
MALEIC HYDRAZIDE JMPR 1976
IDENTITY
Chemical name
1,2-dihydro-3,6-pyridazinedione
Synonyms
MH,6-hydroxy-3(2H)-pyridazone
Structural formula
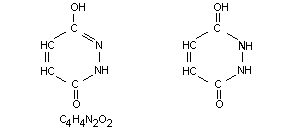 Other information on identity and properties
Composition of the technical product
The technical material contains 99% of maleic hydrazide;
impurities are inorganic salts (e.g. Na2SO4 possibly with minor
amounts of maleic or fumaric acid.
Physical and chemical properties of maleic hydrazide
physical form white crystalline powder
molecular weight 112.1
specific gravity at 25°C 1.60
melting point 292°C(min)
odour faint
volatility non volatile
solubility (approximate) g/100 g solvent at 25°C
distilled water 0.6
alcohol 0.1
acetone 0.1
dimethyl formamide 2.4
xylene less than 0.1
Ph of 0.5% aqueous solution at 25°C: 4.
Maleic hydrazide behaves as a weak monobasic acid. The
alkanolamine and alkali metal salts are moderately soluble in
water.
Formulations used: liquid 30% and 40% a.i.; wettable powder 40%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Maleic hydrazide does not appear to be extensively metabolized
by mammals. In the rabbit, 43-62% of a single oral dose was
excreted unchanged within 48 hrs. Isolation and characterization of
the excreted maleic hydrazide following oral doses of 2 g showed
the excreted product to be the benzylamine salt. No other
metabolites were identified. (Barnes et al, 1957). Incubation of
phenobarbital-induced rat liver microsomes with maleic hydrazide
did not result in any degradation of the compound (Nelson and
Kearney, 1975).
Using maleic hydrazide - 14C labelled material, female rats
were dosed with 0.27, 0.68, or 2.72 mg per rat (equivalent to 10,
25 or 100 µc/rat) by stomach tube. At the low dose level, 65.4% of
the radioactivity was excreted in the urine in 12 hrs and 77.3%
within 6 days.
A further 12.4% of the administered radioactivity was detected
in the faeces over the same period. Rats dosed with 2.5 µc showed
0.12-0.18% expired radioactivity in CO2 over the 72 hour period
after administration. Tissue levels of radioactivity were
statistically insignificant except in the carcass, where <0.001%
of the administered radioactivity was detected. Using paper
chromatography with two different solvent systems, urine from rats
intubated with 100 µc resulted in the identification of unchanged
maleic hydrazide (92-94%) and a maleic hydrazide conjugate (6-8%).
(Mays et al, 1968).
Sixteen rats were given 4g/kg by stomach tube. Urine and
faeces were collected for time intervals 0 to 2, 2 to 4, 4 to 8,
and 8 to 16 days. Two rats/sex were sacrificed after 2, 4, 8, or 16
days post-dosing. In male rats, residues in urine (30,400 mg/kg)
were approximately double those in faeces (17,400 mg/kg) over the
0-2 day period, whereas in females urine residues (1,330 mg/kg)
were much less than in faeces (12,700 mg/kg). In both sexes,
residue levels decreased rapidly, but were still detectable in 8-16
day samples of both urine and faeces. Excretion in females was
slightly slower than in males, however residues were undetectable,
except for traces in the males sacrificed on day 8. (Food Research
Laboratories Inc. 1955).
TOXICOLOGICAL STUDIES
Special Studies on reproduction
Seven groups of approximately 10 male and 10 female rats fed
0, 0.5, 1.0, 2.0 or 5.0 maleic hydrazide as the sodium salt, or 0
or 0.1% maleic hydrazide as the diethanolamine salt were repeatedly
bred using a cycle of 2 weeks pairing, 3 weeks gestation, 3 weeks
lactation and one or two weeks rest prior to remating, for as long
as possible, commencing at 12 weeks on test. The second litters
(F1b) were used to produce the F2 generation which were mated
twice. The F2b generation provided parents for the F3a and b
offspring. Pooled data from the 8 matings of the Fo generation do
not indicate any effects of the sodium salt of maleic hydrazide on
fertility, litter size, gestation index, viability index, or
lactation index. Pup weight at weaning was slightly reduced at the
5.0% level. The diethanolamine salt at the 0.1% level resulted in
reduced fertility, reduced litter size, reduced viability index,
and reduced lactation index. The diethanolamine salt group was
dropped from the study, no attempt being made to produce the F2
generation. Mean data for all generations of rats receiving the
sodium salt do not do not indicate any effects on reproduction,
with respect to fertility, gestation index, viability index, or
lactation index. At the 5.0% level, litter size was reduced in the
F3 generation and weanling weight was reduced in all generations.
(Food Research Laboratories, 1955).
Special studies on mutagenicity
Injection of 0.7% sodium chloride Ringer's solution containing
0.4% maleic hydrazide abdominally in one to two-day old
Drosophila melanogaster males did not result in an increase in
mutation rate. On the other hand, D. melanogaster males fed on
media containing 0.4% maleic hydrazide showed an increased
incidence of lethal mutations in the first brood, although in
subsequent broods, the effect was not apparent (Nasrat, 1965).
In a mouse dominant lethal study, 500 mg maleic hydrazide/kg
did not affect the calculated mutation index. (Epstein & Shafner,
1968).
Andersen et al (1972) tested maleic hydrazide and other
chemicals on eight mutant strains of Salmonella typhimurium on
T4 bacteriophage, and on bacteriophage AP72. In none of these ten
systems was there any ~evidence of mutagenic activity. Maleic
hydrazide was tested by McCann et al (1975, 1976) on several tester
strains of Salmonella. Tests covered a wide dose range, both with
and without liver microsome activation. There was no evidence of
mutagenic activity in this extensive series of tests.
Special studies on carcinogenicity
Swiss mice (ICR/Ha) were injected sub-cutaneously with aqueous
solutions, or tricaprylin suspensions of maleic hydrazide as free
acid (0.4% hydrazine impurity), or with solvent alone in volumes of
0.1, 0.1, 0.2 and 0.2 ml on post-natal days 1, 7, 14 and 21. Total
doses were 3 mg for aqueous solution, and 55 mg for tricaprylin
suspension. (A further group comprising 11 mice all died following
10 mg maleic hydrazide injected on post-natal day 1). Preweaning
mortality was 14% in controls, 5% in mice receiving 3 mg and 53% in
mice receiving 55 mg. Post weaning mortality in males in all groups
was high being 38, 57, and 52% at 0,3 & 55 mg respectively by 49
weeks of age. Decreased weight gain (ca 5%) was noted in the high
dose group. Hepatoma incidence in males at 49 Weeks was 8% in
controls, 18% in the 3 mg group and 73% in the 55 mg group. No
metastases were noted. (Epstein et al, 1967).
Maleic hydrazide was administered by stomach tube in daily
doses of 1000 mg/kg weight to 36 mice of each sex for 3 weeks,
beginning when the animals were 7 days old. Then, 3000 ppm were
mixed directly with the diet, which was fed for approximately 18
months. No significant increase in the incidence of tumors was
observed in comparison with untreated controls (Innes et al.,
1969).
Rats were injected subcutaneously with 1 ml of the
diethanolamine salt (i.e. 5 mg) weekly for 14 months. Of 52 rats so
treated in two experiments, 3 developed sarcomas. No sarcomas
occurred in saline control rats. (Barnes et al, 1957).
Groups of rats and mice were either injected with the
monosodium salt, subcutaneously, once weekly at a dose level of 500
mg,/kg or received 1% maleic hydrazide added to the diet for 100
weeks. Maleic hydrazide did not affect growth or general health in
either species, on either dose regime. One rat receiving maleic
hydrazide by injection (out of a total of 29) developed a sarcoma.
No sarcomas were observed in control rats. Total tumor incidence
was increased in female test groups of both species, fed maleic
hydrazide, the effect being more obvious in the mouse (Barnes et al
1957).
Groups of 25 male and 25 female rats were injected twice
weekly for 65 weeks with arachis oil, water, solvents plus 2 mg
maleic hydrazide or solvents plus 2 mg diethanolamine salt of
maleic hydrazide. A further 39-week observation preceded autopsy.
subcutaneous tumor incidence was increased (4) in the group
receiving arachis oil plus maleic hydrazide when compared with the
arachis oil control group (1). Incidences of tumors in all other
groups were comparable. The increased subcutaneous tumor rate was
attributed to impaired connective tissue repair mechanisms rather
than chemical carcinogenesis induction. (Hunter et al, 1973).
Three groups of 24 male rats were fed basal diet, basal diet
plus 2% maleic hydrazide as the sodium salt, or basal diet plus
0.06% p-dimethylaminoazobenzene for up to 26 weeks. No significant
changes with regard to body weight, desoxyribose nucleic acid
content per liver cell nucleus, average liver cell size, liver
weight, number of cells/liver or DNA/liver were observed with
maleic hydrazide, although the p-dimethylaminoazobenzene affected
all these parameters. Pathological examination of animals fed
maleic hydrazide did not reveal neoplasms, although these occurred
in all rats fed the p-dimethylaminoazobene for more than 10 weeks.
(Mannell & Grice, 1957).
Special studies on mammalian cells in vitro
The effect on mammalian cells was examined by studying the
development of mitosis in fragments of mouse ear epidermis. In
concentrations from 0.0001-0.001 M, maleic hydrazide had no effects
on mitosis or cell division. Maleic hydrazide was also tested upon
skin from the guinea pig's ear, grown in tissue culture. Up to a
concentration of 0.01 M no gross or microscopical effects upon the
explants were noted. The same concentration of maleic hydrazide was
also without effect upon the respiration of skin cells, studied 2
and 22 hr after adding the maleic hydrazide (Barnes et al., 1957).
In vitro studies with mouse thymus cells in culture showed
that maleic hydrazide in concentrations of 0.001 M or more
inhibited growth. Mitotic inhibition occurred at and above 0.0001
M. The ratio of dry mass to DNA content was increased, which was
taken as an indication for inhibition of DNA, but not of protein
synthesis (McCarthy and Epstein, 1968). Mitotic inhibition was also
shown in cultures of human lymphocytes grown in the presence of
0.001-0.01 M. Inhibition was more marked in 72 hr-old cultures than
in fresh ones, probably because of the more active mitotic state in
the former (Timson, 1968).
Special studies on dermal irritation
The diethanolamine salt of maleic hydrazide was applied as a
20% aqueous solution to approximately 10 sq.cm. of abraded, and 10
sq. cm. of intact skin on the backs of 6 rabbits. Repeated
applications were made during a 6-hour period on five consecutive
days on two occasions, separated by a ten-day rest period. No signs
of irritation were noted during the study. (Food Research
Laboratories, 1955).
Special studies on dermal sensitization
Ten guinea pigs were injected intradermally with one dose of
0.5 ml, and 9 subsequent doses of 0.1 ml of a 0.1% aqueous solution
of the diethanolamine salt of maleic hydrazide, dosing being on
alternate days. Two weeks after the last dose, a challenge dose of
0.5 ml was administered intradermally at a different site. No
evidence of sensitization was observed. (Food Research
Laboratories, 1955).
Special studies on eye irritation
Six rabbits received two drops of a 5% solution of the
diethanolamine salt of maleic hydrazide in 5% saline in the right
eye. No signs of irritation were observed in the 14 day
post-treatment observation period. (Food Research Laboratories,
1955).
Special studies on respiratory effects
Deeply anaesthetized rats (ethyl ether) were administered the
diethanolamine salt of maleic hydrazide at various solution
concentrations by instilling small droplets in alternate nostrils
at the moment of inhalation. Doses of 50 to 400 mg diethanolamine
salt were administered (i.e. 15-120 mg maleic hydrazide). At the
400 mg dose level, 2/10 rats died after 10 days. Gross pathological
examination showed haemorrhagic lungs, and in one case, a yellowish
exudate and thoracic adhesions, laboured respiration (7/10) and at
2 weeks, rats (5/7) were also observed at the 400 mg level. (Food
Research Laboratories, 1955).
Special studies on potentiation
The acute toxicity of maleic hydrazide diethanolamine salt in
combination with other pesticides was determined. Pretreatment with
0.1 LD50 potentiated the toxicity of dieldrin and DDT in both
sexes, but decreased the toxicity of diazinon. On the other hand
pretreatment with DDT and dieldrin reduced the toxicity of maleic
hydrazide diethanolamine salt by a factor of two. Diazinon
pretreatment had a different effect, decrease of toxicity in
females, increase in males (Luckens and Wattimena, 1968).
Acute studies
Species Route LD50 References
mg/kg b.w*
Diethanolamine salt Food Research
Rat (fasted) oral 1180 Labs. 1955
Sodium salt
Rat (fasted) oral 5800 ibid
* expressed in terms of maleic hydrazide moiety.
Short term studies
Rat
Groups of 12 male and 12 female rats were fed 0, 0.5, 1.0, 2.0
or 5.0% maleic hydrazide, as the sodium salt, for 12 weeks.
Detailed data are not available. Mean body weight data indicate
that in males, a slight reduction occurs in all groups on maleic
hydrazide and a similar reduction occurs in females at 5.0% dietary
levels. The reductions are stated to be statistically
non-significant. Food utilization, hemoglobin, erythrocyte count,
total and differential leucocyte counts, were comparable in all
groups. Blood sugar was reduced at 5.0%, and non-protein nitrogen
was increased at the same level. Methaemoglobin levels were (if
present) below the limit of detection (0.2g/100 ml) and urinalysis
for sugar, albumin, and microscopic inclusions was unremarkable.
Gross pathology (on 2 males and 2 females at 12 weeks) was normal.
No histological abnormalities were noted. (Food Research
Laboratories, 1955).
A group of 12 male and 12 female rats was fed 1.0% maleic
hydrazide as the diethanolamine salt. Additional, non-contemporary
groups of 12 male and 12 female rats were fed 0.1% or 0% maleic
hydrazide as the diethanolamine salt and a final group of 10 males
and 10 females was fed 1.0% diethanolamine. All rats were on test
for 12 weeks. Body weight was significantly reduced in all test
groups. Mortality was increased markedly in groups receiving 1.0%
diethanolamine, and 1.0% diethanolamine salt of maleic hydrazide.
No further data are available for the group receiving 1.0%
diethanolamine. Food efficiency is stated to be comparable in all
groups except that receiving 1.0% diethanolamine salt of maleic,
hydrazide which is markedly reduced. Haemoglobin and erythrocyte
count are markedly reduced and an increased polymorph/lymphocyte
ratio are noted in the group receiving 1.0% diethanolamine salt of
maleic hydrazide. Mean data on the 0.1% diethanolamine maleic
hydrazide indicate a tendency to reduced haemoglobin levels and
erythrocyte counts, the statistical significance being
non-assessible on the basis of available data. At the 1.0%
diethanolamine maleic hydrazide level, death was preceded by loss
of coordination and muscular control, accompanied by starvation.
Pathological examination showed hyperaemic or haemorrhagic lungs,
and evidence of anaemia. Brain histopathology showed brain oedema.
No microscopic lesions were observed in liver, kidney or lung of
four representative rats of the group. (Food Research Laboratories,
1955).
Dog
Three dogs were given 1 g/kg of the sodium salt of maleic
hydrazide five times weekly for five weeks by gavage. At 5 day
intervals, haemoglobin, erythrocyte count and total and
differential leucocyte counts were determined. Methaemoglobin,
blood sugar and non-protein nitrogen were determined at sacrifice.
Liver, kidney, spleen and bone marrow were examined
histopathologically. No data are presented, but effects on the
parameters listed above are stated to be negligible although
transient eosinophilia, anemia, and leucocytosis were noted
(Food Research Labs, 1955).
Five groups of three mongrel dogs aged between four months and
2 years were fed 0 (1 male, 2 female), 0.5 (2 male, 1 female), 1.0
(1 male, 2 female), 2.0 (2 male, 1 female) % maleic hydrazide as
the sodium salt, or 1.0 (2 male, 1 female)% maleic hydrazide as the
diethanolamine salt in the diet for one year. Body weight and
mortality were unaffected by the sodium salt. The diethanolamine
salt caused loss of body weight, and 2/3 deaths by day 30 on test.
This group was abandoned at day 37 on test. Food intake was
unaffected in groups receiving the sodium salt. Haemoglobin,
erythrocyte count, total and differential leucocyte counts were
comparable in all groups. Blood sugar and non-protein nitrogen did
not show any consistent effects. Gross pathology was comparable in
all dogs. Histopathology on the bone marrow, liver kidney, spleen
and gastrointestinal tract was considered to be within normal
limits. Brains of dogs receiving the diethanolamine salt were
moderately oedematous, and the spinal cord showed early neuronal
degeneration and swelling of myelin sheath. (Food Research Labs.
1955).
Long-term studies
Rat
Five groups of approximately 10 male and 10 female rats were
fed 0, 0.5, 1.0, 2.0, or 5.0% maleic hydrazide as the sodium salt,
for 2 years. A further two noncontemporary groups were fed 0 or
0.1% maleic hydrazide as the diethanolamine salt, also for 2 years.
After 12 weeks on test, rats were bred one to one, within groups,
following a 2 week mating, 3 week gestation, 3 week lactation, 1
week rest cycle throughout the study. (The report states 1 week
rest, or 2 week rest after lactation, in different sections). Body
weight of males in all test groups was slightly reduced up to 1
year, but exceeded controls at 2 years. In females, body weight was
reduced at 5.0% Na salt at 12 weeks only. Mortality was generally
comparable between groups, except for the 0.1% diethanolamine salt
group, where life span was reduced, mortality being increased
between 52 and 96 weeks on test. Haemoglobin and erythrocyte counts
were reduced in the group receiving 0.1% diethanolamine salt, at
all reported time intervals (52,78 and 104 wks). In all other
groups, values were comparable. Total and differential leucocyte
counts were comparable in all groups. Blood glucose and non protein
nitrogen were comparable in all groups receiving the sodium salt at
12 and 24 months. Non protein nitrogen was slightly elevated in the
only measurement taken (24 months) on the sole male and sole female
survivor of the 0.1% diethanolamine salt group. Methaemoglobin was
not detected in any group. Urinalysis was stated to be normal in
all groups. No consistent changes occurred in liver, kidney or
spleen organ/body weight ratios. Tumor incidence was comparable in
all groups. Pathological changes are stated to be attributable to
infections, parasites, or age. (Food Research Laboratories, 1955).
COMMENTS
Maleic hydrazide was evaluated by the IARC in 1974 and it was
concluded that "no carcinogenic effect was observed in adult mice
and rats following oral or subcutaneous administration of maleic
hydrazide. The significance of hepatomas obtained in new born mice
cannot be assessed because of the contamination of maleic anhydride
with hydrazine". A mammalian dominant lethal study was negative.
Reproduction studies indicate an absence of adverse effects of
the sodium salt of maleic hydrazide at 2% of the diet. At 5%, post
natal weight gain in pups is reduced. The diethanolamine salt of
maleic hydrazide, even at 0.1% in the diet, reduces fertility,
litter size, viability index and lactation index. Short term
studies indicate that effects on body weight, and mortality are due
to the diethanolamine moiety. It seems probable that the effects on
reproduction can also be attributed to the same cause although
there are no data available to support this hypothesis. Teratogenic
studies have not been conducted.
A no-effect level was not demonstrated for the diethanolamine
salt in respect to reproduction, short term or long term feeding
studies. Concern was expressed as to the possible use of the
diethanolamine salt of maleic hydrazide without complete
toxicological data being available on the diethanolamine salt. Data
are available on the sodium salt with regard to reproduction in
rat, short term studies in rat and dog and long term studies on
small numbers of rats. The no-effect level in these studies would
appear to be 2% in the diet.
No information was available on the occurrence of hydrazine as
a residue in crops or on the potential for its formation under use
conditions. It was noted that maleic hydrazide and its
ß-D-glucoside were the principal residues found on plants. Data on
the metabolic fate of the ß-D-glucoside in mammals are not
available. Since the carcinogenicity potential cannot be assessed
adequately and the long term rat feeding study is on small numbers
of animals, no toxicological evaluation has been attempted for the
sodium salt of maleic hydrazide. Data are totally inadequate for
toxicological evaluation of the diethanolamine salt.
TOXICOLOGICAL EVALUATION
No acceptable daily intake was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Maleic hydrazide is used as a sprout inhibitor on potatoes at
2-3 kg/ha with a pre-harvest interval (PHI) of 4-6 weeks and on
onions at 2-2.5 kg,/ha with a PHI of 2-4 weeks.
The compound is also used on potatoes, about 10 days before
planting, on young non-bearing Citrus trees, to induce dormancy and
on tobacco for sucker control when in full flower (3 kg a.i./ha).
It is further used on a limited scale as a growth inhibitor on
grass on road verges.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive residue data are available from supervised trials on
onions and potatoes and are summarized in Table 1. Some
supplementary results, together with the limited data available for
apples, carrots and tobacco, are discussed below.
Apples. Pre-harvest spraying of apples with 1000 and 2500 mg/kg
a.i. resulted in residues in the total apple of 1.5 and 3.4 mg/kg
respectively. A considerable part of the residue remained in the
peel: residue levels found in the peel were 4.1 and 10.0 mg/kg
respectively (Hoffman and Carson 1962).
Carrots. Samples taken from plots sprayed at different times
before harvest showed residues of 5.1 mg/kg after a spray
application shortly before harvest and much lower residues, 0.36
mg/kg, when sprayed three weeks earlier (Hoffman and Carson 1962).
Onions. In supervised trials in Poland carried out by Drygus et
al (1968), onions were treated 21 weeks before harvest with maleic
hydrazide at dosages of 12-2.3 kg/ha. The residues after storage
periods of 7-32 weeks ranged from 2.5 to 23 mg/kg. (Table 1.) The
highest residues were found after a storage period of 28 weeks
(i.e. 30 weeks after application) and at the highest application
rate.
Potatoes. Foliar application about 5 weeks before harvest with
sprays containing 0.1, 0.25, 0.5 and 0.75% a.i. resulted in residue
levels in the tuber of 3.1, 15.6, 37.9 and 92.6 mg/kg respectively.
The level in the peelings tended to be higher than in the remainder
of the potato. (Hoffman and Carson 1962). Of 144 samples taken in
the USA from plots treated with maleic hydrazide at 3 kg a.i.
mainly in the period 1950-1960 8% of the samples showed residues
exceeding 30 mg/kg and about 5% contained residues exceeding 40
mg/kg (Uniroyal 1976). Drygas et al (1968) treated potatoes about
8 weeks before normal harvest with maleic, hydrazide at dosage
rates of 1.4 - 2.7 kg/ha. After storage periods of 3 - 24 weeks
residues ranged between 4 and 30 mg/kg (Table 1). The highest
values were found at the highest application rate and after a
storage period of 24 weeks.
Tobacco. After applying maleic hydrazide on tobacco at a rate of
2.25 kg a.i./ha the tobacco leaves contained 37 mg/kg (fresh
weight), whereas the green sucker leaves contained 482 mg/kg.
(Hoffman et al., 1962).
FATE OF RESIDUES
In soil
Mobility. Helweg-Andersen (1971) showed that maleic hydrazide and
its degradation products are only moderately mobile in typical soils.
After application of 5 and 10.1 kg/ha all the maleic hydrazide was
found in the top 10 cm of the soil from 0 to 129 days (the latest
interval checked) under danish field conditions.
Uniroyal (1973-75), in an outdoor test on sandy loam 14C-MH
found only 2.5 - 3.1% of the applied 14C below a depth of 15 cm after
4 - 6´ months.
The diethanolamine salt of maleic hydrazide is rather mobile in
the soil. An aqueous solution of the salt put on top of a soil column
of 1 m was distributed through the whole column within 24 hours. The
initial amount of water was chosen in such way that no water flowed
from the column. Subsequent additional watering removed a considerable
amount of maleic hydrazide from the column (Levi and Crafts 1952).
Persistence. Uniroyal (1973-1975) monitored soil from plots treated
with maleic hydrazide under field conditions. A plot treated for four
consecutive years with 3.4 kg a.i./ha and sampled 10 months after the
last application showed no detectable residues. At another site, soil
treated at the same rate and sampled 4 months after treatment again
contained no detectable residues (lower limit of detection 0.5 mg/kg).
Since the analytical technique used in this case involved caustic
TABLE 1. Residues of maleic hydrazide resulting from supervised trials
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 2 2¨ 2.5 2 3/4 3 4-4.5 7 Ref.
months months months months months months months
onions U.S.A. 1951 1 1 wp 40% 1.7 1
1.5
1951 1 2 wp 40% 7.3 9.8 4.2 7 2.8 1
12.5 11.5 (2.7
11.7) 5.7
3.9
1 2.4 2.7 1
1952 1 2.0 wp 40% 2.5 1
1952 1 2.5 wp 40% 10.0 1
1952 1 3.2 wp 40% 3.0 2.2 1
2.3
Netherlands 1955 1 2.5 liquid 2 2
40% (1.2
4)
1 2.5 liquid 8.1 2
40% (n.d.
12.1)
1.5
(n.d.- 2
1.5
2
days
1964 1 1.8 liquid 7.4 3
30% (6.6
9.0)
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation Ref.
Onions Netherlands 1 2.5 liquid 13.0 9 14 35 44
30% (10.8- days days days days 3
14.2)
U.S.A. 1951- 1 2.3 2.7- 2.3- 3.1 2.5 4
2 11.7 14.5
9 16 23 30 34
weeks weeks weeks weeks weeks
Poland 1968 1 1.2 EC 2.5 6 3 8 4 5
1 1.7 EC 3 4 5 13 13 5
1 2.25 EC 6 8 6 14,23 13 5
Potatoes U.S.A. 1951 1 1.5 wp 40% 1.7 3.3, 3.4 1
4.2 2.0
1951 1 1.8 wp 40% 2.9 1
(1.1-4.
5)
Potatoes U.S.A. 1951 1 2 wp 40% 10.8 3.0, 1
9.8 4.8
1951 1 3 wp 40% 2.7 1
8.7
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 1.5-2 3 - 4 4 - 5 6 - 7 7 - 8 9 -12 Ref.
months months months months months months
1951 1 3 wp 40% 9.8 1.0 2.9 4.7 8.8 6.0 1
3.0 8.6 8.9 8.9 4.7
3.6 9.1 9.4
4.7 2.9
6.0 3.0
11.9
7.4
5.8
1.0
1955 1 2.8 wp 40% 11.3 16.1 10.2 1
14.7 10.8 10.4
4.7 11.1 21.9
3.7 24.2
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 1969 1 2 7.3 6.5 20 1
(4-10) (6-7) 1
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 13
(11-15)
1969 1 3 9 14 9 6 1
(9-24) (7-11)
12.5 1
(8-15) (0-2)
Whole
potato U.S.A. 1951 1 1.6. - 3 10-0- 5.5- 3.0- 10.2 4
16.1 24.2 8.6
32.5 16.3- 27.0
48.9 41.0 32.9 4
11 18 23 32
weeks weeks weeks weeks
Potatoes Poland 1968 1 1.4 EC 8 6 11 9 5
1 2.0 EC 5 4 14 15 5
1 2.7 EC 6 4 10 8,30 5
* With the exception of the data from Poland (Ref. 5), each entry in the Table refers to a separate trial.
References
1. Uniroyal 1973-1975
2. R.I.V. 1955
3. R.I.V. 1964
4. Naugatuck Chem. Comp. 1956.
5. Drygas et al. 1968.
treatment of the soil, it would measure both free and bound maleic
hydrazide owing to hydrolysis of the latter.
Data on the residue extractable with organic solvents show that
half of the applied material is lost in periods varying from less than
one to about six weeks in different soil types. Usually over 90% has
disappeared in about 2-10 weeks. When soil was analysed for
extractable residues more than 3 months after application only traces
of maleic hydrazide were detected, if any. The bound residues in the
soil are degraded less rapidly. Half of the applied maleic hydrazide
is lost over periods varying from one to 14 weeks. Helweg (1975b)
studied the effect of absorbtion on the rate of maleic hydrazide
degradation using activated carbon as a model absorbent. While
absorption caused an initial delay in 14C evolution from 3, 6 14C-MH,
after 4 months almost equal amounts of 14CO2 were evolved from
control soils and those containing activated carbon. In standard soil
types 1 and 2 as recommended for leaching experiments in the Federal
Republic of Germany (Characteristics: organic carbon 2.58 and 1.0%
hydrazide particles <20 µ 10.1 and 19.1% respectively) the initial
residues of 30.5 and 36.1 mg/kg decreased in 8 weeks to 0.76 mg/kg
(2.5%) and 0.44 mg/kg (1.2%) (BASF 1975). In sterile soil the residue
hardly decreased in 6 weeks; in non-sterile soil of the same type an
initial residue of 100 mg/kg decreased in 3 weeks to 5 mg/kg, mainly
as a result of microbial degradation.
Biodegradation. Helweg (1975a , 1975b) found CO2 to be the main
degradation product of maleic hydrazide. From a sandy loam soil
containing 20 mg/kg, kept under laboratory conditions, about 50% of
the 14C was liberated as CO2 within two weeks.
Uniroyal (1973-1975) treated two soil types with 3,6-14C at a
rate of 55 mg/kg (an excessive dosage compared with normal practice).
The 14C release reached 34% of the 14C applied in Connecticut sandy
loam and 57% in Mississippi silt loam after two weeks.
Kaufman and Kalayanova 1975 studied the degradation of MH-14C in
two soils under laboratory conditions, with the results shown in Table
2.
TABLE 2. Laboratory aerobic metabolism of maleic hydrazide.
Cumulative 14CO2 evolved in 23 days
% of applied 14C evolved
Soil rate MH-3,6-14C MH-4,5-14C
kg/ha
Sandy loam 0.56 67.8 43.5
5.6 57.0 40.5
Silty clay 0.56 47.8 17.7
5.6 48.0 18.3
Methanol extraction of the MH-14C treated soil after 29 days
removed only 1-3% of the applied 14C. In addition to maleic
hydrazide, maleimide was identified as a degradation product, showing
the likelihood of an early cleavage of the N-N bond in the degradation
process. No hydrazine formation could be detected in the extracts from
these soils, nor was hydrazine evolved when
p-dimethylaminobenzaldehyde-sulphuric acid solutions were substituted
for CO2 trapping solutions in the Warburg flask. Some of the 14C not
extracted from the soil by methanol could be removed by aqueous
alkali. The humin, and to a lesser extent the humic- and fulvic acid
fractions contained 14C. The authors suggest that the radioactivity
in these fractions could be present as sorbed unchanged maleic
hydrazide, and/or as degradation products incorporated into the
natural organic matter of the soil. There is some evidence that both
sorption and chemical reaction occur. Uniroyal (1973-1975) found that
aqueous base can release unchanged MH-14C from soil-bound residues
not extracted by milder procedures. Helweg, (1975a) found some
evidence for the incorporation of14C into an amino acid fraction of
the soil. Since the microbial degradation of maleic hydrazide produces
CO2 it is very likely that CO2 originating from the degradation of
maleic hydrazide will be incorporated into natural products. It is
evident that CO2 in the major metabolite in maleic hydrazide
degradation, and small amounts of maleimide and natural products have
been detected as intermediate steps in the metabolic pathway. However
the small amounts of 14C products extracted from soil make it
difficult to propose an overall metabolic pathway. On the basis of
some similarity with experiments in which they used Fenton's reagent
as a model of a free radical generating oxidation system, Kaufman and
Kalayanova (1975) tentatively suggest the degradation pathway of
maleic hydrazide in soil shown in Figure 1.
Stoessl 1964 has reported similar products (fumaric, maleic,
succinic, formic and nitric acids) from the photo oxidation of maleic
hydrazide in dilute aqueous solutions in the presence of oxygen. Other
authors e.g. Andreae (1955), Winder and Denneny (1959) and
Povolotskaya (1961) confirm the photo-oxidation reactions of maleic
hydrazide.
Frear (1975) showed that bound residues of 14C-MH in tobacco
root were degraded to 14CO2 and released. About 18% of the 14C-MH
and 3,6-14MH and 0.5% of the 4,5-14C was released as 14CO2 from
tobacco root tissue during 43 days incubation in soil owing to
microbial activity.
In subsequent work it was shown that the bound residue remaining
in tobacco roots after methanol extraction, was partially released by
treatment with aqueous ammonia at 80°C for four days. The 14C
extracted in this way was shown to be unchanged maleic hydrazide.
Noodén (1970) found that the bound maleic hydrazide residue in other
plants consists mainly of unchanged maleic hydrazide.
In plants
Uptake from the soil. In Canada, sandy loam soil was treated with
0.2, 0.5, 1.0 and 5.0 mg/kg maleic hydrazide. Tobacco seedlings were
planted in pots containing the treated soil and grown under glass.
After 8 weeks 10% of the original amount added to the soil remained.
No maleic hydrazide could be detected in the green leaves of the
tobacco plant except in the plants grown on soil treated with the
highest dosage of 5 mg/k The average residue in the leaves of these
was 0.9 mg/kg (Hoffman et al, 1962).
Haeberer et al (1974) showed that even when tobacco was planted
in soil immediately after the application of maleic hydrazide at an
exaggerated rate, no residues were found in the tobacco at harvest.
From these experiments it may be concluded that maleic hydrazide will
not be transferred to next years crop.
Fate in the plant. Frear and Swanson (1975) studied the uptake and
fate of 14C-MH in two flue-cured and two Burley tobacco varieties
grown under glasshouse conditions. It was shown that the
foliar-absorbed maleic hydrazide moves rapidly, both acropetally and
basipetally to actively growing tissues in the tobacco plant including
the roots. Most of the foliar-absorbed maleic hydrazide is transported
to the roots and excreted into the external medium, but a significant
proportion remains in the roots and other tissues as a
methanol-insoluble residue, which was shown to consist largely of
unchanged metabolite of maleic hydrazide. In tobacco the methanol-
soluble metabolite of maleic hydrazide is the ß-D-glucoside of the
phenolic tautomer, 6 hydroxy-3-(2H)-pyridazone.
Towers et al (1958) also found this ß-D-glucoside in tobacco
leaves. They reported that 15% of maleic hydrazide in tobacco leaves.
They reported that 15% of maleic hydrazide was transformed into ito
its ß-D-glucoside when applied to leaf segments. This metabolite was
also seen in apple and willow.
Callaghan (1961) reported the incorporation of maleic hydrazide
into heterochromatin of root-tip colls of Allium cernuum, Vicia
faba and Tradescantia palludosa.
Biswas et al (1967) isolated two unknown transformation products
from tea plants grown in nutrient solution containing 14C-MH. They
speculate on various modes of ring opening, but did not provide
experimental evidence of it.
Noodén (1970) concluded from studies on the uptake of 14C-MH by
roots that maleic hydrazide is bound to cell wall fragments as a
stable complex which is insoluble in 80% ethanol. The bound maleic
hydrazide could be released by heating the tissue with aminoethanol.
Chromatography of the 14C-material released in this way showed that
there was no indication of degradation and the maleic hydrazide was
bound to the cell-walls in an unchanged form.
Other information on identity and properties
Composition of the technical product
The technical material contains 99% of maleic hydrazide;
impurities are inorganic salts (e.g. Na2SO4 possibly with minor
amounts of maleic or fumaric acid.
Physical and chemical properties of maleic hydrazide
physical form white crystalline powder
molecular weight 112.1
specific gravity at 25°C 1.60
melting point 292°C(min)
odour faint
volatility non volatile
solubility (approximate) g/100 g solvent at 25°C
distilled water 0.6
alcohol 0.1
acetone 0.1
dimethyl formamide 2.4
xylene less than 0.1
Ph of 0.5% aqueous solution at 25°C: 4.
Maleic hydrazide behaves as a weak monobasic acid. The
alkanolamine and alkali metal salts are moderately soluble in
water.
Formulations used: liquid 30% and 40% a.i.; wettable powder 40%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Maleic hydrazide does not appear to be extensively metabolized
by mammals. In the rabbit, 43-62% of a single oral dose was
excreted unchanged within 48 hrs. Isolation and characterization of
the excreted maleic hydrazide following oral doses of 2 g showed
the excreted product to be the benzylamine salt. No other
metabolites were identified. (Barnes et al, 1957). Incubation of
phenobarbital-induced rat liver microsomes with maleic hydrazide
did not result in any degradation of the compound (Nelson and
Kearney, 1975).
Using maleic hydrazide - 14C labelled material, female rats
were dosed with 0.27, 0.68, or 2.72 mg per rat (equivalent to 10,
25 or 100 µc/rat) by stomach tube. At the low dose level, 65.4% of
the radioactivity was excreted in the urine in 12 hrs and 77.3%
within 6 days.
A further 12.4% of the administered radioactivity was detected
in the faeces over the same period. Rats dosed with 2.5 µc showed
0.12-0.18% expired radioactivity in CO2 over the 72 hour period
after administration. Tissue levels of radioactivity were
statistically insignificant except in the carcass, where <0.001%
of the administered radioactivity was detected. Using paper
chromatography with two different solvent systems, urine from rats
intubated with 100 µc resulted in the identification of unchanged
maleic hydrazide (92-94%) and a maleic hydrazide conjugate (6-8%).
(Mays et al, 1968).
Sixteen rats were given 4g/kg by stomach tube. Urine and
faeces were collected for time intervals 0 to 2, 2 to 4, 4 to 8,
and 8 to 16 days. Two rats/sex were sacrificed after 2, 4, 8, or 16
days post-dosing. In male rats, residues in urine (30,400 mg/kg)
were approximately double those in faeces (17,400 mg/kg) over the
0-2 day period, whereas in females urine residues (1,330 mg/kg)
were much less than in faeces (12,700 mg/kg). In both sexes,
residue levels decreased rapidly, but were still detectable in 8-16
day samples of both urine and faeces. Excretion in females was
slightly slower than in males, however residues were undetectable,
except for traces in the males sacrificed on day 8. (Food Research
Laboratories Inc. 1955).
TOXICOLOGICAL STUDIES
Special Studies on reproduction
Seven groups of approximately 10 male and 10 female rats fed
0, 0.5, 1.0, 2.0 or 5.0 maleic hydrazide as the sodium salt, or 0
or 0.1% maleic hydrazide as the diethanolamine salt were repeatedly
bred using a cycle of 2 weeks pairing, 3 weeks gestation, 3 weeks
lactation and one or two weeks rest prior to remating, for as long
as possible, commencing at 12 weeks on test. The second litters
(F1b) were used to produce the F2 generation which were mated
twice. The F2b generation provided parents for the F3a and b
offspring. Pooled data from the 8 matings of the Fo generation do
not indicate any effects of the sodium salt of maleic hydrazide on
fertility, litter size, gestation index, viability index, or
lactation index. Pup weight at weaning was slightly reduced at the
5.0% level. The diethanolamine salt at the 0.1% level resulted in
reduced fertility, reduced litter size, reduced viability index,
and reduced lactation index. The diethanolamine salt group was
dropped from the study, no attempt being made to produce the F2
generation. Mean data for all generations of rats receiving the
sodium salt do not do not indicate any effects on reproduction,
with respect to fertility, gestation index, viability index, or
lactation index. At the 5.0% level, litter size was reduced in the
F3 generation and weanling weight was reduced in all generations.
(Food Research Laboratories, 1955).
Special studies on mutagenicity
Injection of 0.7% sodium chloride Ringer's solution containing
0.4% maleic hydrazide abdominally in one to two-day old
Drosophila melanogaster males did not result in an increase in
mutation rate. On the other hand, D. melanogaster males fed on
media containing 0.4% maleic hydrazide showed an increased
incidence of lethal mutations in the first brood, although in
subsequent broods, the effect was not apparent (Nasrat, 1965).
In a mouse dominant lethal study, 500 mg maleic hydrazide/kg
did not affect the calculated mutation index. (Epstein & Shafner,
1968).
Andersen et al (1972) tested maleic hydrazide and other
chemicals on eight mutant strains of Salmonella typhimurium on
T4 bacteriophage, and on bacteriophage AP72. In none of these ten
systems was there any ~evidence of mutagenic activity. Maleic
hydrazide was tested by McCann et al (1975, 1976) on several tester
strains of Salmonella. Tests covered a wide dose range, both with
and without liver microsome activation. There was no evidence of
mutagenic activity in this extensive series of tests.
Special studies on carcinogenicity
Swiss mice (ICR/Ha) were injected sub-cutaneously with aqueous
solutions, or tricaprylin suspensions of maleic hydrazide as free
acid (0.4% hydrazine impurity), or with solvent alone in volumes of
0.1, 0.1, 0.2 and 0.2 ml on post-natal days 1, 7, 14 and 21. Total
doses were 3 mg for aqueous solution, and 55 mg for tricaprylin
suspension. (A further group comprising 11 mice all died following
10 mg maleic hydrazide injected on post-natal day 1). Preweaning
mortality was 14% in controls, 5% in mice receiving 3 mg and 53% in
mice receiving 55 mg. Post weaning mortality in males in all groups
was high being 38, 57, and 52% at 0,3 & 55 mg respectively by 49
weeks of age. Decreased weight gain (ca 5%) was noted in the high
dose group. Hepatoma incidence in males at 49 Weeks was 8% in
controls, 18% in the 3 mg group and 73% in the 55 mg group. No
metastases were noted. (Epstein et al, 1967).
Maleic hydrazide was administered by stomach tube in daily
doses of 1000 mg/kg weight to 36 mice of each sex for 3 weeks,
beginning when the animals were 7 days old. Then, 3000 ppm were
mixed directly with the diet, which was fed for approximately 18
months. No significant increase in the incidence of tumors was
observed in comparison with untreated controls (Innes et al.,
1969).
Rats were injected subcutaneously with 1 ml of the
diethanolamine salt (i.e. 5 mg) weekly for 14 months. Of 52 rats so
treated in two experiments, 3 developed sarcomas. No sarcomas
occurred in saline control rats. (Barnes et al, 1957).
Groups of rats and mice were either injected with the
monosodium salt, subcutaneously, once weekly at a dose level of 500
mg,/kg or received 1% maleic hydrazide added to the diet for 100
weeks. Maleic hydrazide did not affect growth or general health in
either species, on either dose regime. One rat receiving maleic
hydrazide by injection (out of a total of 29) developed a sarcoma.
No sarcomas were observed in control rats. Total tumor incidence
was increased in female test groups of both species, fed maleic
hydrazide, the effect being more obvious in the mouse (Barnes et al
1957).
Groups of 25 male and 25 female rats were injected twice
weekly for 65 weeks with arachis oil, water, solvents plus 2 mg
maleic hydrazide or solvents plus 2 mg diethanolamine salt of
maleic hydrazide. A further 39-week observation preceded autopsy.
subcutaneous tumor incidence was increased (4) in the group
receiving arachis oil plus maleic hydrazide when compared with the
arachis oil control group (1). Incidences of tumors in all other
groups were comparable. The increased subcutaneous tumor rate was
attributed to impaired connective tissue repair mechanisms rather
than chemical carcinogenesis induction. (Hunter et al, 1973).
Three groups of 24 male rats were fed basal diet, basal diet
plus 2% maleic hydrazide as the sodium salt, or basal diet plus
0.06% p-dimethylaminoazobenzene for up to 26 weeks. No significant
changes with regard to body weight, desoxyribose nucleic acid
content per liver cell nucleus, average liver cell size, liver
weight, number of cells/liver or DNA/liver were observed with
maleic hydrazide, although the p-dimethylaminoazobenzene affected
all these parameters. Pathological examination of animals fed
maleic hydrazide did not reveal neoplasms, although these occurred
in all rats fed the p-dimethylaminoazobene for more than 10 weeks.
(Mannell & Grice, 1957).
Special studies on mammalian cells in vitro
The effect on mammalian cells was examined by studying the
development of mitosis in fragments of mouse ear epidermis. In
concentrations from 0.0001-0.001 M, maleic hydrazide had no effects
on mitosis or cell division. Maleic hydrazide was also tested upon
skin from the guinea pig's ear, grown in tissue culture. Up to a
concentration of 0.01 M no gross or microscopical effects upon the
explants were noted. The same concentration of maleic hydrazide was
also without effect upon the respiration of skin cells, studied 2
and 22 hr after adding the maleic hydrazide (Barnes et al., 1957).
In vitro studies with mouse thymus cells in culture showed
that maleic hydrazide in concentrations of 0.001 M or more
inhibited growth. Mitotic inhibition occurred at and above 0.0001
M. The ratio of dry mass to DNA content was increased, which was
taken as an indication for inhibition of DNA, but not of protein
synthesis (McCarthy and Epstein, 1968). Mitotic inhibition was also
shown in cultures of human lymphocytes grown in the presence of
0.001-0.01 M. Inhibition was more marked in 72 hr-old cultures than
in fresh ones, probably because of the more active mitotic state in
the former (Timson, 1968).
Special studies on dermal irritation
The diethanolamine salt of maleic hydrazide was applied as a
20% aqueous solution to approximately 10 sq.cm. of abraded, and 10
sq. cm. of intact skin on the backs of 6 rabbits. Repeated
applications were made during a 6-hour period on five consecutive
days on two occasions, separated by a ten-day rest period. No signs
of irritation were noted during the study. (Food Research
Laboratories, 1955).
Special studies on dermal sensitization
Ten guinea pigs were injected intradermally with one dose of
0.5 ml, and 9 subsequent doses of 0.1 ml of a 0.1% aqueous solution
of the diethanolamine salt of maleic hydrazide, dosing being on
alternate days. Two weeks after the last dose, a challenge dose of
0.5 ml was administered intradermally at a different site. No
evidence of sensitization was observed. (Food Research
Laboratories, 1955).
Special studies on eye irritation
Six rabbits received two drops of a 5% solution of the
diethanolamine salt of maleic hydrazide in 5% saline in the right
eye. No signs of irritation were observed in the 14 day
post-treatment observation period. (Food Research Laboratories,
1955).
Special studies on respiratory effects
Deeply anaesthetized rats (ethyl ether) were administered the
diethanolamine salt of maleic hydrazide at various solution
concentrations by instilling small droplets in alternate nostrils
at the moment of inhalation. Doses of 50 to 400 mg diethanolamine
salt were administered (i.e. 15-120 mg maleic hydrazide). At the
400 mg dose level, 2/10 rats died after 10 days. Gross pathological
examination showed haemorrhagic lungs, and in one case, a yellowish
exudate and thoracic adhesions, laboured respiration (7/10) and at
2 weeks, rats (5/7) were also observed at the 400 mg level. (Food
Research Laboratories, 1955).
Special studies on potentiation
The acute toxicity of maleic hydrazide diethanolamine salt in
combination with other pesticides was determined. Pretreatment with
0.1 LD50 potentiated the toxicity of dieldrin and DDT in both
sexes, but decreased the toxicity of diazinon. On the other hand
pretreatment with DDT and dieldrin reduced the toxicity of maleic
hydrazide diethanolamine salt by a factor of two. Diazinon
pretreatment had a different effect, decrease of toxicity in
females, increase in males (Luckens and Wattimena, 1968).
Acute studies
Species Route LD50 References
mg/kg b.w*
Diethanolamine salt Food Research
Rat (fasted) oral 1180 Labs. 1955
Sodium salt
Rat (fasted) oral 5800 ibid
* expressed in terms of maleic hydrazide moiety.
Short term studies
Rat
Groups of 12 male and 12 female rats were fed 0, 0.5, 1.0, 2.0
or 5.0% maleic hydrazide, as the sodium salt, for 12 weeks.
Detailed data are not available. Mean body weight data indicate
that in males, a slight reduction occurs in all groups on maleic
hydrazide and a similar reduction occurs in females at 5.0% dietary
levels. The reductions are stated to be statistically
non-significant. Food utilization, hemoglobin, erythrocyte count,
total and differential leucocyte counts, were comparable in all
groups. Blood sugar was reduced at 5.0%, and non-protein nitrogen
was increased at the same level. Methaemoglobin levels were (if
present) below the limit of detection (0.2g/100 ml) and urinalysis
for sugar, albumin, and microscopic inclusions was unremarkable.
Gross pathology (on 2 males and 2 females at 12 weeks) was normal.
No histological abnormalities were noted. (Food Research
Laboratories, 1955).
A group of 12 male and 12 female rats was fed 1.0% maleic
hydrazide as the diethanolamine salt. Additional, non-contemporary
groups of 12 male and 12 female rats were fed 0.1% or 0% maleic
hydrazide as the diethanolamine salt and a final group of 10 males
and 10 females was fed 1.0% diethanolamine. All rats were on test
for 12 weeks. Body weight was significantly reduced in all test
groups. Mortality was increased markedly in groups receiving 1.0%
diethanolamine, and 1.0% diethanolamine salt of maleic hydrazide.
No further data are available for the group receiving 1.0%
diethanolamine. Food efficiency is stated to be comparable in all
groups except that receiving 1.0% diethanolamine salt of maleic,
hydrazide which is markedly reduced. Haemoglobin and erythrocyte
count are markedly reduced and an increased polymorph/lymphocyte
ratio are noted in the group receiving 1.0% diethanolamine salt of
maleic hydrazide. Mean data on the 0.1% diethanolamine maleic
hydrazide indicate a tendency to reduced haemoglobin levels and
erythrocyte counts, the statistical significance being
non-assessible on the basis of available data. At the 1.0%
diethanolamine maleic hydrazide level, death was preceded by loss
of coordination and muscular control, accompanied by starvation.
Pathological examination showed hyperaemic or haemorrhagic lungs,
and evidence of anaemia. Brain histopathology showed brain oedema.
No microscopic lesions were observed in liver, kidney or lung of
four representative rats of the group. (Food Research Laboratories,
1955).
Dog
Three dogs were given 1 g/kg of the sodium salt of maleic
hydrazide five times weekly for five weeks by gavage. At 5 day
intervals, haemoglobin, erythrocyte count and total and
differential leucocyte counts were determined. Methaemoglobin,
blood sugar and non-protein nitrogen were determined at sacrifice.
Liver, kidney, spleen and bone marrow were examined
histopathologically. No data are presented, but effects on the
parameters listed above are stated to be negligible although
transient eosinophilia, anemia, and leucocytosis were noted
(Food Research Labs, 1955).
Five groups of three mongrel dogs aged between four months and
2 years were fed 0 (1 male, 2 female), 0.5 (2 male, 1 female), 1.0
(1 male, 2 female), 2.0 (2 male, 1 female) % maleic hydrazide as
the sodium salt, or 1.0 (2 male, 1 female)% maleic hydrazide as the
diethanolamine salt in the diet for one year. Body weight and
mortality were unaffected by the sodium salt. The diethanolamine
salt caused loss of body weight, and 2/3 deaths by day 30 on test.
This group was abandoned at day 37 on test. Food intake was
unaffected in groups receiving the sodium salt. Haemoglobin,
erythrocyte count, total and differential leucocyte counts were
comparable in all groups. Blood sugar and non-protein nitrogen did
not show any consistent effects. Gross pathology was comparable in
all dogs. Histopathology on the bone marrow, liver kidney, spleen
and gastrointestinal tract was considered to be within normal
limits. Brains of dogs receiving the diethanolamine salt were
moderately oedematous, and the spinal cord showed early neuronal
degeneration and swelling of myelin sheath. (Food Research Labs.
1955).
Long-term studies
Rat
Five groups of approximately 10 male and 10 female rats were
fed 0, 0.5, 1.0, 2.0, or 5.0% maleic hydrazide as the sodium salt,
for 2 years. A further two noncontemporary groups were fed 0 or
0.1% maleic hydrazide as the diethanolamine salt, also for 2 years.
After 12 weeks on test, rats were bred one to one, within groups,
following a 2 week mating, 3 week gestation, 3 week lactation, 1
week rest cycle throughout the study. (The report states 1 week
rest, or 2 week rest after lactation, in different sections). Body
weight of males in all test groups was slightly reduced up to 1
year, but exceeded controls at 2 years. In females, body weight was
reduced at 5.0% Na salt at 12 weeks only. Mortality was generally
comparable between groups, except for the 0.1% diethanolamine salt
group, where life span was reduced, mortality being increased
between 52 and 96 weeks on test. Haemoglobin and erythrocyte counts
were reduced in the group receiving 0.1% diethanolamine salt, at
all reported time intervals (52,78 and 104 wks). In all other
groups, values were comparable. Total and differential leucocyte
counts were comparable in all groups. Blood glucose and non protein
nitrogen were comparable in all groups receiving the sodium salt at
12 and 24 months. Non protein nitrogen was slightly elevated in the
only measurement taken (24 months) on the sole male and sole female
survivor of the 0.1% diethanolamine salt group. Methaemoglobin was
not detected in any group. Urinalysis was stated to be normal in
all groups. No consistent changes occurred in liver, kidney or
spleen organ/body weight ratios. Tumor incidence was comparable in
all groups. Pathological changes are stated to be attributable to
infections, parasites, or age. (Food Research Laboratories, 1955).
COMMENTS
Maleic hydrazide was evaluated by the IARC in 1974 and it was
concluded that "no carcinogenic effect was observed in adult mice
and rats following oral or subcutaneous administration of maleic
hydrazide. The significance of hepatomas obtained in new born mice
cannot be assessed because of the contamination of maleic anhydride
with hydrazine". A mammalian dominant lethal study was negative.
Reproduction studies indicate an absence of adverse effects of
the sodium salt of maleic hydrazide at 2% of the diet. At 5%, post
natal weight gain in pups is reduced. The diethanolamine salt of
maleic hydrazide, even at 0.1% in the diet, reduces fertility,
litter size, viability index and lactation index. Short term
studies indicate that effects on body weight, and mortality are due
to the diethanolamine moiety. It seems probable that the effects on
reproduction can also be attributed to the same cause although
there are no data available to support this hypothesis. Teratogenic
studies have not been conducted.
A no-effect level was not demonstrated for the diethanolamine
salt in respect to reproduction, short term or long term feeding
studies. Concern was expressed as to the possible use of the
diethanolamine salt of maleic hydrazide without complete
toxicological data being available on the diethanolamine salt. Data
are available on the sodium salt with regard to reproduction in
rat, short term studies in rat and dog and long term studies on
small numbers of rats. The no-effect level in these studies would
appear to be 2% in the diet.
No information was available on the occurrence of hydrazine as
a residue in crops or on the potential for its formation under use
conditions. It was noted that maleic hydrazide and its
ß-D-glucoside were the principal residues found on plants. Data on
the metabolic fate of the ß-D-glucoside in mammals are not
available. Since the carcinogenicity potential cannot be assessed
adequately and the long term rat feeding study is on small numbers
of animals, no toxicological evaluation has been attempted for the
sodium salt of maleic hydrazide. Data are totally inadequate for
toxicological evaluation of the diethanolamine salt.
TOXICOLOGICAL EVALUATION
No acceptable daily intake was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Maleic hydrazide is used as a sprout inhibitor on potatoes at
2-3 kg/ha with a pre-harvest interval (PHI) of 4-6 weeks and on
onions at 2-2.5 kg,/ha with a PHI of 2-4 weeks.
The compound is also used on potatoes, about 10 days before
planting, on young non-bearing Citrus trees, to induce dormancy and
on tobacco for sucker control when in full flower (3 kg a.i./ha).
It is further used on a limited scale as a growth inhibitor on
grass on road verges.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive residue data are available from supervised trials on
onions and potatoes and are summarized in Table 1. Some
supplementary results, together with the limited data available for
apples, carrots and tobacco, are discussed below.
Apples. Pre-harvest spraying of apples with 1000 and 2500 mg/kg
a.i. resulted in residues in the total apple of 1.5 and 3.4 mg/kg
respectively. A considerable part of the residue remained in the
peel: residue levels found in the peel were 4.1 and 10.0 mg/kg
respectively (Hoffman and Carson 1962).
Carrots. Samples taken from plots sprayed at different times
before harvest showed residues of 5.1 mg/kg after a spray
application shortly before harvest and much lower residues, 0.36
mg/kg, when sprayed three weeks earlier (Hoffman and Carson 1962).
Onions. In supervised trials in Poland carried out by Drygus et
al (1968), onions were treated 21 weeks before harvest with maleic
hydrazide at dosages of 12-2.3 kg/ha. The residues after storage
periods of 7-32 weeks ranged from 2.5 to 23 mg/kg. (Table 1.) The
highest residues were found after a storage period of 28 weeks
(i.e. 30 weeks after application) and at the highest application
rate.
Potatoes. Foliar application about 5 weeks before harvest with
sprays containing 0.1, 0.25, 0.5 and 0.75% a.i. resulted in residue
levels in the tuber of 3.1, 15.6, 37.9 and 92.6 mg/kg respectively.
The level in the peelings tended to be higher than in the remainder
of the potato. (Hoffman and Carson 1962). Of 144 samples taken in
the USA from plots treated with maleic hydrazide at 3 kg a.i.
mainly in the period 1950-1960 8% of the samples showed residues
exceeding 30 mg/kg and about 5% contained residues exceeding 40
mg/kg (Uniroyal 1976). Drygas et al (1968) treated potatoes about
8 weeks before normal harvest with maleic, hydrazide at dosage
rates of 1.4 - 2.7 kg/ha. After storage periods of 3 - 24 weeks
residues ranged between 4 and 30 mg/kg (Table 1). The highest
values were found at the highest application rate and after a
storage period of 24 weeks.
Tobacco. After applying maleic hydrazide on tobacco at a rate of
2.25 kg a.i./ha the tobacco leaves contained 37 mg/kg (fresh
weight), whereas the green sucker leaves contained 482 mg/kg.
(Hoffman et al., 1962).
FATE OF RESIDUES
In soil
Mobility. Helweg-Andersen (1971) showed that maleic hydrazide and
its degradation products are only moderately mobile in typical soils.
After application of 5 and 10.1 kg/ha all the maleic hydrazide was
found in the top 10 cm of the soil from 0 to 129 days (the latest
interval checked) under danish field conditions.
Uniroyal (1973-75), in an outdoor test on sandy loam 14C-MH
found only 2.5 - 3.1% of the applied 14C below a depth of 15 cm after
4 - 6´ months.
The diethanolamine salt of maleic hydrazide is rather mobile in
the soil. An aqueous solution of the salt put on top of a soil column
of 1 m was distributed through the whole column within 24 hours. The
initial amount of water was chosen in such way that no water flowed
from the column. Subsequent additional watering removed a considerable
amount of maleic hydrazide from the column (Levi and Crafts 1952).
Persistence. Uniroyal (1973-1975) monitored soil from plots treated
with maleic hydrazide under field conditions. A plot treated for four
consecutive years with 3.4 kg a.i./ha and sampled 10 months after the
last application showed no detectable residues. At another site, soil
treated at the same rate and sampled 4 months after treatment again
contained no detectable residues (lower limit of detection 0.5 mg/kg).
Since the analytical technique used in this case involved caustic
TABLE 1. Residues of maleic hydrazide resulting from supervised trials
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 2 2¨ 2.5 2 3/4 3 4-4.5 7 Ref.
months months months months months months months
onions U.S.A. 1951 1 1 wp 40% 1.7 1
1.5
1951 1 2 wp 40% 7.3 9.8 4.2 7 2.8 1
12.5 11.5 (2.7
11.7) 5.7
3.9
1 2.4 2.7 1
1952 1 2.0 wp 40% 2.5 1
1952 1 2.5 wp 40% 10.0 1
1952 1 3.2 wp 40% 3.0 2.2 1
2.3
Netherlands 1955 1 2.5 liquid 2 2
40% (1.2
4)
1 2.5 liquid 8.1 2
40% (n.d.
12.1)
1.5
(n.d.- 2
1.5
2
days
1964 1 1.8 liquid 7.4 3
30% (6.6
9.0)
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation Ref.
Onions Netherlands 1 2.5 liquid 13.0 9 14 35 44
30% (10.8- days days days days 3
14.2)
U.S.A. 1951- 1 2.3 2.7- 2.3- 3.1 2.5 4
2 11.7 14.5
9 16 23 30 34
weeks weeks weeks weeks weeks
Poland 1968 1 1.2 EC 2.5 6 3 8 4 5
1 1.7 EC 3 4 5 13 13 5
1 2.25 EC 6 8 6 14,23 13 5
Potatoes U.S.A. 1951 1 1.5 wp 40% 1.7 3.3, 3.4 1
4.2 2.0
1951 1 1.8 wp 40% 2.9 1
(1.1-4.
5)
Potatoes U.S.A. 1951 1 2 wp 40% 10.8 3.0, 1
9.8 4.8
1951 1 3 wp 40% 2.7 1
8.7
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 1.5-2 3 - 4 4 - 5 6 - 7 7 - 8 9 -12 Ref.
months months months months months months
1951 1 3 wp 40% 9.8 1.0 2.9 4.7 8.8 6.0 1
3.0 8.6 8.9 8.9 4.7
3.6 9.1 9.4
4.7 2.9
6.0 3.0
11.9
7.4
5.8
1.0
1955 1 2.8 wp 40% 11.3 16.1 10.2 1
14.7 10.8 10.4
4.7 11.1 21.9
3.7 24.2
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 1969 1 2 7.3 6.5 20 1
(4-10) (6-7) 1
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 13
(11-15)
1969 1 3 9 14 9 6 1
(9-24) (7-11)
12.5 1
(8-15) (0-2)
Whole
potato U.S.A. 1951 1 1.6. - 3 10-0- 5.5- 3.0- 10.2 4
16.1 24.2 8.6
32.5 16.3- 27.0
48.9 41.0 32.9 4
11 18 23 32
weeks weeks weeks weeks
Potatoes Poland 1968 1 1.4 EC 8 6 11 9 5
1 2.0 EC 5 4 14 15 5
1 2.7 EC 6 4 10 8,30 5
* With the exception of the data from Poland (Ref. 5), each entry in the Table refers to a separate trial.
References
1. Uniroyal 1973-1975
2. R.I.V. 1955
3. R.I.V. 1964
4. Naugatuck Chem. Comp. 1956.
5. Drygas et al. 1968.
treatment of the soil, it would measure both free and bound maleic
hydrazide owing to hydrolysis of the latter.
Data on the residue extractable with organic solvents show that
half of the applied material is lost in periods varying from less than
one to about six weeks in different soil types. Usually over 90% has
disappeared in about 2-10 weeks. When soil was analysed for
extractable residues more than 3 months after application only traces
of maleic hydrazide were detected, if any. The bound residues in the
soil are degraded less rapidly. Half of the applied maleic hydrazide
is lost over periods varying from one to 14 weeks. Helweg (1975b)
studied the effect of absorbtion on the rate of maleic hydrazide
degradation using activated carbon as a model absorbent. While
absorption caused an initial delay in 14C evolution from 3, 6 14C-MH,
after 4 months almost equal amounts of 14CO2 were evolved from
control soils and those containing activated carbon. In standard soil
types 1 and 2 as recommended for leaching experiments in the Federal
Republic of Germany (Characteristics: organic carbon 2.58 and 1.0%
hydrazide particles <20 µ 10.1 and 19.1% respectively) the initial
residues of 30.5 and 36.1 mg/kg decreased in 8 weeks to 0.76 mg/kg
(2.5%) and 0.44 mg/kg (1.2%) (BASF 1975). In sterile soil the residue
hardly decreased in 6 weeks; in non-sterile soil of the same type an
initial residue of 100 mg/kg decreased in 3 weeks to 5 mg/kg, mainly
as a result of microbial degradation.
Biodegradation. Helweg (1975a , 1975b) found CO2 to be the main
degradation product of maleic hydrazide. From a sandy loam soil
containing 20 mg/kg, kept under laboratory conditions, about 50% of
the 14C was liberated as CO2 within two weeks.
Uniroyal (1973-1975) treated two soil types with 3,6-14C at a
rate of 55 mg/kg (an excessive dosage compared with normal practice).
The 14C release reached 34% of the 14C applied in Connecticut sandy
loam and 57% in Mississippi silt loam after two weeks.
Kaufman and Kalayanova 1975 studied the degradation of MH-14C in
two soils under laboratory conditions, with the results shown in Table
2.
TABLE 2. Laboratory aerobic metabolism of maleic hydrazide.
Cumulative 14CO2 evolved in 23 days
% of applied 14C evolved
Soil rate MH-3,6-14C MH-4,5-14C
kg/ha
Sandy loam 0.56 67.8 43.5
5.6 57.0 40.5
Silty clay 0.56 47.8 17.7
5.6 48.0 18.3
Methanol extraction of the MH-14C treated soil after 29 days
removed only 1-3% of the applied 14C. In addition to maleic
hydrazide, maleimide was identified as a degradation product, showing
the likelihood of an early cleavage of the N-N bond in the degradation
process. No hydrazine formation could be detected in the extracts from
these soils, nor was hydrazine evolved when
p-dimethylaminobenzaldehyde-sulphuric acid solutions were substituted
for CO2 trapping solutions in the Warburg flask. Some of the 14C not
extracted from the soil by methanol could be removed by aqueous
alkali. The humin, and to a lesser extent the humic- and fulvic acid
fractions contained 14C. The authors suggest that the radioactivity
in these fractions could be present as sorbed unchanged maleic
hydrazide, and/or as degradation products incorporated into the
natural organic matter of the soil. There is some evidence that both
sorption and chemical reaction occur. Uniroyal (1973-1975) found that
aqueous base can release unchanged MH-14C from soil-bound residues
not extracted by milder procedures. Helweg, (1975a) found some
evidence for the incorporation of14C into an amino acid fraction of
the soil. Since the microbial degradation of maleic hydrazide produces
CO2 it is very likely that CO2 originating from the degradation of
maleic hydrazide will be incorporated into natural products. It is
evident that CO2 in the major metabolite in maleic hydrazide
degradation, and small amounts of maleimide and natural products have
been detected as intermediate steps in the metabolic pathway. However
the small amounts of 14C products extracted from soil make it
difficult to propose an overall metabolic pathway. On the basis of
some similarity with experiments in which they used Fenton's reagent
as a model of a free radical generating oxidation system, Kaufman and
Kalayanova (1975) tentatively suggest the degradation pathway of
maleic hydrazide in soil shown in Figure 1.
Stoessl 1964 has reported similar products (fumaric, maleic,
succinic, formic and nitric acids) from the photo oxidation of maleic
hydrazide in dilute aqueous solutions in the presence of oxygen. Other
authors e.g. Andreae (1955), Winder and Denneny (1959) and
Povolotskaya (1961) confirm the photo-oxidation reactions of maleic
hydrazide.
Frear (1975) showed that bound residues of 14C-MH in tobacco
root were degraded to 14CO2 and released. About 18% of the 14C-MH
and 3,6-14MH and 0.5% of the 4,5-14C was released as 14CO2 from
tobacco root tissue during 43 days incubation in soil owing to
microbial activity.
In subsequent work it was shown that the bound residue remaining
in tobacco roots after methanol extraction, was partially released by
treatment with aqueous ammonia at 80°C for four days. The 14C
extracted in this way was shown to be unchanged maleic hydrazide.
Noodén (1970) found that the bound maleic hydrazide residue in other
plants consists mainly of unchanged maleic hydrazide.
In plants
Uptake from the soil. In Canada, sandy loam soil was treated with
0.2, 0.5, 1.0 and 5.0 mg/kg maleic hydrazide. Tobacco seedlings were
planted in pots containing the treated soil and grown under glass.
After 8 weeks 10% of the original amount added to the soil remained.
No maleic hydrazide could be detected in the green leaves of the
tobacco plant except in the plants grown on soil treated with the
highest dosage of 5 mg/k The average residue in the leaves of these
was 0.9 mg/kg (Hoffman et al, 1962).
Haeberer et al (1974) showed that even when tobacco was planted
in soil immediately after the application of maleic hydrazide at an
exaggerated rate, no residues were found in the tobacco at harvest.
From these experiments it may be concluded that maleic hydrazide will
not be transferred to next years crop.
Fate in the plant. Frear and Swanson (1975) studied the uptake and
fate of 14C-MH in two flue-cured and two Burley tobacco varieties
grown under glasshouse conditions. It was shown that the
foliar-absorbed maleic hydrazide moves rapidly, both acropetally and
basipetally to actively growing tissues in the tobacco plant including
the roots. Most of the foliar-absorbed maleic hydrazide is transported
to the roots and excreted into the external medium, but a significant
proportion remains in the roots and other tissues as a
methanol-insoluble residue, which was shown to consist largely of
unchanged metabolite of maleic hydrazide. In tobacco the methanol-
soluble metabolite of maleic hydrazide is the ß-D-glucoside of the
phenolic tautomer, 6 hydroxy-3-(2H)-pyridazone.
Towers et al (1958) also found this ß-D-glucoside in tobacco
leaves. They reported that 15% of maleic hydrazide in tobacco leaves.
They reported that 15% of maleic hydrazide was transformed into ito
its ß-D-glucoside when applied to leaf segments. This metabolite was
also seen in apple and willow.
Callaghan (1961) reported the incorporation of maleic hydrazide
into heterochromatin of root-tip colls of Allium cernuum, Vicia
faba and Tradescantia palludosa.
Biswas et al (1967) isolated two unknown transformation products
from tea plants grown in nutrient solution containing 14C-MH. They
speculate on various modes of ring opening, but did not provide
experimental evidence of it.
Noodén (1970) concluded from studies on the uptake of 14C-MH by
roots that maleic hydrazide is bound to cell wall fragments as a
stable complex which is insoluble in 80% ethanol. The bound maleic
hydrazide could be released by heating the tissue with aminoethanol.
Chromatography of the 14C-material released in this way showed that
there was no indication of degradation and the maleic hydrazide was
bound to the cell-walls in an unchanged form.
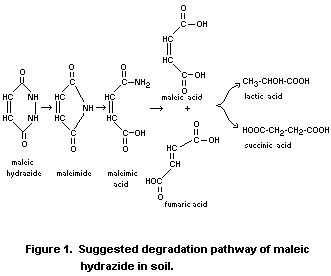 From the above studies on tobacco and other plants it may be
concluded that the principal residues are unchanged maleic hydrazide
(free or bound) and its, ß-D-glucoside. Cleavage of the N-N bond and
opening of the ring structure does not appear to be a significant
metabolic pathway in tobacco or in other crops studied.
Effect of maleic hydrazide on biochemical processes in the plant.
Patterson et al (1952) showed that a foliar spray of 2500 mg/l, about
6 weeks before harvest caused a decrease in reducing and non-reducing
sugars in tubers stored seven months at 7° C. Similar though less
striking decreases were evident when tubers were stored at 13°C.
In other experiments (Gooding and Hubbard, 1956) no effect on the
accumulation of reducing sugars or sucrose was found when potatoes
were stored under cool conditions (0.5 - 4°C) following a pre-harvest
foliar application.
In storage, processing and cooking
Household cooking. Maleic hydrazide is fairly stable during
household cooking. After cooking onions for half an hour, 80% of the
original residue could still be detected. Of this about 25% remained
in the onion and about 75% was found, in the cooking water. (R.I.V.
Netherlands, 1964).
Carry-over in cigarettes and cigarette-smoke. Cigarettes made from
tobacco treated with 2.25 kg a.i./ha contained 10-30 mg/kg maleic
hydrazide. When cigarettes containing 30 mg/kg maleic hydrazide were
smoked in an automatic smoking machine 93% of the original residue was
decomposed or transferred to the side stream. In smoke from cigarettes
containing 10 mg/kg maleic hydrazide and smoked in a similar way, no
maleic hydrazide could be detected in the mainstream smoke. In a third
experiment in which 14C-MH was infused into cigarettes at a rate of
105 mg/kg, 25% of the radioactivity was found in the mainstream
(unpublished experiments of Stone (1957) referred to by Guthrie and
Bowery, 1967).
Residues in food moving in commerce
No information was available to the Meeting.
METHODS OF RESIDUE ANALYSIS
A spectrophotometric method for the determination of maleic
hydrazide residues in plant tissues was originally developed by Wood
(1953) and improved by Lane et al (1958). The sample is boiled in
caustic solution to drive off interfering volatile bases. Distillation
with zinc in a stream of nitrogen then expels hydrazine, which is
reacted in acid solution with p-dimethylamino-benzaldehyde. The yellow
reaction product is measured spectro photometrically. Although the
method is not very sensitive (limit of determination 0.5 -1 mg/kg), it
may be adaptable to regulatory purposes.
Hoffman (1961) and Hoffman et al (1962) describe further
modifications of the Wood method for the spectrophotometric
determination of maleic hydrazide residues.
Haeberer et al (1974) and Haeberer and Chortyk (1974) developed a
rapid quantitative GLC method for non-bound maleic hydrazide residues.
The plant material is extracted with dimethylformamide or directly
with N,O-bis (trimethylsilyl) acetamide. The bis-(trimethysilyl)
derivative is formed by heating for 30 min. at 100°C and measured by
flame ionization gas chromatography. Interfering plant substances are
removed by TLC on alumina with ethyl acetate as developing solvent.
The recovery of maleic hydrazide added to tobacco powder at the rate
of 0.5-10 mg/kg was 94-108%.
NATIONAL TOLERANCES
The national tolerances listed in Table 3 were reported to the
Meeting.
TABLE 3. National tolerances for maleic hydrazide reported to
the Meeting
Country Commodity Tolerance
mg/kg
Argentine potatoes 50
lettuce 0.1
Canada potatoes 50
beets, carrots, swedes
(rutabagas) 30
onions 15
Netherlands onions 15
other vegetables, fruits,
other agricultural
commodities 0*
U.S.A. potato chips 160**
potatoes 50
onions (dry bulb) 15
* The limit of determination = 1 mg/kg
** By weight in finished product.
APPRAISAL
Maleic hydrazide is used in various countries on an extensive
scale as an inhibitor of sprouting in onions. It is also used in a few
countries on potatoes for a similar purpose and on tobacco for
checking sucker growth. On both onions and potatoes the material is
applied as a pre-harvest spray, when biological processes in the
aerial parts of the crops are still very active. Maleic, hydrazide
penetrates extensively into the plant and is transported in the phloem
to actively growing tissues including the bulbs and tubers. Residues
persist in these parts sufficiently to induce dormancy and hamper
sprouting for fairly long periods.
Extensive information from various countries on residues from
supervised trials on onions and from two countries on residues in
potatoes was provided. Limited data were available on residues in
apples, carrots and tobacco, including data on residues in cigarettes
and cigarette smoke.
Considerable information was available on the metabolic pathways
of maleic hydrazide in soil and plants. There is some evidence that
the biodegradation of maleic hydrazide in the soil caused by bacteria
and other micro-organisms leads via maleimide and maleimic acid to
naturally occurring acids such as maleic and fumario, which may be
converted to lactic and succinic acids and finally to CO2. From
studies on tobacco and other plants it appears that the principal
residues in plants consist of unchanged maleic hydrazide, either free
or strongly bound to cell-wall fragments, and the B-D-glucoside of the
phenolic tautomer ß-hydrozy-3-(2H)-pyridazone. Cleavage of the N-N
bond and opening of the ring does not appear to be a significant
metabolic pathway in the crops studied.
Maleic hydrazide residues are fairly stable during cooking. After
normal household cooking of onions about 20% of the residue remained
in the onion and 60% was found in the cooking water.
It was shown that during the manufacture of potato chips, drying
and frying does not lead to any appreciable lose of maleic hydrazide.
Owing to the loss of water during the manufacturing process the
concentration of maleic hydrazide residues in the manufactured product
is higher than in the fresh potatoes.
When cigarettes made from field-treated tobacco and containing 30
and 10 mg/kg maleic hydrazide were smoked in a smoking machine, 93% of
the original residue was decomposed or transferred to the side stream
of the 30 mg/kg level and no maleic hydrazide could be detected in the
mainstream smoke at the 10 mg/kg level.
A fairly specific spectrophotometric method of analysis is
available. Although the method is not very sensitive (limit of
determination 0.5 - 1 mg/kg) it may be suitable for adaptation to
regulatory purposes.
Recently a rapid quantitative GLC method has been developed to
determine the bis(trimethylsilyl) derivative by flame ionisation gas
chromatography. It is uncertain whether the method can be adapted to
include not only the free maleic hydrazide residue but also bound
unchanged maleic hydrazide and its -D-glucoside and whether it can be
used for crops other than tobacco.
EVALUATION
As no ADI was allocated, maximum residue limits could not be
recommended. Data were sufficient to record guideline levels for
onions and potatoes.
The following guideline levels are recommended. They refer to the
sum of free and bound unchanged maleic hydrazide and its
ß-D-glucoside.
Commodity mg/kg
Potatoes 50
Onions 15
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be allocated and
maximum residue limits can be recommended).
1. The results of the carcinogenicity study with rats which is
currently in progress.
2. Teratogenicity study with the sodium salt or the free acid.
3. Further studies clarifying the situation relating to the possible
presence of hydrazine in or on crops.
4. Residue data on other crops for which usage recommendations are
made including tobacco, carrots and swedes and similar crops.
5. Data on the fate of maleic hydrazide and its metabolites in
livestock animals and residues in products of animal origin after
feeding commodities containing maleic hydrazide residues, e.g.
potatoes.
6. Data on the effect of cooking on residues in potatoes and the
effect on residues of different methods of industrial processing
in the manufacture of various potato products, e.g. potato chips,
dried potatoes and potato starch. Data on residues in the wastes
from these products intended for feeding purposes.
7. Further development of the gas-chromatographic method to make it
suitable for regulatory purposes.
8. More information on the carry-over of maleic hydrazide from raw
into cured tobacco and into cigarette smoke.
Desirable
1. Studies on the metabolism of the beta-D-glucoside of maleic
hydrazide.
REFERENCES
Anderson, K.S. et al J. Agric. Chem. 20 649-655.
1972
Andreae, W.A. The Photoinduced Oxidation of Manganous Ions. Arc.
1955 Biochem. Biophys. 55: 684-586.
Baker, J.E. A Study of the Action of Maleic Hydrazide on Processes
1961 of Tobacco and other Plants. Physiologia Plantarum
14: 76-88.
Barnes, J.M., Magee, P.N., Boyland E., Haddow, A., Passey, R.D.,
1957 Bullough, W.S., Cruickshank, C.N.D., Salaman,
M.H., and Williams, R.T. The non-toxicity of
maleic hydrazide for mammalian tissues. Nature,
180, 62-64.
BASF Unpublished report: Verhalten des
1975 Pflanzenschutzmittelwirkstoffes (Maleinhydrazid)
im Boden.
Biswas, P.H., O. Hull and B.D. Mayberry. Metabolism, of Maleic
1967 Hydrazide in Tea, Camellia sinensis.
Physiologia Plantarum 20 : 819-824.
Callaghan, J.J. and P.Grun. Incorporation of 14C labeled
1961 Maleic Hydrazide into Root-tip cells of Allium
cerassum, Vicia faba and Tradescantia
paludosa, J. Biophys. Biochem. Cytol. 10:
567-575.
Drygas, M. D., Sikorska and B. Leszczynska Zmiany Zawortosci
1968 Hydrazydu Maleinowego HM/ Ziemniakach i Cebuli w
Okresic ich Przechowywania Biul Instytutu Ochiony
Roslin 1968 207-213.
Epstein, S.S., Andrea, J., Jaffe, H., Joshi, S., Falk, H., and
1967 Mantel, N. Carcinogenicity of the herbicide,
maleic hydrazide. Nature, 215, 1388-1390.
Epstein, S.D. and Shafner, H. Chemical mutagens in the
1968 environment. Nature, 219, 385-387.
Field, R.J. and A.J. Peel. The Metabolism and Radial Movement
1971 of Growth Regulators and Herbicides in Willow
stems. New Phytol. 70: 743-749.
Food Research Laboratories Inc. Report on toxicological studies
1955 of chemical additives, 3. Maleic hydrazide
(1,2-dihydropyridazine-3,6 dione). Submitted by
Uniroyal Chemical. (Unpublished).
Frear, D.S. and H.R. Swanson. Behaviour and Fate of (14C)
1975 Maleic Hydrazide in Tobacco Plants. U.S. Dept. of
Agric., Agric. Research Service, Metabolism and
Radiation Lab., Fargo, North Dakota 58102.
Freed, V.H. and M.L. Montgomery. The Metabolism of Herbicides
1963 by Plants and Soil. Residue Reviews 3: 1-19.
Gooding, E.B.G. and A.W.Hubbard. The Effect of certain
1956 Sprout-depressant Treatments on Sugar accumulation
in stored Potatoes. J.Sci.Food Agric. 7: 574-577.
Guthrie, F.E. and T.G. Bowery. Pesticide Residues on Tobacco.
1967 Residue Reviews 19: 31-57.
Haeberer, A.F., W.S. Schlotzhauer, O.T.Chortyk. A rapid Quantitative
1974 Method for Maleic hydrazide. J. Agric. Food Chem.
22: 328-330.
Haeberer, A.F. and O.T.Chortyk. Rapid Determination, of Maleic
1974 Hydrazide in Cigarette Smoke Condensate and
Particulate Matter. J. Agric. Food Chem. 22:
1135-1137.
Helweg-Anderson, A. Persistence of Maleic Hydrazide in Soil
1971 and its Influence on CO2 liberation. Tidsskrift
for Planteavl 7: 96-113.
Hellweg-Andersen, A. Abbau des Maleinhydrazids und Einflusz auf
1971 die Respiration in der Erde. Zeitschrift f.
Planzenkultur 75: 84-89.
Helweg, A. Degradation of 14C labeled Maleic Hydrazide in
1975 Soil as Influenced by Sterilization, Concentration
and Pre-treatment. Weed Research 15: 53-58.
Helweg, A. Degradation of 14C Maleic Hydrazide in Soil as
1975 Influenced by Absorbtion on Activated Carbon. Weed
Research 15: 129-133.
Hoffman, I. Spectrophotometric Determination of Maleic Hydrazide
1961 in Tobaccos. J. Ass. Off. Agr. Chem. 44: 723-725.
Hoffman, I. and R.B. Carson. Determination and Distribution of
1962 Maleic Hydrazide in Vegetables and Fruits. J.
Ass.Off. Agr. Chem. 45: 788-789.
Hoffman,. and E.V.Parups. Mode of Action of Maleic Hydrazide
1964 in Relation to Residues in Crops and Soils.
Residue Reviews 7: 96-113.
Hoffman, I., E.V. Parups and R.B. Carson. Analysis for Maleic
1962 Hydrazide. Part I. Detection and Determination in
Dried Green Tobacco Leaves and Suckers. Part II
Determination and Persistence in Soils. J. Agr.
Food Chem. 10: 453-455.
Hunter, B., Mawdesley-Thomas, L.E. and Worden, A.N. The
administration of maleic hydrazide and its
diethanolamine salt to rats. Toxicology 1,
301-307.
Innes, J.R.M. et al Bioassay of pesticides and industrial chemicals
1969 for tumorigenicity in mice: a preliminary note. J.
Nat. Cancer Inst. 42, 1101.
Kaufman, D.D., and N. A. Kalayanova. Maleic Hydrazide Degradation in
1975 Soil. U.S.Dept. of Agriculture, Agric. Research
Service, Beltsville Agricultural Research Centre,
Beltsville, Maryland 20725, U.S.A.
Lane, J.R. Collaborative Study of Maleic Hydrazide Residue
1963 Analysis. J. Ass. Off. Agr. Chem. 46(2): 261-268,
744-748.
Lane, J.R., D.K. Gullstrom and J.E. Newell. Extension of the Residue
1958 Methods for 1,2-dihydro-3,6-pyridazinedione
(maleic hydrazide) and N-1-naphtylphtalamic acid
(Alanap). J. Agr. Food Chem. 6: 671-674.
Levi, E. and A.S. Crafts. Toxicity of maleic hydrazide in California
Soils. Hilgardia 21: 431-463.
Luckens, M.M. and Wattimena, J.R. Acute toxicity of combinations
1968 of maleic, hydrazide diethanolamine and selected
insecticides. Toxicol. Appl. Pharmacol. 12:
287-288 (Abstr.no. 7).
Mannell, W.A. and Grice H.C. A comparative study of maleic hydrazide
1957 and p-dimenthylaminoazobenzene on rat liver. Can.
J. Biochem. Physiol. 35, 1233-1240.
Mays, D.L., Born, G.S. Christian, J.E. and Liska, B.L. Fate of
1968 C14-Maleic hydrazide in rats. J. Ag. Fd. Chem.
16, 356-7.
McCann J., Choi, E., Yamanslic, E., and Ames, B.N. Proc. Natl.
1975 Academy of Science 72 (12) 5135-5139.
McCann, J., Choi, E., Yamanslic, E., and Ames, B.N.
Ibid 73 (3) 950-954.
McCarthy, R.E. and Epstein, S.S. Cytochemical and cytogenetic
1968 effects of maleic hydrazide on cultured mammali
cells. Life Sci. 7:1-6.
Nasrat, G.E. Maleic hydrazide, a chemical mutagen in
1965 Drosophila melanogaster. Nature, 207, 439.
Nelson, J.O., and Kearney, P.C. Metabolism of maleic hydrazide
1975 by hepatic microsomes from phenobarbital induced
rats. U.S.D.A. report.
Naugatuck Chem. Comp. Unpublished Data on Maleic Hydrazide
1956 summarised by the Secretary of the Scientific
Subcommittee on Poisenous Substances used in
Agriculture and Food Storage. American Residue
data of Maleic Hydrazide in Onions and Potatoes.
Noodén, L.D. Metabolism and Binding of 14C-Maleic Hydrazide.
1970 Plant Physiol. 45: 46-52.
Patterson, D.R., S.H. Wittwer et al. 1952. The effect of pre-harvest
foliar sprays of maleic hydrazide on sprout
inhibition and storage quality of potatoes. Plant
Physiol. 27: 135-142.
Povolotskaya, K.L. Mechanism of the Action of Maleic Hydrazide
1961 in Plants. Izvest. Akad. Nauk SSSR 26, Ser.
Biol.no. 2: 250-255.
R.I.V. Unpublished data of the National Institute of Public
1955. 1964. Health Utrecht/Bilthoven, The Netherlands,
provided to JMPR 1976.
Stoessl, A. Photolysis of Maleic Hydrazide. Chem. Ind.:
1964 580-581.
Timson, J. The effects of maleic hydrazide on the mitosis of
1968 phyto-hemagglutinin stimulated human lymphocytes
cultured in vitro. Caryologia 21:157 (Cited in Fd.
Cosm.Toxicol, 8: 104-105, 1970).
Towers, G.H.N., Hutchinson, A. and Andreae, W.A. Formation of a
1958 Glucoside of Maleic Hydrazide in Plants. Nature
181:1535-1536.
Uniroyal Chem. Unpublished data on Maleic Hydrazide provided
1973-1975 to JMPR 1976.
Winder, F.G. and Denneny, J.M. Metal-Catalyzed Auto, Oxidation
1959 of Isoniazid. Biochem. J. 73:500-507.
Wood, P.R. Determination of Maleic Hydrazide Residues in Plant
1953 and Animal Tissues. Anal. Chem. 25:1879-1883.
From the above studies on tobacco and other plants it may be
concluded that the principal residues are unchanged maleic hydrazide
(free or bound) and its, ß-D-glucoside. Cleavage of the N-N bond and
opening of the ring structure does not appear to be a significant
metabolic pathway in tobacco or in other crops studied.
Effect of maleic hydrazide on biochemical processes in the plant.
Patterson et al (1952) showed that a foliar spray of 2500 mg/l, about
6 weeks before harvest caused a decrease in reducing and non-reducing
sugars in tubers stored seven months at 7° C. Similar though less
striking decreases were evident when tubers were stored at 13°C.
In other experiments (Gooding and Hubbard, 1956) no effect on the
accumulation of reducing sugars or sucrose was found when potatoes
were stored under cool conditions (0.5 - 4°C) following a pre-harvest
foliar application.
In storage, processing and cooking
Household cooking. Maleic hydrazide is fairly stable during
household cooking. After cooking onions for half an hour, 80% of the
original residue could still be detected. Of this about 25% remained
in the onion and about 75% was found, in the cooking water. (R.I.V.
Netherlands, 1964).
Carry-over in cigarettes and cigarette-smoke. Cigarettes made from
tobacco treated with 2.25 kg a.i./ha contained 10-30 mg/kg maleic
hydrazide. When cigarettes containing 30 mg/kg maleic hydrazide were
smoked in an automatic smoking machine 93% of the original residue was
decomposed or transferred to the side stream. In smoke from cigarettes
containing 10 mg/kg maleic hydrazide and smoked in a similar way, no
maleic hydrazide could be detected in the mainstream smoke. In a third
experiment in which 14C-MH was infused into cigarettes at a rate of
105 mg/kg, 25% of the radioactivity was found in the mainstream
(unpublished experiments of Stone (1957) referred to by Guthrie and
Bowery, 1967).
Residues in food moving in commerce
No information was available to the Meeting.
METHODS OF RESIDUE ANALYSIS
A spectrophotometric method for the determination of maleic
hydrazide residues in plant tissues was originally developed by Wood
(1953) and improved by Lane et al (1958). The sample is boiled in
caustic solution to drive off interfering volatile bases. Distillation
with zinc in a stream of nitrogen then expels hydrazine, which is
reacted in acid solution with p-dimethylamino-benzaldehyde. The yellow
reaction product is measured spectro photometrically. Although the
method is not very sensitive (limit of determination 0.5 -1 mg/kg), it
may be adaptable to regulatory purposes.
Hoffman (1961) and Hoffman et al (1962) describe further
modifications of the Wood method for the spectrophotometric
determination of maleic hydrazide residues.
Haeberer et al (1974) and Haeberer and Chortyk (1974) developed a
rapid quantitative GLC method for non-bound maleic hydrazide residues.
The plant material is extracted with dimethylformamide or directly
with N,O-bis (trimethylsilyl) acetamide. The bis-(trimethysilyl)
derivative is formed by heating for 30 min. at 100°C and measured by
flame ionization gas chromatography. Interfering plant substances are
removed by TLC on alumina with ethyl acetate as developing solvent.
The recovery of maleic hydrazide added to tobacco powder at the rate
of 0.5-10 mg/kg was 94-108%.
NATIONAL TOLERANCES
The national tolerances listed in Table 3 were reported to the
Meeting.
TABLE 3. National tolerances for maleic hydrazide reported to
the Meeting
Country Commodity Tolerance
mg/kg
Argentine potatoes 50
lettuce 0.1
Canada potatoes 50
beets, carrots, swedes
(rutabagas) 30
onions 15
Netherlands onions 15
other vegetables, fruits,
other agricultural
commodities 0*
U.S.A. potato chips 160**
potatoes 50
onions (dry bulb) 15
* The limit of determination = 1 mg/kg
** By weight in finished product.
APPRAISAL
Maleic hydrazide is used in various countries on an extensive
scale as an inhibitor of sprouting in onions. It is also used in a few
countries on potatoes for a similar purpose and on tobacco for
checking sucker growth. On both onions and potatoes the material is
applied as a pre-harvest spray, when biological processes in the
aerial parts of the crops are still very active. Maleic, hydrazide
penetrates extensively into the plant and is transported in the phloem
to actively growing tissues including the bulbs and tubers. Residues
persist in these parts sufficiently to induce dormancy and hamper
sprouting for fairly long periods.
Extensive information from various countries on residues from
supervised trials on onions and from two countries on residues in
potatoes was provided. Limited data were available on residues in
apples, carrots and tobacco, including data on residues in cigarettes
and cigarette smoke.
Considerable information was available on the metabolic pathways
of maleic hydrazide in soil and plants. There is some evidence that
the biodegradation of maleic hydrazide in the soil caused by bacteria
and other micro-organisms leads via maleimide and maleimic acid to
naturally occurring acids such as maleic and fumario, which may be
converted to lactic and succinic acids and finally to CO2. From
studies on tobacco and other plants it appears that the principal
residues in plants consist of unchanged maleic hydrazide, either free
or strongly bound to cell-wall fragments, and the B-D-glucoside of the
phenolic tautomer ß-hydrozy-3-(2H)-pyridazone. Cleavage of the N-N
bond and opening of the ring does not appear to be a significant
metabolic pathway in the crops studied.
Maleic hydrazide residues are fairly stable during cooking. After
normal household cooking of onions about 20% of the residue remained
in the onion and 60% was found in the cooking water.
It was shown that during the manufacture of potato chips, drying
and frying does not lead to any appreciable lose of maleic hydrazide.
Owing to the loss of water during the manufacturing process the
concentration of maleic hydrazide residues in the manufactured product
is higher than in the fresh potatoes.
When cigarettes made from field-treated tobacco and containing 30
and 10 mg/kg maleic hydrazide were smoked in a smoking machine, 93% of
the original residue was decomposed or transferred to the side stream
of the 30 mg/kg level and no maleic hydrazide could be detected in the
mainstream smoke at the 10 mg/kg level.
A fairly specific spectrophotometric method of analysis is
available. Although the method is not very sensitive (limit of
determination 0.5 - 1 mg/kg) it may be suitable for adaptation to
regulatory purposes.
Recently a rapid quantitative GLC method has been developed to
determine the bis(trimethylsilyl) derivative by flame ionisation gas
chromatography. It is uncertain whether the method can be adapted to
include not only the free maleic hydrazide residue but also bound
unchanged maleic hydrazide and its -D-glucoside and whether it can be
used for crops other than tobacco.
EVALUATION
As no ADI was allocated, maximum residue limits could not be
recommended. Data were sufficient to record guideline levels for
onions and potatoes.
The following guideline levels are recommended. They refer to the
sum of free and bound unchanged maleic hydrazide and its
ß-D-glucoside.
Commodity mg/kg
Potatoes 50
Onions 15
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be allocated and
maximum residue limits can be recommended).
1. The results of the carcinogenicity study with rats which is
currently in progress.
2. Teratogenicity study with the sodium salt or the free acid.
3. Further studies clarifying the situation relating to the possible
presence of hydrazine in or on crops.
4. Residue data on other crops for which usage recommendations are
made including tobacco, carrots and swedes and similar crops.
5. Data on the fate of maleic hydrazide and its metabolites in
livestock animals and residues in products of animal origin after
feeding commodities containing maleic hydrazide residues, e.g.
potatoes.
6. Data on the effect of cooking on residues in potatoes and the
effect on residues of different methods of industrial processing
in the manufacture of various potato products, e.g. potato chips,
dried potatoes and potato starch. Data on residues in the wastes
from these products intended for feeding purposes.
7. Further development of the gas-chromatographic method to make it
suitable for regulatory purposes.
8. More information on the carry-over of maleic hydrazide from raw
into cured tobacco and into cigarette smoke.
Desirable
1. Studies on the metabolism of the beta-D-glucoside of maleic
hydrazide.
REFERENCES
Anderson, K.S. et al J. Agric. Chem. 20 649-655.
1972
Andreae, W.A. The Photoinduced Oxidation of Manganous Ions. Arc.
1955 Biochem. Biophys. 55: 684-586.
Baker, J.E. A Study of the Action of Maleic Hydrazide on Processes
1961 of Tobacco and other Plants. Physiologia Plantarum
14: 76-88.
Barnes, J.M., Magee, P.N., Boyland E., Haddow, A., Passey, R.D.,
1957 Bullough, W.S., Cruickshank, C.N.D., Salaman,
M.H., and Williams, R.T. The non-toxicity of
maleic hydrazide for mammalian tissues. Nature,
180, 62-64.
BASF Unpublished report: Verhalten des
1975 Pflanzenschutzmittelwirkstoffes (Maleinhydrazid)
im Boden.
Biswas, P.H., O. Hull and B.D. Mayberry. Metabolism, of Maleic
1967 Hydrazide in Tea, Camellia sinensis.
Physiologia Plantarum 20 : 819-824.
Callaghan, J.J. and P.Grun. Incorporation of 14C labeled
1961 Maleic Hydrazide into Root-tip cells of Allium
cerassum, Vicia faba and Tradescantia
paludosa, J. Biophys. Biochem. Cytol. 10:
567-575.
Drygas, M. D., Sikorska and B. Leszczynska Zmiany Zawortosci
1968 Hydrazydu Maleinowego HM/ Ziemniakach i Cebuli w
Okresic ich Przechowywania Biul Instytutu Ochiony
Roslin 1968 207-213.
Epstein, S.S., Andrea, J., Jaffe, H., Joshi, S., Falk, H., and
1967 Mantel, N. Carcinogenicity of the herbicide,
maleic hydrazide. Nature, 215, 1388-1390.
Epstein, S.D. and Shafner, H. Chemical mutagens in the
1968 environment. Nature, 219, 385-387.
Field, R.J. and A.J. Peel. The Metabolism and Radial Movement
1971 of Growth Regulators and Herbicides in Willow
stems. New Phytol. 70: 743-749.
Food Research Laboratories Inc. Report on toxicological studies
1955 of chemical additives, 3. Maleic hydrazide
(1,2-dihydropyridazine-3,6 dione). Submitted by
Uniroyal Chemical. (Unpublished).
Frear, D.S. and H.R. Swanson. Behaviour and Fate of (14C)
1975 Maleic Hydrazide in Tobacco Plants. U.S. Dept. of
Agric., Agric. Research Service, Metabolism and
Radiation Lab., Fargo, North Dakota 58102.
Freed, V.H. and M.L. Montgomery. The Metabolism of Herbicides
1963 by Plants and Soil. Residue Reviews 3: 1-19.
Gooding, E.B.G. and A.W.Hubbard. The Effect of certain
1956 Sprout-depressant Treatments on Sugar accumulation
in stored Potatoes. J.Sci.Food Agric. 7: 574-577.
Guthrie, F.E. and T.G. Bowery. Pesticide Residues on Tobacco.
1967 Residue Reviews 19: 31-57.
Haeberer, A.F., W.S. Schlotzhauer, O.T.Chortyk. A rapid Quantitative
1974 Method for Maleic hydrazide. J. Agric. Food Chem.
22: 328-330.
Haeberer, A.F. and O.T.Chortyk. Rapid Determination, of Maleic
1974 Hydrazide in Cigarette Smoke Condensate and
Particulate Matter. J. Agric. Food Chem. 22:
1135-1137.
Helweg-Anderson, A. Persistence of Maleic Hydrazide in Soil
1971 and its Influence on CO2 liberation. Tidsskrift
for Planteavl 7: 96-113.
Hellweg-Andersen, A. Abbau des Maleinhydrazids und Einflusz auf
1971 die Respiration in der Erde. Zeitschrift f.
Planzenkultur 75: 84-89.
Helweg, A. Degradation of 14C labeled Maleic Hydrazide in
1975 Soil as Influenced by Sterilization, Concentration
and Pre-treatment. Weed Research 15: 53-58.
Helweg, A. Degradation of 14C Maleic Hydrazide in Soil as
1975 Influenced by Absorbtion on Activated Carbon. Weed
Research 15: 129-133.
Hoffman, I. Spectrophotometric Determination of Maleic Hydrazide
1961 in Tobaccos. J. Ass. Off. Agr. Chem. 44: 723-725.
Hoffman, I. and R.B. Carson. Determination and Distribution of
1962 Maleic Hydrazide in Vegetables and Fruits. J.
Ass.Off. Agr. Chem. 45: 788-789.
Hoffman,. and E.V.Parups. Mode of Action of Maleic Hydrazide
1964 in Relation to Residues in Crops and Soils.
Residue Reviews 7: 96-113.
Hoffman, I., E.V. Parups and R.B. Carson. Analysis for Maleic
1962 Hydrazide. Part I. Detection and Determination in
Dried Green Tobacco Leaves and Suckers. Part II
Determination and Persistence in Soils. J. Agr.
Food Chem. 10: 453-455.
Hunter, B., Mawdesley-Thomas, L.E. and Worden, A.N. The
administration of maleic hydrazide and its
diethanolamine salt to rats. Toxicology 1,
301-307.
Innes, J.R.M. et al Bioassay of pesticides and industrial chemicals
1969 for tumorigenicity in mice: a preliminary note. J.
Nat. Cancer Inst. 42, 1101.
Kaufman, D.D., and N. A. Kalayanova. Maleic Hydrazide Degradation in
1975 Soil. U.S.Dept. of Agriculture, Agric. Research
Service, Beltsville Agricultural Research Centre,
Beltsville, Maryland 20725, U.S.A.
Lane, J.R. Collaborative Study of Maleic Hydrazide Residue
1963 Analysis. J. Ass. Off. Agr. Chem. 46(2): 261-268,
744-748.
Lane, J.R., D.K. Gullstrom and J.E. Newell. Extension of the Residue
1958 Methods for 1,2-dihydro-3,6-pyridazinedione
(maleic hydrazide) and N-1-naphtylphtalamic acid
(Alanap). J. Agr. Food Chem. 6: 671-674.
Levi, E. and A.S. Crafts. Toxicity of maleic hydrazide in California
Soils. Hilgardia 21: 431-463.
Luckens, M.M. and Wattimena, J.R. Acute toxicity of combinations
1968 of maleic, hydrazide diethanolamine and selected
insecticides. Toxicol. Appl. Pharmacol. 12:
287-288 (Abstr.no. 7).
Mannell, W.A. and Grice H.C. A comparative study of maleic hydrazide
1957 and p-dimenthylaminoazobenzene on rat liver. Can.
J. Biochem. Physiol. 35, 1233-1240.
Mays, D.L., Born, G.S. Christian, J.E. and Liska, B.L. Fate of
1968 C14-Maleic hydrazide in rats. J. Ag. Fd. Chem.
16, 356-7.
McCann J., Choi, E., Yamanslic, E., and Ames, B.N. Proc. Natl.
1975 Academy of Science 72 (12) 5135-5139.
McCann, J., Choi, E., Yamanslic, E., and Ames, B.N.
Ibid 73 (3) 950-954.
McCarthy, R.E. and Epstein, S.S. Cytochemical and cytogenetic
1968 effects of maleic hydrazide on cultured mammali
cells. Life Sci. 7:1-6.
Nasrat, G.E. Maleic hydrazide, a chemical mutagen in
1965 Drosophila melanogaster. Nature, 207, 439.
Nelson, J.O., and Kearney, P.C. Metabolism of maleic hydrazide
1975 by hepatic microsomes from phenobarbital induced
rats. U.S.D.A. report.
Naugatuck Chem. Comp. Unpublished Data on Maleic Hydrazide
1956 summarised by the Secretary of the Scientific
Subcommittee on Poisenous Substances used in
Agriculture and Food Storage. American Residue
data of Maleic Hydrazide in Onions and Potatoes.
Noodén, L.D. Metabolism and Binding of 14C-Maleic Hydrazide.
1970 Plant Physiol. 45: 46-52.
Patterson, D.R., S.H. Wittwer et al. 1952. The effect of pre-harvest
foliar sprays of maleic hydrazide on sprout
inhibition and storage quality of potatoes. Plant
Physiol. 27: 135-142.
Povolotskaya, K.L. Mechanism of the Action of Maleic Hydrazide
1961 in Plants. Izvest. Akad. Nauk SSSR 26, Ser.
Biol.no. 2: 250-255.
R.I.V. Unpublished data of the National Institute of Public
1955. 1964. Health Utrecht/Bilthoven, The Netherlands,
provided to JMPR 1976.
Stoessl, A. Photolysis of Maleic Hydrazide. Chem. Ind.:
1964 580-581.
Timson, J. The effects of maleic hydrazide on the mitosis of
1968 phyto-hemagglutinin stimulated human lymphocytes
cultured in vitro. Caryologia 21:157 (Cited in Fd.
Cosm.Toxicol, 8: 104-105, 1970).
Towers, G.H.N., Hutchinson, A. and Andreae, W.A. Formation of a
1958 Glucoside of Maleic Hydrazide in Plants. Nature
181:1535-1536.
Uniroyal Chem. Unpublished data on Maleic Hydrazide provided
1973-1975 to JMPR 1976.
Winder, F.G. and Denneny, J.M. Metal-Catalyzed Auto, Oxidation
1959 of Isoniazid. Biochem. J. 73:500-507.
Wood, P.R. Determination of Maleic Hydrazide Residues in Plant
1953 and Animal Tissues. Anal. Chem. 25:1879-1883.
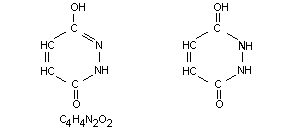 Other information on identity and properties
Composition of the technical product
The technical material contains 99% of maleic hydrazide;
impurities are inorganic salts (e.g. Na2SO4 possibly with minor
amounts of maleic or fumaric acid.
Physical and chemical properties of maleic hydrazide
physical form white crystalline powder
molecular weight 112.1
specific gravity at 25°C 1.60
melting point 292°C(min)
odour faint
volatility non volatile
solubility (approximate) g/100 g solvent at 25°C
distilled water 0.6
alcohol 0.1
acetone 0.1
dimethyl formamide 2.4
xylene less than 0.1
Ph of 0.5% aqueous solution at 25°C: 4.
Maleic hydrazide behaves as a weak monobasic acid. The
alkanolamine and alkali metal salts are moderately soluble in
water.
Formulations used: liquid 30% and 40% a.i.; wettable powder 40%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Maleic hydrazide does not appear to be extensively metabolized
by mammals. In the rabbit, 43-62% of a single oral dose was
excreted unchanged within 48 hrs. Isolation and characterization of
the excreted maleic hydrazide following oral doses of 2 g showed
the excreted product to be the benzylamine salt. No other
metabolites were identified. (Barnes et al, 1957). Incubation of
phenobarbital-induced rat liver microsomes with maleic hydrazide
did not result in any degradation of the compound (Nelson and
Kearney, 1975).
Using maleic hydrazide - 14C labelled material, female rats
were dosed with 0.27, 0.68, or 2.72 mg per rat (equivalent to 10,
25 or 100 µc/rat) by stomach tube. At the low dose level, 65.4% of
the radioactivity was excreted in the urine in 12 hrs and 77.3%
within 6 days.
A further 12.4% of the administered radioactivity was detected
in the faeces over the same period. Rats dosed with 2.5 µc showed
0.12-0.18% expired radioactivity in CO2 over the 72 hour period
after administration. Tissue levels of radioactivity were
statistically insignificant except in the carcass, where <0.001%
of the administered radioactivity was detected. Using paper
chromatography with two different solvent systems, urine from rats
intubated with 100 µc resulted in the identification of unchanged
maleic hydrazide (92-94%) and a maleic hydrazide conjugate (6-8%).
(Mays et al, 1968).
Sixteen rats were given 4g/kg by stomach tube. Urine and
faeces were collected for time intervals 0 to 2, 2 to 4, 4 to 8,
and 8 to 16 days. Two rats/sex were sacrificed after 2, 4, 8, or 16
days post-dosing. In male rats, residues in urine (30,400 mg/kg)
were approximately double those in faeces (17,400 mg/kg) over the
0-2 day period, whereas in females urine residues (1,330 mg/kg)
were much less than in faeces (12,700 mg/kg). In both sexes,
residue levels decreased rapidly, but were still detectable in 8-16
day samples of both urine and faeces. Excretion in females was
slightly slower than in males, however residues were undetectable,
except for traces in the males sacrificed on day 8. (Food Research
Laboratories Inc. 1955).
TOXICOLOGICAL STUDIES
Special Studies on reproduction
Seven groups of approximately 10 male and 10 female rats fed
0, 0.5, 1.0, 2.0 or 5.0 maleic hydrazide as the sodium salt, or 0
or 0.1% maleic hydrazide as the diethanolamine salt were repeatedly
bred using a cycle of 2 weeks pairing, 3 weeks gestation, 3 weeks
lactation and one or two weeks rest prior to remating, for as long
as possible, commencing at 12 weeks on test. The second litters
(F1b) were used to produce the F2 generation which were mated
twice. The F2b generation provided parents for the F3a and b
offspring. Pooled data from the 8 matings of the Fo generation do
not indicate any effects of the sodium salt of maleic hydrazide on
fertility, litter size, gestation index, viability index, or
lactation index. Pup weight at weaning was slightly reduced at the
5.0% level. The diethanolamine salt at the 0.1% level resulted in
reduced fertility, reduced litter size, reduced viability index,
and reduced lactation index. The diethanolamine salt group was
dropped from the study, no attempt being made to produce the F2
generation. Mean data for all generations of rats receiving the
sodium salt do not do not indicate any effects on reproduction,
with respect to fertility, gestation index, viability index, or
lactation index. At the 5.0% level, litter size was reduced in the
F3 generation and weanling weight was reduced in all generations.
(Food Research Laboratories, 1955).
Special studies on mutagenicity
Injection of 0.7% sodium chloride Ringer's solution containing
0.4% maleic hydrazide abdominally in one to two-day old
Drosophila melanogaster males did not result in an increase in
mutation rate. On the other hand, D. melanogaster males fed on
media containing 0.4% maleic hydrazide showed an increased
incidence of lethal mutations in the first brood, although in
subsequent broods, the effect was not apparent (Nasrat, 1965).
In a mouse dominant lethal study, 500 mg maleic hydrazide/kg
did not affect the calculated mutation index. (Epstein & Shafner,
1968).
Andersen et al (1972) tested maleic hydrazide and other
chemicals on eight mutant strains of Salmonella typhimurium on
T4 bacteriophage, and on bacteriophage AP72. In none of these ten
systems was there any ~evidence of mutagenic activity. Maleic
hydrazide was tested by McCann et al (1975, 1976) on several tester
strains of Salmonella. Tests covered a wide dose range, both with
and without liver microsome activation. There was no evidence of
mutagenic activity in this extensive series of tests.
Special studies on carcinogenicity
Swiss mice (ICR/Ha) were injected sub-cutaneously with aqueous
solutions, or tricaprylin suspensions of maleic hydrazide as free
acid (0.4% hydrazine impurity), or with solvent alone in volumes of
0.1, 0.1, 0.2 and 0.2 ml on post-natal days 1, 7, 14 and 21. Total
doses were 3 mg for aqueous solution, and 55 mg for tricaprylin
suspension. (A further group comprising 11 mice all died following
10 mg maleic hydrazide injected on post-natal day 1). Preweaning
mortality was 14% in controls, 5% in mice receiving 3 mg and 53% in
mice receiving 55 mg. Post weaning mortality in males in all groups
was high being 38, 57, and 52% at 0,3 & 55 mg respectively by 49
weeks of age. Decreased weight gain (ca 5%) was noted in the high
dose group. Hepatoma incidence in males at 49 Weeks was 8% in
controls, 18% in the 3 mg group and 73% in the 55 mg group. No
metastases were noted. (Epstein et al, 1967).
Maleic hydrazide was administered by stomach tube in daily
doses of 1000 mg/kg weight to 36 mice of each sex for 3 weeks,
beginning when the animals were 7 days old. Then, 3000 ppm were
mixed directly with the diet, which was fed for approximately 18
months. No significant increase in the incidence of tumors was
observed in comparison with untreated controls (Innes et al.,
1969).
Rats were injected subcutaneously with 1 ml of the
diethanolamine salt (i.e. 5 mg) weekly for 14 months. Of 52 rats so
treated in two experiments, 3 developed sarcomas. No sarcomas
occurred in saline control rats. (Barnes et al, 1957).
Groups of rats and mice were either injected with the
monosodium salt, subcutaneously, once weekly at a dose level of 500
mg,/kg or received 1% maleic hydrazide added to the diet for 100
weeks. Maleic hydrazide did not affect growth or general health in
either species, on either dose regime. One rat receiving maleic
hydrazide by injection (out of a total of 29) developed a sarcoma.
No sarcomas were observed in control rats. Total tumor incidence
was increased in female test groups of both species, fed maleic
hydrazide, the effect being more obvious in the mouse (Barnes et al
1957).
Groups of 25 male and 25 female rats were injected twice
weekly for 65 weeks with arachis oil, water, solvents plus 2 mg
maleic hydrazide or solvents plus 2 mg diethanolamine salt of
maleic hydrazide. A further 39-week observation preceded autopsy.
subcutaneous tumor incidence was increased (4) in the group
receiving arachis oil plus maleic hydrazide when compared with the
arachis oil control group (1). Incidences of tumors in all other
groups were comparable. The increased subcutaneous tumor rate was
attributed to impaired connective tissue repair mechanisms rather
than chemical carcinogenesis induction. (Hunter et al, 1973).
Three groups of 24 male rats were fed basal diet, basal diet
plus 2% maleic hydrazide as the sodium salt, or basal diet plus
0.06% p-dimethylaminoazobenzene for up to 26 weeks. No significant
changes with regard to body weight, desoxyribose nucleic acid
content per liver cell nucleus, average liver cell size, liver
weight, number of cells/liver or DNA/liver were observed with
maleic hydrazide, although the p-dimethylaminoazobenzene affected
all these parameters. Pathological examination of animals fed
maleic hydrazide did not reveal neoplasms, although these occurred
in all rats fed the p-dimethylaminoazobene for more than 10 weeks.
(Mannell & Grice, 1957).
Special studies on mammalian cells in vitro
The effect on mammalian cells was examined by studying the
development of mitosis in fragments of mouse ear epidermis. In
concentrations from 0.0001-0.001 M, maleic hydrazide had no effects
on mitosis or cell division. Maleic hydrazide was also tested upon
skin from the guinea pig's ear, grown in tissue culture. Up to a
concentration of 0.01 M no gross or microscopical effects upon the
explants were noted. The same concentration of maleic hydrazide was
also without effect upon the respiration of skin cells, studied 2
and 22 hr after adding the maleic hydrazide (Barnes et al., 1957).
In vitro studies with mouse thymus cells in culture showed
that maleic hydrazide in concentrations of 0.001 M or more
inhibited growth. Mitotic inhibition occurred at and above 0.0001
M. The ratio of dry mass to DNA content was increased, which was
taken as an indication for inhibition of DNA, but not of protein
synthesis (McCarthy and Epstein, 1968). Mitotic inhibition was also
shown in cultures of human lymphocytes grown in the presence of
0.001-0.01 M. Inhibition was more marked in 72 hr-old cultures than
in fresh ones, probably because of the more active mitotic state in
the former (Timson, 1968).
Special studies on dermal irritation
The diethanolamine salt of maleic hydrazide was applied as a
20% aqueous solution to approximately 10 sq.cm. of abraded, and 10
sq. cm. of intact skin on the backs of 6 rabbits. Repeated
applications were made during a 6-hour period on five consecutive
days on two occasions, separated by a ten-day rest period. No signs
of irritation were noted during the study. (Food Research
Laboratories, 1955).
Special studies on dermal sensitization
Ten guinea pigs were injected intradermally with one dose of
0.5 ml, and 9 subsequent doses of 0.1 ml of a 0.1% aqueous solution
of the diethanolamine salt of maleic hydrazide, dosing being on
alternate days. Two weeks after the last dose, a challenge dose of
0.5 ml was administered intradermally at a different site. No
evidence of sensitization was observed. (Food Research
Laboratories, 1955).
Special studies on eye irritation
Six rabbits received two drops of a 5% solution of the
diethanolamine salt of maleic hydrazide in 5% saline in the right
eye. No signs of irritation were observed in the 14 day
post-treatment observation period. (Food Research Laboratories,
1955).
Special studies on respiratory effects
Deeply anaesthetized rats (ethyl ether) were administered the
diethanolamine salt of maleic hydrazide at various solution
concentrations by instilling small droplets in alternate nostrils
at the moment of inhalation. Doses of 50 to 400 mg diethanolamine
salt were administered (i.e. 15-120 mg maleic hydrazide). At the
400 mg dose level, 2/10 rats died after 10 days. Gross pathological
examination showed haemorrhagic lungs, and in one case, a yellowish
exudate and thoracic adhesions, laboured respiration (7/10) and at
2 weeks, rats (5/7) were also observed at the 400 mg level. (Food
Research Laboratories, 1955).
Special studies on potentiation
The acute toxicity of maleic hydrazide diethanolamine salt in
combination with other pesticides was determined. Pretreatment with
0.1 LD50 potentiated the toxicity of dieldrin and DDT in both
sexes, but decreased the toxicity of diazinon. On the other hand
pretreatment with DDT and dieldrin reduced the toxicity of maleic
hydrazide diethanolamine salt by a factor of two. Diazinon
pretreatment had a different effect, decrease of toxicity in
females, increase in males (Luckens and Wattimena, 1968).
Acute studies
Species Route LD50 References
mg/kg b.w*
Diethanolamine salt Food Research
Rat (fasted) oral 1180 Labs. 1955
Sodium salt
Rat (fasted) oral 5800 ibid
* expressed in terms of maleic hydrazide moiety.
Short term studies
Rat
Groups of 12 male and 12 female rats were fed 0, 0.5, 1.0, 2.0
or 5.0% maleic hydrazide, as the sodium salt, for 12 weeks.
Detailed data are not available. Mean body weight data indicate
that in males, a slight reduction occurs in all groups on maleic
hydrazide and a similar reduction occurs in females at 5.0% dietary
levels. The reductions are stated to be statistically
non-significant. Food utilization, hemoglobin, erythrocyte count,
total and differential leucocyte counts, were comparable in all
groups. Blood sugar was reduced at 5.0%, and non-protein nitrogen
was increased at the same level. Methaemoglobin levels were (if
present) below the limit of detection (0.2g/100 ml) and urinalysis
for sugar, albumin, and microscopic inclusions was unremarkable.
Gross pathology (on 2 males and 2 females at 12 weeks) was normal.
No histological abnormalities were noted. (Food Research
Laboratories, 1955).
A group of 12 male and 12 female rats was fed 1.0% maleic
hydrazide as the diethanolamine salt. Additional, non-contemporary
groups of 12 male and 12 female rats were fed 0.1% or 0% maleic
hydrazide as the diethanolamine salt and a final group of 10 males
and 10 females was fed 1.0% diethanolamine. All rats were on test
for 12 weeks. Body weight was significantly reduced in all test
groups. Mortality was increased markedly in groups receiving 1.0%
diethanolamine, and 1.0% diethanolamine salt of maleic hydrazide.
No further data are available for the group receiving 1.0%
diethanolamine. Food efficiency is stated to be comparable in all
groups except that receiving 1.0% diethanolamine salt of maleic,
hydrazide which is markedly reduced. Haemoglobin and erythrocyte
count are markedly reduced and an increased polymorph/lymphocyte
ratio are noted in the group receiving 1.0% diethanolamine salt of
maleic hydrazide. Mean data on the 0.1% diethanolamine maleic
hydrazide indicate a tendency to reduced haemoglobin levels and
erythrocyte counts, the statistical significance being
non-assessible on the basis of available data. At the 1.0%
diethanolamine maleic hydrazide level, death was preceded by loss
of coordination and muscular control, accompanied by starvation.
Pathological examination showed hyperaemic or haemorrhagic lungs,
and evidence of anaemia. Brain histopathology showed brain oedema.
No microscopic lesions were observed in liver, kidney or lung of
four representative rats of the group. (Food Research Laboratories,
1955).
Dog
Three dogs were given 1 g/kg of the sodium salt of maleic
hydrazide five times weekly for five weeks by gavage. At 5 day
intervals, haemoglobin, erythrocyte count and total and
differential leucocyte counts were determined. Methaemoglobin,
blood sugar and non-protein nitrogen were determined at sacrifice.
Liver, kidney, spleen and bone marrow were examined
histopathologically. No data are presented, but effects on the
parameters listed above are stated to be negligible although
transient eosinophilia, anemia, and leucocytosis were noted
(Food Research Labs, 1955).
Five groups of three mongrel dogs aged between four months and
2 years were fed 0 (1 male, 2 female), 0.5 (2 male, 1 female), 1.0
(1 male, 2 female), 2.0 (2 male, 1 female) % maleic hydrazide as
the sodium salt, or 1.0 (2 male, 1 female)% maleic hydrazide as the
diethanolamine salt in the diet for one year. Body weight and
mortality were unaffected by the sodium salt. The diethanolamine
salt caused loss of body weight, and 2/3 deaths by day 30 on test.
This group was abandoned at day 37 on test. Food intake was
unaffected in groups receiving the sodium salt. Haemoglobin,
erythrocyte count, total and differential leucocyte counts were
comparable in all groups. Blood sugar and non-protein nitrogen did
not show any consistent effects. Gross pathology was comparable in
all dogs. Histopathology on the bone marrow, liver kidney, spleen
and gastrointestinal tract was considered to be within normal
limits. Brains of dogs receiving the diethanolamine salt were
moderately oedematous, and the spinal cord showed early neuronal
degeneration and swelling of myelin sheath. (Food Research Labs.
1955).
Long-term studies
Rat
Five groups of approximately 10 male and 10 female rats were
fed 0, 0.5, 1.0, 2.0, or 5.0% maleic hydrazide as the sodium salt,
for 2 years. A further two noncontemporary groups were fed 0 or
0.1% maleic hydrazide as the diethanolamine salt, also for 2 years.
After 12 weeks on test, rats were bred one to one, within groups,
following a 2 week mating, 3 week gestation, 3 week lactation, 1
week rest cycle throughout the study. (The report states 1 week
rest, or 2 week rest after lactation, in different sections). Body
weight of males in all test groups was slightly reduced up to 1
year, but exceeded controls at 2 years. In females, body weight was
reduced at 5.0% Na salt at 12 weeks only. Mortality was generally
comparable between groups, except for the 0.1% diethanolamine salt
group, where life span was reduced, mortality being increased
between 52 and 96 weeks on test. Haemoglobin and erythrocyte counts
were reduced in the group receiving 0.1% diethanolamine salt, at
all reported time intervals (52,78 and 104 wks). In all other
groups, values were comparable. Total and differential leucocyte
counts were comparable in all groups. Blood glucose and non protein
nitrogen were comparable in all groups receiving the sodium salt at
12 and 24 months. Non protein nitrogen was slightly elevated in the
only measurement taken (24 months) on the sole male and sole female
survivor of the 0.1% diethanolamine salt group. Methaemoglobin was
not detected in any group. Urinalysis was stated to be normal in
all groups. No consistent changes occurred in liver, kidney or
spleen organ/body weight ratios. Tumor incidence was comparable in
all groups. Pathological changes are stated to be attributable to
infections, parasites, or age. (Food Research Laboratories, 1955).
COMMENTS
Maleic hydrazide was evaluated by the IARC in 1974 and it was
concluded that "no carcinogenic effect was observed in adult mice
and rats following oral or subcutaneous administration of maleic
hydrazide. The significance of hepatomas obtained in new born mice
cannot be assessed because of the contamination of maleic anhydride
with hydrazine". A mammalian dominant lethal study was negative.
Reproduction studies indicate an absence of adverse effects of
the sodium salt of maleic hydrazide at 2% of the diet. At 5%, post
natal weight gain in pups is reduced. The diethanolamine salt of
maleic hydrazide, even at 0.1% in the diet, reduces fertility,
litter size, viability index and lactation index. Short term
studies indicate that effects on body weight, and mortality are due
to the diethanolamine moiety. It seems probable that the effects on
reproduction can also be attributed to the same cause although
there are no data available to support this hypothesis. Teratogenic
studies have not been conducted.
A no-effect level was not demonstrated for the diethanolamine
salt in respect to reproduction, short term or long term feeding
studies. Concern was expressed as to the possible use of the
diethanolamine salt of maleic hydrazide without complete
toxicological data being available on the diethanolamine salt. Data
are available on the sodium salt with regard to reproduction in
rat, short term studies in rat and dog and long term studies on
small numbers of rats. The no-effect level in these studies would
appear to be 2% in the diet.
No information was available on the occurrence of hydrazine as
a residue in crops or on the potential for its formation under use
conditions. It was noted that maleic hydrazide and its
ß-D-glucoside were the principal residues found on plants. Data on
the metabolic fate of the ß-D-glucoside in mammals are not
available. Since the carcinogenicity potential cannot be assessed
adequately and the long term rat feeding study is on small numbers
of animals, no toxicological evaluation has been attempted for the
sodium salt of maleic hydrazide. Data are totally inadequate for
toxicological evaluation of the diethanolamine salt.
TOXICOLOGICAL EVALUATION
No acceptable daily intake was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Maleic hydrazide is used as a sprout inhibitor on potatoes at
2-3 kg/ha with a pre-harvest interval (PHI) of 4-6 weeks and on
onions at 2-2.5 kg,/ha with a PHI of 2-4 weeks.
The compound is also used on potatoes, about 10 days before
planting, on young non-bearing Citrus trees, to induce dormancy and
on tobacco for sucker control when in full flower (3 kg a.i./ha).
It is further used on a limited scale as a growth inhibitor on
grass on road verges.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive residue data are available from supervised trials on
onions and potatoes and are summarized in Table 1. Some
supplementary results, together with the limited data available for
apples, carrots and tobacco, are discussed below.
Apples. Pre-harvest spraying of apples with 1000 and 2500 mg/kg
a.i. resulted in residues in the total apple of 1.5 and 3.4 mg/kg
respectively. A considerable part of the residue remained in the
peel: residue levels found in the peel were 4.1 and 10.0 mg/kg
respectively (Hoffman and Carson 1962).
Carrots. Samples taken from plots sprayed at different times
before harvest showed residues of 5.1 mg/kg after a spray
application shortly before harvest and much lower residues, 0.36
mg/kg, when sprayed three weeks earlier (Hoffman and Carson 1962).
Onions. In supervised trials in Poland carried out by Drygus et
al (1968), onions were treated 21 weeks before harvest with maleic
hydrazide at dosages of 12-2.3 kg/ha. The residues after storage
periods of 7-32 weeks ranged from 2.5 to 23 mg/kg. (Table 1.) The
highest residues were found after a storage period of 28 weeks
(i.e. 30 weeks after application) and at the highest application
rate.
Potatoes. Foliar application about 5 weeks before harvest with
sprays containing 0.1, 0.25, 0.5 and 0.75% a.i. resulted in residue
levels in the tuber of 3.1, 15.6, 37.9 and 92.6 mg/kg respectively.
The level in the peelings tended to be higher than in the remainder
of the potato. (Hoffman and Carson 1962). Of 144 samples taken in
the USA from plots treated with maleic hydrazide at 3 kg a.i.
mainly in the period 1950-1960 8% of the samples showed residues
exceeding 30 mg/kg and about 5% contained residues exceeding 40
mg/kg (Uniroyal 1976). Drygas et al (1968) treated potatoes about
8 weeks before normal harvest with maleic, hydrazide at dosage
rates of 1.4 - 2.7 kg/ha. After storage periods of 3 - 24 weeks
residues ranged between 4 and 30 mg/kg (Table 1). The highest
values were found at the highest application rate and after a
storage period of 24 weeks.
Tobacco. After applying maleic hydrazide on tobacco at a rate of
2.25 kg a.i./ha the tobacco leaves contained 37 mg/kg (fresh
weight), whereas the green sucker leaves contained 482 mg/kg.
(Hoffman et al., 1962).
FATE OF RESIDUES
In soil
Mobility. Helweg-Andersen (1971) showed that maleic hydrazide and
its degradation products are only moderately mobile in typical soils.
After application of 5 and 10.1 kg/ha all the maleic hydrazide was
found in the top 10 cm of the soil from 0 to 129 days (the latest
interval checked) under danish field conditions.
Uniroyal (1973-75), in an outdoor test on sandy loam 14C-MH
found only 2.5 - 3.1% of the applied 14C below a depth of 15 cm after
4 - 6´ months.
The diethanolamine salt of maleic hydrazide is rather mobile in
the soil. An aqueous solution of the salt put on top of a soil column
of 1 m was distributed through the whole column within 24 hours. The
initial amount of water was chosen in such way that no water flowed
from the column. Subsequent additional watering removed a considerable
amount of maleic hydrazide from the column (Levi and Crafts 1952).
Persistence. Uniroyal (1973-1975) monitored soil from plots treated
with maleic hydrazide under field conditions. A plot treated for four
consecutive years with 3.4 kg a.i./ha and sampled 10 months after the
last application showed no detectable residues. At another site, soil
treated at the same rate and sampled 4 months after treatment again
contained no detectable residues (lower limit of detection 0.5 mg/kg).
Since the analytical technique used in this case involved caustic
TABLE 1. Residues of maleic hydrazide resulting from supervised trials
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 2 2¨ 2.5 2 3/4 3 4-4.5 7 Ref.
months months months months months months months
onions U.S.A. 1951 1 1 wp 40% 1.7 1
1.5
1951 1 2 wp 40% 7.3 9.8 4.2 7 2.8 1
12.5 11.5 (2.7
11.7) 5.7
3.9
1 2.4 2.7 1
1952 1 2.0 wp 40% 2.5 1
1952 1 2.5 wp 40% 10.0 1
1952 1 3.2 wp 40% 3.0 2.2 1
2.3
Netherlands 1955 1 2.5 liquid 2 2
40% (1.2
4)
1 2.5 liquid 8.1 2
40% (n.d.
12.1)
1.5
(n.d.- 2
1.5
2
days
1964 1 1.8 liquid 7.4 3
30% (6.6
9.0)
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation Ref.
Onions Netherlands 1 2.5 liquid 13.0 9 14 35 44
30% (10.8- days days days days 3
14.2)
U.S.A. 1951- 1 2.3 2.7- 2.3- 3.1 2.5 4
2 11.7 14.5
9 16 23 30 34
weeks weeks weeks weeks weeks
Poland 1968 1 1.2 EC 2.5 6 3 8 4 5
1 1.7 EC 3 4 5 13 13 5
1 2.25 EC 6 8 6 14,23 13 5
Potatoes U.S.A. 1951 1 1.5 wp 40% 1.7 3.3, 3.4 1
4.2 2.0
1951 1 1.8 wp 40% 2.9 1
(1.1-4.
5)
Potatoes U.S.A. 1951 1 2 wp 40% 10.8 3.0, 1
9.8 4.8
1951 1 3 wp 40% 2.7 1
8.7
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 1.5-2 3 - 4 4 - 5 6 - 7 7 - 8 9 -12 Ref.
months months months months months months
1951 1 3 wp 40% 9.8 1.0 2.9 4.7 8.8 6.0 1
3.0 8.6 8.9 8.9 4.7
3.6 9.1 9.4
4.7 2.9
6.0 3.0
11.9
7.4
5.8
1.0
1955 1 2.8 wp 40% 11.3 16.1 10.2 1
14.7 10.8 10.4
4.7 11.1 21.9
3.7 24.2
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 1969 1 2 7.3 6.5 20 1
(4-10) (6-7) 1
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 13
(11-15)
1969 1 3 9 14 9 6 1
(9-24) (7-11)
12.5 1
(8-15) (0-2)
Whole
potato U.S.A. 1951 1 1.6. - 3 10-0- 5.5- 3.0- 10.2 4
16.1 24.2 8.6
32.5 16.3- 27.0
48.9 41.0 32.9 4
11 18 23 32
weeks weeks weeks weeks
Potatoes Poland 1968 1 1.4 EC 8 6 11 9 5
1 2.0 EC 5 4 14 15 5
1 2.7 EC 6 4 10 8,30 5
* With the exception of the data from Poland (Ref. 5), each entry in the Table refers to a separate trial.
References
1. Uniroyal 1973-1975
2. R.I.V. 1955
3. R.I.V. 1964
4. Naugatuck Chem. Comp. 1956.
5. Drygas et al. 1968.
treatment of the soil, it would measure both free and bound maleic
hydrazide owing to hydrolysis of the latter.
Data on the residue extractable with organic solvents show that
half of the applied material is lost in periods varying from less than
one to about six weeks in different soil types. Usually over 90% has
disappeared in about 2-10 weeks. When soil was analysed for
extractable residues more than 3 months after application only traces
of maleic hydrazide were detected, if any. The bound residues in the
soil are degraded less rapidly. Half of the applied maleic hydrazide
is lost over periods varying from one to 14 weeks. Helweg (1975b)
studied the effect of absorbtion on the rate of maleic hydrazide
degradation using activated carbon as a model absorbent. While
absorption caused an initial delay in 14C evolution from 3, 6 14C-MH,
after 4 months almost equal amounts of 14CO2 were evolved from
control soils and those containing activated carbon. In standard soil
types 1 and 2 as recommended for leaching experiments in the Federal
Republic of Germany (Characteristics: organic carbon 2.58 and 1.0%
hydrazide particles <20 µ 10.1 and 19.1% respectively) the initial
residues of 30.5 and 36.1 mg/kg decreased in 8 weeks to 0.76 mg/kg
(2.5%) and 0.44 mg/kg (1.2%) (BASF 1975). In sterile soil the residue
hardly decreased in 6 weeks; in non-sterile soil of the same type an
initial residue of 100 mg/kg decreased in 3 weeks to 5 mg/kg, mainly
as a result of microbial degradation.
Biodegradation. Helweg (1975a , 1975b) found CO2 to be the main
degradation product of maleic hydrazide. From a sandy loam soil
containing 20 mg/kg, kept under laboratory conditions, about 50% of
the 14C was liberated as CO2 within two weeks.
Uniroyal (1973-1975) treated two soil types with 3,6-14C at a
rate of 55 mg/kg (an excessive dosage compared with normal practice).
The 14C release reached 34% of the 14C applied in Connecticut sandy
loam and 57% in Mississippi silt loam after two weeks.
Kaufman and Kalayanova 1975 studied the degradation of MH-14C in
two soils under laboratory conditions, with the results shown in Table
2.
TABLE 2. Laboratory aerobic metabolism of maleic hydrazide.
Cumulative 14CO2 evolved in 23 days
% of applied 14C evolved
Soil rate MH-3,6-14C MH-4,5-14C
kg/ha
Sandy loam 0.56 67.8 43.5
5.6 57.0 40.5
Silty clay 0.56 47.8 17.7
5.6 48.0 18.3
Methanol extraction of the MH-14C treated soil after 29 days
removed only 1-3% of the applied 14C. In addition to maleic
hydrazide, maleimide was identified as a degradation product, showing
the likelihood of an early cleavage of the N-N bond in the degradation
process. No hydrazine formation could be detected in the extracts from
these soils, nor was hydrazine evolved when
p-dimethylaminobenzaldehyde-sulphuric acid solutions were substituted
for CO2 trapping solutions in the Warburg flask. Some of the 14C not
extracted from the soil by methanol could be removed by aqueous
alkali. The humin, and to a lesser extent the humic- and fulvic acid
fractions contained 14C. The authors suggest that the radioactivity
in these fractions could be present as sorbed unchanged maleic
hydrazide, and/or as degradation products incorporated into the
natural organic matter of the soil. There is some evidence that both
sorption and chemical reaction occur. Uniroyal (1973-1975) found that
aqueous base can release unchanged MH-14C from soil-bound residues
not extracted by milder procedures. Helweg, (1975a) found some
evidence for the incorporation of14C into an amino acid fraction of
the soil. Since the microbial degradation of maleic hydrazide produces
CO2 it is very likely that CO2 originating from the degradation of
maleic hydrazide will be incorporated into natural products. It is
evident that CO2 in the major metabolite in maleic hydrazide
degradation, and small amounts of maleimide and natural products have
been detected as intermediate steps in the metabolic pathway. However
the small amounts of 14C products extracted from soil make it
difficult to propose an overall metabolic pathway. On the basis of
some similarity with experiments in which they used Fenton's reagent
as a model of a free radical generating oxidation system, Kaufman and
Kalayanova (1975) tentatively suggest the degradation pathway of
maleic hydrazide in soil shown in Figure 1.
Stoessl 1964 has reported similar products (fumaric, maleic,
succinic, formic and nitric acids) from the photo oxidation of maleic
hydrazide in dilute aqueous solutions in the presence of oxygen. Other
authors e.g. Andreae (1955), Winder and Denneny (1959) and
Povolotskaya (1961) confirm the photo-oxidation reactions of maleic
hydrazide.
Frear (1975) showed that bound residues of 14C-MH in tobacco
root were degraded to 14CO2 and released. About 18% of the 14C-MH
and 3,6-14MH and 0.5% of the 4,5-14C was released as 14CO2 from
tobacco root tissue during 43 days incubation in soil owing to
microbial activity.
In subsequent work it was shown that the bound residue remaining
in tobacco roots after methanol extraction, was partially released by
treatment with aqueous ammonia at 80°C for four days. The 14C
extracted in this way was shown to be unchanged maleic hydrazide.
Noodén (1970) found that the bound maleic hydrazide residue in other
plants consists mainly of unchanged maleic hydrazide.
In plants
Uptake from the soil. In Canada, sandy loam soil was treated with
0.2, 0.5, 1.0 and 5.0 mg/kg maleic hydrazide. Tobacco seedlings were
planted in pots containing the treated soil and grown under glass.
After 8 weeks 10% of the original amount added to the soil remained.
No maleic hydrazide could be detected in the green leaves of the
tobacco plant except in the plants grown on soil treated with the
highest dosage of 5 mg/k The average residue in the leaves of these
was 0.9 mg/kg (Hoffman et al, 1962).
Haeberer et al (1974) showed that even when tobacco was planted
in soil immediately after the application of maleic hydrazide at an
exaggerated rate, no residues were found in the tobacco at harvest.
From these experiments it may be concluded that maleic hydrazide will
not be transferred to next years crop.
Fate in the plant. Frear and Swanson (1975) studied the uptake and
fate of 14C-MH in two flue-cured and two Burley tobacco varieties
grown under glasshouse conditions. It was shown that the
foliar-absorbed maleic hydrazide moves rapidly, both acropetally and
basipetally to actively growing tissues in the tobacco plant including
the roots. Most of the foliar-absorbed maleic hydrazide is transported
to the roots and excreted into the external medium, but a significant
proportion remains in the roots and other tissues as a
methanol-insoluble residue, which was shown to consist largely of
unchanged metabolite of maleic hydrazide. In tobacco the methanol-
soluble metabolite of maleic hydrazide is the ß-D-glucoside of the
phenolic tautomer, 6 hydroxy-3-(2H)-pyridazone.
Towers et al (1958) also found this ß-D-glucoside in tobacco
leaves. They reported that 15% of maleic hydrazide in tobacco leaves.
They reported that 15% of maleic hydrazide was transformed into ito
its ß-D-glucoside when applied to leaf segments. This metabolite was
also seen in apple and willow.
Callaghan (1961) reported the incorporation of maleic hydrazide
into heterochromatin of root-tip colls of Allium cernuum, Vicia
faba and Tradescantia palludosa.
Biswas et al (1967) isolated two unknown transformation products
from tea plants grown in nutrient solution containing 14C-MH. They
speculate on various modes of ring opening, but did not provide
experimental evidence of it.
Noodén (1970) concluded from studies on the uptake of 14C-MH by
roots that maleic hydrazide is bound to cell wall fragments as a
stable complex which is insoluble in 80% ethanol. The bound maleic
hydrazide could be released by heating the tissue with aminoethanol.
Chromatography of the 14C-material released in this way showed that
there was no indication of degradation and the maleic hydrazide was
bound to the cell-walls in an unchanged form.
Other information on identity and properties
Composition of the technical product
The technical material contains 99% of maleic hydrazide;
impurities are inorganic salts (e.g. Na2SO4 possibly with minor
amounts of maleic or fumaric acid.
Physical and chemical properties of maleic hydrazide
physical form white crystalline powder
molecular weight 112.1
specific gravity at 25°C 1.60
melting point 292°C(min)
odour faint
volatility non volatile
solubility (approximate) g/100 g solvent at 25°C
distilled water 0.6
alcohol 0.1
acetone 0.1
dimethyl formamide 2.4
xylene less than 0.1
Ph of 0.5% aqueous solution at 25°C: 4.
Maleic hydrazide behaves as a weak monobasic acid. The
alkanolamine and alkali metal salts are moderately soluble in
water.
Formulations used: liquid 30% and 40% a.i.; wettable powder 40%.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Maleic hydrazide does not appear to be extensively metabolized
by mammals. In the rabbit, 43-62% of a single oral dose was
excreted unchanged within 48 hrs. Isolation and characterization of
the excreted maleic hydrazide following oral doses of 2 g showed
the excreted product to be the benzylamine salt. No other
metabolites were identified. (Barnes et al, 1957). Incubation of
phenobarbital-induced rat liver microsomes with maleic hydrazide
did not result in any degradation of the compound (Nelson and
Kearney, 1975).
Using maleic hydrazide - 14C labelled material, female rats
were dosed with 0.27, 0.68, or 2.72 mg per rat (equivalent to 10,
25 or 100 µc/rat) by stomach tube. At the low dose level, 65.4% of
the radioactivity was excreted in the urine in 12 hrs and 77.3%
within 6 days.
A further 12.4% of the administered radioactivity was detected
in the faeces over the same period. Rats dosed with 2.5 µc showed
0.12-0.18% expired radioactivity in CO2 over the 72 hour period
after administration. Tissue levels of radioactivity were
statistically insignificant except in the carcass, where <0.001%
of the administered radioactivity was detected. Using paper
chromatography with two different solvent systems, urine from rats
intubated with 100 µc resulted in the identification of unchanged
maleic hydrazide (92-94%) and a maleic hydrazide conjugate (6-8%).
(Mays et al, 1968).
Sixteen rats were given 4g/kg by stomach tube. Urine and
faeces were collected for time intervals 0 to 2, 2 to 4, 4 to 8,
and 8 to 16 days. Two rats/sex were sacrificed after 2, 4, 8, or 16
days post-dosing. In male rats, residues in urine (30,400 mg/kg)
were approximately double those in faeces (17,400 mg/kg) over the
0-2 day period, whereas in females urine residues (1,330 mg/kg)
were much less than in faeces (12,700 mg/kg). In both sexes,
residue levels decreased rapidly, but were still detectable in 8-16
day samples of both urine and faeces. Excretion in females was
slightly slower than in males, however residues were undetectable,
except for traces in the males sacrificed on day 8. (Food Research
Laboratories Inc. 1955).
TOXICOLOGICAL STUDIES
Special Studies on reproduction
Seven groups of approximately 10 male and 10 female rats fed
0, 0.5, 1.0, 2.0 or 5.0 maleic hydrazide as the sodium salt, or 0
or 0.1% maleic hydrazide as the diethanolamine salt were repeatedly
bred using a cycle of 2 weeks pairing, 3 weeks gestation, 3 weeks
lactation and one or two weeks rest prior to remating, for as long
as possible, commencing at 12 weeks on test. The second litters
(F1b) were used to produce the F2 generation which were mated
twice. The F2b generation provided parents for the F3a and b
offspring. Pooled data from the 8 matings of the Fo generation do
not indicate any effects of the sodium salt of maleic hydrazide on
fertility, litter size, gestation index, viability index, or
lactation index. Pup weight at weaning was slightly reduced at the
5.0% level. The diethanolamine salt at the 0.1% level resulted in
reduced fertility, reduced litter size, reduced viability index,
and reduced lactation index. The diethanolamine salt group was
dropped from the study, no attempt being made to produce the F2
generation. Mean data for all generations of rats receiving the
sodium salt do not do not indicate any effects on reproduction,
with respect to fertility, gestation index, viability index, or
lactation index. At the 5.0% level, litter size was reduced in the
F3 generation and weanling weight was reduced in all generations.
(Food Research Laboratories, 1955).
Special studies on mutagenicity
Injection of 0.7% sodium chloride Ringer's solution containing
0.4% maleic hydrazide abdominally in one to two-day old
Drosophila melanogaster males did not result in an increase in
mutation rate. On the other hand, D. melanogaster males fed on
media containing 0.4% maleic hydrazide showed an increased
incidence of lethal mutations in the first brood, although in
subsequent broods, the effect was not apparent (Nasrat, 1965).
In a mouse dominant lethal study, 500 mg maleic hydrazide/kg
did not affect the calculated mutation index. (Epstein & Shafner,
1968).
Andersen et al (1972) tested maleic hydrazide and other
chemicals on eight mutant strains of Salmonella typhimurium on
T4 bacteriophage, and on bacteriophage AP72. In none of these ten
systems was there any ~evidence of mutagenic activity. Maleic
hydrazide was tested by McCann et al (1975, 1976) on several tester
strains of Salmonella. Tests covered a wide dose range, both with
and without liver microsome activation. There was no evidence of
mutagenic activity in this extensive series of tests.
Special studies on carcinogenicity
Swiss mice (ICR/Ha) were injected sub-cutaneously with aqueous
solutions, or tricaprylin suspensions of maleic hydrazide as free
acid (0.4% hydrazine impurity), or with solvent alone in volumes of
0.1, 0.1, 0.2 and 0.2 ml on post-natal days 1, 7, 14 and 21. Total
doses were 3 mg for aqueous solution, and 55 mg for tricaprylin
suspension. (A further group comprising 11 mice all died following
10 mg maleic hydrazide injected on post-natal day 1). Preweaning
mortality was 14% in controls, 5% in mice receiving 3 mg and 53% in
mice receiving 55 mg. Post weaning mortality in males in all groups
was high being 38, 57, and 52% at 0,3 & 55 mg respectively by 49
weeks of age. Decreased weight gain (ca 5%) was noted in the high
dose group. Hepatoma incidence in males at 49 Weeks was 8% in
controls, 18% in the 3 mg group and 73% in the 55 mg group. No
metastases were noted. (Epstein et al, 1967).
Maleic hydrazide was administered by stomach tube in daily
doses of 1000 mg/kg weight to 36 mice of each sex for 3 weeks,
beginning when the animals were 7 days old. Then, 3000 ppm were
mixed directly with the diet, which was fed for approximately 18
months. No significant increase in the incidence of tumors was
observed in comparison with untreated controls (Innes et al.,
1969).
Rats were injected subcutaneously with 1 ml of the
diethanolamine salt (i.e. 5 mg) weekly for 14 months. Of 52 rats so
treated in two experiments, 3 developed sarcomas. No sarcomas
occurred in saline control rats. (Barnes et al, 1957).
Groups of rats and mice were either injected with the
monosodium salt, subcutaneously, once weekly at a dose level of 500
mg,/kg or received 1% maleic hydrazide added to the diet for 100
weeks. Maleic hydrazide did not affect growth or general health in
either species, on either dose regime. One rat receiving maleic
hydrazide by injection (out of a total of 29) developed a sarcoma.
No sarcomas were observed in control rats. Total tumor incidence
was increased in female test groups of both species, fed maleic
hydrazide, the effect being more obvious in the mouse (Barnes et al
1957).
Groups of 25 male and 25 female rats were injected twice
weekly for 65 weeks with arachis oil, water, solvents plus 2 mg
maleic hydrazide or solvents plus 2 mg diethanolamine salt of
maleic hydrazide. A further 39-week observation preceded autopsy.
subcutaneous tumor incidence was increased (4) in the group
receiving arachis oil plus maleic hydrazide when compared with the
arachis oil control group (1). Incidences of tumors in all other
groups were comparable. The increased subcutaneous tumor rate was
attributed to impaired connective tissue repair mechanisms rather
than chemical carcinogenesis induction. (Hunter et al, 1973).
Three groups of 24 male rats were fed basal diet, basal diet
plus 2% maleic hydrazide as the sodium salt, or basal diet plus
0.06% p-dimethylaminoazobenzene for up to 26 weeks. No significant
changes with regard to body weight, desoxyribose nucleic acid
content per liver cell nucleus, average liver cell size, liver
weight, number of cells/liver or DNA/liver were observed with
maleic hydrazide, although the p-dimethylaminoazobenzene affected
all these parameters. Pathological examination of animals fed
maleic hydrazide did not reveal neoplasms, although these occurred
in all rats fed the p-dimethylaminoazobene for more than 10 weeks.
(Mannell & Grice, 1957).
Special studies on mammalian cells in vitro
The effect on mammalian cells was examined by studying the
development of mitosis in fragments of mouse ear epidermis. In
concentrations from 0.0001-0.001 M, maleic hydrazide had no effects
on mitosis or cell division. Maleic hydrazide was also tested upon
skin from the guinea pig's ear, grown in tissue culture. Up to a
concentration of 0.01 M no gross or microscopical effects upon the
explants were noted. The same concentration of maleic hydrazide was
also without effect upon the respiration of skin cells, studied 2
and 22 hr after adding the maleic hydrazide (Barnes et al., 1957).
In vitro studies with mouse thymus cells in culture showed
that maleic hydrazide in concentrations of 0.001 M or more
inhibited growth. Mitotic inhibition occurred at and above 0.0001
M. The ratio of dry mass to DNA content was increased, which was
taken as an indication for inhibition of DNA, but not of protein
synthesis (McCarthy and Epstein, 1968). Mitotic inhibition was also
shown in cultures of human lymphocytes grown in the presence of
0.001-0.01 M. Inhibition was more marked in 72 hr-old cultures than
in fresh ones, probably because of the more active mitotic state in
the former (Timson, 1968).
Special studies on dermal irritation
The diethanolamine salt of maleic hydrazide was applied as a
20% aqueous solution to approximately 10 sq.cm. of abraded, and 10
sq. cm. of intact skin on the backs of 6 rabbits. Repeated
applications were made during a 6-hour period on five consecutive
days on two occasions, separated by a ten-day rest period. No signs
of irritation were noted during the study. (Food Research
Laboratories, 1955).
Special studies on dermal sensitization
Ten guinea pigs were injected intradermally with one dose of
0.5 ml, and 9 subsequent doses of 0.1 ml of a 0.1% aqueous solution
of the diethanolamine salt of maleic hydrazide, dosing being on
alternate days. Two weeks after the last dose, a challenge dose of
0.5 ml was administered intradermally at a different site. No
evidence of sensitization was observed. (Food Research
Laboratories, 1955).
Special studies on eye irritation
Six rabbits received two drops of a 5% solution of the
diethanolamine salt of maleic hydrazide in 5% saline in the right
eye. No signs of irritation were observed in the 14 day
post-treatment observation period. (Food Research Laboratories,
1955).
Special studies on respiratory effects
Deeply anaesthetized rats (ethyl ether) were administered the
diethanolamine salt of maleic hydrazide at various solution
concentrations by instilling small droplets in alternate nostrils
at the moment of inhalation. Doses of 50 to 400 mg diethanolamine
salt were administered (i.e. 15-120 mg maleic hydrazide). At the
400 mg dose level, 2/10 rats died after 10 days. Gross pathological
examination showed haemorrhagic lungs, and in one case, a yellowish
exudate and thoracic adhesions, laboured respiration (7/10) and at
2 weeks, rats (5/7) were also observed at the 400 mg level. (Food
Research Laboratories, 1955).
Special studies on potentiation
The acute toxicity of maleic hydrazide diethanolamine salt in
combination with other pesticides was determined. Pretreatment with
0.1 LD50 potentiated the toxicity of dieldrin and DDT in both
sexes, but decreased the toxicity of diazinon. On the other hand
pretreatment with DDT and dieldrin reduced the toxicity of maleic
hydrazide diethanolamine salt by a factor of two. Diazinon
pretreatment had a different effect, decrease of toxicity in
females, increase in males (Luckens and Wattimena, 1968).
Acute studies
Species Route LD50 References
mg/kg b.w*
Diethanolamine salt Food Research
Rat (fasted) oral 1180 Labs. 1955
Sodium salt
Rat (fasted) oral 5800 ibid
* expressed in terms of maleic hydrazide moiety.
Short term studies
Rat
Groups of 12 male and 12 female rats were fed 0, 0.5, 1.0, 2.0
or 5.0% maleic hydrazide, as the sodium salt, for 12 weeks.
Detailed data are not available. Mean body weight data indicate
that in males, a slight reduction occurs in all groups on maleic
hydrazide and a similar reduction occurs in females at 5.0% dietary
levels. The reductions are stated to be statistically
non-significant. Food utilization, hemoglobin, erythrocyte count,
total and differential leucocyte counts, were comparable in all
groups. Blood sugar was reduced at 5.0%, and non-protein nitrogen
was increased at the same level. Methaemoglobin levels were (if
present) below the limit of detection (0.2g/100 ml) and urinalysis
for sugar, albumin, and microscopic inclusions was unremarkable.
Gross pathology (on 2 males and 2 females at 12 weeks) was normal.
No histological abnormalities were noted. (Food Research
Laboratories, 1955).
A group of 12 male and 12 female rats was fed 1.0% maleic
hydrazide as the diethanolamine salt. Additional, non-contemporary
groups of 12 male and 12 female rats were fed 0.1% or 0% maleic
hydrazide as the diethanolamine salt and a final group of 10 males
and 10 females was fed 1.0% diethanolamine. All rats were on test
for 12 weeks. Body weight was significantly reduced in all test
groups. Mortality was increased markedly in groups receiving 1.0%
diethanolamine, and 1.0% diethanolamine salt of maleic hydrazide.
No further data are available for the group receiving 1.0%
diethanolamine. Food efficiency is stated to be comparable in all
groups except that receiving 1.0% diethanolamine salt of maleic,
hydrazide which is markedly reduced. Haemoglobin and erythrocyte
count are markedly reduced and an increased polymorph/lymphocyte
ratio are noted in the group receiving 1.0% diethanolamine salt of
maleic hydrazide. Mean data on the 0.1% diethanolamine maleic
hydrazide indicate a tendency to reduced haemoglobin levels and
erythrocyte counts, the statistical significance being
non-assessible on the basis of available data. At the 1.0%
diethanolamine maleic hydrazide level, death was preceded by loss
of coordination and muscular control, accompanied by starvation.
Pathological examination showed hyperaemic or haemorrhagic lungs,
and evidence of anaemia. Brain histopathology showed brain oedema.
No microscopic lesions were observed in liver, kidney or lung of
four representative rats of the group. (Food Research Laboratories,
1955).
Dog
Three dogs were given 1 g/kg of the sodium salt of maleic
hydrazide five times weekly for five weeks by gavage. At 5 day
intervals, haemoglobin, erythrocyte count and total and
differential leucocyte counts were determined. Methaemoglobin,
blood sugar and non-protein nitrogen were determined at sacrifice.
Liver, kidney, spleen and bone marrow were examined
histopathologically. No data are presented, but effects on the
parameters listed above are stated to be negligible although
transient eosinophilia, anemia, and leucocytosis were noted
(Food Research Labs, 1955).
Five groups of three mongrel dogs aged between four months and
2 years were fed 0 (1 male, 2 female), 0.5 (2 male, 1 female), 1.0
(1 male, 2 female), 2.0 (2 male, 1 female) % maleic hydrazide as
the sodium salt, or 1.0 (2 male, 1 female)% maleic hydrazide as the
diethanolamine salt in the diet for one year. Body weight and
mortality were unaffected by the sodium salt. The diethanolamine
salt caused loss of body weight, and 2/3 deaths by day 30 on test.
This group was abandoned at day 37 on test. Food intake was
unaffected in groups receiving the sodium salt. Haemoglobin,
erythrocyte count, total and differential leucocyte counts were
comparable in all groups. Blood sugar and non-protein nitrogen did
not show any consistent effects. Gross pathology was comparable in
all dogs. Histopathology on the bone marrow, liver kidney, spleen
and gastrointestinal tract was considered to be within normal
limits. Brains of dogs receiving the diethanolamine salt were
moderately oedematous, and the spinal cord showed early neuronal
degeneration and swelling of myelin sheath. (Food Research Labs.
1955).
Long-term studies
Rat
Five groups of approximately 10 male and 10 female rats were
fed 0, 0.5, 1.0, 2.0, or 5.0% maleic hydrazide as the sodium salt,
for 2 years. A further two noncontemporary groups were fed 0 or
0.1% maleic hydrazide as the diethanolamine salt, also for 2 years.
After 12 weeks on test, rats were bred one to one, within groups,
following a 2 week mating, 3 week gestation, 3 week lactation, 1
week rest cycle throughout the study. (The report states 1 week
rest, or 2 week rest after lactation, in different sections). Body
weight of males in all test groups was slightly reduced up to 1
year, but exceeded controls at 2 years. In females, body weight was
reduced at 5.0% Na salt at 12 weeks only. Mortality was generally
comparable between groups, except for the 0.1% diethanolamine salt
group, where life span was reduced, mortality being increased
between 52 and 96 weeks on test. Haemoglobin and erythrocyte counts
were reduced in the group receiving 0.1% diethanolamine salt, at
all reported time intervals (52,78 and 104 wks). In all other
groups, values were comparable. Total and differential leucocyte
counts were comparable in all groups. Blood glucose and non protein
nitrogen were comparable in all groups receiving the sodium salt at
12 and 24 months. Non protein nitrogen was slightly elevated in the
only measurement taken (24 months) on the sole male and sole female
survivor of the 0.1% diethanolamine salt group. Methaemoglobin was
not detected in any group. Urinalysis was stated to be normal in
all groups. No consistent changes occurred in liver, kidney or
spleen organ/body weight ratios. Tumor incidence was comparable in
all groups. Pathological changes are stated to be attributable to
infections, parasites, or age. (Food Research Laboratories, 1955).
COMMENTS
Maleic hydrazide was evaluated by the IARC in 1974 and it was
concluded that "no carcinogenic effect was observed in adult mice
and rats following oral or subcutaneous administration of maleic
hydrazide. The significance of hepatomas obtained in new born mice
cannot be assessed because of the contamination of maleic anhydride
with hydrazine". A mammalian dominant lethal study was negative.
Reproduction studies indicate an absence of adverse effects of
the sodium salt of maleic hydrazide at 2% of the diet. At 5%, post
natal weight gain in pups is reduced. The diethanolamine salt of
maleic hydrazide, even at 0.1% in the diet, reduces fertility,
litter size, viability index and lactation index. Short term
studies indicate that effects on body weight, and mortality are due
to the diethanolamine moiety. It seems probable that the effects on
reproduction can also be attributed to the same cause although
there are no data available to support this hypothesis. Teratogenic
studies have not been conducted.
A no-effect level was not demonstrated for the diethanolamine
salt in respect to reproduction, short term or long term feeding
studies. Concern was expressed as to the possible use of the
diethanolamine salt of maleic hydrazide without complete
toxicological data being available on the diethanolamine salt. Data
are available on the sodium salt with regard to reproduction in
rat, short term studies in rat and dog and long term studies on
small numbers of rats. The no-effect level in these studies would
appear to be 2% in the diet.
No information was available on the occurrence of hydrazine as
a residue in crops or on the potential for its formation under use
conditions. It was noted that maleic hydrazide and its
ß-D-glucoside were the principal residues found on plants. Data on
the metabolic fate of the ß-D-glucoside in mammals are not
available. Since the carcinogenicity potential cannot be assessed
adequately and the long term rat feeding study is on small numbers
of animals, no toxicological evaluation has been attempted for the
sodium salt of maleic hydrazide. Data are totally inadequate for
toxicological evaluation of the diethanolamine salt.
TOXICOLOGICAL EVALUATION
No acceptable daily intake was allocated.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Maleic hydrazide is used as a sprout inhibitor on potatoes at
2-3 kg/ha with a pre-harvest interval (PHI) of 4-6 weeks and on
onions at 2-2.5 kg,/ha with a PHI of 2-4 weeks.
The compound is also used on potatoes, about 10 days before
planting, on young non-bearing Citrus trees, to induce dormancy and
on tobacco for sucker control when in full flower (3 kg a.i./ha).
It is further used on a limited scale as a growth inhibitor on
grass on road verges.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive residue data are available from supervised trials on
onions and potatoes and are summarized in Table 1. Some
supplementary results, together with the limited data available for
apples, carrots and tobacco, are discussed below.
Apples. Pre-harvest spraying of apples with 1000 and 2500 mg/kg
a.i. resulted in residues in the total apple of 1.5 and 3.4 mg/kg
respectively. A considerable part of the residue remained in the
peel: residue levels found in the peel were 4.1 and 10.0 mg/kg
respectively (Hoffman and Carson 1962).
Carrots. Samples taken from plots sprayed at different times
before harvest showed residues of 5.1 mg/kg after a spray
application shortly before harvest and much lower residues, 0.36
mg/kg, when sprayed three weeks earlier (Hoffman and Carson 1962).
Onions. In supervised trials in Poland carried out by Drygus et
al (1968), onions were treated 21 weeks before harvest with maleic
hydrazide at dosages of 12-2.3 kg/ha. The residues after storage
periods of 7-32 weeks ranged from 2.5 to 23 mg/kg. (Table 1.) The
highest residues were found after a storage period of 28 weeks
(i.e. 30 weeks after application) and at the highest application
rate.
Potatoes. Foliar application about 5 weeks before harvest with
sprays containing 0.1, 0.25, 0.5 and 0.75% a.i. resulted in residue
levels in the tuber of 3.1, 15.6, 37.9 and 92.6 mg/kg respectively.
The level in the peelings tended to be higher than in the remainder
of the potato. (Hoffman and Carson 1962). Of 144 samples taken in
the USA from plots treated with maleic hydrazide at 3 kg a.i.
mainly in the period 1950-1960 8% of the samples showed residues
exceeding 30 mg/kg and about 5% contained residues exceeding 40
mg/kg (Uniroyal 1976). Drygas et al (1968) treated potatoes about
8 weeks before normal harvest with maleic, hydrazide at dosage
rates of 1.4 - 2.7 kg/ha. After storage periods of 3 - 24 weeks
residues ranged between 4 and 30 mg/kg (Table 1). The highest
values were found at the highest application rate and after a
storage period of 24 weeks.
Tobacco. After applying maleic hydrazide on tobacco at a rate of
2.25 kg a.i./ha the tobacco leaves contained 37 mg/kg (fresh
weight), whereas the green sucker leaves contained 482 mg/kg.
(Hoffman et al., 1962).
FATE OF RESIDUES
In soil
Mobility. Helweg-Andersen (1971) showed that maleic hydrazide and
its degradation products are only moderately mobile in typical soils.
After application of 5 and 10.1 kg/ha all the maleic hydrazide was
found in the top 10 cm of the soil from 0 to 129 days (the latest
interval checked) under danish field conditions.
Uniroyal (1973-75), in an outdoor test on sandy loam 14C-MH
found only 2.5 - 3.1% of the applied 14C below a depth of 15 cm after
4 - 6´ months.
The diethanolamine salt of maleic hydrazide is rather mobile in
the soil. An aqueous solution of the salt put on top of a soil column
of 1 m was distributed through the whole column within 24 hours. The
initial amount of water was chosen in such way that no water flowed
from the column. Subsequent additional watering removed a considerable
amount of maleic hydrazide from the column (Levi and Crafts 1952).
Persistence. Uniroyal (1973-1975) monitored soil from plots treated
with maleic hydrazide under field conditions. A plot treated for four
consecutive years with 3.4 kg a.i./ha and sampled 10 months after the
last application showed no detectable residues. At another site, soil
treated at the same rate and sampled 4 months after treatment again
contained no detectable residues (lower limit of detection 0.5 mg/kg).
Since the analytical technique used in this case involved caustic
TABLE 1. Residues of maleic hydrazide resulting from supervised trials
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 2 2¨ 2.5 2 3/4 3 4-4.5 7 Ref.
months months months months months months months
onions U.S.A. 1951 1 1 wp 40% 1.7 1
1.5
1951 1 2 wp 40% 7.3 9.8 4.2 7 2.8 1
12.5 11.5 (2.7
11.7) 5.7
3.9
1 2.4 2.7 1
1952 1 2.0 wp 40% 2.5 1
1952 1 2.5 wp 40% 10.0 1
1952 1 3.2 wp 40% 3.0 2.2 1
2.3
Netherlands 1955 1 2.5 liquid 2 2
40% (1.2
4)
1 2.5 liquid 8.1 2
40% (n.d.
12.1)
1.5
(n.d.- 2
1.5
2
days
1964 1 1.8 liquid 7.4 3
30% (6.6
9.0)
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation Ref.
Onions Netherlands 1 2.5 liquid 13.0 9 14 35 44
30% (10.8- days days days days 3
14.2)
U.S.A. 1951- 1 2.3 2.7- 2.3- 3.1 2.5 4
2 11.7 14.5
9 16 23 30 34
weeks weeks weeks weeks weeks
Poland 1968 1 1.2 EC 2.5 6 3 8 4 5
1 1.7 EC 3 4 5 13 13 5
1 2.25 EC 6 8 6 14,23 13 5
Potatoes U.S.A. 1951 1 1.5 wp 40% 1.7 3.3, 3.4 1
4.2 2.0
1951 1 1.8 wp 40% 2.9 1
(1.1-4.
5)
Potatoes U.S.A. 1951 1 2 wp 40% 10.8 3.0, 1
9.8 4.8
1951 1 3 wp 40% 2.7 1
8.7
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 1.5-2 3 - 4 4 - 5 6 - 7 7 - 8 9 -12 Ref.
months months months months months months
1951 1 3 wp 40% 9.8 1.0 2.9 4.7 8.8 6.0 1
3.0 8.6 8.9 8.9 4.7
3.6 9.1 9.4
4.7 2.9
6.0 3.0
11.9
7.4
5.8
1.0
1955 1 2.8 wp 40% 11.3 16.1 10.2 1
14.7 10.8 10.4
4.7 11.1 21.9
3.7 24.2
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 1969 1 2 7.3 6.5 20 1
(4-10) (6-7) 1
TABLE 1. (Cont'd.)
Application Residues, mean and range mg/kg*, at intervals after
application (pre-harvest- + storage period)
Crop Country Year
no kg/a.i./ha formulation 14-18 29-30 35 40-45 Ref.
days days days days
Potatoes U.S.A. 13
(11-15)
1969 1 3 9 14 9 6 1
(9-24) (7-11)
12.5 1
(8-15) (0-2)
Whole
potato U.S.A. 1951 1 1.6. - 3 10-0- 5.5- 3.0- 10.2 4
16.1 24.2 8.6
32.5 16.3- 27.0
48.9 41.0 32.9 4
11 18 23 32
weeks weeks weeks weeks
Potatoes Poland 1968 1 1.4 EC 8 6 11 9 5
1 2.0 EC 5 4 14 15 5
1 2.7 EC 6 4 10 8,30 5
* With the exception of the data from Poland (Ref. 5), each entry in the Table refers to a separate trial.
References
1. Uniroyal 1973-1975
2. R.I.V. 1955
3. R.I.V. 1964
4. Naugatuck Chem. Comp. 1956.
5. Drygas et al. 1968.
treatment of the soil, it would measure both free and bound maleic
hydrazide owing to hydrolysis of the latter.
Data on the residue extractable with organic solvents show that
half of the applied material is lost in periods varying from less than
one to about six weeks in different soil types. Usually over 90% has
disappeared in about 2-10 weeks. When soil was analysed for
extractable residues more than 3 months after application only traces
of maleic hydrazide were detected, if any. The bound residues in the
soil are degraded less rapidly. Half of the applied maleic hydrazide
is lost over periods varying from one to 14 weeks. Helweg (1975b)
studied the effect of absorbtion on the rate of maleic hydrazide
degradation using activated carbon as a model absorbent. While
absorption caused an initial delay in 14C evolution from 3, 6 14C-MH,
after 4 months almost equal amounts of 14CO2 were evolved from
control soils and those containing activated carbon. In standard soil
types 1 and 2 as recommended for leaching experiments in the Federal
Republic of Germany (Characteristics: organic carbon 2.58 and 1.0%
hydrazide particles <20 µ 10.1 and 19.1% respectively) the initial
residues of 30.5 and 36.1 mg/kg decreased in 8 weeks to 0.76 mg/kg
(2.5%) and 0.44 mg/kg (1.2%) (BASF 1975). In sterile soil the residue
hardly decreased in 6 weeks; in non-sterile soil of the same type an
initial residue of 100 mg/kg decreased in 3 weeks to 5 mg/kg, mainly
as a result of microbial degradation.
Biodegradation. Helweg (1975a , 1975b) found CO2 to be the main
degradation product of maleic hydrazide. From a sandy loam soil
containing 20 mg/kg, kept under laboratory conditions, about 50% of
the 14C was liberated as CO2 within two weeks.
Uniroyal (1973-1975) treated two soil types with 3,6-14C at a
rate of 55 mg/kg (an excessive dosage compared with normal practice).
The 14C release reached 34% of the 14C applied in Connecticut sandy
loam and 57% in Mississippi silt loam after two weeks.
Kaufman and Kalayanova 1975 studied the degradation of MH-14C in
two soils under laboratory conditions, with the results shown in Table
2.
TABLE 2. Laboratory aerobic metabolism of maleic hydrazide.
Cumulative 14CO2 evolved in 23 days
% of applied 14C evolved
Soil rate MH-3,6-14C MH-4,5-14C
kg/ha
Sandy loam 0.56 67.8 43.5
5.6 57.0 40.5
Silty clay 0.56 47.8 17.7
5.6 48.0 18.3
Methanol extraction of the MH-14C treated soil after 29 days
removed only 1-3% of the applied 14C. In addition to maleic
hydrazide, maleimide was identified as a degradation product, showing
the likelihood of an early cleavage of the N-N bond in the degradation
process. No hydrazine formation could be detected in the extracts from
these soils, nor was hydrazine evolved when
p-dimethylaminobenzaldehyde-sulphuric acid solutions were substituted
for CO2 trapping solutions in the Warburg flask. Some of the 14C not
extracted from the soil by methanol could be removed by aqueous
alkali. The humin, and to a lesser extent the humic- and fulvic acid
fractions contained 14C. The authors suggest that the radioactivity
in these fractions could be present as sorbed unchanged maleic
hydrazide, and/or as degradation products incorporated into the
natural organic matter of the soil. There is some evidence that both
sorption and chemical reaction occur. Uniroyal (1973-1975) found that
aqueous base can release unchanged MH-14C from soil-bound residues
not extracted by milder procedures. Helweg, (1975a) found some
evidence for the incorporation of14C into an amino acid fraction of
the soil. Since the microbial degradation of maleic hydrazide produces
CO2 it is very likely that CO2 originating from the degradation of
maleic hydrazide will be incorporated into natural products. It is
evident that CO2 in the major metabolite in maleic hydrazide
degradation, and small amounts of maleimide and natural products have
been detected as intermediate steps in the metabolic pathway. However
the small amounts of 14C products extracted from soil make it
difficult to propose an overall metabolic pathway. On the basis of
some similarity with experiments in which they used Fenton's reagent
as a model of a free radical generating oxidation system, Kaufman and
Kalayanova (1975) tentatively suggest the degradation pathway of
maleic hydrazide in soil shown in Figure 1.
Stoessl 1964 has reported similar products (fumaric, maleic,
succinic, formic and nitric acids) from the photo oxidation of maleic
hydrazide in dilute aqueous solutions in the presence of oxygen. Other
authors e.g. Andreae (1955), Winder and Denneny (1959) and
Povolotskaya (1961) confirm the photo-oxidation reactions of maleic
hydrazide.
Frear (1975) showed that bound residues of 14C-MH in tobacco
root were degraded to 14CO2 and released. About 18% of the 14C-MH
and 3,6-14MH and 0.5% of the 4,5-14C was released as 14CO2 from
tobacco root tissue during 43 days incubation in soil owing to
microbial activity.
In subsequent work it was shown that the bound residue remaining
in tobacco roots after methanol extraction, was partially released by
treatment with aqueous ammonia at 80°C for four days. The 14C
extracted in this way was shown to be unchanged maleic hydrazide.
Noodén (1970) found that the bound maleic hydrazide residue in other
plants consists mainly of unchanged maleic hydrazide.
In plants
Uptake from the soil. In Canada, sandy loam soil was treated with
0.2, 0.5, 1.0 and 5.0 mg/kg maleic hydrazide. Tobacco seedlings were
planted in pots containing the treated soil and grown under glass.
After 8 weeks 10% of the original amount added to the soil remained.
No maleic hydrazide could be detected in the green leaves of the
tobacco plant except in the plants grown on soil treated with the
highest dosage of 5 mg/k The average residue in the leaves of these
was 0.9 mg/kg (Hoffman et al, 1962).
Haeberer et al (1974) showed that even when tobacco was planted
in soil immediately after the application of maleic hydrazide at an
exaggerated rate, no residues were found in the tobacco at harvest.
From these experiments it may be concluded that maleic hydrazide will
not be transferred to next years crop.
Fate in the plant. Frear and Swanson (1975) studied the uptake and
fate of 14C-MH in two flue-cured and two Burley tobacco varieties
grown under glasshouse conditions. It was shown that the
foliar-absorbed maleic hydrazide moves rapidly, both acropetally and
basipetally to actively growing tissues in the tobacco plant including
the roots. Most of the foliar-absorbed maleic hydrazide is transported
to the roots and excreted into the external medium, but a significant
proportion remains in the roots and other tissues as a
methanol-insoluble residue, which was shown to consist largely of
unchanged metabolite of maleic hydrazide. In tobacco the methanol-
soluble metabolite of maleic hydrazide is the ß-D-glucoside of the
phenolic tautomer, 6 hydroxy-3-(2H)-pyridazone.
Towers et al (1958) also found this ß-D-glucoside in tobacco
leaves. They reported that 15% of maleic hydrazide in tobacco leaves.
They reported that 15% of maleic hydrazide was transformed into ito
its ß-D-glucoside when applied to leaf segments. This metabolite was
also seen in apple and willow.
Callaghan (1961) reported the incorporation of maleic hydrazide
into heterochromatin of root-tip colls of Allium cernuum, Vicia
faba and Tradescantia palludosa.
Biswas et al (1967) isolated two unknown transformation products
from tea plants grown in nutrient solution containing 14C-MH. They
speculate on various modes of ring opening, but did not provide
experimental evidence of it.
Noodén (1970) concluded from studies on the uptake of 14C-MH by
roots that maleic hydrazide is bound to cell wall fragments as a
stable complex which is insoluble in 80% ethanol. The bound maleic
hydrazide could be released by heating the tissue with aminoethanol.
Chromatography of the 14C-material released in this way showed that
there was no indication of degradation and the maleic hydrazide was
bound to the cell-walls in an unchanged form.
 From the above studies on tobacco and other plants it may be
concluded that the principal residues are unchanged maleic hydrazide
(free or bound) and its, ß-D-glucoside. Cleavage of the N-N bond and
opening of the ring structure does not appear to be a significant
metabolic pathway in tobacco or in other crops studied.
Effect of maleic hydrazide on biochemical processes in the plant.
Patterson et al (1952) showed that a foliar spray of 2500 mg/l, about
6 weeks before harvest caused a decrease in reducing and non-reducing
sugars in tubers stored seven months at 7° C. Similar though less
striking decreases were evident when tubers were stored at 13°C.
In other experiments (Gooding and Hubbard, 1956) no effect on the
accumulation of reducing sugars or sucrose was found when potatoes
were stored under cool conditions (0.5 - 4°C) following a pre-harvest
foliar application.
In storage, processing and cooking
Household cooking. Maleic hydrazide is fairly stable during
household cooking. After cooking onions for half an hour, 80% of the
original residue could still be detected. Of this about 25% remained
in the onion and about 75% was found, in the cooking water. (R.I.V.
Netherlands, 1964).
Carry-over in cigarettes and cigarette-smoke. Cigarettes made from
tobacco treated with 2.25 kg a.i./ha contained 10-30 mg/kg maleic
hydrazide. When cigarettes containing 30 mg/kg maleic hydrazide were
smoked in an automatic smoking machine 93% of the original residue was
decomposed or transferred to the side stream. In smoke from cigarettes
containing 10 mg/kg maleic hydrazide and smoked in a similar way, no
maleic hydrazide could be detected in the mainstream smoke. In a third
experiment in which 14C-MH was infused into cigarettes at a rate of
105 mg/kg, 25% of the radioactivity was found in the mainstream
(unpublished experiments of Stone (1957) referred to by Guthrie and
Bowery, 1967).
Residues in food moving in commerce
No information was available to the Meeting.
METHODS OF RESIDUE ANALYSIS
A spectrophotometric method for the determination of maleic
hydrazide residues in plant tissues was originally developed by Wood
(1953) and improved by Lane et al (1958). The sample is boiled in
caustic solution to drive off interfering volatile bases. Distillation
with zinc in a stream of nitrogen then expels hydrazine, which is
reacted in acid solution with p-dimethylamino-benzaldehyde. The yellow
reaction product is measured spectro photometrically. Although the
method is not very sensitive (limit of determination 0.5 -1 mg/kg), it
may be adaptable to regulatory purposes.
Hoffman (1961) and Hoffman et al (1962) describe further
modifications of the Wood method for the spectrophotometric
determination of maleic hydrazide residues.
Haeberer et al (1974) and Haeberer and Chortyk (1974) developed a
rapid quantitative GLC method for non-bound maleic hydrazide residues.
The plant material is extracted with dimethylformamide or directly
with N,O-bis (trimethylsilyl) acetamide. The bis-(trimethysilyl)
derivative is formed by heating for 30 min. at 100°C and measured by
flame ionization gas chromatography. Interfering plant substances are
removed by TLC on alumina with ethyl acetate as developing solvent.
The recovery of maleic hydrazide added to tobacco powder at the rate
of 0.5-10 mg/kg was 94-108%.
NATIONAL TOLERANCES
The national tolerances listed in Table 3 were reported to the
Meeting.
TABLE 3. National tolerances for maleic hydrazide reported to
the Meeting
Country Commodity Tolerance
mg/kg
Argentine potatoes 50
lettuce 0.1
Canada potatoes 50
beets, carrots, swedes
(rutabagas) 30
onions 15
Netherlands onions 15
other vegetables, fruits,
other agricultural
commodities 0*
U.S.A. potato chips 160**
potatoes 50
onions (dry bulb) 15
* The limit of determination = 1 mg/kg
** By weight in finished product.
APPRAISAL
Maleic hydrazide is used in various countries on an extensive
scale as an inhibitor of sprouting in onions. It is also used in a few
countries on potatoes for a similar purpose and on tobacco for
checking sucker growth. On both onions and potatoes the material is
applied as a pre-harvest spray, when biological processes in the
aerial parts of the crops are still very active. Maleic, hydrazide
penetrates extensively into the plant and is transported in the phloem
to actively growing tissues including the bulbs and tubers. Residues
persist in these parts sufficiently to induce dormancy and hamper
sprouting for fairly long periods.
Extensive information from various countries on residues from
supervised trials on onions and from two countries on residues in
potatoes was provided. Limited data were available on residues in
apples, carrots and tobacco, including data on residues in cigarettes
and cigarette smoke.
Considerable information was available on the metabolic pathways
of maleic hydrazide in soil and plants. There is some evidence that
the biodegradation of maleic hydrazide in the soil caused by bacteria
and other micro-organisms leads via maleimide and maleimic acid to
naturally occurring acids such as maleic and fumario, which may be
converted to lactic and succinic acids and finally to CO2. From
studies on tobacco and other plants it appears that the principal
residues in plants consist of unchanged maleic hydrazide, either free
or strongly bound to cell-wall fragments, and the B-D-glucoside of the
phenolic tautomer ß-hydrozy-3-(2H)-pyridazone. Cleavage of the N-N
bond and opening of the ring does not appear to be a significant
metabolic pathway in the crops studied.
Maleic hydrazide residues are fairly stable during cooking. After
normal household cooking of onions about 20% of the residue remained
in the onion and 60% was found in the cooking water.
It was shown that during the manufacture of potato chips, drying
and frying does not lead to any appreciable lose of maleic hydrazide.
Owing to the loss of water during the manufacturing process the
concentration of maleic hydrazide residues in the manufactured product
is higher than in the fresh potatoes.
When cigarettes made from field-treated tobacco and containing 30
and 10 mg/kg maleic hydrazide were smoked in a smoking machine, 93% of
the original residue was decomposed or transferred to the side stream
of the 30 mg/kg level and no maleic hydrazide could be detected in the
mainstream smoke at the 10 mg/kg level.
A fairly specific spectrophotometric method of analysis is
available. Although the method is not very sensitive (limit of
determination 0.5 - 1 mg/kg) it may be suitable for adaptation to
regulatory purposes.
Recently a rapid quantitative GLC method has been developed to
determine the bis(trimethylsilyl) derivative by flame ionisation gas
chromatography. It is uncertain whether the method can be adapted to
include not only the free maleic hydrazide residue but also bound
unchanged maleic hydrazide and its -D-glucoside and whether it can be
used for crops other than tobacco.
EVALUATION
As no ADI was allocated, maximum residue limits could not be
recommended. Data were sufficient to record guideline levels for
onions and potatoes.
The following guideline levels are recommended. They refer to the
sum of free and bound unchanged maleic hydrazide and its
ß-D-glucoside.
Commodity mg/kg
Potatoes 50
Onions 15
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be allocated and
maximum residue limits can be recommended).
1. The results of the carcinogenicity study with rats which is
currently in progress.
2. Teratogenicity study with the sodium salt or the free acid.
3. Further studies clarifying the situation relating to the possible
presence of hydrazine in or on crops.
4. Residue data on other crops for which usage recommendations are
made including tobacco, carrots and swedes and similar crops.
5. Data on the fate of maleic hydrazide and its metabolites in
livestock animals and residues in products of animal origin after
feeding commodities containing maleic hydrazide residues, e.g.
potatoes.
6. Data on the effect of cooking on residues in potatoes and the
effect on residues of different methods of industrial processing
in the manufacture of various potato products, e.g. potato chips,
dried potatoes and potato starch. Data on residues in the wastes
from these products intended for feeding purposes.
7. Further development of the gas-chromatographic method to make it
suitable for regulatory purposes.
8. More information on the carry-over of maleic hydrazide from raw
into cured tobacco and into cigarette smoke.
Desirable
1. Studies on the metabolism of the beta-D-glucoside of maleic
hydrazide.
REFERENCES
Anderson, K.S. et al J. Agric. Chem. 20 649-655.
1972
Andreae, W.A. The Photoinduced Oxidation of Manganous Ions. Arc.
1955 Biochem. Biophys. 55: 684-586.
Baker, J.E. A Study of the Action of Maleic Hydrazide on Processes
1961 of Tobacco and other Plants. Physiologia Plantarum
14: 76-88.
Barnes, J.M., Magee, P.N., Boyland E., Haddow, A., Passey, R.D.,
1957 Bullough, W.S., Cruickshank, C.N.D., Salaman,
M.H., and Williams, R.T. The non-toxicity of
maleic hydrazide for mammalian tissues. Nature,
180, 62-64.
BASF Unpublished report: Verhalten des
1975 Pflanzenschutzmittelwirkstoffes (Maleinhydrazid)
im Boden.
Biswas, P.H., O. Hull and B.D. Mayberry. Metabolism, of Maleic
1967 Hydrazide in Tea, Camellia sinensis.
Physiologia Plantarum 20 : 819-824.
Callaghan, J.J. and P.Grun. Incorporation of 14C labeled
1961 Maleic Hydrazide into Root-tip cells of Allium
cerassum, Vicia faba and Tradescantia
paludosa, J. Biophys. Biochem. Cytol. 10:
567-575.
Drygas, M. D., Sikorska and B. Leszczynska Zmiany Zawortosci
1968 Hydrazydu Maleinowego HM/ Ziemniakach i Cebuli w
Okresic ich Przechowywania Biul Instytutu Ochiony
Roslin 1968 207-213.
Epstein, S.S., Andrea, J., Jaffe, H., Joshi, S., Falk, H., and
1967 Mantel, N. Carcinogenicity of the herbicide,
maleic hydrazide. Nature, 215, 1388-1390.
Epstein, S.D. and Shafner, H. Chemical mutagens in the
1968 environment. Nature, 219, 385-387.
Field, R.J. and A.J. Peel. The Metabolism and Radial Movement
1971 of Growth Regulators and Herbicides in Willow
stems. New Phytol. 70: 743-749.
Food Research Laboratories Inc. Report on toxicological studies
1955 of chemical additives, 3. Maleic hydrazide
(1,2-dihydropyridazine-3,6 dione). Submitted by
Uniroyal Chemical. (Unpublished).
Frear, D.S. and H.R. Swanson. Behaviour and Fate of (14C)
1975 Maleic Hydrazide in Tobacco Plants. U.S. Dept. of
Agric., Agric. Research Service, Metabolism and
Radiation Lab., Fargo, North Dakota 58102.
Freed, V.H. and M.L. Montgomery. The Metabolism of Herbicides
1963 by Plants and Soil. Residue Reviews 3: 1-19.
Gooding, E.B.G. and A.W.Hubbard. The Effect of certain
1956 Sprout-depressant Treatments on Sugar accumulation
in stored Potatoes. J.Sci.Food Agric. 7: 574-577.
Guthrie, F.E. and T.G. Bowery. Pesticide Residues on Tobacco.
1967 Residue Reviews 19: 31-57.
Haeberer, A.F., W.S. Schlotzhauer, O.T.Chortyk. A rapid Quantitative
1974 Method for Maleic hydrazide. J. Agric. Food Chem.
22: 328-330.
Haeberer, A.F. and O.T.Chortyk. Rapid Determination, of Maleic
1974 Hydrazide in Cigarette Smoke Condensate and
Particulate Matter. J. Agric. Food Chem. 22:
1135-1137.
Helweg-Anderson, A. Persistence of Maleic Hydrazide in Soil
1971 and its Influence on CO2 liberation. Tidsskrift
for Planteavl 7: 96-113.
Hellweg-Andersen, A. Abbau des Maleinhydrazids und Einflusz auf
1971 die Respiration in der Erde. Zeitschrift f.
Planzenkultur 75: 84-89.
Helweg, A. Degradation of 14C labeled Maleic Hydrazide in
1975 Soil as Influenced by Sterilization, Concentration
and Pre-treatment. Weed Research 15: 53-58.
Helweg, A. Degradation of 14C Maleic Hydrazide in Soil as
1975 Influenced by Absorbtion on Activated Carbon. Weed
Research 15: 129-133.
Hoffman, I. Spectrophotometric Determination of Maleic Hydrazide
1961 in Tobaccos. J. Ass. Off. Agr. Chem. 44: 723-725.
Hoffman, I. and R.B. Carson. Determination and Distribution of
1962 Maleic Hydrazide in Vegetables and Fruits. J.
Ass.Off. Agr. Chem. 45: 788-789.
Hoffman,. and E.V.Parups. Mode of Action of Maleic Hydrazide
1964 in Relation to Residues in Crops and Soils.
Residue Reviews 7: 96-113.
Hoffman, I., E.V. Parups and R.B. Carson. Analysis for Maleic
1962 Hydrazide. Part I. Detection and Determination in
Dried Green Tobacco Leaves and Suckers. Part II
Determination and Persistence in Soils. J. Agr.
Food Chem. 10: 453-455.
Hunter, B., Mawdesley-Thomas, L.E. and Worden, A.N. The
administration of maleic hydrazide and its
diethanolamine salt to rats. Toxicology 1,
301-307.
Innes, J.R.M. et al Bioassay of pesticides and industrial chemicals
1969 for tumorigenicity in mice: a preliminary note. J.
Nat. Cancer Inst. 42, 1101.
Kaufman, D.D., and N. A. Kalayanova. Maleic Hydrazide Degradation in
1975 Soil. U.S.Dept. of Agriculture, Agric. Research
Service, Beltsville Agricultural Research Centre,
Beltsville, Maryland 20725, U.S.A.
Lane, J.R. Collaborative Study of Maleic Hydrazide Residue
1963 Analysis. J. Ass. Off. Agr. Chem. 46(2): 261-268,
744-748.
Lane, J.R., D.K. Gullstrom and J.E. Newell. Extension of the Residue
1958 Methods for 1,2-dihydro-3,6-pyridazinedione
(maleic hydrazide) and N-1-naphtylphtalamic acid
(Alanap). J. Agr. Food Chem. 6: 671-674.
Levi, E. and A.S. Crafts. Toxicity of maleic hydrazide in California
Soils. Hilgardia 21: 431-463.
Luckens, M.M. and Wattimena, J.R. Acute toxicity of combinations
1968 of maleic, hydrazide diethanolamine and selected
insecticides. Toxicol. Appl. Pharmacol. 12:
287-288 (Abstr.no. 7).
Mannell, W.A. and Grice H.C. A comparative study of maleic hydrazide
1957 and p-dimenthylaminoazobenzene on rat liver. Can.
J. Biochem. Physiol. 35, 1233-1240.
Mays, D.L., Born, G.S. Christian, J.E. and Liska, B.L. Fate of
1968 C14-Maleic hydrazide in rats. J. Ag. Fd. Chem.
16, 356-7.
McCann J., Choi, E., Yamanslic, E., and Ames, B.N. Proc. Natl.
1975 Academy of Science 72 (12) 5135-5139.
McCann, J., Choi, E., Yamanslic, E., and Ames, B.N.
Ibid 73 (3) 950-954.
McCarthy, R.E. and Epstein, S.S. Cytochemical and cytogenetic
1968 effects of maleic hydrazide on cultured mammali
cells. Life Sci. 7:1-6.
Nasrat, G.E. Maleic hydrazide, a chemical mutagen in
1965 Drosophila melanogaster. Nature, 207, 439.
Nelson, J.O., and Kearney, P.C. Metabolism of maleic hydrazide
1975 by hepatic microsomes from phenobarbital induced
rats. U.S.D.A. report.
Naugatuck Chem. Comp. Unpublished Data on Maleic Hydrazide
1956 summarised by the Secretary of the Scientific
Subcommittee on Poisenous Substances used in
Agriculture and Food Storage. American Residue
data of Maleic Hydrazide in Onions and Potatoes.
Noodén, L.D. Metabolism and Binding of 14C-Maleic Hydrazide.
1970 Plant Physiol. 45: 46-52.
Patterson, D.R., S.H. Wittwer et al. 1952. The effect of pre-harvest
foliar sprays of maleic hydrazide on sprout
inhibition and storage quality of potatoes. Plant
Physiol. 27: 135-142.
Povolotskaya, K.L. Mechanism of the Action of Maleic Hydrazide
1961 in Plants. Izvest. Akad. Nauk SSSR 26, Ser.
Biol.no. 2: 250-255.
R.I.V. Unpublished data of the National Institute of Public
1955. 1964. Health Utrecht/Bilthoven, The Netherlands,
provided to JMPR 1976.
Stoessl, A. Photolysis of Maleic Hydrazide. Chem. Ind.:
1964 580-581.
Timson, J. The effects of maleic hydrazide on the mitosis of
1968 phyto-hemagglutinin stimulated human lymphocytes
cultured in vitro. Caryologia 21:157 (Cited in Fd.
Cosm.Toxicol, 8: 104-105, 1970).
Towers, G.H.N., Hutchinson, A. and Andreae, W.A. Formation of a
1958 Glucoside of Maleic Hydrazide in Plants. Nature
181:1535-1536.
Uniroyal Chem. Unpublished data on Maleic Hydrazide provided
1973-1975 to JMPR 1976.
Winder, F.G. and Denneny, J.M. Metal-Catalyzed Auto, Oxidation
1959 of Isoniazid. Biochem. J. 73:500-507.
Wood, P.R. Determination of Maleic Hydrazide Residues in Plant
1953 and Animal Tissues. Anal. Chem. 25:1879-1883.
From the above studies on tobacco and other plants it may be
concluded that the principal residues are unchanged maleic hydrazide
(free or bound) and its, ß-D-glucoside. Cleavage of the N-N bond and
opening of the ring structure does not appear to be a significant
metabolic pathway in tobacco or in other crops studied.
Effect of maleic hydrazide on biochemical processes in the plant.
Patterson et al (1952) showed that a foliar spray of 2500 mg/l, about
6 weeks before harvest caused a decrease in reducing and non-reducing
sugars in tubers stored seven months at 7° C. Similar though less
striking decreases were evident when tubers were stored at 13°C.
In other experiments (Gooding and Hubbard, 1956) no effect on the
accumulation of reducing sugars or sucrose was found when potatoes
were stored under cool conditions (0.5 - 4°C) following a pre-harvest
foliar application.
In storage, processing and cooking
Household cooking. Maleic hydrazide is fairly stable during
household cooking. After cooking onions for half an hour, 80% of the
original residue could still be detected. Of this about 25% remained
in the onion and about 75% was found, in the cooking water. (R.I.V.
Netherlands, 1964).
Carry-over in cigarettes and cigarette-smoke. Cigarettes made from
tobacco treated with 2.25 kg a.i./ha contained 10-30 mg/kg maleic
hydrazide. When cigarettes containing 30 mg/kg maleic hydrazide were
smoked in an automatic smoking machine 93% of the original residue was
decomposed or transferred to the side stream. In smoke from cigarettes
containing 10 mg/kg maleic hydrazide and smoked in a similar way, no
maleic hydrazide could be detected in the mainstream smoke. In a third
experiment in which 14C-MH was infused into cigarettes at a rate of
105 mg/kg, 25% of the radioactivity was found in the mainstream
(unpublished experiments of Stone (1957) referred to by Guthrie and
Bowery, 1967).
Residues in food moving in commerce
No information was available to the Meeting.
METHODS OF RESIDUE ANALYSIS
A spectrophotometric method for the determination of maleic
hydrazide residues in plant tissues was originally developed by Wood
(1953) and improved by Lane et al (1958). The sample is boiled in
caustic solution to drive off interfering volatile bases. Distillation
with zinc in a stream of nitrogen then expels hydrazine, which is
reacted in acid solution with p-dimethylamino-benzaldehyde. The yellow
reaction product is measured spectro photometrically. Although the
method is not very sensitive (limit of determination 0.5 -1 mg/kg), it
may be adaptable to regulatory purposes.
Hoffman (1961) and Hoffman et al (1962) describe further
modifications of the Wood method for the spectrophotometric
determination of maleic hydrazide residues.
Haeberer et al (1974) and Haeberer and Chortyk (1974) developed a
rapid quantitative GLC method for non-bound maleic hydrazide residues.
The plant material is extracted with dimethylformamide or directly
with N,O-bis (trimethylsilyl) acetamide. The bis-(trimethysilyl)
derivative is formed by heating for 30 min. at 100°C and measured by
flame ionization gas chromatography. Interfering plant substances are
removed by TLC on alumina with ethyl acetate as developing solvent.
The recovery of maleic hydrazide added to tobacco powder at the rate
of 0.5-10 mg/kg was 94-108%.
NATIONAL TOLERANCES
The national tolerances listed in Table 3 were reported to the
Meeting.
TABLE 3. National tolerances for maleic hydrazide reported to
the Meeting
Country Commodity Tolerance
mg/kg
Argentine potatoes 50
lettuce 0.1
Canada potatoes 50
beets, carrots, swedes
(rutabagas) 30
onions 15
Netherlands onions 15
other vegetables, fruits,
other agricultural
commodities 0*
U.S.A. potato chips 160**
potatoes 50
onions (dry bulb) 15
* The limit of determination = 1 mg/kg
** By weight in finished product.
APPRAISAL
Maleic hydrazide is used in various countries on an extensive
scale as an inhibitor of sprouting in onions. It is also used in a few
countries on potatoes for a similar purpose and on tobacco for
checking sucker growth. On both onions and potatoes the material is
applied as a pre-harvest spray, when biological processes in the
aerial parts of the crops are still very active. Maleic, hydrazide
penetrates extensively into the plant and is transported in the phloem
to actively growing tissues including the bulbs and tubers. Residues
persist in these parts sufficiently to induce dormancy and hamper
sprouting for fairly long periods.
Extensive information from various countries on residues from
supervised trials on onions and from two countries on residues in
potatoes was provided. Limited data were available on residues in
apples, carrots and tobacco, including data on residues in cigarettes
and cigarette smoke.
Considerable information was available on the metabolic pathways
of maleic hydrazide in soil and plants. There is some evidence that
the biodegradation of maleic hydrazide in the soil caused by bacteria
and other micro-organisms leads via maleimide and maleimic acid to
naturally occurring acids such as maleic and fumario, which may be
converted to lactic and succinic acids and finally to CO2. From
studies on tobacco and other plants it appears that the principal
residues in plants consist of unchanged maleic hydrazide, either free
or strongly bound to cell-wall fragments, and the B-D-glucoside of the
phenolic tautomer ß-hydrozy-3-(2H)-pyridazone. Cleavage of the N-N
bond and opening of the ring does not appear to be a significant
metabolic pathway in the crops studied.
Maleic hydrazide residues are fairly stable during cooking. After
normal household cooking of onions about 20% of the residue remained
in the onion and 60% was found in the cooking water.
It was shown that during the manufacture of potato chips, drying
and frying does not lead to any appreciable lose of maleic hydrazide.
Owing to the loss of water during the manufacturing process the
concentration of maleic hydrazide residues in the manufactured product
is higher than in the fresh potatoes.
When cigarettes made from field-treated tobacco and containing 30
and 10 mg/kg maleic hydrazide were smoked in a smoking machine, 93% of
the original residue was decomposed or transferred to the side stream
of the 30 mg/kg level and no maleic hydrazide could be detected in the
mainstream smoke at the 10 mg/kg level.
A fairly specific spectrophotometric method of analysis is
available. Although the method is not very sensitive (limit of
determination 0.5 - 1 mg/kg) it may be suitable for adaptation to
regulatory purposes.
Recently a rapid quantitative GLC method has been developed to
determine the bis(trimethylsilyl) derivative by flame ionisation gas
chromatography. It is uncertain whether the method can be adapted to
include not only the free maleic hydrazide residue but also bound
unchanged maleic hydrazide and its -D-glucoside and whether it can be
used for crops other than tobacco.
EVALUATION
As no ADI was allocated, maximum residue limits could not be
recommended. Data were sufficient to record guideline levels for
onions and potatoes.
The following guideline levels are recommended. They refer to the
sum of free and bound unchanged maleic hydrazide and its
ß-D-glucoside.
Commodity mg/kg
Potatoes 50
Onions 15
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be allocated and
maximum residue limits can be recommended).
1. The results of the carcinogenicity study with rats which is
currently in progress.
2. Teratogenicity study with the sodium salt or the free acid.
3. Further studies clarifying the situation relating to the possible
presence of hydrazine in or on crops.
4. Residue data on other crops for which usage recommendations are
made including tobacco, carrots and swedes and similar crops.
5. Data on the fate of maleic hydrazide and its metabolites in
livestock animals and residues in products of animal origin after
feeding commodities containing maleic hydrazide residues, e.g.
potatoes.
6. Data on the effect of cooking on residues in potatoes and the
effect on residues of different methods of industrial processing
in the manufacture of various potato products, e.g. potato chips,
dried potatoes and potato starch. Data on residues in the wastes
from these products intended for feeding purposes.
7. Further development of the gas-chromatographic method to make it
suitable for regulatory purposes.
8. More information on the carry-over of maleic hydrazide from raw
into cured tobacco and into cigarette smoke.
Desirable
1. Studies on the metabolism of the beta-D-glucoside of maleic
hydrazide.
REFERENCES
Anderson, K.S. et al J. Agric. Chem. 20 649-655.
1972
Andreae, W.A. The Photoinduced Oxidation of Manganous Ions. Arc.
1955 Biochem. Biophys. 55: 684-586.
Baker, J.E. A Study of the Action of Maleic Hydrazide on Processes
1961 of Tobacco and other Plants. Physiologia Plantarum
14: 76-88.
Barnes, J.M., Magee, P.N., Boyland E., Haddow, A., Passey, R.D.,
1957 Bullough, W.S., Cruickshank, C.N.D., Salaman,
M.H., and Williams, R.T. The non-toxicity of
maleic hydrazide for mammalian tissues. Nature,
180, 62-64.
BASF Unpublished report: Verhalten des
1975 Pflanzenschutzmittelwirkstoffes (Maleinhydrazid)
im Boden.
Biswas, P.H., O. Hull and B.D. Mayberry. Metabolism, of Maleic
1967 Hydrazide in Tea, Camellia sinensis.
Physiologia Plantarum 20 : 819-824.
Callaghan, J.J. and P.Grun. Incorporation of 14C labeled
1961 Maleic Hydrazide into Root-tip cells of Allium
cerassum, Vicia faba and Tradescantia
paludosa, J. Biophys. Biochem. Cytol. 10:
567-575.
Drygas, M. D., Sikorska and B. Leszczynska Zmiany Zawortosci
1968 Hydrazydu Maleinowego HM/ Ziemniakach i Cebuli w
Okresic ich Przechowywania Biul Instytutu Ochiony
Roslin 1968 207-213.
Epstein, S.S., Andrea, J., Jaffe, H., Joshi, S., Falk, H., and
1967 Mantel, N. Carcinogenicity of the herbicide,
maleic hydrazide. Nature, 215, 1388-1390.
Epstein, S.D. and Shafner, H. Chemical mutagens in the
1968 environment. Nature, 219, 385-387.
Field, R.J. and A.J. Peel. The Metabolism and Radial Movement
1971 of Growth Regulators and Herbicides in Willow
stems. New Phytol. 70: 743-749.
Food Research Laboratories Inc. Report on toxicological studies
1955 of chemical additives, 3. Maleic hydrazide
(1,2-dihydropyridazine-3,6 dione). Submitted by
Uniroyal Chemical. (Unpublished).
Frear, D.S. and H.R. Swanson. Behaviour and Fate of (14C)
1975 Maleic Hydrazide in Tobacco Plants. U.S. Dept. of
Agric., Agric. Research Service, Metabolism and
Radiation Lab., Fargo, North Dakota 58102.
Freed, V.H. and M.L. Montgomery. The Metabolism of Herbicides
1963 by Plants and Soil. Residue Reviews 3: 1-19.
Gooding, E.B.G. and A.W.Hubbard. The Effect of certain
1956 Sprout-depressant Treatments on Sugar accumulation
in stored Potatoes. J.Sci.Food Agric. 7: 574-577.
Guthrie, F.E. and T.G. Bowery. Pesticide Residues on Tobacco.
1967 Residue Reviews 19: 31-57.
Haeberer, A.F., W.S. Schlotzhauer, O.T.Chortyk. A rapid Quantitative
1974 Method for Maleic hydrazide. J. Agric. Food Chem.
22: 328-330.
Haeberer, A.F. and O.T.Chortyk. Rapid Determination, of Maleic
1974 Hydrazide in Cigarette Smoke Condensate and
Particulate Matter. J. Agric. Food Chem. 22:
1135-1137.
Helweg-Anderson, A. Persistence of Maleic Hydrazide in Soil
1971 and its Influence on CO2 liberation. Tidsskrift
for Planteavl 7: 96-113.
Hellweg-Andersen, A. Abbau des Maleinhydrazids und Einflusz auf
1971 die Respiration in der Erde. Zeitschrift f.
Planzenkultur 75: 84-89.
Helweg, A. Degradation of 14C labeled Maleic Hydrazide in
1975 Soil as Influenced by Sterilization, Concentration
and Pre-treatment. Weed Research 15: 53-58.
Helweg, A. Degradation of 14C Maleic Hydrazide in Soil as
1975 Influenced by Absorbtion on Activated Carbon. Weed
Research 15: 129-133.
Hoffman, I. Spectrophotometric Determination of Maleic Hydrazide
1961 in Tobaccos. J. Ass. Off. Agr. Chem. 44: 723-725.
Hoffman, I. and R.B. Carson. Determination and Distribution of
1962 Maleic Hydrazide in Vegetables and Fruits. J.
Ass.Off. Agr. Chem. 45: 788-789.
Hoffman,. and E.V.Parups. Mode of Action of Maleic Hydrazide
1964 in Relation to Residues in Crops and Soils.
Residue Reviews 7: 96-113.
Hoffman, I., E.V. Parups and R.B. Carson. Analysis for Maleic
1962 Hydrazide. Part I. Detection and Determination in
Dried Green Tobacco Leaves and Suckers. Part II
Determination and Persistence in Soils. J. Agr.
Food Chem. 10: 453-455.
Hunter, B., Mawdesley-Thomas, L.E. and Worden, A.N. The
administration of maleic hydrazide and its
diethanolamine salt to rats. Toxicology 1,
301-307.
Innes, J.R.M. et al Bioassay of pesticides and industrial chemicals
1969 for tumorigenicity in mice: a preliminary note. J.
Nat. Cancer Inst. 42, 1101.
Kaufman, D.D., and N. A. Kalayanova. Maleic Hydrazide Degradation in
1975 Soil. U.S.Dept. of Agriculture, Agric. Research
Service, Beltsville Agricultural Research Centre,
Beltsville, Maryland 20725, U.S.A.
Lane, J.R. Collaborative Study of Maleic Hydrazide Residue
1963 Analysis. J. Ass. Off. Agr. Chem. 46(2): 261-268,
744-748.
Lane, J.R., D.K. Gullstrom and J.E. Newell. Extension of the Residue
1958 Methods for 1,2-dihydro-3,6-pyridazinedione
(maleic hydrazide) and N-1-naphtylphtalamic acid
(Alanap). J. Agr. Food Chem. 6: 671-674.
Levi, E. and A.S. Crafts. Toxicity of maleic hydrazide in California
Soils. Hilgardia 21: 431-463.
Luckens, M.M. and Wattimena, J.R. Acute toxicity of combinations
1968 of maleic, hydrazide diethanolamine and selected
insecticides. Toxicol. Appl. Pharmacol. 12:
287-288 (Abstr.no. 7).
Mannell, W.A. and Grice H.C. A comparative study of maleic hydrazide
1957 and p-dimenthylaminoazobenzene on rat liver. Can.
J. Biochem. Physiol. 35, 1233-1240.
Mays, D.L., Born, G.S. Christian, J.E. and Liska, B.L. Fate of
1968 C14-Maleic hydrazide in rats. J. Ag. Fd. Chem.
16, 356-7.
McCann J., Choi, E., Yamanslic, E., and Ames, B.N. Proc. Natl.
1975 Academy of Science 72 (12) 5135-5139.
McCann, J., Choi, E., Yamanslic, E., and Ames, B.N.
Ibid 73 (3) 950-954.
McCarthy, R.E. and Epstein, S.S. Cytochemical and cytogenetic
1968 effects of maleic hydrazide on cultured mammali
cells. Life Sci. 7:1-6.
Nasrat, G.E. Maleic hydrazide, a chemical mutagen in
1965 Drosophila melanogaster. Nature, 207, 439.
Nelson, J.O., and Kearney, P.C. Metabolism of maleic hydrazide
1975 by hepatic microsomes from phenobarbital induced
rats. U.S.D.A. report.
Naugatuck Chem. Comp. Unpublished Data on Maleic Hydrazide
1956 summarised by the Secretary of the Scientific
Subcommittee on Poisenous Substances used in
Agriculture and Food Storage. American Residue
data of Maleic Hydrazide in Onions and Potatoes.
Noodén, L.D. Metabolism and Binding of 14C-Maleic Hydrazide.
1970 Plant Physiol. 45: 46-52.
Patterson, D.R., S.H. Wittwer et al. 1952. The effect of pre-harvest
foliar sprays of maleic hydrazide on sprout
inhibition and storage quality of potatoes. Plant
Physiol. 27: 135-142.
Povolotskaya, K.L. Mechanism of the Action of Maleic Hydrazide
1961 in Plants. Izvest. Akad. Nauk SSSR 26, Ser.
Biol.no. 2: 250-255.
R.I.V. Unpublished data of the National Institute of Public
1955. 1964. Health Utrecht/Bilthoven, The Netherlands,
provided to JMPR 1976.
Stoessl, A. Photolysis of Maleic Hydrazide. Chem. Ind.:
1964 580-581.
Timson, J. The effects of maleic hydrazide on the mitosis of
1968 phyto-hemagglutinin stimulated human lymphocytes
cultured in vitro. Caryologia 21:157 (Cited in Fd.
Cosm.Toxicol, 8: 104-105, 1970).
Towers, G.H.N., Hutchinson, A. and Andreae, W.A. Formation of a
1958 Glucoside of Maleic Hydrazide in Plants. Nature
181:1535-1536.
Uniroyal Chem. Unpublished data on Maleic Hydrazide provided
1973-1975 to JMPR 1976.
Winder, F.G. and Denneny, J.M. Metal-Catalyzed Auto, Oxidation
1959 of Isoniazid. Biochem. J. 73:500-507.
Wood, P.R. Determination of Maleic Hydrazide Residues in Plant
1953 and Animal Tissues. Anal. Chem. 25:1879-1883.
