
BIORESMETHRIN JMPR 1976
Explanation
Bioresmethrin was evaluated in 1975 (FAO/WHO 1976b) and a
monograph covering "Identity" and "Residues in food and their
evaluation" was published. Toxicological information was not then
available and therefore no ADI could be recommended. The 1975 Meeting
indicated that full Toxicological data was required and that the
following information was desirable.
1. Further information on the level and fate of bioresmethrin on
different classes of raw grains.
2. Residue data from supervised trials on other stored commodities,
including nuts, peanuts, lentils, dried fruit and dried
vegetables.
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in food at
the point of consumption following the use of bioresmethrin for
the control of various stored-product pests.
5. Improved procedures for the determination of bioresmethrin
residues in fruit and vegetables as well as stored products.
Information on many of these topics was received and evaluated by
the Meeting and the following monograph addendum is provided.
IDENTITY AND PROPERTIES
Bioresmethrin was developed under grants from the National
Research and Development Corporation by scientists at Rothamsted
Experimental Station in the United Kingdom. At least five principal
companies have been licenced to manufacture, formulate or distribute
bioresmethrin insecticides in different parts of the world and for
different fields of use.
The effective performance of bioresmethrin and its novelty, being
the first synthetic pyrethroid with properties comparable to natural
pyrethrins, has stimulated many independent scientists and government
research workers to study the chemistry, metabolism, biological
effects and fate of bioresmethrin. Much of this information is
available in published literature and in addition the Meeting had
access to the results of a number of on-going studies.
Because the (+) cis isomer of bioresmethrin is acutely somewhat
more toxic than bioresmethrin there is interest in whether this isomer
could be a significant component of technical bioresmethrin. In the
manufacturing process the optical isomers of chrysanthemic acid are
separated by procedures which include crystallisation and it is
understood that substantially all of the (+) cis isomer is removed
before the esterification reaction.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Bioresmethrin is rapidly absorbed (following oral administration)
from the gut and widely distributed in the body within 3 hours. Whole
body radioautographic techniques monitored the distribution of
3H-bioresmethrin following oral or intravenous administration to
rats. At 24 hours following oral administration, most tissues showed
greatly reduced residual radioactivity but concentration in fatty and
other tissues was high (adipose tissue, mesenteric skin, testis,
epididimus, lachrymal gland and connective tissue). The excretion of
3H activity into the bile duct was demonstrated with rats surgically
cannulated to collect the bile. Shortly after iv3 treatment (50%
after 24 hours and 60% after 72 hours) 3H was found in the bile.
After iv administration a large amount was recovered in faeces
suggesting significant enterohepatic circulation (Farebrother, 1973).
Following oral administration to rats (14C-carboxyl label, 0.87
mg/kg), bioresmethrin was slowly eliminated from the body with only
73% of the administered dose accounted for in faeces (32%) and urine
(41%) after 6 days (means from two experiments). The highest residue
after 6 days was found in the fat: other tissues contained 0.01 mg/kg.
Residues were observed in other tissues analyzed following
administration of bioresmethrin (alcohol label): Fat > blood
> lung > kidney > liver > heart > muscle > spleen >> brain.
After two weeks excretion was not complete (Ueda, et al., 1975b).
Qualitative identification of several metabolites of bioresmethrin was
performed. No unmetabolized bioresmethrin was excreted. The
metabolites most slowly excreted (persisting longest in the body)
arise from the alcohol-labelled bioresmethrin. Those arising from the
acid moiety are rapidly excreted.
Biotransformation
Information on the fate of bioresmethrin in laboratory animals
was discussed by the 1975 Joint Meeting (FAO/WHO, 1976b). Some of this
is repeated in the extended discussion which follows.
The biodegradation of bioresmethrin is a complex phenomenon with
several reactions occurring simultaneously and taking place at various
positions in the molecule. The initial step in the metabolism is
cleavage at the ester linkage, a reaction found to be catalyzed by
esterases localized in the liver microsome. Transisomerization was
reported with bioresmethrin but was apparently limited to
isomerization of degradation products, not observed with the parent
molecule and seen only when bioresmethrin was administered at low
levels. Transisomerization was not noted on administration of higher
levels by ip injection (low dose = 1 mg/kg; high dose = 3 gm/rat over
a period of 3 days administered 2X/day). Transisomerization occurred
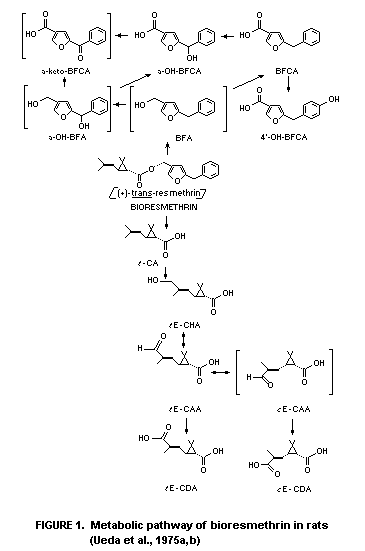
only with the acid portion of the molecule as observed in Figure 1
(tE-CAA cE-CDA), which shows the probable metabolic route for both
the acid and alcohol moieties of bioresmethrin in rats.
In vitro studies with rat and mouse liver preparations
suggested mouse liver esterases hydrolyze bioresmethrin
((+)-trans-resmethrin isomer) rapidly relative to the corresponding
cis-isomers ((+)-or (-)-cis-resmethrin) while microsomal enzymes
oxidize the (+)-trans-isomer, bioresmethrin, more slowly than the
cis-isomers (Ueda et al., 1975a). When administered to rats
bioresmethrin undergoes a complicated series of reactions involving
initial ester cleavage and subsequent oxidation with or without
conjugation of both the acid and alcohol metabolites.
Bioresmethrin is degraded by ester cleavage and the alcohol
moiety is oxidized to 5-benzyl-3-furylmethanol (BFA),
5-benzyl-3-furoic acid (BFCA), 4'-hydroxy BFCA and alpha-hydroxy BFCA
(alpha-OH-BFCA). The chrysanthemate (acid) moiety undergoes oxidation
from trans-chrysanthemic acid (tE-CHA) to
2,2-dimethyl3-(2'hydroxymethyl-1'-propenyl)cyclopropanecarboxylic acid
(tE-CHA) (oxidative metabolism at the methyl group of the isobutenyl
side chain trans (E) to the cyclopropane). This is further oxidized
through the formyl derivative (CAA) to the dicarboxylic acid isomers
(tE-CDA and cE-CDA). It is at the CAA oxidation stage where
isomerization may occur through the proposed aldehyde (cE-CAA)
intermediate to (cE-CDA) the cis-dicarboxylic acid (Ueda et al.,
1975b). This metabolic sequence may also account for the consideration
of Verschoyle and Barnes (1972) that as a delay in signs of poisoning
was evident following iv administration, bioresmethrin may be
converted in vivo to a toxic metabolite. The presence of
(+)-trans-CA,-BFA and BFCA as metabolites, which are more toxic than
bioresmethrin, may account for their observation and conclusions. The
metabolic sequence is very similar qualitatively to that observed with
resmethrin although much less complicated because of the lack of
isomeric products (Miyamoto et al., 1971) and because of the
specificity of certain isomers to enzymatic degradation by selected
routes as mentioned above (Ueda et al., 1975a, b; Abernethy and
Casida, 1973).
TOXICOLOGICAL STUDIES
Special study on teratology
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin in doses of 0, 10, 20, 40 and 80 mg/kg by oral gavage
daily from day 8-16 of gestation. The does were sacrificed on day 28
and examined for implantation, live and dead fetuses, resorption sites
and abnormalities (after staining a representative number for skeletal
examination). There was no apparent effect on parents in the study as
 growth and gestation were unaffected. There was an increase in dead
fetuses at the highest dose and a large number of resorption sites
noted at all treatment levels. There were a number of deformed fetuses
observed but the total numbers were not sufficient for an adequate
statistical evaluation. The deformities included straight tail,
crossed hind limbs and unilateral union of 6th and 7th ribs at the
sternal end. An overall fetal loss was observed at all dose levels
(primarily because of the large number of resorption sites recorded).
(Waldron, 1969).
Special studies on potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin and/or piperonyl butoxide alone and in
combinations at doses approximating the acute ip LD50 value. No
potentiation of the acute toxicity was observed in this study. In all
cases with bioresmethrin combinations, the observed LD50 values were
equal to or exceeded the expected value (Wallwork and Malone, 1971).
Special studies on irritancy and sensitization
Groups of adult guinea pigs (6 males/group) were tested with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or
2,4-dinitrochloro-benzene (DNCB) (0.1 ml of a (1% w/v) formulation in
mineral oil) to assess the sensitization properties. The test
substance was applied to the ears for 4 days. On day 7, 0.2 ml was
applied dermally and the degree of irritation recorded. As expected,
the DNCB was an irritant while bioresmethrin showed only traces of
erythema suggesting a low potential for sensitization and irritation
(Chesher and Malone, 1970b).
Instillation of technical bioresmethrin into the eye of rabbits
(0.1 ml) produced no irritation or corneal damage. A group of six
rabbits was treated with no indication of ocular hazard (Chesher and
Malone, 1970c).
Acute toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork, et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chesher and Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
f inhalation >872 mg/m3 Wallwork & Malone
(24 hr exposure)
f dermal >10000 Wallwork et al., 1970
LD50
Species Sex Route (mg/kg) Reference
Mouse f oral >10000 Wallwork et al., 1970
m oral 3100 Ueda, et al., 1975b
f ip 5359 Wallwork et al., 1973
m ip >1500 Ueda et al., 1975b
Rabbit
Chicken oral >10000 Wallwork et al., 1970
Chesher & Malone, 1970a
Signs of poisoning: After 2 or more hours following oral
administration, tremors occurred; animals were sensitive to each other
and aggressive. The final stages of poisoning consisted of convulsive
twitching, prostration, coma, and death normally between 3 and 24
hours.
Mode of action: The nervous systems of both vertebrates and
invertebrates are equally sensitive and respond in a similar manner to
pyrethins (both natural and synthetic). The uncoordinated tremors of
the rat are similar to those signs of poisoning generally associated
with organochlorine pesticides.
Acute toxicity of metabolites
LD50
(mg/kg*)
Metabolite ip Oral
1) (+)-trans-resmethrin (bioresmethrin) >1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-cis-resmethrin 1 320
(5-benzyl-3-furylmethyl (-)-cis-
chrysanthemate)
3) (+)-trans-CA (t-CA) 98 280
(*)-trans-chrysanthemic acid
4) (+)-cis-CA (c-CA)1 600
(+)-cis-chrysanthemic acid
5) (+)-trans-CDA. (tE-CDA) 408
(+)-trans-chrysanthemumdicarboxylic acid
LD50
(mg/kg*)
Metabolite ip Oral
6) BFA 75 310
5-benzyl-3-furylmethanol
7) BFCA
5-benzyl-3-furoic acid 46
*LD50 - male mouse 24-hour toxicity following intraperitoneal
injection.
1 Cis isomers (numbers 2 and 4) are not metabolites of
bioresmethrin.
Cis isomers are metabolites in degradation of resmethrin.
(Ueda et al., 1975b; Miyamoto, 1975, 1976)
Short-term studies
Rat
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage daily, six days per week for three weeks at doses of
0, 1000 and 2000 mg/kg body weight. There was no mortality
attributable to bioresmethrin. There was a slight reduction in weight
at 2000 mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and hematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated).
Groups of rats (18 males and 18 females/group) were fed
bioresmethrin in the diet at concentrations of 0, 400, 1200 and 8000
ppm (the latter dose group was fed 4000 ppm for 30 days and the level
increased thereafter) for 91 days. There was no mortality observed in
this study. Food consumption was normal and food conversion was
unaffected by bioresmethrin in the diet. Growth was reduced at the
highest dose level which was accompanied by changes in blood chemistry
parameters indicating liver dysfunction (SAP, SGOT, and urinary
nitrogen increased at 90 days, glucose content reduced). Depression of
red blood cell count was observed at 1200 ppm although no consistent
parallel changes were seen in hemoglobin content or packed cell
volume. Urinalyses were normal. Gross and microscopic analysis of
tissues and organs showed an increase in liver weight at 4000 ppm and
a decrease in several other organs (spleen, heart, brain, thymus.
prostate, ovary and uterus at 1200 and 4000/8000 ppm fatty
infiltration of liver was seen on microscopic examination. A no-effect
level in this study would be 400 ppm equivalent to an average daily
intake of 32.8 to 36.1 mg/kg body weight for males and females
respectively (Wallwork et al., 1971).
Dog
Groups of dogs (2 of each sex/group) were administered
bioresmethrin by gavage, daily at dose levels of 0 and 500 mg/kg body
weight for 7 days followed by a dose increase to 1000 mg/kg for a
further 14 days. There were no effects noted in this test with respect
to mortality, behaviour, body weight changes, hematology, blood
chemistry or urinalysis parameters or on electrocardiograph
measurements. Short-term administration for three weeks at an oral
dose of 1000 mg/kg was uneventful in the parameters measured (Malone
and Chesher, 1970).
In a continuation of the above trial, after a two-week interval
on control diets, dogs were administered bioresmethrin by gavage for 7
days at a dose of 2000 mg/kg body weight. Again no significant effects
were noted in the parameters recorded above (Chesher and Malone,
1970b).
Groups of dogs (3 male and 3 female/group) were administered
bioresmethrin by gavage in gelatin capsule, daily for 90 days at
dosage levels of 0, 25, 80 and 250 mg/kg (the high dose was increased
to 500 mg/kg in week 7). There was no mortality. Growth, food
consumption and calculated food utilization parameters were normal.
Clinical biochemistry, ophthalmological and urinalysis parameters were
normal at all intervals (30, 60 and 90 days) examined. In the high
dose group, reduced RBC count, hemoglobin content and packed cell
volume values were noted. BUN was slightly increased only at the high
dose after 12 weeks. There were no adverse effects noted on gross or
microscopic examination of tissues and organs (including bioresmethrin
bone marrow). A no-effect level was observed to be 80 mg/kg
(equivalent to an average of 1600 ppm in the diet) (Noel et al.,
1971).
OBSERVATIONS IN MAN
None.
COMMENTS
Bioresmethrin is the common name for the
(+)-trans-chrysanthemate ester of 5-benzyl-3-furylmethanol, a
synthetic pyrethroid insecticide. Bioresmethrin is a component of
another synthetic pyrethroid, resmethrin, whose toxicological
properties have not been evaluated. Resmethrin consists of a mixture
of isomers having the empirical formula C22H2603. The mixture is
made up of approximately 65% trans-(bioresmethrin, approximately one
half of this fraction, is the (+)-trans-isomer) and 35%
cis-isomers.
In general, the (-)-isomers have low biological activity. The
(+)-cis-isomer of bioresmethrin is acutely more toxic than
bioresmethrin. Bioresmethrin is relatively non-toxic on an acute basis
and readily degradable. In animals, it is absorbed rapidly from the
gut and translocated partially to the lipid portions of the body.
Bioresmethrin is excreted slowly, probably owing to its lipophilicity.
It has been shown to reflect a delay in the onset of acute signs of
poisoning possibly owing to delay in ester cleavage which results in
more toxic (hydrolysis) products. Bioresmethrin is metabolised by
oxidation and hydrolysis occurring at various sites in the molecule.
There is no evidence for isomerisation of bioresmethrin to the
(+)-cis-isomer which might result in greater toxicity.
Several short term toxicology studies in rat and dog are
available. Bioresmethrin is not a teratogen although at high levels it
has been shown to induce some fetal abnormalities and fetal mortality.
Bioresmethrin is nonirritating and does not induce a sensitisation
reaction. In short term studies at high doses, thymic atrophy was
noted over a 3 week test period. This was accompanied by increased
liver size. In rats reduced growth, dysfunction, and fatty
infiltration of the liver were observed in a 90 day study at dietary
levels of 1200 ppm and above. In dogs, several hematological
parameters were affected at dose levels of 250 and 500 mg/kg, as noted
over a 90 day period. No-effect levels were noted based on short term
studies only. There were no observations in man available for
consideration.
Although short term studies were available along with several
special studies which did not specifically raise any unusual
toxicological factors, the absence of data from long term studies
precluded the Meeting from estimating an ADI for man. In concurrence
with previous conclusions, the Meeting expressed its need for
evaluation of long term studies in its consideration of an ADI for
man. This is especially important in the case of bioresmethrin, owing
to the fact that this synthetic pyrethroid is the first of a chemical
class of pesticides projected for extensive use in future. Although
bioresmethrin effects have been observed only at relatively high dose
levels in short term studies, the potential for adverse toxicological
effects in long term studies needs to be evaluated.
TOXICOLOGICAL EVALUATION
No ADI for man was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The high potency and broad spectrum activity of bioresmethrin
could be expected to lead to a wide field of use. Susceptibility to
rapid degradation by light and the availability of several other
synthetic pyrethroid insecticides with much superior stability to
light has however almost completely discouraged interest in
bioresmethrin for use against pests of fruit, vegetable or forage
crops.
In addition to the widespread use of bioresmethrin against pests
of households and of public health there has been considerable
interest in its use for the control of insect pests of stored
products, particularly the lesser grain borer, Rhyzopertha
domenica, which is not controlled by acceptable rates of
organophosphorus insecticides.
The 1975 monograph (FAO/WHO, 1976b) reviewed many scientific
papers on the properties and usefulness of bioresmethrin against
stored product pests. Since the preparation of the monograph a number
of additional studies have been completed.
Desmarchelier (1976) showed that combinations of pyrethroids,
which are particularly effective against Rhyzopertha domenica, and
organophosphorus insecticides which are effective against Tribolium
castaneum controlled both species for as long as the pyrethroid
alone controlled Rhyzopertha domenica and the organophosphorus
compound alone controlled Tribolium castaneum. Bioresmethrin was by
far the best of the many pyrethroid compounds examined.
Bengston et al (1976a) reporting extensive field trial to compare
a range of grain protectant insecticides showed that none of the five
organophosphorus insecticides evaluated was adequate to control
Rhyzopertha domenica, which lived and reproduced in all samples
including those drawn immediately after insecticide application.
Bengston et al. (1976b) demonstrated, in commercial scale trials,
the outstanding performance of several organophosphorus grain
protectants when combined with bioresmethrin. Complete protection was
obtained against all strains of all species of stored product pests
and reproduction was completely inhibited for many months.
The development of organophosphorus- and fumigant-resistant
strains of many stored product pests (Champ and Dyte, 1975) in most
countries and in all continents points to the urgency of having
available acceptable insecticides which have different properties and
modes of action. Supplies of natural-pyrethrum are not adequate to
meet the developing emergency.
Australian authorities have approved the use of bioresmethrin at
the rate of 2 mg/kg for the control of Rhyzopertha domenica and
other insect pests of wheat, oats and barley to protect valuable
stocks of grain and to ensure compliance with the phytosanitary
requirements of grain-importing countries. It is understood that
similar action has been taken in a number of other countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatments
No further information on the level or fate of bioresmethrin
residues on fruit or vegetables has become available. In view of the
advances which have been made with the development of several other
pyrethroid insecticides with outstanding efficacy against a wide range
of pests combined with a high level of stability to light it appears
unlikely that bioresmethrin will be developed or utilised for
pre-harvest application.
Post-harvest treatments
Extensive commercial scale trials were carried out in Australia
during 1976 at 20 sites involving 42 silos each containing from 2,000
to 8,000 tonnes of wheat. The wheat had been placed in storage
directly from the harvest. The temperature of the grain ranged from
27° to 39°C and the moisture content from 9.2% to 12.0%.
Pirimiphos-methyl or fenitrothion was applied in the form of a dilute
emulsion at the rate of 1 litre/tonne to give a deposit of 5 mg/kg of
pirimiphos-methyl or 10 mg/kg of fenitrothion. Bioresmethrin emulsion
was added at the same time to give a nominal deposit 1 mg/kg. The
amount deposited was estimated from the quantity of emulsion used.
Samples were withdrawn for bioassay and for chemical analysis at
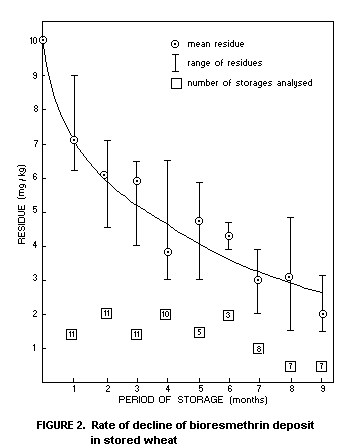
regular intervals for 9 months. The temperature of the grain was
recorded over this period. Figure 2 illustrates the rate of decline of
the bioresmethrin deposit with time (Desmarchelier et al., 1976a). The
variations in the amount found at any one interval after application
are not particularly great but are no doubt due to variations in
temperature, grain moisture and amount of bioresmethrin applied at
each separate site.
These data confirm earlier studies under laboratory conditions
which indicate that the half-life of bioresmethrin in stored grain is
directly dependent on temperature. At 30-35°C the half-life is 8-10
weeks whilst at 20°C it exceeds 26 weeks. This degree of stability is
greater than that shown by malathion under similar conditions and
increases the effectiveness of bioresmethrin as a grain protectant
insecticide. Because the stability is combined with potency against
the target species it is not necessary to use concentrations above 2
mg/kg even on grain stored at relatively high temperatures.
FATE OF RESIDUES
In animals
The metabolism of bioresmethrin in the rat and mouse is described
above ("Biochemical aspects")
In grain
One of the questions remaining in doubt at the 1975 Meeting was
the fate of bioresmethrin on different classes of raw grain.
Preliminary results of an on-going official study in Australia
indicate that there is no significant difference in the stability
(persistence) of bioresmethrin deposits on different types of raw
grain held at 25°C under comparable moisture regimes (Desmarchelier,
growth and gestation were unaffected. There was an increase in dead
fetuses at the highest dose and a large number of resorption sites
noted at all treatment levels. There were a number of deformed fetuses
observed but the total numbers were not sufficient for an adequate
statistical evaluation. The deformities included straight tail,
crossed hind limbs and unilateral union of 6th and 7th ribs at the
sternal end. An overall fetal loss was observed at all dose levels
(primarily because of the large number of resorption sites recorded).
(Waldron, 1969).
Special studies on potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin and/or piperonyl butoxide alone and in
combinations at doses approximating the acute ip LD50 value. No
potentiation of the acute toxicity was observed in this study. In all
cases with bioresmethrin combinations, the observed LD50 values were
equal to or exceeded the expected value (Wallwork and Malone, 1971).
Special studies on irritancy and sensitization
Groups of adult guinea pigs (6 males/group) were tested with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or
2,4-dinitrochloro-benzene (DNCB) (0.1 ml of a (1% w/v) formulation in
mineral oil) to assess the sensitization properties. The test
substance was applied to the ears for 4 days. On day 7, 0.2 ml was
applied dermally and the degree of irritation recorded. As expected,
the DNCB was an irritant while bioresmethrin showed only traces of
erythema suggesting a low potential for sensitization and irritation
(Chesher and Malone, 1970b).
Instillation of technical bioresmethrin into the eye of rabbits
(0.1 ml) produced no irritation or corneal damage. A group of six
rabbits was treated with no indication of ocular hazard (Chesher and
Malone, 1970c).
Acute toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork, et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chesher and Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
f inhalation >872 mg/m3 Wallwork & Malone
(24 hr exposure)
f dermal >10000 Wallwork et al., 1970
LD50
Species Sex Route (mg/kg) Reference
Mouse f oral >10000 Wallwork et al., 1970
m oral 3100 Ueda, et al., 1975b
f ip 5359 Wallwork et al., 1973
m ip >1500 Ueda et al., 1975b
Rabbit
Chicken oral >10000 Wallwork et al., 1970
Chesher & Malone, 1970a
Signs of poisoning: After 2 or more hours following oral
administration, tremors occurred; animals were sensitive to each other
and aggressive. The final stages of poisoning consisted of convulsive
twitching, prostration, coma, and death normally between 3 and 24
hours.
Mode of action: The nervous systems of both vertebrates and
invertebrates are equally sensitive and respond in a similar manner to
pyrethins (both natural and synthetic). The uncoordinated tremors of
the rat are similar to those signs of poisoning generally associated
with organochlorine pesticides.
Acute toxicity of metabolites
LD50
(mg/kg*)
Metabolite ip Oral
1) (+)-trans-resmethrin (bioresmethrin) >1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-cis-resmethrin 1 320
(5-benzyl-3-furylmethyl (-)-cis-
chrysanthemate)
3) (+)-trans-CA (t-CA) 98 280
(*)-trans-chrysanthemic acid
4) (+)-cis-CA (c-CA)1 600
(+)-cis-chrysanthemic acid
5) (+)-trans-CDA. (tE-CDA) 408
(+)-trans-chrysanthemumdicarboxylic acid
LD50
(mg/kg*)
Metabolite ip Oral
6) BFA 75 310
5-benzyl-3-furylmethanol
7) BFCA
5-benzyl-3-furoic acid 46
*LD50 - male mouse 24-hour toxicity following intraperitoneal
injection.
1 Cis isomers (numbers 2 and 4) are not metabolites of
bioresmethrin.
Cis isomers are metabolites in degradation of resmethrin.
(Ueda et al., 1975b; Miyamoto, 1975, 1976)
Short-term studies
Rat
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage daily, six days per week for three weeks at doses of
0, 1000 and 2000 mg/kg body weight. There was no mortality
attributable to bioresmethrin. There was a slight reduction in weight
at 2000 mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and hematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated).
Groups of rats (18 males and 18 females/group) were fed
bioresmethrin in the diet at concentrations of 0, 400, 1200 and 8000
ppm (the latter dose group was fed 4000 ppm for 30 days and the level
increased thereafter) for 91 days. There was no mortality observed in
this study. Food consumption was normal and food conversion was
unaffected by bioresmethrin in the diet. Growth was reduced at the
highest dose level which was accompanied by changes in blood chemistry
parameters indicating liver dysfunction (SAP, SGOT, and urinary
nitrogen increased at 90 days, glucose content reduced). Depression of
red blood cell count was observed at 1200 ppm although no consistent
parallel changes were seen in hemoglobin content or packed cell
volume. Urinalyses were normal. Gross and microscopic analysis of
tissues and organs showed an increase in liver weight at 4000 ppm and
a decrease in several other organs (spleen, heart, brain, thymus.
prostate, ovary and uterus at 1200 and 4000/8000 ppm fatty
infiltration of liver was seen on microscopic examination. A no-effect
level in this study would be 400 ppm equivalent to an average daily
intake of 32.8 to 36.1 mg/kg body weight for males and females
respectively (Wallwork et al., 1971).
Dog
Groups of dogs (2 of each sex/group) were administered
bioresmethrin by gavage, daily at dose levels of 0 and 500 mg/kg body
weight for 7 days followed by a dose increase to 1000 mg/kg for a
further 14 days. There were no effects noted in this test with respect
to mortality, behaviour, body weight changes, hematology, blood
chemistry or urinalysis parameters or on electrocardiograph
measurements. Short-term administration for three weeks at an oral
dose of 1000 mg/kg was uneventful in the parameters measured (Malone
and Chesher, 1970).
In a continuation of the above trial, after a two-week interval
on control diets, dogs were administered bioresmethrin by gavage for 7
days at a dose of 2000 mg/kg body weight. Again no significant effects
were noted in the parameters recorded above (Chesher and Malone,
1970b).
Groups of dogs (3 male and 3 female/group) were administered
bioresmethrin by gavage in gelatin capsule, daily for 90 days at
dosage levels of 0, 25, 80 and 250 mg/kg (the high dose was increased
to 500 mg/kg in week 7). There was no mortality. Growth, food
consumption and calculated food utilization parameters were normal.
Clinical biochemistry, ophthalmological and urinalysis parameters were
normal at all intervals (30, 60 and 90 days) examined. In the high
dose group, reduced RBC count, hemoglobin content and packed cell
volume values were noted. BUN was slightly increased only at the high
dose after 12 weeks. There were no adverse effects noted on gross or
microscopic examination of tissues and organs (including bioresmethrin
bone marrow). A no-effect level was observed to be 80 mg/kg
(equivalent to an average of 1600 ppm in the diet) (Noel et al.,
1971).
OBSERVATIONS IN MAN
None.
COMMENTS
Bioresmethrin is the common name for the
(+)-trans-chrysanthemate ester of 5-benzyl-3-furylmethanol, a
synthetic pyrethroid insecticide. Bioresmethrin is a component of
another synthetic pyrethroid, resmethrin, whose toxicological
properties have not been evaluated. Resmethrin consists of a mixture
of isomers having the empirical formula C22H2603. The mixture is
made up of approximately 65% trans-(bioresmethrin, approximately one
half of this fraction, is the (+)-trans-isomer) and 35%
cis-isomers.
In general, the (-)-isomers have low biological activity. The
(+)-cis-isomer of bioresmethrin is acutely more toxic than
bioresmethrin. Bioresmethrin is relatively non-toxic on an acute basis
and readily degradable. In animals, it is absorbed rapidly from the
gut and translocated partially to the lipid portions of the body.
Bioresmethrin is excreted slowly, probably owing to its lipophilicity.
It has been shown to reflect a delay in the onset of acute signs of
poisoning possibly owing to delay in ester cleavage which results in
more toxic (hydrolysis) products. Bioresmethrin is metabolised by
oxidation and hydrolysis occurring at various sites in the molecule.
There is no evidence for isomerisation of bioresmethrin to the
(+)-cis-isomer which might result in greater toxicity.
Several short term toxicology studies in rat and dog are
available. Bioresmethrin is not a teratogen although at high levels it
has been shown to induce some fetal abnormalities and fetal mortality.
Bioresmethrin is nonirritating and does not induce a sensitisation
reaction. In short term studies at high doses, thymic atrophy was
noted over a 3 week test period. This was accompanied by increased
liver size. In rats reduced growth, dysfunction, and fatty
infiltration of the liver were observed in a 90 day study at dietary
levels of 1200 ppm and above. In dogs, several hematological
parameters were affected at dose levels of 250 and 500 mg/kg, as noted
over a 90 day period. No-effect levels were noted based on short term
studies only. There were no observations in man available for
consideration.
Although short term studies were available along with several
special studies which did not specifically raise any unusual
toxicological factors, the absence of data from long term studies
precluded the Meeting from estimating an ADI for man. In concurrence
with previous conclusions, the Meeting expressed its need for
evaluation of long term studies in its consideration of an ADI for
man. This is especially important in the case of bioresmethrin, owing
to the fact that this synthetic pyrethroid is the first of a chemical
class of pesticides projected for extensive use in future. Although
bioresmethrin effects have been observed only at relatively high dose
levels in short term studies, the potential for adverse toxicological
effects in long term studies needs to be evaluated.
TOXICOLOGICAL EVALUATION
No ADI for man was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The high potency and broad spectrum activity of bioresmethrin
could be expected to lead to a wide field of use. Susceptibility to
rapid degradation by light and the availability of several other
synthetic pyrethroid insecticides with much superior stability to
light has however almost completely discouraged interest in
bioresmethrin for use against pests of fruit, vegetable or forage
crops.
In addition to the widespread use of bioresmethrin against pests
of households and of public health there has been considerable
interest in its use for the control of insect pests of stored
products, particularly the lesser grain borer, Rhyzopertha
domenica, which is not controlled by acceptable rates of
organophosphorus insecticides.
The 1975 monograph (FAO/WHO, 1976b) reviewed many scientific
papers on the properties and usefulness of bioresmethrin against
stored product pests. Since the preparation of the monograph a number
of additional studies have been completed.
Desmarchelier (1976) showed that combinations of pyrethroids,
which are particularly effective against Rhyzopertha domenica, and
organophosphorus insecticides which are effective against Tribolium
castaneum controlled both species for as long as the pyrethroid
alone controlled Rhyzopertha domenica and the organophosphorus
compound alone controlled Tribolium castaneum. Bioresmethrin was by
far the best of the many pyrethroid compounds examined.
Bengston et al (1976a) reporting extensive field trial to compare
a range of grain protectant insecticides showed that none of the five
organophosphorus insecticides evaluated was adequate to control
Rhyzopertha domenica, which lived and reproduced in all samples
including those drawn immediately after insecticide application.
Bengston et al. (1976b) demonstrated, in commercial scale trials,
the outstanding performance of several organophosphorus grain
protectants when combined with bioresmethrin. Complete protection was
obtained against all strains of all species of stored product pests
and reproduction was completely inhibited for many months.
The development of organophosphorus- and fumigant-resistant
strains of many stored product pests (Champ and Dyte, 1975) in most
countries and in all continents points to the urgency of having
available acceptable insecticides which have different properties and
modes of action. Supplies of natural-pyrethrum are not adequate to
meet the developing emergency.
Australian authorities have approved the use of bioresmethrin at
the rate of 2 mg/kg for the control of Rhyzopertha domenica and
other insect pests of wheat, oats and barley to protect valuable
stocks of grain and to ensure compliance with the phytosanitary
requirements of grain-importing countries. It is understood that
similar action has been taken in a number of other countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatments
No further information on the level or fate of bioresmethrin
residues on fruit or vegetables has become available. In view of the
advances which have been made with the development of several other
pyrethroid insecticides with outstanding efficacy against a wide range
of pests combined with a high level of stability to light it appears
unlikely that bioresmethrin will be developed or utilised for
pre-harvest application.
Post-harvest treatments
Extensive commercial scale trials were carried out in Australia
during 1976 at 20 sites involving 42 silos each containing from 2,000
to 8,000 tonnes of wheat. The wheat had been placed in storage
directly from the harvest. The temperature of the grain ranged from
27° to 39°C and the moisture content from 9.2% to 12.0%.
Pirimiphos-methyl or fenitrothion was applied in the form of a dilute
emulsion at the rate of 1 litre/tonne to give a deposit of 5 mg/kg of
pirimiphos-methyl or 10 mg/kg of fenitrothion. Bioresmethrin emulsion
was added at the same time to give a nominal deposit 1 mg/kg. The
amount deposited was estimated from the quantity of emulsion used.
Samples were withdrawn for bioassay and for chemical analysis at
regular intervals for 9 months. The temperature of the grain was
recorded over this period. Figure 2 illustrates the rate of decline of
the bioresmethrin deposit with time (Desmarchelier et al., 1976a). The
variations in the amount found at any one interval after application
are not particularly great but are no doubt due to variations in
temperature, grain moisture and amount of bioresmethrin applied at
each separate site.
These data confirm earlier studies under laboratory conditions
which indicate that the half-life of bioresmethrin in stored grain is
directly dependent on temperature. At 30-35°C the half-life is 8-10
weeks whilst at 20°C it exceeds 26 weeks. This degree of stability is
greater than that shown by malathion under similar conditions and
increases the effectiveness of bioresmethrin as a grain protectant
insecticide. Because the stability is combined with potency against
the target species it is not necessary to use concentrations above 2
mg/kg even on grain stored at relatively high temperatures.
FATE OF RESIDUES
In animals
The metabolism of bioresmethrin in the rat and mouse is described
above ("Biochemical aspects")
In grain
One of the questions remaining in doubt at the 1975 Meeting was
the fate of bioresmethrin on different classes of raw grain.
Preliminary results of an on-going official study in Australia
indicate that there is no significant difference in the stability
(persistence) of bioresmethrin deposits on different types of raw
grain held at 25°C under comparable moisture regimes (Desmarchelier,
 1976b). Twenty kg quantities of barley, oats, rice in husk, husked
rice, polished rice and wheat were treated under standardised
conditions with a dilute emulsion of bioresmethrin/piperonyl butoxide
(1:5) at a rate designed to deposit 7 mg bioresmethrin per kg of
grain. The amount applied was deliberately high to enable the effects
of long term storage and processing to be compared and to ensure that
analytical difficulties would not interfere. The grain was held in a
constant temperature room at 25°C and was sampled after 3 and 6
months.
Table 1 compares the level of bioresmethrin found in each type of
grain after 3 and 6 months in storage.
TABLE 1. Effect of storage at 25°C on bioresmethrin residues on
various grains
Residue*, mg/kg
after storage for
Grain moisture % 3 months 6 months
Barley 13.0 3.5 1.75
Oats 12 3.5 2.0
Rice in husk 13 1.5 1.5
Husked rice 12.5 3.5 1.5
Polished rice 12.7 3.5 1.5
Wheat 11.0 3.5 2.0
* Original rate of application = 7.0 mg/kg
In processing and cooking
The 1975 Meeting indicated that further information on the level
and fate of residues in food at the point of consumption was
desirable. Studies involving barley, oats, rice and wheat were
initiated in Australia and preliminary results were made available to
the present Meeting (Desmarchelier et al., 1976b). In these tests, a
portion of the barley, oats, husked rice, milled rice and wheat in the
storage experiment described above was withdrawn for processing and
cooking after 3 months. The barley was subjected to a simple malting
process after which the bioresmethrin content had declined from 3.5
mg/kg to 1.5 mg/kg. The oats were crushed and were then boiled for 15
minutes in the smallest quantity of water that would cover the grains.
The residue level fell from 3.5 mg/kg to 1.5 mg/kg during cooking. The
husked and the polished rice were likewise boiled in minimal
quantities of water for 15 minutes after which the residue level had
declined from 3.5 mg/kg to 2.5 and 2.0 mg/kg respectively. A portion
of the same husked rice was found to contain only 0.5 mg/kg of
bioresmethrin after being cooked for 25 minutes.
The rice in husk, after 6 months storage, was subjected to
milling, firstly for the removal of husk and then for the removal of
bran, followed by polishing. Removing the husk reduced the
bioresmethrin level from 1.5 mg/kg to 0.25 mg/kg. Further milling and
polishing reduced the residue on the polished rice to 0.1 mg/kg.
The treated barley which had been in storage for 6 months was
subjected to a standard commercial malting process. There was no
reduction of germination and the prepared malt contained only 0.35
mg/kg of bioresmethrin, a reduction from 1.75 mg/kg on the barley
before malting. Table 2 summarizes the results.
METHODS OF RESIDUE ANALYSIS
The methods available for residue analysis were reviewed in 1975
(FAO/WHO 1976b). Since then several further papers have been
published.
Simonaitis and Cail (1975) used a gas chromatographic method with
flame ionisation detection for the determination of resmethrin in
maize, cornmeal, flour and wheat. The minimum detectable concentration
of resmethrin was 0.2 mg/kg (determined on a standard solution of
resmethrin) and an average recovery of 83% was achieved on the four
commodities studied over the range 0.2 to 3.2 mg/kg. Reproducibility
was claimed to be good. Desmarchelier (1976a) applied four detection
systems to the determination of pyrethroids on grains. The first was
that of Andrews (1974), a TLC method for which a minimum detectable
concentration of 0.06 mg/kg was claimed in the original paper when
estimating resmethrin in foliage. The second was that of Heath (1972),
a GLC method used for formulation analysis. No limit of determination
is quoted. The third was based on the spectrophotometric method of
McClellan (1964). The limit of determination is given as 0.5 mg/kg
since below this value background interference from the commodity
becomes relatively large. The fourth was a mixture of the first and
third. Recoveries by all four methods on wheat, bran, pollard, flour
and bread when fortified with 0.5 to 4.0 mg/kg bioresmethrin were
between 90 and 102%. All four methods gave comparable results for any
one sample. The limit of detection for methods one and four was 0.2 to
2.0 µg. This could be improved to 0.02 to 0.05 µg by purification of
the commodity extract. The author points out that the two major
problems with all four of the methods investigated were the relative
insensitivity and lack of selectivity as compared for example with the
electron capture GLC of chlorinated compounds and the flame
photometric detection of phosphorus compounds respectively.
TABLE 2. Fate of bioresmethrin in various grains processed after storage at 25°C
Residue Residue
Storage after after
period, storage processing
Grain (months) (mg/kg)* Processing (mg/kg)*
Barley 3 3.5 primitive malting 1.5
Barley 6 1.75 commercial malting 0.35
Oats 3 3.5 boil 15 minutes 1.5
Rice in husk 6 1.5 husked 0.25
milled/polished 0.1
Husked rice 3 3.5 boiled 15 minutes 2.5
boiled 25 minutes 0.5
Polished rice 3 3.5 boiled 15 minutes 2.0
Wheat 3 3.5 bran 5.5
shorts 3.5
flour 2.5
white bread <0.1
wholemeal bread 1.0
* Original rate of application = 7.0 mg/kg.
A method for the determination of bioresmethrin residues on wheat
by high-speed liquid chromatography has been developed by Gunew
(1976). This is claimed to be highly specific and to have a limit of
determination of 0.02 mg/kg.
Bioresmethrin is extracted from the wheat sample using 25%
acetone in hexane with intermittent shaking for 24 hours. The raw
extract is cleaned up on a column of alumina, and after evaporation of
the eluate, addition of O-chloroaniline as internal standard, and
volume adjustment an aliquot is injected into a microparticulate
silica gel column fitted with a pre-column. A variable wavelength
spectrophotometer set at 225 nm is used as detector. Use is made of
peak height ratios to determine the concentration of bioresmethrin in
the wheat.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The Meeting was aware that the following national tolerances had
been established.
Australia
raw grain 5 mg/kg
milled products from grain 5 mg/kg
cooked cereal products including bread 0.05* mg/kg
France
raw grain 4 mg/kg
*at or about limit of determination
APPRAISAL
Following the evaluation of bioresmethrin by the 1975 Joint
Meeting a number of additional items of information that were
considered desirable have been made available.
It appears that bioresmethrin will not be developed for use on
fruit and vegetable crops or for direct application to livestock.
However there is an important and major use as a grain protectant
insecticide on the whole range of stored grain. Extensive information
on the level and fate of bioresmethrin on several types of raw grain
and processed cereals has been evaluated and has been published in
this and the previous (1975) monograph. The new information confirms
and extends that previously available.
New methods for the analysis of bioresmethrin residues on raw
grain have been published. These have not been evaluated for
regulatory purposes.
The further information evaluated by the Meeting has confirmed
the guideline levels recorded previously and has satisfied the needs
of the Meeting for the information recorded as desirable in 1975 for
the evaluation of residues in food. No additional guideline levels are
recorded.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Long-term studies.
REFERENCES
Abernathy, C.O., and Casida, J.E. Pyrethroid insecticides; esterase
1973 cleavage in relation to selective toxicity.
Science, 179: 1235-36.
Andrews. Resmethrin residues in foliage after aerial application.
1974 Pesticides Monitoring Journal, 8(1): 50-52.
Bengston, M. et al. Interim report of Australian Wheat Board
1976a Working Party an Grain Protectants. Report to
Australian Wheat Board, May 1976. (To be
published).
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J. M.,
1976 Hart, R.G., Phillips, M., Snelson, J.T., and
Sticks, R. Field trials to compare
chlorpyrefos-methyl, fenitrothion,
pirimiphos-methyl, malathion, and methacrifos for
control of malathion-resistant insects infesting
wheat in Australia. J. Stored Prod. Res. (In
press).
Champ, B., and Dyte, C. E. Report of the FAO Global Survey of
1976 Pesticide Susceptibility of Stored Grain Pests.
FAO Plant Protection Series No. 5.
Chesher, B.C., and Malone, J.C. Acute toxicity tests with NRDC 107
1970a in hens. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, the Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, B.C. Sensitization study with NRDC 107
1970b in guinea pigs. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, J.C. Ocular irritancy of NRDC 107 in
1970c rabbits. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone. J.C. Toxicity to dogs of NRDC 107
1971b (continuation). Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.D., and Malone, J.C. Acute toxicity of NRDC 107 by the
1971a intravenous route. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W.,
1976a Moore, B., Phillips, M., Snelson. J.T., and
Sticka, R. Preliminary report of pilot trials in 5
states with combinations of pirimiphos
methyl/bioresmethrin and
fenitrothion/bioresmethrin. Report of Australian
Wheat Board Working Party on Grain Protectants (To
be published).
Desmarchelier, J.M. Selective treatments. including combinations of
1976 pyrethroid and organophosphorus insecticides, for
control of stored product Coleoptera at two
temperatures. J. Stored Prod. Res. (In press).
Desmarchelier, J.M. Analysis of pyrethroids on grains. J. Stored
1976a Prod. Res., 12: 245-252.
Desmarchelier, J.M. Stability of bioresmethrin deposits on various
1976b stored grains held at different temperatures.
Report of CSIRO Division of Entomology to
Australian Wheat Board Working Group on Grain
Protectants (To be published).
FAO/WHO. 1975 Evaluations of Some Pesticide Residues in Foods.
1976b FAO Agricultural Series, 1976 No. World Health
Organization Tech. Ref. Ser., No.
Farebrother, D.A. NRDC 107 -whole body radioautographal study
1973 in albino rats. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Glomot, R. Etude de la toxicite chronique de 3 semaines Undated
chez le rat du RU 11484 (NRDC 107). Unpublished
report from the Roussel Uclaf, submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Glomot. R., and Chevalier, B. Etude de la toxicite argue du RU 11484
1969 (NRDC 107). Unpublished report from Roussel Uclaf,
submitted to the World Health Organization by The
Wellcome Foundation Ltd.
Gunew, D.S. Bioresmethrin in Analytical Methods for Pesticides
1976 and Plant-Growth Regulators. Vol. X, ed. by G.
Zweig and G. Sherma, Academic Press, N.Y.
Heath, R.R. Rapid method for analysing resmethrin content of
1972 formulations and determining the cis-trans ratio.
J. Assoc. Off. Anal. Chem., 55: 1328-30
Malone, J.C., and Chesher, B.C. Toxicity to dogs of NRDC 107.
1970 Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
McClellan, D.B. Pyrethrin - Pyrethrin I and Pyrethrin II in
1964 Analytical Method for Pesticides, Plant Growth
Regulators and Food Additives. Vol. II, p. 400-413
ed. G. Zweig, Academic Press, N.Y.
Miyamoto, J. Terminal residues of bioresmethrin. Submission to IUPAC
1975-76 Terminal Residues Commission, Madrid, September
1975; Leverkusen, September, 1976
Miyamoto, J., Nishida, T., and Udea, K. Metabolic fate of resmethrin,
1971 5-benzyl-3-furyl methyl-d1-trans-chrysanthemate
in the rat. Pesticide Biochem. & Physiol., 1:
293-306.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., and Spicer,
1971 E.J.F. Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished report
from the Huntington Research Centre, submitted to
the World Health Organization by the Wellcome
Foundation Ltd.
Simonaitis, R.A. and Cail, R.S. J. Assoc. Offic. Anal. Chem.,
1975 58(5). 1032-1036.
Ueda, K., Gaughan, L.C., and Casida, J.E. Metabolism of four
1975a resmethrin isomers by liver microsomes. Pesticide
Biochem. Physiol., 5: 280-294.
Ueda, K., Gaughan. L.C., and Casida, J.E. Metabolism of (+)-trans
1975b and (+)-cis resmethrin in rats. J. Agr. Food
Chem., 23(1): 106-115.
Verschoyle, R.D., and Barnes, J.M. Toxicity of natural and synthetic
1972 pyrethrins/rats. Pesticide Biochem. & Physiol.,
2(3): 308-11.
Waldron, M.M. Foetal toxicity study - 95 H 56 - given orally
1969 in the rabbit. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Wallwork, L., Chesher, B.C., and Malone, J.C. Toxicity of NRDC 107
1970 to laboratory animals. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Wallwork, L.M., Clampitt, R.B., and Malone, J.C. NRDC 107, rat
1971 oral 91 day toxicity study. Unpublished report
from the Cooper Technical Bureau, Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Wallwork, L.M. and Malone, J.C. Potentiation toxicity studies with
1971 NRDC 107, bioallethrin and piperonyl butoxide.
Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
Wallwork, L.M., and Malone, J.C. Bioresmethrin; Preliminary
1972 24-hour rat inhalation study. Unpublished report
from the Cooper Technical Bureau. Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by the Wellcome
Foundation Ltd.
1976b). Twenty kg quantities of barley, oats, rice in husk, husked
rice, polished rice and wheat were treated under standardised
conditions with a dilute emulsion of bioresmethrin/piperonyl butoxide
(1:5) at a rate designed to deposit 7 mg bioresmethrin per kg of
grain. The amount applied was deliberately high to enable the effects
of long term storage and processing to be compared and to ensure that
analytical difficulties would not interfere. The grain was held in a
constant temperature room at 25°C and was sampled after 3 and 6
months.
Table 1 compares the level of bioresmethrin found in each type of
grain after 3 and 6 months in storage.
TABLE 1. Effect of storage at 25°C on bioresmethrin residues on
various grains
Residue*, mg/kg
after storage for
Grain moisture % 3 months 6 months
Barley 13.0 3.5 1.75
Oats 12 3.5 2.0
Rice in husk 13 1.5 1.5
Husked rice 12.5 3.5 1.5
Polished rice 12.7 3.5 1.5
Wheat 11.0 3.5 2.0
* Original rate of application = 7.0 mg/kg
In processing and cooking
The 1975 Meeting indicated that further information on the level
and fate of residues in food at the point of consumption was
desirable. Studies involving barley, oats, rice and wheat were
initiated in Australia and preliminary results were made available to
the present Meeting (Desmarchelier et al., 1976b). In these tests, a
portion of the barley, oats, husked rice, milled rice and wheat in the
storage experiment described above was withdrawn for processing and
cooking after 3 months. The barley was subjected to a simple malting
process after which the bioresmethrin content had declined from 3.5
mg/kg to 1.5 mg/kg. The oats were crushed and were then boiled for 15
minutes in the smallest quantity of water that would cover the grains.
The residue level fell from 3.5 mg/kg to 1.5 mg/kg during cooking. The
husked and the polished rice were likewise boiled in minimal
quantities of water for 15 minutes after which the residue level had
declined from 3.5 mg/kg to 2.5 and 2.0 mg/kg respectively. A portion
of the same husked rice was found to contain only 0.5 mg/kg of
bioresmethrin after being cooked for 25 minutes.
The rice in husk, after 6 months storage, was subjected to
milling, firstly for the removal of husk and then for the removal of
bran, followed by polishing. Removing the husk reduced the
bioresmethrin level from 1.5 mg/kg to 0.25 mg/kg. Further milling and
polishing reduced the residue on the polished rice to 0.1 mg/kg.
The treated barley which had been in storage for 6 months was
subjected to a standard commercial malting process. There was no
reduction of germination and the prepared malt contained only 0.35
mg/kg of bioresmethrin, a reduction from 1.75 mg/kg on the barley
before malting. Table 2 summarizes the results.
METHODS OF RESIDUE ANALYSIS
The methods available for residue analysis were reviewed in 1975
(FAO/WHO 1976b). Since then several further papers have been
published.
Simonaitis and Cail (1975) used a gas chromatographic method with
flame ionisation detection for the determination of resmethrin in
maize, cornmeal, flour and wheat. The minimum detectable concentration
of resmethrin was 0.2 mg/kg (determined on a standard solution of
resmethrin) and an average recovery of 83% was achieved on the four
commodities studied over the range 0.2 to 3.2 mg/kg. Reproducibility
was claimed to be good. Desmarchelier (1976a) applied four detection
systems to the determination of pyrethroids on grains. The first was
that of Andrews (1974), a TLC method for which a minimum detectable
concentration of 0.06 mg/kg was claimed in the original paper when
estimating resmethrin in foliage. The second was that of Heath (1972),
a GLC method used for formulation analysis. No limit of determination
is quoted. The third was based on the spectrophotometric method of
McClellan (1964). The limit of determination is given as 0.5 mg/kg
since below this value background interference from the commodity
becomes relatively large. The fourth was a mixture of the first and
third. Recoveries by all four methods on wheat, bran, pollard, flour
and bread when fortified with 0.5 to 4.0 mg/kg bioresmethrin were
between 90 and 102%. All four methods gave comparable results for any
one sample. The limit of detection for methods one and four was 0.2 to
2.0 µg. This could be improved to 0.02 to 0.05 µg by purification of
the commodity extract. The author points out that the two major
problems with all four of the methods investigated were the relative
insensitivity and lack of selectivity as compared for example with the
electron capture GLC of chlorinated compounds and the flame
photometric detection of phosphorus compounds respectively.
TABLE 2. Fate of bioresmethrin in various grains processed after storage at 25°C
Residue Residue
Storage after after
period, storage processing
Grain (months) (mg/kg)* Processing (mg/kg)*
Barley 3 3.5 primitive malting 1.5
Barley 6 1.75 commercial malting 0.35
Oats 3 3.5 boil 15 minutes 1.5
Rice in husk 6 1.5 husked 0.25
milled/polished 0.1
Husked rice 3 3.5 boiled 15 minutes 2.5
boiled 25 minutes 0.5
Polished rice 3 3.5 boiled 15 minutes 2.0
Wheat 3 3.5 bran 5.5
shorts 3.5
flour 2.5
white bread <0.1
wholemeal bread 1.0
* Original rate of application = 7.0 mg/kg.
A method for the determination of bioresmethrin residues on wheat
by high-speed liquid chromatography has been developed by Gunew
(1976). This is claimed to be highly specific and to have a limit of
determination of 0.02 mg/kg.
Bioresmethrin is extracted from the wheat sample using 25%
acetone in hexane with intermittent shaking for 24 hours. The raw
extract is cleaned up on a column of alumina, and after evaporation of
the eluate, addition of O-chloroaniline as internal standard, and
volume adjustment an aliquot is injected into a microparticulate
silica gel column fitted with a pre-column. A variable wavelength
spectrophotometer set at 225 nm is used as detector. Use is made of
peak height ratios to determine the concentration of bioresmethrin in
the wheat.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The Meeting was aware that the following national tolerances had
been established.
Australia
raw grain 5 mg/kg
milled products from grain 5 mg/kg
cooked cereal products including bread 0.05* mg/kg
France
raw grain 4 mg/kg
*at or about limit of determination
APPRAISAL
Following the evaluation of bioresmethrin by the 1975 Joint
Meeting a number of additional items of information that were
considered desirable have been made available.
It appears that bioresmethrin will not be developed for use on
fruit and vegetable crops or for direct application to livestock.
However there is an important and major use as a grain protectant
insecticide on the whole range of stored grain. Extensive information
on the level and fate of bioresmethrin on several types of raw grain
and processed cereals has been evaluated and has been published in
this and the previous (1975) monograph. The new information confirms
and extends that previously available.
New methods for the analysis of bioresmethrin residues on raw
grain have been published. These have not been evaluated for
regulatory purposes.
The further information evaluated by the Meeting has confirmed
the guideline levels recorded previously and has satisfied the needs
of the Meeting for the information recorded as desirable in 1975 for
the evaluation of residues in food. No additional guideline levels are
recorded.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Long-term studies.
REFERENCES
Abernathy, C.O., and Casida, J.E. Pyrethroid insecticides; esterase
1973 cleavage in relation to selective toxicity.
Science, 179: 1235-36.
Andrews. Resmethrin residues in foliage after aerial application.
1974 Pesticides Monitoring Journal, 8(1): 50-52.
Bengston, M. et al. Interim report of Australian Wheat Board
1976a Working Party an Grain Protectants. Report to
Australian Wheat Board, May 1976. (To be
published).
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J. M.,
1976 Hart, R.G., Phillips, M., Snelson, J.T., and
Sticks, R. Field trials to compare
chlorpyrefos-methyl, fenitrothion,
pirimiphos-methyl, malathion, and methacrifos for
control of malathion-resistant insects infesting
wheat in Australia. J. Stored Prod. Res. (In
press).
Champ, B., and Dyte, C. E. Report of the FAO Global Survey of
1976 Pesticide Susceptibility of Stored Grain Pests.
FAO Plant Protection Series No. 5.
Chesher, B.C., and Malone, J.C. Acute toxicity tests with NRDC 107
1970a in hens. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, the Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, B.C. Sensitization study with NRDC 107
1970b in guinea pigs. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, J.C. Ocular irritancy of NRDC 107 in
1970c rabbits. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone. J.C. Toxicity to dogs of NRDC 107
1971b (continuation). Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.D., and Malone, J.C. Acute toxicity of NRDC 107 by the
1971a intravenous route. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W.,
1976a Moore, B., Phillips, M., Snelson. J.T., and
Sticka, R. Preliminary report of pilot trials in 5
states with combinations of pirimiphos
methyl/bioresmethrin and
fenitrothion/bioresmethrin. Report of Australian
Wheat Board Working Party on Grain Protectants (To
be published).
Desmarchelier, J.M. Selective treatments. including combinations of
1976 pyrethroid and organophosphorus insecticides, for
control of stored product Coleoptera at two
temperatures. J. Stored Prod. Res. (In press).
Desmarchelier, J.M. Analysis of pyrethroids on grains. J. Stored
1976a Prod. Res., 12: 245-252.
Desmarchelier, J.M. Stability of bioresmethrin deposits on various
1976b stored grains held at different temperatures.
Report of CSIRO Division of Entomology to
Australian Wheat Board Working Group on Grain
Protectants (To be published).
FAO/WHO. 1975 Evaluations of Some Pesticide Residues in Foods.
1976b FAO Agricultural Series, 1976 No. World Health
Organization Tech. Ref. Ser., No.
Farebrother, D.A. NRDC 107 -whole body radioautographal study
1973 in albino rats. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Glomot, R. Etude de la toxicite chronique de 3 semaines Undated
chez le rat du RU 11484 (NRDC 107). Unpublished
report from the Roussel Uclaf, submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Glomot. R., and Chevalier, B. Etude de la toxicite argue du RU 11484
1969 (NRDC 107). Unpublished report from Roussel Uclaf,
submitted to the World Health Organization by The
Wellcome Foundation Ltd.
Gunew, D.S. Bioresmethrin in Analytical Methods for Pesticides
1976 and Plant-Growth Regulators. Vol. X, ed. by G.
Zweig and G. Sherma, Academic Press, N.Y.
Heath, R.R. Rapid method for analysing resmethrin content of
1972 formulations and determining the cis-trans ratio.
J. Assoc. Off. Anal. Chem., 55: 1328-30
Malone, J.C., and Chesher, B.C. Toxicity to dogs of NRDC 107.
1970 Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
McClellan, D.B. Pyrethrin - Pyrethrin I and Pyrethrin II in
1964 Analytical Method for Pesticides, Plant Growth
Regulators and Food Additives. Vol. II, p. 400-413
ed. G. Zweig, Academic Press, N.Y.
Miyamoto, J. Terminal residues of bioresmethrin. Submission to IUPAC
1975-76 Terminal Residues Commission, Madrid, September
1975; Leverkusen, September, 1976
Miyamoto, J., Nishida, T., and Udea, K. Metabolic fate of resmethrin,
1971 5-benzyl-3-furyl methyl-d1-trans-chrysanthemate
in the rat. Pesticide Biochem. & Physiol., 1:
293-306.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., and Spicer,
1971 E.J.F. Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished report
from the Huntington Research Centre, submitted to
the World Health Organization by the Wellcome
Foundation Ltd.
Simonaitis, R.A. and Cail, R.S. J. Assoc. Offic. Anal. Chem.,
1975 58(5). 1032-1036.
Ueda, K., Gaughan, L.C., and Casida, J.E. Metabolism of four
1975a resmethrin isomers by liver microsomes. Pesticide
Biochem. Physiol., 5: 280-294.
Ueda, K., Gaughan. L.C., and Casida, J.E. Metabolism of (+)-trans
1975b and (+)-cis resmethrin in rats. J. Agr. Food
Chem., 23(1): 106-115.
Verschoyle, R.D., and Barnes, J.M. Toxicity of natural and synthetic
1972 pyrethrins/rats. Pesticide Biochem. & Physiol.,
2(3): 308-11.
Waldron, M.M. Foetal toxicity study - 95 H 56 - given orally
1969 in the rabbit. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Wallwork, L., Chesher, B.C., and Malone, J.C. Toxicity of NRDC 107
1970 to laboratory animals. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Wallwork, L.M., Clampitt, R.B., and Malone, J.C. NRDC 107, rat
1971 oral 91 day toxicity study. Unpublished report
from the Cooper Technical Bureau, Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Wallwork, L.M. and Malone, J.C. Potentiation toxicity studies with
1971 NRDC 107, bioallethrin and piperonyl butoxide.
Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
Wallwork, L.M., and Malone, J.C. Bioresmethrin; Preliminary
1972 24-hour rat inhalation study. Unpublished report
from the Cooper Technical Bureau. Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by the Wellcome
Foundation Ltd.
 growth and gestation were unaffected. There was an increase in dead
fetuses at the highest dose and a large number of resorption sites
noted at all treatment levels. There were a number of deformed fetuses
observed but the total numbers were not sufficient for an adequate
statistical evaluation. The deformities included straight tail,
crossed hind limbs and unilateral union of 6th and 7th ribs at the
sternal end. An overall fetal loss was observed at all dose levels
(primarily because of the large number of resorption sites recorded).
(Waldron, 1969).
Special studies on potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin and/or piperonyl butoxide alone and in
combinations at doses approximating the acute ip LD50 value. No
potentiation of the acute toxicity was observed in this study. In all
cases with bioresmethrin combinations, the observed LD50 values were
equal to or exceeded the expected value (Wallwork and Malone, 1971).
Special studies on irritancy and sensitization
Groups of adult guinea pigs (6 males/group) were tested with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or
2,4-dinitrochloro-benzene (DNCB) (0.1 ml of a (1% w/v) formulation in
mineral oil) to assess the sensitization properties. The test
substance was applied to the ears for 4 days. On day 7, 0.2 ml was
applied dermally and the degree of irritation recorded. As expected,
the DNCB was an irritant while bioresmethrin showed only traces of
erythema suggesting a low potential for sensitization and irritation
(Chesher and Malone, 1970b).
Instillation of technical bioresmethrin into the eye of rabbits
(0.1 ml) produced no irritation or corneal damage. A group of six
rabbits was treated with no indication of ocular hazard (Chesher and
Malone, 1970c).
Acute toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork, et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chesher and Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
f inhalation >872 mg/m3 Wallwork & Malone
(24 hr exposure)
f dermal >10000 Wallwork et al., 1970
LD50
Species Sex Route (mg/kg) Reference
Mouse f oral >10000 Wallwork et al., 1970
m oral 3100 Ueda, et al., 1975b
f ip 5359 Wallwork et al., 1973
m ip >1500 Ueda et al., 1975b
Rabbit
Chicken oral >10000 Wallwork et al., 1970
Chesher & Malone, 1970a
Signs of poisoning: After 2 or more hours following oral
administration, tremors occurred; animals were sensitive to each other
and aggressive. The final stages of poisoning consisted of convulsive
twitching, prostration, coma, and death normally between 3 and 24
hours.
Mode of action: The nervous systems of both vertebrates and
invertebrates are equally sensitive and respond in a similar manner to
pyrethins (both natural and synthetic). The uncoordinated tremors of
the rat are similar to those signs of poisoning generally associated
with organochlorine pesticides.
Acute toxicity of metabolites
LD50
(mg/kg*)
Metabolite ip Oral
1) (+)-trans-resmethrin (bioresmethrin) >1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-cis-resmethrin 1 320
(5-benzyl-3-furylmethyl (-)-cis-
chrysanthemate)
3) (+)-trans-CA (t-CA) 98 280
(*)-trans-chrysanthemic acid
4) (+)-cis-CA (c-CA)1 600
(+)-cis-chrysanthemic acid
5) (+)-trans-CDA. (tE-CDA) 408
(+)-trans-chrysanthemumdicarboxylic acid
LD50
(mg/kg*)
Metabolite ip Oral
6) BFA 75 310
5-benzyl-3-furylmethanol
7) BFCA
5-benzyl-3-furoic acid 46
*LD50 - male mouse 24-hour toxicity following intraperitoneal
injection.
1 Cis isomers (numbers 2 and 4) are not metabolites of
bioresmethrin.
Cis isomers are metabolites in degradation of resmethrin.
(Ueda et al., 1975b; Miyamoto, 1975, 1976)
Short-term studies
Rat
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage daily, six days per week for three weeks at doses of
0, 1000 and 2000 mg/kg body weight. There was no mortality
attributable to bioresmethrin. There was a slight reduction in weight
at 2000 mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and hematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated).
Groups of rats (18 males and 18 females/group) were fed
bioresmethrin in the diet at concentrations of 0, 400, 1200 and 8000
ppm (the latter dose group was fed 4000 ppm for 30 days and the level
increased thereafter) for 91 days. There was no mortality observed in
this study. Food consumption was normal and food conversion was
unaffected by bioresmethrin in the diet. Growth was reduced at the
highest dose level which was accompanied by changes in blood chemistry
parameters indicating liver dysfunction (SAP, SGOT, and urinary
nitrogen increased at 90 days, glucose content reduced). Depression of
red blood cell count was observed at 1200 ppm although no consistent
parallel changes were seen in hemoglobin content or packed cell
volume. Urinalyses were normal. Gross and microscopic analysis of
tissues and organs showed an increase in liver weight at 4000 ppm and
a decrease in several other organs (spleen, heart, brain, thymus.
prostate, ovary and uterus at 1200 and 4000/8000 ppm fatty
infiltration of liver was seen on microscopic examination. A no-effect
level in this study would be 400 ppm equivalent to an average daily
intake of 32.8 to 36.1 mg/kg body weight for males and females
respectively (Wallwork et al., 1971).
Dog
Groups of dogs (2 of each sex/group) were administered
bioresmethrin by gavage, daily at dose levels of 0 and 500 mg/kg body
weight for 7 days followed by a dose increase to 1000 mg/kg for a
further 14 days. There were no effects noted in this test with respect
to mortality, behaviour, body weight changes, hematology, blood
chemistry or urinalysis parameters or on electrocardiograph
measurements. Short-term administration for three weeks at an oral
dose of 1000 mg/kg was uneventful in the parameters measured (Malone
and Chesher, 1970).
In a continuation of the above trial, after a two-week interval
on control diets, dogs were administered bioresmethrin by gavage for 7
days at a dose of 2000 mg/kg body weight. Again no significant effects
were noted in the parameters recorded above (Chesher and Malone,
1970b).
Groups of dogs (3 male and 3 female/group) were administered
bioresmethrin by gavage in gelatin capsule, daily for 90 days at
dosage levels of 0, 25, 80 and 250 mg/kg (the high dose was increased
to 500 mg/kg in week 7). There was no mortality. Growth, food
consumption and calculated food utilization parameters were normal.
Clinical biochemistry, ophthalmological and urinalysis parameters were
normal at all intervals (30, 60 and 90 days) examined. In the high
dose group, reduced RBC count, hemoglobin content and packed cell
volume values were noted. BUN was slightly increased only at the high
dose after 12 weeks. There were no adverse effects noted on gross or
microscopic examination of tissues and organs (including bioresmethrin
bone marrow). A no-effect level was observed to be 80 mg/kg
(equivalent to an average of 1600 ppm in the diet) (Noel et al.,
1971).
OBSERVATIONS IN MAN
None.
COMMENTS
Bioresmethrin is the common name for the
(+)-trans-chrysanthemate ester of 5-benzyl-3-furylmethanol, a
synthetic pyrethroid insecticide. Bioresmethrin is a component of
another synthetic pyrethroid, resmethrin, whose toxicological
properties have not been evaluated. Resmethrin consists of a mixture
of isomers having the empirical formula C22H2603. The mixture is
made up of approximately 65% trans-(bioresmethrin, approximately one
half of this fraction, is the (+)-trans-isomer) and 35%
cis-isomers.
In general, the (-)-isomers have low biological activity. The
(+)-cis-isomer of bioresmethrin is acutely more toxic than
bioresmethrin. Bioresmethrin is relatively non-toxic on an acute basis
and readily degradable. In animals, it is absorbed rapidly from the
gut and translocated partially to the lipid portions of the body.
Bioresmethrin is excreted slowly, probably owing to its lipophilicity.
It has been shown to reflect a delay in the onset of acute signs of
poisoning possibly owing to delay in ester cleavage which results in
more toxic (hydrolysis) products. Bioresmethrin is metabolised by
oxidation and hydrolysis occurring at various sites in the molecule.
There is no evidence for isomerisation of bioresmethrin to the
(+)-cis-isomer which might result in greater toxicity.
Several short term toxicology studies in rat and dog are
available. Bioresmethrin is not a teratogen although at high levels it
has been shown to induce some fetal abnormalities and fetal mortality.
Bioresmethrin is nonirritating and does not induce a sensitisation
reaction. In short term studies at high doses, thymic atrophy was
noted over a 3 week test period. This was accompanied by increased
liver size. In rats reduced growth, dysfunction, and fatty
infiltration of the liver were observed in a 90 day study at dietary
levels of 1200 ppm and above. In dogs, several hematological
parameters were affected at dose levels of 250 and 500 mg/kg, as noted
over a 90 day period. No-effect levels were noted based on short term
studies only. There were no observations in man available for
consideration.
Although short term studies were available along with several
special studies which did not specifically raise any unusual
toxicological factors, the absence of data from long term studies
precluded the Meeting from estimating an ADI for man. In concurrence
with previous conclusions, the Meeting expressed its need for
evaluation of long term studies in its consideration of an ADI for
man. This is especially important in the case of bioresmethrin, owing
to the fact that this synthetic pyrethroid is the first of a chemical
class of pesticides projected for extensive use in future. Although
bioresmethrin effects have been observed only at relatively high dose
levels in short term studies, the potential for adverse toxicological
effects in long term studies needs to be evaluated.
TOXICOLOGICAL EVALUATION
No ADI for man was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The high potency and broad spectrum activity of bioresmethrin
could be expected to lead to a wide field of use. Susceptibility to
rapid degradation by light and the availability of several other
synthetic pyrethroid insecticides with much superior stability to
light has however almost completely discouraged interest in
bioresmethrin for use against pests of fruit, vegetable or forage
crops.
In addition to the widespread use of bioresmethrin against pests
of households and of public health there has been considerable
interest in its use for the control of insect pests of stored
products, particularly the lesser grain borer, Rhyzopertha
domenica, which is not controlled by acceptable rates of
organophosphorus insecticides.
The 1975 monograph (FAO/WHO, 1976b) reviewed many scientific
papers on the properties and usefulness of bioresmethrin against
stored product pests. Since the preparation of the monograph a number
of additional studies have been completed.
Desmarchelier (1976) showed that combinations of pyrethroids,
which are particularly effective against Rhyzopertha domenica, and
organophosphorus insecticides which are effective against Tribolium
castaneum controlled both species for as long as the pyrethroid
alone controlled Rhyzopertha domenica and the organophosphorus
compound alone controlled Tribolium castaneum. Bioresmethrin was by
far the best of the many pyrethroid compounds examined.
Bengston et al (1976a) reporting extensive field trial to compare
a range of grain protectant insecticides showed that none of the five
organophosphorus insecticides evaluated was adequate to control
Rhyzopertha domenica, which lived and reproduced in all samples
including those drawn immediately after insecticide application.
Bengston et al. (1976b) demonstrated, in commercial scale trials,
the outstanding performance of several organophosphorus grain
protectants when combined with bioresmethrin. Complete protection was
obtained against all strains of all species of stored product pests
and reproduction was completely inhibited for many months.
The development of organophosphorus- and fumigant-resistant
strains of many stored product pests (Champ and Dyte, 1975) in most
countries and in all continents points to the urgency of having
available acceptable insecticides which have different properties and
modes of action. Supplies of natural-pyrethrum are not adequate to
meet the developing emergency.
Australian authorities have approved the use of bioresmethrin at
the rate of 2 mg/kg for the control of Rhyzopertha domenica and
other insect pests of wheat, oats and barley to protect valuable
stocks of grain and to ensure compliance with the phytosanitary
requirements of grain-importing countries. It is understood that
similar action has been taken in a number of other countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatments
No further information on the level or fate of bioresmethrin
residues on fruit or vegetables has become available. In view of the
advances which have been made with the development of several other
pyrethroid insecticides with outstanding efficacy against a wide range
of pests combined with a high level of stability to light it appears
unlikely that bioresmethrin will be developed or utilised for
pre-harvest application.
Post-harvest treatments
Extensive commercial scale trials were carried out in Australia
during 1976 at 20 sites involving 42 silos each containing from 2,000
to 8,000 tonnes of wheat. The wheat had been placed in storage
directly from the harvest. The temperature of the grain ranged from
27° to 39°C and the moisture content from 9.2% to 12.0%.
Pirimiphos-methyl or fenitrothion was applied in the form of a dilute
emulsion at the rate of 1 litre/tonne to give a deposit of 5 mg/kg of
pirimiphos-methyl or 10 mg/kg of fenitrothion. Bioresmethrin emulsion
was added at the same time to give a nominal deposit 1 mg/kg. The
amount deposited was estimated from the quantity of emulsion used.
Samples were withdrawn for bioassay and for chemical analysis at
regular intervals for 9 months. The temperature of the grain was
recorded over this period. Figure 2 illustrates the rate of decline of
the bioresmethrin deposit with time (Desmarchelier et al., 1976a). The
variations in the amount found at any one interval after application
are not particularly great but are no doubt due to variations in
temperature, grain moisture and amount of bioresmethrin applied at
each separate site.
These data confirm earlier studies under laboratory conditions
which indicate that the half-life of bioresmethrin in stored grain is
directly dependent on temperature. At 30-35°C the half-life is 8-10
weeks whilst at 20°C it exceeds 26 weeks. This degree of stability is
greater than that shown by malathion under similar conditions and
increases the effectiveness of bioresmethrin as a grain protectant
insecticide. Because the stability is combined with potency against
the target species it is not necessary to use concentrations above 2
mg/kg even on grain stored at relatively high temperatures.
FATE OF RESIDUES
In animals
The metabolism of bioresmethrin in the rat and mouse is described
above ("Biochemical aspects")
In grain
One of the questions remaining in doubt at the 1975 Meeting was
the fate of bioresmethrin on different classes of raw grain.
Preliminary results of an on-going official study in Australia
indicate that there is no significant difference in the stability
(persistence) of bioresmethrin deposits on different types of raw
grain held at 25°C under comparable moisture regimes (Desmarchelier,
growth and gestation were unaffected. There was an increase in dead
fetuses at the highest dose and a large number of resorption sites
noted at all treatment levels. There were a number of deformed fetuses
observed but the total numbers were not sufficient for an adequate
statistical evaluation. The deformities included straight tail,
crossed hind limbs and unilateral union of 6th and 7th ribs at the
sternal end. An overall fetal loss was observed at all dose levels
(primarily because of the large number of resorption sites recorded).
(Waldron, 1969).
Special studies on potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin and/or piperonyl butoxide alone and in
combinations at doses approximating the acute ip LD50 value. No
potentiation of the acute toxicity was observed in this study. In all
cases with bioresmethrin combinations, the observed LD50 values were
equal to or exceeded the expected value (Wallwork and Malone, 1971).
Special studies on irritancy and sensitization
Groups of adult guinea pigs (6 males/group) were tested with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or
2,4-dinitrochloro-benzene (DNCB) (0.1 ml of a (1% w/v) formulation in
mineral oil) to assess the sensitization properties. The test
substance was applied to the ears for 4 days. On day 7, 0.2 ml was
applied dermally and the degree of irritation recorded. As expected,
the DNCB was an irritant while bioresmethrin showed only traces of
erythema suggesting a low potential for sensitization and irritation
(Chesher and Malone, 1970b).
Instillation of technical bioresmethrin into the eye of rabbits
(0.1 ml) produced no irritation or corneal damage. A group of six
rabbits was treated with no indication of ocular hazard (Chesher and
Malone, 1970c).
Acute toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork, et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chesher and Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
f inhalation >872 mg/m3 Wallwork & Malone
(24 hr exposure)
f dermal >10000 Wallwork et al., 1970
LD50
Species Sex Route (mg/kg) Reference
Mouse f oral >10000 Wallwork et al., 1970
m oral 3100 Ueda, et al., 1975b
f ip 5359 Wallwork et al., 1973
m ip >1500 Ueda et al., 1975b
Rabbit
Chicken oral >10000 Wallwork et al., 1970
Chesher & Malone, 1970a
Signs of poisoning: After 2 or more hours following oral
administration, tremors occurred; animals were sensitive to each other
and aggressive. The final stages of poisoning consisted of convulsive
twitching, prostration, coma, and death normally between 3 and 24
hours.
Mode of action: The nervous systems of both vertebrates and
invertebrates are equally sensitive and respond in a similar manner to
pyrethins (both natural and synthetic). The uncoordinated tremors of
the rat are similar to those signs of poisoning generally associated
with organochlorine pesticides.
Acute toxicity of metabolites
LD50
(mg/kg*)
Metabolite ip Oral
1) (+)-trans-resmethrin (bioresmethrin) >1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-cis-resmethrin 1 320
(5-benzyl-3-furylmethyl (-)-cis-
chrysanthemate)
3) (+)-trans-CA (t-CA) 98 280
(*)-trans-chrysanthemic acid
4) (+)-cis-CA (c-CA)1 600
(+)-cis-chrysanthemic acid
5) (+)-trans-CDA. (tE-CDA) 408
(+)-trans-chrysanthemumdicarboxylic acid
LD50
(mg/kg*)
Metabolite ip Oral
6) BFA 75 310
5-benzyl-3-furylmethanol
7) BFCA
5-benzyl-3-furoic acid 46
*LD50 - male mouse 24-hour toxicity following intraperitoneal
injection.
1 Cis isomers (numbers 2 and 4) are not metabolites of
bioresmethrin.
Cis isomers are metabolites in degradation of resmethrin.
(Ueda et al., 1975b; Miyamoto, 1975, 1976)
Short-term studies
Rat
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage daily, six days per week for three weeks at doses of
0, 1000 and 2000 mg/kg body weight. There was no mortality
attributable to bioresmethrin. There was a slight reduction in weight
at 2000 mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and hematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated).
Groups of rats (18 males and 18 females/group) were fed
bioresmethrin in the diet at concentrations of 0, 400, 1200 and 8000
ppm (the latter dose group was fed 4000 ppm for 30 days and the level
increased thereafter) for 91 days. There was no mortality observed in
this study. Food consumption was normal and food conversion was
unaffected by bioresmethrin in the diet. Growth was reduced at the
highest dose level which was accompanied by changes in blood chemistry
parameters indicating liver dysfunction (SAP, SGOT, and urinary
nitrogen increased at 90 days, glucose content reduced). Depression of
red blood cell count was observed at 1200 ppm although no consistent
parallel changes were seen in hemoglobin content or packed cell
volume. Urinalyses were normal. Gross and microscopic analysis of
tissues and organs showed an increase in liver weight at 4000 ppm and
a decrease in several other organs (spleen, heart, brain, thymus.
prostate, ovary and uterus at 1200 and 4000/8000 ppm fatty
infiltration of liver was seen on microscopic examination. A no-effect
level in this study would be 400 ppm equivalent to an average daily
intake of 32.8 to 36.1 mg/kg body weight for males and females
respectively (Wallwork et al., 1971).
Dog
Groups of dogs (2 of each sex/group) were administered
bioresmethrin by gavage, daily at dose levels of 0 and 500 mg/kg body
weight for 7 days followed by a dose increase to 1000 mg/kg for a
further 14 days. There were no effects noted in this test with respect
to mortality, behaviour, body weight changes, hematology, blood
chemistry or urinalysis parameters or on electrocardiograph
measurements. Short-term administration for three weeks at an oral
dose of 1000 mg/kg was uneventful in the parameters measured (Malone
and Chesher, 1970).
In a continuation of the above trial, after a two-week interval
on control diets, dogs were administered bioresmethrin by gavage for 7
days at a dose of 2000 mg/kg body weight. Again no significant effects
were noted in the parameters recorded above (Chesher and Malone,
1970b).
Groups of dogs (3 male and 3 female/group) were administered
bioresmethrin by gavage in gelatin capsule, daily for 90 days at
dosage levels of 0, 25, 80 and 250 mg/kg (the high dose was increased
to 500 mg/kg in week 7). There was no mortality. Growth, food
consumption and calculated food utilization parameters were normal.
Clinical biochemistry, ophthalmological and urinalysis parameters were
normal at all intervals (30, 60 and 90 days) examined. In the high
dose group, reduced RBC count, hemoglobin content and packed cell
volume values were noted. BUN was slightly increased only at the high
dose after 12 weeks. There were no adverse effects noted on gross or
microscopic examination of tissues and organs (including bioresmethrin
bone marrow). A no-effect level was observed to be 80 mg/kg
(equivalent to an average of 1600 ppm in the diet) (Noel et al.,
1971).
OBSERVATIONS IN MAN
None.
COMMENTS
Bioresmethrin is the common name for the
(+)-trans-chrysanthemate ester of 5-benzyl-3-furylmethanol, a
synthetic pyrethroid insecticide. Bioresmethrin is a component of
another synthetic pyrethroid, resmethrin, whose toxicological
properties have not been evaluated. Resmethrin consists of a mixture
of isomers having the empirical formula C22H2603. The mixture is
made up of approximately 65% trans-(bioresmethrin, approximately one
half of this fraction, is the (+)-trans-isomer) and 35%
cis-isomers.
In general, the (-)-isomers have low biological activity. The
(+)-cis-isomer of bioresmethrin is acutely more toxic than
bioresmethrin. Bioresmethrin is relatively non-toxic on an acute basis
and readily degradable. In animals, it is absorbed rapidly from the
gut and translocated partially to the lipid portions of the body.
Bioresmethrin is excreted slowly, probably owing to its lipophilicity.
It has been shown to reflect a delay in the onset of acute signs of
poisoning possibly owing to delay in ester cleavage which results in
more toxic (hydrolysis) products. Bioresmethrin is metabolised by
oxidation and hydrolysis occurring at various sites in the molecule.
There is no evidence for isomerisation of bioresmethrin to the
(+)-cis-isomer which might result in greater toxicity.
Several short term toxicology studies in rat and dog are
available. Bioresmethrin is not a teratogen although at high levels it
has been shown to induce some fetal abnormalities and fetal mortality.
Bioresmethrin is nonirritating and does not induce a sensitisation
reaction. In short term studies at high doses, thymic atrophy was
noted over a 3 week test period. This was accompanied by increased
liver size. In rats reduced growth, dysfunction, and fatty
infiltration of the liver were observed in a 90 day study at dietary
levels of 1200 ppm and above. In dogs, several hematological
parameters were affected at dose levels of 250 and 500 mg/kg, as noted
over a 90 day period. No-effect levels were noted based on short term
studies only. There were no observations in man available for
consideration.
Although short term studies were available along with several
special studies which did not specifically raise any unusual
toxicological factors, the absence of data from long term studies
precluded the Meeting from estimating an ADI for man. In concurrence
with previous conclusions, the Meeting expressed its need for
evaluation of long term studies in its consideration of an ADI for
man. This is especially important in the case of bioresmethrin, owing
to the fact that this synthetic pyrethroid is the first of a chemical
class of pesticides projected for extensive use in future. Although
bioresmethrin effects have been observed only at relatively high dose
levels in short term studies, the potential for adverse toxicological
effects in long term studies needs to be evaluated.
TOXICOLOGICAL EVALUATION
No ADI for man was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The high potency and broad spectrum activity of bioresmethrin
could be expected to lead to a wide field of use. Susceptibility to
rapid degradation by light and the availability of several other
synthetic pyrethroid insecticides with much superior stability to
light has however almost completely discouraged interest in
bioresmethrin for use against pests of fruit, vegetable or forage
crops.
In addition to the widespread use of bioresmethrin against pests
of households and of public health there has been considerable
interest in its use for the control of insect pests of stored
products, particularly the lesser grain borer, Rhyzopertha
domenica, which is not controlled by acceptable rates of
organophosphorus insecticides.
The 1975 monograph (FAO/WHO, 1976b) reviewed many scientific
papers on the properties and usefulness of bioresmethrin against
stored product pests. Since the preparation of the monograph a number
of additional studies have been completed.
Desmarchelier (1976) showed that combinations of pyrethroids,
which are particularly effective against Rhyzopertha domenica, and
organophosphorus insecticides which are effective against Tribolium
castaneum controlled both species for as long as the pyrethroid
alone controlled Rhyzopertha domenica and the organophosphorus
compound alone controlled Tribolium castaneum. Bioresmethrin was by
far the best of the many pyrethroid compounds examined.
Bengston et al (1976a) reporting extensive field trial to compare
a range of grain protectant insecticides showed that none of the five
organophosphorus insecticides evaluated was adequate to control
Rhyzopertha domenica, which lived and reproduced in all samples
including those drawn immediately after insecticide application.
Bengston et al. (1976b) demonstrated, in commercial scale trials,
the outstanding performance of several organophosphorus grain
protectants when combined with bioresmethrin. Complete protection was
obtained against all strains of all species of stored product pests
and reproduction was completely inhibited for many months.
The development of organophosphorus- and fumigant-resistant
strains of many stored product pests (Champ and Dyte, 1975) in most
countries and in all continents points to the urgency of having
available acceptable insecticides which have different properties and
modes of action. Supplies of natural-pyrethrum are not adequate to
meet the developing emergency.
Australian authorities have approved the use of bioresmethrin at
the rate of 2 mg/kg for the control of Rhyzopertha domenica and
other insect pests of wheat, oats and barley to protect valuable
stocks of grain and to ensure compliance with the phytosanitary
requirements of grain-importing countries. It is understood that
similar action has been taken in a number of other countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatments
No further information on the level or fate of bioresmethrin
residues on fruit or vegetables has become available. In view of the
advances which have been made with the development of several other
pyrethroid insecticides with outstanding efficacy against a wide range
of pests combined with a high level of stability to light it appears
unlikely that bioresmethrin will be developed or utilised for
pre-harvest application.
Post-harvest treatments
Extensive commercial scale trials were carried out in Australia
during 1976 at 20 sites involving 42 silos each containing from 2,000
to 8,000 tonnes of wheat. The wheat had been placed in storage
directly from the harvest. The temperature of the grain ranged from
27° to 39°C and the moisture content from 9.2% to 12.0%.
Pirimiphos-methyl or fenitrothion was applied in the form of a dilute
emulsion at the rate of 1 litre/tonne to give a deposit of 5 mg/kg of
pirimiphos-methyl or 10 mg/kg of fenitrothion. Bioresmethrin emulsion
was added at the same time to give a nominal deposit 1 mg/kg. The
amount deposited was estimated from the quantity of emulsion used.
Samples were withdrawn for bioassay and for chemical analysis at
regular intervals for 9 months. The temperature of the grain was
recorded over this period. Figure 2 illustrates the rate of decline of
the bioresmethrin deposit with time (Desmarchelier et al., 1976a). The
variations in the amount found at any one interval after application
are not particularly great but are no doubt due to variations in
temperature, grain moisture and amount of bioresmethrin applied at
each separate site.
These data confirm earlier studies under laboratory conditions
which indicate that the half-life of bioresmethrin in stored grain is
directly dependent on temperature. At 30-35°C the half-life is 8-10
weeks whilst at 20°C it exceeds 26 weeks. This degree of stability is
greater than that shown by malathion under similar conditions and
increases the effectiveness of bioresmethrin as a grain protectant
insecticide. Because the stability is combined with potency against
the target species it is not necessary to use concentrations above 2
mg/kg even on grain stored at relatively high temperatures.
FATE OF RESIDUES
In animals
The metabolism of bioresmethrin in the rat and mouse is described
above ("Biochemical aspects")
In grain
One of the questions remaining in doubt at the 1975 Meeting was
the fate of bioresmethrin on different classes of raw grain.
Preliminary results of an on-going official study in Australia
indicate that there is no significant difference in the stability
(persistence) of bioresmethrin deposits on different types of raw
grain held at 25°C under comparable moisture regimes (Desmarchelier,
 1976b). Twenty kg quantities of barley, oats, rice in husk, husked
rice, polished rice and wheat were treated under standardised
conditions with a dilute emulsion of bioresmethrin/piperonyl butoxide
(1:5) at a rate designed to deposit 7 mg bioresmethrin per kg of
grain. The amount applied was deliberately high to enable the effects
of long term storage and processing to be compared and to ensure that
analytical difficulties would not interfere. The grain was held in a
constant temperature room at 25°C and was sampled after 3 and 6
months.
Table 1 compares the level of bioresmethrin found in each type of
grain after 3 and 6 months in storage.
TABLE 1. Effect of storage at 25°C on bioresmethrin residues on
various grains
Residue*, mg/kg
after storage for
Grain moisture % 3 months 6 months
Barley 13.0 3.5 1.75
Oats 12 3.5 2.0
Rice in husk 13 1.5 1.5
Husked rice 12.5 3.5 1.5
Polished rice 12.7 3.5 1.5
Wheat 11.0 3.5 2.0
* Original rate of application = 7.0 mg/kg
In processing and cooking
The 1975 Meeting indicated that further information on the level
and fate of residues in food at the point of consumption was
desirable. Studies involving barley, oats, rice and wheat were
initiated in Australia and preliminary results were made available to
the present Meeting (Desmarchelier et al., 1976b). In these tests, a
portion of the barley, oats, husked rice, milled rice and wheat in the
storage experiment described above was withdrawn for processing and
cooking after 3 months. The barley was subjected to a simple malting
process after which the bioresmethrin content had declined from 3.5
mg/kg to 1.5 mg/kg. The oats were crushed and were then boiled for 15
minutes in the smallest quantity of water that would cover the grains.
The residue level fell from 3.5 mg/kg to 1.5 mg/kg during cooking. The
husked and the polished rice were likewise boiled in minimal
quantities of water for 15 minutes after which the residue level had
declined from 3.5 mg/kg to 2.5 and 2.0 mg/kg respectively. A portion
of the same husked rice was found to contain only 0.5 mg/kg of
bioresmethrin after being cooked for 25 minutes.
The rice in husk, after 6 months storage, was subjected to
milling, firstly for the removal of husk and then for the removal of
bran, followed by polishing. Removing the husk reduced the
bioresmethrin level from 1.5 mg/kg to 0.25 mg/kg. Further milling and
polishing reduced the residue on the polished rice to 0.1 mg/kg.
The treated barley which had been in storage for 6 months was
subjected to a standard commercial malting process. There was no
reduction of germination and the prepared malt contained only 0.35
mg/kg of bioresmethrin, a reduction from 1.75 mg/kg on the barley
before malting. Table 2 summarizes the results.
METHODS OF RESIDUE ANALYSIS
The methods available for residue analysis were reviewed in 1975
(FAO/WHO 1976b). Since then several further papers have been
published.
Simonaitis and Cail (1975) used a gas chromatographic method with
flame ionisation detection for the determination of resmethrin in
maize, cornmeal, flour and wheat. The minimum detectable concentration
of resmethrin was 0.2 mg/kg (determined on a standard solution of
resmethrin) and an average recovery of 83% was achieved on the four
commodities studied over the range 0.2 to 3.2 mg/kg. Reproducibility
was claimed to be good. Desmarchelier (1976a) applied four detection
systems to the determination of pyrethroids on grains. The first was
that of Andrews (1974), a TLC method for which a minimum detectable
concentration of 0.06 mg/kg was claimed in the original paper when
estimating resmethrin in foliage. The second was that of Heath (1972),
a GLC method used for formulation analysis. No limit of determination
is quoted. The third was based on the spectrophotometric method of
McClellan (1964). The limit of determination is given as 0.5 mg/kg
since below this value background interference from the commodity
becomes relatively large. The fourth was a mixture of the first and
third. Recoveries by all four methods on wheat, bran, pollard, flour
and bread when fortified with 0.5 to 4.0 mg/kg bioresmethrin were
between 90 and 102%. All four methods gave comparable results for any
one sample. The limit of detection for methods one and four was 0.2 to
2.0 µg. This could be improved to 0.02 to 0.05 µg by purification of
the commodity extract. The author points out that the two major
problems with all four of the methods investigated were the relative
insensitivity and lack of selectivity as compared for example with the
electron capture GLC of chlorinated compounds and the flame
photometric detection of phosphorus compounds respectively.
TABLE 2. Fate of bioresmethrin in various grains processed after storage at 25°C
Residue Residue
Storage after after
period, storage processing
Grain (months) (mg/kg)* Processing (mg/kg)*
Barley 3 3.5 primitive malting 1.5
Barley 6 1.75 commercial malting 0.35
Oats 3 3.5 boil 15 minutes 1.5
Rice in husk 6 1.5 husked 0.25
milled/polished 0.1
Husked rice 3 3.5 boiled 15 minutes 2.5
boiled 25 minutes 0.5
Polished rice 3 3.5 boiled 15 minutes 2.0
Wheat 3 3.5 bran 5.5
shorts 3.5
flour 2.5
white bread <0.1
wholemeal bread 1.0
* Original rate of application = 7.0 mg/kg.
A method for the determination of bioresmethrin residues on wheat
by high-speed liquid chromatography has been developed by Gunew
(1976). This is claimed to be highly specific and to have a limit of
determination of 0.02 mg/kg.
Bioresmethrin is extracted from the wheat sample using 25%
acetone in hexane with intermittent shaking for 24 hours. The raw
extract is cleaned up on a column of alumina, and after evaporation of
the eluate, addition of O-chloroaniline as internal standard, and
volume adjustment an aliquot is injected into a microparticulate
silica gel column fitted with a pre-column. A variable wavelength
spectrophotometer set at 225 nm is used as detector. Use is made of
peak height ratios to determine the concentration of bioresmethrin in
the wheat.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The Meeting was aware that the following national tolerances had
been established.
Australia
raw grain 5 mg/kg
milled products from grain 5 mg/kg
cooked cereal products including bread 0.05* mg/kg
France
raw grain 4 mg/kg
*at or about limit of determination
APPRAISAL
Following the evaluation of bioresmethrin by the 1975 Joint
Meeting a number of additional items of information that were
considered desirable have been made available.
It appears that bioresmethrin will not be developed for use on
fruit and vegetable crops or for direct application to livestock.
However there is an important and major use as a grain protectant
insecticide on the whole range of stored grain. Extensive information
on the level and fate of bioresmethrin on several types of raw grain
and processed cereals has been evaluated and has been published in
this and the previous (1975) monograph. The new information confirms
and extends that previously available.
New methods for the analysis of bioresmethrin residues on raw
grain have been published. These have not been evaluated for
regulatory purposes.
The further information evaluated by the Meeting has confirmed
the guideline levels recorded previously and has satisfied the needs
of the Meeting for the information recorded as desirable in 1975 for
the evaluation of residues in food. No additional guideline levels are
recorded.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Long-term studies.
REFERENCES
Abernathy, C.O., and Casida, J.E. Pyrethroid insecticides; esterase
1973 cleavage in relation to selective toxicity.
Science, 179: 1235-36.
Andrews. Resmethrin residues in foliage after aerial application.
1974 Pesticides Monitoring Journal, 8(1): 50-52.
Bengston, M. et al. Interim report of Australian Wheat Board
1976a Working Party an Grain Protectants. Report to
Australian Wheat Board, May 1976. (To be
published).
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J. M.,
1976 Hart, R.G., Phillips, M., Snelson, J.T., and
Sticks, R. Field trials to compare
chlorpyrefos-methyl, fenitrothion,
pirimiphos-methyl, malathion, and methacrifos for
control of malathion-resistant insects infesting
wheat in Australia. J. Stored Prod. Res. (In
press).
Champ, B., and Dyte, C. E. Report of the FAO Global Survey of
1976 Pesticide Susceptibility of Stored Grain Pests.
FAO Plant Protection Series No. 5.
Chesher, B.C., and Malone, J.C. Acute toxicity tests with NRDC 107
1970a in hens. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, the Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, B.C. Sensitization study with NRDC 107
1970b in guinea pigs. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, J.C. Ocular irritancy of NRDC 107 in
1970c rabbits. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone. J.C. Toxicity to dogs of NRDC 107
1971b (continuation). Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.D., and Malone, J.C. Acute toxicity of NRDC 107 by the
1971a intravenous route. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W.,
1976a Moore, B., Phillips, M., Snelson. J.T., and
Sticka, R. Preliminary report of pilot trials in 5
states with combinations of pirimiphos
methyl/bioresmethrin and
fenitrothion/bioresmethrin. Report of Australian
Wheat Board Working Party on Grain Protectants (To
be published).
Desmarchelier, J.M. Selective treatments. including combinations of
1976 pyrethroid and organophosphorus insecticides, for
control of stored product Coleoptera at two
temperatures. J. Stored Prod. Res. (In press).
Desmarchelier, J.M. Analysis of pyrethroids on grains. J. Stored
1976a Prod. Res., 12: 245-252.
Desmarchelier, J.M. Stability of bioresmethrin deposits on various
1976b stored grains held at different temperatures.
Report of CSIRO Division of Entomology to
Australian Wheat Board Working Group on Grain
Protectants (To be published).
FAO/WHO. 1975 Evaluations of Some Pesticide Residues in Foods.
1976b FAO Agricultural Series, 1976 No. World Health
Organization Tech. Ref. Ser., No.
Farebrother, D.A. NRDC 107 -whole body radioautographal study
1973 in albino rats. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Glomot, R. Etude de la toxicite chronique de 3 semaines Undated
chez le rat du RU 11484 (NRDC 107). Unpublished
report from the Roussel Uclaf, submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Glomot. R., and Chevalier, B. Etude de la toxicite argue du RU 11484
1969 (NRDC 107). Unpublished report from Roussel Uclaf,
submitted to the World Health Organization by The
Wellcome Foundation Ltd.
Gunew, D.S. Bioresmethrin in Analytical Methods for Pesticides
1976 and Plant-Growth Regulators. Vol. X, ed. by G.
Zweig and G. Sherma, Academic Press, N.Y.
Heath, R.R. Rapid method for analysing resmethrin content of
1972 formulations and determining the cis-trans ratio.
J. Assoc. Off. Anal. Chem., 55: 1328-30
Malone, J.C., and Chesher, B.C. Toxicity to dogs of NRDC 107.
1970 Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
McClellan, D.B. Pyrethrin - Pyrethrin I and Pyrethrin II in
1964 Analytical Method for Pesticides, Plant Growth
Regulators and Food Additives. Vol. II, p. 400-413
ed. G. Zweig, Academic Press, N.Y.
Miyamoto, J. Terminal residues of bioresmethrin. Submission to IUPAC
1975-76 Terminal Residues Commission, Madrid, September
1975; Leverkusen, September, 1976
Miyamoto, J., Nishida, T., and Udea, K. Metabolic fate of resmethrin,
1971 5-benzyl-3-furyl methyl-d1-trans-chrysanthemate
in the rat. Pesticide Biochem. & Physiol., 1:
293-306.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., and Spicer,
1971 E.J.F. Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished report
from the Huntington Research Centre, submitted to
the World Health Organization by the Wellcome
Foundation Ltd.
Simonaitis, R.A. and Cail, R.S. J. Assoc. Offic. Anal. Chem.,
1975 58(5). 1032-1036.
Ueda, K., Gaughan, L.C., and Casida, J.E. Metabolism of four
1975a resmethrin isomers by liver microsomes. Pesticide
Biochem. Physiol., 5: 280-294.
Ueda, K., Gaughan. L.C., and Casida, J.E. Metabolism of (+)-trans
1975b and (+)-cis resmethrin in rats. J. Agr. Food
Chem., 23(1): 106-115.
Verschoyle, R.D., and Barnes, J.M. Toxicity of natural and synthetic
1972 pyrethrins/rats. Pesticide Biochem. & Physiol.,
2(3): 308-11.
Waldron, M.M. Foetal toxicity study - 95 H 56 - given orally
1969 in the rabbit. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Wallwork, L., Chesher, B.C., and Malone, J.C. Toxicity of NRDC 107
1970 to laboratory animals. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Wallwork, L.M., Clampitt, R.B., and Malone, J.C. NRDC 107, rat
1971 oral 91 day toxicity study. Unpublished report
from the Cooper Technical Bureau, Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Wallwork, L.M. and Malone, J.C. Potentiation toxicity studies with
1971 NRDC 107, bioallethrin and piperonyl butoxide.
Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
Wallwork, L.M., and Malone, J.C. Bioresmethrin; Preliminary
1972 24-hour rat inhalation study. Unpublished report
from the Cooper Technical Bureau. Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by the Wellcome
Foundation Ltd.
1976b). Twenty kg quantities of barley, oats, rice in husk, husked
rice, polished rice and wheat were treated under standardised
conditions with a dilute emulsion of bioresmethrin/piperonyl butoxide
(1:5) at a rate designed to deposit 7 mg bioresmethrin per kg of
grain. The amount applied was deliberately high to enable the effects
of long term storage and processing to be compared and to ensure that
analytical difficulties would not interfere. The grain was held in a
constant temperature room at 25°C and was sampled after 3 and 6
months.
Table 1 compares the level of bioresmethrin found in each type of
grain after 3 and 6 months in storage.
TABLE 1. Effect of storage at 25°C on bioresmethrin residues on
various grains
Residue*, mg/kg
after storage for
Grain moisture % 3 months 6 months
Barley 13.0 3.5 1.75
Oats 12 3.5 2.0
Rice in husk 13 1.5 1.5
Husked rice 12.5 3.5 1.5
Polished rice 12.7 3.5 1.5
Wheat 11.0 3.5 2.0
* Original rate of application = 7.0 mg/kg
In processing and cooking
The 1975 Meeting indicated that further information on the level
and fate of residues in food at the point of consumption was
desirable. Studies involving barley, oats, rice and wheat were
initiated in Australia and preliminary results were made available to
the present Meeting (Desmarchelier et al., 1976b). In these tests, a
portion of the barley, oats, husked rice, milled rice and wheat in the
storage experiment described above was withdrawn for processing and
cooking after 3 months. The barley was subjected to a simple malting
process after which the bioresmethrin content had declined from 3.5
mg/kg to 1.5 mg/kg. The oats were crushed and were then boiled for 15
minutes in the smallest quantity of water that would cover the grains.
The residue level fell from 3.5 mg/kg to 1.5 mg/kg during cooking. The
husked and the polished rice were likewise boiled in minimal
quantities of water for 15 minutes after which the residue level had
declined from 3.5 mg/kg to 2.5 and 2.0 mg/kg respectively. A portion
of the same husked rice was found to contain only 0.5 mg/kg of
bioresmethrin after being cooked for 25 minutes.
The rice in husk, after 6 months storage, was subjected to
milling, firstly for the removal of husk and then for the removal of
bran, followed by polishing. Removing the husk reduced the
bioresmethrin level from 1.5 mg/kg to 0.25 mg/kg. Further milling and
polishing reduced the residue on the polished rice to 0.1 mg/kg.
The treated barley which had been in storage for 6 months was
subjected to a standard commercial malting process. There was no
reduction of germination and the prepared malt contained only 0.35
mg/kg of bioresmethrin, a reduction from 1.75 mg/kg on the barley
before malting. Table 2 summarizes the results.
METHODS OF RESIDUE ANALYSIS
The methods available for residue analysis were reviewed in 1975
(FAO/WHO 1976b). Since then several further papers have been
published.
Simonaitis and Cail (1975) used a gas chromatographic method with
flame ionisation detection for the determination of resmethrin in
maize, cornmeal, flour and wheat. The minimum detectable concentration
of resmethrin was 0.2 mg/kg (determined on a standard solution of
resmethrin) and an average recovery of 83% was achieved on the four
commodities studied over the range 0.2 to 3.2 mg/kg. Reproducibility
was claimed to be good. Desmarchelier (1976a) applied four detection
systems to the determination of pyrethroids on grains. The first was
that of Andrews (1974), a TLC method for which a minimum detectable
concentration of 0.06 mg/kg was claimed in the original paper when
estimating resmethrin in foliage. The second was that of Heath (1972),
a GLC method used for formulation analysis. No limit of determination
is quoted. The third was based on the spectrophotometric method of
McClellan (1964). The limit of determination is given as 0.5 mg/kg
since below this value background interference from the commodity
becomes relatively large. The fourth was a mixture of the first and
third. Recoveries by all four methods on wheat, bran, pollard, flour
and bread when fortified with 0.5 to 4.0 mg/kg bioresmethrin were
between 90 and 102%. All four methods gave comparable results for any
one sample. The limit of detection for methods one and four was 0.2 to
2.0 µg. This could be improved to 0.02 to 0.05 µg by purification of
the commodity extract. The author points out that the two major
problems with all four of the methods investigated were the relative
insensitivity and lack of selectivity as compared for example with the
electron capture GLC of chlorinated compounds and the flame
photometric detection of phosphorus compounds respectively.
TABLE 2. Fate of bioresmethrin in various grains processed after storage at 25°C
Residue Residue
Storage after after
period, storage processing
Grain (months) (mg/kg)* Processing (mg/kg)*
Barley 3 3.5 primitive malting 1.5
Barley 6 1.75 commercial malting 0.35
Oats 3 3.5 boil 15 minutes 1.5
Rice in husk 6 1.5 husked 0.25
milled/polished 0.1
Husked rice 3 3.5 boiled 15 minutes 2.5
boiled 25 minutes 0.5
Polished rice 3 3.5 boiled 15 minutes 2.0
Wheat 3 3.5 bran 5.5
shorts 3.5
flour 2.5
white bread <0.1
wholemeal bread 1.0
* Original rate of application = 7.0 mg/kg.
A method for the determination of bioresmethrin residues on wheat
by high-speed liquid chromatography has been developed by Gunew
(1976). This is claimed to be highly specific and to have a limit of
determination of 0.02 mg/kg.
Bioresmethrin is extracted from the wheat sample using 25%
acetone in hexane with intermittent shaking for 24 hours. The raw
extract is cleaned up on a column of alumina, and after evaporation of
the eluate, addition of O-chloroaniline as internal standard, and
volume adjustment an aliquot is injected into a microparticulate
silica gel column fitted with a pre-column. A variable wavelength
spectrophotometer set at 225 nm is used as detector. Use is made of
peak height ratios to determine the concentration of bioresmethrin in
the wheat.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The Meeting was aware that the following national tolerances had
been established.
Australia
raw grain 5 mg/kg
milled products from grain 5 mg/kg
cooked cereal products including bread 0.05* mg/kg
France
raw grain 4 mg/kg
*at or about limit of determination
APPRAISAL
Following the evaluation of bioresmethrin by the 1975 Joint
Meeting a number of additional items of information that were
considered desirable have been made available.
It appears that bioresmethrin will not be developed for use on
fruit and vegetable crops or for direct application to livestock.
However there is an important and major use as a grain protectant
insecticide on the whole range of stored grain. Extensive information
on the level and fate of bioresmethrin on several types of raw grain
and processed cereals has been evaluated and has been published in
this and the previous (1975) monograph. The new information confirms
and extends that previously available.
New methods for the analysis of bioresmethrin residues on raw
grain have been published. These have not been evaluated for
regulatory purposes.
The further information evaluated by the Meeting has confirmed
the guideline levels recorded previously and has satisfied the needs
of the Meeting for the information recorded as desirable in 1975 for
the evaluation of residues in food. No additional guideline levels are
recorded.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Long-term studies.
REFERENCES
Abernathy, C.O., and Casida, J.E. Pyrethroid insecticides; esterase
1973 cleavage in relation to selective toxicity.
Science, 179: 1235-36.
Andrews. Resmethrin residues in foliage after aerial application.
1974 Pesticides Monitoring Journal, 8(1): 50-52.
Bengston, M. et al. Interim report of Australian Wheat Board
1976a Working Party an Grain Protectants. Report to
Australian Wheat Board, May 1976. (To be
published).
Bengston, M., Connell, M., Crook, I.D., Desmarchelier, J. M.,
1976 Hart, R.G., Phillips, M., Snelson, J.T., and
Sticks, R. Field trials to compare
chlorpyrefos-methyl, fenitrothion,
pirimiphos-methyl, malathion, and methacrifos for
control of malathion-resistant insects infesting
wheat in Australia. J. Stored Prod. Res. (In
press).
Champ, B., and Dyte, C. E. Report of the FAO Global Survey of
1976 Pesticide Susceptibility of Stored Grain Pests.
FAO Plant Protection Series No. 5.
Chesher, B.C., and Malone, J.C. Acute toxicity tests with NRDC 107
1970a in hens. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, the Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, B.C. Sensitization study with NRDC 107
1970b in guinea pigs. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone, J.C. Ocular irritancy of NRDC 107 in
1970c rabbits. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.C., and Malone. J.C. Toxicity to dogs of NRDC 107
1971b (continuation). Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Chesher, B.D., and Malone, J.C. Acute toxicity of NRDC 107 by the
1971a intravenous route. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W.,
1976a Moore, B., Phillips, M., Snelson. J.T., and
Sticka, R. Preliminary report of pilot trials in 5
states with combinations of pirimiphos
methyl/bioresmethrin and
fenitrothion/bioresmethrin. Report of Australian
Wheat Board Working Party on Grain Protectants (To
be published).
Desmarchelier, J.M. Selective treatments. including combinations of
1976 pyrethroid and organophosphorus insecticides, for
control of stored product Coleoptera at two
temperatures. J. Stored Prod. Res. (In press).
Desmarchelier, J.M. Analysis of pyrethroids on grains. J. Stored
1976a Prod. Res., 12: 245-252.
Desmarchelier, J.M. Stability of bioresmethrin deposits on various
1976b stored grains held at different temperatures.
Report of CSIRO Division of Entomology to
Australian Wheat Board Working Group on Grain
Protectants (To be published).
FAO/WHO. 1975 Evaluations of Some Pesticide Residues in Foods.
1976b FAO Agricultural Series, 1976 No. World Health
Organization Tech. Ref. Ser., No.
Farebrother, D.A. NRDC 107 -whole body radioautographal study
1973 in albino rats. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Glomot, R. Etude de la toxicite chronique de 3 semaines Undated
chez le rat du RU 11484 (NRDC 107). Unpublished
report from the Roussel Uclaf, submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Glomot. R., and Chevalier, B. Etude de la toxicite argue du RU 11484
1969 (NRDC 107). Unpublished report from Roussel Uclaf,
submitted to the World Health Organization by The
Wellcome Foundation Ltd.
Gunew, D.S. Bioresmethrin in Analytical Methods for Pesticides
1976 and Plant-Growth Regulators. Vol. X, ed. by G.
Zweig and G. Sherma, Academic Press, N.Y.
Heath, R.R. Rapid method for analysing resmethrin content of
1972 formulations and determining the cis-trans ratio.
J. Assoc. Off. Anal. Chem., 55: 1328-30
Malone, J.C., and Chesher, B.C. Toxicity to dogs of NRDC 107.
1970 Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
McClellan, D.B. Pyrethrin - Pyrethrin I and Pyrethrin II in
1964 Analytical Method for Pesticides, Plant Growth
Regulators and Food Additives. Vol. II, p. 400-413
ed. G. Zweig, Academic Press, N.Y.
Miyamoto, J. Terminal residues of bioresmethrin. Submission to IUPAC
1975-76 Terminal Residues Commission, Madrid, September
1975; Leverkusen, September, 1976
Miyamoto, J., Nishida, T., and Udea, K. Metabolic fate of resmethrin,
1971 5-benzyl-3-furyl methyl-d1-trans-chrysanthemate
in the rat. Pesticide Biochem. & Physiol., 1:
293-306.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., and Spicer,
1971 E.J.F. Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished report
from the Huntington Research Centre, submitted to
the World Health Organization by the Wellcome
Foundation Ltd.
Simonaitis, R.A. and Cail, R.S. J. Assoc. Offic. Anal. Chem.,
1975 58(5). 1032-1036.
Ueda, K., Gaughan, L.C., and Casida, J.E. Metabolism of four
1975a resmethrin isomers by liver microsomes. Pesticide
Biochem. Physiol., 5: 280-294.
Ueda, K., Gaughan. L.C., and Casida, J.E. Metabolism of (+)-trans
1975b and (+)-cis resmethrin in rats. J. Agr. Food
Chem., 23(1): 106-115.
Verschoyle, R.D., and Barnes, J.M. Toxicity of natural and synthetic
1972 pyrethrins/rats. Pesticide Biochem. & Physiol.,
2(3): 308-11.
Waldron, M.M. Foetal toxicity study - 95 H 56 - given orally
1969 in the rabbit. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd., submitted to the World Health
Organization by The Wellcome Foundation Ltd.
Wallwork, L., Chesher, B.C., and Malone, J.C. Toxicity of NRDC 107
1970 to laboratory animals. Unpublished report from the
Cooper Technical Bureau, Berkhamstead, The
Wellcome Foundation Ltd., submitted to the World
Health Organization by The Wellcome Foundation
Ltd.
Wallwork, L.M., Clampitt, R.B., and Malone, J.C. NRDC 107, rat
1971 oral 91 day toxicity study. Unpublished report
from the Cooper Technical Bureau, Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by The Wellcome
Foundation Ltd.
Wallwork, L.M. and Malone, J.C. Potentiation toxicity studies with
1971 NRDC 107, bioallethrin and piperonyl butoxide.
Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation
Ltd., submitted to the World Health Organization
by The Wellcome Foundation Ltd.
Wallwork, L.M., and Malone, J.C. Bioresmethrin; Preliminary
1972 24-hour rat inhalation study. Unpublished report
from the Cooper Technical Bureau. Berkhamstead,
The Wellcome Foundation Ltd., submitted to the
World Health Organization by the Wellcome
Foundation Ltd.
