
DIALIFOS JMPR 1976
IDENTITY
Chemical name
S-(2-chloro-1-phthalimidoethyl) 0,0-diethyl phosphorodithioate
Synonyms
Dialifor (adopted as a common name by the American National
Standards Institute), AC 14503, Torak(R)
Structural formula
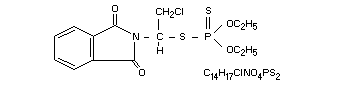 Other information on identity and properties
State: white crystals (pure)
brown crystals (isolated technical)
brown liquid (commercial technical)
Commercial technical dialifos is produced in the form of an
80% solution of technical dialifos in a xylene-range aromatic
solvent. This solution is adjusted to a minimum dialifos content of
72% by weight. Technical dialifos in crystalline form is not an
item of commerce.
Melting point: 67°C (pure)
Vapour pressure: < 10-4 mm Hg 35°C
Solubility: water 0.18 mg/kg
essentially insoluble in vegetable oils,
alcohol and glycols
soluble in acetone, chloroform, xylene and
ethyl ether
Hydrolytic stability in ethanol/water (2:8) at 25°C:
half life of 119 hours at pH 6
half life of 2.5 hours at pH 8
Dialifos is formulated as emulsifiable concentrates in the range 25
to 50% or as a 50% wettable powder.
Purity
TABLE 1. Impurities (maximum levels in commercial technical dialifos
Compound Maximum level (%w/w)
O,O-diethyl S-(1-phthalimidoethyl
phosphorodithioate
("norchlorodialifos") 5
dialifos oxygen analogue <1
N-(2-hydroxyethyl) phthalimide <.3
N-(2-acetoxyethyl) phthalimide <.3
N-(1,2,2-trichloroethyl)phthalimide 1
N-(1,2-dichloroethyl)phthalimide 1
N-(2-chlorovinyl)phthalimide 1
OOO-triethyl phosphorthioate 3
OOS-triethyl phosphorodithioate 3
Unidentified 2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on enzymes and other biochemical parameters
The in vitro inhibitory effect of dialifos (technical) on
rat plasma and erythrocyte cholinesterase was studied. The
enzyme-inhibitor complex was formed within five minutes and was
stable during the incubation period. Linear reaction rates were
also demonstrated, confirming the reliability of the electrometric
assay procedure for cholinesterase activity. The concentration of
dialifos required to obtain a 50% inhibition of plasma
cholinesterase was 2.55 µg/ml and 0.31 µg/ml for erythrocyte
cholinesterase. The in vitro oxidation of dialifos with a
peroxide-acetic acid system decreased the 50% inhibition
concentration to 0.83 µg/ml for plasma cholinesterase and 0.015
µg/ml for erythrocyte cholinesterase (Lazanas et al 1966a, 1966b).
A study was conducted to investigate the effect of dialifos on
aliphatic esterases in comparison with cholinesterase. Groups of 6
female rats were fed levels from 0.5 to 50 ppm dialifos
(technical). Three animals at each dietary level were killed after
1 and 3 weeks and measurements of the rate of enzymic hydrolysis of
diethyl succinate and tributyrin by the liver and serum, and
cholinesterase activity of the brain were conducted. The dietary
levels producing 50% inhibition of tributyrinase activity of liver
and serum were 4.7 and 10.3 ppm respectively after 3 weeks. Diethyl
succinate hydrolysis in the liver and serum was inhibited by 50% at
dietary levels of 13.5 and 12.5 ppm after 1 week and 16.0 and 12.0
ppm after 3 weeks respectively. Brain cholinesterase was
significantly reduced at 50 ppm after 3 weeks. In a preliminary
study liver and serum cholinesterase were shown to be appreciably
inhibited after 1 week at dietary levels of 25 ppm. The
cholinesterase activity of all the tissues was inhibited to a
lesser extent than the aliesterase activity (DuBois et al, 1969).
TOXICOLOGICAL STUDIES
Special studies on antidotes
The acute oral LD50 of dialifos was determined in groups of
male rats (1) without antidote; (2) treated with atropine sulphate
(17.5 mg/kg); (3) treated with 2-PAM (50 mg/kg); (4) treated with
a combination of atropine sulphate and 2-PAM at 17.5 and 50 mg/kg
respectively. Antidotes were administered intramuscularly 30
minutes after dialifos. The LD50 for the groups were 71, 140, 267
and 177 mg/kg respectively. The onset of symptoms and time of death
were delayed in the treated groups. Atropine sulphate and 2-PAM
would appear to be effective antidotes for dialifos (Mastri et al,
1968).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed dialifos
(technical) in the diet at dosage levels of 0, 0.05 and 0.5 mg/kg
body weight/day and subjected to a standard 2 litter, 3 generation
reproduction study. The following parameters were measured: body
weight, mortality, symptoms, hematology, urinalysis, organ weights
and gross and histopathology of all parental generations after the
second weaning as well as plasma and erythrocyte cholinesterase
activities of all parental generations and litters. In addition,
the following reproduction indices were measured: mating, pregnancy,
fertility, parturition and lactation. With respect to progeny mean
litter size, number of stillborn and viable pups and pup body weights
were recorded. Plasma and erythrocyte cholinesterase depression were
observed primarily in the female of all parental groups and
weanlings fed 0.5 mg/kg body weight. There were random non-dose
related effects in some reproductive parameters of both test groups
when compared to controls. No compound-related gross or
histopathological findings were observed. (Arnold et al, 1968).
Special studies on teratology
Rabbit
Groups of 10 pregnant rabbits were administered dialifos
(technical) via gelatin capsule at dosage levels of 0, 1 and 3
mg/kg from day 6 to day 18, and 10 and 25 mg/kg from day 6 to day
16 of gestation. Thalidomide was given at a dosage of 75 mg/kg/day.
Does were sacrificed on day 29 of gestation. Offspring were placed
in an incubator for 24 hours to determine viability and then
sacrificed for skeletal examination. Parental weight loss occurred
at 3, 10 and 25 mg/kg. Mortality was increased at 10 mg/kg (7/10)
and 25 mg/kg (10/10). Hemorrhage in the small intestine was
observed in the two highest dose groups. Litter size was reduced
and fetal resorption increased at 10 and 25 mg/kg while the
viability index was decreased at all dosage levels. Four
abnormalities (3 umbilical hernia, 1 partial acranius) were noted
at 3 mg/kg, however no abnormalities were observed at 10 mg/kg. The
thalidomide group showed clubbing of the extremities (30/49)
(Kennedy et al, 1966). This study was repeated in a similar fashion
using the same strain of rabbit, administered 0, 1, or 3 mg
dialifos (technical)/kg from day 6 to day 18 of gestation. No
adverse effects were observed with respect to maternal body weight,
survival or behaviour. Number of implantation sites and litter size
were reduced and the number of resorption sites increased in
treated groups when compared to corresponding controls. This was
considered to be due to physical manipulation of these animals
rather than a possible toxic effect of the test material. The
viability index was comparable to controls, and no skeletal or
external abnormalities were found in any of the progeny receiving
dialifos (Jackson et al, 1966b).
Groups of 17 female rabbits were treated from day 6 through
day 18 of gestation with daily doses of either 0, 1.0 or 3.0 mg/kg
norchlorodialifos via gelatin capsule. Thalidomide was administered
concurrently at 37.5 mg/kg body weight as a positive control.
Rabbits were sacrificed on gestation day 29. Young were removed by
caesarean section and placed in an incubator for 24 hours. They
were then sacrificed and examined for external skeletal and soft
tissue abnormalities. At 3 mg/kg the animals lost weight during the
dosing period. No effect in body weight gain was exhibited at 1
mg/kg. No deaths or unusual reactions were observed. The number of
live fetuses and fetal survival were reduced at 3 mg/kg. No
significant differences were observed in 24 hour viability index
or fetal body weights between control and norchlorodialifos treated
animals. No compound-related internal or skeletal abnormalities were
noted (Ladd et al, 1972).
Hamster
Pregnant hamsters were dosed orally with dialifos once on day
7 or 8 of gestation or several times on days 6-8 or days 6-10 of
gestation. The total administered dose varied from 100-500 mg/kg.
On day 15 of gestation the surviving hamsters were sacrificed and
the fetuses removed. The usual parameters for teratogenicity were
examined. From the mortality data of the dams it was concluded that
most of the doses were > LD50. The lowest single dose of 100
mg/kg body weight produced malformations in 4/98 fetuses, 3 with
limb defects and one with umbilical hernia (Robens, 1970).
Monkey
Ten pregnant stumptailed macaques were dosed with dialifos
(technical) at 1 mg/kg body weight (the dose causing ca 25% plasma
and erythrocyte cholinesterase depression (Vondruska et al, 1968)
for a period of one week during the critical period of limb
formation. Nine other pregnant females were administered either 5
or 10 mg thalidomide/kg as positive controls. Blood samples were
taken on the 15th, 18th and 21st days of pregnancy and on the last
dosing day for complete hematology and cholinesterase activity.
During the 12th week of gestation, fetuses were recovered by
caesarean section and examined for abnormalities. No hematological
effects were observed. Plasma and erythrocyte cholinesterase
activity for monkeys receiving dialifos were reduced to 65 and 62%
of pre-treatment values. No abortions were observed with dialifos
and all fetuses were normal. Abortions occurred in four females
dosed with thalidomide. A total of five fetuses were recovered from
females treated with thalidomide and all exhibited gross
deformities of the anterior extremities (Vondruska and Fancher,
1969).
Special studies on mutagenicity
Dialifos was tested for mutagenic activity using a series of
microbial indicator organisms (Saccharomyces cerevisiae strain
D4; Salmonella typhimurium strains TA-1535, 1537, 1538) in
qualitative plate tests with and without metabolic activation.
Activation was accomplished by the use of tissue homogenates of
liver, lung and testes from mice, rats and monkeys. The compound
did not exhibit significant genetic activity in any of the assays
performed (Brusick, 1975).
Special studies on neurotoxicity
Groups of 6 hens were given 0 or 464 mg/kg body weight (oral
LD 50) of dialifos (technical). Surviving birds were re-dosed with
the LD50 after 21 days and were observed for an additional 21 days.
Samples of spinal cord, sciatic nerve and brain were taken for
histological examination. A positive control group was dosed with
500 mg/kg body weight TOCP and studied simultaneously with the test
group. No signs of ataxia were noted and no clinical neurotoxic
effects were observed. Birds in the positive control group showed
signs of neurotoxicity approximately 14 days following
administration of the last dose. Histopathology of nervous tissue
was not done (Jackson et al, 1968).
Special studies on potentiation
An acute potentiation study was conducted in order to
determine the degree of interaction between dialifos (technical)
and malathion. The acute oral LD50 values were determined in male
and female rats separately. Doses corresponding to 1/2, 1/4 and 1/8
the LD50 of dialifos were administered orally to animals in
separate groups consisting of 5 male and 5 female rats followed
four hours later by the corresponding fractions of the LD50 of
malathion. No potentiation was observed in female rats. However, in
the case of males, potentiation did occur since 1/8 of the LD50 of
each compound resulted in an LD40 (Schoenig et al, 1966e).
Special studies on eye irritation
Undiluted dialifos when applied directly to the conjunctival
sac of two rabbits was shown to be mildly irritating in both
unwashed and washed eyes. When dialifos was applied as a 5%
suspension in propylene glycol, similar results were observed
(Schoenig et al, 1966d).
Special studies on dermal irritation
When undiluted dialifos was applied to shaved and abraded skin
of two rabbits, a slight irritating effect was noted after 24
hours. Mortality precluded a 72-hour reading (Schoenig et al,
1966d).
Acute toxicity
TABLE 2. Acute toxicity of dialifos
Animal Sex Route Solvent LD50 Reference
mg/kg bw
rat M oral propylene 53 Schoenig et al
glycol 1966d
M oral propylene 43 ibid
glycol
M oral 1% methyl 71 ibid
cellulose
F oral propylene 5 ibid
glycol
mouse M oral propylene 39 Schoenig et al
glycol 1966d
F oral propylene 65 ibid
glycol
rabbit M oral propylene 58 ibid
glycol
F oral propylene 71 ibid
glycol
F oral maize oil 35 Jackson et al
1966a
M dermal xylene 145 Schoenig et al
1966d
dog M oral propylene 97 Schoenig et al
glycol 1966d
chicken F oral maize oil 464 Jackson et al
1968
The impurities of dialifos were isolated. The acute toxicities
of the impurities and pure compound were compared.
TABLE 3. Acute toxicity of pure dialifos compared with its
impurities
LD50
Animal Sex Route Solvent mg/kg bw Reference
pure dialifos
rat M oral propylene 69 Schoenig et al
glycol 1966a
F oral propylene 5 ibid
glycol
impurities
rat M oral propylene 327 Schoenig et al
glycol 1966b
F oral propylene 218 ibid
glycol
TABLE 4. Acute toxicity of dialifos formulations
LD50
Animal Sex Route Solvent mg/kg bw Reference
Emulsifiable concentrate - 47%
rat M oral water 51 Nomura 1970
F oral water 19 ibid
M oral water 62 Ueda 1968
F oral water 21 ibid
M&F Inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
TABLE 4. (Cont'd.)
LD50
Animal Sex Route Solvent mg/kg bw Reference
mouse M oral water 99 Nomura 1970
F oral water 90 ibid
M&F inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
rabbit M dermal water 327 Mastri et al
1969a
guinea M&F inhalation water saturated Hathaway et al
pig (4 hr) vapour 1969a
(LC50)
Wettable Powder - 50%
rabbit M dermal water 735 Mastri et al
1969b
rat M&F inhalation 0.5 mg/l Hathaway et al
(2 hr) (LC50) 1969b
mouse M&F inhalation 1.0 mg/l ibid
(2 hr) LC50)
guinea M&F inhalation 1.0 mg/l ibid
pig (2 hr) LC50)
TABLE 5. Acute toxicity of norchlorodialifos
LD50
Animal Sex Route Solvent mg/kg bw Reference
rat M oral maize oil 81 Gabriel 1972
F oral maize oil 22
rabbit M dermal - > 1000 Gabriel 1972
Short term studies
Rabbit - dermal
Dialifos 4 lbs/gal (0.48 kg/l) emulsifiable solution was
applied to the clipped backs of groups of 3 male and 3 female
rabbits at dosages of 0.3, 1, 5 and 25 mg/kg. The test material was
applied for a period of 6 hours/day, 5 days/week until 22
applications were made. An untreated control group received no
dermal application. At dosage levels of 25 and 5 mg/kg, growth was
depressed with inhibition of plasma, erythrocyte and brain
cholinesterase activity. A slight acanthosis of the epidermis
suggesting slight irritation was observed. There was a slight but
questionable depression of plasma and brain cholinesterase at 1 mg/kg.
No adverse effects were observed in any of the parameters studies at
0.3 mg/kg body weight (Mastri et al, 1969c; Mastri et al, 1970).
Rat and guinea pig - inhalation
Groups of rats and guinea pigs (6 males and 6 females of each
species) were exposed for 6 hours/day, 6 days/week for 2 weeks to
an aerosol of dialifos generated from an acetone solution of a 4
lb/gal (0.48 kg/1) emulsifiable formulation at concentrations of 0,
2.8, 7.7 and 20.0 µg/liter. Half the animals were sacrificed for
pathological examination after the last exposure. The remaining
animals were sacrificed after a further two week observation
period. Plasma, erythrocyte and brain cholinesterase activities
were determined only in the rat. Some mortality, ataxia, dyspnea,
marked plasma and slight erythrocyte cholinesterase depression were
observed at 20 µg/liter. No adverse effects were noted at exposure
levels of 7.7 and 2.8 µg/liter (Hathaway et al, 1969c; Hathaway et
al, 1970).
Mouse - oral
Groups of 5 male and 5 female mice were administered dialifos
dissolved in lard oil by gavage 7 days a week at dosages of 1, 2.5,
5 and 10 mg/kg/day for 90 days. Two control groups were used in
this study, one receiving no treatment, the other an equivalent
amount of lard oil to the treated groups. One half of the animals
were sacrificed after 30 days and the remainder at 90 days. The
following parameters were measured: body weight, hematology, organ
weights, gross and histopathology. Cholinesterase determinations
were not conducted. Decreased growth rate and liver necrosis were
observed in male mice receiving 10 mg/kg body weight. No
significant effects were observed at 5 mg/kg body weight or less
(Nomura, 1970).
Rat - oral
Dialifos dissolved in lard oil was administered by gavage 7
days a week at dosages of 1.0, 2.5, 5 and 10 mg/kg/day for males
and 0.15, 0.375, 0.75 and 1.5 mg/kg/ day for females for 90 days.
Control groups received either no treatment or an equivalent amount
of lard oil. One half of the animals were sacrificed at 30 days and
the remainder at 90 days. Observations on the following parameters
were made: body weight, hematology, organ weights, gross and
microscopic pathology. Serum cholinesterase measurements were made
after 30 days. Serum cholinesterase was depressed at 2.5 mg/kg and
above in males and 0.375 mg/kg and above in females. At the upper
dosage levels in both male and female, increased mortality and
liver necrosis were observed (Nomura, 1970).
Dialifos was administered orally to groups of 3 male and 3
female rats at 0, 0.5, 1.0, 2.5, 5.0, 10.0, 25.0 and 50.0 mg/kg/day
for 14 days. Plasma cholinesterase was inhibited at 0.5 mg/kg/day
and above, erythrocyte cholinesterase at 1.0 mg/kg and above, and
brain cholinesterase at 2.5 mg/kg and above. There was increased
mortality at 10.0 mg/kg and above. Gross pathological findings
among test animals were comparable to those of controls (Wolf and
Calandra, 1965).
Dialifos was fed at dietary levels of 0, 10, 25, 50 and 100
ppm to groups of 10 male and 10 female rats for 90 days.
Erythrocyte cholinesterase activity was decreased at all dietary
levels. Plasma cholinesterase activity was depressed among females
at all dietary levels and among males at 25 ppm and above. Brain
cholinesterase activity of males was depressed at 100 ppm while
among females depression was noted at 25 ppm and above. At 100 ppm,
body weight gain and food consumption were reduced; a
compound-related increase in mortality was noted; BUN and serum A/G
ratios were increased. Hematology, organ weights, gross and
histopathology revealed no compound-related effects (Wolf et al,
1966b).
Groups of 21 male and 21 female rats were fed diets containing
dialifos at levels of 0, 0.3, 1, 3, 10 and 30 ppm for 13 weeks.
After 1, 3, 6 and 13 weeks of feeding, 3 rats/sex/groups were
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. The remaining rats were then fed the control diet
and a similar number of animals sacrificed at 1 and 4 weeks
post-treatment. Plasma and erythrocyte cholinesterase activities
were depressed at 10 and 30 ppm while brain cholinesterase was
depressed at 30 ppm. After 1 week recovery, plasma and erythrocyte
cholinesterase were still depressed at the upper level. Values for
all three parameters were normal after a 4 week recovery period.
Since there was great variability in plasma cholinesterase
activity after 3 weeks and anomolous results after 13 weeks in
erythrocyte cholinesterase values, an additional study was
conducted. Groups of 12 male and 12 female rats were fed as above
for 13 weeks. Determination of plasma, erythrocyte and brain
cholinesterase activity was carried out at 3 and 13 weeks. All the
parameters measured were depressed at 30 ppm. Erythrocyte
cholinesterase was also decreased at 10 ppm. A no-effect level of
3 ppm was demonstrated (Wolf et al 1966a; 1966c).
Norchloro-dialifos was fed to groups of 10 male and 10 female
rats at dietary levels of 0, 50 and 200 ppm for 90 days. Body
weight gain and food consumption were decreased among females at
both test levels. There was a slight decrease in reticulocytes in
both sexes at 200 ppm. Significant depressions in erythrocyte,
plasma and brain cholinesterase activities were observed among male
and female rats from both test groups. No compound-related effects
were noted with respect to mortality, blood chemistry, urinalysis
organ weights, gross and microscopic pathology (Smith et al 1972b).
An additional study was designed in a comparable manner in
which groups of 9 male and 9 female rats were fed dietary levels of
norchloro-dialifos of 0, 1, 3 and 10 ppm for 90 days. After 3, 6
and 13 weeks, 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. After 13 weeks decreases in both plasma and
erythrocyte cholinesterase activities were observed at 3 and 10
ppm. Brain cholinesterase activity was slightly depressed at 3 ppm
among males, while females of all groups showed a non-dose related
decrease (Smith et al 1972a).
Dog - oral
Groups of 1 male and 1 female dogs were fed diets containing
0, 20, 100 and 500 ppm of dialifos for 14 days. Plasma and
erythrocyte cholinesterase activities were depressed at 20 ppm and
above. There was food rejection at all levels with weight loss at
500 ppm (Schoenig et al, 1966a).
Dialifos (technical) was administered in the diet to groups of
2 male and 2 female dogs at levels of 0, 1, 3, 10, 30 and 100 ppm
for 98 days. Plasma and erythrocyte cholinesterase activities were
not depressed when measured at 0, 7, 21, 42 and 90 days at dietary
levels of 1 and 3 ppm or less respectively. A slight reduction in
brain cholinesterase activity was noted at the upper dose level.
Body weight gain, food consumption, hematology, clinical chemistry,
urinalysis, organ weights, gross and histopathology were within
normal limits (Baran et al, 1966).
Groups of 3 male and 3 female dogs were fed dialifos
(technical) which was incorporated into the diet to give a dose
level of 0, 50 ,or 200 ppm for 2 years. The upper level was reduced
to 100 ppm after 21 days. After 1 year, 1 male and 1 female/group
were sacrificed. Most dogs showed weight loss and symptoms
associated with cholinesterase depression while receiving 200 ppm.
Cholinesterase activity was reduced at 50 and 100 ppm in plasma and
erythrocytes at 6, 12 and 24 months. Brain cholinesterase appeared
to be slightly depressed at 100 ppm. No compound-related effects
were observed with respect to food consumption, mortality,
hematology, clinical chemistry, organ function tests, organ
weights, gross and histopathology (Baran et al, 1968).
Tissue residue studies were performed on body tissues of dogs
fed dialifos (technical) for 2 years. Samples of muscle, fat,
liver, kidney and heart of each dog were examined. Barely
detectable residues of dialifos were found in muscle, liver, kidney
and heart samples, residues ranging from 0.003 to 0.005 mg/kg.
Slightly higher levels were detected in the fat samples. These
averaged 0.022 mg/kg at the 50 ppm and 0.032 mg/kg at the 100 ppm
feeding levels. The oxygen analogue of dialifos was not detected in
any of the tissues examined (Ford 1970c).
Chicken - oral
Replicate groups of 3 laying hens were fed 0, 0.375 and 1.25
mg/kg/day of dialifos (technical) for 8 weeks. Erythrocyte and
plasma cholinesterase activities were determined prior to and at 1,
2, 4 and 8 weeks following the initiation of treatment.
Cholinesterase activities were not affected. No toxic symptoms were
observed and no effect was noted on daily food consumption, body
weight or egg production (Sedivy 1968c).
Cattle - oral
Groups of 3 beef animals (180 kg) were fed 0, 0.1 and, O.2
mg/kg/day of dialifos for 28 days. One animal of each group was
sacrificed after 12 days of treatment, and the remaining animals at
28 days for collection of tissue samples. There was no
treatment-related effect with respect to plasma or erythrocyte
cholinesterase activity. Residue levels in fat, kidney, liver and
muscle were generally less than 0.001 mg/kg. The highest residue
level detected was 0.003 mg/kg in the fat of animals fed 0.2 mg/kg
(Taylor, 1969).
Three beef animals (180 kg) were fed diets containing
approximately 2.2 mg/kg/day; a fourth animal served as a control.
At the end of each feeding period of 2, 4 and 8 weeks, one animal
was sacrificed for tissue residue studies. Plasma and erythrocyte
cholinesterase determinations were made at weekly intervals.
Residues in the range of 0.1 to 0.3 mg/kg were found in liver and
fatty tissues. Lower residues ( <0.1 mg/kg) were detected in
muscle and kidney. No evidence for the build-up of the residue was
observed when the feeding period was doubled. The oxygen analogue
of dialifos was not detected to any significant degree in the
tissues analysed. Erythrocyte cholinesterase activity was markedly
inhibited, while plasma inhibition was only slightly affected.
(Ford 1970a; Sedivy 1968a).
Four holstein milk cows were administered a daily dose of
dialifos of 1.35 mg/kg for 21 days. Weekly plasma and erythrocyte
cholinesterase determinations were made and milk samples collected
for residue analysis. No overt signs of toxicity were observed and
milk production was not altered. A marked inhibition of erythrocyte
cholinesterase was noted. The slight reduction in plasma
cholinesterase was questionable. An average dialifos residue of 5
mg/kg was detected in milk samples which quickly disappeared when
the administration of dialifos was terminated. No oxygen analogue
was found in any of the samples (Ford 1970b., Sedivy 1968b).
Long-term studies
Rat
Groups of 30 male and 30 female rats were fed dietary levels
of 0, 0, 20 and 50 ppm of dialifos (technical) for 2 years. After
6, 12 and 24 months 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and cholinesterase determinations.
Erythrocyte cholinesterase activity was depressed in all animals of
both test levels at all examination intervals. Plasma
cholinesterase activity was decreased among females at both test
levels while males exhibited depression at 50 ppm after 6, 12 and
18 months but not at 24 months. Brain cholinesterase activity was
depressed at the higher intake level. No significant differences
between treated and control animals were observed with respect to
body weight gain, food consumption, mortality, hematology, blood
chemistry, urinalysis, organ weights or gross and histopathology.
There was no increase on the incidence of tumours in the test
animals (Wolf et al 1968).
Dialifos was fed at dietary levels of 0, 1, 3 and 10 ppm to
groups of 30 male and 30 female rats for 1 year. Five males and 5
females/group were randomly selected and sacrificed after 3, 6, 9
and 18 months for plasma, erythrocyte and brain cholinesterase
determinations as well as gross and histopathological examination.
Plasma cholinesterase in females and erythrocyte cholinesterase in
both sexes were decreased at the dietary intake of 10 ppm. Brain
cholinesterase was not affected. Body weight gain was slightly
decreased in males fed 10 ppm. There were no compound-related
effects in mortality, organ weights, gross and histopathology
(Regna et al 1975).
A small number of animals from the one year oral toxicity
study reported above were continued on the appropriate diet for an
additional year. The small number of surviving animals on test
makes the interpretation of the data difficult. However, no
significant changes were noted in body weights, food consumption,
mortality, behavioural reactions, plasma, erythrocyte and brain
cholinesterase, gross and histopathology. No evidence of increased
tumour incidence or production of unusual tumour types was observed
in the treated animals (Marias et al, 1975).
OBSERVATIONS IN MAN - ORAL
Dialifos was administered orally to 3 male and 3 female
volunteers in gelatin capsules in daily single doses of 0.01 (2
weeks), 0.03 (4 weeks) and 0.1 mg/kg (10 days) with 2 males and 2
females receiving placebos. After an appropriate recovery period
four of the test subjects were used as controls and the 4 control
subjects became part of the total of 6 test subjects in a second
study. In this study dialifos was administered orally in gelatin
capsules 3 times a day to simulate 3 meals a day in dosage levels
of 0.01 (2 weeks), 0.02 (4 weeks), 0.03 (8 weeks) and 0.05 (4
weeks) mg/kg. In both studies, plasma and erythrocyte
cholinesterase, plasma ali-esterases (tributyrinase and diethyl
succinase) were measured before dosing and at intervals during the
study and recovery periods. Periodically, other observations were
made which included hematology, blood pressure, pulse rate, pupil
size, light reflex, eye accommodation, chest sounds, muscle tone,
kneejerk, tongue tremor and finger tremor. No significant
inhibition of any of the enzyme systems tested was observed at
dosage levels of 0.01, 0.02 and 0.03 mg/kg when given either in single
or divided daily dose. Plasma cholinesterase and tributyrinase
were slightly depressed while no effect was observed
on erythrocyte cholinesterase or diethyl succinase at 0.05 and 0.1
mg/kg. Recovery was essentially complete in 14-21 days. The other
parameters measured were not affected (Greco et al, 1970).
COMMENTS
In mammals dialifos is an acutely toxic organophosphorus ester
which would appear to be rapidly absorbed as shown by the rapid
onset of cholinergic symptoms of poisoning. No data were available
with respect to absorption, metabolism and excretion in animals.
Teratogenic and reproduction studies indicated no adverse
effects at doses below those which were toxic to the parents.
Mutagenic activity was not observed in the microbial indicator
organisms used in the study. No apparent signs of neurotoxicity
were observed in hens given large doses of dialifos.
In several short-and long-term studies in the mouse, rat, dog,
hen, monkey, cattle and man, dialifos was shown to be an active
cholinesterase inhibitor. Plasma cholinesterase was inhibited to a
greater extent than either erythrocyte or brain cholinesterase with
no marked difference between animal species.
The major impurity of technical dialifos, 00-diethyl
S-(1-phthalimidoethyl) phosphorodithioate ("norchlordialifos"), was
as toxic as dialifos as indicated by acute, short-term and
teratogenic studies.
No-effect levels in the rat, dog and man have been established
on the basis of the most sensitive parameter, the depression of
plasma cholinesterase activity. The data were sufficient to
recommend an acceptable daily intake for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat 3 ppm in the diet equivalent to 0.15 mg/kg bw
Dog 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man 0.03 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Dialifos is an acaricide and insecticide used in wettable
powder and emulsion sprays on citrus, apples, pears, grapes,
pecans, rapeseed, cotton, potatoes and sugar beets. Dosages, number
of treatments, and use restrictions vary widely with the target
pest and the residue tolerances in the country in which it is used.
A summary of various national use patterns is shown in Table
6. The table is based primarily on information provided by the
basic manufacturer of dialifos. Country statements furnished to the
Meeting indicate that South Africa has cancelled registered uses
for dialifos, that use in the Netherlands is curtailed because of
resistance problems, and that Australia has no registered uses but
has applications for use on some fruits pending.
TABLE 6. National use patterns of dialifos
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
Citrus
U.S.A. 30-60g/100 l 7 2 treatments per
year
Turkey 1.0-1.5 kg/ha 28 2 treatments per
growing season
Japan 1.7-3.3 kg/ha 45
Grapes
U.S.A. 1.1 kg/ha 25 one treatment after
shot-berry stage
and no more than
2 per year
France 0.75-1.0 kg/ha 28 one spray per
growing season
(before Aug 1)
Apples, Pears
U.S.A. 60-90 g/100 l 60 not more than 5
cover sprays
Netherlands 0.75-1.0 kg/ha* 8 weeks
TABLE 6. (Cont'd.)
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
France 1.0 kg/ha 4 weeks
Germany 1.0 kg/ha 4 weeks
Italy 1.0 kg/ha 4 weeks
Belgium 1.0 kg/ha 6 weeks
Switzerland 1.0 kg/ha 6 weeks
New Zealand pre-blossom spray
only (provisional
registration)
U.K. 0.06%(high vol.) 3 weeks not more than 3
sprays per season
1.0 kg/ha(low vol.)
Pecans
U.S.A. 60 g/100 l -- apply before husk
split
Rapeseed
Germany 288 g/ha apply only during
flowering
* Report decreased use because of resistance.
It was also reported that dialifos is used on potatoes (500g/ha)
and sugar beets (700-750 g/ha) in Europe and on cotton in Turkey and
Africa at rates of 100-125 g/ha.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials in the U.S.A., Canada, Japan
Australia, the Netherlands, Republic of South Africa, West Germany and
Italy were made available to the Meeting through a submission by the
basic manufacturer (Hercules, 1976) and country statements. The
findings are discussed separately by commodity. Unless otherwise
noted, residue values are for dialifos per se.
Citrus
Data from supervised trials in the U.S.A. are summarized in Table
7 and Figure 1.
Other information on identity and properties
State: white crystals (pure)
brown crystals (isolated technical)
brown liquid (commercial technical)
Commercial technical dialifos is produced in the form of an
80% solution of technical dialifos in a xylene-range aromatic
solvent. This solution is adjusted to a minimum dialifos content of
72% by weight. Technical dialifos in crystalline form is not an
item of commerce.
Melting point: 67°C (pure)
Vapour pressure: < 10-4 mm Hg 35°C
Solubility: water 0.18 mg/kg
essentially insoluble in vegetable oils,
alcohol and glycols
soluble in acetone, chloroform, xylene and
ethyl ether
Hydrolytic stability in ethanol/water (2:8) at 25°C:
half life of 119 hours at pH 6
half life of 2.5 hours at pH 8
Dialifos is formulated as emulsifiable concentrates in the range 25
to 50% or as a 50% wettable powder.
Purity
TABLE 1. Impurities (maximum levels in commercial technical dialifos
Compound Maximum level (%w/w)
O,O-diethyl S-(1-phthalimidoethyl
phosphorodithioate
("norchlorodialifos") 5
dialifos oxygen analogue <1
N-(2-hydroxyethyl) phthalimide <.3
N-(2-acetoxyethyl) phthalimide <.3
N-(1,2,2-trichloroethyl)phthalimide 1
N-(1,2-dichloroethyl)phthalimide 1
N-(2-chlorovinyl)phthalimide 1
OOO-triethyl phosphorthioate 3
OOS-triethyl phosphorodithioate 3
Unidentified 2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on enzymes and other biochemical parameters
The in vitro inhibitory effect of dialifos (technical) on
rat plasma and erythrocyte cholinesterase was studied. The
enzyme-inhibitor complex was formed within five minutes and was
stable during the incubation period. Linear reaction rates were
also demonstrated, confirming the reliability of the electrometric
assay procedure for cholinesterase activity. The concentration of
dialifos required to obtain a 50% inhibition of plasma
cholinesterase was 2.55 µg/ml and 0.31 µg/ml for erythrocyte
cholinesterase. The in vitro oxidation of dialifos with a
peroxide-acetic acid system decreased the 50% inhibition
concentration to 0.83 µg/ml for plasma cholinesterase and 0.015
µg/ml for erythrocyte cholinesterase (Lazanas et al 1966a, 1966b).
A study was conducted to investigate the effect of dialifos on
aliphatic esterases in comparison with cholinesterase. Groups of 6
female rats were fed levels from 0.5 to 50 ppm dialifos
(technical). Three animals at each dietary level were killed after
1 and 3 weeks and measurements of the rate of enzymic hydrolysis of
diethyl succinate and tributyrin by the liver and serum, and
cholinesterase activity of the brain were conducted. The dietary
levels producing 50% inhibition of tributyrinase activity of liver
and serum were 4.7 and 10.3 ppm respectively after 3 weeks. Diethyl
succinate hydrolysis in the liver and serum was inhibited by 50% at
dietary levels of 13.5 and 12.5 ppm after 1 week and 16.0 and 12.0
ppm after 3 weeks respectively. Brain cholinesterase was
significantly reduced at 50 ppm after 3 weeks. In a preliminary
study liver and serum cholinesterase were shown to be appreciably
inhibited after 1 week at dietary levels of 25 ppm. The
cholinesterase activity of all the tissues was inhibited to a
lesser extent than the aliesterase activity (DuBois et al, 1969).
TOXICOLOGICAL STUDIES
Special studies on antidotes
The acute oral LD50 of dialifos was determined in groups of
male rats (1) without antidote; (2) treated with atropine sulphate
(17.5 mg/kg); (3) treated with 2-PAM (50 mg/kg); (4) treated with
a combination of atropine sulphate and 2-PAM at 17.5 and 50 mg/kg
respectively. Antidotes were administered intramuscularly 30
minutes after dialifos. The LD50 for the groups were 71, 140, 267
and 177 mg/kg respectively. The onset of symptoms and time of death
were delayed in the treated groups. Atropine sulphate and 2-PAM
would appear to be effective antidotes for dialifos (Mastri et al,
1968).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed dialifos
(technical) in the diet at dosage levels of 0, 0.05 and 0.5 mg/kg
body weight/day and subjected to a standard 2 litter, 3 generation
reproduction study. The following parameters were measured: body
weight, mortality, symptoms, hematology, urinalysis, organ weights
and gross and histopathology of all parental generations after the
second weaning as well as plasma and erythrocyte cholinesterase
activities of all parental generations and litters. In addition,
the following reproduction indices were measured: mating, pregnancy,
fertility, parturition and lactation. With respect to progeny mean
litter size, number of stillborn and viable pups and pup body weights
were recorded. Plasma and erythrocyte cholinesterase depression were
observed primarily in the female of all parental groups and
weanlings fed 0.5 mg/kg body weight. There were random non-dose
related effects in some reproductive parameters of both test groups
when compared to controls. No compound-related gross or
histopathological findings were observed. (Arnold et al, 1968).
Special studies on teratology
Rabbit
Groups of 10 pregnant rabbits were administered dialifos
(technical) via gelatin capsule at dosage levels of 0, 1 and 3
mg/kg from day 6 to day 18, and 10 and 25 mg/kg from day 6 to day
16 of gestation. Thalidomide was given at a dosage of 75 mg/kg/day.
Does were sacrificed on day 29 of gestation. Offspring were placed
in an incubator for 24 hours to determine viability and then
sacrificed for skeletal examination. Parental weight loss occurred
at 3, 10 and 25 mg/kg. Mortality was increased at 10 mg/kg (7/10)
and 25 mg/kg (10/10). Hemorrhage in the small intestine was
observed in the two highest dose groups. Litter size was reduced
and fetal resorption increased at 10 and 25 mg/kg while the
viability index was decreased at all dosage levels. Four
abnormalities (3 umbilical hernia, 1 partial acranius) were noted
at 3 mg/kg, however no abnormalities were observed at 10 mg/kg. The
thalidomide group showed clubbing of the extremities (30/49)
(Kennedy et al, 1966). This study was repeated in a similar fashion
using the same strain of rabbit, administered 0, 1, or 3 mg
dialifos (technical)/kg from day 6 to day 18 of gestation. No
adverse effects were observed with respect to maternal body weight,
survival or behaviour. Number of implantation sites and litter size
were reduced and the number of resorption sites increased in
treated groups when compared to corresponding controls. This was
considered to be due to physical manipulation of these animals
rather than a possible toxic effect of the test material. The
viability index was comparable to controls, and no skeletal or
external abnormalities were found in any of the progeny receiving
dialifos (Jackson et al, 1966b).
Groups of 17 female rabbits were treated from day 6 through
day 18 of gestation with daily doses of either 0, 1.0 or 3.0 mg/kg
norchlorodialifos via gelatin capsule. Thalidomide was administered
concurrently at 37.5 mg/kg body weight as a positive control.
Rabbits were sacrificed on gestation day 29. Young were removed by
caesarean section and placed in an incubator for 24 hours. They
were then sacrificed and examined for external skeletal and soft
tissue abnormalities. At 3 mg/kg the animals lost weight during the
dosing period. No effect in body weight gain was exhibited at 1
mg/kg. No deaths or unusual reactions were observed. The number of
live fetuses and fetal survival were reduced at 3 mg/kg. No
significant differences were observed in 24 hour viability index
or fetal body weights between control and norchlorodialifos treated
animals. No compound-related internal or skeletal abnormalities were
noted (Ladd et al, 1972).
Hamster
Pregnant hamsters were dosed orally with dialifos once on day
7 or 8 of gestation or several times on days 6-8 or days 6-10 of
gestation. The total administered dose varied from 100-500 mg/kg.
On day 15 of gestation the surviving hamsters were sacrificed and
the fetuses removed. The usual parameters for teratogenicity were
examined. From the mortality data of the dams it was concluded that
most of the doses were > LD50. The lowest single dose of 100
mg/kg body weight produced malformations in 4/98 fetuses, 3 with
limb defects and one with umbilical hernia (Robens, 1970).
Monkey
Ten pregnant stumptailed macaques were dosed with dialifos
(technical) at 1 mg/kg body weight (the dose causing ca 25% plasma
and erythrocyte cholinesterase depression (Vondruska et al, 1968)
for a period of one week during the critical period of limb
formation. Nine other pregnant females were administered either 5
or 10 mg thalidomide/kg as positive controls. Blood samples were
taken on the 15th, 18th and 21st days of pregnancy and on the last
dosing day for complete hematology and cholinesterase activity.
During the 12th week of gestation, fetuses were recovered by
caesarean section and examined for abnormalities. No hematological
effects were observed. Plasma and erythrocyte cholinesterase
activity for monkeys receiving dialifos were reduced to 65 and 62%
of pre-treatment values. No abortions were observed with dialifos
and all fetuses were normal. Abortions occurred in four females
dosed with thalidomide. A total of five fetuses were recovered from
females treated with thalidomide and all exhibited gross
deformities of the anterior extremities (Vondruska and Fancher,
1969).
Special studies on mutagenicity
Dialifos was tested for mutagenic activity using a series of
microbial indicator organisms (Saccharomyces cerevisiae strain
D4; Salmonella typhimurium strains TA-1535, 1537, 1538) in
qualitative plate tests with and without metabolic activation.
Activation was accomplished by the use of tissue homogenates of
liver, lung and testes from mice, rats and monkeys. The compound
did not exhibit significant genetic activity in any of the assays
performed (Brusick, 1975).
Special studies on neurotoxicity
Groups of 6 hens were given 0 or 464 mg/kg body weight (oral
LD 50) of dialifos (technical). Surviving birds were re-dosed with
the LD50 after 21 days and were observed for an additional 21 days.
Samples of spinal cord, sciatic nerve and brain were taken for
histological examination. A positive control group was dosed with
500 mg/kg body weight TOCP and studied simultaneously with the test
group. No signs of ataxia were noted and no clinical neurotoxic
effects were observed. Birds in the positive control group showed
signs of neurotoxicity approximately 14 days following
administration of the last dose. Histopathology of nervous tissue
was not done (Jackson et al, 1968).
Special studies on potentiation
An acute potentiation study was conducted in order to
determine the degree of interaction between dialifos (technical)
and malathion. The acute oral LD50 values were determined in male
and female rats separately. Doses corresponding to 1/2, 1/4 and 1/8
the LD50 of dialifos were administered orally to animals in
separate groups consisting of 5 male and 5 female rats followed
four hours later by the corresponding fractions of the LD50 of
malathion. No potentiation was observed in female rats. However, in
the case of males, potentiation did occur since 1/8 of the LD50 of
each compound resulted in an LD40 (Schoenig et al, 1966e).
Special studies on eye irritation
Undiluted dialifos when applied directly to the conjunctival
sac of two rabbits was shown to be mildly irritating in both
unwashed and washed eyes. When dialifos was applied as a 5%
suspension in propylene glycol, similar results were observed
(Schoenig et al, 1966d).
Special studies on dermal irritation
When undiluted dialifos was applied to shaved and abraded skin
of two rabbits, a slight irritating effect was noted after 24
hours. Mortality precluded a 72-hour reading (Schoenig et al,
1966d).
Acute toxicity
TABLE 2. Acute toxicity of dialifos
Animal Sex Route Solvent LD50 Reference
mg/kg bw
rat M oral propylene 53 Schoenig et al
glycol 1966d
M oral propylene 43 ibid
glycol
M oral 1% methyl 71 ibid
cellulose
F oral propylene 5 ibid
glycol
mouse M oral propylene 39 Schoenig et al
glycol 1966d
F oral propylene 65 ibid
glycol
rabbit M oral propylene 58 ibid
glycol
F oral propylene 71 ibid
glycol
F oral maize oil 35 Jackson et al
1966a
M dermal xylene 145 Schoenig et al
1966d
dog M oral propylene 97 Schoenig et al
glycol 1966d
chicken F oral maize oil 464 Jackson et al
1968
The impurities of dialifos were isolated. The acute toxicities
of the impurities and pure compound were compared.
TABLE 3. Acute toxicity of pure dialifos compared with its
impurities
LD50
Animal Sex Route Solvent mg/kg bw Reference
pure dialifos
rat M oral propylene 69 Schoenig et al
glycol 1966a
F oral propylene 5 ibid
glycol
impurities
rat M oral propylene 327 Schoenig et al
glycol 1966b
F oral propylene 218 ibid
glycol
TABLE 4. Acute toxicity of dialifos formulations
LD50
Animal Sex Route Solvent mg/kg bw Reference
Emulsifiable concentrate - 47%
rat M oral water 51 Nomura 1970
F oral water 19 ibid
M oral water 62 Ueda 1968
F oral water 21 ibid
M&F Inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
TABLE 4. (Cont'd.)
LD50
Animal Sex Route Solvent mg/kg bw Reference
mouse M oral water 99 Nomura 1970
F oral water 90 ibid
M&F inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
rabbit M dermal water 327 Mastri et al
1969a
guinea M&F inhalation water saturated Hathaway et al
pig (4 hr) vapour 1969a
(LC50)
Wettable Powder - 50%
rabbit M dermal water 735 Mastri et al
1969b
rat M&F inhalation 0.5 mg/l Hathaway et al
(2 hr) (LC50) 1969b
mouse M&F inhalation 1.0 mg/l ibid
(2 hr) LC50)
guinea M&F inhalation 1.0 mg/l ibid
pig (2 hr) LC50)
TABLE 5. Acute toxicity of norchlorodialifos
LD50
Animal Sex Route Solvent mg/kg bw Reference
rat M oral maize oil 81 Gabriel 1972
F oral maize oil 22
rabbit M dermal - > 1000 Gabriel 1972
Short term studies
Rabbit - dermal
Dialifos 4 lbs/gal (0.48 kg/l) emulsifiable solution was
applied to the clipped backs of groups of 3 male and 3 female
rabbits at dosages of 0.3, 1, 5 and 25 mg/kg. The test material was
applied for a period of 6 hours/day, 5 days/week until 22
applications were made. An untreated control group received no
dermal application. At dosage levels of 25 and 5 mg/kg, growth was
depressed with inhibition of plasma, erythrocyte and brain
cholinesterase activity. A slight acanthosis of the epidermis
suggesting slight irritation was observed. There was a slight but
questionable depression of plasma and brain cholinesterase at 1 mg/kg.
No adverse effects were observed in any of the parameters studies at
0.3 mg/kg body weight (Mastri et al, 1969c; Mastri et al, 1970).
Rat and guinea pig - inhalation
Groups of rats and guinea pigs (6 males and 6 females of each
species) were exposed for 6 hours/day, 6 days/week for 2 weeks to
an aerosol of dialifos generated from an acetone solution of a 4
lb/gal (0.48 kg/1) emulsifiable formulation at concentrations of 0,
2.8, 7.7 and 20.0 µg/liter. Half the animals were sacrificed for
pathological examination after the last exposure. The remaining
animals were sacrificed after a further two week observation
period. Plasma, erythrocyte and brain cholinesterase activities
were determined only in the rat. Some mortality, ataxia, dyspnea,
marked plasma and slight erythrocyte cholinesterase depression were
observed at 20 µg/liter. No adverse effects were noted at exposure
levels of 7.7 and 2.8 µg/liter (Hathaway et al, 1969c; Hathaway et
al, 1970).
Mouse - oral
Groups of 5 male and 5 female mice were administered dialifos
dissolved in lard oil by gavage 7 days a week at dosages of 1, 2.5,
5 and 10 mg/kg/day for 90 days. Two control groups were used in
this study, one receiving no treatment, the other an equivalent
amount of lard oil to the treated groups. One half of the animals
were sacrificed after 30 days and the remainder at 90 days. The
following parameters were measured: body weight, hematology, organ
weights, gross and histopathology. Cholinesterase determinations
were not conducted. Decreased growth rate and liver necrosis were
observed in male mice receiving 10 mg/kg body weight. No
significant effects were observed at 5 mg/kg body weight or less
(Nomura, 1970).
Rat - oral
Dialifos dissolved in lard oil was administered by gavage 7
days a week at dosages of 1.0, 2.5, 5 and 10 mg/kg/day for males
and 0.15, 0.375, 0.75 and 1.5 mg/kg/ day for females for 90 days.
Control groups received either no treatment or an equivalent amount
of lard oil. One half of the animals were sacrificed at 30 days and
the remainder at 90 days. Observations on the following parameters
were made: body weight, hematology, organ weights, gross and
microscopic pathology. Serum cholinesterase measurements were made
after 30 days. Serum cholinesterase was depressed at 2.5 mg/kg and
above in males and 0.375 mg/kg and above in females. At the upper
dosage levels in both male and female, increased mortality and
liver necrosis were observed (Nomura, 1970).
Dialifos was administered orally to groups of 3 male and 3
female rats at 0, 0.5, 1.0, 2.5, 5.0, 10.0, 25.0 and 50.0 mg/kg/day
for 14 days. Plasma cholinesterase was inhibited at 0.5 mg/kg/day
and above, erythrocyte cholinesterase at 1.0 mg/kg and above, and
brain cholinesterase at 2.5 mg/kg and above. There was increased
mortality at 10.0 mg/kg and above. Gross pathological findings
among test animals were comparable to those of controls (Wolf and
Calandra, 1965).
Dialifos was fed at dietary levels of 0, 10, 25, 50 and 100
ppm to groups of 10 male and 10 female rats for 90 days.
Erythrocyte cholinesterase activity was decreased at all dietary
levels. Plasma cholinesterase activity was depressed among females
at all dietary levels and among males at 25 ppm and above. Brain
cholinesterase activity of males was depressed at 100 ppm while
among females depression was noted at 25 ppm and above. At 100 ppm,
body weight gain and food consumption were reduced; a
compound-related increase in mortality was noted; BUN and serum A/G
ratios were increased. Hematology, organ weights, gross and
histopathology revealed no compound-related effects (Wolf et al,
1966b).
Groups of 21 male and 21 female rats were fed diets containing
dialifos at levels of 0, 0.3, 1, 3, 10 and 30 ppm for 13 weeks.
After 1, 3, 6 and 13 weeks of feeding, 3 rats/sex/groups were
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. The remaining rats were then fed the control diet
and a similar number of animals sacrificed at 1 and 4 weeks
post-treatment. Plasma and erythrocyte cholinesterase activities
were depressed at 10 and 30 ppm while brain cholinesterase was
depressed at 30 ppm. After 1 week recovery, plasma and erythrocyte
cholinesterase were still depressed at the upper level. Values for
all three parameters were normal after a 4 week recovery period.
Since there was great variability in plasma cholinesterase
activity after 3 weeks and anomolous results after 13 weeks in
erythrocyte cholinesterase values, an additional study was
conducted. Groups of 12 male and 12 female rats were fed as above
for 13 weeks. Determination of plasma, erythrocyte and brain
cholinesterase activity was carried out at 3 and 13 weeks. All the
parameters measured were depressed at 30 ppm. Erythrocyte
cholinesterase was also decreased at 10 ppm. A no-effect level of
3 ppm was demonstrated (Wolf et al 1966a; 1966c).
Norchloro-dialifos was fed to groups of 10 male and 10 female
rats at dietary levels of 0, 50 and 200 ppm for 90 days. Body
weight gain and food consumption were decreased among females at
both test levels. There was a slight decrease in reticulocytes in
both sexes at 200 ppm. Significant depressions in erythrocyte,
plasma and brain cholinesterase activities were observed among male
and female rats from both test groups. No compound-related effects
were noted with respect to mortality, blood chemistry, urinalysis
organ weights, gross and microscopic pathology (Smith et al 1972b).
An additional study was designed in a comparable manner in
which groups of 9 male and 9 female rats were fed dietary levels of
norchloro-dialifos of 0, 1, 3 and 10 ppm for 90 days. After 3, 6
and 13 weeks, 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. After 13 weeks decreases in both plasma and
erythrocyte cholinesterase activities were observed at 3 and 10
ppm. Brain cholinesterase activity was slightly depressed at 3 ppm
among males, while females of all groups showed a non-dose related
decrease (Smith et al 1972a).
Dog - oral
Groups of 1 male and 1 female dogs were fed diets containing
0, 20, 100 and 500 ppm of dialifos for 14 days. Plasma and
erythrocyte cholinesterase activities were depressed at 20 ppm and
above. There was food rejection at all levels with weight loss at
500 ppm (Schoenig et al, 1966a).
Dialifos (technical) was administered in the diet to groups of
2 male and 2 female dogs at levels of 0, 1, 3, 10, 30 and 100 ppm
for 98 days. Plasma and erythrocyte cholinesterase activities were
not depressed when measured at 0, 7, 21, 42 and 90 days at dietary
levels of 1 and 3 ppm or less respectively. A slight reduction in
brain cholinesterase activity was noted at the upper dose level.
Body weight gain, food consumption, hematology, clinical chemistry,
urinalysis, organ weights, gross and histopathology were within
normal limits (Baran et al, 1966).
Groups of 3 male and 3 female dogs were fed dialifos
(technical) which was incorporated into the diet to give a dose
level of 0, 50 ,or 200 ppm for 2 years. The upper level was reduced
to 100 ppm after 21 days. After 1 year, 1 male and 1 female/group
were sacrificed. Most dogs showed weight loss and symptoms
associated with cholinesterase depression while receiving 200 ppm.
Cholinesterase activity was reduced at 50 and 100 ppm in plasma and
erythrocytes at 6, 12 and 24 months. Brain cholinesterase appeared
to be slightly depressed at 100 ppm. No compound-related effects
were observed with respect to food consumption, mortality,
hematology, clinical chemistry, organ function tests, organ
weights, gross and histopathology (Baran et al, 1968).
Tissue residue studies were performed on body tissues of dogs
fed dialifos (technical) for 2 years. Samples of muscle, fat,
liver, kidney and heart of each dog were examined. Barely
detectable residues of dialifos were found in muscle, liver, kidney
and heart samples, residues ranging from 0.003 to 0.005 mg/kg.
Slightly higher levels were detected in the fat samples. These
averaged 0.022 mg/kg at the 50 ppm and 0.032 mg/kg at the 100 ppm
feeding levels. The oxygen analogue of dialifos was not detected in
any of the tissues examined (Ford 1970c).
Chicken - oral
Replicate groups of 3 laying hens were fed 0, 0.375 and 1.25
mg/kg/day of dialifos (technical) for 8 weeks. Erythrocyte and
plasma cholinesterase activities were determined prior to and at 1,
2, 4 and 8 weeks following the initiation of treatment.
Cholinesterase activities were not affected. No toxic symptoms were
observed and no effect was noted on daily food consumption, body
weight or egg production (Sedivy 1968c).
Cattle - oral
Groups of 3 beef animals (180 kg) were fed 0, 0.1 and, O.2
mg/kg/day of dialifos for 28 days. One animal of each group was
sacrificed after 12 days of treatment, and the remaining animals at
28 days for collection of tissue samples. There was no
treatment-related effect with respect to plasma or erythrocyte
cholinesterase activity. Residue levels in fat, kidney, liver and
muscle were generally less than 0.001 mg/kg. The highest residue
level detected was 0.003 mg/kg in the fat of animals fed 0.2 mg/kg
(Taylor, 1969).
Three beef animals (180 kg) were fed diets containing
approximately 2.2 mg/kg/day; a fourth animal served as a control.
At the end of each feeding period of 2, 4 and 8 weeks, one animal
was sacrificed for tissue residue studies. Plasma and erythrocyte
cholinesterase determinations were made at weekly intervals.
Residues in the range of 0.1 to 0.3 mg/kg were found in liver and
fatty tissues. Lower residues ( <0.1 mg/kg) were detected in
muscle and kidney. No evidence for the build-up of the residue was
observed when the feeding period was doubled. The oxygen analogue
of dialifos was not detected to any significant degree in the
tissues analysed. Erythrocyte cholinesterase activity was markedly
inhibited, while plasma inhibition was only slightly affected.
(Ford 1970a; Sedivy 1968a).
Four holstein milk cows were administered a daily dose of
dialifos of 1.35 mg/kg for 21 days. Weekly plasma and erythrocyte
cholinesterase determinations were made and milk samples collected
for residue analysis. No overt signs of toxicity were observed and
milk production was not altered. A marked inhibition of erythrocyte
cholinesterase was noted. The slight reduction in plasma
cholinesterase was questionable. An average dialifos residue of 5
mg/kg was detected in milk samples which quickly disappeared when
the administration of dialifos was terminated. No oxygen analogue
was found in any of the samples (Ford 1970b., Sedivy 1968b).
Long-term studies
Rat
Groups of 30 male and 30 female rats were fed dietary levels
of 0, 0, 20 and 50 ppm of dialifos (technical) for 2 years. After
6, 12 and 24 months 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and cholinesterase determinations.
Erythrocyte cholinesterase activity was depressed in all animals of
both test levels at all examination intervals. Plasma
cholinesterase activity was decreased among females at both test
levels while males exhibited depression at 50 ppm after 6, 12 and
18 months but not at 24 months. Brain cholinesterase activity was
depressed at the higher intake level. No significant differences
between treated and control animals were observed with respect to
body weight gain, food consumption, mortality, hematology, blood
chemistry, urinalysis, organ weights or gross and histopathology.
There was no increase on the incidence of tumours in the test
animals (Wolf et al 1968).
Dialifos was fed at dietary levels of 0, 1, 3 and 10 ppm to
groups of 30 male and 30 female rats for 1 year. Five males and 5
females/group were randomly selected and sacrificed after 3, 6, 9
and 18 months for plasma, erythrocyte and brain cholinesterase
determinations as well as gross and histopathological examination.
Plasma cholinesterase in females and erythrocyte cholinesterase in
both sexes were decreased at the dietary intake of 10 ppm. Brain
cholinesterase was not affected. Body weight gain was slightly
decreased in males fed 10 ppm. There were no compound-related
effects in mortality, organ weights, gross and histopathology
(Regna et al 1975).
A small number of animals from the one year oral toxicity
study reported above were continued on the appropriate diet for an
additional year. The small number of surviving animals on test
makes the interpretation of the data difficult. However, no
significant changes were noted in body weights, food consumption,
mortality, behavioural reactions, plasma, erythrocyte and brain
cholinesterase, gross and histopathology. No evidence of increased
tumour incidence or production of unusual tumour types was observed
in the treated animals (Marias et al, 1975).
OBSERVATIONS IN MAN - ORAL
Dialifos was administered orally to 3 male and 3 female
volunteers in gelatin capsules in daily single doses of 0.01 (2
weeks), 0.03 (4 weeks) and 0.1 mg/kg (10 days) with 2 males and 2
females receiving placebos. After an appropriate recovery period
four of the test subjects were used as controls and the 4 control
subjects became part of the total of 6 test subjects in a second
study. In this study dialifos was administered orally in gelatin
capsules 3 times a day to simulate 3 meals a day in dosage levels
of 0.01 (2 weeks), 0.02 (4 weeks), 0.03 (8 weeks) and 0.05 (4
weeks) mg/kg. In both studies, plasma and erythrocyte
cholinesterase, plasma ali-esterases (tributyrinase and diethyl
succinase) were measured before dosing and at intervals during the
study and recovery periods. Periodically, other observations were
made which included hematology, blood pressure, pulse rate, pupil
size, light reflex, eye accommodation, chest sounds, muscle tone,
kneejerk, tongue tremor and finger tremor. No significant
inhibition of any of the enzyme systems tested was observed at
dosage levels of 0.01, 0.02 and 0.03 mg/kg when given either in single
or divided daily dose. Plasma cholinesterase and tributyrinase
were slightly depressed while no effect was observed
on erythrocyte cholinesterase or diethyl succinase at 0.05 and 0.1
mg/kg. Recovery was essentially complete in 14-21 days. The other
parameters measured were not affected (Greco et al, 1970).
COMMENTS
In mammals dialifos is an acutely toxic organophosphorus ester
which would appear to be rapidly absorbed as shown by the rapid
onset of cholinergic symptoms of poisoning. No data were available
with respect to absorption, metabolism and excretion in animals.
Teratogenic and reproduction studies indicated no adverse
effects at doses below those which were toxic to the parents.
Mutagenic activity was not observed in the microbial indicator
organisms used in the study. No apparent signs of neurotoxicity
were observed in hens given large doses of dialifos.
In several short-and long-term studies in the mouse, rat, dog,
hen, monkey, cattle and man, dialifos was shown to be an active
cholinesterase inhibitor. Plasma cholinesterase was inhibited to a
greater extent than either erythrocyte or brain cholinesterase with
no marked difference between animal species.
The major impurity of technical dialifos, 00-diethyl
S-(1-phthalimidoethyl) phosphorodithioate ("norchlordialifos"), was
as toxic as dialifos as indicated by acute, short-term and
teratogenic studies.
No-effect levels in the rat, dog and man have been established
on the basis of the most sensitive parameter, the depression of
plasma cholinesterase activity. The data were sufficient to
recommend an acceptable daily intake for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat 3 ppm in the diet equivalent to 0.15 mg/kg bw
Dog 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man 0.03 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Dialifos is an acaricide and insecticide used in wettable
powder and emulsion sprays on citrus, apples, pears, grapes,
pecans, rapeseed, cotton, potatoes and sugar beets. Dosages, number
of treatments, and use restrictions vary widely with the target
pest and the residue tolerances in the country in which it is used.
A summary of various national use patterns is shown in Table
6. The table is based primarily on information provided by the
basic manufacturer of dialifos. Country statements furnished to the
Meeting indicate that South Africa has cancelled registered uses
for dialifos, that use in the Netherlands is curtailed because of
resistance problems, and that Australia has no registered uses but
has applications for use on some fruits pending.
TABLE 6. National use patterns of dialifos
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
Citrus
U.S.A. 30-60g/100 l 7 2 treatments per
year
Turkey 1.0-1.5 kg/ha 28 2 treatments per
growing season
Japan 1.7-3.3 kg/ha 45
Grapes
U.S.A. 1.1 kg/ha 25 one treatment after
shot-berry stage
and no more than
2 per year
France 0.75-1.0 kg/ha 28 one spray per
growing season
(before Aug 1)
Apples, Pears
U.S.A. 60-90 g/100 l 60 not more than 5
cover sprays
Netherlands 0.75-1.0 kg/ha* 8 weeks
TABLE 6. (Cont'd.)
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
France 1.0 kg/ha 4 weeks
Germany 1.0 kg/ha 4 weeks
Italy 1.0 kg/ha 4 weeks
Belgium 1.0 kg/ha 6 weeks
Switzerland 1.0 kg/ha 6 weeks
New Zealand pre-blossom spray
only (provisional
registration)
U.K. 0.06%(high vol.) 3 weeks not more than 3
sprays per season
1.0 kg/ha(low vol.)
Pecans
U.S.A. 60 g/100 l -- apply before husk
split
Rapeseed
Germany 288 g/ha apply only during
flowering
* Report decreased use because of resistance.
It was also reported that dialifos is used on potatoes (500g/ha)
and sugar beets (700-750 g/ha) in Europe and on cotton in Turkey and
Africa at rates of 100-125 g/ha.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials in the U.S.A., Canada, Japan
Australia, the Netherlands, Republic of South Africa, West Germany and
Italy were made available to the Meeting through a submission by the
basic manufacturer (Hercules, 1976) and country statements. The
findings are discussed separately by commodity. Unless otherwise
noted, residue values are for dialifos per se.
Citrus
Data from supervised trials in the U.S.A. are summarized in Table
7 and Figure 1.
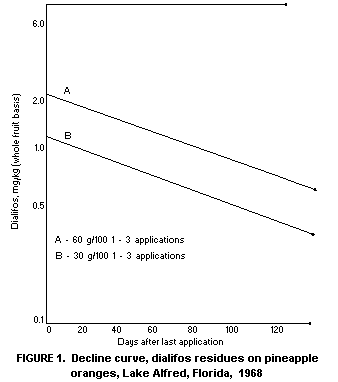 TABLE 7. Dialifos residues in citrus fruits (U.S.A., whole fruit basis).
Dialifos, mg/kg
0.025% a.i.spray 0.05% a.i. spray
Fruit (no. of treatments) Fruit (no. of treatments)
Days after last Valencia Pineapple Valencia Pineapple
treatment Oranges(2) Oranges(3) Grapefruit (3) Lemons (3) Oranges(2) Oranges(3) Grapefruit (3) Lemons (3)
3 0.74 1.4 0.62 0.24 1.2 1.7 1.9 1.2 1.6 0.38
7 0.76 0.93 0.63 0.17 1.3 1.8 2.1 1.3 1.6 0.36
7 0.69 - - - 1.2 - - - - -
56 0.58 0.73 0.54 0.08 0.87 1.2 1.4 0.99 1.6 0.24
84 0.36 0.59 0.64 - 0.61 0.88 1.1 1.1 1.4 -
In supervised trials with oranges in Japan, residues 90-183 days
after a single treatment at 1.6-2.0 kg/ha averaged 0.38 mg/kg in the
peel and 0.01 mg/kg in both whole fruit and juice.
Grapes
Decline studies on grapes showed initial deposits of about 2
mg/kg at the recommended 1.1 kg/ha rate, steady decline for 25-30 days
to about 0.4 mg/kg, and then a levelling off with little additional
loss until harvest. The pattern of residue decline coincides with the
growth of the grape, there being practically no growth dilution over
the last 20-25 days before harvest. Field trials in W. Germany with
analyses at the Central Institute for Nutrition and Food Research,
Holland, were in good agreement with the Californian data (Anon,
1974). Residues found in several varieties of grape after 35 days are
shown in Table 8 (Hercules, 1976).
TABLE 8. Harvest residues of dialifos on grapes, 35 days after
treatment (California)
Residue, mg/kg after treatment at (kg/ha)
Year Variety 2.2 1.7 1.1
1968 Thompson - - 0.98
1969 Thompson 2.1 - 0.7
1971 Emperor 0.5 - 0.4
1971 Carignane 0.28 - 0.25
1972 Thompson 0.88 0.40 0.34
1972 Ruby Red 0.55 0.29 0.23
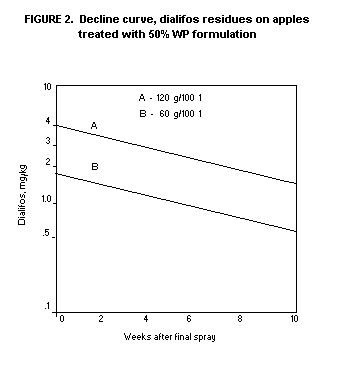
Apples, pears
Supervised trials were conducted at 15 locations in Canada and
the U.S.A. and in South Africa, the Netherlands, and Australia
(Hercules, 1976; Anon., 1969, 1972). No striking differences were
noted with respect to geographic location. Both WP and EC formulations
were used and both high volume and low volume applications made.
Typical initial deposits from recommended rates were about 2-3 mg/kg
with a very slow decline rate. Figure 2 shows a composite decline
curve based on residue values from 9 test locations.
TABLE 7. Dialifos residues in citrus fruits (U.S.A., whole fruit basis).
Dialifos, mg/kg
0.025% a.i.spray 0.05% a.i. spray
Fruit (no. of treatments) Fruit (no. of treatments)
Days after last Valencia Pineapple Valencia Pineapple
treatment Oranges(2) Oranges(3) Grapefruit (3) Lemons (3) Oranges(2) Oranges(3) Grapefruit (3) Lemons (3)
3 0.74 1.4 0.62 0.24 1.2 1.7 1.9 1.2 1.6 0.38
7 0.76 0.93 0.63 0.17 1.3 1.8 2.1 1.3 1.6 0.36
7 0.69 - - - 1.2 - - - - -
56 0.58 0.73 0.54 0.08 0.87 1.2 1.4 0.99 1.6 0.24
84 0.36 0.59 0.64 - 0.61 0.88 1.1 1.1 1.4 -
In supervised trials with oranges in Japan, residues 90-183 days
after a single treatment at 1.6-2.0 kg/ha averaged 0.38 mg/kg in the
peel and 0.01 mg/kg in both whole fruit and juice.
Grapes
Decline studies on grapes showed initial deposits of about 2
mg/kg at the recommended 1.1 kg/ha rate, steady decline for 25-30 days
to about 0.4 mg/kg, and then a levelling off with little additional
loss until harvest. The pattern of residue decline coincides with the
growth of the grape, there being practically no growth dilution over
the last 20-25 days before harvest. Field trials in W. Germany with
analyses at the Central Institute for Nutrition and Food Research,
Holland, were in good agreement with the Californian data (Anon,
1974). Residues found in several varieties of grape after 35 days are
shown in Table 8 (Hercules, 1976).
TABLE 8. Harvest residues of dialifos on grapes, 35 days after
treatment (California)
Residue, mg/kg after treatment at (kg/ha)
Year Variety 2.2 1.7 1.1
1968 Thompson - - 0.98
1969 Thompson 2.1 - 0.7
1971 Emperor 0.5 - 0.4
1971 Carignane 0.28 - 0.25
1972 Thompson 0.88 0.40 0.34
1972 Ruby Red 0.55 0.29 0.23
Apples, pears
Supervised trials were conducted at 15 locations in Canada and
the U.S.A. and in South Africa, the Netherlands, and Australia
(Hercules, 1976; Anon., 1969, 1972). No striking differences were
noted with respect to geographic location. Both WP and EC formulations
were used and both high volume and low volume applications made.
Typical initial deposits from recommended rates were about 2-3 mg/kg
with a very slow decline rate. Figure 2 shows a composite decline
curve based on residue values from 9 test locations.
 Pecans
Analysis of nut meats treated according to label directions
showed no residues above the limit of detection of the method (0.005
mg/kg). Residues were found on outer husks, but there was no
penetration.
Rapeseed
Supervised field trials in W. Germany in 1972 showed only trace
residues (0.01-0.02 mg/kg) in seeds receiving multiple treatments at
the rate of 270 g/ha. Extraction was by surface stripping, a technique
which may not be effective in removing residues absorbed into an oily
seed.
Sugar beets
Field trials in Italy in 1973 showed residues averaging 0.09
mg/kg on roots and 1.17 on tops following 4 applications at 675 g/ha.
Cotton
Results from limited trials at 2 locations in the U.S.A. were
made available. The report contained a total of 4 analyses of treated
samples. Residues in the range of 0.02-0.06 mg/kg were found on
undelinted seed.
Potatoes
Field trials in the Netherlands showed only trace residues
(<0.002 to 0.004 mg/kg) in treated potatoes.
Processed products from treated raw commodities
Data were made available on some processed food and feed products
derived from the raw commodities discussed in the previous section.
The findings are summarized by commodity.
Citrus pulp
Two pilot plant studies showed an average concentration factor of
5.3 in processing oranges to dried pulp. That is, whole oranges
bearing residue levels at the national maximum residue limit of 3
mg/kg would contribute residues approximating 16 mg/kg to the
commercial cattle feed (dried citrus pulp). See also discussion on
potential transfer to meat, milk, eggs.
Grape juice and pomace
Low level residues (maximum 0.02 mg/kg) occurred in juice
expressed from grapes bearing 1 mg/kg, most of the residue remaining
in the pomace. There is about a 4 fold concentration factor between
whole grape and wet pomace.
Raisins
The residues found are summarized in Table 9.
TABLE 9. Dialifos residue in raisins
Dosage Pre-harvest Dialifos, mg/kg
rate No. of interval,
Year kg/ha applications days Processed(2) Unprocessed(1)
1968 0.6 2 62 0.26 0.45
1.1 2 62 0.84 0.91
1970 1.1 2 41 - 1.2
1971 1.1 1 58 - 1.6
1.1+2.2 2 36 - 4.3
1973 1.1 3 40 0.98 -
1973 1.1 2 40 0.76 -
1973 1.1 2 70 0.20 -
(1) Sun dried
(2) Sun dried, cleaned, fumigated, moisture adjusted
Apple pomace
U.S. data indicate a concentration factor of about 16 in
processing from whole apples to dried apple pomace, a commercial
cattle feed.
No data were available on edible oils from cottonseed or rapeseed
or the respective oilseed meals.
FATE OF RESIDUES
Potential for residues in meat, milk, poultry, and eggs
As far as could be determined by the Meeting, there are no
registered uses for dialifos on primary forage crops. There are a
number of commodities from which processed by-products or offal may be
incorporated into animal feeds. This would include dried citrus pulp
and molasses, grape pomace, apple pomace, rapeseed and cottonseed
meal, and sugar beet pulp.
There were sufficient data from controlled feeding experiments
with cattle and poultry to relate dialifos intake levels to residue
levels in milk and meat. However, there was only fragmentary
information available on residue levels in the feed items and on the
proportions in which these feed items occur in animal rations in
national use patterns.
Metabolism studies in ruminants indicate that dialifos is
metabolized to diethyl phosphorodithioate and phthalic acid
derivatives. The metabolic pathways are similar to those of phosmet, a
closely related compound. Some dialifos per se transfers from feeds to
meat fat and to milk. At a feeding level of 45 mg/kg to lactating
cows, maximum residues approximating 0.25 mg/kg appeared in milk (fat
basis). Beef calves were fed at 2, 4 and 10 mg/kg levels. At the
highest feeding level the tissue residue levels were 0.4 mg/kg.
A poultry feeding study was also available. Birds were fed at
levels of 3.7 and 11 ppm in the total feed for up to 8 weeks.
Residues, if any, found in eggs were negligible. Trace residues
occurred in tissues.
National tolerances for dialifos in meat, milk and eggs have been
established in the U.S. to provide for residues resulting from feed
uses of citrus pulp and apple and grape pomace.
In plants
Studies available on dialifos suggest that the principal route of
degradation on plants is by hydrolysis to diethyl phosphorodithioate,
phthalic acid, phthalic acid and phthalimide. The oxon of dialifos has
been detected at trace levels but was not regarded as a significant
component of the terminal residue. This is consistent with the known
metabolic patterns of the related compound phosmet. Photolysis studies
disclosed similar degradation products after exposure to UV radiation
or sunlight. Dialifos per se is the residue of concern on crops.
Radio-tracer studies indicate only a minor tendency to translocate
from foliar deposits or to be absorbed into fruit pulp. Residues are
primarily in the peel or rind. Once residues are fixed in the peel or
on the surface of a fruit, they tend to be extremely persistent, as
shown in the decline curves (Figures 1 and 2) for citrus and apples.
In processing and cooking
No data were available on the effects of cooking. Some limited
information was provided on the reduction of residues on oranges in
commercial processing. 23% of the initial residue on oranges was
removed by washing and an additional 32% loss occurred in the rigorous
processing to dried citrus pulp. There was no evidence submitted of
occurrence in food at the time of consumption or findings of dialifos
in national "total diet" surveys.
METHODS OF RESIDUE ANALYSES
A TLC-cholinesterase inhibition method has been described as
suitable for residues of dialifos and its oxygen analogue in foods
(Mendoza 1968). However, the method of choice for regulatory purposes
would be the gas chromatographic (GC) method developed by the basic
manufacturer. The method is used with either a thermionic or flame
photometric detector and with alternative clean-up steps for analyses
of crops, meat and milk. (Pesticides Analytical Manual, Vol. II,
1967). The method has been validated in government laboratories for
regulatory purposes on crops, meat, and milk. The estimated limit of
determination is 0.01 mg/kg in citrus, 0.005 mg/kg in meat and meat
fat and 0.06 mg/kg in milk fat. The GC method has been validated only
for dialifos per se, but is said to be capable of detecting the oxygen
analogue at greatly reduced sensitivity (limit of determination about
ten times that of dialifos).
The widely used multi-residue screening method employing
acetonitrile extraction and Florisil cleanup (Pesticides Analytical
Manual, Vol. I, 1968) recovers dialifos quantitatively through the
non-fatty sample clean-up procedure, but only partially by the
procedure for fatty samples. Dialifos oxygen analogue it not detected
by either clean-up used with the PAM I method. The behaviour of
dialifos through the multi-residue method of Storherr for
organophosphorus insecticides (PAM I, Section 232.2) has not been
studied. It is known however that the closely related compound phosmet
is quantitatively recovered through the Storherr (charcoal column)
method and it is likely that dialifos would be recovered also.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table
10.
TABLE 10. National tolerances for dialifos reported to the Meeting
Pre-harvest Tolerance
Country Crop interval (mg/kg)
(days)
Germany Apples, pears 28 0.5
Netherlands " 56 0.3
Switzerland " 42 0.3
U.S.A. Citrus 7 3
Apples, pears 60 1.5
Grapes 25 1
Pecans - 0.01
Meat, fat & meat products
of cattle, goats & sheep 0.15
Milk fat 0.15
Meat, fat and meat
by-products of poultry 0.05
Eggs 0.01
* Tolerances are for the sum of dialifos and its oxygen analogue.
APPRAISAL
The acaricide/insecticide dialifos has been in use since the late
1960's on a variety of fruits, nuts, and field crops. It is reported
to be currently used in ten countries and national maximum residue
limits have been established in four of these countries.
The residue of principal concern on harvested crops is dialifos
per se. It tends to be very persistent but is not appreciably
translocated or absorbed into fruits. The oxon of dialifos has been
detected at trace levels but is not a significant residue component.
The principal route of degradation is by hydrolysis to diethyl
phosphorodithioate, and phthalic acid, phthalamic acid and
phthalimide.
The pesticide exhibits some tendency to transfer from animal
feeds to meat and milk. Data were available to show residue levels
expected in some by-product feed items such as dried citrus pulp, but
not in all. National tolerances have been established in the U.S.A.
for residues in meat, milk, and eggs to regulate residues in these
items resulting from the use of treated apple pomace, grape pomace,
and citrus pulp in animal feeds.
While there were adequate data available to support the
recommendations for the maximum residue limits given below, there was
not sufficient information on residues or national use patterns to
support recommendations for potatoes, rape, sugar beets, stone fruits,
berries or cotton.
RECOMMENDATIONS
The following maximum residue limits are recommended for the sum
of dialifos and its oxygen analogue, expressed as dialifos.
Interval on which
recommendation is
Commodity Limit, mg/kg based (days)
Apple pomace (dried) 40
Citrus pulp(dried) 15
Grape pomace (dried) 20
Citrus 3 7
Raisins 2
Apples, pears 2 60
Grapes 1 35
Fat of meat of cattle and
sheep, milk (fat basis) 0.2
Eggs, pecans 0.01*
Meat and fat of poultry 0.05*
*Level at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Metabolism studies in one or more animal species.
2. Further observations in man.
3. Additional information on national use patterns and supervised
residue trials on potatoes, rape, sugar beets and cotton.
REFERENCES
Anon Residues of dialifor when used against red
1969 spider mites on apples in Holland. 1969 Study.
Report of Central Institute for Nutrition and Food
Research, Zeist, Holland. Summarized translation
by Ir. L. Veegans of Hercules BV, The Hague,
Holland. Undated.
Anon Report No. 3968. Residues of dialifor when
1972 used against red spider mites on apples in
Holland. 1972 Study. Central Institute for
Nutrition and Food Research, Zeist, Holland.
Summarized translation by Ir. L. Veegens of
Hercules BV, The Hague, Holland. Undated.
Anon Report No. R4331. Residues of dialifor in
1974 grapes. Central Institute for Nutrition and Food
Research, Zeist, Holland, February 1974.
Arnold, D., Kodras, R., Fancher, O.E. Three generation reproduction
1968 studies in albino rats - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Baran, J., Fancher, O.E. & Calandra, J.C. 98-day sub-acute oral
1966 toxicity of AC14503 technical - Beagle dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Baran, J., Vondruska, J.F., & Fancher. O.E. Two-year chronic oral
1968 toxicity of AC14503 - Beagle dogs. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Bourke, J.B. et al. The metabolism of Torak. Report of the
1970 New York Agricultural Experiment Station, Geneva,
New York, January 1970. (Unpublished)
Brusick, D. Mutagenic evaluation of compound X19943-99-1
1975 Torak. Report from Litton Bionetics, submitted by
Hercules Incorporated. (Unpublished)
DuBois, K.P., Flynn, M., Root, M., & Su, M. Effects of Hercules
1969 AC14503 on aliesterases and cholinesterase -
weanling female Holtzman rats. Report from
Toxicity Laboratories - University of Chicago,
submitted by Hercules Incorporated. (Unpublished)
Ford, J.J. Tissue residue studies on beef calves fed a diet
1970a containing Torak. Submitted by Hercules Research
Center. (Unpublished)
Ford, J.J. Torak residues in milk - dairy cows. Submitted by
1970b Hercules Research Center. (Unpublished)
Ford, J.J. Tissue residue studies on Beagle dogs maintained
1970c for two years on Torak dosed diets. Submitted by
Hercules Research Center. (Unpublished)
Gabriel, K.L. Acute oral and acute dermal toxicity studies
1972 in rats of Hercules X15260-51-2. Report from
Biosearch, Inc., submitted by Hercules
Incorporated. (Unpublished)
Greco, R.A., Palazzolo, R.J., & Fancher, O.E. Effects of
1970 AC14503 on plasma and erythrocyte cholinesterase
and plasma aliesterase activity in human
volunteers. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Acute vapor
1969a inhalation toxicity study on Torak 47%
emulsifiable concentrate in albino rats, guinea
pigs, and mice. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher. O.E. Acute dust
1969b inhalation toxicity study on Torak 50% wettable
powder in albino rats, guinea pigs, and mice.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E Two-week subacute
1969c aerosol inhalation toxicity study on Torak
emulsified concentrate - albino rats and guinea
pigs. Report from Industrial Bio-Test Laboratories
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Two-week subacute
1970 aerosol inhalation toxicity study on Torak
emulsifiable concentrate - albino rats and guinea
pigs. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hercules "Torak" (dialifor) submitted to FAO/WHO by Hercules
1976 Incorporated, July 30, 1976.
Jackson, G.L., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966a study on technical AC14503 female Dutch belted
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Jackson, G., Kennedy, G., Fancher, O.E., Calandra, J.C. Rabbit
1966b teratogenic study AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Jackson, G., Palazzolo, R.J., & Fancher, O.E. Neuro toxicity
1968 study - chickens - AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Kennedy, G., Jackson, G.L., Fancher, O.E., & Calandra, J.C. Rabbit
1966 teratogenic study - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Ladd, R., Jenkins, D.H., Wright, P.L., & Keplinger, M.L.
1972 Teratogenic study with Norchloro-Torak in albino
rabbits. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. The in vitro
1966a inhibitory effect of AC14503 on cholinesterases -
rat plasma. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. Addendum:
1966b The in vitro inhibitory effect of oxidized
AC14503 on rat plasma and erythrocyte
cholinesterase activity. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., Keplinger, M.L.
1975 Two-year chronic oral toxicity study with Torak
technical in albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Study on the efficacy
1968 of atropine sulfate and 2-PAM C1 as antidotes for
AC14503 (technical) intoxication - male albino
rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished).
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal
1969a toxicity study on Torak (Hercules 14503) in male
albino rabbits (emulsified concentrate). Report
from Industrial Bio-Test Laboratories, submitted
by Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal toxicity
1969b study on Torak (Hercules 14503) in male albino
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day subacute
1969c dermal toxicity study on Torak emulsifiable
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day sub-acute
1970 dermal toxicity study on Torak emulsified
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mendoza, C.E., Wales, P.J., Mcleod, H.A., & McKinley, W.P.
1968 "Enzymatic detection of 10 organophosphorous
pesticides and carbaryl on thin-layer
chromatograms: An evaluation of indoxyl,
substituted indoxyl and 1-naphthyl acetates as
substrates for esterases," Analyst, London, 93:
34-38.
Nomura. Torak (C14H17O4NS2PCl) acute toxicity studies
1970 - male and female mice and rats. Report from
Kumamoto University, submitted by Hercules
Incorporated. (Unpublished)
PAM I Pesticide Analytical Manual Vol. I, 2nd Ed.
1968 revised; U.S. Department of Health, Education and
Welfare, Food and Drug Administration.
PAM II Pesticide Analytical Manual Vol. II, revised;
1967 U.S. Department of Health, Education and Welfare,
Food and Drug Administration.
Reyna, M.S., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L.
1975 1-year chronic oral toxicity study with Torak
technical in albino rats. Report from industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Robens, J.F. Teratogenic activity of several phthalimide derivatives
1970 in the golden hamster. Toxicol. Appl. Pharmacol.
16: 24-34.
Schoenig, G., & Fancher, O.E. Acute oral toxicity of recrystallized
1966a AC14503 - albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute oral toxicity
1966b of column residue from Technical AC14503 male and
female albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. 14-day subacute
1966c oral toxicity of technical AC14503 - mongrel dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966d studies on technical AC14503 - mice, albino
rabbits, dogs, and rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute potentiation
1966e of AC14503 with malathion. Report from Industrial
Bio-Test Laboratories submitted by Hercules
Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503 on
1968a beef animals. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968b on dairy cows. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968c on poultry. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972a 90-day cholinesterase study with Norchloro-Torak
in albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972b 90-day subacute oral toxicity study with
Norchloro-Torak in albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
St. John, L.E. et al. Metabolism studies with Torak insecticide
1971 in a dairy cow. Department of Entomology Pesticide
Residue Laboratory, Cornell Univ. J. agr. Fd.
Chem. 19 (5): 900-903.
Taylor, R.E. Cholinesterase and tissue residue study using
1969 Hercules 14503 on beef animals. Report from Harris
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Ueda, K. Report on acute toxicological test - rats. Report 1968
from Tokyo Dental University, submitted by
Hercules Incorporated. (Unpublished)
Vondruska, J.F., Keplinger, M.L., & Fancher, O.E. Status Summary
1968 - Evaluation of AC14503 upon primate
cholinesterase levels in stumptailed monkeys.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Vondruska, J.F. & Fancher, O.E. Teratologic study of compound
1969 AC14503 in stumptailed monkeys. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C. & Calandra. J.C. 14-day oral toxicity of AC14503
1965 - albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Wolf, C., Fancher, O.E., & Calandra, J.C. Effects of AC14503
1966a on cholinesterase activity in the albino rat.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. 90-day subacute oral
1966b toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. Effects of AC14503
1966c on cholinesterase activity on the albino rat
(Additional determinations). Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Keplinger, M.L., & Fancher, O.E. Two-year chronic oral
1968 toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Pecans
Analysis of nut meats treated according to label directions
showed no residues above the limit of detection of the method (0.005
mg/kg). Residues were found on outer husks, but there was no
penetration.
Rapeseed
Supervised field trials in W. Germany in 1972 showed only trace
residues (0.01-0.02 mg/kg) in seeds receiving multiple treatments at
the rate of 270 g/ha. Extraction was by surface stripping, a technique
which may not be effective in removing residues absorbed into an oily
seed.
Sugar beets
Field trials in Italy in 1973 showed residues averaging 0.09
mg/kg on roots and 1.17 on tops following 4 applications at 675 g/ha.
Cotton
Results from limited trials at 2 locations in the U.S.A. were
made available. The report contained a total of 4 analyses of treated
samples. Residues in the range of 0.02-0.06 mg/kg were found on
undelinted seed.
Potatoes
Field trials in the Netherlands showed only trace residues
(<0.002 to 0.004 mg/kg) in treated potatoes.
Processed products from treated raw commodities
Data were made available on some processed food and feed products
derived from the raw commodities discussed in the previous section.
The findings are summarized by commodity.
Citrus pulp
Two pilot plant studies showed an average concentration factor of
5.3 in processing oranges to dried pulp. That is, whole oranges
bearing residue levels at the national maximum residue limit of 3
mg/kg would contribute residues approximating 16 mg/kg to the
commercial cattle feed (dried citrus pulp). See also discussion on
potential transfer to meat, milk, eggs.
Grape juice and pomace
Low level residues (maximum 0.02 mg/kg) occurred in juice
expressed from grapes bearing 1 mg/kg, most of the residue remaining
in the pomace. There is about a 4 fold concentration factor between
whole grape and wet pomace.
Raisins
The residues found are summarized in Table 9.
TABLE 9. Dialifos residue in raisins
Dosage Pre-harvest Dialifos, mg/kg
rate No. of interval,
Year kg/ha applications days Processed(2) Unprocessed(1)
1968 0.6 2 62 0.26 0.45
1.1 2 62 0.84 0.91
1970 1.1 2 41 - 1.2
1971 1.1 1 58 - 1.6
1.1+2.2 2 36 - 4.3
1973 1.1 3 40 0.98 -
1973 1.1 2 40 0.76 -
1973 1.1 2 70 0.20 -
(1) Sun dried
(2) Sun dried, cleaned, fumigated, moisture adjusted
Apple pomace
U.S. data indicate a concentration factor of about 16 in
processing from whole apples to dried apple pomace, a commercial
cattle feed.
No data were available on edible oils from cottonseed or rapeseed
or the respective oilseed meals.
FATE OF RESIDUES
Potential for residues in meat, milk, poultry, and eggs
As far as could be determined by the Meeting, there are no
registered uses for dialifos on primary forage crops. There are a
number of commodities from which processed by-products or offal may be
incorporated into animal feeds. This would include dried citrus pulp
and molasses, grape pomace, apple pomace, rapeseed and cottonseed
meal, and sugar beet pulp.
There were sufficient data from controlled feeding experiments
with cattle and poultry to relate dialifos intake levels to residue
levels in milk and meat. However, there was only fragmentary
information available on residue levels in the feed items and on the
proportions in which these feed items occur in animal rations in
national use patterns.
Metabolism studies in ruminants indicate that dialifos is
metabolized to diethyl phosphorodithioate and phthalic acid
derivatives. The metabolic pathways are similar to those of phosmet, a
closely related compound. Some dialifos per se transfers from feeds to
meat fat and to milk. At a feeding level of 45 mg/kg to lactating
cows, maximum residues approximating 0.25 mg/kg appeared in milk (fat
basis). Beef calves were fed at 2, 4 and 10 mg/kg levels. At the
highest feeding level the tissue residue levels were 0.4 mg/kg.
A poultry feeding study was also available. Birds were fed at
levels of 3.7 and 11 ppm in the total feed for up to 8 weeks.
Residues, if any, found in eggs were negligible. Trace residues
occurred in tissues.
National tolerances for dialifos in meat, milk and eggs have been
established in the U.S. to provide for residues resulting from feed
uses of citrus pulp and apple and grape pomace.
In plants
Studies available on dialifos suggest that the principal route of
degradation on plants is by hydrolysis to diethyl phosphorodithioate,
phthalic acid, phthalic acid and phthalimide. The oxon of dialifos has
been detected at trace levels but was not regarded as a significant
component of the terminal residue. This is consistent with the known
metabolic patterns of the related compound phosmet. Photolysis studies
disclosed similar degradation products after exposure to UV radiation
or sunlight. Dialifos per se is the residue of concern on crops.
Radio-tracer studies indicate only a minor tendency to translocate
from foliar deposits or to be absorbed into fruit pulp. Residues are
primarily in the peel or rind. Once residues are fixed in the peel or
on the surface of a fruit, they tend to be extremely persistent, as
shown in the decline curves (Figures 1 and 2) for citrus and apples.
In processing and cooking
No data were available on the effects of cooking. Some limited
information was provided on the reduction of residues on oranges in
commercial processing. 23% of the initial residue on oranges was
removed by washing and an additional 32% loss occurred in the rigorous
processing to dried citrus pulp. There was no evidence submitted of
occurrence in food at the time of consumption or findings of dialifos
in national "total diet" surveys.
METHODS OF RESIDUE ANALYSES
A TLC-cholinesterase inhibition method has been described as
suitable for residues of dialifos and its oxygen analogue in foods
(Mendoza 1968). However, the method of choice for regulatory purposes
would be the gas chromatographic (GC) method developed by the basic
manufacturer. The method is used with either a thermionic or flame
photometric detector and with alternative clean-up steps for analyses
of crops, meat and milk. (Pesticides Analytical Manual, Vol. II,
1967). The method has been validated in government laboratories for
regulatory purposes on crops, meat, and milk. The estimated limit of
determination is 0.01 mg/kg in citrus, 0.005 mg/kg in meat and meat
fat and 0.06 mg/kg in milk fat. The GC method has been validated only
for dialifos per se, but is said to be capable of detecting the oxygen
analogue at greatly reduced sensitivity (limit of determination about
ten times that of dialifos).
The widely used multi-residue screening method employing
acetonitrile extraction and Florisil cleanup (Pesticides Analytical
Manual, Vol. I, 1968) recovers dialifos quantitatively through the
non-fatty sample clean-up procedure, but only partially by the
procedure for fatty samples. Dialifos oxygen analogue it not detected
by either clean-up used with the PAM I method. The behaviour of
dialifos through the multi-residue method of Storherr for
organophosphorus insecticides (PAM I, Section 232.2) has not been
studied. It is known however that the closely related compound phosmet
is quantitatively recovered through the Storherr (charcoal column)
method and it is likely that dialifos would be recovered also.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table
10.
TABLE 10. National tolerances for dialifos reported to the Meeting
Pre-harvest Tolerance
Country Crop interval (mg/kg)
(days)
Germany Apples, pears 28 0.5
Netherlands " 56 0.3
Switzerland " 42 0.3
U.S.A. Citrus 7 3
Apples, pears 60 1.5
Grapes 25 1
Pecans - 0.01
Meat, fat & meat products
of cattle, goats & sheep 0.15
Milk fat 0.15
Meat, fat and meat
by-products of poultry 0.05
Eggs 0.01
* Tolerances are for the sum of dialifos and its oxygen analogue.
APPRAISAL
The acaricide/insecticide dialifos has been in use since the late
1960's on a variety of fruits, nuts, and field crops. It is reported
to be currently used in ten countries and national maximum residue
limits have been established in four of these countries.
The residue of principal concern on harvested crops is dialifos
per se. It tends to be very persistent but is not appreciably
translocated or absorbed into fruits. The oxon of dialifos has been
detected at trace levels but is not a significant residue component.
The principal route of degradation is by hydrolysis to diethyl
phosphorodithioate, and phthalic acid, phthalamic acid and
phthalimide.
The pesticide exhibits some tendency to transfer from animal
feeds to meat and milk. Data were available to show residue levels
expected in some by-product feed items such as dried citrus pulp, but
not in all. National tolerances have been established in the U.S.A.
for residues in meat, milk, and eggs to regulate residues in these
items resulting from the use of treated apple pomace, grape pomace,
and citrus pulp in animal feeds.
While there were adequate data available to support the
recommendations for the maximum residue limits given below, there was
not sufficient information on residues or national use patterns to
support recommendations for potatoes, rape, sugar beets, stone fruits,
berries or cotton.
RECOMMENDATIONS
The following maximum residue limits are recommended for the sum
of dialifos and its oxygen analogue, expressed as dialifos.
Interval on which
recommendation is
Commodity Limit, mg/kg based (days)
Apple pomace (dried) 40
Citrus pulp(dried) 15
Grape pomace (dried) 20
Citrus 3 7
Raisins 2
Apples, pears 2 60
Grapes 1 35
Fat of meat of cattle and
sheep, milk (fat basis) 0.2
Eggs, pecans 0.01*
Meat and fat of poultry 0.05*
*Level at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Metabolism studies in one or more animal species.
2. Further observations in man.
3. Additional information on national use patterns and supervised
residue trials on potatoes, rape, sugar beets and cotton.
REFERENCES
Anon Residues of dialifor when used against red
1969 spider mites on apples in Holland. 1969 Study.
Report of Central Institute for Nutrition and Food
Research, Zeist, Holland. Summarized translation
by Ir. L. Veegans of Hercules BV, The Hague,
Holland. Undated.
Anon Report No. 3968. Residues of dialifor when
1972 used against red spider mites on apples in
Holland. 1972 Study. Central Institute for
Nutrition and Food Research, Zeist, Holland.
Summarized translation by Ir. L. Veegens of
Hercules BV, The Hague, Holland. Undated.
Anon Report No. R4331. Residues of dialifor in
1974 grapes. Central Institute for Nutrition and Food
Research, Zeist, Holland, February 1974.
Arnold, D., Kodras, R., Fancher, O.E. Three generation reproduction
1968 studies in albino rats - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Baran, J., Fancher, O.E. & Calandra, J.C. 98-day sub-acute oral
1966 toxicity of AC14503 technical - Beagle dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Baran, J., Vondruska, J.F., & Fancher. O.E. Two-year chronic oral
1968 toxicity of AC14503 - Beagle dogs. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Bourke, J.B. et al. The metabolism of Torak. Report of the
1970 New York Agricultural Experiment Station, Geneva,
New York, January 1970. (Unpublished)
Brusick, D. Mutagenic evaluation of compound X19943-99-1
1975 Torak. Report from Litton Bionetics, submitted by
Hercules Incorporated. (Unpublished)
DuBois, K.P., Flynn, M., Root, M., & Su, M. Effects of Hercules
1969 AC14503 on aliesterases and cholinesterase -
weanling female Holtzman rats. Report from
Toxicity Laboratories - University of Chicago,
submitted by Hercules Incorporated. (Unpublished)
Ford, J.J. Tissue residue studies on beef calves fed a diet
1970a containing Torak. Submitted by Hercules Research
Center. (Unpublished)
Ford, J.J. Torak residues in milk - dairy cows. Submitted by
1970b Hercules Research Center. (Unpublished)
Ford, J.J. Tissue residue studies on Beagle dogs maintained
1970c for two years on Torak dosed diets. Submitted by
Hercules Research Center. (Unpublished)
Gabriel, K.L. Acute oral and acute dermal toxicity studies
1972 in rats of Hercules X15260-51-2. Report from
Biosearch, Inc., submitted by Hercules
Incorporated. (Unpublished)
Greco, R.A., Palazzolo, R.J., & Fancher, O.E. Effects of
1970 AC14503 on plasma and erythrocyte cholinesterase
and plasma aliesterase activity in human
volunteers. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Acute vapor
1969a inhalation toxicity study on Torak 47%
emulsifiable concentrate in albino rats, guinea
pigs, and mice. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher. O.E. Acute dust
1969b inhalation toxicity study on Torak 50% wettable
powder in albino rats, guinea pigs, and mice.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E Two-week subacute
1969c aerosol inhalation toxicity study on Torak
emulsified concentrate - albino rats and guinea
pigs. Report from Industrial Bio-Test Laboratories
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Two-week subacute
1970 aerosol inhalation toxicity study on Torak
emulsifiable concentrate - albino rats and guinea
pigs. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hercules "Torak" (dialifor) submitted to FAO/WHO by Hercules
1976 Incorporated, July 30, 1976.
Jackson, G.L., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966a study on technical AC14503 female Dutch belted
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Jackson, G., Kennedy, G., Fancher, O.E., Calandra, J.C. Rabbit
1966b teratogenic study AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Jackson, G., Palazzolo, R.J., & Fancher, O.E. Neuro toxicity
1968 study - chickens - AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Kennedy, G., Jackson, G.L., Fancher, O.E., & Calandra, J.C. Rabbit
1966 teratogenic study - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Ladd, R., Jenkins, D.H., Wright, P.L., & Keplinger, M.L.
1972 Teratogenic study with Norchloro-Torak in albino
rabbits. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. The in vitro
1966a inhibitory effect of AC14503 on cholinesterases -
rat plasma. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. Addendum:
1966b The in vitro inhibitory effect of oxidized
AC14503 on rat plasma and erythrocyte
cholinesterase activity. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., Keplinger, M.L.
1975 Two-year chronic oral toxicity study with Torak
technical in albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Study on the efficacy
1968 of atropine sulfate and 2-PAM C1 as antidotes for
AC14503 (technical) intoxication - male albino
rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished).
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal
1969a toxicity study on Torak (Hercules 14503) in male
albino rabbits (emulsified concentrate). Report
from Industrial Bio-Test Laboratories, submitted
by Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal toxicity
1969b study on Torak (Hercules 14503) in male albino
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day subacute
1969c dermal toxicity study on Torak emulsifiable
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day sub-acute
1970 dermal toxicity study on Torak emulsified
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mendoza, C.E., Wales, P.J., Mcleod, H.A., & McKinley, W.P.
1968 "Enzymatic detection of 10 organophosphorous
pesticides and carbaryl on thin-layer
chromatograms: An evaluation of indoxyl,
substituted indoxyl and 1-naphthyl acetates as
substrates for esterases," Analyst, London, 93:
34-38.
Nomura. Torak (C14H17O4NS2PCl) acute toxicity studies
1970 - male and female mice and rats. Report from
Kumamoto University, submitted by Hercules
Incorporated. (Unpublished)
PAM I Pesticide Analytical Manual Vol. I, 2nd Ed.
1968 revised; U.S. Department of Health, Education and
Welfare, Food and Drug Administration.
PAM II Pesticide Analytical Manual Vol. II, revised;
1967 U.S. Department of Health, Education and Welfare,
Food and Drug Administration.
Reyna, M.S., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L.
1975 1-year chronic oral toxicity study with Torak
technical in albino rats. Report from industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Robens, J.F. Teratogenic activity of several phthalimide derivatives
1970 in the golden hamster. Toxicol. Appl. Pharmacol.
16: 24-34.
Schoenig, G., & Fancher, O.E. Acute oral toxicity of recrystallized
1966a AC14503 - albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute oral toxicity
1966b of column residue from Technical AC14503 male and
female albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. 14-day subacute
1966c oral toxicity of technical AC14503 - mongrel dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966d studies on technical AC14503 - mice, albino
rabbits, dogs, and rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute potentiation
1966e of AC14503 with malathion. Report from Industrial
Bio-Test Laboratories submitted by Hercules
Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503 on
1968a beef animals. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968b on dairy cows. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968c on poultry. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972a 90-day cholinesterase study with Norchloro-Torak
in albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972b 90-day subacute oral toxicity study with
Norchloro-Torak in albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
St. John, L.E. et al. Metabolism studies with Torak insecticide
1971 in a dairy cow. Department of Entomology Pesticide
Residue Laboratory, Cornell Univ. J. agr. Fd.
Chem. 19 (5): 900-903.
Taylor, R.E. Cholinesterase and tissue residue study using
1969 Hercules 14503 on beef animals. Report from Harris
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Ueda, K. Report on acute toxicological test - rats. Report 1968
from Tokyo Dental University, submitted by
Hercules Incorporated. (Unpublished)
Vondruska, J.F., Keplinger, M.L., & Fancher, O.E. Status Summary
1968 - Evaluation of AC14503 upon primate
cholinesterase levels in stumptailed monkeys.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Vondruska, J.F. & Fancher, O.E. Teratologic study of compound
1969 AC14503 in stumptailed monkeys. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C. & Calandra. J.C. 14-day oral toxicity of AC14503
1965 - albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Wolf, C., Fancher, O.E., & Calandra, J.C. Effects of AC14503
1966a on cholinesterase activity in the albino rat.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. 90-day subacute oral
1966b toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. Effects of AC14503
1966c on cholinesterase activity on the albino rat
(Additional determinations). Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Keplinger, M.L., & Fancher, O.E. Two-year chronic oral
1968 toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
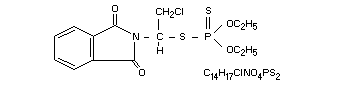 Other information on identity and properties
State: white crystals (pure)
brown crystals (isolated technical)
brown liquid (commercial technical)
Commercial technical dialifos is produced in the form of an
80% solution of technical dialifos in a xylene-range aromatic
solvent. This solution is adjusted to a minimum dialifos content of
72% by weight. Technical dialifos in crystalline form is not an
item of commerce.
Melting point: 67°C (pure)
Vapour pressure: < 10-4 mm Hg 35°C
Solubility: water 0.18 mg/kg
essentially insoluble in vegetable oils,
alcohol and glycols
soluble in acetone, chloroform, xylene and
ethyl ether
Hydrolytic stability in ethanol/water (2:8) at 25°C:
half life of 119 hours at pH 6
half life of 2.5 hours at pH 8
Dialifos is formulated as emulsifiable concentrates in the range 25
to 50% or as a 50% wettable powder.
Purity
TABLE 1. Impurities (maximum levels in commercial technical dialifos
Compound Maximum level (%w/w)
O,O-diethyl S-(1-phthalimidoethyl
phosphorodithioate
("norchlorodialifos") 5
dialifos oxygen analogue <1
N-(2-hydroxyethyl) phthalimide <.3
N-(2-acetoxyethyl) phthalimide <.3
N-(1,2,2-trichloroethyl)phthalimide 1
N-(1,2-dichloroethyl)phthalimide 1
N-(2-chlorovinyl)phthalimide 1
OOO-triethyl phosphorthioate 3
OOS-triethyl phosphorodithioate 3
Unidentified 2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on enzymes and other biochemical parameters
The in vitro inhibitory effect of dialifos (technical) on
rat plasma and erythrocyte cholinesterase was studied. The
enzyme-inhibitor complex was formed within five minutes and was
stable during the incubation period. Linear reaction rates were
also demonstrated, confirming the reliability of the electrometric
assay procedure for cholinesterase activity. The concentration of
dialifos required to obtain a 50% inhibition of plasma
cholinesterase was 2.55 µg/ml and 0.31 µg/ml for erythrocyte
cholinesterase. The in vitro oxidation of dialifos with a
peroxide-acetic acid system decreased the 50% inhibition
concentration to 0.83 µg/ml for plasma cholinesterase and 0.015
µg/ml for erythrocyte cholinesterase (Lazanas et al 1966a, 1966b).
A study was conducted to investigate the effect of dialifos on
aliphatic esterases in comparison with cholinesterase. Groups of 6
female rats were fed levels from 0.5 to 50 ppm dialifos
(technical). Three animals at each dietary level were killed after
1 and 3 weeks and measurements of the rate of enzymic hydrolysis of
diethyl succinate and tributyrin by the liver and serum, and
cholinesterase activity of the brain were conducted. The dietary
levels producing 50% inhibition of tributyrinase activity of liver
and serum were 4.7 and 10.3 ppm respectively after 3 weeks. Diethyl
succinate hydrolysis in the liver and serum was inhibited by 50% at
dietary levels of 13.5 and 12.5 ppm after 1 week and 16.0 and 12.0
ppm after 3 weeks respectively. Brain cholinesterase was
significantly reduced at 50 ppm after 3 weeks. In a preliminary
study liver and serum cholinesterase were shown to be appreciably
inhibited after 1 week at dietary levels of 25 ppm. The
cholinesterase activity of all the tissues was inhibited to a
lesser extent than the aliesterase activity (DuBois et al, 1969).
TOXICOLOGICAL STUDIES
Special studies on antidotes
The acute oral LD50 of dialifos was determined in groups of
male rats (1) without antidote; (2) treated with atropine sulphate
(17.5 mg/kg); (3) treated with 2-PAM (50 mg/kg); (4) treated with
a combination of atropine sulphate and 2-PAM at 17.5 and 50 mg/kg
respectively. Antidotes were administered intramuscularly 30
minutes after dialifos. The LD50 for the groups were 71, 140, 267
and 177 mg/kg respectively. The onset of symptoms and time of death
were delayed in the treated groups. Atropine sulphate and 2-PAM
would appear to be effective antidotes for dialifos (Mastri et al,
1968).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed dialifos
(technical) in the diet at dosage levels of 0, 0.05 and 0.5 mg/kg
body weight/day and subjected to a standard 2 litter, 3 generation
reproduction study. The following parameters were measured: body
weight, mortality, symptoms, hematology, urinalysis, organ weights
and gross and histopathology of all parental generations after the
second weaning as well as plasma and erythrocyte cholinesterase
activities of all parental generations and litters. In addition,
the following reproduction indices were measured: mating, pregnancy,
fertility, parturition and lactation. With respect to progeny mean
litter size, number of stillborn and viable pups and pup body weights
were recorded. Plasma and erythrocyte cholinesterase depression were
observed primarily in the female of all parental groups and
weanlings fed 0.5 mg/kg body weight. There were random non-dose
related effects in some reproductive parameters of both test groups
when compared to controls. No compound-related gross or
histopathological findings were observed. (Arnold et al, 1968).
Special studies on teratology
Rabbit
Groups of 10 pregnant rabbits were administered dialifos
(technical) via gelatin capsule at dosage levels of 0, 1 and 3
mg/kg from day 6 to day 18, and 10 and 25 mg/kg from day 6 to day
16 of gestation. Thalidomide was given at a dosage of 75 mg/kg/day.
Does were sacrificed on day 29 of gestation. Offspring were placed
in an incubator for 24 hours to determine viability and then
sacrificed for skeletal examination. Parental weight loss occurred
at 3, 10 and 25 mg/kg. Mortality was increased at 10 mg/kg (7/10)
and 25 mg/kg (10/10). Hemorrhage in the small intestine was
observed in the two highest dose groups. Litter size was reduced
and fetal resorption increased at 10 and 25 mg/kg while the
viability index was decreased at all dosage levels. Four
abnormalities (3 umbilical hernia, 1 partial acranius) were noted
at 3 mg/kg, however no abnormalities were observed at 10 mg/kg. The
thalidomide group showed clubbing of the extremities (30/49)
(Kennedy et al, 1966). This study was repeated in a similar fashion
using the same strain of rabbit, administered 0, 1, or 3 mg
dialifos (technical)/kg from day 6 to day 18 of gestation. No
adverse effects were observed with respect to maternal body weight,
survival or behaviour. Number of implantation sites and litter size
were reduced and the number of resorption sites increased in
treated groups when compared to corresponding controls. This was
considered to be due to physical manipulation of these animals
rather than a possible toxic effect of the test material. The
viability index was comparable to controls, and no skeletal or
external abnormalities were found in any of the progeny receiving
dialifos (Jackson et al, 1966b).
Groups of 17 female rabbits were treated from day 6 through
day 18 of gestation with daily doses of either 0, 1.0 or 3.0 mg/kg
norchlorodialifos via gelatin capsule. Thalidomide was administered
concurrently at 37.5 mg/kg body weight as a positive control.
Rabbits were sacrificed on gestation day 29. Young were removed by
caesarean section and placed in an incubator for 24 hours. They
were then sacrificed and examined for external skeletal and soft
tissue abnormalities. At 3 mg/kg the animals lost weight during the
dosing period. No effect in body weight gain was exhibited at 1
mg/kg. No deaths or unusual reactions were observed. The number of
live fetuses and fetal survival were reduced at 3 mg/kg. No
significant differences were observed in 24 hour viability index
or fetal body weights between control and norchlorodialifos treated
animals. No compound-related internal or skeletal abnormalities were
noted (Ladd et al, 1972).
Hamster
Pregnant hamsters were dosed orally with dialifos once on day
7 or 8 of gestation or several times on days 6-8 or days 6-10 of
gestation. The total administered dose varied from 100-500 mg/kg.
On day 15 of gestation the surviving hamsters were sacrificed and
the fetuses removed. The usual parameters for teratogenicity were
examined. From the mortality data of the dams it was concluded that
most of the doses were > LD50. The lowest single dose of 100
mg/kg body weight produced malformations in 4/98 fetuses, 3 with
limb defects and one with umbilical hernia (Robens, 1970).
Monkey
Ten pregnant stumptailed macaques were dosed with dialifos
(technical) at 1 mg/kg body weight (the dose causing ca 25% plasma
and erythrocyte cholinesterase depression (Vondruska et al, 1968)
for a period of one week during the critical period of limb
formation. Nine other pregnant females were administered either 5
or 10 mg thalidomide/kg as positive controls. Blood samples were
taken on the 15th, 18th and 21st days of pregnancy and on the last
dosing day for complete hematology and cholinesterase activity.
During the 12th week of gestation, fetuses were recovered by
caesarean section and examined for abnormalities. No hematological
effects were observed. Plasma and erythrocyte cholinesterase
activity for monkeys receiving dialifos were reduced to 65 and 62%
of pre-treatment values. No abortions were observed with dialifos
and all fetuses were normal. Abortions occurred in four females
dosed with thalidomide. A total of five fetuses were recovered from
females treated with thalidomide and all exhibited gross
deformities of the anterior extremities (Vondruska and Fancher,
1969).
Special studies on mutagenicity
Dialifos was tested for mutagenic activity using a series of
microbial indicator organisms (Saccharomyces cerevisiae strain
D4; Salmonella typhimurium strains TA-1535, 1537, 1538) in
qualitative plate tests with and without metabolic activation.
Activation was accomplished by the use of tissue homogenates of
liver, lung and testes from mice, rats and monkeys. The compound
did not exhibit significant genetic activity in any of the assays
performed (Brusick, 1975).
Special studies on neurotoxicity
Groups of 6 hens were given 0 or 464 mg/kg body weight (oral
LD 50) of dialifos (technical). Surviving birds were re-dosed with
the LD50 after 21 days and were observed for an additional 21 days.
Samples of spinal cord, sciatic nerve and brain were taken for
histological examination. A positive control group was dosed with
500 mg/kg body weight TOCP and studied simultaneously with the test
group. No signs of ataxia were noted and no clinical neurotoxic
effects were observed. Birds in the positive control group showed
signs of neurotoxicity approximately 14 days following
administration of the last dose. Histopathology of nervous tissue
was not done (Jackson et al, 1968).
Special studies on potentiation
An acute potentiation study was conducted in order to
determine the degree of interaction between dialifos (technical)
and malathion. The acute oral LD50 values were determined in male
and female rats separately. Doses corresponding to 1/2, 1/4 and 1/8
the LD50 of dialifos were administered orally to animals in
separate groups consisting of 5 male and 5 female rats followed
four hours later by the corresponding fractions of the LD50 of
malathion. No potentiation was observed in female rats. However, in
the case of males, potentiation did occur since 1/8 of the LD50 of
each compound resulted in an LD40 (Schoenig et al, 1966e).
Special studies on eye irritation
Undiluted dialifos when applied directly to the conjunctival
sac of two rabbits was shown to be mildly irritating in both
unwashed and washed eyes. When dialifos was applied as a 5%
suspension in propylene glycol, similar results were observed
(Schoenig et al, 1966d).
Special studies on dermal irritation
When undiluted dialifos was applied to shaved and abraded skin
of two rabbits, a slight irritating effect was noted after 24
hours. Mortality precluded a 72-hour reading (Schoenig et al,
1966d).
Acute toxicity
TABLE 2. Acute toxicity of dialifos
Animal Sex Route Solvent LD50 Reference
mg/kg bw
rat M oral propylene 53 Schoenig et al
glycol 1966d
M oral propylene 43 ibid
glycol
M oral 1% methyl 71 ibid
cellulose
F oral propylene 5 ibid
glycol
mouse M oral propylene 39 Schoenig et al
glycol 1966d
F oral propylene 65 ibid
glycol
rabbit M oral propylene 58 ibid
glycol
F oral propylene 71 ibid
glycol
F oral maize oil 35 Jackson et al
1966a
M dermal xylene 145 Schoenig et al
1966d
dog M oral propylene 97 Schoenig et al
glycol 1966d
chicken F oral maize oil 464 Jackson et al
1968
The impurities of dialifos were isolated. The acute toxicities
of the impurities and pure compound were compared.
TABLE 3. Acute toxicity of pure dialifos compared with its
impurities
LD50
Animal Sex Route Solvent mg/kg bw Reference
pure dialifos
rat M oral propylene 69 Schoenig et al
glycol 1966a
F oral propylene 5 ibid
glycol
impurities
rat M oral propylene 327 Schoenig et al
glycol 1966b
F oral propylene 218 ibid
glycol
TABLE 4. Acute toxicity of dialifos formulations
LD50
Animal Sex Route Solvent mg/kg bw Reference
Emulsifiable concentrate - 47%
rat M oral water 51 Nomura 1970
F oral water 19 ibid
M oral water 62 Ueda 1968
F oral water 21 ibid
M&F Inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
TABLE 4. (Cont'd.)
LD50
Animal Sex Route Solvent mg/kg bw Reference
mouse M oral water 99 Nomura 1970
F oral water 90 ibid
M&F inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
rabbit M dermal water 327 Mastri et al
1969a
guinea M&F inhalation water saturated Hathaway et al
pig (4 hr) vapour 1969a
(LC50)
Wettable Powder - 50%
rabbit M dermal water 735 Mastri et al
1969b
rat M&F inhalation 0.5 mg/l Hathaway et al
(2 hr) (LC50) 1969b
mouse M&F inhalation 1.0 mg/l ibid
(2 hr) LC50)
guinea M&F inhalation 1.0 mg/l ibid
pig (2 hr) LC50)
TABLE 5. Acute toxicity of norchlorodialifos
LD50
Animal Sex Route Solvent mg/kg bw Reference
rat M oral maize oil 81 Gabriel 1972
F oral maize oil 22
rabbit M dermal - > 1000 Gabriel 1972
Short term studies
Rabbit - dermal
Dialifos 4 lbs/gal (0.48 kg/l) emulsifiable solution was
applied to the clipped backs of groups of 3 male and 3 female
rabbits at dosages of 0.3, 1, 5 and 25 mg/kg. The test material was
applied for a period of 6 hours/day, 5 days/week until 22
applications were made. An untreated control group received no
dermal application. At dosage levels of 25 and 5 mg/kg, growth was
depressed with inhibition of plasma, erythrocyte and brain
cholinesterase activity. A slight acanthosis of the epidermis
suggesting slight irritation was observed. There was a slight but
questionable depression of plasma and brain cholinesterase at 1 mg/kg.
No adverse effects were observed in any of the parameters studies at
0.3 mg/kg body weight (Mastri et al, 1969c; Mastri et al, 1970).
Rat and guinea pig - inhalation
Groups of rats and guinea pigs (6 males and 6 females of each
species) were exposed for 6 hours/day, 6 days/week for 2 weeks to
an aerosol of dialifos generated from an acetone solution of a 4
lb/gal (0.48 kg/1) emulsifiable formulation at concentrations of 0,
2.8, 7.7 and 20.0 µg/liter. Half the animals were sacrificed for
pathological examination after the last exposure. The remaining
animals were sacrificed after a further two week observation
period. Plasma, erythrocyte and brain cholinesterase activities
were determined only in the rat. Some mortality, ataxia, dyspnea,
marked plasma and slight erythrocyte cholinesterase depression were
observed at 20 µg/liter. No adverse effects were noted at exposure
levels of 7.7 and 2.8 µg/liter (Hathaway et al, 1969c; Hathaway et
al, 1970).
Mouse - oral
Groups of 5 male and 5 female mice were administered dialifos
dissolved in lard oil by gavage 7 days a week at dosages of 1, 2.5,
5 and 10 mg/kg/day for 90 days. Two control groups were used in
this study, one receiving no treatment, the other an equivalent
amount of lard oil to the treated groups. One half of the animals
were sacrificed after 30 days and the remainder at 90 days. The
following parameters were measured: body weight, hematology, organ
weights, gross and histopathology. Cholinesterase determinations
were not conducted. Decreased growth rate and liver necrosis were
observed in male mice receiving 10 mg/kg body weight. No
significant effects were observed at 5 mg/kg body weight or less
(Nomura, 1970).
Rat - oral
Dialifos dissolved in lard oil was administered by gavage 7
days a week at dosages of 1.0, 2.5, 5 and 10 mg/kg/day for males
and 0.15, 0.375, 0.75 and 1.5 mg/kg/ day for females for 90 days.
Control groups received either no treatment or an equivalent amount
of lard oil. One half of the animals were sacrificed at 30 days and
the remainder at 90 days. Observations on the following parameters
were made: body weight, hematology, organ weights, gross and
microscopic pathology. Serum cholinesterase measurements were made
after 30 days. Serum cholinesterase was depressed at 2.5 mg/kg and
above in males and 0.375 mg/kg and above in females. At the upper
dosage levels in both male and female, increased mortality and
liver necrosis were observed (Nomura, 1970).
Dialifos was administered orally to groups of 3 male and 3
female rats at 0, 0.5, 1.0, 2.5, 5.0, 10.0, 25.0 and 50.0 mg/kg/day
for 14 days. Plasma cholinesterase was inhibited at 0.5 mg/kg/day
and above, erythrocyte cholinesterase at 1.0 mg/kg and above, and
brain cholinesterase at 2.5 mg/kg and above. There was increased
mortality at 10.0 mg/kg and above. Gross pathological findings
among test animals were comparable to those of controls (Wolf and
Calandra, 1965).
Dialifos was fed at dietary levels of 0, 10, 25, 50 and 100
ppm to groups of 10 male and 10 female rats for 90 days.
Erythrocyte cholinesterase activity was decreased at all dietary
levels. Plasma cholinesterase activity was depressed among females
at all dietary levels and among males at 25 ppm and above. Brain
cholinesterase activity of males was depressed at 100 ppm while
among females depression was noted at 25 ppm and above. At 100 ppm,
body weight gain and food consumption were reduced; a
compound-related increase in mortality was noted; BUN and serum A/G
ratios were increased. Hematology, organ weights, gross and
histopathology revealed no compound-related effects (Wolf et al,
1966b).
Groups of 21 male and 21 female rats were fed diets containing
dialifos at levels of 0, 0.3, 1, 3, 10 and 30 ppm for 13 weeks.
After 1, 3, 6 and 13 weeks of feeding, 3 rats/sex/groups were
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. The remaining rats were then fed the control diet
and a similar number of animals sacrificed at 1 and 4 weeks
post-treatment. Plasma and erythrocyte cholinesterase activities
were depressed at 10 and 30 ppm while brain cholinesterase was
depressed at 30 ppm. After 1 week recovery, plasma and erythrocyte
cholinesterase were still depressed at the upper level. Values for
all three parameters were normal after a 4 week recovery period.
Since there was great variability in plasma cholinesterase
activity after 3 weeks and anomolous results after 13 weeks in
erythrocyte cholinesterase values, an additional study was
conducted. Groups of 12 male and 12 female rats were fed as above
for 13 weeks. Determination of plasma, erythrocyte and brain
cholinesterase activity was carried out at 3 and 13 weeks. All the
parameters measured were depressed at 30 ppm. Erythrocyte
cholinesterase was also decreased at 10 ppm. A no-effect level of
3 ppm was demonstrated (Wolf et al 1966a; 1966c).
Norchloro-dialifos was fed to groups of 10 male and 10 female
rats at dietary levels of 0, 50 and 200 ppm for 90 days. Body
weight gain and food consumption were decreased among females at
both test levels. There was a slight decrease in reticulocytes in
both sexes at 200 ppm. Significant depressions in erythrocyte,
plasma and brain cholinesterase activities were observed among male
and female rats from both test groups. No compound-related effects
were noted with respect to mortality, blood chemistry, urinalysis
organ weights, gross and microscopic pathology (Smith et al 1972b).
An additional study was designed in a comparable manner in
which groups of 9 male and 9 female rats were fed dietary levels of
norchloro-dialifos of 0, 1, 3 and 10 ppm for 90 days. After 3, 6
and 13 weeks, 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. After 13 weeks decreases in both plasma and
erythrocyte cholinesterase activities were observed at 3 and 10
ppm. Brain cholinesterase activity was slightly depressed at 3 ppm
among males, while females of all groups showed a non-dose related
decrease (Smith et al 1972a).
Dog - oral
Groups of 1 male and 1 female dogs were fed diets containing
0, 20, 100 and 500 ppm of dialifos for 14 days. Plasma and
erythrocyte cholinesterase activities were depressed at 20 ppm and
above. There was food rejection at all levels with weight loss at
500 ppm (Schoenig et al, 1966a).
Dialifos (technical) was administered in the diet to groups of
2 male and 2 female dogs at levels of 0, 1, 3, 10, 30 and 100 ppm
for 98 days. Plasma and erythrocyte cholinesterase activities were
not depressed when measured at 0, 7, 21, 42 and 90 days at dietary
levels of 1 and 3 ppm or less respectively. A slight reduction in
brain cholinesterase activity was noted at the upper dose level.
Body weight gain, food consumption, hematology, clinical chemistry,
urinalysis, organ weights, gross and histopathology were within
normal limits (Baran et al, 1966).
Groups of 3 male and 3 female dogs were fed dialifos
(technical) which was incorporated into the diet to give a dose
level of 0, 50 ,or 200 ppm for 2 years. The upper level was reduced
to 100 ppm after 21 days. After 1 year, 1 male and 1 female/group
were sacrificed. Most dogs showed weight loss and symptoms
associated with cholinesterase depression while receiving 200 ppm.
Cholinesterase activity was reduced at 50 and 100 ppm in plasma and
erythrocytes at 6, 12 and 24 months. Brain cholinesterase appeared
to be slightly depressed at 100 ppm. No compound-related effects
were observed with respect to food consumption, mortality,
hematology, clinical chemistry, organ function tests, organ
weights, gross and histopathology (Baran et al, 1968).
Tissue residue studies were performed on body tissues of dogs
fed dialifos (technical) for 2 years. Samples of muscle, fat,
liver, kidney and heart of each dog were examined. Barely
detectable residues of dialifos were found in muscle, liver, kidney
and heart samples, residues ranging from 0.003 to 0.005 mg/kg.
Slightly higher levels were detected in the fat samples. These
averaged 0.022 mg/kg at the 50 ppm and 0.032 mg/kg at the 100 ppm
feeding levels. The oxygen analogue of dialifos was not detected in
any of the tissues examined (Ford 1970c).
Chicken - oral
Replicate groups of 3 laying hens were fed 0, 0.375 and 1.25
mg/kg/day of dialifos (technical) for 8 weeks. Erythrocyte and
plasma cholinesterase activities were determined prior to and at 1,
2, 4 and 8 weeks following the initiation of treatment.
Cholinesterase activities were not affected. No toxic symptoms were
observed and no effect was noted on daily food consumption, body
weight or egg production (Sedivy 1968c).
Cattle - oral
Groups of 3 beef animals (180 kg) were fed 0, 0.1 and, O.2
mg/kg/day of dialifos for 28 days. One animal of each group was
sacrificed after 12 days of treatment, and the remaining animals at
28 days for collection of tissue samples. There was no
treatment-related effect with respect to plasma or erythrocyte
cholinesterase activity. Residue levels in fat, kidney, liver and
muscle were generally less than 0.001 mg/kg. The highest residue
level detected was 0.003 mg/kg in the fat of animals fed 0.2 mg/kg
(Taylor, 1969).
Three beef animals (180 kg) were fed diets containing
approximately 2.2 mg/kg/day; a fourth animal served as a control.
At the end of each feeding period of 2, 4 and 8 weeks, one animal
was sacrificed for tissue residue studies. Plasma and erythrocyte
cholinesterase determinations were made at weekly intervals.
Residues in the range of 0.1 to 0.3 mg/kg were found in liver and
fatty tissues. Lower residues ( <0.1 mg/kg) were detected in
muscle and kidney. No evidence for the build-up of the residue was
observed when the feeding period was doubled. The oxygen analogue
of dialifos was not detected to any significant degree in the
tissues analysed. Erythrocyte cholinesterase activity was markedly
inhibited, while plasma inhibition was only slightly affected.
(Ford 1970a; Sedivy 1968a).
Four holstein milk cows were administered a daily dose of
dialifos of 1.35 mg/kg for 21 days. Weekly plasma and erythrocyte
cholinesterase determinations were made and milk samples collected
for residue analysis. No overt signs of toxicity were observed and
milk production was not altered. A marked inhibition of erythrocyte
cholinesterase was noted. The slight reduction in plasma
cholinesterase was questionable. An average dialifos residue of 5
mg/kg was detected in milk samples which quickly disappeared when
the administration of dialifos was terminated. No oxygen analogue
was found in any of the samples (Ford 1970b., Sedivy 1968b).
Long-term studies
Rat
Groups of 30 male and 30 female rats were fed dietary levels
of 0, 0, 20 and 50 ppm of dialifos (technical) for 2 years. After
6, 12 and 24 months 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and cholinesterase determinations.
Erythrocyte cholinesterase activity was depressed in all animals of
both test levels at all examination intervals. Plasma
cholinesterase activity was decreased among females at both test
levels while males exhibited depression at 50 ppm after 6, 12 and
18 months but not at 24 months. Brain cholinesterase activity was
depressed at the higher intake level. No significant differences
between treated and control animals were observed with respect to
body weight gain, food consumption, mortality, hematology, blood
chemistry, urinalysis, organ weights or gross and histopathology.
There was no increase on the incidence of tumours in the test
animals (Wolf et al 1968).
Dialifos was fed at dietary levels of 0, 1, 3 and 10 ppm to
groups of 30 male and 30 female rats for 1 year. Five males and 5
females/group were randomly selected and sacrificed after 3, 6, 9
and 18 months for plasma, erythrocyte and brain cholinesterase
determinations as well as gross and histopathological examination.
Plasma cholinesterase in females and erythrocyte cholinesterase in
both sexes were decreased at the dietary intake of 10 ppm. Brain
cholinesterase was not affected. Body weight gain was slightly
decreased in males fed 10 ppm. There were no compound-related
effects in mortality, organ weights, gross and histopathology
(Regna et al 1975).
A small number of animals from the one year oral toxicity
study reported above were continued on the appropriate diet for an
additional year. The small number of surviving animals on test
makes the interpretation of the data difficult. However, no
significant changes were noted in body weights, food consumption,
mortality, behavioural reactions, plasma, erythrocyte and brain
cholinesterase, gross and histopathology. No evidence of increased
tumour incidence or production of unusual tumour types was observed
in the treated animals (Marias et al, 1975).
OBSERVATIONS IN MAN - ORAL
Dialifos was administered orally to 3 male and 3 female
volunteers in gelatin capsules in daily single doses of 0.01 (2
weeks), 0.03 (4 weeks) and 0.1 mg/kg (10 days) with 2 males and 2
females receiving placebos. After an appropriate recovery period
four of the test subjects were used as controls and the 4 control
subjects became part of the total of 6 test subjects in a second
study. In this study dialifos was administered orally in gelatin
capsules 3 times a day to simulate 3 meals a day in dosage levels
of 0.01 (2 weeks), 0.02 (4 weeks), 0.03 (8 weeks) and 0.05 (4
weeks) mg/kg. In both studies, plasma and erythrocyte
cholinesterase, plasma ali-esterases (tributyrinase and diethyl
succinase) were measured before dosing and at intervals during the
study and recovery periods. Periodically, other observations were
made which included hematology, blood pressure, pulse rate, pupil
size, light reflex, eye accommodation, chest sounds, muscle tone,
kneejerk, tongue tremor and finger tremor. No significant
inhibition of any of the enzyme systems tested was observed at
dosage levels of 0.01, 0.02 and 0.03 mg/kg when given either in single
or divided daily dose. Plasma cholinesterase and tributyrinase
were slightly depressed while no effect was observed
on erythrocyte cholinesterase or diethyl succinase at 0.05 and 0.1
mg/kg. Recovery was essentially complete in 14-21 days. The other
parameters measured were not affected (Greco et al, 1970).
COMMENTS
In mammals dialifos is an acutely toxic organophosphorus ester
which would appear to be rapidly absorbed as shown by the rapid
onset of cholinergic symptoms of poisoning. No data were available
with respect to absorption, metabolism and excretion in animals.
Teratogenic and reproduction studies indicated no adverse
effects at doses below those which were toxic to the parents.
Mutagenic activity was not observed in the microbial indicator
organisms used in the study. No apparent signs of neurotoxicity
were observed in hens given large doses of dialifos.
In several short-and long-term studies in the mouse, rat, dog,
hen, monkey, cattle and man, dialifos was shown to be an active
cholinesterase inhibitor. Plasma cholinesterase was inhibited to a
greater extent than either erythrocyte or brain cholinesterase with
no marked difference between animal species.
The major impurity of technical dialifos, 00-diethyl
S-(1-phthalimidoethyl) phosphorodithioate ("norchlordialifos"), was
as toxic as dialifos as indicated by acute, short-term and
teratogenic studies.
No-effect levels in the rat, dog and man have been established
on the basis of the most sensitive parameter, the depression of
plasma cholinesterase activity. The data were sufficient to
recommend an acceptable daily intake for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat 3 ppm in the diet equivalent to 0.15 mg/kg bw
Dog 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man 0.03 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Dialifos is an acaricide and insecticide used in wettable
powder and emulsion sprays on citrus, apples, pears, grapes,
pecans, rapeseed, cotton, potatoes and sugar beets. Dosages, number
of treatments, and use restrictions vary widely with the target
pest and the residue tolerances in the country in which it is used.
A summary of various national use patterns is shown in Table
6. The table is based primarily on information provided by the
basic manufacturer of dialifos. Country statements furnished to the
Meeting indicate that South Africa has cancelled registered uses
for dialifos, that use in the Netherlands is curtailed because of
resistance problems, and that Australia has no registered uses but
has applications for use on some fruits pending.
TABLE 6. National use patterns of dialifos
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
Citrus
U.S.A. 30-60g/100 l 7 2 treatments per
year
Turkey 1.0-1.5 kg/ha 28 2 treatments per
growing season
Japan 1.7-3.3 kg/ha 45
Grapes
U.S.A. 1.1 kg/ha 25 one treatment after
shot-berry stage
and no more than
2 per year
France 0.75-1.0 kg/ha 28 one spray per
growing season
(before Aug 1)
Apples, Pears
U.S.A. 60-90 g/100 l 60 not more than 5
cover sprays
Netherlands 0.75-1.0 kg/ha* 8 weeks
TABLE 6. (Cont'd.)
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
France 1.0 kg/ha 4 weeks
Germany 1.0 kg/ha 4 weeks
Italy 1.0 kg/ha 4 weeks
Belgium 1.0 kg/ha 6 weeks
Switzerland 1.0 kg/ha 6 weeks
New Zealand pre-blossom spray
only (provisional
registration)
U.K. 0.06%(high vol.) 3 weeks not more than 3
sprays per season
1.0 kg/ha(low vol.)
Pecans
U.S.A. 60 g/100 l -- apply before husk
split
Rapeseed
Germany 288 g/ha apply only during
flowering
* Report decreased use because of resistance.
It was also reported that dialifos is used on potatoes (500g/ha)
and sugar beets (700-750 g/ha) in Europe and on cotton in Turkey and
Africa at rates of 100-125 g/ha.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials in the U.S.A., Canada, Japan
Australia, the Netherlands, Republic of South Africa, West Germany and
Italy were made available to the Meeting through a submission by the
basic manufacturer (Hercules, 1976) and country statements. The
findings are discussed separately by commodity. Unless otherwise
noted, residue values are for dialifos per se.
Citrus
Data from supervised trials in the U.S.A. are summarized in Table
7 and Figure 1.
Other information on identity and properties
State: white crystals (pure)
brown crystals (isolated technical)
brown liquid (commercial technical)
Commercial technical dialifos is produced in the form of an
80% solution of technical dialifos in a xylene-range aromatic
solvent. This solution is adjusted to a minimum dialifos content of
72% by weight. Technical dialifos in crystalline form is not an
item of commerce.
Melting point: 67°C (pure)
Vapour pressure: < 10-4 mm Hg 35°C
Solubility: water 0.18 mg/kg
essentially insoluble in vegetable oils,
alcohol and glycols
soluble in acetone, chloroform, xylene and
ethyl ether
Hydrolytic stability in ethanol/water (2:8) at 25°C:
half life of 119 hours at pH 6
half life of 2.5 hours at pH 8
Dialifos is formulated as emulsifiable concentrates in the range 25
to 50% or as a 50% wettable powder.
Purity
TABLE 1. Impurities (maximum levels in commercial technical dialifos
Compound Maximum level (%w/w)
O,O-diethyl S-(1-phthalimidoethyl
phosphorodithioate
("norchlorodialifos") 5
dialifos oxygen analogue <1
N-(2-hydroxyethyl) phthalimide <.3
N-(2-acetoxyethyl) phthalimide <.3
N-(1,2,2-trichloroethyl)phthalimide 1
N-(1,2-dichloroethyl)phthalimide 1
N-(2-chlorovinyl)phthalimide 1
OOO-triethyl phosphorthioate 3
OOS-triethyl phosphorodithioate 3
Unidentified 2
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on enzymes and other biochemical parameters
The in vitro inhibitory effect of dialifos (technical) on
rat plasma and erythrocyte cholinesterase was studied. The
enzyme-inhibitor complex was formed within five minutes and was
stable during the incubation period. Linear reaction rates were
also demonstrated, confirming the reliability of the electrometric
assay procedure for cholinesterase activity. The concentration of
dialifos required to obtain a 50% inhibition of plasma
cholinesterase was 2.55 µg/ml and 0.31 µg/ml for erythrocyte
cholinesterase. The in vitro oxidation of dialifos with a
peroxide-acetic acid system decreased the 50% inhibition
concentration to 0.83 µg/ml for plasma cholinesterase and 0.015
µg/ml for erythrocyte cholinesterase (Lazanas et al 1966a, 1966b).
A study was conducted to investigate the effect of dialifos on
aliphatic esterases in comparison with cholinesterase. Groups of 6
female rats were fed levels from 0.5 to 50 ppm dialifos
(technical). Three animals at each dietary level were killed after
1 and 3 weeks and measurements of the rate of enzymic hydrolysis of
diethyl succinate and tributyrin by the liver and serum, and
cholinesterase activity of the brain were conducted. The dietary
levels producing 50% inhibition of tributyrinase activity of liver
and serum were 4.7 and 10.3 ppm respectively after 3 weeks. Diethyl
succinate hydrolysis in the liver and serum was inhibited by 50% at
dietary levels of 13.5 and 12.5 ppm after 1 week and 16.0 and 12.0
ppm after 3 weeks respectively. Brain cholinesterase was
significantly reduced at 50 ppm after 3 weeks. In a preliminary
study liver and serum cholinesterase were shown to be appreciably
inhibited after 1 week at dietary levels of 25 ppm. The
cholinesterase activity of all the tissues was inhibited to a
lesser extent than the aliesterase activity (DuBois et al, 1969).
TOXICOLOGICAL STUDIES
Special studies on antidotes
The acute oral LD50 of dialifos was determined in groups of
male rats (1) without antidote; (2) treated with atropine sulphate
(17.5 mg/kg); (3) treated with 2-PAM (50 mg/kg); (4) treated with
a combination of atropine sulphate and 2-PAM at 17.5 and 50 mg/kg
respectively. Antidotes were administered intramuscularly 30
minutes after dialifos. The LD50 for the groups were 71, 140, 267
and 177 mg/kg respectively. The onset of symptoms and time of death
were delayed in the treated groups. Atropine sulphate and 2-PAM
would appear to be effective antidotes for dialifos (Mastri et al,
1968).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed dialifos
(technical) in the diet at dosage levels of 0, 0.05 and 0.5 mg/kg
body weight/day and subjected to a standard 2 litter, 3 generation
reproduction study. The following parameters were measured: body
weight, mortality, symptoms, hematology, urinalysis, organ weights
and gross and histopathology of all parental generations after the
second weaning as well as plasma and erythrocyte cholinesterase
activities of all parental generations and litters. In addition,
the following reproduction indices were measured: mating, pregnancy,
fertility, parturition and lactation. With respect to progeny mean
litter size, number of stillborn and viable pups and pup body weights
were recorded. Plasma and erythrocyte cholinesterase depression were
observed primarily in the female of all parental groups and
weanlings fed 0.5 mg/kg body weight. There were random non-dose
related effects in some reproductive parameters of both test groups
when compared to controls. No compound-related gross or
histopathological findings were observed. (Arnold et al, 1968).
Special studies on teratology
Rabbit
Groups of 10 pregnant rabbits were administered dialifos
(technical) via gelatin capsule at dosage levels of 0, 1 and 3
mg/kg from day 6 to day 18, and 10 and 25 mg/kg from day 6 to day
16 of gestation. Thalidomide was given at a dosage of 75 mg/kg/day.
Does were sacrificed on day 29 of gestation. Offspring were placed
in an incubator for 24 hours to determine viability and then
sacrificed for skeletal examination. Parental weight loss occurred
at 3, 10 and 25 mg/kg. Mortality was increased at 10 mg/kg (7/10)
and 25 mg/kg (10/10). Hemorrhage in the small intestine was
observed in the two highest dose groups. Litter size was reduced
and fetal resorption increased at 10 and 25 mg/kg while the
viability index was decreased at all dosage levels. Four
abnormalities (3 umbilical hernia, 1 partial acranius) were noted
at 3 mg/kg, however no abnormalities were observed at 10 mg/kg. The
thalidomide group showed clubbing of the extremities (30/49)
(Kennedy et al, 1966). This study was repeated in a similar fashion
using the same strain of rabbit, administered 0, 1, or 3 mg
dialifos (technical)/kg from day 6 to day 18 of gestation. No
adverse effects were observed with respect to maternal body weight,
survival or behaviour. Number of implantation sites and litter size
were reduced and the number of resorption sites increased in
treated groups when compared to corresponding controls. This was
considered to be due to physical manipulation of these animals
rather than a possible toxic effect of the test material. The
viability index was comparable to controls, and no skeletal or
external abnormalities were found in any of the progeny receiving
dialifos (Jackson et al, 1966b).
Groups of 17 female rabbits were treated from day 6 through
day 18 of gestation with daily doses of either 0, 1.0 or 3.0 mg/kg
norchlorodialifos via gelatin capsule. Thalidomide was administered
concurrently at 37.5 mg/kg body weight as a positive control.
Rabbits were sacrificed on gestation day 29. Young were removed by
caesarean section and placed in an incubator for 24 hours. They
were then sacrificed and examined for external skeletal and soft
tissue abnormalities. At 3 mg/kg the animals lost weight during the
dosing period. No effect in body weight gain was exhibited at 1
mg/kg. No deaths or unusual reactions were observed. The number of
live fetuses and fetal survival were reduced at 3 mg/kg. No
significant differences were observed in 24 hour viability index
or fetal body weights between control and norchlorodialifos treated
animals. No compound-related internal or skeletal abnormalities were
noted (Ladd et al, 1972).
Hamster
Pregnant hamsters were dosed orally with dialifos once on day
7 or 8 of gestation or several times on days 6-8 or days 6-10 of
gestation. The total administered dose varied from 100-500 mg/kg.
On day 15 of gestation the surviving hamsters were sacrificed and
the fetuses removed. The usual parameters for teratogenicity were
examined. From the mortality data of the dams it was concluded that
most of the doses were > LD50. The lowest single dose of 100
mg/kg body weight produced malformations in 4/98 fetuses, 3 with
limb defects and one with umbilical hernia (Robens, 1970).
Monkey
Ten pregnant stumptailed macaques were dosed with dialifos
(technical) at 1 mg/kg body weight (the dose causing ca 25% plasma
and erythrocyte cholinesterase depression (Vondruska et al, 1968)
for a period of one week during the critical period of limb
formation. Nine other pregnant females were administered either 5
or 10 mg thalidomide/kg as positive controls. Blood samples were
taken on the 15th, 18th and 21st days of pregnancy and on the last
dosing day for complete hematology and cholinesterase activity.
During the 12th week of gestation, fetuses were recovered by
caesarean section and examined for abnormalities. No hematological
effects were observed. Plasma and erythrocyte cholinesterase
activity for monkeys receiving dialifos were reduced to 65 and 62%
of pre-treatment values. No abortions were observed with dialifos
and all fetuses were normal. Abortions occurred in four females
dosed with thalidomide. A total of five fetuses were recovered from
females treated with thalidomide and all exhibited gross
deformities of the anterior extremities (Vondruska and Fancher,
1969).
Special studies on mutagenicity
Dialifos was tested for mutagenic activity using a series of
microbial indicator organisms (Saccharomyces cerevisiae strain
D4; Salmonella typhimurium strains TA-1535, 1537, 1538) in
qualitative plate tests with and without metabolic activation.
Activation was accomplished by the use of tissue homogenates of
liver, lung and testes from mice, rats and monkeys. The compound
did not exhibit significant genetic activity in any of the assays
performed (Brusick, 1975).
Special studies on neurotoxicity
Groups of 6 hens were given 0 or 464 mg/kg body weight (oral
LD 50) of dialifos (technical). Surviving birds were re-dosed with
the LD50 after 21 days and were observed for an additional 21 days.
Samples of spinal cord, sciatic nerve and brain were taken for
histological examination. A positive control group was dosed with
500 mg/kg body weight TOCP and studied simultaneously with the test
group. No signs of ataxia were noted and no clinical neurotoxic
effects were observed. Birds in the positive control group showed
signs of neurotoxicity approximately 14 days following
administration of the last dose. Histopathology of nervous tissue
was not done (Jackson et al, 1968).
Special studies on potentiation
An acute potentiation study was conducted in order to
determine the degree of interaction between dialifos (technical)
and malathion. The acute oral LD50 values were determined in male
and female rats separately. Doses corresponding to 1/2, 1/4 and 1/8
the LD50 of dialifos were administered orally to animals in
separate groups consisting of 5 male and 5 female rats followed
four hours later by the corresponding fractions of the LD50 of
malathion. No potentiation was observed in female rats. However, in
the case of males, potentiation did occur since 1/8 of the LD50 of
each compound resulted in an LD40 (Schoenig et al, 1966e).
Special studies on eye irritation
Undiluted dialifos when applied directly to the conjunctival
sac of two rabbits was shown to be mildly irritating in both
unwashed and washed eyes. When dialifos was applied as a 5%
suspension in propylene glycol, similar results were observed
(Schoenig et al, 1966d).
Special studies on dermal irritation
When undiluted dialifos was applied to shaved and abraded skin
of two rabbits, a slight irritating effect was noted after 24
hours. Mortality precluded a 72-hour reading (Schoenig et al,
1966d).
Acute toxicity
TABLE 2. Acute toxicity of dialifos
Animal Sex Route Solvent LD50 Reference
mg/kg bw
rat M oral propylene 53 Schoenig et al
glycol 1966d
M oral propylene 43 ibid
glycol
M oral 1% methyl 71 ibid
cellulose
F oral propylene 5 ibid
glycol
mouse M oral propylene 39 Schoenig et al
glycol 1966d
F oral propylene 65 ibid
glycol
rabbit M oral propylene 58 ibid
glycol
F oral propylene 71 ibid
glycol
F oral maize oil 35 Jackson et al
1966a
M dermal xylene 145 Schoenig et al
1966d
dog M oral propylene 97 Schoenig et al
glycol 1966d
chicken F oral maize oil 464 Jackson et al
1968
The impurities of dialifos were isolated. The acute toxicities
of the impurities and pure compound were compared.
TABLE 3. Acute toxicity of pure dialifos compared with its
impurities
LD50
Animal Sex Route Solvent mg/kg bw Reference
pure dialifos
rat M oral propylene 69 Schoenig et al
glycol 1966a
F oral propylene 5 ibid
glycol
impurities
rat M oral propylene 327 Schoenig et al
glycol 1966b
F oral propylene 218 ibid
glycol
TABLE 4. Acute toxicity of dialifos formulations
LD50
Animal Sex Route Solvent mg/kg bw Reference
Emulsifiable concentrate - 47%
rat M oral water 51 Nomura 1970
F oral water 19 ibid
M oral water 62 Ueda 1968
F oral water 21 ibid
M&F Inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
TABLE 4. (Cont'd.)
LD50
Animal Sex Route Solvent mg/kg bw Reference
mouse M oral water 99 Nomura 1970
F oral water 90 ibid
M&F inhalation water saturated
(4 hr) vapour(LC50) Hathaway et al
1969a
rabbit M dermal water 327 Mastri et al
1969a
guinea M&F inhalation water saturated Hathaway et al
pig (4 hr) vapour 1969a
(LC50)
Wettable Powder - 50%
rabbit M dermal water 735 Mastri et al
1969b
rat M&F inhalation 0.5 mg/l Hathaway et al
(2 hr) (LC50) 1969b
mouse M&F inhalation 1.0 mg/l ibid
(2 hr) LC50)
guinea M&F inhalation 1.0 mg/l ibid
pig (2 hr) LC50)
TABLE 5. Acute toxicity of norchlorodialifos
LD50
Animal Sex Route Solvent mg/kg bw Reference
rat M oral maize oil 81 Gabriel 1972
F oral maize oil 22
rabbit M dermal - > 1000 Gabriel 1972
Short term studies
Rabbit - dermal
Dialifos 4 lbs/gal (0.48 kg/l) emulsifiable solution was
applied to the clipped backs of groups of 3 male and 3 female
rabbits at dosages of 0.3, 1, 5 and 25 mg/kg. The test material was
applied for a period of 6 hours/day, 5 days/week until 22
applications were made. An untreated control group received no
dermal application. At dosage levels of 25 and 5 mg/kg, growth was
depressed with inhibition of plasma, erythrocyte and brain
cholinesterase activity. A slight acanthosis of the epidermis
suggesting slight irritation was observed. There was a slight but
questionable depression of plasma and brain cholinesterase at 1 mg/kg.
No adverse effects were observed in any of the parameters studies at
0.3 mg/kg body weight (Mastri et al, 1969c; Mastri et al, 1970).
Rat and guinea pig - inhalation
Groups of rats and guinea pigs (6 males and 6 females of each
species) were exposed for 6 hours/day, 6 days/week for 2 weeks to
an aerosol of dialifos generated from an acetone solution of a 4
lb/gal (0.48 kg/1) emulsifiable formulation at concentrations of 0,
2.8, 7.7 and 20.0 µg/liter. Half the animals were sacrificed for
pathological examination after the last exposure. The remaining
animals were sacrificed after a further two week observation
period. Plasma, erythrocyte and brain cholinesterase activities
were determined only in the rat. Some mortality, ataxia, dyspnea,
marked plasma and slight erythrocyte cholinesterase depression were
observed at 20 µg/liter. No adverse effects were noted at exposure
levels of 7.7 and 2.8 µg/liter (Hathaway et al, 1969c; Hathaway et
al, 1970).
Mouse - oral
Groups of 5 male and 5 female mice were administered dialifos
dissolved in lard oil by gavage 7 days a week at dosages of 1, 2.5,
5 and 10 mg/kg/day for 90 days. Two control groups were used in
this study, one receiving no treatment, the other an equivalent
amount of lard oil to the treated groups. One half of the animals
were sacrificed after 30 days and the remainder at 90 days. The
following parameters were measured: body weight, hematology, organ
weights, gross and histopathology. Cholinesterase determinations
were not conducted. Decreased growth rate and liver necrosis were
observed in male mice receiving 10 mg/kg body weight. No
significant effects were observed at 5 mg/kg body weight or less
(Nomura, 1970).
Rat - oral
Dialifos dissolved in lard oil was administered by gavage 7
days a week at dosages of 1.0, 2.5, 5 and 10 mg/kg/day for males
and 0.15, 0.375, 0.75 and 1.5 mg/kg/ day for females for 90 days.
Control groups received either no treatment or an equivalent amount
of lard oil. One half of the animals were sacrificed at 30 days and
the remainder at 90 days. Observations on the following parameters
were made: body weight, hematology, organ weights, gross and
microscopic pathology. Serum cholinesterase measurements were made
after 30 days. Serum cholinesterase was depressed at 2.5 mg/kg and
above in males and 0.375 mg/kg and above in females. At the upper
dosage levels in both male and female, increased mortality and
liver necrosis were observed (Nomura, 1970).
Dialifos was administered orally to groups of 3 male and 3
female rats at 0, 0.5, 1.0, 2.5, 5.0, 10.0, 25.0 and 50.0 mg/kg/day
for 14 days. Plasma cholinesterase was inhibited at 0.5 mg/kg/day
and above, erythrocyte cholinesterase at 1.0 mg/kg and above, and
brain cholinesterase at 2.5 mg/kg and above. There was increased
mortality at 10.0 mg/kg and above. Gross pathological findings
among test animals were comparable to those of controls (Wolf and
Calandra, 1965).
Dialifos was fed at dietary levels of 0, 10, 25, 50 and 100
ppm to groups of 10 male and 10 female rats for 90 days.
Erythrocyte cholinesterase activity was decreased at all dietary
levels. Plasma cholinesterase activity was depressed among females
at all dietary levels and among males at 25 ppm and above. Brain
cholinesterase activity of males was depressed at 100 ppm while
among females depression was noted at 25 ppm and above. At 100 ppm,
body weight gain and food consumption were reduced; a
compound-related increase in mortality was noted; BUN and serum A/G
ratios were increased. Hematology, organ weights, gross and
histopathology revealed no compound-related effects (Wolf et al,
1966b).
Groups of 21 male and 21 female rats were fed diets containing
dialifos at levels of 0, 0.3, 1, 3, 10 and 30 ppm for 13 weeks.
After 1, 3, 6 and 13 weeks of feeding, 3 rats/sex/groups were
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. The remaining rats were then fed the control diet
and a similar number of animals sacrificed at 1 and 4 weeks
post-treatment. Plasma and erythrocyte cholinesterase activities
were depressed at 10 and 30 ppm while brain cholinesterase was
depressed at 30 ppm. After 1 week recovery, plasma and erythrocyte
cholinesterase were still depressed at the upper level. Values for
all three parameters were normal after a 4 week recovery period.
Since there was great variability in plasma cholinesterase
activity after 3 weeks and anomolous results after 13 weeks in
erythrocyte cholinesterase values, an additional study was
conducted. Groups of 12 male and 12 female rats were fed as above
for 13 weeks. Determination of plasma, erythrocyte and brain
cholinesterase activity was carried out at 3 and 13 weeks. All the
parameters measured were depressed at 30 ppm. Erythrocyte
cholinesterase was also decreased at 10 ppm. A no-effect level of
3 ppm was demonstrated (Wolf et al 1966a; 1966c).
Norchloro-dialifos was fed to groups of 10 male and 10 female
rats at dietary levels of 0, 50 and 200 ppm for 90 days. Body
weight gain and food consumption were decreased among females at
both test levels. There was a slight decrease in reticulocytes in
both sexes at 200 ppm. Significant depressions in erythrocyte,
plasma and brain cholinesterase activities were observed among male
and female rats from both test groups. No compound-related effects
were noted with respect to mortality, blood chemistry, urinalysis
organ weights, gross and microscopic pathology (Smith et al 1972b).
An additional study was designed in a comparable manner in
which groups of 9 male and 9 female rats were fed dietary levels of
norchloro-dialifos of 0, 1, 3 and 10 ppm for 90 days. After 3, 6
and 13 weeks, 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and brain cholinesterase
determinations. After 13 weeks decreases in both plasma and
erythrocyte cholinesterase activities were observed at 3 and 10
ppm. Brain cholinesterase activity was slightly depressed at 3 ppm
among males, while females of all groups showed a non-dose related
decrease (Smith et al 1972a).
Dog - oral
Groups of 1 male and 1 female dogs were fed diets containing
0, 20, 100 and 500 ppm of dialifos for 14 days. Plasma and
erythrocyte cholinesterase activities were depressed at 20 ppm and
above. There was food rejection at all levels with weight loss at
500 ppm (Schoenig et al, 1966a).
Dialifos (technical) was administered in the diet to groups of
2 male and 2 female dogs at levels of 0, 1, 3, 10, 30 and 100 ppm
for 98 days. Plasma and erythrocyte cholinesterase activities were
not depressed when measured at 0, 7, 21, 42 and 90 days at dietary
levels of 1 and 3 ppm or less respectively. A slight reduction in
brain cholinesterase activity was noted at the upper dose level.
Body weight gain, food consumption, hematology, clinical chemistry,
urinalysis, organ weights, gross and histopathology were within
normal limits (Baran et al, 1966).
Groups of 3 male and 3 female dogs were fed dialifos
(technical) which was incorporated into the diet to give a dose
level of 0, 50 ,or 200 ppm for 2 years. The upper level was reduced
to 100 ppm after 21 days. After 1 year, 1 male and 1 female/group
were sacrificed. Most dogs showed weight loss and symptoms
associated with cholinesterase depression while receiving 200 ppm.
Cholinesterase activity was reduced at 50 and 100 ppm in plasma and
erythrocytes at 6, 12 and 24 months. Brain cholinesterase appeared
to be slightly depressed at 100 ppm. No compound-related effects
were observed with respect to food consumption, mortality,
hematology, clinical chemistry, organ function tests, organ
weights, gross and histopathology (Baran et al, 1968).
Tissue residue studies were performed on body tissues of dogs
fed dialifos (technical) for 2 years. Samples of muscle, fat,
liver, kidney and heart of each dog were examined. Barely
detectable residues of dialifos were found in muscle, liver, kidney
and heart samples, residues ranging from 0.003 to 0.005 mg/kg.
Slightly higher levels were detected in the fat samples. These
averaged 0.022 mg/kg at the 50 ppm and 0.032 mg/kg at the 100 ppm
feeding levels. The oxygen analogue of dialifos was not detected in
any of the tissues examined (Ford 1970c).
Chicken - oral
Replicate groups of 3 laying hens were fed 0, 0.375 and 1.25
mg/kg/day of dialifos (technical) for 8 weeks. Erythrocyte and
plasma cholinesterase activities were determined prior to and at 1,
2, 4 and 8 weeks following the initiation of treatment.
Cholinesterase activities were not affected. No toxic symptoms were
observed and no effect was noted on daily food consumption, body
weight or egg production (Sedivy 1968c).
Cattle - oral
Groups of 3 beef animals (180 kg) were fed 0, 0.1 and, O.2
mg/kg/day of dialifos for 28 days. One animal of each group was
sacrificed after 12 days of treatment, and the remaining animals at
28 days for collection of tissue samples. There was no
treatment-related effect with respect to plasma or erythrocyte
cholinesterase activity. Residue levels in fat, kidney, liver and
muscle were generally less than 0.001 mg/kg. The highest residue
level detected was 0.003 mg/kg in the fat of animals fed 0.2 mg/kg
(Taylor, 1969).
Three beef animals (180 kg) were fed diets containing
approximately 2.2 mg/kg/day; a fourth animal served as a control.
At the end of each feeding period of 2, 4 and 8 weeks, one animal
was sacrificed for tissue residue studies. Plasma and erythrocyte
cholinesterase determinations were made at weekly intervals.
Residues in the range of 0.1 to 0.3 mg/kg were found in liver and
fatty tissues. Lower residues ( <0.1 mg/kg) were detected in
muscle and kidney. No evidence for the build-up of the residue was
observed when the feeding period was doubled. The oxygen analogue
of dialifos was not detected to any significant degree in the
tissues analysed. Erythrocyte cholinesterase activity was markedly
inhibited, while plasma inhibition was only slightly affected.
(Ford 1970a; Sedivy 1968a).
Four holstein milk cows were administered a daily dose of
dialifos of 1.35 mg/kg for 21 days. Weekly plasma and erythrocyte
cholinesterase determinations were made and milk samples collected
for residue analysis. No overt signs of toxicity were observed and
milk production was not altered. A marked inhibition of erythrocyte
cholinesterase was noted. The slight reduction in plasma
cholinesterase was questionable. An average dialifos residue of 5
mg/kg was detected in milk samples which quickly disappeared when
the administration of dialifos was terminated. No oxygen analogue
was found in any of the samples (Ford 1970b., Sedivy 1968b).
Long-term studies
Rat
Groups of 30 male and 30 female rats were fed dietary levels
of 0, 0, 20 and 50 ppm of dialifos (technical) for 2 years. After
6, 12 and 24 months 3 rats/sex/group were randomly selected and
sacrificed for plasma, erythrocyte and cholinesterase determinations.
Erythrocyte cholinesterase activity was depressed in all animals of
both test levels at all examination intervals. Plasma
cholinesterase activity was decreased among females at both test
levels while males exhibited depression at 50 ppm after 6, 12 and
18 months but not at 24 months. Brain cholinesterase activity was
depressed at the higher intake level. No significant differences
between treated and control animals were observed with respect to
body weight gain, food consumption, mortality, hematology, blood
chemistry, urinalysis, organ weights or gross and histopathology.
There was no increase on the incidence of tumours in the test
animals (Wolf et al 1968).
Dialifos was fed at dietary levels of 0, 1, 3 and 10 ppm to
groups of 30 male and 30 female rats for 1 year. Five males and 5
females/group were randomly selected and sacrificed after 3, 6, 9
and 18 months for plasma, erythrocyte and brain cholinesterase
determinations as well as gross and histopathological examination.
Plasma cholinesterase in females and erythrocyte cholinesterase in
both sexes were decreased at the dietary intake of 10 ppm. Brain
cholinesterase was not affected. Body weight gain was slightly
decreased in males fed 10 ppm. There were no compound-related
effects in mortality, organ weights, gross and histopathology
(Regna et al 1975).
A small number of animals from the one year oral toxicity
study reported above were continued on the appropriate diet for an
additional year. The small number of surviving animals on test
makes the interpretation of the data difficult. However, no
significant changes were noted in body weights, food consumption,
mortality, behavioural reactions, plasma, erythrocyte and brain
cholinesterase, gross and histopathology. No evidence of increased
tumour incidence or production of unusual tumour types was observed
in the treated animals (Marias et al, 1975).
OBSERVATIONS IN MAN - ORAL
Dialifos was administered orally to 3 male and 3 female
volunteers in gelatin capsules in daily single doses of 0.01 (2
weeks), 0.03 (4 weeks) and 0.1 mg/kg (10 days) with 2 males and 2
females receiving placebos. After an appropriate recovery period
four of the test subjects were used as controls and the 4 control
subjects became part of the total of 6 test subjects in a second
study. In this study dialifos was administered orally in gelatin
capsules 3 times a day to simulate 3 meals a day in dosage levels
of 0.01 (2 weeks), 0.02 (4 weeks), 0.03 (8 weeks) and 0.05 (4
weeks) mg/kg. In both studies, plasma and erythrocyte
cholinesterase, plasma ali-esterases (tributyrinase and diethyl
succinase) were measured before dosing and at intervals during the
study and recovery periods. Periodically, other observations were
made which included hematology, blood pressure, pulse rate, pupil
size, light reflex, eye accommodation, chest sounds, muscle tone,
kneejerk, tongue tremor and finger tremor. No significant
inhibition of any of the enzyme systems tested was observed at
dosage levels of 0.01, 0.02 and 0.03 mg/kg when given either in single
or divided daily dose. Plasma cholinesterase and tributyrinase
were slightly depressed while no effect was observed
on erythrocyte cholinesterase or diethyl succinase at 0.05 and 0.1
mg/kg. Recovery was essentially complete in 14-21 days. The other
parameters measured were not affected (Greco et al, 1970).
COMMENTS
In mammals dialifos is an acutely toxic organophosphorus ester
which would appear to be rapidly absorbed as shown by the rapid
onset of cholinergic symptoms of poisoning. No data were available
with respect to absorption, metabolism and excretion in animals.
Teratogenic and reproduction studies indicated no adverse
effects at doses below those which were toxic to the parents.
Mutagenic activity was not observed in the microbial indicator
organisms used in the study. No apparent signs of neurotoxicity
were observed in hens given large doses of dialifos.
In several short-and long-term studies in the mouse, rat, dog,
hen, monkey, cattle and man, dialifos was shown to be an active
cholinesterase inhibitor. Plasma cholinesterase was inhibited to a
greater extent than either erythrocyte or brain cholinesterase with
no marked difference between animal species.
The major impurity of technical dialifos, 00-diethyl
S-(1-phthalimidoethyl) phosphorodithioate ("norchlordialifos"), was
as toxic as dialifos as indicated by acute, short-term and
teratogenic studies.
No-effect levels in the rat, dog and man have been established
on the basis of the most sensitive parameter, the depression of
plasma cholinesterase activity. The data were sufficient to
recommend an acceptable daily intake for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat 3 ppm in the diet equivalent to 0.15 mg/kg bw
Dog 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man 0.03 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.003 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Dialifos is an acaricide and insecticide used in wettable
powder and emulsion sprays on citrus, apples, pears, grapes,
pecans, rapeseed, cotton, potatoes and sugar beets. Dosages, number
of treatments, and use restrictions vary widely with the target
pest and the residue tolerances in the country in which it is used.
A summary of various national use patterns is shown in Table
6. The table is based primarily on information provided by the
basic manufacturer of dialifos. Country statements furnished to the
Meeting indicate that South Africa has cancelled registered uses
for dialifos, that use in the Netherlands is curtailed because of
resistance problems, and that Australia has no registered uses but
has applications for use on some fruits pending.
TABLE 6. National use patterns of dialifos
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
Citrus
U.S.A. 30-60g/100 l 7 2 treatments per
year
Turkey 1.0-1.5 kg/ha 28 2 treatments per
growing season
Japan 1.7-3.3 kg/ha 45
Grapes
U.S.A. 1.1 kg/ha 25 one treatment after
shot-berry stage
and no more than
2 per year
France 0.75-1.0 kg/ha 28 one spray per
growing season
(before Aug 1)
Apples, Pears
U.S.A. 60-90 g/100 l 60 not more than 5
cover sprays
Netherlands 0.75-1.0 kg/ha* 8 weeks
TABLE 6. (Cont'd.)
Application Pre-harvest Other
Country rate (a.i.) interval (days) Restrictions
France 1.0 kg/ha 4 weeks
Germany 1.0 kg/ha 4 weeks
Italy 1.0 kg/ha 4 weeks
Belgium 1.0 kg/ha 6 weeks
Switzerland 1.0 kg/ha 6 weeks
New Zealand pre-blossom spray
only (provisional
registration)
U.K. 0.06%(high vol.) 3 weeks not more than 3
sprays per season
1.0 kg/ha(low vol.)
Pecans
U.S.A. 60 g/100 l -- apply before husk
split
Rapeseed
Germany 288 g/ha apply only during
flowering
* Report decreased use because of resistance.
It was also reported that dialifos is used on potatoes (500g/ha)
and sugar beets (700-750 g/ha) in Europe and on cotton in Turkey and
Africa at rates of 100-125 g/ha.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials in the U.S.A., Canada, Japan
Australia, the Netherlands, Republic of South Africa, West Germany and
Italy were made available to the Meeting through a submission by the
basic manufacturer (Hercules, 1976) and country statements. The
findings are discussed separately by commodity. Unless otherwise
noted, residue values are for dialifos per se.
Citrus
Data from supervised trials in the U.S.A. are summarized in Table
7 and Figure 1.
 TABLE 7. Dialifos residues in citrus fruits (U.S.A., whole fruit basis).
Dialifos, mg/kg
0.025% a.i.spray 0.05% a.i. spray
Fruit (no. of treatments) Fruit (no. of treatments)
Days after last Valencia Pineapple Valencia Pineapple
treatment Oranges(2) Oranges(3) Grapefruit (3) Lemons (3) Oranges(2) Oranges(3) Grapefruit (3) Lemons (3)
3 0.74 1.4 0.62 0.24 1.2 1.7 1.9 1.2 1.6 0.38
7 0.76 0.93 0.63 0.17 1.3 1.8 2.1 1.3 1.6 0.36
7 0.69 - - - 1.2 - - - - -
56 0.58 0.73 0.54 0.08 0.87 1.2 1.4 0.99 1.6 0.24
84 0.36 0.59 0.64 - 0.61 0.88 1.1 1.1 1.4 -
In supervised trials with oranges in Japan, residues 90-183 days
after a single treatment at 1.6-2.0 kg/ha averaged 0.38 mg/kg in the
peel and 0.01 mg/kg in both whole fruit and juice.
Grapes
Decline studies on grapes showed initial deposits of about 2
mg/kg at the recommended 1.1 kg/ha rate, steady decline for 25-30 days
to about 0.4 mg/kg, and then a levelling off with little additional
loss until harvest. The pattern of residue decline coincides with the
growth of the grape, there being practically no growth dilution over
the last 20-25 days before harvest. Field trials in W. Germany with
analyses at the Central Institute for Nutrition and Food Research,
Holland, were in good agreement with the Californian data (Anon,
1974). Residues found in several varieties of grape after 35 days are
shown in Table 8 (Hercules, 1976).
TABLE 8. Harvest residues of dialifos on grapes, 35 days after
treatment (California)
Residue, mg/kg after treatment at (kg/ha)
Year Variety 2.2 1.7 1.1
1968 Thompson - - 0.98
1969 Thompson 2.1 - 0.7
1971 Emperor 0.5 - 0.4
1971 Carignane 0.28 - 0.25
1972 Thompson 0.88 0.40 0.34
1972 Ruby Red 0.55 0.29 0.23
Apples, pears
Supervised trials were conducted at 15 locations in Canada and
the U.S.A. and in South Africa, the Netherlands, and Australia
(Hercules, 1976; Anon., 1969, 1972). No striking differences were
noted with respect to geographic location. Both WP and EC formulations
were used and both high volume and low volume applications made.
Typical initial deposits from recommended rates were about 2-3 mg/kg
with a very slow decline rate. Figure 2 shows a composite decline
curve based on residue values from 9 test locations.
TABLE 7. Dialifos residues in citrus fruits (U.S.A., whole fruit basis).
Dialifos, mg/kg
0.025% a.i.spray 0.05% a.i. spray
Fruit (no. of treatments) Fruit (no. of treatments)
Days after last Valencia Pineapple Valencia Pineapple
treatment Oranges(2) Oranges(3) Grapefruit (3) Lemons (3) Oranges(2) Oranges(3) Grapefruit (3) Lemons (3)
3 0.74 1.4 0.62 0.24 1.2 1.7 1.9 1.2 1.6 0.38
7 0.76 0.93 0.63 0.17 1.3 1.8 2.1 1.3 1.6 0.36
7 0.69 - - - 1.2 - - - - -
56 0.58 0.73 0.54 0.08 0.87 1.2 1.4 0.99 1.6 0.24
84 0.36 0.59 0.64 - 0.61 0.88 1.1 1.1 1.4 -
In supervised trials with oranges in Japan, residues 90-183 days
after a single treatment at 1.6-2.0 kg/ha averaged 0.38 mg/kg in the
peel and 0.01 mg/kg in both whole fruit and juice.
Grapes
Decline studies on grapes showed initial deposits of about 2
mg/kg at the recommended 1.1 kg/ha rate, steady decline for 25-30 days
to about 0.4 mg/kg, and then a levelling off with little additional
loss until harvest. The pattern of residue decline coincides with the
growth of the grape, there being practically no growth dilution over
the last 20-25 days before harvest. Field trials in W. Germany with
analyses at the Central Institute for Nutrition and Food Research,
Holland, were in good agreement with the Californian data (Anon,
1974). Residues found in several varieties of grape after 35 days are
shown in Table 8 (Hercules, 1976).
TABLE 8. Harvest residues of dialifos on grapes, 35 days after
treatment (California)
Residue, mg/kg after treatment at (kg/ha)
Year Variety 2.2 1.7 1.1
1968 Thompson - - 0.98
1969 Thompson 2.1 - 0.7
1971 Emperor 0.5 - 0.4
1971 Carignane 0.28 - 0.25
1972 Thompson 0.88 0.40 0.34
1972 Ruby Red 0.55 0.29 0.23
Apples, pears
Supervised trials were conducted at 15 locations in Canada and
the U.S.A. and in South Africa, the Netherlands, and Australia
(Hercules, 1976; Anon., 1969, 1972). No striking differences were
noted with respect to geographic location. Both WP and EC formulations
were used and both high volume and low volume applications made.
Typical initial deposits from recommended rates were about 2-3 mg/kg
with a very slow decline rate. Figure 2 shows a composite decline
curve based on residue values from 9 test locations.
 Pecans
Analysis of nut meats treated according to label directions
showed no residues above the limit of detection of the method (0.005
mg/kg). Residues were found on outer husks, but there was no
penetration.
Rapeseed
Supervised field trials in W. Germany in 1972 showed only trace
residues (0.01-0.02 mg/kg) in seeds receiving multiple treatments at
the rate of 270 g/ha. Extraction was by surface stripping, a technique
which may not be effective in removing residues absorbed into an oily
seed.
Sugar beets
Field trials in Italy in 1973 showed residues averaging 0.09
mg/kg on roots and 1.17 on tops following 4 applications at 675 g/ha.
Cotton
Results from limited trials at 2 locations in the U.S.A. were
made available. The report contained a total of 4 analyses of treated
samples. Residues in the range of 0.02-0.06 mg/kg were found on
undelinted seed.
Potatoes
Field trials in the Netherlands showed only trace residues
(<0.002 to 0.004 mg/kg) in treated potatoes.
Processed products from treated raw commodities
Data were made available on some processed food and feed products
derived from the raw commodities discussed in the previous section.
The findings are summarized by commodity.
Citrus pulp
Two pilot plant studies showed an average concentration factor of
5.3 in processing oranges to dried pulp. That is, whole oranges
bearing residue levels at the national maximum residue limit of 3
mg/kg would contribute residues approximating 16 mg/kg to the
commercial cattle feed (dried citrus pulp). See also discussion on
potential transfer to meat, milk, eggs.
Grape juice and pomace
Low level residues (maximum 0.02 mg/kg) occurred in juice
expressed from grapes bearing 1 mg/kg, most of the residue remaining
in the pomace. There is about a 4 fold concentration factor between
whole grape and wet pomace.
Raisins
The residues found are summarized in Table 9.
TABLE 9. Dialifos residue in raisins
Dosage Pre-harvest Dialifos, mg/kg
rate No. of interval,
Year kg/ha applications days Processed(2) Unprocessed(1)
1968 0.6 2 62 0.26 0.45
1.1 2 62 0.84 0.91
1970 1.1 2 41 - 1.2
1971 1.1 1 58 - 1.6
1.1+2.2 2 36 - 4.3
1973 1.1 3 40 0.98 -
1973 1.1 2 40 0.76 -
1973 1.1 2 70 0.20 -
(1) Sun dried
(2) Sun dried, cleaned, fumigated, moisture adjusted
Apple pomace
U.S. data indicate a concentration factor of about 16 in
processing from whole apples to dried apple pomace, a commercial
cattle feed.
No data were available on edible oils from cottonseed or rapeseed
or the respective oilseed meals.
FATE OF RESIDUES
Potential for residues in meat, milk, poultry, and eggs
As far as could be determined by the Meeting, there are no
registered uses for dialifos on primary forage crops. There are a
number of commodities from which processed by-products or offal may be
incorporated into animal feeds. This would include dried citrus pulp
and molasses, grape pomace, apple pomace, rapeseed and cottonseed
meal, and sugar beet pulp.
There were sufficient data from controlled feeding experiments
with cattle and poultry to relate dialifos intake levels to residue
levels in milk and meat. However, there was only fragmentary
information available on residue levels in the feed items and on the
proportions in which these feed items occur in animal rations in
national use patterns.
Metabolism studies in ruminants indicate that dialifos is
metabolized to diethyl phosphorodithioate and phthalic acid
derivatives. The metabolic pathways are similar to those of phosmet, a
closely related compound. Some dialifos per se transfers from feeds to
meat fat and to milk. At a feeding level of 45 mg/kg to lactating
cows, maximum residues approximating 0.25 mg/kg appeared in milk (fat
basis). Beef calves were fed at 2, 4 and 10 mg/kg levels. At the
highest feeding level the tissue residue levels were 0.4 mg/kg.
A poultry feeding study was also available. Birds were fed at
levels of 3.7 and 11 ppm in the total feed for up to 8 weeks.
Residues, if any, found in eggs were negligible. Trace residues
occurred in tissues.
National tolerances for dialifos in meat, milk and eggs have been
established in the U.S. to provide for residues resulting from feed
uses of citrus pulp and apple and grape pomace.
In plants
Studies available on dialifos suggest that the principal route of
degradation on plants is by hydrolysis to diethyl phosphorodithioate,
phthalic acid, phthalic acid and phthalimide. The oxon of dialifos has
been detected at trace levels but was not regarded as a significant
component of the terminal residue. This is consistent with the known
metabolic patterns of the related compound phosmet. Photolysis studies
disclosed similar degradation products after exposure to UV radiation
or sunlight. Dialifos per se is the residue of concern on crops.
Radio-tracer studies indicate only a minor tendency to translocate
from foliar deposits or to be absorbed into fruit pulp. Residues are
primarily in the peel or rind. Once residues are fixed in the peel or
on the surface of a fruit, they tend to be extremely persistent, as
shown in the decline curves (Figures 1 and 2) for citrus and apples.
In processing and cooking
No data were available on the effects of cooking. Some limited
information was provided on the reduction of residues on oranges in
commercial processing. 23% of the initial residue on oranges was
removed by washing and an additional 32% loss occurred in the rigorous
processing to dried citrus pulp. There was no evidence submitted of
occurrence in food at the time of consumption or findings of dialifos
in national "total diet" surveys.
METHODS OF RESIDUE ANALYSES
A TLC-cholinesterase inhibition method has been described as
suitable for residues of dialifos and its oxygen analogue in foods
(Mendoza 1968). However, the method of choice for regulatory purposes
would be the gas chromatographic (GC) method developed by the basic
manufacturer. The method is used with either a thermionic or flame
photometric detector and with alternative clean-up steps for analyses
of crops, meat and milk. (Pesticides Analytical Manual, Vol. II,
1967). The method has been validated in government laboratories for
regulatory purposes on crops, meat, and milk. The estimated limit of
determination is 0.01 mg/kg in citrus, 0.005 mg/kg in meat and meat
fat and 0.06 mg/kg in milk fat. The GC method has been validated only
for dialifos per se, but is said to be capable of detecting the oxygen
analogue at greatly reduced sensitivity (limit of determination about
ten times that of dialifos).
The widely used multi-residue screening method employing
acetonitrile extraction and Florisil cleanup (Pesticides Analytical
Manual, Vol. I, 1968) recovers dialifos quantitatively through the
non-fatty sample clean-up procedure, but only partially by the
procedure for fatty samples. Dialifos oxygen analogue it not detected
by either clean-up used with the PAM I method. The behaviour of
dialifos through the multi-residue method of Storherr for
organophosphorus insecticides (PAM I, Section 232.2) has not been
studied. It is known however that the closely related compound phosmet
is quantitatively recovered through the Storherr (charcoal column)
method and it is likely that dialifos would be recovered also.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table
10.
TABLE 10. National tolerances for dialifos reported to the Meeting
Pre-harvest Tolerance
Country Crop interval (mg/kg)
(days)
Germany Apples, pears 28 0.5
Netherlands " 56 0.3
Switzerland " 42 0.3
U.S.A. Citrus 7 3
Apples, pears 60 1.5
Grapes 25 1
Pecans - 0.01
Meat, fat & meat products
of cattle, goats & sheep 0.15
Milk fat 0.15
Meat, fat and meat
by-products of poultry 0.05
Eggs 0.01
* Tolerances are for the sum of dialifos and its oxygen analogue.
APPRAISAL
The acaricide/insecticide dialifos has been in use since the late
1960's on a variety of fruits, nuts, and field crops. It is reported
to be currently used in ten countries and national maximum residue
limits have been established in four of these countries.
The residue of principal concern on harvested crops is dialifos
per se. It tends to be very persistent but is not appreciably
translocated or absorbed into fruits. The oxon of dialifos has been
detected at trace levels but is not a significant residue component.
The principal route of degradation is by hydrolysis to diethyl
phosphorodithioate, and phthalic acid, phthalamic acid and
phthalimide.
The pesticide exhibits some tendency to transfer from animal
feeds to meat and milk. Data were available to show residue levels
expected in some by-product feed items such as dried citrus pulp, but
not in all. National tolerances have been established in the U.S.A.
for residues in meat, milk, and eggs to regulate residues in these
items resulting from the use of treated apple pomace, grape pomace,
and citrus pulp in animal feeds.
While there were adequate data available to support the
recommendations for the maximum residue limits given below, there was
not sufficient information on residues or national use patterns to
support recommendations for potatoes, rape, sugar beets, stone fruits,
berries or cotton.
RECOMMENDATIONS
The following maximum residue limits are recommended for the sum
of dialifos and its oxygen analogue, expressed as dialifos.
Interval on which
recommendation is
Commodity Limit, mg/kg based (days)
Apple pomace (dried) 40
Citrus pulp(dried) 15
Grape pomace (dried) 20
Citrus 3 7
Raisins 2
Apples, pears 2 60
Grapes 1 35
Fat of meat of cattle and
sheep, milk (fat basis) 0.2
Eggs, pecans 0.01*
Meat and fat of poultry 0.05*
*Level at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Metabolism studies in one or more animal species.
2. Further observations in man.
3. Additional information on national use patterns and supervised
residue trials on potatoes, rape, sugar beets and cotton.
REFERENCES
Anon Residues of dialifor when used against red
1969 spider mites on apples in Holland. 1969 Study.
Report of Central Institute for Nutrition and Food
Research, Zeist, Holland. Summarized translation
by Ir. L. Veegans of Hercules BV, The Hague,
Holland. Undated.
Anon Report No. 3968. Residues of dialifor when
1972 used against red spider mites on apples in
Holland. 1972 Study. Central Institute for
Nutrition and Food Research, Zeist, Holland.
Summarized translation by Ir. L. Veegens of
Hercules BV, The Hague, Holland. Undated.
Anon Report No. R4331. Residues of dialifor in
1974 grapes. Central Institute for Nutrition and Food
Research, Zeist, Holland, February 1974.
Arnold, D., Kodras, R., Fancher, O.E. Three generation reproduction
1968 studies in albino rats - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Baran, J., Fancher, O.E. & Calandra, J.C. 98-day sub-acute oral
1966 toxicity of AC14503 technical - Beagle dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Baran, J., Vondruska, J.F., & Fancher. O.E. Two-year chronic oral
1968 toxicity of AC14503 - Beagle dogs. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Bourke, J.B. et al. The metabolism of Torak. Report of the
1970 New York Agricultural Experiment Station, Geneva,
New York, January 1970. (Unpublished)
Brusick, D. Mutagenic evaluation of compound X19943-99-1
1975 Torak. Report from Litton Bionetics, submitted by
Hercules Incorporated. (Unpublished)
DuBois, K.P., Flynn, M., Root, M., & Su, M. Effects of Hercules
1969 AC14503 on aliesterases and cholinesterase -
weanling female Holtzman rats. Report from
Toxicity Laboratories - University of Chicago,
submitted by Hercules Incorporated. (Unpublished)
Ford, J.J. Tissue residue studies on beef calves fed a diet
1970a containing Torak. Submitted by Hercules Research
Center. (Unpublished)
Ford, J.J. Torak residues in milk - dairy cows. Submitted by
1970b Hercules Research Center. (Unpublished)
Ford, J.J. Tissue residue studies on Beagle dogs maintained
1970c for two years on Torak dosed diets. Submitted by
Hercules Research Center. (Unpublished)
Gabriel, K.L. Acute oral and acute dermal toxicity studies
1972 in rats of Hercules X15260-51-2. Report from
Biosearch, Inc., submitted by Hercules
Incorporated. (Unpublished)
Greco, R.A., Palazzolo, R.J., & Fancher, O.E. Effects of
1970 AC14503 on plasma and erythrocyte cholinesterase
and plasma aliesterase activity in human
volunteers. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Acute vapor
1969a inhalation toxicity study on Torak 47%
emulsifiable concentrate in albino rats, guinea
pigs, and mice. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher. O.E. Acute dust
1969b inhalation toxicity study on Torak 50% wettable
powder in albino rats, guinea pigs, and mice.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E Two-week subacute
1969c aerosol inhalation toxicity study on Torak
emulsified concentrate - albino rats and guinea
pigs. Report from Industrial Bio-Test Laboratories
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Two-week subacute
1970 aerosol inhalation toxicity study on Torak
emulsifiable concentrate - albino rats and guinea
pigs. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hercules "Torak" (dialifor) submitted to FAO/WHO by Hercules
1976 Incorporated, July 30, 1976.
Jackson, G.L., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966a study on technical AC14503 female Dutch belted
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Jackson, G., Kennedy, G., Fancher, O.E., Calandra, J.C. Rabbit
1966b teratogenic study AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Jackson, G., Palazzolo, R.J., & Fancher, O.E. Neuro toxicity
1968 study - chickens - AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Kennedy, G., Jackson, G.L., Fancher, O.E., & Calandra, J.C. Rabbit
1966 teratogenic study - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Ladd, R., Jenkins, D.H., Wright, P.L., & Keplinger, M.L.
1972 Teratogenic study with Norchloro-Torak in albino
rabbits. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. The in vitro
1966a inhibitory effect of AC14503 on cholinesterases -
rat plasma. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. Addendum:
1966b The in vitro inhibitory effect of oxidized
AC14503 on rat plasma and erythrocyte
cholinesterase activity. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., Keplinger, M.L.
1975 Two-year chronic oral toxicity study with Torak
technical in albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Study on the efficacy
1968 of atropine sulfate and 2-PAM C1 as antidotes for
AC14503 (technical) intoxication - male albino
rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished).
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal
1969a toxicity study on Torak (Hercules 14503) in male
albino rabbits (emulsified concentrate). Report
from Industrial Bio-Test Laboratories, submitted
by Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal toxicity
1969b study on Torak (Hercules 14503) in male albino
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day subacute
1969c dermal toxicity study on Torak emulsifiable
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day sub-acute
1970 dermal toxicity study on Torak emulsified
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mendoza, C.E., Wales, P.J., Mcleod, H.A., & McKinley, W.P.
1968 "Enzymatic detection of 10 organophosphorous
pesticides and carbaryl on thin-layer
chromatograms: An evaluation of indoxyl,
substituted indoxyl and 1-naphthyl acetates as
substrates for esterases," Analyst, London, 93:
34-38.
Nomura. Torak (C14H17O4NS2PCl) acute toxicity studies
1970 - male and female mice and rats. Report from
Kumamoto University, submitted by Hercules
Incorporated. (Unpublished)
PAM I Pesticide Analytical Manual Vol. I, 2nd Ed.
1968 revised; U.S. Department of Health, Education and
Welfare, Food and Drug Administration.
PAM II Pesticide Analytical Manual Vol. II, revised;
1967 U.S. Department of Health, Education and Welfare,
Food and Drug Administration.
Reyna, M.S., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L.
1975 1-year chronic oral toxicity study with Torak
technical in albino rats. Report from industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Robens, J.F. Teratogenic activity of several phthalimide derivatives
1970 in the golden hamster. Toxicol. Appl. Pharmacol.
16: 24-34.
Schoenig, G., & Fancher, O.E. Acute oral toxicity of recrystallized
1966a AC14503 - albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute oral toxicity
1966b of column residue from Technical AC14503 male and
female albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. 14-day subacute
1966c oral toxicity of technical AC14503 - mongrel dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966d studies on technical AC14503 - mice, albino
rabbits, dogs, and rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute potentiation
1966e of AC14503 with malathion. Report from Industrial
Bio-Test Laboratories submitted by Hercules
Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503 on
1968a beef animals. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968b on dairy cows. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968c on poultry. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972a 90-day cholinesterase study with Norchloro-Torak
in albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972b 90-day subacute oral toxicity study with
Norchloro-Torak in albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
St. John, L.E. et al. Metabolism studies with Torak insecticide
1971 in a dairy cow. Department of Entomology Pesticide
Residue Laboratory, Cornell Univ. J. agr. Fd.
Chem. 19 (5): 900-903.
Taylor, R.E. Cholinesterase and tissue residue study using
1969 Hercules 14503 on beef animals. Report from Harris
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Ueda, K. Report on acute toxicological test - rats. Report 1968
from Tokyo Dental University, submitted by
Hercules Incorporated. (Unpublished)
Vondruska, J.F., Keplinger, M.L., & Fancher, O.E. Status Summary
1968 - Evaluation of AC14503 upon primate
cholinesterase levels in stumptailed monkeys.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Vondruska, J.F. & Fancher, O.E. Teratologic study of compound
1969 AC14503 in stumptailed monkeys. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C. & Calandra. J.C. 14-day oral toxicity of AC14503
1965 - albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Wolf, C., Fancher, O.E., & Calandra, J.C. Effects of AC14503
1966a on cholinesterase activity in the albino rat.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. 90-day subacute oral
1966b toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. Effects of AC14503
1966c on cholinesterase activity on the albino rat
(Additional determinations). Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Keplinger, M.L., & Fancher, O.E. Two-year chronic oral
1968 toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Pecans
Analysis of nut meats treated according to label directions
showed no residues above the limit of detection of the method (0.005
mg/kg). Residues were found on outer husks, but there was no
penetration.
Rapeseed
Supervised field trials in W. Germany in 1972 showed only trace
residues (0.01-0.02 mg/kg) in seeds receiving multiple treatments at
the rate of 270 g/ha. Extraction was by surface stripping, a technique
which may not be effective in removing residues absorbed into an oily
seed.
Sugar beets
Field trials in Italy in 1973 showed residues averaging 0.09
mg/kg on roots and 1.17 on tops following 4 applications at 675 g/ha.
Cotton
Results from limited trials at 2 locations in the U.S.A. were
made available. The report contained a total of 4 analyses of treated
samples. Residues in the range of 0.02-0.06 mg/kg were found on
undelinted seed.
Potatoes
Field trials in the Netherlands showed only trace residues
(<0.002 to 0.004 mg/kg) in treated potatoes.
Processed products from treated raw commodities
Data were made available on some processed food and feed products
derived from the raw commodities discussed in the previous section.
The findings are summarized by commodity.
Citrus pulp
Two pilot plant studies showed an average concentration factor of
5.3 in processing oranges to dried pulp. That is, whole oranges
bearing residue levels at the national maximum residue limit of 3
mg/kg would contribute residues approximating 16 mg/kg to the
commercial cattle feed (dried citrus pulp). See also discussion on
potential transfer to meat, milk, eggs.
Grape juice and pomace
Low level residues (maximum 0.02 mg/kg) occurred in juice
expressed from grapes bearing 1 mg/kg, most of the residue remaining
in the pomace. There is about a 4 fold concentration factor between
whole grape and wet pomace.
Raisins
The residues found are summarized in Table 9.
TABLE 9. Dialifos residue in raisins
Dosage Pre-harvest Dialifos, mg/kg
rate No. of interval,
Year kg/ha applications days Processed(2) Unprocessed(1)
1968 0.6 2 62 0.26 0.45
1.1 2 62 0.84 0.91
1970 1.1 2 41 - 1.2
1971 1.1 1 58 - 1.6
1.1+2.2 2 36 - 4.3
1973 1.1 3 40 0.98 -
1973 1.1 2 40 0.76 -
1973 1.1 2 70 0.20 -
(1) Sun dried
(2) Sun dried, cleaned, fumigated, moisture adjusted
Apple pomace
U.S. data indicate a concentration factor of about 16 in
processing from whole apples to dried apple pomace, a commercial
cattle feed.
No data were available on edible oils from cottonseed or rapeseed
or the respective oilseed meals.
FATE OF RESIDUES
Potential for residues in meat, milk, poultry, and eggs
As far as could be determined by the Meeting, there are no
registered uses for dialifos on primary forage crops. There are a
number of commodities from which processed by-products or offal may be
incorporated into animal feeds. This would include dried citrus pulp
and molasses, grape pomace, apple pomace, rapeseed and cottonseed
meal, and sugar beet pulp.
There were sufficient data from controlled feeding experiments
with cattle and poultry to relate dialifos intake levels to residue
levels in milk and meat. However, there was only fragmentary
information available on residue levels in the feed items and on the
proportions in which these feed items occur in animal rations in
national use patterns.
Metabolism studies in ruminants indicate that dialifos is
metabolized to diethyl phosphorodithioate and phthalic acid
derivatives. The metabolic pathways are similar to those of phosmet, a
closely related compound. Some dialifos per se transfers from feeds to
meat fat and to milk. At a feeding level of 45 mg/kg to lactating
cows, maximum residues approximating 0.25 mg/kg appeared in milk (fat
basis). Beef calves were fed at 2, 4 and 10 mg/kg levels. At the
highest feeding level the tissue residue levels were 0.4 mg/kg.
A poultry feeding study was also available. Birds were fed at
levels of 3.7 and 11 ppm in the total feed for up to 8 weeks.
Residues, if any, found in eggs were negligible. Trace residues
occurred in tissues.
National tolerances for dialifos in meat, milk and eggs have been
established in the U.S. to provide for residues resulting from feed
uses of citrus pulp and apple and grape pomace.
In plants
Studies available on dialifos suggest that the principal route of
degradation on plants is by hydrolysis to diethyl phosphorodithioate,
phthalic acid, phthalic acid and phthalimide. The oxon of dialifos has
been detected at trace levels but was not regarded as a significant
component of the terminal residue. This is consistent with the known
metabolic patterns of the related compound phosmet. Photolysis studies
disclosed similar degradation products after exposure to UV radiation
or sunlight. Dialifos per se is the residue of concern on crops.
Radio-tracer studies indicate only a minor tendency to translocate
from foliar deposits or to be absorbed into fruit pulp. Residues are
primarily in the peel or rind. Once residues are fixed in the peel or
on the surface of a fruit, they tend to be extremely persistent, as
shown in the decline curves (Figures 1 and 2) for citrus and apples.
In processing and cooking
No data were available on the effects of cooking. Some limited
information was provided on the reduction of residues on oranges in
commercial processing. 23% of the initial residue on oranges was
removed by washing and an additional 32% loss occurred in the rigorous
processing to dried citrus pulp. There was no evidence submitted of
occurrence in food at the time of consumption or findings of dialifos
in national "total diet" surveys.
METHODS OF RESIDUE ANALYSES
A TLC-cholinesterase inhibition method has been described as
suitable for residues of dialifos and its oxygen analogue in foods
(Mendoza 1968). However, the method of choice for regulatory purposes
would be the gas chromatographic (GC) method developed by the basic
manufacturer. The method is used with either a thermionic or flame
photometric detector and with alternative clean-up steps for analyses
of crops, meat and milk. (Pesticides Analytical Manual, Vol. II,
1967). The method has been validated in government laboratories for
regulatory purposes on crops, meat, and milk. The estimated limit of
determination is 0.01 mg/kg in citrus, 0.005 mg/kg in meat and meat
fat and 0.06 mg/kg in milk fat. The GC method has been validated only
for dialifos per se, but is said to be capable of detecting the oxygen
analogue at greatly reduced sensitivity (limit of determination about
ten times that of dialifos).
The widely used multi-residue screening method employing
acetonitrile extraction and Florisil cleanup (Pesticides Analytical
Manual, Vol. I, 1968) recovers dialifos quantitatively through the
non-fatty sample clean-up procedure, but only partially by the
procedure for fatty samples. Dialifos oxygen analogue it not detected
by either clean-up used with the PAM I method. The behaviour of
dialifos through the multi-residue method of Storherr for
organophosphorus insecticides (PAM I, Section 232.2) has not been
studied. It is known however that the closely related compound phosmet
is quantitatively recovered through the Storherr (charcoal column)
method and it is likely that dialifos would be recovered also.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the Meeting are shown in Table
10.
TABLE 10. National tolerances for dialifos reported to the Meeting
Pre-harvest Tolerance
Country Crop interval (mg/kg)
(days)
Germany Apples, pears 28 0.5
Netherlands " 56 0.3
Switzerland " 42 0.3
U.S.A. Citrus 7 3
Apples, pears 60 1.5
Grapes 25 1
Pecans - 0.01
Meat, fat & meat products
of cattle, goats & sheep 0.15
Milk fat 0.15
Meat, fat and meat
by-products of poultry 0.05
Eggs 0.01
* Tolerances are for the sum of dialifos and its oxygen analogue.
APPRAISAL
The acaricide/insecticide dialifos has been in use since the late
1960's on a variety of fruits, nuts, and field crops. It is reported
to be currently used in ten countries and national maximum residue
limits have been established in four of these countries.
The residue of principal concern on harvested crops is dialifos
per se. It tends to be very persistent but is not appreciably
translocated or absorbed into fruits. The oxon of dialifos has been
detected at trace levels but is not a significant residue component.
The principal route of degradation is by hydrolysis to diethyl
phosphorodithioate, and phthalic acid, phthalamic acid and
phthalimide.
The pesticide exhibits some tendency to transfer from animal
feeds to meat and milk. Data were available to show residue levels
expected in some by-product feed items such as dried citrus pulp, but
not in all. National tolerances have been established in the U.S.A.
for residues in meat, milk, and eggs to regulate residues in these
items resulting from the use of treated apple pomace, grape pomace,
and citrus pulp in animal feeds.
While there were adequate data available to support the
recommendations for the maximum residue limits given below, there was
not sufficient information on residues or national use patterns to
support recommendations for potatoes, rape, sugar beets, stone fruits,
berries or cotton.
RECOMMENDATIONS
The following maximum residue limits are recommended for the sum
of dialifos and its oxygen analogue, expressed as dialifos.
Interval on which
recommendation is
Commodity Limit, mg/kg based (days)
Apple pomace (dried) 40
Citrus pulp(dried) 15
Grape pomace (dried) 20
Citrus 3 7
Raisins 2
Apples, pears 2 60
Grapes 1 35
Fat of meat of cattle and
sheep, milk (fat basis) 0.2
Eggs, pecans 0.01*
Meat and fat of poultry 0.05*
*Level at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Metabolism studies in one or more animal species.
2. Further observations in man.
3. Additional information on national use patterns and supervised
residue trials on potatoes, rape, sugar beets and cotton.
REFERENCES
Anon Residues of dialifor when used against red
1969 spider mites on apples in Holland. 1969 Study.
Report of Central Institute for Nutrition and Food
Research, Zeist, Holland. Summarized translation
by Ir. L. Veegans of Hercules BV, The Hague,
Holland. Undated.
Anon Report No. 3968. Residues of dialifor when
1972 used against red spider mites on apples in
Holland. 1972 Study. Central Institute for
Nutrition and Food Research, Zeist, Holland.
Summarized translation by Ir. L. Veegens of
Hercules BV, The Hague, Holland. Undated.
Anon Report No. R4331. Residues of dialifor in
1974 grapes. Central Institute for Nutrition and Food
Research, Zeist, Holland, February 1974.
Arnold, D., Kodras, R., Fancher, O.E. Three generation reproduction
1968 studies in albino rats - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Baran, J., Fancher, O.E. & Calandra, J.C. 98-day sub-acute oral
1966 toxicity of AC14503 technical - Beagle dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Baran, J., Vondruska, J.F., & Fancher. O.E. Two-year chronic oral
1968 toxicity of AC14503 - Beagle dogs. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Bourke, J.B. et al. The metabolism of Torak. Report of the
1970 New York Agricultural Experiment Station, Geneva,
New York, January 1970. (Unpublished)
Brusick, D. Mutagenic evaluation of compound X19943-99-1
1975 Torak. Report from Litton Bionetics, submitted by
Hercules Incorporated. (Unpublished)
DuBois, K.P., Flynn, M., Root, M., & Su, M. Effects of Hercules
1969 AC14503 on aliesterases and cholinesterase -
weanling female Holtzman rats. Report from
Toxicity Laboratories - University of Chicago,
submitted by Hercules Incorporated. (Unpublished)
Ford, J.J. Tissue residue studies on beef calves fed a diet
1970a containing Torak. Submitted by Hercules Research
Center. (Unpublished)
Ford, J.J. Torak residues in milk - dairy cows. Submitted by
1970b Hercules Research Center. (Unpublished)
Ford, J.J. Tissue residue studies on Beagle dogs maintained
1970c for two years on Torak dosed diets. Submitted by
Hercules Research Center. (Unpublished)
Gabriel, K.L. Acute oral and acute dermal toxicity studies
1972 in rats of Hercules X15260-51-2. Report from
Biosearch, Inc., submitted by Hercules
Incorporated. (Unpublished)
Greco, R.A., Palazzolo, R.J., & Fancher, O.E. Effects of
1970 AC14503 on plasma and erythrocyte cholinesterase
and plasma aliesterase activity in human
volunteers. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Acute vapor
1969a inhalation toxicity study on Torak 47%
emulsifiable concentrate in albino rats, guinea
pigs, and mice. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher. O.E. Acute dust
1969b inhalation toxicity study on Torak 50% wettable
powder in albino rats, guinea pigs, and mice.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E Two-week subacute
1969c aerosol inhalation toxicity study on Torak
emulsified concentrate - albino rats and guinea
pigs. Report from Industrial Bio-Test Laboratories
submitted by Hercules Incorporated. (Unpublished)
Hathaway, D., Keplinger, M.L., & Fancher, O.E. Two-week subacute
1970 aerosol inhalation toxicity study on Torak
emulsifiable concentrate - albino rats and guinea
pigs. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Hercules "Torak" (dialifor) submitted to FAO/WHO by Hercules
1976 Incorporated, July 30, 1976.
Jackson, G.L., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966a study on technical AC14503 female Dutch belted
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Jackson, G., Kennedy, G., Fancher, O.E., Calandra, J.C. Rabbit
1966b teratogenic study AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Jackson, G., Palazzolo, R.J., & Fancher, O.E. Neuro toxicity
1968 study - chickens - AC14503. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Kennedy, G., Jackson, G.L., Fancher, O.E., & Calandra, J.C. Rabbit
1966 teratogenic study - AC14503. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Ladd, R., Jenkins, D.H., Wright, P.L., & Keplinger, M.L.
1972 Teratogenic study with Norchloro-Torak in albino
rabbits. Report from Industrial Bio Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. The in vitro
1966a inhibitory effect of AC14503 on cholinesterases -
rat plasma. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Lazanas, J.C., Fancher, O.E., & Calandra, J.C. Addendum:
1966b The in vitro inhibitory effect of oxidized
AC14503 on rat plasma and erythrocyte
cholinesterase activity. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., Keplinger, M.L.
1975 Two-year chronic oral toxicity study with Torak
technical in albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Study on the efficacy
1968 of atropine sulfate and 2-PAM C1 as antidotes for
AC14503 (technical) intoxication - male albino
rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished).
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal
1969a toxicity study on Torak (Hercules 14503) in male
albino rabbits (emulsified concentrate). Report
from Industrial Bio-Test Laboratories, submitted
by Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. Acute dermal toxicity
1969b study on Torak (Hercules 14503) in male albino
rabbits. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day subacute
1969c dermal toxicity study on Torak emulsifiable
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mastri, C., Keplinger, M.L., & Fancher, O.E. 30-day sub-acute
1970 dermal toxicity study on Torak emulsified
concentrate in albino rabbits. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Mendoza, C.E., Wales, P.J., Mcleod, H.A., & McKinley, W.P.
1968 "Enzymatic detection of 10 organophosphorous
pesticides and carbaryl on thin-layer
chromatograms: An evaluation of indoxyl,
substituted indoxyl and 1-naphthyl acetates as
substrates for esterases," Analyst, London, 93:
34-38.
Nomura. Torak (C14H17O4NS2PCl) acute toxicity studies
1970 - male and female mice and rats. Report from
Kumamoto University, submitted by Hercules
Incorporated. (Unpublished)
PAM I Pesticide Analytical Manual Vol. I, 2nd Ed.
1968 revised; U.S. Department of Health, Education and
Welfare, Food and Drug Administration.
PAM II Pesticide Analytical Manual Vol. II, revised;
1967 U.S. Department of Health, Education and Welfare,
Food and Drug Administration.
Reyna, M.S., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L.
1975 1-year chronic oral toxicity study with Torak
technical in albino rats. Report from industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Robens, J.F. Teratogenic activity of several phthalimide derivatives
1970 in the golden hamster. Toxicol. Appl. Pharmacol.
16: 24-34.
Schoenig, G., & Fancher, O.E. Acute oral toxicity of recrystallized
1966a AC14503 - albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute oral toxicity
1966b of column residue from Technical AC14503 male and
female albino rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. 14-day subacute
1966c oral toxicity of technical AC14503 - mongrel dogs.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute toxicity
1966d studies on technical AC14503 - mice, albino
rabbits, dogs, and rats. Report from Industrial
Bio-Test Laboratories, submitted by Hercules
Incorporated. (Unpublished)
Schoenig, G., Fancher, O.E., & Calandra, J.C. Acute potentiation
1966e of AC14503 with malathion. Report from Industrial
Bio-Test Laboratories submitted by Hercules
Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503 on
1968a beef animals. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968b on dairy cows. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Sedivy, W.E. Study on the effects of Hercules compound 14503
1968c on poultry. Report from Harris Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972a 90-day cholinesterase study with Norchloro-Torak
in albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Smith, P.S., Reyna, M.S., Kennedy, G.L., & Keplinger, M.L.
1972b 90-day subacute oral toxicity study with
Norchloro-Torak in albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
St. John, L.E. et al. Metabolism studies with Torak insecticide
1971 in a dairy cow. Department of Entomology Pesticide
Residue Laboratory, Cornell Univ. J. agr. Fd.
Chem. 19 (5): 900-903.
Taylor, R.E. Cholinesterase and tissue residue study using
1969 Hercules 14503 on beef animals. Report from Harris
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Ueda, K. Report on acute toxicological test - rats. Report 1968
from Tokyo Dental University, submitted by
Hercules Incorporated. (Unpublished)
Vondruska, J.F., Keplinger, M.L., & Fancher, O.E. Status Summary
1968 - Evaluation of AC14503 upon primate
cholinesterase levels in stumptailed monkeys.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Vondruska, J.F. & Fancher, O.E. Teratologic study of compound
1969 AC14503 in stumptailed monkeys. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C. & Calandra. J.C. 14-day oral toxicity of AC14503
1965 - albino rats. Report from Industrial Bio-Test
Laboratories, submitted by Hercules Incorporated.
(Unpublished)
Wolf, C., Fancher, O.E., & Calandra, J.C. Effects of AC14503
1966a on cholinesterase activity in the albino rat.
Report from Industrial Bio-Test Laboratories,
submitted by Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. 90-day subacute oral
1966b toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Fancher, O.E. & Calandra, J.C. Effects of AC14503
1966c on cholinesterase activity on the albino rat
(Additional determinations). Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
Wolf, C., Keplinger, M.L., & Fancher, O.E. Two-year chronic oral
1968 toxicity of AC14503 - albino rats. Report from
Industrial Bio-Test Laboratories, submitted by
Hercules Incorporated. (Unpublished)
