
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
IMAZALIL
Explanation
Imazalil was reviewed by the Joint Meeting in 1977 (FAO, 1978) and
a temporary ADI for man was estimated to be 0-0.01 mg/kg body
weight. Imazalil is a moderately toxic fungicide from the group of
N-substituted imidazoles. Studies in rats have suggested that
imazalil is rapidly absorbed, distributed, metabolized, and
excreted following oral administration, with the major quantities
of metabolites occurring in urine and faeces within 3 days of
administration. Imazalil does not bioaccumulate. Tissue residues
are observed predominantly in those parts of the body associated
with biodegradation and elimination. Imazalil is not mutagenic as
evidenced by a dominant lethal study in mice and is not teratogenic
in rats. Long-term studies in the rat and a 2-year study in the
dog were believed to be inadequate because of unresolved pathology
questions concerning rat kidney and dog liver.
Based on these considerations, a temporary ADI was allocated and
further work was requested with respect to additional long-term,
short-term, acute toxicity, pharmacokinetic and metabolic studies.
Additional data (and a further interpretation of the previously
reviewed data) were submitted, reviewed, and are considered in this
monograph addendum.
Further metabolic studies with imazalil labelled with 14C and
further information on the nature and level of metabolites and
degradation products in plants were considered desirable.
A study was set up in order to provide information on the metabolic
fate of imazalil on growing banana plants. The results of
experiments have been submitted to the Joint Meeting for
evaluation. Additional information was obtained on use pattern and
national tolerances.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
A single lactating goat was orally administered imazalil (3H,
tritium-labelled) twice a day at a daily dose of 1.0 mg/kg body
weight for 3.5 days. Twenty-four hours after the final dose, the
animal was sacrificed and tissue residues were determined.
Qualitative and quantitative evaluations for tissue and excreted
residues were made.
Within 24 hours of the last dose, 96.6% of the administered
imazalil was excreted, predominantly in the urine (70%).
Approximately 0.14% of the administered radioactivity was secreted
in milk. (A slight translocation of the tritium isotope to water
may have made this milk residue higher than the actual imazalil
residue). Tissue residues were extremely low, not exceeding 0.03
mg/kg. Over the course of the study, an attempt was made to
determine whether translocation of the radioisotope tritium had
occurred with water. It was observed that very little tritiated
water was recovered, confirming the stability of the radioisotope
in the metabolism study. Within 36-hours, a steady state appeared
to be reached with respect to residues in milk and the plasma level
of imazalil. The steady-state concentrations in all cases were
extremely low. In urine, approximately 15% of the radioactivity
was present as free base metabolites and 14% as conjugated basic
metabolites. About 30% of the urinary metabolites were acidic,
polar products. The metabolic sequence with respect to the goat
was almost identical to that previously proposed for the rat.
After the last dose, plasma radioactivity decreased rapidly with
the half-life of approximately 19 hours. Highest tissue levels
were measured in the GI tract and in tissues associated with
biodegradation and excretion.
It was concluded that imazalil is rapidly absorbed and eliminated
from the body (within 24 hours). Approximately 70% of the residue
was found in urine, 26% in the faeces, small fractions were
observed in milk and as a residue in tissues (tissues predominantly
related to the excretory system). The steady-state condition was
rapidly reached on multiple dosing and residue data did not suggest
bioaccumulation (Meuldermans et al, 1979).
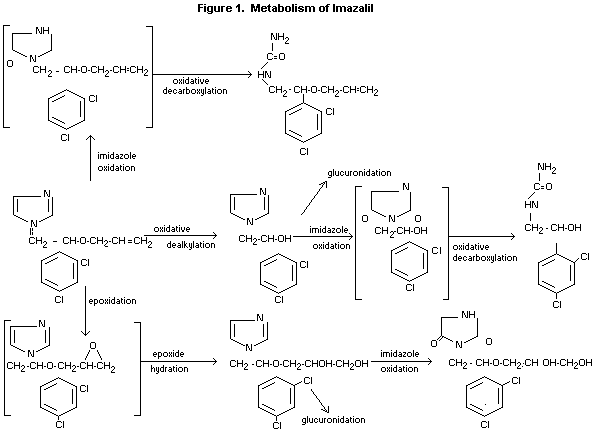
Biotransformation
Studies on the metabolic fate of imazalil (predominantly in
excreta) showed that imazalil was extensively metabolised with only
about 3% of the total administered dose excreted as unchanged
imazalil in the faeces. More than 10 acidic urinary metabolites
were detected. Five major basic metabolites were characterised.
Figure 1 demonstrates a qualitative evaluation of the metabolism of
imazalil.
Imazalil was metabolised extensively in the goat with only
approximately 3% of the parent chemical recovered unchanged,
predominantly in the faeces. Five major basic metabolites were
characterised resulting from oxidation and degradation of the
imidazole ring, from epoxidation and subsequent hydration of the
molecule; and oxidative O-dealkylation (Meuldermans et al, 1979).
The same qualitative metabolic picture with imazalil and its
congeners has been seen in various mammalian species. In man and
the rat, metabolism of a drug similar in chemical structure to
imazalil (econazole) was reported to be similar. Additionally, the
same pathway was observed in monkey and dog as well as rats with
respect to other chemicals of this class of N-substituted imidazoles
(Heykants, 1978).
From all the data on imazalil and on the congeners of imazalil, it
appears that the metabolic fate in all mammalian species is
comparable.
 TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female rats/group) were fed imazalil
in the diet at dosage levels of 0, 50, 200 or 800 mg/kg and
subjected to a standard 3-generation, 2-litter per generation,
reproduction study. Observations included: growth, food
consumption, mortality, and the standard indices of reproduction
(mating, fertility, gestation, viability and lactation).
The reproductive performance of rats was unaffected by imazalil at
any dose level. There was no maternal mortality and the parental
generations were unaffected by imazalil. No foetal abnormalities
nor embryonic effects were noted. Litter size, weight and survival
were normal. There were no effects on the reproductive performance
of rats continuously fed imazalil in the diet at levels up to and
including 800 mg/kg (Marsboom, 1978).
Acute toxicity
Rat
Chemical Form Sex LD50
mg/kg (CL)
Nitrate M 343 (262-448)
F 288 (221-377)
Sulphate M 355 (272-464)
F 309 (237-404)
Acetate M 371 (284-485)
F 309 (237-404)
Free Base M 343 (262-448)
F 227 (174-297)
All data were generated with adult rats, administered imazalil
orally as an aqueous suspension. The acute signs of poisoning were
the same for each chemical form of imazalil. These included:
ataxia, piloerection, hypotonia, hypothermia, exophthalmia,
tremors, salivation, lacrimation, diuresis, diarrhoea, palpable
ptosis, and loss of the righting reflex (Niemegeers, 1979).
Long-term studies
Rat
Groups of rats (50 male and 50 female Wistar rats/group) were
administered imazalil in the diet at dosage levels of 0, 25, 100 or
400 mg/kg for 24 months. The animals were 3-4 months of age at the
initiation of the carcinogenicity study.
Animals were examined daily for behaviourial changes and toxicity.
At the conclusion of the study, gross and microscopic examination
was performed on all surviving animals. During the course of the
study, examinations were performed on animals that had died or were
sacrificed in extremis. Microscopic examinations were performed on
the major tissues and organs and on all lesions that were noted
during the course of gross examination.
There was substantial mortality over the course of the study, and
very few animals survived the full 24 months. It was considered
that this was due to the fact that the animals were 3-4 months old
at the start of the study and that they were not maintained under
SPF-conditions. Evaluation of the data, relating to the time on
study at which animals died, did not suggest that imazalil
contributed to the increased mortality or the accelerated death
rate. There were no dose-related events noted on gross pathology.
There were no significant differences with respect to the overall
tumour rate or the individual types of tumours reported in any of
the does groups. There is no evidence that imazalil was
responsible for tumour formation in any organ or tissue examined in
the study (Marsboom and Herin, 1979a).
Mice
Groups of mice (50 male and 50 female albino, Swiss mice/group)
were administered imazalil in the drinking water for 18 months at
dosage levels of 0, 6.25, 25, or 100 mg/kg. It was approximated
that the dietary levels reflect an intake of 0, 2.5, 10 or 40 mg/kg
body weight based upon an assumption that each mouse drinks
approximately 100 ml/week.
All animals were examined daily for behaviour abnormalities and
clinical toxicity. At the termination of the study, gross and
microscopic examinations were performed on all surviving animals.
A complete examination was performed on animals dying during the
course of the study or that had to be sacrificed before
termination. Gross and microscopic examinations were made on
selected tissues and organs. (Gross examinations did not include
organ-weight data). In addition, any tissue that was suspect of
unusual lesion was examined histologically.
There was no apparent dose-related effect on survival or on the
time that mortality occurred. At the conclusion of 18 months,
there was a substantial number of animals (greater than 25% in all
cases) surviving. There were no significant differences with
respect to survival in any of the test groups when compared to
control values. There were no dose-related effects on health,
behaviour, or appearance. An examination of the data on the
occurrence of tumours in animals dying or sacrificed at the end
of the study showed that there were no substantial differences
between groups or with control values. Imazalil in the drinking
water did not influence the occurrence of tumours. It was
concluded that under the conditions of this assay, imazalil was
not a tumourigen or a carcinogen in mice (Marsboom and Herin,
1979b).
RESIDUES IN FOOD
USE PATTERN
Crop/ Formulation and mode Concentration
disease of application ai
Gherkin (outdoors) 20 EC, 0.025% 0.05 kg/ha
powdery mildew
Barley
Pyrenophora spp. liquid, 2.4% 48 mg/kg seed
Fusarium
Wheat, barley seed dressing
oats, rye 50 mg/kg
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of recent trials are summarised in Table 1.
TABLE 1. Residues resulting from supervised trials in the Netherlands
Crop Year of Application Residues in mg/kg, at intervals
trial (days) after (last) application
Number of Rate per Formulation
treatments treatment
kg ai/ha 3 4 ± 60
wheat 1976 1 0.125 liquid <0.011
wheat-straw 1976 1 0.125 200 g/l <0.11
gherkin 1977 1 0.1 liquid <0.011
(glasshouse) 200 g/l
" 1977 1 0.15 200 g/l 0.04
(0.03-0.05)
1 Limit of determination
FATE OF RESIDUES
Eleven growing banana plants with a minimum of six leaves were used in
the study. One of the banana plants (No. 1) was not treated and
served for providing blank samples. The others were sprayed uniformly
from above with a distilled water solution of 3N-imazalil-sulphate
labelled specially on the asymmetric carbon. The plants were treated
once, four, five or nine times consecutively at two-week intervals.
The sampling of the individual plants was carried out as follows:
One plant (No. 1) was sampled without any previous treatment, serving
as a blank. Plants Nos. 2, 3 and 4 were sampled two hours (the time
necessary for drying), four days and fourteen days respectively after
a single treatment, Plant No. 5 fourteen days after the fourth
treatment, plants Nos. 6, 7 and 8 two hours, four days and fourteen
days respectively after the fifth treatment and plants Nos. 9, 10 and
11 two hours, four days and fourteen days respectively after the ninth
treatment.
Five different parts of the plants were investigated: mature leaves,
new leaves emerged after the last treatment, psuedostem, rhizome and
roots (petioles were considered part; of the psuedostem).
The roots were out from the rhizome and the soil was washed off with
water.
The various parts of plants were minced and thereafter homogenised in
water (1/5, w/v).
The radioactivity levels of samples were measured in the aliquots of
the homogenates immediately after homogenisation.
The leaf homogenates of the treated plants and the spiked blank
homogenate were subjected to repeated extraction. The radioactivity
of the various extracts was measured. Some of the extracts were
analysed with on-line radio HPLC after sample clean up on Sep-pak
TMC18 cartridges. A part of leaf and a part of cigar leaf of plant
No. 11 were prepared for autoradiography.
The radioactivity levels in the various plant samples indicated fairly
uniform sprayings and the absence of tritiated water or other volatile
radioactive compounds. The leaves contained 95.2-100% of the total
radioactivity recovered in the plants treated one to nine times. The
radioactivity was practically all concentrated at the upper surface of
the leaf tested with autoradiography. Transport of the radioactivity
to the roots (0.32% maximally) or the rhizome (2.54% maximally) was
minimal. In the leaves emerged after the last treatment less than
0.03% of the radioactivity recovered in the whole plant was detected.
The metabolite pattern in the leaf homogenates was investigated after
extraction.
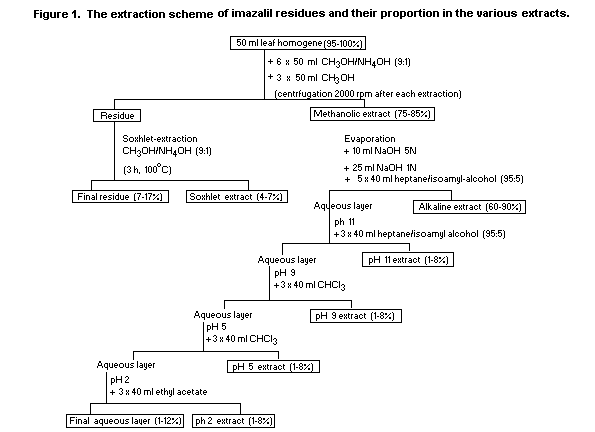
The scheme of extraction and the percentage ratio of the total
radioactivity found in various extracts are shown in Figure 1.
The differences between the various homogenates of the leaves of
plants that were sprayed once, four, five or nine times were only
minor. Attempts to extract the radioactivity from the final residues
by soxhlet with chloroform, ethyl acetate and acetone or by batch
extraction with 19% trichloroacetic acid failed.
Analyses of various plant extracts on radio-HPLC revealed that the
main part of radio-activity originated from imazalil and its
metabolite alpha-(2,4-dichloro-phenyl)-1H-imidazole-1-ethanol
(R14821). Unchanged imazalil constituted 33.8, 46.9, 37.1, and 22.3%
and R 14821 23.4, 22.2, 27.9 and 37.8% of the radioactivity in the
alkaline extracts of the leaves of plants Nos. 8, 9, 10 and 11
respectively. These results indicated that imazalil was degraded
slowly as a function of time and that R 14821 was the main degradation
product which was found in the pH11 extract too. Although the
radioactivity in the extracts of pH 11, pH 9, ph 5 and pH 2 amounted
to 15.38% of the alkaline extract, only a few small radioactivity
peaks could be detected, which may be formed at least partly
artificially from imazalil and R 14821 as similar peak patterns
appeared on the chromatograms of the extracts of blank homogenate
spiked with these compounds.
The remaining part of the radioactivity might be explained with the
presence of a large number of minor metabolites. R 14821 (45.4%) and
imazalil (20.7%) were also major radioactive compounds in the soxhlet
extract.
The parent compound accounted for altogether about 20 and 15% of the
total radioactivity while the proportion of R 14821 was 15% and 25% in
the leaves of plants Nos. 8 and 11 respectively.
A mixture of unidentified polar acid metabolites (7% of the total
radioactivity) was found in both the pH 2 extract and the final
aqueous layer. From 7-17% of the radioactivity in the leaf
homogenates could not be extracted.
The rest of the radioactivity, 35 to 45%, was due to a very large
number of minor metabolites (Meuldermans, W. et al, undated).
EVALUATION
COMMENTS AND APPRAISAL
Imazalil was reviewed by the 1977 Joint Meeting and a temporary ADI
was estimated to be 0-0.01 mg/kg bw/day. Concern was expressed over
the pathological changes observed in rat kidney and dog liver in
two-year studies. On the basis of a re-evaluation by an independent
pathologist the meeting concluded there was no difference between the
control and the treated animals.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female rats/group) were fed imazalil
in the diet at dosage levels of 0, 50, 200 or 800 mg/kg and
subjected to a standard 3-generation, 2-litter per generation,
reproduction study. Observations included: growth, food
consumption, mortality, and the standard indices of reproduction
(mating, fertility, gestation, viability and lactation).
The reproductive performance of rats was unaffected by imazalil at
any dose level. There was no maternal mortality and the parental
generations were unaffected by imazalil. No foetal abnormalities
nor embryonic effects were noted. Litter size, weight and survival
were normal. There were no effects on the reproductive performance
of rats continuously fed imazalil in the diet at levels up to and
including 800 mg/kg (Marsboom, 1978).
Acute toxicity
Rat
Chemical Form Sex LD50
mg/kg (CL)
Nitrate M 343 (262-448)
F 288 (221-377)
Sulphate M 355 (272-464)
F 309 (237-404)
Acetate M 371 (284-485)
F 309 (237-404)
Free Base M 343 (262-448)
F 227 (174-297)
All data were generated with adult rats, administered imazalil
orally as an aqueous suspension. The acute signs of poisoning were
the same for each chemical form of imazalil. These included:
ataxia, piloerection, hypotonia, hypothermia, exophthalmia,
tremors, salivation, lacrimation, diuresis, diarrhoea, palpable
ptosis, and loss of the righting reflex (Niemegeers, 1979).
Long-term studies
Rat
Groups of rats (50 male and 50 female Wistar rats/group) were
administered imazalil in the diet at dosage levels of 0, 25, 100 or
400 mg/kg for 24 months. The animals were 3-4 months of age at the
initiation of the carcinogenicity study.
Animals were examined daily for behaviourial changes and toxicity.
At the conclusion of the study, gross and microscopic examination
was performed on all surviving animals. During the course of the
study, examinations were performed on animals that had died or were
sacrificed in extremis. Microscopic examinations were performed on
the major tissues and organs and on all lesions that were noted
during the course of gross examination.
There was substantial mortality over the course of the study, and
very few animals survived the full 24 months. It was considered
that this was due to the fact that the animals were 3-4 months old
at the start of the study and that they were not maintained under
SPF-conditions. Evaluation of the data, relating to the time on
study at which animals died, did not suggest that imazalil
contributed to the increased mortality or the accelerated death
rate. There were no dose-related events noted on gross pathology.
There were no significant differences with respect to the overall
tumour rate or the individual types of tumours reported in any of
the does groups. There is no evidence that imazalil was
responsible for tumour formation in any organ or tissue examined in
the study (Marsboom and Herin, 1979a).
Mice
Groups of mice (50 male and 50 female albino, Swiss mice/group)
were administered imazalil in the drinking water for 18 months at
dosage levels of 0, 6.25, 25, or 100 mg/kg. It was approximated
that the dietary levels reflect an intake of 0, 2.5, 10 or 40 mg/kg
body weight based upon an assumption that each mouse drinks
approximately 100 ml/week.
All animals were examined daily for behaviour abnormalities and
clinical toxicity. At the termination of the study, gross and
microscopic examinations were performed on all surviving animals.
A complete examination was performed on animals dying during the
course of the study or that had to be sacrificed before
termination. Gross and microscopic examinations were made on
selected tissues and organs. (Gross examinations did not include
organ-weight data). In addition, any tissue that was suspect of
unusual lesion was examined histologically.
There was no apparent dose-related effect on survival or on the
time that mortality occurred. At the conclusion of 18 months,
there was a substantial number of animals (greater than 25% in all
cases) surviving. There were no significant differences with
respect to survival in any of the test groups when compared to
control values. There were no dose-related effects on health,
behaviour, or appearance. An examination of the data on the
occurrence of tumours in animals dying or sacrificed at the end
of the study showed that there were no substantial differences
between groups or with control values. Imazalil in the drinking
water did not influence the occurrence of tumours. It was
concluded that under the conditions of this assay, imazalil was
not a tumourigen or a carcinogen in mice (Marsboom and Herin,
1979b).
RESIDUES IN FOOD
USE PATTERN
Crop/ Formulation and mode Concentration
disease of application ai
Gherkin (outdoors) 20 EC, 0.025% 0.05 kg/ha
powdery mildew
Barley
Pyrenophora spp. liquid, 2.4% 48 mg/kg seed
Fusarium
Wheat, barley seed dressing
oats, rye 50 mg/kg
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of recent trials are summarised in Table 1.
TABLE 1. Residues resulting from supervised trials in the Netherlands
Crop Year of Application Residues in mg/kg, at intervals
trial (days) after (last) application
Number of Rate per Formulation
treatments treatment
kg ai/ha 3 4 ± 60
wheat 1976 1 0.125 liquid <0.011
wheat-straw 1976 1 0.125 200 g/l <0.11
gherkin 1977 1 0.1 liquid <0.011
(glasshouse) 200 g/l
" 1977 1 0.15 200 g/l 0.04
(0.03-0.05)
1 Limit of determination
FATE OF RESIDUES
Eleven growing banana plants with a minimum of six leaves were used in
the study. One of the banana plants (No. 1) was not treated and
served for providing blank samples. The others were sprayed uniformly
from above with a distilled water solution of 3N-imazalil-sulphate
labelled specially on the asymmetric carbon. The plants were treated
once, four, five or nine times consecutively at two-week intervals.
The sampling of the individual plants was carried out as follows:
One plant (No. 1) was sampled without any previous treatment, serving
as a blank. Plants Nos. 2, 3 and 4 were sampled two hours (the time
necessary for drying), four days and fourteen days respectively after
a single treatment, Plant No. 5 fourteen days after the fourth
treatment, plants Nos. 6, 7 and 8 two hours, four days and fourteen
days respectively after the fifth treatment and plants Nos. 9, 10 and
11 two hours, four days and fourteen days respectively after the ninth
treatment.
Five different parts of the plants were investigated: mature leaves,
new leaves emerged after the last treatment, psuedostem, rhizome and
roots (petioles were considered part; of the psuedostem).
The roots were out from the rhizome and the soil was washed off with
water.
The various parts of plants were minced and thereafter homogenised in
water (1/5, w/v).
The radioactivity levels of samples were measured in the aliquots of
the homogenates immediately after homogenisation.
The leaf homogenates of the treated plants and the spiked blank
homogenate were subjected to repeated extraction. The radioactivity
of the various extracts was measured. Some of the extracts were
analysed with on-line radio HPLC after sample clean up on Sep-pak
TMC18 cartridges. A part of leaf and a part of cigar leaf of plant
No. 11 were prepared for autoradiography.
The radioactivity levels in the various plant samples indicated fairly
uniform sprayings and the absence of tritiated water or other volatile
radioactive compounds. The leaves contained 95.2-100% of the total
radioactivity recovered in the plants treated one to nine times. The
radioactivity was practically all concentrated at the upper surface of
the leaf tested with autoradiography. Transport of the radioactivity
to the roots (0.32% maximally) or the rhizome (2.54% maximally) was
minimal. In the leaves emerged after the last treatment less than
0.03% of the radioactivity recovered in the whole plant was detected.
The metabolite pattern in the leaf homogenates was investigated after
extraction.
The scheme of extraction and the percentage ratio of the total
radioactivity found in various extracts are shown in Figure 1.
The differences between the various homogenates of the leaves of
plants that were sprayed once, four, five or nine times were only
minor. Attempts to extract the radioactivity from the final residues
by soxhlet with chloroform, ethyl acetate and acetone or by batch
extraction with 19% trichloroacetic acid failed.
Analyses of various plant extracts on radio-HPLC revealed that the
main part of radio-activity originated from imazalil and its
metabolite alpha-(2,4-dichloro-phenyl)-1H-imidazole-1-ethanol
(R14821). Unchanged imazalil constituted 33.8, 46.9, 37.1, and 22.3%
and R 14821 23.4, 22.2, 27.9 and 37.8% of the radioactivity in the
alkaline extracts of the leaves of plants Nos. 8, 9, 10 and 11
respectively. These results indicated that imazalil was degraded
slowly as a function of time and that R 14821 was the main degradation
product which was found in the pH11 extract too. Although the
radioactivity in the extracts of pH 11, pH 9, ph 5 and pH 2 amounted
to 15.38% of the alkaline extract, only a few small radioactivity
peaks could be detected, which may be formed at least partly
artificially from imazalil and R 14821 as similar peak patterns
appeared on the chromatograms of the extracts of blank homogenate
spiked with these compounds.
The remaining part of the radioactivity might be explained with the
presence of a large number of minor metabolites. R 14821 (45.4%) and
imazalil (20.7%) were also major radioactive compounds in the soxhlet
extract.
The parent compound accounted for altogether about 20 and 15% of the
total radioactivity while the proportion of R 14821 was 15% and 25% in
the leaves of plants Nos. 8 and 11 respectively.
A mixture of unidentified polar acid metabolites (7% of the total
radioactivity) was found in both the pH 2 extract and the final
aqueous layer. From 7-17% of the radioactivity in the leaf
homogenates could not be extracted.
The rest of the radioactivity, 35 to 45%, was due to a very large
number of minor metabolites (Meuldermans, W. et al, undated).
EVALUATION
COMMENTS AND APPRAISAL
Imazalil was reviewed by the 1977 Joint Meeting and a temporary ADI
was estimated to be 0-0.01 mg/kg bw/day. Concern was expressed over
the pathological changes observed in rat kidney and dog liver in
two-year studies. On the basis of a re-evaluation by an independent
pathologist the meeting concluded there was no difference between the
control and the treated animals.
 New data submitted to the meeting with respect to long-term
carcinogenesis studies in two species was found to be inadequate to
meet the current criteria for appropriate carcinogenicity studies.
The survival rate of rats for 24 months was poor, and a carcinogenic
evaluation could not be made with this species. In contrast to the
rat study however there was a significant number of survivors in the
mouse study.
The metabolic profile of imazalil especially with respect to salt
formulations appears to be well defined. Imazalil did not affect
reproduction in rats.
A temporary acceptable daily intake for man was reaffirmed on the
basis of studies in rat and dog. In several respects the long-term
studies reported to the meeting have been inadequate to evaluate fully
the toxicological profile. The Meeting felt that while no immediate
problems exist, a further adequately performed long-term study is
required. Additionally, short-term mutagenicity tests should be
performed.
Additional information has now been provided on the metabolic fate of
3H-labelled imazalil on banana plants grown in a greenhouse, and on
the results of supervised trials.
The major part of the radioactivity (95.2-100%) recovered in the
plants was present on the surface and in the upper layers of the
leaves. Translocation of the radioactivity from the sprayed areas to
the rest of the plant was minimal, since the rhizome, roots and new
leaves that emerged after the last treatment showed very little or no
radioactivity. The parent compound formed 15% of the total
radioactivity recovered in the leaves of plants treated nine times,
while the single major metabolite,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol accounted for 25%.
The rest of the radioactivity was due to a large number of
unidentified minor metabolites, each representing at most only a few
percent of the radioactivity. A considerable part of the unidentified
material might consist of degradation products formed during the
analytical procedure.
The results of the metabolism studies and the supervised trials are in
agreement with the findings discussed in the 1977 evaluation, and
support the recommendations made then.
Level causing no toxicological effect
Rat: 5 mg/kg bw/day
Dog: 1.24 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day
FURTHER WORK OR INFORMATION
Required (by 1984)
1. An adequate long-term study on rats to define fully a no-effect
level.
2. Short-term tests to evaluate the mutagenic potential.
REFERENCES
Heykants, J.J.P. On the metabolism of imazalil and related compounds
in animals and man. A review. (1978) Unpublished summary review of
published and unpublished metabolism data from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, H. Oral three-generation study in Wistar rats. (1978)
Unpublished report from Janssen Pharmaceutica submitted to the World
Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in Wistar rats.
(1979a) Unpublished report (No. 667) from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in albino Swiss
mice. (1979b) Unpublished report (No. 666) from Janssen Pharmaceutica,
submitted to the World Organization by Janssen Pharmaceutica.
Meuldermans, W., Swijsen, E., Hendricks, J., Wolstenborghs, R.,
Lauwers, W. and Heykants, J. (1979) Unpublished report from Janssen
Pharmaceutica submitted to the World Health Organization by Janssen
Pharmaceutica.
Meuldermans, W. et al. The metabolic fate of imazalil on banana
plants. Janssen Research Products Int. Service. R 23979/21.
Niemegears, C.J.E. Comparative acute oral toxicity studies of the
different salts of imazalil in rats. (1979) Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Janssen Pharmaceutica.
New data submitted to the meeting with respect to long-term
carcinogenesis studies in two species was found to be inadequate to
meet the current criteria for appropriate carcinogenicity studies.
The survival rate of rats for 24 months was poor, and a carcinogenic
evaluation could not be made with this species. In contrast to the
rat study however there was a significant number of survivors in the
mouse study.
The metabolic profile of imazalil especially with respect to salt
formulations appears to be well defined. Imazalil did not affect
reproduction in rats.
A temporary acceptable daily intake for man was reaffirmed on the
basis of studies in rat and dog. In several respects the long-term
studies reported to the meeting have been inadequate to evaluate fully
the toxicological profile. The Meeting felt that while no immediate
problems exist, a further adequately performed long-term study is
required. Additionally, short-term mutagenicity tests should be
performed.
Additional information has now been provided on the metabolic fate of
3H-labelled imazalil on banana plants grown in a greenhouse, and on
the results of supervised trials.
The major part of the radioactivity (95.2-100%) recovered in the
plants was present on the surface and in the upper layers of the
leaves. Translocation of the radioactivity from the sprayed areas to
the rest of the plant was minimal, since the rhizome, roots and new
leaves that emerged after the last treatment showed very little or no
radioactivity. The parent compound formed 15% of the total
radioactivity recovered in the leaves of plants treated nine times,
while the single major metabolite,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol accounted for 25%.
The rest of the radioactivity was due to a large number of
unidentified minor metabolites, each representing at most only a few
percent of the radioactivity. A considerable part of the unidentified
material might consist of degradation products formed during the
analytical procedure.
The results of the metabolism studies and the supervised trials are in
agreement with the findings discussed in the 1977 evaluation, and
support the recommendations made then.
Level causing no toxicological effect
Rat: 5 mg/kg bw/day
Dog: 1.24 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day
FURTHER WORK OR INFORMATION
Required (by 1984)
1. An adequate long-term study on rats to define fully a no-effect
level.
2. Short-term tests to evaluate the mutagenic potential.
REFERENCES
Heykants, J.J.P. On the metabolism of imazalil and related compounds
in animals and man. A review. (1978) Unpublished summary review of
published and unpublished metabolism data from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, H. Oral three-generation study in Wistar rats. (1978)
Unpublished report from Janssen Pharmaceutica submitted to the World
Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in Wistar rats.
(1979a) Unpublished report (No. 667) from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in albino Swiss
mice. (1979b) Unpublished report (No. 666) from Janssen Pharmaceutica,
submitted to the World Organization by Janssen Pharmaceutica.
Meuldermans, W., Swijsen, E., Hendricks, J., Wolstenborghs, R.,
Lauwers, W. and Heykants, J. (1979) Unpublished report from Janssen
Pharmaceutica submitted to the World Health Organization by Janssen
Pharmaceutica.
Meuldermans, W. et al. The metabolic fate of imazalil on banana
plants. Janssen Research Products Int. Service. R 23979/21.
Niemegears, C.J.E. Comparative acute oral toxicity studies of the
different salts of imazalil in rats. (1979) Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Janssen Pharmaceutica.
 TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female rats/group) were fed imazalil
in the diet at dosage levels of 0, 50, 200 or 800 mg/kg and
subjected to a standard 3-generation, 2-litter per generation,
reproduction study. Observations included: growth, food
consumption, mortality, and the standard indices of reproduction
(mating, fertility, gestation, viability and lactation).
The reproductive performance of rats was unaffected by imazalil at
any dose level. There was no maternal mortality and the parental
generations were unaffected by imazalil. No foetal abnormalities
nor embryonic effects were noted. Litter size, weight and survival
were normal. There were no effects on the reproductive performance
of rats continuously fed imazalil in the diet at levels up to and
including 800 mg/kg (Marsboom, 1978).
Acute toxicity
Rat
Chemical Form Sex LD50
mg/kg (CL)
Nitrate M 343 (262-448)
F 288 (221-377)
Sulphate M 355 (272-464)
F 309 (237-404)
Acetate M 371 (284-485)
F 309 (237-404)
Free Base M 343 (262-448)
F 227 (174-297)
All data were generated with adult rats, administered imazalil
orally as an aqueous suspension. The acute signs of poisoning were
the same for each chemical form of imazalil. These included:
ataxia, piloerection, hypotonia, hypothermia, exophthalmia,
tremors, salivation, lacrimation, diuresis, diarrhoea, palpable
ptosis, and loss of the righting reflex (Niemegeers, 1979).
Long-term studies
Rat
Groups of rats (50 male and 50 female Wistar rats/group) were
administered imazalil in the diet at dosage levels of 0, 25, 100 or
400 mg/kg for 24 months. The animals were 3-4 months of age at the
initiation of the carcinogenicity study.
Animals were examined daily for behaviourial changes and toxicity.
At the conclusion of the study, gross and microscopic examination
was performed on all surviving animals. During the course of the
study, examinations were performed on animals that had died or were
sacrificed in extremis. Microscopic examinations were performed on
the major tissues and organs and on all lesions that were noted
during the course of gross examination.
There was substantial mortality over the course of the study, and
very few animals survived the full 24 months. It was considered
that this was due to the fact that the animals were 3-4 months old
at the start of the study and that they were not maintained under
SPF-conditions. Evaluation of the data, relating to the time on
study at which animals died, did not suggest that imazalil
contributed to the increased mortality or the accelerated death
rate. There were no dose-related events noted on gross pathology.
There were no significant differences with respect to the overall
tumour rate or the individual types of tumours reported in any of
the does groups. There is no evidence that imazalil was
responsible for tumour formation in any organ or tissue examined in
the study (Marsboom and Herin, 1979a).
Mice
Groups of mice (50 male and 50 female albino, Swiss mice/group)
were administered imazalil in the drinking water for 18 months at
dosage levels of 0, 6.25, 25, or 100 mg/kg. It was approximated
that the dietary levels reflect an intake of 0, 2.5, 10 or 40 mg/kg
body weight based upon an assumption that each mouse drinks
approximately 100 ml/week.
All animals were examined daily for behaviour abnormalities and
clinical toxicity. At the termination of the study, gross and
microscopic examinations were performed on all surviving animals.
A complete examination was performed on animals dying during the
course of the study or that had to be sacrificed before
termination. Gross and microscopic examinations were made on
selected tissues and organs. (Gross examinations did not include
organ-weight data). In addition, any tissue that was suspect of
unusual lesion was examined histologically.
There was no apparent dose-related effect on survival or on the
time that mortality occurred. At the conclusion of 18 months,
there was a substantial number of animals (greater than 25% in all
cases) surviving. There were no significant differences with
respect to survival in any of the test groups when compared to
control values. There were no dose-related effects on health,
behaviour, or appearance. An examination of the data on the
occurrence of tumours in animals dying or sacrificed at the end
of the study showed that there were no substantial differences
between groups or with control values. Imazalil in the drinking
water did not influence the occurrence of tumours. It was
concluded that under the conditions of this assay, imazalil was
not a tumourigen or a carcinogen in mice (Marsboom and Herin,
1979b).
RESIDUES IN FOOD
USE PATTERN
Crop/ Formulation and mode Concentration
disease of application ai
Gherkin (outdoors) 20 EC, 0.025% 0.05 kg/ha
powdery mildew
Barley
Pyrenophora spp. liquid, 2.4% 48 mg/kg seed
Fusarium
Wheat, barley seed dressing
oats, rye 50 mg/kg
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of recent trials are summarised in Table 1.
TABLE 1. Residues resulting from supervised trials in the Netherlands
Crop Year of Application Residues in mg/kg, at intervals
trial (days) after (last) application
Number of Rate per Formulation
treatments treatment
kg ai/ha 3 4 ± 60
wheat 1976 1 0.125 liquid <0.011
wheat-straw 1976 1 0.125 200 g/l <0.11
gherkin 1977 1 0.1 liquid <0.011
(glasshouse) 200 g/l
" 1977 1 0.15 200 g/l 0.04
(0.03-0.05)
1 Limit of determination
FATE OF RESIDUES
Eleven growing banana plants with a minimum of six leaves were used in
the study. One of the banana plants (No. 1) was not treated and
served for providing blank samples. The others were sprayed uniformly
from above with a distilled water solution of 3N-imazalil-sulphate
labelled specially on the asymmetric carbon. The plants were treated
once, four, five or nine times consecutively at two-week intervals.
The sampling of the individual plants was carried out as follows:
One plant (No. 1) was sampled without any previous treatment, serving
as a blank. Plants Nos. 2, 3 and 4 were sampled two hours (the time
necessary for drying), four days and fourteen days respectively after
a single treatment, Plant No. 5 fourteen days after the fourth
treatment, plants Nos. 6, 7 and 8 two hours, four days and fourteen
days respectively after the fifth treatment and plants Nos. 9, 10 and
11 two hours, four days and fourteen days respectively after the ninth
treatment.
Five different parts of the plants were investigated: mature leaves,
new leaves emerged after the last treatment, psuedostem, rhizome and
roots (petioles were considered part; of the psuedostem).
The roots were out from the rhizome and the soil was washed off with
water.
The various parts of plants were minced and thereafter homogenised in
water (1/5, w/v).
The radioactivity levels of samples were measured in the aliquots of
the homogenates immediately after homogenisation.
The leaf homogenates of the treated plants and the spiked blank
homogenate were subjected to repeated extraction. The radioactivity
of the various extracts was measured. Some of the extracts were
analysed with on-line radio HPLC after sample clean up on Sep-pak
TMC18 cartridges. A part of leaf and a part of cigar leaf of plant
No. 11 were prepared for autoradiography.
The radioactivity levels in the various plant samples indicated fairly
uniform sprayings and the absence of tritiated water or other volatile
radioactive compounds. The leaves contained 95.2-100% of the total
radioactivity recovered in the plants treated one to nine times. The
radioactivity was practically all concentrated at the upper surface of
the leaf tested with autoradiography. Transport of the radioactivity
to the roots (0.32% maximally) or the rhizome (2.54% maximally) was
minimal. In the leaves emerged after the last treatment less than
0.03% of the radioactivity recovered in the whole plant was detected.
The metabolite pattern in the leaf homogenates was investigated after
extraction.
The scheme of extraction and the percentage ratio of the total
radioactivity found in various extracts are shown in Figure 1.
The differences between the various homogenates of the leaves of
plants that were sprayed once, four, five or nine times were only
minor. Attempts to extract the radioactivity from the final residues
by soxhlet with chloroform, ethyl acetate and acetone or by batch
extraction with 19% trichloroacetic acid failed.
Analyses of various plant extracts on radio-HPLC revealed that the
main part of radio-activity originated from imazalil and its
metabolite alpha-(2,4-dichloro-phenyl)-1H-imidazole-1-ethanol
(R14821). Unchanged imazalil constituted 33.8, 46.9, 37.1, and 22.3%
and R 14821 23.4, 22.2, 27.9 and 37.8% of the radioactivity in the
alkaline extracts of the leaves of plants Nos. 8, 9, 10 and 11
respectively. These results indicated that imazalil was degraded
slowly as a function of time and that R 14821 was the main degradation
product which was found in the pH11 extract too. Although the
radioactivity in the extracts of pH 11, pH 9, ph 5 and pH 2 amounted
to 15.38% of the alkaline extract, only a few small radioactivity
peaks could be detected, which may be formed at least partly
artificially from imazalil and R 14821 as similar peak patterns
appeared on the chromatograms of the extracts of blank homogenate
spiked with these compounds.
The remaining part of the radioactivity might be explained with the
presence of a large number of minor metabolites. R 14821 (45.4%) and
imazalil (20.7%) were also major radioactive compounds in the soxhlet
extract.
The parent compound accounted for altogether about 20 and 15% of the
total radioactivity while the proportion of R 14821 was 15% and 25% in
the leaves of plants Nos. 8 and 11 respectively.
A mixture of unidentified polar acid metabolites (7% of the total
radioactivity) was found in both the pH 2 extract and the final
aqueous layer. From 7-17% of the radioactivity in the leaf
homogenates could not be extracted.
The rest of the radioactivity, 35 to 45%, was due to a very large
number of minor metabolites (Meuldermans, W. et al, undated).
EVALUATION
COMMENTS AND APPRAISAL
Imazalil was reviewed by the 1977 Joint Meeting and a temporary ADI
was estimated to be 0-0.01 mg/kg bw/day. Concern was expressed over
the pathological changes observed in rat kidney and dog liver in
two-year studies. On the basis of a re-evaluation by an independent
pathologist the meeting concluded there was no difference between the
control and the treated animals.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Groups of rats (10 male and 20 female rats/group) were fed imazalil
in the diet at dosage levels of 0, 50, 200 or 800 mg/kg and
subjected to a standard 3-generation, 2-litter per generation,
reproduction study. Observations included: growth, food
consumption, mortality, and the standard indices of reproduction
(mating, fertility, gestation, viability and lactation).
The reproductive performance of rats was unaffected by imazalil at
any dose level. There was no maternal mortality and the parental
generations were unaffected by imazalil. No foetal abnormalities
nor embryonic effects were noted. Litter size, weight and survival
were normal. There were no effects on the reproductive performance
of rats continuously fed imazalil in the diet at levels up to and
including 800 mg/kg (Marsboom, 1978).
Acute toxicity
Rat
Chemical Form Sex LD50
mg/kg (CL)
Nitrate M 343 (262-448)
F 288 (221-377)
Sulphate M 355 (272-464)
F 309 (237-404)
Acetate M 371 (284-485)
F 309 (237-404)
Free Base M 343 (262-448)
F 227 (174-297)
All data were generated with adult rats, administered imazalil
orally as an aqueous suspension. The acute signs of poisoning were
the same for each chemical form of imazalil. These included:
ataxia, piloerection, hypotonia, hypothermia, exophthalmia,
tremors, salivation, lacrimation, diuresis, diarrhoea, palpable
ptosis, and loss of the righting reflex (Niemegeers, 1979).
Long-term studies
Rat
Groups of rats (50 male and 50 female Wistar rats/group) were
administered imazalil in the diet at dosage levels of 0, 25, 100 or
400 mg/kg for 24 months. The animals were 3-4 months of age at the
initiation of the carcinogenicity study.
Animals were examined daily for behaviourial changes and toxicity.
At the conclusion of the study, gross and microscopic examination
was performed on all surviving animals. During the course of the
study, examinations were performed on animals that had died or were
sacrificed in extremis. Microscopic examinations were performed on
the major tissues and organs and on all lesions that were noted
during the course of gross examination.
There was substantial mortality over the course of the study, and
very few animals survived the full 24 months. It was considered
that this was due to the fact that the animals were 3-4 months old
at the start of the study and that they were not maintained under
SPF-conditions. Evaluation of the data, relating to the time on
study at which animals died, did not suggest that imazalil
contributed to the increased mortality or the accelerated death
rate. There were no dose-related events noted on gross pathology.
There were no significant differences with respect to the overall
tumour rate or the individual types of tumours reported in any of
the does groups. There is no evidence that imazalil was
responsible for tumour formation in any organ or tissue examined in
the study (Marsboom and Herin, 1979a).
Mice
Groups of mice (50 male and 50 female albino, Swiss mice/group)
were administered imazalil in the drinking water for 18 months at
dosage levels of 0, 6.25, 25, or 100 mg/kg. It was approximated
that the dietary levels reflect an intake of 0, 2.5, 10 or 40 mg/kg
body weight based upon an assumption that each mouse drinks
approximately 100 ml/week.
All animals were examined daily for behaviour abnormalities and
clinical toxicity. At the termination of the study, gross and
microscopic examinations were performed on all surviving animals.
A complete examination was performed on animals dying during the
course of the study or that had to be sacrificed before
termination. Gross and microscopic examinations were made on
selected tissues and organs. (Gross examinations did not include
organ-weight data). In addition, any tissue that was suspect of
unusual lesion was examined histologically.
There was no apparent dose-related effect on survival or on the
time that mortality occurred. At the conclusion of 18 months,
there was a substantial number of animals (greater than 25% in all
cases) surviving. There were no significant differences with
respect to survival in any of the test groups when compared to
control values. There were no dose-related effects on health,
behaviour, or appearance. An examination of the data on the
occurrence of tumours in animals dying or sacrificed at the end
of the study showed that there were no substantial differences
between groups or with control values. Imazalil in the drinking
water did not influence the occurrence of tumours. It was
concluded that under the conditions of this assay, imazalil was
not a tumourigen or a carcinogen in mice (Marsboom and Herin,
1979b).
RESIDUES IN FOOD
USE PATTERN
Crop/ Formulation and mode Concentration
disease of application ai
Gherkin (outdoors) 20 EC, 0.025% 0.05 kg/ha
powdery mildew
Barley
Pyrenophora spp. liquid, 2.4% 48 mg/kg seed
Fusarium
Wheat, barley seed dressing
oats, rye 50 mg/kg
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of recent trials are summarised in Table 1.
TABLE 1. Residues resulting from supervised trials in the Netherlands
Crop Year of Application Residues in mg/kg, at intervals
trial (days) after (last) application
Number of Rate per Formulation
treatments treatment
kg ai/ha 3 4 ± 60
wheat 1976 1 0.125 liquid <0.011
wheat-straw 1976 1 0.125 200 g/l <0.11
gherkin 1977 1 0.1 liquid <0.011
(glasshouse) 200 g/l
" 1977 1 0.15 200 g/l 0.04
(0.03-0.05)
1 Limit of determination
FATE OF RESIDUES
Eleven growing banana plants with a minimum of six leaves were used in
the study. One of the banana plants (No. 1) was not treated and
served for providing blank samples. The others were sprayed uniformly
from above with a distilled water solution of 3N-imazalil-sulphate
labelled specially on the asymmetric carbon. The plants were treated
once, four, five or nine times consecutively at two-week intervals.
The sampling of the individual plants was carried out as follows:
One plant (No. 1) was sampled without any previous treatment, serving
as a blank. Plants Nos. 2, 3 and 4 were sampled two hours (the time
necessary for drying), four days and fourteen days respectively after
a single treatment, Plant No. 5 fourteen days after the fourth
treatment, plants Nos. 6, 7 and 8 two hours, four days and fourteen
days respectively after the fifth treatment and plants Nos. 9, 10 and
11 two hours, four days and fourteen days respectively after the ninth
treatment.
Five different parts of the plants were investigated: mature leaves,
new leaves emerged after the last treatment, psuedostem, rhizome and
roots (petioles were considered part; of the psuedostem).
The roots were out from the rhizome and the soil was washed off with
water.
The various parts of plants were minced and thereafter homogenised in
water (1/5, w/v).
The radioactivity levels of samples were measured in the aliquots of
the homogenates immediately after homogenisation.
The leaf homogenates of the treated plants and the spiked blank
homogenate were subjected to repeated extraction. The radioactivity
of the various extracts was measured. Some of the extracts were
analysed with on-line radio HPLC after sample clean up on Sep-pak
TMC18 cartridges. A part of leaf and a part of cigar leaf of plant
No. 11 were prepared for autoradiography.
The radioactivity levels in the various plant samples indicated fairly
uniform sprayings and the absence of tritiated water or other volatile
radioactive compounds. The leaves contained 95.2-100% of the total
radioactivity recovered in the plants treated one to nine times. The
radioactivity was practically all concentrated at the upper surface of
the leaf tested with autoradiography. Transport of the radioactivity
to the roots (0.32% maximally) or the rhizome (2.54% maximally) was
minimal. In the leaves emerged after the last treatment less than
0.03% of the radioactivity recovered in the whole plant was detected.
The metabolite pattern in the leaf homogenates was investigated after
extraction.
The scheme of extraction and the percentage ratio of the total
radioactivity found in various extracts are shown in Figure 1.
The differences between the various homogenates of the leaves of
plants that were sprayed once, four, five or nine times were only
minor. Attempts to extract the radioactivity from the final residues
by soxhlet with chloroform, ethyl acetate and acetone or by batch
extraction with 19% trichloroacetic acid failed.
Analyses of various plant extracts on radio-HPLC revealed that the
main part of radio-activity originated from imazalil and its
metabolite alpha-(2,4-dichloro-phenyl)-1H-imidazole-1-ethanol
(R14821). Unchanged imazalil constituted 33.8, 46.9, 37.1, and 22.3%
and R 14821 23.4, 22.2, 27.9 and 37.8% of the radioactivity in the
alkaline extracts of the leaves of plants Nos. 8, 9, 10 and 11
respectively. These results indicated that imazalil was degraded
slowly as a function of time and that R 14821 was the main degradation
product which was found in the pH11 extract too. Although the
radioactivity in the extracts of pH 11, pH 9, ph 5 and pH 2 amounted
to 15.38% of the alkaline extract, only a few small radioactivity
peaks could be detected, which may be formed at least partly
artificially from imazalil and R 14821 as similar peak patterns
appeared on the chromatograms of the extracts of blank homogenate
spiked with these compounds.
The remaining part of the radioactivity might be explained with the
presence of a large number of minor metabolites. R 14821 (45.4%) and
imazalil (20.7%) were also major radioactive compounds in the soxhlet
extract.
The parent compound accounted for altogether about 20 and 15% of the
total radioactivity while the proportion of R 14821 was 15% and 25% in
the leaves of plants Nos. 8 and 11 respectively.
A mixture of unidentified polar acid metabolites (7% of the total
radioactivity) was found in both the pH 2 extract and the final
aqueous layer. From 7-17% of the radioactivity in the leaf
homogenates could not be extracted.
The rest of the radioactivity, 35 to 45%, was due to a very large
number of minor metabolites (Meuldermans, W. et al, undated).
EVALUATION
COMMENTS AND APPRAISAL
Imazalil was reviewed by the 1977 Joint Meeting and a temporary ADI
was estimated to be 0-0.01 mg/kg bw/day. Concern was expressed over
the pathological changes observed in rat kidney and dog liver in
two-year studies. On the basis of a re-evaluation by an independent
pathologist the meeting concluded there was no difference between the
control and the treated animals.
 New data submitted to the meeting with respect to long-term
carcinogenesis studies in two species was found to be inadequate to
meet the current criteria for appropriate carcinogenicity studies.
The survival rate of rats for 24 months was poor, and a carcinogenic
evaluation could not be made with this species. In contrast to the
rat study however there was a significant number of survivors in the
mouse study.
The metabolic profile of imazalil especially with respect to salt
formulations appears to be well defined. Imazalil did not affect
reproduction in rats.
A temporary acceptable daily intake for man was reaffirmed on the
basis of studies in rat and dog. In several respects the long-term
studies reported to the meeting have been inadequate to evaluate fully
the toxicological profile. The Meeting felt that while no immediate
problems exist, a further adequately performed long-term study is
required. Additionally, short-term mutagenicity tests should be
performed.
Additional information has now been provided on the metabolic fate of
3H-labelled imazalil on banana plants grown in a greenhouse, and on
the results of supervised trials.
The major part of the radioactivity (95.2-100%) recovered in the
plants was present on the surface and in the upper layers of the
leaves. Translocation of the radioactivity from the sprayed areas to
the rest of the plant was minimal, since the rhizome, roots and new
leaves that emerged after the last treatment showed very little or no
radioactivity. The parent compound formed 15% of the total
radioactivity recovered in the leaves of plants treated nine times,
while the single major metabolite,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol accounted for 25%.
The rest of the radioactivity was due to a large number of
unidentified minor metabolites, each representing at most only a few
percent of the radioactivity. A considerable part of the unidentified
material might consist of degradation products formed during the
analytical procedure.
The results of the metabolism studies and the supervised trials are in
agreement with the findings discussed in the 1977 evaluation, and
support the recommendations made then.
Level causing no toxicological effect
Rat: 5 mg/kg bw/day
Dog: 1.24 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day
FURTHER WORK OR INFORMATION
Required (by 1984)
1. An adequate long-term study on rats to define fully a no-effect
level.
2. Short-term tests to evaluate the mutagenic potential.
REFERENCES
Heykants, J.J.P. On the metabolism of imazalil and related compounds
in animals and man. A review. (1978) Unpublished summary review of
published and unpublished metabolism data from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, H. Oral three-generation study in Wistar rats. (1978)
Unpublished report from Janssen Pharmaceutica submitted to the World
Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in Wistar rats.
(1979a) Unpublished report (No. 667) from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in albino Swiss
mice. (1979b) Unpublished report (No. 666) from Janssen Pharmaceutica,
submitted to the World Organization by Janssen Pharmaceutica.
Meuldermans, W., Swijsen, E., Hendricks, J., Wolstenborghs, R.,
Lauwers, W. and Heykants, J. (1979) Unpublished report from Janssen
Pharmaceutica submitted to the World Health Organization by Janssen
Pharmaceutica.
Meuldermans, W. et al. The metabolic fate of imazalil on banana
plants. Janssen Research Products Int. Service. R 23979/21.
Niemegears, C.J.E. Comparative acute oral toxicity studies of the
different salts of imazalil in rats. (1979) Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Janssen Pharmaceutica.
New data submitted to the meeting with respect to long-term
carcinogenesis studies in two species was found to be inadequate to
meet the current criteria for appropriate carcinogenicity studies.
The survival rate of rats for 24 months was poor, and a carcinogenic
evaluation could not be made with this species. In contrast to the
rat study however there was a significant number of survivors in the
mouse study.
The metabolic profile of imazalil especially with respect to salt
formulations appears to be well defined. Imazalil did not affect
reproduction in rats.
A temporary acceptable daily intake for man was reaffirmed on the
basis of studies in rat and dog. In several respects the long-term
studies reported to the meeting have been inadequate to evaluate fully
the toxicological profile. The Meeting felt that while no immediate
problems exist, a further adequately performed long-term study is
required. Additionally, short-term mutagenicity tests should be
performed.
Additional information has now been provided on the metabolic fate of
3H-labelled imazalil on banana plants grown in a greenhouse, and on
the results of supervised trials.
The major part of the radioactivity (95.2-100%) recovered in the
plants was present on the surface and in the upper layers of the
leaves. Translocation of the radioactivity from the sprayed areas to
the rest of the plant was minimal, since the rhizome, roots and new
leaves that emerged after the last treatment showed very little or no
radioactivity. The parent compound formed 15% of the total
radioactivity recovered in the leaves of plants treated nine times,
while the single major metabolite,
alpha-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol accounted for 25%.
The rest of the radioactivity was due to a large number of
unidentified minor metabolites, each representing at most only a few
percent of the radioactivity. A considerable part of the unidentified
material might consist of degradation products formed during the
analytical procedure.
The results of the metabolism studies and the supervised trials are in
agreement with the findings discussed in the 1977 evaluation, and
support the recommendations made then.
Level causing no toxicological effect
Rat: 5 mg/kg bw/day
Dog: 1.24 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day
FURTHER WORK OR INFORMATION
Required (by 1984)
1. An adequate long-term study on rats to define fully a no-effect
level.
2. Short-term tests to evaluate the mutagenic potential.
REFERENCES
Heykants, J.J.P. On the metabolism of imazalil and related compounds
in animals and man. A review. (1978) Unpublished summary review of
published and unpublished metabolism data from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, H. Oral three-generation study in Wistar rats. (1978)
Unpublished report from Janssen Pharmaceutica submitted to the World
Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in Wistar rats.
(1979a) Unpublished report (No. 667) from Janssen Pharmaceutica
submitted to the World Health Organization by Janssen Pharmaceutica.
Marsboom, R. and Herin, V. Oral carcinogenicity study in albino Swiss
mice. (1979b) Unpublished report (No. 666) from Janssen Pharmaceutica,
submitted to the World Organization by Janssen Pharmaceutica.
Meuldermans, W., Swijsen, E., Hendricks, J., Wolstenborghs, R.,
Lauwers, W. and Heykants, J. (1979) Unpublished report from Janssen
Pharmaceutica submitted to the World Health Organization by Janssen
Pharmaceutica.
Meuldermans, W. et al. The metabolic fate of imazalil on banana
plants. Janssen Research Products Int. Service. R 23979/21.
Niemegears, C.J.E. Comparative acute oral toxicity studies of the
different salts of imazalil in rats. (1979) Unpublished report from
Janssen Pharmaceutica, submitted to the World Health Organization by
Janssen Pharmaceutica.
