
FENBUTATIN OXIDE JMPR 1977
IDENTITY
Fenbutatin oxide is a British Standard recommended common name and a
proposed ISO Standard Common Name.
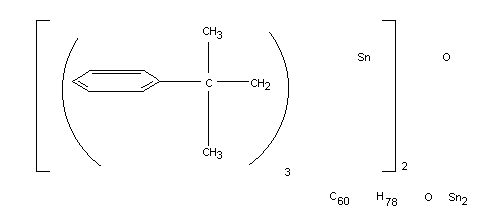
Chemical name
IUPAC: bis [tris(2-methyl-2-phenylproply)tin] oxide
Chemical Abstracts: hexakis(2-methyl-2-phenylpropyl)distannoxane
American Chemical Society: hexakis (ßß-dimethylphenethyl)distannoxane
Synonyms
SD 14114, SD 14114 -U, Torque(R), Vendex(R).
Structural formula
 Other information on identity and properties
(a) Composition of the technical Product:
The technical material contains 97% w/w minimum of fenbutation oxide;
the main impurities are related organo-tin compounds.
(b) Physical and chemical properties
Physical state: crystaline solid
Molecular weight: 1053
Melting point: 138-139°C
Volatility: not volatile
Solubility: insoluble in water, (approx. 5x10-6 g/1 at 23°C)
slightly soluble in most organic solvents;
solubility at 23°C in acetone approx 6 g/l in
dichlororomethane 370 g/l in benzene 140 g/l
Stability: stable to sunlight. Water hydrolyses fenbutatin
oxide to tris-(2-methyl-2-phmylpropyl)tin
hydroxide; this in reconverted to the parent
compound slowly at room temperature and rapidly at
98°C.
Formulations:
Wettable powder 500 g a.i./kg and suspension concentrate 500 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Groups of 2 male and 2 female rats were fed an average of 19.5 ppm
119Sn-labelled fenbutatin oxide (with or without a 3 day withdrawal
period before killing following 3 or 6 day exposure) after 1, 3 or 6
days to study the absorption, distribution and excretion of
fenbutatin. Most of the administered 119Sn was rapidly excreted in the
faeces following multiple dosing (80-90% of the administered 119Sn).
Essentially no 119Sn was excreted via the urine. The GI tract
contained approximately 30, 15 and 5% of the administered 119Sn for
animals sacrificed after 1, 3 or 6 days of dosing. The highest tissue
concentrations of 119Sn were found in liver (0.015-0.09 mg/kg),
kidneys, (0.015 0.055 mg/kg) and fat (< 0.01-0.065 mg/kg). No 119Sn
was detected in brain or bone tissue (Loeffler, 1972). See also the
section "Fate of residues", "In animals".
Biotransformation
Analysis of the faeces from rats dosed with 119Sn-labelled fenbutatin
oxide indicated that ß, ß-dimethylphenethylotamoic acid and 1, 1, 3,
3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxdistannoxane are the
major metabolites (Loeffler, 1972).
Effects on enzymes and other biochemical parameters
In starch gel electrophoretic studies on sera from rate fed either 0
or 600 ppm in the diet for 6 months (2 buffer systems) control and
test animal sera were identical. The effect of 1-phenylanatine or heat
was similar on both sera. Comparisons with intestinal, bone, liver and
kidney alkaline phosphatase indicated the serum alkaline phosphatase
was similar to intestinal alkaline phosphatase. Further studies on rat
sera derived from animals fed 0 or 600 ppm in the diet for one year,
utilizing heat inactivation, selective substrates and inhibitors,
confirmed the absence of any difference between control and test
animal sera, as well an confirming the similarity of the serum
alkaline phosphatase to intestinal alkaline phosphatase (Pickering,
1973a). In a further series of studies by the elevation of serum
alkaline phosphatase observed in rate after administration of 300 ppm
fenbutatin oxide in the diet for 90 to 120 days may shown to be
completely reversible following one months, withdrawal from exposure.
Limited data indicate that the reversal of elevated serum alkaline
phosphataze in the test rate may be completed within 7 days of
withdrawal of the fenbutatin oxide (Pickering, 1973b).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Following preliminary studies to determine suitable dose levels and to
confirm the induction of brain oedema by triethyltin bromide, a
comparative oedma assay of five organo-tin compounds (including
fenbutatin oxide, and its metabolite 1, 1, 3,
3-tetrakis-(ß, ß-dimethylphenethyl)-1, 3-dihydroxydistannoxane) for
oedma formation in the central nervous system of rats, was undertaken.
Groups of 15 male rats were intubated with corn oil (10 mg/kg, 40
mg/kg triethyltin bromide, 1000 mg/kg, fenbutatin oxide, 100 mg/kg 1,
1, 3, 3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxydistannoxane
and sizable doses of two other tin compounds. The triothyltin bromide
group showed increased brain water content after 48 hrs. Seven of ten
brains examined at 24 and 48 hrs showed spongeosis of white matter in
the cerebellum and sometimes of the corpus callosum and pons. No
histological changes were observed in 4 rats killed after 6 hrs.
Fenbutatin oxide (24 and 48 hr. kills), and the metabolite (6, 24 and
48 hr kills) did not show either increased water content or abnormal
brain histology, although rats on both compounds showed signs of
intoxication (Samuels and Dix, 1972),
Special studies on mutagenicity
Micro-organisms
In plate tests using Serratia marcesens, strains HY/alpha 13, a
histidine-requiring auxotroph, HY/alpha 21, a leucine-requiring
auxotroph, and CV/r c3/alpha 742, a thiamine-requiring spontaneous
auxotroph, 50 µl hexane solution (which resulted in a precipitate of
fenbutatin oxide on the agar plate) did not increase the reversion
rate in any strain, nor was growth inhibited, (Dean, et al., 1972a).
Neither conversion rate nor cell survival was affected in
Saccharomyces cerevisiae following exposure for 5 or 16 hrs in pH7
phosphate buffer containing 0.0025 or 0.005 µg fenbutatin oxide/ml
solution. In the same system, 20 µg/ml in suspension was ineffective
in altering conversion rate or cell survival (Dean et al., 1972a).
Mice
Groups of 8 male and 8 female mice were treated with 0.005 or 1000 mg
fenbutatin oxide/kg, in 1% carbomethyl cellulose. Four
animals/sex/group were killed 90 minutes after i.p. colcemid
administration and femurs were removed. One hundred bone marrow
cells/animal were examined. No significant differences in incidence of
chromosomal aberrations were observed (Dean et al., 1972b).
In three or four host-mediated assay experiments using Saccharomyces
cerevisiae and dose levels of 250 or 500 mg fenbutatin oxide/kg,
conversion rate was unaffected. In the fourth replicate, conversion
rate was marginally increased at 250 but not at 500 mg/kg (Dean et
al., 1972a)
In an 8 week dominant lethal study, 8 male mice/group were dosed once
with 250 or 500 mg fenbutatin oxide/kg, and were then mated with 3
females each week. The control group comprised 16 untreated males. No
significant differences in percentage pregnancies, implant incidence
or early resorption rate were noted between control and fenbutatin
oxide treated groups. A positive control group of 8 male mice (200 mg
Endoxan) showed reduced pregnancy rate and increased resorption and
decreased implant rates during the first 3 matings after, dosing.
(Dean and Doak, 1972c).
Special studies on teratology
Three groups of 15 pregnant rabbits were dosed by gelatin capsules on
days 6-18 inclusive of gestation (mating day = day 0), with 3 or 10 mg
fenbutatin oxide/kg or with 37.5 mg thalidomide/kg. Twenty-six rabbits
were used as capsule-only treated controls. Rabbits were killed on day
28 of pregnancy. A repeat experiment differed only in that 20 pregnant
females/dose level and 30 controls were used, autopsy being on day 29.
Foetuses were incubated for 24 hrs. following removal from the uterus,
after which approximately 1/3 were examined for visceral
abnormalities, and 2/3 for skeletal defects. Prior to clearing for
skeletal examination, viscera from these animals were also examined
for defects. Although some maternal deaths occurredy these were random
between groups. Mean live litter size, resorption rate, foetal loss,
survival of incubated foetuses, and incidence of anomalies were not
affected by fenbutatin oxide at either close level. Cromn-rump length
and foetal weight were comparable to controls except at 3 mg/kg in the
repeat experiment, where a significant increase was noted. The
positive control group behaved as expected (Dix and Wilson, 1973).
Special study on reproduction
In a standard 3 generation, 2 litter/generation reproduction study,
groups of 10 male and 20 female rats were fed O, 50, 100 or 300 ppm
fenbutatin oxide (985 purity). It should be noted that rate of the Fo
generation commenced on diet and were mated at 100 days of age, and
that litters were culled to 10 pups/litter on post-partum day 5.
Necropsy was performed on 10 male and 10 female F3b weanlings from
the 0 and 300 ppm, and on 5 weanlings/sex from the 50 and 100 ppm
groups. In the 300 PPM group, parents and pups were smaller, hyper
active and irritable; mean litter size was slightly reduced in the
F1b litters, and in the two F3 generations survival to weaning was
reduced at 300 ppm and testicular organ/body weight ratio was reduced
in the F3b weanlings. Necropsies of F2b adults did not show any
compound-related changes. No consistent effects were observed at 50 or
100 ppm with respect to fertility, gestation, viability or lactation
indices, litter size, litter weight, or necropsy of F3b weanlings
Mine et al., 1973).
Special studies on carcinogenicity
Groups of 48 male and 48 female Carworth Farm No. 1 strain mice were
fed 50, 100, 100 or 600 ppm fenbutatin oxide in the diet for 18
months. A control group comprised 96 males and 96 females. All animals
were examined grossly at death. Microscopic examination was undertaken
on all animals dying during the study and on all 600 ppm mice, on
10/mice/sex at 50, 100 and 300 PPM, and on 45 controls/sex at 18
months. Survival rate was comparable in all groups and exceeded 75%.
There was a high incidence of tumours in all groups. However there was
no indication of compound-or dose-related tumor induction (Granville
et al., 1973a).
Acute Toxicity
TABLE 1a. Acute toxicity of fenbutatin oxide
Species Route Sex LD50(mg/kg) Reference
Mouse Oral - 1450(1223-1659) Simpson 1972a
Rat " - 2631(1929-4659)
Dog Oral(capsule) - >1500
Rabbit Oral - 1500-3000
Rat Dermal - >1000
In mice and rats, death occurred within 10 days. Signs were dyspnoeal
stained coat and transient diarrhoea. In dogs, vomiting and diarrhoea
occurred. In rabbits convulsions occurred at the nighest dose, with
death within 24 hrs. Lower doses caused death up to 18 days after
dosing. Weight loss was still apparent 3 weeks after dosing, Autopsy
revealed damage to the gastric mucosa, massive fatty changes in liver,
reduced spermatogenesis, and multinucleated spermatid production
(Simpson, 1972a).
TABLE 1b. Acute toxicity of 50% wettable powder
Species Route Sex LD50 (mg/kg) Reference
Rat Oral - 1000 Simpson 1972a
Dermal - >1000
TABLE 1c. Acute toxicity of metabolite*
Species Route Sex LD50(mg/kg) Reference
Mouse Oral M 420 Natoff, 1973
F 622
Rat Oral M,F 242
Rabbit Oral M >675
F 300-450
Rat Oral - 132(102-176) Simpson & Dix,
Mouse Oral - 1051(824-1440) 1972c
* 1, 1, 3, 3-tetrakis(ß,ß -dimethylphenethyl)-1,3-dihydroxydistannoxane.
The signs of intoxication in all species were severe diarrhoea,
followed by loss of body weight which was recorded for rabbits, and
inanition and emaciation in all species. Deaths occurred up to 16 days
after dosing the mice, 14 days after dosing the rats and 26 days after
dosing the rabbits. The observation period for rabbits was curtailed
at 28 days.
Short term studies
Rat
Five groups of 5 male and 5 female rats were fed 0, 30, 100, 300 or
1000 ppm fenbutatin oxide in the diet for 5 weeks. Body weight was
significantly reduced, as was food intake at 300 and 1000 ppm.
Reductions in absolute brain, heart, liver, spleen and kidney weights
were noted in both sexes at 1000 ppm. Spleen weight was reduced in
males at 300 ppm and brain, liver, spleen and kidney weight was
reduced at 300 ppm in females. Serum alkaline phosphatase was
increased in both sexes and SGPT levels were elevated in females at
1000 ppm (Simpson, 1972a).
Three groups of five male rats were used in a pair feeding study, one
group being a normal control, the second being fed 600 ppm, and the
third, pair-fed at the food intake levels of the 600 ppm group, The
study ran for 5 weeks. Results (daily body weights) indicated that the
reduction in body weight at 600 ppm can be totally accounted for by
reduced food intake (Simpson, 1972b).
Groups of 6 male and 6 female rats were fed 50, 100, 300 or 600 ppm
fenbutatin oxide (97%) in diet for 3 months. Concurrent controls
comprised 12 male and 12 female rats. Health and behaviour were
comparable in all groups. Body weight gain was reduced in males at 600
ppmv and in females at 300 and 600 ppm. Food intake was reduced in
males at 600 ppm, and in females during the first 3 or 4 weeks of the
study at 300 and 600 ppm. Absolute organ weight of liver and kidney
were reduced in both sexes, heart in females, and brain and spleen in
males at 600 ppm. At 300 ppm, liver, spleen and kidney absolute
weights were reduced in males. Organ/body weight ratios were elevated
for brain in males at 600 ppm, and in females at 300 and 600 ppm.
Heart and liver weight ratios were elevated in females at 600 and
heart at 300 ppm. Testes weight ratio was elevated in males at 300 and
600 ppm. Haematology (haemoglobin, PCV, RBC, WBC, RCV, MOH,
prothrobmin time and KOCT) was comparable in all groups. Clinical
chemistry determinations were comparable for total protein, SGOT, and
electrolytes (K, Na, Cl). BUN and SAP in males were elevated at 300
and 600 ppm in males, as was SGPT at 300 PPm. In females BUN was
elevated at 100 ppm and above. Serum protein fractions were comparable
in all groups. No compound-related gross or histopathological effects
were noted, (Simpson and Thorpe, 1973a).
Three groups of rats, 20/sex/control and 10/sex/test group, were fed
0, 3, 10, 30, 100 or 300 ppm of 99% 1, 1, 3, 3-tetrakis (ß,
ß-dimethylphenethyl)-1,3-dihydroxydistannoxane for 90 days. Health and
behaviour were comparable in all groups. Body weights of 300 ppm males
were significantly decreased throughout the study. This was also true
for the females in the 30, 100 and 300 ppm group but the suppression
was not significant. Slight reductions in haemoglobin, erythrocyte
count and packed cell volume occurred in the 300 ppm males. No changes
were noted in any of the other groups of either sex. The other
clinical values of animals killed after 13 weeks showed no
dose-related differences when compared to the control values. Gross
and microscopic examination of a wide range of tissues from all
control, 100 and 300 ppm animals revealed no consistent changes
associated with exposure to the metabolite. Organ weights of males at
13 weeks did not differ from control weights, the organ body weight
ratios were increased for brain, heart, liver, kidneys and testes in
this group and for the liver in the 100 ppm male group. Brain, spleen
and kidney weights were reduced in the 300 ppm females, (Simpson and
Dix, 1972c).
Dog
Five groups of 2 male and 2 female beagle dogs were orally dosed
(gelatin capsules) with 0, 10, 30, 100 or 300 mg fenbutatin oxide/kg
for 5 weeks. Slight vomiting and diarrhoea occurred at 100 and 300
mg/kg, which was associated with decreased body weigh. At 3O mg/kg and
above, serum alkaline phosphate was elevated. No pathological changes
were observed (Simpson, 1972a).
Groups of dogs (8/sex/control and 4/sex/test group) were administered,
by capsule, 0, 2.5, 5, 15, 30 or 60 mg/kg 97% fenbutatin oxide daily
for 2 years. Blood samples were taken every six weeks to measure BUN,
glucose, plasma protein, sodium, potassium, chloride, SGPT, SGOT, and
alkaline phosphatage. Every 12 weeks hemoglobin, packed cell volume,
RBC, WBC, differential WBC counts, prothrombin and
kaolin-cephalin-coagulation times were determined. Urine analysis was
carried out every 3 months. Emesis and diarrhoea occurred in most of
the dosed dogs. These effects continued in some dogs in the 30 and 60
mg/kg/day groups throughout the study, although at a reduced frequency
towards the end of the study. Lower dosage groups showed none of these
effects after the first year. Convulsions occurred in 5 dogs,
predominantly female, during the second year; control, one female; 2.5
mg/kg/day, one female; 15 mg/kg/day, one female and one male; and 30
mg/kg/day, one female. None of the 60 mg/kg/day animals exhibited this
effect. EEG's with simultaneous single lead monitoring of EEG's of one
female each in the 0, 2.5 and 30 mg/kg/day groups revealed a normal
EEG for the control dog and the other 2 dogs showed normal waking
rhythms but abnormal activity during light sleep. The investigators
contend that the lack of dose response relationship suggest a
diagnosis of spontaneous epilepsy not related to exposure to
fenbutatin oxide. General health and behaviour of all other dogs were
similar to control dogs. Significant reductions in rate of weight
gains occurred in both sexes in the toxicity, long term 60 mg/kg/day
group. Males receiving 30 mg/kg/day exhibited no significant rate of
gain. This effect was not evident in females of this group.
Haematological and clinical chemistry and urine data showed no
differences between control and test groups. Necropsies revealed no
gross changes. Microscopic examination, including frozen sections of
liver and kidney stained for lipid, revealed no compound-related
pathological changes in a wide range of tissues. Organ weights were
not affected by exposure to the test compound (Granville and Dix,
1973b).
Long term studies
Rat
Groups of rats (114/sex/control and 72/sex/test group) were fed 0, 50,
100, 300 or 600 ppm 97% fenbutatin oxide for two years. Rats were
killed at 3 and 12 months (12/sex/control and 6 sex/test group) and at
6 months (24/sex/control and 12/sex/test group). Organ weights of
brain, heart, liver, spleen, kidney and testes were recorded and a
wide variety of tissues examined from all rats dying or killed at
interim periods, and from all rats from the 50 and 100 ppm groups.
Blood determinations were made, at each kill period, for haemoglobin,
PCV, RBC, WBC and WBC differential counts; prothrombin and
kaolin-cephalin-coagulation times; BUN; total protein, potassium
sodium and chloride; SGPT, SGOT and alkaline phosphatase. Also serum
proteins were fractionated electrophoretically and estimates made of
albumin and alpha, - ß, and gamma-globin concentrations.
Fenbutatin oxide rendered the diet unpalatable to both sexes causing,
in the early months of the study, significant reductions in food
consumption and body weight gains. Males were more affected than
females. For the remainder of the study all groups exhibited rates of
gain to the control except both sexes receiving 300 and 600 ppm, which
exhibited reduced body weight reduced body weights throughout the
study. BUN levels were increased at the higher levels for the first 6
months but remained within normal limits for the remainder of the
study. Serum alkaline phosphatase activity was increased in both sexes
at 300 and 600 ppm throughout the study, in the 100 ppm males at 6
months, and in the 100 ppm females at 3 months and 2 years. All other
haemotological and clinical chemistry values remained within normal
limits at all times. Survival and behaviour were not affected by
administration of the compound. No compound-related tissue lesions nor
any increase in tumor incidence were found. Organ weights revealed no
compound-related differences except kidney weight reduction in all
treated males and the 600 ppm females at 2 years. No specific
compound-related lesions were seen in the kidney of any treated group.
Absolute and relative testes weights, unaccompanied by hypertrophy,
were noted in the 300 and 600 ppm groups at 2 years. (Simpson et al.,
1973b).
COMMENTS
Fenbutatin oxide is moderately toxic by the oral route producing
severe gastrointestinal irritation. Acute oral administration to
rabbits reduced spermatogenesis and resulted in multi-nucleated
spermatid production. It is excreted rapidly, mostly unchanged in the
faeces, indicating that it is poorly absorbed. In the rat it is
metabolized to ß, ß-dimethylphenethylstannonio acid (SD 33608) and 1,
1, 3, 3-tetrakis-(ß, ß-dimethylphenethyl)-1,3-dihydrox3rdistannoxane.
The latter metabolite and fenbutatin oxide failed to produce oedema of
the central nervous system of the rat at 100 and 1000 mg/kg
respectively. Fenbutatin oxide produced no genotoxic effects in
several in vitro and in vivo systems. Adequate short- and
long-term studies are available in rats and dogs as well as teratology
study study in rabbits, a multi-generation reproduction study in rats,
and a carcinogenicity study in mice. No-effect levels were
demonstrated in rats and dogs and an acceptable daily intake for
humans was established on the basis of these studies. The no-effect
level was established as 15 mg/kg because of emesis and diarrhoea in
dog, and because of the known toxic effects of organic compounds on
the brain a safety factor of 100 was used.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in diet equivalent to 2.5 mg/kg body weight.
Dog: 15 mg/kg body weight.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.03 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenbutatin oxide is used as a specific mitioide against a wide range
of phytophagous mites. It is recommended especially for the control of
the mobile stages on a wide variety of crops, including pome and stone
fruits, citrus fruits, grapes, berry fruits, vegetables and
ornamentals. Recommendations for use on fruit crops and the
recommended pre-harvest intervals are given in Table 2.
The compound is in general used as a foliar spray in a concentration
range of 0.02 -0.05% a.i., corresponding to approx. 0.4 -1.5 kg
a.i./ha. Most applications are required while the fruits are
developing; the number of applications varies with the crop and
regional conditions.
The pre-harvest intervals are seldom less than 7 days, except on some
glasshouse-grown fruits or vegetables; in most instances the
pre-harvest intervals recommended are at least 14 days.
TABLE 2. Use pattern and recommended pre-harvest intervals of fenbutatin oxide
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Apples, Panonychus ulmi 0.0125-0.05% depending Australia 2
Tetranychus urticae on pest and volume of Belgium 28
Tetranychus mc. danieli spray liquid used. Denmark 28
Paratetranychus spp. For concentrated sprays France 7
Bryobia rabrioculus maximum 1.5 kg a. i./ha. Fed. Rep. of
Germany 14
Eotetranychus carpini Not more than 3 applications the
borealis whilst trees are bearing fruit. Netherlands 42
Italy 30
Aculus schlechtendali Mexico 14
Portugal 21
S. Africa 14
3 days
on fruit
for
canning
Spain 21
Switzerland 21
USA 14
Pears Panonychus ulmi As for apples As for apples
Tetranychus mc. danieli
Tetranychus urticae
Epitremeris pyri
Citrus Panonychus citri 0.015-0.05 % for Italy 60
Eotetranychus banksi concentrated sprays a Mexico 14
Eotetranychus yumensis maximum of 2 kg/ha is S. Africa 7
Tetranychus urticae recommended. Not more Spain 21
Brevipalpus californicus than 4 treatments in USA 7
Phyllocoptruta oleivora one year.
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Cherries Tetranychus urticae 0.02-0.05%; maximum two Italy 30
applications whilst trees Switzerland 21
are bearing fruits. the
Concentrated sprays max. Netherlands 42
1.5 kg a.i./ha.
Peaches Tetranychus urticae 0.02-0.05% maximum two Australia 2
Panonychus ulmi treatments whilst trees Denmark 28
are bearing fruit. France 7
Concentrated sprays up to Italy 30
1 kg a.i./ha.
S. Africa 21
3 days
on fruit
for
canning.
Spain 21
Switzerland 21
Plums Tetranychus urticae 0.02-0.05% Italy 30
Panonychus ulmi Concentrated sprays up Switzerland 21
to 1 kg a.i./ha. the
Netherlands 42
Grapes Panonychus ulmi 0.02-0.05% France 7
Concentrated sprays maximum Fed. Rep. of
rate up to 1 kg a.i./ha. Germany 28
Italy 45
Spain 21
Switzerland 21
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Strawberries Tetranychus
urticae 0.025-0.05% the
Netherlands only before
blossoming
and/or after
harvest.
Bell-peppers Tetranychus 0.02-0.04% the 3
urticae Netherlands (glasshouse)
Cucumbers Tetranychus 0.02-0.05% Italy 30
urticae the
Netherlands 3::
Tomatoes Tetranychue 0.02-0.5% Italy 30
urtical the
Netherlands 3
(glasshouse)
:: includes gherkins glasshouse and outdoors; cucumbers and melons glasshouse only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained on residues from supervised trials
carried out in various countries on a variety of fruits and fruits of
vegetables as follows.
Citrus fruits: grapefruit, lemons, oranges
Pome fruits: apples, pears
Stone fruits: cherries, peaches, plums
Berry fruits: grapes, strawberries
Fruits of vegetables: bell-peppers, cucumbers, gherkins,
melons, tomatoes
The data obtained are summarized in Tables 3-11 (Shell International,
1971-76). In most of the trials fenbutatin oxide was applied as a 50%
wettable powdery but in a few instances a suspension concentrate
containing 500 g a.i./l was used (referred to as SC in Tables). The
application rates in most of the trials are in conformity with good
agricultural practice. In, some instances higher dosages were also
applied; these excessive rates are indicated in the Tables.
It has been demonstrated, using radio-labelled fenbutatin oxide, that
the residues do not to any great extent penetrate through the akin of
treated fruit (see also section "Fate of residues"). The residues
found when analysing the whole fruit were mainly on or in the skin.
This is also illustrated in samples from some field experiments which
were examined. before and after removal of the peel.
Pome fruits
Apples
The data in Table 3 were mainly obtained from samples of crops treated
according to typical use conditions. The results show considerable
variation according to local conditions.
In most instances residues in whole fruit were below 3 mg/kg
fanbutatin oxide from up to 3 treatments made at recommended rates, up
to 0.05% or 1.5 kg a.i./ha, where pre-harvest intervals were 14 days.
In a few instances however residues at harvest exceeded this figure,
but were essentially below 5 mg/kg.
Some samples were anlaysed after removal of the peel, a common
practice in processing such as preparation for canning or drying.
Residues in the pulp were usually below the limits of determination.
Pears
The data in Table 4 show that residues arising from recommended
applications (up to three treatments at up to 0.05 or 1.5 kg a.i./ha)
in whole fruit harvested 2 weeks after the last application were less
than 4 mg/kg, with the exception of one trial in the USA. The analysis
of samples of peeled pears showed that residues of fenbutatin oxide
seldom exceeded 10% of the levels in the whole fruit.
Citrus fruits
Residue data were obtained on grapefruit, lemons and oranges from the
USA, Brazil, Spain and Italy (see Table 5). Most of the results show
residues below 4 mg/kg; only in a few cases have higher residues been
found, in general not exceeding 5 mg/kg.
Removal of the peel and analysis of the pulp and/or juice has shown
that the residues were mainly on the skin. In pulp and juice residues
were below the limit of determination, 0.02-0.05 mg/kg. Only one
sample of juice analysed contained a higher residue (0.08 ME/1). The
residue in the pulp did not generally exceed 30% of the level in the
whole fruit.
Stone fruits
Cherries
Data obtained on cherries (see Table 6) indicate that following
recommended treatments (up to 0.05% a.i.; minimum pre-harvest interval
7 days) residues were not above 4 mg/kg in whole fruit without stones,
except in one trial in USA where a residue of 4.2 mg/kg was recorded 4
weeks after treatment.
Peaches
Residues of fenbutatin oxide an peaches treated according to the
recommendations (up to 0.05% a.i.) with a minimum pre-harvest interval
of 14-21 days were in most cases below 5 mg/kg, but sometimes higher.
It is unlikely that after applying the miticide according to good
agricultural practice the residue level at harvest would exceed 7
mg/kg. Residues in the fruit after peeling were considerably lower,
and did not exceed 0.2 mg/kg. Details are given in Table 7.
Plums
The limited data available (Table 8) indicate that residues at harvest
are unlikely to exceed 3 mg/kg in the whole fruit without stone after
recommended treatments, i.e. up to 0.05% a.i. and a minimum
pre-harvest interval of 7 days.
Grapes
Residues of fenbutatin oxide (Table 9) following treatment of vines
according to the recommendation (up to 0.5% or 1 kg. a.i./ha), were
about 4 mg/kg after 1 week and 0.6-3.2 mg/kg after 3 weeks (excepting
one erratic figure of 6 mg/kg from a trial carried out in the Federal
Republic of Germany).
TABLE 3. Residues of fenbutatin oxide in apples resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Apples
Granny Smith Australia 1973 1 19 WP 50% 1.6 1.3 0.45 0.95
" " 1973 1 25 " " 2.5 2.5
Josephine 1974 1 19 " " 0.94 0.79
Granny Smith 1975 2 20 " " 1.3 1.5 2.2 1.6
" " 1975 2 20 sc 500g/l 1.4 1.4 1.1 0.9
" " 1975 2 30 " " 1.8 2.0 2.1 2.0
Mcintosh Canada 1976 1 25 WP 50% 0.97 0.90 0.67 0.45
" 1976 1 19 " " 0.55
Cortland 1976 1 19 " " 0.15
Cardinal France 1974 1 30 0.3 " " 0.26 0.04
" 1974 1 50 0.5 " " 0.43 0.09
Melrose 1975 1 75 0.6 " " 0.07
Cox's O.P. Fed. Rep. of
Germany 1974 3 25 " " 1.1 0.82 0.66 0.64 0.21
James Grieves 1974 3 25 " " 1.5 0.83 0.82 0.60 0.53
Golden Delicious 1974 3 25 " " 1.4 0.78 0.82 0.88 0.53
" " 1974 3 25 " " 1.4 0.87 0.61 0.53 0.24
Golden Parmane 1974 4 1.0 " " 1.9 1.1 1.1 0.74 1.1
" " 1974 3 1.0 " " 0.82 0.82 0.35 0.27 0.32
Goudreinet Netherlands 1974 2 25 " " 0.30 0.26
" 1974 3 25 " " 0.22 0.28
" 1975 2 30 " " 0.23 0.35
" 1975 2 30 " " 0.28 0.37
Golden Delicious Italy 1975 1 30 " " 1.2 0.98 0.
Stark Delicious 1975 1 30 " " 0.93 0.65 0.60
Granny Smith New Zealand 1975 5 19 " " 0.75 0.60 0.55 0.40
Starking Portugal 1974 2 0.6 " " 0.30 0.68
" 1974 2 1.0 " " 0.75 1.40
" 1974 2 0.6 " " 0.29 0.80
" 1974 2 1.0 " " 0.63 0.80
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Granny Smith South Africa 1976 1 30 WP 50% 1.8 1.3 1.0 0.95
" " 1976 1 50 " " 2.5 2.4 1.5 1.2
" " 1974 1 100 " " 2.4 2.9
(4
days)
Jonathan Switzerland 1974 3 0.5 0.55 0.43 0.35
(10
days)
Worcester U.K. 1974 2 25 " " 0.3
Worcester U.K. 1974 2 42+25 wp+sc 0.3
York Imperial U.S.A. 1974 5 25 1.25 wp 50% 0.93
1974 5 25 1.25 " " 1.3
1974 5 50 2.5 " " 2.6
1974 5 50 2.5 " " 2.2
Newton U.S.A. 1973 1 38 " " 1.6
(Oregon) (1.5-1.7)
Red delicious U.S.A. 1973 4 19 1.5 0.6-0.94
(Oregon)
1973 4 38 3 1.6-1.5
1973 4 30 1.5 0.25-0.84
Red delicious " 1973 4 3.0 " " 2.1-2.0
Newton Pippin (California) 1972 1 25 " " 0.26
" " 1972 1 50 " " 0.60
" " 1972 1 100 " " 1.5
Golden delicious (Washington) 1971 4 12.5 0.75 " " 0.9 0.8 0.8 0.7
" " 1971 4 25 1.5 " " 1.7 1.4 1.0 1.5
" " 1971 4 50 3.0 3.7 3.5 3.3 2.6
" " 1972 4 12.5 0.75 " " 1.6 1.5 1.5
" " 1972 4 25 1.5 " " 3.5 2.5 3.1
" " 1972 4 50 3.0 " " 7.9 4.7 5.0 3.3
" " Michigan 1971 3 12.5 0.25 " " 1.2 1.0 0.85 0.95 0.90
" " 1971 3 25 0.5 " " 3.5 2.5 3.3 3.7 2.4
" " 1971 3 50 1.0 " " 6.1 6.0 4.0 3.7 3.7
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
" " 1972 4 12.5 0.5 " " 1.8 1.4-1.1 1.1-1.6 0.64-0.68
" " 1972 4 25 1.5 " " 2.9-2.2 1.3-1.8 2.2-1.8 1.1 -0.9
1972 4 50 2.0 " " 3.4-6.0 5.4-6.6 5.3-7.0 2.8-4.1
Ben Davis New York 1972 4 12.5 0.75 wp 50% 2.3-2.6 2.2-2.2 2.1-1.5 1.3-1.6
" " 1972 4 25 1.5 " " 5.6-5.4 3.5-4.2 2.8-3.6 3.0-2.5
" " 1972 4 50 3.0 " " 8.5-8.0 5.6-6.0 5.6-6.2 5.7-3.8
Rome 1973 4 12.5 " " 1.8 1.0 1.1 1.4
1973 4 19 " " 2.2 1.4 1.6 1.2
1973 4 38 " " 2.3 2.8 3.2 2.7
wp = wettable powder
sc = suspension concentrate
TABLE 4. Residues or fenbutatin oxide in pears resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No rate formulation 0-2 3 7-9 14 19-21 28-31 32-35
g/1001 kg/ha
Pears
Josephine Australia 1974 1 19 WP 50% 0.5 0.4 0.3 0.5
William 1973 1 19 " " 0.8 1.1 0.65 0.73
" 1973 1 25 " " 1.6 1.2
Packam 1975 2 20 sc 500g/l 1.5 1.6 1.5 1.3
" 1975 2 30 " " 2.3 1.4 1.6 1.2
" 1975 2 20 WP 50% 1.4 1.2 1.3 1.2
William France 1976 1 60 0.3 " " 0.17 0.11 0.03
" 1976 1 100 0.5 1.07 0.16 0.10
Packhams Tr. S. Africa 1976 1 30 " " 1.7 1.6 1.4 1.9
" 1976 1 50 " " 3.6 3.1 2.7 1.6
Winter Melis S. Africa 1974 1 100 " " 3.8 2.5
(5
days)
Kieffer USA (Mich.) 1971 3 12.5 0.38 " " 3.0 1.7 1.6 1.0
" 1971 3 25 0.75 " " 6.0 5.0 1.9 1.1
" 1971 3 50 1.5 " " 4.7 4.0 3.0
Bartlett (Wash) 1971 2 12.5 0.25 " " 1.5 1.3 0.9
" 1971 2 25 0.5 " " 2.7 2.3 1.8
" 1971 2 50 1.0 " " 5.3 4.7 4.3
" 1973 4 12.5 0.75 " " 0.17 0.18
" 1973 4 25 1.5 " " 0.25 0.54
" 1973 4 503 3.0e " " 1.4 1.9
" (Calif) 1973 1 25 1.0 " " 1.8 1.1 1.1 1.0
" 1973 1 50 2.0 " " 2.4 1.4 2.1 1.8
" (New York) 1971 3 12.5 0.75 " " 1.2
" 1971 3 25 1.5 " " 2.2
" 1971 3 50 3.0 " " 3.5
Bosc 1972 3 12.5 0.38 " " 4.2 2.3 1.2 0.9
" 3 25 0.75 " " 6.0 4.6 2.5 2.1
" 3 50 1.5 " " 8.0 6.3 4.6 3.8
e= excessive application
Strawberries
The limited data available indicate that residues following
recommended application 0.05% a.i, minimum pre-harvest interval 7
days), would be unlikely to exceed 4 mg/kg (Table 10):
Fruit of vegetables
The very limited data available (Table 11) suggest that residues
arising from recommended treatments (up to 0.05% a.i., minimum
pre-harvest interval 3 days) are unlikely to exceed 1 mg/kg.
FATE OF RESIDUES
In animals
Provided fenbutatin oxide is used according to good agricultural
practice no detectable residues will occur in products of animal
origin, since crops which are generally used for animal feed are
rarely treated with this acaricide. In some countries, particularly in
the USA, cattle are occasionally given processed fruit pulp as a part
of their diet. Data are available showing that cattle eating feed
containing residues of fenbutatin oxide will not produce milk or meat
containing appreciable residues. See also the section
"Biochemical aspects".
Cattle feeding studies
An experiment, was carried out in which three lactating Guernsey cows
were fed on a diet containing 119Sn-labelled fenbutatin oxide
(Shell, 1976). The quantity given was equivalent to 34 ppm fenbutatin
oxide in the whole ration, which consisted of 5 kg compounded feed and
10 kg alfalfa cubes per animal per day, and was given twice daily
during a 21 day period. Milk samples were taken from each animal at
each milking, and the total radio-activity determined in each sample.
No radio-activity above background level was found, indicating that
total residues in whole milk were equivalent to less than 0.01 mg/kg
fenbutatin oxide. The animals were slaughtered 12 hours after the
final feeding and the radio-activity was determined in samples of fat,
muscle, brain, kidney and liver. No residues were detected in brain,
muscle, fat or liver at a limit of determination equivalent to 0.02
mg/kg fenbutatin oxide. Similar results were obtained with the kidney
samples from 2 of the animals, but the third was found to contain
radio-activity equivalent to 0.03 mg/kg fenbutatin oxide, i.e. just
above the limit of determination in these tissues.
TABLE 5. Residues of fenbutatin oxide in citrus fruits resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Oranges
Pera Natal Brazil 1975 1 30 WP 50% 0.13 0.05
1975 2 30 " " 0.25
1975 1 30 " " 0.27 0.08
1975 2 30 " " 0.42
1975 1 30 " " 0.50 0.30 0.17
1975 2 30 " " 0.75 0.38
1975 1 30 " " 0.38 0.22 0.08
1975 2 30 " " 0.62 0.35
Oranges S. Africa 1975 1 30 sc500g/l
1.0 1.1 0.65 0.65
1975 1 30 " " 0.8 0.60 0.45 0.40
Oranges Calif. 1972 2 25 1.2 wp 50% 3.0 3.3 1.9 2.1 2.2 1.8 2.5 2.4 1.6 1.7
Valencia 1972 2 50 2.4 " " 5.6 6.0 5.0 4.4 4.2 5.0 4.4 3.8 4.0 3.8
1972 2 100 4.8 " " 10.5 11.0 9.5 9.5 7.5 7.5 6.2 5.5
1971 1 25 0.44 " " 1.1 1.3 0.65 0.82 0.8 1.0 0.88 0.96 0.52 0.74
1971 1 50 1. " " 1.8 3.0 1.5 2.0 1.5 1.7 1.4 1.8 1.5 1.8
(Florida) 1972 4 12.5 0.9 " " 0.56 0.62 0.50 0.75 0.74 0.75 0.64 0.75
1972 4 25 1.9 " " 1.5 1.6 1.5 1.5 1.5 2.0 1.5 1.7
1972 4 50 3.6 " " 3.8 4.0 3.2 4.1 3.1 3.2 3.6 3.1
Lemon
Nostrana Italy 1975 1 30 " " 0.9 0.5
Lisbon USA
(Ariz.) 1974 2 19 1.4 " " 0.81 0.23 0.22 0.34
1974 2 19 1.4 " " 2.8 2.0 1.2 1.6
1974 2 32e 3.5 " " 1.6 0.32 0.30 1.2
1974 2 32e 3.5 " " 5.6 5.8 2.9 2.0
(Calif) 1973 3 12.5 0.37 " " 0.40 0.25 0.20 0.30 0.15 0.30 <0.05 <0.05 (0.05)
1973 3 25 0.75 " " 0.95 0.70 0.25 0.40 0.30 0.30 <0.05 <0.05 (0.05)
1973 3 50 1.5 " " 1.0 0.90 0.75 0.70 0.35 0.05 0.05 <0.05 0.05 0.20
TABLE 5. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Grapefruit
Marsh USA
(Calif) 1971 1 25 1.8 " " 0.9 1.3 0.6 0.9 0.8 0.9 0.6 1.0 0.5 0.6
1971 1 50e 3.6 " " 1.2 2.0 0.9 1.4 1.4 2.0 1.4 1.6 1.3 1.6
Red Blush 1972 2 12.5 0.2 " " 0.8 0.7 0.45 0.45 0.86 0.74 0.36 0.44 (0.42 0.36)
1972 2 25 0.4 " " 1.6 1.7 1.3 1.5 0.9 0.7 (0.90 0.68)
1972 2 50 0.8 " " 3.2 2.9 2.5 2.6 1.9 1.5 (1.7 2.5)
Marsh (Florida) 1972 3 12.5 1.0 " " 0.3 0.4 (0.4 0.5)
1972 4 12.5 1.0 " " 0.7 0.7 0.7 0.7 (0.7 0.7)
1972 3 25 1.9 " " 1.1 1.2 (0.4 0.4)
1972 4 25 1.9 " " 1.5 2.8 1.7 1.8 (1.4 1.1)
1972 3 50e 3.8e " " 3.2 3.4 (1.3 1.7)
1972 4 50e 3.8e " " 3.4 4.8 2.8 4.0 (1.8 3.0)
1971 3 12.5 " " 0.71 0.65 0.72 0.65 0.52 0.44
1971 3 25 " " 1.3 1.3 1.8 1.6 1.0 0.75
1971 3 50 " " 3.3 3.3 3.8 3.1 1.8 1.5
e= excessive application
TABLE 6. Residues of fenbutatin oxide in stone fruits resulting from supervised trials
Crop Country Year No g/100 1kg/ha formulation 0.2 7 14 21 28-31
Cherries
(sweet and sour)
Biggerau Fed. Rep.
Germany 1977 3 25 WP 50% 0.70 0.21 0.09
Rote Leber 1977 3 25 " " 1.5 0.17 0.04 0.02
Schattenmorelle 1977 3 25 " " 1.1 0.41 0.22 0.09
Morella 1977 1 25 0.3 " " 0.90 0.50
1977 1 50 0.6 " " 1.10 1.15
Bing USA
(Calif) 1976 1 25 1.1 " " 1.7
1976 1 50 2.2 " " 3.2
Sour USA
(Mich) 1974 2 12.5 0.96
1974 2 25 2.3 1.5 2.2 3.0
1974 2 50 4.1 3.6 3.9 4.2
USA
(Wash) 1974 2 25 1.2 1.4 2.0
1974 2 50 2.4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
Cherries
Bing USA
(Wash) 1974 2 25 1-2 1.4 2.0
1974 2 50 2-4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
TABLE 7. Residues of fenbutatin oxide in peaches resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Peach
Tatura
Aurora
Australia 1975 2 20 sc500/gl 2.2
1975 2 30 " " 3.6
1975 2 20 wp 50% 2.7
Cornish 1973 1 19 " " 1.3 1.9 2.5 0.71
1973 1 25 1.8 1.4
Golden Queen 1974 1 19 1.4 <0.1 1.0
1974 1 19 2.2
1974 2 19 3.5
Alberta Canada 1976 2 2.6 0.63
Royal Gold France 1977 1 50 0.75 0.87
Audenot 1973 1 0.5 3.7 2.6 1.9
Alberta 1973 1 0.5 1.6 1.6 1.5 1.3 0.65
Red. Aven
P.G. 1974 1 0.5 2.0 1.7 0.41
Vellutata Italy 1974 1 30 0.4 0.3 0.5
Netturina N7 1974 1 30 0.5 0.3 0.5
Golden Queen New
Zealand 1975 1 19 0.8 0.65 0.45
Kakamus S. Africa 1976 1 30 7 5.9 4.0
" 1976 1 50 12 7.0 6.0
Sunblest USA
(Calif) 1973 3 50 1.1 4.3 3.0
1973 3 20 4.3 1.0
1973 3 50 7.3 5.7 8.0 2.6
Red Haven (Oregon) 1973 3 19 0.3 3.9 2.8 1.9 1.5
(S.
Carolina) 1973 3 38 0.6 7.7 8.2 4.6 5.8
TABLE 7. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Roter
Ingelheimer Fed. Rep.
of
Germany 1977 3 25 0.25 9.0 3.4 3.4 2.0 1.8 1.3
Rekord
a. Alfter 1977 3 25 0.25 8.1 4.7 2.5 2.2 1.5
Madame
Roenait 1977 3 25 0.25 8.1 3.1 2.5 2.3 3.3
TABLE 8. Residues of fenbutatin oxide in plums resulting from supervised trials
Application Residues in mg/kg at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 3-4 7 14 21 25-28 32-35
Plums
Auerbacher Fed.
Rep.
Germany 1977 3 25 0.25 WP 50% 0.15 0.15 0.15 0.10
Bühler 1977 3 25 0.25 0.25 0.10 0.15 0.10
Czar Netherlands 1977 1 25 1.75 sc500/gl 0.3 0.2 0.1 <0.1 <0.1
1977 1 50 3.5 " " 0.5 0.4 0.2 0.1 0.1
Relsey S. Africa 1976 1 30 wp 50% 1.2 1.3 0.8 0.9
" 1976 1 50 " " 2.2 2.2 1.8 1.0
Stanley USA
(New York) 1974 2 12.5 0.31
1974 2 25 " " 0.50
1974 2 50 " " 0.49
French
Prunes (Calif) 1974 2 12.5 " " 0.27 0.35
1974 2 25 " " 0.92 0.96
1974 2 50 " " 2.8 2.3 1.8
Early
Halian (Wash) 1974 2 25 " " 0.19 0.02 0.11 0.6
50 " " 0.29 0.73 0.47 0.58
100 " " 1.03 1.05 0.67 0.69
TABLE 9. Residues of fenbutatin oxide in grapes resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
rate
Crop Country Year No g/100 1kg/ha formulation 0-3 7 13-14 21 28-31 42-45 56-
Grapes
Semillion France 1973 1 30 wp 50 0.7
1 50 " " 0.9
Grenache 1974 1 0.5 " "
Merlot Rouge 1974 1 0.5 " "
Lavallet 1975 1 50 " " 0.27
Grenache 1975 1 50 0.50
Siebel 1975 1 125e " " 1.1
Müller Fed. Rep.
of Germany 1975 3 50 " " 4.7 3.9 2.5 2.6 1.2
Kerner 1975 3 50 " " 4.8 2.1 2.7 3.0 1.1
Müller 1975 3 50 " " 4.9 3.3 3.4 2.3 2.9
Sylvaner 1975 3 50 " " 2.7 1.9 1.6 3.2 1.4
Riesling 1975 3 50 " " 4.5 1.8 1.6 0.6
Trollinger 1975 3 50 " " 1.3 1.2 1.1 3.2 2.3
Sprühsurgunder 1975 3 50 " " 7.1 6.3 6.2 6.3
Barbera Italy 1975 1 30 " " 7.1 1.3 1.1 0.45
Nebbiolo 1975 1 30 " " 1.8 1.1 0.68
Chasselas Switzerland 1975 1 100e " " 2.1
Rabier USA
(Calif) 1973 3 25 0.5 " " 0.90 0.68 0.16 0.17
1973 3 50 1.0 " " 2.0 0.62 3.0 2.3
Mission 1973 3 0.5 " " 1.0 0.45 0.97 0.65
1973 3 50 1.0 2.2 0.47 0.67 1.2
Thompson 1973 3 25 0.5 1.5 1.5 1.8 1.0
1973 3 50 1.0 3.9 1.8 1.4 0.66
Concord (Michigan) 1974 3 50 1.1 3.1 2.0 2.2 1.8
1974 3 75 1.7 5.6 2.8 3.4 2.7
50 1.1 2.8
100 2.2 2.9
e= excessive application
TABLE 10. Residues of fenbutatin oxide in strawberries resulting from supervised trials
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Strawberries
Gorella (g) Belgium 1976 1 25 0.9 WP 50% 0.2 0.1 <0.1 <0.1
Volla (g) 1976 1 25 0.9 " " 0.40 0.15 0.15 <0.1 <0.1 <0.1
France 1977 1 30 " " 0.35 0.20
Mexico 1977 1 0.55 sc 500g/l 2.3 2.0 0.9
1.1 " " 7.0 4.0 5.9
Netherlands 1977 1 25 wp 50% 0.50
1977 2 25 " " 0.65
1977 1 25 " " 0.15
1977 2 25 " " 0.30
1977 1 25 sc 500g/l 0.65
1977 2 25 " " 1.1
1977 1 25 " " 0.25
1977 2 25 " " 0.50
Parfaite S. Africa 1977 1 25 0.13 " " 0.5 0.2 0.2
1 38 0.19 " " 0.8 0.5 0.5
- (g) UK(Sussex) 1975 1 25 wp 50% 0.7 0.7 0.5 <0.1
- (Devon) 1975 1 25 " " 0.4 0.2 0.5 0.2
Tioga USA(Calif) 1974 1 50 1.1 " " 1.4*
1974 1 100 2.2 " " 2.4*
Heidi 1973 2 80 2.2 " " 4.9* 4.6 3.4
1973 2 160e 4.4e " " 4.8* 5.9 2.9
G - 4 1973 4 160e 4.4e " " 4.0* 3.3 3.0
Tioga (Florida) 1974 6 40 0.55 " " 1.2* 1.4 0.3
Catskil USA
(Wisc) 1975 2 100 1.1 " " 1.6* 0.49
2 200 2.2 " " 2.2* 1.5
Northwest (Wash) 1975 1 50 0.55 " " 0.70 1.1 0.98
1 100 1.1 " " 1.1 1.2 1.83
1 200 2.2 " " 2.4 4.3 3.1
TABLE 10. (Continued)
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Raritan (New Jersey) 2 50 0.55 " " 0.86 0.2 0.60
2 100 1.1 " " 1.4 1.3 0.60
2 200e 2.2e " " 5.0 1.9
(g) = glasshouse or plastic cover
e = excessive application
* = residue after 1 day
TABLE 11. Residues of fenbutatin oxide in fruits of vegetables resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 2-3 4-5 7 10 14 30
cucumbers(g) Netherlands 1974 1 25 wp 50% 0.2 0.1 0.1 <0.1
1974 1 25 " " 0.2 0.2 0.2 <0.1
gherkins(g) 1974 1 0.75 1.8 0.8 0.7 0.9
melons France 1977 1 30 < 0.01 <0.01
sweet
peppers(g) Belgium 1976 1 1.2 1.0 0.6
sweet
peppers(g) Netherlands 1976 1 0.5 0.4 0.6 0.4 0.1
tomatoes(g) Netherlands 1976 1 0.5 0.2 0.4 0.4 0.4
tomatoes Italy 1974 2 30 0.5 0.3
1974 2 30 0.2
tomatoes(g) UK 1975 1 0.2 0.3 0.4
(g) = glasshouse of plastic cover
In a second experiment (Shell, 1973) 3 Guernsey cows were similarly
given a daily ration containing 34 mg/kg 119Sn-labelled fenbutatin
oxide for 21 days. Excretion of the ingested fenbutatin oxide was
mainly via the faeces but small amounts, up to 3% of the daily intake,
were excreted in the urine. As in the previous experiments no residues
were found in the milk (limit of determination 0.02 mg/kg) and none in
the brain, honey fat or most of the muscle samples taken. However
radio-activity averaging 0.27 mg/kg calculated as fenbutatinoxide was
found in the kidney and 0.34 mg/kg in the liver of all three animals.
Radio activity equivalent to 0.08 mg/kg fenbutatin oxide was found in
lone lung sample and a just measurable residue equivalent to 0.04
mg/kg in one muscle sample (gastrocnemius). The limit of determination
was equivalent to 0.04 mg/kg fenbutatin oxide in all the tissues
(Shell, 1973). Residue data from field trials indicate that residues
of fenbutatin oxide in apple pomace from apples containing a residue
of 4 mg/kg may approach 25 mg/kg (Shell, 1972-74).
The data from the cattle-feeding studies indicate that cattle fed
normal amounts (up to 25% of the total diet) of apple pomace made from
apples treated with fenbutatin oxide according to good agricultural
practice are unlikely to yield milk containing detectable amounts of
the acaricide. No measurable residues are likely in muscle tissues,
but they might occasionally occur at low levels (<0.2 mg/kg) in
liver and kidney.
In plants
Fenbutatin oxide is slowly lost after application to crops. The
degradation to in-organic tin occurs through successive loss of the
phenyldimethylethyl groups. The principal organotin degradation
product found on crops is 1,1,3,3,tetrakis(ß, ß-dimethylphenethyl)-
1,3-dihydroxydistannoxane, referred to subsequently as SD 31723(Shell,
1972).
In studies of the fate of fenbutatin oxide on leaves and fruit of
apples and citrus (Shell, 1972, 1974a), small apple trees grown in a
lath-house were treated one to three times with 119Sn-labelled
fenbutatin oxide with approximately 40µg at each application.
Recoveries of 119Sn 2 to 33 days after the last application were
84-96% of the amount applied. Virtually all the radio-activity in the
fruit was found to be on the outer surface of the skin. Substantial
amounts of the radio-activity were removed by rinsing with organic
solvents, or simply by siping the fruit-skin with paper tissues. It
was shown that less than 5% of the total radio-activity present in the
fruit originated from the principal breakdown product SD 31723 and
less than 0.7% from other organotin metabolites including (ß, ß-
dimethylphenethystannoic acid polymer (SD 33608). The structural
formula of the metabolites SD 31723 and SD 33608 are shown on the
following page. Inorganic tin accounted for nearly 10% of the total
119Sn on the apples (Shell, 1972).
Other information on identity and properties
(a) Composition of the technical Product:
The technical material contains 97% w/w minimum of fenbutation oxide;
the main impurities are related organo-tin compounds.
(b) Physical and chemical properties
Physical state: crystaline solid
Molecular weight: 1053
Melting point: 138-139°C
Volatility: not volatile
Solubility: insoluble in water, (approx. 5x10-6 g/1 at 23°C)
slightly soluble in most organic solvents;
solubility at 23°C in acetone approx 6 g/l in
dichlororomethane 370 g/l in benzene 140 g/l
Stability: stable to sunlight. Water hydrolyses fenbutatin
oxide to tris-(2-methyl-2-phmylpropyl)tin
hydroxide; this in reconverted to the parent
compound slowly at room temperature and rapidly at
98°C.
Formulations:
Wettable powder 500 g a.i./kg and suspension concentrate 500 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Groups of 2 male and 2 female rats were fed an average of 19.5 ppm
119Sn-labelled fenbutatin oxide (with or without a 3 day withdrawal
period before killing following 3 or 6 day exposure) after 1, 3 or 6
days to study the absorption, distribution and excretion of
fenbutatin. Most of the administered 119Sn was rapidly excreted in the
faeces following multiple dosing (80-90% of the administered 119Sn).
Essentially no 119Sn was excreted via the urine. The GI tract
contained approximately 30, 15 and 5% of the administered 119Sn for
animals sacrificed after 1, 3 or 6 days of dosing. The highest tissue
concentrations of 119Sn were found in liver (0.015-0.09 mg/kg),
kidneys, (0.015 0.055 mg/kg) and fat (< 0.01-0.065 mg/kg). No 119Sn
was detected in brain or bone tissue (Loeffler, 1972). See also the
section "Fate of residues", "In animals".
Biotransformation
Analysis of the faeces from rats dosed with 119Sn-labelled fenbutatin
oxide indicated that ß, ß-dimethylphenethylotamoic acid and 1, 1, 3,
3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxdistannoxane are the
major metabolites (Loeffler, 1972).
Effects on enzymes and other biochemical parameters
In starch gel electrophoretic studies on sera from rate fed either 0
or 600 ppm in the diet for 6 months (2 buffer systems) control and
test animal sera were identical. The effect of 1-phenylanatine or heat
was similar on both sera. Comparisons with intestinal, bone, liver and
kidney alkaline phosphatase indicated the serum alkaline phosphatase
was similar to intestinal alkaline phosphatase. Further studies on rat
sera derived from animals fed 0 or 600 ppm in the diet for one year,
utilizing heat inactivation, selective substrates and inhibitors,
confirmed the absence of any difference between control and test
animal sera, as well an confirming the similarity of the serum
alkaline phosphatase to intestinal alkaline phosphatase (Pickering,
1973a). In a further series of studies by the elevation of serum
alkaline phosphatase observed in rate after administration of 300 ppm
fenbutatin oxide in the diet for 90 to 120 days may shown to be
completely reversible following one months, withdrawal from exposure.
Limited data indicate that the reversal of elevated serum alkaline
phosphataze in the test rate may be completed within 7 days of
withdrawal of the fenbutatin oxide (Pickering, 1973b).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Following preliminary studies to determine suitable dose levels and to
confirm the induction of brain oedema by triethyltin bromide, a
comparative oedma assay of five organo-tin compounds (including
fenbutatin oxide, and its metabolite 1, 1, 3,
3-tetrakis-(ß, ß-dimethylphenethyl)-1, 3-dihydroxydistannoxane) for
oedma formation in the central nervous system of rats, was undertaken.
Groups of 15 male rats were intubated with corn oil (10 mg/kg, 40
mg/kg triethyltin bromide, 1000 mg/kg, fenbutatin oxide, 100 mg/kg 1,
1, 3, 3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxydistannoxane
and sizable doses of two other tin compounds. The triothyltin bromide
group showed increased brain water content after 48 hrs. Seven of ten
brains examined at 24 and 48 hrs showed spongeosis of white matter in
the cerebellum and sometimes of the corpus callosum and pons. No
histological changes were observed in 4 rats killed after 6 hrs.
Fenbutatin oxide (24 and 48 hr. kills), and the metabolite (6, 24 and
48 hr kills) did not show either increased water content or abnormal
brain histology, although rats on both compounds showed signs of
intoxication (Samuels and Dix, 1972),
Special studies on mutagenicity
Micro-organisms
In plate tests using Serratia marcesens, strains HY/alpha 13, a
histidine-requiring auxotroph, HY/alpha 21, a leucine-requiring
auxotroph, and CV/r c3/alpha 742, a thiamine-requiring spontaneous
auxotroph, 50 µl hexane solution (which resulted in a precipitate of
fenbutatin oxide on the agar plate) did not increase the reversion
rate in any strain, nor was growth inhibited, (Dean, et al., 1972a).
Neither conversion rate nor cell survival was affected in
Saccharomyces cerevisiae following exposure for 5 or 16 hrs in pH7
phosphate buffer containing 0.0025 or 0.005 µg fenbutatin oxide/ml
solution. In the same system, 20 µg/ml in suspension was ineffective
in altering conversion rate or cell survival (Dean et al., 1972a).
Mice
Groups of 8 male and 8 female mice were treated with 0.005 or 1000 mg
fenbutatin oxide/kg, in 1% carbomethyl cellulose. Four
animals/sex/group were killed 90 minutes after i.p. colcemid
administration and femurs were removed. One hundred bone marrow
cells/animal were examined. No significant differences in incidence of
chromosomal aberrations were observed (Dean et al., 1972b).
In three or four host-mediated assay experiments using Saccharomyces
cerevisiae and dose levels of 250 or 500 mg fenbutatin oxide/kg,
conversion rate was unaffected. In the fourth replicate, conversion
rate was marginally increased at 250 but not at 500 mg/kg (Dean et
al., 1972a)
In an 8 week dominant lethal study, 8 male mice/group were dosed once
with 250 or 500 mg fenbutatin oxide/kg, and were then mated with 3
females each week. The control group comprised 16 untreated males. No
significant differences in percentage pregnancies, implant incidence
or early resorption rate were noted between control and fenbutatin
oxide treated groups. A positive control group of 8 male mice (200 mg
Endoxan) showed reduced pregnancy rate and increased resorption and
decreased implant rates during the first 3 matings after, dosing.
(Dean and Doak, 1972c).
Special studies on teratology
Three groups of 15 pregnant rabbits were dosed by gelatin capsules on
days 6-18 inclusive of gestation (mating day = day 0), with 3 or 10 mg
fenbutatin oxide/kg or with 37.5 mg thalidomide/kg. Twenty-six rabbits
were used as capsule-only treated controls. Rabbits were killed on day
28 of pregnancy. A repeat experiment differed only in that 20 pregnant
females/dose level and 30 controls were used, autopsy being on day 29.
Foetuses were incubated for 24 hrs. following removal from the uterus,
after which approximately 1/3 were examined for visceral
abnormalities, and 2/3 for skeletal defects. Prior to clearing for
skeletal examination, viscera from these animals were also examined
for defects. Although some maternal deaths occurredy these were random
between groups. Mean live litter size, resorption rate, foetal loss,
survival of incubated foetuses, and incidence of anomalies were not
affected by fenbutatin oxide at either close level. Cromn-rump length
and foetal weight were comparable to controls except at 3 mg/kg in the
repeat experiment, where a significant increase was noted. The
positive control group behaved as expected (Dix and Wilson, 1973).
Special study on reproduction
In a standard 3 generation, 2 litter/generation reproduction study,
groups of 10 male and 20 female rats were fed O, 50, 100 or 300 ppm
fenbutatin oxide (985 purity). It should be noted that rate of the Fo
generation commenced on diet and were mated at 100 days of age, and
that litters were culled to 10 pups/litter on post-partum day 5.
Necropsy was performed on 10 male and 10 female F3b weanlings from
the 0 and 300 ppm, and on 5 weanlings/sex from the 50 and 100 ppm
groups. In the 300 PPM group, parents and pups were smaller, hyper
active and irritable; mean litter size was slightly reduced in the
F1b litters, and in the two F3 generations survival to weaning was
reduced at 300 ppm and testicular organ/body weight ratio was reduced
in the F3b weanlings. Necropsies of F2b adults did not show any
compound-related changes. No consistent effects were observed at 50 or
100 ppm with respect to fertility, gestation, viability or lactation
indices, litter size, litter weight, or necropsy of F3b weanlings
Mine et al., 1973).
Special studies on carcinogenicity
Groups of 48 male and 48 female Carworth Farm No. 1 strain mice were
fed 50, 100, 100 or 600 ppm fenbutatin oxide in the diet for 18
months. A control group comprised 96 males and 96 females. All animals
were examined grossly at death. Microscopic examination was undertaken
on all animals dying during the study and on all 600 ppm mice, on
10/mice/sex at 50, 100 and 300 PPM, and on 45 controls/sex at 18
months. Survival rate was comparable in all groups and exceeded 75%.
There was a high incidence of tumours in all groups. However there was
no indication of compound-or dose-related tumor induction (Granville
et al., 1973a).
Acute Toxicity
TABLE 1a. Acute toxicity of fenbutatin oxide
Species Route Sex LD50(mg/kg) Reference
Mouse Oral - 1450(1223-1659) Simpson 1972a
Rat " - 2631(1929-4659)
Dog Oral(capsule) - >1500
Rabbit Oral - 1500-3000
Rat Dermal - >1000
In mice and rats, death occurred within 10 days. Signs were dyspnoeal
stained coat and transient diarrhoea. In dogs, vomiting and diarrhoea
occurred. In rabbits convulsions occurred at the nighest dose, with
death within 24 hrs. Lower doses caused death up to 18 days after
dosing. Weight loss was still apparent 3 weeks after dosing, Autopsy
revealed damage to the gastric mucosa, massive fatty changes in liver,
reduced spermatogenesis, and multinucleated spermatid production
(Simpson, 1972a).
TABLE 1b. Acute toxicity of 50% wettable powder
Species Route Sex LD50 (mg/kg) Reference
Rat Oral - 1000 Simpson 1972a
Dermal - >1000
TABLE 1c. Acute toxicity of metabolite*
Species Route Sex LD50(mg/kg) Reference
Mouse Oral M 420 Natoff, 1973
F 622
Rat Oral M,F 242
Rabbit Oral M >675
F 300-450
Rat Oral - 132(102-176) Simpson & Dix,
Mouse Oral - 1051(824-1440) 1972c
* 1, 1, 3, 3-tetrakis(ß,ß -dimethylphenethyl)-1,3-dihydroxydistannoxane.
The signs of intoxication in all species were severe diarrhoea,
followed by loss of body weight which was recorded for rabbits, and
inanition and emaciation in all species. Deaths occurred up to 16 days
after dosing the mice, 14 days after dosing the rats and 26 days after
dosing the rabbits. The observation period for rabbits was curtailed
at 28 days.
Short term studies
Rat
Five groups of 5 male and 5 female rats were fed 0, 30, 100, 300 or
1000 ppm fenbutatin oxide in the diet for 5 weeks. Body weight was
significantly reduced, as was food intake at 300 and 1000 ppm.
Reductions in absolute brain, heart, liver, spleen and kidney weights
were noted in both sexes at 1000 ppm. Spleen weight was reduced in
males at 300 ppm and brain, liver, spleen and kidney weight was
reduced at 300 ppm in females. Serum alkaline phosphatase was
increased in both sexes and SGPT levels were elevated in females at
1000 ppm (Simpson, 1972a).
Three groups of five male rats were used in a pair feeding study, one
group being a normal control, the second being fed 600 ppm, and the
third, pair-fed at the food intake levels of the 600 ppm group, The
study ran for 5 weeks. Results (daily body weights) indicated that the
reduction in body weight at 600 ppm can be totally accounted for by
reduced food intake (Simpson, 1972b).
Groups of 6 male and 6 female rats were fed 50, 100, 300 or 600 ppm
fenbutatin oxide (97%) in diet for 3 months. Concurrent controls
comprised 12 male and 12 female rats. Health and behaviour were
comparable in all groups. Body weight gain was reduced in males at 600
ppmv and in females at 300 and 600 ppm. Food intake was reduced in
males at 600 ppm, and in females during the first 3 or 4 weeks of the
study at 300 and 600 ppm. Absolute organ weight of liver and kidney
were reduced in both sexes, heart in females, and brain and spleen in
males at 600 ppm. At 300 ppm, liver, spleen and kidney absolute
weights were reduced in males. Organ/body weight ratios were elevated
for brain in males at 600 ppm, and in females at 300 and 600 ppm.
Heart and liver weight ratios were elevated in females at 600 and
heart at 300 ppm. Testes weight ratio was elevated in males at 300 and
600 ppm. Haematology (haemoglobin, PCV, RBC, WBC, RCV, MOH,
prothrobmin time and KOCT) was comparable in all groups. Clinical
chemistry determinations were comparable for total protein, SGOT, and
electrolytes (K, Na, Cl). BUN and SAP in males were elevated at 300
and 600 ppm in males, as was SGPT at 300 PPm. In females BUN was
elevated at 100 ppm and above. Serum protein fractions were comparable
in all groups. No compound-related gross or histopathological effects
were noted, (Simpson and Thorpe, 1973a).
Three groups of rats, 20/sex/control and 10/sex/test group, were fed
0, 3, 10, 30, 100 or 300 ppm of 99% 1, 1, 3, 3-tetrakis (ß,
ß-dimethylphenethyl)-1,3-dihydroxydistannoxane for 90 days. Health and
behaviour were comparable in all groups. Body weights of 300 ppm males
were significantly decreased throughout the study. This was also true
for the females in the 30, 100 and 300 ppm group but the suppression
was not significant. Slight reductions in haemoglobin, erythrocyte
count and packed cell volume occurred in the 300 ppm males. No changes
were noted in any of the other groups of either sex. The other
clinical values of animals killed after 13 weeks showed no
dose-related differences when compared to the control values. Gross
and microscopic examination of a wide range of tissues from all
control, 100 and 300 ppm animals revealed no consistent changes
associated with exposure to the metabolite. Organ weights of males at
13 weeks did not differ from control weights, the organ body weight
ratios were increased for brain, heart, liver, kidneys and testes in
this group and for the liver in the 100 ppm male group. Brain, spleen
and kidney weights were reduced in the 300 ppm females, (Simpson and
Dix, 1972c).
Dog
Five groups of 2 male and 2 female beagle dogs were orally dosed
(gelatin capsules) with 0, 10, 30, 100 or 300 mg fenbutatin oxide/kg
for 5 weeks. Slight vomiting and diarrhoea occurred at 100 and 300
mg/kg, which was associated with decreased body weigh. At 3O mg/kg and
above, serum alkaline phosphate was elevated. No pathological changes
were observed (Simpson, 1972a).
Groups of dogs (8/sex/control and 4/sex/test group) were administered,
by capsule, 0, 2.5, 5, 15, 30 or 60 mg/kg 97% fenbutatin oxide daily
for 2 years. Blood samples were taken every six weeks to measure BUN,
glucose, plasma protein, sodium, potassium, chloride, SGPT, SGOT, and
alkaline phosphatage. Every 12 weeks hemoglobin, packed cell volume,
RBC, WBC, differential WBC counts, prothrombin and
kaolin-cephalin-coagulation times were determined. Urine analysis was
carried out every 3 months. Emesis and diarrhoea occurred in most of
the dosed dogs. These effects continued in some dogs in the 30 and 60
mg/kg/day groups throughout the study, although at a reduced frequency
towards the end of the study. Lower dosage groups showed none of these
effects after the first year. Convulsions occurred in 5 dogs,
predominantly female, during the second year; control, one female; 2.5
mg/kg/day, one female; 15 mg/kg/day, one female and one male; and 30
mg/kg/day, one female. None of the 60 mg/kg/day animals exhibited this
effect. EEG's with simultaneous single lead monitoring of EEG's of one
female each in the 0, 2.5 and 30 mg/kg/day groups revealed a normal
EEG for the control dog and the other 2 dogs showed normal waking
rhythms but abnormal activity during light sleep. The investigators
contend that the lack of dose response relationship suggest a
diagnosis of spontaneous epilepsy not related to exposure to
fenbutatin oxide. General health and behaviour of all other dogs were
similar to control dogs. Significant reductions in rate of weight
gains occurred in both sexes in the toxicity, long term 60 mg/kg/day
group. Males receiving 30 mg/kg/day exhibited no significant rate of
gain. This effect was not evident in females of this group.
Haematological and clinical chemistry and urine data showed no
differences between control and test groups. Necropsies revealed no
gross changes. Microscopic examination, including frozen sections of
liver and kidney stained for lipid, revealed no compound-related
pathological changes in a wide range of tissues. Organ weights were
not affected by exposure to the test compound (Granville and Dix,
1973b).
Long term studies
Rat
Groups of rats (114/sex/control and 72/sex/test group) were fed 0, 50,
100, 300 or 600 ppm 97% fenbutatin oxide for two years. Rats were
killed at 3 and 12 months (12/sex/control and 6 sex/test group) and at
6 months (24/sex/control and 12/sex/test group). Organ weights of
brain, heart, liver, spleen, kidney and testes were recorded and a
wide variety of tissues examined from all rats dying or killed at
interim periods, and from all rats from the 50 and 100 ppm groups.
Blood determinations were made, at each kill period, for haemoglobin,
PCV, RBC, WBC and WBC differential counts; prothrombin and
kaolin-cephalin-coagulation times; BUN; total protein, potassium
sodium and chloride; SGPT, SGOT and alkaline phosphatase. Also serum
proteins were fractionated electrophoretically and estimates made of
albumin and alpha, - ß, and gamma-globin concentrations.
Fenbutatin oxide rendered the diet unpalatable to both sexes causing,
in the early months of the study, significant reductions in food
consumption and body weight gains. Males were more affected than
females. For the remainder of the study all groups exhibited rates of
gain to the control except both sexes receiving 300 and 600 ppm, which
exhibited reduced body weight reduced body weights throughout the
study. BUN levels were increased at the higher levels for the first 6
months but remained within normal limits for the remainder of the
study. Serum alkaline phosphatase activity was increased in both sexes
at 300 and 600 ppm throughout the study, in the 100 ppm males at 6
months, and in the 100 ppm females at 3 months and 2 years. All other
haemotological and clinical chemistry values remained within normal
limits at all times. Survival and behaviour were not affected by
administration of the compound. No compound-related tissue lesions nor
any increase in tumor incidence were found. Organ weights revealed no
compound-related differences except kidney weight reduction in all
treated males and the 600 ppm females at 2 years. No specific
compound-related lesions were seen in the kidney of any treated group.
Absolute and relative testes weights, unaccompanied by hypertrophy,
were noted in the 300 and 600 ppm groups at 2 years. (Simpson et al.,
1973b).
COMMENTS
Fenbutatin oxide is moderately toxic by the oral route producing
severe gastrointestinal irritation. Acute oral administration to
rabbits reduced spermatogenesis and resulted in multi-nucleated
spermatid production. It is excreted rapidly, mostly unchanged in the
faeces, indicating that it is poorly absorbed. In the rat it is
metabolized to ß, ß-dimethylphenethylstannonio acid (SD 33608) and 1,
1, 3, 3-tetrakis-(ß, ß-dimethylphenethyl)-1,3-dihydrox3rdistannoxane.
The latter metabolite and fenbutatin oxide failed to produce oedema of
the central nervous system of the rat at 100 and 1000 mg/kg
respectively. Fenbutatin oxide produced no genotoxic effects in
several in vitro and in vivo systems. Adequate short- and
long-term studies are available in rats and dogs as well as teratology
study study in rabbits, a multi-generation reproduction study in rats,
and a carcinogenicity study in mice. No-effect levels were
demonstrated in rats and dogs and an acceptable daily intake for
humans was established on the basis of these studies. The no-effect
level was established as 15 mg/kg because of emesis and diarrhoea in
dog, and because of the known toxic effects of organic compounds on
the brain a safety factor of 100 was used.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in diet equivalent to 2.5 mg/kg body weight.
Dog: 15 mg/kg body weight.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.03 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenbutatin oxide is used as a specific mitioide against a wide range
of phytophagous mites. It is recommended especially for the control of
the mobile stages on a wide variety of crops, including pome and stone
fruits, citrus fruits, grapes, berry fruits, vegetables and
ornamentals. Recommendations for use on fruit crops and the
recommended pre-harvest intervals are given in Table 2.
The compound is in general used as a foliar spray in a concentration
range of 0.02 -0.05% a.i., corresponding to approx. 0.4 -1.5 kg
a.i./ha. Most applications are required while the fruits are
developing; the number of applications varies with the crop and
regional conditions.
The pre-harvest intervals are seldom less than 7 days, except on some
glasshouse-grown fruits or vegetables; in most instances the
pre-harvest intervals recommended are at least 14 days.
TABLE 2. Use pattern and recommended pre-harvest intervals of fenbutatin oxide
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Apples, Panonychus ulmi 0.0125-0.05% depending Australia 2
Tetranychus urticae on pest and volume of Belgium 28
Tetranychus mc. danieli spray liquid used. Denmark 28
Paratetranychus spp. For concentrated sprays France 7
Bryobia rabrioculus maximum 1.5 kg a. i./ha. Fed. Rep. of
Germany 14
Eotetranychus carpini Not more than 3 applications the
borealis whilst trees are bearing fruit. Netherlands 42
Italy 30
Aculus schlechtendali Mexico 14
Portugal 21
S. Africa 14
3 days
on fruit
for
canning
Spain 21
Switzerland 21
USA 14
Pears Panonychus ulmi As for apples As for apples
Tetranychus mc. danieli
Tetranychus urticae
Epitremeris pyri
Citrus Panonychus citri 0.015-0.05 % for Italy 60
Eotetranychus banksi concentrated sprays a Mexico 14
Eotetranychus yumensis maximum of 2 kg/ha is S. Africa 7
Tetranychus urticae recommended. Not more Spain 21
Brevipalpus californicus than 4 treatments in USA 7
Phyllocoptruta oleivora one year.
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Cherries Tetranychus urticae 0.02-0.05%; maximum two Italy 30
applications whilst trees Switzerland 21
are bearing fruits. the
Concentrated sprays max. Netherlands 42
1.5 kg a.i./ha.
Peaches Tetranychus urticae 0.02-0.05% maximum two Australia 2
Panonychus ulmi treatments whilst trees Denmark 28
are bearing fruit. France 7
Concentrated sprays up to Italy 30
1 kg a.i./ha.
S. Africa 21
3 days
on fruit
for
canning.
Spain 21
Switzerland 21
Plums Tetranychus urticae 0.02-0.05% Italy 30
Panonychus ulmi Concentrated sprays up Switzerland 21
to 1 kg a.i./ha. the
Netherlands 42
Grapes Panonychus ulmi 0.02-0.05% France 7
Concentrated sprays maximum Fed. Rep. of
rate up to 1 kg a.i./ha. Germany 28
Italy 45
Spain 21
Switzerland 21
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Strawberries Tetranychus
urticae 0.025-0.05% the
Netherlands only before
blossoming
and/or after
harvest.
Bell-peppers Tetranychus 0.02-0.04% the 3
urticae Netherlands (glasshouse)
Cucumbers Tetranychus 0.02-0.05% Italy 30
urticae the
Netherlands 3::
Tomatoes Tetranychue 0.02-0.5% Italy 30
urtical the
Netherlands 3
(glasshouse)
:: includes gherkins glasshouse and outdoors; cucumbers and melons glasshouse only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained on residues from supervised trials
carried out in various countries on a variety of fruits and fruits of
vegetables as follows.
Citrus fruits: grapefruit, lemons, oranges
Pome fruits: apples, pears
Stone fruits: cherries, peaches, plums
Berry fruits: grapes, strawberries
Fruits of vegetables: bell-peppers, cucumbers, gherkins,
melons, tomatoes
The data obtained are summarized in Tables 3-11 (Shell International,
1971-76). In most of the trials fenbutatin oxide was applied as a 50%
wettable powdery but in a few instances a suspension concentrate
containing 500 g a.i./l was used (referred to as SC in Tables). The
application rates in most of the trials are in conformity with good
agricultural practice. In, some instances higher dosages were also
applied; these excessive rates are indicated in the Tables.
It has been demonstrated, using radio-labelled fenbutatin oxide, that
the residues do not to any great extent penetrate through the akin of
treated fruit (see also section "Fate of residues"). The residues
found when analysing the whole fruit were mainly on or in the skin.
This is also illustrated in samples from some field experiments which
were examined. before and after removal of the peel.
Pome fruits
Apples
The data in Table 3 were mainly obtained from samples of crops treated
according to typical use conditions. The results show considerable
variation according to local conditions.
In most instances residues in whole fruit were below 3 mg/kg
fanbutatin oxide from up to 3 treatments made at recommended rates, up
to 0.05% or 1.5 kg a.i./ha, where pre-harvest intervals were 14 days.
In a few instances however residues at harvest exceeded this figure,
but were essentially below 5 mg/kg.
Some samples were anlaysed after removal of the peel, a common
practice in processing such as preparation for canning or drying.
Residues in the pulp were usually below the limits of determination.
Pears
The data in Table 4 show that residues arising from recommended
applications (up to three treatments at up to 0.05 or 1.5 kg a.i./ha)
in whole fruit harvested 2 weeks after the last application were less
than 4 mg/kg, with the exception of one trial in the USA. The analysis
of samples of peeled pears showed that residues of fenbutatin oxide
seldom exceeded 10% of the levels in the whole fruit.
Citrus fruits
Residue data were obtained on grapefruit, lemons and oranges from the
USA, Brazil, Spain and Italy (see Table 5). Most of the results show
residues below 4 mg/kg; only in a few cases have higher residues been
found, in general not exceeding 5 mg/kg.
Removal of the peel and analysis of the pulp and/or juice has shown
that the residues were mainly on the skin. In pulp and juice residues
were below the limit of determination, 0.02-0.05 mg/kg. Only one
sample of juice analysed contained a higher residue (0.08 ME/1). The
residue in the pulp did not generally exceed 30% of the level in the
whole fruit.
Stone fruits
Cherries
Data obtained on cherries (see Table 6) indicate that following
recommended treatments (up to 0.05% a.i.; minimum pre-harvest interval
7 days) residues were not above 4 mg/kg in whole fruit without stones,
except in one trial in USA where a residue of 4.2 mg/kg was recorded 4
weeks after treatment.
Peaches
Residues of fenbutatin oxide an peaches treated according to the
recommendations (up to 0.05% a.i.) with a minimum pre-harvest interval
of 14-21 days were in most cases below 5 mg/kg, but sometimes higher.
It is unlikely that after applying the miticide according to good
agricultural practice the residue level at harvest would exceed 7
mg/kg. Residues in the fruit after peeling were considerably lower,
and did not exceed 0.2 mg/kg. Details are given in Table 7.
Plums
The limited data available (Table 8) indicate that residues at harvest
are unlikely to exceed 3 mg/kg in the whole fruit without stone after
recommended treatments, i.e. up to 0.05% a.i. and a minimum
pre-harvest interval of 7 days.
Grapes
Residues of fenbutatin oxide (Table 9) following treatment of vines
according to the recommendation (up to 0.5% or 1 kg. a.i./ha), were
about 4 mg/kg after 1 week and 0.6-3.2 mg/kg after 3 weeks (excepting
one erratic figure of 6 mg/kg from a trial carried out in the Federal
Republic of Germany).
TABLE 3. Residues of fenbutatin oxide in apples resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Apples
Granny Smith Australia 1973 1 19 WP 50% 1.6 1.3 0.45 0.95
" " 1973 1 25 " " 2.5 2.5
Josephine 1974 1 19 " " 0.94 0.79
Granny Smith 1975 2 20 " " 1.3 1.5 2.2 1.6
" " 1975 2 20 sc 500g/l 1.4 1.4 1.1 0.9
" " 1975 2 30 " " 1.8 2.0 2.1 2.0
Mcintosh Canada 1976 1 25 WP 50% 0.97 0.90 0.67 0.45
" 1976 1 19 " " 0.55
Cortland 1976 1 19 " " 0.15
Cardinal France 1974 1 30 0.3 " " 0.26 0.04
" 1974 1 50 0.5 " " 0.43 0.09
Melrose 1975 1 75 0.6 " " 0.07
Cox's O.P. Fed. Rep. of
Germany 1974 3 25 " " 1.1 0.82 0.66 0.64 0.21
James Grieves 1974 3 25 " " 1.5 0.83 0.82 0.60 0.53
Golden Delicious 1974 3 25 " " 1.4 0.78 0.82 0.88 0.53
" " 1974 3 25 " " 1.4 0.87 0.61 0.53 0.24
Golden Parmane 1974 4 1.0 " " 1.9 1.1 1.1 0.74 1.1
" " 1974 3 1.0 " " 0.82 0.82 0.35 0.27 0.32
Goudreinet Netherlands 1974 2 25 " " 0.30 0.26
" 1974 3 25 " " 0.22 0.28
" 1975 2 30 " " 0.23 0.35
" 1975 2 30 " " 0.28 0.37
Golden Delicious Italy 1975 1 30 " " 1.2 0.98 0.
Stark Delicious 1975 1 30 " " 0.93 0.65 0.60
Granny Smith New Zealand 1975 5 19 " " 0.75 0.60 0.55 0.40
Starking Portugal 1974 2 0.6 " " 0.30 0.68
" 1974 2 1.0 " " 0.75 1.40
" 1974 2 0.6 " " 0.29 0.80
" 1974 2 1.0 " " 0.63 0.80
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Granny Smith South Africa 1976 1 30 WP 50% 1.8 1.3 1.0 0.95
" " 1976 1 50 " " 2.5 2.4 1.5 1.2
" " 1974 1 100 " " 2.4 2.9
(4
days)
Jonathan Switzerland 1974 3 0.5 0.55 0.43 0.35
(10
days)
Worcester U.K. 1974 2 25 " " 0.3
Worcester U.K. 1974 2 42+25 wp+sc 0.3
York Imperial U.S.A. 1974 5 25 1.25 wp 50% 0.93
1974 5 25 1.25 " " 1.3
1974 5 50 2.5 " " 2.6
1974 5 50 2.5 " " 2.2
Newton U.S.A. 1973 1 38 " " 1.6
(Oregon) (1.5-1.7)
Red delicious U.S.A. 1973 4 19 1.5 0.6-0.94
(Oregon)
1973 4 38 3 1.6-1.5
1973 4 30 1.5 0.25-0.84
Red delicious " 1973 4 3.0 " " 2.1-2.0
Newton Pippin (California) 1972 1 25 " " 0.26
" " 1972 1 50 " " 0.60
" " 1972 1 100 " " 1.5
Golden delicious (Washington) 1971 4 12.5 0.75 " " 0.9 0.8 0.8 0.7
" " 1971 4 25 1.5 " " 1.7 1.4 1.0 1.5
" " 1971 4 50 3.0 3.7 3.5 3.3 2.6
" " 1972 4 12.5 0.75 " " 1.6 1.5 1.5
" " 1972 4 25 1.5 " " 3.5 2.5 3.1
" " 1972 4 50 3.0 " " 7.9 4.7 5.0 3.3
" " Michigan 1971 3 12.5 0.25 " " 1.2 1.0 0.85 0.95 0.90
" " 1971 3 25 0.5 " " 3.5 2.5 3.3 3.7 2.4
" " 1971 3 50 1.0 " " 6.1 6.0 4.0 3.7 3.7
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
" " 1972 4 12.5 0.5 " " 1.8 1.4-1.1 1.1-1.6 0.64-0.68
" " 1972 4 25 1.5 " " 2.9-2.2 1.3-1.8 2.2-1.8 1.1 -0.9
1972 4 50 2.0 " " 3.4-6.0 5.4-6.6 5.3-7.0 2.8-4.1
Ben Davis New York 1972 4 12.5 0.75 wp 50% 2.3-2.6 2.2-2.2 2.1-1.5 1.3-1.6
" " 1972 4 25 1.5 " " 5.6-5.4 3.5-4.2 2.8-3.6 3.0-2.5
" " 1972 4 50 3.0 " " 8.5-8.0 5.6-6.0 5.6-6.2 5.7-3.8
Rome 1973 4 12.5 " " 1.8 1.0 1.1 1.4
1973 4 19 " " 2.2 1.4 1.6 1.2
1973 4 38 " " 2.3 2.8 3.2 2.7
wp = wettable powder
sc = suspension concentrate
TABLE 4. Residues or fenbutatin oxide in pears resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No rate formulation 0-2 3 7-9 14 19-21 28-31 32-35
g/1001 kg/ha
Pears
Josephine Australia 1974 1 19 WP 50% 0.5 0.4 0.3 0.5
William 1973 1 19 " " 0.8 1.1 0.65 0.73
" 1973 1 25 " " 1.6 1.2
Packam 1975 2 20 sc 500g/l 1.5 1.6 1.5 1.3
" 1975 2 30 " " 2.3 1.4 1.6 1.2
" 1975 2 20 WP 50% 1.4 1.2 1.3 1.2
William France 1976 1 60 0.3 " " 0.17 0.11 0.03
" 1976 1 100 0.5 1.07 0.16 0.10
Packhams Tr. S. Africa 1976 1 30 " " 1.7 1.6 1.4 1.9
" 1976 1 50 " " 3.6 3.1 2.7 1.6
Winter Melis S. Africa 1974 1 100 " " 3.8 2.5
(5
days)
Kieffer USA (Mich.) 1971 3 12.5 0.38 " " 3.0 1.7 1.6 1.0
" 1971 3 25 0.75 " " 6.0 5.0 1.9 1.1
" 1971 3 50 1.5 " " 4.7 4.0 3.0
Bartlett (Wash) 1971 2 12.5 0.25 " " 1.5 1.3 0.9
" 1971 2 25 0.5 " " 2.7 2.3 1.8
" 1971 2 50 1.0 " " 5.3 4.7 4.3
" 1973 4 12.5 0.75 " " 0.17 0.18
" 1973 4 25 1.5 " " 0.25 0.54
" 1973 4 503 3.0e " " 1.4 1.9
" (Calif) 1973 1 25 1.0 " " 1.8 1.1 1.1 1.0
" 1973 1 50 2.0 " " 2.4 1.4 2.1 1.8
" (New York) 1971 3 12.5 0.75 " " 1.2
" 1971 3 25 1.5 " " 2.2
" 1971 3 50 3.0 " " 3.5
Bosc 1972 3 12.5 0.38 " " 4.2 2.3 1.2 0.9
" 3 25 0.75 " " 6.0 4.6 2.5 2.1
" 3 50 1.5 " " 8.0 6.3 4.6 3.8
e= excessive application
Strawberries
The limited data available indicate that residues following
recommended application 0.05% a.i, minimum pre-harvest interval 7
days), would be unlikely to exceed 4 mg/kg (Table 10):
Fruit of vegetables
The very limited data available (Table 11) suggest that residues
arising from recommended treatments (up to 0.05% a.i., minimum
pre-harvest interval 3 days) are unlikely to exceed 1 mg/kg.
FATE OF RESIDUES
In animals
Provided fenbutatin oxide is used according to good agricultural
practice no detectable residues will occur in products of animal
origin, since crops which are generally used for animal feed are
rarely treated with this acaricide. In some countries, particularly in
the USA, cattle are occasionally given processed fruit pulp as a part
of their diet. Data are available showing that cattle eating feed
containing residues of fenbutatin oxide will not produce milk or meat
containing appreciable residues. See also the section
"Biochemical aspects".
Cattle feeding studies
An experiment, was carried out in which three lactating Guernsey cows
were fed on a diet containing 119Sn-labelled fenbutatin oxide
(Shell, 1976). The quantity given was equivalent to 34 ppm fenbutatin
oxide in the whole ration, which consisted of 5 kg compounded feed and
10 kg alfalfa cubes per animal per day, and was given twice daily
during a 21 day period. Milk samples were taken from each animal at
each milking, and the total radio-activity determined in each sample.
No radio-activity above background level was found, indicating that
total residues in whole milk were equivalent to less than 0.01 mg/kg
fenbutatin oxide. The animals were slaughtered 12 hours after the
final feeding and the radio-activity was determined in samples of fat,
muscle, brain, kidney and liver. No residues were detected in brain,
muscle, fat or liver at a limit of determination equivalent to 0.02
mg/kg fenbutatin oxide. Similar results were obtained with the kidney
samples from 2 of the animals, but the third was found to contain
radio-activity equivalent to 0.03 mg/kg fenbutatin oxide, i.e. just
above the limit of determination in these tissues.
TABLE 5. Residues of fenbutatin oxide in citrus fruits resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Oranges
Pera Natal Brazil 1975 1 30 WP 50% 0.13 0.05
1975 2 30 " " 0.25
1975 1 30 " " 0.27 0.08
1975 2 30 " " 0.42
1975 1 30 " " 0.50 0.30 0.17
1975 2 30 " " 0.75 0.38
1975 1 30 " " 0.38 0.22 0.08
1975 2 30 " " 0.62 0.35
Oranges S. Africa 1975 1 30 sc500g/l
1.0 1.1 0.65 0.65
1975 1 30 " " 0.8 0.60 0.45 0.40
Oranges Calif. 1972 2 25 1.2 wp 50% 3.0 3.3 1.9 2.1 2.2 1.8 2.5 2.4 1.6 1.7
Valencia 1972 2 50 2.4 " " 5.6 6.0 5.0 4.4 4.2 5.0 4.4 3.8 4.0 3.8
1972 2 100 4.8 " " 10.5 11.0 9.5 9.5 7.5 7.5 6.2 5.5
1971 1 25 0.44 " " 1.1 1.3 0.65 0.82 0.8 1.0 0.88 0.96 0.52 0.74
1971 1 50 1. " " 1.8 3.0 1.5 2.0 1.5 1.7 1.4 1.8 1.5 1.8
(Florida) 1972 4 12.5 0.9 " " 0.56 0.62 0.50 0.75 0.74 0.75 0.64 0.75
1972 4 25 1.9 " " 1.5 1.6 1.5 1.5 1.5 2.0 1.5 1.7
1972 4 50 3.6 " " 3.8 4.0 3.2 4.1 3.1 3.2 3.6 3.1
Lemon
Nostrana Italy 1975 1 30 " " 0.9 0.5
Lisbon USA
(Ariz.) 1974 2 19 1.4 " " 0.81 0.23 0.22 0.34
1974 2 19 1.4 " " 2.8 2.0 1.2 1.6
1974 2 32e 3.5 " " 1.6 0.32 0.30 1.2
1974 2 32e 3.5 " " 5.6 5.8 2.9 2.0
(Calif) 1973 3 12.5 0.37 " " 0.40 0.25 0.20 0.30 0.15 0.30 <0.05 <0.05 (0.05)
1973 3 25 0.75 " " 0.95 0.70 0.25 0.40 0.30 0.30 <0.05 <0.05 (0.05)
1973 3 50 1.5 " " 1.0 0.90 0.75 0.70 0.35 0.05 0.05 <0.05 0.05 0.20
TABLE 5. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Grapefruit
Marsh USA
(Calif) 1971 1 25 1.8 " " 0.9 1.3 0.6 0.9 0.8 0.9 0.6 1.0 0.5 0.6
1971 1 50e 3.6 " " 1.2 2.0 0.9 1.4 1.4 2.0 1.4 1.6 1.3 1.6
Red Blush 1972 2 12.5 0.2 " " 0.8 0.7 0.45 0.45 0.86 0.74 0.36 0.44 (0.42 0.36)
1972 2 25 0.4 " " 1.6 1.7 1.3 1.5 0.9 0.7 (0.90 0.68)
1972 2 50 0.8 " " 3.2 2.9 2.5 2.6 1.9 1.5 (1.7 2.5)
Marsh (Florida) 1972 3 12.5 1.0 " " 0.3 0.4 (0.4 0.5)
1972 4 12.5 1.0 " " 0.7 0.7 0.7 0.7 (0.7 0.7)
1972 3 25 1.9 " " 1.1 1.2 (0.4 0.4)
1972 4 25 1.9 " " 1.5 2.8 1.7 1.8 (1.4 1.1)
1972 3 50e 3.8e " " 3.2 3.4 (1.3 1.7)
1972 4 50e 3.8e " " 3.4 4.8 2.8 4.0 (1.8 3.0)
1971 3 12.5 " " 0.71 0.65 0.72 0.65 0.52 0.44
1971 3 25 " " 1.3 1.3 1.8 1.6 1.0 0.75
1971 3 50 " " 3.3 3.3 3.8 3.1 1.8 1.5
e= excessive application
TABLE 6. Residues of fenbutatin oxide in stone fruits resulting from supervised trials
Crop Country Year No g/100 1kg/ha formulation 0.2 7 14 21 28-31
Cherries
(sweet and sour)
Biggerau Fed. Rep.
Germany 1977 3 25 WP 50% 0.70 0.21 0.09
Rote Leber 1977 3 25 " " 1.5 0.17 0.04 0.02
Schattenmorelle 1977 3 25 " " 1.1 0.41 0.22 0.09
Morella 1977 1 25 0.3 " " 0.90 0.50
1977 1 50 0.6 " " 1.10 1.15
Bing USA
(Calif) 1976 1 25 1.1 " " 1.7
1976 1 50 2.2 " " 3.2
Sour USA
(Mich) 1974 2 12.5 0.96
1974 2 25 2.3 1.5 2.2 3.0
1974 2 50 4.1 3.6 3.9 4.2
USA
(Wash) 1974 2 25 1.2 1.4 2.0
1974 2 50 2.4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
Cherries
Bing USA
(Wash) 1974 2 25 1-2 1.4 2.0
1974 2 50 2-4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
TABLE 7. Residues of fenbutatin oxide in peaches resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Peach
Tatura
Aurora
Australia 1975 2 20 sc500/gl 2.2
1975 2 30 " " 3.6
1975 2 20 wp 50% 2.7
Cornish 1973 1 19 " " 1.3 1.9 2.5 0.71
1973 1 25 1.8 1.4
Golden Queen 1974 1 19 1.4 <0.1 1.0
1974 1 19 2.2
1974 2 19 3.5
Alberta Canada 1976 2 2.6 0.63
Royal Gold France 1977 1 50 0.75 0.87
Audenot 1973 1 0.5 3.7 2.6 1.9
Alberta 1973 1 0.5 1.6 1.6 1.5 1.3 0.65
Red. Aven
P.G. 1974 1 0.5 2.0 1.7 0.41
Vellutata Italy 1974 1 30 0.4 0.3 0.5
Netturina N7 1974 1 30 0.5 0.3 0.5
Golden Queen New
Zealand 1975 1 19 0.8 0.65 0.45
Kakamus S. Africa 1976 1 30 7 5.9 4.0
" 1976 1 50 12 7.0 6.0
Sunblest USA
(Calif) 1973 3 50 1.1 4.3 3.0
1973 3 20 4.3 1.0
1973 3 50 7.3 5.7 8.0 2.6
Red Haven (Oregon) 1973 3 19 0.3 3.9 2.8 1.9 1.5
(S.
Carolina) 1973 3 38 0.6 7.7 8.2 4.6 5.8
TABLE 7. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Roter
Ingelheimer Fed. Rep.
of
Germany 1977 3 25 0.25 9.0 3.4 3.4 2.0 1.8 1.3
Rekord
a. Alfter 1977 3 25 0.25 8.1 4.7 2.5 2.2 1.5
Madame
Roenait 1977 3 25 0.25 8.1 3.1 2.5 2.3 3.3
TABLE 8. Residues of fenbutatin oxide in plums resulting from supervised trials
Application Residues in mg/kg at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 3-4 7 14 21 25-28 32-35
Plums
Auerbacher Fed.
Rep.
Germany 1977 3 25 0.25 WP 50% 0.15 0.15 0.15 0.10
Bühler 1977 3 25 0.25 0.25 0.10 0.15 0.10
Czar Netherlands 1977 1 25 1.75 sc500/gl 0.3 0.2 0.1 <0.1 <0.1
1977 1 50 3.5 " " 0.5 0.4 0.2 0.1 0.1
Relsey S. Africa 1976 1 30 wp 50% 1.2 1.3 0.8 0.9
" 1976 1 50 " " 2.2 2.2 1.8 1.0
Stanley USA
(New York) 1974 2 12.5 0.31
1974 2 25 " " 0.50
1974 2 50 " " 0.49
French
Prunes (Calif) 1974 2 12.5 " " 0.27 0.35
1974 2 25 " " 0.92 0.96
1974 2 50 " " 2.8 2.3 1.8
Early
Halian (Wash) 1974 2 25 " " 0.19 0.02 0.11 0.6
50 " " 0.29 0.73 0.47 0.58
100 " " 1.03 1.05 0.67 0.69
TABLE 9. Residues of fenbutatin oxide in grapes resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
rate
Crop Country Year No g/100 1kg/ha formulation 0-3 7 13-14 21 28-31 42-45 56-
Grapes
Semillion France 1973 1 30 wp 50 0.7
1 50 " " 0.9
Grenache 1974 1 0.5 " "
Merlot Rouge 1974 1 0.5 " "
Lavallet 1975 1 50 " " 0.27
Grenache 1975 1 50 0.50
Siebel 1975 1 125e " " 1.1
Müller Fed. Rep.
of Germany 1975 3 50 " " 4.7 3.9 2.5 2.6 1.2
Kerner 1975 3 50 " " 4.8 2.1 2.7 3.0 1.1
Müller 1975 3 50 " " 4.9 3.3 3.4 2.3 2.9
Sylvaner 1975 3 50 " " 2.7 1.9 1.6 3.2 1.4
Riesling 1975 3 50 " " 4.5 1.8 1.6 0.6
Trollinger 1975 3 50 " " 1.3 1.2 1.1 3.2 2.3
Sprühsurgunder 1975 3 50 " " 7.1 6.3 6.2 6.3
Barbera Italy 1975 1 30 " " 7.1 1.3 1.1 0.45
Nebbiolo 1975 1 30 " " 1.8 1.1 0.68
Chasselas Switzerland 1975 1 100e " " 2.1
Rabier USA
(Calif) 1973 3 25 0.5 " " 0.90 0.68 0.16 0.17
1973 3 50 1.0 " " 2.0 0.62 3.0 2.3
Mission 1973 3 0.5 " " 1.0 0.45 0.97 0.65
1973 3 50 1.0 2.2 0.47 0.67 1.2
Thompson 1973 3 25 0.5 1.5 1.5 1.8 1.0
1973 3 50 1.0 3.9 1.8 1.4 0.66
Concord (Michigan) 1974 3 50 1.1 3.1 2.0 2.2 1.8
1974 3 75 1.7 5.6 2.8 3.4 2.7
50 1.1 2.8
100 2.2 2.9
e= excessive application
TABLE 10. Residues of fenbutatin oxide in strawberries resulting from supervised trials
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Strawberries
Gorella (g) Belgium 1976 1 25 0.9 WP 50% 0.2 0.1 <0.1 <0.1
Volla (g) 1976 1 25 0.9 " " 0.40 0.15 0.15 <0.1 <0.1 <0.1
France 1977 1 30 " " 0.35 0.20
Mexico 1977 1 0.55 sc 500g/l 2.3 2.0 0.9
1.1 " " 7.0 4.0 5.9
Netherlands 1977 1 25 wp 50% 0.50
1977 2 25 " " 0.65
1977 1 25 " " 0.15
1977 2 25 " " 0.30
1977 1 25 sc 500g/l 0.65
1977 2 25 " " 1.1
1977 1 25 " " 0.25
1977 2 25 " " 0.50
Parfaite S. Africa 1977 1 25 0.13 " " 0.5 0.2 0.2
1 38 0.19 " " 0.8 0.5 0.5
- (g) UK(Sussex) 1975 1 25 wp 50% 0.7 0.7 0.5 <0.1
- (Devon) 1975 1 25 " " 0.4 0.2 0.5 0.2
Tioga USA(Calif) 1974 1 50 1.1 " " 1.4*
1974 1 100 2.2 " " 2.4*
Heidi 1973 2 80 2.2 " " 4.9* 4.6 3.4
1973 2 160e 4.4e " " 4.8* 5.9 2.9
G - 4 1973 4 160e 4.4e " " 4.0* 3.3 3.0
Tioga (Florida) 1974 6 40 0.55 " " 1.2* 1.4 0.3
Catskil USA
(Wisc) 1975 2 100 1.1 " " 1.6* 0.49
2 200 2.2 " " 2.2* 1.5
Northwest (Wash) 1975 1 50 0.55 " " 0.70 1.1 0.98
1 100 1.1 " " 1.1 1.2 1.83
1 200 2.2 " " 2.4 4.3 3.1
TABLE 10. (Continued)
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Raritan (New Jersey) 2 50 0.55 " " 0.86 0.2 0.60
2 100 1.1 " " 1.4 1.3 0.60
2 200e 2.2e " " 5.0 1.9
(g) = glasshouse or plastic cover
e = excessive application
* = residue after 1 day
TABLE 11. Residues of fenbutatin oxide in fruits of vegetables resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 2-3 4-5 7 10 14 30
cucumbers(g) Netherlands 1974 1 25 wp 50% 0.2 0.1 0.1 <0.1
1974 1 25 " " 0.2 0.2 0.2 <0.1
gherkins(g) 1974 1 0.75 1.8 0.8 0.7 0.9
melons France 1977 1 30 < 0.01 <0.01
sweet
peppers(g) Belgium 1976 1 1.2 1.0 0.6
sweet
peppers(g) Netherlands 1976 1 0.5 0.4 0.6 0.4 0.1
tomatoes(g) Netherlands 1976 1 0.5 0.2 0.4 0.4 0.4
tomatoes Italy 1974 2 30 0.5 0.3
1974 2 30 0.2
tomatoes(g) UK 1975 1 0.2 0.3 0.4
(g) = glasshouse of plastic cover
In a second experiment (Shell, 1973) 3 Guernsey cows were similarly
given a daily ration containing 34 mg/kg 119Sn-labelled fenbutatin
oxide for 21 days. Excretion of the ingested fenbutatin oxide was
mainly via the faeces but small amounts, up to 3% of the daily intake,
were excreted in the urine. As in the previous experiments no residues
were found in the milk (limit of determination 0.02 mg/kg) and none in
the brain, honey fat or most of the muscle samples taken. However
radio-activity averaging 0.27 mg/kg calculated as fenbutatinoxide was
found in the kidney and 0.34 mg/kg in the liver of all three animals.
Radio activity equivalent to 0.08 mg/kg fenbutatin oxide was found in
lone lung sample and a just measurable residue equivalent to 0.04
mg/kg in one muscle sample (gastrocnemius). The limit of determination
was equivalent to 0.04 mg/kg fenbutatin oxide in all the tissues
(Shell, 1973). Residue data from field trials indicate that residues
of fenbutatin oxide in apple pomace from apples containing a residue
of 4 mg/kg may approach 25 mg/kg (Shell, 1972-74).
The data from the cattle-feeding studies indicate that cattle fed
normal amounts (up to 25% of the total diet) of apple pomace made from
apples treated with fenbutatin oxide according to good agricultural
practice are unlikely to yield milk containing detectable amounts of
the acaricide. No measurable residues are likely in muscle tissues,
but they might occasionally occur at low levels (<0.2 mg/kg) in
liver and kidney.
In plants
Fenbutatin oxide is slowly lost after application to crops. The
degradation to in-organic tin occurs through successive loss of the
phenyldimethylethyl groups. The principal organotin degradation
product found on crops is 1,1,3,3,tetrakis(ß, ß-dimethylphenethyl)-
1,3-dihydroxydistannoxane, referred to subsequently as SD 31723(Shell,
1972).
In studies of the fate of fenbutatin oxide on leaves and fruit of
apples and citrus (Shell, 1972, 1974a), small apple trees grown in a
lath-house were treated one to three times with 119Sn-labelled
fenbutatin oxide with approximately 40µg at each application.
Recoveries of 119Sn 2 to 33 days after the last application were
84-96% of the amount applied. Virtually all the radio-activity in the
fruit was found to be on the outer surface of the skin. Substantial
amounts of the radio-activity were removed by rinsing with organic
solvents, or simply by siping the fruit-skin with paper tissues. It
was shown that less than 5% of the total radio-activity present in the
fruit originated from the principal breakdown product SD 31723 and
less than 0.7% from other organotin metabolites including (ß, ß-
dimethylphenethystannoic acid polymer (SD 33608). The structural
formula of the metabolites SD 31723 and SD 33608 are shown on the
following page. Inorganic tin accounted for nearly 10% of the total
119Sn on the apples (Shell, 1972).
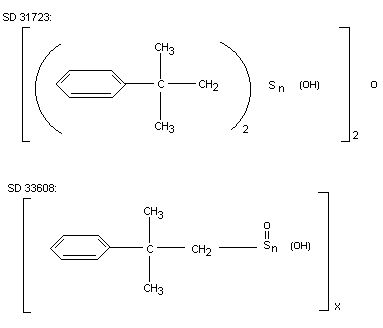 In another experiment apples were treated in a similar way either with
the parent chemical or with SD 31723 and analysed six weeks later. The
result indicated that the rate of breakdown of SD 31723 exceeded that
of fenbutatin oxide itself. It was concluded that fenbutatin oxide is
degraded firstly to SD 31723, and to SD 33608 and eventually to
inorganic tin compounds. Since SD 31723 was degraded faster than the
parent compound, it is unlikely to build up treated crops and would
therefore not be expected to form an appreciable part of any residue
present.
Similar experiments were carried out on oranges (Shell, 1974a). Leaves
were treated with formulated 119Sn-labelled fenbutatin oxide at a
concentration of 39.5 mg/1 and analysed at intervals up to nine months
after treatment. 40 days after the treatment 65% of the applied
radio-activity was still present in the leaves, of which 71% was
unchanged fenbutatin oxide, 3.4% SD 31723, 0.5% SD 33608 and the rest
inorganic tin. At the end of the 9-month period the total
radio-activity present had declined to about 11% of that applied, and
of this almost 60% was still present as fenbutatin oxide; SD 31723
accounted for about 3% and SD 33608 for less than 1% whilst inorganic
tin made up the remainder (Shell, 1974a).
In other experiments oranges with a diameter of 3-4 cm. were similarly
treated at a rate corresponding to 0.2/kg on the anticipated weight at
harvest 6 months later. Some of the oranges were again treated prior
to harvest to give a total dose corresponding to 0.44 mg/kg on harvest
weight and left on the tree for a further 2 months.
The fruit after picking was separated into peel, juice and pulp which
were separately analysed. No radio-activity was detected in the juice
or in the pulp of any of the samples, at the limit of determination
assessed to be 0.001 mg/kg (calculated as fenbutatin oxide). Residues
in the peel of the fruit harvested 6 months after a single treatment
averaged 0.095 mg/kg and 2 months after a second treatment 0.172
mg/kg.
In both instances approximately 6% of the residue was soluble in
organic solvents and where analysed was found to consist largely
(about 90%) of unchanged fenbutatin oxide (Shell, 1974a).
These results therefore confirmed those obtained on apples.
Residue data from SD 31723 in crops
An analytical method for the routine determination of SD 31723 was
used on numerous samples taken from crops treated in the field with
fenbutatin oxide. Residues on SD 31723 were generally below the limit
of determination (usually in these analyses 0.1-0.2 mg/kg, except in
one set of strawberry samples, where the limit was 0.04 mg/kg).
Positive residues were found only in one trial with peaches, where
levels up to 0.3 mg/kg occurred; the residue was solely in the skin.
These data are in line with the observations in other experiments that
residues from fenbutatin oxide are sometimes higher in peaches than in
the other crops studied (Shell, 1976).
In storage and processing
Since fenbutatin oxide is moderately stable, insoluble in water and
remaining largely on the exterior of treated crops, it is clear that
processing which affects the surface of the crop may considerably
reduce the residue levels; some details are given below.
Washing Experiments with apples and citrus showed that fenbutatin
residues may be considerably reduced by washing. 60% or more of the
original residue was removed by this process.
Peeling The removal of the outer peel of the fruit or other
commodity reduces the residue by at least 75%, depending on the
commodity.
Juice extraction Data on residues in extracted citrus juice are
generally below the limit of determination (0.02-0.05 mg/l). In only
one instance was a small residue of fenbutatin oxide (0.08 mg/1)
found.
Apple juice has been obtained with similarly low residues (Shell,
1972-1974). From studies with the radio-labelled compound, residues
would not be expected to occur in the interior of the apples and the
data on juice confirm that the residues on the surface of the fruit
are not extracted to an appreciable extent with the juice during its
preparation. No residues of the main metabolite SD 31723 have been
found in any juice from treated fruits (Shell, 1972, 1974a).
Vinification Wine from grapes containing up to 5.6 mg/kg of
fenbutatin oxide in the whole fruit contained less than 0.02 mg/1, the
limit of determination (Shell, 1974b, 1975). Similarly the breakdown
product SD 31723 was not found in the wine at the limit of
determination of 0.1 mg/1 (Shell, 1975).
In soil
Residue degradation in sterile and non-sterile soils. The residue
degradation of 119Sn-labelled fenbutatin oxide was studied in a
steam-sterilized and unsterilized Hanford sandy loam (particles 2
11.1%; between 2 and 20 21.9%; 20 67%; organic material 0.8-1%; pH
4.6-6.5). 10 mg/kg 119Sn-labelled fenbutatin oxide was mixed into
both soils which were stored in the same room at ambient temperature.
Almost all the 119Sn was organosoluble. Fenbutatin oxide decreased
initially faster in sterile than in normal soil. However the rate of
decrease later became less in sterile than in live soils. It is
suggested that steam sterilisation activates catalytically active
sites on the soil so that more molecules can be degraded initially.
The subsequent decrease in rate as compared with live soils indicates
that microbial degradation, which occurred in the latter became the
main factor in degradation. No accumulation of degradation products
except 119Sn could be 254 fenbutatin oxide measured (Shell, 1973b)
see Table 12.
Metabolism in soil under aerobic and anaerobic conditions
In other experiment in which 10 mg/kg 119Sn-labelled fenbutatin
oxide was mixed into the same soil, the residue degradation was
studied under aerobic and anaerobic conditions. One set of soil
samples was kept under aerobic conditions at ambient temperatures in a
glasshouse; another set was kept under nitrogen after an initial 30
days aerobic period. The amount of solvent-extractable radio-activity,
which was nearly all 119Sn-fenbutatin oxide, decreased slowly during
the 180 days of the experiment. No significant differences could be
found between the rates of degradation under aerobic and anaerobic
conditions (Shell, 1973a).
TABLE 12. Radio-activity extracted from sterile and non sterile soil at intervals after treatment
with fenbutatin oxide
119Sn as mg/kg fenbutatin oxide equivalents
Days after sterile soil live soil
treatment
0 10.1 10.1
30 9.3 9.8
60 9.2 9.1
90 9.0 8.6
120 8.7 8.5
180 8.5 7.9(Shell, 1973b)
The degradation of 14C-labelled fenbutatin oxide was also studied in
Hanford sandy loam under outdoor field conditions. The compound was
applied at a rate of 1.5 kg/ha either as a single application or as
repeated applications, each at the same rate, at six-monthly
intervals. The degradation followed first order kinetics during the
first 12 months and accelerated considerably during the next six
months so that one-half of the 14C-fenbutatin still present after 12
months was degraded during the following 6 months. A similar increase
in the degradation rate was also found in the experiments in which
14C-fenbutatin oxide was applied repeatedly. Since the environmental
conditions did not vary significantly from one year to the other, it
is suggested that adaptation of micro-organisms to the compound was
the primary cause of the increase. No accumulation of the degradation
products SD 31723 or SD 33608 could be detected at any time (Shell,
1974).
Under the field conditions 14C-labelled fenbutatin oxide applied to
the soil surface remained mainly in the top 7.5 cm. No measurable
amounts or very low residues were found in the 22.5-30 cm layer (Table
13).
TABLE 13. Carbon-14 extractable from soil after a single application of fenbutatin
oxide
Depth of soil 14C, as mg/kg fenbutation oxide equivalents after
0 4 months 12 months 18 months
0-7´ cm 1.51 1.04 0.66 0.27
7´-15 cm -.- 0.06 0.06 0.03
15-22´ cm -.- 0.05 < 0.02 0.04
22´-30 cm -.- 0.03 < 0.02 <0.02
Mobility in soil and leaching
A sample of Hanford loam, which had been treated with 14C-labelled
fenbutatin oxide and aged outdoors for 4 months, was placed on top of
a column of the same but untreated soil, 30 cm long. The soil was
saturated with water and leached by applying a total of 50 cm water
over a period of 51 days. The 14C content in each of the daily
effluent samples was below the detection limit of 0.002 mg/kg
equivalents of fenbutatin oxide. The soil on top of the column
contained 1.22 mg/kg equivalents of fenbutatin oxide initially and
1.16 mg/kg at the end of the experiments.
The four 7.5 cm. layers of the column contained 14C equivalent
respectively to 0.02, 0.017, < 0.01 and < 0.01 mg/kg of fenbutatin
oxide at the end of the experiments (Loeffler, 1973).
In air and UV light
Thin layers of 119Sn fenbutatin oxide on a glass surface when
exposed to sunlight slowly decomposed to the derivative SD 31723 and
to more polar compounds such as inorganic tin salts. After 230 hours
of exposure, 81% of the initial 119Sn deposit was extractable by
organic solvents and 6% by aqueous solvents. In this sample 76% of the
organo-soluble 119Sn consisted of unchanged fenbutatin oxide and
21.3% of the degradation product SD 31723 (Shell, 1973d).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE, OR AT
CONSUMPTION
No information available.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods of analysis for fenbutatin oxide residues
on fruit crops and fruits or vegetables have been developed (Shell,
undated, 19746).
Three modifications of a basic method are available. The finely
divided crop sample is extracted with methylene chloride,
concentrated, diluted with hexane and treated with hydrochloric acid,
to form a chloro-derivative. The resultant solution is then cleaned up
on an acid alumina column before determination by GLC. The method has
been adapted for use on samples of milky animal tissues and other
materials (Shell, undated). The modifications differ only in detail
and the choice of the method is dependent on the equipment and
solvents available, the commodities to be analysed and the analyst's
personal preference. Limits of determination are in general 0.01
- 0.05 mg/kg.
Tests have been carried out to determine the loss of residue during
storage at deep-freeze temperatures (-15°C) whilst awaiting analysis.
No changes in residue levels could be detected during a period of
16-18 months (Shell, 19740).
Two methods have also been developed for the determination of the
principal breakdown product of fenbutatin oxide, SD 31723. Both
involve extraction of the finely divided sample with methylene
chloride as for the parent compound. The extracts are subjected to
clean-up on an alumina or silica gel column and the eluates are
concentrated and analysed by a thin layer chromatographic technique.
After development the plates are treated with a suitable reagent to
visualize the tin-containing products. The determinations are
completed by visual comparison of the colour and size of the spots
with standards run under the same conditions. A limit of determination
of 0.1-0.2 mg/kg SD 31723 can be achieved (Shell, undated, undated).
APPRAISAL
Fenbutatin oxide is used as an acaricide as a foliar application on a
considerable scale on a wide variety of fruits and fruiting vegetables
in various countries.
Fenbutatin oxide is mainly marketed as a 50% wettable powdery but also
as a suspension concentrate. The rates of application vary depending
on crop, pest and method of application; normal rates are usually 1-2
kg a.i./ha.
The recommended pre-harvest intervals in various countries vary
considerably for one and the same crop.
Residue data from supervised trials were obtained from several
countries. Residues do not penetrate through the skin of treated fruit
to any great extent, and in the pulp are therefore generally below the
limit of determination.
Extensive data are available on the fate of the residues in plants and
on the levels of residues in products of animal origin arising from
the feeding of treated commodities including the pulp of treated
fruits. On apples after 33 days the remaining residue consists of
about 85% unchanged parent compound, 5% of the principal organotin
metabolite SD 31723 and about 10% of inorganic tin.
The degradation of the main metabolite SD 31723 is faster than that of
the parent compound and thus the metabolite will in general not form
an appreciable part of any residue present in practical conditions.
Occasionally residues not exceeding 0.3 mg/kg of the parent compound
could be found in the kidneys of cows fed with treated feeds and it
was calculated that these residues mould not exceed 0.2 mg/kg in
practical circumstances; in meat and milk, residues were undetectable
(i.e. below 0.02 mg/kg).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national maximum residue limits for fenbutation oxide are
established or under consideration:
Country Commodity maximum residue limit, mg/kg
Australia Apples, pears, peaches 3
Belgium Apples, pears 1
Fed. Rep. of Germany Pome fruit 2
The Netherlands Apples, pears, cherries,
plums 1
Blackberries,
raspberries, strawberries,
black- and red currants 0.2
gherkins (glasshouse
and outdoors) 1
Cucumbers* , melons*,
bell-peppprs *,
eggplant *, tomatoes * 0.3
Italy Apples, pears, apricots,
cherries, peaches,
plums, citrus fruits,
grapes, cucumbers,
tomatoes 0.5
South Africa Apples, pears, peaches,
citrus fruits 2
Switzerland Pome fruit, stone fruit, grapes 1.5
U.S.A. Apples, pears, citrus fruits 4
Liver and kidney of cattle,
goats, horses, pigs and sheep 0.3
Dried apple pomace 20
Dried citrus pulp 7
*glasshouse crops
Fenbutatin oxide applied in field conditions to the soil remains in
the upper layers and is slowly degraded, forming non-mobile
metabolites. Washing fruits and vegetables removes 60% or more of the
original residue, and removal of the outer peel of apples or citrus
reduces the residue by at least 75%.
Gas-chromatographic methods for residue analysis for fenbutatin oxide
in fruit crops, fruits of vegetables, milk and animal tissues are
available, which are suitable or can be adapted for regulatory
purposes. Methods for the determination of the principal metabolite
have also been developed.
RECOMMENDATIONS
The following maximum residue limits for fenbutatin oxide are
recommended. They refer to fenbutatin oxide, excluding any
metabolites.
Commodity Limit, mg/kg Pre-harvest interval
on which
recommendations
are based
Dried apple pomace 20 -
Dried citrus pulp 7 -
Peaches 7 14-21
Apples and pears 5 14
Cherries (sour and sweet) 5 14-21
Citrus fruits 5 7-14
Plums 3 7-14
Strawberries 3 7-14
Fruits of vegetables
(cucumbers, eggplants,
gherkins, melons, sweet
peppers, tomatoes) 1 3-7
Liver and kidney of cattle,
goats, horses, pigs and
sheep 0.2
Meat of cattle, goats,
horses,
pigs and sheep 0.02*
Milk 0.02*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the EEG findings in the dog.
2. Elucidation of species differences in toxic effects on rabbit
testes.
REFERENCES
Dean, B.J., Doak, S.M.A. and Funnell, J. (1972a) Toxicity studies with
SD 14114: genetic studies with SD 14114 in micro-organisms in vitro
and in the host-mediated assay. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Dean, B.J., Doak, S.M., Perquin, B.D. and Senner, K. (1972b) Toxicity
studies with SD 14114: the cytogenetic investigation of bone marrow
cells of mice after a single oral dose of SD 14114. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
the Shell Chemical Company.
Dean, B.J. and Doak, S.M. (1972c) Toxicity studies with SD 14114: the
effects of a single oral dose of SD 14114 on dominant lethal mutations
in male mice. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Company.
Dix, K.M. and Wilson, A.B. (1973) Toxicity studies with SD 14114:
Teratological studies in rabbits given SD 14114 orally. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Granville, G.C., Simpson, B.J. (1973a) and Doak, S.M. Toxicity studies
on the pesticide SD 14114: an 18 month study in mice. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by Shell Chemical Company.
Granville, G.C. and Dix, K.M. (1973b) Toxicity studies on the
pesticide SD 14114: Two year oral toxicity test in dogs. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Hine, C.H., Eisenlord, G. and Loguvan, G.S. (1973) Results of
reproduction study of rats fed diets containing SD 14114-V over three
generations. Unpublished report from Hine Laboratories Inc. submitted
to the World Health Organization by the Shell Chemical Company.
Loeffler, J.E. (1972) The fate of Ingested 119Sn SD 14114 in Rats.
Unpublished report from Shell Development Company, Biological Sciences
Research Center submitted to the World Health Organization by Shell
Chemical Co.
Loeffler, J.E. (1973) Leaching of 14C-SD 14114 from Soil.
Unpublished report TIR-22-112-73, from Shell International.
Natoff I.L. (1973) Toxicity studies on the pesticide SD 14114: acute
oral toxicity studies in the mouse, rat, and rabbit of the metabolite
SD 31723. Unpublished report from Tunstall Laboratory submitted to the
World Health Organization by the Shell Chemical Company.
Pickering, R.G. (1973a) Toxicity studies on the pesticide SD 14114:
Studies on the origin of the increase serum alkaline phosphatase
activity (SAP) in rats fed SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Pickering, R.G. (1973b) Toxicity studies on the pesticide SD 14114:
The reversibility of the increase in rat serum alkaline phosphatase
(SAP) activity produced by SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Samules, G.M.R. and Dix, K.M. (1972) Toxicology of the Pesticide SD
14114: Comparative oldema assay of organotin compounds for oldema
formation in the central nervous system of rate. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
Shell Chemical Company.
Shell Chimie S.A. (1972-1974) Reports TIR-26-131-72, TIR-26-197-73 and
TIR-26-101-74; unpublished.
Shell Chimie S.A. (1973) Reports TIR-26-119-73 and TIR-22-126-73,
unpublished.
Shell Chimie S.A. (1975) Reports BEGR 0019.75 and BEGR 0020.75,
unpublished.
Shell Chirnie S.A. (1976) Report BEGR. 0068.76, unpublished.
Shell Development Co. G.L.C. Methods for residue analysis of
fenbutatin oxide. Unpublished methods MMS-R-345-1, MMS-R-391-1 and
WAMS 215-1 (SAMS-1).
Shell Development Co. (1972) Fate of 119Sn (SD 14114) on apple
trees. Unpublished report.
Shell Development Co. (1972, 1973) Reports TIR-26-113-72 and
TIR-26-197-73 and TIR-26-101-74, unpublished.
Shell Development Co. (1974a) Fate of 119Sn (SD 14114) on Orange
leaves and Oranges. Unpublished report.
Shell Development Co. (1974b) Reports TIR-24-178-74 and TIR-24-238-74,
unpublished.
Shell Development Co. (1974c) Report TIR-26-116-74. Unpublished
report.
Shell International (1971,1976) Residue data from Shell Chimie S.A.,
Shell Development Go. and Shell Research Ltd., Unpublished,
Shell International (1973a) 119Sn-SD 14114 Metabolism Study in Soil
under Aerobic and Anaerobic conditions. Unpublished reports.
Shell International (1973b) 119Sn-SD 14114 Metabolism study in
sterilized and unsterilized soil. Unpublished report.
Shell International (1973d) Photochemical Degradation of SD
14114-119Sn on a glass surface. Unpublished report TIR-22-113-73.
Shell International (1974) Degradation of 14C-SD 14114 after
exposure on soil for 18 month. Unpublished report TIR-22-102-74.
Shell Research Ltd. Residue analysis of SD 14114 (fenbutatin oxide)
and its main metabolite SD 31723. Unpublished method WAMS 212-1 (SAMS
212-1).
Simpson B.J. (1972a) The toxicity of the pesticide SD 14114: Summary
of results of preliminary experiments. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J. (1972b) Toxicity studies on the pesticide SD 14114:
Effect of SD 14114, incorporated in the diet, on the growth rate of
male rats. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Co.
Simpson B.J. and Dix, K.M. (1972c) Toxicity studies on the pesticide
SD 14114: acute and 90 day feeding studies in the rat of the
metabolite SD 31723. Unpublished report from Tunstall Laboratory
submitted to the World Health Organization by the Shell Chemical
Company.
Simpson B.J. and Thorpel B. (1973a) Toxicity studies on the pesticide
SD 14114: Three month feeding study in rats. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J., Grainville, G. and Doak, S.M. (1973b) Toxicity studies
on the pesticide SD 14114: Two year oral experiment in rats.
Unpublished report from Tunstall Laboratory submitted to the World
Health Organization by the Shell Chemical Company.
In another experiment apples were treated in a similar way either with
the parent chemical or with SD 31723 and analysed six weeks later. The
result indicated that the rate of breakdown of SD 31723 exceeded that
of fenbutatin oxide itself. It was concluded that fenbutatin oxide is
degraded firstly to SD 31723, and to SD 33608 and eventually to
inorganic tin compounds. Since SD 31723 was degraded faster than the
parent compound, it is unlikely to build up treated crops and would
therefore not be expected to form an appreciable part of any residue
present.
Similar experiments were carried out on oranges (Shell, 1974a). Leaves
were treated with formulated 119Sn-labelled fenbutatin oxide at a
concentration of 39.5 mg/1 and analysed at intervals up to nine months
after treatment. 40 days after the treatment 65% of the applied
radio-activity was still present in the leaves, of which 71% was
unchanged fenbutatin oxide, 3.4% SD 31723, 0.5% SD 33608 and the rest
inorganic tin. At the end of the 9-month period the total
radio-activity present had declined to about 11% of that applied, and
of this almost 60% was still present as fenbutatin oxide; SD 31723
accounted for about 3% and SD 33608 for less than 1% whilst inorganic
tin made up the remainder (Shell, 1974a).
In other experiments oranges with a diameter of 3-4 cm. were similarly
treated at a rate corresponding to 0.2/kg on the anticipated weight at
harvest 6 months later. Some of the oranges were again treated prior
to harvest to give a total dose corresponding to 0.44 mg/kg on harvest
weight and left on the tree for a further 2 months.
The fruit after picking was separated into peel, juice and pulp which
were separately analysed. No radio-activity was detected in the juice
or in the pulp of any of the samples, at the limit of determination
assessed to be 0.001 mg/kg (calculated as fenbutatin oxide). Residues
in the peel of the fruit harvested 6 months after a single treatment
averaged 0.095 mg/kg and 2 months after a second treatment 0.172
mg/kg.
In both instances approximately 6% of the residue was soluble in
organic solvents and where analysed was found to consist largely
(about 90%) of unchanged fenbutatin oxide (Shell, 1974a).
These results therefore confirmed those obtained on apples.
Residue data from SD 31723 in crops
An analytical method for the routine determination of SD 31723 was
used on numerous samples taken from crops treated in the field with
fenbutatin oxide. Residues on SD 31723 were generally below the limit
of determination (usually in these analyses 0.1-0.2 mg/kg, except in
one set of strawberry samples, where the limit was 0.04 mg/kg).
Positive residues were found only in one trial with peaches, where
levels up to 0.3 mg/kg occurred; the residue was solely in the skin.
These data are in line with the observations in other experiments that
residues from fenbutatin oxide are sometimes higher in peaches than in
the other crops studied (Shell, 1976).
In storage and processing
Since fenbutatin oxide is moderately stable, insoluble in water and
remaining largely on the exterior of treated crops, it is clear that
processing which affects the surface of the crop may considerably
reduce the residue levels; some details are given below.
Washing Experiments with apples and citrus showed that fenbutatin
residues may be considerably reduced by washing. 60% or more of the
original residue was removed by this process.
Peeling The removal of the outer peel of the fruit or other
commodity reduces the residue by at least 75%, depending on the
commodity.
Juice extraction Data on residues in extracted citrus juice are
generally below the limit of determination (0.02-0.05 mg/l). In only
one instance was a small residue of fenbutatin oxide (0.08 mg/1)
found.
Apple juice has been obtained with similarly low residues (Shell,
1972-1974). From studies with the radio-labelled compound, residues
would not be expected to occur in the interior of the apples and the
data on juice confirm that the residues on the surface of the fruit
are not extracted to an appreciable extent with the juice during its
preparation. No residues of the main metabolite SD 31723 have been
found in any juice from treated fruits (Shell, 1972, 1974a).
Vinification Wine from grapes containing up to 5.6 mg/kg of
fenbutatin oxide in the whole fruit contained less than 0.02 mg/1, the
limit of determination (Shell, 1974b, 1975). Similarly the breakdown
product SD 31723 was not found in the wine at the limit of
determination of 0.1 mg/1 (Shell, 1975).
In soil
Residue degradation in sterile and non-sterile soils. The residue
degradation of 119Sn-labelled fenbutatin oxide was studied in a
steam-sterilized and unsterilized Hanford sandy loam (particles 2
11.1%; between 2 and 20 21.9%; 20 67%; organic material 0.8-1%; pH
4.6-6.5). 10 mg/kg 119Sn-labelled fenbutatin oxide was mixed into
both soils which were stored in the same room at ambient temperature.
Almost all the 119Sn was organosoluble. Fenbutatin oxide decreased
initially faster in sterile than in normal soil. However the rate of
decrease later became less in sterile than in live soils. It is
suggested that steam sterilisation activates catalytically active
sites on the soil so that more molecules can be degraded initially.
The subsequent decrease in rate as compared with live soils indicates
that microbial degradation, which occurred in the latter became the
main factor in degradation. No accumulation of degradation products
except 119Sn could be 254 fenbutatin oxide measured (Shell, 1973b)
see Table 12.
Metabolism in soil under aerobic and anaerobic conditions
In other experiment in which 10 mg/kg 119Sn-labelled fenbutatin
oxide was mixed into the same soil, the residue degradation was
studied under aerobic and anaerobic conditions. One set of soil
samples was kept under aerobic conditions at ambient temperatures in a
glasshouse; another set was kept under nitrogen after an initial 30
days aerobic period. The amount of solvent-extractable radio-activity,
which was nearly all 119Sn-fenbutatin oxide, decreased slowly during
the 180 days of the experiment. No significant differences could be
found between the rates of degradation under aerobic and anaerobic
conditions (Shell, 1973a).
TABLE 12. Radio-activity extracted from sterile and non sterile soil at intervals after treatment
with fenbutatin oxide
119Sn as mg/kg fenbutatin oxide equivalents
Days after sterile soil live soil
treatment
0 10.1 10.1
30 9.3 9.8
60 9.2 9.1
90 9.0 8.6
120 8.7 8.5
180 8.5 7.9(Shell, 1973b)
The degradation of 14C-labelled fenbutatin oxide was also studied in
Hanford sandy loam under outdoor field conditions. The compound was
applied at a rate of 1.5 kg/ha either as a single application or as
repeated applications, each at the same rate, at six-monthly
intervals. The degradation followed first order kinetics during the
first 12 months and accelerated considerably during the next six
months so that one-half of the 14C-fenbutatin still present after 12
months was degraded during the following 6 months. A similar increase
in the degradation rate was also found in the experiments in which
14C-fenbutatin oxide was applied repeatedly. Since the environmental
conditions did not vary significantly from one year to the other, it
is suggested that adaptation of micro-organisms to the compound was
the primary cause of the increase. No accumulation of the degradation
products SD 31723 or SD 33608 could be detected at any time (Shell,
1974).
Under the field conditions 14C-labelled fenbutatin oxide applied to
the soil surface remained mainly in the top 7.5 cm. No measurable
amounts or very low residues were found in the 22.5-30 cm layer (Table
13).
TABLE 13. Carbon-14 extractable from soil after a single application of fenbutatin
oxide
Depth of soil 14C, as mg/kg fenbutation oxide equivalents after
0 4 months 12 months 18 months
0-7´ cm 1.51 1.04 0.66 0.27
7´-15 cm -.- 0.06 0.06 0.03
15-22´ cm -.- 0.05 < 0.02 0.04
22´-30 cm -.- 0.03 < 0.02 <0.02
Mobility in soil and leaching
A sample of Hanford loam, which had been treated with 14C-labelled
fenbutatin oxide and aged outdoors for 4 months, was placed on top of
a column of the same but untreated soil, 30 cm long. The soil was
saturated with water and leached by applying a total of 50 cm water
over a period of 51 days. The 14C content in each of the daily
effluent samples was below the detection limit of 0.002 mg/kg
equivalents of fenbutatin oxide. The soil on top of the column
contained 1.22 mg/kg equivalents of fenbutatin oxide initially and
1.16 mg/kg at the end of the experiments.
The four 7.5 cm. layers of the column contained 14C equivalent
respectively to 0.02, 0.017, < 0.01 and < 0.01 mg/kg of fenbutatin
oxide at the end of the experiments (Loeffler, 1973).
In air and UV light
Thin layers of 119Sn fenbutatin oxide on a glass surface when
exposed to sunlight slowly decomposed to the derivative SD 31723 and
to more polar compounds such as inorganic tin salts. After 230 hours
of exposure, 81% of the initial 119Sn deposit was extractable by
organic solvents and 6% by aqueous solvents. In this sample 76% of the
organo-soluble 119Sn consisted of unchanged fenbutatin oxide and
21.3% of the degradation product SD 31723 (Shell, 1973d).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE, OR AT
CONSUMPTION
No information available.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods of analysis for fenbutatin oxide residues
on fruit crops and fruits or vegetables have been developed (Shell,
undated, 19746).
Three modifications of a basic method are available. The finely
divided crop sample is extracted with methylene chloride,
concentrated, diluted with hexane and treated with hydrochloric acid,
to form a chloro-derivative. The resultant solution is then cleaned up
on an acid alumina column before determination by GLC. The method has
been adapted for use on samples of milky animal tissues and other
materials (Shell, undated). The modifications differ only in detail
and the choice of the method is dependent on the equipment and
solvents available, the commodities to be analysed and the analyst's
personal preference. Limits of determination are in general 0.01
- 0.05 mg/kg.
Tests have been carried out to determine the loss of residue during
storage at deep-freeze temperatures (-15°C) whilst awaiting analysis.
No changes in residue levels could be detected during a period of
16-18 months (Shell, 19740).
Two methods have also been developed for the determination of the
principal breakdown product of fenbutatin oxide, SD 31723. Both
involve extraction of the finely divided sample with methylene
chloride as for the parent compound. The extracts are subjected to
clean-up on an alumina or silica gel column and the eluates are
concentrated and analysed by a thin layer chromatographic technique.
After development the plates are treated with a suitable reagent to
visualize the tin-containing products. The determinations are
completed by visual comparison of the colour and size of the spots
with standards run under the same conditions. A limit of determination
of 0.1-0.2 mg/kg SD 31723 can be achieved (Shell, undated, undated).
APPRAISAL
Fenbutatin oxide is used as an acaricide as a foliar application on a
considerable scale on a wide variety of fruits and fruiting vegetables
in various countries.
Fenbutatin oxide is mainly marketed as a 50% wettable powdery but also
as a suspension concentrate. The rates of application vary depending
on crop, pest and method of application; normal rates are usually 1-2
kg a.i./ha.
The recommended pre-harvest intervals in various countries vary
considerably for one and the same crop.
Residue data from supervised trials were obtained from several
countries. Residues do not penetrate through the skin of treated fruit
to any great extent, and in the pulp are therefore generally below the
limit of determination.
Extensive data are available on the fate of the residues in plants and
on the levels of residues in products of animal origin arising from
the feeding of treated commodities including the pulp of treated
fruits. On apples after 33 days the remaining residue consists of
about 85% unchanged parent compound, 5% of the principal organotin
metabolite SD 31723 and about 10% of inorganic tin.
The degradation of the main metabolite SD 31723 is faster than that of
the parent compound and thus the metabolite will in general not form
an appreciable part of any residue present in practical conditions.
Occasionally residues not exceeding 0.3 mg/kg of the parent compound
could be found in the kidneys of cows fed with treated feeds and it
was calculated that these residues mould not exceed 0.2 mg/kg in
practical circumstances; in meat and milk, residues were undetectable
(i.e. below 0.02 mg/kg).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national maximum residue limits for fenbutation oxide are
established or under consideration:
Country Commodity maximum residue limit, mg/kg
Australia Apples, pears, peaches 3
Belgium Apples, pears 1
Fed. Rep. of Germany Pome fruit 2
The Netherlands Apples, pears, cherries,
plums 1
Blackberries,
raspberries, strawberries,
black- and red currants 0.2
gherkins (glasshouse
and outdoors) 1
Cucumbers* , melons*,
bell-peppprs *,
eggplant *, tomatoes * 0.3
Italy Apples, pears, apricots,
cherries, peaches,
plums, citrus fruits,
grapes, cucumbers,
tomatoes 0.5
South Africa Apples, pears, peaches,
citrus fruits 2
Switzerland Pome fruit, stone fruit, grapes 1.5
U.S.A. Apples, pears, citrus fruits 4
Liver and kidney of cattle,
goats, horses, pigs and sheep 0.3
Dried apple pomace 20
Dried citrus pulp 7
*glasshouse crops
Fenbutatin oxide applied in field conditions to the soil remains in
the upper layers and is slowly degraded, forming non-mobile
metabolites. Washing fruits and vegetables removes 60% or more of the
original residue, and removal of the outer peel of apples or citrus
reduces the residue by at least 75%.
Gas-chromatographic methods for residue analysis for fenbutatin oxide
in fruit crops, fruits of vegetables, milk and animal tissues are
available, which are suitable or can be adapted for regulatory
purposes. Methods for the determination of the principal metabolite
have also been developed.
RECOMMENDATIONS
The following maximum residue limits for fenbutatin oxide are
recommended. They refer to fenbutatin oxide, excluding any
metabolites.
Commodity Limit, mg/kg Pre-harvest interval
on which
recommendations
are based
Dried apple pomace 20 -
Dried citrus pulp 7 -
Peaches 7 14-21
Apples and pears 5 14
Cherries (sour and sweet) 5 14-21
Citrus fruits 5 7-14
Plums 3 7-14
Strawberries 3 7-14
Fruits of vegetables
(cucumbers, eggplants,
gherkins, melons, sweet
peppers, tomatoes) 1 3-7
Liver and kidney of cattle,
goats, horses, pigs and
sheep 0.2
Meat of cattle, goats,
horses,
pigs and sheep 0.02*
Milk 0.02*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the EEG findings in the dog.
2. Elucidation of species differences in toxic effects on rabbit
testes.
REFERENCES
Dean, B.J., Doak, S.M.A. and Funnell, J. (1972a) Toxicity studies with
SD 14114: genetic studies with SD 14114 in micro-organisms in vitro
and in the host-mediated assay. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Dean, B.J., Doak, S.M., Perquin, B.D. and Senner, K. (1972b) Toxicity
studies with SD 14114: the cytogenetic investigation of bone marrow
cells of mice after a single oral dose of SD 14114. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
the Shell Chemical Company.
Dean, B.J. and Doak, S.M. (1972c) Toxicity studies with SD 14114: the
effects of a single oral dose of SD 14114 on dominant lethal mutations
in male mice. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Company.
Dix, K.M. and Wilson, A.B. (1973) Toxicity studies with SD 14114:
Teratological studies in rabbits given SD 14114 orally. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Granville, G.C., Simpson, B.J. (1973a) and Doak, S.M. Toxicity studies
on the pesticide SD 14114: an 18 month study in mice. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by Shell Chemical Company.
Granville, G.C. and Dix, K.M. (1973b) Toxicity studies on the
pesticide SD 14114: Two year oral toxicity test in dogs. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Hine, C.H., Eisenlord, G. and Loguvan, G.S. (1973) Results of
reproduction study of rats fed diets containing SD 14114-V over three
generations. Unpublished report from Hine Laboratories Inc. submitted
to the World Health Organization by the Shell Chemical Company.
Loeffler, J.E. (1972) The fate of Ingested 119Sn SD 14114 in Rats.
Unpublished report from Shell Development Company, Biological Sciences
Research Center submitted to the World Health Organization by Shell
Chemical Co.
Loeffler, J.E. (1973) Leaching of 14C-SD 14114 from Soil.
Unpublished report TIR-22-112-73, from Shell International.
Natoff I.L. (1973) Toxicity studies on the pesticide SD 14114: acute
oral toxicity studies in the mouse, rat, and rabbit of the metabolite
SD 31723. Unpublished report from Tunstall Laboratory submitted to the
World Health Organization by the Shell Chemical Company.
Pickering, R.G. (1973a) Toxicity studies on the pesticide SD 14114:
Studies on the origin of the increase serum alkaline phosphatase
activity (SAP) in rats fed SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Pickering, R.G. (1973b) Toxicity studies on the pesticide SD 14114:
The reversibility of the increase in rat serum alkaline phosphatase
(SAP) activity produced by SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Samules, G.M.R. and Dix, K.M. (1972) Toxicology of the Pesticide SD
14114: Comparative oldema assay of organotin compounds for oldema
formation in the central nervous system of rate. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
Shell Chemical Company.
Shell Chimie S.A. (1972-1974) Reports TIR-26-131-72, TIR-26-197-73 and
TIR-26-101-74; unpublished.
Shell Chimie S.A. (1973) Reports TIR-26-119-73 and TIR-22-126-73,
unpublished.
Shell Chimie S.A. (1975) Reports BEGR 0019.75 and BEGR 0020.75,
unpublished.
Shell Chirnie S.A. (1976) Report BEGR. 0068.76, unpublished.
Shell Development Co. G.L.C. Methods for residue analysis of
fenbutatin oxide. Unpublished methods MMS-R-345-1, MMS-R-391-1 and
WAMS 215-1 (SAMS-1).
Shell Development Co. (1972) Fate of 119Sn (SD 14114) on apple
trees. Unpublished report.
Shell Development Co. (1972, 1973) Reports TIR-26-113-72 and
TIR-26-197-73 and TIR-26-101-74, unpublished.
Shell Development Co. (1974a) Fate of 119Sn (SD 14114) on Orange
leaves and Oranges. Unpublished report.
Shell Development Co. (1974b) Reports TIR-24-178-74 and TIR-24-238-74,
unpublished.
Shell Development Co. (1974c) Report TIR-26-116-74. Unpublished
report.
Shell International (1971,1976) Residue data from Shell Chimie S.A.,
Shell Development Go. and Shell Research Ltd., Unpublished,
Shell International (1973a) 119Sn-SD 14114 Metabolism Study in Soil
under Aerobic and Anaerobic conditions. Unpublished reports.
Shell International (1973b) 119Sn-SD 14114 Metabolism study in
sterilized and unsterilized soil. Unpublished report.
Shell International (1973d) Photochemical Degradation of SD
14114-119Sn on a glass surface. Unpublished report TIR-22-113-73.
Shell International (1974) Degradation of 14C-SD 14114 after
exposure on soil for 18 month. Unpublished report TIR-22-102-74.
Shell Research Ltd. Residue analysis of SD 14114 (fenbutatin oxide)
and its main metabolite SD 31723. Unpublished method WAMS 212-1 (SAMS
212-1).
Simpson B.J. (1972a) The toxicity of the pesticide SD 14114: Summary
of results of preliminary experiments. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J. (1972b) Toxicity studies on the pesticide SD 14114:
Effect of SD 14114, incorporated in the diet, on the growth rate of
male rats. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Co.
Simpson B.J. and Dix, K.M. (1972c) Toxicity studies on the pesticide
SD 14114: acute and 90 day feeding studies in the rat of the
metabolite SD 31723. Unpublished report from Tunstall Laboratory
submitted to the World Health Organization by the Shell Chemical
Company.
Simpson B.J. and Thorpel B. (1973a) Toxicity studies on the pesticide
SD 14114: Three month feeding study in rats. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J., Grainville, G. and Doak, S.M. (1973b) Toxicity studies
on the pesticide SD 14114: Two year oral experiment in rats.
Unpublished report from Tunstall Laboratory submitted to the World
Health Organization by the Shell Chemical Company.
 Other information on identity and properties
(a) Composition of the technical Product:
The technical material contains 97% w/w minimum of fenbutation oxide;
the main impurities are related organo-tin compounds.
(b) Physical and chemical properties
Physical state: crystaline solid
Molecular weight: 1053
Melting point: 138-139°C
Volatility: not volatile
Solubility: insoluble in water, (approx. 5x10-6 g/1 at 23°C)
slightly soluble in most organic solvents;
solubility at 23°C in acetone approx 6 g/l in
dichlororomethane 370 g/l in benzene 140 g/l
Stability: stable to sunlight. Water hydrolyses fenbutatin
oxide to tris-(2-methyl-2-phmylpropyl)tin
hydroxide; this in reconverted to the parent
compound slowly at room temperature and rapidly at
98°C.
Formulations:
Wettable powder 500 g a.i./kg and suspension concentrate 500 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Groups of 2 male and 2 female rats were fed an average of 19.5 ppm
119Sn-labelled fenbutatin oxide (with or without a 3 day withdrawal
period before killing following 3 or 6 day exposure) after 1, 3 or 6
days to study the absorption, distribution and excretion of
fenbutatin. Most of the administered 119Sn was rapidly excreted in the
faeces following multiple dosing (80-90% of the administered 119Sn).
Essentially no 119Sn was excreted via the urine. The GI tract
contained approximately 30, 15 and 5% of the administered 119Sn for
animals sacrificed after 1, 3 or 6 days of dosing. The highest tissue
concentrations of 119Sn were found in liver (0.015-0.09 mg/kg),
kidneys, (0.015 0.055 mg/kg) and fat (< 0.01-0.065 mg/kg). No 119Sn
was detected in brain or bone tissue (Loeffler, 1972). See also the
section "Fate of residues", "In animals".
Biotransformation
Analysis of the faeces from rats dosed with 119Sn-labelled fenbutatin
oxide indicated that ß, ß-dimethylphenethylotamoic acid and 1, 1, 3,
3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxdistannoxane are the
major metabolites (Loeffler, 1972).
Effects on enzymes and other biochemical parameters
In starch gel electrophoretic studies on sera from rate fed either 0
or 600 ppm in the diet for 6 months (2 buffer systems) control and
test animal sera were identical. The effect of 1-phenylanatine or heat
was similar on both sera. Comparisons with intestinal, bone, liver and
kidney alkaline phosphatase indicated the serum alkaline phosphatase
was similar to intestinal alkaline phosphatase. Further studies on rat
sera derived from animals fed 0 or 600 ppm in the diet for one year,
utilizing heat inactivation, selective substrates and inhibitors,
confirmed the absence of any difference between control and test
animal sera, as well an confirming the similarity of the serum
alkaline phosphatase to intestinal alkaline phosphatase (Pickering,
1973a). In a further series of studies by the elevation of serum
alkaline phosphatase observed in rate after administration of 300 ppm
fenbutatin oxide in the diet for 90 to 120 days may shown to be
completely reversible following one months, withdrawal from exposure.
Limited data indicate that the reversal of elevated serum alkaline
phosphataze in the test rate may be completed within 7 days of
withdrawal of the fenbutatin oxide (Pickering, 1973b).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Following preliminary studies to determine suitable dose levels and to
confirm the induction of brain oedema by triethyltin bromide, a
comparative oedma assay of five organo-tin compounds (including
fenbutatin oxide, and its metabolite 1, 1, 3,
3-tetrakis-(ß, ß-dimethylphenethyl)-1, 3-dihydroxydistannoxane) for
oedma formation in the central nervous system of rats, was undertaken.
Groups of 15 male rats were intubated with corn oil (10 mg/kg, 40
mg/kg triethyltin bromide, 1000 mg/kg, fenbutatin oxide, 100 mg/kg 1,
1, 3, 3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxydistannoxane
and sizable doses of two other tin compounds. The triothyltin bromide
group showed increased brain water content after 48 hrs. Seven of ten
brains examined at 24 and 48 hrs showed spongeosis of white matter in
the cerebellum and sometimes of the corpus callosum and pons. No
histological changes were observed in 4 rats killed after 6 hrs.
Fenbutatin oxide (24 and 48 hr. kills), and the metabolite (6, 24 and
48 hr kills) did not show either increased water content or abnormal
brain histology, although rats on both compounds showed signs of
intoxication (Samuels and Dix, 1972),
Special studies on mutagenicity
Micro-organisms
In plate tests using Serratia marcesens, strains HY/alpha 13, a
histidine-requiring auxotroph, HY/alpha 21, a leucine-requiring
auxotroph, and CV/r c3/alpha 742, a thiamine-requiring spontaneous
auxotroph, 50 µl hexane solution (which resulted in a precipitate of
fenbutatin oxide on the agar plate) did not increase the reversion
rate in any strain, nor was growth inhibited, (Dean, et al., 1972a).
Neither conversion rate nor cell survival was affected in
Saccharomyces cerevisiae following exposure for 5 or 16 hrs in pH7
phosphate buffer containing 0.0025 or 0.005 µg fenbutatin oxide/ml
solution. In the same system, 20 µg/ml in suspension was ineffective
in altering conversion rate or cell survival (Dean et al., 1972a).
Mice
Groups of 8 male and 8 female mice were treated with 0.005 or 1000 mg
fenbutatin oxide/kg, in 1% carbomethyl cellulose. Four
animals/sex/group were killed 90 minutes after i.p. colcemid
administration and femurs were removed. One hundred bone marrow
cells/animal were examined. No significant differences in incidence of
chromosomal aberrations were observed (Dean et al., 1972b).
In three or four host-mediated assay experiments using Saccharomyces
cerevisiae and dose levels of 250 or 500 mg fenbutatin oxide/kg,
conversion rate was unaffected. In the fourth replicate, conversion
rate was marginally increased at 250 but not at 500 mg/kg (Dean et
al., 1972a)
In an 8 week dominant lethal study, 8 male mice/group were dosed once
with 250 or 500 mg fenbutatin oxide/kg, and were then mated with 3
females each week. The control group comprised 16 untreated males. No
significant differences in percentage pregnancies, implant incidence
or early resorption rate were noted between control and fenbutatin
oxide treated groups. A positive control group of 8 male mice (200 mg
Endoxan) showed reduced pregnancy rate and increased resorption and
decreased implant rates during the first 3 matings after, dosing.
(Dean and Doak, 1972c).
Special studies on teratology
Three groups of 15 pregnant rabbits were dosed by gelatin capsules on
days 6-18 inclusive of gestation (mating day = day 0), with 3 or 10 mg
fenbutatin oxide/kg or with 37.5 mg thalidomide/kg. Twenty-six rabbits
were used as capsule-only treated controls. Rabbits were killed on day
28 of pregnancy. A repeat experiment differed only in that 20 pregnant
females/dose level and 30 controls were used, autopsy being on day 29.
Foetuses were incubated for 24 hrs. following removal from the uterus,
after which approximately 1/3 were examined for visceral
abnormalities, and 2/3 for skeletal defects. Prior to clearing for
skeletal examination, viscera from these animals were also examined
for defects. Although some maternal deaths occurredy these were random
between groups. Mean live litter size, resorption rate, foetal loss,
survival of incubated foetuses, and incidence of anomalies were not
affected by fenbutatin oxide at either close level. Cromn-rump length
and foetal weight were comparable to controls except at 3 mg/kg in the
repeat experiment, where a significant increase was noted. The
positive control group behaved as expected (Dix and Wilson, 1973).
Special study on reproduction
In a standard 3 generation, 2 litter/generation reproduction study,
groups of 10 male and 20 female rats were fed O, 50, 100 or 300 ppm
fenbutatin oxide (985 purity). It should be noted that rate of the Fo
generation commenced on diet and were mated at 100 days of age, and
that litters were culled to 10 pups/litter on post-partum day 5.
Necropsy was performed on 10 male and 10 female F3b weanlings from
the 0 and 300 ppm, and on 5 weanlings/sex from the 50 and 100 ppm
groups. In the 300 PPM group, parents and pups were smaller, hyper
active and irritable; mean litter size was slightly reduced in the
F1b litters, and in the two F3 generations survival to weaning was
reduced at 300 ppm and testicular organ/body weight ratio was reduced
in the F3b weanlings. Necropsies of F2b adults did not show any
compound-related changes. No consistent effects were observed at 50 or
100 ppm with respect to fertility, gestation, viability or lactation
indices, litter size, litter weight, or necropsy of F3b weanlings
Mine et al., 1973).
Special studies on carcinogenicity
Groups of 48 male and 48 female Carworth Farm No. 1 strain mice were
fed 50, 100, 100 or 600 ppm fenbutatin oxide in the diet for 18
months. A control group comprised 96 males and 96 females. All animals
were examined grossly at death. Microscopic examination was undertaken
on all animals dying during the study and on all 600 ppm mice, on
10/mice/sex at 50, 100 and 300 PPM, and on 45 controls/sex at 18
months. Survival rate was comparable in all groups and exceeded 75%.
There was a high incidence of tumours in all groups. However there was
no indication of compound-or dose-related tumor induction (Granville
et al., 1973a).
Acute Toxicity
TABLE 1a. Acute toxicity of fenbutatin oxide
Species Route Sex LD50(mg/kg) Reference
Mouse Oral - 1450(1223-1659) Simpson 1972a
Rat " - 2631(1929-4659)
Dog Oral(capsule) - >1500
Rabbit Oral - 1500-3000
Rat Dermal - >1000
In mice and rats, death occurred within 10 days. Signs were dyspnoeal
stained coat and transient diarrhoea. In dogs, vomiting and diarrhoea
occurred. In rabbits convulsions occurred at the nighest dose, with
death within 24 hrs. Lower doses caused death up to 18 days after
dosing. Weight loss was still apparent 3 weeks after dosing, Autopsy
revealed damage to the gastric mucosa, massive fatty changes in liver,
reduced spermatogenesis, and multinucleated spermatid production
(Simpson, 1972a).
TABLE 1b. Acute toxicity of 50% wettable powder
Species Route Sex LD50 (mg/kg) Reference
Rat Oral - 1000 Simpson 1972a
Dermal - >1000
TABLE 1c. Acute toxicity of metabolite*
Species Route Sex LD50(mg/kg) Reference
Mouse Oral M 420 Natoff, 1973
F 622
Rat Oral M,F 242
Rabbit Oral M >675
F 300-450
Rat Oral - 132(102-176) Simpson & Dix,
Mouse Oral - 1051(824-1440) 1972c
* 1, 1, 3, 3-tetrakis(ß,ß -dimethylphenethyl)-1,3-dihydroxydistannoxane.
The signs of intoxication in all species were severe diarrhoea,
followed by loss of body weight which was recorded for rabbits, and
inanition and emaciation in all species. Deaths occurred up to 16 days
after dosing the mice, 14 days after dosing the rats and 26 days after
dosing the rabbits. The observation period for rabbits was curtailed
at 28 days.
Short term studies
Rat
Five groups of 5 male and 5 female rats were fed 0, 30, 100, 300 or
1000 ppm fenbutatin oxide in the diet for 5 weeks. Body weight was
significantly reduced, as was food intake at 300 and 1000 ppm.
Reductions in absolute brain, heart, liver, spleen and kidney weights
were noted in both sexes at 1000 ppm. Spleen weight was reduced in
males at 300 ppm and brain, liver, spleen and kidney weight was
reduced at 300 ppm in females. Serum alkaline phosphatase was
increased in both sexes and SGPT levels were elevated in females at
1000 ppm (Simpson, 1972a).
Three groups of five male rats were used in a pair feeding study, one
group being a normal control, the second being fed 600 ppm, and the
third, pair-fed at the food intake levels of the 600 ppm group, The
study ran for 5 weeks. Results (daily body weights) indicated that the
reduction in body weight at 600 ppm can be totally accounted for by
reduced food intake (Simpson, 1972b).
Groups of 6 male and 6 female rats were fed 50, 100, 300 or 600 ppm
fenbutatin oxide (97%) in diet for 3 months. Concurrent controls
comprised 12 male and 12 female rats. Health and behaviour were
comparable in all groups. Body weight gain was reduced in males at 600
ppmv and in females at 300 and 600 ppm. Food intake was reduced in
males at 600 ppm, and in females during the first 3 or 4 weeks of the
study at 300 and 600 ppm. Absolute organ weight of liver and kidney
were reduced in both sexes, heart in females, and brain and spleen in
males at 600 ppm. At 300 ppm, liver, spleen and kidney absolute
weights were reduced in males. Organ/body weight ratios were elevated
for brain in males at 600 ppm, and in females at 300 and 600 ppm.
Heart and liver weight ratios were elevated in females at 600 and
heart at 300 ppm. Testes weight ratio was elevated in males at 300 and
600 ppm. Haematology (haemoglobin, PCV, RBC, WBC, RCV, MOH,
prothrobmin time and KOCT) was comparable in all groups. Clinical
chemistry determinations were comparable for total protein, SGOT, and
electrolytes (K, Na, Cl). BUN and SAP in males were elevated at 300
and 600 ppm in males, as was SGPT at 300 PPm. In females BUN was
elevated at 100 ppm and above. Serum protein fractions were comparable
in all groups. No compound-related gross or histopathological effects
were noted, (Simpson and Thorpe, 1973a).
Three groups of rats, 20/sex/control and 10/sex/test group, were fed
0, 3, 10, 30, 100 or 300 ppm of 99% 1, 1, 3, 3-tetrakis (ß,
ß-dimethylphenethyl)-1,3-dihydroxydistannoxane for 90 days. Health and
behaviour were comparable in all groups. Body weights of 300 ppm males
were significantly decreased throughout the study. This was also true
for the females in the 30, 100 and 300 ppm group but the suppression
was not significant. Slight reductions in haemoglobin, erythrocyte
count and packed cell volume occurred in the 300 ppm males. No changes
were noted in any of the other groups of either sex. The other
clinical values of animals killed after 13 weeks showed no
dose-related differences when compared to the control values. Gross
and microscopic examination of a wide range of tissues from all
control, 100 and 300 ppm animals revealed no consistent changes
associated with exposure to the metabolite. Organ weights of males at
13 weeks did not differ from control weights, the organ body weight
ratios were increased for brain, heart, liver, kidneys and testes in
this group and for the liver in the 100 ppm male group. Brain, spleen
and kidney weights were reduced in the 300 ppm females, (Simpson and
Dix, 1972c).
Dog
Five groups of 2 male and 2 female beagle dogs were orally dosed
(gelatin capsules) with 0, 10, 30, 100 or 300 mg fenbutatin oxide/kg
for 5 weeks. Slight vomiting and diarrhoea occurred at 100 and 300
mg/kg, which was associated with decreased body weigh. At 3O mg/kg and
above, serum alkaline phosphate was elevated. No pathological changes
were observed (Simpson, 1972a).
Groups of dogs (8/sex/control and 4/sex/test group) were administered,
by capsule, 0, 2.5, 5, 15, 30 or 60 mg/kg 97% fenbutatin oxide daily
for 2 years. Blood samples were taken every six weeks to measure BUN,
glucose, plasma protein, sodium, potassium, chloride, SGPT, SGOT, and
alkaline phosphatage. Every 12 weeks hemoglobin, packed cell volume,
RBC, WBC, differential WBC counts, prothrombin and
kaolin-cephalin-coagulation times were determined. Urine analysis was
carried out every 3 months. Emesis and diarrhoea occurred in most of
the dosed dogs. These effects continued in some dogs in the 30 and 60
mg/kg/day groups throughout the study, although at a reduced frequency
towards the end of the study. Lower dosage groups showed none of these
effects after the first year. Convulsions occurred in 5 dogs,
predominantly female, during the second year; control, one female; 2.5
mg/kg/day, one female; 15 mg/kg/day, one female and one male; and 30
mg/kg/day, one female. None of the 60 mg/kg/day animals exhibited this
effect. EEG's with simultaneous single lead monitoring of EEG's of one
female each in the 0, 2.5 and 30 mg/kg/day groups revealed a normal
EEG for the control dog and the other 2 dogs showed normal waking
rhythms but abnormal activity during light sleep. The investigators
contend that the lack of dose response relationship suggest a
diagnosis of spontaneous epilepsy not related to exposure to
fenbutatin oxide. General health and behaviour of all other dogs were
similar to control dogs. Significant reductions in rate of weight
gains occurred in both sexes in the toxicity, long term 60 mg/kg/day
group. Males receiving 30 mg/kg/day exhibited no significant rate of
gain. This effect was not evident in females of this group.
Haematological and clinical chemistry and urine data showed no
differences between control and test groups. Necropsies revealed no
gross changes. Microscopic examination, including frozen sections of
liver and kidney stained for lipid, revealed no compound-related
pathological changes in a wide range of tissues. Organ weights were
not affected by exposure to the test compound (Granville and Dix,
1973b).
Long term studies
Rat
Groups of rats (114/sex/control and 72/sex/test group) were fed 0, 50,
100, 300 or 600 ppm 97% fenbutatin oxide for two years. Rats were
killed at 3 and 12 months (12/sex/control and 6 sex/test group) and at
6 months (24/sex/control and 12/sex/test group). Organ weights of
brain, heart, liver, spleen, kidney and testes were recorded and a
wide variety of tissues examined from all rats dying or killed at
interim periods, and from all rats from the 50 and 100 ppm groups.
Blood determinations were made, at each kill period, for haemoglobin,
PCV, RBC, WBC and WBC differential counts; prothrombin and
kaolin-cephalin-coagulation times; BUN; total protein, potassium
sodium and chloride; SGPT, SGOT and alkaline phosphatase. Also serum
proteins were fractionated electrophoretically and estimates made of
albumin and alpha, - ß, and gamma-globin concentrations.
Fenbutatin oxide rendered the diet unpalatable to both sexes causing,
in the early months of the study, significant reductions in food
consumption and body weight gains. Males were more affected than
females. For the remainder of the study all groups exhibited rates of
gain to the control except both sexes receiving 300 and 600 ppm, which
exhibited reduced body weight reduced body weights throughout the
study. BUN levels were increased at the higher levels for the first 6
months but remained within normal limits for the remainder of the
study. Serum alkaline phosphatase activity was increased in both sexes
at 300 and 600 ppm throughout the study, in the 100 ppm males at 6
months, and in the 100 ppm females at 3 months and 2 years. All other
haemotological and clinical chemistry values remained within normal
limits at all times. Survival and behaviour were not affected by
administration of the compound. No compound-related tissue lesions nor
any increase in tumor incidence were found. Organ weights revealed no
compound-related differences except kidney weight reduction in all
treated males and the 600 ppm females at 2 years. No specific
compound-related lesions were seen in the kidney of any treated group.
Absolute and relative testes weights, unaccompanied by hypertrophy,
were noted in the 300 and 600 ppm groups at 2 years. (Simpson et al.,
1973b).
COMMENTS
Fenbutatin oxide is moderately toxic by the oral route producing
severe gastrointestinal irritation. Acute oral administration to
rabbits reduced spermatogenesis and resulted in multi-nucleated
spermatid production. It is excreted rapidly, mostly unchanged in the
faeces, indicating that it is poorly absorbed. In the rat it is
metabolized to ß, ß-dimethylphenethylstannonio acid (SD 33608) and 1,
1, 3, 3-tetrakis-(ß, ß-dimethylphenethyl)-1,3-dihydrox3rdistannoxane.
The latter metabolite and fenbutatin oxide failed to produce oedema of
the central nervous system of the rat at 100 and 1000 mg/kg
respectively. Fenbutatin oxide produced no genotoxic effects in
several in vitro and in vivo systems. Adequate short- and
long-term studies are available in rats and dogs as well as teratology
study study in rabbits, a multi-generation reproduction study in rats,
and a carcinogenicity study in mice. No-effect levels were
demonstrated in rats and dogs and an acceptable daily intake for
humans was established on the basis of these studies. The no-effect
level was established as 15 mg/kg because of emesis and diarrhoea in
dog, and because of the known toxic effects of organic compounds on
the brain a safety factor of 100 was used.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in diet equivalent to 2.5 mg/kg body weight.
Dog: 15 mg/kg body weight.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.03 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenbutatin oxide is used as a specific mitioide against a wide range
of phytophagous mites. It is recommended especially for the control of
the mobile stages on a wide variety of crops, including pome and stone
fruits, citrus fruits, grapes, berry fruits, vegetables and
ornamentals. Recommendations for use on fruit crops and the
recommended pre-harvest intervals are given in Table 2.
The compound is in general used as a foliar spray in a concentration
range of 0.02 -0.05% a.i., corresponding to approx. 0.4 -1.5 kg
a.i./ha. Most applications are required while the fruits are
developing; the number of applications varies with the crop and
regional conditions.
The pre-harvest intervals are seldom less than 7 days, except on some
glasshouse-grown fruits or vegetables; in most instances the
pre-harvest intervals recommended are at least 14 days.
TABLE 2. Use pattern and recommended pre-harvest intervals of fenbutatin oxide
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Apples, Panonychus ulmi 0.0125-0.05% depending Australia 2
Tetranychus urticae on pest and volume of Belgium 28
Tetranychus mc. danieli spray liquid used. Denmark 28
Paratetranychus spp. For concentrated sprays France 7
Bryobia rabrioculus maximum 1.5 kg a. i./ha. Fed. Rep. of
Germany 14
Eotetranychus carpini Not more than 3 applications the
borealis whilst trees are bearing fruit. Netherlands 42
Italy 30
Aculus schlechtendali Mexico 14
Portugal 21
S. Africa 14
3 days
on fruit
for
canning
Spain 21
Switzerland 21
USA 14
Pears Panonychus ulmi As for apples As for apples
Tetranychus mc. danieli
Tetranychus urticae
Epitremeris pyri
Citrus Panonychus citri 0.015-0.05 % for Italy 60
Eotetranychus banksi concentrated sprays a Mexico 14
Eotetranychus yumensis maximum of 2 kg/ha is S. Africa 7
Tetranychus urticae recommended. Not more Spain 21
Brevipalpus californicus than 4 treatments in USA 7
Phyllocoptruta oleivora one year.
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Cherries Tetranychus urticae 0.02-0.05%; maximum two Italy 30
applications whilst trees Switzerland 21
are bearing fruits. the
Concentrated sprays max. Netherlands 42
1.5 kg a.i./ha.
Peaches Tetranychus urticae 0.02-0.05% maximum two Australia 2
Panonychus ulmi treatments whilst trees Denmark 28
are bearing fruit. France 7
Concentrated sprays up to Italy 30
1 kg a.i./ha.
S. Africa 21
3 days
on fruit
for
canning.
Spain 21
Switzerland 21
Plums Tetranychus urticae 0.02-0.05% Italy 30
Panonychus ulmi Concentrated sprays up Switzerland 21
to 1 kg a.i./ha. the
Netherlands 42
Grapes Panonychus ulmi 0.02-0.05% France 7
Concentrated sprays maximum Fed. Rep. of
rate up to 1 kg a.i./ha. Germany 28
Italy 45
Spain 21
Switzerland 21
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Strawberries Tetranychus
urticae 0.025-0.05% the
Netherlands only before
blossoming
and/or after
harvest.
Bell-peppers Tetranychus 0.02-0.04% the 3
urticae Netherlands (glasshouse)
Cucumbers Tetranychus 0.02-0.05% Italy 30
urticae the
Netherlands 3::
Tomatoes Tetranychue 0.02-0.5% Italy 30
urtical the
Netherlands 3
(glasshouse)
:: includes gherkins glasshouse and outdoors; cucumbers and melons glasshouse only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained on residues from supervised trials
carried out in various countries on a variety of fruits and fruits of
vegetables as follows.
Citrus fruits: grapefruit, lemons, oranges
Pome fruits: apples, pears
Stone fruits: cherries, peaches, plums
Berry fruits: grapes, strawberries
Fruits of vegetables: bell-peppers, cucumbers, gherkins,
melons, tomatoes
The data obtained are summarized in Tables 3-11 (Shell International,
1971-76). In most of the trials fenbutatin oxide was applied as a 50%
wettable powdery but in a few instances a suspension concentrate
containing 500 g a.i./l was used (referred to as SC in Tables). The
application rates in most of the trials are in conformity with good
agricultural practice. In, some instances higher dosages were also
applied; these excessive rates are indicated in the Tables.
It has been demonstrated, using radio-labelled fenbutatin oxide, that
the residues do not to any great extent penetrate through the akin of
treated fruit (see also section "Fate of residues"). The residues
found when analysing the whole fruit were mainly on or in the skin.
This is also illustrated in samples from some field experiments which
were examined. before and after removal of the peel.
Pome fruits
Apples
The data in Table 3 were mainly obtained from samples of crops treated
according to typical use conditions. The results show considerable
variation according to local conditions.
In most instances residues in whole fruit were below 3 mg/kg
fanbutatin oxide from up to 3 treatments made at recommended rates, up
to 0.05% or 1.5 kg a.i./ha, where pre-harvest intervals were 14 days.
In a few instances however residues at harvest exceeded this figure,
but were essentially below 5 mg/kg.
Some samples were anlaysed after removal of the peel, a common
practice in processing such as preparation for canning or drying.
Residues in the pulp were usually below the limits of determination.
Pears
The data in Table 4 show that residues arising from recommended
applications (up to three treatments at up to 0.05 or 1.5 kg a.i./ha)
in whole fruit harvested 2 weeks after the last application were less
than 4 mg/kg, with the exception of one trial in the USA. The analysis
of samples of peeled pears showed that residues of fenbutatin oxide
seldom exceeded 10% of the levels in the whole fruit.
Citrus fruits
Residue data were obtained on grapefruit, lemons and oranges from the
USA, Brazil, Spain and Italy (see Table 5). Most of the results show
residues below 4 mg/kg; only in a few cases have higher residues been
found, in general not exceeding 5 mg/kg.
Removal of the peel and analysis of the pulp and/or juice has shown
that the residues were mainly on the skin. In pulp and juice residues
were below the limit of determination, 0.02-0.05 mg/kg. Only one
sample of juice analysed contained a higher residue (0.08 ME/1). The
residue in the pulp did not generally exceed 30% of the level in the
whole fruit.
Stone fruits
Cherries
Data obtained on cherries (see Table 6) indicate that following
recommended treatments (up to 0.05% a.i.; minimum pre-harvest interval
7 days) residues were not above 4 mg/kg in whole fruit without stones,
except in one trial in USA where a residue of 4.2 mg/kg was recorded 4
weeks after treatment.
Peaches
Residues of fenbutatin oxide an peaches treated according to the
recommendations (up to 0.05% a.i.) with a minimum pre-harvest interval
of 14-21 days were in most cases below 5 mg/kg, but sometimes higher.
It is unlikely that after applying the miticide according to good
agricultural practice the residue level at harvest would exceed 7
mg/kg. Residues in the fruit after peeling were considerably lower,
and did not exceed 0.2 mg/kg. Details are given in Table 7.
Plums
The limited data available (Table 8) indicate that residues at harvest
are unlikely to exceed 3 mg/kg in the whole fruit without stone after
recommended treatments, i.e. up to 0.05% a.i. and a minimum
pre-harvest interval of 7 days.
Grapes
Residues of fenbutatin oxide (Table 9) following treatment of vines
according to the recommendation (up to 0.5% or 1 kg. a.i./ha), were
about 4 mg/kg after 1 week and 0.6-3.2 mg/kg after 3 weeks (excepting
one erratic figure of 6 mg/kg from a trial carried out in the Federal
Republic of Germany).
TABLE 3. Residues of fenbutatin oxide in apples resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Apples
Granny Smith Australia 1973 1 19 WP 50% 1.6 1.3 0.45 0.95
" " 1973 1 25 " " 2.5 2.5
Josephine 1974 1 19 " " 0.94 0.79
Granny Smith 1975 2 20 " " 1.3 1.5 2.2 1.6
" " 1975 2 20 sc 500g/l 1.4 1.4 1.1 0.9
" " 1975 2 30 " " 1.8 2.0 2.1 2.0
Mcintosh Canada 1976 1 25 WP 50% 0.97 0.90 0.67 0.45
" 1976 1 19 " " 0.55
Cortland 1976 1 19 " " 0.15
Cardinal France 1974 1 30 0.3 " " 0.26 0.04
" 1974 1 50 0.5 " " 0.43 0.09
Melrose 1975 1 75 0.6 " " 0.07
Cox's O.P. Fed. Rep. of
Germany 1974 3 25 " " 1.1 0.82 0.66 0.64 0.21
James Grieves 1974 3 25 " " 1.5 0.83 0.82 0.60 0.53
Golden Delicious 1974 3 25 " " 1.4 0.78 0.82 0.88 0.53
" " 1974 3 25 " " 1.4 0.87 0.61 0.53 0.24
Golden Parmane 1974 4 1.0 " " 1.9 1.1 1.1 0.74 1.1
" " 1974 3 1.0 " " 0.82 0.82 0.35 0.27 0.32
Goudreinet Netherlands 1974 2 25 " " 0.30 0.26
" 1974 3 25 " " 0.22 0.28
" 1975 2 30 " " 0.23 0.35
" 1975 2 30 " " 0.28 0.37
Golden Delicious Italy 1975 1 30 " " 1.2 0.98 0.
Stark Delicious 1975 1 30 " " 0.93 0.65 0.60
Granny Smith New Zealand 1975 5 19 " " 0.75 0.60 0.55 0.40
Starking Portugal 1974 2 0.6 " " 0.30 0.68
" 1974 2 1.0 " " 0.75 1.40
" 1974 2 0.6 " " 0.29 0.80
" 1974 2 1.0 " " 0.63 0.80
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Granny Smith South Africa 1976 1 30 WP 50% 1.8 1.3 1.0 0.95
" " 1976 1 50 " " 2.5 2.4 1.5 1.2
" " 1974 1 100 " " 2.4 2.9
(4
days)
Jonathan Switzerland 1974 3 0.5 0.55 0.43 0.35
(10
days)
Worcester U.K. 1974 2 25 " " 0.3
Worcester U.K. 1974 2 42+25 wp+sc 0.3
York Imperial U.S.A. 1974 5 25 1.25 wp 50% 0.93
1974 5 25 1.25 " " 1.3
1974 5 50 2.5 " " 2.6
1974 5 50 2.5 " " 2.2
Newton U.S.A. 1973 1 38 " " 1.6
(Oregon) (1.5-1.7)
Red delicious U.S.A. 1973 4 19 1.5 0.6-0.94
(Oregon)
1973 4 38 3 1.6-1.5
1973 4 30 1.5 0.25-0.84
Red delicious " 1973 4 3.0 " " 2.1-2.0
Newton Pippin (California) 1972 1 25 " " 0.26
" " 1972 1 50 " " 0.60
" " 1972 1 100 " " 1.5
Golden delicious (Washington) 1971 4 12.5 0.75 " " 0.9 0.8 0.8 0.7
" " 1971 4 25 1.5 " " 1.7 1.4 1.0 1.5
" " 1971 4 50 3.0 3.7 3.5 3.3 2.6
" " 1972 4 12.5 0.75 " " 1.6 1.5 1.5
" " 1972 4 25 1.5 " " 3.5 2.5 3.1
" " 1972 4 50 3.0 " " 7.9 4.7 5.0 3.3
" " Michigan 1971 3 12.5 0.25 " " 1.2 1.0 0.85 0.95 0.90
" " 1971 3 25 0.5 " " 3.5 2.5 3.3 3.7 2.4
" " 1971 3 50 1.0 " " 6.1 6.0 4.0 3.7 3.7
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
" " 1972 4 12.5 0.5 " " 1.8 1.4-1.1 1.1-1.6 0.64-0.68
" " 1972 4 25 1.5 " " 2.9-2.2 1.3-1.8 2.2-1.8 1.1 -0.9
1972 4 50 2.0 " " 3.4-6.0 5.4-6.6 5.3-7.0 2.8-4.1
Ben Davis New York 1972 4 12.5 0.75 wp 50% 2.3-2.6 2.2-2.2 2.1-1.5 1.3-1.6
" " 1972 4 25 1.5 " " 5.6-5.4 3.5-4.2 2.8-3.6 3.0-2.5
" " 1972 4 50 3.0 " " 8.5-8.0 5.6-6.0 5.6-6.2 5.7-3.8
Rome 1973 4 12.5 " " 1.8 1.0 1.1 1.4
1973 4 19 " " 2.2 1.4 1.6 1.2
1973 4 38 " " 2.3 2.8 3.2 2.7
wp = wettable powder
sc = suspension concentrate
TABLE 4. Residues or fenbutatin oxide in pears resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No rate formulation 0-2 3 7-9 14 19-21 28-31 32-35
g/1001 kg/ha
Pears
Josephine Australia 1974 1 19 WP 50% 0.5 0.4 0.3 0.5
William 1973 1 19 " " 0.8 1.1 0.65 0.73
" 1973 1 25 " " 1.6 1.2
Packam 1975 2 20 sc 500g/l 1.5 1.6 1.5 1.3
" 1975 2 30 " " 2.3 1.4 1.6 1.2
" 1975 2 20 WP 50% 1.4 1.2 1.3 1.2
William France 1976 1 60 0.3 " " 0.17 0.11 0.03
" 1976 1 100 0.5 1.07 0.16 0.10
Packhams Tr. S. Africa 1976 1 30 " " 1.7 1.6 1.4 1.9
" 1976 1 50 " " 3.6 3.1 2.7 1.6
Winter Melis S. Africa 1974 1 100 " " 3.8 2.5
(5
days)
Kieffer USA (Mich.) 1971 3 12.5 0.38 " " 3.0 1.7 1.6 1.0
" 1971 3 25 0.75 " " 6.0 5.0 1.9 1.1
" 1971 3 50 1.5 " " 4.7 4.0 3.0
Bartlett (Wash) 1971 2 12.5 0.25 " " 1.5 1.3 0.9
" 1971 2 25 0.5 " " 2.7 2.3 1.8
" 1971 2 50 1.0 " " 5.3 4.7 4.3
" 1973 4 12.5 0.75 " " 0.17 0.18
" 1973 4 25 1.5 " " 0.25 0.54
" 1973 4 503 3.0e " " 1.4 1.9
" (Calif) 1973 1 25 1.0 " " 1.8 1.1 1.1 1.0
" 1973 1 50 2.0 " " 2.4 1.4 2.1 1.8
" (New York) 1971 3 12.5 0.75 " " 1.2
" 1971 3 25 1.5 " " 2.2
" 1971 3 50 3.0 " " 3.5
Bosc 1972 3 12.5 0.38 " " 4.2 2.3 1.2 0.9
" 3 25 0.75 " " 6.0 4.6 2.5 2.1
" 3 50 1.5 " " 8.0 6.3 4.6 3.8
e= excessive application
Strawberries
The limited data available indicate that residues following
recommended application 0.05% a.i, minimum pre-harvest interval 7
days), would be unlikely to exceed 4 mg/kg (Table 10):
Fruit of vegetables
The very limited data available (Table 11) suggest that residues
arising from recommended treatments (up to 0.05% a.i., minimum
pre-harvest interval 3 days) are unlikely to exceed 1 mg/kg.
FATE OF RESIDUES
In animals
Provided fenbutatin oxide is used according to good agricultural
practice no detectable residues will occur in products of animal
origin, since crops which are generally used for animal feed are
rarely treated with this acaricide. In some countries, particularly in
the USA, cattle are occasionally given processed fruit pulp as a part
of their diet. Data are available showing that cattle eating feed
containing residues of fenbutatin oxide will not produce milk or meat
containing appreciable residues. See also the section
"Biochemical aspects".
Cattle feeding studies
An experiment, was carried out in which three lactating Guernsey cows
were fed on a diet containing 119Sn-labelled fenbutatin oxide
(Shell, 1976). The quantity given was equivalent to 34 ppm fenbutatin
oxide in the whole ration, which consisted of 5 kg compounded feed and
10 kg alfalfa cubes per animal per day, and was given twice daily
during a 21 day period. Milk samples were taken from each animal at
each milking, and the total radio-activity determined in each sample.
No radio-activity above background level was found, indicating that
total residues in whole milk were equivalent to less than 0.01 mg/kg
fenbutatin oxide. The animals were slaughtered 12 hours after the
final feeding and the radio-activity was determined in samples of fat,
muscle, brain, kidney and liver. No residues were detected in brain,
muscle, fat or liver at a limit of determination equivalent to 0.02
mg/kg fenbutatin oxide. Similar results were obtained with the kidney
samples from 2 of the animals, but the third was found to contain
radio-activity equivalent to 0.03 mg/kg fenbutatin oxide, i.e. just
above the limit of determination in these tissues.
TABLE 5. Residues of fenbutatin oxide in citrus fruits resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Oranges
Pera Natal Brazil 1975 1 30 WP 50% 0.13 0.05
1975 2 30 " " 0.25
1975 1 30 " " 0.27 0.08
1975 2 30 " " 0.42
1975 1 30 " " 0.50 0.30 0.17
1975 2 30 " " 0.75 0.38
1975 1 30 " " 0.38 0.22 0.08
1975 2 30 " " 0.62 0.35
Oranges S. Africa 1975 1 30 sc500g/l
1.0 1.1 0.65 0.65
1975 1 30 " " 0.8 0.60 0.45 0.40
Oranges Calif. 1972 2 25 1.2 wp 50% 3.0 3.3 1.9 2.1 2.2 1.8 2.5 2.4 1.6 1.7
Valencia 1972 2 50 2.4 " " 5.6 6.0 5.0 4.4 4.2 5.0 4.4 3.8 4.0 3.8
1972 2 100 4.8 " " 10.5 11.0 9.5 9.5 7.5 7.5 6.2 5.5
1971 1 25 0.44 " " 1.1 1.3 0.65 0.82 0.8 1.0 0.88 0.96 0.52 0.74
1971 1 50 1. " " 1.8 3.0 1.5 2.0 1.5 1.7 1.4 1.8 1.5 1.8
(Florida) 1972 4 12.5 0.9 " " 0.56 0.62 0.50 0.75 0.74 0.75 0.64 0.75
1972 4 25 1.9 " " 1.5 1.6 1.5 1.5 1.5 2.0 1.5 1.7
1972 4 50 3.6 " " 3.8 4.0 3.2 4.1 3.1 3.2 3.6 3.1
Lemon
Nostrana Italy 1975 1 30 " " 0.9 0.5
Lisbon USA
(Ariz.) 1974 2 19 1.4 " " 0.81 0.23 0.22 0.34
1974 2 19 1.4 " " 2.8 2.0 1.2 1.6
1974 2 32e 3.5 " " 1.6 0.32 0.30 1.2
1974 2 32e 3.5 " " 5.6 5.8 2.9 2.0
(Calif) 1973 3 12.5 0.37 " " 0.40 0.25 0.20 0.30 0.15 0.30 <0.05 <0.05 (0.05)
1973 3 25 0.75 " " 0.95 0.70 0.25 0.40 0.30 0.30 <0.05 <0.05 (0.05)
1973 3 50 1.5 " " 1.0 0.90 0.75 0.70 0.35 0.05 0.05 <0.05 0.05 0.20
TABLE 5. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Grapefruit
Marsh USA
(Calif) 1971 1 25 1.8 " " 0.9 1.3 0.6 0.9 0.8 0.9 0.6 1.0 0.5 0.6
1971 1 50e 3.6 " " 1.2 2.0 0.9 1.4 1.4 2.0 1.4 1.6 1.3 1.6
Red Blush 1972 2 12.5 0.2 " " 0.8 0.7 0.45 0.45 0.86 0.74 0.36 0.44 (0.42 0.36)
1972 2 25 0.4 " " 1.6 1.7 1.3 1.5 0.9 0.7 (0.90 0.68)
1972 2 50 0.8 " " 3.2 2.9 2.5 2.6 1.9 1.5 (1.7 2.5)
Marsh (Florida) 1972 3 12.5 1.0 " " 0.3 0.4 (0.4 0.5)
1972 4 12.5 1.0 " " 0.7 0.7 0.7 0.7 (0.7 0.7)
1972 3 25 1.9 " " 1.1 1.2 (0.4 0.4)
1972 4 25 1.9 " " 1.5 2.8 1.7 1.8 (1.4 1.1)
1972 3 50e 3.8e " " 3.2 3.4 (1.3 1.7)
1972 4 50e 3.8e " " 3.4 4.8 2.8 4.0 (1.8 3.0)
1971 3 12.5 " " 0.71 0.65 0.72 0.65 0.52 0.44
1971 3 25 " " 1.3 1.3 1.8 1.6 1.0 0.75
1971 3 50 " " 3.3 3.3 3.8 3.1 1.8 1.5
e= excessive application
TABLE 6. Residues of fenbutatin oxide in stone fruits resulting from supervised trials
Crop Country Year No g/100 1kg/ha formulation 0.2 7 14 21 28-31
Cherries
(sweet and sour)
Biggerau Fed. Rep.
Germany 1977 3 25 WP 50% 0.70 0.21 0.09
Rote Leber 1977 3 25 " " 1.5 0.17 0.04 0.02
Schattenmorelle 1977 3 25 " " 1.1 0.41 0.22 0.09
Morella 1977 1 25 0.3 " " 0.90 0.50
1977 1 50 0.6 " " 1.10 1.15
Bing USA
(Calif) 1976 1 25 1.1 " " 1.7
1976 1 50 2.2 " " 3.2
Sour USA
(Mich) 1974 2 12.5 0.96
1974 2 25 2.3 1.5 2.2 3.0
1974 2 50 4.1 3.6 3.9 4.2
USA
(Wash) 1974 2 25 1.2 1.4 2.0
1974 2 50 2.4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
Cherries
Bing USA
(Wash) 1974 2 25 1-2 1.4 2.0
1974 2 50 2-4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
TABLE 7. Residues of fenbutatin oxide in peaches resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Peach
Tatura
Aurora
Australia 1975 2 20 sc500/gl 2.2
1975 2 30 " " 3.6
1975 2 20 wp 50% 2.7
Cornish 1973 1 19 " " 1.3 1.9 2.5 0.71
1973 1 25 1.8 1.4
Golden Queen 1974 1 19 1.4 <0.1 1.0
1974 1 19 2.2
1974 2 19 3.5
Alberta Canada 1976 2 2.6 0.63
Royal Gold France 1977 1 50 0.75 0.87
Audenot 1973 1 0.5 3.7 2.6 1.9
Alberta 1973 1 0.5 1.6 1.6 1.5 1.3 0.65
Red. Aven
P.G. 1974 1 0.5 2.0 1.7 0.41
Vellutata Italy 1974 1 30 0.4 0.3 0.5
Netturina N7 1974 1 30 0.5 0.3 0.5
Golden Queen New
Zealand 1975 1 19 0.8 0.65 0.45
Kakamus S. Africa 1976 1 30 7 5.9 4.0
" 1976 1 50 12 7.0 6.0
Sunblest USA
(Calif) 1973 3 50 1.1 4.3 3.0
1973 3 20 4.3 1.0
1973 3 50 7.3 5.7 8.0 2.6
Red Haven (Oregon) 1973 3 19 0.3 3.9 2.8 1.9 1.5
(S.
Carolina) 1973 3 38 0.6 7.7 8.2 4.6 5.8
TABLE 7. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Roter
Ingelheimer Fed. Rep.
of
Germany 1977 3 25 0.25 9.0 3.4 3.4 2.0 1.8 1.3
Rekord
a. Alfter 1977 3 25 0.25 8.1 4.7 2.5 2.2 1.5
Madame
Roenait 1977 3 25 0.25 8.1 3.1 2.5 2.3 3.3
TABLE 8. Residues of fenbutatin oxide in plums resulting from supervised trials
Application Residues in mg/kg at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 3-4 7 14 21 25-28 32-35
Plums
Auerbacher Fed.
Rep.
Germany 1977 3 25 0.25 WP 50% 0.15 0.15 0.15 0.10
Bühler 1977 3 25 0.25 0.25 0.10 0.15 0.10
Czar Netherlands 1977 1 25 1.75 sc500/gl 0.3 0.2 0.1 <0.1 <0.1
1977 1 50 3.5 " " 0.5 0.4 0.2 0.1 0.1
Relsey S. Africa 1976 1 30 wp 50% 1.2 1.3 0.8 0.9
" 1976 1 50 " " 2.2 2.2 1.8 1.0
Stanley USA
(New York) 1974 2 12.5 0.31
1974 2 25 " " 0.50
1974 2 50 " " 0.49
French
Prunes (Calif) 1974 2 12.5 " " 0.27 0.35
1974 2 25 " " 0.92 0.96
1974 2 50 " " 2.8 2.3 1.8
Early
Halian (Wash) 1974 2 25 " " 0.19 0.02 0.11 0.6
50 " " 0.29 0.73 0.47 0.58
100 " " 1.03 1.05 0.67 0.69
TABLE 9. Residues of fenbutatin oxide in grapes resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
rate
Crop Country Year No g/100 1kg/ha formulation 0-3 7 13-14 21 28-31 42-45 56-
Grapes
Semillion France 1973 1 30 wp 50 0.7
1 50 " " 0.9
Grenache 1974 1 0.5 " "
Merlot Rouge 1974 1 0.5 " "
Lavallet 1975 1 50 " " 0.27
Grenache 1975 1 50 0.50
Siebel 1975 1 125e " " 1.1
Müller Fed. Rep.
of Germany 1975 3 50 " " 4.7 3.9 2.5 2.6 1.2
Kerner 1975 3 50 " " 4.8 2.1 2.7 3.0 1.1
Müller 1975 3 50 " " 4.9 3.3 3.4 2.3 2.9
Sylvaner 1975 3 50 " " 2.7 1.9 1.6 3.2 1.4
Riesling 1975 3 50 " " 4.5 1.8 1.6 0.6
Trollinger 1975 3 50 " " 1.3 1.2 1.1 3.2 2.3
Sprühsurgunder 1975 3 50 " " 7.1 6.3 6.2 6.3
Barbera Italy 1975 1 30 " " 7.1 1.3 1.1 0.45
Nebbiolo 1975 1 30 " " 1.8 1.1 0.68
Chasselas Switzerland 1975 1 100e " " 2.1
Rabier USA
(Calif) 1973 3 25 0.5 " " 0.90 0.68 0.16 0.17
1973 3 50 1.0 " " 2.0 0.62 3.0 2.3
Mission 1973 3 0.5 " " 1.0 0.45 0.97 0.65
1973 3 50 1.0 2.2 0.47 0.67 1.2
Thompson 1973 3 25 0.5 1.5 1.5 1.8 1.0
1973 3 50 1.0 3.9 1.8 1.4 0.66
Concord (Michigan) 1974 3 50 1.1 3.1 2.0 2.2 1.8
1974 3 75 1.7 5.6 2.8 3.4 2.7
50 1.1 2.8
100 2.2 2.9
e= excessive application
TABLE 10. Residues of fenbutatin oxide in strawberries resulting from supervised trials
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Strawberries
Gorella (g) Belgium 1976 1 25 0.9 WP 50% 0.2 0.1 <0.1 <0.1
Volla (g) 1976 1 25 0.9 " " 0.40 0.15 0.15 <0.1 <0.1 <0.1
France 1977 1 30 " " 0.35 0.20
Mexico 1977 1 0.55 sc 500g/l 2.3 2.0 0.9
1.1 " " 7.0 4.0 5.9
Netherlands 1977 1 25 wp 50% 0.50
1977 2 25 " " 0.65
1977 1 25 " " 0.15
1977 2 25 " " 0.30
1977 1 25 sc 500g/l 0.65
1977 2 25 " " 1.1
1977 1 25 " " 0.25
1977 2 25 " " 0.50
Parfaite S. Africa 1977 1 25 0.13 " " 0.5 0.2 0.2
1 38 0.19 " " 0.8 0.5 0.5
- (g) UK(Sussex) 1975 1 25 wp 50% 0.7 0.7 0.5 <0.1
- (Devon) 1975 1 25 " " 0.4 0.2 0.5 0.2
Tioga USA(Calif) 1974 1 50 1.1 " " 1.4*
1974 1 100 2.2 " " 2.4*
Heidi 1973 2 80 2.2 " " 4.9* 4.6 3.4
1973 2 160e 4.4e " " 4.8* 5.9 2.9
G - 4 1973 4 160e 4.4e " " 4.0* 3.3 3.0
Tioga (Florida) 1974 6 40 0.55 " " 1.2* 1.4 0.3
Catskil USA
(Wisc) 1975 2 100 1.1 " " 1.6* 0.49
2 200 2.2 " " 2.2* 1.5
Northwest (Wash) 1975 1 50 0.55 " " 0.70 1.1 0.98
1 100 1.1 " " 1.1 1.2 1.83
1 200 2.2 " " 2.4 4.3 3.1
TABLE 10. (Continued)
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Raritan (New Jersey) 2 50 0.55 " " 0.86 0.2 0.60
2 100 1.1 " " 1.4 1.3 0.60
2 200e 2.2e " " 5.0 1.9
(g) = glasshouse or plastic cover
e = excessive application
* = residue after 1 day
TABLE 11. Residues of fenbutatin oxide in fruits of vegetables resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 2-3 4-5 7 10 14 30
cucumbers(g) Netherlands 1974 1 25 wp 50% 0.2 0.1 0.1 <0.1
1974 1 25 " " 0.2 0.2 0.2 <0.1
gherkins(g) 1974 1 0.75 1.8 0.8 0.7 0.9
melons France 1977 1 30 < 0.01 <0.01
sweet
peppers(g) Belgium 1976 1 1.2 1.0 0.6
sweet
peppers(g) Netherlands 1976 1 0.5 0.4 0.6 0.4 0.1
tomatoes(g) Netherlands 1976 1 0.5 0.2 0.4 0.4 0.4
tomatoes Italy 1974 2 30 0.5 0.3
1974 2 30 0.2
tomatoes(g) UK 1975 1 0.2 0.3 0.4
(g) = glasshouse of plastic cover
In a second experiment (Shell, 1973) 3 Guernsey cows were similarly
given a daily ration containing 34 mg/kg 119Sn-labelled fenbutatin
oxide for 21 days. Excretion of the ingested fenbutatin oxide was
mainly via the faeces but small amounts, up to 3% of the daily intake,
were excreted in the urine. As in the previous experiments no residues
were found in the milk (limit of determination 0.02 mg/kg) and none in
the brain, honey fat or most of the muscle samples taken. However
radio-activity averaging 0.27 mg/kg calculated as fenbutatinoxide was
found in the kidney and 0.34 mg/kg in the liver of all three animals.
Radio activity equivalent to 0.08 mg/kg fenbutatin oxide was found in
lone lung sample and a just measurable residue equivalent to 0.04
mg/kg in one muscle sample (gastrocnemius). The limit of determination
was equivalent to 0.04 mg/kg fenbutatin oxide in all the tissues
(Shell, 1973). Residue data from field trials indicate that residues
of fenbutatin oxide in apple pomace from apples containing a residue
of 4 mg/kg may approach 25 mg/kg (Shell, 1972-74).
The data from the cattle-feeding studies indicate that cattle fed
normal amounts (up to 25% of the total diet) of apple pomace made from
apples treated with fenbutatin oxide according to good agricultural
practice are unlikely to yield milk containing detectable amounts of
the acaricide. No measurable residues are likely in muscle tissues,
but they might occasionally occur at low levels (<0.2 mg/kg) in
liver and kidney.
In plants
Fenbutatin oxide is slowly lost after application to crops. The
degradation to in-organic tin occurs through successive loss of the
phenyldimethylethyl groups. The principal organotin degradation
product found on crops is 1,1,3,3,tetrakis(ß, ß-dimethylphenethyl)-
1,3-dihydroxydistannoxane, referred to subsequently as SD 31723(Shell,
1972).
In studies of the fate of fenbutatin oxide on leaves and fruit of
apples and citrus (Shell, 1972, 1974a), small apple trees grown in a
lath-house were treated one to three times with 119Sn-labelled
fenbutatin oxide with approximately 40µg at each application.
Recoveries of 119Sn 2 to 33 days after the last application were
84-96% of the amount applied. Virtually all the radio-activity in the
fruit was found to be on the outer surface of the skin. Substantial
amounts of the radio-activity were removed by rinsing with organic
solvents, or simply by siping the fruit-skin with paper tissues. It
was shown that less than 5% of the total radio-activity present in the
fruit originated from the principal breakdown product SD 31723 and
less than 0.7% from other organotin metabolites including (ß, ß-
dimethylphenethystannoic acid polymer (SD 33608). The structural
formula of the metabolites SD 31723 and SD 33608 are shown on the
following page. Inorganic tin accounted for nearly 10% of the total
119Sn on the apples (Shell, 1972).
Other information on identity and properties
(a) Composition of the technical Product:
The technical material contains 97% w/w minimum of fenbutation oxide;
the main impurities are related organo-tin compounds.
(b) Physical and chemical properties
Physical state: crystaline solid
Molecular weight: 1053
Melting point: 138-139°C
Volatility: not volatile
Solubility: insoluble in water, (approx. 5x10-6 g/1 at 23°C)
slightly soluble in most organic solvents;
solubility at 23°C in acetone approx 6 g/l in
dichlororomethane 370 g/l in benzene 140 g/l
Stability: stable to sunlight. Water hydrolyses fenbutatin
oxide to tris-(2-methyl-2-phmylpropyl)tin
hydroxide; this in reconverted to the parent
compound slowly at room temperature and rapidly at
98°C.
Formulations:
Wettable powder 500 g a.i./kg and suspension concentrate 500 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Groups of 2 male and 2 female rats were fed an average of 19.5 ppm
119Sn-labelled fenbutatin oxide (with or without a 3 day withdrawal
period before killing following 3 or 6 day exposure) after 1, 3 or 6
days to study the absorption, distribution and excretion of
fenbutatin. Most of the administered 119Sn was rapidly excreted in the
faeces following multiple dosing (80-90% of the administered 119Sn).
Essentially no 119Sn was excreted via the urine. The GI tract
contained approximately 30, 15 and 5% of the administered 119Sn for
animals sacrificed after 1, 3 or 6 days of dosing. The highest tissue
concentrations of 119Sn were found in liver (0.015-0.09 mg/kg),
kidneys, (0.015 0.055 mg/kg) and fat (< 0.01-0.065 mg/kg). No 119Sn
was detected in brain or bone tissue (Loeffler, 1972). See also the
section "Fate of residues", "In animals".
Biotransformation
Analysis of the faeces from rats dosed with 119Sn-labelled fenbutatin
oxide indicated that ß, ß-dimethylphenethylotamoic acid and 1, 1, 3,
3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxdistannoxane are the
major metabolites (Loeffler, 1972).
Effects on enzymes and other biochemical parameters
In starch gel electrophoretic studies on sera from rate fed either 0
or 600 ppm in the diet for 6 months (2 buffer systems) control and
test animal sera were identical. The effect of 1-phenylanatine or heat
was similar on both sera. Comparisons with intestinal, bone, liver and
kidney alkaline phosphatase indicated the serum alkaline phosphatase
was similar to intestinal alkaline phosphatase. Further studies on rat
sera derived from animals fed 0 or 600 ppm in the diet for one year,
utilizing heat inactivation, selective substrates and inhibitors,
confirmed the absence of any difference between control and test
animal sera, as well an confirming the similarity of the serum
alkaline phosphatase to intestinal alkaline phosphatase (Pickering,
1973a). In a further series of studies by the elevation of serum
alkaline phosphatase observed in rate after administration of 300 ppm
fenbutatin oxide in the diet for 90 to 120 days may shown to be
completely reversible following one months, withdrawal from exposure.
Limited data indicate that the reversal of elevated serum alkaline
phosphataze in the test rate may be completed within 7 days of
withdrawal of the fenbutatin oxide (Pickering, 1973b).
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Following preliminary studies to determine suitable dose levels and to
confirm the induction of brain oedema by triethyltin bromide, a
comparative oedma assay of five organo-tin compounds (including
fenbutatin oxide, and its metabolite 1, 1, 3,
3-tetrakis-(ß, ß-dimethylphenethyl)-1, 3-dihydroxydistannoxane) for
oedma formation in the central nervous system of rats, was undertaken.
Groups of 15 male rats were intubated with corn oil (10 mg/kg, 40
mg/kg triethyltin bromide, 1000 mg/kg, fenbutatin oxide, 100 mg/kg 1,
1, 3, 3-tetrakis (ß, ß-dimethylphenethyl)-1,3-dihydroxydistannoxane
and sizable doses of two other tin compounds. The triothyltin bromide
group showed increased brain water content after 48 hrs. Seven of ten
brains examined at 24 and 48 hrs showed spongeosis of white matter in
the cerebellum and sometimes of the corpus callosum and pons. No
histological changes were observed in 4 rats killed after 6 hrs.
Fenbutatin oxide (24 and 48 hr. kills), and the metabolite (6, 24 and
48 hr kills) did not show either increased water content or abnormal
brain histology, although rats on both compounds showed signs of
intoxication (Samuels and Dix, 1972),
Special studies on mutagenicity
Micro-organisms
In plate tests using Serratia marcesens, strains HY/alpha 13, a
histidine-requiring auxotroph, HY/alpha 21, a leucine-requiring
auxotroph, and CV/r c3/alpha 742, a thiamine-requiring spontaneous
auxotroph, 50 µl hexane solution (which resulted in a precipitate of
fenbutatin oxide on the agar plate) did not increase the reversion
rate in any strain, nor was growth inhibited, (Dean, et al., 1972a).
Neither conversion rate nor cell survival was affected in
Saccharomyces cerevisiae following exposure for 5 or 16 hrs in pH7
phosphate buffer containing 0.0025 or 0.005 µg fenbutatin oxide/ml
solution. In the same system, 20 µg/ml in suspension was ineffective
in altering conversion rate or cell survival (Dean et al., 1972a).
Mice
Groups of 8 male and 8 female mice were treated with 0.005 or 1000 mg
fenbutatin oxide/kg, in 1% carbomethyl cellulose. Four
animals/sex/group were killed 90 minutes after i.p. colcemid
administration and femurs were removed. One hundred bone marrow
cells/animal were examined. No significant differences in incidence of
chromosomal aberrations were observed (Dean et al., 1972b).
In three or four host-mediated assay experiments using Saccharomyces
cerevisiae and dose levels of 250 or 500 mg fenbutatin oxide/kg,
conversion rate was unaffected. In the fourth replicate, conversion
rate was marginally increased at 250 but not at 500 mg/kg (Dean et
al., 1972a)
In an 8 week dominant lethal study, 8 male mice/group were dosed once
with 250 or 500 mg fenbutatin oxide/kg, and were then mated with 3
females each week. The control group comprised 16 untreated males. No
significant differences in percentage pregnancies, implant incidence
or early resorption rate were noted between control and fenbutatin
oxide treated groups. A positive control group of 8 male mice (200 mg
Endoxan) showed reduced pregnancy rate and increased resorption and
decreased implant rates during the first 3 matings after, dosing.
(Dean and Doak, 1972c).
Special studies on teratology
Three groups of 15 pregnant rabbits were dosed by gelatin capsules on
days 6-18 inclusive of gestation (mating day = day 0), with 3 or 10 mg
fenbutatin oxide/kg or with 37.5 mg thalidomide/kg. Twenty-six rabbits
were used as capsule-only treated controls. Rabbits were killed on day
28 of pregnancy. A repeat experiment differed only in that 20 pregnant
females/dose level and 30 controls were used, autopsy being on day 29.
Foetuses were incubated for 24 hrs. following removal from the uterus,
after which approximately 1/3 were examined for visceral
abnormalities, and 2/3 for skeletal defects. Prior to clearing for
skeletal examination, viscera from these animals were also examined
for defects. Although some maternal deaths occurredy these were random
between groups. Mean live litter size, resorption rate, foetal loss,
survival of incubated foetuses, and incidence of anomalies were not
affected by fenbutatin oxide at either close level. Cromn-rump length
and foetal weight were comparable to controls except at 3 mg/kg in the
repeat experiment, where a significant increase was noted. The
positive control group behaved as expected (Dix and Wilson, 1973).
Special study on reproduction
In a standard 3 generation, 2 litter/generation reproduction study,
groups of 10 male and 20 female rats were fed O, 50, 100 or 300 ppm
fenbutatin oxide (985 purity). It should be noted that rate of the Fo
generation commenced on diet and were mated at 100 days of age, and
that litters were culled to 10 pups/litter on post-partum day 5.
Necropsy was performed on 10 male and 10 female F3b weanlings from
the 0 and 300 ppm, and on 5 weanlings/sex from the 50 and 100 ppm
groups. In the 300 PPM group, parents and pups were smaller, hyper
active and irritable; mean litter size was slightly reduced in the
F1b litters, and in the two F3 generations survival to weaning was
reduced at 300 ppm and testicular organ/body weight ratio was reduced
in the F3b weanlings. Necropsies of F2b adults did not show any
compound-related changes. No consistent effects were observed at 50 or
100 ppm with respect to fertility, gestation, viability or lactation
indices, litter size, litter weight, or necropsy of F3b weanlings
Mine et al., 1973).
Special studies on carcinogenicity
Groups of 48 male and 48 female Carworth Farm No. 1 strain mice were
fed 50, 100, 100 or 600 ppm fenbutatin oxide in the diet for 18
months. A control group comprised 96 males and 96 females. All animals
were examined grossly at death. Microscopic examination was undertaken
on all animals dying during the study and on all 600 ppm mice, on
10/mice/sex at 50, 100 and 300 PPM, and on 45 controls/sex at 18
months. Survival rate was comparable in all groups and exceeded 75%.
There was a high incidence of tumours in all groups. However there was
no indication of compound-or dose-related tumor induction (Granville
et al., 1973a).
Acute Toxicity
TABLE 1a. Acute toxicity of fenbutatin oxide
Species Route Sex LD50(mg/kg) Reference
Mouse Oral - 1450(1223-1659) Simpson 1972a
Rat " - 2631(1929-4659)
Dog Oral(capsule) - >1500
Rabbit Oral - 1500-3000
Rat Dermal - >1000
In mice and rats, death occurred within 10 days. Signs were dyspnoeal
stained coat and transient diarrhoea. In dogs, vomiting and diarrhoea
occurred. In rabbits convulsions occurred at the nighest dose, with
death within 24 hrs. Lower doses caused death up to 18 days after
dosing. Weight loss was still apparent 3 weeks after dosing, Autopsy
revealed damage to the gastric mucosa, massive fatty changes in liver,
reduced spermatogenesis, and multinucleated spermatid production
(Simpson, 1972a).
TABLE 1b. Acute toxicity of 50% wettable powder
Species Route Sex LD50 (mg/kg) Reference
Rat Oral - 1000 Simpson 1972a
Dermal - >1000
TABLE 1c. Acute toxicity of metabolite*
Species Route Sex LD50(mg/kg) Reference
Mouse Oral M 420 Natoff, 1973
F 622
Rat Oral M,F 242
Rabbit Oral M >675
F 300-450
Rat Oral - 132(102-176) Simpson & Dix,
Mouse Oral - 1051(824-1440) 1972c
* 1, 1, 3, 3-tetrakis(ß,ß -dimethylphenethyl)-1,3-dihydroxydistannoxane.
The signs of intoxication in all species were severe diarrhoea,
followed by loss of body weight which was recorded for rabbits, and
inanition and emaciation in all species. Deaths occurred up to 16 days
after dosing the mice, 14 days after dosing the rats and 26 days after
dosing the rabbits. The observation period for rabbits was curtailed
at 28 days.
Short term studies
Rat
Five groups of 5 male and 5 female rats were fed 0, 30, 100, 300 or
1000 ppm fenbutatin oxide in the diet for 5 weeks. Body weight was
significantly reduced, as was food intake at 300 and 1000 ppm.
Reductions in absolute brain, heart, liver, spleen and kidney weights
were noted in both sexes at 1000 ppm. Spleen weight was reduced in
males at 300 ppm and brain, liver, spleen and kidney weight was
reduced at 300 ppm in females. Serum alkaline phosphatase was
increased in both sexes and SGPT levels were elevated in females at
1000 ppm (Simpson, 1972a).
Three groups of five male rats were used in a pair feeding study, one
group being a normal control, the second being fed 600 ppm, and the
third, pair-fed at the food intake levels of the 600 ppm group, The
study ran for 5 weeks. Results (daily body weights) indicated that the
reduction in body weight at 600 ppm can be totally accounted for by
reduced food intake (Simpson, 1972b).
Groups of 6 male and 6 female rats were fed 50, 100, 300 or 600 ppm
fenbutatin oxide (97%) in diet for 3 months. Concurrent controls
comprised 12 male and 12 female rats. Health and behaviour were
comparable in all groups. Body weight gain was reduced in males at 600
ppmv and in females at 300 and 600 ppm. Food intake was reduced in
males at 600 ppm, and in females during the first 3 or 4 weeks of the
study at 300 and 600 ppm. Absolute organ weight of liver and kidney
were reduced in both sexes, heart in females, and brain and spleen in
males at 600 ppm. At 300 ppm, liver, spleen and kidney absolute
weights were reduced in males. Organ/body weight ratios were elevated
for brain in males at 600 ppm, and in females at 300 and 600 ppm.
Heart and liver weight ratios were elevated in females at 600 and
heart at 300 ppm. Testes weight ratio was elevated in males at 300 and
600 ppm. Haematology (haemoglobin, PCV, RBC, WBC, RCV, MOH,
prothrobmin time and KOCT) was comparable in all groups. Clinical
chemistry determinations were comparable for total protein, SGOT, and
electrolytes (K, Na, Cl). BUN and SAP in males were elevated at 300
and 600 ppm in males, as was SGPT at 300 PPm. In females BUN was
elevated at 100 ppm and above. Serum protein fractions were comparable
in all groups. No compound-related gross or histopathological effects
were noted, (Simpson and Thorpe, 1973a).
Three groups of rats, 20/sex/control and 10/sex/test group, were fed
0, 3, 10, 30, 100 or 300 ppm of 99% 1, 1, 3, 3-tetrakis (ß,
ß-dimethylphenethyl)-1,3-dihydroxydistannoxane for 90 days. Health and
behaviour were comparable in all groups. Body weights of 300 ppm males
were significantly decreased throughout the study. This was also true
for the females in the 30, 100 and 300 ppm group but the suppression
was not significant. Slight reductions in haemoglobin, erythrocyte
count and packed cell volume occurred in the 300 ppm males. No changes
were noted in any of the other groups of either sex. The other
clinical values of animals killed after 13 weeks showed no
dose-related differences when compared to the control values. Gross
and microscopic examination of a wide range of tissues from all
control, 100 and 300 ppm animals revealed no consistent changes
associated with exposure to the metabolite. Organ weights of males at
13 weeks did not differ from control weights, the organ body weight
ratios were increased for brain, heart, liver, kidneys and testes in
this group and for the liver in the 100 ppm male group. Brain, spleen
and kidney weights were reduced in the 300 ppm females, (Simpson and
Dix, 1972c).
Dog
Five groups of 2 male and 2 female beagle dogs were orally dosed
(gelatin capsules) with 0, 10, 30, 100 or 300 mg fenbutatin oxide/kg
for 5 weeks. Slight vomiting and diarrhoea occurred at 100 and 300
mg/kg, which was associated with decreased body weigh. At 3O mg/kg and
above, serum alkaline phosphate was elevated. No pathological changes
were observed (Simpson, 1972a).
Groups of dogs (8/sex/control and 4/sex/test group) were administered,
by capsule, 0, 2.5, 5, 15, 30 or 60 mg/kg 97% fenbutatin oxide daily
for 2 years. Blood samples were taken every six weeks to measure BUN,
glucose, plasma protein, sodium, potassium, chloride, SGPT, SGOT, and
alkaline phosphatage. Every 12 weeks hemoglobin, packed cell volume,
RBC, WBC, differential WBC counts, prothrombin and
kaolin-cephalin-coagulation times were determined. Urine analysis was
carried out every 3 months. Emesis and diarrhoea occurred in most of
the dosed dogs. These effects continued in some dogs in the 30 and 60
mg/kg/day groups throughout the study, although at a reduced frequency
towards the end of the study. Lower dosage groups showed none of these
effects after the first year. Convulsions occurred in 5 dogs,
predominantly female, during the second year; control, one female; 2.5
mg/kg/day, one female; 15 mg/kg/day, one female and one male; and 30
mg/kg/day, one female. None of the 60 mg/kg/day animals exhibited this
effect. EEG's with simultaneous single lead monitoring of EEG's of one
female each in the 0, 2.5 and 30 mg/kg/day groups revealed a normal
EEG for the control dog and the other 2 dogs showed normal waking
rhythms but abnormal activity during light sleep. The investigators
contend that the lack of dose response relationship suggest a
diagnosis of spontaneous epilepsy not related to exposure to
fenbutatin oxide. General health and behaviour of all other dogs were
similar to control dogs. Significant reductions in rate of weight
gains occurred in both sexes in the toxicity, long term 60 mg/kg/day
group. Males receiving 30 mg/kg/day exhibited no significant rate of
gain. This effect was not evident in females of this group.
Haematological and clinical chemistry and urine data showed no
differences between control and test groups. Necropsies revealed no
gross changes. Microscopic examination, including frozen sections of
liver and kidney stained for lipid, revealed no compound-related
pathological changes in a wide range of tissues. Organ weights were
not affected by exposure to the test compound (Granville and Dix,
1973b).
Long term studies
Rat
Groups of rats (114/sex/control and 72/sex/test group) were fed 0, 50,
100, 300 or 600 ppm 97% fenbutatin oxide for two years. Rats were
killed at 3 and 12 months (12/sex/control and 6 sex/test group) and at
6 months (24/sex/control and 12/sex/test group). Organ weights of
brain, heart, liver, spleen, kidney and testes were recorded and a
wide variety of tissues examined from all rats dying or killed at
interim periods, and from all rats from the 50 and 100 ppm groups.
Blood determinations were made, at each kill period, for haemoglobin,
PCV, RBC, WBC and WBC differential counts; prothrombin and
kaolin-cephalin-coagulation times; BUN; total protein, potassium
sodium and chloride; SGPT, SGOT and alkaline phosphatase. Also serum
proteins were fractionated electrophoretically and estimates made of
albumin and alpha, - ß, and gamma-globin concentrations.
Fenbutatin oxide rendered the diet unpalatable to both sexes causing,
in the early months of the study, significant reductions in food
consumption and body weight gains. Males were more affected than
females. For the remainder of the study all groups exhibited rates of
gain to the control except both sexes receiving 300 and 600 ppm, which
exhibited reduced body weight reduced body weights throughout the
study. BUN levels were increased at the higher levels for the first 6
months but remained within normal limits for the remainder of the
study. Serum alkaline phosphatase activity was increased in both sexes
at 300 and 600 ppm throughout the study, in the 100 ppm males at 6
months, and in the 100 ppm females at 3 months and 2 years. All other
haemotological and clinical chemistry values remained within normal
limits at all times. Survival and behaviour were not affected by
administration of the compound. No compound-related tissue lesions nor
any increase in tumor incidence were found. Organ weights revealed no
compound-related differences except kidney weight reduction in all
treated males and the 600 ppm females at 2 years. No specific
compound-related lesions were seen in the kidney of any treated group.
Absolute and relative testes weights, unaccompanied by hypertrophy,
were noted in the 300 and 600 ppm groups at 2 years. (Simpson et al.,
1973b).
COMMENTS
Fenbutatin oxide is moderately toxic by the oral route producing
severe gastrointestinal irritation. Acute oral administration to
rabbits reduced spermatogenesis and resulted in multi-nucleated
spermatid production. It is excreted rapidly, mostly unchanged in the
faeces, indicating that it is poorly absorbed. In the rat it is
metabolized to ß, ß-dimethylphenethylstannonio acid (SD 33608) and 1,
1, 3, 3-tetrakis-(ß, ß-dimethylphenethyl)-1,3-dihydrox3rdistannoxane.
The latter metabolite and fenbutatin oxide failed to produce oedema of
the central nervous system of the rat at 100 and 1000 mg/kg
respectively. Fenbutatin oxide produced no genotoxic effects in
several in vitro and in vivo systems. Adequate short- and
long-term studies are available in rats and dogs as well as teratology
study study in rabbits, a multi-generation reproduction study in rats,
and a carcinogenicity study in mice. No-effect levels were
demonstrated in rats and dogs and an acceptable daily intake for
humans was established on the basis of these studies. The no-effect
level was established as 15 mg/kg because of emesis and diarrhoea in
dog, and because of the known toxic effects of organic compounds on
the brain a safety factor of 100 was used.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in diet equivalent to 2.5 mg/kg body weight.
Dog: 15 mg/kg body weight.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.03 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenbutatin oxide is used as a specific mitioide against a wide range
of phytophagous mites. It is recommended especially for the control of
the mobile stages on a wide variety of crops, including pome and stone
fruits, citrus fruits, grapes, berry fruits, vegetables and
ornamentals. Recommendations for use on fruit crops and the
recommended pre-harvest intervals are given in Table 2.
The compound is in general used as a foliar spray in a concentration
range of 0.02 -0.05% a.i., corresponding to approx. 0.4 -1.5 kg
a.i./ha. Most applications are required while the fruits are
developing; the number of applications varies with the crop and
regional conditions.
The pre-harvest intervals are seldom less than 7 days, except on some
glasshouse-grown fruits or vegetables; in most instances the
pre-harvest intervals recommended are at least 14 days.
TABLE 2. Use pattern and recommended pre-harvest intervals of fenbutatin oxide
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Apples, Panonychus ulmi 0.0125-0.05% depending Australia 2
Tetranychus urticae on pest and volume of Belgium 28
Tetranychus mc. danieli spray liquid used. Denmark 28
Paratetranychus spp. For concentrated sprays France 7
Bryobia rabrioculus maximum 1.5 kg a. i./ha. Fed. Rep. of
Germany 14
Eotetranychus carpini Not more than 3 applications the
borealis whilst trees are bearing fruit. Netherlands 42
Italy 30
Aculus schlechtendali Mexico 14
Portugal 21
S. Africa 14
3 days
on fruit
for
canning
Spain 21
Switzerland 21
USA 14
Pears Panonychus ulmi As for apples As for apples
Tetranychus mc. danieli
Tetranychus urticae
Epitremeris pyri
Citrus Panonychus citri 0.015-0.05 % for Italy 60
Eotetranychus banksi concentrated sprays a Mexico 14
Eotetranychus yumensis maximum of 2 kg/ha is S. Africa 7
Tetranychus urticae recommended. Not more Spain 21
Brevipalpus californicus than 4 treatments in USA 7
Phyllocoptruta oleivora one year.
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Cherries Tetranychus urticae 0.02-0.05%; maximum two Italy 30
applications whilst trees Switzerland 21
are bearing fruits. the
Concentrated sprays max. Netherlands 42
1.5 kg a.i./ha.
Peaches Tetranychus urticae 0.02-0.05% maximum two Australia 2
Panonychus ulmi treatments whilst trees Denmark 28
are bearing fruit. France 7
Concentrated sprays up to Italy 30
1 kg a.i./ha.
S. Africa 21
3 days
on fruit
for
canning.
Spain 21
Switzerland 21
Plums Tetranychus urticae 0.02-0.05% Italy 30
Panonychus ulmi Concentrated sprays up Switzerland 21
to 1 kg a.i./ha. the
Netherlands 42
Grapes Panonychus ulmi 0.02-0.05% France 7
Concentrated sprays maximum Fed. Rep. of
rate up to 1 kg a.i./ha. Germany 28
Italy 45
Spain 21
Switzerland 21
TABLE 2. (Continued)
Crop Pest Application rate, a.i. Recommended
pre-harvest
intervals
Country Days
Strawberries Tetranychus
urticae 0.025-0.05% the
Netherlands only before
blossoming
and/or after
harvest.
Bell-peppers Tetranychus 0.02-0.04% the 3
urticae Netherlands (glasshouse)
Cucumbers Tetranychus 0.02-0.05% Italy 30
urticae the
Netherlands 3::
Tomatoes Tetranychue 0.02-0.5% Italy 30
urtical the
Netherlands 3
(glasshouse)
:: includes gherkins glasshouse and outdoors; cucumbers and melons glasshouse only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained on residues from supervised trials
carried out in various countries on a variety of fruits and fruits of
vegetables as follows.
Citrus fruits: grapefruit, lemons, oranges
Pome fruits: apples, pears
Stone fruits: cherries, peaches, plums
Berry fruits: grapes, strawberries
Fruits of vegetables: bell-peppers, cucumbers, gherkins,
melons, tomatoes
The data obtained are summarized in Tables 3-11 (Shell International,
1971-76). In most of the trials fenbutatin oxide was applied as a 50%
wettable powdery but in a few instances a suspension concentrate
containing 500 g a.i./l was used (referred to as SC in Tables). The
application rates in most of the trials are in conformity with good
agricultural practice. In, some instances higher dosages were also
applied; these excessive rates are indicated in the Tables.
It has been demonstrated, using radio-labelled fenbutatin oxide, that
the residues do not to any great extent penetrate through the akin of
treated fruit (see also section "Fate of residues"). The residues
found when analysing the whole fruit were mainly on or in the skin.
This is also illustrated in samples from some field experiments which
were examined. before and after removal of the peel.
Pome fruits
Apples
The data in Table 3 were mainly obtained from samples of crops treated
according to typical use conditions. The results show considerable
variation according to local conditions.
In most instances residues in whole fruit were below 3 mg/kg
fanbutatin oxide from up to 3 treatments made at recommended rates, up
to 0.05% or 1.5 kg a.i./ha, where pre-harvest intervals were 14 days.
In a few instances however residues at harvest exceeded this figure,
but were essentially below 5 mg/kg.
Some samples were anlaysed after removal of the peel, a common
practice in processing such as preparation for canning or drying.
Residues in the pulp were usually below the limits of determination.
Pears
The data in Table 4 show that residues arising from recommended
applications (up to three treatments at up to 0.05 or 1.5 kg a.i./ha)
in whole fruit harvested 2 weeks after the last application were less
than 4 mg/kg, with the exception of one trial in the USA. The analysis
of samples of peeled pears showed that residues of fenbutatin oxide
seldom exceeded 10% of the levels in the whole fruit.
Citrus fruits
Residue data were obtained on grapefruit, lemons and oranges from the
USA, Brazil, Spain and Italy (see Table 5). Most of the results show
residues below 4 mg/kg; only in a few cases have higher residues been
found, in general not exceeding 5 mg/kg.
Removal of the peel and analysis of the pulp and/or juice has shown
that the residues were mainly on the skin. In pulp and juice residues
were below the limit of determination, 0.02-0.05 mg/kg. Only one
sample of juice analysed contained a higher residue (0.08 ME/1). The
residue in the pulp did not generally exceed 30% of the level in the
whole fruit.
Stone fruits
Cherries
Data obtained on cherries (see Table 6) indicate that following
recommended treatments (up to 0.05% a.i.; minimum pre-harvest interval
7 days) residues were not above 4 mg/kg in whole fruit without stones,
except in one trial in USA where a residue of 4.2 mg/kg was recorded 4
weeks after treatment.
Peaches
Residues of fenbutatin oxide an peaches treated according to the
recommendations (up to 0.05% a.i.) with a minimum pre-harvest interval
of 14-21 days were in most cases below 5 mg/kg, but sometimes higher.
It is unlikely that after applying the miticide according to good
agricultural practice the residue level at harvest would exceed 7
mg/kg. Residues in the fruit after peeling were considerably lower,
and did not exceed 0.2 mg/kg. Details are given in Table 7.
Plums
The limited data available (Table 8) indicate that residues at harvest
are unlikely to exceed 3 mg/kg in the whole fruit without stone after
recommended treatments, i.e. up to 0.05% a.i. and a minimum
pre-harvest interval of 7 days.
Grapes
Residues of fenbutatin oxide (Table 9) following treatment of vines
according to the recommendation (up to 0.5% or 1 kg. a.i./ha), were
about 4 mg/kg after 1 week and 0.6-3.2 mg/kg after 3 weeks (excepting
one erratic figure of 6 mg/kg from a trial carried out in the Federal
Republic of Germany).
TABLE 3. Residues of fenbutatin oxide in apples resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Apples
Granny Smith Australia 1973 1 19 WP 50% 1.6 1.3 0.45 0.95
" " 1973 1 25 " " 2.5 2.5
Josephine 1974 1 19 " " 0.94 0.79
Granny Smith 1975 2 20 " " 1.3 1.5 2.2 1.6
" " 1975 2 20 sc 500g/l 1.4 1.4 1.1 0.9
" " 1975 2 30 " " 1.8 2.0 2.1 2.0
Mcintosh Canada 1976 1 25 WP 50% 0.97 0.90 0.67 0.45
" 1976 1 19 " " 0.55
Cortland 1976 1 19 " " 0.15
Cardinal France 1974 1 30 0.3 " " 0.26 0.04
" 1974 1 50 0.5 " " 0.43 0.09
Melrose 1975 1 75 0.6 " " 0.07
Cox's O.P. Fed. Rep. of
Germany 1974 3 25 " " 1.1 0.82 0.66 0.64 0.21
James Grieves 1974 3 25 " " 1.5 0.83 0.82 0.60 0.53
Golden Delicious 1974 3 25 " " 1.4 0.78 0.82 0.88 0.53
" " 1974 3 25 " " 1.4 0.87 0.61 0.53 0.24
Golden Parmane 1974 4 1.0 " " 1.9 1.1 1.1 0.74 1.1
" " 1974 3 1.0 " " 0.82 0.82 0.35 0.27 0.32
Goudreinet Netherlands 1974 2 25 " " 0.30 0.26
" 1974 3 25 " " 0.22 0.28
" 1975 2 30 " " 0.23 0.35
" 1975 2 30 " " 0.28 0.37
Golden Delicious Italy 1975 1 30 " " 1.2 0.98 0.
Stark Delicious 1975 1 30 " " 0.93 0.65 0.60
Granny Smith New Zealand 1975 5 19 " " 0.75 0.60 0.55 0.40
Starking Portugal 1974 2 0.6 " " 0.30 0.68
" 1974 2 1.0 " " 0.75 1.40
" 1974 2 0.6 " " 0.29 0.80
" 1974 2 1.0 " " 0.63 0.80
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
Granny Smith South Africa 1976 1 30 WP 50% 1.8 1.3 1.0 0.95
" " 1976 1 50 " " 2.5 2.4 1.5 1.2
" " 1974 1 100 " " 2.4 2.9
(4
days)
Jonathan Switzerland 1974 3 0.5 0.55 0.43 0.35
(10
days)
Worcester U.K. 1974 2 25 " " 0.3
Worcester U.K. 1974 2 42+25 wp+sc 0.3
York Imperial U.S.A. 1974 5 25 1.25 wp 50% 0.93
1974 5 25 1.25 " " 1.3
1974 5 50 2.5 " " 2.6
1974 5 50 2.5 " " 2.2
Newton U.S.A. 1973 1 38 " " 1.6
(Oregon) (1.5-1.7)
Red delicious U.S.A. 1973 4 19 1.5 0.6-0.94
(Oregon)
1973 4 38 3 1.6-1.5
1973 4 30 1.5 0.25-0.84
Red delicious " 1973 4 3.0 " " 2.1-2.0
Newton Pippin (California) 1972 1 25 " " 0.26
" " 1972 1 50 " " 0.60
" " 1972 1 100 " " 1.5
Golden delicious (Washington) 1971 4 12.5 0.75 " " 0.9 0.8 0.8 0.7
" " 1971 4 25 1.5 " " 1.7 1.4 1.0 1.5
" " 1971 4 50 3.0 3.7 3.5 3.3 2.6
" " 1972 4 12.5 0.75 " " 1.6 1.5 1.5
" " 1972 4 25 1.5 " " 3.5 2.5 3.1
" " 1972 4 50 3.0 " " 7.9 4.7 5.0 3.3
" " Michigan 1971 3 12.5 0.25 " " 1.2 1.0 0.85 0.95 0.90
" " 1971 3 25 0.5 " " 3.5 2.5 3.3 3.7 2.4
" " 1971 3 50 1.0 " " 6.1 6.0 4.0 3.7 3.7
TABLE 3. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. rate formulation.
g/100 1 kg/ha 0-2 3 7-9 14 19-21 28 32-35
" " 1972 4 12.5 0.5 " " 1.8 1.4-1.1 1.1-1.6 0.64-0.68
" " 1972 4 25 1.5 " " 2.9-2.2 1.3-1.8 2.2-1.8 1.1 -0.9
1972 4 50 2.0 " " 3.4-6.0 5.4-6.6 5.3-7.0 2.8-4.1
Ben Davis New York 1972 4 12.5 0.75 wp 50% 2.3-2.6 2.2-2.2 2.1-1.5 1.3-1.6
" " 1972 4 25 1.5 " " 5.6-5.4 3.5-4.2 2.8-3.6 3.0-2.5
" " 1972 4 50 3.0 " " 8.5-8.0 5.6-6.0 5.6-6.2 5.7-3.8
Rome 1973 4 12.5 " " 1.8 1.0 1.1 1.4
1973 4 19 " " 2.2 1.4 1.6 1.2
1973 4 38 " " 2.3 2.8 3.2 2.7
wp = wettable powder
sc = suspension concentrate
TABLE 4. Residues or fenbutatin oxide in pears resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No rate formulation 0-2 3 7-9 14 19-21 28-31 32-35
g/1001 kg/ha
Pears
Josephine Australia 1974 1 19 WP 50% 0.5 0.4 0.3 0.5
William 1973 1 19 " " 0.8 1.1 0.65 0.73
" 1973 1 25 " " 1.6 1.2
Packam 1975 2 20 sc 500g/l 1.5 1.6 1.5 1.3
" 1975 2 30 " " 2.3 1.4 1.6 1.2
" 1975 2 20 WP 50% 1.4 1.2 1.3 1.2
William France 1976 1 60 0.3 " " 0.17 0.11 0.03
" 1976 1 100 0.5 1.07 0.16 0.10
Packhams Tr. S. Africa 1976 1 30 " " 1.7 1.6 1.4 1.9
" 1976 1 50 " " 3.6 3.1 2.7 1.6
Winter Melis S. Africa 1974 1 100 " " 3.8 2.5
(5
days)
Kieffer USA (Mich.) 1971 3 12.5 0.38 " " 3.0 1.7 1.6 1.0
" 1971 3 25 0.75 " " 6.0 5.0 1.9 1.1
" 1971 3 50 1.5 " " 4.7 4.0 3.0
Bartlett (Wash) 1971 2 12.5 0.25 " " 1.5 1.3 0.9
" 1971 2 25 0.5 " " 2.7 2.3 1.8
" 1971 2 50 1.0 " " 5.3 4.7 4.3
" 1973 4 12.5 0.75 " " 0.17 0.18
" 1973 4 25 1.5 " " 0.25 0.54
" 1973 4 503 3.0e " " 1.4 1.9
" (Calif) 1973 1 25 1.0 " " 1.8 1.1 1.1 1.0
" 1973 1 50 2.0 " " 2.4 1.4 2.1 1.8
" (New York) 1971 3 12.5 0.75 " " 1.2
" 1971 3 25 1.5 " " 2.2
" 1971 3 50 3.0 " " 3.5
Bosc 1972 3 12.5 0.38 " " 4.2 2.3 1.2 0.9
" 3 25 0.75 " " 6.0 4.6 2.5 2.1
" 3 50 1.5 " " 8.0 6.3 4.6 3.8
e= excessive application
Strawberries
The limited data available indicate that residues following
recommended application 0.05% a.i, minimum pre-harvest interval 7
days), would be unlikely to exceed 4 mg/kg (Table 10):
Fruit of vegetables
The very limited data available (Table 11) suggest that residues
arising from recommended treatments (up to 0.05% a.i., minimum
pre-harvest interval 3 days) are unlikely to exceed 1 mg/kg.
FATE OF RESIDUES
In animals
Provided fenbutatin oxide is used according to good agricultural
practice no detectable residues will occur in products of animal
origin, since crops which are generally used for animal feed are
rarely treated with this acaricide. In some countries, particularly in
the USA, cattle are occasionally given processed fruit pulp as a part
of their diet. Data are available showing that cattle eating feed
containing residues of fenbutatin oxide will not produce milk or meat
containing appreciable residues. See also the section
"Biochemical aspects".
Cattle feeding studies
An experiment, was carried out in which three lactating Guernsey cows
were fed on a diet containing 119Sn-labelled fenbutatin oxide
(Shell, 1976). The quantity given was equivalent to 34 ppm fenbutatin
oxide in the whole ration, which consisted of 5 kg compounded feed and
10 kg alfalfa cubes per animal per day, and was given twice daily
during a 21 day period. Milk samples were taken from each animal at
each milking, and the total radio-activity determined in each sample.
No radio-activity above background level was found, indicating that
total residues in whole milk were equivalent to less than 0.01 mg/kg
fenbutatin oxide. The animals were slaughtered 12 hours after the
final feeding and the radio-activity was determined in samples of fat,
muscle, brain, kidney and liver. No residues were detected in brain,
muscle, fat or liver at a limit of determination equivalent to 0.02
mg/kg fenbutatin oxide. Similar results were obtained with the kidney
samples from 2 of the animals, but the third was found to contain
radio-activity equivalent to 0.03 mg/kg fenbutatin oxide, i.e. just
above the limit of determination in these tissues.
TABLE 5. Residues of fenbutatin oxide in citrus fruits resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Oranges
Pera Natal Brazil 1975 1 30 WP 50% 0.13 0.05
1975 2 30 " " 0.25
1975 1 30 " " 0.27 0.08
1975 2 30 " " 0.42
1975 1 30 " " 0.50 0.30 0.17
1975 2 30 " " 0.75 0.38
1975 1 30 " " 0.38 0.22 0.08
1975 2 30 " " 0.62 0.35
Oranges S. Africa 1975 1 30 sc500g/l
1.0 1.1 0.65 0.65
1975 1 30 " " 0.8 0.60 0.45 0.40
Oranges Calif. 1972 2 25 1.2 wp 50% 3.0 3.3 1.9 2.1 2.2 1.8 2.5 2.4 1.6 1.7
Valencia 1972 2 50 2.4 " " 5.6 6.0 5.0 4.4 4.2 5.0 4.4 3.8 4.0 3.8
1972 2 100 4.8 " " 10.5 11.0 9.5 9.5 7.5 7.5 6.2 5.5
1971 1 25 0.44 " " 1.1 1.3 0.65 0.82 0.8 1.0 0.88 0.96 0.52 0.74
1971 1 50 1. " " 1.8 3.0 1.5 2.0 1.5 1.7 1.4 1.8 1.5 1.8
(Florida) 1972 4 12.5 0.9 " " 0.56 0.62 0.50 0.75 0.74 0.75 0.64 0.75
1972 4 25 1.9 " " 1.5 1.6 1.5 1.5 1.5 2.0 1.5 1.7
1972 4 50 3.6 " " 3.8 4.0 3.2 4.1 3.1 3.2 3.6 3.1
Lemon
Nostrana Italy 1975 1 30 " " 0.9 0.5
Lisbon USA
(Ariz.) 1974 2 19 1.4 " " 0.81 0.23 0.22 0.34
1974 2 19 1.4 " " 2.8 2.0 1.2 1.6
1974 2 32e 3.5 " " 1.6 0.32 0.30 1.2
1974 2 32e 3.5 " " 5.6 5.8 2.9 2.0
(Calif) 1973 3 12.5 0.37 " " 0.40 0.25 0.20 0.30 0.15 0.30 <0.05 <0.05 (0.05)
1973 3 25 0.75 " " 0.95 0.70 0.25 0.40 0.30 0.30 <0.05 <0.05 (0.05)
1973 3 50 1.5 " " 1.0 0.90 0.75 0.70 0.35 0.05 0.05 <0.05 0.05 0.20
TABLE 5. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 7 14 21 28-31 32-35 58-64
(45)
Grapefruit
Marsh USA
(Calif) 1971 1 25 1.8 " " 0.9 1.3 0.6 0.9 0.8 0.9 0.6 1.0 0.5 0.6
1971 1 50e 3.6 " " 1.2 2.0 0.9 1.4 1.4 2.0 1.4 1.6 1.3 1.6
Red Blush 1972 2 12.5 0.2 " " 0.8 0.7 0.45 0.45 0.86 0.74 0.36 0.44 (0.42 0.36)
1972 2 25 0.4 " " 1.6 1.7 1.3 1.5 0.9 0.7 (0.90 0.68)
1972 2 50 0.8 " " 3.2 2.9 2.5 2.6 1.9 1.5 (1.7 2.5)
Marsh (Florida) 1972 3 12.5 1.0 " " 0.3 0.4 (0.4 0.5)
1972 4 12.5 1.0 " " 0.7 0.7 0.7 0.7 (0.7 0.7)
1972 3 25 1.9 " " 1.1 1.2 (0.4 0.4)
1972 4 25 1.9 " " 1.5 2.8 1.7 1.8 (1.4 1.1)
1972 3 50e 3.8e " " 3.2 3.4 (1.3 1.7)
1972 4 50e 3.8e " " 3.4 4.8 2.8 4.0 (1.8 3.0)
1971 3 12.5 " " 0.71 0.65 0.72 0.65 0.52 0.44
1971 3 25 " " 1.3 1.3 1.8 1.6 1.0 0.75
1971 3 50 " " 3.3 3.3 3.8 3.1 1.8 1.5
e= excessive application
TABLE 6. Residues of fenbutatin oxide in stone fruits resulting from supervised trials
Crop Country Year No g/100 1kg/ha formulation 0.2 7 14 21 28-31
Cherries
(sweet and sour)
Biggerau Fed. Rep.
Germany 1977 3 25 WP 50% 0.70 0.21 0.09
Rote Leber 1977 3 25 " " 1.5 0.17 0.04 0.02
Schattenmorelle 1977 3 25 " " 1.1 0.41 0.22 0.09
Morella 1977 1 25 0.3 " " 0.90 0.50
1977 1 50 0.6 " " 1.10 1.15
Bing USA
(Calif) 1976 1 25 1.1 " " 1.7
1976 1 50 2.2 " " 3.2
Sour USA
(Mich) 1974 2 12.5 0.96
1974 2 25 2.3 1.5 2.2 3.0
1974 2 50 4.1 3.6 3.9 4.2
USA
(Wash) 1974 2 25 1.2 1.4 2.0
1974 2 50 2.4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
Cherries
Bing USA
(Wash) 1974 2 25 1-2 1.4 2.0
1974 2 50 2-4 1.7 1.7
1974 2 100c 4-5 3.6 3.8
TABLE 7. Residues of fenbutatin oxide in peaches resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Peach
Tatura
Aurora
Australia 1975 2 20 sc500/gl 2.2
1975 2 30 " " 3.6
1975 2 20 wp 50% 2.7
Cornish 1973 1 19 " " 1.3 1.9 2.5 0.71
1973 1 25 1.8 1.4
Golden Queen 1974 1 19 1.4 <0.1 1.0
1974 1 19 2.2
1974 2 19 3.5
Alberta Canada 1976 2 2.6 0.63
Royal Gold France 1977 1 50 0.75 0.87
Audenot 1973 1 0.5 3.7 2.6 1.9
Alberta 1973 1 0.5 1.6 1.6 1.5 1.3 0.65
Red. Aven
P.G. 1974 1 0.5 2.0 1.7 0.41
Vellutata Italy 1974 1 30 0.4 0.3 0.5
Netturina N7 1974 1 30 0.5 0.3 0.5
Golden Queen New
Zealand 1975 1 19 0.8 0.65 0.45
Kakamus S. Africa 1976 1 30 7 5.9 4.0
" 1976 1 50 12 7.0 6.0
Sunblest USA
(Calif) 1973 3 50 1.1 4.3 3.0
1973 3 20 4.3 1.0
1973 3 50 7.3 5.7 8.0 2.6
Red Haven (Oregon) 1973 3 19 0.3 3.9 2.8 1.9 1.5
(S.
Carolina) 1973 3 38 0.6 7.7 8.2 4.6 5.8
TABLE 7. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No g/100 1kg/ha formulation 0-3 7-8 13-14 20-21 28-30 35 42
Roter
Ingelheimer Fed. Rep.
of
Germany 1977 3 25 0.25 9.0 3.4 3.4 2.0 1.8 1.3
Rekord
a. Alfter 1977 3 25 0.25 8.1 4.7 2.5 2.2 1.5
Madame
Roenait 1977 3 25 0.25 8.1 3.1 2.5 2.3 3.3
TABLE 8. Residues of fenbutatin oxide in plums resulting from supervised trials
Application Residues in mg/kg at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 3-4 7 14 21 25-28 32-35
Plums
Auerbacher Fed.
Rep.
Germany 1977 3 25 0.25 WP 50% 0.15 0.15 0.15 0.10
Bühler 1977 3 25 0.25 0.25 0.10 0.15 0.10
Czar Netherlands 1977 1 25 1.75 sc500/gl 0.3 0.2 0.1 <0.1 <0.1
1977 1 50 3.5 " " 0.5 0.4 0.2 0.1 0.1
Relsey S. Africa 1976 1 30 wp 50% 1.2 1.3 0.8 0.9
" 1976 1 50 " " 2.2 2.2 1.8 1.0
Stanley USA
(New York) 1974 2 12.5 0.31
1974 2 25 " " 0.50
1974 2 50 " " 0.49
French
Prunes (Calif) 1974 2 12.5 " " 0.27 0.35
1974 2 25 " " 0.92 0.96
1974 2 50 " " 2.8 2.3 1.8
Early
Halian (Wash) 1974 2 25 " " 0.19 0.02 0.11 0.6
50 " " 0.29 0.73 0.47 0.58
100 " " 1.03 1.05 0.67 0.69
TABLE 9. Residues of fenbutatin oxide in grapes resulting from supervised trials
Application Residues (mg/kg) at intervals(days) after application
rate
Crop Country Year No g/100 1kg/ha formulation 0-3 7 13-14 21 28-31 42-45 56-
Grapes
Semillion France 1973 1 30 wp 50 0.7
1 50 " " 0.9
Grenache 1974 1 0.5 " "
Merlot Rouge 1974 1 0.5 " "
Lavallet 1975 1 50 " " 0.27
Grenache 1975 1 50 0.50
Siebel 1975 1 125e " " 1.1
Müller Fed. Rep.
of Germany 1975 3 50 " " 4.7 3.9 2.5 2.6 1.2
Kerner 1975 3 50 " " 4.8 2.1 2.7 3.0 1.1
Müller 1975 3 50 " " 4.9 3.3 3.4 2.3 2.9
Sylvaner 1975 3 50 " " 2.7 1.9 1.6 3.2 1.4
Riesling 1975 3 50 " " 4.5 1.8 1.6 0.6
Trollinger 1975 3 50 " " 1.3 1.2 1.1 3.2 2.3
Sprühsurgunder 1975 3 50 " " 7.1 6.3 6.2 6.3
Barbera Italy 1975 1 30 " " 7.1 1.3 1.1 0.45
Nebbiolo 1975 1 30 " " 1.8 1.1 0.68
Chasselas Switzerland 1975 1 100e " " 2.1
Rabier USA
(Calif) 1973 3 25 0.5 " " 0.90 0.68 0.16 0.17
1973 3 50 1.0 " " 2.0 0.62 3.0 2.3
Mission 1973 3 0.5 " " 1.0 0.45 0.97 0.65
1973 3 50 1.0 2.2 0.47 0.67 1.2
Thompson 1973 3 25 0.5 1.5 1.5 1.8 1.0
1973 3 50 1.0 3.9 1.8 1.4 0.66
Concord (Michigan) 1974 3 50 1.1 3.1 2.0 2.2 1.8
1974 3 75 1.7 5.6 2.8 3.4 2.7
50 1.1 2.8
100 2.2 2.9
e= excessive application
TABLE 10. Residues of fenbutatin oxide in strawberries resulting from supervised trials
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Strawberries
Gorella (g) Belgium 1976 1 25 0.9 WP 50% 0.2 0.1 <0.1 <0.1
Volla (g) 1976 1 25 0.9 " " 0.40 0.15 0.15 <0.1 <0.1 <0.1
France 1977 1 30 " " 0.35 0.20
Mexico 1977 1 0.55 sc 500g/l 2.3 2.0 0.9
1.1 " " 7.0 4.0 5.9
Netherlands 1977 1 25 wp 50% 0.50
1977 2 25 " " 0.65
1977 1 25 " " 0.15
1977 2 25 " " 0.30
1977 1 25 sc 500g/l 0.65
1977 2 25 " " 1.1
1977 1 25 " " 0.25
1977 2 25 " " 0.50
Parfaite S. Africa 1977 1 25 0.13 " " 0.5 0.2 0.2
1 38 0.19 " " 0.8 0.5 0.5
- (g) UK(Sussex) 1975 1 25 wp 50% 0.7 0.7 0.5 <0.1
- (Devon) 1975 1 25 " " 0.4 0.2 0.5 0.2
Tioga USA(Calif) 1974 1 50 1.1 " " 1.4*
1974 1 100 2.2 " " 2.4*
Heidi 1973 2 80 2.2 " " 4.9* 4.6 3.4
1973 2 160e 4.4e " " 4.8* 5.9 2.9
G - 4 1973 4 160e 4.4e " " 4.0* 3.3 3.0
Tioga (Florida) 1974 6 40 0.55 " " 1.2* 1.4 0.3
Catskil USA
(Wisc) 1975 2 100 1.1 " " 1.6* 0.49
2 200 2.2 " " 2.2* 1.5
Northwest (Wash) 1975 1 50 0.55 " " 0.70 1.1 0.98
1 100 1.1 " " 1.1 1.2 1.83
1 200 2.2 " " 2.4 4.3 3.1
TABLE 10. (Continued)
Application Residues (mg/kg) at intervals (days)-after application
Rate
Crop Country Year No g/100 1 kg/ha formulation 0-2 3-4 5-7 10-14 28-32 35-38 42
Raritan (New Jersey) 2 50 0.55 " " 0.86 0.2 0.60
2 100 1.1 " " 1.4 1.3 0.60
2 200e 2.2e " " 5.0 1.9
(g) = glasshouse or plastic cover
e = excessive application
* = residue after 1 day
TABLE 11. Residues of fenbutatin oxide in fruits of vegetables resulting from supervised trials
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year No g/100 1 kg/ha formulation 0 2-3 4-5 7 10 14 30
cucumbers(g) Netherlands 1974 1 25 wp 50% 0.2 0.1 0.1 <0.1
1974 1 25 " " 0.2 0.2 0.2 <0.1
gherkins(g) 1974 1 0.75 1.8 0.8 0.7 0.9
melons France 1977 1 30 < 0.01 <0.01
sweet
peppers(g) Belgium 1976 1 1.2 1.0 0.6
sweet
peppers(g) Netherlands 1976 1 0.5 0.4 0.6 0.4 0.1
tomatoes(g) Netherlands 1976 1 0.5 0.2 0.4 0.4 0.4
tomatoes Italy 1974 2 30 0.5 0.3
1974 2 30 0.2
tomatoes(g) UK 1975 1 0.2 0.3 0.4
(g) = glasshouse of plastic cover
In a second experiment (Shell, 1973) 3 Guernsey cows were similarly
given a daily ration containing 34 mg/kg 119Sn-labelled fenbutatin
oxide for 21 days. Excretion of the ingested fenbutatin oxide was
mainly via the faeces but small amounts, up to 3% of the daily intake,
were excreted in the urine. As in the previous experiments no residues
were found in the milk (limit of determination 0.02 mg/kg) and none in
the brain, honey fat or most of the muscle samples taken. However
radio-activity averaging 0.27 mg/kg calculated as fenbutatinoxide was
found in the kidney and 0.34 mg/kg in the liver of all three animals.
Radio activity equivalent to 0.08 mg/kg fenbutatin oxide was found in
lone lung sample and a just measurable residue equivalent to 0.04
mg/kg in one muscle sample (gastrocnemius). The limit of determination
was equivalent to 0.04 mg/kg fenbutatin oxide in all the tissues
(Shell, 1973). Residue data from field trials indicate that residues
of fenbutatin oxide in apple pomace from apples containing a residue
of 4 mg/kg may approach 25 mg/kg (Shell, 1972-74).
The data from the cattle-feeding studies indicate that cattle fed
normal amounts (up to 25% of the total diet) of apple pomace made from
apples treated with fenbutatin oxide according to good agricultural
practice are unlikely to yield milk containing detectable amounts of
the acaricide. No measurable residues are likely in muscle tissues,
but they might occasionally occur at low levels (<0.2 mg/kg) in
liver and kidney.
In plants
Fenbutatin oxide is slowly lost after application to crops. The
degradation to in-organic tin occurs through successive loss of the
phenyldimethylethyl groups. The principal organotin degradation
product found on crops is 1,1,3,3,tetrakis(ß, ß-dimethylphenethyl)-
1,3-dihydroxydistannoxane, referred to subsequently as SD 31723(Shell,
1972).
In studies of the fate of fenbutatin oxide on leaves and fruit of
apples and citrus (Shell, 1972, 1974a), small apple trees grown in a
lath-house were treated one to three times with 119Sn-labelled
fenbutatin oxide with approximately 40µg at each application.
Recoveries of 119Sn 2 to 33 days after the last application were
84-96% of the amount applied. Virtually all the radio-activity in the
fruit was found to be on the outer surface of the skin. Substantial
amounts of the radio-activity were removed by rinsing with organic
solvents, or simply by siping the fruit-skin with paper tissues. It
was shown that less than 5% of the total radio-activity present in the
fruit originated from the principal breakdown product SD 31723 and
less than 0.7% from other organotin metabolites including (ß, ß-
dimethylphenethystannoic acid polymer (SD 33608). The structural
formula of the metabolites SD 31723 and SD 33608 are shown on the
following page. Inorganic tin accounted for nearly 10% of the total
119Sn on the apples (Shell, 1972).
 In another experiment apples were treated in a similar way either with
the parent chemical or with SD 31723 and analysed six weeks later. The
result indicated that the rate of breakdown of SD 31723 exceeded that
of fenbutatin oxide itself. It was concluded that fenbutatin oxide is
degraded firstly to SD 31723, and to SD 33608 and eventually to
inorganic tin compounds. Since SD 31723 was degraded faster than the
parent compound, it is unlikely to build up treated crops and would
therefore not be expected to form an appreciable part of any residue
present.
Similar experiments were carried out on oranges (Shell, 1974a). Leaves
were treated with formulated 119Sn-labelled fenbutatin oxide at a
concentration of 39.5 mg/1 and analysed at intervals up to nine months
after treatment. 40 days after the treatment 65% of the applied
radio-activity was still present in the leaves, of which 71% was
unchanged fenbutatin oxide, 3.4% SD 31723, 0.5% SD 33608 and the rest
inorganic tin. At the end of the 9-month period the total
radio-activity present had declined to about 11% of that applied, and
of this almost 60% was still present as fenbutatin oxide; SD 31723
accounted for about 3% and SD 33608 for less than 1% whilst inorganic
tin made up the remainder (Shell, 1974a).
In other experiments oranges with a diameter of 3-4 cm. were similarly
treated at a rate corresponding to 0.2/kg on the anticipated weight at
harvest 6 months later. Some of the oranges were again treated prior
to harvest to give a total dose corresponding to 0.44 mg/kg on harvest
weight and left on the tree for a further 2 months.
The fruit after picking was separated into peel, juice and pulp which
were separately analysed. No radio-activity was detected in the juice
or in the pulp of any of the samples, at the limit of determination
assessed to be 0.001 mg/kg (calculated as fenbutatin oxide). Residues
in the peel of the fruit harvested 6 months after a single treatment
averaged 0.095 mg/kg and 2 months after a second treatment 0.172
mg/kg.
In both instances approximately 6% of the residue was soluble in
organic solvents and where analysed was found to consist largely
(about 90%) of unchanged fenbutatin oxide (Shell, 1974a).
These results therefore confirmed those obtained on apples.
Residue data from SD 31723 in crops
An analytical method for the routine determination of SD 31723 was
used on numerous samples taken from crops treated in the field with
fenbutatin oxide. Residues on SD 31723 were generally below the limit
of determination (usually in these analyses 0.1-0.2 mg/kg, except in
one set of strawberry samples, where the limit was 0.04 mg/kg).
Positive residues were found only in one trial with peaches, where
levels up to 0.3 mg/kg occurred; the residue was solely in the skin.
These data are in line with the observations in other experiments that
residues from fenbutatin oxide are sometimes higher in peaches than in
the other crops studied (Shell, 1976).
In storage and processing
Since fenbutatin oxide is moderately stable, insoluble in water and
remaining largely on the exterior of treated crops, it is clear that
processing which affects the surface of the crop may considerably
reduce the residue levels; some details are given below.
Washing Experiments with apples and citrus showed that fenbutatin
residues may be considerably reduced by washing. 60% or more of the
original residue was removed by this process.
Peeling The removal of the outer peel of the fruit or other
commodity reduces the residue by at least 75%, depending on the
commodity.
Juice extraction Data on residues in extracted citrus juice are
generally below the limit of determination (0.02-0.05 mg/l). In only
one instance was a small residue of fenbutatin oxide (0.08 mg/1)
found.
Apple juice has been obtained with similarly low residues (Shell,
1972-1974). From studies with the radio-labelled compound, residues
would not be expected to occur in the interior of the apples and the
data on juice confirm that the residues on the surface of the fruit
are not extracted to an appreciable extent with the juice during its
preparation. No residues of the main metabolite SD 31723 have been
found in any juice from treated fruits (Shell, 1972, 1974a).
Vinification Wine from grapes containing up to 5.6 mg/kg of
fenbutatin oxide in the whole fruit contained less than 0.02 mg/1, the
limit of determination (Shell, 1974b, 1975). Similarly the breakdown
product SD 31723 was not found in the wine at the limit of
determination of 0.1 mg/1 (Shell, 1975).
In soil
Residue degradation in sterile and non-sterile soils. The residue
degradation of 119Sn-labelled fenbutatin oxide was studied in a
steam-sterilized and unsterilized Hanford sandy loam (particles 2
11.1%; between 2 and 20 21.9%; 20 67%; organic material 0.8-1%; pH
4.6-6.5). 10 mg/kg 119Sn-labelled fenbutatin oxide was mixed into
both soils which were stored in the same room at ambient temperature.
Almost all the 119Sn was organosoluble. Fenbutatin oxide decreased
initially faster in sterile than in normal soil. However the rate of
decrease later became less in sterile than in live soils. It is
suggested that steam sterilisation activates catalytically active
sites on the soil so that more molecules can be degraded initially.
The subsequent decrease in rate as compared with live soils indicates
that microbial degradation, which occurred in the latter became the
main factor in degradation. No accumulation of degradation products
except 119Sn could be 254 fenbutatin oxide measured (Shell, 1973b)
see Table 12.
Metabolism in soil under aerobic and anaerobic conditions
In other experiment in which 10 mg/kg 119Sn-labelled fenbutatin
oxide was mixed into the same soil, the residue degradation was
studied under aerobic and anaerobic conditions. One set of soil
samples was kept under aerobic conditions at ambient temperatures in a
glasshouse; another set was kept under nitrogen after an initial 30
days aerobic period. The amount of solvent-extractable radio-activity,
which was nearly all 119Sn-fenbutatin oxide, decreased slowly during
the 180 days of the experiment. No significant differences could be
found between the rates of degradation under aerobic and anaerobic
conditions (Shell, 1973a).
TABLE 12. Radio-activity extracted from sterile and non sterile soil at intervals after treatment
with fenbutatin oxide
119Sn as mg/kg fenbutatin oxide equivalents
Days after sterile soil live soil
treatment
0 10.1 10.1
30 9.3 9.8
60 9.2 9.1
90 9.0 8.6
120 8.7 8.5
180 8.5 7.9(Shell, 1973b)
The degradation of 14C-labelled fenbutatin oxide was also studied in
Hanford sandy loam under outdoor field conditions. The compound was
applied at a rate of 1.5 kg/ha either as a single application or as
repeated applications, each at the same rate, at six-monthly
intervals. The degradation followed first order kinetics during the
first 12 months and accelerated considerably during the next six
months so that one-half of the 14C-fenbutatin still present after 12
months was degraded during the following 6 months. A similar increase
in the degradation rate was also found in the experiments in which
14C-fenbutatin oxide was applied repeatedly. Since the environmental
conditions did not vary significantly from one year to the other, it
is suggested that adaptation of micro-organisms to the compound was
the primary cause of the increase. No accumulation of the degradation
products SD 31723 or SD 33608 could be detected at any time (Shell,
1974).
Under the field conditions 14C-labelled fenbutatin oxide applied to
the soil surface remained mainly in the top 7.5 cm. No measurable
amounts or very low residues were found in the 22.5-30 cm layer (Table
13).
TABLE 13. Carbon-14 extractable from soil after a single application of fenbutatin
oxide
Depth of soil 14C, as mg/kg fenbutation oxide equivalents after
0 4 months 12 months 18 months
0-7´ cm 1.51 1.04 0.66 0.27
7´-15 cm -.- 0.06 0.06 0.03
15-22´ cm -.- 0.05 < 0.02 0.04
22´-30 cm -.- 0.03 < 0.02 <0.02
Mobility in soil and leaching
A sample of Hanford loam, which had been treated with 14C-labelled
fenbutatin oxide and aged outdoors for 4 months, was placed on top of
a column of the same but untreated soil, 30 cm long. The soil was
saturated with water and leached by applying a total of 50 cm water
over a period of 51 days. The 14C content in each of the daily
effluent samples was below the detection limit of 0.002 mg/kg
equivalents of fenbutatin oxide. The soil on top of the column
contained 1.22 mg/kg equivalents of fenbutatin oxide initially and
1.16 mg/kg at the end of the experiments.
The four 7.5 cm. layers of the column contained 14C equivalent
respectively to 0.02, 0.017, < 0.01 and < 0.01 mg/kg of fenbutatin
oxide at the end of the experiments (Loeffler, 1973).
In air and UV light
Thin layers of 119Sn fenbutatin oxide on a glass surface when
exposed to sunlight slowly decomposed to the derivative SD 31723 and
to more polar compounds such as inorganic tin salts. After 230 hours
of exposure, 81% of the initial 119Sn deposit was extractable by
organic solvents and 6% by aqueous solvents. In this sample 76% of the
organo-soluble 119Sn consisted of unchanged fenbutatin oxide and
21.3% of the degradation product SD 31723 (Shell, 1973d).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE, OR AT
CONSUMPTION
No information available.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods of analysis for fenbutatin oxide residues
on fruit crops and fruits or vegetables have been developed (Shell,
undated, 19746).
Three modifications of a basic method are available. The finely
divided crop sample is extracted with methylene chloride,
concentrated, diluted with hexane and treated with hydrochloric acid,
to form a chloro-derivative. The resultant solution is then cleaned up
on an acid alumina column before determination by GLC. The method has
been adapted for use on samples of milky animal tissues and other
materials (Shell, undated). The modifications differ only in detail
and the choice of the method is dependent on the equipment and
solvents available, the commodities to be analysed and the analyst's
personal preference. Limits of determination are in general 0.01
- 0.05 mg/kg.
Tests have been carried out to determine the loss of residue during
storage at deep-freeze temperatures (-15°C) whilst awaiting analysis.
No changes in residue levels could be detected during a period of
16-18 months (Shell, 19740).
Two methods have also been developed for the determination of the
principal breakdown product of fenbutatin oxide, SD 31723. Both
involve extraction of the finely divided sample with methylene
chloride as for the parent compound. The extracts are subjected to
clean-up on an alumina or silica gel column and the eluates are
concentrated and analysed by a thin layer chromatographic technique.
After development the plates are treated with a suitable reagent to
visualize the tin-containing products. The determinations are
completed by visual comparison of the colour and size of the spots
with standards run under the same conditions. A limit of determination
of 0.1-0.2 mg/kg SD 31723 can be achieved (Shell, undated, undated).
APPRAISAL
Fenbutatin oxide is used as an acaricide as a foliar application on a
considerable scale on a wide variety of fruits and fruiting vegetables
in various countries.
Fenbutatin oxide is mainly marketed as a 50% wettable powdery but also
as a suspension concentrate. The rates of application vary depending
on crop, pest and method of application; normal rates are usually 1-2
kg a.i./ha.
The recommended pre-harvest intervals in various countries vary
considerably for one and the same crop.
Residue data from supervised trials were obtained from several
countries. Residues do not penetrate through the skin of treated fruit
to any great extent, and in the pulp are therefore generally below the
limit of determination.
Extensive data are available on the fate of the residues in plants and
on the levels of residues in products of animal origin arising from
the feeding of treated commodities including the pulp of treated
fruits. On apples after 33 days the remaining residue consists of
about 85% unchanged parent compound, 5% of the principal organotin
metabolite SD 31723 and about 10% of inorganic tin.
The degradation of the main metabolite SD 31723 is faster than that of
the parent compound and thus the metabolite will in general not form
an appreciable part of any residue present in practical conditions.
Occasionally residues not exceeding 0.3 mg/kg of the parent compound
could be found in the kidneys of cows fed with treated feeds and it
was calculated that these residues mould not exceed 0.2 mg/kg in
practical circumstances; in meat and milk, residues were undetectable
(i.e. below 0.02 mg/kg).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national maximum residue limits for fenbutation oxide are
established or under consideration:
Country Commodity maximum residue limit, mg/kg
Australia Apples, pears, peaches 3
Belgium Apples, pears 1
Fed. Rep. of Germany Pome fruit 2
The Netherlands Apples, pears, cherries,
plums 1
Blackberries,
raspberries, strawberries,
black- and red currants 0.2
gherkins (glasshouse
and outdoors) 1
Cucumbers* , melons*,
bell-peppprs *,
eggplant *, tomatoes * 0.3
Italy Apples, pears, apricots,
cherries, peaches,
plums, citrus fruits,
grapes, cucumbers,
tomatoes 0.5
South Africa Apples, pears, peaches,
citrus fruits 2
Switzerland Pome fruit, stone fruit, grapes 1.5
U.S.A. Apples, pears, citrus fruits 4
Liver and kidney of cattle,
goats, horses, pigs and sheep 0.3
Dried apple pomace 20
Dried citrus pulp 7
*glasshouse crops
Fenbutatin oxide applied in field conditions to the soil remains in
the upper layers and is slowly degraded, forming non-mobile
metabolites. Washing fruits and vegetables removes 60% or more of the
original residue, and removal of the outer peel of apples or citrus
reduces the residue by at least 75%.
Gas-chromatographic methods for residue analysis for fenbutatin oxide
in fruit crops, fruits of vegetables, milk and animal tissues are
available, which are suitable or can be adapted for regulatory
purposes. Methods for the determination of the principal metabolite
have also been developed.
RECOMMENDATIONS
The following maximum residue limits for fenbutatin oxide are
recommended. They refer to fenbutatin oxide, excluding any
metabolites.
Commodity Limit, mg/kg Pre-harvest interval
on which
recommendations
are based
Dried apple pomace 20 -
Dried citrus pulp 7 -
Peaches 7 14-21
Apples and pears 5 14
Cherries (sour and sweet) 5 14-21
Citrus fruits 5 7-14
Plums 3 7-14
Strawberries 3 7-14
Fruits of vegetables
(cucumbers, eggplants,
gherkins, melons, sweet
peppers, tomatoes) 1 3-7
Liver and kidney of cattle,
goats, horses, pigs and
sheep 0.2
Meat of cattle, goats,
horses,
pigs and sheep 0.02*
Milk 0.02*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the EEG findings in the dog.
2. Elucidation of species differences in toxic effects on rabbit
testes.
REFERENCES
Dean, B.J., Doak, S.M.A. and Funnell, J. (1972a) Toxicity studies with
SD 14114: genetic studies with SD 14114 in micro-organisms in vitro
and in the host-mediated assay. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Dean, B.J., Doak, S.M., Perquin, B.D. and Senner, K. (1972b) Toxicity
studies with SD 14114: the cytogenetic investigation of bone marrow
cells of mice after a single oral dose of SD 14114. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
the Shell Chemical Company.
Dean, B.J. and Doak, S.M. (1972c) Toxicity studies with SD 14114: the
effects of a single oral dose of SD 14114 on dominant lethal mutations
in male mice. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Company.
Dix, K.M. and Wilson, A.B. (1973) Toxicity studies with SD 14114:
Teratological studies in rabbits given SD 14114 orally. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Granville, G.C., Simpson, B.J. (1973a) and Doak, S.M. Toxicity studies
on the pesticide SD 14114: an 18 month study in mice. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by Shell Chemical Company.
Granville, G.C. and Dix, K.M. (1973b) Toxicity studies on the
pesticide SD 14114: Two year oral toxicity test in dogs. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Hine, C.H., Eisenlord, G. and Loguvan, G.S. (1973) Results of
reproduction study of rats fed diets containing SD 14114-V over three
generations. Unpublished report from Hine Laboratories Inc. submitted
to the World Health Organization by the Shell Chemical Company.
Loeffler, J.E. (1972) The fate of Ingested 119Sn SD 14114 in Rats.
Unpublished report from Shell Development Company, Biological Sciences
Research Center submitted to the World Health Organization by Shell
Chemical Co.
Loeffler, J.E. (1973) Leaching of 14C-SD 14114 from Soil.
Unpublished report TIR-22-112-73, from Shell International.
Natoff I.L. (1973) Toxicity studies on the pesticide SD 14114: acute
oral toxicity studies in the mouse, rat, and rabbit of the metabolite
SD 31723. Unpublished report from Tunstall Laboratory submitted to the
World Health Organization by the Shell Chemical Company.
Pickering, R.G. (1973a) Toxicity studies on the pesticide SD 14114:
Studies on the origin of the increase serum alkaline phosphatase
activity (SAP) in rats fed SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Pickering, R.G. (1973b) Toxicity studies on the pesticide SD 14114:
The reversibility of the increase in rat serum alkaline phosphatase
(SAP) activity produced by SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Samules, G.M.R. and Dix, K.M. (1972) Toxicology of the Pesticide SD
14114: Comparative oldema assay of organotin compounds for oldema
formation in the central nervous system of rate. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
Shell Chemical Company.
Shell Chimie S.A. (1972-1974) Reports TIR-26-131-72, TIR-26-197-73 and
TIR-26-101-74; unpublished.
Shell Chimie S.A. (1973) Reports TIR-26-119-73 and TIR-22-126-73,
unpublished.
Shell Chimie S.A. (1975) Reports BEGR 0019.75 and BEGR 0020.75,
unpublished.
Shell Chirnie S.A. (1976) Report BEGR. 0068.76, unpublished.
Shell Development Co. G.L.C. Methods for residue analysis of
fenbutatin oxide. Unpublished methods MMS-R-345-1, MMS-R-391-1 and
WAMS 215-1 (SAMS-1).
Shell Development Co. (1972) Fate of 119Sn (SD 14114) on apple
trees. Unpublished report.
Shell Development Co. (1972, 1973) Reports TIR-26-113-72 and
TIR-26-197-73 and TIR-26-101-74, unpublished.
Shell Development Co. (1974a) Fate of 119Sn (SD 14114) on Orange
leaves and Oranges. Unpublished report.
Shell Development Co. (1974b) Reports TIR-24-178-74 and TIR-24-238-74,
unpublished.
Shell Development Co. (1974c) Report TIR-26-116-74. Unpublished
report.
Shell International (1971,1976) Residue data from Shell Chimie S.A.,
Shell Development Go. and Shell Research Ltd., Unpublished,
Shell International (1973a) 119Sn-SD 14114 Metabolism Study in Soil
under Aerobic and Anaerobic conditions. Unpublished reports.
Shell International (1973b) 119Sn-SD 14114 Metabolism study in
sterilized and unsterilized soil. Unpublished report.
Shell International (1973d) Photochemical Degradation of SD
14114-119Sn on a glass surface. Unpublished report TIR-22-113-73.
Shell International (1974) Degradation of 14C-SD 14114 after
exposure on soil for 18 month. Unpublished report TIR-22-102-74.
Shell Research Ltd. Residue analysis of SD 14114 (fenbutatin oxide)
and its main metabolite SD 31723. Unpublished method WAMS 212-1 (SAMS
212-1).
Simpson B.J. (1972a) The toxicity of the pesticide SD 14114: Summary
of results of preliminary experiments. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J. (1972b) Toxicity studies on the pesticide SD 14114:
Effect of SD 14114, incorporated in the diet, on the growth rate of
male rats. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Co.
Simpson B.J. and Dix, K.M. (1972c) Toxicity studies on the pesticide
SD 14114: acute and 90 day feeding studies in the rat of the
metabolite SD 31723. Unpublished report from Tunstall Laboratory
submitted to the World Health Organization by the Shell Chemical
Company.
Simpson B.J. and Thorpel B. (1973a) Toxicity studies on the pesticide
SD 14114: Three month feeding study in rats. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J., Grainville, G. and Doak, S.M. (1973b) Toxicity studies
on the pesticide SD 14114: Two year oral experiment in rats.
Unpublished report from Tunstall Laboratory submitted to the World
Health Organization by the Shell Chemical Company.
In another experiment apples were treated in a similar way either with
the parent chemical or with SD 31723 and analysed six weeks later. The
result indicated that the rate of breakdown of SD 31723 exceeded that
of fenbutatin oxide itself. It was concluded that fenbutatin oxide is
degraded firstly to SD 31723, and to SD 33608 and eventually to
inorganic tin compounds. Since SD 31723 was degraded faster than the
parent compound, it is unlikely to build up treated crops and would
therefore not be expected to form an appreciable part of any residue
present.
Similar experiments were carried out on oranges (Shell, 1974a). Leaves
were treated with formulated 119Sn-labelled fenbutatin oxide at a
concentration of 39.5 mg/1 and analysed at intervals up to nine months
after treatment. 40 days after the treatment 65% of the applied
radio-activity was still present in the leaves, of which 71% was
unchanged fenbutatin oxide, 3.4% SD 31723, 0.5% SD 33608 and the rest
inorganic tin. At the end of the 9-month period the total
radio-activity present had declined to about 11% of that applied, and
of this almost 60% was still present as fenbutatin oxide; SD 31723
accounted for about 3% and SD 33608 for less than 1% whilst inorganic
tin made up the remainder (Shell, 1974a).
In other experiments oranges with a diameter of 3-4 cm. were similarly
treated at a rate corresponding to 0.2/kg on the anticipated weight at
harvest 6 months later. Some of the oranges were again treated prior
to harvest to give a total dose corresponding to 0.44 mg/kg on harvest
weight and left on the tree for a further 2 months.
The fruit after picking was separated into peel, juice and pulp which
were separately analysed. No radio-activity was detected in the juice
or in the pulp of any of the samples, at the limit of determination
assessed to be 0.001 mg/kg (calculated as fenbutatin oxide). Residues
in the peel of the fruit harvested 6 months after a single treatment
averaged 0.095 mg/kg and 2 months after a second treatment 0.172
mg/kg.
In both instances approximately 6% of the residue was soluble in
organic solvents and where analysed was found to consist largely
(about 90%) of unchanged fenbutatin oxide (Shell, 1974a).
These results therefore confirmed those obtained on apples.
Residue data from SD 31723 in crops
An analytical method for the routine determination of SD 31723 was
used on numerous samples taken from crops treated in the field with
fenbutatin oxide. Residues on SD 31723 were generally below the limit
of determination (usually in these analyses 0.1-0.2 mg/kg, except in
one set of strawberry samples, where the limit was 0.04 mg/kg).
Positive residues were found only in one trial with peaches, where
levels up to 0.3 mg/kg occurred; the residue was solely in the skin.
These data are in line with the observations in other experiments that
residues from fenbutatin oxide are sometimes higher in peaches than in
the other crops studied (Shell, 1976).
In storage and processing
Since fenbutatin oxide is moderately stable, insoluble in water and
remaining largely on the exterior of treated crops, it is clear that
processing which affects the surface of the crop may considerably
reduce the residue levels; some details are given below.
Washing Experiments with apples and citrus showed that fenbutatin
residues may be considerably reduced by washing. 60% or more of the
original residue was removed by this process.
Peeling The removal of the outer peel of the fruit or other
commodity reduces the residue by at least 75%, depending on the
commodity.
Juice extraction Data on residues in extracted citrus juice are
generally below the limit of determination (0.02-0.05 mg/l). In only
one instance was a small residue of fenbutatin oxide (0.08 mg/1)
found.
Apple juice has been obtained with similarly low residues (Shell,
1972-1974). From studies with the radio-labelled compound, residues
would not be expected to occur in the interior of the apples and the
data on juice confirm that the residues on the surface of the fruit
are not extracted to an appreciable extent with the juice during its
preparation. No residues of the main metabolite SD 31723 have been
found in any juice from treated fruits (Shell, 1972, 1974a).
Vinification Wine from grapes containing up to 5.6 mg/kg of
fenbutatin oxide in the whole fruit contained less than 0.02 mg/1, the
limit of determination (Shell, 1974b, 1975). Similarly the breakdown
product SD 31723 was not found in the wine at the limit of
determination of 0.1 mg/1 (Shell, 1975).
In soil
Residue degradation in sterile and non-sterile soils. The residue
degradation of 119Sn-labelled fenbutatin oxide was studied in a
steam-sterilized and unsterilized Hanford sandy loam (particles 2
11.1%; between 2 and 20 21.9%; 20 67%; organic material 0.8-1%; pH
4.6-6.5). 10 mg/kg 119Sn-labelled fenbutatin oxide was mixed into
both soils which were stored in the same room at ambient temperature.
Almost all the 119Sn was organosoluble. Fenbutatin oxide decreased
initially faster in sterile than in normal soil. However the rate of
decrease later became less in sterile than in live soils. It is
suggested that steam sterilisation activates catalytically active
sites on the soil so that more molecules can be degraded initially.
The subsequent decrease in rate as compared with live soils indicates
that microbial degradation, which occurred in the latter became the
main factor in degradation. No accumulation of degradation products
except 119Sn could be 254 fenbutatin oxide measured (Shell, 1973b)
see Table 12.
Metabolism in soil under aerobic and anaerobic conditions
In other experiment in which 10 mg/kg 119Sn-labelled fenbutatin
oxide was mixed into the same soil, the residue degradation was
studied under aerobic and anaerobic conditions. One set of soil
samples was kept under aerobic conditions at ambient temperatures in a
glasshouse; another set was kept under nitrogen after an initial 30
days aerobic period. The amount of solvent-extractable radio-activity,
which was nearly all 119Sn-fenbutatin oxide, decreased slowly during
the 180 days of the experiment. No significant differences could be
found between the rates of degradation under aerobic and anaerobic
conditions (Shell, 1973a).
TABLE 12. Radio-activity extracted from sterile and non sterile soil at intervals after treatment
with fenbutatin oxide
119Sn as mg/kg fenbutatin oxide equivalents
Days after sterile soil live soil
treatment
0 10.1 10.1
30 9.3 9.8
60 9.2 9.1
90 9.0 8.6
120 8.7 8.5
180 8.5 7.9(Shell, 1973b)
The degradation of 14C-labelled fenbutatin oxide was also studied in
Hanford sandy loam under outdoor field conditions. The compound was
applied at a rate of 1.5 kg/ha either as a single application or as
repeated applications, each at the same rate, at six-monthly
intervals. The degradation followed first order kinetics during the
first 12 months and accelerated considerably during the next six
months so that one-half of the 14C-fenbutatin still present after 12
months was degraded during the following 6 months. A similar increase
in the degradation rate was also found in the experiments in which
14C-fenbutatin oxide was applied repeatedly. Since the environmental
conditions did not vary significantly from one year to the other, it
is suggested that adaptation of micro-organisms to the compound was
the primary cause of the increase. No accumulation of the degradation
products SD 31723 or SD 33608 could be detected at any time (Shell,
1974).
Under the field conditions 14C-labelled fenbutatin oxide applied to
the soil surface remained mainly in the top 7.5 cm. No measurable
amounts or very low residues were found in the 22.5-30 cm layer (Table
13).
TABLE 13. Carbon-14 extractable from soil after a single application of fenbutatin
oxide
Depth of soil 14C, as mg/kg fenbutation oxide equivalents after
0 4 months 12 months 18 months
0-7´ cm 1.51 1.04 0.66 0.27
7´-15 cm -.- 0.06 0.06 0.03
15-22´ cm -.- 0.05 < 0.02 0.04
22´-30 cm -.- 0.03 < 0.02 <0.02
Mobility in soil and leaching
A sample of Hanford loam, which had been treated with 14C-labelled
fenbutatin oxide and aged outdoors for 4 months, was placed on top of
a column of the same but untreated soil, 30 cm long. The soil was
saturated with water and leached by applying a total of 50 cm water
over a period of 51 days. The 14C content in each of the daily
effluent samples was below the detection limit of 0.002 mg/kg
equivalents of fenbutatin oxide. The soil on top of the column
contained 1.22 mg/kg equivalents of fenbutatin oxide initially and
1.16 mg/kg at the end of the experiments.
The four 7.5 cm. layers of the column contained 14C equivalent
respectively to 0.02, 0.017, < 0.01 and < 0.01 mg/kg of fenbutatin
oxide at the end of the experiments (Loeffler, 1973).
In air and UV light
Thin layers of 119Sn fenbutatin oxide on a glass surface when
exposed to sunlight slowly decomposed to the derivative SD 31723 and
to more polar compounds such as inorganic tin salts. After 230 hours
of exposure, 81% of the initial 119Sn deposit was extractable by
organic solvents and 6% by aqueous solvents. In this sample 76% of the
organo-soluble 119Sn consisted of unchanged fenbutatin oxide and
21.3% of the degradation product SD 31723 (Shell, 1973d).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE, OR AT
CONSUMPTION
No information available.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods of analysis for fenbutatin oxide residues
on fruit crops and fruits or vegetables have been developed (Shell,
undated, 19746).
Three modifications of a basic method are available. The finely
divided crop sample is extracted with methylene chloride,
concentrated, diluted with hexane and treated with hydrochloric acid,
to form a chloro-derivative. The resultant solution is then cleaned up
on an acid alumina column before determination by GLC. The method has
been adapted for use on samples of milky animal tissues and other
materials (Shell, undated). The modifications differ only in detail
and the choice of the method is dependent on the equipment and
solvents available, the commodities to be analysed and the analyst's
personal preference. Limits of determination are in general 0.01
- 0.05 mg/kg.
Tests have been carried out to determine the loss of residue during
storage at deep-freeze temperatures (-15°C) whilst awaiting analysis.
No changes in residue levels could be detected during a period of
16-18 months (Shell, 19740).
Two methods have also been developed for the determination of the
principal breakdown product of fenbutatin oxide, SD 31723. Both
involve extraction of the finely divided sample with methylene
chloride as for the parent compound. The extracts are subjected to
clean-up on an alumina or silica gel column and the eluates are
concentrated and analysed by a thin layer chromatographic technique.
After development the plates are treated with a suitable reagent to
visualize the tin-containing products. The determinations are
completed by visual comparison of the colour and size of the spots
with standards run under the same conditions. A limit of determination
of 0.1-0.2 mg/kg SD 31723 can be achieved (Shell, undated, undated).
APPRAISAL
Fenbutatin oxide is used as an acaricide as a foliar application on a
considerable scale on a wide variety of fruits and fruiting vegetables
in various countries.
Fenbutatin oxide is mainly marketed as a 50% wettable powdery but also
as a suspension concentrate. The rates of application vary depending
on crop, pest and method of application; normal rates are usually 1-2
kg a.i./ha.
The recommended pre-harvest intervals in various countries vary
considerably for one and the same crop.
Residue data from supervised trials were obtained from several
countries. Residues do not penetrate through the skin of treated fruit
to any great extent, and in the pulp are therefore generally below the
limit of determination.
Extensive data are available on the fate of the residues in plants and
on the levels of residues in products of animal origin arising from
the feeding of treated commodities including the pulp of treated
fruits. On apples after 33 days the remaining residue consists of
about 85% unchanged parent compound, 5% of the principal organotin
metabolite SD 31723 and about 10% of inorganic tin.
The degradation of the main metabolite SD 31723 is faster than that of
the parent compound and thus the metabolite will in general not form
an appreciable part of any residue present in practical conditions.
Occasionally residues not exceeding 0.3 mg/kg of the parent compound
could be found in the kidneys of cows fed with treated feeds and it
was calculated that these residues mould not exceed 0.2 mg/kg in
practical circumstances; in meat and milk, residues were undetectable
(i.e. below 0.02 mg/kg).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national maximum residue limits for fenbutation oxide are
established or under consideration:
Country Commodity maximum residue limit, mg/kg
Australia Apples, pears, peaches 3
Belgium Apples, pears 1
Fed. Rep. of Germany Pome fruit 2
The Netherlands Apples, pears, cherries,
plums 1
Blackberries,
raspberries, strawberries,
black- and red currants 0.2
gherkins (glasshouse
and outdoors) 1
Cucumbers* , melons*,
bell-peppprs *,
eggplant *, tomatoes * 0.3
Italy Apples, pears, apricots,
cherries, peaches,
plums, citrus fruits,
grapes, cucumbers,
tomatoes 0.5
South Africa Apples, pears, peaches,
citrus fruits 2
Switzerland Pome fruit, stone fruit, grapes 1.5
U.S.A. Apples, pears, citrus fruits 4
Liver and kidney of cattle,
goats, horses, pigs and sheep 0.3
Dried apple pomace 20
Dried citrus pulp 7
*glasshouse crops
Fenbutatin oxide applied in field conditions to the soil remains in
the upper layers and is slowly degraded, forming non-mobile
metabolites. Washing fruits and vegetables removes 60% or more of the
original residue, and removal of the outer peel of apples or citrus
reduces the residue by at least 75%.
Gas-chromatographic methods for residue analysis for fenbutatin oxide
in fruit crops, fruits of vegetables, milk and animal tissues are
available, which are suitable or can be adapted for regulatory
purposes. Methods for the determination of the principal metabolite
have also been developed.
RECOMMENDATIONS
The following maximum residue limits for fenbutatin oxide are
recommended. They refer to fenbutatin oxide, excluding any
metabolites.
Commodity Limit, mg/kg Pre-harvest interval
on which
recommendations
are based
Dried apple pomace 20 -
Dried citrus pulp 7 -
Peaches 7 14-21
Apples and pears 5 14
Cherries (sour and sweet) 5 14-21
Citrus fruits 5 7-14
Plums 3 7-14
Strawberries 3 7-14
Fruits of vegetables
(cucumbers, eggplants,
gherkins, melons, sweet
peppers, tomatoes) 1 3-7
Liver and kidney of cattle,
goats, horses, pigs and
sheep 0.2
Meat of cattle, goats,
horses,
pigs and sheep 0.02*
Milk 0.02*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the EEG findings in the dog.
2. Elucidation of species differences in toxic effects on rabbit
testes.
REFERENCES
Dean, B.J., Doak, S.M.A. and Funnell, J. (1972a) Toxicity studies with
SD 14114: genetic studies with SD 14114 in micro-organisms in vitro
and in the host-mediated assay. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Dean, B.J., Doak, S.M., Perquin, B.D. and Senner, K. (1972b) Toxicity
studies with SD 14114: the cytogenetic investigation of bone marrow
cells of mice after a single oral dose of SD 14114. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
the Shell Chemical Company.
Dean, B.J. and Doak, S.M. (1972c) Toxicity studies with SD 14114: the
effects of a single oral dose of SD 14114 on dominant lethal mutations
in male mice. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Company.
Dix, K.M. and Wilson, A.B. (1973) Toxicity studies with SD 14114:
Teratological studies in rabbits given SD 14114 orally. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Granville, G.C., Simpson, B.J. (1973a) and Doak, S.M. Toxicity studies
on the pesticide SD 14114: an 18 month study in mice. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by Shell Chemical Company.
Granville, G.C. and Dix, K.M. (1973b) Toxicity studies on the
pesticide SD 14114: Two year oral toxicity test in dogs. Unpublished
report from Tunstall Laboratory submitted to the World Health
Organization by the Shell Chemical Company.
Hine, C.H., Eisenlord, G. and Loguvan, G.S. (1973) Results of
reproduction study of rats fed diets containing SD 14114-V over three
generations. Unpublished report from Hine Laboratories Inc. submitted
to the World Health Organization by the Shell Chemical Company.
Loeffler, J.E. (1972) The fate of Ingested 119Sn SD 14114 in Rats.
Unpublished report from Shell Development Company, Biological Sciences
Research Center submitted to the World Health Organization by Shell
Chemical Co.
Loeffler, J.E. (1973) Leaching of 14C-SD 14114 from Soil.
Unpublished report TIR-22-112-73, from Shell International.
Natoff I.L. (1973) Toxicity studies on the pesticide SD 14114: acute
oral toxicity studies in the mouse, rat, and rabbit of the metabolite
SD 31723. Unpublished report from Tunstall Laboratory submitted to the
World Health Organization by the Shell Chemical Company.
Pickering, R.G. (1973a) Toxicity studies on the pesticide SD 14114:
Studies on the origin of the increase serum alkaline phosphatase
activity (SAP) in rats fed SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Pickering, R.G. (1973b) Toxicity studies on the pesticide SD 14114:
The reversibility of the increase in rat serum alkaline phosphatase
(SAP) activity produced by SD 14114. Unpublished report from Tunstall
Laboratory submitted to the World Health Organization by the Shell
Chemical Company.
Samules, G.M.R. and Dix, K.M. (1972) Toxicology of the Pesticide SD
14114: Comparative oldema assay of organotin compounds for oldema
formation in the central nervous system of rate. Unpublished report
from Tunstall Laboratory submitted to the World Health Organization by
Shell Chemical Company.
Shell Chimie S.A. (1972-1974) Reports TIR-26-131-72, TIR-26-197-73 and
TIR-26-101-74; unpublished.
Shell Chimie S.A. (1973) Reports TIR-26-119-73 and TIR-22-126-73,
unpublished.
Shell Chimie S.A. (1975) Reports BEGR 0019.75 and BEGR 0020.75,
unpublished.
Shell Chirnie S.A. (1976) Report BEGR. 0068.76, unpublished.
Shell Development Co. G.L.C. Methods for residue analysis of
fenbutatin oxide. Unpublished methods MMS-R-345-1, MMS-R-391-1 and
WAMS 215-1 (SAMS-1).
Shell Development Co. (1972) Fate of 119Sn (SD 14114) on apple
trees. Unpublished report.
Shell Development Co. (1972, 1973) Reports TIR-26-113-72 and
TIR-26-197-73 and TIR-26-101-74, unpublished.
Shell Development Co. (1974a) Fate of 119Sn (SD 14114) on Orange
leaves and Oranges. Unpublished report.
Shell Development Co. (1974b) Reports TIR-24-178-74 and TIR-24-238-74,
unpublished.
Shell Development Co. (1974c) Report TIR-26-116-74. Unpublished
report.
Shell International (1971,1976) Residue data from Shell Chimie S.A.,
Shell Development Go. and Shell Research Ltd., Unpublished,
Shell International (1973a) 119Sn-SD 14114 Metabolism Study in Soil
under Aerobic and Anaerobic conditions. Unpublished reports.
Shell International (1973b) 119Sn-SD 14114 Metabolism study in
sterilized and unsterilized soil. Unpublished report.
Shell International (1973d) Photochemical Degradation of SD
14114-119Sn on a glass surface. Unpublished report TIR-22-113-73.
Shell International (1974) Degradation of 14C-SD 14114 after
exposure on soil for 18 month. Unpublished report TIR-22-102-74.
Shell Research Ltd. Residue analysis of SD 14114 (fenbutatin oxide)
and its main metabolite SD 31723. Unpublished method WAMS 212-1 (SAMS
212-1).
Simpson B.J. (1972a) The toxicity of the pesticide SD 14114: Summary
of results of preliminary experiments. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J. (1972b) Toxicity studies on the pesticide SD 14114:
Effect of SD 14114, incorporated in the diet, on the growth rate of
male rats. Unpublished report from Tunstall Laboratory submitted to
the World Health Organization by the Shell Chemical Co.
Simpson B.J. and Dix, K.M. (1972c) Toxicity studies on the pesticide
SD 14114: acute and 90 day feeding studies in the rat of the
metabolite SD 31723. Unpublished report from Tunstall Laboratory
submitted to the World Health Organization by the Shell Chemical
Company.
Simpson B.J. and Thorpel B. (1973a) Toxicity studies on the pesticide
SD 14114: Three month feeding study in rats. Unpublished report from
Tunstall Laboratory submitted to the World Health Organization by the
Shell Chemical Company.
Simpson B.J., Grainville, G. and Doak, S.M. (1973b) Toxicity studies
on the pesticide SD 14114: Two year oral experiment in rats.
Unpublished report from Tunstall Laboratory submitted to the World
Health Organization by the Shell Chemical Company.
