
TRIFORINE JMPR 1978
Explanation
Limited information on triforine was reviewed by the 1977
Meeting (FAO/WHO, 1978b). No ADI could be allocated owing to lack
of data. Information which has now become available is evaluated in
the following monograph addendum.
IDENTITY
The following information supplements that given in FAO/WHO,
1978b.
Alternative chemical name
N,N'-[1,4 piperazinediyl-bis-(2,2,2-trichloroethylidine)] -
bis-[formamide]
Synonyms
WOS 524, Denarin (R)
Other information on identity and properties
Melting point: approx. 155°C (decomposition)
Vapor pressure: 2,6 . 10-7 Torr at 25°C
Stability: Stable under normal conditions
Solubility:
Water: approx. 6 ppm at room temperature;
low solubility in most common organic
and inorganic solvents
Purity
The technical product contains 99 ± 2% pure triforine.
Impurities
max. 05% N-(1-formamido-2,2,2-trichloro)
ethyl-N'-formylpiperazine
max. 0.5% N-(1-formamido-2,2,2-trichloro)
ethyl-piperazine
max. 0.05% piperazine
max. 0.2% water
max. 0.1% chloride
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion
Following oral and intraperitoneal administration of
(3H)-labelled triforine to rats at doses of 2.3 mg per animal
approximately 74% of the oral administration dose were excreted in
the urine and 17% in the faeces, whereas after intraperitoneal
administration, 46% and 7% were excreted in urine and faeces
respectively within 24 hours. 18% of an orally administered dose
and 23% of an intraperitoneally administered dose were excreted in
the bile in 24 hours. Only 0.2% of the administered radioactivity
was detected 150 hours after per os application in liver, kidney,
lung, heart and stomach (Darda, 1971).
In another more extended study triforine (3H)-labelled in the
piperazine ring or (14C)-labelled in both sides chains was orally
administered to rats at various dose levels. Determination of blood
level, renal, faecal and biliary excretion were performed after
application of 9 - 15 mg/kg b.w, whereas renal and faecal excretion
measurements were also carried out under the application of
increased doses, of 25 - 200 mg/kg b.w. Following (3H)-triforine
application the maximum blood level reached after 4 hours was 1.3%.
Within 24 hours 74% of the applied activity was eliminated in
urine, 17% in faeces, thus confirming the results of the previous
study. A total of 1.9% of the applied radioactivity was excreted in
the urine as tritiated water within 6 days. Following
(14C)-triforine administration 52% of the dose was eliminated with
urine and approximately 40% in faeces within 24 hours. The
different excretion pattern of the radioactivities after (3H)- and
(14C)-triforine respectively indicates a side chain degradation of
the triforine molecule. After treatment with increased doses of
triforine the excretion rates of the radioactivity were comparable
and biliary excretion studies confirmed the results obtained in the
first study (Darda, 1977). As major urinary metabolite in rats the
compound N-[2,2,2-trichloro-1-(piperazine-1-yl) ethyl] -formamide
could be identified, the occurrence of it confirms the degradation
by cleavage of one side chain of the triforine molecule. In faeces
a small portion of unchanged triforine was found. Presumably
metabolites of the side chains, originating from the bile are also
eliminated with the faeces (Darda, 1977).
TOXICOLOGICAL STUDIES
Special study on mutagenicity
In vitro studies using the Salmonella typhimurium strains TA
100 and TA 98 as Indicator organisms gave no indication of a
mutagenic action of the test-substance with or without metabolic
activation (Obemeier and Frohberg, 1977).
Special study on reproduction
In a three-generation study groups of 10 male and 20 females
were administered 0, 100, 500 and 2500 ppm triforine in the diet.
The treatment had no adverse effect on the general health condition
of dame and pups. No marked intergroup differences in body weight
development was found, including the weight gains in females during
the periods of gestation and laceration. Reproduction and other
parameters were not influenced. Single malformations were
distributed over all groups including control. Autopsy and organ
weight determinations of a part of animals of the F3b generation
did not reveal abnormalities except an increase in absolute spleen
weights of male animals at 2500 ppm; no histopathological findings
were detected in any of the dose groups that could be connected
with the administration of the test compound (Niggeschulze et al.,
1974).
Special study on teratogenicity
Groups of 20 pregnant rats were orally dosed with 100, 400,
800 and 1600 mg/kg b.w. from day 6 through 15 of gestation. The
dams tolerated doses of up to 1600 mg/kg without signs of
incompatibility. Reduced body weight compared to control was found
in females treated with 1600 mg/kg determined on day 19 of
gestation. At 1600 mg/kg various differences in the parameters
compared to control were observed. The number of fetuses was
reduced and accordingly an increase in the number of resorptions
was found. 3 fetuses were dead (0 in the control group). Moreover
post-implantation loss was increased. Treatment with 800 mg/kg and
above caused an increased number of variations consisting mainly in
retarded ossifications of the sternebrae. No malformations were
found in any of the dose groups. The results of this study show
that triforine may cause embryotoxic, but no teratogenic effects
(Leuschner, 1972).
Special study on carcinogenicity
See also long term study.
Groups of NMRI-EMD-SPF mice, each consisting of 40 male and 40
female animals were fed a diet containing the test compound at
concentrations of 0, 30, 150 and 750 ppm for a period of 81 weeks.
The feeding did not influence the general appearance, behaviour,
body weight gain, food consumption, mean life duration. No
intergroup difference was evident with respect to incidences,
location and type of tumours (Hofmann et al., 1975).
Acute Toxicity
Species Sex Route LD50 References
mg/kg
Rat M F oral > 16'000 Frohberg et al., 1973
Rat M F dermal > 10'000 Frohberg et al., 1973
Rat M F inhalation > 4'500 Bullock et al., 1975
(LC50 1 hours) mg/m3air
Mouse M F oral > 6'000 Muacevic, 1968
Dog M F oral > 2'000 Muacevic, 1969
Quail M F oral > 6'000 Muacevic, 1970
Rabbits tolerated the single instillation of 0.1 g triforine
into the conjunctival sack without any signs of irritation
(Frohberg et al., 1973).
Short term studies
Rat
Groups of rats, each consisting of 10 male and 10 female
animals, received doses of 2 ml/kg b.w. triforine at concentrations
of 0.5 and 1.5% by dermal application to the abraded or intact skin
for up to 21 days. The daily exposure time was 4 hours. Slight
reddening and swelling (max. degree 1 according to Draize),
presumably treatment-independent, was observed in the treated as
well as in control animals at the application site. No abnormal
findings were found as regards general appearance, body weight
gain, food consumption, haematological parameters, clinical
chemistry, macroscopic and microscopic examinations of organ and
tissues (Leuschner et al., 1972).
Over a period of 13 weeks groups of 30-50 male and female
animals were maintained on a diet supplemented with 0, 2500, 7000
and 20'000 ppm triforine. Part of the animals of the highest dose
group was observed during a recovery period of 6 weeks after
cessation of treatment. At 20'000 ppm in male animals only the mean
body weight gain was reduced. Haematological investigations
revealed in all dose groups a reversible decrease in the
erythrocyte count, haemotocrit value and an increase in the number
of reticulocytes, particularly in females. Doses of 7000 ppm and
above caused additionally siderosis in kidney and lungs (Stötzer et
al., 1971a).
In a supplementary study lasting also for 13 weeks rats (30
animals per dose level) were fed dosages of 0, 100 and 500 ppm
triforine. The feeding did not influence the mean body weight gain,
food consumption, values of haematological and clinical chemistry
examinations. No alteration in the organ weights was observed that
could be attributed to treatment, nor were there any abnormal
macroscopic or microscopic findings in the organs or tissues
examined (Stötzer et al., 1971b).
Groups of rats each consisting of 10 male and 10 female
animals were maintained on a diet containing the test compound at
concentrations of 0, 25, 125, 626 and 3125 ppm for a period of 26
weeks. The only substance-induced changes were an increase in the
reticulocyte count, and reduction of the number of erythrocytes as
well as haemotocrit value at 625 ppm and above. A dose-dependent
increase of the absolute and relative liver weights in female
animals of all treatment groups was found. As was demonstrated in
a complementary study, where in addition two other groups of
control animals used in previous experiments were used for the
calculations of normal liver weight values, the increased liver
weights of treated animals only were significantly different from
the control value in the 3125 ppm dose group. At autopsy no
abnormal findings were detected, whereas the histological
examination revealed an increase in the siderin deposits in liver
and spleen from 625 ppm onwards (Stötzer et al., 1972a).
Dog
Over a period of 13 weeks 8 animals (4 male and 4 female) per
dose level were fed with a diet supplemented with triforine at
dietary levels of 0, 3500, 10'000 and 30'000 ppm. Another 8 dogs
treated with 30'000 ppm were observed during a recovery period of
6 weeks. Doses of 3500 ppm and above caused reduction in the number
of erythrocytes, haematocrit value and Hb content and an increase
in the reticulocyte count. In the recovery period these changes
returned to normal. Slight reversible dose-dependent increases of
the SAP and bilirubin values were found at 10'000 ppm and above,
whereas cholesterol was increased in all dose groups.
Macropathological examination revealed no substance-induced
alterations; variations in the organ weight showed no clear
dose-relationship. The microscopic examination of tissues showed
changes in the heart in several treated animals consisting of fatty
infiltration of the myocardial fibres. Hemosiderosis of the
Kupffer's cells of the liver was found in all treatment groups. A
dose-relationship in frequency was evident. It was not possible to
achieve a no effect level in this trial (Stötzer et al., 1971c).
A supplementary 13-week feeding study using dose levels of 0,
100, 600 and 3500 ppm was performed with groups of 8 animals (4 of
each sex). The general appearance, body weight gain, food
consumption and the results of urinalyses were not influenced by
the treatment. At 3500 ppm the of erythrocytes haematocrit value
and the Hb content were reduced, whereas no significant increase in
the reticulocyte count was found at any dose level. The parameters
of clinical chemistry including glucose, SGPT, BUN, SAP, bilirubin
and cholesterol values did not differ from normal, whereas at 3500
ppm a decrease of the total serum protein level was observed.
Autopsy revealed no abnormalities, the relative spleen weights
however were significantly increased at 3500 ppm. At 600 ppm and
3500 ppm a dose-dependent increase in the frequency of iron positive
pigment deposits was detected in the Kupffer's cells of the liver,
in the spleen and bone marrow (Leuschner et al., 1971).
Groups of 4 beagle dogs (2 of each sex) obtained food
supplemented with 0, 10, 40, 100 and 1000 ppm of the test compound
for a period of 6 months. The only changes that are considered to
be treatment-related were a slight increase in erythropoiesis in
the bone marrow at 1000 ppm and an increase in the mitotic index of
the erythropoietic system in one animal of this dose group.
Moreover increased cholesterol values and hemosiderosis of the
Kupffer's cells with increased frequency at 1000 ppm were
demonstrated. Fatty infiltration of liver of cells was found in
some treated animals (Stötzer et al., 1972b).
Triforine was fed over a period of 104 weeks to groups of 8
beagle dogs (4 males and 4 females) at dietary concentrations of 0,
10, 40, 100 and 1000 ppm. No intergroup differences were evident
concerning mean body weight gain, food consumption, general
appearance behaviour and also parameters of the haematological
examinations. Only at the highest dose level of 1000 ppm changes
were observed that could be associated with the administration of
the test compound. These changes included an increase in the number
of cells of the erythropoietic system, and a corresponding decrease
in cells of the granulopoietic system in the bone marrow. A mitotic
index above normal was found in one animal of this dose group.
Increased siderosis of the Kupffer's cells of the liver compared to
the control was also evident at the highest dose level, whereas no
marked differences between control and treated animals could be
demonstrated as regards the iron content in spleen. Bone marrow
examinations revealed siderosis in two animals of the 1000 ppm
group.
The results of urinalyses showed slight proteinuria in most of
the treated animals after 78 weeks of treatment. These changes were
not accompanied by histopathological alterations of the kidney and
are therefore not considered to be substance-induced (von
Sandersleben et al., 1974).
Long term studies
Rat
Triforine was fed over a period of 104 weeks to groups of
70-100 rats (35-50 of each sex) at dietary concentrations of 0, 25,
125, 625 and 3125 ppm. Mortality, mean body weight gain, food
consumption, general appearance and behaviour were not affected by
the treatment. After 6 weeks of feeding haematological changes were
found consisting of reduction of the red blood count. haematocrit
value and an increase in the number of reticulocytes; all these
transient differences indicating a slight anaemia reached
significance only in the animals fed with 3125 ppm compared to
control. Results of clinical chemistry examinations and urinalyses
were within normal control limits. The macroscopic investigations
as well as organ weight determinations did not reveal changes of
organs that could be attributed to treatment. Concerning the
histopathological alterations of tissues siderin deposits in spleen
were detected in control as well as in treated animal; no clear
dose-dependent intergroup differences in frequency and intensity
of siderosis was evident. Moreover fatty changes of the epithelium
and of several Kupffer's cells of the liver were found in all groups
without evidence of a definite relation to the dose administered.
In some animals of all groups changes of the adrenals occurred,
consisting of cavernous dilatation and thrombosed sinuses. However,
there was no clear dose dependency, thus making a relationship to the
treatment very unlikely. No intergroup differences of tumour
incidences were observed. Some nodular hyperplasia of small bile
ducts, occurred in 3 out of 40 animals in the control group, in 7
out of 30 at 25 ppm, in 5 out of 30 at 125 ppm, in 8 out of 30 at
625 ppm and in 12 animals out of 40 at 3125 ppm, thus indicating
slightly higher frequencies in treated animals (Hill et al., 1974).
COMMENTS
Triforine is rapidly absorbed. It is metabolised and excreted
in urine within 24 hours. No mutagenic activity was demonstrated in
in vitro tests. A three-generation study was negative at dietary
doses levels up to 2500 ppm failed to show any adverse affect on
reproduction. In a teratogenicity study, some embryotoxic but no
teratogenic effects were noted.
In young rats doses of 625 ppm and above induced a transient
slight haemolytic anaemia, accompanied by an increase in siderin
deposits in liver and spleen. In a long-term study an rats, some
signs of haemolytic anaemia were observed only at 3125 ppm, the
highest dose level tested. A no-effect level of 625 ppm was found.
In a two year dog study a no-effect level of 100 ppm was observed.
Increased erythropoiesis in bone marrow and haemosiderosis of the
Kupffer's cells in liver occurred at dietary levels of 1000 ppm.
No tumorigenic activity was noted in any study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 625 ppm in the diet equivalent to 30 mg/kg bw
Dog: 100 ppm in the diet equivalent to 2.5 mg/kg bw
Estimate of acceptable daily intake for man
0 - 0.02 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Triforine is a fungicide with locally systemic (translaminar)
action for the control of powdery mildews, scab, rusts, monilia,
and foliar diseases in fruit, vegetables, cereals, hops, soybeans,
grapevine, cotton and ornamentals.
Pre-harvest treatments
Triforine is registered or can be sold by permission in many
countries. It is used as an emulsifiable concentrate (190g
triforine/litre) and a liquid seed dressing. Triforine is
recommended as follows (highest recommended dosage of active
ingredient):
Stone fruit 23.75 g/100 l
Pome fruit 23.75 g/100 l
Soft fruit (berries) 28.5 g/100 l
Cucurbits 28.5 g/100 l
Beans 28.5 g/100 l
Tomatoes 285 g/ha
Peppers
Eggplant
Cereals 285 g/ha
Hops 19 g/100 l
Soybeans 285
Grapevine 28.5 g/100 l
Cotton 285
Ornamentals 28.5 g/100 l
For seed treatment rates between 200 and 300 g ai per 100 kg
seed are recommended.
The following pre-harvest intervals have been established (by
May 1978):
Vegetables (in general):
8 days Yugoslavia
14 days Hungary
Melons and cucumbers:
0 days France
2 days United Kingdom
3 days Denmark, Federal Republic of Germany,
Israel, Netherlands, South Africa,
Switzerland
4 days Norway
7 days German Democratic Republic, Spain
Scorzonera:
7 days Netherlands
21 days Switzerland
28 days Belgium
Tomatoes:
3 days Israel
Eggplants:
3 days Israel
Peppers:
3 days Israel
Beans:
3 days South Africa
Leek:
28 days Belgium
Cereals:
0 days France
15 days Spain
21 days Denmark, Hungary
28 days Belgium
49 days *) Federal Republic of Germany
56 days German Democratic Republic
Fruit (in general):
0 days France
14 days Hungary, Yugoslavia
Pome fruit:
0 days South Africa
7 days United Kingdom
14 days Federal Republic of Germany, Israel
21 days German Democratic Republic
28 days Czechoslovakia
Apples:
21 days Switzerland
Stone fruit:
0 days Australia
1 day New Zealand
Apricots:
14 days Israel
Cherries:
14 days Federal Republic of Germany
Almonds:
14 days Israel
Mango:
14 days Israel
Soft fruit (in general):
14 days Federal Republic of Germany
Black Currants:
14 days United Kingdom
*) these pre-harvest intervals are based not on residues found
but on the interval between last application necessary against
rust or powdery mildew and harvest
Hops:
10 days Federal Republic of Germany
Tobacco:
7 days Yugoslavia
Post-harvest treatments
For dip treatment of stone fruit a concentration of 250-1000
mg a.i./kg is recommended by the manufacturer. In Australia a
post-harvest dip of 14.3 g/100 l (0.014%) immediately after harvest
is recommended for stone fruit.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residues resulting from supervised trials in a wide range
of crops in many countries are shown in Tables 1 and 2. Residues
decline rapidly and rarely exceed 1 mg/kg on most fruits and
vegetables as soon as 3 days after the last application. For cereals,
the need for treatment close to harvest is rare and residues at
harvest are less than 0.1 mg/kg in grain.
FATE OF RESIDUES
In animals
Absorption, distribution and excretion in mammals
In trials in which single doses of 3H triforine (labelled in
the piperazine ring) were administered orally and intraperitoneally
to male FW 49 rats good but slow absorption and rapid excretion
were observed, the (metabolised) active principle being excreted
quantitatively. Within 24 hours 90.8% of the orally administered
dose had been excreted with the urine and the faeces. Following
oral administration the maximum blood level was reached after 4
hours (1.3% of the administered dose in the total blood); after
i.p. injection the maximum level was reached 30 minutes after
application (3% in the total blood). Whereas after oral
administration 77.5% was excreted with the urine and 18.7% with the
faeces, the ration following i-p. injection was 61%: 11%. The
biliary excretion, which is practically finished within 30 hours
after oral administration but persists longer after i.p. injection,
amounted to 19.1 and 24.5% respectively. After i.p. injection the
delayed renal excretion and varying excretion rate indicates an
enterohepatic circulation, which was excluded in the case of oral
administration. This might explain the fact that even 150 hours
after i.p. injection residual activities of 0.7% were still to be
found in the liver and a total residual activity of 0.2% in other
organs (kidneys, spleen, lung, heart, stomach), whereas after oral
administration only traces - probably tritiated water - were to be
detected (Darda 1971, 1977).
TABLE 1. Triforine residues in various crops
Residues (mg/kg) at intervals (days) after last application
Number of 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 45-50
Crop Country Dosage rate applications
Fruit and
Vegetables
Apples Fed. Rep. Germ. 23.75 g/100 l 7 0.94 0.55 0.73 0.56 0.43
" " " 23.75 g/100 l 10 1.38 0.93 0.68 0.75
Great Britain 47.5 g/100 l 10 1.33 0.95 0.40 0.49 0.27
South Africa 23.75 g/100 l 1 0.33 0.27 0.29 0.24 0.24
" " 25g ai/100 l 9 0.43
" " 25g ai/100 l 8 0.21
" " 25g ai/100 l 12 0.25
" " 25g ai/100 l 8 0.17
" " 25g ai/100 l 8 0.10
" " 25g ai/100 l 7 0.72 0.58 0.60
" " 37.5g ai/100l 8 0.27
" " 37.5g ai/100l 8 0.16
USA 22,4 g/100 l 10 0.30 0.22 0.10 0.15
Finland 1.3 g/tree 3 0.06
" 0.05 g/tree 5 0.02
New Zealand 25g ai/100 l 9 0.38 0.34 0.23 0.21
Beans Fed. Rep. Germ. 28.5 g/100 l 6 0.23- 0.09- 0.06- 0.04-
0.36 0.10 0.09 0.06
" " " 28.5 g/100 l 3 0.83 0.39 0.19 0.13
" " " 23.75 g/100 l 2 1.38 0.19 0.06 0.03 0.02
South Africa 0.285 kg/ha 4 1.52 0.55 0.55 0.42 0.01
Finland 0.9 kg/ha 1 0.005
" 0.9+0.45 kg/ha 2 0.015
TABLE 1. (Cont'd.)
Residues (mg/kg) at intervals (days) after last application
Number of 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 45-50
Crop Country Dosage rate applications
Brussels
Sprouts Netherlands 0.3 kg ai/ha 1 0.15 0.1
Red currants Finland 2 g/bush 2 3.1
" 0.34 g/bush 2 0.15
" 0.3+0.1 g/bush 2 0.13
Black
currents Fed. Rep. Germ. 28.5 g/100 l 3 2.10 0.69 0.56 0.31 0.23
" " " 28.5 g/100 l 5 5.60 3.18 1.14 0.89
" " " 28.5 g/100 l 4 2.39 0.71 0.59 0.19
Finland 0.34 g/bush 2 0.2
" 0.4 g/bush 2 0.2
Blueberries USA 0.3 kg/ha 1 0.75 0.55 0.25 0.16 0.05
USA 12.9 g/100 l 1 1.83 0.99 1.12 0.48
USA 23.9 g/100 l 1 3.10 2.22 2.47 0.87
Cherries Fed. Rep. Germ. 28.5 b/100 l 4 0.95 0.76 0.45 0.18 0.13
" " " 28.5 g/100 l 7 1.41 0.58 0.28 0.21
" " " 28.5 g/100 l 6 0.96 0.62 0.64 0.33
USA 17.9 g/100 l 5 1.06 0.15 0.20
" 17.9 g/100 l 5 1.15 0.76 0.51
Cucumbers Fed. Rep. Germ. 28.5 g/100 l 8 0.94 0.68 0.39 0.11
(glasshouse) " " " 28.5 g/100 l 6 0.15 0.08 0.06 0.03
" " " 23.75 g/100 l 5 0.38 0.19 0.13 0.09 0.04 0.03
Great Britain 19 g/100 l 3 0.28 0.21
TABLE 1. (Cont'd.)
Residues (mg/kg) at intervals (days) after last application
Number of 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 45-50
Crop Country Dosage rate applications
Norway 0.03% ai 3 0.15
" 0.03% ai 4 0.4
" 0.03% ai 4 0.2
" 0.03% ai 4 0.25
(field) Netherlands 450-600g ai/ha 5 0.45 0.24
New Zealand 190g ai/ha 4 0.4 0.2
South Africa 30g ai/100 l 1 0.05 0.02 0.02 0.02 0.02 0.02
Gherkins Netherlands 450-600g ai/ha 5 0.51 0.28
Gooseberries Fed. Rep. Germ. 23.75 g/100 l 5 0.58 0.45 0.46 0.30 0.19
Finland 0.15 g/bush 2 0.25
" 0.4 g/bush 2 0.3
Peaches Fed. Rep. Germ. 23.75 g/100 l 5 1.42 1.22 0.50 0.16
" " " 19 g/100 l 3 0.48 0.92 0.64 0.58
USA 17.9 g/100 l 1 1.10 0.26 0.11
" 17.9 g/100 l 3 1.71- 1.44- 0.02-
2.06 1.71 0.97
" 23.9 g/100 l 9 4.19 2.88 0.56 0.27 0.19
Australia 0.025%, 11 1/tree 7 1.93 1.53
" 0.025%, 9 1/tree 6 2.34 0.81 0.26
Peaches Australia 300 mg/kg 1 2.04 0.98 1.96
(post- " 200 mg/kg 1 1.20 0.88 0.65
harvest or " 150 mg/kg 1 1.06 2. 0.54
mature fruit) " 100 mg/kg 1 0.47 0.39 0.43
" 300 mg/kg 1
" 200 mg/kg 1 9.53 1.10
" 150 mg/kg 1 6.34 0.59
" 100 mg/kg 1 2.74 0.81
TABLE 1. (Cont'd.)
Residues (mg/kg) at intervals (days) after last application
Number of 0 1 2-3 4-5 6-8 9-12 13-16 21-24 27-30 45-50
Crop Country Dosage rate applications
(post- " 300 mg/kg 1 8.45
harvest " 200 mg/kg 1 5.65
dip) " 150 mg/kg 1 2.97
" 100 mg/kg 1 2.48
New Zealand 20g ai/100 l 1 0.48
" " 15g ai/100 l 1 0.58
" " 20g ai/100 l 16 0.92 0.35 0.14
Plums Fed. Rep. Germ. 23.75 g/100 l 3 0.91 0.41 0.23 0.21 0.20
" " " 23.75 g/100 l 3 0.38 0.18 0.10 0.10 0.10
Prunes USA 11.95 g or 3 0.38- 0.28- 0.20-
23.9 g/100 l 0.58 0.49 0.43
Pumpkins South Africa 28.5 g/100 l 1 0.22 0.20 0.20 0.17 0.15
Squashes Israel 9.5 g/100 l 4 0.11 0.06 0.04
Strawberries Italy 23.75 g/100 l 4 0.10 0.07 0.06 0.06 0.06
Finland 750 g/ha 3 0.4
" 750 g/ha 2 0.06
" 600 g/ha 3 0.1
Tomatoes Israel 10.45 g/100 l 2-3 0.18 0.11 0.08 0.06
Tamarillo New Zealand 19g ai/100 l 5 0.01
TABLE 2. Triforine residues in cereals
Number of Residues (mg/kg) at intervals (days) after last application
Cereals Country Dosage Rate Applications
0 7 13-14 20-21 27-31 35 41-45 56-67 77-99
Barley
green Fed. Rep. Germ. 0.38 kg/ha 1 30.9 2.6 0.2 0.1 < 0.1 < 0.1 <0.1
plants
grain " " " 0.38 kg/ha 1 < 0.01
straw " " " 0.38 kg/ha 1 < 0.01
green " " " 0.38 kg/ha 1 28.4 1.2 0.35 0.12 0.1 < 0.1 < 0.1
plants
grain " " " 0.38 kg/ha 1 < 0.01
straw " " " 0.38 kg/ha 1 0.02
green pl. Great Britain 0.18 kg/ha 1 1.84- 0.66- 0.38- 0.18- <0.02
2.27 0.68 0.50 0.21
grain " " 0.36 kg/ha 1 0.04
straw " " 0.36 kg/ha 1 0.05
grain New Zealand 0.24 kg/ha 1 0.2
" " 0.24 kg/ha 1 0.3
Oats
green pl. Fed. Rep. Germ 0.285 kg/ha 2 6.10 1.08 0.20 0.21
TABLE 2. (Cont'd.)
Number of Residues (mg/kg) at intervals (days) after last application
Cereals Country Dosage Rate Applications
0 7 13-14 20-21 27-31 35 41-45 56-67 77-99
grain " " " 0.285 kg/ha 2 < 0.01
straw " " " 0.285 kg/ha 2 0.16
0.285 kg/ha 2
Rye
green pl. Fed. Rep. Germ. 0.285 kg/ha 1 7.52 0.84 0.73 0.53
grain. " " " 0.285 kg/ha 1 < 0.01
straw " " " 0.285 kg/ha 1 0.29
Wheat
grain Fed. Rep. Germ. 0.38 kg/ha 2 0.03
grain " " " 0.38 kg/ha 2 0.02
grain Finland 0.228 kg/ha 1 < 0.01
grain Great Britain 0.34 kg/ha 1 0.03
grain Netherlands 0.19 kg/ha 3 < 0.01
straw " 0.19 kg/ha 3 0.31
In another trial 14C-triforine (labelled side chains) was
suspended in tylose and administered to rats. After oral
administration the maximum blood level was reached after 4 hours
(2.5% of the administered dose). 52.5% was excreted with the urine
within 24 hours and 54.9% within a period of 3 days; 39.5% had been
excreted with the faeces after 24 hours.
There were no changes in the ratio of urinary to faecal
excretion up to a dosage of 200 mg/kg. A maximum of 15-20%
unchanged triforine was found in the faeces (Darda 1977).
Biotransformation in mammals
The main portion of the fungicide absorbed is eliminated
through the kidneys after complete biotransformation. A minor part
is excreted with the bile in the form of several unidentified
metabolites, some of which may be reabsorbed.
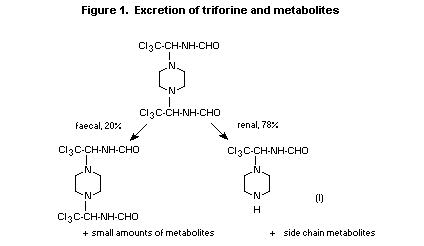
The calculation of absorption can be done most easily with the
3H data (intact piperazine ring). Because 78% of the 3H label is
found in the urine, mainly as N-[2,2,2-trichloro-1-(piperazin-1-yl)
ethyl]formamide (I, Figure 1) and a part of the 3H-material eliminated
with the bile is probably excreted with the faeces, it can be
concluded that at least 80% of orally administered triforine is
absorbed by the rat. The partial examination for labelled substances
in the faeces showed that a portion of the faecal radioactivity was
attributable to unchanged triforine. Presumably metabolites of the
triforine side chains, originating from the bile, are also eliminated
with the faeces (14C-labelled metabolites).
From the studies of triforine hydrolysis, it would be expected
that a small fraction of the applied triforine is degraded before
absorption, leading to labelled side chain products being excreted
with the faeces. This would explain the difference between 3H - and
14C-radioactivities observed in the faeces.
The quantitative elimination of triforine over 24 h does not
indicate accumulation of the compound or its metabolites in rats. The
principal course of metabolism of triforine to (I) is confirmed by the
quantitative distribution of the two isotopes (3H/14C) in urinary
excretion (Darda, 1977).
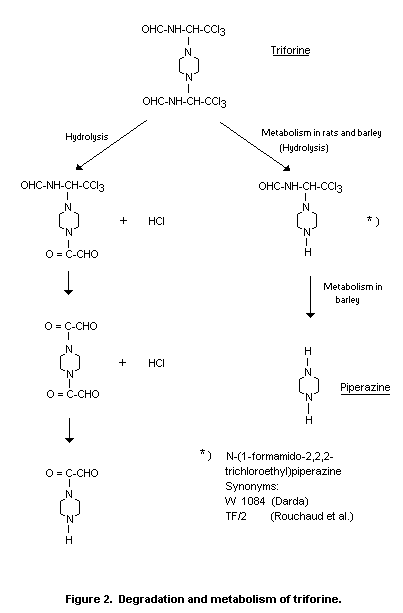
 In plants
Extensive studies have been carried out by Rouchaud et al.,
(1977a,b,c; 1978a,b,c) on the metabolism of triforine and piperazine
in barley plants (grown both in pots in glasshouses and in the field)
and on the characterization of bound and soluble residues derived from
triforine in barley. In these studies triforine was labelled with
tritium 3H) uniformly in the piperazine ring and piperazine was
labelled with 14C in the 2,5 ring positions.
In barley treated with 3H-triforine, 15 and 30 days after
treatment, unchanged triforin, amounted to 57.5 and 43.2%
respectively. Of three soluble metabolites detected, piperazine (0.3
and 4.0%) and N-(1-formamido-2,2,2-trichloroethyl)piperazine (12.9 and
8.4%) were identified, the latter for the first time as a metabolite
in the plant. Identification was by TLC with four solvent systems. The
solid residue contained 23.1 and 38.2% of bound label after 15 and 30
days respectively.
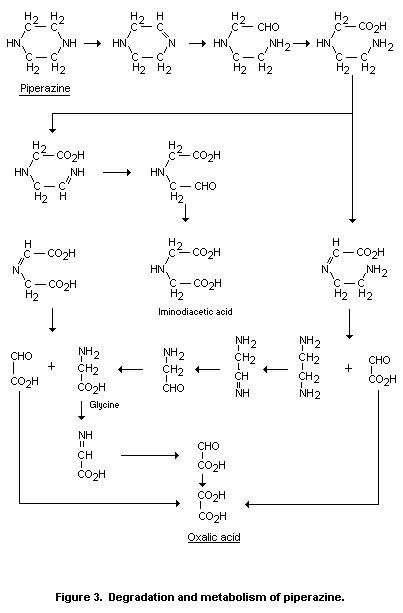
The shoots of barley plants root-treated with 2,5-14C-Piperazine
were analysed 30 days after treatment. Methanol extraction left a
solid residue which contained 31.9% of 14C (all percentages refer to
the total 14C incorporated into the shoots); further extraction with
acidified methanol and dimethyl sulphoxide dissolved 3.2% and 5.8% of
the 14C respectively. The initial methanol extract contained
radioactive piperazine (16.8%), iminodiacetic acid (8.6%), glycine
(15.4%), oxalic acid (7.2%) and unidentified compounds (20.1%). These
results show that piperazine is certainly not the end-product of the
metabolism of triforine in barley.
The results of these studies and of those by Darda in mammals are
summarized in Figures 2 and 3.
In soil
Triforine is absorbed in the upper soil layers and contamination
of ground-water is unlikely. Breakdown in soils is approximately 50%
hydrolytic and 50% microbial. The half-life is approximately 3 weeks.
No adverse influence on soil micro-organisms or earthworms has been
found. (Celamerck, Technical Information Bulletin, Triforine).
In storage and processing
No information.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not yet available to the Meeting on levels or incidence
of triforine residues in food moving in commerce or in food at the
time of consumption. Triforine is not detected by the existing
multi-residue methods of analysis and therefore is not included in
national food and feed monitoring programme.
In plants
Extensive studies have been carried out by Rouchaud et al.,
(1977a,b,c; 1978a,b,c) on the metabolism of triforine and piperazine
in barley plants (grown both in pots in glasshouses and in the field)
and on the characterization of bound and soluble residues derived from
triforine in barley. In these studies triforine was labelled with
tritium 3H) uniformly in the piperazine ring and piperazine was
labelled with 14C in the 2,5 ring positions.
In barley treated with 3H-triforine, 15 and 30 days after
treatment, unchanged triforin, amounted to 57.5 and 43.2%
respectively. Of three soluble metabolites detected, piperazine (0.3
and 4.0%) and N-(1-formamido-2,2,2-trichloroethyl)piperazine (12.9 and
8.4%) were identified, the latter for the first time as a metabolite
in the plant. Identification was by TLC with four solvent systems. The
solid residue contained 23.1 and 38.2% of bound label after 15 and 30
days respectively.
The shoots of barley plants root-treated with 2,5-14C-Piperazine
were analysed 30 days after treatment. Methanol extraction left a
solid residue which contained 31.9% of 14C (all percentages refer to
the total 14C incorporated into the shoots); further extraction with
acidified methanol and dimethyl sulphoxide dissolved 3.2% and 5.8% of
the 14C respectively. The initial methanol extract contained
radioactive piperazine (16.8%), iminodiacetic acid (8.6%), glycine
(15.4%), oxalic acid (7.2%) and unidentified compounds (20.1%). These
results show that piperazine is certainly not the end-product of the
metabolism of triforine in barley.
The results of these studies and of those by Darda in mammals are
summarized in Figures 2 and 3.
In soil
Triforine is absorbed in the upper soil layers and contamination
of ground-water is unlikely. Breakdown in soils is approximately 50%
hydrolytic and 50% microbial. The half-life is approximately 3 weeks.
No adverse influence on soil micro-organisms or earthworms has been
found. (Celamerck, Technical Information Bulletin, Triforine).
In storage and processing
No information.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not yet available to the Meeting on levels or incidence
of triforine residues in food moving in commerce or in food at the
time of consumption. Triforine is not detected by the existing
multi-residue methods of analysis and therefore is not included in
national food and feed monitoring programme.
 METHODS OF RESIDUE ANALYSIS
The manufacturer has developed a method of analysis for triforine
residues in cereals (green plants, straw, grain), fruit, meat
(including liver and kidney), milk, vegetables (including hops), soil
and water (Celamerck, 1976). The sample is blended with acetone. After
removal of the acetone by distillation the triforine in the remaining
aqueous phase is partitioned into toluene, the toluene is evaporated
and the triforine degraded by heating with dilute sulphuric acid. The
chloral hydrate thus formed is distilled into cold water, extracted
into ethyl formate, and determined by gas chromatography with an
electron capture detector, 1,2-dibromoethane is used as an internal
standard. When 0.02-2 mg/kg triforine is added to untreated samples,
recovery amounts to 70-85%. The detection limit is about 0.005-0.01
mg/kg. Although this method determines only unchanged triforine and
not its metabolites, this does not seem a serious deficiency in view
of the metabolism in barley. Of the soluble metabolites after 15 days,
N-(1-formamido-2,2,2-trichloroethyl) piperazine (TF/2) accounted for
only 13% of the total radioactivity.
A procedure was developed by Rouchaud et al., (1977) to measure
the total amount of compounds containing the piperazine ring in barley
plants treated with triforine. In this elegant but complex procedure a
sample of straw or grains is extracted with chloroform and the
chloroform is extracted with 0.1N aq. HC1. The residual chloroform
is concentrated (Concentrate A). The HC1 extract is brought to pH4
with aq, NaOH and freeze-dried (Concentrate B). Part of Concentrate A
is then analysed for chloral hydrate by the GLO procedure described in
the previous paragraph. Part of Concentrate B (freeze-dried) is shaken
with aq. NaOH and chloroform and the chloroform phase is analysed by
GLC for piperazine. The solid residue from the extraction is heated
with aq. 4N HC1, centrifuged, brought to pH4 with NaOH,
freeze-dried, and analysed the same way as Concentrate B. The three
results are added to give the total amount of compounds containing the
piperazine ring. Further analysis of Concentrates A and B by
preparative TLC and GLC gives the amounts of triforine, TF/2, and
piperazine. This method appears highly suitable for research on
triforine distribution, disappearance, and metabolism, but would be
difficult to use for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
A list of national MRLs available to the Meeting is given in
Table 3.
TABLE 3. National MRLs reported to the meeting
Country Crop mg/kg
Australia stone fruit 10
Belgium grain 0.1
leek 0.01
TABLE 3. (Cont'd.)
Country Crop mg/kg
scorzonera 0.01
Federal Republic of Germany cereals 0.2
cucumbers 1.0
pome fruit 1.5
cherries 1.5
soft fruit 1.5
tomatoes 0.5
German Democratic Republic pome fruit 1.0
stone fruit 1.0
soft fruit 1.0
fruit vegetables 0.5
leafy vegetables 1.0
cereals 0.2
hops 1.0
Hungary apple 0.5
others 0.2
Israel general 0.4
Netherlands cucumbers 0.4
gherkins 0.4
melons 0.4
Brussels sprouts 0.2
grain 0.2
New Zealand stone fruit 3.0
pip fruit 0.5
cereals 0.5
South Africa cucurbits 0.5
pome fruit 2.0
beans 1.0
Switzerland apple 0.2
cucumbers 0.3
scorzonera 0.3
APPRAISAL
Triforine is a fungicide with locally systemic (translaminar)
action which is effective for the control of powdery mildews, scab,
rusts, monilia and foliar diseases in fruit, vegetables, cereals,
hops, soybeans, grape vine, cotton and ornamentals. It is marketed as
an emulsifiable concentrate containing 190 g ai/l and as a liquid seed
dressing. It also has some use as a post-harvest dip for peaches. The
technical product is 99 ± 2% pure triforine.
Residue data were available on 19 crops and from 11 countries,
mainly the Federal Republic of Germany, USA and Australia,
representing a wide range of climatic conditions and agricultural
practices. When applied at recommended rates, residues tend to be low
(2 mg/kg or less) even on the day of completion of spraying, and are
reduced rapidly by weathering and metabolism.
Very extensive information is available on the fate of triforine
residues in plants (barley) and to a lesser extent in mammals. The
primary soluble degradation products in plants are
N-(1-formamido-2,2,2-trichloroethyl)piperazine and piperazine.
Approximately 40% of the residue 30 days after treatment consists of
bound residues. In rates, 90.8% of orally administered radiolabelled
triforine is excreted in the urine and faeces within 24 hours. The
metabolites in rats are similar to those in barley. There is no
tendency for residues to accumulate in the fat of animals.
An analytical method is available for triforine residues in
crops, meat, soil and water. Residues are extracted with acetone,
hydrolysed by mineral acid to yield 2 moles of chloral hydrate for
each mole of triforine and distilled to isolate the chloral hydrate,
which is then determined by gas chromatography with electron capture
detection. The detection limit for most samples is in the range
0.005-0.01 mg/kg with recoveries of 70-90% or better. The method is
suitable for use in regulatory analysis.
RECOMMENDATIONS
The following maximum residue limits are recommended. They refer
to triforine determined as chloral hydrate and expressed as triforine.
Commodity Limit, mg/kg
Peaches 5
Apples 2
Cherries 2
Plums 2
Beans 1
Blueberries 1
Gooseberries 1
Red currants 1
Black currants 1
Strawberries 1
Commodity Limit, mg/kg
Cucurbits 0.5
Tomatoes 0.5
Brussels sprouts 0.2
Raw cereals 0.1
Tamarillo 0.02
FURTHER WORK OR INFORMATION
Desirable
1. Information from supervised trials on residues in leafy
vegetables, root vegetables (scorzonera), pod vegetables, eggplants,
peppers, grapes and hops.
2. Results of work in progress to determine residues in meat and
milk of goats administered triforine in the diet (estimated to be
available by June, 1980).
REFERENCES
Anonymous Information on triforine from Australia.
(1978)
Anonymous Information on triforine from Finland*
(1978)
Anonymous Information on triforine from Norway.,
(1978)
Anonymous Information on triforine from the Netherlands.
(1978)
Anonymous Information on triforine from New Zealand.
(1978)
Anonymous Information on triforine from the Republic of South Africa.
(1978)
Bourke, J.B. Nelsen, T.R. and Eichler, D.E. Residues and
(1977) disappearance of triforine from various crops. J.
Agri. Food Chem. 25, 36.
Bullock, C.H., Narcisse, K., McGregor, J.M. and Spencer, J.A. The
(1975) acute rat inhalation study with Cela W 524
Technical. Unpublished report from Environmental
Health and Toxicology, San Francisco, USA, dated
October 29, 1975, No. 812/XXI:126, submitted by
Celmarck.
CELAMERCK Information on Identity and Use pattern of triforine.
(1978)
CELAMERCK Determination of triforine residues. Unpublished report,
(1976) RU 3.26/12/10, Ingelheim, W. Germany.
CELAMERCK Triforine (=CELA W524). Technical Information Bulletin.
(1978)
CELAMERCK Saprol R (Funginex, Triforine 20) Technical Information
(1978) Bulletin.
Darda, S. Biochemical Investigations with W 524 (3H) in rats.
(1971) Unpublished report from Boehringer Sohn, Germany,
dated October 27, No. T 38 E/71, submitted by
Celamerck.
Darda, S. Absorption, Metabolism and Excretion of the Fungicide
(1977) Triforine in the Rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D., Ost, W., Wotscholsowsky,
(1977) M. Hydrolysis and photolysis of the fungicide
triforine. Pestic. Sci. 8, 183-192.
Eicheler, D. Uber das abbauverhalten von triforine. Oargestellt
(1972) an einigen ausgenwählten kulturplfanzen
(Degradation of triforine in selected crops)
Mededelingen Fakulteit Landbouw-weteuschappen Gent
37 (2) 831-840.
Frohberg, H., von Eberstein, M. and Weisse, G. Triforine (W 524)
(1978) trials for acute toxicity in rats after oral
administration and dermal application and for
primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt, dated March 20,
1973, No. 4/36/73, submitted by Celamerck.
Hill, K., Köllmer, H., Weisse, I., Frölke, W., Guénard, J. and
(1971) Stötzer, H. Chronic toxicity tests with the
compound W 524-XX in rats using oral
administration - duration 2 years. Unpublished
report from Boehringer Sohn, dated June 1974, No.
T 27 E/74, submitted by Celamerck.
Hofmann, A., Oelrich, I., Sumi, N., Weisse, G., Köllmer, H.,
(1975) Weisse, I., Frohberg, H. and Stötzer, H. W 524
(Triforine), 81-week carcinogenicity study in mice
(substance administered in food). Unpublished
report from E. Merck, Darmstadt, dated September
14, 1975, No. 4/76/75, submitted by Celamerck.
Leuschner, F. The effects of W 524 on pregnant rats and their
(1972) fetuses following oral administration and dermal
application and for primary mucosal irritation in
rabbits. Unpublished report from E. Merck,,
Darmstadt, dated March 20, 1973, No. 4/36/73,
submitted by Celamerck.
Leuschner, F., Seuschner, A., Schwerdtfeger, W. and Dontenwill,
(1971) W. 13 weeks oral toxicity study in beagle dogs
with W 524. Unpublished report from Laboratorium
für Pharmakologie und Toxikologie, Hamburg, dated
March 31, 1971, No. T 13 E/71, submitted by
Celamerck.
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Dontenwill,
(1972) W. 21-day toxicity tests with the compound W 524
in Sprague-Dawley rats using dermal application.
Unpublished report from Laboratorium für
Pharmokologie und Toxikologie, Hamburg, dated
April 4, 1972, No. T 17 E/72, submitted by
Celamerck.
Muacevic, G. Results of the range-finding pharmacological
(1968) investigation. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated February 5, 1968,
submitted by Celamerck.
Muacevic, G. Results of range-finding pharmacological investigation.
(1969) Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated February 18, 1969,
submitted by Celamerck.
Muacevic, G. Acute toxicological investigation. Unpublished report
(1970) from Boehringer Sohn. Ingelheim, Germany, dated
September 9, 1970, submitted by Celamerck.
Nijgeschulze, A., Hill, K. and Stötzer, H. Three-generation study
(1974) with the fungicide W 524 in rats. Unpublished
report from Boehringer Sohn, Germany, dated May
31, 1974, No. T 26 E/74, submitted by Celamerck.
Obermeier, J., and Frohberg, H. Triforine-trial for mutagenic
potential in bacteria with (1977) and without
added metabolising system. Unpublished report from
E. Merck, Darmstadt, Germany, dated September 8,
1977, No. 4/136/77, submitted by Celamerck.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of the
(1977a) Fungicide Triforine in Barley Plants. Pestic, Sci.
7, 115-127.
Rouchaud, J., Decallone, J.R. and Meyer, J.A. Residues of Triforine
(1977b) and its metabolites in barley grain and straw.
Bull. Environ. Contam. Toxicol. 18, 742.
Rouchaud, J.P. Analysis of piperazine, of the piperazine generating
(1977c) fungicide triforine, and of triforine's
metabolites in barley plants. Bull. Environ.
Contam. Toxicol. 18(2): 184-189.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of
(1978a) (2,5-14C)Piperazine in Barley Plants. Pestic.
Sci. 9, 139-145.
Rouchaud, J., Decallone, J.R. and Meyer, J. The nature of bound
(1978b) residues derived from triforine in barley plants.
Pestic. Sci. 9, 74-78.
Rouchaud, J., Moons, C., Decallone, J.R. and Meyer, J.A.
(1978c) Metabolism of (3H)-Triforine in barley grown in
the field. The unbound radioactive residue.
Université Catholique de Louvain, Laboratorie de
Phytopathologie, 3, Place Croix du Sud, 1348
Louvain-la-Nevve, Belgium. Unpublished manuscript.
von Sandersleben, J., Herbet, M., Weisse, I., Frölke, W., Guénard,
(1974) J. and Stötzer, H. Chronic toxicity test of the
substance W 524-XX on beagles, oral application,
over 104 weeks. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated March 20, 1974,
submitted by Celamerck.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J.
(1971a) and Tilov, T. Testing of the subacute toxicity of
the substance W 524 in rats following oral
administration, Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated April 22, 1971,
No. T 14E, submitted by Celamerck.
Stötzer H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W.,
(1971b) Guénard, J. and Tilov, T. Testing of the subacute
toxicity of the substance W 524 in rats following
oral administration. Unpublished report from
Boehringer Sohn, Ingelheim, Germany, dated May 16,
1971, No. T 15 E, submitted by Celamerck.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke,
(1971c) W., Guénard, J. and von Sandersleben, J. Testing
of the subacute toxicity of the substance W 524 in
dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany,
dated February 5, 1971, No. T 12 E, submitted by
Celamerck.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke,
(1972a) W., Guénard, J. and Tilov, T. Chronic toxicity
studies with the substance W 524-XX in rats using
oral administration - duration 26 weeks.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated, November 28, 1972,
submitted by Celamerck.
Stötzer H., Herbst, M., Weisse, I., Frölke, W., Guénard, J.,
(1972b) and von Sandersleben, J. Chronic toxicity studies
with the substance W 524-XX in beagle dogs using
oral administration - duration 6 months.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated October 17, 1972,
submitted by Celamerck.
Zucks, A., deVires, F.W. and Oalbers, A.M.J. Uptake distribution
(1976) and metabolic fate of 3H-triforine in plants.
Pestic. Sci. 7, 115-127.
METHODS OF RESIDUE ANALYSIS
The manufacturer has developed a method of analysis for triforine
residues in cereals (green plants, straw, grain), fruit, meat
(including liver and kidney), milk, vegetables (including hops), soil
and water (Celamerck, 1976). The sample is blended with acetone. After
removal of the acetone by distillation the triforine in the remaining
aqueous phase is partitioned into toluene, the toluene is evaporated
and the triforine degraded by heating with dilute sulphuric acid. The
chloral hydrate thus formed is distilled into cold water, extracted
into ethyl formate, and determined by gas chromatography with an
electron capture detector, 1,2-dibromoethane is used as an internal
standard. When 0.02-2 mg/kg triforine is added to untreated samples,
recovery amounts to 70-85%. The detection limit is about 0.005-0.01
mg/kg. Although this method determines only unchanged triforine and
not its metabolites, this does not seem a serious deficiency in view
of the metabolism in barley. Of the soluble metabolites after 15 days,
N-(1-formamido-2,2,2-trichloroethyl) piperazine (TF/2) accounted for
only 13% of the total radioactivity.
A procedure was developed by Rouchaud et al., (1977) to measure
the total amount of compounds containing the piperazine ring in barley
plants treated with triforine. In this elegant but complex procedure a
sample of straw or grains is extracted with chloroform and the
chloroform is extracted with 0.1N aq. HC1. The residual chloroform
is concentrated (Concentrate A). The HC1 extract is brought to pH4
with aq, NaOH and freeze-dried (Concentrate B). Part of Concentrate A
is then analysed for chloral hydrate by the GLO procedure described in
the previous paragraph. Part of Concentrate B (freeze-dried) is shaken
with aq. NaOH and chloroform and the chloroform phase is analysed by
GLC for piperazine. The solid residue from the extraction is heated
with aq. 4N HC1, centrifuged, brought to pH4 with NaOH,
freeze-dried, and analysed the same way as Concentrate B. The three
results are added to give the total amount of compounds containing the
piperazine ring. Further analysis of Concentrates A and B by
preparative TLC and GLC gives the amounts of triforine, TF/2, and
piperazine. This method appears highly suitable for research on
triforine distribution, disappearance, and metabolism, but would be
difficult to use for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
A list of national MRLs available to the Meeting is given in
Table 3.
TABLE 3. National MRLs reported to the meeting
Country Crop mg/kg
Australia stone fruit 10
Belgium grain 0.1
leek 0.01
TABLE 3. (Cont'd.)
Country Crop mg/kg
scorzonera 0.01
Federal Republic of Germany cereals 0.2
cucumbers 1.0
pome fruit 1.5
cherries 1.5
soft fruit 1.5
tomatoes 0.5
German Democratic Republic pome fruit 1.0
stone fruit 1.0
soft fruit 1.0
fruit vegetables 0.5
leafy vegetables 1.0
cereals 0.2
hops 1.0
Hungary apple 0.5
others 0.2
Israel general 0.4
Netherlands cucumbers 0.4
gherkins 0.4
melons 0.4
Brussels sprouts 0.2
grain 0.2
New Zealand stone fruit 3.0
pip fruit 0.5
cereals 0.5
South Africa cucurbits 0.5
pome fruit 2.0
beans 1.0
Switzerland apple 0.2
cucumbers 0.3
scorzonera 0.3
APPRAISAL
Triforine is a fungicide with locally systemic (translaminar)
action which is effective for the control of powdery mildews, scab,
rusts, monilia and foliar diseases in fruit, vegetables, cereals,
hops, soybeans, grape vine, cotton and ornamentals. It is marketed as
an emulsifiable concentrate containing 190 g ai/l and as a liquid seed
dressing. It also has some use as a post-harvest dip for peaches. The
technical product is 99 ± 2% pure triforine.
Residue data were available on 19 crops and from 11 countries,
mainly the Federal Republic of Germany, USA and Australia,
representing a wide range of climatic conditions and agricultural
practices. When applied at recommended rates, residues tend to be low
(2 mg/kg or less) even on the day of completion of spraying, and are
reduced rapidly by weathering and metabolism.
Very extensive information is available on the fate of triforine
residues in plants (barley) and to a lesser extent in mammals. The
primary soluble degradation products in plants are
N-(1-formamido-2,2,2-trichloroethyl)piperazine and piperazine.
Approximately 40% of the residue 30 days after treatment consists of
bound residues. In rates, 90.8% of orally administered radiolabelled
triforine is excreted in the urine and faeces within 24 hours. The
metabolites in rats are similar to those in barley. There is no
tendency for residues to accumulate in the fat of animals.
An analytical method is available for triforine residues in
crops, meat, soil and water. Residues are extracted with acetone,
hydrolysed by mineral acid to yield 2 moles of chloral hydrate for
each mole of triforine and distilled to isolate the chloral hydrate,
which is then determined by gas chromatography with electron capture
detection. The detection limit for most samples is in the range
0.005-0.01 mg/kg with recoveries of 70-90% or better. The method is
suitable for use in regulatory analysis.
RECOMMENDATIONS
The following maximum residue limits are recommended. They refer
to triforine determined as chloral hydrate and expressed as triforine.
Commodity Limit, mg/kg
Peaches 5
Apples 2
Cherries 2
Plums 2
Beans 1
Blueberries 1
Gooseberries 1
Red currants 1
Black currants 1
Strawberries 1
Commodity Limit, mg/kg
Cucurbits 0.5
Tomatoes 0.5
Brussels sprouts 0.2
Raw cereals 0.1
Tamarillo 0.02
FURTHER WORK OR INFORMATION
Desirable
1. Information from supervised trials on residues in leafy
vegetables, root vegetables (scorzonera), pod vegetables, eggplants,
peppers, grapes and hops.
2. Results of work in progress to determine residues in meat and
milk of goats administered triforine in the diet (estimated to be
available by June, 1980).
REFERENCES
Anonymous Information on triforine from Australia.
(1978)
Anonymous Information on triforine from Finland*
(1978)
Anonymous Information on triforine from Norway.,
(1978)
Anonymous Information on triforine from the Netherlands.
(1978)
Anonymous Information on triforine from New Zealand.
(1978)
Anonymous Information on triforine from the Republic of South Africa.
(1978)
Bourke, J.B. Nelsen, T.R. and Eichler, D.E. Residues and
(1977) disappearance of triforine from various crops. J.
Agri. Food Chem. 25, 36.
Bullock, C.H., Narcisse, K., McGregor, J.M. and Spencer, J.A. The
(1975) acute rat inhalation study with Cela W 524
Technical. Unpublished report from Environmental
Health and Toxicology, San Francisco, USA, dated
October 29, 1975, No. 812/XXI:126, submitted by
Celmarck.
CELAMERCK Information on Identity and Use pattern of triforine.
(1978)
CELAMERCK Determination of triforine residues. Unpublished report,
(1976) RU 3.26/12/10, Ingelheim, W. Germany.
CELAMERCK Triforine (=CELA W524). Technical Information Bulletin.
(1978)
CELAMERCK Saprol R (Funginex, Triforine 20) Technical Information
(1978) Bulletin.
Darda, S. Biochemical Investigations with W 524 (3H) in rats.
(1971) Unpublished report from Boehringer Sohn, Germany,
dated October 27, No. T 38 E/71, submitted by
Celamerck.
Darda, S. Absorption, Metabolism and Excretion of the Fungicide
(1977) Triforine in the Rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D., Ost, W., Wotscholsowsky,
(1977) M. Hydrolysis and photolysis of the fungicide
triforine. Pestic. Sci. 8, 183-192.
Eicheler, D. Uber das abbauverhalten von triforine. Oargestellt
(1972) an einigen ausgenwählten kulturplfanzen
(Degradation of triforine in selected crops)
Mededelingen Fakulteit Landbouw-weteuschappen Gent
37 (2) 831-840.
Frohberg, H., von Eberstein, M. and Weisse, G. Triforine (W 524)
(1978) trials for acute toxicity in rats after oral
administration and dermal application and for
primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt, dated March 20,
1973, No. 4/36/73, submitted by Celamerck.
Hill, K., Köllmer, H., Weisse, I., Frölke, W., Guénard, J. and
(1971) Stötzer, H. Chronic toxicity tests with the
compound W 524-XX in rats using oral
administration - duration 2 years. Unpublished
report from Boehringer Sohn, dated June 1974, No.
T 27 E/74, submitted by Celamerck.
Hofmann, A., Oelrich, I., Sumi, N., Weisse, G., Köllmer, H.,
(1975) Weisse, I., Frohberg, H. and Stötzer, H. W 524
(Triforine), 81-week carcinogenicity study in mice
(substance administered in food). Unpublished
report from E. Merck, Darmstadt, dated September
14, 1975, No. 4/76/75, submitted by Celamerck.
Leuschner, F. The effects of W 524 on pregnant rats and their
(1972) fetuses following oral administration and dermal
application and for primary mucosal irritation in
rabbits. Unpublished report from E. Merck,,
Darmstadt, dated March 20, 1973, No. 4/36/73,
submitted by Celamerck.
Leuschner, F., Seuschner, A., Schwerdtfeger, W. and Dontenwill,
(1971) W. 13 weeks oral toxicity study in beagle dogs
with W 524. Unpublished report from Laboratorium
für Pharmakologie und Toxikologie, Hamburg, dated
March 31, 1971, No. T 13 E/71, submitted by
Celamerck.
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Dontenwill,
(1972) W. 21-day toxicity tests with the compound W 524
in Sprague-Dawley rats using dermal application.
Unpublished report from Laboratorium für
Pharmokologie und Toxikologie, Hamburg, dated
April 4, 1972, No. T 17 E/72, submitted by
Celamerck.
Muacevic, G. Results of the range-finding pharmacological
(1968) investigation. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated February 5, 1968,
submitted by Celamerck.
Muacevic, G. Results of range-finding pharmacological investigation.
(1969) Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated February 18, 1969,
submitted by Celamerck.
Muacevic, G. Acute toxicological investigation. Unpublished report
(1970) from Boehringer Sohn. Ingelheim, Germany, dated
September 9, 1970, submitted by Celamerck.
Nijgeschulze, A., Hill, K. and Stötzer, H. Three-generation study
(1974) with the fungicide W 524 in rats. Unpublished
report from Boehringer Sohn, Germany, dated May
31, 1974, No. T 26 E/74, submitted by Celamerck.
Obermeier, J., and Frohberg, H. Triforine-trial for mutagenic
potential in bacteria with (1977) and without
added metabolising system. Unpublished report from
E. Merck, Darmstadt, Germany, dated September 8,
1977, No. 4/136/77, submitted by Celamerck.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of the
(1977a) Fungicide Triforine in Barley Plants. Pestic, Sci.
7, 115-127.
Rouchaud, J., Decallone, J.R. and Meyer, J.A. Residues of Triforine
(1977b) and its metabolites in barley grain and straw.
Bull. Environ. Contam. Toxicol. 18, 742.
Rouchaud, J.P. Analysis of piperazine, of the piperazine generating
(1977c) fungicide triforine, and of triforine's
metabolites in barley plants. Bull. Environ.
Contam. Toxicol. 18(2): 184-189.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of
(1978a) (2,5-14C)Piperazine in Barley Plants. Pestic.
Sci. 9, 139-145.
Rouchaud, J., Decallone, J.R. and Meyer, J. The nature of bound
(1978b) residues derived from triforine in barley plants.
Pestic. Sci. 9, 74-78.
Rouchaud, J., Moons, C., Decallone, J.R. and Meyer, J.A.
(1978c) Metabolism of (3H)-Triforine in barley grown in
the field. The unbound radioactive residue.
Université Catholique de Louvain, Laboratorie de
Phytopathologie, 3, Place Croix du Sud, 1348
Louvain-la-Nevve, Belgium. Unpublished manuscript.
von Sandersleben, J., Herbet, M., Weisse, I., Frölke, W., Guénard,
(1974) J. and Stötzer, H. Chronic toxicity test of the
substance W 524-XX on beagles, oral application,
over 104 weeks. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated March 20, 1974,
submitted by Celamerck.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J.
(1971a) and Tilov, T. Testing of the subacute toxicity of
the substance W 524 in rats following oral
administration, Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated April 22, 1971,
No. T 14E, submitted by Celamerck.
Stötzer H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W.,
(1971b) Guénard, J. and Tilov, T. Testing of the subacute
toxicity of the substance W 524 in rats following
oral administration. Unpublished report from
Boehringer Sohn, Ingelheim, Germany, dated May 16,
1971, No. T 15 E, submitted by Celamerck.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke,
(1971c) W., Guénard, J. and von Sandersleben, J. Testing
of the subacute toxicity of the substance W 524 in
dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany,
dated February 5, 1971, No. T 12 E, submitted by
Celamerck.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke,
(1972a) W., Guénard, J. and Tilov, T. Chronic toxicity
studies with the substance W 524-XX in rats using
oral administration - duration 26 weeks.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated, November 28, 1972,
submitted by Celamerck.
Stötzer H., Herbst, M., Weisse, I., Frölke, W., Guénard, J.,
(1972b) and von Sandersleben, J. Chronic toxicity studies
with the substance W 524-XX in beagle dogs using
oral administration - duration 6 months.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated October 17, 1972,
submitted by Celamerck.
Zucks, A., deVires, F.W. and Oalbers, A.M.J. Uptake distribution
(1976) and metabolic fate of 3H-triforine in plants.
Pestic. Sci. 7, 115-127.
 In plants
Extensive studies have been carried out by Rouchaud et al.,
(1977a,b,c; 1978a,b,c) on the metabolism of triforine and piperazine
in barley plants (grown both in pots in glasshouses and in the field)
and on the characterization of bound and soluble residues derived from
triforine in barley. In these studies triforine was labelled with
tritium 3H) uniformly in the piperazine ring and piperazine was
labelled with 14C in the 2,5 ring positions.
In barley treated with 3H-triforine, 15 and 30 days after
treatment, unchanged triforin, amounted to 57.5 and 43.2%
respectively. Of three soluble metabolites detected, piperazine (0.3
and 4.0%) and N-(1-formamido-2,2,2-trichloroethyl)piperazine (12.9 and
8.4%) were identified, the latter for the first time as a metabolite
in the plant. Identification was by TLC with four solvent systems. The
solid residue contained 23.1 and 38.2% of bound label after 15 and 30
days respectively.
The shoots of barley plants root-treated with 2,5-14C-Piperazine
were analysed 30 days after treatment. Methanol extraction left a
solid residue which contained 31.9% of 14C (all percentages refer to
the total 14C incorporated into the shoots); further extraction with
acidified methanol and dimethyl sulphoxide dissolved 3.2% and 5.8% of
the 14C respectively. The initial methanol extract contained
radioactive piperazine (16.8%), iminodiacetic acid (8.6%), glycine
(15.4%), oxalic acid (7.2%) and unidentified compounds (20.1%). These
results show that piperazine is certainly not the end-product of the
metabolism of triforine in barley.
The results of these studies and of those by Darda in mammals are
summarized in Figures 2 and 3.
In soil
Triforine is absorbed in the upper soil layers and contamination
of ground-water is unlikely. Breakdown in soils is approximately 50%
hydrolytic and 50% microbial. The half-life is approximately 3 weeks.
No adverse influence on soil micro-organisms or earthworms has been
found. (Celamerck, Technical Information Bulletin, Triforine).
In storage and processing
No information.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not yet available to the Meeting on levels or incidence
of triforine residues in food moving in commerce or in food at the
time of consumption. Triforine is not detected by the existing
multi-residue methods of analysis and therefore is not included in
national food and feed monitoring programme.
In plants
Extensive studies have been carried out by Rouchaud et al.,
(1977a,b,c; 1978a,b,c) on the metabolism of triforine and piperazine
in barley plants (grown both in pots in glasshouses and in the field)
and on the characterization of bound and soluble residues derived from
triforine in barley. In these studies triforine was labelled with
tritium 3H) uniformly in the piperazine ring and piperazine was
labelled with 14C in the 2,5 ring positions.
In barley treated with 3H-triforine, 15 and 30 days after
treatment, unchanged triforin, amounted to 57.5 and 43.2%
respectively. Of three soluble metabolites detected, piperazine (0.3
and 4.0%) and N-(1-formamido-2,2,2-trichloroethyl)piperazine (12.9 and
8.4%) were identified, the latter for the first time as a metabolite
in the plant. Identification was by TLC with four solvent systems. The
solid residue contained 23.1 and 38.2% of bound label after 15 and 30
days respectively.
The shoots of barley plants root-treated with 2,5-14C-Piperazine
were analysed 30 days after treatment. Methanol extraction left a
solid residue which contained 31.9% of 14C (all percentages refer to
the total 14C incorporated into the shoots); further extraction with
acidified methanol and dimethyl sulphoxide dissolved 3.2% and 5.8% of
the 14C respectively. The initial methanol extract contained
radioactive piperazine (16.8%), iminodiacetic acid (8.6%), glycine
(15.4%), oxalic acid (7.2%) and unidentified compounds (20.1%). These
results show that piperazine is certainly not the end-product of the
metabolism of triforine in barley.
The results of these studies and of those by Darda in mammals are
summarized in Figures 2 and 3.
In soil
Triforine is absorbed in the upper soil layers and contamination
of ground-water is unlikely. Breakdown in soils is approximately 50%
hydrolytic and 50% microbial. The half-life is approximately 3 weeks.
No adverse influence on soil micro-organisms or earthworms has been
found. (Celamerck, Technical Information Bulletin, Triforine).
In storage and processing
No information.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Data were not yet available to the Meeting on levels or incidence
of triforine residues in food moving in commerce or in food at the
time of consumption. Triforine is not detected by the existing
multi-residue methods of analysis and therefore is not included in
national food and feed monitoring programme.

 METHODS OF RESIDUE ANALYSIS
The manufacturer has developed a method of analysis for triforine
residues in cereals (green plants, straw, grain), fruit, meat
(including liver and kidney), milk, vegetables (including hops), soil
and water (Celamerck, 1976). The sample is blended with acetone. After
removal of the acetone by distillation the triforine in the remaining
aqueous phase is partitioned into toluene, the toluene is evaporated
and the triforine degraded by heating with dilute sulphuric acid. The
chloral hydrate thus formed is distilled into cold water, extracted
into ethyl formate, and determined by gas chromatography with an
electron capture detector, 1,2-dibromoethane is used as an internal
standard. When 0.02-2 mg/kg triforine is added to untreated samples,
recovery amounts to 70-85%. The detection limit is about 0.005-0.01
mg/kg. Although this method determines only unchanged triforine and
not its metabolites, this does not seem a serious deficiency in view
of the metabolism in barley. Of the soluble metabolites after 15 days,
N-(1-formamido-2,2,2-trichloroethyl) piperazine (TF/2) accounted for
only 13% of the total radioactivity.
A procedure was developed by Rouchaud et al., (1977) to measure
the total amount of compounds containing the piperazine ring in barley
plants treated with triforine. In this elegant but complex procedure a
sample of straw or grains is extracted with chloroform and the
chloroform is extracted with 0.1N aq. HC1. The residual chloroform
is concentrated (Concentrate A). The HC1 extract is brought to pH4
with aq, NaOH and freeze-dried (Concentrate B). Part of Concentrate A
is then analysed for chloral hydrate by the GLO procedure described in
the previous paragraph. Part of Concentrate B (freeze-dried) is shaken
with aq. NaOH and chloroform and the chloroform phase is analysed by
GLC for piperazine. The solid residue from the extraction is heated
with aq. 4N HC1, centrifuged, brought to pH4 with NaOH,
freeze-dried, and analysed the same way as Concentrate B. The three
results are added to give the total amount of compounds containing the
piperazine ring. Further analysis of Concentrates A and B by
preparative TLC and GLC gives the amounts of triforine, TF/2, and
piperazine. This method appears highly suitable for research on
triforine distribution, disappearance, and metabolism, but would be
difficult to use for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
A list of national MRLs available to the Meeting is given in
Table 3.
TABLE 3. National MRLs reported to the meeting
Country Crop mg/kg
Australia stone fruit 10
Belgium grain 0.1
leek 0.01
TABLE 3. (Cont'd.)
Country Crop mg/kg
scorzonera 0.01
Federal Republic of Germany cereals 0.2
cucumbers 1.0
pome fruit 1.5
cherries 1.5
soft fruit 1.5
tomatoes 0.5
German Democratic Republic pome fruit 1.0
stone fruit 1.0
soft fruit 1.0
fruit vegetables 0.5
leafy vegetables 1.0
cereals 0.2
hops 1.0
Hungary apple 0.5
others 0.2
Israel general 0.4
Netherlands cucumbers 0.4
gherkins 0.4
melons 0.4
Brussels sprouts 0.2
grain 0.2
New Zealand stone fruit 3.0
pip fruit 0.5
cereals 0.5
South Africa cucurbits 0.5
pome fruit 2.0
beans 1.0
Switzerland apple 0.2
cucumbers 0.3
scorzonera 0.3
APPRAISAL
Triforine is a fungicide with locally systemic (translaminar)
action which is effective for the control of powdery mildews, scab,
rusts, monilia and foliar diseases in fruit, vegetables, cereals,
hops, soybeans, grape vine, cotton and ornamentals. It is marketed as
an emulsifiable concentrate containing 190 g ai/l and as a liquid seed
dressing. It also has some use as a post-harvest dip for peaches. The
technical product is 99 ± 2% pure triforine.
Residue data were available on 19 crops and from 11 countries,
mainly the Federal Republic of Germany, USA and Australia,
representing a wide range of climatic conditions and agricultural
practices. When applied at recommended rates, residues tend to be low
(2 mg/kg or less) even on the day of completion of spraying, and are
reduced rapidly by weathering and metabolism.
Very extensive information is available on the fate of triforine
residues in plants (barley) and to a lesser extent in mammals. The
primary soluble degradation products in plants are
N-(1-formamido-2,2,2-trichloroethyl)piperazine and piperazine.
Approximately 40% of the residue 30 days after treatment consists of
bound residues. In rates, 90.8% of orally administered radiolabelled
triforine is excreted in the urine and faeces within 24 hours. The
metabolites in rats are similar to those in barley. There is no
tendency for residues to accumulate in the fat of animals.
An analytical method is available for triforine residues in
crops, meat, soil and water. Residues are extracted with acetone,
hydrolysed by mineral acid to yield 2 moles of chloral hydrate for
each mole of triforine and distilled to isolate the chloral hydrate,
which is then determined by gas chromatography with electron capture
detection. The detection limit for most samples is in the range
0.005-0.01 mg/kg with recoveries of 70-90% or better. The method is
suitable for use in regulatory analysis.
RECOMMENDATIONS
The following maximum residue limits are recommended. They refer
to triforine determined as chloral hydrate and expressed as triforine.
Commodity Limit, mg/kg
Peaches 5
Apples 2
Cherries 2
Plums 2
Beans 1
Blueberries 1
Gooseberries 1
Red currants 1
Black currants 1
Strawberries 1
Commodity Limit, mg/kg
Cucurbits 0.5
Tomatoes 0.5
Brussels sprouts 0.2
Raw cereals 0.1
Tamarillo 0.02
FURTHER WORK OR INFORMATION
Desirable
1. Information from supervised trials on residues in leafy
vegetables, root vegetables (scorzonera), pod vegetables, eggplants,
peppers, grapes and hops.
2. Results of work in progress to determine residues in meat and
milk of goats administered triforine in the diet (estimated to be
available by June, 1980).
REFERENCES
Anonymous Information on triforine from Australia.
(1978)
Anonymous Information on triforine from Finland*
(1978)
Anonymous Information on triforine from Norway.,
(1978)
Anonymous Information on triforine from the Netherlands.
(1978)
Anonymous Information on triforine from New Zealand.
(1978)
Anonymous Information on triforine from the Republic of South Africa.
(1978)
Bourke, J.B. Nelsen, T.R. and Eichler, D.E. Residues and
(1977) disappearance of triforine from various crops. J.
Agri. Food Chem. 25, 36.
Bullock, C.H., Narcisse, K., McGregor, J.M. and Spencer, J.A. The
(1975) acute rat inhalation study with Cela W 524
Technical. Unpublished report from Environmental
Health and Toxicology, San Francisco, USA, dated
October 29, 1975, No. 812/XXI:126, submitted by
Celmarck.
CELAMERCK Information on Identity and Use pattern of triforine.
(1978)
CELAMERCK Determination of triforine residues. Unpublished report,
(1976) RU 3.26/12/10, Ingelheim, W. Germany.
CELAMERCK Triforine (=CELA W524). Technical Information Bulletin.
(1978)
CELAMERCK Saprol R (Funginex, Triforine 20) Technical Information
(1978) Bulletin.
Darda, S. Biochemical Investigations with W 524 (3H) in rats.
(1971) Unpublished report from Boehringer Sohn, Germany,
dated October 27, No. T 38 E/71, submitted by
Celamerck.
Darda, S. Absorption, Metabolism and Excretion of the Fungicide
(1977) Triforine in the Rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D., Ost, W., Wotscholsowsky,
(1977) M. Hydrolysis and photolysis of the fungicide
triforine. Pestic. Sci. 8, 183-192.
Eicheler, D. Uber das abbauverhalten von triforine. Oargestellt
(1972) an einigen ausgenwählten kulturplfanzen
(Degradation of triforine in selected crops)
Mededelingen Fakulteit Landbouw-weteuschappen Gent
37 (2) 831-840.
Frohberg, H., von Eberstein, M. and Weisse, G. Triforine (W 524)
(1978) trials for acute toxicity in rats after oral
administration and dermal application and for
primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt, dated March 20,
1973, No. 4/36/73, submitted by Celamerck.
Hill, K., Köllmer, H., Weisse, I., Frölke, W., Guénard, J. and
(1971) Stötzer, H. Chronic toxicity tests with the
compound W 524-XX in rats using oral
administration - duration 2 years. Unpublished
report from Boehringer Sohn, dated June 1974, No.
T 27 E/74, submitted by Celamerck.
Hofmann, A., Oelrich, I., Sumi, N., Weisse, G., Köllmer, H.,
(1975) Weisse, I., Frohberg, H. and Stötzer, H. W 524
(Triforine), 81-week carcinogenicity study in mice
(substance administered in food). Unpublished
report from E. Merck, Darmstadt, dated September
14, 1975, No. 4/76/75, submitted by Celamerck.
Leuschner, F. The effects of W 524 on pregnant rats and their
(1972) fetuses following oral administration and dermal
application and for primary mucosal irritation in
rabbits. Unpublished report from E. Merck,,
Darmstadt, dated March 20, 1973, No. 4/36/73,
submitted by Celamerck.
Leuschner, F., Seuschner, A., Schwerdtfeger, W. and Dontenwill,
(1971) W. 13 weeks oral toxicity study in beagle dogs
with W 524. Unpublished report from Laboratorium
für Pharmakologie und Toxikologie, Hamburg, dated
March 31, 1971, No. T 13 E/71, submitted by
Celamerck.
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Dontenwill,
(1972) W. 21-day toxicity tests with the compound W 524
in Sprague-Dawley rats using dermal application.
Unpublished report from Laboratorium für
Pharmokologie und Toxikologie, Hamburg, dated
April 4, 1972, No. T 17 E/72, submitted by
Celamerck.
Muacevic, G. Results of the range-finding pharmacological
(1968) investigation. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated February 5, 1968,
submitted by Celamerck.
Muacevic, G. Results of range-finding pharmacological investigation.
(1969) Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated February 18, 1969,
submitted by Celamerck.
Muacevic, G. Acute toxicological investigation. Unpublished report
(1970) from Boehringer Sohn. Ingelheim, Germany, dated
September 9, 1970, submitted by Celamerck.
Nijgeschulze, A., Hill, K. and Stötzer, H. Three-generation study
(1974) with the fungicide W 524 in rats. Unpublished
report from Boehringer Sohn, Germany, dated May
31, 1974, No. T 26 E/74, submitted by Celamerck.
Obermeier, J., and Frohberg, H. Triforine-trial for mutagenic
potential in bacteria with (1977) and without
added metabolising system. Unpublished report from
E. Merck, Darmstadt, Germany, dated September 8,
1977, No. 4/136/77, submitted by Celamerck.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of the
(1977a) Fungicide Triforine in Barley Plants. Pestic, Sci.
7, 115-127.
Rouchaud, J., Decallone, J.R. and Meyer, J.A. Residues of Triforine
(1977b) and its metabolites in barley grain and straw.
Bull. Environ. Contam. Toxicol. 18, 742.
Rouchaud, J.P. Analysis of piperazine, of the piperazine generating
(1977c) fungicide triforine, and of triforine's
metabolites in barley plants. Bull. Environ.
Contam. Toxicol. 18(2): 184-189.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of
(1978a) (2,5-14C)Piperazine in Barley Plants. Pestic.
Sci. 9, 139-145.
Rouchaud, J., Decallone, J.R. and Meyer, J. The nature of bound
(1978b) residues derived from triforine in barley plants.
Pestic. Sci. 9, 74-78.
Rouchaud, J., Moons, C., Decallone, J.R. and Meyer, J.A.
(1978c) Metabolism of (3H)-Triforine in barley grown in
the field. The unbound radioactive residue.
Université Catholique de Louvain, Laboratorie de
Phytopathologie, 3, Place Croix du Sud, 1348
Louvain-la-Nevve, Belgium. Unpublished manuscript.
von Sandersleben, J., Herbet, M., Weisse, I., Frölke, W., Guénard,
(1974) J. and Stötzer, H. Chronic toxicity test of the
substance W 524-XX on beagles, oral application,
over 104 weeks. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated March 20, 1974,
submitted by Celamerck.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J.
(1971a) and Tilov, T. Testing of the subacute toxicity of
the substance W 524 in rats following oral
administration, Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated April 22, 1971,
No. T 14E, submitted by Celamerck.
Stötzer H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W.,
(1971b) Guénard, J. and Tilov, T. Testing of the subacute
toxicity of the substance W 524 in rats following
oral administration. Unpublished report from
Boehringer Sohn, Ingelheim, Germany, dated May 16,
1971, No. T 15 E, submitted by Celamerck.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke,
(1971c) W., Guénard, J. and von Sandersleben, J. Testing
of the subacute toxicity of the substance W 524 in
dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany,
dated February 5, 1971, No. T 12 E, submitted by
Celamerck.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke,
(1972a) W., Guénard, J. and Tilov, T. Chronic toxicity
studies with the substance W 524-XX in rats using
oral administration - duration 26 weeks.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated, November 28, 1972,
submitted by Celamerck.
Stötzer H., Herbst, M., Weisse, I., Frölke, W., Guénard, J.,
(1972b) and von Sandersleben, J. Chronic toxicity studies
with the substance W 524-XX in beagle dogs using
oral administration - duration 6 months.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated October 17, 1972,
submitted by Celamerck.
Zucks, A., deVires, F.W. and Oalbers, A.M.J. Uptake distribution
(1976) and metabolic fate of 3H-triforine in plants.
Pestic. Sci. 7, 115-127.
METHODS OF RESIDUE ANALYSIS
The manufacturer has developed a method of analysis for triforine
residues in cereals (green plants, straw, grain), fruit, meat
(including liver and kidney), milk, vegetables (including hops), soil
and water (Celamerck, 1976). The sample is blended with acetone. After
removal of the acetone by distillation the triforine in the remaining
aqueous phase is partitioned into toluene, the toluene is evaporated
and the triforine degraded by heating with dilute sulphuric acid. The
chloral hydrate thus formed is distilled into cold water, extracted
into ethyl formate, and determined by gas chromatography with an
electron capture detector, 1,2-dibromoethane is used as an internal
standard. When 0.02-2 mg/kg triforine is added to untreated samples,
recovery amounts to 70-85%. The detection limit is about 0.005-0.01
mg/kg. Although this method determines only unchanged triforine and
not its metabolites, this does not seem a serious deficiency in view
of the metabolism in barley. Of the soluble metabolites after 15 days,
N-(1-formamido-2,2,2-trichloroethyl) piperazine (TF/2) accounted for
only 13% of the total radioactivity.
A procedure was developed by Rouchaud et al., (1977) to measure
the total amount of compounds containing the piperazine ring in barley
plants treated with triforine. In this elegant but complex procedure a
sample of straw or grains is extracted with chloroform and the
chloroform is extracted with 0.1N aq. HC1. The residual chloroform
is concentrated (Concentrate A). The HC1 extract is brought to pH4
with aq, NaOH and freeze-dried (Concentrate B). Part of Concentrate A
is then analysed for chloral hydrate by the GLO procedure described in
the previous paragraph. Part of Concentrate B (freeze-dried) is shaken
with aq. NaOH and chloroform and the chloroform phase is analysed by
GLC for piperazine. The solid residue from the extraction is heated
with aq. 4N HC1, centrifuged, brought to pH4 with NaOH,
freeze-dried, and analysed the same way as Concentrate B. The three
results are added to give the total amount of compounds containing the
piperazine ring. Further analysis of Concentrates A and B by
preparative TLC and GLC gives the amounts of triforine, TF/2, and
piperazine. This method appears highly suitable for research on
triforine distribution, disappearance, and metabolism, but would be
difficult to use for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
A list of national MRLs available to the Meeting is given in
Table 3.
TABLE 3. National MRLs reported to the meeting
Country Crop mg/kg
Australia stone fruit 10
Belgium grain 0.1
leek 0.01
TABLE 3. (Cont'd.)
Country Crop mg/kg
scorzonera 0.01
Federal Republic of Germany cereals 0.2
cucumbers 1.0
pome fruit 1.5
cherries 1.5
soft fruit 1.5
tomatoes 0.5
German Democratic Republic pome fruit 1.0
stone fruit 1.0
soft fruit 1.0
fruit vegetables 0.5
leafy vegetables 1.0
cereals 0.2
hops 1.0
Hungary apple 0.5
others 0.2
Israel general 0.4
Netherlands cucumbers 0.4
gherkins 0.4
melons 0.4
Brussels sprouts 0.2
grain 0.2
New Zealand stone fruit 3.0
pip fruit 0.5
cereals 0.5
South Africa cucurbits 0.5
pome fruit 2.0
beans 1.0
Switzerland apple 0.2
cucumbers 0.3
scorzonera 0.3
APPRAISAL
Triforine is a fungicide with locally systemic (translaminar)
action which is effective for the control of powdery mildews, scab,
rusts, monilia and foliar diseases in fruit, vegetables, cereals,
hops, soybeans, grape vine, cotton and ornamentals. It is marketed as
an emulsifiable concentrate containing 190 g ai/l and as a liquid seed
dressing. It also has some use as a post-harvest dip for peaches. The
technical product is 99 ± 2% pure triforine.
Residue data were available on 19 crops and from 11 countries,
mainly the Federal Republic of Germany, USA and Australia,
representing a wide range of climatic conditions and agricultural
practices. When applied at recommended rates, residues tend to be low
(2 mg/kg or less) even on the day of completion of spraying, and are
reduced rapidly by weathering and metabolism.
Very extensive information is available on the fate of triforine
residues in plants (barley) and to a lesser extent in mammals. The
primary soluble degradation products in plants are
N-(1-formamido-2,2,2-trichloroethyl)piperazine and piperazine.
Approximately 40% of the residue 30 days after treatment consists of
bound residues. In rates, 90.8% of orally administered radiolabelled
triforine is excreted in the urine and faeces within 24 hours. The
metabolites in rats are similar to those in barley. There is no
tendency for residues to accumulate in the fat of animals.
An analytical method is available for triforine residues in
crops, meat, soil and water. Residues are extracted with acetone,
hydrolysed by mineral acid to yield 2 moles of chloral hydrate for
each mole of triforine and distilled to isolate the chloral hydrate,
which is then determined by gas chromatography with electron capture
detection. The detection limit for most samples is in the range
0.005-0.01 mg/kg with recoveries of 70-90% or better. The method is
suitable for use in regulatory analysis.
RECOMMENDATIONS
The following maximum residue limits are recommended. They refer
to triforine determined as chloral hydrate and expressed as triforine.
Commodity Limit, mg/kg
Peaches 5
Apples 2
Cherries 2
Plums 2
Beans 1
Blueberries 1
Gooseberries 1
Red currants 1
Black currants 1
Strawberries 1
Commodity Limit, mg/kg
Cucurbits 0.5
Tomatoes 0.5
Brussels sprouts 0.2
Raw cereals 0.1
Tamarillo 0.02
FURTHER WORK OR INFORMATION
Desirable
1. Information from supervised trials on residues in leafy
vegetables, root vegetables (scorzonera), pod vegetables, eggplants,
peppers, grapes and hops.
2. Results of work in progress to determine residues in meat and
milk of goats administered triforine in the diet (estimated to be
available by June, 1980).
REFERENCES
Anonymous Information on triforine from Australia.
(1978)
Anonymous Information on triforine from Finland*
(1978)
Anonymous Information on triforine from Norway.,
(1978)
Anonymous Information on triforine from the Netherlands.
(1978)
Anonymous Information on triforine from New Zealand.
(1978)
Anonymous Information on triforine from the Republic of South Africa.
(1978)
Bourke, J.B. Nelsen, T.R. and Eichler, D.E. Residues and
(1977) disappearance of triforine from various crops. J.
Agri. Food Chem. 25, 36.
Bullock, C.H., Narcisse, K., McGregor, J.M. and Spencer, J.A. The
(1975) acute rat inhalation study with Cela W 524
Technical. Unpublished report from Environmental
Health and Toxicology, San Francisco, USA, dated
October 29, 1975, No. 812/XXI:126, submitted by
Celmarck.
CELAMERCK Information on Identity and Use pattern of triforine.
(1978)
CELAMERCK Determination of triforine residues. Unpublished report,
(1976) RU 3.26/12/10, Ingelheim, W. Germany.
CELAMERCK Triforine (=CELA W524). Technical Information Bulletin.
(1978)
CELAMERCK Saprol R (Funginex, Triforine 20) Technical Information
(1978) Bulletin.
Darda, S. Biochemical Investigations with W 524 (3H) in rats.
(1971) Unpublished report from Boehringer Sohn, Germany,
dated October 27, No. T 38 E/71, submitted by
Celamerck.
Darda, S. Absorption, Metabolism and Excretion of the Fungicide
(1977) Triforine in the Rat. Pestic. Sci. 8, 193-202.
Darda, S., Darskus, R.L., Eichler, D., Ost, W., Wotscholsowsky,
(1977) M. Hydrolysis and photolysis of the fungicide
triforine. Pestic. Sci. 8, 183-192.
Eicheler, D. Uber das abbauverhalten von triforine. Oargestellt
(1972) an einigen ausgenwählten kulturplfanzen
(Degradation of triforine in selected crops)
Mededelingen Fakulteit Landbouw-weteuschappen Gent
37 (2) 831-840.
Frohberg, H., von Eberstein, M. and Weisse, G. Triforine (W 524)
(1978) trials for acute toxicity in rats after oral
administration and dermal application and for
primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt, dated March 20,
1973, No. 4/36/73, submitted by Celamerck.
Hill, K., Köllmer, H., Weisse, I., Frölke, W., Guénard, J. and
(1971) Stötzer, H. Chronic toxicity tests with the
compound W 524-XX in rats using oral
administration - duration 2 years. Unpublished
report from Boehringer Sohn, dated June 1974, No.
T 27 E/74, submitted by Celamerck.
Hofmann, A., Oelrich, I., Sumi, N., Weisse, G., Köllmer, H.,
(1975) Weisse, I., Frohberg, H. and Stötzer, H. W 524
(Triforine), 81-week carcinogenicity study in mice
(substance administered in food). Unpublished
report from E. Merck, Darmstadt, dated September
14, 1975, No. 4/76/75, submitted by Celamerck.
Leuschner, F. The effects of W 524 on pregnant rats and their
(1972) fetuses following oral administration and dermal
application and for primary mucosal irritation in
rabbits. Unpublished report from E. Merck,,
Darmstadt, dated March 20, 1973, No. 4/36/73,
submitted by Celamerck.
Leuschner, F., Seuschner, A., Schwerdtfeger, W. and Dontenwill,
(1971) W. 13 weeks oral toxicity study in beagle dogs
with W 524. Unpublished report from Laboratorium
für Pharmakologie und Toxikologie, Hamburg, dated
March 31, 1971, No. T 13 E/71, submitted by
Celamerck.
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Dontenwill,
(1972) W. 21-day toxicity tests with the compound W 524
in Sprague-Dawley rats using dermal application.
Unpublished report from Laboratorium für
Pharmokologie und Toxikologie, Hamburg, dated
April 4, 1972, No. T 17 E/72, submitted by
Celamerck.
Muacevic, G. Results of the range-finding pharmacological
(1968) investigation. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated February 5, 1968,
submitted by Celamerck.
Muacevic, G. Results of range-finding pharmacological investigation.
(1969) Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated February 18, 1969,
submitted by Celamerck.
Muacevic, G. Acute toxicological investigation. Unpublished report
(1970) from Boehringer Sohn. Ingelheim, Germany, dated
September 9, 1970, submitted by Celamerck.
Nijgeschulze, A., Hill, K. and Stötzer, H. Three-generation study
(1974) with the fungicide W 524 in rats. Unpublished
report from Boehringer Sohn, Germany, dated May
31, 1974, No. T 26 E/74, submitted by Celamerck.
Obermeier, J., and Frohberg, H. Triforine-trial for mutagenic
potential in bacteria with (1977) and without
added metabolising system. Unpublished report from
E. Merck, Darmstadt, Germany, dated September 8,
1977, No. 4/136/77, submitted by Celamerck.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of the
(1977a) Fungicide Triforine in Barley Plants. Pestic, Sci.
7, 115-127.
Rouchaud, J., Decallone, J.R. and Meyer, J.A. Residues of Triforine
(1977b) and its metabolites in barley grain and straw.
Bull. Environ. Contam. Toxicol. 18, 742.
Rouchaud, J.P. Analysis of piperazine, of the piperazine generating
(1977c) fungicide triforine, and of triforine's
metabolites in barley plants. Bull. Environ.
Contam. Toxicol. 18(2): 184-189.
Rouchaud, J.P., Decallone, J.R. and Meyer, J.A. Metabolism of
(1978a) (2,5-14C)Piperazine in Barley Plants. Pestic.
Sci. 9, 139-145.
Rouchaud, J., Decallone, J.R. and Meyer, J. The nature of bound
(1978b) residues derived from triforine in barley plants.
Pestic. Sci. 9, 74-78.
Rouchaud, J., Moons, C., Decallone, J.R. and Meyer, J.A.
(1978c) Metabolism of (3H)-Triforine in barley grown in
the field. The unbound radioactive residue.
Université Catholique de Louvain, Laboratorie de
Phytopathologie, 3, Place Croix du Sud, 1348
Louvain-la-Nevve, Belgium. Unpublished manuscript.
von Sandersleben, J., Herbet, M., Weisse, I., Frölke, W., Guénard,
(1974) J. and Stötzer, H. Chronic toxicity test of the
substance W 524-XX on beagles, oral application,
over 104 weeks. Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated March 20, 1974,
submitted by Celamerck.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J.
(1971a) and Tilov, T. Testing of the subacute toxicity of
the substance W 524 in rats following oral
administration, Unpublished report from Boehringer
Sohn, Ingelheim, Germany, dated April 22, 1971,
No. T 14E, submitted by Celamerck.
Stötzer H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W.,
(1971b) Guénard, J. and Tilov, T. Testing of the subacute
toxicity of the substance W 524 in rats following
oral administration. Unpublished report from
Boehringer Sohn, Ingelheim, Germany, dated May 16,
1971, No. T 15 E, submitted by Celamerck.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke,
(1971c) W., Guénard, J. and von Sandersleben, J. Testing
of the subacute toxicity of the substance W 524 in
dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany,
dated February 5, 1971, No. T 12 E, submitted by
Celamerck.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke,
(1972a) W., Guénard, J. and Tilov, T. Chronic toxicity
studies with the substance W 524-XX in rats using
oral administration - duration 26 weeks.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated, November 28, 1972,
submitted by Celamerck.
Stötzer H., Herbst, M., Weisse, I., Frölke, W., Guénard, J.,
(1972b) and von Sandersleben, J. Chronic toxicity studies
with the substance W 524-XX in beagle dogs using
oral administration - duration 6 months.
Unpublished report from Boehringer Sohn,
Ingelheim, Germany, dated October 17, 1972,
submitted by Celamerck.
Zucks, A., deVires, F.W. and Oalbers, A.M.J. Uptake distribution
(1976) and metabolic fate of 3H-triforine in plants.
Pestic. Sci. 7, 115-127.
