
FENAMIPHOS JMPR 1974
IDENTITY
Fenamiphos is a draft ISO common name.
Chemical name
O-ethyl O-(3-methyl-4-methylthiophenyl) isopropylphosphormidate.
O-ethyl 4-(methylthio)-m-tolyl isopropylphosphoramidate.
Synonyms
Nemacur(R), phenamiphos, Bayer 68138.
Structural Formula
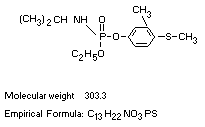 Molecular weight 303.3
Empirical Formula: C13H22NO3PS
Other information on identity and properties
State: brown semi-solid (technical)
white crystals (pure)
Melting Point: 46°C (technical)
49°C (Pure)
Vapour pressure: 10-6mm Hg (at 30°C)
Solubility: in water approx. 700 mg/l at 20°C
slightly soluble in most organic
solvents
Specific gravity (melt): 1.14
Hydrolytic stability in propanol/water (1:1) at 40°C:
40% degradation after 14 days at pH 2
no degradation after 50 days at pH 7
half life of 31.5 hours at pH 11.3
Formulations: 5% Granular, 10% Granular, 400 E.C. (Emulsifiable
concentration 400 g/l)
Purity
The minimum purity is 87%. Maximum levels of the individual
impurities are shown in Table 1.
TABLE 1 Impurities (maximum levels) in technical fenamiphos
Compound Maximum
level (%w/w)
O,O-diethyl isopropylphosphoramidate 3.0
4-methylthiocresol 0.5
O,O-bis[(4-methylthio)-3-ethylphenyl]
isopropylphosphoramidate 2.0
O,O-diethyl O-[(4-methylthio)-3-methylphenyl]
phosphate 1.0
O-ethyl O-[(4,6-bismethylthio)-3-methylphenyl]
isopropylphosphoramidate 3.0
O-ethyl O,O-bis-[(4-methylthio)-3-methylphenyl]
phosphate 8.0
O-ethyl O-[(4-methylthio)-3-methylphenyl] phosphate 1.0
water 0.3
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformations
The two major metabolites identified following injection into
bean, tomato, peanut and potato stems were O-ethyl
O-4-methylsulfinyl-m-tolyl isopropylphosphoramidate and O-ethyl
O-4-methylsulfonyl-m-tolyl isopropylphosphoramidate, the sulphoxide
and sulphone of the thio-ether (Waggoner, 1972). A third metabolite
(apparently a more potent inhibitor of cholinesterase) was
not identified but was considered to be a further metabolite
resulting from the sulphoxide. Further details of this study are
given in the section "Fate of residues in plants". Small residues of
the unknown metabolite may be of toxicological significance because
of the potent anticholinesterase properties.
The metabolic fate of labelled fenamiphos was studied in vivo
in rats and in vitro with rat liver microsomes. The compounds were
labelled in the ethyl (14C) isopropyl, (14C) or the thiomethyl (3H)
positions. In vivo fenamiphos is excreted within 12-15 hours
following a single oral dose of 2 mg/kg. In the in vitro studies a
small quantity of an unknown metabolite possibly resulting from the
N-dealkylation of fenamiphos was observed (Khasawinah and Flint,
1972). Apart from this minor component, metabolism in animals and
plants follows the same pattern: oxidation of the thioether to the
sulphoxide and sulphone, dearylation to yield the methyl thioether
phenol (or its sulphoxide and sulphide) and potentially dealkylation
of the ethyl, isopropyl or isopropylamide moiety of the phosphate
ester. Treatment of rats with fenamiphos sulphoxide or sulphone
produced the same excretion pattern and almost identical urinary
metabolites (Gronberg, 1969).
When fenamiphos sulphoxide was given to a lactating cow (Gronberg
et al., 1974) the identified components of the residue were
fenamiphos, its sulphoxide and its desisopropyl derivative, and the
phenols of phenamiphos, its sulphoxide and its sulphone. An
unidentified metabolite was also detected. Quantitative details are
given in the section "Fate of residues in animals".
Effects on enzymes and other biochemical parameters
Fenamiphos, as are other organophosphate esters, is an inhibitor
of cholinesterase enzymes. The in vitro I50 values were as follows:
Rat serum - 5.1 x 10-5M.
Rat erythrocyte - 6.3 x 10-4M.
Rat brain - 2.1 x 10-4M.
The maximum inhibition of whole blood cholinesterase in vivo
occurred in rats three hours after dosing. In vivo sensitivity of
cholinesterase reflects the in vitro I50 values with plasma
cholinesterase being more sensitive to inhibition than the erythrocyte
enzyme (Löser and Kimmerle, 1971). The metabolites of fenamiphos are
more active inhibitors than the parent molecule (Waggoner, 1972). In
vitro inhibition of horse serum cholinesterase showed relative
values for fenamiphos < sulfoxide = sulfone < sulfoxide metabolite.
In vitro I50 values of chicken and monkey liver aliesterase
(triacetin hydrolysis) and monkey cholinesterase are as follows:
Chicken (aliesterase): 4.2 x 10-5M.
Monkey (aliesterase): 1.0 x 10-6M.
Monkey (cholinesterase): 4.6 x 10-6M.
(Coulston and Wills, 1974)
On the basis of some preliminary electrophysiological studies
with the cat, it was estimated that a threshold dose of fenamiphos
might fall in the range of 0.8 to 1.5 mg/kg (Coulston and Wills,
1974).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of Charles River random bred Swiss white mice (125 males
and 125 females/group) were fed fenamiphos in the diet at 0, 25 and 50
ppm for eighteen months in a study specifically designed to evaluate
the carcinogenic potential of the compound. Additional groups of mice
were fed 10 ppm N-nitrosodiethylamine for eighteen months (125 males
and 125 females); 40 ppm for six months (60 males and 60 females); and
1000 ppm benzidine (100 females) for eighteen months as positive
controls. Fenamiphos did not affect the incidence or pattern of
mortality. Clinical chemistry studies performed at 18 months were
normal. No signs of tumour formation were observed on gross or
microscopic examination which were attributed to fenamiphos (Smith et
al., 1972).
Special studies on mutagenicity
Mouse
Groups of twelve male mice were administered fenamiphos
intraperitoneally at 0, 0.25 and 0.50 mg/kg and mated for six
consecutive weeks to virgin females in a standard dominant lethal
test. A positive reference (methyl methanesulphonate) was used in this
experiment. Fenamiphos did not induce alterations in male germinal
cells which would be expected to lead to an early embryo mortality
(Arnold et al., 1971).
Special studies on neurotoxicity
Hen
Groups of hens (eight per group) were fed fenamiphos in the diet
at levels of 0, 1, 3, 10, and 30 ppm for thirty days. At the end of
the feeding experiment some animals were sacrificed and the remainder
were observed for four weeks for neurological signs of poisoning. Food
consumption was depressed at 30 ppm and the average body weight and
growth of the animals at this same level was reduced. Cholinesterase
(whole blood) depression was observed at thirty days at 3, 10 and 30
ppm, although cholinergic signs of poisoning were not observed at any
time. There were no indications of delayed neurotoxic effects and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with H&E) did not indicate delayed neurological involvement
with fenamiphos (Kimmerle, 1970; Spicer, 1970).
Groups of hens (ten per group) were orally administered an LD50
dose of fenamiphos (5.0 mg/kg). The birds were observed for three
weeks and sacrificed. No evidence of delayed neurotoxicity was
observed either clinically or histologically while positive signs were
observed with TOCP (Kimmerle, 1971; Spicer, 1971).
Special studies on potentiation
Rat
Male rats were tested orally with fenamiphos (SRA 3886) in
combination with disulfoton or E 154 and no potentiation of the acute
toxicity was noted (Kimmerle, 1972c).
Special studies on reproduction
Rat
A standard three generation, two litters per generation,
reproduction study was performed with fenamiphos. Four groups of
animals, consisting of ten male and twenty female rats per group, were
fed fenamiphos at 0, 3, 10, and 30 ppm in the diet throughout the
study period including mating, gestation, and suckling. Immediately
after birth, pups were examined for malformations prior to either
preparing for another generation or sacrifice. Five weanling rats per
group of the F3b generation were sacrificed and macroscopic and
microscopic examinations performed on the major tissues and organs.
There were no apparent differences with regard to reproduction
parameters including fertility, litter size, lactation index, growth
of young, or on examination for malformation in any of the animals
exposed to fenamiphos levels up to and including 30 ppm in the diet.
It was concluded that fenamiphos had no influence on the reproduction
of rats at dietary levels up to and including 30 ppm (Löser, 1972c;
Cherry et al., 1972).
Special studies on teratogenicity
Rabbit
Groups of fifteen artificially inseminated rabbits were orally
administered fenamiphos at levels of 0, 0.2, and 0.4 mg/kg/day.
Positive control groups (oral treatment with thalidomide (37.5
mg/kg/day)) were concurrently run with this experiment. All animals
were treated from day 6 through 18 of gestation and sacrificed on day
29. At day 29 the young were examined for 24 hours, sacrificed and
examined after skeletal staining. At the high level of treatment of
fenamiphos, 2/12 instances of abortion and 2/12 premature deliveries
were observed. No external or internal abnormalities were observed in
the foetuses following treatment. With the exception of the animals at
the high level of treatment where a few aborted or delivered
prematurely, no effects on reproduction were observed with fenamiphos
(Ladd et al., 1971).
Acute toxicity
TABLE 2 Acute toxicity of fenamiphos
LD50
Animal Sex Route (mg/kg) References
Rat M oral 2.4-15.6 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Rat F oral 2.3-19.4 Kimmerle, 1972c
Crawford and Anderson, 1974
DuBois et al., 1967
Rat M ip 3.0-3.7 ibid.
Rat F ip 4.2-4.9 Löser and Kimmerle, 1971
Rat M dermal 73-500 DuBois et al., 1967
Rat F dermal 84-154 Kimmerle and Solmecke, 1971a
Mouse M oral 22.7 Löser and Kimmerle, 1971
Mouse M&F ip 3.4 DuBois at al., 1967
Guinea Pig M oral 56-100 ibid.
Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Guinea Pig M ip 17.3 DuBois et al., 1967
TABLE 2 (Cont'd.)
LD50
Animal Sex Route (mg/kg) References
Rabbit M oral 10-17.5 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Cat M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Dog M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Hen F oral 5.3 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
TABLE 3 Acute toxicity of metabolites and impurities of fenamiphos
LD50
Species Sex Route (mg/kg) References
Fenamiphos-sulphoxide
Rat F oral 10-25 Thyssen, 1974a
Fenamiphos-solphone
Rat F oral 10-25 ibid.
4-methylthio-m-cresol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 1333>2500 ibid.
Thyssen, 1974c
3-methyl-4-methylthiophenol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 500-1000 Thyssen, 1974d
1175 Crawford and Anderson, 1974
3-methyl-4-methanesulphonylphenol
Rat M oral 1250 ibid
Rat F oral 1000 Thyssen, 1974e
1854 Crawford and Anderson, 1974
Impurities identified as components of the technical mixture
(aryldiamide, diarylamide, diaryl ethyl ester, diethyl ester,
diethylmonamide, di-SCH3 compound, ethyl aryl ester, ethyldiamide,
and 4-methylthio-3-cresol) were tested for their acute toxic
properties to rats. At a dosage of 1.5 times the acute LD50 of
fenamiphos none of the materials were toxic (Crawford and Anderson,
1973b).
Technical fenamiphos administered to the skin of rabbits as an
acetone solution at 50 mg/kg resulted in a slight erythema and was not
considered as a primary irritant. Technical fenamiphos applied to the
conjunctival sac of rabbits (100 mg/kg as a crystalline material) was
irritating to the eye. This irritation was believed to be a mechanical
rather than a physiological effect (Crawford and Anderson, 1971).
A 15% granular formulation was examined for dermal and inhalation
toxicity properties and at the highest level examined (200 mg/kg and
20 mg/l respectively) no toxicity was observed (Crawford et al.,
1970b; Crawford and Anderson, 1972a). The rat oral LD50 of a 15%
granular formulation was 66.6 mg/kg for male rats and 62.7 mg/kg for
female rats (DuBois and Kinoshita, 1970). A 5% or 10% granular
formulation, administered at up to 500 mg/kg to the backs of rats
resulted in depressed cholinesterase activity values. Mortality was
not observed at the maximum dose tested and it was considered that the
granular formulation was not a dermal hazard although the material was
rapidly absorbed into the body (Kimmerle and Solmecke, 1971b). The
acute dermal toxicity and dermal and eye irritation properties of a
liquid formulation (37.4% a.i.) were tested in rabbits. The acute
LD50 was 75.7 mg/kg and 71.5 mg/kg to male and female rabbits
respectively (Crawford et al., 1970a; Crawford and Anderson, 1972b).
When applied to intact and abraded skin at a dose of 0.25 ml (0.085 mg
a.i.), fenamiphos was not found to be a primary irritant. Fenamiphos
(0.1 ml, equivalent to 0.034 mg a.i.) was an irritant when applied to
the conjunctival sac of rabbits. Control formulation data were not
reported (Crawford and Anderson, 1973c). The acute rat oral LD50 of a
35% liquid formulation was calculated to be 24.8 mg/kg (equivalent to
8.7 mg fenamiphos /kg). Inhalation toxicity studies with this
preparation using a small particle size distribution (1-3 microns)
resulted in an LC50 value of 0.175 and 0.091 mg/l following one and
four hour exposure respectively (DuBois and Kinoshita, 1971). Studies
with rodents administered fenamiphos by inhalation at doses up to 0.23
mg/l for one to four hours exposure as a static spray showed that
rabbits and guinea pigs were more tolerant of the acute effects than
rats and mice. A one hour LC50 value for mice was 0.06 mg/l and for
rats 0.23 mg/l while rabbits and guinea pigs were unaffected at these
concentrations (Kimmerle and Solmecke 1971a; 1972b). Acute oral
administration of 2.5 mg/kg to rabbits had no effect on several liver
function tests including BSP, SGPT and SDH activity at 1 and 24 hours
and seven days after treatment (Kimmerle and Solmecke 1971a).
Studies performed on male rats indicated that, following
poisoning, the administration of atropine and/or 2-PAM or Toxogonin
would reduce the acute lethal dose by a factor of approximately two.
As with other organophosphorus compounds, rapid administration of
atropine and oxime reactivator following poisoning would afford some
protection and would alleviate the cholinergic signs of poisoning
(Kimmerle, 1972a; DuBois et al., 1967).
Short term studies
Rat
Groups of rats (fifteen males and fifteen females per group,
thirty males and thirty females were used as controls) were fed
fenamiphos in the diet at 0, 4, 8, 16, and 32 ppm for twelve weeks.
Male and female rats from the highest dose group exhibited signs of
cholinergic stimulation in the first two months of the test.
Behavioral differences in all other groups were not apparent. There
were no differences from the control in the average of any group with
regard to food consumption and growth. With the exception of
cholinesterase inhibition, there was no apparent effect on group
averages of haematological parameters examined. Plasma cholinesterase
depression was observed at 8 ppm. A no-effect level, based upon plasma
cholinesterase, was observed at 4 ppm. Gross and microscopic
pathological examination was performed on tissues and organs at the
conclusion of the experiment. A slightly increased liver weight,
observed in males at 16 and 32 ppm, was not reflected in the
calculation of relative organ-to-body weight ratios nor in the
histological examination. Based on cholinesterase depression, 4 ppm in
the diet was a no-effect level in this experiment (Löser, 1968b;
Mawdesley-Thomas and Urwin, 1970a).
Groups of male rats (15 per group) were administered fenamiphos
orally or by intraperitoneal injection daily, 5 days/week for 60 days.
The animals all survived a dose range of 1.5 (ip) to 1.7 (oral)
mg/kg/day with no evidence of a cumulative toxicological effect due to
fenamiphos (Kimmerle and Solmecke, 1971a). In an earlier study female
rats survived daily ip administration of 1 mg/kg for 60 days while 40%
of those administered 2 mg/kg did not survive. All rats died at 3
mg/kg administered by intraperitoneal injection (DuBois and Flynn,
1968). These studies indicate little if any cumulative toxicity.
Dog
Groups of beagle dogs (two males and two females/group) were fed
fenamiphos in the diet at 0, 2, 6, and 18 ppm for three months.
Behavioral abnormalities, evidenced by signs of cholinergic
stimulation, were evident at 18 ppm in the diet and growth of females
at this high level was reduced. Average haematological and clinical
chemistry values including liver function tests, kidney function tests
and urinalyses were not apparently affected by fenamiphos in the diet.
Averages of plasma and red blood cell cholinesterase values were
depressed at 4 ppm in the plasma of both males and females. At 2 ppm
no effect on the average red cell cholinesterase values was found
while plasma cholinesterase was depressed (20-30%) in both sexes.
Gross morphological examination of tissues and organs at the
conclusion of the study indicated no abnormal effects of fenamiphos in
the diet (Löser, 1968a).
Groups of beagle dogs (two males and two females/dose, three of
each sex were used in the control group) were fed fenamiphos in the
diet at levels of 0, 1, 2 and 5 ppm for twelve weeks. There was no
effect of feeding fenamiphos at levels up to and including 5 ppm in
the diet on the average of any of the parameters examined including
haematology, liver function tests, clinical chemistry tests, kidney
function tests, and gross and microscopic examination of tissues and
organs. As with other studies, cholinesterase was the only parameter
significantly affected, with the females being more susceptible than
the males and the plasma being more sensitive to inhibition than red
blood cell. Erythrocyte cholinesterase was unchanged at 2 ppm while
plasma cholinesterase was slightly depressed. One ppm in the diet had
no effect on plasma cholinesterase. As the depression of plasma
cholinesterase was minimal and transient at 2 ppm it might be
considered that this would be a no-effect level in this experiment
(Löser, 1969; Mawdesley-Thomas and Urwin, 1970b).
As an extension of the previous short term study in dogs (Löser,
1969), an additional two male and two female dogs were fed fenamiphos
in the diet at 10 ppm for three months. Two male and two female
animals were also maintained as a control. There was no mortality over
the course of the experiment and a very slight deviation in the
average body weight of both males and females was noted. Because of
the small sample population, the slight weight differences cannot be
fully evaluated. There was no effect of feeding fenamiphos on any of
the haematological parameters examined. Urinalysis, clearance
examinations, blood sugar and cholesterol were normal over the course
of the experiment. Cholinesterase activity was significantly depressed
in both plasma and erythrocytes and in both male and female dogs. In
addition, several abnormalities were noted with regard to some of the
serum enzyme parameters related to liver function after three months
of feeding, especially in male dogs. Alkaline phosphatase and SGPT
activity were significantly increased. Following gross and microscopic
examination of tissues and organs, no significant differences were
noted between the control and those animals fed 10 ppm. The abnormal
findings in some enzyme parameters related to liver functions,
predominantly in males at three months of feeding 10 ppm in the diet,
were not accompanied by pathological findings at autopsy nor confirmed
in another test where fenamiphos was fed at levels of 18 ppm in the
diet. They are considered a transient occurrence and not directly
related to the presence of fenamiphos in the diet (Löser, 1970;
Thompson et al., 1972a).
Groups of pure bred beagle dogs (four males and four females per
group) were fed fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5,
and 10 ppm for two years. There were no significant effects noted with
fenamiphos at any dietary level with regard to growth, food
consumption or any of the standard clinical and physiological
examinations made during the course of the study. One dog died after
being fed 0.5 ppm in the diet. This dog was considered to have died of
pneumonia and its death was not related to administration of
fenamiphos. Gross and histological examinations of all tissues and
organs at the conclusion of study did not reveal any abnormal
developments considered to have been a result of feeding fenamiphos.
The only significant physiological effect observed was
anti-cholinesterase activity which was marginally depressed in plasma
at 2 ppm. As with other species, the plasma appears to be the most
sensitive indicator of exposure with a no-effect level being 1 ppm in
the diet (equivalent to 0.029-0.036 mg/kg/day) (Löser, 1972b; Thompson
et al., 1972b).
Long term studies
Rats
Groups of SPF derived Wistar strain (forty males and forty
females per group, eighty males and eighty females used as controls)
were fed fenamiphos in the diet, at concentrations of 0, 3, 10, and 30
ppm for two years. Behavioral abnormalities were evident, as signs of
cholinergic stimulation, only during the first six weeks of feeding at
30 ppm. After six weeks of feeding, the obvious anticholinesterase
effect disappeared and was not evident for the remainder of the two
year study. There were no differences in averages at any feeding level
on growth, mortality, food consumption, haematological values,
clinical chemistry values or liver and kidney function tests. Urine
examinations as well as blood sugar and cholesterin values were
normal. Thyroid weights and thyroid-to-body weight ratios of females
were heavier than normal at 30 ppm. All other major tissues and organs
appeared normal under gross and microscopic examination. The thyroid
weight in females at 30 ppm was high but was not accompanied by an
abnormal tumour development, goitre or by unusual histological
findings.
Cholinesterase determinations indicated that plasma
cholinesterase was the most sensitive parameter. At 10 ppm in the
diet, fenamiphos inhibited plasma cholinesterase. No significant
effects were observed at 3 ppm in the diet (equivalent to 0.17-0.23
mg/kg bw/day) (Löser, 1972a; Cherry and Newman, 1973).
COMMENTS
Fenamiphos, an acutely toxic organophosphate ester, is rapidly
absorbed as evidenced by rapid onset of cholinergic signs of
poisoning. In animals fenamiphos is rapidly degraded, predominantly
through oxidation to acutely toxic products. Fenamiphos does not
appear to affect biochemical systems other than cholinesterase.
Testing for the delayed neurotoxic effect commonly seen with TOCP was
negative. Short term and long term dietary studies in both rats and
dogs have indicated, with the exception of cholinesterase depression,
no unusual effects. In long term studies with rats, transient
behavioural abnormalities were observed during the first six weeks of
feeding, which disappeared and were not evident for the remainder of
the study. Female rats are more susceptible than males to
cholinesterase depression. Plasma cholinesterase depression is a
better indicator of exposure than red blood cell cholinesterase. Based
on plasma cholinesterase depression, a no-effect level in a two-year
rat study is considered to be 3 ppm in the diet and in a two year dog
study, 1 ppm in the diet. Long term studies in rats and mice reported
to the Meeting did not show a carcinogenic effect. Although there are
no studies available in man, sufficient data are available and the
Meeting allocated an ADI using the more sensitive species, the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 ppm in the diet equivalent to 0.17 mg/kg bw.
Dog: 1 ppm in the diet equivalent to 0.029 mg/kg bw.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0006 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenamiphos is an organophosphorus compound with a systemic action
and a powerful nematicidal effect. It is active against root-knot,
cyst-forming and free-living nematodes and has important applications
against sucking insect pests and spider mites. Fenamiphos is
commercially used in the USA, Australia, Spain, the Cameroons,
Caribbean, Chile, Columbia, Costa Rica, Ecuador, Guatemala, Ivory
Coast, Jamaica, Martinique, Mexico, Nicaragua, San Salvador and
Uruguay. Registration proceedings and trials are in progress in
several other countries.
Fenamiphos is formulated as 5 and 10% granules and as a 40%
emulsifiable concentrate.
Pre-harvest treatments
Fenamiphos is formulated as a granular product and as an
emulsifiable concentrate. Both formulations are recommended chiefly
for treatments in banana, pineapple, vegetable (tomatoes, brassicas,
peppers, eggplants), tobacco, coffee, groundnut and citrus crops.
Application is usually made to the soil at sowing, planting and
transplanting, and in established crops. The general dose rate is 5 to
10 kg active ingredient per hectare for broadcast (overall)
application, but is reduced for band application according to band
width. Furthermore, the emulsifiable concentrate is suitable for dip
treatments of banana seedlings and for foliar spraying of pineapples.
Details of uses and recommendations are given in Table 4.
TABLE 4 Use Pattern of fenamiphos (All applications are pre-harvest against nematodes)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Bananas 1.5-2.5 g/m2 At planting G
3.0 g/plant Soil treatment in
established crop G 3-4
4-5 kg/ha In irrigation
canals or direct
to soil EC 2
1-1.5 g/corm Dip treatment
pre-planting plus
soil application EC
2 or 1.5 g/
planting position
(but no more than
total of 3 g/
planting position) G
Pineapples 10-40 kg/ha Broadcast treatment
pre-planting of
crowns and slips G
5-20 kg/ha Broadcast treatment
pre-planting of
crowns and slips
in combination with G
3-5 kg/ha foliar spraying 3 in
post-planting LC established
crop
15 kg/ha Foliar spray
application at
planting, repeat
after an interval
of 2 months EC 2
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Tomatoes 1-2.5 g/m2 Immediately before
or after sowing G
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Brassicas 5-8 kg/ha Broadcast/overall
application G,
pre-planting EC
Tobacco 1-2.5 g/m2 Before or immediately
after sowing or G
0.1-0.2 g/plant at transplanting or
in established crop G 1
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Coffee 1-2.5 g/m2 Seedbed treatment G
1.5-2.5 g/shrub In established crop G 2-3
Groundnuts 3-5 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Cucurbitaceae 0.15-0.2 g/ Band application
planting hole or at sowing G
1.5-2.5 kg/ha
Peppers 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Eggplant 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Citrus 1-1.5 g/m2 Seedbed treatment G
2-3 g/tree Treatment in nursery G
1.5-3 g/tree In established crop G, LC 2-3
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Potatoes 5-10 kg/ha Broadcast/overall G, EC,
treatment pre-planting LC 1
Pyrethrum 5-8 kg/ha Broadcast application
at planting G
Ornamentals 1.5-3 g/m2 In established crops G
Cotton 1.7-3.5 kg/ha Band application
(band width of G,
30-45 cm.) at sowing LC
Beets 3-(6) kg/ha Band application
immediately after
sowing or during G,
emergence EC
Soybeans 5-8 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Beans 5-8 kg/ha Broadcast application
pre-sowing G 1
Grapes 2-3 g/plant G 1
Strawberries 10 kg/ha Broadcast application
pre-planting G
300 g/1000 Post-planting or G
metres of row post-harvest
Lawns 10-20 kg/ha Broadcast application G
1 G = Granules; EC = Emulsifiable concentrate; LC = Liquid concentrate.
Post-harvest treatments
No recommended uses.
Other uses
Fenamiphos is recommended for use on ornamentals (granular
formulation).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of fenamiphos together with its sulphoxide and
sulphone resulting from supervised trials in a wide range of crops are
shown in Table 5. In most crops, residues were below 0.05 mg/kg. The
exceptions were grapes, lettuce, potatoes, strawberries and tomatoes
which contained up to about 0.1, 0.5, 1, 0.7 and 0.3 mg/kg
respectively. Residues in tobacco were much higher, with respective
maximum levels in green, cured and fermented tobacco of about 12, 140
and 100 mg/kg.
FATE OF RESIDUES
In animals
A feeding study was carried out on a lactating dairy cow to
determine whether fenamiphos sulphoxide, found in plants as the major
metabolite, would be incorporated into milk and animal tissues either
as the intact compound or in metabolized form (Gronberg et al., 1974.
See also "Biotransformations"). A single dose of 0.8 mg/kg body
weight, corresponding to 27 ppm in the diet, of ring-14C fenamiphos
sulphoxide was administered. Four hours after application the label
was distributed as follows. Urine 39%, faeces <0.1%, rumen 47%,
tissues 1.4%, bile 0.1%, omasum, abomasum and milk <0.1% each; total
recovery 88%. The following compounds were found: fenamiphos (I),
fenamiphos sulphoxide (II), desisopropyl-fenamiphos (III), phenol of
fenamiphos (IV), phenol of fenamiphos sulphoxide (V), phenol of
fenamiphos sulphone (VI) and "unknown" (VII). The levels of these
residues in tissues and milk are shown in Table 6.
Of the dose, 18% was reduced to the parent fenamiphos, apparently
by microorganisms. At 4 hours 82% of the activity in whole blood and
87% of the activity in urine was identified as conjugated phenols.
The residues of fenamiphos in poultry eggs and tissues were
determined by Gronberg et al. (1973). Laying hens were fed for 14 days
with carbon-14 ring-labelled fenamiphos in feed at 0.06, 0.18 and 0.65
ppm concentration. During this time, residues in eggs were below the
detection limit of 0.003 mg/kg. At slaughter, all tissue residues from
birds given feed containing 0.65 ppm were below the limit of
determination of 0.003 to 0.005 mg/kg, although very low residues were
detectable in some tissues.
TABLE 5 Residues of fenamiphos and metabolites resulting from supervised trials
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Apples Australia 10% G 22.4 334 1 n.d.2 n.d.2
Bananas, Central
peel America 10% G 2.8-12 g
a.i./plant 1-195 41 <0.025-0.3 <0.025
pulp <0.025 <0.025
Bananas, Ivory Coast 5% G 10 g a.i./plant 1-14 4 <0.02 <0.02
peel
pulp <0.02 <0.02
peel 28-88 3 0.06-0.1 0.07
pulp 0.03-0.08 0.05
Broccoli USA 3% G;
15% G 6.72 65-101 8 <0.01-0.04 0.02
15% G 10.0 92 1 0.2 0.2
Brussels
Sprouts USA 3% SC;
15% G 6.72-10.0 107-133 5 <0.01-0.02 <0.01
Cabbage USA 3% SC;
15% G 6.72-10.0 55-108 14 <0.01-0.02 0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 40% EC 4.5 42-106 6 n.d.2 n.d.2
Carrots USA 15% G 6.72 96-160 9 <0.01-0.05 0.01
Cauliflower USA 3% SC;
15% G 6.72-10.0 97-111 12 <0.01-0.04 0.02
Coffee Brazil - 1-2 g a.i./plant 14-15 6 <0.02-0.07 0.05
beans, Central
green America 28-30 6 <0.02-0.09 0.05
56-62 4 <0.02-0.05 0.03
90 2 0.03; 0.05 0.04
Cottonseed USA 3% SC;
15% G 1.12 157-196 10 <0.01 <0.01
Cotton USA 3% SC;
gin trash 15% G 1.12 161-196 8 <0.01 <0.01
Cotton USA 3% SC;
foliage 15% G 1.12 143-148 4 <0.01-0.1 0.04
Grapefruit USA 3% SC;
peel 15% G 33.6 - 50.4 183-184 1 <0.01 <0.01
pulp <0.01 <0.01
whole fruit <0.01 <0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Grapes Australia 5% G
43% EC 5.6 - 14.7 145-147 2 n.d.2 n.d.2
22.0 145-147 1 0.03 0.03
44.0 145-147 1 0.08 0.08
Leaf-lettuce USA 3% SC 11.2 46 1 0.3 0.3
Head USA
lettuce, 3% SC;
head 15% G 11.2 61-72 4 0.02-0.1 0.08
wrapper leaves 0.09-0.5 0.3
discard leaves 0.09-6.5 1.9
Lemons, USA
whole fruit 3% SC 33.6 184 1 0.01 0.01
Limes, USA 3% SC;
whole fruit 15% G 33.6 147 2 <0.01 <0.01
Melons Guatemala
Australia 5% G
Egypt 40% EC 1.6-10.0 56-112 5 n.d2-0.05 n.d.2
Oranges, USA 3% SC; 22.4-50.4 182-366 4 <0.01-0.1 0.04
peel 10% G;
15% G
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
pulp 4 <0.01 <0.01
whole fruit 5 <0.01-0.02 0.01
whole fruit Australia 5% G;
40% EC 11.2-22.4 21-139 10 n.d.2 n.d.2
Peanuts, USA 3% SC;
kernels 10% G 4.51-13.45 122-148 5 <0.01-0.02 <0.01
shells (0.01-0.2 0.06
vines 98-148 5 0.02-4.3 2.3
Pineapples Hawaii, USA 3% SC;
10% G 44.8-112 238-658 10 <0.01-0.03 <0.01
3% SC
(foliar spray) 5.6 1-29 20 <0.02-4.0 0.5
Potatoes USA 3% SC;
15% G (row
appl) 6.1 41-58 3 <0.02-1.2 0.7
71-106 7 <0.01-1.0 0.15
Potatoes, USA, Canada, 3% SC;
whole Fed. Rep. of 15% G
Germany, UK, (broadcast
Australia 4-5-11.2 67-203 23 n.d.2-0.45 0.07
peel 1 0.07 0.07
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
flesh 1 0.07 0.07
Sweet USA 3% SC;
Potatoes 15% G
(broadcast) 6.72 108-143 8 <0.01-0.04 0.01
3% SC;
15% G
(row appl) 4.0-6.1 108-143 8 <0.01-0.06 0.01
Soybeans, USA 3% SC;
bean, green 15% G 11.2 75-117 12 <0.01-0.09 0.04
vine, green 75-117 12 <0.01-12 2.9
bean, dry 131-159 11 <0.01-0.03 0.01
Strawberries Australia 5% G
40% EC 16.5-22.5 36-121 6 0.03-0.7 0.2
Sugarbeets, USA 3% SC;
tops 15% G 6.72-10.0 119-187 9 <0.01-0.08 0.01
beets <0.01-0.03 0.01
Tangerines, USA 3% SC;
peel 15% G 33.6 247-251 2 0.07; 0.3 0.2
pulp 2 <0.01; 0.03 0.02
whole fruit 1 0.03 0.03
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Tobacco Australia 40% EC 50-95 4 0-5-4.8 1.9
Tobacco, USA 3% SC; 6.72 4 3 0.9-7.8 3.5
green 15% G 7 3 0.8-7.0 2.9
11 3 0.8-3.3 1.8
14 3 1.4-12 5.2
21 3 1.2-4.9. 2.5
28 3 0.4-4.2 1.7
35 3 0.25-3.6 1.4
36-148 13 0.02-4.8 1.0
air cured 88-106 2 3.2; 4.2 3.7
flue cured 85 2 11; 13 12
cured 75-91 6 4.5-140 33
aged 75-92 3 5.7-16 11
fermented 89 1 98 98
Tomatoes USA, 3% SC;
Canada 15% G 11.2-16.8 61-89 10 <0.01-0.3 0.1
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 5% G;
40% w/v 9.8-11.2 78-161 5 <0.05 <0.05
Fed. Rep, of
Germany 5% G 9.8-12.2 79-161 5 n.d.3 n.d.3
1 EC = emulsifiable concentrate; G = granules; SC = spraying concentrate. n.d. = <0.01.
2 <0.01 mg/kg.
3 <0.05 mg/kg.
TABLE 6 Residues, mg/kg, in tissue and milk of cow fed fenamiphos
sulphoxide (Gronberg et al., 1974)
Compound1 Fat Meat Liver Kidney Milk
I 0.004 n.d.2 0.006 n.d. n.d.
II n.d. n.d. 0.002 n.d. 0.002
III 0.002 n.d. 0.005 0.16 0.004
IV 0.005 0.001 0.029 0.86 0.005
V 0.003 0.003 0.021 0.36 0.025
VI n.d. n.d. n.d. 0.01 0.004
VII 0.002 0.005 0.036 0.25 0.020
1 For identities of compounds, see text.
2 n.d., not detectable, = <0.001 mg/kg.
In plants
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thiooxidation of the parent compound to
the sulphoxide and sulphone. Both metabolites are detoxified by
hydrolysis, generally followed by glucosylation of the phenolic
products.
Fenamiphos-14C,3H was injected into the stems of beans,
tomatoes, peanuts and potatoes and the plants were harvested at 7, 14,
21 and 28 days (Waggoner, 1972. See also "Biotransformations"). Two
major metabolites were identified by TLC, isotopic ratios, IR, and MS
as the sulphoxide and sulphone of fenamiphos. The sulphoxide was the
major metabolite, accounting for 33-41% of the residue, followed by
the sulphone (31-33%), both after 7 days' treatment. Two minor
metabolites containing intact phosphoramidate structures accounted for
not more than 1.5 and 0.2% of the total residue.
Khasawinah studied fenamiphos metabolism in carrots (1973a),
tomatoes (1973b), cabbages (1973c), soybeans (1972b) and tobacco
plants (1971) following soil application, and in snap beans (1972a)
after both soil treatment and stem injection; 14C-ring, 14C-1-ethyl
and 3H-methylthio labelled fenamiphos were used.
Carrots readily absorbed and metabolized fenamiphos and its major
soil foliage and 14C from the ethyl group was detected in the foliage
and carrots. This 14C was probably incorporated into lipids,
pigments, sugars and structural elements such as cellulose.
Water-soluble residues were mainly conjugates of the sulphoxide and
sulphone phenols. No parent fenamiphos was detected. Residues of the
sulphoxide and sulphone were below 0.06 mg/kg in both carrots and
foliage. At the end of the growing period, soil residues were 2.7
mg/kg fenamiphos equivalents (one third of the amount applied) of
which 65% consisted of the sulphoxide and sulphone in the ratio 4:1.
Results with tomatoes were similar. Sulphoxide and sulphone
residues reached a peak of 0.2-0.3 mg/kg 30-40 days after treatment,
and no parent compound was detected later than 40 days. The results
showed the stability of the thio-methyl-ring linkage.
Comparable results were obtained with head cabbage. An
organosoluble polar metabolite with an apparently intact
phosphoramidate structure was detected soon after treatment and
declined gradually to insignificant levels.
In tobacco plants the sulphoxide reached a peak in less than one
week, and then decreased as the sulphone increased. At the end of the
growing period the ratio of sulphoxide to sulphone was about 3:2.
Soybeans were planted in soil that had been treated with
14C-ethyl, 3H-methylthio fenamiphos and cropped with tobacco for one
growing season. The soil at soybean planting contained 0.65 mg/kg
fenamiphos sulphoxide, 0.07 mg/kg fenamiphos sulphone and 0.18 mg/kg
fenamiphos equivalents as unextractable material. Nineteen days old
soybean seedlings contained 16.9 mg/kg (fresh weight basis) of these
two soil metabolites in a sulphoxide: sulphone ratio of 73:17. In the
mature plant (150-day growing period), the total concentration of the
two metabolites, found in equal proportions, was 3.5 mg/kg in the dry
leaves and 0.19 mg/kg in the dry beans. The water-soluble
radioactivity consisted mainly of the glucose conjugate of the
sulphone phenol. The insoluble radioactivity was not characterized.
The fate of 14C1-ethyl and 3H-methylthio fenamiphos was
investigated in snap beans following stem injection and soil treatment
in systems designed for the recovery of the applied nematicide. The
study demonstrated volatile 14C radioactivity, presumably as CO2.
40% of the stem-injected and 12% of the soil-applied fenamiphos was
recovered in this form. Volatile tritium radioactivity was less, 7.4%
and 1.3% of the amounts applied by stem-injection and soil treatment,
respectively. The metabolites formed by both methods of application
were fenamiphos sulphoxide, fenamiphos sulphone and the sulphoxide and
sulphone phenols in the free form or conjugated with glucose.
The metabolism of similarly labelled fenamiphos applied by stem
injection, soil drench or spray to fruited pineapple plants was
studied by Flint (1973). Soil treatment at 20 pounds a.i./acre
resulted in maximum total radioactive residues in fruit of 0.086 mg/kg
fenamiphos equivalents at 90 days after treatment. Metabolites
identified in fruit and foliage were identical to those previously
found in several other plant and animal systems.
In soil and water
Khasawinah (1970) treated the top 1.5 cm of a sandy loam soil, in
which tobacco was growing, with labelled fenamiphos. One third of the
applied amount was taken up by the growing plants during an 11-week
period. The major product in the soil was the sulphoxide, with small
quantities of the sulphone. No hydrolysis products of the parent
compound or its oxidation products were detected in the soil extracts.
None of the radioactivity was leached into the drainage water, and all
of it remained in the root zone. About 22% of the radioactivity
remaining in the soil after 177 days was bound to clay particles and
was unextractable.
Fenamiphos was studied for soil runoff, leaching, adsorption, and
stability in water by Flint et al. (1971) and Church (1971). It was
moderately adsorbed by sandy loam and silt loam soil. Fenamiphos
residues in runoff water were less than 3.4% of the applied compound
over a 37-day interval during which 2.3 to 3.7 inches of irrigation
were applied; the application rate of fenamiphos was 22.5 kg a.i./ha.
The leaching of fenamiphos from the three types of soil was inversely
proportional to the adsorptivity of the soils. It was estimated that
111 inches of rainfall would be needed for clay loam soil and 75
inches for sandy loam to leach fenamiphos to a depth of 12 inches.
Fenamiphos was relatively stable in aqueous systems at pH 5 and 7
in the laboratory but declined rapidly with a half-life of 4.8 days in
a simulated field pond at PH 7 and average temperature of 29°C.
The leaching of "aged" fenamiphos residues in sandy loam soil was
investigated by Tweedy et al. (1974). Ring-14C fenamiphos was
incubated with sandy loam soil under greenhouse conditions for 30 days
and then applied to the top of a sandy loam soil column 2.9 inches in
diameter and 12 inches high. Distilled water, equivalent to 0.5
acre-inch/day was added daily for 45 days. Of the radioactivity
recovered from the system, 77.5% was found in the top fourth of the
column and only 2.3% was recovered in the leachate. The majority of
the activity, 65%, apparently did not move at all.
The metabolism of fenamiphos soil residues under aerobic and
anaerobic conditions was investigated by Shaw and Flint (1974).
Fenamiphos, labelled with tritium in the methylthio group or with
carbon-14 uniformly throughout the aromatic ring, was used in this
study. The results indicated that anaerobic conditions arrested
further degradation while aerobic incubation allowed continued
metabolism. Essentially no 14C or 3H radioactivity was lost during
incubation indicating stability of the aromatic ring system. The major
metabolite was fenamiphos sulphoxide with some further thio-oxidation
and hydrolysis observed under aerobic conditions.
The effect of fenamiphos on soil microbial populations was
studied by Houseworth and Tweedy (1972) using Indiana clay loam and
Commerce silt loam soils. The soils were treated with 50 and 250 mg/kg
of the pesticide and maintained at 50% field moisture capacity for 56
days. At intervals, samples of soil were removed from each treatment
and assayed for populations of bacteria, fungi and Actinomycetes.
Fenamiphos had no significant effect on them under the conditions of
the experiment.
The photodecomposition of thin films of fenamiphos was
investigated by Khasawinah and Sandie (1974). Thin films of dual
labelled ethyl-1-14C, methylthio-3H fenamiphos were coated on glass
plates and silica gel coated glass plates and exposed in the open air
for about three weeks. Fenamiphos was rapidly oxidized to its
sulphoxide and then to the sulphone. Some hydrolysis of the sulphone
occurred after long intervals, especially on glass.
The results of some studies in the USA of the persistence of
fenamiphos and its oxidation products in various types of soil were
available to the Meeting. They are reproduced in Table 7.
In storage and processing
The effect of alkali refining and steam deodorization on residues
in cottonseed and soybean oils was studied in laboratory simulation
experiments by Thornton (1973a) and Olson (1972b, 1974). In
cottonseed, the two processes in combination removed 98.5% of the
fenamiphos, 98.4% of the sulphoxide and 88% of the sulphone. Since the
sulphone would constitute only a small proportion of a naturally
occurring residue, the combined processes would remove nearly all the
residue. In soybeans, the effects of the processes on sulphoxide
residues was studied, as this is the main component of the residue
after long post-treatment intervals. The simulated refining process
removed 96% of the sulphoxide, and deodorization removed 38%. A
hypothetical residue of 0.42 mg/kg should therefore be reduced to 0.01
mg/kg by the two steps.
The effect of citrus processing on residues of fenamiphos
sulphoxide was investigated by Olson (1972a). Commercial citrus
processing involves liming and drying of the chopped solids in the
preparation of cattle feed. Fenamiphos sulphoxide, the main metabolite
in crops, was resistant to the liming step but 39% of the residue was
removed by drying. To prepare citrus oil, an aqueous emulsion is
centrifuged vigorously. 99% of any fenamiphos sulphoxide present in
the emulsion would be removed from the oil by this procedure.
TABLE 7 Persistence of fenamiphos + sulphoxide + sulphone in various soils in the USA1
Residue, phenamiphos + sulphoxide + sulphone, mg/kg, after interval, days2
0 30-40 80-100 180-200 360-400
Soil Range Mean Range Mean Range Mean Range Mean Range Mean
Silt loam 1.2-15 7.7 5.4-12 9.4 0.8-15 6.9 0.1-6.5 2.5 0.1-2.5 1.1
Clay 1.0-1.7 1.3 3.7 3.7 1.5-2.7 2.1 0.6-1.3 1.0 0.6-0.7 0.7
Sand muck 5.1-6.3 5.7 5.1-5.8 5.4 0.1 0.1 0.7-1.0 0.8 0.4-0.6 0.5
Sand 5.5 5.5 0.3-0.6 0.5 0.1-0.2 0.2 0.06-0.07 0.07 0.03-0.04 0.04
Sand clay 6.8-8.5 7.7 3.0-4.9 3.9 0.5-1.2 0.8 0.1 0.1 0.1 0.1
Fox sand 4.3-9.2 6.7 6.8-8.0 7.4 3.1-3.2 3.1 1.0-1.3 1.1 0.9-1.0 0.9
Peat 15-16.5 16 17-20 18 12.5-17 15 6.8-8.8 7.4 6.3-7.2 6.8
Low humus 13-17 15 4.6-6.6 5.5 3.6-6.6 5.1 1.2-3.3 2.2 0.3-0.6 0.4
1 Fenamiphos was added to soils at 10mg/kg level as granules or spray concentrate.
2 Usually two or three analyses at each interval.
Olson (1970a) studied the fate of residues on pineapple crowns.
The fresh crowns were dipped in an aqueous emulsion of spraying
concentrate containing 0.12% fenamiphos, and allowed to drain dry. The
surface residue immediately after treatment amounted to about 30 mg
per crown, of which about half could be removed by extracting with
water. Seven days after treatment, aqueous extraction removed about
one-third of the original residue. Surface extraction with benzene,
however, removed virtually all the initial deposit (29 mg per crown)
but only 27% (8 mg) after 7 days.
Storage of broccoli, carrots, peanut kernels, pineapple fruit and
potatoes at -18° to -23°C for two years showed no significant loss of
fenamiphos, the sulphoxide or the sulphone (Anonymous, 1973).
There was no significant loss of residues (parent + sulphoxide +
sulphone) in field-treated potatoes or of fenamiphos from potatoes
fortified in the laboratory when cooked at 100°C for 30 minutes in
sealed jars (Thornton, 1972).
METHODS OF RESIDUE ANALYSIS
A specific gas-chromatographic procedure for the determination of
residues of fenamiphos and sulphoxide and sulphone in various crops,
animal tissues and milk is described by Thornton (1969, 1971a).
Following initial extraction, the extract is oxidized with potassium
permanganate to convert fenamiphos and its sulphoxide to the sulphone.
The sulphone is then determined by gas chromatography with alkali
flame ionisation detection. Recoveries from a variety of crops and
animal tissues fortified with fenamiphos or its metabolites at the
blending step were generally between 75 and 110%. Interference studies
by Thornton (1971b, 1973b) and Olson (1970d, 1971b,c) have shown the
method to be specific in the presence of other possible
organophosphorus compounds. The limit of detection is about
0.02 mg/kg.
The determination of residues of fenamiphos and its metabolites
in tobacco and tobacco smoke was successfully investigated by Olson
(1971a) with the Thornton (1971a) method. This method should be
suitable for regulatory purposes.
The determination of fenamiphos residues in soil by thermionic
emission gas chromatography is described by Olson (1970b). The soil
sample is extracted in a Soxhlet apparatus, using a 1:1
chloroform-methanol solvent mixture. The solvent is evaporated and the
residue oxidized and chromatographed. Recoveries of fenamiphos and its
metabolites were 80-107%. The sensitivity of the method is
approximately 0.02 mg/kg. Olson (1970c) has also extended the
procedure to a number of other organophosphorus pesticides.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table 8.
TABLE 8 National tolerances reported to the Meeting
Safety
Tolerance Interval
Count Crop mg/kg (days)
Australia Tomatoes, brassicas,
cucurbits, lettuce, citrus,
pineapples, grapes,
bananas, ginger No residue
Spain Tobacco sugarbeets, etc.
(industrial purpose),
bananas 60
USA Bananas, citrus 0.1 180
APPRAISAL
Fenamiphos is a systemic nematicide which gives good control of
root-knot cyst forming and free living nematodes. It is also effective
against sucking insect pests and spider mites. It is formulated as 5%
and 10% granules and as an emulsifiable concentrate (40%). Both
formulations are recommended chiefly for treating bananas, pineapples,
tomatoes, brassicas, eggplant, tobacco, groundnuts, citrus crops,
ornamentals and pyrethrum. It is chiefly applied to the soil at sowing
or planting. The general dose rate is 5-10 kg active ingredient/ha for
broadcast application, but is reduced for band application. The
emulsifiable concentrate is especially suitable for dip treatment of
banana seedlings and for foliar spraying of pineapples. Fenamiphos is
commercially used in Australia, USA, some Central and South American
countries, Spain, the Cameroons and the Ivory Coast.
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thio-oxidation of the parent compound
to the sulphoxide and sulphone. In general the major part of the
residue in crops and soil is the sulphoxide, followed by the sulphone.
Both are relatively persistent. Both metabolites are detoxified by
hydrolysis, usually followed by conjugation of the phenolic products
as glycosides. Little or none of the parent fenamiphos seems to be
present in most crops at harvest.
Micro-organisms in soil do not seen to be affected by fenamiphos.
The dissipation of residues in soil depends on the type of soil,
increasing in the order peat < silt loam < clay = sand. Fenamiphos
is only very slowly leached from soil.
Following application in accordance with good agricultural
practice, the maximum residue levels of fenamiphos including its
sulphoxide and sulphone in Brussels sprouts, cotton seed, cabbage,
carrots, cauliflower, grapefruit, lemons, limes, melons, oranges
(pulp), peanut kernels, pineapples, soybeans (dried) and sugar beets
are at or below 0.05 mg/kg. Higher residues are found in some other
crops. Typical maximum residues are: grapes 0.1 ppm, lettuce 0.5 ppm,
potatoes 1 ppm, strawberries 0.7 ppm, tomatoes 0.3 ppm and tobacco
(green) 12 ppm, (cured) 140 ppm (fermented) 100 ppm. Data for
fenamiphos residues in tobacco smoke are not available.
Processing cottonseed or soybeans to oil removed 98-99% of the
residues and processing citrus to cattle feed removed about 40%.
Cooking potatoes had no significant effect. In a laboratory experiment
approximately one third of the fenamiphos originally applied was
extractable with water from the surface of the crowns of pineapples
seven days after dipping in an aqueous emulsion containing 0.12%
fenamiphos.
Residues in eggs and tissues from hens fed for 14 days with 14C
ring-labelled fenamiphos in feed at 0.65 ppm were below 0.005 mg/kg.
Residues in milk and tissues of a dairy cow fed once with a dose
corresponding to 27 ppm fenamiphos sulfoxide in the diet were below
0.01 ppm.
Specific gas-chromatographic procedures for the determination of
residues of fenamiphos, its sulphoxide and sulphone in various crops,
animal tissues, milk and soil have been elaborated. The initial
extract is oxidized with permanganate to convert fenamiphos and its
sulphoxide to the sulphone. The sulphone is determined with an alkali
flame ionisation or flame photometric detector. Limits of detection
are about 0.02 ppm, and recoveries higher than 75%. The methods should
be suitable for regulatory purposes.
RECOMMENDATIONS
The following limits refer to fenamiphos including its sulphoxide
and sulphone, expressed as fenamiphos.
TOLERANCES
Tolerance
Crop (mg/kg)
Bananas, coffee beans (green and
roasted, grapes, sweet potatoes 0.1
Broccoli, Brussels sprouts, cabbage,
carrots, cauliflower, citrus fruits,
cottonseed, melons, peanut kernels,
pineapples, soybeans (dried), sugar beets 0.05*
* At or about the limit of determination.
TEMPORARY TOLERANCES
Temporary
tolerance
Crop (mg/kg)
Potatoes, tomatoes 0.2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further data on which to judge the residues in or on potatoes and
tomatoes.
2. Adequate residue data on which to base recommendations for
tolerances for other crops (e.g. beans, cucumbers, lettuce,
peppers and strawberries).
DESIRABLE
1. Brain cholinesterase and behavioural studies in animals exposed
to low levels for extended periods.
2. Observations in man.
3. Additional studies on the potentiation effects with other
organophosphorus pesticides.
4. Residue data for raw agricultural products moving in commerce.
5. Further residue data for different crops from supervised trials
including data on rates and frequencies of application, soil,
foliar or other treatment and pre-harvest intervals, especially
for broccoli and tangerines.
REFERENCES
Anonymous. (1973) The effect of frozen storage at 0 to -10°F on
Nemacur residues in peanut meat, broccoli, carrot roots, potatoes,
pineapple fruit. Chemagro Report No. 36132, 36318, 36319, 36320,
39094.
Arnold, D., Keplinger M. and Fancher, O. (1971) Mutagenic study with
Nemacur(R) (BAY 68138) Technical in albino mice. Report from
Industrial BioTest Laboratories, Inc., submitted by Bayer AG.
(Unpublished).
Cherry, C., Urwin, C. and Newman, A. (1972) Pathology report of BAY
68,138. Rat breeding study. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished).
Cherry, C. and Newman, A. (1973) Pathology report of Bayer 68,138.
Chronic toxicological studies in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Church, D.D. (1971) Nemacur - leaching, runoff and water stability.
Chemagro Report No. 26 849.
Coulston, F. and Wills, J.H. (1974) Summary of research of the
Institute of Comparative and Human Toxicology, Albany Medical College,
on fenamiphos. Report submitted by Bayer AG. (Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970a) The skin and eye
irritating properties of BAY 68,138 3 lb/gal S.C. to rabbits. Report
from Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970b) The acute inhalation
toxicity of Nemacur(R) 15% granular to rats. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C, and Anderson, R. (1971) The skin and eye irritating
properties of Bay 68138 technical to rabbits. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1972a) The acute dermal toxicity of
Nemacur(R) technical to rabbits. Report from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (unpublished).
Crawford C. and Anderson, R. (1972b) The acute dermal toxicity of
Nemacur(R) 3 lbs/gal. spray concentrate to rabbits. Report from
Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford C. and Anderson, R. (1973a) The dermal toxicity of Nemacur(R)
15% granules to rats. Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1973b) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R) with
analytical grade Nemacur(R). Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford C. and Anderson, R. (1973c) The eye and skin irritancy of
Nemacur(R) 2 lb/gal spray concentrate to rabbits. Report submitted by
Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1974) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Report from Chemagro Division of Baychem Corporation, submitted by
Bayer AG. (Unpublished).
DuBois, K., Flynn, M. and Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Report from University of
Chicago. (Unpublished).
DuBois, K. and Flynn, M. (1968) The subacute parenteral toxicity of
BAY 68138 to rats. Report from University of Chicago, submitted by
Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1970) Acute oral and dermal toxicity of
a granular formulation of BAY 68138. Report from University of
Chicago, submitted by Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1971) The acute oral and inhalation
toxicity of a Nemacur(R) (BAY 68138) formulation to rats. Report from
University of Chicago submitted by Bayer AG. (Unpublished).
Flint, D.R. (1973) The metabolism of Nemacur in pineapple. Chemagro
Report No. 39 119.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour, J., II. (1971) The
mobility and persistence of Nemacur in soil and water. Chemagro Report
No. 29 974.
Gronberg, R.R. (1969) The metabolic fate of
ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate (BAY 68138),
ethyl-4-(methylsulfinyl)-m-tolyl isopropylphosphoramidate (BAY 68138
sulfoxide) and ethyl-4-(methylsulfonyl)-m-tolyl-isopropylphosphoramidate
(BAY 68138 sulfone) in white rats. Report No. 26759 from Chemagro
Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Gronberg, R.R., Flint, D.R. and Pither, K. (1974) The metabolic fate
of Nemacur sulfoxide administered orally to a lactating dairy cow.
Report No. 41104 from the Chemagro Division of Baychem. Corp.,
submitted by Bayer AG. (Unpublished).
Gronberg, R.R., Simmons, C.E. and Shaw, H.R., II. (1973) Residues of
Nemacur in poultry eggs and tissue. Report No. 35995 from the Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Houseworth, L.D., and Tweedy, B.G. (1972) Effect of Nemacur on
microbial populations. Chemagro Report No. 34 990.
Khasawinah, A.M. (1970) The fate of Nemacur in soil. Chemagro Report
No. 28 796.
Khasawinah, A.M. (1971) Metabolism of Nemacur in tobacco plants.
Chemagro Report No. 29 142.
Khasawinah, A.M. (1972a) Metabolism of Nemacur in snap beans grown in
closed glass chambers. Chemagro Report No. 34 992.
Khasawinah, A.M. (1972b) The uptake and metabolism of Nemacur soil
residues by soybean plants. Chemagro Report No. 35012.
Khasawinah, A.M. (1973a) Metabolism of Nemacur in carrots. Chemagro
Report No. 36005.
Khasawinah, A.M. (1973b) Metabolism of Nemacur in tomatoes. Chemagro
Report No. 38501.
Khasawinah, A.M. (1973c) The metabolism of Nemacur in cabbage.
Chemagro Report No. 39120.
Khasawinah, A.M. and Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Report No. 34217 from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (Unpublished).
Khasawinah, A.M. and Sandie, F.E. (1974) Photodecomposition of thin
films of Nemacur. Chemagro Report No. 39217.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on chickens.
Report from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Report from
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. and Solmecke, B. (1971a) BAY 68138 toxicological studies.
Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. and Solmecke, B. (1971b) Granular formulation. Acute
dermal toxicity on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972a) Antidotal experiments on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972c) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Report from Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Ladd, R., Jenkins, D., Keplinger, M. and Fancher, O. (1971)
Teratogenic study with Nemacur(R) technical in albino rabbits. Report
from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Löser, E. (1968a) Subchronic toxicological studies on dogs. Report
from the Institute for Toxicology, Farbenfabriken Bayer AG.
(Unpublished).
Löser, E. (1968b) Subchronic toxicological studies on rats. Report
from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Löser, E. (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Report from the Institute for Toxicology, submitted by
Bayer AG. (Unpublished).
Löser, E. (1970) Subchronic toxicological studies on dogs. Report from
the Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Löser, E. and Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachr. Bayer,
24(1):69-113.
Löser, E. (1972a) Chronic toxicological studies on rats (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished)
Löser, E. (1972c) Generation studies on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Olson, T.J. (1970a) Residues on the surface of fresh pineapple crowns
treated with an aqueous emulsion of S.C. formulation of Nemacur.
Chemagro Report No. 28014.
Olson, T.J. (1970b) Determination of Nemacur residues in soil by
thermionic emission gas chromatography. Chemagro Report No. 28731.
Olson, T.J. (1970c) Determination of Dasanit, Guthion, Metasystox R,
Nemacur and trichlorfon in soil by thermionic emission gas
chromatography. Chemagro Report No. 27835.
Olson, T.J. (1970d) An interference study for the determination of
residues of Nemacur on pineapple. Chemagro Report No. 28723.
Olson, T.J. (1971a) Determination of Nemacur residues in tobacco and
tobacco smoke. Chemagro Report No. 30448.
Olson, T.J. (1971b) An interference study for the determination of
residues of Nemacur on cotton and tomatoes. Chemagro Report No. 30095.
Olson, T.J. (1971c) An interference study for the Nemacur crop residue
method for bananas. Chemagro Report No. 31041.
Olson, T.J. (1972a) The effect of citrus processing on residues of
Nemacur sulfoxide. Chemagro Report No. 34050.
Olson, T.J. (1972b) Effect of commercial soybean oil processing on
residues of Nemacur sulfoxide. Chemagro Report No. 33461.
Olson, T.J. (1974) The effect of alkali refining on Nemacur residues
in cottonseed oil. Chemagro Report No. 40113.
Shaw, H.R. and Flint, D.R. (1974) The metabolism of Nemacur soil
residues under aerobic and anaerobic conditions. Chemagro Report No.
39909.
Smith, P., Wright, P. and Keplinger, M. (1972) Eighteen month
carcinogenic study with Nemacur(R) (BAY 68138) in Swiss white mice.
Report from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Report from the Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Spicer, J. (1971) Pathology report of BAY 68138. Hen study. Report
from Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C., Newman, A. and Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C. Newman, A. and Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Report from Huntingdon Research Centre, submitted by Bayer AG.
(Unpublished).
Thornton, J.S. (1969) A gas chromatographic method for the
determination of Nemacur and metabolite residues in crops. Chemagro
Report No. 25402.
Thornton, J.S. (1971a) Determination of residues of Nemacur and its
metabolites in plant and animal tissues. J. agr. Food Chem., 19
(5):890-893.
Thornton, J.S. (1971b) An interference study for the residue method
for Nemacur and metabolites. Chemagro Report No. 25533.
Thornton, J.S. (1972) Effect of cooking on residues of Nemacur in
potatoes. Chemagro Report No. 35314.
Thornton, J.S. (1973a) Effect of alkali refining and deodorization on
residues of Nemacur and metabolites in cottonseed oil. Chemagro Report
No. 35345.
Thornton, J.S. (1973b) An interference study for the Nemacur residue
method. I. Additional compounds not previously tested. Chemagro Report
No. 36 142.
Thyssen. (1974a) Nemacursulfoxid, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974b) Nemacursulfon, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974c) 4-methyl-mercapto-m-Kresol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974d) 3-methyl-4-methyl-mercaptophenol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974e) 3-methyl-4 methan-sulfonyl phenol, Akute Toxizitat
bei Ratten. Report from Institute for Toxicology, submitted by Bayer
AG. (Unpublished).
Tweedy, B.G. and Houseworth, L.D. (1974) Leaching of "aged" Nemacur
residues in sandy loam soil. Chemagro Report No. 40 506.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl
4-(methylthio)-M-tolyl isopropylphosphoramidate] and identification
of two metabolites in plants. J. agr. Food Chem., 20(1):157-160.
Molecular weight 303.3
Empirical Formula: C13H22NO3PS
Other information on identity and properties
State: brown semi-solid (technical)
white crystals (pure)
Melting Point: 46°C (technical)
49°C (Pure)
Vapour pressure: 10-6mm Hg (at 30°C)
Solubility: in water approx. 700 mg/l at 20°C
slightly soluble in most organic
solvents
Specific gravity (melt): 1.14
Hydrolytic stability in propanol/water (1:1) at 40°C:
40% degradation after 14 days at pH 2
no degradation after 50 days at pH 7
half life of 31.5 hours at pH 11.3
Formulations: 5% Granular, 10% Granular, 400 E.C. (Emulsifiable
concentration 400 g/l)
Purity
The minimum purity is 87%. Maximum levels of the individual
impurities are shown in Table 1.
TABLE 1 Impurities (maximum levels) in technical fenamiphos
Compound Maximum
level (%w/w)
O,O-diethyl isopropylphosphoramidate 3.0
4-methylthiocresol 0.5
O,O-bis[(4-methylthio)-3-ethylphenyl]
isopropylphosphoramidate 2.0
O,O-diethyl O-[(4-methylthio)-3-methylphenyl]
phosphate 1.0
O-ethyl O-[(4,6-bismethylthio)-3-methylphenyl]
isopropylphosphoramidate 3.0
O-ethyl O,O-bis-[(4-methylthio)-3-methylphenyl]
phosphate 8.0
O-ethyl O-[(4-methylthio)-3-methylphenyl] phosphate 1.0
water 0.3
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformations
The two major metabolites identified following injection into
bean, tomato, peanut and potato stems were O-ethyl
O-4-methylsulfinyl-m-tolyl isopropylphosphoramidate and O-ethyl
O-4-methylsulfonyl-m-tolyl isopropylphosphoramidate, the sulphoxide
and sulphone of the thio-ether (Waggoner, 1972). A third metabolite
(apparently a more potent inhibitor of cholinesterase) was
not identified but was considered to be a further metabolite
resulting from the sulphoxide. Further details of this study are
given in the section "Fate of residues in plants". Small residues of
the unknown metabolite may be of toxicological significance because
of the potent anticholinesterase properties.
The metabolic fate of labelled fenamiphos was studied in vivo
in rats and in vitro with rat liver microsomes. The compounds were
labelled in the ethyl (14C) isopropyl, (14C) or the thiomethyl (3H)
positions. In vivo fenamiphos is excreted within 12-15 hours
following a single oral dose of 2 mg/kg. In the in vitro studies a
small quantity of an unknown metabolite possibly resulting from the
N-dealkylation of fenamiphos was observed (Khasawinah and Flint,
1972). Apart from this minor component, metabolism in animals and
plants follows the same pattern: oxidation of the thioether to the
sulphoxide and sulphone, dearylation to yield the methyl thioether
phenol (or its sulphoxide and sulphide) and potentially dealkylation
of the ethyl, isopropyl or isopropylamide moiety of the phosphate
ester. Treatment of rats with fenamiphos sulphoxide or sulphone
produced the same excretion pattern and almost identical urinary
metabolites (Gronberg, 1969).
When fenamiphos sulphoxide was given to a lactating cow (Gronberg
et al., 1974) the identified components of the residue were
fenamiphos, its sulphoxide and its desisopropyl derivative, and the
phenols of phenamiphos, its sulphoxide and its sulphone. An
unidentified metabolite was also detected. Quantitative details are
given in the section "Fate of residues in animals".
Effects on enzymes and other biochemical parameters
Fenamiphos, as are other organophosphate esters, is an inhibitor
of cholinesterase enzymes. The in vitro I50 values were as follows:
Rat serum - 5.1 x 10-5M.
Rat erythrocyte - 6.3 x 10-4M.
Rat brain - 2.1 x 10-4M.
The maximum inhibition of whole blood cholinesterase in vivo
occurred in rats three hours after dosing. In vivo sensitivity of
cholinesterase reflects the in vitro I50 values with plasma
cholinesterase being more sensitive to inhibition than the erythrocyte
enzyme (Löser and Kimmerle, 1971). The metabolites of fenamiphos are
more active inhibitors than the parent molecule (Waggoner, 1972). In
vitro inhibition of horse serum cholinesterase showed relative
values for fenamiphos < sulfoxide = sulfone < sulfoxide metabolite.
In vitro I50 values of chicken and monkey liver aliesterase
(triacetin hydrolysis) and monkey cholinesterase are as follows:
Chicken (aliesterase): 4.2 x 10-5M.
Monkey (aliesterase): 1.0 x 10-6M.
Monkey (cholinesterase): 4.6 x 10-6M.
(Coulston and Wills, 1974)
On the basis of some preliminary electrophysiological studies
with the cat, it was estimated that a threshold dose of fenamiphos
might fall in the range of 0.8 to 1.5 mg/kg (Coulston and Wills,
1974).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of Charles River random bred Swiss white mice (125 males
and 125 females/group) were fed fenamiphos in the diet at 0, 25 and 50
ppm for eighteen months in a study specifically designed to evaluate
the carcinogenic potential of the compound. Additional groups of mice
were fed 10 ppm N-nitrosodiethylamine for eighteen months (125 males
and 125 females); 40 ppm for six months (60 males and 60 females); and
1000 ppm benzidine (100 females) for eighteen months as positive
controls. Fenamiphos did not affect the incidence or pattern of
mortality. Clinical chemistry studies performed at 18 months were
normal. No signs of tumour formation were observed on gross or
microscopic examination which were attributed to fenamiphos (Smith et
al., 1972).
Special studies on mutagenicity
Mouse
Groups of twelve male mice were administered fenamiphos
intraperitoneally at 0, 0.25 and 0.50 mg/kg and mated for six
consecutive weeks to virgin females in a standard dominant lethal
test. A positive reference (methyl methanesulphonate) was used in this
experiment. Fenamiphos did not induce alterations in male germinal
cells which would be expected to lead to an early embryo mortality
(Arnold et al., 1971).
Special studies on neurotoxicity
Hen
Groups of hens (eight per group) were fed fenamiphos in the diet
at levels of 0, 1, 3, 10, and 30 ppm for thirty days. At the end of
the feeding experiment some animals were sacrificed and the remainder
were observed for four weeks for neurological signs of poisoning. Food
consumption was depressed at 30 ppm and the average body weight and
growth of the animals at this same level was reduced. Cholinesterase
(whole blood) depression was observed at thirty days at 3, 10 and 30
ppm, although cholinergic signs of poisoning were not observed at any
time. There were no indications of delayed neurotoxic effects and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with H&E) did not indicate delayed neurological involvement
with fenamiphos (Kimmerle, 1970; Spicer, 1970).
Groups of hens (ten per group) were orally administered an LD50
dose of fenamiphos (5.0 mg/kg). The birds were observed for three
weeks and sacrificed. No evidence of delayed neurotoxicity was
observed either clinically or histologically while positive signs were
observed with TOCP (Kimmerle, 1971; Spicer, 1971).
Special studies on potentiation
Rat
Male rats were tested orally with fenamiphos (SRA 3886) in
combination with disulfoton or E 154 and no potentiation of the acute
toxicity was noted (Kimmerle, 1972c).
Special studies on reproduction
Rat
A standard three generation, two litters per generation,
reproduction study was performed with fenamiphos. Four groups of
animals, consisting of ten male and twenty female rats per group, were
fed fenamiphos at 0, 3, 10, and 30 ppm in the diet throughout the
study period including mating, gestation, and suckling. Immediately
after birth, pups were examined for malformations prior to either
preparing for another generation or sacrifice. Five weanling rats per
group of the F3b generation were sacrificed and macroscopic and
microscopic examinations performed on the major tissues and organs.
There were no apparent differences with regard to reproduction
parameters including fertility, litter size, lactation index, growth
of young, or on examination for malformation in any of the animals
exposed to fenamiphos levels up to and including 30 ppm in the diet.
It was concluded that fenamiphos had no influence on the reproduction
of rats at dietary levels up to and including 30 ppm (Löser, 1972c;
Cherry et al., 1972).
Special studies on teratogenicity
Rabbit
Groups of fifteen artificially inseminated rabbits were orally
administered fenamiphos at levels of 0, 0.2, and 0.4 mg/kg/day.
Positive control groups (oral treatment with thalidomide (37.5
mg/kg/day)) were concurrently run with this experiment. All animals
were treated from day 6 through 18 of gestation and sacrificed on day
29. At day 29 the young were examined for 24 hours, sacrificed and
examined after skeletal staining. At the high level of treatment of
fenamiphos, 2/12 instances of abortion and 2/12 premature deliveries
were observed. No external or internal abnormalities were observed in
the foetuses following treatment. With the exception of the animals at
the high level of treatment where a few aborted or delivered
prematurely, no effects on reproduction were observed with fenamiphos
(Ladd et al., 1971).
Acute toxicity
TABLE 2 Acute toxicity of fenamiphos
LD50
Animal Sex Route (mg/kg) References
Rat M oral 2.4-15.6 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Rat F oral 2.3-19.4 Kimmerle, 1972c
Crawford and Anderson, 1974
DuBois et al., 1967
Rat M ip 3.0-3.7 ibid.
Rat F ip 4.2-4.9 Löser and Kimmerle, 1971
Rat M dermal 73-500 DuBois et al., 1967
Rat F dermal 84-154 Kimmerle and Solmecke, 1971a
Mouse M oral 22.7 Löser and Kimmerle, 1971
Mouse M&F ip 3.4 DuBois at al., 1967
Guinea Pig M oral 56-100 ibid.
Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Guinea Pig M ip 17.3 DuBois et al., 1967
TABLE 2 (Cont'd.)
LD50
Animal Sex Route (mg/kg) References
Rabbit M oral 10-17.5 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Cat M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Dog M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Hen F oral 5.3 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
TABLE 3 Acute toxicity of metabolites and impurities of fenamiphos
LD50
Species Sex Route (mg/kg) References
Fenamiphos-sulphoxide
Rat F oral 10-25 Thyssen, 1974a
Fenamiphos-solphone
Rat F oral 10-25 ibid.
4-methylthio-m-cresol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 1333>2500 ibid.
Thyssen, 1974c
3-methyl-4-methylthiophenol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 500-1000 Thyssen, 1974d
1175 Crawford and Anderson, 1974
3-methyl-4-methanesulphonylphenol
Rat M oral 1250 ibid
Rat F oral 1000 Thyssen, 1974e
1854 Crawford and Anderson, 1974
Impurities identified as components of the technical mixture
(aryldiamide, diarylamide, diaryl ethyl ester, diethyl ester,
diethylmonamide, di-SCH3 compound, ethyl aryl ester, ethyldiamide,
and 4-methylthio-3-cresol) were tested for their acute toxic
properties to rats. At a dosage of 1.5 times the acute LD50 of
fenamiphos none of the materials were toxic (Crawford and Anderson,
1973b).
Technical fenamiphos administered to the skin of rabbits as an
acetone solution at 50 mg/kg resulted in a slight erythema and was not
considered as a primary irritant. Technical fenamiphos applied to the
conjunctival sac of rabbits (100 mg/kg as a crystalline material) was
irritating to the eye. This irritation was believed to be a mechanical
rather than a physiological effect (Crawford and Anderson, 1971).
A 15% granular formulation was examined for dermal and inhalation
toxicity properties and at the highest level examined (200 mg/kg and
20 mg/l respectively) no toxicity was observed (Crawford et al.,
1970b; Crawford and Anderson, 1972a). The rat oral LD50 of a 15%
granular formulation was 66.6 mg/kg for male rats and 62.7 mg/kg for
female rats (DuBois and Kinoshita, 1970). A 5% or 10% granular
formulation, administered at up to 500 mg/kg to the backs of rats
resulted in depressed cholinesterase activity values. Mortality was
not observed at the maximum dose tested and it was considered that the
granular formulation was not a dermal hazard although the material was
rapidly absorbed into the body (Kimmerle and Solmecke, 1971b). The
acute dermal toxicity and dermal and eye irritation properties of a
liquid formulation (37.4% a.i.) were tested in rabbits. The acute
LD50 was 75.7 mg/kg and 71.5 mg/kg to male and female rabbits
respectively (Crawford et al., 1970a; Crawford and Anderson, 1972b).
When applied to intact and abraded skin at a dose of 0.25 ml (0.085 mg
a.i.), fenamiphos was not found to be a primary irritant. Fenamiphos
(0.1 ml, equivalent to 0.034 mg a.i.) was an irritant when applied to
the conjunctival sac of rabbits. Control formulation data were not
reported (Crawford and Anderson, 1973c). The acute rat oral LD50 of a
35% liquid formulation was calculated to be 24.8 mg/kg (equivalent to
8.7 mg fenamiphos /kg). Inhalation toxicity studies with this
preparation using a small particle size distribution (1-3 microns)
resulted in an LC50 value of 0.175 and 0.091 mg/l following one and
four hour exposure respectively (DuBois and Kinoshita, 1971). Studies
with rodents administered fenamiphos by inhalation at doses up to 0.23
mg/l for one to four hours exposure as a static spray showed that
rabbits and guinea pigs were more tolerant of the acute effects than
rats and mice. A one hour LC50 value for mice was 0.06 mg/l and for
rats 0.23 mg/l while rabbits and guinea pigs were unaffected at these
concentrations (Kimmerle and Solmecke 1971a; 1972b). Acute oral
administration of 2.5 mg/kg to rabbits had no effect on several liver
function tests including BSP, SGPT and SDH activity at 1 and 24 hours
and seven days after treatment (Kimmerle and Solmecke 1971a).
Studies performed on male rats indicated that, following
poisoning, the administration of atropine and/or 2-PAM or Toxogonin
would reduce the acute lethal dose by a factor of approximately two.
As with other organophosphorus compounds, rapid administration of
atropine and oxime reactivator following poisoning would afford some
protection and would alleviate the cholinergic signs of poisoning
(Kimmerle, 1972a; DuBois et al., 1967).
Short term studies
Rat
Groups of rats (fifteen males and fifteen females per group,
thirty males and thirty females were used as controls) were fed
fenamiphos in the diet at 0, 4, 8, 16, and 32 ppm for twelve weeks.
Male and female rats from the highest dose group exhibited signs of
cholinergic stimulation in the first two months of the test.
Behavioral differences in all other groups were not apparent. There
were no differences from the control in the average of any group with
regard to food consumption and growth. With the exception of
cholinesterase inhibition, there was no apparent effect on group
averages of haematological parameters examined. Plasma cholinesterase
depression was observed at 8 ppm. A no-effect level, based upon plasma
cholinesterase, was observed at 4 ppm. Gross and microscopic
pathological examination was performed on tissues and organs at the
conclusion of the experiment. A slightly increased liver weight,
observed in males at 16 and 32 ppm, was not reflected in the
calculation of relative organ-to-body weight ratios nor in the
histological examination. Based on cholinesterase depression, 4 ppm in
the diet was a no-effect level in this experiment (Löser, 1968b;
Mawdesley-Thomas and Urwin, 1970a).
Groups of male rats (15 per group) were administered fenamiphos
orally or by intraperitoneal injection daily, 5 days/week for 60 days.
The animals all survived a dose range of 1.5 (ip) to 1.7 (oral)
mg/kg/day with no evidence of a cumulative toxicological effect due to
fenamiphos (Kimmerle and Solmecke, 1971a). In an earlier study female
rats survived daily ip administration of 1 mg/kg for 60 days while 40%
of those administered 2 mg/kg did not survive. All rats died at 3
mg/kg administered by intraperitoneal injection (DuBois and Flynn,
1968). These studies indicate little if any cumulative toxicity.
Dog
Groups of beagle dogs (two males and two females/group) were fed
fenamiphos in the diet at 0, 2, 6, and 18 ppm for three months.
Behavioral abnormalities, evidenced by signs of cholinergic
stimulation, were evident at 18 ppm in the diet and growth of females
at this high level was reduced. Average haematological and clinical
chemistry values including liver function tests, kidney function tests
and urinalyses were not apparently affected by fenamiphos in the diet.
Averages of plasma and red blood cell cholinesterase values were
depressed at 4 ppm in the plasma of both males and females. At 2 ppm
no effect on the average red cell cholinesterase values was found
while plasma cholinesterase was depressed (20-30%) in both sexes.
Gross morphological examination of tissues and organs at the
conclusion of the study indicated no abnormal effects of fenamiphos in
the diet (Löser, 1968a).
Groups of beagle dogs (two males and two females/dose, three of
each sex were used in the control group) were fed fenamiphos in the
diet at levels of 0, 1, 2 and 5 ppm for twelve weeks. There was no
effect of feeding fenamiphos at levels up to and including 5 ppm in
the diet on the average of any of the parameters examined including
haematology, liver function tests, clinical chemistry tests, kidney
function tests, and gross and microscopic examination of tissues and
organs. As with other studies, cholinesterase was the only parameter
significantly affected, with the females being more susceptible than
the males and the plasma being more sensitive to inhibition than red
blood cell. Erythrocyte cholinesterase was unchanged at 2 ppm while
plasma cholinesterase was slightly depressed. One ppm in the diet had
no effect on plasma cholinesterase. As the depression of plasma
cholinesterase was minimal and transient at 2 ppm it might be
considered that this would be a no-effect level in this experiment
(Löser, 1969; Mawdesley-Thomas and Urwin, 1970b).
As an extension of the previous short term study in dogs (Löser,
1969), an additional two male and two female dogs were fed fenamiphos
in the diet at 10 ppm for three months. Two male and two female
animals were also maintained as a control. There was no mortality over
the course of the experiment and a very slight deviation in the
average body weight of both males and females was noted. Because of
the small sample population, the slight weight differences cannot be
fully evaluated. There was no effect of feeding fenamiphos on any of
the haematological parameters examined. Urinalysis, clearance
examinations, blood sugar and cholesterol were normal over the course
of the experiment. Cholinesterase activity was significantly depressed
in both plasma and erythrocytes and in both male and female dogs. In
addition, several abnormalities were noted with regard to some of the
serum enzyme parameters related to liver function after three months
of feeding, especially in male dogs. Alkaline phosphatase and SGPT
activity were significantly increased. Following gross and microscopic
examination of tissues and organs, no significant differences were
noted between the control and those animals fed 10 ppm. The abnormal
findings in some enzyme parameters related to liver functions,
predominantly in males at three months of feeding 10 ppm in the diet,
were not accompanied by pathological findings at autopsy nor confirmed
in another test where fenamiphos was fed at levels of 18 ppm in the
diet. They are considered a transient occurrence and not directly
related to the presence of fenamiphos in the diet (Löser, 1970;
Thompson et al., 1972a).
Groups of pure bred beagle dogs (four males and four females per
group) were fed fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5,
and 10 ppm for two years. There were no significant effects noted with
fenamiphos at any dietary level with regard to growth, food
consumption or any of the standard clinical and physiological
examinations made during the course of the study. One dog died after
being fed 0.5 ppm in the diet. This dog was considered to have died of
pneumonia and its death was not related to administration of
fenamiphos. Gross and histological examinations of all tissues and
organs at the conclusion of study did not reveal any abnormal
developments considered to have been a result of feeding fenamiphos.
The only significant physiological effect observed was
anti-cholinesterase activity which was marginally depressed in plasma
at 2 ppm. As with other species, the plasma appears to be the most
sensitive indicator of exposure with a no-effect level being 1 ppm in
the diet (equivalent to 0.029-0.036 mg/kg/day) (Löser, 1972b; Thompson
et al., 1972b).
Long term studies
Rats
Groups of SPF derived Wistar strain (forty males and forty
females per group, eighty males and eighty females used as controls)
were fed fenamiphos in the diet, at concentrations of 0, 3, 10, and 30
ppm for two years. Behavioral abnormalities were evident, as signs of
cholinergic stimulation, only during the first six weeks of feeding at
30 ppm. After six weeks of feeding, the obvious anticholinesterase
effect disappeared and was not evident for the remainder of the two
year study. There were no differences in averages at any feeding level
on growth, mortality, food consumption, haematological values,
clinical chemistry values or liver and kidney function tests. Urine
examinations as well as blood sugar and cholesterin values were
normal. Thyroid weights and thyroid-to-body weight ratios of females
were heavier than normal at 30 ppm. All other major tissues and organs
appeared normal under gross and microscopic examination. The thyroid
weight in females at 30 ppm was high but was not accompanied by an
abnormal tumour development, goitre or by unusual histological
findings.
Cholinesterase determinations indicated that plasma
cholinesterase was the most sensitive parameter. At 10 ppm in the
diet, fenamiphos inhibited plasma cholinesterase. No significant
effects were observed at 3 ppm in the diet (equivalent to 0.17-0.23
mg/kg bw/day) (Löser, 1972a; Cherry and Newman, 1973).
COMMENTS
Fenamiphos, an acutely toxic organophosphate ester, is rapidly
absorbed as evidenced by rapid onset of cholinergic signs of
poisoning. In animals fenamiphos is rapidly degraded, predominantly
through oxidation to acutely toxic products. Fenamiphos does not
appear to affect biochemical systems other than cholinesterase.
Testing for the delayed neurotoxic effect commonly seen with TOCP was
negative. Short term and long term dietary studies in both rats and
dogs have indicated, with the exception of cholinesterase depression,
no unusual effects. In long term studies with rats, transient
behavioural abnormalities were observed during the first six weeks of
feeding, which disappeared and were not evident for the remainder of
the study. Female rats are more susceptible than males to
cholinesterase depression. Plasma cholinesterase depression is a
better indicator of exposure than red blood cell cholinesterase. Based
on plasma cholinesterase depression, a no-effect level in a two-year
rat study is considered to be 3 ppm in the diet and in a two year dog
study, 1 ppm in the diet. Long term studies in rats and mice reported
to the Meeting did not show a carcinogenic effect. Although there are
no studies available in man, sufficient data are available and the
Meeting allocated an ADI using the more sensitive species, the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 ppm in the diet equivalent to 0.17 mg/kg bw.
Dog: 1 ppm in the diet equivalent to 0.029 mg/kg bw.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0006 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenamiphos is an organophosphorus compound with a systemic action
and a powerful nematicidal effect. It is active against root-knot,
cyst-forming and free-living nematodes and has important applications
against sucking insect pests and spider mites. Fenamiphos is
commercially used in the USA, Australia, Spain, the Cameroons,
Caribbean, Chile, Columbia, Costa Rica, Ecuador, Guatemala, Ivory
Coast, Jamaica, Martinique, Mexico, Nicaragua, San Salvador and
Uruguay. Registration proceedings and trials are in progress in
several other countries.
Fenamiphos is formulated as 5 and 10% granules and as a 40%
emulsifiable concentrate.
Pre-harvest treatments
Fenamiphos is formulated as a granular product and as an
emulsifiable concentrate. Both formulations are recommended chiefly
for treatments in banana, pineapple, vegetable (tomatoes, brassicas,
peppers, eggplants), tobacco, coffee, groundnut and citrus crops.
Application is usually made to the soil at sowing, planting and
transplanting, and in established crops. The general dose rate is 5 to
10 kg active ingredient per hectare for broadcast (overall)
application, but is reduced for band application according to band
width. Furthermore, the emulsifiable concentrate is suitable for dip
treatments of banana seedlings and for foliar spraying of pineapples.
Details of uses and recommendations are given in Table 4.
TABLE 4 Use Pattern of fenamiphos (All applications are pre-harvest against nematodes)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Bananas 1.5-2.5 g/m2 At planting G
3.0 g/plant Soil treatment in
established crop G 3-4
4-5 kg/ha In irrigation
canals or direct
to soil EC 2
1-1.5 g/corm Dip treatment
pre-planting plus
soil application EC
2 or 1.5 g/
planting position
(but no more than
total of 3 g/
planting position) G
Pineapples 10-40 kg/ha Broadcast treatment
pre-planting of
crowns and slips G
5-20 kg/ha Broadcast treatment
pre-planting of
crowns and slips
in combination with G
3-5 kg/ha foliar spraying 3 in
post-planting LC established
crop
15 kg/ha Foliar spray
application at
planting, repeat
after an interval
of 2 months EC 2
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Tomatoes 1-2.5 g/m2 Immediately before
or after sowing G
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Brassicas 5-8 kg/ha Broadcast/overall
application G,
pre-planting EC
Tobacco 1-2.5 g/m2 Before or immediately
after sowing or G
0.1-0.2 g/plant at transplanting or
in established crop G 1
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Coffee 1-2.5 g/m2 Seedbed treatment G
1.5-2.5 g/shrub In established crop G 2-3
Groundnuts 3-5 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Cucurbitaceae 0.15-0.2 g/ Band application
planting hole or at sowing G
1.5-2.5 kg/ha
Peppers 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Eggplant 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Citrus 1-1.5 g/m2 Seedbed treatment G
2-3 g/tree Treatment in nursery G
1.5-3 g/tree In established crop G, LC 2-3
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Potatoes 5-10 kg/ha Broadcast/overall G, EC,
treatment pre-planting LC 1
Pyrethrum 5-8 kg/ha Broadcast application
at planting G
Ornamentals 1.5-3 g/m2 In established crops G
Cotton 1.7-3.5 kg/ha Band application
(band width of G,
30-45 cm.) at sowing LC
Beets 3-(6) kg/ha Band application
immediately after
sowing or during G,
emergence EC
Soybeans 5-8 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Beans 5-8 kg/ha Broadcast application
pre-sowing G 1
Grapes 2-3 g/plant G 1
Strawberries 10 kg/ha Broadcast application
pre-planting G
300 g/1000 Post-planting or G
metres of row post-harvest
Lawns 10-20 kg/ha Broadcast application G
1 G = Granules; EC = Emulsifiable concentrate; LC = Liquid concentrate.
Post-harvest treatments
No recommended uses.
Other uses
Fenamiphos is recommended for use on ornamentals (granular
formulation).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of fenamiphos together with its sulphoxide and
sulphone resulting from supervised trials in a wide range of crops are
shown in Table 5. In most crops, residues were below 0.05 mg/kg. The
exceptions were grapes, lettuce, potatoes, strawberries and tomatoes
which contained up to about 0.1, 0.5, 1, 0.7 and 0.3 mg/kg
respectively. Residues in tobacco were much higher, with respective
maximum levels in green, cured and fermented tobacco of about 12, 140
and 100 mg/kg.
FATE OF RESIDUES
In animals
A feeding study was carried out on a lactating dairy cow to
determine whether fenamiphos sulphoxide, found in plants as the major
metabolite, would be incorporated into milk and animal tissues either
as the intact compound or in metabolized form (Gronberg et al., 1974.
See also "Biotransformations"). A single dose of 0.8 mg/kg body
weight, corresponding to 27 ppm in the diet, of ring-14C fenamiphos
sulphoxide was administered. Four hours after application the label
was distributed as follows. Urine 39%, faeces <0.1%, rumen 47%,
tissues 1.4%, bile 0.1%, omasum, abomasum and milk <0.1% each; total
recovery 88%. The following compounds were found: fenamiphos (I),
fenamiphos sulphoxide (II), desisopropyl-fenamiphos (III), phenol of
fenamiphos (IV), phenol of fenamiphos sulphoxide (V), phenol of
fenamiphos sulphone (VI) and "unknown" (VII). The levels of these
residues in tissues and milk are shown in Table 6.
Of the dose, 18% was reduced to the parent fenamiphos, apparently
by microorganisms. At 4 hours 82% of the activity in whole blood and
87% of the activity in urine was identified as conjugated phenols.
The residues of fenamiphos in poultry eggs and tissues were
determined by Gronberg et al. (1973). Laying hens were fed for 14 days
with carbon-14 ring-labelled fenamiphos in feed at 0.06, 0.18 and 0.65
ppm concentration. During this time, residues in eggs were below the
detection limit of 0.003 mg/kg. At slaughter, all tissue residues from
birds given feed containing 0.65 ppm were below the limit of
determination of 0.003 to 0.005 mg/kg, although very low residues were
detectable in some tissues.
TABLE 5 Residues of fenamiphos and metabolites resulting from supervised trials
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Apples Australia 10% G 22.4 334 1 n.d.2 n.d.2
Bananas, Central
peel America 10% G 2.8-12 g
a.i./plant 1-195 41 <0.025-0.3 <0.025
pulp <0.025 <0.025
Bananas, Ivory Coast 5% G 10 g a.i./plant 1-14 4 <0.02 <0.02
peel
pulp <0.02 <0.02
peel 28-88 3 0.06-0.1 0.07
pulp 0.03-0.08 0.05
Broccoli USA 3% G;
15% G 6.72 65-101 8 <0.01-0.04 0.02
15% G 10.0 92 1 0.2 0.2
Brussels
Sprouts USA 3% SC;
15% G 6.72-10.0 107-133 5 <0.01-0.02 <0.01
Cabbage USA 3% SC;
15% G 6.72-10.0 55-108 14 <0.01-0.02 0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 40% EC 4.5 42-106 6 n.d.2 n.d.2
Carrots USA 15% G 6.72 96-160 9 <0.01-0.05 0.01
Cauliflower USA 3% SC;
15% G 6.72-10.0 97-111 12 <0.01-0.04 0.02
Coffee Brazil - 1-2 g a.i./plant 14-15 6 <0.02-0.07 0.05
beans, Central
green America 28-30 6 <0.02-0.09 0.05
56-62 4 <0.02-0.05 0.03
90 2 0.03; 0.05 0.04
Cottonseed USA 3% SC;
15% G 1.12 157-196 10 <0.01 <0.01
Cotton USA 3% SC;
gin trash 15% G 1.12 161-196 8 <0.01 <0.01
Cotton USA 3% SC;
foliage 15% G 1.12 143-148 4 <0.01-0.1 0.04
Grapefruit USA 3% SC;
peel 15% G 33.6 - 50.4 183-184 1 <0.01 <0.01
pulp <0.01 <0.01
whole fruit <0.01 <0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Grapes Australia 5% G
43% EC 5.6 - 14.7 145-147 2 n.d.2 n.d.2
22.0 145-147 1 0.03 0.03
44.0 145-147 1 0.08 0.08
Leaf-lettuce USA 3% SC 11.2 46 1 0.3 0.3
Head USA
lettuce, 3% SC;
head 15% G 11.2 61-72 4 0.02-0.1 0.08
wrapper leaves 0.09-0.5 0.3
discard leaves 0.09-6.5 1.9
Lemons, USA
whole fruit 3% SC 33.6 184 1 0.01 0.01
Limes, USA 3% SC;
whole fruit 15% G 33.6 147 2 <0.01 <0.01
Melons Guatemala
Australia 5% G
Egypt 40% EC 1.6-10.0 56-112 5 n.d2-0.05 n.d.2
Oranges, USA 3% SC; 22.4-50.4 182-366 4 <0.01-0.1 0.04
peel 10% G;
15% G
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
pulp 4 <0.01 <0.01
whole fruit 5 <0.01-0.02 0.01
whole fruit Australia 5% G;
40% EC 11.2-22.4 21-139 10 n.d.2 n.d.2
Peanuts, USA 3% SC;
kernels 10% G 4.51-13.45 122-148 5 <0.01-0.02 <0.01
shells (0.01-0.2 0.06
vines 98-148 5 0.02-4.3 2.3
Pineapples Hawaii, USA 3% SC;
10% G 44.8-112 238-658 10 <0.01-0.03 <0.01
3% SC
(foliar spray) 5.6 1-29 20 <0.02-4.0 0.5
Potatoes USA 3% SC;
15% G (row
appl) 6.1 41-58 3 <0.02-1.2 0.7
71-106 7 <0.01-1.0 0.15
Potatoes, USA, Canada, 3% SC;
whole Fed. Rep. of 15% G
Germany, UK, (broadcast
Australia 4-5-11.2 67-203 23 n.d.2-0.45 0.07
peel 1 0.07 0.07
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
flesh 1 0.07 0.07
Sweet USA 3% SC;
Potatoes 15% G
(broadcast) 6.72 108-143 8 <0.01-0.04 0.01
3% SC;
15% G
(row appl) 4.0-6.1 108-143 8 <0.01-0.06 0.01
Soybeans, USA 3% SC;
bean, green 15% G 11.2 75-117 12 <0.01-0.09 0.04
vine, green 75-117 12 <0.01-12 2.9
bean, dry 131-159 11 <0.01-0.03 0.01
Strawberries Australia 5% G
40% EC 16.5-22.5 36-121 6 0.03-0.7 0.2
Sugarbeets, USA 3% SC;
tops 15% G 6.72-10.0 119-187 9 <0.01-0.08 0.01
beets <0.01-0.03 0.01
Tangerines, USA 3% SC;
peel 15% G 33.6 247-251 2 0.07; 0.3 0.2
pulp 2 <0.01; 0.03 0.02
whole fruit 1 0.03 0.03
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Tobacco Australia 40% EC 50-95 4 0-5-4.8 1.9
Tobacco, USA 3% SC; 6.72 4 3 0.9-7.8 3.5
green 15% G 7 3 0.8-7.0 2.9
11 3 0.8-3.3 1.8
14 3 1.4-12 5.2
21 3 1.2-4.9. 2.5
28 3 0.4-4.2 1.7
35 3 0.25-3.6 1.4
36-148 13 0.02-4.8 1.0
air cured 88-106 2 3.2; 4.2 3.7
flue cured 85 2 11; 13 12
cured 75-91 6 4.5-140 33
aged 75-92 3 5.7-16 11
fermented 89 1 98 98
Tomatoes USA, 3% SC;
Canada 15% G 11.2-16.8 61-89 10 <0.01-0.3 0.1
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 5% G;
40% w/v 9.8-11.2 78-161 5 <0.05 <0.05
Fed. Rep, of
Germany 5% G 9.8-12.2 79-161 5 n.d.3 n.d.3
1 EC = emulsifiable concentrate; G = granules; SC = spraying concentrate. n.d. = <0.01.
2 <0.01 mg/kg.
3 <0.05 mg/kg.
TABLE 6 Residues, mg/kg, in tissue and milk of cow fed fenamiphos
sulphoxide (Gronberg et al., 1974)
Compound1 Fat Meat Liver Kidney Milk
I 0.004 n.d.2 0.006 n.d. n.d.
II n.d. n.d. 0.002 n.d. 0.002
III 0.002 n.d. 0.005 0.16 0.004
IV 0.005 0.001 0.029 0.86 0.005
V 0.003 0.003 0.021 0.36 0.025
VI n.d. n.d. n.d. 0.01 0.004
VII 0.002 0.005 0.036 0.25 0.020
1 For identities of compounds, see text.
2 n.d., not detectable, = <0.001 mg/kg.
In plants
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thiooxidation of the parent compound to
the sulphoxide and sulphone. Both metabolites are detoxified by
hydrolysis, generally followed by glucosylation of the phenolic
products.
Fenamiphos-14C,3H was injected into the stems of beans,
tomatoes, peanuts and potatoes and the plants were harvested at 7, 14,
21 and 28 days (Waggoner, 1972. See also "Biotransformations"). Two
major metabolites were identified by TLC, isotopic ratios, IR, and MS
as the sulphoxide and sulphone of fenamiphos. The sulphoxide was the
major metabolite, accounting for 33-41% of the residue, followed by
the sulphone (31-33%), both after 7 days' treatment. Two minor
metabolites containing intact phosphoramidate structures accounted for
not more than 1.5 and 0.2% of the total residue.
Khasawinah studied fenamiphos metabolism in carrots (1973a),
tomatoes (1973b), cabbages (1973c), soybeans (1972b) and tobacco
plants (1971) following soil application, and in snap beans (1972a)
after both soil treatment and stem injection; 14C-ring, 14C-1-ethyl
and 3H-methylthio labelled fenamiphos were used.
Carrots readily absorbed and metabolized fenamiphos and its major
soil foliage and 14C from the ethyl group was detected in the foliage
and carrots. This 14C was probably incorporated into lipids,
pigments, sugars and structural elements such as cellulose.
Water-soluble residues were mainly conjugates of the sulphoxide and
sulphone phenols. No parent fenamiphos was detected. Residues of the
sulphoxide and sulphone were below 0.06 mg/kg in both carrots and
foliage. At the end of the growing period, soil residues were 2.7
mg/kg fenamiphos equivalents (one third of the amount applied) of
which 65% consisted of the sulphoxide and sulphone in the ratio 4:1.
Results with tomatoes were similar. Sulphoxide and sulphone
residues reached a peak of 0.2-0.3 mg/kg 30-40 days after treatment,
and no parent compound was detected later than 40 days. The results
showed the stability of the thio-methyl-ring linkage.
Comparable results were obtained with head cabbage. An
organosoluble polar metabolite with an apparently intact
phosphoramidate structure was detected soon after treatment and
declined gradually to insignificant levels.
In tobacco plants the sulphoxide reached a peak in less than one
week, and then decreased as the sulphone increased. At the end of the
growing period the ratio of sulphoxide to sulphone was about 3:2.
Soybeans were planted in soil that had been treated with
14C-ethyl, 3H-methylthio fenamiphos and cropped with tobacco for one
growing season. The soil at soybean planting contained 0.65 mg/kg
fenamiphos sulphoxide, 0.07 mg/kg fenamiphos sulphone and 0.18 mg/kg
fenamiphos equivalents as unextractable material. Nineteen days old
soybean seedlings contained 16.9 mg/kg (fresh weight basis) of these
two soil metabolites in a sulphoxide: sulphone ratio of 73:17. In the
mature plant (150-day growing period), the total concentration of the
two metabolites, found in equal proportions, was 3.5 mg/kg in the dry
leaves and 0.19 mg/kg in the dry beans. The water-soluble
radioactivity consisted mainly of the glucose conjugate of the
sulphone phenol. The insoluble radioactivity was not characterized.
The fate of 14C1-ethyl and 3H-methylthio fenamiphos was
investigated in snap beans following stem injection and soil treatment
in systems designed for the recovery of the applied nematicide. The
study demonstrated volatile 14C radioactivity, presumably as CO2.
40% of the stem-injected and 12% of the soil-applied fenamiphos was
recovered in this form. Volatile tritium radioactivity was less, 7.4%
and 1.3% of the amounts applied by stem-injection and soil treatment,
respectively. The metabolites formed by both methods of application
were fenamiphos sulphoxide, fenamiphos sulphone and the sulphoxide and
sulphone phenols in the free form or conjugated with glucose.
The metabolism of similarly labelled fenamiphos applied by stem
injection, soil drench or spray to fruited pineapple plants was
studied by Flint (1973). Soil treatment at 20 pounds a.i./acre
resulted in maximum total radioactive residues in fruit of 0.086 mg/kg
fenamiphos equivalents at 90 days after treatment. Metabolites
identified in fruit and foliage were identical to those previously
found in several other plant and animal systems.
In soil and water
Khasawinah (1970) treated the top 1.5 cm of a sandy loam soil, in
which tobacco was growing, with labelled fenamiphos. One third of the
applied amount was taken up by the growing plants during an 11-week
period. The major product in the soil was the sulphoxide, with small
quantities of the sulphone. No hydrolysis products of the parent
compound or its oxidation products were detected in the soil extracts.
None of the radioactivity was leached into the drainage water, and all
of it remained in the root zone. About 22% of the radioactivity
remaining in the soil after 177 days was bound to clay particles and
was unextractable.
Fenamiphos was studied for soil runoff, leaching, adsorption, and
stability in water by Flint et al. (1971) and Church (1971). It was
moderately adsorbed by sandy loam and silt loam soil. Fenamiphos
residues in runoff water were less than 3.4% of the applied compound
over a 37-day interval during which 2.3 to 3.7 inches of irrigation
were applied; the application rate of fenamiphos was 22.5 kg a.i./ha.
The leaching of fenamiphos from the three types of soil was inversely
proportional to the adsorptivity of the soils. It was estimated that
111 inches of rainfall would be needed for clay loam soil and 75
inches for sandy loam to leach fenamiphos to a depth of 12 inches.
Fenamiphos was relatively stable in aqueous systems at pH 5 and 7
in the laboratory but declined rapidly with a half-life of 4.8 days in
a simulated field pond at PH 7 and average temperature of 29°C.
The leaching of "aged" fenamiphos residues in sandy loam soil was
investigated by Tweedy et al. (1974). Ring-14C fenamiphos was
incubated with sandy loam soil under greenhouse conditions for 30 days
and then applied to the top of a sandy loam soil column 2.9 inches in
diameter and 12 inches high. Distilled water, equivalent to 0.5
acre-inch/day was added daily for 45 days. Of the radioactivity
recovered from the system, 77.5% was found in the top fourth of the
column and only 2.3% was recovered in the leachate. The majority of
the activity, 65%, apparently did not move at all.
The metabolism of fenamiphos soil residues under aerobic and
anaerobic conditions was investigated by Shaw and Flint (1974).
Fenamiphos, labelled with tritium in the methylthio group or with
carbon-14 uniformly throughout the aromatic ring, was used in this
study. The results indicated that anaerobic conditions arrested
further degradation while aerobic incubation allowed continued
metabolism. Essentially no 14C or 3H radioactivity was lost during
incubation indicating stability of the aromatic ring system. The major
metabolite was fenamiphos sulphoxide with some further thio-oxidation
and hydrolysis observed under aerobic conditions.
The effect of fenamiphos on soil microbial populations was
studied by Houseworth and Tweedy (1972) using Indiana clay loam and
Commerce silt loam soils. The soils were treated with 50 and 250 mg/kg
of the pesticide and maintained at 50% field moisture capacity for 56
days. At intervals, samples of soil were removed from each treatment
and assayed for populations of bacteria, fungi and Actinomycetes.
Fenamiphos had no significant effect on them under the conditions of
the experiment.
The photodecomposition of thin films of fenamiphos was
investigated by Khasawinah and Sandie (1974). Thin films of dual
labelled ethyl-1-14C, methylthio-3H fenamiphos were coated on glass
plates and silica gel coated glass plates and exposed in the open air
for about three weeks. Fenamiphos was rapidly oxidized to its
sulphoxide and then to the sulphone. Some hydrolysis of the sulphone
occurred after long intervals, especially on glass.
The results of some studies in the USA of the persistence of
fenamiphos and its oxidation products in various types of soil were
available to the Meeting. They are reproduced in Table 7.
In storage and processing
The effect of alkali refining and steam deodorization on residues
in cottonseed and soybean oils was studied in laboratory simulation
experiments by Thornton (1973a) and Olson (1972b, 1974). In
cottonseed, the two processes in combination removed 98.5% of the
fenamiphos, 98.4% of the sulphoxide and 88% of the sulphone. Since the
sulphone would constitute only a small proportion of a naturally
occurring residue, the combined processes would remove nearly all the
residue. In soybeans, the effects of the processes on sulphoxide
residues was studied, as this is the main component of the residue
after long post-treatment intervals. The simulated refining process
removed 96% of the sulphoxide, and deodorization removed 38%. A
hypothetical residue of 0.42 mg/kg should therefore be reduced to 0.01
mg/kg by the two steps.
The effect of citrus processing on residues of fenamiphos
sulphoxide was investigated by Olson (1972a). Commercial citrus
processing involves liming and drying of the chopped solids in the
preparation of cattle feed. Fenamiphos sulphoxide, the main metabolite
in crops, was resistant to the liming step but 39% of the residue was
removed by drying. To prepare citrus oil, an aqueous emulsion is
centrifuged vigorously. 99% of any fenamiphos sulphoxide present in
the emulsion would be removed from the oil by this procedure.
TABLE 7 Persistence of fenamiphos + sulphoxide + sulphone in various soils in the USA1
Residue, phenamiphos + sulphoxide + sulphone, mg/kg, after interval, days2
0 30-40 80-100 180-200 360-400
Soil Range Mean Range Mean Range Mean Range Mean Range Mean
Silt loam 1.2-15 7.7 5.4-12 9.4 0.8-15 6.9 0.1-6.5 2.5 0.1-2.5 1.1
Clay 1.0-1.7 1.3 3.7 3.7 1.5-2.7 2.1 0.6-1.3 1.0 0.6-0.7 0.7
Sand muck 5.1-6.3 5.7 5.1-5.8 5.4 0.1 0.1 0.7-1.0 0.8 0.4-0.6 0.5
Sand 5.5 5.5 0.3-0.6 0.5 0.1-0.2 0.2 0.06-0.07 0.07 0.03-0.04 0.04
Sand clay 6.8-8.5 7.7 3.0-4.9 3.9 0.5-1.2 0.8 0.1 0.1 0.1 0.1
Fox sand 4.3-9.2 6.7 6.8-8.0 7.4 3.1-3.2 3.1 1.0-1.3 1.1 0.9-1.0 0.9
Peat 15-16.5 16 17-20 18 12.5-17 15 6.8-8.8 7.4 6.3-7.2 6.8
Low humus 13-17 15 4.6-6.6 5.5 3.6-6.6 5.1 1.2-3.3 2.2 0.3-0.6 0.4
1 Fenamiphos was added to soils at 10mg/kg level as granules or spray concentrate.
2 Usually two or three analyses at each interval.
Olson (1970a) studied the fate of residues on pineapple crowns.
The fresh crowns were dipped in an aqueous emulsion of spraying
concentrate containing 0.12% fenamiphos, and allowed to drain dry. The
surface residue immediately after treatment amounted to about 30 mg
per crown, of which about half could be removed by extracting with
water. Seven days after treatment, aqueous extraction removed about
one-third of the original residue. Surface extraction with benzene,
however, removed virtually all the initial deposit (29 mg per crown)
but only 27% (8 mg) after 7 days.
Storage of broccoli, carrots, peanut kernels, pineapple fruit and
potatoes at -18° to -23°C for two years showed no significant loss of
fenamiphos, the sulphoxide or the sulphone (Anonymous, 1973).
There was no significant loss of residues (parent + sulphoxide +
sulphone) in field-treated potatoes or of fenamiphos from potatoes
fortified in the laboratory when cooked at 100°C for 30 minutes in
sealed jars (Thornton, 1972).
METHODS OF RESIDUE ANALYSIS
A specific gas-chromatographic procedure for the determination of
residues of fenamiphos and sulphoxide and sulphone in various crops,
animal tissues and milk is described by Thornton (1969, 1971a).
Following initial extraction, the extract is oxidized with potassium
permanganate to convert fenamiphos and its sulphoxide to the sulphone.
The sulphone is then determined by gas chromatography with alkali
flame ionisation detection. Recoveries from a variety of crops and
animal tissues fortified with fenamiphos or its metabolites at the
blending step were generally between 75 and 110%. Interference studies
by Thornton (1971b, 1973b) and Olson (1970d, 1971b,c) have shown the
method to be specific in the presence of other possible
organophosphorus compounds. The limit of detection is about
0.02 mg/kg.
The determination of residues of fenamiphos and its metabolites
in tobacco and tobacco smoke was successfully investigated by Olson
(1971a) with the Thornton (1971a) method. This method should be
suitable for regulatory purposes.
The determination of fenamiphos residues in soil by thermionic
emission gas chromatography is described by Olson (1970b). The soil
sample is extracted in a Soxhlet apparatus, using a 1:1
chloroform-methanol solvent mixture. The solvent is evaporated and the
residue oxidized and chromatographed. Recoveries of fenamiphos and its
metabolites were 80-107%. The sensitivity of the method is
approximately 0.02 mg/kg. Olson (1970c) has also extended the
procedure to a number of other organophosphorus pesticides.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table 8.
TABLE 8 National tolerances reported to the Meeting
Safety
Tolerance Interval
Count Crop mg/kg (days)
Australia Tomatoes, brassicas,
cucurbits, lettuce, citrus,
pineapples, grapes,
bananas, ginger No residue
Spain Tobacco sugarbeets, etc.
(industrial purpose),
bananas 60
USA Bananas, citrus 0.1 180
APPRAISAL
Fenamiphos is a systemic nematicide which gives good control of
root-knot cyst forming and free living nematodes. It is also effective
against sucking insect pests and spider mites. It is formulated as 5%
and 10% granules and as an emulsifiable concentrate (40%). Both
formulations are recommended chiefly for treating bananas, pineapples,
tomatoes, brassicas, eggplant, tobacco, groundnuts, citrus crops,
ornamentals and pyrethrum. It is chiefly applied to the soil at sowing
or planting. The general dose rate is 5-10 kg active ingredient/ha for
broadcast application, but is reduced for band application. The
emulsifiable concentrate is especially suitable for dip treatment of
banana seedlings and for foliar spraying of pineapples. Fenamiphos is
commercially used in Australia, USA, some Central and South American
countries, Spain, the Cameroons and the Ivory Coast.
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thio-oxidation of the parent compound
to the sulphoxide and sulphone. In general the major part of the
residue in crops and soil is the sulphoxide, followed by the sulphone.
Both are relatively persistent. Both metabolites are detoxified by
hydrolysis, usually followed by conjugation of the phenolic products
as glycosides. Little or none of the parent fenamiphos seems to be
present in most crops at harvest.
Micro-organisms in soil do not seen to be affected by fenamiphos.
The dissipation of residues in soil depends on the type of soil,
increasing in the order peat < silt loam < clay = sand. Fenamiphos
is only very slowly leached from soil.
Following application in accordance with good agricultural
practice, the maximum residue levels of fenamiphos including its
sulphoxide and sulphone in Brussels sprouts, cotton seed, cabbage,
carrots, cauliflower, grapefruit, lemons, limes, melons, oranges
(pulp), peanut kernels, pineapples, soybeans (dried) and sugar beets
are at or below 0.05 mg/kg. Higher residues are found in some other
crops. Typical maximum residues are: grapes 0.1 ppm, lettuce 0.5 ppm,
potatoes 1 ppm, strawberries 0.7 ppm, tomatoes 0.3 ppm and tobacco
(green) 12 ppm, (cured) 140 ppm (fermented) 100 ppm. Data for
fenamiphos residues in tobacco smoke are not available.
Processing cottonseed or soybeans to oil removed 98-99% of the
residues and processing citrus to cattle feed removed about 40%.
Cooking potatoes had no significant effect. In a laboratory experiment
approximately one third of the fenamiphos originally applied was
extractable with water from the surface of the crowns of pineapples
seven days after dipping in an aqueous emulsion containing 0.12%
fenamiphos.
Residues in eggs and tissues from hens fed for 14 days with 14C
ring-labelled fenamiphos in feed at 0.65 ppm were below 0.005 mg/kg.
Residues in milk and tissues of a dairy cow fed once with a dose
corresponding to 27 ppm fenamiphos sulfoxide in the diet were below
0.01 ppm.
Specific gas-chromatographic procedures for the determination of
residues of fenamiphos, its sulphoxide and sulphone in various crops,
animal tissues, milk and soil have been elaborated. The initial
extract is oxidized with permanganate to convert fenamiphos and its
sulphoxide to the sulphone. The sulphone is determined with an alkali
flame ionisation or flame photometric detector. Limits of detection
are about 0.02 ppm, and recoveries higher than 75%. The methods should
be suitable for regulatory purposes.
RECOMMENDATIONS
The following limits refer to fenamiphos including its sulphoxide
and sulphone, expressed as fenamiphos.
TOLERANCES
Tolerance
Crop (mg/kg)
Bananas, coffee beans (green and
roasted, grapes, sweet potatoes 0.1
Broccoli, Brussels sprouts, cabbage,
carrots, cauliflower, citrus fruits,
cottonseed, melons, peanut kernels,
pineapples, soybeans (dried), sugar beets 0.05*
* At or about the limit of determination.
TEMPORARY TOLERANCES
Temporary
tolerance
Crop (mg/kg)
Potatoes, tomatoes 0.2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further data on which to judge the residues in or on potatoes and
tomatoes.
2. Adequate residue data on which to base recommendations for
tolerances for other crops (e.g. beans, cucumbers, lettuce,
peppers and strawberries).
DESIRABLE
1. Brain cholinesterase and behavioural studies in animals exposed
to low levels for extended periods.
2. Observations in man.
3. Additional studies on the potentiation effects with other
organophosphorus pesticides.
4. Residue data for raw agricultural products moving in commerce.
5. Further residue data for different crops from supervised trials
including data on rates and frequencies of application, soil,
foliar or other treatment and pre-harvest intervals, especially
for broccoli and tangerines.
REFERENCES
Anonymous. (1973) The effect of frozen storage at 0 to -10°F on
Nemacur residues in peanut meat, broccoli, carrot roots, potatoes,
pineapple fruit. Chemagro Report No. 36132, 36318, 36319, 36320,
39094.
Arnold, D., Keplinger M. and Fancher, O. (1971) Mutagenic study with
Nemacur(R) (BAY 68138) Technical in albino mice. Report from
Industrial BioTest Laboratories, Inc., submitted by Bayer AG.
(Unpublished).
Cherry, C., Urwin, C. and Newman, A. (1972) Pathology report of BAY
68,138. Rat breeding study. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished).
Cherry, C. and Newman, A. (1973) Pathology report of Bayer 68,138.
Chronic toxicological studies in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Church, D.D. (1971) Nemacur - leaching, runoff and water stability.
Chemagro Report No. 26 849.
Coulston, F. and Wills, J.H. (1974) Summary of research of the
Institute of Comparative and Human Toxicology, Albany Medical College,
on fenamiphos. Report submitted by Bayer AG. (Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970a) The skin and eye
irritating properties of BAY 68,138 3 lb/gal S.C. to rabbits. Report
from Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970b) The acute inhalation
toxicity of Nemacur(R) 15% granular to rats. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C, and Anderson, R. (1971) The skin and eye irritating
properties of Bay 68138 technical to rabbits. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1972a) The acute dermal toxicity of
Nemacur(R) technical to rabbits. Report from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (unpublished).
Crawford C. and Anderson, R. (1972b) The acute dermal toxicity of
Nemacur(R) 3 lbs/gal. spray concentrate to rabbits. Report from
Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford C. and Anderson, R. (1973a) The dermal toxicity of Nemacur(R)
15% granules to rats. Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1973b) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R) with
analytical grade Nemacur(R). Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford C. and Anderson, R. (1973c) The eye and skin irritancy of
Nemacur(R) 2 lb/gal spray concentrate to rabbits. Report submitted by
Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1974) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Report from Chemagro Division of Baychem Corporation, submitted by
Bayer AG. (Unpublished).
DuBois, K., Flynn, M. and Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Report from University of
Chicago. (Unpublished).
DuBois, K. and Flynn, M. (1968) The subacute parenteral toxicity of
BAY 68138 to rats. Report from University of Chicago, submitted by
Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1970) Acute oral and dermal toxicity of
a granular formulation of BAY 68138. Report from University of
Chicago, submitted by Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1971) The acute oral and inhalation
toxicity of a Nemacur(R) (BAY 68138) formulation to rats. Report from
University of Chicago submitted by Bayer AG. (Unpublished).
Flint, D.R. (1973) The metabolism of Nemacur in pineapple. Chemagro
Report No. 39 119.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour, J., II. (1971) The
mobility and persistence of Nemacur in soil and water. Chemagro Report
No. 29 974.
Gronberg, R.R. (1969) The metabolic fate of
ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate (BAY 68138),
ethyl-4-(methylsulfinyl)-m-tolyl isopropylphosphoramidate (BAY 68138
sulfoxide) and ethyl-4-(methylsulfonyl)-m-tolyl-isopropylphosphoramidate
(BAY 68138 sulfone) in white rats. Report No. 26759 from Chemagro
Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Gronberg, R.R., Flint, D.R. and Pither, K. (1974) The metabolic fate
of Nemacur sulfoxide administered orally to a lactating dairy cow.
Report No. 41104 from the Chemagro Division of Baychem. Corp.,
submitted by Bayer AG. (Unpublished).
Gronberg, R.R., Simmons, C.E. and Shaw, H.R., II. (1973) Residues of
Nemacur in poultry eggs and tissue. Report No. 35995 from the Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Houseworth, L.D., and Tweedy, B.G. (1972) Effect of Nemacur on
microbial populations. Chemagro Report No. 34 990.
Khasawinah, A.M. (1970) The fate of Nemacur in soil. Chemagro Report
No. 28 796.
Khasawinah, A.M. (1971) Metabolism of Nemacur in tobacco plants.
Chemagro Report No. 29 142.
Khasawinah, A.M. (1972a) Metabolism of Nemacur in snap beans grown in
closed glass chambers. Chemagro Report No. 34 992.
Khasawinah, A.M. (1972b) The uptake and metabolism of Nemacur soil
residues by soybean plants. Chemagro Report No. 35012.
Khasawinah, A.M. (1973a) Metabolism of Nemacur in carrots. Chemagro
Report No. 36005.
Khasawinah, A.M. (1973b) Metabolism of Nemacur in tomatoes. Chemagro
Report No. 38501.
Khasawinah, A.M. (1973c) The metabolism of Nemacur in cabbage.
Chemagro Report No. 39120.
Khasawinah, A.M. and Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Report No. 34217 from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (Unpublished).
Khasawinah, A.M. and Sandie, F.E. (1974) Photodecomposition of thin
films of Nemacur. Chemagro Report No. 39217.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on chickens.
Report from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Report from
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. and Solmecke, B. (1971a) BAY 68138 toxicological studies.
Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. and Solmecke, B. (1971b) Granular formulation. Acute
dermal toxicity on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972a) Antidotal experiments on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972c) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Report from Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Ladd, R., Jenkins, D., Keplinger, M. and Fancher, O. (1971)
Teratogenic study with Nemacur(R) technical in albino rabbits. Report
from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Löser, E. (1968a) Subchronic toxicological studies on dogs. Report
from the Institute for Toxicology, Farbenfabriken Bayer AG.
(Unpublished).
Löser, E. (1968b) Subchronic toxicological studies on rats. Report
from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Löser, E. (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Report from the Institute for Toxicology, submitted by
Bayer AG. (Unpublished).
Löser, E. (1970) Subchronic toxicological studies on dogs. Report from
the Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Löser, E. and Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachr. Bayer,
24(1):69-113.
Löser, E. (1972a) Chronic toxicological studies on rats (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished)
Löser, E. (1972c) Generation studies on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Olson, T.J. (1970a) Residues on the surface of fresh pineapple crowns
treated with an aqueous emulsion of S.C. formulation of Nemacur.
Chemagro Report No. 28014.
Olson, T.J. (1970b) Determination of Nemacur residues in soil by
thermionic emission gas chromatography. Chemagro Report No. 28731.
Olson, T.J. (1970c) Determination of Dasanit, Guthion, Metasystox R,
Nemacur and trichlorfon in soil by thermionic emission gas
chromatography. Chemagro Report No. 27835.
Olson, T.J. (1970d) An interference study for the determination of
residues of Nemacur on pineapple. Chemagro Report No. 28723.
Olson, T.J. (1971a) Determination of Nemacur residues in tobacco and
tobacco smoke. Chemagro Report No. 30448.
Olson, T.J. (1971b) An interference study for the determination of
residues of Nemacur on cotton and tomatoes. Chemagro Report No. 30095.
Olson, T.J. (1971c) An interference study for the Nemacur crop residue
method for bananas. Chemagro Report No. 31041.
Olson, T.J. (1972a) The effect of citrus processing on residues of
Nemacur sulfoxide. Chemagro Report No. 34050.
Olson, T.J. (1972b) Effect of commercial soybean oil processing on
residues of Nemacur sulfoxide. Chemagro Report No. 33461.
Olson, T.J. (1974) The effect of alkali refining on Nemacur residues
in cottonseed oil. Chemagro Report No. 40113.
Shaw, H.R. and Flint, D.R. (1974) The metabolism of Nemacur soil
residues under aerobic and anaerobic conditions. Chemagro Report No.
39909.
Smith, P., Wright, P. and Keplinger, M. (1972) Eighteen month
carcinogenic study with Nemacur(R) (BAY 68138) in Swiss white mice.
Report from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Report from the Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Spicer, J. (1971) Pathology report of BAY 68138. Hen study. Report
from Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C., Newman, A. and Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C. Newman, A. and Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Report from Huntingdon Research Centre, submitted by Bayer AG.
(Unpublished).
Thornton, J.S. (1969) A gas chromatographic method for the
determination of Nemacur and metabolite residues in crops. Chemagro
Report No. 25402.
Thornton, J.S. (1971a) Determination of residues of Nemacur and its
metabolites in plant and animal tissues. J. agr. Food Chem., 19
(5):890-893.
Thornton, J.S. (1971b) An interference study for the residue method
for Nemacur and metabolites. Chemagro Report No. 25533.
Thornton, J.S. (1972) Effect of cooking on residues of Nemacur in
potatoes. Chemagro Report No. 35314.
Thornton, J.S. (1973a) Effect of alkali refining and deodorization on
residues of Nemacur and metabolites in cottonseed oil. Chemagro Report
No. 35345.
Thornton, J.S. (1973b) An interference study for the Nemacur residue
method. I. Additional compounds not previously tested. Chemagro Report
No. 36 142.
Thyssen. (1974a) Nemacursulfoxid, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974b) Nemacursulfon, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974c) 4-methyl-mercapto-m-Kresol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974d) 3-methyl-4-methyl-mercaptophenol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974e) 3-methyl-4 methan-sulfonyl phenol, Akute Toxizitat
bei Ratten. Report from Institute for Toxicology, submitted by Bayer
AG. (Unpublished).
Tweedy, B.G. and Houseworth, L.D. (1974) Leaching of "aged" Nemacur
residues in sandy loam soil. Chemagro Report No. 40 506.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl
4-(methylthio)-M-tolyl isopropylphosphoramidate] and identification
of two metabolites in plants. J. agr. Food Chem., 20(1):157-160.
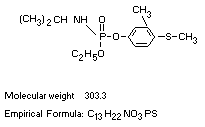 Molecular weight 303.3
Empirical Formula: C13H22NO3PS
Other information on identity and properties
State: brown semi-solid (technical)
white crystals (pure)
Melting Point: 46°C (technical)
49°C (Pure)
Vapour pressure: 10-6mm Hg (at 30°C)
Solubility: in water approx. 700 mg/l at 20°C
slightly soluble in most organic
solvents
Specific gravity (melt): 1.14
Hydrolytic stability in propanol/water (1:1) at 40°C:
40% degradation after 14 days at pH 2
no degradation after 50 days at pH 7
half life of 31.5 hours at pH 11.3
Formulations: 5% Granular, 10% Granular, 400 E.C. (Emulsifiable
concentration 400 g/l)
Purity
The minimum purity is 87%. Maximum levels of the individual
impurities are shown in Table 1.
TABLE 1 Impurities (maximum levels) in technical fenamiphos
Compound Maximum
level (%w/w)
O,O-diethyl isopropylphosphoramidate 3.0
4-methylthiocresol 0.5
O,O-bis[(4-methylthio)-3-ethylphenyl]
isopropylphosphoramidate 2.0
O,O-diethyl O-[(4-methylthio)-3-methylphenyl]
phosphate 1.0
O-ethyl O-[(4,6-bismethylthio)-3-methylphenyl]
isopropylphosphoramidate 3.0
O-ethyl O,O-bis-[(4-methylthio)-3-methylphenyl]
phosphate 8.0
O-ethyl O-[(4-methylthio)-3-methylphenyl] phosphate 1.0
water 0.3
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformations
The two major metabolites identified following injection into
bean, tomato, peanut and potato stems were O-ethyl
O-4-methylsulfinyl-m-tolyl isopropylphosphoramidate and O-ethyl
O-4-methylsulfonyl-m-tolyl isopropylphosphoramidate, the sulphoxide
and sulphone of the thio-ether (Waggoner, 1972). A third metabolite
(apparently a more potent inhibitor of cholinesterase) was
not identified but was considered to be a further metabolite
resulting from the sulphoxide. Further details of this study are
given in the section "Fate of residues in plants". Small residues of
the unknown metabolite may be of toxicological significance because
of the potent anticholinesterase properties.
The metabolic fate of labelled fenamiphos was studied in vivo
in rats and in vitro with rat liver microsomes. The compounds were
labelled in the ethyl (14C) isopropyl, (14C) or the thiomethyl (3H)
positions. In vivo fenamiphos is excreted within 12-15 hours
following a single oral dose of 2 mg/kg. In the in vitro studies a
small quantity of an unknown metabolite possibly resulting from the
N-dealkylation of fenamiphos was observed (Khasawinah and Flint,
1972). Apart from this minor component, metabolism in animals and
plants follows the same pattern: oxidation of the thioether to the
sulphoxide and sulphone, dearylation to yield the methyl thioether
phenol (or its sulphoxide and sulphide) and potentially dealkylation
of the ethyl, isopropyl or isopropylamide moiety of the phosphate
ester. Treatment of rats with fenamiphos sulphoxide or sulphone
produced the same excretion pattern and almost identical urinary
metabolites (Gronberg, 1969).
When fenamiphos sulphoxide was given to a lactating cow (Gronberg
et al., 1974) the identified components of the residue were
fenamiphos, its sulphoxide and its desisopropyl derivative, and the
phenols of phenamiphos, its sulphoxide and its sulphone. An
unidentified metabolite was also detected. Quantitative details are
given in the section "Fate of residues in animals".
Effects on enzymes and other biochemical parameters
Fenamiphos, as are other organophosphate esters, is an inhibitor
of cholinesterase enzymes. The in vitro I50 values were as follows:
Rat serum - 5.1 x 10-5M.
Rat erythrocyte - 6.3 x 10-4M.
Rat brain - 2.1 x 10-4M.
The maximum inhibition of whole blood cholinesterase in vivo
occurred in rats three hours after dosing. In vivo sensitivity of
cholinesterase reflects the in vitro I50 values with plasma
cholinesterase being more sensitive to inhibition than the erythrocyte
enzyme (Löser and Kimmerle, 1971). The metabolites of fenamiphos are
more active inhibitors than the parent molecule (Waggoner, 1972). In
vitro inhibition of horse serum cholinesterase showed relative
values for fenamiphos < sulfoxide = sulfone < sulfoxide metabolite.
In vitro I50 values of chicken and monkey liver aliesterase
(triacetin hydrolysis) and monkey cholinesterase are as follows:
Chicken (aliesterase): 4.2 x 10-5M.
Monkey (aliesterase): 1.0 x 10-6M.
Monkey (cholinesterase): 4.6 x 10-6M.
(Coulston and Wills, 1974)
On the basis of some preliminary electrophysiological studies
with the cat, it was estimated that a threshold dose of fenamiphos
might fall in the range of 0.8 to 1.5 mg/kg (Coulston and Wills,
1974).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of Charles River random bred Swiss white mice (125 males
and 125 females/group) were fed fenamiphos in the diet at 0, 25 and 50
ppm for eighteen months in a study specifically designed to evaluate
the carcinogenic potential of the compound. Additional groups of mice
were fed 10 ppm N-nitrosodiethylamine for eighteen months (125 males
and 125 females); 40 ppm for six months (60 males and 60 females); and
1000 ppm benzidine (100 females) for eighteen months as positive
controls. Fenamiphos did not affect the incidence or pattern of
mortality. Clinical chemistry studies performed at 18 months were
normal. No signs of tumour formation were observed on gross or
microscopic examination which were attributed to fenamiphos (Smith et
al., 1972).
Special studies on mutagenicity
Mouse
Groups of twelve male mice were administered fenamiphos
intraperitoneally at 0, 0.25 and 0.50 mg/kg and mated for six
consecutive weeks to virgin females in a standard dominant lethal
test. A positive reference (methyl methanesulphonate) was used in this
experiment. Fenamiphos did not induce alterations in male germinal
cells which would be expected to lead to an early embryo mortality
(Arnold et al., 1971).
Special studies on neurotoxicity
Hen
Groups of hens (eight per group) were fed fenamiphos in the diet
at levels of 0, 1, 3, 10, and 30 ppm for thirty days. At the end of
the feeding experiment some animals were sacrificed and the remainder
were observed for four weeks for neurological signs of poisoning. Food
consumption was depressed at 30 ppm and the average body weight and
growth of the animals at this same level was reduced. Cholinesterase
(whole blood) depression was observed at thirty days at 3, 10 and 30
ppm, although cholinergic signs of poisoning were not observed at any
time. There were no indications of delayed neurotoxic effects and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with H&E) did not indicate delayed neurological involvement
with fenamiphos (Kimmerle, 1970; Spicer, 1970).
Groups of hens (ten per group) were orally administered an LD50
dose of fenamiphos (5.0 mg/kg). The birds were observed for three
weeks and sacrificed. No evidence of delayed neurotoxicity was
observed either clinically or histologically while positive signs were
observed with TOCP (Kimmerle, 1971; Spicer, 1971).
Special studies on potentiation
Rat
Male rats were tested orally with fenamiphos (SRA 3886) in
combination with disulfoton or E 154 and no potentiation of the acute
toxicity was noted (Kimmerle, 1972c).
Special studies on reproduction
Rat
A standard three generation, two litters per generation,
reproduction study was performed with fenamiphos. Four groups of
animals, consisting of ten male and twenty female rats per group, were
fed fenamiphos at 0, 3, 10, and 30 ppm in the diet throughout the
study period including mating, gestation, and suckling. Immediately
after birth, pups were examined for malformations prior to either
preparing for another generation or sacrifice. Five weanling rats per
group of the F3b generation were sacrificed and macroscopic and
microscopic examinations performed on the major tissues and organs.
There were no apparent differences with regard to reproduction
parameters including fertility, litter size, lactation index, growth
of young, or on examination for malformation in any of the animals
exposed to fenamiphos levels up to and including 30 ppm in the diet.
It was concluded that fenamiphos had no influence on the reproduction
of rats at dietary levels up to and including 30 ppm (Löser, 1972c;
Cherry et al., 1972).
Special studies on teratogenicity
Rabbit
Groups of fifteen artificially inseminated rabbits were orally
administered fenamiphos at levels of 0, 0.2, and 0.4 mg/kg/day.
Positive control groups (oral treatment with thalidomide (37.5
mg/kg/day)) were concurrently run with this experiment. All animals
were treated from day 6 through 18 of gestation and sacrificed on day
29. At day 29 the young were examined for 24 hours, sacrificed and
examined after skeletal staining. At the high level of treatment of
fenamiphos, 2/12 instances of abortion and 2/12 premature deliveries
were observed. No external or internal abnormalities were observed in
the foetuses following treatment. With the exception of the animals at
the high level of treatment where a few aborted or delivered
prematurely, no effects on reproduction were observed with fenamiphos
(Ladd et al., 1971).
Acute toxicity
TABLE 2 Acute toxicity of fenamiphos
LD50
Animal Sex Route (mg/kg) References
Rat M oral 2.4-15.6 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Rat F oral 2.3-19.4 Kimmerle, 1972c
Crawford and Anderson, 1974
DuBois et al., 1967
Rat M ip 3.0-3.7 ibid.
Rat F ip 4.2-4.9 Löser and Kimmerle, 1971
Rat M dermal 73-500 DuBois et al., 1967
Rat F dermal 84-154 Kimmerle and Solmecke, 1971a
Mouse M oral 22.7 Löser and Kimmerle, 1971
Mouse M&F ip 3.4 DuBois at al., 1967
Guinea Pig M oral 56-100 ibid.
Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Guinea Pig M ip 17.3 DuBois et al., 1967
TABLE 2 (Cont'd.)
LD50
Animal Sex Route (mg/kg) References
Rabbit M oral 10-17.5 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Cat M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Dog M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Hen F oral 5.3 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
TABLE 3 Acute toxicity of metabolites and impurities of fenamiphos
LD50
Species Sex Route (mg/kg) References
Fenamiphos-sulphoxide
Rat F oral 10-25 Thyssen, 1974a
Fenamiphos-solphone
Rat F oral 10-25 ibid.
4-methylthio-m-cresol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 1333>2500 ibid.
Thyssen, 1974c
3-methyl-4-methylthiophenol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 500-1000 Thyssen, 1974d
1175 Crawford and Anderson, 1974
3-methyl-4-methanesulphonylphenol
Rat M oral 1250 ibid
Rat F oral 1000 Thyssen, 1974e
1854 Crawford and Anderson, 1974
Impurities identified as components of the technical mixture
(aryldiamide, diarylamide, diaryl ethyl ester, diethyl ester,
diethylmonamide, di-SCH3 compound, ethyl aryl ester, ethyldiamide,
and 4-methylthio-3-cresol) were tested for their acute toxic
properties to rats. At a dosage of 1.5 times the acute LD50 of
fenamiphos none of the materials were toxic (Crawford and Anderson,
1973b).
Technical fenamiphos administered to the skin of rabbits as an
acetone solution at 50 mg/kg resulted in a slight erythema and was not
considered as a primary irritant. Technical fenamiphos applied to the
conjunctival sac of rabbits (100 mg/kg as a crystalline material) was
irritating to the eye. This irritation was believed to be a mechanical
rather than a physiological effect (Crawford and Anderson, 1971).
A 15% granular formulation was examined for dermal and inhalation
toxicity properties and at the highest level examined (200 mg/kg and
20 mg/l respectively) no toxicity was observed (Crawford et al.,
1970b; Crawford and Anderson, 1972a). The rat oral LD50 of a 15%
granular formulation was 66.6 mg/kg for male rats and 62.7 mg/kg for
female rats (DuBois and Kinoshita, 1970). A 5% or 10% granular
formulation, administered at up to 500 mg/kg to the backs of rats
resulted in depressed cholinesterase activity values. Mortality was
not observed at the maximum dose tested and it was considered that the
granular formulation was not a dermal hazard although the material was
rapidly absorbed into the body (Kimmerle and Solmecke, 1971b). The
acute dermal toxicity and dermal and eye irritation properties of a
liquid formulation (37.4% a.i.) were tested in rabbits. The acute
LD50 was 75.7 mg/kg and 71.5 mg/kg to male and female rabbits
respectively (Crawford et al., 1970a; Crawford and Anderson, 1972b).
When applied to intact and abraded skin at a dose of 0.25 ml (0.085 mg
a.i.), fenamiphos was not found to be a primary irritant. Fenamiphos
(0.1 ml, equivalent to 0.034 mg a.i.) was an irritant when applied to
the conjunctival sac of rabbits. Control formulation data were not
reported (Crawford and Anderson, 1973c). The acute rat oral LD50 of a
35% liquid formulation was calculated to be 24.8 mg/kg (equivalent to
8.7 mg fenamiphos /kg). Inhalation toxicity studies with this
preparation using a small particle size distribution (1-3 microns)
resulted in an LC50 value of 0.175 and 0.091 mg/l following one and
four hour exposure respectively (DuBois and Kinoshita, 1971). Studies
with rodents administered fenamiphos by inhalation at doses up to 0.23
mg/l for one to four hours exposure as a static spray showed that
rabbits and guinea pigs were more tolerant of the acute effects than
rats and mice. A one hour LC50 value for mice was 0.06 mg/l and for
rats 0.23 mg/l while rabbits and guinea pigs were unaffected at these
concentrations (Kimmerle and Solmecke 1971a; 1972b). Acute oral
administration of 2.5 mg/kg to rabbits had no effect on several liver
function tests including BSP, SGPT and SDH activity at 1 and 24 hours
and seven days after treatment (Kimmerle and Solmecke 1971a).
Studies performed on male rats indicated that, following
poisoning, the administration of atropine and/or 2-PAM or Toxogonin
would reduce the acute lethal dose by a factor of approximately two.
As with other organophosphorus compounds, rapid administration of
atropine and oxime reactivator following poisoning would afford some
protection and would alleviate the cholinergic signs of poisoning
(Kimmerle, 1972a; DuBois et al., 1967).
Short term studies
Rat
Groups of rats (fifteen males and fifteen females per group,
thirty males and thirty females were used as controls) were fed
fenamiphos in the diet at 0, 4, 8, 16, and 32 ppm for twelve weeks.
Male and female rats from the highest dose group exhibited signs of
cholinergic stimulation in the first two months of the test.
Behavioral differences in all other groups were not apparent. There
were no differences from the control in the average of any group with
regard to food consumption and growth. With the exception of
cholinesterase inhibition, there was no apparent effect on group
averages of haematological parameters examined. Plasma cholinesterase
depression was observed at 8 ppm. A no-effect level, based upon plasma
cholinesterase, was observed at 4 ppm. Gross and microscopic
pathological examination was performed on tissues and organs at the
conclusion of the experiment. A slightly increased liver weight,
observed in males at 16 and 32 ppm, was not reflected in the
calculation of relative organ-to-body weight ratios nor in the
histological examination. Based on cholinesterase depression, 4 ppm in
the diet was a no-effect level in this experiment (Löser, 1968b;
Mawdesley-Thomas and Urwin, 1970a).
Groups of male rats (15 per group) were administered fenamiphos
orally or by intraperitoneal injection daily, 5 days/week for 60 days.
The animals all survived a dose range of 1.5 (ip) to 1.7 (oral)
mg/kg/day with no evidence of a cumulative toxicological effect due to
fenamiphos (Kimmerle and Solmecke, 1971a). In an earlier study female
rats survived daily ip administration of 1 mg/kg for 60 days while 40%
of those administered 2 mg/kg did not survive. All rats died at 3
mg/kg administered by intraperitoneal injection (DuBois and Flynn,
1968). These studies indicate little if any cumulative toxicity.
Dog
Groups of beagle dogs (two males and two females/group) were fed
fenamiphos in the diet at 0, 2, 6, and 18 ppm for three months.
Behavioral abnormalities, evidenced by signs of cholinergic
stimulation, were evident at 18 ppm in the diet and growth of females
at this high level was reduced. Average haematological and clinical
chemistry values including liver function tests, kidney function tests
and urinalyses were not apparently affected by fenamiphos in the diet.
Averages of plasma and red blood cell cholinesterase values were
depressed at 4 ppm in the plasma of both males and females. At 2 ppm
no effect on the average red cell cholinesterase values was found
while plasma cholinesterase was depressed (20-30%) in both sexes.
Gross morphological examination of tissues and organs at the
conclusion of the study indicated no abnormal effects of fenamiphos in
the diet (Löser, 1968a).
Groups of beagle dogs (two males and two females/dose, three of
each sex were used in the control group) were fed fenamiphos in the
diet at levels of 0, 1, 2 and 5 ppm for twelve weeks. There was no
effect of feeding fenamiphos at levels up to and including 5 ppm in
the diet on the average of any of the parameters examined including
haematology, liver function tests, clinical chemistry tests, kidney
function tests, and gross and microscopic examination of tissues and
organs. As with other studies, cholinesterase was the only parameter
significantly affected, with the females being more susceptible than
the males and the plasma being more sensitive to inhibition than red
blood cell. Erythrocyte cholinesterase was unchanged at 2 ppm while
plasma cholinesterase was slightly depressed. One ppm in the diet had
no effect on plasma cholinesterase. As the depression of plasma
cholinesterase was minimal and transient at 2 ppm it might be
considered that this would be a no-effect level in this experiment
(Löser, 1969; Mawdesley-Thomas and Urwin, 1970b).
As an extension of the previous short term study in dogs (Löser,
1969), an additional two male and two female dogs were fed fenamiphos
in the diet at 10 ppm for three months. Two male and two female
animals were also maintained as a control. There was no mortality over
the course of the experiment and a very slight deviation in the
average body weight of both males and females was noted. Because of
the small sample population, the slight weight differences cannot be
fully evaluated. There was no effect of feeding fenamiphos on any of
the haematological parameters examined. Urinalysis, clearance
examinations, blood sugar and cholesterol were normal over the course
of the experiment. Cholinesterase activity was significantly depressed
in both plasma and erythrocytes and in both male and female dogs. In
addition, several abnormalities were noted with regard to some of the
serum enzyme parameters related to liver function after three months
of feeding, especially in male dogs. Alkaline phosphatase and SGPT
activity were significantly increased. Following gross and microscopic
examination of tissues and organs, no significant differences were
noted between the control and those animals fed 10 ppm. The abnormal
findings in some enzyme parameters related to liver functions,
predominantly in males at three months of feeding 10 ppm in the diet,
were not accompanied by pathological findings at autopsy nor confirmed
in another test where fenamiphos was fed at levels of 18 ppm in the
diet. They are considered a transient occurrence and not directly
related to the presence of fenamiphos in the diet (Löser, 1970;
Thompson et al., 1972a).
Groups of pure bred beagle dogs (four males and four females per
group) were fed fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5,
and 10 ppm for two years. There were no significant effects noted with
fenamiphos at any dietary level with regard to growth, food
consumption or any of the standard clinical and physiological
examinations made during the course of the study. One dog died after
being fed 0.5 ppm in the diet. This dog was considered to have died of
pneumonia and its death was not related to administration of
fenamiphos. Gross and histological examinations of all tissues and
organs at the conclusion of study did not reveal any abnormal
developments considered to have been a result of feeding fenamiphos.
The only significant physiological effect observed was
anti-cholinesterase activity which was marginally depressed in plasma
at 2 ppm. As with other species, the plasma appears to be the most
sensitive indicator of exposure with a no-effect level being 1 ppm in
the diet (equivalent to 0.029-0.036 mg/kg/day) (Löser, 1972b; Thompson
et al., 1972b).
Long term studies
Rats
Groups of SPF derived Wistar strain (forty males and forty
females per group, eighty males and eighty females used as controls)
were fed fenamiphos in the diet, at concentrations of 0, 3, 10, and 30
ppm for two years. Behavioral abnormalities were evident, as signs of
cholinergic stimulation, only during the first six weeks of feeding at
30 ppm. After six weeks of feeding, the obvious anticholinesterase
effect disappeared and was not evident for the remainder of the two
year study. There were no differences in averages at any feeding level
on growth, mortality, food consumption, haematological values,
clinical chemistry values or liver and kidney function tests. Urine
examinations as well as blood sugar and cholesterin values were
normal. Thyroid weights and thyroid-to-body weight ratios of females
were heavier than normal at 30 ppm. All other major tissues and organs
appeared normal under gross and microscopic examination. The thyroid
weight in females at 30 ppm was high but was not accompanied by an
abnormal tumour development, goitre or by unusual histological
findings.
Cholinesterase determinations indicated that plasma
cholinesterase was the most sensitive parameter. At 10 ppm in the
diet, fenamiphos inhibited plasma cholinesterase. No significant
effects were observed at 3 ppm in the diet (equivalent to 0.17-0.23
mg/kg bw/day) (Löser, 1972a; Cherry and Newman, 1973).
COMMENTS
Fenamiphos, an acutely toxic organophosphate ester, is rapidly
absorbed as evidenced by rapid onset of cholinergic signs of
poisoning. In animals fenamiphos is rapidly degraded, predominantly
through oxidation to acutely toxic products. Fenamiphos does not
appear to affect biochemical systems other than cholinesterase.
Testing for the delayed neurotoxic effect commonly seen with TOCP was
negative. Short term and long term dietary studies in both rats and
dogs have indicated, with the exception of cholinesterase depression,
no unusual effects. In long term studies with rats, transient
behavioural abnormalities were observed during the first six weeks of
feeding, which disappeared and were not evident for the remainder of
the study. Female rats are more susceptible than males to
cholinesterase depression. Plasma cholinesterase depression is a
better indicator of exposure than red blood cell cholinesterase. Based
on plasma cholinesterase depression, a no-effect level in a two-year
rat study is considered to be 3 ppm in the diet and in a two year dog
study, 1 ppm in the diet. Long term studies in rats and mice reported
to the Meeting did not show a carcinogenic effect. Although there are
no studies available in man, sufficient data are available and the
Meeting allocated an ADI using the more sensitive species, the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 ppm in the diet equivalent to 0.17 mg/kg bw.
Dog: 1 ppm in the diet equivalent to 0.029 mg/kg bw.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0006 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenamiphos is an organophosphorus compound with a systemic action
and a powerful nematicidal effect. It is active against root-knot,
cyst-forming and free-living nematodes and has important applications
against sucking insect pests and spider mites. Fenamiphos is
commercially used in the USA, Australia, Spain, the Cameroons,
Caribbean, Chile, Columbia, Costa Rica, Ecuador, Guatemala, Ivory
Coast, Jamaica, Martinique, Mexico, Nicaragua, San Salvador and
Uruguay. Registration proceedings and trials are in progress in
several other countries.
Fenamiphos is formulated as 5 and 10% granules and as a 40%
emulsifiable concentrate.
Pre-harvest treatments
Fenamiphos is formulated as a granular product and as an
emulsifiable concentrate. Both formulations are recommended chiefly
for treatments in banana, pineapple, vegetable (tomatoes, brassicas,
peppers, eggplants), tobacco, coffee, groundnut and citrus crops.
Application is usually made to the soil at sowing, planting and
transplanting, and in established crops. The general dose rate is 5 to
10 kg active ingredient per hectare for broadcast (overall)
application, but is reduced for band application according to band
width. Furthermore, the emulsifiable concentrate is suitable for dip
treatments of banana seedlings and for foliar spraying of pineapples.
Details of uses and recommendations are given in Table 4.
TABLE 4 Use Pattern of fenamiphos (All applications are pre-harvest against nematodes)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Bananas 1.5-2.5 g/m2 At planting G
3.0 g/plant Soil treatment in
established crop G 3-4
4-5 kg/ha In irrigation
canals or direct
to soil EC 2
1-1.5 g/corm Dip treatment
pre-planting plus
soil application EC
2 or 1.5 g/
planting position
(but no more than
total of 3 g/
planting position) G
Pineapples 10-40 kg/ha Broadcast treatment
pre-planting of
crowns and slips G
5-20 kg/ha Broadcast treatment
pre-planting of
crowns and slips
in combination with G
3-5 kg/ha foliar spraying 3 in
post-planting LC established
crop
15 kg/ha Foliar spray
application at
planting, repeat
after an interval
of 2 months EC 2
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Tomatoes 1-2.5 g/m2 Immediately before
or after sowing G
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Brassicas 5-8 kg/ha Broadcast/overall
application G,
pre-planting EC
Tobacco 1-2.5 g/m2 Before or immediately
after sowing or G
0.1-0.2 g/plant at transplanting or
in established crop G 1
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Coffee 1-2.5 g/m2 Seedbed treatment G
1.5-2.5 g/shrub In established crop G 2-3
Groundnuts 3-5 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Cucurbitaceae 0.15-0.2 g/ Band application
planting hole or at sowing G
1.5-2.5 kg/ha
Peppers 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Eggplant 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Citrus 1-1.5 g/m2 Seedbed treatment G
2-3 g/tree Treatment in nursery G
1.5-3 g/tree In established crop G, LC 2-3
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Potatoes 5-10 kg/ha Broadcast/overall G, EC,
treatment pre-planting LC 1
Pyrethrum 5-8 kg/ha Broadcast application
at planting G
Ornamentals 1.5-3 g/m2 In established crops G
Cotton 1.7-3.5 kg/ha Band application
(band width of G,
30-45 cm.) at sowing LC
Beets 3-(6) kg/ha Band application
immediately after
sowing or during G,
emergence EC
Soybeans 5-8 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Beans 5-8 kg/ha Broadcast application
pre-sowing G 1
Grapes 2-3 g/plant G 1
Strawberries 10 kg/ha Broadcast application
pre-planting G
300 g/1000 Post-planting or G
metres of row post-harvest
Lawns 10-20 kg/ha Broadcast application G
1 G = Granules; EC = Emulsifiable concentrate; LC = Liquid concentrate.
Post-harvest treatments
No recommended uses.
Other uses
Fenamiphos is recommended for use on ornamentals (granular
formulation).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of fenamiphos together with its sulphoxide and
sulphone resulting from supervised trials in a wide range of crops are
shown in Table 5. In most crops, residues were below 0.05 mg/kg. The
exceptions were grapes, lettuce, potatoes, strawberries and tomatoes
which contained up to about 0.1, 0.5, 1, 0.7 and 0.3 mg/kg
respectively. Residues in tobacco were much higher, with respective
maximum levels in green, cured and fermented tobacco of about 12, 140
and 100 mg/kg.
FATE OF RESIDUES
In animals
A feeding study was carried out on a lactating dairy cow to
determine whether fenamiphos sulphoxide, found in plants as the major
metabolite, would be incorporated into milk and animal tissues either
as the intact compound or in metabolized form (Gronberg et al., 1974.
See also "Biotransformations"). A single dose of 0.8 mg/kg body
weight, corresponding to 27 ppm in the diet, of ring-14C fenamiphos
sulphoxide was administered. Four hours after application the label
was distributed as follows. Urine 39%, faeces <0.1%, rumen 47%,
tissues 1.4%, bile 0.1%, omasum, abomasum and milk <0.1% each; total
recovery 88%. The following compounds were found: fenamiphos (I),
fenamiphos sulphoxide (II), desisopropyl-fenamiphos (III), phenol of
fenamiphos (IV), phenol of fenamiphos sulphoxide (V), phenol of
fenamiphos sulphone (VI) and "unknown" (VII). The levels of these
residues in tissues and milk are shown in Table 6.
Of the dose, 18% was reduced to the parent fenamiphos, apparently
by microorganisms. At 4 hours 82% of the activity in whole blood and
87% of the activity in urine was identified as conjugated phenols.
The residues of fenamiphos in poultry eggs and tissues were
determined by Gronberg et al. (1973). Laying hens were fed for 14 days
with carbon-14 ring-labelled fenamiphos in feed at 0.06, 0.18 and 0.65
ppm concentration. During this time, residues in eggs were below the
detection limit of 0.003 mg/kg. At slaughter, all tissue residues from
birds given feed containing 0.65 ppm were below the limit of
determination of 0.003 to 0.005 mg/kg, although very low residues were
detectable in some tissues.
TABLE 5 Residues of fenamiphos and metabolites resulting from supervised trials
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Apples Australia 10% G 22.4 334 1 n.d.2 n.d.2
Bananas, Central
peel America 10% G 2.8-12 g
a.i./plant 1-195 41 <0.025-0.3 <0.025
pulp <0.025 <0.025
Bananas, Ivory Coast 5% G 10 g a.i./plant 1-14 4 <0.02 <0.02
peel
pulp <0.02 <0.02
peel 28-88 3 0.06-0.1 0.07
pulp 0.03-0.08 0.05
Broccoli USA 3% G;
15% G 6.72 65-101 8 <0.01-0.04 0.02
15% G 10.0 92 1 0.2 0.2
Brussels
Sprouts USA 3% SC;
15% G 6.72-10.0 107-133 5 <0.01-0.02 <0.01
Cabbage USA 3% SC;
15% G 6.72-10.0 55-108 14 <0.01-0.02 0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 40% EC 4.5 42-106 6 n.d.2 n.d.2
Carrots USA 15% G 6.72 96-160 9 <0.01-0.05 0.01
Cauliflower USA 3% SC;
15% G 6.72-10.0 97-111 12 <0.01-0.04 0.02
Coffee Brazil - 1-2 g a.i./plant 14-15 6 <0.02-0.07 0.05
beans, Central
green America 28-30 6 <0.02-0.09 0.05
56-62 4 <0.02-0.05 0.03
90 2 0.03; 0.05 0.04
Cottonseed USA 3% SC;
15% G 1.12 157-196 10 <0.01 <0.01
Cotton USA 3% SC;
gin trash 15% G 1.12 161-196 8 <0.01 <0.01
Cotton USA 3% SC;
foliage 15% G 1.12 143-148 4 <0.01-0.1 0.04
Grapefruit USA 3% SC;
peel 15% G 33.6 - 50.4 183-184 1 <0.01 <0.01
pulp <0.01 <0.01
whole fruit <0.01 <0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Grapes Australia 5% G
43% EC 5.6 - 14.7 145-147 2 n.d.2 n.d.2
22.0 145-147 1 0.03 0.03
44.0 145-147 1 0.08 0.08
Leaf-lettuce USA 3% SC 11.2 46 1 0.3 0.3
Head USA
lettuce, 3% SC;
head 15% G 11.2 61-72 4 0.02-0.1 0.08
wrapper leaves 0.09-0.5 0.3
discard leaves 0.09-6.5 1.9
Lemons, USA
whole fruit 3% SC 33.6 184 1 0.01 0.01
Limes, USA 3% SC;
whole fruit 15% G 33.6 147 2 <0.01 <0.01
Melons Guatemala
Australia 5% G
Egypt 40% EC 1.6-10.0 56-112 5 n.d2-0.05 n.d.2
Oranges, USA 3% SC; 22.4-50.4 182-366 4 <0.01-0.1 0.04
peel 10% G;
15% G
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
pulp 4 <0.01 <0.01
whole fruit 5 <0.01-0.02 0.01
whole fruit Australia 5% G;
40% EC 11.2-22.4 21-139 10 n.d.2 n.d.2
Peanuts, USA 3% SC;
kernels 10% G 4.51-13.45 122-148 5 <0.01-0.02 <0.01
shells (0.01-0.2 0.06
vines 98-148 5 0.02-4.3 2.3
Pineapples Hawaii, USA 3% SC;
10% G 44.8-112 238-658 10 <0.01-0.03 <0.01
3% SC
(foliar spray) 5.6 1-29 20 <0.02-4.0 0.5
Potatoes USA 3% SC;
15% G (row
appl) 6.1 41-58 3 <0.02-1.2 0.7
71-106 7 <0.01-1.0 0.15
Potatoes, USA, Canada, 3% SC;
whole Fed. Rep. of 15% G
Germany, UK, (broadcast
Australia 4-5-11.2 67-203 23 n.d.2-0.45 0.07
peel 1 0.07 0.07
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
flesh 1 0.07 0.07
Sweet USA 3% SC;
Potatoes 15% G
(broadcast) 6.72 108-143 8 <0.01-0.04 0.01
3% SC;
15% G
(row appl) 4.0-6.1 108-143 8 <0.01-0.06 0.01
Soybeans, USA 3% SC;
bean, green 15% G 11.2 75-117 12 <0.01-0.09 0.04
vine, green 75-117 12 <0.01-12 2.9
bean, dry 131-159 11 <0.01-0.03 0.01
Strawberries Australia 5% G
40% EC 16.5-22.5 36-121 6 0.03-0.7 0.2
Sugarbeets, USA 3% SC;
tops 15% G 6.72-10.0 119-187 9 <0.01-0.08 0.01
beets <0.01-0.03 0.01
Tangerines, USA 3% SC;
peel 15% G 33.6 247-251 2 0.07; 0.3 0.2
pulp 2 <0.01; 0.03 0.02
whole fruit 1 0.03 0.03
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Tobacco Australia 40% EC 50-95 4 0-5-4.8 1.9
Tobacco, USA 3% SC; 6.72 4 3 0.9-7.8 3.5
green 15% G 7 3 0.8-7.0 2.9
11 3 0.8-3.3 1.8
14 3 1.4-12 5.2
21 3 1.2-4.9. 2.5
28 3 0.4-4.2 1.7
35 3 0.25-3.6 1.4
36-148 13 0.02-4.8 1.0
air cured 88-106 2 3.2; 4.2 3.7
flue cured 85 2 11; 13 12
cured 75-91 6 4.5-140 33
aged 75-92 3 5.7-16 11
fermented 89 1 98 98
Tomatoes USA, 3% SC;
Canada 15% G 11.2-16.8 61-89 10 <0.01-0.3 0.1
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 5% G;
40% w/v 9.8-11.2 78-161 5 <0.05 <0.05
Fed. Rep, of
Germany 5% G 9.8-12.2 79-161 5 n.d.3 n.d.3
1 EC = emulsifiable concentrate; G = granules; SC = spraying concentrate. n.d. = <0.01.
2 <0.01 mg/kg.
3 <0.05 mg/kg.
TABLE 6 Residues, mg/kg, in tissue and milk of cow fed fenamiphos
sulphoxide (Gronberg et al., 1974)
Compound1 Fat Meat Liver Kidney Milk
I 0.004 n.d.2 0.006 n.d. n.d.
II n.d. n.d. 0.002 n.d. 0.002
III 0.002 n.d. 0.005 0.16 0.004
IV 0.005 0.001 0.029 0.86 0.005
V 0.003 0.003 0.021 0.36 0.025
VI n.d. n.d. n.d. 0.01 0.004
VII 0.002 0.005 0.036 0.25 0.020
1 For identities of compounds, see text.
2 n.d., not detectable, = <0.001 mg/kg.
In plants
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thiooxidation of the parent compound to
the sulphoxide and sulphone. Both metabolites are detoxified by
hydrolysis, generally followed by glucosylation of the phenolic
products.
Fenamiphos-14C,3H was injected into the stems of beans,
tomatoes, peanuts and potatoes and the plants were harvested at 7, 14,
21 and 28 days (Waggoner, 1972. See also "Biotransformations"). Two
major metabolites were identified by TLC, isotopic ratios, IR, and MS
as the sulphoxide and sulphone of fenamiphos. The sulphoxide was the
major metabolite, accounting for 33-41% of the residue, followed by
the sulphone (31-33%), both after 7 days' treatment. Two minor
metabolites containing intact phosphoramidate structures accounted for
not more than 1.5 and 0.2% of the total residue.
Khasawinah studied fenamiphos metabolism in carrots (1973a),
tomatoes (1973b), cabbages (1973c), soybeans (1972b) and tobacco
plants (1971) following soil application, and in snap beans (1972a)
after both soil treatment and stem injection; 14C-ring, 14C-1-ethyl
and 3H-methylthio labelled fenamiphos were used.
Carrots readily absorbed and metabolized fenamiphos and its major
soil foliage and 14C from the ethyl group was detected in the foliage
and carrots. This 14C was probably incorporated into lipids,
pigments, sugars and structural elements such as cellulose.
Water-soluble residues were mainly conjugates of the sulphoxide and
sulphone phenols. No parent fenamiphos was detected. Residues of the
sulphoxide and sulphone were below 0.06 mg/kg in both carrots and
foliage. At the end of the growing period, soil residues were 2.7
mg/kg fenamiphos equivalents (one third of the amount applied) of
which 65% consisted of the sulphoxide and sulphone in the ratio 4:1.
Results with tomatoes were similar. Sulphoxide and sulphone
residues reached a peak of 0.2-0.3 mg/kg 30-40 days after treatment,
and no parent compound was detected later than 40 days. The results
showed the stability of the thio-methyl-ring linkage.
Comparable results were obtained with head cabbage. An
organosoluble polar metabolite with an apparently intact
phosphoramidate structure was detected soon after treatment and
declined gradually to insignificant levels.
In tobacco plants the sulphoxide reached a peak in less than one
week, and then decreased as the sulphone increased. At the end of the
growing period the ratio of sulphoxide to sulphone was about 3:2.
Soybeans were planted in soil that had been treated with
14C-ethyl, 3H-methylthio fenamiphos and cropped with tobacco for one
growing season. The soil at soybean planting contained 0.65 mg/kg
fenamiphos sulphoxide, 0.07 mg/kg fenamiphos sulphone and 0.18 mg/kg
fenamiphos equivalents as unextractable material. Nineteen days old
soybean seedlings contained 16.9 mg/kg (fresh weight basis) of these
two soil metabolites in a sulphoxide: sulphone ratio of 73:17. In the
mature plant (150-day growing period), the total concentration of the
two metabolites, found in equal proportions, was 3.5 mg/kg in the dry
leaves and 0.19 mg/kg in the dry beans. The water-soluble
radioactivity consisted mainly of the glucose conjugate of the
sulphone phenol. The insoluble radioactivity was not characterized.
The fate of 14C1-ethyl and 3H-methylthio fenamiphos was
investigated in snap beans following stem injection and soil treatment
in systems designed for the recovery of the applied nematicide. The
study demonstrated volatile 14C radioactivity, presumably as CO2.
40% of the stem-injected and 12% of the soil-applied fenamiphos was
recovered in this form. Volatile tritium radioactivity was less, 7.4%
and 1.3% of the amounts applied by stem-injection and soil treatment,
respectively. The metabolites formed by both methods of application
were fenamiphos sulphoxide, fenamiphos sulphone and the sulphoxide and
sulphone phenols in the free form or conjugated with glucose.
The metabolism of similarly labelled fenamiphos applied by stem
injection, soil drench or spray to fruited pineapple plants was
studied by Flint (1973). Soil treatment at 20 pounds a.i./acre
resulted in maximum total radioactive residues in fruit of 0.086 mg/kg
fenamiphos equivalents at 90 days after treatment. Metabolites
identified in fruit and foliage were identical to those previously
found in several other plant and animal systems.
In soil and water
Khasawinah (1970) treated the top 1.5 cm of a sandy loam soil, in
which tobacco was growing, with labelled fenamiphos. One third of the
applied amount was taken up by the growing plants during an 11-week
period. The major product in the soil was the sulphoxide, with small
quantities of the sulphone. No hydrolysis products of the parent
compound or its oxidation products were detected in the soil extracts.
None of the radioactivity was leached into the drainage water, and all
of it remained in the root zone. About 22% of the radioactivity
remaining in the soil after 177 days was bound to clay particles and
was unextractable.
Fenamiphos was studied for soil runoff, leaching, adsorption, and
stability in water by Flint et al. (1971) and Church (1971). It was
moderately adsorbed by sandy loam and silt loam soil. Fenamiphos
residues in runoff water were less than 3.4% of the applied compound
over a 37-day interval during which 2.3 to 3.7 inches of irrigation
were applied; the application rate of fenamiphos was 22.5 kg a.i./ha.
The leaching of fenamiphos from the three types of soil was inversely
proportional to the adsorptivity of the soils. It was estimated that
111 inches of rainfall would be needed for clay loam soil and 75
inches for sandy loam to leach fenamiphos to a depth of 12 inches.
Fenamiphos was relatively stable in aqueous systems at pH 5 and 7
in the laboratory but declined rapidly with a half-life of 4.8 days in
a simulated field pond at PH 7 and average temperature of 29°C.
The leaching of "aged" fenamiphos residues in sandy loam soil was
investigated by Tweedy et al. (1974). Ring-14C fenamiphos was
incubated with sandy loam soil under greenhouse conditions for 30 days
and then applied to the top of a sandy loam soil column 2.9 inches in
diameter and 12 inches high. Distilled water, equivalent to 0.5
acre-inch/day was added daily for 45 days. Of the radioactivity
recovered from the system, 77.5% was found in the top fourth of the
column and only 2.3% was recovered in the leachate. The majority of
the activity, 65%, apparently did not move at all.
The metabolism of fenamiphos soil residues under aerobic and
anaerobic conditions was investigated by Shaw and Flint (1974).
Fenamiphos, labelled with tritium in the methylthio group or with
carbon-14 uniformly throughout the aromatic ring, was used in this
study. The results indicated that anaerobic conditions arrested
further degradation while aerobic incubation allowed continued
metabolism. Essentially no 14C or 3H radioactivity was lost during
incubation indicating stability of the aromatic ring system. The major
metabolite was fenamiphos sulphoxide with some further thio-oxidation
and hydrolysis observed under aerobic conditions.
The effect of fenamiphos on soil microbial populations was
studied by Houseworth and Tweedy (1972) using Indiana clay loam and
Commerce silt loam soils. The soils were treated with 50 and 250 mg/kg
of the pesticide and maintained at 50% field moisture capacity for 56
days. At intervals, samples of soil were removed from each treatment
and assayed for populations of bacteria, fungi and Actinomycetes.
Fenamiphos had no significant effect on them under the conditions of
the experiment.
The photodecomposition of thin films of fenamiphos was
investigated by Khasawinah and Sandie (1974). Thin films of dual
labelled ethyl-1-14C, methylthio-3H fenamiphos were coated on glass
plates and silica gel coated glass plates and exposed in the open air
for about three weeks. Fenamiphos was rapidly oxidized to its
sulphoxide and then to the sulphone. Some hydrolysis of the sulphone
occurred after long intervals, especially on glass.
The results of some studies in the USA of the persistence of
fenamiphos and its oxidation products in various types of soil were
available to the Meeting. They are reproduced in Table 7.
In storage and processing
The effect of alkali refining and steam deodorization on residues
in cottonseed and soybean oils was studied in laboratory simulation
experiments by Thornton (1973a) and Olson (1972b, 1974). In
cottonseed, the two processes in combination removed 98.5% of the
fenamiphos, 98.4% of the sulphoxide and 88% of the sulphone. Since the
sulphone would constitute only a small proportion of a naturally
occurring residue, the combined processes would remove nearly all the
residue. In soybeans, the effects of the processes on sulphoxide
residues was studied, as this is the main component of the residue
after long post-treatment intervals. The simulated refining process
removed 96% of the sulphoxide, and deodorization removed 38%. A
hypothetical residue of 0.42 mg/kg should therefore be reduced to 0.01
mg/kg by the two steps.
The effect of citrus processing on residues of fenamiphos
sulphoxide was investigated by Olson (1972a). Commercial citrus
processing involves liming and drying of the chopped solids in the
preparation of cattle feed. Fenamiphos sulphoxide, the main metabolite
in crops, was resistant to the liming step but 39% of the residue was
removed by drying. To prepare citrus oil, an aqueous emulsion is
centrifuged vigorously. 99% of any fenamiphos sulphoxide present in
the emulsion would be removed from the oil by this procedure.
TABLE 7 Persistence of fenamiphos + sulphoxide + sulphone in various soils in the USA1
Residue, phenamiphos + sulphoxide + sulphone, mg/kg, after interval, days2
0 30-40 80-100 180-200 360-400
Soil Range Mean Range Mean Range Mean Range Mean Range Mean
Silt loam 1.2-15 7.7 5.4-12 9.4 0.8-15 6.9 0.1-6.5 2.5 0.1-2.5 1.1
Clay 1.0-1.7 1.3 3.7 3.7 1.5-2.7 2.1 0.6-1.3 1.0 0.6-0.7 0.7
Sand muck 5.1-6.3 5.7 5.1-5.8 5.4 0.1 0.1 0.7-1.0 0.8 0.4-0.6 0.5
Sand 5.5 5.5 0.3-0.6 0.5 0.1-0.2 0.2 0.06-0.07 0.07 0.03-0.04 0.04
Sand clay 6.8-8.5 7.7 3.0-4.9 3.9 0.5-1.2 0.8 0.1 0.1 0.1 0.1
Fox sand 4.3-9.2 6.7 6.8-8.0 7.4 3.1-3.2 3.1 1.0-1.3 1.1 0.9-1.0 0.9
Peat 15-16.5 16 17-20 18 12.5-17 15 6.8-8.8 7.4 6.3-7.2 6.8
Low humus 13-17 15 4.6-6.6 5.5 3.6-6.6 5.1 1.2-3.3 2.2 0.3-0.6 0.4
1 Fenamiphos was added to soils at 10mg/kg level as granules or spray concentrate.
2 Usually two or three analyses at each interval.
Olson (1970a) studied the fate of residues on pineapple crowns.
The fresh crowns were dipped in an aqueous emulsion of spraying
concentrate containing 0.12% fenamiphos, and allowed to drain dry. The
surface residue immediately after treatment amounted to about 30 mg
per crown, of which about half could be removed by extracting with
water. Seven days after treatment, aqueous extraction removed about
one-third of the original residue. Surface extraction with benzene,
however, removed virtually all the initial deposit (29 mg per crown)
but only 27% (8 mg) after 7 days.
Storage of broccoli, carrots, peanut kernels, pineapple fruit and
potatoes at -18° to -23°C for two years showed no significant loss of
fenamiphos, the sulphoxide or the sulphone (Anonymous, 1973).
There was no significant loss of residues (parent + sulphoxide +
sulphone) in field-treated potatoes or of fenamiphos from potatoes
fortified in the laboratory when cooked at 100°C for 30 minutes in
sealed jars (Thornton, 1972).
METHODS OF RESIDUE ANALYSIS
A specific gas-chromatographic procedure for the determination of
residues of fenamiphos and sulphoxide and sulphone in various crops,
animal tissues and milk is described by Thornton (1969, 1971a).
Following initial extraction, the extract is oxidized with potassium
permanganate to convert fenamiphos and its sulphoxide to the sulphone.
The sulphone is then determined by gas chromatography with alkali
flame ionisation detection. Recoveries from a variety of crops and
animal tissues fortified with fenamiphos or its metabolites at the
blending step were generally between 75 and 110%. Interference studies
by Thornton (1971b, 1973b) and Olson (1970d, 1971b,c) have shown the
method to be specific in the presence of other possible
organophosphorus compounds. The limit of detection is about
0.02 mg/kg.
The determination of residues of fenamiphos and its metabolites
in tobacco and tobacco smoke was successfully investigated by Olson
(1971a) with the Thornton (1971a) method. This method should be
suitable for regulatory purposes.
The determination of fenamiphos residues in soil by thermionic
emission gas chromatography is described by Olson (1970b). The soil
sample is extracted in a Soxhlet apparatus, using a 1:1
chloroform-methanol solvent mixture. The solvent is evaporated and the
residue oxidized and chromatographed. Recoveries of fenamiphos and its
metabolites were 80-107%. The sensitivity of the method is
approximately 0.02 mg/kg. Olson (1970c) has also extended the
procedure to a number of other organophosphorus pesticides.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table 8.
TABLE 8 National tolerances reported to the Meeting
Safety
Tolerance Interval
Count Crop mg/kg (days)
Australia Tomatoes, brassicas,
cucurbits, lettuce, citrus,
pineapples, grapes,
bananas, ginger No residue
Spain Tobacco sugarbeets, etc.
(industrial purpose),
bananas 60
USA Bananas, citrus 0.1 180
APPRAISAL
Fenamiphos is a systemic nematicide which gives good control of
root-knot cyst forming and free living nematodes. It is also effective
against sucking insect pests and spider mites. It is formulated as 5%
and 10% granules and as an emulsifiable concentrate (40%). Both
formulations are recommended chiefly for treating bananas, pineapples,
tomatoes, brassicas, eggplant, tobacco, groundnuts, citrus crops,
ornamentals and pyrethrum. It is chiefly applied to the soil at sowing
or planting. The general dose rate is 5-10 kg active ingredient/ha for
broadcast application, but is reduced for band application. The
emulsifiable concentrate is especially suitable for dip treatment of
banana seedlings and for foliar spraying of pineapples. Fenamiphos is
commercially used in Australia, USA, some Central and South American
countries, Spain, the Cameroons and the Ivory Coast.
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thio-oxidation of the parent compound
to the sulphoxide and sulphone. In general the major part of the
residue in crops and soil is the sulphoxide, followed by the sulphone.
Both are relatively persistent. Both metabolites are detoxified by
hydrolysis, usually followed by conjugation of the phenolic products
as glycosides. Little or none of the parent fenamiphos seems to be
present in most crops at harvest.
Micro-organisms in soil do not seen to be affected by fenamiphos.
The dissipation of residues in soil depends on the type of soil,
increasing in the order peat < silt loam < clay = sand. Fenamiphos
is only very slowly leached from soil.
Following application in accordance with good agricultural
practice, the maximum residue levels of fenamiphos including its
sulphoxide and sulphone in Brussels sprouts, cotton seed, cabbage,
carrots, cauliflower, grapefruit, lemons, limes, melons, oranges
(pulp), peanut kernels, pineapples, soybeans (dried) and sugar beets
are at or below 0.05 mg/kg. Higher residues are found in some other
crops. Typical maximum residues are: grapes 0.1 ppm, lettuce 0.5 ppm,
potatoes 1 ppm, strawberries 0.7 ppm, tomatoes 0.3 ppm and tobacco
(green) 12 ppm, (cured) 140 ppm (fermented) 100 ppm. Data for
fenamiphos residues in tobacco smoke are not available.
Processing cottonseed or soybeans to oil removed 98-99% of the
residues and processing citrus to cattle feed removed about 40%.
Cooking potatoes had no significant effect. In a laboratory experiment
approximately one third of the fenamiphos originally applied was
extractable with water from the surface of the crowns of pineapples
seven days after dipping in an aqueous emulsion containing 0.12%
fenamiphos.
Residues in eggs and tissues from hens fed for 14 days with 14C
ring-labelled fenamiphos in feed at 0.65 ppm were below 0.005 mg/kg.
Residues in milk and tissues of a dairy cow fed once with a dose
corresponding to 27 ppm fenamiphos sulfoxide in the diet were below
0.01 ppm.
Specific gas-chromatographic procedures for the determination of
residues of fenamiphos, its sulphoxide and sulphone in various crops,
animal tissues, milk and soil have been elaborated. The initial
extract is oxidized with permanganate to convert fenamiphos and its
sulphoxide to the sulphone. The sulphone is determined with an alkali
flame ionisation or flame photometric detector. Limits of detection
are about 0.02 ppm, and recoveries higher than 75%. The methods should
be suitable for regulatory purposes.
RECOMMENDATIONS
The following limits refer to fenamiphos including its sulphoxide
and sulphone, expressed as fenamiphos.
TOLERANCES
Tolerance
Crop (mg/kg)
Bananas, coffee beans (green and
roasted, grapes, sweet potatoes 0.1
Broccoli, Brussels sprouts, cabbage,
carrots, cauliflower, citrus fruits,
cottonseed, melons, peanut kernels,
pineapples, soybeans (dried), sugar beets 0.05*
* At or about the limit of determination.
TEMPORARY TOLERANCES
Temporary
tolerance
Crop (mg/kg)
Potatoes, tomatoes 0.2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further data on which to judge the residues in or on potatoes and
tomatoes.
2. Adequate residue data on which to base recommendations for
tolerances for other crops (e.g. beans, cucumbers, lettuce,
peppers and strawberries).
DESIRABLE
1. Brain cholinesterase and behavioural studies in animals exposed
to low levels for extended periods.
2. Observations in man.
3. Additional studies on the potentiation effects with other
organophosphorus pesticides.
4. Residue data for raw agricultural products moving in commerce.
5. Further residue data for different crops from supervised trials
including data on rates and frequencies of application, soil,
foliar or other treatment and pre-harvest intervals, especially
for broccoli and tangerines.
REFERENCES
Anonymous. (1973) The effect of frozen storage at 0 to -10°F on
Nemacur residues in peanut meat, broccoli, carrot roots, potatoes,
pineapple fruit. Chemagro Report No. 36132, 36318, 36319, 36320,
39094.
Arnold, D., Keplinger M. and Fancher, O. (1971) Mutagenic study with
Nemacur(R) (BAY 68138) Technical in albino mice. Report from
Industrial BioTest Laboratories, Inc., submitted by Bayer AG.
(Unpublished).
Cherry, C., Urwin, C. and Newman, A. (1972) Pathology report of BAY
68,138. Rat breeding study. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished).
Cherry, C. and Newman, A. (1973) Pathology report of Bayer 68,138.
Chronic toxicological studies in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Church, D.D. (1971) Nemacur - leaching, runoff and water stability.
Chemagro Report No. 26 849.
Coulston, F. and Wills, J.H. (1974) Summary of research of the
Institute of Comparative and Human Toxicology, Albany Medical College,
on fenamiphos. Report submitted by Bayer AG. (Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970a) The skin and eye
irritating properties of BAY 68,138 3 lb/gal S.C. to rabbits. Report
from Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970b) The acute inhalation
toxicity of Nemacur(R) 15% granular to rats. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C, and Anderson, R. (1971) The skin and eye irritating
properties of Bay 68138 technical to rabbits. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1972a) The acute dermal toxicity of
Nemacur(R) technical to rabbits. Report from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (unpublished).
Crawford C. and Anderson, R. (1972b) The acute dermal toxicity of
Nemacur(R) 3 lbs/gal. spray concentrate to rabbits. Report from
Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford C. and Anderson, R. (1973a) The dermal toxicity of Nemacur(R)
15% granules to rats. Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1973b) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R) with
analytical grade Nemacur(R). Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford C. and Anderson, R. (1973c) The eye and skin irritancy of
Nemacur(R) 2 lb/gal spray concentrate to rabbits. Report submitted by
Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1974) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Report from Chemagro Division of Baychem Corporation, submitted by
Bayer AG. (Unpublished).
DuBois, K., Flynn, M. and Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Report from University of
Chicago. (Unpublished).
DuBois, K. and Flynn, M. (1968) The subacute parenteral toxicity of
BAY 68138 to rats. Report from University of Chicago, submitted by
Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1970) Acute oral and dermal toxicity of
a granular formulation of BAY 68138. Report from University of
Chicago, submitted by Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1971) The acute oral and inhalation
toxicity of a Nemacur(R) (BAY 68138) formulation to rats. Report from
University of Chicago submitted by Bayer AG. (Unpublished).
Flint, D.R. (1973) The metabolism of Nemacur in pineapple. Chemagro
Report No. 39 119.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour, J., II. (1971) The
mobility and persistence of Nemacur in soil and water. Chemagro Report
No. 29 974.
Gronberg, R.R. (1969) The metabolic fate of
ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate (BAY 68138),
ethyl-4-(methylsulfinyl)-m-tolyl isopropylphosphoramidate (BAY 68138
sulfoxide) and ethyl-4-(methylsulfonyl)-m-tolyl-isopropylphosphoramidate
(BAY 68138 sulfone) in white rats. Report No. 26759 from Chemagro
Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Gronberg, R.R., Flint, D.R. and Pither, K. (1974) The metabolic fate
of Nemacur sulfoxide administered orally to a lactating dairy cow.
Report No. 41104 from the Chemagro Division of Baychem. Corp.,
submitted by Bayer AG. (Unpublished).
Gronberg, R.R., Simmons, C.E. and Shaw, H.R., II. (1973) Residues of
Nemacur in poultry eggs and tissue. Report No. 35995 from the Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Houseworth, L.D., and Tweedy, B.G. (1972) Effect of Nemacur on
microbial populations. Chemagro Report No. 34 990.
Khasawinah, A.M. (1970) The fate of Nemacur in soil. Chemagro Report
No. 28 796.
Khasawinah, A.M. (1971) Metabolism of Nemacur in tobacco plants.
Chemagro Report No. 29 142.
Khasawinah, A.M. (1972a) Metabolism of Nemacur in snap beans grown in
closed glass chambers. Chemagro Report No. 34 992.
Khasawinah, A.M. (1972b) The uptake and metabolism of Nemacur soil
residues by soybean plants. Chemagro Report No. 35012.
Khasawinah, A.M. (1973a) Metabolism of Nemacur in carrots. Chemagro
Report No. 36005.
Khasawinah, A.M. (1973b) Metabolism of Nemacur in tomatoes. Chemagro
Report No. 38501.
Khasawinah, A.M. (1973c) The metabolism of Nemacur in cabbage.
Chemagro Report No. 39120.
Khasawinah, A.M. and Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Report No. 34217 from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (Unpublished).
Khasawinah, A.M. and Sandie, F.E. (1974) Photodecomposition of thin
films of Nemacur. Chemagro Report No. 39217.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on chickens.
Report from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Report from
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. and Solmecke, B. (1971a) BAY 68138 toxicological studies.
Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. and Solmecke, B. (1971b) Granular formulation. Acute
dermal toxicity on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972a) Antidotal experiments on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972c) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Report from Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Ladd, R., Jenkins, D., Keplinger, M. and Fancher, O. (1971)
Teratogenic study with Nemacur(R) technical in albino rabbits. Report
from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Löser, E. (1968a) Subchronic toxicological studies on dogs. Report
from the Institute for Toxicology, Farbenfabriken Bayer AG.
(Unpublished).
Löser, E. (1968b) Subchronic toxicological studies on rats. Report
from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Löser, E. (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Report from the Institute for Toxicology, submitted by
Bayer AG. (Unpublished).
Löser, E. (1970) Subchronic toxicological studies on dogs. Report from
the Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Löser, E. and Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachr. Bayer,
24(1):69-113.
Löser, E. (1972a) Chronic toxicological studies on rats (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished)
Löser, E. (1972c) Generation studies on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Olson, T.J. (1970a) Residues on the surface of fresh pineapple crowns
treated with an aqueous emulsion of S.C. formulation of Nemacur.
Chemagro Report No. 28014.
Olson, T.J. (1970b) Determination of Nemacur residues in soil by
thermionic emission gas chromatography. Chemagro Report No. 28731.
Olson, T.J. (1970c) Determination of Dasanit, Guthion, Metasystox R,
Nemacur and trichlorfon in soil by thermionic emission gas
chromatography. Chemagro Report No. 27835.
Olson, T.J. (1970d) An interference study for the determination of
residues of Nemacur on pineapple. Chemagro Report No. 28723.
Olson, T.J. (1971a) Determination of Nemacur residues in tobacco and
tobacco smoke. Chemagro Report No. 30448.
Olson, T.J. (1971b) An interference study for the determination of
residues of Nemacur on cotton and tomatoes. Chemagro Report No. 30095.
Olson, T.J. (1971c) An interference study for the Nemacur crop residue
method for bananas. Chemagro Report No. 31041.
Olson, T.J. (1972a) The effect of citrus processing on residues of
Nemacur sulfoxide. Chemagro Report No. 34050.
Olson, T.J. (1972b) Effect of commercial soybean oil processing on
residues of Nemacur sulfoxide. Chemagro Report No. 33461.
Olson, T.J. (1974) The effect of alkali refining on Nemacur residues
in cottonseed oil. Chemagro Report No. 40113.
Shaw, H.R. and Flint, D.R. (1974) The metabolism of Nemacur soil
residues under aerobic and anaerobic conditions. Chemagro Report No.
39909.
Smith, P., Wright, P. and Keplinger, M. (1972) Eighteen month
carcinogenic study with Nemacur(R) (BAY 68138) in Swiss white mice.
Report from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Report from the Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Spicer, J. (1971) Pathology report of BAY 68138. Hen study. Report
from Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C., Newman, A. and Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C. Newman, A. and Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Report from Huntingdon Research Centre, submitted by Bayer AG.
(Unpublished).
Thornton, J.S. (1969) A gas chromatographic method for the
determination of Nemacur and metabolite residues in crops. Chemagro
Report No. 25402.
Thornton, J.S. (1971a) Determination of residues of Nemacur and its
metabolites in plant and animal tissues. J. agr. Food Chem., 19
(5):890-893.
Thornton, J.S. (1971b) An interference study for the residue method
for Nemacur and metabolites. Chemagro Report No. 25533.
Thornton, J.S. (1972) Effect of cooking on residues of Nemacur in
potatoes. Chemagro Report No. 35314.
Thornton, J.S. (1973a) Effect of alkali refining and deodorization on
residues of Nemacur and metabolites in cottonseed oil. Chemagro Report
No. 35345.
Thornton, J.S. (1973b) An interference study for the Nemacur residue
method. I. Additional compounds not previously tested. Chemagro Report
No. 36 142.
Thyssen. (1974a) Nemacursulfoxid, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974b) Nemacursulfon, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974c) 4-methyl-mercapto-m-Kresol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974d) 3-methyl-4-methyl-mercaptophenol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974e) 3-methyl-4 methan-sulfonyl phenol, Akute Toxizitat
bei Ratten. Report from Institute for Toxicology, submitted by Bayer
AG. (Unpublished).
Tweedy, B.G. and Houseworth, L.D. (1974) Leaching of "aged" Nemacur
residues in sandy loam soil. Chemagro Report No. 40 506.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl
4-(methylthio)-M-tolyl isopropylphosphoramidate] and identification
of two metabolites in plants. J. agr. Food Chem., 20(1):157-160.
Molecular weight 303.3
Empirical Formula: C13H22NO3PS
Other information on identity and properties
State: brown semi-solid (technical)
white crystals (pure)
Melting Point: 46°C (technical)
49°C (Pure)
Vapour pressure: 10-6mm Hg (at 30°C)
Solubility: in water approx. 700 mg/l at 20°C
slightly soluble in most organic
solvents
Specific gravity (melt): 1.14
Hydrolytic stability in propanol/water (1:1) at 40°C:
40% degradation after 14 days at pH 2
no degradation after 50 days at pH 7
half life of 31.5 hours at pH 11.3
Formulations: 5% Granular, 10% Granular, 400 E.C. (Emulsifiable
concentration 400 g/l)
Purity
The minimum purity is 87%. Maximum levels of the individual
impurities are shown in Table 1.
TABLE 1 Impurities (maximum levels) in technical fenamiphos
Compound Maximum
level (%w/w)
O,O-diethyl isopropylphosphoramidate 3.0
4-methylthiocresol 0.5
O,O-bis[(4-methylthio)-3-ethylphenyl]
isopropylphosphoramidate 2.0
O,O-diethyl O-[(4-methylthio)-3-methylphenyl]
phosphate 1.0
O-ethyl O-[(4,6-bismethylthio)-3-methylphenyl]
isopropylphosphoramidate 3.0
O-ethyl O,O-bis-[(4-methylthio)-3-methylphenyl]
phosphate 8.0
O-ethyl O-[(4-methylthio)-3-methylphenyl] phosphate 1.0
water 0.3
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformations
The two major metabolites identified following injection into
bean, tomato, peanut and potato stems were O-ethyl
O-4-methylsulfinyl-m-tolyl isopropylphosphoramidate and O-ethyl
O-4-methylsulfonyl-m-tolyl isopropylphosphoramidate, the sulphoxide
and sulphone of the thio-ether (Waggoner, 1972). A third metabolite
(apparently a more potent inhibitor of cholinesterase) was
not identified but was considered to be a further metabolite
resulting from the sulphoxide. Further details of this study are
given in the section "Fate of residues in plants". Small residues of
the unknown metabolite may be of toxicological significance because
of the potent anticholinesterase properties.
The metabolic fate of labelled fenamiphos was studied in vivo
in rats and in vitro with rat liver microsomes. The compounds were
labelled in the ethyl (14C) isopropyl, (14C) or the thiomethyl (3H)
positions. In vivo fenamiphos is excreted within 12-15 hours
following a single oral dose of 2 mg/kg. In the in vitro studies a
small quantity of an unknown metabolite possibly resulting from the
N-dealkylation of fenamiphos was observed (Khasawinah and Flint,
1972). Apart from this minor component, metabolism in animals and
plants follows the same pattern: oxidation of the thioether to the
sulphoxide and sulphone, dearylation to yield the methyl thioether
phenol (or its sulphoxide and sulphide) and potentially dealkylation
of the ethyl, isopropyl or isopropylamide moiety of the phosphate
ester. Treatment of rats with fenamiphos sulphoxide or sulphone
produced the same excretion pattern and almost identical urinary
metabolites (Gronberg, 1969).
When fenamiphos sulphoxide was given to a lactating cow (Gronberg
et al., 1974) the identified components of the residue were
fenamiphos, its sulphoxide and its desisopropyl derivative, and the
phenols of phenamiphos, its sulphoxide and its sulphone. An
unidentified metabolite was also detected. Quantitative details are
given in the section "Fate of residues in animals".
Effects on enzymes and other biochemical parameters
Fenamiphos, as are other organophosphate esters, is an inhibitor
of cholinesterase enzymes. The in vitro I50 values were as follows:
Rat serum - 5.1 x 10-5M.
Rat erythrocyte - 6.3 x 10-4M.
Rat brain - 2.1 x 10-4M.
The maximum inhibition of whole blood cholinesterase in vivo
occurred in rats three hours after dosing. In vivo sensitivity of
cholinesterase reflects the in vitro I50 values with plasma
cholinesterase being more sensitive to inhibition than the erythrocyte
enzyme (Löser and Kimmerle, 1971). The metabolites of fenamiphos are
more active inhibitors than the parent molecule (Waggoner, 1972). In
vitro inhibition of horse serum cholinesterase showed relative
values for fenamiphos < sulfoxide = sulfone < sulfoxide metabolite.
In vitro I50 values of chicken and monkey liver aliesterase
(triacetin hydrolysis) and monkey cholinesterase are as follows:
Chicken (aliesterase): 4.2 x 10-5M.
Monkey (aliesterase): 1.0 x 10-6M.
Monkey (cholinesterase): 4.6 x 10-6M.
(Coulston and Wills, 1974)
On the basis of some preliminary electrophysiological studies
with the cat, it was estimated that a threshold dose of fenamiphos
might fall in the range of 0.8 to 1.5 mg/kg (Coulston and Wills,
1974).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of Charles River random bred Swiss white mice (125 males
and 125 females/group) were fed fenamiphos in the diet at 0, 25 and 50
ppm for eighteen months in a study specifically designed to evaluate
the carcinogenic potential of the compound. Additional groups of mice
were fed 10 ppm N-nitrosodiethylamine for eighteen months (125 males
and 125 females); 40 ppm for six months (60 males and 60 females); and
1000 ppm benzidine (100 females) for eighteen months as positive
controls. Fenamiphos did not affect the incidence or pattern of
mortality. Clinical chemistry studies performed at 18 months were
normal. No signs of tumour formation were observed on gross or
microscopic examination which were attributed to fenamiphos (Smith et
al., 1972).
Special studies on mutagenicity
Mouse
Groups of twelve male mice were administered fenamiphos
intraperitoneally at 0, 0.25 and 0.50 mg/kg and mated for six
consecutive weeks to virgin females in a standard dominant lethal
test. A positive reference (methyl methanesulphonate) was used in this
experiment. Fenamiphos did not induce alterations in male germinal
cells which would be expected to lead to an early embryo mortality
(Arnold et al., 1971).
Special studies on neurotoxicity
Hen
Groups of hens (eight per group) were fed fenamiphos in the diet
at levels of 0, 1, 3, 10, and 30 ppm for thirty days. At the end of
the feeding experiment some animals were sacrificed and the remainder
were observed for four weeks for neurological signs of poisoning. Food
consumption was depressed at 30 ppm and the average body weight and
growth of the animals at this same level was reduced. Cholinesterase
(whole blood) depression was observed at thirty days at 3, 10 and 30
ppm, although cholinergic signs of poisoning were not observed at any
time. There were no indications of delayed neurotoxic effects and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with H&E) did not indicate delayed neurological involvement
with fenamiphos (Kimmerle, 1970; Spicer, 1970).
Groups of hens (ten per group) were orally administered an LD50
dose of fenamiphos (5.0 mg/kg). The birds were observed for three
weeks and sacrificed. No evidence of delayed neurotoxicity was
observed either clinically or histologically while positive signs were
observed with TOCP (Kimmerle, 1971; Spicer, 1971).
Special studies on potentiation
Rat
Male rats were tested orally with fenamiphos (SRA 3886) in
combination with disulfoton or E 154 and no potentiation of the acute
toxicity was noted (Kimmerle, 1972c).
Special studies on reproduction
Rat
A standard three generation, two litters per generation,
reproduction study was performed with fenamiphos. Four groups of
animals, consisting of ten male and twenty female rats per group, were
fed fenamiphos at 0, 3, 10, and 30 ppm in the diet throughout the
study period including mating, gestation, and suckling. Immediately
after birth, pups were examined for malformations prior to either
preparing for another generation or sacrifice. Five weanling rats per
group of the F3b generation were sacrificed and macroscopic and
microscopic examinations performed on the major tissues and organs.
There were no apparent differences with regard to reproduction
parameters including fertility, litter size, lactation index, growth
of young, or on examination for malformation in any of the animals
exposed to fenamiphos levels up to and including 30 ppm in the diet.
It was concluded that fenamiphos had no influence on the reproduction
of rats at dietary levels up to and including 30 ppm (Löser, 1972c;
Cherry et al., 1972).
Special studies on teratogenicity
Rabbit
Groups of fifteen artificially inseminated rabbits were orally
administered fenamiphos at levels of 0, 0.2, and 0.4 mg/kg/day.
Positive control groups (oral treatment with thalidomide (37.5
mg/kg/day)) were concurrently run with this experiment. All animals
were treated from day 6 through 18 of gestation and sacrificed on day
29. At day 29 the young were examined for 24 hours, sacrificed and
examined after skeletal staining. At the high level of treatment of
fenamiphos, 2/12 instances of abortion and 2/12 premature deliveries
were observed. No external or internal abnormalities were observed in
the foetuses following treatment. With the exception of the animals at
the high level of treatment where a few aborted or delivered
prematurely, no effects on reproduction were observed with fenamiphos
(Ladd et al., 1971).
Acute toxicity
TABLE 2 Acute toxicity of fenamiphos
LD50
Animal Sex Route (mg/kg) References
Rat M oral 2.4-15.6 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Rat F oral 2.3-19.4 Kimmerle, 1972c
Crawford and Anderson, 1974
DuBois et al., 1967
Rat M ip 3.0-3.7 ibid.
Rat F ip 4.2-4.9 Löser and Kimmerle, 1971
Rat M dermal 73-500 DuBois et al., 1967
Rat F dermal 84-154 Kimmerle and Solmecke, 1971a
Mouse M oral 22.7 Löser and Kimmerle, 1971
Mouse M&F ip 3.4 DuBois at al., 1967
Guinea Pig M oral 56-100 ibid.
Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Guinea Pig M ip 17.3 DuBois et al., 1967
TABLE 2 (Cont'd.)
LD50
Animal Sex Route (mg/kg) References
Rabbit M oral 10-17.5 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Cat M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Dog M oral Ca.10 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
Hen F oral 5.3 Löser and Kimmerle, 1971
Kimmerle and Solmecke, 1971a
TABLE 3 Acute toxicity of metabolites and impurities of fenamiphos
LD50
Species Sex Route (mg/kg) References
Fenamiphos-sulphoxide
Rat F oral 10-25 Thyssen, 1974a
Fenamiphos-solphone
Rat F oral 10-25 ibid.
4-methylthio-m-cresol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 1333>2500 ibid.
Thyssen, 1974c
3-methyl-4-methylthiophenol
Rat M oral 1418 Crawford and Anderson, 1974
Rat F oral 500-1000 Thyssen, 1974d
1175 Crawford and Anderson, 1974
3-methyl-4-methanesulphonylphenol
Rat M oral 1250 ibid
Rat F oral 1000 Thyssen, 1974e
1854 Crawford and Anderson, 1974
Impurities identified as components of the technical mixture
(aryldiamide, diarylamide, diaryl ethyl ester, diethyl ester,
diethylmonamide, di-SCH3 compound, ethyl aryl ester, ethyldiamide,
and 4-methylthio-3-cresol) were tested for their acute toxic
properties to rats. At a dosage of 1.5 times the acute LD50 of
fenamiphos none of the materials were toxic (Crawford and Anderson,
1973b).
Technical fenamiphos administered to the skin of rabbits as an
acetone solution at 50 mg/kg resulted in a slight erythema and was not
considered as a primary irritant. Technical fenamiphos applied to the
conjunctival sac of rabbits (100 mg/kg as a crystalline material) was
irritating to the eye. This irritation was believed to be a mechanical
rather than a physiological effect (Crawford and Anderson, 1971).
A 15% granular formulation was examined for dermal and inhalation
toxicity properties and at the highest level examined (200 mg/kg and
20 mg/l respectively) no toxicity was observed (Crawford et al.,
1970b; Crawford and Anderson, 1972a). The rat oral LD50 of a 15%
granular formulation was 66.6 mg/kg for male rats and 62.7 mg/kg for
female rats (DuBois and Kinoshita, 1970). A 5% or 10% granular
formulation, administered at up to 500 mg/kg to the backs of rats
resulted in depressed cholinesterase activity values. Mortality was
not observed at the maximum dose tested and it was considered that the
granular formulation was not a dermal hazard although the material was
rapidly absorbed into the body (Kimmerle and Solmecke, 1971b). The
acute dermal toxicity and dermal and eye irritation properties of a
liquid formulation (37.4% a.i.) were tested in rabbits. The acute
LD50 was 75.7 mg/kg and 71.5 mg/kg to male and female rabbits
respectively (Crawford et al., 1970a; Crawford and Anderson, 1972b).
When applied to intact and abraded skin at a dose of 0.25 ml (0.085 mg
a.i.), fenamiphos was not found to be a primary irritant. Fenamiphos
(0.1 ml, equivalent to 0.034 mg a.i.) was an irritant when applied to
the conjunctival sac of rabbits. Control formulation data were not
reported (Crawford and Anderson, 1973c). The acute rat oral LD50 of a
35% liquid formulation was calculated to be 24.8 mg/kg (equivalent to
8.7 mg fenamiphos /kg). Inhalation toxicity studies with this
preparation using a small particle size distribution (1-3 microns)
resulted in an LC50 value of 0.175 and 0.091 mg/l following one and
four hour exposure respectively (DuBois and Kinoshita, 1971). Studies
with rodents administered fenamiphos by inhalation at doses up to 0.23
mg/l for one to four hours exposure as a static spray showed that
rabbits and guinea pigs were more tolerant of the acute effects than
rats and mice. A one hour LC50 value for mice was 0.06 mg/l and for
rats 0.23 mg/l while rabbits and guinea pigs were unaffected at these
concentrations (Kimmerle and Solmecke 1971a; 1972b). Acute oral
administration of 2.5 mg/kg to rabbits had no effect on several liver
function tests including BSP, SGPT and SDH activity at 1 and 24 hours
and seven days after treatment (Kimmerle and Solmecke 1971a).
Studies performed on male rats indicated that, following
poisoning, the administration of atropine and/or 2-PAM or Toxogonin
would reduce the acute lethal dose by a factor of approximately two.
As with other organophosphorus compounds, rapid administration of
atropine and oxime reactivator following poisoning would afford some
protection and would alleviate the cholinergic signs of poisoning
(Kimmerle, 1972a; DuBois et al., 1967).
Short term studies
Rat
Groups of rats (fifteen males and fifteen females per group,
thirty males and thirty females were used as controls) were fed
fenamiphos in the diet at 0, 4, 8, 16, and 32 ppm for twelve weeks.
Male and female rats from the highest dose group exhibited signs of
cholinergic stimulation in the first two months of the test.
Behavioral differences in all other groups were not apparent. There
were no differences from the control in the average of any group with
regard to food consumption and growth. With the exception of
cholinesterase inhibition, there was no apparent effect on group
averages of haematological parameters examined. Plasma cholinesterase
depression was observed at 8 ppm. A no-effect level, based upon plasma
cholinesterase, was observed at 4 ppm. Gross and microscopic
pathological examination was performed on tissues and organs at the
conclusion of the experiment. A slightly increased liver weight,
observed in males at 16 and 32 ppm, was not reflected in the
calculation of relative organ-to-body weight ratios nor in the
histological examination. Based on cholinesterase depression, 4 ppm in
the diet was a no-effect level in this experiment (Löser, 1968b;
Mawdesley-Thomas and Urwin, 1970a).
Groups of male rats (15 per group) were administered fenamiphos
orally or by intraperitoneal injection daily, 5 days/week for 60 days.
The animals all survived a dose range of 1.5 (ip) to 1.7 (oral)
mg/kg/day with no evidence of a cumulative toxicological effect due to
fenamiphos (Kimmerle and Solmecke, 1971a). In an earlier study female
rats survived daily ip administration of 1 mg/kg for 60 days while 40%
of those administered 2 mg/kg did not survive. All rats died at 3
mg/kg administered by intraperitoneal injection (DuBois and Flynn,
1968). These studies indicate little if any cumulative toxicity.
Dog
Groups of beagle dogs (two males and two females/group) were fed
fenamiphos in the diet at 0, 2, 6, and 18 ppm for three months.
Behavioral abnormalities, evidenced by signs of cholinergic
stimulation, were evident at 18 ppm in the diet and growth of females
at this high level was reduced. Average haematological and clinical
chemistry values including liver function tests, kidney function tests
and urinalyses were not apparently affected by fenamiphos in the diet.
Averages of plasma and red blood cell cholinesterase values were
depressed at 4 ppm in the plasma of both males and females. At 2 ppm
no effect on the average red cell cholinesterase values was found
while plasma cholinesterase was depressed (20-30%) in both sexes.
Gross morphological examination of tissues and organs at the
conclusion of the study indicated no abnormal effects of fenamiphos in
the diet (Löser, 1968a).
Groups of beagle dogs (two males and two females/dose, three of
each sex were used in the control group) were fed fenamiphos in the
diet at levels of 0, 1, 2 and 5 ppm for twelve weeks. There was no
effect of feeding fenamiphos at levels up to and including 5 ppm in
the diet on the average of any of the parameters examined including
haematology, liver function tests, clinical chemistry tests, kidney
function tests, and gross and microscopic examination of tissues and
organs. As with other studies, cholinesterase was the only parameter
significantly affected, with the females being more susceptible than
the males and the plasma being more sensitive to inhibition than red
blood cell. Erythrocyte cholinesterase was unchanged at 2 ppm while
plasma cholinesterase was slightly depressed. One ppm in the diet had
no effect on plasma cholinesterase. As the depression of plasma
cholinesterase was minimal and transient at 2 ppm it might be
considered that this would be a no-effect level in this experiment
(Löser, 1969; Mawdesley-Thomas and Urwin, 1970b).
As an extension of the previous short term study in dogs (Löser,
1969), an additional two male and two female dogs were fed fenamiphos
in the diet at 10 ppm for three months. Two male and two female
animals were also maintained as a control. There was no mortality over
the course of the experiment and a very slight deviation in the
average body weight of both males and females was noted. Because of
the small sample population, the slight weight differences cannot be
fully evaluated. There was no effect of feeding fenamiphos on any of
the haematological parameters examined. Urinalysis, clearance
examinations, blood sugar and cholesterol were normal over the course
of the experiment. Cholinesterase activity was significantly depressed
in both plasma and erythrocytes and in both male and female dogs. In
addition, several abnormalities were noted with regard to some of the
serum enzyme parameters related to liver function after three months
of feeding, especially in male dogs. Alkaline phosphatase and SGPT
activity were significantly increased. Following gross and microscopic
examination of tissues and organs, no significant differences were
noted between the control and those animals fed 10 ppm. The abnormal
findings in some enzyme parameters related to liver functions,
predominantly in males at three months of feeding 10 ppm in the diet,
were not accompanied by pathological findings at autopsy nor confirmed
in another test where fenamiphos was fed at levels of 18 ppm in the
diet. They are considered a transient occurrence and not directly
related to the presence of fenamiphos in the diet (Löser, 1970;
Thompson et al., 1972a).
Groups of pure bred beagle dogs (four males and four females per
group) were fed fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5,
and 10 ppm for two years. There were no significant effects noted with
fenamiphos at any dietary level with regard to growth, food
consumption or any of the standard clinical and physiological
examinations made during the course of the study. One dog died after
being fed 0.5 ppm in the diet. This dog was considered to have died of
pneumonia and its death was not related to administration of
fenamiphos. Gross and histological examinations of all tissues and
organs at the conclusion of study did not reveal any abnormal
developments considered to have been a result of feeding fenamiphos.
The only significant physiological effect observed was
anti-cholinesterase activity which was marginally depressed in plasma
at 2 ppm. As with other species, the plasma appears to be the most
sensitive indicator of exposure with a no-effect level being 1 ppm in
the diet (equivalent to 0.029-0.036 mg/kg/day) (Löser, 1972b; Thompson
et al., 1972b).
Long term studies
Rats
Groups of SPF derived Wistar strain (forty males and forty
females per group, eighty males and eighty females used as controls)
were fed fenamiphos in the diet, at concentrations of 0, 3, 10, and 30
ppm for two years. Behavioral abnormalities were evident, as signs of
cholinergic stimulation, only during the first six weeks of feeding at
30 ppm. After six weeks of feeding, the obvious anticholinesterase
effect disappeared and was not evident for the remainder of the two
year study. There were no differences in averages at any feeding level
on growth, mortality, food consumption, haematological values,
clinical chemistry values or liver and kidney function tests. Urine
examinations as well as blood sugar and cholesterin values were
normal. Thyroid weights and thyroid-to-body weight ratios of females
were heavier than normal at 30 ppm. All other major tissues and organs
appeared normal under gross and microscopic examination. The thyroid
weight in females at 30 ppm was high but was not accompanied by an
abnormal tumour development, goitre or by unusual histological
findings.
Cholinesterase determinations indicated that plasma
cholinesterase was the most sensitive parameter. At 10 ppm in the
diet, fenamiphos inhibited plasma cholinesterase. No significant
effects were observed at 3 ppm in the diet (equivalent to 0.17-0.23
mg/kg bw/day) (Löser, 1972a; Cherry and Newman, 1973).
COMMENTS
Fenamiphos, an acutely toxic organophosphate ester, is rapidly
absorbed as evidenced by rapid onset of cholinergic signs of
poisoning. In animals fenamiphos is rapidly degraded, predominantly
through oxidation to acutely toxic products. Fenamiphos does not
appear to affect biochemical systems other than cholinesterase.
Testing for the delayed neurotoxic effect commonly seen with TOCP was
negative. Short term and long term dietary studies in both rats and
dogs have indicated, with the exception of cholinesterase depression,
no unusual effects. In long term studies with rats, transient
behavioural abnormalities were observed during the first six weeks of
feeding, which disappeared and were not evident for the remainder of
the study. Female rats are more susceptible than males to
cholinesterase depression. Plasma cholinesterase depression is a
better indicator of exposure than red blood cell cholinesterase. Based
on plasma cholinesterase depression, a no-effect level in a two-year
rat study is considered to be 3 ppm in the diet and in a two year dog
study, 1 ppm in the diet. Long term studies in rats and mice reported
to the Meeting did not show a carcinogenic effect. Although there are
no studies available in man, sufficient data are available and the
Meeting allocated an ADI using the more sensitive species, the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 ppm in the diet equivalent to 0.17 mg/kg bw.
Dog: 1 ppm in the diet equivalent to 0.029 mg/kg bw.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0006 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenamiphos is an organophosphorus compound with a systemic action
and a powerful nematicidal effect. It is active against root-knot,
cyst-forming and free-living nematodes and has important applications
against sucking insect pests and spider mites. Fenamiphos is
commercially used in the USA, Australia, Spain, the Cameroons,
Caribbean, Chile, Columbia, Costa Rica, Ecuador, Guatemala, Ivory
Coast, Jamaica, Martinique, Mexico, Nicaragua, San Salvador and
Uruguay. Registration proceedings and trials are in progress in
several other countries.
Fenamiphos is formulated as 5 and 10% granules and as a 40%
emulsifiable concentrate.
Pre-harvest treatments
Fenamiphos is formulated as a granular product and as an
emulsifiable concentrate. Both formulations are recommended chiefly
for treatments in banana, pineapple, vegetable (tomatoes, brassicas,
peppers, eggplants), tobacco, coffee, groundnut and citrus crops.
Application is usually made to the soil at sowing, planting and
transplanting, and in established crops. The general dose rate is 5 to
10 kg active ingredient per hectare for broadcast (overall)
application, but is reduced for band application according to band
width. Furthermore, the emulsifiable concentrate is suitable for dip
treatments of banana seedlings and for foliar spraying of pineapples.
Details of uses and recommendations are given in Table 4.
TABLE 4 Use Pattern of fenamiphos (All applications are pre-harvest against nematodes)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Bananas 1.5-2.5 g/m2 At planting G
3.0 g/plant Soil treatment in
established crop G 3-4
4-5 kg/ha In irrigation
canals or direct
to soil EC 2
1-1.5 g/corm Dip treatment
pre-planting plus
soil application EC
2 or 1.5 g/
planting position
(but no more than
total of 3 g/
planting position) G
Pineapples 10-40 kg/ha Broadcast treatment
pre-planting of
crowns and slips G
5-20 kg/ha Broadcast treatment
pre-planting of
crowns and slips
in combination with G
3-5 kg/ha foliar spraying 3 in
post-planting LC established
crop
15 kg/ha Foliar spray
application at
planting, repeat
after an interval
of 2 months EC 2
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Tomatoes 1-2.5 g/m2 Immediately before
or after sowing G
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Brassicas 5-8 kg/ha Broadcast/overall
application G,
pre-planting EC
Tobacco 1-2.5 g/m2 Before or immediately
after sowing or G
0.1-0.2 g/plant at transplanting or
in established crop G 1
5-10 kg/ha Broadcast/overall
application G,
pre-planting EC
Coffee 1-2.5 g/m2 Seedbed treatment G
1.5-2.5 g/shrub In established crop G 2-3
Groundnuts 3-5 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Cucurbitaceae 0.15-0.2 g/ Band application
planting hole or at sowing G
1.5-2.5 kg/ha
Peppers 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Eggplant 5-8 kg/ha Broadcast application
at sowing or at
transplanting G 1
Citrus 1-1.5 g/m2 Seedbed treatment G
2-3 g/tree Treatment in nursery G
1.5-3 g/tree In established crop G, LC 2-3
TABLE 4 (Cont'd.)
No. of
Application rate treatments
Crop a.i. Type of Application Formulation1 per year
Potatoes 5-10 kg/ha Broadcast/overall G, EC,
treatment pre-planting LC 1
Pyrethrum 5-8 kg/ha Broadcast application
at planting G
Ornamentals 1.5-3 g/m2 In established crops G
Cotton 1.7-3.5 kg/ha Band application
(band width of G,
30-45 cm.) at sowing LC
Beets 3-(6) kg/ha Band application
immediately after
sowing or during G,
emergence EC
Soybeans 5-8 kg/ha Broadcast/overall
application G,
pre-sowing LC 1
Beans 5-8 kg/ha Broadcast application
pre-sowing G 1
Grapes 2-3 g/plant G 1
Strawberries 10 kg/ha Broadcast application
pre-planting G
300 g/1000 Post-planting or G
metres of row post-harvest
Lawns 10-20 kg/ha Broadcast application G
1 G = Granules; EC = Emulsifiable concentrate; LC = Liquid concentrate.
Post-harvest treatments
No recommended uses.
Other uses
Fenamiphos is recommended for use on ornamentals (granular
formulation).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues of fenamiphos together with its sulphoxide and
sulphone resulting from supervised trials in a wide range of crops are
shown in Table 5. In most crops, residues were below 0.05 mg/kg. The
exceptions were grapes, lettuce, potatoes, strawberries and tomatoes
which contained up to about 0.1, 0.5, 1, 0.7 and 0.3 mg/kg
respectively. Residues in tobacco were much higher, with respective
maximum levels in green, cured and fermented tobacco of about 12, 140
and 100 mg/kg.
FATE OF RESIDUES
In animals
A feeding study was carried out on a lactating dairy cow to
determine whether fenamiphos sulphoxide, found in plants as the major
metabolite, would be incorporated into milk and animal tissues either
as the intact compound or in metabolized form (Gronberg et al., 1974.
See also "Biotransformations"). A single dose of 0.8 mg/kg body
weight, corresponding to 27 ppm in the diet, of ring-14C fenamiphos
sulphoxide was administered. Four hours after application the label
was distributed as follows. Urine 39%, faeces <0.1%, rumen 47%,
tissues 1.4%, bile 0.1%, omasum, abomasum and milk <0.1% each; total
recovery 88%. The following compounds were found: fenamiphos (I),
fenamiphos sulphoxide (II), desisopropyl-fenamiphos (III), phenol of
fenamiphos (IV), phenol of fenamiphos sulphoxide (V), phenol of
fenamiphos sulphone (VI) and "unknown" (VII). The levels of these
residues in tissues and milk are shown in Table 6.
Of the dose, 18% was reduced to the parent fenamiphos, apparently
by microorganisms. At 4 hours 82% of the activity in whole blood and
87% of the activity in urine was identified as conjugated phenols.
The residues of fenamiphos in poultry eggs and tissues were
determined by Gronberg et al. (1973). Laying hens were fed for 14 days
with carbon-14 ring-labelled fenamiphos in feed at 0.06, 0.18 and 0.65
ppm concentration. During this time, residues in eggs were below the
detection limit of 0.003 mg/kg. At slaughter, all tissue residues from
birds given feed containing 0.65 ppm were below the limit of
determination of 0.003 to 0.005 mg/kg, although very low residues were
detectable in some tissues.
TABLE 5 Residues of fenamiphos and metabolites resulting from supervised trials
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Apples Australia 10% G 22.4 334 1 n.d.2 n.d.2
Bananas, Central
peel America 10% G 2.8-12 g
a.i./plant 1-195 41 <0.025-0.3 <0.025
pulp <0.025 <0.025
Bananas, Ivory Coast 5% G 10 g a.i./plant 1-14 4 <0.02 <0.02
peel
pulp <0.02 <0.02
peel 28-88 3 0.06-0.1 0.07
pulp 0.03-0.08 0.05
Broccoli USA 3% G;
15% G 6.72 65-101 8 <0.01-0.04 0.02
15% G 10.0 92 1 0.2 0.2
Brussels
Sprouts USA 3% SC;
15% G 6.72-10.0 107-133 5 <0.01-0.02 <0.01
Cabbage USA 3% SC;
15% G 6.72-10.0 55-108 14 <0.01-0.02 0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 40% EC 4.5 42-106 6 n.d.2 n.d.2
Carrots USA 15% G 6.72 96-160 9 <0.01-0.05 0.01
Cauliflower USA 3% SC;
15% G 6.72-10.0 97-111 12 <0.01-0.04 0.02
Coffee Brazil - 1-2 g a.i./plant 14-15 6 <0.02-0.07 0.05
beans, Central
green America 28-30 6 <0.02-0.09 0.05
56-62 4 <0.02-0.05 0.03
90 2 0.03; 0.05 0.04
Cottonseed USA 3% SC;
15% G 1.12 157-196 10 <0.01 <0.01
Cotton USA 3% SC;
gin trash 15% G 1.12 161-196 8 <0.01 <0.01
Cotton USA 3% SC;
foliage 15% G 1.12 143-148 4 <0.01-0.1 0.04
Grapefruit USA 3% SC;
peel 15% G 33.6 - 50.4 183-184 1 <0.01 <0.01
pulp <0.01 <0.01
whole fruit <0.01 <0.01
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Grapes Australia 5% G
43% EC 5.6 - 14.7 145-147 2 n.d.2 n.d.2
22.0 145-147 1 0.03 0.03
44.0 145-147 1 0.08 0.08
Leaf-lettuce USA 3% SC 11.2 46 1 0.3 0.3
Head USA
lettuce, 3% SC;
head 15% G 11.2 61-72 4 0.02-0.1 0.08
wrapper leaves 0.09-0.5 0.3
discard leaves 0.09-6.5 1.9
Lemons, USA
whole fruit 3% SC 33.6 184 1 0.01 0.01
Limes, USA 3% SC;
whole fruit 15% G 33.6 147 2 <0.01 <0.01
Melons Guatemala
Australia 5% G
Egypt 40% EC 1.6-10.0 56-112 5 n.d2-0.05 n.d.2
Oranges, USA 3% SC; 22.4-50.4 182-366 4 <0.01-0.1 0.04
peel 10% G;
15% G
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
pulp 4 <0.01 <0.01
whole fruit 5 <0.01-0.02 0.01
whole fruit Australia 5% G;
40% EC 11.2-22.4 21-139 10 n.d.2 n.d.2
Peanuts, USA 3% SC;
kernels 10% G 4.51-13.45 122-148 5 <0.01-0.02 <0.01
shells (0.01-0.2 0.06
vines 98-148 5 0.02-4.3 2.3
Pineapples Hawaii, USA 3% SC;
10% G 44.8-112 238-658 10 <0.01-0.03 <0.01
3% SC
(foliar spray) 5.6 1-29 20 <0.02-4.0 0.5
Potatoes USA 3% SC;
15% G (row
appl) 6.1 41-58 3 <0.02-1.2 0.7
71-106 7 <0.01-1.0 0.15
Potatoes, USA, Canada, 3% SC;
whole Fed. Rep. of 15% G
Germany, UK, (broadcast
Australia 4-5-11.2 67-203 23 n.d.2-0.45 0.07
peel 1 0.07 0.07
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
flesh 1 0.07 0.07
Sweet USA 3% SC;
Potatoes 15% G
(broadcast) 6.72 108-143 8 <0.01-0.04 0.01
3% SC;
15% G
(row appl) 4.0-6.1 108-143 8 <0.01-0.06 0.01
Soybeans, USA 3% SC;
bean, green 15% G 11.2 75-117 12 <0.01-0.09 0.04
vine, green 75-117 12 <0.01-12 2.9
bean, dry 131-159 11 <0.01-0.03 0.01
Strawberries Australia 5% G
40% EC 16.5-22.5 36-121 6 0.03-0.7 0.2
Sugarbeets, USA 3% SC;
tops 15% G 6.72-10.0 119-187 9 <0.01-0.08 0.01
beets <0.01-0.03 0.01
Tangerines, USA 3% SC;
peel 15% G 33.6 247-251 2 0.07; 0.3 0.2
pulp 2 <0.01; 0.03 0.02
whole fruit 1 0.03 0.03
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Tobacco Australia 40% EC 50-95 4 0-5-4.8 1.9
Tobacco, USA 3% SC; 6.72 4 3 0.9-7.8 3.5
green 15% G 7 3 0.8-7.0 2.9
11 3 0.8-3.3 1.8
14 3 1.4-12 5.2
21 3 1.2-4.9. 2.5
28 3 0.4-4.2 1.7
35 3 0.25-3.6 1.4
36-148 13 0.02-4.8 1.0
air cured 88-106 2 3.2; 4.2 3.7
flue cured 85 2 11; 13 12
cured 75-91 6 4.5-140 33
aged 75-92 3 5.7-16 11
fermented 89 1 98 98
Tomatoes USA, 3% SC;
Canada 15% G 11.2-16.8 61-89 10 <0.01-0.3 0.1
TABLE 5 (Cont'd.)
Rate of Residues, fenamiphos and
application Interval No. of sulphoxide and sulphone, mg/kg
Crop Country Formulation1 (kg a.i./ha) (days) analyses Range Average
Australia 5% G;
40% w/v 9.8-11.2 78-161 5 <0.05 <0.05
Fed. Rep, of
Germany 5% G 9.8-12.2 79-161 5 n.d.3 n.d.3
1 EC = emulsifiable concentrate; G = granules; SC = spraying concentrate. n.d. = <0.01.
2 <0.01 mg/kg.
3 <0.05 mg/kg.
TABLE 6 Residues, mg/kg, in tissue and milk of cow fed fenamiphos
sulphoxide (Gronberg et al., 1974)
Compound1 Fat Meat Liver Kidney Milk
I 0.004 n.d.2 0.006 n.d. n.d.
II n.d. n.d. 0.002 n.d. 0.002
III 0.002 n.d. 0.005 0.16 0.004
IV 0.005 0.001 0.029 0.86 0.005
V 0.003 0.003 0.021 0.36 0.025
VI n.d. n.d. n.d. 0.01 0.004
VII 0.002 0.005 0.036 0.25 0.020
1 For identities of compounds, see text.
2 n.d., not detectable, = <0.001 mg/kg.
In plants
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thiooxidation of the parent compound to
the sulphoxide and sulphone. Both metabolites are detoxified by
hydrolysis, generally followed by glucosylation of the phenolic
products.
Fenamiphos-14C,3H was injected into the stems of beans,
tomatoes, peanuts and potatoes and the plants were harvested at 7, 14,
21 and 28 days (Waggoner, 1972. See also "Biotransformations"). Two
major metabolites were identified by TLC, isotopic ratios, IR, and MS
as the sulphoxide and sulphone of fenamiphos. The sulphoxide was the
major metabolite, accounting for 33-41% of the residue, followed by
the sulphone (31-33%), both after 7 days' treatment. Two minor
metabolites containing intact phosphoramidate structures accounted for
not more than 1.5 and 0.2% of the total residue.
Khasawinah studied fenamiphos metabolism in carrots (1973a),
tomatoes (1973b), cabbages (1973c), soybeans (1972b) and tobacco
plants (1971) following soil application, and in snap beans (1972a)
after both soil treatment and stem injection; 14C-ring, 14C-1-ethyl
and 3H-methylthio labelled fenamiphos were used.
Carrots readily absorbed and metabolized fenamiphos and its major
soil foliage and 14C from the ethyl group was detected in the foliage
and carrots. This 14C was probably incorporated into lipids,
pigments, sugars and structural elements such as cellulose.
Water-soluble residues were mainly conjugates of the sulphoxide and
sulphone phenols. No parent fenamiphos was detected. Residues of the
sulphoxide and sulphone were below 0.06 mg/kg in both carrots and
foliage. At the end of the growing period, soil residues were 2.7
mg/kg fenamiphos equivalents (one third of the amount applied) of
which 65% consisted of the sulphoxide and sulphone in the ratio 4:1.
Results with tomatoes were similar. Sulphoxide and sulphone
residues reached a peak of 0.2-0.3 mg/kg 30-40 days after treatment,
and no parent compound was detected later than 40 days. The results
showed the stability of the thio-methyl-ring linkage.
Comparable results were obtained with head cabbage. An
organosoluble polar metabolite with an apparently intact
phosphoramidate structure was detected soon after treatment and
declined gradually to insignificant levels.
In tobacco plants the sulphoxide reached a peak in less than one
week, and then decreased as the sulphone increased. At the end of the
growing period the ratio of sulphoxide to sulphone was about 3:2.
Soybeans were planted in soil that had been treated with
14C-ethyl, 3H-methylthio fenamiphos and cropped with tobacco for one
growing season. The soil at soybean planting contained 0.65 mg/kg
fenamiphos sulphoxide, 0.07 mg/kg fenamiphos sulphone and 0.18 mg/kg
fenamiphos equivalents as unextractable material. Nineteen days old
soybean seedlings contained 16.9 mg/kg (fresh weight basis) of these
two soil metabolites in a sulphoxide: sulphone ratio of 73:17. In the
mature plant (150-day growing period), the total concentration of the
two metabolites, found in equal proportions, was 3.5 mg/kg in the dry
leaves and 0.19 mg/kg in the dry beans. The water-soluble
radioactivity consisted mainly of the glucose conjugate of the
sulphone phenol. The insoluble radioactivity was not characterized.
The fate of 14C1-ethyl and 3H-methylthio fenamiphos was
investigated in snap beans following stem injection and soil treatment
in systems designed for the recovery of the applied nematicide. The
study demonstrated volatile 14C radioactivity, presumably as CO2.
40% of the stem-injected and 12% of the soil-applied fenamiphos was
recovered in this form. Volatile tritium radioactivity was less, 7.4%
and 1.3% of the amounts applied by stem-injection and soil treatment,
respectively. The metabolites formed by both methods of application
were fenamiphos sulphoxide, fenamiphos sulphone and the sulphoxide and
sulphone phenols in the free form or conjugated with glucose.
The metabolism of similarly labelled fenamiphos applied by stem
injection, soil drench or spray to fruited pineapple plants was
studied by Flint (1973). Soil treatment at 20 pounds a.i./acre
resulted in maximum total radioactive residues in fruit of 0.086 mg/kg
fenamiphos equivalents at 90 days after treatment. Metabolites
identified in fruit and foliage were identical to those previously
found in several other plant and animal systems.
In soil and water
Khasawinah (1970) treated the top 1.5 cm of a sandy loam soil, in
which tobacco was growing, with labelled fenamiphos. One third of the
applied amount was taken up by the growing plants during an 11-week
period. The major product in the soil was the sulphoxide, with small
quantities of the sulphone. No hydrolysis products of the parent
compound or its oxidation products were detected in the soil extracts.
None of the radioactivity was leached into the drainage water, and all
of it remained in the root zone. About 22% of the radioactivity
remaining in the soil after 177 days was bound to clay particles and
was unextractable.
Fenamiphos was studied for soil runoff, leaching, adsorption, and
stability in water by Flint et al. (1971) and Church (1971). It was
moderately adsorbed by sandy loam and silt loam soil. Fenamiphos
residues in runoff water were less than 3.4% of the applied compound
over a 37-day interval during which 2.3 to 3.7 inches of irrigation
were applied; the application rate of fenamiphos was 22.5 kg a.i./ha.
The leaching of fenamiphos from the three types of soil was inversely
proportional to the adsorptivity of the soils. It was estimated that
111 inches of rainfall would be needed for clay loam soil and 75
inches for sandy loam to leach fenamiphos to a depth of 12 inches.
Fenamiphos was relatively stable in aqueous systems at pH 5 and 7
in the laboratory but declined rapidly with a half-life of 4.8 days in
a simulated field pond at PH 7 and average temperature of 29°C.
The leaching of "aged" fenamiphos residues in sandy loam soil was
investigated by Tweedy et al. (1974). Ring-14C fenamiphos was
incubated with sandy loam soil under greenhouse conditions for 30 days
and then applied to the top of a sandy loam soil column 2.9 inches in
diameter and 12 inches high. Distilled water, equivalent to 0.5
acre-inch/day was added daily for 45 days. Of the radioactivity
recovered from the system, 77.5% was found in the top fourth of the
column and only 2.3% was recovered in the leachate. The majority of
the activity, 65%, apparently did not move at all.
The metabolism of fenamiphos soil residues under aerobic and
anaerobic conditions was investigated by Shaw and Flint (1974).
Fenamiphos, labelled with tritium in the methylthio group or with
carbon-14 uniformly throughout the aromatic ring, was used in this
study. The results indicated that anaerobic conditions arrested
further degradation while aerobic incubation allowed continued
metabolism. Essentially no 14C or 3H radioactivity was lost during
incubation indicating stability of the aromatic ring system. The major
metabolite was fenamiphos sulphoxide with some further thio-oxidation
and hydrolysis observed under aerobic conditions.
The effect of fenamiphos on soil microbial populations was
studied by Houseworth and Tweedy (1972) using Indiana clay loam and
Commerce silt loam soils. The soils were treated with 50 and 250 mg/kg
of the pesticide and maintained at 50% field moisture capacity for 56
days. At intervals, samples of soil were removed from each treatment
and assayed for populations of bacteria, fungi and Actinomycetes.
Fenamiphos had no significant effect on them under the conditions of
the experiment.
The photodecomposition of thin films of fenamiphos was
investigated by Khasawinah and Sandie (1974). Thin films of dual
labelled ethyl-1-14C, methylthio-3H fenamiphos were coated on glass
plates and silica gel coated glass plates and exposed in the open air
for about three weeks. Fenamiphos was rapidly oxidized to its
sulphoxide and then to the sulphone. Some hydrolysis of the sulphone
occurred after long intervals, especially on glass.
The results of some studies in the USA of the persistence of
fenamiphos and its oxidation products in various types of soil were
available to the Meeting. They are reproduced in Table 7.
In storage and processing
The effect of alkali refining and steam deodorization on residues
in cottonseed and soybean oils was studied in laboratory simulation
experiments by Thornton (1973a) and Olson (1972b, 1974). In
cottonseed, the two processes in combination removed 98.5% of the
fenamiphos, 98.4% of the sulphoxide and 88% of the sulphone. Since the
sulphone would constitute only a small proportion of a naturally
occurring residue, the combined processes would remove nearly all the
residue. In soybeans, the effects of the processes on sulphoxide
residues was studied, as this is the main component of the residue
after long post-treatment intervals. The simulated refining process
removed 96% of the sulphoxide, and deodorization removed 38%. A
hypothetical residue of 0.42 mg/kg should therefore be reduced to 0.01
mg/kg by the two steps.
The effect of citrus processing on residues of fenamiphos
sulphoxide was investigated by Olson (1972a). Commercial citrus
processing involves liming and drying of the chopped solids in the
preparation of cattle feed. Fenamiphos sulphoxide, the main metabolite
in crops, was resistant to the liming step but 39% of the residue was
removed by drying. To prepare citrus oil, an aqueous emulsion is
centrifuged vigorously. 99% of any fenamiphos sulphoxide present in
the emulsion would be removed from the oil by this procedure.
TABLE 7 Persistence of fenamiphos + sulphoxide + sulphone in various soils in the USA1
Residue, phenamiphos + sulphoxide + sulphone, mg/kg, after interval, days2
0 30-40 80-100 180-200 360-400
Soil Range Mean Range Mean Range Mean Range Mean Range Mean
Silt loam 1.2-15 7.7 5.4-12 9.4 0.8-15 6.9 0.1-6.5 2.5 0.1-2.5 1.1
Clay 1.0-1.7 1.3 3.7 3.7 1.5-2.7 2.1 0.6-1.3 1.0 0.6-0.7 0.7
Sand muck 5.1-6.3 5.7 5.1-5.8 5.4 0.1 0.1 0.7-1.0 0.8 0.4-0.6 0.5
Sand 5.5 5.5 0.3-0.6 0.5 0.1-0.2 0.2 0.06-0.07 0.07 0.03-0.04 0.04
Sand clay 6.8-8.5 7.7 3.0-4.9 3.9 0.5-1.2 0.8 0.1 0.1 0.1 0.1
Fox sand 4.3-9.2 6.7 6.8-8.0 7.4 3.1-3.2 3.1 1.0-1.3 1.1 0.9-1.0 0.9
Peat 15-16.5 16 17-20 18 12.5-17 15 6.8-8.8 7.4 6.3-7.2 6.8
Low humus 13-17 15 4.6-6.6 5.5 3.6-6.6 5.1 1.2-3.3 2.2 0.3-0.6 0.4
1 Fenamiphos was added to soils at 10mg/kg level as granules or spray concentrate.
2 Usually two or three analyses at each interval.
Olson (1970a) studied the fate of residues on pineapple crowns.
The fresh crowns were dipped in an aqueous emulsion of spraying
concentrate containing 0.12% fenamiphos, and allowed to drain dry. The
surface residue immediately after treatment amounted to about 30 mg
per crown, of which about half could be removed by extracting with
water. Seven days after treatment, aqueous extraction removed about
one-third of the original residue. Surface extraction with benzene,
however, removed virtually all the initial deposit (29 mg per crown)
but only 27% (8 mg) after 7 days.
Storage of broccoli, carrots, peanut kernels, pineapple fruit and
potatoes at -18° to -23°C for two years showed no significant loss of
fenamiphos, the sulphoxide or the sulphone (Anonymous, 1973).
There was no significant loss of residues (parent + sulphoxide +
sulphone) in field-treated potatoes or of fenamiphos from potatoes
fortified in the laboratory when cooked at 100°C for 30 minutes in
sealed jars (Thornton, 1972).
METHODS OF RESIDUE ANALYSIS
A specific gas-chromatographic procedure for the determination of
residues of fenamiphos and sulphoxide and sulphone in various crops,
animal tissues and milk is described by Thornton (1969, 1971a).
Following initial extraction, the extract is oxidized with potassium
permanganate to convert fenamiphos and its sulphoxide to the sulphone.
The sulphone is then determined by gas chromatography with alkali
flame ionisation detection. Recoveries from a variety of crops and
animal tissues fortified with fenamiphos or its metabolites at the
blending step were generally between 75 and 110%. Interference studies
by Thornton (1971b, 1973b) and Olson (1970d, 1971b,c) have shown the
method to be specific in the presence of other possible
organophosphorus compounds. The limit of detection is about
0.02 mg/kg.
The determination of residues of fenamiphos and its metabolites
in tobacco and tobacco smoke was successfully investigated by Olson
(1971a) with the Thornton (1971a) method. This method should be
suitable for regulatory purposes.
The determination of fenamiphos residues in soil by thermionic
emission gas chromatography is described by Olson (1970b). The soil
sample is extracted in a Soxhlet apparatus, using a 1:1
chloroform-methanol solvent mixture. The solvent is evaporated and the
residue oxidized and chromatographed. Recoveries of fenamiphos and its
metabolites were 80-107%. The sensitivity of the method is
approximately 0.02 mg/kg. Olson (1970c) has also extended the
procedure to a number of other organophosphorus pesticides.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table 8.
TABLE 8 National tolerances reported to the Meeting
Safety
Tolerance Interval
Count Crop mg/kg (days)
Australia Tomatoes, brassicas,
cucurbits, lettuce, citrus,
pineapples, grapes,
bananas, ginger No residue
Spain Tobacco sugarbeets, etc.
(industrial purpose),
bananas 60
USA Bananas, citrus 0.1 180
APPRAISAL
Fenamiphos is a systemic nematicide which gives good control of
root-knot cyst forming and free living nematodes. It is also effective
against sucking insect pests and spider mites. It is formulated as 5%
and 10% granules and as an emulsifiable concentrate (40%). Both
formulations are recommended chiefly for treating bananas, pineapples,
tomatoes, brassicas, eggplant, tobacco, groundnuts, citrus crops,
ornamentals and pyrethrum. It is chiefly applied to the soil at sowing
or planting. The general dose rate is 5-10 kg active ingredient/ha for
broadcast application, but is reduced for band application. The
emulsifiable concentrate is especially suitable for dip treatment of
banana seedlings and for foliar spraying of pineapples. Fenamiphos is
commercially used in Australia, USA, some Central and South American
countries, Spain, the Cameroons and the Ivory Coast.
Various investigations of the metabolism of fenamiphos in plants
and soil demonstrate the rapid thio-oxidation of the parent compound
to the sulphoxide and sulphone. In general the major part of the
residue in crops and soil is the sulphoxide, followed by the sulphone.
Both are relatively persistent. Both metabolites are detoxified by
hydrolysis, usually followed by conjugation of the phenolic products
as glycosides. Little or none of the parent fenamiphos seems to be
present in most crops at harvest.
Micro-organisms in soil do not seen to be affected by fenamiphos.
The dissipation of residues in soil depends on the type of soil,
increasing in the order peat < silt loam < clay = sand. Fenamiphos
is only very slowly leached from soil.
Following application in accordance with good agricultural
practice, the maximum residue levels of fenamiphos including its
sulphoxide and sulphone in Brussels sprouts, cotton seed, cabbage,
carrots, cauliflower, grapefruit, lemons, limes, melons, oranges
(pulp), peanut kernels, pineapples, soybeans (dried) and sugar beets
are at or below 0.05 mg/kg. Higher residues are found in some other
crops. Typical maximum residues are: grapes 0.1 ppm, lettuce 0.5 ppm,
potatoes 1 ppm, strawberries 0.7 ppm, tomatoes 0.3 ppm and tobacco
(green) 12 ppm, (cured) 140 ppm (fermented) 100 ppm. Data for
fenamiphos residues in tobacco smoke are not available.
Processing cottonseed or soybeans to oil removed 98-99% of the
residues and processing citrus to cattle feed removed about 40%.
Cooking potatoes had no significant effect. In a laboratory experiment
approximately one third of the fenamiphos originally applied was
extractable with water from the surface of the crowns of pineapples
seven days after dipping in an aqueous emulsion containing 0.12%
fenamiphos.
Residues in eggs and tissues from hens fed for 14 days with 14C
ring-labelled fenamiphos in feed at 0.65 ppm were below 0.005 mg/kg.
Residues in milk and tissues of a dairy cow fed once with a dose
corresponding to 27 ppm fenamiphos sulfoxide in the diet were below
0.01 ppm.
Specific gas-chromatographic procedures for the determination of
residues of fenamiphos, its sulphoxide and sulphone in various crops,
animal tissues, milk and soil have been elaborated. The initial
extract is oxidized with permanganate to convert fenamiphos and its
sulphoxide to the sulphone. The sulphone is determined with an alkali
flame ionisation or flame photometric detector. Limits of detection
are about 0.02 ppm, and recoveries higher than 75%. The methods should
be suitable for regulatory purposes.
RECOMMENDATIONS
The following limits refer to fenamiphos including its sulphoxide
and sulphone, expressed as fenamiphos.
TOLERANCES
Tolerance
Crop (mg/kg)
Bananas, coffee beans (green and
roasted, grapes, sweet potatoes 0.1
Broccoli, Brussels sprouts, cabbage,
carrots, cauliflower, citrus fruits,
cottonseed, melons, peanut kernels,
pineapples, soybeans (dried), sugar beets 0.05*
* At or about the limit of determination.
TEMPORARY TOLERANCES
Temporary
tolerance
Crop (mg/kg)
Potatoes, tomatoes 0.2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further data on which to judge the residues in or on potatoes and
tomatoes.
2. Adequate residue data on which to base recommendations for
tolerances for other crops (e.g. beans, cucumbers, lettuce,
peppers and strawberries).
DESIRABLE
1. Brain cholinesterase and behavioural studies in animals exposed
to low levels for extended periods.
2. Observations in man.
3. Additional studies on the potentiation effects with other
organophosphorus pesticides.
4. Residue data for raw agricultural products moving in commerce.
5. Further residue data for different crops from supervised trials
including data on rates and frequencies of application, soil,
foliar or other treatment and pre-harvest intervals, especially
for broccoli and tangerines.
REFERENCES
Anonymous. (1973) The effect of frozen storage at 0 to -10°F on
Nemacur residues in peanut meat, broccoli, carrot roots, potatoes,
pineapple fruit. Chemagro Report No. 36132, 36318, 36319, 36320,
39094.
Arnold, D., Keplinger M. and Fancher, O. (1971) Mutagenic study with
Nemacur(R) (BAY 68138) Technical in albino mice. Report from
Industrial BioTest Laboratories, Inc., submitted by Bayer AG.
(Unpublished).
Cherry, C., Urwin, C. and Newman, A. (1972) Pathology report of BAY
68,138. Rat breeding study. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished).
Cherry, C. and Newman, A. (1973) Pathology report of Bayer 68,138.
Chronic toxicological studies in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Church, D.D. (1971) Nemacur - leaching, runoff and water stability.
Chemagro Report No. 26 849.
Coulston, F. and Wills, J.H. (1974) Summary of research of the
Institute of Comparative and Human Toxicology, Albany Medical College,
on fenamiphos. Report submitted by Bayer AG. (Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970a) The skin and eye
irritating properties of BAY 68,138 3 lb/gal S.C. to rabbits. Report
from Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford, C., Anderson, R. and Nelson, D. (1970b) The acute inhalation
toxicity of Nemacur(R) 15% granular to rats. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C, and Anderson, R. (1971) The skin and eye irritating
properties of Bay 68138 technical to rabbits. Report from Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1972a) The acute dermal toxicity of
Nemacur(R) technical to rabbits. Report from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (unpublished).
Crawford C. and Anderson, R. (1972b) The acute dermal toxicity of
Nemacur(R) 3 lbs/gal. spray concentrate to rabbits. Report from
Chemagro Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Crawford C. and Anderson, R. (1973a) The dermal toxicity of Nemacur(R)
15% granules to rats. Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1973b) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R) with
analytical grade Nemacur(R). Report from Chemagro Division of Baychem
Corporation, submitted by Bayer AG. (Unpublished).
Crawford C. and Anderson, R. (1973c) The eye and skin irritancy of
Nemacur(R) 2 lb/gal spray concentrate to rabbits. Report submitted by
Bayer AG. (Unpublished).
Crawford, C. and Anderson, R. (1974) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Report from Chemagro Division of Baychem Corporation, submitted by
Bayer AG. (Unpublished).
DuBois, K., Flynn, M. and Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Report from University of
Chicago. (Unpublished).
DuBois, K. and Flynn, M. (1968) The subacute parenteral toxicity of
BAY 68138 to rats. Report from University of Chicago, submitted by
Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1970) Acute oral and dermal toxicity of
a granular formulation of BAY 68138. Report from University of
Chicago, submitted by Bayer AG. (Unpublished).
DuBois, K. and Kinoshita, F. (1971) The acute oral and inhalation
toxicity of a Nemacur(R) (BAY 68138) formulation to rats. Report from
University of Chicago submitted by Bayer AG. (Unpublished).
Flint, D.R. (1973) The metabolism of Nemacur in pineapple. Chemagro
Report No. 39 119.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour, J., II. (1971) The
mobility and persistence of Nemacur in soil and water. Chemagro Report
No. 29 974.
Gronberg, R.R. (1969) The metabolic fate of
ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate (BAY 68138),
ethyl-4-(methylsulfinyl)-m-tolyl isopropylphosphoramidate (BAY 68138
sulfoxide) and ethyl-4-(methylsulfonyl)-m-tolyl-isopropylphosphoramidate
(BAY 68138 sulfone) in white rats. Report No. 26759 from Chemagro
Division of Baychem Corporation, submitted by Bayer AG.
(Unpublished).
Gronberg, R.R., Flint, D.R. and Pither, K. (1974) The metabolic fate
of Nemacur sulfoxide administered orally to a lactating dairy cow.
Report No. 41104 from the Chemagro Division of Baychem. Corp.,
submitted by Bayer AG. (Unpublished).
Gronberg, R.R., Simmons, C.E. and Shaw, H.R., II. (1973) Residues of
Nemacur in poultry eggs and tissue. Report No. 35995 from the Chemagro
Division of Baychem Corporation, submitted by Bayer AG. (Unpublished).
Houseworth, L.D., and Tweedy, B.G. (1972) Effect of Nemacur on
microbial populations. Chemagro Report No. 34 990.
Khasawinah, A.M. (1970) The fate of Nemacur in soil. Chemagro Report
No. 28 796.
Khasawinah, A.M. (1971) Metabolism of Nemacur in tobacco plants.
Chemagro Report No. 29 142.
Khasawinah, A.M. (1972a) Metabolism of Nemacur in snap beans grown in
closed glass chambers. Chemagro Report No. 34 992.
Khasawinah, A.M. (1972b) The uptake and metabolism of Nemacur soil
residues by soybean plants. Chemagro Report No. 35012.
Khasawinah, A.M. (1973a) Metabolism of Nemacur in carrots. Chemagro
Report No. 36005.
Khasawinah, A.M. (1973b) Metabolism of Nemacur in tomatoes. Chemagro
Report No. 38501.
Khasawinah, A.M. (1973c) The metabolism of Nemacur in cabbage.
Chemagro Report No. 39120.
Khasawinah, A.M. and Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Report No. 34217 from Chemagro Division of
Baychem Corporation, submitted by Bayer AG. (Unpublished).
Khasawinah, A.M. and Sandie, F.E. (1974) Photodecomposition of thin
films of Nemacur. Chemagro Report No. 39217.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on chickens.
Report from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Report from
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. and Solmecke, B. (1971a) BAY 68138 toxicological studies.
Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Kimmerle, G. and Solmecke, B. (1971b) Granular formulation. Acute
dermal toxicity on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972a) Antidotal experiments on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Kimmerle, G. (1972c) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Report from Institute for Toxicology,
submitted by Bayer AG. (Unpublished).
Ladd, R., Jenkins, D., Keplinger, M. and Fancher, O. (1971)
Teratogenic study with Nemacur(R) technical in albino rabbits. Report
from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Löser, E. (1968a) Subchronic toxicological studies on dogs. Report
from the Institute for Toxicology, Farbenfabriken Bayer AG.
(Unpublished).
Löser, E. (1968b) Subchronic toxicological studies on rats. Report
from the Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Löser, E. (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Report from the Institute for Toxicology, submitted by
Bayer AG. (Unpublished).
Löser, E. (1970) Subchronic toxicological studies on dogs. Report from
the Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Löser, E. and Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachr. Bayer,
24(1):69-113.
Löser, E. (1972a) Chronic toxicological studies on rats (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Report from the Institute for Toxicology,
submitted by Bayer AG. (Unpublished)
Löser, E. (1972c) Generation studies on rats. Report from the
Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Mawdesley-Thomas, L. and Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Olson, T.J. (1970a) Residues on the surface of fresh pineapple crowns
treated with an aqueous emulsion of S.C. formulation of Nemacur.
Chemagro Report No. 28014.
Olson, T.J. (1970b) Determination of Nemacur residues in soil by
thermionic emission gas chromatography. Chemagro Report No. 28731.
Olson, T.J. (1970c) Determination of Dasanit, Guthion, Metasystox R,
Nemacur and trichlorfon in soil by thermionic emission gas
chromatography. Chemagro Report No. 27835.
Olson, T.J. (1970d) An interference study for the determination of
residues of Nemacur on pineapple. Chemagro Report No. 28723.
Olson, T.J. (1971a) Determination of Nemacur residues in tobacco and
tobacco smoke. Chemagro Report No. 30448.
Olson, T.J. (1971b) An interference study for the determination of
residues of Nemacur on cotton and tomatoes. Chemagro Report No. 30095.
Olson, T.J. (1971c) An interference study for the Nemacur crop residue
method for bananas. Chemagro Report No. 31041.
Olson, T.J. (1972a) The effect of citrus processing on residues of
Nemacur sulfoxide. Chemagro Report No. 34050.
Olson, T.J. (1972b) Effect of commercial soybean oil processing on
residues of Nemacur sulfoxide. Chemagro Report No. 33461.
Olson, T.J. (1974) The effect of alkali refining on Nemacur residues
in cottonseed oil. Chemagro Report No. 40113.
Shaw, H.R. and Flint, D.R. (1974) The metabolism of Nemacur soil
residues under aerobic and anaerobic conditions. Chemagro Report No.
39909.
Smith, P., Wright, P. and Keplinger, M. (1972) Eighteen month
carcinogenic study with Nemacur(R) (BAY 68138) in Swiss white mice.
Report from Industrial BioTest Laboratories, submitted by Bayer AG.
(Unpublished).
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Report from the Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished).
Spicer, J. (1971) Pathology report of BAY 68138. Hen study. Report
from Huntingdon Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C., Newman, A. and Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Report from the Huntingdon
Research Centre, submitted by Bayer AG. (Unpublished).
Thompson, C. Newman, A. and Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Report from Huntingdon Research Centre, submitted by Bayer AG.
(Unpublished).
Thornton, J.S. (1969) A gas chromatographic method for the
determination of Nemacur and metabolite residues in crops. Chemagro
Report No. 25402.
Thornton, J.S. (1971a) Determination of residues of Nemacur and its
metabolites in plant and animal tissues. J. agr. Food Chem., 19
(5):890-893.
Thornton, J.S. (1971b) An interference study for the residue method
for Nemacur and metabolites. Chemagro Report No. 25533.
Thornton, J.S. (1972) Effect of cooking on residues of Nemacur in
potatoes. Chemagro Report No. 35314.
Thornton, J.S. (1973a) Effect of alkali refining and deodorization on
residues of Nemacur and metabolites in cottonseed oil. Chemagro Report
No. 35345.
Thornton, J.S. (1973b) An interference study for the Nemacur residue
method. I. Additional compounds not previously tested. Chemagro Report
No. 36 142.
Thyssen. (1974a) Nemacursulfoxid, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974b) Nemacursulfon, Akute Toxizitat bei Ratten. Report
from Institute for Toxicology, submitted by Bayer AG. (Unpublished).
Thyssen. (1974c) 4-methyl-mercapto-m-Kresol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974d) 3-methyl-4-methyl-mercaptophenol, Akute Toxizitat bei
Ratten. Report from Institute for Toxicology, submitted by Bayer AG.
(Unpublished).
Thyssen. (1974e) 3-methyl-4 methan-sulfonyl phenol, Akute Toxizitat
bei Ratten. Report from Institute for Toxicology, submitted by Bayer
AG. (Unpublished).
Tweedy, B.G. and Houseworth, L.D. (1974) Leaching of "aged" Nemacur
residues in sandy loam soil. Chemagro Report No. 40 506.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl
4-(methylthio)-M-tolyl isopropylphosphoramidate] and identification
of two metabolites in plants. J. agr. Food Chem., 20(1):157-160.
