
CARTAP JMPR 1978
Explanation
This pesticide was previously evaluated by the 1976 Meeting
(FAO/WHO, 1977b), when a temporary ADI was established and some
temporary MRLs were recommended and various requirements for
additional information were recorded. Some of this information had
since become available for evaluation.
Attention had also been drawn to the need to re-examine the
MRL recommended for dried green tea to take account of higher
residues found in tea grown in the shade.
Special explanatory note
Certain of the temporary MRLs, although listed correctly in
the Report of the 1976 Meeting (FAO/WHO 1977a), were incorrectly
recorded in the Monograph (FAO/WHO 1977b). The correct figures are
listed again at the end of this Monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Groups of rats (5 female, SD-JCL Strain/group) and mice (4
male, ICR/JCL Strain/group) were administered 35S-cartap HCL by
gavage at a dose of 20 mg/kg body weight. In studies on the
metabolic fate, cartap was previously shown to have been rapidly
absorbed and eliminated in urine within 24 hours following a single
acute oral dose. In extensions of previous studies rats and mice
were shown to excrete cartap rapidly. In urine, 94 and 89% of the
administered dose was excreted within 24 hours in rats and mice,
respectively. There were no apparent differences noted in the
absorption and excretion in the two rodent species with respect to
both qualitative and quantitative recovery of urinary metabolites.
Cartap was rapidly absorbed, metabolized and excreted in both rats
and mice. Several metabolites were identified confirming the
principle routes of degradation. There was no storage of cartap or
its metabolites in the body as might be expected with this
predominantly water-soluble chemical. (Fujita et al., 1971)
Biodegradation
In preliminary experiments where cartap was administered by
intravenous injection it was found to be rapidly metabolized
through two principle routes of degradation, hydrolysis and
oxidation (Fujita et al., 1971). Further studies on the absorption
and metabolism of cartap administered orally to rats has confirmed
the presence of metabolites and the principle routes of
degradation-hydrolysis of the carbonyl carbon and oxidation of
the sulfur atom to the sulfoxide (S O), sulfone (SO2) and
ultimately the sulfate (SO3H) (Kamesaki et al., 1976a).
These studies in rats confirmed and expanded the original
conclusions by elucidating an extensive degradation pattern.
Approximately 85% of the administered oral dose to rats was
found in urine within 48 hours after treatment. Approximately 75%
of the recovered radioactivity was found as nonpolar lipophilic
components, 23% were polar components and 1% was found as sulfates.
On the following page is a probable pathway for the metabolic fate
of cartap which accounts for approximately 70% of the observed
products. An additional 10% of the administered oral dose of cartap
was characterized as 2-methylsulfinyl-3-methylthioprop-1-ene (7.2%)
and 2,3-di(methylsulfinyl) prop-1-ene (2.0%), two liposoluble
metabolites representing further degradation of the molecule (The
origin of these products is unclear based on the structural
configuration of cartap. These structures, although consistent with
the IR and NMR spectral evaluation are difficult to imagine as
coming from the cartap molecule).
Cartap is readily hydrolyzed to nereistoxin, a naturally
occurring insecticidal substance isolated from the marine segmented
worms, Lumbrineris heteropoda. Extensive information is available
on the chemistry and synthesis of nereistoxin and its derivatives,
of which cartap hydrochloride is the most potent. Nereistoxin is in
an apparent equilibrium with its dihydronereistoxin derivative. In
mammals, nereistoxin is methylated and oxidized at the sulfur atom
as well as undergoing a series of oxidative demethylation reactions
at the dimethylaminomoiety. A small (> 1%) quantity of products
was observed to correspond to sulfates.
In rice plants grown in hydroponic solutions or under
similated field conditions containing 35S cartap, absorption and
distribution was rapid with cartap accumulating in all parts of the
plant. Extensive degradation of cartap was found to occur rapidly
with incorporation of 35S into natural components
(sulfur-containing amino acids). Prior to complete degradation,
cartap was observed to be degraded through a series of similar
oxidative pathways as in mammals with the principle exception being
the methylation reaction, as generally occurs in mammals, did not
appear to occur in plants. The pathway of metabolism in plants thus
results in a series of metabolites that substantially differs from
that seen in mammals. Nereistoxin appears to predominant in plants
and undergoes sulfur oxidation to the oxide (sulfinyl) and
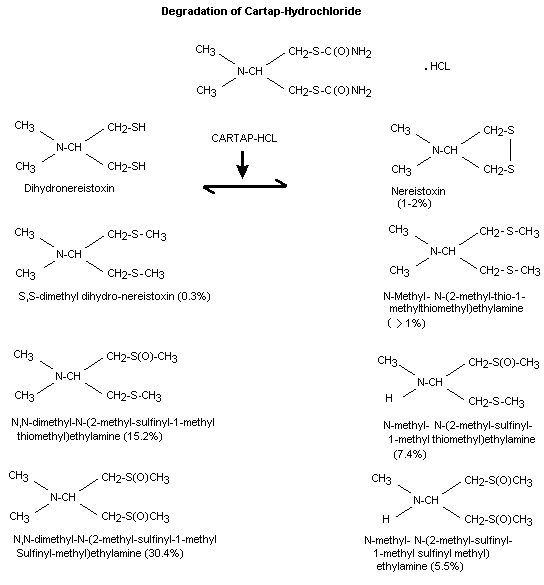 dioxide (sulfonyl) and sulfate as well as N-demethylation. Thus,
the metabolic pathway in plants appears to result in terminal
residues that may be substantially different from those observed in
mammals. This difference appears to result from the initial
equilibrium established with nereistoxin and dihydronereistoxin
both of which follow different pathways in plants and animals to
their ultimate degradation (Kodo et al. 1970; Kamesaki et al.,
1974; Kamesaki et al., 1976b; 1976c., and Sugita et al., 1978).
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Mice
Groups of pregnant mice (CD-1 Strain, 25 mice/group, 24 mice
were used at the highest dose level) were administered an aqueous
solution of cartap by gavage from day 6 through day 15 of gestation
at dosage levels of 0, 10, 25 and 50 mg/kg body weight. The control
was administered a volume of 10 ml water/kg body weight. On day 18
of pregnancy each animal was sacrificed and examinations made of
viable and nonviable fetuses including an examinations of pre and
post-implantation losses. Fetuses were examined for somatic and
skeletal abnormalities.
Maternal weight gain was slightly depressed at the highest
dose level employed, 50 mg/kg/day. There were no differences from
control values with respect to post-implantation losses, litter
size and fetal abnormalities. Cartap appeared to exert no
teratogenic or fetotoxic effects in mice at levels up to and
including that which has been shown to have an adverse effect on
maternal well-being (Tesh et al., 1976a).
Rat
In a similar study to evaluate the teratological potential of
cartap in the rat, groups of pregnant rats (CD-Strain, 20-22 rats
per group), were administered an aqueous solution in cartap at
dosage levels of 0, 10, 25 and 50 mg/kg/day from day 6 to day 15 of
gestation. Rats were sacrificed on day 21 of pregnancy and again
examined for the presence of viable and nonviable fetuses to
determine pre-implantation and post-implanation loss.
Mortality was observed at the highest level with one dam found
dead on day 10 of gestation. There were no apparent effects noted
with respect to maternal growth. The average litter size of the
treated groups were similar to or greater than those noted in the
control. Fetal weight at the highest level of treatment was
slightly reduced. Mean fetal weight was also reduced at the two
intermediate levels although this reduction was not significant.
There were no indications of post-implantation losses. Somatic and
skeletal abnormalities, found in treated groups, were not believed
to have occurred as a result of the administration of cartap, as
they occurred to the same degree in controls. An incidence of
subcutaneous edema slightly in excess of that noted in the control
group was noted in fetuses of the highest dose group. It was
reported that the subcutaneous edema incidence in both the cartap
and control group was considerably higher than noted when
evaluating a larger control population. It was considered that
this effect did not arise from the presence of cartap. A second
effect identified as perimeningeal cavitation in a slight excess
over that noted with the control was noted of the highest dose
group. However, it has been pointed out that perimeningeal
cavitation does exist in control animals and the range of this
occurrence varies widely. It was also considered that this effect
was not attributed to the presence of cartap. A slight reduction
in ossification was noted with respect to the size of the
interior fontanelle and the number of carpels/tarsels. This effect
seemed to correspond to the marginal depression in fetal, weight
observed at the high dose level. It was concluded that daily oral
administration of cartap through the period of organogenesis at
dose levels of 50 mg/kg body weight resulted in a slight reduction
in fetal weight and a marginal retardation in development. There
was no indication that fetal survival was affected or that
malformations were observed (Tesh et al., 1976b).
Special Studies on Mutagenesis
The mutagenic potential of cartap was evaluated using several
strains microorganisms as testor strains. The results of a
rec-assay, evaluating DNA damaging capacity in Bacillus
subtilis (H-17 and M-45 strains) was negative. Reverse mutation
tests, with and without metabolic activation systems using E.
coli and Salmonella typhimurium (TA 98, TA 100, TA 1535, TA
1537 and TA 1538) were all negative. A host mediated assay using
the G 46 strain J.S. typhimurium in the mouse was also negative
(Sherasu et al., 1976).
In vivo cytogenetic effects of cartap were examined using
bone marrow cells of adult male CF-1 Mice and Wistar rats. There
were no increases of chromosomal aberration in cells of rodents
treated with a variety of test programs evaluating cartap. These
tests include doses of 0, 10, 100 and 150 mg/kg/day to mice or 0,
10 and 100 mg/kg/day to rats administered as a single dose or 5
daily doses. In addition, cartap was administered to weanling male
rats orally at 0 or 200 mg/kg/bw or by extraperitoneal injection 0
or 30 mg/kg/bw. In all cases, a positive control of
triethylene-melamine (0.5 mg/kg) or furylfuramide (240 mg/kg) was
employed. A dominant lethal test was performed where male mice were
administered 100 mg/kg/day either as a single or as 5 multiple
daily oral doses. All results showed a complete lack of any
mutagenic activity under the experimental conditions (Kikuchi, et
al, 1976).
COMMENTS
The 1976 Joint Meeting estimated a Temporary Acceptable Daily
Intake for man of 0.05 mg/kg based upon long term studies in two
rodent species. Further data required included detailed studies of
metabolism, further studies of teratogenic and mutagenic potential
and a feeding study in a non-rodent species. Results of additional
studies on metabolism in plants and animals and teratogenicity
tests were made available.
The detailed metabolism studies indicated that Cartap follows
a different route of metabolism in plants and animals and it is
suggested that selective toxicological studies be conducted on
those terminal plant metabolites that differ from the metabolites
found in animals.
New test data on teratogenic potential of cartap, administered
during the full period or organogenesis in mice and rats did not
reveal teratogenic activity at doses above the no effect observed
level established by the 1976 Joint Meeting. Negative results were
obtained in several mutagenicity assays.
In view of these new data which reduced concerns expressed at
the 1976 Joint Meeting the present meeting was able to estimate an
acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 mg/kg bw/day
Dog: 10 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Tea
Of the two cultural methods for growing tea in Japan, the
shade culture expectedly resulted in higher residues than the
open-field culture (Table 1). This is due mainly to the unstable
nature of cartap in sunlight. Residues ranged from 3.3 to 17.4
mg/kg with the shade culture 10 days after the last application
when cartap was applied twice at 1.0 kg a.i./ha. At 14 days after
the last application, the residues ranged from 2.7 to 15.0 mg/kg.
With single applications, a maximum residue of 14.0 mg/kg was
obtained 10 days after the last application. The highest residue
obtained under open field condition was 1.4 mg/kg 10 days after the
last application when cartap was applied twice. Cartap being water
soluble, about 76% of the insecticide is extracted by hot water.
Table 1. Comparative residues of cartap hydrochloride in green tea grown in shade or sunlight.
(50% soluble powder formulation used)
Situation No of Application Rate, Cartap (mg/kg) at intervals (days) after last application
trials (kg ai/ha No) 10 14 15 20 21 27 28
Open field 4 1.0 1 0.37 0.56 0.16
(0.48)
0.73 0.81 0.68
0.49 0.6 0.64
(0.42) (0.43)
0.92 1.12 0.57
Shade 4 1.0 1 7.08 6.60 1.66 0.92
(6.87) (6.12) (1.30) (0.97)
1.76 2.31 0.60 0.36
(1.77) (2.37) (0.60) (0.33)
14 8.51 4.26 2.0
(8.3) (5.8) (2.8) (1.4)
3.55 3.96 0.92 0.58
(2.2) (2.5) (0.7) (0.4)
Open field 4 1.0 2 1.26 1.12 0.22
(0.97)
0.83 0.52 0.45
(0.71)
0.76 0.64 0.38
(0.52)
1.36 1.84 0.56
Shade 4 1.0 2 9.38 7.40
(8.75) (5.50)
3.30 2.66
(2.45) (2.21)
17.4 15.0
(10.6) (9.1)
6.38 5.64
(4.2) (3.4)
Figures in parenthesis are residue equivalent equivalents extracted by hot water
FATE OF RESIDUES
In animals
When cows were fed with 2 and 10 ppm of cartap hydrochloride in
the concentrate part of their ration for 30 consecutive days, some
indications of a transient accumulation of cartap-derived compounds in
milk were observed at the higher feeding level. At the 2 ppm feeding
level, the low apparent residues were attributed to analytical
interferences since similar levels and frequency of occurrence were
also observed in the control group. At the higher feeding level, mean
maximum residues of 33 µg/kg were observed after 7 days treatment.
There was a subsequent decline of residues and no cartap was detected
by the end of the treatment period or during the 30 days recovery
period immediately following (Table 2).
In muscle and liver, the maximum estimated levels of
cartap-derived compounds observed at the end of the treatment period
were 18 µg/kg in muscle and 14 µg/kg in liver, expresse as cartap.
However, the level in liver could be an over estimate because of
interference. No residues could be found in either tissue by the end
of the recovery period. In the kidney, the concentration of residues
appeared to be related to dietary concentration. At the 2 ppm feeding
level, the samples from two animals showed 19 and 33 µg/kg residues at
the end of the treatment period and none the end of the recovery
period. At 10 the ppm level, the residues were 49 and 41 g/kg at the
end of the treatment period declining to 41 and 13 µg/kg, 7 and 30
days respectively after cessation of treatment. No residues were
detected in the fat (Table 3). (Takeda 1978a)
In plants and soil
The absorption and distribution of 35S-labelled cartap
hydrochloride in rice plants grown under various hydroponic conditions
were studied by autoradiography. After 35S was absorbed from the
roots, it was distributed throughout the laminae and high
concentrations were observed in the leaf sheaths. Accumulation
occurred in the leaf apices. Absorption through the leaf sheaths was
also observed, and was found to occur faster in the young leaves than
in the older leaves. Distribution occurred through the vascular
tissues. When applied to the leaves, the insecticide diffused from the
applied region to the leaf apices and accumulated in the leaf sheaths.
35S was detected in the digestive organs, neural tissue and spiracles
of the intoxicated rice stem borers (Kodo et al., 1970).
In 66-day old rice seedlings, the major region of absorption was
observed to be the roots. As much as 15% of the applied
35S-radioactivity was found to have been taken up by the plant,
mainly through the roots. As much as 500 mg/kg accumulated in the rice
plant seedlings after 6 days immersion (Kamesaki et al 1974).
Table 2. Residues in milk of cows fed cartap hydrochloride in the diet.
Cartap, mg/kg, at interval(days)after start of feeding
Cartap in feed Animal
concentrate, ppm number 0 2 4 7 14 22 26 30* 37 51 60
0 3 L T T T T ND ND ND - - -
(control) 11 L T ND T ND ND ND ND - - -
15 ND T T ND T ND ND ND ND ND ND
16 ND ND ND I ND ND ND ND ND - -
2 4 ND L ND T T ND T ND - - -
13 ND ND ND ND T T ND ND ND ND ND
17 ND T ND ND T T ND ND ND - -
20 ND ND ND T ND ND ND - - -
10 1 T 0.02 ND 0.04 0.03 0.02 ND ND - - -
6 T T 0.4 0.04 0.03 0.03 ND ND ND ND ND
7 ND L 0.4 0.02 0.03 T T ND - - -
14 ND 0.02 T 0.03 0.02 0.02 ND ND ND - -
* End of feeding period
All results corrected for 62% recovery.
ND denotes "none detected".
L denotes "sample lost".
I denotes "insufficient sample".
- denotes animal sacrificed.
T denotes less than 0.015 mg/kg.
Table 3. Residues in tissues of cows fed cartap hydrochloride in the diet.
Cartap in feed Animal Time of Cartap, mg/kg in
concentrate, ppm number sacrifice
(days)
Kidney Muscle Liver Fat
0 3 30 ND ND T ND
(control) 11 30 ND ND T ND
16 37 ND ND T ND
15 60 ND ND T ND
Z 4 30 0.02 0.02 0.01 ND
20 30 0.03 NL 0.01 ND
17 37 ND T 0.01 ND
13 60 ND T T ND
10 1 30 0.05 T T ND
7 30 0.04 T 0.01 ND
14 37 0.04 T ND ND
6 60 0.01 T ND ND
All results corrected for appropriate recovery.
ND denotes "none detected"; T denotes 0.01 mg/kg
When paddy cultivation conditions were simulated, 35S-labelled
cartap hydrochloride was rapidly absorbed, a maximum level in most
tissues being reached after 3 days (Table 4). Metabolism to
water-soluble components occurred readily. Accumulation of 35-S in
the panicle was also observed (Kamesaki et al., 1976a). It was
subsequently found by Kameseki et al., (1976b) that under conventional
field practice, the amount of 35S-radioactivity was high in the hull
and rice bran but low in the milled rice. Most of the metabolites were
water soluble and many were amphoteric. Paper chromatography showed
three of the metabolites to have the same Rf values as methionine
sulphoxide, methionine sulphone and S-methyl cysteine Sulphoxide.
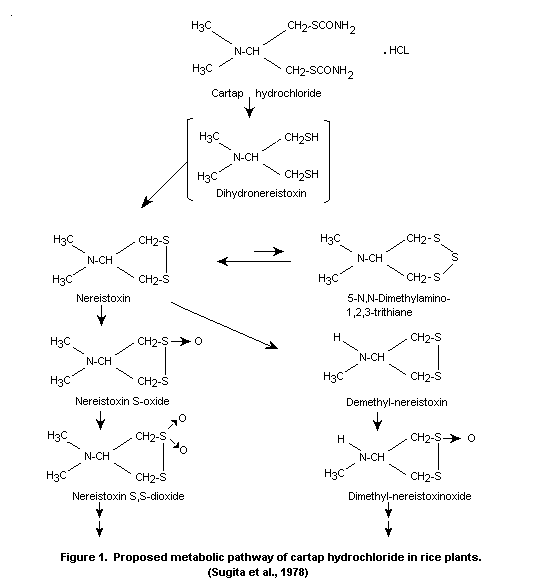
Recently, the formation of seven metabolites was confirmed by Sugita
et al., (1978) who proposed the metabolic pathway of cartap in rice
shown in Figure 1.
In paddy soil, Kamesaki et al., (1976a) found that residues
gradually decrease with time but the individual values cannot readily
be correlated because the samples were taken from different pots.
In water
The 35S-radioactivity from labelled cartap hydrochloride rapidly
declined after application to simulated paddy water as seen in Table 5
(Kamesaki et al., 1976a).
Table 4. Absorption of radioactive components by plant parts.
Days after % of applied radioactivity (mean of duplicates) found in
application
Root Sheath Blade Culm Neck Panicle
1 9.1 27.8 29.5 20.4 4.0 9.3
3 3.6 27.6 48.3 7.1 2.7 10.8
6 5.1 21.8 51.5 5.6 2.8 13.2
13 2.5 18.3 57.5 4.5 1.8 15.4
20 2.1 16.8 54.5 4.0 2.3 20.3
27 3.1 18.2 50.8 4.6 2.9 20.5
34 2.2 19.8 52.1 5.6 2.6 17.6
41 1.5 19.3 54.4 5.4 2.7 16.7
Table 5. 35S radioactivity from labelled cartap
hydrochloride in simulated paddy water (x 105 dmp/pot)
Days after Average of duplicates
application (counts/min. X 10-5)
1 789.5
2 330.9
3 217.6
5 111.8
6 111.4
7 115.1
8 74.2
9 55.6
12 33.5
13 27.3
15 9.3
16 5.7
In processing and cooking
The effect of cooking on cartap residues in rice was determined
by Inoue (1977). It was found that washing the soaked, air-dried,
polished rice before cooking reduced cartap, residues by about one
third. No significant reduction in residues was obtained by boiling.
METHODS OF RESIDUE ANALYSIS
The attention of the Meeting was drawn to difficulties found by
some experienced analysts in established laboratories in using the gas
chromatographic procedure for cartap. The stability of the reference
standards is thought to contribute to the problem.
APPRAISAL
Cartap was previously evaluated in 1976 (FAO/WHO, 1977b). The
manufacturer has submitted additional data in response to the
requirements of that meeting.
Photodecomposition appears to contribute significantly to the
decomposition of the insecticide. This explains the higher cartap,
residues found in green tea grown under shade compared to those in
plants grown in the open field.
In dairy cows, intake results in transient accumulation of the
insecticide in milk. Accumulation was also observed in the kidney but
not in the liver, muscle or fat.
dioxide (sulfonyl) and sulfate as well as N-demethylation. Thus,
the metabolic pathway in plants appears to result in terminal
residues that may be substantially different from those observed in
mammals. This difference appears to result from the initial
equilibrium established with nereistoxin and dihydronereistoxin
both of which follow different pathways in plants and animals to
their ultimate degradation (Kodo et al. 1970; Kamesaki et al.,
1974; Kamesaki et al., 1976b; 1976c., and Sugita et al., 1978).
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Mice
Groups of pregnant mice (CD-1 Strain, 25 mice/group, 24 mice
were used at the highest dose level) were administered an aqueous
solution of cartap by gavage from day 6 through day 15 of gestation
at dosage levels of 0, 10, 25 and 50 mg/kg body weight. The control
was administered a volume of 10 ml water/kg body weight. On day 18
of pregnancy each animal was sacrificed and examinations made of
viable and nonviable fetuses including an examinations of pre and
post-implantation losses. Fetuses were examined for somatic and
skeletal abnormalities.
Maternal weight gain was slightly depressed at the highest
dose level employed, 50 mg/kg/day. There were no differences from
control values with respect to post-implantation losses, litter
size and fetal abnormalities. Cartap appeared to exert no
teratogenic or fetotoxic effects in mice at levels up to and
including that which has been shown to have an adverse effect on
maternal well-being (Tesh et al., 1976a).
Rat
In a similar study to evaluate the teratological potential of
cartap in the rat, groups of pregnant rats (CD-Strain, 20-22 rats
per group), were administered an aqueous solution in cartap at
dosage levels of 0, 10, 25 and 50 mg/kg/day from day 6 to day 15 of
gestation. Rats were sacrificed on day 21 of pregnancy and again
examined for the presence of viable and nonviable fetuses to
determine pre-implantation and post-implanation loss.
Mortality was observed at the highest level with one dam found
dead on day 10 of gestation. There were no apparent effects noted
with respect to maternal growth. The average litter size of the
treated groups were similar to or greater than those noted in the
control. Fetal weight at the highest level of treatment was
slightly reduced. Mean fetal weight was also reduced at the two
intermediate levels although this reduction was not significant.
There were no indications of post-implantation losses. Somatic and
skeletal abnormalities, found in treated groups, were not believed
to have occurred as a result of the administration of cartap, as
they occurred to the same degree in controls. An incidence of
subcutaneous edema slightly in excess of that noted in the control
group was noted in fetuses of the highest dose group. It was
reported that the subcutaneous edema incidence in both the cartap
and control group was considerably higher than noted when
evaluating a larger control population. It was considered that
this effect did not arise from the presence of cartap. A second
effect identified as perimeningeal cavitation in a slight excess
over that noted with the control was noted of the highest dose
group. However, it has been pointed out that perimeningeal
cavitation does exist in control animals and the range of this
occurrence varies widely. It was also considered that this effect
was not attributed to the presence of cartap. A slight reduction
in ossification was noted with respect to the size of the
interior fontanelle and the number of carpels/tarsels. This effect
seemed to correspond to the marginal depression in fetal, weight
observed at the high dose level. It was concluded that daily oral
administration of cartap through the period of organogenesis at
dose levels of 50 mg/kg body weight resulted in a slight reduction
in fetal weight and a marginal retardation in development. There
was no indication that fetal survival was affected or that
malformations were observed (Tesh et al., 1976b).
Special Studies on Mutagenesis
The mutagenic potential of cartap was evaluated using several
strains microorganisms as testor strains. The results of a
rec-assay, evaluating DNA damaging capacity in Bacillus
subtilis (H-17 and M-45 strains) was negative. Reverse mutation
tests, with and without metabolic activation systems using E.
coli and Salmonella typhimurium (TA 98, TA 100, TA 1535, TA
1537 and TA 1538) were all negative. A host mediated assay using
the G 46 strain J.S. typhimurium in the mouse was also negative
(Sherasu et al., 1976).
In vivo cytogenetic effects of cartap were examined using
bone marrow cells of adult male CF-1 Mice and Wistar rats. There
were no increases of chromosomal aberration in cells of rodents
treated with a variety of test programs evaluating cartap. These
tests include doses of 0, 10, 100 and 150 mg/kg/day to mice or 0,
10 and 100 mg/kg/day to rats administered as a single dose or 5
daily doses. In addition, cartap was administered to weanling male
rats orally at 0 or 200 mg/kg/bw or by extraperitoneal injection 0
or 30 mg/kg/bw. In all cases, a positive control of
triethylene-melamine (0.5 mg/kg) or furylfuramide (240 mg/kg) was
employed. A dominant lethal test was performed where male mice were
administered 100 mg/kg/day either as a single or as 5 multiple
daily oral doses. All results showed a complete lack of any
mutagenic activity under the experimental conditions (Kikuchi, et
al, 1976).
COMMENTS
The 1976 Joint Meeting estimated a Temporary Acceptable Daily
Intake for man of 0.05 mg/kg based upon long term studies in two
rodent species. Further data required included detailed studies of
metabolism, further studies of teratogenic and mutagenic potential
and a feeding study in a non-rodent species. Results of additional
studies on metabolism in plants and animals and teratogenicity
tests were made available.
The detailed metabolism studies indicated that Cartap follows
a different route of metabolism in plants and animals and it is
suggested that selective toxicological studies be conducted on
those terminal plant metabolites that differ from the metabolites
found in animals.
New test data on teratogenic potential of cartap, administered
during the full period or organogenesis in mice and rats did not
reveal teratogenic activity at doses above the no effect observed
level established by the 1976 Joint Meeting. Negative results were
obtained in several mutagenicity assays.
In view of these new data which reduced concerns expressed at
the 1976 Joint Meeting the present meeting was able to estimate an
acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 mg/kg bw/day
Dog: 10 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Tea
Of the two cultural methods for growing tea in Japan, the
shade culture expectedly resulted in higher residues than the
open-field culture (Table 1). This is due mainly to the unstable
nature of cartap in sunlight. Residues ranged from 3.3 to 17.4
mg/kg with the shade culture 10 days after the last application
when cartap was applied twice at 1.0 kg a.i./ha. At 14 days after
the last application, the residues ranged from 2.7 to 15.0 mg/kg.
With single applications, a maximum residue of 14.0 mg/kg was
obtained 10 days after the last application. The highest residue
obtained under open field condition was 1.4 mg/kg 10 days after the
last application when cartap was applied twice. Cartap being water
soluble, about 76% of the insecticide is extracted by hot water.
Table 1. Comparative residues of cartap hydrochloride in green tea grown in shade or sunlight.
(50% soluble powder formulation used)
Situation No of Application Rate, Cartap (mg/kg) at intervals (days) after last application
trials (kg ai/ha No) 10 14 15 20 21 27 28
Open field 4 1.0 1 0.37 0.56 0.16
(0.48)
0.73 0.81 0.68
0.49 0.6 0.64
(0.42) (0.43)
0.92 1.12 0.57
Shade 4 1.0 1 7.08 6.60 1.66 0.92
(6.87) (6.12) (1.30) (0.97)
1.76 2.31 0.60 0.36
(1.77) (2.37) (0.60) (0.33)
14 8.51 4.26 2.0
(8.3) (5.8) (2.8) (1.4)
3.55 3.96 0.92 0.58
(2.2) (2.5) (0.7) (0.4)
Open field 4 1.0 2 1.26 1.12 0.22
(0.97)
0.83 0.52 0.45
(0.71)
0.76 0.64 0.38
(0.52)
1.36 1.84 0.56
Shade 4 1.0 2 9.38 7.40
(8.75) (5.50)
3.30 2.66
(2.45) (2.21)
17.4 15.0
(10.6) (9.1)
6.38 5.64
(4.2) (3.4)
Figures in parenthesis are residue equivalent equivalents extracted by hot water
FATE OF RESIDUES
In animals
When cows were fed with 2 and 10 ppm of cartap hydrochloride in
the concentrate part of their ration for 30 consecutive days, some
indications of a transient accumulation of cartap-derived compounds in
milk were observed at the higher feeding level. At the 2 ppm feeding
level, the low apparent residues were attributed to analytical
interferences since similar levels and frequency of occurrence were
also observed in the control group. At the higher feeding level, mean
maximum residues of 33 µg/kg were observed after 7 days treatment.
There was a subsequent decline of residues and no cartap was detected
by the end of the treatment period or during the 30 days recovery
period immediately following (Table 2).
In muscle and liver, the maximum estimated levels of
cartap-derived compounds observed at the end of the treatment period
were 18 µg/kg in muscle and 14 µg/kg in liver, expresse as cartap.
However, the level in liver could be an over estimate because of
interference. No residues could be found in either tissue by the end
of the recovery period. In the kidney, the concentration of residues
appeared to be related to dietary concentration. At the 2 ppm feeding
level, the samples from two animals showed 19 and 33 µg/kg residues at
the end of the treatment period and none the end of the recovery
period. At 10 the ppm level, the residues were 49 and 41 g/kg at the
end of the treatment period declining to 41 and 13 µg/kg, 7 and 30
days respectively after cessation of treatment. No residues were
detected in the fat (Table 3). (Takeda 1978a)
In plants and soil
The absorption and distribution of 35S-labelled cartap
hydrochloride in rice plants grown under various hydroponic conditions
were studied by autoradiography. After 35S was absorbed from the
roots, it was distributed throughout the laminae and high
concentrations were observed in the leaf sheaths. Accumulation
occurred in the leaf apices. Absorption through the leaf sheaths was
also observed, and was found to occur faster in the young leaves than
in the older leaves. Distribution occurred through the vascular
tissues. When applied to the leaves, the insecticide diffused from the
applied region to the leaf apices and accumulated in the leaf sheaths.
35S was detected in the digestive organs, neural tissue and spiracles
of the intoxicated rice stem borers (Kodo et al., 1970).
In 66-day old rice seedlings, the major region of absorption was
observed to be the roots. As much as 15% of the applied
35S-radioactivity was found to have been taken up by the plant,
mainly through the roots. As much as 500 mg/kg accumulated in the rice
plant seedlings after 6 days immersion (Kamesaki et al 1974).
Table 2. Residues in milk of cows fed cartap hydrochloride in the diet.
Cartap, mg/kg, at interval(days)after start of feeding
Cartap in feed Animal
concentrate, ppm number 0 2 4 7 14 22 26 30* 37 51 60
0 3 L T T T T ND ND ND - - -
(control) 11 L T ND T ND ND ND ND - - -
15 ND T T ND T ND ND ND ND ND ND
16 ND ND ND I ND ND ND ND ND - -
2 4 ND L ND T T ND T ND - - -
13 ND ND ND ND T T ND ND ND ND ND
17 ND T ND ND T T ND ND ND - -
20 ND ND ND T ND ND ND - - -
10 1 T 0.02 ND 0.04 0.03 0.02 ND ND - - -
6 T T 0.4 0.04 0.03 0.03 ND ND ND ND ND
7 ND L 0.4 0.02 0.03 T T ND - - -
14 ND 0.02 T 0.03 0.02 0.02 ND ND ND - -
* End of feeding period
All results corrected for 62% recovery.
ND denotes "none detected".
L denotes "sample lost".
I denotes "insufficient sample".
- denotes animal sacrificed.
T denotes less than 0.015 mg/kg.
Table 3. Residues in tissues of cows fed cartap hydrochloride in the diet.
Cartap in feed Animal Time of Cartap, mg/kg in
concentrate, ppm number sacrifice
(days)
Kidney Muscle Liver Fat
0 3 30 ND ND T ND
(control) 11 30 ND ND T ND
16 37 ND ND T ND
15 60 ND ND T ND
Z 4 30 0.02 0.02 0.01 ND
20 30 0.03 NL 0.01 ND
17 37 ND T 0.01 ND
13 60 ND T T ND
10 1 30 0.05 T T ND
7 30 0.04 T 0.01 ND
14 37 0.04 T ND ND
6 60 0.01 T ND ND
All results corrected for appropriate recovery.
ND denotes "none detected"; T denotes 0.01 mg/kg
When paddy cultivation conditions were simulated, 35S-labelled
cartap hydrochloride was rapidly absorbed, a maximum level in most
tissues being reached after 3 days (Table 4). Metabolism to
water-soluble components occurred readily. Accumulation of 35-S in
the panicle was also observed (Kamesaki et al., 1976a). It was
subsequently found by Kameseki et al., (1976b) that under conventional
field practice, the amount of 35S-radioactivity was high in the hull
and rice bran but low in the milled rice. Most of the metabolites were
water soluble and many were amphoteric. Paper chromatography showed
three of the metabolites to have the same Rf values as methionine
sulphoxide, methionine sulphone and S-methyl cysteine Sulphoxide.
Recently, the formation of seven metabolites was confirmed by Sugita
et al., (1978) who proposed the metabolic pathway of cartap in rice
shown in Figure 1.
In paddy soil, Kamesaki et al., (1976a) found that residues
gradually decrease with time but the individual values cannot readily
be correlated because the samples were taken from different pots.
In water
The 35S-radioactivity from labelled cartap hydrochloride rapidly
declined after application to simulated paddy water as seen in Table 5
(Kamesaki et al., 1976a).
Table 4. Absorption of radioactive components by plant parts.
Days after % of applied radioactivity (mean of duplicates) found in
application
Root Sheath Blade Culm Neck Panicle
1 9.1 27.8 29.5 20.4 4.0 9.3
3 3.6 27.6 48.3 7.1 2.7 10.8
6 5.1 21.8 51.5 5.6 2.8 13.2
13 2.5 18.3 57.5 4.5 1.8 15.4
20 2.1 16.8 54.5 4.0 2.3 20.3
27 3.1 18.2 50.8 4.6 2.9 20.5
34 2.2 19.8 52.1 5.6 2.6 17.6
41 1.5 19.3 54.4 5.4 2.7 16.7
Table 5. 35S radioactivity from labelled cartap
hydrochloride in simulated paddy water (x 105 dmp/pot)
Days after Average of duplicates
application (counts/min. X 10-5)
1 789.5
2 330.9
3 217.6
5 111.8
6 111.4
7 115.1
8 74.2
9 55.6
12 33.5
13 27.3
15 9.3
16 5.7
In processing and cooking
The effect of cooking on cartap residues in rice was determined
by Inoue (1977). It was found that washing the soaked, air-dried,
polished rice before cooking reduced cartap, residues by about one
third. No significant reduction in residues was obtained by boiling.
METHODS OF RESIDUE ANALYSIS
The attention of the Meeting was drawn to difficulties found by
some experienced analysts in established laboratories in using the gas
chromatographic procedure for cartap. The stability of the reference
standards is thought to contribute to the problem.
APPRAISAL
Cartap was previously evaluated in 1976 (FAO/WHO, 1977b). The
manufacturer has submitted additional data in response to the
requirements of that meeting.
Photodecomposition appears to contribute significantly to the
decomposition of the insecticide. This explains the higher cartap,
residues found in green tea grown under shade compared to those in
plants grown in the open field.
In dairy cows, intake results in transient accumulation of the
insecticide in milk. Accumulation was also observed in the kidney but
not in the liver, muscle or fat.
 In rice, diffusion through the roots and leaves occurs with
accumulation of the insecticide in the leaf sheathe and panicles.
Absorption into the plant was rapid. Degradation in soil and water was
also rapid. Seven metabolites, mainly hydrolysis products, were
identified in rice plants.
Washing rice prior to cooking reduced cartap residues by about
one third but no significant reduction was obtained by cooking.
Some problems have been reported in analysis by the GLC
procedure, probably owing to the unstable nature of the analytical
standard.
RECOMMENDATIONS
With the allocation of an ADI, the previous temporary MRLs are
converted to MRLs. The limit for tea is amended as shown. The other
tabulated limits were recommended in 1976, but were incorrectly
recorded in the text of the 1976 monograph (FAO/WHO, 1977b). They were
correctly recorded both in the Annex to FAO/WHO, 1977b and in Annex 1
to the report of the 1976 Meeting (FAO/WHO, 1977a).
Commodity MRL (mg/kg)
Tea (green, dry) 20
Cabbage 0.2
Chestnuts (seed including pericarp) 0.1
Ginger 0.1
Potatoes 0.1
Rice (hulled) 0.1
Sweet corn 0.1
FURTHER WORK OR INFORMATION
Desirable
1. Information on use patterns and residue level in additional food
crop for which the compound is used in order to act additional
MRLs.
2. Further inter-laboratory information on evaluation of the method
of analysis.
3. Short-term feeding studies on terminal plant metabolites that are
not found in animals.
REFERENCES
Fujita T., Y. Shirakawa, K. Iwamoto and K. Konishi. Fate of Cartap
(1971) in Animals (II) Investigation of Urinary
Metabolites in Rats and Mice. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Inoue, M. Effect of Cooking on Cartap Residue. Takeda Chemical
Industries, Ltd., Sept. 14 (unpublished).
Kamesaki, S., Y. Inoue, K. Konishi Y. Kono, Y. Oshiko and M. Sakai
(1974) Fate of Cartap in Plants (II). Absorption and
Distribution in Rice Plants (2). Unpublished
report submitted by Takeda Chemical Industries,
Ltd.
Kamesaki, S., K. Konishi, Y. Shirakawa and T. Fujita Fate of Cartap
(1976a) in Animals (III). Isolation and Identification of
Urinary Metabolites in Rats. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishil Y. Kono, Y. Ohiko and M. Sakai
(1976b) Fate of Cartap in Plants (III). Distribution and
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishi, Y. Kono, Y. Oshiko, M. Sakai
(1976c) Fate of Cartap in Plants (IV). Isolation of
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kikuchi, Y., Hitotsumachi, S., Yamamoto, K.I. Mutagenicity Tests
(1976) on Cartap hydrochloride. In vitro cytogenetic
and dominant lethal tests in mammals. J. of the
Takeda Research Laboratories 33: 257-63.
Unpublished report submitted by Takeda Chemical
Industries, Ltd.
Kodo, I., K. Nishi, T. Toga and N. Tan Fate of Cartap in Plants
(1970) (I). Absorption and Distribution in Rice Plants
(1). Unpublished report submitted by Takeda
Chemical Industries, Ltd.
Shirasu, Y., Moriya, M. and Watanabe, K. Assessment of the
(1976) mutagenicity of cartap hydrochloride by various
bacterial test systems. Unpublished report from
the Dept. of Toxicology, Institute of
Environmental Toxicology submitted by Fabeda
Chemical Industries Ltd.
Sugita N., A. Takabatake and N. Tan Fate of Cartap In Plants
(1978) (V). Primary Metabolites in Rice Plants.
Unpublished report submitted by Takeda Chemical
Industries, Ltd. to the WHO.
Sugita N., A. Takabatake and N. Tan Fate of Cartap in Plants
(1978) (V). Primary Metabolites in Rice Plants. Takeda
Chemical Industries, Ltd., April 17 (unpublished).
Tesh, J.M. S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Tesh, J.M., S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Takeda Chemical Industries, Ltd. 7A-7 (Cartap): Milk and Tissue
(1978a) Residues following repeated dietary administration
to dairy cows over thirty days with a maximum
period of thirty days respite from treatment. LSR
Report No. 78/TCL8/112 (unpublished).
Takeda Chemical Industries, Ltd. Comments to information on the Fate
(1978b) of Residue in manufactured Green Tea
(unpublished).
In rice, diffusion through the roots and leaves occurs with
accumulation of the insecticide in the leaf sheathe and panicles.
Absorption into the plant was rapid. Degradation in soil and water was
also rapid. Seven metabolites, mainly hydrolysis products, were
identified in rice plants.
Washing rice prior to cooking reduced cartap residues by about
one third but no significant reduction was obtained by cooking.
Some problems have been reported in analysis by the GLC
procedure, probably owing to the unstable nature of the analytical
standard.
RECOMMENDATIONS
With the allocation of an ADI, the previous temporary MRLs are
converted to MRLs. The limit for tea is amended as shown. The other
tabulated limits were recommended in 1976, but were incorrectly
recorded in the text of the 1976 monograph (FAO/WHO, 1977b). They were
correctly recorded both in the Annex to FAO/WHO, 1977b and in Annex 1
to the report of the 1976 Meeting (FAO/WHO, 1977a).
Commodity MRL (mg/kg)
Tea (green, dry) 20
Cabbage 0.2
Chestnuts (seed including pericarp) 0.1
Ginger 0.1
Potatoes 0.1
Rice (hulled) 0.1
Sweet corn 0.1
FURTHER WORK OR INFORMATION
Desirable
1. Information on use patterns and residue level in additional food
crop for which the compound is used in order to act additional
MRLs.
2. Further inter-laboratory information on evaluation of the method
of analysis.
3. Short-term feeding studies on terminal plant metabolites that are
not found in animals.
REFERENCES
Fujita T., Y. Shirakawa, K. Iwamoto and K. Konishi. Fate of Cartap
(1971) in Animals (II) Investigation of Urinary
Metabolites in Rats and Mice. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Inoue, M. Effect of Cooking on Cartap Residue. Takeda Chemical
Industries, Ltd., Sept. 14 (unpublished).
Kamesaki, S., Y. Inoue, K. Konishi Y. Kono, Y. Oshiko and M. Sakai
(1974) Fate of Cartap in Plants (II). Absorption and
Distribution in Rice Plants (2). Unpublished
report submitted by Takeda Chemical Industries,
Ltd.
Kamesaki, S., K. Konishi, Y. Shirakawa and T. Fujita Fate of Cartap
(1976a) in Animals (III). Isolation and Identification of
Urinary Metabolites in Rats. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishil Y. Kono, Y. Ohiko and M. Sakai
(1976b) Fate of Cartap in Plants (III). Distribution and
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishi, Y. Kono, Y. Oshiko, M. Sakai
(1976c) Fate of Cartap in Plants (IV). Isolation of
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kikuchi, Y., Hitotsumachi, S., Yamamoto, K.I. Mutagenicity Tests
(1976) on Cartap hydrochloride. In vitro cytogenetic
and dominant lethal tests in mammals. J. of the
Takeda Research Laboratories 33: 257-63.
Unpublished report submitted by Takeda Chemical
Industries, Ltd.
Kodo, I., K. Nishi, T. Toga and N. Tan Fate of Cartap in Plants
(1970) (I). Absorption and Distribution in Rice Plants
(1). Unpublished report submitted by Takeda
Chemical Industries, Ltd.
Shirasu, Y., Moriya, M. and Watanabe, K. Assessment of the
(1976) mutagenicity of cartap hydrochloride by various
bacterial test systems. Unpublished report from
the Dept. of Toxicology, Institute of
Environmental Toxicology submitted by Fabeda
Chemical Industries Ltd.
Sugita N., A. Takabatake and N. Tan Fate of Cartap In Plants
(1978) (V). Primary Metabolites in Rice Plants.
Unpublished report submitted by Takeda Chemical
Industries, Ltd. to the WHO.
Sugita N., A. Takabatake and N. Tan Fate of Cartap in Plants
(1978) (V). Primary Metabolites in Rice Plants. Takeda
Chemical Industries, Ltd., April 17 (unpublished).
Tesh, J.M. S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Tesh, J.M., S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Takeda Chemical Industries, Ltd. 7A-7 (Cartap): Milk and Tissue
(1978a) Residues following repeated dietary administration
to dairy cows over thirty days with a maximum
period of thirty days respite from treatment. LSR
Report No. 78/TCL8/112 (unpublished).
Takeda Chemical Industries, Ltd. Comments to information on the Fate
(1978b) of Residue in manufactured Green Tea
(unpublished).
 dioxide (sulfonyl) and sulfate as well as N-demethylation. Thus,
the metabolic pathway in plants appears to result in terminal
residues that may be substantially different from those observed in
mammals. This difference appears to result from the initial
equilibrium established with nereistoxin and dihydronereistoxin
both of which follow different pathways in plants and animals to
their ultimate degradation (Kodo et al. 1970; Kamesaki et al.,
1974; Kamesaki et al., 1976b; 1976c., and Sugita et al., 1978).
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Mice
Groups of pregnant mice (CD-1 Strain, 25 mice/group, 24 mice
were used at the highest dose level) were administered an aqueous
solution of cartap by gavage from day 6 through day 15 of gestation
at dosage levels of 0, 10, 25 and 50 mg/kg body weight. The control
was administered a volume of 10 ml water/kg body weight. On day 18
of pregnancy each animal was sacrificed and examinations made of
viable and nonviable fetuses including an examinations of pre and
post-implantation losses. Fetuses were examined for somatic and
skeletal abnormalities.
Maternal weight gain was slightly depressed at the highest
dose level employed, 50 mg/kg/day. There were no differences from
control values with respect to post-implantation losses, litter
size and fetal abnormalities. Cartap appeared to exert no
teratogenic or fetotoxic effects in mice at levels up to and
including that which has been shown to have an adverse effect on
maternal well-being (Tesh et al., 1976a).
Rat
In a similar study to evaluate the teratological potential of
cartap in the rat, groups of pregnant rats (CD-Strain, 20-22 rats
per group), were administered an aqueous solution in cartap at
dosage levels of 0, 10, 25 and 50 mg/kg/day from day 6 to day 15 of
gestation. Rats were sacrificed on day 21 of pregnancy and again
examined for the presence of viable and nonviable fetuses to
determine pre-implantation and post-implanation loss.
Mortality was observed at the highest level with one dam found
dead on day 10 of gestation. There were no apparent effects noted
with respect to maternal growth. The average litter size of the
treated groups were similar to or greater than those noted in the
control. Fetal weight at the highest level of treatment was
slightly reduced. Mean fetal weight was also reduced at the two
intermediate levels although this reduction was not significant.
There were no indications of post-implantation losses. Somatic and
skeletal abnormalities, found in treated groups, were not believed
to have occurred as a result of the administration of cartap, as
they occurred to the same degree in controls. An incidence of
subcutaneous edema slightly in excess of that noted in the control
group was noted in fetuses of the highest dose group. It was
reported that the subcutaneous edema incidence in both the cartap
and control group was considerably higher than noted when
evaluating a larger control population. It was considered that
this effect did not arise from the presence of cartap. A second
effect identified as perimeningeal cavitation in a slight excess
over that noted with the control was noted of the highest dose
group. However, it has been pointed out that perimeningeal
cavitation does exist in control animals and the range of this
occurrence varies widely. It was also considered that this effect
was not attributed to the presence of cartap. A slight reduction
in ossification was noted with respect to the size of the
interior fontanelle and the number of carpels/tarsels. This effect
seemed to correspond to the marginal depression in fetal, weight
observed at the high dose level. It was concluded that daily oral
administration of cartap through the period of organogenesis at
dose levels of 50 mg/kg body weight resulted in a slight reduction
in fetal weight and a marginal retardation in development. There
was no indication that fetal survival was affected or that
malformations were observed (Tesh et al., 1976b).
Special Studies on Mutagenesis
The mutagenic potential of cartap was evaluated using several
strains microorganisms as testor strains. The results of a
rec-assay, evaluating DNA damaging capacity in Bacillus
subtilis (H-17 and M-45 strains) was negative. Reverse mutation
tests, with and without metabolic activation systems using E.
coli and Salmonella typhimurium (TA 98, TA 100, TA 1535, TA
1537 and TA 1538) were all negative. A host mediated assay using
the G 46 strain J.S. typhimurium in the mouse was also negative
(Sherasu et al., 1976).
In vivo cytogenetic effects of cartap were examined using
bone marrow cells of adult male CF-1 Mice and Wistar rats. There
were no increases of chromosomal aberration in cells of rodents
treated with a variety of test programs evaluating cartap. These
tests include doses of 0, 10, 100 and 150 mg/kg/day to mice or 0,
10 and 100 mg/kg/day to rats administered as a single dose or 5
daily doses. In addition, cartap was administered to weanling male
rats orally at 0 or 200 mg/kg/bw or by extraperitoneal injection 0
or 30 mg/kg/bw. In all cases, a positive control of
triethylene-melamine (0.5 mg/kg) or furylfuramide (240 mg/kg) was
employed. A dominant lethal test was performed where male mice were
administered 100 mg/kg/day either as a single or as 5 multiple
daily oral doses. All results showed a complete lack of any
mutagenic activity under the experimental conditions (Kikuchi, et
al, 1976).
COMMENTS
The 1976 Joint Meeting estimated a Temporary Acceptable Daily
Intake for man of 0.05 mg/kg based upon long term studies in two
rodent species. Further data required included detailed studies of
metabolism, further studies of teratogenic and mutagenic potential
and a feeding study in a non-rodent species. Results of additional
studies on metabolism in plants and animals and teratogenicity
tests were made available.
The detailed metabolism studies indicated that Cartap follows
a different route of metabolism in plants and animals and it is
suggested that selective toxicological studies be conducted on
those terminal plant metabolites that differ from the metabolites
found in animals.
New test data on teratogenic potential of cartap, administered
during the full period or organogenesis in mice and rats did not
reveal teratogenic activity at doses above the no effect observed
level established by the 1976 Joint Meeting. Negative results were
obtained in several mutagenicity assays.
In view of these new data which reduced concerns expressed at
the 1976 Joint Meeting the present meeting was able to estimate an
acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 mg/kg bw/day
Dog: 10 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Tea
Of the two cultural methods for growing tea in Japan, the
shade culture expectedly resulted in higher residues than the
open-field culture (Table 1). This is due mainly to the unstable
nature of cartap in sunlight. Residues ranged from 3.3 to 17.4
mg/kg with the shade culture 10 days after the last application
when cartap was applied twice at 1.0 kg a.i./ha. At 14 days after
the last application, the residues ranged from 2.7 to 15.0 mg/kg.
With single applications, a maximum residue of 14.0 mg/kg was
obtained 10 days after the last application. The highest residue
obtained under open field condition was 1.4 mg/kg 10 days after the
last application when cartap was applied twice. Cartap being water
soluble, about 76% of the insecticide is extracted by hot water.
Table 1. Comparative residues of cartap hydrochloride in green tea grown in shade or sunlight.
(50% soluble powder formulation used)
Situation No of Application Rate, Cartap (mg/kg) at intervals (days) after last application
trials (kg ai/ha No) 10 14 15 20 21 27 28
Open field 4 1.0 1 0.37 0.56 0.16
(0.48)
0.73 0.81 0.68
0.49 0.6 0.64
(0.42) (0.43)
0.92 1.12 0.57
Shade 4 1.0 1 7.08 6.60 1.66 0.92
(6.87) (6.12) (1.30) (0.97)
1.76 2.31 0.60 0.36
(1.77) (2.37) (0.60) (0.33)
14 8.51 4.26 2.0
(8.3) (5.8) (2.8) (1.4)
3.55 3.96 0.92 0.58
(2.2) (2.5) (0.7) (0.4)
Open field 4 1.0 2 1.26 1.12 0.22
(0.97)
0.83 0.52 0.45
(0.71)
0.76 0.64 0.38
(0.52)
1.36 1.84 0.56
Shade 4 1.0 2 9.38 7.40
(8.75) (5.50)
3.30 2.66
(2.45) (2.21)
17.4 15.0
(10.6) (9.1)
6.38 5.64
(4.2) (3.4)
Figures in parenthesis are residue equivalent equivalents extracted by hot water
FATE OF RESIDUES
In animals
When cows were fed with 2 and 10 ppm of cartap hydrochloride in
the concentrate part of their ration for 30 consecutive days, some
indications of a transient accumulation of cartap-derived compounds in
milk were observed at the higher feeding level. At the 2 ppm feeding
level, the low apparent residues were attributed to analytical
interferences since similar levels and frequency of occurrence were
also observed in the control group. At the higher feeding level, mean
maximum residues of 33 µg/kg were observed after 7 days treatment.
There was a subsequent decline of residues and no cartap was detected
by the end of the treatment period or during the 30 days recovery
period immediately following (Table 2).
In muscle and liver, the maximum estimated levels of
cartap-derived compounds observed at the end of the treatment period
were 18 µg/kg in muscle and 14 µg/kg in liver, expresse as cartap.
However, the level in liver could be an over estimate because of
interference. No residues could be found in either tissue by the end
of the recovery period. In the kidney, the concentration of residues
appeared to be related to dietary concentration. At the 2 ppm feeding
level, the samples from two animals showed 19 and 33 µg/kg residues at
the end of the treatment period and none the end of the recovery
period. At 10 the ppm level, the residues were 49 and 41 g/kg at the
end of the treatment period declining to 41 and 13 µg/kg, 7 and 30
days respectively after cessation of treatment. No residues were
detected in the fat (Table 3). (Takeda 1978a)
In plants and soil
The absorption and distribution of 35S-labelled cartap
hydrochloride in rice plants grown under various hydroponic conditions
were studied by autoradiography. After 35S was absorbed from the
roots, it was distributed throughout the laminae and high
concentrations were observed in the leaf sheaths. Accumulation
occurred in the leaf apices. Absorption through the leaf sheaths was
also observed, and was found to occur faster in the young leaves than
in the older leaves. Distribution occurred through the vascular
tissues. When applied to the leaves, the insecticide diffused from the
applied region to the leaf apices and accumulated in the leaf sheaths.
35S was detected in the digestive organs, neural tissue and spiracles
of the intoxicated rice stem borers (Kodo et al., 1970).
In 66-day old rice seedlings, the major region of absorption was
observed to be the roots. As much as 15% of the applied
35S-radioactivity was found to have been taken up by the plant,
mainly through the roots. As much as 500 mg/kg accumulated in the rice
plant seedlings after 6 days immersion (Kamesaki et al 1974).
Table 2. Residues in milk of cows fed cartap hydrochloride in the diet.
Cartap, mg/kg, at interval(days)after start of feeding
Cartap in feed Animal
concentrate, ppm number 0 2 4 7 14 22 26 30* 37 51 60
0 3 L T T T T ND ND ND - - -
(control) 11 L T ND T ND ND ND ND - - -
15 ND T T ND T ND ND ND ND ND ND
16 ND ND ND I ND ND ND ND ND - -
2 4 ND L ND T T ND T ND - - -
13 ND ND ND ND T T ND ND ND ND ND
17 ND T ND ND T T ND ND ND - -
20 ND ND ND T ND ND ND - - -
10 1 T 0.02 ND 0.04 0.03 0.02 ND ND - - -
6 T T 0.4 0.04 0.03 0.03 ND ND ND ND ND
7 ND L 0.4 0.02 0.03 T T ND - - -
14 ND 0.02 T 0.03 0.02 0.02 ND ND ND - -
* End of feeding period
All results corrected for 62% recovery.
ND denotes "none detected".
L denotes "sample lost".
I denotes "insufficient sample".
- denotes animal sacrificed.
T denotes less than 0.015 mg/kg.
Table 3. Residues in tissues of cows fed cartap hydrochloride in the diet.
Cartap in feed Animal Time of Cartap, mg/kg in
concentrate, ppm number sacrifice
(days)
Kidney Muscle Liver Fat
0 3 30 ND ND T ND
(control) 11 30 ND ND T ND
16 37 ND ND T ND
15 60 ND ND T ND
Z 4 30 0.02 0.02 0.01 ND
20 30 0.03 NL 0.01 ND
17 37 ND T 0.01 ND
13 60 ND T T ND
10 1 30 0.05 T T ND
7 30 0.04 T 0.01 ND
14 37 0.04 T ND ND
6 60 0.01 T ND ND
All results corrected for appropriate recovery.
ND denotes "none detected"; T denotes 0.01 mg/kg
When paddy cultivation conditions were simulated, 35S-labelled
cartap hydrochloride was rapidly absorbed, a maximum level in most
tissues being reached after 3 days (Table 4). Metabolism to
water-soluble components occurred readily. Accumulation of 35-S in
the panicle was also observed (Kamesaki et al., 1976a). It was
subsequently found by Kameseki et al., (1976b) that under conventional
field practice, the amount of 35S-radioactivity was high in the hull
and rice bran but low in the milled rice. Most of the metabolites were
water soluble and many were amphoteric. Paper chromatography showed
three of the metabolites to have the same Rf values as methionine
sulphoxide, methionine sulphone and S-methyl cysteine Sulphoxide.
Recently, the formation of seven metabolites was confirmed by Sugita
et al., (1978) who proposed the metabolic pathway of cartap in rice
shown in Figure 1.
In paddy soil, Kamesaki et al., (1976a) found that residues
gradually decrease with time but the individual values cannot readily
be correlated because the samples were taken from different pots.
In water
The 35S-radioactivity from labelled cartap hydrochloride rapidly
declined after application to simulated paddy water as seen in Table 5
(Kamesaki et al., 1976a).
Table 4. Absorption of radioactive components by plant parts.
Days after % of applied radioactivity (mean of duplicates) found in
application
Root Sheath Blade Culm Neck Panicle
1 9.1 27.8 29.5 20.4 4.0 9.3
3 3.6 27.6 48.3 7.1 2.7 10.8
6 5.1 21.8 51.5 5.6 2.8 13.2
13 2.5 18.3 57.5 4.5 1.8 15.4
20 2.1 16.8 54.5 4.0 2.3 20.3
27 3.1 18.2 50.8 4.6 2.9 20.5
34 2.2 19.8 52.1 5.6 2.6 17.6
41 1.5 19.3 54.4 5.4 2.7 16.7
Table 5. 35S radioactivity from labelled cartap
hydrochloride in simulated paddy water (x 105 dmp/pot)
Days after Average of duplicates
application (counts/min. X 10-5)
1 789.5
2 330.9
3 217.6
5 111.8
6 111.4
7 115.1
8 74.2
9 55.6
12 33.5
13 27.3
15 9.3
16 5.7
In processing and cooking
The effect of cooking on cartap residues in rice was determined
by Inoue (1977). It was found that washing the soaked, air-dried,
polished rice before cooking reduced cartap, residues by about one
third. No significant reduction in residues was obtained by boiling.
METHODS OF RESIDUE ANALYSIS
The attention of the Meeting was drawn to difficulties found by
some experienced analysts in established laboratories in using the gas
chromatographic procedure for cartap. The stability of the reference
standards is thought to contribute to the problem.
APPRAISAL
Cartap was previously evaluated in 1976 (FAO/WHO, 1977b). The
manufacturer has submitted additional data in response to the
requirements of that meeting.
Photodecomposition appears to contribute significantly to the
decomposition of the insecticide. This explains the higher cartap,
residues found in green tea grown under shade compared to those in
plants grown in the open field.
In dairy cows, intake results in transient accumulation of the
insecticide in milk. Accumulation was also observed in the kidney but
not in the liver, muscle or fat.
dioxide (sulfonyl) and sulfate as well as N-demethylation. Thus,
the metabolic pathway in plants appears to result in terminal
residues that may be substantially different from those observed in
mammals. This difference appears to result from the initial
equilibrium established with nereistoxin and dihydronereistoxin
both of which follow different pathways in plants and animals to
their ultimate degradation (Kodo et al. 1970; Kamesaki et al.,
1974; Kamesaki et al., 1976b; 1976c., and Sugita et al., 1978).
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Mice
Groups of pregnant mice (CD-1 Strain, 25 mice/group, 24 mice
were used at the highest dose level) were administered an aqueous
solution of cartap by gavage from day 6 through day 15 of gestation
at dosage levels of 0, 10, 25 and 50 mg/kg body weight. The control
was administered a volume of 10 ml water/kg body weight. On day 18
of pregnancy each animal was sacrificed and examinations made of
viable and nonviable fetuses including an examinations of pre and
post-implantation losses. Fetuses were examined for somatic and
skeletal abnormalities.
Maternal weight gain was slightly depressed at the highest
dose level employed, 50 mg/kg/day. There were no differences from
control values with respect to post-implantation losses, litter
size and fetal abnormalities. Cartap appeared to exert no
teratogenic or fetotoxic effects in mice at levels up to and
including that which has been shown to have an adverse effect on
maternal well-being (Tesh et al., 1976a).
Rat
In a similar study to evaluate the teratological potential of
cartap in the rat, groups of pregnant rats (CD-Strain, 20-22 rats
per group), were administered an aqueous solution in cartap at
dosage levels of 0, 10, 25 and 50 mg/kg/day from day 6 to day 15 of
gestation. Rats were sacrificed on day 21 of pregnancy and again
examined for the presence of viable and nonviable fetuses to
determine pre-implantation and post-implanation loss.
Mortality was observed at the highest level with one dam found
dead on day 10 of gestation. There were no apparent effects noted
with respect to maternal growth. The average litter size of the
treated groups were similar to or greater than those noted in the
control. Fetal weight at the highest level of treatment was
slightly reduced. Mean fetal weight was also reduced at the two
intermediate levels although this reduction was not significant.
There were no indications of post-implantation losses. Somatic and
skeletal abnormalities, found in treated groups, were not believed
to have occurred as a result of the administration of cartap, as
they occurred to the same degree in controls. An incidence of
subcutaneous edema slightly in excess of that noted in the control
group was noted in fetuses of the highest dose group. It was
reported that the subcutaneous edema incidence in both the cartap
and control group was considerably higher than noted when
evaluating a larger control population. It was considered that
this effect did not arise from the presence of cartap. A second
effect identified as perimeningeal cavitation in a slight excess
over that noted with the control was noted of the highest dose
group. However, it has been pointed out that perimeningeal
cavitation does exist in control animals and the range of this
occurrence varies widely. It was also considered that this effect
was not attributed to the presence of cartap. A slight reduction
in ossification was noted with respect to the size of the
interior fontanelle and the number of carpels/tarsels. This effect
seemed to correspond to the marginal depression in fetal, weight
observed at the high dose level. It was concluded that daily oral
administration of cartap through the period of organogenesis at
dose levels of 50 mg/kg body weight resulted in a slight reduction
in fetal weight and a marginal retardation in development. There
was no indication that fetal survival was affected or that
malformations were observed (Tesh et al., 1976b).
Special Studies on Mutagenesis
The mutagenic potential of cartap was evaluated using several
strains microorganisms as testor strains. The results of a
rec-assay, evaluating DNA damaging capacity in Bacillus
subtilis (H-17 and M-45 strains) was negative. Reverse mutation
tests, with and without metabolic activation systems using E.
coli and Salmonella typhimurium (TA 98, TA 100, TA 1535, TA
1537 and TA 1538) were all negative. A host mediated assay using
the G 46 strain J.S. typhimurium in the mouse was also negative
(Sherasu et al., 1976).
In vivo cytogenetic effects of cartap were examined using
bone marrow cells of adult male CF-1 Mice and Wistar rats. There
were no increases of chromosomal aberration in cells of rodents
treated with a variety of test programs evaluating cartap. These
tests include doses of 0, 10, 100 and 150 mg/kg/day to mice or 0,
10 and 100 mg/kg/day to rats administered as a single dose or 5
daily doses. In addition, cartap was administered to weanling male
rats orally at 0 or 200 mg/kg/bw or by extraperitoneal injection 0
or 30 mg/kg/bw. In all cases, a positive control of
triethylene-melamine (0.5 mg/kg) or furylfuramide (240 mg/kg) was
employed. A dominant lethal test was performed where male mice were
administered 100 mg/kg/day either as a single or as 5 multiple
daily oral doses. All results showed a complete lack of any
mutagenic activity under the experimental conditions (Kikuchi, et
al, 1976).
COMMENTS
The 1976 Joint Meeting estimated a Temporary Acceptable Daily
Intake for man of 0.05 mg/kg based upon long term studies in two
rodent species. Further data required included detailed studies of
metabolism, further studies of teratogenic and mutagenic potential
and a feeding study in a non-rodent species. Results of additional
studies on metabolism in plants and animals and teratogenicity
tests were made available.
The detailed metabolism studies indicated that Cartap follows
a different route of metabolism in plants and animals and it is
suggested that selective toxicological studies be conducted on
those terminal plant metabolites that differ from the metabolites
found in animals.
New test data on teratogenic potential of cartap, administered
during the full period or organogenesis in mice and rats did not
reveal teratogenic activity at doses above the no effect observed
level established by the 1976 Joint Meeting. Negative results were
obtained in several mutagenicity assays.
In view of these new data which reduced concerns expressed at
the 1976 Joint Meeting the present meeting was able to estimate an
acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 mg/kg bw/day
Dog: 10 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Tea
Of the two cultural methods for growing tea in Japan, the
shade culture expectedly resulted in higher residues than the
open-field culture (Table 1). This is due mainly to the unstable
nature of cartap in sunlight. Residues ranged from 3.3 to 17.4
mg/kg with the shade culture 10 days after the last application
when cartap was applied twice at 1.0 kg a.i./ha. At 14 days after
the last application, the residues ranged from 2.7 to 15.0 mg/kg.
With single applications, a maximum residue of 14.0 mg/kg was
obtained 10 days after the last application. The highest residue
obtained under open field condition was 1.4 mg/kg 10 days after the
last application when cartap was applied twice. Cartap being water
soluble, about 76% of the insecticide is extracted by hot water.
Table 1. Comparative residues of cartap hydrochloride in green tea grown in shade or sunlight.
(50% soluble powder formulation used)
Situation No of Application Rate, Cartap (mg/kg) at intervals (days) after last application
trials (kg ai/ha No) 10 14 15 20 21 27 28
Open field 4 1.0 1 0.37 0.56 0.16
(0.48)
0.73 0.81 0.68
0.49 0.6 0.64
(0.42) (0.43)
0.92 1.12 0.57
Shade 4 1.0 1 7.08 6.60 1.66 0.92
(6.87) (6.12) (1.30) (0.97)
1.76 2.31 0.60 0.36
(1.77) (2.37) (0.60) (0.33)
14 8.51 4.26 2.0
(8.3) (5.8) (2.8) (1.4)
3.55 3.96 0.92 0.58
(2.2) (2.5) (0.7) (0.4)
Open field 4 1.0 2 1.26 1.12 0.22
(0.97)
0.83 0.52 0.45
(0.71)
0.76 0.64 0.38
(0.52)
1.36 1.84 0.56
Shade 4 1.0 2 9.38 7.40
(8.75) (5.50)
3.30 2.66
(2.45) (2.21)
17.4 15.0
(10.6) (9.1)
6.38 5.64
(4.2) (3.4)
Figures in parenthesis are residue equivalent equivalents extracted by hot water
FATE OF RESIDUES
In animals
When cows were fed with 2 and 10 ppm of cartap hydrochloride in
the concentrate part of their ration for 30 consecutive days, some
indications of a transient accumulation of cartap-derived compounds in
milk were observed at the higher feeding level. At the 2 ppm feeding
level, the low apparent residues were attributed to analytical
interferences since similar levels and frequency of occurrence were
also observed in the control group. At the higher feeding level, mean
maximum residues of 33 µg/kg were observed after 7 days treatment.
There was a subsequent decline of residues and no cartap was detected
by the end of the treatment period or during the 30 days recovery
period immediately following (Table 2).
In muscle and liver, the maximum estimated levels of
cartap-derived compounds observed at the end of the treatment period
were 18 µg/kg in muscle and 14 µg/kg in liver, expresse as cartap.
However, the level in liver could be an over estimate because of
interference. No residues could be found in either tissue by the end
of the recovery period. In the kidney, the concentration of residues
appeared to be related to dietary concentration. At the 2 ppm feeding
level, the samples from two animals showed 19 and 33 µg/kg residues at
the end of the treatment period and none the end of the recovery
period. At 10 the ppm level, the residues were 49 and 41 g/kg at the
end of the treatment period declining to 41 and 13 µg/kg, 7 and 30
days respectively after cessation of treatment. No residues were
detected in the fat (Table 3). (Takeda 1978a)
In plants and soil
The absorption and distribution of 35S-labelled cartap
hydrochloride in rice plants grown under various hydroponic conditions
were studied by autoradiography. After 35S was absorbed from the
roots, it was distributed throughout the laminae and high
concentrations were observed in the leaf sheaths. Accumulation
occurred in the leaf apices. Absorption through the leaf sheaths was
also observed, and was found to occur faster in the young leaves than
in the older leaves. Distribution occurred through the vascular
tissues. When applied to the leaves, the insecticide diffused from the
applied region to the leaf apices and accumulated in the leaf sheaths.
35S was detected in the digestive organs, neural tissue and spiracles
of the intoxicated rice stem borers (Kodo et al., 1970).
In 66-day old rice seedlings, the major region of absorption was
observed to be the roots. As much as 15% of the applied
35S-radioactivity was found to have been taken up by the plant,
mainly through the roots. As much as 500 mg/kg accumulated in the rice
plant seedlings after 6 days immersion (Kamesaki et al 1974).
Table 2. Residues in milk of cows fed cartap hydrochloride in the diet.
Cartap, mg/kg, at interval(days)after start of feeding
Cartap in feed Animal
concentrate, ppm number 0 2 4 7 14 22 26 30* 37 51 60
0 3 L T T T T ND ND ND - - -
(control) 11 L T ND T ND ND ND ND - - -
15 ND T T ND T ND ND ND ND ND ND
16 ND ND ND I ND ND ND ND ND - -
2 4 ND L ND T T ND T ND - - -
13 ND ND ND ND T T ND ND ND ND ND
17 ND T ND ND T T ND ND ND - -
20 ND ND ND T ND ND ND - - -
10 1 T 0.02 ND 0.04 0.03 0.02 ND ND - - -
6 T T 0.4 0.04 0.03 0.03 ND ND ND ND ND
7 ND L 0.4 0.02 0.03 T T ND - - -
14 ND 0.02 T 0.03 0.02 0.02 ND ND ND - -
* End of feeding period
All results corrected for 62% recovery.
ND denotes "none detected".
L denotes "sample lost".
I denotes "insufficient sample".
- denotes animal sacrificed.
T denotes less than 0.015 mg/kg.
Table 3. Residues in tissues of cows fed cartap hydrochloride in the diet.
Cartap in feed Animal Time of Cartap, mg/kg in
concentrate, ppm number sacrifice
(days)
Kidney Muscle Liver Fat
0 3 30 ND ND T ND
(control) 11 30 ND ND T ND
16 37 ND ND T ND
15 60 ND ND T ND
Z 4 30 0.02 0.02 0.01 ND
20 30 0.03 NL 0.01 ND
17 37 ND T 0.01 ND
13 60 ND T T ND
10 1 30 0.05 T T ND
7 30 0.04 T 0.01 ND
14 37 0.04 T ND ND
6 60 0.01 T ND ND
All results corrected for appropriate recovery.
ND denotes "none detected"; T denotes 0.01 mg/kg
When paddy cultivation conditions were simulated, 35S-labelled
cartap hydrochloride was rapidly absorbed, a maximum level in most
tissues being reached after 3 days (Table 4). Metabolism to
water-soluble components occurred readily. Accumulation of 35-S in
the panicle was also observed (Kamesaki et al., 1976a). It was
subsequently found by Kameseki et al., (1976b) that under conventional
field practice, the amount of 35S-radioactivity was high in the hull
and rice bran but low in the milled rice. Most of the metabolites were
water soluble and many were amphoteric. Paper chromatography showed
three of the metabolites to have the same Rf values as methionine
sulphoxide, methionine sulphone and S-methyl cysteine Sulphoxide.
Recently, the formation of seven metabolites was confirmed by Sugita
et al., (1978) who proposed the metabolic pathway of cartap in rice
shown in Figure 1.
In paddy soil, Kamesaki et al., (1976a) found that residues
gradually decrease with time but the individual values cannot readily
be correlated because the samples were taken from different pots.
In water
The 35S-radioactivity from labelled cartap hydrochloride rapidly
declined after application to simulated paddy water as seen in Table 5
(Kamesaki et al., 1976a).
Table 4. Absorption of radioactive components by plant parts.
Days after % of applied radioactivity (mean of duplicates) found in
application
Root Sheath Blade Culm Neck Panicle
1 9.1 27.8 29.5 20.4 4.0 9.3
3 3.6 27.6 48.3 7.1 2.7 10.8
6 5.1 21.8 51.5 5.6 2.8 13.2
13 2.5 18.3 57.5 4.5 1.8 15.4
20 2.1 16.8 54.5 4.0 2.3 20.3
27 3.1 18.2 50.8 4.6 2.9 20.5
34 2.2 19.8 52.1 5.6 2.6 17.6
41 1.5 19.3 54.4 5.4 2.7 16.7
Table 5. 35S radioactivity from labelled cartap
hydrochloride in simulated paddy water (x 105 dmp/pot)
Days after Average of duplicates
application (counts/min. X 10-5)
1 789.5
2 330.9
3 217.6
5 111.8
6 111.4
7 115.1
8 74.2
9 55.6
12 33.5
13 27.3
15 9.3
16 5.7
In processing and cooking
The effect of cooking on cartap residues in rice was determined
by Inoue (1977). It was found that washing the soaked, air-dried,
polished rice before cooking reduced cartap, residues by about one
third. No significant reduction in residues was obtained by boiling.
METHODS OF RESIDUE ANALYSIS
The attention of the Meeting was drawn to difficulties found by
some experienced analysts in established laboratories in using the gas
chromatographic procedure for cartap. The stability of the reference
standards is thought to contribute to the problem.
APPRAISAL
Cartap was previously evaluated in 1976 (FAO/WHO, 1977b). The
manufacturer has submitted additional data in response to the
requirements of that meeting.
Photodecomposition appears to contribute significantly to the
decomposition of the insecticide. This explains the higher cartap,
residues found in green tea grown under shade compared to those in
plants grown in the open field.
In dairy cows, intake results in transient accumulation of the
insecticide in milk. Accumulation was also observed in the kidney but
not in the liver, muscle or fat.
 In rice, diffusion through the roots and leaves occurs with
accumulation of the insecticide in the leaf sheathe and panicles.
Absorption into the plant was rapid. Degradation in soil and water was
also rapid. Seven metabolites, mainly hydrolysis products, were
identified in rice plants.
Washing rice prior to cooking reduced cartap residues by about
one third but no significant reduction was obtained by cooking.
Some problems have been reported in analysis by the GLC
procedure, probably owing to the unstable nature of the analytical
standard.
RECOMMENDATIONS
With the allocation of an ADI, the previous temporary MRLs are
converted to MRLs. The limit for tea is amended as shown. The other
tabulated limits were recommended in 1976, but were incorrectly
recorded in the text of the 1976 monograph (FAO/WHO, 1977b). They were
correctly recorded both in the Annex to FAO/WHO, 1977b and in Annex 1
to the report of the 1976 Meeting (FAO/WHO, 1977a).
Commodity MRL (mg/kg)
Tea (green, dry) 20
Cabbage 0.2
Chestnuts (seed including pericarp) 0.1
Ginger 0.1
Potatoes 0.1
Rice (hulled) 0.1
Sweet corn 0.1
FURTHER WORK OR INFORMATION
Desirable
1. Information on use patterns and residue level in additional food
crop for which the compound is used in order to act additional
MRLs.
2. Further inter-laboratory information on evaluation of the method
of analysis.
3. Short-term feeding studies on terminal plant metabolites that are
not found in animals.
REFERENCES
Fujita T., Y. Shirakawa, K. Iwamoto and K. Konishi. Fate of Cartap
(1971) in Animals (II) Investigation of Urinary
Metabolites in Rats and Mice. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Inoue, M. Effect of Cooking on Cartap Residue. Takeda Chemical
Industries, Ltd., Sept. 14 (unpublished).
Kamesaki, S., Y. Inoue, K. Konishi Y. Kono, Y. Oshiko and M. Sakai
(1974) Fate of Cartap in Plants (II). Absorption and
Distribution in Rice Plants (2). Unpublished
report submitted by Takeda Chemical Industries,
Ltd.
Kamesaki, S., K. Konishi, Y. Shirakawa and T. Fujita Fate of Cartap
(1976a) in Animals (III). Isolation and Identification of
Urinary Metabolites in Rats. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishil Y. Kono, Y. Ohiko and M. Sakai
(1976b) Fate of Cartap in Plants (III). Distribution and
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishi, Y. Kono, Y. Oshiko, M. Sakai
(1976c) Fate of Cartap in Plants (IV). Isolation of
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kikuchi, Y., Hitotsumachi, S., Yamamoto, K.I. Mutagenicity Tests
(1976) on Cartap hydrochloride. In vitro cytogenetic
and dominant lethal tests in mammals. J. of the
Takeda Research Laboratories 33: 257-63.
Unpublished report submitted by Takeda Chemical
Industries, Ltd.
Kodo, I., K. Nishi, T. Toga and N. Tan Fate of Cartap in Plants
(1970) (I). Absorption and Distribution in Rice Plants
(1). Unpublished report submitted by Takeda
Chemical Industries, Ltd.
Shirasu, Y., Moriya, M. and Watanabe, K. Assessment of the
(1976) mutagenicity of cartap hydrochloride by various
bacterial test systems. Unpublished report from
the Dept. of Toxicology, Institute of
Environmental Toxicology submitted by Fabeda
Chemical Industries Ltd.
Sugita N., A. Takabatake and N. Tan Fate of Cartap In Plants
(1978) (V). Primary Metabolites in Rice Plants.
Unpublished report submitted by Takeda Chemical
Industries, Ltd. to the WHO.
Sugita N., A. Takabatake and N. Tan Fate of Cartap in Plants
(1978) (V). Primary Metabolites in Rice Plants. Takeda
Chemical Industries, Ltd., April 17 (unpublished).
Tesh, J.M. S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Tesh, J.M., S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Takeda Chemical Industries, Ltd. 7A-7 (Cartap): Milk and Tissue
(1978a) Residues following repeated dietary administration
to dairy cows over thirty days with a maximum
period of thirty days respite from treatment. LSR
Report No. 78/TCL8/112 (unpublished).
Takeda Chemical Industries, Ltd. Comments to information on the Fate
(1978b) of Residue in manufactured Green Tea
(unpublished).
In rice, diffusion through the roots and leaves occurs with
accumulation of the insecticide in the leaf sheathe and panicles.
Absorption into the plant was rapid. Degradation in soil and water was
also rapid. Seven metabolites, mainly hydrolysis products, were
identified in rice plants.
Washing rice prior to cooking reduced cartap residues by about
one third but no significant reduction was obtained by cooking.
Some problems have been reported in analysis by the GLC
procedure, probably owing to the unstable nature of the analytical
standard.
RECOMMENDATIONS
With the allocation of an ADI, the previous temporary MRLs are
converted to MRLs. The limit for tea is amended as shown. The other
tabulated limits were recommended in 1976, but were incorrectly
recorded in the text of the 1976 monograph (FAO/WHO, 1977b). They were
correctly recorded both in the Annex to FAO/WHO, 1977b and in Annex 1
to the report of the 1976 Meeting (FAO/WHO, 1977a).
Commodity MRL (mg/kg)
Tea (green, dry) 20
Cabbage 0.2
Chestnuts (seed including pericarp) 0.1
Ginger 0.1
Potatoes 0.1
Rice (hulled) 0.1
Sweet corn 0.1
FURTHER WORK OR INFORMATION
Desirable
1. Information on use patterns and residue level in additional food
crop for which the compound is used in order to act additional
MRLs.
2. Further inter-laboratory information on evaluation of the method
of analysis.
3. Short-term feeding studies on terminal plant metabolites that are
not found in animals.
REFERENCES
Fujita T., Y. Shirakawa, K. Iwamoto and K. Konishi. Fate of Cartap
(1971) in Animals (II) Investigation of Urinary
Metabolites in Rats and Mice. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Inoue, M. Effect of Cooking on Cartap Residue. Takeda Chemical
Industries, Ltd., Sept. 14 (unpublished).
Kamesaki, S., Y. Inoue, K. Konishi Y. Kono, Y. Oshiko and M. Sakai
(1974) Fate of Cartap in Plants (II). Absorption and
Distribution in Rice Plants (2). Unpublished
report submitted by Takeda Chemical Industries,
Ltd.
Kamesaki, S., K. Konishi, Y. Shirakawa and T. Fujita Fate of Cartap
(1976a) in Animals (III). Isolation and Identification of
Urinary Metabolites in Rats. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishil Y. Kono, Y. Ohiko and M. Sakai
(1976b) Fate of Cartap in Plants (III). Distribution and
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kamesaki, S., Y. Inoue, K. Konishi, Y. Kono, Y. Oshiko, M. Sakai
(1976c) Fate of Cartap in Plants (IV). Isolation of
Metabolites in Rice Plants. Unpublished report
submitted by Takeda Chemical Industries, Ltd.
Kikuchi, Y., Hitotsumachi, S., Yamamoto, K.I. Mutagenicity Tests
(1976) on Cartap hydrochloride. In vitro cytogenetic
and dominant lethal tests in mammals. J. of the
Takeda Research Laboratories 33: 257-63.
Unpublished report submitted by Takeda Chemical
Industries, Ltd.
Kodo, I., K. Nishi, T. Toga and N. Tan Fate of Cartap in Plants
(1970) (I). Absorption and Distribution in Rice Plants
(1). Unpublished report submitted by Takeda
Chemical Industries, Ltd.
Shirasu, Y., Moriya, M. and Watanabe, K. Assessment of the
(1976) mutagenicity of cartap hydrochloride by various
bacterial test systems. Unpublished report from
the Dept. of Toxicology, Institute of
Environmental Toxicology submitted by Fabeda
Chemical Industries Ltd.
Sugita N., A. Takabatake and N. Tan Fate of Cartap In Plants
(1978) (V). Primary Metabolites in Rice Plants.
Unpublished report submitted by Takeda Chemical
Industries, Ltd. to the WHO.
Sugita N., A. Takabatake and N. Tan Fate of Cartap in Plants
(1978) (V). Primary Metabolites in Rice Plants. Takeda
Chemical Industries, Ltd., April 17 (unpublished).
Tesh, J.M. S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Tesh, J.M., S.A. Tesh and M.E. Earthy TA-7 Effects Upon Pregnancy
(1976b) in the Rat. Unpublished study from Life Sciences
Research submitted by Takeda Chemical Industries,
Ltd.
Takeda Chemical Industries, Ltd. 7A-7 (Cartap): Milk and Tissue
(1978a) Residues following repeated dietary administration
to dairy cows over thirty days with a maximum
period of thirty days respite from treatment. LSR
Report No. 78/TCL8/112 (unpublished).
Takeda Chemical Industries, Ltd. Comments to information on the Fate
(1978b) of Residue in manufactured Green Tea
(unpublished).
