
CHLORPYRIFOS-METHYL JMPR 1975
IDENTITY
Chemical name
O,O-Dimethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate
Synonyms
DOWCOR 214, ENT 27520, OMS 1155, RELDANR, DOWRELDANR
ZERTELLR
Structural formula
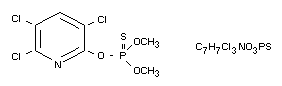 Other information on identity and properties
(Brust, 1969; Kenaga, 1971)
Molecular weight : 322.6
State : Granular crystalline solid
Colour and odour : White/mild mercaptan
Melting point : 45.5-46.5°C
Vapour pressure : 7.40 × 10-7mm Hg at 0°C
4.22 × 10-5mm Hg at 25°C
1.8 × 10-4mm Hg at 35°C
Solubility (g/100g : Acetone 640
at 23°C)
Acetonitrile 680
Benzene 520
Carbon disulfide 430
Carbon tetrachloride 280
Chloroform 330
Diethyl ether 480
Ethanol 30
Methanol 30
n-Octanol 20
Hexane 23
Water 0.0004
The compound readily dissolves in
dimethylformamide, N-methylpyrrolidine,
tetrahydrofuran, xylene, methylene
chloride and 1,1,1-trichloroethane.
Stability : Chlorpyrifos-methyl is reported to be
hydrolyzed by water, the rate being
dependent on temperature and pH.
Half-lives at 25°C vary from 22.7 to
9.4 days in tap water and buffered
distilled water within the Ph range
4.2-8.0.
This hydrolysis is enhanced by traces
of copper ions due to chelation
(Meikle, 1973). The major products of
hydrolysis are
3,5,6-trichloro-2-pyridinol and
O,O-dimethyl phosphate.
Photodecomposition of evaporated
chlorpyrifos-methyl in humid air is
also reported, with the formation of
3,5,6-trichloro-2-pyridinol,
hydroxylated pyridinol derivatives and
oxidation products of quinone
structures (Smith & Taylor, 1972).
Purity : The crystalline compound is stated to
be 99.8% ± 0.1%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 2,6 C14 ring labelled chlorpyrifos-methyl was administered
as a single oral dose (16 mg/kg) to rats, the radioactivity was
rapidly absorbed and excreted. After 72 hours, 90-93% of the
radioactivity was eliminated from the body, 83-85% in urine, 7-9% in
faeces and 0.23-0.43% in respired air. Maximum blood levels (2.4-3.7%)
were reached five hours after treatment. The maximum amount of the
dose remaining in the tissues by 72 hours was 0.65-1.3 ppm. Individual
tissue levels were low (less than 1 ppm). Urinary metabolites were
shown to be 3,5,6 trichloro-2-pyridinol and unidentified activity at
the origin by thin layer chromatography. The possibility of urinary
conjugates was not studied (Branson and Litchfield, 1971a).
The distribution of a single oral dose of C14 labelled
chlorpyrifos-methyl in the body of the young rat was investigated by
whole body autoradiographic techniques. Whole body autoradiograms were
taken at 0.5, 1, 3, 5, 7, 24, 72 and 120 hours after treatment. The
highest level was found initially in the blood. The dispersion in the
various tissues reached a peak at three hours. Residues were extremely
low in fat, liver, kidney and intra-intestinal faeces 72 hours after
administration and completely eliminated from the body by 120 hours
(Nakajima et al., 1974).
Biotransformation
The metabolism of 2,6 C14 ring labelled chlorpyrifos-methyl was
studied in sheep and rats using radioactive counting,
mass-spectroscopy, infrared spectroscopy, column, thin-layer and gas
liquid chromatography techniques. Two ewes were dosed orally with 100
mg/kg by gelatin capsule. Recoveries of radioactivity after four days
were 69.2% in urine, 12.5% in faeces and 2% in tissues. No C14O2 was
detected. Radioactivity in the urine was shown to be the glucuronide
of 3,5,6 trichloro-2-pyridinol 41.6%, o-methyl-o-trimethylsily-o-(3,
5,6 trichloro-2 -pyridyl) phosphorothionate 38% and three minor
unknown fractions 5.2%. In the faeces, 34% of the faecal radioactivity
consisted of parent compound and 55.3% made up primarily of
o-methyl-o-hydrogen-(3,5,6 trichloro-2-pyridyl) phosphorothionate and
3,5,6 trichloro-2-pyridinol. The three fractions found in the urine
were present in the plasma. Detectable amounts of radioactivity (0.32
to 11.8 ppm) were found in the tissues after 96 hours, the highest
level being found in visceral fat (Branson and Litchfield, 1971; Bakke
and Price, 1975).
Ten male rats were given a single oral dose (10 mg) of 2,6 C14
ring labelled chlorpyrifos-methyl. After 48 hours the radioactivity in
the urine consisted of three components, the glucuronide of 3,5,6
trichloro-2-pyridinol (68.6%), o-methyl-o-hydrogen-o (3,5,6
trichloro-2-pyridyl) phosphorothionate (17.8%) and 3,5,6
trichloro-2-pyridinol (13.8%). Faecal metabolites were not
characterized. No respiratory C14O2 was detected (Bakke and Price,
1975).
The principal metabolite of chlorpyrifos-methyl has been shown to
be 3,5,6-trichloro-2-pyridinol. The metabolism of this metabolite in
rats was summarized by FAO/WHO (1973) when the pesticide chlorpyrifos
was evaluated. It was rapidly absorbed and excreted primarily in the
urine and faeces. Small tissue residues (<0.3 ppm) were present and
occurred mainly in biological systems involved with urinary excretion,
i.e. liver, kidney and blood. Residues of the pyridinol in fat were
trivial, which is consistent with its much greater polarity (FAO/WHO,
1973; Smith et al., 1970).
In a similar study in sheep a single oral dose 2,6 C14 ring
labelled 2,5,6 trichloro-2-pyridinol was shown to be rapidly absorbed
and excreted in the urine and faeces. The only radioactive component
detected in the plasma and urine was the glucuronide of the pyridinol
and in faeces the unchanged pyridinol. No C14O2 was detected in
respired air. Residues in tissues after 96 hours were mainly in the
digestive and excretory tracts and was non-detectable in fat, muscle,
heart and other tissues (Bakke and Price, 1975).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
Chlorpyrifos-methyl was administered to groups of Sprague-Dawley
CD strain rats at dietary levels of 0, 0.1 and 1.0 mg/kg/day for 104
weeks. There were no overt signs of reaction to the test compound.
Body weight gain, food consumption, efficiency of food utilization
were comparable between groups and within normal limits. The changes
observed on gross and histopathological examination were those
consistent with the age and strain of rat employed. There was no
compound-related effect an the incidence of spontaneous tumours
(Hunter et al., 1974b).
Special studies on neurotoxicity
Chlorpyrifos-methyl was administered orally to adult hens at 4860
mg/kg body weight and were observed for 21 days. Survivors were given
an intramuscular injection of atropine (10 mg/kg) and PAM (50 mg/kg)
and redosed with 4455 mg/kg chlorpyrifos-methyl and observed for a
further 21 days. No signs of ataxia were detected. The birds were not
examined histologically for nervous systems lesions. TOCP was used as
a positive control (Ross et al., 1975).
Special studies on reproduction
Rat
In a three generation reproduction study (two litters per
generation) three groups of 10 male and 20 female rats received
chlorpyrifos-methyl at dietary levels of 0, 1.0 or 3.0 mg/kg body
weight/day. No treatment related effects on behaviour, survival, body
weight gain or food consumption were observed in parental animals.
Fertility, gestation, viability and lactation indices were comparable
to control values. Pup weights of the second litter, third generation
were significantly less than control at 0, 4 and 21 days postpartum.
There was no effect on sex ratio. In adults of third generation,
plasma cholinesterase activity was decreased in both sexes at both
dosage levels. Erythrocyte cholinesterase depression was observed at 1
mg/kg in females and in both sexes at the highest dose level. Brain
cholinesterase activity was not affected. Gross and histopathological
examination of control and high dose parental animals of the third
generation did not reveal any treatment related abnormalities
(Thompson et al., 1975).
Special studies on teratogenicity
Mouse
Chlorpyrifos-methyl was administered orally to mice at dosage
levels of 0, 20, 100 and 250 mg/kg body weight/day from day 7 through
day 13 of gestation. Animals were sacrificed on day 18 and pups
removed by caesarean section. No significant differences between the
control and treated groups were observed in regard to number of
implants and number of deaths. Body weights were lower in both males
and females at 250 mg/kg. An increased incidence of cleft palate and a
delay of ossification of the cervicovertebral body was observed at 250
mg/kg. When pregnant mice were dosed with a single oral dose of 1000
mg/kg on the seventh or eleventh day of pregnancy, there was no effect
on mortality or body weights of the foetuses. Skeletal abnormalities
were observed (exencephalia, cleft palate, liberation of bone fragment
of cervicovertabral arch) in a few cases especially on treatment on
seventh day of gestation. No control data was presented in the 1000
mg/kg body weight study. In a further study, pregnant mice were
treated as before with 0, 20, 100 and 250 mg chlorpyrifos-methyl/kg
body weight/day on day 7 through day 13 of gestation. Females were
allowed to deliver their pups. Body weights at birth and after three
weeks were dose dependent, being lower on the higher treatment. No
significant morphological abnormalities were detected (Esaki et al.,
1973).
Pregnant rats received 0, 20, 100 and 250 mg/kg body weight of
chlorpyrifos-methyl from day 9 to day 15 of gestation. On day 20
animals were laparotomized and embryos removed. No reproductive or
teratogenic effects were observed. In a similar study the pregnant
rats were allowed to deliver their progeny. Body weight at birth
tended to be higher in the treated groups but no differences observed
when weaned at four weeks. No teratogenic effects related to treatment
were noted (Esaki et al., 1973).
Chlorpyrifos-ethyl was administered to pregnant rats at 0, 50,
100 or 200 mg/kg body weight/day on days 6 through 15 of gestation.
Animals were killed on day 21 of gestation and foetuses removed by
caesarean section. No significant effects were observed in the number
of corpora lutea per pregnant female, the number of resorption sites
or the number of live foetuses. No dose-related effects were observed
in the incidence of runts, subcutaneous oedema and dilated renal
pelvis. Evidence of delayed ossification of sternabrae was observed at
all treatment levels with an increase in lumbar spurs at the high dose
level. The administration of 200 mg chlorpyrifos-methyl/kg body weight
for 10 days caused a significant decrease in plasma and red blood cell
cholinesterase activity in maternal blood as well as in a homogenate
of foetal tissues (Schwetz et al. 1973;Schwetz, 1974a, b).
Acute toxicity
LD50
Species Sex Route Solvent (mg/kg) Reference
Rat M oral CMC 3 733 Hasegawa at al., 1973a
F oral CMC 3 597 "
M oral corn oil 2 472 "
F oral corn oil 1 828 "
M&F oral corn oil 1 700 Davis and Collins, 1975
M oral corn oil 2 140 Litchfield and Norris, 1969
F oral corn oil 1 090 "
M oral corn oil 2 140 Olson, 1964a
F oral corn oil 1 630 "
F oral corn oil >1 000 Olson et al., 1963
F oral corn oil 3 600 Esaki et al., 1973
M&F dermal CMC >4 827 Hasegawa et al., 1973a
M&F dermal corn oil >3 713 "
LD50
Species Sex Route Solvent (mg/kg) Reference
Mouse M oral corn oil 2 254 "
F oral corn oil 2 032 "
M oral arachis oil 1 122 WHO, 1966
F oral corn oil 2 440 Esaki et al., 1973
M&F dermal CMC >2 856 Hasegawa at al., 1973a
Cavy M oral corn oil 2 250 Olson, 1964a
Rabbit M&F oral corn oil approx.
2 000 "
Chicks M oral corn oil >7 950 Olsen, 1964a
(capsule)
Chicken F oral corn oil 4 860 Ross et al., 1975
(capsule)
M&F oral corn oil 7 532 Ross and Roberts, 1974
(gavage)
oral corn oil 8 000 "
(capsule)
Undiluted chlorpyrifos-methyl was applied directly to
conjunctival sac of rabbits. There were signs of conjunctival
irritation which subsided after 24-48 hours. No corneal injury was
discernible (Olson and Taylor, 1964b).
No significant skin reaction occurred when undiluted
chlorpyrifos-methyl was applied to shaved and abraded skin of the
rabbit for prolonged periods (Olson and Taylor, 1964b; United States
Army, 1973).
Cholinesterase activity was reduced by 90% and all animals died
within 24 hours when rats were treated orally with chlorpyrifos-methyl
(4 g/kg) and subcutaneously with various levels of atropine sulfate.
Rats were treated with a sublethal oral dose of chlorpyrifos-methyl in
conjunction with subcutaneous injections of atropine sulfate and PAM
(25, 50 and 100 mg/kg) and glutathione (100, 300, 500 and 700 mg/kg)
or combinations of each. Symptoms of anticholinesterase activity were
observed and total blood, erythrocyte, plasma and brain cholinesterase
levels determined. The antidotes were effective especially at the
upper dose levels in reducing the toxic symptoms. The combination of
atropine sulfate with PAM or glutathione was more effective than
atropine sulfate alone (Hayashi et al., 1973).
Chlorpyrifos-methyl was administered to groups of rats at 5 x
LD50 (8.5 g/kg). At first signs of anticholinesterase activity one
group was given atropine sulfate (17.5 mg/kg) intramuscularly and a
further group atropine sulfate (17.5 mg/kg) and PAM (50 mg/kg)
intramuscularly. A further administration of antidote was given to all
rats seven hours after treatment. All treated rats died within 24
hours, untreated controls died within eight hours (Davies and Collins,
1975).
Short-term studies
Avian species
Mallard ducks, bobwhite and Japanese quail were fed various
levels of chlorpyrifos-methyl for five days and observed for a further
three days. The following LC50 values were determined.
Bobwhite quail - 1835 ppm, Japanese quail ->5000 ppm, mallard
duck - 2500-5000 ppm. Body weight and food consumption were reduced
for bobwhite and Japanese quail at 2500 and 1250 ppm respectively and
at 5000 ppm for the mallard duck. In a similar study, mallard ducks
were fed graded levels of chlorpyrifos-methyl for five days. Whole
blood cholinesterase activity was inhibited at dietary levels of 78
ppm and greater. Brain cholinesterase was not affected at the highest
level tested (Shellenberger, 1970).
Mouse
Chlorpyrifos-methyl was administered orally to mice at 0, 100,
250, 500 and 1000 mg/kg body weight/day for 14 days. No effect on
mortality or significant changes in body weight at 100 and 250 mg/kg
were observed. After four doses, there was 100% mortality at 1000
mg/kg. At 500 mg/kg, 40% mortality occurred in 14 days and a
noticeable decrease in body weight (Esaki et al., 1973).
Groups of male and female mice were fed standard diets containing
0, 0.5, 1, 5, 10, 20, 30, 40 and 100 ppm for a period of six months.
No differences were observed in mortality, food intake, body weight
gain, organ weights and urinalysis. Haematological, biochemical and
pathohistological parameters were considered normal in groups given
less than 40 ppm. Plasma and erythrocyte cholinesterase activities
were lower at dosage levels greater than 20 ppm. Brain cholinesterase
was decreased in males given 40 and 100 ppm and in females given 40
ppm (Hasegawa et al., 1973c).
Rat
Chlorpyrifos-methyl was administered orally to rats at 20, 100
and 500 mg/kg body weight/day for 14 days. No significant effect on
mortality or body weight gain was shown. At 500 mg/kg hypertrophy of
the liver and heart were observed (Esaki et al., 1973).
Groups of female rats were fed chlorpyrifos-methyl in the diet
for 14 days at 0, 1.6, 6, 25, 95, 500 and 2130 mg/kg body weight/day.
Plasma and red blood cell cholinesterase was depressed at 6 mg/kg and
in brain at 95 mg/kg. Organ to body weight ratios of liver, kidney and
spleen were not significantly different from control but brain to body
weight ratio was increased at 2130 mg/kg. No compound-related gross or
histopathological lesions were observed (United States Army, 1973).
Groups of six male and female rats consumed in their diet 0,
0.08, 0.8, 8.0, 40, 80 and 160 mg chlorpyrifos-methyl/kg body
weight/day for 90 days. Plasma cholinesterase activity was depressed
in males at 8 mg/kg and above and in females at 0.8 mg/kg and above.
The incidence of erythrocyte and brain cholinesterase depression was
significantly increased at 8.0 mg/kg/day and above for both male and
female. Liver weight to body weight ratio of male rats was increased
at 40, 80 and 160 mg/kg. Apart from small aggregates of lymphocytes
and plasma cells in the periportal areas of the liver, no changes were
noted in gross or histopathology (Steinberg, 1971).
Chlorpyrifos-methyl was fed at dietary levels of 0, 0.5, 1, 5,
10, 20, 30, 40 and 100 ppm to groups of male and female Wistar rats
for six months. No significant differences were noted in mortality,
food intake, weight gain, organ weights and urinalysis.
Haematological, biochemical and pathological findings were not
affected at 40 ppm or less. Serum and erythrocyte cholinesterase
activity was decreased at 30 ppm and above at one, three and six
months (Hasegawa et al., 1973b).
Groups of five male and five female rats received 0, 0.2, 1.0 and
5.0 mg chlorpyrifos-methyl/kg body weight/day by stomach tube daily,
six days/week, for six weeks. Plasma cholinesterase activity was
depressed in females at 5 mg/kg. Erythrocyte and brain cholinesterase
activities were not significantly reduced. Growth, haematology, blood
chemistry and liver microsomal mixed function oxidase activities were
not affected (Coulston and Griffin, 1975).
Dog
Groups of dogs (two males and two females/group) were fed
chlorpyrifos-methyl in the diet for 92 days at dose levels of 0, 0.3,
1, 3 and 10 mg/kg body weight/day. No treatment-related effects were
observed in behaviour, food consumption, body weight, hematology,
clinical chemistry, urinalysis, organ weight: body weight ratios,
gross and histopathology. Plasma cholinesterase activity at all test
levels was depressed after one, four and 12 weeks of treatment.
Inhibition of erythrocyte cholinesterase activity was evident at 1.0,
3.0 and 10.0 mg/kg body weight/day. The activity of brain
cholinesterase was not affected (Sparschu et al., 1971).
A supplemental study was conducted in which groups of dogs (two
males and two females/group) were fed chlorpyrifos-methyl in the diet
at dose levels of 0, 0.03 and 0.1 mg/kg body weight/day for 125 days.
There was no significant depression of plasma or erythrocyte
cholinesterase activity. Behaviour was normal and body weight and food
consumption considered to be within normal limits (Humiston et al.,
1972).
Groups of dogs (seven males and seven females/group) were fed
chlorpyrifos-methyl at dietary levels of 0, 0.03, 0.1, 1.0 and 3.0
mg/kg/day for 104 weeks with an interim sacrifice of three males and
three females/group at 26 weeks. No significant effects were observed
with respect to mortality, behaviour, food and water consumption,
ophthalmology, haematology, blood biochemistry, organ to body weight
ratio or gross and histopathology at either 26 or 104 weeks. Treated
animals gained less weight than controls due to some weight loss
during the latter part of the study. Body weights, however, were
considered within normal limits. Cholinesterase activity was depressed
in red blood cell and plasma at 3.0 mg/kg and in plasma at 1.0 mg/kg.
There was no reduction in brain cholinesterase activity at 26 or 104
weeks (Rivett et al., 1974).
Monkey
Chlorpyrifos-methyl was administered by stomach tube daily, six
days/week for six months to five groups of rhesus monkeys, three male
and three female group at dose levels of 0, 0.1, 0.2, 1.0 and 5.0
mg/kg body weight/day. Plasma cholinesterase was depressed in those
animals receiving 1.0 and 5.0 mg/kg. Inhibition of erythrocyte
cholinesterase was evident at 5.0 mg/kg. Brain cholinesterase was not
affected at any test level. No treatment related effects were noted in
growth, haematology, blood chemistry, mixed function oxidase activity
of the liver, relative organ weights, gross or histopathology
(Coulston and Griffin, 1975).
Cow
Groups of four cows were fed for 42 days silage corn which had
been sprayed 83 days previously with chlorpyrifos-methyl. The diets
contained 0, 0.35, 0.87 and 1.85 ppm chlorpyrifos-methyl and 0, 0.44,
0.79 and 1.75 ppm 3,5,6 trichloro-2-pyridinol. Residue intakes
amounted to 0.009, 0.022 and 0.054 mg chlorpyrifos-methyl and 0.012,
0.020 and 0.051 mg 3,5,6 trichloro-2-pyridinol/kg body weight. No
adverse effects were observed in silage intake, milk production, blood
cholinesterase activity or body weight gains. Urine, faeces and milk
contained trace amounts of chlorpyrifos-methyl and 3,5,6
trichloro-2-pyridinol. One week after treated feed withdrawn these
residue were not detectable (Johnson et al., 1974).
Long-term studies
Rat
Groups of 55 male and 55 female rats were administered
chlorpyrifos-methyl in the diet at 0, 0.03, 0.1, 1.0 and 3.0 mg/kg
body weight/day for 104 weeks. After five weeks of treatment five
males and five female/group were sacrificed for cholinesterase
determination. Twenty-six weeks after treatment 10 males and 10
females/group were killed for interim study and the size of each group
was reduced to 30 males and 30 females after 52 weeks. Red blood cell
cholinesterase activity was depressed in both sexes at 1.0 and 3.0
mg/kg/day. Plasma cholinesterase activity was reduced consistently in
females at 1.0 and 3.0 mg/kg/day; males showing little effect during
the first year after which it was significantly depressed at 3.0
mg/kg/day. Brain cholinesterase levels were not affected.
Chlorpyrifos-methyl produced no significant effect on behaviour,
mortality, body weight gain, food consumption and efficiency of food
utilization. Haematologic, blood biochemical and urinalysis
determinations were within normal limits and revealed no abnormal
changes. No effect attributable to treatment was observed in organ
weights or on gross and histopathologic examination. Tumour incidence
was comparable between groups (Hunter et al., 1974a).
Observations in man
Fourteen male volunteers were divided into two treatment groups
of five men each and a control group of four men. Chlorpyrifos-methyl
was administered by gelatin capsule in a single daily dose of 0, 0.03
and 0.1 mg/kg body weight/day for four weeks. Plasma and erythrocyte
cholinesterase activities were not depressed at levels tested.
Haematology, blood chemistry, urinalysis, blood pressure, pulse and
ophthalmology were not affected by treatment (Coulston et al., 1975).
COMMENTS
In mammals, chlorpyrifos-methyl is rapidly absorbed and
metabolized the principal metabolite being
3,5,6-trichloro-2-pyridinol. The parent compound and metabolite are
excreted primarily in the urine and faeces and are not stored to any
extent in the body. This metabolite has also been shown to occur in
plants.
Teratogenic studies in the rat revealed no adverse effects at
dietary intake levels of up to 250 mg/kg. In an inadequately conducted
and reported study in the mouse there appeared to be a tendency for a
weak teratogenic response at 250 mg/kg. No compound-related
abnormalities were noted at 100 mg/kg.
No adverse effects were noted in the reproductive capacity of the
rat over three generations at dietary levels of up to 3 mg/kg.
Mutagenic studies have not been reported.
No apparent clinical evidence of neurotoxicity was observed in
hens given a large dose of chlorpyrifos-methyl. Histological
examination of nervous tissue was not conducted. In various short- and
long-term studies in the rat, dog, mouse, monkey, mallard duck,
bobwhite and Japanese quail, chlorpyrifos-methyl was shown to be an
active cholinesterase inhibitor. Plasma cholinesterase activity was
inhibited to a greater extent than either erythrocyte or brain
cholinesterase.
In the long-term study and specific tumorigenic study in the rat,
no compound-related effect or increase in tumour incidence was
observed.
No effect levels were demonstrated in the rat, dog, monkey and
man on the basis of the most sensitive parameter plasma cholinesterase
inhibition. The no-effect level in man was confirmed by two
independent studies. The data were considered sufficient to allocate
an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg
Dog: 0.1 mg/kg
Monkey: 0.2 mg/kg
Man: 0.1 mg/kg
Estimation of acceptable daily intake
0-0.01 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of chlorpyrifos-methyl for insect control in fruit,
vegetable and cereal plants and in grain storage is recorded from the
following countries: Egypt, France, Japan, Korea, and Turkey. In these
countries it is marketed as dusts and/or granules and as emulsifiable
concentrates containing 2-3% a.i. and 25-40% a.i. respectively.
As a relatively new chemical chlorpyrifos-methyl is marketed as a
broad-spectrum organophosphorus insecticide of relatively low toxicity
and persistence, although it shows reasonably good stability in
stored, dry products such as grains (Schulten, 1973) and dried fruits
(Soderstrom and Armstrong, 1974). In these products it controls a wide
range of beetles, weevils, moths and mites, including several such
species which may have developed resistance towards other
insecticides, e.g. malathion. Using spray techniques or admixing
procedures to treat the stored grain products, chlorpyrifos-methyl is
usually applied at the rate of 5-10 mg a.i. per kg.
In several of the countries mentioned, chlorpyrifos-methyl is
approved for wider uses in agriculture and horticulture against pests
of economic importance connected to fruit and vegetable growing
(Nakayama et al., 1973). In laboratory tests it is further found to be
effective against a wide range of household pests, including spiders,
houseflies, cockroaches, house crickets etc, (Snetsinger, 1972).
A number of recommended practical applications is listed in Table
1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on chlorpyrifos-methyl residues resulting from a number of
the above uses have been presented by the originating company (Kenaga,
1975). The data derived from field trials and controlled experimental
work under a variety of geographical and climatic conditions is as
follows.
In plants
Cereal plants and grass
Leuck et al. (1975) found chlorpyrifos-methyl to be relatively
non-persistent in field trials where coastal bermuda grass and corn
were sprayed once with 0.56, 1.12 and 2.24 kg a.i. per ha (see Table
2). In both crops and at all three levels chlorpyrifos-methyl
diminished rapidly at rates corresponding to half-lives of about two
to three days. The hydrolysis product, 3,5,6-trichloro-2-pyridinol,
was more persistent and in some cases actually increased up to a
maximum during the first few days after application. Thereafter the
pyridinol disappeared following a first order scheme with estimated
half-life values of 9-12 days.
TABLE 1. Some use patterns of chlorpyrifos-methyl on plants and grain
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Australia Emulsifiable concentrate Wheat 5.0 mg/kg
Egypt Emuls. conc., 24% (w/v) Spodoptera littoralis and 0.9 kg/ha or Foliar broadcast spray
S. exigua on vegetables 90 g/litre
and clover
France Emuls. conc., 24% (w/v) Stored grain pests 2.5 mg/kg
Japan Dust, 2% (w/w) Rice stemborer 0.6-0.8 kg/ha Max. 4 applic. per season
Micro-granule, 3% (w/w) Rice stemborer 0.9-1.2 kg/ha Broadcast appl. Max. 4 x
per season
Granule, 5% (w/w) Rice stemborer 1.5-2.0 kg/ha Submerged appl. Max. 4 x
per season
Emuls. conc., 25% (w/v) Rice stemborer 0.1-0.375 kg/ha Max. 4 sprays/season:
rice and cabbage
Diamondback moth, Pieria
rapae, aphids on tobacco Max. 2 sprays/season:
Cutworm on cabbage, chinese cabbage and
chinese cabbage, radish radish
Korea Granule, 3% (w/w) Rice stemborer, plant 0.9-1.2 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice.
Tobacco cutworm, mole
cricket on tobacco and
vegetables
TABLE 1. (Cont'd)
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Korea Emuls. conc., 25% (w/v) Rice stemborer, plant 0.25-0.35 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice
Tobacco cutworm, mole
cricket on tobacco and
vegetables
Sudan Emuls. conc., 24% (w/v) Pests of apricots
Turkey Emuls. conc., 24% (w/v) Spodoptera litteralis and 0.9 kg/ha Foliar broadcast spray
Heliothis obsoleta in
cotton and vegetables
Aphids on melons
USA Emuls. conc. Wheat 5-10 mg/kg
Johnson et al. (1974) in an earlier experiment sprayed corn in
the dent stage with chlorpyrifos-methyl at the same dosage rates. In
this case, the corn was harvested and ensiled for forage purposes one
day after the treatment (Table 2). The initial losses of
chlorpyrifos-methyl from the time of application through harvesting
and ensiling amounted to 62-79%. After the ensiling, however, residues
became relatively stable and they disappeared only slowly with
formation of the pyridinol. From the first day until the eighty-third
day, on average for the three treatments, 60% of the
chlorpyrifos-methyl disappeared while the pyridinol increased 421%.
TABLE 2. Residues of chlorpyrifos-methyl and pyridinol in grass and corn
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Bermudagrass, 0.56 0 5.5 1.07
coastal 7 0.1 0.73
(Leuck et al., 1975) 21 <0.01 0.32
1.12 0 12.5 2.21
7 0.21 1.81
21 0.01 0.58
2.24 0 28.4 4.48
7 0.45 4.08
21 0.09 1.24
Corn, growing 0.56 0 2.2 0.11
(Leuck et al., 1975) 7 0.11 0.17
21 0.03 0.09
1.12 0 8.2 0.41
7 0.29 0.54
21 0.07 0.21
2.24 0 20.4 0.78
7 1.43 1.38
21 0.20 0.60
TABLE 2. (Cont'd.)
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Forage corn, 0.56 0 2.22 0.11
ensiled at day 1 1 0.75 0.13
(Johnson et al., 27 0.51 0.48
1974) 55 0.47 0.49
83 0.34 0.70
125 0.34 0.27
1.12 0 8.20 0.41
1 1.92 0.34
27 1.42 1.09
55 1.27 1.40
83 0.72 1.74
125 0.77 0.35
2.24 0 20.40 0.78
1 4.08 0.69
27 2.98 2.50
55 2.31 2.28
83 1.64 3.34
125 1.80 1.13
Kubota (1975) has reported on field trials with growing rice. As
shown in Table 3, residues at the time of harvest were not detectable
or at most insignificant when spraying was carried out 4-17 days
before harvest.
Stored grain products
White and LaHue (1974), in their experiments showing effective
protection against several grain insects, e.g. rice weevil, lesser
grain borers, flour beetle, sawtooth grain beetle, and foreign grain
beetle, also followed the dissipation of chlorpyrifos-methyl in stored
wheat, sorghum and maize. Wheat at 79°C and containing 14.6% moisture
was treated with 4.8, 6.6 and 10.4 mg/kg chlorpyrifos-methyl. The
residue half-life was >6 months, 2-3 months and <1 month,
respectively. Maize and sorghum tested under similar conditions at
about 6-7 mg/kg had a residue half-life of about 2-3 months (Table 4).
TABLE 3. Residues of Chlorpyrifos-methyl in rice*
Application
Rate Days after Number of
Formulation (kg/ha) Frequency last treatment samples Residues, mg/kg
3% dust 1.2 2 × 35-42 8 <0.005-<0.005(n.d.)
4 × 14-42 8 <0.005-<0.005(n.d.)
6 × 11 8 <0.005-<0.005(n.d.)
5% granule 2 2 × 53-57 8 <0.005-<0.005(n.d.)
4 × 53-57 8 <0.005-<0.005(n.d.)
6 × 17-32 8 <0.005-0.012
25% e.c. 0.30-0.375 2 × 32-53 8 <0.005-<0.005(n.d.)
4 × 24-53 8 <0.005-<0.005(n.d.)
6 × 4-32 8 0.005-0.013
* From Kubota (1975).
TABLE 4. Residues of chlorpyrifos-methyl in stored grains in the United States of America*
Residue, mg/kg
Grain 0 days 5 days 1 2 3 4 5 6 8 9 months
Wheat (26°C, 4.8 4.6 3.5 3.0 2.8 3.3 - 3.3 - -
14.6% moisture) 6.6 5.5 5.0 2.8 3.2 - - 1.6 - -
10.4 6.2 4.1 2.6 2.3 - - 4.4 - -
Corn
(13.6% moisture) 6.70 - - - - 3.2 2.5 2.3 1.9 1.8
Sorghum - - - - 3.5 3.3 2.7 2.6 2.2 1.9
* From White and LaHue (1974).
In laboratory experiments, Bulla and LaHue (1975) tested the
disappearance rates of chlorpyrifos-methyl on the same three grains,
kept for six months at 80°F in covered glass jars with regulated
moisture contents from 10 to 16%. With overall half-life rates varying
from one to six months they found a decreasing persistence in the
sequence: maize > wheat > sorghum. Grains containing 14 or 16%
moisture degraded chlorpyrifos-methyl about 1.5-2 times faster than
those containing 10 or 12%.
In similar Australian experiments under commercial conditions,
Deahl and Tucker (1974) found that chlorpyrifos-methyl at initial
concentrations of 5.0 and 6.2 mg/kg declined at rates corresponding to
half-lives of the order of three to five months (see Table 5). The
average half-life at 31°C was 15-16 weeks with indications of a
reduced breakdown at lower temperatures (Desmarchelier, 1975a).
From these extensive trials, Desmarchelier et al. (1975b and c)
also reported the rate of loss of residues of chlorpyrifos-methyl on
white wheat containing 11% moisture at 25°C when stored in glass jars.
These tests indicate that under the laboratory conditions stated,
chlorpyrifos-methyl decays at a predictable first-order rate,
irrespective of concentration or age of the residues.
Morel and Galet (1975) report tests conducted in France on wheat
containing 15.2% moisture and 2.35 and 1.17 mg/kg chlorpyrifos-methyl
which was stored for various periods of time at 25°C for residue
analyses. Under these conditions half-lives of chlorpyrifos-methyl
were estimated to be about six weeks. Residue levels six months after
treatment were in the range of 0.2-0.4 mg/kg.
Apples and peaches
Residue levels on apples and peaches have been established under
Japanese conditions following spraying at recommended dosage rates,
i.e. about 1-1.5 kg/ha. The results of these trials are shown in Table
6 which indicates levels in apples below 0.3-0.5 mg/kg if harvested
about two weeks after the last treatment. The results for peaches do
not show signs of penetration of residues through the peel into the
fruit pulp.
Vegetable plants
Residue data from experiments with chlorpyrifos-methyl
applications on vegetable plants are shown in Tables 7 and 8. The
experiments derive from different geographical regions and cover
mostly foliar applications on the following crops: artichoke, beans,
cabbage, chinese cabbage, eggplant, lettuce, pepper, radish, tea and
tomatoes.
TABLE 5. Residues of chlorpyrifos-methyl during silo-storage, Australia*
Residue, mg/kg after interval (weeks)
Depth of
Treatment silo 1 6 11 16 22 26
6.2 mg/kg 0.1 m 4.5 3.1 2.3 2.1 1.9 1.5
(Queensland 1.5 m 3.4 3.6 2.1 2.3 1.8 1.5
experiment) 6.0 m 3.2 3.0 2.4 2.2 2.0 -
Average 3.7 3.2 2.3 2.2 1.9 1.5
3 8 13
5.0 mg/kg 0.1 m 4.1 3.9 1.8
(N.S. Wales 1.5 m 4.1 3.7 2.4
experiment) 6.0 m 3.3 3.1 2.4
Average 3.8 3.6 2.2
* From Deahl and Tucker (1974).
TABLE 6. Residues of chlorpyrifos-methyl in apples and peaches, Japan*
Preharvest Number of Number of Range of
Application interval applications samples results (mg/kg)
Apples
1.25-1.5 10 days 1-2 × 6 0.22-0.353
kg/ha 20 days 1-2 × 7 0.201-0.310
30 days 1-2 × 4 0.174-0.186
10 days 4 × 9 0.182-0.798
20 days 4 × 6 0.128-0.225
30 days 4 × 4 0.162-0.257
10 days 6 × 4 0.489-0.936
20 days 6 × 7 0.152-0.333
30 days 6 × 4 0.250-0.478
Peaches Peel Pulp
0.9 kg/ha 21 days 2 × 6 <0.03-0.26 <0.005
30 days 2 × 8 <0.03-0.05 <0.005
21 days 4 × 6 <0.03-0.11 <0.005
30 days 4 × 8 <0.03-0.05 <0.005
21 days 6 × 6 <0.03-0.10 <0.005
30 days 6 × 8 <0.03-0.06 <0.005
* From Kubota (1975).
TABLE 7. Residues of chlorpyrifos-methyl in vegetables, Europe/Near East
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Artichoke 48 g/100 l 7 days 2 0.05 France1
(aver.)
14 days 2 0.03
(aver.)
Beans 1.2 kg/ha 0 days 1 0.32 Turkey2
7 days 1 0.01
14 days 1 0.02
Beans, 48 g/100 l 7 days 5 <0.02 France1
french (aver.)
14 days 3 <0.02
(aver.)
Eggplant 1.2 kg/ha 0 days 1 0.06 Turkey2
7 days 1 0.01
14 days 1 0.01
Lettuce 48 g/100 l 7 days 4 0.78 France1
(aver.)
14 days 2 0.04
(aver.)
Pepper 1.2 kg/ha 0 days 1 2.28 Egypt3
1 day 1 0.58
2 days 1 0.25
3 days 1 0.20
5 days 1 0.09
7 days 1 0.10
9 days 1 0.04
TABLE 7. (continued)
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Tomato 0.1% until 3 days 1 1.90 England4
run-off 6 days 1 0.90
(greenhouse) 9 days 1 0.40
13 days 1 0.09
1.2 kg/ha 0 days 1 0.31 Turkey2
7 days 1 0.04
14 days 1 0.05
48 g/100 l 7 days 6 0.21 France1
(aver.)
14 days 6 0.04
(aver.)
1 Hascouet (1974).
2 Hollick and Collison (1972a).
3 Hollick and Collison (1972b).
4 Wirth et al. (1971).
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Cabbage 0.45 kg/ha 7 days 2 × 8 <0.002-0.002
14 days 2 × 8 <0.002
7 days 4 × 8 <0.002
14 days 4 × 8 <0.002
Chinese 0.25 kg/ha 14 days 4 × 2 <0.005
cabbage
0.375 kg/ha 7 days 2 × 7 0.027-0.220
14 days 2 × 7 <0.005-0.100
7 days 4 × 7 0.036-0.175
14 days 4 × 5 0.039-0.056
0.50 kg/ha 7 days 2 × 4 0.019-0.025
14 days 2 × 4 0.004-0.006
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Chinese 7 days 4 × 4 0.001-0.002
cabbage 14 days 4 × 4 0.003-0.007
Eggplant 0.75 kg/ha 3 days 2 × 4 0.002-0.003
7 days 2 × 4 0.001
14 days 2 × 4 <0.002
3 days 4 × 4 0.002-0.004
7 days 4 × 4 0.001
14 days 4 × 4 <0.002
Pepper, 0.50 kg/ha 3 days 2 × 4 0.011-0.171
green 7 days 2 × 4 0.004-0.028
14 days 2 × 4 <0.002-0.012
Pepper, 3 days 4 × 4 0.013-0.129
green 7 days 4 × 4 0.003-0.060
(cont'd) 14 days 4 × 4 <0.003-0.021
Leaves Root
Radish 0.2-0.3 kg/ha 7 days 2 × 4 0.024-0.324 <0.002-0.069
28 days 2 × 4 0.003-0.009 0.005-0.008
7 days 3 × 2 0.109 <0.001-0.003
28 days 3 × 4 0.024-0.130 <0.001-0.001
7 days 4 × 4 0.040-0.299 <0.002-0.081
28 days 4 × 4 0.003-0.010 0.013-0.020
7 days 6 × 2 0.075 <0.002-0.002
14 days 6 × 4 0.019-0.040 0.002-0.003
Tea 0.50 kg/ha 7 days 1 × 6 <0.001-0.065
14 days 1 × 6 <0.001-0.047
21 days 1 × 6 <0.003
27 days 1 × 6 <0.003
7 days 2 × 6 <0.001-0.026
0.75 kg/ha 7 days 1 × 7 <0.001-0.091
14 days 1 × 6 <0.001-0.056
21 days 1 × 6 <0.001-0.047
7 days 2 × 6 <0.003-0.085
* From Kubota (1975).
The levels of chlorpyrifos-methyl residues on these crops at the
time of harvest, i.e. from 7 to 14 days after the last treatment are
generally at or below 0.1 mg/kg.
FATE OF RESIDUES
In plants
Residues of chlorpyrifos-methyl in or on plants are assumed to
behave similarly to chlorpyrifos Of FAD/WHO, 1973) without
translocation in plants to any significant extent.
The principal hydrolysis product of chlorpyrifos-methyl in
plants, animals and soil, 3,5,6-trichloro-2-pyridinol, may or may not
be taken up by plants depending on pH. The free pyridinol, which is
the predominant form of the compound below pH 6.0, is essentially
insoluble in water. In radio-labelled studies, soil and nutrient
culture experiments have shown its uptake by plants to be
insignificant (Smith et al., 1967). At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the sodium salt) as well as rate of absorption by the
plant are enhanced compared with the free pyridinol. Although still at
very low levels, the sodium salt of the pyridinol enters the plant
more than five times as fast as the free pyridinol. It undergoes
metabolism with the liberation of chloride and the formation of
several unidentified water soluble decomposition products.
In cereal-products
Residues of chlorpyrifos-methyl in treated grains have been
followed from the time of application through milling and baking
procedures in several experiments (see Table 9). Bulla and LaHue
(1975) fractionated wheat containing 6.3 mg/kg chlorpyrifos-methyl and
found that most of the residues after three and six months of "ageing"
were still concentrated in the outer grain fractions, such as "red
dog" and bran. The residues in flour were reduced to below 10% of the
original concentration (i.e. to 0.53 and 0.39 mg/kg, respectively).
Bread baking reduced the remaining residues further by about 60%.
Similar results have been found by Bengtson et al. (1975) who
after 11 and 22 weeks of ageing also found considerable reduction from
grain residues to milled flour and baked bread, the latter showing
below 0.25 mg/kg residual chlorpyrifos-methyl.
Morel and Galet (1975) analysed wheat which was milled from 6 to
17 weeks after treatment of commercially stored grains with 1.6-1.8
mg/kg of chlorpyrifos-methyl. The distribution of residues in various
milled fractions is shown in Table 9. About 90% of the residues
remained in the outer bran and course middlings.
TABLE 9. Chlorpyrifos-methyl residues in milled wheat fractions and bread
Residues, mg/kg, in
Bread
Ageing Pollard
period Whole wheat "Red dog" Bran (or middling) Flour Wholemeal White
USA (Bulla and LaHue, 1975)
3 months 6.3 mg/kg* 6.5 5.0 0.53 0.21
6 months 6.3 mg/kg* 3.3 3.0 0.39 0.16
France (Morel and Gallet, 1975)
6 weeks 4.3 1.4 0.26
9 weeks 1.6-1.8 mg/kg* 2.96 1.46 0.22
14 weeks 3.92 1.60 0.29
17 weeks 3.55 1.61 0.23
Australia (Bengtson et al., 1975 and Desmarchelier, 1975a)
6 weeks 6.7-6.9 mg/kg - 7.6-8.8 2.6-3.2 0.38-0.38 1.11-1.17 0.25-0.28
11 weeks 3.6 mg/kg - 9.3 9.0 0.6 - 0.2
11 weeks 2.0 mg/kg - 5.2 4.6 <0.10 - <0.12
22 weeks 1.8 mg/kg - 6.6 3.6 0.4 - 0.13
* Treatment levels.
In animals and animal products
Insects
Although direct studies have not been carried out with
chlorpyrifos-methyl to the same extent as for its ethyl analogue,
chlorpyrifos (cf. FAO/WHO, 1973), there is evidence that the general
metabolic pathways in living organisms are closely parallel for the
two compounds, e.g. the principal metabolic products in some insects
(tobacco budworm, cf. Whitten and Bull, 1974) are
3,5,6-trichloro-2-pyridinol mad O,O-dimethyl phosphate produced by
microsomal oxidase activity which apparently is indicated also
catalyses the formation of the P=O analogue of chlorpyrifos-methyl.
Soluble, non-oxidative hydrolytic enzyme systems are likewise
believed to act in detoxification mechanisms through O-dealkylation
(see Figure 1).
Absorption studies in these insects suggest that the slightly
higher toxicity of chlorpyrifos-methyl compared to chlorpyrifos is
probably connected with a faster rate of absorption of the former.
Cow
In their previously mentioned studies of chlorpyrifos-methyl in
ensiled forage corn, Johnson et al. (1974) fed silage corn containing
0.35, 0.87 and 1.87 mg/kg chlorpyrifos-methyl and 0.44, 0.79 and 1.75
mg/kg 3,5,6-trichloro-2-pyridinol to cows for 42 days. This dietary
residue intake was equivalent to an average of 0.09, 0.022 and 0.054
mg chlorpyrifos-methyl and 0.012, 0.20 and 0.051 mg of the pyridinol
per kg of body weight per day, respectively.
Residue analyses showed no trace of the P=O analogue of
chlorpyrifos-methyl in any samples including milk, urine and faeces.,
The principal excretory route for chlorpyrifos-methyl was by means of
the faeces. Only at the 0.054 mg/kg day dosage did a trace of
chlorpyrifos-methyl (0.001-0.002 mg/kg) appear in the milk with none
in the urine. The principal excretory route for the pyridinol was via
the urine. Some excretion of the pyridinol occurred also in the faeces
(up to 0.36 mg/kg and in the milk (up to 0.008 mg/kg) at the 0.054
mg/kg/day rate. These studies did not include analyses of animal meat
or fat.
In soils
Regoli et al. (1974) studied the aerobic degradation of
ring-labelled C14-chlorpyrifos-methyl in the laboratory at 1 mg/kg in
two soils (silty clay loam and loam containing 4.2% and 0.8% organic
carbon respectively) incubated at 15°, 25° and 35°C and with moisture
at 32% or 100% of field capacity for up to 428 days. All incubations
were conducted in closed, aerated containers so that a balance of
radioactivity added to each sample could be drawn.
Other information on identity and properties
(Brust, 1969; Kenaga, 1971)
Molecular weight : 322.6
State : Granular crystalline solid
Colour and odour : White/mild mercaptan
Melting point : 45.5-46.5°C
Vapour pressure : 7.40 × 10-7mm Hg at 0°C
4.22 × 10-5mm Hg at 25°C
1.8 × 10-4mm Hg at 35°C
Solubility (g/100g : Acetone 640
at 23°C)
Acetonitrile 680
Benzene 520
Carbon disulfide 430
Carbon tetrachloride 280
Chloroform 330
Diethyl ether 480
Ethanol 30
Methanol 30
n-Octanol 20
Hexane 23
Water 0.0004
The compound readily dissolves in
dimethylformamide, N-methylpyrrolidine,
tetrahydrofuran, xylene, methylene
chloride and 1,1,1-trichloroethane.
Stability : Chlorpyrifos-methyl is reported to be
hydrolyzed by water, the rate being
dependent on temperature and pH.
Half-lives at 25°C vary from 22.7 to
9.4 days in tap water and buffered
distilled water within the Ph range
4.2-8.0.
This hydrolysis is enhanced by traces
of copper ions due to chelation
(Meikle, 1973). The major products of
hydrolysis are
3,5,6-trichloro-2-pyridinol and
O,O-dimethyl phosphate.
Photodecomposition of evaporated
chlorpyrifos-methyl in humid air is
also reported, with the formation of
3,5,6-trichloro-2-pyridinol,
hydroxylated pyridinol derivatives and
oxidation products of quinone
structures (Smith & Taylor, 1972).
Purity : The crystalline compound is stated to
be 99.8% ± 0.1%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 2,6 C14 ring labelled chlorpyrifos-methyl was administered
as a single oral dose (16 mg/kg) to rats, the radioactivity was
rapidly absorbed and excreted. After 72 hours, 90-93% of the
radioactivity was eliminated from the body, 83-85% in urine, 7-9% in
faeces and 0.23-0.43% in respired air. Maximum blood levels (2.4-3.7%)
were reached five hours after treatment. The maximum amount of the
dose remaining in the tissues by 72 hours was 0.65-1.3 ppm. Individual
tissue levels were low (less than 1 ppm). Urinary metabolites were
shown to be 3,5,6 trichloro-2-pyridinol and unidentified activity at
the origin by thin layer chromatography. The possibility of urinary
conjugates was not studied (Branson and Litchfield, 1971a).
The distribution of a single oral dose of C14 labelled
chlorpyrifos-methyl in the body of the young rat was investigated by
whole body autoradiographic techniques. Whole body autoradiograms were
taken at 0.5, 1, 3, 5, 7, 24, 72 and 120 hours after treatment. The
highest level was found initially in the blood. The dispersion in the
various tissues reached a peak at three hours. Residues were extremely
low in fat, liver, kidney and intra-intestinal faeces 72 hours after
administration and completely eliminated from the body by 120 hours
(Nakajima et al., 1974).
Biotransformation
The metabolism of 2,6 C14 ring labelled chlorpyrifos-methyl was
studied in sheep and rats using radioactive counting,
mass-spectroscopy, infrared spectroscopy, column, thin-layer and gas
liquid chromatography techniques. Two ewes were dosed orally with 100
mg/kg by gelatin capsule. Recoveries of radioactivity after four days
were 69.2% in urine, 12.5% in faeces and 2% in tissues. No C14O2 was
detected. Radioactivity in the urine was shown to be the glucuronide
of 3,5,6 trichloro-2-pyridinol 41.6%, o-methyl-o-trimethylsily-o-(3,
5,6 trichloro-2 -pyridyl) phosphorothionate 38% and three minor
unknown fractions 5.2%. In the faeces, 34% of the faecal radioactivity
consisted of parent compound and 55.3% made up primarily of
o-methyl-o-hydrogen-(3,5,6 trichloro-2-pyridyl) phosphorothionate and
3,5,6 trichloro-2-pyridinol. The three fractions found in the urine
were present in the plasma. Detectable amounts of radioactivity (0.32
to 11.8 ppm) were found in the tissues after 96 hours, the highest
level being found in visceral fat (Branson and Litchfield, 1971; Bakke
and Price, 1975).
Ten male rats were given a single oral dose (10 mg) of 2,6 C14
ring labelled chlorpyrifos-methyl. After 48 hours the radioactivity in
the urine consisted of three components, the glucuronide of 3,5,6
trichloro-2-pyridinol (68.6%), o-methyl-o-hydrogen-o (3,5,6
trichloro-2-pyridyl) phosphorothionate (17.8%) and 3,5,6
trichloro-2-pyridinol (13.8%). Faecal metabolites were not
characterized. No respiratory C14O2 was detected (Bakke and Price,
1975).
The principal metabolite of chlorpyrifos-methyl has been shown to
be 3,5,6-trichloro-2-pyridinol. The metabolism of this metabolite in
rats was summarized by FAO/WHO (1973) when the pesticide chlorpyrifos
was evaluated. It was rapidly absorbed and excreted primarily in the
urine and faeces. Small tissue residues (<0.3 ppm) were present and
occurred mainly in biological systems involved with urinary excretion,
i.e. liver, kidney and blood. Residues of the pyridinol in fat were
trivial, which is consistent with its much greater polarity (FAO/WHO,
1973; Smith et al., 1970).
In a similar study in sheep a single oral dose 2,6 C14 ring
labelled 2,5,6 trichloro-2-pyridinol was shown to be rapidly absorbed
and excreted in the urine and faeces. The only radioactive component
detected in the plasma and urine was the glucuronide of the pyridinol
and in faeces the unchanged pyridinol. No C14O2 was detected in
respired air. Residues in tissues after 96 hours were mainly in the
digestive and excretory tracts and was non-detectable in fat, muscle,
heart and other tissues (Bakke and Price, 1975).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
Chlorpyrifos-methyl was administered to groups of Sprague-Dawley
CD strain rats at dietary levels of 0, 0.1 and 1.0 mg/kg/day for 104
weeks. There were no overt signs of reaction to the test compound.
Body weight gain, food consumption, efficiency of food utilization
were comparable between groups and within normal limits. The changes
observed on gross and histopathological examination were those
consistent with the age and strain of rat employed. There was no
compound-related effect an the incidence of spontaneous tumours
(Hunter et al., 1974b).
Special studies on neurotoxicity
Chlorpyrifos-methyl was administered orally to adult hens at 4860
mg/kg body weight and were observed for 21 days. Survivors were given
an intramuscular injection of atropine (10 mg/kg) and PAM (50 mg/kg)
and redosed with 4455 mg/kg chlorpyrifos-methyl and observed for a
further 21 days. No signs of ataxia were detected. The birds were not
examined histologically for nervous systems lesions. TOCP was used as
a positive control (Ross et al., 1975).
Special studies on reproduction
Rat
In a three generation reproduction study (two litters per
generation) three groups of 10 male and 20 female rats received
chlorpyrifos-methyl at dietary levels of 0, 1.0 or 3.0 mg/kg body
weight/day. No treatment related effects on behaviour, survival, body
weight gain or food consumption were observed in parental animals.
Fertility, gestation, viability and lactation indices were comparable
to control values. Pup weights of the second litter, third generation
were significantly less than control at 0, 4 and 21 days postpartum.
There was no effect on sex ratio. In adults of third generation,
plasma cholinesterase activity was decreased in both sexes at both
dosage levels. Erythrocyte cholinesterase depression was observed at 1
mg/kg in females and in both sexes at the highest dose level. Brain
cholinesterase activity was not affected. Gross and histopathological
examination of control and high dose parental animals of the third
generation did not reveal any treatment related abnormalities
(Thompson et al., 1975).
Special studies on teratogenicity
Mouse
Chlorpyrifos-methyl was administered orally to mice at dosage
levels of 0, 20, 100 and 250 mg/kg body weight/day from day 7 through
day 13 of gestation. Animals were sacrificed on day 18 and pups
removed by caesarean section. No significant differences between the
control and treated groups were observed in regard to number of
implants and number of deaths. Body weights were lower in both males
and females at 250 mg/kg. An increased incidence of cleft palate and a
delay of ossification of the cervicovertebral body was observed at 250
mg/kg. When pregnant mice were dosed with a single oral dose of 1000
mg/kg on the seventh or eleventh day of pregnancy, there was no effect
on mortality or body weights of the foetuses. Skeletal abnormalities
were observed (exencephalia, cleft palate, liberation of bone fragment
of cervicovertabral arch) in a few cases especially on treatment on
seventh day of gestation. No control data was presented in the 1000
mg/kg body weight study. In a further study, pregnant mice were
treated as before with 0, 20, 100 and 250 mg chlorpyrifos-methyl/kg
body weight/day on day 7 through day 13 of gestation. Females were
allowed to deliver their pups. Body weights at birth and after three
weeks were dose dependent, being lower on the higher treatment. No
significant morphological abnormalities were detected (Esaki et al.,
1973).
Pregnant rats received 0, 20, 100 and 250 mg/kg body weight of
chlorpyrifos-methyl from day 9 to day 15 of gestation. On day 20
animals were laparotomized and embryos removed. No reproductive or
teratogenic effects were observed. In a similar study the pregnant
rats were allowed to deliver their progeny. Body weight at birth
tended to be higher in the treated groups but no differences observed
when weaned at four weeks. No teratogenic effects related to treatment
were noted (Esaki et al., 1973).
Chlorpyrifos-ethyl was administered to pregnant rats at 0, 50,
100 or 200 mg/kg body weight/day on days 6 through 15 of gestation.
Animals were killed on day 21 of gestation and foetuses removed by
caesarean section. No significant effects were observed in the number
of corpora lutea per pregnant female, the number of resorption sites
or the number of live foetuses. No dose-related effects were observed
in the incidence of runts, subcutaneous oedema and dilated renal
pelvis. Evidence of delayed ossification of sternabrae was observed at
all treatment levels with an increase in lumbar spurs at the high dose
level. The administration of 200 mg chlorpyrifos-methyl/kg body weight
for 10 days caused a significant decrease in plasma and red blood cell
cholinesterase activity in maternal blood as well as in a homogenate
of foetal tissues (Schwetz et al. 1973;Schwetz, 1974a, b).
Acute toxicity
LD50
Species Sex Route Solvent (mg/kg) Reference
Rat M oral CMC 3 733 Hasegawa at al., 1973a
F oral CMC 3 597 "
M oral corn oil 2 472 "
F oral corn oil 1 828 "
M&F oral corn oil 1 700 Davis and Collins, 1975
M oral corn oil 2 140 Litchfield and Norris, 1969
F oral corn oil 1 090 "
M oral corn oil 2 140 Olson, 1964a
F oral corn oil 1 630 "
F oral corn oil >1 000 Olson et al., 1963
F oral corn oil 3 600 Esaki et al., 1973
M&F dermal CMC >4 827 Hasegawa et al., 1973a
M&F dermal corn oil >3 713 "
LD50
Species Sex Route Solvent (mg/kg) Reference
Mouse M oral corn oil 2 254 "
F oral corn oil 2 032 "
M oral arachis oil 1 122 WHO, 1966
F oral corn oil 2 440 Esaki et al., 1973
M&F dermal CMC >2 856 Hasegawa at al., 1973a
Cavy M oral corn oil 2 250 Olson, 1964a
Rabbit M&F oral corn oil approx.
2 000 "
Chicks M oral corn oil >7 950 Olsen, 1964a
(capsule)
Chicken F oral corn oil 4 860 Ross et al., 1975
(capsule)
M&F oral corn oil 7 532 Ross and Roberts, 1974
(gavage)
oral corn oil 8 000 "
(capsule)
Undiluted chlorpyrifos-methyl was applied directly to
conjunctival sac of rabbits. There were signs of conjunctival
irritation which subsided after 24-48 hours. No corneal injury was
discernible (Olson and Taylor, 1964b).
No significant skin reaction occurred when undiluted
chlorpyrifos-methyl was applied to shaved and abraded skin of the
rabbit for prolonged periods (Olson and Taylor, 1964b; United States
Army, 1973).
Cholinesterase activity was reduced by 90% and all animals died
within 24 hours when rats were treated orally with chlorpyrifos-methyl
(4 g/kg) and subcutaneously with various levels of atropine sulfate.
Rats were treated with a sublethal oral dose of chlorpyrifos-methyl in
conjunction with subcutaneous injections of atropine sulfate and PAM
(25, 50 and 100 mg/kg) and glutathione (100, 300, 500 and 700 mg/kg)
or combinations of each. Symptoms of anticholinesterase activity were
observed and total blood, erythrocyte, plasma and brain cholinesterase
levels determined. The antidotes were effective especially at the
upper dose levels in reducing the toxic symptoms. The combination of
atropine sulfate with PAM or glutathione was more effective than
atropine sulfate alone (Hayashi et al., 1973).
Chlorpyrifos-methyl was administered to groups of rats at 5 x
LD50 (8.5 g/kg). At first signs of anticholinesterase activity one
group was given atropine sulfate (17.5 mg/kg) intramuscularly and a
further group atropine sulfate (17.5 mg/kg) and PAM (50 mg/kg)
intramuscularly. A further administration of antidote was given to all
rats seven hours after treatment. All treated rats died within 24
hours, untreated controls died within eight hours (Davies and Collins,
1975).
Short-term studies
Avian species
Mallard ducks, bobwhite and Japanese quail were fed various
levels of chlorpyrifos-methyl for five days and observed for a further
three days. The following LC50 values were determined.
Bobwhite quail - 1835 ppm, Japanese quail ->5000 ppm, mallard
duck - 2500-5000 ppm. Body weight and food consumption were reduced
for bobwhite and Japanese quail at 2500 and 1250 ppm respectively and
at 5000 ppm for the mallard duck. In a similar study, mallard ducks
were fed graded levels of chlorpyrifos-methyl for five days. Whole
blood cholinesterase activity was inhibited at dietary levels of 78
ppm and greater. Brain cholinesterase was not affected at the highest
level tested (Shellenberger, 1970).
Mouse
Chlorpyrifos-methyl was administered orally to mice at 0, 100,
250, 500 and 1000 mg/kg body weight/day for 14 days. No effect on
mortality or significant changes in body weight at 100 and 250 mg/kg
were observed. After four doses, there was 100% mortality at 1000
mg/kg. At 500 mg/kg, 40% mortality occurred in 14 days and a
noticeable decrease in body weight (Esaki et al., 1973).
Groups of male and female mice were fed standard diets containing
0, 0.5, 1, 5, 10, 20, 30, 40 and 100 ppm for a period of six months.
No differences were observed in mortality, food intake, body weight
gain, organ weights and urinalysis. Haematological, biochemical and
pathohistological parameters were considered normal in groups given
less than 40 ppm. Plasma and erythrocyte cholinesterase activities
were lower at dosage levels greater than 20 ppm. Brain cholinesterase
was decreased in males given 40 and 100 ppm and in females given 40
ppm (Hasegawa et al., 1973c).
Rat
Chlorpyrifos-methyl was administered orally to rats at 20, 100
and 500 mg/kg body weight/day for 14 days. No significant effect on
mortality or body weight gain was shown. At 500 mg/kg hypertrophy of
the liver and heart were observed (Esaki et al., 1973).
Groups of female rats were fed chlorpyrifos-methyl in the diet
for 14 days at 0, 1.6, 6, 25, 95, 500 and 2130 mg/kg body weight/day.
Plasma and red blood cell cholinesterase was depressed at 6 mg/kg and
in brain at 95 mg/kg. Organ to body weight ratios of liver, kidney and
spleen were not significantly different from control but brain to body
weight ratio was increased at 2130 mg/kg. No compound-related gross or
histopathological lesions were observed (United States Army, 1973).
Groups of six male and female rats consumed in their diet 0,
0.08, 0.8, 8.0, 40, 80 and 160 mg chlorpyrifos-methyl/kg body
weight/day for 90 days. Plasma cholinesterase activity was depressed
in males at 8 mg/kg and above and in females at 0.8 mg/kg and above.
The incidence of erythrocyte and brain cholinesterase depression was
significantly increased at 8.0 mg/kg/day and above for both male and
female. Liver weight to body weight ratio of male rats was increased
at 40, 80 and 160 mg/kg. Apart from small aggregates of lymphocytes
and plasma cells in the periportal areas of the liver, no changes were
noted in gross or histopathology (Steinberg, 1971).
Chlorpyrifos-methyl was fed at dietary levels of 0, 0.5, 1, 5,
10, 20, 30, 40 and 100 ppm to groups of male and female Wistar rats
for six months. No significant differences were noted in mortality,
food intake, weight gain, organ weights and urinalysis.
Haematological, biochemical and pathological findings were not
affected at 40 ppm or less. Serum and erythrocyte cholinesterase
activity was decreased at 30 ppm and above at one, three and six
months (Hasegawa et al., 1973b).
Groups of five male and five female rats received 0, 0.2, 1.0 and
5.0 mg chlorpyrifos-methyl/kg body weight/day by stomach tube daily,
six days/week, for six weeks. Plasma cholinesterase activity was
depressed in females at 5 mg/kg. Erythrocyte and brain cholinesterase
activities were not significantly reduced. Growth, haematology, blood
chemistry and liver microsomal mixed function oxidase activities were
not affected (Coulston and Griffin, 1975).
Dog
Groups of dogs (two males and two females/group) were fed
chlorpyrifos-methyl in the diet for 92 days at dose levels of 0, 0.3,
1, 3 and 10 mg/kg body weight/day. No treatment-related effects were
observed in behaviour, food consumption, body weight, hematology,
clinical chemistry, urinalysis, organ weight: body weight ratios,
gross and histopathology. Plasma cholinesterase activity at all test
levels was depressed after one, four and 12 weeks of treatment.
Inhibition of erythrocyte cholinesterase activity was evident at 1.0,
3.0 and 10.0 mg/kg body weight/day. The activity of brain
cholinesterase was not affected (Sparschu et al., 1971).
A supplemental study was conducted in which groups of dogs (two
males and two females/group) were fed chlorpyrifos-methyl in the diet
at dose levels of 0, 0.03 and 0.1 mg/kg body weight/day for 125 days.
There was no significant depression of plasma or erythrocyte
cholinesterase activity. Behaviour was normal and body weight and food
consumption considered to be within normal limits (Humiston et al.,
1972).
Groups of dogs (seven males and seven females/group) were fed
chlorpyrifos-methyl at dietary levels of 0, 0.03, 0.1, 1.0 and 3.0
mg/kg/day for 104 weeks with an interim sacrifice of three males and
three females/group at 26 weeks. No significant effects were observed
with respect to mortality, behaviour, food and water consumption,
ophthalmology, haematology, blood biochemistry, organ to body weight
ratio or gross and histopathology at either 26 or 104 weeks. Treated
animals gained less weight than controls due to some weight loss
during the latter part of the study. Body weights, however, were
considered within normal limits. Cholinesterase activity was depressed
in red blood cell and plasma at 3.0 mg/kg and in plasma at 1.0 mg/kg.
There was no reduction in brain cholinesterase activity at 26 or 104
weeks (Rivett et al., 1974).
Monkey
Chlorpyrifos-methyl was administered by stomach tube daily, six
days/week for six months to five groups of rhesus monkeys, three male
and three female group at dose levels of 0, 0.1, 0.2, 1.0 and 5.0
mg/kg body weight/day. Plasma cholinesterase was depressed in those
animals receiving 1.0 and 5.0 mg/kg. Inhibition of erythrocyte
cholinesterase was evident at 5.0 mg/kg. Brain cholinesterase was not
affected at any test level. No treatment related effects were noted in
growth, haematology, blood chemistry, mixed function oxidase activity
of the liver, relative organ weights, gross or histopathology
(Coulston and Griffin, 1975).
Cow
Groups of four cows were fed for 42 days silage corn which had
been sprayed 83 days previously with chlorpyrifos-methyl. The diets
contained 0, 0.35, 0.87 and 1.85 ppm chlorpyrifos-methyl and 0, 0.44,
0.79 and 1.75 ppm 3,5,6 trichloro-2-pyridinol. Residue intakes
amounted to 0.009, 0.022 and 0.054 mg chlorpyrifos-methyl and 0.012,
0.020 and 0.051 mg 3,5,6 trichloro-2-pyridinol/kg body weight. No
adverse effects were observed in silage intake, milk production, blood
cholinesterase activity or body weight gains. Urine, faeces and milk
contained trace amounts of chlorpyrifos-methyl and 3,5,6
trichloro-2-pyridinol. One week after treated feed withdrawn these
residue were not detectable (Johnson et al., 1974).
Long-term studies
Rat
Groups of 55 male and 55 female rats were administered
chlorpyrifos-methyl in the diet at 0, 0.03, 0.1, 1.0 and 3.0 mg/kg
body weight/day for 104 weeks. After five weeks of treatment five
males and five female/group were sacrificed for cholinesterase
determination. Twenty-six weeks after treatment 10 males and 10
females/group were killed for interim study and the size of each group
was reduced to 30 males and 30 females after 52 weeks. Red blood cell
cholinesterase activity was depressed in both sexes at 1.0 and 3.0
mg/kg/day. Plasma cholinesterase activity was reduced consistently in
females at 1.0 and 3.0 mg/kg/day; males showing little effect during
the first year after which it was significantly depressed at 3.0
mg/kg/day. Brain cholinesterase levels were not affected.
Chlorpyrifos-methyl produced no significant effect on behaviour,
mortality, body weight gain, food consumption and efficiency of food
utilization. Haematologic, blood biochemical and urinalysis
determinations were within normal limits and revealed no abnormal
changes. No effect attributable to treatment was observed in organ
weights or on gross and histopathologic examination. Tumour incidence
was comparable between groups (Hunter et al., 1974a).
Observations in man
Fourteen male volunteers were divided into two treatment groups
of five men each and a control group of four men. Chlorpyrifos-methyl
was administered by gelatin capsule in a single daily dose of 0, 0.03
and 0.1 mg/kg body weight/day for four weeks. Plasma and erythrocyte
cholinesterase activities were not depressed at levels tested.
Haematology, blood chemistry, urinalysis, blood pressure, pulse and
ophthalmology were not affected by treatment (Coulston et al., 1975).
COMMENTS
In mammals, chlorpyrifos-methyl is rapidly absorbed and
metabolized the principal metabolite being
3,5,6-trichloro-2-pyridinol. The parent compound and metabolite are
excreted primarily in the urine and faeces and are not stored to any
extent in the body. This metabolite has also been shown to occur in
plants.
Teratogenic studies in the rat revealed no adverse effects at
dietary intake levels of up to 250 mg/kg. In an inadequately conducted
and reported study in the mouse there appeared to be a tendency for a
weak teratogenic response at 250 mg/kg. No compound-related
abnormalities were noted at 100 mg/kg.
No adverse effects were noted in the reproductive capacity of the
rat over three generations at dietary levels of up to 3 mg/kg.
Mutagenic studies have not been reported.
No apparent clinical evidence of neurotoxicity was observed in
hens given a large dose of chlorpyrifos-methyl. Histological
examination of nervous tissue was not conducted. In various short- and
long-term studies in the rat, dog, mouse, monkey, mallard duck,
bobwhite and Japanese quail, chlorpyrifos-methyl was shown to be an
active cholinesterase inhibitor. Plasma cholinesterase activity was
inhibited to a greater extent than either erythrocyte or brain
cholinesterase.
In the long-term study and specific tumorigenic study in the rat,
no compound-related effect or increase in tumour incidence was
observed.
No effect levels were demonstrated in the rat, dog, monkey and
man on the basis of the most sensitive parameter plasma cholinesterase
inhibition. The no-effect level in man was confirmed by two
independent studies. The data were considered sufficient to allocate
an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg
Dog: 0.1 mg/kg
Monkey: 0.2 mg/kg
Man: 0.1 mg/kg
Estimation of acceptable daily intake
0-0.01 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of chlorpyrifos-methyl for insect control in fruit,
vegetable and cereal plants and in grain storage is recorded from the
following countries: Egypt, France, Japan, Korea, and Turkey. In these
countries it is marketed as dusts and/or granules and as emulsifiable
concentrates containing 2-3% a.i. and 25-40% a.i. respectively.
As a relatively new chemical chlorpyrifos-methyl is marketed as a
broad-spectrum organophosphorus insecticide of relatively low toxicity
and persistence, although it shows reasonably good stability in
stored, dry products such as grains (Schulten, 1973) and dried fruits
(Soderstrom and Armstrong, 1974). In these products it controls a wide
range of beetles, weevils, moths and mites, including several such
species which may have developed resistance towards other
insecticides, e.g. malathion. Using spray techniques or admixing
procedures to treat the stored grain products, chlorpyrifos-methyl is
usually applied at the rate of 5-10 mg a.i. per kg.
In several of the countries mentioned, chlorpyrifos-methyl is
approved for wider uses in agriculture and horticulture against pests
of economic importance connected to fruit and vegetable growing
(Nakayama et al., 1973). In laboratory tests it is further found to be
effective against a wide range of household pests, including spiders,
houseflies, cockroaches, house crickets etc, (Snetsinger, 1972).
A number of recommended practical applications is listed in Table
1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on chlorpyrifos-methyl residues resulting from a number of
the above uses have been presented by the originating company (Kenaga,
1975). The data derived from field trials and controlled experimental
work under a variety of geographical and climatic conditions is as
follows.
In plants
Cereal plants and grass
Leuck et al. (1975) found chlorpyrifos-methyl to be relatively
non-persistent in field trials where coastal bermuda grass and corn
were sprayed once with 0.56, 1.12 and 2.24 kg a.i. per ha (see Table
2). In both crops and at all three levels chlorpyrifos-methyl
diminished rapidly at rates corresponding to half-lives of about two
to three days. The hydrolysis product, 3,5,6-trichloro-2-pyridinol,
was more persistent and in some cases actually increased up to a
maximum during the first few days after application. Thereafter the
pyridinol disappeared following a first order scheme with estimated
half-life values of 9-12 days.
TABLE 1. Some use patterns of chlorpyrifos-methyl on plants and grain
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Australia Emulsifiable concentrate Wheat 5.0 mg/kg
Egypt Emuls. conc., 24% (w/v) Spodoptera littoralis and 0.9 kg/ha or Foliar broadcast spray
S. exigua on vegetables 90 g/litre
and clover
France Emuls. conc., 24% (w/v) Stored grain pests 2.5 mg/kg
Japan Dust, 2% (w/w) Rice stemborer 0.6-0.8 kg/ha Max. 4 applic. per season
Micro-granule, 3% (w/w) Rice stemborer 0.9-1.2 kg/ha Broadcast appl. Max. 4 x
per season
Granule, 5% (w/w) Rice stemborer 1.5-2.0 kg/ha Submerged appl. Max. 4 x
per season
Emuls. conc., 25% (w/v) Rice stemborer 0.1-0.375 kg/ha Max. 4 sprays/season:
rice and cabbage
Diamondback moth, Pieria
rapae, aphids on tobacco Max. 2 sprays/season:
Cutworm on cabbage, chinese cabbage and
chinese cabbage, radish radish
Korea Granule, 3% (w/w) Rice stemborer, plant 0.9-1.2 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice.
Tobacco cutworm, mole
cricket on tobacco and
vegetables
TABLE 1. (Cont'd)
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Korea Emuls. conc., 25% (w/v) Rice stemborer, plant 0.25-0.35 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice
Tobacco cutworm, mole
cricket on tobacco and
vegetables
Sudan Emuls. conc., 24% (w/v) Pests of apricots
Turkey Emuls. conc., 24% (w/v) Spodoptera litteralis and 0.9 kg/ha Foliar broadcast spray
Heliothis obsoleta in
cotton and vegetables
Aphids on melons
USA Emuls. conc. Wheat 5-10 mg/kg
Johnson et al. (1974) in an earlier experiment sprayed corn in
the dent stage with chlorpyrifos-methyl at the same dosage rates. In
this case, the corn was harvested and ensiled for forage purposes one
day after the treatment (Table 2). The initial losses of
chlorpyrifos-methyl from the time of application through harvesting
and ensiling amounted to 62-79%. After the ensiling, however, residues
became relatively stable and they disappeared only slowly with
formation of the pyridinol. From the first day until the eighty-third
day, on average for the three treatments, 60% of the
chlorpyrifos-methyl disappeared while the pyridinol increased 421%.
TABLE 2. Residues of chlorpyrifos-methyl and pyridinol in grass and corn
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Bermudagrass, 0.56 0 5.5 1.07
coastal 7 0.1 0.73
(Leuck et al., 1975) 21 <0.01 0.32
1.12 0 12.5 2.21
7 0.21 1.81
21 0.01 0.58
2.24 0 28.4 4.48
7 0.45 4.08
21 0.09 1.24
Corn, growing 0.56 0 2.2 0.11
(Leuck et al., 1975) 7 0.11 0.17
21 0.03 0.09
1.12 0 8.2 0.41
7 0.29 0.54
21 0.07 0.21
2.24 0 20.4 0.78
7 1.43 1.38
21 0.20 0.60
TABLE 2. (Cont'd.)
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Forage corn, 0.56 0 2.22 0.11
ensiled at day 1 1 0.75 0.13
(Johnson et al., 27 0.51 0.48
1974) 55 0.47 0.49
83 0.34 0.70
125 0.34 0.27
1.12 0 8.20 0.41
1 1.92 0.34
27 1.42 1.09
55 1.27 1.40
83 0.72 1.74
125 0.77 0.35
2.24 0 20.40 0.78
1 4.08 0.69
27 2.98 2.50
55 2.31 2.28
83 1.64 3.34
125 1.80 1.13
Kubota (1975) has reported on field trials with growing rice. As
shown in Table 3, residues at the time of harvest were not detectable
or at most insignificant when spraying was carried out 4-17 days
before harvest.
Stored grain products
White and LaHue (1974), in their experiments showing effective
protection against several grain insects, e.g. rice weevil, lesser
grain borers, flour beetle, sawtooth grain beetle, and foreign grain
beetle, also followed the dissipation of chlorpyrifos-methyl in stored
wheat, sorghum and maize. Wheat at 79°C and containing 14.6% moisture
was treated with 4.8, 6.6 and 10.4 mg/kg chlorpyrifos-methyl. The
residue half-life was >6 months, 2-3 months and <1 month,
respectively. Maize and sorghum tested under similar conditions at
about 6-7 mg/kg had a residue half-life of about 2-3 months (Table 4).
TABLE 3. Residues of Chlorpyrifos-methyl in rice*
Application
Rate Days after Number of
Formulation (kg/ha) Frequency last treatment samples Residues, mg/kg
3% dust 1.2 2 × 35-42 8 <0.005-<0.005(n.d.)
4 × 14-42 8 <0.005-<0.005(n.d.)
6 × 11 8 <0.005-<0.005(n.d.)
5% granule 2 2 × 53-57 8 <0.005-<0.005(n.d.)
4 × 53-57 8 <0.005-<0.005(n.d.)
6 × 17-32 8 <0.005-0.012
25% e.c. 0.30-0.375 2 × 32-53 8 <0.005-<0.005(n.d.)
4 × 24-53 8 <0.005-<0.005(n.d.)
6 × 4-32 8 0.005-0.013
* From Kubota (1975).
TABLE 4. Residues of chlorpyrifos-methyl in stored grains in the United States of America*
Residue, mg/kg
Grain 0 days 5 days 1 2 3 4 5 6 8 9 months
Wheat (26°C, 4.8 4.6 3.5 3.0 2.8 3.3 - 3.3 - -
14.6% moisture) 6.6 5.5 5.0 2.8 3.2 - - 1.6 - -
10.4 6.2 4.1 2.6 2.3 - - 4.4 - -
Corn
(13.6% moisture) 6.70 - - - - 3.2 2.5 2.3 1.9 1.8
Sorghum - - - - 3.5 3.3 2.7 2.6 2.2 1.9
* From White and LaHue (1974).
In laboratory experiments, Bulla and LaHue (1975) tested the
disappearance rates of chlorpyrifos-methyl on the same three grains,
kept for six months at 80°F in covered glass jars with regulated
moisture contents from 10 to 16%. With overall half-life rates varying
from one to six months they found a decreasing persistence in the
sequence: maize > wheat > sorghum. Grains containing 14 or 16%
moisture degraded chlorpyrifos-methyl about 1.5-2 times faster than
those containing 10 or 12%.
In similar Australian experiments under commercial conditions,
Deahl and Tucker (1974) found that chlorpyrifos-methyl at initial
concentrations of 5.0 and 6.2 mg/kg declined at rates corresponding to
half-lives of the order of three to five months (see Table 5). The
average half-life at 31°C was 15-16 weeks with indications of a
reduced breakdown at lower temperatures (Desmarchelier, 1975a).
From these extensive trials, Desmarchelier et al. (1975b and c)
also reported the rate of loss of residues of chlorpyrifos-methyl on
white wheat containing 11% moisture at 25°C when stored in glass jars.
These tests indicate that under the laboratory conditions stated,
chlorpyrifos-methyl decays at a predictable first-order rate,
irrespective of concentration or age of the residues.
Morel and Galet (1975) report tests conducted in France on wheat
containing 15.2% moisture and 2.35 and 1.17 mg/kg chlorpyrifos-methyl
which was stored for various periods of time at 25°C for residue
analyses. Under these conditions half-lives of chlorpyrifos-methyl
were estimated to be about six weeks. Residue levels six months after
treatment were in the range of 0.2-0.4 mg/kg.
Apples and peaches
Residue levels on apples and peaches have been established under
Japanese conditions following spraying at recommended dosage rates,
i.e. about 1-1.5 kg/ha. The results of these trials are shown in Table
6 which indicates levels in apples below 0.3-0.5 mg/kg if harvested
about two weeks after the last treatment. The results for peaches do
not show signs of penetration of residues through the peel into the
fruit pulp.
Vegetable plants
Residue data from experiments with chlorpyrifos-methyl
applications on vegetable plants are shown in Tables 7 and 8. The
experiments derive from different geographical regions and cover
mostly foliar applications on the following crops: artichoke, beans,
cabbage, chinese cabbage, eggplant, lettuce, pepper, radish, tea and
tomatoes.
TABLE 5. Residues of chlorpyrifos-methyl during silo-storage, Australia*
Residue, mg/kg after interval (weeks)
Depth of
Treatment silo 1 6 11 16 22 26
6.2 mg/kg 0.1 m 4.5 3.1 2.3 2.1 1.9 1.5
(Queensland 1.5 m 3.4 3.6 2.1 2.3 1.8 1.5
experiment) 6.0 m 3.2 3.0 2.4 2.2 2.0 -
Average 3.7 3.2 2.3 2.2 1.9 1.5
3 8 13
5.0 mg/kg 0.1 m 4.1 3.9 1.8
(N.S. Wales 1.5 m 4.1 3.7 2.4
experiment) 6.0 m 3.3 3.1 2.4
Average 3.8 3.6 2.2
* From Deahl and Tucker (1974).
TABLE 6. Residues of chlorpyrifos-methyl in apples and peaches, Japan*
Preharvest Number of Number of Range of
Application interval applications samples results (mg/kg)
Apples
1.25-1.5 10 days 1-2 × 6 0.22-0.353
kg/ha 20 days 1-2 × 7 0.201-0.310
30 days 1-2 × 4 0.174-0.186
10 days 4 × 9 0.182-0.798
20 days 4 × 6 0.128-0.225
30 days 4 × 4 0.162-0.257
10 days 6 × 4 0.489-0.936
20 days 6 × 7 0.152-0.333
30 days 6 × 4 0.250-0.478
Peaches Peel Pulp
0.9 kg/ha 21 days 2 × 6 <0.03-0.26 <0.005
30 days 2 × 8 <0.03-0.05 <0.005
21 days 4 × 6 <0.03-0.11 <0.005
30 days 4 × 8 <0.03-0.05 <0.005
21 days 6 × 6 <0.03-0.10 <0.005
30 days 6 × 8 <0.03-0.06 <0.005
* From Kubota (1975).
TABLE 7. Residues of chlorpyrifos-methyl in vegetables, Europe/Near East
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Artichoke 48 g/100 l 7 days 2 0.05 France1
(aver.)
14 days 2 0.03
(aver.)
Beans 1.2 kg/ha 0 days 1 0.32 Turkey2
7 days 1 0.01
14 days 1 0.02
Beans, 48 g/100 l 7 days 5 <0.02 France1
french (aver.)
14 days 3 <0.02
(aver.)
Eggplant 1.2 kg/ha 0 days 1 0.06 Turkey2
7 days 1 0.01
14 days 1 0.01
Lettuce 48 g/100 l 7 days 4 0.78 France1
(aver.)
14 days 2 0.04
(aver.)
Pepper 1.2 kg/ha 0 days 1 2.28 Egypt3
1 day 1 0.58
2 days 1 0.25
3 days 1 0.20
5 days 1 0.09
7 days 1 0.10
9 days 1 0.04
TABLE 7. (continued)
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Tomato 0.1% until 3 days 1 1.90 England4
run-off 6 days 1 0.90
(greenhouse) 9 days 1 0.40
13 days 1 0.09
1.2 kg/ha 0 days 1 0.31 Turkey2
7 days 1 0.04
14 days 1 0.05
48 g/100 l 7 days 6 0.21 France1
(aver.)
14 days 6 0.04
(aver.)
1 Hascouet (1974).
2 Hollick and Collison (1972a).
3 Hollick and Collison (1972b).
4 Wirth et al. (1971).
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Cabbage 0.45 kg/ha 7 days 2 × 8 <0.002-0.002
14 days 2 × 8 <0.002
7 days 4 × 8 <0.002
14 days 4 × 8 <0.002
Chinese 0.25 kg/ha 14 days 4 × 2 <0.005
cabbage
0.375 kg/ha 7 days 2 × 7 0.027-0.220
14 days 2 × 7 <0.005-0.100
7 days 4 × 7 0.036-0.175
14 days 4 × 5 0.039-0.056
0.50 kg/ha 7 days 2 × 4 0.019-0.025
14 days 2 × 4 0.004-0.006
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Chinese 7 days 4 × 4 0.001-0.002
cabbage 14 days 4 × 4 0.003-0.007
Eggplant 0.75 kg/ha 3 days 2 × 4 0.002-0.003
7 days 2 × 4 0.001
14 days 2 × 4 <0.002
3 days 4 × 4 0.002-0.004
7 days 4 × 4 0.001
14 days 4 × 4 <0.002
Pepper, 0.50 kg/ha 3 days 2 × 4 0.011-0.171
green 7 days 2 × 4 0.004-0.028
14 days 2 × 4 <0.002-0.012
Pepper, 3 days 4 × 4 0.013-0.129
green 7 days 4 × 4 0.003-0.060
(cont'd) 14 days 4 × 4 <0.003-0.021
Leaves Root
Radish 0.2-0.3 kg/ha 7 days 2 × 4 0.024-0.324 <0.002-0.069
28 days 2 × 4 0.003-0.009 0.005-0.008
7 days 3 × 2 0.109 <0.001-0.003
28 days 3 × 4 0.024-0.130 <0.001-0.001
7 days 4 × 4 0.040-0.299 <0.002-0.081
28 days 4 × 4 0.003-0.010 0.013-0.020
7 days 6 × 2 0.075 <0.002-0.002
14 days 6 × 4 0.019-0.040 0.002-0.003
Tea 0.50 kg/ha 7 days 1 × 6 <0.001-0.065
14 days 1 × 6 <0.001-0.047
21 days 1 × 6 <0.003
27 days 1 × 6 <0.003
7 days 2 × 6 <0.001-0.026
0.75 kg/ha 7 days 1 × 7 <0.001-0.091
14 days 1 × 6 <0.001-0.056
21 days 1 × 6 <0.001-0.047
7 days 2 × 6 <0.003-0.085
* From Kubota (1975).
The levels of chlorpyrifos-methyl residues on these crops at the
time of harvest, i.e. from 7 to 14 days after the last treatment are
generally at or below 0.1 mg/kg.
FATE OF RESIDUES
In plants
Residues of chlorpyrifos-methyl in or on plants are assumed to
behave similarly to chlorpyrifos Of FAD/WHO, 1973) without
translocation in plants to any significant extent.
The principal hydrolysis product of chlorpyrifos-methyl in
plants, animals and soil, 3,5,6-trichloro-2-pyridinol, may or may not
be taken up by plants depending on pH. The free pyridinol, which is
the predominant form of the compound below pH 6.0, is essentially
insoluble in water. In radio-labelled studies, soil and nutrient
culture experiments have shown its uptake by plants to be
insignificant (Smith et al., 1967). At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the sodium salt) as well as rate of absorption by the
plant are enhanced compared with the free pyridinol. Although still at
very low levels, the sodium salt of the pyridinol enters the plant
more than five times as fast as the free pyridinol. It undergoes
metabolism with the liberation of chloride and the formation of
several unidentified water soluble decomposition products.
In cereal-products
Residues of chlorpyrifos-methyl in treated grains have been
followed from the time of application through milling and baking
procedures in several experiments (see Table 9). Bulla and LaHue
(1975) fractionated wheat containing 6.3 mg/kg chlorpyrifos-methyl and
found that most of the residues after three and six months of "ageing"
were still concentrated in the outer grain fractions, such as "red
dog" and bran. The residues in flour were reduced to below 10% of the
original concentration (i.e. to 0.53 and 0.39 mg/kg, respectively).
Bread baking reduced the remaining residues further by about 60%.
Similar results have been found by Bengtson et al. (1975) who
after 11 and 22 weeks of ageing also found considerable reduction from
grain residues to milled flour and baked bread, the latter showing
below 0.25 mg/kg residual chlorpyrifos-methyl.
Morel and Galet (1975) analysed wheat which was milled from 6 to
17 weeks after treatment of commercially stored grains with 1.6-1.8
mg/kg of chlorpyrifos-methyl. The distribution of residues in various
milled fractions is shown in Table 9. About 90% of the residues
remained in the outer bran and course middlings.
TABLE 9. Chlorpyrifos-methyl residues in milled wheat fractions and bread
Residues, mg/kg, in
Bread
Ageing Pollard
period Whole wheat "Red dog" Bran (or middling) Flour Wholemeal White
USA (Bulla and LaHue, 1975)
3 months 6.3 mg/kg* 6.5 5.0 0.53 0.21
6 months 6.3 mg/kg* 3.3 3.0 0.39 0.16
France (Morel and Gallet, 1975)
6 weeks 4.3 1.4 0.26
9 weeks 1.6-1.8 mg/kg* 2.96 1.46 0.22
14 weeks 3.92 1.60 0.29
17 weeks 3.55 1.61 0.23
Australia (Bengtson et al., 1975 and Desmarchelier, 1975a)
6 weeks 6.7-6.9 mg/kg - 7.6-8.8 2.6-3.2 0.38-0.38 1.11-1.17 0.25-0.28
11 weeks 3.6 mg/kg - 9.3 9.0 0.6 - 0.2
11 weeks 2.0 mg/kg - 5.2 4.6 <0.10 - <0.12
22 weeks 1.8 mg/kg - 6.6 3.6 0.4 - 0.13
* Treatment levels.
In animals and animal products
Insects
Although direct studies have not been carried out with
chlorpyrifos-methyl to the same extent as for its ethyl analogue,
chlorpyrifos (cf. FAO/WHO, 1973), there is evidence that the general
metabolic pathways in living organisms are closely parallel for the
two compounds, e.g. the principal metabolic products in some insects
(tobacco budworm, cf. Whitten and Bull, 1974) are
3,5,6-trichloro-2-pyridinol mad O,O-dimethyl phosphate produced by
microsomal oxidase activity which apparently is indicated also
catalyses the formation of the P=O analogue of chlorpyrifos-methyl.
Soluble, non-oxidative hydrolytic enzyme systems are likewise
believed to act in detoxification mechanisms through O-dealkylation
(see Figure 1).
Absorption studies in these insects suggest that the slightly
higher toxicity of chlorpyrifos-methyl compared to chlorpyrifos is
probably connected with a faster rate of absorption of the former.
Cow
In their previously mentioned studies of chlorpyrifos-methyl in
ensiled forage corn, Johnson et al. (1974) fed silage corn containing
0.35, 0.87 and 1.87 mg/kg chlorpyrifos-methyl and 0.44, 0.79 and 1.75
mg/kg 3,5,6-trichloro-2-pyridinol to cows for 42 days. This dietary
residue intake was equivalent to an average of 0.09, 0.022 and 0.054
mg chlorpyrifos-methyl and 0.012, 0.20 and 0.051 mg of the pyridinol
per kg of body weight per day, respectively.
Residue analyses showed no trace of the P=O analogue of
chlorpyrifos-methyl in any samples including milk, urine and faeces.,
The principal excretory route for chlorpyrifos-methyl was by means of
the faeces. Only at the 0.054 mg/kg day dosage did a trace of
chlorpyrifos-methyl (0.001-0.002 mg/kg) appear in the milk with none
in the urine. The principal excretory route for the pyridinol was via
the urine. Some excretion of the pyridinol occurred also in the faeces
(up to 0.36 mg/kg and in the milk (up to 0.008 mg/kg) at the 0.054
mg/kg/day rate. These studies did not include analyses of animal meat
or fat.
In soils
Regoli et al. (1974) studied the aerobic degradation of
ring-labelled C14-chlorpyrifos-methyl in the laboratory at 1 mg/kg in
two soils (silty clay loam and loam containing 4.2% and 0.8% organic
carbon respectively) incubated at 15°, 25° and 35°C and with moisture
at 32% or 100% of field capacity for up to 428 days. All incubations
were conducted in closed, aerated containers so that a balance of
radioactivity added to each sample could be drawn.
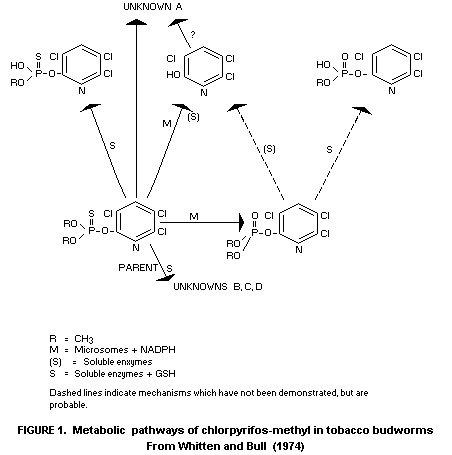 An overall average of 100.4% recovery of added activity was
obtained for samples over the entire study and it was found that the
major metabolite in soil was 3,5,6-trichloro-2-pyridinol which is
further degraded to CO2 followed by lesser amounts of
3,5,6-trichloro-2-methoxy-pyridine. The authors speculated whether the
methoxy-compound is formed through direct methylation of
chlorpyrifos-methyl or from the pyridinol through microbial activity,
but the former route, involving subsequent conversion of the
methoxypyridine to the pyridinol seems more likely as no other
decomposition-products could be traced.
The breakdown of chlorpyrifos-methyl to
3,5,6-trichloro-2-pyridinol is a rapid process if conditions are
favourable for microbial and hydrolytic activity. The time required
for 50% breakdown ranged from 1.5 to 2.0 days in the high organic soil
at 25-35°C and 100% moisture to 33.3-17.7 days in the low organic soil
at 15°C and 32% moisture. The calculated times required for 90%
degradation ranged from 20 to 30 days under favourable conditions to
500-1600 days under less favourable. Thus in practice
chlorpyrifos-methyl could hardly persist in soils and give rise to
traceable carry-over during crop rotations.
Hamaker (1974) studied the soil adsorption of
C14-chlorpyrifos-methyl by analysing both the soil and the
supernatant from slurries of soil in 1 mg/l solutions (one part of
soil:four parts of solution). The soils contained organic carbon
ranging from 0.28 to 5.76%.
The adsorption by these soils ranged from 46 to 99% after 24
hours and the average distribution ratio between soil organic carbon
and water was found to be 3300 after four hours and 4600 after 24
hours. This is less by one-third than in the case of chlorpyrifos but
still represents a strong adsorption to soil.
The hydrolysis product (3,5,6-trichloro-2-pyridinol) is absorbed
to a smaller degree than the parent compound showing a distribution
ratio of 714 between soil and water. In the conclusions of the author,
however, this still represents a sufficiently strong adsorption to
make leaching of the compound from soils an unlikely process.
Kubota (1975) has reported on both field trials and laboratory
tests determining residue disappearance from rice paddy soils over
periods of 30 days (Table 10). The half-life periods in the field
tests varied from five to about 20 days, while the half-life was less
than three days when chlorpyrifos-methyl was applied directly to the
soil in the laboratory.
A DOWCO 214 formulation applied to bacterial cultures in amounts
corresponding to normal soil application showed no influence on the
bacterial activity measured as the O2-uptake under incubation of
Azotobacter vinelandii (MacRae and Celo, 1974). Neither was the
nitrogen fixation of this strain as measured by the reduction of
acetylene to ethylene affected, even at concentrations 100 times the
practical application rates (Wood and MacRae, 1974).
TABLE 10. Disappearance of chlorpyrifos-methyl in Japanese soils*
Formulation/ Dosage Days after Number of Range of
treatment rate application samples residues (mg/kg)
3% dust 1.8 kg/ha 0 days 4 1.98-2.94
10 days 4 0.41-1.47
20 days 4 0.10-0.33
30 days 4 <0.05-0.17
3% dust 0.18 kg/ha 0 days 4 0.171-0.236
10 days 4 0.121-0.183
20 days 4 0.059-0.089
30 days 4 0.007-0.014
96% DOWCO 214 5 mg/kg 0 days 4 4.02-4.10
(laboratory
test) 3 days 4 0.82-1.47
10 days 4 0.26-0.39
30 days 4 0.06-0.31
* From Kubota (1974).
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods for the determination of
chlorpyrifos-methyl and its principal metabolite,
3,5,6-trichloro-2-pyridinol, have been described in numerous
modifications, mostly varying in the detailed procedures of extraction
and clean-up (Deahl and Tucker (1974), Desmarchelier (1975b) for
grains; Hollick and Collison (1972a and b) for various vegetables;
Johnson et al. (1974) for corn, milk, urine and faeces; Kubota (1975)
for a variety of vegetables, rice and soils; McKellar et al. (1970,
1971a, b and c) for grains, grass, bovine tissues land milk; Regoli et
al. (1974) for soils; Wirth et al. (1971) for tomatoes).
Of the two compounds, the pyridinol which usually appears as a
hydrolytic product and mostly in non-fatty tissues is of less
importance in the analysis for regulatory purposes. The determination
of the P=O analogue of chlorpyrifos-methyl may or may not be included
by the above procedures (cf. for instance Johnson et al. (1974)), but
it is usually not encountered in animal or plant tissues.
Generally speaking, the principle of determination is based on
extraction with dichloromethane (from wet media), acetone or methanol
(from wet media and soils) or hexane (from dry or fatty tissues)
followed by partitioning procedures (acetone: CH2Cl2 and/or hexane:
acetonitrile) with eventual further clean-up on florisil, silica gel
or alumina.
The final determination of chlorpyrifos-methyl (and its oxygen
analogue) is made from acetone solution by GLC utilizing a phosphorus
specific detector of the FPD- or AFID types. For the pyridinol
metabolite the determination is made according to McKellar and
Dishburger (1970) on GLC with a nickel (Ni63) EC-detector after
derivatization with N,O-bis(trimethylsilyl)acetamide.
The analytical sensitivities reported as limits of determination
for these methods range from 0.001 to 0.02 mg/kg of
chlorpyrifos-ethyl, about 0.01 mg/kg of the O-analogue and 0.001-0.05
mg/kg of the pyridinol in various substrates and with recoveries of
85.9-100.4% for all the compounds.
The general work chart for the analysis of chlorpyrifos (FAO/WHO
(1973) page 192) also seems to apply for the analysis of
chlorpyrifos-methyl and the general scheme should be adaptable for
regulatory purposes as part of a multiresidue analysis system.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Crop Tolerance, mg/kg (PHI)
Japan Rice 0.03 (60 days)
Chinese cabbage,
radish 0.03 (30 days)
Cabbage 0.03 (7 days)
Tobacco 0.03
APPRAISAL
Chlorpyrifos-methyl is an organophosphorus insecticide of
relatively low toxicity and with broad spectrum effects against a
number of insects of great economic interest as well as several
species of household insects. Since its introduction it has gained
particular and fairly wide interest as a potential grain protectant.
Information on the chemical and physical properties of
chlorpyrifos-methyl has been presented to the Meeting. The compound is
available in crystalline form with a purity of 99.8%. It is relatively
easily hydrolysed with the formation of 3,5,6-trichloro-2-pyridinol
which is also the major metabolite in plants, animals and soils.
It is registered and marketed in some countries as dusts,
granules and emulsifiable concentrates for pre-harvest agricultural
and horticultural purposes and for post-harvest grain treatments.
Recommended dosage rates vary from 0.1 to 2.0 kg/ha under field
conditions and from 2.5 to 10 mg/kg in grain storage.
Residue data from supervised trials and experimental tests with
chlorpyrifos-methyl in several countries have been made available by
the manufacturer in support of registrations, including the
establishment of tolerances. These data include residue levels and
disappearance rates in several vegetable crops, fruits and cereal
plants as well as stored grain products, including wheat, corn and
sorghum grains.
While generally of low persistence, chlorpyrifos-methyl is
relatively stable and evidences prolonged protection against insects
in grains and stored, dried products. The half-life of
chlorpyrifos-methyl in stored wheat averages three to five months
under practical conditions, but at higher moisture levels and higher
temperatures the rate of decomposition increases. Conversely at low
temperatures and low humidities the effective life is expected to be
prolonged.
Evidence of the fate of residues during the milling, processing
and baking of cereals is demonstrated in several trials. Residues in
grains are to a great extent confined to the outer layers and
concentrated in bran and milling offals. Correspondingly they are
diminished to low levels (10% of the original deposits or lower) in
flour and further reduced to half of this in baked bread.
Feeding trials with cows indicate that both chlorpyrifos-methyl
and its pyridinol moiety may give rise to trace amounts (below 0.01
mg/kg) in milk, but that the main excretory route for the parent
compound is via the faeces and for the metabolite via the urine.
Residue studies on meat products, fat or eggs are not available.
Several soil studies have been made on chlorpyrifos-methyl which
confirms that hydrolysis to 3,5,6-trichloro-2-pyridinol is a fairly
rapid process under normal conditions and that total residues of
chlorpyrifos-methyl could hardly persist in soils or give rise to
traceable carry-over in crop rotation. Neither run-off nor leaching of
the compounds from the site of application is likely to occur.
Specific gas-chromatographic methods of analysis are available
for the determination of chlorpyrifos-methyl and
3,5,6-trichloro-2-pyridinol. The analytical procedure is closely
parallel to that for the ethyl analogue, chlorpyrifos, and should be
adaptable for regulatory purposes as part of multiresidue analysis.
Residues of chlorpyrifos-methyl may occur as its main metabolite,
3,5,6-trichloro-2-pyridinol. The amounts of this, however, are
generally low in relation to the original deposits.
RECOMMENDATIONS
The following maximum residue limits based on the parent compound
are recommended. They are not likely to be exceeded when following
good agricultural practices. Determination of the level of
chlorpyrifos-methyl parent compound will adequately control the amount
of total residue resulting from the use of this insecticide.
Maximum Pre-harvest interval
residue on which
limit recommendations
Commodity mg/kg are based (days)
Bran 20
Raw grains (wheat, corn, sorghum) 10
Flour, wholemeal bread 2
Apples, peaches 0.5 14
Tomatoes 0.5 3-5
White bread 0.5
Artichoke, beans, cabbage,
chinese cabbage, eggplant, 0.1 7-14
lettuce (outdoor), peppers,
radish, tea (green)
Rice (pre-harvest treatment) 0.1 21
Milk 0.01
FURTHER WORK OR INFORMATION
REQUIRED (before additional maximum residue limits can be
recommended)
1. Information on residues in animal tissues, fat and eggs
following feeding of chlorpyrifos-methyl residues in animal
feeds.
DESIRABLE
1. Appropriate mutagenic study.
2. Neurotoxicity study with histological examination of nervous
tissues.
3. Information on evidence of residues in commerce.
4. Further information on residue disappearance in practical
grain storage at low temperatures and low humidities.
REFERENCES
Bakke, J. E. and Price, C. E. (1975) Metabolism of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl)phosphorothionate and
3,5,6-trichloro-2-pyridinol in sheep and rats. Metabolism and
Radiation Laboratory, A.R.S., U.S.D.A. Fargo, North Dakota, June 10.
Personal communication to the Dow Chemical Company submitted to the
World Health Organization by the Dow Chemical Co., Midland, Michigan,
USA.
Bengtson, M., Connel, M., Desmarchelier, J., Philips, M., Snelson, J.
and Sticka, R. (1975) Evaluation of four new grain protectants. Report
to the Australian Wheat Board 1975. (To be published) (See
Desmarchelier, 1975c, Table 16).
Branson, D.R. and Litchfield, N.H. (1971) Absorption excretion and
distribution of
O,O-dimethyl-O-3,5,6-trichloro-2-6,14-2-pyridyl-phosphorothioate
(C14-Dowco 214) in rats. Unpublished report from the
Dow Chemical Company submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Brust, H.F. (1969) A summary of chemical and physical properties of
O,O-dimethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report OL 3083 from The Dow Chemical Co., Midland,
Michigan, September 12, 6 pp.
Bulla, L.A. Jr. and LaHue, D.W. (1975) Progress report studies on the
development of new grain protectant treatment - chlorpyrifos-methyl.
Report to The Dow Chemical Co., Michigan from U.S.D.A., A.R.S. Grain
Marketing Research Center, Manhattan, Kansas, February 18, 12 pp.
Coulston, F. and Griffin, T.B. (1975) A safety evaluation of Dowco 214
in rats and rhesus monkeys. Unpublished report, from the Institute of
Comparative and Human Toxicology, Albany Medical College submitted to
the World Health Organization by the Dow Chemical Co., Midland,
Michigan, USA.
Coulston, F., Rosenblum, I. and Griffin, T.B. (1975) Study of
chlorpyrifos-methyl in human volunteers. Unpublished report from
Institute of Comparative and Human Toxicology, Albany Medical College
and the International Center of Environmental Safety, Holloman AFB,
New Mexico submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Davies, R.E. and Collins, C.J. (1975) Response to oxime reactivating
agent in rats acutely poisoned with Reldan technical. Unpublished
report from the Huntingdon Research Centre submitted to the World
Health Organization by The Dow Chemical Co., Midland, Michigan, USA.
Deahl, H.Z. and Tucker, K.E.B. (1974) Residue studies on wheat stored
in vertical concrete silos in Queensland and N.S.W., Australia.
Unpublished report GHF-P-024 from Dow Chemical (Australia) Ltd.,
Altona, Victoria, November 18.
Desmarchelier, J.M. (1975a) Draft report No. 329-4-23 on 1973/74 grain
protectant trials to Australian Wheat Board, Working Party on Grain
Protectants. Chemical analysis - Residues and fate of protectants in
grain. CSIRO, Canberra.
Desmarchelier, J.M. (1975b) Quantitative measurement of rate of loss
of insecticidal activity on treated wheat due to lack of availability
to insects. In press. Communicated to Dow Chemical (Australia) Ltd.,
Sydney, June, 5 pp,
Desmarchelier, J.M. (1975c) An interim report on residues in wheat
treated with chlorpyrifos-methyl-grain protectant field trial, Tammin,
Western Australia. Communication to Dow Chemical (Australia) Ltd.,
Sydney, May 19, 16 pp.
Esaki, K., Tanioka, Y., Tsukada, M., Izumiyama, K. and Ohshio, K.
(1973) Effects of DOWCO 214 against fetuses of experimental animals.
Exper. Animal Central Laboratories, Kawasaki, Japan, Oct. 20.
Unpublished report submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Hamaker, J.W. (1974) Soil absorption of chlorpyrifos-methyl and its
hydrolysis products. Unpublished report GS 1343 (revised) from Dow
Chemical USA, Walnut Creek, California, February 20,
Hascouet, X. (1974) Determination of residues of chlorpyrifos and
chlorpyrifos methyl in vegetables after foliar treatment. Report to
The Dow Chemical Company, France. Ministry of Agriculture, C.N.R.A.,
Versailles, August 5.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E. and Kitagaki, T. (1973a) (Hokkaido Inst. of
Pub. Health, Sapporo, Japan). Studies on the safety of the newly
introduced organophosphorus insecticide, chlorpyrifos-methyl. Part 1.
Acute toxicity of chlorpyrifos-methyl in rats and mice. Hokkaidoritsu
Eisei Kenkyusho-Ho (Rep. Hokkaido Inst. Pub. Health), 23:57-61.
Japanese. English Summary in Pesticide Abstracts 7(4):74-0891, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973b) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of the newly introduced organophosphorus insecticide,
chlorpyrifos-methyl, Part 2. Subacute (6 months) toxicity of
chlorpyrifos-methyl in rats. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23:62-70. Japanese. English Summary in
Pesticide Abstracts 7(4):74-0892, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973c) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of newly introduced organophosphorus insecticide,
chlorpyrifos-methyl. Part 3. Subacute (six months) toxicity of
chlorpyrifos-methyl in mice. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23: 71-80. Japanese. English Summary in
Pesticide Abstracts, 7(4):74-0893, 1974.
Hayashi, E., Nakamura, I., Terada, Y., Kunimoto, M. and Yamada, S.
(1973) Therapeutic experiment on DOWCO 214 poisoned rats. Unpublished
report from Shizuaka Pharmacology College, Shizuoka, Japan, September
15. Submitted to the World Health Organization by The Dow Chemical
Co., Midland, Michigan, USA.
Hollick, C.B. and Collison, R.J. (1972a) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in egg plants, tomatoes, beans and peppers treated with NOLTRAN
insecticide in Turkey., Unpublished report GHE-P 011 from Dow Chemical
Company Limited, King's Lynn, England, August 23.
Hollick, C.B. and Collison, R.J. (1972b) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in peppers treated with NOLTRAN insecticide in Egypt. Unpublished
report CHE-P 102 from Dow Chemical Company Limited, King's Lynn,
England, October 16.
Humiston, C.G., Wade, C.E. and McCollister, S.B. (1972) Results of a
supplemental dietary feeding study of DOWCO 214 in dogs. Unpublished
report from The Dow Chemical Co., submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Cherry, C.P.
(1974a) DOWCO 214 - Toxicity following dietary administration to rats
for two years. Unpublished report from the Huntingdon Research Centre,
Huntingdon, England, submitted to the World Health Organization by The
Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Graham, C. and Brooks, P.N. (1974b) Tumorigenicity
screening of DOWCO 214 in the rat (final report 0-104 weeks).
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by the Dow
Chemical Co., Midland, Michigan, USA.
Johnson, J.C. Jr, Jones, R.L., Leuck, D.B., Bowman, M.C. and Knox,
F.E. (1974) Persistence of chlorpyrifos-methyl in corn silage and
effects of feeding dairy cows the treated silage. J. Dairy Sci.
57(1):467-73.
Kenaga, E.E. (1971) Some physicial, chemical, and insecticidal
properties of some O,O-dialkyl O-(3,5,6-trichloro-2-pyridyl)
phosphates and phosphorothioates. Bull. World Health Org.,
44:225-228.
Kenaga, E.E. (1975) Chlorpyrifos-methyl. Information on identity, use,
residues, analysis and tolerances. Submission from The Dow Chemical
Company, Midland, Michigan, prepared for the FAO/WHO 1975 Joint
Meeting, June 1975.
Kubota, Y. (1974) Summary tables of chlorpyrifos-methyl residue data
for crops and soil in Japan. Reports submitted by Dow Chemical
International Ltd., Tokyo, Japan, February 6.
Leuck, D.B., Jones, R.L. and Bowman, M.C. (1975) Chlorpyrifos-methyl
insecticide residues: their analysis and persistence in coastal
Bermudagrass and forage corn. J. Econ. Entoml., 68(3):287-90.
Litchfield, N. and Norris, J. (1969) O-O-dimethyl-o-3,5,6
trichloro-2-pyridyl phosphorothioate (Dowco 214). Unpublished report
from The Dow Chemical Co., submitted to the World Health Organization
by the Dow Chemical Co., Midland, Michigan, USA.
MacRae, I.C. and Celo, J.S. (1974) The effects of organo-phosphorus
pesticides on the respiration of Azotobacter vinelandii. Soil Biol.
Biochem., 6, 109-111.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography, method ACR 70.19 of the Dow Chemical Company, USA,
December 14.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-2-pyridinol in milk and cream by gas chromatography.
Method ACR. 71.2 of the Dow Chemical Company, USA, February 2.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-2-pyridinol in grass by gas chromatography. Method
ACR. 71.5 of the Dow Chemical Company, USA, May 6.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-2-pyridinol in corn grain, forage and stover by gas
chromatography. Method 71.19 of the Dow Chemical Company, USA,
September 27.
Meikle, R.W. (1973) Hydrolysis rate of DOWCO 214 in buffered distilled
water and in tap water. Unpublished report GS-1316 from the Dow
Chemical Company, USA, Walnut Creek, California, April 16.
Morel, J.L. and Galet, G. (1975) Results of residue analysis carried
out in France in grains treated with chlorpyrifos-methyl until May
1975. Communication from Dow Chemical Europe S.A. (report in press).
Nakajima, E., Shigehara, E. and Shindo, S. (1974) Whole body
autoradiography in rats medicated with the organophosphoric
insecticide DOWCO 214-14C. Unpublished report from the Drug
Metabolism Res. Lab., Sankyo Co. Ltd., Shinagawa, Tokyo, Japan
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Nakayama, I., Kuroda, T., Kitagaki, T., Shinohara, H. and Otokozawa,
T. (1973) Bagworms: effectiveness of several insecticide formulations
against the larvae of three species. J. Econ. Entomol.,
66(4):941-43.
Olson, K.J. (1964a) Results of toxicological tests on
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report from the Biochemical Research Laboratory, The Dow
Chemical Company, Midland, Michigan, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Olson, K.J. and Taylor, M.L. (1964b) Results of range finding
toxicological tests on O,O-dimethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate. Unpublished report from the Biochemical Research
Laboratory, The Dow Chemical Company, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Regoli, A.J., Laskowski, D.A. and Kurihara, N.H. (1974) Aerobic
degradation of chlorpyrifos-methyl in soil. Unpublished report GS-1367
from The Dow Chemical Company, Midland, Michigan, July 12.
Rivett, K.F., Sortwell, R.J., Heywood, R., Street, A.E. and Cherry,
C.P. (1974) Dowco 214 dietary toxicity studies in beagle dogs.
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Ross, D.B. and Roberts, N.L. (1974) The acute toxicity (LD50) of
Reldan (Dowco 214) to the chicken. Report DWC 234/74822. Unpublished
report from the Huntingdon Research Centre, Huntingdon, England,
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Ross, D.B., Burroughs, S.J. and Roberts, N.L. (1975) Examination of
chlorpyrifos-methyl (Dowco 214) for neurotoxicity in the domestic hen.
Report DWC 225/75401. Unpublished report from the Huntingdon Research
Centre, Huntingdon, England, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Schulten, G.G.M. (1973) Further insecticide trials on the control of
Ephestia cautella in stacks of bagged maize in Malawi. Intern. Pest
Control, March/April, p. 18-20.
Schwetz, B.A., Keeler, P.A., Pernell, H.C., Smith, F.A., Humiston,
C.G. and Gehring, P.J. (1973) A study of the prenatal toxicity of
Dowco 214 in rats. Report #NB T35. 12-46193-9, Unpublished report from
The Dow Chemical Company, submitted to the World Health Organization
by The Dow Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974a) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health organization by The Dow
Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974b) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Shellenberger, T.E. (1970) Toxicological evaluations of DOWCO 214 with
wildlife and DOWCO 179 with Mallard ducklings. Unpublished report from
the Gulf Research Institute, New Iberia, Louisiana, submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967) Investigations on
DURSBAN insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agric. Fd. Chem.,
15(5):870-77.
Smith, G.N. and Taylor, Y. (1972) Photodecomposition of DOWCO 214.
Unpublished report NBE-2 from The Dow Chemical Company, Midland,
Michigan, January 21.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Unpublished report
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Snetsinger, R. and Carroll, D. (1972) Evaluation of Dursban and DOWCO
214. Pest Control, 40(5):24-32.
Soderstrom, E.L. and Armstrong, J.W. (1974) Chlorpyrifos-methyl: A
protectant for raisins. J. Econ. Entomol., 68(1), p. 132.
Sparschu, G.L., Humiston, C.G., McCollister, S.B. and Wade, C.E.
(1971) Results of 92-day dietary feeding studies on DOWCO 214 in dogs.
Unpublished report from The Dow Chemical Company submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Steinberg, M. (1971) Summary of findings from 90-day feeding study
with DOWCO 214. Department of the Army. U.S. Army Hygiene Agency,
Aberdeen Proving Ground, Maryland. October 5, 8 pp. (Personal
communication).
U.S. Department of the Army. (1973) Preliminary assessment of relative
toxicity of candidate louse toxicant DOWCO 214 (ENT-27520)
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate evaluation
of animal exposure data and recommendations concerning a sleeve test
program. Study No. 51-19-68/74. November 1968 - May 1972. U.S. Army
Environmental Hygiene Agency. USAEHA-LT. Aberdeen Proving Ground,
Maryland, 38 pp.
Thompson, D.J., Dyke, I.L. and Warner, S.D. (1975) Results of a three
generation, two litter reproduction study on
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Dowco
214) in the rat. Unpublished report from Dow Chemical Corporation,
Department of Pathology and Toxicology, Zionsville, Indiana, submitted
to the World Health Organization by The Dow Chemical Co., Midland,
Michigan, USA.
White, G.S. and LaHue, D. (1974) Preliminary information concerning
DOWCO 214 treatment of wheat grain. Communicated by The Dow Chemical
Company, Midland, Michigan from the U.S.D.A., A.R.S. Grain Marketing
Research Center, Manhattan, Kansas, January 14.
Whitten, C.J. and Bull, D.L. (1974) Comparative toxicity, absorption
and metabolism of chlorpyrifos and its dimethyl homologue in
methyl-parathion resistant and susceptible tobacco budworms. Pest.
Biochem. & Physiol., 4:266-74.
Wirth, M., Würth, E. and Engeler, V. (1971) A residue study: DOWCO 179
and DOWCO 214 on tomatoes in a greenhouse. Unpublished report GHE-P
005 from Dow Chemical Company Limited, King's Lynn, England, December
16.
Wood, P.A. and MacRae, I.C. (1974) The effect of several
organophosphorus insecticides upon the acetylene-reduction activity of
Azotobacter vinelandii. Bull. Environ. Contamin. & Toxicol.,
12(1):26-31.
World Health Organization. (1966) WHO insecticide evaluation and
testing programme. Stage 1 mammalian toxicity report. OMS 1155.
An overall average of 100.4% recovery of added activity was
obtained for samples over the entire study and it was found that the
major metabolite in soil was 3,5,6-trichloro-2-pyridinol which is
further degraded to CO2 followed by lesser amounts of
3,5,6-trichloro-2-methoxy-pyridine. The authors speculated whether the
methoxy-compound is formed through direct methylation of
chlorpyrifos-methyl or from the pyridinol through microbial activity,
but the former route, involving subsequent conversion of the
methoxypyridine to the pyridinol seems more likely as no other
decomposition-products could be traced.
The breakdown of chlorpyrifos-methyl to
3,5,6-trichloro-2-pyridinol is a rapid process if conditions are
favourable for microbial and hydrolytic activity. The time required
for 50% breakdown ranged from 1.5 to 2.0 days in the high organic soil
at 25-35°C and 100% moisture to 33.3-17.7 days in the low organic soil
at 15°C and 32% moisture. The calculated times required for 90%
degradation ranged from 20 to 30 days under favourable conditions to
500-1600 days under less favourable. Thus in practice
chlorpyrifos-methyl could hardly persist in soils and give rise to
traceable carry-over during crop rotations.
Hamaker (1974) studied the soil adsorption of
C14-chlorpyrifos-methyl by analysing both the soil and the
supernatant from slurries of soil in 1 mg/l solutions (one part of
soil:four parts of solution). The soils contained organic carbon
ranging from 0.28 to 5.76%.
The adsorption by these soils ranged from 46 to 99% after 24
hours and the average distribution ratio between soil organic carbon
and water was found to be 3300 after four hours and 4600 after 24
hours. This is less by one-third than in the case of chlorpyrifos but
still represents a strong adsorption to soil.
The hydrolysis product (3,5,6-trichloro-2-pyridinol) is absorbed
to a smaller degree than the parent compound showing a distribution
ratio of 714 between soil and water. In the conclusions of the author,
however, this still represents a sufficiently strong adsorption to
make leaching of the compound from soils an unlikely process.
Kubota (1975) has reported on both field trials and laboratory
tests determining residue disappearance from rice paddy soils over
periods of 30 days (Table 10). The half-life periods in the field
tests varied from five to about 20 days, while the half-life was less
than three days when chlorpyrifos-methyl was applied directly to the
soil in the laboratory.
A DOWCO 214 formulation applied to bacterial cultures in amounts
corresponding to normal soil application showed no influence on the
bacterial activity measured as the O2-uptake under incubation of
Azotobacter vinelandii (MacRae and Celo, 1974). Neither was the
nitrogen fixation of this strain as measured by the reduction of
acetylene to ethylene affected, even at concentrations 100 times the
practical application rates (Wood and MacRae, 1974).
TABLE 10. Disappearance of chlorpyrifos-methyl in Japanese soils*
Formulation/ Dosage Days after Number of Range of
treatment rate application samples residues (mg/kg)
3% dust 1.8 kg/ha 0 days 4 1.98-2.94
10 days 4 0.41-1.47
20 days 4 0.10-0.33
30 days 4 <0.05-0.17
3% dust 0.18 kg/ha 0 days 4 0.171-0.236
10 days 4 0.121-0.183
20 days 4 0.059-0.089
30 days 4 0.007-0.014
96% DOWCO 214 5 mg/kg 0 days 4 4.02-4.10
(laboratory
test) 3 days 4 0.82-1.47
10 days 4 0.26-0.39
30 days 4 0.06-0.31
* From Kubota (1974).
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods for the determination of
chlorpyrifos-methyl and its principal metabolite,
3,5,6-trichloro-2-pyridinol, have been described in numerous
modifications, mostly varying in the detailed procedures of extraction
and clean-up (Deahl and Tucker (1974), Desmarchelier (1975b) for
grains; Hollick and Collison (1972a and b) for various vegetables;
Johnson et al. (1974) for corn, milk, urine and faeces; Kubota (1975)
for a variety of vegetables, rice and soils; McKellar et al. (1970,
1971a, b and c) for grains, grass, bovine tissues land milk; Regoli et
al. (1974) for soils; Wirth et al. (1971) for tomatoes).
Of the two compounds, the pyridinol which usually appears as a
hydrolytic product and mostly in non-fatty tissues is of less
importance in the analysis for regulatory purposes. The determination
of the P=O analogue of chlorpyrifos-methyl may or may not be included
by the above procedures (cf. for instance Johnson et al. (1974)), but
it is usually not encountered in animal or plant tissues.
Generally speaking, the principle of determination is based on
extraction with dichloromethane (from wet media), acetone or methanol
(from wet media and soils) or hexane (from dry or fatty tissues)
followed by partitioning procedures (acetone: CH2Cl2 and/or hexane:
acetonitrile) with eventual further clean-up on florisil, silica gel
or alumina.
The final determination of chlorpyrifos-methyl (and its oxygen
analogue) is made from acetone solution by GLC utilizing a phosphorus
specific detector of the FPD- or AFID types. For the pyridinol
metabolite the determination is made according to McKellar and
Dishburger (1970) on GLC with a nickel (Ni63) EC-detector after
derivatization with N,O-bis(trimethylsilyl)acetamide.
The analytical sensitivities reported as limits of determination
for these methods range from 0.001 to 0.02 mg/kg of
chlorpyrifos-ethyl, about 0.01 mg/kg of the O-analogue and 0.001-0.05
mg/kg of the pyridinol in various substrates and with recoveries of
85.9-100.4% for all the compounds.
The general work chart for the analysis of chlorpyrifos (FAO/WHO
(1973) page 192) also seems to apply for the analysis of
chlorpyrifos-methyl and the general scheme should be adaptable for
regulatory purposes as part of a multiresidue analysis system.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Crop Tolerance, mg/kg (PHI)
Japan Rice 0.03 (60 days)
Chinese cabbage,
radish 0.03 (30 days)
Cabbage 0.03 (7 days)
Tobacco 0.03
APPRAISAL
Chlorpyrifos-methyl is an organophosphorus insecticide of
relatively low toxicity and with broad spectrum effects against a
number of insects of great economic interest as well as several
species of household insects. Since its introduction it has gained
particular and fairly wide interest as a potential grain protectant.
Information on the chemical and physical properties of
chlorpyrifos-methyl has been presented to the Meeting. The compound is
available in crystalline form with a purity of 99.8%. It is relatively
easily hydrolysed with the formation of 3,5,6-trichloro-2-pyridinol
which is also the major metabolite in plants, animals and soils.
It is registered and marketed in some countries as dusts,
granules and emulsifiable concentrates for pre-harvest agricultural
and horticultural purposes and for post-harvest grain treatments.
Recommended dosage rates vary from 0.1 to 2.0 kg/ha under field
conditions and from 2.5 to 10 mg/kg in grain storage.
Residue data from supervised trials and experimental tests with
chlorpyrifos-methyl in several countries have been made available by
the manufacturer in support of registrations, including the
establishment of tolerances. These data include residue levels and
disappearance rates in several vegetable crops, fruits and cereal
plants as well as stored grain products, including wheat, corn and
sorghum grains.
While generally of low persistence, chlorpyrifos-methyl is
relatively stable and evidences prolonged protection against insects
in grains and stored, dried products. The half-life of
chlorpyrifos-methyl in stored wheat averages three to five months
under practical conditions, but at higher moisture levels and higher
temperatures the rate of decomposition increases. Conversely at low
temperatures and low humidities the effective life is expected to be
prolonged.
Evidence of the fate of residues during the milling, processing
and baking of cereals is demonstrated in several trials. Residues in
grains are to a great extent confined to the outer layers and
concentrated in bran and milling offals. Correspondingly they are
diminished to low levels (10% of the original deposits or lower) in
flour and further reduced to half of this in baked bread.
Feeding trials with cows indicate that both chlorpyrifos-methyl
and its pyridinol moiety may give rise to trace amounts (below 0.01
mg/kg) in milk, but that the main excretory route for the parent
compound is via the faeces and for the metabolite via the urine.
Residue studies on meat products, fat or eggs are not available.
Several soil studies have been made on chlorpyrifos-methyl which
confirms that hydrolysis to 3,5,6-trichloro-2-pyridinol is a fairly
rapid process under normal conditions and that total residues of
chlorpyrifos-methyl could hardly persist in soils or give rise to
traceable carry-over in crop rotation. Neither run-off nor leaching of
the compounds from the site of application is likely to occur.
Specific gas-chromatographic methods of analysis are available
for the determination of chlorpyrifos-methyl and
3,5,6-trichloro-2-pyridinol. The analytical procedure is closely
parallel to that for the ethyl analogue, chlorpyrifos, and should be
adaptable for regulatory purposes as part of multiresidue analysis.
Residues of chlorpyrifos-methyl may occur as its main metabolite,
3,5,6-trichloro-2-pyridinol. The amounts of this, however, are
generally low in relation to the original deposits.
RECOMMENDATIONS
The following maximum residue limits based on the parent compound
are recommended. They are not likely to be exceeded when following
good agricultural practices. Determination of the level of
chlorpyrifos-methyl parent compound will adequately control the amount
of total residue resulting from the use of this insecticide.
Maximum Pre-harvest interval
residue on which
limit recommendations
Commodity mg/kg are based (days)
Bran 20
Raw grains (wheat, corn, sorghum) 10
Flour, wholemeal bread 2
Apples, peaches 0.5 14
Tomatoes 0.5 3-5
White bread 0.5
Artichoke, beans, cabbage,
chinese cabbage, eggplant, 0.1 7-14
lettuce (outdoor), peppers,
radish, tea (green)
Rice (pre-harvest treatment) 0.1 21
Milk 0.01
FURTHER WORK OR INFORMATION
REQUIRED (before additional maximum residue limits can be
recommended)
1. Information on residues in animal tissues, fat and eggs
following feeding of chlorpyrifos-methyl residues in animal
feeds.
DESIRABLE
1. Appropriate mutagenic study.
2. Neurotoxicity study with histological examination of nervous
tissues.
3. Information on evidence of residues in commerce.
4. Further information on residue disappearance in practical
grain storage at low temperatures and low humidities.
REFERENCES
Bakke, J. E. and Price, C. E. (1975) Metabolism of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl)phosphorothionate and
3,5,6-trichloro-2-pyridinol in sheep and rats. Metabolism and
Radiation Laboratory, A.R.S., U.S.D.A. Fargo, North Dakota, June 10.
Personal communication to the Dow Chemical Company submitted to the
World Health Organization by the Dow Chemical Co., Midland, Michigan,
USA.
Bengtson, M., Connel, M., Desmarchelier, J., Philips, M., Snelson, J.
and Sticka, R. (1975) Evaluation of four new grain protectants. Report
to the Australian Wheat Board 1975. (To be published) (See
Desmarchelier, 1975c, Table 16).
Branson, D.R. and Litchfield, N.H. (1971) Absorption excretion and
distribution of
O,O-dimethyl-O-3,5,6-trichloro-2-6,14-2-pyridyl-phosphorothioate
(C14-Dowco 214) in rats. Unpublished report from the
Dow Chemical Company submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Brust, H.F. (1969) A summary of chemical and physical properties of
O,O-dimethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report OL 3083 from The Dow Chemical Co., Midland,
Michigan, September 12, 6 pp.
Bulla, L.A. Jr. and LaHue, D.W. (1975) Progress report studies on the
development of new grain protectant treatment - chlorpyrifos-methyl.
Report to The Dow Chemical Co., Michigan from U.S.D.A., A.R.S. Grain
Marketing Research Center, Manhattan, Kansas, February 18, 12 pp.
Coulston, F. and Griffin, T.B. (1975) A safety evaluation of Dowco 214
in rats and rhesus monkeys. Unpublished report, from the Institute of
Comparative and Human Toxicology, Albany Medical College submitted to
the World Health Organization by the Dow Chemical Co., Midland,
Michigan, USA.
Coulston, F., Rosenblum, I. and Griffin, T.B. (1975) Study of
chlorpyrifos-methyl in human volunteers. Unpublished report from
Institute of Comparative and Human Toxicology, Albany Medical College
and the International Center of Environmental Safety, Holloman AFB,
New Mexico submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Davies, R.E. and Collins, C.J. (1975) Response to oxime reactivating
agent in rats acutely poisoned with Reldan technical. Unpublished
report from the Huntingdon Research Centre submitted to the World
Health Organization by The Dow Chemical Co., Midland, Michigan, USA.
Deahl, H.Z. and Tucker, K.E.B. (1974) Residue studies on wheat stored
in vertical concrete silos in Queensland and N.S.W., Australia.
Unpublished report GHF-P-024 from Dow Chemical (Australia) Ltd.,
Altona, Victoria, November 18.
Desmarchelier, J.M. (1975a) Draft report No. 329-4-23 on 1973/74 grain
protectant trials to Australian Wheat Board, Working Party on Grain
Protectants. Chemical analysis - Residues and fate of protectants in
grain. CSIRO, Canberra.
Desmarchelier, J.M. (1975b) Quantitative measurement of rate of loss
of insecticidal activity on treated wheat due to lack of availability
to insects. In press. Communicated to Dow Chemical (Australia) Ltd.,
Sydney, June, 5 pp,
Desmarchelier, J.M. (1975c) An interim report on residues in wheat
treated with chlorpyrifos-methyl-grain protectant field trial, Tammin,
Western Australia. Communication to Dow Chemical (Australia) Ltd.,
Sydney, May 19, 16 pp.
Esaki, K., Tanioka, Y., Tsukada, M., Izumiyama, K. and Ohshio, K.
(1973) Effects of DOWCO 214 against fetuses of experimental animals.
Exper. Animal Central Laboratories, Kawasaki, Japan, Oct. 20.
Unpublished report submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Hamaker, J.W. (1974) Soil absorption of chlorpyrifos-methyl and its
hydrolysis products. Unpublished report GS 1343 (revised) from Dow
Chemical USA, Walnut Creek, California, February 20,
Hascouet, X. (1974) Determination of residues of chlorpyrifos and
chlorpyrifos methyl in vegetables after foliar treatment. Report to
The Dow Chemical Company, France. Ministry of Agriculture, C.N.R.A.,
Versailles, August 5.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E. and Kitagaki, T. (1973a) (Hokkaido Inst. of
Pub. Health, Sapporo, Japan). Studies on the safety of the newly
introduced organophosphorus insecticide, chlorpyrifos-methyl. Part 1.
Acute toxicity of chlorpyrifos-methyl in rats and mice. Hokkaidoritsu
Eisei Kenkyusho-Ho (Rep. Hokkaido Inst. Pub. Health), 23:57-61.
Japanese. English Summary in Pesticide Abstracts 7(4):74-0891, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973b) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of the newly introduced organophosphorus insecticide,
chlorpyrifos-methyl, Part 2. Subacute (6 months) toxicity of
chlorpyrifos-methyl in rats. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23:62-70. Japanese. English Summary in
Pesticide Abstracts 7(4):74-0892, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973c) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of newly introduced organophosphorus insecticide,
chlorpyrifos-methyl. Part 3. Subacute (six months) toxicity of
chlorpyrifos-methyl in mice. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23: 71-80. Japanese. English Summary in
Pesticide Abstracts, 7(4):74-0893, 1974.
Hayashi, E., Nakamura, I., Terada, Y., Kunimoto, M. and Yamada, S.
(1973) Therapeutic experiment on DOWCO 214 poisoned rats. Unpublished
report from Shizuaka Pharmacology College, Shizuoka, Japan, September
15. Submitted to the World Health Organization by The Dow Chemical
Co., Midland, Michigan, USA.
Hollick, C.B. and Collison, R.J. (1972a) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in egg plants, tomatoes, beans and peppers treated with NOLTRAN
insecticide in Turkey., Unpublished report GHE-P 011 from Dow Chemical
Company Limited, King's Lynn, England, August 23.
Hollick, C.B. and Collison, R.J. (1972b) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in peppers treated with NOLTRAN insecticide in Egypt. Unpublished
report CHE-P 102 from Dow Chemical Company Limited, King's Lynn,
England, October 16.
Humiston, C.G., Wade, C.E. and McCollister, S.B. (1972) Results of a
supplemental dietary feeding study of DOWCO 214 in dogs. Unpublished
report from The Dow Chemical Co., submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Cherry, C.P.
(1974a) DOWCO 214 - Toxicity following dietary administration to rats
for two years. Unpublished report from the Huntingdon Research Centre,
Huntingdon, England, submitted to the World Health Organization by The
Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Graham, C. and Brooks, P.N. (1974b) Tumorigenicity
screening of DOWCO 214 in the rat (final report 0-104 weeks).
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by the Dow
Chemical Co., Midland, Michigan, USA.
Johnson, J.C. Jr, Jones, R.L., Leuck, D.B., Bowman, M.C. and Knox,
F.E. (1974) Persistence of chlorpyrifos-methyl in corn silage and
effects of feeding dairy cows the treated silage. J. Dairy Sci.
57(1):467-73.
Kenaga, E.E. (1971) Some physicial, chemical, and insecticidal
properties of some O,O-dialkyl O-(3,5,6-trichloro-2-pyridyl)
phosphates and phosphorothioates. Bull. World Health Org.,
44:225-228.
Kenaga, E.E. (1975) Chlorpyrifos-methyl. Information on identity, use,
residues, analysis and tolerances. Submission from The Dow Chemical
Company, Midland, Michigan, prepared for the FAO/WHO 1975 Joint
Meeting, June 1975.
Kubota, Y. (1974) Summary tables of chlorpyrifos-methyl residue data
for crops and soil in Japan. Reports submitted by Dow Chemical
International Ltd., Tokyo, Japan, February 6.
Leuck, D.B., Jones, R.L. and Bowman, M.C. (1975) Chlorpyrifos-methyl
insecticide residues: their analysis and persistence in coastal
Bermudagrass and forage corn. J. Econ. Entoml., 68(3):287-90.
Litchfield, N. and Norris, J. (1969) O-O-dimethyl-o-3,5,6
trichloro-2-pyridyl phosphorothioate (Dowco 214). Unpublished report
from The Dow Chemical Co., submitted to the World Health Organization
by the Dow Chemical Co., Midland, Michigan, USA.
MacRae, I.C. and Celo, J.S. (1974) The effects of organo-phosphorus
pesticides on the respiration of Azotobacter vinelandii. Soil Biol.
Biochem., 6, 109-111.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography, method ACR 70.19 of the Dow Chemical Company, USA,
December 14.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-2-pyridinol in milk and cream by gas chromatography.
Method ACR. 71.2 of the Dow Chemical Company, USA, February 2.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-2-pyridinol in grass by gas chromatography. Method
ACR. 71.5 of the Dow Chemical Company, USA, May 6.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-2-pyridinol in corn grain, forage and stover by gas
chromatography. Method 71.19 of the Dow Chemical Company, USA,
September 27.
Meikle, R.W. (1973) Hydrolysis rate of DOWCO 214 in buffered distilled
water and in tap water. Unpublished report GS-1316 from the Dow
Chemical Company, USA, Walnut Creek, California, April 16.
Morel, J.L. and Galet, G. (1975) Results of residue analysis carried
out in France in grains treated with chlorpyrifos-methyl until May
1975. Communication from Dow Chemical Europe S.A. (report in press).
Nakajima, E., Shigehara, E. and Shindo, S. (1974) Whole body
autoradiography in rats medicated with the organophosphoric
insecticide DOWCO 214-14C. Unpublished report from the Drug
Metabolism Res. Lab., Sankyo Co. Ltd., Shinagawa, Tokyo, Japan
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Nakayama, I., Kuroda, T., Kitagaki, T., Shinohara, H. and Otokozawa,
T. (1973) Bagworms: effectiveness of several insecticide formulations
against the larvae of three species. J. Econ. Entomol.,
66(4):941-43.
Olson, K.J. (1964a) Results of toxicological tests on
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report from the Biochemical Research Laboratory, The Dow
Chemical Company, Midland, Michigan, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Olson, K.J. and Taylor, M.L. (1964b) Results of range finding
toxicological tests on O,O-dimethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate. Unpublished report from the Biochemical Research
Laboratory, The Dow Chemical Company, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Regoli, A.J., Laskowski, D.A. and Kurihara, N.H. (1974) Aerobic
degradation of chlorpyrifos-methyl in soil. Unpublished report GS-1367
from The Dow Chemical Company, Midland, Michigan, July 12.
Rivett, K.F., Sortwell, R.J., Heywood, R., Street, A.E. and Cherry,
C.P. (1974) Dowco 214 dietary toxicity studies in beagle dogs.
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Ross, D.B. and Roberts, N.L. (1974) The acute toxicity (LD50) of
Reldan (Dowco 214) to the chicken. Report DWC 234/74822. Unpublished
report from the Huntingdon Research Centre, Huntingdon, England,
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Ross, D.B., Burroughs, S.J. and Roberts, N.L. (1975) Examination of
chlorpyrifos-methyl (Dowco 214) for neurotoxicity in the domestic hen.
Report DWC 225/75401. Unpublished report from the Huntingdon Research
Centre, Huntingdon, England, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Schulten, G.G.M. (1973) Further insecticide trials on the control of
Ephestia cautella in stacks of bagged maize in Malawi. Intern. Pest
Control, March/April, p. 18-20.
Schwetz, B.A., Keeler, P.A., Pernell, H.C., Smith, F.A., Humiston,
C.G. and Gehring, P.J. (1973) A study of the prenatal toxicity of
Dowco 214 in rats. Report #NB T35. 12-46193-9, Unpublished report from
The Dow Chemical Company, submitted to the World Health Organization
by The Dow Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974a) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health organization by The Dow
Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974b) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Shellenberger, T.E. (1970) Toxicological evaluations of DOWCO 214 with
wildlife and DOWCO 179 with Mallard ducklings. Unpublished report from
the Gulf Research Institute, New Iberia, Louisiana, submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967) Investigations on
DURSBAN insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agric. Fd. Chem.,
15(5):870-77.
Smith, G.N. and Taylor, Y. (1972) Photodecomposition of DOWCO 214.
Unpublished report NBE-2 from The Dow Chemical Company, Midland,
Michigan, January 21.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Unpublished report
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Snetsinger, R. and Carroll, D. (1972) Evaluation of Dursban and DOWCO
214. Pest Control, 40(5):24-32.
Soderstrom, E.L. and Armstrong, J.W. (1974) Chlorpyrifos-methyl: A
protectant for raisins. J. Econ. Entomol., 68(1), p. 132.
Sparschu, G.L., Humiston, C.G., McCollister, S.B. and Wade, C.E.
(1971) Results of 92-day dietary feeding studies on DOWCO 214 in dogs.
Unpublished report from The Dow Chemical Company submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Steinberg, M. (1971) Summary of findings from 90-day feeding study
with DOWCO 214. Department of the Army. U.S. Army Hygiene Agency,
Aberdeen Proving Ground, Maryland. October 5, 8 pp. (Personal
communication).
U.S. Department of the Army. (1973) Preliminary assessment of relative
toxicity of candidate louse toxicant DOWCO 214 (ENT-27520)
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate evaluation
of animal exposure data and recommendations concerning a sleeve test
program. Study No. 51-19-68/74. November 1968 - May 1972. U.S. Army
Environmental Hygiene Agency. USAEHA-LT. Aberdeen Proving Ground,
Maryland, 38 pp.
Thompson, D.J., Dyke, I.L. and Warner, S.D. (1975) Results of a three
generation, two litter reproduction study on
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Dowco
214) in the rat. Unpublished report from Dow Chemical Corporation,
Department of Pathology and Toxicology, Zionsville, Indiana, submitted
to the World Health Organization by The Dow Chemical Co., Midland,
Michigan, USA.
White, G.S. and LaHue, D. (1974) Preliminary information concerning
DOWCO 214 treatment of wheat grain. Communicated by The Dow Chemical
Company, Midland, Michigan from the U.S.D.A., A.R.S. Grain Marketing
Research Center, Manhattan, Kansas, January 14.
Whitten, C.J. and Bull, D.L. (1974) Comparative toxicity, absorption
and metabolism of chlorpyrifos and its dimethyl homologue in
methyl-parathion resistant and susceptible tobacco budworms. Pest.
Biochem. & Physiol., 4:266-74.
Wirth, M., Würth, E. and Engeler, V. (1971) A residue study: DOWCO 179
and DOWCO 214 on tomatoes in a greenhouse. Unpublished report GHE-P
005 from Dow Chemical Company Limited, King's Lynn, England, December
16.
Wood, P.A. and MacRae, I.C. (1974) The effect of several
organophosphorus insecticides upon the acetylene-reduction activity of
Azotobacter vinelandii. Bull. Environ. Contamin. & Toxicol.,
12(1):26-31.
World Health Organization. (1966) WHO insecticide evaluation and
testing programme. Stage 1 mammalian toxicity report. OMS 1155.
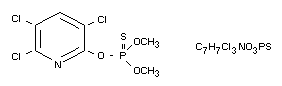 Other information on identity and properties
(Brust, 1969; Kenaga, 1971)
Molecular weight : 322.6
State : Granular crystalline solid
Colour and odour : White/mild mercaptan
Melting point : 45.5-46.5°C
Vapour pressure : 7.40 × 10-7mm Hg at 0°C
4.22 × 10-5mm Hg at 25°C
1.8 × 10-4mm Hg at 35°C
Solubility (g/100g : Acetone 640
at 23°C)
Acetonitrile 680
Benzene 520
Carbon disulfide 430
Carbon tetrachloride 280
Chloroform 330
Diethyl ether 480
Ethanol 30
Methanol 30
n-Octanol 20
Hexane 23
Water 0.0004
The compound readily dissolves in
dimethylformamide, N-methylpyrrolidine,
tetrahydrofuran, xylene, methylene
chloride and 1,1,1-trichloroethane.
Stability : Chlorpyrifos-methyl is reported to be
hydrolyzed by water, the rate being
dependent on temperature and pH.
Half-lives at 25°C vary from 22.7 to
9.4 days in tap water and buffered
distilled water within the Ph range
4.2-8.0.
This hydrolysis is enhanced by traces
of copper ions due to chelation
(Meikle, 1973). The major products of
hydrolysis are
3,5,6-trichloro-2-pyridinol and
O,O-dimethyl phosphate.
Photodecomposition of evaporated
chlorpyrifos-methyl in humid air is
also reported, with the formation of
3,5,6-trichloro-2-pyridinol,
hydroxylated pyridinol derivatives and
oxidation products of quinone
structures (Smith & Taylor, 1972).
Purity : The crystalline compound is stated to
be 99.8% ± 0.1%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 2,6 C14 ring labelled chlorpyrifos-methyl was administered
as a single oral dose (16 mg/kg) to rats, the radioactivity was
rapidly absorbed and excreted. After 72 hours, 90-93% of the
radioactivity was eliminated from the body, 83-85% in urine, 7-9% in
faeces and 0.23-0.43% in respired air. Maximum blood levels (2.4-3.7%)
were reached five hours after treatment. The maximum amount of the
dose remaining in the tissues by 72 hours was 0.65-1.3 ppm. Individual
tissue levels were low (less than 1 ppm). Urinary metabolites were
shown to be 3,5,6 trichloro-2-pyridinol and unidentified activity at
the origin by thin layer chromatography. The possibility of urinary
conjugates was not studied (Branson and Litchfield, 1971a).
The distribution of a single oral dose of C14 labelled
chlorpyrifos-methyl in the body of the young rat was investigated by
whole body autoradiographic techniques. Whole body autoradiograms were
taken at 0.5, 1, 3, 5, 7, 24, 72 and 120 hours after treatment. The
highest level was found initially in the blood. The dispersion in the
various tissues reached a peak at three hours. Residues were extremely
low in fat, liver, kidney and intra-intestinal faeces 72 hours after
administration and completely eliminated from the body by 120 hours
(Nakajima et al., 1974).
Biotransformation
The metabolism of 2,6 C14 ring labelled chlorpyrifos-methyl was
studied in sheep and rats using radioactive counting,
mass-spectroscopy, infrared spectroscopy, column, thin-layer and gas
liquid chromatography techniques. Two ewes were dosed orally with 100
mg/kg by gelatin capsule. Recoveries of radioactivity after four days
were 69.2% in urine, 12.5% in faeces and 2% in tissues. No C14O2 was
detected. Radioactivity in the urine was shown to be the glucuronide
of 3,5,6 trichloro-2-pyridinol 41.6%, o-methyl-o-trimethylsily-o-(3,
5,6 trichloro-2 -pyridyl) phosphorothionate 38% and three minor
unknown fractions 5.2%. In the faeces, 34% of the faecal radioactivity
consisted of parent compound and 55.3% made up primarily of
o-methyl-o-hydrogen-(3,5,6 trichloro-2-pyridyl) phosphorothionate and
3,5,6 trichloro-2-pyridinol. The three fractions found in the urine
were present in the plasma. Detectable amounts of radioactivity (0.32
to 11.8 ppm) were found in the tissues after 96 hours, the highest
level being found in visceral fat (Branson and Litchfield, 1971; Bakke
and Price, 1975).
Ten male rats were given a single oral dose (10 mg) of 2,6 C14
ring labelled chlorpyrifos-methyl. After 48 hours the radioactivity in
the urine consisted of three components, the glucuronide of 3,5,6
trichloro-2-pyridinol (68.6%), o-methyl-o-hydrogen-o (3,5,6
trichloro-2-pyridyl) phosphorothionate (17.8%) and 3,5,6
trichloro-2-pyridinol (13.8%). Faecal metabolites were not
characterized. No respiratory C14O2 was detected (Bakke and Price,
1975).
The principal metabolite of chlorpyrifos-methyl has been shown to
be 3,5,6-trichloro-2-pyridinol. The metabolism of this metabolite in
rats was summarized by FAO/WHO (1973) when the pesticide chlorpyrifos
was evaluated. It was rapidly absorbed and excreted primarily in the
urine and faeces. Small tissue residues (<0.3 ppm) were present and
occurred mainly in biological systems involved with urinary excretion,
i.e. liver, kidney and blood. Residues of the pyridinol in fat were
trivial, which is consistent with its much greater polarity (FAO/WHO,
1973; Smith et al., 1970).
In a similar study in sheep a single oral dose 2,6 C14 ring
labelled 2,5,6 trichloro-2-pyridinol was shown to be rapidly absorbed
and excreted in the urine and faeces. The only radioactive component
detected in the plasma and urine was the glucuronide of the pyridinol
and in faeces the unchanged pyridinol. No C14O2 was detected in
respired air. Residues in tissues after 96 hours were mainly in the
digestive and excretory tracts and was non-detectable in fat, muscle,
heart and other tissues (Bakke and Price, 1975).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
Chlorpyrifos-methyl was administered to groups of Sprague-Dawley
CD strain rats at dietary levels of 0, 0.1 and 1.0 mg/kg/day for 104
weeks. There were no overt signs of reaction to the test compound.
Body weight gain, food consumption, efficiency of food utilization
were comparable between groups and within normal limits. The changes
observed on gross and histopathological examination were those
consistent with the age and strain of rat employed. There was no
compound-related effect an the incidence of spontaneous tumours
(Hunter et al., 1974b).
Special studies on neurotoxicity
Chlorpyrifos-methyl was administered orally to adult hens at 4860
mg/kg body weight and were observed for 21 days. Survivors were given
an intramuscular injection of atropine (10 mg/kg) and PAM (50 mg/kg)
and redosed with 4455 mg/kg chlorpyrifos-methyl and observed for a
further 21 days. No signs of ataxia were detected. The birds were not
examined histologically for nervous systems lesions. TOCP was used as
a positive control (Ross et al., 1975).
Special studies on reproduction
Rat
In a three generation reproduction study (two litters per
generation) three groups of 10 male and 20 female rats received
chlorpyrifos-methyl at dietary levels of 0, 1.0 or 3.0 mg/kg body
weight/day. No treatment related effects on behaviour, survival, body
weight gain or food consumption were observed in parental animals.
Fertility, gestation, viability and lactation indices were comparable
to control values. Pup weights of the second litter, third generation
were significantly less than control at 0, 4 and 21 days postpartum.
There was no effect on sex ratio. In adults of third generation,
plasma cholinesterase activity was decreased in both sexes at both
dosage levels. Erythrocyte cholinesterase depression was observed at 1
mg/kg in females and in both sexes at the highest dose level. Brain
cholinesterase activity was not affected. Gross and histopathological
examination of control and high dose parental animals of the third
generation did not reveal any treatment related abnormalities
(Thompson et al., 1975).
Special studies on teratogenicity
Mouse
Chlorpyrifos-methyl was administered orally to mice at dosage
levels of 0, 20, 100 and 250 mg/kg body weight/day from day 7 through
day 13 of gestation. Animals were sacrificed on day 18 and pups
removed by caesarean section. No significant differences between the
control and treated groups were observed in regard to number of
implants and number of deaths. Body weights were lower in both males
and females at 250 mg/kg. An increased incidence of cleft palate and a
delay of ossification of the cervicovertebral body was observed at 250
mg/kg. When pregnant mice were dosed with a single oral dose of 1000
mg/kg on the seventh or eleventh day of pregnancy, there was no effect
on mortality or body weights of the foetuses. Skeletal abnormalities
were observed (exencephalia, cleft palate, liberation of bone fragment
of cervicovertabral arch) in a few cases especially on treatment on
seventh day of gestation. No control data was presented in the 1000
mg/kg body weight study. In a further study, pregnant mice were
treated as before with 0, 20, 100 and 250 mg chlorpyrifos-methyl/kg
body weight/day on day 7 through day 13 of gestation. Females were
allowed to deliver their pups. Body weights at birth and after three
weeks were dose dependent, being lower on the higher treatment. No
significant morphological abnormalities were detected (Esaki et al.,
1973).
Pregnant rats received 0, 20, 100 and 250 mg/kg body weight of
chlorpyrifos-methyl from day 9 to day 15 of gestation. On day 20
animals were laparotomized and embryos removed. No reproductive or
teratogenic effects were observed. In a similar study the pregnant
rats were allowed to deliver their progeny. Body weight at birth
tended to be higher in the treated groups but no differences observed
when weaned at four weeks. No teratogenic effects related to treatment
were noted (Esaki et al., 1973).
Chlorpyrifos-ethyl was administered to pregnant rats at 0, 50,
100 or 200 mg/kg body weight/day on days 6 through 15 of gestation.
Animals were killed on day 21 of gestation and foetuses removed by
caesarean section. No significant effects were observed in the number
of corpora lutea per pregnant female, the number of resorption sites
or the number of live foetuses. No dose-related effects were observed
in the incidence of runts, subcutaneous oedema and dilated renal
pelvis. Evidence of delayed ossification of sternabrae was observed at
all treatment levels with an increase in lumbar spurs at the high dose
level. The administration of 200 mg chlorpyrifos-methyl/kg body weight
for 10 days caused a significant decrease in plasma and red blood cell
cholinesterase activity in maternal blood as well as in a homogenate
of foetal tissues (Schwetz et al. 1973;Schwetz, 1974a, b).
Acute toxicity
LD50
Species Sex Route Solvent (mg/kg) Reference
Rat M oral CMC 3 733 Hasegawa at al., 1973a
F oral CMC 3 597 "
M oral corn oil 2 472 "
F oral corn oil 1 828 "
M&F oral corn oil 1 700 Davis and Collins, 1975
M oral corn oil 2 140 Litchfield and Norris, 1969
F oral corn oil 1 090 "
M oral corn oil 2 140 Olson, 1964a
F oral corn oil 1 630 "
F oral corn oil >1 000 Olson et al., 1963
F oral corn oil 3 600 Esaki et al., 1973
M&F dermal CMC >4 827 Hasegawa et al., 1973a
M&F dermal corn oil >3 713 "
LD50
Species Sex Route Solvent (mg/kg) Reference
Mouse M oral corn oil 2 254 "
F oral corn oil 2 032 "
M oral arachis oil 1 122 WHO, 1966
F oral corn oil 2 440 Esaki et al., 1973
M&F dermal CMC >2 856 Hasegawa at al., 1973a
Cavy M oral corn oil 2 250 Olson, 1964a
Rabbit M&F oral corn oil approx.
2 000 "
Chicks M oral corn oil >7 950 Olsen, 1964a
(capsule)
Chicken F oral corn oil 4 860 Ross et al., 1975
(capsule)
M&F oral corn oil 7 532 Ross and Roberts, 1974
(gavage)
oral corn oil 8 000 "
(capsule)
Undiluted chlorpyrifos-methyl was applied directly to
conjunctival sac of rabbits. There were signs of conjunctival
irritation which subsided after 24-48 hours. No corneal injury was
discernible (Olson and Taylor, 1964b).
No significant skin reaction occurred when undiluted
chlorpyrifos-methyl was applied to shaved and abraded skin of the
rabbit for prolonged periods (Olson and Taylor, 1964b; United States
Army, 1973).
Cholinesterase activity was reduced by 90% and all animals died
within 24 hours when rats were treated orally with chlorpyrifos-methyl
(4 g/kg) and subcutaneously with various levels of atropine sulfate.
Rats were treated with a sublethal oral dose of chlorpyrifos-methyl in
conjunction with subcutaneous injections of atropine sulfate and PAM
(25, 50 and 100 mg/kg) and glutathione (100, 300, 500 and 700 mg/kg)
or combinations of each. Symptoms of anticholinesterase activity were
observed and total blood, erythrocyte, plasma and brain cholinesterase
levels determined. The antidotes were effective especially at the
upper dose levels in reducing the toxic symptoms. The combination of
atropine sulfate with PAM or glutathione was more effective than
atropine sulfate alone (Hayashi et al., 1973).
Chlorpyrifos-methyl was administered to groups of rats at 5 x
LD50 (8.5 g/kg). At first signs of anticholinesterase activity one
group was given atropine sulfate (17.5 mg/kg) intramuscularly and a
further group atropine sulfate (17.5 mg/kg) and PAM (50 mg/kg)
intramuscularly. A further administration of antidote was given to all
rats seven hours after treatment. All treated rats died within 24
hours, untreated controls died within eight hours (Davies and Collins,
1975).
Short-term studies
Avian species
Mallard ducks, bobwhite and Japanese quail were fed various
levels of chlorpyrifos-methyl for five days and observed for a further
three days. The following LC50 values were determined.
Bobwhite quail - 1835 ppm, Japanese quail ->5000 ppm, mallard
duck - 2500-5000 ppm. Body weight and food consumption were reduced
for bobwhite and Japanese quail at 2500 and 1250 ppm respectively and
at 5000 ppm for the mallard duck. In a similar study, mallard ducks
were fed graded levels of chlorpyrifos-methyl for five days. Whole
blood cholinesterase activity was inhibited at dietary levels of 78
ppm and greater. Brain cholinesterase was not affected at the highest
level tested (Shellenberger, 1970).
Mouse
Chlorpyrifos-methyl was administered orally to mice at 0, 100,
250, 500 and 1000 mg/kg body weight/day for 14 days. No effect on
mortality or significant changes in body weight at 100 and 250 mg/kg
were observed. After four doses, there was 100% mortality at 1000
mg/kg. At 500 mg/kg, 40% mortality occurred in 14 days and a
noticeable decrease in body weight (Esaki et al., 1973).
Groups of male and female mice were fed standard diets containing
0, 0.5, 1, 5, 10, 20, 30, 40 and 100 ppm for a period of six months.
No differences were observed in mortality, food intake, body weight
gain, organ weights and urinalysis. Haematological, biochemical and
pathohistological parameters were considered normal in groups given
less than 40 ppm. Plasma and erythrocyte cholinesterase activities
were lower at dosage levels greater than 20 ppm. Brain cholinesterase
was decreased in males given 40 and 100 ppm and in females given 40
ppm (Hasegawa et al., 1973c).
Rat
Chlorpyrifos-methyl was administered orally to rats at 20, 100
and 500 mg/kg body weight/day for 14 days. No significant effect on
mortality or body weight gain was shown. At 500 mg/kg hypertrophy of
the liver and heart were observed (Esaki et al., 1973).
Groups of female rats were fed chlorpyrifos-methyl in the diet
for 14 days at 0, 1.6, 6, 25, 95, 500 and 2130 mg/kg body weight/day.
Plasma and red blood cell cholinesterase was depressed at 6 mg/kg and
in brain at 95 mg/kg. Organ to body weight ratios of liver, kidney and
spleen were not significantly different from control but brain to body
weight ratio was increased at 2130 mg/kg. No compound-related gross or
histopathological lesions were observed (United States Army, 1973).
Groups of six male and female rats consumed in their diet 0,
0.08, 0.8, 8.0, 40, 80 and 160 mg chlorpyrifos-methyl/kg body
weight/day for 90 days. Plasma cholinesterase activity was depressed
in males at 8 mg/kg and above and in females at 0.8 mg/kg and above.
The incidence of erythrocyte and brain cholinesterase depression was
significantly increased at 8.0 mg/kg/day and above for both male and
female. Liver weight to body weight ratio of male rats was increased
at 40, 80 and 160 mg/kg. Apart from small aggregates of lymphocytes
and plasma cells in the periportal areas of the liver, no changes were
noted in gross or histopathology (Steinberg, 1971).
Chlorpyrifos-methyl was fed at dietary levels of 0, 0.5, 1, 5,
10, 20, 30, 40 and 100 ppm to groups of male and female Wistar rats
for six months. No significant differences were noted in mortality,
food intake, weight gain, organ weights and urinalysis.
Haematological, biochemical and pathological findings were not
affected at 40 ppm or less. Serum and erythrocyte cholinesterase
activity was decreased at 30 ppm and above at one, three and six
months (Hasegawa et al., 1973b).
Groups of five male and five female rats received 0, 0.2, 1.0 and
5.0 mg chlorpyrifos-methyl/kg body weight/day by stomach tube daily,
six days/week, for six weeks. Plasma cholinesterase activity was
depressed in females at 5 mg/kg. Erythrocyte and brain cholinesterase
activities were not significantly reduced. Growth, haematology, blood
chemistry and liver microsomal mixed function oxidase activities were
not affected (Coulston and Griffin, 1975).
Dog
Groups of dogs (two males and two females/group) were fed
chlorpyrifos-methyl in the diet for 92 days at dose levels of 0, 0.3,
1, 3 and 10 mg/kg body weight/day. No treatment-related effects were
observed in behaviour, food consumption, body weight, hematology,
clinical chemistry, urinalysis, organ weight: body weight ratios,
gross and histopathology. Plasma cholinesterase activity at all test
levels was depressed after one, four and 12 weeks of treatment.
Inhibition of erythrocyte cholinesterase activity was evident at 1.0,
3.0 and 10.0 mg/kg body weight/day. The activity of brain
cholinesterase was not affected (Sparschu et al., 1971).
A supplemental study was conducted in which groups of dogs (two
males and two females/group) were fed chlorpyrifos-methyl in the diet
at dose levels of 0, 0.03 and 0.1 mg/kg body weight/day for 125 days.
There was no significant depression of plasma or erythrocyte
cholinesterase activity. Behaviour was normal and body weight and food
consumption considered to be within normal limits (Humiston et al.,
1972).
Groups of dogs (seven males and seven females/group) were fed
chlorpyrifos-methyl at dietary levels of 0, 0.03, 0.1, 1.0 and 3.0
mg/kg/day for 104 weeks with an interim sacrifice of three males and
three females/group at 26 weeks. No significant effects were observed
with respect to mortality, behaviour, food and water consumption,
ophthalmology, haematology, blood biochemistry, organ to body weight
ratio or gross and histopathology at either 26 or 104 weeks. Treated
animals gained less weight than controls due to some weight loss
during the latter part of the study. Body weights, however, were
considered within normal limits. Cholinesterase activity was depressed
in red blood cell and plasma at 3.0 mg/kg and in plasma at 1.0 mg/kg.
There was no reduction in brain cholinesterase activity at 26 or 104
weeks (Rivett et al., 1974).
Monkey
Chlorpyrifos-methyl was administered by stomach tube daily, six
days/week for six months to five groups of rhesus monkeys, three male
and three female group at dose levels of 0, 0.1, 0.2, 1.0 and 5.0
mg/kg body weight/day. Plasma cholinesterase was depressed in those
animals receiving 1.0 and 5.0 mg/kg. Inhibition of erythrocyte
cholinesterase was evident at 5.0 mg/kg. Brain cholinesterase was not
affected at any test level. No treatment related effects were noted in
growth, haematology, blood chemistry, mixed function oxidase activity
of the liver, relative organ weights, gross or histopathology
(Coulston and Griffin, 1975).
Cow
Groups of four cows were fed for 42 days silage corn which had
been sprayed 83 days previously with chlorpyrifos-methyl. The diets
contained 0, 0.35, 0.87 and 1.85 ppm chlorpyrifos-methyl and 0, 0.44,
0.79 and 1.75 ppm 3,5,6 trichloro-2-pyridinol. Residue intakes
amounted to 0.009, 0.022 and 0.054 mg chlorpyrifos-methyl and 0.012,
0.020 and 0.051 mg 3,5,6 trichloro-2-pyridinol/kg body weight. No
adverse effects were observed in silage intake, milk production, blood
cholinesterase activity or body weight gains. Urine, faeces and milk
contained trace amounts of chlorpyrifos-methyl and 3,5,6
trichloro-2-pyridinol. One week after treated feed withdrawn these
residue were not detectable (Johnson et al., 1974).
Long-term studies
Rat
Groups of 55 male and 55 female rats were administered
chlorpyrifos-methyl in the diet at 0, 0.03, 0.1, 1.0 and 3.0 mg/kg
body weight/day for 104 weeks. After five weeks of treatment five
males and five female/group were sacrificed for cholinesterase
determination. Twenty-six weeks after treatment 10 males and 10
females/group were killed for interim study and the size of each group
was reduced to 30 males and 30 females after 52 weeks. Red blood cell
cholinesterase activity was depressed in both sexes at 1.0 and 3.0
mg/kg/day. Plasma cholinesterase activity was reduced consistently in
females at 1.0 and 3.0 mg/kg/day; males showing little effect during
the first year after which it was significantly depressed at 3.0
mg/kg/day. Brain cholinesterase levels were not affected.
Chlorpyrifos-methyl produced no significant effect on behaviour,
mortality, body weight gain, food consumption and efficiency of food
utilization. Haematologic, blood biochemical and urinalysis
determinations were within normal limits and revealed no abnormal
changes. No effect attributable to treatment was observed in organ
weights or on gross and histopathologic examination. Tumour incidence
was comparable between groups (Hunter et al., 1974a).
Observations in man
Fourteen male volunteers were divided into two treatment groups
of five men each and a control group of four men. Chlorpyrifos-methyl
was administered by gelatin capsule in a single daily dose of 0, 0.03
and 0.1 mg/kg body weight/day for four weeks. Plasma and erythrocyte
cholinesterase activities were not depressed at levels tested.
Haematology, blood chemistry, urinalysis, blood pressure, pulse and
ophthalmology were not affected by treatment (Coulston et al., 1975).
COMMENTS
In mammals, chlorpyrifos-methyl is rapidly absorbed and
metabolized the principal metabolite being
3,5,6-trichloro-2-pyridinol. The parent compound and metabolite are
excreted primarily in the urine and faeces and are not stored to any
extent in the body. This metabolite has also been shown to occur in
plants.
Teratogenic studies in the rat revealed no adverse effects at
dietary intake levels of up to 250 mg/kg. In an inadequately conducted
and reported study in the mouse there appeared to be a tendency for a
weak teratogenic response at 250 mg/kg. No compound-related
abnormalities were noted at 100 mg/kg.
No adverse effects were noted in the reproductive capacity of the
rat over three generations at dietary levels of up to 3 mg/kg.
Mutagenic studies have not been reported.
No apparent clinical evidence of neurotoxicity was observed in
hens given a large dose of chlorpyrifos-methyl. Histological
examination of nervous tissue was not conducted. In various short- and
long-term studies in the rat, dog, mouse, monkey, mallard duck,
bobwhite and Japanese quail, chlorpyrifos-methyl was shown to be an
active cholinesterase inhibitor. Plasma cholinesterase activity was
inhibited to a greater extent than either erythrocyte or brain
cholinesterase.
In the long-term study and specific tumorigenic study in the rat,
no compound-related effect or increase in tumour incidence was
observed.
No effect levels were demonstrated in the rat, dog, monkey and
man on the basis of the most sensitive parameter plasma cholinesterase
inhibition. The no-effect level in man was confirmed by two
independent studies. The data were considered sufficient to allocate
an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg
Dog: 0.1 mg/kg
Monkey: 0.2 mg/kg
Man: 0.1 mg/kg
Estimation of acceptable daily intake
0-0.01 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of chlorpyrifos-methyl for insect control in fruit,
vegetable and cereal plants and in grain storage is recorded from the
following countries: Egypt, France, Japan, Korea, and Turkey. In these
countries it is marketed as dusts and/or granules and as emulsifiable
concentrates containing 2-3% a.i. and 25-40% a.i. respectively.
As a relatively new chemical chlorpyrifos-methyl is marketed as a
broad-spectrum organophosphorus insecticide of relatively low toxicity
and persistence, although it shows reasonably good stability in
stored, dry products such as grains (Schulten, 1973) and dried fruits
(Soderstrom and Armstrong, 1974). In these products it controls a wide
range of beetles, weevils, moths and mites, including several such
species which may have developed resistance towards other
insecticides, e.g. malathion. Using spray techniques or admixing
procedures to treat the stored grain products, chlorpyrifos-methyl is
usually applied at the rate of 5-10 mg a.i. per kg.
In several of the countries mentioned, chlorpyrifos-methyl is
approved for wider uses in agriculture and horticulture against pests
of economic importance connected to fruit and vegetable growing
(Nakayama et al., 1973). In laboratory tests it is further found to be
effective against a wide range of household pests, including spiders,
houseflies, cockroaches, house crickets etc, (Snetsinger, 1972).
A number of recommended practical applications is listed in Table
1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on chlorpyrifos-methyl residues resulting from a number of
the above uses have been presented by the originating company (Kenaga,
1975). The data derived from field trials and controlled experimental
work under a variety of geographical and climatic conditions is as
follows.
In plants
Cereal plants and grass
Leuck et al. (1975) found chlorpyrifos-methyl to be relatively
non-persistent in field trials where coastal bermuda grass and corn
were sprayed once with 0.56, 1.12 and 2.24 kg a.i. per ha (see Table
2). In both crops and at all three levels chlorpyrifos-methyl
diminished rapidly at rates corresponding to half-lives of about two
to three days. The hydrolysis product, 3,5,6-trichloro-2-pyridinol,
was more persistent and in some cases actually increased up to a
maximum during the first few days after application. Thereafter the
pyridinol disappeared following a first order scheme with estimated
half-life values of 9-12 days.
TABLE 1. Some use patterns of chlorpyrifos-methyl on plants and grain
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Australia Emulsifiable concentrate Wheat 5.0 mg/kg
Egypt Emuls. conc., 24% (w/v) Spodoptera littoralis and 0.9 kg/ha or Foliar broadcast spray
S. exigua on vegetables 90 g/litre
and clover
France Emuls. conc., 24% (w/v) Stored grain pests 2.5 mg/kg
Japan Dust, 2% (w/w) Rice stemborer 0.6-0.8 kg/ha Max. 4 applic. per season
Micro-granule, 3% (w/w) Rice stemborer 0.9-1.2 kg/ha Broadcast appl. Max. 4 x
per season
Granule, 5% (w/w) Rice stemborer 1.5-2.0 kg/ha Submerged appl. Max. 4 x
per season
Emuls. conc., 25% (w/v) Rice stemborer 0.1-0.375 kg/ha Max. 4 sprays/season:
rice and cabbage
Diamondback moth, Pieria
rapae, aphids on tobacco Max. 2 sprays/season:
Cutworm on cabbage, chinese cabbage and
chinese cabbage, radish radish
Korea Granule, 3% (w/w) Rice stemborer, plant 0.9-1.2 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice.
Tobacco cutworm, mole
cricket on tobacco and
vegetables
TABLE 1. (Cont'd)
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Korea Emuls. conc., 25% (w/v) Rice stemborer, plant 0.25-0.35 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice
Tobacco cutworm, mole
cricket on tobacco and
vegetables
Sudan Emuls. conc., 24% (w/v) Pests of apricots
Turkey Emuls. conc., 24% (w/v) Spodoptera litteralis and 0.9 kg/ha Foliar broadcast spray
Heliothis obsoleta in
cotton and vegetables
Aphids on melons
USA Emuls. conc. Wheat 5-10 mg/kg
Johnson et al. (1974) in an earlier experiment sprayed corn in
the dent stage with chlorpyrifos-methyl at the same dosage rates. In
this case, the corn was harvested and ensiled for forage purposes one
day after the treatment (Table 2). The initial losses of
chlorpyrifos-methyl from the time of application through harvesting
and ensiling amounted to 62-79%. After the ensiling, however, residues
became relatively stable and they disappeared only slowly with
formation of the pyridinol. From the first day until the eighty-third
day, on average for the three treatments, 60% of the
chlorpyrifos-methyl disappeared while the pyridinol increased 421%.
TABLE 2. Residues of chlorpyrifos-methyl and pyridinol in grass and corn
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Bermudagrass, 0.56 0 5.5 1.07
coastal 7 0.1 0.73
(Leuck et al., 1975) 21 <0.01 0.32
1.12 0 12.5 2.21
7 0.21 1.81
21 0.01 0.58
2.24 0 28.4 4.48
7 0.45 4.08
21 0.09 1.24
Corn, growing 0.56 0 2.2 0.11
(Leuck et al., 1975) 7 0.11 0.17
21 0.03 0.09
1.12 0 8.2 0.41
7 0.29 0.54
21 0.07 0.21
2.24 0 20.4 0.78
7 1.43 1.38
21 0.20 0.60
TABLE 2. (Cont'd.)
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Forage corn, 0.56 0 2.22 0.11
ensiled at day 1 1 0.75 0.13
(Johnson et al., 27 0.51 0.48
1974) 55 0.47 0.49
83 0.34 0.70
125 0.34 0.27
1.12 0 8.20 0.41
1 1.92 0.34
27 1.42 1.09
55 1.27 1.40
83 0.72 1.74
125 0.77 0.35
2.24 0 20.40 0.78
1 4.08 0.69
27 2.98 2.50
55 2.31 2.28
83 1.64 3.34
125 1.80 1.13
Kubota (1975) has reported on field trials with growing rice. As
shown in Table 3, residues at the time of harvest were not detectable
or at most insignificant when spraying was carried out 4-17 days
before harvest.
Stored grain products
White and LaHue (1974), in their experiments showing effective
protection against several grain insects, e.g. rice weevil, lesser
grain borers, flour beetle, sawtooth grain beetle, and foreign grain
beetle, also followed the dissipation of chlorpyrifos-methyl in stored
wheat, sorghum and maize. Wheat at 79°C and containing 14.6% moisture
was treated with 4.8, 6.6 and 10.4 mg/kg chlorpyrifos-methyl. The
residue half-life was >6 months, 2-3 months and <1 month,
respectively. Maize and sorghum tested under similar conditions at
about 6-7 mg/kg had a residue half-life of about 2-3 months (Table 4).
TABLE 3. Residues of Chlorpyrifos-methyl in rice*
Application
Rate Days after Number of
Formulation (kg/ha) Frequency last treatment samples Residues, mg/kg
3% dust 1.2 2 × 35-42 8 <0.005-<0.005(n.d.)
4 × 14-42 8 <0.005-<0.005(n.d.)
6 × 11 8 <0.005-<0.005(n.d.)
5% granule 2 2 × 53-57 8 <0.005-<0.005(n.d.)
4 × 53-57 8 <0.005-<0.005(n.d.)
6 × 17-32 8 <0.005-0.012
25% e.c. 0.30-0.375 2 × 32-53 8 <0.005-<0.005(n.d.)
4 × 24-53 8 <0.005-<0.005(n.d.)
6 × 4-32 8 0.005-0.013
* From Kubota (1975).
TABLE 4. Residues of chlorpyrifos-methyl in stored grains in the United States of America*
Residue, mg/kg
Grain 0 days 5 days 1 2 3 4 5 6 8 9 months
Wheat (26°C, 4.8 4.6 3.5 3.0 2.8 3.3 - 3.3 - -
14.6% moisture) 6.6 5.5 5.0 2.8 3.2 - - 1.6 - -
10.4 6.2 4.1 2.6 2.3 - - 4.4 - -
Corn
(13.6% moisture) 6.70 - - - - 3.2 2.5 2.3 1.9 1.8
Sorghum - - - - 3.5 3.3 2.7 2.6 2.2 1.9
* From White and LaHue (1974).
In laboratory experiments, Bulla and LaHue (1975) tested the
disappearance rates of chlorpyrifos-methyl on the same three grains,
kept for six months at 80°F in covered glass jars with regulated
moisture contents from 10 to 16%. With overall half-life rates varying
from one to six months they found a decreasing persistence in the
sequence: maize > wheat > sorghum. Grains containing 14 or 16%
moisture degraded chlorpyrifos-methyl about 1.5-2 times faster than
those containing 10 or 12%.
In similar Australian experiments under commercial conditions,
Deahl and Tucker (1974) found that chlorpyrifos-methyl at initial
concentrations of 5.0 and 6.2 mg/kg declined at rates corresponding to
half-lives of the order of three to five months (see Table 5). The
average half-life at 31°C was 15-16 weeks with indications of a
reduced breakdown at lower temperatures (Desmarchelier, 1975a).
From these extensive trials, Desmarchelier et al. (1975b and c)
also reported the rate of loss of residues of chlorpyrifos-methyl on
white wheat containing 11% moisture at 25°C when stored in glass jars.
These tests indicate that under the laboratory conditions stated,
chlorpyrifos-methyl decays at a predictable first-order rate,
irrespective of concentration or age of the residues.
Morel and Galet (1975) report tests conducted in France on wheat
containing 15.2% moisture and 2.35 and 1.17 mg/kg chlorpyrifos-methyl
which was stored for various periods of time at 25°C for residue
analyses. Under these conditions half-lives of chlorpyrifos-methyl
were estimated to be about six weeks. Residue levels six months after
treatment were in the range of 0.2-0.4 mg/kg.
Apples and peaches
Residue levels on apples and peaches have been established under
Japanese conditions following spraying at recommended dosage rates,
i.e. about 1-1.5 kg/ha. The results of these trials are shown in Table
6 which indicates levels in apples below 0.3-0.5 mg/kg if harvested
about two weeks after the last treatment. The results for peaches do
not show signs of penetration of residues through the peel into the
fruit pulp.
Vegetable plants
Residue data from experiments with chlorpyrifos-methyl
applications on vegetable plants are shown in Tables 7 and 8. The
experiments derive from different geographical regions and cover
mostly foliar applications on the following crops: artichoke, beans,
cabbage, chinese cabbage, eggplant, lettuce, pepper, radish, tea and
tomatoes.
TABLE 5. Residues of chlorpyrifos-methyl during silo-storage, Australia*
Residue, mg/kg after interval (weeks)
Depth of
Treatment silo 1 6 11 16 22 26
6.2 mg/kg 0.1 m 4.5 3.1 2.3 2.1 1.9 1.5
(Queensland 1.5 m 3.4 3.6 2.1 2.3 1.8 1.5
experiment) 6.0 m 3.2 3.0 2.4 2.2 2.0 -
Average 3.7 3.2 2.3 2.2 1.9 1.5
3 8 13
5.0 mg/kg 0.1 m 4.1 3.9 1.8
(N.S. Wales 1.5 m 4.1 3.7 2.4
experiment) 6.0 m 3.3 3.1 2.4
Average 3.8 3.6 2.2
* From Deahl and Tucker (1974).
TABLE 6. Residues of chlorpyrifos-methyl in apples and peaches, Japan*
Preharvest Number of Number of Range of
Application interval applications samples results (mg/kg)
Apples
1.25-1.5 10 days 1-2 × 6 0.22-0.353
kg/ha 20 days 1-2 × 7 0.201-0.310
30 days 1-2 × 4 0.174-0.186
10 days 4 × 9 0.182-0.798
20 days 4 × 6 0.128-0.225
30 days 4 × 4 0.162-0.257
10 days 6 × 4 0.489-0.936
20 days 6 × 7 0.152-0.333
30 days 6 × 4 0.250-0.478
Peaches Peel Pulp
0.9 kg/ha 21 days 2 × 6 <0.03-0.26 <0.005
30 days 2 × 8 <0.03-0.05 <0.005
21 days 4 × 6 <0.03-0.11 <0.005
30 days 4 × 8 <0.03-0.05 <0.005
21 days 6 × 6 <0.03-0.10 <0.005
30 days 6 × 8 <0.03-0.06 <0.005
* From Kubota (1975).
TABLE 7. Residues of chlorpyrifos-methyl in vegetables, Europe/Near East
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Artichoke 48 g/100 l 7 days 2 0.05 France1
(aver.)
14 days 2 0.03
(aver.)
Beans 1.2 kg/ha 0 days 1 0.32 Turkey2
7 days 1 0.01
14 days 1 0.02
Beans, 48 g/100 l 7 days 5 <0.02 France1
french (aver.)
14 days 3 <0.02
(aver.)
Eggplant 1.2 kg/ha 0 days 1 0.06 Turkey2
7 days 1 0.01
14 days 1 0.01
Lettuce 48 g/100 l 7 days 4 0.78 France1
(aver.)
14 days 2 0.04
(aver.)
Pepper 1.2 kg/ha 0 days 1 2.28 Egypt3
1 day 1 0.58
2 days 1 0.25
3 days 1 0.20
5 days 1 0.09
7 days 1 0.10
9 days 1 0.04
TABLE 7. (continued)
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Tomato 0.1% until 3 days 1 1.90 England4
run-off 6 days 1 0.90
(greenhouse) 9 days 1 0.40
13 days 1 0.09
1.2 kg/ha 0 days 1 0.31 Turkey2
7 days 1 0.04
14 days 1 0.05
48 g/100 l 7 days 6 0.21 France1
(aver.)
14 days 6 0.04
(aver.)
1 Hascouet (1974).
2 Hollick and Collison (1972a).
3 Hollick and Collison (1972b).
4 Wirth et al. (1971).
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Cabbage 0.45 kg/ha 7 days 2 × 8 <0.002-0.002
14 days 2 × 8 <0.002
7 days 4 × 8 <0.002
14 days 4 × 8 <0.002
Chinese 0.25 kg/ha 14 days 4 × 2 <0.005
cabbage
0.375 kg/ha 7 days 2 × 7 0.027-0.220
14 days 2 × 7 <0.005-0.100
7 days 4 × 7 0.036-0.175
14 days 4 × 5 0.039-0.056
0.50 kg/ha 7 days 2 × 4 0.019-0.025
14 days 2 × 4 0.004-0.006
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Chinese 7 days 4 × 4 0.001-0.002
cabbage 14 days 4 × 4 0.003-0.007
Eggplant 0.75 kg/ha 3 days 2 × 4 0.002-0.003
7 days 2 × 4 0.001
14 days 2 × 4 <0.002
3 days 4 × 4 0.002-0.004
7 days 4 × 4 0.001
14 days 4 × 4 <0.002
Pepper, 0.50 kg/ha 3 days 2 × 4 0.011-0.171
green 7 days 2 × 4 0.004-0.028
14 days 2 × 4 <0.002-0.012
Pepper, 3 days 4 × 4 0.013-0.129
green 7 days 4 × 4 0.003-0.060
(cont'd) 14 days 4 × 4 <0.003-0.021
Leaves Root
Radish 0.2-0.3 kg/ha 7 days 2 × 4 0.024-0.324 <0.002-0.069
28 days 2 × 4 0.003-0.009 0.005-0.008
7 days 3 × 2 0.109 <0.001-0.003
28 days 3 × 4 0.024-0.130 <0.001-0.001
7 days 4 × 4 0.040-0.299 <0.002-0.081
28 days 4 × 4 0.003-0.010 0.013-0.020
7 days 6 × 2 0.075 <0.002-0.002
14 days 6 × 4 0.019-0.040 0.002-0.003
Tea 0.50 kg/ha 7 days 1 × 6 <0.001-0.065
14 days 1 × 6 <0.001-0.047
21 days 1 × 6 <0.003
27 days 1 × 6 <0.003
7 days 2 × 6 <0.001-0.026
0.75 kg/ha 7 days 1 × 7 <0.001-0.091
14 days 1 × 6 <0.001-0.056
21 days 1 × 6 <0.001-0.047
7 days 2 × 6 <0.003-0.085
* From Kubota (1975).
The levels of chlorpyrifos-methyl residues on these crops at the
time of harvest, i.e. from 7 to 14 days after the last treatment are
generally at or below 0.1 mg/kg.
FATE OF RESIDUES
In plants
Residues of chlorpyrifos-methyl in or on plants are assumed to
behave similarly to chlorpyrifos Of FAD/WHO, 1973) without
translocation in plants to any significant extent.
The principal hydrolysis product of chlorpyrifos-methyl in
plants, animals and soil, 3,5,6-trichloro-2-pyridinol, may or may not
be taken up by plants depending on pH. The free pyridinol, which is
the predominant form of the compound below pH 6.0, is essentially
insoluble in water. In radio-labelled studies, soil and nutrient
culture experiments have shown its uptake by plants to be
insignificant (Smith et al., 1967). At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the sodium salt) as well as rate of absorption by the
plant are enhanced compared with the free pyridinol. Although still at
very low levels, the sodium salt of the pyridinol enters the plant
more than five times as fast as the free pyridinol. It undergoes
metabolism with the liberation of chloride and the formation of
several unidentified water soluble decomposition products.
In cereal-products
Residues of chlorpyrifos-methyl in treated grains have been
followed from the time of application through milling and baking
procedures in several experiments (see Table 9). Bulla and LaHue
(1975) fractionated wheat containing 6.3 mg/kg chlorpyrifos-methyl and
found that most of the residues after three and six months of "ageing"
were still concentrated in the outer grain fractions, such as "red
dog" and bran. The residues in flour were reduced to below 10% of the
original concentration (i.e. to 0.53 and 0.39 mg/kg, respectively).
Bread baking reduced the remaining residues further by about 60%.
Similar results have been found by Bengtson et al. (1975) who
after 11 and 22 weeks of ageing also found considerable reduction from
grain residues to milled flour and baked bread, the latter showing
below 0.25 mg/kg residual chlorpyrifos-methyl.
Morel and Galet (1975) analysed wheat which was milled from 6 to
17 weeks after treatment of commercially stored grains with 1.6-1.8
mg/kg of chlorpyrifos-methyl. The distribution of residues in various
milled fractions is shown in Table 9. About 90% of the residues
remained in the outer bran and course middlings.
TABLE 9. Chlorpyrifos-methyl residues in milled wheat fractions and bread
Residues, mg/kg, in
Bread
Ageing Pollard
period Whole wheat "Red dog" Bran (or middling) Flour Wholemeal White
USA (Bulla and LaHue, 1975)
3 months 6.3 mg/kg* 6.5 5.0 0.53 0.21
6 months 6.3 mg/kg* 3.3 3.0 0.39 0.16
France (Morel and Gallet, 1975)
6 weeks 4.3 1.4 0.26
9 weeks 1.6-1.8 mg/kg* 2.96 1.46 0.22
14 weeks 3.92 1.60 0.29
17 weeks 3.55 1.61 0.23
Australia (Bengtson et al., 1975 and Desmarchelier, 1975a)
6 weeks 6.7-6.9 mg/kg - 7.6-8.8 2.6-3.2 0.38-0.38 1.11-1.17 0.25-0.28
11 weeks 3.6 mg/kg - 9.3 9.0 0.6 - 0.2
11 weeks 2.0 mg/kg - 5.2 4.6 <0.10 - <0.12
22 weeks 1.8 mg/kg - 6.6 3.6 0.4 - 0.13
* Treatment levels.
In animals and animal products
Insects
Although direct studies have not been carried out with
chlorpyrifos-methyl to the same extent as for its ethyl analogue,
chlorpyrifos (cf. FAO/WHO, 1973), there is evidence that the general
metabolic pathways in living organisms are closely parallel for the
two compounds, e.g. the principal metabolic products in some insects
(tobacco budworm, cf. Whitten and Bull, 1974) are
3,5,6-trichloro-2-pyridinol mad O,O-dimethyl phosphate produced by
microsomal oxidase activity which apparently is indicated also
catalyses the formation of the P=O analogue of chlorpyrifos-methyl.
Soluble, non-oxidative hydrolytic enzyme systems are likewise
believed to act in detoxification mechanisms through O-dealkylation
(see Figure 1).
Absorption studies in these insects suggest that the slightly
higher toxicity of chlorpyrifos-methyl compared to chlorpyrifos is
probably connected with a faster rate of absorption of the former.
Cow
In their previously mentioned studies of chlorpyrifos-methyl in
ensiled forage corn, Johnson et al. (1974) fed silage corn containing
0.35, 0.87 and 1.87 mg/kg chlorpyrifos-methyl and 0.44, 0.79 and 1.75
mg/kg 3,5,6-trichloro-2-pyridinol to cows for 42 days. This dietary
residue intake was equivalent to an average of 0.09, 0.022 and 0.054
mg chlorpyrifos-methyl and 0.012, 0.20 and 0.051 mg of the pyridinol
per kg of body weight per day, respectively.
Residue analyses showed no trace of the P=O analogue of
chlorpyrifos-methyl in any samples including milk, urine and faeces.,
The principal excretory route for chlorpyrifos-methyl was by means of
the faeces. Only at the 0.054 mg/kg day dosage did a trace of
chlorpyrifos-methyl (0.001-0.002 mg/kg) appear in the milk with none
in the urine. The principal excretory route for the pyridinol was via
the urine. Some excretion of the pyridinol occurred also in the faeces
(up to 0.36 mg/kg and in the milk (up to 0.008 mg/kg) at the 0.054
mg/kg/day rate. These studies did not include analyses of animal meat
or fat.
In soils
Regoli et al. (1974) studied the aerobic degradation of
ring-labelled C14-chlorpyrifos-methyl in the laboratory at 1 mg/kg in
two soils (silty clay loam and loam containing 4.2% and 0.8% organic
carbon respectively) incubated at 15°, 25° and 35°C and with moisture
at 32% or 100% of field capacity for up to 428 days. All incubations
were conducted in closed, aerated containers so that a balance of
radioactivity added to each sample could be drawn.
Other information on identity and properties
(Brust, 1969; Kenaga, 1971)
Molecular weight : 322.6
State : Granular crystalline solid
Colour and odour : White/mild mercaptan
Melting point : 45.5-46.5°C
Vapour pressure : 7.40 × 10-7mm Hg at 0°C
4.22 × 10-5mm Hg at 25°C
1.8 × 10-4mm Hg at 35°C
Solubility (g/100g : Acetone 640
at 23°C)
Acetonitrile 680
Benzene 520
Carbon disulfide 430
Carbon tetrachloride 280
Chloroform 330
Diethyl ether 480
Ethanol 30
Methanol 30
n-Octanol 20
Hexane 23
Water 0.0004
The compound readily dissolves in
dimethylformamide, N-methylpyrrolidine,
tetrahydrofuran, xylene, methylene
chloride and 1,1,1-trichloroethane.
Stability : Chlorpyrifos-methyl is reported to be
hydrolyzed by water, the rate being
dependent on temperature and pH.
Half-lives at 25°C vary from 22.7 to
9.4 days in tap water and buffered
distilled water within the Ph range
4.2-8.0.
This hydrolysis is enhanced by traces
of copper ions due to chelation
(Meikle, 1973). The major products of
hydrolysis are
3,5,6-trichloro-2-pyridinol and
O,O-dimethyl phosphate.
Photodecomposition of evaporated
chlorpyrifos-methyl in humid air is
also reported, with the formation of
3,5,6-trichloro-2-pyridinol,
hydroxylated pyridinol derivatives and
oxidation products of quinone
structures (Smith & Taylor, 1972).
Purity : The crystalline compound is stated to
be 99.8% ± 0.1%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When 2,6 C14 ring labelled chlorpyrifos-methyl was administered
as a single oral dose (16 mg/kg) to rats, the radioactivity was
rapidly absorbed and excreted. After 72 hours, 90-93% of the
radioactivity was eliminated from the body, 83-85% in urine, 7-9% in
faeces and 0.23-0.43% in respired air. Maximum blood levels (2.4-3.7%)
were reached five hours after treatment. The maximum amount of the
dose remaining in the tissues by 72 hours was 0.65-1.3 ppm. Individual
tissue levels were low (less than 1 ppm). Urinary metabolites were
shown to be 3,5,6 trichloro-2-pyridinol and unidentified activity at
the origin by thin layer chromatography. The possibility of urinary
conjugates was not studied (Branson and Litchfield, 1971a).
The distribution of a single oral dose of C14 labelled
chlorpyrifos-methyl in the body of the young rat was investigated by
whole body autoradiographic techniques. Whole body autoradiograms were
taken at 0.5, 1, 3, 5, 7, 24, 72 and 120 hours after treatment. The
highest level was found initially in the blood. The dispersion in the
various tissues reached a peak at three hours. Residues were extremely
low in fat, liver, kidney and intra-intestinal faeces 72 hours after
administration and completely eliminated from the body by 120 hours
(Nakajima et al., 1974).
Biotransformation
The metabolism of 2,6 C14 ring labelled chlorpyrifos-methyl was
studied in sheep and rats using radioactive counting,
mass-spectroscopy, infrared spectroscopy, column, thin-layer and gas
liquid chromatography techniques. Two ewes were dosed orally with 100
mg/kg by gelatin capsule. Recoveries of radioactivity after four days
were 69.2% in urine, 12.5% in faeces and 2% in tissues. No C14O2 was
detected. Radioactivity in the urine was shown to be the glucuronide
of 3,5,6 trichloro-2-pyridinol 41.6%, o-methyl-o-trimethylsily-o-(3,
5,6 trichloro-2 -pyridyl) phosphorothionate 38% and three minor
unknown fractions 5.2%. In the faeces, 34% of the faecal radioactivity
consisted of parent compound and 55.3% made up primarily of
o-methyl-o-hydrogen-(3,5,6 trichloro-2-pyridyl) phosphorothionate and
3,5,6 trichloro-2-pyridinol. The three fractions found in the urine
were present in the plasma. Detectable amounts of radioactivity (0.32
to 11.8 ppm) were found in the tissues after 96 hours, the highest
level being found in visceral fat (Branson and Litchfield, 1971; Bakke
and Price, 1975).
Ten male rats were given a single oral dose (10 mg) of 2,6 C14
ring labelled chlorpyrifos-methyl. After 48 hours the radioactivity in
the urine consisted of three components, the glucuronide of 3,5,6
trichloro-2-pyridinol (68.6%), o-methyl-o-hydrogen-o (3,5,6
trichloro-2-pyridyl) phosphorothionate (17.8%) and 3,5,6
trichloro-2-pyridinol (13.8%). Faecal metabolites were not
characterized. No respiratory C14O2 was detected (Bakke and Price,
1975).
The principal metabolite of chlorpyrifos-methyl has been shown to
be 3,5,6-trichloro-2-pyridinol. The metabolism of this metabolite in
rats was summarized by FAO/WHO (1973) when the pesticide chlorpyrifos
was evaluated. It was rapidly absorbed and excreted primarily in the
urine and faeces. Small tissue residues (<0.3 ppm) were present and
occurred mainly in biological systems involved with urinary excretion,
i.e. liver, kidney and blood. Residues of the pyridinol in fat were
trivial, which is consistent with its much greater polarity (FAO/WHO,
1973; Smith et al., 1970).
In a similar study in sheep a single oral dose 2,6 C14 ring
labelled 2,5,6 trichloro-2-pyridinol was shown to be rapidly absorbed
and excreted in the urine and faeces. The only radioactive component
detected in the plasma and urine was the glucuronide of the pyridinol
and in faeces the unchanged pyridinol. No C14O2 was detected in
respired air. Residues in tissues after 96 hours were mainly in the
digestive and excretory tracts and was non-detectable in fat, muscle,
heart and other tissues (Bakke and Price, 1975).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
Chlorpyrifos-methyl was administered to groups of Sprague-Dawley
CD strain rats at dietary levels of 0, 0.1 and 1.0 mg/kg/day for 104
weeks. There were no overt signs of reaction to the test compound.
Body weight gain, food consumption, efficiency of food utilization
were comparable between groups and within normal limits. The changes
observed on gross and histopathological examination were those
consistent with the age and strain of rat employed. There was no
compound-related effect an the incidence of spontaneous tumours
(Hunter et al., 1974b).
Special studies on neurotoxicity
Chlorpyrifos-methyl was administered orally to adult hens at 4860
mg/kg body weight and were observed for 21 days. Survivors were given
an intramuscular injection of atropine (10 mg/kg) and PAM (50 mg/kg)
and redosed with 4455 mg/kg chlorpyrifos-methyl and observed for a
further 21 days. No signs of ataxia were detected. The birds were not
examined histologically for nervous systems lesions. TOCP was used as
a positive control (Ross et al., 1975).
Special studies on reproduction
Rat
In a three generation reproduction study (two litters per
generation) three groups of 10 male and 20 female rats received
chlorpyrifos-methyl at dietary levels of 0, 1.0 or 3.0 mg/kg body
weight/day. No treatment related effects on behaviour, survival, body
weight gain or food consumption were observed in parental animals.
Fertility, gestation, viability and lactation indices were comparable
to control values. Pup weights of the second litter, third generation
were significantly less than control at 0, 4 and 21 days postpartum.
There was no effect on sex ratio. In adults of third generation,
plasma cholinesterase activity was decreased in both sexes at both
dosage levels. Erythrocyte cholinesterase depression was observed at 1
mg/kg in females and in both sexes at the highest dose level. Brain
cholinesterase activity was not affected. Gross and histopathological
examination of control and high dose parental animals of the third
generation did not reveal any treatment related abnormalities
(Thompson et al., 1975).
Special studies on teratogenicity
Mouse
Chlorpyrifos-methyl was administered orally to mice at dosage
levels of 0, 20, 100 and 250 mg/kg body weight/day from day 7 through
day 13 of gestation. Animals were sacrificed on day 18 and pups
removed by caesarean section. No significant differences between the
control and treated groups were observed in regard to number of
implants and number of deaths. Body weights were lower in both males
and females at 250 mg/kg. An increased incidence of cleft palate and a
delay of ossification of the cervicovertebral body was observed at 250
mg/kg. When pregnant mice were dosed with a single oral dose of 1000
mg/kg on the seventh or eleventh day of pregnancy, there was no effect
on mortality or body weights of the foetuses. Skeletal abnormalities
were observed (exencephalia, cleft palate, liberation of bone fragment
of cervicovertabral arch) in a few cases especially on treatment on
seventh day of gestation. No control data was presented in the 1000
mg/kg body weight study. In a further study, pregnant mice were
treated as before with 0, 20, 100 and 250 mg chlorpyrifos-methyl/kg
body weight/day on day 7 through day 13 of gestation. Females were
allowed to deliver their pups. Body weights at birth and after three
weeks were dose dependent, being lower on the higher treatment. No
significant morphological abnormalities were detected (Esaki et al.,
1973).
Pregnant rats received 0, 20, 100 and 250 mg/kg body weight of
chlorpyrifos-methyl from day 9 to day 15 of gestation. On day 20
animals were laparotomized and embryos removed. No reproductive or
teratogenic effects were observed. In a similar study the pregnant
rats were allowed to deliver their progeny. Body weight at birth
tended to be higher in the treated groups but no differences observed
when weaned at four weeks. No teratogenic effects related to treatment
were noted (Esaki et al., 1973).
Chlorpyrifos-ethyl was administered to pregnant rats at 0, 50,
100 or 200 mg/kg body weight/day on days 6 through 15 of gestation.
Animals were killed on day 21 of gestation and foetuses removed by
caesarean section. No significant effects were observed in the number
of corpora lutea per pregnant female, the number of resorption sites
or the number of live foetuses. No dose-related effects were observed
in the incidence of runts, subcutaneous oedema and dilated renal
pelvis. Evidence of delayed ossification of sternabrae was observed at
all treatment levels with an increase in lumbar spurs at the high dose
level. The administration of 200 mg chlorpyrifos-methyl/kg body weight
for 10 days caused a significant decrease in plasma and red blood cell
cholinesterase activity in maternal blood as well as in a homogenate
of foetal tissues (Schwetz et al. 1973;Schwetz, 1974a, b).
Acute toxicity
LD50
Species Sex Route Solvent (mg/kg) Reference
Rat M oral CMC 3 733 Hasegawa at al., 1973a
F oral CMC 3 597 "
M oral corn oil 2 472 "
F oral corn oil 1 828 "
M&F oral corn oil 1 700 Davis and Collins, 1975
M oral corn oil 2 140 Litchfield and Norris, 1969
F oral corn oil 1 090 "
M oral corn oil 2 140 Olson, 1964a
F oral corn oil 1 630 "
F oral corn oil >1 000 Olson et al., 1963
F oral corn oil 3 600 Esaki et al., 1973
M&F dermal CMC >4 827 Hasegawa et al., 1973a
M&F dermal corn oil >3 713 "
LD50
Species Sex Route Solvent (mg/kg) Reference
Mouse M oral corn oil 2 254 "
F oral corn oil 2 032 "
M oral arachis oil 1 122 WHO, 1966
F oral corn oil 2 440 Esaki et al., 1973
M&F dermal CMC >2 856 Hasegawa at al., 1973a
Cavy M oral corn oil 2 250 Olson, 1964a
Rabbit M&F oral corn oil approx.
2 000 "
Chicks M oral corn oil >7 950 Olsen, 1964a
(capsule)
Chicken F oral corn oil 4 860 Ross et al., 1975
(capsule)
M&F oral corn oil 7 532 Ross and Roberts, 1974
(gavage)
oral corn oil 8 000 "
(capsule)
Undiluted chlorpyrifos-methyl was applied directly to
conjunctival sac of rabbits. There were signs of conjunctival
irritation which subsided after 24-48 hours. No corneal injury was
discernible (Olson and Taylor, 1964b).
No significant skin reaction occurred when undiluted
chlorpyrifos-methyl was applied to shaved and abraded skin of the
rabbit for prolonged periods (Olson and Taylor, 1964b; United States
Army, 1973).
Cholinesterase activity was reduced by 90% and all animals died
within 24 hours when rats were treated orally with chlorpyrifos-methyl
(4 g/kg) and subcutaneously with various levels of atropine sulfate.
Rats were treated with a sublethal oral dose of chlorpyrifos-methyl in
conjunction with subcutaneous injections of atropine sulfate and PAM
(25, 50 and 100 mg/kg) and glutathione (100, 300, 500 and 700 mg/kg)
or combinations of each. Symptoms of anticholinesterase activity were
observed and total blood, erythrocyte, plasma and brain cholinesterase
levels determined. The antidotes were effective especially at the
upper dose levels in reducing the toxic symptoms. The combination of
atropine sulfate with PAM or glutathione was more effective than
atropine sulfate alone (Hayashi et al., 1973).
Chlorpyrifos-methyl was administered to groups of rats at 5 x
LD50 (8.5 g/kg). At first signs of anticholinesterase activity one
group was given atropine sulfate (17.5 mg/kg) intramuscularly and a
further group atropine sulfate (17.5 mg/kg) and PAM (50 mg/kg)
intramuscularly. A further administration of antidote was given to all
rats seven hours after treatment. All treated rats died within 24
hours, untreated controls died within eight hours (Davies and Collins,
1975).
Short-term studies
Avian species
Mallard ducks, bobwhite and Japanese quail were fed various
levels of chlorpyrifos-methyl for five days and observed for a further
three days. The following LC50 values were determined.
Bobwhite quail - 1835 ppm, Japanese quail ->5000 ppm, mallard
duck - 2500-5000 ppm. Body weight and food consumption were reduced
for bobwhite and Japanese quail at 2500 and 1250 ppm respectively and
at 5000 ppm for the mallard duck. In a similar study, mallard ducks
were fed graded levels of chlorpyrifos-methyl for five days. Whole
blood cholinesterase activity was inhibited at dietary levels of 78
ppm and greater. Brain cholinesterase was not affected at the highest
level tested (Shellenberger, 1970).
Mouse
Chlorpyrifos-methyl was administered orally to mice at 0, 100,
250, 500 and 1000 mg/kg body weight/day for 14 days. No effect on
mortality or significant changes in body weight at 100 and 250 mg/kg
were observed. After four doses, there was 100% mortality at 1000
mg/kg. At 500 mg/kg, 40% mortality occurred in 14 days and a
noticeable decrease in body weight (Esaki et al., 1973).
Groups of male and female mice were fed standard diets containing
0, 0.5, 1, 5, 10, 20, 30, 40 and 100 ppm for a period of six months.
No differences were observed in mortality, food intake, body weight
gain, organ weights and urinalysis. Haematological, biochemical and
pathohistological parameters were considered normal in groups given
less than 40 ppm. Plasma and erythrocyte cholinesterase activities
were lower at dosage levels greater than 20 ppm. Brain cholinesterase
was decreased in males given 40 and 100 ppm and in females given 40
ppm (Hasegawa et al., 1973c).
Rat
Chlorpyrifos-methyl was administered orally to rats at 20, 100
and 500 mg/kg body weight/day for 14 days. No significant effect on
mortality or body weight gain was shown. At 500 mg/kg hypertrophy of
the liver and heart were observed (Esaki et al., 1973).
Groups of female rats were fed chlorpyrifos-methyl in the diet
for 14 days at 0, 1.6, 6, 25, 95, 500 and 2130 mg/kg body weight/day.
Plasma and red blood cell cholinesterase was depressed at 6 mg/kg and
in brain at 95 mg/kg. Organ to body weight ratios of liver, kidney and
spleen were not significantly different from control but brain to body
weight ratio was increased at 2130 mg/kg. No compound-related gross or
histopathological lesions were observed (United States Army, 1973).
Groups of six male and female rats consumed in their diet 0,
0.08, 0.8, 8.0, 40, 80 and 160 mg chlorpyrifos-methyl/kg body
weight/day for 90 days. Plasma cholinesterase activity was depressed
in males at 8 mg/kg and above and in females at 0.8 mg/kg and above.
The incidence of erythrocyte and brain cholinesterase depression was
significantly increased at 8.0 mg/kg/day and above for both male and
female. Liver weight to body weight ratio of male rats was increased
at 40, 80 and 160 mg/kg. Apart from small aggregates of lymphocytes
and plasma cells in the periportal areas of the liver, no changes were
noted in gross or histopathology (Steinberg, 1971).
Chlorpyrifos-methyl was fed at dietary levels of 0, 0.5, 1, 5,
10, 20, 30, 40 and 100 ppm to groups of male and female Wistar rats
for six months. No significant differences were noted in mortality,
food intake, weight gain, organ weights and urinalysis.
Haematological, biochemical and pathological findings were not
affected at 40 ppm or less. Serum and erythrocyte cholinesterase
activity was decreased at 30 ppm and above at one, three and six
months (Hasegawa et al., 1973b).
Groups of five male and five female rats received 0, 0.2, 1.0 and
5.0 mg chlorpyrifos-methyl/kg body weight/day by stomach tube daily,
six days/week, for six weeks. Plasma cholinesterase activity was
depressed in females at 5 mg/kg. Erythrocyte and brain cholinesterase
activities were not significantly reduced. Growth, haematology, blood
chemistry and liver microsomal mixed function oxidase activities were
not affected (Coulston and Griffin, 1975).
Dog
Groups of dogs (two males and two females/group) were fed
chlorpyrifos-methyl in the diet for 92 days at dose levels of 0, 0.3,
1, 3 and 10 mg/kg body weight/day. No treatment-related effects were
observed in behaviour, food consumption, body weight, hematology,
clinical chemistry, urinalysis, organ weight: body weight ratios,
gross and histopathology. Plasma cholinesterase activity at all test
levels was depressed after one, four and 12 weeks of treatment.
Inhibition of erythrocyte cholinesterase activity was evident at 1.0,
3.0 and 10.0 mg/kg body weight/day. The activity of brain
cholinesterase was not affected (Sparschu et al., 1971).
A supplemental study was conducted in which groups of dogs (two
males and two females/group) were fed chlorpyrifos-methyl in the diet
at dose levels of 0, 0.03 and 0.1 mg/kg body weight/day for 125 days.
There was no significant depression of plasma or erythrocyte
cholinesterase activity. Behaviour was normal and body weight and food
consumption considered to be within normal limits (Humiston et al.,
1972).
Groups of dogs (seven males and seven females/group) were fed
chlorpyrifos-methyl at dietary levels of 0, 0.03, 0.1, 1.0 and 3.0
mg/kg/day for 104 weeks with an interim sacrifice of three males and
three females/group at 26 weeks. No significant effects were observed
with respect to mortality, behaviour, food and water consumption,
ophthalmology, haematology, blood biochemistry, organ to body weight
ratio or gross and histopathology at either 26 or 104 weeks. Treated
animals gained less weight than controls due to some weight loss
during the latter part of the study. Body weights, however, were
considered within normal limits. Cholinesterase activity was depressed
in red blood cell and plasma at 3.0 mg/kg and in plasma at 1.0 mg/kg.
There was no reduction in brain cholinesterase activity at 26 or 104
weeks (Rivett et al., 1974).
Monkey
Chlorpyrifos-methyl was administered by stomach tube daily, six
days/week for six months to five groups of rhesus monkeys, three male
and three female group at dose levels of 0, 0.1, 0.2, 1.0 and 5.0
mg/kg body weight/day. Plasma cholinesterase was depressed in those
animals receiving 1.0 and 5.0 mg/kg. Inhibition of erythrocyte
cholinesterase was evident at 5.0 mg/kg. Brain cholinesterase was not
affected at any test level. No treatment related effects were noted in
growth, haematology, blood chemistry, mixed function oxidase activity
of the liver, relative organ weights, gross or histopathology
(Coulston and Griffin, 1975).
Cow
Groups of four cows were fed for 42 days silage corn which had
been sprayed 83 days previously with chlorpyrifos-methyl. The diets
contained 0, 0.35, 0.87 and 1.85 ppm chlorpyrifos-methyl and 0, 0.44,
0.79 and 1.75 ppm 3,5,6 trichloro-2-pyridinol. Residue intakes
amounted to 0.009, 0.022 and 0.054 mg chlorpyrifos-methyl and 0.012,
0.020 and 0.051 mg 3,5,6 trichloro-2-pyridinol/kg body weight. No
adverse effects were observed in silage intake, milk production, blood
cholinesterase activity or body weight gains. Urine, faeces and milk
contained trace amounts of chlorpyrifos-methyl and 3,5,6
trichloro-2-pyridinol. One week after treated feed withdrawn these
residue were not detectable (Johnson et al., 1974).
Long-term studies
Rat
Groups of 55 male and 55 female rats were administered
chlorpyrifos-methyl in the diet at 0, 0.03, 0.1, 1.0 and 3.0 mg/kg
body weight/day for 104 weeks. After five weeks of treatment five
males and five female/group were sacrificed for cholinesterase
determination. Twenty-six weeks after treatment 10 males and 10
females/group were killed for interim study and the size of each group
was reduced to 30 males and 30 females after 52 weeks. Red blood cell
cholinesterase activity was depressed in both sexes at 1.0 and 3.0
mg/kg/day. Plasma cholinesterase activity was reduced consistently in
females at 1.0 and 3.0 mg/kg/day; males showing little effect during
the first year after which it was significantly depressed at 3.0
mg/kg/day. Brain cholinesterase levels were not affected.
Chlorpyrifos-methyl produced no significant effect on behaviour,
mortality, body weight gain, food consumption and efficiency of food
utilization. Haematologic, blood biochemical and urinalysis
determinations were within normal limits and revealed no abnormal
changes. No effect attributable to treatment was observed in organ
weights or on gross and histopathologic examination. Tumour incidence
was comparable between groups (Hunter et al., 1974a).
Observations in man
Fourteen male volunteers were divided into two treatment groups
of five men each and a control group of four men. Chlorpyrifos-methyl
was administered by gelatin capsule in a single daily dose of 0, 0.03
and 0.1 mg/kg body weight/day for four weeks. Plasma and erythrocyte
cholinesterase activities were not depressed at levels tested.
Haematology, blood chemistry, urinalysis, blood pressure, pulse and
ophthalmology were not affected by treatment (Coulston et al., 1975).
COMMENTS
In mammals, chlorpyrifos-methyl is rapidly absorbed and
metabolized the principal metabolite being
3,5,6-trichloro-2-pyridinol. The parent compound and metabolite are
excreted primarily in the urine and faeces and are not stored to any
extent in the body. This metabolite has also been shown to occur in
plants.
Teratogenic studies in the rat revealed no adverse effects at
dietary intake levels of up to 250 mg/kg. In an inadequately conducted
and reported study in the mouse there appeared to be a tendency for a
weak teratogenic response at 250 mg/kg. No compound-related
abnormalities were noted at 100 mg/kg.
No adverse effects were noted in the reproductive capacity of the
rat over three generations at dietary levels of up to 3 mg/kg.
Mutagenic studies have not been reported.
No apparent clinical evidence of neurotoxicity was observed in
hens given a large dose of chlorpyrifos-methyl. Histological
examination of nervous tissue was not conducted. In various short- and
long-term studies in the rat, dog, mouse, monkey, mallard duck,
bobwhite and Japanese quail, chlorpyrifos-methyl was shown to be an
active cholinesterase inhibitor. Plasma cholinesterase activity was
inhibited to a greater extent than either erythrocyte or brain
cholinesterase.
In the long-term study and specific tumorigenic study in the rat,
no compound-related effect or increase in tumour incidence was
observed.
No effect levels were demonstrated in the rat, dog, monkey and
man on the basis of the most sensitive parameter plasma cholinesterase
inhibition. The no-effect level in man was confirmed by two
independent studies. The data were considered sufficient to allocate
an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg
Dog: 0.1 mg/kg
Monkey: 0.2 mg/kg
Man: 0.1 mg/kg
Estimation of acceptable daily intake
0-0.01 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of chlorpyrifos-methyl for insect control in fruit,
vegetable and cereal plants and in grain storage is recorded from the
following countries: Egypt, France, Japan, Korea, and Turkey. In these
countries it is marketed as dusts and/or granules and as emulsifiable
concentrates containing 2-3% a.i. and 25-40% a.i. respectively.
As a relatively new chemical chlorpyrifos-methyl is marketed as a
broad-spectrum organophosphorus insecticide of relatively low toxicity
and persistence, although it shows reasonably good stability in
stored, dry products such as grains (Schulten, 1973) and dried fruits
(Soderstrom and Armstrong, 1974). In these products it controls a wide
range of beetles, weevils, moths and mites, including several such
species which may have developed resistance towards other
insecticides, e.g. malathion. Using spray techniques or admixing
procedures to treat the stored grain products, chlorpyrifos-methyl is
usually applied at the rate of 5-10 mg a.i. per kg.
In several of the countries mentioned, chlorpyrifos-methyl is
approved for wider uses in agriculture and horticulture against pests
of economic importance connected to fruit and vegetable growing
(Nakayama et al., 1973). In laboratory tests it is further found to be
effective against a wide range of household pests, including spiders,
houseflies, cockroaches, house crickets etc, (Snetsinger, 1972).
A number of recommended practical applications is listed in Table
1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on chlorpyrifos-methyl residues resulting from a number of
the above uses have been presented by the originating company (Kenaga,
1975). The data derived from field trials and controlled experimental
work under a variety of geographical and climatic conditions is as
follows.
In plants
Cereal plants and grass
Leuck et al. (1975) found chlorpyrifos-methyl to be relatively
non-persistent in field trials where coastal bermuda grass and corn
were sprayed once with 0.56, 1.12 and 2.24 kg a.i. per ha (see Table
2). In both crops and at all three levels chlorpyrifos-methyl
diminished rapidly at rates corresponding to half-lives of about two
to three days. The hydrolysis product, 3,5,6-trichloro-2-pyridinol,
was more persistent and in some cases actually increased up to a
maximum during the first few days after application. Thereafter the
pyridinol disappeared following a first order scheme with estimated
half-life values of 9-12 days.
TABLE 1. Some use patterns of chlorpyrifos-methyl on plants and grain
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Australia Emulsifiable concentrate Wheat 5.0 mg/kg
Egypt Emuls. conc., 24% (w/v) Spodoptera littoralis and 0.9 kg/ha or Foliar broadcast spray
S. exigua on vegetables 90 g/litre
and clover
France Emuls. conc., 24% (w/v) Stored grain pests 2.5 mg/kg
Japan Dust, 2% (w/w) Rice stemborer 0.6-0.8 kg/ha Max. 4 applic. per season
Micro-granule, 3% (w/w) Rice stemborer 0.9-1.2 kg/ha Broadcast appl. Max. 4 x
per season
Granule, 5% (w/w) Rice stemborer 1.5-2.0 kg/ha Submerged appl. Max. 4 x
per season
Emuls. conc., 25% (w/v) Rice stemborer 0.1-0.375 kg/ha Max. 4 sprays/season:
rice and cabbage
Diamondback moth, Pieria
rapae, aphids on tobacco Max. 2 sprays/season:
Cutworm on cabbage, chinese cabbage and
chinese cabbage, radish radish
Korea Granule, 3% (w/w) Rice stemborer, plant 0.9-1.2 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice.
Tobacco cutworm, mole
cricket on tobacco and
vegetables
TABLE 1. (Cont'd)
Insecticide use Use rate Method and number
Country Formulation principal pest/crop (active ing.) of applications
Korea Emuls. conc., 25% (w/v) Rice stemborer, plant 0.25-0.35 kg/ha 2-3 appl. per season
hopper, leaf hopper on
rice
Tobacco cutworm, mole
cricket on tobacco and
vegetables
Sudan Emuls. conc., 24% (w/v) Pests of apricots
Turkey Emuls. conc., 24% (w/v) Spodoptera litteralis and 0.9 kg/ha Foliar broadcast spray
Heliothis obsoleta in
cotton and vegetables
Aphids on melons
USA Emuls. conc. Wheat 5-10 mg/kg
Johnson et al. (1974) in an earlier experiment sprayed corn in
the dent stage with chlorpyrifos-methyl at the same dosage rates. In
this case, the corn was harvested and ensiled for forage purposes one
day after the treatment (Table 2). The initial losses of
chlorpyrifos-methyl from the time of application through harvesting
and ensiling amounted to 62-79%. After the ensiling, however, residues
became relatively stable and they disappeared only slowly with
formation of the pyridinol. From the first day until the eighty-third
day, on average for the three treatments, 60% of the
chlorpyrifos-methyl disappeared while the pyridinol increased 421%.
TABLE 2. Residues of chlorpyrifos-methyl and pyridinol in grass and corn
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Bermudagrass, 0.56 0 5.5 1.07
coastal 7 0.1 0.73
(Leuck et al., 1975) 21 <0.01 0.32
1.12 0 12.5 2.21
7 0.21 1.81
21 0.01 0.58
2.24 0 28.4 4.48
7 0.45 4.08
21 0.09 1.24
Corn, growing 0.56 0 2.2 0.11
(Leuck et al., 1975) 7 0.11 0.17
21 0.03 0.09
1.12 0 8.2 0.41
7 0.29 0.54
21 0.07 0.21
2.24 0 20.4 0.78
7 1.43 1.38
21 0.20 0.60
TABLE 2. (Cont'd.)
Residues (mg/kg)
Treatment Days after
Crop kg/ha treatment Chlorpyrifos-methyl Trichloro-2-pyridinol
Forage corn, 0.56 0 2.22 0.11
ensiled at day 1 1 0.75 0.13
(Johnson et al., 27 0.51 0.48
1974) 55 0.47 0.49
83 0.34 0.70
125 0.34 0.27
1.12 0 8.20 0.41
1 1.92 0.34
27 1.42 1.09
55 1.27 1.40
83 0.72 1.74
125 0.77 0.35
2.24 0 20.40 0.78
1 4.08 0.69
27 2.98 2.50
55 2.31 2.28
83 1.64 3.34
125 1.80 1.13
Kubota (1975) has reported on field trials with growing rice. As
shown in Table 3, residues at the time of harvest were not detectable
or at most insignificant when spraying was carried out 4-17 days
before harvest.
Stored grain products
White and LaHue (1974), in their experiments showing effective
protection against several grain insects, e.g. rice weevil, lesser
grain borers, flour beetle, sawtooth grain beetle, and foreign grain
beetle, also followed the dissipation of chlorpyrifos-methyl in stored
wheat, sorghum and maize. Wheat at 79°C and containing 14.6% moisture
was treated with 4.8, 6.6 and 10.4 mg/kg chlorpyrifos-methyl. The
residue half-life was >6 months, 2-3 months and <1 month,
respectively. Maize and sorghum tested under similar conditions at
about 6-7 mg/kg had a residue half-life of about 2-3 months (Table 4).
TABLE 3. Residues of Chlorpyrifos-methyl in rice*
Application
Rate Days after Number of
Formulation (kg/ha) Frequency last treatment samples Residues, mg/kg
3% dust 1.2 2 × 35-42 8 <0.005-<0.005(n.d.)
4 × 14-42 8 <0.005-<0.005(n.d.)
6 × 11 8 <0.005-<0.005(n.d.)
5% granule 2 2 × 53-57 8 <0.005-<0.005(n.d.)
4 × 53-57 8 <0.005-<0.005(n.d.)
6 × 17-32 8 <0.005-0.012
25% e.c. 0.30-0.375 2 × 32-53 8 <0.005-<0.005(n.d.)
4 × 24-53 8 <0.005-<0.005(n.d.)
6 × 4-32 8 0.005-0.013
* From Kubota (1975).
TABLE 4. Residues of chlorpyrifos-methyl in stored grains in the United States of America*
Residue, mg/kg
Grain 0 days 5 days 1 2 3 4 5 6 8 9 months
Wheat (26°C, 4.8 4.6 3.5 3.0 2.8 3.3 - 3.3 - -
14.6% moisture) 6.6 5.5 5.0 2.8 3.2 - - 1.6 - -
10.4 6.2 4.1 2.6 2.3 - - 4.4 - -
Corn
(13.6% moisture) 6.70 - - - - 3.2 2.5 2.3 1.9 1.8
Sorghum - - - - 3.5 3.3 2.7 2.6 2.2 1.9
* From White and LaHue (1974).
In laboratory experiments, Bulla and LaHue (1975) tested the
disappearance rates of chlorpyrifos-methyl on the same three grains,
kept for six months at 80°F in covered glass jars with regulated
moisture contents from 10 to 16%. With overall half-life rates varying
from one to six months they found a decreasing persistence in the
sequence: maize > wheat > sorghum. Grains containing 14 or 16%
moisture degraded chlorpyrifos-methyl about 1.5-2 times faster than
those containing 10 or 12%.
In similar Australian experiments under commercial conditions,
Deahl and Tucker (1974) found that chlorpyrifos-methyl at initial
concentrations of 5.0 and 6.2 mg/kg declined at rates corresponding to
half-lives of the order of three to five months (see Table 5). The
average half-life at 31°C was 15-16 weeks with indications of a
reduced breakdown at lower temperatures (Desmarchelier, 1975a).
From these extensive trials, Desmarchelier et al. (1975b and c)
also reported the rate of loss of residues of chlorpyrifos-methyl on
white wheat containing 11% moisture at 25°C when stored in glass jars.
These tests indicate that under the laboratory conditions stated,
chlorpyrifos-methyl decays at a predictable first-order rate,
irrespective of concentration or age of the residues.
Morel and Galet (1975) report tests conducted in France on wheat
containing 15.2% moisture and 2.35 and 1.17 mg/kg chlorpyrifos-methyl
which was stored for various periods of time at 25°C for residue
analyses. Under these conditions half-lives of chlorpyrifos-methyl
were estimated to be about six weeks. Residue levels six months after
treatment were in the range of 0.2-0.4 mg/kg.
Apples and peaches
Residue levels on apples and peaches have been established under
Japanese conditions following spraying at recommended dosage rates,
i.e. about 1-1.5 kg/ha. The results of these trials are shown in Table
6 which indicates levels in apples below 0.3-0.5 mg/kg if harvested
about two weeks after the last treatment. The results for peaches do
not show signs of penetration of residues through the peel into the
fruit pulp.
Vegetable plants
Residue data from experiments with chlorpyrifos-methyl
applications on vegetable plants are shown in Tables 7 and 8. The
experiments derive from different geographical regions and cover
mostly foliar applications on the following crops: artichoke, beans,
cabbage, chinese cabbage, eggplant, lettuce, pepper, radish, tea and
tomatoes.
TABLE 5. Residues of chlorpyrifos-methyl during silo-storage, Australia*
Residue, mg/kg after interval (weeks)
Depth of
Treatment silo 1 6 11 16 22 26
6.2 mg/kg 0.1 m 4.5 3.1 2.3 2.1 1.9 1.5
(Queensland 1.5 m 3.4 3.6 2.1 2.3 1.8 1.5
experiment) 6.0 m 3.2 3.0 2.4 2.2 2.0 -
Average 3.7 3.2 2.3 2.2 1.9 1.5
3 8 13
5.0 mg/kg 0.1 m 4.1 3.9 1.8
(N.S. Wales 1.5 m 4.1 3.7 2.4
experiment) 6.0 m 3.3 3.1 2.4
Average 3.8 3.6 2.2
* From Deahl and Tucker (1974).
TABLE 6. Residues of chlorpyrifos-methyl in apples and peaches, Japan*
Preharvest Number of Number of Range of
Application interval applications samples results (mg/kg)
Apples
1.25-1.5 10 days 1-2 × 6 0.22-0.353
kg/ha 20 days 1-2 × 7 0.201-0.310
30 days 1-2 × 4 0.174-0.186
10 days 4 × 9 0.182-0.798
20 days 4 × 6 0.128-0.225
30 days 4 × 4 0.162-0.257
10 days 6 × 4 0.489-0.936
20 days 6 × 7 0.152-0.333
30 days 6 × 4 0.250-0.478
Peaches Peel Pulp
0.9 kg/ha 21 days 2 × 6 <0.03-0.26 <0.005
30 days 2 × 8 <0.03-0.05 <0.005
21 days 4 × 6 <0.03-0.11 <0.005
30 days 4 × 8 <0.03-0.05 <0.005
21 days 6 × 6 <0.03-0.10 <0.005
30 days 6 × 8 <0.03-0.06 <0.005
* From Kubota (1975).
TABLE 7. Residues of chlorpyrifos-methyl in vegetables, Europe/Near East
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Artichoke 48 g/100 l 7 days 2 0.05 France1
(aver.)
14 days 2 0.03
(aver.)
Beans 1.2 kg/ha 0 days 1 0.32 Turkey2
7 days 1 0.01
14 days 1 0.02
Beans, 48 g/100 l 7 days 5 <0.02 France1
french (aver.)
14 days 3 <0.02
(aver.)
Eggplant 1.2 kg/ha 0 days 1 0.06 Turkey2
7 days 1 0.01
14 days 1 0.01
Lettuce 48 g/100 l 7 days 4 0.78 France1
(aver.)
14 days 2 0.04
(aver.)
Pepper 1.2 kg/ha 0 days 1 2.28 Egypt3
1 day 1 0.58
2 days 1 0.25
3 days 1 0.20
5 days 1 0.09
7 days 1 0.10
9 days 1 0.04
TABLE 7. (continued)
Preharvest Number of Residues Country and
Crop Dosage rate interval applications (mg/kg) reference
Tomato 0.1% until 3 days 1 1.90 England4
run-off 6 days 1 0.90
(greenhouse) 9 days 1 0.40
13 days 1 0.09
1.2 kg/ha 0 days 1 0.31 Turkey2
7 days 1 0.04
14 days 1 0.05
48 g/100 l 7 days 6 0.21 France1
(aver.)
14 days 6 0.04
(aver.)
1 Hascouet (1974).
2 Hollick and Collison (1972a).
3 Hollick and Collison (1972b).
4 Wirth et al. (1971).
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Cabbage 0.45 kg/ha 7 days 2 × 8 <0.002-0.002
14 days 2 × 8 <0.002
7 days 4 × 8 <0.002
14 days 4 × 8 <0.002
Chinese 0.25 kg/ha 14 days 4 × 2 <0.005
cabbage
0.375 kg/ha 7 days 2 × 7 0.027-0.220
14 days 2 × 7 <0.005-0.100
7 days 4 × 7 0.036-0.175
14 days 4 × 5 0.039-0.056
0.50 kg/ha 7 days 2 × 4 0.019-0.025
14 days 2 × 4 0.004-0.006
TABLE 8. Residues of chlorpyrifos-methyl in vegetables, Japan*
Dosage Preharvest Number of Number of Range of
Crop rate interval treatments samples residues (mg/kg)
Chinese 7 days 4 × 4 0.001-0.002
cabbage 14 days 4 × 4 0.003-0.007
Eggplant 0.75 kg/ha 3 days 2 × 4 0.002-0.003
7 days 2 × 4 0.001
14 days 2 × 4 <0.002
3 days 4 × 4 0.002-0.004
7 days 4 × 4 0.001
14 days 4 × 4 <0.002
Pepper, 0.50 kg/ha 3 days 2 × 4 0.011-0.171
green 7 days 2 × 4 0.004-0.028
14 days 2 × 4 <0.002-0.012
Pepper, 3 days 4 × 4 0.013-0.129
green 7 days 4 × 4 0.003-0.060
(cont'd) 14 days 4 × 4 <0.003-0.021
Leaves Root
Radish 0.2-0.3 kg/ha 7 days 2 × 4 0.024-0.324 <0.002-0.069
28 days 2 × 4 0.003-0.009 0.005-0.008
7 days 3 × 2 0.109 <0.001-0.003
28 days 3 × 4 0.024-0.130 <0.001-0.001
7 days 4 × 4 0.040-0.299 <0.002-0.081
28 days 4 × 4 0.003-0.010 0.013-0.020
7 days 6 × 2 0.075 <0.002-0.002
14 days 6 × 4 0.019-0.040 0.002-0.003
Tea 0.50 kg/ha 7 days 1 × 6 <0.001-0.065
14 days 1 × 6 <0.001-0.047
21 days 1 × 6 <0.003
27 days 1 × 6 <0.003
7 days 2 × 6 <0.001-0.026
0.75 kg/ha 7 days 1 × 7 <0.001-0.091
14 days 1 × 6 <0.001-0.056
21 days 1 × 6 <0.001-0.047
7 days 2 × 6 <0.003-0.085
* From Kubota (1975).
The levels of chlorpyrifos-methyl residues on these crops at the
time of harvest, i.e. from 7 to 14 days after the last treatment are
generally at or below 0.1 mg/kg.
FATE OF RESIDUES
In plants
Residues of chlorpyrifos-methyl in or on plants are assumed to
behave similarly to chlorpyrifos Of FAD/WHO, 1973) without
translocation in plants to any significant extent.
The principal hydrolysis product of chlorpyrifos-methyl in
plants, animals and soil, 3,5,6-trichloro-2-pyridinol, may or may not
be taken up by plants depending on pH. The free pyridinol, which is
the predominant form of the compound below pH 6.0, is essentially
insoluble in water. In radio-labelled studies, soil and nutrient
culture experiments have shown its uptake by plants to be
insignificant (Smith et al., 1967). At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the sodium salt) as well as rate of absorption by the
plant are enhanced compared with the free pyridinol. Although still at
very low levels, the sodium salt of the pyridinol enters the plant
more than five times as fast as the free pyridinol. It undergoes
metabolism with the liberation of chloride and the formation of
several unidentified water soluble decomposition products.
In cereal-products
Residues of chlorpyrifos-methyl in treated grains have been
followed from the time of application through milling and baking
procedures in several experiments (see Table 9). Bulla and LaHue
(1975) fractionated wheat containing 6.3 mg/kg chlorpyrifos-methyl and
found that most of the residues after three and six months of "ageing"
were still concentrated in the outer grain fractions, such as "red
dog" and bran. The residues in flour were reduced to below 10% of the
original concentration (i.e. to 0.53 and 0.39 mg/kg, respectively).
Bread baking reduced the remaining residues further by about 60%.
Similar results have been found by Bengtson et al. (1975) who
after 11 and 22 weeks of ageing also found considerable reduction from
grain residues to milled flour and baked bread, the latter showing
below 0.25 mg/kg residual chlorpyrifos-methyl.
Morel and Galet (1975) analysed wheat which was milled from 6 to
17 weeks after treatment of commercially stored grains with 1.6-1.8
mg/kg of chlorpyrifos-methyl. The distribution of residues in various
milled fractions is shown in Table 9. About 90% of the residues
remained in the outer bran and course middlings.
TABLE 9. Chlorpyrifos-methyl residues in milled wheat fractions and bread
Residues, mg/kg, in
Bread
Ageing Pollard
period Whole wheat "Red dog" Bran (or middling) Flour Wholemeal White
USA (Bulla and LaHue, 1975)
3 months 6.3 mg/kg* 6.5 5.0 0.53 0.21
6 months 6.3 mg/kg* 3.3 3.0 0.39 0.16
France (Morel and Gallet, 1975)
6 weeks 4.3 1.4 0.26
9 weeks 1.6-1.8 mg/kg* 2.96 1.46 0.22
14 weeks 3.92 1.60 0.29
17 weeks 3.55 1.61 0.23
Australia (Bengtson et al., 1975 and Desmarchelier, 1975a)
6 weeks 6.7-6.9 mg/kg - 7.6-8.8 2.6-3.2 0.38-0.38 1.11-1.17 0.25-0.28
11 weeks 3.6 mg/kg - 9.3 9.0 0.6 - 0.2
11 weeks 2.0 mg/kg - 5.2 4.6 <0.10 - <0.12
22 weeks 1.8 mg/kg - 6.6 3.6 0.4 - 0.13
* Treatment levels.
In animals and animal products
Insects
Although direct studies have not been carried out with
chlorpyrifos-methyl to the same extent as for its ethyl analogue,
chlorpyrifos (cf. FAO/WHO, 1973), there is evidence that the general
metabolic pathways in living organisms are closely parallel for the
two compounds, e.g. the principal metabolic products in some insects
(tobacco budworm, cf. Whitten and Bull, 1974) are
3,5,6-trichloro-2-pyridinol mad O,O-dimethyl phosphate produced by
microsomal oxidase activity which apparently is indicated also
catalyses the formation of the P=O analogue of chlorpyrifos-methyl.
Soluble, non-oxidative hydrolytic enzyme systems are likewise
believed to act in detoxification mechanisms through O-dealkylation
(see Figure 1).
Absorption studies in these insects suggest that the slightly
higher toxicity of chlorpyrifos-methyl compared to chlorpyrifos is
probably connected with a faster rate of absorption of the former.
Cow
In their previously mentioned studies of chlorpyrifos-methyl in
ensiled forage corn, Johnson et al. (1974) fed silage corn containing
0.35, 0.87 and 1.87 mg/kg chlorpyrifos-methyl and 0.44, 0.79 and 1.75
mg/kg 3,5,6-trichloro-2-pyridinol to cows for 42 days. This dietary
residue intake was equivalent to an average of 0.09, 0.022 and 0.054
mg chlorpyrifos-methyl and 0.012, 0.20 and 0.051 mg of the pyridinol
per kg of body weight per day, respectively.
Residue analyses showed no trace of the P=O analogue of
chlorpyrifos-methyl in any samples including milk, urine and faeces.,
The principal excretory route for chlorpyrifos-methyl was by means of
the faeces. Only at the 0.054 mg/kg day dosage did a trace of
chlorpyrifos-methyl (0.001-0.002 mg/kg) appear in the milk with none
in the urine. The principal excretory route for the pyridinol was via
the urine. Some excretion of the pyridinol occurred also in the faeces
(up to 0.36 mg/kg and in the milk (up to 0.008 mg/kg) at the 0.054
mg/kg/day rate. These studies did not include analyses of animal meat
or fat.
In soils
Regoli et al. (1974) studied the aerobic degradation of
ring-labelled C14-chlorpyrifos-methyl in the laboratory at 1 mg/kg in
two soils (silty clay loam and loam containing 4.2% and 0.8% organic
carbon respectively) incubated at 15°, 25° and 35°C and with moisture
at 32% or 100% of field capacity for up to 428 days. All incubations
were conducted in closed, aerated containers so that a balance of
radioactivity added to each sample could be drawn.
 An overall average of 100.4% recovery of added activity was
obtained for samples over the entire study and it was found that the
major metabolite in soil was 3,5,6-trichloro-2-pyridinol which is
further degraded to CO2 followed by lesser amounts of
3,5,6-trichloro-2-methoxy-pyridine. The authors speculated whether the
methoxy-compound is formed through direct methylation of
chlorpyrifos-methyl or from the pyridinol through microbial activity,
but the former route, involving subsequent conversion of the
methoxypyridine to the pyridinol seems more likely as no other
decomposition-products could be traced.
The breakdown of chlorpyrifos-methyl to
3,5,6-trichloro-2-pyridinol is a rapid process if conditions are
favourable for microbial and hydrolytic activity. The time required
for 50% breakdown ranged from 1.5 to 2.0 days in the high organic soil
at 25-35°C and 100% moisture to 33.3-17.7 days in the low organic soil
at 15°C and 32% moisture. The calculated times required for 90%
degradation ranged from 20 to 30 days under favourable conditions to
500-1600 days under less favourable. Thus in practice
chlorpyrifos-methyl could hardly persist in soils and give rise to
traceable carry-over during crop rotations.
Hamaker (1974) studied the soil adsorption of
C14-chlorpyrifos-methyl by analysing both the soil and the
supernatant from slurries of soil in 1 mg/l solutions (one part of
soil:four parts of solution). The soils contained organic carbon
ranging from 0.28 to 5.76%.
The adsorption by these soils ranged from 46 to 99% after 24
hours and the average distribution ratio between soil organic carbon
and water was found to be 3300 after four hours and 4600 after 24
hours. This is less by one-third than in the case of chlorpyrifos but
still represents a strong adsorption to soil.
The hydrolysis product (3,5,6-trichloro-2-pyridinol) is absorbed
to a smaller degree than the parent compound showing a distribution
ratio of 714 between soil and water. In the conclusions of the author,
however, this still represents a sufficiently strong adsorption to
make leaching of the compound from soils an unlikely process.
Kubota (1975) has reported on both field trials and laboratory
tests determining residue disappearance from rice paddy soils over
periods of 30 days (Table 10). The half-life periods in the field
tests varied from five to about 20 days, while the half-life was less
than three days when chlorpyrifos-methyl was applied directly to the
soil in the laboratory.
A DOWCO 214 formulation applied to bacterial cultures in amounts
corresponding to normal soil application showed no influence on the
bacterial activity measured as the O2-uptake under incubation of
Azotobacter vinelandii (MacRae and Celo, 1974). Neither was the
nitrogen fixation of this strain as measured by the reduction of
acetylene to ethylene affected, even at concentrations 100 times the
practical application rates (Wood and MacRae, 1974).
TABLE 10. Disappearance of chlorpyrifos-methyl in Japanese soils*
Formulation/ Dosage Days after Number of Range of
treatment rate application samples residues (mg/kg)
3% dust 1.8 kg/ha 0 days 4 1.98-2.94
10 days 4 0.41-1.47
20 days 4 0.10-0.33
30 days 4 <0.05-0.17
3% dust 0.18 kg/ha 0 days 4 0.171-0.236
10 days 4 0.121-0.183
20 days 4 0.059-0.089
30 days 4 0.007-0.014
96% DOWCO 214 5 mg/kg 0 days 4 4.02-4.10
(laboratory
test) 3 days 4 0.82-1.47
10 days 4 0.26-0.39
30 days 4 0.06-0.31
* From Kubota (1974).
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods for the determination of
chlorpyrifos-methyl and its principal metabolite,
3,5,6-trichloro-2-pyridinol, have been described in numerous
modifications, mostly varying in the detailed procedures of extraction
and clean-up (Deahl and Tucker (1974), Desmarchelier (1975b) for
grains; Hollick and Collison (1972a and b) for various vegetables;
Johnson et al. (1974) for corn, milk, urine and faeces; Kubota (1975)
for a variety of vegetables, rice and soils; McKellar et al. (1970,
1971a, b and c) for grains, grass, bovine tissues land milk; Regoli et
al. (1974) for soils; Wirth et al. (1971) for tomatoes).
Of the two compounds, the pyridinol which usually appears as a
hydrolytic product and mostly in non-fatty tissues is of less
importance in the analysis for regulatory purposes. The determination
of the P=O analogue of chlorpyrifos-methyl may or may not be included
by the above procedures (cf. for instance Johnson et al. (1974)), but
it is usually not encountered in animal or plant tissues.
Generally speaking, the principle of determination is based on
extraction with dichloromethane (from wet media), acetone or methanol
(from wet media and soils) or hexane (from dry or fatty tissues)
followed by partitioning procedures (acetone: CH2Cl2 and/or hexane:
acetonitrile) with eventual further clean-up on florisil, silica gel
or alumina.
The final determination of chlorpyrifos-methyl (and its oxygen
analogue) is made from acetone solution by GLC utilizing a phosphorus
specific detector of the FPD- or AFID types. For the pyridinol
metabolite the determination is made according to McKellar and
Dishburger (1970) on GLC with a nickel (Ni63) EC-detector after
derivatization with N,O-bis(trimethylsilyl)acetamide.
The analytical sensitivities reported as limits of determination
for these methods range from 0.001 to 0.02 mg/kg of
chlorpyrifos-ethyl, about 0.01 mg/kg of the O-analogue and 0.001-0.05
mg/kg of the pyridinol in various substrates and with recoveries of
85.9-100.4% for all the compounds.
The general work chart for the analysis of chlorpyrifos (FAO/WHO
(1973) page 192) also seems to apply for the analysis of
chlorpyrifos-methyl and the general scheme should be adaptable for
regulatory purposes as part of a multiresidue analysis system.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Crop Tolerance, mg/kg (PHI)
Japan Rice 0.03 (60 days)
Chinese cabbage,
radish 0.03 (30 days)
Cabbage 0.03 (7 days)
Tobacco 0.03
APPRAISAL
Chlorpyrifos-methyl is an organophosphorus insecticide of
relatively low toxicity and with broad spectrum effects against a
number of insects of great economic interest as well as several
species of household insects. Since its introduction it has gained
particular and fairly wide interest as a potential grain protectant.
Information on the chemical and physical properties of
chlorpyrifos-methyl has been presented to the Meeting. The compound is
available in crystalline form with a purity of 99.8%. It is relatively
easily hydrolysed with the formation of 3,5,6-trichloro-2-pyridinol
which is also the major metabolite in plants, animals and soils.
It is registered and marketed in some countries as dusts,
granules and emulsifiable concentrates for pre-harvest agricultural
and horticultural purposes and for post-harvest grain treatments.
Recommended dosage rates vary from 0.1 to 2.0 kg/ha under field
conditions and from 2.5 to 10 mg/kg in grain storage.
Residue data from supervised trials and experimental tests with
chlorpyrifos-methyl in several countries have been made available by
the manufacturer in support of registrations, including the
establishment of tolerances. These data include residue levels and
disappearance rates in several vegetable crops, fruits and cereal
plants as well as stored grain products, including wheat, corn and
sorghum grains.
While generally of low persistence, chlorpyrifos-methyl is
relatively stable and evidences prolonged protection against insects
in grains and stored, dried products. The half-life of
chlorpyrifos-methyl in stored wheat averages three to five months
under practical conditions, but at higher moisture levels and higher
temperatures the rate of decomposition increases. Conversely at low
temperatures and low humidities the effective life is expected to be
prolonged.
Evidence of the fate of residues during the milling, processing
and baking of cereals is demonstrated in several trials. Residues in
grains are to a great extent confined to the outer layers and
concentrated in bran and milling offals. Correspondingly they are
diminished to low levels (10% of the original deposits or lower) in
flour and further reduced to half of this in baked bread.
Feeding trials with cows indicate that both chlorpyrifos-methyl
and its pyridinol moiety may give rise to trace amounts (below 0.01
mg/kg) in milk, but that the main excretory route for the parent
compound is via the faeces and for the metabolite via the urine.
Residue studies on meat products, fat or eggs are not available.
Several soil studies have been made on chlorpyrifos-methyl which
confirms that hydrolysis to 3,5,6-trichloro-2-pyridinol is a fairly
rapid process under normal conditions and that total residues of
chlorpyrifos-methyl could hardly persist in soils or give rise to
traceable carry-over in crop rotation. Neither run-off nor leaching of
the compounds from the site of application is likely to occur.
Specific gas-chromatographic methods of analysis are available
for the determination of chlorpyrifos-methyl and
3,5,6-trichloro-2-pyridinol. The analytical procedure is closely
parallel to that for the ethyl analogue, chlorpyrifos, and should be
adaptable for regulatory purposes as part of multiresidue analysis.
Residues of chlorpyrifos-methyl may occur as its main metabolite,
3,5,6-trichloro-2-pyridinol. The amounts of this, however, are
generally low in relation to the original deposits.
RECOMMENDATIONS
The following maximum residue limits based on the parent compound
are recommended. They are not likely to be exceeded when following
good agricultural practices. Determination of the level of
chlorpyrifos-methyl parent compound will adequately control the amount
of total residue resulting from the use of this insecticide.
Maximum Pre-harvest interval
residue on which
limit recommendations
Commodity mg/kg are based (days)
Bran 20
Raw grains (wheat, corn, sorghum) 10
Flour, wholemeal bread 2
Apples, peaches 0.5 14
Tomatoes 0.5 3-5
White bread 0.5
Artichoke, beans, cabbage,
chinese cabbage, eggplant, 0.1 7-14
lettuce (outdoor), peppers,
radish, tea (green)
Rice (pre-harvest treatment) 0.1 21
Milk 0.01
FURTHER WORK OR INFORMATION
REQUIRED (before additional maximum residue limits can be
recommended)
1. Information on residues in animal tissues, fat and eggs
following feeding of chlorpyrifos-methyl residues in animal
feeds.
DESIRABLE
1. Appropriate mutagenic study.
2. Neurotoxicity study with histological examination of nervous
tissues.
3. Information on evidence of residues in commerce.
4. Further information on residue disappearance in practical
grain storage at low temperatures and low humidities.
REFERENCES
Bakke, J. E. and Price, C. E. (1975) Metabolism of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl)phosphorothionate and
3,5,6-trichloro-2-pyridinol in sheep and rats. Metabolism and
Radiation Laboratory, A.R.S., U.S.D.A. Fargo, North Dakota, June 10.
Personal communication to the Dow Chemical Company submitted to the
World Health Organization by the Dow Chemical Co., Midland, Michigan,
USA.
Bengtson, M., Connel, M., Desmarchelier, J., Philips, M., Snelson, J.
and Sticka, R. (1975) Evaluation of four new grain protectants. Report
to the Australian Wheat Board 1975. (To be published) (See
Desmarchelier, 1975c, Table 16).
Branson, D.R. and Litchfield, N.H. (1971) Absorption excretion and
distribution of
O,O-dimethyl-O-3,5,6-trichloro-2-6,14-2-pyridyl-phosphorothioate
(C14-Dowco 214) in rats. Unpublished report from the
Dow Chemical Company submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Brust, H.F. (1969) A summary of chemical and physical properties of
O,O-dimethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report OL 3083 from The Dow Chemical Co., Midland,
Michigan, September 12, 6 pp.
Bulla, L.A. Jr. and LaHue, D.W. (1975) Progress report studies on the
development of new grain protectant treatment - chlorpyrifos-methyl.
Report to The Dow Chemical Co., Michigan from U.S.D.A., A.R.S. Grain
Marketing Research Center, Manhattan, Kansas, February 18, 12 pp.
Coulston, F. and Griffin, T.B. (1975) A safety evaluation of Dowco 214
in rats and rhesus monkeys. Unpublished report, from the Institute of
Comparative and Human Toxicology, Albany Medical College submitted to
the World Health Organization by the Dow Chemical Co., Midland,
Michigan, USA.
Coulston, F., Rosenblum, I. and Griffin, T.B. (1975) Study of
chlorpyrifos-methyl in human volunteers. Unpublished report from
Institute of Comparative and Human Toxicology, Albany Medical College
and the International Center of Environmental Safety, Holloman AFB,
New Mexico submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Davies, R.E. and Collins, C.J. (1975) Response to oxime reactivating
agent in rats acutely poisoned with Reldan technical. Unpublished
report from the Huntingdon Research Centre submitted to the World
Health Organization by The Dow Chemical Co., Midland, Michigan, USA.
Deahl, H.Z. and Tucker, K.E.B. (1974) Residue studies on wheat stored
in vertical concrete silos in Queensland and N.S.W., Australia.
Unpublished report GHF-P-024 from Dow Chemical (Australia) Ltd.,
Altona, Victoria, November 18.
Desmarchelier, J.M. (1975a) Draft report No. 329-4-23 on 1973/74 grain
protectant trials to Australian Wheat Board, Working Party on Grain
Protectants. Chemical analysis - Residues and fate of protectants in
grain. CSIRO, Canberra.
Desmarchelier, J.M. (1975b) Quantitative measurement of rate of loss
of insecticidal activity on treated wheat due to lack of availability
to insects. In press. Communicated to Dow Chemical (Australia) Ltd.,
Sydney, June, 5 pp,
Desmarchelier, J.M. (1975c) An interim report on residues in wheat
treated with chlorpyrifos-methyl-grain protectant field trial, Tammin,
Western Australia. Communication to Dow Chemical (Australia) Ltd.,
Sydney, May 19, 16 pp.
Esaki, K., Tanioka, Y., Tsukada, M., Izumiyama, K. and Ohshio, K.
(1973) Effects of DOWCO 214 against fetuses of experimental animals.
Exper. Animal Central Laboratories, Kawasaki, Japan, Oct. 20.
Unpublished report submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Hamaker, J.W. (1974) Soil absorption of chlorpyrifos-methyl and its
hydrolysis products. Unpublished report GS 1343 (revised) from Dow
Chemical USA, Walnut Creek, California, February 20,
Hascouet, X. (1974) Determination of residues of chlorpyrifos and
chlorpyrifos methyl in vegetables after foliar treatment. Report to
The Dow Chemical Company, France. Ministry of Agriculture, C.N.R.A.,
Versailles, August 5.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E. and Kitagaki, T. (1973a) (Hokkaido Inst. of
Pub. Health, Sapporo, Japan). Studies on the safety of the newly
introduced organophosphorus insecticide, chlorpyrifos-methyl. Part 1.
Acute toxicity of chlorpyrifos-methyl in rats and mice. Hokkaidoritsu
Eisei Kenkyusho-Ho (Rep. Hokkaido Inst. Pub. Health), 23:57-61.
Japanese. English Summary in Pesticide Abstracts 7(4):74-0891, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973b) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of the newly introduced organophosphorus insecticide,
chlorpyrifos-methyl, Part 2. Subacute (6 months) toxicity of
chlorpyrifos-methyl in rats. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23:62-70. Japanese. English Summary in
Pesticide Abstracts 7(4):74-0892, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973c) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of newly introduced organophosphorus insecticide,
chlorpyrifos-methyl. Part 3. Subacute (six months) toxicity of
chlorpyrifos-methyl in mice. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23: 71-80. Japanese. English Summary in
Pesticide Abstracts, 7(4):74-0893, 1974.
Hayashi, E., Nakamura, I., Terada, Y., Kunimoto, M. and Yamada, S.
(1973) Therapeutic experiment on DOWCO 214 poisoned rats. Unpublished
report from Shizuaka Pharmacology College, Shizuoka, Japan, September
15. Submitted to the World Health Organization by The Dow Chemical
Co., Midland, Michigan, USA.
Hollick, C.B. and Collison, R.J. (1972a) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in egg plants, tomatoes, beans and peppers treated with NOLTRAN
insecticide in Turkey., Unpublished report GHE-P 011 from Dow Chemical
Company Limited, King's Lynn, England, August 23.
Hollick, C.B. and Collison, R.J. (1972b) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in peppers treated with NOLTRAN insecticide in Egypt. Unpublished
report CHE-P 102 from Dow Chemical Company Limited, King's Lynn,
England, October 16.
Humiston, C.G., Wade, C.E. and McCollister, S.B. (1972) Results of a
supplemental dietary feeding study of DOWCO 214 in dogs. Unpublished
report from The Dow Chemical Co., submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Cherry, C.P.
(1974a) DOWCO 214 - Toxicity following dietary administration to rats
for two years. Unpublished report from the Huntingdon Research Centre,
Huntingdon, England, submitted to the World Health Organization by The
Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Graham, C. and Brooks, P.N. (1974b) Tumorigenicity
screening of DOWCO 214 in the rat (final report 0-104 weeks).
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by the Dow
Chemical Co., Midland, Michigan, USA.
Johnson, J.C. Jr, Jones, R.L., Leuck, D.B., Bowman, M.C. and Knox,
F.E. (1974) Persistence of chlorpyrifos-methyl in corn silage and
effects of feeding dairy cows the treated silage. J. Dairy Sci.
57(1):467-73.
Kenaga, E.E. (1971) Some physicial, chemical, and insecticidal
properties of some O,O-dialkyl O-(3,5,6-trichloro-2-pyridyl)
phosphates and phosphorothioates. Bull. World Health Org.,
44:225-228.
Kenaga, E.E. (1975) Chlorpyrifos-methyl. Information on identity, use,
residues, analysis and tolerances. Submission from The Dow Chemical
Company, Midland, Michigan, prepared for the FAO/WHO 1975 Joint
Meeting, June 1975.
Kubota, Y. (1974) Summary tables of chlorpyrifos-methyl residue data
for crops and soil in Japan. Reports submitted by Dow Chemical
International Ltd., Tokyo, Japan, February 6.
Leuck, D.B., Jones, R.L. and Bowman, M.C. (1975) Chlorpyrifos-methyl
insecticide residues: their analysis and persistence in coastal
Bermudagrass and forage corn. J. Econ. Entoml., 68(3):287-90.
Litchfield, N. and Norris, J. (1969) O-O-dimethyl-o-3,5,6
trichloro-2-pyridyl phosphorothioate (Dowco 214). Unpublished report
from The Dow Chemical Co., submitted to the World Health Organization
by the Dow Chemical Co., Midland, Michigan, USA.
MacRae, I.C. and Celo, J.S. (1974) The effects of organo-phosphorus
pesticides on the respiration of Azotobacter vinelandii. Soil Biol.
Biochem., 6, 109-111.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography, method ACR 70.19 of the Dow Chemical Company, USA,
December 14.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-2-pyridinol in milk and cream by gas chromatography.
Method ACR. 71.2 of the Dow Chemical Company, USA, February 2.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-2-pyridinol in grass by gas chromatography. Method
ACR. 71.5 of the Dow Chemical Company, USA, May 6.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-2-pyridinol in corn grain, forage and stover by gas
chromatography. Method 71.19 of the Dow Chemical Company, USA,
September 27.
Meikle, R.W. (1973) Hydrolysis rate of DOWCO 214 in buffered distilled
water and in tap water. Unpublished report GS-1316 from the Dow
Chemical Company, USA, Walnut Creek, California, April 16.
Morel, J.L. and Galet, G. (1975) Results of residue analysis carried
out in France in grains treated with chlorpyrifos-methyl until May
1975. Communication from Dow Chemical Europe S.A. (report in press).
Nakajima, E., Shigehara, E. and Shindo, S. (1974) Whole body
autoradiography in rats medicated with the organophosphoric
insecticide DOWCO 214-14C. Unpublished report from the Drug
Metabolism Res. Lab., Sankyo Co. Ltd., Shinagawa, Tokyo, Japan
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Nakayama, I., Kuroda, T., Kitagaki, T., Shinohara, H. and Otokozawa,
T. (1973) Bagworms: effectiveness of several insecticide formulations
against the larvae of three species. J. Econ. Entomol.,
66(4):941-43.
Olson, K.J. (1964a) Results of toxicological tests on
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report from the Biochemical Research Laboratory, The Dow
Chemical Company, Midland, Michigan, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Olson, K.J. and Taylor, M.L. (1964b) Results of range finding
toxicological tests on O,O-dimethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate. Unpublished report from the Biochemical Research
Laboratory, The Dow Chemical Company, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Regoli, A.J., Laskowski, D.A. and Kurihara, N.H. (1974) Aerobic
degradation of chlorpyrifos-methyl in soil. Unpublished report GS-1367
from The Dow Chemical Company, Midland, Michigan, July 12.
Rivett, K.F., Sortwell, R.J., Heywood, R., Street, A.E. and Cherry,
C.P. (1974) Dowco 214 dietary toxicity studies in beagle dogs.
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Ross, D.B. and Roberts, N.L. (1974) The acute toxicity (LD50) of
Reldan (Dowco 214) to the chicken. Report DWC 234/74822. Unpublished
report from the Huntingdon Research Centre, Huntingdon, England,
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Ross, D.B., Burroughs, S.J. and Roberts, N.L. (1975) Examination of
chlorpyrifos-methyl (Dowco 214) for neurotoxicity in the domestic hen.
Report DWC 225/75401. Unpublished report from the Huntingdon Research
Centre, Huntingdon, England, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Schulten, G.G.M. (1973) Further insecticide trials on the control of
Ephestia cautella in stacks of bagged maize in Malawi. Intern. Pest
Control, March/April, p. 18-20.
Schwetz, B.A., Keeler, P.A., Pernell, H.C., Smith, F.A., Humiston,
C.G. and Gehring, P.J. (1973) A study of the prenatal toxicity of
Dowco 214 in rats. Report #NB T35. 12-46193-9, Unpublished report from
The Dow Chemical Company, submitted to the World Health Organization
by The Dow Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974a) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health organization by The Dow
Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974b) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Shellenberger, T.E. (1970) Toxicological evaluations of DOWCO 214 with
wildlife and DOWCO 179 with Mallard ducklings. Unpublished report from
the Gulf Research Institute, New Iberia, Louisiana, submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967) Investigations on
DURSBAN insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agric. Fd. Chem.,
15(5):870-77.
Smith, G.N. and Taylor, Y. (1972) Photodecomposition of DOWCO 214.
Unpublished report NBE-2 from The Dow Chemical Company, Midland,
Michigan, January 21.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Unpublished report
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Snetsinger, R. and Carroll, D. (1972) Evaluation of Dursban and DOWCO
214. Pest Control, 40(5):24-32.
Soderstrom, E.L. and Armstrong, J.W. (1974) Chlorpyrifos-methyl: A
protectant for raisins. J. Econ. Entomol., 68(1), p. 132.
Sparschu, G.L., Humiston, C.G., McCollister, S.B. and Wade, C.E.
(1971) Results of 92-day dietary feeding studies on DOWCO 214 in dogs.
Unpublished report from The Dow Chemical Company submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Steinberg, M. (1971) Summary of findings from 90-day feeding study
with DOWCO 214. Department of the Army. U.S. Army Hygiene Agency,
Aberdeen Proving Ground, Maryland. October 5, 8 pp. (Personal
communication).
U.S. Department of the Army. (1973) Preliminary assessment of relative
toxicity of candidate louse toxicant DOWCO 214 (ENT-27520)
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate evaluation
of animal exposure data and recommendations concerning a sleeve test
program. Study No. 51-19-68/74. November 1968 - May 1972. U.S. Army
Environmental Hygiene Agency. USAEHA-LT. Aberdeen Proving Ground,
Maryland, 38 pp.
Thompson, D.J., Dyke, I.L. and Warner, S.D. (1975) Results of a three
generation, two litter reproduction study on
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Dowco
214) in the rat. Unpublished report from Dow Chemical Corporation,
Department of Pathology and Toxicology, Zionsville, Indiana, submitted
to the World Health Organization by The Dow Chemical Co., Midland,
Michigan, USA.
White, G.S. and LaHue, D. (1974) Preliminary information concerning
DOWCO 214 treatment of wheat grain. Communicated by The Dow Chemical
Company, Midland, Michigan from the U.S.D.A., A.R.S. Grain Marketing
Research Center, Manhattan, Kansas, January 14.
Whitten, C.J. and Bull, D.L. (1974) Comparative toxicity, absorption
and metabolism of chlorpyrifos and its dimethyl homologue in
methyl-parathion resistant and susceptible tobacco budworms. Pest.
Biochem. & Physiol., 4:266-74.
Wirth, M., Würth, E. and Engeler, V. (1971) A residue study: DOWCO 179
and DOWCO 214 on tomatoes in a greenhouse. Unpublished report GHE-P
005 from Dow Chemical Company Limited, King's Lynn, England, December
16.
Wood, P.A. and MacRae, I.C. (1974) The effect of several
organophosphorus insecticides upon the acetylene-reduction activity of
Azotobacter vinelandii. Bull. Environ. Contamin. & Toxicol.,
12(1):26-31.
World Health Organization. (1966) WHO insecticide evaluation and
testing programme. Stage 1 mammalian toxicity report. OMS 1155.
An overall average of 100.4% recovery of added activity was
obtained for samples over the entire study and it was found that the
major metabolite in soil was 3,5,6-trichloro-2-pyridinol which is
further degraded to CO2 followed by lesser amounts of
3,5,6-trichloro-2-methoxy-pyridine. The authors speculated whether the
methoxy-compound is formed through direct methylation of
chlorpyrifos-methyl or from the pyridinol through microbial activity,
but the former route, involving subsequent conversion of the
methoxypyridine to the pyridinol seems more likely as no other
decomposition-products could be traced.
The breakdown of chlorpyrifos-methyl to
3,5,6-trichloro-2-pyridinol is a rapid process if conditions are
favourable for microbial and hydrolytic activity. The time required
for 50% breakdown ranged from 1.5 to 2.0 days in the high organic soil
at 25-35°C and 100% moisture to 33.3-17.7 days in the low organic soil
at 15°C and 32% moisture. The calculated times required for 90%
degradation ranged from 20 to 30 days under favourable conditions to
500-1600 days under less favourable. Thus in practice
chlorpyrifos-methyl could hardly persist in soils and give rise to
traceable carry-over during crop rotations.
Hamaker (1974) studied the soil adsorption of
C14-chlorpyrifos-methyl by analysing both the soil and the
supernatant from slurries of soil in 1 mg/l solutions (one part of
soil:four parts of solution). The soils contained organic carbon
ranging from 0.28 to 5.76%.
The adsorption by these soils ranged from 46 to 99% after 24
hours and the average distribution ratio between soil organic carbon
and water was found to be 3300 after four hours and 4600 after 24
hours. This is less by one-third than in the case of chlorpyrifos but
still represents a strong adsorption to soil.
The hydrolysis product (3,5,6-trichloro-2-pyridinol) is absorbed
to a smaller degree than the parent compound showing a distribution
ratio of 714 between soil and water. In the conclusions of the author,
however, this still represents a sufficiently strong adsorption to
make leaching of the compound from soils an unlikely process.
Kubota (1975) has reported on both field trials and laboratory
tests determining residue disappearance from rice paddy soils over
periods of 30 days (Table 10). The half-life periods in the field
tests varied from five to about 20 days, while the half-life was less
than three days when chlorpyrifos-methyl was applied directly to the
soil in the laboratory.
A DOWCO 214 formulation applied to bacterial cultures in amounts
corresponding to normal soil application showed no influence on the
bacterial activity measured as the O2-uptake under incubation of
Azotobacter vinelandii (MacRae and Celo, 1974). Neither was the
nitrogen fixation of this strain as measured by the reduction of
acetylene to ethylene affected, even at concentrations 100 times the
practical application rates (Wood and MacRae, 1974).
TABLE 10. Disappearance of chlorpyrifos-methyl in Japanese soils*
Formulation/ Dosage Days after Number of Range of
treatment rate application samples residues (mg/kg)
3% dust 1.8 kg/ha 0 days 4 1.98-2.94
10 days 4 0.41-1.47
20 days 4 0.10-0.33
30 days 4 <0.05-0.17
3% dust 0.18 kg/ha 0 days 4 0.171-0.236
10 days 4 0.121-0.183
20 days 4 0.059-0.089
30 days 4 0.007-0.014
96% DOWCO 214 5 mg/kg 0 days 4 4.02-4.10
(laboratory
test) 3 days 4 0.82-1.47
10 days 4 0.26-0.39
30 days 4 0.06-0.31
* From Kubota (1974).
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods for the determination of
chlorpyrifos-methyl and its principal metabolite,
3,5,6-trichloro-2-pyridinol, have been described in numerous
modifications, mostly varying in the detailed procedures of extraction
and clean-up (Deahl and Tucker (1974), Desmarchelier (1975b) for
grains; Hollick and Collison (1972a and b) for various vegetables;
Johnson et al. (1974) for corn, milk, urine and faeces; Kubota (1975)
for a variety of vegetables, rice and soils; McKellar et al. (1970,
1971a, b and c) for grains, grass, bovine tissues land milk; Regoli et
al. (1974) for soils; Wirth et al. (1971) for tomatoes).
Of the two compounds, the pyridinol which usually appears as a
hydrolytic product and mostly in non-fatty tissues is of less
importance in the analysis for regulatory purposes. The determination
of the P=O analogue of chlorpyrifos-methyl may or may not be included
by the above procedures (cf. for instance Johnson et al. (1974)), but
it is usually not encountered in animal or plant tissues.
Generally speaking, the principle of determination is based on
extraction with dichloromethane (from wet media), acetone or methanol
(from wet media and soils) or hexane (from dry or fatty tissues)
followed by partitioning procedures (acetone: CH2Cl2 and/or hexane:
acetonitrile) with eventual further clean-up on florisil, silica gel
or alumina.
The final determination of chlorpyrifos-methyl (and its oxygen
analogue) is made from acetone solution by GLC utilizing a phosphorus
specific detector of the FPD- or AFID types. For the pyridinol
metabolite the determination is made according to McKellar and
Dishburger (1970) on GLC with a nickel (Ni63) EC-detector after
derivatization with N,O-bis(trimethylsilyl)acetamide.
The analytical sensitivities reported as limits of determination
for these methods range from 0.001 to 0.02 mg/kg of
chlorpyrifos-ethyl, about 0.01 mg/kg of the O-analogue and 0.001-0.05
mg/kg of the pyridinol in various substrates and with recoveries of
85.9-100.4% for all the compounds.
The general work chart for the analysis of chlorpyrifos (FAO/WHO
(1973) page 192) also seems to apply for the analysis of
chlorpyrifos-methyl and the general scheme should be adaptable for
regulatory purposes as part of a multiresidue analysis system.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Crop Tolerance, mg/kg (PHI)
Japan Rice 0.03 (60 days)
Chinese cabbage,
radish 0.03 (30 days)
Cabbage 0.03 (7 days)
Tobacco 0.03
APPRAISAL
Chlorpyrifos-methyl is an organophosphorus insecticide of
relatively low toxicity and with broad spectrum effects against a
number of insects of great economic interest as well as several
species of household insects. Since its introduction it has gained
particular and fairly wide interest as a potential grain protectant.
Information on the chemical and physical properties of
chlorpyrifos-methyl has been presented to the Meeting. The compound is
available in crystalline form with a purity of 99.8%. It is relatively
easily hydrolysed with the formation of 3,5,6-trichloro-2-pyridinol
which is also the major metabolite in plants, animals and soils.
It is registered and marketed in some countries as dusts,
granules and emulsifiable concentrates for pre-harvest agricultural
and horticultural purposes and for post-harvest grain treatments.
Recommended dosage rates vary from 0.1 to 2.0 kg/ha under field
conditions and from 2.5 to 10 mg/kg in grain storage.
Residue data from supervised trials and experimental tests with
chlorpyrifos-methyl in several countries have been made available by
the manufacturer in support of registrations, including the
establishment of tolerances. These data include residue levels and
disappearance rates in several vegetable crops, fruits and cereal
plants as well as stored grain products, including wheat, corn and
sorghum grains.
While generally of low persistence, chlorpyrifos-methyl is
relatively stable and evidences prolonged protection against insects
in grains and stored, dried products. The half-life of
chlorpyrifos-methyl in stored wheat averages three to five months
under practical conditions, but at higher moisture levels and higher
temperatures the rate of decomposition increases. Conversely at low
temperatures and low humidities the effective life is expected to be
prolonged.
Evidence of the fate of residues during the milling, processing
and baking of cereals is demonstrated in several trials. Residues in
grains are to a great extent confined to the outer layers and
concentrated in bran and milling offals. Correspondingly they are
diminished to low levels (10% of the original deposits or lower) in
flour and further reduced to half of this in baked bread.
Feeding trials with cows indicate that both chlorpyrifos-methyl
and its pyridinol moiety may give rise to trace amounts (below 0.01
mg/kg) in milk, but that the main excretory route for the parent
compound is via the faeces and for the metabolite via the urine.
Residue studies on meat products, fat or eggs are not available.
Several soil studies have been made on chlorpyrifos-methyl which
confirms that hydrolysis to 3,5,6-trichloro-2-pyridinol is a fairly
rapid process under normal conditions and that total residues of
chlorpyrifos-methyl could hardly persist in soils or give rise to
traceable carry-over in crop rotation. Neither run-off nor leaching of
the compounds from the site of application is likely to occur.
Specific gas-chromatographic methods of analysis are available
for the determination of chlorpyrifos-methyl and
3,5,6-trichloro-2-pyridinol. The analytical procedure is closely
parallel to that for the ethyl analogue, chlorpyrifos, and should be
adaptable for regulatory purposes as part of multiresidue analysis.
Residues of chlorpyrifos-methyl may occur as its main metabolite,
3,5,6-trichloro-2-pyridinol. The amounts of this, however, are
generally low in relation to the original deposits.
RECOMMENDATIONS
The following maximum residue limits based on the parent compound
are recommended. They are not likely to be exceeded when following
good agricultural practices. Determination of the level of
chlorpyrifos-methyl parent compound will adequately control the amount
of total residue resulting from the use of this insecticide.
Maximum Pre-harvest interval
residue on which
limit recommendations
Commodity mg/kg are based (days)
Bran 20
Raw grains (wheat, corn, sorghum) 10
Flour, wholemeal bread 2
Apples, peaches 0.5 14
Tomatoes 0.5 3-5
White bread 0.5
Artichoke, beans, cabbage,
chinese cabbage, eggplant, 0.1 7-14
lettuce (outdoor), peppers,
radish, tea (green)
Rice (pre-harvest treatment) 0.1 21
Milk 0.01
FURTHER WORK OR INFORMATION
REQUIRED (before additional maximum residue limits can be
recommended)
1. Information on residues in animal tissues, fat and eggs
following feeding of chlorpyrifos-methyl residues in animal
feeds.
DESIRABLE
1. Appropriate mutagenic study.
2. Neurotoxicity study with histological examination of nervous
tissues.
3. Information on evidence of residues in commerce.
4. Further information on residue disappearance in practical
grain storage at low temperatures and low humidities.
REFERENCES
Bakke, J. E. and Price, C. E. (1975) Metabolism of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl)phosphorothionate and
3,5,6-trichloro-2-pyridinol in sheep and rats. Metabolism and
Radiation Laboratory, A.R.S., U.S.D.A. Fargo, North Dakota, June 10.
Personal communication to the Dow Chemical Company submitted to the
World Health Organization by the Dow Chemical Co., Midland, Michigan,
USA.
Bengtson, M., Connel, M., Desmarchelier, J., Philips, M., Snelson, J.
and Sticka, R. (1975) Evaluation of four new grain protectants. Report
to the Australian Wheat Board 1975. (To be published) (See
Desmarchelier, 1975c, Table 16).
Branson, D.R. and Litchfield, N.H. (1971) Absorption excretion and
distribution of
O,O-dimethyl-O-3,5,6-trichloro-2-6,14-2-pyridyl-phosphorothioate
(C14-Dowco 214) in rats. Unpublished report from the
Dow Chemical Company submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Brust, H.F. (1969) A summary of chemical and physical properties of
O,O-dimethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report OL 3083 from The Dow Chemical Co., Midland,
Michigan, September 12, 6 pp.
Bulla, L.A. Jr. and LaHue, D.W. (1975) Progress report studies on the
development of new grain protectant treatment - chlorpyrifos-methyl.
Report to The Dow Chemical Co., Michigan from U.S.D.A., A.R.S. Grain
Marketing Research Center, Manhattan, Kansas, February 18, 12 pp.
Coulston, F. and Griffin, T.B. (1975) A safety evaluation of Dowco 214
in rats and rhesus monkeys. Unpublished report, from the Institute of
Comparative and Human Toxicology, Albany Medical College submitted to
the World Health Organization by the Dow Chemical Co., Midland,
Michigan, USA.
Coulston, F., Rosenblum, I. and Griffin, T.B. (1975) Study of
chlorpyrifos-methyl in human volunteers. Unpublished report from
Institute of Comparative and Human Toxicology, Albany Medical College
and the International Center of Environmental Safety, Holloman AFB,
New Mexico submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Davies, R.E. and Collins, C.J. (1975) Response to oxime reactivating
agent in rats acutely poisoned with Reldan technical. Unpublished
report from the Huntingdon Research Centre submitted to the World
Health Organization by The Dow Chemical Co., Midland, Michigan, USA.
Deahl, H.Z. and Tucker, K.E.B. (1974) Residue studies on wheat stored
in vertical concrete silos in Queensland and N.S.W., Australia.
Unpublished report GHF-P-024 from Dow Chemical (Australia) Ltd.,
Altona, Victoria, November 18.
Desmarchelier, J.M. (1975a) Draft report No. 329-4-23 on 1973/74 grain
protectant trials to Australian Wheat Board, Working Party on Grain
Protectants. Chemical analysis - Residues and fate of protectants in
grain. CSIRO, Canberra.
Desmarchelier, J.M. (1975b) Quantitative measurement of rate of loss
of insecticidal activity on treated wheat due to lack of availability
to insects. In press. Communicated to Dow Chemical (Australia) Ltd.,
Sydney, June, 5 pp,
Desmarchelier, J.M. (1975c) An interim report on residues in wheat
treated with chlorpyrifos-methyl-grain protectant field trial, Tammin,
Western Australia. Communication to Dow Chemical (Australia) Ltd.,
Sydney, May 19, 16 pp.
Esaki, K., Tanioka, Y., Tsukada, M., Izumiyama, K. and Ohshio, K.
(1973) Effects of DOWCO 214 against fetuses of experimental animals.
Exper. Animal Central Laboratories, Kawasaki, Japan, Oct. 20.
Unpublished report submitted to the World Health Organization by the
Dow Chemical Co., Midland, Michigan, USA.
Hamaker, J.W. (1974) Soil absorption of chlorpyrifos-methyl and its
hydrolysis products. Unpublished report GS 1343 (revised) from Dow
Chemical USA, Walnut Creek, California, February 20,
Hascouet, X. (1974) Determination of residues of chlorpyrifos and
chlorpyrifos methyl in vegetables after foliar treatment. Report to
The Dow Chemical Company, France. Ministry of Agriculture, C.N.R.A.,
Versailles, August 5.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E. and Kitagaki, T. (1973a) (Hokkaido Inst. of
Pub. Health, Sapporo, Japan). Studies on the safety of the newly
introduced organophosphorus insecticide, chlorpyrifos-methyl. Part 1.
Acute toxicity of chlorpyrifos-methyl in rats and mice. Hokkaidoritsu
Eisei Kenkyusho-Ho (Rep. Hokkaido Inst. Pub. Health), 23:57-61.
Japanese. English Summary in Pesticide Abstracts 7(4):74-0891, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973b) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of the newly introduced organophosphorus insecticide,
chlorpyrifos-methyl, Part 2. Subacute (6 months) toxicity of
chlorpyrifos-methyl in rats. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23:62-70. Japanese. English Summary in
Pesticide Abstracts 7(4):74-0892, 1974.
Hasegawa, M., Ono, T., Karashimada, T., Sato, H., Kameyama, K.,
Kinoshita, Y., Ogawa, E., Kobayashi, H., Sonoda, M. and Kitagaki, T.
(1973c) (Hokkaido Inst. Pub. Health, Sapporo, Japan). Studies on the
safety of newly introduced organophosphorus insecticide,
chlorpyrifos-methyl. Part 3. Subacute (six months) toxicity of
chlorpyrifos-methyl in mice. Hokkaidoritsu Eisei Kenkyusho-Ho. (Rep.
Hokkaido Inst. Pub. Health), 23: 71-80. Japanese. English Summary in
Pesticide Abstracts, 7(4):74-0893, 1974.
Hayashi, E., Nakamura, I., Terada, Y., Kunimoto, M. and Yamada, S.
(1973) Therapeutic experiment on DOWCO 214 poisoned rats. Unpublished
report from Shizuaka Pharmacology College, Shizuoka, Japan, September
15. Submitted to the World Health Organization by The Dow Chemical
Co., Midland, Michigan, USA.
Hollick, C.B. and Collison, R.J. (1972a) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in egg plants, tomatoes, beans and peppers treated with NOLTRAN
insecticide in Turkey., Unpublished report GHE-P 011 from Dow Chemical
Company Limited, King's Lynn, England, August 23.
Hollick, C.B. and Collison, R.J. (1972b) Determination of residues of
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (DOWCO
214) in peppers treated with NOLTRAN insecticide in Egypt. Unpublished
report CHE-P 102 from Dow Chemical Company Limited, King's Lynn,
England, October 16.
Humiston, C.G., Wade, C.E. and McCollister, S.B. (1972) Results of a
supplemental dietary feeding study of DOWCO 214 in dogs. Unpublished
report from The Dow Chemical Co., submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. and Cherry, C.P.
(1974a) DOWCO 214 - Toxicity following dietary administration to rats
for two years. Unpublished report from the Huntingdon Research Centre,
Huntingdon, England, submitted to the World Health Organization by The
Dow Chemical Co., Midland, Michigan, USA.
Hunter, B., Graham, C. and Brooks, P.N. (1974b) Tumorigenicity
screening of DOWCO 214 in the rat (final report 0-104 weeks).
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by the Dow
Chemical Co., Midland, Michigan, USA.
Johnson, J.C. Jr, Jones, R.L., Leuck, D.B., Bowman, M.C. and Knox,
F.E. (1974) Persistence of chlorpyrifos-methyl in corn silage and
effects of feeding dairy cows the treated silage. J. Dairy Sci.
57(1):467-73.
Kenaga, E.E. (1971) Some physicial, chemical, and insecticidal
properties of some O,O-dialkyl O-(3,5,6-trichloro-2-pyridyl)
phosphates and phosphorothioates. Bull. World Health Org.,
44:225-228.
Kenaga, E.E. (1975) Chlorpyrifos-methyl. Information on identity, use,
residues, analysis and tolerances. Submission from The Dow Chemical
Company, Midland, Michigan, prepared for the FAO/WHO 1975 Joint
Meeting, June 1975.
Kubota, Y. (1974) Summary tables of chlorpyrifos-methyl residue data
for crops and soil in Japan. Reports submitted by Dow Chemical
International Ltd., Tokyo, Japan, February 6.
Leuck, D.B., Jones, R.L. and Bowman, M.C. (1975) Chlorpyrifos-methyl
insecticide residues: their analysis and persistence in coastal
Bermudagrass and forage corn. J. Econ. Entoml., 68(3):287-90.
Litchfield, N. and Norris, J. (1969) O-O-dimethyl-o-3,5,6
trichloro-2-pyridyl phosphorothioate (Dowco 214). Unpublished report
from The Dow Chemical Co., submitted to the World Health Organization
by the Dow Chemical Co., Midland, Michigan, USA.
MacRae, I.C. and Celo, J.S. (1974) The effects of organo-phosphorus
pesticides on the respiration of Azotobacter vinelandii. Soil Biol.
Biochem., 6, 109-111.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography, method ACR 70.19 of the Dow Chemical Company, USA,
December 14.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-2-pyridinol in milk and cream by gas chromatography.
Method ACR. 71.2 of the Dow Chemical Company, USA, February 2.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-2-pyridinol in grass by gas chromatography. Method
ACR. 71.5 of the Dow Chemical Company, USA, May 6.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-2-pyridinol in corn grain, forage and stover by gas
chromatography. Method 71.19 of the Dow Chemical Company, USA,
September 27.
Meikle, R.W. (1973) Hydrolysis rate of DOWCO 214 in buffered distilled
water and in tap water. Unpublished report GS-1316 from the Dow
Chemical Company, USA, Walnut Creek, California, April 16.
Morel, J.L. and Galet, G. (1975) Results of residue analysis carried
out in France in grains treated with chlorpyrifos-methyl until May
1975. Communication from Dow Chemical Europe S.A. (report in press).
Nakajima, E., Shigehara, E. and Shindo, S. (1974) Whole body
autoradiography in rats medicated with the organophosphoric
insecticide DOWCO 214-14C. Unpublished report from the Drug
Metabolism Res. Lab., Sankyo Co. Ltd., Shinagawa, Tokyo, Japan
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Nakayama, I., Kuroda, T., Kitagaki, T., Shinohara, H. and Otokozawa,
T. (1973) Bagworms: effectiveness of several insecticide formulations
against the larvae of three species. J. Econ. Entomol.,
66(4):941-43.
Olson, K.J. (1964a) Results of toxicological tests on
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate.
Unpublished report from the Biochemical Research Laboratory, The Dow
Chemical Company, Midland, Michigan, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Olson, K.J. and Taylor, M.L. (1964b) Results of range finding
toxicological tests on O,O-dimethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate. Unpublished report from the Biochemical Research
Laboratory, The Dow Chemical Company, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Regoli, A.J., Laskowski, D.A. and Kurihara, N.H. (1974) Aerobic
degradation of chlorpyrifos-methyl in soil. Unpublished report GS-1367
from The Dow Chemical Company, Midland, Michigan, July 12.
Rivett, K.F., Sortwell, R.J., Heywood, R., Street, A.E. and Cherry,
C.P. (1974) Dowco 214 dietary toxicity studies in beagle dogs.
Unpublished report from the Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Ross, D.B. and Roberts, N.L. (1974) The acute toxicity (LD50) of
Reldan (Dowco 214) to the chicken. Report DWC 234/74822. Unpublished
report from the Huntingdon Research Centre, Huntingdon, England,
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Ross, D.B., Burroughs, S.J. and Roberts, N.L. (1975) Examination of
chlorpyrifos-methyl (Dowco 214) for neurotoxicity in the domestic hen.
Report DWC 225/75401. Unpublished report from the Huntingdon Research
Centre, Huntingdon, England, submitted to the World Health
Organization by The Dow Chemical Co., Midland, Michigan, USA.
Schulten, G.G.M. (1973) Further insecticide trials on the control of
Ephestia cautella in stacks of bagged maize in Malawi. Intern. Pest
Control, March/April, p. 18-20.
Schwetz, B.A., Keeler, P.A., Pernell, H.C., Smith, F.A., Humiston,
C.G. and Gehring, P.J. (1973) A study of the prenatal toxicity of
Dowco 214 in rats. Report #NB T35. 12-46193-9, Unpublished report from
The Dow Chemical Company, submitted to the World Health Organization
by The Dow Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974a) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health organization by The Dow
Chemical Co., Midland, Michigan, USA.
Schwetz, B.A. (1974b) Personal communication to R.W. Morgan of Dow
Chemical Company submitted to the World Health Organization by The Dow
Chemical Co., Midland, Michigan, USA.
Shellenberger, T.E. (1970) Toxicological evaluations of DOWCO 214 with
wildlife and DOWCO 179 with Mallard ducklings. Unpublished report from
the Gulf Research Institute, New Iberia, Louisiana, submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967) Investigations on
DURSBAN insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agric. Fd. Chem.,
15(5):870-77.
Smith, G.N. and Taylor, Y. (1972) Photodecomposition of DOWCO 214.
Unpublished report NBE-2 from The Dow Chemical Company, Midland,
Michigan, January 21.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Unpublished report
submitted to the World Health Organization by The Dow Chemical Co.,
Midland, Michigan, USA.
Snetsinger, R. and Carroll, D. (1972) Evaluation of Dursban and DOWCO
214. Pest Control, 40(5):24-32.
Soderstrom, E.L. and Armstrong, J.W. (1974) Chlorpyrifos-methyl: A
protectant for raisins. J. Econ. Entomol., 68(1), p. 132.
Sparschu, G.L., Humiston, C.G., McCollister, S.B. and Wade, C.E.
(1971) Results of 92-day dietary feeding studies on DOWCO 214 in dogs.
Unpublished report from The Dow Chemical Company submitted to the
World Health Organization by The Dow Chemical Co., Midland, Michigan,
USA.
Steinberg, M. (1971) Summary of findings from 90-day feeding study
with DOWCO 214. Department of the Army. U.S. Army Hygiene Agency,
Aberdeen Proving Ground, Maryland. October 5, 8 pp. (Personal
communication).
U.S. Department of the Army. (1973) Preliminary assessment of relative
toxicity of candidate louse toxicant DOWCO 214 (ENT-27520)
O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate evaluation
of animal exposure data and recommendations concerning a sleeve test
program. Study No. 51-19-68/74. November 1968 - May 1972. U.S. Army
Environmental Hygiene Agency. USAEHA-LT. Aberdeen Proving Ground,
Maryland, 38 pp.
Thompson, D.J., Dyke, I.L. and Warner, S.D. (1975) Results of a three
generation, two litter reproduction study on
O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Dowco
214) in the rat. Unpublished report from Dow Chemical Corporation,
Department of Pathology and Toxicology, Zionsville, Indiana, submitted
to the World Health Organization by The Dow Chemical Co., Midland,
Michigan, USA.
White, G.S. and LaHue, D. (1974) Preliminary information concerning
DOWCO 214 treatment of wheat grain. Communicated by The Dow Chemical
Company, Midland, Michigan from the U.S.D.A., A.R.S. Grain Marketing
Research Center, Manhattan, Kansas, January 14.
Whitten, C.J. and Bull, D.L. (1974) Comparative toxicity, absorption
and metabolism of chlorpyrifos and its dimethyl homologue in
methyl-parathion resistant and susceptible tobacco budworms. Pest.
Biochem. & Physiol., 4:266-74.
Wirth, M., Würth, E. and Engeler, V. (1971) A residue study: DOWCO 179
and DOWCO 214 on tomatoes in a greenhouse. Unpublished report GHE-P
005 from Dow Chemical Company Limited, King's Lynn, England, December
16.
Wood, P.A. and MacRae, I.C. (1974) The effect of several
organophosphorus insecticides upon the acetylene-reduction activity of
Azotobacter vinelandii. Bull. Environ. Contamin. & Toxicol.,
12(1):26-31.
World Health Organization. (1966) WHO insecticide evaluation and
testing programme. Stage 1 mammalian toxicity report. OMS 1155.
