
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
OXAMYL
IDENTITY
Chemical names
N,N-dimethyl-2-methylcarbanoyloxyimino-2-(methylthio)acetamide
(IUPAC)
Methyl N',N'-dimethyl-N-[(methylcarbamoyl)oxy-i-thio-
oxamimidate S-methyl-1-(dimethylcarbamoyl)-N-[(methylcarbamoyl)
oxy] thioformimidate 2-dimethylamino-1-(methylthio) glyoxal
O-methylcarbamoylmonoxime
Synonyms
VydateTM, D-1410, DPX 1410, Du Pont 1410
Structural formula
Molecular formula: C7H13N3O3S
Molecular weight: 219.3
Description: slightly sulphurous
Melting point: 100-102° changing to a different crystalline
form which melts at 108-110°
Specific gravity: 0.97 25°/4°
Vapour pressure: 2.3 × 10-4 mm Hg at 25°C
3.75 × 10-4 mm Hg at 30°C
8.4 × 10-4 mm Hg at 40°C
7.6 × 10-3 mm Hg at 70°C
Solubility at 25°C g/100 g Solvent
Methanol 144
DMF 108
Acetone 67
Ethanol 33
Cyclohexanone 29
Water 28
Isopropanol 11
Toluene 1
Oxamyl is commercially available as a 24% liquid formulation and a
10% granular formulation. The liquid formulated material is a
clear liquid before colour addition.
No information was available on the manufacturing process, inerts
or technical impurities.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
A male Charles River CD rat was given 50 mg/kg non-radiolabelled
oxamyl in the diet. Thirty-two days later, the rat received by
intragastric intubation 2 ml of peanut oil containing 1.0 mg (3.74
µCi) of [14C]oxamyl. A second rat was preconditioned for 18 days
on a diet containing 150 mg unlabelled oxamyl/kg feed. This animal
was treated like the first with 1.03 mg (5.6 µCi) of [14C]oxamyl.
Both animals were killed after 3 days.
Most of the radiolabelled doses (68-72%) were eliminated within 72
hours. Most of the activity was recovered in the urine (61%, 48%
in each animal respectively), with a lesser amount in the faeces
(6% and 23%). Little, if any, radioactivity appeared in the
expired air (<0.3%). Levels of radioactivity were distributed
throughout the body, but mainly in the hide, carcass, GI-tract and
blood. Total recovery was about 91%.
About 51 and 43% of the original radioactivity in the rat skin,
hair and blood, respectively were found to be present as 14C which
had been reincorporated into natural amino acids (protein). The
remainder of the radioactivity in tissues has not been identified,
but it was not found as known metabolites of oxamyl.
The radioactivity in the urine was present as a very complex
mixture of compounds. Conjugates of methyl-N-hydroxy-N',
N'-dimethyl-1-thiooxamimidate (I), methyl
N-hydroxy-N'-methyl-1-thiooxamimidate (II), N,N-dimethyloxamic acid
(III) and N-methyloxamic acid (IV), present in about equal amounts,
accounted for approximately 79 and 72% of the elimination products
in urine and faeces, respectively. No oxamyl or other
organosoluble metabolites were detected (Harvey and Han, 1978).
A male Charles River CD rat was given a water supply containing
1540 mg/l of non-labelled plant metabolite methyl
N',N-dimethyl-N[(1-glucosyl)oxy]-1-thiooxamimidate (metabolite A).
Eight days later the rat received by intragastric intubation 4 ml
of an aqueous solution containing 0.67 mg (3.77 µCi) of 14C
metabolite A. About 69% of the total 14C was eliminated in 72
hours: 64% in the urine and 5% in the faeces. The major component
(45%) of the urinary radioactivity was identified as unchanged
metabolite (30% of the original dose), 19% as conjugates of I and
II. Conjugates of III an IV account for a substantial part of the
remainder. Extracts of liver, carcass and blood showed no
radioactivity in the form of I, II or V (< 1%). Evidence was
presented that about 65% of the remainder was incorporated in amino
acids. (Harvey and Han 1978).
A male Charles River CD rat received 450 mg/kg unlabelled
N,N-dimethyl-1-cyanoformamide (DMCF), another plant metabolite of
oxamyl in the diet. Seven days later the rat was given, by
intragastric intubation 4 ml of an aqueous solution containing 1.1
mg (10.7 µCi) of 14C-DMCF. Excretion and distribution were
comparable to those after oxamyl or metabolite A treatment. The
results showed no DMCF or other organosoluble 14C-material in the
urine. However 15% of the total radioactivity of the urine was
accounted for by conjugates of III and 7% by IV, (Harvey and Han,
1978).
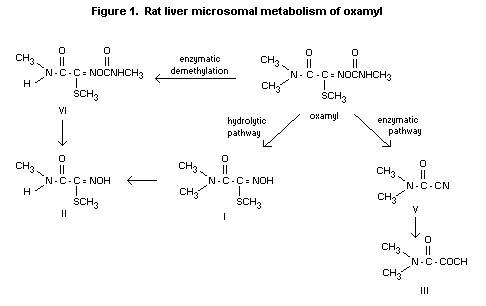
The incubation of oxamyl with rat liver microsomes for 2 hours
resulted in a mixture of six compounds. In addition to intact
oxamyl, substantial amounts of I, III and DMCF (V) were found.
Smaller amounts of the monomethyl analogue of oxamyl (VI) and II
were detected, indicating that N-demethylation was occurring at a
slower rate. The control incubation (no microsomes) produced only
the corresponding oximino compound (I) in approximately the same
amount, suggesting that hydrolysis is not mediated by the liver
enzyme system, but by chemical hydrolysis.
Liver microsome incubation of DMCF (V) produced III as the only
breakdown product. In the incubation of metabolite A, >9O% of the
metabolite A was recovered intact. Likewise, incubation of
metabolite I resulted in less than 1% degradation.
In figure 1 the metabolism of oxamyl by rat liver microsomes is
given (Harvey and Han, 1978; Han and Harvey, undated).
 TOXICOLOGICAL STUDIES
Special studies on cholinesterase activity
Rat
Two groups of 10 male rats were given orally 4.8 mg oxamyl/kg bw.
One group received 50 mg/kg atropine sulphate i.p. immediately
after dosing of oxamyl. Cholinesterase activity in blood was
determined 24 hrs before and 5 min., 4 hrs and 24 hrs after dosing.
The animals without atropine showed a decrease in cholinesterase
after 5 min. with a maximum after 4 hrs. After 24 hrs, the
activity was normal again. Animals that were treated with atropine
showed a slight inhibition initially, but the activity was normal
after 4 hrs. The experiment was repeated with the same result
(Schmoyer and Henry, 1970).
Four groups of 8 ChR-CD rats were fed oxamyl in the diet at dosage
levels of 0 (control), 50, 100 or 150 mg/kg feed during 29 days.
Erythrocyte and plasma AChE were measured at day 1, 7, 21, and 28.
On the 29th day the rats were sacrificed to measure AChE in the
brain.
Eight additional rats were assigned to each group for assay of
brain AChE 14 days after the start of the experiment.
With male animals cholinesterase activity of the plasma was only
marginally decreased in the 150 and 100 mg/kg groups throughout the
study. In the females plasma cholinesterase activity was
dose-related decreased in the 150 and 100 mg/kg groups from day 7
onwards. No clear effects were observed on the AChE activity in
the erythrocytes. At the end of the study cholinesterase activity
in brain tissue of females was dose-related decreased in the 150
and 100 mg/kg groups. After 14 days cholinesterase activity in the
brain was inhibited in the 150 mg/kg group. In summary, 50 mg/kg
in the diet was a no-effect level (Barnes and Aftosmis, 1978).
Special study on delayed neurotoxicity
Chicken
The animals were given various oral doses of oxamyl. The ALD
(approximate lethal dose) was established at 40 mg/kg bw. Two
groups of 5 adult hens received 20 or 40 mg/kg bw respectively
followed by 0.5 mg/kg atropine i.m. No mortality occurred. The
animals were kept for 28 days. They showed marked symptoms of
cholinesterase inhibition but recovery was complete after 12 hours.
As a positive control 1200 mg/kg TOCP was used, followed by
atropine. Two weeks after dosing the animals showed typical signs
of delayed neurotoxicity (Ki Poong Lee, 1970).
Special study on reproduction
Rat
The animals of the long-term feeding study were also used for a
3-generation reproduction study. Groups of 16 males and 16 females
on a diet containing 0, 50, 100 or 150 mg/kg were chosen for
breeding three generations of each two litters. Throughout the
study the litter size, viability and lactation indices and body
weights of weanlings were decreased at 100 and 150 mg/kg. A
marginal effect on the body weight of the weanlings was also
observed at 50 mg/kg. No effect on fertility and gestation indices
was detected. Organ weights of the weanling pups of the F3b
generation showed a slight increase in relative kidney weight at
150 mg/kg. The relative testes weight was increased at 100 and 150
mg/kg. No histopathological abnormalities were found that could be
ascribed to the compound (Sherman et al. 1972).
Special study on mutagenicity
Microorganisms
Oxamyl showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, and reverse mutation tests with and
without a liver activation system using 5 strains of Salmonella
thyphimurium TA and E. coli WP2 hcr. A host-mediated assay
in mice using S. typhimurium G-46 was also negative (Shirasu
et al, 1976).
Special study on teratogenicity
Rat
Groups of 26-28 pregnant females received 0, 50, 100, 150 or 300
mg/kg oxamyl in the diet from day 6-15 of pregnancy. In the
mothers, a dose-related decrease in body weight and food
consumption was found for 100, 150 and 300 mg/kg groups. There
were no effects on number of implantation sites, resorptions and
live foetuses nor on embryonal development. Oxamyl does not show
teratogenic or embryotoxic properties (Culik and Sherman, 1971).
Acute Toxicity
species sex route LD50 in mg/kg bw reference
rat M oral 371 (27-51) Carroll, 1972
rat M oral 1102 (97-185) Gibson, 1973
rat M oral 5.4 (3.7-7.8) Fretz, 1969
rat3 M oral 16 (22) Schmoyer, 1969
rat3 F oral 11 (7-16) Schmoyer, 1969
1 formulation Vydate L (26% ai) in aqueous solution
2 Vydate G (10% ai) in corn oil
3 fasted overnight
Dermal studies
Rabbits
When oxamyl was applied as a suspension in a hydrophillic ointment
for 24 hrs, the ALD (approximate lethal dose) was 2250-5000 mg/kg
bw on the intact skin, but only 90-130 mg/kg on the abraded skin.
When oxamyl was applied as a slurry in propylene glycol the ALD was
130 or 60 mg/kg bw in the intact and abraded skin respectively
(Colburn, 1970).
When Vydate L (27% ai) was applied during 24 hrs the LD50 was 740
± 150 mg/kg bw on active ingredient basis on the intact akin
(Morrow, 1973).
Inhalation studies
Rats
The LC50 (1 h) for oxamyl was 0.035 mg/l air when Vydate L (27% ai)
was used in the test (males only) (Barras 1974). When the
experiment was carried out with oxamyl dust the LC50 (1 h) for male
and female animals was 0.17 and 0.12 mg/l respectively. The
concentration in air was calculated after chemical analysis
(Tayfun, 1969).
Symptoms of intoxication
Fasciculations, tremors, salivation, lacrimation, bulging eyes and
chromodacryorrhoea were found after an acute oral dose of oxamyl.
They can be considered as symptoms of cholinesterase inhibition
(Carroll, 1972).
A good antidotal effect was obtained when rats orally treated with
oxamyl received an i.p. injection with atropine sulphate (Sherman,
1969)
Acute toxicity of oxamyl metabolites in rats
LD50 in
metabolite sex route mg/kg bw reference
methyl N-hydroxy-N,N' dimethyl- M oral 11,000 (=ALD) Fretz, 1968
-1-thiooxamimidate (INA-2213)
methyl N-hydroxy-N' methyl-1- M oral 6,675 (6370- Dale, 1973
thiooxamimidate (IEL-2953) 6690)
N,N-dimethyloxamic acid (IND-2708) M oral 3,540 Barbo, 1972
N,N-dimethyl-1-cyanoformamide M oral 450 (=ALD) Ashley, 1974
(DMCF) (INN-79)
methyl N'-methyl-N [(methyl-carbamoyl) M oral 60 Schmoyer, 1969
oxy]-1-thiooxamimidate (IND-14O9)
glucose conjugate of methyl N'- M oral > 7500 Henry, 1976
methylhydroxy-N',N'-dimethyl
-1-thiooxamimidate (ING-3515)
Short-term studies
Rat
Six male rats were given orally 2.4 mg/kg bw oxamyl for 10 days. No
mortality occurred. Oxamyl does not exhibit cumulative oral toxicity
(Fretz and Sherman, 1968).
Rabbit
Rabbits were treated dermally for 15 days during a period of 4 weeks
with Vydate L (26% ai) in dimethylformamide. Groups of each 5 male
and 5 female animals received 193 mg/kg Vydate L, or 50 mg/kg bw of
oxamyl during 6 hrs/day on the intact or abraded skin. Control
animals received dimethylformamide. All oxamyl treated animals with
abraded skin showed marked signs of cholinesterase inhibition. Only
slight symptoms were seen on the animals with intact skin. No
mortalities occurred. No changes in body weight, organ weight or
histopathology were found that could be ascribed to the treatment.
All animals showed mild erythema of the treated skin (Dion, 1970).
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 100 or 500 mg/kg oxamyl in the diet. After a few days the highest
dose level was reduced to 150 mg/kg because with 500 mg/kg all animals
showed strong symptoms of cholinesterase inhibition. They were given
control diet for 3 days and the symptoms disappeared or became less
severe. Then the animals were given 150 mg/kg. No clinical signs of
toxicity were seen in the remainder of the experiment. The body
weight gain was lower at 100 and 150 mg/kg and slightly affected in
the females of the 50 mg/kg group. The food consumption was lower at
150 mg/kg only. Urinalysis showed a higher number of animals with
blood in urine at 100 and 150 mg/kg and a higher number with protein
at 150 mg/kg. Male rats showed lower absolute organ weights for
heart, kidneys, liver, spleen and thymus at 100 and 150 mg/kg. In the
females this effect was found for the liver at 100 and 150 mg/kg and
the kidneys and lungs at 100 mg/kg. No effects were found on
haematology, clinical chemistry and histopathology. Cholinesterase
activity was not determined. The marginal no-effect level is 50 mg/kg
(Snee et al, 1969).
At the end of the experiment 6 animals per group per sex were used for
reproduction performance. In the F1a and F1b generation the
fertility index and the number of pups/litter were decreased at 100
and 150 mg/kg. The weight of the young at weaning was significantly
lower at all dose levels (Snee et al, 1969).
Dog
Groups of 4 male and 4 female animals each received for 90 days 0, 50,
100 or 150 mg/kg oxamyl in the diet. Only marginal effects were found
with 150 mg/kg. A slight increased activity was found for alkaline
phosphatase after 4 and 13 weeks. In the males the weight of
adrenals, spleen and testes was somewhat decreased. The females
showed a slight increase in thyroid weight and a decrease in adrenal
weight. Histopathologically no abnormalities were found that could be
ascribed to the compound (Holsing, 1969).
Groups of 4 male and 4 female animals each received for 2 years 0, 50,
100 or 150 mg/kg oxamyl (95%) in the diet. After 1 year 1 male and 1
female animal from the control and 150 mg/kg groups were killed.
Haematology, clinical chemistry and urinalysis were carried out after
1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 months. The haemoglobin content,
haematocrit and number of erythrocytes in blood at 150 mg/kg were
found to be lower than the controls on most times of measurement in
both sexes.
Higher values for cholesterol and alkaline phosphatase activity were
found more often at 150 mg/kg in males and females. On some occasions
cholesterol was also higher in the other experimental groups.
No effects on organ weights, cholinesterase or aliesterase activity,
or histopathology were found that could be ascribed to the compound
(Sherman, et al, 1972).
Short term study with DMCF, a metabolite of oxamyl
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 150 or 450 mg/kg N,N-dimethyl-1-cyanoformamide (DMCF) in the diet.
Growth inhibition was found at 450 mg/kg in males and females, and the
food consumption was also lower. At the highest dose level
haemoglobin content, haematocrit and number of erythrocytes were
lower. At 150 mg/kg a slight effect was found on these parameters in
the females or males. In the male animals an increased LDH activity
was found at 450 mg/kg and in the female animals a decreased activity
in GPT, GOT, and LDH at the same dose level. Organ weights were not
reported in this study. Histopathologically an increase was found in
hydronephrosis of the kidneys in the males and minute foci of
mineralization in the kidneys of females at 450 mg/kg. The animals of
the other dose levels were not studied histopathologically. The
no-effect level is probably 50 mg/kg (Kaplan, 1976).
At the end of the experiment 6 animals per group were used for
reproduction performance. With 450 mg/kg a decreased weight of the
young at weaning was observed (Kaplan, 1976).
Long-term studies
Rat
Groups of 36 male and 36 female animals each received for 2 years O,
50, 100 or 150 mg/kg oxamyl (95%) in the diet (2 control groups were
used). After one year of feeding the number of rats of each sex in
each group was reduced to 30. With 100 and 150 mg/kg a decreased body
weight was found with male and female animals that was related to
dose. With 50 mg/kg the male animals showed a slightly lower body
weight, which was apparent after 4 weeks and remained so throughout
the study. Food consumption was lower at 150 mg/kg. Cholinesterase
activity in blood in the 150 mg/kg group was only decreased in the
females after 4 days of feeding and in the males after 8 days, and not
after 1, 6, 12 and 24 months. Aliesterase activity was not affected
throughout the study.
At the end of the experiment relative weights of brain, testes and
adrenals were increased in the males and brain, heart, lungs, kidneys
and adrenals in females at 150 mg/kg. In the females most or these
organ weights were also increased at 100 mg/kg. These changes are
probably connected with the decreased body weight. No haematological,
biochemical or histopathological abnormalities were found that could
be ascribed to the compound (Sherman et al, 1972).
RESIDUES IN FOOD
USE PATTERN
Oxamyl formulations, either as a 24% liquid or 10% granular, are
recommended or registered for use in numerous countries. Food uses
made available to the Meeting by the manufacturer are summarized in
Table 1. Proposed uses are not included but are discussed under
appropriate commodities when residues are discussed.
According to information provided to the Meeting, uses listed for the
United States also apply to Argentina, Barbados, Bolivia, Brazil,
Chile, Colombia, Costa Rica, The Dominican Republic, Ecuador,
Guatemala, Mexico, Nicaragua, Peru, El Salvador, Trinidad and Tobago,
Uruguay and Venezuela unless indicated otherwise in Table 1. Only a
few national governments provided usage information directly to the
meeting.
The liquid formulation is a contact-type, moderately residual
insecticide when applied as a foliar spray. When so applied it is
effective on several species of mites and, by systemic action in many
plants, on nematodes. When oxamyl formulations are applied to the
soil they function as a contact type broad-spectrum nematocide and, by
systemic action, as a miticide/insecticide.
Applications may be pre-planting, at planting, or foliar (to
fruit-bearing or non- fruit-bearing plants). They may be applied in
the furrow or broadcast. Soil incorporation may be mechanical or by
irrigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
There are numerous combinations of type of formulation, method and
time of application suitable for a wide range of pest control needs.
Because of this, the residue level can vary widely, even on the same
commodity. A large volume of data on 29 commodities or groups of
commodities was presented to the meeting; this has been considerably
condensed into Table 2 (Du Pont, 1980).
The analytical method used extracts both oxamyl and its oxime
metabolite (I), converts the oxamyl to the oxime which is quantitised,
so the residue levels quoted are of oxamyl plus the oxime expressed as
oxamyl. Data on residues of the DMCF metabolite are in parentheses.
TABLE 1. Recommended uses for oxamyl1
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation2,3 treatments interval (wks) notes
Apples
non-bearing U.S.A. 0.28-2.2 24% Liq. 52
6.7 - 9 pre-plant, soil incorporation
bearing 0.28-2.2 2 do not graze treated orchards
non-bearing Greece 2-4 10% G 52 soil
1.2 24% G multiple 52 foliage
Bananas Greece 3 g/plt 10% G 3 at planting
2-3 g/plt 2 on plantations (around plant)
0.01% 24% Liq. foliage
Central Amer. 0.9-1.3 24% Liq. 5-8 foliar
Spain 3.8 24% Liq. in irrigation water
Beans Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, broadcast glasshouse and
in open
1.2 24% Liq. 2 2 spray, broadcast glasshouse and
in open
Beets (roots Denmark 0.7-1.8 10% G row, at planting
or tops) 2.5-5 10% G broadcast, before planting
Carrots Greece 3-4 10% G 2 pre-plant, broadcast
2-3 10% G 2 furrow
1.2 24% Liq. 2 2 spray
1.2 24% Liq. multiple 52 foliage
Celery Greece 3-4 10% G 2 preplant broadcast, glasshouse and
in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
USA 0.56-2.2 24% Liq. 2 untrimmed
4.4 24% Liq. 2 pre-plant soil incorporated
Citrus Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
USA up to 1.1 1 do not graze treated orchards
(0.008-0.03%)
Cherries Greece 2-4 10% G 52 soil non-bearing
1.2 24% Liq. multiple 52 foliage non-bearing
Cucumbers Bulgaria 0.024% 24% Liq. multiple 2 foliage (greenhouse)
Czechoslovakia 0.1 g/plt. 1 2 glasshouse
Netherlands 5 10% G glasshouse, shortly before planting
Cucurbits Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2 24% Liq. 2 2 spray, glasshouse and in open
USA 0.56-2.2 24% Liq. 2 untrimmed
Eggplant Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
Onions Denmark 0.7-1.8 10% G row, at planting
2.5-5 10% G broadcast, before planting
United
Kingdom 13-4.9 10% G furrow, at planting
Peaches Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Pears Greece 2.4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
Peas United 2.5 10% G pre-plant
Kingdom
Peppers Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt 10% G 2 2 glasshouse
Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2spray, glasshouse and in open
Pipfruit N. Zealand 0.048-0.06% 24% Liq. 2 1
Potatoes Denmark 2.5-5 10% G 1 broadcast, pre-planting
Greece 3-4 10% G 2 pre-plant, broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Libya 4-5.5 10% G pre-plant, soil
1.7 24% Liq. 3 (foliar) 4 soil, pre-plant and foliar
Mexico 0.24-0.72 24% Liq. multiple 1 foliage
Netherlands 10% G before or at planting
N. Zealand 5 10% G 13 pre-plant broadcast
United
Kingdom 4-5.5 10% G pre-plant, soil
U.S.A. 0.28-1.1 24% Liq. 1 foliar
Squash Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Strawberries Denmark 0.48 24% Liq. 3 after final harvest
Greece 2-4 10% G non-bearing
1-2 24% Liq. multiple non-bearing
Sugarbeets Greece 0.8-1 10% G at planting
0.18 24% G foliage
Netherlands 0.75 10% G at sowing
Spain 0.8 10% G in row
Syria 2.4-3.6 24% Liq. 1 at plant
1.8 24% Liq. 1 foliar
United
Kingdom 0.6-0.9 10% Liq. in furrow at planting
Tomatoes Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt. 10% G 2 2 glasshouse
Denmark 4.8 24% Liq. field, preplant
0.05% 24% Liq. field, preplant
0.07% 24% L1q. 4 wk. after planting
Egypt 1.8 24% Liq. 4
Greece 3-4 10% G 2 preplant broadcast,
glasshouse or in open
2-3 10% G 2 furrow, glasshouse
or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Netherlands 5 10% G glasshouse, shortly before
planting
Jordan 1.8 24% Liq. 2 4 foliar
Libya 3 10% G 1 4
1.7 24% Liq. 2 4
Mexico 0.06-0.12% 24% Liq. 1 (day) foliar
Syria 1.8 24% Liq. 2 foliar (seedlings)
United
Kingdom 0.1 g/plt 10% G 2 2
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
U.S.A. 0.56-1.1 24% Liq. multiple 1 (day) (AL, FL, MD, NJ, PA, P.R.,
SC, VA) foliar
2 (day) (HI) foliar
3 (day) (CA) foliar
4.5-9 24% Liq. 1 (day) (HI, soil, by
drip irrigation)
1 Most listed U.S. uses are special local need registrations, limited to specific geographical areas.
2 G = granular
3 Liq. = liquid
TABLE 2. Oxamyl residues from supervised trials (DMCF in parentheses)
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Apples N. Zealand 1980 1 0.03-0.06% 24% EC 1 0.1-0.2 2
2-3 0.03-0.11 2
6-8 N.D.1 2
12-14 N.D.1 2
U.S.A. 1972-75 2-6 0.28 24% Liq. 2-3 0.19-0.2 (<0.02) 2
(10 states) 4-5 0.21-0.36 (<0.02) 2
9-11 0.11-0.21 (<0.02) 3
2 0.67 24% Liq. 11 0.12 1
1-3 0.76-1.1 24% Liq. 2-3 0.32-0.84 (0.03-0.05) 5
4-5 0.1-0.23 2
6-8 0.09-0.68 (<0.02-0.04) 14
12-14 1.2 1
1-2 1.7-2.2 24% Liq. 2-5 0.31-1.5 3
6-8 0.05-1.4 (<0.02-0.03) 4
12-17 0.14-1.1 (<0.02-0.04) 5
21-30 0.27 1
1 4 24% Liq. 2-3 1.9 1
6-8 1.2-1.4 2
12-14 0.99-1.0 (0.05-0.15) 1
Banana2 Costa 1975-77 1-8 0.9-2.2 24% Liq. 1-10 <0.02 (0.02) 10 bagged or unbagged,
Rica3 (aerial) whole fruit or edible peel
1 0.05 limit of determination
2 Cavendish and Giant Cavendish
3 five farms
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Beans U.S.A. 1975-77
(succulent, green
snap, lima) Beans Foliage-hay
preplant 1 2.2-4.51 10% G 67 <0.02
foliar VA 1 0.28 24% Liq. 2-3 0.1 (<0.04) 1
6-8 0.02 (<0.04)
VA, FL, CA 1-8 0.56 24% Liq. 1 0.05-0.84 (<0.04) 2 2.5 (<0.04)
2-3 <0.02-5.3 (<0.04) 10 1-14 (<0.04-0.08)
6-11 <0.02-0.97 (<0.04) 9 0.24-16 (<0.04)
12-14 <0.02-0.65 (0.04) 8 0.26-5.7
CA, FL 5-8 1.1 24% Liq. 1 1.9 1 6.7 (0.14)
2-3 <0.02-7.7 (<0.04-0.19) 10 3.2-29 (<0.04-0.41)
6-9 <0.02-2.3 (<0.04-0.17) 8 0.59-33 (<0.04-0.36)
11-14 <0.02-1.0 (<0.04-0.09) 10 0.08-15 (0.06-0.20)
CA, FL 5-8 2.2 24% Liq. 1 6.5 (0.35) 1
2-3 0.04-13 (0.04-0.37) 9 77 (0.66)
6-8 0.1-7.3 (<0.04-0.31) 8 0.91-67 (<0.04-0.83)
12-14 0.05-2.0 (<0.04-0.09) 9 0.06-37 (0.06-0.67)
CA 5 4.5 1 8.9 (0.84) 1
2-3 12 (0.54) 1
12-14 0.28 (<0.04) 1
Beans Beans Straw
(dry-pinto, WY 1977 1 0.56 24% Liq. 50 <0.02 (<0.04) 0.04 (<0.04)
navy, dry) 1 1.1 50 <0.02 (<0.04) 0.18 (<0.04)
vines
CA, MI 1973-78 1.1-2.2 10% G 103-111 <0.02 (<0.04) <0.02-0.08 (<0.04)
3.4 (preplant) 111-132 <0.02 (<0.04)
1 12 inch band incorporated
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Range, mg/kg
Beets, red U.S.A. 1973 1 3.4-6.7 G 84 <0.02 soil
2 1.1 Liq. 48 <0.02 foliar
1 3.4-6.7 G 84 <0.02 soil
+ + + +
2 1.1 Liq. <0.02 foliar
Cantaloupe U.S.A.
GA 1971 1 4.5 10% G 80 0.05
4.5 10% G
+ +
1.1 2 Liq. 39 <0.02
No. in range
CA, FL 1976-78 5-8 1.1 (foliar) 2 L 1 0.04-0.25 4
2-3 0.19-0.26 3
4-5 0.05 1
6-8 0.06-0.16 4
FL, IN, CA 1976-78 5-8 1.1 (foliar) 2 L 1 0.1-0.62 (<0.02-0.03) 5
2-3 0.12-0.48 (<0.02-0.04) 4
4-5 0.06-0.19 (<0.02) 2
6-8 0.07-0.59 (<0.02-0.05) 6
AZ, CA, IN 1976-78 5-8 2.2 (foliar) 2 L 1 0.23-0.91 (0.05-0.1) 3
FL 2-3 0.03-0.66 (0.66) 5
6-8 0.05-0.5 (0.04-0.05) 3
IN 1974-78 2-5 4.5-6.7 2 L 1 0.44-0.69 (0.05-0.08) 2
(foliar) 2-3 0.12-0.52 (0.04-0.08) 2
6-8 0.3 (<0.02) 2
41-51 0.02 1
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
IN 1978 5 9-13 2 L 1 0.49-1.4 (0.07-0.2) 2
(foliar) 2-3 0.44-1.4 (0.02-0.09) 2
6-8 0.41-0.87 (0.02-0.8) 2
FL 1972 1 2.2 2 L
+ +
1.1 2 L 31-40 0.18 (<0.02)
Carrot U.S.A.
preplant OH, MI 1975 1 3.4-4.1 unspecified 120-135 <0.02
soil MI, OH, FL 1970-73 1 3.4-6.7 G. or Liq. 103-185 <0.02 (<0.02)
DE
foliar DE 1971 5 0.56 Liq. 18 <0.02 (<0.02)
soil OH 1973-74 1 3.4-6.7 G. or Liq. 122-185
+ + + +
foliar 3-5 0.56-2.2 Liq. 22 or unspecified <0.02 (<0.02)
Celery U.S.A. (FL) 1974 3 0.56 Liq. 4-5 8.1 1
6-8 6.6 1
12-14 0.88 1
1974 5 1.1 Liq. 1 3.6-24 3
4-5 0.99-8.1 (0.81) 3
6-8 0.66-6.6 6
1.5-1.7 (trimmed) 2
12-14 0.12-1.5 4
0.94 (trimmed)
15-20 0.36-0.39 2
0.27-0.33 (trimmed) 2
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1974 3-5 2.2 Liq. 4-5 20 1
12-14 0.86-3.1 3
2.4 (trimmed) 1
15-20 1.3-1.4
grapefruit lemons oranges tangelos tangerines
Citrus2 U.S.A. 1971-75 1-5 0.59-1.11 Liq. 1 0.14-0.25 0.21-0.29 0.13-0.6
AZ,CA,FL,TX (foliar) 2-3 0.03-2.1 0.17-0.25 0.02-1.1
4-5 0.11 0.18 <0.04
6-8 0.03-1.2 0.05-0.17 0.02-0.8
9-11 <0.02 <0.02
12-14 0.03-0.08 0.06-0.37
15-21 <0.04-0.3
2-7 1.1 90% sol. powder 84 0.04
105 0.14
1 1.4-1.51 Liq. 2-3 3.6 0.51
(foliar) 4-5 .42
6-8 1.2-2 0.34
12-17 0.46-0.8 0.06
1-6 2.1-2.2 Liq. 1 1.3 0.39 0.26-0.36
or 3 2.2+4.5+2.2 2-3 <0.02-0.67 0.29 <0.02-0.52 <0.02-0.1
(foliar)1 4-5 <0.02-0.84 0.04 <0.02-0.92 0.06-0.88
<0.02-0.57
6-8 <0.02-0.3 0.04 <0.02-3 0.02-0.24 <0.02-0.4
9-14 <0.02 <0.02-0.87 0.02
21 <0.02
1 presumed on the basis of proposed label
2 DMCF is <0.06 mg/kg in fruit, peel or pulp except for a 0.54 value at high dosage (4-5 days)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1971-75 3 2.2+4.5+4.5 Liq. 1 1.5 0.55 2.-4.8
1-3 4.2-4.5 2-3 0.08-1.1 0.36 0.22-4.4
(foliar)1 4-5 0.07-0.64 0.32 0.25-3.6 0.88
6-8 0.05-0.64 0.36 0.13-2 0.24-0.36
12-14 <0.02 <0.02-0.67
21 0.03
84 0.04
Corn, field USA 1970-75 1 0.56-4.5 Liq. 93-198 <0.02 (<0.02) 27 (kernal or stalk)
(GA,FL,NC) (band or furrow)
Cottonseed USA 1969-75 1 0.06-0.28 Liq. 51-75 <0.02
(AZ,TX,CA,NC) (foliar)1
1-5 0.37-0.56 Liq. 6-8 <0.02-0.09
(foliar)1 12-17 <0.02-0.13
1-5 0.75-1.1 Liq. 1 25 1
(foliar)1 4-5 0.75 1
6-8 0.02-0.17 19
12-17 <0.02-0.18 19
1-5 1.7-2.2 Liq. 6-8 0.03-0.26 12
(foliar)1 12-14 <0.02-0.17 7
15-17 0.02-0.05 2
146 0.02 2
3-5 4-5 Liq. 6-8 0.25-0.64 4
(foliar)1 15-17 0.02 1
Cucumber U.K. 1973 - 5 unspecified unspecified 0.41-0.68 2
U.S.A.
preplant VA 1977 1 2.2-4.5 10% G 51-75 0.15-0.47 (<0.02-0.06) 2
1 9 10% G 76-100 <0.02
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
foliar FL,VA,CA 1973-78 2-7 0.56 2 L 1 0.15-0.36 5
2-3 0.18-0.38 3
6-8 0.18-0.28 4
12-14 0.07 1
31-40 0.02 (<0.02) 1
FL,CA,VA 1973-78 2-7 1.1 2 L 1 0.22-0.54 (0.1-0.14) 5
2-3 0.26-0.39 (0.03-0.15) 3
6-8 0.26-0.38 (0.07-0.19) 5
12-14 0.15 1
31-40 0.03 (0.02)
AR,CA,FL,VA 1974-78 1-7 2.2 2 L 1 0.45-1.1 (0.14-0.19) 4
2-3 0.03-0.7 (0.23-0.24) 4
4-5 <0.02 1
6-8 0.02-0.78 (0.12-0.25) 5
12-14 0.16 (0.02) 1
41-75 0.05-0.08 (<0.02) 2
1972-76 1-7 4.5 2 L 1 0.87-2.2 (0.17-0.13) 2
6-8 0.84-1.8 (0.25-0.35) 2
12-14 0.32 (0.03) 1
31-40 0.02-0.26 (<0.02-0.07) 2
51-75 <0.02 1
glasshouse Netherlands 19791 1 5 10% G 42 0.16-0.28 (av. 0.21)
1 10 10% G 42 0.08-0.33 (av. 0.27)
2 5 250%g/l 14 0.37-0.52 (av. 0.43)
Honey dew USA 6-9 0.56 2 L 1 0.2-0.24 3
FL 1976-78 (foliar) 2-3 0.18-0.28 2
6-8 0.19-0.23 3
FL 1976-78 1.1 2 L 1 0.36-0.4 (<0.02-0.03) 3
(foliar) 2-3 0.39-0.5 (<0.02-0.03) 2
6-8 0.32-0.44(0.02.-0.03) 3
1 CIVO report R 6282 (1979)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
AZ,CA,FL 1976-78 5-9 2.2 2 L 1 0.68-0.71 (0.04) 2
(foliar) 2-3 <0.02-0.68 (0.05) 3
4-5 <0.02 1
6-8 <0.02-0.7 (0.06-0.1) 3
FL 1976 9 4.5 2 L 1 1.0 (0.04) 1
(foliar) 6-8 0.92 (0.06) 1
Onions U.K. 1972 11 0.84-3.4 10% G1 unspecified <0.04
Netherlands 1977 2 0.41 3.3% G 100-150 <0.01 12
nuts hulls hay (dry)
Peanuts U.S.A. 1971-76 1 1.7-5 (soil) 10% G 76-100 0.03 0.07
(OK,TX,VA, 101-150 <0.02 <0.02 <0.02-0.38
GA,NC) 151-200 <0.02-0.04 <0.02-0.1 <0.02-0.04
1 1-4.5 10% G 12-14 <0.02 <0.02 <0.57
+ (soil) + 51-75 <0.02-0.03 <0.02 <0.02-0.07
2-3 0.56-1.7 24% Liq. 76-100 <0.02 0.04 0.91
1 1.7-2.8 24% Liq. 76-100 <0.02 <0.02 <0.26-1.0
(soil) 100-200 <0.02 <0.02
2-3 0.56-1.7 24% Liq. 6-8 <0.02 0.05 0.06
(foliar) 21-30 <0.02 <0.01 0.04
76-100 <0.02
pea pod
Peas U.K. 1.4-2.8 10% G unknown <0.01 <0.01
(preplant)1
11.1 10% G unknown <0.01 0.15
(preplant)1
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Pepper U.S.A. 1975-78 4-18 0.561 24% Liq. 1 0.04-0.88 6
(AL,CA,FL) (foliar) 2-3 0.15-0.58 (<0.02-0.04) 5
4-5 0.47-0.7 (0.08) 3
6-8 0.11-0.7 (<0.02-0.003) 7
4-18 1.1 24% Liq. 1 0.23-1.1 (0.03-0.12) 7
(foliar) 2-3 0.14-1.0 (0.03-0.06) 6
4-5 0.62-1.5 (0.14) 2
6-8 0.15-1.3 (0.03-0.14) 6
5-6 2.2 24% Liq. 1 1.3-3 (0.04-0.08) 3
(foliar) 2-3 0.46-1.9 (0.04-0.46) 2
4-5 1.4 1
6-8 1.2 (0.07) 1
wholefruit bran leaves hay DMCF fruit2
Pineapple U.S.A. 1975-76 1-6 1.1 24% Liq. 13-14 <0.02-0.46 0.04-0.57 0.26-0.55 0.11-0.46
Hawaii 23-27 <0.02-0.43 0.28-1.7 <0.02-0.22 0.14-1.2 (<0.02)
35-42 0.03-0.09 0.06-0.15 (<0.02)
2.2 24% Liq. 13-14 0.03-1.1 0.10-3.5 0.76-1.5 0.27-1.3
23-27 0.02-0.59 1.1-3.2 0.04-1.4 0.55-1.2 (<0.02-0.04)
35-42 <0.02-0.36 0.02-0.82 (<0.02)
4.5 24% Liq. 13-14 0.13-2.5 0.22-3.0 1.9-6.8 0.75-2.3
23-27 <0.02-0.91 2.5-5.2 0.02-0.70 1.9-8.5 (<0.02-0.1)
9 24% Liq. 14 0.14-1.2 0.57-1.7 2.4-7.3
23 0.17-0.46 0.3-1.9 (0.09-0.2)
Potatoes S. Africa 1974 4-5 0.24% 24% Liq. 51-75 <0.002-0.033 plant water and/or foliar
U.K. 1973 13 5.6 10% G3 unspecified <0.02-0.03
1 with and without 0.56 kg ai/ha in transplant water
2 DMCF residues in pineapple leaves were generally compared to those in the fruit
3 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
(soil) USA (DE) 1969-71 1 1.1-3.4 10% G 76-150 <0.02 pre-plant, soil incorporated
NY, FL, MI 1966-75 1 3.4-6.7 10% G 104-106 <0.02
FL 1974-75 1 10.1-13.4 24% Liq. 21-30 <0.02 broadcast, soil incorporated
FL 1974 1 3.4-4 24% Liq. 100-150 <0.02
(foliar) DE 1969-74 3-6 0.21-0.28 24% Liq. 6-20 <0.02
CA, DE 1969-74 3-13 0.43-0.56 24% Liq. 1-5 <0.02
6-8 <0.02-0.10
12-14 <0.02-0.03
18-30 <0.02
CA,DE,FL,MI 1972-75 1-5 1-1.1 24% Liq. 1 <0.02
2-3 <0.02-0.03
4-14 <0.02-0.03
18-30 <0.02
CA, DE 1969-72 1-5 2.2-3.4 24% Liq. 4-5 <0.02
2-3 0.02-0.05
4-5 <0.02-0.02
6-14 <0.02-0.06 high value from low dosage
DE, CA 1969-74 1-5 4.5 24% Liq. 4-5 <0.02
6-8 0.05-0.11
12-14 0.09
(soil + MI 1974 1 + 3.4-6.8+ G + 100-150
foliar) 1.7 1.1-2.2 24% Liq. 6-8 <0.02
CO 1973-74 1 + 3.4-6.7 G + 100-150
3 1.1 24% Liq. 6-40 <0.02-0.02
DE 1971 1 2.2 10% G soil incorporated
2 (foliar) 24% Liq. 18 <0.02
(dose unspecified)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
soil straw
Soybeans U.S.A. (TN,MO 1970-77 1-3 0.56-6.73 10% G 113-185 <0.02-0.051 <0.02-0.052
(dry) VA,AL,FL,NC;
25 tests)
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02
summer 4-8 <0.02
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02-0.06
vining 4-8 <0.02
tops roots
Sugarbeets U.K. 14 0.6-0.95 10% G4 unspecified 0.05 0.04
U.S.A. (GA,NC 1 0.56 Liq. 76-100 <0.02 transplant water
LA,VA,NY) 1+1 0.56+1.1 Liq. 76-100 <0.02 transplant water+foliage
1 4.5-9 G 76-200 <0.02 soil
1 4.5 Liq. 100-150 <0.02 soil
2 Liq. 51-75 <0.02 foliar
1+ 0.56-9 + G + 75-100 <0.02 soil +
2 1.1 Liq. 51-75 <0.02 foliar
Sugarcane S. Africa Data provided were not useful because of uncertainty of dosage rates.
Sweet potato U.S.A. (GA, 1970-74 1 4.5-95 24% Liq. 100-150 <0.02 (<0.02)
NC,VA,ML) preplant-row and broadcast
1 only 1 of 25 results was at 0.05 mg/kg
2 only 1 of 7 results was at 0.05 mg/kg
3 all but one application were at planting or 1 day prior
4 presumed on the basis of proposed label
5 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
Watermelon U.S.A. 1976-78 5-8 0.56 2L (foliar) 1 0.05-0.29 (<0.02)
(FL,CA) 2-3 0.27
6-8 0.03-0.28 (0.02)
FL 1976-78 8-9 1.1 2L (foliar) 1 0.38-0.77 (<0.02)
2-3 0.47 (0.02)
6-8 0.32-0.56 (0.02)
AZ,CA,FL 1976-78 5-9 2.2 2L (foliar) 1 0.1-1.2
2-3 <0.02-0.87 (0.05)
4-5 <0.02
6-8 0.05-0.78 (<0.02-0.1)
Tomato Netherlands1 1979 1 5 10% G 5 0.02-0.11
(glasshouse)
1 10 10% G 5 0.04-0.23
(glasshouse)
2 5 250 g/l 5 0.32-0.57
preplant broadcast
5.6 10% G 51-150 0.11-0.132
U.K. 1974 11.2 10% G 0.05-0.12
post plant broadcast
5.6-6.7 10% G 12-14 0.66
21-30 0.012
9 10% G 21-30 0.092
11.2 10% G 21-30 0.52
preplant + postplant broadcast
5.6+5.6 10% G 12-14 0.152 (0.85)
11 + 5.6 10% G 12-14 0.072
11 + 11 10% G 12-14 0.78
1 CIVO report R 6282 (1979)
2 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
unspecified application method
2.2-5 10% G1 unspecified 0.06-0.19
10.1 0.07-18.7
U.S.A. 1974-75 5-11 0.56 24% Liq.1 1 0.14-0.44 6
FL, 10 (foliar)1 2-3 0.04-0.31 6
locations 4-5 0.08-0.35 6
6-8 0.25 1
5-12 0.84-1.1 24% Liq.1 1 0.26-1.0 (0.21) 11
(foliar)1 2-3 0.1-0.71 10
4-5 0.11-0.55 10
6-8 0.34 1
5-11 2.2 24% Liq.1 1 0.82-1.9 6
(foliar)1 2-3 0.15-0.78 7
4-5 0.24-0.49 6
6-8 0.24 1
5 4.5 24% Liq.1 1 0.57 1
(foliar)1 4-5 0.35 1
6-8 0.37 1
1 0.05 limit of determination
Apples - Maximum oxamyl residues from approved dosage rates were 1.5
mg/kg at 2-5 days after application and 1.2 mg/kg at the interval of 2
weeks recommended for uses on bearing trees although the application
rate at the latter residues level was less than the maximum permitted.
Residues of DMCF ranged from 0.02-0.05 mg/kg from approved use. There
was no data from uses on non-bearing trees although a one-year
interval is applied for that use.
Bananas - Residues of oxamyl or DMCF were <0.02 mg/kg from
application rates up to 1.7 times maximum recommended dosage in whole
fruit (bagged or unbagged) or edible pulp. The data reflect foliar
applications only.
No residue data were provided for non-foliar uses on bananas.
Beans - Although no residue data were available from the single
country for which recommended usage information was available, data
encompassing those rates were available from trials in the U.S. where
similar uses and a 7-day pre-harvest interval have been proposed.
Residues of oxamyl from pre-plant uses on succulent beans were <0.02
mg/kg although such data were quite limited. Maximum residues of
oxamyl from recommended foliar rates were 1.0, 11 and 15 mg/kg in
succulent beans, foliage, and hay respectively and 0.09, 0.15 and 0.20
mg/kg respectively for DMCF, all at a 14-day interval. At 7 days
maximum oxamyl residues were 2.3, 33 and 25 mg/kg respectively on
succulent beans, foliage and hay. A 14-day pre-harvest interval is
recommended for beans but not specified for forage-hay. An 18-day
pre-graze or pre-forage interval is proposed in the U.S.A.
Oxamyl residues on dry beans from rates reflecting recommended uses
were <0.02 mg/kg and were 0.18 and 0.08 mg/kg on straw and vines
respectively, although most such applications were pre-plant.
Residue data from foliar uses are needed on dry bean varieties and
additional residue data from pre-plant uses on succulent beans,
including additional data on feed items from these uses. Such data
should come from other countries as well as from the U.S.A. The
potential for residues in meat and milk warrants complete data on feed
items.
Celery - Good agricultural practice information (both pre-plant and
foliar) was available from 2 countries although of these only residue
data from foliar treatments in the U.S.A. were available. Maximum
residues at the recommended 2-week pre-harvest interval were 3.1 and
2.4 mg/kg respectively for untrimmed and trimmed celery. Residue data
from other countries would be desirable.
Citrus - (grapefruit, lemons, oranges, tangelos, tangerines) - Residue
data on these citrus crops were available from the U.S.A. reflecting
foliar good agricultural practices. No data were available from good
agricultural practices on non-bearing citrus trees. The maximum
residue on citrus from recommended usage was 1.2 mg/kg on grapefruit
from only one application at the recommended 7-day pre-harvest
interval and this at an application rate 0.7 kg ai/ha, thus less than
the maximum 1.2 kg ai/ha recommended. At 1.5 kg ai/ha maximum
residues, again on grapefruit, were 2 mg/kg at 7 days from only one
application. Residues on oranges from application rates 2 times the
maximum recommended rate were 3 mg/kg. Residues from multiple
applications at recommended rates would be expected to be less than 3
mg/kg.
No additional residues of oxamyl were released when citrus samples
were subjected to ß-glucosidase enzyme treatment.
Corn field - Good agricultural practice information was not provided
for corn although a 0.56-2.2 kg ai/ha at planting 24% liquid
formulation use is proposed in the U.S.A. Residues from applications
up to 2 times this rate resulted in oxamyl residues of <0.02 mg/kg in
the kernels and stalk at 93-198 days after treatment. Only 3 analyses
were made on stalks. Additional residue data are needed for forage
and fodder as well as additional residue data from major corn
producing areas.
Cottonseed - Good agricultural practice information was not provided
for oxamyl uses on cotton although uses at 0.028-1.1 kg/ha foliar
applications of a 24% liquid formulation and a 21-day PHI interval
have been proposed in the U.S.A. Residue data reflecting these uses
were available with residues up to 0.17 mg/kg near the proposed 21-day
pre-harvest interval. Residues would not be expected to exceed 0.2
mg/kg. No DCMF residue was detected in cottonseed meal or oil.
Cucurbits - (cantaloupe, cucumbers, honeydew, summer squash, vining
squash, watermelons) - The only available registered use information
for cucurbits were on greenhouse cucumber, in Bulgaria, Czechoslovakia
and the Netherlands and for cucurbits (greenhouse and in the open) in
Greece. No residue data were provided from these countries except the
Netherlands although residue data on the above cucurbits were
available from the USA where pre-plant and foliar liquid formulation
uses with a 1-day pre-harvest interval are proposed, the former at
2.2-4.5 kg ai/ha and the latter proposed use at 0.56-1.1 kg ai/ha.
The proposed foliar treatment rate is therefore comparable to the
Greek spray uses. Some data from pre-plant granular trials in the
U.S.A. were also provided but none for the proposed pre-plant liquid
formulation uses.
Maximum residues on cucurbits at the recommended 1.2 kg ai/ha foliar
rate were 0.77 and 0.48 mg/kg at 1 and 3 days respectively and 0.47
mg/kg for granular pre-plant treatment at 66 days; for a combined
pre-plant-foliar application a maximum residue of 1.24 mg/kg was
observed. Foliar residues on the various cucurbits were generally
comparable at comparable intervals and application rates. Residue
data from the U.K. were of little value because of lack of knowledge
of the interval, formulation and method of application.
Residue data resulting from recommended uses in countries other than
the U.S.A. (including glasshouse uses) are needed as well as
additional data from pre-plant uses (including liquid formulations).
Onions - Only limited data were available. Maximum residues from
apparent at-plant applications at less than the maximum recommended
rates were <0.04 mg/kg at 100-150 days or at intervals unspecified.
Data from other countries are needed.
Peanuts - Information on recommended good agricultural practices for
oxamyl on peanuts was not provided although pre-plant application of a
10% granular formulation at 3.4-5 kg ai/ha and 24% liquid formulation
at 3.4-5.6 kg ai/ha are proposed uses in the U.S.A. No specific
pre-harvest interval is proposed. Also proposed is a 1.1 kg ai/ha
foliar application of the 24% liquid formulation. Residue data from
the U.S.A. reflecting these proposed rates were provided. Maximum
residues were 0.04 mg/kg in hulls and 1.0 mg/kg in hay. Combining
maximum residues from separate soil and foliar uses results in 0.06
mg/kg for nuts, 0.15 for hulls and 1.6 for hay. Residues would not be
expected to exceed 0.1, 0.2 and 2 mg/kg in the nuts, hulls and hay
respectively.
Peas - Only very limited data were available and it was on an
unspecified pea variety. Maximum residues from recommended granular
pre-plant application rates were <0.01 mg/kg in pea or pod although
the interval was not given. At over 4 times the recommended granular
rate residues were <0.01 for the pea and 0.15 mg/kg on the pod, again
the interval was not provided. Additional data are needed on specific
peas and with known intervals.
Bell peppers - No residue data were provided from the three countries
for which recommended uses were provided. However, residue data on
bell peppers provided from field trials in the U.S.A. reflect proposed
uses of 0.56-1.1 kg ai/ha foliar and 0.56 kg ai/ha transplant water
treatments. A one-week pre-harvest interval is proposed as compared
to the two-week interval recommended. The foliar treatment was
reasonably close to the maximum recommended foliar use in Table 1.
Maximum residues from the proposed foliar treatment were 1.3 mg/kg at
7 days (1.7 at recommended rates) after last application or at
intervals greater than 8 days. No data were available from the
proposed transplant water treatment alone. Maximum residues from the
proposed transplant water application with a less than maximum foliar
application was 0.7 mg/kg at 7 days. Combined residues could
therefore be expected to be about 2.4 mg/kg at 7 days. A limit of 3.0
mg/kg would allow for some variability due to the relatively small
number of samples. Residue data from additional countries are
desirable and should include data from glasshouse uses.
Pineapples - Good agricultural practice information was not made
available although a liquid formulation pre-plant use at 4.5-9 kg
ai/ha and a foliar use at 1.1-4.5 kg ai/ha with a 30-day pre-harvest
interval are proposed in the U.S.A. Data reflecting the proposed
foliar use were available with maximum residues of 0.91 mg/kg in whole
fruit (27 days), 5.2 in bran (27 days), 1.4 in leaves at 23 days or
0.82 at 35 days and 8.5 in hay (27 days). Residues would therefore
not be expected to exceed 1.0, 6.0, 1 or 10 mg/kg in the whole fruit,
bran, forage, and hay respectively at the proposed 30-day interval.
Root and tuber vegetables (beets, carrots, sugar beets and sweet
potatoes) - Although good agricultural practice information for root
and tuber crops was available from a number of countries, most of the
residue data on the above root and tuber crops were from trials in the
USA where, in addition to USA-recommended uses in Table 1, additional
uses have been proposed. Of these proposed uses, broadcast and furrow
pre-plant liquid formulation application of 4.5-9 and 2.2-4.5 kg ai/ha
respectively are proposed for carrots and similar pre-plant rates and
a 7-day pre-harvest interval are proposed for potatoes. From
recommended application rates and methods of application, maximum
residues on root and tuber crops were 0.06 mg/kg at 14 days after last
application and from proposed uses, 0.1 mg/kg at 7 dove (both on
potatoes). The majority of residues from recommended or proposed uses
were 0.03 mg/kg or less. Residues for the root and tuber crops for
which data are provided would not be expected to exceed 0.1 mg/kg from
recommended and proposed uses. No data were available from processing
fractions of root crops.
Residue data on root and tuber vegetables resulting from recommended
good agricultural practices from additional countries are desirable.
Soybeans - Good agricultural practice information was not available.
A proposed USA use would allow liquid formation at-plant and pre-plant
treatments at 2.2-4.5 kg ai/ha and data from 7 states reflecting and
up to 1.5 times these proposed uses were available for dry beans and
straw. Maximum residues were 0.05 mg/kg for the dry seeds and straw
at 113-185 days after last application although all but one sample
each for seeds and straw from the 24 tests were <0.02 mg/kg. The
maximum 0.05 mg/kg residue in soybean seed was from an application
rate of one-fourth of the maximum proposed. On this basis residues
from the proposed uses would be expected to be less than 0.2 mg/kg.
No data were available for the oil, meal, hulls, forage or hay,
although a forage restriction is proposed. Although no concentration
data for the oil or meal were available, a 0.33 n-octanol water
partition coefficient would suggest that concentration of residues may
not occur. This would be consistent with fractionation studies on
cottonseed and peanuts.
Sugarcane - Good agricultural practice information was not provided
for oxamyl on sugarcane. The limited residue data from trials in
South Africa were not useful because of uncertainties on dosage rates.
Tomatoes - Good agricultural practice information was available from
eleven countries, although of these residue data were available only
from the United Kingdom and the USA.
The application rates for the U.K. residues data cannot be compared to
the 0.1 g/plt use described in the label provided. Maximum residues
from the trials were 0.85 mg/kg at the U.K. recommended 2-week pre-
harvest interval.
In the USA 1-3 day intervals are allowed for foliar uses. Maximum
residues from foliar uses recommended in the USA (FL) were 1.0 mg/kg
at a one-day interval and 1.9 mg/kg at two times the recommended rate.
No data were available for drip irrigation uses. The data would
indicate that residues would not exceed 1.0 mg/kg from U.K. uses and
USA foliar uses, taking into account their different pre-harvest
intervals. However, the relatively small number of samples at a given
rate would dictate a higher limit. Additional data from these and
other countries reflecting good agricultural practices would be
desirable.
FATE OF RESIDUES
Figure 2 lists the names and structures of oxamyl and related
compounds.
Figure 2. Names and structures of oxamyl and related compounds
COMPOUND NAME STRUCTURE
O O
OXAMYL METHYL N',N'-DIMETHYL-N[METHYL " "
CARBAMOYL)OXY]-1-THIOOXAMIMIDATE (CH3)2N-C-C=NOCNHCH3
'
SCH3
(I) OXIMINO METABOLITE O
METHYL N-HYDROXY-N',N'- "
DIMETHYL-1- (CH3)2N-C-C=NOH
THIOOXAMIMIDATE '
SCH3
(II) METHYL N-HYDROXY-N'-METHYL-1- O
THIOOXAMIMIDATE "
CH3NH-C-C=NOH
'
SCH3
Figure 2 (continued)
COMPOUND NAME STRUCTURE
O
(III) N'N-DIMETHYLOXAMIC ACID "
(CH3)2N-C-COOH
O
(IV) N-METHYLOXAMIC ACID "
CH3NH-C-COOH
O
(V) DMCF "
N,N-DIMETNYL-1-CYANOFORMAMIDE (CH3)2N-C-CN
O O
(VI) METHYL N'-METHYL-N- " "
[(METHYLCARBAMOYL)OXY]-1- CH3NH-C-C=NOCNHCH3
THIOOXAMIMIDATE '
SCH3
O O
" "
(VII) N,N-DIMETHYLOXAMIDE (CH3)2N-C-C-NH2
O
"
METABOLITE A GLUCOSE CONJUGATE OF I (CH3)2N-C-C=NO-GLUCOSE
'
SCH3
O
"
METABOLITE A' GLUCLOSE CONJUGATE OF II CH3NH-C-C=NO-GLUCOSE
'
SCH3
In animals
In a ruminant metabolism study two lactating goats were maintained on
diets containing approximately 10 mg/kg 14C-oxamyl for 10 and 20 days
respectively (Harvey, 1980a). 60-70% of the radioactivity was
eliminated in the urine and faeces, about 6% was expired, 2-3% was
found in milk and an estimated 22% in the entrails and carcass. The
radioactive compounds were not identified. However, there was no
evidence of oxamyl or oximino compound in milk, blood and tissues
(<0.01 ppm).
If calculated as oxamyl, maximum residues would have been 0.4-0.6
mg/kg in milk, and about 1.4 mg/kg in blood and tissues. Little was
found in fat. Milk residues peaked at about 10-14 days but in blood
they continued to rise throughout the feeding. In each case residues
rapidly decreased after withdrawal of the fortified diet.
About 5% of the activity in milk was incorporated into natural
lactose, caesin and lipids. Two additional components were isolated
and accounted for an additional 30% of milk residues.
In a more recent study 14C-labelled oxamyl and selected metabolites
were studied in vitro by incubation in rumen fluid of a Holstein
cow (Belasco and Harvey, 1980). Oxamyl was 41% metabolized within one
hour and 99% within six hours. After 24 hours incubation the oximino
metabolite (I) and DMCF (V) accounted for 80% of the radioactivity (67
and 13% respectively). The remaining radioactivity at 24 hours was
dimethyloxamic acid 5%, dimethyloxamide 10% and 1-2% each of minor
metabolites previously identified in rat metabolism studies.
Metabolite A, a major plant metabolite, was almost totally metabolized
by rumen fluid. This contrasts to rat metabolism where this compound
is largely unchanged in vitro by liver microsomes and only slowly
metabolized in vivo (Harvey and Han, 1978a). About 70% of
metabolite A was metabolized to DMCF by rumen fluid and the remainder
to unidentified components. The DMCF was found to metabolize to
N,N-dimethyloxamide, N,N-dimethyloxamic acid and N-methyloxamic acid.
Only the dimethyloxamide was not previously identified during rat
metabolism studies.
In a livestock-feeding study oxamyl was fed to Guernsey dairy cows at
2, 10 or 20 mg/kg in the diet for 30 days (DuPont, 1973). Recoveries
of added oxamyl were 70% or greater in whole milk, milk fat, or milk
aqueous fractions at 0.02-0.2 mg/kg fortification levels and over 80%
at 0.04-0.40 mg/kg levels in meat. No residues of oxamyl were
detected (<0.02 mg/kg) in any sample of milk or milk fractions,
liver, kidney, lean muscle or subcutaneous fat at any of the feeding
levels.
When these same samples were analysed for DMCF, no residues were found
at any feeding level in milk or tissues (<0.02 mg/kg for milk, <0.04
mg/kg for meat and fat). Recoveries were 67-82% for 0.02-0.05 mg/kg
fortification levels in milk and 46-100% at 0.04-0.2 mg/kg
fortification levels in meat and fat (Du Pont, 1976).
In a poultry-feeding study, adult laying hens were fed diets
containing 0, 1 or 5 mg/kg oxamyl for a four-week period (Zahnow,
1978). Samples of eggs and tissues were collected and analyzed for
oxamyl and DMCF. Oxamyl residues were <0.02 mg/kg in eggs, liver,
muscle and fat and <0.05 mg/kg in skin (due to limited sample
availability). Residues of DMCF were reported as <0.01 mg/kg in
eggs, liver, muscle, fat and skin.
Oxamyl recoveries from eggs averaged 76% at the 0.02 mg/kg
fortification level and at this same level, except for an apparent
error in skin data, averaged 85-97% in meat, tissues, skin and fat.
Recoveries of DMCF were relatively low, averaging 58 and 50% at 0.01
and 0.02 mg/kg fortification levels, respectively, in eggs and 54-63%
in meat, fat and skin tissues at a 0.02 mg/kg fortification level.
In plants
Plant metabolism of oxamyl was studied in tobacco, alfalfa, peanuts,
potatoes, oranges and tomatoes (Harvey, Han and Reiser, 1978).
In one experiment two tobacco plants were each treated on the leaves
with 10 mg 14C-oxamyl and grown in a growth chamber for 7 and 15 days
respectively. 95% of the radioactive material was recovered at 15
days; about 50% was surface residues, 37% extractable leaf internal
residues and 0.1% or less in the roots. About 4-5% were volatile
components. The surface residue was 95% oxamyl and 3% the oximino
compound (I). Of the leaf internal extractable residues, 56% was
oxamyl, 5% oximino compound (I) and 39% was in the polar fraction, 93%
of which was metabolite A and 7% N,N-dimethyloxamic acid. The
seven-day plants were not as extensively analyzed, but to the extent
they were, residues were similar to the 15-day residues with the
proportion of individual residues reflecting the shorter metabolism
period.
Alfalfa received three 0.5 lb. ai/acre 14C-oxamyl spray field
treatments at two-week intervals and was harvested 2.5 weeks after
last treatment. Extractable residues were 75% of applied. Of this,
>90% was metabolite A with only about 0.8% each for oxamyl and the
oximino compound (I).
Peanuts received two foliar field treatments of 14C-oxamyl at 2 lb.
ai/acre. Samples of young plants were taken at four weeks after the
first treatment, immediately before the second treatment, and
additional samples at harvest. There were therefore three fractions
analyzed - young plants, mature hay and mature nuts. In the young
plant 99% of the radioactivity was determined to be a 2:1 ratio of two
metabolites, A and A' (see figure 2). No oxamyl or oximino compound
(I) was detected.
In mature hay ß-glucosidase treatment of a very polar fraction
containing 99% of extractable residues released metabolites A and A',
giving evidence of an initial polysaccharide type compound, i.e.
metabolites A and A' with additional hexose units (see Figure 3).
Also about 1% of the residue was the oximino compound (I) and <0.5%
oxamyl. When unextractable residues (about 40% in mature hay or nuts)
were treated with a mixture of cellulose enzymes, about 60% was
released yielding a fraction characteristic of metabolites A and A'.
This suggests that the initial unextractable residue was a cellulose
of starch-like structure.
No oxamyl or oximino compound could be detected in the nuts. Of
extractable residues about 21% was incorporated into peanut oil
lipids, about 18% into the polysaccharide-type structure found in
mature hay, about 15% was in lipids or as glucose conjugates and about
37% was unextractable. Of the unextractables about 60% was in the
form of the cellulose-starch type structures discussed above.
Potatoes received five foliar field treatments of 14C-oxamyl at 0.5
to 1.0 lb ai/acre giving an oxamyl residue equivalent of 7 mg/kg in
the tuber. Peels contained <1% of the total residue. Free oxamyl or
oximino compound accounted for <3% of the residue, although mild acid
hydrolysis yielded 39% of the radioactivity in the form of oximino
compounds I and II.
Hydrolysis with ß-glucosidase yielded metabolites A and A', suggesting
the initial presence of the polysaccharide conjugates discussed under
mature peanut hay. At least 35% of the 14C was determined to be
incorporated into the glucose of the tuber starch.
Apples were brush-treated in an orchard at 1.0 lb ai/100 gal and 6
weeks later at harvest yielded 0.8-2.0 mg/kg residues, evenly
distributed through the fruit. About 98% of the residue was
extracted, 77% being organosoluble. Of the organosolubles 16% was
oxamyl, 42% oximino compound I, and 17% DMCF. The remaining 23% of
total fruit residue appeared to be in the form of polysaccharide-type
structures.
Oranges were harvested for analysis six weeks after brush application
of 14C-oxamyl to immature oranges at 1.2 lb. ai/100 gal. Residues
were 2.5 mg/kg on a whole fruit basis expressed as oxamyl. Rind
contained 82% of the residue and the juice 18%. On a whole fruit
basis, residues were 9% oxamyl, 6% oximino compound, 20% DMCF, 35%
metabolite A, 22% metabolite A' and 8% unidentified polar metabolites.
Small green tomato fruits were evenly spotted by pipet with 0.37 mg
14C-oxamyl each and the mature fruit harvested two weeks later for
analysis. Residues were 59% oxamyl, 13% oximino compound, 5%
metabolite A, 4% DMCF and 19% polar metabolites and natural products.
The studies on tobacco, peanuts, and potatoes demonstrate the systemic
nature of foliarly applied oxamyl, which readily translocates to the
roots, legumes and tubers respectively.
Figure 3 summarises a major metabolic pathway for oxamyl in plant
tissues. The methylcarbomoyl group is hydrolysed to form the oximino
compound, followed by conjugate formation of the oximino compound with
glucose to form metabolite A, which may undergo demethylation to form
metabolite A'. Metabolite A predominates in short-term studies (2-6
weeks) with metabolite A' identified in moderate-length studies (1-2
months). At harvest little uncomplexed metabolite A or A' is found
but 14C is incorporated into polysaccharide, cellulose or starch-like
structures suggesting the addition of hexose units to metabolites A or
A'.
In addition to the metabolic route shown in Figure 2, oxamyl may also
be completely broken down in plants and incorporated into natural
plant lipids or into the glucose of starch. The metabolite DMCF is
frequently found as a plant residue and so is the oximino compound in
some fruits. No residues of the S-oxide or S,S,-dioxide of oxamyl or
its oxime were observed during these investigations.
TOXICOLOGICAL STUDIES
Special studies on cholinesterase activity
Rat
Two groups of 10 male rats were given orally 4.8 mg oxamyl/kg bw.
One group received 50 mg/kg atropine sulphate i.p. immediately
after dosing of oxamyl. Cholinesterase activity in blood was
determined 24 hrs before and 5 min., 4 hrs and 24 hrs after dosing.
The animals without atropine showed a decrease in cholinesterase
after 5 min. with a maximum after 4 hrs. After 24 hrs, the
activity was normal again. Animals that were treated with atropine
showed a slight inhibition initially, but the activity was normal
after 4 hrs. The experiment was repeated with the same result
(Schmoyer and Henry, 1970).
Four groups of 8 ChR-CD rats were fed oxamyl in the diet at dosage
levels of 0 (control), 50, 100 or 150 mg/kg feed during 29 days.
Erythrocyte and plasma AChE were measured at day 1, 7, 21, and 28.
On the 29th day the rats were sacrificed to measure AChE in the
brain.
Eight additional rats were assigned to each group for assay of
brain AChE 14 days after the start of the experiment.
With male animals cholinesterase activity of the plasma was only
marginally decreased in the 150 and 100 mg/kg groups throughout the
study. In the females plasma cholinesterase activity was
dose-related decreased in the 150 and 100 mg/kg groups from day 7
onwards. No clear effects were observed on the AChE activity in
the erythrocytes. At the end of the study cholinesterase activity
in brain tissue of females was dose-related decreased in the 150
and 100 mg/kg groups. After 14 days cholinesterase activity in the
brain was inhibited in the 150 mg/kg group. In summary, 50 mg/kg
in the diet was a no-effect level (Barnes and Aftosmis, 1978).
Special study on delayed neurotoxicity
Chicken
The animals were given various oral doses of oxamyl. The ALD
(approximate lethal dose) was established at 40 mg/kg bw. Two
groups of 5 adult hens received 20 or 40 mg/kg bw respectively
followed by 0.5 mg/kg atropine i.m. No mortality occurred. The
animals were kept for 28 days. They showed marked symptoms of
cholinesterase inhibition but recovery was complete after 12 hours.
As a positive control 1200 mg/kg TOCP was used, followed by
atropine. Two weeks after dosing the animals showed typical signs
of delayed neurotoxicity (Ki Poong Lee, 1970).
Special study on reproduction
Rat
The animals of the long-term feeding study were also used for a
3-generation reproduction study. Groups of 16 males and 16 females
on a diet containing 0, 50, 100 or 150 mg/kg were chosen for
breeding three generations of each two litters. Throughout the
study the litter size, viability and lactation indices and body
weights of weanlings were decreased at 100 and 150 mg/kg. A
marginal effect on the body weight of the weanlings was also
observed at 50 mg/kg. No effect on fertility and gestation indices
was detected. Organ weights of the weanling pups of the F3b
generation showed a slight increase in relative kidney weight at
150 mg/kg. The relative testes weight was increased at 100 and 150
mg/kg. No histopathological abnormalities were found that could be
ascribed to the compound (Sherman et al. 1972).
Special study on mutagenicity
Microorganisms
Oxamyl showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, and reverse mutation tests with and
without a liver activation system using 5 strains of Salmonella
thyphimurium TA and E. coli WP2 hcr. A host-mediated assay
in mice using S. typhimurium G-46 was also negative (Shirasu
et al, 1976).
Special study on teratogenicity
Rat
Groups of 26-28 pregnant females received 0, 50, 100, 150 or 300
mg/kg oxamyl in the diet from day 6-15 of pregnancy. In the
mothers, a dose-related decrease in body weight and food
consumption was found for 100, 150 and 300 mg/kg groups. There
were no effects on number of implantation sites, resorptions and
live foetuses nor on embryonal development. Oxamyl does not show
teratogenic or embryotoxic properties (Culik and Sherman, 1971).
Acute Toxicity
species sex route LD50 in mg/kg bw reference
rat M oral 371 (27-51) Carroll, 1972
rat M oral 1102 (97-185) Gibson, 1973
rat M oral 5.4 (3.7-7.8) Fretz, 1969
rat3 M oral 16 (22) Schmoyer, 1969
rat3 F oral 11 (7-16) Schmoyer, 1969
1 formulation Vydate L (26% ai) in aqueous solution
2 Vydate G (10% ai) in corn oil
3 fasted overnight
Dermal studies
Rabbits
When oxamyl was applied as a suspension in a hydrophillic ointment
for 24 hrs, the ALD (approximate lethal dose) was 2250-5000 mg/kg
bw on the intact skin, but only 90-130 mg/kg on the abraded skin.
When oxamyl was applied as a slurry in propylene glycol the ALD was
130 or 60 mg/kg bw in the intact and abraded skin respectively
(Colburn, 1970).
When Vydate L (27% ai) was applied during 24 hrs the LD50 was 740
± 150 mg/kg bw on active ingredient basis on the intact akin
(Morrow, 1973).
Inhalation studies
Rats
The LC50 (1 h) for oxamyl was 0.035 mg/l air when Vydate L (27% ai)
was used in the test (males only) (Barras 1974). When the
experiment was carried out with oxamyl dust the LC50 (1 h) for male
and female animals was 0.17 and 0.12 mg/l respectively. The
concentration in air was calculated after chemical analysis
(Tayfun, 1969).
Symptoms of intoxication
Fasciculations, tremors, salivation, lacrimation, bulging eyes and
chromodacryorrhoea were found after an acute oral dose of oxamyl.
They can be considered as symptoms of cholinesterase inhibition
(Carroll, 1972).
A good antidotal effect was obtained when rats orally treated with
oxamyl received an i.p. injection with atropine sulphate (Sherman,
1969)
Acute toxicity of oxamyl metabolites in rats
LD50 in
metabolite sex route mg/kg bw reference
methyl N-hydroxy-N,N' dimethyl- M oral 11,000 (=ALD) Fretz, 1968
-1-thiooxamimidate (INA-2213)
methyl N-hydroxy-N' methyl-1- M oral 6,675 (6370- Dale, 1973
thiooxamimidate (IEL-2953) 6690)
N,N-dimethyloxamic acid (IND-2708) M oral 3,540 Barbo, 1972
N,N-dimethyl-1-cyanoformamide M oral 450 (=ALD) Ashley, 1974
(DMCF) (INN-79)
methyl N'-methyl-N [(methyl-carbamoyl) M oral 60 Schmoyer, 1969
oxy]-1-thiooxamimidate (IND-14O9)
glucose conjugate of methyl N'- M oral > 7500 Henry, 1976
methylhydroxy-N',N'-dimethyl
-1-thiooxamimidate (ING-3515)
Short-term studies
Rat
Six male rats were given orally 2.4 mg/kg bw oxamyl for 10 days. No
mortality occurred. Oxamyl does not exhibit cumulative oral toxicity
(Fretz and Sherman, 1968).
Rabbit
Rabbits were treated dermally for 15 days during a period of 4 weeks
with Vydate L (26% ai) in dimethylformamide. Groups of each 5 male
and 5 female animals received 193 mg/kg Vydate L, or 50 mg/kg bw of
oxamyl during 6 hrs/day on the intact or abraded skin. Control
animals received dimethylformamide. All oxamyl treated animals with
abraded skin showed marked signs of cholinesterase inhibition. Only
slight symptoms were seen on the animals with intact skin. No
mortalities occurred. No changes in body weight, organ weight or
histopathology were found that could be ascribed to the treatment.
All animals showed mild erythema of the treated skin (Dion, 1970).
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 100 or 500 mg/kg oxamyl in the diet. After a few days the highest
dose level was reduced to 150 mg/kg because with 500 mg/kg all animals
showed strong symptoms of cholinesterase inhibition. They were given
control diet for 3 days and the symptoms disappeared or became less
severe. Then the animals were given 150 mg/kg. No clinical signs of
toxicity were seen in the remainder of the experiment. The body
weight gain was lower at 100 and 150 mg/kg and slightly affected in
the females of the 50 mg/kg group. The food consumption was lower at
150 mg/kg only. Urinalysis showed a higher number of animals with
blood in urine at 100 and 150 mg/kg and a higher number with protein
at 150 mg/kg. Male rats showed lower absolute organ weights for
heart, kidneys, liver, spleen and thymus at 100 and 150 mg/kg. In the
females this effect was found for the liver at 100 and 150 mg/kg and
the kidneys and lungs at 100 mg/kg. No effects were found on
haematology, clinical chemistry and histopathology. Cholinesterase
activity was not determined. The marginal no-effect level is 50 mg/kg
(Snee et al, 1969).
At the end of the experiment 6 animals per group per sex were used for
reproduction performance. In the F1a and F1b generation the
fertility index and the number of pups/litter were decreased at 100
and 150 mg/kg. The weight of the young at weaning was significantly
lower at all dose levels (Snee et al, 1969).
Dog
Groups of 4 male and 4 female animals each received for 90 days 0, 50,
100 or 150 mg/kg oxamyl in the diet. Only marginal effects were found
with 150 mg/kg. A slight increased activity was found for alkaline
phosphatase after 4 and 13 weeks. In the males the weight of
adrenals, spleen and testes was somewhat decreased. The females
showed a slight increase in thyroid weight and a decrease in adrenal
weight. Histopathologically no abnormalities were found that could be
ascribed to the compound (Holsing, 1969).
Groups of 4 male and 4 female animals each received for 2 years 0, 50,
100 or 150 mg/kg oxamyl (95%) in the diet. After 1 year 1 male and 1
female animal from the control and 150 mg/kg groups were killed.
Haematology, clinical chemistry and urinalysis were carried out after
1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 months. The haemoglobin content,
haematocrit and number of erythrocytes in blood at 150 mg/kg were
found to be lower than the controls on most times of measurement in
both sexes.
Higher values for cholesterol and alkaline phosphatase activity were
found more often at 150 mg/kg in males and females. On some occasions
cholesterol was also higher in the other experimental groups.
No effects on organ weights, cholinesterase or aliesterase activity,
or histopathology were found that could be ascribed to the compound
(Sherman, et al, 1972).
Short term study with DMCF, a metabolite of oxamyl
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 150 or 450 mg/kg N,N-dimethyl-1-cyanoformamide (DMCF) in the diet.
Growth inhibition was found at 450 mg/kg in males and females, and the
food consumption was also lower. At the highest dose level
haemoglobin content, haematocrit and number of erythrocytes were
lower. At 150 mg/kg a slight effect was found on these parameters in
the females or males. In the male animals an increased LDH activity
was found at 450 mg/kg and in the female animals a decreased activity
in GPT, GOT, and LDH at the same dose level. Organ weights were not
reported in this study. Histopathologically an increase was found in
hydronephrosis of the kidneys in the males and minute foci of
mineralization in the kidneys of females at 450 mg/kg. The animals of
the other dose levels were not studied histopathologically. The
no-effect level is probably 50 mg/kg (Kaplan, 1976).
At the end of the experiment 6 animals per group were used for
reproduction performance. With 450 mg/kg a decreased weight of the
young at weaning was observed (Kaplan, 1976).
Long-term studies
Rat
Groups of 36 male and 36 female animals each received for 2 years O,
50, 100 or 150 mg/kg oxamyl (95%) in the diet (2 control groups were
used). After one year of feeding the number of rats of each sex in
each group was reduced to 30. With 100 and 150 mg/kg a decreased body
weight was found with male and female animals that was related to
dose. With 50 mg/kg the male animals showed a slightly lower body
weight, which was apparent after 4 weeks and remained so throughout
the study. Food consumption was lower at 150 mg/kg. Cholinesterase
activity in blood in the 150 mg/kg group was only decreased in the
females after 4 days of feeding and in the males after 8 days, and not
after 1, 6, 12 and 24 months. Aliesterase activity was not affected
throughout the study.
At the end of the experiment relative weights of brain, testes and
adrenals were increased in the males and brain, heart, lungs, kidneys
and adrenals in females at 150 mg/kg. In the females most or these
organ weights were also increased at 100 mg/kg. These changes are
probably connected with the decreased body weight. No haematological,
biochemical or histopathological abnormalities were found that could
be ascribed to the compound (Sherman et al, 1972).
RESIDUES IN FOOD
USE PATTERN
Oxamyl formulations, either as a 24% liquid or 10% granular, are
recommended or registered for use in numerous countries. Food uses
made available to the Meeting by the manufacturer are summarized in
Table 1. Proposed uses are not included but are discussed under
appropriate commodities when residues are discussed.
According to information provided to the Meeting, uses listed for the
United States also apply to Argentina, Barbados, Bolivia, Brazil,
Chile, Colombia, Costa Rica, The Dominican Republic, Ecuador,
Guatemala, Mexico, Nicaragua, Peru, El Salvador, Trinidad and Tobago,
Uruguay and Venezuela unless indicated otherwise in Table 1. Only a
few national governments provided usage information directly to the
meeting.
The liquid formulation is a contact-type, moderately residual
insecticide when applied as a foliar spray. When so applied it is
effective on several species of mites and, by systemic action in many
plants, on nematodes. When oxamyl formulations are applied to the
soil they function as a contact type broad-spectrum nematocide and, by
systemic action, as a miticide/insecticide.
Applications may be pre-planting, at planting, or foliar (to
fruit-bearing or non- fruit-bearing plants). They may be applied in
the furrow or broadcast. Soil incorporation may be mechanical or by
irrigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
There are numerous combinations of type of formulation, method and
time of application suitable for a wide range of pest control needs.
Because of this, the residue level can vary widely, even on the same
commodity. A large volume of data on 29 commodities or groups of
commodities was presented to the meeting; this has been considerably
condensed into Table 2 (Du Pont, 1980).
The analytical method used extracts both oxamyl and its oxime
metabolite (I), converts the oxamyl to the oxime which is quantitised,
so the residue levels quoted are of oxamyl plus the oxime expressed as
oxamyl. Data on residues of the DMCF metabolite are in parentheses.
TABLE 1. Recommended uses for oxamyl1
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation2,3 treatments interval (wks) notes
Apples
non-bearing U.S.A. 0.28-2.2 24% Liq. 52
6.7 - 9 pre-plant, soil incorporation
bearing 0.28-2.2 2 do not graze treated orchards
non-bearing Greece 2-4 10% G 52 soil
1.2 24% G multiple 52 foliage
Bananas Greece 3 g/plt 10% G 3 at planting
2-3 g/plt 2 on plantations (around plant)
0.01% 24% Liq. foliage
Central Amer. 0.9-1.3 24% Liq. 5-8 foliar
Spain 3.8 24% Liq. in irrigation water
Beans Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, broadcast glasshouse and
in open
1.2 24% Liq. 2 2 spray, broadcast glasshouse and
in open
Beets (roots Denmark 0.7-1.8 10% G row, at planting
or tops) 2.5-5 10% G broadcast, before planting
Carrots Greece 3-4 10% G 2 pre-plant, broadcast
2-3 10% G 2 furrow
1.2 24% Liq. 2 2 spray
1.2 24% Liq. multiple 52 foliage
Celery Greece 3-4 10% G 2 preplant broadcast, glasshouse and
in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
USA 0.56-2.2 24% Liq. 2 untrimmed
4.4 24% Liq. 2 pre-plant soil incorporated
Citrus Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
USA up to 1.1 1 do not graze treated orchards
(0.008-0.03%)
Cherries Greece 2-4 10% G 52 soil non-bearing
1.2 24% Liq. multiple 52 foliage non-bearing
Cucumbers Bulgaria 0.024% 24% Liq. multiple 2 foliage (greenhouse)
Czechoslovakia 0.1 g/plt. 1 2 glasshouse
Netherlands 5 10% G glasshouse, shortly before planting
Cucurbits Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2 24% Liq. 2 2 spray, glasshouse and in open
USA 0.56-2.2 24% Liq. 2 untrimmed
Eggplant Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
Onions Denmark 0.7-1.8 10% G row, at planting
2.5-5 10% G broadcast, before planting
United
Kingdom 13-4.9 10% G furrow, at planting
Peaches Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Pears Greece 2.4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
Peas United 2.5 10% G pre-plant
Kingdom
Peppers Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt 10% G 2 2 glasshouse
Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2spray, glasshouse and in open
Pipfruit N. Zealand 0.048-0.06% 24% Liq. 2 1
Potatoes Denmark 2.5-5 10% G 1 broadcast, pre-planting
Greece 3-4 10% G 2 pre-plant, broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Libya 4-5.5 10% G pre-plant, soil
1.7 24% Liq. 3 (foliar) 4 soil, pre-plant and foliar
Mexico 0.24-0.72 24% Liq. multiple 1 foliage
Netherlands 10% G before or at planting
N. Zealand 5 10% G 13 pre-plant broadcast
United
Kingdom 4-5.5 10% G pre-plant, soil
U.S.A. 0.28-1.1 24% Liq. 1 foliar
Squash Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Strawberries Denmark 0.48 24% Liq. 3 after final harvest
Greece 2-4 10% G non-bearing
1-2 24% Liq. multiple non-bearing
Sugarbeets Greece 0.8-1 10% G at planting
0.18 24% G foliage
Netherlands 0.75 10% G at sowing
Spain 0.8 10% G in row
Syria 2.4-3.6 24% Liq. 1 at plant
1.8 24% Liq. 1 foliar
United
Kingdom 0.6-0.9 10% Liq. in furrow at planting
Tomatoes Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt. 10% G 2 2 glasshouse
Denmark 4.8 24% Liq. field, preplant
0.05% 24% Liq. field, preplant
0.07% 24% L1q. 4 wk. after planting
Egypt 1.8 24% Liq. 4
Greece 3-4 10% G 2 preplant broadcast,
glasshouse or in open
2-3 10% G 2 furrow, glasshouse
or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Netherlands 5 10% G glasshouse, shortly before
planting
Jordan 1.8 24% Liq. 2 4 foliar
Libya 3 10% G 1 4
1.7 24% Liq. 2 4
Mexico 0.06-0.12% 24% Liq. 1 (day) foliar
Syria 1.8 24% Liq. 2 foliar (seedlings)
United
Kingdom 0.1 g/plt 10% G 2 2
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
U.S.A. 0.56-1.1 24% Liq. multiple 1 (day) (AL, FL, MD, NJ, PA, P.R.,
SC, VA) foliar
2 (day) (HI) foliar
3 (day) (CA) foliar
4.5-9 24% Liq. 1 (day) (HI, soil, by
drip irrigation)
1 Most listed U.S. uses are special local need registrations, limited to specific geographical areas.
2 G = granular
3 Liq. = liquid
TABLE 2. Oxamyl residues from supervised trials (DMCF in parentheses)
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Apples N. Zealand 1980 1 0.03-0.06% 24% EC 1 0.1-0.2 2
2-3 0.03-0.11 2
6-8 N.D.1 2
12-14 N.D.1 2
U.S.A. 1972-75 2-6 0.28 24% Liq. 2-3 0.19-0.2 (<0.02) 2
(10 states) 4-5 0.21-0.36 (<0.02) 2
9-11 0.11-0.21 (<0.02) 3
2 0.67 24% Liq. 11 0.12 1
1-3 0.76-1.1 24% Liq. 2-3 0.32-0.84 (0.03-0.05) 5
4-5 0.1-0.23 2
6-8 0.09-0.68 (<0.02-0.04) 14
12-14 1.2 1
1-2 1.7-2.2 24% Liq. 2-5 0.31-1.5 3
6-8 0.05-1.4 (<0.02-0.03) 4
12-17 0.14-1.1 (<0.02-0.04) 5
21-30 0.27 1
1 4 24% Liq. 2-3 1.9 1
6-8 1.2-1.4 2
12-14 0.99-1.0 (0.05-0.15) 1
Banana2 Costa 1975-77 1-8 0.9-2.2 24% Liq. 1-10 <0.02 (0.02) 10 bagged or unbagged,
Rica3 (aerial) whole fruit or edible peel
1 0.05 limit of determination
2 Cavendish and Giant Cavendish
3 five farms
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Beans U.S.A. 1975-77
(succulent, green
snap, lima) Beans Foliage-hay
preplant 1 2.2-4.51 10% G 67 <0.02
foliar VA 1 0.28 24% Liq. 2-3 0.1 (<0.04) 1
6-8 0.02 (<0.04)
VA, FL, CA 1-8 0.56 24% Liq. 1 0.05-0.84 (<0.04) 2 2.5 (<0.04)
2-3 <0.02-5.3 (<0.04) 10 1-14 (<0.04-0.08)
6-11 <0.02-0.97 (<0.04) 9 0.24-16 (<0.04)
12-14 <0.02-0.65 (0.04) 8 0.26-5.7
CA, FL 5-8 1.1 24% Liq. 1 1.9 1 6.7 (0.14)
2-3 <0.02-7.7 (<0.04-0.19) 10 3.2-29 (<0.04-0.41)
6-9 <0.02-2.3 (<0.04-0.17) 8 0.59-33 (<0.04-0.36)
11-14 <0.02-1.0 (<0.04-0.09) 10 0.08-15 (0.06-0.20)
CA, FL 5-8 2.2 24% Liq. 1 6.5 (0.35) 1
2-3 0.04-13 (0.04-0.37) 9 77 (0.66)
6-8 0.1-7.3 (<0.04-0.31) 8 0.91-67 (<0.04-0.83)
12-14 0.05-2.0 (<0.04-0.09) 9 0.06-37 (0.06-0.67)
CA 5 4.5 1 8.9 (0.84) 1
2-3 12 (0.54) 1
12-14 0.28 (<0.04) 1
Beans Beans Straw
(dry-pinto, WY 1977 1 0.56 24% Liq. 50 <0.02 (<0.04) 0.04 (<0.04)
navy, dry) 1 1.1 50 <0.02 (<0.04) 0.18 (<0.04)
vines
CA, MI 1973-78 1.1-2.2 10% G 103-111 <0.02 (<0.04) <0.02-0.08 (<0.04)
3.4 (preplant) 111-132 <0.02 (<0.04)
1 12 inch band incorporated
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Range, mg/kg
Beets, red U.S.A. 1973 1 3.4-6.7 G 84 <0.02 soil
2 1.1 Liq. 48 <0.02 foliar
1 3.4-6.7 G 84 <0.02 soil
+ + + +
2 1.1 Liq. <0.02 foliar
Cantaloupe U.S.A.
GA 1971 1 4.5 10% G 80 0.05
4.5 10% G
+ +
1.1 2 Liq. 39 <0.02
No. in range
CA, FL 1976-78 5-8 1.1 (foliar) 2 L 1 0.04-0.25 4
2-3 0.19-0.26 3
4-5 0.05 1
6-8 0.06-0.16 4
FL, IN, CA 1976-78 5-8 1.1 (foliar) 2 L 1 0.1-0.62 (<0.02-0.03) 5
2-3 0.12-0.48 (<0.02-0.04) 4
4-5 0.06-0.19 (<0.02) 2
6-8 0.07-0.59 (<0.02-0.05) 6
AZ, CA, IN 1976-78 5-8 2.2 (foliar) 2 L 1 0.23-0.91 (0.05-0.1) 3
FL 2-3 0.03-0.66 (0.66) 5
6-8 0.05-0.5 (0.04-0.05) 3
IN 1974-78 2-5 4.5-6.7 2 L 1 0.44-0.69 (0.05-0.08) 2
(foliar) 2-3 0.12-0.52 (0.04-0.08) 2
6-8 0.3 (<0.02) 2
41-51 0.02 1
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
IN 1978 5 9-13 2 L 1 0.49-1.4 (0.07-0.2) 2
(foliar) 2-3 0.44-1.4 (0.02-0.09) 2
6-8 0.41-0.87 (0.02-0.8) 2
FL 1972 1 2.2 2 L
+ +
1.1 2 L 31-40 0.18 (<0.02)
Carrot U.S.A.
preplant OH, MI 1975 1 3.4-4.1 unspecified 120-135 <0.02
soil MI, OH, FL 1970-73 1 3.4-6.7 G. or Liq. 103-185 <0.02 (<0.02)
DE
foliar DE 1971 5 0.56 Liq. 18 <0.02 (<0.02)
soil OH 1973-74 1 3.4-6.7 G. or Liq. 122-185
+ + + +
foliar 3-5 0.56-2.2 Liq. 22 or unspecified <0.02 (<0.02)
Celery U.S.A. (FL) 1974 3 0.56 Liq. 4-5 8.1 1
6-8 6.6 1
12-14 0.88 1
1974 5 1.1 Liq. 1 3.6-24 3
4-5 0.99-8.1 (0.81) 3
6-8 0.66-6.6 6
1.5-1.7 (trimmed) 2
12-14 0.12-1.5 4
0.94 (trimmed)
15-20 0.36-0.39 2
0.27-0.33 (trimmed) 2
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1974 3-5 2.2 Liq. 4-5 20 1
12-14 0.86-3.1 3
2.4 (trimmed) 1
15-20 1.3-1.4
grapefruit lemons oranges tangelos tangerines
Citrus2 U.S.A. 1971-75 1-5 0.59-1.11 Liq. 1 0.14-0.25 0.21-0.29 0.13-0.6
AZ,CA,FL,TX (foliar) 2-3 0.03-2.1 0.17-0.25 0.02-1.1
4-5 0.11 0.18 <0.04
6-8 0.03-1.2 0.05-0.17 0.02-0.8
9-11 <0.02 <0.02
12-14 0.03-0.08 0.06-0.37
15-21 <0.04-0.3
2-7 1.1 90% sol. powder 84 0.04
105 0.14
1 1.4-1.51 Liq. 2-3 3.6 0.51
(foliar) 4-5 .42
6-8 1.2-2 0.34
12-17 0.46-0.8 0.06
1-6 2.1-2.2 Liq. 1 1.3 0.39 0.26-0.36
or 3 2.2+4.5+2.2 2-3 <0.02-0.67 0.29 <0.02-0.52 <0.02-0.1
(foliar)1 4-5 <0.02-0.84 0.04 <0.02-0.92 0.06-0.88
<0.02-0.57
6-8 <0.02-0.3 0.04 <0.02-3 0.02-0.24 <0.02-0.4
9-14 <0.02 <0.02-0.87 0.02
21 <0.02
1 presumed on the basis of proposed label
2 DMCF is <0.06 mg/kg in fruit, peel or pulp except for a 0.54 value at high dosage (4-5 days)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1971-75 3 2.2+4.5+4.5 Liq. 1 1.5 0.55 2.-4.8
1-3 4.2-4.5 2-3 0.08-1.1 0.36 0.22-4.4
(foliar)1 4-5 0.07-0.64 0.32 0.25-3.6 0.88
6-8 0.05-0.64 0.36 0.13-2 0.24-0.36
12-14 <0.02 <0.02-0.67
21 0.03
84 0.04
Corn, field USA 1970-75 1 0.56-4.5 Liq. 93-198 <0.02 (<0.02) 27 (kernal or stalk)
(GA,FL,NC) (band or furrow)
Cottonseed USA 1969-75 1 0.06-0.28 Liq. 51-75 <0.02
(AZ,TX,CA,NC) (foliar)1
1-5 0.37-0.56 Liq. 6-8 <0.02-0.09
(foliar)1 12-17 <0.02-0.13
1-5 0.75-1.1 Liq. 1 25 1
(foliar)1 4-5 0.75 1
6-8 0.02-0.17 19
12-17 <0.02-0.18 19
1-5 1.7-2.2 Liq. 6-8 0.03-0.26 12
(foliar)1 12-14 <0.02-0.17 7
15-17 0.02-0.05 2
146 0.02 2
3-5 4-5 Liq. 6-8 0.25-0.64 4
(foliar)1 15-17 0.02 1
Cucumber U.K. 1973 - 5 unspecified unspecified 0.41-0.68 2
U.S.A.
preplant VA 1977 1 2.2-4.5 10% G 51-75 0.15-0.47 (<0.02-0.06) 2
1 9 10% G 76-100 <0.02
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
foliar FL,VA,CA 1973-78 2-7 0.56 2 L 1 0.15-0.36 5
2-3 0.18-0.38 3
6-8 0.18-0.28 4
12-14 0.07 1
31-40 0.02 (<0.02) 1
FL,CA,VA 1973-78 2-7 1.1 2 L 1 0.22-0.54 (0.1-0.14) 5
2-3 0.26-0.39 (0.03-0.15) 3
6-8 0.26-0.38 (0.07-0.19) 5
12-14 0.15 1
31-40 0.03 (0.02)
AR,CA,FL,VA 1974-78 1-7 2.2 2 L 1 0.45-1.1 (0.14-0.19) 4
2-3 0.03-0.7 (0.23-0.24) 4
4-5 <0.02 1
6-8 0.02-0.78 (0.12-0.25) 5
12-14 0.16 (0.02) 1
41-75 0.05-0.08 (<0.02) 2
1972-76 1-7 4.5 2 L 1 0.87-2.2 (0.17-0.13) 2
6-8 0.84-1.8 (0.25-0.35) 2
12-14 0.32 (0.03) 1
31-40 0.02-0.26 (<0.02-0.07) 2
51-75 <0.02 1
glasshouse Netherlands 19791 1 5 10% G 42 0.16-0.28 (av. 0.21)
1 10 10% G 42 0.08-0.33 (av. 0.27)
2 5 250%g/l 14 0.37-0.52 (av. 0.43)
Honey dew USA 6-9 0.56 2 L 1 0.2-0.24 3
FL 1976-78 (foliar) 2-3 0.18-0.28 2
6-8 0.19-0.23 3
FL 1976-78 1.1 2 L 1 0.36-0.4 (<0.02-0.03) 3
(foliar) 2-3 0.39-0.5 (<0.02-0.03) 2
6-8 0.32-0.44(0.02.-0.03) 3
1 CIVO report R 6282 (1979)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
AZ,CA,FL 1976-78 5-9 2.2 2 L 1 0.68-0.71 (0.04) 2
(foliar) 2-3 <0.02-0.68 (0.05) 3
4-5 <0.02 1
6-8 <0.02-0.7 (0.06-0.1) 3
FL 1976 9 4.5 2 L 1 1.0 (0.04) 1
(foliar) 6-8 0.92 (0.06) 1
Onions U.K. 1972 11 0.84-3.4 10% G1 unspecified <0.04
Netherlands 1977 2 0.41 3.3% G 100-150 <0.01 12
nuts hulls hay (dry)
Peanuts U.S.A. 1971-76 1 1.7-5 (soil) 10% G 76-100 0.03 0.07
(OK,TX,VA, 101-150 <0.02 <0.02 <0.02-0.38
GA,NC) 151-200 <0.02-0.04 <0.02-0.1 <0.02-0.04
1 1-4.5 10% G 12-14 <0.02 <0.02 <0.57
+ (soil) + 51-75 <0.02-0.03 <0.02 <0.02-0.07
2-3 0.56-1.7 24% Liq. 76-100 <0.02 0.04 0.91
1 1.7-2.8 24% Liq. 76-100 <0.02 <0.02 <0.26-1.0
(soil) 100-200 <0.02 <0.02
2-3 0.56-1.7 24% Liq. 6-8 <0.02 0.05 0.06
(foliar) 21-30 <0.02 <0.01 0.04
76-100 <0.02
pea pod
Peas U.K. 1.4-2.8 10% G unknown <0.01 <0.01
(preplant)1
11.1 10% G unknown <0.01 0.15
(preplant)1
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Pepper U.S.A. 1975-78 4-18 0.561 24% Liq. 1 0.04-0.88 6
(AL,CA,FL) (foliar) 2-3 0.15-0.58 (<0.02-0.04) 5
4-5 0.47-0.7 (0.08) 3
6-8 0.11-0.7 (<0.02-0.003) 7
4-18 1.1 24% Liq. 1 0.23-1.1 (0.03-0.12) 7
(foliar) 2-3 0.14-1.0 (0.03-0.06) 6
4-5 0.62-1.5 (0.14) 2
6-8 0.15-1.3 (0.03-0.14) 6
5-6 2.2 24% Liq. 1 1.3-3 (0.04-0.08) 3
(foliar) 2-3 0.46-1.9 (0.04-0.46) 2
4-5 1.4 1
6-8 1.2 (0.07) 1
wholefruit bran leaves hay DMCF fruit2
Pineapple U.S.A. 1975-76 1-6 1.1 24% Liq. 13-14 <0.02-0.46 0.04-0.57 0.26-0.55 0.11-0.46
Hawaii 23-27 <0.02-0.43 0.28-1.7 <0.02-0.22 0.14-1.2 (<0.02)
35-42 0.03-0.09 0.06-0.15 (<0.02)
2.2 24% Liq. 13-14 0.03-1.1 0.10-3.5 0.76-1.5 0.27-1.3
23-27 0.02-0.59 1.1-3.2 0.04-1.4 0.55-1.2 (<0.02-0.04)
35-42 <0.02-0.36 0.02-0.82 (<0.02)
4.5 24% Liq. 13-14 0.13-2.5 0.22-3.0 1.9-6.8 0.75-2.3
23-27 <0.02-0.91 2.5-5.2 0.02-0.70 1.9-8.5 (<0.02-0.1)
9 24% Liq. 14 0.14-1.2 0.57-1.7 2.4-7.3
23 0.17-0.46 0.3-1.9 (0.09-0.2)
Potatoes S. Africa 1974 4-5 0.24% 24% Liq. 51-75 <0.002-0.033 plant water and/or foliar
U.K. 1973 13 5.6 10% G3 unspecified <0.02-0.03
1 with and without 0.56 kg ai/ha in transplant water
2 DMCF residues in pineapple leaves were generally compared to those in the fruit
3 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
(soil) USA (DE) 1969-71 1 1.1-3.4 10% G 76-150 <0.02 pre-plant, soil incorporated
NY, FL, MI 1966-75 1 3.4-6.7 10% G 104-106 <0.02
FL 1974-75 1 10.1-13.4 24% Liq. 21-30 <0.02 broadcast, soil incorporated
FL 1974 1 3.4-4 24% Liq. 100-150 <0.02
(foliar) DE 1969-74 3-6 0.21-0.28 24% Liq. 6-20 <0.02
CA, DE 1969-74 3-13 0.43-0.56 24% Liq. 1-5 <0.02
6-8 <0.02-0.10
12-14 <0.02-0.03
18-30 <0.02
CA,DE,FL,MI 1972-75 1-5 1-1.1 24% Liq. 1 <0.02
2-3 <0.02-0.03
4-14 <0.02-0.03
18-30 <0.02
CA, DE 1969-72 1-5 2.2-3.4 24% Liq. 4-5 <0.02
2-3 0.02-0.05
4-5 <0.02-0.02
6-14 <0.02-0.06 high value from low dosage
DE, CA 1969-74 1-5 4.5 24% Liq. 4-5 <0.02
6-8 0.05-0.11
12-14 0.09
(soil + MI 1974 1 + 3.4-6.8+ G + 100-150
foliar) 1.7 1.1-2.2 24% Liq. 6-8 <0.02
CO 1973-74 1 + 3.4-6.7 G + 100-150
3 1.1 24% Liq. 6-40 <0.02-0.02
DE 1971 1 2.2 10% G soil incorporated
2 (foliar) 24% Liq. 18 <0.02
(dose unspecified)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
soil straw
Soybeans U.S.A. (TN,MO 1970-77 1-3 0.56-6.73 10% G 113-185 <0.02-0.051 <0.02-0.052
(dry) VA,AL,FL,NC;
25 tests)
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02
summer 4-8 <0.02
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02-0.06
vining 4-8 <0.02
tops roots
Sugarbeets U.K. 14 0.6-0.95 10% G4 unspecified 0.05 0.04
U.S.A. (GA,NC 1 0.56 Liq. 76-100 <0.02 transplant water
LA,VA,NY) 1+1 0.56+1.1 Liq. 76-100 <0.02 transplant water+foliage
1 4.5-9 G 76-200 <0.02 soil
1 4.5 Liq. 100-150 <0.02 soil
2 Liq. 51-75 <0.02 foliar
1+ 0.56-9 + G + 75-100 <0.02 soil +
2 1.1 Liq. 51-75 <0.02 foliar
Sugarcane S. Africa Data provided were not useful because of uncertainty of dosage rates.
Sweet potato U.S.A. (GA, 1970-74 1 4.5-95 24% Liq. 100-150 <0.02 (<0.02)
NC,VA,ML) preplant-row and broadcast
1 only 1 of 25 results was at 0.05 mg/kg
2 only 1 of 7 results was at 0.05 mg/kg
3 all but one application were at planting or 1 day prior
4 presumed on the basis of proposed label
5 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
Watermelon U.S.A. 1976-78 5-8 0.56 2L (foliar) 1 0.05-0.29 (<0.02)
(FL,CA) 2-3 0.27
6-8 0.03-0.28 (0.02)
FL 1976-78 8-9 1.1 2L (foliar) 1 0.38-0.77 (<0.02)
2-3 0.47 (0.02)
6-8 0.32-0.56 (0.02)
AZ,CA,FL 1976-78 5-9 2.2 2L (foliar) 1 0.1-1.2
2-3 <0.02-0.87 (0.05)
4-5 <0.02
6-8 0.05-0.78 (<0.02-0.1)
Tomato Netherlands1 1979 1 5 10% G 5 0.02-0.11
(glasshouse)
1 10 10% G 5 0.04-0.23
(glasshouse)
2 5 250 g/l 5 0.32-0.57
preplant broadcast
5.6 10% G 51-150 0.11-0.132
U.K. 1974 11.2 10% G 0.05-0.12
post plant broadcast
5.6-6.7 10% G 12-14 0.66
21-30 0.012
9 10% G 21-30 0.092
11.2 10% G 21-30 0.52
preplant + postplant broadcast
5.6+5.6 10% G 12-14 0.152 (0.85)
11 + 5.6 10% G 12-14 0.072
11 + 11 10% G 12-14 0.78
1 CIVO report R 6282 (1979)
2 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
unspecified application method
2.2-5 10% G1 unspecified 0.06-0.19
10.1 0.07-18.7
U.S.A. 1974-75 5-11 0.56 24% Liq.1 1 0.14-0.44 6
FL, 10 (foliar)1 2-3 0.04-0.31 6
locations 4-5 0.08-0.35 6
6-8 0.25 1
5-12 0.84-1.1 24% Liq.1 1 0.26-1.0 (0.21) 11
(foliar)1 2-3 0.1-0.71 10
4-5 0.11-0.55 10
6-8 0.34 1
5-11 2.2 24% Liq.1 1 0.82-1.9 6
(foliar)1 2-3 0.15-0.78 7
4-5 0.24-0.49 6
6-8 0.24 1
5 4.5 24% Liq.1 1 0.57 1
(foliar)1 4-5 0.35 1
6-8 0.37 1
1 0.05 limit of determination
Apples - Maximum oxamyl residues from approved dosage rates were 1.5
mg/kg at 2-5 days after application and 1.2 mg/kg at the interval of 2
weeks recommended for uses on bearing trees although the application
rate at the latter residues level was less than the maximum permitted.
Residues of DMCF ranged from 0.02-0.05 mg/kg from approved use. There
was no data from uses on non-bearing trees although a one-year
interval is applied for that use.
Bananas - Residues of oxamyl or DMCF were <0.02 mg/kg from
application rates up to 1.7 times maximum recommended dosage in whole
fruit (bagged or unbagged) or edible pulp. The data reflect foliar
applications only.
No residue data were provided for non-foliar uses on bananas.
Beans - Although no residue data were available from the single
country for which recommended usage information was available, data
encompassing those rates were available from trials in the U.S. where
similar uses and a 7-day pre-harvest interval have been proposed.
Residues of oxamyl from pre-plant uses on succulent beans were <0.02
mg/kg although such data were quite limited. Maximum residues of
oxamyl from recommended foliar rates were 1.0, 11 and 15 mg/kg in
succulent beans, foliage, and hay respectively and 0.09, 0.15 and 0.20
mg/kg respectively for DMCF, all at a 14-day interval. At 7 days
maximum oxamyl residues were 2.3, 33 and 25 mg/kg respectively on
succulent beans, foliage and hay. A 14-day pre-harvest interval is
recommended for beans but not specified for forage-hay. An 18-day
pre-graze or pre-forage interval is proposed in the U.S.A.
Oxamyl residues on dry beans from rates reflecting recommended uses
were <0.02 mg/kg and were 0.18 and 0.08 mg/kg on straw and vines
respectively, although most such applications were pre-plant.
Residue data from foliar uses are needed on dry bean varieties and
additional residue data from pre-plant uses on succulent beans,
including additional data on feed items from these uses. Such data
should come from other countries as well as from the U.S.A. The
potential for residues in meat and milk warrants complete data on feed
items.
Celery - Good agricultural practice information (both pre-plant and
foliar) was available from 2 countries although of these only residue
data from foliar treatments in the U.S.A. were available. Maximum
residues at the recommended 2-week pre-harvest interval were 3.1 and
2.4 mg/kg respectively for untrimmed and trimmed celery. Residue data
from other countries would be desirable.
Citrus - (grapefruit, lemons, oranges, tangelos, tangerines) - Residue
data on these citrus crops were available from the U.S.A. reflecting
foliar good agricultural practices. No data were available from good
agricultural practices on non-bearing citrus trees. The maximum
residue on citrus from recommended usage was 1.2 mg/kg on grapefruit
from only one application at the recommended 7-day pre-harvest
interval and this at an application rate 0.7 kg ai/ha, thus less than
the maximum 1.2 kg ai/ha recommended. At 1.5 kg ai/ha maximum
residues, again on grapefruit, were 2 mg/kg at 7 days from only one
application. Residues on oranges from application rates 2 times the
maximum recommended rate were 3 mg/kg. Residues from multiple
applications at recommended rates would be expected to be less than 3
mg/kg.
No additional residues of oxamyl were released when citrus samples
were subjected to ß-glucosidase enzyme treatment.
Corn field - Good agricultural practice information was not provided
for corn although a 0.56-2.2 kg ai/ha at planting 24% liquid
formulation use is proposed in the U.S.A. Residues from applications
up to 2 times this rate resulted in oxamyl residues of <0.02 mg/kg in
the kernels and stalk at 93-198 days after treatment. Only 3 analyses
were made on stalks. Additional residue data are needed for forage
and fodder as well as additional residue data from major corn
producing areas.
Cottonseed - Good agricultural practice information was not provided
for oxamyl uses on cotton although uses at 0.028-1.1 kg/ha foliar
applications of a 24% liquid formulation and a 21-day PHI interval
have been proposed in the U.S.A. Residue data reflecting these uses
were available with residues up to 0.17 mg/kg near the proposed 21-day
pre-harvest interval. Residues would not be expected to exceed 0.2
mg/kg. No DCMF residue was detected in cottonseed meal or oil.
Cucurbits - (cantaloupe, cucumbers, honeydew, summer squash, vining
squash, watermelons) - The only available registered use information
for cucurbits were on greenhouse cucumber, in Bulgaria, Czechoslovakia
and the Netherlands and for cucurbits (greenhouse and in the open) in
Greece. No residue data were provided from these countries except the
Netherlands although residue data on the above cucurbits were
available from the USA where pre-plant and foliar liquid formulation
uses with a 1-day pre-harvest interval are proposed, the former at
2.2-4.5 kg ai/ha and the latter proposed use at 0.56-1.1 kg ai/ha.
The proposed foliar treatment rate is therefore comparable to the
Greek spray uses. Some data from pre-plant granular trials in the
U.S.A. were also provided but none for the proposed pre-plant liquid
formulation uses.
Maximum residues on cucurbits at the recommended 1.2 kg ai/ha foliar
rate were 0.77 and 0.48 mg/kg at 1 and 3 days respectively and 0.47
mg/kg for granular pre-plant treatment at 66 days; for a combined
pre-plant-foliar application a maximum residue of 1.24 mg/kg was
observed. Foliar residues on the various cucurbits were generally
comparable at comparable intervals and application rates. Residue
data from the U.K. were of little value because of lack of knowledge
of the interval, formulation and method of application.
Residue data resulting from recommended uses in countries other than
the U.S.A. (including glasshouse uses) are needed as well as
additional data from pre-plant uses (including liquid formulations).
Onions - Only limited data were available. Maximum residues from
apparent at-plant applications at less than the maximum recommended
rates were <0.04 mg/kg at 100-150 days or at intervals unspecified.
Data from other countries are needed.
Peanuts - Information on recommended good agricultural practices for
oxamyl on peanuts was not provided although pre-plant application of a
10% granular formulation at 3.4-5 kg ai/ha and 24% liquid formulation
at 3.4-5.6 kg ai/ha are proposed uses in the U.S.A. No specific
pre-harvest interval is proposed. Also proposed is a 1.1 kg ai/ha
foliar application of the 24% liquid formulation. Residue data from
the U.S.A. reflecting these proposed rates were provided. Maximum
residues were 0.04 mg/kg in hulls and 1.0 mg/kg in hay. Combining
maximum residues from separate soil and foliar uses results in 0.06
mg/kg for nuts, 0.15 for hulls and 1.6 for hay. Residues would not be
expected to exceed 0.1, 0.2 and 2 mg/kg in the nuts, hulls and hay
respectively.
Peas - Only very limited data were available and it was on an
unspecified pea variety. Maximum residues from recommended granular
pre-plant application rates were <0.01 mg/kg in pea or pod although
the interval was not given. At over 4 times the recommended granular
rate residues were <0.01 for the pea and 0.15 mg/kg on the pod, again
the interval was not provided. Additional data are needed on specific
peas and with known intervals.
Bell peppers - No residue data were provided from the three countries
for which recommended uses were provided. However, residue data on
bell peppers provided from field trials in the U.S.A. reflect proposed
uses of 0.56-1.1 kg ai/ha foliar and 0.56 kg ai/ha transplant water
treatments. A one-week pre-harvest interval is proposed as compared
to the two-week interval recommended. The foliar treatment was
reasonably close to the maximum recommended foliar use in Table 1.
Maximum residues from the proposed foliar treatment were 1.3 mg/kg at
7 days (1.7 at recommended rates) after last application or at
intervals greater than 8 days. No data were available from the
proposed transplant water treatment alone. Maximum residues from the
proposed transplant water application with a less than maximum foliar
application was 0.7 mg/kg at 7 days. Combined residues could
therefore be expected to be about 2.4 mg/kg at 7 days. A limit of 3.0
mg/kg would allow for some variability due to the relatively small
number of samples. Residue data from additional countries are
desirable and should include data from glasshouse uses.
Pineapples - Good agricultural practice information was not made
available although a liquid formulation pre-plant use at 4.5-9 kg
ai/ha and a foliar use at 1.1-4.5 kg ai/ha with a 30-day pre-harvest
interval are proposed in the U.S.A. Data reflecting the proposed
foliar use were available with maximum residues of 0.91 mg/kg in whole
fruit (27 days), 5.2 in bran (27 days), 1.4 in leaves at 23 days or
0.82 at 35 days and 8.5 in hay (27 days). Residues would therefore
not be expected to exceed 1.0, 6.0, 1 or 10 mg/kg in the whole fruit,
bran, forage, and hay respectively at the proposed 30-day interval.
Root and tuber vegetables (beets, carrots, sugar beets and sweet
potatoes) - Although good agricultural practice information for root
and tuber crops was available from a number of countries, most of the
residue data on the above root and tuber crops were from trials in the
USA where, in addition to USA-recommended uses in Table 1, additional
uses have been proposed. Of these proposed uses, broadcast and furrow
pre-plant liquid formulation application of 4.5-9 and 2.2-4.5 kg ai/ha
respectively are proposed for carrots and similar pre-plant rates and
a 7-day pre-harvest interval are proposed for potatoes. From
recommended application rates and methods of application, maximum
residues on root and tuber crops were 0.06 mg/kg at 14 days after last
application and from proposed uses, 0.1 mg/kg at 7 dove (both on
potatoes). The majority of residues from recommended or proposed uses
were 0.03 mg/kg or less. Residues for the root and tuber crops for
which data are provided would not be expected to exceed 0.1 mg/kg from
recommended and proposed uses. No data were available from processing
fractions of root crops.
Residue data on root and tuber vegetables resulting from recommended
good agricultural practices from additional countries are desirable.
Soybeans - Good agricultural practice information was not available.
A proposed USA use would allow liquid formation at-plant and pre-plant
treatments at 2.2-4.5 kg ai/ha and data from 7 states reflecting and
up to 1.5 times these proposed uses were available for dry beans and
straw. Maximum residues were 0.05 mg/kg for the dry seeds and straw
at 113-185 days after last application although all but one sample
each for seeds and straw from the 24 tests were <0.02 mg/kg. The
maximum 0.05 mg/kg residue in soybean seed was from an application
rate of one-fourth of the maximum proposed. On this basis residues
from the proposed uses would be expected to be less than 0.2 mg/kg.
No data were available for the oil, meal, hulls, forage or hay,
although a forage restriction is proposed. Although no concentration
data for the oil or meal were available, a 0.33 n-octanol water
partition coefficient would suggest that concentration of residues may
not occur. This would be consistent with fractionation studies on
cottonseed and peanuts.
Sugarcane - Good agricultural practice information was not provided
for oxamyl on sugarcane. The limited residue data from trials in
South Africa were not useful because of uncertainties on dosage rates.
Tomatoes - Good agricultural practice information was available from
eleven countries, although of these residue data were available only
from the United Kingdom and the USA.
The application rates for the U.K. residues data cannot be compared to
the 0.1 g/plt use described in the label provided. Maximum residues
from the trials were 0.85 mg/kg at the U.K. recommended 2-week pre-
harvest interval.
In the USA 1-3 day intervals are allowed for foliar uses. Maximum
residues from foliar uses recommended in the USA (FL) were 1.0 mg/kg
at a one-day interval and 1.9 mg/kg at two times the recommended rate.
No data were available for drip irrigation uses. The data would
indicate that residues would not exceed 1.0 mg/kg from U.K. uses and
USA foliar uses, taking into account their different pre-harvest
intervals. However, the relatively small number of samples at a given
rate would dictate a higher limit. Additional data from these and
other countries reflecting good agricultural practices would be
desirable.
FATE OF RESIDUES
Figure 2 lists the names and structures of oxamyl and related
compounds.
Figure 2. Names and structures of oxamyl and related compounds
COMPOUND NAME STRUCTURE
O O
OXAMYL METHYL N',N'-DIMETHYL-N[METHYL " "
CARBAMOYL)OXY]-1-THIOOXAMIMIDATE (CH3)2N-C-C=NOCNHCH3
'
SCH3
(I) OXIMINO METABOLITE O
METHYL N-HYDROXY-N',N'- "
DIMETHYL-1- (CH3)2N-C-C=NOH
THIOOXAMIMIDATE '
SCH3
(II) METHYL N-HYDROXY-N'-METHYL-1- O
THIOOXAMIMIDATE "
CH3NH-C-C=NOH
'
SCH3
Figure 2 (continued)
COMPOUND NAME STRUCTURE
O
(III) N'N-DIMETHYLOXAMIC ACID "
(CH3)2N-C-COOH
O
(IV) N-METHYLOXAMIC ACID "
CH3NH-C-COOH
O
(V) DMCF "
N,N-DIMETNYL-1-CYANOFORMAMIDE (CH3)2N-C-CN
O O
(VI) METHYL N'-METHYL-N- " "
[(METHYLCARBAMOYL)OXY]-1- CH3NH-C-C=NOCNHCH3
THIOOXAMIMIDATE '
SCH3
O O
" "
(VII) N,N-DIMETHYLOXAMIDE (CH3)2N-C-C-NH2
O
"
METABOLITE A GLUCOSE CONJUGATE OF I (CH3)2N-C-C=NO-GLUCOSE
'
SCH3
O
"
METABOLITE A' GLUCLOSE CONJUGATE OF II CH3NH-C-C=NO-GLUCOSE
'
SCH3
In animals
In a ruminant metabolism study two lactating goats were maintained on
diets containing approximately 10 mg/kg 14C-oxamyl for 10 and 20 days
respectively (Harvey, 1980a). 60-70% of the radioactivity was
eliminated in the urine and faeces, about 6% was expired, 2-3% was
found in milk and an estimated 22% in the entrails and carcass. The
radioactive compounds were not identified. However, there was no
evidence of oxamyl or oximino compound in milk, blood and tissues
(<0.01 ppm).
If calculated as oxamyl, maximum residues would have been 0.4-0.6
mg/kg in milk, and about 1.4 mg/kg in blood and tissues. Little was
found in fat. Milk residues peaked at about 10-14 days but in blood
they continued to rise throughout the feeding. In each case residues
rapidly decreased after withdrawal of the fortified diet.
About 5% of the activity in milk was incorporated into natural
lactose, caesin and lipids. Two additional components were isolated
and accounted for an additional 30% of milk residues.
In a more recent study 14C-labelled oxamyl and selected metabolites
were studied in vitro by incubation in rumen fluid of a Holstein
cow (Belasco and Harvey, 1980). Oxamyl was 41% metabolized within one
hour and 99% within six hours. After 24 hours incubation the oximino
metabolite (I) and DMCF (V) accounted for 80% of the radioactivity (67
and 13% respectively). The remaining radioactivity at 24 hours was
dimethyloxamic acid 5%, dimethyloxamide 10% and 1-2% each of minor
metabolites previously identified in rat metabolism studies.
Metabolite A, a major plant metabolite, was almost totally metabolized
by rumen fluid. This contrasts to rat metabolism where this compound
is largely unchanged in vitro by liver microsomes and only slowly
metabolized in vivo (Harvey and Han, 1978a). About 70% of
metabolite A was metabolized to DMCF by rumen fluid and the remainder
to unidentified components. The DMCF was found to metabolize to
N,N-dimethyloxamide, N,N-dimethyloxamic acid and N-methyloxamic acid.
Only the dimethyloxamide was not previously identified during rat
metabolism studies.
In a livestock-feeding study oxamyl was fed to Guernsey dairy cows at
2, 10 or 20 mg/kg in the diet for 30 days (DuPont, 1973). Recoveries
of added oxamyl were 70% or greater in whole milk, milk fat, or milk
aqueous fractions at 0.02-0.2 mg/kg fortification levels and over 80%
at 0.04-0.40 mg/kg levels in meat. No residues of oxamyl were
detected (<0.02 mg/kg) in any sample of milk or milk fractions,
liver, kidney, lean muscle or subcutaneous fat at any of the feeding
levels.
When these same samples were analysed for DMCF, no residues were found
at any feeding level in milk or tissues (<0.02 mg/kg for milk, <0.04
mg/kg for meat and fat). Recoveries were 67-82% for 0.02-0.05 mg/kg
fortification levels in milk and 46-100% at 0.04-0.2 mg/kg
fortification levels in meat and fat (Du Pont, 1976).
In a poultry-feeding study, adult laying hens were fed diets
containing 0, 1 or 5 mg/kg oxamyl for a four-week period (Zahnow,
1978). Samples of eggs and tissues were collected and analyzed for
oxamyl and DMCF. Oxamyl residues were <0.02 mg/kg in eggs, liver,
muscle and fat and <0.05 mg/kg in skin (due to limited sample
availability). Residues of DMCF were reported as <0.01 mg/kg in
eggs, liver, muscle, fat and skin.
Oxamyl recoveries from eggs averaged 76% at the 0.02 mg/kg
fortification level and at this same level, except for an apparent
error in skin data, averaged 85-97% in meat, tissues, skin and fat.
Recoveries of DMCF were relatively low, averaging 58 and 50% at 0.01
and 0.02 mg/kg fortification levels, respectively, in eggs and 54-63%
in meat, fat and skin tissues at a 0.02 mg/kg fortification level.
In plants
Plant metabolism of oxamyl was studied in tobacco, alfalfa, peanuts,
potatoes, oranges and tomatoes (Harvey, Han and Reiser, 1978).
In one experiment two tobacco plants were each treated on the leaves
with 10 mg 14C-oxamyl and grown in a growth chamber for 7 and 15 days
respectively. 95% of the radioactive material was recovered at 15
days; about 50% was surface residues, 37% extractable leaf internal
residues and 0.1% or less in the roots. About 4-5% were volatile
components. The surface residue was 95% oxamyl and 3% the oximino
compound (I). Of the leaf internal extractable residues, 56% was
oxamyl, 5% oximino compound (I) and 39% was in the polar fraction, 93%
of which was metabolite A and 7% N,N-dimethyloxamic acid. The
seven-day plants were not as extensively analyzed, but to the extent
they were, residues were similar to the 15-day residues with the
proportion of individual residues reflecting the shorter metabolism
period.
Alfalfa received three 0.5 lb. ai/acre 14C-oxamyl spray field
treatments at two-week intervals and was harvested 2.5 weeks after
last treatment. Extractable residues were 75% of applied. Of this,
>90% was metabolite A with only about 0.8% each for oxamyl and the
oximino compound (I).
Peanuts received two foliar field treatments of 14C-oxamyl at 2 lb.
ai/acre. Samples of young plants were taken at four weeks after the
first treatment, immediately before the second treatment, and
additional samples at harvest. There were therefore three fractions
analyzed - young plants, mature hay and mature nuts. In the young
plant 99% of the radioactivity was determined to be a 2:1 ratio of two
metabolites, A and A' (see figure 2). No oxamyl or oximino compound
(I) was detected.
In mature hay ß-glucosidase treatment of a very polar fraction
containing 99% of extractable residues released metabolites A and A',
giving evidence of an initial polysaccharide type compound, i.e.
metabolites A and A' with additional hexose units (see Figure 3).
Also about 1% of the residue was the oximino compound (I) and <0.5%
oxamyl. When unextractable residues (about 40% in mature hay or nuts)
were treated with a mixture of cellulose enzymes, about 60% was
released yielding a fraction characteristic of metabolites A and A'.
This suggests that the initial unextractable residue was a cellulose
of starch-like structure.
No oxamyl or oximino compound could be detected in the nuts. Of
extractable residues about 21% was incorporated into peanut oil
lipids, about 18% into the polysaccharide-type structure found in
mature hay, about 15% was in lipids or as glucose conjugates and about
37% was unextractable. Of the unextractables about 60% was in the
form of the cellulose-starch type structures discussed above.
Potatoes received five foliar field treatments of 14C-oxamyl at 0.5
to 1.0 lb ai/acre giving an oxamyl residue equivalent of 7 mg/kg in
the tuber. Peels contained <1% of the total residue. Free oxamyl or
oximino compound accounted for <3% of the residue, although mild acid
hydrolysis yielded 39% of the radioactivity in the form of oximino
compounds I and II.
Hydrolysis with ß-glucosidase yielded metabolites A and A', suggesting
the initial presence of the polysaccharide conjugates discussed under
mature peanut hay. At least 35% of the 14C was determined to be
incorporated into the glucose of the tuber starch.
Apples were brush-treated in an orchard at 1.0 lb ai/100 gal and 6
weeks later at harvest yielded 0.8-2.0 mg/kg residues, evenly
distributed through the fruit. About 98% of the residue was
extracted, 77% being organosoluble. Of the organosolubles 16% was
oxamyl, 42% oximino compound I, and 17% DMCF. The remaining 23% of
total fruit residue appeared to be in the form of polysaccharide-type
structures.
Oranges were harvested for analysis six weeks after brush application
of 14C-oxamyl to immature oranges at 1.2 lb. ai/100 gal. Residues
were 2.5 mg/kg on a whole fruit basis expressed as oxamyl. Rind
contained 82% of the residue and the juice 18%. On a whole fruit
basis, residues were 9% oxamyl, 6% oximino compound, 20% DMCF, 35%
metabolite A, 22% metabolite A' and 8% unidentified polar metabolites.
Small green tomato fruits were evenly spotted by pipet with 0.37 mg
14C-oxamyl each and the mature fruit harvested two weeks later for
analysis. Residues were 59% oxamyl, 13% oximino compound, 5%
metabolite A, 4% DMCF and 19% polar metabolites and natural products.
The studies on tobacco, peanuts, and potatoes demonstrate the systemic
nature of foliarly applied oxamyl, which readily translocates to the
roots, legumes and tubers respectively.
Figure 3 summarises a major metabolic pathway for oxamyl in plant
tissues. The methylcarbomoyl group is hydrolysed to form the oximino
compound, followed by conjugate formation of the oximino compound with
glucose to form metabolite A, which may undergo demethylation to form
metabolite A'. Metabolite A predominates in short-term studies (2-6
weeks) with metabolite A' identified in moderate-length studies (1-2
months). At harvest little uncomplexed metabolite A or A' is found
but 14C is incorporated into polysaccharide, cellulose or starch-like
structures suggesting the addition of hexose units to metabolites A or
A'.
In addition to the metabolic route shown in Figure 2, oxamyl may also
be completely broken down in plants and incorporated into natural
plant lipids or into the glucose of starch. The metabolite DMCF is
frequently found as a plant residue and so is the oximino compound in
some fruits. No residues of the S-oxide or S,S,-dioxide of oxamyl or
its oxime were observed during these investigations.
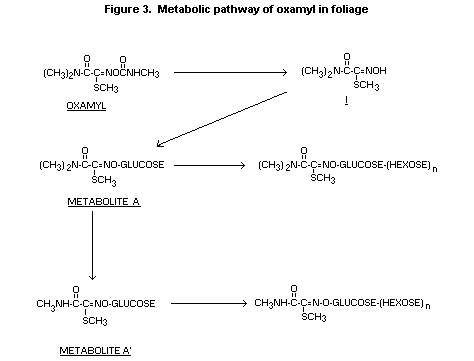 In soil and water
Numerous studies have been conducted on oxamyl soil metabolism,
decomposition, dissipation, adsorption, mobility effect on soil
microorganisms and on dissipation and decomposition in water. In one
compilation of studies the decomposition of oxamyl in soil and water
was investigated with various soils under aerobic and anaerobic
conditions and in distilled or river water at various pHs, with
artificial UV light or sunlight, and in the dark. Also included were
experiments on soil leaching and mobility in various soils (Harvey and
Han, 1978b).
Oxamyl stability was found to be highly pH dependent, hydrolysing to
the oximino compound I only under mildly basic conditions. In
pH-adjusted distilled water 3% and 9% hydrolysis was observed after 24
and 48 hours respectively at a pH of 6.9 and 30% within 6 hours at a
pH of 9.1. At a pH of 4.7 it was completely stable at least through a
4-day period.
In another of these studies 14C-oxamyl was rapidly degraded by soil
under aerobic conditions in a glass metabolism apparatus. The soil
was treated at a rate of 4 lb. oxamyl/acre. After 42 days under
aerobic conditions 51% of the 14C had been liberated as 14CO2, 4%
remained as oxamyl, <1% compound I and 37% as unextractable or
unidentified polar tractions, much of which was found to be
incorporated into soil organic fractions. Under anaerobic conditions
analogous residues at 42 days were 3, 8, 41, and 48% for CO2, oxamyl,
compound I and unextracted or unidentified residues respectively.
A half-life of 11-15 days was found under aerobic conditions in the
laboratory when soil was treated at a rate of 6 mg/kg. Under
anaerobic conditions the half-life was about six days. In another
study the half-life of oxamyl in a loamy sand was 14 days.
A study to the effect of soil moisture at 15°C indicated half-lives of
13-14 days in clay loam or loamy sand and 34-39 days in peaty or humic
loamy sand (Smelt et al, 1979). As soil moisture decreased to the
wilting point there was a gradual decrease in conversion and this
decreased further at below-wilt moisture levels with clay loam. In
humic loamy soil the conversion rate increased sharply at very low
soil moisture content.
In a soil-TLC experiment oxamyl appeared to be moderately to highly
mobile with Rf values of 0.53-1.0 in four soils (Harvey and Han,
1978b). By one system, compounds with Rf values of 0.35-0.64 are
rated as moderately mobile and those with Rf values of 0.96-1.00 as
highly mobile. This finding was consistent with laboratory adsorption
studies on three soils which indicated no adsorption under conditions
of the test. This is also consistent with another laboratory soil
adsorption-leaching study in which oxamyl was found to be highly
mobile with 61-100% eluted through a 45 cm × 5 cm diameter column of
aged and fresh soil with 20 inches of water (Chrazanowski, undated).
Of unleached residues >70% remained in the top five cm.
This high mobility observed in laboratory studies was not confirmed by
field studies. In another of the collection of studies by Harvey and
Han (1978b), volatility losses of 14C from three soil types (to a 15
inch depth) treated at 6 lb. ai 14C-oxamyl/acre were 80-93% of
original treatment at three-five months. Only traces of oxamyl or
compound I (<0.05%) was detected at three-five months in a soil
extract although significant amounts were found at one week and one
month. Unextracted residues accounted for 14% of the original
treatment at three-five months. The leachate from a fine sand had the
highest residues of the three soil types with 14C residues up to 6.8%
of original treatment. Of this 6.8% about 1% was oxamyl, 11%
unidentified polar materials and 88% compound I. Analysis again
indicated 14C incorporation into soil organic fractions.
In another of these studies oxamyl field soil leaching-disappearance
studies revealed residues of 3.2 mg/kg (calculated as oxamyl) in the
top four inches at zero days when treated at 5.7 lb ai/acre. Residues
decreased to 0.13 mg/kg in the top four inches at 30 days with a
half-life of about one week. Residues at the four-eight inch depth
peaked at 0.15 mg/kg at nine days; at the 8-12 inch depth at 0.15
mg/kg in the 9-23 day period; at the 12-18 inch depth at 0.11 mg/kg in
a 9-23 day period; and at 0.06 mg/kg at 23 days at the 18-24 inch
depth. Residues were negligible (<0.04 mg/kg) through a 60-day
interval at the 24-30 inch depth.
Therefore, under practical conditions the field leaching and
dissipation experiments do not support laboratory tests, which
indicate that oxamyl is highly mobile. They indicate some mobility
and rapid dissipation, presumably mostly as CO2, with a half-life in
soil of six to eight days with only trace residues leaching below
15-18 inches within a 2-3 month period. The rapid dissipation
apparently minimizes leaching under field conditions.
In a crop rotation study, cabbage, red beets and sorghum seeds were
grown in the greenhouse in soils treated 30 and 120 days earlier with
14C-oxamyl at 8 lb ai/acre (Harvey, undated). At 30 days 19% of the
original oxamyl remained in the soil. Crops planted therein had at
maturity residues of 0.6-4 mg/kg (calculated as oxamyl) or 0.01 to
0.12 mg/kg of oxamyl plus its hydrolysis product. At 120 days <1% of
the original oxamyl remained. Mature crops from the 120-day soil
contained <0.2 mg/kg residue (calculated as oxamyl), of which <0.02
mg/kg was oxamyl plus its oximino metabolite. No DMCF was detected.
The experiment would suggest that low levels of oxamyl plus its
oximino metabolite could occur in these commodities if they are
planted within 30 days of high soil applications of oxamyl. Similar
experiments in the field on other commodities reflecting maximum use
rates would be desirable, including use of both liquid and granular
commercial formulations.
Two studies were conducted on the possible effects of oxamyl on soil
microorganisms. In one controlled laboratory study 5.0 mg/kg oxamyl
in silt loam soil increased the time for 50% nitrification by 5 days
although total nitrification after 3 weeks was the same as in the
control (Han, undated). No significant effect was observed at a 0.5
mg/kg oxamyl level.
In the other laboratory study the effect on the population and
respiration of microorganisms was studied in soils treated at 10 mg/kg
oxamyl (Peeples, undated). No reduction was observed in the
populations of either fungi or bacteria at one, two, four and eight
weeks when compared to controls. Respiration of soil microorganisms
in these oxamyl treated soils was determined by measurement of CO2
evolution. Oxamyl had no effect on CO2 evolution.
In storage and processing
No information was provided on residues in stored commodities. Data
were provided on the effect of processing or simulated processing
residues in several commodities:
Apple - When apples with 1.2 mg/kg aged residues were processed in the
laboratory to juice and wet and dry pomace (11O°C, 17 hours) residues
were reduced to 0.5, 0.85 mg/kg and none, respectively.
Citrus - No concentration of residues was found in press juice, wet
pulp, or dried pulp when field-treated oranges and grapefruit with
terminal residues of 0.51 and 0.62 mg/kg respectively were analyzed.
Residues of 0.37 and 0.30 mg/kg in the wet pulp of oranges and
grapefruit respectively were essentially destroyed during the drying
process (<0.05 mg/kg remaining).
Cottonseed - In a fractionation study in which cottonseed was
fortified with oxamyl at 0.2 and 0.5 mg/kg, residues did not
concentrate in the meal or oil. Residues recovered in the meal were
5-8% of those on the undelinted seed; no residues were recovered in
the oil. No conversion to DMCF was detected. Fortifications with
metabolite A, the oximino compound I and DMCF also resulted in major
losses of these compounds during fractionation. The details of this
study were not provided to the meeting.
Peanuts - In a laboratory-simulated commercial oil extraction with
refluxing hexane, maximum residues in oil and meal were 10 and 28%,
respectively, of those in peanuts fortified at 0.2 and 2.0 mg/kg.
Pineapple - A simulated commercial processing study demonstrated that
residues in bran may be 0.4-6 times that in the whole fruit. This is
supported by actual residue data in Table 2. A similar concentration
occurs with the metabolite DMCF.
Soybeans - Although no concentration data were available for soybean
oil or meal, a 0.33 n-octanol water-partition coefficient would
suggest that concentration may not occur. This would be consistent
with fractionation studies in cottonseed and peanuts.
Tomatoes - When tomatoes with field-incurred or fortified residues of
1-10 mg/kg oxamyl were ground and concentrated by cooking to a puree
of one half the original weight, residues in the puree in mg/kg were
one half or less of those in tomatoes. Analytical recoveries were
61-110%.
Photodecomposition
Hydrolysis of oxamyl to the oximino compound (I) was accelerated by UV
light in both distilled water and river water, more rapidly in the
latter (Harvey and Han, 1978b). In river water treated with
artificial UV light, residues after seven days were 22% oxamyl, 39%
oximino compound (I) and 3% polar fractions. In the dark, control
residues were 84, 16, 0 and 0% respectively for the same compounds at
ten days.
Oxamyl degradation was enhanced still further when sunlight was used.
Oxamyl decreased from 100% at zero day to zero after about 1.5 days.
Oximino compound (I) decreased from a maximum of about 95% of the
total radioactivity at 1.5 days to about 68% and 50% at seven and 21
days, respectively, while its geometric isomer was increasing from
about 4% at 1.5 days to 32 and 42%, respectively, at seven and 21
days. The oximino compound (I) and its geometric isomer declined
after about 21 days, while the polar fraction increased from about 3%
of the activity at 14 days to 20% at 42 days.
At 42 days 14% of the total radioactivity was N,N-dimethyloxamic acid
and two unidentified polar compounds, which accounted for 3% of the
total. A 17% loss of radioactivity during the six-week study was
attributed to the loss of CO2. No S-oxidation products of oxamyl or
its oximino compound were observed.
RESIDUES IN COMMERCE OR AT CONSUMPTION
No information was provided to the meeting on evidence of residues in
food in commerce or at consumption.
METHODS OF ANALYSIS
Methods are available for the analysis of oxamyl alone, oxamyl plus
its oxime, and for its DMCF metabolise.
A method for the analysis of oxamyl alone uses column chromatography
to separate oxamyl from its oxime (Bromilow, 1976). Oxamyl is
extracted by blending with acetone dichloromethane (1:1) except for
potatoes, in which case only dichloromethane is used. After
concentration the extract is chromatographed on a Florosil column with
acetone as the eluting solvent. The eluant is concentrated to a small
volume for gas chromatographic analysis.
An aliquot of sample and methanolic trimethylphenylammonium hydroxide
is injected into the gas chromatograph for on-column derivatization to
the methoxime derivative. The gas chromatographic column is 0.5%
carbowax 20 M + 5% SE-30 and detection is by a flame photometric
detector equipped with a 394 nm filter. At 0.02-0.04 mg/kg
fortification levels average recoveries ranged between 87-91% on
barley, peas, and potatoes and 69% on tomatoes. Controls were
generally <0.01 mg/kg although a high of 0.15 mg/kg apparent residue
has been observed on pea pods. Recoveries average 87-96% on soils.
The low tomato recoveries were attributed to incomplete extraction of
residues from extract concentrate. On the basis of discussions, which
will follow, it is probable that part of this loss was due to the acid
catalyzed degradation of oxamyl by the highly acidic tomatoes.
In another procedure oxamyl residues are determined as the oximino
metabolite and expressed as oxamyl by using a 1.35 conversion factor
(Holt and Pease, 1976). The method also measures any free oxamyl
oxime and this was confirmed with recoveries of 65 and 57% when
tobacco was fortified with the oxime at 0.2-0.4 mg/kg.
Oxamyl (and its oxime) are extracted from plant and animal tissues and
soil by blending with ethyl acetate. After concentration and
partitioning with hexane the aqueous extract is made alkaline (pH 12)
with 1 N NaOH and partitioned with chloroform, which is discarded.
The aqueous extract is heated to convert oxamyl to its oxime and again
partitioned with chloroform. The aqueous phase is partitioned with
ethyl acetate - methanol (9:1) and concentrated for analysis with a
flame photometric detector equipped with a 394 nm filter. The
chromatographic column is 10% SP-1200/1% H3PO4 on Chromosorb W AW.
This method has been successfully used on over 25 agricultural
commodities as well as on milk, animal tissues, soil, urine and
faeces. Average recoveries generally range from 75-100%. A
sensitivity of about 0.02 mg/kg is generally attainable for most
commodities, although apparent residues of 0.04-0.05 mg/kg may occur
at times in beans, citrus, peanut hay and onions and up to 0.26 mg/kg
on bean foliage.
Successful method trials using the Holt-Pease method were conducted in
laboratories of the Environmental Protection Agency in the United
States. Recoveries were 77-82% at oxamyl-fortification levels of 3
and 6 mg/kg in celery and 0.2-0.4 mg/kg in cottonseed.
Somewhat low recoveries have been observed during analysis of highly
acidic commodities, such as citrus, tomatoes, and peaches. This can
be minimized by making concentrations under alkaline conditions.
A method is also available for the determination of the DMCF
metabolite of oxamyl (Holt, 1976). In this method the sample is
extracted by blending with ethyl acetate and a potassium dihydrogen
phosphate - NaOH buffer. After concentration to an aqueous phase the
sample is partitioned with hexane (which is discarded) and
re-extracted into ethyl acetate, which is concentrated for analysis.
Analysis is on a 5% Carbowax 20 M gas chromatographic column and
detection with a nitrogen - phosphorous detector. Recoveries on
citrus, celery, strawberries and tomatoes averaged 66-100% and 60% on
potatoes when the commodities were fortified at 0.04-2.0 mg/kg DMCF.
Average recoveries were 74-100% on tomato paste, cottonseed oil,
cottonseed meal and tobacco fortified at 0.2-2.5, 0.2-1.0, 0.1-0.50
and 0.05-0.5 mg/kg respectively. The sensitivity is generally
considered to be 0.04 mg/kg.
The buffer was incorporated into the procedure to minimize conversion
of oxamyl or its oxime to DMCF when highly acidic commodities were
analyzed. This conversion was 5-15% without the buffer and, as
discussed earlier, was observed by Holt and Pease (1976), reduced
recoveries during the development and use of their oxamyl method. The
authors also note that under some conditions some thermal degradation
of oxamyl or its oxime to DMCF may occur in gas chromatographic
injection ports with temperatures in excess of 100-110°C.
NATIONAL TOLERANCES REPORTED TO MEETING
Commodities Tolerances (mg/kg)
USA Netherlands Fed. Rep. of
Germany
apples 2
bananas 0.1 (reflecting 0.02 mg/kg in pulp)
celery 3
citrus 3
cottonseed 0.2
cucumbers 1
pineapple (whole) 1
pineapple forage 10
potatoes 0.1 0.05
sugarbeet (roots) 0.05 (provisional)
tomatoes 2 1
EVALUATION
COMMENTS AND APPRAISAL
Oxamyl is a systemic insecticide, miticide and nematicide registered
or approved for use in numerous countries. It is available as a
liquid or granular formulation and is systemic whether applied
foliarly or to the soil. Foliar applications result in nematocidal
action in the roots and likewise soil applications result in
insecticide and miticidal action in the foliage. It is also effective
as a direct contact insecticide, miticide, and nematicide.
Recommended use information for over 30 countries was provided to the
meeting by the manufacturer. Only a few countries officially provided
this information directly to the meeting.
Oxamyl is degraded in animals by two major pathways, hydrolysis to the
oximino compound and enzymic conversion to N,N-dimethyloxamic acid.
Metabolism studies with 14C-oxamyl in two rats showed that only 70%
of the dose was eliminated in the urine and faeces in 72 hours.
Substantial amounts (22% of the dose) were incorporated into the body
tissues, especially the skin and hair, partly by conversion of
14C-metabolites into natural amino acids. The meeting expressed
concern over the high tissue residues and the lack of identification
of much (50%) of this material.
Oxamyl has a high acute toxicity displaying the typical effects of
carbamate insecticides: rapid onset of cholinesterase inhibition
followed by rapid complete recovery. There was no evidence of delayed
neurotoxicity. The acute toxicity of most of the metabolites is lower
than oxamyl. Oxamyl is not mutagenic or teratogenic.
In a 3-generation rat reproduction study, 50 mg/kg of oxamyl in the
feed slightly decreased the body weight of the weanlings.
The dog is somewhat less sensitive than the rat. In a 90-day and
2-year feeding study a no-effect level of 100 mg/kg was observed. In
the long-term study with rats growth inhibition was found at 100 and
150 mg/kg. A slight effect was also observed in the males at 50
mg/kg. Owing to the lack of a clear no-effect level in the rat and
lack of identification of 50% of the tissue residues, only a temporary
ADI, based on the 2-year study in dogs, was allocated.
Residue data were provided on 29 commodities or groups of commodities.
Residues on individual crops were highly variable, reflecting the many
combinations of uses and pest control needs. Because of the many
variables it was necessary to significantly condense detailed
information on residues in table 2. However, the detailed data were
examined by the meeting.
In some cases no residue data were available from countries for which
recommended or approved use information was available but from a
country with proposed uses. In other cases no recommended or approved
use information was available, although residue data were provided
from a country with a proposed use. Estimate of residues were made on
the basis of available data. The basis for those estimates are
discussed.
In plants a major metabolic route is the conversion of oxamyl to its
oxime, which conjugates with glucose to form metabolite A. This in
turn may add additional hexose units to build progressively into
polysaccharide and starch and cellulose, like structures. With time,
demethylation of metabolite A can occur to form metabolite A' which
way also add additional hexose units. In some plants complete
breakdown of oxamyl occurs with incorporation into natural plant
molecules such as glucose and lipids. The metabolite DMCF is a
residue in some plants and the oxime is found in some fruits. No
residues were observed for the S-oxide or S-dioxide of oxamyl.
Surface residues may be up to 50% of the total and most of this is
oxamyl in some plants.
In an in vitro rumen fluid metabolism study oxamyl was rapidly and
almost totally metabolized. The oximino and DMCF metabolites
accounted for 80% of the radioactivity after 24-hour incubation.
These and remaining metabolites were similar to those identified in
the rat metabolism studies.
The major metabolite of plant metabolism, the glucose conjugate of the
oxime, was almost completely metabolized in rumen fluid, mostly to
DMCF. This contrasts to the rat metabolism where this metabolite was
resistant to metabolism. When DMCF was metabolized in the rumen fluid
the same metabolic products identified in the rat were found plus
dimethyloxamide.
In livestock feeding studies at up to 20 mg/kg in the diet of cattle
and up to 5 mg/kg in the diet of poultry, no residues (<0.02 mg/kg)
were found in animal tissues, milk, fat or eggs. No feeding studies
(or metabolism studies) were provided, for non-ruminant livestock
other than poultry. The available data suggest that there is no
likelihood of residues in meat, fat, milk, poultry or eggs from
approved uses on certain crops. However, additional residue data from
recommended uses on other important crops used for animal foodstuffs,
e.g. sugarbeet leaves, are needed.
Oxamyl is stable in acidic to neutral water but rapidly hydrolyses
under alkaline conditions. Ultraviolet light catalyzes the
degradation to the oxime or its geometric isomer and eventually to
polar compounds.
Laboratory studies indicate that oxamyl should be highly mobile in
soils, but field decomposition and leaching studies do not support
this. This is apparently because the relatively rapid degradation
does not allow sufficient time for significant mobility. On the basis
of laboratory metabolism studies a major route of dissipation appears
to be the evolution of CO2, although soil pH is a factor in some
soils. Significant residues seldom leached below an 18 inch depth
under the study conditions. The half-life of oxamyl in soil was
highly variable, ranging from 6 -39 days, depending on variables such
as location, soil type and moisture content.
In crop rotation studies there was evidence that low levels of
residues could occur in follow-up crops where oxamyl has been used in
the soil. These studies were 14C-oxamyl greenhouse studies. Crop
rotation studies under field condition and reflecting recommended
usage would be desirable.
No evidence of a permanent effect on soil microorganisms by the use of
oxamyl was observed.
No data were available on the effect of storage on oxamyl residues or
on residues in food in commerce or at consumption. Studies were
available on the effect of processing on several commodities. No
concentration of residues was noted except in the case of pineapple
bran where a 6-fold concentration occurred.
Two methods of analysis are available. In one, oxamyl is separated
from its oxime and derivatized on a gas chromatographic column to its
methoxime. In the second, oxamyl and its oxime are extracted together
and the oxamyl hydrolysed to its oxime for the gas chromatographic
determination of total oxamyl plus free oxime residue as the oxime.
Although both methods appear to be adequate, the latter would appear
to be the method of choice for enforcement because it has apparently
been much more extensively used and tested. Most of the residue data
provided to the meeting were developed with this procedure. A
disadvantage would be that it also measures the free oxime, which may
not be of major toxicological concern. The oxime is not, however, a
major residue in many plants, although it can be in some fruits.
The meeting examined residue data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
oxamyl wee used in practice and when reported intervals between last
application and harvest were observed,
Level causing no toxicological effect
Dog: 100 mg/kg in the diet, equivalent to 2.5 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. The levels refer to
the sum of oxamyl plus its oxime calculated as oxamyl. Some figures
are temporary (T), because either there is no recommended use or the
data were inadequate to estimate a maximum residue level.
Estimated Maximum Pre-harvest
Residue levels Interval (days)
Commodity (mg/kg)
apples 2 14
banana 0.051
beans, kidney 3 (T) 7
beans, kidney (dry) 0.051 (T) 50
beans, lima 3 (T) 7
celery 3 14
citrus 3 7
maize 0.051 (T) (at planting use)
cottonseed 0.2 21
cucumber 2 1
melon 2 1
summer squash 2 1
watermelons 2 1
peanuts 0.1 (T)
peanut fodder 2 (T)
peppers, bell 3 7
pineapple 1 (T) 30
root and tuber vegetables:
beets 0.1 14
carrots 0.1 14
potatoes 0.1 7
sugar-beets 0.1 7
sweet potatoes 0.1 (T) (apply within one week of
planting)
soybeans (dry) 0.051 (T) (pre-planting)
tomatoes 2 1
1 at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Identification of animal tissue residues.
2. Clarification of the no-effect level in the rat especially in
relation to the marginal effect of 50 mg/kg on the body weight in
several studies.
3. Further data on beans, maize and soybeans for reconsideration of
the temporary recommendations.
4. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
5. Approved use information on those items for which only proposed
uses were available.
6. Further data are required on beans, maize and soybeans for
reconsideration of the temporary recommendations.
7. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
8. Approved use information on those items for which only proposed
uses were available.
Desirable
1. Residue data from short-term feeding studies on pigs.
2. Toxicological observations in man.
3. Additional residue data from trials reflecting GAP in additional
countries; in particular residue data on onions, peas, and sugarcane
for which there are existing use patterns.
4. Information on residues in foods in commerce and at consumption.
5. Information on the effect on oxamyl residues of cooking, processing
or storage of raw agricultural commodities.
6, Crop rotation studies on additional commodities and under field
conditions with applications of commercial formulations (both granular
and liquid) according to maximum recommendations.
REFERENCES
Ashley, W.E. Acute oral test. Unpublished report, dd. 29-8-1974, from
the Haskell Laboratory, report no. 585-74, submitted to WHO by Du Pont
de Nemours and Company.
Barbo, E.C. Oral LD50 test. Unpublished report, dd. 9-10-1972, from
the Haskell Laboratory, report no. 399-72, submitted to WHO by Du Pont
de Nemours and Company.
Barnes, J.R. and Aftosmis, J.G. Cholinesterase tests with oxamyl.
(1978) Unpublished report from Haskell Laboratory. Report no. 270-78,
submitted to WHO by Du Pont de Nemours and Company.
Barras, C.E. Acute inhalation toxicity. Unpublished report, 19-2-1974,
from the Haskell Laboratory, report no. 30-74, submitted to WHO by Du
Pont de Nemours and Company.
Belasco, I.J. and Harvey, J. Jr. In vitro Rumen Metabolism of
14C-Label Oxamyl and Selected Metabolites of Oxamyl. J. Agric. Food
Chem., Vol. 28(4), 689
Bromilow, R.H. Determination of Residues of Oxamyl in Crops and Soils
by Gas-Liquid Chromatography. Analyst, Vol. 101, 982.
Chrazanowski, R.L. Soil Absorption Studies With Vydate(R) Oxamyl
Insecticide/Nematicide. Unpublished study provided to FAO as Vol. 1,
Section I, May 30, 1980, Exhibit 2 by the Biochemicals Department,
Experimental Station, E.I. Du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, 19898.
Carroll, K.S. Oral LD50 test. Unpublished report, dd. 23-8-1972, from
the Haskell Laboratory, report no 327-72, submitted to WHO by Du Pont
de Nemours and Company.
Civo. Gas Chromatographic Analysis of Residues of Oxamyl Residues in
Cucumbers and Tomatoes, Report no. R 6282, Ir., PDA, Olthof (1979)
Central Institute for Nutrition and Research, the Netherlands.
Colburn, C.W. Acute skin absorption toxicity tests on rabbits.
Unpublished report, dd. 30-12-1970, from the Haskell Laboratory,
report no. 282-70, submitted to WHO by Du Pont de Nemours and Company.
Culik, R. and Sherman, H. Teratogenic study in rats with
S-methyl-l-dimethylcarbamoyl-N [(methylcarbamoyl)oxy] thioformimidate
(IND-1410). Unpublished report, dd. 8-1-1971, from the Haskell
Laboratory, report no. 5-71, submitted to WHO by Du Pont de Nemours
and Company.
Dale, N.C. Oral LD50 test. Unpublished report, dd. 30-3-1973, from
the Haskell Laboratory, report no. 126-73, submitted to WHO by Du Pont
de Nemours and Company.
Dion, L.K. Fifteen-exposure dermal study with IND-1410 liquid
S-methyl-l-dimethyl-carbamoyl-H-[(methylcarbamoyl)oxy]
thioformimidate. Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 235-70, submitted to WHO by Du Pont de Nemours
and Company.
Du Pont. Oxamyl Livestock Feeding Studies, Milk and Meat. This
unpublished study, dated January 1973, was provided to FAO as Vol. I,
section 3, May 30, 1980, exhibit 17, by the Biochemicals Department of
Du Pont.
Du Pont. Livestock Feeding study. This unpublished study, dated
4-19-76 was provided to FAO, Vol. I, Section 3, May 30, 1980, Exhibit
18, by the Biochemicals Department of Du Pont.
Du Pont. Residue data were provided to FAO as U.S. data - Vol. I,
Section 4, Exhibits 21-28, May 30, 1980 by the Biochemicals Department
of Du Pont.
Fretz, S.B. Ten-dose subacute oral test. Unpublished report, dd.
25-6-1968, from the Haskell Laboratory, report no. 150-68, submitted
to WHO by Du Pont de Nemours and Company.
Fretz S.B. Acute oral test. Unpublished report, dd. 27-12-1968, from
the Haskell Laboratory, report no. 300-68, submitted to WHO by Du Pont
de Nemours and Company.
Fretz S.B. Oral LD50 test. Unpublished report, dd. 31-7-1969, from
the Haskell Laboratory, report 214-69, submitted to WHO by Du Pont de
Nemours and Company.
Gibson, J.R. Oral LD50 test. Unpublished report, dd. 21-5-73, from
the Haskell Laboratory, report no. 236-73, submitted to WHO by Du Pont
de Nemours and Company.
Han, J. C-Y. and Harvey, J. In vitro rat liver microsomal
metabolism of oxamyl and selected metabolites of oxamyl. (undated)
Unpublished report from Du Pont de Nemours and Company, submitted to
WHO.
Han, J. C-Y. Evaluation of Possible Effects of DPX-1410 on Nitrifying
Bacteria In Two Different Soils. This unpublished undated study was
made available to FAO as Vol. I, Section 1, May 30, 1980, Exhibit 5,
by the Biochemicals Department of Du Pont.
Harvey J. and Han, J. C-Y. Metabolism of oxamyl and selected
metabolites in the rat. J. Agric. Food Chem. 26, 902-910.
Harvey, J. Jr. and Han, J. C-Y. Decomposition of Oxamyl In Soil and
Water. J. Agric. Food Chem, Vol. 26(3), 537.
Harvey, J. Jr., Han J. C-Y. and Reiser, R.W. Metabolism of Oxamyl In
Plants. J. Agric. Food Chem., Vol. 26(3), 529.
Harvey, J. Jr. Metabolism of 14C-Oxamyl in the Lactating Goat. This
unpublished undated study was made available to FAO as Vol. I, Section
2, May 30, 1980, Exhibit 9, by the Biochemicals Department of Du Pont.
Harvey, J. Jr. Crop Rotation Study with 14C-Oxamyl in the Greenhouse.
This unpublished undated study was made available as Vol. I, Section
1, May 30, 1980, Exhibit 3 by the Biochemicals Department of Du Pont.
Henry J.E. Acute oral test (mouse). Unpublished report, dd. 2-6-1976,
from the Haskell Laboratory, report no. 387-76, submitted to WHO by Du
Pont de Nemours and Company.
Holsing, G.C. 13-Week oral administration - dogs, insecticide 1410,
final report. Unpublished report, dd. 10-10-1969, from the Hazleton
Laboratories, Inc., submitted to WHO by Du Pont de Nemours and
Company.
Holt, R.F. Residue Procedure for the Determination of Oxamyl
Metabolite N,N-Dimethyl-l-cyanoformamide (DMCF). This unpublished
method, dated 5-3-76 was made available to FAO as Vol. I, Section 3,
May 30, 1980, Exhibit 16, by the Biochemicals Department of du Pont.
Holt, R.F. and Pease, H.L. Determination of Oxamyl Residues Using
Flame Photometric Gas Chromatography, J. Agric. Food Chem., Vol.
24(2), 263.
Kaplan, A.M. Ninety-day feeding study in rats with
1-cyano-N,N-dimethylformamide (INN-79), metabolite of Vydate.
Unpublished report, dd. 26-8-1976, from the Haskell Laboratory, report
no. 630-76, submitted to WHO by Du Pont do Nemours and Company.
Ki Poong Lee. Oral ald and delayed paralysis test (white leghorn
chickens). Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 234-70, submitted to WHO by Du Pont de Nemours
and Company.
Morrow, R.W. Skin absorption acute toxicity test in rabbits - LD50.
Unpublished report, dd. 27-2-1973, from the Haskell Laboratory, report
no. 94-73, submitted to WHO by Du Pont de Nemours and Company.
Peeples J.L. Effect of Oxamyl on Soil Microorganisms. This unpublished
undated study was made available to FAO as Vol. 1, Section 1, May 30,
1980, Exhibit 6, by the Biochemicals Department of du Pont.
Schmoyer, L.A, Oral LD50 test. Unpublished report, dd. 9-12-1969,
from the Haskell Laboratory, report no. 375-69, submitted to WHO by Du
Pont de Nemours and Company.
Schmoyer, L.A. Acute oral test Unpublished report, dd. 29-12-69, from
the Haskell Laboratory, report no. 411-69, submitted to WHO by Du Pont
de Nemours and Company.
Schmoyer, L.A. and Henry, N.W. Ind-1410 and cholinesterase activity.
Unpublished report, dd. 14-1-1970, from the Haskell Laboratory, report
No. 18-70, submitted to WHO by Du Pont de Nemours and Company.
Sherman, H. Antidote study, Unpublished report, dd. 14-10-1969, from
the Haskell Laboratory, report no. 322-69, submitted to WHO by Du Pont
de Nemours and Company.
Sherman, H., Barnes, J.R. and Aftosmis, J.O. Long-term feeding study
in rats and dogs with
l-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IBD-1410): final report. Unpublished report, d.d.
22-2-1972, from the Haskell Laboratory, report No. 37-72, submitted to
WHO by Du Pont de Nemours and Company.
Shirasu, Y., Moritani, M. and Watanabe, R. Oxamyl mutagenicity study
using bacteria. Unpublished report, dd. 4-6-1976, from the Institute
of Environmental Toxicology, submitted to WHO by Du Pont de Nemours
and Company.
Smelt, J.H., Dekker, A. and Leistra, M. Effect of Soil Moisture
Condition on the Conversion Rate of Oxamyl. Neth. J. Agric. Sc. 27,
191-198.
Snee, D.A., Sherman, H., Barnes, J.R. and Stula, E.F. Ninety-day
feeding study in rats with
1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IND-1410). Unpublished report, dd. 6-10-1969, from the
Haskell Laboratory, report no. 308-69, submitted to WHO by Du Pont de
Nemours and Company.
Tayfun, F.O. One-hour inhalation toxicity. Unpublished report, dd.
22-9-1969, from the Haskell Laboratory, report no. 281-69, submitted
to WHO by Du Pont de Nemours and Company.
Zahnow, E.W. Oxamyl - Chicken and Egg Feeding Study. This unpublished
study, dated 12-4-78, was made available to FAO as Section 3, May 30,
1980, Exhibit 19 by the Biochemicals Department of du Pont.
In soil and water
Numerous studies have been conducted on oxamyl soil metabolism,
decomposition, dissipation, adsorption, mobility effect on soil
microorganisms and on dissipation and decomposition in water. In one
compilation of studies the decomposition of oxamyl in soil and water
was investigated with various soils under aerobic and anaerobic
conditions and in distilled or river water at various pHs, with
artificial UV light or sunlight, and in the dark. Also included were
experiments on soil leaching and mobility in various soils (Harvey and
Han, 1978b).
Oxamyl stability was found to be highly pH dependent, hydrolysing to
the oximino compound I only under mildly basic conditions. In
pH-adjusted distilled water 3% and 9% hydrolysis was observed after 24
and 48 hours respectively at a pH of 6.9 and 30% within 6 hours at a
pH of 9.1. At a pH of 4.7 it was completely stable at least through a
4-day period.
In another of these studies 14C-oxamyl was rapidly degraded by soil
under aerobic conditions in a glass metabolism apparatus. The soil
was treated at a rate of 4 lb. oxamyl/acre. After 42 days under
aerobic conditions 51% of the 14C had been liberated as 14CO2, 4%
remained as oxamyl, <1% compound I and 37% as unextractable or
unidentified polar tractions, much of which was found to be
incorporated into soil organic fractions. Under anaerobic conditions
analogous residues at 42 days were 3, 8, 41, and 48% for CO2, oxamyl,
compound I and unextracted or unidentified residues respectively.
A half-life of 11-15 days was found under aerobic conditions in the
laboratory when soil was treated at a rate of 6 mg/kg. Under
anaerobic conditions the half-life was about six days. In another
study the half-life of oxamyl in a loamy sand was 14 days.
A study to the effect of soil moisture at 15°C indicated half-lives of
13-14 days in clay loam or loamy sand and 34-39 days in peaty or humic
loamy sand (Smelt et al, 1979). As soil moisture decreased to the
wilting point there was a gradual decrease in conversion and this
decreased further at below-wilt moisture levels with clay loam. In
humic loamy soil the conversion rate increased sharply at very low
soil moisture content.
In a soil-TLC experiment oxamyl appeared to be moderately to highly
mobile with Rf values of 0.53-1.0 in four soils (Harvey and Han,
1978b). By one system, compounds with Rf values of 0.35-0.64 are
rated as moderately mobile and those with Rf values of 0.96-1.00 as
highly mobile. This finding was consistent with laboratory adsorption
studies on three soils which indicated no adsorption under conditions
of the test. This is also consistent with another laboratory soil
adsorption-leaching study in which oxamyl was found to be highly
mobile with 61-100% eluted through a 45 cm × 5 cm diameter column of
aged and fresh soil with 20 inches of water (Chrazanowski, undated).
Of unleached residues >70% remained in the top five cm.
This high mobility observed in laboratory studies was not confirmed by
field studies. In another of the collection of studies by Harvey and
Han (1978b), volatility losses of 14C from three soil types (to a 15
inch depth) treated at 6 lb. ai 14C-oxamyl/acre were 80-93% of
original treatment at three-five months. Only traces of oxamyl or
compound I (<0.05%) was detected at three-five months in a soil
extract although significant amounts were found at one week and one
month. Unextracted residues accounted for 14% of the original
treatment at three-five months. The leachate from a fine sand had the
highest residues of the three soil types with 14C residues up to 6.8%
of original treatment. Of this 6.8% about 1% was oxamyl, 11%
unidentified polar materials and 88% compound I. Analysis again
indicated 14C incorporation into soil organic fractions.
In another of these studies oxamyl field soil leaching-disappearance
studies revealed residues of 3.2 mg/kg (calculated as oxamyl) in the
top four inches at zero days when treated at 5.7 lb ai/acre. Residues
decreased to 0.13 mg/kg in the top four inches at 30 days with a
half-life of about one week. Residues at the four-eight inch depth
peaked at 0.15 mg/kg at nine days; at the 8-12 inch depth at 0.15
mg/kg in the 9-23 day period; at the 12-18 inch depth at 0.11 mg/kg in
a 9-23 day period; and at 0.06 mg/kg at 23 days at the 18-24 inch
depth. Residues were negligible (<0.04 mg/kg) through a 60-day
interval at the 24-30 inch depth.
Therefore, under practical conditions the field leaching and
dissipation experiments do not support laboratory tests, which
indicate that oxamyl is highly mobile. They indicate some mobility
and rapid dissipation, presumably mostly as CO2, with a half-life in
soil of six to eight days with only trace residues leaching below
15-18 inches within a 2-3 month period. The rapid dissipation
apparently minimizes leaching under field conditions.
In a crop rotation study, cabbage, red beets and sorghum seeds were
grown in the greenhouse in soils treated 30 and 120 days earlier with
14C-oxamyl at 8 lb ai/acre (Harvey, undated). At 30 days 19% of the
original oxamyl remained in the soil. Crops planted therein had at
maturity residues of 0.6-4 mg/kg (calculated as oxamyl) or 0.01 to
0.12 mg/kg of oxamyl plus its hydrolysis product. At 120 days <1% of
the original oxamyl remained. Mature crops from the 120-day soil
contained <0.2 mg/kg residue (calculated as oxamyl), of which <0.02
mg/kg was oxamyl plus its oximino metabolite. No DMCF was detected.
The experiment would suggest that low levels of oxamyl plus its
oximino metabolite could occur in these commodities if they are
planted within 30 days of high soil applications of oxamyl. Similar
experiments in the field on other commodities reflecting maximum use
rates would be desirable, including use of both liquid and granular
commercial formulations.
Two studies were conducted on the possible effects of oxamyl on soil
microorganisms. In one controlled laboratory study 5.0 mg/kg oxamyl
in silt loam soil increased the time for 50% nitrification by 5 days
although total nitrification after 3 weeks was the same as in the
control (Han, undated). No significant effect was observed at a 0.5
mg/kg oxamyl level.
In the other laboratory study the effect on the population and
respiration of microorganisms was studied in soils treated at 10 mg/kg
oxamyl (Peeples, undated). No reduction was observed in the
populations of either fungi or bacteria at one, two, four and eight
weeks when compared to controls. Respiration of soil microorganisms
in these oxamyl treated soils was determined by measurement of CO2
evolution. Oxamyl had no effect on CO2 evolution.
In storage and processing
No information was provided on residues in stored commodities. Data
were provided on the effect of processing or simulated processing
residues in several commodities:
Apple - When apples with 1.2 mg/kg aged residues were processed in the
laboratory to juice and wet and dry pomace (11O°C, 17 hours) residues
were reduced to 0.5, 0.85 mg/kg and none, respectively.
Citrus - No concentration of residues was found in press juice, wet
pulp, or dried pulp when field-treated oranges and grapefruit with
terminal residues of 0.51 and 0.62 mg/kg respectively were analyzed.
Residues of 0.37 and 0.30 mg/kg in the wet pulp of oranges and
grapefruit respectively were essentially destroyed during the drying
process (<0.05 mg/kg remaining).
Cottonseed - In a fractionation study in which cottonseed was
fortified with oxamyl at 0.2 and 0.5 mg/kg, residues did not
concentrate in the meal or oil. Residues recovered in the meal were
5-8% of those on the undelinted seed; no residues were recovered in
the oil. No conversion to DMCF was detected. Fortifications with
metabolite A, the oximino compound I and DMCF also resulted in major
losses of these compounds during fractionation. The details of this
study were not provided to the meeting.
Peanuts - In a laboratory-simulated commercial oil extraction with
refluxing hexane, maximum residues in oil and meal were 10 and 28%,
respectively, of those in peanuts fortified at 0.2 and 2.0 mg/kg.
Pineapple - A simulated commercial processing study demonstrated that
residues in bran may be 0.4-6 times that in the whole fruit. This is
supported by actual residue data in Table 2. A similar concentration
occurs with the metabolite DMCF.
Soybeans - Although no concentration data were available for soybean
oil or meal, a 0.33 n-octanol water-partition coefficient would
suggest that concentration may not occur. This would be consistent
with fractionation studies in cottonseed and peanuts.
Tomatoes - When tomatoes with field-incurred or fortified residues of
1-10 mg/kg oxamyl were ground and concentrated by cooking to a puree
of one half the original weight, residues in the puree in mg/kg were
one half or less of those in tomatoes. Analytical recoveries were
61-110%.
Photodecomposition
Hydrolysis of oxamyl to the oximino compound (I) was accelerated by UV
light in both distilled water and river water, more rapidly in the
latter (Harvey and Han, 1978b). In river water treated with
artificial UV light, residues after seven days were 22% oxamyl, 39%
oximino compound (I) and 3% polar fractions. In the dark, control
residues were 84, 16, 0 and 0% respectively for the same compounds at
ten days.
Oxamyl degradation was enhanced still further when sunlight was used.
Oxamyl decreased from 100% at zero day to zero after about 1.5 days.
Oximino compound (I) decreased from a maximum of about 95% of the
total radioactivity at 1.5 days to about 68% and 50% at seven and 21
days, respectively, while its geometric isomer was increasing from
about 4% at 1.5 days to 32 and 42%, respectively, at seven and 21
days. The oximino compound (I) and its geometric isomer declined
after about 21 days, while the polar fraction increased from about 3%
of the activity at 14 days to 20% at 42 days.
At 42 days 14% of the total radioactivity was N,N-dimethyloxamic acid
and two unidentified polar compounds, which accounted for 3% of the
total. A 17% loss of radioactivity during the six-week study was
attributed to the loss of CO2. No S-oxidation products of oxamyl or
its oximino compound were observed.
RESIDUES IN COMMERCE OR AT CONSUMPTION
No information was provided to the meeting on evidence of residues in
food in commerce or at consumption.
METHODS OF ANALYSIS
Methods are available for the analysis of oxamyl alone, oxamyl plus
its oxime, and for its DMCF metabolise.
A method for the analysis of oxamyl alone uses column chromatography
to separate oxamyl from its oxime (Bromilow, 1976). Oxamyl is
extracted by blending with acetone dichloromethane (1:1) except for
potatoes, in which case only dichloromethane is used. After
concentration the extract is chromatographed on a Florosil column with
acetone as the eluting solvent. The eluant is concentrated to a small
volume for gas chromatographic analysis.
An aliquot of sample and methanolic trimethylphenylammonium hydroxide
is injected into the gas chromatograph for on-column derivatization to
the methoxime derivative. The gas chromatographic column is 0.5%
carbowax 20 M + 5% SE-30 and detection is by a flame photometric
detector equipped with a 394 nm filter. At 0.02-0.04 mg/kg
fortification levels average recoveries ranged between 87-91% on
barley, peas, and potatoes and 69% on tomatoes. Controls were
generally <0.01 mg/kg although a high of 0.15 mg/kg apparent residue
has been observed on pea pods. Recoveries average 87-96% on soils.
The low tomato recoveries were attributed to incomplete extraction of
residues from extract concentrate. On the basis of discussions, which
will follow, it is probable that part of this loss was due to the acid
catalyzed degradation of oxamyl by the highly acidic tomatoes.
In another procedure oxamyl residues are determined as the oximino
metabolite and expressed as oxamyl by using a 1.35 conversion factor
(Holt and Pease, 1976). The method also measures any free oxamyl
oxime and this was confirmed with recoveries of 65 and 57% when
tobacco was fortified with the oxime at 0.2-0.4 mg/kg.
Oxamyl (and its oxime) are extracted from plant and animal tissues and
soil by blending with ethyl acetate. After concentration and
partitioning with hexane the aqueous extract is made alkaline (pH 12)
with 1 N NaOH and partitioned with chloroform, which is discarded.
The aqueous extract is heated to convert oxamyl to its oxime and again
partitioned with chloroform. The aqueous phase is partitioned with
ethyl acetate - methanol (9:1) and concentrated for analysis with a
flame photometric detector equipped with a 394 nm filter. The
chromatographic column is 10% SP-1200/1% H3PO4 on Chromosorb W AW.
This method has been successfully used on over 25 agricultural
commodities as well as on milk, animal tissues, soil, urine and
faeces. Average recoveries generally range from 75-100%. A
sensitivity of about 0.02 mg/kg is generally attainable for most
commodities, although apparent residues of 0.04-0.05 mg/kg may occur
at times in beans, citrus, peanut hay and onions and up to 0.26 mg/kg
on bean foliage.
Successful method trials using the Holt-Pease method were conducted in
laboratories of the Environmental Protection Agency in the United
States. Recoveries were 77-82% at oxamyl-fortification levels of 3
and 6 mg/kg in celery and 0.2-0.4 mg/kg in cottonseed.
Somewhat low recoveries have been observed during analysis of highly
acidic commodities, such as citrus, tomatoes, and peaches. This can
be minimized by making concentrations under alkaline conditions.
A method is also available for the determination of the DMCF
metabolite of oxamyl (Holt, 1976). In this method the sample is
extracted by blending with ethyl acetate and a potassium dihydrogen
phosphate - NaOH buffer. After concentration to an aqueous phase the
sample is partitioned with hexane (which is discarded) and
re-extracted into ethyl acetate, which is concentrated for analysis.
Analysis is on a 5% Carbowax 20 M gas chromatographic column and
detection with a nitrogen - phosphorous detector. Recoveries on
citrus, celery, strawberries and tomatoes averaged 66-100% and 60% on
potatoes when the commodities were fortified at 0.04-2.0 mg/kg DMCF.
Average recoveries were 74-100% on tomato paste, cottonseed oil,
cottonseed meal and tobacco fortified at 0.2-2.5, 0.2-1.0, 0.1-0.50
and 0.05-0.5 mg/kg respectively. The sensitivity is generally
considered to be 0.04 mg/kg.
The buffer was incorporated into the procedure to minimize conversion
of oxamyl or its oxime to DMCF when highly acidic commodities were
analyzed. This conversion was 5-15% without the buffer and, as
discussed earlier, was observed by Holt and Pease (1976), reduced
recoveries during the development and use of their oxamyl method. The
authors also note that under some conditions some thermal degradation
of oxamyl or its oxime to DMCF may occur in gas chromatographic
injection ports with temperatures in excess of 100-110°C.
NATIONAL TOLERANCES REPORTED TO MEETING
Commodities Tolerances (mg/kg)
USA Netherlands Fed. Rep. of
Germany
apples 2
bananas 0.1 (reflecting 0.02 mg/kg in pulp)
celery 3
citrus 3
cottonseed 0.2
cucumbers 1
pineapple (whole) 1
pineapple forage 10
potatoes 0.1 0.05
sugarbeet (roots) 0.05 (provisional)
tomatoes 2 1
EVALUATION
COMMENTS AND APPRAISAL
Oxamyl is a systemic insecticide, miticide and nematicide registered
or approved for use in numerous countries. It is available as a
liquid or granular formulation and is systemic whether applied
foliarly or to the soil. Foliar applications result in nematocidal
action in the roots and likewise soil applications result in
insecticide and miticidal action in the foliage. It is also effective
as a direct contact insecticide, miticide, and nematicide.
Recommended use information for over 30 countries was provided to the
meeting by the manufacturer. Only a few countries officially provided
this information directly to the meeting.
Oxamyl is degraded in animals by two major pathways, hydrolysis to the
oximino compound and enzymic conversion to N,N-dimethyloxamic acid.
Metabolism studies with 14C-oxamyl in two rats showed that only 70%
of the dose was eliminated in the urine and faeces in 72 hours.
Substantial amounts (22% of the dose) were incorporated into the body
tissues, especially the skin and hair, partly by conversion of
14C-metabolites into natural amino acids. The meeting expressed
concern over the high tissue residues and the lack of identification
of much (50%) of this material.
Oxamyl has a high acute toxicity displaying the typical effects of
carbamate insecticides: rapid onset of cholinesterase inhibition
followed by rapid complete recovery. There was no evidence of delayed
neurotoxicity. The acute toxicity of most of the metabolites is lower
than oxamyl. Oxamyl is not mutagenic or teratogenic.
In a 3-generation rat reproduction study, 50 mg/kg of oxamyl in the
feed slightly decreased the body weight of the weanlings.
The dog is somewhat less sensitive than the rat. In a 90-day and
2-year feeding study a no-effect level of 100 mg/kg was observed. In
the long-term study with rats growth inhibition was found at 100 and
150 mg/kg. A slight effect was also observed in the males at 50
mg/kg. Owing to the lack of a clear no-effect level in the rat and
lack of identification of 50% of the tissue residues, only a temporary
ADI, based on the 2-year study in dogs, was allocated.
Residue data were provided on 29 commodities or groups of commodities.
Residues on individual crops were highly variable, reflecting the many
combinations of uses and pest control needs. Because of the many
variables it was necessary to significantly condense detailed
information on residues in table 2. However, the detailed data were
examined by the meeting.
In some cases no residue data were available from countries for which
recommended or approved use information was available but from a
country with proposed uses. In other cases no recommended or approved
use information was available, although residue data were provided
from a country with a proposed use. Estimate of residues were made on
the basis of available data. The basis for those estimates are
discussed.
In plants a major metabolic route is the conversion of oxamyl to its
oxime, which conjugates with glucose to form metabolite A. This in
turn may add additional hexose units to build progressively into
polysaccharide and starch and cellulose, like structures. With time,
demethylation of metabolite A can occur to form metabolite A' which
way also add additional hexose units. In some plants complete
breakdown of oxamyl occurs with incorporation into natural plant
molecules such as glucose and lipids. The metabolite DMCF is a
residue in some plants and the oxime is found in some fruits. No
residues were observed for the S-oxide or S-dioxide of oxamyl.
Surface residues may be up to 50% of the total and most of this is
oxamyl in some plants.
In an in vitro rumen fluid metabolism study oxamyl was rapidly and
almost totally metabolized. The oximino and DMCF metabolites
accounted for 80% of the radioactivity after 24-hour incubation.
These and remaining metabolites were similar to those identified in
the rat metabolism studies.
The major metabolite of plant metabolism, the glucose conjugate of the
oxime, was almost completely metabolized in rumen fluid, mostly to
DMCF. This contrasts to the rat metabolism where this metabolite was
resistant to metabolism. When DMCF was metabolized in the rumen fluid
the same metabolic products identified in the rat were found plus
dimethyloxamide.
In livestock feeding studies at up to 20 mg/kg in the diet of cattle
and up to 5 mg/kg in the diet of poultry, no residues (<0.02 mg/kg)
were found in animal tissues, milk, fat or eggs. No feeding studies
(or metabolism studies) were provided, for non-ruminant livestock
other than poultry. The available data suggest that there is no
likelihood of residues in meat, fat, milk, poultry or eggs from
approved uses on certain crops. However, additional residue data from
recommended uses on other important crops used for animal foodstuffs,
e.g. sugarbeet leaves, are needed.
Oxamyl is stable in acidic to neutral water but rapidly hydrolyses
under alkaline conditions. Ultraviolet light catalyzes the
degradation to the oxime or its geometric isomer and eventually to
polar compounds.
Laboratory studies indicate that oxamyl should be highly mobile in
soils, but field decomposition and leaching studies do not support
this. This is apparently because the relatively rapid degradation
does not allow sufficient time for significant mobility. On the basis
of laboratory metabolism studies a major route of dissipation appears
to be the evolution of CO2, although soil pH is a factor in some
soils. Significant residues seldom leached below an 18 inch depth
under the study conditions. The half-life of oxamyl in soil was
highly variable, ranging from 6 -39 days, depending on variables such
as location, soil type and moisture content.
In crop rotation studies there was evidence that low levels of
residues could occur in follow-up crops where oxamyl has been used in
the soil. These studies were 14C-oxamyl greenhouse studies. Crop
rotation studies under field condition and reflecting recommended
usage would be desirable.
No evidence of a permanent effect on soil microorganisms by the use of
oxamyl was observed.
No data were available on the effect of storage on oxamyl residues or
on residues in food in commerce or at consumption. Studies were
available on the effect of processing on several commodities. No
concentration of residues was noted except in the case of pineapple
bran where a 6-fold concentration occurred.
Two methods of analysis are available. In one, oxamyl is separated
from its oxime and derivatized on a gas chromatographic column to its
methoxime. In the second, oxamyl and its oxime are extracted together
and the oxamyl hydrolysed to its oxime for the gas chromatographic
determination of total oxamyl plus free oxime residue as the oxime.
Although both methods appear to be adequate, the latter would appear
to be the method of choice for enforcement because it has apparently
been much more extensively used and tested. Most of the residue data
provided to the meeting were developed with this procedure. A
disadvantage would be that it also measures the free oxime, which may
not be of major toxicological concern. The oxime is not, however, a
major residue in many plants, although it can be in some fruits.
The meeting examined residue data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
oxamyl wee used in practice and when reported intervals between last
application and harvest were observed,
Level causing no toxicological effect
Dog: 100 mg/kg in the diet, equivalent to 2.5 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. The levels refer to
the sum of oxamyl plus its oxime calculated as oxamyl. Some figures
are temporary (T), because either there is no recommended use or the
data were inadequate to estimate a maximum residue level.
Estimated Maximum Pre-harvest
Residue levels Interval (days)
Commodity (mg/kg)
apples 2 14
banana 0.051
beans, kidney 3 (T) 7
beans, kidney (dry) 0.051 (T) 50
beans, lima 3 (T) 7
celery 3 14
citrus 3 7
maize 0.051 (T) (at planting use)
cottonseed 0.2 21
cucumber 2 1
melon 2 1
summer squash 2 1
watermelons 2 1
peanuts 0.1 (T)
peanut fodder 2 (T)
peppers, bell 3 7
pineapple 1 (T) 30
root and tuber vegetables:
beets 0.1 14
carrots 0.1 14
potatoes 0.1 7
sugar-beets 0.1 7
sweet potatoes 0.1 (T) (apply within one week of
planting)
soybeans (dry) 0.051 (T) (pre-planting)
tomatoes 2 1
1 at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Identification of animal tissue residues.
2. Clarification of the no-effect level in the rat especially in
relation to the marginal effect of 50 mg/kg on the body weight in
several studies.
3. Further data on beans, maize and soybeans for reconsideration of
the temporary recommendations.
4. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
5. Approved use information on those items for which only proposed
uses were available.
6. Further data are required on beans, maize and soybeans for
reconsideration of the temporary recommendations.
7. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
8. Approved use information on those items for which only proposed
uses were available.
Desirable
1. Residue data from short-term feeding studies on pigs.
2. Toxicological observations in man.
3. Additional residue data from trials reflecting GAP in additional
countries; in particular residue data on onions, peas, and sugarcane
for which there are existing use patterns.
4. Information on residues in foods in commerce and at consumption.
5. Information on the effect on oxamyl residues of cooking, processing
or storage of raw agricultural commodities.
6, Crop rotation studies on additional commodities and under field
conditions with applications of commercial formulations (both granular
and liquid) according to maximum recommendations.
REFERENCES
Ashley, W.E. Acute oral test. Unpublished report, dd. 29-8-1974, from
the Haskell Laboratory, report no. 585-74, submitted to WHO by Du Pont
de Nemours and Company.
Barbo, E.C. Oral LD50 test. Unpublished report, dd. 9-10-1972, from
the Haskell Laboratory, report no. 399-72, submitted to WHO by Du Pont
de Nemours and Company.
Barnes, J.R. and Aftosmis, J.G. Cholinesterase tests with oxamyl.
(1978) Unpublished report from Haskell Laboratory. Report no. 270-78,
submitted to WHO by Du Pont de Nemours and Company.
Barras, C.E. Acute inhalation toxicity. Unpublished report, 19-2-1974,
from the Haskell Laboratory, report no. 30-74, submitted to WHO by Du
Pont de Nemours and Company.
Belasco, I.J. and Harvey, J. Jr. In vitro Rumen Metabolism of
14C-Label Oxamyl and Selected Metabolites of Oxamyl. J. Agric. Food
Chem., Vol. 28(4), 689
Bromilow, R.H. Determination of Residues of Oxamyl in Crops and Soils
by Gas-Liquid Chromatography. Analyst, Vol. 101, 982.
Chrazanowski, R.L. Soil Absorption Studies With Vydate(R) Oxamyl
Insecticide/Nematicide. Unpublished study provided to FAO as Vol. 1,
Section I, May 30, 1980, Exhibit 2 by the Biochemicals Department,
Experimental Station, E.I. Du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, 19898.
Carroll, K.S. Oral LD50 test. Unpublished report, dd. 23-8-1972, from
the Haskell Laboratory, report no 327-72, submitted to WHO by Du Pont
de Nemours and Company.
Civo. Gas Chromatographic Analysis of Residues of Oxamyl Residues in
Cucumbers and Tomatoes, Report no. R 6282, Ir., PDA, Olthof (1979)
Central Institute for Nutrition and Research, the Netherlands.
Colburn, C.W. Acute skin absorption toxicity tests on rabbits.
Unpublished report, dd. 30-12-1970, from the Haskell Laboratory,
report no. 282-70, submitted to WHO by Du Pont de Nemours and Company.
Culik, R. and Sherman, H. Teratogenic study in rats with
S-methyl-l-dimethylcarbamoyl-N [(methylcarbamoyl)oxy] thioformimidate
(IND-1410). Unpublished report, dd. 8-1-1971, from the Haskell
Laboratory, report no. 5-71, submitted to WHO by Du Pont de Nemours
and Company.
Dale, N.C. Oral LD50 test. Unpublished report, dd. 30-3-1973, from
the Haskell Laboratory, report no. 126-73, submitted to WHO by Du Pont
de Nemours and Company.
Dion, L.K. Fifteen-exposure dermal study with IND-1410 liquid
S-methyl-l-dimethyl-carbamoyl-H-[(methylcarbamoyl)oxy]
thioformimidate. Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 235-70, submitted to WHO by Du Pont de Nemours
and Company.
Du Pont. Oxamyl Livestock Feeding Studies, Milk and Meat. This
unpublished study, dated January 1973, was provided to FAO as Vol. I,
section 3, May 30, 1980, exhibit 17, by the Biochemicals Department of
Du Pont.
Du Pont. Livestock Feeding study. This unpublished study, dated
4-19-76 was provided to FAO, Vol. I, Section 3, May 30, 1980, Exhibit
18, by the Biochemicals Department of Du Pont.
Du Pont. Residue data were provided to FAO as U.S. data - Vol. I,
Section 4, Exhibits 21-28, May 30, 1980 by the Biochemicals Department
of Du Pont.
Fretz, S.B. Ten-dose subacute oral test. Unpublished report, dd.
25-6-1968, from the Haskell Laboratory, report no. 150-68, submitted
to WHO by Du Pont de Nemours and Company.
Fretz S.B. Acute oral test. Unpublished report, dd. 27-12-1968, from
the Haskell Laboratory, report no. 300-68, submitted to WHO by Du Pont
de Nemours and Company.
Fretz S.B. Oral LD50 test. Unpublished report, dd. 31-7-1969, from
the Haskell Laboratory, report 214-69, submitted to WHO by Du Pont de
Nemours and Company.
Gibson, J.R. Oral LD50 test. Unpublished report, dd. 21-5-73, from
the Haskell Laboratory, report no. 236-73, submitted to WHO by Du Pont
de Nemours and Company.
Han, J. C-Y. and Harvey, J. In vitro rat liver microsomal
metabolism of oxamyl and selected metabolites of oxamyl. (undated)
Unpublished report from Du Pont de Nemours and Company, submitted to
WHO.
Han, J. C-Y. Evaluation of Possible Effects of DPX-1410 on Nitrifying
Bacteria In Two Different Soils. This unpublished undated study was
made available to FAO as Vol. I, Section 1, May 30, 1980, Exhibit 5,
by the Biochemicals Department of Du Pont.
Harvey J. and Han, J. C-Y. Metabolism of oxamyl and selected
metabolites in the rat. J. Agric. Food Chem. 26, 902-910.
Harvey, J. Jr. and Han, J. C-Y. Decomposition of Oxamyl In Soil and
Water. J. Agric. Food Chem, Vol. 26(3), 537.
Harvey, J. Jr., Han J. C-Y. and Reiser, R.W. Metabolism of Oxamyl In
Plants. J. Agric. Food Chem., Vol. 26(3), 529.
Harvey, J. Jr. Metabolism of 14C-Oxamyl in the Lactating Goat. This
unpublished undated study was made available to FAO as Vol. I, Section
2, May 30, 1980, Exhibit 9, by the Biochemicals Department of Du Pont.
Harvey, J. Jr. Crop Rotation Study with 14C-Oxamyl in the Greenhouse.
This unpublished undated study was made available as Vol. I, Section
1, May 30, 1980, Exhibit 3 by the Biochemicals Department of Du Pont.
Henry J.E. Acute oral test (mouse). Unpublished report, dd. 2-6-1976,
from the Haskell Laboratory, report no. 387-76, submitted to WHO by Du
Pont de Nemours and Company.
Holsing, G.C. 13-Week oral administration - dogs, insecticide 1410,
final report. Unpublished report, dd. 10-10-1969, from the Hazleton
Laboratories, Inc., submitted to WHO by Du Pont de Nemours and
Company.
Holt, R.F. Residue Procedure for the Determination of Oxamyl
Metabolite N,N-Dimethyl-l-cyanoformamide (DMCF). This unpublished
method, dated 5-3-76 was made available to FAO as Vol. I, Section 3,
May 30, 1980, Exhibit 16, by the Biochemicals Department of du Pont.
Holt, R.F. and Pease, H.L. Determination of Oxamyl Residues Using
Flame Photometric Gas Chromatography, J. Agric. Food Chem., Vol.
24(2), 263.
Kaplan, A.M. Ninety-day feeding study in rats with
1-cyano-N,N-dimethylformamide (INN-79), metabolite of Vydate.
Unpublished report, dd. 26-8-1976, from the Haskell Laboratory, report
no. 630-76, submitted to WHO by Du Pont do Nemours and Company.
Ki Poong Lee. Oral ald and delayed paralysis test (white leghorn
chickens). Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 234-70, submitted to WHO by Du Pont de Nemours
and Company.
Morrow, R.W. Skin absorption acute toxicity test in rabbits - LD50.
Unpublished report, dd. 27-2-1973, from the Haskell Laboratory, report
no. 94-73, submitted to WHO by Du Pont de Nemours and Company.
Peeples J.L. Effect of Oxamyl on Soil Microorganisms. This unpublished
undated study was made available to FAO as Vol. 1, Section 1, May 30,
1980, Exhibit 6, by the Biochemicals Department of du Pont.
Schmoyer, L.A, Oral LD50 test. Unpublished report, dd. 9-12-1969,
from the Haskell Laboratory, report no. 375-69, submitted to WHO by Du
Pont de Nemours and Company.
Schmoyer, L.A. Acute oral test Unpublished report, dd. 29-12-69, from
the Haskell Laboratory, report no. 411-69, submitted to WHO by Du Pont
de Nemours and Company.
Schmoyer, L.A. and Henry, N.W. Ind-1410 and cholinesterase activity.
Unpublished report, dd. 14-1-1970, from the Haskell Laboratory, report
No. 18-70, submitted to WHO by Du Pont de Nemours and Company.
Sherman, H. Antidote study, Unpublished report, dd. 14-10-1969, from
the Haskell Laboratory, report no. 322-69, submitted to WHO by Du Pont
de Nemours and Company.
Sherman, H., Barnes, J.R. and Aftosmis, J.O. Long-term feeding study
in rats and dogs with
l-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IBD-1410): final report. Unpublished report, d.d.
22-2-1972, from the Haskell Laboratory, report No. 37-72, submitted to
WHO by Du Pont de Nemours and Company.
Shirasu, Y., Moritani, M. and Watanabe, R. Oxamyl mutagenicity study
using bacteria. Unpublished report, dd. 4-6-1976, from the Institute
of Environmental Toxicology, submitted to WHO by Du Pont de Nemours
and Company.
Smelt, J.H., Dekker, A. and Leistra, M. Effect of Soil Moisture
Condition on the Conversion Rate of Oxamyl. Neth. J. Agric. Sc. 27,
191-198.
Snee, D.A., Sherman, H., Barnes, J.R. and Stula, E.F. Ninety-day
feeding study in rats with
1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IND-1410). Unpublished report, dd. 6-10-1969, from the
Haskell Laboratory, report no. 308-69, submitted to WHO by Du Pont de
Nemours and Company.
Tayfun, F.O. One-hour inhalation toxicity. Unpublished report, dd.
22-9-1969, from the Haskell Laboratory, report no. 281-69, submitted
to WHO by Du Pont de Nemours and Company.
Zahnow, E.W. Oxamyl - Chicken and Egg Feeding Study. This unpublished
study, dated 12-4-78, was made available to FAO as Section 3, May 30,
1980, Exhibit 19 by the Biochemicals Department of du Pont.
 TOXICOLOGICAL STUDIES
Special studies on cholinesterase activity
Rat
Two groups of 10 male rats were given orally 4.8 mg oxamyl/kg bw.
One group received 50 mg/kg atropine sulphate i.p. immediately
after dosing of oxamyl. Cholinesterase activity in blood was
determined 24 hrs before and 5 min., 4 hrs and 24 hrs after dosing.
The animals without atropine showed a decrease in cholinesterase
after 5 min. with a maximum after 4 hrs. After 24 hrs, the
activity was normal again. Animals that were treated with atropine
showed a slight inhibition initially, but the activity was normal
after 4 hrs. The experiment was repeated with the same result
(Schmoyer and Henry, 1970).
Four groups of 8 ChR-CD rats were fed oxamyl in the diet at dosage
levels of 0 (control), 50, 100 or 150 mg/kg feed during 29 days.
Erythrocyte and plasma AChE were measured at day 1, 7, 21, and 28.
On the 29th day the rats were sacrificed to measure AChE in the
brain.
Eight additional rats were assigned to each group for assay of
brain AChE 14 days after the start of the experiment.
With male animals cholinesterase activity of the plasma was only
marginally decreased in the 150 and 100 mg/kg groups throughout the
study. In the females plasma cholinesterase activity was
dose-related decreased in the 150 and 100 mg/kg groups from day 7
onwards. No clear effects were observed on the AChE activity in
the erythrocytes. At the end of the study cholinesterase activity
in brain tissue of females was dose-related decreased in the 150
and 100 mg/kg groups. After 14 days cholinesterase activity in the
brain was inhibited in the 150 mg/kg group. In summary, 50 mg/kg
in the diet was a no-effect level (Barnes and Aftosmis, 1978).
Special study on delayed neurotoxicity
Chicken
The animals were given various oral doses of oxamyl. The ALD
(approximate lethal dose) was established at 40 mg/kg bw. Two
groups of 5 adult hens received 20 or 40 mg/kg bw respectively
followed by 0.5 mg/kg atropine i.m. No mortality occurred. The
animals were kept for 28 days. They showed marked symptoms of
cholinesterase inhibition but recovery was complete after 12 hours.
As a positive control 1200 mg/kg TOCP was used, followed by
atropine. Two weeks after dosing the animals showed typical signs
of delayed neurotoxicity (Ki Poong Lee, 1970).
Special study on reproduction
Rat
The animals of the long-term feeding study were also used for a
3-generation reproduction study. Groups of 16 males and 16 females
on a diet containing 0, 50, 100 or 150 mg/kg were chosen for
breeding three generations of each two litters. Throughout the
study the litter size, viability and lactation indices and body
weights of weanlings were decreased at 100 and 150 mg/kg. A
marginal effect on the body weight of the weanlings was also
observed at 50 mg/kg. No effect on fertility and gestation indices
was detected. Organ weights of the weanling pups of the F3b
generation showed a slight increase in relative kidney weight at
150 mg/kg. The relative testes weight was increased at 100 and 150
mg/kg. No histopathological abnormalities were found that could be
ascribed to the compound (Sherman et al. 1972).
Special study on mutagenicity
Microorganisms
Oxamyl showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, and reverse mutation tests with and
without a liver activation system using 5 strains of Salmonella
thyphimurium TA and E. coli WP2 hcr. A host-mediated assay
in mice using S. typhimurium G-46 was also negative (Shirasu
et al, 1976).
Special study on teratogenicity
Rat
Groups of 26-28 pregnant females received 0, 50, 100, 150 or 300
mg/kg oxamyl in the diet from day 6-15 of pregnancy. In the
mothers, a dose-related decrease in body weight and food
consumption was found for 100, 150 and 300 mg/kg groups. There
were no effects on number of implantation sites, resorptions and
live foetuses nor on embryonal development. Oxamyl does not show
teratogenic or embryotoxic properties (Culik and Sherman, 1971).
Acute Toxicity
species sex route LD50 in mg/kg bw reference
rat M oral 371 (27-51) Carroll, 1972
rat M oral 1102 (97-185) Gibson, 1973
rat M oral 5.4 (3.7-7.8) Fretz, 1969
rat3 M oral 16 (22) Schmoyer, 1969
rat3 F oral 11 (7-16) Schmoyer, 1969
1 formulation Vydate L (26% ai) in aqueous solution
2 Vydate G (10% ai) in corn oil
3 fasted overnight
Dermal studies
Rabbits
When oxamyl was applied as a suspension in a hydrophillic ointment
for 24 hrs, the ALD (approximate lethal dose) was 2250-5000 mg/kg
bw on the intact skin, but only 90-130 mg/kg on the abraded skin.
When oxamyl was applied as a slurry in propylene glycol the ALD was
130 or 60 mg/kg bw in the intact and abraded skin respectively
(Colburn, 1970).
When Vydate L (27% ai) was applied during 24 hrs the LD50 was 740
± 150 mg/kg bw on active ingredient basis on the intact akin
(Morrow, 1973).
Inhalation studies
Rats
The LC50 (1 h) for oxamyl was 0.035 mg/l air when Vydate L (27% ai)
was used in the test (males only) (Barras 1974). When the
experiment was carried out with oxamyl dust the LC50 (1 h) for male
and female animals was 0.17 and 0.12 mg/l respectively. The
concentration in air was calculated after chemical analysis
(Tayfun, 1969).
Symptoms of intoxication
Fasciculations, tremors, salivation, lacrimation, bulging eyes and
chromodacryorrhoea were found after an acute oral dose of oxamyl.
They can be considered as symptoms of cholinesterase inhibition
(Carroll, 1972).
A good antidotal effect was obtained when rats orally treated with
oxamyl received an i.p. injection with atropine sulphate (Sherman,
1969)
Acute toxicity of oxamyl metabolites in rats
LD50 in
metabolite sex route mg/kg bw reference
methyl N-hydroxy-N,N' dimethyl- M oral 11,000 (=ALD) Fretz, 1968
-1-thiooxamimidate (INA-2213)
methyl N-hydroxy-N' methyl-1- M oral 6,675 (6370- Dale, 1973
thiooxamimidate (IEL-2953) 6690)
N,N-dimethyloxamic acid (IND-2708) M oral 3,540 Barbo, 1972
N,N-dimethyl-1-cyanoformamide M oral 450 (=ALD) Ashley, 1974
(DMCF) (INN-79)
methyl N'-methyl-N [(methyl-carbamoyl) M oral 60 Schmoyer, 1969
oxy]-1-thiooxamimidate (IND-14O9)
glucose conjugate of methyl N'- M oral > 7500 Henry, 1976
methylhydroxy-N',N'-dimethyl
-1-thiooxamimidate (ING-3515)
Short-term studies
Rat
Six male rats were given orally 2.4 mg/kg bw oxamyl for 10 days. No
mortality occurred. Oxamyl does not exhibit cumulative oral toxicity
(Fretz and Sherman, 1968).
Rabbit
Rabbits were treated dermally for 15 days during a period of 4 weeks
with Vydate L (26% ai) in dimethylformamide. Groups of each 5 male
and 5 female animals received 193 mg/kg Vydate L, or 50 mg/kg bw of
oxamyl during 6 hrs/day on the intact or abraded skin. Control
animals received dimethylformamide. All oxamyl treated animals with
abraded skin showed marked signs of cholinesterase inhibition. Only
slight symptoms were seen on the animals with intact skin. No
mortalities occurred. No changes in body weight, organ weight or
histopathology were found that could be ascribed to the treatment.
All animals showed mild erythema of the treated skin (Dion, 1970).
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 100 or 500 mg/kg oxamyl in the diet. After a few days the highest
dose level was reduced to 150 mg/kg because with 500 mg/kg all animals
showed strong symptoms of cholinesterase inhibition. They were given
control diet for 3 days and the symptoms disappeared or became less
severe. Then the animals were given 150 mg/kg. No clinical signs of
toxicity were seen in the remainder of the experiment. The body
weight gain was lower at 100 and 150 mg/kg and slightly affected in
the females of the 50 mg/kg group. The food consumption was lower at
150 mg/kg only. Urinalysis showed a higher number of animals with
blood in urine at 100 and 150 mg/kg and a higher number with protein
at 150 mg/kg. Male rats showed lower absolute organ weights for
heart, kidneys, liver, spleen and thymus at 100 and 150 mg/kg. In the
females this effect was found for the liver at 100 and 150 mg/kg and
the kidneys and lungs at 100 mg/kg. No effects were found on
haematology, clinical chemistry and histopathology. Cholinesterase
activity was not determined. The marginal no-effect level is 50 mg/kg
(Snee et al, 1969).
At the end of the experiment 6 animals per group per sex were used for
reproduction performance. In the F1a and F1b generation the
fertility index and the number of pups/litter were decreased at 100
and 150 mg/kg. The weight of the young at weaning was significantly
lower at all dose levels (Snee et al, 1969).
Dog
Groups of 4 male and 4 female animals each received for 90 days 0, 50,
100 or 150 mg/kg oxamyl in the diet. Only marginal effects were found
with 150 mg/kg. A slight increased activity was found for alkaline
phosphatase after 4 and 13 weeks. In the males the weight of
adrenals, spleen and testes was somewhat decreased. The females
showed a slight increase in thyroid weight and a decrease in adrenal
weight. Histopathologically no abnormalities were found that could be
ascribed to the compound (Holsing, 1969).
Groups of 4 male and 4 female animals each received for 2 years 0, 50,
100 or 150 mg/kg oxamyl (95%) in the diet. After 1 year 1 male and 1
female animal from the control and 150 mg/kg groups were killed.
Haematology, clinical chemistry and urinalysis were carried out after
1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 months. The haemoglobin content,
haematocrit and number of erythrocytes in blood at 150 mg/kg were
found to be lower than the controls on most times of measurement in
both sexes.
Higher values for cholesterol and alkaline phosphatase activity were
found more often at 150 mg/kg in males and females. On some occasions
cholesterol was also higher in the other experimental groups.
No effects on organ weights, cholinesterase or aliesterase activity,
or histopathology were found that could be ascribed to the compound
(Sherman, et al, 1972).
Short term study with DMCF, a metabolite of oxamyl
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 150 or 450 mg/kg N,N-dimethyl-1-cyanoformamide (DMCF) in the diet.
Growth inhibition was found at 450 mg/kg in males and females, and the
food consumption was also lower. At the highest dose level
haemoglobin content, haematocrit and number of erythrocytes were
lower. At 150 mg/kg a slight effect was found on these parameters in
the females or males. In the male animals an increased LDH activity
was found at 450 mg/kg and in the female animals a decreased activity
in GPT, GOT, and LDH at the same dose level. Organ weights were not
reported in this study. Histopathologically an increase was found in
hydronephrosis of the kidneys in the males and minute foci of
mineralization in the kidneys of females at 450 mg/kg. The animals of
the other dose levels were not studied histopathologically. The
no-effect level is probably 50 mg/kg (Kaplan, 1976).
At the end of the experiment 6 animals per group were used for
reproduction performance. With 450 mg/kg a decreased weight of the
young at weaning was observed (Kaplan, 1976).
Long-term studies
Rat
Groups of 36 male and 36 female animals each received for 2 years O,
50, 100 or 150 mg/kg oxamyl (95%) in the diet (2 control groups were
used). After one year of feeding the number of rats of each sex in
each group was reduced to 30. With 100 and 150 mg/kg a decreased body
weight was found with male and female animals that was related to
dose. With 50 mg/kg the male animals showed a slightly lower body
weight, which was apparent after 4 weeks and remained so throughout
the study. Food consumption was lower at 150 mg/kg. Cholinesterase
activity in blood in the 150 mg/kg group was only decreased in the
females after 4 days of feeding and in the males after 8 days, and not
after 1, 6, 12 and 24 months. Aliesterase activity was not affected
throughout the study.
At the end of the experiment relative weights of brain, testes and
adrenals were increased in the males and brain, heart, lungs, kidneys
and adrenals in females at 150 mg/kg. In the females most or these
organ weights were also increased at 100 mg/kg. These changes are
probably connected with the decreased body weight. No haematological,
biochemical or histopathological abnormalities were found that could
be ascribed to the compound (Sherman et al, 1972).
RESIDUES IN FOOD
USE PATTERN
Oxamyl formulations, either as a 24% liquid or 10% granular, are
recommended or registered for use in numerous countries. Food uses
made available to the Meeting by the manufacturer are summarized in
Table 1. Proposed uses are not included but are discussed under
appropriate commodities when residues are discussed.
According to information provided to the Meeting, uses listed for the
United States also apply to Argentina, Barbados, Bolivia, Brazil,
Chile, Colombia, Costa Rica, The Dominican Republic, Ecuador,
Guatemala, Mexico, Nicaragua, Peru, El Salvador, Trinidad and Tobago,
Uruguay and Venezuela unless indicated otherwise in Table 1. Only a
few national governments provided usage information directly to the
meeting.
The liquid formulation is a contact-type, moderately residual
insecticide when applied as a foliar spray. When so applied it is
effective on several species of mites and, by systemic action in many
plants, on nematodes. When oxamyl formulations are applied to the
soil they function as a contact type broad-spectrum nematocide and, by
systemic action, as a miticide/insecticide.
Applications may be pre-planting, at planting, or foliar (to
fruit-bearing or non- fruit-bearing plants). They may be applied in
the furrow or broadcast. Soil incorporation may be mechanical or by
irrigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
There are numerous combinations of type of formulation, method and
time of application suitable for a wide range of pest control needs.
Because of this, the residue level can vary widely, even on the same
commodity. A large volume of data on 29 commodities or groups of
commodities was presented to the meeting; this has been considerably
condensed into Table 2 (Du Pont, 1980).
The analytical method used extracts both oxamyl and its oxime
metabolite (I), converts the oxamyl to the oxime which is quantitised,
so the residue levels quoted are of oxamyl plus the oxime expressed as
oxamyl. Data on residues of the DMCF metabolite are in parentheses.
TABLE 1. Recommended uses for oxamyl1
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation2,3 treatments interval (wks) notes
Apples
non-bearing U.S.A. 0.28-2.2 24% Liq. 52
6.7 - 9 pre-plant, soil incorporation
bearing 0.28-2.2 2 do not graze treated orchards
non-bearing Greece 2-4 10% G 52 soil
1.2 24% G multiple 52 foliage
Bananas Greece 3 g/plt 10% G 3 at planting
2-3 g/plt 2 on plantations (around plant)
0.01% 24% Liq. foliage
Central Amer. 0.9-1.3 24% Liq. 5-8 foliar
Spain 3.8 24% Liq. in irrigation water
Beans Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, broadcast glasshouse and
in open
1.2 24% Liq. 2 2 spray, broadcast glasshouse and
in open
Beets (roots Denmark 0.7-1.8 10% G row, at planting
or tops) 2.5-5 10% G broadcast, before planting
Carrots Greece 3-4 10% G 2 pre-plant, broadcast
2-3 10% G 2 furrow
1.2 24% Liq. 2 2 spray
1.2 24% Liq. multiple 52 foliage
Celery Greece 3-4 10% G 2 preplant broadcast, glasshouse and
in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
USA 0.56-2.2 24% Liq. 2 untrimmed
4.4 24% Liq. 2 pre-plant soil incorporated
Citrus Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
USA up to 1.1 1 do not graze treated orchards
(0.008-0.03%)
Cherries Greece 2-4 10% G 52 soil non-bearing
1.2 24% Liq. multiple 52 foliage non-bearing
Cucumbers Bulgaria 0.024% 24% Liq. multiple 2 foliage (greenhouse)
Czechoslovakia 0.1 g/plt. 1 2 glasshouse
Netherlands 5 10% G glasshouse, shortly before planting
Cucurbits Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2 24% Liq. 2 2 spray, glasshouse and in open
USA 0.56-2.2 24% Liq. 2 untrimmed
Eggplant Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
Onions Denmark 0.7-1.8 10% G row, at planting
2.5-5 10% G broadcast, before planting
United
Kingdom 13-4.9 10% G furrow, at planting
Peaches Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Pears Greece 2.4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
Peas United 2.5 10% G pre-plant
Kingdom
Peppers Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt 10% G 2 2 glasshouse
Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2spray, glasshouse and in open
Pipfruit N. Zealand 0.048-0.06% 24% Liq. 2 1
Potatoes Denmark 2.5-5 10% G 1 broadcast, pre-planting
Greece 3-4 10% G 2 pre-plant, broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Libya 4-5.5 10% G pre-plant, soil
1.7 24% Liq. 3 (foliar) 4 soil, pre-plant and foliar
Mexico 0.24-0.72 24% Liq. multiple 1 foliage
Netherlands 10% G before or at planting
N. Zealand 5 10% G 13 pre-plant broadcast
United
Kingdom 4-5.5 10% G pre-plant, soil
U.S.A. 0.28-1.1 24% Liq. 1 foliar
Squash Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Strawberries Denmark 0.48 24% Liq. 3 after final harvest
Greece 2-4 10% G non-bearing
1-2 24% Liq. multiple non-bearing
Sugarbeets Greece 0.8-1 10% G at planting
0.18 24% G foliage
Netherlands 0.75 10% G at sowing
Spain 0.8 10% G in row
Syria 2.4-3.6 24% Liq. 1 at plant
1.8 24% Liq. 1 foliar
United
Kingdom 0.6-0.9 10% Liq. in furrow at planting
Tomatoes Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt. 10% G 2 2 glasshouse
Denmark 4.8 24% Liq. field, preplant
0.05% 24% Liq. field, preplant
0.07% 24% L1q. 4 wk. after planting
Egypt 1.8 24% Liq. 4
Greece 3-4 10% G 2 preplant broadcast,
glasshouse or in open
2-3 10% G 2 furrow, glasshouse
or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Netherlands 5 10% G glasshouse, shortly before
planting
Jordan 1.8 24% Liq. 2 4 foliar
Libya 3 10% G 1 4
1.7 24% Liq. 2 4
Mexico 0.06-0.12% 24% Liq. 1 (day) foliar
Syria 1.8 24% Liq. 2 foliar (seedlings)
United
Kingdom 0.1 g/plt 10% G 2 2
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
U.S.A. 0.56-1.1 24% Liq. multiple 1 (day) (AL, FL, MD, NJ, PA, P.R.,
SC, VA) foliar
2 (day) (HI) foliar
3 (day) (CA) foliar
4.5-9 24% Liq. 1 (day) (HI, soil, by
drip irrigation)
1 Most listed U.S. uses are special local need registrations, limited to specific geographical areas.
2 G = granular
3 Liq. = liquid
TABLE 2. Oxamyl residues from supervised trials (DMCF in parentheses)
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Apples N. Zealand 1980 1 0.03-0.06% 24% EC 1 0.1-0.2 2
2-3 0.03-0.11 2
6-8 N.D.1 2
12-14 N.D.1 2
U.S.A. 1972-75 2-6 0.28 24% Liq. 2-3 0.19-0.2 (<0.02) 2
(10 states) 4-5 0.21-0.36 (<0.02) 2
9-11 0.11-0.21 (<0.02) 3
2 0.67 24% Liq. 11 0.12 1
1-3 0.76-1.1 24% Liq. 2-3 0.32-0.84 (0.03-0.05) 5
4-5 0.1-0.23 2
6-8 0.09-0.68 (<0.02-0.04) 14
12-14 1.2 1
1-2 1.7-2.2 24% Liq. 2-5 0.31-1.5 3
6-8 0.05-1.4 (<0.02-0.03) 4
12-17 0.14-1.1 (<0.02-0.04) 5
21-30 0.27 1
1 4 24% Liq. 2-3 1.9 1
6-8 1.2-1.4 2
12-14 0.99-1.0 (0.05-0.15) 1
Banana2 Costa 1975-77 1-8 0.9-2.2 24% Liq. 1-10 <0.02 (0.02) 10 bagged or unbagged,
Rica3 (aerial) whole fruit or edible peel
1 0.05 limit of determination
2 Cavendish and Giant Cavendish
3 five farms
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Beans U.S.A. 1975-77
(succulent, green
snap, lima) Beans Foliage-hay
preplant 1 2.2-4.51 10% G 67 <0.02
foliar VA 1 0.28 24% Liq. 2-3 0.1 (<0.04) 1
6-8 0.02 (<0.04)
VA, FL, CA 1-8 0.56 24% Liq. 1 0.05-0.84 (<0.04) 2 2.5 (<0.04)
2-3 <0.02-5.3 (<0.04) 10 1-14 (<0.04-0.08)
6-11 <0.02-0.97 (<0.04) 9 0.24-16 (<0.04)
12-14 <0.02-0.65 (0.04) 8 0.26-5.7
CA, FL 5-8 1.1 24% Liq. 1 1.9 1 6.7 (0.14)
2-3 <0.02-7.7 (<0.04-0.19) 10 3.2-29 (<0.04-0.41)
6-9 <0.02-2.3 (<0.04-0.17) 8 0.59-33 (<0.04-0.36)
11-14 <0.02-1.0 (<0.04-0.09) 10 0.08-15 (0.06-0.20)
CA, FL 5-8 2.2 24% Liq. 1 6.5 (0.35) 1
2-3 0.04-13 (0.04-0.37) 9 77 (0.66)
6-8 0.1-7.3 (<0.04-0.31) 8 0.91-67 (<0.04-0.83)
12-14 0.05-2.0 (<0.04-0.09) 9 0.06-37 (0.06-0.67)
CA 5 4.5 1 8.9 (0.84) 1
2-3 12 (0.54) 1
12-14 0.28 (<0.04) 1
Beans Beans Straw
(dry-pinto, WY 1977 1 0.56 24% Liq. 50 <0.02 (<0.04) 0.04 (<0.04)
navy, dry) 1 1.1 50 <0.02 (<0.04) 0.18 (<0.04)
vines
CA, MI 1973-78 1.1-2.2 10% G 103-111 <0.02 (<0.04) <0.02-0.08 (<0.04)
3.4 (preplant) 111-132 <0.02 (<0.04)
1 12 inch band incorporated
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Range, mg/kg
Beets, red U.S.A. 1973 1 3.4-6.7 G 84 <0.02 soil
2 1.1 Liq. 48 <0.02 foliar
1 3.4-6.7 G 84 <0.02 soil
+ + + +
2 1.1 Liq. <0.02 foliar
Cantaloupe U.S.A.
GA 1971 1 4.5 10% G 80 0.05
4.5 10% G
+ +
1.1 2 Liq. 39 <0.02
No. in range
CA, FL 1976-78 5-8 1.1 (foliar) 2 L 1 0.04-0.25 4
2-3 0.19-0.26 3
4-5 0.05 1
6-8 0.06-0.16 4
FL, IN, CA 1976-78 5-8 1.1 (foliar) 2 L 1 0.1-0.62 (<0.02-0.03) 5
2-3 0.12-0.48 (<0.02-0.04) 4
4-5 0.06-0.19 (<0.02) 2
6-8 0.07-0.59 (<0.02-0.05) 6
AZ, CA, IN 1976-78 5-8 2.2 (foliar) 2 L 1 0.23-0.91 (0.05-0.1) 3
FL 2-3 0.03-0.66 (0.66) 5
6-8 0.05-0.5 (0.04-0.05) 3
IN 1974-78 2-5 4.5-6.7 2 L 1 0.44-0.69 (0.05-0.08) 2
(foliar) 2-3 0.12-0.52 (0.04-0.08) 2
6-8 0.3 (<0.02) 2
41-51 0.02 1
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
IN 1978 5 9-13 2 L 1 0.49-1.4 (0.07-0.2) 2
(foliar) 2-3 0.44-1.4 (0.02-0.09) 2
6-8 0.41-0.87 (0.02-0.8) 2
FL 1972 1 2.2 2 L
+ +
1.1 2 L 31-40 0.18 (<0.02)
Carrot U.S.A.
preplant OH, MI 1975 1 3.4-4.1 unspecified 120-135 <0.02
soil MI, OH, FL 1970-73 1 3.4-6.7 G. or Liq. 103-185 <0.02 (<0.02)
DE
foliar DE 1971 5 0.56 Liq. 18 <0.02 (<0.02)
soil OH 1973-74 1 3.4-6.7 G. or Liq. 122-185
+ + + +
foliar 3-5 0.56-2.2 Liq. 22 or unspecified <0.02 (<0.02)
Celery U.S.A. (FL) 1974 3 0.56 Liq. 4-5 8.1 1
6-8 6.6 1
12-14 0.88 1
1974 5 1.1 Liq. 1 3.6-24 3
4-5 0.99-8.1 (0.81) 3
6-8 0.66-6.6 6
1.5-1.7 (trimmed) 2
12-14 0.12-1.5 4
0.94 (trimmed)
15-20 0.36-0.39 2
0.27-0.33 (trimmed) 2
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1974 3-5 2.2 Liq. 4-5 20 1
12-14 0.86-3.1 3
2.4 (trimmed) 1
15-20 1.3-1.4
grapefruit lemons oranges tangelos tangerines
Citrus2 U.S.A. 1971-75 1-5 0.59-1.11 Liq. 1 0.14-0.25 0.21-0.29 0.13-0.6
AZ,CA,FL,TX (foliar) 2-3 0.03-2.1 0.17-0.25 0.02-1.1
4-5 0.11 0.18 <0.04
6-8 0.03-1.2 0.05-0.17 0.02-0.8
9-11 <0.02 <0.02
12-14 0.03-0.08 0.06-0.37
15-21 <0.04-0.3
2-7 1.1 90% sol. powder 84 0.04
105 0.14
1 1.4-1.51 Liq. 2-3 3.6 0.51
(foliar) 4-5 .42
6-8 1.2-2 0.34
12-17 0.46-0.8 0.06
1-6 2.1-2.2 Liq. 1 1.3 0.39 0.26-0.36
or 3 2.2+4.5+2.2 2-3 <0.02-0.67 0.29 <0.02-0.52 <0.02-0.1
(foliar)1 4-5 <0.02-0.84 0.04 <0.02-0.92 0.06-0.88
<0.02-0.57
6-8 <0.02-0.3 0.04 <0.02-3 0.02-0.24 <0.02-0.4
9-14 <0.02 <0.02-0.87 0.02
21 <0.02
1 presumed on the basis of proposed label
2 DMCF is <0.06 mg/kg in fruit, peel or pulp except for a 0.54 value at high dosage (4-5 days)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1971-75 3 2.2+4.5+4.5 Liq. 1 1.5 0.55 2.-4.8
1-3 4.2-4.5 2-3 0.08-1.1 0.36 0.22-4.4
(foliar)1 4-5 0.07-0.64 0.32 0.25-3.6 0.88
6-8 0.05-0.64 0.36 0.13-2 0.24-0.36
12-14 <0.02 <0.02-0.67
21 0.03
84 0.04
Corn, field USA 1970-75 1 0.56-4.5 Liq. 93-198 <0.02 (<0.02) 27 (kernal or stalk)
(GA,FL,NC) (band or furrow)
Cottonseed USA 1969-75 1 0.06-0.28 Liq. 51-75 <0.02
(AZ,TX,CA,NC) (foliar)1
1-5 0.37-0.56 Liq. 6-8 <0.02-0.09
(foliar)1 12-17 <0.02-0.13
1-5 0.75-1.1 Liq. 1 25 1
(foliar)1 4-5 0.75 1
6-8 0.02-0.17 19
12-17 <0.02-0.18 19
1-5 1.7-2.2 Liq. 6-8 0.03-0.26 12
(foliar)1 12-14 <0.02-0.17 7
15-17 0.02-0.05 2
146 0.02 2
3-5 4-5 Liq. 6-8 0.25-0.64 4
(foliar)1 15-17 0.02 1
Cucumber U.K. 1973 - 5 unspecified unspecified 0.41-0.68 2
U.S.A.
preplant VA 1977 1 2.2-4.5 10% G 51-75 0.15-0.47 (<0.02-0.06) 2
1 9 10% G 76-100 <0.02
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
foliar FL,VA,CA 1973-78 2-7 0.56 2 L 1 0.15-0.36 5
2-3 0.18-0.38 3
6-8 0.18-0.28 4
12-14 0.07 1
31-40 0.02 (<0.02) 1
FL,CA,VA 1973-78 2-7 1.1 2 L 1 0.22-0.54 (0.1-0.14) 5
2-3 0.26-0.39 (0.03-0.15) 3
6-8 0.26-0.38 (0.07-0.19) 5
12-14 0.15 1
31-40 0.03 (0.02)
AR,CA,FL,VA 1974-78 1-7 2.2 2 L 1 0.45-1.1 (0.14-0.19) 4
2-3 0.03-0.7 (0.23-0.24) 4
4-5 <0.02 1
6-8 0.02-0.78 (0.12-0.25) 5
12-14 0.16 (0.02) 1
41-75 0.05-0.08 (<0.02) 2
1972-76 1-7 4.5 2 L 1 0.87-2.2 (0.17-0.13) 2
6-8 0.84-1.8 (0.25-0.35) 2
12-14 0.32 (0.03) 1
31-40 0.02-0.26 (<0.02-0.07) 2
51-75 <0.02 1
glasshouse Netherlands 19791 1 5 10% G 42 0.16-0.28 (av. 0.21)
1 10 10% G 42 0.08-0.33 (av. 0.27)
2 5 250%g/l 14 0.37-0.52 (av. 0.43)
Honey dew USA 6-9 0.56 2 L 1 0.2-0.24 3
FL 1976-78 (foliar) 2-3 0.18-0.28 2
6-8 0.19-0.23 3
FL 1976-78 1.1 2 L 1 0.36-0.4 (<0.02-0.03) 3
(foliar) 2-3 0.39-0.5 (<0.02-0.03) 2
6-8 0.32-0.44(0.02.-0.03) 3
1 CIVO report R 6282 (1979)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
AZ,CA,FL 1976-78 5-9 2.2 2 L 1 0.68-0.71 (0.04) 2
(foliar) 2-3 <0.02-0.68 (0.05) 3
4-5 <0.02 1
6-8 <0.02-0.7 (0.06-0.1) 3
FL 1976 9 4.5 2 L 1 1.0 (0.04) 1
(foliar) 6-8 0.92 (0.06) 1
Onions U.K. 1972 11 0.84-3.4 10% G1 unspecified <0.04
Netherlands 1977 2 0.41 3.3% G 100-150 <0.01 12
nuts hulls hay (dry)
Peanuts U.S.A. 1971-76 1 1.7-5 (soil) 10% G 76-100 0.03 0.07
(OK,TX,VA, 101-150 <0.02 <0.02 <0.02-0.38
GA,NC) 151-200 <0.02-0.04 <0.02-0.1 <0.02-0.04
1 1-4.5 10% G 12-14 <0.02 <0.02 <0.57
+ (soil) + 51-75 <0.02-0.03 <0.02 <0.02-0.07
2-3 0.56-1.7 24% Liq. 76-100 <0.02 0.04 0.91
1 1.7-2.8 24% Liq. 76-100 <0.02 <0.02 <0.26-1.0
(soil) 100-200 <0.02 <0.02
2-3 0.56-1.7 24% Liq. 6-8 <0.02 0.05 0.06
(foliar) 21-30 <0.02 <0.01 0.04
76-100 <0.02
pea pod
Peas U.K. 1.4-2.8 10% G unknown <0.01 <0.01
(preplant)1
11.1 10% G unknown <0.01 0.15
(preplant)1
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Pepper U.S.A. 1975-78 4-18 0.561 24% Liq. 1 0.04-0.88 6
(AL,CA,FL) (foliar) 2-3 0.15-0.58 (<0.02-0.04) 5
4-5 0.47-0.7 (0.08) 3
6-8 0.11-0.7 (<0.02-0.003) 7
4-18 1.1 24% Liq. 1 0.23-1.1 (0.03-0.12) 7
(foliar) 2-3 0.14-1.0 (0.03-0.06) 6
4-5 0.62-1.5 (0.14) 2
6-8 0.15-1.3 (0.03-0.14) 6
5-6 2.2 24% Liq. 1 1.3-3 (0.04-0.08) 3
(foliar) 2-3 0.46-1.9 (0.04-0.46) 2
4-5 1.4 1
6-8 1.2 (0.07) 1
wholefruit bran leaves hay DMCF fruit2
Pineapple U.S.A. 1975-76 1-6 1.1 24% Liq. 13-14 <0.02-0.46 0.04-0.57 0.26-0.55 0.11-0.46
Hawaii 23-27 <0.02-0.43 0.28-1.7 <0.02-0.22 0.14-1.2 (<0.02)
35-42 0.03-0.09 0.06-0.15 (<0.02)
2.2 24% Liq. 13-14 0.03-1.1 0.10-3.5 0.76-1.5 0.27-1.3
23-27 0.02-0.59 1.1-3.2 0.04-1.4 0.55-1.2 (<0.02-0.04)
35-42 <0.02-0.36 0.02-0.82 (<0.02)
4.5 24% Liq. 13-14 0.13-2.5 0.22-3.0 1.9-6.8 0.75-2.3
23-27 <0.02-0.91 2.5-5.2 0.02-0.70 1.9-8.5 (<0.02-0.1)
9 24% Liq. 14 0.14-1.2 0.57-1.7 2.4-7.3
23 0.17-0.46 0.3-1.9 (0.09-0.2)
Potatoes S. Africa 1974 4-5 0.24% 24% Liq. 51-75 <0.002-0.033 plant water and/or foliar
U.K. 1973 13 5.6 10% G3 unspecified <0.02-0.03
1 with and without 0.56 kg ai/ha in transplant water
2 DMCF residues in pineapple leaves were generally compared to those in the fruit
3 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
(soil) USA (DE) 1969-71 1 1.1-3.4 10% G 76-150 <0.02 pre-plant, soil incorporated
NY, FL, MI 1966-75 1 3.4-6.7 10% G 104-106 <0.02
FL 1974-75 1 10.1-13.4 24% Liq. 21-30 <0.02 broadcast, soil incorporated
FL 1974 1 3.4-4 24% Liq. 100-150 <0.02
(foliar) DE 1969-74 3-6 0.21-0.28 24% Liq. 6-20 <0.02
CA, DE 1969-74 3-13 0.43-0.56 24% Liq. 1-5 <0.02
6-8 <0.02-0.10
12-14 <0.02-0.03
18-30 <0.02
CA,DE,FL,MI 1972-75 1-5 1-1.1 24% Liq. 1 <0.02
2-3 <0.02-0.03
4-14 <0.02-0.03
18-30 <0.02
CA, DE 1969-72 1-5 2.2-3.4 24% Liq. 4-5 <0.02
2-3 0.02-0.05
4-5 <0.02-0.02
6-14 <0.02-0.06 high value from low dosage
DE, CA 1969-74 1-5 4.5 24% Liq. 4-5 <0.02
6-8 0.05-0.11
12-14 0.09
(soil + MI 1974 1 + 3.4-6.8+ G + 100-150
foliar) 1.7 1.1-2.2 24% Liq. 6-8 <0.02
CO 1973-74 1 + 3.4-6.7 G + 100-150
3 1.1 24% Liq. 6-40 <0.02-0.02
DE 1971 1 2.2 10% G soil incorporated
2 (foliar) 24% Liq. 18 <0.02
(dose unspecified)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
soil straw
Soybeans U.S.A. (TN,MO 1970-77 1-3 0.56-6.73 10% G 113-185 <0.02-0.051 <0.02-0.052
(dry) VA,AL,FL,NC;
25 tests)
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02
summer 4-8 <0.02
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02-0.06
vining 4-8 <0.02
tops roots
Sugarbeets U.K. 14 0.6-0.95 10% G4 unspecified 0.05 0.04
U.S.A. (GA,NC 1 0.56 Liq. 76-100 <0.02 transplant water
LA,VA,NY) 1+1 0.56+1.1 Liq. 76-100 <0.02 transplant water+foliage
1 4.5-9 G 76-200 <0.02 soil
1 4.5 Liq. 100-150 <0.02 soil
2 Liq. 51-75 <0.02 foliar
1+ 0.56-9 + G + 75-100 <0.02 soil +
2 1.1 Liq. 51-75 <0.02 foliar
Sugarcane S. Africa Data provided were not useful because of uncertainty of dosage rates.
Sweet potato U.S.A. (GA, 1970-74 1 4.5-95 24% Liq. 100-150 <0.02 (<0.02)
NC,VA,ML) preplant-row and broadcast
1 only 1 of 25 results was at 0.05 mg/kg
2 only 1 of 7 results was at 0.05 mg/kg
3 all but one application were at planting or 1 day prior
4 presumed on the basis of proposed label
5 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
Watermelon U.S.A. 1976-78 5-8 0.56 2L (foliar) 1 0.05-0.29 (<0.02)
(FL,CA) 2-3 0.27
6-8 0.03-0.28 (0.02)
FL 1976-78 8-9 1.1 2L (foliar) 1 0.38-0.77 (<0.02)
2-3 0.47 (0.02)
6-8 0.32-0.56 (0.02)
AZ,CA,FL 1976-78 5-9 2.2 2L (foliar) 1 0.1-1.2
2-3 <0.02-0.87 (0.05)
4-5 <0.02
6-8 0.05-0.78 (<0.02-0.1)
Tomato Netherlands1 1979 1 5 10% G 5 0.02-0.11
(glasshouse)
1 10 10% G 5 0.04-0.23
(glasshouse)
2 5 250 g/l 5 0.32-0.57
preplant broadcast
5.6 10% G 51-150 0.11-0.132
U.K. 1974 11.2 10% G 0.05-0.12
post plant broadcast
5.6-6.7 10% G 12-14 0.66
21-30 0.012
9 10% G 21-30 0.092
11.2 10% G 21-30 0.52
preplant + postplant broadcast
5.6+5.6 10% G 12-14 0.152 (0.85)
11 + 5.6 10% G 12-14 0.072
11 + 11 10% G 12-14 0.78
1 CIVO report R 6282 (1979)
2 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
unspecified application method
2.2-5 10% G1 unspecified 0.06-0.19
10.1 0.07-18.7
U.S.A. 1974-75 5-11 0.56 24% Liq.1 1 0.14-0.44 6
FL, 10 (foliar)1 2-3 0.04-0.31 6
locations 4-5 0.08-0.35 6
6-8 0.25 1
5-12 0.84-1.1 24% Liq.1 1 0.26-1.0 (0.21) 11
(foliar)1 2-3 0.1-0.71 10
4-5 0.11-0.55 10
6-8 0.34 1
5-11 2.2 24% Liq.1 1 0.82-1.9 6
(foliar)1 2-3 0.15-0.78 7
4-5 0.24-0.49 6
6-8 0.24 1
5 4.5 24% Liq.1 1 0.57 1
(foliar)1 4-5 0.35 1
6-8 0.37 1
1 0.05 limit of determination
Apples - Maximum oxamyl residues from approved dosage rates were 1.5
mg/kg at 2-5 days after application and 1.2 mg/kg at the interval of 2
weeks recommended for uses on bearing trees although the application
rate at the latter residues level was less than the maximum permitted.
Residues of DMCF ranged from 0.02-0.05 mg/kg from approved use. There
was no data from uses on non-bearing trees although a one-year
interval is applied for that use.
Bananas - Residues of oxamyl or DMCF were <0.02 mg/kg from
application rates up to 1.7 times maximum recommended dosage in whole
fruit (bagged or unbagged) or edible pulp. The data reflect foliar
applications only.
No residue data were provided for non-foliar uses on bananas.
Beans - Although no residue data were available from the single
country for which recommended usage information was available, data
encompassing those rates were available from trials in the U.S. where
similar uses and a 7-day pre-harvest interval have been proposed.
Residues of oxamyl from pre-plant uses on succulent beans were <0.02
mg/kg although such data were quite limited. Maximum residues of
oxamyl from recommended foliar rates were 1.0, 11 and 15 mg/kg in
succulent beans, foliage, and hay respectively and 0.09, 0.15 and 0.20
mg/kg respectively for DMCF, all at a 14-day interval. At 7 days
maximum oxamyl residues were 2.3, 33 and 25 mg/kg respectively on
succulent beans, foliage and hay. A 14-day pre-harvest interval is
recommended for beans but not specified for forage-hay. An 18-day
pre-graze or pre-forage interval is proposed in the U.S.A.
Oxamyl residues on dry beans from rates reflecting recommended uses
were <0.02 mg/kg and were 0.18 and 0.08 mg/kg on straw and vines
respectively, although most such applications were pre-plant.
Residue data from foliar uses are needed on dry bean varieties and
additional residue data from pre-plant uses on succulent beans,
including additional data on feed items from these uses. Such data
should come from other countries as well as from the U.S.A. The
potential for residues in meat and milk warrants complete data on feed
items.
Celery - Good agricultural practice information (both pre-plant and
foliar) was available from 2 countries although of these only residue
data from foliar treatments in the U.S.A. were available. Maximum
residues at the recommended 2-week pre-harvest interval were 3.1 and
2.4 mg/kg respectively for untrimmed and trimmed celery. Residue data
from other countries would be desirable.
Citrus - (grapefruit, lemons, oranges, tangelos, tangerines) - Residue
data on these citrus crops were available from the U.S.A. reflecting
foliar good agricultural practices. No data were available from good
agricultural practices on non-bearing citrus trees. The maximum
residue on citrus from recommended usage was 1.2 mg/kg on grapefruit
from only one application at the recommended 7-day pre-harvest
interval and this at an application rate 0.7 kg ai/ha, thus less than
the maximum 1.2 kg ai/ha recommended. At 1.5 kg ai/ha maximum
residues, again on grapefruit, were 2 mg/kg at 7 days from only one
application. Residues on oranges from application rates 2 times the
maximum recommended rate were 3 mg/kg. Residues from multiple
applications at recommended rates would be expected to be less than 3
mg/kg.
No additional residues of oxamyl were released when citrus samples
were subjected to ß-glucosidase enzyme treatment.
Corn field - Good agricultural practice information was not provided
for corn although a 0.56-2.2 kg ai/ha at planting 24% liquid
formulation use is proposed in the U.S.A. Residues from applications
up to 2 times this rate resulted in oxamyl residues of <0.02 mg/kg in
the kernels and stalk at 93-198 days after treatment. Only 3 analyses
were made on stalks. Additional residue data are needed for forage
and fodder as well as additional residue data from major corn
producing areas.
Cottonseed - Good agricultural practice information was not provided
for oxamyl uses on cotton although uses at 0.028-1.1 kg/ha foliar
applications of a 24% liquid formulation and a 21-day PHI interval
have been proposed in the U.S.A. Residue data reflecting these uses
were available with residues up to 0.17 mg/kg near the proposed 21-day
pre-harvest interval. Residues would not be expected to exceed 0.2
mg/kg. No DCMF residue was detected in cottonseed meal or oil.
Cucurbits - (cantaloupe, cucumbers, honeydew, summer squash, vining
squash, watermelons) - The only available registered use information
for cucurbits were on greenhouse cucumber, in Bulgaria, Czechoslovakia
and the Netherlands and for cucurbits (greenhouse and in the open) in
Greece. No residue data were provided from these countries except the
Netherlands although residue data on the above cucurbits were
available from the USA where pre-plant and foliar liquid formulation
uses with a 1-day pre-harvest interval are proposed, the former at
2.2-4.5 kg ai/ha and the latter proposed use at 0.56-1.1 kg ai/ha.
The proposed foliar treatment rate is therefore comparable to the
Greek spray uses. Some data from pre-plant granular trials in the
U.S.A. were also provided but none for the proposed pre-plant liquid
formulation uses.
Maximum residues on cucurbits at the recommended 1.2 kg ai/ha foliar
rate were 0.77 and 0.48 mg/kg at 1 and 3 days respectively and 0.47
mg/kg for granular pre-plant treatment at 66 days; for a combined
pre-plant-foliar application a maximum residue of 1.24 mg/kg was
observed. Foliar residues on the various cucurbits were generally
comparable at comparable intervals and application rates. Residue
data from the U.K. were of little value because of lack of knowledge
of the interval, formulation and method of application.
Residue data resulting from recommended uses in countries other than
the U.S.A. (including glasshouse uses) are needed as well as
additional data from pre-plant uses (including liquid formulations).
Onions - Only limited data were available. Maximum residues from
apparent at-plant applications at less than the maximum recommended
rates were <0.04 mg/kg at 100-150 days or at intervals unspecified.
Data from other countries are needed.
Peanuts - Information on recommended good agricultural practices for
oxamyl on peanuts was not provided although pre-plant application of a
10% granular formulation at 3.4-5 kg ai/ha and 24% liquid formulation
at 3.4-5.6 kg ai/ha are proposed uses in the U.S.A. No specific
pre-harvest interval is proposed. Also proposed is a 1.1 kg ai/ha
foliar application of the 24% liquid formulation. Residue data from
the U.S.A. reflecting these proposed rates were provided. Maximum
residues were 0.04 mg/kg in hulls and 1.0 mg/kg in hay. Combining
maximum residues from separate soil and foliar uses results in 0.06
mg/kg for nuts, 0.15 for hulls and 1.6 for hay. Residues would not be
expected to exceed 0.1, 0.2 and 2 mg/kg in the nuts, hulls and hay
respectively.
Peas - Only very limited data were available and it was on an
unspecified pea variety. Maximum residues from recommended granular
pre-plant application rates were <0.01 mg/kg in pea or pod although
the interval was not given. At over 4 times the recommended granular
rate residues were <0.01 for the pea and 0.15 mg/kg on the pod, again
the interval was not provided. Additional data are needed on specific
peas and with known intervals.
Bell peppers - No residue data were provided from the three countries
for which recommended uses were provided. However, residue data on
bell peppers provided from field trials in the U.S.A. reflect proposed
uses of 0.56-1.1 kg ai/ha foliar and 0.56 kg ai/ha transplant water
treatments. A one-week pre-harvest interval is proposed as compared
to the two-week interval recommended. The foliar treatment was
reasonably close to the maximum recommended foliar use in Table 1.
Maximum residues from the proposed foliar treatment were 1.3 mg/kg at
7 days (1.7 at recommended rates) after last application or at
intervals greater than 8 days. No data were available from the
proposed transplant water treatment alone. Maximum residues from the
proposed transplant water application with a less than maximum foliar
application was 0.7 mg/kg at 7 days. Combined residues could
therefore be expected to be about 2.4 mg/kg at 7 days. A limit of 3.0
mg/kg would allow for some variability due to the relatively small
number of samples. Residue data from additional countries are
desirable and should include data from glasshouse uses.
Pineapples - Good agricultural practice information was not made
available although a liquid formulation pre-plant use at 4.5-9 kg
ai/ha and a foliar use at 1.1-4.5 kg ai/ha with a 30-day pre-harvest
interval are proposed in the U.S.A. Data reflecting the proposed
foliar use were available with maximum residues of 0.91 mg/kg in whole
fruit (27 days), 5.2 in bran (27 days), 1.4 in leaves at 23 days or
0.82 at 35 days and 8.5 in hay (27 days). Residues would therefore
not be expected to exceed 1.0, 6.0, 1 or 10 mg/kg in the whole fruit,
bran, forage, and hay respectively at the proposed 30-day interval.
Root and tuber vegetables (beets, carrots, sugar beets and sweet
potatoes) - Although good agricultural practice information for root
and tuber crops was available from a number of countries, most of the
residue data on the above root and tuber crops were from trials in the
USA where, in addition to USA-recommended uses in Table 1, additional
uses have been proposed. Of these proposed uses, broadcast and furrow
pre-plant liquid formulation application of 4.5-9 and 2.2-4.5 kg ai/ha
respectively are proposed for carrots and similar pre-plant rates and
a 7-day pre-harvest interval are proposed for potatoes. From
recommended application rates and methods of application, maximum
residues on root and tuber crops were 0.06 mg/kg at 14 days after last
application and from proposed uses, 0.1 mg/kg at 7 dove (both on
potatoes). The majority of residues from recommended or proposed uses
were 0.03 mg/kg or less. Residues for the root and tuber crops for
which data are provided would not be expected to exceed 0.1 mg/kg from
recommended and proposed uses. No data were available from processing
fractions of root crops.
Residue data on root and tuber vegetables resulting from recommended
good agricultural practices from additional countries are desirable.
Soybeans - Good agricultural practice information was not available.
A proposed USA use would allow liquid formation at-plant and pre-plant
treatments at 2.2-4.5 kg ai/ha and data from 7 states reflecting and
up to 1.5 times these proposed uses were available for dry beans and
straw. Maximum residues were 0.05 mg/kg for the dry seeds and straw
at 113-185 days after last application although all but one sample
each for seeds and straw from the 24 tests were <0.02 mg/kg. The
maximum 0.05 mg/kg residue in soybean seed was from an application
rate of one-fourth of the maximum proposed. On this basis residues
from the proposed uses would be expected to be less than 0.2 mg/kg.
No data were available for the oil, meal, hulls, forage or hay,
although a forage restriction is proposed. Although no concentration
data for the oil or meal were available, a 0.33 n-octanol water
partition coefficient would suggest that concentration of residues may
not occur. This would be consistent with fractionation studies on
cottonseed and peanuts.
Sugarcane - Good agricultural practice information was not provided
for oxamyl on sugarcane. The limited residue data from trials in
South Africa were not useful because of uncertainties on dosage rates.
Tomatoes - Good agricultural practice information was available from
eleven countries, although of these residue data were available only
from the United Kingdom and the USA.
The application rates for the U.K. residues data cannot be compared to
the 0.1 g/plt use described in the label provided. Maximum residues
from the trials were 0.85 mg/kg at the U.K. recommended 2-week pre-
harvest interval.
In the USA 1-3 day intervals are allowed for foliar uses. Maximum
residues from foliar uses recommended in the USA (FL) were 1.0 mg/kg
at a one-day interval and 1.9 mg/kg at two times the recommended rate.
No data were available for drip irrigation uses. The data would
indicate that residues would not exceed 1.0 mg/kg from U.K. uses and
USA foliar uses, taking into account their different pre-harvest
intervals. However, the relatively small number of samples at a given
rate would dictate a higher limit. Additional data from these and
other countries reflecting good agricultural practices would be
desirable.
FATE OF RESIDUES
Figure 2 lists the names and structures of oxamyl and related
compounds.
Figure 2. Names and structures of oxamyl and related compounds
COMPOUND NAME STRUCTURE
O O
OXAMYL METHYL N',N'-DIMETHYL-N[METHYL " "
CARBAMOYL)OXY]-1-THIOOXAMIMIDATE (CH3)2N-C-C=NOCNHCH3
'
SCH3
(I) OXIMINO METABOLITE O
METHYL N-HYDROXY-N',N'- "
DIMETHYL-1- (CH3)2N-C-C=NOH
THIOOXAMIMIDATE '
SCH3
(II) METHYL N-HYDROXY-N'-METHYL-1- O
THIOOXAMIMIDATE "
CH3NH-C-C=NOH
'
SCH3
Figure 2 (continued)
COMPOUND NAME STRUCTURE
O
(III) N'N-DIMETHYLOXAMIC ACID "
(CH3)2N-C-COOH
O
(IV) N-METHYLOXAMIC ACID "
CH3NH-C-COOH
O
(V) DMCF "
N,N-DIMETNYL-1-CYANOFORMAMIDE (CH3)2N-C-CN
O O
(VI) METHYL N'-METHYL-N- " "
[(METHYLCARBAMOYL)OXY]-1- CH3NH-C-C=NOCNHCH3
THIOOXAMIMIDATE '
SCH3
O O
" "
(VII) N,N-DIMETHYLOXAMIDE (CH3)2N-C-C-NH2
O
"
METABOLITE A GLUCOSE CONJUGATE OF I (CH3)2N-C-C=NO-GLUCOSE
'
SCH3
O
"
METABOLITE A' GLUCLOSE CONJUGATE OF II CH3NH-C-C=NO-GLUCOSE
'
SCH3
In animals
In a ruminant metabolism study two lactating goats were maintained on
diets containing approximately 10 mg/kg 14C-oxamyl for 10 and 20 days
respectively (Harvey, 1980a). 60-70% of the radioactivity was
eliminated in the urine and faeces, about 6% was expired, 2-3% was
found in milk and an estimated 22% in the entrails and carcass. The
radioactive compounds were not identified. However, there was no
evidence of oxamyl or oximino compound in milk, blood and tissues
(<0.01 ppm).
If calculated as oxamyl, maximum residues would have been 0.4-0.6
mg/kg in milk, and about 1.4 mg/kg in blood and tissues. Little was
found in fat. Milk residues peaked at about 10-14 days but in blood
they continued to rise throughout the feeding. In each case residues
rapidly decreased after withdrawal of the fortified diet.
About 5% of the activity in milk was incorporated into natural
lactose, caesin and lipids. Two additional components were isolated
and accounted for an additional 30% of milk residues.
In a more recent study 14C-labelled oxamyl and selected metabolites
were studied in vitro by incubation in rumen fluid of a Holstein
cow (Belasco and Harvey, 1980). Oxamyl was 41% metabolized within one
hour and 99% within six hours. After 24 hours incubation the oximino
metabolite (I) and DMCF (V) accounted for 80% of the radioactivity (67
and 13% respectively). The remaining radioactivity at 24 hours was
dimethyloxamic acid 5%, dimethyloxamide 10% and 1-2% each of minor
metabolites previously identified in rat metabolism studies.
Metabolite A, a major plant metabolite, was almost totally metabolized
by rumen fluid. This contrasts to rat metabolism where this compound
is largely unchanged in vitro by liver microsomes and only slowly
metabolized in vivo (Harvey and Han, 1978a). About 70% of
metabolite A was metabolized to DMCF by rumen fluid and the remainder
to unidentified components. The DMCF was found to metabolize to
N,N-dimethyloxamide, N,N-dimethyloxamic acid and N-methyloxamic acid.
Only the dimethyloxamide was not previously identified during rat
metabolism studies.
In a livestock-feeding study oxamyl was fed to Guernsey dairy cows at
2, 10 or 20 mg/kg in the diet for 30 days (DuPont, 1973). Recoveries
of added oxamyl were 70% or greater in whole milk, milk fat, or milk
aqueous fractions at 0.02-0.2 mg/kg fortification levels and over 80%
at 0.04-0.40 mg/kg levels in meat. No residues of oxamyl were
detected (<0.02 mg/kg) in any sample of milk or milk fractions,
liver, kidney, lean muscle or subcutaneous fat at any of the feeding
levels.
When these same samples were analysed for DMCF, no residues were found
at any feeding level in milk or tissues (<0.02 mg/kg for milk, <0.04
mg/kg for meat and fat). Recoveries were 67-82% for 0.02-0.05 mg/kg
fortification levels in milk and 46-100% at 0.04-0.2 mg/kg
fortification levels in meat and fat (Du Pont, 1976).
In a poultry-feeding study, adult laying hens were fed diets
containing 0, 1 or 5 mg/kg oxamyl for a four-week period (Zahnow,
1978). Samples of eggs and tissues were collected and analyzed for
oxamyl and DMCF. Oxamyl residues were <0.02 mg/kg in eggs, liver,
muscle and fat and <0.05 mg/kg in skin (due to limited sample
availability). Residues of DMCF were reported as <0.01 mg/kg in
eggs, liver, muscle, fat and skin.
Oxamyl recoveries from eggs averaged 76% at the 0.02 mg/kg
fortification level and at this same level, except for an apparent
error in skin data, averaged 85-97% in meat, tissues, skin and fat.
Recoveries of DMCF were relatively low, averaging 58 and 50% at 0.01
and 0.02 mg/kg fortification levels, respectively, in eggs and 54-63%
in meat, fat and skin tissues at a 0.02 mg/kg fortification level.
In plants
Plant metabolism of oxamyl was studied in tobacco, alfalfa, peanuts,
potatoes, oranges and tomatoes (Harvey, Han and Reiser, 1978).
In one experiment two tobacco plants were each treated on the leaves
with 10 mg 14C-oxamyl and grown in a growth chamber for 7 and 15 days
respectively. 95% of the radioactive material was recovered at 15
days; about 50% was surface residues, 37% extractable leaf internal
residues and 0.1% or less in the roots. About 4-5% were volatile
components. The surface residue was 95% oxamyl and 3% the oximino
compound (I). Of the leaf internal extractable residues, 56% was
oxamyl, 5% oximino compound (I) and 39% was in the polar fraction, 93%
of which was metabolite A and 7% N,N-dimethyloxamic acid. The
seven-day plants were not as extensively analyzed, but to the extent
they were, residues were similar to the 15-day residues with the
proportion of individual residues reflecting the shorter metabolism
period.
Alfalfa received three 0.5 lb. ai/acre 14C-oxamyl spray field
treatments at two-week intervals and was harvested 2.5 weeks after
last treatment. Extractable residues were 75% of applied. Of this,
>90% was metabolite A with only about 0.8% each for oxamyl and the
oximino compound (I).
Peanuts received two foliar field treatments of 14C-oxamyl at 2 lb.
ai/acre. Samples of young plants were taken at four weeks after the
first treatment, immediately before the second treatment, and
additional samples at harvest. There were therefore three fractions
analyzed - young plants, mature hay and mature nuts. In the young
plant 99% of the radioactivity was determined to be a 2:1 ratio of two
metabolites, A and A' (see figure 2). No oxamyl or oximino compound
(I) was detected.
In mature hay ß-glucosidase treatment of a very polar fraction
containing 99% of extractable residues released metabolites A and A',
giving evidence of an initial polysaccharide type compound, i.e.
metabolites A and A' with additional hexose units (see Figure 3).
Also about 1% of the residue was the oximino compound (I) and <0.5%
oxamyl. When unextractable residues (about 40% in mature hay or nuts)
were treated with a mixture of cellulose enzymes, about 60% was
released yielding a fraction characteristic of metabolites A and A'.
This suggests that the initial unextractable residue was a cellulose
of starch-like structure.
No oxamyl or oximino compound could be detected in the nuts. Of
extractable residues about 21% was incorporated into peanut oil
lipids, about 18% into the polysaccharide-type structure found in
mature hay, about 15% was in lipids or as glucose conjugates and about
37% was unextractable. Of the unextractables about 60% was in the
form of the cellulose-starch type structures discussed above.
Potatoes received five foliar field treatments of 14C-oxamyl at 0.5
to 1.0 lb ai/acre giving an oxamyl residue equivalent of 7 mg/kg in
the tuber. Peels contained <1% of the total residue. Free oxamyl or
oximino compound accounted for <3% of the residue, although mild acid
hydrolysis yielded 39% of the radioactivity in the form of oximino
compounds I and II.
Hydrolysis with ß-glucosidase yielded metabolites A and A', suggesting
the initial presence of the polysaccharide conjugates discussed under
mature peanut hay. At least 35% of the 14C was determined to be
incorporated into the glucose of the tuber starch.
Apples were brush-treated in an orchard at 1.0 lb ai/100 gal and 6
weeks later at harvest yielded 0.8-2.0 mg/kg residues, evenly
distributed through the fruit. About 98% of the residue was
extracted, 77% being organosoluble. Of the organosolubles 16% was
oxamyl, 42% oximino compound I, and 17% DMCF. The remaining 23% of
total fruit residue appeared to be in the form of polysaccharide-type
structures.
Oranges were harvested for analysis six weeks after brush application
of 14C-oxamyl to immature oranges at 1.2 lb. ai/100 gal. Residues
were 2.5 mg/kg on a whole fruit basis expressed as oxamyl. Rind
contained 82% of the residue and the juice 18%. On a whole fruit
basis, residues were 9% oxamyl, 6% oximino compound, 20% DMCF, 35%
metabolite A, 22% metabolite A' and 8% unidentified polar metabolites.
Small green tomato fruits were evenly spotted by pipet with 0.37 mg
14C-oxamyl each and the mature fruit harvested two weeks later for
analysis. Residues were 59% oxamyl, 13% oximino compound, 5%
metabolite A, 4% DMCF and 19% polar metabolites and natural products.
The studies on tobacco, peanuts, and potatoes demonstrate the systemic
nature of foliarly applied oxamyl, which readily translocates to the
roots, legumes and tubers respectively.
Figure 3 summarises a major metabolic pathway for oxamyl in plant
tissues. The methylcarbomoyl group is hydrolysed to form the oximino
compound, followed by conjugate formation of the oximino compound with
glucose to form metabolite A, which may undergo demethylation to form
metabolite A'. Metabolite A predominates in short-term studies (2-6
weeks) with metabolite A' identified in moderate-length studies (1-2
months). At harvest little uncomplexed metabolite A or A' is found
but 14C is incorporated into polysaccharide, cellulose or starch-like
structures suggesting the addition of hexose units to metabolites A or
A'.
In addition to the metabolic route shown in Figure 2, oxamyl may also
be completely broken down in plants and incorporated into natural
plant lipids or into the glucose of starch. The metabolite DMCF is
frequently found as a plant residue and so is the oximino compound in
some fruits. No residues of the S-oxide or S,S,-dioxide of oxamyl or
its oxime were observed during these investigations.
TOXICOLOGICAL STUDIES
Special studies on cholinesterase activity
Rat
Two groups of 10 male rats were given orally 4.8 mg oxamyl/kg bw.
One group received 50 mg/kg atropine sulphate i.p. immediately
after dosing of oxamyl. Cholinesterase activity in blood was
determined 24 hrs before and 5 min., 4 hrs and 24 hrs after dosing.
The animals without atropine showed a decrease in cholinesterase
after 5 min. with a maximum after 4 hrs. After 24 hrs, the
activity was normal again. Animals that were treated with atropine
showed a slight inhibition initially, but the activity was normal
after 4 hrs. The experiment was repeated with the same result
(Schmoyer and Henry, 1970).
Four groups of 8 ChR-CD rats were fed oxamyl in the diet at dosage
levels of 0 (control), 50, 100 or 150 mg/kg feed during 29 days.
Erythrocyte and plasma AChE were measured at day 1, 7, 21, and 28.
On the 29th day the rats were sacrificed to measure AChE in the
brain.
Eight additional rats were assigned to each group for assay of
brain AChE 14 days after the start of the experiment.
With male animals cholinesterase activity of the plasma was only
marginally decreased in the 150 and 100 mg/kg groups throughout the
study. In the females plasma cholinesterase activity was
dose-related decreased in the 150 and 100 mg/kg groups from day 7
onwards. No clear effects were observed on the AChE activity in
the erythrocytes. At the end of the study cholinesterase activity
in brain tissue of females was dose-related decreased in the 150
and 100 mg/kg groups. After 14 days cholinesterase activity in the
brain was inhibited in the 150 mg/kg group. In summary, 50 mg/kg
in the diet was a no-effect level (Barnes and Aftosmis, 1978).
Special study on delayed neurotoxicity
Chicken
The animals were given various oral doses of oxamyl. The ALD
(approximate lethal dose) was established at 40 mg/kg bw. Two
groups of 5 adult hens received 20 or 40 mg/kg bw respectively
followed by 0.5 mg/kg atropine i.m. No mortality occurred. The
animals were kept for 28 days. They showed marked symptoms of
cholinesterase inhibition but recovery was complete after 12 hours.
As a positive control 1200 mg/kg TOCP was used, followed by
atropine. Two weeks after dosing the animals showed typical signs
of delayed neurotoxicity (Ki Poong Lee, 1970).
Special study on reproduction
Rat
The animals of the long-term feeding study were also used for a
3-generation reproduction study. Groups of 16 males and 16 females
on a diet containing 0, 50, 100 or 150 mg/kg were chosen for
breeding three generations of each two litters. Throughout the
study the litter size, viability and lactation indices and body
weights of weanlings were decreased at 100 and 150 mg/kg. A
marginal effect on the body weight of the weanlings was also
observed at 50 mg/kg. No effect on fertility and gestation indices
was detected. Organ weights of the weanling pups of the F3b
generation showed a slight increase in relative kidney weight at
150 mg/kg. The relative testes weight was increased at 100 and 150
mg/kg. No histopathological abnormalities were found that could be
ascribed to the compound (Sherman et al. 1972).
Special study on mutagenicity
Microorganisms
Oxamyl showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, and reverse mutation tests with and
without a liver activation system using 5 strains of Salmonella
thyphimurium TA and E. coli WP2 hcr. A host-mediated assay
in mice using S. typhimurium G-46 was also negative (Shirasu
et al, 1976).
Special study on teratogenicity
Rat
Groups of 26-28 pregnant females received 0, 50, 100, 150 or 300
mg/kg oxamyl in the diet from day 6-15 of pregnancy. In the
mothers, a dose-related decrease in body weight and food
consumption was found for 100, 150 and 300 mg/kg groups. There
were no effects on number of implantation sites, resorptions and
live foetuses nor on embryonal development. Oxamyl does not show
teratogenic or embryotoxic properties (Culik and Sherman, 1971).
Acute Toxicity
species sex route LD50 in mg/kg bw reference
rat M oral 371 (27-51) Carroll, 1972
rat M oral 1102 (97-185) Gibson, 1973
rat M oral 5.4 (3.7-7.8) Fretz, 1969
rat3 M oral 16 (22) Schmoyer, 1969
rat3 F oral 11 (7-16) Schmoyer, 1969
1 formulation Vydate L (26% ai) in aqueous solution
2 Vydate G (10% ai) in corn oil
3 fasted overnight
Dermal studies
Rabbits
When oxamyl was applied as a suspension in a hydrophillic ointment
for 24 hrs, the ALD (approximate lethal dose) was 2250-5000 mg/kg
bw on the intact skin, but only 90-130 mg/kg on the abraded skin.
When oxamyl was applied as a slurry in propylene glycol the ALD was
130 or 60 mg/kg bw in the intact and abraded skin respectively
(Colburn, 1970).
When Vydate L (27% ai) was applied during 24 hrs the LD50 was 740
± 150 mg/kg bw on active ingredient basis on the intact akin
(Morrow, 1973).
Inhalation studies
Rats
The LC50 (1 h) for oxamyl was 0.035 mg/l air when Vydate L (27% ai)
was used in the test (males only) (Barras 1974). When the
experiment was carried out with oxamyl dust the LC50 (1 h) for male
and female animals was 0.17 and 0.12 mg/l respectively. The
concentration in air was calculated after chemical analysis
(Tayfun, 1969).
Symptoms of intoxication
Fasciculations, tremors, salivation, lacrimation, bulging eyes and
chromodacryorrhoea were found after an acute oral dose of oxamyl.
They can be considered as symptoms of cholinesterase inhibition
(Carroll, 1972).
A good antidotal effect was obtained when rats orally treated with
oxamyl received an i.p. injection with atropine sulphate (Sherman,
1969)
Acute toxicity of oxamyl metabolites in rats
LD50 in
metabolite sex route mg/kg bw reference
methyl N-hydroxy-N,N' dimethyl- M oral 11,000 (=ALD) Fretz, 1968
-1-thiooxamimidate (INA-2213)
methyl N-hydroxy-N' methyl-1- M oral 6,675 (6370- Dale, 1973
thiooxamimidate (IEL-2953) 6690)
N,N-dimethyloxamic acid (IND-2708) M oral 3,540 Barbo, 1972
N,N-dimethyl-1-cyanoformamide M oral 450 (=ALD) Ashley, 1974
(DMCF) (INN-79)
methyl N'-methyl-N [(methyl-carbamoyl) M oral 60 Schmoyer, 1969
oxy]-1-thiooxamimidate (IND-14O9)
glucose conjugate of methyl N'- M oral > 7500 Henry, 1976
methylhydroxy-N',N'-dimethyl
-1-thiooxamimidate (ING-3515)
Short-term studies
Rat
Six male rats were given orally 2.4 mg/kg bw oxamyl for 10 days. No
mortality occurred. Oxamyl does not exhibit cumulative oral toxicity
(Fretz and Sherman, 1968).
Rabbit
Rabbits were treated dermally for 15 days during a period of 4 weeks
with Vydate L (26% ai) in dimethylformamide. Groups of each 5 male
and 5 female animals received 193 mg/kg Vydate L, or 50 mg/kg bw of
oxamyl during 6 hrs/day on the intact or abraded skin. Control
animals received dimethylformamide. All oxamyl treated animals with
abraded skin showed marked signs of cholinesterase inhibition. Only
slight symptoms were seen on the animals with intact skin. No
mortalities occurred. No changes in body weight, organ weight or
histopathology were found that could be ascribed to the treatment.
All animals showed mild erythema of the treated skin (Dion, 1970).
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 100 or 500 mg/kg oxamyl in the diet. After a few days the highest
dose level was reduced to 150 mg/kg because with 500 mg/kg all animals
showed strong symptoms of cholinesterase inhibition. They were given
control diet for 3 days and the symptoms disappeared or became less
severe. Then the animals were given 150 mg/kg. No clinical signs of
toxicity were seen in the remainder of the experiment. The body
weight gain was lower at 100 and 150 mg/kg and slightly affected in
the females of the 50 mg/kg group. The food consumption was lower at
150 mg/kg only. Urinalysis showed a higher number of animals with
blood in urine at 100 and 150 mg/kg and a higher number with protein
at 150 mg/kg. Male rats showed lower absolute organ weights for
heart, kidneys, liver, spleen and thymus at 100 and 150 mg/kg. In the
females this effect was found for the liver at 100 and 150 mg/kg and
the kidneys and lungs at 100 mg/kg. No effects were found on
haematology, clinical chemistry and histopathology. Cholinesterase
activity was not determined. The marginal no-effect level is 50 mg/kg
(Snee et al, 1969).
At the end of the experiment 6 animals per group per sex were used for
reproduction performance. In the F1a and F1b generation the
fertility index and the number of pups/litter were decreased at 100
and 150 mg/kg. The weight of the young at weaning was significantly
lower at all dose levels (Snee et al, 1969).
Dog
Groups of 4 male and 4 female animals each received for 90 days 0, 50,
100 or 150 mg/kg oxamyl in the diet. Only marginal effects were found
with 150 mg/kg. A slight increased activity was found for alkaline
phosphatase after 4 and 13 weeks. In the males the weight of
adrenals, spleen and testes was somewhat decreased. The females
showed a slight increase in thyroid weight and a decrease in adrenal
weight. Histopathologically no abnormalities were found that could be
ascribed to the compound (Holsing, 1969).
Groups of 4 male and 4 female animals each received for 2 years 0, 50,
100 or 150 mg/kg oxamyl (95%) in the diet. After 1 year 1 male and 1
female animal from the control and 150 mg/kg groups were killed.
Haematology, clinical chemistry and urinalysis were carried out after
1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 months. The haemoglobin content,
haematocrit and number of erythrocytes in blood at 150 mg/kg were
found to be lower than the controls on most times of measurement in
both sexes.
Higher values for cholesterol and alkaline phosphatase activity were
found more often at 150 mg/kg in males and females. On some occasions
cholesterol was also higher in the other experimental groups.
No effects on organ weights, cholinesterase or aliesterase activity,
or histopathology were found that could be ascribed to the compound
(Sherman, et al, 1972).
Short term study with DMCF, a metabolite of oxamyl
Rat
Groups of 16 male and 16 female animals each received for 90 days 0,
50, 150 or 450 mg/kg N,N-dimethyl-1-cyanoformamide (DMCF) in the diet.
Growth inhibition was found at 450 mg/kg in males and females, and the
food consumption was also lower. At the highest dose level
haemoglobin content, haematocrit and number of erythrocytes were
lower. At 150 mg/kg a slight effect was found on these parameters in
the females or males. In the male animals an increased LDH activity
was found at 450 mg/kg and in the female animals a decreased activity
in GPT, GOT, and LDH at the same dose level. Organ weights were not
reported in this study. Histopathologically an increase was found in
hydronephrosis of the kidneys in the males and minute foci of
mineralization in the kidneys of females at 450 mg/kg. The animals of
the other dose levels were not studied histopathologically. The
no-effect level is probably 50 mg/kg (Kaplan, 1976).
At the end of the experiment 6 animals per group were used for
reproduction performance. With 450 mg/kg a decreased weight of the
young at weaning was observed (Kaplan, 1976).
Long-term studies
Rat
Groups of 36 male and 36 female animals each received for 2 years O,
50, 100 or 150 mg/kg oxamyl (95%) in the diet (2 control groups were
used). After one year of feeding the number of rats of each sex in
each group was reduced to 30. With 100 and 150 mg/kg a decreased body
weight was found with male and female animals that was related to
dose. With 50 mg/kg the male animals showed a slightly lower body
weight, which was apparent after 4 weeks and remained so throughout
the study. Food consumption was lower at 150 mg/kg. Cholinesterase
activity in blood in the 150 mg/kg group was only decreased in the
females after 4 days of feeding and in the males after 8 days, and not
after 1, 6, 12 and 24 months. Aliesterase activity was not affected
throughout the study.
At the end of the experiment relative weights of brain, testes and
adrenals were increased in the males and brain, heart, lungs, kidneys
and adrenals in females at 150 mg/kg. In the females most or these
organ weights were also increased at 100 mg/kg. These changes are
probably connected with the decreased body weight. No haematological,
biochemical or histopathological abnormalities were found that could
be ascribed to the compound (Sherman et al, 1972).
RESIDUES IN FOOD
USE PATTERN
Oxamyl formulations, either as a 24% liquid or 10% granular, are
recommended or registered for use in numerous countries. Food uses
made available to the Meeting by the manufacturer are summarized in
Table 1. Proposed uses are not included but are discussed under
appropriate commodities when residues are discussed.
According to information provided to the Meeting, uses listed for the
United States also apply to Argentina, Barbados, Bolivia, Brazil,
Chile, Colombia, Costa Rica, The Dominican Republic, Ecuador,
Guatemala, Mexico, Nicaragua, Peru, El Salvador, Trinidad and Tobago,
Uruguay and Venezuela unless indicated otherwise in Table 1. Only a
few national governments provided usage information directly to the
meeting.
The liquid formulation is a contact-type, moderately residual
insecticide when applied as a foliar spray. When so applied it is
effective on several species of mites and, by systemic action in many
plants, on nematodes. When oxamyl formulations are applied to the
soil they function as a contact type broad-spectrum nematocide and, by
systemic action, as a miticide/insecticide.
Applications may be pre-planting, at planting, or foliar (to
fruit-bearing or non- fruit-bearing plants). They may be applied in
the furrow or broadcast. Soil incorporation may be mechanical or by
irrigation.
RESIDUES RESULTING FROM SUPERVISED TRIALS
There are numerous combinations of type of formulation, method and
time of application suitable for a wide range of pest control needs.
Because of this, the residue level can vary widely, even on the same
commodity. A large volume of data on 29 commodities or groups of
commodities was presented to the meeting; this has been considerably
condensed into Table 2 (Du Pont, 1980).
The analytical method used extracts both oxamyl and its oxime
metabolite (I), converts the oxamyl to the oxime which is quantitised,
so the residue levels quoted are of oxamyl plus the oxime expressed as
oxamyl. Data on residues of the DMCF metabolite are in parentheses.
TABLE 1. Recommended uses for oxamyl1
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation2,3 treatments interval (wks) notes
Apples
non-bearing U.S.A. 0.28-2.2 24% Liq. 52
6.7 - 9 pre-plant, soil incorporation
bearing 0.28-2.2 2 do not graze treated orchards
non-bearing Greece 2-4 10% G 52 soil
1.2 24% G multiple 52 foliage
Bananas Greece 3 g/plt 10% G 3 at planting
2-3 g/plt 2 on plantations (around plant)
0.01% 24% Liq. foliage
Central Amer. 0.9-1.3 24% Liq. 5-8 foliar
Spain 3.8 24% Liq. in irrigation water
Beans Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, broadcast glasshouse and
in open
1.2 24% Liq. 2 2 spray, broadcast glasshouse and
in open
Beets (roots Denmark 0.7-1.8 10% G row, at planting
or tops) 2.5-5 10% G broadcast, before planting
Carrots Greece 3-4 10% G 2 pre-plant, broadcast
2-3 10% G 2 furrow
1.2 24% Liq. 2 2 spray
1.2 24% Liq. multiple 52 foliage
Celery Greece 3-4 10% G 2 preplant broadcast, glasshouse and
in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
USA 0.56-2.2 24% Liq. 2 untrimmed
4.4 24% Liq. 2 pre-plant soil incorporated
Citrus Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
USA up to 1.1 1 do not graze treated orchards
(0.008-0.03%)
Cherries Greece 2-4 10% G 52 soil non-bearing
1.2 24% Liq. multiple 52 foliage non-bearing
Cucumbers Bulgaria 0.024% 24% Liq. multiple 2 foliage (greenhouse)
Czechoslovakia 0.1 g/plt. 1 2 glasshouse
Netherlands 5 10% G glasshouse, shortly before planting
Cucurbits Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2 24% Liq. 2 2 spray, glasshouse and in open
USA 0.56-2.2 24% Liq. 2 untrimmed
Eggplant Greece 3-4 10% G 2 pre-plant broadcast,
glasshouse and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse and in open
Onions Denmark 0.7-1.8 10% G row, at planting
2.5-5 10% G broadcast, before planting
United
Kingdom 13-4.9 10% G furrow, at planting
Peaches Greece 2-4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Pears Greece 2.4 10% G 52 soil, non-bearing
1.2 24% Liq. multiple 52 foliage, non-bearing
Peas United 2.5 10% G pre-plant
Kingdom
Peppers Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt 10% G 2 2 glasshouse
Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
and in open
2-3 10% G 2 furrow, glasshouse and in open
1.2-1.4 24% Liq. 2 2spray, glasshouse and in open
Pipfruit N. Zealand 0.048-0.06% 24% Liq. 2 1
Potatoes Denmark 2.5-5 10% G 1 broadcast, pre-planting
Greece 3-4 10% G 2 pre-plant, broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Libya 4-5.5 10% G pre-plant, soil
1.7 24% Liq. 3 (foliar) 4 soil, pre-plant and foliar
Mexico 0.24-0.72 24% Liq. multiple 1 foliage
Netherlands 10% G before or at planting
N. Zealand 5 10% G 13 pre-plant broadcast
United
Kingdom 4-5.5 10% G pre-plant, soil
U.S.A. 0.28-1.1 24% Liq. 1 foliar
Squash Greece 3-4 10% G 2 pre-plant broadcast, glasshouse
or in open
2-3 10% G 2 furrow, glasshouse or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
Strawberries Denmark 0.48 24% Liq. 3 after final harvest
Greece 2-4 10% G non-bearing
1-2 24% Liq. multiple non-bearing
Sugarbeets Greece 0.8-1 10% G at planting
0.18 24% G foliage
Netherlands 0.75 10% G at sowing
Spain 0.8 10% G in row
Syria 2.4-3.6 24% Liq. 1 at plant
1.8 24% Liq. 1 foliar
United
Kingdom 0.6-0.9 10% Liq. in furrow at planting
Tomatoes Bulgaria 0.024% 24% Liq. multiple 2 foliage, glasshouse
Czechoslovakia 0.1 g/plt. 10% G 2 2 glasshouse
Denmark 4.8 24% Liq. field, preplant
0.05% 24% Liq. field, preplant
0.07% 24% L1q. 4 wk. after planting
Egypt 1.8 24% Liq. 4
Greece 3-4 10% G 2 preplant broadcast,
glasshouse or in open
2-3 10% G 2 furrow, glasshouse
or in open
1.2-1.4 24% Liq. 2 2 spray, glasshouse or in open
Netherlands 5 10% G glasshouse, shortly before
planting
Jordan 1.8 24% Liq. 2 4 foliar
Libya 3 10% G 1 4
1.7 24% Liq. 2 4
Mexico 0.06-0.12% 24% Liq. 1 (day) foliar
Syria 1.8 24% Liq. 2 foliar (seedlings)
United
Kingdom 0.1 g/plt 10% G 2 2
TABLE 1. Continued...
Rate, kg No. of pre-harvest
Crop Country or % ai Formulation treatments interval (wks) notes
U.S.A. 0.56-1.1 24% Liq. multiple 1 (day) (AL, FL, MD, NJ, PA, P.R.,
SC, VA) foliar
2 (day) (HI) foliar
3 (day) (CA) foliar
4.5-9 24% Liq. 1 (day) (HI, soil, by
drip irrigation)
1 Most listed U.S. uses are special local need registrations, limited to specific geographical areas.
2 G = granular
3 Liq. = liquid
TABLE 2. Oxamyl residues from supervised trials (DMCF in parentheses)
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Apples N. Zealand 1980 1 0.03-0.06% 24% EC 1 0.1-0.2 2
2-3 0.03-0.11 2
6-8 N.D.1 2
12-14 N.D.1 2
U.S.A. 1972-75 2-6 0.28 24% Liq. 2-3 0.19-0.2 (<0.02) 2
(10 states) 4-5 0.21-0.36 (<0.02) 2
9-11 0.11-0.21 (<0.02) 3
2 0.67 24% Liq. 11 0.12 1
1-3 0.76-1.1 24% Liq. 2-3 0.32-0.84 (0.03-0.05) 5
4-5 0.1-0.23 2
6-8 0.09-0.68 (<0.02-0.04) 14
12-14 1.2 1
1-2 1.7-2.2 24% Liq. 2-5 0.31-1.5 3
6-8 0.05-1.4 (<0.02-0.03) 4
12-17 0.14-1.1 (<0.02-0.04) 5
21-30 0.27 1
1 4 24% Liq. 2-3 1.9 1
6-8 1.2-1.4 2
12-14 0.99-1.0 (0.05-0.15) 1
Banana2 Costa 1975-77 1-8 0.9-2.2 24% Liq. 1-10 <0.02 (0.02) 10 bagged or unbagged,
Rica3 (aerial) whole fruit or edible peel
1 0.05 limit of determination
2 Cavendish and Giant Cavendish
3 five farms
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Beans U.S.A. 1975-77
(succulent, green
snap, lima) Beans Foliage-hay
preplant 1 2.2-4.51 10% G 67 <0.02
foliar VA 1 0.28 24% Liq. 2-3 0.1 (<0.04) 1
6-8 0.02 (<0.04)
VA, FL, CA 1-8 0.56 24% Liq. 1 0.05-0.84 (<0.04) 2 2.5 (<0.04)
2-3 <0.02-5.3 (<0.04) 10 1-14 (<0.04-0.08)
6-11 <0.02-0.97 (<0.04) 9 0.24-16 (<0.04)
12-14 <0.02-0.65 (0.04) 8 0.26-5.7
CA, FL 5-8 1.1 24% Liq. 1 1.9 1 6.7 (0.14)
2-3 <0.02-7.7 (<0.04-0.19) 10 3.2-29 (<0.04-0.41)
6-9 <0.02-2.3 (<0.04-0.17) 8 0.59-33 (<0.04-0.36)
11-14 <0.02-1.0 (<0.04-0.09) 10 0.08-15 (0.06-0.20)
CA, FL 5-8 2.2 24% Liq. 1 6.5 (0.35) 1
2-3 0.04-13 (0.04-0.37) 9 77 (0.66)
6-8 0.1-7.3 (<0.04-0.31) 8 0.91-67 (<0.04-0.83)
12-14 0.05-2.0 (<0.04-0.09) 9 0.06-37 (0.06-0.67)
CA 5 4.5 1 8.9 (0.84) 1
2-3 12 (0.54) 1
12-14 0.28 (<0.04) 1
Beans Beans Straw
(dry-pinto, WY 1977 1 0.56 24% Liq. 50 <0.02 (<0.04) 0.04 (<0.04)
navy, dry) 1 1.1 50 <0.02 (<0.04) 0.18 (<0.04)
vines
CA, MI 1973-78 1.1-2.2 10% G 103-111 <0.02 (<0.04) <0.02-0.08 (<0.04)
3.4 (preplant) 111-132 <0.02 (<0.04)
1 12 inch band incorporated
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Range, mg/kg
Beets, red U.S.A. 1973 1 3.4-6.7 G 84 <0.02 soil
2 1.1 Liq. 48 <0.02 foliar
1 3.4-6.7 G 84 <0.02 soil
+ + + +
2 1.1 Liq. <0.02 foliar
Cantaloupe U.S.A.
GA 1971 1 4.5 10% G 80 0.05
4.5 10% G
+ +
1.1 2 Liq. 39 <0.02
No. in range
CA, FL 1976-78 5-8 1.1 (foliar) 2 L 1 0.04-0.25 4
2-3 0.19-0.26 3
4-5 0.05 1
6-8 0.06-0.16 4
FL, IN, CA 1976-78 5-8 1.1 (foliar) 2 L 1 0.1-0.62 (<0.02-0.03) 5
2-3 0.12-0.48 (<0.02-0.04) 4
4-5 0.06-0.19 (<0.02) 2
6-8 0.07-0.59 (<0.02-0.05) 6
AZ, CA, IN 1976-78 5-8 2.2 (foliar) 2 L 1 0.23-0.91 (0.05-0.1) 3
FL 2-3 0.03-0.66 (0.66) 5
6-8 0.05-0.5 (0.04-0.05) 3
IN 1974-78 2-5 4.5-6.7 2 L 1 0.44-0.69 (0.05-0.08) 2
(foliar) 2-3 0.12-0.52 (0.04-0.08) 2
6-8 0.3 (<0.02) 2
41-51 0.02 1
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
IN 1978 5 9-13 2 L 1 0.49-1.4 (0.07-0.2) 2
(foliar) 2-3 0.44-1.4 (0.02-0.09) 2
6-8 0.41-0.87 (0.02-0.8) 2
FL 1972 1 2.2 2 L
+ +
1.1 2 L 31-40 0.18 (<0.02)
Carrot U.S.A.
preplant OH, MI 1975 1 3.4-4.1 unspecified 120-135 <0.02
soil MI, OH, FL 1970-73 1 3.4-6.7 G. or Liq. 103-185 <0.02 (<0.02)
DE
foliar DE 1971 5 0.56 Liq. 18 <0.02 (<0.02)
soil OH 1973-74 1 3.4-6.7 G. or Liq. 122-185
+ + + +
foliar 3-5 0.56-2.2 Liq. 22 or unspecified <0.02 (<0.02)
Celery U.S.A. (FL) 1974 3 0.56 Liq. 4-5 8.1 1
6-8 6.6 1
12-14 0.88 1
1974 5 1.1 Liq. 1 3.6-24 3
4-5 0.99-8.1 (0.81) 3
6-8 0.66-6.6 6
1.5-1.7 (trimmed) 2
12-14 0.12-1.5 4
0.94 (trimmed)
15-20 0.36-0.39 2
0.27-0.33 (trimmed) 2
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1974 3-5 2.2 Liq. 4-5 20 1
12-14 0.86-3.1 3
2.4 (trimmed) 1
15-20 1.3-1.4
grapefruit lemons oranges tangelos tangerines
Citrus2 U.S.A. 1971-75 1-5 0.59-1.11 Liq. 1 0.14-0.25 0.21-0.29 0.13-0.6
AZ,CA,FL,TX (foliar) 2-3 0.03-2.1 0.17-0.25 0.02-1.1
4-5 0.11 0.18 <0.04
6-8 0.03-1.2 0.05-0.17 0.02-0.8
9-11 <0.02 <0.02
12-14 0.03-0.08 0.06-0.37
15-21 <0.04-0.3
2-7 1.1 90% sol. powder 84 0.04
105 0.14
1 1.4-1.51 Liq. 2-3 3.6 0.51
(foliar) 4-5 .42
6-8 1.2-2 0.34
12-17 0.46-0.8 0.06
1-6 2.1-2.2 Liq. 1 1.3 0.39 0.26-0.36
or 3 2.2+4.5+2.2 2-3 <0.02-0.67 0.29 <0.02-0.52 <0.02-0.1
(foliar)1 4-5 <0.02-0.84 0.04 <0.02-0.92 0.06-0.88
<0.02-0.57
6-8 <0.02-0.3 0.04 <0.02-3 0.02-0.24 <0.02-0.4
9-14 <0.02 <0.02-0.87 0.02
21 <0.02
1 presumed on the basis of proposed label
2 DMCF is <0.06 mg/kg in fruit, peel or pulp except for a 0.54 value at high dosage (4-5 days)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
1971-75 3 2.2+4.5+4.5 Liq. 1 1.5 0.55 2.-4.8
1-3 4.2-4.5 2-3 0.08-1.1 0.36 0.22-4.4
(foliar)1 4-5 0.07-0.64 0.32 0.25-3.6 0.88
6-8 0.05-0.64 0.36 0.13-2 0.24-0.36
12-14 <0.02 <0.02-0.67
21 0.03
84 0.04
Corn, field USA 1970-75 1 0.56-4.5 Liq. 93-198 <0.02 (<0.02) 27 (kernal or stalk)
(GA,FL,NC) (band or furrow)
Cottonseed USA 1969-75 1 0.06-0.28 Liq. 51-75 <0.02
(AZ,TX,CA,NC) (foliar)1
1-5 0.37-0.56 Liq. 6-8 <0.02-0.09
(foliar)1 12-17 <0.02-0.13
1-5 0.75-1.1 Liq. 1 25 1
(foliar)1 4-5 0.75 1
6-8 0.02-0.17 19
12-17 <0.02-0.18 19
1-5 1.7-2.2 Liq. 6-8 0.03-0.26 12
(foliar)1 12-14 <0.02-0.17 7
15-17 0.02-0.05 2
146 0.02 2
3-5 4-5 Liq. 6-8 0.25-0.64 4
(foliar)1 15-17 0.02 1
Cucumber U.K. 1973 - 5 unspecified unspecified 0.41-0.68 2
U.S.A.
preplant VA 1977 1 2.2-4.5 10% G 51-75 0.15-0.47 (<0.02-0.06) 2
1 9 10% G 76-100 <0.02
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
foliar FL,VA,CA 1973-78 2-7 0.56 2 L 1 0.15-0.36 5
2-3 0.18-0.38 3
6-8 0.18-0.28 4
12-14 0.07 1
31-40 0.02 (<0.02) 1
FL,CA,VA 1973-78 2-7 1.1 2 L 1 0.22-0.54 (0.1-0.14) 5
2-3 0.26-0.39 (0.03-0.15) 3
6-8 0.26-0.38 (0.07-0.19) 5
12-14 0.15 1
31-40 0.03 (0.02)
AR,CA,FL,VA 1974-78 1-7 2.2 2 L 1 0.45-1.1 (0.14-0.19) 4
2-3 0.03-0.7 (0.23-0.24) 4
4-5 <0.02 1
6-8 0.02-0.78 (0.12-0.25) 5
12-14 0.16 (0.02) 1
41-75 0.05-0.08 (<0.02) 2
1972-76 1-7 4.5 2 L 1 0.87-2.2 (0.17-0.13) 2
6-8 0.84-1.8 (0.25-0.35) 2
12-14 0.32 (0.03) 1
31-40 0.02-0.26 (<0.02-0.07) 2
51-75 <0.02 1
glasshouse Netherlands 19791 1 5 10% G 42 0.16-0.28 (av. 0.21)
1 10 10% G 42 0.08-0.33 (av. 0.27)
2 5 250%g/l 14 0.37-0.52 (av. 0.43)
Honey dew USA 6-9 0.56 2 L 1 0.2-0.24 3
FL 1976-78 (foliar) 2-3 0.18-0.28 2
6-8 0.19-0.23 3
FL 1976-78 1.1 2 L 1 0.36-0.4 (<0.02-0.03) 3
(foliar) 2-3 0.39-0.5 (<0.02-0.03) 2
6-8 0.32-0.44(0.02.-0.03) 3
1 CIVO report R 6282 (1979)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
AZ,CA,FL 1976-78 5-9 2.2 2 L 1 0.68-0.71 (0.04) 2
(foliar) 2-3 <0.02-0.68 (0.05) 3
4-5 <0.02 1
6-8 <0.02-0.7 (0.06-0.1) 3
FL 1976 9 4.5 2 L 1 1.0 (0.04) 1
(foliar) 6-8 0.92 (0.06) 1
Onions U.K. 1972 11 0.84-3.4 10% G1 unspecified <0.04
Netherlands 1977 2 0.41 3.3% G 100-150 <0.01 12
nuts hulls hay (dry)
Peanuts U.S.A. 1971-76 1 1.7-5 (soil) 10% G 76-100 0.03 0.07
(OK,TX,VA, 101-150 <0.02 <0.02 <0.02-0.38
GA,NC) 151-200 <0.02-0.04 <0.02-0.1 <0.02-0.04
1 1-4.5 10% G 12-14 <0.02 <0.02 <0.57
+ (soil) + 51-75 <0.02-0.03 <0.02 <0.02-0.07
2-3 0.56-1.7 24% Liq. 76-100 <0.02 0.04 0.91
1 1.7-2.8 24% Liq. 76-100 <0.02 <0.02 <0.26-1.0
(soil) 100-200 <0.02 <0.02
2-3 0.56-1.7 24% Liq. 6-8 <0.02 0.05 0.06
(foliar) 21-30 <0.02 <0.01 0.04
76-100 <0.02
pea pod
Peas U.K. 1.4-2.8 10% G unknown <0.01 <0.01
(preplant)1
11.1 10% G unknown <0.01 0.15
(preplant)1
1 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
Pepper U.S.A. 1975-78 4-18 0.561 24% Liq. 1 0.04-0.88 6
(AL,CA,FL) (foliar) 2-3 0.15-0.58 (<0.02-0.04) 5
4-5 0.47-0.7 (0.08) 3
6-8 0.11-0.7 (<0.02-0.003) 7
4-18 1.1 24% Liq. 1 0.23-1.1 (0.03-0.12) 7
(foliar) 2-3 0.14-1.0 (0.03-0.06) 6
4-5 0.62-1.5 (0.14) 2
6-8 0.15-1.3 (0.03-0.14) 6
5-6 2.2 24% Liq. 1 1.3-3 (0.04-0.08) 3
(foliar) 2-3 0.46-1.9 (0.04-0.46) 2
4-5 1.4 1
6-8 1.2 (0.07) 1
wholefruit bran leaves hay DMCF fruit2
Pineapple U.S.A. 1975-76 1-6 1.1 24% Liq. 13-14 <0.02-0.46 0.04-0.57 0.26-0.55 0.11-0.46
Hawaii 23-27 <0.02-0.43 0.28-1.7 <0.02-0.22 0.14-1.2 (<0.02)
35-42 0.03-0.09 0.06-0.15 (<0.02)
2.2 24% Liq. 13-14 0.03-1.1 0.10-3.5 0.76-1.5 0.27-1.3
23-27 0.02-0.59 1.1-3.2 0.04-1.4 0.55-1.2 (<0.02-0.04)
35-42 <0.02-0.36 0.02-0.82 (<0.02)
4.5 24% Liq. 13-14 0.13-2.5 0.22-3.0 1.9-6.8 0.75-2.3
23-27 <0.02-0.91 2.5-5.2 0.02-0.70 1.9-8.5 (<0.02-0.1)
9 24% Liq. 14 0.14-1.2 0.57-1.7 2.4-7.3
23 0.17-0.46 0.3-1.9 (0.09-0.2)
Potatoes S. Africa 1974 4-5 0.24% 24% Liq. 51-75 <0.002-0.033 plant water and/or foliar
U.K. 1973 13 5.6 10% G3 unspecified <0.02-0.03
1 with and without 0.56 kg ai/ha in transplant water
2 DMCF residues in pineapple leaves were generally compared to those in the fruit
3 presumed on the basis of proposed label
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
(soil) USA (DE) 1969-71 1 1.1-3.4 10% G 76-150 <0.02 pre-plant, soil incorporated
NY, FL, MI 1966-75 1 3.4-6.7 10% G 104-106 <0.02
FL 1974-75 1 10.1-13.4 24% Liq. 21-30 <0.02 broadcast, soil incorporated
FL 1974 1 3.4-4 24% Liq. 100-150 <0.02
(foliar) DE 1969-74 3-6 0.21-0.28 24% Liq. 6-20 <0.02
CA, DE 1969-74 3-13 0.43-0.56 24% Liq. 1-5 <0.02
6-8 <0.02-0.10
12-14 <0.02-0.03
18-30 <0.02
CA,DE,FL,MI 1972-75 1-5 1-1.1 24% Liq. 1 <0.02
2-3 <0.02-0.03
4-14 <0.02-0.03
18-30 <0.02
CA, DE 1969-72 1-5 2.2-3.4 24% Liq. 4-5 <0.02
2-3 0.02-0.05
4-5 <0.02-0.02
6-14 <0.02-0.06 high value from low dosage
DE, CA 1969-74 1-5 4.5 24% Liq. 4-5 <0.02
6-8 0.05-0.11
12-14 0.09
(soil + MI 1974 1 + 3.4-6.8+ G + 100-150
foliar) 1.7 1.1-2.2 24% Liq. 6-8 <0.02
CO 1973-74 1 + 3.4-6.7 G + 100-150
3 1.1 24% Liq. 6-40 <0.02-0.02
DE 1971 1 2.2 10% G soil incorporated
2 (foliar) 24% Liq. 18 <0.02
(dose unspecified)
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
soil straw
Soybeans U.S.A. (TN,MO 1970-77 1-3 0.56-6.73 10% G 113-185 <0.02-0.051 <0.02-0.052
(dry) VA,AL,FL,NC;
25 tests)
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02
summer 4-8 <0.02
Squash, U.S.A. 1976 5 2.2 (foliar) 2 L 2-3 <0.02-0.06
vining 4-8 <0.02
tops roots
Sugarbeets U.K. 14 0.6-0.95 10% G4 unspecified 0.05 0.04
U.S.A. (GA,NC 1 0.56 Liq. 76-100 <0.02 transplant water
LA,VA,NY) 1+1 0.56+1.1 Liq. 76-100 <0.02 transplant water+foliage
1 4.5-9 G 76-200 <0.02 soil
1 4.5 Liq. 100-150 <0.02 soil
2 Liq. 51-75 <0.02 foliar
1+ 0.56-9 + G + 75-100 <0.02 soil +
2 1.1 Liq. 51-75 <0.02 foliar
Sugarcane S. Africa Data provided were not useful because of uncertainty of dosage rates.
Sweet potato U.S.A. (GA, 1970-74 1 4.5-95 24% Liq. 100-150 <0.02 (<0.02)
NC,VA,ML) preplant-row and broadcast
1 only 1 of 25 results was at 0.05 mg/kg
2 only 1 of 7 results was at 0.05 mg/kg
3 all but one application were at planting or 1 day prior
4 presumed on the basis of proposed label
5 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg
Watermelon U.S.A. 1976-78 5-8 0.56 2L (foliar) 1 0.05-0.29 (<0.02)
(FL,CA) 2-3 0.27
6-8 0.03-0.28 (0.02)
FL 1976-78 8-9 1.1 2L (foliar) 1 0.38-0.77 (<0.02)
2-3 0.47 (0.02)
6-8 0.32-0.56 (0.02)
AZ,CA,FL 1976-78 5-9 2.2 2L (foliar) 1 0.1-1.2
2-3 <0.02-0.87 (0.05)
4-5 <0.02
6-8 0.05-0.78 (<0.02-0.1)
Tomato Netherlands1 1979 1 5 10% G 5 0.02-0.11
(glasshouse)
1 10 10% G 5 0.04-0.23
(glasshouse)
2 5 250 g/l 5 0.32-0.57
preplant broadcast
5.6 10% G 51-150 0.11-0.132
U.K. 1974 11.2 10% G 0.05-0.12
post plant broadcast
5.6-6.7 10% G 12-14 0.66
21-30 0.012
9 10% G 21-30 0.092
11.2 10% G 21-30 0.52
preplant + postplant broadcast
5.6+5.6 10% G 12-14 0.152 (0.85)
11 + 5.6 10% G 12-14 0.072
11 + 11 10% G 12-14 0.78
1 CIVO report R 6282 (1979)
2 under glass
TABLE 2. Continued...
Rate, kg Interval
ai/ha or last application No. in
Crop Country Year No. % ai Formulation to harvest (days) Range, mg/kg range
unspecified application method
2.2-5 10% G1 unspecified 0.06-0.19
10.1 0.07-18.7
U.S.A. 1974-75 5-11 0.56 24% Liq.1 1 0.14-0.44 6
FL, 10 (foliar)1 2-3 0.04-0.31 6
locations 4-5 0.08-0.35 6
6-8 0.25 1
5-12 0.84-1.1 24% Liq.1 1 0.26-1.0 (0.21) 11
(foliar)1 2-3 0.1-0.71 10
4-5 0.11-0.55 10
6-8 0.34 1
5-11 2.2 24% Liq.1 1 0.82-1.9 6
(foliar)1 2-3 0.15-0.78 7
4-5 0.24-0.49 6
6-8 0.24 1
5 4.5 24% Liq.1 1 0.57 1
(foliar)1 4-5 0.35 1
6-8 0.37 1
1 0.05 limit of determination
Apples - Maximum oxamyl residues from approved dosage rates were 1.5
mg/kg at 2-5 days after application and 1.2 mg/kg at the interval of 2
weeks recommended for uses on bearing trees although the application
rate at the latter residues level was less than the maximum permitted.
Residues of DMCF ranged from 0.02-0.05 mg/kg from approved use. There
was no data from uses on non-bearing trees although a one-year
interval is applied for that use.
Bananas - Residues of oxamyl or DMCF were <0.02 mg/kg from
application rates up to 1.7 times maximum recommended dosage in whole
fruit (bagged or unbagged) or edible pulp. The data reflect foliar
applications only.
No residue data were provided for non-foliar uses on bananas.
Beans - Although no residue data were available from the single
country for which recommended usage information was available, data
encompassing those rates were available from trials in the U.S. where
similar uses and a 7-day pre-harvest interval have been proposed.
Residues of oxamyl from pre-plant uses on succulent beans were <0.02
mg/kg although such data were quite limited. Maximum residues of
oxamyl from recommended foliar rates were 1.0, 11 and 15 mg/kg in
succulent beans, foliage, and hay respectively and 0.09, 0.15 and 0.20
mg/kg respectively for DMCF, all at a 14-day interval. At 7 days
maximum oxamyl residues were 2.3, 33 and 25 mg/kg respectively on
succulent beans, foliage and hay. A 14-day pre-harvest interval is
recommended for beans but not specified for forage-hay. An 18-day
pre-graze or pre-forage interval is proposed in the U.S.A.
Oxamyl residues on dry beans from rates reflecting recommended uses
were <0.02 mg/kg and were 0.18 and 0.08 mg/kg on straw and vines
respectively, although most such applications were pre-plant.
Residue data from foliar uses are needed on dry bean varieties and
additional residue data from pre-plant uses on succulent beans,
including additional data on feed items from these uses. Such data
should come from other countries as well as from the U.S.A. The
potential for residues in meat and milk warrants complete data on feed
items.
Celery - Good agricultural practice information (both pre-plant and
foliar) was available from 2 countries although of these only residue
data from foliar treatments in the U.S.A. were available. Maximum
residues at the recommended 2-week pre-harvest interval were 3.1 and
2.4 mg/kg respectively for untrimmed and trimmed celery. Residue data
from other countries would be desirable.
Citrus - (grapefruit, lemons, oranges, tangelos, tangerines) - Residue
data on these citrus crops were available from the U.S.A. reflecting
foliar good agricultural practices. No data were available from good
agricultural practices on non-bearing citrus trees. The maximum
residue on citrus from recommended usage was 1.2 mg/kg on grapefruit
from only one application at the recommended 7-day pre-harvest
interval and this at an application rate 0.7 kg ai/ha, thus less than
the maximum 1.2 kg ai/ha recommended. At 1.5 kg ai/ha maximum
residues, again on grapefruit, were 2 mg/kg at 7 days from only one
application. Residues on oranges from application rates 2 times the
maximum recommended rate were 3 mg/kg. Residues from multiple
applications at recommended rates would be expected to be less than 3
mg/kg.
No additional residues of oxamyl were released when citrus samples
were subjected to ß-glucosidase enzyme treatment.
Corn field - Good agricultural practice information was not provided
for corn although a 0.56-2.2 kg ai/ha at planting 24% liquid
formulation use is proposed in the U.S.A. Residues from applications
up to 2 times this rate resulted in oxamyl residues of <0.02 mg/kg in
the kernels and stalk at 93-198 days after treatment. Only 3 analyses
were made on stalks. Additional residue data are needed for forage
and fodder as well as additional residue data from major corn
producing areas.
Cottonseed - Good agricultural practice information was not provided
for oxamyl uses on cotton although uses at 0.028-1.1 kg/ha foliar
applications of a 24% liquid formulation and a 21-day PHI interval
have been proposed in the U.S.A. Residue data reflecting these uses
were available with residues up to 0.17 mg/kg near the proposed 21-day
pre-harvest interval. Residues would not be expected to exceed 0.2
mg/kg. No DCMF residue was detected in cottonseed meal or oil.
Cucurbits - (cantaloupe, cucumbers, honeydew, summer squash, vining
squash, watermelons) - The only available registered use information
for cucurbits were on greenhouse cucumber, in Bulgaria, Czechoslovakia
and the Netherlands and for cucurbits (greenhouse and in the open) in
Greece. No residue data were provided from these countries except the
Netherlands although residue data on the above cucurbits were
available from the USA where pre-plant and foliar liquid formulation
uses with a 1-day pre-harvest interval are proposed, the former at
2.2-4.5 kg ai/ha and the latter proposed use at 0.56-1.1 kg ai/ha.
The proposed foliar treatment rate is therefore comparable to the
Greek spray uses. Some data from pre-plant granular trials in the
U.S.A. were also provided but none for the proposed pre-plant liquid
formulation uses.
Maximum residues on cucurbits at the recommended 1.2 kg ai/ha foliar
rate were 0.77 and 0.48 mg/kg at 1 and 3 days respectively and 0.47
mg/kg for granular pre-plant treatment at 66 days; for a combined
pre-plant-foliar application a maximum residue of 1.24 mg/kg was
observed. Foliar residues on the various cucurbits were generally
comparable at comparable intervals and application rates. Residue
data from the U.K. were of little value because of lack of knowledge
of the interval, formulation and method of application.
Residue data resulting from recommended uses in countries other than
the U.S.A. (including glasshouse uses) are needed as well as
additional data from pre-plant uses (including liquid formulations).
Onions - Only limited data were available. Maximum residues from
apparent at-plant applications at less than the maximum recommended
rates were <0.04 mg/kg at 100-150 days or at intervals unspecified.
Data from other countries are needed.
Peanuts - Information on recommended good agricultural practices for
oxamyl on peanuts was not provided although pre-plant application of a
10% granular formulation at 3.4-5 kg ai/ha and 24% liquid formulation
at 3.4-5.6 kg ai/ha are proposed uses in the U.S.A. No specific
pre-harvest interval is proposed. Also proposed is a 1.1 kg ai/ha
foliar application of the 24% liquid formulation. Residue data from
the U.S.A. reflecting these proposed rates were provided. Maximum
residues were 0.04 mg/kg in hulls and 1.0 mg/kg in hay. Combining
maximum residues from separate soil and foliar uses results in 0.06
mg/kg for nuts, 0.15 for hulls and 1.6 for hay. Residues would not be
expected to exceed 0.1, 0.2 and 2 mg/kg in the nuts, hulls and hay
respectively.
Peas - Only very limited data were available and it was on an
unspecified pea variety. Maximum residues from recommended granular
pre-plant application rates were <0.01 mg/kg in pea or pod although
the interval was not given. At over 4 times the recommended granular
rate residues were <0.01 for the pea and 0.15 mg/kg on the pod, again
the interval was not provided. Additional data are needed on specific
peas and with known intervals.
Bell peppers - No residue data were provided from the three countries
for which recommended uses were provided. However, residue data on
bell peppers provided from field trials in the U.S.A. reflect proposed
uses of 0.56-1.1 kg ai/ha foliar and 0.56 kg ai/ha transplant water
treatments. A one-week pre-harvest interval is proposed as compared
to the two-week interval recommended. The foliar treatment was
reasonably close to the maximum recommended foliar use in Table 1.
Maximum residues from the proposed foliar treatment were 1.3 mg/kg at
7 days (1.7 at recommended rates) after last application or at
intervals greater than 8 days. No data were available from the
proposed transplant water treatment alone. Maximum residues from the
proposed transplant water application with a less than maximum foliar
application was 0.7 mg/kg at 7 days. Combined residues could
therefore be expected to be about 2.4 mg/kg at 7 days. A limit of 3.0
mg/kg would allow for some variability due to the relatively small
number of samples. Residue data from additional countries are
desirable and should include data from glasshouse uses.
Pineapples - Good agricultural practice information was not made
available although a liquid formulation pre-plant use at 4.5-9 kg
ai/ha and a foliar use at 1.1-4.5 kg ai/ha with a 30-day pre-harvest
interval are proposed in the U.S.A. Data reflecting the proposed
foliar use were available with maximum residues of 0.91 mg/kg in whole
fruit (27 days), 5.2 in bran (27 days), 1.4 in leaves at 23 days or
0.82 at 35 days and 8.5 in hay (27 days). Residues would therefore
not be expected to exceed 1.0, 6.0, 1 or 10 mg/kg in the whole fruit,
bran, forage, and hay respectively at the proposed 30-day interval.
Root and tuber vegetables (beets, carrots, sugar beets and sweet
potatoes) - Although good agricultural practice information for root
and tuber crops was available from a number of countries, most of the
residue data on the above root and tuber crops were from trials in the
USA where, in addition to USA-recommended uses in Table 1, additional
uses have been proposed. Of these proposed uses, broadcast and furrow
pre-plant liquid formulation application of 4.5-9 and 2.2-4.5 kg ai/ha
respectively are proposed for carrots and similar pre-plant rates and
a 7-day pre-harvest interval are proposed for potatoes. From
recommended application rates and methods of application, maximum
residues on root and tuber crops were 0.06 mg/kg at 14 days after last
application and from proposed uses, 0.1 mg/kg at 7 dove (both on
potatoes). The majority of residues from recommended or proposed uses
were 0.03 mg/kg or less. Residues for the root and tuber crops for
which data are provided would not be expected to exceed 0.1 mg/kg from
recommended and proposed uses. No data were available from processing
fractions of root crops.
Residue data on root and tuber vegetables resulting from recommended
good agricultural practices from additional countries are desirable.
Soybeans - Good agricultural practice information was not available.
A proposed USA use would allow liquid formation at-plant and pre-plant
treatments at 2.2-4.5 kg ai/ha and data from 7 states reflecting and
up to 1.5 times these proposed uses were available for dry beans and
straw. Maximum residues were 0.05 mg/kg for the dry seeds and straw
at 113-185 days after last application although all but one sample
each for seeds and straw from the 24 tests were <0.02 mg/kg. The
maximum 0.05 mg/kg residue in soybean seed was from an application
rate of one-fourth of the maximum proposed. On this basis residues
from the proposed uses would be expected to be less than 0.2 mg/kg.
No data were available for the oil, meal, hulls, forage or hay,
although a forage restriction is proposed. Although no concentration
data for the oil or meal were available, a 0.33 n-octanol water
partition coefficient would suggest that concentration of residues may
not occur. This would be consistent with fractionation studies on
cottonseed and peanuts.
Sugarcane - Good agricultural practice information was not provided
for oxamyl on sugarcane. The limited residue data from trials in
South Africa were not useful because of uncertainties on dosage rates.
Tomatoes - Good agricultural practice information was available from
eleven countries, although of these residue data were available only
from the United Kingdom and the USA.
The application rates for the U.K. residues data cannot be compared to
the 0.1 g/plt use described in the label provided. Maximum residues
from the trials were 0.85 mg/kg at the U.K. recommended 2-week pre-
harvest interval.
In the USA 1-3 day intervals are allowed for foliar uses. Maximum
residues from foliar uses recommended in the USA (FL) were 1.0 mg/kg
at a one-day interval and 1.9 mg/kg at two times the recommended rate.
No data were available for drip irrigation uses. The data would
indicate that residues would not exceed 1.0 mg/kg from U.K. uses and
USA foliar uses, taking into account their different pre-harvest
intervals. However, the relatively small number of samples at a given
rate would dictate a higher limit. Additional data from these and
other countries reflecting good agricultural practices would be
desirable.
FATE OF RESIDUES
Figure 2 lists the names and structures of oxamyl and related
compounds.
Figure 2. Names and structures of oxamyl and related compounds
COMPOUND NAME STRUCTURE
O O
OXAMYL METHYL N',N'-DIMETHYL-N[METHYL " "
CARBAMOYL)OXY]-1-THIOOXAMIMIDATE (CH3)2N-C-C=NOCNHCH3
'
SCH3
(I) OXIMINO METABOLITE O
METHYL N-HYDROXY-N',N'- "
DIMETHYL-1- (CH3)2N-C-C=NOH
THIOOXAMIMIDATE '
SCH3
(II) METHYL N-HYDROXY-N'-METHYL-1- O
THIOOXAMIMIDATE "
CH3NH-C-C=NOH
'
SCH3
Figure 2 (continued)
COMPOUND NAME STRUCTURE
O
(III) N'N-DIMETHYLOXAMIC ACID "
(CH3)2N-C-COOH
O
(IV) N-METHYLOXAMIC ACID "
CH3NH-C-COOH
O
(V) DMCF "
N,N-DIMETNYL-1-CYANOFORMAMIDE (CH3)2N-C-CN
O O
(VI) METHYL N'-METHYL-N- " "
[(METHYLCARBAMOYL)OXY]-1- CH3NH-C-C=NOCNHCH3
THIOOXAMIMIDATE '
SCH3
O O
" "
(VII) N,N-DIMETHYLOXAMIDE (CH3)2N-C-C-NH2
O
"
METABOLITE A GLUCOSE CONJUGATE OF I (CH3)2N-C-C=NO-GLUCOSE
'
SCH3
O
"
METABOLITE A' GLUCLOSE CONJUGATE OF II CH3NH-C-C=NO-GLUCOSE
'
SCH3
In animals
In a ruminant metabolism study two lactating goats were maintained on
diets containing approximately 10 mg/kg 14C-oxamyl for 10 and 20 days
respectively (Harvey, 1980a). 60-70% of the radioactivity was
eliminated in the urine and faeces, about 6% was expired, 2-3% was
found in milk and an estimated 22% in the entrails and carcass. The
radioactive compounds were not identified. However, there was no
evidence of oxamyl or oximino compound in milk, blood and tissues
(<0.01 ppm).
If calculated as oxamyl, maximum residues would have been 0.4-0.6
mg/kg in milk, and about 1.4 mg/kg in blood and tissues. Little was
found in fat. Milk residues peaked at about 10-14 days but in blood
they continued to rise throughout the feeding. In each case residues
rapidly decreased after withdrawal of the fortified diet.
About 5% of the activity in milk was incorporated into natural
lactose, caesin and lipids. Two additional components were isolated
and accounted for an additional 30% of milk residues.
In a more recent study 14C-labelled oxamyl and selected metabolites
were studied in vitro by incubation in rumen fluid of a Holstein
cow (Belasco and Harvey, 1980). Oxamyl was 41% metabolized within one
hour and 99% within six hours. After 24 hours incubation the oximino
metabolite (I) and DMCF (V) accounted for 80% of the radioactivity (67
and 13% respectively). The remaining radioactivity at 24 hours was
dimethyloxamic acid 5%, dimethyloxamide 10% and 1-2% each of minor
metabolites previously identified in rat metabolism studies.
Metabolite A, a major plant metabolite, was almost totally metabolized
by rumen fluid. This contrasts to rat metabolism where this compound
is largely unchanged in vitro by liver microsomes and only slowly
metabolized in vivo (Harvey and Han, 1978a). About 70% of
metabolite A was metabolized to DMCF by rumen fluid and the remainder
to unidentified components. The DMCF was found to metabolize to
N,N-dimethyloxamide, N,N-dimethyloxamic acid and N-methyloxamic acid.
Only the dimethyloxamide was not previously identified during rat
metabolism studies.
In a livestock-feeding study oxamyl was fed to Guernsey dairy cows at
2, 10 or 20 mg/kg in the diet for 30 days (DuPont, 1973). Recoveries
of added oxamyl were 70% or greater in whole milk, milk fat, or milk
aqueous fractions at 0.02-0.2 mg/kg fortification levels and over 80%
at 0.04-0.40 mg/kg levels in meat. No residues of oxamyl were
detected (<0.02 mg/kg) in any sample of milk or milk fractions,
liver, kidney, lean muscle or subcutaneous fat at any of the feeding
levels.
When these same samples were analysed for DMCF, no residues were found
at any feeding level in milk or tissues (<0.02 mg/kg for milk, <0.04
mg/kg for meat and fat). Recoveries were 67-82% for 0.02-0.05 mg/kg
fortification levels in milk and 46-100% at 0.04-0.2 mg/kg
fortification levels in meat and fat (Du Pont, 1976).
In a poultry-feeding study, adult laying hens were fed diets
containing 0, 1 or 5 mg/kg oxamyl for a four-week period (Zahnow,
1978). Samples of eggs and tissues were collected and analyzed for
oxamyl and DMCF. Oxamyl residues were <0.02 mg/kg in eggs, liver,
muscle and fat and <0.05 mg/kg in skin (due to limited sample
availability). Residues of DMCF were reported as <0.01 mg/kg in
eggs, liver, muscle, fat and skin.
Oxamyl recoveries from eggs averaged 76% at the 0.02 mg/kg
fortification level and at this same level, except for an apparent
error in skin data, averaged 85-97% in meat, tissues, skin and fat.
Recoveries of DMCF were relatively low, averaging 58 and 50% at 0.01
and 0.02 mg/kg fortification levels, respectively, in eggs and 54-63%
in meat, fat and skin tissues at a 0.02 mg/kg fortification level.
In plants
Plant metabolism of oxamyl was studied in tobacco, alfalfa, peanuts,
potatoes, oranges and tomatoes (Harvey, Han and Reiser, 1978).
In one experiment two tobacco plants were each treated on the leaves
with 10 mg 14C-oxamyl and grown in a growth chamber for 7 and 15 days
respectively. 95% of the radioactive material was recovered at 15
days; about 50% was surface residues, 37% extractable leaf internal
residues and 0.1% or less in the roots. About 4-5% were volatile
components. The surface residue was 95% oxamyl and 3% the oximino
compound (I). Of the leaf internal extractable residues, 56% was
oxamyl, 5% oximino compound (I) and 39% was in the polar fraction, 93%
of which was metabolite A and 7% N,N-dimethyloxamic acid. The
seven-day plants were not as extensively analyzed, but to the extent
they were, residues were similar to the 15-day residues with the
proportion of individual residues reflecting the shorter metabolism
period.
Alfalfa received three 0.5 lb. ai/acre 14C-oxamyl spray field
treatments at two-week intervals and was harvested 2.5 weeks after
last treatment. Extractable residues were 75% of applied. Of this,
>90% was metabolite A with only about 0.8% each for oxamyl and the
oximino compound (I).
Peanuts received two foliar field treatments of 14C-oxamyl at 2 lb.
ai/acre. Samples of young plants were taken at four weeks after the
first treatment, immediately before the second treatment, and
additional samples at harvest. There were therefore three fractions
analyzed - young plants, mature hay and mature nuts. In the young
plant 99% of the radioactivity was determined to be a 2:1 ratio of two
metabolites, A and A' (see figure 2). No oxamyl or oximino compound
(I) was detected.
In mature hay ß-glucosidase treatment of a very polar fraction
containing 99% of extractable residues released metabolites A and A',
giving evidence of an initial polysaccharide type compound, i.e.
metabolites A and A' with additional hexose units (see Figure 3).
Also about 1% of the residue was the oximino compound (I) and <0.5%
oxamyl. When unextractable residues (about 40% in mature hay or nuts)
were treated with a mixture of cellulose enzymes, about 60% was
released yielding a fraction characteristic of metabolites A and A'.
This suggests that the initial unextractable residue was a cellulose
of starch-like structure.
No oxamyl or oximino compound could be detected in the nuts. Of
extractable residues about 21% was incorporated into peanut oil
lipids, about 18% into the polysaccharide-type structure found in
mature hay, about 15% was in lipids or as glucose conjugates and about
37% was unextractable. Of the unextractables about 60% was in the
form of the cellulose-starch type structures discussed above.
Potatoes received five foliar field treatments of 14C-oxamyl at 0.5
to 1.0 lb ai/acre giving an oxamyl residue equivalent of 7 mg/kg in
the tuber. Peels contained <1% of the total residue. Free oxamyl or
oximino compound accounted for <3% of the residue, although mild acid
hydrolysis yielded 39% of the radioactivity in the form of oximino
compounds I and II.
Hydrolysis with ß-glucosidase yielded metabolites A and A', suggesting
the initial presence of the polysaccharide conjugates discussed under
mature peanut hay. At least 35% of the 14C was determined to be
incorporated into the glucose of the tuber starch.
Apples were brush-treated in an orchard at 1.0 lb ai/100 gal and 6
weeks later at harvest yielded 0.8-2.0 mg/kg residues, evenly
distributed through the fruit. About 98% of the residue was
extracted, 77% being organosoluble. Of the organosolubles 16% was
oxamyl, 42% oximino compound I, and 17% DMCF. The remaining 23% of
total fruit residue appeared to be in the form of polysaccharide-type
structures.
Oranges were harvested for analysis six weeks after brush application
of 14C-oxamyl to immature oranges at 1.2 lb. ai/100 gal. Residues
were 2.5 mg/kg on a whole fruit basis expressed as oxamyl. Rind
contained 82% of the residue and the juice 18%. On a whole fruit
basis, residues were 9% oxamyl, 6% oximino compound, 20% DMCF, 35%
metabolite A, 22% metabolite A' and 8% unidentified polar metabolites.
Small green tomato fruits were evenly spotted by pipet with 0.37 mg
14C-oxamyl each and the mature fruit harvested two weeks later for
analysis. Residues were 59% oxamyl, 13% oximino compound, 5%
metabolite A, 4% DMCF and 19% polar metabolites and natural products.
The studies on tobacco, peanuts, and potatoes demonstrate the systemic
nature of foliarly applied oxamyl, which readily translocates to the
roots, legumes and tubers respectively.
Figure 3 summarises a major metabolic pathway for oxamyl in plant
tissues. The methylcarbomoyl group is hydrolysed to form the oximino
compound, followed by conjugate formation of the oximino compound with
glucose to form metabolite A, which may undergo demethylation to form
metabolite A'. Metabolite A predominates in short-term studies (2-6
weeks) with metabolite A' identified in moderate-length studies (1-2
months). At harvest little uncomplexed metabolite A or A' is found
but 14C is incorporated into polysaccharide, cellulose or starch-like
structures suggesting the addition of hexose units to metabolites A or
A'.
In addition to the metabolic route shown in Figure 2, oxamyl may also
be completely broken down in plants and incorporated into natural
plant lipids or into the glucose of starch. The metabolite DMCF is
frequently found as a plant residue and so is the oximino compound in
some fruits. No residues of the S-oxide or S,S,-dioxide of oxamyl or
its oxime were observed during these investigations.
 In soil and water
Numerous studies have been conducted on oxamyl soil metabolism,
decomposition, dissipation, adsorption, mobility effect on soil
microorganisms and on dissipation and decomposition in water. In one
compilation of studies the decomposition of oxamyl in soil and water
was investigated with various soils under aerobic and anaerobic
conditions and in distilled or river water at various pHs, with
artificial UV light or sunlight, and in the dark. Also included were
experiments on soil leaching and mobility in various soils (Harvey and
Han, 1978b).
Oxamyl stability was found to be highly pH dependent, hydrolysing to
the oximino compound I only under mildly basic conditions. In
pH-adjusted distilled water 3% and 9% hydrolysis was observed after 24
and 48 hours respectively at a pH of 6.9 and 30% within 6 hours at a
pH of 9.1. At a pH of 4.7 it was completely stable at least through a
4-day period.
In another of these studies 14C-oxamyl was rapidly degraded by soil
under aerobic conditions in a glass metabolism apparatus. The soil
was treated at a rate of 4 lb. oxamyl/acre. After 42 days under
aerobic conditions 51% of the 14C had been liberated as 14CO2, 4%
remained as oxamyl, <1% compound I and 37% as unextractable or
unidentified polar tractions, much of which was found to be
incorporated into soil organic fractions. Under anaerobic conditions
analogous residues at 42 days were 3, 8, 41, and 48% for CO2, oxamyl,
compound I and unextracted or unidentified residues respectively.
A half-life of 11-15 days was found under aerobic conditions in the
laboratory when soil was treated at a rate of 6 mg/kg. Under
anaerobic conditions the half-life was about six days. In another
study the half-life of oxamyl in a loamy sand was 14 days.
A study to the effect of soil moisture at 15°C indicated half-lives of
13-14 days in clay loam or loamy sand and 34-39 days in peaty or humic
loamy sand (Smelt et al, 1979). As soil moisture decreased to the
wilting point there was a gradual decrease in conversion and this
decreased further at below-wilt moisture levels with clay loam. In
humic loamy soil the conversion rate increased sharply at very low
soil moisture content.
In a soil-TLC experiment oxamyl appeared to be moderately to highly
mobile with Rf values of 0.53-1.0 in four soils (Harvey and Han,
1978b). By one system, compounds with Rf values of 0.35-0.64 are
rated as moderately mobile and those with Rf values of 0.96-1.00 as
highly mobile. This finding was consistent with laboratory adsorption
studies on three soils which indicated no adsorption under conditions
of the test. This is also consistent with another laboratory soil
adsorption-leaching study in which oxamyl was found to be highly
mobile with 61-100% eluted through a 45 cm × 5 cm diameter column of
aged and fresh soil with 20 inches of water (Chrazanowski, undated).
Of unleached residues >70% remained in the top five cm.
This high mobility observed in laboratory studies was not confirmed by
field studies. In another of the collection of studies by Harvey and
Han (1978b), volatility losses of 14C from three soil types (to a 15
inch depth) treated at 6 lb. ai 14C-oxamyl/acre were 80-93% of
original treatment at three-five months. Only traces of oxamyl or
compound I (<0.05%) was detected at three-five months in a soil
extract although significant amounts were found at one week and one
month. Unextracted residues accounted for 14% of the original
treatment at three-five months. The leachate from a fine sand had the
highest residues of the three soil types with 14C residues up to 6.8%
of original treatment. Of this 6.8% about 1% was oxamyl, 11%
unidentified polar materials and 88% compound I. Analysis again
indicated 14C incorporation into soil organic fractions.
In another of these studies oxamyl field soil leaching-disappearance
studies revealed residues of 3.2 mg/kg (calculated as oxamyl) in the
top four inches at zero days when treated at 5.7 lb ai/acre. Residues
decreased to 0.13 mg/kg in the top four inches at 30 days with a
half-life of about one week. Residues at the four-eight inch depth
peaked at 0.15 mg/kg at nine days; at the 8-12 inch depth at 0.15
mg/kg in the 9-23 day period; at the 12-18 inch depth at 0.11 mg/kg in
a 9-23 day period; and at 0.06 mg/kg at 23 days at the 18-24 inch
depth. Residues were negligible (<0.04 mg/kg) through a 60-day
interval at the 24-30 inch depth.
Therefore, under practical conditions the field leaching and
dissipation experiments do not support laboratory tests, which
indicate that oxamyl is highly mobile. They indicate some mobility
and rapid dissipation, presumably mostly as CO2, with a half-life in
soil of six to eight days with only trace residues leaching below
15-18 inches within a 2-3 month period. The rapid dissipation
apparently minimizes leaching under field conditions.
In a crop rotation study, cabbage, red beets and sorghum seeds were
grown in the greenhouse in soils treated 30 and 120 days earlier with
14C-oxamyl at 8 lb ai/acre (Harvey, undated). At 30 days 19% of the
original oxamyl remained in the soil. Crops planted therein had at
maturity residues of 0.6-4 mg/kg (calculated as oxamyl) or 0.01 to
0.12 mg/kg of oxamyl plus its hydrolysis product. At 120 days <1% of
the original oxamyl remained. Mature crops from the 120-day soil
contained <0.2 mg/kg residue (calculated as oxamyl), of which <0.02
mg/kg was oxamyl plus its oximino metabolite. No DMCF was detected.
The experiment would suggest that low levels of oxamyl plus its
oximino metabolite could occur in these commodities if they are
planted within 30 days of high soil applications of oxamyl. Similar
experiments in the field on other commodities reflecting maximum use
rates would be desirable, including use of both liquid and granular
commercial formulations.
Two studies were conducted on the possible effects of oxamyl on soil
microorganisms. In one controlled laboratory study 5.0 mg/kg oxamyl
in silt loam soil increased the time for 50% nitrification by 5 days
although total nitrification after 3 weeks was the same as in the
control (Han, undated). No significant effect was observed at a 0.5
mg/kg oxamyl level.
In the other laboratory study the effect on the population and
respiration of microorganisms was studied in soils treated at 10 mg/kg
oxamyl (Peeples, undated). No reduction was observed in the
populations of either fungi or bacteria at one, two, four and eight
weeks when compared to controls. Respiration of soil microorganisms
in these oxamyl treated soils was determined by measurement of CO2
evolution. Oxamyl had no effect on CO2 evolution.
In storage and processing
No information was provided on residues in stored commodities. Data
were provided on the effect of processing or simulated processing
residues in several commodities:
Apple - When apples with 1.2 mg/kg aged residues were processed in the
laboratory to juice and wet and dry pomace (11O°C, 17 hours) residues
were reduced to 0.5, 0.85 mg/kg and none, respectively.
Citrus - No concentration of residues was found in press juice, wet
pulp, or dried pulp when field-treated oranges and grapefruit with
terminal residues of 0.51 and 0.62 mg/kg respectively were analyzed.
Residues of 0.37 and 0.30 mg/kg in the wet pulp of oranges and
grapefruit respectively were essentially destroyed during the drying
process (<0.05 mg/kg remaining).
Cottonseed - In a fractionation study in which cottonseed was
fortified with oxamyl at 0.2 and 0.5 mg/kg, residues did not
concentrate in the meal or oil. Residues recovered in the meal were
5-8% of those on the undelinted seed; no residues were recovered in
the oil. No conversion to DMCF was detected. Fortifications with
metabolite A, the oximino compound I and DMCF also resulted in major
losses of these compounds during fractionation. The details of this
study were not provided to the meeting.
Peanuts - In a laboratory-simulated commercial oil extraction with
refluxing hexane, maximum residues in oil and meal were 10 and 28%,
respectively, of those in peanuts fortified at 0.2 and 2.0 mg/kg.
Pineapple - A simulated commercial processing study demonstrated that
residues in bran may be 0.4-6 times that in the whole fruit. This is
supported by actual residue data in Table 2. A similar concentration
occurs with the metabolite DMCF.
Soybeans - Although no concentration data were available for soybean
oil or meal, a 0.33 n-octanol water-partition coefficient would
suggest that concentration may not occur. This would be consistent
with fractionation studies in cottonseed and peanuts.
Tomatoes - When tomatoes with field-incurred or fortified residues of
1-10 mg/kg oxamyl were ground and concentrated by cooking to a puree
of one half the original weight, residues in the puree in mg/kg were
one half or less of those in tomatoes. Analytical recoveries were
61-110%.
Photodecomposition
Hydrolysis of oxamyl to the oximino compound (I) was accelerated by UV
light in both distilled water and river water, more rapidly in the
latter (Harvey and Han, 1978b). In river water treated with
artificial UV light, residues after seven days were 22% oxamyl, 39%
oximino compound (I) and 3% polar fractions. In the dark, control
residues were 84, 16, 0 and 0% respectively for the same compounds at
ten days.
Oxamyl degradation was enhanced still further when sunlight was used.
Oxamyl decreased from 100% at zero day to zero after about 1.5 days.
Oximino compound (I) decreased from a maximum of about 95% of the
total radioactivity at 1.5 days to about 68% and 50% at seven and 21
days, respectively, while its geometric isomer was increasing from
about 4% at 1.5 days to 32 and 42%, respectively, at seven and 21
days. The oximino compound (I) and its geometric isomer declined
after about 21 days, while the polar fraction increased from about 3%
of the activity at 14 days to 20% at 42 days.
At 42 days 14% of the total radioactivity was N,N-dimethyloxamic acid
and two unidentified polar compounds, which accounted for 3% of the
total. A 17% loss of radioactivity during the six-week study was
attributed to the loss of CO2. No S-oxidation products of oxamyl or
its oximino compound were observed.
RESIDUES IN COMMERCE OR AT CONSUMPTION
No information was provided to the meeting on evidence of residues in
food in commerce or at consumption.
METHODS OF ANALYSIS
Methods are available for the analysis of oxamyl alone, oxamyl plus
its oxime, and for its DMCF metabolise.
A method for the analysis of oxamyl alone uses column chromatography
to separate oxamyl from its oxime (Bromilow, 1976). Oxamyl is
extracted by blending with acetone dichloromethane (1:1) except for
potatoes, in which case only dichloromethane is used. After
concentration the extract is chromatographed on a Florosil column with
acetone as the eluting solvent. The eluant is concentrated to a small
volume for gas chromatographic analysis.
An aliquot of sample and methanolic trimethylphenylammonium hydroxide
is injected into the gas chromatograph for on-column derivatization to
the methoxime derivative. The gas chromatographic column is 0.5%
carbowax 20 M + 5% SE-30 and detection is by a flame photometric
detector equipped with a 394 nm filter. At 0.02-0.04 mg/kg
fortification levels average recoveries ranged between 87-91% on
barley, peas, and potatoes and 69% on tomatoes. Controls were
generally <0.01 mg/kg although a high of 0.15 mg/kg apparent residue
has been observed on pea pods. Recoveries average 87-96% on soils.
The low tomato recoveries were attributed to incomplete extraction of
residues from extract concentrate. On the basis of discussions, which
will follow, it is probable that part of this loss was due to the acid
catalyzed degradation of oxamyl by the highly acidic tomatoes.
In another procedure oxamyl residues are determined as the oximino
metabolite and expressed as oxamyl by using a 1.35 conversion factor
(Holt and Pease, 1976). The method also measures any free oxamyl
oxime and this was confirmed with recoveries of 65 and 57% when
tobacco was fortified with the oxime at 0.2-0.4 mg/kg.
Oxamyl (and its oxime) are extracted from plant and animal tissues and
soil by blending with ethyl acetate. After concentration and
partitioning with hexane the aqueous extract is made alkaline (pH 12)
with 1 N NaOH and partitioned with chloroform, which is discarded.
The aqueous extract is heated to convert oxamyl to its oxime and again
partitioned with chloroform. The aqueous phase is partitioned with
ethyl acetate - methanol (9:1) and concentrated for analysis with a
flame photometric detector equipped with a 394 nm filter. The
chromatographic column is 10% SP-1200/1% H3PO4 on Chromosorb W AW.
This method has been successfully used on over 25 agricultural
commodities as well as on milk, animal tissues, soil, urine and
faeces. Average recoveries generally range from 75-100%. A
sensitivity of about 0.02 mg/kg is generally attainable for most
commodities, although apparent residues of 0.04-0.05 mg/kg may occur
at times in beans, citrus, peanut hay and onions and up to 0.26 mg/kg
on bean foliage.
Successful method trials using the Holt-Pease method were conducted in
laboratories of the Environmental Protection Agency in the United
States. Recoveries were 77-82% at oxamyl-fortification levels of 3
and 6 mg/kg in celery and 0.2-0.4 mg/kg in cottonseed.
Somewhat low recoveries have been observed during analysis of highly
acidic commodities, such as citrus, tomatoes, and peaches. This can
be minimized by making concentrations under alkaline conditions.
A method is also available for the determination of the DMCF
metabolite of oxamyl (Holt, 1976). In this method the sample is
extracted by blending with ethyl acetate and a potassium dihydrogen
phosphate - NaOH buffer. After concentration to an aqueous phase the
sample is partitioned with hexane (which is discarded) and
re-extracted into ethyl acetate, which is concentrated for analysis.
Analysis is on a 5% Carbowax 20 M gas chromatographic column and
detection with a nitrogen - phosphorous detector. Recoveries on
citrus, celery, strawberries and tomatoes averaged 66-100% and 60% on
potatoes when the commodities were fortified at 0.04-2.0 mg/kg DMCF.
Average recoveries were 74-100% on tomato paste, cottonseed oil,
cottonseed meal and tobacco fortified at 0.2-2.5, 0.2-1.0, 0.1-0.50
and 0.05-0.5 mg/kg respectively. The sensitivity is generally
considered to be 0.04 mg/kg.
The buffer was incorporated into the procedure to minimize conversion
of oxamyl or its oxime to DMCF when highly acidic commodities were
analyzed. This conversion was 5-15% without the buffer and, as
discussed earlier, was observed by Holt and Pease (1976), reduced
recoveries during the development and use of their oxamyl method. The
authors also note that under some conditions some thermal degradation
of oxamyl or its oxime to DMCF may occur in gas chromatographic
injection ports with temperatures in excess of 100-110°C.
NATIONAL TOLERANCES REPORTED TO MEETING
Commodities Tolerances (mg/kg)
USA Netherlands Fed. Rep. of
Germany
apples 2
bananas 0.1 (reflecting 0.02 mg/kg in pulp)
celery 3
citrus 3
cottonseed 0.2
cucumbers 1
pineapple (whole) 1
pineapple forage 10
potatoes 0.1 0.05
sugarbeet (roots) 0.05 (provisional)
tomatoes 2 1
EVALUATION
COMMENTS AND APPRAISAL
Oxamyl is a systemic insecticide, miticide and nematicide registered
or approved for use in numerous countries. It is available as a
liquid or granular formulation and is systemic whether applied
foliarly or to the soil. Foliar applications result in nematocidal
action in the roots and likewise soil applications result in
insecticide and miticidal action in the foliage. It is also effective
as a direct contact insecticide, miticide, and nematicide.
Recommended use information for over 30 countries was provided to the
meeting by the manufacturer. Only a few countries officially provided
this information directly to the meeting.
Oxamyl is degraded in animals by two major pathways, hydrolysis to the
oximino compound and enzymic conversion to N,N-dimethyloxamic acid.
Metabolism studies with 14C-oxamyl in two rats showed that only 70%
of the dose was eliminated in the urine and faeces in 72 hours.
Substantial amounts (22% of the dose) were incorporated into the body
tissues, especially the skin and hair, partly by conversion of
14C-metabolites into natural amino acids. The meeting expressed
concern over the high tissue residues and the lack of identification
of much (50%) of this material.
Oxamyl has a high acute toxicity displaying the typical effects of
carbamate insecticides: rapid onset of cholinesterase inhibition
followed by rapid complete recovery. There was no evidence of delayed
neurotoxicity. The acute toxicity of most of the metabolites is lower
than oxamyl. Oxamyl is not mutagenic or teratogenic.
In a 3-generation rat reproduction study, 50 mg/kg of oxamyl in the
feed slightly decreased the body weight of the weanlings.
The dog is somewhat less sensitive than the rat. In a 90-day and
2-year feeding study a no-effect level of 100 mg/kg was observed. In
the long-term study with rats growth inhibition was found at 100 and
150 mg/kg. A slight effect was also observed in the males at 50
mg/kg. Owing to the lack of a clear no-effect level in the rat and
lack of identification of 50% of the tissue residues, only a temporary
ADI, based on the 2-year study in dogs, was allocated.
Residue data were provided on 29 commodities or groups of commodities.
Residues on individual crops were highly variable, reflecting the many
combinations of uses and pest control needs. Because of the many
variables it was necessary to significantly condense detailed
information on residues in table 2. However, the detailed data were
examined by the meeting.
In some cases no residue data were available from countries for which
recommended or approved use information was available but from a
country with proposed uses. In other cases no recommended or approved
use information was available, although residue data were provided
from a country with a proposed use. Estimate of residues were made on
the basis of available data. The basis for those estimates are
discussed.
In plants a major metabolic route is the conversion of oxamyl to its
oxime, which conjugates with glucose to form metabolite A. This in
turn may add additional hexose units to build progressively into
polysaccharide and starch and cellulose, like structures. With time,
demethylation of metabolite A can occur to form metabolite A' which
way also add additional hexose units. In some plants complete
breakdown of oxamyl occurs with incorporation into natural plant
molecules such as glucose and lipids. The metabolite DMCF is a
residue in some plants and the oxime is found in some fruits. No
residues were observed for the S-oxide or S-dioxide of oxamyl.
Surface residues may be up to 50% of the total and most of this is
oxamyl in some plants.
In an in vitro rumen fluid metabolism study oxamyl was rapidly and
almost totally metabolized. The oximino and DMCF metabolites
accounted for 80% of the radioactivity after 24-hour incubation.
These and remaining metabolites were similar to those identified in
the rat metabolism studies.
The major metabolite of plant metabolism, the glucose conjugate of the
oxime, was almost completely metabolized in rumen fluid, mostly to
DMCF. This contrasts to the rat metabolism where this metabolite was
resistant to metabolism. When DMCF was metabolized in the rumen fluid
the same metabolic products identified in the rat were found plus
dimethyloxamide.
In livestock feeding studies at up to 20 mg/kg in the diet of cattle
and up to 5 mg/kg in the diet of poultry, no residues (<0.02 mg/kg)
were found in animal tissues, milk, fat or eggs. No feeding studies
(or metabolism studies) were provided, for non-ruminant livestock
other than poultry. The available data suggest that there is no
likelihood of residues in meat, fat, milk, poultry or eggs from
approved uses on certain crops. However, additional residue data from
recommended uses on other important crops used for animal foodstuffs,
e.g. sugarbeet leaves, are needed.
Oxamyl is stable in acidic to neutral water but rapidly hydrolyses
under alkaline conditions. Ultraviolet light catalyzes the
degradation to the oxime or its geometric isomer and eventually to
polar compounds.
Laboratory studies indicate that oxamyl should be highly mobile in
soils, but field decomposition and leaching studies do not support
this. This is apparently because the relatively rapid degradation
does not allow sufficient time for significant mobility. On the basis
of laboratory metabolism studies a major route of dissipation appears
to be the evolution of CO2, although soil pH is a factor in some
soils. Significant residues seldom leached below an 18 inch depth
under the study conditions. The half-life of oxamyl in soil was
highly variable, ranging from 6 -39 days, depending on variables such
as location, soil type and moisture content.
In crop rotation studies there was evidence that low levels of
residues could occur in follow-up crops where oxamyl has been used in
the soil. These studies were 14C-oxamyl greenhouse studies. Crop
rotation studies under field condition and reflecting recommended
usage would be desirable.
No evidence of a permanent effect on soil microorganisms by the use of
oxamyl was observed.
No data were available on the effect of storage on oxamyl residues or
on residues in food in commerce or at consumption. Studies were
available on the effect of processing on several commodities. No
concentration of residues was noted except in the case of pineapple
bran where a 6-fold concentration occurred.
Two methods of analysis are available. In one, oxamyl is separated
from its oxime and derivatized on a gas chromatographic column to its
methoxime. In the second, oxamyl and its oxime are extracted together
and the oxamyl hydrolysed to its oxime for the gas chromatographic
determination of total oxamyl plus free oxime residue as the oxime.
Although both methods appear to be adequate, the latter would appear
to be the method of choice for enforcement because it has apparently
been much more extensively used and tested. Most of the residue data
provided to the meeting were developed with this procedure. A
disadvantage would be that it also measures the free oxime, which may
not be of major toxicological concern. The oxime is not, however, a
major residue in many plants, although it can be in some fruits.
The meeting examined residue data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
oxamyl wee used in practice and when reported intervals between last
application and harvest were observed,
Level causing no toxicological effect
Dog: 100 mg/kg in the diet, equivalent to 2.5 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. The levels refer to
the sum of oxamyl plus its oxime calculated as oxamyl. Some figures
are temporary (T), because either there is no recommended use or the
data were inadequate to estimate a maximum residue level.
Estimated Maximum Pre-harvest
Residue levels Interval (days)
Commodity (mg/kg)
apples 2 14
banana 0.051
beans, kidney 3 (T) 7
beans, kidney (dry) 0.051 (T) 50
beans, lima 3 (T) 7
celery 3 14
citrus 3 7
maize 0.051 (T) (at planting use)
cottonseed 0.2 21
cucumber 2 1
melon 2 1
summer squash 2 1
watermelons 2 1
peanuts 0.1 (T)
peanut fodder 2 (T)
peppers, bell 3 7
pineapple 1 (T) 30
root and tuber vegetables:
beets 0.1 14
carrots 0.1 14
potatoes 0.1 7
sugar-beets 0.1 7
sweet potatoes 0.1 (T) (apply within one week of
planting)
soybeans (dry) 0.051 (T) (pre-planting)
tomatoes 2 1
1 at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Identification of animal tissue residues.
2. Clarification of the no-effect level in the rat especially in
relation to the marginal effect of 50 mg/kg on the body weight in
several studies.
3. Further data on beans, maize and soybeans for reconsideration of
the temporary recommendations.
4. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
5. Approved use information on those items for which only proposed
uses were available.
6. Further data are required on beans, maize and soybeans for
reconsideration of the temporary recommendations.
7. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
8. Approved use information on those items for which only proposed
uses were available.
Desirable
1. Residue data from short-term feeding studies on pigs.
2. Toxicological observations in man.
3. Additional residue data from trials reflecting GAP in additional
countries; in particular residue data on onions, peas, and sugarcane
for which there are existing use patterns.
4. Information on residues in foods in commerce and at consumption.
5. Information on the effect on oxamyl residues of cooking, processing
or storage of raw agricultural commodities.
6, Crop rotation studies on additional commodities and under field
conditions with applications of commercial formulations (both granular
and liquid) according to maximum recommendations.
REFERENCES
Ashley, W.E. Acute oral test. Unpublished report, dd. 29-8-1974, from
the Haskell Laboratory, report no. 585-74, submitted to WHO by Du Pont
de Nemours and Company.
Barbo, E.C. Oral LD50 test. Unpublished report, dd. 9-10-1972, from
the Haskell Laboratory, report no. 399-72, submitted to WHO by Du Pont
de Nemours and Company.
Barnes, J.R. and Aftosmis, J.G. Cholinesterase tests with oxamyl.
(1978) Unpublished report from Haskell Laboratory. Report no. 270-78,
submitted to WHO by Du Pont de Nemours and Company.
Barras, C.E. Acute inhalation toxicity. Unpublished report, 19-2-1974,
from the Haskell Laboratory, report no. 30-74, submitted to WHO by Du
Pont de Nemours and Company.
Belasco, I.J. and Harvey, J. Jr. In vitro Rumen Metabolism of
14C-Label Oxamyl and Selected Metabolites of Oxamyl. J. Agric. Food
Chem., Vol. 28(4), 689
Bromilow, R.H. Determination of Residues of Oxamyl in Crops and Soils
by Gas-Liquid Chromatography. Analyst, Vol. 101, 982.
Chrazanowski, R.L. Soil Absorption Studies With Vydate(R) Oxamyl
Insecticide/Nematicide. Unpublished study provided to FAO as Vol. 1,
Section I, May 30, 1980, Exhibit 2 by the Biochemicals Department,
Experimental Station, E.I. Du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, 19898.
Carroll, K.S. Oral LD50 test. Unpublished report, dd. 23-8-1972, from
the Haskell Laboratory, report no 327-72, submitted to WHO by Du Pont
de Nemours and Company.
Civo. Gas Chromatographic Analysis of Residues of Oxamyl Residues in
Cucumbers and Tomatoes, Report no. R 6282, Ir., PDA, Olthof (1979)
Central Institute for Nutrition and Research, the Netherlands.
Colburn, C.W. Acute skin absorption toxicity tests on rabbits.
Unpublished report, dd. 30-12-1970, from the Haskell Laboratory,
report no. 282-70, submitted to WHO by Du Pont de Nemours and Company.
Culik, R. and Sherman, H. Teratogenic study in rats with
S-methyl-l-dimethylcarbamoyl-N [(methylcarbamoyl)oxy] thioformimidate
(IND-1410). Unpublished report, dd. 8-1-1971, from the Haskell
Laboratory, report no. 5-71, submitted to WHO by Du Pont de Nemours
and Company.
Dale, N.C. Oral LD50 test. Unpublished report, dd. 30-3-1973, from
the Haskell Laboratory, report no. 126-73, submitted to WHO by Du Pont
de Nemours and Company.
Dion, L.K. Fifteen-exposure dermal study with IND-1410 liquid
S-methyl-l-dimethyl-carbamoyl-H-[(methylcarbamoyl)oxy]
thioformimidate. Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 235-70, submitted to WHO by Du Pont de Nemours
and Company.
Du Pont. Oxamyl Livestock Feeding Studies, Milk and Meat. This
unpublished study, dated January 1973, was provided to FAO as Vol. I,
section 3, May 30, 1980, exhibit 17, by the Biochemicals Department of
Du Pont.
Du Pont. Livestock Feeding study. This unpublished study, dated
4-19-76 was provided to FAO, Vol. I, Section 3, May 30, 1980, Exhibit
18, by the Biochemicals Department of Du Pont.
Du Pont. Residue data were provided to FAO as U.S. data - Vol. I,
Section 4, Exhibits 21-28, May 30, 1980 by the Biochemicals Department
of Du Pont.
Fretz, S.B. Ten-dose subacute oral test. Unpublished report, dd.
25-6-1968, from the Haskell Laboratory, report no. 150-68, submitted
to WHO by Du Pont de Nemours and Company.
Fretz S.B. Acute oral test. Unpublished report, dd. 27-12-1968, from
the Haskell Laboratory, report no. 300-68, submitted to WHO by Du Pont
de Nemours and Company.
Fretz S.B. Oral LD50 test. Unpublished report, dd. 31-7-1969, from
the Haskell Laboratory, report 214-69, submitted to WHO by Du Pont de
Nemours and Company.
Gibson, J.R. Oral LD50 test. Unpublished report, dd. 21-5-73, from
the Haskell Laboratory, report no. 236-73, submitted to WHO by Du Pont
de Nemours and Company.
Han, J. C-Y. and Harvey, J. In vitro rat liver microsomal
metabolism of oxamyl and selected metabolites of oxamyl. (undated)
Unpublished report from Du Pont de Nemours and Company, submitted to
WHO.
Han, J. C-Y. Evaluation of Possible Effects of DPX-1410 on Nitrifying
Bacteria In Two Different Soils. This unpublished undated study was
made available to FAO as Vol. I, Section 1, May 30, 1980, Exhibit 5,
by the Biochemicals Department of Du Pont.
Harvey J. and Han, J. C-Y. Metabolism of oxamyl and selected
metabolites in the rat. J. Agric. Food Chem. 26, 902-910.
Harvey, J. Jr. and Han, J. C-Y. Decomposition of Oxamyl In Soil and
Water. J. Agric. Food Chem, Vol. 26(3), 537.
Harvey, J. Jr., Han J. C-Y. and Reiser, R.W. Metabolism of Oxamyl In
Plants. J. Agric. Food Chem., Vol. 26(3), 529.
Harvey, J. Jr. Metabolism of 14C-Oxamyl in the Lactating Goat. This
unpublished undated study was made available to FAO as Vol. I, Section
2, May 30, 1980, Exhibit 9, by the Biochemicals Department of Du Pont.
Harvey, J. Jr. Crop Rotation Study with 14C-Oxamyl in the Greenhouse.
This unpublished undated study was made available as Vol. I, Section
1, May 30, 1980, Exhibit 3 by the Biochemicals Department of Du Pont.
Henry J.E. Acute oral test (mouse). Unpublished report, dd. 2-6-1976,
from the Haskell Laboratory, report no. 387-76, submitted to WHO by Du
Pont de Nemours and Company.
Holsing, G.C. 13-Week oral administration - dogs, insecticide 1410,
final report. Unpublished report, dd. 10-10-1969, from the Hazleton
Laboratories, Inc., submitted to WHO by Du Pont de Nemours and
Company.
Holt, R.F. Residue Procedure for the Determination of Oxamyl
Metabolite N,N-Dimethyl-l-cyanoformamide (DMCF). This unpublished
method, dated 5-3-76 was made available to FAO as Vol. I, Section 3,
May 30, 1980, Exhibit 16, by the Biochemicals Department of du Pont.
Holt, R.F. and Pease, H.L. Determination of Oxamyl Residues Using
Flame Photometric Gas Chromatography, J. Agric. Food Chem., Vol.
24(2), 263.
Kaplan, A.M. Ninety-day feeding study in rats with
1-cyano-N,N-dimethylformamide (INN-79), metabolite of Vydate.
Unpublished report, dd. 26-8-1976, from the Haskell Laboratory, report
no. 630-76, submitted to WHO by Du Pont do Nemours and Company.
Ki Poong Lee. Oral ald and delayed paralysis test (white leghorn
chickens). Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 234-70, submitted to WHO by Du Pont de Nemours
and Company.
Morrow, R.W. Skin absorption acute toxicity test in rabbits - LD50.
Unpublished report, dd. 27-2-1973, from the Haskell Laboratory, report
no. 94-73, submitted to WHO by Du Pont de Nemours and Company.
Peeples J.L. Effect of Oxamyl on Soil Microorganisms. This unpublished
undated study was made available to FAO as Vol. 1, Section 1, May 30,
1980, Exhibit 6, by the Biochemicals Department of du Pont.
Schmoyer, L.A, Oral LD50 test. Unpublished report, dd. 9-12-1969,
from the Haskell Laboratory, report no. 375-69, submitted to WHO by Du
Pont de Nemours and Company.
Schmoyer, L.A. Acute oral test Unpublished report, dd. 29-12-69, from
the Haskell Laboratory, report no. 411-69, submitted to WHO by Du Pont
de Nemours and Company.
Schmoyer, L.A. and Henry, N.W. Ind-1410 and cholinesterase activity.
Unpublished report, dd. 14-1-1970, from the Haskell Laboratory, report
No. 18-70, submitted to WHO by Du Pont de Nemours and Company.
Sherman, H. Antidote study, Unpublished report, dd. 14-10-1969, from
the Haskell Laboratory, report no. 322-69, submitted to WHO by Du Pont
de Nemours and Company.
Sherman, H., Barnes, J.R. and Aftosmis, J.O. Long-term feeding study
in rats and dogs with
l-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IBD-1410): final report. Unpublished report, d.d.
22-2-1972, from the Haskell Laboratory, report No. 37-72, submitted to
WHO by Du Pont de Nemours and Company.
Shirasu, Y., Moritani, M. and Watanabe, R. Oxamyl mutagenicity study
using bacteria. Unpublished report, dd. 4-6-1976, from the Institute
of Environmental Toxicology, submitted to WHO by Du Pont de Nemours
and Company.
Smelt, J.H., Dekker, A. and Leistra, M. Effect of Soil Moisture
Condition on the Conversion Rate of Oxamyl. Neth. J. Agric. Sc. 27,
191-198.
Snee, D.A., Sherman, H., Barnes, J.R. and Stula, E.F. Ninety-day
feeding study in rats with
1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IND-1410). Unpublished report, dd. 6-10-1969, from the
Haskell Laboratory, report no. 308-69, submitted to WHO by Du Pont de
Nemours and Company.
Tayfun, F.O. One-hour inhalation toxicity. Unpublished report, dd.
22-9-1969, from the Haskell Laboratory, report no. 281-69, submitted
to WHO by Du Pont de Nemours and Company.
Zahnow, E.W. Oxamyl - Chicken and Egg Feeding Study. This unpublished
study, dated 12-4-78, was made available to FAO as Section 3, May 30,
1980, Exhibit 19 by the Biochemicals Department of du Pont.
In soil and water
Numerous studies have been conducted on oxamyl soil metabolism,
decomposition, dissipation, adsorption, mobility effect on soil
microorganisms and on dissipation and decomposition in water. In one
compilation of studies the decomposition of oxamyl in soil and water
was investigated with various soils under aerobic and anaerobic
conditions and in distilled or river water at various pHs, with
artificial UV light or sunlight, and in the dark. Also included were
experiments on soil leaching and mobility in various soils (Harvey and
Han, 1978b).
Oxamyl stability was found to be highly pH dependent, hydrolysing to
the oximino compound I only under mildly basic conditions. In
pH-adjusted distilled water 3% and 9% hydrolysis was observed after 24
and 48 hours respectively at a pH of 6.9 and 30% within 6 hours at a
pH of 9.1. At a pH of 4.7 it was completely stable at least through a
4-day period.
In another of these studies 14C-oxamyl was rapidly degraded by soil
under aerobic conditions in a glass metabolism apparatus. The soil
was treated at a rate of 4 lb. oxamyl/acre. After 42 days under
aerobic conditions 51% of the 14C had been liberated as 14CO2, 4%
remained as oxamyl, <1% compound I and 37% as unextractable or
unidentified polar tractions, much of which was found to be
incorporated into soil organic fractions. Under anaerobic conditions
analogous residues at 42 days were 3, 8, 41, and 48% for CO2, oxamyl,
compound I and unextracted or unidentified residues respectively.
A half-life of 11-15 days was found under aerobic conditions in the
laboratory when soil was treated at a rate of 6 mg/kg. Under
anaerobic conditions the half-life was about six days. In another
study the half-life of oxamyl in a loamy sand was 14 days.
A study to the effect of soil moisture at 15°C indicated half-lives of
13-14 days in clay loam or loamy sand and 34-39 days in peaty or humic
loamy sand (Smelt et al, 1979). As soil moisture decreased to the
wilting point there was a gradual decrease in conversion and this
decreased further at below-wilt moisture levels with clay loam. In
humic loamy soil the conversion rate increased sharply at very low
soil moisture content.
In a soil-TLC experiment oxamyl appeared to be moderately to highly
mobile with Rf values of 0.53-1.0 in four soils (Harvey and Han,
1978b). By one system, compounds with Rf values of 0.35-0.64 are
rated as moderately mobile and those with Rf values of 0.96-1.00 as
highly mobile. This finding was consistent with laboratory adsorption
studies on three soils which indicated no adsorption under conditions
of the test. This is also consistent with another laboratory soil
adsorption-leaching study in which oxamyl was found to be highly
mobile with 61-100% eluted through a 45 cm × 5 cm diameter column of
aged and fresh soil with 20 inches of water (Chrazanowski, undated).
Of unleached residues >70% remained in the top five cm.
This high mobility observed in laboratory studies was not confirmed by
field studies. In another of the collection of studies by Harvey and
Han (1978b), volatility losses of 14C from three soil types (to a 15
inch depth) treated at 6 lb. ai 14C-oxamyl/acre were 80-93% of
original treatment at three-five months. Only traces of oxamyl or
compound I (<0.05%) was detected at three-five months in a soil
extract although significant amounts were found at one week and one
month. Unextracted residues accounted for 14% of the original
treatment at three-five months. The leachate from a fine sand had the
highest residues of the three soil types with 14C residues up to 6.8%
of original treatment. Of this 6.8% about 1% was oxamyl, 11%
unidentified polar materials and 88% compound I. Analysis again
indicated 14C incorporation into soil organic fractions.
In another of these studies oxamyl field soil leaching-disappearance
studies revealed residues of 3.2 mg/kg (calculated as oxamyl) in the
top four inches at zero days when treated at 5.7 lb ai/acre. Residues
decreased to 0.13 mg/kg in the top four inches at 30 days with a
half-life of about one week. Residues at the four-eight inch depth
peaked at 0.15 mg/kg at nine days; at the 8-12 inch depth at 0.15
mg/kg in the 9-23 day period; at the 12-18 inch depth at 0.11 mg/kg in
a 9-23 day period; and at 0.06 mg/kg at 23 days at the 18-24 inch
depth. Residues were negligible (<0.04 mg/kg) through a 60-day
interval at the 24-30 inch depth.
Therefore, under practical conditions the field leaching and
dissipation experiments do not support laboratory tests, which
indicate that oxamyl is highly mobile. They indicate some mobility
and rapid dissipation, presumably mostly as CO2, with a half-life in
soil of six to eight days with only trace residues leaching below
15-18 inches within a 2-3 month period. The rapid dissipation
apparently minimizes leaching under field conditions.
In a crop rotation study, cabbage, red beets and sorghum seeds were
grown in the greenhouse in soils treated 30 and 120 days earlier with
14C-oxamyl at 8 lb ai/acre (Harvey, undated). At 30 days 19% of the
original oxamyl remained in the soil. Crops planted therein had at
maturity residues of 0.6-4 mg/kg (calculated as oxamyl) or 0.01 to
0.12 mg/kg of oxamyl plus its hydrolysis product. At 120 days <1% of
the original oxamyl remained. Mature crops from the 120-day soil
contained <0.2 mg/kg residue (calculated as oxamyl), of which <0.02
mg/kg was oxamyl plus its oximino metabolite. No DMCF was detected.
The experiment would suggest that low levels of oxamyl plus its
oximino metabolite could occur in these commodities if they are
planted within 30 days of high soil applications of oxamyl. Similar
experiments in the field on other commodities reflecting maximum use
rates would be desirable, including use of both liquid and granular
commercial formulations.
Two studies were conducted on the possible effects of oxamyl on soil
microorganisms. In one controlled laboratory study 5.0 mg/kg oxamyl
in silt loam soil increased the time for 50% nitrification by 5 days
although total nitrification after 3 weeks was the same as in the
control (Han, undated). No significant effect was observed at a 0.5
mg/kg oxamyl level.
In the other laboratory study the effect on the population and
respiration of microorganisms was studied in soils treated at 10 mg/kg
oxamyl (Peeples, undated). No reduction was observed in the
populations of either fungi or bacteria at one, two, four and eight
weeks when compared to controls. Respiration of soil microorganisms
in these oxamyl treated soils was determined by measurement of CO2
evolution. Oxamyl had no effect on CO2 evolution.
In storage and processing
No information was provided on residues in stored commodities. Data
were provided on the effect of processing or simulated processing
residues in several commodities:
Apple - When apples with 1.2 mg/kg aged residues were processed in the
laboratory to juice and wet and dry pomace (11O°C, 17 hours) residues
were reduced to 0.5, 0.85 mg/kg and none, respectively.
Citrus - No concentration of residues was found in press juice, wet
pulp, or dried pulp when field-treated oranges and grapefruit with
terminal residues of 0.51 and 0.62 mg/kg respectively were analyzed.
Residues of 0.37 and 0.30 mg/kg in the wet pulp of oranges and
grapefruit respectively were essentially destroyed during the drying
process (<0.05 mg/kg remaining).
Cottonseed - In a fractionation study in which cottonseed was
fortified with oxamyl at 0.2 and 0.5 mg/kg, residues did not
concentrate in the meal or oil. Residues recovered in the meal were
5-8% of those on the undelinted seed; no residues were recovered in
the oil. No conversion to DMCF was detected. Fortifications with
metabolite A, the oximino compound I and DMCF also resulted in major
losses of these compounds during fractionation. The details of this
study were not provided to the meeting.
Peanuts - In a laboratory-simulated commercial oil extraction with
refluxing hexane, maximum residues in oil and meal were 10 and 28%,
respectively, of those in peanuts fortified at 0.2 and 2.0 mg/kg.
Pineapple - A simulated commercial processing study demonstrated that
residues in bran may be 0.4-6 times that in the whole fruit. This is
supported by actual residue data in Table 2. A similar concentration
occurs with the metabolite DMCF.
Soybeans - Although no concentration data were available for soybean
oil or meal, a 0.33 n-octanol water-partition coefficient would
suggest that concentration may not occur. This would be consistent
with fractionation studies in cottonseed and peanuts.
Tomatoes - When tomatoes with field-incurred or fortified residues of
1-10 mg/kg oxamyl were ground and concentrated by cooking to a puree
of one half the original weight, residues in the puree in mg/kg were
one half or less of those in tomatoes. Analytical recoveries were
61-110%.
Photodecomposition
Hydrolysis of oxamyl to the oximino compound (I) was accelerated by UV
light in both distilled water and river water, more rapidly in the
latter (Harvey and Han, 1978b). In river water treated with
artificial UV light, residues after seven days were 22% oxamyl, 39%
oximino compound (I) and 3% polar fractions. In the dark, control
residues were 84, 16, 0 and 0% respectively for the same compounds at
ten days.
Oxamyl degradation was enhanced still further when sunlight was used.
Oxamyl decreased from 100% at zero day to zero after about 1.5 days.
Oximino compound (I) decreased from a maximum of about 95% of the
total radioactivity at 1.5 days to about 68% and 50% at seven and 21
days, respectively, while its geometric isomer was increasing from
about 4% at 1.5 days to 32 and 42%, respectively, at seven and 21
days. The oximino compound (I) and its geometric isomer declined
after about 21 days, while the polar fraction increased from about 3%
of the activity at 14 days to 20% at 42 days.
At 42 days 14% of the total radioactivity was N,N-dimethyloxamic acid
and two unidentified polar compounds, which accounted for 3% of the
total. A 17% loss of radioactivity during the six-week study was
attributed to the loss of CO2. No S-oxidation products of oxamyl or
its oximino compound were observed.
RESIDUES IN COMMERCE OR AT CONSUMPTION
No information was provided to the meeting on evidence of residues in
food in commerce or at consumption.
METHODS OF ANALYSIS
Methods are available for the analysis of oxamyl alone, oxamyl plus
its oxime, and for its DMCF metabolise.
A method for the analysis of oxamyl alone uses column chromatography
to separate oxamyl from its oxime (Bromilow, 1976). Oxamyl is
extracted by blending with acetone dichloromethane (1:1) except for
potatoes, in which case only dichloromethane is used. After
concentration the extract is chromatographed on a Florosil column with
acetone as the eluting solvent. The eluant is concentrated to a small
volume for gas chromatographic analysis.
An aliquot of sample and methanolic trimethylphenylammonium hydroxide
is injected into the gas chromatograph for on-column derivatization to
the methoxime derivative. The gas chromatographic column is 0.5%
carbowax 20 M + 5% SE-30 and detection is by a flame photometric
detector equipped with a 394 nm filter. At 0.02-0.04 mg/kg
fortification levels average recoveries ranged between 87-91% on
barley, peas, and potatoes and 69% on tomatoes. Controls were
generally <0.01 mg/kg although a high of 0.15 mg/kg apparent residue
has been observed on pea pods. Recoveries average 87-96% on soils.
The low tomato recoveries were attributed to incomplete extraction of
residues from extract concentrate. On the basis of discussions, which
will follow, it is probable that part of this loss was due to the acid
catalyzed degradation of oxamyl by the highly acidic tomatoes.
In another procedure oxamyl residues are determined as the oximino
metabolite and expressed as oxamyl by using a 1.35 conversion factor
(Holt and Pease, 1976). The method also measures any free oxamyl
oxime and this was confirmed with recoveries of 65 and 57% when
tobacco was fortified with the oxime at 0.2-0.4 mg/kg.
Oxamyl (and its oxime) are extracted from plant and animal tissues and
soil by blending with ethyl acetate. After concentration and
partitioning with hexane the aqueous extract is made alkaline (pH 12)
with 1 N NaOH and partitioned with chloroform, which is discarded.
The aqueous extract is heated to convert oxamyl to its oxime and again
partitioned with chloroform. The aqueous phase is partitioned with
ethyl acetate - methanol (9:1) and concentrated for analysis with a
flame photometric detector equipped with a 394 nm filter. The
chromatographic column is 10% SP-1200/1% H3PO4 on Chromosorb W AW.
This method has been successfully used on over 25 agricultural
commodities as well as on milk, animal tissues, soil, urine and
faeces. Average recoveries generally range from 75-100%. A
sensitivity of about 0.02 mg/kg is generally attainable for most
commodities, although apparent residues of 0.04-0.05 mg/kg may occur
at times in beans, citrus, peanut hay and onions and up to 0.26 mg/kg
on bean foliage.
Successful method trials using the Holt-Pease method were conducted in
laboratories of the Environmental Protection Agency in the United
States. Recoveries were 77-82% at oxamyl-fortification levels of 3
and 6 mg/kg in celery and 0.2-0.4 mg/kg in cottonseed.
Somewhat low recoveries have been observed during analysis of highly
acidic commodities, such as citrus, tomatoes, and peaches. This can
be minimized by making concentrations under alkaline conditions.
A method is also available for the determination of the DMCF
metabolite of oxamyl (Holt, 1976). In this method the sample is
extracted by blending with ethyl acetate and a potassium dihydrogen
phosphate - NaOH buffer. After concentration to an aqueous phase the
sample is partitioned with hexane (which is discarded) and
re-extracted into ethyl acetate, which is concentrated for analysis.
Analysis is on a 5% Carbowax 20 M gas chromatographic column and
detection with a nitrogen - phosphorous detector. Recoveries on
citrus, celery, strawberries and tomatoes averaged 66-100% and 60% on
potatoes when the commodities were fortified at 0.04-2.0 mg/kg DMCF.
Average recoveries were 74-100% on tomato paste, cottonseed oil,
cottonseed meal and tobacco fortified at 0.2-2.5, 0.2-1.0, 0.1-0.50
and 0.05-0.5 mg/kg respectively. The sensitivity is generally
considered to be 0.04 mg/kg.
The buffer was incorporated into the procedure to minimize conversion
of oxamyl or its oxime to DMCF when highly acidic commodities were
analyzed. This conversion was 5-15% without the buffer and, as
discussed earlier, was observed by Holt and Pease (1976), reduced
recoveries during the development and use of their oxamyl method. The
authors also note that under some conditions some thermal degradation
of oxamyl or its oxime to DMCF may occur in gas chromatographic
injection ports with temperatures in excess of 100-110°C.
NATIONAL TOLERANCES REPORTED TO MEETING
Commodities Tolerances (mg/kg)
USA Netherlands Fed. Rep. of
Germany
apples 2
bananas 0.1 (reflecting 0.02 mg/kg in pulp)
celery 3
citrus 3
cottonseed 0.2
cucumbers 1
pineapple (whole) 1
pineapple forage 10
potatoes 0.1 0.05
sugarbeet (roots) 0.05 (provisional)
tomatoes 2 1
EVALUATION
COMMENTS AND APPRAISAL
Oxamyl is a systemic insecticide, miticide and nematicide registered
or approved for use in numerous countries. It is available as a
liquid or granular formulation and is systemic whether applied
foliarly or to the soil. Foliar applications result in nematocidal
action in the roots and likewise soil applications result in
insecticide and miticidal action in the foliage. It is also effective
as a direct contact insecticide, miticide, and nematicide.
Recommended use information for over 30 countries was provided to the
meeting by the manufacturer. Only a few countries officially provided
this information directly to the meeting.
Oxamyl is degraded in animals by two major pathways, hydrolysis to the
oximino compound and enzymic conversion to N,N-dimethyloxamic acid.
Metabolism studies with 14C-oxamyl in two rats showed that only 70%
of the dose was eliminated in the urine and faeces in 72 hours.
Substantial amounts (22% of the dose) were incorporated into the body
tissues, especially the skin and hair, partly by conversion of
14C-metabolites into natural amino acids. The meeting expressed
concern over the high tissue residues and the lack of identification
of much (50%) of this material.
Oxamyl has a high acute toxicity displaying the typical effects of
carbamate insecticides: rapid onset of cholinesterase inhibition
followed by rapid complete recovery. There was no evidence of delayed
neurotoxicity. The acute toxicity of most of the metabolites is lower
than oxamyl. Oxamyl is not mutagenic or teratogenic.
In a 3-generation rat reproduction study, 50 mg/kg of oxamyl in the
feed slightly decreased the body weight of the weanlings.
The dog is somewhat less sensitive than the rat. In a 90-day and
2-year feeding study a no-effect level of 100 mg/kg was observed. In
the long-term study with rats growth inhibition was found at 100 and
150 mg/kg. A slight effect was also observed in the males at 50
mg/kg. Owing to the lack of a clear no-effect level in the rat and
lack of identification of 50% of the tissue residues, only a temporary
ADI, based on the 2-year study in dogs, was allocated.
Residue data were provided on 29 commodities or groups of commodities.
Residues on individual crops were highly variable, reflecting the many
combinations of uses and pest control needs. Because of the many
variables it was necessary to significantly condense detailed
information on residues in table 2. However, the detailed data were
examined by the meeting.
In some cases no residue data were available from countries for which
recommended or approved use information was available but from a
country with proposed uses. In other cases no recommended or approved
use information was available, although residue data were provided
from a country with a proposed use. Estimate of residues were made on
the basis of available data. The basis for those estimates are
discussed.
In plants a major metabolic route is the conversion of oxamyl to its
oxime, which conjugates with glucose to form metabolite A. This in
turn may add additional hexose units to build progressively into
polysaccharide and starch and cellulose, like structures. With time,
demethylation of metabolite A can occur to form metabolite A' which
way also add additional hexose units. In some plants complete
breakdown of oxamyl occurs with incorporation into natural plant
molecules such as glucose and lipids. The metabolite DMCF is a
residue in some plants and the oxime is found in some fruits. No
residues were observed for the S-oxide or S-dioxide of oxamyl.
Surface residues may be up to 50% of the total and most of this is
oxamyl in some plants.
In an in vitro rumen fluid metabolism study oxamyl was rapidly and
almost totally metabolized. The oximino and DMCF metabolites
accounted for 80% of the radioactivity after 24-hour incubation.
These and remaining metabolites were similar to those identified in
the rat metabolism studies.
The major metabolite of plant metabolism, the glucose conjugate of the
oxime, was almost completely metabolized in rumen fluid, mostly to
DMCF. This contrasts to the rat metabolism where this metabolite was
resistant to metabolism. When DMCF was metabolized in the rumen fluid
the same metabolic products identified in the rat were found plus
dimethyloxamide.
In livestock feeding studies at up to 20 mg/kg in the diet of cattle
and up to 5 mg/kg in the diet of poultry, no residues (<0.02 mg/kg)
were found in animal tissues, milk, fat or eggs. No feeding studies
(or metabolism studies) were provided, for non-ruminant livestock
other than poultry. The available data suggest that there is no
likelihood of residues in meat, fat, milk, poultry or eggs from
approved uses on certain crops. However, additional residue data from
recommended uses on other important crops used for animal foodstuffs,
e.g. sugarbeet leaves, are needed.
Oxamyl is stable in acidic to neutral water but rapidly hydrolyses
under alkaline conditions. Ultraviolet light catalyzes the
degradation to the oxime or its geometric isomer and eventually to
polar compounds.
Laboratory studies indicate that oxamyl should be highly mobile in
soils, but field decomposition and leaching studies do not support
this. This is apparently because the relatively rapid degradation
does not allow sufficient time for significant mobility. On the basis
of laboratory metabolism studies a major route of dissipation appears
to be the evolution of CO2, although soil pH is a factor in some
soils. Significant residues seldom leached below an 18 inch depth
under the study conditions. The half-life of oxamyl in soil was
highly variable, ranging from 6 -39 days, depending on variables such
as location, soil type and moisture content.
In crop rotation studies there was evidence that low levels of
residues could occur in follow-up crops where oxamyl has been used in
the soil. These studies were 14C-oxamyl greenhouse studies. Crop
rotation studies under field condition and reflecting recommended
usage would be desirable.
No evidence of a permanent effect on soil microorganisms by the use of
oxamyl was observed.
No data were available on the effect of storage on oxamyl residues or
on residues in food in commerce or at consumption. Studies were
available on the effect of processing on several commodities. No
concentration of residues was noted except in the case of pineapple
bran where a 6-fold concentration occurred.
Two methods of analysis are available. In one, oxamyl is separated
from its oxime and derivatized on a gas chromatographic column to its
methoxime. In the second, oxamyl and its oxime are extracted together
and the oxamyl hydrolysed to its oxime for the gas chromatographic
determination of total oxamyl plus free oxime residue as the oxime.
Although both methods appear to be adequate, the latter would appear
to be the method of choice for enforcement because it has apparently
been much more extensively used and tested. Most of the residue data
provided to the meeting were developed with this procedure. A
disadvantage would be that it also measures the free oxime, which may
not be of major toxicological concern. The oxime is not, however, a
major residue in many plants, although it can be in some fruits.
The meeting examined residue data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels that were likely to occur when
oxamyl wee used in practice and when reported intervals between last
application and harvest were observed,
Level causing no toxicological effect
Dog: 100 mg/kg in the diet, equivalent to 2.5 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. The levels refer to
the sum of oxamyl plus its oxime calculated as oxamyl. Some figures
are temporary (T), because either there is no recommended use or the
data were inadequate to estimate a maximum residue level.
Estimated Maximum Pre-harvest
Residue levels Interval (days)
Commodity (mg/kg)
apples 2 14
banana 0.051
beans, kidney 3 (T) 7
beans, kidney (dry) 0.051 (T) 50
beans, lima 3 (T) 7
celery 3 14
citrus 3 7
maize 0.051 (T) (at planting use)
cottonseed 0.2 21
cucumber 2 1
melon 2 1
summer squash 2 1
watermelons 2 1
peanuts 0.1 (T)
peanut fodder 2 (T)
peppers, bell 3 7
pineapple 1 (T) 30
root and tuber vegetables:
beets 0.1 14
carrots 0.1 14
potatoes 0.1 7
sugar-beets 0.1 7
sweet potatoes 0.1 (T) (apply within one week of
planting)
soybeans (dry) 0.051 (T) (pre-planting)
tomatoes 2 1
1 at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Identification of animal tissue residues.
2. Clarification of the no-effect level in the rat especially in
relation to the marginal effect of 50 mg/kg on the body weight in
several studies.
3. Further data on beans, maize and soybeans for reconsideration of
the temporary recommendations.
4. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
5. Approved use information on those items for which only proposed
uses were available.
6. Further data are required on beans, maize and soybeans for
reconsideration of the temporary recommendations.
7. Additional data on materials used for animal feedstuffs, e.g.
sugarbeet leaves, maize fodder and bean fodder.
8. Approved use information on those items for which only proposed
uses were available.
Desirable
1. Residue data from short-term feeding studies on pigs.
2. Toxicological observations in man.
3. Additional residue data from trials reflecting GAP in additional
countries; in particular residue data on onions, peas, and sugarcane
for which there are existing use patterns.
4. Information on residues in foods in commerce and at consumption.
5. Information on the effect on oxamyl residues of cooking, processing
or storage of raw agricultural commodities.
6, Crop rotation studies on additional commodities and under field
conditions with applications of commercial formulations (both granular
and liquid) according to maximum recommendations.
REFERENCES
Ashley, W.E. Acute oral test. Unpublished report, dd. 29-8-1974, from
the Haskell Laboratory, report no. 585-74, submitted to WHO by Du Pont
de Nemours and Company.
Barbo, E.C. Oral LD50 test. Unpublished report, dd. 9-10-1972, from
the Haskell Laboratory, report no. 399-72, submitted to WHO by Du Pont
de Nemours and Company.
Barnes, J.R. and Aftosmis, J.G. Cholinesterase tests with oxamyl.
(1978) Unpublished report from Haskell Laboratory. Report no. 270-78,
submitted to WHO by Du Pont de Nemours and Company.
Barras, C.E. Acute inhalation toxicity. Unpublished report, 19-2-1974,
from the Haskell Laboratory, report no. 30-74, submitted to WHO by Du
Pont de Nemours and Company.
Belasco, I.J. and Harvey, J. Jr. In vitro Rumen Metabolism of
14C-Label Oxamyl and Selected Metabolites of Oxamyl. J. Agric. Food
Chem., Vol. 28(4), 689
Bromilow, R.H. Determination of Residues of Oxamyl in Crops and Soils
by Gas-Liquid Chromatography. Analyst, Vol. 101, 982.
Chrazanowski, R.L. Soil Absorption Studies With Vydate(R) Oxamyl
Insecticide/Nematicide. Unpublished study provided to FAO as Vol. 1,
Section I, May 30, 1980, Exhibit 2 by the Biochemicals Department,
Experimental Station, E.I. Du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, 19898.
Carroll, K.S. Oral LD50 test. Unpublished report, dd. 23-8-1972, from
the Haskell Laboratory, report no 327-72, submitted to WHO by Du Pont
de Nemours and Company.
Civo. Gas Chromatographic Analysis of Residues of Oxamyl Residues in
Cucumbers and Tomatoes, Report no. R 6282, Ir., PDA, Olthof (1979)
Central Institute for Nutrition and Research, the Netherlands.
Colburn, C.W. Acute skin absorption toxicity tests on rabbits.
Unpublished report, dd. 30-12-1970, from the Haskell Laboratory,
report no. 282-70, submitted to WHO by Du Pont de Nemours and Company.
Culik, R. and Sherman, H. Teratogenic study in rats with
S-methyl-l-dimethylcarbamoyl-N [(methylcarbamoyl)oxy] thioformimidate
(IND-1410). Unpublished report, dd. 8-1-1971, from the Haskell
Laboratory, report no. 5-71, submitted to WHO by Du Pont de Nemours
and Company.
Dale, N.C. Oral LD50 test. Unpublished report, dd. 30-3-1973, from
the Haskell Laboratory, report no. 126-73, submitted to WHO by Du Pont
de Nemours and Company.
Dion, L.K. Fifteen-exposure dermal study with IND-1410 liquid
S-methyl-l-dimethyl-carbamoyl-H-[(methylcarbamoyl)oxy]
thioformimidate. Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 235-70, submitted to WHO by Du Pont de Nemours
and Company.
Du Pont. Oxamyl Livestock Feeding Studies, Milk and Meat. This
unpublished study, dated January 1973, was provided to FAO as Vol. I,
section 3, May 30, 1980, exhibit 17, by the Biochemicals Department of
Du Pont.
Du Pont. Livestock Feeding study. This unpublished study, dated
4-19-76 was provided to FAO, Vol. I, Section 3, May 30, 1980, Exhibit
18, by the Biochemicals Department of Du Pont.
Du Pont. Residue data were provided to FAO as U.S. data - Vol. I,
Section 4, Exhibits 21-28, May 30, 1980 by the Biochemicals Department
of Du Pont.
Fretz, S.B. Ten-dose subacute oral test. Unpublished report, dd.
25-6-1968, from the Haskell Laboratory, report no. 150-68, submitted
to WHO by Du Pont de Nemours and Company.
Fretz S.B. Acute oral test. Unpublished report, dd. 27-12-1968, from
the Haskell Laboratory, report no. 300-68, submitted to WHO by Du Pont
de Nemours and Company.
Fretz S.B. Oral LD50 test. Unpublished report, dd. 31-7-1969, from
the Haskell Laboratory, report 214-69, submitted to WHO by Du Pont de
Nemours and Company.
Gibson, J.R. Oral LD50 test. Unpublished report, dd. 21-5-73, from
the Haskell Laboratory, report no. 236-73, submitted to WHO by Du Pont
de Nemours and Company.
Han, J. C-Y. and Harvey, J. In vitro rat liver microsomal
metabolism of oxamyl and selected metabolites of oxamyl. (undated)
Unpublished report from Du Pont de Nemours and Company, submitted to
WHO.
Han, J. C-Y. Evaluation of Possible Effects of DPX-1410 on Nitrifying
Bacteria In Two Different Soils. This unpublished undated study was
made available to FAO as Vol. I, Section 1, May 30, 1980, Exhibit 5,
by the Biochemicals Department of Du Pont.
Harvey J. and Han, J. C-Y. Metabolism of oxamyl and selected
metabolites in the rat. J. Agric. Food Chem. 26, 902-910.
Harvey, J. Jr. and Han, J. C-Y. Decomposition of Oxamyl In Soil and
Water. J. Agric. Food Chem, Vol. 26(3), 537.
Harvey, J. Jr., Han J. C-Y. and Reiser, R.W. Metabolism of Oxamyl In
Plants. J. Agric. Food Chem., Vol. 26(3), 529.
Harvey, J. Jr. Metabolism of 14C-Oxamyl in the Lactating Goat. This
unpublished undated study was made available to FAO as Vol. I, Section
2, May 30, 1980, Exhibit 9, by the Biochemicals Department of Du Pont.
Harvey, J. Jr. Crop Rotation Study with 14C-Oxamyl in the Greenhouse.
This unpublished undated study was made available as Vol. I, Section
1, May 30, 1980, Exhibit 3 by the Biochemicals Department of Du Pont.
Henry J.E. Acute oral test (mouse). Unpublished report, dd. 2-6-1976,
from the Haskell Laboratory, report no. 387-76, submitted to WHO by Du
Pont de Nemours and Company.
Holsing, G.C. 13-Week oral administration - dogs, insecticide 1410,
final report. Unpublished report, dd. 10-10-1969, from the Hazleton
Laboratories, Inc., submitted to WHO by Du Pont de Nemours and
Company.
Holt, R.F. Residue Procedure for the Determination of Oxamyl
Metabolite N,N-Dimethyl-l-cyanoformamide (DMCF). This unpublished
method, dated 5-3-76 was made available to FAO as Vol. I, Section 3,
May 30, 1980, Exhibit 16, by the Biochemicals Department of du Pont.
Holt, R.F. and Pease, H.L. Determination of Oxamyl Residues Using
Flame Photometric Gas Chromatography, J. Agric. Food Chem., Vol.
24(2), 263.
Kaplan, A.M. Ninety-day feeding study in rats with
1-cyano-N,N-dimethylformamide (INN-79), metabolite of Vydate.
Unpublished report, dd. 26-8-1976, from the Haskell Laboratory, report
no. 630-76, submitted to WHO by Du Pont do Nemours and Company.
Ki Poong Lee. Oral ald and delayed paralysis test (white leghorn
chickens). Unpublished report, dd. 4-6-1970, from the Haskell
Laboratory, report no. 234-70, submitted to WHO by Du Pont de Nemours
and Company.
Morrow, R.W. Skin absorption acute toxicity test in rabbits - LD50.
Unpublished report, dd. 27-2-1973, from the Haskell Laboratory, report
no. 94-73, submitted to WHO by Du Pont de Nemours and Company.
Peeples J.L. Effect of Oxamyl on Soil Microorganisms. This unpublished
undated study was made available to FAO as Vol. 1, Section 1, May 30,
1980, Exhibit 6, by the Biochemicals Department of du Pont.
Schmoyer, L.A, Oral LD50 test. Unpublished report, dd. 9-12-1969,
from the Haskell Laboratory, report no. 375-69, submitted to WHO by Du
Pont de Nemours and Company.
Schmoyer, L.A. Acute oral test Unpublished report, dd. 29-12-69, from
the Haskell Laboratory, report no. 411-69, submitted to WHO by Du Pont
de Nemours and Company.
Schmoyer, L.A. and Henry, N.W. Ind-1410 and cholinesterase activity.
Unpublished report, dd. 14-1-1970, from the Haskell Laboratory, report
No. 18-70, submitted to WHO by Du Pont de Nemours and Company.
Sherman, H. Antidote study, Unpublished report, dd. 14-10-1969, from
the Haskell Laboratory, report no. 322-69, submitted to WHO by Du Pont
de Nemours and Company.
Sherman, H., Barnes, J.R. and Aftosmis, J.O. Long-term feeding study
in rats and dogs with
l-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IBD-1410): final report. Unpublished report, d.d.
22-2-1972, from the Haskell Laboratory, report No. 37-72, submitted to
WHO by Du Pont de Nemours and Company.
Shirasu, Y., Moritani, M. and Watanabe, R. Oxamyl mutagenicity study
using bacteria. Unpublished report, dd. 4-6-1976, from the Institute
of Environmental Toxicology, submitted to WHO by Du Pont de Nemours
and Company.
Smelt, J.H., Dekker, A. and Leistra, M. Effect of Soil Moisture
Condition on the Conversion Rate of Oxamyl. Neth. J. Agric. Sc. 27,
191-198.
Snee, D.A., Sherman, H., Barnes, J.R. and Stula, E.F. Ninety-day
feeding study in rats with
1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid,
methyl ester (IND-1410). Unpublished report, dd. 6-10-1969, from the
Haskell Laboratory, report no. 308-69, submitted to WHO by Du Pont de
Nemours and Company.
Tayfun, F.O. One-hour inhalation toxicity. Unpublished report, dd.
22-9-1969, from the Haskell Laboratory, report no. 281-69, submitted
to WHO by Du Pont de Nemours and Company.
Zahnow, E.W. Oxamyl - Chicken and Egg Feeding Study. This unpublished
study, dated 12-4-78, was made available to FAO as Section 3, May 30,
1980, Exhibit 19 by the Biochemicals Department of du Pont.
