
LEPTOPHOS JMPR 1975
Explanation
Leptophos was evaluated with regard to data on residues in food
by the 1974 Joint Meeting (FAO/WHO, 1975). Arising from the
requirements of that Meeting for further information, data were
obtained on residues from supervised trials in Canada, Japan, India
and New Zealand on a large number of crops including fruit, small
grains, rice, maize, sugar beet, potatoes, sunflower seed, rape seed,
flax seed, tea, tobacco and some vegetable crops. Such information was
required by the 1974 Joint Meeting from countries other than the
United States of America.
Some information became available on residues in waste of
agricultural crops which may be used as animal feed e.g. sugar beet
leaves, straw and chaff of cereal crops, including rice and maize
fodder.
Additional data became available on the fate of leptophos
residues in apples, cabbage, tobacco and soil from studies with 14C
phenyl-and 14C-phenoxy labelled leptophos. Data on the fate of
leptophos residues during processing of cotton seed and sunflower seed
were also provided: some of the by-products of this processing may be
used in animal feed. New data were obtained from model studies with
14C leptophos in a simple food chain in which water, Daphnia magna
and the common bluegill, were included.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Absorption, distribution and excretion
Leptophos is rapidly absorbed and eliminated from the body
following acute oral administration (Kennedy et al., 1970). Within 96
hours following an oral dose of 0.8 mg/kg, rats were found to have
eliminated ring (probably phenyl) labelled leptophos in the urine
(80-88% of the dose) and faeces (11-12% of the dose). Small quantities
were noted in several tissues. Within 24 hours 75 to 81% of the
recovered radioactivity (ranging from 98-103% of the dose) was
observed in urine. In two further studies on the excretion of
leptophos following acute oral dosing, it was again observed that
there was rapid elimination via urine and faeces (Kennedy and
Keplinger, 1971, 1972). Using phenoxy-labelled leptophos results were
extremely variable. Urinary excretion in both males and females varied
from 8-85% of the recovered dose while in the faeces a range of 8-84%
of the excreted dose was noted within 96 hours. These studies using a
total of four rats of each sex showed extremes in biological variation
with some animals excreting more in urine than faeces and others
reversing the trend. In one instance a possible sex difference in
excretion was suggested. In all studies, rapid excretion was observed
with the major quantity eliminated within 24 hours. Tissue levels
contained little radioactivity after 96 hours. Skin and fat, where
analysed, showed levels of 1% of an acutely administered oral dose. In
adipose tissue, the major residue was leptophos with traces of the
phenol observed (Tarka, 1973).
In studies with rats administered leptophos as a single oral dose
ranging from 0.2 to 0.5 mg/kg or in five consecutive doses, recovery
of all radio-labelled material was rapid; within five days of the
final dose with a urine:faeces ratio of 80:20. Tissue concentrations
at five days following the last dose (in the multiple dosed animals)
were very low with the highest being liver (<0.25% of the dose
administered). No attempt was made to characterize the tissue
metabolites (Badie and Whitacre, 1975).
In mice, rapid elimination was again noted following oral
intubation. Differences in elimination were noted with different
14C-positional isomers. The phenyl-label elimination pattern was
notably slower than the phenoxy-labelled elimination. In all cases
rapid elimination was observed predominantly in the urine (90-96% of
the recovered dose) with minor mounts noted in faeces (Holmstead et
al., 1973).
Biotransformation
Characterization of radio-labelled components in rat and mouse
urine and tissues was performed in conjunction with several
14C-distribution studies (Kennedy et al., 1970a; Kennedy and
Keplinger, 1971a; Tarka, 1973; Holmstead et al., 1973; Badie and
Whitacre, 1975). In three studies, no intact leptophos was observed in
urine which was known to contain considerable radioactivity. In
contrast, leptophos was observed in faeces. Leptophos is apparently
metabolized and excreted in urine as several components, the structure
of several of which were suggested. In mice following oral
administration of 25-50 mg/kg, small (1-2%) quantities of leptophos
and the oxon were observed in faeces. Other components in urine
included: O-methyl phenyl phosphonate (a major component in rat),
O-methyl phenyl phosphonothioic acid (a major component in mice);
leptophos phenol, and phenyl phosphonic acid. The major rat metabolite
in urine differs from the major mouse metabolite in urine possibly
reflecting species differences in metabolism. Small quantities of
materials in urine (designated unknown) were found to chromatograph
with known standards of O-(4-bromo-2, 5-dichlorophenyl)
phenyl-phosphonic acid. Impurities found in leptophos, including the
desbromo derivative, the desbromo monochloro derivative and the
S-methyl isomeride were not reported in these studies.
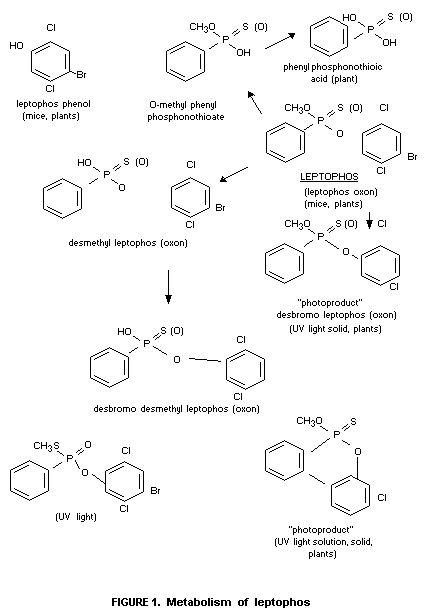
Studies in plants indicated that leptophos was slowly absorbed
following a foliar treatment with the major quantity found to remain
on the leaf surface (Holmstead et al., 1973). Studies with several
leaf types (bean - lettuce) showed that residues diminished rapidly on
both types of surfaces (Schroeder, 1971). The primary mechanism by
which leptophos was lost was presumed to be by volatilization.
Qualitatively, leptophos was metabolized to products similar to those
found with the mouse. Phenyl phosphonate derivatives were also
recovered from plant surfaces,
In vitro studies using artificial sources of sunlight to
degrade leptophos in acetone solution resulted in rapid degradation to
a desbromo derivative followed by dehalagenation to a
desbromo-deschloro leptophos which was reported to rearrange to a
stable aryl ring structure (Schwemmer, 1971). As a solid, leptophos
degrades slowly by photolysis in sunlight (March and Fukuto, 1975).
Several products which were isolated include: desbromo leptophos;
leptophos oxon; phenyl phosphonic acids, chlorinated phenol and traces
of the S-methyl isomeride (although this latter and possible other
products may have originated as impurities in the technical material
used). A flow diagram of the suggested metabolic scheme is seen in
Fig. 1.
Effects on enzymes and other biochemical parameters
Leptophos is a weak anticholinesterase agent as demonstrated by
several short-term studies although it is metabolized to the oxon
which is a relatively potent inhibitor. Leptophos oxon has also been
demonstrated to be an inhibitor of the mipafox-sensitive esterase
found in hen brain that is sensitive to inhibition by compounds known
to induce delayed neurotoxicity (Johnson, 1975). The bimolecular rate
constant was found to be 106 and 1 × 105 Mole-1 for brain and RBC
cholinesterase respectively. Brain cholinesterase was inhibited by
leptophos and leptophos oxon. Following 30 minute preincubation, I50
values for both compounds were 2 × 10-4M. and 2 × 10-7M. respectively
calculated. I50 values for the desphenoxy derivative and the phenol
were <10-3M. (Hassan, 1975).
Bioaccumulation
The biological fate of leptophos was studied using a model
terrestrial-aquatic ecosystem (Sanborn and Metcalf, 1975). In this
model environment, leptophos was observed to bioaccumulate in higher
animal species. In comparison with several other organophosphates,
leptophos was the most stable and showed a tendency towards
persistence and bioaccumulation.
 In further studies of the biomagnification of leptophos, it was
observed that an apparent equilibrium was established in fish exposed
to leptophos for periods of time. After about one week of exposure
during which time leptophos accumulates there is no additional
build-up in tissues. These data also suggest that ingestion of
leptophos - contaminated invertebrates by fish does not increase the
fish residue suggesting that biomagnification is not a significant
factor (Sleight and Macek, 1973; Johnson, 1973).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Leptophos oxon
Rat
Groups of rats (25 males and 25 females/group) were subdivided
into two categories: individually housed (10 rats of each sex per
group) and group housed (15 rats of each sex per group). These groups
were fed a control diet and one containing leptophos oxon for 28 days.
The leptophos oxon diet was increased at weekly intervals from 200 ppm
to 300 ppm to 500 ppm and finally to 800 ppm. Growth (as evidenced by
body weight) was reduced in the latter stages of the test in females
while food consumption was normal. Spleen weight in males and females
was reduced at 7, 21 and 28 days. Cholinesterase activity of RBC and
brain was depressed at all test intervals. Plasma cholinesterase was
depressed in females at all intervals while in males the depression
became evident after 14 days. No effects were noted on survival,
haematology, blood chemistry, urinalyses or on microscopic examination
of tissues and organs (Plank et al., 1971).
Groups of female rats (15 rats/group) were fed leptophos oxon for
four weeks at dose levels of 0, 1, 5, 10 and 20 ppm. Measurements of
red blood cell and plasma cholinesterase activity were made at 14 and
28 days. Plasma cholinesterase was unaffected while erythrocyte
cholinesterase was depressed at 10 ppm and above at 14 days. At the 28
day interval reduction of activity was not significant (Smith et al.,
1971).
Groups of rats (15 males and 15 females/group) were fed leptophos
oxon for 90 days at dose levels of 0, 25, 50 and 500 ppm. Inhibition
of plasma cholinesterase activity was noted at all dose levels in
females while males were affected at 500 ppm only at the 84 day test
interval. Inhibition of erythrocyte and brain cholinesterase activity
was similar in both sexes and was notable only at 500 ppm. No effects
were noted on mortality, growth, food consumption, haematology
parameters, clinical chemistry parameters, urinalyses, and on gross
and microscopic examination of organs and tissues (Plank et al.
1971c).
Leptophos phenol
Rat
Groups of rats (25 of each sex/group) were divided into two
subgroups and housed individually (10 of each sex/group) or housed
together (15 of each sex/group). The sexes were not mixed. Two dietary
groups were used; a control and a test group which received a dietary
level of leptophos phenol of 3500 ppm for one week. This was increased
to 5000 ppm for the second week, to 8000 ppm for the third week and
finally to 13 000 ppm for the fourth week. There were no effects on
food consumption, growth, haematology, blood chemistry including
cholinesterase or on growth and microscopic examination of tissues and
organs (Plank et al,, 1971a).
Groups of rats (15 males and 15 females/group) were fed leptophos
phenol at dietary levels of 0, 300, 1000 and 10 000 ppm (3000 ppm for
days 1-49) for 90 days. At 10 000 ppm there was a significant increase
in liver weight in females. In males a decreased spleen weight was
noted at all doses tested (none was significantly different from
control values although the decrease was evident in ratios calculated
from body and brain weight data). No effects were observed on
survival, food consumption, growth, haematology, blood chemistry,
urinalyses or on gross or microscopic examination of tissues or organs
(other than the gross effects on liver and spleen). A report of the
microscopic examination of liver and spleen indicated no abnormalities
(Plank et al., 1971b).
Special studies on mutagenicity
Mouse
A dominant lethal study was conducted where groups of male mice
(8 mice/group) were administered a single oral or intraperitoneal dose
of 0, 15 or 30 mg leptophos/kg. The males were mated with three
females per week for six weeks during the normal period of
spermatogenesis. Positive control studies were performed with methyl
methane-sulfonate. There were no effects of leptophos on mating
performance or on reproduction parameters including preimplantation
loss, early resorption or embryo viability. Leptophos under the
conditions of this test did not induce mutagenic changes in male
germinal cells (Arnold et al., 1971).
Special studies on neurotoxicity
Chickens
Groups of white leghorn hens (6 hens/group) were orally
administered leptophos at doses of 100, 200 and 400 mg/kg on day 0 and
14. Observations on clinical conditions were made and at the
conclusion of the study (day 28) survivors were sacrificed and nervous
tissue examined microscopically for indications of myelin disruption.
No clinical signs of delayed neurotoxicity were noted in this
study following the first dosing. Three hens of six tested at the high
level died 6-14 days after the second dose. These hens had lost a
considerable portion of their body weight before death. At 200 mg/kg
one of six died and one other showed clinical signs of ataxia.
Microscopic examination of H & E and Luxol Fast Blue stained sections
of nerve did not reveal myelin degeneration (Stephens et al., 1969).
Groups of white leghorn hens (10 hens/group) were administered
leptophos orally at doses of 0, 5, 10, 15, 30, 50, 75, 100 and 200
mg/kg at days 0 and 21. A positive control was also used in this study
(Tri-o-Cresyl phosphate, 500 mg/kg - orally at day 0). A number of
animals showing clinical signs of ataxia were sacrificed at day 42.
Gross and microscopic examination of brain, spinal cord and sciatic
nerves were performed. Examinations were made of H & E stained
sections and tissues embedded in ParaplastR and stained with Luxol
Fast Blue - Holmes Silver Nitrate. Both light and electron microscopic
examinations were made in this study.
A positive delayed neurological condition was clinically evident
in this study with both TOCP and leptophos. Loss of body weight is
generally observed clinically in animals with delayed peripheral
ataxia and this was observed with the TOCP group in 6 of 10 hens
weighed at day 18. In the leptophos groups, weight loss was noted in 1
of 10 hens at 75 mg/kg, in 3 of 10 at 100 mg/kg, in 5 of 10 hens at
200 mg/kg. This weight loss was accompanied by signs of peripheral
neuritis in various hens of the three upper group levels (3 hens at 75
mg/kg, 5 hens at 100 mg/kg and the majority of hens at 200 mg/kg).
Light and/or electron microscopic sections of nerve tissue and muscle
preparations indicated retrograde degeneration of 8 of 10 TOCP hens
and 5 of 10 hens at 200 mg/kg. No other positive signs of degeneration
were observed. At acute oral dose levels of 75 mg/kg and above,
leptophos was shown to induce a clinical neuropathy in hens (Fletcher
et al., 1975).
Other studies have confirmed that, at high acute oral doses,
leptophos will induce delayed neurotoxicity and clinical signs of
neuropathy (Abou-Donia et al., 1974; Abou-Donia and Preissig, 1975;
Kimmerle, 1972). In male chickens, 2 of 9 tested were ataxic between
9-13 days after oral dosing with 180 mg/kg. No neurotoxic effects were
noted at dose levels below 180 mg/kg. Further studies showed that 200
mg/kg and above resulted in ataxia. A 30% formulation of emulsifiable
concentrate administered to hens by oral or IV injection resulted in
positive clinical neuropathy at dose levels above 100 mg/kg.
Special studies on reproduction
Rat
In two studies, groups of rats (8 males and 16 females/group
there - were two control groups of 8 males and 16 females each) were
fed leptophos in the diet at levels of 0, 10, 30, 40 and 60 ppm and
subjected to a standard three generation, two litter per generation
reproduction study. After two litters of the first generation were
born, the 60 ppm level was discontinued because of pup mortality. A
second study was initiated at 0 and 5 ppm. The 5 ppm dose was changed
after one generation to the 40 ppm level (noted above) and maintained
at this level for two further generations.
Parental body weight and reproductive performance was similar in
the 60 ppm group to the control group. Reproductive indices, including
mating, pregnancy, fertility and parturition showed no differences
with respect to control and all treated rats. Survival data on pups
including viability and lactation indices were significantly decreased
at 60 ppm but were similar to control values at 40, 30 and 10 ppm.
Mean body weight data for pups at weaning at all dose levels
(including 60 ppm) were similar. A no-effect level for rat
reproduction is 30 ppm (Haley et al., 1973, 1972).
Special studies on teratogenecity
Rabbit
Groups of pregnant New Zealand rabbits (10-13 rabbits per group)
were administered leptophos orally from day 6 to day 18 of gestation
at dose levels of 0, 1 and 3 mg/kg. Thalidomide was administered over
this same period at a dose of 37.5 mg/kg to a group of rabbits
designated as a positive control. No effects were noted on the growth
of does. Skeletal or somatic abnormalities were noted with
thalidomide. Leptophos at a dose of 3 mg/kg administered orally to
rabbits during organogenesis elicited no teratological response (Ladd
et al., 1971).
Signs of poisoning are typical of acute parasympathomimetic
stimulation seen by other anticholinesterase organophosphates. These
include: hypoactivity, tremors, muscular weakness, ruffed fur,
diarrhoea, exophthalmia, haemorrhagic conjunctivitis and salivation.
Acute Toxicity
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M Tech (white mass) Oral 31.6 Wazeter, 1965
M Tech (white powder) Oral 59.0 " 1966
M Tech (98.5% white powder) Oral 54.5 " 1968
M Tech (90% brown granule) Oral 37.1 " 1968a
M Tech (99.6% white powder) Oral 56.6 " 1971
M Tech (90% white crystal) Oral 44.7 " 1970
M Tech (90% grey granule) Oral 52.8 " 1971a
M & F Tech (94%) Oral 20.0 Kretchmar et al., 1971
M & F Tech (90%) Oral 90.5 Mastri et al., 1969
M & F (1 day old) Tech 90% Oral 12.0 Kretchmar et al., 1971a
M & F (5 day old) Tech 90% Oral 15.0 Kretchmar et al., 1971a
F Tech (98.5% white powder) Oral 24.3 Wazeter, 1968
F Tech (90% brown granule) Oral 26.1 " 1968
F Tech (99.6% white powder) Oral 40.1 " 1971
F Tech (90% grey granule) Oral 42.9 " 1971a
Rabbit M & F Tech (90% white crystal) Dermal 10 000 " 1970
M & F Tech (90% brown powder) Dermal 800 " 1968a
" 1968b
Chicken F Tech Oral 225 Fletcher et al., 1975
Technical leptophos is an eye irritant when instilled into the conjuctival sac of rabbits (Wazeter, 1968b, 1970).
Leptophos is not an irritant when tested on normal or abraded rabbit skin (Wazeter, 1970).
Acute toxicity formulations
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M 3 EC (yellow oil) Oral 271 Wazeter, 1970a
M 30 EC (yellow oil) Oral 271 " 1970a
M 29.5 EC Oral 400 Mastri et al., 1969a
M 35.7 EC Oral 735 " 1969a
M 5 G Oral 2 263 Wazeter, 1971b
M 50 WP (grey powder) Oral 121 " 1971c
M 2 EC (yellow liquid) Oral 178 " 1971d
M 3 EC (34.7%) Oral 121 " 1971e
M 3% Dust (grey dust) Oral 1 780 " 1971i
M 3 ULV (32.1%) Oral 192 " 1970b
M 5 G Inhal 26.9 mg/L Wazeter, 1971b
M 3 ULV (32.1%) Inhal 200 mg/L " 1970b
M & F 3 EC 4 hr
Inhal 2.7 mg/L Hathaway et al., 1968
M 3% dust 4 hr
Inhal 33 mg/L Wazeter, 1971i
M 50 WP Inhal 19 mg/L " 1971c
M 2 EC Inhal 200 mg/L " 1971d
M 3 EC Inhal 200 mg/L " 197le
F 29.5 EC Oral 218 mg/L Mastri et al., 1969a
F 36.7 EC Oral 327 mg/L " 1969a
Rabbit M & F 2.7 EC Dermal 2 000 Wazeter and Goldenthal, 1973
Leptophos formulations are an eye irritant when instilled into the conjunctival sac (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i). However, one
formulation tested (3 ULV - 32.1% leptophos) was not an eye irritant (Wazeter, 1970b). Leptophos fomulations are not a skin irritant when tested
on normal or abraded rabbit skin (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i).
Acute toxicity - metabolites and possible contaminants
LD50
Compound Species Sex Route (mg/kg) Reference
Leptophos oxon Rat M & F Oral 119 Mastri et al., 1969b
Rat M & F Oral 43 Kretchmar et al., 1972
Phenylphosphonic
acid Rat M Oral 2 480 Mastri et al., 1969b
2 241 " 1969c
S-methyl isomeride Rat F Oral 735 Kretchmar et al., 1972a
O.O-bis
(2,5-di-chloro-4-brom-phenyl)
phenylphosphono-thioate Rat M Oral 4 640 Wazeter, 1971f
O.O-dimethyl
phenyl-phos-phonothioate Rat M Oral 287 " 1971g
O-methyl phenyl
phosphonic acid Rat M Oral 3 690 " 1971h
O-(2,3-dichloro-4-bromophenyl) Rat M Oral 59.5 Wazeter and Goldenthal, 1972
phenylphos-phonothioate Rat F Oral 37.5 Wazeter and Goldenthal, 1972
Leptophos phenol Rat M & F Oral 2 654 Mastri et al., 1969b
Antidotal studies
Administration of atropine sulfate and 2-PAM alone and in
combination resulted in an increase in the LD50 value suggesting that
these agents might be successful in antidotal therapy (Kretchmar et
al., 1971). The combination of atropine and 2-PAM was no more
successful than the administration of either agent alone.
Antidote LD50 (mg/kg) (95% C.L.)
None 20.0 (11.8-34.0)
Atropine Sulfate (IM)
10 mg/kg 1.5 & 5 hr
after poisoning 54.0 (41.2-70.7)
2-PAM
1.5 hr after poisoning 38.5 (33.8-43.9)
Atropine + 2-PAM 40.0 (27.6-58.0)
Short-term studies
Rat
Groups of rats (2 males and 2 females/group) were administered
leptophos by oral intubation of 10 days at dose levels equivalent to
dietary intakes of 0, 3, 10, 30 and 100 ppm. Mortality was evident at
100 ppm. At the conclusion of the study the animals were sacrificed
and cholinesterase determinations run using brain, plasma and red
blood cells.
No depression of plasma cholinesterase was noted. The RBC and
brain cholinesterase activity was depressed at 30 ppm and above in all
surviving animals (Wazeter, 1968c).
Groups of rats (10 males and 10 females/group) were fed dietary
levels of leptophos of 0, 100, 250 and 500 ppm for 28 days. A second
series of female animals (10 rats/group) were fed levels of 0, 50 and
75 ppm for 14 days in a cholinesterase depression range-finding test.
A dose-dependent depression of cholinesterase activity was
observed in all tissues at all dose intervals and times tested in the
initial high level study. Activity of the most susceptible
cholinesterase source (female RBC) was slightly depressed 32% and 22%
at 75 and 500 ppm respectively after seven days of feeding. The plasma
cholinesterase activity in the initial study was not inhibited until
the two week test interval after which it steadily declined (Plank, et
al., 1970).
Groups of rats (10 male and 10 female rats/group were fed
leptophos in the diet at dose levels of 0, 1, 5 and 10 ppm for 90
days. Growth, food consumption and behaviour were not affected by
leptophos. No changes were noted in haematology, blood, clinical
chemistry or urinalysis parameters measured. Cholinesterase activity
in RBC, plasma and brain were normal at 90 days of testing as were the
40 day examinations of RBC and plasma cholinesterase. Gross and
microscopic examination of tissues and organs showed no
leptophos-related lesions (Wazeter, 1969).
Dog
Groups of dogs (four male and four female beagle dogs/group) were
fed leptophos in the diet for 90 days at dose levels of 0, 10 and 30
ppm. Behaviour, growth and food consumption were not affected over
this test interval. No effects were noted on haematology parameters on
blood, clinical chemistry on urinalyses or on gross and microscopic
analyses of tissues and organs. A no-effect level was 30 ppm (Lindberg
et al., 1969).
Groups of beagle dogs (4 males and 4 female/group) were fed
leptophos in the diet at dosage levels of 0, 10, 20, 30 and 60 ppm for
two years (the 60 ppm group had been fed a diet containing 5 ppm for
180 days which was then increased to 60 ppm for the remainder of the
study. No mortality occurred over the course of the study and growth,
food consumption and behaviour was normal at all levels. No
leptophos-related effects were noted on haematology parameters,
clinical chemistry values (including blood and brain cholinesterase
values) or on gross and microscopic examination of tissues and organs.
Leptophos in the diet at 60 ppm had no effect on any parameter
recorded in this study (Hartke et al., 1971).
Chicken
Groups of white leghorn chickens (4 male and 20 females/group)
were fed leptophos in the diet at dosage levels of 0, 0.03, 0,10, and
0.30 ppm for four weeks. Eggs were collected and incubated during the
latter 14 days of treatment. The chicks were observed for 14 days
post-hatching having been fed the leptophos diet corresponding to the
parent. There were no treatment related differences in body weight or
egg production and size of eggs. Egg hatchability, chick viability,
and growth was not affected by leptophos. No abnormal behaviour or
signs of poisoning were observed in this study in either adults or
chicks (Perkins and Singh, 1971). Analysis of tissue residues
(including eggs and adipose tissue) did not indicate the presence of
residues of leptophos, the oxon, the phenol or a photo-product
(Suzuki, 1971).
Steer
Groups of steers (three steers/each treatment group and one
steer/control group) were fed leptophos in the diet at levels of 0,
15, 45 and 150 ppm for four weeks. At 28 days the control and two each
of the treatment groups were sacrificed. The remainder were allowed to
consume normal untreated food for a further 14 days after which they
were sacrificed. Daily observations showed no abnormal behaviour or
toxic signs of poisoning. No gross or microscopic lesions were
observed at the conclusion of the study. Depression of the whole blood
and plasma cholinesterase was evident at the higher dose levels over
the 28 day feeding interval. After seven days on a control ration the
depressed values returned to normal. Tissue residues of leptophos and
the oxon were low in liver, kidney, muscle and brain. In fat,
leptophos was present after 28 days in measurable levels which were
dose dependent. Following the 14 day recovery intervals, these
residues in fat were considerably reduced (Perkins and Singh, 1971a).
Cow
Groups of lactating dairy cows (three cows/group fed leptophos
and one cow/control group) were fed leptophos in the diet at
calculated dose levels of 0, 5, 15 and 50 ppm (actual values fed as
assessed by chemical analysis were 0, 3.2, 10.0 and 37.4 ppm) for 28
days and maintained for a further 14 days on control diets to measure
residue reductions and recovery of normal values. Appearance,
behaviour and general condition were normal over the study. Body
weights of several animals tested with leptophos were reduced over the
study. Milk production was reduced in the low and intermediate group
but not at the high level of leptophos. Cholinesterase depression was
not noted in this study (Fink, 1971). Traces of leptophos were noted
in fat at the conclusion of the 28 day feeding interval at levels
ranging from 0.2 to 0.5 ppm in the high (37.4 ppm) dose level. After
two weeks, these residues were reduced to 0.2 ppm (Suzuki, 1971a).
Long-term studies
Mouse
Groups of Swiss white mice (65 males and 65 females/group) were
fed leptophos in the diet for 18 months at dose levels of 0, 50, 100
ppm to assess a carcinogenic potential in mice. Two positive control
groups were fed N-nitrosodiethylamine at levels of 10 ppm (50 males
and 50 females) and 40 ppm (15 males and 15 females). After six
months, the mice of the high positive control group and 15 of each sex
in the leptophos group were sacrificed and subjected to gross and
microscopic examination for tumour formation. At 18 months all
remaining animals were sacrificed and 10 animals of each sex (or
group) were examined.
The positive control sacrificed at six months (40 ppm) showed
definitive evidence of lung adenoma or carcinoma. At 18 months, the
positive control (10 ppm) again showed a positive response to the
carcinogen. No evidence of lung lesions was noted at 100 ppm leptophos
in the diet. There were no leptophos-related lesions or tumours in any
of the tissues and organs examined in this study (Smith et al., 1973).
Rat
Groups of Charles River albino rats (50 males and 50
females/group) were fed leptophos in the diet for two years at
concentrations of 0, 10, 20, 30 and 60 ppm (the 60 ppm were initially
fed 5 ppm for the first seven months). Mortality was unaffected by
leptophos in the diet (although at the conclusion of the study very
few animals in all groups were alive). Food consumption and growth
were similarly not affected. There were no differences from control
values in the haematology, blood chemistry or urine parameters
examined. Gross and microscopic examination of tissues and organs
failed to indicate any pathologic disorders noted in controls.
Cholinesterase activity measured in brain over the first 90 days
showed no reduction in activity at any dose tested. Erythrocyte
cholinesterase activity was depressed at all levels tested. A
significant reduction (below 25%) was noted at the 60 ppm level. A
no-effect level based on RBC cholinesterase depression is 30 ppm
(Smith et al., 1971a).
Comments
Leptophos, an anticholinesterase organophosphonothioate ester
recommended for use in agriculture as an insecticide, has a moderate
acute oral toxicity. Leptophos is rapidly absorbed, metabolized and
excreted within 24 hours following single acute oral dosing in
animals, primarily via the urine. No tissue residues were observed
except for adipose tissue which maintains traces of leptophos for
extended periods following acute dosing. The metabolism of leptophos
includes oxidative and hydrolytic processes, generally resulting in
less toxic products (except the oxon and the dichlorodesbromo
derivatives). All materials noted as field residues have been found as
metabolites in animals.
Leptophos (and its metabolites) is a relatively persistent
pesticide. Artificial environmental studies using model systems
suggest that leptophos may bioaccumulate in the food chain.
Sufficient toxicological data have been reported including long
and short-term studies with rodents and dogs, reproduction studies
(including teratogenesis and mutagenesis studies); and carcinogenesis
studies. The only significant adverse toxicological parameters
reported in these studies have been esterase depression, pup mortality
in the reproduction study at high levels and delayed neurotoxicity in
hens. Short-term studies on the metabolites have shown no substantial
toxicological effect on rodents except for esterase depression with
the oxon metabolite.
Based on the no-effect level observed in two year studies in rats
and dogs and the no-effect level in acute avian studies for delayed
neurotoxicity, a temporary ADI was estimated. An exceptionally high
safety factor was used to allocate the ADI reflecting concern over the
positive neurotoxicity results. There was less concern over the
significant delayed neurotoxicity as a factor in evaluating the
toxicological hazard of food residues than in its evaluation of
delayed neurotoxicity as a hazard to occupationally exposed
individuals. The occurrence of the delayed neurotoxicity was, however,
considered to be highly significant in the toxicological evaluation of
leptophos. It was decided that delayed neurotoxicity should be
considered as an additional toxicological parameter, showing a
dose-response relationship, which permits an evaluation to be made of
a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 30 ppm in the diet equivalent to 1.5 g/kg bw
Dog: 60 ppm in the diet equivalent to 2.1 mg/kg bw
Hen: 50 mg/kg (based on delayed neurotoxicity)
Estimate of temporary acceptable daily intake
0-0.001 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in crops
Extensive data were obtained on residues from supervised trials
in which crops were treated according to recommended dosage rates and
pre-harvest intervals. In most trials separate residue figures were
given for the parent leptophos and the metabolites leptophos-oxon,
desbromo-leptophos and the phenol. Leptophos residues consisted mainly
of the parent compound with much lower levels of the metabolites
mentioned, as in the trials recorded in the 1974 monograph of
leptophos. In the following tables therefore, a single figure is shown
referring to the sum of leptophos, its oxygen analogue and
desbromo-leptophos expressed as leptophos.
In a number of trials "apparent" oxon residues were found. As
explained in the 1974 monograph, these consist of bis-(2-ethylhexyl)
phthalate, a component of plastic laboratory equipment. In the
following Tables these apparent oxon residues are omitted.
Comments on the data
Apples. Residue data from Canada (three locations in Quebec and
one in Ontario) show considerable residue levels, up to 2 mg/kg, after
a pre-harvest interval of 6-10 weeks. Further data on apples are given
in the section "Fate of residues".
Vegetables
Beans. After pre-harvest intervals of 37-40 days residues in
beans were either not detectable (Canada) or very low, at or below
0.02 mg/kg (Japan).
Brassicas. No detectable residues were found in broccoli,
cabbage or cauliflower after pre-harvest intervals of about 60 days,
but still considerable residues after intervals of 25-30 days. These
data confirm those available from the United States of America at the
1974 Joint Meeting.
In supervised trials in Japan residue levels in the edible part
of Chinese cabbage after a pre-harvest interval of 14-31 days were in
the range 0.1-0.2 mg/kg.
Celery. Residue levels were very low, 0.01-0.02 mg/kg in three
locations in Ontario after a pre-harvest interval of 61 days. In other
trials residues ranged from 0.02-0.05 mg/kg after a similar interval
and levels up to 0.5 mg/kg were found after an interval of 42 days.
Lettuce. Residue levels in Canadian trials after a pre-harvest
interval of 21-30 days were slightly lower than in the experiments
evaluated at the 1974 Joint Meeting from the United States of America.
After normal trimming for market, residues did not exceed 0.5 mg/kg.
Onions, peas, potatoes. Residues were low after the normal
pre-harvest intervals for these crops. In green onions residues were
at or below 0.1 mg/kg after a pre-harvest interval of about 60 days
and in peas <0.01 mg/kg after 30 days. In the vines however, residues
ranged from 1.1-10.7 mg/kg 22-30 days after treatment. In potatoes in
New Zealand no detectable residues were found after 21 days. This is
in conformity with the United States of America data evaluated by the
1974 Joint Meeting.
Cereal crops
Wheat, oats and barley. Residue levels were below 0.1 mg/kg
after a pre-harvest interval of 60 days in most of the trials; in some
trials levels reached about 0.5 mg/kg in these crops.
Maize. Residues in kernels and forage were generally low (0.05
mg/kg or below) in Canadian experiments, as in the United States of
America data evaluated by the 1974 Meeting.
Rice. After pre-harvest intervals of about 30 days residue
levels in inhulled rice ranged from 0.1-0.5 mg/kg and in leaves and
straw from 6 to 20 mg/kg.
Sugar beet. Data from residue trials in Canada and Japan were
evaluated. After a pre-harvest interval of about 50 days, residues in
the roots did not exceed 0.05 mg/kg and those in the tops ranged from
<0.02-0.5 mg/kg.
FATE OF RESIDUES
In plants
Data were obtained from three new studies on the fate of
leptophos residues in apples, cabbage and tobacco carried out with
14C-phenoxy- and 14C-phenyl-leptophos. The results generally agreed
with those of the trials evaluated by the 1974 Joint Meeting.
In the experiment on apples it was shown that the parent
leptophos was the principal residue 6 and 14 days after treatment,
with approximately 10% of the extractable radioactivity as
leptophos-oxon. Less than 1% of 14C was unextractable from the apple
pulp. Fourteen days after treatment, about half of the total
14C-phenyl residue remained on the surface of the apples and up to
20% of this surface residue remained at the origin during TLC on
silica gel.
Only small mounts of 4-bromo-2,5-dichlorophenol, phenylphosphonic
acid, O-methyl phenylphosphonothioate and methyl phenylphosphonate
were extracted from the apple tissue (Whiteacre and Schnur, 1974).
TABLE 1. Nature of 14C compounds extracted from the surface of
apples treated with 14C-phenoxy and C-phenyl-leptophos
% of total surface radio carbon
14C-phenyl 14C-phenoxy
Metabolites Days after treatment Days after treatment
0 6 14 0 6 14
leptophos 98.6 80.0 77.8 98.4 89.6 92.3
leptophos oxon 1.4 1.7 2.0 1.4 1.0 1.5
4-bromo-2,5-dichlorophenol - - - <0.01 <0.01 <0.01
polar compounds
at origin of <0.01 18.2 20.2 1.6 9.0 6.2
TLC plate
Total 100.0 99.9 100.0 101.4 99.6 100.0
In the experiment on cabbage (Diaz, 1974) it was shown that on
this crop also, the parent leptophos is the principal residue 4, 7 and
14 days after treatment; only small amounts of leptophos oxon and free
4-bromo-2,5-dichlorophenol were present. A small amount of 4-bromo-2,
5-dichlorophenol was conjugated; no significant amounts of O-methyl
phenylphosphonothioate, methyl phenylphosphonate or phenylphosphonic
acid were found.
In soil
A new study was carried out on the behaviour and fate of
14C-phenoxy-and 14C-phenyl-leptophos in soil (Rieck, 1974), giving
additional information on the mount of "bound" residue (not
extractable with normal extraction procedures). Columns of a sandy
loam soil consisting of a one inch depth of treated soil on top of a
four inch depth of untreated soil were kept under aerobic and
anaerobic conditions and watered to 80% of field capacity. Two columns
were analysed initially and the others were watered with half inch
depth of water at weekly intervals and the leacheate collected. Soil
columns were analysed after 15, 30, 60, 90 and 120 days.
It was found (Table 2) that the bound radiocarbon (defined as
unextractable by 24 hours soxhlet extraction with 80% methanol)
increased with time at all soil depths. The radioactivity in the top
2.5 cm (treated) section decreased with time from 92% of the total to
42% after 120 days. The total 14C in the levels below this remained
fairly constant during the experiment.
TABLE 2. Distribution of total and bound radioactivity in aerobic
sandy loam soil
14C-leptophos equivalent on dry weight basis,*
found at depth
0 - 2-1/2 cm 2-1/2 - 7-1/2 cm 7-1/2 - 12-1/2 cm
Time
(days) total bound total bound total bound
0 9.16 0.02 0.23 0 0.05 0.0
15 8.33 0.39 0.06 0 0.14 0.02
30 6.33 0.69 0.13 0.06 0.04 0.03
60 5.24 0.98 0.19 0.09 0.09 0.05
90 4.75 1.47 0.20 0.14 0.07 0.07
120 4.19 1.84 0.19 0.14 0.04 0.04
* to calculate in terms of percentage of the amount applied, move
the decimal point one place to the right.
Under the conditions of the experiment the downward movement of
leptophos and its metabolites was insignificant. The only
14C-labelled compounds extractable from the lower layers were parent
leptophos, 4-bromo-2,5-dichlorophenol and methyl phenylphosphonate.
The total leacheate collected during the study contained only 0.46% of
the total activity applied to the upper soil layer.
In storage and processing
Sunflower and cotton seed
The nature and magnitude of residues in processed fractions of
sunflower seed were evaluated in three studies in Canada, In one study
the seed processed had a parent leptophos residue of 0.06 mg/kg. The
meal, crude oil and refined oil fractions showed residues of 0.03,
0.18 and 0.20 mg/kg parent compound respectively. No residues were
determined in the hulls, soapstock or finished oil. In another study
in which the crop was treated with 0.3 kg/ha the residue in the seed
was 0.19 mg/kg. No residues were measured in the hulls, meal, crude
oil, refined oil, finished oil or soapstock.
In a similar study in Australia on the processing of cotton seed,
in which the crop was treated with 0.9 kg/ha, after 78 days the level
in the seed was 6.33 mg/kg total leptophos residue. The following
residues were found in the processed fractions.
mg/kg mg/kg
seed 6.3 refined oil 32.4
hulls 2.62 finished oil 0.07
meal 0.13 soapstock 3.70
crude oil 40.0
Tea
Tea was brewed for 3-10 minutes according to a standard
procedure. In one study in which tea was treated with 1.3 kg
leptophos/ha and harvested and brewed (three minutes brew) on the same
day, the brew contained 0.05 mg/kg leptophos and 0.07 mg/kg of
4-bromo-2,5-dichlorophenol. In a brew of tea harvested 3-7 and 14 days
after treatment no leptophos or metabolites except up to 0.02 mg/kg of
the phenol were found. Sun-dried tea harvested at 0 and 7 days after
treatment and brewed for three minutes showed no residues of leptophos
or its metabolites, except 0.06 mg/kg of the phenol in the brew at day
0 and 0.01 mg/kg of the phenol in the brew after seven days.
TABLE 3. Leptophos: Residues, include the parent compound and the main metabolites, leptophos oxon and desbromo-leptophos.
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Fruit crops
Apple New Zealand 1972 11 46 g/100 l wp 45% 2.65 1.95 2.02 2.08 2.28 V143
1972 11 60 g/100 l wp 45% 3.71 4.16 1.89 1.66 1.83 V143
New Zealand 1972 11 46 g/100 l wp 45% 2.82 1.45 1.45 1.43 1.78 V144
1972 11 60 g/100 l wp 45% 2.69 1.56 2.23 1.35 1.69 V144
Australia 1971/ 8 60 g/100 l wp 45% 1.55 1.28 2.27 1.50 V144
(Tasmania) 1972
8 60 g/100 l wp 45% 2.52 4.28 2.90 3.80 V144
Australia 1972 8 60 g/100 l wp 45% 4.06-7.29 5.50-9.03 4.33-6.85 3.56-4.18 V144
(Tasmania)
Australia 1972 5 60 g/100 l wp 45% 0.51-2.54 1.77-1.82 V144
(NSW)
Australia 1970 5 90 g/100 l wp 45% 0.36-0.66 V73
(NSW) 1970 5 120 g/100 l wp 45% 0.65-0.93 V73
Australia 1971 4 120 g/100 l wp 45% 0.30-0.48 V173
(Tasmania)
6 120 g/100 l wp 45% 0.65-0.83 V173
Canada 1973 4 110 g/100 l wp 45% 1.51 V178
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Apple Canada 1973 3 72 g/100 l wp 45% 0.76 V178
(cont'd)
Canada 1973 4 2 kg/ha wp 40% 3.83 V178
rate
kg a.i./ha 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Vegetables
Beans Canada 1974 1 1 EC 32% <0.01 Braun
et al.,
1975
1 2 EC 32% <0.01 "
Broccoli Canada 1974 1 1 wp 45% 0.08 "
1 2 wp 45% 0.13 "
Cabbage Canada 1974 1 1 wp 45% <0.01 "
1 2 wp 45% <0.01 "
Canada 1974 8 0.6 EC 0.71 V 176
0.06
EC 0.23 0.15 V 190
Carrots Canada 1974 1 0.5 EC 32% 0.07 0.02 0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Carrots 1 1 EC 32% 0.10 0.03 0.02
(cont'd) 1 2 EC 32% 0.12 0.03 Braun
et al.,
1975
Canada 1974 2 0.5 EC 32% 0.16 0.06 0.13 Braun
et al.,
2 1 EC 32% 0.32 0.06 0.05 "
2 2 EC 32% 0.34 0.11 0.14 "
Canada 1974 1 1 EC 0.07 V 210
1 1 EC 0.05 V 210
1 2 EC 0.33 V 210
1 2 EC 0.07 V 210
Cauliflower Canada 1974 1 1 wp <0.01 Braun
et al.,
1975
1 2 wp <0.01 "
0/14 21/23 28/30 35/40 42/46 49/56 63/
Vegetables
Celery Canada 1974 1 0.5 wp 0.10 <0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Celery 1 1 wp 0.20 0.02 "
(cont'd)
1 2 wp 0.40 0.04 "
(Stalks) Canada 1974 1 1 EC 0.01 V 208
1 2 EC 0.02 V 208
1 1 EC <0.03 V 208
1 2 EC <0.03 V 208
Lettuce Canada 1974 1 0.5 EC 0.20 0.01 Braun
(outdoors) et al.,
1 1 EC 0.37 0.12 "
1 2 EC 0.25 0.04 "
Onions Canada 1974 1 0.5 EC 0.04 <0.01 "
(green)
1 1 EC 0.08 0.02 "
1 2 EC 0.27 0.07 "
2 0.5 EC 0.08 0.02 "
2 1 EC 0.27 0.03 "
2 2 EC 0.42 0.09 "
Peas
(Pods) Canada 1974 1 1 EC 0.01 "
2 EC 0.01 "
Canada 1974 2 1 EC 0.02 Braun
et al.,
2 EC 0.03 1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Peas
(cont'd)
(Vines) Canada 1974 1 1 EC 2.06 Braun
et al.,
1975
2 EC 1.10 "
2 1 EC 10.65 "
2 EC 7.48 "
Rutabaga Canada 1974 1 0.5 EC 0.01 <0.01 "
1 1 EC 0.01 0.01 "
1 2 EC 0.02 <0.01 "
2 0.5 EC <0.01 0.01 "
2 1 EC 0.04 0.02 "
2 2 EC 0.06 0.02 "
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage
Chinese Japan 1971 1 0.35 EC 34% 0.09-0.10 Miyagi,
1971
3 0.35 EC 34% 0.09-0.10
1 0.35 EC 34% 0.08-0.10
3 0.35 EC 34% 0.10-0.12
1 0.50 EC 34% 0.06-0.07
3 0.50 EC 34% 0.06-0.08
1 0.50 EC 34% 0.05-0.06
0.12-0.14
Japan 1970 1 0.35 EC 34% 0.08-0.09 Miyagi,
1971
3 0.35 EC 34% 0.09-0.09
1 0.35 EC 34% 0.09
3 0.35 EC 34% 0.08-0.10
1 0.5 EC 34%
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage 3 0.5 EC 34%
(cont'd) 1 0.5 EC 34%
3 0.5 EC 34%
Potatoes New Zealand 1972 1 0.44 wp 45% <0.05 <0.05 <0.05
Japan 1972 2 0.35 <0.005 <0.005 V 142
3 <0.005 <0.005
Residues in mg/kg, at intervals (days) after application
21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Field crops
Barley Canada 1973 1 0.5 2.17 V 173
Grain 0.64
Grain Canada 1973 1 0.5 0.11
Straw 0.5 0.12
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Grain Canada 1.0 0.14 V 155
Straw 1.0 0.62
Oats Canada 1973 1 0.5
Grain <0.01 V 159
Stubble <0.01
Wheat Canada 1973 1 0.5 0.37
Grain 1 0.36 0.03 V 173
Bran 0.04
Flour <0.01
Canada 1970 1 0.5
Grain <0.01 V 113
Straw 0.01
+ chaff
Canada 1970 1 1
Grain <0.01 V 113
Straw 0.17
+ chaff
Wheat Canada 1972 1 0.5
Grain 0.72 V 155
Straw 0.28
1972 1 1
Grain 0.24
Straw 1.32
Maize
Sweetcorn Canada 1974 1 1 EC
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/56 63/72 75/90 Ref.
Maize
(cont'd)
Cobs <0.01 <0.01 Braun
et al.,
Forrage <0.01 <0.01 1975
1 2 EC
Cobs <0.01 <0.01
Forrage <0.01 <0.01
2 1 EC
Cobs <0.01 <0.01
Forrage <0.01 0.31
2 2 EC
Cobs <0.01 <0.01
Forrage <0.01 0.02
28/33 75/92
Fieldcorn
forrage Canada 1974 1 0.5 EC 0.06 V 158
Forrage 1 1 EC 0.04
Sunflower Canada 1973 1 1 EC V 155
Hulls 0.01
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/33 35/40 42/46 49/56 63/72 75/92 Ref.
Sunflower Canada
(Cont'd)
Meal 0.03
Crude oil 0.18
Refined oil 0.20
Finished oil <0.01
Sunflower
seed Canada 1972 1 0.5 EC <0.01 V 174
Rape seed Canada 1973 1 0.5 EC 0.11 V 183
1 1 EC <0.01
0.5 EC 0.19 V 177
0.5 EC 0.22
0.25 EC 0.07
0.4 EC 0.04
Flax seed Canada 1973 1 0.5 0.09 0.07 V 174
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice Japan 1973 5 0.35 EC
Unhulled 0.20 Niigata,
1973
Straw 15.0-16.8
6 0.35 EC
Unhulled 0.21
Straw 15.9-16.7
6 0.35 EC
Unhulled 0.32
Straw 16.9-17.3
5 0.35 EC
Unhulled 0.11
Straw 22.4-23.3
6 0.35 EC
Unhulled 0.08
Straw 30.5-31.7
6 0.35 EC
Unhulled 0.03-0.05
Straw 6.15-8.33
5 0.8 dust 2%
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.11
Straw 7.20-7.80
Japan 1973 6 0.8 dust 2% Niigata,
1973
Unhulled 0.10
Straw 7.20-7.40
6 0.8 dust 2%
Unhulled 0.15-0.16
Straw 11.6-12.4
5 0.8 dust 2%
Unhulled 0.12
Straw 4.15-5.30
6 0.8 dust 2%
Unhulled 0.08-8.09
Straw 9.4-10.8
6 0.8 dust 2%
Unhulled 0.10
Straw 11.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 1.59-2.82
6 0.6-0.8 dust 2%
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.10
Straw 10.2-10.3
Japan 1973 6 0.6-0.8 dust 2% Niigata,
1973
Unhulled 0.20
Straw 13.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 11.2-11.9
6 0.6-0.8 dust 2%
Unhulled 0.07-0.08
Straw 10.3-10.9
6 0.6-0.8 dust 2%
Unhulled 0.09-0.10
Straw 19.3-19.7
Tea (dry
manuf.) India 1973 2 1.3 EC 93.4 1.77 3.57 0.29 V 203
Assam
*1973 2 1.3 EC 77.3 0.11 3.67 0.23
*1973 1 1.3 EC 2.80-3.77
1973 1 1.3 EC 1.36-1.94
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet Canada 1973 1 0.5 EC
Roots) 0.05 0.05 V 170
)
Tops ) 0.09 0.07 V 170
Roots 1973 2 0.5 EC <0.02
Roots 2 1.0 EC <0.02
Roots 2 1.0 EC <0.02
Roots) 1 1.0 EC <0.02 0.04
Tops ) <0.02 <0.02
Japan 1972 3 0.35 EC Hokkaido,
Roots <0.02 <0.02 exp. sta.
1972
Tops 0.43-0.59 0.17-0.30
1972 5 0.35
Roots 0.01 <0.01
Tops 1.36-1.72 0.66-0.84
1972 3 0.35
Roots <0.01 <0.01
Tops 0.59-0.61 0.25-0.27
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet
(cont'd)
1972 5 0.35
Roots 0.01 <0.01
Tops 0.93-1.02 0.34-0.39
Japan 1971 3 0.35 Hokkaido,
Roots <0.01 <0.01 exp. sta.
1972
Tops 0.59-0.66 0.34-0.39
5 0.35
Roots 0.01 0.01
Tops 1.75-1.87 0.93-0.98
1971 3 0.35
Roots <0.01 <0.01
Tops 0.64-0.69 0.26-0.32
5 0.35
Roots <0.01 <0.01
Tops 1.17-1.20 0.39-0.46
* Sun-dried.
In a 10 minutes brew of sun-dried tea harvested seven days after
treatment no residues of parent leptophos, leptophos-oxon or
desbromoleptophos was found. The levels of the phenol ranged between
<0.01 mg/kg and 0.06 mg/kg.
In a food chain
Data were obtained in two model studies (Anonymous, 1973;
Johnson, 1975) with 14C-leptophos in a simple food chain in which
water, Daphnia magna and the common blue gill (Lepomis macrochirus
rafinesque) were included.
Daphnia magna accumulated leptophos rather rapidly from the
water. In less than four hours leptophos in daphnia reached a constant
level about 1500 times that in the surrounding water. Blue gills
feeding on Daphnia magna exposed to leptophos reached a plateau about
1/10 of that in the daphnia.
It is obvious that the most significant route of accumulation of
leptophos residues by fish will be by direct uptake from water.
Blue gills exposed to leptophos in water only (not in the food)
contained mean 14C residues in the tissues of 0.179 mg/kg, about 750
times the mean concentration of 14C-leptophos in the water, after
10-42 days' exposure. When blue gills were exposed to both aqueous and
dietary 14C-leptophos for 10-28 days the residue level was almost
identical (0.170 mg/kg, about 770 times the concentration in the
water).
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours. The bio-transfer
rate of leptophos in the Daphnia-blue gill food chain was only 6.5% of
that of DDT, a typical bioaccumulating compound.
APPRAISAL
Leptophos was evaluated with regard to agricultural data by the
1974 Joint Meeting (FAO/WHO,1975). Arising from the list of
requirements, information was obtained on residues from supervised
trials in various areas on fruit crops (apples and pears), small
grains, rice, sugar beet, potatoes and on some vegetable crops.
Some information became available on residues in waste of
agricultural crops which may be used for feeding purposes, e.g. sugar
beet leaves, straw and chaff of cereal crops, including rice.
Additional data were obtained on the fate of leptophos residues
in apples, cabbage, lettuce and tomatoes from studies with 14C-phenyl
and 14C-phenoxy-labelled leptophos. Additional data became available
on the fate of 14C-phenoxy- and 14C-phenyl-labelled leptophos in
soil. It was shown in this study that the bound fraction increases
with time at all soil depths. The total 14C in the upper 2.5 cm layer
decreased with time from 92% of the applied radioactivity to 42% after
120 days; the total 14C in the lower layers was fairly constant
during that period.
New data were obtained from model studies with 14C-labelled
leptophos in a simple food chain which included water, Daphnia magna
and the common blue gill. Daphnia magna accumulated leptophos rather
rapidly from the water; in less than four hours leptophos plateaued in
Daphnia to levels about 1500 times the level in the surrounding water.
Blue gills feeding on Daphnia magna exposed to leptophos reached a
plateau one-tenth of that of the daphnids.
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours.
The bio-transfer rate of leptophos in the Daphnia-blue gill food
chain was less than one-tenth of that of DDT, which is recognized as a
bioaccumulating compound.
The new data available confirm the limits recorded at the 1974
Joint Meeting on cabbage, broccoli, lettuce, tomatoes, potatoes,
cotton seed and maize.
In the light of the temporary ADI established at the present
Meeting these limits are now recommended as temporary maximum residue
limits, and replace the guideline levels recorded in the 1974
monograph. Additional temporary maximum residue limits are
recommended.
Recommendations
The maximum residue limits refer to the sum of leptophos, its
oxygen analogue and desbromo-leptophos, expressed as leptophos.
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Apples, pears 2 42-60
Broccoli, Brussels sprouts,
cabbage 2 28-50
Lettuce 2 28 (outdoor)
Tomatoes 2 7
Cotton seed oil (crude) 1 28
Raw cereals (wheat, oats,
barley) 0.5 60
Rice (hulled) 0.5 30
(Cont'd)
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Cotton seed, cotton seed meal 0.2 28
Carrots 0.2 60
Potatoes 0.1 21
Maize, fieldcorn (kernels) 0.05 7
Sweet corn (kernels and cobs,
husks and silk removed) 0.05 3-7
Sunflower seed 0.05 100
Sugar beet (roots) 0.05 50-60
Sugar beet (tops) 0.5
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1978)
1. Studies on the kinetics of accumulation of leptophos in storage
depots of two species, preferably non-rodent to investigate the
potential buildup in man to ultimate threshold levels.
2. Studies on the species variation for delayed neurotoxicity with
leptophos to assess its ultimate effect on man.
3. Epidemiological studies on people in exposed occupations in
agriculture or industry.
4. Further long-term low-level feeding studies in a species
susceptible to the delayed neurotoxic syndrome.
5. Residue data on the major crops for which national tolerances or
use recommendations exist, e.g., tea, vegetables not included in the
recommendations, and citrus fruits.
6. Residues in those parts of agricultural commodities which are
used either as such or as agricultural waste for animal feed.
DESIRABLE
1. Improved methodology for assessing the delayed neurotoxic
syndrome.
2. Studies on the mechanism of neurotoxic action of leptophos.
Further investigations into mechanism of action of TOCP and other
neurotoxic agents as compared to leptophos.
3. Data on current use patterns in countries outside the United
States of America and Canada and on residue levels resulting from
those uses.
REFERENCES
Anonymous. (1973) Exposure of 14C-leptophos to daphnia magna and
bluegill sunfish (lepomis macrochiris). Accumulation, distribution,
elimination and bio-concentration in the food chain. Unpublished
report from Bionomics, Inc., submitted to FAO by Velsicol Chemical
Corporation.
Abou-Donia, M.B., Othman, M.A., Tantawy, G., Khalil, A.Z. and Shawer,
M.F. (1974) Neurotoxic effect of leptophos. Experimentia, 30:63-64.
Abou-Donia, M.B. and Pressig, S. (1975) Studies on delayed
neurotoxicity produced by leptophos. Proc. Fed. Amer. Soc. Exptl.
Biol., 34:810.
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Phosvel (VCS-506) in albino mice. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Badie, M. and Whitacre, D.M. (1975) Metabolism of 14C-leptophos and
14C-4-bromo-2,5-dichlorophenol in rats: A multiple dosing study.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Braun, E., McEwen, F.L., Frank, R. and Ritcey, G. (1975) Residues of
leptophos and its metabolites following application to various crop
plants. J. Agr. Food Chem., 23(1):90-95.
Braun, E., McEwen, F.L., and Frank, R. (1974) Residues of
chlorpyriphos and leptophos in three field tested vegetable crops.
Canad. J. Plant Sci., 55:133-137.
Diaz, L.I. (1974) Radiotracer study of leptophos on cabbage. Velsicol
Chem. Corp. Lab. rep. 194. (Unpublished)
Dorough, H.W. (1974) Fate of phosvel-14C in burley tobacco. Progress
report of Department of Entomology, University of Kentucky, Lexington,
provided to Velsicol. (Unpublished)
Fink, R.J. (1971) Continuous feeding and residue study - cows with
technical Phosvel (VCS-506). Unpublished report from Hazleton
Laboratories, submitted to the World Health Organization by Velsicol
Chemical Corporation.
Fletcher, D., Jenkins, D.H., Kinoshita, P.R., Gordon, D.E. and
Keplinger, M.L. (1975) Neurotoxicity study with technical leptophos in
chickens. Unpublished report from Industrial Bio-Test Laboratories,
Inc., submitted to the World Health Organization by Velsicol Chemical
Corporation.
Haley, S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1972) Three
generation reproduction study with VCS-506 in albino rats. Unpublished
report by Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Haley, S., Kennedy, G.L. and Keplinger, M.L. (1973) Three-generation
reproduction study with VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Hartke, K., Lindberg, D.C., Wright, P.L. and Keplinger, M.L. (1971)
Two-year chronic oral toxicity study with VCS-506 technical in beagle
dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Hassan, A. (1975) Personal communication to the World Health
Organization.
Hathaway, D., Keplinger, M.L. and Fancher, O.E. (1968) Acute aerosol
inhalation toxicity study on VCS-506, 3 EC. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Hokkaido. (1972) Phosphel residue analysis on sugar beet. National
Hokkaido. Agr. Exp. Sta. Residue data provided to Velsicol.
(Unpublished)
Holmstead, R.L., Fukuto, T.R. and March, R.B. (1973) The metabolism of
O-(4-bromo-2,5-dichlorophenyl) O-methyl phenylphosphonothioate
(leptophos) in white mice and cotton plants. Archives of Environmental
Contamination and Toxicology. Vol. 1, No. 2, 133-147.
Johnson, B.T. (1973) Leptophos accumulation through a simple food
chain. Unpublished report from the United States Department of the
Interior, submitted to the World Health organization by Velsicol
Chemical Corporation.
Johnson, M.K. (1975) The delayed neuropathy caused by some
organophosphorus esters: Mechanism and challenge. CRC Critical Reviews
in Toxicology, June.
Kennedy, G. and Keplinger, M.L. (1971) Absorption, distribution, and
excretion study with Phosvel in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1971a) Characterization of 14C in
excreta of rats following oral administration of Phosvel. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1972) Absorption, distribution, and
excretion study with VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970) Distribution and
excretion study of VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970a)
Characterization of 14C in urine of rats following oral
administration of 14C-VCS-506. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kimmerle, G. (1972) Acute neurotoxicity studies in hens. Unpublished
report from Farbenfabriken Bayer, submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1971) Study of the
efficacy of atropine sulfate and 2-PAM as antidotes for VCS-506
intoxication in albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C., Keplinger, M.L. and Fancher, O.E. (1971a)
Acute oral (intragastric) toxicity studies with VCS-506 in one day old
and five day old albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1972) Acute oral
toxicity study with oxon of VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Kretchmar, B,, Mastri, C. and Keplinger, M.L. (1972a) Acute oral range
finding toxicity study with S-isomeride in female albino rats.
Unpublished report from industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L. (1971)
Teratogenic study with Phosvel in albino rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health organization by Velsicol Chemical Corporation.
Lindburg, D., Vondruska, J.F. and Fancher, O.E. (1969) Ninety-day
subacute oral toxicity of VCS-506 in beagle dogs. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
March, R.B. and Fukuto, T.R. (1975) Reports on studies on the
photolysis and metabolism of Phosvel. Unpublished report from the
University of California, submitted to the World Health Organization
by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969) Acute oral
toxicity study on VCS-506 technical in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L, and Fancher, O.E. (1969a) Acute oral
toxicity study on two formulations of VCS-506 E.C. in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity on four metabolites of VCS-506 in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on phenylphosphonic acid in albino rats. Unpublished
report from industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Miyagi. (1971) Phosphel residues on chinese cabbage. Miyagi Pref. Agr.
Exp. Sta. and Aichi Pref. Agr. Exp. Sta. Residues data provided to
Velsicol.
Niigata. (1973) Phosphel residue analysis on rice. Niigata Pref. Agr.
Exp. Sta. and Shizuoka Pref. Agr. Exp. Sta. Data provided to Velsicol.
(Unpublished)
Perkins, C.T. and Singh, V.K. (1971) Chicken toxicity and residue
study. Unpublished report from Bio/Toxicological Research
Laboratories, Inc., submitted to the World Health organization by
Velsicol Chemical Corporation.
Perkins, C.T. and Singh, V.K. (1971a) Beef animal feeding study.
Unpublished report from Bio/Toxicological Research Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J., Keplinger, M.L. and Fancher, O.E. (1970) Cholinesterase
study of VCS-506 in albino rats. Unpublished report from International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971) Twenty-eight day
target organ study with VCS-506 oxon metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971a) Twenty-eight
day target organ study with VCS-506 phenol metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971b)
Ninety-day subacute oral toxicity, study with VCS-506 Phenol
metabolite in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971c)
Ninety-day subacute oral toxicity study with VCS-506 oxon metabolite
in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Rieck, C.E. (1974) Soil metabolism of 14C-leptophos. Report of Agr.
Dept., University of Kentucky, Lexington, provided to Velsicol.
(Unpublished)
Sanborn, A. and Metcalf, R.L. (1975) The fate of leptophos (Phosvel)
in a terrestrial-aquatic ecosystem. Unpublished report from the
University of Illinois submitted to the World Health Organization by
Velsicol Chemical Corporation.
Schroeder, C. (1971) The radiocarbon distribution of 14C labelled
Phosvel in lettuce and green beans. Unpublished report from WARF
Institute submitted to the World Health Organization by Velsicol
Chemical Corporation.
Schwemmer, B. (1971) UV photolysis of leptophos in solution.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Sleight, B.H. and Macek, K.J. (1973) Exposure of 14C-leptophos to
Daphnia magna and bluegill sunfish (Lepomis macrochirus):
Accumulation, distribution, elimination, and bioconcentration in the
food chain. Unpublished report from Bionomics, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971)
Twenty-eight day cholinesterase study with VCS-506 oxon metabolite in
female albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc. submitted to the World Health Organization by
Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971a)
Two-year chronic oral toxicity study with VCS-506 technical in albino
rats. Unpublished report from Industrial Bio-Test Laboratories, Inc.
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Smith, P.S., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. (1973)
Eighteen-month carcinogenic study with VCS-506 technical in Swiss
white mice. Unpublished report from Industrial Bio-Test Laboratories,
Inc. submitted to the World Health Organization by Velsicol Chemical
Corporation.
Stephens, J.L., Jenkins, D.H. and Fancher, O.E. (1969) Demyelination
study-chickens, VCS-506 Technical. Unpublished report from industrial
Bio-Test Laboratories, Inc. submitted to the World Health Organization
by Velsicol Chemical Corporation.
Suzuki, H. (1971) Phosvel-chicken feeding study. Unpublished report
from Velsicol Chemical Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Suzuki, H. (1971a) Phosvel-dairy cattle feeding study. Unpublished
report from Velsicol Chemical Corporation submitted to the World
Health Organization by Velsicol Chemical Corporation.
Tarka, S.M. Jr. (1973) Identification of radioactivity in the fat of
rats administered 14C-phenoxy-labelled leptophos. Unpublished report
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1965) Acute oral toxicity (LD50) in the albino rat.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1966) Acute oral toxicity (LD50) in male albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968) Comparative acute oral toxicity in male albino
rats. Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968a) Acute toxicity studies in the albino rabbit.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968b) Acute toxicity study in the rabbit. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1968c) Ten-day subacute oral toxicity study in rats for
analysis of plasma, erythrocyte and brain cholinesterase activity.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1969) Ninety-day feeding study in albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970a) Acute oral toxicity (LD50) in the male albino
rat. Unpublished report from international Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970b) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971a) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971b) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971c) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971d) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (197le) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971f) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971g) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971h) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971i) Acute toxicity studies in rats and rabbits.
Unpublished study by International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1972) Acute oral toxicity (LD50)
in male and female albino rats. Unpublished report by International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1973) Acute dermal toxicity
(LD50) in male and female albino rabbits. Unpublished report from
International Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Whitacre, D.M. and Schnur, P.D. (1974) Metabolic fate of 14C-labelled
leptophos on apples. Velsicol Chem. Corp. Lab. rep. No. 195.
(Unpublished)
In further studies of the biomagnification of leptophos, it was
observed that an apparent equilibrium was established in fish exposed
to leptophos for periods of time. After about one week of exposure
during which time leptophos accumulates there is no additional
build-up in tissues. These data also suggest that ingestion of
leptophos - contaminated invertebrates by fish does not increase the
fish residue suggesting that biomagnification is not a significant
factor (Sleight and Macek, 1973; Johnson, 1973).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Leptophos oxon
Rat
Groups of rats (25 males and 25 females/group) were subdivided
into two categories: individually housed (10 rats of each sex per
group) and group housed (15 rats of each sex per group). These groups
were fed a control diet and one containing leptophos oxon for 28 days.
The leptophos oxon diet was increased at weekly intervals from 200 ppm
to 300 ppm to 500 ppm and finally to 800 ppm. Growth (as evidenced by
body weight) was reduced in the latter stages of the test in females
while food consumption was normal. Spleen weight in males and females
was reduced at 7, 21 and 28 days. Cholinesterase activity of RBC and
brain was depressed at all test intervals. Plasma cholinesterase was
depressed in females at all intervals while in males the depression
became evident after 14 days. No effects were noted on survival,
haematology, blood chemistry, urinalyses or on microscopic examination
of tissues and organs (Plank et al., 1971).
Groups of female rats (15 rats/group) were fed leptophos oxon for
four weeks at dose levels of 0, 1, 5, 10 and 20 ppm. Measurements of
red blood cell and plasma cholinesterase activity were made at 14 and
28 days. Plasma cholinesterase was unaffected while erythrocyte
cholinesterase was depressed at 10 ppm and above at 14 days. At the 28
day interval reduction of activity was not significant (Smith et al.,
1971).
Groups of rats (15 males and 15 females/group) were fed leptophos
oxon for 90 days at dose levels of 0, 25, 50 and 500 ppm. Inhibition
of plasma cholinesterase activity was noted at all dose levels in
females while males were affected at 500 ppm only at the 84 day test
interval. Inhibition of erythrocyte and brain cholinesterase activity
was similar in both sexes and was notable only at 500 ppm. No effects
were noted on mortality, growth, food consumption, haematology
parameters, clinical chemistry parameters, urinalyses, and on gross
and microscopic examination of organs and tissues (Plank et al.
1971c).
Leptophos phenol
Rat
Groups of rats (25 of each sex/group) were divided into two
subgroups and housed individually (10 of each sex/group) or housed
together (15 of each sex/group). The sexes were not mixed. Two dietary
groups were used; a control and a test group which received a dietary
level of leptophos phenol of 3500 ppm for one week. This was increased
to 5000 ppm for the second week, to 8000 ppm for the third week and
finally to 13 000 ppm for the fourth week. There were no effects on
food consumption, growth, haematology, blood chemistry including
cholinesterase or on growth and microscopic examination of tissues and
organs (Plank et al,, 1971a).
Groups of rats (15 males and 15 females/group) were fed leptophos
phenol at dietary levels of 0, 300, 1000 and 10 000 ppm (3000 ppm for
days 1-49) for 90 days. At 10 000 ppm there was a significant increase
in liver weight in females. In males a decreased spleen weight was
noted at all doses tested (none was significantly different from
control values although the decrease was evident in ratios calculated
from body and brain weight data). No effects were observed on
survival, food consumption, growth, haematology, blood chemistry,
urinalyses or on gross or microscopic examination of tissues or organs
(other than the gross effects on liver and spleen). A report of the
microscopic examination of liver and spleen indicated no abnormalities
(Plank et al., 1971b).
Special studies on mutagenicity
Mouse
A dominant lethal study was conducted where groups of male mice
(8 mice/group) were administered a single oral or intraperitoneal dose
of 0, 15 or 30 mg leptophos/kg. The males were mated with three
females per week for six weeks during the normal period of
spermatogenesis. Positive control studies were performed with methyl
methane-sulfonate. There were no effects of leptophos on mating
performance or on reproduction parameters including preimplantation
loss, early resorption or embryo viability. Leptophos under the
conditions of this test did not induce mutagenic changes in male
germinal cells (Arnold et al., 1971).
Special studies on neurotoxicity
Chickens
Groups of white leghorn hens (6 hens/group) were orally
administered leptophos at doses of 100, 200 and 400 mg/kg on day 0 and
14. Observations on clinical conditions were made and at the
conclusion of the study (day 28) survivors were sacrificed and nervous
tissue examined microscopically for indications of myelin disruption.
No clinical signs of delayed neurotoxicity were noted in this
study following the first dosing. Three hens of six tested at the high
level died 6-14 days after the second dose. These hens had lost a
considerable portion of their body weight before death. At 200 mg/kg
one of six died and one other showed clinical signs of ataxia.
Microscopic examination of H & E and Luxol Fast Blue stained sections
of nerve did not reveal myelin degeneration (Stephens et al., 1969).
Groups of white leghorn hens (10 hens/group) were administered
leptophos orally at doses of 0, 5, 10, 15, 30, 50, 75, 100 and 200
mg/kg at days 0 and 21. A positive control was also used in this study
(Tri-o-Cresyl phosphate, 500 mg/kg - orally at day 0). A number of
animals showing clinical signs of ataxia were sacrificed at day 42.
Gross and microscopic examination of brain, spinal cord and sciatic
nerves were performed. Examinations were made of H & E stained
sections and tissues embedded in ParaplastR and stained with Luxol
Fast Blue - Holmes Silver Nitrate. Both light and electron microscopic
examinations were made in this study.
A positive delayed neurological condition was clinically evident
in this study with both TOCP and leptophos. Loss of body weight is
generally observed clinically in animals with delayed peripheral
ataxia and this was observed with the TOCP group in 6 of 10 hens
weighed at day 18. In the leptophos groups, weight loss was noted in 1
of 10 hens at 75 mg/kg, in 3 of 10 at 100 mg/kg, in 5 of 10 hens at
200 mg/kg. This weight loss was accompanied by signs of peripheral
neuritis in various hens of the three upper group levels (3 hens at 75
mg/kg, 5 hens at 100 mg/kg and the majority of hens at 200 mg/kg).
Light and/or electron microscopic sections of nerve tissue and muscle
preparations indicated retrograde degeneration of 8 of 10 TOCP hens
and 5 of 10 hens at 200 mg/kg. No other positive signs of degeneration
were observed. At acute oral dose levels of 75 mg/kg and above,
leptophos was shown to induce a clinical neuropathy in hens (Fletcher
et al., 1975).
Other studies have confirmed that, at high acute oral doses,
leptophos will induce delayed neurotoxicity and clinical signs of
neuropathy (Abou-Donia et al., 1974; Abou-Donia and Preissig, 1975;
Kimmerle, 1972). In male chickens, 2 of 9 tested were ataxic between
9-13 days after oral dosing with 180 mg/kg. No neurotoxic effects were
noted at dose levels below 180 mg/kg. Further studies showed that 200
mg/kg and above resulted in ataxia. A 30% formulation of emulsifiable
concentrate administered to hens by oral or IV injection resulted in
positive clinical neuropathy at dose levels above 100 mg/kg.
Special studies on reproduction
Rat
In two studies, groups of rats (8 males and 16 females/group
there - were two control groups of 8 males and 16 females each) were
fed leptophos in the diet at levels of 0, 10, 30, 40 and 60 ppm and
subjected to a standard three generation, two litter per generation
reproduction study. After two litters of the first generation were
born, the 60 ppm level was discontinued because of pup mortality. A
second study was initiated at 0 and 5 ppm. The 5 ppm dose was changed
after one generation to the 40 ppm level (noted above) and maintained
at this level for two further generations.
Parental body weight and reproductive performance was similar in
the 60 ppm group to the control group. Reproductive indices, including
mating, pregnancy, fertility and parturition showed no differences
with respect to control and all treated rats. Survival data on pups
including viability and lactation indices were significantly decreased
at 60 ppm but were similar to control values at 40, 30 and 10 ppm.
Mean body weight data for pups at weaning at all dose levels
(including 60 ppm) were similar. A no-effect level for rat
reproduction is 30 ppm (Haley et al., 1973, 1972).
Special studies on teratogenecity
Rabbit
Groups of pregnant New Zealand rabbits (10-13 rabbits per group)
were administered leptophos orally from day 6 to day 18 of gestation
at dose levels of 0, 1 and 3 mg/kg. Thalidomide was administered over
this same period at a dose of 37.5 mg/kg to a group of rabbits
designated as a positive control. No effects were noted on the growth
of does. Skeletal or somatic abnormalities were noted with
thalidomide. Leptophos at a dose of 3 mg/kg administered orally to
rabbits during organogenesis elicited no teratological response (Ladd
et al., 1971).
Signs of poisoning are typical of acute parasympathomimetic
stimulation seen by other anticholinesterase organophosphates. These
include: hypoactivity, tremors, muscular weakness, ruffed fur,
diarrhoea, exophthalmia, haemorrhagic conjunctivitis and salivation.
Acute Toxicity
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M Tech (white mass) Oral 31.6 Wazeter, 1965
M Tech (white powder) Oral 59.0 " 1966
M Tech (98.5% white powder) Oral 54.5 " 1968
M Tech (90% brown granule) Oral 37.1 " 1968a
M Tech (99.6% white powder) Oral 56.6 " 1971
M Tech (90% white crystal) Oral 44.7 " 1970
M Tech (90% grey granule) Oral 52.8 " 1971a
M & F Tech (94%) Oral 20.0 Kretchmar et al., 1971
M & F Tech (90%) Oral 90.5 Mastri et al., 1969
M & F (1 day old) Tech 90% Oral 12.0 Kretchmar et al., 1971a
M & F (5 day old) Tech 90% Oral 15.0 Kretchmar et al., 1971a
F Tech (98.5% white powder) Oral 24.3 Wazeter, 1968
F Tech (90% brown granule) Oral 26.1 " 1968
F Tech (99.6% white powder) Oral 40.1 " 1971
F Tech (90% grey granule) Oral 42.9 " 1971a
Rabbit M & F Tech (90% white crystal) Dermal 10 000 " 1970
M & F Tech (90% brown powder) Dermal 800 " 1968a
" 1968b
Chicken F Tech Oral 225 Fletcher et al., 1975
Technical leptophos is an eye irritant when instilled into the conjuctival sac of rabbits (Wazeter, 1968b, 1970).
Leptophos is not an irritant when tested on normal or abraded rabbit skin (Wazeter, 1970).
Acute toxicity formulations
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M 3 EC (yellow oil) Oral 271 Wazeter, 1970a
M 30 EC (yellow oil) Oral 271 " 1970a
M 29.5 EC Oral 400 Mastri et al., 1969a
M 35.7 EC Oral 735 " 1969a
M 5 G Oral 2 263 Wazeter, 1971b
M 50 WP (grey powder) Oral 121 " 1971c
M 2 EC (yellow liquid) Oral 178 " 1971d
M 3 EC (34.7%) Oral 121 " 1971e
M 3% Dust (grey dust) Oral 1 780 " 1971i
M 3 ULV (32.1%) Oral 192 " 1970b
M 5 G Inhal 26.9 mg/L Wazeter, 1971b
M 3 ULV (32.1%) Inhal 200 mg/L " 1970b
M & F 3 EC 4 hr
Inhal 2.7 mg/L Hathaway et al., 1968
M 3% dust 4 hr
Inhal 33 mg/L Wazeter, 1971i
M 50 WP Inhal 19 mg/L " 1971c
M 2 EC Inhal 200 mg/L " 1971d
M 3 EC Inhal 200 mg/L " 197le
F 29.5 EC Oral 218 mg/L Mastri et al., 1969a
F 36.7 EC Oral 327 mg/L " 1969a
Rabbit M & F 2.7 EC Dermal 2 000 Wazeter and Goldenthal, 1973
Leptophos formulations are an eye irritant when instilled into the conjunctival sac (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i). However, one
formulation tested (3 ULV - 32.1% leptophos) was not an eye irritant (Wazeter, 1970b). Leptophos fomulations are not a skin irritant when tested
on normal or abraded rabbit skin (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i).
Acute toxicity - metabolites and possible contaminants
LD50
Compound Species Sex Route (mg/kg) Reference
Leptophos oxon Rat M & F Oral 119 Mastri et al., 1969b
Rat M & F Oral 43 Kretchmar et al., 1972
Phenylphosphonic
acid Rat M Oral 2 480 Mastri et al., 1969b
2 241 " 1969c
S-methyl isomeride Rat F Oral 735 Kretchmar et al., 1972a
O.O-bis
(2,5-di-chloro-4-brom-phenyl)
phenylphosphono-thioate Rat M Oral 4 640 Wazeter, 1971f
O.O-dimethyl
phenyl-phos-phonothioate Rat M Oral 287 " 1971g
O-methyl phenyl
phosphonic acid Rat M Oral 3 690 " 1971h
O-(2,3-dichloro-4-bromophenyl) Rat M Oral 59.5 Wazeter and Goldenthal, 1972
phenylphos-phonothioate Rat F Oral 37.5 Wazeter and Goldenthal, 1972
Leptophos phenol Rat M & F Oral 2 654 Mastri et al., 1969b
Antidotal studies
Administration of atropine sulfate and 2-PAM alone and in
combination resulted in an increase in the LD50 value suggesting that
these agents might be successful in antidotal therapy (Kretchmar et
al., 1971). The combination of atropine and 2-PAM was no more
successful than the administration of either agent alone.
Antidote LD50 (mg/kg) (95% C.L.)
None 20.0 (11.8-34.0)
Atropine Sulfate (IM)
10 mg/kg 1.5 & 5 hr
after poisoning 54.0 (41.2-70.7)
2-PAM
1.5 hr after poisoning 38.5 (33.8-43.9)
Atropine + 2-PAM 40.0 (27.6-58.0)
Short-term studies
Rat
Groups of rats (2 males and 2 females/group) were administered
leptophos by oral intubation of 10 days at dose levels equivalent to
dietary intakes of 0, 3, 10, 30 and 100 ppm. Mortality was evident at
100 ppm. At the conclusion of the study the animals were sacrificed
and cholinesterase determinations run using brain, plasma and red
blood cells.
No depression of plasma cholinesterase was noted. The RBC and
brain cholinesterase activity was depressed at 30 ppm and above in all
surviving animals (Wazeter, 1968c).
Groups of rats (10 males and 10 females/group) were fed dietary
levels of leptophos of 0, 100, 250 and 500 ppm for 28 days. A second
series of female animals (10 rats/group) were fed levels of 0, 50 and
75 ppm for 14 days in a cholinesterase depression range-finding test.
A dose-dependent depression of cholinesterase activity was
observed in all tissues at all dose intervals and times tested in the
initial high level study. Activity of the most susceptible
cholinesterase source (female RBC) was slightly depressed 32% and 22%
at 75 and 500 ppm respectively after seven days of feeding. The plasma
cholinesterase activity in the initial study was not inhibited until
the two week test interval after which it steadily declined (Plank, et
al., 1970).
Groups of rats (10 male and 10 female rats/group were fed
leptophos in the diet at dose levels of 0, 1, 5 and 10 ppm for 90
days. Growth, food consumption and behaviour were not affected by
leptophos. No changes were noted in haematology, blood, clinical
chemistry or urinalysis parameters measured. Cholinesterase activity
in RBC, plasma and brain were normal at 90 days of testing as were the
40 day examinations of RBC and plasma cholinesterase. Gross and
microscopic examination of tissues and organs showed no
leptophos-related lesions (Wazeter, 1969).
Dog
Groups of dogs (four male and four female beagle dogs/group) were
fed leptophos in the diet for 90 days at dose levels of 0, 10 and 30
ppm. Behaviour, growth and food consumption were not affected over
this test interval. No effects were noted on haematology parameters on
blood, clinical chemistry on urinalyses or on gross and microscopic
analyses of tissues and organs. A no-effect level was 30 ppm (Lindberg
et al., 1969).
Groups of beagle dogs (4 males and 4 female/group) were fed
leptophos in the diet at dosage levels of 0, 10, 20, 30 and 60 ppm for
two years (the 60 ppm group had been fed a diet containing 5 ppm for
180 days which was then increased to 60 ppm for the remainder of the
study. No mortality occurred over the course of the study and growth,
food consumption and behaviour was normal at all levels. No
leptophos-related effects were noted on haematology parameters,
clinical chemistry values (including blood and brain cholinesterase
values) or on gross and microscopic examination of tissues and organs.
Leptophos in the diet at 60 ppm had no effect on any parameter
recorded in this study (Hartke et al., 1971).
Chicken
Groups of white leghorn chickens (4 male and 20 females/group)
were fed leptophos in the diet at dosage levels of 0, 0.03, 0,10, and
0.30 ppm for four weeks. Eggs were collected and incubated during the
latter 14 days of treatment. The chicks were observed for 14 days
post-hatching having been fed the leptophos diet corresponding to the
parent. There were no treatment related differences in body weight or
egg production and size of eggs. Egg hatchability, chick viability,
and growth was not affected by leptophos. No abnormal behaviour or
signs of poisoning were observed in this study in either adults or
chicks (Perkins and Singh, 1971). Analysis of tissue residues
(including eggs and adipose tissue) did not indicate the presence of
residues of leptophos, the oxon, the phenol or a photo-product
(Suzuki, 1971).
Steer
Groups of steers (three steers/each treatment group and one
steer/control group) were fed leptophos in the diet at levels of 0,
15, 45 and 150 ppm for four weeks. At 28 days the control and two each
of the treatment groups were sacrificed. The remainder were allowed to
consume normal untreated food for a further 14 days after which they
were sacrificed. Daily observations showed no abnormal behaviour or
toxic signs of poisoning. No gross or microscopic lesions were
observed at the conclusion of the study. Depression of the whole blood
and plasma cholinesterase was evident at the higher dose levels over
the 28 day feeding interval. After seven days on a control ration the
depressed values returned to normal. Tissue residues of leptophos and
the oxon were low in liver, kidney, muscle and brain. In fat,
leptophos was present after 28 days in measurable levels which were
dose dependent. Following the 14 day recovery intervals, these
residues in fat were considerably reduced (Perkins and Singh, 1971a).
Cow
Groups of lactating dairy cows (three cows/group fed leptophos
and one cow/control group) were fed leptophos in the diet at
calculated dose levels of 0, 5, 15 and 50 ppm (actual values fed as
assessed by chemical analysis were 0, 3.2, 10.0 and 37.4 ppm) for 28
days and maintained for a further 14 days on control diets to measure
residue reductions and recovery of normal values. Appearance,
behaviour and general condition were normal over the study. Body
weights of several animals tested with leptophos were reduced over the
study. Milk production was reduced in the low and intermediate group
but not at the high level of leptophos. Cholinesterase depression was
not noted in this study (Fink, 1971). Traces of leptophos were noted
in fat at the conclusion of the 28 day feeding interval at levels
ranging from 0.2 to 0.5 ppm in the high (37.4 ppm) dose level. After
two weeks, these residues were reduced to 0.2 ppm (Suzuki, 1971a).
Long-term studies
Mouse
Groups of Swiss white mice (65 males and 65 females/group) were
fed leptophos in the diet for 18 months at dose levels of 0, 50, 100
ppm to assess a carcinogenic potential in mice. Two positive control
groups were fed N-nitrosodiethylamine at levels of 10 ppm (50 males
and 50 females) and 40 ppm (15 males and 15 females). After six
months, the mice of the high positive control group and 15 of each sex
in the leptophos group were sacrificed and subjected to gross and
microscopic examination for tumour formation. At 18 months all
remaining animals were sacrificed and 10 animals of each sex (or
group) were examined.
The positive control sacrificed at six months (40 ppm) showed
definitive evidence of lung adenoma or carcinoma. At 18 months, the
positive control (10 ppm) again showed a positive response to the
carcinogen. No evidence of lung lesions was noted at 100 ppm leptophos
in the diet. There were no leptophos-related lesions or tumours in any
of the tissues and organs examined in this study (Smith et al., 1973).
Rat
Groups of Charles River albino rats (50 males and 50
females/group) were fed leptophos in the diet for two years at
concentrations of 0, 10, 20, 30 and 60 ppm (the 60 ppm were initially
fed 5 ppm for the first seven months). Mortality was unaffected by
leptophos in the diet (although at the conclusion of the study very
few animals in all groups were alive). Food consumption and growth
were similarly not affected. There were no differences from control
values in the haematology, blood chemistry or urine parameters
examined. Gross and microscopic examination of tissues and organs
failed to indicate any pathologic disorders noted in controls.
Cholinesterase activity measured in brain over the first 90 days
showed no reduction in activity at any dose tested. Erythrocyte
cholinesterase activity was depressed at all levels tested. A
significant reduction (below 25%) was noted at the 60 ppm level. A
no-effect level based on RBC cholinesterase depression is 30 ppm
(Smith et al., 1971a).
Comments
Leptophos, an anticholinesterase organophosphonothioate ester
recommended for use in agriculture as an insecticide, has a moderate
acute oral toxicity. Leptophos is rapidly absorbed, metabolized and
excreted within 24 hours following single acute oral dosing in
animals, primarily via the urine. No tissue residues were observed
except for adipose tissue which maintains traces of leptophos for
extended periods following acute dosing. The metabolism of leptophos
includes oxidative and hydrolytic processes, generally resulting in
less toxic products (except the oxon and the dichlorodesbromo
derivatives). All materials noted as field residues have been found as
metabolites in animals.
Leptophos (and its metabolites) is a relatively persistent
pesticide. Artificial environmental studies using model systems
suggest that leptophos may bioaccumulate in the food chain.
Sufficient toxicological data have been reported including long
and short-term studies with rodents and dogs, reproduction studies
(including teratogenesis and mutagenesis studies); and carcinogenesis
studies. The only significant adverse toxicological parameters
reported in these studies have been esterase depression, pup mortality
in the reproduction study at high levels and delayed neurotoxicity in
hens. Short-term studies on the metabolites have shown no substantial
toxicological effect on rodents except for esterase depression with
the oxon metabolite.
Based on the no-effect level observed in two year studies in rats
and dogs and the no-effect level in acute avian studies for delayed
neurotoxicity, a temporary ADI was estimated. An exceptionally high
safety factor was used to allocate the ADI reflecting concern over the
positive neurotoxicity results. There was less concern over the
significant delayed neurotoxicity as a factor in evaluating the
toxicological hazard of food residues than in its evaluation of
delayed neurotoxicity as a hazard to occupationally exposed
individuals. The occurrence of the delayed neurotoxicity was, however,
considered to be highly significant in the toxicological evaluation of
leptophos. It was decided that delayed neurotoxicity should be
considered as an additional toxicological parameter, showing a
dose-response relationship, which permits an evaluation to be made of
a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 30 ppm in the diet equivalent to 1.5 g/kg bw
Dog: 60 ppm in the diet equivalent to 2.1 mg/kg bw
Hen: 50 mg/kg (based on delayed neurotoxicity)
Estimate of temporary acceptable daily intake
0-0.001 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in crops
Extensive data were obtained on residues from supervised trials
in which crops were treated according to recommended dosage rates and
pre-harvest intervals. In most trials separate residue figures were
given for the parent leptophos and the metabolites leptophos-oxon,
desbromo-leptophos and the phenol. Leptophos residues consisted mainly
of the parent compound with much lower levels of the metabolites
mentioned, as in the trials recorded in the 1974 monograph of
leptophos. In the following tables therefore, a single figure is shown
referring to the sum of leptophos, its oxygen analogue and
desbromo-leptophos expressed as leptophos.
In a number of trials "apparent" oxon residues were found. As
explained in the 1974 monograph, these consist of bis-(2-ethylhexyl)
phthalate, a component of plastic laboratory equipment. In the
following Tables these apparent oxon residues are omitted.
Comments on the data
Apples. Residue data from Canada (three locations in Quebec and
one in Ontario) show considerable residue levels, up to 2 mg/kg, after
a pre-harvest interval of 6-10 weeks. Further data on apples are given
in the section "Fate of residues".
Vegetables
Beans. After pre-harvest intervals of 37-40 days residues in
beans were either not detectable (Canada) or very low, at or below
0.02 mg/kg (Japan).
Brassicas. No detectable residues were found in broccoli,
cabbage or cauliflower after pre-harvest intervals of about 60 days,
but still considerable residues after intervals of 25-30 days. These
data confirm those available from the United States of America at the
1974 Joint Meeting.
In supervised trials in Japan residue levels in the edible part
of Chinese cabbage after a pre-harvest interval of 14-31 days were in
the range 0.1-0.2 mg/kg.
Celery. Residue levels were very low, 0.01-0.02 mg/kg in three
locations in Ontario after a pre-harvest interval of 61 days. In other
trials residues ranged from 0.02-0.05 mg/kg after a similar interval
and levels up to 0.5 mg/kg were found after an interval of 42 days.
Lettuce. Residue levels in Canadian trials after a pre-harvest
interval of 21-30 days were slightly lower than in the experiments
evaluated at the 1974 Joint Meeting from the United States of America.
After normal trimming for market, residues did not exceed 0.5 mg/kg.
Onions, peas, potatoes. Residues were low after the normal
pre-harvest intervals for these crops. In green onions residues were
at or below 0.1 mg/kg after a pre-harvest interval of about 60 days
and in peas <0.01 mg/kg after 30 days. In the vines however, residues
ranged from 1.1-10.7 mg/kg 22-30 days after treatment. In potatoes in
New Zealand no detectable residues were found after 21 days. This is
in conformity with the United States of America data evaluated by the
1974 Joint Meeting.
Cereal crops
Wheat, oats and barley. Residue levels were below 0.1 mg/kg
after a pre-harvest interval of 60 days in most of the trials; in some
trials levels reached about 0.5 mg/kg in these crops.
Maize. Residues in kernels and forage were generally low (0.05
mg/kg or below) in Canadian experiments, as in the United States of
America data evaluated by the 1974 Meeting.
Rice. After pre-harvest intervals of about 30 days residue
levels in inhulled rice ranged from 0.1-0.5 mg/kg and in leaves and
straw from 6 to 20 mg/kg.
Sugar beet. Data from residue trials in Canada and Japan were
evaluated. After a pre-harvest interval of about 50 days, residues in
the roots did not exceed 0.05 mg/kg and those in the tops ranged from
<0.02-0.5 mg/kg.
FATE OF RESIDUES
In plants
Data were obtained from three new studies on the fate of
leptophos residues in apples, cabbage and tobacco carried out with
14C-phenoxy- and 14C-phenyl-leptophos. The results generally agreed
with those of the trials evaluated by the 1974 Joint Meeting.
In the experiment on apples it was shown that the parent
leptophos was the principal residue 6 and 14 days after treatment,
with approximately 10% of the extractable radioactivity as
leptophos-oxon. Less than 1% of 14C was unextractable from the apple
pulp. Fourteen days after treatment, about half of the total
14C-phenyl residue remained on the surface of the apples and up to
20% of this surface residue remained at the origin during TLC on
silica gel.
Only small mounts of 4-bromo-2,5-dichlorophenol, phenylphosphonic
acid, O-methyl phenylphosphonothioate and methyl phenylphosphonate
were extracted from the apple tissue (Whiteacre and Schnur, 1974).
TABLE 1. Nature of 14C compounds extracted from the surface of
apples treated with 14C-phenoxy and C-phenyl-leptophos
% of total surface radio carbon
14C-phenyl 14C-phenoxy
Metabolites Days after treatment Days after treatment
0 6 14 0 6 14
leptophos 98.6 80.0 77.8 98.4 89.6 92.3
leptophos oxon 1.4 1.7 2.0 1.4 1.0 1.5
4-bromo-2,5-dichlorophenol - - - <0.01 <0.01 <0.01
polar compounds
at origin of <0.01 18.2 20.2 1.6 9.0 6.2
TLC plate
Total 100.0 99.9 100.0 101.4 99.6 100.0
In the experiment on cabbage (Diaz, 1974) it was shown that on
this crop also, the parent leptophos is the principal residue 4, 7 and
14 days after treatment; only small amounts of leptophos oxon and free
4-bromo-2,5-dichlorophenol were present. A small amount of 4-bromo-2,
5-dichlorophenol was conjugated; no significant amounts of O-methyl
phenylphosphonothioate, methyl phenylphosphonate or phenylphosphonic
acid were found.
In soil
A new study was carried out on the behaviour and fate of
14C-phenoxy-and 14C-phenyl-leptophos in soil (Rieck, 1974), giving
additional information on the mount of "bound" residue (not
extractable with normal extraction procedures). Columns of a sandy
loam soil consisting of a one inch depth of treated soil on top of a
four inch depth of untreated soil were kept under aerobic and
anaerobic conditions and watered to 80% of field capacity. Two columns
were analysed initially and the others were watered with half inch
depth of water at weekly intervals and the leacheate collected. Soil
columns were analysed after 15, 30, 60, 90 and 120 days.
It was found (Table 2) that the bound radiocarbon (defined as
unextractable by 24 hours soxhlet extraction with 80% methanol)
increased with time at all soil depths. The radioactivity in the top
2.5 cm (treated) section decreased with time from 92% of the total to
42% after 120 days. The total 14C in the levels below this remained
fairly constant during the experiment.
TABLE 2. Distribution of total and bound radioactivity in aerobic
sandy loam soil
14C-leptophos equivalent on dry weight basis,*
found at depth
0 - 2-1/2 cm 2-1/2 - 7-1/2 cm 7-1/2 - 12-1/2 cm
Time
(days) total bound total bound total bound
0 9.16 0.02 0.23 0 0.05 0.0
15 8.33 0.39 0.06 0 0.14 0.02
30 6.33 0.69 0.13 0.06 0.04 0.03
60 5.24 0.98 0.19 0.09 0.09 0.05
90 4.75 1.47 0.20 0.14 0.07 0.07
120 4.19 1.84 0.19 0.14 0.04 0.04
* to calculate in terms of percentage of the amount applied, move
the decimal point one place to the right.
Under the conditions of the experiment the downward movement of
leptophos and its metabolites was insignificant. The only
14C-labelled compounds extractable from the lower layers were parent
leptophos, 4-bromo-2,5-dichlorophenol and methyl phenylphosphonate.
The total leacheate collected during the study contained only 0.46% of
the total activity applied to the upper soil layer.
In storage and processing
Sunflower and cotton seed
The nature and magnitude of residues in processed fractions of
sunflower seed were evaluated in three studies in Canada, In one study
the seed processed had a parent leptophos residue of 0.06 mg/kg. The
meal, crude oil and refined oil fractions showed residues of 0.03,
0.18 and 0.20 mg/kg parent compound respectively. No residues were
determined in the hulls, soapstock or finished oil. In another study
in which the crop was treated with 0.3 kg/ha the residue in the seed
was 0.19 mg/kg. No residues were measured in the hulls, meal, crude
oil, refined oil, finished oil or soapstock.
In a similar study in Australia on the processing of cotton seed,
in which the crop was treated with 0.9 kg/ha, after 78 days the level
in the seed was 6.33 mg/kg total leptophos residue. The following
residues were found in the processed fractions.
mg/kg mg/kg
seed 6.3 refined oil 32.4
hulls 2.62 finished oil 0.07
meal 0.13 soapstock 3.70
crude oil 40.0
Tea
Tea was brewed for 3-10 minutes according to a standard
procedure. In one study in which tea was treated with 1.3 kg
leptophos/ha and harvested and brewed (three minutes brew) on the same
day, the brew contained 0.05 mg/kg leptophos and 0.07 mg/kg of
4-bromo-2,5-dichlorophenol. In a brew of tea harvested 3-7 and 14 days
after treatment no leptophos or metabolites except up to 0.02 mg/kg of
the phenol were found. Sun-dried tea harvested at 0 and 7 days after
treatment and brewed for three minutes showed no residues of leptophos
or its metabolites, except 0.06 mg/kg of the phenol in the brew at day
0 and 0.01 mg/kg of the phenol in the brew after seven days.
TABLE 3. Leptophos: Residues, include the parent compound and the main metabolites, leptophos oxon and desbromo-leptophos.
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Fruit crops
Apple New Zealand 1972 11 46 g/100 l wp 45% 2.65 1.95 2.02 2.08 2.28 V143
1972 11 60 g/100 l wp 45% 3.71 4.16 1.89 1.66 1.83 V143
New Zealand 1972 11 46 g/100 l wp 45% 2.82 1.45 1.45 1.43 1.78 V144
1972 11 60 g/100 l wp 45% 2.69 1.56 2.23 1.35 1.69 V144
Australia 1971/ 8 60 g/100 l wp 45% 1.55 1.28 2.27 1.50 V144
(Tasmania) 1972
8 60 g/100 l wp 45% 2.52 4.28 2.90 3.80 V144
Australia 1972 8 60 g/100 l wp 45% 4.06-7.29 5.50-9.03 4.33-6.85 3.56-4.18 V144
(Tasmania)
Australia 1972 5 60 g/100 l wp 45% 0.51-2.54 1.77-1.82 V144
(NSW)
Australia 1970 5 90 g/100 l wp 45% 0.36-0.66 V73
(NSW) 1970 5 120 g/100 l wp 45% 0.65-0.93 V73
Australia 1971 4 120 g/100 l wp 45% 0.30-0.48 V173
(Tasmania)
6 120 g/100 l wp 45% 0.65-0.83 V173
Canada 1973 4 110 g/100 l wp 45% 1.51 V178
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Apple Canada 1973 3 72 g/100 l wp 45% 0.76 V178
(cont'd)
Canada 1973 4 2 kg/ha wp 40% 3.83 V178
rate
kg a.i./ha 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Vegetables
Beans Canada 1974 1 1 EC 32% <0.01 Braun
et al.,
1975
1 2 EC 32% <0.01 "
Broccoli Canada 1974 1 1 wp 45% 0.08 "
1 2 wp 45% 0.13 "
Cabbage Canada 1974 1 1 wp 45% <0.01 "
1 2 wp 45% <0.01 "
Canada 1974 8 0.6 EC 0.71 V 176
0.06
EC 0.23 0.15 V 190
Carrots Canada 1974 1 0.5 EC 32% 0.07 0.02 0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Carrots 1 1 EC 32% 0.10 0.03 0.02
(cont'd) 1 2 EC 32% 0.12 0.03 Braun
et al.,
1975
Canada 1974 2 0.5 EC 32% 0.16 0.06 0.13 Braun
et al.,
2 1 EC 32% 0.32 0.06 0.05 "
2 2 EC 32% 0.34 0.11 0.14 "
Canada 1974 1 1 EC 0.07 V 210
1 1 EC 0.05 V 210
1 2 EC 0.33 V 210
1 2 EC 0.07 V 210
Cauliflower Canada 1974 1 1 wp <0.01 Braun
et al.,
1975
1 2 wp <0.01 "
0/14 21/23 28/30 35/40 42/46 49/56 63/
Vegetables
Celery Canada 1974 1 0.5 wp 0.10 <0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Celery 1 1 wp 0.20 0.02 "
(cont'd)
1 2 wp 0.40 0.04 "
(Stalks) Canada 1974 1 1 EC 0.01 V 208
1 2 EC 0.02 V 208
1 1 EC <0.03 V 208
1 2 EC <0.03 V 208
Lettuce Canada 1974 1 0.5 EC 0.20 0.01 Braun
(outdoors) et al.,
1 1 EC 0.37 0.12 "
1 2 EC 0.25 0.04 "
Onions Canada 1974 1 0.5 EC 0.04 <0.01 "
(green)
1 1 EC 0.08 0.02 "
1 2 EC 0.27 0.07 "
2 0.5 EC 0.08 0.02 "
2 1 EC 0.27 0.03 "
2 2 EC 0.42 0.09 "
Peas
(Pods) Canada 1974 1 1 EC 0.01 "
2 EC 0.01 "
Canada 1974 2 1 EC 0.02 Braun
et al.,
2 EC 0.03 1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Peas
(cont'd)
(Vines) Canada 1974 1 1 EC 2.06 Braun
et al.,
1975
2 EC 1.10 "
2 1 EC 10.65 "
2 EC 7.48 "
Rutabaga Canada 1974 1 0.5 EC 0.01 <0.01 "
1 1 EC 0.01 0.01 "
1 2 EC 0.02 <0.01 "
2 0.5 EC <0.01 0.01 "
2 1 EC 0.04 0.02 "
2 2 EC 0.06 0.02 "
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage
Chinese Japan 1971 1 0.35 EC 34% 0.09-0.10 Miyagi,
1971
3 0.35 EC 34% 0.09-0.10
1 0.35 EC 34% 0.08-0.10
3 0.35 EC 34% 0.10-0.12
1 0.50 EC 34% 0.06-0.07
3 0.50 EC 34% 0.06-0.08
1 0.50 EC 34% 0.05-0.06
0.12-0.14
Japan 1970 1 0.35 EC 34% 0.08-0.09 Miyagi,
1971
3 0.35 EC 34% 0.09-0.09
1 0.35 EC 34% 0.09
3 0.35 EC 34% 0.08-0.10
1 0.5 EC 34%
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage 3 0.5 EC 34%
(cont'd) 1 0.5 EC 34%
3 0.5 EC 34%
Potatoes New Zealand 1972 1 0.44 wp 45% <0.05 <0.05 <0.05
Japan 1972 2 0.35 <0.005 <0.005 V 142
3 <0.005 <0.005
Residues in mg/kg, at intervals (days) after application
21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Field crops
Barley Canada 1973 1 0.5 2.17 V 173
Grain 0.64
Grain Canada 1973 1 0.5 0.11
Straw 0.5 0.12
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Grain Canada 1.0 0.14 V 155
Straw 1.0 0.62
Oats Canada 1973 1 0.5
Grain <0.01 V 159
Stubble <0.01
Wheat Canada 1973 1 0.5 0.37
Grain 1 0.36 0.03 V 173
Bran 0.04
Flour <0.01
Canada 1970 1 0.5
Grain <0.01 V 113
Straw 0.01
+ chaff
Canada 1970 1 1
Grain <0.01 V 113
Straw 0.17
+ chaff
Wheat Canada 1972 1 0.5
Grain 0.72 V 155
Straw 0.28
1972 1 1
Grain 0.24
Straw 1.32
Maize
Sweetcorn Canada 1974 1 1 EC
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/56 63/72 75/90 Ref.
Maize
(cont'd)
Cobs <0.01 <0.01 Braun
et al.,
Forrage <0.01 <0.01 1975
1 2 EC
Cobs <0.01 <0.01
Forrage <0.01 <0.01
2 1 EC
Cobs <0.01 <0.01
Forrage <0.01 0.31
2 2 EC
Cobs <0.01 <0.01
Forrage <0.01 0.02
28/33 75/92
Fieldcorn
forrage Canada 1974 1 0.5 EC 0.06 V 158
Forrage 1 1 EC 0.04
Sunflower Canada 1973 1 1 EC V 155
Hulls 0.01
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/33 35/40 42/46 49/56 63/72 75/92 Ref.
Sunflower Canada
(Cont'd)
Meal 0.03
Crude oil 0.18
Refined oil 0.20
Finished oil <0.01
Sunflower
seed Canada 1972 1 0.5 EC <0.01 V 174
Rape seed Canada 1973 1 0.5 EC 0.11 V 183
1 1 EC <0.01
0.5 EC 0.19 V 177
0.5 EC 0.22
0.25 EC 0.07
0.4 EC 0.04
Flax seed Canada 1973 1 0.5 0.09 0.07 V 174
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice Japan 1973 5 0.35 EC
Unhulled 0.20 Niigata,
1973
Straw 15.0-16.8
6 0.35 EC
Unhulled 0.21
Straw 15.9-16.7
6 0.35 EC
Unhulled 0.32
Straw 16.9-17.3
5 0.35 EC
Unhulled 0.11
Straw 22.4-23.3
6 0.35 EC
Unhulled 0.08
Straw 30.5-31.7
6 0.35 EC
Unhulled 0.03-0.05
Straw 6.15-8.33
5 0.8 dust 2%
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.11
Straw 7.20-7.80
Japan 1973 6 0.8 dust 2% Niigata,
1973
Unhulled 0.10
Straw 7.20-7.40
6 0.8 dust 2%
Unhulled 0.15-0.16
Straw 11.6-12.4
5 0.8 dust 2%
Unhulled 0.12
Straw 4.15-5.30
6 0.8 dust 2%
Unhulled 0.08-8.09
Straw 9.4-10.8
6 0.8 dust 2%
Unhulled 0.10
Straw 11.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 1.59-2.82
6 0.6-0.8 dust 2%
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.10
Straw 10.2-10.3
Japan 1973 6 0.6-0.8 dust 2% Niigata,
1973
Unhulled 0.20
Straw 13.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 11.2-11.9
6 0.6-0.8 dust 2%
Unhulled 0.07-0.08
Straw 10.3-10.9
6 0.6-0.8 dust 2%
Unhulled 0.09-0.10
Straw 19.3-19.7
Tea (dry
manuf.) India 1973 2 1.3 EC 93.4 1.77 3.57 0.29 V 203
Assam
*1973 2 1.3 EC 77.3 0.11 3.67 0.23
*1973 1 1.3 EC 2.80-3.77
1973 1 1.3 EC 1.36-1.94
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet Canada 1973 1 0.5 EC
Roots) 0.05 0.05 V 170
)
Tops ) 0.09 0.07 V 170
Roots 1973 2 0.5 EC <0.02
Roots 2 1.0 EC <0.02
Roots 2 1.0 EC <0.02
Roots) 1 1.0 EC <0.02 0.04
Tops ) <0.02 <0.02
Japan 1972 3 0.35 EC Hokkaido,
Roots <0.02 <0.02 exp. sta.
1972
Tops 0.43-0.59 0.17-0.30
1972 5 0.35
Roots 0.01 <0.01
Tops 1.36-1.72 0.66-0.84
1972 3 0.35
Roots <0.01 <0.01
Tops 0.59-0.61 0.25-0.27
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet
(cont'd)
1972 5 0.35
Roots 0.01 <0.01
Tops 0.93-1.02 0.34-0.39
Japan 1971 3 0.35 Hokkaido,
Roots <0.01 <0.01 exp. sta.
1972
Tops 0.59-0.66 0.34-0.39
5 0.35
Roots 0.01 0.01
Tops 1.75-1.87 0.93-0.98
1971 3 0.35
Roots <0.01 <0.01
Tops 0.64-0.69 0.26-0.32
5 0.35
Roots <0.01 <0.01
Tops 1.17-1.20 0.39-0.46
* Sun-dried.
In a 10 minutes brew of sun-dried tea harvested seven days after
treatment no residues of parent leptophos, leptophos-oxon or
desbromoleptophos was found. The levels of the phenol ranged between
<0.01 mg/kg and 0.06 mg/kg.
In a food chain
Data were obtained in two model studies (Anonymous, 1973;
Johnson, 1975) with 14C-leptophos in a simple food chain in which
water, Daphnia magna and the common blue gill (Lepomis macrochirus
rafinesque) were included.
Daphnia magna accumulated leptophos rather rapidly from the
water. In less than four hours leptophos in daphnia reached a constant
level about 1500 times that in the surrounding water. Blue gills
feeding on Daphnia magna exposed to leptophos reached a plateau about
1/10 of that in the daphnia.
It is obvious that the most significant route of accumulation of
leptophos residues by fish will be by direct uptake from water.
Blue gills exposed to leptophos in water only (not in the food)
contained mean 14C residues in the tissues of 0.179 mg/kg, about 750
times the mean concentration of 14C-leptophos in the water, after
10-42 days' exposure. When blue gills were exposed to both aqueous and
dietary 14C-leptophos for 10-28 days the residue level was almost
identical (0.170 mg/kg, about 770 times the concentration in the
water).
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours. The bio-transfer
rate of leptophos in the Daphnia-blue gill food chain was only 6.5% of
that of DDT, a typical bioaccumulating compound.
APPRAISAL
Leptophos was evaluated with regard to agricultural data by the
1974 Joint Meeting (FAO/WHO,1975). Arising from the list of
requirements, information was obtained on residues from supervised
trials in various areas on fruit crops (apples and pears), small
grains, rice, sugar beet, potatoes and on some vegetable crops.
Some information became available on residues in waste of
agricultural crops which may be used for feeding purposes, e.g. sugar
beet leaves, straw and chaff of cereal crops, including rice.
Additional data were obtained on the fate of leptophos residues
in apples, cabbage, lettuce and tomatoes from studies with 14C-phenyl
and 14C-phenoxy-labelled leptophos. Additional data became available
on the fate of 14C-phenoxy- and 14C-phenyl-labelled leptophos in
soil. It was shown in this study that the bound fraction increases
with time at all soil depths. The total 14C in the upper 2.5 cm layer
decreased with time from 92% of the applied radioactivity to 42% after
120 days; the total 14C in the lower layers was fairly constant
during that period.
New data were obtained from model studies with 14C-labelled
leptophos in a simple food chain which included water, Daphnia magna
and the common blue gill. Daphnia magna accumulated leptophos rather
rapidly from the water; in less than four hours leptophos plateaued in
Daphnia to levels about 1500 times the level in the surrounding water.
Blue gills feeding on Daphnia magna exposed to leptophos reached a
plateau one-tenth of that of the daphnids.
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours.
The bio-transfer rate of leptophos in the Daphnia-blue gill food
chain was less than one-tenth of that of DDT, which is recognized as a
bioaccumulating compound.
The new data available confirm the limits recorded at the 1974
Joint Meeting on cabbage, broccoli, lettuce, tomatoes, potatoes,
cotton seed and maize.
In the light of the temporary ADI established at the present
Meeting these limits are now recommended as temporary maximum residue
limits, and replace the guideline levels recorded in the 1974
monograph. Additional temporary maximum residue limits are
recommended.
Recommendations
The maximum residue limits refer to the sum of leptophos, its
oxygen analogue and desbromo-leptophos, expressed as leptophos.
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Apples, pears 2 42-60
Broccoli, Brussels sprouts,
cabbage 2 28-50
Lettuce 2 28 (outdoor)
Tomatoes 2 7
Cotton seed oil (crude) 1 28
Raw cereals (wheat, oats,
barley) 0.5 60
Rice (hulled) 0.5 30
(Cont'd)
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Cotton seed, cotton seed meal 0.2 28
Carrots 0.2 60
Potatoes 0.1 21
Maize, fieldcorn (kernels) 0.05 7
Sweet corn (kernels and cobs,
husks and silk removed) 0.05 3-7
Sunflower seed 0.05 100
Sugar beet (roots) 0.05 50-60
Sugar beet (tops) 0.5
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1978)
1. Studies on the kinetics of accumulation of leptophos in storage
depots of two species, preferably non-rodent to investigate the
potential buildup in man to ultimate threshold levels.
2. Studies on the species variation for delayed neurotoxicity with
leptophos to assess its ultimate effect on man.
3. Epidemiological studies on people in exposed occupations in
agriculture or industry.
4. Further long-term low-level feeding studies in a species
susceptible to the delayed neurotoxic syndrome.
5. Residue data on the major crops for which national tolerances or
use recommendations exist, e.g., tea, vegetables not included in the
recommendations, and citrus fruits.
6. Residues in those parts of agricultural commodities which are
used either as such or as agricultural waste for animal feed.
DESIRABLE
1. Improved methodology for assessing the delayed neurotoxic
syndrome.
2. Studies on the mechanism of neurotoxic action of leptophos.
Further investigations into mechanism of action of TOCP and other
neurotoxic agents as compared to leptophos.
3. Data on current use patterns in countries outside the United
States of America and Canada and on residue levels resulting from
those uses.
REFERENCES
Anonymous. (1973) Exposure of 14C-leptophos to daphnia magna and
bluegill sunfish (lepomis macrochiris). Accumulation, distribution,
elimination and bio-concentration in the food chain. Unpublished
report from Bionomics, Inc., submitted to FAO by Velsicol Chemical
Corporation.
Abou-Donia, M.B., Othman, M.A., Tantawy, G., Khalil, A.Z. and Shawer,
M.F. (1974) Neurotoxic effect of leptophos. Experimentia, 30:63-64.
Abou-Donia, M.B. and Pressig, S. (1975) Studies on delayed
neurotoxicity produced by leptophos. Proc. Fed. Amer. Soc. Exptl.
Biol., 34:810.
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Phosvel (VCS-506) in albino mice. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Badie, M. and Whitacre, D.M. (1975) Metabolism of 14C-leptophos and
14C-4-bromo-2,5-dichlorophenol in rats: A multiple dosing study.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Braun, E., McEwen, F.L., Frank, R. and Ritcey, G. (1975) Residues of
leptophos and its metabolites following application to various crop
plants. J. Agr. Food Chem., 23(1):90-95.
Braun, E., McEwen, F.L., and Frank, R. (1974) Residues of
chlorpyriphos and leptophos in three field tested vegetable crops.
Canad. J. Plant Sci., 55:133-137.
Diaz, L.I. (1974) Radiotracer study of leptophos on cabbage. Velsicol
Chem. Corp. Lab. rep. 194. (Unpublished)
Dorough, H.W. (1974) Fate of phosvel-14C in burley tobacco. Progress
report of Department of Entomology, University of Kentucky, Lexington,
provided to Velsicol. (Unpublished)
Fink, R.J. (1971) Continuous feeding and residue study - cows with
technical Phosvel (VCS-506). Unpublished report from Hazleton
Laboratories, submitted to the World Health Organization by Velsicol
Chemical Corporation.
Fletcher, D., Jenkins, D.H., Kinoshita, P.R., Gordon, D.E. and
Keplinger, M.L. (1975) Neurotoxicity study with technical leptophos in
chickens. Unpublished report from Industrial Bio-Test Laboratories,
Inc., submitted to the World Health Organization by Velsicol Chemical
Corporation.
Haley, S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1972) Three
generation reproduction study with VCS-506 in albino rats. Unpublished
report by Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Haley, S., Kennedy, G.L. and Keplinger, M.L. (1973) Three-generation
reproduction study with VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Hartke, K., Lindberg, D.C., Wright, P.L. and Keplinger, M.L. (1971)
Two-year chronic oral toxicity study with VCS-506 technical in beagle
dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Hassan, A. (1975) Personal communication to the World Health
Organization.
Hathaway, D., Keplinger, M.L. and Fancher, O.E. (1968) Acute aerosol
inhalation toxicity study on VCS-506, 3 EC. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Hokkaido. (1972) Phosphel residue analysis on sugar beet. National
Hokkaido. Agr. Exp. Sta. Residue data provided to Velsicol.
(Unpublished)
Holmstead, R.L., Fukuto, T.R. and March, R.B. (1973) The metabolism of
O-(4-bromo-2,5-dichlorophenyl) O-methyl phenylphosphonothioate
(leptophos) in white mice and cotton plants. Archives of Environmental
Contamination and Toxicology. Vol. 1, No. 2, 133-147.
Johnson, B.T. (1973) Leptophos accumulation through a simple food
chain. Unpublished report from the United States Department of the
Interior, submitted to the World Health organization by Velsicol
Chemical Corporation.
Johnson, M.K. (1975) The delayed neuropathy caused by some
organophosphorus esters: Mechanism and challenge. CRC Critical Reviews
in Toxicology, June.
Kennedy, G. and Keplinger, M.L. (1971) Absorption, distribution, and
excretion study with Phosvel in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1971a) Characterization of 14C in
excreta of rats following oral administration of Phosvel. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1972) Absorption, distribution, and
excretion study with VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970) Distribution and
excretion study of VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970a)
Characterization of 14C in urine of rats following oral
administration of 14C-VCS-506. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kimmerle, G. (1972) Acute neurotoxicity studies in hens. Unpublished
report from Farbenfabriken Bayer, submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1971) Study of the
efficacy of atropine sulfate and 2-PAM as antidotes for VCS-506
intoxication in albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C., Keplinger, M.L. and Fancher, O.E. (1971a)
Acute oral (intragastric) toxicity studies with VCS-506 in one day old
and five day old albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1972) Acute oral
toxicity study with oxon of VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Kretchmar, B,, Mastri, C. and Keplinger, M.L. (1972a) Acute oral range
finding toxicity study with S-isomeride in female albino rats.
Unpublished report from industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L. (1971)
Teratogenic study with Phosvel in albino rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health organization by Velsicol Chemical Corporation.
Lindburg, D., Vondruska, J.F. and Fancher, O.E. (1969) Ninety-day
subacute oral toxicity of VCS-506 in beagle dogs. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
March, R.B. and Fukuto, T.R. (1975) Reports on studies on the
photolysis and metabolism of Phosvel. Unpublished report from the
University of California, submitted to the World Health Organization
by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969) Acute oral
toxicity study on VCS-506 technical in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L, and Fancher, O.E. (1969a) Acute oral
toxicity study on two formulations of VCS-506 E.C. in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity on four metabolites of VCS-506 in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on phenylphosphonic acid in albino rats. Unpublished
report from industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Miyagi. (1971) Phosphel residues on chinese cabbage. Miyagi Pref. Agr.
Exp. Sta. and Aichi Pref. Agr. Exp. Sta. Residues data provided to
Velsicol.
Niigata. (1973) Phosphel residue analysis on rice. Niigata Pref. Agr.
Exp. Sta. and Shizuoka Pref. Agr. Exp. Sta. Data provided to Velsicol.
(Unpublished)
Perkins, C.T. and Singh, V.K. (1971) Chicken toxicity and residue
study. Unpublished report from Bio/Toxicological Research
Laboratories, Inc., submitted to the World Health organization by
Velsicol Chemical Corporation.
Perkins, C.T. and Singh, V.K. (1971a) Beef animal feeding study.
Unpublished report from Bio/Toxicological Research Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J., Keplinger, M.L. and Fancher, O.E. (1970) Cholinesterase
study of VCS-506 in albino rats. Unpublished report from International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971) Twenty-eight day
target organ study with VCS-506 oxon metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971a) Twenty-eight
day target organ study with VCS-506 phenol metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971b)
Ninety-day subacute oral toxicity, study with VCS-506 Phenol
metabolite in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971c)
Ninety-day subacute oral toxicity study with VCS-506 oxon metabolite
in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Rieck, C.E. (1974) Soil metabolism of 14C-leptophos. Report of Agr.
Dept., University of Kentucky, Lexington, provided to Velsicol.
(Unpublished)
Sanborn, A. and Metcalf, R.L. (1975) The fate of leptophos (Phosvel)
in a terrestrial-aquatic ecosystem. Unpublished report from the
University of Illinois submitted to the World Health Organization by
Velsicol Chemical Corporation.
Schroeder, C. (1971) The radiocarbon distribution of 14C labelled
Phosvel in lettuce and green beans. Unpublished report from WARF
Institute submitted to the World Health Organization by Velsicol
Chemical Corporation.
Schwemmer, B. (1971) UV photolysis of leptophos in solution.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Sleight, B.H. and Macek, K.J. (1973) Exposure of 14C-leptophos to
Daphnia magna and bluegill sunfish (Lepomis macrochirus):
Accumulation, distribution, elimination, and bioconcentration in the
food chain. Unpublished report from Bionomics, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971)
Twenty-eight day cholinesterase study with VCS-506 oxon metabolite in
female albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc. submitted to the World Health Organization by
Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971a)
Two-year chronic oral toxicity study with VCS-506 technical in albino
rats. Unpublished report from Industrial Bio-Test Laboratories, Inc.
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Smith, P.S., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. (1973)
Eighteen-month carcinogenic study with VCS-506 technical in Swiss
white mice. Unpublished report from Industrial Bio-Test Laboratories,
Inc. submitted to the World Health Organization by Velsicol Chemical
Corporation.
Stephens, J.L., Jenkins, D.H. and Fancher, O.E. (1969) Demyelination
study-chickens, VCS-506 Technical. Unpublished report from industrial
Bio-Test Laboratories, Inc. submitted to the World Health Organization
by Velsicol Chemical Corporation.
Suzuki, H. (1971) Phosvel-chicken feeding study. Unpublished report
from Velsicol Chemical Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Suzuki, H. (1971a) Phosvel-dairy cattle feeding study. Unpublished
report from Velsicol Chemical Corporation submitted to the World
Health Organization by Velsicol Chemical Corporation.
Tarka, S.M. Jr. (1973) Identification of radioactivity in the fat of
rats administered 14C-phenoxy-labelled leptophos. Unpublished report
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1965) Acute oral toxicity (LD50) in the albino rat.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1966) Acute oral toxicity (LD50) in male albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968) Comparative acute oral toxicity in male albino
rats. Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968a) Acute toxicity studies in the albino rabbit.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968b) Acute toxicity study in the rabbit. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1968c) Ten-day subacute oral toxicity study in rats for
analysis of plasma, erythrocyte and brain cholinesterase activity.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1969) Ninety-day feeding study in albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970a) Acute oral toxicity (LD50) in the male albino
rat. Unpublished report from international Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970b) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971a) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971b) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971c) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971d) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (197le) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971f) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971g) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971h) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971i) Acute toxicity studies in rats and rabbits.
Unpublished study by International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1972) Acute oral toxicity (LD50)
in male and female albino rats. Unpublished report by International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1973) Acute dermal toxicity
(LD50) in male and female albino rabbits. Unpublished report from
International Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Whitacre, D.M. and Schnur, P.D. (1974) Metabolic fate of 14C-labelled
leptophos on apples. Velsicol Chem. Corp. Lab. rep. No. 195.
(Unpublished)
 In further studies of the biomagnification of leptophos, it was
observed that an apparent equilibrium was established in fish exposed
to leptophos for periods of time. After about one week of exposure
during which time leptophos accumulates there is no additional
build-up in tissues. These data also suggest that ingestion of
leptophos - contaminated invertebrates by fish does not increase the
fish residue suggesting that biomagnification is not a significant
factor (Sleight and Macek, 1973; Johnson, 1973).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Leptophos oxon
Rat
Groups of rats (25 males and 25 females/group) were subdivided
into two categories: individually housed (10 rats of each sex per
group) and group housed (15 rats of each sex per group). These groups
were fed a control diet and one containing leptophos oxon for 28 days.
The leptophos oxon diet was increased at weekly intervals from 200 ppm
to 300 ppm to 500 ppm and finally to 800 ppm. Growth (as evidenced by
body weight) was reduced in the latter stages of the test in females
while food consumption was normal. Spleen weight in males and females
was reduced at 7, 21 and 28 days. Cholinesterase activity of RBC and
brain was depressed at all test intervals. Plasma cholinesterase was
depressed in females at all intervals while in males the depression
became evident after 14 days. No effects were noted on survival,
haematology, blood chemistry, urinalyses or on microscopic examination
of tissues and organs (Plank et al., 1971).
Groups of female rats (15 rats/group) were fed leptophos oxon for
four weeks at dose levels of 0, 1, 5, 10 and 20 ppm. Measurements of
red blood cell and plasma cholinesterase activity were made at 14 and
28 days. Plasma cholinesterase was unaffected while erythrocyte
cholinesterase was depressed at 10 ppm and above at 14 days. At the 28
day interval reduction of activity was not significant (Smith et al.,
1971).
Groups of rats (15 males and 15 females/group) were fed leptophos
oxon for 90 days at dose levels of 0, 25, 50 and 500 ppm. Inhibition
of plasma cholinesterase activity was noted at all dose levels in
females while males were affected at 500 ppm only at the 84 day test
interval. Inhibition of erythrocyte and brain cholinesterase activity
was similar in both sexes and was notable only at 500 ppm. No effects
were noted on mortality, growth, food consumption, haematology
parameters, clinical chemistry parameters, urinalyses, and on gross
and microscopic examination of organs and tissues (Plank et al.
1971c).
Leptophos phenol
Rat
Groups of rats (25 of each sex/group) were divided into two
subgroups and housed individually (10 of each sex/group) or housed
together (15 of each sex/group). The sexes were not mixed. Two dietary
groups were used; a control and a test group which received a dietary
level of leptophos phenol of 3500 ppm for one week. This was increased
to 5000 ppm for the second week, to 8000 ppm for the third week and
finally to 13 000 ppm for the fourth week. There were no effects on
food consumption, growth, haematology, blood chemistry including
cholinesterase or on growth and microscopic examination of tissues and
organs (Plank et al,, 1971a).
Groups of rats (15 males and 15 females/group) were fed leptophos
phenol at dietary levels of 0, 300, 1000 and 10 000 ppm (3000 ppm for
days 1-49) for 90 days. At 10 000 ppm there was a significant increase
in liver weight in females. In males a decreased spleen weight was
noted at all doses tested (none was significantly different from
control values although the decrease was evident in ratios calculated
from body and brain weight data). No effects were observed on
survival, food consumption, growth, haematology, blood chemistry,
urinalyses or on gross or microscopic examination of tissues or organs
(other than the gross effects on liver and spleen). A report of the
microscopic examination of liver and spleen indicated no abnormalities
(Plank et al., 1971b).
Special studies on mutagenicity
Mouse
A dominant lethal study was conducted where groups of male mice
(8 mice/group) were administered a single oral or intraperitoneal dose
of 0, 15 or 30 mg leptophos/kg. The males were mated with three
females per week for six weeks during the normal period of
spermatogenesis. Positive control studies were performed with methyl
methane-sulfonate. There were no effects of leptophos on mating
performance or on reproduction parameters including preimplantation
loss, early resorption or embryo viability. Leptophos under the
conditions of this test did not induce mutagenic changes in male
germinal cells (Arnold et al., 1971).
Special studies on neurotoxicity
Chickens
Groups of white leghorn hens (6 hens/group) were orally
administered leptophos at doses of 100, 200 and 400 mg/kg on day 0 and
14. Observations on clinical conditions were made and at the
conclusion of the study (day 28) survivors were sacrificed and nervous
tissue examined microscopically for indications of myelin disruption.
No clinical signs of delayed neurotoxicity were noted in this
study following the first dosing. Three hens of six tested at the high
level died 6-14 days after the second dose. These hens had lost a
considerable portion of their body weight before death. At 200 mg/kg
one of six died and one other showed clinical signs of ataxia.
Microscopic examination of H & E and Luxol Fast Blue stained sections
of nerve did not reveal myelin degeneration (Stephens et al., 1969).
Groups of white leghorn hens (10 hens/group) were administered
leptophos orally at doses of 0, 5, 10, 15, 30, 50, 75, 100 and 200
mg/kg at days 0 and 21. A positive control was also used in this study
(Tri-o-Cresyl phosphate, 500 mg/kg - orally at day 0). A number of
animals showing clinical signs of ataxia were sacrificed at day 42.
Gross and microscopic examination of brain, spinal cord and sciatic
nerves were performed. Examinations were made of H & E stained
sections and tissues embedded in ParaplastR and stained with Luxol
Fast Blue - Holmes Silver Nitrate. Both light and electron microscopic
examinations were made in this study.
A positive delayed neurological condition was clinically evident
in this study with both TOCP and leptophos. Loss of body weight is
generally observed clinically in animals with delayed peripheral
ataxia and this was observed with the TOCP group in 6 of 10 hens
weighed at day 18. In the leptophos groups, weight loss was noted in 1
of 10 hens at 75 mg/kg, in 3 of 10 at 100 mg/kg, in 5 of 10 hens at
200 mg/kg. This weight loss was accompanied by signs of peripheral
neuritis in various hens of the three upper group levels (3 hens at 75
mg/kg, 5 hens at 100 mg/kg and the majority of hens at 200 mg/kg).
Light and/or electron microscopic sections of nerve tissue and muscle
preparations indicated retrograde degeneration of 8 of 10 TOCP hens
and 5 of 10 hens at 200 mg/kg. No other positive signs of degeneration
were observed. At acute oral dose levels of 75 mg/kg and above,
leptophos was shown to induce a clinical neuropathy in hens (Fletcher
et al., 1975).
Other studies have confirmed that, at high acute oral doses,
leptophos will induce delayed neurotoxicity and clinical signs of
neuropathy (Abou-Donia et al., 1974; Abou-Donia and Preissig, 1975;
Kimmerle, 1972). In male chickens, 2 of 9 tested were ataxic between
9-13 days after oral dosing with 180 mg/kg. No neurotoxic effects were
noted at dose levels below 180 mg/kg. Further studies showed that 200
mg/kg and above resulted in ataxia. A 30% formulation of emulsifiable
concentrate administered to hens by oral or IV injection resulted in
positive clinical neuropathy at dose levels above 100 mg/kg.
Special studies on reproduction
Rat
In two studies, groups of rats (8 males and 16 females/group
there - were two control groups of 8 males and 16 females each) were
fed leptophos in the diet at levels of 0, 10, 30, 40 and 60 ppm and
subjected to a standard three generation, two litter per generation
reproduction study. After two litters of the first generation were
born, the 60 ppm level was discontinued because of pup mortality. A
second study was initiated at 0 and 5 ppm. The 5 ppm dose was changed
after one generation to the 40 ppm level (noted above) and maintained
at this level for two further generations.
Parental body weight and reproductive performance was similar in
the 60 ppm group to the control group. Reproductive indices, including
mating, pregnancy, fertility and parturition showed no differences
with respect to control and all treated rats. Survival data on pups
including viability and lactation indices were significantly decreased
at 60 ppm but were similar to control values at 40, 30 and 10 ppm.
Mean body weight data for pups at weaning at all dose levels
(including 60 ppm) were similar. A no-effect level for rat
reproduction is 30 ppm (Haley et al., 1973, 1972).
Special studies on teratogenecity
Rabbit
Groups of pregnant New Zealand rabbits (10-13 rabbits per group)
were administered leptophos orally from day 6 to day 18 of gestation
at dose levels of 0, 1 and 3 mg/kg. Thalidomide was administered over
this same period at a dose of 37.5 mg/kg to a group of rabbits
designated as a positive control. No effects were noted on the growth
of does. Skeletal or somatic abnormalities were noted with
thalidomide. Leptophos at a dose of 3 mg/kg administered orally to
rabbits during organogenesis elicited no teratological response (Ladd
et al., 1971).
Signs of poisoning are typical of acute parasympathomimetic
stimulation seen by other anticholinesterase organophosphates. These
include: hypoactivity, tremors, muscular weakness, ruffed fur,
diarrhoea, exophthalmia, haemorrhagic conjunctivitis and salivation.
Acute Toxicity
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M Tech (white mass) Oral 31.6 Wazeter, 1965
M Tech (white powder) Oral 59.0 " 1966
M Tech (98.5% white powder) Oral 54.5 " 1968
M Tech (90% brown granule) Oral 37.1 " 1968a
M Tech (99.6% white powder) Oral 56.6 " 1971
M Tech (90% white crystal) Oral 44.7 " 1970
M Tech (90% grey granule) Oral 52.8 " 1971a
M & F Tech (94%) Oral 20.0 Kretchmar et al., 1971
M & F Tech (90%) Oral 90.5 Mastri et al., 1969
M & F (1 day old) Tech 90% Oral 12.0 Kretchmar et al., 1971a
M & F (5 day old) Tech 90% Oral 15.0 Kretchmar et al., 1971a
F Tech (98.5% white powder) Oral 24.3 Wazeter, 1968
F Tech (90% brown granule) Oral 26.1 " 1968
F Tech (99.6% white powder) Oral 40.1 " 1971
F Tech (90% grey granule) Oral 42.9 " 1971a
Rabbit M & F Tech (90% white crystal) Dermal 10 000 " 1970
M & F Tech (90% brown powder) Dermal 800 " 1968a
" 1968b
Chicken F Tech Oral 225 Fletcher et al., 1975
Technical leptophos is an eye irritant when instilled into the conjuctival sac of rabbits (Wazeter, 1968b, 1970).
Leptophos is not an irritant when tested on normal or abraded rabbit skin (Wazeter, 1970).
Acute toxicity formulations
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M 3 EC (yellow oil) Oral 271 Wazeter, 1970a
M 30 EC (yellow oil) Oral 271 " 1970a
M 29.5 EC Oral 400 Mastri et al., 1969a
M 35.7 EC Oral 735 " 1969a
M 5 G Oral 2 263 Wazeter, 1971b
M 50 WP (grey powder) Oral 121 " 1971c
M 2 EC (yellow liquid) Oral 178 " 1971d
M 3 EC (34.7%) Oral 121 " 1971e
M 3% Dust (grey dust) Oral 1 780 " 1971i
M 3 ULV (32.1%) Oral 192 " 1970b
M 5 G Inhal 26.9 mg/L Wazeter, 1971b
M 3 ULV (32.1%) Inhal 200 mg/L " 1970b
M & F 3 EC 4 hr
Inhal 2.7 mg/L Hathaway et al., 1968
M 3% dust 4 hr
Inhal 33 mg/L Wazeter, 1971i
M 50 WP Inhal 19 mg/L " 1971c
M 2 EC Inhal 200 mg/L " 1971d
M 3 EC Inhal 200 mg/L " 197le
F 29.5 EC Oral 218 mg/L Mastri et al., 1969a
F 36.7 EC Oral 327 mg/L " 1969a
Rabbit M & F 2.7 EC Dermal 2 000 Wazeter and Goldenthal, 1973
Leptophos formulations are an eye irritant when instilled into the conjunctival sac (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i). However, one
formulation tested (3 ULV - 32.1% leptophos) was not an eye irritant (Wazeter, 1970b). Leptophos fomulations are not a skin irritant when tested
on normal or abraded rabbit skin (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i).
Acute toxicity - metabolites and possible contaminants
LD50
Compound Species Sex Route (mg/kg) Reference
Leptophos oxon Rat M & F Oral 119 Mastri et al., 1969b
Rat M & F Oral 43 Kretchmar et al., 1972
Phenylphosphonic
acid Rat M Oral 2 480 Mastri et al., 1969b
2 241 " 1969c
S-methyl isomeride Rat F Oral 735 Kretchmar et al., 1972a
O.O-bis
(2,5-di-chloro-4-brom-phenyl)
phenylphosphono-thioate Rat M Oral 4 640 Wazeter, 1971f
O.O-dimethyl
phenyl-phos-phonothioate Rat M Oral 287 " 1971g
O-methyl phenyl
phosphonic acid Rat M Oral 3 690 " 1971h
O-(2,3-dichloro-4-bromophenyl) Rat M Oral 59.5 Wazeter and Goldenthal, 1972
phenylphos-phonothioate Rat F Oral 37.5 Wazeter and Goldenthal, 1972
Leptophos phenol Rat M & F Oral 2 654 Mastri et al., 1969b
Antidotal studies
Administration of atropine sulfate and 2-PAM alone and in
combination resulted in an increase in the LD50 value suggesting that
these agents might be successful in antidotal therapy (Kretchmar et
al., 1971). The combination of atropine and 2-PAM was no more
successful than the administration of either agent alone.
Antidote LD50 (mg/kg) (95% C.L.)
None 20.0 (11.8-34.0)
Atropine Sulfate (IM)
10 mg/kg 1.5 & 5 hr
after poisoning 54.0 (41.2-70.7)
2-PAM
1.5 hr after poisoning 38.5 (33.8-43.9)
Atropine + 2-PAM 40.0 (27.6-58.0)
Short-term studies
Rat
Groups of rats (2 males and 2 females/group) were administered
leptophos by oral intubation of 10 days at dose levels equivalent to
dietary intakes of 0, 3, 10, 30 and 100 ppm. Mortality was evident at
100 ppm. At the conclusion of the study the animals were sacrificed
and cholinesterase determinations run using brain, plasma and red
blood cells.
No depression of plasma cholinesterase was noted. The RBC and
brain cholinesterase activity was depressed at 30 ppm and above in all
surviving animals (Wazeter, 1968c).
Groups of rats (10 males and 10 females/group) were fed dietary
levels of leptophos of 0, 100, 250 and 500 ppm for 28 days. A second
series of female animals (10 rats/group) were fed levels of 0, 50 and
75 ppm for 14 days in a cholinesterase depression range-finding test.
A dose-dependent depression of cholinesterase activity was
observed in all tissues at all dose intervals and times tested in the
initial high level study. Activity of the most susceptible
cholinesterase source (female RBC) was slightly depressed 32% and 22%
at 75 and 500 ppm respectively after seven days of feeding. The plasma
cholinesterase activity in the initial study was not inhibited until
the two week test interval after which it steadily declined (Plank, et
al., 1970).
Groups of rats (10 male and 10 female rats/group were fed
leptophos in the diet at dose levels of 0, 1, 5 and 10 ppm for 90
days. Growth, food consumption and behaviour were not affected by
leptophos. No changes were noted in haematology, blood, clinical
chemistry or urinalysis parameters measured. Cholinesterase activity
in RBC, plasma and brain were normal at 90 days of testing as were the
40 day examinations of RBC and plasma cholinesterase. Gross and
microscopic examination of tissues and organs showed no
leptophos-related lesions (Wazeter, 1969).
Dog
Groups of dogs (four male and four female beagle dogs/group) were
fed leptophos in the diet for 90 days at dose levels of 0, 10 and 30
ppm. Behaviour, growth and food consumption were not affected over
this test interval. No effects were noted on haematology parameters on
blood, clinical chemistry on urinalyses or on gross and microscopic
analyses of tissues and organs. A no-effect level was 30 ppm (Lindberg
et al., 1969).
Groups of beagle dogs (4 males and 4 female/group) were fed
leptophos in the diet at dosage levels of 0, 10, 20, 30 and 60 ppm for
two years (the 60 ppm group had been fed a diet containing 5 ppm for
180 days which was then increased to 60 ppm for the remainder of the
study. No mortality occurred over the course of the study and growth,
food consumption and behaviour was normal at all levels. No
leptophos-related effects were noted on haematology parameters,
clinical chemistry values (including blood and brain cholinesterase
values) or on gross and microscopic examination of tissues and organs.
Leptophos in the diet at 60 ppm had no effect on any parameter
recorded in this study (Hartke et al., 1971).
Chicken
Groups of white leghorn chickens (4 male and 20 females/group)
were fed leptophos in the diet at dosage levels of 0, 0.03, 0,10, and
0.30 ppm for four weeks. Eggs were collected and incubated during the
latter 14 days of treatment. The chicks were observed for 14 days
post-hatching having been fed the leptophos diet corresponding to the
parent. There were no treatment related differences in body weight or
egg production and size of eggs. Egg hatchability, chick viability,
and growth was not affected by leptophos. No abnormal behaviour or
signs of poisoning were observed in this study in either adults or
chicks (Perkins and Singh, 1971). Analysis of tissue residues
(including eggs and adipose tissue) did not indicate the presence of
residues of leptophos, the oxon, the phenol or a photo-product
(Suzuki, 1971).
Steer
Groups of steers (three steers/each treatment group and one
steer/control group) were fed leptophos in the diet at levels of 0,
15, 45 and 150 ppm for four weeks. At 28 days the control and two each
of the treatment groups were sacrificed. The remainder were allowed to
consume normal untreated food for a further 14 days after which they
were sacrificed. Daily observations showed no abnormal behaviour or
toxic signs of poisoning. No gross or microscopic lesions were
observed at the conclusion of the study. Depression of the whole blood
and plasma cholinesterase was evident at the higher dose levels over
the 28 day feeding interval. After seven days on a control ration the
depressed values returned to normal. Tissue residues of leptophos and
the oxon were low in liver, kidney, muscle and brain. In fat,
leptophos was present after 28 days in measurable levels which were
dose dependent. Following the 14 day recovery intervals, these
residues in fat were considerably reduced (Perkins and Singh, 1971a).
Cow
Groups of lactating dairy cows (three cows/group fed leptophos
and one cow/control group) were fed leptophos in the diet at
calculated dose levels of 0, 5, 15 and 50 ppm (actual values fed as
assessed by chemical analysis were 0, 3.2, 10.0 and 37.4 ppm) for 28
days and maintained for a further 14 days on control diets to measure
residue reductions and recovery of normal values. Appearance,
behaviour and general condition were normal over the study. Body
weights of several animals tested with leptophos were reduced over the
study. Milk production was reduced in the low and intermediate group
but not at the high level of leptophos. Cholinesterase depression was
not noted in this study (Fink, 1971). Traces of leptophos were noted
in fat at the conclusion of the 28 day feeding interval at levels
ranging from 0.2 to 0.5 ppm in the high (37.4 ppm) dose level. After
two weeks, these residues were reduced to 0.2 ppm (Suzuki, 1971a).
Long-term studies
Mouse
Groups of Swiss white mice (65 males and 65 females/group) were
fed leptophos in the diet for 18 months at dose levels of 0, 50, 100
ppm to assess a carcinogenic potential in mice. Two positive control
groups were fed N-nitrosodiethylamine at levels of 10 ppm (50 males
and 50 females) and 40 ppm (15 males and 15 females). After six
months, the mice of the high positive control group and 15 of each sex
in the leptophos group were sacrificed and subjected to gross and
microscopic examination for tumour formation. At 18 months all
remaining animals were sacrificed and 10 animals of each sex (or
group) were examined.
The positive control sacrificed at six months (40 ppm) showed
definitive evidence of lung adenoma or carcinoma. At 18 months, the
positive control (10 ppm) again showed a positive response to the
carcinogen. No evidence of lung lesions was noted at 100 ppm leptophos
in the diet. There were no leptophos-related lesions or tumours in any
of the tissues and organs examined in this study (Smith et al., 1973).
Rat
Groups of Charles River albino rats (50 males and 50
females/group) were fed leptophos in the diet for two years at
concentrations of 0, 10, 20, 30 and 60 ppm (the 60 ppm were initially
fed 5 ppm for the first seven months). Mortality was unaffected by
leptophos in the diet (although at the conclusion of the study very
few animals in all groups were alive). Food consumption and growth
were similarly not affected. There were no differences from control
values in the haematology, blood chemistry or urine parameters
examined. Gross and microscopic examination of tissues and organs
failed to indicate any pathologic disorders noted in controls.
Cholinesterase activity measured in brain over the first 90 days
showed no reduction in activity at any dose tested. Erythrocyte
cholinesterase activity was depressed at all levels tested. A
significant reduction (below 25%) was noted at the 60 ppm level. A
no-effect level based on RBC cholinesterase depression is 30 ppm
(Smith et al., 1971a).
Comments
Leptophos, an anticholinesterase organophosphonothioate ester
recommended for use in agriculture as an insecticide, has a moderate
acute oral toxicity. Leptophos is rapidly absorbed, metabolized and
excreted within 24 hours following single acute oral dosing in
animals, primarily via the urine. No tissue residues were observed
except for adipose tissue which maintains traces of leptophos for
extended periods following acute dosing. The metabolism of leptophos
includes oxidative and hydrolytic processes, generally resulting in
less toxic products (except the oxon and the dichlorodesbromo
derivatives). All materials noted as field residues have been found as
metabolites in animals.
Leptophos (and its metabolites) is a relatively persistent
pesticide. Artificial environmental studies using model systems
suggest that leptophos may bioaccumulate in the food chain.
Sufficient toxicological data have been reported including long
and short-term studies with rodents and dogs, reproduction studies
(including teratogenesis and mutagenesis studies); and carcinogenesis
studies. The only significant adverse toxicological parameters
reported in these studies have been esterase depression, pup mortality
in the reproduction study at high levels and delayed neurotoxicity in
hens. Short-term studies on the metabolites have shown no substantial
toxicological effect on rodents except for esterase depression with
the oxon metabolite.
Based on the no-effect level observed in two year studies in rats
and dogs and the no-effect level in acute avian studies for delayed
neurotoxicity, a temporary ADI was estimated. An exceptionally high
safety factor was used to allocate the ADI reflecting concern over the
positive neurotoxicity results. There was less concern over the
significant delayed neurotoxicity as a factor in evaluating the
toxicological hazard of food residues than in its evaluation of
delayed neurotoxicity as a hazard to occupationally exposed
individuals. The occurrence of the delayed neurotoxicity was, however,
considered to be highly significant in the toxicological evaluation of
leptophos. It was decided that delayed neurotoxicity should be
considered as an additional toxicological parameter, showing a
dose-response relationship, which permits an evaluation to be made of
a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 30 ppm in the diet equivalent to 1.5 g/kg bw
Dog: 60 ppm in the diet equivalent to 2.1 mg/kg bw
Hen: 50 mg/kg (based on delayed neurotoxicity)
Estimate of temporary acceptable daily intake
0-0.001 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in crops
Extensive data were obtained on residues from supervised trials
in which crops were treated according to recommended dosage rates and
pre-harvest intervals. In most trials separate residue figures were
given for the parent leptophos and the metabolites leptophos-oxon,
desbromo-leptophos and the phenol. Leptophos residues consisted mainly
of the parent compound with much lower levels of the metabolites
mentioned, as in the trials recorded in the 1974 monograph of
leptophos. In the following tables therefore, a single figure is shown
referring to the sum of leptophos, its oxygen analogue and
desbromo-leptophos expressed as leptophos.
In a number of trials "apparent" oxon residues were found. As
explained in the 1974 monograph, these consist of bis-(2-ethylhexyl)
phthalate, a component of plastic laboratory equipment. In the
following Tables these apparent oxon residues are omitted.
Comments on the data
Apples. Residue data from Canada (three locations in Quebec and
one in Ontario) show considerable residue levels, up to 2 mg/kg, after
a pre-harvest interval of 6-10 weeks. Further data on apples are given
in the section "Fate of residues".
Vegetables
Beans. After pre-harvest intervals of 37-40 days residues in
beans were either not detectable (Canada) or very low, at or below
0.02 mg/kg (Japan).
Brassicas. No detectable residues were found in broccoli,
cabbage or cauliflower after pre-harvest intervals of about 60 days,
but still considerable residues after intervals of 25-30 days. These
data confirm those available from the United States of America at the
1974 Joint Meeting.
In supervised trials in Japan residue levels in the edible part
of Chinese cabbage after a pre-harvest interval of 14-31 days were in
the range 0.1-0.2 mg/kg.
Celery. Residue levels were very low, 0.01-0.02 mg/kg in three
locations in Ontario after a pre-harvest interval of 61 days. In other
trials residues ranged from 0.02-0.05 mg/kg after a similar interval
and levels up to 0.5 mg/kg were found after an interval of 42 days.
Lettuce. Residue levels in Canadian trials after a pre-harvest
interval of 21-30 days were slightly lower than in the experiments
evaluated at the 1974 Joint Meeting from the United States of America.
After normal trimming for market, residues did not exceed 0.5 mg/kg.
Onions, peas, potatoes. Residues were low after the normal
pre-harvest intervals for these crops. In green onions residues were
at or below 0.1 mg/kg after a pre-harvest interval of about 60 days
and in peas <0.01 mg/kg after 30 days. In the vines however, residues
ranged from 1.1-10.7 mg/kg 22-30 days after treatment. In potatoes in
New Zealand no detectable residues were found after 21 days. This is
in conformity with the United States of America data evaluated by the
1974 Joint Meeting.
Cereal crops
Wheat, oats and barley. Residue levels were below 0.1 mg/kg
after a pre-harvest interval of 60 days in most of the trials; in some
trials levels reached about 0.5 mg/kg in these crops.
Maize. Residues in kernels and forage were generally low (0.05
mg/kg or below) in Canadian experiments, as in the United States of
America data evaluated by the 1974 Meeting.
Rice. After pre-harvest intervals of about 30 days residue
levels in inhulled rice ranged from 0.1-0.5 mg/kg and in leaves and
straw from 6 to 20 mg/kg.
Sugar beet. Data from residue trials in Canada and Japan were
evaluated. After a pre-harvest interval of about 50 days, residues in
the roots did not exceed 0.05 mg/kg and those in the tops ranged from
<0.02-0.5 mg/kg.
FATE OF RESIDUES
In plants
Data were obtained from three new studies on the fate of
leptophos residues in apples, cabbage and tobacco carried out with
14C-phenoxy- and 14C-phenyl-leptophos. The results generally agreed
with those of the trials evaluated by the 1974 Joint Meeting.
In the experiment on apples it was shown that the parent
leptophos was the principal residue 6 and 14 days after treatment,
with approximately 10% of the extractable radioactivity as
leptophos-oxon. Less than 1% of 14C was unextractable from the apple
pulp. Fourteen days after treatment, about half of the total
14C-phenyl residue remained on the surface of the apples and up to
20% of this surface residue remained at the origin during TLC on
silica gel.
Only small mounts of 4-bromo-2,5-dichlorophenol, phenylphosphonic
acid, O-methyl phenylphosphonothioate and methyl phenylphosphonate
were extracted from the apple tissue (Whiteacre and Schnur, 1974).
TABLE 1. Nature of 14C compounds extracted from the surface of
apples treated with 14C-phenoxy and C-phenyl-leptophos
% of total surface radio carbon
14C-phenyl 14C-phenoxy
Metabolites Days after treatment Days after treatment
0 6 14 0 6 14
leptophos 98.6 80.0 77.8 98.4 89.6 92.3
leptophos oxon 1.4 1.7 2.0 1.4 1.0 1.5
4-bromo-2,5-dichlorophenol - - - <0.01 <0.01 <0.01
polar compounds
at origin of <0.01 18.2 20.2 1.6 9.0 6.2
TLC plate
Total 100.0 99.9 100.0 101.4 99.6 100.0
In the experiment on cabbage (Diaz, 1974) it was shown that on
this crop also, the parent leptophos is the principal residue 4, 7 and
14 days after treatment; only small amounts of leptophos oxon and free
4-bromo-2,5-dichlorophenol were present. A small amount of 4-bromo-2,
5-dichlorophenol was conjugated; no significant amounts of O-methyl
phenylphosphonothioate, methyl phenylphosphonate or phenylphosphonic
acid were found.
In soil
A new study was carried out on the behaviour and fate of
14C-phenoxy-and 14C-phenyl-leptophos in soil (Rieck, 1974), giving
additional information on the mount of "bound" residue (not
extractable with normal extraction procedures). Columns of a sandy
loam soil consisting of a one inch depth of treated soil on top of a
four inch depth of untreated soil were kept under aerobic and
anaerobic conditions and watered to 80% of field capacity. Two columns
were analysed initially and the others were watered with half inch
depth of water at weekly intervals and the leacheate collected. Soil
columns were analysed after 15, 30, 60, 90 and 120 days.
It was found (Table 2) that the bound radiocarbon (defined as
unextractable by 24 hours soxhlet extraction with 80% methanol)
increased with time at all soil depths. The radioactivity in the top
2.5 cm (treated) section decreased with time from 92% of the total to
42% after 120 days. The total 14C in the levels below this remained
fairly constant during the experiment.
TABLE 2. Distribution of total and bound radioactivity in aerobic
sandy loam soil
14C-leptophos equivalent on dry weight basis,*
found at depth
0 - 2-1/2 cm 2-1/2 - 7-1/2 cm 7-1/2 - 12-1/2 cm
Time
(days) total bound total bound total bound
0 9.16 0.02 0.23 0 0.05 0.0
15 8.33 0.39 0.06 0 0.14 0.02
30 6.33 0.69 0.13 0.06 0.04 0.03
60 5.24 0.98 0.19 0.09 0.09 0.05
90 4.75 1.47 0.20 0.14 0.07 0.07
120 4.19 1.84 0.19 0.14 0.04 0.04
* to calculate in terms of percentage of the amount applied, move
the decimal point one place to the right.
Under the conditions of the experiment the downward movement of
leptophos and its metabolites was insignificant. The only
14C-labelled compounds extractable from the lower layers were parent
leptophos, 4-bromo-2,5-dichlorophenol and methyl phenylphosphonate.
The total leacheate collected during the study contained only 0.46% of
the total activity applied to the upper soil layer.
In storage and processing
Sunflower and cotton seed
The nature and magnitude of residues in processed fractions of
sunflower seed were evaluated in three studies in Canada, In one study
the seed processed had a parent leptophos residue of 0.06 mg/kg. The
meal, crude oil and refined oil fractions showed residues of 0.03,
0.18 and 0.20 mg/kg parent compound respectively. No residues were
determined in the hulls, soapstock or finished oil. In another study
in which the crop was treated with 0.3 kg/ha the residue in the seed
was 0.19 mg/kg. No residues were measured in the hulls, meal, crude
oil, refined oil, finished oil or soapstock.
In a similar study in Australia on the processing of cotton seed,
in which the crop was treated with 0.9 kg/ha, after 78 days the level
in the seed was 6.33 mg/kg total leptophos residue. The following
residues were found in the processed fractions.
mg/kg mg/kg
seed 6.3 refined oil 32.4
hulls 2.62 finished oil 0.07
meal 0.13 soapstock 3.70
crude oil 40.0
Tea
Tea was brewed for 3-10 minutes according to a standard
procedure. In one study in which tea was treated with 1.3 kg
leptophos/ha and harvested and brewed (three minutes brew) on the same
day, the brew contained 0.05 mg/kg leptophos and 0.07 mg/kg of
4-bromo-2,5-dichlorophenol. In a brew of tea harvested 3-7 and 14 days
after treatment no leptophos or metabolites except up to 0.02 mg/kg of
the phenol were found. Sun-dried tea harvested at 0 and 7 days after
treatment and brewed for three minutes showed no residues of leptophos
or its metabolites, except 0.06 mg/kg of the phenol in the brew at day
0 and 0.01 mg/kg of the phenol in the brew after seven days.
TABLE 3. Leptophos: Residues, include the parent compound and the main metabolites, leptophos oxon and desbromo-leptophos.
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Fruit crops
Apple New Zealand 1972 11 46 g/100 l wp 45% 2.65 1.95 2.02 2.08 2.28 V143
1972 11 60 g/100 l wp 45% 3.71 4.16 1.89 1.66 1.83 V143
New Zealand 1972 11 46 g/100 l wp 45% 2.82 1.45 1.45 1.43 1.78 V144
1972 11 60 g/100 l wp 45% 2.69 1.56 2.23 1.35 1.69 V144
Australia 1971/ 8 60 g/100 l wp 45% 1.55 1.28 2.27 1.50 V144
(Tasmania) 1972
8 60 g/100 l wp 45% 2.52 4.28 2.90 3.80 V144
Australia 1972 8 60 g/100 l wp 45% 4.06-7.29 5.50-9.03 4.33-6.85 3.56-4.18 V144
(Tasmania)
Australia 1972 5 60 g/100 l wp 45% 0.51-2.54 1.77-1.82 V144
(NSW)
Australia 1970 5 90 g/100 l wp 45% 0.36-0.66 V73
(NSW) 1970 5 120 g/100 l wp 45% 0.65-0.93 V73
Australia 1971 4 120 g/100 l wp 45% 0.30-0.48 V173
(Tasmania)
6 120 g/100 l wp 45% 0.65-0.83 V173
Canada 1973 4 110 g/100 l wp 45% 1.51 V178
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Apple Canada 1973 3 72 g/100 l wp 45% 0.76 V178
(cont'd)
Canada 1973 4 2 kg/ha wp 40% 3.83 V178
rate
kg a.i./ha 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Vegetables
Beans Canada 1974 1 1 EC 32% <0.01 Braun
et al.,
1975
1 2 EC 32% <0.01 "
Broccoli Canada 1974 1 1 wp 45% 0.08 "
1 2 wp 45% 0.13 "
Cabbage Canada 1974 1 1 wp 45% <0.01 "
1 2 wp 45% <0.01 "
Canada 1974 8 0.6 EC 0.71 V 176
0.06
EC 0.23 0.15 V 190
Carrots Canada 1974 1 0.5 EC 32% 0.07 0.02 0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Carrots 1 1 EC 32% 0.10 0.03 0.02
(cont'd) 1 2 EC 32% 0.12 0.03 Braun
et al.,
1975
Canada 1974 2 0.5 EC 32% 0.16 0.06 0.13 Braun
et al.,
2 1 EC 32% 0.32 0.06 0.05 "
2 2 EC 32% 0.34 0.11 0.14 "
Canada 1974 1 1 EC 0.07 V 210
1 1 EC 0.05 V 210
1 2 EC 0.33 V 210
1 2 EC 0.07 V 210
Cauliflower Canada 1974 1 1 wp <0.01 Braun
et al.,
1975
1 2 wp <0.01 "
0/14 21/23 28/30 35/40 42/46 49/56 63/
Vegetables
Celery Canada 1974 1 0.5 wp 0.10 <0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Celery 1 1 wp 0.20 0.02 "
(cont'd)
1 2 wp 0.40 0.04 "
(Stalks) Canada 1974 1 1 EC 0.01 V 208
1 2 EC 0.02 V 208
1 1 EC <0.03 V 208
1 2 EC <0.03 V 208
Lettuce Canada 1974 1 0.5 EC 0.20 0.01 Braun
(outdoors) et al.,
1 1 EC 0.37 0.12 "
1 2 EC 0.25 0.04 "
Onions Canada 1974 1 0.5 EC 0.04 <0.01 "
(green)
1 1 EC 0.08 0.02 "
1 2 EC 0.27 0.07 "
2 0.5 EC 0.08 0.02 "
2 1 EC 0.27 0.03 "
2 2 EC 0.42 0.09 "
Peas
(Pods) Canada 1974 1 1 EC 0.01 "
2 EC 0.01 "
Canada 1974 2 1 EC 0.02 Braun
et al.,
2 EC 0.03 1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Peas
(cont'd)
(Vines) Canada 1974 1 1 EC 2.06 Braun
et al.,
1975
2 EC 1.10 "
2 1 EC 10.65 "
2 EC 7.48 "
Rutabaga Canada 1974 1 0.5 EC 0.01 <0.01 "
1 1 EC 0.01 0.01 "
1 2 EC 0.02 <0.01 "
2 0.5 EC <0.01 0.01 "
2 1 EC 0.04 0.02 "
2 2 EC 0.06 0.02 "
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage
Chinese Japan 1971 1 0.35 EC 34% 0.09-0.10 Miyagi,
1971
3 0.35 EC 34% 0.09-0.10
1 0.35 EC 34% 0.08-0.10
3 0.35 EC 34% 0.10-0.12
1 0.50 EC 34% 0.06-0.07
3 0.50 EC 34% 0.06-0.08
1 0.50 EC 34% 0.05-0.06
0.12-0.14
Japan 1970 1 0.35 EC 34% 0.08-0.09 Miyagi,
1971
3 0.35 EC 34% 0.09-0.09
1 0.35 EC 34% 0.09
3 0.35 EC 34% 0.08-0.10
1 0.5 EC 34%
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage 3 0.5 EC 34%
(cont'd) 1 0.5 EC 34%
3 0.5 EC 34%
Potatoes New Zealand 1972 1 0.44 wp 45% <0.05 <0.05 <0.05
Japan 1972 2 0.35 <0.005 <0.005 V 142
3 <0.005 <0.005
Residues in mg/kg, at intervals (days) after application
21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Field crops
Barley Canada 1973 1 0.5 2.17 V 173
Grain 0.64
Grain Canada 1973 1 0.5 0.11
Straw 0.5 0.12
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Grain Canada 1.0 0.14 V 155
Straw 1.0 0.62
Oats Canada 1973 1 0.5
Grain <0.01 V 159
Stubble <0.01
Wheat Canada 1973 1 0.5 0.37
Grain 1 0.36 0.03 V 173
Bran 0.04
Flour <0.01
Canada 1970 1 0.5
Grain <0.01 V 113
Straw 0.01
+ chaff
Canada 1970 1 1
Grain <0.01 V 113
Straw 0.17
+ chaff
Wheat Canada 1972 1 0.5
Grain 0.72 V 155
Straw 0.28
1972 1 1
Grain 0.24
Straw 1.32
Maize
Sweetcorn Canada 1974 1 1 EC
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/56 63/72 75/90 Ref.
Maize
(cont'd)
Cobs <0.01 <0.01 Braun
et al.,
Forrage <0.01 <0.01 1975
1 2 EC
Cobs <0.01 <0.01
Forrage <0.01 <0.01
2 1 EC
Cobs <0.01 <0.01
Forrage <0.01 0.31
2 2 EC
Cobs <0.01 <0.01
Forrage <0.01 0.02
28/33 75/92
Fieldcorn
forrage Canada 1974 1 0.5 EC 0.06 V 158
Forrage 1 1 EC 0.04
Sunflower Canada 1973 1 1 EC V 155
Hulls 0.01
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/33 35/40 42/46 49/56 63/72 75/92 Ref.
Sunflower Canada
(Cont'd)
Meal 0.03
Crude oil 0.18
Refined oil 0.20
Finished oil <0.01
Sunflower
seed Canada 1972 1 0.5 EC <0.01 V 174
Rape seed Canada 1973 1 0.5 EC 0.11 V 183
1 1 EC <0.01
0.5 EC 0.19 V 177
0.5 EC 0.22
0.25 EC 0.07
0.4 EC 0.04
Flax seed Canada 1973 1 0.5 0.09 0.07 V 174
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice Japan 1973 5 0.35 EC
Unhulled 0.20 Niigata,
1973
Straw 15.0-16.8
6 0.35 EC
Unhulled 0.21
Straw 15.9-16.7
6 0.35 EC
Unhulled 0.32
Straw 16.9-17.3
5 0.35 EC
Unhulled 0.11
Straw 22.4-23.3
6 0.35 EC
Unhulled 0.08
Straw 30.5-31.7
6 0.35 EC
Unhulled 0.03-0.05
Straw 6.15-8.33
5 0.8 dust 2%
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.11
Straw 7.20-7.80
Japan 1973 6 0.8 dust 2% Niigata,
1973
Unhulled 0.10
Straw 7.20-7.40
6 0.8 dust 2%
Unhulled 0.15-0.16
Straw 11.6-12.4
5 0.8 dust 2%
Unhulled 0.12
Straw 4.15-5.30
6 0.8 dust 2%
Unhulled 0.08-8.09
Straw 9.4-10.8
6 0.8 dust 2%
Unhulled 0.10
Straw 11.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 1.59-2.82
6 0.6-0.8 dust 2%
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.10
Straw 10.2-10.3
Japan 1973 6 0.6-0.8 dust 2% Niigata,
1973
Unhulled 0.20
Straw 13.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 11.2-11.9
6 0.6-0.8 dust 2%
Unhulled 0.07-0.08
Straw 10.3-10.9
6 0.6-0.8 dust 2%
Unhulled 0.09-0.10
Straw 19.3-19.7
Tea (dry
manuf.) India 1973 2 1.3 EC 93.4 1.77 3.57 0.29 V 203
Assam
*1973 2 1.3 EC 77.3 0.11 3.67 0.23
*1973 1 1.3 EC 2.80-3.77
1973 1 1.3 EC 1.36-1.94
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet Canada 1973 1 0.5 EC
Roots) 0.05 0.05 V 170
)
Tops ) 0.09 0.07 V 170
Roots 1973 2 0.5 EC <0.02
Roots 2 1.0 EC <0.02
Roots 2 1.0 EC <0.02
Roots) 1 1.0 EC <0.02 0.04
Tops ) <0.02 <0.02
Japan 1972 3 0.35 EC Hokkaido,
Roots <0.02 <0.02 exp. sta.
1972
Tops 0.43-0.59 0.17-0.30
1972 5 0.35
Roots 0.01 <0.01
Tops 1.36-1.72 0.66-0.84
1972 3 0.35
Roots <0.01 <0.01
Tops 0.59-0.61 0.25-0.27
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet
(cont'd)
1972 5 0.35
Roots 0.01 <0.01
Tops 0.93-1.02 0.34-0.39
Japan 1971 3 0.35 Hokkaido,
Roots <0.01 <0.01 exp. sta.
1972
Tops 0.59-0.66 0.34-0.39
5 0.35
Roots 0.01 0.01
Tops 1.75-1.87 0.93-0.98
1971 3 0.35
Roots <0.01 <0.01
Tops 0.64-0.69 0.26-0.32
5 0.35
Roots <0.01 <0.01
Tops 1.17-1.20 0.39-0.46
* Sun-dried.
In a 10 minutes brew of sun-dried tea harvested seven days after
treatment no residues of parent leptophos, leptophos-oxon or
desbromoleptophos was found. The levels of the phenol ranged between
<0.01 mg/kg and 0.06 mg/kg.
In a food chain
Data were obtained in two model studies (Anonymous, 1973;
Johnson, 1975) with 14C-leptophos in a simple food chain in which
water, Daphnia magna and the common blue gill (Lepomis macrochirus
rafinesque) were included.
Daphnia magna accumulated leptophos rather rapidly from the
water. In less than four hours leptophos in daphnia reached a constant
level about 1500 times that in the surrounding water. Blue gills
feeding on Daphnia magna exposed to leptophos reached a plateau about
1/10 of that in the daphnia.
It is obvious that the most significant route of accumulation of
leptophos residues by fish will be by direct uptake from water.
Blue gills exposed to leptophos in water only (not in the food)
contained mean 14C residues in the tissues of 0.179 mg/kg, about 750
times the mean concentration of 14C-leptophos in the water, after
10-42 days' exposure. When blue gills were exposed to both aqueous and
dietary 14C-leptophos for 10-28 days the residue level was almost
identical (0.170 mg/kg, about 770 times the concentration in the
water).
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours. The bio-transfer
rate of leptophos in the Daphnia-blue gill food chain was only 6.5% of
that of DDT, a typical bioaccumulating compound.
APPRAISAL
Leptophos was evaluated with regard to agricultural data by the
1974 Joint Meeting (FAO/WHO,1975). Arising from the list of
requirements, information was obtained on residues from supervised
trials in various areas on fruit crops (apples and pears), small
grains, rice, sugar beet, potatoes and on some vegetable crops.
Some information became available on residues in waste of
agricultural crops which may be used for feeding purposes, e.g. sugar
beet leaves, straw and chaff of cereal crops, including rice.
Additional data were obtained on the fate of leptophos residues
in apples, cabbage, lettuce and tomatoes from studies with 14C-phenyl
and 14C-phenoxy-labelled leptophos. Additional data became available
on the fate of 14C-phenoxy- and 14C-phenyl-labelled leptophos in
soil. It was shown in this study that the bound fraction increases
with time at all soil depths. The total 14C in the upper 2.5 cm layer
decreased with time from 92% of the applied radioactivity to 42% after
120 days; the total 14C in the lower layers was fairly constant
during that period.
New data were obtained from model studies with 14C-labelled
leptophos in a simple food chain which included water, Daphnia magna
and the common blue gill. Daphnia magna accumulated leptophos rather
rapidly from the water; in less than four hours leptophos plateaued in
Daphnia to levels about 1500 times the level in the surrounding water.
Blue gills feeding on Daphnia magna exposed to leptophos reached a
plateau one-tenth of that of the daphnids.
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours.
The bio-transfer rate of leptophos in the Daphnia-blue gill food
chain was less than one-tenth of that of DDT, which is recognized as a
bioaccumulating compound.
The new data available confirm the limits recorded at the 1974
Joint Meeting on cabbage, broccoli, lettuce, tomatoes, potatoes,
cotton seed and maize.
In the light of the temporary ADI established at the present
Meeting these limits are now recommended as temporary maximum residue
limits, and replace the guideline levels recorded in the 1974
monograph. Additional temporary maximum residue limits are
recommended.
Recommendations
The maximum residue limits refer to the sum of leptophos, its
oxygen analogue and desbromo-leptophos, expressed as leptophos.
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Apples, pears 2 42-60
Broccoli, Brussels sprouts,
cabbage 2 28-50
Lettuce 2 28 (outdoor)
Tomatoes 2 7
Cotton seed oil (crude) 1 28
Raw cereals (wheat, oats,
barley) 0.5 60
Rice (hulled) 0.5 30
(Cont'd)
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Cotton seed, cotton seed meal 0.2 28
Carrots 0.2 60
Potatoes 0.1 21
Maize, fieldcorn (kernels) 0.05 7
Sweet corn (kernels and cobs,
husks and silk removed) 0.05 3-7
Sunflower seed 0.05 100
Sugar beet (roots) 0.05 50-60
Sugar beet (tops) 0.5
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1978)
1. Studies on the kinetics of accumulation of leptophos in storage
depots of two species, preferably non-rodent to investigate the
potential buildup in man to ultimate threshold levels.
2. Studies on the species variation for delayed neurotoxicity with
leptophos to assess its ultimate effect on man.
3. Epidemiological studies on people in exposed occupations in
agriculture or industry.
4. Further long-term low-level feeding studies in a species
susceptible to the delayed neurotoxic syndrome.
5. Residue data on the major crops for which national tolerances or
use recommendations exist, e.g., tea, vegetables not included in the
recommendations, and citrus fruits.
6. Residues in those parts of agricultural commodities which are
used either as such or as agricultural waste for animal feed.
DESIRABLE
1. Improved methodology for assessing the delayed neurotoxic
syndrome.
2. Studies on the mechanism of neurotoxic action of leptophos.
Further investigations into mechanism of action of TOCP and other
neurotoxic agents as compared to leptophos.
3. Data on current use patterns in countries outside the United
States of America and Canada and on residue levels resulting from
those uses.
REFERENCES
Anonymous. (1973) Exposure of 14C-leptophos to daphnia magna and
bluegill sunfish (lepomis macrochiris). Accumulation, distribution,
elimination and bio-concentration in the food chain. Unpublished
report from Bionomics, Inc., submitted to FAO by Velsicol Chemical
Corporation.
Abou-Donia, M.B., Othman, M.A., Tantawy, G., Khalil, A.Z. and Shawer,
M.F. (1974) Neurotoxic effect of leptophos. Experimentia, 30:63-64.
Abou-Donia, M.B. and Pressig, S. (1975) Studies on delayed
neurotoxicity produced by leptophos. Proc. Fed. Amer. Soc. Exptl.
Biol., 34:810.
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Phosvel (VCS-506) in albino mice. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Badie, M. and Whitacre, D.M. (1975) Metabolism of 14C-leptophos and
14C-4-bromo-2,5-dichlorophenol in rats: A multiple dosing study.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Braun, E., McEwen, F.L., Frank, R. and Ritcey, G. (1975) Residues of
leptophos and its metabolites following application to various crop
plants. J. Agr. Food Chem., 23(1):90-95.
Braun, E., McEwen, F.L., and Frank, R. (1974) Residues of
chlorpyriphos and leptophos in three field tested vegetable crops.
Canad. J. Plant Sci., 55:133-137.
Diaz, L.I. (1974) Radiotracer study of leptophos on cabbage. Velsicol
Chem. Corp. Lab. rep. 194. (Unpublished)
Dorough, H.W. (1974) Fate of phosvel-14C in burley tobacco. Progress
report of Department of Entomology, University of Kentucky, Lexington,
provided to Velsicol. (Unpublished)
Fink, R.J. (1971) Continuous feeding and residue study - cows with
technical Phosvel (VCS-506). Unpublished report from Hazleton
Laboratories, submitted to the World Health Organization by Velsicol
Chemical Corporation.
Fletcher, D., Jenkins, D.H., Kinoshita, P.R., Gordon, D.E. and
Keplinger, M.L. (1975) Neurotoxicity study with technical leptophos in
chickens. Unpublished report from Industrial Bio-Test Laboratories,
Inc., submitted to the World Health Organization by Velsicol Chemical
Corporation.
Haley, S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1972) Three
generation reproduction study with VCS-506 in albino rats. Unpublished
report by Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Haley, S., Kennedy, G.L. and Keplinger, M.L. (1973) Three-generation
reproduction study with VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Hartke, K., Lindberg, D.C., Wright, P.L. and Keplinger, M.L. (1971)
Two-year chronic oral toxicity study with VCS-506 technical in beagle
dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Hassan, A. (1975) Personal communication to the World Health
Organization.
Hathaway, D., Keplinger, M.L. and Fancher, O.E. (1968) Acute aerosol
inhalation toxicity study on VCS-506, 3 EC. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Hokkaido. (1972) Phosphel residue analysis on sugar beet. National
Hokkaido. Agr. Exp. Sta. Residue data provided to Velsicol.
(Unpublished)
Holmstead, R.L., Fukuto, T.R. and March, R.B. (1973) The metabolism of
O-(4-bromo-2,5-dichlorophenyl) O-methyl phenylphosphonothioate
(leptophos) in white mice and cotton plants. Archives of Environmental
Contamination and Toxicology. Vol. 1, No. 2, 133-147.
Johnson, B.T. (1973) Leptophos accumulation through a simple food
chain. Unpublished report from the United States Department of the
Interior, submitted to the World Health organization by Velsicol
Chemical Corporation.
Johnson, M.K. (1975) The delayed neuropathy caused by some
organophosphorus esters: Mechanism and challenge. CRC Critical Reviews
in Toxicology, June.
Kennedy, G. and Keplinger, M.L. (1971) Absorption, distribution, and
excretion study with Phosvel in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1971a) Characterization of 14C in
excreta of rats following oral administration of Phosvel. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1972) Absorption, distribution, and
excretion study with VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970) Distribution and
excretion study of VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970a)
Characterization of 14C in urine of rats following oral
administration of 14C-VCS-506. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kimmerle, G. (1972) Acute neurotoxicity studies in hens. Unpublished
report from Farbenfabriken Bayer, submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1971) Study of the
efficacy of atropine sulfate and 2-PAM as antidotes for VCS-506
intoxication in albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C., Keplinger, M.L. and Fancher, O.E. (1971a)
Acute oral (intragastric) toxicity studies with VCS-506 in one day old
and five day old albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1972) Acute oral
toxicity study with oxon of VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Kretchmar, B,, Mastri, C. and Keplinger, M.L. (1972a) Acute oral range
finding toxicity study with S-isomeride in female albino rats.
Unpublished report from industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L. (1971)
Teratogenic study with Phosvel in albino rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health organization by Velsicol Chemical Corporation.
Lindburg, D., Vondruska, J.F. and Fancher, O.E. (1969) Ninety-day
subacute oral toxicity of VCS-506 in beagle dogs. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
March, R.B. and Fukuto, T.R. (1975) Reports on studies on the
photolysis and metabolism of Phosvel. Unpublished report from the
University of California, submitted to the World Health Organization
by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969) Acute oral
toxicity study on VCS-506 technical in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L, and Fancher, O.E. (1969a) Acute oral
toxicity study on two formulations of VCS-506 E.C. in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity on four metabolites of VCS-506 in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on phenylphosphonic acid in albino rats. Unpublished
report from industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Miyagi. (1971) Phosphel residues on chinese cabbage. Miyagi Pref. Agr.
Exp. Sta. and Aichi Pref. Agr. Exp. Sta. Residues data provided to
Velsicol.
Niigata. (1973) Phosphel residue analysis on rice. Niigata Pref. Agr.
Exp. Sta. and Shizuoka Pref. Agr. Exp. Sta. Data provided to Velsicol.
(Unpublished)
Perkins, C.T. and Singh, V.K. (1971) Chicken toxicity and residue
study. Unpublished report from Bio/Toxicological Research
Laboratories, Inc., submitted to the World Health organization by
Velsicol Chemical Corporation.
Perkins, C.T. and Singh, V.K. (1971a) Beef animal feeding study.
Unpublished report from Bio/Toxicological Research Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J., Keplinger, M.L. and Fancher, O.E. (1970) Cholinesterase
study of VCS-506 in albino rats. Unpublished report from International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971) Twenty-eight day
target organ study with VCS-506 oxon metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971a) Twenty-eight
day target organ study with VCS-506 phenol metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971b)
Ninety-day subacute oral toxicity, study with VCS-506 Phenol
metabolite in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971c)
Ninety-day subacute oral toxicity study with VCS-506 oxon metabolite
in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Rieck, C.E. (1974) Soil metabolism of 14C-leptophos. Report of Agr.
Dept., University of Kentucky, Lexington, provided to Velsicol.
(Unpublished)
Sanborn, A. and Metcalf, R.L. (1975) The fate of leptophos (Phosvel)
in a terrestrial-aquatic ecosystem. Unpublished report from the
University of Illinois submitted to the World Health Organization by
Velsicol Chemical Corporation.
Schroeder, C. (1971) The radiocarbon distribution of 14C labelled
Phosvel in lettuce and green beans. Unpublished report from WARF
Institute submitted to the World Health Organization by Velsicol
Chemical Corporation.
Schwemmer, B. (1971) UV photolysis of leptophos in solution.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Sleight, B.H. and Macek, K.J. (1973) Exposure of 14C-leptophos to
Daphnia magna and bluegill sunfish (Lepomis macrochirus):
Accumulation, distribution, elimination, and bioconcentration in the
food chain. Unpublished report from Bionomics, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971)
Twenty-eight day cholinesterase study with VCS-506 oxon metabolite in
female albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc. submitted to the World Health Organization by
Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971a)
Two-year chronic oral toxicity study with VCS-506 technical in albino
rats. Unpublished report from Industrial Bio-Test Laboratories, Inc.
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Smith, P.S., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. (1973)
Eighteen-month carcinogenic study with VCS-506 technical in Swiss
white mice. Unpublished report from Industrial Bio-Test Laboratories,
Inc. submitted to the World Health Organization by Velsicol Chemical
Corporation.
Stephens, J.L., Jenkins, D.H. and Fancher, O.E. (1969) Demyelination
study-chickens, VCS-506 Technical. Unpublished report from industrial
Bio-Test Laboratories, Inc. submitted to the World Health Organization
by Velsicol Chemical Corporation.
Suzuki, H. (1971) Phosvel-chicken feeding study. Unpublished report
from Velsicol Chemical Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Suzuki, H. (1971a) Phosvel-dairy cattle feeding study. Unpublished
report from Velsicol Chemical Corporation submitted to the World
Health Organization by Velsicol Chemical Corporation.
Tarka, S.M. Jr. (1973) Identification of radioactivity in the fat of
rats administered 14C-phenoxy-labelled leptophos. Unpublished report
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1965) Acute oral toxicity (LD50) in the albino rat.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1966) Acute oral toxicity (LD50) in male albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968) Comparative acute oral toxicity in male albino
rats. Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968a) Acute toxicity studies in the albino rabbit.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968b) Acute toxicity study in the rabbit. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1968c) Ten-day subacute oral toxicity study in rats for
analysis of plasma, erythrocyte and brain cholinesterase activity.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1969) Ninety-day feeding study in albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970a) Acute oral toxicity (LD50) in the male albino
rat. Unpublished report from international Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970b) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971a) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971b) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971c) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971d) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (197le) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971f) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971g) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971h) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971i) Acute toxicity studies in rats and rabbits.
Unpublished study by International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1972) Acute oral toxicity (LD50)
in male and female albino rats. Unpublished report by International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1973) Acute dermal toxicity
(LD50) in male and female albino rabbits. Unpublished report from
International Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Whitacre, D.M. and Schnur, P.D. (1974) Metabolic fate of 14C-labelled
leptophos on apples. Velsicol Chem. Corp. Lab. rep. No. 195.
(Unpublished)
In further studies of the biomagnification of leptophos, it was
observed that an apparent equilibrium was established in fish exposed
to leptophos for periods of time. After about one week of exposure
during which time leptophos accumulates there is no additional
build-up in tissues. These data also suggest that ingestion of
leptophos - contaminated invertebrates by fish does not increase the
fish residue suggesting that biomagnification is not a significant
factor (Sleight and Macek, 1973; Johnson, 1973).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Leptophos oxon
Rat
Groups of rats (25 males and 25 females/group) were subdivided
into two categories: individually housed (10 rats of each sex per
group) and group housed (15 rats of each sex per group). These groups
were fed a control diet and one containing leptophos oxon for 28 days.
The leptophos oxon diet was increased at weekly intervals from 200 ppm
to 300 ppm to 500 ppm and finally to 800 ppm. Growth (as evidenced by
body weight) was reduced in the latter stages of the test in females
while food consumption was normal. Spleen weight in males and females
was reduced at 7, 21 and 28 days. Cholinesterase activity of RBC and
brain was depressed at all test intervals. Plasma cholinesterase was
depressed in females at all intervals while in males the depression
became evident after 14 days. No effects were noted on survival,
haematology, blood chemistry, urinalyses or on microscopic examination
of tissues and organs (Plank et al., 1971).
Groups of female rats (15 rats/group) were fed leptophos oxon for
four weeks at dose levels of 0, 1, 5, 10 and 20 ppm. Measurements of
red blood cell and plasma cholinesterase activity were made at 14 and
28 days. Plasma cholinesterase was unaffected while erythrocyte
cholinesterase was depressed at 10 ppm and above at 14 days. At the 28
day interval reduction of activity was not significant (Smith et al.,
1971).
Groups of rats (15 males and 15 females/group) were fed leptophos
oxon for 90 days at dose levels of 0, 25, 50 and 500 ppm. Inhibition
of plasma cholinesterase activity was noted at all dose levels in
females while males were affected at 500 ppm only at the 84 day test
interval. Inhibition of erythrocyte and brain cholinesterase activity
was similar in both sexes and was notable only at 500 ppm. No effects
were noted on mortality, growth, food consumption, haematology
parameters, clinical chemistry parameters, urinalyses, and on gross
and microscopic examination of organs and tissues (Plank et al.
1971c).
Leptophos phenol
Rat
Groups of rats (25 of each sex/group) were divided into two
subgroups and housed individually (10 of each sex/group) or housed
together (15 of each sex/group). The sexes were not mixed. Two dietary
groups were used; a control and a test group which received a dietary
level of leptophos phenol of 3500 ppm for one week. This was increased
to 5000 ppm for the second week, to 8000 ppm for the third week and
finally to 13 000 ppm for the fourth week. There were no effects on
food consumption, growth, haematology, blood chemistry including
cholinesterase or on growth and microscopic examination of tissues and
organs (Plank et al,, 1971a).
Groups of rats (15 males and 15 females/group) were fed leptophos
phenol at dietary levels of 0, 300, 1000 and 10 000 ppm (3000 ppm for
days 1-49) for 90 days. At 10 000 ppm there was a significant increase
in liver weight in females. In males a decreased spleen weight was
noted at all doses tested (none was significantly different from
control values although the decrease was evident in ratios calculated
from body and brain weight data). No effects were observed on
survival, food consumption, growth, haematology, blood chemistry,
urinalyses or on gross or microscopic examination of tissues or organs
(other than the gross effects on liver and spleen). A report of the
microscopic examination of liver and spleen indicated no abnormalities
(Plank et al., 1971b).
Special studies on mutagenicity
Mouse
A dominant lethal study was conducted where groups of male mice
(8 mice/group) were administered a single oral or intraperitoneal dose
of 0, 15 or 30 mg leptophos/kg. The males were mated with three
females per week for six weeks during the normal period of
spermatogenesis. Positive control studies were performed with methyl
methane-sulfonate. There were no effects of leptophos on mating
performance or on reproduction parameters including preimplantation
loss, early resorption or embryo viability. Leptophos under the
conditions of this test did not induce mutagenic changes in male
germinal cells (Arnold et al., 1971).
Special studies on neurotoxicity
Chickens
Groups of white leghorn hens (6 hens/group) were orally
administered leptophos at doses of 100, 200 and 400 mg/kg on day 0 and
14. Observations on clinical conditions were made and at the
conclusion of the study (day 28) survivors were sacrificed and nervous
tissue examined microscopically for indications of myelin disruption.
No clinical signs of delayed neurotoxicity were noted in this
study following the first dosing. Three hens of six tested at the high
level died 6-14 days after the second dose. These hens had lost a
considerable portion of their body weight before death. At 200 mg/kg
one of six died and one other showed clinical signs of ataxia.
Microscopic examination of H & E and Luxol Fast Blue stained sections
of nerve did not reveal myelin degeneration (Stephens et al., 1969).
Groups of white leghorn hens (10 hens/group) were administered
leptophos orally at doses of 0, 5, 10, 15, 30, 50, 75, 100 and 200
mg/kg at days 0 and 21. A positive control was also used in this study
(Tri-o-Cresyl phosphate, 500 mg/kg - orally at day 0). A number of
animals showing clinical signs of ataxia were sacrificed at day 42.
Gross and microscopic examination of brain, spinal cord and sciatic
nerves were performed. Examinations were made of H & E stained
sections and tissues embedded in ParaplastR and stained with Luxol
Fast Blue - Holmes Silver Nitrate. Both light and electron microscopic
examinations were made in this study.
A positive delayed neurological condition was clinically evident
in this study with both TOCP and leptophos. Loss of body weight is
generally observed clinically in animals with delayed peripheral
ataxia and this was observed with the TOCP group in 6 of 10 hens
weighed at day 18. In the leptophos groups, weight loss was noted in 1
of 10 hens at 75 mg/kg, in 3 of 10 at 100 mg/kg, in 5 of 10 hens at
200 mg/kg. This weight loss was accompanied by signs of peripheral
neuritis in various hens of the three upper group levels (3 hens at 75
mg/kg, 5 hens at 100 mg/kg and the majority of hens at 200 mg/kg).
Light and/or electron microscopic sections of nerve tissue and muscle
preparations indicated retrograde degeneration of 8 of 10 TOCP hens
and 5 of 10 hens at 200 mg/kg. No other positive signs of degeneration
were observed. At acute oral dose levels of 75 mg/kg and above,
leptophos was shown to induce a clinical neuropathy in hens (Fletcher
et al., 1975).
Other studies have confirmed that, at high acute oral doses,
leptophos will induce delayed neurotoxicity and clinical signs of
neuropathy (Abou-Donia et al., 1974; Abou-Donia and Preissig, 1975;
Kimmerle, 1972). In male chickens, 2 of 9 tested were ataxic between
9-13 days after oral dosing with 180 mg/kg. No neurotoxic effects were
noted at dose levels below 180 mg/kg. Further studies showed that 200
mg/kg and above resulted in ataxia. A 30% formulation of emulsifiable
concentrate administered to hens by oral or IV injection resulted in
positive clinical neuropathy at dose levels above 100 mg/kg.
Special studies on reproduction
Rat
In two studies, groups of rats (8 males and 16 females/group
there - were two control groups of 8 males and 16 females each) were
fed leptophos in the diet at levels of 0, 10, 30, 40 and 60 ppm and
subjected to a standard three generation, two litter per generation
reproduction study. After two litters of the first generation were
born, the 60 ppm level was discontinued because of pup mortality. A
second study was initiated at 0 and 5 ppm. The 5 ppm dose was changed
after one generation to the 40 ppm level (noted above) and maintained
at this level for two further generations.
Parental body weight and reproductive performance was similar in
the 60 ppm group to the control group. Reproductive indices, including
mating, pregnancy, fertility and parturition showed no differences
with respect to control and all treated rats. Survival data on pups
including viability and lactation indices were significantly decreased
at 60 ppm but were similar to control values at 40, 30 and 10 ppm.
Mean body weight data for pups at weaning at all dose levels
(including 60 ppm) were similar. A no-effect level for rat
reproduction is 30 ppm (Haley et al., 1973, 1972).
Special studies on teratogenecity
Rabbit
Groups of pregnant New Zealand rabbits (10-13 rabbits per group)
were administered leptophos orally from day 6 to day 18 of gestation
at dose levels of 0, 1 and 3 mg/kg. Thalidomide was administered over
this same period at a dose of 37.5 mg/kg to a group of rabbits
designated as a positive control. No effects were noted on the growth
of does. Skeletal or somatic abnormalities were noted with
thalidomide. Leptophos at a dose of 3 mg/kg administered orally to
rabbits during organogenesis elicited no teratological response (Ladd
et al., 1971).
Signs of poisoning are typical of acute parasympathomimetic
stimulation seen by other anticholinesterase organophosphates. These
include: hypoactivity, tremors, muscular weakness, ruffed fur,
diarrhoea, exophthalmia, haemorrhagic conjunctivitis and salivation.
Acute Toxicity
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M Tech (white mass) Oral 31.6 Wazeter, 1965
M Tech (white powder) Oral 59.0 " 1966
M Tech (98.5% white powder) Oral 54.5 " 1968
M Tech (90% brown granule) Oral 37.1 " 1968a
M Tech (99.6% white powder) Oral 56.6 " 1971
M Tech (90% white crystal) Oral 44.7 " 1970
M Tech (90% grey granule) Oral 52.8 " 1971a
M & F Tech (94%) Oral 20.0 Kretchmar et al., 1971
M & F Tech (90%) Oral 90.5 Mastri et al., 1969
M & F (1 day old) Tech 90% Oral 12.0 Kretchmar et al., 1971a
M & F (5 day old) Tech 90% Oral 15.0 Kretchmar et al., 1971a
F Tech (98.5% white powder) Oral 24.3 Wazeter, 1968
F Tech (90% brown granule) Oral 26.1 " 1968
F Tech (99.6% white powder) Oral 40.1 " 1971
F Tech (90% grey granule) Oral 42.9 " 1971a
Rabbit M & F Tech (90% white crystal) Dermal 10 000 " 1970
M & F Tech (90% brown powder) Dermal 800 " 1968a
" 1968b
Chicken F Tech Oral 225 Fletcher et al., 1975
Technical leptophos is an eye irritant when instilled into the conjuctival sac of rabbits (Wazeter, 1968b, 1970).
Leptophos is not an irritant when tested on normal or abraded rabbit skin (Wazeter, 1970).
Acute toxicity formulations
LD50
Species Sex Formulation Route (mg/kg) Reference
Rat M 3 EC (yellow oil) Oral 271 Wazeter, 1970a
M 30 EC (yellow oil) Oral 271 " 1970a
M 29.5 EC Oral 400 Mastri et al., 1969a
M 35.7 EC Oral 735 " 1969a
M 5 G Oral 2 263 Wazeter, 1971b
M 50 WP (grey powder) Oral 121 " 1971c
M 2 EC (yellow liquid) Oral 178 " 1971d
M 3 EC (34.7%) Oral 121 " 1971e
M 3% Dust (grey dust) Oral 1 780 " 1971i
M 3 ULV (32.1%) Oral 192 " 1970b
M 5 G Inhal 26.9 mg/L Wazeter, 1971b
M 3 ULV (32.1%) Inhal 200 mg/L " 1970b
M & F 3 EC 4 hr
Inhal 2.7 mg/L Hathaway et al., 1968
M 3% dust 4 hr
Inhal 33 mg/L Wazeter, 1971i
M 50 WP Inhal 19 mg/L " 1971c
M 2 EC Inhal 200 mg/L " 1971d
M 3 EC Inhal 200 mg/L " 197le
F 29.5 EC Oral 218 mg/L Mastri et al., 1969a
F 36.7 EC Oral 327 mg/L " 1969a
Rabbit M & F 2.7 EC Dermal 2 000 Wazeter and Goldenthal, 1973
Leptophos formulations are an eye irritant when instilled into the conjunctival sac (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i). However, one
formulation tested (3 ULV - 32.1% leptophos) was not an eye irritant (Wazeter, 1970b). Leptophos fomulations are not a skin irritant when tested
on normal or abraded rabbit skin (Wazeter, 1971b, 1971c, 1971d, 1971e, 1971i).
Acute toxicity - metabolites and possible contaminants
LD50
Compound Species Sex Route (mg/kg) Reference
Leptophos oxon Rat M & F Oral 119 Mastri et al., 1969b
Rat M & F Oral 43 Kretchmar et al., 1972
Phenylphosphonic
acid Rat M Oral 2 480 Mastri et al., 1969b
2 241 " 1969c
S-methyl isomeride Rat F Oral 735 Kretchmar et al., 1972a
O.O-bis
(2,5-di-chloro-4-brom-phenyl)
phenylphosphono-thioate Rat M Oral 4 640 Wazeter, 1971f
O.O-dimethyl
phenyl-phos-phonothioate Rat M Oral 287 " 1971g
O-methyl phenyl
phosphonic acid Rat M Oral 3 690 " 1971h
O-(2,3-dichloro-4-bromophenyl) Rat M Oral 59.5 Wazeter and Goldenthal, 1972
phenylphos-phonothioate Rat F Oral 37.5 Wazeter and Goldenthal, 1972
Leptophos phenol Rat M & F Oral 2 654 Mastri et al., 1969b
Antidotal studies
Administration of atropine sulfate and 2-PAM alone and in
combination resulted in an increase in the LD50 value suggesting that
these agents might be successful in antidotal therapy (Kretchmar et
al., 1971). The combination of atropine and 2-PAM was no more
successful than the administration of either agent alone.
Antidote LD50 (mg/kg) (95% C.L.)
None 20.0 (11.8-34.0)
Atropine Sulfate (IM)
10 mg/kg 1.5 & 5 hr
after poisoning 54.0 (41.2-70.7)
2-PAM
1.5 hr after poisoning 38.5 (33.8-43.9)
Atropine + 2-PAM 40.0 (27.6-58.0)
Short-term studies
Rat
Groups of rats (2 males and 2 females/group) were administered
leptophos by oral intubation of 10 days at dose levels equivalent to
dietary intakes of 0, 3, 10, 30 and 100 ppm. Mortality was evident at
100 ppm. At the conclusion of the study the animals were sacrificed
and cholinesterase determinations run using brain, plasma and red
blood cells.
No depression of plasma cholinesterase was noted. The RBC and
brain cholinesterase activity was depressed at 30 ppm and above in all
surviving animals (Wazeter, 1968c).
Groups of rats (10 males and 10 females/group) were fed dietary
levels of leptophos of 0, 100, 250 and 500 ppm for 28 days. A second
series of female animals (10 rats/group) were fed levels of 0, 50 and
75 ppm for 14 days in a cholinesterase depression range-finding test.
A dose-dependent depression of cholinesterase activity was
observed in all tissues at all dose intervals and times tested in the
initial high level study. Activity of the most susceptible
cholinesterase source (female RBC) was slightly depressed 32% and 22%
at 75 and 500 ppm respectively after seven days of feeding. The plasma
cholinesterase activity in the initial study was not inhibited until
the two week test interval after which it steadily declined (Plank, et
al., 1970).
Groups of rats (10 male and 10 female rats/group were fed
leptophos in the diet at dose levels of 0, 1, 5 and 10 ppm for 90
days. Growth, food consumption and behaviour were not affected by
leptophos. No changes were noted in haematology, blood, clinical
chemistry or urinalysis parameters measured. Cholinesterase activity
in RBC, plasma and brain were normal at 90 days of testing as were the
40 day examinations of RBC and plasma cholinesterase. Gross and
microscopic examination of tissues and organs showed no
leptophos-related lesions (Wazeter, 1969).
Dog
Groups of dogs (four male and four female beagle dogs/group) were
fed leptophos in the diet for 90 days at dose levels of 0, 10 and 30
ppm. Behaviour, growth and food consumption were not affected over
this test interval. No effects were noted on haematology parameters on
blood, clinical chemistry on urinalyses or on gross and microscopic
analyses of tissues and organs. A no-effect level was 30 ppm (Lindberg
et al., 1969).
Groups of beagle dogs (4 males and 4 female/group) were fed
leptophos in the diet at dosage levels of 0, 10, 20, 30 and 60 ppm for
two years (the 60 ppm group had been fed a diet containing 5 ppm for
180 days which was then increased to 60 ppm for the remainder of the
study. No mortality occurred over the course of the study and growth,
food consumption and behaviour was normal at all levels. No
leptophos-related effects were noted on haematology parameters,
clinical chemistry values (including blood and brain cholinesterase
values) or on gross and microscopic examination of tissues and organs.
Leptophos in the diet at 60 ppm had no effect on any parameter
recorded in this study (Hartke et al., 1971).
Chicken
Groups of white leghorn chickens (4 male and 20 females/group)
were fed leptophos in the diet at dosage levels of 0, 0.03, 0,10, and
0.30 ppm for four weeks. Eggs were collected and incubated during the
latter 14 days of treatment. The chicks were observed for 14 days
post-hatching having been fed the leptophos diet corresponding to the
parent. There were no treatment related differences in body weight or
egg production and size of eggs. Egg hatchability, chick viability,
and growth was not affected by leptophos. No abnormal behaviour or
signs of poisoning were observed in this study in either adults or
chicks (Perkins and Singh, 1971). Analysis of tissue residues
(including eggs and adipose tissue) did not indicate the presence of
residues of leptophos, the oxon, the phenol or a photo-product
(Suzuki, 1971).
Steer
Groups of steers (three steers/each treatment group and one
steer/control group) were fed leptophos in the diet at levels of 0,
15, 45 and 150 ppm for four weeks. At 28 days the control and two each
of the treatment groups were sacrificed. The remainder were allowed to
consume normal untreated food for a further 14 days after which they
were sacrificed. Daily observations showed no abnormal behaviour or
toxic signs of poisoning. No gross or microscopic lesions were
observed at the conclusion of the study. Depression of the whole blood
and plasma cholinesterase was evident at the higher dose levels over
the 28 day feeding interval. After seven days on a control ration the
depressed values returned to normal. Tissue residues of leptophos and
the oxon were low in liver, kidney, muscle and brain. In fat,
leptophos was present after 28 days in measurable levels which were
dose dependent. Following the 14 day recovery intervals, these
residues in fat were considerably reduced (Perkins and Singh, 1971a).
Cow
Groups of lactating dairy cows (three cows/group fed leptophos
and one cow/control group) were fed leptophos in the diet at
calculated dose levels of 0, 5, 15 and 50 ppm (actual values fed as
assessed by chemical analysis were 0, 3.2, 10.0 and 37.4 ppm) for 28
days and maintained for a further 14 days on control diets to measure
residue reductions and recovery of normal values. Appearance,
behaviour and general condition were normal over the study. Body
weights of several animals tested with leptophos were reduced over the
study. Milk production was reduced in the low and intermediate group
but not at the high level of leptophos. Cholinesterase depression was
not noted in this study (Fink, 1971). Traces of leptophos were noted
in fat at the conclusion of the 28 day feeding interval at levels
ranging from 0.2 to 0.5 ppm in the high (37.4 ppm) dose level. After
two weeks, these residues were reduced to 0.2 ppm (Suzuki, 1971a).
Long-term studies
Mouse
Groups of Swiss white mice (65 males and 65 females/group) were
fed leptophos in the diet for 18 months at dose levels of 0, 50, 100
ppm to assess a carcinogenic potential in mice. Two positive control
groups were fed N-nitrosodiethylamine at levels of 10 ppm (50 males
and 50 females) and 40 ppm (15 males and 15 females). After six
months, the mice of the high positive control group and 15 of each sex
in the leptophos group were sacrificed and subjected to gross and
microscopic examination for tumour formation. At 18 months all
remaining animals were sacrificed and 10 animals of each sex (or
group) were examined.
The positive control sacrificed at six months (40 ppm) showed
definitive evidence of lung adenoma or carcinoma. At 18 months, the
positive control (10 ppm) again showed a positive response to the
carcinogen. No evidence of lung lesions was noted at 100 ppm leptophos
in the diet. There were no leptophos-related lesions or tumours in any
of the tissues and organs examined in this study (Smith et al., 1973).
Rat
Groups of Charles River albino rats (50 males and 50
females/group) were fed leptophos in the diet for two years at
concentrations of 0, 10, 20, 30 and 60 ppm (the 60 ppm were initially
fed 5 ppm for the first seven months). Mortality was unaffected by
leptophos in the diet (although at the conclusion of the study very
few animals in all groups were alive). Food consumption and growth
were similarly not affected. There were no differences from control
values in the haematology, blood chemistry or urine parameters
examined. Gross and microscopic examination of tissues and organs
failed to indicate any pathologic disorders noted in controls.
Cholinesterase activity measured in brain over the first 90 days
showed no reduction in activity at any dose tested. Erythrocyte
cholinesterase activity was depressed at all levels tested. A
significant reduction (below 25%) was noted at the 60 ppm level. A
no-effect level based on RBC cholinesterase depression is 30 ppm
(Smith et al., 1971a).
Comments
Leptophos, an anticholinesterase organophosphonothioate ester
recommended for use in agriculture as an insecticide, has a moderate
acute oral toxicity. Leptophos is rapidly absorbed, metabolized and
excreted within 24 hours following single acute oral dosing in
animals, primarily via the urine. No tissue residues were observed
except for adipose tissue which maintains traces of leptophos for
extended periods following acute dosing. The metabolism of leptophos
includes oxidative and hydrolytic processes, generally resulting in
less toxic products (except the oxon and the dichlorodesbromo
derivatives). All materials noted as field residues have been found as
metabolites in animals.
Leptophos (and its metabolites) is a relatively persistent
pesticide. Artificial environmental studies using model systems
suggest that leptophos may bioaccumulate in the food chain.
Sufficient toxicological data have been reported including long
and short-term studies with rodents and dogs, reproduction studies
(including teratogenesis and mutagenesis studies); and carcinogenesis
studies. The only significant adverse toxicological parameters
reported in these studies have been esterase depression, pup mortality
in the reproduction study at high levels and delayed neurotoxicity in
hens. Short-term studies on the metabolites have shown no substantial
toxicological effect on rodents except for esterase depression with
the oxon metabolite.
Based on the no-effect level observed in two year studies in rats
and dogs and the no-effect level in acute avian studies for delayed
neurotoxicity, a temporary ADI was estimated. An exceptionally high
safety factor was used to allocate the ADI reflecting concern over the
positive neurotoxicity results. There was less concern over the
significant delayed neurotoxicity as a factor in evaluating the
toxicological hazard of food residues than in its evaluation of
delayed neurotoxicity as a hazard to occupationally exposed
individuals. The occurrence of the delayed neurotoxicity was, however,
considered to be highly significant in the toxicological evaluation of
leptophos. It was decided that delayed neurotoxicity should be
considered as an additional toxicological parameter, showing a
dose-response relationship, which permits an evaluation to be made of
a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 30 ppm in the diet equivalent to 1.5 g/kg bw
Dog: 60 ppm in the diet equivalent to 2.1 mg/kg bw
Hen: 50 mg/kg (based on delayed neurotoxicity)
Estimate of temporary acceptable daily intake
0-0.001 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in crops
Extensive data were obtained on residues from supervised trials
in which crops were treated according to recommended dosage rates and
pre-harvest intervals. In most trials separate residue figures were
given for the parent leptophos and the metabolites leptophos-oxon,
desbromo-leptophos and the phenol. Leptophos residues consisted mainly
of the parent compound with much lower levels of the metabolites
mentioned, as in the trials recorded in the 1974 monograph of
leptophos. In the following tables therefore, a single figure is shown
referring to the sum of leptophos, its oxygen analogue and
desbromo-leptophos expressed as leptophos.
In a number of trials "apparent" oxon residues were found. As
explained in the 1974 monograph, these consist of bis-(2-ethylhexyl)
phthalate, a component of plastic laboratory equipment. In the
following Tables these apparent oxon residues are omitted.
Comments on the data
Apples. Residue data from Canada (three locations in Quebec and
one in Ontario) show considerable residue levels, up to 2 mg/kg, after
a pre-harvest interval of 6-10 weeks. Further data on apples are given
in the section "Fate of residues".
Vegetables
Beans. After pre-harvest intervals of 37-40 days residues in
beans were either not detectable (Canada) or very low, at or below
0.02 mg/kg (Japan).
Brassicas. No detectable residues were found in broccoli,
cabbage or cauliflower after pre-harvest intervals of about 60 days,
but still considerable residues after intervals of 25-30 days. These
data confirm those available from the United States of America at the
1974 Joint Meeting.
In supervised trials in Japan residue levels in the edible part
of Chinese cabbage after a pre-harvest interval of 14-31 days were in
the range 0.1-0.2 mg/kg.
Celery. Residue levels were very low, 0.01-0.02 mg/kg in three
locations in Ontario after a pre-harvest interval of 61 days. In other
trials residues ranged from 0.02-0.05 mg/kg after a similar interval
and levels up to 0.5 mg/kg were found after an interval of 42 days.
Lettuce. Residue levels in Canadian trials after a pre-harvest
interval of 21-30 days were slightly lower than in the experiments
evaluated at the 1974 Joint Meeting from the United States of America.
After normal trimming for market, residues did not exceed 0.5 mg/kg.
Onions, peas, potatoes. Residues were low after the normal
pre-harvest intervals for these crops. In green onions residues were
at or below 0.1 mg/kg after a pre-harvest interval of about 60 days
and in peas <0.01 mg/kg after 30 days. In the vines however, residues
ranged from 1.1-10.7 mg/kg 22-30 days after treatment. In potatoes in
New Zealand no detectable residues were found after 21 days. This is
in conformity with the United States of America data evaluated by the
1974 Joint Meeting.
Cereal crops
Wheat, oats and barley. Residue levels were below 0.1 mg/kg
after a pre-harvest interval of 60 days in most of the trials; in some
trials levels reached about 0.5 mg/kg in these crops.
Maize. Residues in kernels and forage were generally low (0.05
mg/kg or below) in Canadian experiments, as in the United States of
America data evaluated by the 1974 Meeting.
Rice. After pre-harvest intervals of about 30 days residue
levels in inhulled rice ranged from 0.1-0.5 mg/kg and in leaves and
straw from 6 to 20 mg/kg.
Sugar beet. Data from residue trials in Canada and Japan were
evaluated. After a pre-harvest interval of about 50 days, residues in
the roots did not exceed 0.05 mg/kg and those in the tops ranged from
<0.02-0.5 mg/kg.
FATE OF RESIDUES
In plants
Data were obtained from three new studies on the fate of
leptophos residues in apples, cabbage and tobacco carried out with
14C-phenoxy- and 14C-phenyl-leptophos. The results generally agreed
with those of the trials evaluated by the 1974 Joint Meeting.
In the experiment on apples it was shown that the parent
leptophos was the principal residue 6 and 14 days after treatment,
with approximately 10% of the extractable radioactivity as
leptophos-oxon. Less than 1% of 14C was unextractable from the apple
pulp. Fourteen days after treatment, about half of the total
14C-phenyl residue remained on the surface of the apples and up to
20% of this surface residue remained at the origin during TLC on
silica gel.
Only small mounts of 4-bromo-2,5-dichlorophenol, phenylphosphonic
acid, O-methyl phenylphosphonothioate and methyl phenylphosphonate
were extracted from the apple tissue (Whiteacre and Schnur, 1974).
TABLE 1. Nature of 14C compounds extracted from the surface of
apples treated with 14C-phenoxy and C-phenyl-leptophos
% of total surface radio carbon
14C-phenyl 14C-phenoxy
Metabolites Days after treatment Days after treatment
0 6 14 0 6 14
leptophos 98.6 80.0 77.8 98.4 89.6 92.3
leptophos oxon 1.4 1.7 2.0 1.4 1.0 1.5
4-bromo-2,5-dichlorophenol - - - <0.01 <0.01 <0.01
polar compounds
at origin of <0.01 18.2 20.2 1.6 9.0 6.2
TLC plate
Total 100.0 99.9 100.0 101.4 99.6 100.0
In the experiment on cabbage (Diaz, 1974) it was shown that on
this crop also, the parent leptophos is the principal residue 4, 7 and
14 days after treatment; only small amounts of leptophos oxon and free
4-bromo-2,5-dichlorophenol were present. A small amount of 4-bromo-2,
5-dichlorophenol was conjugated; no significant amounts of O-methyl
phenylphosphonothioate, methyl phenylphosphonate or phenylphosphonic
acid were found.
In soil
A new study was carried out on the behaviour and fate of
14C-phenoxy-and 14C-phenyl-leptophos in soil (Rieck, 1974), giving
additional information on the mount of "bound" residue (not
extractable with normal extraction procedures). Columns of a sandy
loam soil consisting of a one inch depth of treated soil on top of a
four inch depth of untreated soil were kept under aerobic and
anaerobic conditions and watered to 80% of field capacity. Two columns
were analysed initially and the others were watered with half inch
depth of water at weekly intervals and the leacheate collected. Soil
columns were analysed after 15, 30, 60, 90 and 120 days.
It was found (Table 2) that the bound radiocarbon (defined as
unextractable by 24 hours soxhlet extraction with 80% methanol)
increased with time at all soil depths. The radioactivity in the top
2.5 cm (treated) section decreased with time from 92% of the total to
42% after 120 days. The total 14C in the levels below this remained
fairly constant during the experiment.
TABLE 2. Distribution of total and bound radioactivity in aerobic
sandy loam soil
14C-leptophos equivalent on dry weight basis,*
found at depth
0 - 2-1/2 cm 2-1/2 - 7-1/2 cm 7-1/2 - 12-1/2 cm
Time
(days) total bound total bound total bound
0 9.16 0.02 0.23 0 0.05 0.0
15 8.33 0.39 0.06 0 0.14 0.02
30 6.33 0.69 0.13 0.06 0.04 0.03
60 5.24 0.98 0.19 0.09 0.09 0.05
90 4.75 1.47 0.20 0.14 0.07 0.07
120 4.19 1.84 0.19 0.14 0.04 0.04
* to calculate in terms of percentage of the amount applied, move
the decimal point one place to the right.
Under the conditions of the experiment the downward movement of
leptophos and its metabolites was insignificant. The only
14C-labelled compounds extractable from the lower layers were parent
leptophos, 4-bromo-2,5-dichlorophenol and methyl phenylphosphonate.
The total leacheate collected during the study contained only 0.46% of
the total activity applied to the upper soil layer.
In storage and processing
Sunflower and cotton seed
The nature and magnitude of residues in processed fractions of
sunflower seed were evaluated in three studies in Canada, In one study
the seed processed had a parent leptophos residue of 0.06 mg/kg. The
meal, crude oil and refined oil fractions showed residues of 0.03,
0.18 and 0.20 mg/kg parent compound respectively. No residues were
determined in the hulls, soapstock or finished oil. In another study
in which the crop was treated with 0.3 kg/ha the residue in the seed
was 0.19 mg/kg. No residues were measured in the hulls, meal, crude
oil, refined oil, finished oil or soapstock.
In a similar study in Australia on the processing of cotton seed,
in which the crop was treated with 0.9 kg/ha, after 78 days the level
in the seed was 6.33 mg/kg total leptophos residue. The following
residues were found in the processed fractions.
mg/kg mg/kg
seed 6.3 refined oil 32.4
hulls 2.62 finished oil 0.07
meal 0.13 soapstock 3.70
crude oil 40.0
Tea
Tea was brewed for 3-10 minutes according to a standard
procedure. In one study in which tea was treated with 1.3 kg
leptophos/ha and harvested and brewed (three minutes brew) on the same
day, the brew contained 0.05 mg/kg leptophos and 0.07 mg/kg of
4-bromo-2,5-dichlorophenol. In a brew of tea harvested 3-7 and 14 days
after treatment no leptophos or metabolites except up to 0.02 mg/kg of
the phenol were found. Sun-dried tea harvested at 0 and 7 days after
treatment and brewed for three minutes showed no residues of leptophos
or its metabolites, except 0.06 mg/kg of the phenol in the brew at day
0 and 0.01 mg/kg of the phenol in the brew after seven days.
TABLE 3. Leptophos: Residues, include the parent compound and the main metabolites, leptophos oxon and desbromo-leptophos.
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Fruit crops
Apple New Zealand 1972 11 46 g/100 l wp 45% 2.65 1.95 2.02 2.08 2.28 V143
1972 11 60 g/100 l wp 45% 3.71 4.16 1.89 1.66 1.83 V143
New Zealand 1972 11 46 g/100 l wp 45% 2.82 1.45 1.45 1.43 1.78 V144
1972 11 60 g/100 l wp 45% 2.69 1.56 2.23 1.35 1.69 V144
Australia 1971/ 8 60 g/100 l wp 45% 1.55 1.28 2.27 1.50 V144
(Tasmania) 1972
8 60 g/100 l wp 45% 2.52 4.28 2.90 3.80 V144
Australia 1972 8 60 g/100 l wp 45% 4.06-7.29 5.50-9.03 4.33-6.85 3.56-4.18 V144
(Tasmania)
Australia 1972 5 60 g/100 l wp 45% 0.51-2.54 1.77-1.82 V144
(NSW)
Australia 1970 5 90 g/100 l wp 45% 0.36-0.66 V73
(NSW) 1970 5 120 g/100 l wp 45% 0.65-0.93 V73
Australia 1971 4 120 g/100 l wp 45% 0.30-0.48 V173
(Tasmania)
6 120 g/100 l wp 45% 0.65-0.83 V173
Canada 1973 4 110 g/100 l wp 45% 1.51 V178
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate kg a.i./
Crop Country Year No. ha or g/100 l formulation 0/1 7/8 14/16 21 28/30 35/37 57/63 Ref.
Apple Canada 1973 3 72 g/100 l wp 45% 0.76 V178
(cont'd)
Canada 1973 4 2 kg/ha wp 40% 3.83 V178
rate
kg a.i./ha 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Vegetables
Beans Canada 1974 1 1 EC 32% <0.01 Braun
et al.,
1975
1 2 EC 32% <0.01 "
Broccoli Canada 1974 1 1 wp 45% 0.08 "
1 2 wp 45% 0.13 "
Cabbage Canada 1974 1 1 wp 45% <0.01 "
1 2 wp 45% <0.01 "
Canada 1974 8 0.6 EC 0.71 V 176
0.06
EC 0.23 0.15 V 190
Carrots Canada 1974 1 0.5 EC 32% 0.07 0.02 0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/25 28/32 35/38 42/50 53/58 63/67 70/80
Carrots 1 1 EC 32% 0.10 0.03 0.02
(cont'd) 1 2 EC 32% 0.12 0.03 Braun
et al.,
1975
Canada 1974 2 0.5 EC 32% 0.16 0.06 0.13 Braun
et al.,
2 1 EC 32% 0.32 0.06 0.05 "
2 2 EC 32% 0.34 0.11 0.14 "
Canada 1974 1 1 EC 0.07 V 210
1 1 EC 0.05 V 210
1 2 EC 0.33 V 210
1 2 EC 0.07 V 210
Cauliflower Canada 1974 1 1 wp <0.01 Braun
et al.,
1975
1 2 wp <0.01 "
0/14 21/23 28/30 35/40 42/46 49/56 63/
Vegetables
Celery Canada 1974 1 0.5 wp 0.10 <0.01 Braun
et al.,
1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Celery 1 1 wp 0.20 0.02 "
(cont'd)
1 2 wp 0.40 0.04 "
(Stalks) Canada 1974 1 1 EC 0.01 V 208
1 2 EC 0.02 V 208
1 1 EC <0.03 V 208
1 2 EC <0.03 V 208
Lettuce Canada 1974 1 0.5 EC 0.20 0.01 Braun
(outdoors) et al.,
1 1 EC 0.37 0.12 "
1 2 EC 0.25 0.04 "
Onions Canada 1974 1 0.5 EC 0.04 <0.01 "
(green)
1 1 EC 0.08 0.02 "
1 2 EC 0.27 0.07 "
2 0.5 EC 0.08 0.02 "
2 1 EC 0.27 0.03 "
2 2 EC 0.42 0.09 "
Peas
(Pods) Canada 1974 1 1 EC 0.01 "
2 EC 0.01 "
Canada 1974 2 1 EC 0.02 Braun
et al.,
2 EC 0.03 1975
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0/14 21/23 28/30 35/40 42/46 49/56 63/
Peas
(cont'd)
(Vines) Canada 1974 1 1 EC 2.06 Braun
et al.,
1975
2 EC 1.10 "
2 1 EC 10.65 "
2 EC 7.48 "
Rutabaga Canada 1974 1 0.5 EC 0.01 <0.01 "
1 1 EC 0.01 0.01 "
1 2 EC 0.02 <0.01 "
2 0.5 EC <0.01 0.01 "
2 1 EC 0.04 0.02 "
2 2 EC 0.06 0.02 "
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage
Chinese Japan 1971 1 0.35 EC 34% 0.09-0.10 Miyagi,
1971
3 0.35 EC 34% 0.09-0.10
1 0.35 EC 34% 0.08-0.10
3 0.35 EC 34% 0.10-0.12
1 0.50 EC 34% 0.06-0.07
3 0.50 EC 34% 0.06-0.08
1 0.50 EC 34% 0.05-0.06
0.12-0.14
Japan 1970 1 0.35 EC 34% 0.08-0.09 Miyagi,
1971
3 0.35 EC 34% 0.09-0.09
1 0.35 EC 34% 0.09
3 0.35 EC 34% 0.08-0.10
1 0.5 EC 34%
TABLE 3. (continued)
Application
Pre-harvest interval in days
rate
Crop Country Year No. kg a.i./ha formulation 7 14 21
Cabbage 3 0.5 EC 34%
(cont'd) 1 0.5 EC 34%
3 0.5 EC 34%
Potatoes New Zealand 1972 1 0.44 wp 45% <0.05 <0.05 <0.05
Japan 1972 2 0.35 <0.005 <0.005 V 142
3 <0.005 <0.005
Residues in mg/kg, at intervals (days) after application
21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Field crops
Barley Canada 1973 1 0.5 2.17 V 173
Grain 0.64
Grain Canada 1973 1 0.5 0.11
Straw 0.5 0.12
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/46 63/72 75/90 Ref.
Grain Canada 1.0 0.14 V 155
Straw 1.0 0.62
Oats Canada 1973 1 0.5
Grain <0.01 V 159
Stubble <0.01
Wheat Canada 1973 1 0.5 0.37
Grain 1 0.36 0.03 V 173
Bran 0.04
Flour <0.01
Canada 1970 1 0.5
Grain <0.01 V 113
Straw 0.01
+ chaff
Canada 1970 1 1
Grain <0.01 V 113
Straw 0.17
+ chaff
Wheat Canada 1972 1 0.5
Grain 0.72 V 155
Straw 0.28
1972 1 1
Grain 0.24
Straw 1.32
Maize
Sweetcorn Canada 1974 1 1 EC
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/30 35/40 42/46 49/56 63/72 75/90 Ref.
Maize
(cont'd)
Cobs <0.01 <0.01 Braun
et al.,
Forrage <0.01 <0.01 1975
1 2 EC
Cobs <0.01 <0.01
Forrage <0.01 <0.01
2 1 EC
Cobs <0.01 <0.01
Forrage <0.01 0.31
2 2 EC
Cobs <0.01 <0.01
Forrage <0.01 0.02
28/33 75/92
Fieldcorn
forrage Canada 1974 1 0.5 EC 0.06 V 158
Forrage 1 1 EC 0.04
Sunflower Canada 1973 1 1 EC V 155
Hulls 0.01
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 21/24 28/33 35/40 42/46 49/56 63/72 75/92 Ref.
Sunflower Canada
(Cont'd)
Meal 0.03
Crude oil 0.18
Refined oil 0.20
Finished oil <0.01
Sunflower
seed Canada 1972 1 0.5 EC <0.01 V 174
Rape seed Canada 1973 1 0.5 EC 0.11 V 183
1 1 EC <0.01
0.5 EC 0.19 V 177
0.5 EC 0.22
0.25 EC 0.07
0.4 EC 0.04
Flax seed Canada 1973 1 0.5 0.09 0.07 V 174
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice Japan 1973 5 0.35 EC
Unhulled 0.20 Niigata,
1973
Straw 15.0-16.8
6 0.35 EC
Unhulled 0.21
Straw 15.9-16.7
6 0.35 EC
Unhulled 0.32
Straw 16.9-17.3
5 0.35 EC
Unhulled 0.11
Straw 22.4-23.3
6 0.35 EC
Unhulled 0.08
Straw 30.5-31.7
6 0.35 EC
Unhulled 0.03-0.05
Straw 6.15-8.33
5 0.8 dust 2%
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.11
Straw 7.20-7.80
Japan 1973 6 0.8 dust 2% Niigata,
1973
Unhulled 0.10
Straw 7.20-7.40
6 0.8 dust 2%
Unhulled 0.15-0.16
Straw 11.6-12.4
5 0.8 dust 2%
Unhulled 0.12
Straw 4.15-5.30
6 0.8 dust 2%
Unhulled 0.08-8.09
Straw 9.4-10.8
6 0.8 dust 2%
Unhulled 0.10
Straw 11.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 1.59-2.82
6 0.6-0.8 dust 2%
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 0 3 7 15 20 30 Ref.
Rice (cont'd)
Unhulled 0.10
Straw 10.2-10.3
Japan 1973 6 0.6-0.8 dust 2% Niigata,
1973
Unhulled 0.20
Straw 13.6
5 0.6-0.8 dust 2%
Unhulled 0.11
Straw 11.2-11.9
6 0.6-0.8 dust 2%
Unhulled 0.07-0.08
Straw 10.3-10.9
6 0.6-0.8 dust 2%
Unhulled 0.09-0.10
Straw 19.3-19.7
Tea (dry
manuf.) India 1973 2 1.3 EC 93.4 1.77 3.57 0.29 V 203
Assam
*1973 2 1.3 EC 77.3 0.11 3.67 0.23
*1973 1 1.3 EC 2.80-3.77
1973 1 1.3 EC 1.36-1.94
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet Canada 1973 1 0.5 EC
Roots) 0.05 0.05 V 170
)
Tops ) 0.09 0.07 V 170
Roots 1973 2 0.5 EC <0.02
Roots 2 1.0 EC <0.02
Roots 2 1.0 EC <0.02
Roots) 1 1.0 EC <0.02 0.04
Tops ) <0.02 <0.02
Japan 1972 3 0.35 EC Hokkaido,
Roots <0.02 <0.02 exp. sta.
1972
Tops 0.43-0.59 0.17-0.30
1972 5 0.35
Roots 0.01 <0.01
Tops 1.36-1.72 0.66-0.84
1972 3 0.35
Roots <0.01 <0.01
Tops 0.59-0.61 0.25-0.27
TABLE 3. (continued)
Application
Residues in mg/kg, at intervals (days) after application
rate
Crop Country Year No. kg a.i./ha formulation 30/33 38/45 50/52 75/80 Ref.
Sugarbeet
(cont'd)
1972 5 0.35
Roots 0.01 <0.01
Tops 0.93-1.02 0.34-0.39
Japan 1971 3 0.35 Hokkaido,
Roots <0.01 <0.01 exp. sta.
1972
Tops 0.59-0.66 0.34-0.39
5 0.35
Roots 0.01 0.01
Tops 1.75-1.87 0.93-0.98
1971 3 0.35
Roots <0.01 <0.01
Tops 0.64-0.69 0.26-0.32
5 0.35
Roots <0.01 <0.01
Tops 1.17-1.20 0.39-0.46
* Sun-dried.
In a 10 minutes brew of sun-dried tea harvested seven days after
treatment no residues of parent leptophos, leptophos-oxon or
desbromoleptophos was found. The levels of the phenol ranged between
<0.01 mg/kg and 0.06 mg/kg.
In a food chain
Data were obtained in two model studies (Anonymous, 1973;
Johnson, 1975) with 14C-leptophos in a simple food chain in which
water, Daphnia magna and the common blue gill (Lepomis macrochirus
rafinesque) were included.
Daphnia magna accumulated leptophos rather rapidly from the
water. In less than four hours leptophos in daphnia reached a constant
level about 1500 times that in the surrounding water. Blue gills
feeding on Daphnia magna exposed to leptophos reached a plateau about
1/10 of that in the daphnia.
It is obvious that the most significant route of accumulation of
leptophos residues by fish will be by direct uptake from water.
Blue gills exposed to leptophos in water only (not in the food)
contained mean 14C residues in the tissues of 0.179 mg/kg, about 750
times the mean concentration of 14C-leptophos in the water, after
10-42 days' exposure. When blue gills were exposed to both aqueous and
dietary 14C-leptophos for 10-28 days the residue level was almost
identical (0.170 mg/kg, about 770 times the concentration in the
water).
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours. The bio-transfer
rate of leptophos in the Daphnia-blue gill food chain was only 6.5% of
that of DDT, a typical bioaccumulating compound.
APPRAISAL
Leptophos was evaluated with regard to agricultural data by the
1974 Joint Meeting (FAO/WHO,1975). Arising from the list of
requirements, information was obtained on residues from supervised
trials in various areas on fruit crops (apples and pears), small
grains, rice, sugar beet, potatoes and on some vegetable crops.
Some information became available on residues in waste of
agricultural crops which may be used for feeding purposes, e.g. sugar
beet leaves, straw and chaff of cereal crops, including rice.
Additional data were obtained on the fate of leptophos residues
in apples, cabbage, lettuce and tomatoes from studies with 14C-phenyl
and 14C-phenoxy-labelled leptophos. Additional data became available
on the fate of 14C-phenoxy- and 14C-phenyl-labelled leptophos in
soil. It was shown in this study that the bound fraction increases
with time at all soil depths. The total 14C in the upper 2.5 cm layer
decreased with time from 92% of the applied radioactivity to 42% after
120 days; the total 14C in the lower layers was fairly constant
during that period.
New data were obtained from model studies with 14C-labelled
leptophos in a simple food chain which included water, Daphnia magna
and the common blue gill. Daphnia magna accumulated leptophos rather
rapidly from the water; in less than four hours leptophos plateaued in
Daphnia to levels about 1500 times the level in the surrounding water.
Blue gills feeding on Daphnia magna exposed to leptophos reached a
plateau one-tenth of that of the daphnids.
When Daphnia were transferred to insecticide-free water, their
leptophos level decreased by 50% in about 19 hours.
The bio-transfer rate of leptophos in the Daphnia-blue gill food
chain was less than one-tenth of that of DDT, which is recognized as a
bioaccumulating compound.
The new data available confirm the limits recorded at the 1974
Joint Meeting on cabbage, broccoli, lettuce, tomatoes, potatoes,
cotton seed and maize.
In the light of the temporary ADI established at the present
Meeting these limits are now recommended as temporary maximum residue
limits, and replace the guideline levels recorded in the 1974
monograph. Additional temporary maximum residue limits are
recommended.
Recommendations
The maximum residue limits refer to the sum of leptophos, its
oxygen analogue and desbromo-leptophos, expressed as leptophos.
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Apples, pears 2 42-60
Broccoli, Brussels sprouts,
cabbage 2 28-50
Lettuce 2 28 (outdoor)
Tomatoes 2 7
Cotton seed oil (crude) 1 28
Raw cereals (wheat, oats,
barley) 0.5 60
Rice (hulled) 0.5 30
(Cont'd)
Maximum Pre-harvest
residue interval on which
Commodity limit recommendation
mg/kg is based
Cotton seed, cotton seed meal 0.2 28
Carrots 0.2 60
Potatoes 0.1 21
Maize, fieldcorn (kernels) 0.05 7
Sweet corn (kernels and cobs,
husks and silk removed) 0.05 3-7
Sunflower seed 0.05 100
Sugar beet (roots) 0.05 50-60
Sugar beet (tops) 0.5
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1978)
1. Studies on the kinetics of accumulation of leptophos in storage
depots of two species, preferably non-rodent to investigate the
potential buildup in man to ultimate threshold levels.
2. Studies on the species variation for delayed neurotoxicity with
leptophos to assess its ultimate effect on man.
3. Epidemiological studies on people in exposed occupations in
agriculture or industry.
4. Further long-term low-level feeding studies in a species
susceptible to the delayed neurotoxic syndrome.
5. Residue data on the major crops for which national tolerances or
use recommendations exist, e.g., tea, vegetables not included in the
recommendations, and citrus fruits.
6. Residues in those parts of agricultural commodities which are
used either as such or as agricultural waste for animal feed.
DESIRABLE
1. Improved methodology for assessing the delayed neurotoxic
syndrome.
2. Studies on the mechanism of neurotoxic action of leptophos.
Further investigations into mechanism of action of TOCP and other
neurotoxic agents as compared to leptophos.
3. Data on current use patterns in countries outside the United
States of America and Canada and on residue levels resulting from
those uses.
REFERENCES
Anonymous. (1973) Exposure of 14C-leptophos to daphnia magna and
bluegill sunfish (lepomis macrochiris). Accumulation, distribution,
elimination and bio-concentration in the food chain. Unpublished
report from Bionomics, Inc., submitted to FAO by Velsicol Chemical
Corporation.
Abou-Donia, M.B., Othman, M.A., Tantawy, G., Khalil, A.Z. and Shawer,
M.F. (1974) Neurotoxic effect of leptophos. Experimentia, 30:63-64.
Abou-Donia, M.B. and Pressig, S. (1975) Studies on delayed
neurotoxicity produced by leptophos. Proc. Fed. Amer. Soc. Exptl.
Biol., 34:810.
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1971)
Mutagenic study with Phosvel (VCS-506) in albino mice. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Badie, M. and Whitacre, D.M. (1975) Metabolism of 14C-leptophos and
14C-4-bromo-2,5-dichlorophenol in rats: A multiple dosing study.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Braun, E., McEwen, F.L., Frank, R. and Ritcey, G. (1975) Residues of
leptophos and its metabolites following application to various crop
plants. J. Agr. Food Chem., 23(1):90-95.
Braun, E., McEwen, F.L., and Frank, R. (1974) Residues of
chlorpyriphos and leptophos in three field tested vegetable crops.
Canad. J. Plant Sci., 55:133-137.
Diaz, L.I. (1974) Radiotracer study of leptophos on cabbage. Velsicol
Chem. Corp. Lab. rep. 194. (Unpublished)
Dorough, H.W. (1974) Fate of phosvel-14C in burley tobacco. Progress
report of Department of Entomology, University of Kentucky, Lexington,
provided to Velsicol. (Unpublished)
Fink, R.J. (1971) Continuous feeding and residue study - cows with
technical Phosvel (VCS-506). Unpublished report from Hazleton
Laboratories, submitted to the World Health Organization by Velsicol
Chemical Corporation.
Fletcher, D., Jenkins, D.H., Kinoshita, P.R., Gordon, D.E. and
Keplinger, M.L. (1975) Neurotoxicity study with technical leptophos in
chickens. Unpublished report from Industrial Bio-Test Laboratories,
Inc., submitted to the World Health Organization by Velsicol Chemical
Corporation.
Haley, S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1972) Three
generation reproduction study with VCS-506 in albino rats. Unpublished
report by Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Haley, S., Kennedy, G.L. and Keplinger, M.L. (1973) Three-generation
reproduction study with VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Hartke, K., Lindberg, D.C., Wright, P.L. and Keplinger, M.L. (1971)
Two-year chronic oral toxicity study with VCS-506 technical in beagle
dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Hassan, A. (1975) Personal communication to the World Health
Organization.
Hathaway, D., Keplinger, M.L. and Fancher, O.E. (1968) Acute aerosol
inhalation toxicity study on VCS-506, 3 EC. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Hokkaido. (1972) Phosphel residue analysis on sugar beet. National
Hokkaido. Agr. Exp. Sta. Residue data provided to Velsicol.
(Unpublished)
Holmstead, R.L., Fukuto, T.R. and March, R.B. (1973) The metabolism of
O-(4-bromo-2,5-dichlorophenyl) O-methyl phenylphosphonothioate
(leptophos) in white mice and cotton plants. Archives of Environmental
Contamination and Toxicology. Vol. 1, No. 2, 133-147.
Johnson, B.T. (1973) Leptophos accumulation through a simple food
chain. Unpublished report from the United States Department of the
Interior, submitted to the World Health organization by Velsicol
Chemical Corporation.
Johnson, M.K. (1975) The delayed neuropathy caused by some
organophosphorus esters: Mechanism and challenge. CRC Critical Reviews
in Toxicology, June.
Kennedy, G. and Keplinger, M.L. (1971) Absorption, distribution, and
excretion study with Phosvel in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1971a) Characterization of 14C in
excreta of rats following oral administration of Phosvel. Unpublished
report from Industrial Bio-Test Laboratories, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Kennedy, G. and Keplinger, M.L. (1972) Absorption, distribution, and
excretion study with VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970) Distribution and
excretion study of VCS-506 in albino rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kennedy, G., Keplinger, M.L. and Fancher, O.E. (1970a)
Characterization of 14C in urine of rats following oral
administration of 14C-VCS-506. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kimmerle, G. (1972) Acute neurotoxicity studies in hens. Unpublished
report from Farbenfabriken Bayer, submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1971) Study of the
efficacy of atropine sulfate and 2-PAM as antidotes for VCS-506
intoxication in albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C., Keplinger, M.L. and Fancher, O.E. (1971a)
Acute oral (intragastric) toxicity studies with VCS-506 in one day old
and five day old albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World Health
Organization by Velsicol Chemical Corporation.
Kretchmar, B., Mastri, C. and Keplinger, M.L. (1972) Acute oral
toxicity study with oxon of VCS-506 in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Kretchmar, B,, Mastri, C. and Keplinger, M.L. (1972a) Acute oral range
finding toxicity study with S-isomeride in female albino rats.
Unpublished report from industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L. (1971)
Teratogenic study with Phosvel in albino rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health organization by Velsicol Chemical Corporation.
Lindburg, D., Vondruska, J.F. and Fancher, O.E. (1969) Ninety-day
subacute oral toxicity of VCS-506 in beagle dogs. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
March, R.B. and Fukuto, T.R. (1975) Reports on studies on the
photolysis and metabolism of Phosvel. Unpublished report from the
University of California, submitted to the World Health Organization
by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969) Acute oral
toxicity study on VCS-506 technical in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger, M.L, and Fancher, O.E. (1969a) Acute oral
toxicity study on two formulations of VCS-506 E.C. in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity on four metabolites of VCS-506 in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Mastri, C., Keplinger M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on phenylphosphonic acid in albino rats. Unpublished
report from industrial Bio-Test Laboratories, Inc., submitted to the
World Health Organization by Velsicol Chemical Corporation.
Miyagi. (1971) Phosphel residues on chinese cabbage. Miyagi Pref. Agr.
Exp. Sta. and Aichi Pref. Agr. Exp. Sta. Residues data provided to
Velsicol.
Niigata. (1973) Phosphel residue analysis on rice. Niigata Pref. Agr.
Exp. Sta. and Shizuoka Pref. Agr. Exp. Sta. Data provided to Velsicol.
(Unpublished)
Perkins, C.T. and Singh, V.K. (1971) Chicken toxicity and residue
study. Unpublished report from Bio/Toxicological Research
Laboratories, Inc., submitted to the World Health organization by
Velsicol Chemical Corporation.
Perkins, C.T. and Singh, V.K. (1971a) Beef animal feeding study.
Unpublished report from Bio/Toxicological Research Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J., Keplinger, M.L. and Fancher, O.E. (1970) Cholinesterase
study of VCS-506 in albino rats. Unpublished report from International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971) Twenty-eight day
target organ study with VCS-506 oxon metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Keplinger, M.L. and Fancher, O.E. (1971a) Twenty-eight
day target organ study with VCS-506 phenol metabolite in albino rats.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971b)
Ninety-day subacute oral toxicity, study with VCS-506 Phenol
metabolite in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Plank, J.B., Wright, P.L., Keplinger, M.L. and Fancher, O.E. (1971c)
Ninety-day subacute oral toxicity study with VCS-506 oxon metabolite
in albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health Organization by
Velsicol Chemical Corporation.
Rieck, C.E. (1974) Soil metabolism of 14C-leptophos. Report of Agr.
Dept., University of Kentucky, Lexington, provided to Velsicol.
(Unpublished)
Sanborn, A. and Metcalf, R.L. (1975) The fate of leptophos (Phosvel)
in a terrestrial-aquatic ecosystem. Unpublished report from the
University of Illinois submitted to the World Health Organization by
Velsicol Chemical Corporation.
Schroeder, C. (1971) The radiocarbon distribution of 14C labelled
Phosvel in lettuce and green beans. Unpublished report from WARF
Institute submitted to the World Health Organization by Velsicol
Chemical Corporation.
Schwemmer, B. (1971) UV photolysis of leptophos in solution.
Unpublished report submitted to the World Health Organization by
Velsicol Chemical Corporation.
Sleight, B.H. and Macek, K.J. (1973) Exposure of 14C-leptophos to
Daphnia magna and bluegill sunfish (Lepomis macrochirus):
Accumulation, distribution, elimination, and bioconcentration in the
food chain. Unpublished report from Bionomics, Inc. submitted to the
World Health Organization by Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971)
Twenty-eight day cholinesterase study with VCS-506 oxon metabolite in
female albino rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc. submitted to the World Health Organization by
Velsicol Chemical Corporation.
Smith, P.S., Plank, J.B., Wright, P.L. and Keplinger, M.L. (1971a)
Two-year chronic oral toxicity study with VCS-506 technical in albino
rats. Unpublished report from Industrial Bio-Test Laboratories, Inc.
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Smith, P.S., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. (1973)
Eighteen-month carcinogenic study with VCS-506 technical in Swiss
white mice. Unpublished report from Industrial Bio-Test Laboratories,
Inc. submitted to the World Health Organization by Velsicol Chemical
Corporation.
Stephens, J.L., Jenkins, D.H. and Fancher, O.E. (1969) Demyelination
study-chickens, VCS-506 Technical. Unpublished report from industrial
Bio-Test Laboratories, Inc. submitted to the World Health Organization
by Velsicol Chemical Corporation.
Suzuki, H. (1971) Phosvel-chicken feeding study. Unpublished report
from Velsicol Chemical Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Suzuki, H. (1971a) Phosvel-dairy cattle feeding study. Unpublished
report from Velsicol Chemical Corporation submitted to the World
Health Organization by Velsicol Chemical Corporation.
Tarka, S.M. Jr. (1973) Identification of radioactivity in the fat of
rats administered 14C-phenoxy-labelled leptophos. Unpublished report
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1965) Acute oral toxicity (LD50) in the albino rat.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1966) Acute oral toxicity (LD50) in male albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968) Comparative acute oral toxicity in male albino
rats. Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968a) Acute toxicity studies in the albino rabbit.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1968b) Acute toxicity study in the rabbit. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1968c) Ten-day subacute oral toxicity study in rats for
analysis of plasma, erythrocyte and brain cholinesterase activity.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1969) Ninety-day feeding study in albino rats.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970a) Acute oral toxicity (LD50) in the male albino
rat. Unpublished report from international Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1970b) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971a) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971b) Acute oral toxicity (LD50) in male and female
albino rats. Unpublished report from International Research and
Development Corporation submitted to the World Health Organization by
Velsicol Chemical Corporation.
Wazeter, F.X. (1971c) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971d) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (197le) Acute toxicity studies in rats and rabbits.
Unpublished report from International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. (1971f) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971g) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971h) Acute oral LD50 in albino rats. Unpublished
report from International Research and Development Corporation
submitted to the World Health Organization by Velsicol Chemical
Corporation.
Wazeter, F.X. (1971i) Acute toxicity studies in rats and rabbits.
Unpublished study by International Research and Development
Corporation submitted to the World Health Organization by Velsicol
Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1972) Acute oral toxicity (LD50)
in male and female albino rats. Unpublished report by International
Research and Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Wazeter, F.X. and Goldenthal, E.I. (1973) Acute dermal toxicity
(LD50) in male and female albino rabbits. Unpublished report from
International Development Corporation submitted to the World Health
Organization by Velsicol Chemical Corporation.
Whitacre, D.M. and Schnur, P.D. (1974) Metabolic fate of 14C-labelled
leptophos on apples. Velsicol Chem. Corp. Lab. rep. No. 195.
(Unpublished)
