
EDIFENPHOS JMPR 1976
IDENTITY
Chemical name
O-ethyl SS-diphenyl phosphorodithioate
Synonyms
EDDP, SRA 7847, Bayer 78418, Hinosan(R)
Structural formula
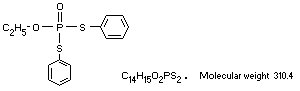 C14H15O2PS2. Molecular weight 310.4
Other information on identity and properties
Physical and chemical properties
Physical state: the technical product has a yellow to
light brown colour.
Boiling point: 154° at 0.01 mm Hg.
Specific gravity: 1.23 at 20°/4°C.
Vapour pressure: 10-4 to 10-2 mm Hg at 20-100°C.
Solubility: insoluble in water, soluble in acetone
and xylene.
Stability: half-life values: 49 hours at 25°C and
pH 9; 1135 hours at 25°C and pH 7.
Composition of the technical product
The technical product contains a minimum of 87% of edifenphos.
The main impurities are:
SSS-triphenyl phosphorotrithioate max 7%
OO-diethyl S-phenyl phosphorothioate max 3.5%
diphenyl sulphide max 2.5%
toluene max 1.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Edifenphos is rapidly absorbed following acute oral
administration to rats. Within 8 hours of dosing the major part of
an 35S-dose was absorbed. Within 72 hours of the administered dose,
96% in rats and > 97% in mice was excreted in the urine and feces.
There were no significant sex differences noted in rats, while in
mice females excreted somewhat more in the feces than corresponding
males. Apart from this no sex differences in absorption and
excretion were noted. Tissue residues (35S-labelled) in both rats
and mice were extremely low at 72 hours. Residues in tissues
following acute or sub-acute (10-daily) doses were generally low
and associated with tissues concerned with distribution and
excretion. Of the 6 tissues examined at various intervals after
dosing (from 1 to 72 hours) the following sequence reflects tissue
distribution: liver > kidney > lung > heart >> blood = brain.
This same qualitative relationship of tissue distribution was
reflected over the time for maximum tissue clearance (Ueyema, et
al., 1976).
Absorption of a single oral dose of edifenphos (0.12 mg/kg
b.w.) in a lactating cow was slow than seen with the rat, mouse,
dog and even lactating goat. In the cow, the blood residue reached
a maximum 20 hours after a single oral dose. Thirty percent of the
administered dose was excreted during the first day after dosing
and another 30% during the following 4 days. Twenty seven percent
of the dose was excreted in the feces in 5 days and < 3% was
observed as a residue in milk (not above 0.09 mg/kg edifenphos
equivalents at any time). In a study where a cow was administered
5 consecutive oral daily doses of edifenphos (0.12 mg/kg. b.w.),
blood levels of edifenphos (expressed as equivalents) increased from
0.05 mg/kg after the first application to 0.2 ppm after the fifth.
A residue plateau was not observed during this time. In urine and
milk, the increase of total residues was less pronounced and seemed
to level off after the third application at a concentration of 4 and
0.18 mg/kg respectively (Pither and Gronberg, 1975). Residues in the
tissues at slaughter, 20 hours after the last dose, ranged from 0.13 to
0.67 mg/kg edifenphos equivalents.
Biotransformation
Qualitative determination of the fate of edifenphos in rats
and dogs was reported following a single oral dose. No edifenphos
was observed in urine but rather the hydrolysis products were
observed both free and conjugated. A difference was observed
between male and female rats where two unidentified products were
observed in female urine that were not seen in males. (Eben and
Kimmerle, 1972). In contrast, no such differences with respect to
sex were noted in another study using a 35S-labelled product. In
rats, dogs, mice and goats, a similar pattern of metabolic
breakdown was reported. The metabolic pathway followed in animals,
rice plant and the rice blast fungus, together with photochemical
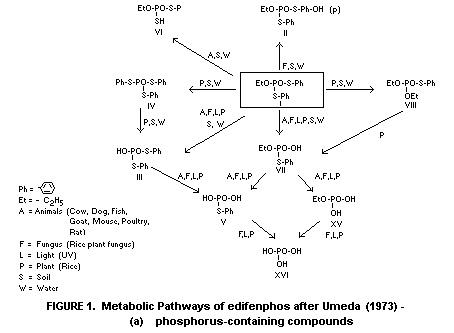
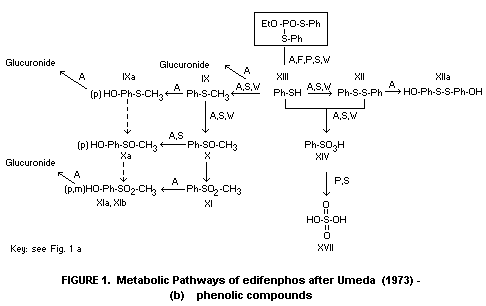
and weathering reactions, are illustrated in Figures 1(a) and 1(b).
All radioactivity in the urine of rats and mice consisted of
conjugated or water soluble metabolites. The major metabolite in
rats was the des-S-phenyl hydrolysis product while in mice the
mono-S-phenyl phosphorothioate was prevalent (further dealkylation
of the major rat metabolite). In feces, small (< 2%) quantities of
edifenphos were noted as well as diphenyl disulfide (Ueyama et al.,
1976).
Metabolism in other animals, plants and fungi is discussed in
the section "Fate of residues".
Effects on Enzymes and Other Biochemical Parameters
Rabbits were orally administered edifenphos and liver function
tests performed at various times (up to 7 days) after poisoning. No
effects were noted on SGPT or SDH activity or BSP retention
following a dose of 50 mg/kg, while there were effects reported at
100 mg/kg (Kimmerle and Lorke, 1967).
A single oral application of edifenphos to rats (150 mg/kg)
increased liver weight 24 hours after the treatment. While no
changes were observed in body weight, relative liver weight changes
were observed at lower doses (> 25 mg/kg) within 24 hours. One
week after treatment the highest treatment group still showed an
increased relative liver weight while other groups appeared to have
recovered. In this study, changes in liver size were not reflected
in changes in certain liver function tests (SGOT activity, SGPT
activity, Cholesterol content and glucuronidase activity). Slight
changes were noted at 24 hours in the A/G ratio, S-protein content,
certain drug metabolizing enzyme assays (EPN detoxication but not
aniline hydroxylation or aminopyrine demethylation) and, as expected,
cholinesterase activity. Within 7 days these liver changes had
recovered and values were normal. Gross and microscopic examination
did not suggest a pathological condition.
Cholinesterase depression measured at 24 hours after treatment
was dose dependent and sensitivity was as follows: RBC>plasma>
brain. At 7 days after treatment, recovery of plasma cholinesterase
was complete while RBC and brain continued to show a reduction at
the higher dose levels (Anonymous, 1976b).
Acute signs of poisoning reflect acute depression of
cholinesterase activity. Within 3 hours of following oral
intubation of edifenphos (25-50 mg/kg - male rats) significant
depression of whole blood cholinesterase was observed. After a dose
of 10 mg/kg the enzyme activity was not depressed. At higher levels
the effects were persistent, lasting for three days. All enzyme
activity was normal 7 days following acute exposure (Kimmerle /
Lorke, 1967).
In vitro studies with rat brain also demonstrated the
sensitivity of this cholinesterase source to edifenphos with an I50
of 1.05 x 10-6 being noted. In vivo inhibition following
poisoning was rapid and the duration of recovery was long with full
recovery not seen until 2-3 weeks after treatment. While there was
an obvious sex difference seen in acute toxicity, the recovery of
cholinesterase activity did not reflect a sex difference. The rate
of recovery of cholinesterase (brain, serum or submaxillary gland)
following an LD50 dose was the same in both males and females.
In addition to its effects on acetylcholinesterase, edifenphos
was found to be an effective inhibitor of aliesterase activity.
Dietary administration of edifenphos at levels resulting in 50%
inhibition of liver and serum aliesterase activity in rats are seen
in Table 1. A dietary level of 5 ppm for one week reduced esterase
activity (those hydrolyzing tributyrin) by 50% in the liver.
TABLE 1. Dietary Levels (ppm) Inducing-50% Aliesterase
Inhibition in Rats
Substrate Male Female
Liver Serum Liver Serum
Tributyrin 4.9 8.4 5.4 9.5
Diethylsuccinate 18.4 10.2 11.5 7.4
(Chen, et al. 1972)
C14H15O2PS2. Molecular weight 310.4
Other information on identity and properties
Physical and chemical properties
Physical state: the technical product has a yellow to
light brown colour.
Boiling point: 154° at 0.01 mm Hg.
Specific gravity: 1.23 at 20°/4°C.
Vapour pressure: 10-4 to 10-2 mm Hg at 20-100°C.
Solubility: insoluble in water, soluble in acetone
and xylene.
Stability: half-life values: 49 hours at 25°C and
pH 9; 1135 hours at 25°C and pH 7.
Composition of the technical product
The technical product contains a minimum of 87% of edifenphos.
The main impurities are:
SSS-triphenyl phosphorotrithioate max 7%
OO-diethyl S-phenyl phosphorothioate max 3.5%
diphenyl sulphide max 2.5%
toluene max 1.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Edifenphos is rapidly absorbed following acute oral
administration to rats. Within 8 hours of dosing the major part of
an 35S-dose was absorbed. Within 72 hours of the administered dose,
96% in rats and > 97% in mice was excreted in the urine and feces.
There were no significant sex differences noted in rats, while in
mice females excreted somewhat more in the feces than corresponding
males. Apart from this no sex differences in absorption and
excretion were noted. Tissue residues (35S-labelled) in both rats
and mice were extremely low at 72 hours. Residues in tissues
following acute or sub-acute (10-daily) doses were generally low
and associated with tissues concerned with distribution and
excretion. Of the 6 tissues examined at various intervals after
dosing (from 1 to 72 hours) the following sequence reflects tissue
distribution: liver > kidney > lung > heart >> blood = brain.
This same qualitative relationship of tissue distribution was
reflected over the time for maximum tissue clearance (Ueyema, et
al., 1976).
Absorption of a single oral dose of edifenphos (0.12 mg/kg
b.w.) in a lactating cow was slow than seen with the rat, mouse,
dog and even lactating goat. In the cow, the blood residue reached
a maximum 20 hours after a single oral dose. Thirty percent of the
administered dose was excreted during the first day after dosing
and another 30% during the following 4 days. Twenty seven percent
of the dose was excreted in the feces in 5 days and < 3% was
observed as a residue in milk (not above 0.09 mg/kg edifenphos
equivalents at any time). In a study where a cow was administered
5 consecutive oral daily doses of edifenphos (0.12 mg/kg. b.w.),
blood levels of edifenphos (expressed as equivalents) increased from
0.05 mg/kg after the first application to 0.2 ppm after the fifth.
A residue plateau was not observed during this time. In urine and
milk, the increase of total residues was less pronounced and seemed
to level off after the third application at a concentration of 4 and
0.18 mg/kg respectively (Pither and Gronberg, 1975). Residues in the
tissues at slaughter, 20 hours after the last dose, ranged from 0.13 to
0.67 mg/kg edifenphos equivalents.
Biotransformation
Qualitative determination of the fate of edifenphos in rats
and dogs was reported following a single oral dose. No edifenphos
was observed in urine but rather the hydrolysis products were
observed both free and conjugated. A difference was observed
between male and female rats where two unidentified products were
observed in female urine that were not seen in males. (Eben and
Kimmerle, 1972). In contrast, no such differences with respect to
sex were noted in another study using a 35S-labelled product. In
rats, dogs, mice and goats, a similar pattern of metabolic
breakdown was reported. The metabolic pathway followed in animals,
rice plant and the rice blast fungus, together with photochemical
and weathering reactions, are illustrated in Figures 1(a) and 1(b).
All radioactivity in the urine of rats and mice consisted of
conjugated or water soluble metabolites. The major metabolite in
rats was the des-S-phenyl hydrolysis product while in mice the
mono-S-phenyl phosphorothioate was prevalent (further dealkylation
of the major rat metabolite). In feces, small (< 2%) quantities of
edifenphos were noted as well as diphenyl disulfide (Ueyama et al.,
1976).
Metabolism in other animals, plants and fungi is discussed in
the section "Fate of residues".
Effects on Enzymes and Other Biochemical Parameters
Rabbits were orally administered edifenphos and liver function
tests performed at various times (up to 7 days) after poisoning. No
effects were noted on SGPT or SDH activity or BSP retention
following a dose of 50 mg/kg, while there were effects reported at
100 mg/kg (Kimmerle and Lorke, 1967).
A single oral application of edifenphos to rats (150 mg/kg)
increased liver weight 24 hours after the treatment. While no
changes were observed in body weight, relative liver weight changes
were observed at lower doses (> 25 mg/kg) within 24 hours. One
week after treatment the highest treatment group still showed an
increased relative liver weight while other groups appeared to have
recovered. In this study, changes in liver size were not reflected
in changes in certain liver function tests (SGOT activity, SGPT
activity, Cholesterol content and glucuronidase activity). Slight
changes were noted at 24 hours in the A/G ratio, S-protein content,
certain drug metabolizing enzyme assays (EPN detoxication but not
aniline hydroxylation or aminopyrine demethylation) and, as expected,
cholinesterase activity. Within 7 days these liver changes had
recovered and values were normal. Gross and microscopic examination
did not suggest a pathological condition.
Cholinesterase depression measured at 24 hours after treatment
was dose dependent and sensitivity was as follows: RBC>plasma>
brain. At 7 days after treatment, recovery of plasma cholinesterase
was complete while RBC and brain continued to show a reduction at
the higher dose levels (Anonymous, 1976b).
Acute signs of poisoning reflect acute depression of
cholinesterase activity. Within 3 hours of following oral
intubation of edifenphos (25-50 mg/kg - male rats) significant
depression of whole blood cholinesterase was observed. After a dose
of 10 mg/kg the enzyme activity was not depressed. At higher levels
the effects were persistent, lasting for three days. All enzyme
activity was normal 7 days following acute exposure (Kimmerle /
Lorke, 1967).
In vitro studies with rat brain also demonstrated the
sensitivity of this cholinesterase source to edifenphos with an I50
of 1.05 x 10-6 being noted. In vivo inhibition following
poisoning was rapid and the duration of recovery was long with full
recovery not seen until 2-3 weeks after treatment. While there was
an obvious sex difference seen in acute toxicity, the recovery of
cholinesterase activity did not reflect a sex difference. The rate
of recovery of cholinesterase (brain, serum or submaxillary gland)
following an LD50 dose was the same in both males and females.
In addition to its effects on acetylcholinesterase, edifenphos
was found to be an effective inhibitor of aliesterase activity.
Dietary administration of edifenphos at levels resulting in 50%
inhibition of liver and serum aliesterase activity in rats are seen
in Table 1. A dietary level of 5 ppm for one week reduced esterase
activity (those hydrolyzing tributyrin) by 50% in the liver.
TABLE 1. Dietary Levels (ppm) Inducing-50% Aliesterase
Inhibition in Rats
Substrate Male Female
Liver Serum Liver Serum
Tributyrin 4.9 8.4 5.4 9.5
Diethylsuccinate 18.4 10.2 11.5 7.4
(Chen, et al. 1972)
 Cholinesterase depression in rats was observed following acute
inhalation exposure to aerosolized edifenphos. Plasma and RBC
cholinesterase depression was seen following exposure to 30 mg/m3
for 4 hours (threshold level). No depression was noted at an
exposure concentration of 9 mg/m3 for 4 hours (Kimmerle, 1975b).
TOXICOLOGICAL STUDIES
Special Studies on Teratology
Groups of rats (20 mated females/group) were administered
edifenphos by oral gavage at doses of 0, 5, 15 and 30 mg/kg body
weight from day 6 to 15 of gestation. All animals were sacrificed
and pups delivered by Caesarean Section. Increased mortality
reduced growth and an unhealthy appearance were observed at the two
highest dose levels. At the highest dose level, 3 litters were
resorbed. There were no abortions and with the exception of the 3
resorbed litters, the numbers of implantations, fetuses and
resorptions did not differ from control values. The weights of
fetuses were not different from controls and the nature and
frequency of somatic and skeletal variations were within normal
limits. There were no malformations and no indications of a
teratogenic potential was noted in this study (Lorke, 1971).
Groups of rabbits (11-13 mated does/group) were administered
edifenphos by oral gavage at dose levels of 0, 3, 6 and 12 mg/kg
body weight from day 6 to 18 of gestation. On day 29 of gestation,
the does were sacrificed and Caesarean Section performed. There was
no mortality although the two highest dose levels resulted in
reduced growth (the highest level treatment showed a significant
weight loss). In the 6 mg/kg group one doe aborted and one resorbed
all fetuses. Implantation recorded for the two highest doses was
reduced. However, the significance of this was not apparent as
treatment did not begin until 6 days after fertilization and
edifenphos treatment should not have affected this parameter. There
were no differences in the number of fetuses in the 0 and 3 mg/kg
dose while the upper two doses had fewer fetuses. The weight of
fetuses and placenta of the treated group did not differ from
control values. Malformations were not observed on gross or
skeletal examination of fetuses. As suggested with rats, edifenphos
did not induce a teratological response in rabbits (Machemer,
1976).
Special Studies on Reproduction
Groups of rats (10 males and 20 females/group) were fed
edifenphos in the diet at concentrations of 0, 5, 15 and 150 ppm
and mated to begin a standard 3 generation, 2 litter per
generation, reproduction study. Reproductive indices included:
fertility, gestation, viability and lactation measured for all 6
litters. There was no mortality observed and the highest dietary
concentration did not affect fertility. Litter size was reduced and
in one instance the litter weight was reduced at the 150 ppm level.
Slight effects (not statistically significant) were noted on
viability and lactation indices in the high dose group. Histological
examination of the F3b generation gave no indication of adverse
effects on the major tissues and organs. A no effect-level in this
study would be 15 ppm (Loser, 1976).
Groups of rats (20 females/group) were fed edifenphos in the
diet at dosage levels of 0, 5, 15 and 50 ppm, mated and subjected
to a standard 3 generation, 2 litters per generation, reproduction
study, In combination with the study, additional groups of 5
females from the P and F1b and 10 females from the F2b were
sacrificed at day 20 of gestation for an examination of fetuses for
any teratological events. Additionally, offspring of 5 female rats
from individual groups of the F1b, F2b and 10 females from the P3b
were maintained after weaning at the same dietary concentrations of
edifenphos listed above for periods ranging from 1 to 6 months.
These short term studies were designed to evaluate growth and
development of rats exposed initially in utero and thereafter for
short periods to dietary levels of edifenphos.
Growth of parental generations was unaffected by the presence
of edifenphos in the diet. Slight reduction of male food intake and
body weight at 50 ppm was noted in the first parental generation.
The reduced food consumption was observed in the F2 and F3 parents
accompanied by slight reduction of growth. The reduced growth was
predominant in those animals fed after weaning for prolonged
periods.
There were no significant differences noted in the
reproduction indices over the course of the three generations.
Edifenphos did not affect the ability of the rats to reproduce,
maintain pregnancy, deliver, nurse and wean normal sized litters
over the course of three generations. A teratological examination
of fetuses delivered by Caesarean Section prior to term did not
show any abnormalities related to somatic or skeletal defects. In
the first generation the 50 ppm dosed group reflected a slight
retardation in the degree of bone ossification not always noted in
the two lower doses or the controls. There were no significant
differences in implants, resorptions, live fetuses or in the weight
of fetuses.
In those weanlings from the F1b, F2b and F3b litters that were
maintained on diets containing edifenphos for 1 to 6 months after
weaning, growth was not substantially affected. Slight reductions
of growth were noted at 50 ppm accompanied by reduced food intake.
Hematological examinations made at the conclusion of each time
interval were normal. Liver function tests including SGOT and SGPT
activity and cholinesterase determinations using plasma, RBC and
brain were normal. Gross and microscopic examination of tissues and
organs of the animals maintained after weaning showed only on
consistent event attributable to edifenphos in the diet. An
enlarged kidney in the 50 ppm group fed for 3-6 months was
accompanied by changes (saccular necrosis) in the tubular
epithelium noted on microscopic examination. No dose related
changes were noted in a variety of tissues and organs that could be
attributable to edifenphos in the diet (Anonymous, 1976a).
Special Study on Mutagenicity
Mice
A dominant lethal study using male mice was performed with
edifenphos. Groups of mice (12 males/group) were administered
edifenphos by a single intraperitoneal injection at doses of 0, 50
and 100 mg/kg. A positive control (MMS, methyl methansulfonate, 100
mg/kg) was included in the study. The males were mated with three
untreated virgin females. The females were changed weekly for 6
consecutive weeks to evaluate maturation of male mouse germ cells.
One week after breeding, the females were sacrificed and examined
for implantation sites, resorption sites and embryos.
There were no deaths as a result of treatments with
edifenphos. Except for a slight reduction in the mating index at
week 6 in the 100 mg/kg dose group, there was no effect on the
ability to mate or fertilise the females. There were no
dose-related effects noted with respect to implantation, resorption
or embryo viability. There was no increase in pre-implantation loss
or mutuation rate although the positive control showed a
significant increase in early resorptions, attesting to the species
susceptibility. Edifenphos did not demonstrate a mutagenic
potential by the dominant lethal test in mice (Arnold, et al.
1971).
Microorganisms
When examined in vitro with various strains of
microorganisms (B. subtilis, E. coli and S. typhimurium
edifenphos did not induce a mutagenic response. In two studies with
the aid of a B. subtilis strain lacking the recombination
repair system, S. typhimurium and E. coli with either a
histidine or tryptophane deficiency, edifenphos, examined for its
ability to induce mutations, was found to be negative. Positive and
negative controls were used in these assays to assure sensitivity
to the mutagenic activity and to provide a baseline of operation.
In addition, liver homogenates (fortified with cofactors necessary
for microsomal oxidation) were used to activate, if possible,
edifenphos to an active mutagen. In studies with reversion colonies
in combination with a microsomal activation system, edifenphos did
not induce mutations (Shirasu et al., 1976a; 1976b; Yatomi, 1975).
A host mediated assay was performed where groups of 5 mice
were orally administered two doses of 0, 50 or 100 mg edifenphos/kg
(within 24 hours). After the second dose, a microbiological test
system of S. typhimurium was injected into the peritoneal
cavity, removed after 3 hours and examined for revertant colonies.
In this assay, edifenphos was negative although a positive control
of dimethylnitrosamine (50 mg/kg, oral administration) induced
reversion at a substantial rate (Shirasu. et al., 1976a).
Special Studies on Potentiation
Oral administration of equitoxic doses (LD50) of edifenphos
with fenthion or propoxur did not result in potentiation of the
acute toxicity of the combination. (Kimmerle, 1967).
A greater than additive toxicity, observed with a combination
of malathion and edifenphos, suggested a potentiating effect with
these two pesticides (Kimmerle and Lorke, 1967). Further studies on
toxic interaction of two organophosphorus pesticides were
undertaken where rats fed 0, 5 or 25 ppm edifenphos in the diet
were administered malathion by ip injection at a sublethal dose
(400 mg/kg). The increased mortality observed in the two groups fed
edifenphos suggested a significant potentiating effect on malathion
toxicity (Chen, et al., 1972).
Special Studies on Antidotes
Following acute oral administration of edifenphos to male
rats, antidotal administration (ip injection) of atropine (50
mg/kg) alone or in combination with 2-PAM or BH6 (50 or 20 mg/kg,
respectively) resulted in a slight reduction in the acute toxicity
(less than 2 fold) (Kimmerle and Lorke, 1967).
Special Studies on Neurotoxicity
Edifenphos was administered to hens by oral intubation or
intraperitoneal injection at dose levels up to 1000 mg/kg. The
surviving animals were examined for 42 days with no signs of
delayed neurotoxicity (Kimmerle and Lorke, 1967).
Groups of hens were administered edifenphos by oral intubation
at the LD50 level (547 mg/kg body weight) together with atropine,
50 mg/kg administered by ip injection. Delayed neurotoxic signs of
poisoning were not seen, but were observed in all hens treated
orally with TOCP (350 mg/kg). The hens were observed for 3 weeks
and sacrificed (Kimmerle, 1971). Histological examination of
portions of the central and peripheral nervous system of the
edifenphos-treated hens was negative with respect to axon or myelin
disruption. The TOCP-treated hens showed axon and neuron
degeneration in the spinal cord (brain and peripheral nerve were
not affected) (Spicer, 1971a).
In an effort to evaluate the relationship of delayed
neurotoxicity and copper imbalance in hens, an examination of
several organophosphates inducing a delayed neurotoxicity was made.
Edifenphos as one of the negative compounds did not induce
neurotoxicity and did not increase copper and ceruloplasmin levels
as did several of the positive neurotoxic agents (Kimmerle and
Loser, 1974).
Groups of hens (8 hens/group) were fed edifenphos in the diet
for 30 days at concentrations of 0, 100, 250, 500 and 1000 ppm.
Growth was depressed at 500 ppm and above. Whole blood
cholinesterase was depressed after 30 days only in the highest dose
group. Animals allowed to recover on control diets for a 4 week
period recovered body weight and cholinesterase depression was
reduced. Neurotoxic clinical signs were not observed (Kimmerle,
1969). Histological examination of brain, spinal cord and
peripheral nerve showed no evidence of myelin degeneration (Spicer,
1971b).
Acute Toxicity
TABLE 2. Acute toxicity of edifenphos
LD50
Species Sex Route (mg/kg) Reference
Rat M oral 119-340 Kimmerle, 1967;
Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 48-81 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974;
Chen, et al., 1972
dermal
(24 hour exposure) 184-413 Kimmerle, 1972a Thyssen &
Kimmerle, 1974
(4 hour exposure) 1,230 Kimmerle & Lorke, 1967
(7 hour exposure) 600 Kimmerle & Lorke, 1967
F oral 63-150 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 26-55 Thyssen & Kimmerle, 1974;
Kimmerle & Lorke, 1967;
Chen, et al., 1972
dermal
(24 hour exposure) 86-135 Kimmerle, 1972a,
Thyssen & Kimmerle, 1974
Mice M oral 392 Thyssen & Kimmerle, 1974
TABLE 2. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
Mice F oral 218-295 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Guinea Pig oral 350-400 Kimmerle & Lorke, 1967
Rabbit F oral 250-400 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Cat oral >250 Kimmerle & Lorke, 1967
Note -- In some instances the range of values is reflective of
differences in solvents used in the test. The lower values were
obtained with Lutrol while the higher values were obtained with
ethanol: propylene glycol mixtures. In all cases there appears to be a
significant sex difference with females more susceptible than males.
Signs of poisoning were typical of cholinergic stimulation (tremors,
salivation, labored respiration, etc.) appearing 2-3 hours after acute
poisoning and persisting for up to 2 days with rats and up to a week
with mice and rabbits.
Inhalation Toxicity
Acute aerosol inhalation toxicity tests (both static and dynamic
flow) were performed with groups of rats using various exposure times.
Edifenphos technical or a 50% EC formulation (Hinosan(R)) was used in
several studies. Data expressed as the technical product do not show
significant differences in toxicity between the two forms of
edifenphos. No indication of particle size distribution was given in
these data.
Dynamic tests
Results of dynamic tests are given in Table 3.
TABLE 3. Inhalation toxicity of edifenphos (dynamic tests)
Exposure LD50
Species Sex Time (mg/m3) Reference
Rat M 1 hour >898-1310 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b;
Kimmerle & Lorke, 1967
TABLE 3. (Cont'd.)
Exposure LD50
Species Sex Time (mg/m3) Reference
4 hour 362 - 775 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b.,
Kimmerle, 1975b;
Kimmerle & Lorke, 1967
F 1 hour > 1017-1046 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b
4 hour 230 - 320 Thyssen & Kimmerle, 1974;
Kimmerle, 1975b;
Kimmerle, 1972b
Static Tests
Groups of rats, mice, rabbits and guinea pigs were exposed to
static concentrations of edifenphos ranging from 108 to 398 mg/m3 for
up to 4 hours. In the 1 hour exposure, no toxicity was recorded (only
one dose level was tested) but mice exhibited toxic signs of exposure.
In the 4 hour exposure the susceptibility of rats, rabbits and mice
was the same with 7/10 rats, 2/3 rabbits and 12/20 mice dying at a
concentration of 269 mg/m3. Only 1/5 guinea pigs died at this
exposure. No deaths were reported at a concentration of 101 mg/m3 for
the 4 hour exposure (Kimmerle & Lorke, 1967).
Edifenphos did not induce a primary dermal irritation when
applied to the skin of rabbits' ears for up to 24 hours or to the
conjunctival sac of rabbits. The 50 EC formulation of Hinosan(R)
irritated both the conjunctiva and skin (1 hour exposure). The primary
irritation was attributed to the formulation ingredients and not
technical edifenphos (Kimmerle and Lorke, 1967; Thyssen and Kimmerle,
1974).
Toxicity of metabolites
Acute toxicities of edifenphos metabolites to rats are shown in
Table 4.
TABLE 4. Acute toxicity of edifenphos metabolites to rats
Compound Route LD50
(mg/kg)
O-ethyl S-phenyl oral M >500
S-(4-hydroxyphenyl)- F >1000
phosphorodithioate
SS-diphenyl oral M 1000-2000
phosphorodithioate F 1000-2000
O-ethyl S-phenyl oral M 1000-2000
phosphorothioate F 616
O-ethyl S-phenyl oral M approx. 1000
S-(3-hydroxyphenyl) F approx. 500
phosphorodithioate
(Lamb & Matzkanin, 1976)
Short-term studies
Mice
Groups of ICR mice (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. In general, growth was not affected by dietary
edifenphos. Food consumption, behaviour, physical condition and
mortality were unaffected. Plasma cholinesterase depression was noted
at 30 ppm with RBC and brain depressed only at 1000 ppm. Hematology,
urinalysis and clinical chemistry parameters were normal. On gross
examination of tissues and organs, spleen and liver enlargement was
observed at 1000 ppm. Microscopic examinations showed no abnormalities
in spleen although liver and adrenal changes were noted at 300 ppm and
above. In liver, hypertrophy of hepatocytes and deposition of
phospholipid were observed. Adrenal hypertrophy and pigment deposition
were noted at the two highest doses (Imamichi, et al., 1973b). A
no-effect level of 10 ppm was observed equivalent to 1.53 mg/kg body
weight.
Rats
Groups of rats (10 males and 10 females/group) were exposed to
edifenphos by inhalation at concentrations of 0, 4 (range of 3-15), 10
(8-12) and 27 (24-30) mg/m3 air for 12 weeks, 5 days/week for 6
hours/day. The range of particle size was 0.5 to 1.5 µ with 97% of the
particles having a mean mass diameter of 1 µ (a fully respirable
particle). There was no mortality although females exposed to 27
mg/m3 were observed to be adversely affected. Growth was normal at
all levels of exposure as were clinical chemistry, hematology and
urinalysis parameters. Cholinesterase (brain, plasma and RBC) activity
was depressed at 10 mg/m3 and above. No effects were noted at 4
mg/m3. Gross and microscopic analysis of tissues and organs were not
affected by edifenphos in the diet. Female adrenal weight was
increased at the highest dose level but no abnormalities were detected
on microscopic examination (Kimmerle, 1975; Mohr, 1975).
Groups of rats (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. There was no mortality during this study but
growth was inhibited at 1000 ppm in both males and females. In
contrast to the reduced growth, food consumption was normal at all
dose levels. Several hematological parameters were affected at 1000
ppm, namely reduced RBC count, hemoglobin content and hematocrit value
primarily in males. Liver function was reduced at 1000 ppm (SGOT), 100
ppm (SGPT) and at 30 PPM (SAP) predominantly in males, with the
reduction not as marked in females. Urinalysis parameters were
unaffected. Cholinesterase depression was noted at 300 ppm in males
(brain and RBC) and 100 ppm in females (brain and RBC), plasma was
depressed in females at 10 ppm. Gross examination revealed enlarged
liver (1000 ppm) and kidney (300 ppm). Microscopic examination of
tissues showed hypertrophy and fatty degeneration of hepatocytes at
100 ppm and above. No pathological changes were noted in liver at 30
ppm. At 100 ppm and above, kidney and adrenal changes were noted. At
high levels a toxic nephritis was observed and the kidney and adrenal
glands were hypertrophic. In the male, kidney degeneration was
prevalent while in the females the adrenal was damaged. Gross changes
noted in brain, pituitary and thyroid of males at high dose levels
were not accompanied by pathological changes (Imamichi, 1973a).
Groups of rats (15 males and 15 females/group) were fed
edifenphos in the diet at dosage levels of 0, 1, 2, 5, 10 and 150 ppm
for 3 months. Growth was affected by edifenphos and there were no
differences in behaviour, mortality or appearance. Food consumption in
the highest dose group was slightly reduced. Hematological
examinations were normal at 1 and 3 months as were liver function and
urinalysis tests measured at the same time intervals. Cholinesterase
depression was observed in the highest dose group (in both RBC and
plasma) within one week of initiation of the feeding trial. The
depression was constant and there was no indication of a cumulative
effect relating to edifenphos. Gross and microscopic analysis of
tissues and organs, performed at the conclusion of the study, showed
no dose-related gross change and no significant microscopically
observable alteration (Loser, 1972; Urwin and Newman, 1972).
Groups of rats (10 males/group) were administered edifenphos by
oral intubation, daily for 14 days at dose rates of 0, 12.5, 50 and
100 mg/kg. Mortality was not reported but a slight decrease in average
body weight was reported at 100 mg/kg. A significant increase in
absolute and relative liver weight was reported at all dose levels at
24 hours. The livers had recovered to normal size within 7 days of the
end of treatment. Cholinesterase was depressed at all levels with the
following order of sensitivity: RBC > plasma > brain. One week after
treatment RBC and brain cholinesterase activity were depressed at all
dose levels. Liver function tests were slightly affected (SGOT
reduced) but were normal within 7 days. EPN detoxication by liver
microsomes was stimulated at all dose levels (aniline hydroxylase and
aminopyrine demethylase were unaffected) and recovery was not complete
at 7 days. Microscopic examination of liver, adrenals and kidney
showed no adverse effects (Anonymous, 1976c).
Groups of rats (15 females/group) were administered edifenphos
orally (0, 5, 10, 20, 33.5 and 50 mg/kg) or by intraperitoneal
injection (0, 1.75, 3.5, 7, 10, 11.5 and 17.5 mg/kg), daily 5 weeks
per week for 60 days. Oral administration of 50 mg/kg daily resulted
in no mortality or cumulative toxicity although signs of poisoning
were seen. No signs of poisoning were evident at 10 mg/kg/day
following oral dosing. With daily ip injections, no substantial
mortality (1/15 deaths) was observed at 7 mg/kg (11/15 died at 11.5
mg/kg). Signs of poisoning were not evident at 3.5 mg/kg. These
results suggest a minimal cumulative toxicological effect (Kimmerle
and Lorke, 1967).
Dog
Groups of dogs (2 male and 2 female/group, controls had 3 of each
sex) were fed edifenphos in the diet at dosage levels of 0, 3, 10 and
30 ppm for 90 days. There was no mortality over the course of the
study and growth, food consumption, behaviour and general appearance
were normal. Clinical chemistry, hematology and urinalysis values were
normal at one and 3 months (except for a slight increase of SDH
activity at only one month in both males and females at 30 ppm). Blood
cholinesterase activity was depressed in both plasma and RBC (to the
same extent in both enzyme sources) at the highest dose. As with
rodent studies, there was no evidence of cumulative activity over the
3 month interval. Gross and microscopic examination of tissues and
organs revealed no adverse effects of edifenphos in the diet. A slight
increase in the absolute and relative weight of the spleen at 30 ppm
was not accompanied by microscopic lesions (other than minimal)
congestion and probable hemosiderin deposition) (Loser, 1969; Spicer
and Urwin, 1971).
Groups of dogs (4 male and 4 female/group) were fed edifenphos in
the diet for two years at dose levels of 0, 7, and 20 ppm. A fourth
group was fed a diet containing 60 ppm for 1 year after which the
concentration was increased to 80 ppm for 5 months and raised to 120
ppm for the remaining 7 months of study. There was no mortality and
behaviour and physical appearance were normal and unaffected by
edifenphos in the diet. Growth and food consumption was normal at the
20 ppm level although it was slightly depressed at the high dose
regime. At the high level food consumption was slow, diet rejection
was observed and body weight gain depressed. There was no effect noted
on ophthalmological examination, urinary or hematology parameters or
on reflex testing. Some liver function test parameters were abnormal
(data showed SAP increased although SGPT was normal). This change was
accompanied by an increased gross and relative liver weight noted at
the time of sacrifice. Cholinesterase activity was depressed in the
high dose group in both plasma and RBC of males and females. Brain
cholinesterase was unaffected at any dose level. Gross and microscopic
examination of tissues and organs did not suggest an adverse effect of
edifenphos fed in the diet at concentrations up to and including 120
ppm. The inductive effect noted on the liver (increased organ weight
accompanied by increased phosphatase activity) was attributed to an
adaptation reaction noted with many drugs and chemicals. Microscopic
examination of liver did not show changes attributable to the presence
of dietary edifenphos which would account for the increased liver
weight. A no-effect level was observed to be 20 ppm equivalent to a
daily intake of 0.58 mg/kg body weight (Hoffmann, 1976; Gallagher and
Practice, 1976).
Long Term Studies
Rat
Groups of rats (50 male and 50 female rats/group, 100 of each sex
were used as controls) were fed edifenphos in the diet at
concentrations of 0, 2, 5, 15 and 150 ppm for two years. Mortality was
unaffected during the two years. Growth, behaviour and physical
appearance were normal in all groups. Hematology, blood chemistry and
urinalysis parameters were normal. A slight decrease in alkaline
phosphatase at 150 ppm was considered to be within the normal range
for the animals. Cholinesterase depression was observed in plasma and
RBC of both sexes at 150 ppm. Brain cholinesterase was depressed only
in females at 150 ppm. Gross and microscopic examinations were
performed on surviving animals. No abnormal, dose-related events were
recorded in the gross examination. A slight increase in adrenal size
of males at 15 ppm and above was not reflected in changes observed
under microscopic examination. Histopathological examination of
tissues and organs revealed luminal deposits of calcium salts in the
medulary tubules of male rats fed 15 and 150 ppm diets accompanied in
the high dose group by an increased incidence of glomerulonephrosis in
males. These changes were not noted in females. A complete examination
of tumours was performed revealing no indication of carcinogenic
potential. The frequency of both benign and malignant tumors was
typical of the rat strain used in the study. A no-effect dietary
level, based on the microscopic examination observing slight kidney
changes, was 5 ppm equivalent to 0.30 mg/kg body weight (Loser, 1976;
Offer and Prentice, 1976).
COMMENTS
Edifenphos, an organophosphorus ester, is used in agriculture as
a fungicide to control rice diseases. Following oral administration it
is rapidly absorbed, distributed, metabolised and excreted in mammals.
The well defined metabolic sequence observed in plants and animals
appears to be the same. In metabolism studies and other studies in
several species of animals there were no indications of any
culminative effects.
Biochemical parameters affected by acute and subacute
administration of edifenphos include cholinesterase depression and
changes in certain liver enzyme values reflecting changes in basic
liver function. Edifenphos is not a potent cholinesterase inhibitor.
However, the recovery of depressed enzyme activity is not rapid
suggesting that regeneration of cholinesterase activity is dependent
on enzyme synthesis rather than reversal of an enzyme-inhibitor
complex. A sensitive biochemical parameter was aliesterase activity of
liver and serum with a level of 5 ppm of edifenphos in the diet
showing 50% inhibition. In special studies for teratogenic and
mutagenic potential, no effects of edifenphos were noted on several
species. At high levels, reproduction was slightly impaired but at
levels that did not affect maternal well-being reproduction parameters
were unaffected. Edifenphos appeared to potentiate the acute effects
of malathion as might be expected from the data on depression of
aliesterase activity. There is no evidence that edifenphos would
induce a delayed neurotoxic response in hens.
Edifenphos is moderately toxic on acute exposure to a variety of
mammalian species with signs of poisoning typical of
parasympathomimetic agents. The acute signs of poisoning, although
modified slightly by atropine and reactivators, were not completely
controlled.
In all cases, the acute toxicity of metabolites was greatly
reduced with respect to the acute toxicity of the parent compound. In
both short and long term studies in rats and dogs, the most sensitive
parameters affected were cholinesterase depression, liver enlargement
and deposits of kidney salts in males on long term studies. Based on
short term studies in mice and dogs and long term studies in rats a
no-effect level was suggested and a temporary ADI for man was
recommended. The dietary studies in rat (both short term and long
term) showing liver and serum aliesterase depression and kidney salt
build-up in males were used as a basis for evaluating the no-effect
level. While 5 ppm was a level causing 50% inhibition of aliesterase
activity the significance of this effect was unclear and has been
taken to suggest "exposure" rather than "toxicological effect".
The liver involvement noted in many studies precluded
consideration of a finite ADI until a specific carcinogenic study is
performed using a species susceptible to liver changes that might
relate to hepatic carcinoma. As these changes noted in this study
resemble those effects seen with other compounds including chlorinated
hydrocarbons, such studies are required. The lack of carcinogenic
potential noted in the 2 years, on a limited number of rats, was
reassuring that the compound is not a potent carcinogen in this
species. However, it was considered in the 2 year rat study that the
highest dose level and the small group size were not sufficient to
fully evaluate the potential for carcinogenicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm in the diet equivalent to 1.53 mg/kg bw
Dog: 20 ppm in the diet equivalent to 0.58 mg/kg bw
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg bw
Estimate of temporary acceptable daily intake for man
0 - 0.003 mp/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Edifenphos is an organophosphorus compound with a fairly
selective action against rice blast caused by Piricularia oryzae,
one of the major rice diseases. It has both a protective and curative
action against the fungus attack. There is some evidence that the
compound interferes with chitin formation in the fungal cell wall, a
process which dose not occur in higher plants. This mode of action may
therefore explain the selective action of the fungicide.
The compound is also effective against a few other fungus
diseases of rice, e.g. ear blight caused by Cochliobolus
miyabeanus, Hormodendrum and against some insect pests, e.g. leaf
hoppers.
USE PATTERN
Edifenphos is authorised or recommended and sold in various
countries in Asia including Japan, in Central and South America and in
Europe, e.g. Italy. The product is marketed as emulsifiable
concentrates with various concentrations of edifenphos and as a dust.
In most countries two to four treatments are applied at dosage rates
of 300-800 g a.i./ha, with a pre-harvest interval of 21 days. The
recommended preharvest interval in Italy is 60 days.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
Japan and in some South American countries, namely Colombia, Mexico,
Peru and El Salvador. A summary of these data is given in Table 5.
FATE OF RESIDUES
The metabolic and other reactions of edifenphos exposed to
plants, animals, fungi, soil, light and water are shown in Figures
1(a) and 1(b) taken from Umeda (1972) with some modifications.
TABLE 5. Residues of edifenphos (parent compound) in rice resulting from supervised trials
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
Rice Japan 1967 2 0.36 EC 30% <0.02
hulled 3 0.36 EC 30% <0.02
4 0.36 EC 30% <0.02
3 0.6 dust 1.5% <0.02
4 0.6 dust 1.5% <0.02
1969 4 0.6 dust 1.5% <0.01
5 0.6 dust 1.5% 0.015
3 0.6 dust 1.5% <0.01
4 0.6 dust 1.5% < 0.01
3 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% 0.01
Rice Japan 1969 2 0.36 EC 30% <0.01
polished 3 0.45 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 2.5% 0.01
3 0.5 dust 2.5% 0.01
Rice
(in husk Peru 1969 2 0.5 EC 0.07
(straw 0.15
(in husk Colombia 1969 2 0.5 EC 0.1
(straw 0.25
(in husk El Salvador 1969 2 0.5 EC 0.45
(straw 1.4
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
(in husk Mexico 1969 2 0.5 EC 0.2
(straw 0.05
15/20 21/25 26/30 31/35 36/40 41/50 51/60
Rice Japan 1970 2 0.45 EC <0.01 <0.01
hulled 4 0.45 EC <0.01
2 0.2-0.45 EC <0.01 <0.01
4 0.2-0.45 EC <0.01
2 0.45 <0.01 <0.01
4 0.45 <0.01
(hulled 1973 2 0.3-0.45 EC 0.02
(straw 0.62
(hulled 1973 3 0.3-0.45 EC 0.01
(straw 0.32
hulled 1970 2 0.6 dust 0.03 0.03
4 0.6 dust 0.01
1971 3 0.75 dust 0.03 0.01
4 0.75 dust 0.02
1970 2 0.6-0.8 dust <0.01 0.01
4 0.6-0.8 dust <0.01
1970 2 0.6-0.8 dust <0.01 <0.01
4 0.6-0.8 dust <0.01
Rice Japan 1970 2 0.6 dust 0.01 0.01
hulled 4 0.6 dust 0.02
2 1 dust 0.01 0.01
4 1 dust 0.01
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 15/20 21/25 26/30 31/35 36/40 41/50 51/60
(hulled 1972 2 <0.01 <0.01
(straw 0.48 0.26
(hulled 1972 3 1 dust <0.01
(straw 0.93
(hulled 1972 2 1 dust <0.01
(straw 0.18
(hulled 3 1 dust 0.02 <0.01
(straw 3.81 0.24
(hulled 1973 2 1 dust <0.01
(straw 0.67
(hulled 1973 3 1 dust 0.01
(straw 0.74
(hulled 1973 2 1 dust <0.01
(straw 0.72
(hulled 3 1 dust 0.1
straw) 2.12
Rice
(hulled Japan 1972 2 1 dust 0.01
(straw 2.31
(hulled
(straw 3 1 dust 0.02
5.31
(hulled 3 1 dust <0.01
(straw 3.79
In plants
Extensive data are available on absorption, translocation,
accumulation and metabolism in the rice plant. (Ishizuka et al 1973,
1974; Nitokuno 1971; Takase et al 1973; Tomizawa et al 1972; Uesugi et
al 1972, Ueyama et al 1973, Umeda et al 1972).
Absorption and translocation
Izhizuka et al (1973, 1974) found that when edifenphos was
sprayed on rice leaves 60% was lost from the surface in five days and
75% in ten days. It was shown that about 70% of the edifenphos applied
had penetrated the leaf tissue within two hours.
Only small quantities were translocated from the site of
application, and translocation from roots to shoots was also slight.
Hence the loss of edifenphos was mainly from the leaf surfaces by
volatization and decomposition, rather than by metabolism inside the
plant.
When the flag leaf of a rice plant was painted with 32P-labelled
edifenphos some translocation of the chemical to the lower part of the
leaf and to the ears was observed (Ishizuka et al 1973, 1974; Nitokuno
1971; Umeda 1972). Since no complex of edifenphos with
photosynthesized plant products was found, it appears that edifenphos
is translocated without prior metabolism. The concentration of
edifenphos in plant parts is in the order: leaves and stems >> husks
>> hulled rice.
Metabolism
Takase et al (1973) studied the metabolic fate of edifenphos
after spraying rice plants at various growth stages. The
hexane-soluble components, which were analysed throughout the
experimental period, consisted, mainly of edifenphos parent compound
(83.2-89.3% of the radioactivity in the fraction). The major
components in the water soluble fraction were identified as O-ethyl
S-phenyl phosphorothioate (metabolite VII, Figure 1(a)) and
SS-diphenyl phosphorodithioate, (III) each representing about 30% of
the total 32p in the water-soluble fraction. With the lapse of time,
ethyl phosphate (XV) was formed.
Ueyama (1973) detected nine metabolites after applying
35S-labelled edifenphos to rice leaves. Three were identified as
metabolites IV, VIII and XII (Figures 1(a) and 1(b)). It is probable
that the formation of VIII and XII involved the exchange of phenylthio
and ethoxy radicals between molecules of edifenphos.
A high proportion of S-phenyl phosphorothioate (V) and small
amounts of O-ethyl S-phenyl phosphorothioate (VII) were found among
the water-soluble metabolites. Results are shown in Table 6.
TABLE 6. Toluene extracted metabolites in and on rice plants treated with 35S-edifenphos (after Ueyama)
Metabolites, 35S counts expressed as edifenphos equivalents, mg/kg
Growth stage Part of Period after
at application Plant application Identified* Not-identified
I IV VIII XII A B C E F
Tillering leaf 1 hour 35 4.3 2.9 1.1 0.72 1
blade
6 hour 18 1.1 1.2 0.59 0.02 0.33
1 day 20 2.8 1.1 1.4 0.05 0.46
3 day 17 1.1 0.73 0.16 0.26 1.2
7 day 1.2 0.26 1.5 0.13 0.07 trace trace
15 day 0.79 0.22 - 0.18 <0.01 0.01
Tillering leaf 1 hour 0.73 trace 3.3 - trace
sheath 6 hour 0.84 trace 1.0 trace trace
1 day 0.79 trace 0.33 0.33 trace
3 day 0.65 trace 0.36 - 0.02
7 day 0.04 0.01 0.05 0.04 <0.01 trace
15 day trace - trace
Heading leaf 1 hour 42 10 1.2 trace 0.27 0.05 0.05 0.33
blade 6 hour 31 8.2 3.2 trace 0.27 0.13 0.13 0.89
1 day 9.8 2.5 1.1 0.12 0.06 0.01 0.03 0.15
3 day 7.8 1.6 0.55 trace 0.03 0.02 0.12 0.29
7 day 3.2 0.19 - 0.02 <0.01 0.03 0.37
15 day 0.40 0.38 - 0.18 0.24 0.30
* For identities of numbered metabolites, see Figures 1(a) and 1(b) (I = parent compound)
In animals
Pither and Gronberg (1975) administered 0.12 mg/kg of edifenphos
as a single oral dose and as a daily dose for 5 days to dairy cows.
Results are described above ("Evaluation for acceptable daily intake -
- Absorption, distribution and excretion").
Strankowski et al (1976) identified metabolites of 14C ring-
labelled edifenphos in the muscle, liver, kidney, fat, milk and urine
of dairy cows. Methyl phenyl sulfone (XI, Figure 1(b)) was the
principal metabolite in both milk and tissues representing 95% of the
organo-soluble radio-carbon in the milk and from 70% (kidney) to 99%
in the tissues. Small amounts of metabolites Xa, XIa, XIb, together
with other minor metabolites were also found (Table 7).
TABLE 7. Distribution of edifenphos and its metabolites in the
tissues and milk of dairy cows (Strankowski, 1976).
Residue, % of total radioactivity in organic extract,
as metabolite no.*
Tissue I IX X Xa XI XIa Xlb XIIa
muscle - - - - 85 2 - -
fat - 1 - - 99 - - -
liver <1 <1 <1 4 84 <1 <1 <1
kidney <1 - <1 2 70 2 9 <1
milk - - 1 3 93 1 1 -
* For identities of numbered metabolites see Figure 1(b),
(I = edifenphos).
Hermann and Gronberg (1976) administered 3 mg/kg 14C-edifenphos
orally to laying hens. After 96 hours 76% of the activity had been
excreted. Methyl phenyl sulfone (XI) was the only metabolite detected
in all the tissues and in egg, but a second metabolite was found in
kidneys and was identified as either IX, XII or XIII (Figure 1(b)).
The total radiocarbon residues at intervals of 6-72 hours after dosing
are given in Table 8.
TABLE 8. Total radiocarbon from 14C-edifenphos in tissues of
laying hens
Radioactivity expressed as edifenphos,
mg/kg*, after interval, hours
Tissue 6 24 48 72
Muscle (breast) 0.66 0.73 0.57 0.21
Muscle (thigh) 0.69 0.75 0.59 0.19
Skin 0.79 0.74 0.58 0.21
Fat 0.97 1.00 0.80 0.30
Liver 1.72 1.45 1.17 0.51
Kidney 1.83 1.29 0.96 0.39
Gizzard 0.72 0.76 0.56 0.21
Heart 0.82 0.89 0.66 0.26
* Values are mean figures of single analyses of tissues from each of
four hens.
Ueyama and Takas (1975) determined the residue levels in goat
milk after administration of edifenphos at the rate of 1 mg/kg/day for
ten consecutive days. No edifenphos could be detected in the milk.
After a single oral dose of 10 mg/kg, 0.0006-0.0008 mg/kg in
edifenphos could be detected within 24 hours.
Lamb and Roney (1976a) exposed crayfish to 14C-edifenphos
concentrations of 10 µg/l (i.e. 10-8) for 14 days and determined 14C
residues during the exposure period and a 28 day withdrawal period.
14C residues, expressed as edifenphos, accumulated to a maximum level
of 0.2 mg/kg. About half of the accumulated 14C was lost within 7
days of withdrawal.
The same authors (1976b) exposed Channel catfish (Ictalurus
punctatus) continuously for 28 days to 10-12 µg/l of 14C
ring-labelled edifenphos. 14C residues accumulated to a maximum of 1.2
mg/kg edifenphos equivalents in ten days, representing an accumulation
factor of 104-117. The non-edible portions (head, viscera and scales)
contained 75% of the extractable residues. The acetonitrile fraction
(81% of the total extractable residue) consisted of edifenphos 44%,
metabolite VI (Figure 1(a)) 10%, VII 19% and VIII 27%. During the
withdrawal period about 50% of the accumulated 14C residues were
excreted in five hours and about 85% within four days.
In fungi
Uesugi and Tomizawa (1971) studied the metabolism of edifenphos
in mycelia of the rice blast (Pericularia oryzae) with 32P-, 35S-
and non-labelled edifenphos. The main metabolic pathway consists in
the hydrolysis of one P-S linkage, followed by that of the other P-S
or the ethyl ester bond, finally yielding phosphoric acid. Another
hydroxylated intermediate, metabolite II (Figure 1(a)) was also found.
No significant differences in route or rate of metabolism were
found between susceptible and resistant strains of the fungus.
In storage, processing and cooking
The residue is mainly located on the rice husk, hence during
milling and polishing most of the residue is removed. Takase et al
(1973) estimated the distribution of 32P-labelled edifenphos in
milling fractions (Table 9).
The residues at harvest in the husks consisted largely of
edifenphos metabolites, although small quantities of the parent
compound were found (Table 10).
Residues of edifenphos from foliar application (0.63 kg/ha, 2
applications) were found primarily in straw and seed hulls 37 days
after the second application. The residue in the hulls ranged from
0.02-0.29 mg/kg. Trace amounts were found in the bran, but no residue
was detectable in the milled and polished rice (Chemagro, 1971).
On exposure to light
A marked degradation of 35S-edifenphos by UV light was found on
irradation in aqueous or hexane solution or as a thin film. In hexane,
the formation of water-soluble degradation products was low. The
metabolites III, VIII, XV and XVI were identified (Murai et al, 1976).
In water and soil
Shaw (1976b) and Shaw and Murphy (1976) studied the hydrolysis of
14C ring-labelled edifenphos at concentrations of 1 and 10 mg/kg in
aqueous solutions buffered at pH 3, 6 and 9 and at temperatures of 25,
35 and 45 degrees C. The hydrolysis followed first order kinetics and
its rate increased with temperature and with increasing pH. The same
major hydrolysis products were formed under all conditions, namely
compounds VII and XII (Figure 1(a) and (b)) with traces of II, III,
IV, VI, VIII and XIV.
TABLE 9. Distribution of 32P-labelled edifenphos and its metabolites in milling fractions of rice
Radioactivity (32P) expressed as edifenphos, mg,/kg
Number of treatments 1 2 3 1
Pre-harvest interval,days 13 1 24 8
Milling fraction Hexane- Water- Hexane- Water- Hexane- Water- Hexane- Water-
soluble soluble soluble soluble soluble soluble soluble soluble
husks 0.11 0.04 0.17 0.12 0.28 0.14 2.13 0.34
hulled rice trace 0.02 trace 0.01 0.06 0.03 0.31 0.05
polished rice 0.01 0.01 0.01 0.01 trace trace trace -
TABLE 10. Distribution of radio-activity from 32P-edifenphos in rice
husk.
Residue, % of total radio-activity
3 applications, 1 application,
Compound* latest 24 days 8 day pre-harvest
pre-harvest harvest
Unknown
Water-soluble metabolite 9.6 13.4
III 10.9 19.4
VII 37.6 29.1
XV, XVI 41.8 28.1
* For identities of metabolites, see figures 1(a) and 1(b).
Shaw (1976a) incubated 14C ring-labelled edifenphos in pond
water (pH 8.3) containing soil for 62 days after irradiation with UV
light and sunlight, with alternating 12-hour periods of light and
darkness. In these conditions the half-life of edifenphos was 16.8
hours. The main products were compounds VII and XIV and one or more of
compounds IX, XII and XIII. Traces of II, III, IV, X and XI were also
found. These products were found in both the water and the bottom
sludge.
Flint and Shaw (1975) estimated the half-life of edifenphos in an
outdoor simulated pond system at 20°C. Under these conditions the
half-life was 29 hours in the water and about 60 hours in the bottom
sludge.
Edifenphos is almost immobile when applied to soil. Leaching and
soil adsorption were inversely correlated and the latter was
proportional to the soil organic matter content.
When columns of sand and sandy loam soil containing edifenphos at
a level equivalent to 0.5 kg/ha were eluted with water at a rate
representing 200 mm of irrigation in two days, no edifenphos was found
in the eluate (Bayer, 1974 a, b). The half-life of edifenphos was
about 23 days in the sandy loam (pH 5.2, organic carbon 0.57% and
particles < 20 µ 19.5%), but only 6-8 days in the sand (pH 6.8,
organic bound carbon 2.50% and particles < 20 µ 10.1%). In further
laboratory experiments simulating the flooded soil conditions
associated with rice growing (Nitokuno, 1972), edifenphos was degraded
rapidly during the first 24 hours. The half-life was about 19 hours in
a clay soil and 2 1/2 days in a silt loam. Subsequent degradation was
slower in both soil types.
Tomizama (1975) reports similar results obtained in a laboratory
degradation experiment. Under the anaerobic conditions of the
experiment about half the edifenphos was degraded during the first 3 -
5 days. From the sixth day onwards the rate of degradation was
considerably slower.
Studies by McNamara (1976) and McNamara and Close (1976) were
made on the persistence and degradation of 14C ring-labelled
edifenphos in loam and silt loam under aerobic conditions and in loam
under anaerobic conditions. The half-lives were 1 day in the aerobic
silt loam and 3 days in loam under both aerobic and anaerobic
conditions. The organo-soluble radioactivity decreased to 10-20% of
that applied within 21 days in all samples. In aerobic soils the
decrease was mainly due to volatilization and binding to the soil. In
aerobic loam about 20% of the total radioactivity was lost by
volatilization in 14 days, and in silt loam 35% in 21 days.
In aerobic soil the main organo-soluble radioactive residues were
edifenphos, its initial hydrolytic products III and VII, and
derivatives of thiophenol (XIII): diphenyl disulphide XII,
benzenesulphonic acid XIV, methyl phenyl sulphoxide X and methyl
phenyl sulphone XI. The two major terminal metabolites were X and XI.
In anaerobic loam the metabolic pathway proceeded via thiophenol
(XIII) to diphenyl disulphide XII, and benzenesulphonic acid XIV. Only
small amounts of methylated products were found.
In a model ecosystem
Edifenphos was fairly rapidly degraded in a model ecosystem, the
half-life being 60 hours. The main metabolic features were the
conversion of edifenphos to compounds IV and VII and the rapid
oxidation of the diphenyl disulphide released from these compounds. It
was found that sulphur atoms derived from the diphenyl sulphide were
incorporated into sulphuric acid and then into cell constituents,
(Tomizawa and Kazano, 1975).
METHODS OF RESIDUE ANALYSIS
Vogeler (1968) developed a gas-chromatographic method for the
determination of edifenphos residues in rice, using an alkali
thermionic or flame photometric detector. The rice sample is macerated
and extracted with acetone and the filtrate evaporated to dryness. The
residue is cleaned up by acetonitrile - petroleum ether partition and
by chromatography on a Florisil column. Recoveries from control
samples fortified with 0.05-1 mg/kg ranged from 70-80%. The limit of
determination is about 0.05 mg/kg. Thornton (1971) checked several
organophosphorus compounds which are authorised or recommended for use
on rice for interference with Vogeler's method. They were tested at
levels equivalent to the highest recommended dose rate on rice in the
USA. The compounds tested were dichlorvos, fensulfothion, fenthion,
malathion, naled, parathion, parathion-methyl, phorate and TEPP. None
showed any interference.
Takase et al (1971) described a modified gaschromatographic
method for the analysis of edifenphos in rice. Extraction is with
acetonitrile and the residue is cleaned up by partitioning into
acetonitrile and n-hexane followed by TLC on alumina. The residue is
determined with a flame photometric detector. Recoveries of added
levels of 0.1 to 0.2 mg/kg ranged from 96-102%. The limit of
determination is 0.005 mg/kg or lower.
Stanley (1972) adapted and modified the Vogeler method to
determine edifenphos residues in milk and cattle tissues. For milk the
method involves initial blending with acetone, followed by separation
of edifenphos from the aqueous solution by partitioning into
chloroform. Clean-up is by partition between Skellysolve B and
acetonitrile and chromatography on a Florisil column. Animal tissues
are extracted with acetonitrile, partitioned with Skellysolve B, and
cleaned up on a Florisil column. The edifenphos parent compound is
determined by gas chromatography with a thermionic detector. The limit
of determination of edifenphos in milk is 0.002 mg/kg and in cattle
tissues 0.02 mg/kg.
Recoveries from milk spiked with 0.005-0.5 mg/kg ranged from 77
to 110%, and in various cattle tissues spiked with 0.05-0.1 mg/kg from
82 to 118%.
Sandie and Gronberg (1975) adapted the Stanley (1972) method for
the analysis of edifenphos in poultry and eggs. For poultry tissues
the method involves extraction, partition between acetonitrile and
hexane, and gas chromatography with a thermionic detector. Eggs are
extracted with acetone, and clean-up is by successive aqueous
acetone-chloroform and acetonitrile - hexane partitions, followed by
silica gel chromatography. Recoveries were 84-107% from tissues at
levels of 0.05 and 0.1 mg/kg and 86-97% from eggs at 0.01 mg/kg. The
limits of determination were 0.01 mg/kg in poultry tissues and
0.001 mg/kg in eggs.
Minor (1976) examined possible interferences by other
organophosphorus pesticides in the determination of edifenphos in
cattle and poultry tissues and eggs by the methods of Sandie and
Gronberg (1975) and Stanley (1972). He found that edifenphos could be
adequately separated from all the 25 organophosphorus pesticides with
a USA tolerance in milk if the Stanley Florisil column and the Sandie
and Gronberg silica gel column were used successively in the clean-up
procedure.
NATIONAL TOLERANCES
National tolerances reported to the meeting are given in Table
11. They refer to the parent compound only.
TABLE 11. National tolerances reported to the Meeting
Country Commodity Tolerance, Recommended
mg/kg pre-harvest
interval, days
Italy rice, hulled 0.05 60
Japan rice, hulled 0.03 21
Mexico rice, hulled 20
U.S.A. rice, hulled 0.1*
rice, hulls 0.3
* temporary tolerances valid until May 1977
APPRAISAL
Edifenphos is an organophosphorus compound with a rather
selective action against the rice blast caused by Piricularia
oryzae, one of the major rice diseases, against which it shows both
a protective and curative action. There is some evidence that the
compound interferes with the formation of chitin in the fungus
cell-wall, a process which does not occur in higher plants. This may
therefore explain the selective action of the fungicide. Edifenphos is
also effective against a few other rice diseases and some insect
pests, e.g. leafhoppers.
The pesticide is used on an extensive scale on rice, as EC
formulations and as a dust, in Asian countries including Japan, in
Central and South America and in some mediterranean countries, e.g.
Italy. In most countries 2 - 4 treatments are applied at 300 - 800 g
ai/ha, generally with a pre-harvest interval of 21 days.
Extensive data were obtained on the fate of edifenphos residues
in soil, water, rice plants and livestock animals such as cattle,
goats and poultry. Degradation pathways in plants, soil and animals
are similar, although there are quantative differences.
In a study with 35S labelled edifenphos on rice, nine
metabolites were found in a toluene extract from leaves: the three
main products were OO-diethyl S-phenyl phosphorothioate, SSS-triphenyl
phosphorotrithioate and diphenyl disulphide. A large proportion of
S-phenyl phosphorodithioate and small amounts of O-ethyl S-phenyl
phosphorothioate were found as water-soluble metabolites. In a study
with 32p-labelled edifenphos, SS-diphenyl phosphorodithioate was
identified as another major water-soluble metabolite.
In a cattle feeding study with ring-labelled 14C-edifenphos it
was shown that methyl phenyl sulphone was the principle metabolite
both in milk and in animal tissues such as muscle, liver and kidney:
in milk it amounted to about 93% of the radioactivity extracted. The
parent compound, together with m- and p-hydroxyphenyl methyl sulphone
and p-hydroxyphenyl methyl sulphoxide were found, but only in small
amounts.
After administering a single dose of 14C-labelled edifenphos
orally to laying hens (3 mg/kg), 76% of the radioactivity was excreted
in 96 hours. A single metabolite, methyl phenyl sulphone, was
identified in all tissues and eggs.
More than half of the residue from spraying edifenphos onto rice
leaves was dissipated in five days. Only small quantities were
translocated from the site of application. Translocation of edifenphos
from the roots to the shoots was also only slight.
Residues in rice at harvest are rather low. After the recommended
pre-harvest interval of 21 days, the residues of edifenphos and its
main metabolites in hulled rice following an application of
32P-labelled edifenphos to rice plants were of the order of 0.03 and
0.06 mg/kg edifenphos equivalents of water-soluble and hexane-soluble
metabolites respectively. Only traces were found in polished rice. In
the rice husks the residues were about 0.15 and 0.3 mg/kg edifenphos
equivalents of water-soluble and hexane-soluble 32P respectively.
Gas-chromatographic methods of analysis with alkali flame or
flame photometric detection are available. Recoveries in rice were
70-80% and the limit of determination ranged from 0.005 to 0.05 mg/kg
edifenphos parent compound. Nearly all organophosphorus pesticides
registered and/or recommended for use on rice were checked for
interference. None of the compounds interfered when tested at levels
that would be reached by treatments at the highest recommended
dosages, provided appropriate clean-up procedures were used. These
methods should be suitable for or adaptable to regulatory purposes.
Some of the GLC methods mentioned are suitable for the analysis
of edifenphos residues in cattle tissues, milk, poultry and eggs. The
limit of determination is 0.01 - 0.02 mg/kg parent compound.
Recoveries at levels of 0.01 - 0.1 mg/kg ranged from about 80 to 100%.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the parent compound edifenphos only.
Commodity Limit, mg/kg
Rice (in husk) 0.2
Rice (hulled) 0.05
Rice (polished) 0.01*
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Further studies to examine the hepatic involvement observed in
several animal species.
Desirable
1. Observations in man (relative to occupational exposure).
2. More information on residues of edifenphos and its main
metabolites on rice in husk at harvest.
3. A method of residue analysis suitable for edifenphos together
with its main metabolites.
REFERENCES
Anonymous Hinosan (Edifenphos)/Generation Study on Rats.
1976a Unpublished report from Nihon Tokushu Noyaku Seizo
Co., Ltd., submitted to the WHO by Bayer A.G.
* at or about the limit of determination
Anonymous Special Toxicity Test of Hinosan - I (single
1976b p.o. application). Unpublished report from Nihon
Tokushu Noyaku Seizo Co., Ltd., submitted by Bayer
A.G.
Anonymous Special Toxicity Test of Hinosan - II (14 day
1976c repeat p.o. application). Unpublished report from
Nihon Tokushu Noyaku Seizo Co., Ltd., submitted by
Bayer A.G.
Arnold, D. Mutagenic Study with Hinosan (BAY 78 418) in
1971 Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted by Bayer
A.G.
Bayer, A.G. Pflanzenschnitzmittle - Rückstunde in
1974a Sickerwasser Sickelwasser (edifenphos).
Unpublished. Reports Bayer A.G. No. 8503/8504/74.
Bayer, A.G. Verhalten des Pflanzenschutzmittelwirkstuffes
1974b edifenphos in Boden. Unpublished report Bayer A.G.
No. 8506/74.
Chen, R.S., Kinoshita, F.K. and DuBois, K.P. Acute Toxicity and
1972 Antiesterase Action of O-Ethyl-S, S-diphenyl
Phosphorodithioate (Hinosan). Tox. Appl. Pharm. 23
519-527.
Eben, A. and Kimmerle, G. Metabolism of Hinosan Active Ingredient
1972 in Rats and Dogs. Unpublished report No. 3687 from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Flint, D.R., Shaw, II, H.R. The Mobility and Persistence of Hinosan
1975 in Soil and Water. Chemagro Report No. 43 663.
Gallagher, P.J. and Prentice D.E. Pathology Report of Hinosan
1976 (SRA7847) Chronic Toxicity study in Dogs over two
years. Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Hermann, P.A. and Gronberg, R.R. Excretion and Metabolism of Hinosan
1976 in Poultry. Chemagro Report No. 49 023.
Hoffmann, K. SRA7847 (Active Ingredient of Hinosan) Chronic Toxicity
1976 Study on Dogs. Unpublished report from Institut
für Toxikologie, Bayer, A.G., submitted to the WHO
by Bayer A.G.
Imamichi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, X.,
1973a Ikadai, H., Mori, H., Furusawa, U. and Saito, K.
Hinosan Subchronic Toxicological Studies on Rats.
Unpublished report from Nippon Vet. and Zoo Techn.
College, Tokyo, Japan, submitted to the WHO by
Bayer A.G.
Imamachi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, K.,
1973b Tauchi, K., Ikadai, H., Mori, H., Furusawa, U. and
Saito, K. Hinosan-Subchronic Toxicological Studies
on Mice. Unpublished report from Nippon Vet. and
Zoo Techn. College, Tokyo, Japan, submitted to the
WHO by Bayer A.G.
Ishizuka, K., Hosaka, H., Takase, I., Tan, K.E., Hirata, H.
1974 Metabolism and Accumulation of Pesticides in Rice
Plants. Comp. Studies Food Environ. Contam.;
Inter. Atomic Energy Agency, Vienna, 363 - 372.
Kimmerle, G. Bayer 78 418 (SRA7848)/Potenzierungsversuche.
1967 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Bay 78 418/Subcronische Neurotoxizitats-versuche
1969 bei Huhnern. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Kimmerle, G. Hinosan/Acute Neurotoxicity Studies on Hens.
1971 Unpublished report from Institut für Toxikologie,
Bayer A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Dermal Toxicity of Hinosan Active Ingredient to Rats.
1972a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Acute Inhalation Experiments with Hinosan Active
1972b Ingredient on Rats. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Subchronic Inhalation Toxicity Study on Rats.
1975a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Acute Inhalation Toxicity Study on Rats.
1975b Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. and Lorke, D. Toxikologische Untersuchungen mit dem
1967 Wirkstoff, Bayer 78 418. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. and Loser, E. Delayed Neurotoxicity of Organophosphorus
1974 Compounds and Copper Concentration in the Serum of
Hens. EQS Environmental Quality and Safety
3:172-178.
Lamb, D.W. and Matskanin, C.W. The Acute Oral Toxicity of Hinosan
1976 Metabolites to Rats. Unpublished report from
Chemagro Agr. Div. Mobay Chem., Corp., submitted
to the WHO by Bayer A.G.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976a in Chanuel Catfish Exposed to Hinosan-14C.
Chemagro Report No. 46 379.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976b in Catfish Exposed to Hinosan 14C. Chemagro
Report No. 48 832.
Loeffler, W.W. The Effect of Processing on Pesticide Residues.
1972 Chemagro Report No. 34 176.
Lorke, D. Hinosan/Studies of Product for Possible Embryotoxic
1971 and Teratogenic Effects on Rats. Unpublished
report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Subchronische toxikologische Untersuchungen
1969 an Hunden. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Loser, E. SRA7847/Subchronic Toxicological Studies on Rats.
1972 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Generation Study on Rats. Unpublished report
1976a from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Chronic Toxicity Study on Rats. Unpublished
1976b report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Machemer, L. Hinosan Active Ingredient (SRA7847)/Studies of
1976 Embryotoxic and Teratogenic Effects on Rabbits
following Oral Administration. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Minor, R.G. An Interference Study for the Residue Methods for
1976 Hinosan in Milk, Eggs and Animal Tissues. Chemagro
Report No. 48 819.
Mohr, U. Subchronic Inhalation Toxicity Study on Rats,
1975 Histopathological Findings. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Murai, T., Uesugi, Y., Tomisawa, C. and Tanaka, T.
1976 Photo-Degradation of Pesticide (I), Degradation of
Organophosphorus Fungicides EDDP and IBP by
Ultraviolet Light. Chemagro Report No. 45 169.
Murphy, J.J., Feldhas, L., Simmons, C.E., Thornton, J.S. and
1976 Gronberg, R.R. Total Radioactive Residues of
(14C) Hinosan in Rotational Crops. Chemagro
Report No. 48 826.
McNamara, F.T. Persistence and Metabolism of Hinosan in Soil.
1976 Chemagro Report No. 49 025.
McNamara, F.T. and Close, C.L. Persistence and Metabolism
1976 of Hinosan in Soil under Aerobic Conditions.
Chemagro Report No. 46 402.
Nitokuno Hinosan, a Fungicide for Control of Rice Blast,
1971 Chem. Econ. Engin. Rev. 3 (1), 57-59.
Nitokuno Gas Chromatographic Determination of Residues of
1972 Hinosan (O-ethyl S,S-diphenyl phosphorodithiolate)
in Rice Grains. Unpublished report.
Nitokuno Special Toxicity Test of Hinosan I. (single p.o.
1976 application). Unpublished report of Nihon Tokushu
Noyaku, Seizo Co., submitted by Bayer A.G. to the
JMPR.
Offer, J.M. and Prentice, D.E. Pathology Report of Bay 78 418
1976 (Hinosan) Chronic Toxicity Testing on Rats.
Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Pither, K.M. and Bronberg, R.R. Residue Levels in Lactating
1975 Dairy Cattle following Oral Administration of
Hinosan-ring-Ul-14C. Unpublished report No. 44
845 from Chemagro Agr. Div. Mobay Chem. Corp.,
submitted to the WHO by Bayer A.G.
Sandie, F.E. and Gronberg, R.R. A Gas Chromatographic Method
1975 for the Determination of Residues of Hinosan in
Poultry Tissue and Eggs. Chemagro Report No. 45
987.
Shaw, II, H.R. Hydrolysis of Hinosan in Pond Water Containing Soil.
1976a Chemagro Report No. 48 829.
Shaw, II, H.R. Hydrolysis of Hinosan in Buffered Aqueous Solution.
1976b Chemagro Report No. 48 831.
Shaw, II, H.R. and Murphy, J.J. Hydrolysis of Hinosan in Buffered
1976 Aqueous Solution. Chemagro Report No. 46 403.
Shirasu, Y., Moriya, M. and Kato, K. Report of the Mutagenicity
1976a Study of Hinosan (EddP, edifenphos). Unpublished
report from the Department of Toxicology,
Institute of Environmental Toxicology, Tokyo,
Japan, submitted to the WHO by Bayer A.G.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. and Kada, T.
1976b Mutagenicity Screening of Pesticides in the
Microbial System. Mutation Research 40:19-30.
Spicer, E.J.F. Pathology Report of Bay 78 418/Hen Study.
1971a Unpublished report from Huntingdon Research
Central submitted to the WHO by Bayer A.G.
Spicer, E.J.F. Pathology Report of Bay 78 418/Subchronic
1971b Neurotoxicity Study in Hens. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Spicer, E.J.F. and Erwin, C. Pathology Report of Bay 78 418/
1971 Subchronic Toxicity Studies in Dogs. Unpublished
report from Huntingdon Research Centre, submitted
to the WHO by Bayer A.G.
Stanley, C.W. Gas Chromatographic Method for the Determination
1972 of Residues of Hinosan in Animal Tissues and Milk.
Chemagro Report No. 32 070.
Strankowski, K.J., Pither, K.M. and Murphy, J.J. Metabolism of
1976 Hinosan by a Lactating Dairy Cow. Unpublished
report No. 48 846 from Chemagro Agr. Div. Mobay
Chem. Corp., submitted to the WHO by Bayer A.G.
Takase, I., Nakamura, S., Tsuda, H. and Ueyama, I. The Gas
1971 Chromatographic Determination of Pesticides. (Part
4). Cleanup Method for Organophosphorus Pesticide
Residues in Rice Grain. Noyaku Seisan Gijutsu
(Pesticide and Technique) 23:31-35.
Takase, I., Tan, K.E. and Ishizuka, K. Metabolic Transformation
1973 and Accumulation of O-Ethyl S,S-Diphenyl
Phosphorodithiolate (Hinosan) in Rice Plants. Agr.
Biol. Chem. 37(7): 1563-1571.
Thornton, J.S. A Study of the Possible Interferences of Other
1971 Pesticides with the Gas Chromatographic Residue
Method for Hinosan. Chemagro Report No. 29 702.
Thyssen, J. and Kimmerle, G. Hinosan 50 E.C./Toxicological Studies.
1974 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Tomizawa, C. Degradation of Organophosphorus Pesticides in Soils
1975 with Special Reference to Unaerobic Soil
Conditions. Environ. Qual. Safety 4:117-127.
Tomizawa, C., Uesugi, Y. and Murai, T. Fats and Significance of
1972 Fungicides in Rice. Radiotracer Studies Chem. Res.
Food. Agric.; Intern. Atomic Energy Agency,
Vienna, 103-105.
Tomizawa, C. and Kazano, H. Biological Accumulation of Pesticides
1975 in an Ecosystem. Rev. Plant Protec. Res.
8:41-54.
Uesugi, Y. and Tomizawa, C. Metabolism of O-Ethyl S,S Diphenyl
1971 Phosphorodithiolate (Hinosan) by Mycelial Cells of
Pyricularia Oryzae. Agric. Boil. Chem. 35
(6):941-949.
Uesugi, Y. Tomizawa, C. and Murai, T. Degradation of
1972 Organophosphorus Fungicides. Environ. Toxicol.
Pest. Academic Press, 327-339.
Ueyama, I., Uesugi, Y., Tomizawa, C. and Murai, T. Metabolic
1973 Fate of O-Ethyl S,S-Diphenyl Phosphorodithiolate
(Hinosan) in Rice Plant. Agr. Biol. Chem.
37(7):1543-1551.
Ueyama, I. and Takase, I. Metabolic Behaviour of O-Ethyl
1975 S,S-Diphenyl Phosphorodithiolate (edifenphos) in
Female Goat. Agr. Biol. Chem. 39(9):1719-1727.
Ueyama, I., Takase, I. and Tomizawa, C. The Metabolism of Edifenphos
1976 (Hinosan) in Mice and Rats. Unpublished report No.
1046 from Nithon Tokushu Noyaku Seizo Co. .td.
Agr. Chem. Inst. and National Institute of Agr.
Sciences, submitted to the WHO by Bayer A.G.
Umeda, Y. Anti-Blast Fungicide "Hinosan" and its Biological
1972 Properties and Metabolism in the Rice Plant. Japan
Pest. Inform. No. 10, 85-88.
Umeda, Y. Hinosan, a Fungicide for Control of Rice Blast.
1973 Japan Pest Inform. No. 17, 25-28.
Urwin, C. and Newman, A.J. Pathology Report of SRA7847/3
1972 Month Feeding Study in Rats. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Vogeler, K. Caschromatographische Methods zur Bestimmung von
1968 Hinosan-Rückständen in Reis. Pflanzenschutz
Nachr., Bayer 21 (3):321-325.
Yatomi, A. Mutagenicity Test on Bacteria. Unpublished report
1975 from Nihon Tokushu Seizo K.K., submitted to the
WHO by Bayer A.G.
Cholinesterase depression in rats was observed following acute
inhalation exposure to aerosolized edifenphos. Plasma and RBC
cholinesterase depression was seen following exposure to 30 mg/m3
for 4 hours (threshold level). No depression was noted at an
exposure concentration of 9 mg/m3 for 4 hours (Kimmerle, 1975b).
TOXICOLOGICAL STUDIES
Special Studies on Teratology
Groups of rats (20 mated females/group) were administered
edifenphos by oral gavage at doses of 0, 5, 15 and 30 mg/kg body
weight from day 6 to 15 of gestation. All animals were sacrificed
and pups delivered by Caesarean Section. Increased mortality
reduced growth and an unhealthy appearance were observed at the two
highest dose levels. At the highest dose level, 3 litters were
resorbed. There were no abortions and with the exception of the 3
resorbed litters, the numbers of implantations, fetuses and
resorptions did not differ from control values. The weights of
fetuses were not different from controls and the nature and
frequency of somatic and skeletal variations were within normal
limits. There were no malformations and no indications of a
teratogenic potential was noted in this study (Lorke, 1971).
Groups of rabbits (11-13 mated does/group) were administered
edifenphos by oral gavage at dose levels of 0, 3, 6 and 12 mg/kg
body weight from day 6 to 18 of gestation. On day 29 of gestation,
the does were sacrificed and Caesarean Section performed. There was
no mortality although the two highest dose levels resulted in
reduced growth (the highest level treatment showed a significant
weight loss). In the 6 mg/kg group one doe aborted and one resorbed
all fetuses. Implantation recorded for the two highest doses was
reduced. However, the significance of this was not apparent as
treatment did not begin until 6 days after fertilization and
edifenphos treatment should not have affected this parameter. There
were no differences in the number of fetuses in the 0 and 3 mg/kg
dose while the upper two doses had fewer fetuses. The weight of
fetuses and placenta of the treated group did not differ from
control values. Malformations were not observed on gross or
skeletal examination of fetuses. As suggested with rats, edifenphos
did not induce a teratological response in rabbits (Machemer,
1976).
Special Studies on Reproduction
Groups of rats (10 males and 20 females/group) were fed
edifenphos in the diet at concentrations of 0, 5, 15 and 150 ppm
and mated to begin a standard 3 generation, 2 litter per
generation, reproduction study. Reproductive indices included:
fertility, gestation, viability and lactation measured for all 6
litters. There was no mortality observed and the highest dietary
concentration did not affect fertility. Litter size was reduced and
in one instance the litter weight was reduced at the 150 ppm level.
Slight effects (not statistically significant) were noted on
viability and lactation indices in the high dose group. Histological
examination of the F3b generation gave no indication of adverse
effects on the major tissues and organs. A no effect-level in this
study would be 15 ppm (Loser, 1976).
Groups of rats (20 females/group) were fed edifenphos in the
diet at dosage levels of 0, 5, 15 and 50 ppm, mated and subjected
to a standard 3 generation, 2 litters per generation, reproduction
study, In combination with the study, additional groups of 5
females from the P and F1b and 10 females from the F2b were
sacrificed at day 20 of gestation for an examination of fetuses for
any teratological events. Additionally, offspring of 5 female rats
from individual groups of the F1b, F2b and 10 females from the P3b
were maintained after weaning at the same dietary concentrations of
edifenphos listed above for periods ranging from 1 to 6 months.
These short term studies were designed to evaluate growth and
development of rats exposed initially in utero and thereafter for
short periods to dietary levels of edifenphos.
Growth of parental generations was unaffected by the presence
of edifenphos in the diet. Slight reduction of male food intake and
body weight at 50 ppm was noted in the first parental generation.
The reduced food consumption was observed in the F2 and F3 parents
accompanied by slight reduction of growth. The reduced growth was
predominant in those animals fed after weaning for prolonged
periods.
There were no significant differences noted in the
reproduction indices over the course of the three generations.
Edifenphos did not affect the ability of the rats to reproduce,
maintain pregnancy, deliver, nurse and wean normal sized litters
over the course of three generations. A teratological examination
of fetuses delivered by Caesarean Section prior to term did not
show any abnormalities related to somatic or skeletal defects. In
the first generation the 50 ppm dosed group reflected a slight
retardation in the degree of bone ossification not always noted in
the two lower doses or the controls. There were no significant
differences in implants, resorptions, live fetuses or in the weight
of fetuses.
In those weanlings from the F1b, F2b and F3b litters that were
maintained on diets containing edifenphos for 1 to 6 months after
weaning, growth was not substantially affected. Slight reductions
of growth were noted at 50 ppm accompanied by reduced food intake.
Hematological examinations made at the conclusion of each time
interval were normal. Liver function tests including SGOT and SGPT
activity and cholinesterase determinations using plasma, RBC and
brain were normal. Gross and microscopic examination of tissues and
organs of the animals maintained after weaning showed only on
consistent event attributable to edifenphos in the diet. An
enlarged kidney in the 50 ppm group fed for 3-6 months was
accompanied by changes (saccular necrosis) in the tubular
epithelium noted on microscopic examination. No dose related
changes were noted in a variety of tissues and organs that could be
attributable to edifenphos in the diet (Anonymous, 1976a).
Special Study on Mutagenicity
Mice
A dominant lethal study using male mice was performed with
edifenphos. Groups of mice (12 males/group) were administered
edifenphos by a single intraperitoneal injection at doses of 0, 50
and 100 mg/kg. A positive control (MMS, methyl methansulfonate, 100
mg/kg) was included in the study. The males were mated with three
untreated virgin females. The females were changed weekly for 6
consecutive weeks to evaluate maturation of male mouse germ cells.
One week after breeding, the females were sacrificed and examined
for implantation sites, resorption sites and embryos.
There were no deaths as a result of treatments with
edifenphos. Except for a slight reduction in the mating index at
week 6 in the 100 mg/kg dose group, there was no effect on the
ability to mate or fertilise the females. There were no
dose-related effects noted with respect to implantation, resorption
or embryo viability. There was no increase in pre-implantation loss
or mutuation rate although the positive control showed a
significant increase in early resorptions, attesting to the species
susceptibility. Edifenphos did not demonstrate a mutagenic
potential by the dominant lethal test in mice (Arnold, et al.
1971).
Microorganisms
When examined in vitro with various strains of
microorganisms (B. subtilis, E. coli and S. typhimurium
edifenphos did not induce a mutagenic response. In two studies with
the aid of a B. subtilis strain lacking the recombination
repair system, S. typhimurium and E. coli with either a
histidine or tryptophane deficiency, edifenphos, examined for its
ability to induce mutations, was found to be negative. Positive and
negative controls were used in these assays to assure sensitivity
to the mutagenic activity and to provide a baseline of operation.
In addition, liver homogenates (fortified with cofactors necessary
for microsomal oxidation) were used to activate, if possible,
edifenphos to an active mutagen. In studies with reversion colonies
in combination with a microsomal activation system, edifenphos did
not induce mutations (Shirasu et al., 1976a; 1976b; Yatomi, 1975).
A host mediated assay was performed where groups of 5 mice
were orally administered two doses of 0, 50 or 100 mg edifenphos/kg
(within 24 hours). After the second dose, a microbiological test
system of S. typhimurium was injected into the peritoneal
cavity, removed after 3 hours and examined for revertant colonies.
In this assay, edifenphos was negative although a positive control
of dimethylnitrosamine (50 mg/kg, oral administration) induced
reversion at a substantial rate (Shirasu. et al., 1976a).
Special Studies on Potentiation
Oral administration of equitoxic doses (LD50) of edifenphos
with fenthion or propoxur did not result in potentiation of the
acute toxicity of the combination. (Kimmerle, 1967).
A greater than additive toxicity, observed with a combination
of malathion and edifenphos, suggested a potentiating effect with
these two pesticides (Kimmerle and Lorke, 1967). Further studies on
toxic interaction of two organophosphorus pesticides were
undertaken where rats fed 0, 5 or 25 ppm edifenphos in the diet
were administered malathion by ip injection at a sublethal dose
(400 mg/kg). The increased mortality observed in the two groups fed
edifenphos suggested a significant potentiating effect on malathion
toxicity (Chen, et al., 1972).
Special Studies on Antidotes
Following acute oral administration of edifenphos to male
rats, antidotal administration (ip injection) of atropine (50
mg/kg) alone or in combination with 2-PAM or BH6 (50 or 20 mg/kg,
respectively) resulted in a slight reduction in the acute toxicity
(less than 2 fold) (Kimmerle and Lorke, 1967).
Special Studies on Neurotoxicity
Edifenphos was administered to hens by oral intubation or
intraperitoneal injection at dose levels up to 1000 mg/kg. The
surviving animals were examined for 42 days with no signs of
delayed neurotoxicity (Kimmerle and Lorke, 1967).
Groups of hens were administered edifenphos by oral intubation
at the LD50 level (547 mg/kg body weight) together with atropine,
50 mg/kg administered by ip injection. Delayed neurotoxic signs of
poisoning were not seen, but were observed in all hens treated
orally with TOCP (350 mg/kg). The hens were observed for 3 weeks
and sacrificed (Kimmerle, 1971). Histological examination of
portions of the central and peripheral nervous system of the
edifenphos-treated hens was negative with respect to axon or myelin
disruption. The TOCP-treated hens showed axon and neuron
degeneration in the spinal cord (brain and peripheral nerve were
not affected) (Spicer, 1971a).
In an effort to evaluate the relationship of delayed
neurotoxicity and copper imbalance in hens, an examination of
several organophosphates inducing a delayed neurotoxicity was made.
Edifenphos as one of the negative compounds did not induce
neurotoxicity and did not increase copper and ceruloplasmin levels
as did several of the positive neurotoxic agents (Kimmerle and
Loser, 1974).
Groups of hens (8 hens/group) were fed edifenphos in the diet
for 30 days at concentrations of 0, 100, 250, 500 and 1000 ppm.
Growth was depressed at 500 ppm and above. Whole blood
cholinesterase was depressed after 30 days only in the highest dose
group. Animals allowed to recover on control diets for a 4 week
period recovered body weight and cholinesterase depression was
reduced. Neurotoxic clinical signs were not observed (Kimmerle,
1969). Histological examination of brain, spinal cord and
peripheral nerve showed no evidence of myelin degeneration (Spicer,
1971b).
Acute Toxicity
TABLE 2. Acute toxicity of edifenphos
LD50
Species Sex Route (mg/kg) Reference
Rat M oral 119-340 Kimmerle, 1967;
Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 48-81 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974;
Chen, et al., 1972
dermal
(24 hour exposure) 184-413 Kimmerle, 1972a Thyssen &
Kimmerle, 1974
(4 hour exposure) 1,230 Kimmerle & Lorke, 1967
(7 hour exposure) 600 Kimmerle & Lorke, 1967
F oral 63-150 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 26-55 Thyssen & Kimmerle, 1974;
Kimmerle & Lorke, 1967;
Chen, et al., 1972
dermal
(24 hour exposure) 86-135 Kimmerle, 1972a,
Thyssen & Kimmerle, 1974
Mice M oral 392 Thyssen & Kimmerle, 1974
TABLE 2. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
Mice F oral 218-295 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Guinea Pig oral 350-400 Kimmerle & Lorke, 1967
Rabbit F oral 250-400 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Cat oral >250 Kimmerle & Lorke, 1967
Note -- In some instances the range of values is reflective of
differences in solvents used in the test. The lower values were
obtained with Lutrol while the higher values were obtained with
ethanol: propylene glycol mixtures. In all cases there appears to be a
significant sex difference with females more susceptible than males.
Signs of poisoning were typical of cholinergic stimulation (tremors,
salivation, labored respiration, etc.) appearing 2-3 hours after acute
poisoning and persisting for up to 2 days with rats and up to a week
with mice and rabbits.
Inhalation Toxicity
Acute aerosol inhalation toxicity tests (both static and dynamic
flow) were performed with groups of rats using various exposure times.
Edifenphos technical or a 50% EC formulation (Hinosan(R)) was used in
several studies. Data expressed as the technical product do not show
significant differences in toxicity between the two forms of
edifenphos. No indication of particle size distribution was given in
these data.
Dynamic tests
Results of dynamic tests are given in Table 3.
TABLE 3. Inhalation toxicity of edifenphos (dynamic tests)
Exposure LD50
Species Sex Time (mg/m3) Reference
Rat M 1 hour >898-1310 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b;
Kimmerle & Lorke, 1967
TABLE 3. (Cont'd.)
Exposure LD50
Species Sex Time (mg/m3) Reference
4 hour 362 - 775 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b.,
Kimmerle, 1975b;
Kimmerle & Lorke, 1967
F 1 hour > 1017-1046 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b
4 hour 230 - 320 Thyssen & Kimmerle, 1974;
Kimmerle, 1975b;
Kimmerle, 1972b
Static Tests
Groups of rats, mice, rabbits and guinea pigs were exposed to
static concentrations of edifenphos ranging from 108 to 398 mg/m3 for
up to 4 hours. In the 1 hour exposure, no toxicity was recorded (only
one dose level was tested) but mice exhibited toxic signs of exposure.
In the 4 hour exposure the susceptibility of rats, rabbits and mice
was the same with 7/10 rats, 2/3 rabbits and 12/20 mice dying at a
concentration of 269 mg/m3. Only 1/5 guinea pigs died at this
exposure. No deaths were reported at a concentration of 101 mg/m3 for
the 4 hour exposure (Kimmerle & Lorke, 1967).
Edifenphos did not induce a primary dermal irritation when
applied to the skin of rabbits' ears for up to 24 hours or to the
conjunctival sac of rabbits. The 50 EC formulation of Hinosan(R)
irritated both the conjunctiva and skin (1 hour exposure). The primary
irritation was attributed to the formulation ingredients and not
technical edifenphos (Kimmerle and Lorke, 1967; Thyssen and Kimmerle,
1974).
Toxicity of metabolites
Acute toxicities of edifenphos metabolites to rats are shown in
Table 4.
TABLE 4. Acute toxicity of edifenphos metabolites to rats
Compound Route LD50
(mg/kg)
O-ethyl S-phenyl oral M >500
S-(4-hydroxyphenyl)- F >1000
phosphorodithioate
SS-diphenyl oral M 1000-2000
phosphorodithioate F 1000-2000
O-ethyl S-phenyl oral M 1000-2000
phosphorothioate F 616
O-ethyl S-phenyl oral M approx. 1000
S-(3-hydroxyphenyl) F approx. 500
phosphorodithioate
(Lamb & Matzkanin, 1976)
Short-term studies
Mice
Groups of ICR mice (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. In general, growth was not affected by dietary
edifenphos. Food consumption, behaviour, physical condition and
mortality were unaffected. Plasma cholinesterase depression was noted
at 30 ppm with RBC and brain depressed only at 1000 ppm. Hematology,
urinalysis and clinical chemistry parameters were normal. On gross
examination of tissues and organs, spleen and liver enlargement was
observed at 1000 ppm. Microscopic examinations showed no abnormalities
in spleen although liver and adrenal changes were noted at 300 ppm and
above. In liver, hypertrophy of hepatocytes and deposition of
phospholipid were observed. Adrenal hypertrophy and pigment deposition
were noted at the two highest doses (Imamichi, et al., 1973b). A
no-effect level of 10 ppm was observed equivalent to 1.53 mg/kg body
weight.
Rats
Groups of rats (10 males and 10 females/group) were exposed to
edifenphos by inhalation at concentrations of 0, 4 (range of 3-15), 10
(8-12) and 27 (24-30) mg/m3 air for 12 weeks, 5 days/week for 6
hours/day. The range of particle size was 0.5 to 1.5 µ with 97% of the
particles having a mean mass diameter of 1 µ (a fully respirable
particle). There was no mortality although females exposed to 27
mg/m3 were observed to be adversely affected. Growth was normal at
all levels of exposure as were clinical chemistry, hematology and
urinalysis parameters. Cholinesterase (brain, plasma and RBC) activity
was depressed at 10 mg/m3 and above. No effects were noted at 4
mg/m3. Gross and microscopic analysis of tissues and organs were not
affected by edifenphos in the diet. Female adrenal weight was
increased at the highest dose level but no abnormalities were detected
on microscopic examination (Kimmerle, 1975; Mohr, 1975).
Groups of rats (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. There was no mortality during this study but
growth was inhibited at 1000 ppm in both males and females. In
contrast to the reduced growth, food consumption was normal at all
dose levels. Several hematological parameters were affected at 1000
ppm, namely reduced RBC count, hemoglobin content and hematocrit value
primarily in males. Liver function was reduced at 1000 ppm (SGOT), 100
ppm (SGPT) and at 30 PPM (SAP) predominantly in males, with the
reduction not as marked in females. Urinalysis parameters were
unaffected. Cholinesterase depression was noted at 300 ppm in males
(brain and RBC) and 100 ppm in females (brain and RBC), plasma was
depressed in females at 10 ppm. Gross examination revealed enlarged
liver (1000 ppm) and kidney (300 ppm). Microscopic examination of
tissues showed hypertrophy and fatty degeneration of hepatocytes at
100 ppm and above. No pathological changes were noted in liver at 30
ppm. At 100 ppm and above, kidney and adrenal changes were noted. At
high levels a toxic nephritis was observed and the kidney and adrenal
glands were hypertrophic. In the male, kidney degeneration was
prevalent while in the females the adrenal was damaged. Gross changes
noted in brain, pituitary and thyroid of males at high dose levels
were not accompanied by pathological changes (Imamichi, 1973a).
Groups of rats (15 males and 15 females/group) were fed
edifenphos in the diet at dosage levels of 0, 1, 2, 5, 10 and 150 ppm
for 3 months. Growth was affected by edifenphos and there were no
differences in behaviour, mortality or appearance. Food consumption in
the highest dose group was slightly reduced. Hematological
examinations were normal at 1 and 3 months as were liver function and
urinalysis tests measured at the same time intervals. Cholinesterase
depression was observed in the highest dose group (in both RBC and
plasma) within one week of initiation of the feeding trial. The
depression was constant and there was no indication of a cumulative
effect relating to edifenphos. Gross and microscopic analysis of
tissues and organs, performed at the conclusion of the study, showed
no dose-related gross change and no significant microscopically
observable alteration (Loser, 1972; Urwin and Newman, 1972).
Groups of rats (10 males/group) were administered edifenphos by
oral intubation, daily for 14 days at dose rates of 0, 12.5, 50 and
100 mg/kg. Mortality was not reported but a slight decrease in average
body weight was reported at 100 mg/kg. A significant increase in
absolute and relative liver weight was reported at all dose levels at
24 hours. The livers had recovered to normal size within 7 days of the
end of treatment. Cholinesterase was depressed at all levels with the
following order of sensitivity: RBC > plasma > brain. One week after
treatment RBC and brain cholinesterase activity were depressed at all
dose levels. Liver function tests were slightly affected (SGOT
reduced) but were normal within 7 days. EPN detoxication by liver
microsomes was stimulated at all dose levels (aniline hydroxylase and
aminopyrine demethylase were unaffected) and recovery was not complete
at 7 days. Microscopic examination of liver, adrenals and kidney
showed no adverse effects (Anonymous, 1976c).
Groups of rats (15 females/group) were administered edifenphos
orally (0, 5, 10, 20, 33.5 and 50 mg/kg) or by intraperitoneal
injection (0, 1.75, 3.5, 7, 10, 11.5 and 17.5 mg/kg), daily 5 weeks
per week for 60 days. Oral administration of 50 mg/kg daily resulted
in no mortality or cumulative toxicity although signs of poisoning
were seen. No signs of poisoning were evident at 10 mg/kg/day
following oral dosing. With daily ip injections, no substantial
mortality (1/15 deaths) was observed at 7 mg/kg (11/15 died at 11.5
mg/kg). Signs of poisoning were not evident at 3.5 mg/kg. These
results suggest a minimal cumulative toxicological effect (Kimmerle
and Lorke, 1967).
Dog
Groups of dogs (2 male and 2 female/group, controls had 3 of each
sex) were fed edifenphos in the diet at dosage levels of 0, 3, 10 and
30 ppm for 90 days. There was no mortality over the course of the
study and growth, food consumption, behaviour and general appearance
were normal. Clinical chemistry, hematology and urinalysis values were
normal at one and 3 months (except for a slight increase of SDH
activity at only one month in both males and females at 30 ppm). Blood
cholinesterase activity was depressed in both plasma and RBC (to the
same extent in both enzyme sources) at the highest dose. As with
rodent studies, there was no evidence of cumulative activity over the
3 month interval. Gross and microscopic examination of tissues and
organs revealed no adverse effects of edifenphos in the diet. A slight
increase in the absolute and relative weight of the spleen at 30 ppm
was not accompanied by microscopic lesions (other than minimal)
congestion and probable hemosiderin deposition) (Loser, 1969; Spicer
and Urwin, 1971).
Groups of dogs (4 male and 4 female/group) were fed edifenphos in
the diet for two years at dose levels of 0, 7, and 20 ppm. A fourth
group was fed a diet containing 60 ppm for 1 year after which the
concentration was increased to 80 ppm for 5 months and raised to 120
ppm for the remaining 7 months of study. There was no mortality and
behaviour and physical appearance were normal and unaffected by
edifenphos in the diet. Growth and food consumption was normal at the
20 ppm level although it was slightly depressed at the high dose
regime. At the high level food consumption was slow, diet rejection
was observed and body weight gain depressed. There was no effect noted
on ophthalmological examination, urinary or hematology parameters or
on reflex testing. Some liver function test parameters were abnormal
(data showed SAP increased although SGPT was normal). This change was
accompanied by an increased gross and relative liver weight noted at
the time of sacrifice. Cholinesterase activity was depressed in the
high dose group in both plasma and RBC of males and females. Brain
cholinesterase was unaffected at any dose level. Gross and microscopic
examination of tissues and organs did not suggest an adverse effect of
edifenphos fed in the diet at concentrations up to and including 120
ppm. The inductive effect noted on the liver (increased organ weight
accompanied by increased phosphatase activity) was attributed to an
adaptation reaction noted with many drugs and chemicals. Microscopic
examination of liver did not show changes attributable to the presence
of dietary edifenphos which would account for the increased liver
weight. A no-effect level was observed to be 20 ppm equivalent to a
daily intake of 0.58 mg/kg body weight (Hoffmann, 1976; Gallagher and
Practice, 1976).
Long Term Studies
Rat
Groups of rats (50 male and 50 female rats/group, 100 of each sex
were used as controls) were fed edifenphos in the diet at
concentrations of 0, 2, 5, 15 and 150 ppm for two years. Mortality was
unaffected during the two years. Growth, behaviour and physical
appearance were normal in all groups. Hematology, blood chemistry and
urinalysis parameters were normal. A slight decrease in alkaline
phosphatase at 150 ppm was considered to be within the normal range
for the animals. Cholinesterase depression was observed in plasma and
RBC of both sexes at 150 ppm. Brain cholinesterase was depressed only
in females at 150 ppm. Gross and microscopic examinations were
performed on surviving animals. No abnormal, dose-related events were
recorded in the gross examination. A slight increase in adrenal size
of males at 15 ppm and above was not reflected in changes observed
under microscopic examination. Histopathological examination of
tissues and organs revealed luminal deposits of calcium salts in the
medulary tubules of male rats fed 15 and 150 ppm diets accompanied in
the high dose group by an increased incidence of glomerulonephrosis in
males. These changes were not noted in females. A complete examination
of tumours was performed revealing no indication of carcinogenic
potential. The frequency of both benign and malignant tumors was
typical of the rat strain used in the study. A no-effect dietary
level, based on the microscopic examination observing slight kidney
changes, was 5 ppm equivalent to 0.30 mg/kg body weight (Loser, 1976;
Offer and Prentice, 1976).
COMMENTS
Edifenphos, an organophosphorus ester, is used in agriculture as
a fungicide to control rice diseases. Following oral administration it
is rapidly absorbed, distributed, metabolised and excreted in mammals.
The well defined metabolic sequence observed in plants and animals
appears to be the same. In metabolism studies and other studies in
several species of animals there were no indications of any
culminative effects.
Biochemical parameters affected by acute and subacute
administration of edifenphos include cholinesterase depression and
changes in certain liver enzyme values reflecting changes in basic
liver function. Edifenphos is not a potent cholinesterase inhibitor.
However, the recovery of depressed enzyme activity is not rapid
suggesting that regeneration of cholinesterase activity is dependent
on enzyme synthesis rather than reversal of an enzyme-inhibitor
complex. A sensitive biochemical parameter was aliesterase activity of
liver and serum with a level of 5 ppm of edifenphos in the diet
showing 50% inhibition. In special studies for teratogenic and
mutagenic potential, no effects of edifenphos were noted on several
species. At high levels, reproduction was slightly impaired but at
levels that did not affect maternal well-being reproduction parameters
were unaffected. Edifenphos appeared to potentiate the acute effects
of malathion as might be expected from the data on depression of
aliesterase activity. There is no evidence that edifenphos would
induce a delayed neurotoxic response in hens.
Edifenphos is moderately toxic on acute exposure to a variety of
mammalian species with signs of poisoning typical of
parasympathomimetic agents. The acute signs of poisoning, although
modified slightly by atropine and reactivators, were not completely
controlled.
In all cases, the acute toxicity of metabolites was greatly
reduced with respect to the acute toxicity of the parent compound. In
both short and long term studies in rats and dogs, the most sensitive
parameters affected were cholinesterase depression, liver enlargement
and deposits of kidney salts in males on long term studies. Based on
short term studies in mice and dogs and long term studies in rats a
no-effect level was suggested and a temporary ADI for man was
recommended. The dietary studies in rat (both short term and long
term) showing liver and serum aliesterase depression and kidney salt
build-up in males were used as a basis for evaluating the no-effect
level. While 5 ppm was a level causing 50% inhibition of aliesterase
activity the significance of this effect was unclear and has been
taken to suggest "exposure" rather than "toxicological effect".
The liver involvement noted in many studies precluded
consideration of a finite ADI until a specific carcinogenic study is
performed using a species susceptible to liver changes that might
relate to hepatic carcinoma. As these changes noted in this study
resemble those effects seen with other compounds including chlorinated
hydrocarbons, such studies are required. The lack of carcinogenic
potential noted in the 2 years, on a limited number of rats, was
reassuring that the compound is not a potent carcinogen in this
species. However, it was considered in the 2 year rat study that the
highest dose level and the small group size were not sufficient to
fully evaluate the potential for carcinogenicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm in the diet equivalent to 1.53 mg/kg bw
Dog: 20 ppm in the diet equivalent to 0.58 mg/kg bw
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg bw
Estimate of temporary acceptable daily intake for man
0 - 0.003 mp/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Edifenphos is an organophosphorus compound with a fairly
selective action against rice blast caused by Piricularia oryzae,
one of the major rice diseases. It has both a protective and curative
action against the fungus attack. There is some evidence that the
compound interferes with chitin formation in the fungal cell wall, a
process which dose not occur in higher plants. This mode of action may
therefore explain the selective action of the fungicide.
The compound is also effective against a few other fungus
diseases of rice, e.g. ear blight caused by Cochliobolus
miyabeanus, Hormodendrum and against some insect pests, e.g. leaf
hoppers.
USE PATTERN
Edifenphos is authorised or recommended and sold in various
countries in Asia including Japan, in Central and South America and in
Europe, e.g. Italy. The product is marketed as emulsifiable
concentrates with various concentrations of edifenphos and as a dust.
In most countries two to four treatments are applied at dosage rates
of 300-800 g a.i./ha, with a pre-harvest interval of 21 days. The
recommended preharvest interval in Italy is 60 days.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
Japan and in some South American countries, namely Colombia, Mexico,
Peru and El Salvador. A summary of these data is given in Table 5.
FATE OF RESIDUES
The metabolic and other reactions of edifenphos exposed to
plants, animals, fungi, soil, light and water are shown in Figures
1(a) and 1(b) taken from Umeda (1972) with some modifications.
TABLE 5. Residues of edifenphos (parent compound) in rice resulting from supervised trials
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
Rice Japan 1967 2 0.36 EC 30% <0.02
hulled 3 0.36 EC 30% <0.02
4 0.36 EC 30% <0.02
3 0.6 dust 1.5% <0.02
4 0.6 dust 1.5% <0.02
1969 4 0.6 dust 1.5% <0.01
5 0.6 dust 1.5% 0.015
3 0.6 dust 1.5% <0.01
4 0.6 dust 1.5% < 0.01
3 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% 0.01
Rice Japan 1969 2 0.36 EC 30% <0.01
polished 3 0.45 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 2.5% 0.01
3 0.5 dust 2.5% 0.01
Rice
(in husk Peru 1969 2 0.5 EC 0.07
(straw 0.15
(in husk Colombia 1969 2 0.5 EC 0.1
(straw 0.25
(in husk El Salvador 1969 2 0.5 EC 0.45
(straw 1.4
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
(in husk Mexico 1969 2 0.5 EC 0.2
(straw 0.05
15/20 21/25 26/30 31/35 36/40 41/50 51/60
Rice Japan 1970 2 0.45 EC <0.01 <0.01
hulled 4 0.45 EC <0.01
2 0.2-0.45 EC <0.01 <0.01
4 0.2-0.45 EC <0.01
2 0.45 <0.01 <0.01
4 0.45 <0.01
(hulled 1973 2 0.3-0.45 EC 0.02
(straw 0.62
(hulled 1973 3 0.3-0.45 EC 0.01
(straw 0.32
hulled 1970 2 0.6 dust 0.03 0.03
4 0.6 dust 0.01
1971 3 0.75 dust 0.03 0.01
4 0.75 dust 0.02
1970 2 0.6-0.8 dust <0.01 0.01
4 0.6-0.8 dust <0.01
1970 2 0.6-0.8 dust <0.01 <0.01
4 0.6-0.8 dust <0.01
Rice Japan 1970 2 0.6 dust 0.01 0.01
hulled 4 0.6 dust 0.02
2 1 dust 0.01 0.01
4 1 dust 0.01
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 15/20 21/25 26/30 31/35 36/40 41/50 51/60
(hulled 1972 2 <0.01 <0.01
(straw 0.48 0.26
(hulled 1972 3 1 dust <0.01
(straw 0.93
(hulled 1972 2 1 dust <0.01
(straw 0.18
(hulled 3 1 dust 0.02 <0.01
(straw 3.81 0.24
(hulled 1973 2 1 dust <0.01
(straw 0.67
(hulled 1973 3 1 dust 0.01
(straw 0.74
(hulled 1973 2 1 dust <0.01
(straw 0.72
(hulled 3 1 dust 0.1
straw) 2.12
Rice
(hulled Japan 1972 2 1 dust 0.01
(straw 2.31
(hulled
(straw 3 1 dust 0.02
5.31
(hulled 3 1 dust <0.01
(straw 3.79
In plants
Extensive data are available on absorption, translocation,
accumulation and metabolism in the rice plant. (Ishizuka et al 1973,
1974; Nitokuno 1971; Takase et al 1973; Tomizawa et al 1972; Uesugi et
al 1972, Ueyama et al 1973, Umeda et al 1972).
Absorption and translocation
Izhizuka et al (1973, 1974) found that when edifenphos was
sprayed on rice leaves 60% was lost from the surface in five days and
75% in ten days. It was shown that about 70% of the edifenphos applied
had penetrated the leaf tissue within two hours.
Only small quantities were translocated from the site of
application, and translocation from roots to shoots was also slight.
Hence the loss of edifenphos was mainly from the leaf surfaces by
volatization and decomposition, rather than by metabolism inside the
plant.
When the flag leaf of a rice plant was painted with 32P-labelled
edifenphos some translocation of the chemical to the lower part of the
leaf and to the ears was observed (Ishizuka et al 1973, 1974; Nitokuno
1971; Umeda 1972). Since no complex of edifenphos with
photosynthesized plant products was found, it appears that edifenphos
is translocated without prior metabolism. The concentration of
edifenphos in plant parts is in the order: leaves and stems >> husks
>> hulled rice.
Metabolism
Takase et al (1973) studied the metabolic fate of edifenphos
after spraying rice plants at various growth stages. The
hexane-soluble components, which were analysed throughout the
experimental period, consisted, mainly of edifenphos parent compound
(83.2-89.3% of the radioactivity in the fraction). The major
components in the water soluble fraction were identified as O-ethyl
S-phenyl phosphorothioate (metabolite VII, Figure 1(a)) and
SS-diphenyl phosphorodithioate, (III) each representing about 30% of
the total 32p in the water-soluble fraction. With the lapse of time,
ethyl phosphate (XV) was formed.
Ueyama (1973) detected nine metabolites after applying
35S-labelled edifenphos to rice leaves. Three were identified as
metabolites IV, VIII and XII (Figures 1(a) and 1(b)). It is probable
that the formation of VIII and XII involved the exchange of phenylthio
and ethoxy radicals between molecules of edifenphos.
A high proportion of S-phenyl phosphorothioate (V) and small
amounts of O-ethyl S-phenyl phosphorothioate (VII) were found among
the water-soluble metabolites. Results are shown in Table 6.
TABLE 6. Toluene extracted metabolites in and on rice plants treated with 35S-edifenphos (after Ueyama)
Metabolites, 35S counts expressed as edifenphos equivalents, mg/kg
Growth stage Part of Period after
at application Plant application Identified* Not-identified
I IV VIII XII A B C E F
Tillering leaf 1 hour 35 4.3 2.9 1.1 0.72 1
blade
6 hour 18 1.1 1.2 0.59 0.02 0.33
1 day 20 2.8 1.1 1.4 0.05 0.46
3 day 17 1.1 0.73 0.16 0.26 1.2
7 day 1.2 0.26 1.5 0.13 0.07 trace trace
15 day 0.79 0.22 - 0.18 <0.01 0.01
Tillering leaf 1 hour 0.73 trace 3.3 - trace
sheath 6 hour 0.84 trace 1.0 trace trace
1 day 0.79 trace 0.33 0.33 trace
3 day 0.65 trace 0.36 - 0.02
7 day 0.04 0.01 0.05 0.04 <0.01 trace
15 day trace - trace
Heading leaf 1 hour 42 10 1.2 trace 0.27 0.05 0.05 0.33
blade 6 hour 31 8.2 3.2 trace 0.27 0.13 0.13 0.89
1 day 9.8 2.5 1.1 0.12 0.06 0.01 0.03 0.15
3 day 7.8 1.6 0.55 trace 0.03 0.02 0.12 0.29
7 day 3.2 0.19 - 0.02 <0.01 0.03 0.37
15 day 0.40 0.38 - 0.18 0.24 0.30
* For identities of numbered metabolites, see Figures 1(a) and 1(b) (I = parent compound)
In animals
Pither and Gronberg (1975) administered 0.12 mg/kg of edifenphos
as a single oral dose and as a daily dose for 5 days to dairy cows.
Results are described above ("Evaluation for acceptable daily intake -
- Absorption, distribution and excretion").
Strankowski et al (1976) identified metabolites of 14C ring-
labelled edifenphos in the muscle, liver, kidney, fat, milk and urine
of dairy cows. Methyl phenyl sulfone (XI, Figure 1(b)) was the
principal metabolite in both milk and tissues representing 95% of the
organo-soluble radio-carbon in the milk and from 70% (kidney) to 99%
in the tissues. Small amounts of metabolites Xa, XIa, XIb, together
with other minor metabolites were also found (Table 7).
TABLE 7. Distribution of edifenphos and its metabolites in the
tissues and milk of dairy cows (Strankowski, 1976).
Residue, % of total radioactivity in organic extract,
as metabolite no.*
Tissue I IX X Xa XI XIa Xlb XIIa
muscle - - - - 85 2 - -
fat - 1 - - 99 - - -
liver <1 <1 <1 4 84 <1 <1 <1
kidney <1 - <1 2 70 2 9 <1
milk - - 1 3 93 1 1 -
* For identities of numbered metabolites see Figure 1(b),
(I = edifenphos).
Hermann and Gronberg (1976) administered 3 mg/kg 14C-edifenphos
orally to laying hens. After 96 hours 76% of the activity had been
excreted. Methyl phenyl sulfone (XI) was the only metabolite detected
in all the tissues and in egg, but a second metabolite was found in
kidneys and was identified as either IX, XII or XIII (Figure 1(b)).
The total radiocarbon residues at intervals of 6-72 hours after dosing
are given in Table 8.
TABLE 8. Total radiocarbon from 14C-edifenphos in tissues of
laying hens
Radioactivity expressed as edifenphos,
mg/kg*, after interval, hours
Tissue 6 24 48 72
Muscle (breast) 0.66 0.73 0.57 0.21
Muscle (thigh) 0.69 0.75 0.59 0.19
Skin 0.79 0.74 0.58 0.21
Fat 0.97 1.00 0.80 0.30
Liver 1.72 1.45 1.17 0.51
Kidney 1.83 1.29 0.96 0.39
Gizzard 0.72 0.76 0.56 0.21
Heart 0.82 0.89 0.66 0.26
* Values are mean figures of single analyses of tissues from each of
four hens.
Ueyama and Takas (1975) determined the residue levels in goat
milk after administration of edifenphos at the rate of 1 mg/kg/day for
ten consecutive days. No edifenphos could be detected in the milk.
After a single oral dose of 10 mg/kg, 0.0006-0.0008 mg/kg in
edifenphos could be detected within 24 hours.
Lamb and Roney (1976a) exposed crayfish to 14C-edifenphos
concentrations of 10 µg/l (i.e. 10-8) for 14 days and determined 14C
residues during the exposure period and a 28 day withdrawal period.
14C residues, expressed as edifenphos, accumulated to a maximum level
of 0.2 mg/kg. About half of the accumulated 14C was lost within 7
days of withdrawal.
The same authors (1976b) exposed Channel catfish (Ictalurus
punctatus) continuously for 28 days to 10-12 µg/l of 14C
ring-labelled edifenphos. 14C residues accumulated to a maximum of 1.2
mg/kg edifenphos equivalents in ten days, representing an accumulation
factor of 104-117. The non-edible portions (head, viscera and scales)
contained 75% of the extractable residues. The acetonitrile fraction
(81% of the total extractable residue) consisted of edifenphos 44%,
metabolite VI (Figure 1(a)) 10%, VII 19% and VIII 27%. During the
withdrawal period about 50% of the accumulated 14C residues were
excreted in five hours and about 85% within four days.
In fungi
Uesugi and Tomizawa (1971) studied the metabolism of edifenphos
in mycelia of the rice blast (Pericularia oryzae) with 32P-, 35S-
and non-labelled edifenphos. The main metabolic pathway consists in
the hydrolysis of one P-S linkage, followed by that of the other P-S
or the ethyl ester bond, finally yielding phosphoric acid. Another
hydroxylated intermediate, metabolite II (Figure 1(a)) was also found.
No significant differences in route or rate of metabolism were
found between susceptible and resistant strains of the fungus.
In storage, processing and cooking
The residue is mainly located on the rice husk, hence during
milling and polishing most of the residue is removed. Takase et al
(1973) estimated the distribution of 32P-labelled edifenphos in
milling fractions (Table 9).
The residues at harvest in the husks consisted largely of
edifenphos metabolites, although small quantities of the parent
compound were found (Table 10).
Residues of edifenphos from foliar application (0.63 kg/ha, 2
applications) were found primarily in straw and seed hulls 37 days
after the second application. The residue in the hulls ranged from
0.02-0.29 mg/kg. Trace amounts were found in the bran, but no residue
was detectable in the milled and polished rice (Chemagro, 1971).
On exposure to light
A marked degradation of 35S-edifenphos by UV light was found on
irradation in aqueous or hexane solution or as a thin film. In hexane,
the formation of water-soluble degradation products was low. The
metabolites III, VIII, XV and XVI were identified (Murai et al, 1976).
In water and soil
Shaw (1976b) and Shaw and Murphy (1976) studied the hydrolysis of
14C ring-labelled edifenphos at concentrations of 1 and 10 mg/kg in
aqueous solutions buffered at pH 3, 6 and 9 and at temperatures of 25,
35 and 45 degrees C. The hydrolysis followed first order kinetics and
its rate increased with temperature and with increasing pH. The same
major hydrolysis products were formed under all conditions, namely
compounds VII and XII (Figure 1(a) and (b)) with traces of II, III,
IV, VI, VIII and XIV.
TABLE 9. Distribution of 32P-labelled edifenphos and its metabolites in milling fractions of rice
Radioactivity (32P) expressed as edifenphos, mg,/kg
Number of treatments 1 2 3 1
Pre-harvest interval,days 13 1 24 8
Milling fraction Hexane- Water- Hexane- Water- Hexane- Water- Hexane- Water-
soluble soluble soluble soluble soluble soluble soluble soluble
husks 0.11 0.04 0.17 0.12 0.28 0.14 2.13 0.34
hulled rice trace 0.02 trace 0.01 0.06 0.03 0.31 0.05
polished rice 0.01 0.01 0.01 0.01 trace trace trace -
TABLE 10. Distribution of radio-activity from 32P-edifenphos in rice
husk.
Residue, % of total radio-activity
3 applications, 1 application,
Compound* latest 24 days 8 day pre-harvest
pre-harvest harvest
Unknown
Water-soluble metabolite 9.6 13.4
III 10.9 19.4
VII 37.6 29.1
XV, XVI 41.8 28.1
* For identities of metabolites, see figures 1(a) and 1(b).
Shaw (1976a) incubated 14C ring-labelled edifenphos in pond
water (pH 8.3) containing soil for 62 days after irradiation with UV
light and sunlight, with alternating 12-hour periods of light and
darkness. In these conditions the half-life of edifenphos was 16.8
hours. The main products were compounds VII and XIV and one or more of
compounds IX, XII and XIII. Traces of II, III, IV, X and XI were also
found. These products were found in both the water and the bottom
sludge.
Flint and Shaw (1975) estimated the half-life of edifenphos in an
outdoor simulated pond system at 20°C. Under these conditions the
half-life was 29 hours in the water and about 60 hours in the bottom
sludge.
Edifenphos is almost immobile when applied to soil. Leaching and
soil adsorption were inversely correlated and the latter was
proportional to the soil organic matter content.
When columns of sand and sandy loam soil containing edifenphos at
a level equivalent to 0.5 kg/ha were eluted with water at a rate
representing 200 mm of irrigation in two days, no edifenphos was found
in the eluate (Bayer, 1974 a, b). The half-life of edifenphos was
about 23 days in the sandy loam (pH 5.2, organic carbon 0.57% and
particles < 20 µ 19.5%), but only 6-8 days in the sand (pH 6.8,
organic bound carbon 2.50% and particles < 20 µ 10.1%). In further
laboratory experiments simulating the flooded soil conditions
associated with rice growing (Nitokuno, 1972), edifenphos was degraded
rapidly during the first 24 hours. The half-life was about 19 hours in
a clay soil and 2 1/2 days in a silt loam. Subsequent degradation was
slower in both soil types.
Tomizama (1975) reports similar results obtained in a laboratory
degradation experiment. Under the anaerobic conditions of the
experiment about half the edifenphos was degraded during the first 3 -
5 days. From the sixth day onwards the rate of degradation was
considerably slower.
Studies by McNamara (1976) and McNamara and Close (1976) were
made on the persistence and degradation of 14C ring-labelled
edifenphos in loam and silt loam under aerobic conditions and in loam
under anaerobic conditions. The half-lives were 1 day in the aerobic
silt loam and 3 days in loam under both aerobic and anaerobic
conditions. The organo-soluble radioactivity decreased to 10-20% of
that applied within 21 days in all samples. In aerobic soils the
decrease was mainly due to volatilization and binding to the soil. In
aerobic loam about 20% of the total radioactivity was lost by
volatilization in 14 days, and in silt loam 35% in 21 days.
In aerobic soil the main organo-soluble radioactive residues were
edifenphos, its initial hydrolytic products III and VII, and
derivatives of thiophenol (XIII): diphenyl disulphide XII,
benzenesulphonic acid XIV, methyl phenyl sulphoxide X and methyl
phenyl sulphone XI. The two major terminal metabolites were X and XI.
In anaerobic loam the metabolic pathway proceeded via thiophenol
(XIII) to diphenyl disulphide XII, and benzenesulphonic acid XIV. Only
small amounts of methylated products were found.
In a model ecosystem
Edifenphos was fairly rapidly degraded in a model ecosystem, the
half-life being 60 hours. The main metabolic features were the
conversion of edifenphos to compounds IV and VII and the rapid
oxidation of the diphenyl disulphide released from these compounds. It
was found that sulphur atoms derived from the diphenyl sulphide were
incorporated into sulphuric acid and then into cell constituents,
(Tomizawa and Kazano, 1975).
METHODS OF RESIDUE ANALYSIS
Vogeler (1968) developed a gas-chromatographic method for the
determination of edifenphos residues in rice, using an alkali
thermionic or flame photometric detector. The rice sample is macerated
and extracted with acetone and the filtrate evaporated to dryness. The
residue is cleaned up by acetonitrile - petroleum ether partition and
by chromatography on a Florisil column. Recoveries from control
samples fortified with 0.05-1 mg/kg ranged from 70-80%. The limit of
determination is about 0.05 mg/kg. Thornton (1971) checked several
organophosphorus compounds which are authorised or recommended for use
on rice for interference with Vogeler's method. They were tested at
levels equivalent to the highest recommended dose rate on rice in the
USA. The compounds tested were dichlorvos, fensulfothion, fenthion,
malathion, naled, parathion, parathion-methyl, phorate and TEPP. None
showed any interference.
Takase et al (1971) described a modified gaschromatographic
method for the analysis of edifenphos in rice. Extraction is with
acetonitrile and the residue is cleaned up by partitioning into
acetonitrile and n-hexane followed by TLC on alumina. The residue is
determined with a flame photometric detector. Recoveries of added
levels of 0.1 to 0.2 mg/kg ranged from 96-102%. The limit of
determination is 0.005 mg/kg or lower.
Stanley (1972) adapted and modified the Vogeler method to
determine edifenphos residues in milk and cattle tissues. For milk the
method involves initial blending with acetone, followed by separation
of edifenphos from the aqueous solution by partitioning into
chloroform. Clean-up is by partition between Skellysolve B and
acetonitrile and chromatography on a Florisil column. Animal tissues
are extracted with acetonitrile, partitioned with Skellysolve B, and
cleaned up on a Florisil column. The edifenphos parent compound is
determined by gas chromatography with a thermionic detector. The limit
of determination of edifenphos in milk is 0.002 mg/kg and in cattle
tissues 0.02 mg/kg.
Recoveries from milk spiked with 0.005-0.5 mg/kg ranged from 77
to 110%, and in various cattle tissues spiked with 0.05-0.1 mg/kg from
82 to 118%.
Sandie and Gronberg (1975) adapted the Stanley (1972) method for
the analysis of edifenphos in poultry and eggs. For poultry tissues
the method involves extraction, partition between acetonitrile and
hexane, and gas chromatography with a thermionic detector. Eggs are
extracted with acetone, and clean-up is by successive aqueous
acetone-chloroform and acetonitrile - hexane partitions, followed by
silica gel chromatography. Recoveries were 84-107% from tissues at
levels of 0.05 and 0.1 mg/kg and 86-97% from eggs at 0.01 mg/kg. The
limits of determination were 0.01 mg/kg in poultry tissues and
0.001 mg/kg in eggs.
Minor (1976) examined possible interferences by other
organophosphorus pesticides in the determination of edifenphos in
cattle and poultry tissues and eggs by the methods of Sandie and
Gronberg (1975) and Stanley (1972). He found that edifenphos could be
adequately separated from all the 25 organophosphorus pesticides with
a USA tolerance in milk if the Stanley Florisil column and the Sandie
and Gronberg silica gel column were used successively in the clean-up
procedure.
NATIONAL TOLERANCES
National tolerances reported to the meeting are given in Table
11. They refer to the parent compound only.
TABLE 11. National tolerances reported to the Meeting
Country Commodity Tolerance, Recommended
mg/kg pre-harvest
interval, days
Italy rice, hulled 0.05 60
Japan rice, hulled 0.03 21
Mexico rice, hulled 20
U.S.A. rice, hulled 0.1*
rice, hulls 0.3
* temporary tolerances valid until May 1977
APPRAISAL
Edifenphos is an organophosphorus compound with a rather
selective action against the rice blast caused by Piricularia
oryzae, one of the major rice diseases, against which it shows both
a protective and curative action. There is some evidence that the
compound interferes with the formation of chitin in the fungus
cell-wall, a process which does not occur in higher plants. This may
therefore explain the selective action of the fungicide. Edifenphos is
also effective against a few other rice diseases and some insect
pests, e.g. leafhoppers.
The pesticide is used on an extensive scale on rice, as EC
formulations and as a dust, in Asian countries including Japan, in
Central and South America and in some mediterranean countries, e.g.
Italy. In most countries 2 - 4 treatments are applied at 300 - 800 g
ai/ha, generally with a pre-harvest interval of 21 days.
Extensive data were obtained on the fate of edifenphos residues
in soil, water, rice plants and livestock animals such as cattle,
goats and poultry. Degradation pathways in plants, soil and animals
are similar, although there are quantative differences.
In a study with 35S labelled edifenphos on rice, nine
metabolites were found in a toluene extract from leaves: the three
main products were OO-diethyl S-phenyl phosphorothioate, SSS-triphenyl
phosphorotrithioate and diphenyl disulphide. A large proportion of
S-phenyl phosphorodithioate and small amounts of O-ethyl S-phenyl
phosphorothioate were found as water-soluble metabolites. In a study
with 32p-labelled edifenphos, SS-diphenyl phosphorodithioate was
identified as another major water-soluble metabolite.
In a cattle feeding study with ring-labelled 14C-edifenphos it
was shown that methyl phenyl sulphone was the principle metabolite
both in milk and in animal tissues such as muscle, liver and kidney:
in milk it amounted to about 93% of the radioactivity extracted. The
parent compound, together with m- and p-hydroxyphenyl methyl sulphone
and p-hydroxyphenyl methyl sulphoxide were found, but only in small
amounts.
After administering a single dose of 14C-labelled edifenphos
orally to laying hens (3 mg/kg), 76% of the radioactivity was excreted
in 96 hours. A single metabolite, methyl phenyl sulphone, was
identified in all tissues and eggs.
More than half of the residue from spraying edifenphos onto rice
leaves was dissipated in five days. Only small quantities were
translocated from the site of application. Translocation of edifenphos
from the roots to the shoots was also only slight.
Residues in rice at harvest are rather low. After the recommended
pre-harvest interval of 21 days, the residues of edifenphos and its
main metabolites in hulled rice following an application of
32P-labelled edifenphos to rice plants were of the order of 0.03 and
0.06 mg/kg edifenphos equivalents of water-soluble and hexane-soluble
metabolites respectively. Only traces were found in polished rice. In
the rice husks the residues were about 0.15 and 0.3 mg/kg edifenphos
equivalents of water-soluble and hexane-soluble 32P respectively.
Gas-chromatographic methods of analysis with alkali flame or
flame photometric detection are available. Recoveries in rice were
70-80% and the limit of determination ranged from 0.005 to 0.05 mg/kg
edifenphos parent compound. Nearly all organophosphorus pesticides
registered and/or recommended for use on rice were checked for
interference. None of the compounds interfered when tested at levels
that would be reached by treatments at the highest recommended
dosages, provided appropriate clean-up procedures were used. These
methods should be suitable for or adaptable to regulatory purposes.
Some of the GLC methods mentioned are suitable for the analysis
of edifenphos residues in cattle tissues, milk, poultry and eggs. The
limit of determination is 0.01 - 0.02 mg/kg parent compound.
Recoveries at levels of 0.01 - 0.1 mg/kg ranged from about 80 to 100%.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the parent compound edifenphos only.
Commodity Limit, mg/kg
Rice (in husk) 0.2
Rice (hulled) 0.05
Rice (polished) 0.01*
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Further studies to examine the hepatic involvement observed in
several animal species.
Desirable
1. Observations in man (relative to occupational exposure).
2. More information on residues of edifenphos and its main
metabolites on rice in husk at harvest.
3. A method of residue analysis suitable for edifenphos together
with its main metabolites.
REFERENCES
Anonymous Hinosan (Edifenphos)/Generation Study on Rats.
1976a Unpublished report from Nihon Tokushu Noyaku Seizo
Co., Ltd., submitted to the WHO by Bayer A.G.
* at or about the limit of determination
Anonymous Special Toxicity Test of Hinosan - I (single
1976b p.o. application). Unpublished report from Nihon
Tokushu Noyaku Seizo Co., Ltd., submitted by Bayer
A.G.
Anonymous Special Toxicity Test of Hinosan - II (14 day
1976c repeat p.o. application). Unpublished report from
Nihon Tokushu Noyaku Seizo Co., Ltd., submitted by
Bayer A.G.
Arnold, D. Mutagenic Study with Hinosan (BAY 78 418) in
1971 Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted by Bayer
A.G.
Bayer, A.G. Pflanzenschnitzmittle - Rückstunde in
1974a Sickerwasser Sickelwasser (edifenphos).
Unpublished. Reports Bayer A.G. No. 8503/8504/74.
Bayer, A.G. Verhalten des Pflanzenschutzmittelwirkstuffes
1974b edifenphos in Boden. Unpublished report Bayer A.G.
No. 8506/74.
Chen, R.S., Kinoshita, F.K. and DuBois, K.P. Acute Toxicity and
1972 Antiesterase Action of O-Ethyl-S, S-diphenyl
Phosphorodithioate (Hinosan). Tox. Appl. Pharm. 23
519-527.
Eben, A. and Kimmerle, G. Metabolism of Hinosan Active Ingredient
1972 in Rats and Dogs. Unpublished report No. 3687 from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Flint, D.R., Shaw, II, H.R. The Mobility and Persistence of Hinosan
1975 in Soil and Water. Chemagro Report No. 43 663.
Gallagher, P.J. and Prentice D.E. Pathology Report of Hinosan
1976 (SRA7847) Chronic Toxicity study in Dogs over two
years. Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Hermann, P.A. and Gronberg, R.R. Excretion and Metabolism of Hinosan
1976 in Poultry. Chemagro Report No. 49 023.
Hoffmann, K. SRA7847 (Active Ingredient of Hinosan) Chronic Toxicity
1976 Study on Dogs. Unpublished report from Institut
für Toxikologie, Bayer, A.G., submitted to the WHO
by Bayer A.G.
Imamichi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, X.,
1973a Ikadai, H., Mori, H., Furusawa, U. and Saito, K.
Hinosan Subchronic Toxicological Studies on Rats.
Unpublished report from Nippon Vet. and Zoo Techn.
College, Tokyo, Japan, submitted to the WHO by
Bayer A.G.
Imamachi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, K.,
1973b Tauchi, K., Ikadai, H., Mori, H., Furusawa, U. and
Saito, K. Hinosan-Subchronic Toxicological Studies
on Mice. Unpublished report from Nippon Vet. and
Zoo Techn. College, Tokyo, Japan, submitted to the
WHO by Bayer A.G.
Ishizuka, K., Hosaka, H., Takase, I., Tan, K.E., Hirata, H.
1974 Metabolism and Accumulation of Pesticides in Rice
Plants. Comp. Studies Food Environ. Contam.;
Inter. Atomic Energy Agency, Vienna, 363 - 372.
Kimmerle, G. Bayer 78 418 (SRA7848)/Potenzierungsversuche.
1967 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Bay 78 418/Subcronische Neurotoxizitats-versuche
1969 bei Huhnern. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Kimmerle, G. Hinosan/Acute Neurotoxicity Studies on Hens.
1971 Unpublished report from Institut für Toxikologie,
Bayer A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Dermal Toxicity of Hinosan Active Ingredient to Rats.
1972a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Acute Inhalation Experiments with Hinosan Active
1972b Ingredient on Rats. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Subchronic Inhalation Toxicity Study on Rats.
1975a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Acute Inhalation Toxicity Study on Rats.
1975b Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. and Lorke, D. Toxikologische Untersuchungen mit dem
1967 Wirkstoff, Bayer 78 418. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. and Loser, E. Delayed Neurotoxicity of Organophosphorus
1974 Compounds and Copper Concentration in the Serum of
Hens. EQS Environmental Quality and Safety
3:172-178.
Lamb, D.W. and Matskanin, C.W. The Acute Oral Toxicity of Hinosan
1976 Metabolites to Rats. Unpublished report from
Chemagro Agr. Div. Mobay Chem., Corp., submitted
to the WHO by Bayer A.G.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976a in Chanuel Catfish Exposed to Hinosan-14C.
Chemagro Report No. 46 379.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976b in Catfish Exposed to Hinosan 14C. Chemagro
Report No. 48 832.
Loeffler, W.W. The Effect of Processing on Pesticide Residues.
1972 Chemagro Report No. 34 176.
Lorke, D. Hinosan/Studies of Product for Possible Embryotoxic
1971 and Teratogenic Effects on Rats. Unpublished
report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Subchronische toxikologische Untersuchungen
1969 an Hunden. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Loser, E. SRA7847/Subchronic Toxicological Studies on Rats.
1972 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Generation Study on Rats. Unpublished report
1976a from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Chronic Toxicity Study on Rats. Unpublished
1976b report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Machemer, L. Hinosan Active Ingredient (SRA7847)/Studies of
1976 Embryotoxic and Teratogenic Effects on Rabbits
following Oral Administration. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Minor, R.G. An Interference Study for the Residue Methods for
1976 Hinosan in Milk, Eggs and Animal Tissues. Chemagro
Report No. 48 819.
Mohr, U. Subchronic Inhalation Toxicity Study on Rats,
1975 Histopathological Findings. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Murai, T., Uesugi, Y., Tomisawa, C. and Tanaka, T.
1976 Photo-Degradation of Pesticide (I), Degradation of
Organophosphorus Fungicides EDDP and IBP by
Ultraviolet Light. Chemagro Report No. 45 169.
Murphy, J.J., Feldhas, L., Simmons, C.E., Thornton, J.S. and
1976 Gronberg, R.R. Total Radioactive Residues of
(14C) Hinosan in Rotational Crops. Chemagro
Report No. 48 826.
McNamara, F.T. Persistence and Metabolism of Hinosan in Soil.
1976 Chemagro Report No. 49 025.
McNamara, F.T. and Close, C.L. Persistence and Metabolism
1976 of Hinosan in Soil under Aerobic Conditions.
Chemagro Report No. 46 402.
Nitokuno Hinosan, a Fungicide for Control of Rice Blast,
1971 Chem. Econ. Engin. Rev. 3 (1), 57-59.
Nitokuno Gas Chromatographic Determination of Residues of
1972 Hinosan (O-ethyl S,S-diphenyl phosphorodithiolate)
in Rice Grains. Unpublished report.
Nitokuno Special Toxicity Test of Hinosan I. (single p.o.
1976 application). Unpublished report of Nihon Tokushu
Noyaku, Seizo Co., submitted by Bayer A.G. to the
JMPR.
Offer, J.M. and Prentice, D.E. Pathology Report of Bay 78 418
1976 (Hinosan) Chronic Toxicity Testing on Rats.
Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Pither, K.M. and Bronberg, R.R. Residue Levels in Lactating
1975 Dairy Cattle following Oral Administration of
Hinosan-ring-Ul-14C. Unpublished report No. 44
845 from Chemagro Agr. Div. Mobay Chem. Corp.,
submitted to the WHO by Bayer A.G.
Sandie, F.E. and Gronberg, R.R. A Gas Chromatographic Method
1975 for the Determination of Residues of Hinosan in
Poultry Tissue and Eggs. Chemagro Report No. 45
987.
Shaw, II, H.R. Hydrolysis of Hinosan in Pond Water Containing Soil.
1976a Chemagro Report No. 48 829.
Shaw, II, H.R. Hydrolysis of Hinosan in Buffered Aqueous Solution.
1976b Chemagro Report No. 48 831.
Shaw, II, H.R. and Murphy, J.J. Hydrolysis of Hinosan in Buffered
1976 Aqueous Solution. Chemagro Report No. 46 403.
Shirasu, Y., Moriya, M. and Kato, K. Report of the Mutagenicity
1976a Study of Hinosan (EddP, edifenphos). Unpublished
report from the Department of Toxicology,
Institute of Environmental Toxicology, Tokyo,
Japan, submitted to the WHO by Bayer A.G.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. and Kada, T.
1976b Mutagenicity Screening of Pesticides in the
Microbial System. Mutation Research 40:19-30.
Spicer, E.J.F. Pathology Report of Bay 78 418/Hen Study.
1971a Unpublished report from Huntingdon Research
Central submitted to the WHO by Bayer A.G.
Spicer, E.J.F. Pathology Report of Bay 78 418/Subchronic
1971b Neurotoxicity Study in Hens. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Spicer, E.J.F. and Erwin, C. Pathology Report of Bay 78 418/
1971 Subchronic Toxicity Studies in Dogs. Unpublished
report from Huntingdon Research Centre, submitted
to the WHO by Bayer A.G.
Stanley, C.W. Gas Chromatographic Method for the Determination
1972 of Residues of Hinosan in Animal Tissues and Milk.
Chemagro Report No. 32 070.
Strankowski, K.J., Pither, K.M. and Murphy, J.J. Metabolism of
1976 Hinosan by a Lactating Dairy Cow. Unpublished
report No. 48 846 from Chemagro Agr. Div. Mobay
Chem. Corp., submitted to the WHO by Bayer A.G.
Takase, I., Nakamura, S., Tsuda, H. and Ueyama, I. The Gas
1971 Chromatographic Determination of Pesticides. (Part
4). Cleanup Method for Organophosphorus Pesticide
Residues in Rice Grain. Noyaku Seisan Gijutsu
(Pesticide and Technique) 23:31-35.
Takase, I., Tan, K.E. and Ishizuka, K. Metabolic Transformation
1973 and Accumulation of O-Ethyl S,S-Diphenyl
Phosphorodithiolate (Hinosan) in Rice Plants. Agr.
Biol. Chem. 37(7): 1563-1571.
Thornton, J.S. A Study of the Possible Interferences of Other
1971 Pesticides with the Gas Chromatographic Residue
Method for Hinosan. Chemagro Report No. 29 702.
Thyssen, J. and Kimmerle, G. Hinosan 50 E.C./Toxicological Studies.
1974 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Tomizawa, C. Degradation of Organophosphorus Pesticides in Soils
1975 with Special Reference to Unaerobic Soil
Conditions. Environ. Qual. Safety 4:117-127.
Tomizawa, C., Uesugi, Y. and Murai, T. Fats and Significance of
1972 Fungicides in Rice. Radiotracer Studies Chem. Res.
Food. Agric.; Intern. Atomic Energy Agency,
Vienna, 103-105.
Tomizawa, C. and Kazano, H. Biological Accumulation of Pesticides
1975 in an Ecosystem. Rev. Plant Protec. Res.
8:41-54.
Uesugi, Y. and Tomizawa, C. Metabolism of O-Ethyl S,S Diphenyl
1971 Phosphorodithiolate (Hinosan) by Mycelial Cells of
Pyricularia Oryzae. Agric. Boil. Chem. 35
(6):941-949.
Uesugi, Y. Tomizawa, C. and Murai, T. Degradation of
1972 Organophosphorus Fungicides. Environ. Toxicol.
Pest. Academic Press, 327-339.
Ueyama, I., Uesugi, Y., Tomizawa, C. and Murai, T. Metabolic
1973 Fate of O-Ethyl S,S-Diphenyl Phosphorodithiolate
(Hinosan) in Rice Plant. Agr. Biol. Chem.
37(7):1543-1551.
Ueyama, I. and Takase, I. Metabolic Behaviour of O-Ethyl
1975 S,S-Diphenyl Phosphorodithiolate (edifenphos) in
Female Goat. Agr. Biol. Chem. 39(9):1719-1727.
Ueyama, I., Takase, I. and Tomizawa, C. The Metabolism of Edifenphos
1976 (Hinosan) in Mice and Rats. Unpublished report No.
1046 from Nithon Tokushu Noyaku Seizo Co. .td.
Agr. Chem. Inst. and National Institute of Agr.
Sciences, submitted to the WHO by Bayer A.G.
Umeda, Y. Anti-Blast Fungicide "Hinosan" and its Biological
1972 Properties and Metabolism in the Rice Plant. Japan
Pest. Inform. No. 10, 85-88.
Umeda, Y. Hinosan, a Fungicide for Control of Rice Blast.
1973 Japan Pest Inform. No. 17, 25-28.
Urwin, C. and Newman, A.J. Pathology Report of SRA7847/3
1972 Month Feeding Study in Rats. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Vogeler, K. Caschromatographische Methods zur Bestimmung von
1968 Hinosan-Rückständen in Reis. Pflanzenschutz
Nachr., Bayer 21 (3):321-325.
Yatomi, A. Mutagenicity Test on Bacteria. Unpublished report
1975 from Nihon Tokushu Seizo K.K., submitted to the
WHO by Bayer A.G.
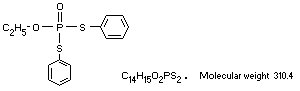 C14H15O2PS2. Molecular weight 310.4
Other information on identity and properties
Physical and chemical properties
Physical state: the technical product has a yellow to
light brown colour.
Boiling point: 154° at 0.01 mm Hg.
Specific gravity: 1.23 at 20°/4°C.
Vapour pressure: 10-4 to 10-2 mm Hg at 20-100°C.
Solubility: insoluble in water, soluble in acetone
and xylene.
Stability: half-life values: 49 hours at 25°C and
pH 9; 1135 hours at 25°C and pH 7.
Composition of the technical product
The technical product contains a minimum of 87% of edifenphos.
The main impurities are:
SSS-triphenyl phosphorotrithioate max 7%
OO-diethyl S-phenyl phosphorothioate max 3.5%
diphenyl sulphide max 2.5%
toluene max 1.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Edifenphos is rapidly absorbed following acute oral
administration to rats. Within 8 hours of dosing the major part of
an 35S-dose was absorbed. Within 72 hours of the administered dose,
96% in rats and > 97% in mice was excreted in the urine and feces.
There were no significant sex differences noted in rats, while in
mice females excreted somewhat more in the feces than corresponding
males. Apart from this no sex differences in absorption and
excretion were noted. Tissue residues (35S-labelled) in both rats
and mice were extremely low at 72 hours. Residues in tissues
following acute or sub-acute (10-daily) doses were generally low
and associated with tissues concerned with distribution and
excretion. Of the 6 tissues examined at various intervals after
dosing (from 1 to 72 hours) the following sequence reflects tissue
distribution: liver > kidney > lung > heart >> blood = brain.
This same qualitative relationship of tissue distribution was
reflected over the time for maximum tissue clearance (Ueyema, et
al., 1976).
Absorption of a single oral dose of edifenphos (0.12 mg/kg
b.w.) in a lactating cow was slow than seen with the rat, mouse,
dog and even lactating goat. In the cow, the blood residue reached
a maximum 20 hours after a single oral dose. Thirty percent of the
administered dose was excreted during the first day after dosing
and another 30% during the following 4 days. Twenty seven percent
of the dose was excreted in the feces in 5 days and < 3% was
observed as a residue in milk (not above 0.09 mg/kg edifenphos
equivalents at any time). In a study where a cow was administered
5 consecutive oral daily doses of edifenphos (0.12 mg/kg. b.w.),
blood levels of edifenphos (expressed as equivalents) increased from
0.05 mg/kg after the first application to 0.2 ppm after the fifth.
A residue plateau was not observed during this time. In urine and
milk, the increase of total residues was less pronounced and seemed
to level off after the third application at a concentration of 4 and
0.18 mg/kg respectively (Pither and Gronberg, 1975). Residues in the
tissues at slaughter, 20 hours after the last dose, ranged from 0.13 to
0.67 mg/kg edifenphos equivalents.
Biotransformation
Qualitative determination of the fate of edifenphos in rats
and dogs was reported following a single oral dose. No edifenphos
was observed in urine but rather the hydrolysis products were
observed both free and conjugated. A difference was observed
between male and female rats where two unidentified products were
observed in female urine that were not seen in males. (Eben and
Kimmerle, 1972). In contrast, no such differences with respect to
sex were noted in another study using a 35S-labelled product. In
rats, dogs, mice and goats, a similar pattern of metabolic
breakdown was reported. The metabolic pathway followed in animals,
rice plant and the rice blast fungus, together with photochemical
and weathering reactions, are illustrated in Figures 1(a) and 1(b).
All radioactivity in the urine of rats and mice consisted of
conjugated or water soluble metabolites. The major metabolite in
rats was the des-S-phenyl hydrolysis product while in mice the
mono-S-phenyl phosphorothioate was prevalent (further dealkylation
of the major rat metabolite). In feces, small (< 2%) quantities of
edifenphos were noted as well as diphenyl disulfide (Ueyama et al.,
1976).
Metabolism in other animals, plants and fungi is discussed in
the section "Fate of residues".
Effects on Enzymes and Other Biochemical Parameters
Rabbits were orally administered edifenphos and liver function
tests performed at various times (up to 7 days) after poisoning. No
effects were noted on SGPT or SDH activity or BSP retention
following a dose of 50 mg/kg, while there were effects reported at
100 mg/kg (Kimmerle and Lorke, 1967).
A single oral application of edifenphos to rats (150 mg/kg)
increased liver weight 24 hours after the treatment. While no
changes were observed in body weight, relative liver weight changes
were observed at lower doses (> 25 mg/kg) within 24 hours. One
week after treatment the highest treatment group still showed an
increased relative liver weight while other groups appeared to have
recovered. In this study, changes in liver size were not reflected
in changes in certain liver function tests (SGOT activity, SGPT
activity, Cholesterol content and glucuronidase activity). Slight
changes were noted at 24 hours in the A/G ratio, S-protein content,
certain drug metabolizing enzyme assays (EPN detoxication but not
aniline hydroxylation or aminopyrine demethylation) and, as expected,
cholinesterase activity. Within 7 days these liver changes had
recovered and values were normal. Gross and microscopic examination
did not suggest a pathological condition.
Cholinesterase depression measured at 24 hours after treatment
was dose dependent and sensitivity was as follows: RBC>plasma>
brain. At 7 days after treatment, recovery of plasma cholinesterase
was complete while RBC and brain continued to show a reduction at
the higher dose levels (Anonymous, 1976b).
Acute signs of poisoning reflect acute depression of
cholinesterase activity. Within 3 hours of following oral
intubation of edifenphos (25-50 mg/kg - male rats) significant
depression of whole blood cholinesterase was observed. After a dose
of 10 mg/kg the enzyme activity was not depressed. At higher levels
the effects were persistent, lasting for three days. All enzyme
activity was normal 7 days following acute exposure (Kimmerle /
Lorke, 1967).
In vitro studies with rat brain also demonstrated the
sensitivity of this cholinesterase source to edifenphos with an I50
of 1.05 x 10-6 being noted. In vivo inhibition following
poisoning was rapid and the duration of recovery was long with full
recovery not seen until 2-3 weeks after treatment. While there was
an obvious sex difference seen in acute toxicity, the recovery of
cholinesterase activity did not reflect a sex difference. The rate
of recovery of cholinesterase (brain, serum or submaxillary gland)
following an LD50 dose was the same in both males and females.
In addition to its effects on acetylcholinesterase, edifenphos
was found to be an effective inhibitor of aliesterase activity.
Dietary administration of edifenphos at levels resulting in 50%
inhibition of liver and serum aliesterase activity in rats are seen
in Table 1. A dietary level of 5 ppm for one week reduced esterase
activity (those hydrolyzing tributyrin) by 50% in the liver.
TABLE 1. Dietary Levels (ppm) Inducing-50% Aliesterase
Inhibition in Rats
Substrate Male Female
Liver Serum Liver Serum
Tributyrin 4.9 8.4 5.4 9.5
Diethylsuccinate 18.4 10.2 11.5 7.4
(Chen, et al. 1972)
C14H15O2PS2. Molecular weight 310.4
Other information on identity and properties
Physical and chemical properties
Physical state: the technical product has a yellow to
light brown colour.
Boiling point: 154° at 0.01 mm Hg.
Specific gravity: 1.23 at 20°/4°C.
Vapour pressure: 10-4 to 10-2 mm Hg at 20-100°C.
Solubility: insoluble in water, soluble in acetone
and xylene.
Stability: half-life values: 49 hours at 25°C and
pH 9; 1135 hours at 25°C and pH 7.
Composition of the technical product
The technical product contains a minimum of 87% of edifenphos.
The main impurities are:
SSS-triphenyl phosphorotrithioate max 7%
OO-diethyl S-phenyl phosphorothioate max 3.5%
diphenyl sulphide max 2.5%
toluene max 1.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
Edifenphos is rapidly absorbed following acute oral
administration to rats. Within 8 hours of dosing the major part of
an 35S-dose was absorbed. Within 72 hours of the administered dose,
96% in rats and > 97% in mice was excreted in the urine and feces.
There were no significant sex differences noted in rats, while in
mice females excreted somewhat more in the feces than corresponding
males. Apart from this no sex differences in absorption and
excretion were noted. Tissue residues (35S-labelled) in both rats
and mice were extremely low at 72 hours. Residues in tissues
following acute or sub-acute (10-daily) doses were generally low
and associated with tissues concerned with distribution and
excretion. Of the 6 tissues examined at various intervals after
dosing (from 1 to 72 hours) the following sequence reflects tissue
distribution: liver > kidney > lung > heart >> blood = brain.
This same qualitative relationship of tissue distribution was
reflected over the time for maximum tissue clearance (Ueyema, et
al., 1976).
Absorption of a single oral dose of edifenphos (0.12 mg/kg
b.w.) in a lactating cow was slow than seen with the rat, mouse,
dog and even lactating goat. In the cow, the blood residue reached
a maximum 20 hours after a single oral dose. Thirty percent of the
administered dose was excreted during the first day after dosing
and another 30% during the following 4 days. Twenty seven percent
of the dose was excreted in the feces in 5 days and < 3% was
observed as a residue in milk (not above 0.09 mg/kg edifenphos
equivalents at any time). In a study where a cow was administered
5 consecutive oral daily doses of edifenphos (0.12 mg/kg. b.w.),
blood levels of edifenphos (expressed as equivalents) increased from
0.05 mg/kg after the first application to 0.2 ppm after the fifth.
A residue plateau was not observed during this time. In urine and
milk, the increase of total residues was less pronounced and seemed
to level off after the third application at a concentration of 4 and
0.18 mg/kg respectively (Pither and Gronberg, 1975). Residues in the
tissues at slaughter, 20 hours after the last dose, ranged from 0.13 to
0.67 mg/kg edifenphos equivalents.
Biotransformation
Qualitative determination of the fate of edifenphos in rats
and dogs was reported following a single oral dose. No edifenphos
was observed in urine but rather the hydrolysis products were
observed both free and conjugated. A difference was observed
between male and female rats where two unidentified products were
observed in female urine that were not seen in males. (Eben and
Kimmerle, 1972). In contrast, no such differences with respect to
sex were noted in another study using a 35S-labelled product. In
rats, dogs, mice and goats, a similar pattern of metabolic
breakdown was reported. The metabolic pathway followed in animals,
rice plant and the rice blast fungus, together with photochemical
and weathering reactions, are illustrated in Figures 1(a) and 1(b).
All radioactivity in the urine of rats and mice consisted of
conjugated or water soluble metabolites. The major metabolite in
rats was the des-S-phenyl hydrolysis product while in mice the
mono-S-phenyl phosphorothioate was prevalent (further dealkylation
of the major rat metabolite). In feces, small (< 2%) quantities of
edifenphos were noted as well as diphenyl disulfide (Ueyama et al.,
1976).
Metabolism in other animals, plants and fungi is discussed in
the section "Fate of residues".
Effects on Enzymes and Other Biochemical Parameters
Rabbits were orally administered edifenphos and liver function
tests performed at various times (up to 7 days) after poisoning. No
effects were noted on SGPT or SDH activity or BSP retention
following a dose of 50 mg/kg, while there were effects reported at
100 mg/kg (Kimmerle and Lorke, 1967).
A single oral application of edifenphos to rats (150 mg/kg)
increased liver weight 24 hours after the treatment. While no
changes were observed in body weight, relative liver weight changes
were observed at lower doses (> 25 mg/kg) within 24 hours. One
week after treatment the highest treatment group still showed an
increased relative liver weight while other groups appeared to have
recovered. In this study, changes in liver size were not reflected
in changes in certain liver function tests (SGOT activity, SGPT
activity, Cholesterol content and glucuronidase activity). Slight
changes were noted at 24 hours in the A/G ratio, S-protein content,
certain drug metabolizing enzyme assays (EPN detoxication but not
aniline hydroxylation or aminopyrine demethylation) and, as expected,
cholinesterase activity. Within 7 days these liver changes had
recovered and values were normal. Gross and microscopic examination
did not suggest a pathological condition.
Cholinesterase depression measured at 24 hours after treatment
was dose dependent and sensitivity was as follows: RBC>plasma>
brain. At 7 days after treatment, recovery of plasma cholinesterase
was complete while RBC and brain continued to show a reduction at
the higher dose levels (Anonymous, 1976b).
Acute signs of poisoning reflect acute depression of
cholinesterase activity. Within 3 hours of following oral
intubation of edifenphos (25-50 mg/kg - male rats) significant
depression of whole blood cholinesterase was observed. After a dose
of 10 mg/kg the enzyme activity was not depressed. At higher levels
the effects were persistent, lasting for three days. All enzyme
activity was normal 7 days following acute exposure (Kimmerle /
Lorke, 1967).
In vitro studies with rat brain also demonstrated the
sensitivity of this cholinesterase source to edifenphos with an I50
of 1.05 x 10-6 being noted. In vivo inhibition following
poisoning was rapid and the duration of recovery was long with full
recovery not seen until 2-3 weeks after treatment. While there was
an obvious sex difference seen in acute toxicity, the recovery of
cholinesterase activity did not reflect a sex difference. The rate
of recovery of cholinesterase (brain, serum or submaxillary gland)
following an LD50 dose was the same in both males and females.
In addition to its effects on acetylcholinesterase, edifenphos
was found to be an effective inhibitor of aliesterase activity.
Dietary administration of edifenphos at levels resulting in 50%
inhibition of liver and serum aliesterase activity in rats are seen
in Table 1. A dietary level of 5 ppm for one week reduced esterase
activity (those hydrolyzing tributyrin) by 50% in the liver.
TABLE 1. Dietary Levels (ppm) Inducing-50% Aliesterase
Inhibition in Rats
Substrate Male Female
Liver Serum Liver Serum
Tributyrin 4.9 8.4 5.4 9.5
Diethylsuccinate 18.4 10.2 11.5 7.4
(Chen, et al. 1972)

 Cholinesterase depression in rats was observed following acute
inhalation exposure to aerosolized edifenphos. Plasma and RBC
cholinesterase depression was seen following exposure to 30 mg/m3
for 4 hours (threshold level). No depression was noted at an
exposure concentration of 9 mg/m3 for 4 hours (Kimmerle, 1975b).
TOXICOLOGICAL STUDIES
Special Studies on Teratology
Groups of rats (20 mated females/group) were administered
edifenphos by oral gavage at doses of 0, 5, 15 and 30 mg/kg body
weight from day 6 to 15 of gestation. All animals were sacrificed
and pups delivered by Caesarean Section. Increased mortality
reduced growth and an unhealthy appearance were observed at the two
highest dose levels. At the highest dose level, 3 litters were
resorbed. There were no abortions and with the exception of the 3
resorbed litters, the numbers of implantations, fetuses and
resorptions did not differ from control values. The weights of
fetuses were not different from controls and the nature and
frequency of somatic and skeletal variations were within normal
limits. There were no malformations and no indications of a
teratogenic potential was noted in this study (Lorke, 1971).
Groups of rabbits (11-13 mated does/group) were administered
edifenphos by oral gavage at dose levels of 0, 3, 6 and 12 mg/kg
body weight from day 6 to 18 of gestation. On day 29 of gestation,
the does were sacrificed and Caesarean Section performed. There was
no mortality although the two highest dose levels resulted in
reduced growth (the highest level treatment showed a significant
weight loss). In the 6 mg/kg group one doe aborted and one resorbed
all fetuses. Implantation recorded for the two highest doses was
reduced. However, the significance of this was not apparent as
treatment did not begin until 6 days after fertilization and
edifenphos treatment should not have affected this parameter. There
were no differences in the number of fetuses in the 0 and 3 mg/kg
dose while the upper two doses had fewer fetuses. The weight of
fetuses and placenta of the treated group did not differ from
control values. Malformations were not observed on gross or
skeletal examination of fetuses. As suggested with rats, edifenphos
did not induce a teratological response in rabbits (Machemer,
1976).
Special Studies on Reproduction
Groups of rats (10 males and 20 females/group) were fed
edifenphos in the diet at concentrations of 0, 5, 15 and 150 ppm
and mated to begin a standard 3 generation, 2 litter per
generation, reproduction study. Reproductive indices included:
fertility, gestation, viability and lactation measured for all 6
litters. There was no mortality observed and the highest dietary
concentration did not affect fertility. Litter size was reduced and
in one instance the litter weight was reduced at the 150 ppm level.
Slight effects (not statistically significant) were noted on
viability and lactation indices in the high dose group. Histological
examination of the F3b generation gave no indication of adverse
effects on the major tissues and organs. A no effect-level in this
study would be 15 ppm (Loser, 1976).
Groups of rats (20 females/group) were fed edifenphos in the
diet at dosage levels of 0, 5, 15 and 50 ppm, mated and subjected
to a standard 3 generation, 2 litters per generation, reproduction
study, In combination with the study, additional groups of 5
females from the P and F1b and 10 females from the F2b were
sacrificed at day 20 of gestation for an examination of fetuses for
any teratological events. Additionally, offspring of 5 female rats
from individual groups of the F1b, F2b and 10 females from the P3b
were maintained after weaning at the same dietary concentrations of
edifenphos listed above for periods ranging from 1 to 6 months.
These short term studies were designed to evaluate growth and
development of rats exposed initially in utero and thereafter for
short periods to dietary levels of edifenphos.
Growth of parental generations was unaffected by the presence
of edifenphos in the diet. Slight reduction of male food intake and
body weight at 50 ppm was noted in the first parental generation.
The reduced food consumption was observed in the F2 and F3 parents
accompanied by slight reduction of growth. The reduced growth was
predominant in those animals fed after weaning for prolonged
periods.
There were no significant differences noted in the
reproduction indices over the course of the three generations.
Edifenphos did not affect the ability of the rats to reproduce,
maintain pregnancy, deliver, nurse and wean normal sized litters
over the course of three generations. A teratological examination
of fetuses delivered by Caesarean Section prior to term did not
show any abnormalities related to somatic or skeletal defects. In
the first generation the 50 ppm dosed group reflected a slight
retardation in the degree of bone ossification not always noted in
the two lower doses or the controls. There were no significant
differences in implants, resorptions, live fetuses or in the weight
of fetuses.
In those weanlings from the F1b, F2b and F3b litters that were
maintained on diets containing edifenphos for 1 to 6 months after
weaning, growth was not substantially affected. Slight reductions
of growth were noted at 50 ppm accompanied by reduced food intake.
Hematological examinations made at the conclusion of each time
interval were normal. Liver function tests including SGOT and SGPT
activity and cholinesterase determinations using plasma, RBC and
brain were normal. Gross and microscopic examination of tissues and
organs of the animals maintained after weaning showed only on
consistent event attributable to edifenphos in the diet. An
enlarged kidney in the 50 ppm group fed for 3-6 months was
accompanied by changes (saccular necrosis) in the tubular
epithelium noted on microscopic examination. No dose related
changes were noted in a variety of tissues and organs that could be
attributable to edifenphos in the diet (Anonymous, 1976a).
Special Study on Mutagenicity
Mice
A dominant lethal study using male mice was performed with
edifenphos. Groups of mice (12 males/group) were administered
edifenphos by a single intraperitoneal injection at doses of 0, 50
and 100 mg/kg. A positive control (MMS, methyl methansulfonate, 100
mg/kg) was included in the study. The males were mated with three
untreated virgin females. The females were changed weekly for 6
consecutive weeks to evaluate maturation of male mouse germ cells.
One week after breeding, the females were sacrificed and examined
for implantation sites, resorption sites and embryos.
There were no deaths as a result of treatments with
edifenphos. Except for a slight reduction in the mating index at
week 6 in the 100 mg/kg dose group, there was no effect on the
ability to mate or fertilise the females. There were no
dose-related effects noted with respect to implantation, resorption
or embryo viability. There was no increase in pre-implantation loss
or mutuation rate although the positive control showed a
significant increase in early resorptions, attesting to the species
susceptibility. Edifenphos did not demonstrate a mutagenic
potential by the dominant lethal test in mice (Arnold, et al.
1971).
Microorganisms
When examined in vitro with various strains of
microorganisms (B. subtilis, E. coli and S. typhimurium
edifenphos did not induce a mutagenic response. In two studies with
the aid of a B. subtilis strain lacking the recombination
repair system, S. typhimurium and E. coli with either a
histidine or tryptophane deficiency, edifenphos, examined for its
ability to induce mutations, was found to be negative. Positive and
negative controls were used in these assays to assure sensitivity
to the mutagenic activity and to provide a baseline of operation.
In addition, liver homogenates (fortified with cofactors necessary
for microsomal oxidation) were used to activate, if possible,
edifenphos to an active mutagen. In studies with reversion colonies
in combination with a microsomal activation system, edifenphos did
not induce mutations (Shirasu et al., 1976a; 1976b; Yatomi, 1975).
A host mediated assay was performed where groups of 5 mice
were orally administered two doses of 0, 50 or 100 mg edifenphos/kg
(within 24 hours). After the second dose, a microbiological test
system of S. typhimurium was injected into the peritoneal
cavity, removed after 3 hours and examined for revertant colonies.
In this assay, edifenphos was negative although a positive control
of dimethylnitrosamine (50 mg/kg, oral administration) induced
reversion at a substantial rate (Shirasu. et al., 1976a).
Special Studies on Potentiation
Oral administration of equitoxic doses (LD50) of edifenphos
with fenthion or propoxur did not result in potentiation of the
acute toxicity of the combination. (Kimmerle, 1967).
A greater than additive toxicity, observed with a combination
of malathion and edifenphos, suggested a potentiating effect with
these two pesticides (Kimmerle and Lorke, 1967). Further studies on
toxic interaction of two organophosphorus pesticides were
undertaken where rats fed 0, 5 or 25 ppm edifenphos in the diet
were administered malathion by ip injection at a sublethal dose
(400 mg/kg). The increased mortality observed in the two groups fed
edifenphos suggested a significant potentiating effect on malathion
toxicity (Chen, et al., 1972).
Special Studies on Antidotes
Following acute oral administration of edifenphos to male
rats, antidotal administration (ip injection) of atropine (50
mg/kg) alone or in combination with 2-PAM or BH6 (50 or 20 mg/kg,
respectively) resulted in a slight reduction in the acute toxicity
(less than 2 fold) (Kimmerle and Lorke, 1967).
Special Studies on Neurotoxicity
Edifenphos was administered to hens by oral intubation or
intraperitoneal injection at dose levels up to 1000 mg/kg. The
surviving animals were examined for 42 days with no signs of
delayed neurotoxicity (Kimmerle and Lorke, 1967).
Groups of hens were administered edifenphos by oral intubation
at the LD50 level (547 mg/kg body weight) together with atropine,
50 mg/kg administered by ip injection. Delayed neurotoxic signs of
poisoning were not seen, but were observed in all hens treated
orally with TOCP (350 mg/kg). The hens were observed for 3 weeks
and sacrificed (Kimmerle, 1971). Histological examination of
portions of the central and peripheral nervous system of the
edifenphos-treated hens was negative with respect to axon or myelin
disruption. The TOCP-treated hens showed axon and neuron
degeneration in the spinal cord (brain and peripheral nerve were
not affected) (Spicer, 1971a).
In an effort to evaluate the relationship of delayed
neurotoxicity and copper imbalance in hens, an examination of
several organophosphates inducing a delayed neurotoxicity was made.
Edifenphos as one of the negative compounds did not induce
neurotoxicity and did not increase copper and ceruloplasmin levels
as did several of the positive neurotoxic agents (Kimmerle and
Loser, 1974).
Groups of hens (8 hens/group) were fed edifenphos in the diet
for 30 days at concentrations of 0, 100, 250, 500 and 1000 ppm.
Growth was depressed at 500 ppm and above. Whole blood
cholinesterase was depressed after 30 days only in the highest dose
group. Animals allowed to recover on control diets for a 4 week
period recovered body weight and cholinesterase depression was
reduced. Neurotoxic clinical signs were not observed (Kimmerle,
1969). Histological examination of brain, spinal cord and
peripheral nerve showed no evidence of myelin degeneration (Spicer,
1971b).
Acute Toxicity
TABLE 2. Acute toxicity of edifenphos
LD50
Species Sex Route (mg/kg) Reference
Rat M oral 119-340 Kimmerle, 1967;
Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 48-81 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974;
Chen, et al., 1972
dermal
(24 hour exposure) 184-413 Kimmerle, 1972a Thyssen &
Kimmerle, 1974
(4 hour exposure) 1,230 Kimmerle & Lorke, 1967
(7 hour exposure) 600 Kimmerle & Lorke, 1967
F oral 63-150 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 26-55 Thyssen & Kimmerle, 1974;
Kimmerle & Lorke, 1967;
Chen, et al., 1972
dermal
(24 hour exposure) 86-135 Kimmerle, 1972a,
Thyssen & Kimmerle, 1974
Mice M oral 392 Thyssen & Kimmerle, 1974
TABLE 2. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
Mice F oral 218-295 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Guinea Pig oral 350-400 Kimmerle & Lorke, 1967
Rabbit F oral 250-400 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Cat oral >250 Kimmerle & Lorke, 1967
Note -- In some instances the range of values is reflective of
differences in solvents used in the test. The lower values were
obtained with Lutrol while the higher values were obtained with
ethanol: propylene glycol mixtures. In all cases there appears to be a
significant sex difference with females more susceptible than males.
Signs of poisoning were typical of cholinergic stimulation (tremors,
salivation, labored respiration, etc.) appearing 2-3 hours after acute
poisoning and persisting for up to 2 days with rats and up to a week
with mice and rabbits.
Inhalation Toxicity
Acute aerosol inhalation toxicity tests (both static and dynamic
flow) were performed with groups of rats using various exposure times.
Edifenphos technical or a 50% EC formulation (Hinosan(R)) was used in
several studies. Data expressed as the technical product do not show
significant differences in toxicity between the two forms of
edifenphos. No indication of particle size distribution was given in
these data.
Dynamic tests
Results of dynamic tests are given in Table 3.
TABLE 3. Inhalation toxicity of edifenphos (dynamic tests)
Exposure LD50
Species Sex Time (mg/m3) Reference
Rat M 1 hour >898-1310 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b;
Kimmerle & Lorke, 1967
TABLE 3. (Cont'd.)
Exposure LD50
Species Sex Time (mg/m3) Reference
4 hour 362 - 775 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b.,
Kimmerle, 1975b;
Kimmerle & Lorke, 1967
F 1 hour > 1017-1046 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b
4 hour 230 - 320 Thyssen & Kimmerle, 1974;
Kimmerle, 1975b;
Kimmerle, 1972b
Static Tests
Groups of rats, mice, rabbits and guinea pigs were exposed to
static concentrations of edifenphos ranging from 108 to 398 mg/m3 for
up to 4 hours. In the 1 hour exposure, no toxicity was recorded (only
one dose level was tested) but mice exhibited toxic signs of exposure.
In the 4 hour exposure the susceptibility of rats, rabbits and mice
was the same with 7/10 rats, 2/3 rabbits and 12/20 mice dying at a
concentration of 269 mg/m3. Only 1/5 guinea pigs died at this
exposure. No deaths were reported at a concentration of 101 mg/m3 for
the 4 hour exposure (Kimmerle & Lorke, 1967).
Edifenphos did not induce a primary dermal irritation when
applied to the skin of rabbits' ears for up to 24 hours or to the
conjunctival sac of rabbits. The 50 EC formulation of Hinosan(R)
irritated both the conjunctiva and skin (1 hour exposure). The primary
irritation was attributed to the formulation ingredients and not
technical edifenphos (Kimmerle and Lorke, 1967; Thyssen and Kimmerle,
1974).
Toxicity of metabolites
Acute toxicities of edifenphos metabolites to rats are shown in
Table 4.
TABLE 4. Acute toxicity of edifenphos metabolites to rats
Compound Route LD50
(mg/kg)
O-ethyl S-phenyl oral M >500
S-(4-hydroxyphenyl)- F >1000
phosphorodithioate
SS-diphenyl oral M 1000-2000
phosphorodithioate F 1000-2000
O-ethyl S-phenyl oral M 1000-2000
phosphorothioate F 616
O-ethyl S-phenyl oral M approx. 1000
S-(3-hydroxyphenyl) F approx. 500
phosphorodithioate
(Lamb & Matzkanin, 1976)
Short-term studies
Mice
Groups of ICR mice (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. In general, growth was not affected by dietary
edifenphos. Food consumption, behaviour, physical condition and
mortality were unaffected. Plasma cholinesterase depression was noted
at 30 ppm with RBC and brain depressed only at 1000 ppm. Hematology,
urinalysis and clinical chemistry parameters were normal. On gross
examination of tissues and organs, spleen and liver enlargement was
observed at 1000 ppm. Microscopic examinations showed no abnormalities
in spleen although liver and adrenal changes were noted at 300 ppm and
above. In liver, hypertrophy of hepatocytes and deposition of
phospholipid were observed. Adrenal hypertrophy and pigment deposition
were noted at the two highest doses (Imamichi, et al., 1973b). A
no-effect level of 10 ppm was observed equivalent to 1.53 mg/kg body
weight.
Rats
Groups of rats (10 males and 10 females/group) were exposed to
edifenphos by inhalation at concentrations of 0, 4 (range of 3-15), 10
(8-12) and 27 (24-30) mg/m3 air for 12 weeks, 5 days/week for 6
hours/day. The range of particle size was 0.5 to 1.5 µ with 97% of the
particles having a mean mass diameter of 1 µ (a fully respirable
particle). There was no mortality although females exposed to 27
mg/m3 were observed to be adversely affected. Growth was normal at
all levels of exposure as were clinical chemistry, hematology and
urinalysis parameters. Cholinesterase (brain, plasma and RBC) activity
was depressed at 10 mg/m3 and above. No effects were noted at 4
mg/m3. Gross and microscopic analysis of tissues and organs were not
affected by edifenphos in the diet. Female adrenal weight was
increased at the highest dose level but no abnormalities were detected
on microscopic examination (Kimmerle, 1975; Mohr, 1975).
Groups of rats (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. There was no mortality during this study but
growth was inhibited at 1000 ppm in both males and females. In
contrast to the reduced growth, food consumption was normal at all
dose levels. Several hematological parameters were affected at 1000
ppm, namely reduced RBC count, hemoglobin content and hematocrit value
primarily in males. Liver function was reduced at 1000 ppm (SGOT), 100
ppm (SGPT) and at 30 PPM (SAP) predominantly in males, with the
reduction not as marked in females. Urinalysis parameters were
unaffected. Cholinesterase depression was noted at 300 ppm in males
(brain and RBC) and 100 ppm in females (brain and RBC), plasma was
depressed in females at 10 ppm. Gross examination revealed enlarged
liver (1000 ppm) and kidney (300 ppm). Microscopic examination of
tissues showed hypertrophy and fatty degeneration of hepatocytes at
100 ppm and above. No pathological changes were noted in liver at 30
ppm. At 100 ppm and above, kidney and adrenal changes were noted. At
high levels a toxic nephritis was observed and the kidney and adrenal
glands were hypertrophic. In the male, kidney degeneration was
prevalent while in the females the adrenal was damaged. Gross changes
noted in brain, pituitary and thyroid of males at high dose levels
were not accompanied by pathological changes (Imamichi, 1973a).
Groups of rats (15 males and 15 females/group) were fed
edifenphos in the diet at dosage levels of 0, 1, 2, 5, 10 and 150 ppm
for 3 months. Growth was affected by edifenphos and there were no
differences in behaviour, mortality or appearance. Food consumption in
the highest dose group was slightly reduced. Hematological
examinations were normal at 1 and 3 months as were liver function and
urinalysis tests measured at the same time intervals. Cholinesterase
depression was observed in the highest dose group (in both RBC and
plasma) within one week of initiation of the feeding trial. The
depression was constant and there was no indication of a cumulative
effect relating to edifenphos. Gross and microscopic analysis of
tissues and organs, performed at the conclusion of the study, showed
no dose-related gross change and no significant microscopically
observable alteration (Loser, 1972; Urwin and Newman, 1972).
Groups of rats (10 males/group) were administered edifenphos by
oral intubation, daily for 14 days at dose rates of 0, 12.5, 50 and
100 mg/kg. Mortality was not reported but a slight decrease in average
body weight was reported at 100 mg/kg. A significant increase in
absolute and relative liver weight was reported at all dose levels at
24 hours. The livers had recovered to normal size within 7 days of the
end of treatment. Cholinesterase was depressed at all levels with the
following order of sensitivity: RBC > plasma > brain. One week after
treatment RBC and brain cholinesterase activity were depressed at all
dose levels. Liver function tests were slightly affected (SGOT
reduced) but were normal within 7 days. EPN detoxication by liver
microsomes was stimulated at all dose levels (aniline hydroxylase and
aminopyrine demethylase were unaffected) and recovery was not complete
at 7 days. Microscopic examination of liver, adrenals and kidney
showed no adverse effects (Anonymous, 1976c).
Groups of rats (15 females/group) were administered edifenphos
orally (0, 5, 10, 20, 33.5 and 50 mg/kg) or by intraperitoneal
injection (0, 1.75, 3.5, 7, 10, 11.5 and 17.5 mg/kg), daily 5 weeks
per week for 60 days. Oral administration of 50 mg/kg daily resulted
in no mortality or cumulative toxicity although signs of poisoning
were seen. No signs of poisoning were evident at 10 mg/kg/day
following oral dosing. With daily ip injections, no substantial
mortality (1/15 deaths) was observed at 7 mg/kg (11/15 died at 11.5
mg/kg). Signs of poisoning were not evident at 3.5 mg/kg. These
results suggest a minimal cumulative toxicological effect (Kimmerle
and Lorke, 1967).
Dog
Groups of dogs (2 male and 2 female/group, controls had 3 of each
sex) were fed edifenphos in the diet at dosage levels of 0, 3, 10 and
30 ppm for 90 days. There was no mortality over the course of the
study and growth, food consumption, behaviour and general appearance
were normal. Clinical chemistry, hematology and urinalysis values were
normal at one and 3 months (except for a slight increase of SDH
activity at only one month in both males and females at 30 ppm). Blood
cholinesterase activity was depressed in both plasma and RBC (to the
same extent in both enzyme sources) at the highest dose. As with
rodent studies, there was no evidence of cumulative activity over the
3 month interval. Gross and microscopic examination of tissues and
organs revealed no adverse effects of edifenphos in the diet. A slight
increase in the absolute and relative weight of the spleen at 30 ppm
was not accompanied by microscopic lesions (other than minimal)
congestion and probable hemosiderin deposition) (Loser, 1969; Spicer
and Urwin, 1971).
Groups of dogs (4 male and 4 female/group) were fed edifenphos in
the diet for two years at dose levels of 0, 7, and 20 ppm. A fourth
group was fed a diet containing 60 ppm for 1 year after which the
concentration was increased to 80 ppm for 5 months and raised to 120
ppm for the remaining 7 months of study. There was no mortality and
behaviour and physical appearance were normal and unaffected by
edifenphos in the diet. Growth and food consumption was normal at the
20 ppm level although it was slightly depressed at the high dose
regime. At the high level food consumption was slow, diet rejection
was observed and body weight gain depressed. There was no effect noted
on ophthalmological examination, urinary or hematology parameters or
on reflex testing. Some liver function test parameters were abnormal
(data showed SAP increased although SGPT was normal). This change was
accompanied by an increased gross and relative liver weight noted at
the time of sacrifice. Cholinesterase activity was depressed in the
high dose group in both plasma and RBC of males and females. Brain
cholinesterase was unaffected at any dose level. Gross and microscopic
examination of tissues and organs did not suggest an adverse effect of
edifenphos fed in the diet at concentrations up to and including 120
ppm. The inductive effect noted on the liver (increased organ weight
accompanied by increased phosphatase activity) was attributed to an
adaptation reaction noted with many drugs and chemicals. Microscopic
examination of liver did not show changes attributable to the presence
of dietary edifenphos which would account for the increased liver
weight. A no-effect level was observed to be 20 ppm equivalent to a
daily intake of 0.58 mg/kg body weight (Hoffmann, 1976; Gallagher and
Practice, 1976).
Long Term Studies
Rat
Groups of rats (50 male and 50 female rats/group, 100 of each sex
were used as controls) were fed edifenphos in the diet at
concentrations of 0, 2, 5, 15 and 150 ppm for two years. Mortality was
unaffected during the two years. Growth, behaviour and physical
appearance were normal in all groups. Hematology, blood chemistry and
urinalysis parameters were normal. A slight decrease in alkaline
phosphatase at 150 ppm was considered to be within the normal range
for the animals. Cholinesterase depression was observed in plasma and
RBC of both sexes at 150 ppm. Brain cholinesterase was depressed only
in females at 150 ppm. Gross and microscopic examinations were
performed on surviving animals. No abnormal, dose-related events were
recorded in the gross examination. A slight increase in adrenal size
of males at 15 ppm and above was not reflected in changes observed
under microscopic examination. Histopathological examination of
tissues and organs revealed luminal deposits of calcium salts in the
medulary tubules of male rats fed 15 and 150 ppm diets accompanied in
the high dose group by an increased incidence of glomerulonephrosis in
males. These changes were not noted in females. A complete examination
of tumours was performed revealing no indication of carcinogenic
potential. The frequency of both benign and malignant tumors was
typical of the rat strain used in the study. A no-effect dietary
level, based on the microscopic examination observing slight kidney
changes, was 5 ppm equivalent to 0.30 mg/kg body weight (Loser, 1976;
Offer and Prentice, 1976).
COMMENTS
Edifenphos, an organophosphorus ester, is used in agriculture as
a fungicide to control rice diseases. Following oral administration it
is rapidly absorbed, distributed, metabolised and excreted in mammals.
The well defined metabolic sequence observed in plants and animals
appears to be the same. In metabolism studies and other studies in
several species of animals there were no indications of any
culminative effects.
Biochemical parameters affected by acute and subacute
administration of edifenphos include cholinesterase depression and
changes in certain liver enzyme values reflecting changes in basic
liver function. Edifenphos is not a potent cholinesterase inhibitor.
However, the recovery of depressed enzyme activity is not rapid
suggesting that regeneration of cholinesterase activity is dependent
on enzyme synthesis rather than reversal of an enzyme-inhibitor
complex. A sensitive biochemical parameter was aliesterase activity of
liver and serum with a level of 5 ppm of edifenphos in the diet
showing 50% inhibition. In special studies for teratogenic and
mutagenic potential, no effects of edifenphos were noted on several
species. At high levels, reproduction was slightly impaired but at
levels that did not affect maternal well-being reproduction parameters
were unaffected. Edifenphos appeared to potentiate the acute effects
of malathion as might be expected from the data on depression of
aliesterase activity. There is no evidence that edifenphos would
induce a delayed neurotoxic response in hens.
Edifenphos is moderately toxic on acute exposure to a variety of
mammalian species with signs of poisoning typical of
parasympathomimetic agents. The acute signs of poisoning, although
modified slightly by atropine and reactivators, were not completely
controlled.
In all cases, the acute toxicity of metabolites was greatly
reduced with respect to the acute toxicity of the parent compound. In
both short and long term studies in rats and dogs, the most sensitive
parameters affected were cholinesterase depression, liver enlargement
and deposits of kidney salts in males on long term studies. Based on
short term studies in mice and dogs and long term studies in rats a
no-effect level was suggested and a temporary ADI for man was
recommended. The dietary studies in rat (both short term and long
term) showing liver and serum aliesterase depression and kidney salt
build-up in males were used as a basis for evaluating the no-effect
level. While 5 ppm was a level causing 50% inhibition of aliesterase
activity the significance of this effect was unclear and has been
taken to suggest "exposure" rather than "toxicological effect".
The liver involvement noted in many studies precluded
consideration of a finite ADI until a specific carcinogenic study is
performed using a species susceptible to liver changes that might
relate to hepatic carcinoma. As these changes noted in this study
resemble those effects seen with other compounds including chlorinated
hydrocarbons, such studies are required. The lack of carcinogenic
potential noted in the 2 years, on a limited number of rats, was
reassuring that the compound is not a potent carcinogen in this
species. However, it was considered in the 2 year rat study that the
highest dose level and the small group size were not sufficient to
fully evaluate the potential for carcinogenicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm in the diet equivalent to 1.53 mg/kg bw
Dog: 20 ppm in the diet equivalent to 0.58 mg/kg bw
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg bw
Estimate of temporary acceptable daily intake for man
0 - 0.003 mp/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Edifenphos is an organophosphorus compound with a fairly
selective action against rice blast caused by Piricularia oryzae,
one of the major rice diseases. It has both a protective and curative
action against the fungus attack. There is some evidence that the
compound interferes with chitin formation in the fungal cell wall, a
process which dose not occur in higher plants. This mode of action may
therefore explain the selective action of the fungicide.
The compound is also effective against a few other fungus
diseases of rice, e.g. ear blight caused by Cochliobolus
miyabeanus, Hormodendrum and against some insect pests, e.g. leaf
hoppers.
USE PATTERN
Edifenphos is authorised or recommended and sold in various
countries in Asia including Japan, in Central and South America and in
Europe, e.g. Italy. The product is marketed as emulsifiable
concentrates with various concentrations of edifenphos and as a dust.
In most countries two to four treatments are applied at dosage rates
of 300-800 g a.i./ha, with a pre-harvest interval of 21 days. The
recommended preharvest interval in Italy is 60 days.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
Japan and in some South American countries, namely Colombia, Mexico,
Peru and El Salvador. A summary of these data is given in Table 5.
FATE OF RESIDUES
The metabolic and other reactions of edifenphos exposed to
plants, animals, fungi, soil, light and water are shown in Figures
1(a) and 1(b) taken from Umeda (1972) with some modifications.
TABLE 5. Residues of edifenphos (parent compound) in rice resulting from supervised trials
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
Rice Japan 1967 2 0.36 EC 30% <0.02
hulled 3 0.36 EC 30% <0.02
4 0.36 EC 30% <0.02
3 0.6 dust 1.5% <0.02
4 0.6 dust 1.5% <0.02
1969 4 0.6 dust 1.5% <0.01
5 0.6 dust 1.5% 0.015
3 0.6 dust 1.5% <0.01
4 0.6 dust 1.5% < 0.01
3 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% 0.01
Rice Japan 1969 2 0.36 EC 30% <0.01
polished 3 0.45 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 2.5% 0.01
3 0.5 dust 2.5% 0.01
Rice
(in husk Peru 1969 2 0.5 EC 0.07
(straw 0.15
(in husk Colombia 1969 2 0.5 EC 0.1
(straw 0.25
(in husk El Salvador 1969 2 0.5 EC 0.45
(straw 1.4
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
(in husk Mexico 1969 2 0.5 EC 0.2
(straw 0.05
15/20 21/25 26/30 31/35 36/40 41/50 51/60
Rice Japan 1970 2 0.45 EC <0.01 <0.01
hulled 4 0.45 EC <0.01
2 0.2-0.45 EC <0.01 <0.01
4 0.2-0.45 EC <0.01
2 0.45 <0.01 <0.01
4 0.45 <0.01
(hulled 1973 2 0.3-0.45 EC 0.02
(straw 0.62
(hulled 1973 3 0.3-0.45 EC 0.01
(straw 0.32
hulled 1970 2 0.6 dust 0.03 0.03
4 0.6 dust 0.01
1971 3 0.75 dust 0.03 0.01
4 0.75 dust 0.02
1970 2 0.6-0.8 dust <0.01 0.01
4 0.6-0.8 dust <0.01
1970 2 0.6-0.8 dust <0.01 <0.01
4 0.6-0.8 dust <0.01
Rice Japan 1970 2 0.6 dust 0.01 0.01
hulled 4 0.6 dust 0.02
2 1 dust 0.01 0.01
4 1 dust 0.01
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 15/20 21/25 26/30 31/35 36/40 41/50 51/60
(hulled 1972 2 <0.01 <0.01
(straw 0.48 0.26
(hulled 1972 3 1 dust <0.01
(straw 0.93
(hulled 1972 2 1 dust <0.01
(straw 0.18
(hulled 3 1 dust 0.02 <0.01
(straw 3.81 0.24
(hulled 1973 2 1 dust <0.01
(straw 0.67
(hulled 1973 3 1 dust 0.01
(straw 0.74
(hulled 1973 2 1 dust <0.01
(straw 0.72
(hulled 3 1 dust 0.1
straw) 2.12
Rice
(hulled Japan 1972 2 1 dust 0.01
(straw 2.31
(hulled
(straw 3 1 dust 0.02
5.31
(hulled 3 1 dust <0.01
(straw 3.79
In plants
Extensive data are available on absorption, translocation,
accumulation and metabolism in the rice plant. (Ishizuka et al 1973,
1974; Nitokuno 1971; Takase et al 1973; Tomizawa et al 1972; Uesugi et
al 1972, Ueyama et al 1973, Umeda et al 1972).
Absorption and translocation
Izhizuka et al (1973, 1974) found that when edifenphos was
sprayed on rice leaves 60% was lost from the surface in five days and
75% in ten days. It was shown that about 70% of the edifenphos applied
had penetrated the leaf tissue within two hours.
Only small quantities were translocated from the site of
application, and translocation from roots to shoots was also slight.
Hence the loss of edifenphos was mainly from the leaf surfaces by
volatization and decomposition, rather than by metabolism inside the
plant.
When the flag leaf of a rice plant was painted with 32P-labelled
edifenphos some translocation of the chemical to the lower part of the
leaf and to the ears was observed (Ishizuka et al 1973, 1974; Nitokuno
1971; Umeda 1972). Since no complex of edifenphos with
photosynthesized plant products was found, it appears that edifenphos
is translocated without prior metabolism. The concentration of
edifenphos in plant parts is in the order: leaves and stems >> husks
>> hulled rice.
Metabolism
Takase et al (1973) studied the metabolic fate of edifenphos
after spraying rice plants at various growth stages. The
hexane-soluble components, which were analysed throughout the
experimental period, consisted, mainly of edifenphos parent compound
(83.2-89.3% of the radioactivity in the fraction). The major
components in the water soluble fraction were identified as O-ethyl
S-phenyl phosphorothioate (metabolite VII, Figure 1(a)) and
SS-diphenyl phosphorodithioate, (III) each representing about 30% of
the total 32p in the water-soluble fraction. With the lapse of time,
ethyl phosphate (XV) was formed.
Ueyama (1973) detected nine metabolites after applying
35S-labelled edifenphos to rice leaves. Three were identified as
metabolites IV, VIII and XII (Figures 1(a) and 1(b)). It is probable
that the formation of VIII and XII involved the exchange of phenylthio
and ethoxy radicals between molecules of edifenphos.
A high proportion of S-phenyl phosphorothioate (V) and small
amounts of O-ethyl S-phenyl phosphorothioate (VII) were found among
the water-soluble metabolites. Results are shown in Table 6.
TABLE 6. Toluene extracted metabolites in and on rice plants treated with 35S-edifenphos (after Ueyama)
Metabolites, 35S counts expressed as edifenphos equivalents, mg/kg
Growth stage Part of Period after
at application Plant application Identified* Not-identified
I IV VIII XII A B C E F
Tillering leaf 1 hour 35 4.3 2.9 1.1 0.72 1
blade
6 hour 18 1.1 1.2 0.59 0.02 0.33
1 day 20 2.8 1.1 1.4 0.05 0.46
3 day 17 1.1 0.73 0.16 0.26 1.2
7 day 1.2 0.26 1.5 0.13 0.07 trace trace
15 day 0.79 0.22 - 0.18 <0.01 0.01
Tillering leaf 1 hour 0.73 trace 3.3 - trace
sheath 6 hour 0.84 trace 1.0 trace trace
1 day 0.79 trace 0.33 0.33 trace
3 day 0.65 trace 0.36 - 0.02
7 day 0.04 0.01 0.05 0.04 <0.01 trace
15 day trace - trace
Heading leaf 1 hour 42 10 1.2 trace 0.27 0.05 0.05 0.33
blade 6 hour 31 8.2 3.2 trace 0.27 0.13 0.13 0.89
1 day 9.8 2.5 1.1 0.12 0.06 0.01 0.03 0.15
3 day 7.8 1.6 0.55 trace 0.03 0.02 0.12 0.29
7 day 3.2 0.19 - 0.02 <0.01 0.03 0.37
15 day 0.40 0.38 - 0.18 0.24 0.30
* For identities of numbered metabolites, see Figures 1(a) and 1(b) (I = parent compound)
In animals
Pither and Gronberg (1975) administered 0.12 mg/kg of edifenphos
as a single oral dose and as a daily dose for 5 days to dairy cows.
Results are described above ("Evaluation for acceptable daily intake -
- Absorption, distribution and excretion").
Strankowski et al (1976) identified metabolites of 14C ring-
labelled edifenphos in the muscle, liver, kidney, fat, milk and urine
of dairy cows. Methyl phenyl sulfone (XI, Figure 1(b)) was the
principal metabolite in both milk and tissues representing 95% of the
organo-soluble radio-carbon in the milk and from 70% (kidney) to 99%
in the tissues. Small amounts of metabolites Xa, XIa, XIb, together
with other minor metabolites were also found (Table 7).
TABLE 7. Distribution of edifenphos and its metabolites in the
tissues and milk of dairy cows (Strankowski, 1976).
Residue, % of total radioactivity in organic extract,
as metabolite no.*
Tissue I IX X Xa XI XIa Xlb XIIa
muscle - - - - 85 2 - -
fat - 1 - - 99 - - -
liver <1 <1 <1 4 84 <1 <1 <1
kidney <1 - <1 2 70 2 9 <1
milk - - 1 3 93 1 1 -
* For identities of numbered metabolites see Figure 1(b),
(I = edifenphos).
Hermann and Gronberg (1976) administered 3 mg/kg 14C-edifenphos
orally to laying hens. After 96 hours 76% of the activity had been
excreted. Methyl phenyl sulfone (XI) was the only metabolite detected
in all the tissues and in egg, but a second metabolite was found in
kidneys and was identified as either IX, XII or XIII (Figure 1(b)).
The total radiocarbon residues at intervals of 6-72 hours after dosing
are given in Table 8.
TABLE 8. Total radiocarbon from 14C-edifenphos in tissues of
laying hens
Radioactivity expressed as edifenphos,
mg/kg*, after interval, hours
Tissue 6 24 48 72
Muscle (breast) 0.66 0.73 0.57 0.21
Muscle (thigh) 0.69 0.75 0.59 0.19
Skin 0.79 0.74 0.58 0.21
Fat 0.97 1.00 0.80 0.30
Liver 1.72 1.45 1.17 0.51
Kidney 1.83 1.29 0.96 0.39
Gizzard 0.72 0.76 0.56 0.21
Heart 0.82 0.89 0.66 0.26
* Values are mean figures of single analyses of tissues from each of
four hens.
Ueyama and Takas (1975) determined the residue levels in goat
milk after administration of edifenphos at the rate of 1 mg/kg/day for
ten consecutive days. No edifenphos could be detected in the milk.
After a single oral dose of 10 mg/kg, 0.0006-0.0008 mg/kg in
edifenphos could be detected within 24 hours.
Lamb and Roney (1976a) exposed crayfish to 14C-edifenphos
concentrations of 10 µg/l (i.e. 10-8) for 14 days and determined 14C
residues during the exposure period and a 28 day withdrawal period.
14C residues, expressed as edifenphos, accumulated to a maximum level
of 0.2 mg/kg. About half of the accumulated 14C was lost within 7
days of withdrawal.
The same authors (1976b) exposed Channel catfish (Ictalurus
punctatus) continuously for 28 days to 10-12 µg/l of 14C
ring-labelled edifenphos. 14C residues accumulated to a maximum of 1.2
mg/kg edifenphos equivalents in ten days, representing an accumulation
factor of 104-117. The non-edible portions (head, viscera and scales)
contained 75% of the extractable residues. The acetonitrile fraction
(81% of the total extractable residue) consisted of edifenphos 44%,
metabolite VI (Figure 1(a)) 10%, VII 19% and VIII 27%. During the
withdrawal period about 50% of the accumulated 14C residues were
excreted in five hours and about 85% within four days.
In fungi
Uesugi and Tomizawa (1971) studied the metabolism of edifenphos
in mycelia of the rice blast (Pericularia oryzae) with 32P-, 35S-
and non-labelled edifenphos. The main metabolic pathway consists in
the hydrolysis of one P-S linkage, followed by that of the other P-S
or the ethyl ester bond, finally yielding phosphoric acid. Another
hydroxylated intermediate, metabolite II (Figure 1(a)) was also found.
No significant differences in route or rate of metabolism were
found between susceptible and resistant strains of the fungus.
In storage, processing and cooking
The residue is mainly located on the rice husk, hence during
milling and polishing most of the residue is removed. Takase et al
(1973) estimated the distribution of 32P-labelled edifenphos in
milling fractions (Table 9).
The residues at harvest in the husks consisted largely of
edifenphos metabolites, although small quantities of the parent
compound were found (Table 10).
Residues of edifenphos from foliar application (0.63 kg/ha, 2
applications) were found primarily in straw and seed hulls 37 days
after the second application. The residue in the hulls ranged from
0.02-0.29 mg/kg. Trace amounts were found in the bran, but no residue
was detectable in the milled and polished rice (Chemagro, 1971).
On exposure to light
A marked degradation of 35S-edifenphos by UV light was found on
irradation in aqueous or hexane solution or as a thin film. In hexane,
the formation of water-soluble degradation products was low. The
metabolites III, VIII, XV and XVI were identified (Murai et al, 1976).
In water and soil
Shaw (1976b) and Shaw and Murphy (1976) studied the hydrolysis of
14C ring-labelled edifenphos at concentrations of 1 and 10 mg/kg in
aqueous solutions buffered at pH 3, 6 and 9 and at temperatures of 25,
35 and 45 degrees C. The hydrolysis followed first order kinetics and
its rate increased with temperature and with increasing pH. The same
major hydrolysis products were formed under all conditions, namely
compounds VII and XII (Figure 1(a) and (b)) with traces of II, III,
IV, VI, VIII and XIV.
TABLE 9. Distribution of 32P-labelled edifenphos and its metabolites in milling fractions of rice
Radioactivity (32P) expressed as edifenphos, mg,/kg
Number of treatments 1 2 3 1
Pre-harvest interval,days 13 1 24 8
Milling fraction Hexane- Water- Hexane- Water- Hexane- Water- Hexane- Water-
soluble soluble soluble soluble soluble soluble soluble soluble
husks 0.11 0.04 0.17 0.12 0.28 0.14 2.13 0.34
hulled rice trace 0.02 trace 0.01 0.06 0.03 0.31 0.05
polished rice 0.01 0.01 0.01 0.01 trace trace trace -
TABLE 10. Distribution of radio-activity from 32P-edifenphos in rice
husk.
Residue, % of total radio-activity
3 applications, 1 application,
Compound* latest 24 days 8 day pre-harvest
pre-harvest harvest
Unknown
Water-soluble metabolite 9.6 13.4
III 10.9 19.4
VII 37.6 29.1
XV, XVI 41.8 28.1
* For identities of metabolites, see figures 1(a) and 1(b).
Shaw (1976a) incubated 14C ring-labelled edifenphos in pond
water (pH 8.3) containing soil for 62 days after irradiation with UV
light and sunlight, with alternating 12-hour periods of light and
darkness. In these conditions the half-life of edifenphos was 16.8
hours. The main products were compounds VII and XIV and one or more of
compounds IX, XII and XIII. Traces of II, III, IV, X and XI were also
found. These products were found in both the water and the bottom
sludge.
Flint and Shaw (1975) estimated the half-life of edifenphos in an
outdoor simulated pond system at 20°C. Under these conditions the
half-life was 29 hours in the water and about 60 hours in the bottom
sludge.
Edifenphos is almost immobile when applied to soil. Leaching and
soil adsorption were inversely correlated and the latter was
proportional to the soil organic matter content.
When columns of sand and sandy loam soil containing edifenphos at
a level equivalent to 0.5 kg/ha were eluted with water at a rate
representing 200 mm of irrigation in two days, no edifenphos was found
in the eluate (Bayer, 1974 a, b). The half-life of edifenphos was
about 23 days in the sandy loam (pH 5.2, organic carbon 0.57% and
particles < 20 µ 19.5%), but only 6-8 days in the sand (pH 6.8,
organic bound carbon 2.50% and particles < 20 µ 10.1%). In further
laboratory experiments simulating the flooded soil conditions
associated with rice growing (Nitokuno, 1972), edifenphos was degraded
rapidly during the first 24 hours. The half-life was about 19 hours in
a clay soil and 2 1/2 days in a silt loam. Subsequent degradation was
slower in both soil types.
Tomizama (1975) reports similar results obtained in a laboratory
degradation experiment. Under the anaerobic conditions of the
experiment about half the edifenphos was degraded during the first 3 -
5 days. From the sixth day onwards the rate of degradation was
considerably slower.
Studies by McNamara (1976) and McNamara and Close (1976) were
made on the persistence and degradation of 14C ring-labelled
edifenphos in loam and silt loam under aerobic conditions and in loam
under anaerobic conditions. The half-lives were 1 day in the aerobic
silt loam and 3 days in loam under both aerobic and anaerobic
conditions. The organo-soluble radioactivity decreased to 10-20% of
that applied within 21 days in all samples. In aerobic soils the
decrease was mainly due to volatilization and binding to the soil. In
aerobic loam about 20% of the total radioactivity was lost by
volatilization in 14 days, and in silt loam 35% in 21 days.
In aerobic soil the main organo-soluble radioactive residues were
edifenphos, its initial hydrolytic products III and VII, and
derivatives of thiophenol (XIII): diphenyl disulphide XII,
benzenesulphonic acid XIV, methyl phenyl sulphoxide X and methyl
phenyl sulphone XI. The two major terminal metabolites were X and XI.
In anaerobic loam the metabolic pathway proceeded via thiophenol
(XIII) to diphenyl disulphide XII, and benzenesulphonic acid XIV. Only
small amounts of methylated products were found.
In a model ecosystem
Edifenphos was fairly rapidly degraded in a model ecosystem, the
half-life being 60 hours. The main metabolic features were the
conversion of edifenphos to compounds IV and VII and the rapid
oxidation of the diphenyl disulphide released from these compounds. It
was found that sulphur atoms derived from the diphenyl sulphide were
incorporated into sulphuric acid and then into cell constituents,
(Tomizawa and Kazano, 1975).
METHODS OF RESIDUE ANALYSIS
Vogeler (1968) developed a gas-chromatographic method for the
determination of edifenphos residues in rice, using an alkali
thermionic or flame photometric detector. The rice sample is macerated
and extracted with acetone and the filtrate evaporated to dryness. The
residue is cleaned up by acetonitrile - petroleum ether partition and
by chromatography on a Florisil column. Recoveries from control
samples fortified with 0.05-1 mg/kg ranged from 70-80%. The limit of
determination is about 0.05 mg/kg. Thornton (1971) checked several
organophosphorus compounds which are authorised or recommended for use
on rice for interference with Vogeler's method. They were tested at
levels equivalent to the highest recommended dose rate on rice in the
USA. The compounds tested were dichlorvos, fensulfothion, fenthion,
malathion, naled, parathion, parathion-methyl, phorate and TEPP. None
showed any interference.
Takase et al (1971) described a modified gaschromatographic
method for the analysis of edifenphos in rice. Extraction is with
acetonitrile and the residue is cleaned up by partitioning into
acetonitrile and n-hexane followed by TLC on alumina. The residue is
determined with a flame photometric detector. Recoveries of added
levels of 0.1 to 0.2 mg/kg ranged from 96-102%. The limit of
determination is 0.005 mg/kg or lower.
Stanley (1972) adapted and modified the Vogeler method to
determine edifenphos residues in milk and cattle tissues. For milk the
method involves initial blending with acetone, followed by separation
of edifenphos from the aqueous solution by partitioning into
chloroform. Clean-up is by partition between Skellysolve B and
acetonitrile and chromatography on a Florisil column. Animal tissues
are extracted with acetonitrile, partitioned with Skellysolve B, and
cleaned up on a Florisil column. The edifenphos parent compound is
determined by gas chromatography with a thermionic detector. The limit
of determination of edifenphos in milk is 0.002 mg/kg and in cattle
tissues 0.02 mg/kg.
Recoveries from milk spiked with 0.005-0.5 mg/kg ranged from 77
to 110%, and in various cattle tissues spiked with 0.05-0.1 mg/kg from
82 to 118%.
Sandie and Gronberg (1975) adapted the Stanley (1972) method for
the analysis of edifenphos in poultry and eggs. For poultry tissues
the method involves extraction, partition between acetonitrile and
hexane, and gas chromatography with a thermionic detector. Eggs are
extracted with acetone, and clean-up is by successive aqueous
acetone-chloroform and acetonitrile - hexane partitions, followed by
silica gel chromatography. Recoveries were 84-107% from tissues at
levels of 0.05 and 0.1 mg/kg and 86-97% from eggs at 0.01 mg/kg. The
limits of determination were 0.01 mg/kg in poultry tissues and
0.001 mg/kg in eggs.
Minor (1976) examined possible interferences by other
organophosphorus pesticides in the determination of edifenphos in
cattle and poultry tissues and eggs by the methods of Sandie and
Gronberg (1975) and Stanley (1972). He found that edifenphos could be
adequately separated from all the 25 organophosphorus pesticides with
a USA tolerance in milk if the Stanley Florisil column and the Sandie
and Gronberg silica gel column were used successively in the clean-up
procedure.
NATIONAL TOLERANCES
National tolerances reported to the meeting are given in Table
11. They refer to the parent compound only.
TABLE 11. National tolerances reported to the Meeting
Country Commodity Tolerance, Recommended
mg/kg pre-harvest
interval, days
Italy rice, hulled 0.05 60
Japan rice, hulled 0.03 21
Mexico rice, hulled 20
U.S.A. rice, hulled 0.1*
rice, hulls 0.3
* temporary tolerances valid until May 1977
APPRAISAL
Edifenphos is an organophosphorus compound with a rather
selective action against the rice blast caused by Piricularia
oryzae, one of the major rice diseases, against which it shows both
a protective and curative action. There is some evidence that the
compound interferes with the formation of chitin in the fungus
cell-wall, a process which does not occur in higher plants. This may
therefore explain the selective action of the fungicide. Edifenphos is
also effective against a few other rice diseases and some insect
pests, e.g. leafhoppers.
The pesticide is used on an extensive scale on rice, as EC
formulations and as a dust, in Asian countries including Japan, in
Central and South America and in some mediterranean countries, e.g.
Italy. In most countries 2 - 4 treatments are applied at 300 - 800 g
ai/ha, generally with a pre-harvest interval of 21 days.
Extensive data were obtained on the fate of edifenphos residues
in soil, water, rice plants and livestock animals such as cattle,
goats and poultry. Degradation pathways in plants, soil and animals
are similar, although there are quantative differences.
In a study with 35S labelled edifenphos on rice, nine
metabolites were found in a toluene extract from leaves: the three
main products were OO-diethyl S-phenyl phosphorothioate, SSS-triphenyl
phosphorotrithioate and diphenyl disulphide. A large proportion of
S-phenyl phosphorodithioate and small amounts of O-ethyl S-phenyl
phosphorothioate were found as water-soluble metabolites. In a study
with 32p-labelled edifenphos, SS-diphenyl phosphorodithioate was
identified as another major water-soluble metabolite.
In a cattle feeding study with ring-labelled 14C-edifenphos it
was shown that methyl phenyl sulphone was the principle metabolite
both in milk and in animal tissues such as muscle, liver and kidney:
in milk it amounted to about 93% of the radioactivity extracted. The
parent compound, together with m- and p-hydroxyphenyl methyl sulphone
and p-hydroxyphenyl methyl sulphoxide were found, but only in small
amounts.
After administering a single dose of 14C-labelled edifenphos
orally to laying hens (3 mg/kg), 76% of the radioactivity was excreted
in 96 hours. A single metabolite, methyl phenyl sulphone, was
identified in all tissues and eggs.
More than half of the residue from spraying edifenphos onto rice
leaves was dissipated in five days. Only small quantities were
translocated from the site of application. Translocation of edifenphos
from the roots to the shoots was also only slight.
Residues in rice at harvest are rather low. After the recommended
pre-harvest interval of 21 days, the residues of edifenphos and its
main metabolites in hulled rice following an application of
32P-labelled edifenphos to rice plants were of the order of 0.03 and
0.06 mg/kg edifenphos equivalents of water-soluble and hexane-soluble
metabolites respectively. Only traces were found in polished rice. In
the rice husks the residues were about 0.15 and 0.3 mg/kg edifenphos
equivalents of water-soluble and hexane-soluble 32P respectively.
Gas-chromatographic methods of analysis with alkali flame or
flame photometric detection are available. Recoveries in rice were
70-80% and the limit of determination ranged from 0.005 to 0.05 mg/kg
edifenphos parent compound. Nearly all organophosphorus pesticides
registered and/or recommended for use on rice were checked for
interference. None of the compounds interfered when tested at levels
that would be reached by treatments at the highest recommended
dosages, provided appropriate clean-up procedures were used. These
methods should be suitable for or adaptable to regulatory purposes.
Some of the GLC methods mentioned are suitable for the analysis
of edifenphos residues in cattle tissues, milk, poultry and eggs. The
limit of determination is 0.01 - 0.02 mg/kg parent compound.
Recoveries at levels of 0.01 - 0.1 mg/kg ranged from about 80 to 100%.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the parent compound edifenphos only.
Commodity Limit, mg/kg
Rice (in husk) 0.2
Rice (hulled) 0.05
Rice (polished) 0.01*
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Further studies to examine the hepatic involvement observed in
several animal species.
Desirable
1. Observations in man (relative to occupational exposure).
2. More information on residues of edifenphos and its main
metabolites on rice in husk at harvest.
3. A method of residue analysis suitable for edifenphos together
with its main metabolites.
REFERENCES
Anonymous Hinosan (Edifenphos)/Generation Study on Rats.
1976a Unpublished report from Nihon Tokushu Noyaku Seizo
Co., Ltd., submitted to the WHO by Bayer A.G.
* at or about the limit of determination
Anonymous Special Toxicity Test of Hinosan - I (single
1976b p.o. application). Unpublished report from Nihon
Tokushu Noyaku Seizo Co., Ltd., submitted by Bayer
A.G.
Anonymous Special Toxicity Test of Hinosan - II (14 day
1976c repeat p.o. application). Unpublished report from
Nihon Tokushu Noyaku Seizo Co., Ltd., submitted by
Bayer A.G.
Arnold, D. Mutagenic Study with Hinosan (BAY 78 418) in
1971 Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted by Bayer
A.G.
Bayer, A.G. Pflanzenschnitzmittle - Rückstunde in
1974a Sickerwasser Sickelwasser (edifenphos).
Unpublished. Reports Bayer A.G. No. 8503/8504/74.
Bayer, A.G. Verhalten des Pflanzenschutzmittelwirkstuffes
1974b edifenphos in Boden. Unpublished report Bayer A.G.
No. 8506/74.
Chen, R.S., Kinoshita, F.K. and DuBois, K.P. Acute Toxicity and
1972 Antiesterase Action of O-Ethyl-S, S-diphenyl
Phosphorodithioate (Hinosan). Tox. Appl. Pharm. 23
519-527.
Eben, A. and Kimmerle, G. Metabolism of Hinosan Active Ingredient
1972 in Rats and Dogs. Unpublished report No. 3687 from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Flint, D.R., Shaw, II, H.R. The Mobility and Persistence of Hinosan
1975 in Soil and Water. Chemagro Report No. 43 663.
Gallagher, P.J. and Prentice D.E. Pathology Report of Hinosan
1976 (SRA7847) Chronic Toxicity study in Dogs over two
years. Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Hermann, P.A. and Gronberg, R.R. Excretion and Metabolism of Hinosan
1976 in Poultry. Chemagro Report No. 49 023.
Hoffmann, K. SRA7847 (Active Ingredient of Hinosan) Chronic Toxicity
1976 Study on Dogs. Unpublished report from Institut
für Toxikologie, Bayer, A.G., submitted to the WHO
by Bayer A.G.
Imamichi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, X.,
1973a Ikadai, H., Mori, H., Furusawa, U. and Saito, K.
Hinosan Subchronic Toxicological Studies on Rats.
Unpublished report from Nippon Vet. and Zoo Techn.
College, Tokyo, Japan, submitted to the WHO by
Bayer A.G.
Imamachi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, K.,
1973b Tauchi, K., Ikadai, H., Mori, H., Furusawa, U. and
Saito, K. Hinosan-Subchronic Toxicological Studies
on Mice. Unpublished report from Nippon Vet. and
Zoo Techn. College, Tokyo, Japan, submitted to the
WHO by Bayer A.G.
Ishizuka, K., Hosaka, H., Takase, I., Tan, K.E., Hirata, H.
1974 Metabolism and Accumulation of Pesticides in Rice
Plants. Comp. Studies Food Environ. Contam.;
Inter. Atomic Energy Agency, Vienna, 363 - 372.
Kimmerle, G. Bayer 78 418 (SRA7848)/Potenzierungsversuche.
1967 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Bay 78 418/Subcronische Neurotoxizitats-versuche
1969 bei Huhnern. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Kimmerle, G. Hinosan/Acute Neurotoxicity Studies on Hens.
1971 Unpublished report from Institut für Toxikologie,
Bayer A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Dermal Toxicity of Hinosan Active Ingredient to Rats.
1972a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Acute Inhalation Experiments with Hinosan Active
1972b Ingredient on Rats. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Subchronic Inhalation Toxicity Study on Rats.
1975a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Acute Inhalation Toxicity Study on Rats.
1975b Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. and Lorke, D. Toxikologische Untersuchungen mit dem
1967 Wirkstoff, Bayer 78 418. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. and Loser, E. Delayed Neurotoxicity of Organophosphorus
1974 Compounds and Copper Concentration in the Serum of
Hens. EQS Environmental Quality and Safety
3:172-178.
Lamb, D.W. and Matskanin, C.W. The Acute Oral Toxicity of Hinosan
1976 Metabolites to Rats. Unpublished report from
Chemagro Agr. Div. Mobay Chem., Corp., submitted
to the WHO by Bayer A.G.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976a in Chanuel Catfish Exposed to Hinosan-14C.
Chemagro Report No. 46 379.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976b in Catfish Exposed to Hinosan 14C. Chemagro
Report No. 48 832.
Loeffler, W.W. The Effect of Processing on Pesticide Residues.
1972 Chemagro Report No. 34 176.
Lorke, D. Hinosan/Studies of Product for Possible Embryotoxic
1971 and Teratogenic Effects on Rats. Unpublished
report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Subchronische toxikologische Untersuchungen
1969 an Hunden. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Loser, E. SRA7847/Subchronic Toxicological Studies on Rats.
1972 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Generation Study on Rats. Unpublished report
1976a from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Chronic Toxicity Study on Rats. Unpublished
1976b report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Machemer, L. Hinosan Active Ingredient (SRA7847)/Studies of
1976 Embryotoxic and Teratogenic Effects on Rabbits
following Oral Administration. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Minor, R.G. An Interference Study for the Residue Methods for
1976 Hinosan in Milk, Eggs and Animal Tissues. Chemagro
Report No. 48 819.
Mohr, U. Subchronic Inhalation Toxicity Study on Rats,
1975 Histopathological Findings. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Murai, T., Uesugi, Y., Tomisawa, C. and Tanaka, T.
1976 Photo-Degradation of Pesticide (I), Degradation of
Organophosphorus Fungicides EDDP and IBP by
Ultraviolet Light. Chemagro Report No. 45 169.
Murphy, J.J., Feldhas, L., Simmons, C.E., Thornton, J.S. and
1976 Gronberg, R.R. Total Radioactive Residues of
(14C) Hinosan in Rotational Crops. Chemagro
Report No. 48 826.
McNamara, F.T. Persistence and Metabolism of Hinosan in Soil.
1976 Chemagro Report No. 49 025.
McNamara, F.T. and Close, C.L. Persistence and Metabolism
1976 of Hinosan in Soil under Aerobic Conditions.
Chemagro Report No. 46 402.
Nitokuno Hinosan, a Fungicide for Control of Rice Blast,
1971 Chem. Econ. Engin. Rev. 3 (1), 57-59.
Nitokuno Gas Chromatographic Determination of Residues of
1972 Hinosan (O-ethyl S,S-diphenyl phosphorodithiolate)
in Rice Grains. Unpublished report.
Nitokuno Special Toxicity Test of Hinosan I. (single p.o.
1976 application). Unpublished report of Nihon Tokushu
Noyaku, Seizo Co., submitted by Bayer A.G. to the
JMPR.
Offer, J.M. and Prentice, D.E. Pathology Report of Bay 78 418
1976 (Hinosan) Chronic Toxicity Testing on Rats.
Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Pither, K.M. and Bronberg, R.R. Residue Levels in Lactating
1975 Dairy Cattle following Oral Administration of
Hinosan-ring-Ul-14C. Unpublished report No. 44
845 from Chemagro Agr. Div. Mobay Chem. Corp.,
submitted to the WHO by Bayer A.G.
Sandie, F.E. and Gronberg, R.R. A Gas Chromatographic Method
1975 for the Determination of Residues of Hinosan in
Poultry Tissue and Eggs. Chemagro Report No. 45
987.
Shaw, II, H.R. Hydrolysis of Hinosan in Pond Water Containing Soil.
1976a Chemagro Report No. 48 829.
Shaw, II, H.R. Hydrolysis of Hinosan in Buffered Aqueous Solution.
1976b Chemagro Report No. 48 831.
Shaw, II, H.R. and Murphy, J.J. Hydrolysis of Hinosan in Buffered
1976 Aqueous Solution. Chemagro Report No. 46 403.
Shirasu, Y., Moriya, M. and Kato, K. Report of the Mutagenicity
1976a Study of Hinosan (EddP, edifenphos). Unpublished
report from the Department of Toxicology,
Institute of Environmental Toxicology, Tokyo,
Japan, submitted to the WHO by Bayer A.G.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. and Kada, T.
1976b Mutagenicity Screening of Pesticides in the
Microbial System. Mutation Research 40:19-30.
Spicer, E.J.F. Pathology Report of Bay 78 418/Hen Study.
1971a Unpublished report from Huntingdon Research
Central submitted to the WHO by Bayer A.G.
Spicer, E.J.F. Pathology Report of Bay 78 418/Subchronic
1971b Neurotoxicity Study in Hens. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Spicer, E.J.F. and Erwin, C. Pathology Report of Bay 78 418/
1971 Subchronic Toxicity Studies in Dogs. Unpublished
report from Huntingdon Research Centre, submitted
to the WHO by Bayer A.G.
Stanley, C.W. Gas Chromatographic Method for the Determination
1972 of Residues of Hinosan in Animal Tissues and Milk.
Chemagro Report No. 32 070.
Strankowski, K.J., Pither, K.M. and Murphy, J.J. Metabolism of
1976 Hinosan by a Lactating Dairy Cow. Unpublished
report No. 48 846 from Chemagro Agr. Div. Mobay
Chem. Corp., submitted to the WHO by Bayer A.G.
Takase, I., Nakamura, S., Tsuda, H. and Ueyama, I. The Gas
1971 Chromatographic Determination of Pesticides. (Part
4). Cleanup Method for Organophosphorus Pesticide
Residues in Rice Grain. Noyaku Seisan Gijutsu
(Pesticide and Technique) 23:31-35.
Takase, I., Tan, K.E. and Ishizuka, K. Metabolic Transformation
1973 and Accumulation of O-Ethyl S,S-Diphenyl
Phosphorodithiolate (Hinosan) in Rice Plants. Agr.
Biol. Chem. 37(7): 1563-1571.
Thornton, J.S. A Study of the Possible Interferences of Other
1971 Pesticides with the Gas Chromatographic Residue
Method for Hinosan. Chemagro Report No. 29 702.
Thyssen, J. and Kimmerle, G. Hinosan 50 E.C./Toxicological Studies.
1974 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Tomizawa, C. Degradation of Organophosphorus Pesticides in Soils
1975 with Special Reference to Unaerobic Soil
Conditions. Environ. Qual. Safety 4:117-127.
Tomizawa, C., Uesugi, Y. and Murai, T. Fats and Significance of
1972 Fungicides in Rice. Radiotracer Studies Chem. Res.
Food. Agric.; Intern. Atomic Energy Agency,
Vienna, 103-105.
Tomizawa, C. and Kazano, H. Biological Accumulation of Pesticides
1975 in an Ecosystem. Rev. Plant Protec. Res.
8:41-54.
Uesugi, Y. and Tomizawa, C. Metabolism of O-Ethyl S,S Diphenyl
1971 Phosphorodithiolate (Hinosan) by Mycelial Cells of
Pyricularia Oryzae. Agric. Boil. Chem. 35
(6):941-949.
Uesugi, Y. Tomizawa, C. and Murai, T. Degradation of
1972 Organophosphorus Fungicides. Environ. Toxicol.
Pest. Academic Press, 327-339.
Ueyama, I., Uesugi, Y., Tomizawa, C. and Murai, T. Metabolic
1973 Fate of O-Ethyl S,S-Diphenyl Phosphorodithiolate
(Hinosan) in Rice Plant. Agr. Biol. Chem.
37(7):1543-1551.
Ueyama, I. and Takase, I. Metabolic Behaviour of O-Ethyl
1975 S,S-Diphenyl Phosphorodithiolate (edifenphos) in
Female Goat. Agr. Biol. Chem. 39(9):1719-1727.
Ueyama, I., Takase, I. and Tomizawa, C. The Metabolism of Edifenphos
1976 (Hinosan) in Mice and Rats. Unpublished report No.
1046 from Nithon Tokushu Noyaku Seizo Co. .td.
Agr. Chem. Inst. and National Institute of Agr.
Sciences, submitted to the WHO by Bayer A.G.
Umeda, Y. Anti-Blast Fungicide "Hinosan" and its Biological
1972 Properties and Metabolism in the Rice Plant. Japan
Pest. Inform. No. 10, 85-88.
Umeda, Y. Hinosan, a Fungicide for Control of Rice Blast.
1973 Japan Pest Inform. No. 17, 25-28.
Urwin, C. and Newman, A.J. Pathology Report of SRA7847/3
1972 Month Feeding Study in Rats. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Vogeler, K. Caschromatographische Methods zur Bestimmung von
1968 Hinosan-Rückständen in Reis. Pflanzenschutz
Nachr., Bayer 21 (3):321-325.
Yatomi, A. Mutagenicity Test on Bacteria. Unpublished report
1975 from Nihon Tokushu Seizo K.K., submitted to the
WHO by Bayer A.G.
Cholinesterase depression in rats was observed following acute
inhalation exposure to aerosolized edifenphos. Plasma and RBC
cholinesterase depression was seen following exposure to 30 mg/m3
for 4 hours (threshold level). No depression was noted at an
exposure concentration of 9 mg/m3 for 4 hours (Kimmerle, 1975b).
TOXICOLOGICAL STUDIES
Special Studies on Teratology
Groups of rats (20 mated females/group) were administered
edifenphos by oral gavage at doses of 0, 5, 15 and 30 mg/kg body
weight from day 6 to 15 of gestation. All animals were sacrificed
and pups delivered by Caesarean Section. Increased mortality
reduced growth and an unhealthy appearance were observed at the two
highest dose levels. At the highest dose level, 3 litters were
resorbed. There were no abortions and with the exception of the 3
resorbed litters, the numbers of implantations, fetuses and
resorptions did not differ from control values. The weights of
fetuses were not different from controls and the nature and
frequency of somatic and skeletal variations were within normal
limits. There were no malformations and no indications of a
teratogenic potential was noted in this study (Lorke, 1971).
Groups of rabbits (11-13 mated does/group) were administered
edifenphos by oral gavage at dose levels of 0, 3, 6 and 12 mg/kg
body weight from day 6 to 18 of gestation. On day 29 of gestation,
the does were sacrificed and Caesarean Section performed. There was
no mortality although the two highest dose levels resulted in
reduced growth (the highest level treatment showed a significant
weight loss). In the 6 mg/kg group one doe aborted and one resorbed
all fetuses. Implantation recorded for the two highest doses was
reduced. However, the significance of this was not apparent as
treatment did not begin until 6 days after fertilization and
edifenphos treatment should not have affected this parameter. There
were no differences in the number of fetuses in the 0 and 3 mg/kg
dose while the upper two doses had fewer fetuses. The weight of
fetuses and placenta of the treated group did not differ from
control values. Malformations were not observed on gross or
skeletal examination of fetuses. As suggested with rats, edifenphos
did not induce a teratological response in rabbits (Machemer,
1976).
Special Studies on Reproduction
Groups of rats (10 males and 20 females/group) were fed
edifenphos in the diet at concentrations of 0, 5, 15 and 150 ppm
and mated to begin a standard 3 generation, 2 litter per
generation, reproduction study. Reproductive indices included:
fertility, gestation, viability and lactation measured for all 6
litters. There was no mortality observed and the highest dietary
concentration did not affect fertility. Litter size was reduced and
in one instance the litter weight was reduced at the 150 ppm level.
Slight effects (not statistically significant) were noted on
viability and lactation indices in the high dose group. Histological
examination of the F3b generation gave no indication of adverse
effects on the major tissues and organs. A no effect-level in this
study would be 15 ppm (Loser, 1976).
Groups of rats (20 females/group) were fed edifenphos in the
diet at dosage levels of 0, 5, 15 and 50 ppm, mated and subjected
to a standard 3 generation, 2 litters per generation, reproduction
study, In combination with the study, additional groups of 5
females from the P and F1b and 10 females from the F2b were
sacrificed at day 20 of gestation for an examination of fetuses for
any teratological events. Additionally, offspring of 5 female rats
from individual groups of the F1b, F2b and 10 females from the P3b
were maintained after weaning at the same dietary concentrations of
edifenphos listed above for periods ranging from 1 to 6 months.
These short term studies were designed to evaluate growth and
development of rats exposed initially in utero and thereafter for
short periods to dietary levels of edifenphos.
Growth of parental generations was unaffected by the presence
of edifenphos in the diet. Slight reduction of male food intake and
body weight at 50 ppm was noted in the first parental generation.
The reduced food consumption was observed in the F2 and F3 parents
accompanied by slight reduction of growth. The reduced growth was
predominant in those animals fed after weaning for prolonged
periods.
There were no significant differences noted in the
reproduction indices over the course of the three generations.
Edifenphos did not affect the ability of the rats to reproduce,
maintain pregnancy, deliver, nurse and wean normal sized litters
over the course of three generations. A teratological examination
of fetuses delivered by Caesarean Section prior to term did not
show any abnormalities related to somatic or skeletal defects. In
the first generation the 50 ppm dosed group reflected a slight
retardation in the degree of bone ossification not always noted in
the two lower doses or the controls. There were no significant
differences in implants, resorptions, live fetuses or in the weight
of fetuses.
In those weanlings from the F1b, F2b and F3b litters that were
maintained on diets containing edifenphos for 1 to 6 months after
weaning, growth was not substantially affected. Slight reductions
of growth were noted at 50 ppm accompanied by reduced food intake.
Hematological examinations made at the conclusion of each time
interval were normal. Liver function tests including SGOT and SGPT
activity and cholinesterase determinations using plasma, RBC and
brain were normal. Gross and microscopic examination of tissues and
organs of the animals maintained after weaning showed only on
consistent event attributable to edifenphos in the diet. An
enlarged kidney in the 50 ppm group fed for 3-6 months was
accompanied by changes (saccular necrosis) in the tubular
epithelium noted on microscopic examination. No dose related
changes were noted in a variety of tissues and organs that could be
attributable to edifenphos in the diet (Anonymous, 1976a).
Special Study on Mutagenicity
Mice
A dominant lethal study using male mice was performed with
edifenphos. Groups of mice (12 males/group) were administered
edifenphos by a single intraperitoneal injection at doses of 0, 50
and 100 mg/kg. A positive control (MMS, methyl methansulfonate, 100
mg/kg) was included in the study. The males were mated with three
untreated virgin females. The females were changed weekly for 6
consecutive weeks to evaluate maturation of male mouse germ cells.
One week after breeding, the females were sacrificed and examined
for implantation sites, resorption sites and embryos.
There were no deaths as a result of treatments with
edifenphos. Except for a slight reduction in the mating index at
week 6 in the 100 mg/kg dose group, there was no effect on the
ability to mate or fertilise the females. There were no
dose-related effects noted with respect to implantation, resorption
or embryo viability. There was no increase in pre-implantation loss
or mutuation rate although the positive control showed a
significant increase in early resorptions, attesting to the species
susceptibility. Edifenphos did not demonstrate a mutagenic
potential by the dominant lethal test in mice (Arnold, et al.
1971).
Microorganisms
When examined in vitro with various strains of
microorganisms (B. subtilis, E. coli and S. typhimurium
edifenphos did not induce a mutagenic response. In two studies with
the aid of a B. subtilis strain lacking the recombination
repair system, S. typhimurium and E. coli with either a
histidine or tryptophane deficiency, edifenphos, examined for its
ability to induce mutations, was found to be negative. Positive and
negative controls were used in these assays to assure sensitivity
to the mutagenic activity and to provide a baseline of operation.
In addition, liver homogenates (fortified with cofactors necessary
for microsomal oxidation) were used to activate, if possible,
edifenphos to an active mutagen. In studies with reversion colonies
in combination with a microsomal activation system, edifenphos did
not induce mutations (Shirasu et al., 1976a; 1976b; Yatomi, 1975).
A host mediated assay was performed where groups of 5 mice
were orally administered two doses of 0, 50 or 100 mg edifenphos/kg
(within 24 hours). After the second dose, a microbiological test
system of S. typhimurium was injected into the peritoneal
cavity, removed after 3 hours and examined for revertant colonies.
In this assay, edifenphos was negative although a positive control
of dimethylnitrosamine (50 mg/kg, oral administration) induced
reversion at a substantial rate (Shirasu. et al., 1976a).
Special Studies on Potentiation
Oral administration of equitoxic doses (LD50) of edifenphos
with fenthion or propoxur did not result in potentiation of the
acute toxicity of the combination. (Kimmerle, 1967).
A greater than additive toxicity, observed with a combination
of malathion and edifenphos, suggested a potentiating effect with
these two pesticides (Kimmerle and Lorke, 1967). Further studies on
toxic interaction of two organophosphorus pesticides were
undertaken where rats fed 0, 5 or 25 ppm edifenphos in the diet
were administered malathion by ip injection at a sublethal dose
(400 mg/kg). The increased mortality observed in the two groups fed
edifenphos suggested a significant potentiating effect on malathion
toxicity (Chen, et al., 1972).
Special Studies on Antidotes
Following acute oral administration of edifenphos to male
rats, antidotal administration (ip injection) of atropine (50
mg/kg) alone or in combination with 2-PAM or BH6 (50 or 20 mg/kg,
respectively) resulted in a slight reduction in the acute toxicity
(less than 2 fold) (Kimmerle and Lorke, 1967).
Special Studies on Neurotoxicity
Edifenphos was administered to hens by oral intubation or
intraperitoneal injection at dose levels up to 1000 mg/kg. The
surviving animals were examined for 42 days with no signs of
delayed neurotoxicity (Kimmerle and Lorke, 1967).
Groups of hens were administered edifenphos by oral intubation
at the LD50 level (547 mg/kg body weight) together with atropine,
50 mg/kg administered by ip injection. Delayed neurotoxic signs of
poisoning were not seen, but were observed in all hens treated
orally with TOCP (350 mg/kg). The hens were observed for 3 weeks
and sacrificed (Kimmerle, 1971). Histological examination of
portions of the central and peripheral nervous system of the
edifenphos-treated hens was negative with respect to axon or myelin
disruption. The TOCP-treated hens showed axon and neuron
degeneration in the spinal cord (brain and peripheral nerve were
not affected) (Spicer, 1971a).
In an effort to evaluate the relationship of delayed
neurotoxicity and copper imbalance in hens, an examination of
several organophosphates inducing a delayed neurotoxicity was made.
Edifenphos as one of the negative compounds did not induce
neurotoxicity and did not increase copper and ceruloplasmin levels
as did several of the positive neurotoxic agents (Kimmerle and
Loser, 1974).
Groups of hens (8 hens/group) were fed edifenphos in the diet
for 30 days at concentrations of 0, 100, 250, 500 and 1000 ppm.
Growth was depressed at 500 ppm and above. Whole blood
cholinesterase was depressed after 30 days only in the highest dose
group. Animals allowed to recover on control diets for a 4 week
period recovered body weight and cholinesterase depression was
reduced. Neurotoxic clinical signs were not observed (Kimmerle,
1969). Histological examination of brain, spinal cord and
peripheral nerve showed no evidence of myelin degeneration (Spicer,
1971b).
Acute Toxicity
TABLE 2. Acute toxicity of edifenphos
LD50
Species Sex Route (mg/kg) Reference
Rat M oral 119-340 Kimmerle, 1967;
Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 48-81 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974;
Chen, et al., 1972
dermal
(24 hour exposure) 184-413 Kimmerle, 1972a Thyssen &
Kimmerle, 1974
(4 hour exposure) 1,230 Kimmerle & Lorke, 1967
(7 hour exposure) 600 Kimmerle & Lorke, 1967
F oral 63-150 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
ip 26-55 Thyssen & Kimmerle, 1974;
Kimmerle & Lorke, 1967;
Chen, et al., 1972
dermal
(24 hour exposure) 86-135 Kimmerle, 1972a,
Thyssen & Kimmerle, 1974
Mice M oral 392 Thyssen & Kimmerle, 1974
TABLE 2. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
Mice F oral 218-295 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Guinea Pig oral 350-400 Kimmerle & Lorke, 1967
Rabbit F oral 250-400 Kimmerle & Lorke, 1967;
Thyssen & Kimmerle, 1974
Cat oral >250 Kimmerle & Lorke, 1967
Note -- In some instances the range of values is reflective of
differences in solvents used in the test. The lower values were
obtained with Lutrol while the higher values were obtained with
ethanol: propylene glycol mixtures. In all cases there appears to be a
significant sex difference with females more susceptible than males.
Signs of poisoning were typical of cholinergic stimulation (tremors,
salivation, labored respiration, etc.) appearing 2-3 hours after acute
poisoning and persisting for up to 2 days with rats and up to a week
with mice and rabbits.
Inhalation Toxicity
Acute aerosol inhalation toxicity tests (both static and dynamic
flow) were performed with groups of rats using various exposure times.
Edifenphos technical or a 50% EC formulation (Hinosan(R)) was used in
several studies. Data expressed as the technical product do not show
significant differences in toxicity between the two forms of
edifenphos. No indication of particle size distribution was given in
these data.
Dynamic tests
Results of dynamic tests are given in Table 3.
TABLE 3. Inhalation toxicity of edifenphos (dynamic tests)
Exposure LD50
Species Sex Time (mg/m3) Reference
Rat M 1 hour >898-1310 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b;
Kimmerle & Lorke, 1967
TABLE 3. (Cont'd.)
Exposure LD50
Species Sex Time (mg/m3) Reference
4 hour 362 - 775 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b.,
Kimmerle, 1975b;
Kimmerle & Lorke, 1967
F 1 hour > 1017-1046 Thyssen & Kimmerle, 1974;
Kimmerle, 1972b
4 hour 230 - 320 Thyssen & Kimmerle, 1974;
Kimmerle, 1975b;
Kimmerle, 1972b
Static Tests
Groups of rats, mice, rabbits and guinea pigs were exposed to
static concentrations of edifenphos ranging from 108 to 398 mg/m3 for
up to 4 hours. In the 1 hour exposure, no toxicity was recorded (only
one dose level was tested) but mice exhibited toxic signs of exposure.
In the 4 hour exposure the susceptibility of rats, rabbits and mice
was the same with 7/10 rats, 2/3 rabbits and 12/20 mice dying at a
concentration of 269 mg/m3. Only 1/5 guinea pigs died at this
exposure. No deaths were reported at a concentration of 101 mg/m3 for
the 4 hour exposure (Kimmerle & Lorke, 1967).
Edifenphos did not induce a primary dermal irritation when
applied to the skin of rabbits' ears for up to 24 hours or to the
conjunctival sac of rabbits. The 50 EC formulation of Hinosan(R)
irritated both the conjunctiva and skin (1 hour exposure). The primary
irritation was attributed to the formulation ingredients and not
technical edifenphos (Kimmerle and Lorke, 1967; Thyssen and Kimmerle,
1974).
Toxicity of metabolites
Acute toxicities of edifenphos metabolites to rats are shown in
Table 4.
TABLE 4. Acute toxicity of edifenphos metabolites to rats
Compound Route LD50
(mg/kg)
O-ethyl S-phenyl oral M >500
S-(4-hydroxyphenyl)- F >1000
phosphorodithioate
SS-diphenyl oral M 1000-2000
phosphorodithioate F 1000-2000
O-ethyl S-phenyl oral M 1000-2000
phosphorothioate F 616
O-ethyl S-phenyl oral M approx. 1000
S-(3-hydroxyphenyl) F approx. 500
phosphorodithioate
(Lamb & Matzkanin, 1976)
Short-term studies
Mice
Groups of ICR mice (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. In general, growth was not affected by dietary
edifenphos. Food consumption, behaviour, physical condition and
mortality were unaffected. Plasma cholinesterase depression was noted
at 30 ppm with RBC and brain depressed only at 1000 ppm. Hematology,
urinalysis and clinical chemistry parameters were normal. On gross
examination of tissues and organs, spleen and liver enlargement was
observed at 1000 ppm. Microscopic examinations showed no abnormalities
in spleen although liver and adrenal changes were noted at 300 ppm and
above. In liver, hypertrophy of hepatocytes and deposition of
phospholipid were observed. Adrenal hypertrophy and pigment deposition
were noted at the two highest doses (Imamichi, et al., 1973b). A
no-effect level of 10 ppm was observed equivalent to 1.53 mg/kg body
weight.
Rats
Groups of rats (10 males and 10 females/group) were exposed to
edifenphos by inhalation at concentrations of 0, 4 (range of 3-15), 10
(8-12) and 27 (24-30) mg/m3 air for 12 weeks, 5 days/week for 6
hours/day. The range of particle size was 0.5 to 1.5 µ with 97% of the
particles having a mean mass diameter of 1 µ (a fully respirable
particle). There was no mortality although females exposed to 27
mg/m3 were observed to be adversely affected. Growth was normal at
all levels of exposure as were clinical chemistry, hematology and
urinalysis parameters. Cholinesterase (brain, plasma and RBC) activity
was depressed at 10 mg/m3 and above. No effects were noted at 4
mg/m3. Gross and microscopic analysis of tissues and organs were not
affected by edifenphos in the diet. Female adrenal weight was
increased at the highest dose level but no abnormalities were detected
on microscopic examination (Kimmerle, 1975; Mohr, 1975).
Groups of rats (10 males and 10 females/group) were fed
edifenphos in the diet for 3 months at dosage levels of 0, 3, 10, 30,
100, 300 and 1000 ppm. There was no mortality during this study but
growth was inhibited at 1000 ppm in both males and females. In
contrast to the reduced growth, food consumption was normal at all
dose levels. Several hematological parameters were affected at 1000
ppm, namely reduced RBC count, hemoglobin content and hematocrit value
primarily in males. Liver function was reduced at 1000 ppm (SGOT), 100
ppm (SGPT) and at 30 PPM (SAP) predominantly in males, with the
reduction not as marked in females. Urinalysis parameters were
unaffected. Cholinesterase depression was noted at 300 ppm in males
(brain and RBC) and 100 ppm in females (brain and RBC), plasma was
depressed in females at 10 ppm. Gross examination revealed enlarged
liver (1000 ppm) and kidney (300 ppm). Microscopic examination of
tissues showed hypertrophy and fatty degeneration of hepatocytes at
100 ppm and above. No pathological changes were noted in liver at 30
ppm. At 100 ppm and above, kidney and adrenal changes were noted. At
high levels a toxic nephritis was observed and the kidney and adrenal
glands were hypertrophic. In the male, kidney degeneration was
prevalent while in the females the adrenal was damaged. Gross changes
noted in brain, pituitary and thyroid of males at high dose levels
were not accompanied by pathological changes (Imamichi, 1973a).
Groups of rats (15 males and 15 females/group) were fed
edifenphos in the diet at dosage levels of 0, 1, 2, 5, 10 and 150 ppm
for 3 months. Growth was affected by edifenphos and there were no
differences in behaviour, mortality or appearance. Food consumption in
the highest dose group was slightly reduced. Hematological
examinations were normal at 1 and 3 months as were liver function and
urinalysis tests measured at the same time intervals. Cholinesterase
depression was observed in the highest dose group (in both RBC and
plasma) within one week of initiation of the feeding trial. The
depression was constant and there was no indication of a cumulative
effect relating to edifenphos. Gross and microscopic analysis of
tissues and organs, performed at the conclusion of the study, showed
no dose-related gross change and no significant microscopically
observable alteration (Loser, 1972; Urwin and Newman, 1972).
Groups of rats (10 males/group) were administered edifenphos by
oral intubation, daily for 14 days at dose rates of 0, 12.5, 50 and
100 mg/kg. Mortality was not reported but a slight decrease in average
body weight was reported at 100 mg/kg. A significant increase in
absolute and relative liver weight was reported at all dose levels at
24 hours. The livers had recovered to normal size within 7 days of the
end of treatment. Cholinesterase was depressed at all levels with the
following order of sensitivity: RBC > plasma > brain. One week after
treatment RBC and brain cholinesterase activity were depressed at all
dose levels. Liver function tests were slightly affected (SGOT
reduced) but were normal within 7 days. EPN detoxication by liver
microsomes was stimulated at all dose levels (aniline hydroxylase and
aminopyrine demethylase were unaffected) and recovery was not complete
at 7 days. Microscopic examination of liver, adrenals and kidney
showed no adverse effects (Anonymous, 1976c).
Groups of rats (15 females/group) were administered edifenphos
orally (0, 5, 10, 20, 33.5 and 50 mg/kg) or by intraperitoneal
injection (0, 1.75, 3.5, 7, 10, 11.5 and 17.5 mg/kg), daily 5 weeks
per week for 60 days. Oral administration of 50 mg/kg daily resulted
in no mortality or cumulative toxicity although signs of poisoning
were seen. No signs of poisoning were evident at 10 mg/kg/day
following oral dosing. With daily ip injections, no substantial
mortality (1/15 deaths) was observed at 7 mg/kg (11/15 died at 11.5
mg/kg). Signs of poisoning were not evident at 3.5 mg/kg. These
results suggest a minimal cumulative toxicological effect (Kimmerle
and Lorke, 1967).
Dog
Groups of dogs (2 male and 2 female/group, controls had 3 of each
sex) were fed edifenphos in the diet at dosage levels of 0, 3, 10 and
30 ppm for 90 days. There was no mortality over the course of the
study and growth, food consumption, behaviour and general appearance
were normal. Clinical chemistry, hematology and urinalysis values were
normal at one and 3 months (except for a slight increase of SDH
activity at only one month in both males and females at 30 ppm). Blood
cholinesterase activity was depressed in both plasma and RBC (to the
same extent in both enzyme sources) at the highest dose. As with
rodent studies, there was no evidence of cumulative activity over the
3 month interval. Gross and microscopic examination of tissues and
organs revealed no adverse effects of edifenphos in the diet. A slight
increase in the absolute and relative weight of the spleen at 30 ppm
was not accompanied by microscopic lesions (other than minimal)
congestion and probable hemosiderin deposition) (Loser, 1969; Spicer
and Urwin, 1971).
Groups of dogs (4 male and 4 female/group) were fed edifenphos in
the diet for two years at dose levels of 0, 7, and 20 ppm. A fourth
group was fed a diet containing 60 ppm for 1 year after which the
concentration was increased to 80 ppm for 5 months and raised to 120
ppm for the remaining 7 months of study. There was no mortality and
behaviour and physical appearance were normal and unaffected by
edifenphos in the diet. Growth and food consumption was normal at the
20 ppm level although it was slightly depressed at the high dose
regime. At the high level food consumption was slow, diet rejection
was observed and body weight gain depressed. There was no effect noted
on ophthalmological examination, urinary or hematology parameters or
on reflex testing. Some liver function test parameters were abnormal
(data showed SAP increased although SGPT was normal). This change was
accompanied by an increased gross and relative liver weight noted at
the time of sacrifice. Cholinesterase activity was depressed in the
high dose group in both plasma and RBC of males and females. Brain
cholinesterase was unaffected at any dose level. Gross and microscopic
examination of tissues and organs did not suggest an adverse effect of
edifenphos fed in the diet at concentrations up to and including 120
ppm. The inductive effect noted on the liver (increased organ weight
accompanied by increased phosphatase activity) was attributed to an
adaptation reaction noted with many drugs and chemicals. Microscopic
examination of liver did not show changes attributable to the presence
of dietary edifenphos which would account for the increased liver
weight. A no-effect level was observed to be 20 ppm equivalent to a
daily intake of 0.58 mg/kg body weight (Hoffmann, 1976; Gallagher and
Practice, 1976).
Long Term Studies
Rat
Groups of rats (50 male and 50 female rats/group, 100 of each sex
were used as controls) were fed edifenphos in the diet at
concentrations of 0, 2, 5, 15 and 150 ppm for two years. Mortality was
unaffected during the two years. Growth, behaviour and physical
appearance were normal in all groups. Hematology, blood chemistry and
urinalysis parameters were normal. A slight decrease in alkaline
phosphatase at 150 ppm was considered to be within the normal range
for the animals. Cholinesterase depression was observed in plasma and
RBC of both sexes at 150 ppm. Brain cholinesterase was depressed only
in females at 150 ppm. Gross and microscopic examinations were
performed on surviving animals. No abnormal, dose-related events were
recorded in the gross examination. A slight increase in adrenal size
of males at 15 ppm and above was not reflected in changes observed
under microscopic examination. Histopathological examination of
tissues and organs revealed luminal deposits of calcium salts in the
medulary tubules of male rats fed 15 and 150 ppm diets accompanied in
the high dose group by an increased incidence of glomerulonephrosis in
males. These changes were not noted in females. A complete examination
of tumours was performed revealing no indication of carcinogenic
potential. The frequency of both benign and malignant tumors was
typical of the rat strain used in the study. A no-effect dietary
level, based on the microscopic examination observing slight kidney
changes, was 5 ppm equivalent to 0.30 mg/kg body weight (Loser, 1976;
Offer and Prentice, 1976).
COMMENTS
Edifenphos, an organophosphorus ester, is used in agriculture as
a fungicide to control rice diseases. Following oral administration it
is rapidly absorbed, distributed, metabolised and excreted in mammals.
The well defined metabolic sequence observed in plants and animals
appears to be the same. In metabolism studies and other studies in
several species of animals there were no indications of any
culminative effects.
Biochemical parameters affected by acute and subacute
administration of edifenphos include cholinesterase depression and
changes in certain liver enzyme values reflecting changes in basic
liver function. Edifenphos is not a potent cholinesterase inhibitor.
However, the recovery of depressed enzyme activity is not rapid
suggesting that regeneration of cholinesterase activity is dependent
on enzyme synthesis rather than reversal of an enzyme-inhibitor
complex. A sensitive biochemical parameter was aliesterase activity of
liver and serum with a level of 5 ppm of edifenphos in the diet
showing 50% inhibition. In special studies for teratogenic and
mutagenic potential, no effects of edifenphos were noted on several
species. At high levels, reproduction was slightly impaired but at
levels that did not affect maternal well-being reproduction parameters
were unaffected. Edifenphos appeared to potentiate the acute effects
of malathion as might be expected from the data on depression of
aliesterase activity. There is no evidence that edifenphos would
induce a delayed neurotoxic response in hens.
Edifenphos is moderately toxic on acute exposure to a variety of
mammalian species with signs of poisoning typical of
parasympathomimetic agents. The acute signs of poisoning, although
modified slightly by atropine and reactivators, were not completely
controlled.
In all cases, the acute toxicity of metabolites was greatly
reduced with respect to the acute toxicity of the parent compound. In
both short and long term studies in rats and dogs, the most sensitive
parameters affected were cholinesterase depression, liver enlargement
and deposits of kidney salts in males on long term studies. Based on
short term studies in mice and dogs and long term studies in rats a
no-effect level was suggested and a temporary ADI for man was
recommended. The dietary studies in rat (both short term and long
term) showing liver and serum aliesterase depression and kidney salt
build-up in males were used as a basis for evaluating the no-effect
level. While 5 ppm was a level causing 50% inhibition of aliesterase
activity the significance of this effect was unclear and has been
taken to suggest "exposure" rather than "toxicological effect".
The liver involvement noted in many studies precluded
consideration of a finite ADI until a specific carcinogenic study is
performed using a species susceptible to liver changes that might
relate to hepatic carcinoma. As these changes noted in this study
resemble those effects seen with other compounds including chlorinated
hydrocarbons, such studies are required. The lack of carcinogenic
potential noted in the 2 years, on a limited number of rats, was
reassuring that the compound is not a potent carcinogen in this
species. However, it was considered in the 2 year rat study that the
highest dose level and the small group size were not sufficient to
fully evaluate the potential for carcinogenicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm in the diet equivalent to 1.53 mg/kg bw
Dog: 20 ppm in the diet equivalent to 0.58 mg/kg bw
Rat: 5 ppm in the diet equivalent to 0.25 mg/kg bw
Estimate of temporary acceptable daily intake for man
0 - 0.003 mp/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Edifenphos is an organophosphorus compound with a fairly
selective action against rice blast caused by Piricularia oryzae,
one of the major rice diseases. It has both a protective and curative
action against the fungus attack. There is some evidence that the
compound interferes with chitin formation in the fungal cell wall, a
process which dose not occur in higher plants. This mode of action may
therefore explain the selective action of the fungicide.
The compound is also effective against a few other fungus
diseases of rice, e.g. ear blight caused by Cochliobolus
miyabeanus, Hormodendrum and against some insect pests, e.g. leaf
hoppers.
USE PATTERN
Edifenphos is authorised or recommended and sold in various
countries in Asia including Japan, in Central and South America and in
Europe, e.g. Italy. The product is marketed as emulsifiable
concentrates with various concentrations of edifenphos and as a dust.
In most countries two to four treatments are applied at dosage rates
of 300-800 g a.i./ha, with a pre-harvest interval of 21 days. The
recommended preharvest interval in Italy is 60 days.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
Japan and in some South American countries, namely Colombia, Mexico,
Peru and El Salvador. A summary of these data is given in Table 5.
FATE OF RESIDUES
The metabolic and other reactions of edifenphos exposed to
plants, animals, fungi, soil, light and water are shown in Figures
1(a) and 1(b) taken from Umeda (1972) with some modifications.
TABLE 5. Residues of edifenphos (parent compound) in rice resulting from supervised trials
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
Rice Japan 1967 2 0.36 EC 30% <0.02
hulled 3 0.36 EC 30% <0.02
4 0.36 EC 30% <0.02
3 0.6 dust 1.5% <0.02
4 0.6 dust 1.5% <0.02
1969 4 0.6 dust 1.5% <0.01
5 0.6 dust 1.5% 0.015
3 0.6 dust 1.5% <0.01
4 0.6 dust 1.5% < 0.01
3 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% 0.01
Rice Japan 1969 2 0.36 EC 30% <0.01
polished 3 0.45 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
4 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 1.5% <0.01
3 0.75 dust 2.5% 0.01
3 0.5 dust 2.5% 0.01
Rice
(in husk Peru 1969 2 0.5 EC 0.07
(straw 0.15
(in husk Colombia 1969 2 0.5 EC 0.1
(straw 0.25
(in husk El Salvador 1969 2 0.5 EC 0.45
(straw 1.4
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 7 13/16 20/23 25/27 30/34 36/39 40/42
(in husk Mexico 1969 2 0.5 EC 0.2
(straw 0.05
15/20 21/25 26/30 31/35 36/40 41/50 51/60
Rice Japan 1970 2 0.45 EC <0.01 <0.01
hulled 4 0.45 EC <0.01
2 0.2-0.45 EC <0.01 <0.01
4 0.2-0.45 EC <0.01
2 0.45 <0.01 <0.01
4 0.45 <0.01
(hulled 1973 2 0.3-0.45 EC 0.02
(straw 0.62
(hulled 1973 3 0.3-0.45 EC 0.01
(straw 0.32
hulled 1970 2 0.6 dust 0.03 0.03
4 0.6 dust 0.01
1971 3 0.75 dust 0.03 0.01
4 0.75 dust 0.02
1970 2 0.6-0.8 dust <0.01 0.01
4 0.6-0.8 dust <0.01
1970 2 0.6-0.8 dust <0.01 <0.01
4 0.6-0.8 dust <0.01
Rice Japan 1970 2 0.6 dust 0.01 0.01
hulled 4 0.6 dust 0.02
2 1 dust 0.01 0.01
4 1 dust 0.01
TABLE 5. (Cont'd.)
Application Residues in mg/kg, at intervals (days) after application
Crop Country Year rate Formulation
no kg ai./ha 15/20 21/25 26/30 31/35 36/40 41/50 51/60
(hulled 1972 2 <0.01 <0.01
(straw 0.48 0.26
(hulled 1972 3 1 dust <0.01
(straw 0.93
(hulled 1972 2 1 dust <0.01
(straw 0.18
(hulled 3 1 dust 0.02 <0.01
(straw 3.81 0.24
(hulled 1973 2 1 dust <0.01
(straw 0.67
(hulled 1973 3 1 dust 0.01
(straw 0.74
(hulled 1973 2 1 dust <0.01
(straw 0.72
(hulled 3 1 dust 0.1
straw) 2.12
Rice
(hulled Japan 1972 2 1 dust 0.01
(straw 2.31
(hulled
(straw 3 1 dust 0.02
5.31
(hulled 3 1 dust <0.01
(straw 3.79
In plants
Extensive data are available on absorption, translocation,
accumulation and metabolism in the rice plant. (Ishizuka et al 1973,
1974; Nitokuno 1971; Takase et al 1973; Tomizawa et al 1972; Uesugi et
al 1972, Ueyama et al 1973, Umeda et al 1972).
Absorption and translocation
Izhizuka et al (1973, 1974) found that when edifenphos was
sprayed on rice leaves 60% was lost from the surface in five days and
75% in ten days. It was shown that about 70% of the edifenphos applied
had penetrated the leaf tissue within two hours.
Only small quantities were translocated from the site of
application, and translocation from roots to shoots was also slight.
Hence the loss of edifenphos was mainly from the leaf surfaces by
volatization and decomposition, rather than by metabolism inside the
plant.
When the flag leaf of a rice plant was painted with 32P-labelled
edifenphos some translocation of the chemical to the lower part of the
leaf and to the ears was observed (Ishizuka et al 1973, 1974; Nitokuno
1971; Umeda 1972). Since no complex of edifenphos with
photosynthesized plant products was found, it appears that edifenphos
is translocated without prior metabolism. The concentration of
edifenphos in plant parts is in the order: leaves and stems >> husks
>> hulled rice.
Metabolism
Takase et al (1973) studied the metabolic fate of edifenphos
after spraying rice plants at various growth stages. The
hexane-soluble components, which were analysed throughout the
experimental period, consisted, mainly of edifenphos parent compound
(83.2-89.3% of the radioactivity in the fraction). The major
components in the water soluble fraction were identified as O-ethyl
S-phenyl phosphorothioate (metabolite VII, Figure 1(a)) and
SS-diphenyl phosphorodithioate, (III) each representing about 30% of
the total 32p in the water-soluble fraction. With the lapse of time,
ethyl phosphate (XV) was formed.
Ueyama (1973) detected nine metabolites after applying
35S-labelled edifenphos to rice leaves. Three were identified as
metabolites IV, VIII and XII (Figures 1(a) and 1(b)). It is probable
that the formation of VIII and XII involved the exchange of phenylthio
and ethoxy radicals between molecules of edifenphos.
A high proportion of S-phenyl phosphorothioate (V) and small
amounts of O-ethyl S-phenyl phosphorothioate (VII) were found among
the water-soluble metabolites. Results are shown in Table 6.
TABLE 6. Toluene extracted metabolites in and on rice plants treated with 35S-edifenphos (after Ueyama)
Metabolites, 35S counts expressed as edifenphos equivalents, mg/kg
Growth stage Part of Period after
at application Plant application Identified* Not-identified
I IV VIII XII A B C E F
Tillering leaf 1 hour 35 4.3 2.9 1.1 0.72 1
blade
6 hour 18 1.1 1.2 0.59 0.02 0.33
1 day 20 2.8 1.1 1.4 0.05 0.46
3 day 17 1.1 0.73 0.16 0.26 1.2
7 day 1.2 0.26 1.5 0.13 0.07 trace trace
15 day 0.79 0.22 - 0.18 <0.01 0.01
Tillering leaf 1 hour 0.73 trace 3.3 - trace
sheath 6 hour 0.84 trace 1.0 trace trace
1 day 0.79 trace 0.33 0.33 trace
3 day 0.65 trace 0.36 - 0.02
7 day 0.04 0.01 0.05 0.04 <0.01 trace
15 day trace - trace
Heading leaf 1 hour 42 10 1.2 trace 0.27 0.05 0.05 0.33
blade 6 hour 31 8.2 3.2 trace 0.27 0.13 0.13 0.89
1 day 9.8 2.5 1.1 0.12 0.06 0.01 0.03 0.15
3 day 7.8 1.6 0.55 trace 0.03 0.02 0.12 0.29
7 day 3.2 0.19 - 0.02 <0.01 0.03 0.37
15 day 0.40 0.38 - 0.18 0.24 0.30
* For identities of numbered metabolites, see Figures 1(a) and 1(b) (I = parent compound)
In animals
Pither and Gronberg (1975) administered 0.12 mg/kg of edifenphos
as a single oral dose and as a daily dose for 5 days to dairy cows.
Results are described above ("Evaluation for acceptable daily intake -
- Absorption, distribution and excretion").
Strankowski et al (1976) identified metabolites of 14C ring-
labelled edifenphos in the muscle, liver, kidney, fat, milk and urine
of dairy cows. Methyl phenyl sulfone (XI, Figure 1(b)) was the
principal metabolite in both milk and tissues representing 95% of the
organo-soluble radio-carbon in the milk and from 70% (kidney) to 99%
in the tissues. Small amounts of metabolites Xa, XIa, XIb, together
with other minor metabolites were also found (Table 7).
TABLE 7. Distribution of edifenphos and its metabolites in the
tissues and milk of dairy cows (Strankowski, 1976).
Residue, % of total radioactivity in organic extract,
as metabolite no.*
Tissue I IX X Xa XI XIa Xlb XIIa
muscle - - - - 85 2 - -
fat - 1 - - 99 - - -
liver <1 <1 <1 4 84 <1 <1 <1
kidney <1 - <1 2 70 2 9 <1
milk - - 1 3 93 1 1 -
* For identities of numbered metabolites see Figure 1(b),
(I = edifenphos).
Hermann and Gronberg (1976) administered 3 mg/kg 14C-edifenphos
orally to laying hens. After 96 hours 76% of the activity had been
excreted. Methyl phenyl sulfone (XI) was the only metabolite detected
in all the tissues and in egg, but a second metabolite was found in
kidneys and was identified as either IX, XII or XIII (Figure 1(b)).
The total radiocarbon residues at intervals of 6-72 hours after dosing
are given in Table 8.
TABLE 8. Total radiocarbon from 14C-edifenphos in tissues of
laying hens
Radioactivity expressed as edifenphos,
mg/kg*, after interval, hours
Tissue 6 24 48 72
Muscle (breast) 0.66 0.73 0.57 0.21
Muscle (thigh) 0.69 0.75 0.59 0.19
Skin 0.79 0.74 0.58 0.21
Fat 0.97 1.00 0.80 0.30
Liver 1.72 1.45 1.17 0.51
Kidney 1.83 1.29 0.96 0.39
Gizzard 0.72 0.76 0.56 0.21
Heart 0.82 0.89 0.66 0.26
* Values are mean figures of single analyses of tissues from each of
four hens.
Ueyama and Takas (1975) determined the residue levels in goat
milk after administration of edifenphos at the rate of 1 mg/kg/day for
ten consecutive days. No edifenphos could be detected in the milk.
After a single oral dose of 10 mg/kg, 0.0006-0.0008 mg/kg in
edifenphos could be detected within 24 hours.
Lamb and Roney (1976a) exposed crayfish to 14C-edifenphos
concentrations of 10 µg/l (i.e. 10-8) for 14 days and determined 14C
residues during the exposure period and a 28 day withdrawal period.
14C residues, expressed as edifenphos, accumulated to a maximum level
of 0.2 mg/kg. About half of the accumulated 14C was lost within 7
days of withdrawal.
The same authors (1976b) exposed Channel catfish (Ictalurus
punctatus) continuously for 28 days to 10-12 µg/l of 14C
ring-labelled edifenphos. 14C residues accumulated to a maximum of 1.2
mg/kg edifenphos equivalents in ten days, representing an accumulation
factor of 104-117. The non-edible portions (head, viscera and scales)
contained 75% of the extractable residues. The acetonitrile fraction
(81% of the total extractable residue) consisted of edifenphos 44%,
metabolite VI (Figure 1(a)) 10%, VII 19% and VIII 27%. During the
withdrawal period about 50% of the accumulated 14C residues were
excreted in five hours and about 85% within four days.
In fungi
Uesugi and Tomizawa (1971) studied the metabolism of edifenphos
in mycelia of the rice blast (Pericularia oryzae) with 32P-, 35S-
and non-labelled edifenphos. The main metabolic pathway consists in
the hydrolysis of one P-S linkage, followed by that of the other P-S
or the ethyl ester bond, finally yielding phosphoric acid. Another
hydroxylated intermediate, metabolite II (Figure 1(a)) was also found.
No significant differences in route or rate of metabolism were
found between susceptible and resistant strains of the fungus.
In storage, processing and cooking
The residue is mainly located on the rice husk, hence during
milling and polishing most of the residue is removed. Takase et al
(1973) estimated the distribution of 32P-labelled edifenphos in
milling fractions (Table 9).
The residues at harvest in the husks consisted largely of
edifenphos metabolites, although small quantities of the parent
compound were found (Table 10).
Residues of edifenphos from foliar application (0.63 kg/ha, 2
applications) were found primarily in straw and seed hulls 37 days
after the second application. The residue in the hulls ranged from
0.02-0.29 mg/kg. Trace amounts were found in the bran, but no residue
was detectable in the milled and polished rice (Chemagro, 1971).
On exposure to light
A marked degradation of 35S-edifenphos by UV light was found on
irradation in aqueous or hexane solution or as a thin film. In hexane,
the formation of water-soluble degradation products was low. The
metabolites III, VIII, XV and XVI were identified (Murai et al, 1976).
In water and soil
Shaw (1976b) and Shaw and Murphy (1976) studied the hydrolysis of
14C ring-labelled edifenphos at concentrations of 1 and 10 mg/kg in
aqueous solutions buffered at pH 3, 6 and 9 and at temperatures of 25,
35 and 45 degrees C. The hydrolysis followed first order kinetics and
its rate increased with temperature and with increasing pH. The same
major hydrolysis products were formed under all conditions, namely
compounds VII and XII (Figure 1(a) and (b)) with traces of II, III,
IV, VI, VIII and XIV.
TABLE 9. Distribution of 32P-labelled edifenphos and its metabolites in milling fractions of rice
Radioactivity (32P) expressed as edifenphos, mg,/kg
Number of treatments 1 2 3 1
Pre-harvest interval,days 13 1 24 8
Milling fraction Hexane- Water- Hexane- Water- Hexane- Water- Hexane- Water-
soluble soluble soluble soluble soluble soluble soluble soluble
husks 0.11 0.04 0.17 0.12 0.28 0.14 2.13 0.34
hulled rice trace 0.02 trace 0.01 0.06 0.03 0.31 0.05
polished rice 0.01 0.01 0.01 0.01 trace trace trace -
TABLE 10. Distribution of radio-activity from 32P-edifenphos in rice
husk.
Residue, % of total radio-activity
3 applications, 1 application,
Compound* latest 24 days 8 day pre-harvest
pre-harvest harvest
Unknown
Water-soluble metabolite 9.6 13.4
III 10.9 19.4
VII 37.6 29.1
XV, XVI 41.8 28.1
* For identities of metabolites, see figures 1(a) and 1(b).
Shaw (1976a) incubated 14C ring-labelled edifenphos in pond
water (pH 8.3) containing soil for 62 days after irradiation with UV
light and sunlight, with alternating 12-hour periods of light and
darkness. In these conditions the half-life of edifenphos was 16.8
hours. The main products were compounds VII and XIV and one or more of
compounds IX, XII and XIII. Traces of II, III, IV, X and XI were also
found. These products were found in both the water and the bottom
sludge.
Flint and Shaw (1975) estimated the half-life of edifenphos in an
outdoor simulated pond system at 20°C. Under these conditions the
half-life was 29 hours in the water and about 60 hours in the bottom
sludge.
Edifenphos is almost immobile when applied to soil. Leaching and
soil adsorption were inversely correlated and the latter was
proportional to the soil organic matter content.
When columns of sand and sandy loam soil containing edifenphos at
a level equivalent to 0.5 kg/ha were eluted with water at a rate
representing 200 mm of irrigation in two days, no edifenphos was found
in the eluate (Bayer, 1974 a, b). The half-life of edifenphos was
about 23 days in the sandy loam (pH 5.2, organic carbon 0.57% and
particles < 20 µ 19.5%), but only 6-8 days in the sand (pH 6.8,
organic bound carbon 2.50% and particles < 20 µ 10.1%). In further
laboratory experiments simulating the flooded soil conditions
associated with rice growing (Nitokuno, 1972), edifenphos was degraded
rapidly during the first 24 hours. The half-life was about 19 hours in
a clay soil and 2 1/2 days in a silt loam. Subsequent degradation was
slower in both soil types.
Tomizama (1975) reports similar results obtained in a laboratory
degradation experiment. Under the anaerobic conditions of the
experiment about half the edifenphos was degraded during the first 3 -
5 days. From the sixth day onwards the rate of degradation was
considerably slower.
Studies by McNamara (1976) and McNamara and Close (1976) were
made on the persistence and degradation of 14C ring-labelled
edifenphos in loam and silt loam under aerobic conditions and in loam
under anaerobic conditions. The half-lives were 1 day in the aerobic
silt loam and 3 days in loam under both aerobic and anaerobic
conditions. The organo-soluble radioactivity decreased to 10-20% of
that applied within 21 days in all samples. In aerobic soils the
decrease was mainly due to volatilization and binding to the soil. In
aerobic loam about 20% of the total radioactivity was lost by
volatilization in 14 days, and in silt loam 35% in 21 days.
In aerobic soil the main organo-soluble radioactive residues were
edifenphos, its initial hydrolytic products III and VII, and
derivatives of thiophenol (XIII): diphenyl disulphide XII,
benzenesulphonic acid XIV, methyl phenyl sulphoxide X and methyl
phenyl sulphone XI. The two major terminal metabolites were X and XI.
In anaerobic loam the metabolic pathway proceeded via thiophenol
(XIII) to diphenyl disulphide XII, and benzenesulphonic acid XIV. Only
small amounts of methylated products were found.
In a model ecosystem
Edifenphos was fairly rapidly degraded in a model ecosystem, the
half-life being 60 hours. The main metabolic features were the
conversion of edifenphos to compounds IV and VII and the rapid
oxidation of the diphenyl disulphide released from these compounds. It
was found that sulphur atoms derived from the diphenyl sulphide were
incorporated into sulphuric acid and then into cell constituents,
(Tomizawa and Kazano, 1975).
METHODS OF RESIDUE ANALYSIS
Vogeler (1968) developed a gas-chromatographic method for the
determination of edifenphos residues in rice, using an alkali
thermionic or flame photometric detector. The rice sample is macerated
and extracted with acetone and the filtrate evaporated to dryness. The
residue is cleaned up by acetonitrile - petroleum ether partition and
by chromatography on a Florisil column. Recoveries from control
samples fortified with 0.05-1 mg/kg ranged from 70-80%. The limit of
determination is about 0.05 mg/kg. Thornton (1971) checked several
organophosphorus compounds which are authorised or recommended for use
on rice for interference with Vogeler's method. They were tested at
levels equivalent to the highest recommended dose rate on rice in the
USA. The compounds tested were dichlorvos, fensulfothion, fenthion,
malathion, naled, parathion, parathion-methyl, phorate and TEPP. None
showed any interference.
Takase et al (1971) described a modified gaschromatographic
method for the analysis of edifenphos in rice. Extraction is with
acetonitrile and the residue is cleaned up by partitioning into
acetonitrile and n-hexane followed by TLC on alumina. The residue is
determined with a flame photometric detector. Recoveries of added
levels of 0.1 to 0.2 mg/kg ranged from 96-102%. The limit of
determination is 0.005 mg/kg or lower.
Stanley (1972) adapted and modified the Vogeler method to
determine edifenphos residues in milk and cattle tissues. For milk the
method involves initial blending with acetone, followed by separation
of edifenphos from the aqueous solution by partitioning into
chloroform. Clean-up is by partition between Skellysolve B and
acetonitrile and chromatography on a Florisil column. Animal tissues
are extracted with acetonitrile, partitioned with Skellysolve B, and
cleaned up on a Florisil column. The edifenphos parent compound is
determined by gas chromatography with a thermionic detector. The limit
of determination of edifenphos in milk is 0.002 mg/kg and in cattle
tissues 0.02 mg/kg.
Recoveries from milk spiked with 0.005-0.5 mg/kg ranged from 77
to 110%, and in various cattle tissues spiked with 0.05-0.1 mg/kg from
82 to 118%.
Sandie and Gronberg (1975) adapted the Stanley (1972) method for
the analysis of edifenphos in poultry and eggs. For poultry tissues
the method involves extraction, partition between acetonitrile and
hexane, and gas chromatography with a thermionic detector. Eggs are
extracted with acetone, and clean-up is by successive aqueous
acetone-chloroform and acetonitrile - hexane partitions, followed by
silica gel chromatography. Recoveries were 84-107% from tissues at
levels of 0.05 and 0.1 mg/kg and 86-97% from eggs at 0.01 mg/kg. The
limits of determination were 0.01 mg/kg in poultry tissues and
0.001 mg/kg in eggs.
Minor (1976) examined possible interferences by other
organophosphorus pesticides in the determination of edifenphos in
cattle and poultry tissues and eggs by the methods of Sandie and
Gronberg (1975) and Stanley (1972). He found that edifenphos could be
adequately separated from all the 25 organophosphorus pesticides with
a USA tolerance in milk if the Stanley Florisil column and the Sandie
and Gronberg silica gel column were used successively in the clean-up
procedure.
NATIONAL TOLERANCES
National tolerances reported to the meeting are given in Table
11. They refer to the parent compound only.
TABLE 11. National tolerances reported to the Meeting
Country Commodity Tolerance, Recommended
mg/kg pre-harvest
interval, days
Italy rice, hulled 0.05 60
Japan rice, hulled 0.03 21
Mexico rice, hulled 20
U.S.A. rice, hulled 0.1*
rice, hulls 0.3
* temporary tolerances valid until May 1977
APPRAISAL
Edifenphos is an organophosphorus compound with a rather
selective action against the rice blast caused by Piricularia
oryzae, one of the major rice diseases, against which it shows both
a protective and curative action. There is some evidence that the
compound interferes with the formation of chitin in the fungus
cell-wall, a process which does not occur in higher plants. This may
therefore explain the selective action of the fungicide. Edifenphos is
also effective against a few other rice diseases and some insect
pests, e.g. leafhoppers.
The pesticide is used on an extensive scale on rice, as EC
formulations and as a dust, in Asian countries including Japan, in
Central and South America and in some mediterranean countries, e.g.
Italy. In most countries 2 - 4 treatments are applied at 300 - 800 g
ai/ha, generally with a pre-harvest interval of 21 days.
Extensive data were obtained on the fate of edifenphos residues
in soil, water, rice plants and livestock animals such as cattle,
goats and poultry. Degradation pathways in plants, soil and animals
are similar, although there are quantative differences.
In a study with 35S labelled edifenphos on rice, nine
metabolites were found in a toluene extract from leaves: the three
main products were OO-diethyl S-phenyl phosphorothioate, SSS-triphenyl
phosphorotrithioate and diphenyl disulphide. A large proportion of
S-phenyl phosphorodithioate and small amounts of O-ethyl S-phenyl
phosphorothioate were found as water-soluble metabolites. In a study
with 32p-labelled edifenphos, SS-diphenyl phosphorodithioate was
identified as another major water-soluble metabolite.
In a cattle feeding study with ring-labelled 14C-edifenphos it
was shown that methyl phenyl sulphone was the principle metabolite
both in milk and in animal tissues such as muscle, liver and kidney:
in milk it amounted to about 93% of the radioactivity extracted. The
parent compound, together with m- and p-hydroxyphenyl methyl sulphone
and p-hydroxyphenyl methyl sulphoxide were found, but only in small
amounts.
After administering a single dose of 14C-labelled edifenphos
orally to laying hens (3 mg/kg), 76% of the radioactivity was excreted
in 96 hours. A single metabolite, methyl phenyl sulphone, was
identified in all tissues and eggs.
More than half of the residue from spraying edifenphos onto rice
leaves was dissipated in five days. Only small quantities were
translocated from the site of application. Translocation of edifenphos
from the roots to the shoots was also only slight.
Residues in rice at harvest are rather low. After the recommended
pre-harvest interval of 21 days, the residues of edifenphos and its
main metabolites in hulled rice following an application of
32P-labelled edifenphos to rice plants were of the order of 0.03 and
0.06 mg/kg edifenphos equivalents of water-soluble and hexane-soluble
metabolites respectively. Only traces were found in polished rice. In
the rice husks the residues were about 0.15 and 0.3 mg/kg edifenphos
equivalents of water-soluble and hexane-soluble 32P respectively.
Gas-chromatographic methods of analysis with alkali flame or
flame photometric detection are available. Recoveries in rice were
70-80% and the limit of determination ranged from 0.005 to 0.05 mg/kg
edifenphos parent compound. Nearly all organophosphorus pesticides
registered and/or recommended for use on rice were checked for
interference. None of the compounds interfered when tested at levels
that would be reached by treatments at the highest recommended
dosages, provided appropriate clean-up procedures were used. These
methods should be suitable for or adaptable to regulatory purposes.
Some of the GLC methods mentioned are suitable for the analysis
of edifenphos residues in cattle tissues, milk, poultry and eggs. The
limit of determination is 0.01 - 0.02 mg/kg parent compound.
Recoveries at levels of 0.01 - 0.1 mg/kg ranged from about 80 to 100%.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the parent compound edifenphos only.
Commodity Limit, mg/kg
Rice (in husk) 0.2
Rice (hulled) 0.05
Rice (polished) 0.01*
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Further studies to examine the hepatic involvement observed in
several animal species.
Desirable
1. Observations in man (relative to occupational exposure).
2. More information on residues of edifenphos and its main
metabolites on rice in husk at harvest.
3. A method of residue analysis suitable for edifenphos together
with its main metabolites.
REFERENCES
Anonymous Hinosan (Edifenphos)/Generation Study on Rats.
1976a Unpublished report from Nihon Tokushu Noyaku Seizo
Co., Ltd., submitted to the WHO by Bayer A.G.
* at or about the limit of determination
Anonymous Special Toxicity Test of Hinosan - I (single
1976b p.o. application). Unpublished report from Nihon
Tokushu Noyaku Seizo Co., Ltd., submitted by Bayer
A.G.
Anonymous Special Toxicity Test of Hinosan - II (14 day
1976c repeat p.o. application). Unpublished report from
Nihon Tokushu Noyaku Seizo Co., Ltd., submitted by
Bayer A.G.
Arnold, D. Mutagenic Study with Hinosan (BAY 78 418) in
1971 Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted by Bayer
A.G.
Bayer, A.G. Pflanzenschnitzmittle - Rückstunde in
1974a Sickerwasser Sickelwasser (edifenphos).
Unpublished. Reports Bayer A.G. No. 8503/8504/74.
Bayer, A.G. Verhalten des Pflanzenschutzmittelwirkstuffes
1974b edifenphos in Boden. Unpublished report Bayer A.G.
No. 8506/74.
Chen, R.S., Kinoshita, F.K. and DuBois, K.P. Acute Toxicity and
1972 Antiesterase Action of O-Ethyl-S, S-diphenyl
Phosphorodithioate (Hinosan). Tox. Appl. Pharm. 23
519-527.
Eben, A. and Kimmerle, G. Metabolism of Hinosan Active Ingredient
1972 in Rats and Dogs. Unpublished report No. 3687 from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Flint, D.R., Shaw, II, H.R. The Mobility and Persistence of Hinosan
1975 in Soil and Water. Chemagro Report No. 43 663.
Gallagher, P.J. and Prentice D.E. Pathology Report of Hinosan
1976 (SRA7847) Chronic Toxicity study in Dogs over two
years. Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Hermann, P.A. and Gronberg, R.R. Excretion and Metabolism of Hinosan
1976 in Poultry. Chemagro Report No. 49 023.
Hoffmann, K. SRA7847 (Active Ingredient of Hinosan) Chronic Toxicity
1976 Study on Dogs. Unpublished report from Institut
für Toxikologie, Bayer, A.G., submitted to the WHO
by Bayer A.G.
Imamichi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, X.,
1973a Ikadai, H., Mori, H., Furusawa, U. and Saito, K.
Hinosan Subchronic Toxicological Studies on Rats.
Unpublished report from Nippon Vet. and Zoo Techn.
College, Tokyo, Japan, submitted to the WHO by
Bayer A.G.
Imamachi, T., Sudo, T., Uematsu, Y., Igarashi, A., Suzuki, K.,
1973b Tauchi, K., Ikadai, H., Mori, H., Furusawa, U. and
Saito, K. Hinosan-Subchronic Toxicological Studies
on Mice. Unpublished report from Nippon Vet. and
Zoo Techn. College, Tokyo, Japan, submitted to the
WHO by Bayer A.G.
Ishizuka, K., Hosaka, H., Takase, I., Tan, K.E., Hirata, H.
1974 Metabolism and Accumulation of Pesticides in Rice
Plants. Comp. Studies Food Environ. Contam.;
Inter. Atomic Energy Agency, Vienna, 363 - 372.
Kimmerle, G. Bayer 78 418 (SRA7848)/Potenzierungsversuche.
1967 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Bay 78 418/Subcronische Neurotoxizitats-versuche
1969 bei Huhnern. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Kimmerle, G. Hinosan/Acute Neurotoxicity Studies on Hens.
1971 Unpublished report from Institut für Toxikologie,
Bayer A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Dermal Toxicity of Hinosan Active Ingredient to Rats.
1972a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. Acute Inhalation Experiments with Hinosan Active
1972b Ingredient on Rats. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Subchronic Inhalation Toxicity Study on Rats.
1975a Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. SRA7847/Acute Inhalation Toxicity Study on Rats.
1975b Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Kimmerle, G. and Lorke, D. Toxikologische Untersuchungen mit dem
1967 Wirkstoff, Bayer 78 418. Unpublished report from
Institut für Toxikologie, Bayer, A.G., submitted
to the WHO by Bayer A.G.
Kimmerle, G. and Loser, E. Delayed Neurotoxicity of Organophosphorus
1974 Compounds and Copper Concentration in the Serum of
Hens. EQS Environmental Quality and Safety
3:172-178.
Lamb, D.W. and Matskanin, C.W. The Acute Oral Toxicity of Hinosan
1976 Metabolites to Rats. Unpublished report from
Chemagro Agr. Div. Mobay Chem., Corp., submitted
to the WHO by Bayer A.G.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976a in Chanuel Catfish Exposed to Hinosan-14C.
Chemagro Report No. 46 379.
Lamb, D.W. and Roney, D.J. Accumulation and Persistence of Residues
1976b in Catfish Exposed to Hinosan 14C. Chemagro
Report No. 48 832.
Loeffler, W.W. The Effect of Processing on Pesticide Residues.
1972 Chemagro Report No. 34 176.
Lorke, D. Hinosan/Studies of Product for Possible Embryotoxic
1971 and Teratogenic Effects on Rats. Unpublished
report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Subchronische toxikologische Untersuchungen
1969 an Hunden. Unpublished report from Institut für
Toxikologie, Bayer, A.G., submitted to the WHO by
Bayer A.G.
Loser, E. SRA7847/Subchronic Toxicological Studies on Rats.
1972 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Generation Study on Rats. Unpublished report
1976a from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Loser, E. Bay 78 418/Chronic Toxicity Study on Rats. Unpublished
1976b report from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Machemer, L. Hinosan Active Ingredient (SRA7847)/Studies of
1976 Embryotoxic and Teratogenic Effects on Rabbits
following Oral Administration. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Minor, R.G. An Interference Study for the Residue Methods for
1976 Hinosan in Milk, Eggs and Animal Tissues. Chemagro
Report No. 48 819.
Mohr, U. Subchronic Inhalation Toxicity Study on Rats,
1975 Histopathological Findings. Unpublished report
from Institut für Toxikologie, Bayer, A.G.,
submitted to the WHO by Bayer A.G.
Murai, T., Uesugi, Y., Tomisawa, C. and Tanaka, T.
1976 Photo-Degradation of Pesticide (I), Degradation of
Organophosphorus Fungicides EDDP and IBP by
Ultraviolet Light. Chemagro Report No. 45 169.
Murphy, J.J., Feldhas, L., Simmons, C.E., Thornton, J.S. and
1976 Gronberg, R.R. Total Radioactive Residues of
(14C) Hinosan in Rotational Crops. Chemagro
Report No. 48 826.
McNamara, F.T. Persistence and Metabolism of Hinosan in Soil.
1976 Chemagro Report No. 49 025.
McNamara, F.T. and Close, C.L. Persistence and Metabolism
1976 of Hinosan in Soil under Aerobic Conditions.
Chemagro Report No. 46 402.
Nitokuno Hinosan, a Fungicide for Control of Rice Blast,
1971 Chem. Econ. Engin. Rev. 3 (1), 57-59.
Nitokuno Gas Chromatographic Determination of Residues of
1972 Hinosan (O-ethyl S,S-diphenyl phosphorodithiolate)
in Rice Grains. Unpublished report.
Nitokuno Special Toxicity Test of Hinosan I. (single p.o.
1976 application). Unpublished report of Nihon Tokushu
Noyaku, Seizo Co., submitted by Bayer A.G. to the
JMPR.
Offer, J.M. and Prentice, D.E. Pathology Report of Bay 78 418
1976 (Hinosan) Chronic Toxicity Testing on Rats.
Unpublished report from Huntingdon Research
Centre, submitted to the WHO by Bayer A.G.
Pither, K.M. and Bronberg, R.R. Residue Levels in Lactating
1975 Dairy Cattle following Oral Administration of
Hinosan-ring-Ul-14C. Unpublished report No. 44
845 from Chemagro Agr. Div. Mobay Chem. Corp.,
submitted to the WHO by Bayer A.G.
Sandie, F.E. and Gronberg, R.R. A Gas Chromatographic Method
1975 for the Determination of Residues of Hinosan in
Poultry Tissue and Eggs. Chemagro Report No. 45
987.
Shaw, II, H.R. Hydrolysis of Hinosan in Pond Water Containing Soil.
1976a Chemagro Report No. 48 829.
Shaw, II, H.R. Hydrolysis of Hinosan in Buffered Aqueous Solution.
1976b Chemagro Report No. 48 831.
Shaw, II, H.R. and Murphy, J.J. Hydrolysis of Hinosan in Buffered
1976 Aqueous Solution. Chemagro Report No. 46 403.
Shirasu, Y., Moriya, M. and Kato, K. Report of the Mutagenicity
1976a Study of Hinosan (EddP, edifenphos). Unpublished
report from the Department of Toxicology,
Institute of Environmental Toxicology, Tokyo,
Japan, submitted to the WHO by Bayer A.G.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. and Kada, T.
1976b Mutagenicity Screening of Pesticides in the
Microbial System. Mutation Research 40:19-30.
Spicer, E.J.F. Pathology Report of Bay 78 418/Hen Study.
1971a Unpublished report from Huntingdon Research
Central submitted to the WHO by Bayer A.G.
Spicer, E.J.F. Pathology Report of Bay 78 418/Subchronic
1971b Neurotoxicity Study in Hens. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Spicer, E.J.F. and Erwin, C. Pathology Report of Bay 78 418/
1971 Subchronic Toxicity Studies in Dogs. Unpublished
report from Huntingdon Research Centre, submitted
to the WHO by Bayer A.G.
Stanley, C.W. Gas Chromatographic Method for the Determination
1972 of Residues of Hinosan in Animal Tissues and Milk.
Chemagro Report No. 32 070.
Strankowski, K.J., Pither, K.M. and Murphy, J.J. Metabolism of
1976 Hinosan by a Lactating Dairy Cow. Unpublished
report No. 48 846 from Chemagro Agr. Div. Mobay
Chem. Corp., submitted to the WHO by Bayer A.G.
Takase, I., Nakamura, S., Tsuda, H. and Ueyama, I. The Gas
1971 Chromatographic Determination of Pesticides. (Part
4). Cleanup Method for Organophosphorus Pesticide
Residues in Rice Grain. Noyaku Seisan Gijutsu
(Pesticide and Technique) 23:31-35.
Takase, I., Tan, K.E. and Ishizuka, K. Metabolic Transformation
1973 and Accumulation of O-Ethyl S,S-Diphenyl
Phosphorodithiolate (Hinosan) in Rice Plants. Agr.
Biol. Chem. 37(7): 1563-1571.
Thornton, J.S. A Study of the Possible Interferences of Other
1971 Pesticides with the Gas Chromatographic Residue
Method for Hinosan. Chemagro Report No. 29 702.
Thyssen, J. and Kimmerle, G. Hinosan 50 E.C./Toxicological Studies.
1974 Unpublished report from Institut für Toxikologie,
Bayer, A.G., submitted to the WHO by Bayer A.G.
Tomizawa, C. Degradation of Organophosphorus Pesticides in Soils
1975 with Special Reference to Unaerobic Soil
Conditions. Environ. Qual. Safety 4:117-127.
Tomizawa, C., Uesugi, Y. and Murai, T. Fats and Significance of
1972 Fungicides in Rice. Radiotracer Studies Chem. Res.
Food. Agric.; Intern. Atomic Energy Agency,
Vienna, 103-105.
Tomizawa, C. and Kazano, H. Biological Accumulation of Pesticides
1975 in an Ecosystem. Rev. Plant Protec. Res.
8:41-54.
Uesugi, Y. and Tomizawa, C. Metabolism of O-Ethyl S,S Diphenyl
1971 Phosphorodithiolate (Hinosan) by Mycelial Cells of
Pyricularia Oryzae. Agric. Boil. Chem. 35
(6):941-949.
Uesugi, Y. Tomizawa, C. and Murai, T. Degradation of
1972 Organophosphorus Fungicides. Environ. Toxicol.
Pest. Academic Press, 327-339.
Ueyama, I., Uesugi, Y., Tomizawa, C. and Murai, T. Metabolic
1973 Fate of O-Ethyl S,S-Diphenyl Phosphorodithiolate
(Hinosan) in Rice Plant. Agr. Biol. Chem.
37(7):1543-1551.
Ueyama, I. and Takase, I. Metabolic Behaviour of O-Ethyl
1975 S,S-Diphenyl Phosphorodithiolate (edifenphos) in
Female Goat. Agr. Biol. Chem. 39(9):1719-1727.
Ueyama, I., Takase, I. and Tomizawa, C. The Metabolism of Edifenphos
1976 (Hinosan) in Mice and Rats. Unpublished report No.
1046 from Nithon Tokushu Noyaku Seizo Co. .td.
Agr. Chem. Inst. and National Institute of Agr.
Sciences, submitted to the WHO by Bayer A.G.
Umeda, Y. Anti-Blast Fungicide "Hinosan" and its Biological
1972 Properties and Metabolism in the Rice Plant. Japan
Pest. Inform. No. 10, 85-88.
Umeda, Y. Hinosan, a Fungicide for Control of Rice Blast.
1973 Japan Pest Inform. No. 17, 25-28.
Urwin, C. and Newman, A.J. Pathology Report of SRA7847/3
1972 Month Feeding Study in Rats. Unpublished report
from Huntingdon Research Centre, submitted to the
WHO by Bayer A.G.
Vogeler, K. Caschromatographische Methods zur Bestimmung von
1968 Hinosan-Rückständen in Reis. Pflanzenschutz
Nachr., Bayer 21 (3):321-325.
Yatomi, A. Mutagenicity Test on Bacteria. Unpublished report
1975 from Nihon Tokushu Seizo K.K., submitted to the
WHO by Bayer A.G.
