
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
MECARBAM
IDENTITY
Chemical name (IUPAC)
S-(N-ethoxycarbonyl-N-methylcarbamoylmethyl) O,O-diethyl
phosphorodithioate
Synonyms Murfotox(R), P474, MC474, Pestan(R), Afos.(R),
Murphotox(R), Murotox(R)
Structural formula
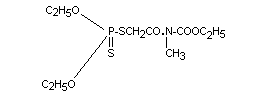 Molecular formula C10H20O5NPS2
Molecular weight 329.4
Other information on identity and properties
Mecarbam is a colourless oily liquid; the technical material may be
pale yellow to brown. Technical mecarbam contains >85%, typically
92-95%, of mecarbam with small amounts of triethyldithiophosphate
and S-(ethoxycarbonyl) diethyl dithiophosphate as impurities. Its
freezing point is 9°C, specific gravity is 1.222/20°C, refractive
index nD20 is 1.5138, volatility is 6 × 10-6 g/litre of air at
40°C. It is slightly soluble in water, soluble in hexane (4%) and
kerosene (2%) and miscible in all proportions with alcohols,
ketones, aromatic hydrocarbons and chlorinated hydrocarbons. It is
stable at normal temperatures but hydrolyses at pH below 3 to yield
(C2H5O)2PSSCH2COOH, CO2, C2H5OH and NH2CH3.
The most widely used formulation is the 68% emulsifiable
concentrate, but similar 50%, 90% and 100% formulations are
available and dust and wettable powder preparations have also been
marketed.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
Groups of rats were administered mecarbam (approximately 10 mg/kg
body weight 14C-ethoxy-labelled in dimethyl sulphoxide). Mecarbam
was rapidly absorbed following oral dosing and was rapidly
excreted, predominantly in the urine. Within 48 hours, 92% of the
radioactivity was excreted in urine, 2% in faeces and approximately
5% was eliminated as CO2. There was no accumulation of
radioactivity in any of the tissues examined. The highest
concentrations of mecarbam in this study were found in the major
metabolising and excretory tissues and organs (Aryton and Harnby,
1977a).
Further studies on the absorption and distribution of mecarbam,
using dimethylformamide as a vehicle, suggested that mecarbam,
while slowly absorbed from the GI tract following an acute oral
dose, is rapidly distributed through the body and excreted
primarily through the urine. Following an oral dose of 10 mg/kg
body weight, it was observed that the 14C-radioactivity did not
fully leave the stomach until 24-48 hours following administration
(McCulloch, 1980).
In a further experiment to demonstrate the rapid removal of
mecarbam following absorption an intravenous dose of mecarbam was
introduced into the tail vein of male rats. Blood samples were
taken in periods up to 6 hours following treatment. Residues
declined rapidly during the first 40 minutes and slower thereafter,
demonstrating a biphasic excretion curve. At the end of 6 hours,
radioactivity in the blood had diminished to a point where it
almost reached background levels (Ayrton and Harnby, 1977b).
Biotransformation
No data available.
TOXICOLOGICAL STUDIES
Special Studies on Potentiation
Mecarbam was orally administered to male rats alone and in
combination (1:1 w/w suspension in propylene glycol) with three
other organophosphate insecticides (malathion, demeton-methyl,
demeton-S-methyl sulphoxide and dimethoate). The relationship of
the observed LD50 with respect to that calculated from the acute
toxicity curves indicated that there was no acute potentiation or
additive effect of the presence of mecarbam with the other
cholinergic insecticides tested. Demeton-methyl and dimethoate,
under the conditions of this test, appear to be somewhat protective
with respect to the toxicity of mecarbam. In no case was
potentiation observed (Middleton, 1960).
Special studies on reproduction
Groups of rats (25 male and 25 female rats/group) were fed mecarbam
in their diet at dosage levels of 0, 2, or 50 mg/kg and subjected
to a two-generation, two-litter per generation reproduction test.
Technical mecarbam was used with an active ingredient of 83.2% and
the dietary levels were corrected to account for this
concentration. A modified teratology bioassay was incorporated
into this reproduction study where 5 females per group were set
aside and examined to determine day 0 of gestation. The animals
were fed mecarbam throughout gestation at the above dosage levels.
On day 19 or 20 of gestation some animals were sacrificed and the
foetuses delivered by caesarean section. This modified teratology
programme was performed with the second litter of each generation.
The remaining animals of the second litter of the second generation
were maintained for a three-month observation period to evaluate
post-natal effects. In the modified teratology examination soft
tissue and skeletal examinations were performed on all foetuses.
All reproduction data were subjected to statistical analysis.
The presence of mecarbam in the diet at 50 mg/kg resulted in a
depression of growth of both males and females in both parental
groups. Food consumption was not affected. In addition to growth
reduction, the animals receiving the high dose showed clinical
signs of toxicity with respect to an increased incidence of rough
coat and hunched appearance, signs which have been noted in other
dietary studies as being associated with mecarbam toxicity. There
was no compound-related mortality observed over the course of the
study attributable to the presence of mecarbam in the diet.
Examination of reproduction data failed to show any significant
effects with respect to both litters of both generations
administered 2 mg/kg in the diet. In contrast, the 50 mg/kg
dietary level affected a number of reproduction parameters
including: a depression of numbers of pups born alive; an increased
number of animals dying during lactation; a decreased number of
animals ultimately weaned; and the weight of the weaned animals was
significantly reduced. These effects were present in both litters
of both generations. The surviving animals, in addition to being
smaller, appeared unhealthy (were cold and somewhat cyanotic).
Evaluation of those foetuses removed by caesarean section for
potential teratogenic effects failed to show any compound-related
differences from controls. While there was a reduced number of
implantation sites, ovarian corpora lutea, and an increased number
of resorption sites at 50 mg/kg, there were no teratogenic
occurrences in any of the foetuses examined with respect to either
somatic or skeletal abnormalities.
The presence of mecarbam in the diet at 50 mg/kg had a significant
effect on both maternal and foetal development and well being but
was not teratogenic (Rutter, 1974).
Special studies on Neurotoxicity
Preliminary studies to define the delayed neurotoxic potential of
mecarbam were performed with adult hens. The hens were
administered mecarbam subcutaneously, one dose per week for three
consecutive weeks at dosage levels corresponding to multiples of
the acute LD50. The animals were not treated with atropine and
survival was low. The surviving animals were observed over a
period of time extending to nine months. A small sample of the
surviving hens were subjected to histological examinations of
nervous tissue while a larger sample was examined macroscopically
for gross alterations.
In this preliminary trial, there was no evidence of delayed
neurotoxicity induced by the multiple administration of mecarbam
and the examination, either by gross or histological techniques,
was negative (Anonymous, 1960).
Two formulations of mecarbam (Pestcombi(R) and Testan(R)) were
examined for their delayed neurotoxic potential with adult hens.
Pestcombi(R) contained mecarbam (22%) and EPN (30%) while Testan(R)
contained mecarbam alone (28%). Adult hens were administered these
materials orally as a single maximum tolerated dose and were
observed for clinical signs of delayed neurotoxicity for 21 days.
When no clinical signs of ataxia were observed, the animals were
further treated in a similar manner and again observed for an
additional 21 days. At the conclusion of the 42-day experimental
period, several of the survivors were sacrificed for microscopic
examination of central and peripheral nerve tissue and skeletal
muscle following H and E staining.
There was no clinical sign of ataxia or histological evidence that
mecarbam alone or in combination with EPN induced a clinical
delayed neurotoxicity in hens (Shillam, 1968).
Acute Toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat M Oral 23-53 Tomich, 1958a, 1959, 1965;
Davies and Collins, 1975
F Oral 31-33 Tomich, 1959, 1962; Davies and
Collins, 1975
M Dermal >1222 Middleton, 1960;
Inhalation (1-5 µm 0.7 mg/l Berczy and Binns, 1971
particle size) (6 hr LC50)
Mouse M IP 120-130 Tomich, 1958a; Anony, 1962
M SC 600 Tomich, 1958
M Oral 106 Child, 1958
M IV 67 Tomich, 1968b
Guinea Pig Oral 65 Tomich (no date)
Dermal >1222 Middleton, 1960
Rabbit Oral 60 Tomich (no date)
Dermal 229 Middleton, 1960
Cat Oral <50 Tomich (no date)
Sheep Oral 20-25 Nicolas Inst., 1966
Further acute studies in rats using formulated products, rather than
technical mecarbam, demonstrated a sex difference with respect to
acute toxicity (the females were more sensitive than the males).
Additionally, these studies showed that concentrated formulated
products appear to be more toxic than technical mecarbam probably due
to an increased absorption of the active ingredient as a result of the
presence of insecticidally-inert formulation products (Davies and
Halliday, 1971; Kynoch et al, 1980a; 1980b).
Signs of poisoning
The signs of acute poisoning resulting from mecarbam administration
are similar to those seen with other cholinergic organophosphate
esters. These signs include: salivation, lacrimation (sometimes
bloody), piloerection, hunched posture, abnormal gait, changes in the
respiratory rate, and fine and coarse body tremors. Death occurred in
poisoned rats from one hour to three days following dosing. Autopsy
showed congestion and haemorrhage of the lungs and pale color in the
liver, kidney, and spleen. Following subacute administration recovery
of survivors, as judged by external appearance and behaviour, was
slow. Recovery was complete within eight days of dosing.
Special studies on antidotes
As with other cholinergic organophosphates, 2-PAM was found to exert a
protective effect on acute cholinergic signs of poisoning (Middleton,
1960). Atropine was not studied with respect to its antidotal effects
on the acute toxicity of mecarbam.
Short-term studies
Rat inhalation
Groups of rats (4 male and 4 female/group) were exposed to mecarbam by
inhalation. Exposures were carried out for 6 hours/day for a total of
14 exposure days over a 3-week experiment time. The aerosol
particulate was fully respirable and generated at concentrations of 0,
2, 10 and 50 mg/l air.
The animals were observed daily and growth was recorded at twice
weekly intervals. At the conclusion of the study urinalysis,
haematology, and clinical blood chemistry parameters were evaluated.
All rats were sacrificed, and gross and microscopic examination of
tissues and organs was performed.
Although respiratory irritation was observed in the two higher dose
levels, the exposure to mecarbam did not appear to result in adverse
behaviourial effects and growth was not affected. At the high dose
level, tremors were noted, and the animals became aggressive in the
second week of exposure. Urinalysis, haematology, and clinical
chemistry parameters were unaffected by exposure to mecarbam. At the
highest dose level, erythrocyte (but not plasma) cholinesterase
activity was depressed in male rats. Females did not show a
significant cholinesterase depression at any dose level although at 50
mg/l, the activity was slightly depressed.
Gross and microscopic examination of the lungs and major tissues and
organs showed no changes in morphology that would be attributable to
the presence of mecarbam. Based upon the conditions of this
experiment, the rat was shown to tolerate a concentration of 10 mg/l
air for a daily 6-hour exposure over a three-week test interval
(Berczy et al, 1972).
Cow
Groups of lactating dairy cows (4 cows/group) were administered
mecarbam in their diet at dosage levels of 0, 2, and 10 mg/kg for 30
days. The study was designed to evaluate the presence of residues in
milk and meat and to examine excretion and body burdens of mecarbam in
this species.
There was no mortality or signs of poisoning evident over the course
of the study. There were no changes in body weight or in milk
production. Although mecarbam was present in low levels in the milk
of cows administered mecarbam at both dietary levels, there was no
build up or evidence of bioaccumulation. The levels of mecarbam in
milk did not exceed 10 mg/kg. At the conclusion of the study, animals
were sacrificed at various intervals up to 1 week and 1 month
following cessation of treatment. There was no substantial evidence
of mecarbam in tissues and organs in any of the animals receiving
dietary administration. From this study, it may be concluded that
mecarbam, at levels of 10 mg/kg in the diet, does not elicit a toxic
reaction in cows, is not bioaccumulated, nor is it found as a residue
in meat, although low levels may be excreted in milk (Hepworth and
Brown, 1978).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
mecarbam in the diet at dosage levels of 0, 10, 20 or 50 mg/kg for 6
months. The respective dietary intake of mecarbam corresponding to
the food intake data was 0, 0.35, 0.80 or 1.78 mg/kg body weight.
The animals were observed daily for abnormal signs of behaviour and
toxicity. Data on food consumption and growth were recorded weekly.
At periodic intervals during the course of the study, the control and
high-dose group animals were examined with respect to haematological
and blood biochemistry parameters. Urinalyses were performed at the
same time intervals (with the exception of the last time interval, at
the conclusion of the study). Cholinesterase determinations were
performed by the electrometric measurement method examining red blood
cell and plasma enzyme activity. These analyses were performed
periodically during the course of the study. At the conclusion of the
study, the animals were sacrificed and, in addition to RBC and plasma,
brain cholinesterase activity was measured.
At the completion of the study, all animals were sacrificed and a
gross examination was made of selected tissues and organs.
Microscopic examinations were performed on tissues and organs of the
control and 50 mg/kg dose group.
There was no mortality over the 6-month trial. Food consumption and
growth were normal at all dose levels. Urinalyses and blood chemistry
values were within normal limits in all dose groups. Although several
haematological parameters did show some significant differences for
control values periodically during the course of the study, there was
no dose relationship nor time relationship noted with these findings.
RBC and plasma cholinesterase activity depression was evident in both
males and females in the high dose group. Plasma cholinesterase
appeared to be the more sensitive parameter and was reduced in both
males and females at 20 mg/kg. At this dose level, only females
showed a reduced red blood cell cholinesterase activity (noted only at
the conclusion of the study). In general, females appear to show a
slightly greater level of cholinesterase depression than males. Brain
cholinesterase, evaluated at the conclusion of the study, was
depressed significantly at 50 mg/kg in both males and females and at
20 mg/kg in males only. There was no depression of cholinesterase
activity in either sex at 10 mg/kg.
Gross and microscopic examination of tissues and organs at the
conclusion of the study did not show gross or microscopic
abnormalities attributable to the presence of mecarbam. It was
concluded that there were no pathological conditions induced over the
course of this study (Ward et al, 1979).
Groups of dogs (4 male and 4 female beagle dogs/group) were
administered mecarbam in the diet at dosage levels of 0, 1, 5 or 50
mg/kg for 2 years. Mecarbam was introduced into the dry diet, which
was prepared weekly.
All animals were examined daily for changes in behaviour and toxic
signs of poisoning. Growth and food consumption data were recorded
thereafter. At 0, 26, 68 and 104 weeks, haematology, clinical
chemistry, and urinalysis examinations were performed on all animals.
At 26 weeks, one male and one female of each group was sacrificed and
subjected to gross and microscopic examination of tissues and organs.
At the conclusion of the study, all animals were sacrificed and a
similar evaluation was made. Additionally, at the conclusion of the
study, brain cholinesterase analyses were performed on all animals.
There was no mortality over the course of the study. Growth and food
consumption data were comparable to control values at all time
intervals examined. Urinalysis, haematological and clinical
biochemistry data, with the exception of cholinesterase values, showed
no abnormalities attributable to the presence of mecarbam. Inhibition
of plasma and erythrocyte cholinesterase was noted at the highest dose
level in all animals at all intervals examined. Occasional slight
reduction of cholinesterase activity at 5 mg/kg was observed
periodically in the experiment. At the conclusion of the study, brain
cholinesterase depression was evident only at 50 mg/kg.
Gross and microscopic examination of a wide variety of tissues and
organs of animals sacrificed at the conclusion and during the course
of the study failed to detect any compound related tissue damage in
the series of tissues examined. Occasional spontaneous lesions and
incidental findings were not attributable to the presence of mecarbam
in the diet. A no-effect level, based upon cholinesterase depression
(which appears to be the most sensitive parameter) is 5 mg/kg in the
diet equivalent to a daily dietary intake of 0.15 mg/kg body weight
(based upon actual food consumption in both males and females) (Rutter
and Voelker, 1975).
Rat
Groups of rats (15 female rats per group) were administered mecarbam
in the diet at dosage levels of 0, 1, 5, 10 or 50 g/kg for six months.
Animals were examined daily and growth and food consumption data were
recorded weekly. Ophthalmological examination of animals in the
control and 50 mg/kg groups were made at periodic intervals.
Urinalyses, haematology, and blood chemistry parameters were recorded
at periodic intervals during the course of the 26-week experiment.
Plasma, red blood cell, and brain cholinesterase activity were
measured using the electrometric procedure.
At the conclusion of the 26-week feeding trial, all animals were
sacrificed and gross examinations of tissues and organs was performed
on all animals. Microscopic tissue examinations were performed on all
animals from the control and high dose group.
There was no mortality observed over the course of the study.
Clinical signs of poisoning commonly seen as muscular tremors and a
general unkempt appearance were observed at 50 mg/kg. Data on growth
and food consumption did not show a significant effect of mecarbam at
any dose level over the course of the study. Urinalysis, blood
chemistry, and biochemistry parameters (with the exception of
cholinesterase activity) were affected by mecarbam. Cholinesterase
depression was generally more pronounced in red blood cells than in
plasma. Enzyme depression was obvious at the two higher dose levels.
At the conclusion of the study, brain cholinesterase was substantially
depressed at two highest dose levels.
There were no adverse effects noted on ophthalmological examination.
Gross and microscopic examination of tissues and organs at the
conclusion of the study failed to show any adverse effects that could
be attributable to the presence of mecarbam in the diet (Rivett et
al, 1972).
Long-term study
Rat
Groups of rats (25 male and 25 female Sprague-Dawley rats/group) were
fed mecarbam in the diet at dosage levels of O, 1, 5, or 50 mg/kg for
104 weeks. (Based on an active ingredient of 83%, this corresponds to
dietary levels of 0, 0.83, 4.15, and 41.5 mg/kg). The animals were
observed daily for mortality and changes in behaviour. Growth and
food consumption were reported weekly for 10 weeks, biweekly for 15
weeks, and monthly thereafter to the termination of the study. Groups
of 5 rats per sex were sacrificed periodically for clinical chemistry,
haematology, and urinalysis. At 26 weeks and at the termination of
the experiment, animals were sacrificed and gross and microscopic
examination of tissues and organs were performed. Microscopic
examinations of the control and the high dose group animals were
performed in detail at the conclusion of the study. Animals of the
low and mid-dose groups were also examined microscopically with
respect to kidney and liver tissue. In all cases, unusual lesions and
tissue masses were examined microscopically, when observed.
During the first 10 weeks of the study, there was no mortality and no
unusual effects noted with respect to behaviour. A hunched appearance
was observed with increasing frequency at week 10, more frequently in
the treated animals of the high-dose group than in the control or
lower-dosed animals. At the conclusion of the study, all surviving
males and most of the surviving females had the hunched appearance.
Over the course of the study, mortality was observed in the treated
animals more frequently than in the control group although the
differences were not significant statistically (the number of animals
used in the study precludes an adequate evaluation of the increased
mortality induced by mecarbam). Growth was affected predominantly in
the males at 50 mg/kg. There were no effects noted with respect to
food consumption, clinical chemistry studies (with the exception of
cholinesterase), haematology, or urinalysis parameters. A
dose-related reduction of cholinesterase was observed. This reduction
was significant at 50 mg/kg in both males and females. At 104 weeks,
cholinesterase activity was substantially depressed in the high-dose
group in both males and females.
Gross and microscopic examination of tissues and organs at the 26-week
and 104-week intervals did not show compound-related changes at any
dietary dose level. There was a high incidence of spontaneous lesions
and neoplasms which were similar in both treated and control groups.
There was no indication that mecarbam induced a carcinogenic change in
any of the tissues examined. Respiratory disease was present in most
animals and was believed responsible for the reduction of surviving
animals at the conclusion of the study.
Based on cholinesterase depression, a no-effect level of 5 mg/kg was
recognised as being equivalent to a dosage level of 0.21 mg/kg body
weight (Rutter and Banas, 1975).
Observations in Man
None
RESIDUES IN FOOD
USE PATTERN
Mecarbam is an insecticide effective against plant sucking insects
such as aphids, leafhoppers, mealybugs, scale insects, lace bugs,
thrips and stem and fruit mining pests. It is also an effective
acaricide in those few areas where resistance to organophosphorus
compounds is absent, although resistant whitefly can be controlled by
mecarbam.
The principal use of mecarbam is for control of scale insects and
mealybugs in citrus crops, with lesser usage against the same pests on
vines, olives and deciduous fruits. Applications on citrus fruits are
usually made between petal fall and harvest during the period of
movement of the larvae after release from the parent. At this time
its control of other pests, such as aphids and whitefly, can also be
useful. Applications can also be made in mixtures with summer or
winter oils.
It acts as a contact and stomach insecticide. It is absorbed through
the leaf surface, but is only slowly translocated in the plant sap
from leaf application. It is systemic by root absorption and treated
plants remain toxic to insects for about 12 days. The formulations
are compatible with most commonly-used spray chemicals. Transient
phytotoxic effects have been observed on young, soft plants in
greenhouses; more mature plants have not usually shown these symptoms
but a wettable powder formulation has been tested for greenhouse use.
Normal rates of use range from 0.03 to 0.075% ai for high volume
spraying; low volume treatment requires appropriately higher
concentrations. Table 1 lists the crops treated and the pests
controlled.
TABLE 1. Crops treated and pests controlled.
Crop Pest
Apples aphids
codling moth
lace bug
scale insects
Brassicas cabbage root fly
Carrots carrot fly
Cashew whitefly
Cherries aphids
lace bugs
fruit fly
Citrus scale insects
mealybugs
mites
whitefly
Olives olive fly
scale insects
Peaches aphids
scale insects
mites
Pears aphids
leaf hoppers
lace bugs
pear sucker
mites
Pistachio nuts mites
scale insects
Plums aphids
leaf hoppers
mites
Rice leaf hoppers
plant hoppers
leaf miners
rice stem maggot
Vines vine moth
mites
mealybugs
Glasshouse crops whitefly
Open blossoms should not be sprayed and edible crops should not be
harvested within 14 days from spray treatment. Table 2 sets out
information on recommended rates of use and pre-harvest intervals for
various crops in seven countries. Use on olives is discouraged other
than very early in the season when the oil content is minimal. Use on
vegetable crops is very limited.
TABLE 2. Recommended rates of use and pre-harvest intervals
% a.i. Maximum Pre-harvest
Country Formulation in spray Crop No. of Interval
Treatments
Egypt 68% e.c. 0.1 citrus - -
68% e.c. 0.1 fruit trees - -
Cyprus 68% e.c. 0.034-0.1 citrus - 14
68% e.c. 0.34-0.085 apples, almonds, repeat at -
peaches, pears, 10-15 days
cherries
Greece 68% e.c. 0.051-0.085 citrus - 14
68% e.c. 0.051-0.085 fruit trees 14 -
12% in 0.06 fruit trees - -
oil.
Iran 68% e.c. 0.051-0.085 citrus - 14
Japan 25% e.c. 0.025-0.043 citrus 4 30
Morocco 50% e.c. 0.05-0.06 citrus - -
50% e.c. 0.0375-0.085 peaches, plums, - -
apples, pears,
vines
S. Africa 90% e.c. 0.0495 citrus 2 90
90% e.c. 0.054 apples, pears 4 14
plums, peaches,
apricots.
FATE OF RESIDUES
In plants
The degradation of mecarbam applied to dwarf bean plants has been
examined (Murphy Chem. Ltd., 1977) following different methods of
mecarbam application, root uptake and foliar absorption. The results
indicated that mecarbam is slowly translocated from the foliage to the
roots but is rapidly metabolised in both places. At least four
metabolites were indicated (three identified) resulting from either
oxidative or hydrolytic attack. Levels of mecarbam and its
metabolites declined from 10.4 to 0.05 mg/kg in 26 days after foliar
application; all levels observed in the roots were low, the highest
being 0.5 mg/kg at 5 days. Root uptake studies showed rapid
degradation of mecarbam and only low levels were found in the foliage.
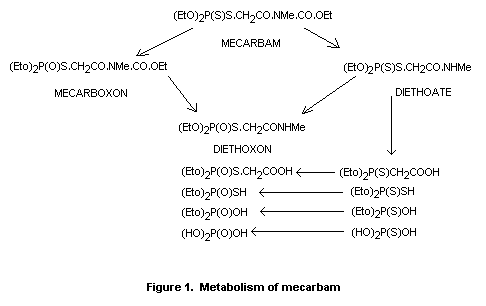
Figure 1 illustrates the metabolic formation of mecarboxon, diethoate
and diethoxon from mecarbam.
Molecular formula C10H20O5NPS2
Molecular weight 329.4
Other information on identity and properties
Mecarbam is a colourless oily liquid; the technical material may be
pale yellow to brown. Technical mecarbam contains >85%, typically
92-95%, of mecarbam with small amounts of triethyldithiophosphate
and S-(ethoxycarbonyl) diethyl dithiophosphate as impurities. Its
freezing point is 9°C, specific gravity is 1.222/20°C, refractive
index nD20 is 1.5138, volatility is 6 × 10-6 g/litre of air at
40°C. It is slightly soluble in water, soluble in hexane (4%) and
kerosene (2%) and miscible in all proportions with alcohols,
ketones, aromatic hydrocarbons and chlorinated hydrocarbons. It is
stable at normal temperatures but hydrolyses at pH below 3 to yield
(C2H5O)2PSSCH2COOH, CO2, C2H5OH and NH2CH3.
The most widely used formulation is the 68% emulsifiable
concentrate, but similar 50%, 90% and 100% formulations are
available and dust and wettable powder preparations have also been
marketed.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
Groups of rats were administered mecarbam (approximately 10 mg/kg
body weight 14C-ethoxy-labelled in dimethyl sulphoxide). Mecarbam
was rapidly absorbed following oral dosing and was rapidly
excreted, predominantly in the urine. Within 48 hours, 92% of the
radioactivity was excreted in urine, 2% in faeces and approximately
5% was eliminated as CO2. There was no accumulation of
radioactivity in any of the tissues examined. The highest
concentrations of mecarbam in this study were found in the major
metabolising and excretory tissues and organs (Aryton and Harnby,
1977a).
Further studies on the absorption and distribution of mecarbam,
using dimethylformamide as a vehicle, suggested that mecarbam,
while slowly absorbed from the GI tract following an acute oral
dose, is rapidly distributed through the body and excreted
primarily through the urine. Following an oral dose of 10 mg/kg
body weight, it was observed that the 14C-radioactivity did not
fully leave the stomach until 24-48 hours following administration
(McCulloch, 1980).
In a further experiment to demonstrate the rapid removal of
mecarbam following absorption an intravenous dose of mecarbam was
introduced into the tail vein of male rats. Blood samples were
taken in periods up to 6 hours following treatment. Residues
declined rapidly during the first 40 minutes and slower thereafter,
demonstrating a biphasic excretion curve. At the end of 6 hours,
radioactivity in the blood had diminished to a point where it
almost reached background levels (Ayrton and Harnby, 1977b).
Biotransformation
No data available.
TOXICOLOGICAL STUDIES
Special Studies on Potentiation
Mecarbam was orally administered to male rats alone and in
combination (1:1 w/w suspension in propylene glycol) with three
other organophosphate insecticides (malathion, demeton-methyl,
demeton-S-methyl sulphoxide and dimethoate). The relationship of
the observed LD50 with respect to that calculated from the acute
toxicity curves indicated that there was no acute potentiation or
additive effect of the presence of mecarbam with the other
cholinergic insecticides tested. Demeton-methyl and dimethoate,
under the conditions of this test, appear to be somewhat protective
with respect to the toxicity of mecarbam. In no case was
potentiation observed (Middleton, 1960).
Special studies on reproduction
Groups of rats (25 male and 25 female rats/group) were fed mecarbam
in their diet at dosage levels of 0, 2, or 50 mg/kg and subjected
to a two-generation, two-litter per generation reproduction test.
Technical mecarbam was used with an active ingredient of 83.2% and
the dietary levels were corrected to account for this
concentration. A modified teratology bioassay was incorporated
into this reproduction study where 5 females per group were set
aside and examined to determine day 0 of gestation. The animals
were fed mecarbam throughout gestation at the above dosage levels.
On day 19 or 20 of gestation some animals were sacrificed and the
foetuses delivered by caesarean section. This modified teratology
programme was performed with the second litter of each generation.
The remaining animals of the second litter of the second generation
were maintained for a three-month observation period to evaluate
post-natal effects. In the modified teratology examination soft
tissue and skeletal examinations were performed on all foetuses.
All reproduction data were subjected to statistical analysis.
The presence of mecarbam in the diet at 50 mg/kg resulted in a
depression of growth of both males and females in both parental
groups. Food consumption was not affected. In addition to growth
reduction, the animals receiving the high dose showed clinical
signs of toxicity with respect to an increased incidence of rough
coat and hunched appearance, signs which have been noted in other
dietary studies as being associated with mecarbam toxicity. There
was no compound-related mortality observed over the course of the
study attributable to the presence of mecarbam in the diet.
Examination of reproduction data failed to show any significant
effects with respect to both litters of both generations
administered 2 mg/kg in the diet. In contrast, the 50 mg/kg
dietary level affected a number of reproduction parameters
including: a depression of numbers of pups born alive; an increased
number of animals dying during lactation; a decreased number of
animals ultimately weaned; and the weight of the weaned animals was
significantly reduced. These effects were present in both litters
of both generations. The surviving animals, in addition to being
smaller, appeared unhealthy (were cold and somewhat cyanotic).
Evaluation of those foetuses removed by caesarean section for
potential teratogenic effects failed to show any compound-related
differences from controls. While there was a reduced number of
implantation sites, ovarian corpora lutea, and an increased number
of resorption sites at 50 mg/kg, there were no teratogenic
occurrences in any of the foetuses examined with respect to either
somatic or skeletal abnormalities.
The presence of mecarbam in the diet at 50 mg/kg had a significant
effect on both maternal and foetal development and well being but
was not teratogenic (Rutter, 1974).
Special studies on Neurotoxicity
Preliminary studies to define the delayed neurotoxic potential of
mecarbam were performed with adult hens. The hens were
administered mecarbam subcutaneously, one dose per week for three
consecutive weeks at dosage levels corresponding to multiples of
the acute LD50. The animals were not treated with atropine and
survival was low. The surviving animals were observed over a
period of time extending to nine months. A small sample of the
surviving hens were subjected to histological examinations of
nervous tissue while a larger sample was examined macroscopically
for gross alterations.
In this preliminary trial, there was no evidence of delayed
neurotoxicity induced by the multiple administration of mecarbam
and the examination, either by gross or histological techniques,
was negative (Anonymous, 1960).
Two formulations of mecarbam (Pestcombi(R) and Testan(R)) were
examined for their delayed neurotoxic potential with adult hens.
Pestcombi(R) contained mecarbam (22%) and EPN (30%) while Testan(R)
contained mecarbam alone (28%). Adult hens were administered these
materials orally as a single maximum tolerated dose and were
observed for clinical signs of delayed neurotoxicity for 21 days.
When no clinical signs of ataxia were observed, the animals were
further treated in a similar manner and again observed for an
additional 21 days. At the conclusion of the 42-day experimental
period, several of the survivors were sacrificed for microscopic
examination of central and peripheral nerve tissue and skeletal
muscle following H and E staining.
There was no clinical sign of ataxia or histological evidence that
mecarbam alone or in combination with EPN induced a clinical
delayed neurotoxicity in hens (Shillam, 1968).
Acute Toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat M Oral 23-53 Tomich, 1958a, 1959, 1965;
Davies and Collins, 1975
F Oral 31-33 Tomich, 1959, 1962; Davies and
Collins, 1975
M Dermal >1222 Middleton, 1960;
Inhalation (1-5 µm 0.7 mg/l Berczy and Binns, 1971
particle size) (6 hr LC50)
Mouse M IP 120-130 Tomich, 1958a; Anony, 1962
M SC 600 Tomich, 1958
M Oral 106 Child, 1958
M IV 67 Tomich, 1968b
Guinea Pig Oral 65 Tomich (no date)
Dermal >1222 Middleton, 1960
Rabbit Oral 60 Tomich (no date)
Dermal 229 Middleton, 1960
Cat Oral <50 Tomich (no date)
Sheep Oral 20-25 Nicolas Inst., 1966
Further acute studies in rats using formulated products, rather than
technical mecarbam, demonstrated a sex difference with respect to
acute toxicity (the females were more sensitive than the males).
Additionally, these studies showed that concentrated formulated
products appear to be more toxic than technical mecarbam probably due
to an increased absorption of the active ingredient as a result of the
presence of insecticidally-inert formulation products (Davies and
Halliday, 1971; Kynoch et al, 1980a; 1980b).
Signs of poisoning
The signs of acute poisoning resulting from mecarbam administration
are similar to those seen with other cholinergic organophosphate
esters. These signs include: salivation, lacrimation (sometimes
bloody), piloerection, hunched posture, abnormal gait, changes in the
respiratory rate, and fine and coarse body tremors. Death occurred in
poisoned rats from one hour to three days following dosing. Autopsy
showed congestion and haemorrhage of the lungs and pale color in the
liver, kidney, and spleen. Following subacute administration recovery
of survivors, as judged by external appearance and behaviour, was
slow. Recovery was complete within eight days of dosing.
Special studies on antidotes
As with other cholinergic organophosphates, 2-PAM was found to exert a
protective effect on acute cholinergic signs of poisoning (Middleton,
1960). Atropine was not studied with respect to its antidotal effects
on the acute toxicity of mecarbam.
Short-term studies
Rat inhalation
Groups of rats (4 male and 4 female/group) were exposed to mecarbam by
inhalation. Exposures were carried out for 6 hours/day for a total of
14 exposure days over a 3-week experiment time. The aerosol
particulate was fully respirable and generated at concentrations of 0,
2, 10 and 50 mg/l air.
The animals were observed daily and growth was recorded at twice
weekly intervals. At the conclusion of the study urinalysis,
haematology, and clinical blood chemistry parameters were evaluated.
All rats were sacrificed, and gross and microscopic examination of
tissues and organs was performed.
Although respiratory irritation was observed in the two higher dose
levels, the exposure to mecarbam did not appear to result in adverse
behaviourial effects and growth was not affected. At the high dose
level, tremors were noted, and the animals became aggressive in the
second week of exposure. Urinalysis, haematology, and clinical
chemistry parameters were unaffected by exposure to mecarbam. At the
highest dose level, erythrocyte (but not plasma) cholinesterase
activity was depressed in male rats. Females did not show a
significant cholinesterase depression at any dose level although at 50
mg/l, the activity was slightly depressed.
Gross and microscopic examination of the lungs and major tissues and
organs showed no changes in morphology that would be attributable to
the presence of mecarbam. Based upon the conditions of this
experiment, the rat was shown to tolerate a concentration of 10 mg/l
air for a daily 6-hour exposure over a three-week test interval
(Berczy et al, 1972).
Cow
Groups of lactating dairy cows (4 cows/group) were administered
mecarbam in their diet at dosage levels of 0, 2, and 10 mg/kg for 30
days. The study was designed to evaluate the presence of residues in
milk and meat and to examine excretion and body burdens of mecarbam in
this species.
There was no mortality or signs of poisoning evident over the course
of the study. There were no changes in body weight or in milk
production. Although mecarbam was present in low levels in the milk
of cows administered mecarbam at both dietary levels, there was no
build up or evidence of bioaccumulation. The levels of mecarbam in
milk did not exceed 10 mg/kg. At the conclusion of the study, animals
were sacrificed at various intervals up to 1 week and 1 month
following cessation of treatment. There was no substantial evidence
of mecarbam in tissues and organs in any of the animals receiving
dietary administration. From this study, it may be concluded that
mecarbam, at levels of 10 mg/kg in the diet, does not elicit a toxic
reaction in cows, is not bioaccumulated, nor is it found as a residue
in meat, although low levels may be excreted in milk (Hepworth and
Brown, 1978).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
mecarbam in the diet at dosage levels of 0, 10, 20 or 50 mg/kg for 6
months. The respective dietary intake of mecarbam corresponding to
the food intake data was 0, 0.35, 0.80 or 1.78 mg/kg body weight.
The animals were observed daily for abnormal signs of behaviour and
toxicity. Data on food consumption and growth were recorded weekly.
At periodic intervals during the course of the study, the control and
high-dose group animals were examined with respect to haematological
and blood biochemistry parameters. Urinalyses were performed at the
same time intervals (with the exception of the last time interval, at
the conclusion of the study). Cholinesterase determinations were
performed by the electrometric measurement method examining red blood
cell and plasma enzyme activity. These analyses were performed
periodically during the course of the study. At the conclusion of the
study, the animals were sacrificed and, in addition to RBC and plasma,
brain cholinesterase activity was measured.
At the completion of the study, all animals were sacrificed and a
gross examination was made of selected tissues and organs.
Microscopic examinations were performed on tissues and organs of the
control and 50 mg/kg dose group.
There was no mortality over the 6-month trial. Food consumption and
growth were normal at all dose levels. Urinalyses and blood chemistry
values were within normal limits in all dose groups. Although several
haematological parameters did show some significant differences for
control values periodically during the course of the study, there was
no dose relationship nor time relationship noted with these findings.
RBC and plasma cholinesterase activity depression was evident in both
males and females in the high dose group. Plasma cholinesterase
appeared to be the more sensitive parameter and was reduced in both
males and females at 20 mg/kg. At this dose level, only females
showed a reduced red blood cell cholinesterase activity (noted only at
the conclusion of the study). In general, females appear to show a
slightly greater level of cholinesterase depression than males. Brain
cholinesterase, evaluated at the conclusion of the study, was
depressed significantly at 50 mg/kg in both males and females and at
20 mg/kg in males only. There was no depression of cholinesterase
activity in either sex at 10 mg/kg.
Gross and microscopic examination of tissues and organs at the
conclusion of the study did not show gross or microscopic
abnormalities attributable to the presence of mecarbam. It was
concluded that there were no pathological conditions induced over the
course of this study (Ward et al, 1979).
Groups of dogs (4 male and 4 female beagle dogs/group) were
administered mecarbam in the diet at dosage levels of 0, 1, 5 or 50
mg/kg for 2 years. Mecarbam was introduced into the dry diet, which
was prepared weekly.
All animals were examined daily for changes in behaviour and toxic
signs of poisoning. Growth and food consumption data were recorded
thereafter. At 0, 26, 68 and 104 weeks, haematology, clinical
chemistry, and urinalysis examinations were performed on all animals.
At 26 weeks, one male and one female of each group was sacrificed and
subjected to gross and microscopic examination of tissues and organs.
At the conclusion of the study, all animals were sacrificed and a
similar evaluation was made. Additionally, at the conclusion of the
study, brain cholinesterase analyses were performed on all animals.
There was no mortality over the course of the study. Growth and food
consumption data were comparable to control values at all time
intervals examined. Urinalysis, haematological and clinical
biochemistry data, with the exception of cholinesterase values, showed
no abnormalities attributable to the presence of mecarbam. Inhibition
of plasma and erythrocyte cholinesterase was noted at the highest dose
level in all animals at all intervals examined. Occasional slight
reduction of cholinesterase activity at 5 mg/kg was observed
periodically in the experiment. At the conclusion of the study, brain
cholinesterase depression was evident only at 50 mg/kg.
Gross and microscopic examination of a wide variety of tissues and
organs of animals sacrificed at the conclusion and during the course
of the study failed to detect any compound related tissue damage in
the series of tissues examined. Occasional spontaneous lesions and
incidental findings were not attributable to the presence of mecarbam
in the diet. A no-effect level, based upon cholinesterase depression
(which appears to be the most sensitive parameter) is 5 mg/kg in the
diet equivalent to a daily dietary intake of 0.15 mg/kg body weight
(based upon actual food consumption in both males and females) (Rutter
and Voelker, 1975).
Rat
Groups of rats (15 female rats per group) were administered mecarbam
in the diet at dosage levels of 0, 1, 5, 10 or 50 g/kg for six months.
Animals were examined daily and growth and food consumption data were
recorded weekly. Ophthalmological examination of animals in the
control and 50 mg/kg groups were made at periodic intervals.
Urinalyses, haematology, and blood chemistry parameters were recorded
at periodic intervals during the course of the 26-week experiment.
Plasma, red blood cell, and brain cholinesterase activity were
measured using the electrometric procedure.
At the conclusion of the 26-week feeding trial, all animals were
sacrificed and gross examinations of tissues and organs was performed
on all animals. Microscopic tissue examinations were performed on all
animals from the control and high dose group.
There was no mortality observed over the course of the study.
Clinical signs of poisoning commonly seen as muscular tremors and a
general unkempt appearance were observed at 50 mg/kg. Data on growth
and food consumption did not show a significant effect of mecarbam at
any dose level over the course of the study. Urinalysis, blood
chemistry, and biochemistry parameters (with the exception of
cholinesterase activity) were affected by mecarbam. Cholinesterase
depression was generally more pronounced in red blood cells than in
plasma. Enzyme depression was obvious at the two higher dose levels.
At the conclusion of the study, brain cholinesterase was substantially
depressed at two highest dose levels.
There were no adverse effects noted on ophthalmological examination.
Gross and microscopic examination of tissues and organs at the
conclusion of the study failed to show any adverse effects that could
be attributable to the presence of mecarbam in the diet (Rivett et
al, 1972).
Long-term study
Rat
Groups of rats (25 male and 25 female Sprague-Dawley rats/group) were
fed mecarbam in the diet at dosage levels of O, 1, 5, or 50 mg/kg for
104 weeks. (Based on an active ingredient of 83%, this corresponds to
dietary levels of 0, 0.83, 4.15, and 41.5 mg/kg). The animals were
observed daily for mortality and changes in behaviour. Growth and
food consumption were reported weekly for 10 weeks, biweekly for 15
weeks, and monthly thereafter to the termination of the study. Groups
of 5 rats per sex were sacrificed periodically for clinical chemistry,
haematology, and urinalysis. At 26 weeks and at the termination of
the experiment, animals were sacrificed and gross and microscopic
examination of tissues and organs were performed. Microscopic
examinations of the control and the high dose group animals were
performed in detail at the conclusion of the study. Animals of the
low and mid-dose groups were also examined microscopically with
respect to kidney and liver tissue. In all cases, unusual lesions and
tissue masses were examined microscopically, when observed.
During the first 10 weeks of the study, there was no mortality and no
unusual effects noted with respect to behaviour. A hunched appearance
was observed with increasing frequency at week 10, more frequently in
the treated animals of the high-dose group than in the control or
lower-dosed animals. At the conclusion of the study, all surviving
males and most of the surviving females had the hunched appearance.
Over the course of the study, mortality was observed in the treated
animals more frequently than in the control group although the
differences were not significant statistically (the number of animals
used in the study precludes an adequate evaluation of the increased
mortality induced by mecarbam). Growth was affected predominantly in
the males at 50 mg/kg. There were no effects noted with respect to
food consumption, clinical chemistry studies (with the exception of
cholinesterase), haematology, or urinalysis parameters. A
dose-related reduction of cholinesterase was observed. This reduction
was significant at 50 mg/kg in both males and females. At 104 weeks,
cholinesterase activity was substantially depressed in the high-dose
group in both males and females.
Gross and microscopic examination of tissues and organs at the 26-week
and 104-week intervals did not show compound-related changes at any
dietary dose level. There was a high incidence of spontaneous lesions
and neoplasms which were similar in both treated and control groups.
There was no indication that mecarbam induced a carcinogenic change in
any of the tissues examined. Respiratory disease was present in most
animals and was believed responsible for the reduction of surviving
animals at the conclusion of the study.
Based on cholinesterase depression, a no-effect level of 5 mg/kg was
recognised as being equivalent to a dosage level of 0.21 mg/kg body
weight (Rutter and Banas, 1975).
Observations in Man
None
RESIDUES IN FOOD
USE PATTERN
Mecarbam is an insecticide effective against plant sucking insects
such as aphids, leafhoppers, mealybugs, scale insects, lace bugs,
thrips and stem and fruit mining pests. It is also an effective
acaricide in those few areas where resistance to organophosphorus
compounds is absent, although resistant whitefly can be controlled by
mecarbam.
The principal use of mecarbam is for control of scale insects and
mealybugs in citrus crops, with lesser usage against the same pests on
vines, olives and deciduous fruits. Applications on citrus fruits are
usually made between petal fall and harvest during the period of
movement of the larvae after release from the parent. At this time
its control of other pests, such as aphids and whitefly, can also be
useful. Applications can also be made in mixtures with summer or
winter oils.
It acts as a contact and stomach insecticide. It is absorbed through
the leaf surface, but is only slowly translocated in the plant sap
from leaf application. It is systemic by root absorption and treated
plants remain toxic to insects for about 12 days. The formulations
are compatible with most commonly-used spray chemicals. Transient
phytotoxic effects have been observed on young, soft plants in
greenhouses; more mature plants have not usually shown these symptoms
but a wettable powder formulation has been tested for greenhouse use.
Normal rates of use range from 0.03 to 0.075% ai for high volume
spraying; low volume treatment requires appropriately higher
concentrations. Table 1 lists the crops treated and the pests
controlled.
TABLE 1. Crops treated and pests controlled.
Crop Pest
Apples aphids
codling moth
lace bug
scale insects
Brassicas cabbage root fly
Carrots carrot fly
Cashew whitefly
Cherries aphids
lace bugs
fruit fly
Citrus scale insects
mealybugs
mites
whitefly
Olives olive fly
scale insects
Peaches aphids
scale insects
mites
Pears aphids
leaf hoppers
lace bugs
pear sucker
mites
Pistachio nuts mites
scale insects
Plums aphids
leaf hoppers
mites
Rice leaf hoppers
plant hoppers
leaf miners
rice stem maggot
Vines vine moth
mites
mealybugs
Glasshouse crops whitefly
Open blossoms should not be sprayed and edible crops should not be
harvested within 14 days from spray treatment. Table 2 sets out
information on recommended rates of use and pre-harvest intervals for
various crops in seven countries. Use on olives is discouraged other
than very early in the season when the oil content is minimal. Use on
vegetable crops is very limited.
TABLE 2. Recommended rates of use and pre-harvest intervals
% a.i. Maximum Pre-harvest
Country Formulation in spray Crop No. of Interval
Treatments
Egypt 68% e.c. 0.1 citrus - -
68% e.c. 0.1 fruit trees - -
Cyprus 68% e.c. 0.034-0.1 citrus - 14
68% e.c. 0.34-0.085 apples, almonds, repeat at -
peaches, pears, 10-15 days
cherries
Greece 68% e.c. 0.051-0.085 citrus - 14
68% e.c. 0.051-0.085 fruit trees 14 -
12% in 0.06 fruit trees - -
oil.
Iran 68% e.c. 0.051-0.085 citrus - 14
Japan 25% e.c. 0.025-0.043 citrus 4 30
Morocco 50% e.c. 0.05-0.06 citrus - -
50% e.c. 0.0375-0.085 peaches, plums, - -
apples, pears,
vines
S. Africa 90% e.c. 0.0495 citrus 2 90
90% e.c. 0.054 apples, pears 4 14
plums, peaches,
apricots.
FATE OF RESIDUES
In plants
The degradation of mecarbam applied to dwarf bean plants has been
examined (Murphy Chem. Ltd., 1977) following different methods of
mecarbam application, root uptake and foliar absorption. The results
indicated that mecarbam is slowly translocated from the foliage to the
roots but is rapidly metabolised in both places. At least four
metabolites were indicated (three identified) resulting from either
oxidative or hydrolytic attack. Levels of mecarbam and its
metabolites declined from 10.4 to 0.05 mg/kg in 26 days after foliar
application; all levels observed in the roots were low, the highest
being 0.5 mg/kg at 5 days. Root uptake studies showed rapid
degradation of mecarbam and only low levels were found in the foliage.
Figure 1 illustrates the metabolic formation of mecarboxon, diethoate
and diethoxon from mecarbam.
 In soils
Mecarbam added to loam and to sandy soil was studied under sterile and
nonsterile conditions (Murphy Chem. Ltd., 1977). Analysis showed that
the pesticide has a short life, levels dropping from the applied 7.5
mg/kg to less than 0.4 mg/kg in 50 days in loam soil and to O.1 mg/kg
in 34 days in sandy soil. Degradation was shown to be mainly by
chemical rather than by microbial action since similar degradation was
obtained with soil that had been sterilised by autoclaving.
In cooking
The principal use of mecarbam is on citrus crops, and most residue
resides in the peel or rind, not in the flesh of the fruit. Since the
peel is consumed in marmalade, the fate of residues on processing
oranges into this preserve has been studied (Murphy Chem. Ltd., 1970).
The yield of home-made marmalade is about 2-3 times the weight of
fruit used. Batches of marmalade were prepared using a normal
domestic recipe, except that the peel was finely shredded at -30°C to
minimise possible losses of mecarbam. Oranges containing
field-incurred residues and untreated oranges to which known amounts
of mecarbam had been added were used in these tests. The
field-treated fruit had residues of 2.4 mg/kg (10.7 mg/kg in the peel)
and the added mecarbam was at a level of 2.7 mg/kg immediately before
cooking. Levels of mecarbam in the marmalade ranged from 0.14 to 0.22
mg/kg, giving an average loss on cooking of over 80%. The maximum
level of mecarbam to be expected in marmalade prepared from treated
fruit is of the order of 0.05 to 0.1 mg/kg.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
Data was available from supervised trials of mecarbam on various types
of oranges grown in Japan, South Africa, Egypt, Israel and Morocco
(Murphy Chem. Ltd., 1980). In all of the studies only negligible
amounts of mecarbam (less than 0.03 mg/kg) were found in the flesh of
the fruit. Residues on the peel, however, ranged up to about 10 mg/kg
at the recommended 14 and 30 day pre-harvest intervals and up to 5
mg/kg at about 90 days. Residues of mecarbam in the peel of
grapefruit were about 0.5 mg/kg at 34 days after treatment, with not
more than 0.01 mg/kg in the flesh of the fruit.
The principal metabolites were also determined on treated oranges
resulting from three trials carried out in Egypt; the results are
given in Table 3 (Murphy Chem. Ltd., 1980). At 14 days after
treatment, the parent mecarbam remains the predominant residue in the
peel, the three metabolites together comprising only 5 to 10% of the
total residue.
TABLE 3. Mecarbam and metabolite residues in oranges grown in Egypt,
1975 (mg/kg)
No. of days Peel Flesh
after
application Mecarbam Mecarboxon Diethoate Diethoxon Mecarbam
0 5.95 0.08 0.20 1.39 0.10
7 7.0 0.17 0.22 0.31 0.05
14 6.95 0.28 0.17 0.25 0.02
28 5.45 0.22 0.13 0.11 0.01
Untreated <0.05 - - - <0.002
0 18.3 0.028 0.032 0.054 0.12
7 14.0 0.68 0.036 0.050 0.05
14 5.35 0.20 0.04 0.004 0.03
Untreated <0.05 - - - <0.002
0 14.4 0.103 0.024 0.30 0.06
In all three trials mecarbam was applied at a final spray concentration
of 0.1% ai.
Other crops
A limited amount of data was presented on mecarbam residues in olives,
grapes, pears and various vegetable crops. The analytical results
were not easy to interpret but it appears that treated olives
containing up to 1.5 mg/kg can yield olive oil containing up to 8.5
mg/kg of mecarbam. Residues in grapes and pears at 14 days or more
after application were generally less than 0.3 mg/kg; no evidence of
occurrence of metabolites was available (Murphy Chem. Ltd., 1980).
The data on residues on cabbage, cauliflowers, broccoli, Brussels
sprouts, celery, carrots, onions, turnips, sugarbeet and lucerne had
mostly been obtained during the 1960s when analytical procedures were
less specific or accurate than current methods. Results reported were
very inconsistent and in many instances the control values were equal
to or greater than the observed levels in treated crops, typically
about 0.3 mg/kg on leafy vegetables.
The data on these vegetable crops were not adequate to support
recommendations for maximum residue levels.
METHODS OF RESIDUE ANALYSIS
Mecarbam is extracted from crops with hexane, cleaned up by solvent
partition and/or column chromatography and determined by GLC with
either electron capture or phosphorus specific detection (Lynch,
1976). The procedure should be suitable for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
Available information on national MRLs is given in Table 4.
TABLE 4. National MRLs for mecarbam reported to the meeting
Country Commodity MRL (mg/kg)
Belgium Fruit, vegetables (not potatoes) 1
Netherlands Fruit, vegetables (not potatoes) 1
South Africa Apples, apricots, peaches, pears,
plums 0.05
Citrus 0.05
EVALUATION
COMMENTS AND APPRAISAL
Mecarbam is an organophosphorus insecticide effective against plant
sucking pests such as aphids, scale insects, etc., and also acts as an
acaricide. Its principal use is for the control of scale insects and
mealybugs on citrus fruits, with lesser uses against the same range of
insects on vines, olives and deciduous fruits; it has also been used
on vegetables.
Mecarbam is rapidly absorbed following acute oral ingestion, with
rapid distribution and elimination from the body. Mecarbam does not
bioaccumulate in the body on repeated administration. The metabolism
has not been elucidated.
Mecarbam elicits parasympathomimetic signs of poisoning following high
doses. Antidotal studies with mecarbam are deficient, although it is
presumed that atropine may be effective.
Mecarbam appears not to induce a delayed neurotoxic response in hens
although studies were not fully acceptable. Mecarbam does not affect
reproduction in rats at levels below those at which dams might be
affected. Limited information derived from a teratology bioassay
combined with the reproduction study did not suggest mecarbam to be
teratogenic. A more complete teratogenicity bioassay, specifically to
evaluate this effect, is required. There are no data available to
assess the mutagenic potential of mecarbam. There are no specific
studies to assess the carcinogenic potential although long-term
studies in rats alleviate concerns in this direction. Adequate
short-term dietary studies in dogs and long-term dietary studies in
rats demonstrate that the principal effects attributable to mecarbam
in the diet are reflective of cholinesterase depression. In both
species a no-effect level has been noted.
On the basis of these levels a temporary acceptable daily intake was
allocated and research suggested to complete the data base.
Mecarbam is systemic by root absorption and absorbed through the leaf
surface. The three main metabolites are mecarboxon, diethoate and
diethoxon, but these form only a small part of the total residue,
which consists mainly of the parent mecarbam and remains in the peel
of citrus fruits. Preparation of marmalade from treated fruit results
in residue losses of about 80%. Available residue data were adequate
for oranges but were scanty and difficult to interpret for other
fruits, olives or vegetables. A gas chromatographic method of
analysis is available which can be adapted for regulatory purposes.
Level causing no toxicological effect
Rat: 5 mg/kg in the diet equivalent to 0.21 mg/kg bw/day
Dog: 5 mg/kg in the diet equivalent to 0.15 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day
RECOMMENDATIONS OF RESIDUES LIMITS
From the data available the meeting was able to estimate a maximum
residue level for oranges only.
The meeting concluded that the level is suitable for establishing a
temporary maximum residue limit. The level applies to the parent
compound, excluding any metabolites.
Commodity Estimated maximum Treatment-harvest interval
residue level (mg/kg) on which level is based
oranges 2 14
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Complete metabolic studies in laboratory animals.
2. A complete teratogenicity bioassay.
3. Studies to define the mutagenic potential.
4. Studies in hens or other appropriate species to define the
potential for delayed neurotoxicity.
5. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
6. Data on the use of mecarbam on vegetables and deciduous fruits
together with residue data from supervised trials.
7. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
8. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
9. Data an use of mecarbam on vegetables and deciduous fruits,
together with residue data from supervised trials.
10. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
Desirable
1. Studies on antidotes.
2. Epidemiological data on potential for adverse effects resulting
from occupational exposure.
REFERENCES
Anonymous. Neurotoxicity to hens. (1960) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Anonymous. Oral LD50 in mice. Murphy Compound P. 474/17. (1962)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Aryton J. and Harnby G. Preliminary metabolic studies on Mecarbam
(S(N-ethoxycarbonyl-N-methylcarbamoyl-methyl)diethyl
phosphorothiolothioate). (1977a) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical Ltd. to the World
Health Organization.
Aryton J. and Harnby, G. Blood levels of 14C following an intravenous
administration of 14C Mecarbam to rats. (1977b) Unpublished report
from Glaxo Laboratories,Ltd., submitted by Murphy Chemical, Ltd. to
the World Health Organization.
Berczy, Z.S. and Binns, R. Acute inhalation toxicity to the rat of
Mecarbam. (1971) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield
R.A.S. (1972) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Child K.J. Acute oral toxicity of P. 474/1 to male mice. (1958)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Davies, R.E. and Collins, C.J. Acute oral toxicity to rats of Mecarbam
(Murfotox). (1975) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Davies, R.D. and Halliday, J.C. Acute oral toxicity to rats of
Murfotox liquid (64% ai). (1971) Unpublished report from Huntingdon
Research Centre submitted by Murphy Chemical Ltd. to The World Health
Organization.
Hepworth, P.L. and Brown, P.M. Mecarbam: Milk and tissue residues
following repeated dietary administration to dairy cows over thirty
days with a maximum period of thirty days respite from treatments.
(1978) Unpublished report from Life Sciences Research (to Takeda
Chemical Industries, Ltd.), submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 50% EC. (1980a) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical Ltd. to The World Health
Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 25% wp. (1980b) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Lynch, V.P. Mecarbam; in Volume 8 of Analytical Methods for Pesticides
and Plant Growth Regulators, Ed G. Zweig and G. Sherma, Academic
Press, N.Y. p. 135-140.
McCulloch, R.J. The distribution of 14C Mecarbam in rats.
Autoradiographic studies (Report no. 77261). (1980) Unpublished report
from Glaxo Research, Ltd. submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Middleton, T.R. Potentiation of Mecarbam by other insecticides.
(1960a) Unpublished report from Glaxo Laboratories, Ltd, submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Middleton, T.R. Percutaneous toxicity of Mecarbam to rats. (1960b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Murphy Chem. Ltd. Fate of mecarbam residues in oranges when converted
to marmalade. Murphy Chem. Ltd., unpublished report, 27 November 1970.
Murphy Chem. Ltd. Mecarbam 2M; metabolism studies in plants and soil.
Murphy Chem. Ltd., unpublished report No. 77/32. (1977).
Murphy Chem. Ltd. Mecarbam residues data. Volume 3, Murphy Chem. Ltd.,
unpublished report. (1980)
Nicholas Institute. Mecarbam toxicity to sheep. (1966) Unpublished
report from the Nicholas Institute, submitted by Murphy Chemical, Ltd.
to The World Health Organization.
Rivett, K.F., Batham, P., Street, A.E., Haywood, R. and Newman, A.J.
(1972) Unpublished report from the Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Rutter, H.A., Jr. Mecarbam. Two-generation reproductive study in rats.
(1974) Unpublished report from Hazelton Laboratories American, Ltd.
(to Takeda Chemical Industries, Ltd.), submitted by Murphy Chemical,
Ltd. to The World Health Organization.
Rutter, H.A., Jr. and Banas, D.A. 104-Week dietary administration in
rats - Mecarbam - final report. (1975) Unpublished report from
Hazelton Laboratories American, Ltd. (to Takeda Chemical Industries,
Ltd.), submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Rutter, H.A., Jr. and Voelker, R.W. Two-year dietary toxicity study in
dogs. Mecarbam. Final Report. (1975) Unpublished report from Hazelton
Laboratories American, Ltd. (to Takeda Chemical Industries Ltd.),
submitted to The World Health Organization.
Robinson, D. and Shillam, K.W.G. Neurotoxicity of Pestoombi(R) and
Pestan(R) in the domestic hen. (1968) Unpublished reports from
Huntingdon Research Centre, submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Tomich, E.G. Acute oral toxicities of P.474 to guinea pigs, rabbits
and cats. Unpublished undated report from Glaxo Laboratories, Ltd.,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute intraperitoneal and subcutaneous toxicities to male
mice and acute oral toxicity to male rats. (1958a) Unpublished report
from Glaxo Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to
The World Health Organization.
Tomich E.G. P.474/5 acute intravenous toxicity (male mice). (1958b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute oral toxicity to female rats. P.474/5 and P.474/7.
(1959) Unpublished report from Glaxo Laboratories, Ltd., submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound P. 414/17. (1962)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound Mecarbam batch 416
(lab stripped). (1965) Unpublished report from Glaxo Laboratories,
Ltd., submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Ward, R.J., Kellett, B.S., Hadlow, C.J. and Lancaster, M.G. Mecarbam.
Oral toxicity to dogs (dietary administration for 6 months). (1979)
Unpublished report of studies performed at Manston Research Center,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
In soils
Mecarbam added to loam and to sandy soil was studied under sterile and
nonsterile conditions (Murphy Chem. Ltd., 1977). Analysis showed that
the pesticide has a short life, levels dropping from the applied 7.5
mg/kg to less than 0.4 mg/kg in 50 days in loam soil and to O.1 mg/kg
in 34 days in sandy soil. Degradation was shown to be mainly by
chemical rather than by microbial action since similar degradation was
obtained with soil that had been sterilised by autoclaving.
In cooking
The principal use of mecarbam is on citrus crops, and most residue
resides in the peel or rind, not in the flesh of the fruit. Since the
peel is consumed in marmalade, the fate of residues on processing
oranges into this preserve has been studied (Murphy Chem. Ltd., 1970).
The yield of home-made marmalade is about 2-3 times the weight of
fruit used. Batches of marmalade were prepared using a normal
domestic recipe, except that the peel was finely shredded at -30°C to
minimise possible losses of mecarbam. Oranges containing
field-incurred residues and untreated oranges to which known amounts
of mecarbam had been added were used in these tests. The
field-treated fruit had residues of 2.4 mg/kg (10.7 mg/kg in the peel)
and the added mecarbam was at a level of 2.7 mg/kg immediately before
cooking. Levels of mecarbam in the marmalade ranged from 0.14 to 0.22
mg/kg, giving an average loss on cooking of over 80%. The maximum
level of mecarbam to be expected in marmalade prepared from treated
fruit is of the order of 0.05 to 0.1 mg/kg.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
Data was available from supervised trials of mecarbam on various types
of oranges grown in Japan, South Africa, Egypt, Israel and Morocco
(Murphy Chem. Ltd., 1980). In all of the studies only negligible
amounts of mecarbam (less than 0.03 mg/kg) were found in the flesh of
the fruit. Residues on the peel, however, ranged up to about 10 mg/kg
at the recommended 14 and 30 day pre-harvest intervals and up to 5
mg/kg at about 90 days. Residues of mecarbam in the peel of
grapefruit were about 0.5 mg/kg at 34 days after treatment, with not
more than 0.01 mg/kg in the flesh of the fruit.
The principal metabolites were also determined on treated oranges
resulting from three trials carried out in Egypt; the results are
given in Table 3 (Murphy Chem. Ltd., 1980). At 14 days after
treatment, the parent mecarbam remains the predominant residue in the
peel, the three metabolites together comprising only 5 to 10% of the
total residue.
TABLE 3. Mecarbam and metabolite residues in oranges grown in Egypt,
1975 (mg/kg)
No. of days Peel Flesh
after
application Mecarbam Mecarboxon Diethoate Diethoxon Mecarbam
0 5.95 0.08 0.20 1.39 0.10
7 7.0 0.17 0.22 0.31 0.05
14 6.95 0.28 0.17 0.25 0.02
28 5.45 0.22 0.13 0.11 0.01
Untreated <0.05 - - - <0.002
0 18.3 0.028 0.032 0.054 0.12
7 14.0 0.68 0.036 0.050 0.05
14 5.35 0.20 0.04 0.004 0.03
Untreated <0.05 - - - <0.002
0 14.4 0.103 0.024 0.30 0.06
In all three trials mecarbam was applied at a final spray concentration
of 0.1% ai.
Other crops
A limited amount of data was presented on mecarbam residues in olives,
grapes, pears and various vegetable crops. The analytical results
were not easy to interpret but it appears that treated olives
containing up to 1.5 mg/kg can yield olive oil containing up to 8.5
mg/kg of mecarbam. Residues in grapes and pears at 14 days or more
after application were generally less than 0.3 mg/kg; no evidence of
occurrence of metabolites was available (Murphy Chem. Ltd., 1980).
The data on residues on cabbage, cauliflowers, broccoli, Brussels
sprouts, celery, carrots, onions, turnips, sugarbeet and lucerne had
mostly been obtained during the 1960s when analytical procedures were
less specific or accurate than current methods. Results reported were
very inconsistent and in many instances the control values were equal
to or greater than the observed levels in treated crops, typically
about 0.3 mg/kg on leafy vegetables.
The data on these vegetable crops were not adequate to support
recommendations for maximum residue levels.
METHODS OF RESIDUE ANALYSIS
Mecarbam is extracted from crops with hexane, cleaned up by solvent
partition and/or column chromatography and determined by GLC with
either electron capture or phosphorus specific detection (Lynch,
1976). The procedure should be suitable for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
Available information on national MRLs is given in Table 4.
TABLE 4. National MRLs for mecarbam reported to the meeting
Country Commodity MRL (mg/kg)
Belgium Fruit, vegetables (not potatoes) 1
Netherlands Fruit, vegetables (not potatoes) 1
South Africa Apples, apricots, peaches, pears,
plums 0.05
Citrus 0.05
EVALUATION
COMMENTS AND APPRAISAL
Mecarbam is an organophosphorus insecticide effective against plant
sucking pests such as aphids, scale insects, etc., and also acts as an
acaricide. Its principal use is for the control of scale insects and
mealybugs on citrus fruits, with lesser uses against the same range of
insects on vines, olives and deciduous fruits; it has also been used
on vegetables.
Mecarbam is rapidly absorbed following acute oral ingestion, with
rapid distribution and elimination from the body. Mecarbam does not
bioaccumulate in the body on repeated administration. The metabolism
has not been elucidated.
Mecarbam elicits parasympathomimetic signs of poisoning following high
doses. Antidotal studies with mecarbam are deficient, although it is
presumed that atropine may be effective.
Mecarbam appears not to induce a delayed neurotoxic response in hens
although studies were not fully acceptable. Mecarbam does not affect
reproduction in rats at levels below those at which dams might be
affected. Limited information derived from a teratology bioassay
combined with the reproduction study did not suggest mecarbam to be
teratogenic. A more complete teratogenicity bioassay, specifically to
evaluate this effect, is required. There are no data available to
assess the mutagenic potential of mecarbam. There are no specific
studies to assess the carcinogenic potential although long-term
studies in rats alleviate concerns in this direction. Adequate
short-term dietary studies in dogs and long-term dietary studies in
rats demonstrate that the principal effects attributable to mecarbam
in the diet are reflective of cholinesterase depression. In both
species a no-effect level has been noted.
On the basis of these levels a temporary acceptable daily intake was
allocated and research suggested to complete the data base.
Mecarbam is systemic by root absorption and absorbed through the leaf
surface. The three main metabolites are mecarboxon, diethoate and
diethoxon, but these form only a small part of the total residue,
which consists mainly of the parent mecarbam and remains in the peel
of citrus fruits. Preparation of marmalade from treated fruit results
in residue losses of about 80%. Available residue data were adequate
for oranges but were scanty and difficult to interpret for other
fruits, olives or vegetables. A gas chromatographic method of
analysis is available which can be adapted for regulatory purposes.
Level causing no toxicological effect
Rat: 5 mg/kg in the diet equivalent to 0.21 mg/kg bw/day
Dog: 5 mg/kg in the diet equivalent to 0.15 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day
RECOMMENDATIONS OF RESIDUES LIMITS
From the data available the meeting was able to estimate a maximum
residue level for oranges only.
The meeting concluded that the level is suitable for establishing a
temporary maximum residue limit. The level applies to the parent
compound, excluding any metabolites.
Commodity Estimated maximum Treatment-harvest interval
residue level (mg/kg) on which level is based
oranges 2 14
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Complete metabolic studies in laboratory animals.
2. A complete teratogenicity bioassay.
3. Studies to define the mutagenic potential.
4. Studies in hens or other appropriate species to define the
potential for delayed neurotoxicity.
5. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
6. Data on the use of mecarbam on vegetables and deciduous fruits
together with residue data from supervised trials.
7. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
8. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
9. Data an use of mecarbam on vegetables and deciduous fruits,
together with residue data from supervised trials.
10. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
Desirable
1. Studies on antidotes.
2. Epidemiological data on potential for adverse effects resulting
from occupational exposure.
REFERENCES
Anonymous. Neurotoxicity to hens. (1960) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Anonymous. Oral LD50 in mice. Murphy Compound P. 474/17. (1962)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Aryton J. and Harnby G. Preliminary metabolic studies on Mecarbam
(S(N-ethoxycarbonyl-N-methylcarbamoyl-methyl)diethyl
phosphorothiolothioate). (1977a) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical Ltd. to the World
Health Organization.
Aryton J. and Harnby, G. Blood levels of 14C following an intravenous
administration of 14C Mecarbam to rats. (1977b) Unpublished report
from Glaxo Laboratories,Ltd., submitted by Murphy Chemical, Ltd. to
the World Health Organization.
Berczy, Z.S. and Binns, R. Acute inhalation toxicity to the rat of
Mecarbam. (1971) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield
R.A.S. (1972) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Child K.J. Acute oral toxicity of P. 474/1 to male mice. (1958)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Davies, R.E. and Collins, C.J. Acute oral toxicity to rats of Mecarbam
(Murfotox). (1975) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Davies, R.D. and Halliday, J.C. Acute oral toxicity to rats of
Murfotox liquid (64% ai). (1971) Unpublished report from Huntingdon
Research Centre submitted by Murphy Chemical Ltd. to The World Health
Organization.
Hepworth, P.L. and Brown, P.M. Mecarbam: Milk and tissue residues
following repeated dietary administration to dairy cows over thirty
days with a maximum period of thirty days respite from treatments.
(1978) Unpublished report from Life Sciences Research (to Takeda
Chemical Industries, Ltd.), submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 50% EC. (1980a) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical Ltd. to The World Health
Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 25% wp. (1980b) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Lynch, V.P. Mecarbam; in Volume 8 of Analytical Methods for Pesticides
and Plant Growth Regulators, Ed G. Zweig and G. Sherma, Academic
Press, N.Y. p. 135-140.
McCulloch, R.J. The distribution of 14C Mecarbam in rats.
Autoradiographic studies (Report no. 77261). (1980) Unpublished report
from Glaxo Research, Ltd. submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Middleton, T.R. Potentiation of Mecarbam by other insecticides.
(1960a) Unpublished report from Glaxo Laboratories, Ltd, submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Middleton, T.R. Percutaneous toxicity of Mecarbam to rats. (1960b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Murphy Chem. Ltd. Fate of mecarbam residues in oranges when converted
to marmalade. Murphy Chem. Ltd., unpublished report, 27 November 1970.
Murphy Chem. Ltd. Mecarbam 2M; metabolism studies in plants and soil.
Murphy Chem. Ltd., unpublished report No. 77/32. (1977).
Murphy Chem. Ltd. Mecarbam residues data. Volume 3, Murphy Chem. Ltd.,
unpublished report. (1980)
Nicholas Institute. Mecarbam toxicity to sheep. (1966) Unpublished
report from the Nicholas Institute, submitted by Murphy Chemical, Ltd.
to The World Health Organization.
Rivett, K.F., Batham, P., Street, A.E., Haywood, R. and Newman, A.J.
(1972) Unpublished report from the Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Rutter, H.A., Jr. Mecarbam. Two-generation reproductive study in rats.
(1974) Unpublished report from Hazelton Laboratories American, Ltd.
(to Takeda Chemical Industries, Ltd.), submitted by Murphy Chemical,
Ltd. to The World Health Organization.
Rutter, H.A., Jr. and Banas, D.A. 104-Week dietary administration in
rats - Mecarbam - final report. (1975) Unpublished report from
Hazelton Laboratories American, Ltd. (to Takeda Chemical Industries,
Ltd.), submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Rutter, H.A., Jr. and Voelker, R.W. Two-year dietary toxicity study in
dogs. Mecarbam. Final Report. (1975) Unpublished report from Hazelton
Laboratories American, Ltd. (to Takeda Chemical Industries Ltd.),
submitted to The World Health Organization.
Robinson, D. and Shillam, K.W.G. Neurotoxicity of Pestoombi(R) and
Pestan(R) in the domestic hen. (1968) Unpublished reports from
Huntingdon Research Centre, submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Tomich, E.G. Acute oral toxicities of P.474 to guinea pigs, rabbits
and cats. Unpublished undated report from Glaxo Laboratories, Ltd.,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute intraperitoneal and subcutaneous toxicities to male
mice and acute oral toxicity to male rats. (1958a) Unpublished report
from Glaxo Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to
The World Health Organization.
Tomich E.G. P.474/5 acute intravenous toxicity (male mice). (1958b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute oral toxicity to female rats. P.474/5 and P.474/7.
(1959) Unpublished report from Glaxo Laboratories, Ltd., submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound P. 414/17. (1962)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound Mecarbam batch 416
(lab stripped). (1965) Unpublished report from Glaxo Laboratories,
Ltd., submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Ward, R.J., Kellett, B.S., Hadlow, C.J. and Lancaster, M.G. Mecarbam.
Oral toxicity to dogs (dietary administration for 6 months). (1979)
Unpublished report of studies performed at Manston Research Center,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
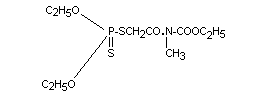 Molecular formula C10H20O5NPS2
Molecular weight 329.4
Other information on identity and properties
Mecarbam is a colourless oily liquid; the technical material may be
pale yellow to brown. Technical mecarbam contains >85%, typically
92-95%, of mecarbam with small amounts of triethyldithiophosphate
and S-(ethoxycarbonyl) diethyl dithiophosphate as impurities. Its
freezing point is 9°C, specific gravity is 1.222/20°C, refractive
index nD20 is 1.5138, volatility is 6 × 10-6 g/litre of air at
40°C. It is slightly soluble in water, soluble in hexane (4%) and
kerosene (2%) and miscible in all proportions with alcohols,
ketones, aromatic hydrocarbons and chlorinated hydrocarbons. It is
stable at normal temperatures but hydrolyses at pH below 3 to yield
(C2H5O)2PSSCH2COOH, CO2, C2H5OH and NH2CH3.
The most widely used formulation is the 68% emulsifiable
concentrate, but similar 50%, 90% and 100% formulations are
available and dust and wettable powder preparations have also been
marketed.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
Groups of rats were administered mecarbam (approximately 10 mg/kg
body weight 14C-ethoxy-labelled in dimethyl sulphoxide). Mecarbam
was rapidly absorbed following oral dosing and was rapidly
excreted, predominantly in the urine. Within 48 hours, 92% of the
radioactivity was excreted in urine, 2% in faeces and approximately
5% was eliminated as CO2. There was no accumulation of
radioactivity in any of the tissues examined. The highest
concentrations of mecarbam in this study were found in the major
metabolising and excretory tissues and organs (Aryton and Harnby,
1977a).
Further studies on the absorption and distribution of mecarbam,
using dimethylformamide as a vehicle, suggested that mecarbam,
while slowly absorbed from the GI tract following an acute oral
dose, is rapidly distributed through the body and excreted
primarily through the urine. Following an oral dose of 10 mg/kg
body weight, it was observed that the 14C-radioactivity did not
fully leave the stomach until 24-48 hours following administration
(McCulloch, 1980).
In a further experiment to demonstrate the rapid removal of
mecarbam following absorption an intravenous dose of mecarbam was
introduced into the tail vein of male rats. Blood samples were
taken in periods up to 6 hours following treatment. Residues
declined rapidly during the first 40 minutes and slower thereafter,
demonstrating a biphasic excretion curve. At the end of 6 hours,
radioactivity in the blood had diminished to a point where it
almost reached background levels (Ayrton and Harnby, 1977b).
Biotransformation
No data available.
TOXICOLOGICAL STUDIES
Special Studies on Potentiation
Mecarbam was orally administered to male rats alone and in
combination (1:1 w/w suspension in propylene glycol) with three
other organophosphate insecticides (malathion, demeton-methyl,
demeton-S-methyl sulphoxide and dimethoate). The relationship of
the observed LD50 with respect to that calculated from the acute
toxicity curves indicated that there was no acute potentiation or
additive effect of the presence of mecarbam with the other
cholinergic insecticides tested. Demeton-methyl and dimethoate,
under the conditions of this test, appear to be somewhat protective
with respect to the toxicity of mecarbam. In no case was
potentiation observed (Middleton, 1960).
Special studies on reproduction
Groups of rats (25 male and 25 female rats/group) were fed mecarbam
in their diet at dosage levels of 0, 2, or 50 mg/kg and subjected
to a two-generation, two-litter per generation reproduction test.
Technical mecarbam was used with an active ingredient of 83.2% and
the dietary levels were corrected to account for this
concentration. A modified teratology bioassay was incorporated
into this reproduction study where 5 females per group were set
aside and examined to determine day 0 of gestation. The animals
were fed mecarbam throughout gestation at the above dosage levels.
On day 19 or 20 of gestation some animals were sacrificed and the
foetuses delivered by caesarean section. This modified teratology
programme was performed with the second litter of each generation.
The remaining animals of the second litter of the second generation
were maintained for a three-month observation period to evaluate
post-natal effects. In the modified teratology examination soft
tissue and skeletal examinations were performed on all foetuses.
All reproduction data were subjected to statistical analysis.
The presence of mecarbam in the diet at 50 mg/kg resulted in a
depression of growth of both males and females in both parental
groups. Food consumption was not affected. In addition to growth
reduction, the animals receiving the high dose showed clinical
signs of toxicity with respect to an increased incidence of rough
coat and hunched appearance, signs which have been noted in other
dietary studies as being associated with mecarbam toxicity. There
was no compound-related mortality observed over the course of the
study attributable to the presence of mecarbam in the diet.
Examination of reproduction data failed to show any significant
effects with respect to both litters of both generations
administered 2 mg/kg in the diet. In contrast, the 50 mg/kg
dietary level affected a number of reproduction parameters
including: a depression of numbers of pups born alive; an increased
number of animals dying during lactation; a decreased number of
animals ultimately weaned; and the weight of the weaned animals was
significantly reduced. These effects were present in both litters
of both generations. The surviving animals, in addition to being
smaller, appeared unhealthy (were cold and somewhat cyanotic).
Evaluation of those foetuses removed by caesarean section for
potential teratogenic effects failed to show any compound-related
differences from controls. While there was a reduced number of
implantation sites, ovarian corpora lutea, and an increased number
of resorption sites at 50 mg/kg, there were no teratogenic
occurrences in any of the foetuses examined with respect to either
somatic or skeletal abnormalities.
The presence of mecarbam in the diet at 50 mg/kg had a significant
effect on both maternal and foetal development and well being but
was not teratogenic (Rutter, 1974).
Special studies on Neurotoxicity
Preliminary studies to define the delayed neurotoxic potential of
mecarbam were performed with adult hens. The hens were
administered mecarbam subcutaneously, one dose per week for three
consecutive weeks at dosage levels corresponding to multiples of
the acute LD50. The animals were not treated with atropine and
survival was low. The surviving animals were observed over a
period of time extending to nine months. A small sample of the
surviving hens were subjected to histological examinations of
nervous tissue while a larger sample was examined macroscopically
for gross alterations.
In this preliminary trial, there was no evidence of delayed
neurotoxicity induced by the multiple administration of mecarbam
and the examination, either by gross or histological techniques,
was negative (Anonymous, 1960).
Two formulations of mecarbam (Pestcombi(R) and Testan(R)) were
examined for their delayed neurotoxic potential with adult hens.
Pestcombi(R) contained mecarbam (22%) and EPN (30%) while Testan(R)
contained mecarbam alone (28%). Adult hens were administered these
materials orally as a single maximum tolerated dose and were
observed for clinical signs of delayed neurotoxicity for 21 days.
When no clinical signs of ataxia were observed, the animals were
further treated in a similar manner and again observed for an
additional 21 days. At the conclusion of the 42-day experimental
period, several of the survivors were sacrificed for microscopic
examination of central and peripheral nerve tissue and skeletal
muscle following H and E staining.
There was no clinical sign of ataxia or histological evidence that
mecarbam alone or in combination with EPN induced a clinical
delayed neurotoxicity in hens (Shillam, 1968).
Acute Toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat M Oral 23-53 Tomich, 1958a, 1959, 1965;
Davies and Collins, 1975
F Oral 31-33 Tomich, 1959, 1962; Davies and
Collins, 1975
M Dermal >1222 Middleton, 1960;
Inhalation (1-5 µm 0.7 mg/l Berczy and Binns, 1971
particle size) (6 hr LC50)
Mouse M IP 120-130 Tomich, 1958a; Anony, 1962
M SC 600 Tomich, 1958
M Oral 106 Child, 1958
M IV 67 Tomich, 1968b
Guinea Pig Oral 65 Tomich (no date)
Dermal >1222 Middleton, 1960
Rabbit Oral 60 Tomich (no date)
Dermal 229 Middleton, 1960
Cat Oral <50 Tomich (no date)
Sheep Oral 20-25 Nicolas Inst., 1966
Further acute studies in rats using formulated products, rather than
technical mecarbam, demonstrated a sex difference with respect to
acute toxicity (the females were more sensitive than the males).
Additionally, these studies showed that concentrated formulated
products appear to be more toxic than technical mecarbam probably due
to an increased absorption of the active ingredient as a result of the
presence of insecticidally-inert formulation products (Davies and
Halliday, 1971; Kynoch et al, 1980a; 1980b).
Signs of poisoning
The signs of acute poisoning resulting from mecarbam administration
are similar to those seen with other cholinergic organophosphate
esters. These signs include: salivation, lacrimation (sometimes
bloody), piloerection, hunched posture, abnormal gait, changes in the
respiratory rate, and fine and coarse body tremors. Death occurred in
poisoned rats from one hour to three days following dosing. Autopsy
showed congestion and haemorrhage of the lungs and pale color in the
liver, kidney, and spleen. Following subacute administration recovery
of survivors, as judged by external appearance and behaviour, was
slow. Recovery was complete within eight days of dosing.
Special studies on antidotes
As with other cholinergic organophosphates, 2-PAM was found to exert a
protective effect on acute cholinergic signs of poisoning (Middleton,
1960). Atropine was not studied with respect to its antidotal effects
on the acute toxicity of mecarbam.
Short-term studies
Rat inhalation
Groups of rats (4 male and 4 female/group) were exposed to mecarbam by
inhalation. Exposures were carried out for 6 hours/day for a total of
14 exposure days over a 3-week experiment time. The aerosol
particulate was fully respirable and generated at concentrations of 0,
2, 10 and 50 mg/l air.
The animals were observed daily and growth was recorded at twice
weekly intervals. At the conclusion of the study urinalysis,
haematology, and clinical blood chemistry parameters were evaluated.
All rats were sacrificed, and gross and microscopic examination of
tissues and organs was performed.
Although respiratory irritation was observed in the two higher dose
levels, the exposure to mecarbam did not appear to result in adverse
behaviourial effects and growth was not affected. At the high dose
level, tremors were noted, and the animals became aggressive in the
second week of exposure. Urinalysis, haematology, and clinical
chemistry parameters were unaffected by exposure to mecarbam. At the
highest dose level, erythrocyte (but not plasma) cholinesterase
activity was depressed in male rats. Females did not show a
significant cholinesterase depression at any dose level although at 50
mg/l, the activity was slightly depressed.
Gross and microscopic examination of the lungs and major tissues and
organs showed no changes in morphology that would be attributable to
the presence of mecarbam. Based upon the conditions of this
experiment, the rat was shown to tolerate a concentration of 10 mg/l
air for a daily 6-hour exposure over a three-week test interval
(Berczy et al, 1972).
Cow
Groups of lactating dairy cows (4 cows/group) were administered
mecarbam in their diet at dosage levels of 0, 2, and 10 mg/kg for 30
days. The study was designed to evaluate the presence of residues in
milk and meat and to examine excretion and body burdens of mecarbam in
this species.
There was no mortality or signs of poisoning evident over the course
of the study. There were no changes in body weight or in milk
production. Although mecarbam was present in low levels in the milk
of cows administered mecarbam at both dietary levels, there was no
build up or evidence of bioaccumulation. The levels of mecarbam in
milk did not exceed 10 mg/kg. At the conclusion of the study, animals
were sacrificed at various intervals up to 1 week and 1 month
following cessation of treatment. There was no substantial evidence
of mecarbam in tissues and organs in any of the animals receiving
dietary administration. From this study, it may be concluded that
mecarbam, at levels of 10 mg/kg in the diet, does not elicit a toxic
reaction in cows, is not bioaccumulated, nor is it found as a residue
in meat, although low levels may be excreted in milk (Hepworth and
Brown, 1978).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
mecarbam in the diet at dosage levels of 0, 10, 20 or 50 mg/kg for 6
months. The respective dietary intake of mecarbam corresponding to
the food intake data was 0, 0.35, 0.80 or 1.78 mg/kg body weight.
The animals were observed daily for abnormal signs of behaviour and
toxicity. Data on food consumption and growth were recorded weekly.
At periodic intervals during the course of the study, the control and
high-dose group animals were examined with respect to haematological
and blood biochemistry parameters. Urinalyses were performed at the
same time intervals (with the exception of the last time interval, at
the conclusion of the study). Cholinesterase determinations were
performed by the electrometric measurement method examining red blood
cell and plasma enzyme activity. These analyses were performed
periodically during the course of the study. At the conclusion of the
study, the animals were sacrificed and, in addition to RBC and plasma,
brain cholinesterase activity was measured.
At the completion of the study, all animals were sacrificed and a
gross examination was made of selected tissues and organs.
Microscopic examinations were performed on tissues and organs of the
control and 50 mg/kg dose group.
There was no mortality over the 6-month trial. Food consumption and
growth were normal at all dose levels. Urinalyses and blood chemistry
values were within normal limits in all dose groups. Although several
haematological parameters did show some significant differences for
control values periodically during the course of the study, there was
no dose relationship nor time relationship noted with these findings.
RBC and plasma cholinesterase activity depression was evident in both
males and females in the high dose group. Plasma cholinesterase
appeared to be the more sensitive parameter and was reduced in both
males and females at 20 mg/kg. At this dose level, only females
showed a reduced red blood cell cholinesterase activity (noted only at
the conclusion of the study). In general, females appear to show a
slightly greater level of cholinesterase depression than males. Brain
cholinesterase, evaluated at the conclusion of the study, was
depressed significantly at 50 mg/kg in both males and females and at
20 mg/kg in males only. There was no depression of cholinesterase
activity in either sex at 10 mg/kg.
Gross and microscopic examination of tissues and organs at the
conclusion of the study did not show gross or microscopic
abnormalities attributable to the presence of mecarbam. It was
concluded that there were no pathological conditions induced over the
course of this study (Ward et al, 1979).
Groups of dogs (4 male and 4 female beagle dogs/group) were
administered mecarbam in the diet at dosage levels of 0, 1, 5 or 50
mg/kg for 2 years. Mecarbam was introduced into the dry diet, which
was prepared weekly.
All animals were examined daily for changes in behaviour and toxic
signs of poisoning. Growth and food consumption data were recorded
thereafter. At 0, 26, 68 and 104 weeks, haematology, clinical
chemistry, and urinalysis examinations were performed on all animals.
At 26 weeks, one male and one female of each group was sacrificed and
subjected to gross and microscopic examination of tissues and organs.
At the conclusion of the study, all animals were sacrificed and a
similar evaluation was made. Additionally, at the conclusion of the
study, brain cholinesterase analyses were performed on all animals.
There was no mortality over the course of the study. Growth and food
consumption data were comparable to control values at all time
intervals examined. Urinalysis, haematological and clinical
biochemistry data, with the exception of cholinesterase values, showed
no abnormalities attributable to the presence of mecarbam. Inhibition
of plasma and erythrocyte cholinesterase was noted at the highest dose
level in all animals at all intervals examined. Occasional slight
reduction of cholinesterase activity at 5 mg/kg was observed
periodically in the experiment. At the conclusion of the study, brain
cholinesterase depression was evident only at 50 mg/kg.
Gross and microscopic examination of a wide variety of tissues and
organs of animals sacrificed at the conclusion and during the course
of the study failed to detect any compound related tissue damage in
the series of tissues examined. Occasional spontaneous lesions and
incidental findings were not attributable to the presence of mecarbam
in the diet. A no-effect level, based upon cholinesterase depression
(which appears to be the most sensitive parameter) is 5 mg/kg in the
diet equivalent to a daily dietary intake of 0.15 mg/kg body weight
(based upon actual food consumption in both males and females) (Rutter
and Voelker, 1975).
Rat
Groups of rats (15 female rats per group) were administered mecarbam
in the diet at dosage levels of 0, 1, 5, 10 or 50 g/kg for six months.
Animals were examined daily and growth and food consumption data were
recorded weekly. Ophthalmological examination of animals in the
control and 50 mg/kg groups were made at periodic intervals.
Urinalyses, haematology, and blood chemistry parameters were recorded
at periodic intervals during the course of the 26-week experiment.
Plasma, red blood cell, and brain cholinesterase activity were
measured using the electrometric procedure.
At the conclusion of the 26-week feeding trial, all animals were
sacrificed and gross examinations of tissues and organs was performed
on all animals. Microscopic tissue examinations were performed on all
animals from the control and high dose group.
There was no mortality observed over the course of the study.
Clinical signs of poisoning commonly seen as muscular tremors and a
general unkempt appearance were observed at 50 mg/kg. Data on growth
and food consumption did not show a significant effect of mecarbam at
any dose level over the course of the study. Urinalysis, blood
chemistry, and biochemistry parameters (with the exception of
cholinesterase activity) were affected by mecarbam. Cholinesterase
depression was generally more pronounced in red blood cells than in
plasma. Enzyme depression was obvious at the two higher dose levels.
At the conclusion of the study, brain cholinesterase was substantially
depressed at two highest dose levels.
There were no adverse effects noted on ophthalmological examination.
Gross and microscopic examination of tissues and organs at the
conclusion of the study failed to show any adverse effects that could
be attributable to the presence of mecarbam in the diet (Rivett et
al, 1972).
Long-term study
Rat
Groups of rats (25 male and 25 female Sprague-Dawley rats/group) were
fed mecarbam in the diet at dosage levels of O, 1, 5, or 50 mg/kg for
104 weeks. (Based on an active ingredient of 83%, this corresponds to
dietary levels of 0, 0.83, 4.15, and 41.5 mg/kg). The animals were
observed daily for mortality and changes in behaviour. Growth and
food consumption were reported weekly for 10 weeks, biweekly for 15
weeks, and monthly thereafter to the termination of the study. Groups
of 5 rats per sex were sacrificed periodically for clinical chemistry,
haematology, and urinalysis. At 26 weeks and at the termination of
the experiment, animals were sacrificed and gross and microscopic
examination of tissues and organs were performed. Microscopic
examinations of the control and the high dose group animals were
performed in detail at the conclusion of the study. Animals of the
low and mid-dose groups were also examined microscopically with
respect to kidney and liver tissue. In all cases, unusual lesions and
tissue masses were examined microscopically, when observed.
During the first 10 weeks of the study, there was no mortality and no
unusual effects noted with respect to behaviour. A hunched appearance
was observed with increasing frequency at week 10, more frequently in
the treated animals of the high-dose group than in the control or
lower-dosed animals. At the conclusion of the study, all surviving
males and most of the surviving females had the hunched appearance.
Over the course of the study, mortality was observed in the treated
animals more frequently than in the control group although the
differences were not significant statistically (the number of animals
used in the study precludes an adequate evaluation of the increased
mortality induced by mecarbam). Growth was affected predominantly in
the males at 50 mg/kg. There were no effects noted with respect to
food consumption, clinical chemistry studies (with the exception of
cholinesterase), haematology, or urinalysis parameters. A
dose-related reduction of cholinesterase was observed. This reduction
was significant at 50 mg/kg in both males and females. At 104 weeks,
cholinesterase activity was substantially depressed in the high-dose
group in both males and females.
Gross and microscopic examination of tissues and organs at the 26-week
and 104-week intervals did not show compound-related changes at any
dietary dose level. There was a high incidence of spontaneous lesions
and neoplasms which were similar in both treated and control groups.
There was no indication that mecarbam induced a carcinogenic change in
any of the tissues examined. Respiratory disease was present in most
animals and was believed responsible for the reduction of surviving
animals at the conclusion of the study.
Based on cholinesterase depression, a no-effect level of 5 mg/kg was
recognised as being equivalent to a dosage level of 0.21 mg/kg body
weight (Rutter and Banas, 1975).
Observations in Man
None
RESIDUES IN FOOD
USE PATTERN
Mecarbam is an insecticide effective against plant sucking insects
such as aphids, leafhoppers, mealybugs, scale insects, lace bugs,
thrips and stem and fruit mining pests. It is also an effective
acaricide in those few areas where resistance to organophosphorus
compounds is absent, although resistant whitefly can be controlled by
mecarbam.
The principal use of mecarbam is for control of scale insects and
mealybugs in citrus crops, with lesser usage against the same pests on
vines, olives and deciduous fruits. Applications on citrus fruits are
usually made between petal fall and harvest during the period of
movement of the larvae after release from the parent. At this time
its control of other pests, such as aphids and whitefly, can also be
useful. Applications can also be made in mixtures with summer or
winter oils.
It acts as a contact and stomach insecticide. It is absorbed through
the leaf surface, but is only slowly translocated in the plant sap
from leaf application. It is systemic by root absorption and treated
plants remain toxic to insects for about 12 days. The formulations
are compatible with most commonly-used spray chemicals. Transient
phytotoxic effects have been observed on young, soft plants in
greenhouses; more mature plants have not usually shown these symptoms
but a wettable powder formulation has been tested for greenhouse use.
Normal rates of use range from 0.03 to 0.075% ai for high volume
spraying; low volume treatment requires appropriately higher
concentrations. Table 1 lists the crops treated and the pests
controlled.
TABLE 1. Crops treated and pests controlled.
Crop Pest
Apples aphids
codling moth
lace bug
scale insects
Brassicas cabbage root fly
Carrots carrot fly
Cashew whitefly
Cherries aphids
lace bugs
fruit fly
Citrus scale insects
mealybugs
mites
whitefly
Olives olive fly
scale insects
Peaches aphids
scale insects
mites
Pears aphids
leaf hoppers
lace bugs
pear sucker
mites
Pistachio nuts mites
scale insects
Plums aphids
leaf hoppers
mites
Rice leaf hoppers
plant hoppers
leaf miners
rice stem maggot
Vines vine moth
mites
mealybugs
Glasshouse crops whitefly
Open blossoms should not be sprayed and edible crops should not be
harvested within 14 days from spray treatment. Table 2 sets out
information on recommended rates of use and pre-harvest intervals for
various crops in seven countries. Use on olives is discouraged other
than very early in the season when the oil content is minimal. Use on
vegetable crops is very limited.
TABLE 2. Recommended rates of use and pre-harvest intervals
% a.i. Maximum Pre-harvest
Country Formulation in spray Crop No. of Interval
Treatments
Egypt 68% e.c. 0.1 citrus - -
68% e.c. 0.1 fruit trees - -
Cyprus 68% e.c. 0.034-0.1 citrus - 14
68% e.c. 0.34-0.085 apples, almonds, repeat at -
peaches, pears, 10-15 days
cherries
Greece 68% e.c. 0.051-0.085 citrus - 14
68% e.c. 0.051-0.085 fruit trees 14 -
12% in 0.06 fruit trees - -
oil.
Iran 68% e.c. 0.051-0.085 citrus - 14
Japan 25% e.c. 0.025-0.043 citrus 4 30
Morocco 50% e.c. 0.05-0.06 citrus - -
50% e.c. 0.0375-0.085 peaches, plums, - -
apples, pears,
vines
S. Africa 90% e.c. 0.0495 citrus 2 90
90% e.c. 0.054 apples, pears 4 14
plums, peaches,
apricots.
FATE OF RESIDUES
In plants
The degradation of mecarbam applied to dwarf bean plants has been
examined (Murphy Chem. Ltd., 1977) following different methods of
mecarbam application, root uptake and foliar absorption. The results
indicated that mecarbam is slowly translocated from the foliage to the
roots but is rapidly metabolised in both places. At least four
metabolites were indicated (three identified) resulting from either
oxidative or hydrolytic attack. Levels of mecarbam and its
metabolites declined from 10.4 to 0.05 mg/kg in 26 days after foliar
application; all levels observed in the roots were low, the highest
being 0.5 mg/kg at 5 days. Root uptake studies showed rapid
degradation of mecarbam and only low levels were found in the foliage.
Figure 1 illustrates the metabolic formation of mecarboxon, diethoate
and diethoxon from mecarbam.
Molecular formula C10H20O5NPS2
Molecular weight 329.4
Other information on identity and properties
Mecarbam is a colourless oily liquid; the technical material may be
pale yellow to brown. Technical mecarbam contains >85%, typically
92-95%, of mecarbam with small amounts of triethyldithiophosphate
and S-(ethoxycarbonyl) diethyl dithiophosphate as impurities. Its
freezing point is 9°C, specific gravity is 1.222/20°C, refractive
index nD20 is 1.5138, volatility is 6 × 10-6 g/litre of air at
40°C. It is slightly soluble in water, soluble in hexane (4%) and
kerosene (2%) and miscible in all proportions with alcohols,
ketones, aromatic hydrocarbons and chlorinated hydrocarbons. It is
stable at normal temperatures but hydrolyses at pH below 3 to yield
(C2H5O)2PSSCH2COOH, CO2, C2H5OH and NH2CH3.
The most widely used formulation is the 68% emulsifiable
concentrate, but similar 50%, 90% and 100% formulations are
available and dust and wettable powder preparations have also been
marketed.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
Groups of rats were administered mecarbam (approximately 10 mg/kg
body weight 14C-ethoxy-labelled in dimethyl sulphoxide). Mecarbam
was rapidly absorbed following oral dosing and was rapidly
excreted, predominantly in the urine. Within 48 hours, 92% of the
radioactivity was excreted in urine, 2% in faeces and approximately
5% was eliminated as CO2. There was no accumulation of
radioactivity in any of the tissues examined. The highest
concentrations of mecarbam in this study were found in the major
metabolising and excretory tissues and organs (Aryton and Harnby,
1977a).
Further studies on the absorption and distribution of mecarbam,
using dimethylformamide as a vehicle, suggested that mecarbam,
while slowly absorbed from the GI tract following an acute oral
dose, is rapidly distributed through the body and excreted
primarily through the urine. Following an oral dose of 10 mg/kg
body weight, it was observed that the 14C-radioactivity did not
fully leave the stomach until 24-48 hours following administration
(McCulloch, 1980).
In a further experiment to demonstrate the rapid removal of
mecarbam following absorption an intravenous dose of mecarbam was
introduced into the tail vein of male rats. Blood samples were
taken in periods up to 6 hours following treatment. Residues
declined rapidly during the first 40 minutes and slower thereafter,
demonstrating a biphasic excretion curve. At the end of 6 hours,
radioactivity in the blood had diminished to a point where it
almost reached background levels (Ayrton and Harnby, 1977b).
Biotransformation
No data available.
TOXICOLOGICAL STUDIES
Special Studies on Potentiation
Mecarbam was orally administered to male rats alone and in
combination (1:1 w/w suspension in propylene glycol) with three
other organophosphate insecticides (malathion, demeton-methyl,
demeton-S-methyl sulphoxide and dimethoate). The relationship of
the observed LD50 with respect to that calculated from the acute
toxicity curves indicated that there was no acute potentiation or
additive effect of the presence of mecarbam with the other
cholinergic insecticides tested. Demeton-methyl and dimethoate,
under the conditions of this test, appear to be somewhat protective
with respect to the toxicity of mecarbam. In no case was
potentiation observed (Middleton, 1960).
Special studies on reproduction
Groups of rats (25 male and 25 female rats/group) were fed mecarbam
in their diet at dosage levels of 0, 2, or 50 mg/kg and subjected
to a two-generation, two-litter per generation reproduction test.
Technical mecarbam was used with an active ingredient of 83.2% and
the dietary levels were corrected to account for this
concentration. A modified teratology bioassay was incorporated
into this reproduction study where 5 females per group were set
aside and examined to determine day 0 of gestation. The animals
were fed mecarbam throughout gestation at the above dosage levels.
On day 19 or 20 of gestation some animals were sacrificed and the
foetuses delivered by caesarean section. This modified teratology
programme was performed with the second litter of each generation.
The remaining animals of the second litter of the second generation
were maintained for a three-month observation period to evaluate
post-natal effects. In the modified teratology examination soft
tissue and skeletal examinations were performed on all foetuses.
All reproduction data were subjected to statistical analysis.
The presence of mecarbam in the diet at 50 mg/kg resulted in a
depression of growth of both males and females in both parental
groups. Food consumption was not affected. In addition to growth
reduction, the animals receiving the high dose showed clinical
signs of toxicity with respect to an increased incidence of rough
coat and hunched appearance, signs which have been noted in other
dietary studies as being associated with mecarbam toxicity. There
was no compound-related mortality observed over the course of the
study attributable to the presence of mecarbam in the diet.
Examination of reproduction data failed to show any significant
effects with respect to both litters of both generations
administered 2 mg/kg in the diet. In contrast, the 50 mg/kg
dietary level affected a number of reproduction parameters
including: a depression of numbers of pups born alive; an increased
number of animals dying during lactation; a decreased number of
animals ultimately weaned; and the weight of the weaned animals was
significantly reduced. These effects were present in both litters
of both generations. The surviving animals, in addition to being
smaller, appeared unhealthy (were cold and somewhat cyanotic).
Evaluation of those foetuses removed by caesarean section for
potential teratogenic effects failed to show any compound-related
differences from controls. While there was a reduced number of
implantation sites, ovarian corpora lutea, and an increased number
of resorption sites at 50 mg/kg, there were no teratogenic
occurrences in any of the foetuses examined with respect to either
somatic or skeletal abnormalities.
The presence of mecarbam in the diet at 50 mg/kg had a significant
effect on both maternal and foetal development and well being but
was not teratogenic (Rutter, 1974).
Special studies on Neurotoxicity
Preliminary studies to define the delayed neurotoxic potential of
mecarbam were performed with adult hens. The hens were
administered mecarbam subcutaneously, one dose per week for three
consecutive weeks at dosage levels corresponding to multiples of
the acute LD50. The animals were not treated with atropine and
survival was low. The surviving animals were observed over a
period of time extending to nine months. A small sample of the
surviving hens were subjected to histological examinations of
nervous tissue while a larger sample was examined macroscopically
for gross alterations.
In this preliminary trial, there was no evidence of delayed
neurotoxicity induced by the multiple administration of mecarbam
and the examination, either by gross or histological techniques,
was negative (Anonymous, 1960).
Two formulations of mecarbam (Pestcombi(R) and Testan(R)) were
examined for their delayed neurotoxic potential with adult hens.
Pestcombi(R) contained mecarbam (22%) and EPN (30%) while Testan(R)
contained mecarbam alone (28%). Adult hens were administered these
materials orally as a single maximum tolerated dose and were
observed for clinical signs of delayed neurotoxicity for 21 days.
When no clinical signs of ataxia were observed, the animals were
further treated in a similar manner and again observed for an
additional 21 days. At the conclusion of the 42-day experimental
period, several of the survivors were sacrificed for microscopic
examination of central and peripheral nerve tissue and skeletal
muscle following H and E staining.
There was no clinical sign of ataxia or histological evidence that
mecarbam alone or in combination with EPN induced a clinical
delayed neurotoxicity in hens (Shillam, 1968).
Acute Toxicity
LD50
Species Sex Route (mg/kg) Reference
Rat M Oral 23-53 Tomich, 1958a, 1959, 1965;
Davies and Collins, 1975
F Oral 31-33 Tomich, 1959, 1962; Davies and
Collins, 1975
M Dermal >1222 Middleton, 1960;
Inhalation (1-5 µm 0.7 mg/l Berczy and Binns, 1971
particle size) (6 hr LC50)
Mouse M IP 120-130 Tomich, 1958a; Anony, 1962
M SC 600 Tomich, 1958
M Oral 106 Child, 1958
M IV 67 Tomich, 1968b
Guinea Pig Oral 65 Tomich (no date)
Dermal >1222 Middleton, 1960
Rabbit Oral 60 Tomich (no date)
Dermal 229 Middleton, 1960
Cat Oral <50 Tomich (no date)
Sheep Oral 20-25 Nicolas Inst., 1966
Further acute studies in rats using formulated products, rather than
technical mecarbam, demonstrated a sex difference with respect to
acute toxicity (the females were more sensitive than the males).
Additionally, these studies showed that concentrated formulated
products appear to be more toxic than technical mecarbam probably due
to an increased absorption of the active ingredient as a result of the
presence of insecticidally-inert formulation products (Davies and
Halliday, 1971; Kynoch et al, 1980a; 1980b).
Signs of poisoning
The signs of acute poisoning resulting from mecarbam administration
are similar to those seen with other cholinergic organophosphate
esters. These signs include: salivation, lacrimation (sometimes
bloody), piloerection, hunched posture, abnormal gait, changes in the
respiratory rate, and fine and coarse body tremors. Death occurred in
poisoned rats from one hour to three days following dosing. Autopsy
showed congestion and haemorrhage of the lungs and pale color in the
liver, kidney, and spleen. Following subacute administration recovery
of survivors, as judged by external appearance and behaviour, was
slow. Recovery was complete within eight days of dosing.
Special studies on antidotes
As with other cholinergic organophosphates, 2-PAM was found to exert a
protective effect on acute cholinergic signs of poisoning (Middleton,
1960). Atropine was not studied with respect to its antidotal effects
on the acute toxicity of mecarbam.
Short-term studies
Rat inhalation
Groups of rats (4 male and 4 female/group) were exposed to mecarbam by
inhalation. Exposures were carried out for 6 hours/day for a total of
14 exposure days over a 3-week experiment time. The aerosol
particulate was fully respirable and generated at concentrations of 0,
2, 10 and 50 mg/l air.
The animals were observed daily and growth was recorded at twice
weekly intervals. At the conclusion of the study urinalysis,
haematology, and clinical blood chemistry parameters were evaluated.
All rats were sacrificed, and gross and microscopic examination of
tissues and organs was performed.
Although respiratory irritation was observed in the two higher dose
levels, the exposure to mecarbam did not appear to result in adverse
behaviourial effects and growth was not affected. At the high dose
level, tremors were noted, and the animals became aggressive in the
second week of exposure. Urinalysis, haematology, and clinical
chemistry parameters were unaffected by exposure to mecarbam. At the
highest dose level, erythrocyte (but not plasma) cholinesterase
activity was depressed in male rats. Females did not show a
significant cholinesterase depression at any dose level although at 50
mg/l, the activity was slightly depressed.
Gross and microscopic examination of the lungs and major tissues and
organs showed no changes in morphology that would be attributable to
the presence of mecarbam. Based upon the conditions of this
experiment, the rat was shown to tolerate a concentration of 10 mg/l
air for a daily 6-hour exposure over a three-week test interval
(Berczy et al, 1972).
Cow
Groups of lactating dairy cows (4 cows/group) were administered
mecarbam in their diet at dosage levels of 0, 2, and 10 mg/kg for 30
days. The study was designed to evaluate the presence of residues in
milk and meat and to examine excretion and body burdens of mecarbam in
this species.
There was no mortality or signs of poisoning evident over the course
of the study. There were no changes in body weight or in milk
production. Although mecarbam was present in low levels in the milk
of cows administered mecarbam at both dietary levels, there was no
build up or evidence of bioaccumulation. The levels of mecarbam in
milk did not exceed 10 mg/kg. At the conclusion of the study, animals
were sacrificed at various intervals up to 1 week and 1 month
following cessation of treatment. There was no substantial evidence
of mecarbam in tissues and organs in any of the animals receiving
dietary administration. From this study, it may be concluded that
mecarbam, at levels of 10 mg/kg in the diet, does not elicit a toxic
reaction in cows, is not bioaccumulated, nor is it found as a residue
in meat, although low levels may be excreted in milk (Hepworth and
Brown, 1978).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
mecarbam in the diet at dosage levels of 0, 10, 20 or 50 mg/kg for 6
months. The respective dietary intake of mecarbam corresponding to
the food intake data was 0, 0.35, 0.80 or 1.78 mg/kg body weight.
The animals were observed daily for abnormal signs of behaviour and
toxicity. Data on food consumption and growth were recorded weekly.
At periodic intervals during the course of the study, the control and
high-dose group animals were examined with respect to haematological
and blood biochemistry parameters. Urinalyses were performed at the
same time intervals (with the exception of the last time interval, at
the conclusion of the study). Cholinesterase determinations were
performed by the electrometric measurement method examining red blood
cell and plasma enzyme activity. These analyses were performed
periodically during the course of the study. At the conclusion of the
study, the animals were sacrificed and, in addition to RBC and plasma,
brain cholinesterase activity was measured.
At the completion of the study, all animals were sacrificed and a
gross examination was made of selected tissues and organs.
Microscopic examinations were performed on tissues and organs of the
control and 50 mg/kg dose group.
There was no mortality over the 6-month trial. Food consumption and
growth were normal at all dose levels. Urinalyses and blood chemistry
values were within normal limits in all dose groups. Although several
haematological parameters did show some significant differences for
control values periodically during the course of the study, there was
no dose relationship nor time relationship noted with these findings.
RBC and plasma cholinesterase activity depression was evident in both
males and females in the high dose group. Plasma cholinesterase
appeared to be the more sensitive parameter and was reduced in both
males and females at 20 mg/kg. At this dose level, only females
showed a reduced red blood cell cholinesterase activity (noted only at
the conclusion of the study). In general, females appear to show a
slightly greater level of cholinesterase depression than males. Brain
cholinesterase, evaluated at the conclusion of the study, was
depressed significantly at 50 mg/kg in both males and females and at
20 mg/kg in males only. There was no depression of cholinesterase
activity in either sex at 10 mg/kg.
Gross and microscopic examination of tissues and organs at the
conclusion of the study did not show gross or microscopic
abnormalities attributable to the presence of mecarbam. It was
concluded that there were no pathological conditions induced over the
course of this study (Ward et al, 1979).
Groups of dogs (4 male and 4 female beagle dogs/group) were
administered mecarbam in the diet at dosage levels of 0, 1, 5 or 50
mg/kg for 2 years. Mecarbam was introduced into the dry diet, which
was prepared weekly.
All animals were examined daily for changes in behaviour and toxic
signs of poisoning. Growth and food consumption data were recorded
thereafter. At 0, 26, 68 and 104 weeks, haematology, clinical
chemistry, and urinalysis examinations were performed on all animals.
At 26 weeks, one male and one female of each group was sacrificed and
subjected to gross and microscopic examination of tissues and organs.
At the conclusion of the study, all animals were sacrificed and a
similar evaluation was made. Additionally, at the conclusion of the
study, brain cholinesterase analyses were performed on all animals.
There was no mortality over the course of the study. Growth and food
consumption data were comparable to control values at all time
intervals examined. Urinalysis, haematological and clinical
biochemistry data, with the exception of cholinesterase values, showed
no abnormalities attributable to the presence of mecarbam. Inhibition
of plasma and erythrocyte cholinesterase was noted at the highest dose
level in all animals at all intervals examined. Occasional slight
reduction of cholinesterase activity at 5 mg/kg was observed
periodically in the experiment. At the conclusion of the study, brain
cholinesterase depression was evident only at 50 mg/kg.
Gross and microscopic examination of a wide variety of tissues and
organs of animals sacrificed at the conclusion and during the course
of the study failed to detect any compound related tissue damage in
the series of tissues examined. Occasional spontaneous lesions and
incidental findings were not attributable to the presence of mecarbam
in the diet. A no-effect level, based upon cholinesterase depression
(which appears to be the most sensitive parameter) is 5 mg/kg in the
diet equivalent to a daily dietary intake of 0.15 mg/kg body weight
(based upon actual food consumption in both males and females) (Rutter
and Voelker, 1975).
Rat
Groups of rats (15 female rats per group) were administered mecarbam
in the diet at dosage levels of 0, 1, 5, 10 or 50 g/kg for six months.
Animals were examined daily and growth and food consumption data were
recorded weekly. Ophthalmological examination of animals in the
control and 50 mg/kg groups were made at periodic intervals.
Urinalyses, haematology, and blood chemistry parameters were recorded
at periodic intervals during the course of the 26-week experiment.
Plasma, red blood cell, and brain cholinesterase activity were
measured using the electrometric procedure.
At the conclusion of the 26-week feeding trial, all animals were
sacrificed and gross examinations of tissues and organs was performed
on all animals. Microscopic tissue examinations were performed on all
animals from the control and high dose group.
There was no mortality observed over the course of the study.
Clinical signs of poisoning commonly seen as muscular tremors and a
general unkempt appearance were observed at 50 mg/kg. Data on growth
and food consumption did not show a significant effect of mecarbam at
any dose level over the course of the study. Urinalysis, blood
chemistry, and biochemistry parameters (with the exception of
cholinesterase activity) were affected by mecarbam. Cholinesterase
depression was generally more pronounced in red blood cells than in
plasma. Enzyme depression was obvious at the two higher dose levels.
At the conclusion of the study, brain cholinesterase was substantially
depressed at two highest dose levels.
There were no adverse effects noted on ophthalmological examination.
Gross and microscopic examination of tissues and organs at the
conclusion of the study failed to show any adverse effects that could
be attributable to the presence of mecarbam in the diet (Rivett et
al, 1972).
Long-term study
Rat
Groups of rats (25 male and 25 female Sprague-Dawley rats/group) were
fed mecarbam in the diet at dosage levels of O, 1, 5, or 50 mg/kg for
104 weeks. (Based on an active ingredient of 83%, this corresponds to
dietary levels of 0, 0.83, 4.15, and 41.5 mg/kg). The animals were
observed daily for mortality and changes in behaviour. Growth and
food consumption were reported weekly for 10 weeks, biweekly for 15
weeks, and monthly thereafter to the termination of the study. Groups
of 5 rats per sex were sacrificed periodically for clinical chemistry,
haematology, and urinalysis. At 26 weeks and at the termination of
the experiment, animals were sacrificed and gross and microscopic
examination of tissues and organs were performed. Microscopic
examinations of the control and the high dose group animals were
performed in detail at the conclusion of the study. Animals of the
low and mid-dose groups were also examined microscopically with
respect to kidney and liver tissue. In all cases, unusual lesions and
tissue masses were examined microscopically, when observed.
During the first 10 weeks of the study, there was no mortality and no
unusual effects noted with respect to behaviour. A hunched appearance
was observed with increasing frequency at week 10, more frequently in
the treated animals of the high-dose group than in the control or
lower-dosed animals. At the conclusion of the study, all surviving
males and most of the surviving females had the hunched appearance.
Over the course of the study, mortality was observed in the treated
animals more frequently than in the control group although the
differences were not significant statistically (the number of animals
used in the study precludes an adequate evaluation of the increased
mortality induced by mecarbam). Growth was affected predominantly in
the males at 50 mg/kg. There were no effects noted with respect to
food consumption, clinical chemistry studies (with the exception of
cholinesterase), haematology, or urinalysis parameters. A
dose-related reduction of cholinesterase was observed. This reduction
was significant at 50 mg/kg in both males and females. At 104 weeks,
cholinesterase activity was substantially depressed in the high-dose
group in both males and females.
Gross and microscopic examination of tissues and organs at the 26-week
and 104-week intervals did not show compound-related changes at any
dietary dose level. There was a high incidence of spontaneous lesions
and neoplasms which were similar in both treated and control groups.
There was no indication that mecarbam induced a carcinogenic change in
any of the tissues examined. Respiratory disease was present in most
animals and was believed responsible for the reduction of surviving
animals at the conclusion of the study.
Based on cholinesterase depression, a no-effect level of 5 mg/kg was
recognised as being equivalent to a dosage level of 0.21 mg/kg body
weight (Rutter and Banas, 1975).
Observations in Man
None
RESIDUES IN FOOD
USE PATTERN
Mecarbam is an insecticide effective against plant sucking insects
such as aphids, leafhoppers, mealybugs, scale insects, lace bugs,
thrips and stem and fruit mining pests. It is also an effective
acaricide in those few areas where resistance to organophosphorus
compounds is absent, although resistant whitefly can be controlled by
mecarbam.
The principal use of mecarbam is for control of scale insects and
mealybugs in citrus crops, with lesser usage against the same pests on
vines, olives and deciduous fruits. Applications on citrus fruits are
usually made between petal fall and harvest during the period of
movement of the larvae after release from the parent. At this time
its control of other pests, such as aphids and whitefly, can also be
useful. Applications can also be made in mixtures with summer or
winter oils.
It acts as a contact and stomach insecticide. It is absorbed through
the leaf surface, but is only slowly translocated in the plant sap
from leaf application. It is systemic by root absorption and treated
plants remain toxic to insects for about 12 days. The formulations
are compatible with most commonly-used spray chemicals. Transient
phytotoxic effects have been observed on young, soft plants in
greenhouses; more mature plants have not usually shown these symptoms
but a wettable powder formulation has been tested for greenhouse use.
Normal rates of use range from 0.03 to 0.075% ai for high volume
spraying; low volume treatment requires appropriately higher
concentrations. Table 1 lists the crops treated and the pests
controlled.
TABLE 1. Crops treated and pests controlled.
Crop Pest
Apples aphids
codling moth
lace bug
scale insects
Brassicas cabbage root fly
Carrots carrot fly
Cashew whitefly
Cherries aphids
lace bugs
fruit fly
Citrus scale insects
mealybugs
mites
whitefly
Olives olive fly
scale insects
Peaches aphids
scale insects
mites
Pears aphids
leaf hoppers
lace bugs
pear sucker
mites
Pistachio nuts mites
scale insects
Plums aphids
leaf hoppers
mites
Rice leaf hoppers
plant hoppers
leaf miners
rice stem maggot
Vines vine moth
mites
mealybugs
Glasshouse crops whitefly
Open blossoms should not be sprayed and edible crops should not be
harvested within 14 days from spray treatment. Table 2 sets out
information on recommended rates of use and pre-harvest intervals for
various crops in seven countries. Use on olives is discouraged other
than very early in the season when the oil content is minimal. Use on
vegetable crops is very limited.
TABLE 2. Recommended rates of use and pre-harvest intervals
% a.i. Maximum Pre-harvest
Country Formulation in spray Crop No. of Interval
Treatments
Egypt 68% e.c. 0.1 citrus - -
68% e.c. 0.1 fruit trees - -
Cyprus 68% e.c. 0.034-0.1 citrus - 14
68% e.c. 0.34-0.085 apples, almonds, repeat at -
peaches, pears, 10-15 days
cherries
Greece 68% e.c. 0.051-0.085 citrus - 14
68% e.c. 0.051-0.085 fruit trees 14 -
12% in 0.06 fruit trees - -
oil.
Iran 68% e.c. 0.051-0.085 citrus - 14
Japan 25% e.c. 0.025-0.043 citrus 4 30
Morocco 50% e.c. 0.05-0.06 citrus - -
50% e.c. 0.0375-0.085 peaches, plums, - -
apples, pears,
vines
S. Africa 90% e.c. 0.0495 citrus 2 90
90% e.c. 0.054 apples, pears 4 14
plums, peaches,
apricots.
FATE OF RESIDUES
In plants
The degradation of mecarbam applied to dwarf bean plants has been
examined (Murphy Chem. Ltd., 1977) following different methods of
mecarbam application, root uptake and foliar absorption. The results
indicated that mecarbam is slowly translocated from the foliage to the
roots but is rapidly metabolised in both places. At least four
metabolites were indicated (three identified) resulting from either
oxidative or hydrolytic attack. Levels of mecarbam and its
metabolites declined from 10.4 to 0.05 mg/kg in 26 days after foliar
application; all levels observed in the roots were low, the highest
being 0.5 mg/kg at 5 days. Root uptake studies showed rapid
degradation of mecarbam and only low levels were found in the foliage.
Figure 1 illustrates the metabolic formation of mecarboxon, diethoate
and diethoxon from mecarbam.
 In soils
Mecarbam added to loam and to sandy soil was studied under sterile and
nonsterile conditions (Murphy Chem. Ltd., 1977). Analysis showed that
the pesticide has a short life, levels dropping from the applied 7.5
mg/kg to less than 0.4 mg/kg in 50 days in loam soil and to O.1 mg/kg
in 34 days in sandy soil. Degradation was shown to be mainly by
chemical rather than by microbial action since similar degradation was
obtained with soil that had been sterilised by autoclaving.
In cooking
The principal use of mecarbam is on citrus crops, and most residue
resides in the peel or rind, not in the flesh of the fruit. Since the
peel is consumed in marmalade, the fate of residues on processing
oranges into this preserve has been studied (Murphy Chem. Ltd., 1970).
The yield of home-made marmalade is about 2-3 times the weight of
fruit used. Batches of marmalade were prepared using a normal
domestic recipe, except that the peel was finely shredded at -30°C to
minimise possible losses of mecarbam. Oranges containing
field-incurred residues and untreated oranges to which known amounts
of mecarbam had been added were used in these tests. The
field-treated fruit had residues of 2.4 mg/kg (10.7 mg/kg in the peel)
and the added mecarbam was at a level of 2.7 mg/kg immediately before
cooking. Levels of mecarbam in the marmalade ranged from 0.14 to 0.22
mg/kg, giving an average loss on cooking of over 80%. The maximum
level of mecarbam to be expected in marmalade prepared from treated
fruit is of the order of 0.05 to 0.1 mg/kg.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
Data was available from supervised trials of mecarbam on various types
of oranges grown in Japan, South Africa, Egypt, Israel and Morocco
(Murphy Chem. Ltd., 1980). In all of the studies only negligible
amounts of mecarbam (less than 0.03 mg/kg) were found in the flesh of
the fruit. Residues on the peel, however, ranged up to about 10 mg/kg
at the recommended 14 and 30 day pre-harvest intervals and up to 5
mg/kg at about 90 days. Residues of mecarbam in the peel of
grapefruit were about 0.5 mg/kg at 34 days after treatment, with not
more than 0.01 mg/kg in the flesh of the fruit.
The principal metabolites were also determined on treated oranges
resulting from three trials carried out in Egypt; the results are
given in Table 3 (Murphy Chem. Ltd., 1980). At 14 days after
treatment, the parent mecarbam remains the predominant residue in the
peel, the three metabolites together comprising only 5 to 10% of the
total residue.
TABLE 3. Mecarbam and metabolite residues in oranges grown in Egypt,
1975 (mg/kg)
No. of days Peel Flesh
after
application Mecarbam Mecarboxon Diethoate Diethoxon Mecarbam
0 5.95 0.08 0.20 1.39 0.10
7 7.0 0.17 0.22 0.31 0.05
14 6.95 0.28 0.17 0.25 0.02
28 5.45 0.22 0.13 0.11 0.01
Untreated <0.05 - - - <0.002
0 18.3 0.028 0.032 0.054 0.12
7 14.0 0.68 0.036 0.050 0.05
14 5.35 0.20 0.04 0.004 0.03
Untreated <0.05 - - - <0.002
0 14.4 0.103 0.024 0.30 0.06
In all three trials mecarbam was applied at a final spray concentration
of 0.1% ai.
Other crops
A limited amount of data was presented on mecarbam residues in olives,
grapes, pears and various vegetable crops. The analytical results
were not easy to interpret but it appears that treated olives
containing up to 1.5 mg/kg can yield olive oil containing up to 8.5
mg/kg of mecarbam. Residues in grapes and pears at 14 days or more
after application were generally less than 0.3 mg/kg; no evidence of
occurrence of metabolites was available (Murphy Chem. Ltd., 1980).
The data on residues on cabbage, cauliflowers, broccoli, Brussels
sprouts, celery, carrots, onions, turnips, sugarbeet and lucerne had
mostly been obtained during the 1960s when analytical procedures were
less specific or accurate than current methods. Results reported were
very inconsistent and in many instances the control values were equal
to or greater than the observed levels in treated crops, typically
about 0.3 mg/kg on leafy vegetables.
The data on these vegetable crops were not adequate to support
recommendations for maximum residue levels.
METHODS OF RESIDUE ANALYSIS
Mecarbam is extracted from crops with hexane, cleaned up by solvent
partition and/or column chromatography and determined by GLC with
either electron capture or phosphorus specific detection (Lynch,
1976). The procedure should be suitable for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
Available information on national MRLs is given in Table 4.
TABLE 4. National MRLs for mecarbam reported to the meeting
Country Commodity MRL (mg/kg)
Belgium Fruit, vegetables (not potatoes) 1
Netherlands Fruit, vegetables (not potatoes) 1
South Africa Apples, apricots, peaches, pears,
plums 0.05
Citrus 0.05
EVALUATION
COMMENTS AND APPRAISAL
Mecarbam is an organophosphorus insecticide effective against plant
sucking pests such as aphids, scale insects, etc., and also acts as an
acaricide. Its principal use is for the control of scale insects and
mealybugs on citrus fruits, with lesser uses against the same range of
insects on vines, olives and deciduous fruits; it has also been used
on vegetables.
Mecarbam is rapidly absorbed following acute oral ingestion, with
rapid distribution and elimination from the body. Mecarbam does not
bioaccumulate in the body on repeated administration. The metabolism
has not been elucidated.
Mecarbam elicits parasympathomimetic signs of poisoning following high
doses. Antidotal studies with mecarbam are deficient, although it is
presumed that atropine may be effective.
Mecarbam appears not to induce a delayed neurotoxic response in hens
although studies were not fully acceptable. Mecarbam does not affect
reproduction in rats at levels below those at which dams might be
affected. Limited information derived from a teratology bioassay
combined with the reproduction study did not suggest mecarbam to be
teratogenic. A more complete teratogenicity bioassay, specifically to
evaluate this effect, is required. There are no data available to
assess the mutagenic potential of mecarbam. There are no specific
studies to assess the carcinogenic potential although long-term
studies in rats alleviate concerns in this direction. Adequate
short-term dietary studies in dogs and long-term dietary studies in
rats demonstrate that the principal effects attributable to mecarbam
in the diet are reflective of cholinesterase depression. In both
species a no-effect level has been noted.
On the basis of these levels a temporary acceptable daily intake was
allocated and research suggested to complete the data base.
Mecarbam is systemic by root absorption and absorbed through the leaf
surface. The three main metabolites are mecarboxon, diethoate and
diethoxon, but these form only a small part of the total residue,
which consists mainly of the parent mecarbam and remains in the peel
of citrus fruits. Preparation of marmalade from treated fruit results
in residue losses of about 80%. Available residue data were adequate
for oranges but were scanty and difficult to interpret for other
fruits, olives or vegetables. A gas chromatographic method of
analysis is available which can be adapted for regulatory purposes.
Level causing no toxicological effect
Rat: 5 mg/kg in the diet equivalent to 0.21 mg/kg bw/day
Dog: 5 mg/kg in the diet equivalent to 0.15 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day
RECOMMENDATIONS OF RESIDUES LIMITS
From the data available the meeting was able to estimate a maximum
residue level for oranges only.
The meeting concluded that the level is suitable for establishing a
temporary maximum residue limit. The level applies to the parent
compound, excluding any metabolites.
Commodity Estimated maximum Treatment-harvest interval
residue level (mg/kg) on which level is based
oranges 2 14
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Complete metabolic studies in laboratory animals.
2. A complete teratogenicity bioassay.
3. Studies to define the mutagenic potential.
4. Studies in hens or other appropriate species to define the
potential for delayed neurotoxicity.
5. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
6. Data on the use of mecarbam on vegetables and deciduous fruits
together with residue data from supervised trials.
7. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
8. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
9. Data an use of mecarbam on vegetables and deciduous fruits,
together with residue data from supervised trials.
10. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
Desirable
1. Studies on antidotes.
2. Epidemiological data on potential for adverse effects resulting
from occupational exposure.
REFERENCES
Anonymous. Neurotoxicity to hens. (1960) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Anonymous. Oral LD50 in mice. Murphy Compound P. 474/17. (1962)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Aryton J. and Harnby G. Preliminary metabolic studies on Mecarbam
(S(N-ethoxycarbonyl-N-methylcarbamoyl-methyl)diethyl
phosphorothiolothioate). (1977a) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical Ltd. to the World
Health Organization.
Aryton J. and Harnby, G. Blood levels of 14C following an intravenous
administration of 14C Mecarbam to rats. (1977b) Unpublished report
from Glaxo Laboratories,Ltd., submitted by Murphy Chemical, Ltd. to
the World Health Organization.
Berczy, Z.S. and Binns, R. Acute inhalation toxicity to the rat of
Mecarbam. (1971) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield
R.A.S. (1972) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Child K.J. Acute oral toxicity of P. 474/1 to male mice. (1958)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Davies, R.E. and Collins, C.J. Acute oral toxicity to rats of Mecarbam
(Murfotox). (1975) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Davies, R.D. and Halliday, J.C. Acute oral toxicity to rats of
Murfotox liquid (64% ai). (1971) Unpublished report from Huntingdon
Research Centre submitted by Murphy Chemical Ltd. to The World Health
Organization.
Hepworth, P.L. and Brown, P.M. Mecarbam: Milk and tissue residues
following repeated dietary administration to dairy cows over thirty
days with a maximum period of thirty days respite from treatments.
(1978) Unpublished report from Life Sciences Research (to Takeda
Chemical Industries, Ltd.), submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 50% EC. (1980a) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical Ltd. to The World Health
Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 25% wp. (1980b) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Lynch, V.P. Mecarbam; in Volume 8 of Analytical Methods for Pesticides
and Plant Growth Regulators, Ed G. Zweig and G. Sherma, Academic
Press, N.Y. p. 135-140.
McCulloch, R.J. The distribution of 14C Mecarbam in rats.
Autoradiographic studies (Report no. 77261). (1980) Unpublished report
from Glaxo Research, Ltd. submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Middleton, T.R. Potentiation of Mecarbam by other insecticides.
(1960a) Unpublished report from Glaxo Laboratories, Ltd, submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Middleton, T.R. Percutaneous toxicity of Mecarbam to rats. (1960b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Murphy Chem. Ltd. Fate of mecarbam residues in oranges when converted
to marmalade. Murphy Chem. Ltd., unpublished report, 27 November 1970.
Murphy Chem. Ltd. Mecarbam 2M; metabolism studies in plants and soil.
Murphy Chem. Ltd., unpublished report No. 77/32. (1977).
Murphy Chem. Ltd. Mecarbam residues data. Volume 3, Murphy Chem. Ltd.,
unpublished report. (1980)
Nicholas Institute. Mecarbam toxicity to sheep. (1966) Unpublished
report from the Nicholas Institute, submitted by Murphy Chemical, Ltd.
to The World Health Organization.
Rivett, K.F., Batham, P., Street, A.E., Haywood, R. and Newman, A.J.
(1972) Unpublished report from the Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Rutter, H.A., Jr. Mecarbam. Two-generation reproductive study in rats.
(1974) Unpublished report from Hazelton Laboratories American, Ltd.
(to Takeda Chemical Industries, Ltd.), submitted by Murphy Chemical,
Ltd. to The World Health Organization.
Rutter, H.A., Jr. and Banas, D.A. 104-Week dietary administration in
rats - Mecarbam - final report. (1975) Unpublished report from
Hazelton Laboratories American, Ltd. (to Takeda Chemical Industries,
Ltd.), submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Rutter, H.A., Jr. and Voelker, R.W. Two-year dietary toxicity study in
dogs. Mecarbam. Final Report. (1975) Unpublished report from Hazelton
Laboratories American, Ltd. (to Takeda Chemical Industries Ltd.),
submitted to The World Health Organization.
Robinson, D. and Shillam, K.W.G. Neurotoxicity of Pestoombi(R) and
Pestan(R) in the domestic hen. (1968) Unpublished reports from
Huntingdon Research Centre, submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Tomich, E.G. Acute oral toxicities of P.474 to guinea pigs, rabbits
and cats. Unpublished undated report from Glaxo Laboratories, Ltd.,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute intraperitoneal and subcutaneous toxicities to male
mice and acute oral toxicity to male rats. (1958a) Unpublished report
from Glaxo Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to
The World Health Organization.
Tomich E.G. P.474/5 acute intravenous toxicity (male mice). (1958b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute oral toxicity to female rats. P.474/5 and P.474/7.
(1959) Unpublished report from Glaxo Laboratories, Ltd., submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound P. 414/17. (1962)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound Mecarbam batch 416
(lab stripped). (1965) Unpublished report from Glaxo Laboratories,
Ltd., submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Ward, R.J., Kellett, B.S., Hadlow, C.J. and Lancaster, M.G. Mecarbam.
Oral toxicity to dogs (dietary administration for 6 months). (1979)
Unpublished report of studies performed at Manston Research Center,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
In soils
Mecarbam added to loam and to sandy soil was studied under sterile and
nonsterile conditions (Murphy Chem. Ltd., 1977). Analysis showed that
the pesticide has a short life, levels dropping from the applied 7.5
mg/kg to less than 0.4 mg/kg in 50 days in loam soil and to O.1 mg/kg
in 34 days in sandy soil. Degradation was shown to be mainly by
chemical rather than by microbial action since similar degradation was
obtained with soil that had been sterilised by autoclaving.
In cooking
The principal use of mecarbam is on citrus crops, and most residue
resides in the peel or rind, not in the flesh of the fruit. Since the
peel is consumed in marmalade, the fate of residues on processing
oranges into this preserve has been studied (Murphy Chem. Ltd., 1970).
The yield of home-made marmalade is about 2-3 times the weight of
fruit used. Batches of marmalade were prepared using a normal
domestic recipe, except that the peel was finely shredded at -30°C to
minimise possible losses of mecarbam. Oranges containing
field-incurred residues and untreated oranges to which known amounts
of mecarbam had been added were used in these tests. The
field-treated fruit had residues of 2.4 mg/kg (10.7 mg/kg in the peel)
and the added mecarbam was at a level of 2.7 mg/kg immediately before
cooking. Levels of mecarbam in the marmalade ranged from 0.14 to 0.22
mg/kg, giving an average loss on cooking of over 80%. The maximum
level of mecarbam to be expected in marmalade prepared from treated
fruit is of the order of 0.05 to 0.1 mg/kg.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
Data was available from supervised trials of mecarbam on various types
of oranges grown in Japan, South Africa, Egypt, Israel and Morocco
(Murphy Chem. Ltd., 1980). In all of the studies only negligible
amounts of mecarbam (less than 0.03 mg/kg) were found in the flesh of
the fruit. Residues on the peel, however, ranged up to about 10 mg/kg
at the recommended 14 and 30 day pre-harvest intervals and up to 5
mg/kg at about 90 days. Residues of mecarbam in the peel of
grapefruit were about 0.5 mg/kg at 34 days after treatment, with not
more than 0.01 mg/kg in the flesh of the fruit.
The principal metabolites were also determined on treated oranges
resulting from three trials carried out in Egypt; the results are
given in Table 3 (Murphy Chem. Ltd., 1980). At 14 days after
treatment, the parent mecarbam remains the predominant residue in the
peel, the three metabolites together comprising only 5 to 10% of the
total residue.
TABLE 3. Mecarbam and metabolite residues in oranges grown in Egypt,
1975 (mg/kg)
No. of days Peel Flesh
after
application Mecarbam Mecarboxon Diethoate Diethoxon Mecarbam
0 5.95 0.08 0.20 1.39 0.10
7 7.0 0.17 0.22 0.31 0.05
14 6.95 0.28 0.17 0.25 0.02
28 5.45 0.22 0.13 0.11 0.01
Untreated <0.05 - - - <0.002
0 18.3 0.028 0.032 0.054 0.12
7 14.0 0.68 0.036 0.050 0.05
14 5.35 0.20 0.04 0.004 0.03
Untreated <0.05 - - - <0.002
0 14.4 0.103 0.024 0.30 0.06
In all three trials mecarbam was applied at a final spray concentration
of 0.1% ai.
Other crops
A limited amount of data was presented on mecarbam residues in olives,
grapes, pears and various vegetable crops. The analytical results
were not easy to interpret but it appears that treated olives
containing up to 1.5 mg/kg can yield olive oil containing up to 8.5
mg/kg of mecarbam. Residues in grapes and pears at 14 days or more
after application were generally less than 0.3 mg/kg; no evidence of
occurrence of metabolites was available (Murphy Chem. Ltd., 1980).
The data on residues on cabbage, cauliflowers, broccoli, Brussels
sprouts, celery, carrots, onions, turnips, sugarbeet and lucerne had
mostly been obtained during the 1960s when analytical procedures were
less specific or accurate than current methods. Results reported were
very inconsistent and in many instances the control values were equal
to or greater than the observed levels in treated crops, typically
about 0.3 mg/kg on leafy vegetables.
The data on these vegetable crops were not adequate to support
recommendations for maximum residue levels.
METHODS OF RESIDUE ANALYSIS
Mecarbam is extracted from crops with hexane, cleaned up by solvent
partition and/or column chromatography and determined by GLC with
either electron capture or phosphorus specific detection (Lynch,
1976). The procedure should be suitable for regulatory purposes.
NATIONAL MRLs REPORTED TO THE MEETING
Available information on national MRLs is given in Table 4.
TABLE 4. National MRLs for mecarbam reported to the meeting
Country Commodity MRL (mg/kg)
Belgium Fruit, vegetables (not potatoes) 1
Netherlands Fruit, vegetables (not potatoes) 1
South Africa Apples, apricots, peaches, pears,
plums 0.05
Citrus 0.05
EVALUATION
COMMENTS AND APPRAISAL
Mecarbam is an organophosphorus insecticide effective against plant
sucking pests such as aphids, scale insects, etc., and also acts as an
acaricide. Its principal use is for the control of scale insects and
mealybugs on citrus fruits, with lesser uses against the same range of
insects on vines, olives and deciduous fruits; it has also been used
on vegetables.
Mecarbam is rapidly absorbed following acute oral ingestion, with
rapid distribution and elimination from the body. Mecarbam does not
bioaccumulate in the body on repeated administration. The metabolism
has not been elucidated.
Mecarbam elicits parasympathomimetic signs of poisoning following high
doses. Antidotal studies with mecarbam are deficient, although it is
presumed that atropine may be effective.
Mecarbam appears not to induce a delayed neurotoxic response in hens
although studies were not fully acceptable. Mecarbam does not affect
reproduction in rats at levels below those at which dams might be
affected. Limited information derived from a teratology bioassay
combined with the reproduction study did not suggest mecarbam to be
teratogenic. A more complete teratogenicity bioassay, specifically to
evaluate this effect, is required. There are no data available to
assess the mutagenic potential of mecarbam. There are no specific
studies to assess the carcinogenic potential although long-term
studies in rats alleviate concerns in this direction. Adequate
short-term dietary studies in dogs and long-term dietary studies in
rats demonstrate that the principal effects attributable to mecarbam
in the diet are reflective of cholinesterase depression. In both
species a no-effect level has been noted.
On the basis of these levels a temporary acceptable daily intake was
allocated and research suggested to complete the data base.
Mecarbam is systemic by root absorption and absorbed through the leaf
surface. The three main metabolites are mecarboxon, diethoate and
diethoxon, but these form only a small part of the total residue,
which consists mainly of the parent mecarbam and remains in the peel
of citrus fruits. Preparation of marmalade from treated fruit results
in residue losses of about 80%. Available residue data were adequate
for oranges but were scanty and difficult to interpret for other
fruits, olives or vegetables. A gas chromatographic method of
analysis is available which can be adapted for regulatory purposes.
Level causing no toxicological effect
Rat: 5 mg/kg in the diet equivalent to 0.21 mg/kg bw/day
Dog: 5 mg/kg in the diet equivalent to 0.15 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day
RECOMMENDATIONS OF RESIDUES LIMITS
From the data available the meeting was able to estimate a maximum
residue level for oranges only.
The meeting concluded that the level is suitable for establishing a
temporary maximum residue limit. The level applies to the parent
compound, excluding any metabolites.
Commodity Estimated maximum Treatment-harvest interval
residue level (mg/kg) on which level is based
oranges 2 14
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Complete metabolic studies in laboratory animals.
2. A complete teratogenicity bioassay.
3. Studies to define the mutagenic potential.
4. Studies in hens or other appropriate species to define the
potential for delayed neurotoxicity.
5. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
6. Data on the use of mecarbam on vegetables and deciduous fruits
together with residue data from supervised trials.
7. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
8. Further data on residues of mecarbam in citrus fruits, olives,
olive oil and other fruits to support additional recommendations for
maximum residue levels.
9. Data an use of mecarbam on vegetables and deciduous fruits,
together with residue data from supervised trials.
10. Data on residues occurring in meat or milk from feeding animals
with citrus pulp prepared from treated fruit.
Desirable
1. Studies on antidotes.
2. Epidemiological data on potential for adverse effects resulting
from occupational exposure.
REFERENCES
Anonymous. Neurotoxicity to hens. (1960) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Anonymous. Oral LD50 in mice. Murphy Compound P. 474/17. (1962)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Aryton J. and Harnby G. Preliminary metabolic studies on Mecarbam
(S(N-ethoxycarbonyl-N-methylcarbamoyl-methyl)diethyl
phosphorothiolothioate). (1977a) Unpublished report from Glaxo
Laboratories, Ltd., submitted by Murphy Chemical Ltd. to the World
Health Organization.
Aryton J. and Harnby, G. Blood levels of 14C following an intravenous
administration of 14C Mecarbam to rats. (1977b) Unpublished report
from Glaxo Laboratories,Ltd., submitted by Murphy Chemical, Ltd. to
the World Health Organization.
Berczy, Z.S. and Binns, R. Acute inhalation toxicity to the rat of
Mecarbam. (1971) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield
R.A.S. (1972) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Child K.J. Acute oral toxicity of P. 474/1 to male mice. (1958)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Davies, R.E. and Collins, C.J. Acute oral toxicity to rats of Mecarbam
(Murfotox). (1975) Unpublished report from Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Davies, R.D. and Halliday, J.C. Acute oral toxicity to rats of
Murfotox liquid (64% ai). (1971) Unpublished report from Huntingdon
Research Centre submitted by Murphy Chemical Ltd. to The World Health
Organization.
Hepworth, P.L. and Brown, P.M. Mecarbam: Milk and tissue residues
following repeated dietary administration to dairy cows over thirty
days with a maximum period of thirty days respite from treatments.
(1978) Unpublished report from Life Sciences Research (to Takeda
Chemical Industries, Ltd.), submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 50% EC. (1980a) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical Ltd. to The World Health
Organization.
Kynoch S.R., Charnley, B.N. and Ginty, J.L. Acute oral toxicity to
rats of Murfotox 25% wp. (1980b) Unpublished report from Huntingdon
Research Centre, submitted by Murphy Chemical, Ltd. to The World
Health Organization.
Lynch, V.P. Mecarbam; in Volume 8 of Analytical Methods for Pesticides
and Plant Growth Regulators, Ed G. Zweig and G. Sherma, Academic
Press, N.Y. p. 135-140.
McCulloch, R.J. The distribution of 14C Mecarbam in rats.
Autoradiographic studies (Report no. 77261). (1980) Unpublished report
from Glaxo Research, Ltd. submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Middleton, T.R. Potentiation of Mecarbam by other insecticides.
(1960a) Unpublished report from Glaxo Laboratories, Ltd, submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Middleton, T.R. Percutaneous toxicity of Mecarbam to rats. (1960b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Murphy Chem. Ltd. Fate of mecarbam residues in oranges when converted
to marmalade. Murphy Chem. Ltd., unpublished report, 27 November 1970.
Murphy Chem. Ltd. Mecarbam 2M; metabolism studies in plants and soil.
Murphy Chem. Ltd., unpublished report No. 77/32. (1977).
Murphy Chem. Ltd. Mecarbam residues data. Volume 3, Murphy Chem. Ltd.,
unpublished report. (1980)
Nicholas Institute. Mecarbam toxicity to sheep. (1966) Unpublished
report from the Nicholas Institute, submitted by Murphy Chemical, Ltd.
to The World Health Organization.
Rivett, K.F., Batham, P., Street, A.E., Haywood, R. and Newman, A.J.
(1972) Unpublished report from the Huntingdon Research Centre,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Rutter, H.A., Jr. Mecarbam. Two-generation reproductive study in rats.
(1974) Unpublished report from Hazelton Laboratories American, Ltd.
(to Takeda Chemical Industries, Ltd.), submitted by Murphy Chemical,
Ltd. to The World Health Organization.
Rutter, H.A., Jr. and Banas, D.A. 104-Week dietary administration in
rats - Mecarbam - final report. (1975) Unpublished report from
Hazelton Laboratories American, Ltd. (to Takeda Chemical Industries,
Ltd.), submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Rutter, H.A., Jr. and Voelker, R.W. Two-year dietary toxicity study in
dogs. Mecarbam. Final Report. (1975) Unpublished report from Hazelton
Laboratories American, Ltd. (to Takeda Chemical Industries Ltd.),
submitted to The World Health Organization.
Robinson, D. and Shillam, K.W.G. Neurotoxicity of Pestoombi(R) and
Pestan(R) in the domestic hen. (1968) Unpublished reports from
Huntingdon Research Centre, submitted by Murphy Chemical, Ltd. to The
World Health Organization.
Tomich, E.G. Acute oral toxicities of P.474 to guinea pigs, rabbits
and cats. Unpublished undated report from Glaxo Laboratories, Ltd.,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute intraperitoneal and subcutaneous toxicities to male
mice and acute oral toxicity to male rats. (1958a) Unpublished report
from Glaxo Laboratories, Ltd., submitted by Murphy Chemical, Ltd. to
The World Health Organization.
Tomich E.G. P.474/5 acute intravenous toxicity (male mice). (1958b)
Unpublished report from Glaxo Laboratories, Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Acute oral toxicity to female rats. P.474/5 and P.474/7.
(1959) Unpublished report from Glaxo Laboratories, Ltd., submitted by
Murphy Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound P. 414/17. (1962)
Unpublished report from Glaxo Laboratories Ltd., submitted by Murphy
Chemical, Ltd. to The World Health Organization.
Tomich, E.G. Oral LD50 in rats. Murphy Compound Mecarbam batch 416
(lab stripped). (1965) Unpublished report from Glaxo Laboratories,
Ltd., submitted by Murphy Chemical, Ltd. to The World Health
Organization.
Ward, R.J., Kellett, B.S., Hadlow, C.J. and Lancaster, M.G. Mecarbam.
Oral toxicity to dogs (dietary administration for 6 months). (1979)
Unpublished report of studies performed at Manston Research Center,
submitted by Murphy Chemical, Ltd. to The World Health Organization.
