined to below
detection level within 21 days after withdrawal. It was not determined
whether residues of the 4-hydroxy metabolite would occur in the milk
and tissues of cows if only chlorothalonil were ingested.
Since no data were provided on residues in crops that may be fed
to animals, it was not possible to recommend maximum residue limits
for milk and meat.
Most available multi-residue GLC methods appear to be suitable
for the determination of the parent compound, but chlorothalonil is
not recovered by the original Mills Florisil multi-residue cleanup
procedure (McMahon et al., 1973). The GLC method proposed by Wolfe
and Stallard (1970) for chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile appears to be
suitable for regulatory purposes when the total residue is required.
National tolerances are in effect in a number of countries.
RECOMMENDATIONS
The following maximum residue limits are recommended for
chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, expressed as
chlorothalonil.
TEMPORARY TOLERANCES
Pre-harvest
interval on which
Limit recommendations
Commodity (mg/kg) are based (days)
Peaches 30 7
Currants (black, red and white) 25 3
Celery 15 7
Peppers 10 1
Blackberries, raspberries, 10 7
cherries, chicory sprouts
Collards, kale, endive, 10 14
lettuce (head)
Broccoli, Brussels sprouts, 5 7
cabbage, cauliflower, beans
(green including pod), oranges,
onions, cranberries
Cucumbers, melons, pumpkins, 5 1
squash, tomatoes
Carrots, sweet corn, sugar 1 1
beets
Lima beans, peanuts (whole) 0.5 0
Peanuts (kernel), potatoes 0.1 0
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Additional study to resolve lower limit of kidney effects in rat.
2. Define growth reduction in pups relative to dietary ingestion or
secretion into milk.
3. Data on residues of chlorothalonil and the 4-hydroxy metabolite
in crops that may be fed to animals.
4. The results of feeding studies on dairy cattle understood to be
in progress to determine the level and nature of residues in milk
and tissues.
DESIRABLE
1. Observations in man.
2. Residue data for food moving in commerce.
3. Further information on effects of processing, including household
cooking, on residues.
REFERENCES
Beasley, A. and Leong, K. (1965). Acute inhalation exposure - rats.
Report from Hazelton Laboratories Inc., submitted by The Diamond
Shamrock Chemical Co. (Unpublished)
Blackmore, R. and Shott, L. (1968). Final report four month feeding
study - rats. Report from Hazelton Laboratories, Inc. (Unpublished)
Diamond Shamrock (1971). Documentation on the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Diamond Shamrock (1974). Documentation concerning the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Di Dario, A., Curry, T.L., Thayer, P. and Turner, J.J. (1965). The
biological performance of tetrachloroisophthalonitrile as influenced
by particle size and crystalline form. Phytopathology, 55:1055.
Doyle, R. and Elsea, J. (1963). Acute oral, dermal and eye toxicity
and irritation studies on DAC-2787. Report from Hill Top Research
Institute Inc., submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Duane, W.C. (1970). Biodegradation of Daconil 2787(R). Report
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Duggan, R.E. and Cook, H.R. (1971). National food and feed monitoring
Program. Pestic. Monit. J., 5:37-43.
Gutenmann, W.H. and Lisk, D.J. (1966). Metabolism of Daconil and
Dacthal pesticides in lactating cows. J. Dairy Sci., 49:1272-1276.
Hastings, T.F. and Jessup, D.C. (1974). 3-Generation reproduction
study in albino rats using DAC-3701 in the diet. Report from BIO/TOX
Research Laboratories, Inc. Submitted to WHO by Diamond Shamrock
Chemical Co. (Unpublished)
Holliday, W., Schadeberg, K., Goode, J. and Keplinger, M. (1973).
Twenty-one day subacute aerosol inhalation toxicity study with Bravo
6F(R) in albino rats. Report from Industrial Bio Test Laboratories,
Inc. (Unpublished)
Holsing, G. and Shott, L. (1970). Two year dietary administration
- rats. Report from Hazelton Laboratories Inc. submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Holsing, G. and Voelker, R. (1970). Final report 104 week dietary
administration - dogs. Report from Hazelton Laboratories Inc.
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967a). Absence of 14C movement in crop plant organs
after topical application and soil amendment studies with isotopic
Daconil 2787. Report to Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967b). Movement of 14C in or on roots of crop species
grown in soil amended with isotopic Daconil 2787. Report to Diamond
Shamrock Chemical Co. (Unpublished)
Legator, M. (1974). Mutagenic Testing with DAC 2787. Report from
Division of Genetics, Roger Williams General Hospital and Brown
University, Division of Biological and Medical Sciences submitted by
Diamond Shamrock Chemical Co. (Unpublished
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967).
Comparison of various carbon adsorbents and quantitative elution and
separation of forty-two pesticides from a carbon-Solka Floc cleanup
column. J. Ass. off. analyt. Chem., 50:1216-1228.
McLeod, H.A. and Ritcey, W.R. (1973). Analytical methods for pesticide
residues in foods. Information Canada, Ottawa, Canada.
McMahon, B.M., Sawyer, L.D. and Corneliussen, P.E. (editors) (1973).
Pesticide analytical manual. Volume 1. Methods which detect multiple
residues. Food and Drug Administration, Washington, D.C.
Mills, P.A., Bong, B.A., Kamps, L.R. and Burke, J.A. (1972). Elution
solvent system for Florisil column cleanup in organochlorine pesticide
residue analyses. J. Ass. off. analyt. Chem., 55:39-43.
Osadchuk, M., Romach, M. and McCully, K.A. (1971). Cleanup and
separation procedures for multi-pesticide residue analysis in
monitoring and regulatory laboratories. Proc. Second Intern. IUPAC
Congress Pestic. Chem. Vol. IV, Methods in Residue Analysis, p.
357-383. A.S. Tahori (editor), Gordon and Breach Science Publishers,
New York.
Paynter, O. (1965a). Oral dose range - dogs - final report. Report of
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1965b). Repeated dermal application - rabbits. Report
from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1966). Reproduction - rabbit. Report from Hazelton
Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1967a). Three generation reproduction study - rats.
Report from Hazelton Laboratories Inc. submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1967b). Ten week amino acid feeding study - rats. Report
from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. (1967c). Final report - two year dietary feeding - rats.
Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Busey, W. (1967). Long term (76 weeks) feeding study,
rats, DAC 2787. Final Report from Hazelton Laboratories Inc.
(Unpublished)
Paynter, O. and Crews, L. (1967). Final report - two year dietary
feeding - rats. Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Murphy, J. (1967). Sixteen week dietary feeding -
dogs. Report from Hazelton Laboratories Inc., submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Powers, M. (1965). Acute oral administration - rats. Report from
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Ryer, F.H. and Sullivan, J.B. (1966). Radiotracer metabolism study.
Report from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Skinner, W.A. and Stallard, D.E. (1967). Daconil 2787(R) animal
metabolism studies. Report from and submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Stallard, D.E. and Wolfe, A.L. (1967). The fate of
2,4,5,6-tetrachloroisophthalonitrile (Daconil 2787(R)) in soil.
Report from Diamond Shamrock Chemical Co. (Unpublished)
Sterner, W. and Loveless, L. (1963). A study of the subacute toxicity
of tetrachloroisophthalonitrile to rats. Report from International
Bio-Research, Inc. (Unpublished)
Teeters, W. (1966). In vivo assay of spasmodic activity, mice, DAC
2787. Final report from Hazelton Laboratories Inc. (Unpublished)
Turner, N.J. and Lamont, D. (1965). Control of fungal diseases in the
greenhouse with thermally induced dusts of
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
23:51-54.
Turner, N.J., Limpel, L.E., Battershell, R.D., Bluestone, H., Lamont,
D. (1964). A new foliage protectant fungicide,
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
22:303-310.
Wazeter, F. (1971). Acute oral LD50 in male albino rats. Report from
International Research and Development Corporation. (Unpublished)
Wazeter, F. and Goldenthal, E. (1972). Acute oral toxicity in beagle
dogs. Report from International Research and Development Corporation.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968a). Analysis of tissues and organs
for storage of the Daconil metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968b). The fate of DAC-3701
(4-hydroxy-2,5,6-trichloroisophthalonitrile) in soil. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1969). Residues in milk from cows fed
2,4,5,6-tetra-chloroisophthalonitrile. Diamond Shamrock Chemical Co.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970a). Residues in milk from cows fed
2,5,6-trichloro-4-hydroxyisophthalonitrile. Diamond Shamrock Chemical
Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970b). Residues in tissues of dairy
cows fed Daconil 2787 and 2,5,6-trichloro-4-hydroxyisophthalonitrile.
Diamond Shamrock Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970c). Analytical method for
determination of Daconil 2787 and DAC-3701 residues. Diamond Shamrock
Chemical Co. (Unpublished)
 Other Information on Identity and Properties
Molecular weight: 265.9
State: White crystalline solid
Melting point: 250-251°C
Boiling point: 350°C at 760 mm Hg
Vapour Pressures:
Vapour Pressures Temperatures
mm Hg °C
< 0.1 40
9.2 170
17.4 191
27.3 212
43.3 230
Solubility:
Solvent % by weight at 25°C
acetone 2
AR-60 6
AR-55 3
cyclohexanone 3
dimethyl sulfoxide 2
dimethyl formamide 3
kerosene <1
mineral seal oil <1
methyl ethyl ketone 2
xylene 8
water 0.6 ppm
Stability: Stable under normal temperatures of storage. Chemically
stable in alkaline or acidic aqueous media. Stable to
ultraviolet radiation.
Purity of technical material:
Ingredient Range (%) Average (%)
2,4,5,6-tetrachloro 95.6-98.5 97.6
-1,3-benzenedicarbonitrile
tetrachlorophthalonitrile - <0.1
tetrachloroterephtholonitrile <0.1-1.6 0.5
pentachlorobenzonitrile 0.5-2.5 1.2
partially chlorinated 0.2-1.0 0.4
dicyanobenzenes (all isomers)
unchlorinated dicyanobenzenes <0.1-1.6 0.3
(all isomers)
insolublein xylene <0.1-1.0 0.2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Following acute oral administration to dogs, chlorothalonil (500
mg/kg) was rapidly excreted, mainly unchanged, in the faeces.
Approximately 90% of the administered dose was found in faeces within
72 hours. No chlorothalonil or metabolites were found in blood or
urine when examined using methods having a sensitivity of 1 ppm for
blood and 0.1 ppm for urine (Skinner and Stallard, 1967). Urine and
faecal samples were collected from dogs and rats fed chlorothalonil in
the diet for approximately ten months. In both species excretion of
unchanged chlorothalonil in faeces was proportional to the
concentration in the diet. Rats fed a dose of 0.15% excreted 21% in
the faeces; those fed 1.5% excreted 67%; dogs fed 0.15% excreted
9-15%; those fed 1.5% excreted 67-71%; and those fed 3% excreted
86-86% unchanged chlorothalonil in the faeces. Assays based on total
chlorine content established that recovery in faeces of chlorothalonil
consumed in the diet was complete. The total chlorine assay and
incomplete recovery of chlorothalonil or metabolites from faeces
suggests a biotransformation product excreted with the faeces and not
identified.
Three male and three female weanling rats were fed for 22 days at
5000 ppm in the diet. They were orally administered 14C
chlorothalonil at a dose of 2.8 µ Ci/rat. The dose (1.5 mg) did not
significantly increase the daily input of chlorothalonil. After 264
hours, 95% of the material was recovered, predominantly in faeces
(88%) and urine (5%) with none detected in tissues or as CO2.
Chlorothalonil was not rapidly removed from the animals body (43% in
24 hours; 64% in 48 hours, and 76% in 72 hours) (Ryer and Sullivan,
1966).
Studies on the transformation of chlorothalonil in soil (Stallard
and Wolfe, 1967; Duane, 1970) have isolated and identified a
metabolite of chlorothalonil as
4-hydroxy-2,5,6-trichloroisophthalo-nitrile (DAC-3701).
Other Information on Identity and Properties
Molecular weight: 265.9
State: White crystalline solid
Melting point: 250-251°C
Boiling point: 350°C at 760 mm Hg
Vapour Pressures:
Vapour Pressures Temperatures
mm Hg °C
< 0.1 40
9.2 170
17.4 191
27.3 212
43.3 230
Solubility:
Solvent % by weight at 25°C
acetone 2
AR-60 6
AR-55 3
cyclohexanone 3
dimethyl sulfoxide 2
dimethyl formamide 3
kerosene <1
mineral seal oil <1
methyl ethyl ketone 2
xylene 8
water 0.6 ppm
Stability: Stable under normal temperatures of storage. Chemically
stable in alkaline or acidic aqueous media. Stable to
ultraviolet radiation.
Purity of technical material:
Ingredient Range (%) Average (%)
2,4,5,6-tetrachloro 95.6-98.5 97.6
-1,3-benzenedicarbonitrile
tetrachlorophthalonitrile - <0.1
tetrachloroterephtholonitrile <0.1-1.6 0.5
pentachlorobenzonitrile 0.5-2.5 1.2
partially chlorinated 0.2-1.0 0.4
dicyanobenzenes (all isomers)
unchlorinated dicyanobenzenes <0.1-1.6 0.3
(all isomers)
insolublein xylene <0.1-1.0 0.2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Following acute oral administration to dogs, chlorothalonil (500
mg/kg) was rapidly excreted, mainly unchanged, in the faeces.
Approximately 90% of the administered dose was found in faeces within
72 hours. No chlorothalonil or metabolites were found in blood or
urine when examined using methods having a sensitivity of 1 ppm for
blood and 0.1 ppm for urine (Skinner and Stallard, 1967). Urine and
faecal samples were collected from dogs and rats fed chlorothalonil in
the diet for approximately ten months. In both species excretion of
unchanged chlorothalonil in faeces was proportional to the
concentration in the diet. Rats fed a dose of 0.15% excreted 21% in
the faeces; those fed 1.5% excreted 67%; dogs fed 0.15% excreted
9-15%; those fed 1.5% excreted 67-71%; and those fed 3% excreted
86-86% unchanged chlorothalonil in the faeces. Assays based on total
chlorine content established that recovery in faeces of chlorothalonil
consumed in the diet was complete. The total chlorine assay and
incomplete recovery of chlorothalonil or metabolites from faeces
suggests a biotransformation product excreted with the faeces and not
identified.
Three male and three female weanling rats were fed for 22 days at
5000 ppm in the diet. They were orally administered 14C
chlorothalonil at a dose of 2.8 µ Ci/rat. The dose (1.5 mg) did not
significantly increase the daily input of chlorothalonil. After 264
hours, 95% of the material was recovered, predominantly in faeces
(88%) and urine (5%) with none detected in tissues or as CO2.
Chlorothalonil was not rapidly removed from the animals body (43% in
24 hours; 64% in 48 hours, and 76% in 72 hours) (Ryer and Sullivan,
1966).
Studies on the transformation of chlorothalonil in soil (Stallard
and Wolfe, 1967; Duane, 1970) have isolated and identified a
metabolite of chlorothalonil as
4-hydroxy-2,5,6-trichloroisophthalo-nitrile (DAC-3701).
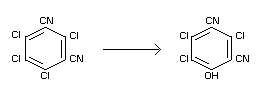 Residues and biotransformations of chlorothalonil in farm animals,
plants and soil are discussed in the sections "Residues resulting from
supervised trials" and "Fate of residues."
Pharmacological effects
Chlorothalonil has a cathartic effect which is apparently due to
irritation of the gastro-intestinal tract. This was evidenced by a
dose related decrease in urinary volume and increase in urine specific
gravity. Studies using rats subjected to levels of 0, 1500, and 15,000
ppm in the diet showed a dose related decrease in retention of an
orally administered dye indicative of a potential laxative effect.
Some experiments utilizing selected animals on feeding studies
suggested an increase in water intake, a decrease in urine volume, and
an increase in fecal moisture which was dose related especially at
high concentrations in the diet (Paynter, 1967c; Skinner and Stallard,
1967).
Chlorothalonil administered orally at one gm/kg to mice increased
intestinal mobility as measured by the percentage of the small
intestine traversed by a charcoal marker within a selected time
interval. The laxative action of chlorothalonil was significantly
reduced by pre-treatment with corn oil but not with atropine or
papaverine (Teeters, 1966).
Residue analyses of tissues and organs of animals fed
chlorothalonil in the diet indicated that there was no accumulation in
any body organ. Small quantities of the 4-hydroxy metabolite were
observed in liver and kidney with no parent compound noted (Wolfe and
Stallard, 1968a).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse
The potential genetic hazard of chlorothalonil was evaluated in
mice by the host mediated assay, in vivo cytogenic testing and the
dominant lethal test. The compound did not produce any measurable
mutagenic response when evaluated in vitro against eight indicator
organisms of the Salmonella typhimurium, histidine auxotroph tester
strains which can be reverted by both base substitution and frame
shift mutagens. In these tester strains, both repair deficient and
repair competent strains were used. When the tester strains were
inoculated into animals treated with chlorothalonil daily for five
days at an oral dose of 6.5 mg/kg (10 animals per treatment), no
increase in the number of mutations over the control were found. The
positive control, dimethylnitrosamine, increased mutation frequency in
mice (ip, 4 mg/kg).
Following oral administration of chlorothalonil daily for five
days at 6.5 mg/kg, an examination of bone marrow for the presence of
cells with micronuclei indicative of cytogenic abnormalities was
negative.
Treated orally for five days at 6.5 mg/kg, two male mice were
mated sequentially with untreated females in a standard dominant
lethal test. No adverse effect on fertility, implantation or foetal
mortality was observed. A positive control (triethylene melamine),
administered ip at 0.5 mg/kg, produced an increase in early death at
the post meiotic period. At the concentration tested in the three
procedures used, chlorothalonil did not produce mutagenic effects
(Legator, 1974).
Special studies on reproduction
Rat
Groups of rats (10 males and 20 females per group) were fed
chlorothalonil in the diet at levels of 0, 0.15, 1.5, and 3.0% in a
standard three generation, two litters per generation, reproduction
study. Because of food refusal and poor weight gain, the test using
the two higher levels was interrupted. After the first litter of pups
(F1a), the high level of treatment which had been reduced to 2% was
discontinued and a new dose level of 0.5% (with another control group)
was substituted. The full three generation reproduction test was
ultimately performed using dosage levels of 0, 0.15, 0.5 and 1.5% in
the diet. At the high discontinued dose level there was a significant
growth depression in nursing litters and an emaciated appearance in
the pups at weaning. There was also a reduction of fertility and
lactation at this maternally toxic level.
Growth suppression in pups was noted at all test intervals
through all three generations. It was considered that while smaller,
pups were still within a normal weight range, no malformations were
observed and reproduction indices were not affected. Necropsy
examination performed on parents and on the terminal F3b generation
revealed gross changes in the kidneys and in the G.I. tract. Gross
changes included enlargement and distention of the caecum and colon,
soft faecal matter, and occasional thickening of the stomach wall.
Microscopic changes were also evident in stomach and kidney.
Chlorothalonil examined at maternally toxic dietary levels did not
have an effect on reproduction in the rat (Paynter, 1967a).
Groups of rats (10 males and 20 females per group) were fed the
chlorothalonil metabolite, 4-hydroxy 2,5,6-trichloroisophthalonitrile,
in the diet at levels of 0, 10, 50, 100 and 200 ppm for 70 days prior
to mating in 3 generation, one litter per generation, reproduction
study. Growth was reduced, clinical chemistry changes (increased SGPT)
were noted and gross and microscopic changes observed at 200 mg/kg.
Reproduction was affected at 100 mg/kg as evidenced by reduced litter
size and weight as well as decreased survival. Milk analysis revealed
the 4-hydroxy metabolite in the stomach curd of 7 day pups in a
concentration comparable to that fed to the parents. No effects were
noted at 50 mg/kg on the reproduction parameters recorded (Hastings
and Jessup, 1974).
Special studies on teratogenicity
Rabbit
Groups of 8 pregnant rabbits were administered chlorothalonil
from day 8 to day 16 of gestation. The initial dose on days eight and
nine (0, 180, and 375 ml/kg/day) was reduced because of decreased food
consumption to 0, 62.5, and 31.25 mg/kg/day respectively for the
remainder of the study. Deaths were noted in the treated groups and
there was a severe weight loss with the treated dose. There was no
apparent effect on the embryo and while there was a considerable
effect on the adults, chlorothalonil is not considered to be a
teratogen (Paynter, 1966).
Acute toxicity
TABLE 1. Acute toxicity of chlorothalonil
Species Route LD50 mg/kg References
Rat Oral 10,000 Powers, 1965
Doyle and Elsea, 1963
Inhalation 4.7 mg/kg Beasley and Leong, 1965
Dog Oral > 5,000 Paynter, 1965a
Rabbit Dermal 10,000 Doyle and Elsea, 1963
Signs of poisoning include depression, diarrhoea and an unkempt
appearance. 3 mg crystalline technical product applied to the
conjunctival sac produced a mild irritation which was probably
mechanical (Doyle and Elsea, 1963).
Acute studies of the 4-hydroxy metabolite showed an oral LD50 in
male rats of 332 mg/kg. An LD50 could not be found in dogs because of
emesis at all dose levels (Wazeter, 1971; Wazeter and Goldenthal,
1972).
Short term studies
Rat (inhalation)
Groups of rats (15 males and 15 females per group) were exposed
to chlorothalonil by inhalation 6 hours/day, 5 days/week for three
weeks. This exposure was at a mean concentration of 12.2 mg/l at a
respirable particle size range of 1-5 microns (91%), 6-25 microns
(9%), and 26-40 microns (<1%). There were no deaths or untoward
behaviour among any of the animals. Growth was normal, and gross and
histological examinations did not reveal any compound-related effects
(Holliday et al., 1973).
Rat (feeding)
Groups of rats (10 males and 10 females per group) were fed diets
containing 5000 ppm chlorothalonil for 4 weeks. In an attempt to
determine the effect of chlorothalonil on absorption and utilization
of protein, fat, and amino acids, 10 amino acids varying in
concentration from 0.2 to 0.9% were added to the diet. On the basis of
a ten week feeding of dietary levels containing chlorothalonil plus
casein and corn oil or chlorothalonil plus amino acids, it was
concluded that chlorothalonil did not interfere directly with the
absorption and utilization of protein, fat, or amino acids. The
reduction in weight gain predominantly noted at this high feeding
level of chlorothalonil was presumably due to catharsis and not to
absorption difficulties (Paynter, 1967b).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of O, 250, 500, 750 and 1500 ppm
for 22 weeks. No mortality was observed and appearance and behaviour
were normal. Growth was slightly reduced at all feeding levels in
males and at 750 and 1500 ppm in the females. Food consumption was
comparable in all groups.
Haematological and urine analysis values were within normal
limits. An increase in liver-kidney weight was noted especially in
males at the two higher dosage levels. Kidney alterations,
characterized by irregular swelling of the tubular epithelium,
epithelial degeneration, and tubular dilation were seen in all test
groups with the males affected to a greater degree than females
(Blackmore and Shott, 1968).
Groups of rats (10 males and 10 females per group) were
administered chlorothalonil by oral gavage at dosage levels of 0, 0.5,
1.0, 2.0, 4.0 and 8.0 gm/kg five days a week for 13 weeks.
Administration of 4.0 gm/kg resulted in a slight, non-significant
reduction of growth, poor general condition, and reduced white blood
count. No significant findings were observed with regard to
elucidating the potential renal toxicity problem observed in longer
studies. Studies performed in this test indicated that chlorothalonil
did not result in a specific abnormality. It was suggested there might
be a decrease in resistance and defence mechanisms, making rats more
susceptible to naturally occurring infections (Sterner and Loveless,
1963).
Rabbit (dermal)
Groups of albino rabbits were daily administered a 75%
formulation of chlorothalonil to either intact or abraded skin, 5 days
per week, for three weeks. (Groups of two males and two females served
as controls; 5 males and 5 females were treated groups.) Animals were
administered chlorothalonil at dose levels of 0, 500, or 1000 mg/kg.
Repeated application of chlorothalonil to the intact or abraded
skin of rabbits resulted in dose-related dermal irritation consisting
of erythema, atonia, and desquamation. The degree of irritation was
more severe to the abraded skin. A number of animals at the high level
showed atypical haematological values in conjunction with diarrhoea
and/or dermal irritation as a result of application of chlorothalonil.
As might be expected, histological examination of the skin revealed
the presence of a moderate degree of acanthosis particularly in the
abraded skin areas, hyperkeratosis, rarely focal parakeratosis, and
slight to moderate leucocytic infiltration. No pathological
abnormalities were noted in other tissues (Paynter, 1965b).
Dog
Groups of dogs (4 males and 4 females per group) were fed
chlorothalonil in the diet at 0, 250, 500 and 750 ppm for sixteen
weeks. There was no mortality and no apparent effect on behaviour or
growth in any of the dogs tested. Haematological, clinical chemistry
and urine analysis values were normal with the exception of slightly
raised PBI values at high dose levels in females. Gross and
microscopic examination of organs and tissues did not reveal any
compound-related abnormalities (Paynter and Murphy, 1967).
Groups of beagle dogs (8 males and 8 females per group) were fed
chlorothalonil in the diet for two years at dosage levels of 0, 60 and
120 ppm. There were no effects noted on behaviour and growth over the
course of the study. Clinical chemistry values including haematology,
biochemistry and urine analysis were comparable to the controls at all
levels of feeding. Gross and microscopic examination of tissues and
organs performed on animals sacrificed at 12 months indicated a
compound-related change in the kidney. Further examination of tissues
and organs at 24 months did not show chlorothalonil-related
abnormalities. A slight degree renal tubule vacuolation in two of four
animals at 120 ppm after two years in the absence of other changes
(urinalyses values) was considered questionable especially as a slight
degree of vacuolation was noted in control as well as other treated
animals (Holsing and Voelker, 1970).
Long term studies
Rat
Groups of rats (35 males and 35 females per group; 70 males and
70 females were utilized for the control group) were fed
chlorothalonil in the diet for two years at levels of 0, 1500, 15,000,
and 30,000 ppm. Because of food refusal and poor weight gain at the
two highest dose levels, the compound was discontinued within one
week. The rats were fed basal diets for two weeks, after which
chlorothalonil administration was resumed and dietary levels increased
until the end of nine weeks when the intermediate group was fed 15,000
ppm. This intermediate group was then continued for the remainder of
the two year period. The high dose level was increased at biweekly
intervals until the sixteenth week when the group treatment was
terminated.
Growth suppression was observed at all levels and was
dose-related. This reduction in growth was reversible, as noted when
the high dose group recovered after being removed from the diet
containing chlorothalonil and fed a control diet. Haematological and
urine analysis values were within the normal range. PBI values were
generally reduced. Chlorothalonil had a cathartic action as evidenced
by increased water consumption and increased weight of faeces. Organ
weight and organ-to-body weight ratios increased for the liver and
kidney at the higher levels. Microscopic examination of the thyroid,
stomach, kidney, and liver revealed pathological changes.
A distinct alteration was produced in the squamous epithelium of
the cardiac portion of the stomach in most of the male and female
rats. The squamous epithelium was rather consistently thickened and
covered by a layer of keratin. In the kidneys of the higher dose
animals, the epithelium of the proximal convoluted tubules was paler
than usual and uniformly enlarged. At the termination of the study the
thyroid glands of the 15,000 ppm animals exhibited an increase in
epithelial pigmentation. Changes in the stomach at all levels included
acanthosis and hyperkeratosis.
At 13 weeks, the kidneys of both sexes fed 1500 ppm were similar
to those of the control. Gross and microscopic pathological
differences especially in males were, however, observed at the one and
two year intervals at this dose level. Liver changes, predominantly in
females, were characterized by an enlargement of the cells in the
nuclei, particularly in the central lobular area, and the formation of
large multinucleated cells in the pericentral area.
No increase in tumor formation was evident. Alterations in the
thyroid and the stomach appeared to be reversible; the alterations in
the liver were slight and confined to the females; the alterations in
the kidney were not reversible (Paynter, 1967c).
When samples of urine and faeces were taken for metabolism
studies (Skinner and Stallard, 1967) from rats from this long-term
study, data were reported for the volume of urine collected from the
rat over the period of sampling. The volume of urine was found to
decrease proportionately as the dietary dose increased. Conversely,
there appeared to be an increase in faecal excretion although the food
consumption remained constant.
Groups of rats (15 males and 15 females per group) were fed
chlorothalonil in the diet at levels of 0, 500, 1000, and 5000 ppm for
76 weeks. There were no apparent effects on behaviour and growth with
the exception of animals at the high level where food refusal was
noted. In a separate paired feeding study, there was no effect of
chlorothalonil on growth or behaviour. Increased liver weight, kidney
weight, and kidney to body weight ratio was apparent at the higher
test intervals. Microscopic examination indicated chiefly tubular
hypertrophy, epithelial irregularity and vacuolation. The degree of
kidney damage was dose-related and appeared to increase in severity in
males. No other adverse effects were noted in this study (Paynter and
Busey, 1967).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of 0 and 5000 ppm for two years.
Growth suppression in both males and females was evident throughout
the two year period. Survival was not affected and haematological,
biochemical and urine analysis values were within a normal range. A
cathartic effect of chlorothalonil was suggested as evidenced by
increased water consumption and increased weight of faecal excretion.
Organ weight and organ-to-body weight ratios, were increased for the
kidney and caecum. Histological examination of the kidneys at one year
again showed evidence of tubular hypertrophy and epithelial
alterations. No significant effects were noted with other tissues and
organs (Paynter and Crews, 1967).
Groups of rats (50 males and 50 females per group) were fed
chlorothalonil in the diet at levels of 0, 4, 10, 20, 30, 40 and 60
ppm for two years. No effects were seen on appearance, behaviour,
growth, food consumption or mortality. Haematological, clinical
chemistry and urine analysis values were normal. Animals sacrificed at
13, 52 and 104 weeks were examined for gross and microscopic defects.
A major lesion observed in studies at higher dose levels, necrosis of
the epithelial lining of the proximal tubules in the deep portion of
the cortex, was observed sporadically in this experiment at the one
and two year intervals. Vacuolation observed at 13 weeks predominantly
in females at 4 mg/kg and above, was not noted at later intervals.
There was no dose-related histological effect noted. A no-effect level
in this study would be 60 ppm (Holsing and Shott, 1970).
COMMENT
Chlorothalonil is rapidly excreted primarily unchanged. A
metabolite in plants and animals, the 4-hydroxy compound, is more
acutely toxic and more persistent than the parent molecule.
Studies performed at maternally toxic levels showed no effects of
chlorothalonil on reproduction. Growth retardation of pups was
believed to be the result of ingestion of treated diet. The results of
a reproduction study with the 4-hydroxy metabolite were negative at
low levels although secretion into the milk was observed. The results
of mutagenesis and teratogenesis tests were negative within the
parameters of the defined studies. Results of long and short term
chlorothalonil studies at high dietary levels to rats and dogs suggest
a toxicological problem associated with the kidney. Kidney changes
were characterized microscopically as hypertrophy, dilation,
cytoplasmic vacuolation, and hyperplasia of the epithelial cells of
proximal tubules and grossly as enlarged, greenish-brown granular
kidneys. No clinical effects were noted although some renal pathology
was suggested by a reduced urine volume. The significance of
histological changes in the kidney at lower dose levels needs
clarification.
No direct observations in man were reported.
Low-level feeding studies in rat and dog showed no effects in rat
at 60 ppm and in dog at 120 ppm forming the basis for allocating a
temporary ADI for man.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Rat: 60 ppm in the diet, equivalent to 3.0 mg/kg bw.
Dog: 120 ppm in the diet, equivalent to 3.0 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Chlorothalonil is a fungicide with broad spectrum activity. It is
effective against many fungus diseases which damage vegetables (rusts,
anthracnose, downy mildews, leaf spots, soft rot, leaf blight, scab,
early blight, late blight, pink rot, powdery mildew, etc.); agronomic
crops (downy mildew, leaf spots, leaf rust, brown spot, blue mold,
etc.), small fruits (anthracnose, gray mold powdery mildew, black rot,
downy mildew, ripe rot, leaf scorch, leaf blight, etc.); tree fruits
and nuts (scab, powdery mildew, black rot, white rot, fly speck, sooty
blotch, bitter rot, fire blight, blossom blight, leaf spot, melanose,
canker, greasy spot, leaf curl, etc.); tropical crops (signatoka,
black pod, coffee berry disease, leaf disease); turf and ornamental
crops. It has been tested on over 60 crops throughout the world and is
non-phytotoxic to most of these crop plants (Diamond Shamrock, 1974).
Literature available to the meeting further confirmed the fungicidal
effectiveness of chlorothalonil in both field and glasshouse tests
(DiDaris et al., 1965; Turner et al., 1964; Turner and Lamont, 1965).
Chlorothalonil is available as a wettable powder, dust and in
tablet form. The following formulations are produced.
Daconil 2787 W-75, a 75% wettable powder designed to be mixed
with water and applied as a spray. Registered for use in the
U.S.A. for turf and ornamentals and in 20 other countries for use
on a variety of crops (Diamond Shamrock, 1971).
Bravo W-75, a 75% wettable powder formulation registered for use
in the U.S.A. on agricultural crops.
Termil tollets - a tollet containing 8 g active ingredient
formulation to be vaporized by a suitable heat source at
temperatures of 315-425°C in a shallow pan.
Exotherm Termil - a powder containing 20% active ingredients
packaged in 100 g cans. Application is made by removing the lid
from the can and igniting the powder to generate smoke.
The recommended application rates for various crops using the
wettable powder formulation Daconil 2787 W-75 are as follows:
Application
Crop rate
Vegetable crops (asparagus, beans, 1000-3400 g/ha
broccoli, Brussels sprouts, cabbage,
cantaloupe, carrot, cauliflower, celery,
chinese cabbage, corn (sweet), pumpkin,
squash, tomato, turnip, watermelon)
Agronomic crops (hop, peanut, spearmint, 1000-2300 g/ha
sugar beet, tobacco)
Small fruits (blackberry, blueberry, 120-480 g/100 litres
currant, grape, raspberry, strawberry)
Tree fruits and nuts (apple, cherry, 120-360 g/100 litres
grapefruit, lemon, orange, peach,
pecan, pear, tangerine)
Tropical crops (banana, cacao, coffee, 240-480 g/100 litres
rubber)
Thermal dusting with Termil tollets or Exotherm Termil is used to
control a variety of diseases of ornamentals and vegetable crops
(asparagus, snap beans, cucumbers and tomatoes) grown in glasshouses,
at an application rate of 7-7.5 g a.i./100 cu.m. of space.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits and vegetables (Diamond Shamrock, 1974). A summary of much of
this information appears in Table 2 together with details of rates and
number of applications and pre-harvest intervals used. The majority of
these data are from trials in the U.S.A. with a few trials from
Canadian locations.
Chlorothalonil residues were detected on most aboveground crops
at the time of harvest. The higher residues occurred on leafy
vegetables, e.g. lettuce, escarole, chicory, spinach, celery and
crucifers including collards and kale, with generally lower levels on
melons, fruits and root crops. High residues were detected also in
lima bean plants, sugar beet tops and peanut hay which may be used for
animal feeds. The amount of fungicide applied, time interval between
last application and harvest, surface area, weight and surface
structure of the crop are factors that affect the level of the
residue. The residue level diminishes with time after application.
Glasshouse trials of thermal dusting were carried out on
cucumbers, leaf lettuce and tomatoes. The residue data are summarized
in Table 3. In general, the residue levels were low, <4 mg/kg,
following a pre-harvest interval of 1 day.
Only negligible residues of the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, DAC 3701 (see
"Biotransformation" and "Fate of Residues"), were detected in most
commodities. Only in the case of peaches were significant residues
(up to 0.5 mg/kg) detected routinely. Peanut hay and lima beans
(including pods) also sometimes showed residues of 0.5-1 mg/kg.
A series of experiments were performed to see if chlorothalonil
or the 4-hydroxy metabolite could be a residue in meat or milk from
feeding these products to cows. Groups of cows (4 cows/group) were fed
chlorothalonil plus the 4-hydroxy metabolite at dosage levels of 0, 25
ppm chlorothalonil plus 0.2 ppm metabolite, 75 ppm chlorothalonil plus
0.6 ppm metabolite, and 250 ppm chlorothalonil plus 2.0 ppm metabolite
for 30 days. Half the animals were sacrificed at the end of the test
and half after a 32 day recovery period. At a sensitivity of 0.02 ppm,
no chlorothalonil was found in milk (Wolfe and Stallard, 1969). A
small quantity of the metabolite was noted in milk: details are given
in Table 4. Residues in the milk reached maxima of 0.20, 0.26 and 0.78
respectively at the 3 feeding levels after about 18 days (Wolfe and
Stallard, 1970a). At the 30-day interval small residues of
chlorothalonil and the metabolite were seen in muscle, fat, liver and
kidney. Residues of the metabolite were respectively 0.51, 2.1, 0.93
and 3.7 mg/kg at the highest feeding level. No residues were seen
after the 32 days recovery period (Wolfe and Stallard, 1970b).
TABLE 2a. Residues of chlorothalonil from supervised trials.
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 12 13 1 9 0-0.7 0.15
(tops removed) 1.7 9 0-7 3 8-9 0.1-3.6 0.3-2.8
1.9 9 2 1 6 1-8-5.8 4.4
2.5 7-10 0-7 4 6-8 0-8.7 0.02-7.3
Broccoli 1.3 9 1-15 3 9 0.01-9.0 0.01-6.0
2.5 6-8 0-12 4 5-9 0-2.6 0-1.4
Brussels
sprouts 1.3 4-5 0-21 4 9-18 0.7-3.5 1.7-2.5
1.7 5 8 1 3 4.6-5.3 5.0
2.5 4-8 0-18 2 3,5 0.9-4.3 1.0-3.5
3.4 5 0-14 4 2-5 2.0-11 3.2-11
Cabbage 1.7 5-9 0 2 3,7 0.8-2.7 1.5-1.6
2.5 7-9 0-7 5 5-9 0-0.2 <0.01-0.4
Cauliflower 0.8 2 33 1 9 0.03-0.09 0.06
2.5 2-10 0-33 5 7-9 0-1.9 0.02-1.2
Cucumber 0.8 4-9 0-6 5 6-9 0-1.1 0.05-0.9
1.3 4-9 0-6 4 6-18 0-1.2 0.1-0.8
1.7 1-12 0-7 12 3-23 0-2.5 0.2-2.4
2.5 4-13 0-14 12 6-9 0-2.8 0.01-1.8
Summer squash 0.8 6-9 0-3 2 5,6 0.07-0.9 0-2-0.3
1.3 6-9 0-3 2 6 0.2-1.2 0.45-0.9
1.7 1-9 0-9 9 3-25 0-2.0 0-1-1.3
2.5 5 0-14 3 3 0.06-0.25 0.07-0.2
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Cantaloupe
(whole melons) 1.7 7-11 0-14 4 5-17 0-1.1 0.02-0.6
2.5 7 4 1 12 0.7-1.8 1.1
(pulp) 2.5 7 0 1 5 0.05-0.1 0.07
Muskmelon
(pulp) 1.3 11 0 1 3 0 0
(rind) 1.3 11 0 1 3 0.03-0.2 0.1
(pulp) 2.5 11 0 1 3 0 0
(rind) 2.5 11 0 1 3 1.4-1.7 1.5
Honeydew melon 1.7 10 0-14 3 5-8 0-1.6 0.03-0.7
2.5 7 0-14 3 6 0.07-0.4 0.1-0.25
Winter squash
(whole) 1.7 5-10 0 2 3 0.04-1.7 0.05-1.4
(pulp) 1.7 5-10 0 2 3,8 0 0
(whole) 2.5 7 7 1 3 0.8-2.3 1.4
Watermelon
(whole) 0.8 8-10 0-14 4 3 0-0.6 0.02-0.35
(rind) 1.3 7 14 1 3 0.2-0.4 0.3
(whole) 1.7 8-10 0-14 4 3 0.03-1.0 0.08-0.5
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(whole) 2.5 8-10 0-14 4 0.05-1.0 0.05-0.8
Pumpkin (whole) 1.7 10 0-7 3 3 0.8-1.8 0.8-1.4
(pulp) 1.7 10 0 1 2 0 0
Tomato 0.6 18 3 1 9 0-0.1 0.05
1.3 6-18 0-14 5 6-18 0.03-5.5 0.05-4.4
1.7 4-10 0-15 17 3-27 0.01-5.5 0.09-3.4
2.5 5-18 0-14 12 3-32 0.2-4.5 0.2-4.5
Peanuts (nuts) 0.8 6 78 1 3 0.03 0.3
1.3 4-12 4-78 11 4-24 0-0.3 0-0.09
(hulls) 1.3 4-12 4-55 9 3-12 0-0.8 0.09-0.5
(hay) 1.3 4-11 4-68 11 2-30 0.96 0.3-89
(nuts) 1.7 6 78 1 3 0.02-0.04 0.03
Potatoes
(whole) 0.8 10 12 2 9 0 0
(peeled) 0.8 13 15 1 6 0 0
(peelings) 0.8 13 15 1 3 0-0.01 0
Potatoes
(whole) 1.3 3-13 12-23 16 35-48 0-0.07 0-0.02
(peeled) 1.3 10-13 0-15 4 33 0-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(peelings) 1.3 13 15 1 4 0-0.05 0.01
(whole) 1.7 5-12 0-23 11 19-44 0-0.06 0-0.01
(peeled) 1.7 5-12 0-13 3 21 0 0
(peelings) 1.7 5-12 0-14 6 14 0-0.15 0.06
(whole) 2.5 10 14 1 9 0-0.01 0
Sugar beets
(roots) 1.3 4 61 1 10 0-0.02 0.01
1.7 4-6 14-41 9 9-27 0-0.5 0-0.3
(tops) 1.7 4-6 14-41 3 9 0-5-17 0.9-11
(roots) 2.5 3-6 14-59 5 5-12 0-1.2 0.02-0.5
(tops) 2.5 3-5 14-59 3 6-17 0.2-32 1.2-16
(roots) 3.4 5 23 1 6 0.03-0.09 0.05
Sweet corn
(kernels
and cob) 1.3 14 14 2 17 0 0
1.7 10 0 1 9 0-0.03 0
2.5 11 0-7 3 5-9 0.01-0.1 0.04
Snap beans 1.7 5-8 0-7 5 5-17 0.04-14 0.1-11
2.5 8 0-7 3 9 0.8-10 2-3-5.4
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Lima beans
(beans) 1.3 10 0 1 3 0-2-0.4 0.3
(plants) 1.3 10 0 1 9 220-535 400
(beans) 1.7 7 0-15 2 5-9 0-0.1 0-0.7
(pod + bean) 1.7 13 0 1 3 10-13 12
(plants) 1.7 4-13 0-15 3 2-9 22-310 47-117
Celery 0.8 5-24 0-14 6 8-9 0.2-21 0.4-18
1.3 9-24 0-14 5 6-9 0.1-52 0.4-29
1.4 8 7-14 2 6-8 0.06-1.7 0.1-1
1.7 9-24 0-14 7 6-9 0.1-53 0.7-34
1.8 8 7-14 2 6 0.1-5.4 0.3-3.4
1.9 26 7-14 2 6-8 0.2-17 0.6-12
2.5 5-14 0-14 5 8-9 0.6-17 1.4-10
3.4 10-18 1-14 4 6-9 2.8-26 4.5-18
Oranges (whole) 1.3 1-14 0-14 3 6 1.8-5.1 2.7-4.3
(peel) 1.3 1 173-397 5 20 0-0.7 0.1
Grapefruit
(whole) 0.4-0.8 1 309 2 6 0.01-0.02 0.01
1.3 1 200-309 3 11 0-0.05 0.02
(peel) 1.3 1-2 22-309 6 6-17 0-0.2 0.06-0.09
Limes (whole) 1.3 1 119-280 2 12 0-0.01 0
Lemons (whole) 1.3 1 280 1 4 0.01-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Tangerines
(whole) 1.3 1 280 1 6 0.01 0.01
Cherries 0.4 1-4 3-10 2 4-12 0-0.2 0.04-0.2
0.6 4 0-24 5 2-13 0.1-11 0.1-9.3
1.0 4 20 1 6 0.1-0.2 0.2
1.3 3-5 2-24 5 5-14 0.7-7.8 1.4-5.7
1.5 4-5 20-24 3 18 1-10 3.1-4.2
Peaches 0.6 4 8 1 3 6-8 6.7
0.8 4 8 1 6 3.3-6.3 5.0
1.0 7 0-14 3 3 6.4-19 8-15
1.3 4-11 6-14 3 3-12 2.8-20 4-16
1.5 6-7 0-25 11 3-18 1.3-45 4-31
1.7 4 8 1 3 27-28 28
2.5 4 8 1 3 32-54 45
Currants 1.0 4 3 1 6 16-20 18
1.3 4 3 1 6 16-20 18
1.5 4 3 1 6 20-23 23
Blackberries 1.3 2 4 1 12 4-11 7.3
1.7 2 23-48 2 12 0.5-2 1.2
1.9 2 4 1 12 3-16 9.6
2.5 2 0-16 3 9 0.3-8 2-4
3.0 2 0-16 4 7-12 0.5-43 1.6-21
3.4 2 0-16 3 9 1.3-9.3 2.5-4
Raspberries 0.4 2 4 1 6 0.05-1 0.3
0.8 2 4 1 6 0.2-2.4 1.2
1.3 2-3 0-8 3 6-12 0.4-6.5 0.7-4.1
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
1.5 2 0-8 2 6 0.6-2.7 0.7-1.7
1.7 3 0-7 3 3 5.6-20 5-7-15
Collards 2.5 3 0-14 4 9 3.3-88 5.7-69
Kale 2.5 3 0-14 4 6 1.5-71 2.8-62
Escarole 1.7 8 0-7 3 4-6 0.4-24 1.3-15
Endive 0.4 5 1 1 2 24-28 26
0.8 5 1 1 3 22-44 31
Chicory 1.7 8 0-7 3 3-5 0.04-30 0.3-24
Lettuce leaf 1.3 3 0 1 8 13-43 24
2.5 3-4 0-7 3 8-9 5-89 13-68
Lettuce head 1.7 4-7 0-14 5 2-12 0.1-100 1.3-86
Spinach 1.3 5 3-8 2 6 4-48 14-29
Turnip greens 1.7 2 2 1 7 5-10 7.2
Onions, green 1.7 3-4 0-7 3 6-8 0.6-11 1.1-7.6
2.5 3-4 0-7 3 6-7 1.3-24 2.0-18
Onions, mature 0.8-1.3 8-9 12-14 2 30 0-0.1 0-0.05
dry 1.7-2.5 5-9 7-9 2 105 0-0.3 0-0.1
Peppers 1.7 9-13 0-7 2 3-9 0.4-9.4 1.2-8.4
2.5 7 0-7 3 9 0.1-3.3 0.5-1.9
TABLE 2b. Residues of hydroxy-metabolite (DAC-3701) from supervised trials
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 8 0-7 2 3,5 0.01-0.06 0.01-0.04
(tops removed) 2.5 7 0 1 6 0.04 0.04
Broccoli 1.3 9 1-15 3 9 0 0
2.5 8 0 1 7 0-0.06 0.01
Brussels sprouts 2.5 8 0 1 5 0.01-0.02 0.02
Cabbage 2.5 9 0 1 6 0 0
Cucumber 0.8 7 0 1 6 0-0.03 < 0.01
1.7 7 0 1 6 0-0.03 < 0.01
2.5 6 0 1 3 0 0
Cantaloupe
(whole melons) 1.7 7 4 1 13 0.02-0.04 0.03
2.5 7 4 1 8 0.02-0.08 0.05
(pulp) 2.5 5 1 1 5 0.08-0.14 0.1
Watermelon
(rind) 1.3 7 14 1 3 0 0
Tomato 1.3 6 0 1 6 0-0.2 0.01
1.7 5 1 1 3 0.06-0.1 0.08
2.5 5-8 0-1 3 11 0.01-0.1 0.02-0.09
Peanuts (nuts) 1.3 4-12 4-55 6 10-20 0 0
(hulls) 1.3 4-12 6-55 5 3-9 0-0.05 0-0.02
TABLE 2b. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Peanuts
(hay) 1.3 4-11 4-55 6 9-33 0-0.6 0.03-0.4
Sugar beets
(roots) 2.5 3 25 2 9 0-0.01 0.01
(tops) 2.5 3 23 1 6 0 0
(roots) 3.4 5 23 1 6 0-0.01 0.01
Lima beans
(pod + bean) 1.7 4 3 1 3 0.8-1.1 1.0
Celery 0.8 20 0 1 4 0.05-0.1 0.05
1.4 8 7-14 2 2-5 0.05-0.2 0.09-0.15
Peaches 0.6 3 0-10 2 3 0.1-0.3 0.2
0.8 2-10 0-10 4 2-5 0-1-0.4 0.2-0.3
1.3 2-4 0-12 4 2-3 0.01-0.5 0.01-0.3
Lettuce head 1.3 4 1-7 2 4 0-0.04 0.01
1.7 4 21 1 6 0-0.04 0.02
Spinach 1.3 5 3 1 8 0.04-0.3 0.1
Turnip greens 1.7 2 2 1 5 0-0.02 0
Onions, mature 0.8-1.3 5-8 14 3-8 3-8 0.0.02 0-0.01
dry 1.7-2.5 8 - 3-5 3-5 0-0.2 0.1-0.2
TABLE 3. Chlorothalonil and DAC-3701 residues in glasshouse crops from supervised trials
Residues (ppm)
Days from No. of Range Means or
Crop and Rate No. of application No. of determinations individual range of
residue g/1000 ft3 applications to harvest trials per trial determinations) means/trial
Cucumbers
chlorothalonil 2 12-15 1 4 3 0.01-0.09 0.03-0.06
DAC-3701 2 12-15 1 4 3 0 0
Lettuce, leaf
chlorothalonil 2 5-12 1 7 3 0.05-3.6 0.05-3.0
Tomatoes
chlorothalonil 2 1-8 1 8 2-3 0.02-0.4 0.03-0.3
1.8 3-8 1 6 4 0.1-3.7 0.2-3.0
2 12-18 1 5 3 0.5-1.4 0.7-1.4
2 1-9 1 9 3 0-0.8 0-0.6
2 8-16 0.5 9 3 0.04-0.7 0.08-0.6
DAC-3701 2 1-8 1 8 2-3 0 0
TABLE 4. DAC-3701 residues (mg/kg) in cows milk during
feeding trial
Residue (mg/kg at 0.2*, 0.6** and 2.0*** ppm
DAC-3071 in diet
Days 0 0.2 0.6 2.0
0 < 0.03 < 0.03 < 0.03 < 0.03
2 < 0.03 < 0.03 < 0.03 0.06
4 0.04 0.04 0.06 0.16
8 0.04 0.08 0.10 0.51
14 < 0.03 0.13 0.16 0.57
18 < 0.03 0.20 0.26 0.74
22 < 0.03 0.11 0.17 0.78
26 < 0.03 0.11 0.13 0.59
30 < 0.03 0.16 0.26 0.74
37 < 0.03 0.06 0.12 0.55
44 < 0.03 0.04 0.16 0.16
51 < 0.03 < 0.03 < 0.03 0.06
60 < 0.03 < 0.03 < 0.03 < 0.03
* +25 ppm chlorothalonil
** +75 ppm chlorothalonil
*** +250 ppm chlorothalonil
Residues of chlorothalonil were not detected in the tissues of
dogs and rats fed levels in the diet of up to 30 000 mg/kg (Wolfe and
Stallard, 1968a) although small quantities of the hydroxy metabolite
were found in the liver and kidney.
No residues of chlorothalonil were detected in milk from a
Holstein cow fed the compound at the 5 ppm level in the ration for
four days (Gutenmann and Lisk, 1966). The method was sensitive to
about 0.03 ppm.
FATE OF RESIDUES
General comments
The disappearance and fate of chlorothalonil residues is
reasonably well documented. The only identified metabolite is the
4-hydroxy compound, DAC-3701, mentioned previously. Although
chlorothalonil residues decrease quite rapidly on plants, the actual
fate is unknown.
In animals
In the experiment by Gutenmann and Lisk (1966) mentioned above,
samples of milk, urine and faeces were collected throughout the
feeding period and for five days thereafter. No residues of
chlorothalonil, acid products of nitrile hydrolysis or conjugates of
acidic or phenolic derivatives were detected. In the same series of
experiments, chlorothalonil disappeared rapidly when incubated with
rumen juice at the 1 ppm level. Two unidentified metabolites were
formed. Feeding studies with dogs and rats showed that chlorothalonil
was rapidly eliminated in the faeces with negligible amounts in the
urine (Skinner and Stallard, 1967). The eliminated material was mostly
unchanged chlorothalonil. Analysis of tissue samples from rats and
dogs from chronic feeding studies of one and two years indicated no
detectable storage of chlorothalonil. A radio-tracer study with rats
confirmed that elimination of chlorothalonil was complete and there
was no significant tissue storage (Ryer and Sullivan, 1966; Skinner
and Stallard, 1967).
Dogs and rats were fed for 2 years on diets containing 1500,
15 000 and 30 000 mg/kg of chlorothalonil (Wolfe and Stallard, 1968a).
Residues were not detected in muscle tissue. Residues of
4-hydroxy-2,5,6-1,3-benzene-dicarbonitrile (DAC-3701) in kidney
tissues of dogs were <1.5 mg/kg and <3.5 mg/kg in the livers of
dogs and rats. Residue levels of the hydroxy metabolite were <0.25
mg/kg in the urine of the dogs and rats.
In the feeding experiment involving both chlorothalonil and
DAC-3701 (Wolfe and Stallard, 1969, 1970a, 1970b; see preceding
section and Table 4), the DAC-3701 residues in the milk accounted for
10-16% of the administered metabolite, but it was not possible to
determine whether part of the milk residue derived from the
chlorothalonil in the diet.
In plants
In studies with ring labelled 14C-chlorothalonil, Kunkel (1967a)
found no evidence of translocation from topical applications on
cucumber cotyledons, cucumber leaves, cucumber hypocotyls, bean
unifolilate leaves or tomato leaves. He also demonstrated that the
labelled compound was not translocated into the aerial parts of corn
or tomato plants when they were cultivated for 23 days in soil treated
with 14C-chlorothalonil. In another study, Kunkel (1967b) showed that
autoradiographic techniques did not demonstrate 14C-movement or
translocation within root systems of sweet corn, cucumber or tomato
grown in soil treated with ring-labelled chlorothalonil. Since
DAC-3701 is a soil metabolite of chlorothalonil and 14C activity was
not translocated from the soil, the experiments also showed that the
4-hydroxy metabolite is not translocated into the root system or
aerial parts of these plants. The residue data from supervised trials
(Table 2) showed that DAC-3701 is a residue following chlorothalonil
treatment. Of the crops tested for DAC-3701 residues, peaches
contained up to 0.5 mg/kg, spinach up to 0.3 mg/kg, onions up to 0.2
mg/kg, celery up to 0.2 mg/kg, cantaloupe up to 0.1 mg/kg and lima
bean plants up to 1 mg/kg. Negligible residues of DAC-3701 (<0.1 ppm)
were reported for carrots, tomatoes, peanuts (except peanut hay),
sugar beets, turnip greens, broccoli, Brussels sprouts, cabbage,
cucumber, watermelon and lettuce.
In soil
Tests conducted under both laboratory and field conditions
demonstrated that chlorothalonil is rapidly degraded in soil (Stallard
and Wolfe, 1967). In the laboratory experiments its half-life in the
types of soils tested ranged from 4 to over 40 days. The degradation
rate increased with increasing organic matter content, moisture
content and temperature but appeared to be independent of pH within
the range 6-8. Chlorothalonil was evidently not lost by
volatilization, since it disappeared rapidly from treated soil
incubated in tightly closed jars.
In the field trials, turf plots in 3 locations in the U.S.A. were
treated with chlorothalonil and the half-life of the chlorothalonil
residues ranged from 26-45 days. The data indicated a decreased
degradation rate during the winter months.
4-Hydroxy-2,5,6-trichloro-1,3-benzenecarbonitrile (DAC-3701) was
the major soil degradation product following treatment with 14C-ring
labelled chlorothalonil and incubation (Duane, 1970). This compound
accounted for over 80% of the radioactivity extracted from the soil.
(Approximately 20% of the radioactivity was not extracted.) A second
degradation product, more polar than DAC-3701 and without a hydroxyl
group, was detected but not identified. During these experiments it
was shown that 14C-chlorothalonil was not volatilized from the soil
and no volatile degradation products were formed.
Laboratory experiments with 5 representative soil types
demonstrated that the half-life values for DAC-3701 ranged from 36
days in a sandy loam type soil to 220 days in clay type soil (Wolfe
and Stallard, 1968b).
Duane (1970) demonstrated that bacteria isolated from soil were
capable of metabolizing chlorothalonil in culture media. Thus it may
be assumed that naturally occurring soil microorganisms are in part
responsible for the rapid loss of chlorothalonil under field
conditions.
In storage and processing
Some data are available on the effect of washing, field trimming
and peeling on the residue levels of chlorothalonil in some
commodities (Diamond Shamrock, 1974). Table 5 summarizes the data on
the effect of washing with water. Residues on carrots, cucumbers,
summer squash, tomatoes, currants and cranberries were reduced by
more than 50%. Washing was least effective with the leafy vegetables.
Significant reduction in chlorothalonil residues results from trimming
cabbage and head lettuce (Table 6). Chlorothalonil residue are
concentrated on the surface and as a result peeling removes
almost all the residues leaving the pulp of many fruits and
vegetables almost residue-free (Table 7). Following processing,
residues of chlorothalonil were not detected ( <0.1 mg/kg) in canned
spinach (Table 8). The fate of any DAC-3701 residues was not
determined.
TABLE 5. Effect of washing with water on residues of
chlorothalonil
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Carrots 4.39-7.34 1.05-3.53 52-76
Cucumbers 0.08-0.45 0.01-0.03 87-95
Squash, summer 0.21-1.33 0.01-0.12 76-97
Tomatoes 0.21-0.82 0.02-0.08 90
Celery 1.04-20.0 0.76-8.18 0-59
Peaches 7.6-15.0 4.9-8.6 35-45
Currants 18.3 2.6 86
Cranberries 0.91 0.25 73
TABLE 5. (Cont'd.)
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Collards 5.73-69.0 1.7-31.3 31-70
Kale 2.8-62.0 1.6-20.0 43-74
Escarole 1.32-4.1 0.63-2.6 36-44
Chicory 0.31-4.0 0.23-1.5 26-62
Lettuce 23.7-67.6 8.9-37.1 45-70
Cauliflower 1.23 0.85 30
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not available to the Meeting to indicate the level and
incidence of chlorothalonil residues in food moving in commerce or in
food at the time of consumption. Chlorothalonil residues were not
determined in the U.S.A. national food and feed monitoring program
(Duggan and Cook, 1971).
METHODS OF RESIDUE ANALYSIS
Residues of chlorothalonil and its metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile DAC-3701), can be
determined by gas-liquid chromotography (GLC) with either electron
capture or microcoulometric (halide) detectors (Wolfe and Stallard,
1970). The parent compound is chromatographed directly but the
metabolite is converted to the methyl ether by reacting with
diazomethane prior to chromatography.
Crop and soil samples are extracted with acidified acetone. The
acetone is removed by distillation and the residues are partitioned
into diethyl ether. The ether extract is cleaned-up on a Florisil
chromatographic column. Chlorothalonil is eluted with 5%
acetone-dichloromethane and DAC-3701 with 50% acetone-dichloromethane.
Following reaction with diazomethane, the methyl ether of DAC-3701 and
chlorothalonil are determined by GLC either by electron capture or
microcoulometric detection. The sensitivity of the method is
TABLE 6. Effect of trimming on residues of chlorothalonil in cabbage and lettuce
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop kg/ha applications to harvest determinations (mg/kg) (mg/kg)
Cabbage
(field trimmed) 1.7 9 0 7 0.77-2.67 1.60
1.7 5 0 3 1.25-1.72 1.47
(market trimmed) 2.5 7-8 0-7 7-9 0.00-0.18 <0.01-0.03
Lettuce
(whole untrimmed
heads) 1.7 4-7 0-7 2-12 11.5-100 33.7-85.5
1.7 4 14 11 0.10-3.0 1.33
(trimmed heads) 1.3 5 1-7 4 0.15-1.98 0.53-0.95
1.7 4 2-14 6 0.07-2.9 0.80-0.90
TABLE 7. Effect of peeling on chlorothalonil residues
Crop Residues (mg/kg)
Cucumbers Peelings - 1.26; Pulp - <0.01
Muskmelon Rind - 0.11-1.50; Pulp - 0.00
Winter squash Whole - 0.05-1.42; Pulp - 0.00
Pumpkin Whole - 1.42; Pulp - 0.00
Cantaloupe Whole - 1.09; Edible portion - 0.07
Peanut Hulls - 0.13-0.51; Meat - 0.00
Potatoes Peelings - 0.00-0.06; Pulp - 0.00
Lima beans Pod and beans - 11.9; Beans - 0.00
Grapefruit Peel - 0.09; Whole - 0.02
Oranges Whole - 2.70-4.29; Pulp - <0.01-0.08;
Juice - 0.00
TABLE 8. Effect of canning on chlorothalonil residues in spinach
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop % applications to harvest analysis (ppm) (ppm)
Spinach
(raw) control 0 - 6 <0.1 <0.1
0.11 5 3 6 16.1-47.8 29.1
0.11 5 8 6 3.7-30.9 13.6
(processed,
canned) control 0 - 3 <0.1 <0.1
0.11 4 16 3 <0.1 <0.1
0.02 mg/kg. Recovery values obtained from fortified crops were greater
than 72 and 76% for chlorothalonil and DAC-3701 respectively. Some
modifications to the method described above are required for animal
tissue (Wolfe and Stallard, 1970b) and milk samples (Wolfe and
Stallard, 1969, 1970c). Average, recoveries for chlorothalonil and
DAC-3701 respectively were:
muscle - 81, 91%
fat - 76, 91%
kidney - 82, 84%
liver - 93 83%
milk - 88, 83%
The sensitivity of the method for milk is 0.02 mg/kg for
chlorothalonil and 0.03 mg/kg for DAC 3701. Chlorothalonil is not
recovered by the Mills Florisil multi-residue procedure but is
recovered (McMahon et al., 1973) in the alternative Florisil elution
system (Mills et al., 1972). Recovery from deactivated Florisil
(Osadchuk et al., 1971) is achieved by elution with 10% ethyl acetate
in hexane (McLeod and Ritcey, 1973).
Chlorothalonil can be recovered through the carbon-cellulose
cleanup procedure of McLeod et al. (1967). Gutenmann and Lisk (1966)
determined chlorothalonil by electron capture GLC in milk, urine and
faeces after extracting with acetone-phosphoric acid and partitioning
into hexane. Possible acid metabolites were determined after
diazomethane esterification of the evaporated acetone extract.
With the exception of the Mills Florisil multiresidue procedure,
the multiresidue procedures discussed above appear to be suitable for
regulatory determination of the parent compound. When the total
residue, chlorothalonil and DAC-3701, is required the GLC method
proposed by Wolfe and Stallard (1970c) can be recommended for
regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Some examples of national tolerances were reported to the Meeting
and are listed in Table 9.
TABLE 9. Examples of national tolerances as reported to the Meeting
Tolerance
Count Commodity (mg/kg)
Australia Beets, carrots, corn, cucumbers,
onions, peppers, potatoes,
tomatoes 7
Peanuts 0.2
TABLE 9. (cont'd)
Tolerance
Count Commodity (mg/kg)
Canada Celery 15
Broccoli, Brussels sprouts,
cabbage, cauliflower, cucumbers,
melons, pumpkins, snap beans,
squash, tomatoes 5
Carrots 1
Peanuts 0.3
Netherlands Potatoes 0.05
Apples, apricots, beets, carrots,
citrus, corn, cucumbers, grapes,
melons, onions, peaches, pears,
peppers, plums, tomatoes 0.01
Switzerland Potatoes 0.05
U.S.A. Celery 15*
Broccoli, Brussels sprouts, cabbage,
cauliflower, cucumbers, melons,
pumpkins, snap beans, squash
(summer and winter), tomatoes 5*
Carrots, sweet corn (kernels plus
cob with husks removed) 1*
Peanuts 0.3*
Potatoes 0.1*
* Including metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzen-edicarbonitrile.
APPRAISAL
Chlorothalonil is a broad-spectrum fungicide with effective
action against many fungus diseases which damage vegetable, tree,
small fruit and other agricultural crops, turf and ornamentals. The
use pattern of chlorothalonil is such that residues remain on most
above-ground crops at the time of harvest. It is desirable to have
some residue of the fungicide on the mature crop to protect it from
disease organisms during shipment.
4-Hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile is a major
metabolite of chlorothalonil in soil and a metabolite in plants, but
only negligible residues were found on most crops investigated.
Chlorothalonil does not translocate into plants from the soil or from
topical application.
Extensive crop residue data indicated that chlorothalonil is a
relatively persistent fungicide. The level of the residue depends on
such factors as rate of the fungicide applied, time interval between
last application and harvest and the surface area, weight and surface
structure of the crop. The residue level diminishes with time after
application, and pre-harvest intervals are therefore recommended for
some crops. Some data are available on the effects of washing,
trimming and peeling on chlorothalonil residues.
Chlorothalonil residues do not occur in the tissues or milk of
cows, but residues of the 4-hydroxy metabolite are found when cows are
fed chlorothalonil and the 4-hydroxy compound together in their
ration. The residue levels depend on the levels fed and reach a steady
value in the milk after 18 days. Residues in milk declined to below
detection level within 21 days after withdrawal. It was not determined
whether residues of the 4-hydroxy metabolite would occur in the milk
and tissues of cows if only chlorothalonil were ingested.
Since no data were provided on residues in crops that may be fed
to animals, it was not possible to recommend maximum residue limits
for milk and meat.
Most available multi-residue GLC methods appear to be suitable
for the determination of the parent compound, but chlorothalonil is
not recovered by the original Mills Florisil multi-residue cleanup
procedure (McMahon et al., 1973). The GLC method proposed by Wolfe
and Stallard (1970) for chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile appears to be
suitable for regulatory purposes when the total residue is required.
National tolerances are in effect in a number of countries.
RECOMMENDATIONS
The following maximum residue limits are recommended for
chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, expressed as
chlorothalonil.
TEMPORARY TOLERANCES
Pre-harvest
interval on which
Limit recommendations
Commodity (mg/kg) are based (days)
Peaches 30 7
Currants (black, red and white) 25 3
Celery 15 7
Peppers 10 1
Blackberries, raspberries, 10 7
cherries, chicory sprouts
Collards, kale, endive, 10 14
lettuce (head)
Broccoli, Brussels sprouts, 5 7
cabbage, cauliflower, beans
(green including pod), oranges,
onions, cranberries
Cucumbers, melons, pumpkins, 5 1
squash, tomatoes
Carrots, sweet corn, sugar 1 1
beets
Lima beans, peanuts (whole) 0.5 0
Peanuts (kernel), potatoes 0.1 0
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Additional study to resolve lower limit of kidney effects in rat.
2. Define growth reduction in pups relative to dietary ingestion or
secretion into milk.
3. Data on residues of chlorothalonil and the 4-hydroxy metabolite
in crops that may be fed to animals.
4. The results of feeding studies on dairy cattle understood to be
in progress to determine the level and nature of residues in milk
and tissues.
DESIRABLE
1. Observations in man.
2. Residue data for food moving in commerce.
3. Further information on effects of processing, including household
cooking, on residues.
REFERENCES
Beasley, A. and Leong, K. (1965). Acute inhalation exposure - rats.
Report from Hazelton Laboratories Inc., submitted by The Diamond
Shamrock Chemical Co. (Unpublished)
Blackmore, R. and Shott, L. (1968). Final report four month feeding
study - rats. Report from Hazelton Laboratories, Inc. (Unpublished)
Diamond Shamrock (1971). Documentation on the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Diamond Shamrock (1974). Documentation concerning the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Di Dario, A., Curry, T.L., Thayer, P. and Turner, J.J. (1965). The
biological performance of tetrachloroisophthalonitrile as influenced
by particle size and crystalline form. Phytopathology, 55:1055.
Doyle, R. and Elsea, J. (1963). Acute oral, dermal and eye toxicity
and irritation studies on DAC-2787. Report from Hill Top Research
Institute Inc., submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Duane, W.C. (1970). Biodegradation of Daconil 2787(R). Report
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Duggan, R.E. and Cook, H.R. (1971). National food and feed monitoring
Program. Pestic. Monit. J., 5:37-43.
Gutenmann, W.H. and Lisk, D.J. (1966). Metabolism of Daconil and
Dacthal pesticides in lactating cows. J. Dairy Sci., 49:1272-1276.
Hastings, T.F. and Jessup, D.C. (1974). 3-Generation reproduction
study in albino rats using DAC-3701 in the diet. Report from BIO/TOX
Research Laboratories, Inc. Submitted to WHO by Diamond Shamrock
Chemical Co. (Unpublished)
Holliday, W., Schadeberg, K., Goode, J. and Keplinger, M. (1973).
Twenty-one day subacute aerosol inhalation toxicity study with Bravo
6F(R) in albino rats. Report from Industrial Bio Test Laboratories,
Inc. (Unpublished)
Holsing, G. and Shott, L. (1970). Two year dietary administration
- rats. Report from Hazelton Laboratories Inc. submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Holsing, G. and Voelker, R. (1970). Final report 104 week dietary
administration - dogs. Report from Hazelton Laboratories Inc.
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967a). Absence of 14C movement in crop plant organs
after topical application and soil amendment studies with isotopic
Daconil 2787. Report to Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967b). Movement of 14C in or on roots of crop species
grown in soil amended with isotopic Daconil 2787. Report to Diamond
Shamrock Chemical Co. (Unpublished)
Legator, M. (1974). Mutagenic Testing with DAC 2787. Report from
Division of Genetics, Roger Williams General Hospital and Brown
University, Division of Biological and Medical Sciences submitted by
Diamond Shamrock Chemical Co. (Unpublished
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967).
Comparison of various carbon adsorbents and quantitative elution and
separation of forty-two pesticides from a carbon-Solka Floc cleanup
column. J. Ass. off. analyt. Chem., 50:1216-1228.
McLeod, H.A. and Ritcey, W.R. (1973). Analytical methods for pesticide
residues in foods. Information Canada, Ottawa, Canada.
McMahon, B.M., Sawyer, L.D. and Corneliussen, P.E. (editors) (1973).
Pesticide analytical manual. Volume 1. Methods which detect multiple
residues. Food and Drug Administration, Washington, D.C.
Mills, P.A., Bong, B.A., Kamps, L.R. and Burke, J.A. (1972). Elution
solvent system for Florisil column cleanup in organochlorine pesticide
residue analyses. J. Ass. off. analyt. Chem., 55:39-43.
Osadchuk, M., Romach, M. and McCully, K.A. (1971). Cleanup and
separation procedures for multi-pesticide residue analysis in
monitoring and regulatory laboratories. Proc. Second Intern. IUPAC
Congress Pestic. Chem. Vol. IV, Methods in Residue Analysis, p.
357-383. A.S. Tahori (editor), Gordon and Breach Science Publishers,
New York.
Paynter, O. (1965a). Oral dose range - dogs - final report. Report of
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1965b). Repeated dermal application - rabbits. Report
from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1966). Reproduction - rabbit. Report from Hazelton
Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1967a). Three generation reproduction study - rats.
Report from Hazelton Laboratories Inc. submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1967b). Ten week amino acid feeding study - rats. Report
from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. (1967c). Final report - two year dietary feeding - rats.
Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Busey, W. (1967). Long term (76 weeks) feeding study,
rats, DAC 2787. Final Report from Hazelton Laboratories Inc.
(Unpublished)
Paynter, O. and Crews, L. (1967). Final report - two year dietary
feeding - rats. Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Murphy, J. (1967). Sixteen week dietary feeding -
dogs. Report from Hazelton Laboratories Inc., submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Powers, M. (1965). Acute oral administration - rats. Report from
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Ryer, F.H. and Sullivan, J.B. (1966). Radiotracer metabolism study.
Report from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Skinner, W.A. and Stallard, D.E. (1967). Daconil 2787(R) animal
metabolism studies. Report from and submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Stallard, D.E. and Wolfe, A.L. (1967). The fate of
2,4,5,6-tetrachloroisophthalonitrile (Daconil 2787(R)) in soil.
Report from Diamond Shamrock Chemical Co. (Unpublished)
Sterner, W. and Loveless, L. (1963). A study of the subacute toxicity
of tetrachloroisophthalonitrile to rats. Report from International
Bio-Research, Inc. (Unpublished)
Teeters, W. (1966). In vivo assay of spasmodic activity, mice, DAC
2787. Final report from Hazelton Laboratories Inc. (Unpublished)
Turner, N.J. and Lamont, D. (1965). Control of fungal diseases in the
greenhouse with thermally induced dusts of
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
23:51-54.
Turner, N.J., Limpel, L.E., Battershell, R.D., Bluestone, H., Lamont,
D. (1964). A new foliage protectant fungicide,
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
22:303-310.
Wazeter, F. (1971). Acute oral LD50 in male albino rats. Report from
International Research and Development Corporation. (Unpublished)
Wazeter, F. and Goldenthal, E. (1972). Acute oral toxicity in beagle
dogs. Report from International Research and Development Corporation.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968a). Analysis of tissues and organs
for storage of the Daconil metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968b). The fate of DAC-3701
(4-hydroxy-2,5,6-trichloroisophthalonitrile) in soil. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1969). Residues in milk from cows fed
2,4,5,6-tetra-chloroisophthalonitrile. Diamond Shamrock Chemical Co.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970a). Residues in milk from cows fed
2,5,6-trichloro-4-hydroxyisophthalonitrile. Diamond Shamrock Chemical
Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970b). Residues in tissues of dairy
cows fed Daconil 2787 and 2,5,6-trichloro-4-hydroxyisophthalonitrile.
Diamond Shamrock Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970c). Analytical method for
determination of Daconil 2787 and DAC-3701 residues. Diamond Shamrock
Chemical Co. (Unpublished)
Residues and biotransformations of chlorothalonil in farm animals,
plants and soil are discussed in the sections "Residues resulting from
supervised trials" and "Fate of residues."
Pharmacological effects
Chlorothalonil has a cathartic effect which is apparently due to
irritation of the gastro-intestinal tract. This was evidenced by a
dose related decrease in urinary volume and increase in urine specific
gravity. Studies using rats subjected to levels of 0, 1500, and 15,000
ppm in the diet showed a dose related decrease in retention of an
orally administered dye indicative of a potential laxative effect.
Some experiments utilizing selected animals on feeding studies
suggested an increase in water intake, a decrease in urine volume, and
an increase in fecal moisture which was dose related especially at
high concentrations in the diet (Paynter, 1967c; Skinner and Stallard,
1967).
Chlorothalonil administered orally at one gm/kg to mice increased
intestinal mobility as measured by the percentage of the small
intestine traversed by a charcoal marker within a selected time
interval. The laxative action of chlorothalonil was significantly
reduced by pre-treatment with corn oil but not with atropine or
papaverine (Teeters, 1966).
Residue analyses of tissues and organs of animals fed
chlorothalonil in the diet indicated that there was no accumulation in
any body organ. Small quantities of the 4-hydroxy metabolite were
observed in liver and kidney with no parent compound noted (Wolfe and
Stallard, 1968a).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse
The potential genetic hazard of chlorothalonil was evaluated in
mice by the host mediated assay, in vivo cytogenic testing and the
dominant lethal test. The compound did not produce any measurable
mutagenic response when evaluated in vitro against eight indicator
organisms of the Salmonella typhimurium, histidine auxotroph tester
strains which can be reverted by both base substitution and frame
shift mutagens. In these tester strains, both repair deficient and
repair competent strains were used. When the tester strains were
inoculated into animals treated with chlorothalonil daily for five
days at an oral dose of 6.5 mg/kg (10 animals per treatment), no
increase in the number of mutations over the control were found. The
positive control, dimethylnitrosamine, increased mutation frequency in
mice (ip, 4 mg/kg).
Following oral administration of chlorothalonil daily for five
days at 6.5 mg/kg, an examination of bone marrow for the presence of
cells with micronuclei indicative of cytogenic abnormalities was
negative.
Treated orally for five days at 6.5 mg/kg, two male mice were
mated sequentially with untreated females in a standard dominant
lethal test. No adverse effect on fertility, implantation or foetal
mortality was observed. A positive control (triethylene melamine),
administered ip at 0.5 mg/kg, produced an increase in early death at
the post meiotic period. At the concentration tested in the three
procedures used, chlorothalonil did not produce mutagenic effects
(Legator, 1974).
Special studies on reproduction
Rat
Groups of rats (10 males and 20 females per group) were fed
chlorothalonil in the diet at levels of 0, 0.15, 1.5, and 3.0% in a
standard three generation, two litters per generation, reproduction
study. Because of food refusal and poor weight gain, the test using
the two higher levels was interrupted. After the first litter of pups
(F1a), the high level of treatment which had been reduced to 2% was
discontinued and a new dose level of 0.5% (with another control group)
was substituted. The full three generation reproduction test was
ultimately performed using dosage levels of 0, 0.15, 0.5 and 1.5% in
the diet. At the high discontinued dose level there was a significant
growth depression in nursing litters and an emaciated appearance in
the pups at weaning. There was also a reduction of fertility and
lactation at this maternally toxic level.
Growth suppression in pups was noted at all test intervals
through all three generations. It was considered that while smaller,
pups were still within a normal weight range, no malformations were
observed and reproduction indices were not affected. Necropsy
examination performed on parents and on the terminal F3b generation
revealed gross changes in the kidneys and in the G.I. tract. Gross
changes included enlargement and distention of the caecum and colon,
soft faecal matter, and occasional thickening of the stomach wall.
Microscopic changes were also evident in stomach and kidney.
Chlorothalonil examined at maternally toxic dietary levels did not
have an effect on reproduction in the rat (Paynter, 1967a).
Groups of rats (10 males and 20 females per group) were fed the
chlorothalonil metabolite, 4-hydroxy 2,5,6-trichloroisophthalonitrile,
in the diet at levels of 0, 10, 50, 100 and 200 ppm for 70 days prior
to mating in 3 generation, one litter per generation, reproduction
study. Growth was reduced, clinical chemistry changes (increased SGPT)
were noted and gross and microscopic changes observed at 200 mg/kg.
Reproduction was affected at 100 mg/kg as evidenced by reduced litter
size and weight as well as decreased survival. Milk analysis revealed
the 4-hydroxy metabolite in the stomach curd of 7 day pups in a
concentration comparable to that fed to the parents. No effects were
noted at 50 mg/kg on the reproduction parameters recorded (Hastings
and Jessup, 1974).
Special studies on teratogenicity
Rabbit
Groups of 8 pregnant rabbits were administered chlorothalonil
from day 8 to day 16 of gestation. The initial dose on days eight and
nine (0, 180, and 375 ml/kg/day) was reduced because of decreased food
consumption to 0, 62.5, and 31.25 mg/kg/day respectively for the
remainder of the study. Deaths were noted in the treated groups and
there was a severe weight loss with the treated dose. There was no
apparent effect on the embryo and while there was a considerable
effect on the adults, chlorothalonil is not considered to be a
teratogen (Paynter, 1966).
Acute toxicity
TABLE 1. Acute toxicity of chlorothalonil
Species Route LD50 mg/kg References
Rat Oral 10,000 Powers, 1965
Doyle and Elsea, 1963
Inhalation 4.7 mg/kg Beasley and Leong, 1965
Dog Oral > 5,000 Paynter, 1965a
Rabbit Dermal 10,000 Doyle and Elsea, 1963
Signs of poisoning include depression, diarrhoea and an unkempt
appearance. 3 mg crystalline technical product applied to the
conjunctival sac produced a mild irritation which was probably
mechanical (Doyle and Elsea, 1963).
Acute studies of the 4-hydroxy metabolite showed an oral LD50 in
male rats of 332 mg/kg. An LD50 could not be found in dogs because of
emesis at all dose levels (Wazeter, 1971; Wazeter and Goldenthal,
1972).
Short term studies
Rat (inhalation)
Groups of rats (15 males and 15 females per group) were exposed
to chlorothalonil by inhalation 6 hours/day, 5 days/week for three
weeks. This exposure was at a mean concentration of 12.2 mg/l at a
respirable particle size range of 1-5 microns (91%), 6-25 microns
(9%), and 26-40 microns (<1%). There were no deaths or untoward
behaviour among any of the animals. Growth was normal, and gross and
histological examinations did not reveal any compound-related effects
(Holliday et al., 1973).
Rat (feeding)
Groups of rats (10 males and 10 females per group) were fed diets
containing 5000 ppm chlorothalonil for 4 weeks. In an attempt to
determine the effect of chlorothalonil on absorption and utilization
of protein, fat, and amino acids, 10 amino acids varying in
concentration from 0.2 to 0.9% were added to the diet. On the basis of
a ten week feeding of dietary levels containing chlorothalonil plus
casein and corn oil or chlorothalonil plus amino acids, it was
concluded that chlorothalonil did not interfere directly with the
absorption and utilization of protein, fat, or amino acids. The
reduction in weight gain predominantly noted at this high feeding
level of chlorothalonil was presumably due to catharsis and not to
absorption difficulties (Paynter, 1967b).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of O, 250, 500, 750 and 1500 ppm
for 22 weeks. No mortality was observed and appearance and behaviour
were normal. Growth was slightly reduced at all feeding levels in
males and at 750 and 1500 ppm in the females. Food consumption was
comparable in all groups.
Haematological and urine analysis values were within normal
limits. An increase in liver-kidney weight was noted especially in
males at the two higher dosage levels. Kidney alterations,
characterized by irregular swelling of the tubular epithelium,
epithelial degeneration, and tubular dilation were seen in all test
groups with the males affected to a greater degree than females
(Blackmore and Shott, 1968).
Groups of rats (10 males and 10 females per group) were
administered chlorothalonil by oral gavage at dosage levels of 0, 0.5,
1.0, 2.0, 4.0 and 8.0 gm/kg five days a week for 13 weeks.
Administration of 4.0 gm/kg resulted in a slight, non-significant
reduction of growth, poor general condition, and reduced white blood
count. No significant findings were observed with regard to
elucidating the potential renal toxicity problem observed in longer
studies. Studies performed in this test indicated that chlorothalonil
did not result in a specific abnormality. It was suggested there might
be a decrease in resistance and defence mechanisms, making rats more
susceptible to naturally occurring infections (Sterner and Loveless,
1963).
Rabbit (dermal)
Groups of albino rabbits were daily administered a 75%
formulation of chlorothalonil to either intact or abraded skin, 5 days
per week, for three weeks. (Groups of two males and two females served
as controls; 5 males and 5 females were treated groups.) Animals were
administered chlorothalonil at dose levels of 0, 500, or 1000 mg/kg.
Repeated application of chlorothalonil to the intact or abraded
skin of rabbits resulted in dose-related dermal irritation consisting
of erythema, atonia, and desquamation. The degree of irritation was
more severe to the abraded skin. A number of animals at the high level
showed atypical haematological values in conjunction with diarrhoea
and/or dermal irritation as a result of application of chlorothalonil.
As might be expected, histological examination of the skin revealed
the presence of a moderate degree of acanthosis particularly in the
abraded skin areas, hyperkeratosis, rarely focal parakeratosis, and
slight to moderate leucocytic infiltration. No pathological
abnormalities were noted in other tissues (Paynter, 1965b).
Dog
Groups of dogs (4 males and 4 females per group) were fed
chlorothalonil in the diet at 0, 250, 500 and 750 ppm for sixteen
weeks. There was no mortality and no apparent effect on behaviour or
growth in any of the dogs tested. Haematological, clinical chemistry
and urine analysis values were normal with the exception of slightly
raised PBI values at high dose levels in females. Gross and
microscopic examination of organs and tissues did not reveal any
compound-related abnormalities (Paynter and Murphy, 1967).
Groups of beagle dogs (8 males and 8 females per group) were fed
chlorothalonil in the diet for two years at dosage levels of 0, 60 and
120 ppm. There were no effects noted on behaviour and growth over the
course of the study. Clinical chemistry values including haematology,
biochemistry and urine analysis were comparable to the controls at all
levels of feeding. Gross and microscopic examination of tissues and
organs performed on animals sacrificed at 12 months indicated a
compound-related change in the kidney. Further examination of tissues
and organs at 24 months did not show chlorothalonil-related
abnormalities. A slight degree renal tubule vacuolation in two of four
animals at 120 ppm after two years in the absence of other changes
(urinalyses values) was considered questionable especially as a slight
degree of vacuolation was noted in control as well as other treated
animals (Holsing and Voelker, 1970).
Long term studies
Rat
Groups of rats (35 males and 35 females per group; 70 males and
70 females were utilized for the control group) were fed
chlorothalonil in the diet for two years at levels of 0, 1500, 15,000,
and 30,000 ppm. Because of food refusal and poor weight gain at the
two highest dose levels, the compound was discontinued within one
week. The rats were fed basal diets for two weeks, after which
chlorothalonil administration was resumed and dietary levels increased
until the end of nine weeks when the intermediate group was fed 15,000
ppm. This intermediate group was then continued for the remainder of
the two year period. The high dose level was increased at biweekly
intervals until the sixteenth week when the group treatment was
terminated.
Growth suppression was observed at all levels and was
dose-related. This reduction in growth was reversible, as noted when
the high dose group recovered after being removed from the diet
containing chlorothalonil and fed a control diet. Haematological and
urine analysis values were within the normal range. PBI values were
generally reduced. Chlorothalonil had a cathartic action as evidenced
by increased water consumption and increased weight of faeces. Organ
weight and organ-to-body weight ratios increased for the liver and
kidney at the higher levels. Microscopic examination of the thyroid,
stomach, kidney, and liver revealed pathological changes.
A distinct alteration was produced in the squamous epithelium of
the cardiac portion of the stomach in most of the male and female
rats. The squamous epithelium was rather consistently thickened and
covered by a layer of keratin. In the kidneys of the higher dose
animals, the epithelium of the proximal convoluted tubules was paler
than usual and uniformly enlarged. At the termination of the study the
thyroid glands of the 15,000 ppm animals exhibited an increase in
epithelial pigmentation. Changes in the stomach at all levels included
acanthosis and hyperkeratosis.
At 13 weeks, the kidneys of both sexes fed 1500 ppm were similar
to those of the control. Gross and microscopic pathological
differences especially in males were, however, observed at the one and
two year intervals at this dose level. Liver changes, predominantly in
females, were characterized by an enlargement of the cells in the
nuclei, particularly in the central lobular area, and the formation of
large multinucleated cells in the pericentral area.
No increase in tumor formation was evident. Alterations in the
thyroid and the stomach appeared to be reversible; the alterations in
the liver were slight and confined to the females; the alterations in
the kidney were not reversible (Paynter, 1967c).
When samples of urine and faeces were taken for metabolism
studies (Skinner and Stallard, 1967) from rats from this long-term
study, data were reported for the volume of urine collected from the
rat over the period of sampling. The volume of urine was found to
decrease proportionately as the dietary dose increased. Conversely,
there appeared to be an increase in faecal excretion although the food
consumption remained constant.
Groups of rats (15 males and 15 females per group) were fed
chlorothalonil in the diet at levels of 0, 500, 1000, and 5000 ppm for
76 weeks. There were no apparent effects on behaviour and growth with
the exception of animals at the high level where food refusal was
noted. In a separate paired feeding study, there was no effect of
chlorothalonil on growth or behaviour. Increased liver weight, kidney
weight, and kidney to body weight ratio was apparent at the higher
test intervals. Microscopic examination indicated chiefly tubular
hypertrophy, epithelial irregularity and vacuolation. The degree of
kidney damage was dose-related and appeared to increase in severity in
males. No other adverse effects were noted in this study (Paynter and
Busey, 1967).
Groups of rats (35 males and 35 females per group) were fed
chlorothalonil in the diet at levels of 0 and 5000 ppm for two years.
Growth suppression in both males and females was evident throughout
the two year period. Survival was not affected and haematological,
biochemical and urine analysis values were within a normal range. A
cathartic effect of chlorothalonil was suggested as evidenced by
increased water consumption and increased weight of faecal excretion.
Organ weight and organ-to-body weight ratios, were increased for the
kidney and caecum. Histological examination of the kidneys at one year
again showed evidence of tubular hypertrophy and epithelial
alterations. No significant effects were noted with other tissues and
organs (Paynter and Crews, 1967).
Groups of rats (50 males and 50 females per group) were fed
chlorothalonil in the diet at levels of 0, 4, 10, 20, 30, 40 and 60
ppm for two years. No effects were seen on appearance, behaviour,
growth, food consumption or mortality. Haematological, clinical
chemistry and urine analysis values were normal. Animals sacrificed at
13, 52 and 104 weeks were examined for gross and microscopic defects.
A major lesion observed in studies at higher dose levels, necrosis of
the epithelial lining of the proximal tubules in the deep portion of
the cortex, was observed sporadically in this experiment at the one
and two year intervals. Vacuolation observed at 13 weeks predominantly
in females at 4 mg/kg and above, was not noted at later intervals.
There was no dose-related histological effect noted. A no-effect level
in this study would be 60 ppm (Holsing and Shott, 1970).
COMMENT
Chlorothalonil is rapidly excreted primarily unchanged. A
metabolite in plants and animals, the 4-hydroxy compound, is more
acutely toxic and more persistent than the parent molecule.
Studies performed at maternally toxic levels showed no effects of
chlorothalonil on reproduction. Growth retardation of pups was
believed to be the result of ingestion of treated diet. The results of
a reproduction study with the 4-hydroxy metabolite were negative at
low levels although secretion into the milk was observed. The results
of mutagenesis and teratogenesis tests were negative within the
parameters of the defined studies. Results of long and short term
chlorothalonil studies at high dietary levels to rats and dogs suggest
a toxicological problem associated with the kidney. Kidney changes
were characterized microscopically as hypertrophy, dilation,
cytoplasmic vacuolation, and hyperplasia of the epithelial cells of
proximal tubules and grossly as enlarged, greenish-brown granular
kidneys. No clinical effects were noted although some renal pathology
was suggested by a reduced urine volume. The significance of
histological changes in the kidney at lower dose levels needs
clarification.
No direct observations in man were reported.
Low-level feeding studies in rat and dog showed no effects in rat
at 60 ppm and in dog at 120 ppm forming the basis for allocating a
temporary ADI for man.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Rat: 60 ppm in the diet, equivalent to 3.0 mg/kg bw.
Dog: 120 ppm in the diet, equivalent to 3.0 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Chlorothalonil is a fungicide with broad spectrum activity. It is
effective against many fungus diseases which damage vegetables (rusts,
anthracnose, downy mildews, leaf spots, soft rot, leaf blight, scab,
early blight, late blight, pink rot, powdery mildew, etc.); agronomic
crops (downy mildew, leaf spots, leaf rust, brown spot, blue mold,
etc.), small fruits (anthracnose, gray mold powdery mildew, black rot,
downy mildew, ripe rot, leaf scorch, leaf blight, etc.); tree fruits
and nuts (scab, powdery mildew, black rot, white rot, fly speck, sooty
blotch, bitter rot, fire blight, blossom blight, leaf spot, melanose,
canker, greasy spot, leaf curl, etc.); tropical crops (signatoka,
black pod, coffee berry disease, leaf disease); turf and ornamental
crops. It has been tested on over 60 crops throughout the world and is
non-phytotoxic to most of these crop plants (Diamond Shamrock, 1974).
Literature available to the meeting further confirmed the fungicidal
effectiveness of chlorothalonil in both field and glasshouse tests
(DiDaris et al., 1965; Turner et al., 1964; Turner and Lamont, 1965).
Chlorothalonil is available as a wettable powder, dust and in
tablet form. The following formulations are produced.
Daconil 2787 W-75, a 75% wettable powder designed to be mixed
with water and applied as a spray. Registered for use in the
U.S.A. for turf and ornamentals and in 20 other countries for use
on a variety of crops (Diamond Shamrock, 1971).
Bravo W-75, a 75% wettable powder formulation registered for use
in the U.S.A. on agricultural crops.
Termil tollets - a tollet containing 8 g active ingredient
formulation to be vaporized by a suitable heat source at
temperatures of 315-425°C in a shallow pan.
Exotherm Termil - a powder containing 20% active ingredients
packaged in 100 g cans. Application is made by removing the lid
from the can and igniting the powder to generate smoke.
The recommended application rates for various crops using the
wettable powder formulation Daconil 2787 W-75 are as follows:
Application
Crop rate
Vegetable crops (asparagus, beans, 1000-3400 g/ha
broccoli, Brussels sprouts, cabbage,
cantaloupe, carrot, cauliflower, celery,
chinese cabbage, corn (sweet), pumpkin,
squash, tomato, turnip, watermelon)
Agronomic crops (hop, peanut, spearmint, 1000-2300 g/ha
sugar beet, tobacco)
Small fruits (blackberry, blueberry, 120-480 g/100 litres
currant, grape, raspberry, strawberry)
Tree fruits and nuts (apple, cherry, 120-360 g/100 litres
grapefruit, lemon, orange, peach,
pecan, pear, tangerine)
Tropical crops (banana, cacao, coffee, 240-480 g/100 litres
rubber)
Thermal dusting with Termil tollets or Exotherm Termil is used to
control a variety of diseases of ornamentals and vegetable crops
(asparagus, snap beans, cucumbers and tomatoes) grown in glasshouses,
at an application rate of 7-7.5 g a.i./100 cu.m. of space.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits and vegetables (Diamond Shamrock, 1974). A summary of much of
this information appears in Table 2 together with details of rates and
number of applications and pre-harvest intervals used. The majority of
these data are from trials in the U.S.A. with a few trials from
Canadian locations.
Chlorothalonil residues were detected on most aboveground crops
at the time of harvest. The higher residues occurred on leafy
vegetables, e.g. lettuce, escarole, chicory, spinach, celery and
crucifers including collards and kale, with generally lower levels on
melons, fruits and root crops. High residues were detected also in
lima bean plants, sugar beet tops and peanut hay which may be used for
animal feeds. The amount of fungicide applied, time interval between
last application and harvest, surface area, weight and surface
structure of the crop are factors that affect the level of the
residue. The residue level diminishes with time after application.
Glasshouse trials of thermal dusting were carried out on
cucumbers, leaf lettuce and tomatoes. The residue data are summarized
in Table 3. In general, the residue levels were low, <4 mg/kg,
following a pre-harvest interval of 1 day.
Only negligible residues of the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, DAC 3701 (see
"Biotransformation" and "Fate of Residues"), were detected in most
commodities. Only in the case of peaches were significant residues
(up to 0.5 mg/kg) detected routinely. Peanut hay and lima beans
(including pods) also sometimes showed residues of 0.5-1 mg/kg.
A series of experiments were performed to see if chlorothalonil
or the 4-hydroxy metabolite could be a residue in meat or milk from
feeding these products to cows. Groups of cows (4 cows/group) were fed
chlorothalonil plus the 4-hydroxy metabolite at dosage levels of 0, 25
ppm chlorothalonil plus 0.2 ppm metabolite, 75 ppm chlorothalonil plus
0.6 ppm metabolite, and 250 ppm chlorothalonil plus 2.0 ppm metabolite
for 30 days. Half the animals were sacrificed at the end of the test
and half after a 32 day recovery period. At a sensitivity of 0.02 ppm,
no chlorothalonil was found in milk (Wolfe and Stallard, 1969). A
small quantity of the metabolite was noted in milk: details are given
in Table 4. Residues in the milk reached maxima of 0.20, 0.26 and 0.78
respectively at the 3 feeding levels after about 18 days (Wolfe and
Stallard, 1970a). At the 30-day interval small residues of
chlorothalonil and the metabolite were seen in muscle, fat, liver and
kidney. Residues of the metabolite were respectively 0.51, 2.1, 0.93
and 3.7 mg/kg at the highest feeding level. No residues were seen
after the 32 days recovery period (Wolfe and Stallard, 1970b).
TABLE 2a. Residues of chlorothalonil from supervised trials.
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 12 13 1 9 0-0.7 0.15
(tops removed) 1.7 9 0-7 3 8-9 0.1-3.6 0.3-2.8
1.9 9 2 1 6 1-8-5.8 4.4
2.5 7-10 0-7 4 6-8 0-8.7 0.02-7.3
Broccoli 1.3 9 1-15 3 9 0.01-9.0 0.01-6.0
2.5 6-8 0-12 4 5-9 0-2.6 0-1.4
Brussels
sprouts 1.3 4-5 0-21 4 9-18 0.7-3.5 1.7-2.5
1.7 5 8 1 3 4.6-5.3 5.0
2.5 4-8 0-18 2 3,5 0.9-4.3 1.0-3.5
3.4 5 0-14 4 2-5 2.0-11 3.2-11
Cabbage 1.7 5-9 0 2 3,7 0.8-2.7 1.5-1.6
2.5 7-9 0-7 5 5-9 0-0.2 <0.01-0.4
Cauliflower 0.8 2 33 1 9 0.03-0.09 0.06
2.5 2-10 0-33 5 7-9 0-1.9 0.02-1.2
Cucumber 0.8 4-9 0-6 5 6-9 0-1.1 0.05-0.9
1.3 4-9 0-6 4 6-18 0-1.2 0.1-0.8
1.7 1-12 0-7 12 3-23 0-2.5 0.2-2.4
2.5 4-13 0-14 12 6-9 0-2.8 0.01-1.8
Summer squash 0.8 6-9 0-3 2 5,6 0.07-0.9 0-2-0.3
1.3 6-9 0-3 2 6 0.2-1.2 0.45-0.9
1.7 1-9 0-9 9 3-25 0-2.0 0-1-1.3
2.5 5 0-14 3 3 0.06-0.25 0.07-0.2
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Cantaloupe
(whole melons) 1.7 7-11 0-14 4 5-17 0-1.1 0.02-0.6
2.5 7 4 1 12 0.7-1.8 1.1
(pulp) 2.5 7 0 1 5 0.05-0.1 0.07
Muskmelon
(pulp) 1.3 11 0 1 3 0 0
(rind) 1.3 11 0 1 3 0.03-0.2 0.1
(pulp) 2.5 11 0 1 3 0 0
(rind) 2.5 11 0 1 3 1.4-1.7 1.5
Honeydew melon 1.7 10 0-14 3 5-8 0-1.6 0.03-0.7
2.5 7 0-14 3 6 0.07-0.4 0.1-0.25
Winter squash
(whole) 1.7 5-10 0 2 3 0.04-1.7 0.05-1.4
(pulp) 1.7 5-10 0 2 3,8 0 0
(whole) 2.5 7 7 1 3 0.8-2.3 1.4
Watermelon
(whole) 0.8 8-10 0-14 4 3 0-0.6 0.02-0.35
(rind) 1.3 7 14 1 3 0.2-0.4 0.3
(whole) 1.7 8-10 0-14 4 3 0.03-1.0 0.08-0.5
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(whole) 2.5 8-10 0-14 4 0.05-1.0 0.05-0.8
Pumpkin (whole) 1.7 10 0-7 3 3 0.8-1.8 0.8-1.4
(pulp) 1.7 10 0 1 2 0 0
Tomato 0.6 18 3 1 9 0-0.1 0.05
1.3 6-18 0-14 5 6-18 0.03-5.5 0.05-4.4
1.7 4-10 0-15 17 3-27 0.01-5.5 0.09-3.4
2.5 5-18 0-14 12 3-32 0.2-4.5 0.2-4.5
Peanuts (nuts) 0.8 6 78 1 3 0.03 0.3
1.3 4-12 4-78 11 4-24 0-0.3 0-0.09
(hulls) 1.3 4-12 4-55 9 3-12 0-0.8 0.09-0.5
(hay) 1.3 4-11 4-68 11 2-30 0.96 0.3-89
(nuts) 1.7 6 78 1 3 0.02-0.04 0.03
Potatoes
(whole) 0.8 10 12 2 9 0 0
(peeled) 0.8 13 15 1 6 0 0
(peelings) 0.8 13 15 1 3 0-0.01 0
Potatoes
(whole) 1.3 3-13 12-23 16 35-48 0-0.07 0-0.02
(peeled) 1.3 10-13 0-15 4 33 0-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
(peelings) 1.3 13 15 1 4 0-0.05 0.01
(whole) 1.7 5-12 0-23 11 19-44 0-0.06 0-0.01
(peeled) 1.7 5-12 0-13 3 21 0 0
(peelings) 1.7 5-12 0-14 6 14 0-0.15 0.06
(whole) 2.5 10 14 1 9 0-0.01 0
Sugar beets
(roots) 1.3 4 61 1 10 0-0.02 0.01
1.7 4-6 14-41 9 9-27 0-0.5 0-0.3
(tops) 1.7 4-6 14-41 3 9 0-5-17 0.9-11
(roots) 2.5 3-6 14-59 5 5-12 0-1.2 0.02-0.5
(tops) 2.5 3-5 14-59 3 6-17 0.2-32 1.2-16
(roots) 3.4 5 23 1 6 0.03-0.09 0.05
Sweet corn
(kernels
and cob) 1.3 14 14 2 17 0 0
1.7 10 0 1 9 0-0.03 0
2.5 11 0-7 3 5-9 0.01-0.1 0.04
Snap beans 1.7 5-8 0-7 5 5-17 0.04-14 0.1-11
2.5 8 0-7 3 9 0.8-10 2-3-5.4
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Lima beans
(beans) 1.3 10 0 1 3 0-2-0.4 0.3
(plants) 1.3 10 0 1 9 220-535 400
(beans) 1.7 7 0-15 2 5-9 0-0.1 0-0.7
(pod + bean) 1.7 13 0 1 3 10-13 12
(plants) 1.7 4-13 0-15 3 2-9 22-310 47-117
Celery 0.8 5-24 0-14 6 8-9 0.2-21 0.4-18
1.3 9-24 0-14 5 6-9 0.1-52 0.4-29
1.4 8 7-14 2 6-8 0.06-1.7 0.1-1
1.7 9-24 0-14 7 6-9 0.1-53 0.7-34
1.8 8 7-14 2 6 0.1-5.4 0.3-3.4
1.9 26 7-14 2 6-8 0.2-17 0.6-12
2.5 5-14 0-14 5 8-9 0.6-17 1.4-10
3.4 10-18 1-14 4 6-9 2.8-26 4.5-18
Oranges (whole) 1.3 1-14 0-14 3 6 1.8-5.1 2.7-4.3
(peel) 1.3 1 173-397 5 20 0-0.7 0.1
Grapefruit
(whole) 0.4-0.8 1 309 2 6 0.01-0.02 0.01
1.3 1 200-309 3 11 0-0.05 0.02
(peel) 1.3 1-2 22-309 6 6-17 0-0.2 0.06-0.09
Limes (whole) 1.3 1 119-280 2 12 0-0.01 0
Lemons (whole) 1.3 1 280 1 4 0.01-0.02 0.01
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Tangerines
(whole) 1.3 1 280 1 6 0.01 0.01
Cherries 0.4 1-4 3-10 2 4-12 0-0.2 0.04-0.2
0.6 4 0-24 5 2-13 0.1-11 0.1-9.3
1.0 4 20 1 6 0.1-0.2 0.2
1.3 3-5 2-24 5 5-14 0.7-7.8 1.4-5.7
1.5 4-5 20-24 3 18 1-10 3.1-4.2
Peaches 0.6 4 8 1 3 6-8 6.7
0.8 4 8 1 6 3.3-6.3 5.0
1.0 7 0-14 3 3 6.4-19 8-15
1.3 4-11 6-14 3 3-12 2.8-20 4-16
1.5 6-7 0-25 11 3-18 1.3-45 4-31
1.7 4 8 1 3 27-28 28
2.5 4 8 1 3 32-54 45
Currants 1.0 4 3 1 6 16-20 18
1.3 4 3 1 6 16-20 18
1.5 4 3 1 6 20-23 23
Blackberries 1.3 2 4 1 12 4-11 7.3
1.7 2 23-48 2 12 0.5-2 1.2
1.9 2 4 1 12 3-16 9.6
2.5 2 0-16 3 9 0.3-8 2-4
3.0 2 0-16 4 7-12 0.5-43 1.6-21
3.4 2 0-16 3 9 1.3-9.3 2.5-4
Raspberries 0.4 2 4 1 6 0.05-1 0.3
0.8 2 4 1 6 0.2-2.4 1.2
1.3 2-3 0-8 3 6-12 0.4-6.5 0.7-4.1
TABLE 2a. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
1.5 2 0-8 2 6 0.6-2.7 0.7-1.7
1.7 3 0-7 3 3 5.6-20 5-7-15
Collards 2.5 3 0-14 4 9 3.3-88 5.7-69
Kale 2.5 3 0-14 4 6 1.5-71 2.8-62
Escarole 1.7 8 0-7 3 4-6 0.4-24 1.3-15
Endive 0.4 5 1 1 2 24-28 26
0.8 5 1 1 3 22-44 31
Chicory 1.7 8 0-7 3 3-5 0.04-30 0.3-24
Lettuce leaf 1.3 3 0 1 8 13-43 24
2.5 3-4 0-7 3 8-9 5-89 13-68
Lettuce head 1.7 4-7 0-14 5 2-12 0.1-100 1.3-86
Spinach 1.3 5 3-8 2 6 4-48 14-29
Turnip greens 1.7 2 2 1 7 5-10 7.2
Onions, green 1.7 3-4 0-7 3 6-8 0.6-11 1.1-7.6
2.5 3-4 0-7 3 6-7 1.3-24 2.0-18
Onions, mature 0.8-1.3 8-9 12-14 2 30 0-0.1 0-0.05
dry 1.7-2.5 5-9 7-9 2 105 0-0.3 0-0.1
Peppers 1.7 9-13 0-7 2 3-9 0.4-9.4 1.2-8.4
2.5 7 0-7 3 9 0.1-3.3 0.5-1.9
TABLE 2b. Residues of hydroxy-metabolite (DAC-3701) from supervised trials
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Carrots 1.3 8 0-7 2 3,5 0.01-0.06 0.01-0.04
(tops removed) 2.5 7 0 1 6 0.04 0.04
Broccoli 1.3 9 1-15 3 9 0 0
2.5 8 0 1 7 0-0.06 0.01
Brussels sprouts 2.5 8 0 1 5 0.01-0.02 0.02
Cabbage 2.5 9 0 1 6 0 0
Cucumber 0.8 7 0 1 6 0-0.03 < 0.01
1.7 7 0 1 6 0-0.03 < 0.01
2.5 6 0 1 3 0 0
Cantaloupe
(whole melons) 1.7 7 4 1 13 0.02-0.04 0.03
2.5 7 4 1 8 0.02-0.08 0.05
(pulp) 2.5 5 1 1 5 0.08-0.14 0.1
Watermelon
(rind) 1.3 7 14 1 3 0 0
Tomato 1.3 6 0 1 6 0-0.2 0.01
1.7 5 1 1 3 0.06-0.1 0.08
2.5 5-8 0-1 3 11 0.01-0.1 0.02-0.09
Peanuts (nuts) 1.3 4-12 4-55 6 10-20 0 0
(hulls) 1.3 4-12 6-55 5 3-9 0-0.05 0-0.02
TABLE 2b. (Cont'd.)
Application Residues. mg/kg
Rate, Pre-harvest No. Range
a.i., interval of Determinations Total (means
Crop kg/ha No. (days) trials per trial range per trial)
Peanuts
(hay) 1.3 4-11 4-55 6 9-33 0-0.6 0.03-0.4
Sugar beets
(roots) 2.5 3 25 2 9 0-0.01 0.01
(tops) 2.5 3 23 1 6 0 0
(roots) 3.4 5 23 1 6 0-0.01 0.01
Lima beans
(pod + bean) 1.7 4 3 1 3 0.8-1.1 1.0
Celery 0.8 20 0 1 4 0.05-0.1 0.05
1.4 8 7-14 2 2-5 0.05-0.2 0.09-0.15
Peaches 0.6 3 0-10 2 3 0.1-0.3 0.2
0.8 2-10 0-10 4 2-5 0-1-0.4 0.2-0.3
1.3 2-4 0-12 4 2-3 0.01-0.5 0.01-0.3
Lettuce head 1.3 4 1-7 2 4 0-0.04 0.01
1.7 4 21 1 6 0-0.04 0.02
Spinach 1.3 5 3 1 8 0.04-0.3 0.1
Turnip greens 1.7 2 2 1 5 0-0.02 0
Onions, mature 0.8-1.3 5-8 14 3-8 3-8 0.0.02 0-0.01
dry 1.7-2.5 8 - 3-5 3-5 0-0.2 0.1-0.2
TABLE 3. Chlorothalonil and DAC-3701 residues in glasshouse crops from supervised trials
Residues (ppm)
Days from No. of Range Means or
Crop and Rate No. of application No. of determinations individual range of
residue g/1000 ft3 applications to harvest trials per trial determinations) means/trial
Cucumbers
chlorothalonil 2 12-15 1 4 3 0.01-0.09 0.03-0.06
DAC-3701 2 12-15 1 4 3 0 0
Lettuce, leaf
chlorothalonil 2 5-12 1 7 3 0.05-3.6 0.05-3.0
Tomatoes
chlorothalonil 2 1-8 1 8 2-3 0.02-0.4 0.03-0.3
1.8 3-8 1 6 4 0.1-3.7 0.2-3.0
2 12-18 1 5 3 0.5-1.4 0.7-1.4
2 1-9 1 9 3 0-0.8 0-0.6
2 8-16 0.5 9 3 0.04-0.7 0.08-0.6
DAC-3701 2 1-8 1 8 2-3 0 0
TABLE 4. DAC-3701 residues (mg/kg) in cows milk during
feeding trial
Residue (mg/kg at 0.2*, 0.6** and 2.0*** ppm
DAC-3071 in diet
Days 0 0.2 0.6 2.0
0 < 0.03 < 0.03 < 0.03 < 0.03
2 < 0.03 < 0.03 < 0.03 0.06
4 0.04 0.04 0.06 0.16
8 0.04 0.08 0.10 0.51
14 < 0.03 0.13 0.16 0.57
18 < 0.03 0.20 0.26 0.74
22 < 0.03 0.11 0.17 0.78
26 < 0.03 0.11 0.13 0.59
30 < 0.03 0.16 0.26 0.74
37 < 0.03 0.06 0.12 0.55
44 < 0.03 0.04 0.16 0.16
51 < 0.03 < 0.03 < 0.03 0.06
60 < 0.03 < 0.03 < 0.03 < 0.03
* +25 ppm chlorothalonil
** +75 ppm chlorothalonil
*** +250 ppm chlorothalonil
Residues of chlorothalonil were not detected in the tissues of
dogs and rats fed levels in the diet of up to 30 000 mg/kg (Wolfe and
Stallard, 1968a) although small quantities of the hydroxy metabolite
were found in the liver and kidney.
No residues of chlorothalonil were detected in milk from a
Holstein cow fed the compound at the 5 ppm level in the ration for
four days (Gutenmann and Lisk, 1966). The method was sensitive to
about 0.03 ppm.
FATE OF RESIDUES
General comments
The disappearance and fate of chlorothalonil residues is
reasonably well documented. The only identified metabolite is the
4-hydroxy compound, DAC-3701, mentioned previously. Although
chlorothalonil residues decrease quite rapidly on plants, the actual
fate is unknown.
In animals
In the experiment by Gutenmann and Lisk (1966) mentioned above,
samples of milk, urine and faeces were collected throughout the
feeding period and for five days thereafter. No residues of
chlorothalonil, acid products of nitrile hydrolysis or conjugates of
acidic or phenolic derivatives were detected. In the same series of
experiments, chlorothalonil disappeared rapidly when incubated with
rumen juice at the 1 ppm level. Two unidentified metabolites were
formed. Feeding studies with dogs and rats showed that chlorothalonil
was rapidly eliminated in the faeces with negligible amounts in the
urine (Skinner and Stallard, 1967). The eliminated material was mostly
unchanged chlorothalonil. Analysis of tissue samples from rats and
dogs from chronic feeding studies of one and two years indicated no
detectable storage of chlorothalonil. A radio-tracer study with rats
confirmed that elimination of chlorothalonil was complete and there
was no significant tissue storage (Ryer and Sullivan, 1966; Skinner
and Stallard, 1967).
Dogs and rats were fed for 2 years on diets containing 1500,
15 000 and 30 000 mg/kg of chlorothalonil (Wolfe and Stallard, 1968a).
Residues were not detected in muscle tissue. Residues of
4-hydroxy-2,5,6-1,3-benzene-dicarbonitrile (DAC-3701) in kidney
tissues of dogs were <1.5 mg/kg and <3.5 mg/kg in the livers of
dogs and rats. Residue levels of the hydroxy metabolite were <0.25
mg/kg in the urine of the dogs and rats.
In the feeding experiment involving both chlorothalonil and
DAC-3701 (Wolfe and Stallard, 1969, 1970a, 1970b; see preceding
section and Table 4), the DAC-3701 residues in the milk accounted for
10-16% of the administered metabolite, but it was not possible to
determine whether part of the milk residue derived from the
chlorothalonil in the diet.
In plants
In studies with ring labelled 14C-chlorothalonil, Kunkel (1967a)
found no evidence of translocation from topical applications on
cucumber cotyledons, cucumber leaves, cucumber hypocotyls, bean
unifolilate leaves or tomato leaves. He also demonstrated that the
labelled compound was not translocated into the aerial parts of corn
or tomato plants when they were cultivated for 23 days in soil treated
with 14C-chlorothalonil. In another study, Kunkel (1967b) showed that
autoradiographic techniques did not demonstrate 14C-movement or
translocation within root systems of sweet corn, cucumber or tomato
grown in soil treated with ring-labelled chlorothalonil. Since
DAC-3701 is a soil metabolite of chlorothalonil and 14C activity was
not translocated from the soil, the experiments also showed that the
4-hydroxy metabolite is not translocated into the root system or
aerial parts of these plants. The residue data from supervised trials
(Table 2) showed that DAC-3701 is a residue following chlorothalonil
treatment. Of the crops tested for DAC-3701 residues, peaches
contained up to 0.5 mg/kg, spinach up to 0.3 mg/kg, onions up to 0.2
mg/kg, celery up to 0.2 mg/kg, cantaloupe up to 0.1 mg/kg and lima
bean plants up to 1 mg/kg. Negligible residues of DAC-3701 (<0.1 ppm)
were reported for carrots, tomatoes, peanuts (except peanut hay),
sugar beets, turnip greens, broccoli, Brussels sprouts, cabbage,
cucumber, watermelon and lettuce.
In soil
Tests conducted under both laboratory and field conditions
demonstrated that chlorothalonil is rapidly degraded in soil (Stallard
and Wolfe, 1967). In the laboratory experiments its half-life in the
types of soils tested ranged from 4 to over 40 days. The degradation
rate increased with increasing organic matter content, moisture
content and temperature but appeared to be independent of pH within
the range 6-8. Chlorothalonil was evidently not lost by
volatilization, since it disappeared rapidly from treated soil
incubated in tightly closed jars.
In the field trials, turf plots in 3 locations in the U.S.A. were
treated with chlorothalonil and the half-life of the chlorothalonil
residues ranged from 26-45 days. The data indicated a decreased
degradation rate during the winter months.
4-Hydroxy-2,5,6-trichloro-1,3-benzenecarbonitrile (DAC-3701) was
the major soil degradation product following treatment with 14C-ring
labelled chlorothalonil and incubation (Duane, 1970). This compound
accounted for over 80% of the radioactivity extracted from the soil.
(Approximately 20% of the radioactivity was not extracted.) A second
degradation product, more polar than DAC-3701 and without a hydroxyl
group, was detected but not identified. During these experiments it
was shown that 14C-chlorothalonil was not volatilized from the soil
and no volatile degradation products were formed.
Laboratory experiments with 5 representative soil types
demonstrated that the half-life values for DAC-3701 ranged from 36
days in a sandy loam type soil to 220 days in clay type soil (Wolfe
and Stallard, 1968b).
Duane (1970) demonstrated that bacteria isolated from soil were
capable of metabolizing chlorothalonil in culture media. Thus it may
be assumed that naturally occurring soil microorganisms are in part
responsible for the rapid loss of chlorothalonil under field
conditions.
In storage and processing
Some data are available on the effect of washing, field trimming
and peeling on the residue levels of chlorothalonil in some
commodities (Diamond Shamrock, 1974). Table 5 summarizes the data on
the effect of washing with water. Residues on carrots, cucumbers,
summer squash, tomatoes, currants and cranberries were reduced by
more than 50%. Washing was least effective with the leafy vegetables.
Significant reduction in chlorothalonil residues results from trimming
cabbage and head lettuce (Table 6). Chlorothalonil residue are
concentrated on the surface and as a result peeling removes
almost all the residues leaving the pulp of many fruits and
vegetables almost residue-free (Table 7). Following processing,
residues of chlorothalonil were not detected ( <0.1 mg/kg) in canned
spinach (Table 8). The fate of any DAC-3701 residues was not
determined.
TABLE 5. Effect of washing with water on residues of
chlorothalonil
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Carrots 4.39-7.34 1.05-3.53 52-76
Cucumbers 0.08-0.45 0.01-0.03 87-95
Squash, summer 0.21-1.33 0.01-0.12 76-97
Tomatoes 0.21-0.82 0.02-0.08 90
Celery 1.04-20.0 0.76-8.18 0-59
Peaches 7.6-15.0 4.9-8.6 35-45
Currants 18.3 2.6 86
Cranberries 0.91 0.25 73
TABLE 5. (Cont'd.)
Residue (mg/kg) Reduction
Crop Unwashed Washed %
Collards 5.73-69.0 1.7-31.3 31-70
Kale 2.8-62.0 1.6-20.0 43-74
Escarole 1.32-4.1 0.63-2.6 36-44
Chicory 0.31-4.0 0.23-1.5 26-62
Lettuce 23.7-67.6 8.9-37.1 45-70
Cauliflower 1.23 0.85 30
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not available to the Meeting to indicate the level and
incidence of chlorothalonil residues in food moving in commerce or in
food at the time of consumption. Chlorothalonil residues were not
determined in the U.S.A. national food and feed monitoring program
(Duggan and Cook, 1971).
METHODS OF RESIDUE ANALYSIS
Residues of chlorothalonil and its metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile DAC-3701), can be
determined by gas-liquid chromotography (GLC) with either electron
capture or microcoulometric (halide) detectors (Wolfe and Stallard,
1970). The parent compound is chromatographed directly but the
metabolite is converted to the methyl ether by reacting with
diazomethane prior to chromatography.
Crop and soil samples are extracted with acidified acetone. The
acetone is removed by distillation and the residues are partitioned
into diethyl ether. The ether extract is cleaned-up on a Florisil
chromatographic column. Chlorothalonil is eluted with 5%
acetone-dichloromethane and DAC-3701 with 50% acetone-dichloromethane.
Following reaction with diazomethane, the methyl ether of DAC-3701 and
chlorothalonil are determined by GLC either by electron capture or
microcoulometric detection. The sensitivity of the method is
TABLE 6. Effect of trimming on residues of chlorothalonil in cabbage and lettuce
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop kg/ha applications to harvest determinations (mg/kg) (mg/kg)
Cabbage
(field trimmed) 1.7 9 0 7 0.77-2.67 1.60
1.7 5 0 3 1.25-1.72 1.47
(market trimmed) 2.5 7-8 0-7 7-9 0.00-0.18 <0.01-0.03
Lettuce
(whole untrimmed
heads) 1.7 4-7 0-7 2-12 11.5-100 33.7-85.5
1.7 4 14 11 0.10-3.0 1.33
(trimmed heads) 1.3 5 1-7 4 0.15-1.98 0.53-0.95
1.7 4 2-14 6 0.07-2.9 0.80-0.90
TABLE 7. Effect of peeling on chlorothalonil residues
Crop Residues (mg/kg)
Cucumbers Peelings - 1.26; Pulp - <0.01
Muskmelon Rind - 0.11-1.50; Pulp - 0.00
Winter squash Whole - 0.05-1.42; Pulp - 0.00
Pumpkin Whole - 1.42; Pulp - 0.00
Cantaloupe Whole - 1.09; Edible portion - 0.07
Peanut Hulls - 0.13-0.51; Meat - 0.00
Potatoes Peelings - 0.00-0.06; Pulp - 0.00
Lima beans Pod and beans - 11.9; Beans - 0.00
Grapefruit Peel - 0.09; Whole - 0.02
Oranges Whole - 2.70-4.29; Pulp - <0.01-0.08;
Juice - 0.00
TABLE 8. Effect of canning on chlorothalonil residues in spinach
Rate, Days from Residue Mean
a.i., No. of application No. of range residue
Crop % applications to harvest analysis (ppm) (ppm)
Spinach
(raw) control 0 - 6 <0.1 <0.1
0.11 5 3 6 16.1-47.8 29.1
0.11 5 8 6 3.7-30.9 13.6
(processed,
canned) control 0 - 3 <0.1 <0.1
0.11 4 16 3 <0.1 <0.1
0.02 mg/kg. Recovery values obtained from fortified crops were greater
than 72 and 76% for chlorothalonil and DAC-3701 respectively. Some
modifications to the method described above are required for animal
tissue (Wolfe and Stallard, 1970b) and milk samples (Wolfe and
Stallard, 1969, 1970c). Average, recoveries for chlorothalonil and
DAC-3701 respectively were:
muscle - 81, 91%
fat - 76, 91%
kidney - 82, 84%
liver - 93 83%
milk - 88, 83%
The sensitivity of the method for milk is 0.02 mg/kg for
chlorothalonil and 0.03 mg/kg for DAC 3701. Chlorothalonil is not
recovered by the Mills Florisil multi-residue procedure but is
recovered (McMahon et al., 1973) in the alternative Florisil elution
system (Mills et al., 1972). Recovery from deactivated Florisil
(Osadchuk et al., 1971) is achieved by elution with 10% ethyl acetate
in hexane (McLeod and Ritcey, 1973).
Chlorothalonil can be recovered through the carbon-cellulose
cleanup procedure of McLeod et al. (1967). Gutenmann and Lisk (1966)
determined chlorothalonil by electron capture GLC in milk, urine and
faeces after extracting with acetone-phosphoric acid and partitioning
into hexane. Possible acid metabolites were determined after
diazomethane esterification of the evaporated acetone extract.
With the exception of the Mills Florisil multiresidue procedure,
the multiresidue procedures discussed above appear to be suitable for
regulatory determination of the parent compound. When the total
residue, chlorothalonil and DAC-3701, is required the GLC method
proposed by Wolfe and Stallard (1970c) can be recommended for
regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Some examples of national tolerances were reported to the Meeting
and are listed in Table 9.
TABLE 9. Examples of national tolerances as reported to the Meeting
Tolerance
Count Commodity (mg/kg)
Australia Beets, carrots, corn, cucumbers,
onions, peppers, potatoes,
tomatoes 7
Peanuts 0.2
TABLE 9. (cont'd)
Tolerance
Count Commodity (mg/kg)
Canada Celery 15
Broccoli, Brussels sprouts,
cabbage, cauliflower, cucumbers,
melons, pumpkins, snap beans,
squash, tomatoes 5
Carrots 1
Peanuts 0.3
Netherlands Potatoes 0.05
Apples, apricots, beets, carrots,
citrus, corn, cucumbers, grapes,
melons, onions, peaches, pears,
peppers, plums, tomatoes 0.01
Switzerland Potatoes 0.05
U.S.A. Celery 15*
Broccoli, Brussels sprouts, cabbage,
cauliflower, cucumbers, melons,
pumpkins, snap beans, squash
(summer and winter), tomatoes 5*
Carrots, sweet corn (kernels plus
cob with husks removed) 1*
Peanuts 0.3*
Potatoes 0.1*
* Including metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzen-edicarbonitrile.
APPRAISAL
Chlorothalonil is a broad-spectrum fungicide with effective
action against many fungus diseases which damage vegetable, tree,
small fruit and other agricultural crops, turf and ornamentals. The
use pattern of chlorothalonil is such that residues remain on most
above-ground crops at the time of harvest. It is desirable to have
some residue of the fungicide on the mature crop to protect it from
disease organisms during shipment.
4-Hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile is a major
metabolite of chlorothalonil in soil and a metabolite in plants, but
only negligible residues were found on most crops investigated.
Chlorothalonil does not translocate into plants from the soil or from
topical application.
Extensive crop residue data indicated that chlorothalonil is a
relatively persistent fungicide. The level of the residue depends on
such factors as rate of the fungicide applied, time interval between
last application and harvest and the surface area, weight and surface
structure of the crop. The residue level diminishes with time after
application, and pre-harvest intervals are therefore recommended for
some crops. Some data are available on the effects of washing,
trimming and peeling on chlorothalonil residues.
Chlorothalonil residues do not occur in the tissues or milk of
cows, but residues of the 4-hydroxy metabolite are found when cows are
fed chlorothalonil and the 4-hydroxy compound together in their
ration. The residue levels depend on the levels fed and reach a steady
value in the milk after 18 days. Residues in milk declined to below
detection level within 21 days after withdrawal. It was not determined
whether residues of the 4-hydroxy metabolite would occur in the milk
and tissues of cows if only chlorothalonil were ingested.
Since no data were provided on residues in crops that may be fed
to animals, it was not possible to recommend maximum residue limits
for milk and meat.
Most available multi-residue GLC methods appear to be suitable
for the determination of the parent compound, but chlorothalonil is
not recovered by the original Mills Florisil multi-residue cleanup
procedure (McMahon et al., 1973). The GLC method proposed by Wolfe
and Stallard (1970) for chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile appears to be
suitable for regulatory purposes when the total residue is required.
National tolerances are in effect in a number of countries.
RECOMMENDATIONS
The following maximum residue limits are recommended for
chlorothalonil and the metabolite
4-hydroxy-2,5,6-trichloro-1,3-benzenedicarbonitrile, expressed as
chlorothalonil.
TEMPORARY TOLERANCES
Pre-harvest
interval on which
Limit recommendations
Commodity (mg/kg) are based (days)
Peaches 30 7
Currants (black, red and white) 25 3
Celery 15 7
Peppers 10 1
Blackberries, raspberries, 10 7
cherries, chicory sprouts
Collards, kale, endive, 10 14
lettuce (head)
Broccoli, Brussels sprouts, 5 7
cabbage, cauliflower, beans
(green including pod), oranges,
onions, cranberries
Cucumbers, melons, pumpkins, 5 1
squash, tomatoes
Carrots, sweet corn, sugar 1 1
beets
Lima beans, peanuts (whole) 0.5 0
Peanuts (kernel), potatoes 0.1 0
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Additional study to resolve lower limit of kidney effects in rat.
2. Define growth reduction in pups relative to dietary ingestion or
secretion into milk.
3. Data on residues of chlorothalonil and the 4-hydroxy metabolite
in crops that may be fed to animals.
4. The results of feeding studies on dairy cattle understood to be
in progress to determine the level and nature of residues in milk
and tissues.
DESIRABLE
1. Observations in man.
2. Residue data for food moving in commerce.
3. Further information on effects of processing, including household
cooking, on residues.
REFERENCES
Beasley, A. and Leong, K. (1965). Acute inhalation exposure - rats.
Report from Hazelton Laboratories Inc., submitted by The Diamond
Shamrock Chemical Co. (Unpublished)
Blackmore, R. and Shott, L. (1968). Final report four month feeding
study - rats. Report from Hazelton Laboratories, Inc. (Unpublished)
Diamond Shamrock (1971). Documentation on the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Diamond Shamrock (1974). Documentation concerning the pesticide,
chlorothalonil. Diamond Shamrock Chemical Co. (Unpublished)
Di Dario, A., Curry, T.L., Thayer, P. and Turner, J.J. (1965). The
biological performance of tetrachloroisophthalonitrile as influenced
by particle size and crystalline form. Phytopathology, 55:1055.
Doyle, R. and Elsea, J. (1963). Acute oral, dermal and eye toxicity
and irritation studies on DAC-2787. Report from Hill Top Research
Institute Inc., submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Duane, W.C. (1970). Biodegradation of Daconil 2787(R). Report
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Duggan, R.E. and Cook, H.R. (1971). National food and feed monitoring
Program. Pestic. Monit. J., 5:37-43.
Gutenmann, W.H. and Lisk, D.J. (1966). Metabolism of Daconil and
Dacthal pesticides in lactating cows. J. Dairy Sci., 49:1272-1276.
Hastings, T.F. and Jessup, D.C. (1974). 3-Generation reproduction
study in albino rats using DAC-3701 in the diet. Report from BIO/TOX
Research Laboratories, Inc. Submitted to WHO by Diamond Shamrock
Chemical Co. (Unpublished)
Holliday, W., Schadeberg, K., Goode, J. and Keplinger, M. (1973).
Twenty-one day subacute aerosol inhalation toxicity study with Bravo
6F(R) in albino rats. Report from Industrial Bio Test Laboratories,
Inc. (Unpublished)
Holsing, G. and Shott, L. (1970). Two year dietary administration
- rats. Report from Hazelton Laboratories Inc. submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Holsing, G. and Voelker, R. (1970). Final report 104 week dietary
administration - dogs. Report from Hazelton Laboratories Inc.
submitted by Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967a). Absence of 14C movement in crop plant organs
after topical application and soil amendment studies with isotopic
Daconil 2787. Report to Diamond Shamrock Chemical Co. (Unpublished)
Kunkel, J.F. (1967b). Movement of 14C in or on roots of crop species
grown in soil amended with isotopic Daconil 2787. Report to Diamond
Shamrock Chemical Co. (Unpublished)
Legator, M. (1974). Mutagenic Testing with DAC 2787. Report from
Division of Genetics, Roger Williams General Hospital and Brown
University, Division of Biological and Medical Sciences submitted by
Diamond Shamrock Chemical Co. (Unpublished
McLeod, H.A., Mendoza, C., Wales, P. and McKinley, W.P. (1967).
Comparison of various carbon adsorbents and quantitative elution and
separation of forty-two pesticides from a carbon-Solka Floc cleanup
column. J. Ass. off. analyt. Chem., 50:1216-1228.
McLeod, H.A. and Ritcey, W.R. (1973). Analytical methods for pesticide
residues in foods. Information Canada, Ottawa, Canada.
McMahon, B.M., Sawyer, L.D. and Corneliussen, P.E. (editors) (1973).
Pesticide analytical manual. Volume 1. Methods which detect multiple
residues. Food and Drug Administration, Washington, D.C.
Mills, P.A., Bong, B.A., Kamps, L.R. and Burke, J.A. (1972). Elution
solvent system for Florisil column cleanup in organochlorine pesticide
residue analyses. J. Ass. off. analyt. Chem., 55:39-43.
Osadchuk, M., Romach, M. and McCully, K.A. (1971). Cleanup and
separation procedures for multi-pesticide residue analysis in
monitoring and regulatory laboratories. Proc. Second Intern. IUPAC
Congress Pestic. Chem. Vol. IV, Methods in Residue Analysis, p.
357-383. A.S. Tahori (editor), Gordon and Breach Science Publishers,
New York.
Paynter, O. (1965a). Oral dose range - dogs - final report. Report of
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1965b). Repeated dermal application - rabbits. Report
from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1966). Reproduction - rabbit. Report from Hazelton
Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Paynter, O. (1967a). Three generation reproduction study - rats.
Report from Hazelton Laboratories Inc. submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Paynter, O. (1967b). Ten week amino acid feeding study - rats. Report
from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. (1967c). Final report - two year dietary feeding - rats.
Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Busey, W. (1967). Long term (76 weeks) feeding study,
rats, DAC 2787. Final Report from Hazelton Laboratories Inc.
(Unpublished)
Paynter, O. and Crews, L. (1967). Final report - two year dietary
feeding - rats. Report from Hazelton Laboratories Inc. (Unpublished)
Paynter, O. and Murphy, J. (1967). Sixteen week dietary feeding -
dogs. Report from Hazelton Laboratories Inc., submitted by Diamond
Shamrock Chemical Co. (Unpublished)
Powers, M. (1965). Acute oral administration - rats. Report from
Hazelton Laboratories Inc. submitted by Diamond Shamrock Chemical Co.
(Unpublished)
Ryer, F.H. and Sullivan, J.B. (1966). Radiotracer metabolism study.
Report from Hazelton Laboratories Inc., submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Skinner, W.A. and Stallard, D.E. (1967). Daconil 2787(R) animal
metabolism studies. Report from and submitted by Diamond Shamrock
Chemical Co. (Unpublished)
Stallard, D.E. and Wolfe, A.L. (1967). The fate of
2,4,5,6-tetrachloroisophthalonitrile (Daconil 2787(R)) in soil.
Report from Diamond Shamrock Chemical Co. (Unpublished)
Sterner, W. and Loveless, L. (1963). A study of the subacute toxicity
of tetrachloroisophthalonitrile to rats. Report from International
Bio-Research, Inc. (Unpublished)
Teeters, W. (1966). In vivo assay of spasmodic activity, mice, DAC
2787. Final report from Hazelton Laboratories Inc. (Unpublished)
Turner, N.J. and Lamont, D. (1965). Control of fungal diseases in the
greenhouse with thermally induced dusts of
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
23:51-54.
Turner, N.J., Limpel, L.E., Battershell, R.D., Bluestone, H., Lamont,
D. (1964). A new foliage protectant fungicide,
tetrachloroisophthalonitrile. Contr. Boyce Thomson Inst. Pl. Res.,
22:303-310.
Wazeter, F. (1971). Acute oral LD50 in male albino rats. Report from
International Research and Development Corporation. (Unpublished)
Wazeter, F. and Goldenthal, E. (1972). Acute oral toxicity in beagle
dogs. Report from International Research and Development Corporation.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968a). Analysis of tissues and organs
for storage of the Daconil metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1968b). The fate of DAC-3701
(4-hydroxy-2,5,6-trichloroisophthalonitrile) in soil. Diamond Shamrock
Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1969). Residues in milk from cows fed
2,4,5,6-tetra-chloroisophthalonitrile. Diamond Shamrock Chemical Co.
(Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970a). Residues in milk from cows fed
2,5,6-trichloro-4-hydroxyisophthalonitrile. Diamond Shamrock Chemical
Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970b). Residues in tissues of dairy
cows fed Daconil 2787 and 2,5,6-trichloro-4-hydroxyisophthalonitrile.
Diamond Shamrock Chemical Co. (Unpublished)
Wolfe, A.L. and Stallard, D.E. (1970c). Analytical method for
determination of Daconil 2787 and DAC-3701 residues. Diamond Shamrock
Chemical Co. (Unpublished)
