
DAMINOZIDE JMPR 1977
IDENTITY
Chemical name
1. N-dimethylaminosuccinamic acid.
2. succinic acid 2,2-dimethylhydrazide
3. butanedioic acid mono (2,2-dimethylhydrazide).
Synonyms
Alar (R); SADH, Kylar, B-Nine (R), B-995, aminocide (obsolete common
name).
Structural formula
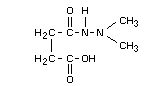 Other information on identity and properties
Daminozide is a white crystalline material of low volatility and
slight odour. It is soluble in polar solvents (g/100 g a 25°C: water
10; methanol 5; acetone 2.5). It is practically insoluble in aromatic
and aliphatic hydrocarbon solvents.
The technical grade product from the primary producer is typically
>99% daminozide. The manufacturing specifications require 98% minimum
and a melting point range of 154°C to 161°C. It is a white powder
which may contain traces of moisture, succinic acid, succinic
anhydride and a salt of daminozide and unsymmetrical dimethyl
hydrazine (UDMH). Volatile impurities are removed by an oven drying
step in the process. Daminozide was formerly manufactured by a process
in which dimethylnitrosamine was a starting reactant. The primary
manufacturer now uses a new process in which dimethylnitrosamine is
not used.
Daminozide is now commercially available only in formulations of
water-soluble powders containing 85% active ingredient and 15% inert
surfactants and mineral salts. Shelf-life studies show no degradation
of the formulations over a period of years. Liquid formulations
formerly produced are no longer commercially available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Daminozide is rapidly excreted and does not bioaccumulate in rats.
Two male and two female rats were treated with a single oral dose of
approximately 5 mg/kg unlabelled daminozide and 96 hours later with a
single 5 mg/kg oral dose of 14C-labelled daminozide (position of 14C
label unspecified) to study the absorption and excretion of
daminozide. After 2 days 69% of the administered dose had been
excreted in the faeces, 24% in the urine and 2.4% expired as 14CO2
Rats sacrificed 2 days after dosing contained an average of 0.35% of
the administered dose in the brain, liver, lung,,heart and spleen with
the majority of this residue in the liver. The rats sacrificed 4 days
after dosing contained only 0.03 or 0.12% of the administered dose in
these organs (Ryer, 1966).
A cow fed a diet containing 25 ppm daminozide for 4 days excreted 81%
of the daminozide in the faeces and 1.2% in the urine (St. John, Jr.
et al., 1969). See also "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on mutagenicity
Groups of male mice (20/group) were fed dietary levels of 0, 10, 300
or 10,000 ppm daminozide for 5 consecutive days to determine dominant
lethal effects. Following treatment, the males were monogamously
paired with untreated females, weekly, for 4 consecutive weeks.
Thirteen days after pairing females were killed and the uterine
contents examined for implantations, viable embryos and early or later
deaths.
No male deaths occurred during the test period and no signs of
compound effect on behavior were noted. During treatment, weight gain
at 10,000 ppm was marginally retarded. No effect was observed on
mating performance or pregnancy rate in any group. Neither
implantation rate nor viable litter size were significantly affected
by any treatment level. Inter-group differences in post-implantation
loss never attained significance (P>0.05) and showed no consistent
dose relationship (Palmer and Lovell, 1973).
Special study on teratogenicity
Groups of female rats (25/control group, 21/test group) were
administered 0, 250 or 500 mg/kg/day daminozide (12.5% w/v) in corn
oil, by gavage, from day 6 through 15 of gestation to determine
teratogenic effects. Daily observations were made and body weights
recorded on days 1, 9, 12 and 15. Records were kept of corpora lutea,
resorption sites, viable foetuses, foetal external and internal
abnormalities and skeletal development. Approximately equal numbers of
foetuses were examined for internal effects, using the Wilson
technique, and skeletal effects using clearing and alizarin staining
techniques.
Resorption sites, expressed as mean/female, were as follows: control,
0.4; 250 mg/kg, 1.1; 500 mg/kg, 0.7. Two females with a total of 10
resorption sites were included in the 250 mg/kg mean. Examination of
foetuses revealed no compound-related effects and none of the other
parameters were affected by compound administration (Keplinger et al.,
1972).
Special study on reproduction
Groups of rats (20/sex/group) were fed dietary levels of 0 or 300 ppm
daminozide, from weaning through three generations. The F0 group was
part of the 2 year feeding study described under "Long term studies".
PUP weights, number born alive or dead and the usual indexes of
reproductive performance were recorded. Data from two litters/3
generations revealed no significant effect on either fertility or
reproductive capacity. The pups survived equally well in the control
and test groups and no effect on growth was evident. Blood and urine
values were comparable to controls in each generation. Organ weights
were not affected nor was any compound-induced histopathology observed
(Oser, 1966).
Acute toxicity
Observed signs or oral intoxication were depression, ptosis, ataxia,
diarrhoea, excessive urination and laboured respiration. Dermal
application produced mild erythema and oedema.
TABLE 1. Acute toxicity of daminozide.
LD 50'
Species Route Sex mg/kg Reference
Rat Oral M 6,810 Anonymous, 1966
Oral M,F 8,400 Carson, 1963
Inhalation M 147 mg/l Carson, 1963
Rabbit Dermal M,F >10,000 Anonymous, 1966
Dermal M,F 16,000 Carson, 1963
Short term studies
Rat
Groups of rats (5 males and 5 females/group; 10 of each sex were
controls) were fed dietary levels of 0, 26, 80, 240, 720 or 2160
mg/kg/day daminozide technical for 90 days. No differences were noted
in body weight gains between any test group and the control gains. No
significant differences in haematological, blood chemistry or urine
values were observed and organ weights and ratio were comparable to
the control group. No gross or microscopic changes occurred in any
group which could be attributed to daminozide administration (Carson,
1964).
Dog
Groups of dogs (6/sex/control and 4/sex/test groups) were fed dietary
levels of 0, 300, 1000 or 3000 ppm daminozide technical for two years.
Body weight gains were within normal limits and comparable to control
gains. Appearance, behaviour, haematological, blood chemistry and
urine analyses of the test groups were also comparable to controls.
Gross necropsies, microscopic examination of tissues and organ weight
data revealed no compound or dose-related effects (Oser, 1966).
Long term studies
Rat
Groups of rats (37/sex/control and 25/sex/test group) were fed dietary
levels of 0, 300, 1000 and 3000 ppm diaminozide technical for two
years.
No differences in appearance or behaviour were observed between
control and test rats during the study. Throughout the study the body
weights gains of the 300 ppm group were slightly less than those of
the controls, but weight gains of the 1000 and 3000 ppm groups were
comparable to the control gains. No haematological or blood chemistry
differences related to compound consumption were noted during the
study. Survival over two years was not affected and was comparable
between all groups. Liver/body weight ratios for both sexes in the 300
and 3000 ppm groups were significantly higher than the control ratio
at 104 weeks. In the 300 ppm male group the mean liver weight was 21%
higher than the mean control liver weight. Nothing in the gross or
microscopic examinations explains this difference. Microscopic
examination of tissues revealed no dose-related effects. Tumors were
both benign and malignant, the former being principally mammary
adenomas or fibromas. In the latter category, reticulum cell sarcomas
predominated in both the test and control groups, the incidence being
not significantly higher at the 3000 ppm level than in the control
group. Several varied types of malignant neoplasms were also noted but
in no cage was there more than one of each type in any group (Oser,
1966).
COMMENTS
Daminozide is not highly acutely toxic as measured by LD50 values in
the g/kg range for oral and dermal studies and is only mildly
irritating to the eyes and skin. It is rapidly excreted and does not
bioaccumulate in mammals. Daminozide did not produce dominant lethal
effects in male mice at 10,000 ppm and no teratological or
reproductive effects were observed at levels of 300 ppm or lower. An
adequate two-year dog study has been conducted.
The long-term rat study was inadequate because of internal conflicts
within the report and because of the inability to evaluate the
carcinogenic potential of daminozide. Information on the
biotransformation of daminozide in animals is needed.
Because of the above concerns no recommendation for an acceptable
daily intake for man can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Daminozide is a plant growth regulator applied only in foliar sprays
to various tree fruits, grapes, vegetables, melons and peanuts.
Depending on the crop and timing of treatment, it is variously
intended to hasten ripening, increase fruit set, enhance fruit colour,
retard stem elongation, control fruit drop, delay maturity, reduce
vine growth or to produce certain other beneficial growth regulating
effects.
Introduced on an experimental basis in the early 1960s, it was first
registered for use on ornamentals. Since that time there have been
numerous reports in the horticultural journals on the efficacy of the
compound as a growth regulator. Use was expanded to include food crops
in 1968 in the U.S.A. Table 2 shows current (1977) registered use
patterns in the U.S.A. Uses in Canada are similar to the U.S. uses.
There was other information available to the Meeting that the product
has been used in Italy, South Africa, Japan, Australia, Federal
Republic of Germany, the Netherlands and the United Kingdom. The
directions for use on the product labelling accepted in those
countries generally conform to the use patterns shown in Table 2.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trials in the U.S.A. on fourteen crops and controlled feeding
experiments with meat and dairy animals (Uniroyal, 1977). The
government of the Netherlands submitted reports of supervised residue
trials on apples and pears. Analyses were by the colorimetric
procedure described below under "Methods of residue analysis".
Analyses of untreated controls (crop blanks) were reported in each
experiment, along with % recovery of daminozide from fortified
controls. Test samples were corrected for crop blank. Recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form where such presentation
is feasible.
Apples
Field trials were conducted at locations in 10 states representing all
of the major apple growing areas of the U.S.A. Some 180 harvest
samples were analyzed in duplicate. The wide range of experimental
conditions in the various field tests and the resulting wide range of
residues found, preclude a tabular summary of data. The residues
ranged from 0.1 mg/kg to as high as 80 mg/kg. If the high values
resulting from exaggerated treatments are excluded, the data indicate
that residues would not be likely to exceed 30 mg/kg when the normal
dosages (Table 2) are applied and the prescribed 60-70 day preharvest
interval is observed.
TABLE 2. Registered use patterns for daminozide in the U.S.A. (1977).
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
sweet cherries hasten 0.1-0.2% 1 2 weeks after full
ripening, bloom
concentrate
maturity
sour cherries " 0.4% 1 "
peaches, hasten 0.1-0.2% 1 13 weeks post-bloom
nectarines ripening, to pit hardening
concentrate
maturity
pears prevent fruit drop 0.1% 1 18 to 24 days before harvest
apples multiple effects(1) 0.075-0.2% 160 to 70 days before harvest
prunes hasten ripening 0.05-0.1% 1 1 month before
harvest
grapes increase fruit set, 0.05-0.1% 1 from 1st bloom to
reduce vine growth (1.4-2.4 kg/ha) full bloom
Brussels uniform sprout 1.9-3.75 kg/ha 1 30 days before
sprouts development harvest
cantaloupes reduce vine growth 1.9 kg/ha 1 2 to 4 leaf stage
TABLE 2. (Continued)
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
tomatoes multiple effects(1) 0.6-2.4 kg/ha 2 before transplanting
4.8 kg/ha 1 after transplant - not
within 7 days
of picking
peanuts multiple effects(1) 1.0 kg/ha 2 at pegging time
0.5 kg/ha 30 days before
harvest
peppers 0.5-2.3 kg/ha 2 at 2-4 leaf stage
(seedlings) and 7-10 days
later
(1) There are 9 separate beneficial effects claimed for apples alone. Other claims are similarly complex. The directions for use of this
product are complex because of many factors (plant vigour, variety, moisture stress, etc.) which can not only modify the desired effect
on the plant but can contribute adverse effects.
The residues from trials in the Netherlands were generally 5 mg/kg or
less. The rates of application in these experiments were somewhat
lower than the rates permitted in some other countries.
Studies designed to measure initial deposits or residue decline rate
(half-life) were not included. The data clearly show, however, that
daminozide is extremely persistent in apples. Significant residues
remain for more than 159 days after the spray application.
Brussels sprouts
Four separate trials were conducted at locations in California and New
York. A single treatment was made at normal and exaggerated rates.
Crop blanks were reported as zero. Recoveries were 87 and 83% at 1 and
10 mg/kg respectively. The data are summarized in Table 3. Under good
agricultural practice the residues would not be likely to exceed 20
mg/kg.
TABLE 3. Residues in Brussels sprouts, mg/kg (single application).
Application rate, Interval, days from treatment
kg a.i./ha 30 33 46
1.9 6.3, 5.2, 6.8 7.1, 1.4, 0.0
3.4 2.8, 1.4
3.8 14.6, 15.5, 13.0 2.3, 2-7, 1.5
6.8* 4.8, 3.2
7.7* 25.4, 24.8, 24.6 5.6, 6.2, 5.2
*excessive dosage
Cantaloupes
Nine residue trials were reported from locations in California,
Arizona, Texas and Michigan. The registered use patterns on melons
provide for treatment when the plant is at the 2-4 leaf stage, at
which point no fruit would be present. The experiments included
exaggerated treatment rates, and samples taken as early as 44 days
after treatment. If these samples were mature melons, fruit must have
been present at the time of treatment (melons take from 85 to 150 days
from planting to maturity). Even under these conditions the residue
values of some 20 samples ranged from < 0.1 to 1.6 mg/kg (Table 4)
compared with apparent daminozide in untreated controls up to 1.1
mg/kg. It would appear that residues to be expected in cantaloupes
from good agricultural practice would be of a lower order than those
found in the tree fruits. It is not likely that residues in excess of
3 mg/kg would occur in cantaloupes. Data were not available on other
melon varieties, but the conclusion regarding cantaloupes could be
extrapolated to other melons.
TABLE 4. Residues in cantaloupes,mg/kg
Interval from No. of Application Rate,
last treatment treatments kg a.i./ha
days 0.47 0.95 1.9 3.8 7.6
44 2* <0.1 1.6
50 1 1.0,1.5 1.5,1.6
57 1 0.6,0.8 1.2,1.6
68 1 1.2 1.6
73 2* <0.1 <0.1
87 1 <0.1 <0.1
115 2* <0.1 <0.1
*Rate given for second treatment only.
Sour cherries
Trials were carried out at several locations in four states
representing the important cherry producing areas in the U.S.A. A
total of 70 test samples and 25 control samples were analyzed. Crop
blanks ranged from 0.1 to 0.64 mg/kg.
The dosages applied in the tests ranged from 25% of the manufacturer's
minimum recommended dosage to twice the maximum recommended rate (See
Table 2). Samples were taken at periods after treatment ranging from
56 to 65 days. This roughly corresponds to the label directions which
call for a single spray 10-14 days after full bloom. The season from
bloom to harvest in the U.S.A. is 80 to 100 days (Magness, 1971).
The sampling schedule in all trials was designed to show residues at
normal harvest. Therefore, no estimates of initial deposit or residue
decline rate are possible. Overall the residues found on mature
cherries ranged from 5.3 to 78 mg/kg. Although some correlation could
be made with application rate, the variation in harvest residues
appeared to be almost random (the data do not lend themselves to
tabulation). If the data from trials with excessive or minimal dosages
were excluded, the residues fall between 10 and 53 mg/kg. It is not
likely that residues under actual (commercial) conditions would ever
exceed 60 mg/kg.
Sweet cherries
Analyses of 176 test samples and 39 control samples from field trials
in five states were made available. The experimental design generally
paralleled that discussed above for sour cherries. That is, the trials
were designed to show residues on harvested cherries treated
approximately as prescribed on the product label (2 weeks after full
bloom). The overall range of residues found (1 to 54 mg/kg) was
comparable to that reported on sour cherries. If only the data from
trials following good agricultural practice (See Table 2) are used,
most samples were in the range of 9-18 mg/kg, with a few higher values
approaching 30 mg/kg. The difference between the residue load expected
on sweet and sour cherries is mainly due to the application rate,
which is 0.2% for sweet and 0.4% for sour cherries. A maximum residue
limit of 30 mg/kg for sweet cherries would therefore be consistent.
Grapes
The residue data are summarized in Table 5. Some 44 samples from field
trials in 4 states were reported. Analyses were in duplicate,
including 8 untreated control samples. All controls were reported as
0.1 mg/kg, apparent daminozide.
The registered use directions for grapes (Table 2 requires treatment
during the period from first bloom to full bloom. The period between
bloom and harvest for grape varieties will differ greatly. In some
varieties it may be as much as 5-6 months (Magness, 1971). It is
interesting to note that in the field trials, the intervals between
spray and harvest were from 102 to 111 days, and probably represent an
early maturing variety, probably Concord. From the standpoint of
residues at harvest, it therefore represents the "worst case". Even
with some exaggerated dosages, all residues were below 10 mg/kg.
TABLE 5. Residues in grapes, single application, 102-111 days from
treatment
Application rate Residue range,
% a.i. mg/kg
0.05 0.4 - 2.5
0.10 1.3 - 5.4
0.20 2.6 - 9.5
Nectarines and peaches
The use directions for these two fruits are the same. They also incur
residues to the same degree, and may be discussed together.
The prescribed cut-off date for sprays is at "pit hardening".
Presumably, this growth stage is recognizable to experienced fruit
growers. As near as can be determined, pit hardening occurs 40 to 80
days before harvest, depending on variety, geographic location and
other factors.
The data made available on nectarines and peaches included a total of
more than 500 analyses including 70 untreated controls. Treatments
were made at the prescribed rate, at excessive rates, and at less than
the prescribed rate. The intervals between treatment and sampling were
variously identified as treatments at "style abscission", pit
hardening, and in some cases by numerical day before harvest. Owing to
the variables in the field tests, a wide range of residues was
reported (1.0 to 93.0 mg/kg).
Interest is mainly in the residues from applications closely
approximating the registered use pattern (Table 2). The distribution
of residues from application of 0.1 to 0.2% sprays in the period from
style abscission (3 weeks post-bloom) to pit hardening in nectarines
and peaches was:
mg/kg <1 1-5 5-10 10-15 15-20 20-25 >25
No. of samples 35 140 48 45 3 7 1
The distribution indicates that residues would not be likely to exceed
30 mg/kg when uses described in Table 2 are followed.
Peanuts
Results of residue trials in Georgia, N. Carolina, Texas, Alabama,
Florida and Oklahoma were submitted to the Meeting. Separate analyses
were made on kernels, hay and hulls.
Kernels
A total of 166 analyses of test samples and 13 of controls were made.
All controls samples were zero except two reported at 3.1 and 2.8
mg/kg. (The analyst noted that contamination was suspected.)
The manufacturer's recommendations are for one treatment of 0.96 kg
a.i./ha at pegging time followed by a second treatment of 0.47 kg
a.i./ha thirty days before harvest. For those field trials in which
the treatment approximated the recommended pattern, the following
residue distribution was obtained:
Range, mg/kg: <5.0 5-10 10-15 15-20 20-25 25-30
Number of samples: 31 20 7 4 4 2
From this skewed distribution pattern it is apparent that maximum
residue limit of 30 mg/kg for kernels would be adequate to cover all
residues from good agricultural practice.
Peanut hay
62 analyses were submitted, 24 of which corresponded to the normal
split dosage pattern mentioned above. Residues in the trials
reflecting recommended usage were mostly in the range of 1-5 mg/kg.
However, three were in the range of 5-10 mg/kg and three were greater
than 10 mg/kg. A maximum residue limit of 20 mg/kg would be adequate.
Peanut hulls
Very limited data were available on peanut hulls. Residues were 2
mg/kg or less. Peanut hulls are not an important item of international
trade. The only interest in this commodity is that the hulls are
incorporated at low levels into some mixed animal feeds.
Peanut meal
Field-treated peanuts which were determined to be carrying 12 mg/kg of
daminozide were processed in a laboratory experiment simulating a
commercial process. The meal presscake was found to contain 30-33
mg/kg. On the basis of this approximately three-fold concentration
factor, peanut kernels bearing the maximum residues of 20 mg/kg would
be expected to produce meal containing about 60 mg/kg.
Pears
Limited data (14 test samples + 7 controls) from Washington and Oregon
were available. Seven samples were from trials in which the
manufacturer's recommended rate was applied (0.1% spray). Residues at
this dosage ranged from 0.8-17.0 mg/kg, with an average of 10.8 mg/kg.
Residues from uses according to good agricultural practice would
probably not exceed 20 mg/kg.
Peppers
Field trials were conducted in Texas, Georgia, Connecticut, Delaware
and Michigan. Samples were collected 77-125 days after treatment.
Single and double applications were made at the prescribed rate and at
four times the prescribed rate. The net difference between the
apparent daminozide in the test samples and the control samples was
less than 1 mg/kg in all cases, the highest residue reported being
0.44 mg/kg. A maximum residue limit of 1 mg/kg would be adequate.
Plums
Residue trials at 6 locations in California were reported to the
Meeting. The plums (fresh prunes) were treated at the prescribed rate
(Table 2) and at rates up to 4 times the prescribed rate. The interval
between treatment and sampling ranged from 34 to 78 days (as compared
with the registered label requirement of a 30 day pre-harvest
interval). Residues from all treatment rates ranged from 3.0 to 71.5
mg/kg. Residues from the normal rates were 3.0 to 43.7 mg/kg. It is
unlikely that residues from normal usage would exceed 50 mg/kg.
Field-treated fresh prunes were analyzed and converted to dried prunes
by the commercial procedure. This involves a 5-10 minute mechanical
wash and drying on trays at 170°F for 30 hours. The maximum
concentration factor (mg/kg in dried prunes: mg/kg in fresh) was found
to be 2.7. On this basis, a prediction can be made that fresh prunes
bearing maximum residues of 50 mg/kg would produce dried prunes
containing 135 mg/kg.
Tomatoes
The registered uses of daminozide on tomatoes include 2 applications
prior to transplanting (greenhouse or outdoors) and one application in
the field no later than 7 days prior to picking (see Table 2). The
application on plants to be transplanted is for all practical purposes
a "no-residue" use. Residue data were made available on tomatoes which
had received both transplant and field treatments at the normal and
excessive rates. All residue trials (7) were carried out in Florida,
on green tomatoes. The only field use for daminozide on tomatoes
permitted in the U.S.A. is on Florida winter tomatoes which are
harvested green and artificially ripened. These residue trials
therefore were highly specialized and do not necessarily reflect
residues which might result from more general usage on tomatoes. The
data are shown in Table 6.
TABLE 6. Residues in tomatoes, mg/kg
Interval from Application Rate*
last treatment kg a.i./ha
Days 4.76 9.52
11 13 68.5
21 9 35
7 6 8
7 8 12.5
18 12 20.5
14 20 45.5
19 15 28
29 22 8
* Field treatment after transplant use.
Alfalfa
There are no national tolerances or registrations for daminozide use
on alfalfa. However, the product has some utility in the culture of
alfalfa seed, and registration is pending in U.S.A. In growing alfalfa
for seed there is some utilization of alfalfa forage, hay, and seed
screenings for animal feeds. The submission to the Meeting therefore
contained some residue data on alfalfa hay, fresh forage, and seed
screenings. Residues on fresh alfalfa ranged from 4-12 mg/kg and on
hay from 2-20 mg/kg. Seed screenings showed a maximum of 1.4 mg/kg. In
view of the specialized nature of the use it would not appear
necessary at this time for the meeting to recommend a maximum residue
limit.
Meat, milk, poultry and eggs
Daminozide is used on a number of crops which yield by-products used
commercially in animal feeds. Primary daminozide sources would be
peanut meal and peanut hay. Some additional contribution to the diet
could occur from peanut hulls and the pomaces resulting from
processing apples, grapes and tomatoes. In recognition of this, the
basic manufacturer submitted to the Meeting some animal feeding,
studies (Uniroyal, 1977).
(a) Cattle were fed at levels of 0, 20, 60 and 200 ppm in the total
diet for 6 weeks. Milk was collected at intervals and the animals
slaughtered at the end of the feeding period. No daminozide residues
were found in any tissues (muscle, fat, liver, kidney) by a method
with a lower limit of detection estimated at 0.2 mg/kg. Trace residues
were found in the milk of animals on the 60 and 200 ppm diets at
levels of 0.05 and 0.07 mg/kg respectively.
(b) Hogs were fed 0, 60 and 300 ppm in the diet (2 animals at each
level) for 31 days. No daminozide residues were detected (40.2 mg/kg)
in muscle or fat. Apparent daminozide residues were found in liver and
kidney, at 0.3 and 0.8 mg/kg respectively, in animals on the highest
feeding level.
(c) Poultry were fed 0, 20, 60 and 200 ppm in the diet (10 hens in
each group) for 31 days. Eggs were composited weekly and on day 31 all
birds were sacrificed. On the final sampling, eggs from the 300 ppm
and 60 ppm feeding levels contained 0.3-0.5 and 0.25 mg/kg
respectively. No detectable residues were found (< 0.2 mg/kg) in eggs
from the 20 ppm feeding level. No residues were found in muscle or fat
of birds on the lower feeding levels. At the 200 ppm level, residues
of 0.4 and 0.7 mg/kg were found respectively in muscle and fat.
Residues of 1 and 1.1 mg/kg were found in liver of birds on the
highest level. Daminozide concentrates in poultry kidney. Values of
1.3, 1.8 and 7.4 mg/kg were reported in kidney for the 3 feeding
levels.
The studies show that residues occur at high feeding levels, but this
must be related to the actual anticipated intake from peanut meal and
peanut hay. Peanut hay would be used to the extent of 25% in beef
cattle diets, 60% in dairy diets, and 10% for pigs. Peanut meal can
make up 15%-beef; 25%-dairy, 10%-poultry and 10%-pigs (Harris, 1975).
After adjusting for the proportion of peanut meal and hay in the total
diet of each species, it is apparent that the residues in milk, eggs
and tissues (except poultry kidney) Will be below the level of
detection of the colorimetric method.
FATE OF RESIDUES
General
Daminozide is readily absorbed and translocated from foliar sprays.
The entrapment of 14CO2 from applications of labelled daminozide
indicates that, under certain conditions, at least part of the applied
pesticide is fully degraded. Other data show that daminozide, in free
or loosely bound form, is the principal residue component and very
persistent in fruits. Many of the conclusions regarding the nature of
the terminal residues rest on characterization by selective solvent
extractions, in vitro experiments and analysis by wet chemical
procedures for predicted metabolites. In view of the known biological
activity of some postulated metabolites of the compound, particularly
nitrosamines, hydrazides and other hydrazine derivatives, further
identification of the residues in harvested fruits and vegetables
would be desirable.
In animals
Rats were fed 14C-labelled daminozide and the excreta monitored. The
majority of the administered dose, apparently occurred in the urine as
unchanged daminozide. As far as can be determined, there has been no
effort to study metabolic pathways in meat or milk animals with radio-
labelled daminozide. However, analyses by the chemical method
(previously discussed) were made for one postulated metabolite (NDMA)
in milk from cows fed 200 ppm daminozide in the diet. No NDMA was
found at a minimum detection level of 0.03 mg/kg. See also the
sections "Biochemical aspects" and "Residues resulting from supervised
trials".
In plants
It has been reported that daminozide decomposes to unsymmetrical
dimethylhydrazine (UDMH) in the plant and that it is the latter
compound which is the active principle through its effect on the plant
enzyme diamine oxidase (Reed, 1963). Another investigator (Dahlgren,
1963) found that maleic acid dimethyl hydrazide (CO11, a related
growth regulator) is hydrolysed to UDMH in aqueous solutions. Dahlgren
noted that daminozide was stable under the same conditions. In
experiments reported by the manufacturer (Uniroyal, 1967) no free UDMH
or succinic acid was detected after attempts (in vitro) to enzymically
hydrolyze daminozide with trypsin, proteinase, papain, ficin and
urease. No evidence was found of hydrolysis to UDMH and succinic acid
in macerates of plant leaves by colorimetric tests for UDMH and paper
chromatographic tests for succinic acid. While the evidence regarding
the occurrence of UDMH is somewhat contradictory it is likely that,
even if UDMH occurs as an intermediate metabolite, its presence would
be fleeting owing to its reactivity and that it would not be present
per se in harvested crops.
The gas-chromatographic method of analysis for nitrosodimethylamine
(NDMA), discussed below ("Methods of residue analysis",) has been used
in studies intended to show the absence of this possible oxidation
product in treated crops (Uniroyal, 1967). No NDMA was detected in
treated apples, tomatoes or peanut foliage by the method, which is
said to be sensitive to 0.002 mg/kg NDMA.
Radiotracer studies were conducted by the U.S. Department of
Agriculture (Uniroyal, 1966) on apple trees in the field and on apple
seedlings in the greenhouse. Daminozide was labelled in the succinic
acid moiety and (in a separate test) in the UDMH group. Activity in
the sprayed seedlings was distributed throughout the plant with
highest activity in the leaves. 20% of the original activity was
released as 14CO2 in the first seven weeks after application.
In the field studies, 15% of the total applied activity remained in
the tree after 100 days. The activity in the apple fruit after 125
days was identified as 75-85% unmetabolized daminozide. Trace amounts
of succinic acid were identified and there was some incorporation of
14CO2 into plant components.
14C-labelled daminozide was used in metabolism studies with peanut
plants (Uniroyal, 1977). Characterization of the activity by selective
solvent extraction indicated that the majority of the activity was
present in bound form, probably as sugar complexes. Daminozide per se
was recoverable from the extracts and it was concluded that residues
present in peanuts in complexed form would be detected by the
colorimetric method of analysis. Analyses of peanut oil for 14C
activity showed residues of about 2-5 mg/kg expressed as daminozide.
Selective solvent extraction of the oil indicated that the activity
was not daminozide but was probably in the form of fatty acid
glycerides.
In soil
Residue decline studies were conducted on sandy loam and clay soils
spiked at 5 and 10 mg/kg. Daminozide per se is not persistent in
soils. The breakdown is probably through microbial action. In the clay
soil, < 0.1 mg/kg was present two days after treatment. The sandy
loam contained 0.1 mg/kg at 2 and 3 weeks respectively at the 5 and 10
mg/kg fortification levels. The compound is very mobile in soils
because of its water solubility.
In processing
Studies show that in the commercial processing of peanuts there is no
appreciable concentration of residues in the refined peanut oil. There
is, however, a 3 fold concentration of residues in peanut meal (e.g.,
peanut meats bearing 20 mg/kg will produce peanut meal bearing 60
mg/kg).
Data on the processing of fresh tomatoes bearing approximately 40
mg/kg into ketchup and tomato paste show that there is essentially no
loss of daminozide residues in processing. (The residues concentrate
to the same degree as the solids do in the concentration process.)
Residues found in tomato paste ranged from 155 to 197 mg/kg. Analyses
for nitrosodimethylamine (NDMA) in tomato paste were negative by the
chemical method with a reported sensitivity of 0.002 mg/kg.
Experiments in which field-treated plums were processed into dried
prunes showed that residues were concentrated in the dried fruit by a
factor of 2.7. See "Residues resulting from supervised trials" for
details of this work and of the effect on residues of processing
peanuts to peanut meal.
There are national tolerances on grapes (10 ppm, U.S.A. and Canada)
but no data were made available on transfer of residues to wine or
grape pomace.
Evidence of residues in food in commerce or at consumption
As noted under "Methods of residue analysis", daminozide is not
detected by any of the multi-residue screening methods currently used
in market basket surveys, monitoring, or government regulatory
programmes. The "specific" colorimetric method has not been employed
in the Canadian or the U.S. Food and Drug Administration surveillance
programme and there is no record of a finding of daminozide in foods
in commerce. In view of the demonstrated stability of daminozide
residues in food processing, and the relatively high levels incurred
in fruits from good agricultural practices, it may be concluded that
residues are occurring in the diet in those countries where there is
substantial use.
METHODS OF RESIDUE ANALYSIS
Daminozide residues in crops are determined by a colorimetric method
(PAM II, 1967). The method involves hydrolysis in boiling 50% sodium
hydroxide to release unsymetrical dimethylhydrazine (UDMH) which is
distilled and reacted with trisodium pentacyanoamine ferroate (TPF) at
pH 5.0. The red colour produced is measured spectrophotometrically at
490 and 600 nm. The difference between the two readings is plotted as
net absorbance against µg of compound in a calibration curve.
Modifications of this basic method permit determination of daminozide
in peanuts, animal tissues, milk and eggs.
The chromogenic agent TPF used for determination of daminozide also
forms colored complexes with nitroso compounds, primary aromatic
amines and aliphatic or aromatic hydrazines (Feigl, 1949). Some
possible metabolites of daminozide would therefore contribute
absorbance which would be additive to that of the daminozide-TPF
complex. If present they would be interpreted as daminozide residues
(but not measured quantitatively).
The method has been tested in government regulatory laboratories on
grapes and animal tissues. Because of large and variable crop blanks
it was concluded that a reliable lower limit of determination for
daminozide in samples of plant origin was no better than 1 mg/kg for
regulatory purposes. The estimated sensitivity for animal products is
0.05 mg/kg for milk and 0.2 mg/kg for tissues and eggs. The government
analyst noted that 2 hours colour development time was required rather
than the 1 hour prescribed in the procedure.
The assumption was made that any free UDMH, or any metabolites which
would yield UDMH under the rigorous caustic hydrolysis, would also be
measured as daminozide by this method. There are no experimental data
to confirm that conclusion. Whether in fact any such metabolites
actually occur in crops at harvest is uncertain (see discussion under
"Fate of residues").
Colorimetric methods are ordinarily not specific. In this case, the
method was tried in the presence of most pesticides used on the
subject crops (in 1968) to gauge interference. Only the fungicide
"Botran" was found to interfere, and this interference can be
eliminated by a modification of the method.
A second colorimetric method of analysis for daminozide in plant
substrates has been published (Edgerton, 1967). This method also
measures UDMH in a hydrolysate, but colour development is with
phosphomolybdic acid. Sensitivity is comparable to the colorimetric
method discussed above.
As noted above, the colorimetric method for the parent compound would
also measure any alkylnitrosamines as daminozide. Because of concern
for the toxic potential of a possible metabolite, nitrosodimethylamine
(NDMA), a "specific" method for NDMA was developed by the manufacturer
(Uniroyal, 1967). This is a GC method in which NDMA is separated from
crop substrate by vacuum distillation, sorbed on polymer beads,
removed from the beads by baking and determined by micro-coulometric
gas chromatography (measurement of NH3). Sensitivity was estimated at
0.002 mg/kg. Recovery experiments at a fortification level of 0.010
mg/kg were reported to be adequate. This method has not been validated
in government laboratories.
Daminozide is not detected by any of the multi-residue screening
procedures used in government market basket surveys and monitoring
programs. There is no record of any government regulatory experience
with the colorimetric method for daminozide. It is questionable
whether by present standards it would be satisfactory for regulatory
purposes, particularly for enforcement of the lower tolerance levels.
As far as can be determined there is no confirmatory method of
analysis available. It would be desirable to have further information
on the reliability of this method, particularly with respect to (a)
whether it actually measures UDMH metabolites in free or conjugated
forms and (b) actual experience with the method in government
laboratories. An independent method, preferably a more specific GC or
HPLC method would be desirable.
APPRAISAL
Daminozide is a growth regulator which has been used on a variety of
fruits and vegetables since 1967. The product produces numerous
beneficial growth regulating effects. It is said to control premature
fruit drop, delay maturity, enhance fruit colour, reduce vine growth,
retard stem elongation and increase fruit set, among other claims.
Information submitted to the Joint Meeting by the primary manufacturer
indicates that the principal uses of the product are in the U.S.A.
Other information made available to the Meeting indicates that
daminozide is also used in Italy, South Africa, Japan, Australia,
NATIONAL TOLERANCES REPORTED TO THE MEETING
Commodity Tolerance, mg/kg
U.S. Canada Australia Netherlands
Fruits and vegetables 10
Sour cherries 55 55
Plums 50 20
Tomatoes 40 0.5
Apples 30 30 30
Nectarines 30
Peaches 30 25 30
Peanuts 30 20 30
Sweet cherries 30 30
Brussels sprouts 20 15
Peanut hay 20
Pears 20 15 30
Grapes 10 10
Peanut hulls 10
Melons 3
Peppers 1
Meat, fat and meat 0.2 0.2
by-products of cattle,
goats, horses, poultry
(except kidney), sheep
Poultry kidney 2
Eggs 0.2 0.2
Milk 0.02 0.05
Canada, Federal Republic of Germany, the United Kingdom and the
Netherlands. National tolerances have been established in the U.S.A.,
Canada, Australia and the Netherlands.
Daminozide was formerly manufactured by a process in which
dimethylnitrosamine was a starting reactant, and occurred as impurity
in the technical grade product. The primary manufacturer informed the
Meeting that a new process has been adopted in which
dimethylnitrosamine is not used. The recommended guideline limits
apply only to daminozide manufactured by the process in which
dimethylnitrosamine is excluded.
The compound is readily absorbed and translocated from foliar sprays.
While there in some evidence of degradation, the parent molecule in
free or loosely bound forms is persistent, particularly in the fruits.
The chemical structure of daminozide suggests the possible presence of
some metabolites of interest, including nitrosamines, hydrazides and
other hydrazine derivatives. No positive findings of these metabolites
were reported. The postulated metabolite N-dimethylnitrosamine was not
detected by a method said to be sensitive to 0.002 mg/kg. Indirect
evidence was reported of the absence of unsymmetrical
dimethylhydrazine (UDMH).
A feeding study with 14C daminozide in rats indicated that the
majority of the administered dose was excreted in the urine as
unchanged daminozide. There was no information on the metabolism
patterns in large animals or on metabolites in meat or milk. In
general, the information available on the fate of daminozide in plants
and animals is not very extensive, particularly with respect to the
nature of the terminal residues.
The analytical method currently available to enforce national
tolerances is a colorimetric method which measures unsymmetrical
dimethyl hydrazine (UDMH) released from daminozide by vigorous caustic
hydrolysis. The chromogenic agent is not specific for UDMH. Other
classes of compounds also produce colour complexes, including
aliphatic hydrazines, nitroso compounds and primary aromatic amines.
It is theorized that complexed daminozide, free UDMH and any other
metabolic products which would yield UDMH would be measured as
daminozide. It has been determined that the method has never been
applied in the U.S. or Canadian official surveillance programmes and
there is no information on experience with the method in other
government laboratories. The method would not appear to be very useful
for regulatory purposes.
Studies on the effects of processing show that the compound is not
destroyed and tends to concentrate in products such as tomato paste
and peanut meal. It has never been reported in foods in commerce (see
above comment on the analytical method). It is not persistent in
soils. Extensive data from supervised residue trials carried out in
the U.S.A. were available. Data on supervised trials on apples and
pears from the Netherlands were also reported. All analyses were by
the colorimetric method discussed above. The data generally showed the
highest residues in fruits, with considerable variability in harvest
residues. The residue values on a given crop followed almost a random
distribution. The experiments on which reports were received were all
designed to show harvest residues from registered use patterns and
therefore permitted no estimates of decline rate.
EVALUATION
As no ADI or temporary ADI could be allocated, no recommendations for
maximum residue limits could be made. Guideline levels are recorded
for the commodities listed below. The usual statements regarding the
intervals on which the levels are based are omitted in this case
because of the highly specialized uses of the product which are
related to stages of growth or maturation.
Commodity Guideline level, mg/kg
Sour cherries 60
Plums 50
Tomatoes 40
Apples, nectarines,
peaches, peanuts
(kernels), pears,
sweet cherries 30
Brussels sprouts 20
Grapes, peanut hay 10
Melons 3
Peppers 1
Eggs, meat 0.2*
Milk 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake for humans (ADI) and
maximum limits (MRL can be established)
1. An adequate long-term rat study.
2. Information on the biotransformation in animals.
Desirable
1. An analytical method more suitable for regulatory purposes than the
present colorimetric method.
2. Information on the occurrence of residues in foods in commerce.
3. Further identification of the terminal residues in crops and in
foods of animal origin.
REFERENCES
Anonymous, (1966) Acute Oral Administration-Male Rats. Unpublished
report from Hazleton Labs., Inc., submitted to the World Health
Organization by the Uniroyal Chemical Company.
Carson S., (1963) Toxicological Examination of Compound B-995.
Unpublished report from Food and Drug Research Labs., submitted to the
World Health Organization by Uniroyal Chemical Company.
Carson S., (1964) Subacute (90 day) Feeding Studies with B-995 in
Rats. Unpublished report from Food and Drug Research Labs., submitted
to the World Health Organization by Uniroyal Chemical Division of
Uniroyal,Corp.
Dahlgren, Dahlgren and Simmerman, (1963) Science, 148, 485; 1963
Edgerton, L.J., Rockey, M., Arnold, H., and Lisk, D.J., (1967)
"Colorimetric Determination of Alar Residues in Apples", J. Agr. Food
Chem. 15, no. 5, p. 812, 1967
Feigl, F., (1949) Chemistry of Specific Selective, and Sensitive
Reactions, p. 377, Academic Press, New York, 1949
Harris, L.E., (1975) "Guide for estimating toxic residues in animal
feeds or diets", U.S. National Technical Information Service,
accession no. PB-243-748-LK
Keplinger, M.L., Kennedy, G.L., and Haley, S., (1972) Teratogenic
Study with Alar in Albino Rats. Unpublished report from Industrial
Bio-Test Labs., submitted to the World Health Organization by the
Uniroyal Chemical Division of Uniroyal, Corp.
Magness, J.R., Markle, G.M., Compton, C.C., (1971) Food and Feed Crops
of the United States, Bull. 828, New Jersey Agricultural Experiment
Station, 1971
Oser, B.L., (1966) Chronic (2 year) Feeding Studies with B-995 in Rats
and Dogs. Unpublished report from Food and Drug Research Labs.,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Palmer, A.K. and Lovell, M.P., (1973) Dominant Lethal Assay of Alar in
the Male Mouse. Unpublished report from Huntingdon Research Center,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Pam, II., (1967) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education and Welfare, Food and Drug
Administration
Reed, (1963) Science, 148, 1097; 1963
Ryer, F.H., (1966) Final Report. Radiotracer Metabolism Study,
Alar-C14. Unpublished report from Hazleton Labs., Inc., submitted to
the World Health Organization by the United States Rubber Company.
St. John, Jr., L.E., Arnold, H. and Lesk, D.J., (1969) Metabolic
Studies with Alar Growth Regulator in the Dairy Cow.
J. Agr. Food Chem., 17, No. 11 116.117.
Uniroyal, (1966) Unpublished information, Uniroyal, Inc.v to U.S. Food
and Drug Administration
Uniroyal, (1967) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1973) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1977) Submission to Joint Meeting, May 17, 1977, Uniroyal,
Inc.
Other information on identity and properties
Daminozide is a white crystalline material of low volatility and
slight odour. It is soluble in polar solvents (g/100 g a 25°C: water
10; methanol 5; acetone 2.5). It is practically insoluble in aromatic
and aliphatic hydrocarbon solvents.
The technical grade product from the primary producer is typically
>99% daminozide. The manufacturing specifications require 98% minimum
and a melting point range of 154°C to 161°C. It is a white powder
which may contain traces of moisture, succinic acid, succinic
anhydride and a salt of daminozide and unsymmetrical dimethyl
hydrazine (UDMH). Volatile impurities are removed by an oven drying
step in the process. Daminozide was formerly manufactured by a process
in which dimethylnitrosamine was a starting reactant. The primary
manufacturer now uses a new process in which dimethylnitrosamine is
not used.
Daminozide is now commercially available only in formulations of
water-soluble powders containing 85% active ingredient and 15% inert
surfactants and mineral salts. Shelf-life studies show no degradation
of the formulations over a period of years. Liquid formulations
formerly produced are no longer commercially available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Daminozide is rapidly excreted and does not bioaccumulate in rats.
Two male and two female rats were treated with a single oral dose of
approximately 5 mg/kg unlabelled daminozide and 96 hours later with a
single 5 mg/kg oral dose of 14C-labelled daminozide (position of 14C
label unspecified) to study the absorption and excretion of
daminozide. After 2 days 69% of the administered dose had been
excreted in the faeces, 24% in the urine and 2.4% expired as 14CO2
Rats sacrificed 2 days after dosing contained an average of 0.35% of
the administered dose in the brain, liver, lung,,heart and spleen with
the majority of this residue in the liver. The rats sacrificed 4 days
after dosing contained only 0.03 or 0.12% of the administered dose in
these organs (Ryer, 1966).
A cow fed a diet containing 25 ppm daminozide for 4 days excreted 81%
of the daminozide in the faeces and 1.2% in the urine (St. John, Jr.
et al., 1969). See also "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on mutagenicity
Groups of male mice (20/group) were fed dietary levels of 0, 10, 300
or 10,000 ppm daminozide for 5 consecutive days to determine dominant
lethal effects. Following treatment, the males were monogamously
paired with untreated females, weekly, for 4 consecutive weeks.
Thirteen days after pairing females were killed and the uterine
contents examined for implantations, viable embryos and early or later
deaths.
No male deaths occurred during the test period and no signs of
compound effect on behavior were noted. During treatment, weight gain
at 10,000 ppm was marginally retarded. No effect was observed on
mating performance or pregnancy rate in any group. Neither
implantation rate nor viable litter size were significantly affected
by any treatment level. Inter-group differences in post-implantation
loss never attained significance (P>0.05) and showed no consistent
dose relationship (Palmer and Lovell, 1973).
Special study on teratogenicity
Groups of female rats (25/control group, 21/test group) were
administered 0, 250 or 500 mg/kg/day daminozide (12.5% w/v) in corn
oil, by gavage, from day 6 through 15 of gestation to determine
teratogenic effects. Daily observations were made and body weights
recorded on days 1, 9, 12 and 15. Records were kept of corpora lutea,
resorption sites, viable foetuses, foetal external and internal
abnormalities and skeletal development. Approximately equal numbers of
foetuses were examined for internal effects, using the Wilson
technique, and skeletal effects using clearing and alizarin staining
techniques.
Resorption sites, expressed as mean/female, were as follows: control,
0.4; 250 mg/kg, 1.1; 500 mg/kg, 0.7. Two females with a total of 10
resorption sites were included in the 250 mg/kg mean. Examination of
foetuses revealed no compound-related effects and none of the other
parameters were affected by compound administration (Keplinger et al.,
1972).
Special study on reproduction
Groups of rats (20/sex/group) were fed dietary levels of 0 or 300 ppm
daminozide, from weaning through three generations. The F0 group was
part of the 2 year feeding study described under "Long term studies".
PUP weights, number born alive or dead and the usual indexes of
reproductive performance were recorded. Data from two litters/3
generations revealed no significant effect on either fertility or
reproductive capacity. The pups survived equally well in the control
and test groups and no effect on growth was evident. Blood and urine
values were comparable to controls in each generation. Organ weights
were not affected nor was any compound-induced histopathology observed
(Oser, 1966).
Acute toxicity
Observed signs or oral intoxication were depression, ptosis, ataxia,
diarrhoea, excessive urination and laboured respiration. Dermal
application produced mild erythema and oedema.
TABLE 1. Acute toxicity of daminozide.
LD 50'
Species Route Sex mg/kg Reference
Rat Oral M 6,810 Anonymous, 1966
Oral M,F 8,400 Carson, 1963
Inhalation M 147 mg/l Carson, 1963
Rabbit Dermal M,F >10,000 Anonymous, 1966
Dermal M,F 16,000 Carson, 1963
Short term studies
Rat
Groups of rats (5 males and 5 females/group; 10 of each sex were
controls) were fed dietary levels of 0, 26, 80, 240, 720 or 2160
mg/kg/day daminozide technical for 90 days. No differences were noted
in body weight gains between any test group and the control gains. No
significant differences in haematological, blood chemistry or urine
values were observed and organ weights and ratio were comparable to
the control group. No gross or microscopic changes occurred in any
group which could be attributed to daminozide administration (Carson,
1964).
Dog
Groups of dogs (6/sex/control and 4/sex/test groups) were fed dietary
levels of 0, 300, 1000 or 3000 ppm daminozide technical for two years.
Body weight gains were within normal limits and comparable to control
gains. Appearance, behaviour, haematological, blood chemistry and
urine analyses of the test groups were also comparable to controls.
Gross necropsies, microscopic examination of tissues and organ weight
data revealed no compound or dose-related effects (Oser, 1966).
Long term studies
Rat
Groups of rats (37/sex/control and 25/sex/test group) were fed dietary
levels of 0, 300, 1000 and 3000 ppm diaminozide technical for two
years.
No differences in appearance or behaviour were observed between
control and test rats during the study. Throughout the study the body
weights gains of the 300 ppm group were slightly less than those of
the controls, but weight gains of the 1000 and 3000 ppm groups were
comparable to the control gains. No haematological or blood chemistry
differences related to compound consumption were noted during the
study. Survival over two years was not affected and was comparable
between all groups. Liver/body weight ratios for both sexes in the 300
and 3000 ppm groups were significantly higher than the control ratio
at 104 weeks. In the 300 ppm male group the mean liver weight was 21%
higher than the mean control liver weight. Nothing in the gross or
microscopic examinations explains this difference. Microscopic
examination of tissues revealed no dose-related effects. Tumors were
both benign and malignant, the former being principally mammary
adenomas or fibromas. In the latter category, reticulum cell sarcomas
predominated in both the test and control groups, the incidence being
not significantly higher at the 3000 ppm level than in the control
group. Several varied types of malignant neoplasms were also noted but
in no cage was there more than one of each type in any group (Oser,
1966).
COMMENTS
Daminozide is not highly acutely toxic as measured by LD50 values in
the g/kg range for oral and dermal studies and is only mildly
irritating to the eyes and skin. It is rapidly excreted and does not
bioaccumulate in mammals. Daminozide did not produce dominant lethal
effects in male mice at 10,000 ppm and no teratological or
reproductive effects were observed at levels of 300 ppm or lower. An
adequate two-year dog study has been conducted.
The long-term rat study was inadequate because of internal conflicts
within the report and because of the inability to evaluate the
carcinogenic potential of daminozide. Information on the
biotransformation of daminozide in animals is needed.
Because of the above concerns no recommendation for an acceptable
daily intake for man can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Daminozide is a plant growth regulator applied only in foliar sprays
to various tree fruits, grapes, vegetables, melons and peanuts.
Depending on the crop and timing of treatment, it is variously
intended to hasten ripening, increase fruit set, enhance fruit colour,
retard stem elongation, control fruit drop, delay maturity, reduce
vine growth or to produce certain other beneficial growth regulating
effects.
Introduced on an experimental basis in the early 1960s, it was first
registered for use on ornamentals. Since that time there have been
numerous reports in the horticultural journals on the efficacy of the
compound as a growth regulator. Use was expanded to include food crops
in 1968 in the U.S.A. Table 2 shows current (1977) registered use
patterns in the U.S.A. Uses in Canada are similar to the U.S. uses.
There was other information available to the Meeting that the product
has been used in Italy, South Africa, Japan, Australia, Federal
Republic of Germany, the Netherlands and the United Kingdom. The
directions for use on the product labelling accepted in those
countries generally conform to the use patterns shown in Table 2.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trials in the U.S.A. on fourteen crops and controlled feeding
experiments with meat and dairy animals (Uniroyal, 1977). The
government of the Netherlands submitted reports of supervised residue
trials on apples and pears. Analyses were by the colorimetric
procedure described below under "Methods of residue analysis".
Analyses of untreated controls (crop blanks) were reported in each
experiment, along with % recovery of daminozide from fortified
controls. Test samples were corrected for crop blank. Recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form where such presentation
is feasible.
Apples
Field trials were conducted at locations in 10 states representing all
of the major apple growing areas of the U.S.A. Some 180 harvest
samples were analyzed in duplicate. The wide range of experimental
conditions in the various field tests and the resulting wide range of
residues found, preclude a tabular summary of data. The residues
ranged from 0.1 mg/kg to as high as 80 mg/kg. If the high values
resulting from exaggerated treatments are excluded, the data indicate
that residues would not be likely to exceed 30 mg/kg when the normal
dosages (Table 2) are applied and the prescribed 60-70 day preharvest
interval is observed.
TABLE 2. Registered use patterns for daminozide in the U.S.A. (1977).
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
sweet cherries hasten 0.1-0.2% 1 2 weeks after full
ripening, bloom
concentrate
maturity
sour cherries " 0.4% 1 "
peaches, hasten 0.1-0.2% 1 13 weeks post-bloom
nectarines ripening, to pit hardening
concentrate
maturity
pears prevent fruit drop 0.1% 1 18 to 24 days before harvest
apples multiple effects(1) 0.075-0.2% 160 to 70 days before harvest
prunes hasten ripening 0.05-0.1% 1 1 month before
harvest
grapes increase fruit set, 0.05-0.1% 1 from 1st bloom to
reduce vine growth (1.4-2.4 kg/ha) full bloom
Brussels uniform sprout 1.9-3.75 kg/ha 1 30 days before
sprouts development harvest
cantaloupes reduce vine growth 1.9 kg/ha 1 2 to 4 leaf stage
TABLE 2. (Continued)
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
tomatoes multiple effects(1) 0.6-2.4 kg/ha 2 before transplanting
4.8 kg/ha 1 after transplant - not
within 7 days
of picking
peanuts multiple effects(1) 1.0 kg/ha 2 at pegging time
0.5 kg/ha 30 days before
harvest
peppers 0.5-2.3 kg/ha 2 at 2-4 leaf stage
(seedlings) and 7-10 days
later
(1) There are 9 separate beneficial effects claimed for apples alone. Other claims are similarly complex. The directions for use of this
product are complex because of many factors (plant vigour, variety, moisture stress, etc.) which can not only modify the desired effect
on the plant but can contribute adverse effects.
The residues from trials in the Netherlands were generally 5 mg/kg or
less. The rates of application in these experiments were somewhat
lower than the rates permitted in some other countries.
Studies designed to measure initial deposits or residue decline rate
(half-life) were not included. The data clearly show, however, that
daminozide is extremely persistent in apples. Significant residues
remain for more than 159 days after the spray application.
Brussels sprouts
Four separate trials were conducted at locations in California and New
York. A single treatment was made at normal and exaggerated rates.
Crop blanks were reported as zero. Recoveries were 87 and 83% at 1 and
10 mg/kg respectively. The data are summarized in Table 3. Under good
agricultural practice the residues would not be likely to exceed 20
mg/kg.
TABLE 3. Residues in Brussels sprouts, mg/kg (single application).
Application rate, Interval, days from treatment
kg a.i./ha 30 33 46
1.9 6.3, 5.2, 6.8 7.1, 1.4, 0.0
3.4 2.8, 1.4
3.8 14.6, 15.5, 13.0 2.3, 2-7, 1.5
6.8* 4.8, 3.2
7.7* 25.4, 24.8, 24.6 5.6, 6.2, 5.2
*excessive dosage
Cantaloupes
Nine residue trials were reported from locations in California,
Arizona, Texas and Michigan. The registered use patterns on melons
provide for treatment when the plant is at the 2-4 leaf stage, at
which point no fruit would be present. The experiments included
exaggerated treatment rates, and samples taken as early as 44 days
after treatment. If these samples were mature melons, fruit must have
been present at the time of treatment (melons take from 85 to 150 days
from planting to maturity). Even under these conditions the residue
values of some 20 samples ranged from < 0.1 to 1.6 mg/kg (Table 4)
compared with apparent daminozide in untreated controls up to 1.1
mg/kg. It would appear that residues to be expected in cantaloupes
from good agricultural practice would be of a lower order than those
found in the tree fruits. It is not likely that residues in excess of
3 mg/kg would occur in cantaloupes. Data were not available on other
melon varieties, but the conclusion regarding cantaloupes could be
extrapolated to other melons.
TABLE 4. Residues in cantaloupes,mg/kg
Interval from No. of Application Rate,
last treatment treatments kg a.i./ha
days 0.47 0.95 1.9 3.8 7.6
44 2* <0.1 1.6
50 1 1.0,1.5 1.5,1.6
57 1 0.6,0.8 1.2,1.6
68 1 1.2 1.6
73 2* <0.1 <0.1
87 1 <0.1 <0.1
115 2* <0.1 <0.1
*Rate given for second treatment only.
Sour cherries
Trials were carried out at several locations in four states
representing the important cherry producing areas in the U.S.A. A
total of 70 test samples and 25 control samples were analyzed. Crop
blanks ranged from 0.1 to 0.64 mg/kg.
The dosages applied in the tests ranged from 25% of the manufacturer's
minimum recommended dosage to twice the maximum recommended rate (See
Table 2). Samples were taken at periods after treatment ranging from
56 to 65 days. This roughly corresponds to the label directions which
call for a single spray 10-14 days after full bloom. The season from
bloom to harvest in the U.S.A. is 80 to 100 days (Magness, 1971).
The sampling schedule in all trials was designed to show residues at
normal harvest. Therefore, no estimates of initial deposit or residue
decline rate are possible. Overall the residues found on mature
cherries ranged from 5.3 to 78 mg/kg. Although some correlation could
be made with application rate, the variation in harvest residues
appeared to be almost random (the data do not lend themselves to
tabulation). If the data from trials with excessive or minimal dosages
were excluded, the residues fall between 10 and 53 mg/kg. It is not
likely that residues under actual (commercial) conditions would ever
exceed 60 mg/kg.
Sweet cherries
Analyses of 176 test samples and 39 control samples from field trials
in five states were made available. The experimental design generally
paralleled that discussed above for sour cherries. That is, the trials
were designed to show residues on harvested cherries treated
approximately as prescribed on the product label (2 weeks after full
bloom). The overall range of residues found (1 to 54 mg/kg) was
comparable to that reported on sour cherries. If only the data from
trials following good agricultural practice (See Table 2) are used,
most samples were in the range of 9-18 mg/kg, with a few higher values
approaching 30 mg/kg. The difference between the residue load expected
on sweet and sour cherries is mainly due to the application rate,
which is 0.2% for sweet and 0.4% for sour cherries. A maximum residue
limit of 30 mg/kg for sweet cherries would therefore be consistent.
Grapes
The residue data are summarized in Table 5. Some 44 samples from field
trials in 4 states were reported. Analyses were in duplicate,
including 8 untreated control samples. All controls were reported as
0.1 mg/kg, apparent daminozide.
The registered use directions for grapes (Table 2 requires treatment
during the period from first bloom to full bloom. The period between
bloom and harvest for grape varieties will differ greatly. In some
varieties it may be as much as 5-6 months (Magness, 1971). It is
interesting to note that in the field trials, the intervals between
spray and harvest were from 102 to 111 days, and probably represent an
early maturing variety, probably Concord. From the standpoint of
residues at harvest, it therefore represents the "worst case". Even
with some exaggerated dosages, all residues were below 10 mg/kg.
TABLE 5. Residues in grapes, single application, 102-111 days from
treatment
Application rate Residue range,
% a.i. mg/kg
0.05 0.4 - 2.5
0.10 1.3 - 5.4
0.20 2.6 - 9.5
Nectarines and peaches
The use directions for these two fruits are the same. They also incur
residues to the same degree, and may be discussed together.
The prescribed cut-off date for sprays is at "pit hardening".
Presumably, this growth stage is recognizable to experienced fruit
growers. As near as can be determined, pit hardening occurs 40 to 80
days before harvest, depending on variety, geographic location and
other factors.
The data made available on nectarines and peaches included a total of
more than 500 analyses including 70 untreated controls. Treatments
were made at the prescribed rate, at excessive rates, and at less than
the prescribed rate. The intervals between treatment and sampling were
variously identified as treatments at "style abscission", pit
hardening, and in some cases by numerical day before harvest. Owing to
the variables in the field tests, a wide range of residues was
reported (1.0 to 93.0 mg/kg).
Interest is mainly in the residues from applications closely
approximating the registered use pattern (Table 2). The distribution
of residues from application of 0.1 to 0.2% sprays in the period from
style abscission (3 weeks post-bloom) to pit hardening in nectarines
and peaches was:
mg/kg <1 1-5 5-10 10-15 15-20 20-25 >25
No. of samples 35 140 48 45 3 7 1
The distribution indicates that residues would not be likely to exceed
30 mg/kg when uses described in Table 2 are followed.
Peanuts
Results of residue trials in Georgia, N. Carolina, Texas, Alabama,
Florida and Oklahoma were submitted to the Meeting. Separate analyses
were made on kernels, hay and hulls.
Kernels
A total of 166 analyses of test samples and 13 of controls were made.
All controls samples were zero except two reported at 3.1 and 2.8
mg/kg. (The analyst noted that contamination was suspected.)
The manufacturer's recommendations are for one treatment of 0.96 kg
a.i./ha at pegging time followed by a second treatment of 0.47 kg
a.i./ha thirty days before harvest. For those field trials in which
the treatment approximated the recommended pattern, the following
residue distribution was obtained:
Range, mg/kg: <5.0 5-10 10-15 15-20 20-25 25-30
Number of samples: 31 20 7 4 4 2
From this skewed distribution pattern it is apparent that maximum
residue limit of 30 mg/kg for kernels would be adequate to cover all
residues from good agricultural practice.
Peanut hay
62 analyses were submitted, 24 of which corresponded to the normal
split dosage pattern mentioned above. Residues in the trials
reflecting recommended usage were mostly in the range of 1-5 mg/kg.
However, three were in the range of 5-10 mg/kg and three were greater
than 10 mg/kg. A maximum residue limit of 20 mg/kg would be adequate.
Peanut hulls
Very limited data were available on peanut hulls. Residues were 2
mg/kg or less. Peanut hulls are not an important item of international
trade. The only interest in this commodity is that the hulls are
incorporated at low levels into some mixed animal feeds.
Peanut meal
Field-treated peanuts which were determined to be carrying 12 mg/kg of
daminozide were processed in a laboratory experiment simulating a
commercial process. The meal presscake was found to contain 30-33
mg/kg. On the basis of this approximately three-fold concentration
factor, peanut kernels bearing the maximum residues of 20 mg/kg would
be expected to produce meal containing about 60 mg/kg.
Pears
Limited data (14 test samples + 7 controls) from Washington and Oregon
were available. Seven samples were from trials in which the
manufacturer's recommended rate was applied (0.1% spray). Residues at
this dosage ranged from 0.8-17.0 mg/kg, with an average of 10.8 mg/kg.
Residues from uses according to good agricultural practice would
probably not exceed 20 mg/kg.
Peppers
Field trials were conducted in Texas, Georgia, Connecticut, Delaware
and Michigan. Samples were collected 77-125 days after treatment.
Single and double applications were made at the prescribed rate and at
four times the prescribed rate. The net difference between the
apparent daminozide in the test samples and the control samples was
less than 1 mg/kg in all cases, the highest residue reported being
0.44 mg/kg. A maximum residue limit of 1 mg/kg would be adequate.
Plums
Residue trials at 6 locations in California were reported to the
Meeting. The plums (fresh prunes) were treated at the prescribed rate
(Table 2) and at rates up to 4 times the prescribed rate. The interval
between treatment and sampling ranged from 34 to 78 days (as compared
with the registered label requirement of a 30 day pre-harvest
interval). Residues from all treatment rates ranged from 3.0 to 71.5
mg/kg. Residues from the normal rates were 3.0 to 43.7 mg/kg. It is
unlikely that residues from normal usage would exceed 50 mg/kg.
Field-treated fresh prunes were analyzed and converted to dried prunes
by the commercial procedure. This involves a 5-10 minute mechanical
wash and drying on trays at 170°F for 30 hours. The maximum
concentration factor (mg/kg in dried prunes: mg/kg in fresh) was found
to be 2.7. On this basis, a prediction can be made that fresh prunes
bearing maximum residues of 50 mg/kg would produce dried prunes
containing 135 mg/kg.
Tomatoes
The registered uses of daminozide on tomatoes include 2 applications
prior to transplanting (greenhouse or outdoors) and one application in
the field no later than 7 days prior to picking (see Table 2). The
application on plants to be transplanted is for all practical purposes
a "no-residue" use. Residue data were made available on tomatoes which
had received both transplant and field treatments at the normal and
excessive rates. All residue trials (7) were carried out in Florida,
on green tomatoes. The only field use for daminozide on tomatoes
permitted in the U.S.A. is on Florida winter tomatoes which are
harvested green and artificially ripened. These residue trials
therefore were highly specialized and do not necessarily reflect
residues which might result from more general usage on tomatoes. The
data are shown in Table 6.
TABLE 6. Residues in tomatoes, mg/kg
Interval from Application Rate*
last treatment kg a.i./ha
Days 4.76 9.52
11 13 68.5
21 9 35
7 6 8
7 8 12.5
18 12 20.5
14 20 45.5
19 15 28
29 22 8
* Field treatment after transplant use.
Alfalfa
There are no national tolerances or registrations for daminozide use
on alfalfa. However, the product has some utility in the culture of
alfalfa seed, and registration is pending in U.S.A. In growing alfalfa
for seed there is some utilization of alfalfa forage, hay, and seed
screenings for animal feeds. The submission to the Meeting therefore
contained some residue data on alfalfa hay, fresh forage, and seed
screenings. Residues on fresh alfalfa ranged from 4-12 mg/kg and on
hay from 2-20 mg/kg. Seed screenings showed a maximum of 1.4 mg/kg. In
view of the specialized nature of the use it would not appear
necessary at this time for the meeting to recommend a maximum residue
limit.
Meat, milk, poultry and eggs
Daminozide is used on a number of crops which yield by-products used
commercially in animal feeds. Primary daminozide sources would be
peanut meal and peanut hay. Some additional contribution to the diet
could occur from peanut hulls and the pomaces resulting from
processing apples, grapes and tomatoes. In recognition of this, the
basic manufacturer submitted to the Meeting some animal feeding,
studies (Uniroyal, 1977).
(a) Cattle were fed at levels of 0, 20, 60 and 200 ppm in the total
diet for 6 weeks. Milk was collected at intervals and the animals
slaughtered at the end of the feeding period. No daminozide residues
were found in any tissues (muscle, fat, liver, kidney) by a method
with a lower limit of detection estimated at 0.2 mg/kg. Trace residues
were found in the milk of animals on the 60 and 200 ppm diets at
levels of 0.05 and 0.07 mg/kg respectively.
(b) Hogs were fed 0, 60 and 300 ppm in the diet (2 animals at each
level) for 31 days. No daminozide residues were detected (40.2 mg/kg)
in muscle or fat. Apparent daminozide residues were found in liver and
kidney, at 0.3 and 0.8 mg/kg respectively, in animals on the highest
feeding level.
(c) Poultry were fed 0, 20, 60 and 200 ppm in the diet (10 hens in
each group) for 31 days. Eggs were composited weekly and on day 31 all
birds were sacrificed. On the final sampling, eggs from the 300 ppm
and 60 ppm feeding levels contained 0.3-0.5 and 0.25 mg/kg
respectively. No detectable residues were found (< 0.2 mg/kg) in eggs
from the 20 ppm feeding level. No residues were found in muscle or fat
of birds on the lower feeding levels. At the 200 ppm level, residues
of 0.4 and 0.7 mg/kg were found respectively in muscle and fat.
Residues of 1 and 1.1 mg/kg were found in liver of birds on the
highest level. Daminozide concentrates in poultry kidney. Values of
1.3, 1.8 and 7.4 mg/kg were reported in kidney for the 3 feeding
levels.
The studies show that residues occur at high feeding levels, but this
must be related to the actual anticipated intake from peanut meal and
peanut hay. Peanut hay would be used to the extent of 25% in beef
cattle diets, 60% in dairy diets, and 10% for pigs. Peanut meal can
make up 15%-beef; 25%-dairy, 10%-poultry and 10%-pigs (Harris, 1975).
After adjusting for the proportion of peanut meal and hay in the total
diet of each species, it is apparent that the residues in milk, eggs
and tissues (except poultry kidney) Will be below the level of
detection of the colorimetric method.
FATE OF RESIDUES
General
Daminozide is readily absorbed and translocated from foliar sprays.
The entrapment of 14CO2 from applications of labelled daminozide
indicates that, under certain conditions, at least part of the applied
pesticide is fully degraded. Other data show that daminozide, in free
or loosely bound form, is the principal residue component and very
persistent in fruits. Many of the conclusions regarding the nature of
the terminal residues rest on characterization by selective solvent
extractions, in vitro experiments and analysis by wet chemical
procedures for predicted metabolites. In view of the known biological
activity of some postulated metabolites of the compound, particularly
nitrosamines, hydrazides and other hydrazine derivatives, further
identification of the residues in harvested fruits and vegetables
would be desirable.
In animals
Rats were fed 14C-labelled daminozide and the excreta monitored. The
majority of the administered dose, apparently occurred in the urine as
unchanged daminozide. As far as can be determined, there has been no
effort to study metabolic pathways in meat or milk animals with radio-
labelled daminozide. However, analyses by the chemical method
(previously discussed) were made for one postulated metabolite (NDMA)
in milk from cows fed 200 ppm daminozide in the diet. No NDMA was
found at a minimum detection level of 0.03 mg/kg. See also the
sections "Biochemical aspects" and "Residues resulting from supervised
trials".
In plants
It has been reported that daminozide decomposes to unsymmetrical
dimethylhydrazine (UDMH) in the plant and that it is the latter
compound which is the active principle through its effect on the plant
enzyme diamine oxidase (Reed, 1963). Another investigator (Dahlgren,
1963) found that maleic acid dimethyl hydrazide (CO11, a related
growth regulator) is hydrolysed to UDMH in aqueous solutions. Dahlgren
noted that daminozide was stable under the same conditions. In
experiments reported by the manufacturer (Uniroyal, 1967) no free UDMH
or succinic acid was detected after attempts (in vitro) to enzymically
hydrolyze daminozide with trypsin, proteinase, papain, ficin and
urease. No evidence was found of hydrolysis to UDMH and succinic acid
in macerates of plant leaves by colorimetric tests for UDMH and paper
chromatographic tests for succinic acid. While the evidence regarding
the occurrence of UDMH is somewhat contradictory it is likely that,
even if UDMH occurs as an intermediate metabolite, its presence would
be fleeting owing to its reactivity and that it would not be present
per se in harvested crops.
The gas-chromatographic method of analysis for nitrosodimethylamine
(NDMA), discussed below ("Methods of residue analysis",) has been used
in studies intended to show the absence of this possible oxidation
product in treated crops (Uniroyal, 1967). No NDMA was detected in
treated apples, tomatoes or peanut foliage by the method, which is
said to be sensitive to 0.002 mg/kg NDMA.
Radiotracer studies were conducted by the U.S. Department of
Agriculture (Uniroyal, 1966) on apple trees in the field and on apple
seedlings in the greenhouse. Daminozide was labelled in the succinic
acid moiety and (in a separate test) in the UDMH group. Activity in
the sprayed seedlings was distributed throughout the plant with
highest activity in the leaves. 20% of the original activity was
released as 14CO2 in the first seven weeks after application.
In the field studies, 15% of the total applied activity remained in
the tree after 100 days. The activity in the apple fruit after 125
days was identified as 75-85% unmetabolized daminozide. Trace amounts
of succinic acid were identified and there was some incorporation of
14CO2 into plant components.
14C-labelled daminozide was used in metabolism studies with peanut
plants (Uniroyal, 1977). Characterization of the activity by selective
solvent extraction indicated that the majority of the activity was
present in bound form, probably as sugar complexes. Daminozide per se
was recoverable from the extracts and it was concluded that residues
present in peanuts in complexed form would be detected by the
colorimetric method of analysis. Analyses of peanut oil for 14C
activity showed residues of about 2-5 mg/kg expressed as daminozide.
Selective solvent extraction of the oil indicated that the activity
was not daminozide but was probably in the form of fatty acid
glycerides.
In soil
Residue decline studies were conducted on sandy loam and clay soils
spiked at 5 and 10 mg/kg. Daminozide per se is not persistent in
soils. The breakdown is probably through microbial action. In the clay
soil, < 0.1 mg/kg was present two days after treatment. The sandy
loam contained 0.1 mg/kg at 2 and 3 weeks respectively at the 5 and 10
mg/kg fortification levels. The compound is very mobile in soils
because of its water solubility.
In processing
Studies show that in the commercial processing of peanuts there is no
appreciable concentration of residues in the refined peanut oil. There
is, however, a 3 fold concentration of residues in peanut meal (e.g.,
peanut meats bearing 20 mg/kg will produce peanut meal bearing 60
mg/kg).
Data on the processing of fresh tomatoes bearing approximately 40
mg/kg into ketchup and tomato paste show that there is essentially no
loss of daminozide residues in processing. (The residues concentrate
to the same degree as the solids do in the concentration process.)
Residues found in tomato paste ranged from 155 to 197 mg/kg. Analyses
for nitrosodimethylamine (NDMA) in tomato paste were negative by the
chemical method with a reported sensitivity of 0.002 mg/kg.
Experiments in which field-treated plums were processed into dried
prunes showed that residues were concentrated in the dried fruit by a
factor of 2.7. See "Residues resulting from supervised trials" for
details of this work and of the effect on residues of processing
peanuts to peanut meal.
There are national tolerances on grapes (10 ppm, U.S.A. and Canada)
but no data were made available on transfer of residues to wine or
grape pomace.
Evidence of residues in food in commerce or at consumption
As noted under "Methods of residue analysis", daminozide is not
detected by any of the multi-residue screening methods currently used
in market basket surveys, monitoring, or government regulatory
programmes. The "specific" colorimetric method has not been employed
in the Canadian or the U.S. Food and Drug Administration surveillance
programme and there is no record of a finding of daminozide in foods
in commerce. In view of the demonstrated stability of daminozide
residues in food processing, and the relatively high levels incurred
in fruits from good agricultural practices, it may be concluded that
residues are occurring in the diet in those countries where there is
substantial use.
METHODS OF RESIDUE ANALYSIS
Daminozide residues in crops are determined by a colorimetric method
(PAM II, 1967). The method involves hydrolysis in boiling 50% sodium
hydroxide to release unsymetrical dimethylhydrazine (UDMH) which is
distilled and reacted with trisodium pentacyanoamine ferroate (TPF) at
pH 5.0. The red colour produced is measured spectrophotometrically at
490 and 600 nm. The difference between the two readings is plotted as
net absorbance against µg of compound in a calibration curve.
Modifications of this basic method permit determination of daminozide
in peanuts, animal tissues, milk and eggs.
The chromogenic agent TPF used for determination of daminozide also
forms colored complexes with nitroso compounds, primary aromatic
amines and aliphatic or aromatic hydrazines (Feigl, 1949). Some
possible metabolites of daminozide would therefore contribute
absorbance which would be additive to that of the daminozide-TPF
complex. If present they would be interpreted as daminozide residues
(but not measured quantitatively).
The method has been tested in government regulatory laboratories on
grapes and animal tissues. Because of large and variable crop blanks
it was concluded that a reliable lower limit of determination for
daminozide in samples of plant origin was no better than 1 mg/kg for
regulatory purposes. The estimated sensitivity for animal products is
0.05 mg/kg for milk and 0.2 mg/kg for tissues and eggs. The government
analyst noted that 2 hours colour development time was required rather
than the 1 hour prescribed in the procedure.
The assumption was made that any free UDMH, or any metabolites which
would yield UDMH under the rigorous caustic hydrolysis, would also be
measured as daminozide by this method. There are no experimental data
to confirm that conclusion. Whether in fact any such metabolites
actually occur in crops at harvest is uncertain (see discussion under
"Fate of residues").
Colorimetric methods are ordinarily not specific. In this case, the
method was tried in the presence of most pesticides used on the
subject crops (in 1968) to gauge interference. Only the fungicide
"Botran" was found to interfere, and this interference can be
eliminated by a modification of the method.
A second colorimetric method of analysis for daminozide in plant
substrates has been published (Edgerton, 1967). This method also
measures UDMH in a hydrolysate, but colour development is with
phosphomolybdic acid. Sensitivity is comparable to the colorimetric
method discussed above.
As noted above, the colorimetric method for the parent compound would
also measure any alkylnitrosamines as daminozide. Because of concern
for the toxic potential of a possible metabolite, nitrosodimethylamine
(NDMA), a "specific" method for NDMA was developed by the manufacturer
(Uniroyal, 1967). This is a GC method in which NDMA is separated from
crop substrate by vacuum distillation, sorbed on polymer beads,
removed from the beads by baking and determined by micro-coulometric
gas chromatography (measurement of NH3). Sensitivity was estimated at
0.002 mg/kg. Recovery experiments at a fortification level of 0.010
mg/kg were reported to be adequate. This method has not been validated
in government laboratories.
Daminozide is not detected by any of the multi-residue screening
procedures used in government market basket surveys and monitoring
programs. There is no record of any government regulatory experience
with the colorimetric method for daminozide. It is questionable
whether by present standards it would be satisfactory for regulatory
purposes, particularly for enforcement of the lower tolerance levels.
As far as can be determined there is no confirmatory method of
analysis available. It would be desirable to have further information
on the reliability of this method, particularly with respect to (a)
whether it actually measures UDMH metabolites in free or conjugated
forms and (b) actual experience with the method in government
laboratories. An independent method, preferably a more specific GC or
HPLC method would be desirable.
APPRAISAL
Daminozide is a growth regulator which has been used on a variety of
fruits and vegetables since 1967. The product produces numerous
beneficial growth regulating effects. It is said to control premature
fruit drop, delay maturity, enhance fruit colour, reduce vine growth,
retard stem elongation and increase fruit set, among other claims.
Information submitted to the Joint Meeting by the primary manufacturer
indicates that the principal uses of the product are in the U.S.A.
Other information made available to the Meeting indicates that
daminozide is also used in Italy, South Africa, Japan, Australia,
NATIONAL TOLERANCES REPORTED TO THE MEETING
Commodity Tolerance, mg/kg
U.S. Canada Australia Netherlands
Fruits and vegetables 10
Sour cherries 55 55
Plums 50 20
Tomatoes 40 0.5
Apples 30 30 30
Nectarines 30
Peaches 30 25 30
Peanuts 30 20 30
Sweet cherries 30 30
Brussels sprouts 20 15
Peanut hay 20
Pears 20 15 30
Grapes 10 10
Peanut hulls 10
Melons 3
Peppers 1
Meat, fat and meat 0.2 0.2
by-products of cattle,
goats, horses, poultry
(except kidney), sheep
Poultry kidney 2
Eggs 0.2 0.2
Milk 0.02 0.05
Canada, Federal Republic of Germany, the United Kingdom and the
Netherlands. National tolerances have been established in the U.S.A.,
Canada, Australia and the Netherlands.
Daminozide was formerly manufactured by a process in which
dimethylnitrosamine was a starting reactant, and occurred as impurity
in the technical grade product. The primary manufacturer informed the
Meeting that a new process has been adopted in which
dimethylnitrosamine is not used. The recommended guideline limits
apply only to daminozide manufactured by the process in which
dimethylnitrosamine is excluded.
The compound is readily absorbed and translocated from foliar sprays.
While there in some evidence of degradation, the parent molecule in
free or loosely bound forms is persistent, particularly in the fruits.
The chemical structure of daminozide suggests the possible presence of
some metabolites of interest, including nitrosamines, hydrazides and
other hydrazine derivatives. No positive findings of these metabolites
were reported. The postulated metabolite N-dimethylnitrosamine was not
detected by a method said to be sensitive to 0.002 mg/kg. Indirect
evidence was reported of the absence of unsymmetrical
dimethylhydrazine (UDMH).
A feeding study with 14C daminozide in rats indicated that the
majority of the administered dose was excreted in the urine as
unchanged daminozide. There was no information on the metabolism
patterns in large animals or on metabolites in meat or milk. In
general, the information available on the fate of daminozide in plants
and animals is not very extensive, particularly with respect to the
nature of the terminal residues.
The analytical method currently available to enforce national
tolerances is a colorimetric method which measures unsymmetrical
dimethyl hydrazine (UDMH) released from daminozide by vigorous caustic
hydrolysis. The chromogenic agent is not specific for UDMH. Other
classes of compounds also produce colour complexes, including
aliphatic hydrazines, nitroso compounds and primary aromatic amines.
It is theorized that complexed daminozide, free UDMH and any other
metabolic products which would yield UDMH would be measured as
daminozide. It has been determined that the method has never been
applied in the U.S. or Canadian official surveillance programmes and
there is no information on experience with the method in other
government laboratories. The method would not appear to be very useful
for regulatory purposes.
Studies on the effects of processing show that the compound is not
destroyed and tends to concentrate in products such as tomato paste
and peanut meal. It has never been reported in foods in commerce (see
above comment on the analytical method). It is not persistent in
soils. Extensive data from supervised residue trials carried out in
the U.S.A. were available. Data on supervised trials on apples and
pears from the Netherlands were also reported. All analyses were by
the colorimetric method discussed above. The data generally showed the
highest residues in fruits, with considerable variability in harvest
residues. The residue values on a given crop followed almost a random
distribution. The experiments on which reports were received were all
designed to show harvest residues from registered use patterns and
therefore permitted no estimates of decline rate.
EVALUATION
As no ADI or temporary ADI could be allocated, no recommendations for
maximum residue limits could be made. Guideline levels are recorded
for the commodities listed below. The usual statements regarding the
intervals on which the levels are based are omitted in this case
because of the highly specialized uses of the product which are
related to stages of growth or maturation.
Commodity Guideline level, mg/kg
Sour cherries 60
Plums 50
Tomatoes 40
Apples, nectarines,
peaches, peanuts
(kernels), pears,
sweet cherries 30
Brussels sprouts 20
Grapes, peanut hay 10
Melons 3
Peppers 1
Eggs, meat 0.2*
Milk 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake for humans (ADI) and
maximum limits (MRL can be established)
1. An adequate long-term rat study.
2. Information on the biotransformation in animals.
Desirable
1. An analytical method more suitable for regulatory purposes than the
present colorimetric method.
2. Information on the occurrence of residues in foods in commerce.
3. Further identification of the terminal residues in crops and in
foods of animal origin.
REFERENCES
Anonymous, (1966) Acute Oral Administration-Male Rats. Unpublished
report from Hazleton Labs., Inc., submitted to the World Health
Organization by the Uniroyal Chemical Company.
Carson S., (1963) Toxicological Examination of Compound B-995.
Unpublished report from Food and Drug Research Labs., submitted to the
World Health Organization by Uniroyal Chemical Company.
Carson S., (1964) Subacute (90 day) Feeding Studies with B-995 in
Rats. Unpublished report from Food and Drug Research Labs., submitted
to the World Health Organization by Uniroyal Chemical Division of
Uniroyal,Corp.
Dahlgren, Dahlgren and Simmerman, (1963) Science, 148, 485; 1963
Edgerton, L.J., Rockey, M., Arnold, H., and Lisk, D.J., (1967)
"Colorimetric Determination of Alar Residues in Apples", J. Agr. Food
Chem. 15, no. 5, p. 812, 1967
Feigl, F., (1949) Chemistry of Specific Selective, and Sensitive
Reactions, p. 377, Academic Press, New York, 1949
Harris, L.E., (1975) "Guide for estimating toxic residues in animal
feeds or diets", U.S. National Technical Information Service,
accession no. PB-243-748-LK
Keplinger, M.L., Kennedy, G.L., and Haley, S., (1972) Teratogenic
Study with Alar in Albino Rats. Unpublished report from Industrial
Bio-Test Labs., submitted to the World Health Organization by the
Uniroyal Chemical Division of Uniroyal, Corp.
Magness, J.R., Markle, G.M., Compton, C.C., (1971) Food and Feed Crops
of the United States, Bull. 828, New Jersey Agricultural Experiment
Station, 1971
Oser, B.L., (1966) Chronic (2 year) Feeding Studies with B-995 in Rats
and Dogs. Unpublished report from Food and Drug Research Labs.,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Palmer, A.K. and Lovell, M.P., (1973) Dominant Lethal Assay of Alar in
the Male Mouse. Unpublished report from Huntingdon Research Center,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Pam, II., (1967) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education and Welfare, Food and Drug
Administration
Reed, (1963) Science, 148, 1097; 1963
Ryer, F.H., (1966) Final Report. Radiotracer Metabolism Study,
Alar-C14. Unpublished report from Hazleton Labs., Inc., submitted to
the World Health Organization by the United States Rubber Company.
St. John, Jr., L.E., Arnold, H. and Lesk, D.J., (1969) Metabolic
Studies with Alar Growth Regulator in the Dairy Cow.
J. Agr. Food Chem., 17, No. 11 116.117.
Uniroyal, (1966) Unpublished information, Uniroyal, Inc.v to U.S. Food
and Drug Administration
Uniroyal, (1967) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1973) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1977) Submission to Joint Meeting, May 17, 1977, Uniroyal,
Inc.
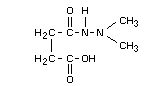 Other information on identity and properties
Daminozide is a white crystalline material of low volatility and
slight odour. It is soluble in polar solvents (g/100 g a 25°C: water
10; methanol 5; acetone 2.5). It is practically insoluble in aromatic
and aliphatic hydrocarbon solvents.
The technical grade product from the primary producer is typically
>99% daminozide. The manufacturing specifications require 98% minimum
and a melting point range of 154°C to 161°C. It is a white powder
which may contain traces of moisture, succinic acid, succinic
anhydride and a salt of daminozide and unsymmetrical dimethyl
hydrazine (UDMH). Volatile impurities are removed by an oven drying
step in the process. Daminozide was formerly manufactured by a process
in which dimethylnitrosamine was a starting reactant. The primary
manufacturer now uses a new process in which dimethylnitrosamine is
not used.
Daminozide is now commercially available only in formulations of
water-soluble powders containing 85% active ingredient and 15% inert
surfactants and mineral salts. Shelf-life studies show no degradation
of the formulations over a period of years. Liquid formulations
formerly produced are no longer commercially available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Daminozide is rapidly excreted and does not bioaccumulate in rats.
Two male and two female rats were treated with a single oral dose of
approximately 5 mg/kg unlabelled daminozide and 96 hours later with a
single 5 mg/kg oral dose of 14C-labelled daminozide (position of 14C
label unspecified) to study the absorption and excretion of
daminozide. After 2 days 69% of the administered dose had been
excreted in the faeces, 24% in the urine and 2.4% expired as 14CO2
Rats sacrificed 2 days after dosing contained an average of 0.35% of
the administered dose in the brain, liver, lung,,heart and spleen with
the majority of this residue in the liver. The rats sacrificed 4 days
after dosing contained only 0.03 or 0.12% of the administered dose in
these organs (Ryer, 1966).
A cow fed a diet containing 25 ppm daminozide for 4 days excreted 81%
of the daminozide in the faeces and 1.2% in the urine (St. John, Jr.
et al., 1969). See also "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on mutagenicity
Groups of male mice (20/group) were fed dietary levels of 0, 10, 300
or 10,000 ppm daminozide for 5 consecutive days to determine dominant
lethal effects. Following treatment, the males were monogamously
paired with untreated females, weekly, for 4 consecutive weeks.
Thirteen days after pairing females were killed and the uterine
contents examined for implantations, viable embryos and early or later
deaths.
No male deaths occurred during the test period and no signs of
compound effect on behavior were noted. During treatment, weight gain
at 10,000 ppm was marginally retarded. No effect was observed on
mating performance or pregnancy rate in any group. Neither
implantation rate nor viable litter size were significantly affected
by any treatment level. Inter-group differences in post-implantation
loss never attained significance (P>0.05) and showed no consistent
dose relationship (Palmer and Lovell, 1973).
Special study on teratogenicity
Groups of female rats (25/control group, 21/test group) were
administered 0, 250 or 500 mg/kg/day daminozide (12.5% w/v) in corn
oil, by gavage, from day 6 through 15 of gestation to determine
teratogenic effects. Daily observations were made and body weights
recorded on days 1, 9, 12 and 15. Records were kept of corpora lutea,
resorption sites, viable foetuses, foetal external and internal
abnormalities and skeletal development. Approximately equal numbers of
foetuses were examined for internal effects, using the Wilson
technique, and skeletal effects using clearing and alizarin staining
techniques.
Resorption sites, expressed as mean/female, were as follows: control,
0.4; 250 mg/kg, 1.1; 500 mg/kg, 0.7. Two females with a total of 10
resorption sites were included in the 250 mg/kg mean. Examination of
foetuses revealed no compound-related effects and none of the other
parameters were affected by compound administration (Keplinger et al.,
1972).
Special study on reproduction
Groups of rats (20/sex/group) were fed dietary levels of 0 or 300 ppm
daminozide, from weaning through three generations. The F0 group was
part of the 2 year feeding study described under "Long term studies".
PUP weights, number born alive or dead and the usual indexes of
reproductive performance were recorded. Data from two litters/3
generations revealed no significant effect on either fertility or
reproductive capacity. The pups survived equally well in the control
and test groups and no effect on growth was evident. Blood and urine
values were comparable to controls in each generation. Organ weights
were not affected nor was any compound-induced histopathology observed
(Oser, 1966).
Acute toxicity
Observed signs or oral intoxication were depression, ptosis, ataxia,
diarrhoea, excessive urination and laboured respiration. Dermal
application produced mild erythema and oedema.
TABLE 1. Acute toxicity of daminozide.
LD 50'
Species Route Sex mg/kg Reference
Rat Oral M 6,810 Anonymous, 1966
Oral M,F 8,400 Carson, 1963
Inhalation M 147 mg/l Carson, 1963
Rabbit Dermal M,F >10,000 Anonymous, 1966
Dermal M,F 16,000 Carson, 1963
Short term studies
Rat
Groups of rats (5 males and 5 females/group; 10 of each sex were
controls) were fed dietary levels of 0, 26, 80, 240, 720 or 2160
mg/kg/day daminozide technical for 90 days. No differences were noted
in body weight gains between any test group and the control gains. No
significant differences in haematological, blood chemistry or urine
values were observed and organ weights and ratio were comparable to
the control group. No gross or microscopic changes occurred in any
group which could be attributed to daminozide administration (Carson,
1964).
Dog
Groups of dogs (6/sex/control and 4/sex/test groups) were fed dietary
levels of 0, 300, 1000 or 3000 ppm daminozide technical for two years.
Body weight gains were within normal limits and comparable to control
gains. Appearance, behaviour, haematological, blood chemistry and
urine analyses of the test groups were also comparable to controls.
Gross necropsies, microscopic examination of tissues and organ weight
data revealed no compound or dose-related effects (Oser, 1966).
Long term studies
Rat
Groups of rats (37/sex/control and 25/sex/test group) were fed dietary
levels of 0, 300, 1000 and 3000 ppm diaminozide technical for two
years.
No differences in appearance or behaviour were observed between
control and test rats during the study. Throughout the study the body
weights gains of the 300 ppm group were slightly less than those of
the controls, but weight gains of the 1000 and 3000 ppm groups were
comparable to the control gains. No haematological or blood chemistry
differences related to compound consumption were noted during the
study. Survival over two years was not affected and was comparable
between all groups. Liver/body weight ratios for both sexes in the 300
and 3000 ppm groups were significantly higher than the control ratio
at 104 weeks. In the 300 ppm male group the mean liver weight was 21%
higher than the mean control liver weight. Nothing in the gross or
microscopic examinations explains this difference. Microscopic
examination of tissues revealed no dose-related effects. Tumors were
both benign and malignant, the former being principally mammary
adenomas or fibromas. In the latter category, reticulum cell sarcomas
predominated in both the test and control groups, the incidence being
not significantly higher at the 3000 ppm level than in the control
group. Several varied types of malignant neoplasms were also noted but
in no cage was there more than one of each type in any group (Oser,
1966).
COMMENTS
Daminozide is not highly acutely toxic as measured by LD50 values in
the g/kg range for oral and dermal studies and is only mildly
irritating to the eyes and skin. It is rapidly excreted and does not
bioaccumulate in mammals. Daminozide did not produce dominant lethal
effects in male mice at 10,000 ppm and no teratological or
reproductive effects were observed at levels of 300 ppm or lower. An
adequate two-year dog study has been conducted.
The long-term rat study was inadequate because of internal conflicts
within the report and because of the inability to evaluate the
carcinogenic potential of daminozide. Information on the
biotransformation of daminozide in animals is needed.
Because of the above concerns no recommendation for an acceptable
daily intake for man can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Daminozide is a plant growth regulator applied only in foliar sprays
to various tree fruits, grapes, vegetables, melons and peanuts.
Depending on the crop and timing of treatment, it is variously
intended to hasten ripening, increase fruit set, enhance fruit colour,
retard stem elongation, control fruit drop, delay maturity, reduce
vine growth or to produce certain other beneficial growth regulating
effects.
Introduced on an experimental basis in the early 1960s, it was first
registered for use on ornamentals. Since that time there have been
numerous reports in the horticultural journals on the efficacy of the
compound as a growth regulator. Use was expanded to include food crops
in 1968 in the U.S.A. Table 2 shows current (1977) registered use
patterns in the U.S.A. Uses in Canada are similar to the U.S. uses.
There was other information available to the Meeting that the product
has been used in Italy, South Africa, Japan, Australia, Federal
Republic of Germany, the Netherlands and the United Kingdom. The
directions for use on the product labelling accepted in those
countries generally conform to the use patterns shown in Table 2.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trials in the U.S.A. on fourteen crops and controlled feeding
experiments with meat and dairy animals (Uniroyal, 1977). The
government of the Netherlands submitted reports of supervised residue
trials on apples and pears. Analyses were by the colorimetric
procedure described below under "Methods of residue analysis".
Analyses of untreated controls (crop blanks) were reported in each
experiment, along with % recovery of daminozide from fortified
controls. Test samples were corrected for crop blank. Recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form where such presentation
is feasible.
Apples
Field trials were conducted at locations in 10 states representing all
of the major apple growing areas of the U.S.A. Some 180 harvest
samples were analyzed in duplicate. The wide range of experimental
conditions in the various field tests and the resulting wide range of
residues found, preclude a tabular summary of data. The residues
ranged from 0.1 mg/kg to as high as 80 mg/kg. If the high values
resulting from exaggerated treatments are excluded, the data indicate
that residues would not be likely to exceed 30 mg/kg when the normal
dosages (Table 2) are applied and the prescribed 60-70 day preharvest
interval is observed.
TABLE 2. Registered use patterns for daminozide in the U.S.A. (1977).
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
sweet cherries hasten 0.1-0.2% 1 2 weeks after full
ripening, bloom
concentrate
maturity
sour cherries " 0.4% 1 "
peaches, hasten 0.1-0.2% 1 13 weeks post-bloom
nectarines ripening, to pit hardening
concentrate
maturity
pears prevent fruit drop 0.1% 1 18 to 24 days before harvest
apples multiple effects(1) 0.075-0.2% 160 to 70 days before harvest
prunes hasten ripening 0.05-0.1% 1 1 month before
harvest
grapes increase fruit set, 0.05-0.1% 1 from 1st bloom to
reduce vine growth (1.4-2.4 kg/ha) full bloom
Brussels uniform sprout 1.9-3.75 kg/ha 1 30 days before
sprouts development harvest
cantaloupes reduce vine growth 1.9 kg/ha 1 2 to 4 leaf stage
TABLE 2. (Continued)
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
tomatoes multiple effects(1) 0.6-2.4 kg/ha 2 before transplanting
4.8 kg/ha 1 after transplant - not
within 7 days
of picking
peanuts multiple effects(1) 1.0 kg/ha 2 at pegging time
0.5 kg/ha 30 days before
harvest
peppers 0.5-2.3 kg/ha 2 at 2-4 leaf stage
(seedlings) and 7-10 days
later
(1) There are 9 separate beneficial effects claimed for apples alone. Other claims are similarly complex. The directions for use of this
product are complex because of many factors (plant vigour, variety, moisture stress, etc.) which can not only modify the desired effect
on the plant but can contribute adverse effects.
The residues from trials in the Netherlands were generally 5 mg/kg or
less. The rates of application in these experiments were somewhat
lower than the rates permitted in some other countries.
Studies designed to measure initial deposits or residue decline rate
(half-life) were not included. The data clearly show, however, that
daminozide is extremely persistent in apples. Significant residues
remain for more than 159 days after the spray application.
Brussels sprouts
Four separate trials were conducted at locations in California and New
York. A single treatment was made at normal and exaggerated rates.
Crop blanks were reported as zero. Recoveries were 87 and 83% at 1 and
10 mg/kg respectively. The data are summarized in Table 3. Under good
agricultural practice the residues would not be likely to exceed 20
mg/kg.
TABLE 3. Residues in Brussels sprouts, mg/kg (single application).
Application rate, Interval, days from treatment
kg a.i./ha 30 33 46
1.9 6.3, 5.2, 6.8 7.1, 1.4, 0.0
3.4 2.8, 1.4
3.8 14.6, 15.5, 13.0 2.3, 2-7, 1.5
6.8* 4.8, 3.2
7.7* 25.4, 24.8, 24.6 5.6, 6.2, 5.2
*excessive dosage
Cantaloupes
Nine residue trials were reported from locations in California,
Arizona, Texas and Michigan. The registered use patterns on melons
provide for treatment when the plant is at the 2-4 leaf stage, at
which point no fruit would be present. The experiments included
exaggerated treatment rates, and samples taken as early as 44 days
after treatment. If these samples were mature melons, fruit must have
been present at the time of treatment (melons take from 85 to 150 days
from planting to maturity). Even under these conditions the residue
values of some 20 samples ranged from < 0.1 to 1.6 mg/kg (Table 4)
compared with apparent daminozide in untreated controls up to 1.1
mg/kg. It would appear that residues to be expected in cantaloupes
from good agricultural practice would be of a lower order than those
found in the tree fruits. It is not likely that residues in excess of
3 mg/kg would occur in cantaloupes. Data were not available on other
melon varieties, but the conclusion regarding cantaloupes could be
extrapolated to other melons.
TABLE 4. Residues in cantaloupes,mg/kg
Interval from No. of Application Rate,
last treatment treatments kg a.i./ha
days 0.47 0.95 1.9 3.8 7.6
44 2* <0.1 1.6
50 1 1.0,1.5 1.5,1.6
57 1 0.6,0.8 1.2,1.6
68 1 1.2 1.6
73 2* <0.1 <0.1
87 1 <0.1 <0.1
115 2* <0.1 <0.1
*Rate given for second treatment only.
Sour cherries
Trials were carried out at several locations in four states
representing the important cherry producing areas in the U.S.A. A
total of 70 test samples and 25 control samples were analyzed. Crop
blanks ranged from 0.1 to 0.64 mg/kg.
The dosages applied in the tests ranged from 25% of the manufacturer's
minimum recommended dosage to twice the maximum recommended rate (See
Table 2). Samples were taken at periods after treatment ranging from
56 to 65 days. This roughly corresponds to the label directions which
call for a single spray 10-14 days after full bloom. The season from
bloom to harvest in the U.S.A. is 80 to 100 days (Magness, 1971).
The sampling schedule in all trials was designed to show residues at
normal harvest. Therefore, no estimates of initial deposit or residue
decline rate are possible. Overall the residues found on mature
cherries ranged from 5.3 to 78 mg/kg. Although some correlation could
be made with application rate, the variation in harvest residues
appeared to be almost random (the data do not lend themselves to
tabulation). If the data from trials with excessive or minimal dosages
were excluded, the residues fall between 10 and 53 mg/kg. It is not
likely that residues under actual (commercial) conditions would ever
exceed 60 mg/kg.
Sweet cherries
Analyses of 176 test samples and 39 control samples from field trials
in five states were made available. The experimental design generally
paralleled that discussed above for sour cherries. That is, the trials
were designed to show residues on harvested cherries treated
approximately as prescribed on the product label (2 weeks after full
bloom). The overall range of residues found (1 to 54 mg/kg) was
comparable to that reported on sour cherries. If only the data from
trials following good agricultural practice (See Table 2) are used,
most samples were in the range of 9-18 mg/kg, with a few higher values
approaching 30 mg/kg. The difference between the residue load expected
on sweet and sour cherries is mainly due to the application rate,
which is 0.2% for sweet and 0.4% for sour cherries. A maximum residue
limit of 30 mg/kg for sweet cherries would therefore be consistent.
Grapes
The residue data are summarized in Table 5. Some 44 samples from field
trials in 4 states were reported. Analyses were in duplicate,
including 8 untreated control samples. All controls were reported as
0.1 mg/kg, apparent daminozide.
The registered use directions for grapes (Table 2 requires treatment
during the period from first bloom to full bloom. The period between
bloom and harvest for grape varieties will differ greatly. In some
varieties it may be as much as 5-6 months (Magness, 1971). It is
interesting to note that in the field trials, the intervals between
spray and harvest were from 102 to 111 days, and probably represent an
early maturing variety, probably Concord. From the standpoint of
residues at harvest, it therefore represents the "worst case". Even
with some exaggerated dosages, all residues were below 10 mg/kg.
TABLE 5. Residues in grapes, single application, 102-111 days from
treatment
Application rate Residue range,
% a.i. mg/kg
0.05 0.4 - 2.5
0.10 1.3 - 5.4
0.20 2.6 - 9.5
Nectarines and peaches
The use directions for these two fruits are the same. They also incur
residues to the same degree, and may be discussed together.
The prescribed cut-off date for sprays is at "pit hardening".
Presumably, this growth stage is recognizable to experienced fruit
growers. As near as can be determined, pit hardening occurs 40 to 80
days before harvest, depending on variety, geographic location and
other factors.
The data made available on nectarines and peaches included a total of
more than 500 analyses including 70 untreated controls. Treatments
were made at the prescribed rate, at excessive rates, and at less than
the prescribed rate. The intervals between treatment and sampling were
variously identified as treatments at "style abscission", pit
hardening, and in some cases by numerical day before harvest. Owing to
the variables in the field tests, a wide range of residues was
reported (1.0 to 93.0 mg/kg).
Interest is mainly in the residues from applications closely
approximating the registered use pattern (Table 2). The distribution
of residues from application of 0.1 to 0.2% sprays in the period from
style abscission (3 weeks post-bloom) to pit hardening in nectarines
and peaches was:
mg/kg <1 1-5 5-10 10-15 15-20 20-25 >25
No. of samples 35 140 48 45 3 7 1
The distribution indicates that residues would not be likely to exceed
30 mg/kg when uses described in Table 2 are followed.
Peanuts
Results of residue trials in Georgia, N. Carolina, Texas, Alabama,
Florida and Oklahoma were submitted to the Meeting. Separate analyses
were made on kernels, hay and hulls.
Kernels
A total of 166 analyses of test samples and 13 of controls were made.
All controls samples were zero except two reported at 3.1 and 2.8
mg/kg. (The analyst noted that contamination was suspected.)
The manufacturer's recommendations are for one treatment of 0.96 kg
a.i./ha at pegging time followed by a second treatment of 0.47 kg
a.i./ha thirty days before harvest. For those field trials in which
the treatment approximated the recommended pattern, the following
residue distribution was obtained:
Range, mg/kg: <5.0 5-10 10-15 15-20 20-25 25-30
Number of samples: 31 20 7 4 4 2
From this skewed distribution pattern it is apparent that maximum
residue limit of 30 mg/kg for kernels would be adequate to cover all
residues from good agricultural practice.
Peanut hay
62 analyses were submitted, 24 of which corresponded to the normal
split dosage pattern mentioned above. Residues in the trials
reflecting recommended usage were mostly in the range of 1-5 mg/kg.
However, three were in the range of 5-10 mg/kg and three were greater
than 10 mg/kg. A maximum residue limit of 20 mg/kg would be adequate.
Peanut hulls
Very limited data were available on peanut hulls. Residues were 2
mg/kg or less. Peanut hulls are not an important item of international
trade. The only interest in this commodity is that the hulls are
incorporated at low levels into some mixed animal feeds.
Peanut meal
Field-treated peanuts which were determined to be carrying 12 mg/kg of
daminozide were processed in a laboratory experiment simulating a
commercial process. The meal presscake was found to contain 30-33
mg/kg. On the basis of this approximately three-fold concentration
factor, peanut kernels bearing the maximum residues of 20 mg/kg would
be expected to produce meal containing about 60 mg/kg.
Pears
Limited data (14 test samples + 7 controls) from Washington and Oregon
were available. Seven samples were from trials in which the
manufacturer's recommended rate was applied (0.1% spray). Residues at
this dosage ranged from 0.8-17.0 mg/kg, with an average of 10.8 mg/kg.
Residues from uses according to good agricultural practice would
probably not exceed 20 mg/kg.
Peppers
Field trials were conducted in Texas, Georgia, Connecticut, Delaware
and Michigan. Samples were collected 77-125 days after treatment.
Single and double applications were made at the prescribed rate and at
four times the prescribed rate. The net difference between the
apparent daminozide in the test samples and the control samples was
less than 1 mg/kg in all cases, the highest residue reported being
0.44 mg/kg. A maximum residue limit of 1 mg/kg would be adequate.
Plums
Residue trials at 6 locations in California were reported to the
Meeting. The plums (fresh prunes) were treated at the prescribed rate
(Table 2) and at rates up to 4 times the prescribed rate. The interval
between treatment and sampling ranged from 34 to 78 days (as compared
with the registered label requirement of a 30 day pre-harvest
interval). Residues from all treatment rates ranged from 3.0 to 71.5
mg/kg. Residues from the normal rates were 3.0 to 43.7 mg/kg. It is
unlikely that residues from normal usage would exceed 50 mg/kg.
Field-treated fresh prunes were analyzed and converted to dried prunes
by the commercial procedure. This involves a 5-10 minute mechanical
wash and drying on trays at 170°F for 30 hours. The maximum
concentration factor (mg/kg in dried prunes: mg/kg in fresh) was found
to be 2.7. On this basis, a prediction can be made that fresh prunes
bearing maximum residues of 50 mg/kg would produce dried prunes
containing 135 mg/kg.
Tomatoes
The registered uses of daminozide on tomatoes include 2 applications
prior to transplanting (greenhouse or outdoors) and one application in
the field no later than 7 days prior to picking (see Table 2). The
application on plants to be transplanted is for all practical purposes
a "no-residue" use. Residue data were made available on tomatoes which
had received both transplant and field treatments at the normal and
excessive rates. All residue trials (7) were carried out in Florida,
on green tomatoes. The only field use for daminozide on tomatoes
permitted in the U.S.A. is on Florida winter tomatoes which are
harvested green and artificially ripened. These residue trials
therefore were highly specialized and do not necessarily reflect
residues which might result from more general usage on tomatoes. The
data are shown in Table 6.
TABLE 6. Residues in tomatoes, mg/kg
Interval from Application Rate*
last treatment kg a.i./ha
Days 4.76 9.52
11 13 68.5
21 9 35
7 6 8
7 8 12.5
18 12 20.5
14 20 45.5
19 15 28
29 22 8
* Field treatment after transplant use.
Alfalfa
There are no national tolerances or registrations for daminozide use
on alfalfa. However, the product has some utility in the culture of
alfalfa seed, and registration is pending in U.S.A. In growing alfalfa
for seed there is some utilization of alfalfa forage, hay, and seed
screenings for animal feeds. The submission to the Meeting therefore
contained some residue data on alfalfa hay, fresh forage, and seed
screenings. Residues on fresh alfalfa ranged from 4-12 mg/kg and on
hay from 2-20 mg/kg. Seed screenings showed a maximum of 1.4 mg/kg. In
view of the specialized nature of the use it would not appear
necessary at this time for the meeting to recommend a maximum residue
limit.
Meat, milk, poultry and eggs
Daminozide is used on a number of crops which yield by-products used
commercially in animal feeds. Primary daminozide sources would be
peanut meal and peanut hay. Some additional contribution to the diet
could occur from peanut hulls and the pomaces resulting from
processing apples, grapes and tomatoes. In recognition of this, the
basic manufacturer submitted to the Meeting some animal feeding,
studies (Uniroyal, 1977).
(a) Cattle were fed at levels of 0, 20, 60 and 200 ppm in the total
diet for 6 weeks. Milk was collected at intervals and the animals
slaughtered at the end of the feeding period. No daminozide residues
were found in any tissues (muscle, fat, liver, kidney) by a method
with a lower limit of detection estimated at 0.2 mg/kg. Trace residues
were found in the milk of animals on the 60 and 200 ppm diets at
levels of 0.05 and 0.07 mg/kg respectively.
(b) Hogs were fed 0, 60 and 300 ppm in the diet (2 animals at each
level) for 31 days. No daminozide residues were detected (40.2 mg/kg)
in muscle or fat. Apparent daminozide residues were found in liver and
kidney, at 0.3 and 0.8 mg/kg respectively, in animals on the highest
feeding level.
(c) Poultry were fed 0, 20, 60 and 200 ppm in the diet (10 hens in
each group) for 31 days. Eggs were composited weekly and on day 31 all
birds were sacrificed. On the final sampling, eggs from the 300 ppm
and 60 ppm feeding levels contained 0.3-0.5 and 0.25 mg/kg
respectively. No detectable residues were found (< 0.2 mg/kg) in eggs
from the 20 ppm feeding level. No residues were found in muscle or fat
of birds on the lower feeding levels. At the 200 ppm level, residues
of 0.4 and 0.7 mg/kg were found respectively in muscle and fat.
Residues of 1 and 1.1 mg/kg were found in liver of birds on the
highest level. Daminozide concentrates in poultry kidney. Values of
1.3, 1.8 and 7.4 mg/kg were reported in kidney for the 3 feeding
levels.
The studies show that residues occur at high feeding levels, but this
must be related to the actual anticipated intake from peanut meal and
peanut hay. Peanut hay would be used to the extent of 25% in beef
cattle diets, 60% in dairy diets, and 10% for pigs. Peanut meal can
make up 15%-beef; 25%-dairy, 10%-poultry and 10%-pigs (Harris, 1975).
After adjusting for the proportion of peanut meal and hay in the total
diet of each species, it is apparent that the residues in milk, eggs
and tissues (except poultry kidney) Will be below the level of
detection of the colorimetric method.
FATE OF RESIDUES
General
Daminozide is readily absorbed and translocated from foliar sprays.
The entrapment of 14CO2 from applications of labelled daminozide
indicates that, under certain conditions, at least part of the applied
pesticide is fully degraded. Other data show that daminozide, in free
or loosely bound form, is the principal residue component and very
persistent in fruits. Many of the conclusions regarding the nature of
the terminal residues rest on characterization by selective solvent
extractions, in vitro experiments and analysis by wet chemical
procedures for predicted metabolites. In view of the known biological
activity of some postulated metabolites of the compound, particularly
nitrosamines, hydrazides and other hydrazine derivatives, further
identification of the residues in harvested fruits and vegetables
would be desirable.
In animals
Rats were fed 14C-labelled daminozide and the excreta monitored. The
majority of the administered dose, apparently occurred in the urine as
unchanged daminozide. As far as can be determined, there has been no
effort to study metabolic pathways in meat or milk animals with radio-
labelled daminozide. However, analyses by the chemical method
(previously discussed) were made for one postulated metabolite (NDMA)
in milk from cows fed 200 ppm daminozide in the diet. No NDMA was
found at a minimum detection level of 0.03 mg/kg. See also the
sections "Biochemical aspects" and "Residues resulting from supervised
trials".
In plants
It has been reported that daminozide decomposes to unsymmetrical
dimethylhydrazine (UDMH) in the plant and that it is the latter
compound which is the active principle through its effect on the plant
enzyme diamine oxidase (Reed, 1963). Another investigator (Dahlgren,
1963) found that maleic acid dimethyl hydrazide (CO11, a related
growth regulator) is hydrolysed to UDMH in aqueous solutions. Dahlgren
noted that daminozide was stable under the same conditions. In
experiments reported by the manufacturer (Uniroyal, 1967) no free UDMH
or succinic acid was detected after attempts (in vitro) to enzymically
hydrolyze daminozide with trypsin, proteinase, papain, ficin and
urease. No evidence was found of hydrolysis to UDMH and succinic acid
in macerates of plant leaves by colorimetric tests for UDMH and paper
chromatographic tests for succinic acid. While the evidence regarding
the occurrence of UDMH is somewhat contradictory it is likely that,
even if UDMH occurs as an intermediate metabolite, its presence would
be fleeting owing to its reactivity and that it would not be present
per se in harvested crops.
The gas-chromatographic method of analysis for nitrosodimethylamine
(NDMA), discussed below ("Methods of residue analysis",) has been used
in studies intended to show the absence of this possible oxidation
product in treated crops (Uniroyal, 1967). No NDMA was detected in
treated apples, tomatoes or peanut foliage by the method, which is
said to be sensitive to 0.002 mg/kg NDMA.
Radiotracer studies were conducted by the U.S. Department of
Agriculture (Uniroyal, 1966) on apple trees in the field and on apple
seedlings in the greenhouse. Daminozide was labelled in the succinic
acid moiety and (in a separate test) in the UDMH group. Activity in
the sprayed seedlings was distributed throughout the plant with
highest activity in the leaves. 20% of the original activity was
released as 14CO2 in the first seven weeks after application.
In the field studies, 15% of the total applied activity remained in
the tree after 100 days. The activity in the apple fruit after 125
days was identified as 75-85% unmetabolized daminozide. Trace amounts
of succinic acid were identified and there was some incorporation of
14CO2 into plant components.
14C-labelled daminozide was used in metabolism studies with peanut
plants (Uniroyal, 1977). Characterization of the activity by selective
solvent extraction indicated that the majority of the activity was
present in bound form, probably as sugar complexes. Daminozide per se
was recoverable from the extracts and it was concluded that residues
present in peanuts in complexed form would be detected by the
colorimetric method of analysis. Analyses of peanut oil for 14C
activity showed residues of about 2-5 mg/kg expressed as daminozide.
Selective solvent extraction of the oil indicated that the activity
was not daminozide but was probably in the form of fatty acid
glycerides.
In soil
Residue decline studies were conducted on sandy loam and clay soils
spiked at 5 and 10 mg/kg. Daminozide per se is not persistent in
soils. The breakdown is probably through microbial action. In the clay
soil, < 0.1 mg/kg was present two days after treatment. The sandy
loam contained 0.1 mg/kg at 2 and 3 weeks respectively at the 5 and 10
mg/kg fortification levels. The compound is very mobile in soils
because of its water solubility.
In processing
Studies show that in the commercial processing of peanuts there is no
appreciable concentration of residues in the refined peanut oil. There
is, however, a 3 fold concentration of residues in peanut meal (e.g.,
peanut meats bearing 20 mg/kg will produce peanut meal bearing 60
mg/kg).
Data on the processing of fresh tomatoes bearing approximately 40
mg/kg into ketchup and tomato paste show that there is essentially no
loss of daminozide residues in processing. (The residues concentrate
to the same degree as the solids do in the concentration process.)
Residues found in tomato paste ranged from 155 to 197 mg/kg. Analyses
for nitrosodimethylamine (NDMA) in tomato paste were negative by the
chemical method with a reported sensitivity of 0.002 mg/kg.
Experiments in which field-treated plums were processed into dried
prunes showed that residues were concentrated in the dried fruit by a
factor of 2.7. See "Residues resulting from supervised trials" for
details of this work and of the effect on residues of processing
peanuts to peanut meal.
There are national tolerances on grapes (10 ppm, U.S.A. and Canada)
but no data were made available on transfer of residues to wine or
grape pomace.
Evidence of residues in food in commerce or at consumption
As noted under "Methods of residue analysis", daminozide is not
detected by any of the multi-residue screening methods currently used
in market basket surveys, monitoring, or government regulatory
programmes. The "specific" colorimetric method has not been employed
in the Canadian or the U.S. Food and Drug Administration surveillance
programme and there is no record of a finding of daminozide in foods
in commerce. In view of the demonstrated stability of daminozide
residues in food processing, and the relatively high levels incurred
in fruits from good agricultural practices, it may be concluded that
residues are occurring in the diet in those countries where there is
substantial use.
METHODS OF RESIDUE ANALYSIS
Daminozide residues in crops are determined by a colorimetric method
(PAM II, 1967). The method involves hydrolysis in boiling 50% sodium
hydroxide to release unsymetrical dimethylhydrazine (UDMH) which is
distilled and reacted with trisodium pentacyanoamine ferroate (TPF) at
pH 5.0. The red colour produced is measured spectrophotometrically at
490 and 600 nm. The difference between the two readings is plotted as
net absorbance against µg of compound in a calibration curve.
Modifications of this basic method permit determination of daminozide
in peanuts, animal tissues, milk and eggs.
The chromogenic agent TPF used for determination of daminozide also
forms colored complexes with nitroso compounds, primary aromatic
amines and aliphatic or aromatic hydrazines (Feigl, 1949). Some
possible metabolites of daminozide would therefore contribute
absorbance which would be additive to that of the daminozide-TPF
complex. If present they would be interpreted as daminozide residues
(but not measured quantitatively).
The method has been tested in government regulatory laboratories on
grapes and animal tissues. Because of large and variable crop blanks
it was concluded that a reliable lower limit of determination for
daminozide in samples of plant origin was no better than 1 mg/kg for
regulatory purposes. The estimated sensitivity for animal products is
0.05 mg/kg for milk and 0.2 mg/kg for tissues and eggs. The government
analyst noted that 2 hours colour development time was required rather
than the 1 hour prescribed in the procedure.
The assumption was made that any free UDMH, or any metabolites which
would yield UDMH under the rigorous caustic hydrolysis, would also be
measured as daminozide by this method. There are no experimental data
to confirm that conclusion. Whether in fact any such metabolites
actually occur in crops at harvest is uncertain (see discussion under
"Fate of residues").
Colorimetric methods are ordinarily not specific. In this case, the
method was tried in the presence of most pesticides used on the
subject crops (in 1968) to gauge interference. Only the fungicide
"Botran" was found to interfere, and this interference can be
eliminated by a modification of the method.
A second colorimetric method of analysis for daminozide in plant
substrates has been published (Edgerton, 1967). This method also
measures UDMH in a hydrolysate, but colour development is with
phosphomolybdic acid. Sensitivity is comparable to the colorimetric
method discussed above.
As noted above, the colorimetric method for the parent compound would
also measure any alkylnitrosamines as daminozide. Because of concern
for the toxic potential of a possible metabolite, nitrosodimethylamine
(NDMA), a "specific" method for NDMA was developed by the manufacturer
(Uniroyal, 1967). This is a GC method in which NDMA is separated from
crop substrate by vacuum distillation, sorbed on polymer beads,
removed from the beads by baking and determined by micro-coulometric
gas chromatography (measurement of NH3). Sensitivity was estimated at
0.002 mg/kg. Recovery experiments at a fortification level of 0.010
mg/kg were reported to be adequate. This method has not been validated
in government laboratories.
Daminozide is not detected by any of the multi-residue screening
procedures used in government market basket surveys and monitoring
programs. There is no record of any government regulatory experience
with the colorimetric method for daminozide. It is questionable
whether by present standards it would be satisfactory for regulatory
purposes, particularly for enforcement of the lower tolerance levels.
As far as can be determined there is no confirmatory method of
analysis available. It would be desirable to have further information
on the reliability of this method, particularly with respect to (a)
whether it actually measures UDMH metabolites in free or conjugated
forms and (b) actual experience with the method in government
laboratories. An independent method, preferably a more specific GC or
HPLC method would be desirable.
APPRAISAL
Daminozide is a growth regulator which has been used on a variety of
fruits and vegetables since 1967. The product produces numerous
beneficial growth regulating effects. It is said to control premature
fruit drop, delay maturity, enhance fruit colour, reduce vine growth,
retard stem elongation and increase fruit set, among other claims.
Information submitted to the Joint Meeting by the primary manufacturer
indicates that the principal uses of the product are in the U.S.A.
Other information made available to the Meeting indicates that
daminozide is also used in Italy, South Africa, Japan, Australia,
NATIONAL TOLERANCES REPORTED TO THE MEETING
Commodity Tolerance, mg/kg
U.S. Canada Australia Netherlands
Fruits and vegetables 10
Sour cherries 55 55
Plums 50 20
Tomatoes 40 0.5
Apples 30 30 30
Nectarines 30
Peaches 30 25 30
Peanuts 30 20 30
Sweet cherries 30 30
Brussels sprouts 20 15
Peanut hay 20
Pears 20 15 30
Grapes 10 10
Peanut hulls 10
Melons 3
Peppers 1
Meat, fat and meat 0.2 0.2
by-products of cattle,
goats, horses, poultry
(except kidney), sheep
Poultry kidney 2
Eggs 0.2 0.2
Milk 0.02 0.05
Canada, Federal Republic of Germany, the United Kingdom and the
Netherlands. National tolerances have been established in the U.S.A.,
Canada, Australia and the Netherlands.
Daminozide was formerly manufactured by a process in which
dimethylnitrosamine was a starting reactant, and occurred as impurity
in the technical grade product. The primary manufacturer informed the
Meeting that a new process has been adopted in which
dimethylnitrosamine is not used. The recommended guideline limits
apply only to daminozide manufactured by the process in which
dimethylnitrosamine is excluded.
The compound is readily absorbed and translocated from foliar sprays.
While there in some evidence of degradation, the parent molecule in
free or loosely bound forms is persistent, particularly in the fruits.
The chemical structure of daminozide suggests the possible presence of
some metabolites of interest, including nitrosamines, hydrazides and
other hydrazine derivatives. No positive findings of these metabolites
were reported. The postulated metabolite N-dimethylnitrosamine was not
detected by a method said to be sensitive to 0.002 mg/kg. Indirect
evidence was reported of the absence of unsymmetrical
dimethylhydrazine (UDMH).
A feeding study with 14C daminozide in rats indicated that the
majority of the administered dose was excreted in the urine as
unchanged daminozide. There was no information on the metabolism
patterns in large animals or on metabolites in meat or milk. In
general, the information available on the fate of daminozide in plants
and animals is not very extensive, particularly with respect to the
nature of the terminal residues.
The analytical method currently available to enforce national
tolerances is a colorimetric method which measures unsymmetrical
dimethyl hydrazine (UDMH) released from daminozide by vigorous caustic
hydrolysis. The chromogenic agent is not specific for UDMH. Other
classes of compounds also produce colour complexes, including
aliphatic hydrazines, nitroso compounds and primary aromatic amines.
It is theorized that complexed daminozide, free UDMH and any other
metabolic products which would yield UDMH would be measured as
daminozide. It has been determined that the method has never been
applied in the U.S. or Canadian official surveillance programmes and
there is no information on experience with the method in other
government laboratories. The method would not appear to be very useful
for regulatory purposes.
Studies on the effects of processing show that the compound is not
destroyed and tends to concentrate in products such as tomato paste
and peanut meal. It has never been reported in foods in commerce (see
above comment on the analytical method). It is not persistent in
soils. Extensive data from supervised residue trials carried out in
the U.S.A. were available. Data on supervised trials on apples and
pears from the Netherlands were also reported. All analyses were by
the colorimetric method discussed above. The data generally showed the
highest residues in fruits, with considerable variability in harvest
residues. The residue values on a given crop followed almost a random
distribution. The experiments on which reports were received were all
designed to show harvest residues from registered use patterns and
therefore permitted no estimates of decline rate.
EVALUATION
As no ADI or temporary ADI could be allocated, no recommendations for
maximum residue limits could be made. Guideline levels are recorded
for the commodities listed below. The usual statements regarding the
intervals on which the levels are based are omitted in this case
because of the highly specialized uses of the product which are
related to stages of growth or maturation.
Commodity Guideline level, mg/kg
Sour cherries 60
Plums 50
Tomatoes 40
Apples, nectarines,
peaches, peanuts
(kernels), pears,
sweet cherries 30
Brussels sprouts 20
Grapes, peanut hay 10
Melons 3
Peppers 1
Eggs, meat 0.2*
Milk 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake for humans (ADI) and
maximum limits (MRL can be established)
1. An adequate long-term rat study.
2. Information on the biotransformation in animals.
Desirable
1. An analytical method more suitable for regulatory purposes than the
present colorimetric method.
2. Information on the occurrence of residues in foods in commerce.
3. Further identification of the terminal residues in crops and in
foods of animal origin.
REFERENCES
Anonymous, (1966) Acute Oral Administration-Male Rats. Unpublished
report from Hazleton Labs., Inc., submitted to the World Health
Organization by the Uniroyal Chemical Company.
Carson S., (1963) Toxicological Examination of Compound B-995.
Unpublished report from Food and Drug Research Labs., submitted to the
World Health Organization by Uniroyal Chemical Company.
Carson S., (1964) Subacute (90 day) Feeding Studies with B-995 in
Rats. Unpublished report from Food and Drug Research Labs., submitted
to the World Health Organization by Uniroyal Chemical Division of
Uniroyal,Corp.
Dahlgren, Dahlgren and Simmerman, (1963) Science, 148, 485; 1963
Edgerton, L.J., Rockey, M., Arnold, H., and Lisk, D.J., (1967)
"Colorimetric Determination of Alar Residues in Apples", J. Agr. Food
Chem. 15, no. 5, p. 812, 1967
Feigl, F., (1949) Chemistry of Specific Selective, and Sensitive
Reactions, p. 377, Academic Press, New York, 1949
Harris, L.E., (1975) "Guide for estimating toxic residues in animal
feeds or diets", U.S. National Technical Information Service,
accession no. PB-243-748-LK
Keplinger, M.L., Kennedy, G.L., and Haley, S., (1972) Teratogenic
Study with Alar in Albino Rats. Unpublished report from Industrial
Bio-Test Labs., submitted to the World Health Organization by the
Uniroyal Chemical Division of Uniroyal, Corp.
Magness, J.R., Markle, G.M., Compton, C.C., (1971) Food and Feed Crops
of the United States, Bull. 828, New Jersey Agricultural Experiment
Station, 1971
Oser, B.L., (1966) Chronic (2 year) Feeding Studies with B-995 in Rats
and Dogs. Unpublished report from Food and Drug Research Labs.,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Palmer, A.K. and Lovell, M.P., (1973) Dominant Lethal Assay of Alar in
the Male Mouse. Unpublished report from Huntingdon Research Center,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Pam, II., (1967) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education and Welfare, Food and Drug
Administration
Reed, (1963) Science, 148, 1097; 1963
Ryer, F.H., (1966) Final Report. Radiotracer Metabolism Study,
Alar-C14. Unpublished report from Hazleton Labs., Inc., submitted to
the World Health Organization by the United States Rubber Company.
St. John, Jr., L.E., Arnold, H. and Lesk, D.J., (1969) Metabolic
Studies with Alar Growth Regulator in the Dairy Cow.
J. Agr. Food Chem., 17, No. 11 116.117.
Uniroyal, (1966) Unpublished information, Uniroyal, Inc.v to U.S. Food
and Drug Administration
Uniroyal, (1967) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1973) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1977) Submission to Joint Meeting, May 17, 1977, Uniroyal,
Inc.
Other information on identity and properties
Daminozide is a white crystalline material of low volatility and
slight odour. It is soluble in polar solvents (g/100 g a 25°C: water
10; methanol 5; acetone 2.5). It is practically insoluble in aromatic
and aliphatic hydrocarbon solvents.
The technical grade product from the primary producer is typically
>99% daminozide. The manufacturing specifications require 98% minimum
and a melting point range of 154°C to 161°C. It is a white powder
which may contain traces of moisture, succinic acid, succinic
anhydride and a salt of daminozide and unsymmetrical dimethyl
hydrazine (UDMH). Volatile impurities are removed by an oven drying
step in the process. Daminozide was formerly manufactured by a process
in which dimethylnitrosamine was a starting reactant. The primary
manufacturer now uses a new process in which dimethylnitrosamine is
not used.
Daminozide is now commercially available only in formulations of
water-soluble powders containing 85% active ingredient and 15% inert
surfactants and mineral salts. Shelf-life studies show no degradation
of the formulations over a period of years. Liquid formulations
formerly produced are no longer commercially available.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Daminozide is rapidly excreted and does not bioaccumulate in rats.
Two male and two female rats were treated with a single oral dose of
approximately 5 mg/kg unlabelled daminozide and 96 hours later with a
single 5 mg/kg oral dose of 14C-labelled daminozide (position of 14C
label unspecified) to study the absorption and excretion of
daminozide. After 2 days 69% of the administered dose had been
excreted in the faeces, 24% in the urine and 2.4% expired as 14CO2
Rats sacrificed 2 days after dosing contained an average of 0.35% of
the administered dose in the brain, liver, lung,,heart and spleen with
the majority of this residue in the liver. The rats sacrificed 4 days
after dosing contained only 0.03 or 0.12% of the administered dose in
these organs (Ryer, 1966).
A cow fed a diet containing 25 ppm daminozide for 4 days excreted 81%
of the daminozide in the faeces and 1.2% in the urine (St. John, Jr.
et al., 1969). See also "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on mutagenicity
Groups of male mice (20/group) were fed dietary levels of 0, 10, 300
or 10,000 ppm daminozide for 5 consecutive days to determine dominant
lethal effects. Following treatment, the males were monogamously
paired with untreated females, weekly, for 4 consecutive weeks.
Thirteen days after pairing females were killed and the uterine
contents examined for implantations, viable embryos and early or later
deaths.
No male deaths occurred during the test period and no signs of
compound effect on behavior were noted. During treatment, weight gain
at 10,000 ppm was marginally retarded. No effect was observed on
mating performance or pregnancy rate in any group. Neither
implantation rate nor viable litter size were significantly affected
by any treatment level. Inter-group differences in post-implantation
loss never attained significance (P>0.05) and showed no consistent
dose relationship (Palmer and Lovell, 1973).
Special study on teratogenicity
Groups of female rats (25/control group, 21/test group) were
administered 0, 250 or 500 mg/kg/day daminozide (12.5% w/v) in corn
oil, by gavage, from day 6 through 15 of gestation to determine
teratogenic effects. Daily observations were made and body weights
recorded on days 1, 9, 12 and 15. Records were kept of corpora lutea,
resorption sites, viable foetuses, foetal external and internal
abnormalities and skeletal development. Approximately equal numbers of
foetuses were examined for internal effects, using the Wilson
technique, and skeletal effects using clearing and alizarin staining
techniques.
Resorption sites, expressed as mean/female, were as follows: control,
0.4; 250 mg/kg, 1.1; 500 mg/kg, 0.7. Two females with a total of 10
resorption sites were included in the 250 mg/kg mean. Examination of
foetuses revealed no compound-related effects and none of the other
parameters were affected by compound administration (Keplinger et al.,
1972).
Special study on reproduction
Groups of rats (20/sex/group) were fed dietary levels of 0 or 300 ppm
daminozide, from weaning through three generations. The F0 group was
part of the 2 year feeding study described under "Long term studies".
PUP weights, number born alive or dead and the usual indexes of
reproductive performance were recorded. Data from two litters/3
generations revealed no significant effect on either fertility or
reproductive capacity. The pups survived equally well in the control
and test groups and no effect on growth was evident. Blood and urine
values were comparable to controls in each generation. Organ weights
were not affected nor was any compound-induced histopathology observed
(Oser, 1966).
Acute toxicity
Observed signs or oral intoxication were depression, ptosis, ataxia,
diarrhoea, excessive urination and laboured respiration. Dermal
application produced mild erythema and oedema.
TABLE 1. Acute toxicity of daminozide.
LD 50'
Species Route Sex mg/kg Reference
Rat Oral M 6,810 Anonymous, 1966
Oral M,F 8,400 Carson, 1963
Inhalation M 147 mg/l Carson, 1963
Rabbit Dermal M,F >10,000 Anonymous, 1966
Dermal M,F 16,000 Carson, 1963
Short term studies
Rat
Groups of rats (5 males and 5 females/group; 10 of each sex were
controls) were fed dietary levels of 0, 26, 80, 240, 720 or 2160
mg/kg/day daminozide technical for 90 days. No differences were noted
in body weight gains between any test group and the control gains. No
significant differences in haematological, blood chemistry or urine
values were observed and organ weights and ratio were comparable to
the control group. No gross or microscopic changes occurred in any
group which could be attributed to daminozide administration (Carson,
1964).
Dog
Groups of dogs (6/sex/control and 4/sex/test groups) were fed dietary
levels of 0, 300, 1000 or 3000 ppm daminozide technical for two years.
Body weight gains were within normal limits and comparable to control
gains. Appearance, behaviour, haematological, blood chemistry and
urine analyses of the test groups were also comparable to controls.
Gross necropsies, microscopic examination of tissues and organ weight
data revealed no compound or dose-related effects (Oser, 1966).
Long term studies
Rat
Groups of rats (37/sex/control and 25/sex/test group) were fed dietary
levels of 0, 300, 1000 and 3000 ppm diaminozide technical for two
years.
No differences in appearance or behaviour were observed between
control and test rats during the study. Throughout the study the body
weights gains of the 300 ppm group were slightly less than those of
the controls, but weight gains of the 1000 and 3000 ppm groups were
comparable to the control gains. No haematological or blood chemistry
differences related to compound consumption were noted during the
study. Survival over two years was not affected and was comparable
between all groups. Liver/body weight ratios for both sexes in the 300
and 3000 ppm groups were significantly higher than the control ratio
at 104 weeks. In the 300 ppm male group the mean liver weight was 21%
higher than the mean control liver weight. Nothing in the gross or
microscopic examinations explains this difference. Microscopic
examination of tissues revealed no dose-related effects. Tumors were
both benign and malignant, the former being principally mammary
adenomas or fibromas. In the latter category, reticulum cell sarcomas
predominated in both the test and control groups, the incidence being
not significantly higher at the 3000 ppm level than in the control
group. Several varied types of malignant neoplasms were also noted but
in no cage was there more than one of each type in any group (Oser,
1966).
COMMENTS
Daminozide is not highly acutely toxic as measured by LD50 values in
the g/kg range for oral and dermal studies and is only mildly
irritating to the eyes and skin. It is rapidly excreted and does not
bioaccumulate in mammals. Daminozide did not produce dominant lethal
effects in male mice at 10,000 ppm and no teratological or
reproductive effects were observed at levels of 300 ppm or lower. An
adequate two-year dog study has been conducted.
The long-term rat study was inadequate because of internal conflicts
within the report and because of the inability to evaluate the
carcinogenic potential of daminozide. Information on the
biotransformation of daminozide in animals is needed.
Because of the above concerns no recommendation for an acceptable
daily intake for man can be made.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Daminozide is a plant growth regulator applied only in foliar sprays
to various tree fruits, grapes, vegetables, melons and peanuts.
Depending on the crop and timing of treatment, it is variously
intended to hasten ripening, increase fruit set, enhance fruit colour,
retard stem elongation, control fruit drop, delay maturity, reduce
vine growth or to produce certain other beneficial growth regulating
effects.
Introduced on an experimental basis in the early 1960s, it was first
registered for use on ornamentals. Since that time there have been
numerous reports in the horticultural journals on the efficacy of the
compound as a growth regulator. Use was expanded to include food crops
in 1968 in the U.S.A. Table 2 shows current (1977) registered use
patterns in the U.S.A. Uses in Canada are similar to the U.S. uses.
There was other information available to the Meeting that the product
has been used in Italy, South Africa, Japan, Australia, Federal
Republic of Germany, the Netherlands and the United Kingdom. The
directions for use on the product labelling accepted in those
countries generally conform to the use patterns shown in Table 2.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trials in the U.S.A. on fourteen crops and controlled feeding
experiments with meat and dairy animals (Uniroyal, 1977). The
government of the Netherlands submitted reports of supervised residue
trials on apples and pears. Analyses were by the colorimetric
procedure described below under "Methods of residue analysis".
Analyses of untreated controls (crop blanks) were reported in each
experiment, along with % recovery of daminozide from fortified
controls. Test samples were corrected for crop blank. Recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form where such presentation
is feasible.
Apples
Field trials were conducted at locations in 10 states representing all
of the major apple growing areas of the U.S.A. Some 180 harvest
samples were analyzed in duplicate. The wide range of experimental
conditions in the various field tests and the resulting wide range of
residues found, preclude a tabular summary of data. The residues
ranged from 0.1 mg/kg to as high as 80 mg/kg. If the high values
resulting from exaggerated treatments are excluded, the data indicate
that residues would not be likely to exceed 30 mg/kg when the normal
dosages (Table 2) are applied and the prescribed 60-70 day preharvest
interval is observed.
TABLE 2. Registered use patterns for daminozide in the U.S.A. (1977).
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
sweet cherries hasten 0.1-0.2% 1 2 weeks after full
ripening, bloom
concentrate
maturity
sour cherries " 0.4% 1 "
peaches, hasten 0.1-0.2% 1 13 weeks post-bloom
nectarines ripening, to pit hardening
concentrate
maturity
pears prevent fruit drop 0.1% 1 18 to 24 days before harvest
apples multiple effects(1) 0.075-0.2% 160 to 70 days before harvest
prunes hasten ripening 0.05-0.1% 1 1 month before
harvest
grapes increase fruit set, 0.05-0.1% 1 from 1st bloom to
reduce vine growth (1.4-2.4 kg/ha) full bloom
Brussels uniform sprout 1.9-3.75 kg/ha 1 30 days before
sprouts development harvest
cantaloupes reduce vine growth 1.9 kg/ha 1 2 to 4 leaf stage
TABLE 2. (Continued)
Crop Purpose Spray No. of Timing or pre-harvest
Application Applications limitation
Rate (a.i.)
tomatoes multiple effects(1) 0.6-2.4 kg/ha 2 before transplanting
4.8 kg/ha 1 after transplant - not
within 7 days
of picking
peanuts multiple effects(1) 1.0 kg/ha 2 at pegging time
0.5 kg/ha 30 days before
harvest
peppers 0.5-2.3 kg/ha 2 at 2-4 leaf stage
(seedlings) and 7-10 days
later
(1) There are 9 separate beneficial effects claimed for apples alone. Other claims are similarly complex. The directions for use of this
product are complex because of many factors (plant vigour, variety, moisture stress, etc.) which can not only modify the desired effect
on the plant but can contribute adverse effects.
The residues from trials in the Netherlands were generally 5 mg/kg or
less. The rates of application in these experiments were somewhat
lower than the rates permitted in some other countries.
Studies designed to measure initial deposits or residue decline rate
(half-life) were not included. The data clearly show, however, that
daminozide is extremely persistent in apples. Significant residues
remain for more than 159 days after the spray application.
Brussels sprouts
Four separate trials were conducted at locations in California and New
York. A single treatment was made at normal and exaggerated rates.
Crop blanks were reported as zero. Recoveries were 87 and 83% at 1 and
10 mg/kg respectively. The data are summarized in Table 3. Under good
agricultural practice the residues would not be likely to exceed 20
mg/kg.
TABLE 3. Residues in Brussels sprouts, mg/kg (single application).
Application rate, Interval, days from treatment
kg a.i./ha 30 33 46
1.9 6.3, 5.2, 6.8 7.1, 1.4, 0.0
3.4 2.8, 1.4
3.8 14.6, 15.5, 13.0 2.3, 2-7, 1.5
6.8* 4.8, 3.2
7.7* 25.4, 24.8, 24.6 5.6, 6.2, 5.2
*excessive dosage
Cantaloupes
Nine residue trials were reported from locations in California,
Arizona, Texas and Michigan. The registered use patterns on melons
provide for treatment when the plant is at the 2-4 leaf stage, at
which point no fruit would be present. The experiments included
exaggerated treatment rates, and samples taken as early as 44 days
after treatment. If these samples were mature melons, fruit must have
been present at the time of treatment (melons take from 85 to 150 days
from planting to maturity). Even under these conditions the residue
values of some 20 samples ranged from < 0.1 to 1.6 mg/kg (Table 4)
compared with apparent daminozide in untreated controls up to 1.1
mg/kg. It would appear that residues to be expected in cantaloupes
from good agricultural practice would be of a lower order than those
found in the tree fruits. It is not likely that residues in excess of
3 mg/kg would occur in cantaloupes. Data were not available on other
melon varieties, but the conclusion regarding cantaloupes could be
extrapolated to other melons.
TABLE 4. Residues in cantaloupes,mg/kg
Interval from No. of Application Rate,
last treatment treatments kg a.i./ha
days 0.47 0.95 1.9 3.8 7.6
44 2* <0.1 1.6
50 1 1.0,1.5 1.5,1.6
57 1 0.6,0.8 1.2,1.6
68 1 1.2 1.6
73 2* <0.1 <0.1
87 1 <0.1 <0.1
115 2* <0.1 <0.1
*Rate given for second treatment only.
Sour cherries
Trials were carried out at several locations in four states
representing the important cherry producing areas in the U.S.A. A
total of 70 test samples and 25 control samples were analyzed. Crop
blanks ranged from 0.1 to 0.64 mg/kg.
The dosages applied in the tests ranged from 25% of the manufacturer's
minimum recommended dosage to twice the maximum recommended rate (See
Table 2). Samples were taken at periods after treatment ranging from
56 to 65 days. This roughly corresponds to the label directions which
call for a single spray 10-14 days after full bloom. The season from
bloom to harvest in the U.S.A. is 80 to 100 days (Magness, 1971).
The sampling schedule in all trials was designed to show residues at
normal harvest. Therefore, no estimates of initial deposit or residue
decline rate are possible. Overall the residues found on mature
cherries ranged from 5.3 to 78 mg/kg. Although some correlation could
be made with application rate, the variation in harvest residues
appeared to be almost random (the data do not lend themselves to
tabulation). If the data from trials with excessive or minimal dosages
were excluded, the residues fall between 10 and 53 mg/kg. It is not
likely that residues under actual (commercial) conditions would ever
exceed 60 mg/kg.
Sweet cherries
Analyses of 176 test samples and 39 control samples from field trials
in five states were made available. The experimental design generally
paralleled that discussed above for sour cherries. That is, the trials
were designed to show residues on harvested cherries treated
approximately as prescribed on the product label (2 weeks after full
bloom). The overall range of residues found (1 to 54 mg/kg) was
comparable to that reported on sour cherries. If only the data from
trials following good agricultural practice (See Table 2) are used,
most samples were in the range of 9-18 mg/kg, with a few higher values
approaching 30 mg/kg. The difference between the residue load expected
on sweet and sour cherries is mainly due to the application rate,
which is 0.2% for sweet and 0.4% for sour cherries. A maximum residue
limit of 30 mg/kg for sweet cherries would therefore be consistent.
Grapes
The residue data are summarized in Table 5. Some 44 samples from field
trials in 4 states were reported. Analyses were in duplicate,
including 8 untreated control samples. All controls were reported as
0.1 mg/kg, apparent daminozide.
The registered use directions for grapes (Table 2 requires treatment
during the period from first bloom to full bloom. The period between
bloom and harvest for grape varieties will differ greatly. In some
varieties it may be as much as 5-6 months (Magness, 1971). It is
interesting to note that in the field trials, the intervals between
spray and harvest were from 102 to 111 days, and probably represent an
early maturing variety, probably Concord. From the standpoint of
residues at harvest, it therefore represents the "worst case". Even
with some exaggerated dosages, all residues were below 10 mg/kg.
TABLE 5. Residues in grapes, single application, 102-111 days from
treatment
Application rate Residue range,
% a.i. mg/kg
0.05 0.4 - 2.5
0.10 1.3 - 5.4
0.20 2.6 - 9.5
Nectarines and peaches
The use directions for these two fruits are the same. They also incur
residues to the same degree, and may be discussed together.
The prescribed cut-off date for sprays is at "pit hardening".
Presumably, this growth stage is recognizable to experienced fruit
growers. As near as can be determined, pit hardening occurs 40 to 80
days before harvest, depending on variety, geographic location and
other factors.
The data made available on nectarines and peaches included a total of
more than 500 analyses including 70 untreated controls. Treatments
were made at the prescribed rate, at excessive rates, and at less than
the prescribed rate. The intervals between treatment and sampling were
variously identified as treatments at "style abscission", pit
hardening, and in some cases by numerical day before harvest. Owing to
the variables in the field tests, a wide range of residues was
reported (1.0 to 93.0 mg/kg).
Interest is mainly in the residues from applications closely
approximating the registered use pattern (Table 2). The distribution
of residues from application of 0.1 to 0.2% sprays in the period from
style abscission (3 weeks post-bloom) to pit hardening in nectarines
and peaches was:
mg/kg <1 1-5 5-10 10-15 15-20 20-25 >25
No. of samples 35 140 48 45 3 7 1
The distribution indicates that residues would not be likely to exceed
30 mg/kg when uses described in Table 2 are followed.
Peanuts
Results of residue trials in Georgia, N. Carolina, Texas, Alabama,
Florida and Oklahoma were submitted to the Meeting. Separate analyses
were made on kernels, hay and hulls.
Kernels
A total of 166 analyses of test samples and 13 of controls were made.
All controls samples were zero except two reported at 3.1 and 2.8
mg/kg. (The analyst noted that contamination was suspected.)
The manufacturer's recommendations are for one treatment of 0.96 kg
a.i./ha at pegging time followed by a second treatment of 0.47 kg
a.i./ha thirty days before harvest. For those field trials in which
the treatment approximated the recommended pattern, the following
residue distribution was obtained:
Range, mg/kg: <5.0 5-10 10-15 15-20 20-25 25-30
Number of samples: 31 20 7 4 4 2
From this skewed distribution pattern it is apparent that maximum
residue limit of 30 mg/kg for kernels would be adequate to cover all
residues from good agricultural practice.
Peanut hay
62 analyses were submitted, 24 of which corresponded to the normal
split dosage pattern mentioned above. Residues in the trials
reflecting recommended usage were mostly in the range of 1-5 mg/kg.
However, three were in the range of 5-10 mg/kg and three were greater
than 10 mg/kg. A maximum residue limit of 20 mg/kg would be adequate.
Peanut hulls
Very limited data were available on peanut hulls. Residues were 2
mg/kg or less. Peanut hulls are not an important item of international
trade. The only interest in this commodity is that the hulls are
incorporated at low levels into some mixed animal feeds.
Peanut meal
Field-treated peanuts which were determined to be carrying 12 mg/kg of
daminozide were processed in a laboratory experiment simulating a
commercial process. The meal presscake was found to contain 30-33
mg/kg. On the basis of this approximately three-fold concentration
factor, peanut kernels bearing the maximum residues of 20 mg/kg would
be expected to produce meal containing about 60 mg/kg.
Pears
Limited data (14 test samples + 7 controls) from Washington and Oregon
were available. Seven samples were from trials in which the
manufacturer's recommended rate was applied (0.1% spray). Residues at
this dosage ranged from 0.8-17.0 mg/kg, with an average of 10.8 mg/kg.
Residues from uses according to good agricultural practice would
probably not exceed 20 mg/kg.
Peppers
Field trials were conducted in Texas, Georgia, Connecticut, Delaware
and Michigan. Samples were collected 77-125 days after treatment.
Single and double applications were made at the prescribed rate and at
four times the prescribed rate. The net difference between the
apparent daminozide in the test samples and the control samples was
less than 1 mg/kg in all cases, the highest residue reported being
0.44 mg/kg. A maximum residue limit of 1 mg/kg would be adequate.
Plums
Residue trials at 6 locations in California were reported to the
Meeting. The plums (fresh prunes) were treated at the prescribed rate
(Table 2) and at rates up to 4 times the prescribed rate. The interval
between treatment and sampling ranged from 34 to 78 days (as compared
with the registered label requirement of a 30 day pre-harvest
interval). Residues from all treatment rates ranged from 3.0 to 71.5
mg/kg. Residues from the normal rates were 3.0 to 43.7 mg/kg. It is
unlikely that residues from normal usage would exceed 50 mg/kg.
Field-treated fresh prunes were analyzed and converted to dried prunes
by the commercial procedure. This involves a 5-10 minute mechanical
wash and drying on trays at 170°F for 30 hours. The maximum
concentration factor (mg/kg in dried prunes: mg/kg in fresh) was found
to be 2.7. On this basis, a prediction can be made that fresh prunes
bearing maximum residues of 50 mg/kg would produce dried prunes
containing 135 mg/kg.
Tomatoes
The registered uses of daminozide on tomatoes include 2 applications
prior to transplanting (greenhouse or outdoors) and one application in
the field no later than 7 days prior to picking (see Table 2). The
application on plants to be transplanted is for all practical purposes
a "no-residue" use. Residue data were made available on tomatoes which
had received both transplant and field treatments at the normal and
excessive rates. All residue trials (7) were carried out in Florida,
on green tomatoes. The only field use for daminozide on tomatoes
permitted in the U.S.A. is on Florida winter tomatoes which are
harvested green and artificially ripened. These residue trials
therefore were highly specialized and do not necessarily reflect
residues which might result from more general usage on tomatoes. The
data are shown in Table 6.
TABLE 6. Residues in tomatoes, mg/kg
Interval from Application Rate*
last treatment kg a.i./ha
Days 4.76 9.52
11 13 68.5
21 9 35
7 6 8
7 8 12.5
18 12 20.5
14 20 45.5
19 15 28
29 22 8
* Field treatment after transplant use.
Alfalfa
There are no national tolerances or registrations for daminozide use
on alfalfa. However, the product has some utility in the culture of
alfalfa seed, and registration is pending in U.S.A. In growing alfalfa
for seed there is some utilization of alfalfa forage, hay, and seed
screenings for animal feeds. The submission to the Meeting therefore
contained some residue data on alfalfa hay, fresh forage, and seed
screenings. Residues on fresh alfalfa ranged from 4-12 mg/kg and on
hay from 2-20 mg/kg. Seed screenings showed a maximum of 1.4 mg/kg. In
view of the specialized nature of the use it would not appear
necessary at this time for the meeting to recommend a maximum residue
limit.
Meat, milk, poultry and eggs
Daminozide is used on a number of crops which yield by-products used
commercially in animal feeds. Primary daminozide sources would be
peanut meal and peanut hay. Some additional contribution to the diet
could occur from peanut hulls and the pomaces resulting from
processing apples, grapes and tomatoes. In recognition of this, the
basic manufacturer submitted to the Meeting some animal feeding,
studies (Uniroyal, 1977).
(a) Cattle were fed at levels of 0, 20, 60 and 200 ppm in the total
diet for 6 weeks. Milk was collected at intervals and the animals
slaughtered at the end of the feeding period. No daminozide residues
were found in any tissues (muscle, fat, liver, kidney) by a method
with a lower limit of detection estimated at 0.2 mg/kg. Trace residues
were found in the milk of animals on the 60 and 200 ppm diets at
levels of 0.05 and 0.07 mg/kg respectively.
(b) Hogs were fed 0, 60 and 300 ppm in the diet (2 animals at each
level) for 31 days. No daminozide residues were detected (40.2 mg/kg)
in muscle or fat. Apparent daminozide residues were found in liver and
kidney, at 0.3 and 0.8 mg/kg respectively, in animals on the highest
feeding level.
(c) Poultry were fed 0, 20, 60 and 200 ppm in the diet (10 hens in
each group) for 31 days. Eggs were composited weekly and on day 31 all
birds were sacrificed. On the final sampling, eggs from the 300 ppm
and 60 ppm feeding levels contained 0.3-0.5 and 0.25 mg/kg
respectively. No detectable residues were found (< 0.2 mg/kg) in eggs
from the 20 ppm feeding level. No residues were found in muscle or fat
of birds on the lower feeding levels. At the 200 ppm level, residues
of 0.4 and 0.7 mg/kg were found respectively in muscle and fat.
Residues of 1 and 1.1 mg/kg were found in liver of birds on the
highest level. Daminozide concentrates in poultry kidney. Values of
1.3, 1.8 and 7.4 mg/kg were reported in kidney for the 3 feeding
levels.
The studies show that residues occur at high feeding levels, but this
must be related to the actual anticipated intake from peanut meal and
peanut hay. Peanut hay would be used to the extent of 25% in beef
cattle diets, 60% in dairy diets, and 10% for pigs. Peanut meal can
make up 15%-beef; 25%-dairy, 10%-poultry and 10%-pigs (Harris, 1975).
After adjusting for the proportion of peanut meal and hay in the total
diet of each species, it is apparent that the residues in milk, eggs
and tissues (except poultry kidney) Will be below the level of
detection of the colorimetric method.
FATE OF RESIDUES
General
Daminozide is readily absorbed and translocated from foliar sprays.
The entrapment of 14CO2 from applications of labelled daminozide
indicates that, under certain conditions, at least part of the applied
pesticide is fully degraded. Other data show that daminozide, in free
or loosely bound form, is the principal residue component and very
persistent in fruits. Many of the conclusions regarding the nature of
the terminal residues rest on characterization by selective solvent
extractions, in vitro experiments and analysis by wet chemical
procedures for predicted metabolites. In view of the known biological
activity of some postulated metabolites of the compound, particularly
nitrosamines, hydrazides and other hydrazine derivatives, further
identification of the residues in harvested fruits and vegetables
would be desirable.
In animals
Rats were fed 14C-labelled daminozide and the excreta monitored. The
majority of the administered dose, apparently occurred in the urine as
unchanged daminozide. As far as can be determined, there has been no
effort to study metabolic pathways in meat or milk animals with radio-
labelled daminozide. However, analyses by the chemical method
(previously discussed) were made for one postulated metabolite (NDMA)
in milk from cows fed 200 ppm daminozide in the diet. No NDMA was
found at a minimum detection level of 0.03 mg/kg. See also the
sections "Biochemical aspects" and "Residues resulting from supervised
trials".
In plants
It has been reported that daminozide decomposes to unsymmetrical
dimethylhydrazine (UDMH) in the plant and that it is the latter
compound which is the active principle through its effect on the plant
enzyme diamine oxidase (Reed, 1963). Another investigator (Dahlgren,
1963) found that maleic acid dimethyl hydrazide (CO11, a related
growth regulator) is hydrolysed to UDMH in aqueous solutions. Dahlgren
noted that daminozide was stable under the same conditions. In
experiments reported by the manufacturer (Uniroyal, 1967) no free UDMH
or succinic acid was detected after attempts (in vitro) to enzymically
hydrolyze daminozide with trypsin, proteinase, papain, ficin and
urease. No evidence was found of hydrolysis to UDMH and succinic acid
in macerates of plant leaves by colorimetric tests for UDMH and paper
chromatographic tests for succinic acid. While the evidence regarding
the occurrence of UDMH is somewhat contradictory it is likely that,
even if UDMH occurs as an intermediate metabolite, its presence would
be fleeting owing to its reactivity and that it would not be present
per se in harvested crops.
The gas-chromatographic method of analysis for nitrosodimethylamine
(NDMA), discussed below ("Methods of residue analysis",) has been used
in studies intended to show the absence of this possible oxidation
product in treated crops (Uniroyal, 1967). No NDMA was detected in
treated apples, tomatoes or peanut foliage by the method, which is
said to be sensitive to 0.002 mg/kg NDMA.
Radiotracer studies were conducted by the U.S. Department of
Agriculture (Uniroyal, 1966) on apple trees in the field and on apple
seedlings in the greenhouse. Daminozide was labelled in the succinic
acid moiety and (in a separate test) in the UDMH group. Activity in
the sprayed seedlings was distributed throughout the plant with
highest activity in the leaves. 20% of the original activity was
released as 14CO2 in the first seven weeks after application.
In the field studies, 15% of the total applied activity remained in
the tree after 100 days. The activity in the apple fruit after 125
days was identified as 75-85% unmetabolized daminozide. Trace amounts
of succinic acid were identified and there was some incorporation of
14CO2 into plant components.
14C-labelled daminozide was used in metabolism studies with peanut
plants (Uniroyal, 1977). Characterization of the activity by selective
solvent extraction indicated that the majority of the activity was
present in bound form, probably as sugar complexes. Daminozide per se
was recoverable from the extracts and it was concluded that residues
present in peanuts in complexed form would be detected by the
colorimetric method of analysis. Analyses of peanut oil for 14C
activity showed residues of about 2-5 mg/kg expressed as daminozide.
Selective solvent extraction of the oil indicated that the activity
was not daminozide but was probably in the form of fatty acid
glycerides.
In soil
Residue decline studies were conducted on sandy loam and clay soils
spiked at 5 and 10 mg/kg. Daminozide per se is not persistent in
soils. The breakdown is probably through microbial action. In the clay
soil, < 0.1 mg/kg was present two days after treatment. The sandy
loam contained 0.1 mg/kg at 2 and 3 weeks respectively at the 5 and 10
mg/kg fortification levels. The compound is very mobile in soils
because of its water solubility.
In processing
Studies show that in the commercial processing of peanuts there is no
appreciable concentration of residues in the refined peanut oil. There
is, however, a 3 fold concentration of residues in peanut meal (e.g.,
peanut meats bearing 20 mg/kg will produce peanut meal bearing 60
mg/kg).
Data on the processing of fresh tomatoes bearing approximately 40
mg/kg into ketchup and tomato paste show that there is essentially no
loss of daminozide residues in processing. (The residues concentrate
to the same degree as the solids do in the concentration process.)
Residues found in tomato paste ranged from 155 to 197 mg/kg. Analyses
for nitrosodimethylamine (NDMA) in tomato paste were negative by the
chemical method with a reported sensitivity of 0.002 mg/kg.
Experiments in which field-treated plums were processed into dried
prunes showed that residues were concentrated in the dried fruit by a
factor of 2.7. See "Residues resulting from supervised trials" for
details of this work and of the effect on residues of processing
peanuts to peanut meal.
There are national tolerances on grapes (10 ppm, U.S.A. and Canada)
but no data were made available on transfer of residues to wine or
grape pomace.
Evidence of residues in food in commerce or at consumption
As noted under "Methods of residue analysis", daminozide is not
detected by any of the multi-residue screening methods currently used
in market basket surveys, monitoring, or government regulatory
programmes. The "specific" colorimetric method has not been employed
in the Canadian or the U.S. Food and Drug Administration surveillance
programme and there is no record of a finding of daminozide in foods
in commerce. In view of the demonstrated stability of daminozide
residues in food processing, and the relatively high levels incurred
in fruits from good agricultural practices, it may be concluded that
residues are occurring in the diet in those countries where there is
substantial use.
METHODS OF RESIDUE ANALYSIS
Daminozide residues in crops are determined by a colorimetric method
(PAM II, 1967). The method involves hydrolysis in boiling 50% sodium
hydroxide to release unsymetrical dimethylhydrazine (UDMH) which is
distilled and reacted with trisodium pentacyanoamine ferroate (TPF) at
pH 5.0. The red colour produced is measured spectrophotometrically at
490 and 600 nm. The difference between the two readings is plotted as
net absorbance against µg of compound in a calibration curve.
Modifications of this basic method permit determination of daminozide
in peanuts, animal tissues, milk and eggs.
The chromogenic agent TPF used for determination of daminozide also
forms colored complexes with nitroso compounds, primary aromatic
amines and aliphatic or aromatic hydrazines (Feigl, 1949). Some
possible metabolites of daminozide would therefore contribute
absorbance which would be additive to that of the daminozide-TPF
complex. If present they would be interpreted as daminozide residues
(but not measured quantitatively).
The method has been tested in government regulatory laboratories on
grapes and animal tissues. Because of large and variable crop blanks
it was concluded that a reliable lower limit of determination for
daminozide in samples of plant origin was no better than 1 mg/kg for
regulatory purposes. The estimated sensitivity for animal products is
0.05 mg/kg for milk and 0.2 mg/kg for tissues and eggs. The government
analyst noted that 2 hours colour development time was required rather
than the 1 hour prescribed in the procedure.
The assumption was made that any free UDMH, or any metabolites which
would yield UDMH under the rigorous caustic hydrolysis, would also be
measured as daminozide by this method. There are no experimental data
to confirm that conclusion. Whether in fact any such metabolites
actually occur in crops at harvest is uncertain (see discussion under
"Fate of residues").
Colorimetric methods are ordinarily not specific. In this case, the
method was tried in the presence of most pesticides used on the
subject crops (in 1968) to gauge interference. Only the fungicide
"Botran" was found to interfere, and this interference can be
eliminated by a modification of the method.
A second colorimetric method of analysis for daminozide in plant
substrates has been published (Edgerton, 1967). This method also
measures UDMH in a hydrolysate, but colour development is with
phosphomolybdic acid. Sensitivity is comparable to the colorimetric
method discussed above.
As noted above, the colorimetric method for the parent compound would
also measure any alkylnitrosamines as daminozide. Because of concern
for the toxic potential of a possible metabolite, nitrosodimethylamine
(NDMA), a "specific" method for NDMA was developed by the manufacturer
(Uniroyal, 1967). This is a GC method in which NDMA is separated from
crop substrate by vacuum distillation, sorbed on polymer beads,
removed from the beads by baking and determined by micro-coulometric
gas chromatography (measurement of NH3). Sensitivity was estimated at
0.002 mg/kg. Recovery experiments at a fortification level of 0.010
mg/kg were reported to be adequate. This method has not been validated
in government laboratories.
Daminozide is not detected by any of the multi-residue screening
procedures used in government market basket surveys and monitoring
programs. There is no record of any government regulatory experience
with the colorimetric method for daminozide. It is questionable
whether by present standards it would be satisfactory for regulatory
purposes, particularly for enforcement of the lower tolerance levels.
As far as can be determined there is no confirmatory method of
analysis available. It would be desirable to have further information
on the reliability of this method, particularly with respect to (a)
whether it actually measures UDMH metabolites in free or conjugated
forms and (b) actual experience with the method in government
laboratories. An independent method, preferably a more specific GC or
HPLC method would be desirable.
APPRAISAL
Daminozide is a growth regulator which has been used on a variety of
fruits and vegetables since 1967. The product produces numerous
beneficial growth regulating effects. It is said to control premature
fruit drop, delay maturity, enhance fruit colour, reduce vine growth,
retard stem elongation and increase fruit set, among other claims.
Information submitted to the Joint Meeting by the primary manufacturer
indicates that the principal uses of the product are in the U.S.A.
Other information made available to the Meeting indicates that
daminozide is also used in Italy, South Africa, Japan, Australia,
NATIONAL TOLERANCES REPORTED TO THE MEETING
Commodity Tolerance, mg/kg
U.S. Canada Australia Netherlands
Fruits and vegetables 10
Sour cherries 55 55
Plums 50 20
Tomatoes 40 0.5
Apples 30 30 30
Nectarines 30
Peaches 30 25 30
Peanuts 30 20 30
Sweet cherries 30 30
Brussels sprouts 20 15
Peanut hay 20
Pears 20 15 30
Grapes 10 10
Peanut hulls 10
Melons 3
Peppers 1
Meat, fat and meat 0.2 0.2
by-products of cattle,
goats, horses, poultry
(except kidney), sheep
Poultry kidney 2
Eggs 0.2 0.2
Milk 0.02 0.05
Canada, Federal Republic of Germany, the United Kingdom and the
Netherlands. National tolerances have been established in the U.S.A.,
Canada, Australia and the Netherlands.
Daminozide was formerly manufactured by a process in which
dimethylnitrosamine was a starting reactant, and occurred as impurity
in the technical grade product. The primary manufacturer informed the
Meeting that a new process has been adopted in which
dimethylnitrosamine is not used. The recommended guideline limits
apply only to daminozide manufactured by the process in which
dimethylnitrosamine is excluded.
The compound is readily absorbed and translocated from foliar sprays.
While there in some evidence of degradation, the parent molecule in
free or loosely bound forms is persistent, particularly in the fruits.
The chemical structure of daminozide suggests the possible presence of
some metabolites of interest, including nitrosamines, hydrazides and
other hydrazine derivatives. No positive findings of these metabolites
were reported. The postulated metabolite N-dimethylnitrosamine was not
detected by a method said to be sensitive to 0.002 mg/kg. Indirect
evidence was reported of the absence of unsymmetrical
dimethylhydrazine (UDMH).
A feeding study with 14C daminozide in rats indicated that the
majority of the administered dose was excreted in the urine as
unchanged daminozide. There was no information on the metabolism
patterns in large animals or on metabolites in meat or milk. In
general, the information available on the fate of daminozide in plants
and animals is not very extensive, particularly with respect to the
nature of the terminal residues.
The analytical method currently available to enforce national
tolerances is a colorimetric method which measures unsymmetrical
dimethyl hydrazine (UDMH) released from daminozide by vigorous caustic
hydrolysis. The chromogenic agent is not specific for UDMH. Other
classes of compounds also produce colour complexes, including
aliphatic hydrazines, nitroso compounds and primary aromatic amines.
It is theorized that complexed daminozide, free UDMH and any other
metabolic products which would yield UDMH would be measured as
daminozide. It has been determined that the method has never been
applied in the U.S. or Canadian official surveillance programmes and
there is no information on experience with the method in other
government laboratories. The method would not appear to be very useful
for regulatory purposes.
Studies on the effects of processing show that the compound is not
destroyed and tends to concentrate in products such as tomato paste
and peanut meal. It has never been reported in foods in commerce (see
above comment on the analytical method). It is not persistent in
soils. Extensive data from supervised residue trials carried out in
the U.S.A. were available. Data on supervised trials on apples and
pears from the Netherlands were also reported. All analyses were by
the colorimetric method discussed above. The data generally showed the
highest residues in fruits, with considerable variability in harvest
residues. The residue values on a given crop followed almost a random
distribution. The experiments on which reports were received were all
designed to show harvest residues from registered use patterns and
therefore permitted no estimates of decline rate.
EVALUATION
As no ADI or temporary ADI could be allocated, no recommendations for
maximum residue limits could be made. Guideline levels are recorded
for the commodities listed below. The usual statements regarding the
intervals on which the levels are based are omitted in this case
because of the highly specialized uses of the product which are
related to stages of growth or maturation.
Commodity Guideline level, mg/kg
Sour cherries 60
Plums 50
Tomatoes 40
Apples, nectarines,
peaches, peanuts
(kernels), pears,
sweet cherries 30
Brussels sprouts 20
Grapes, peanut hay 10
Melons 3
Peppers 1
Eggs, meat 0.2*
Milk 0.05*
*At or about the limit of determination
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake for humans (ADI) and
maximum limits (MRL can be established)
1. An adequate long-term rat study.
2. Information on the biotransformation in animals.
Desirable
1. An analytical method more suitable for regulatory purposes than the
present colorimetric method.
2. Information on the occurrence of residues in foods in commerce.
3. Further identification of the terminal residues in crops and in
foods of animal origin.
REFERENCES
Anonymous, (1966) Acute Oral Administration-Male Rats. Unpublished
report from Hazleton Labs., Inc., submitted to the World Health
Organization by the Uniroyal Chemical Company.
Carson S., (1963) Toxicological Examination of Compound B-995.
Unpublished report from Food and Drug Research Labs., submitted to the
World Health Organization by Uniroyal Chemical Company.
Carson S., (1964) Subacute (90 day) Feeding Studies with B-995 in
Rats. Unpublished report from Food and Drug Research Labs., submitted
to the World Health Organization by Uniroyal Chemical Division of
Uniroyal,Corp.
Dahlgren, Dahlgren and Simmerman, (1963) Science, 148, 485; 1963
Edgerton, L.J., Rockey, M., Arnold, H., and Lisk, D.J., (1967)
"Colorimetric Determination of Alar Residues in Apples", J. Agr. Food
Chem. 15, no. 5, p. 812, 1967
Feigl, F., (1949) Chemistry of Specific Selective, and Sensitive
Reactions, p. 377, Academic Press, New York, 1949
Harris, L.E., (1975) "Guide for estimating toxic residues in animal
feeds or diets", U.S. National Technical Information Service,
accession no. PB-243-748-LK
Keplinger, M.L., Kennedy, G.L., and Haley, S., (1972) Teratogenic
Study with Alar in Albino Rats. Unpublished report from Industrial
Bio-Test Labs., submitted to the World Health Organization by the
Uniroyal Chemical Division of Uniroyal, Corp.
Magness, J.R., Markle, G.M., Compton, C.C., (1971) Food and Feed Crops
of the United States, Bull. 828, New Jersey Agricultural Experiment
Station, 1971
Oser, B.L., (1966) Chronic (2 year) Feeding Studies with B-995 in Rats
and Dogs. Unpublished report from Food and Drug Research Labs.,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Palmer, A.K. and Lovell, M.P., (1973) Dominant Lethal Assay of Alar in
the Male Mouse. Unpublished report from Huntingdon Research Center,
submitted to the World Health Organization by Uniroyal Chemical
Division of Uniroyal, Corp.
Pam, II., (1967) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education and Welfare, Food and Drug
Administration
Reed, (1963) Science, 148, 1097; 1963
Ryer, F.H., (1966) Final Report. Radiotracer Metabolism Study,
Alar-C14. Unpublished report from Hazleton Labs., Inc., submitted to
the World Health Organization by the United States Rubber Company.
St. John, Jr., L.E., Arnold, H. and Lesk, D.J., (1969) Metabolic
Studies with Alar Growth Regulator in the Dairy Cow.
J. Agr. Food Chem., 17, No. 11 116.117.
Uniroyal, (1966) Unpublished information, Uniroyal, Inc.v to U.S. Food
and Drug Administration
Uniroyal, (1967) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1973) Unpublished information, Uniroyal, Inc., to U.S. Food
and Drug Administration
Uniroyal, (1977) Submission to Joint Meeting, May 17, 1977, Uniroyal,
Inc.
