
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
PHENTHOATE
IDENTITY
Chemical name (IUPAC): S-alpha-ethoxycarbonylbenzyl
O,O-dimethyl-phosphorodithioate
Synonyms: Cidial(R), Elsan(R), L 561, ENT
27386, OMS 1075
Structural Formula:
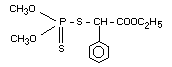 Molecular formula: C12H17O4PS2
Molecular weight: 320.4
Colour: reddish yellow
Melting point: 17.5 ± 0.5°C
Boiling point: the compound decomposes at normal
pressure before reaching the boiling
point.
Vapour pressure: 4 × 10-5 torr at 40°C
20
Density d 1.226
4
20
Refractive index: d /1.552 approx.
D
Flash point (/Cleveland): 165°C approx.
Partition coefficient: Octanol//water log P = 3.69, 3.82 at
two concentrations
Solubility of pure compound: in water at 24°C approx 10 mg/l.
Solubility of technical grade material: miscible in all proportion
with acetone, benzene, carbon disulphide, carbon-tetrachloride,
cyclohexane, cyclohexanone, dioxane, ethanol, ethyl ether,
methanol, methyl cellosolve; soluble at 2O°C 200 mg/l in water, 120
g/l in n-hexane, 17% in ligroin, above 20% in diethylene glycol and
above 100 g/l in petroleum solvent.
Stability of technical material: after one year of storage at room
temperature in the original sealed containers, the decrease of the
active ingredient content is about 1-2% of the original value. The
active ingredient content decreases by 1-4% after one month at
50°C. In water-ethanol 1:1 solutions buffered to pH 3.9, 5.8 and
7.8 the degradation of phenthoate is relatively slight after about
20 days. At pH 9.7 the degradation is approximately 25% after 20
days.
Typical composition of the technical material: 92% ai minimum
Formulations: Cidial E-4, Cidial 50L, Cidial 50% ES, Cidial AS,
Cidial ULV, Elsan 50 EC, Elsan dust (2%), Elsan 40WP
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phenthoate is rapidly absorbed, distributed, and metabolites
excreted following oral administration to female mice (3-4 months
of age). Within 24 hours following administration using either 14C
or 32P-radiolabelled phenthoate, at dosage levels ranging from 17
to 161 mg/kg, all administered radioactivity was recovered,
predominantly in urine (over 90%) and faeces. The major residues
found in urine were water-soluble degradation products (Takade et
al, 1976).
Biotransformation
Phenthoate is metabolised rapidly in the mouse with the major
portion of the molecule degraded and the products appearing in
urine within 24 hours of administration. The metabolic sequence
appears to follow that noted with a variety of other
organophosphorous compounds and includes both oxidative and
hydrolytic degradation (Takade et al, 1976).
The metabolic fate of phenthoate was examined in a cow administered
a daily dose of 20 mg/kg (relative to its dietary intake) for eight
days. Urinary metabolites, isolated over the course of the study,
were identified as phenthoate acid and desmethyl phenthoate acid.
No other metabolic products were identified (Wargo, 1975).
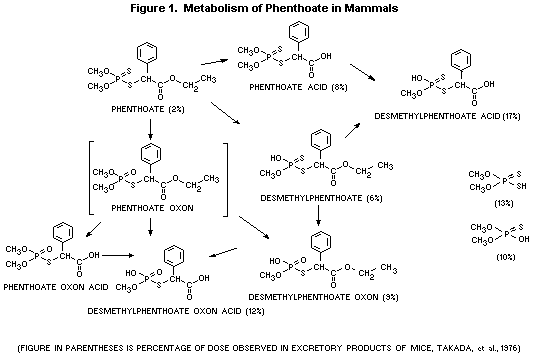
A proposed metabolic pathway for mammalian species is shown in
Figure 1.
Molecular formula: C12H17O4PS2
Molecular weight: 320.4
Colour: reddish yellow
Melting point: 17.5 ± 0.5°C
Boiling point: the compound decomposes at normal
pressure before reaching the boiling
point.
Vapour pressure: 4 × 10-5 torr at 40°C
20
Density d 1.226
4
20
Refractive index: d /1.552 approx.
D
Flash point (/Cleveland): 165°C approx.
Partition coefficient: Octanol//water log P = 3.69, 3.82 at
two concentrations
Solubility of pure compound: in water at 24°C approx 10 mg/l.
Solubility of technical grade material: miscible in all proportion
with acetone, benzene, carbon disulphide, carbon-tetrachloride,
cyclohexane, cyclohexanone, dioxane, ethanol, ethyl ether,
methanol, methyl cellosolve; soluble at 2O°C 200 mg/l in water, 120
g/l in n-hexane, 17% in ligroin, above 20% in diethylene glycol and
above 100 g/l in petroleum solvent.
Stability of technical material: after one year of storage at room
temperature in the original sealed containers, the decrease of the
active ingredient content is about 1-2% of the original value. The
active ingredient content decreases by 1-4% after one month at
50°C. In water-ethanol 1:1 solutions buffered to pH 3.9, 5.8 and
7.8 the degradation of phenthoate is relatively slight after about
20 days. At pH 9.7 the degradation is approximately 25% after 20
days.
Typical composition of the technical material: 92% ai minimum
Formulations: Cidial E-4, Cidial 50L, Cidial 50% ES, Cidial AS,
Cidial ULV, Elsan 50 EC, Elsan dust (2%), Elsan 40WP
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phenthoate is rapidly absorbed, distributed, and metabolites
excreted following oral administration to female mice (3-4 months
of age). Within 24 hours following administration using either 14C
or 32P-radiolabelled phenthoate, at dosage levels ranging from 17
to 161 mg/kg, all administered radioactivity was recovered,
predominantly in urine (over 90%) and faeces. The major residues
found in urine were water-soluble degradation products (Takade et
al, 1976).
Biotransformation
Phenthoate is metabolised rapidly in the mouse with the major
portion of the molecule degraded and the products appearing in
urine within 24 hours of administration. The metabolic sequence
appears to follow that noted with a variety of other
organophosphorous compounds and includes both oxidative and
hydrolytic degradation (Takade et al, 1976).
The metabolic fate of phenthoate was examined in a cow administered
a daily dose of 20 mg/kg (relative to its dietary intake) for eight
days. Urinary metabolites, isolated over the course of the study,
were identified as phenthoate acid and desmethyl phenthoate acid.
No other metabolic products were identified (Wargo, 1975).
A proposed metabolic pathway for mammalian species is shown in
Figure 1.
 Effects on enzymes and other biochemical parameters
Groups of rats (15 males and females/group) were administered
phenthoate orally for 90 days at daily dosage levels of 0, 2.5,
5.0, 12.5 or 25 mg/kg bw. Plasma, erythrocyte, and brain
cholinesterase were the most sensitive, being inhibited about 50%
of normal at the highest dose (plasma was slightly less inhibited).
No significant inhibition was observed at 5 mg/kg (Trabucchi,
1965). Also see Special Studies on Potentiation for studies on the
effects of organophosphate esters on serum and liver
carboxyesterases, enzymes directly involved in the degradation of
phenthoate.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rats
Two almost identical reproduction studies were performed. In both
studies, groups of rats (8 male and 16 female rats/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg
and subjected to a standard 2-litter per generation, 3 generation
reproduction study. Animals were fed phenthoate from 21 days of
age until 100 days of age when they were mated to initiate the
study. The first litters were weaned at 21 days, examined, and
discarded. The parental animals were again mated to provide a
second litter, which became the parental animals for the second
generation. Eight males and 16 females from the second litters of
each generation were selected for the succeeding generation. Gross
pathology examinations were performed on the second litters of each
generation. In one of the studies, gross and microscopic
examinations of selected tissues and organs were conducted on males
and females in the control and high-level group of each parental
generation and on the second litter of the third generation
animals. Additionally, in one of the studies, cholinesterase
activity was measured in male and female animals following weaning
of the second litter. This was conducted on both the parental and
weanling animals with respect to plasma, erythrocyte and brain
cholinesterase activity. Although these two latter parameters were
not evaluated in one reproduction study, gross pathological
examinations were made.
Reproduction indices, including mating, fecundity, male and female
fertility, gestation, and lactation, were calculated and compared
with control values. All pups were examined for physical
abnormalities and viability.
With the exception of a slightly depressed erythrocyte
cholinesterase activity observed at 100 mg/kg in all generations of
both parents and weanlings, there were no significant effects of
phenthoate on any reproduction parameter measured in the study. In
both studies, there were no differences with respect to any of the
indices observed, and it was considered that phenthoate, at dietary
dosage levels up to and including 100 mg/kg displays no reproduction
hazard to rats (Pfeifer et al, 1975; Wright et al, 1975).
Special study on teratogenicity
Groups of rabbits (17 pregnant New Zealand albino rabbits/group)
were administered phenthoate orally at dosages of 0, 3 or 10 mg/kg
body weight from days 6 through 18 of gestation in a standard
teratology bioassay. A positive control with thalidomide (50 mg/kg
bw), administered during gestation, was included in the study. On
day 29 of gestation, all pregnant animals were sacrificed and
foetuses delivered by caesarean section.
There was no mortality attributable to phenthoate. The data
related to reproduction (implantation sites, resorption sites, and
live young) were not affected by phenthoate at the highest dose
level studies. There were no external or internal abnormalities
(teratogenic events) associated with phenthoate treatment of
pregnant rabbits. Growth of the foetuses was unaffected as was
survival of young for 24 hours after delivery. Internal
development, including somatic and skeletal development, was
normal. There were no teratogenic effects noted on the
administration of phenthoate to pregnant rabbits during the
critical period of organogenesis.
In contrast to the lack of teratogenic response with phenthoate,
thalidomide reduced the number of live young, increased the number
of resorption sites, and reduced foetal viability. Several of the
foetuses exhibited external somatic and skeletal abnormalities
suggesting that the strain of rabbit used in the study was
susceptible to eliciting a chemically-induced teratogenic response.
Treatment of rabbits with phenthoate during gestation (foetal
organogenesis) did not induce a teratogenic response (Ladd. et
al, 1975).
Special studies on mutagenicity
Mammalian tests
In two identical studies, groups of male mice (12 mice/group) were
administered phenthoate as a single intraperitoneal injection at
dosage levels of 0, 150 or 300 mg/kg bw. Each animal of each group
was mated with groups of three untreated, virgin females for a
period of one week after which the females were removed and
replaced by another group of females. This procedure continued for
six consecutive weeks, the period required for maturation of the
male germ cell. Females were sacrificed approximately one week
after breeding and the number of implantation sites, resorption
sites, and embryos were recorded. Data were collected with respect
to early and late deaths.
There was no mortality in male mice attributable to the phenthoate
treatment; the high-dose group was slightly hypoactive for 3-4
hours after treatment. Pregnancy rates of females mated to males
receiving 300 mg/kg was slightly lower than the control values for
weeks 4 and 6. The numbers of implantation sites, resorption
sites, and embryos from females mated with treated males were the
same as those from control matings. There were essentially no
differences between treatment groups and controls with respect to
mutation rates indicating that, in this in vivo mutagenicity
study, phenthoate is not a mutagen affecting male germinal cells
(Arnold et al, 1974; 1975).
Microbiological tests
An evaluation of the mutagenicity potential of phenthoate was
performed using the standard Ames assay and other microbial test
systems. Strains of Salmonella typhimurium (TA1535, TA1538,
TA98 and TA100), with and without a metabolic activation system S-9
derived from rat liver-induced with Aroclor 1254, were tested at
concentrations up to and including 2 mg/plate in an effort to
evaluate genetic mutations. Standard positive control chemicals
were used (2-AAF, 2-AA, and B(a)P) in the presence of the metabolic
activation system and (MMNG, 2NF, and 9-AA) in the absence of the
metabolic activation system.
Under the conditions of the experimental assay, phenthoate showed
no mutagenic activity towards these strains of microorganisms
either in the presence or in the absence of an enzymatic metabolic
system. (Carneri, 1979).
A similar study with Salmonella typhimurium was reported
where phenthoate was tested at concentrations up to and including
5 mg/plate, in the presence and absence of a male rat liver
metabolic activation system. In addition, two strains of E.
coli WP-2 were tested for mutagenicity at the same
concentrations. Again, there was no evidence of mutagenicity,
either in the presence or absence of the metabolic activation
system utilised, with either species tested (Shirasu, et al,
1976).
The recombination-capacity of B. subtilis (H-17 and M-45) was
tested following exposure to phenthoate. Phenthoate did not
prohibit growth in this assay, again demonstrating no mutagenic
potential (Shirasu et al, 1976).
A standard, host-mediated assay was performed with mice, utilising
S. typhimurium as an indicator strain. Phenthoate was
administered to groups of 6 mice at dosage levels of O, 100 or 300
mg/kg body weight by oral intubation for two consecutive days.
After the second dosing, the indicator strain was administered to
the peritoneal cavity, recovered and grown and the mutagenic
potential of phenthoate evaluated. The number of reverted colonies
did not increase as a result of in vivo phenthoate treatment
suggesting that, under the conditions of this microbial assay,
phenthoate is not mutagenic (Shirasu et al, 1976).
Special studies on neurotoxicity
Chickens
Groups of adult hens, fasted for 16 hours, were administered
phenthoate orally at dosage levels of 0 or 2,990 mg/kg bw (a
previously observed LD50 value). A positive control (TOCP, 0.5
mg/kg) was also used. Ten animals were employed in each test
group. With the exception of the TOCP treatment, the single dose
was repeated following a 21-day observation period. At the
conclusion of the study (42 days), all surviving birds were
sacrificed and subjected to gross and microscopic examinations for
axon and myelin disruption. These included the brain, sciatic
nerve, and spinal cord.
There were no signs of delayed neurotoxicity observed with
phenthoate following both treatments although half of the animals
died over the course of the study. The positive control animals
(TOCP) were observed to lose weight and on microscopic examination
were found to display myelin and axon disruption in the spinal cord
and sciatic nerve. Axon degeneration and myelin disruption were
not seen in the brain of the TOCP-treated animals. Based on the
results of this study, it was concluded that phenthoate does not
induce a delayed neurotoxic reaction similar to that seen with TOCP
in adult chickens (Fletcher et al, 1976).
Groups of hens (10 adult hens/group) were administered phenthoate
orally at dosage levels of 0, 10, 50, 100 or 750 mg/kg/bw to assess
the delayed-neurotoxic potential of phenthoate. Animals
administered the highest dose level were also administered atropine
and 2-PAM to control cholinergic signs of poisoning. A positive
control of TOCP (1,000 mg/kg) and an untreated negative control
were included in the study.
When mortality was observed at the highest dose level, a second
group of hens was introduced using this dose level. These, too,
were protected from the acute cholinergic signs of poisoning. In
all phenthoate and negative control animals, there were no clinical
or histological signs of delayed neurotoxicity, although acute
signs of cholinergic stimulation were evident with phenthoate. The
positive control animals, administered TOCP, exhibited ataxia,
paralysis, and standard signs of delayed neurotoxicity.
Histological examination of the TOCP animals revealed a significant
number of animals with histological defects in the nervous tissue.
Based upon this bioassay, phenthoate does not induce a
delayed-neurotoxic reaction in hens (Good et al, 1979).
Special studies on potentiation
Pellegrini and Santi (1972) have reported on the potentiating
effect of a series of common organophosphorous compounds found as
impurities in technical phenthoate (as well as other methyl
organophosphate esters). Several of the trimethylphosphate
impurities substantially potentiate the acute toxicity of
phenthoate, probably through interaction at the carboxylic acid
portion of the molecule. Similar results (with respect to increased
acute toxicity when combinations of OP's were tested) were obtained
with malathion, providing evidence for the inhibition of
carboxyesterase activity as a primary mode of action in this
potentiation.
The acute LD50 of phenthoate was seen to change substantially as
the presence of impurities in technical mixture increased. A
highly purified phenthoate technical sample (98.5%) had an oral
LD50 in rats of 4,728 mg/kg body weight. As the content of
impurities increased, the rat oral LD50 value decreased (90.50% =
LD50 of 242 mg/kg; 78.7% = 118 mg/kg; 61.2% = 77.7 mg/kg). Two
impurities, O,S,S-trimethyl phosphorodithioate and O,O,S-trimethyl
phosphorothioate, were found to be extremely active in potentiating
the oral LD50 in rats. Fukuto and his coworkers (Stevens and
Fukuto, 1980; Umetsu et al, 1980; Mallipudi et al, 1980;
Hammond et al, 1980) have recently confirmed this potentiation
and its probable mode of action. In in vitro studies,
hydrolysis of phenthoate by rat liver and serum carboxyesterases
was examined in the presence and absence of these two impurities.
Phenthoate acid was the exclusive degradation product obtained with
the carboxyesterases. In the presence of impurities, the rate of
hydrolysis of phenthoate to phenthoate acid was substantially
diminished, supporting the conclusion that inhibition of
carboxyesterases is the primary cause of potentiation of phenthoate
toxicity.
Special study for carcinogenicity
Mice
Groups of mice (50 male and 50 female (Charles River) mice/group)
were fed phenthoate in the diet at dosage levels of 0, 500 or 1000
mg/kg for 18 months. The animals were examined daily for mortality
and adverse behaviourial reactions. At the conclusion of the
study, growth and microscopic examination of tissues and organs was
performed. Microscopic analysis of 10 animals of each sex surviving
the 18-month treatment was performed, as were microscopic studies
on animals that were sacrificed or had died during the course of
the study. All neoplasms and tissues with suspected neoplasms were
examined histologically. Complete pathological examinations were
made specifically to identify neoplastic lesions.
There were no unusual behaviourial changes or increases in
mortality over the course of the study attributable to the presence
of phenthoate. At the conclusion of the study, histological
examination revealed no treatment-related lesions among any of the
animals. The lesions that did occur were considered to be
naturally occurring, not attributable to the presence of phenthoate
and not unusual for the strain of mice. Although a substantial
number of animals that died were not examined histologically
TABLE 1. Acute Toxicity
Species Route Sex LD50 mg/kg bw Reference
Rat Oral2 M+F 245-440 Salvaneschi, 1968; He & Gera,
1978a; Toyoshima et al, 1978;
Trabucchi, 1965; Pellegrini &
Santi, 1972.
M 270 (231-316) Toyoshima et al, 1978.
F 255 (216-301) Toyoshima et al, 1978.
IP M+F 720 (672-77) Re and Gera, 1975b
M 720 (634-868) Toyoshima et al, 1971.
F 745 (615-901) Toyoshima et al, 1971.
SC M+F >2000 Toyoshima et al, 1971.
Dermal M+F 2100 Re and Gera, 1978c
(1522-2898)
M+F >5000 Toyoshima et al, 1968.
Mouse Oral M+F 360-840 Salvaneschi, 1968; Re and Gera,
1978b; Trabucchi, 1965;
Pellegrini & Santi, 1972.
IP M+F 420-430 Toyoshima et al, 1971.
IV M+F >250 Salvaneschi, 1968.
SC M+F >2000 Toyoshima, et al, 1971.
Dog Oral >500 Salvaneschi, 1968.
ca 500 Trabucchi, 1965.
Guinea pig Oral 377 Salvaneschi, 1968; Pellegrini
& Santi, 1977.
ca 400 Trabucchi, 1965.
Rabbit Oral ca 210 Salvaneschi, 1968; Pellegrini
& Santi, 1972.
Dermal 1830 Re and Gera, 1978d.
(unabraded skin) (1194-1595)
Abraded skin 2220 Re and Gera, 1978d.
(1872-2697)
Hare M 72 Pellegrini & Santi, 1971.
Chicken Oral ca 2551 Salvaneschi, 1968; Pellegrini
& Santi 1972.
2990 Fletcher et al, 1976
1 This value was obtained with technical product of 90.5% purity. A purified
technical product had an LD50 of 2800 mg/kg. (See the section on potentiation
for an explanation).
2 The acute oral toxicity of phenthoate oxon is 63 mg/kg bw (Pellegrini and
Santi, 1972).
because of autolysis, a sufficient number of survivors were
available to evaluate the carcinogenic potential. In this study,
phenthoate was not a carcinogen in mice (Oscarson et al, 1976).
Toxic signs of poisoning were similar to those noted with other
cholinergic organophosphate esters. Mortality occurred within 1-10
hours following acute intoxication. Signs of poisoning included
salivation, lacrimation, slowed or laboured respiration, tachycardia,
exophthalmus, tremors and convulsions followed by death.
No antidotal studies were reported, although it is probable that
atropine and 2-PAM will be therapeutic in the event of acute
overexposure.
Phenthoate (technical product) applied to the skin of rabbits was
found to be non-irritating, inducing a slight erythema 24 hours after
treatment (Re and Gera, 1978e). Formulated phenthoate is extremely
irritating (see short-term dermal studies in rabbits).
As with dermal studies, technical phenthoate, administered to the
conjunctival sac of rabbits, was found to be a non-irritant. However,
a formulation of phenthoate was irritating, increasing in its
irritability properties for 2-3 days after administration and
producing lasting effects for up to 14 days after treatment. While
phenthoate is not an irritant as a technical product, the formulated
products are irritants (Re and Gera, 1975a).
Acute toxicity studies examining the stability of formulated
phenthoate during storage have shown an increased toxicity when the
product was stored at 40°C for 12 months. The acute oral LD50 value
decreased from 258 mg/kg at the inception of the study to 130 mg/kg at
one year. The rate of change of acute toxicity was as shown in Table
2.
TABLE 2. Change of acute toxicity with time
Storage Time LD50
(months) (mg/kg)
0 258 (217-307)
1 282 (185-430)
3 200 (163-244)
6 119
12 130 (100-170)
(Noakes, 1963)
All samples produced similar signs of poisoning but those stored for 6
months or longer had a more rapid onset of toxic signs. The increased
toxicity may be as a result of formation of the trimethylthiophosphate
impurities known to potentiate the acute toxicity of phenthoate (see
Special studies on potentiation).
Short-term studies
Rabbit - dermal
In a series of three short-term dermal studies, a phenthoate
formulation was administered to rabbits and its dermal toxicity
evaluated. Groups of young adult rabbits (5 male and 5 female (3 of
each sex were tested at the two lowest doses) New Zealand albino
rabbits/group) were administered phenthoate (Cidial(R) E-4) dermally,
five days per week for three weeks to intact or abraded skin at dosage
rates of 2, 20, 75, 200, or 400 mg/kg/bw. Negative controls were run
with each test. Animals were observed daily for mortality and growth
was evaluated by body weight changes at weekly intervals. At the
beginning and at the end of the study, haematologic, clinical
chemistry, and urine analyses ware performed. Cholinesterase activity
was measured only in those rabbits treated at dosage levels below 200
mg/kg. Growth and microscopic examinations were performed on a
variety of tissues and organs in all animals. Over the course of the
studies, mortality was reported, primarily in the 400 mg/kg dosage
group. Hyperactivity was noted in all treated animals. It was
observed that at all dosage levels, the phenthoate formulation was
severely irritating to the skin and probably contributed to the
hyperactivity. Muscular fibrillations were observed in all animals at
the highest dose level. Significant body weight loss was observed in
all treated animals at the highest dose level. There were no unusual
effects noted in the haematologic, clinical chemistry, or urinalysis
parameters.
Significant erythrocyte cholinesterase was observed at 75 mg/kg in
both abraded and non-abraded groups. Plasma cholinesterase was only
slightly affected, predominantly toward the end of the treatment
period. The effect of phenthoate on blood cholinesterase appeared to
be cumulative, reaching maximal levels towards the end of the
treatment interval. After the dosing ended, plasma cholinesterase
recovered rapidly while erythrocyte cholinesterase, depressed at the
conclusion of the study, was still approaching normal 21 days after
the last treatment. No inhibition of cholinesterase was observed at
20 mg/kg.
There were no significant gross or microscopic changes noted, with the
exception of dermal changes characterised by pale red erythema, slight
to moderate edema, moderate desquamation and superficial escharosis.
Microscopic examination also revealed dermal changes in the epidermis,
predominantly at the high dose level. There were no other gross or
microscopic changes attributable to the administration of phenthoate
(Brett et al, 1974a; 1974b; Paa et al, 1974).
Groups of rabbits (2 male and 2 female rabbits/group) were
administered phenthoate dermally, to the intact or abraded skin, 5
days/week for 3 weeks at dosage levels of 0, 10, 50 or 100 mg/kg bw.
Growth, physical appearance and behaviour, food consumption, clinical
chemistry, haematology, and urine analyses were performed. At the
conclusion of the study, absolute and relative organ weights were
obtained on gross examination. Microscopic examinations of tissues
and organs were also performed.
Mortality was observed, as 1 male and 1 female administered 100 mg/kg
(intact skin) died during the course of the study. These animals had
shown severe signs of poisoning and mortality was attributable to the
phenthoate treatments.
An apparent anaemia (normocytic) was observed predominantly in the
survivors of the high-dose group. It was characterized by reduced
haematocrit, haemoglobin and red blood cell concentrations. A
dose-related reduction of cholinesterase activity was also noted. Red
blood cell, plasma and brain, cholinesterase activity were depressed
at all dose levels. No other significant effects were noted with
respect to clinical chemistry, haematology, and urinalyses. Organ
weight data as well as gross and microscopic pathology were not
unusual. Other than severe dermatitis, there were no pathological
effects noted in the study (Serota et al, 1979).
Rat - dietary
Groups of rats (10 male and 10 female, Donryu rats/group) were fed
phenthoate in the diet at dosage levels of 0, 5, 10, 30, 100, 300 or
1000 mg/kg for 3 months. Animals were observed daily for behaviourial
changes and growth was recorded at weekly intervals. At the
conclusion of the study, haematological, blood chemistry, and urine
analyses were performed. Animals were sacrificed at 3 months and
gross and microscopic examinations of the tissues and organs were
performed.
While there were no gross toxic signs of poisoning, growth, especially
in male rats fed 1000 mg/kg was reduced. Females receiving 1000 mg/kg
in the diet displayed a slight depression of body weight during the
course of the study. Food consumption data were normal. At 1000
mg/kg, there was a significant change in total and differential white
blood cell counts in both males and females. Urinalysis data, with
the exception of slight changes in sodium excretion values in both
males and females (males increased, females decreased) at 1000 mg/kg,
did not show changes attributable to the presence of phenthoate in the
diet. Slight blood chemistry changes were also observed at 1000 mg/kg
with respect to several parameters (SGOT, BUN, A/G ratios, and
cholinesterase activity). With the exception of cholinesterase
depression, the other clinical chemistry parameters were not
substantially different from control values.
Erythrocyte cholinesterase was significantly depressed in both males
and females at 30 mg/kg and above. At 10 mg/kg, a slight reduction of
activity was reported (less than 25%). Significant inhibition of
plasma cholinesterase was noted only in male rats fed 1000 mg/kg.
There was no significant inhibition of brain cholinesterase at 1000
mg/kg.
Gross and microscopic examinations of tissues and organs showed no
significant effects attributable to the presence of phenthoate at
dosage levels below 1000 mg/kg. At 1000 mg/kg several major organs in
both males and females showed significant weight depression: thymus,
heart, adrenal glands, testes, and ovaries. In males, the weight of
liver, kidney, spleen, and seminal vesicles was decreased while in
females, the weight of the heart, lung, and uterus was decreased. The
gross changes observed at the conclusion of the study were not
reflected in abnormalities noted on microscopic histopathologic
examination.
Based on these studies, a no-effect level is 10 mg/kg (Toyoshima et
al, 1973).
Mice
Groups of mice (10 male and 10 female ICR- mice/group) were
administered phenthoate in the diet at dosage levels of 0, 5, 10, 30,
100, 300 or 1000 mg/kg for 3 months. Growth, as observed by body
weight changes, was recorded on a weekly basis. Food and water
consumption data were recorded and at the conclusion of the study,
animals were sacrificed for haematological, blood chemistry, and urine
analyses. Gross and microscopic examinations of tissues and organs
were performed at the conclusion of the study.
There was no mortality observed over the 3-month interval. Growth was
depressed at 1000 mg/kg, primarily during the initial parts of the
study. After the first month, growth was normal in all test groups.
Food and water consumption were not affected by the presence of
phenthoate. Haematologic values in both males and females showed a
trend towards a reduction in the total and differential leucocyte
count in both males and females. This trend was not significantly
different from control values at dosage levels below 1000 mg/kg.
Urinalyses were normal as were all blood chemistry values with the
exception of cholinesterase activity. Significant plasma
cholinesterase depression was observed only at 1000 mg/kg. At 100
mg/kg and above, erythrocyte cholinesterase was inhibited. Brain
cholinesterase depression was not observed at any dose level.
On gross examination at the conclusion of the study, organ weight
changes were observed at 1000 mg/kg. Male mice showed a significant
depression of the thymus and adrenal glands, while female mice showed
significant depression of the weight of heart, lungs, thymus, liver,
kidneys, spleen, uterus, and ovaries. On histological examination,
the tissues of treated animals did not display pathological changes
attributable to the presence of phenthoate, suggesting that the gross
changes were physiological adaptation to the high dosage of
phenthoate. A no-effect level in the study, based upon cholinesterase
depression in both males and females, is 30 mg/kg in the diet
(Toyoshima et al, 1973).
Dogs
Groups of dogs (4 female and 4 male, beagle dogs/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg for
2 years. Daily examinations were made for clinical signs of poisoning
or adverse behaviour. Growth, as evidenced by body weight, was
recorded weekly as were food consumption data. At periodic intervals
(1, 3, 6, 9, 12, 18, and 24 months), haematology, blood chemistry
(including cholinesterase) and urine analyses were performed. Plasma
and erythrocyte cholinesterase determinations were performed at 2, 4,
6, 9, 13, 26, 78, and 104 weeks and brain cholinesterase analyses were
conducted at the conclusion of the study. At the conclusion of 104
weeks of dietary administration, each dog was sacrificed and gross and
microscopic examinations of tissues and organs were performed.
There was no mortality over the course of the study at any dosage
level. Growth and food consumption were normal. With the exception
of cholinesterase depression, data from blood clinical chemistry
tests, haematological tests, and urinalyses were normal. Erythrocyte
cholinesterase inhibition was observed at 30 mg/kg and above. This
effect was first noted 2 weeks after the initiation of the study and
was relatively constant over the entire study. Plasma and brain
cholinesterase activity were unaffected and considered normal at all
dietary levels. Gross and microscopic examinations of tissues and
organs at the conclusion of the study did not show any significant
effect of the inclusion of phenthoate in the diet at dose levels up to
and including 100 mg/kg. A no-effect level in this study, based on
cholinesterase depression, was 10 mg/kg in the diet equivalent to 0.29
mg/kg bw, a value derived from the average food consumption values
over the 2-year period (Nelson et al, 1972).
Long-term study
Mice
(See special study on carcinogenicity).
Rats
No data available.
RESIDUES IN FOOD
USE PATTERN
Phenthoate is a wide spectrum insecticide for agricultural and
domestic use. It is active against both chewing and sucking insects,
in particular against lepidopterous larvae, soft scales and larvae and
adults of some species of mosquitoes. The pesticide should be applied
in enough water to insure complete coverage of the foliage, branches
and fruit when applied as a ground application.
The registered use patterns reported from Italy, Israel, Japan and
South Africa are summarised in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
The trials were performed in Brazil, Florida, Italy and South Africa
with both the recommended and approximately double dose rates. The
results are summarised in Tables 5 and 6. Treatments carried out with
low volume spraying (about 940 l/ha) or with 0.5% oil additive
resulted in somewhat higher residue than the dilute application, when
the spray was applied until run off to achieve full coverage.
The repeated treatments carried out following the recommended use
pattern did not increase the residue in the fruit significantly. The
colometric method used in 1963-65 experiments showed 3-4 times higher
residues than those obtained with GLC determination of samples
deriving from similarly treated plots at later trials.
The phenthoate residues remain entirely in the peel of fruits. The
pulp did not contain detectable residues (limit of determination
0.02-0-03 mg/kg) at any time after treatment with the exception of two
sets of experiments. In one case about 10-25% of the residue found in
the peel was detected in the pulp at the day of treatment and after 98
days, respectively. However in the other experiment the residue in
the pulp was only about 2.55% of the residue found in the peel and
decreased with time.
The phenthoate oxon was only detected in the peel. Limit of
determination in the pulp was 0.01 mg/kg (Moye). The oxon derivative
amounted to a maximum of 0.12 mg/kg (43%) on lemon, 0.08 mg/kg (15%)
on grapefruit and 0.62 mg/kg (38%) on orange. The values in
parentheses refer to the ratio of oxon to the parent compound. The
results of other trials (Iwata, 1979) indicate similar residue ranges.
The residue, on a whole fruit basis, can be calculated from the
residue measured in the peel taking into account the peel/pulp weight
ratio which is fairly constant for different varieties (Iwata, 1977).
For instance, in the case of valencia orange 1/5 of the peel residue
can be considered as the residue on whole fruit basis, while for the
variety Hamlin, the ratio is 1/4.
Other Crops
A limited number of supervised trials were carried out on apple, pear,
cabbage, lettuce, olives, onions, radish, spinach, sweet potato and
sugarbeet in Italy and Japan in 1962-65. The results of these
experiments were provided to the Joint Meeting for evaluation (Tables
7 and 8). However they were not considered to represent the current
use of phenthoate or to be sufficient for evaluation, thus no
TABLE 3. Registered Uses of Phenthoate
Crop Pest ai [g/100R] Formulation No. of treatments Amount of water l/ha
Citrus Scale insects ) 50 - 100 EC 1 - 2 1000-10000
Aphids ) 40 WP (generally)
Orange leaf miner )
Leaf rollers )
Apple Codling moth 40 - 50 EC 5 - 10 1000-2500
WP 1 - 6 5000
Brassica Large white butterfly 50 - 1000 EC 1 or more up to 1000
Japanese pears Leaf rollers 40 - 50 WP 1 - 6 3000
Fruit moths
Mealy bugs
Nuts Codling moth 70 EC 3 - 4 2000-10000
Olives Black scale 60 - 75 EC 3 - 4 2000-10000
Olive thrips
Onions Thrips 75 EC 1 or more up to 1000
Pears Codling moth 40 - 50 EC 3 - 7 1000-2000
Pear psylla 200 250 200 - 500
Potatoes Potato tuber moth 100 EC 1 or more up to 1000
Rice (paddy) Rice stem borers ) 50 - 100 EC 1 - 4 800 - 1500
Leaf and plant )
hoppers ) 1.65 - 3.3 kg EC 1 30-40 aerial
Rice bugs ) 0.6-0.8/kg/ha. Dust
Rice leaf beetle )
Rice leaf miner )
recommendation was made by the Meeting for the maximum residue levels
in/on these commodities.
FATE OF RESIDUES
In animals
Lactating dairy cattle were administered 14C-labelled phenthoate at
levels of 1, 5 and 20 mg/kg on feed basis (Wargo, Anonym. 1979).
Samples of urine, faeces and milk were collected every day during the
administration for 8, 18, 26 days and withdrawal (7 days).
The animals were slaughtered the following day and the tissue samples
were taken. The total 14C residue measured in various tissues and
calculated as parent compound at a dose rate of 20 mg/kg is given in
Table 9. Muscle and fat did not contain detectable residues at rates
of 1 and 5 mg/kg. The residue in the other tissues also decreased at
the lower rates.
Total 14C extractable residues in liver and kidney were 29% and 51%
respectively, and consisted of organosoluble and watersoluble
metabolites.
The metabolites were separated with various chromatographic methods.
In the liver at least eleven compounds were found, of which the major
component represented 8% of the total 14C residues in liver. One of
the remaining ten was present at a higher concentration than 3%. The
unextractable residue was quantitatively released by acidic
hydrolysis. At each feeding level the cows with a seven-day
withdrawal period demonstrated a significant decrease in residues
compared to the cows sacrificed 24 hours after the last 14C
phenthoate dose. Even the "bound" metabolites were eliminated from
the tissues of kidney and liver.
The 14C residues in milk reached the plateau on the second or third
day of treatment. During the feeding period the total residue
plateaued at the levels given below:
Level in diet Residue in milk, mg/kg
1 mg/kg 0.001-0.003
5 mg/kg 0.008-0.026
20 mg/kg 0.015-0.04
The residue declined after withdrawal of the pesticide from the diet
and approached the limit of determination after 3-6 days.
The 0.04 mg/kg total 14C residue in milk consisted of 7% (0.0028
mg/kg) organosoluble, 16% (O.006 mg/kg) water-soluble compounds and
52% (0.019 mg/kg) milk solids.
The residue level in each fraction was too low for further
investigation.
Neither phenthoate nor its oxon could be detected by conventional
analytical methods based on GLC determination in any of the tissue or
milk samples (limit of determination 0.01 mg/kg for fat and 0.05 mg/kg
for the other samples).
White leghorn laying hens were given feed containing 14C phenthoate
at 1.8, 5.9 or 26 mg/kg for 30 days. The birds were sacrificed on
days 1, 3, 7, 14, 21 and 30 during the treatment and on days 7 and 15
during the withdrawal period (Huhtanen, 1979a). The maximum residue
contents of tissues analyzed are given in table 4.
TABLE 4. Residues of phenthoate in tissues of chickens fed phenthoate
in the diet at various levels for 30 days
Tissue Concentration of phenthoate in diet
1.8 mg/kg 5.9 mg/kg 26 mg/kg
breast muscle 0.333 0.072 0.073
leg muscle 0.018 0.035 0.13
liver 0.024 0.04 0.48
kidney 0.094 0.53 1.3
skin 0.039 0.16 0.38
fat 0.019 0.07 0.16
eggs 0.014 0.058 0.35
limits of detection
for non-fatty tissues 0.0077 0.027 0.066
for fatty tissues 0.016 0.053 0.13
A plateau of the residue was reached in liver and kidney between the
third and seventh day of treatment. In the muscles detectable
residues were found from 21st day of treatment but no residue was
detected during the withdrawal period either in the muscles or in the
eggs. Less than 10% of the extracted material, which accounted for 70
of total radioactivity, was of non-polar character in the kidney. A
large percentage (50%) of the more polar compounds were acidic.
No abnormalities were observed in the organs of the hens and the
production of eggs was not reduced during the treatment period.
Fish residue studies were carried out with channel catfish and
bluegill sunfish in order to determine the biomagnification behaviour
of phenthoate.
In water containing 14C-phenthoate at 10-6 mg/l level the average
concentration ratios from catfish tissue/mud and catfish tissue/water
were 0.17 (0.09-0.33) and 5.76 (2.47-9.97) respectively after 33 days.
Residue levels in contaminated fishes showed a gradual reduction if
the fishes were placed in uncontaminated water (Marshall, 1979).
The uptake and bioaccumulation of 14C-phenthoate residues from soil
aged for 21 days in the aquaria into channel catfish were studied at
fortified soil levels of 0.01 and 1 mg/kg. The residues in the
catfish "meat" were 0.003 mg/kg 28 days after placing them into the
contaminated water (Booth, 1978).
Bluegill sunfish were exposed to 14C-phenthoate at 2.1 × 10-5 mg/l or
at 2 × 10-4 mg/1 in the water of a simulated dynamic water system for
30 days. The average bioaccumulation ratio of 14C was 816 and 901
respectively, while for the edible tissue 240 and 248 were the
calculated values. The residues found in tissues of 14-day withdrawal
samples taken from the higher level tank was only 3% of the level
found after 30 days of exposure (Huhtanen, 1979b).
In plants
Selected leaves and fruit of a Valencia orange tree were treated by
brush application with a solution of 30 mg phenthoate [32P or 14C] in
50 cm of water containing 0.06% emulsifier (Takade, 1977). Samples of
leaves and fruits were taken 3, 7, 10 and 14 days after treatment and
rinsed 5 times with acetone to remove the surface material. After
rinsing with acetone, the fruit was carefully peeled. The leaves,
peel and pulp were cut into small pieces and placed in a vacuum oven
at 40°C for approximately 10 hours. No loss in radioactivity was
detected for the drying process. The dried samples were extracted
with acetone. The metabolites were separated on thin layer plates
using four solvent systems. The total radioactivity of solid
materials was then determined by combustion analysis.
The major portion of phenthoate applied to leaves was lost by
volatilisation. The concentration and distribution of residue in
orange fruit are given in Table 10. Significant loss in total
recovery wan observed in fruit. Less than 1% of the total activity
applied was detected in the orange pulp. The majority of the residue
both in fruit and in leaves was intact phenthoate. The ratio of
phenthoate and its phenthoate oxon is shown in Table 11. Other
metabolites, desmethyl phenthoate, mandelic acid, bis-alpha-ethoxy
carbonylbenzyl disulphide, O,O-dimethyl phosphorodithioic acid, O,O-
dimethyl-phosphorothioic acid and two unidentified compounds amounted
to 0-5.2% individually. Glucosidase, hydrochloric acid and sodium
hydroxide together dissolved about 92-98% of the bound residues in the
peel. The major conjugated metabolites were, in order of importance,
ethyl mandelate, mandelic acid, desmethyl phenthoate and phenthoate
acid (Mallipudi and Fukuto, 1980).
Apple, pear and olive trees were treated with 32P-labelled phenthoate
according to the recommended use pattern (Santi, 1967). The surface
of the fruits was washed with methanol to remove the original active
substance or any other derivatives containing methanol-soluble 32P
still present on the peel (methanol extract).
After washing, the various fruits were homogenised in a blender with
chloroform (chloroform extract).
The plant filter cake was further extracted with water to separate
eventual water-soluble compounds (water extract). The 32P content of
various extracts and of residual plant was determined radiometrically.
The phenthoate equivalents of the radioactive residues found at
certain times after treatments are listed in Table 12.
Column chromatography, thin-layer chromatography and paper
chromatography were used for the separation and identification of the
compounds in the various extracts.
The probable metabolic pathways are shown in Fig. 2.
The repeated treatments of apple trees with a solution containing
0.02% ai (half the dosage usually applied) resulted in qualitatively
similar picture regardless the number of treatments. The phenthoate,
although not systemic, penetrates slightly into the fruits and
undergoes oxidation and hydrolysis; 15-29 days after treatment 95% of
water-soluble residue was phosphoric acid while monomethyl and
dimethyl phosphoric acids were detected in traces. Phenthoate oxon
was detected in traces only 1 day after treatment.
Effects on enzymes and other biochemical parameters
Groups of rats (15 males and females/group) were administered
phenthoate orally for 90 days at daily dosage levels of 0, 2.5,
5.0, 12.5 or 25 mg/kg bw. Plasma, erythrocyte, and brain
cholinesterase were the most sensitive, being inhibited about 50%
of normal at the highest dose (plasma was slightly less inhibited).
No significant inhibition was observed at 5 mg/kg (Trabucchi,
1965). Also see Special Studies on Potentiation for studies on the
effects of organophosphate esters on serum and liver
carboxyesterases, enzymes directly involved in the degradation of
phenthoate.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rats
Two almost identical reproduction studies were performed. In both
studies, groups of rats (8 male and 16 female rats/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg
and subjected to a standard 2-litter per generation, 3 generation
reproduction study. Animals were fed phenthoate from 21 days of
age until 100 days of age when they were mated to initiate the
study. The first litters were weaned at 21 days, examined, and
discarded. The parental animals were again mated to provide a
second litter, which became the parental animals for the second
generation. Eight males and 16 females from the second litters of
each generation were selected for the succeeding generation. Gross
pathology examinations were performed on the second litters of each
generation. In one of the studies, gross and microscopic
examinations of selected tissues and organs were conducted on males
and females in the control and high-level group of each parental
generation and on the second litter of the third generation
animals. Additionally, in one of the studies, cholinesterase
activity was measured in male and female animals following weaning
of the second litter. This was conducted on both the parental and
weanling animals with respect to plasma, erythrocyte and brain
cholinesterase activity. Although these two latter parameters were
not evaluated in one reproduction study, gross pathological
examinations were made.
Reproduction indices, including mating, fecundity, male and female
fertility, gestation, and lactation, were calculated and compared
with control values. All pups were examined for physical
abnormalities and viability.
With the exception of a slightly depressed erythrocyte
cholinesterase activity observed at 100 mg/kg in all generations of
both parents and weanlings, there were no significant effects of
phenthoate on any reproduction parameter measured in the study. In
both studies, there were no differences with respect to any of the
indices observed, and it was considered that phenthoate, at dietary
dosage levels up to and including 100 mg/kg displays no reproduction
hazard to rats (Pfeifer et al, 1975; Wright et al, 1975).
Special study on teratogenicity
Groups of rabbits (17 pregnant New Zealand albino rabbits/group)
were administered phenthoate orally at dosages of 0, 3 or 10 mg/kg
body weight from days 6 through 18 of gestation in a standard
teratology bioassay. A positive control with thalidomide (50 mg/kg
bw), administered during gestation, was included in the study. On
day 29 of gestation, all pregnant animals were sacrificed and
foetuses delivered by caesarean section.
There was no mortality attributable to phenthoate. The data
related to reproduction (implantation sites, resorption sites, and
live young) were not affected by phenthoate at the highest dose
level studies. There were no external or internal abnormalities
(teratogenic events) associated with phenthoate treatment of
pregnant rabbits. Growth of the foetuses was unaffected as was
survival of young for 24 hours after delivery. Internal
development, including somatic and skeletal development, was
normal. There were no teratogenic effects noted on the
administration of phenthoate to pregnant rabbits during the
critical period of organogenesis.
In contrast to the lack of teratogenic response with phenthoate,
thalidomide reduced the number of live young, increased the number
of resorption sites, and reduced foetal viability. Several of the
foetuses exhibited external somatic and skeletal abnormalities
suggesting that the strain of rabbit used in the study was
susceptible to eliciting a chemically-induced teratogenic response.
Treatment of rabbits with phenthoate during gestation (foetal
organogenesis) did not induce a teratogenic response (Ladd. et
al, 1975).
Special studies on mutagenicity
Mammalian tests
In two identical studies, groups of male mice (12 mice/group) were
administered phenthoate as a single intraperitoneal injection at
dosage levels of 0, 150 or 300 mg/kg bw. Each animal of each group
was mated with groups of three untreated, virgin females for a
period of one week after which the females were removed and
replaced by another group of females. This procedure continued for
six consecutive weeks, the period required for maturation of the
male germ cell. Females were sacrificed approximately one week
after breeding and the number of implantation sites, resorption
sites, and embryos were recorded. Data were collected with respect
to early and late deaths.
There was no mortality in male mice attributable to the phenthoate
treatment; the high-dose group was slightly hypoactive for 3-4
hours after treatment. Pregnancy rates of females mated to males
receiving 300 mg/kg was slightly lower than the control values for
weeks 4 and 6. The numbers of implantation sites, resorption
sites, and embryos from females mated with treated males were the
same as those from control matings. There were essentially no
differences between treatment groups and controls with respect to
mutation rates indicating that, in this in vivo mutagenicity
study, phenthoate is not a mutagen affecting male germinal cells
(Arnold et al, 1974; 1975).
Microbiological tests
An evaluation of the mutagenicity potential of phenthoate was
performed using the standard Ames assay and other microbial test
systems. Strains of Salmonella typhimurium (TA1535, TA1538,
TA98 and TA100), with and without a metabolic activation system S-9
derived from rat liver-induced with Aroclor 1254, were tested at
concentrations up to and including 2 mg/plate in an effort to
evaluate genetic mutations. Standard positive control chemicals
were used (2-AAF, 2-AA, and B(a)P) in the presence of the metabolic
activation system and (MMNG, 2NF, and 9-AA) in the absence of the
metabolic activation system.
Under the conditions of the experimental assay, phenthoate showed
no mutagenic activity towards these strains of microorganisms
either in the presence or in the absence of an enzymatic metabolic
system. (Carneri, 1979).
A similar study with Salmonella typhimurium was reported
where phenthoate was tested at concentrations up to and including
5 mg/plate, in the presence and absence of a male rat liver
metabolic activation system. In addition, two strains of E.
coli WP-2 were tested for mutagenicity at the same
concentrations. Again, there was no evidence of mutagenicity,
either in the presence or absence of the metabolic activation
system utilised, with either species tested (Shirasu, et al,
1976).
The recombination-capacity of B. subtilis (H-17 and M-45) was
tested following exposure to phenthoate. Phenthoate did not
prohibit growth in this assay, again demonstrating no mutagenic
potential (Shirasu et al, 1976).
A standard, host-mediated assay was performed with mice, utilising
S. typhimurium as an indicator strain. Phenthoate was
administered to groups of 6 mice at dosage levels of O, 100 or 300
mg/kg body weight by oral intubation for two consecutive days.
After the second dosing, the indicator strain was administered to
the peritoneal cavity, recovered and grown and the mutagenic
potential of phenthoate evaluated. The number of reverted colonies
did not increase as a result of in vivo phenthoate treatment
suggesting that, under the conditions of this microbial assay,
phenthoate is not mutagenic (Shirasu et al, 1976).
Special studies on neurotoxicity
Chickens
Groups of adult hens, fasted for 16 hours, were administered
phenthoate orally at dosage levels of 0 or 2,990 mg/kg bw (a
previously observed LD50 value). A positive control (TOCP, 0.5
mg/kg) was also used. Ten animals were employed in each test
group. With the exception of the TOCP treatment, the single dose
was repeated following a 21-day observation period. At the
conclusion of the study (42 days), all surviving birds were
sacrificed and subjected to gross and microscopic examinations for
axon and myelin disruption. These included the brain, sciatic
nerve, and spinal cord.
There were no signs of delayed neurotoxicity observed with
phenthoate following both treatments although half of the animals
died over the course of the study. The positive control animals
(TOCP) were observed to lose weight and on microscopic examination
were found to display myelin and axon disruption in the spinal cord
and sciatic nerve. Axon degeneration and myelin disruption were
not seen in the brain of the TOCP-treated animals. Based on the
results of this study, it was concluded that phenthoate does not
induce a delayed neurotoxic reaction similar to that seen with TOCP
in adult chickens (Fletcher et al, 1976).
Groups of hens (10 adult hens/group) were administered phenthoate
orally at dosage levels of 0, 10, 50, 100 or 750 mg/kg/bw to assess
the delayed-neurotoxic potential of phenthoate. Animals
administered the highest dose level were also administered atropine
and 2-PAM to control cholinergic signs of poisoning. A positive
control of TOCP (1,000 mg/kg) and an untreated negative control
were included in the study.
When mortality was observed at the highest dose level, a second
group of hens was introduced using this dose level. These, too,
were protected from the acute cholinergic signs of poisoning. In
all phenthoate and negative control animals, there were no clinical
or histological signs of delayed neurotoxicity, although acute
signs of cholinergic stimulation were evident with phenthoate. The
positive control animals, administered TOCP, exhibited ataxia,
paralysis, and standard signs of delayed neurotoxicity.
Histological examination of the TOCP animals revealed a significant
number of animals with histological defects in the nervous tissue.
Based upon this bioassay, phenthoate does not induce a
delayed-neurotoxic reaction in hens (Good et al, 1979).
Special studies on potentiation
Pellegrini and Santi (1972) have reported on the potentiating
effect of a series of common organophosphorous compounds found as
impurities in technical phenthoate (as well as other methyl
organophosphate esters). Several of the trimethylphosphate
impurities substantially potentiate the acute toxicity of
phenthoate, probably through interaction at the carboxylic acid
portion of the molecule. Similar results (with respect to increased
acute toxicity when combinations of OP's were tested) were obtained
with malathion, providing evidence for the inhibition of
carboxyesterase activity as a primary mode of action in this
potentiation.
The acute LD50 of phenthoate was seen to change substantially as
the presence of impurities in technical mixture increased. A
highly purified phenthoate technical sample (98.5%) had an oral
LD50 in rats of 4,728 mg/kg body weight. As the content of
impurities increased, the rat oral LD50 value decreased (90.50% =
LD50 of 242 mg/kg; 78.7% = 118 mg/kg; 61.2% = 77.7 mg/kg). Two
impurities, O,S,S-trimethyl phosphorodithioate and O,O,S-trimethyl
phosphorothioate, were found to be extremely active in potentiating
the oral LD50 in rats. Fukuto and his coworkers (Stevens and
Fukuto, 1980; Umetsu et al, 1980; Mallipudi et al, 1980;
Hammond et al, 1980) have recently confirmed this potentiation
and its probable mode of action. In in vitro studies,
hydrolysis of phenthoate by rat liver and serum carboxyesterases
was examined in the presence and absence of these two impurities.
Phenthoate acid was the exclusive degradation product obtained with
the carboxyesterases. In the presence of impurities, the rate of
hydrolysis of phenthoate to phenthoate acid was substantially
diminished, supporting the conclusion that inhibition of
carboxyesterases is the primary cause of potentiation of phenthoate
toxicity.
Special study for carcinogenicity
Mice
Groups of mice (50 male and 50 female (Charles River) mice/group)
were fed phenthoate in the diet at dosage levels of 0, 500 or 1000
mg/kg for 18 months. The animals were examined daily for mortality
and adverse behaviourial reactions. At the conclusion of the
study, growth and microscopic examination of tissues and organs was
performed. Microscopic analysis of 10 animals of each sex surviving
the 18-month treatment was performed, as were microscopic studies
on animals that were sacrificed or had died during the course of
the study. All neoplasms and tissues with suspected neoplasms were
examined histologically. Complete pathological examinations were
made specifically to identify neoplastic lesions.
There were no unusual behaviourial changes or increases in
mortality over the course of the study attributable to the presence
of phenthoate. At the conclusion of the study, histological
examination revealed no treatment-related lesions among any of the
animals. The lesions that did occur were considered to be
naturally occurring, not attributable to the presence of phenthoate
and not unusual for the strain of mice. Although a substantial
number of animals that died were not examined histologically
TABLE 1. Acute Toxicity
Species Route Sex LD50 mg/kg bw Reference
Rat Oral2 M+F 245-440 Salvaneschi, 1968; He & Gera,
1978a; Toyoshima et al, 1978;
Trabucchi, 1965; Pellegrini &
Santi, 1972.
M 270 (231-316) Toyoshima et al, 1978.
F 255 (216-301) Toyoshima et al, 1978.
IP M+F 720 (672-77) Re and Gera, 1975b
M 720 (634-868) Toyoshima et al, 1971.
F 745 (615-901) Toyoshima et al, 1971.
SC M+F >2000 Toyoshima et al, 1971.
Dermal M+F 2100 Re and Gera, 1978c
(1522-2898)
M+F >5000 Toyoshima et al, 1968.
Mouse Oral M+F 360-840 Salvaneschi, 1968; Re and Gera,
1978b; Trabucchi, 1965;
Pellegrini & Santi, 1972.
IP M+F 420-430 Toyoshima et al, 1971.
IV M+F >250 Salvaneschi, 1968.
SC M+F >2000 Toyoshima, et al, 1971.
Dog Oral >500 Salvaneschi, 1968.
ca 500 Trabucchi, 1965.
Guinea pig Oral 377 Salvaneschi, 1968; Pellegrini
& Santi, 1977.
ca 400 Trabucchi, 1965.
Rabbit Oral ca 210 Salvaneschi, 1968; Pellegrini
& Santi, 1972.
Dermal 1830 Re and Gera, 1978d.
(unabraded skin) (1194-1595)
Abraded skin 2220 Re and Gera, 1978d.
(1872-2697)
Hare M 72 Pellegrini & Santi, 1971.
Chicken Oral ca 2551 Salvaneschi, 1968; Pellegrini
& Santi 1972.
2990 Fletcher et al, 1976
1 This value was obtained with technical product of 90.5% purity. A purified
technical product had an LD50 of 2800 mg/kg. (See the section on potentiation
for an explanation).
2 The acute oral toxicity of phenthoate oxon is 63 mg/kg bw (Pellegrini and
Santi, 1972).
because of autolysis, a sufficient number of survivors were
available to evaluate the carcinogenic potential. In this study,
phenthoate was not a carcinogen in mice (Oscarson et al, 1976).
Toxic signs of poisoning were similar to those noted with other
cholinergic organophosphate esters. Mortality occurred within 1-10
hours following acute intoxication. Signs of poisoning included
salivation, lacrimation, slowed or laboured respiration, tachycardia,
exophthalmus, tremors and convulsions followed by death.
No antidotal studies were reported, although it is probable that
atropine and 2-PAM will be therapeutic in the event of acute
overexposure.
Phenthoate (technical product) applied to the skin of rabbits was
found to be non-irritating, inducing a slight erythema 24 hours after
treatment (Re and Gera, 1978e). Formulated phenthoate is extremely
irritating (see short-term dermal studies in rabbits).
As with dermal studies, technical phenthoate, administered to the
conjunctival sac of rabbits, was found to be a non-irritant. However,
a formulation of phenthoate was irritating, increasing in its
irritability properties for 2-3 days after administration and
producing lasting effects for up to 14 days after treatment. While
phenthoate is not an irritant as a technical product, the formulated
products are irritants (Re and Gera, 1975a).
Acute toxicity studies examining the stability of formulated
phenthoate during storage have shown an increased toxicity when the
product was stored at 40°C for 12 months. The acute oral LD50 value
decreased from 258 mg/kg at the inception of the study to 130 mg/kg at
one year. The rate of change of acute toxicity was as shown in Table
2.
TABLE 2. Change of acute toxicity with time
Storage Time LD50
(months) (mg/kg)
0 258 (217-307)
1 282 (185-430)
3 200 (163-244)
6 119
12 130 (100-170)
(Noakes, 1963)
All samples produced similar signs of poisoning but those stored for 6
months or longer had a more rapid onset of toxic signs. The increased
toxicity may be as a result of formation of the trimethylthiophosphate
impurities known to potentiate the acute toxicity of phenthoate (see
Special studies on potentiation).
Short-term studies
Rabbit - dermal
In a series of three short-term dermal studies, a phenthoate
formulation was administered to rabbits and its dermal toxicity
evaluated. Groups of young adult rabbits (5 male and 5 female (3 of
each sex were tested at the two lowest doses) New Zealand albino
rabbits/group) were administered phenthoate (Cidial(R) E-4) dermally,
five days per week for three weeks to intact or abraded skin at dosage
rates of 2, 20, 75, 200, or 400 mg/kg/bw. Negative controls were run
with each test. Animals were observed daily for mortality and growth
was evaluated by body weight changes at weekly intervals. At the
beginning and at the end of the study, haematologic, clinical
chemistry, and urine analyses ware performed. Cholinesterase activity
was measured only in those rabbits treated at dosage levels below 200
mg/kg. Growth and microscopic examinations were performed on a
variety of tissues and organs in all animals. Over the course of the
studies, mortality was reported, primarily in the 400 mg/kg dosage
group. Hyperactivity was noted in all treated animals. It was
observed that at all dosage levels, the phenthoate formulation was
severely irritating to the skin and probably contributed to the
hyperactivity. Muscular fibrillations were observed in all animals at
the highest dose level. Significant body weight loss was observed in
all treated animals at the highest dose level. There were no unusual
effects noted in the haematologic, clinical chemistry, or urinalysis
parameters.
Significant erythrocyte cholinesterase was observed at 75 mg/kg in
both abraded and non-abraded groups. Plasma cholinesterase was only
slightly affected, predominantly toward the end of the treatment
period. The effect of phenthoate on blood cholinesterase appeared to
be cumulative, reaching maximal levels towards the end of the
treatment interval. After the dosing ended, plasma cholinesterase
recovered rapidly while erythrocyte cholinesterase, depressed at the
conclusion of the study, was still approaching normal 21 days after
the last treatment. No inhibition of cholinesterase was observed at
20 mg/kg.
There were no significant gross or microscopic changes noted, with the
exception of dermal changes characterised by pale red erythema, slight
to moderate edema, moderate desquamation and superficial escharosis.
Microscopic examination also revealed dermal changes in the epidermis,
predominantly at the high dose level. There were no other gross or
microscopic changes attributable to the administration of phenthoate
(Brett et al, 1974a; 1974b; Paa et al, 1974).
Groups of rabbits (2 male and 2 female rabbits/group) were
administered phenthoate dermally, to the intact or abraded skin, 5
days/week for 3 weeks at dosage levels of 0, 10, 50 or 100 mg/kg bw.
Growth, physical appearance and behaviour, food consumption, clinical
chemistry, haematology, and urine analyses were performed. At the
conclusion of the study, absolute and relative organ weights were
obtained on gross examination. Microscopic examinations of tissues
and organs were also performed.
Mortality was observed, as 1 male and 1 female administered 100 mg/kg
(intact skin) died during the course of the study. These animals had
shown severe signs of poisoning and mortality was attributable to the
phenthoate treatments.
An apparent anaemia (normocytic) was observed predominantly in the
survivors of the high-dose group. It was characterized by reduced
haematocrit, haemoglobin and red blood cell concentrations. A
dose-related reduction of cholinesterase activity was also noted. Red
blood cell, plasma and brain, cholinesterase activity were depressed
at all dose levels. No other significant effects were noted with
respect to clinical chemistry, haematology, and urinalyses. Organ
weight data as well as gross and microscopic pathology were not
unusual. Other than severe dermatitis, there were no pathological
effects noted in the study (Serota et al, 1979).
Rat - dietary
Groups of rats (10 male and 10 female, Donryu rats/group) were fed
phenthoate in the diet at dosage levels of 0, 5, 10, 30, 100, 300 or
1000 mg/kg for 3 months. Animals were observed daily for behaviourial
changes and growth was recorded at weekly intervals. At the
conclusion of the study, haematological, blood chemistry, and urine
analyses were performed. Animals were sacrificed at 3 months and
gross and microscopic examinations of the tissues and organs were
performed.
While there were no gross toxic signs of poisoning, growth, especially
in male rats fed 1000 mg/kg was reduced. Females receiving 1000 mg/kg
in the diet displayed a slight depression of body weight during the
course of the study. Food consumption data were normal. At 1000
mg/kg, there was a significant change in total and differential white
blood cell counts in both males and females. Urinalysis data, with
the exception of slight changes in sodium excretion values in both
males and females (males increased, females decreased) at 1000 mg/kg,
did not show changes attributable to the presence of phenthoate in the
diet. Slight blood chemistry changes were also observed at 1000 mg/kg
with respect to several parameters (SGOT, BUN, A/G ratios, and
cholinesterase activity). With the exception of cholinesterase
depression, the other clinical chemistry parameters were not
substantially different from control values.
Erythrocyte cholinesterase was significantly depressed in both males
and females at 30 mg/kg and above. At 10 mg/kg, a slight reduction of
activity was reported (less than 25%). Significant inhibition of
plasma cholinesterase was noted only in male rats fed 1000 mg/kg.
There was no significant inhibition of brain cholinesterase at 1000
mg/kg.
Gross and microscopic examinations of tissues and organs showed no
significant effects attributable to the presence of phenthoate at
dosage levels below 1000 mg/kg. At 1000 mg/kg several major organs in
both males and females showed significant weight depression: thymus,
heart, adrenal glands, testes, and ovaries. In males, the weight of
liver, kidney, spleen, and seminal vesicles was decreased while in
females, the weight of the heart, lung, and uterus was decreased. The
gross changes observed at the conclusion of the study were not
reflected in abnormalities noted on microscopic histopathologic
examination.
Based on these studies, a no-effect level is 10 mg/kg (Toyoshima et
al, 1973).
Mice
Groups of mice (10 male and 10 female ICR- mice/group) were
administered phenthoate in the diet at dosage levels of 0, 5, 10, 30,
100, 300 or 1000 mg/kg for 3 months. Growth, as observed by body
weight changes, was recorded on a weekly basis. Food and water
consumption data were recorded and at the conclusion of the study,
animals were sacrificed for haematological, blood chemistry, and urine
analyses. Gross and microscopic examinations of tissues and organs
were performed at the conclusion of the study.
There was no mortality observed over the 3-month interval. Growth was
depressed at 1000 mg/kg, primarily during the initial parts of the
study. After the first month, growth was normal in all test groups.
Food and water consumption were not affected by the presence of
phenthoate. Haematologic values in both males and females showed a
trend towards a reduction in the total and differential leucocyte
count in both males and females. This trend was not significantly
different from control values at dosage levels below 1000 mg/kg.
Urinalyses were normal as were all blood chemistry values with the
exception of cholinesterase activity. Significant plasma
cholinesterase depression was observed only at 1000 mg/kg. At 100
mg/kg and above, erythrocyte cholinesterase was inhibited. Brain
cholinesterase depression was not observed at any dose level.
On gross examination at the conclusion of the study, organ weight
changes were observed at 1000 mg/kg. Male mice showed a significant
depression of the thymus and adrenal glands, while female mice showed
significant depression of the weight of heart, lungs, thymus, liver,
kidneys, spleen, uterus, and ovaries. On histological examination,
the tissues of treated animals did not display pathological changes
attributable to the presence of phenthoate, suggesting that the gross
changes were physiological adaptation to the high dosage of
phenthoate. A no-effect level in the study, based upon cholinesterase
depression in both males and females, is 30 mg/kg in the diet
(Toyoshima et al, 1973).
Dogs
Groups of dogs (4 female and 4 male, beagle dogs/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg for
2 years. Daily examinations were made for clinical signs of poisoning
or adverse behaviour. Growth, as evidenced by body weight, was
recorded weekly as were food consumption data. At periodic intervals
(1, 3, 6, 9, 12, 18, and 24 months), haematology, blood chemistry
(including cholinesterase) and urine analyses were performed. Plasma
and erythrocyte cholinesterase determinations were performed at 2, 4,
6, 9, 13, 26, 78, and 104 weeks and brain cholinesterase analyses were
conducted at the conclusion of the study. At the conclusion of 104
weeks of dietary administration, each dog was sacrificed and gross and
microscopic examinations of tissues and organs were performed.
There was no mortality over the course of the study at any dosage
level. Growth and food consumption were normal. With the exception
of cholinesterase depression, data from blood clinical chemistry
tests, haematological tests, and urinalyses were normal. Erythrocyte
cholinesterase inhibition was observed at 30 mg/kg and above. This
effect was first noted 2 weeks after the initiation of the study and
was relatively constant over the entire study. Plasma and brain
cholinesterase activity were unaffected and considered normal at all
dietary levels. Gross and microscopic examinations of tissues and
organs at the conclusion of the study did not show any significant
effect of the inclusion of phenthoate in the diet at dose levels up to
and including 100 mg/kg. A no-effect level in this study, based on
cholinesterase depression, was 10 mg/kg in the diet equivalent to 0.29
mg/kg bw, a value derived from the average food consumption values
over the 2-year period (Nelson et al, 1972).
Long-term study
Mice
(See special study on carcinogenicity).
Rats
No data available.
RESIDUES IN FOOD
USE PATTERN
Phenthoate is a wide spectrum insecticide for agricultural and
domestic use. It is active against both chewing and sucking insects,
in particular against lepidopterous larvae, soft scales and larvae and
adults of some species of mosquitoes. The pesticide should be applied
in enough water to insure complete coverage of the foliage, branches
and fruit when applied as a ground application.
The registered use patterns reported from Italy, Israel, Japan and
South Africa are summarised in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
The trials were performed in Brazil, Florida, Italy and South Africa
with both the recommended and approximately double dose rates. The
results are summarised in Tables 5 and 6. Treatments carried out with
low volume spraying (about 940 l/ha) or with 0.5% oil additive
resulted in somewhat higher residue than the dilute application, when
the spray was applied until run off to achieve full coverage.
The repeated treatments carried out following the recommended use
pattern did not increase the residue in the fruit significantly. The
colometric method used in 1963-65 experiments showed 3-4 times higher
residues than those obtained with GLC determination of samples
deriving from similarly treated plots at later trials.
The phenthoate residues remain entirely in the peel of fruits. The
pulp did not contain detectable residues (limit of determination
0.02-0-03 mg/kg) at any time after treatment with the exception of two
sets of experiments. In one case about 10-25% of the residue found in
the peel was detected in the pulp at the day of treatment and after 98
days, respectively. However in the other experiment the residue in
the pulp was only about 2.55% of the residue found in the peel and
decreased with time.
The phenthoate oxon was only detected in the peel. Limit of
determination in the pulp was 0.01 mg/kg (Moye). The oxon derivative
amounted to a maximum of 0.12 mg/kg (43%) on lemon, 0.08 mg/kg (15%)
on grapefruit and 0.62 mg/kg (38%) on orange. The values in
parentheses refer to the ratio of oxon to the parent compound. The
results of other trials (Iwata, 1979) indicate similar residue ranges.
The residue, on a whole fruit basis, can be calculated from the
residue measured in the peel taking into account the peel/pulp weight
ratio which is fairly constant for different varieties (Iwata, 1977).
For instance, in the case of valencia orange 1/5 of the peel residue
can be considered as the residue on whole fruit basis, while for the
variety Hamlin, the ratio is 1/4.
Other Crops
A limited number of supervised trials were carried out on apple, pear,
cabbage, lettuce, olives, onions, radish, spinach, sweet potato and
sugarbeet in Italy and Japan in 1962-65. The results of these
experiments were provided to the Joint Meeting for evaluation (Tables
7 and 8). However they were not considered to represent the current
use of phenthoate or to be sufficient for evaluation, thus no
TABLE 3. Registered Uses of Phenthoate
Crop Pest ai [g/100R] Formulation No. of treatments Amount of water l/ha
Citrus Scale insects ) 50 - 100 EC 1 - 2 1000-10000
Aphids ) 40 WP (generally)
Orange leaf miner )
Leaf rollers )
Apple Codling moth 40 - 50 EC 5 - 10 1000-2500
WP 1 - 6 5000
Brassica Large white butterfly 50 - 1000 EC 1 or more up to 1000
Japanese pears Leaf rollers 40 - 50 WP 1 - 6 3000
Fruit moths
Mealy bugs
Nuts Codling moth 70 EC 3 - 4 2000-10000
Olives Black scale 60 - 75 EC 3 - 4 2000-10000
Olive thrips
Onions Thrips 75 EC 1 or more up to 1000
Pears Codling moth 40 - 50 EC 3 - 7 1000-2000
Pear psylla 200 250 200 - 500
Potatoes Potato tuber moth 100 EC 1 or more up to 1000
Rice (paddy) Rice stem borers ) 50 - 100 EC 1 - 4 800 - 1500
Leaf and plant )
hoppers ) 1.65 - 3.3 kg EC 1 30-40 aerial
Rice bugs ) 0.6-0.8/kg/ha. Dust
Rice leaf beetle )
Rice leaf miner )
recommendation was made by the Meeting for the maximum residue levels
in/on these commodities.
FATE OF RESIDUES
In animals
Lactating dairy cattle were administered 14C-labelled phenthoate at
levels of 1, 5 and 20 mg/kg on feed basis (Wargo, Anonym. 1979).
Samples of urine, faeces and milk were collected every day during the
administration for 8, 18, 26 days and withdrawal (7 days).
The animals were slaughtered the following day and the tissue samples
were taken. The total 14C residue measured in various tissues and
calculated as parent compound at a dose rate of 20 mg/kg is given in
Table 9. Muscle and fat did not contain detectable residues at rates
of 1 and 5 mg/kg. The residue in the other tissues also decreased at
the lower rates.
Total 14C extractable residues in liver and kidney were 29% and 51%
respectively, and consisted of organosoluble and watersoluble
metabolites.
The metabolites were separated with various chromatographic methods.
In the liver at least eleven compounds were found, of which the major
component represented 8% of the total 14C residues in liver. One of
the remaining ten was present at a higher concentration than 3%. The
unextractable residue was quantitatively released by acidic
hydrolysis. At each feeding level the cows with a seven-day
withdrawal period demonstrated a significant decrease in residues
compared to the cows sacrificed 24 hours after the last 14C
phenthoate dose. Even the "bound" metabolites were eliminated from
the tissues of kidney and liver.
The 14C residues in milk reached the plateau on the second or third
day of treatment. During the feeding period the total residue
plateaued at the levels given below:
Level in diet Residue in milk, mg/kg
1 mg/kg 0.001-0.003
5 mg/kg 0.008-0.026
20 mg/kg 0.015-0.04
The residue declined after withdrawal of the pesticide from the diet
and approached the limit of determination after 3-6 days.
The 0.04 mg/kg total 14C residue in milk consisted of 7% (0.0028
mg/kg) organosoluble, 16% (O.006 mg/kg) water-soluble compounds and
52% (0.019 mg/kg) milk solids.
The residue level in each fraction was too low for further
investigation.
Neither phenthoate nor its oxon could be detected by conventional
analytical methods based on GLC determination in any of the tissue or
milk samples (limit of determination 0.01 mg/kg for fat and 0.05 mg/kg
for the other samples).
White leghorn laying hens were given feed containing 14C phenthoate
at 1.8, 5.9 or 26 mg/kg for 30 days. The birds were sacrificed on
days 1, 3, 7, 14, 21 and 30 during the treatment and on days 7 and 15
during the withdrawal period (Huhtanen, 1979a). The maximum residue
contents of tissues analyzed are given in table 4.
TABLE 4. Residues of phenthoate in tissues of chickens fed phenthoate
in the diet at various levels for 30 days
Tissue Concentration of phenthoate in diet
1.8 mg/kg 5.9 mg/kg 26 mg/kg
breast muscle 0.333 0.072 0.073
leg muscle 0.018 0.035 0.13
liver 0.024 0.04 0.48
kidney 0.094 0.53 1.3
skin 0.039 0.16 0.38
fat 0.019 0.07 0.16
eggs 0.014 0.058 0.35
limits of detection
for non-fatty tissues 0.0077 0.027 0.066
for fatty tissues 0.016 0.053 0.13
A plateau of the residue was reached in liver and kidney between the
third and seventh day of treatment. In the muscles detectable
residues were found from 21st day of treatment but no residue was
detected during the withdrawal period either in the muscles or in the
eggs. Less than 10% of the extracted material, which accounted for 70
of total radioactivity, was of non-polar character in the kidney. A
large percentage (50%) of the more polar compounds were acidic.
No abnormalities were observed in the organs of the hens and the
production of eggs was not reduced during the treatment period.
Fish residue studies were carried out with channel catfish and
bluegill sunfish in order to determine the biomagnification behaviour
of phenthoate.
In water containing 14C-phenthoate at 10-6 mg/l level the average
concentration ratios from catfish tissue/mud and catfish tissue/water
were 0.17 (0.09-0.33) and 5.76 (2.47-9.97) respectively after 33 days.
Residue levels in contaminated fishes showed a gradual reduction if
the fishes were placed in uncontaminated water (Marshall, 1979).
The uptake and bioaccumulation of 14C-phenthoate residues from soil
aged for 21 days in the aquaria into channel catfish were studied at
fortified soil levels of 0.01 and 1 mg/kg. The residues in the
catfish "meat" were 0.003 mg/kg 28 days after placing them into the
contaminated water (Booth, 1978).
Bluegill sunfish were exposed to 14C-phenthoate at 2.1 × 10-5 mg/l or
at 2 × 10-4 mg/1 in the water of a simulated dynamic water system for
30 days. The average bioaccumulation ratio of 14C was 816 and 901
respectively, while for the edible tissue 240 and 248 were the
calculated values. The residues found in tissues of 14-day withdrawal
samples taken from the higher level tank was only 3% of the level
found after 30 days of exposure (Huhtanen, 1979b).
In plants
Selected leaves and fruit of a Valencia orange tree were treated by
brush application with a solution of 30 mg phenthoate [32P or 14C] in
50 cm of water containing 0.06% emulsifier (Takade, 1977). Samples of
leaves and fruits were taken 3, 7, 10 and 14 days after treatment and
rinsed 5 times with acetone to remove the surface material. After
rinsing with acetone, the fruit was carefully peeled. The leaves,
peel and pulp were cut into small pieces and placed in a vacuum oven
at 40°C for approximately 10 hours. No loss in radioactivity was
detected for the drying process. The dried samples were extracted
with acetone. The metabolites were separated on thin layer plates
using four solvent systems. The total radioactivity of solid
materials was then determined by combustion analysis.
The major portion of phenthoate applied to leaves was lost by
volatilisation. The concentration and distribution of residue in
orange fruit are given in Table 10. Significant loss in total
recovery wan observed in fruit. Less than 1% of the total activity
applied was detected in the orange pulp. The majority of the residue
both in fruit and in leaves was intact phenthoate. The ratio of
phenthoate and its phenthoate oxon is shown in Table 11. Other
metabolites, desmethyl phenthoate, mandelic acid, bis-alpha-ethoxy
carbonylbenzyl disulphide, O,O-dimethyl phosphorodithioic acid, O,O-
dimethyl-phosphorothioic acid and two unidentified compounds amounted
to 0-5.2% individually. Glucosidase, hydrochloric acid and sodium
hydroxide together dissolved about 92-98% of the bound residues in the
peel. The major conjugated metabolites were, in order of importance,
ethyl mandelate, mandelic acid, desmethyl phenthoate and phenthoate
acid (Mallipudi and Fukuto, 1980).
Apple, pear and olive trees were treated with 32P-labelled phenthoate
according to the recommended use pattern (Santi, 1967). The surface
of the fruits was washed with methanol to remove the original active
substance or any other derivatives containing methanol-soluble 32P
still present on the peel (methanol extract).
After washing, the various fruits were homogenised in a blender with
chloroform (chloroform extract).
The plant filter cake was further extracted with water to separate
eventual water-soluble compounds (water extract). The 32P content of
various extracts and of residual plant was determined radiometrically.
The phenthoate equivalents of the radioactive residues found at
certain times after treatments are listed in Table 12.
Column chromatography, thin-layer chromatography and paper
chromatography were used for the separation and identification of the
compounds in the various extracts.
The probable metabolic pathways are shown in Fig. 2.
The repeated treatments of apple trees with a solution containing
0.02% ai (half the dosage usually applied) resulted in qualitatively
similar picture regardless the number of treatments. The phenthoate,
although not systemic, penetrates slightly into the fruits and
undergoes oxidation and hydrolysis; 15-29 days after treatment 95% of
water-soluble residue was phosphoric acid while monomethyl and
dimethyl phosphoric acids were detected in traces. Phenthoate oxon
was detected in traces only 1 day after treatment.
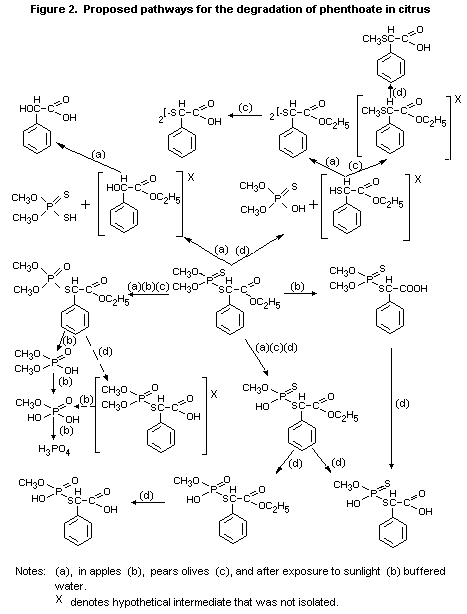 The metabolism of phenthoate in the two varieties of pears showed a
slightly different pattern. Phenthoate acid,
O,O-dimethyl-thio-phosphoric acid, O,O-dimethyl dithio phosphoric
acid, dimethyl phosphoric acid and phosphoric acid were identified and
four other unidentified compounds were found in the "water-soluble"
extract. The quantity of any of them did not reach 0.1 mg/kg
phenthoate equivalent. Intact phenthoate in varying amounts, traces
of P=O derivative and unidentified compounds were found on the surface
of olive at different intervals after treatment. Phenthoate
penetrated into drupes with subsequent hydrolysis and oxidation
process and formation of the same metabolites as found in other
fruits. The processed oil contained only intact phenthoate (0.14
mg/kg), while in olive husks 0.23 mg/kg phenthoate and 3 mg/kg of
other 32P containing compounds were detected.
A 0.05% emulsified solution of 14C-labelled phenthoate was sprayed on
cabbage seedlings and brushed over Hime apple fruits and also
strawberries. Phenthoate remained mostly on the surface and only
3.3-6.7% of the total amount penetrated into the plants. The residue
decreased rapidly both on the surface and in the plants. Eight days
after treatment the residue on the surface of cabbage consisted of 86%
intact phenthoate, 8% bis-alpha-carbethoxy-benzyl disulphide, 1.9%
ethyl mandelate and 1.3% unidentified compound. Phenthoate oxon was
detectable in traces (0.4-0.7%) during the first day after treatment.
Within the cabbage seedlings 8 days after treatment intact phenthoate
was present in an amount of 10.2% and the metabolites identified were
mandelic acid (46.9%), bis-alpha-carbethoxy-benzyl disulphide (6.5%),
phenthoate acid (4.8%), desmethyl phenthoate (3.2%), phenthoate oxon
(1.8%). Six unidentified compounds were separated (total amount
18.8%) of which two accounted for 14.3%. The degradation of
phenthoate in/on apple took place more slowly than in/on cabbage, or
strawberry, while the metabolite patterns were rather similar in case
of apple and strawberry to that found in/on cabbage seedlings (Hirose
et al, 1971).
In soil
The degradation of ring-labelled 14C-phenthoate in a moist (50% of
capacity) loam and silty clay loam soil was studied (Iwata, 1977b).
Phenthoate was rapidly degraded by heat-labile soil enzymes which
converted it to phenthoate acid under both aerobic and anaerobic
conditions, even with a soil treatment of 100 mg/kg. Under aerobic
conditions and in low concentration the phenthoate acid underwent
extensive microbiological degradation to CO2 (up to 50% of the
theoretical value) and polar products. At 100 mg/kg level or under
anaerobic conditions it degraded by first order kinetics, presumably
by simple hydrolysis.
In a loam soil phenthoate at 1.25 and 2.5 mg/kg had no effects on the
rates of degradation of 14C-cellulose or 14C-starch by soil microbes
over a 25-day period as measured by the evolution of 14C. The rate
of nitrification in soil amended with (NH4)2SO4 was likewise
unaffected over a 28 days period (Sikka, 1979).
Phenthoate appears to be relatively persistent under dry soil
conditions (Iwata, 1977a) and in dry dust adhering to plant surfaces
(Iwata, 1975). In two sets of experiments 95% and 62% of the initial
doses remained unchanged after 59 and 75 days respectively.
The results indicate that conditions favouring microbial activity,
such as adequate moisture and warm temperatures, make soil type of
secondary importance for phenthoate degradation.
The fate of residues was also studied under field condition (Moye et
al, undated). Soil samples were taken from the drip-line and middle
at 0-6 and 6-12 cm depths under the orange trees treated with Cidial 4
E solution of 0.03% ai 1, 3, 7, 14 and 28 days after treatment. Four
samples were taken from both depths for each time. The maximum of
phenthoate concentration was observed at the drip-line on the third
day (1 mg/kg, in 0-6 cm and 0.68 mg/kg in 6-12 cm).
The mean residue at 0-6 cm was 0.56 mg/kg which decreased to 0.12
mg/kg 28 days after treatment.
Phenthoate oxon was not detectable in any of the experiments. The
mobility of phenthoate and its soil metabolites were studied by
leaching experiments using sandy soil (organic matter 1.6%), silt loam
(o.m. 6%), sandy loam (o.m. 3.3%), clay loam (o.m. 5.9%) (Anonymous,
1978a). The columns were filled to 25.4 cm with the untreated soils
after prewetting the column with water. The fortified soil (5 cm) was
layered over the untreated soil. For studying the mobility of
metabolites the fortified soils were aged for 30 days under greenhouse
conditions. Water equivalent to 500 mm rain for parent compound and
to 380 mm for metabolise was allowed to pass through the column and
analyzed. The columns were sectioned into four equal lengths for
analyses by the measurement of radioactivity. The upper 15 cm of the
sandy soil contained 72.3% of phenthoate and 85.7% of the metabolites
while the other soils retained 87.3-88.8% and 89.4-90.9% respectively.
The water effluent from the light sandy soil contained 8.32% and 6.62%
of the original activity. From the other soils the 14C activity in
the water effluent amounted to 2.9-6.44% and 3.39-4.21%.
In water
14C phenthoate was added to 0.01 M sodium phosphate buffers (pH 6.0,
7.0 or 8.0) to give a final concentration of 1.5 × 10 -5 M. Aliquots
were taken 3, 7, 10, 14, 21 and 28 days after addition of the 14C
material to the buffer and analyzed an TLC plates subsequent to
appropriate preparation.
32P-phenthoate was added to the buffer solutions in a 1 × 10-4 M
final concentration and was treated similarly to the 14C compound.
All solutions were held at 24.5 ± 1°C in a constant temperature room.
The pH of buffered solutions, containing radiolabelled phenthoate
under similar conditions, was monitored over the experimental period
and no pH change was detectable.
Evidently, phenthoate is fairly stable in water at the indicated pH
values. 45%, 21%, 22% of its initial amount were present after 28
days at pH 6, 7, 8 respectively. Its half life is about 12 days at pH
8.0. Phenthoate acid was observed in greatest amount (30-55%)
irrespective of pH. Hydrolysis of carbonyletoxy moiety appeared to be
fastest at pH 8.0. On the other hand the rate of formation of
desmethyl phenthoate, the second most prominent hydrolysis product,
was fastest at pH 6. Phenthoate oxon was not detected at any time.
Other products indicated in Figure 2 were observed in small amounts
(Takade, 1977).
Photodegradation
The photodegradation of 14C-phenthoate was studied in distilled water
(pH = 7.0±0.5) at concentrations of 8 mg/kg and 5 mg/kg (Anonymous,
1978).
A medium pressure mercury lamp, fitted with a Pyrex 7740 filter that
excludes light of wavelength less than 280 nm, was immersed in the
solution to be irradiated. After 5 and 8 hours of irradiation,
aliquots of the photolysed solutions were taken and analyzed applying
HPLC and TLC techniques. Approximately 50% of phenthoate had degraded
after 6-8 hours of irradiation, while there was no loss in another
phenthoate solution kept in the dark for the same time. Ethyl
mandelate was found to be the major degradation product. In addition
three unknown products were detected but each of them accounted for
less than 10% of the parent compound.
An acetone solution of radiolabelled phenthoate (32P or 14C) was
applied to glass plates, which were exposed to sunlight daily for 7
hours (Takade, 1977).
Air temperature during the exposure ranged from 7.2 to 23.3°C. Under
these conditions approximately 90% of the applied phenthoate was lost
after 40 hrs of exposure by volatilisation.
The unchanged phenthoate gradually decreased in proportion to the
other alteration product during the exposure (see Figure 2).
Phenthoate oxon was identified as the major product. Other products
isolated were desmethyl phenthoate, mandelic acid, bis-[alpha-carboxy)
benzyl]-disulphide, bis[alpha-/carbethoxy/benzyl]disulphide and
O,O-dimethyl phosphorothioic acid. Results of other experiments
indicated that elevated temperatures may speed up the phenthoate oxon
formation and its disappearance. The latter is also facilitated by
rainfall (Nigg and Stamper, 1980).
The degradation products formed upon exposure to sunlight and air on
glass plates or found on citrus leaves and fruits are essentially the
same.
In processing
The effect of processing was studied on orange, lemon and grapefruit
treated in the field twice with Cidial 4 E in solutions of 0.03% or
0.06% ai. The samples were taken 50, 19 and 18 days after last
treatment respectively and were processed with a FMC processor. The
residue ranges found in 4 samples taken at each stage are summarised
in Table 13 (Moye et al, undated).
Little, if any, residue decrease occurred from washing of oranges and
lemons, which is in good agreement with the findings of Iwata et al,
who carried out laboratory washing to simulate packing house
treatment. On the other hand laboratory washing of the grapefruit
removed about 50% of the residue found 3 days after treatment and from
about 25% to less than 10% thereafter.
Phenthoate seems to be concentrated in the waxes and oils of the rind.
Fruit juice contained negligible residues (<0.01 mg/kg). Residues
found in the dried rind, which is used as a cattle feed, show an
approximate 44-58% loss of residue during processing and drying.
The majority of the residue found in/on olives remained in the olive
husk and the processed oil contained only intact phenthoate (4.1% of
total residue) (Santi, 1976).
METHODS OF RESIDUE ANALYSIS
Methods that have been developed for the determination of phenthoate
in crops and animal tissues have been reviewed. Bazzi (undated)
described a method which is based on acetone extraction, liquid-liquid
partition clean up on a Plorex column and gas chromatographic
determination using 3.8% UCCW 982 on Gas Chrom Q packing and a flame
photometric detector. The limit of determination is 0.04 mg/kg or
less depending on the type of sample. The method is suitable for the
determination of phenthoate and its oxon in citrus and their processed
by-products in maize, cattle meat and soil.
Moye et al (undated) found acetone: acetonitrile 1:1 mixture most
efficient for the extraction of phenthoate from soil, fruits and
by-products of citrus processing. OV-101, OV-17 (Iwata et al, 1979)
and OV-225 (Moye et al, undated) liquid phases were used to separate
phenthoate, phenthoate oxon from other organophosphate pesticides also
applied in citrus orchards.
Another method for the determination of residues in animal products
includes extraction by acetonitrile from muscle, kidney and liver, by
acetone and benzene from milk and by heptane from fat; partition with
benzene, hexane and acetonitrile; column chromatography on Floricil;
and GLC determination using 3% OV-101 packing with FPD. Limit of
determination: 0.01 mg/kg for milk, 0.05 mg/kg for tissues. The
recovery at 0.05-0.1 mg/kg level is about 92% for all samples.
The basic method described by Bazzi (undated) in combination with the
other liquid phases and slight modifications proposed by the other
authors is suitable for regulatory purposes.
NATIONAL MRL'S REPORTED TO THE MEETING
Phenthoate is registered in 36 countries all around the world. Some
of the maximum residue limits and pre-harvest intervals were reported
to the meeting.
Pre-harvest
intervals MRL
(days) (mg/kg)
Hungary apple, pear,
sugar-beet 21 0.5
Italy apple, pear, walnut,
vegetables, rice 20 0.3
citrus, olive 60 0.3
Israel oranges, grapefruit 20-30
Japan pear, peach 7 0.1
pumpkin 1 0.1
mandarine, orange 14 0.1
rice (unpolished) 7 0.05
japanese pear 30 0.1
Netherlands citrus fruit 0.5
New Zealand brassica crops 0.7
South Africa citrus 21 1.0
brassica 3 1.0
onion,potato 7 0.1
Sweden citrus 1.0
USA citrus 2.0
TABLE 5. Residues of Phenthoate in oranges
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia USA 1973 1 8.5 50 L 0.8 0.5 0.4 0.3 0.2 0.1 0.1 Iwata, 1977a
(Florida) 1 4.3 50 L 0.5 0.3 0.2 0.1 0.09 0.09 0.07
Valencia USA 1979 1 4.2 4 EC 2.9 2.3 1.5 0.58 0.23 Iwata, 1979
(peel) (Florida) 1 8.4 4 EC 4.0 3.0 2.1 0.85 0.35
1 8.41 4 EC 6.3 4.2 3.0 1.4 0.38
Valencia USA
(peel) (Florida) 1979 2 0.03%3 4 EC 1.65 0.67 0.29 0.38 Moye et al
(pulp) 0.06% 0.03 0.04 0.01 0.01 (undated)
Hamlin Brazil 1979 1 0.1% 50 L 1.38 0.79 0.5 0.33 0.22 0.22-0.12 Camargo,1980
(peel)
Valencia Italy 1965 1 0.06%1,2 50 L 4.9 1.6 1 1.1 0.8 0.6 Anonym, 1965
Moro (peel) Italy 1963 1 0.05%1,2 50 L 3.2 1.8 1.2
Valencia Israel 1973 1 7.5+0.5% oil 50 L 1.66 1.08 0.5 0.2 Greenberg
1 10 50 L 0.71 0.58 0.04 0.09 0.02 et al, 1974
1 20 50 L 2.1 1.33 0.75 0.37 0.12
Shamouti Israel 1973 1 7.5 50 L 1.02 0.98 0.45 0.21
1 7.5+0.5% oil 50 L 0.98 1.08 0.4 0.36
1 15 50 L 1.68 1.41 0.82 0.4
Orange Japan 3 0.05% 50 EC 0.01 0.008 0.004 Anonym, 1980
(pulp) 5 0.05% 0.01 0.009 0.009
3 0.05% 0.02 0.005 0.008
5 0.05% 0.015 0.006 0.005
TABLE 5. Continued...
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia S. Africa 1970 1 0.213% 50 L 3.7 2.05 1.1
1971 1 0.1% 50 L 1.21 0.56 0.32 0.23 Anonym,
1970
Valencia S. Africa 0.37 0.3 0.12
(pulp)
1 Low volume application with a spraying volume of 940 l/ha
2 Colorimetric method was used for the determination
3 Three months elapsed between applications; the maximum residue values found in four replicates are given
TABLE 6. Residues of Phenthoate in Grapefruit and Lemon
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-4 8-11 18-21 33-35 38-46 60
Grapefruit USA 1978 1 3.4 4 EC 0.94 0.77 0.48 Iwata, 1979
(peel) 1 6.7 4 EC 2.1 1.6 0.87
1 6.71 4 EC 3.9 2.5 1.4
1979 1 0.03%2 4 EC 0.9 0.5 0.31
0.06%2 1.8 0.77 0.59
(pulp) USA 1979 2 0.03+0.063,4 4 EC 0.007 0.008 0.011 0.002 <0.002 Moye et al, undated
Whole fruit Israel 1973 1 0.075% 50 L 0.97 0.47 0.23 1.131 Greenberg et al,
1 0-0.75%4 50 L 1.4 0.63 0.39 0.14 1974
1 0.075%+0.5% oil 50 L 0.84 0.79 0.93 0.1
2 0.075%+0.5% oil4 50 L 1.0 1.0 0.74 0.16
1 0.15% 50 L 1.25 1.5 0.41 2.7
1 0.75%4 2.0 2.0 0.57 0.42
Lemon USA 1974 1 7 50 L 1.2 0.8 0.6 0.4 0.3 0.3 Iwata, 1979a
(peel) 1974 1 1.8 50 L 0.5 0.3 0.2 0.2 0.1 0.1
1978 1 4.2 4 EC 1.5 0.9 0.47
1 8.2 4 EC 2.8 1.7 0.78
1 8.21 4 EC 3.8 3.3 2.2
1979 1 5 4 EC 3.7 2.7 1.1
10 4 EC 7 4.5 1.9 Iwata, 1979
10 4 EC 14 11 5.7
Whole fruit S.Africa 1973 1 0.04% 50 L 0.4 0.26 0.08 0.09 Anonym, 1970
0.1% 50 L 1.4 0.96 0.47 0.17
1 Low volume application with a spraying volume of 940 l/ha
2 Sprays were applied to achieve full coverage
3 17 days elapsed between applications
4 The maximum residue values found in four replicates are given.
TABLE 7. Residues of Phenthoate in Apple and Pear
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0 1 4-7 9 14-17 21-26 29-34 39-46
Apple Italy 19621 1 0.04% 50 L 2.8 1.19 0.47 0.22 0.16 0.11 <0.1 Anonym, 1972
1962 1 0.03% 50 L 1.57 0.78 0.34 0.18 0.13 0.1
19621 1 0.04% 50 L 1.6 0.93 0.36 0.1 <0.1
1962 4 0.04% 50 L 1.92 1.21 0.48 0.12 <0.1
Pear Italy 19631 1 0.04% 50 L 1.45 1.1 0.6 0.4 0.25 Anonym, 1973
1963 1 0.04% 50 L 1.9 1.7 0.54 0.3 0.1 <0.1
1963 6 0.05% 50 L 0.64 0.35 0.18
Japanese Japan 3 0.05% 0.3 0.11 0.05 Anonym, 1980
pear 5 0 05% 0.44 0.16 0.09
3 0.05% 0.48 0.16 0.03
4 0.05% 0.58 0.1 0.08
1 Colourimetric method was used for the determination.
TABLE 8. Residues of phenthoate in other crope resulting from supervised trials
Application Residues in mg/kg, at intervals (days) Reference
after application
Crop Country Year No. rate % formulation 0 1 6-7 13-14 20-21 21-26 34 70
volume (1)
Cabbage Italy 1965 2 0.05 50 L <0. 1 <0.1 Anonym.,
6 3.18 0.11 0.09 1965
Olives Italy 1963 1 0.05/3000 50 L 10.9 8.1 0.49 0.49 0.2 Anonym.,
1963
Onion Japan 2 0.05/1500 0.01 Anonym.,
4 0.01 1980
Radish 2 0.05/2000 0.005
4 0.05/1500-2000 0.005 0.005 0.005 0.005
3 0.05/1800
Spinach Japan 2 0 05/1500 0.015
3 0:05/1500 0.018
2 0 05/1500 0.005 0.015
3 0:05/1500 0.007 0.005
Tea/leaf Japan 2 0.05/2000 0.008
3 0.05/2000 0.04
2 0.05/3000 0.04
3 0.05/3000 0.04
Lettuce Japan 2 0.05/2000 0.08 0.08 0.02
4 O.05/2000 0.06 0.05 0.09
TABLE 9. Carbon 14 residues in bovine tissue after feeding at a
20 mg/kg level in the diet
mg/kg in tissues after feeding for
Tissue 8 days 18 days 26 days 26 days
7 days
withdrawal
Muscle 0.013 <0.033 <0.033 <0.033
Fat 0.016 0.056 0.045 <0.045
Kidney 0.324 0.408 0.502 0.116
Liver 0.238 0.228 0.297 0.140
Heart 0.028 0.061 0.087 0.052
Brain 0.036 <0.042 0.054 <0.042
TABLE 10. The penetration of 14C and 32P phenthoate into orange
fruit, and the distribution of radioactivity in the surface wash,
acetone extract and solid residue.
Percentage of the applied radioactivity
recovered at indicated time (days)
0 3 7 10 14
14C-labelled phenthoate
External surface wash 72.5 66.1 9.8 8.1 4.6
Internal acetone extract1 - 5.4 15.6 12.8 7.8
Solid residue1 27.5 21.7 21.6 34.7 28.3
Total recovered 100.0 93.2 47.0 55.6 40.7
32P-labelled phenthoate
External surface wash 96.4 63.4 17.4 13.6 11.6
Internal acetone extract1 - 5.9 22.0 24.2 18.2
Solid residue1 3.6 19.4 13.9 16.3 13.2
Total recovered 100.0 88.7 53.3 54.1 43.0
1 Includes peel only. Radioactivity in pulp was less than 1% of
the applied.
TABLE 11. Amount of 14C metabolites found in the surface wash and
acetone extract of orange leaves after indicated time intervals
Compound found in Percent of recovered radioactivity
indicated material after indicated time (days)
3 7 10 14
Phenthoate
Surface wash 84.4 45.5 35.4 41.6
Acetone extract 4.6 46.1 37.7 17.0
Phenthoate
Surface wash 0 0 2.8 5.1
Acetone extract 0.2 0 7.2 12.4
TABLE 12. 32P Phenthoate equivalents (mg/kg) in treated apples, pears and olives
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Apples: var. Stark Delicious (0.02% solution)
2 hrs. 1.00 0.21 0.17 0.04 - - 1.17
1 day 0.26 traces 0.31 0.06 - - 0.57
15 days 0.02 - 0.08 0.02 0.95 0.80 0.10
29 days 0.00 - 0.00 - 0.96 0.69 0.00
Pears: var. Trionfo di Vienna (0.04% solution)
2 hrs. 1.35 traces 0.19 0.02 - - 1.44
1 day 1.19 traces 0.40 0.05 - - 1.59
4 days 0.33 - 0.26 0.09 - - 0.59
9 days 0.19 - 0.12 0.07 0.43 0.18 0.31
31 days1 0.006 - 0.01 0.02 0.16 0.09 0.016
Pears: var. Passacrassana (0.045% solution)
2 hrs. 1.23 traces 0.03 traces - - 1.26
1 day 1.01 traces 0.07 traces - - 1.08
5 days 0.55 - 0.05 - 0.04 0.02 0.60
25 days1 0.28 - 0.16 - 0.39 0.07 0.44
40 days2 0.20 traces 0.10 traces 0.34 0.14 0.30
TABLE 12. Continued...
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Olives /oils-yielding/: var. Leccino (0.05% solution)
2 hrs. 11.16 traces 0.15 0.01 traces - 11.31
1 day 5.92 traces 1.50 0.36 0.10 - 7.42
9 days 0.36 0.18 0.53 0.38 3.60 0.84 0.89
30 days 0.19 0.03 0.06 0.35 2.58 0.86 0.25
70 days 0.05 - 0.10 0.05 3.23 0.59 0.15
1 Fruit picking
2 Pears of this winter variety were analysed after picking from trees
and stored for 15 days at 19-20°C.
TABLE 13. Phenthoate residue (mg/kg) detected in processed citrus by-products
ORANGE LEMON GRAPEFRUIT
Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon
Unwashed peel 0.24-1.00 0.06-0.09 0.08-0.28 0.06-0.12 0.031-0.74 <0.01
Washed peel 0.23-0.73 0.036-0.069 0.15-0.32 <0.01 0.23-0.51 <0.01-0.09
Unwashed pulp <0.002-0.031 0.01 0.003-0.024 <0.01 0.007-0.031 <0.01
Washed pulp 0.005-0.011 0.01 0.002-0.004 <0.01 0.02-0.09 <0.01
Chopped peel 0.34-0.67 0.01-0.03 0.08-0.26 0.03 0.26-0.49 0.002-0.051
Peel frits 0.32-0.52 0.05-0.15 0.25-0.74 0.05-0.15 1.14-2.45 0.015-0.031
Finisher pulp 0.008-0.015 <0.01 0.008-0.023 <0.01 0.003-0.008 <0.01
Dried rind 0.13-0.43 0.01-0.036 2.2-2.7 0.01 0.72-1.51 0.06-0.21
Press liquor 0.08-0.14 <0.01-0.01 0.008-0.032 0.009-0.017 0.13-0.19 0.01-0.013
Emulsion water 0.004-0.07 <0.004-0.1 0.003-0.009 0.04-0.06 0.006-0.021 <0.01
Fruit juice 0.002-0.003 <0.01 0.004-0.009 <0.01 <0.002 <0.01
After-water rinse <0.002-0.004 <0.01 <0.002-0.005 <0.01 <0.002 <0.01
Pre-water rinse <0.002 <0.01 0.002 <0.01 <0.002 <0.01
Molasses 0.047-0.068 <0.01 0.01-0.03 <0.01 0.037-0.071 0.01
Oil 3.29-14.05 1.18-2.48 7.25-17.25 1.65-2.5 5.4-8.5 0.45-1.1
EVALUATION
COMMENTS AND APPRAISAL
Phenthoate is a wide-spectrum organophosphorus insecticide, mainly
used on citrus at rates of 0.6 to 5 kg ai/ha.
The technical material contains a minimum of 92% of phenthoate.
Detailed analyses of the typical technical material, including the
identity of the minor components, was provided. It is generally
soluble in most polar and non-polar organic solvents and also slightly
soluble in water.
Phenthoate is a cholinergic compound of moderately acute toxicity and
is rapidly absorbed, metabolized and excreted in mammals. The pattern
of metabolism is similar in both plants and animals and is similar to
that noted with other organophosphorus esters. The rate of metabolism
is shown to be significantly affected (with potentiation of toxicity)
by organophosphorus esters commonly found as impurities in the
technical product. The acute toxicity of phenthoate is dependant on
the purity of the technical product, a feature which raised
significant questions about occupational exposure. It was suggested
that a more purified product should be manufactured and used to
alleviate this problem. Formulations that are stored also have the
potential to become more toxic under certain conditions. Although
these factors do not directly affect phenthoate residues in food they
should not be ignored.
Phenthoate is moderately toxic to mammals. The acute LD50 was noted
to vary from 78 to over 4500 mg/kg bw, depending possibly on the
impurities and the species. Antidotal studies are not available.
Phenthoate does not induce a delayed neurotoxic reaction in hens, is
not mutagenic or teratogenic, nor does it interfere with reproduction,
as evidenced by a standardized 3-generation study in rats. In a
series of short-term toxicity studies, cholinesterase depression was
the most significant measure of effect of exposure. In dermal and
ophthalmological studies, phenthoate applied as a formulated product
was irritating. On the basis of available short-term studies, it is
concluded that phenthoate is a potent cholinesterase inhibitor.
A two-year study in the dog showing red blood cell cholinesterase
depression served as the primary basis to evaluate a no-effect level.
Phenthoate is not a carcinogen in mice. A two.year study in rats was
considered invalid and not included in the evaluation.
On the basis of available data, no-effect levels in mammalian species
were determined and a temporary ADI for man was allocated. This
evaluation was made on the assumption that the manufacture of
phenthoate would be by a process that would assure that a product of
at least 92% purity would be marketed. As this was the product on
which toxicological evaluations were made and as impurities have a
substantial toxicological significance this purity restriction was
important.
Phenthoate residues gradually disappear from treated plants within 4
to 10 weeks, most being lost by volatilization. Decomposition of
surface deposits is mainly due to photodegradation, resulting in
phenthoate oxon as the main metabolite. On cabbage, the surface
residue 8 days after application consisted of 86% phenthoate but no
oxon was detected; on orange leaves, 41.6% of the total residue was
phenthoate and 5.1% was the oxon at 14 days after treatment.
Phenthoate is not systemic but does enter the peel of fruit, where it
undergoes hydrolytic cleavage and oxidation. Virtually all of the
residue remains in the peel of treated citrus fruit, the pulp
containing less than 1% of the total residue. Phenthoate oxon has
been detected only in the peel.
The main metabolites in plants, phenthoate oxon, dimethyl phenthoate,
mandelic acid, bis-2-(carbethoxy) benzyl disulphide, O,O-dimethyl
phosphorodithioic acid, O,O-dimethyl phosphorothioic acid and
phenthoate acid, are similar to those secreted by animals. Their
amounts and relative proportions depend on the crop and the time
elapsed since application but, in practice, the parent phenthoate
should be considered as the only residue of importance.
Repeated treatments of citrus fruits or apples according to
recommended usage do not lead to increased residues or changed
metabolite patterns. The residue is contained mostly in the waxes and
oils of the citrus rind and is not decreased significantly by washing;
the fruit juice contains negligible residues. Dried rind as used for
cattle feed can contain 40 to 60% of the residue originally present in
the peel.
Laying hens fed for 30 days with feed containing 1.8 to 2.6 mg/kg of
phenthoate showed no reduction of egg production or any abnormalities.
Residues in the muscle and in eggs were low and disappeared after
withdrawal of the compound from the diet; in kidney and liver levels
reached a plateau between the third and seventh days of treatment.
Neither phenthoate nor its metabolites accumulated in fish kept in
water containing phenthoate at the 10-4 to 10-6 mg/l levels.
Lactating cows given 14C-phenthoate at levels of 1.5 and 20 mg/kg in
the feed excreted the material mainly in the urine and faeces. The
main metabolite was phenthoate acid, which accounted for 45% of the
14C activity of the urine. Residues in milk reached a plateau 2 to 3
days after administration began. The organosoluble fraction of the
residue in milk, possibly phenthoate, phenthoate oxon and unconjugated
metabolites, was very low (0.0028 mg/kg) even at the higher dosage
rate. Meat from the cattle contained traces of residues at the higher
dosage rate only, the muscle tissue and the fat containing the
residues in roughly equal proportions. Residues in various tissues
declined rapidly when the pesticide was withdrawn from the diet; even
the bound (unextractable) metabolites were eliminated from kidney and
liver. Neither phenthoate nor its oxon could be detected in tissue or
milk samples by conventional analytical methods at limits of
determination of 0.05 and 0.01 mg/kg respectively. It was concluded
that feeding cattle with citrus by-products prepared from
phenthoate-treated fruit would not result in detectable residues in
meat or milk.
In soil, phenthoate is rapidly converted to phenthoate acid under both
aerobic and anaerobic conditions, even when as much as 100 mg/kg is
added to the soil. Under conditions favourable for microbiological
activity, such as adequate moisture and warm temperature, the soil
type has no influence on the degradation of phenthoate. On the other
hand, phenthoate persists for a relatively long time under dry soil
conditions. Phenthoate and its metabolites show moderate mobility in
soil, the majority of the residue being contained in the upper 15 cm.
The likelihood of contamination of lower soil layers or underground
waters is very low in view of the high degradability and slow leaching
properties of the residues.
Analytical methods, based on GLC separation on columns of different
polarity with flame photometric detection are available and suitable
for regulatory purposes.
Level-causing no toxicological effect in:
Dog: 10 mg/kg in the diet equivalent to 0.29 mg/kg bw/day.
Mouse: 30 mg/kg in the diet equivalent to 4.5 mg/kg bw/day.
Rat: 10 mg/kg in the diet equivalent to 1.0 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that available information was adequate for the
estimation of maximum residue levels for phenthoate on citrus fruits
only. The data on other crops were not considered adequate for
estimating maximum residue levels. The levels apply to the parent
phenthoate excluding all metabolites.
Commodity Temporary maximum Interval between last
residue level (mg/kg) application and harvest
on which levels are
based
Citrus fruit 1 21
Carcass meat of cattle 0.051
Milk 0.011
Egg 0.051
1 At the limit of determination.
FURTHER WORK OR INFORMATION
Required (by June, 1984)
A chronic toxicity study in an acceptable mammalian species (rodent).
Desirable
1. Further information on impurities in the technical products in
order to evaluate the degree to which these impurities potentiate the
toxicity of phenthoate.
2. Data from supervised trials on other commodities in accordance
with present uses of the compound.
3. Residue data from crops, known to have been treated under
practical conditions, moving in commerce.
REFERENCES
Anonymous. Cidial Residues in Apples. Montedison S.p.A. Unpublished
report no. 77, (1962).
Anonymous. Cidial Residues in Pears, Olives. Montedison S.p.A,
Unpublished report nos.78, 79, (1963).
Anonymous. Cidial Residues in Oranges. Montedison S.p.A., Unpublished
report no 74/b, (1964).
Anonymous. Cidial Residues on Oranges, Cabbages. Montedison S.p.A.
Unpublished reports Nos. 76/b, 80, (1965).
Anonymous. Cidial Residues on Citrus Form Chem. Report Montedison
S.p.A. Unpublished report no. 74/a, (1970).
Anonymous. Determination of Cidial [Ethyl
alpha(/Dimethoxy-phosphinothioyl/thio) benzene acetate] in muscle,
milk, kidney, liver and fat. Thompson-Hayward Chemical Co., Montedison
S.p.A. Unpublished report no. 62, (1975).
Anonymous. Cidial Leaching Study on Parent compound and on its Soil
Degradation Products. Carried out by Thompson-Hayward Chemical
Company. Montedison S.p.A. Unpublished Report no 91, (1978a).
Anonymous. Photodegradation of Phenthoate in Aqueous Solution.
Submitted by Syracuse Research Corporation. Montedison S.p.A.
Unpublished Report no. 93, (1978b).
Anonymous. Interpretation and discussion on the metabolism of Cidial
in the dairy cow. Unpublished Report of Analytical Development
Corporation. Montedison S.p.A. Report No. 65, (1979).
Anonymous. Summary of Residue Data in Japan. Nissan Chemical
Industries Ltd. Unpublished reports, (1980).
Arnold, D., Kennedy, G.L., Kinoshita, F.K. and Keplinger, M.L.
Mutagenic Study with Cidial in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (No. 622-05876), reviewed and
validated by Bob West Associates, Inc. (Nov. 6, 1978), submitted to
the World Health Organization by Montedison.
Arnold, D., Kennedy, G.L. and Keplinger, M.L. Mutagenic Study with
Elsan (Phenthoate) in Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 632-05147), reviewed and validated by
Bob West Associates, Inc. (Feb, 8, 1980), submitted to the World
Health Organization by Montedison.
Bazzi, B. Phenthoate in Zweg G., ed. Analytical Methods for Pesticides
and Plant Growth Regulators. Academic Press, New York, Vol. VIII, p.
159.
Booth G.M. Bioaccumulation Study of Cidial in Channel Catfish.
Montedison S.p.A. Unpublished report no. 87, (1978).
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04413), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04524), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Camargo de., J.L.G. Residues de carbophenothione Phenthoate em cascas
e polpas de laranjos hamlin determinados por cromatografia em fase
gasosa. Dissertatio submitted to Luiz de Queiroz University, Sao
Paulo, 1980.
de Carneri, I. Microbiological Study of Mutagenesis on CRA 133 (GMA
267/78) Genetic Mutation Test in Salmonella Typhimurium (Ames). (1979)
Unpublished report from Farmitalia Carlo Erba, Research and
Development, submitted to the World Health Organization by Montedison.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
Neurotoxicity Study with Elsan in Chickens. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (no. 8580-80635), reviewed and
validated by Bob West Associates, Inc. (Jan. 25, 1980), submitted to
the World Health Organization by Montedison.
Good, J., Cooper, D. and Terrell, Y. Acute Delayed Neurotoxicity Study
of Cidial Technical 96% Batch 104 in Female Chickens. (1979)
Unpublished report from Cannon Laboratories, Inc. (No. 8E1191),
submitted to the World Health Organization by Montedison.
Greenberg, R. et al. Residues and Disappearance of Cidial in Valencia
Oranges. Montedison S.p.a. Unpublished Report No. 73/a, (1974).
Hammond, P.S., Talcott, R.E. and Fukuto, T.R. Metabolism and Mode of
Action of O,O,S-Trimethyl Phosphorothioate in the Rat. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #38, Division of Pesticide Chemistry, American
Chemical Society.
Hathaway, D., Keplinger, M.L. and Fletcher, D.B. Acute Inhalation
Toxicity Study with Phenthoate (Cidial) Technical in Albino Rats.
(1971) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(no. N-9324), reviewed and validated by Bob West Associates, Inc.
(Nov. 6, 1978), submitted to the World Health Organization by
Montedison.
Hirose, M. et al. Residue Degradation and Metabolism of 14C Labelled
Elsan(R) (O,O-Dimethyl-S-alpha-carboetoxy-benzyl phosphorodithioate)
in Cabbage, Hime Apples and Strawberries. (In Japanese). Scientific
Pest. Control 36: 43-51.
Huhtanen, K. Poultry Tissue and Egg Study with Cidial. Cammon
Laboratories Inc. Montedison S.p.A. Unpublished Report Nos. 84, 85,
(1979a).
Huhtanen, K. Accumulation of 14C Cidial in Bluegill Sunfish Exposed
in Dynamic Flow System. Cammon Laboratories, Inc. Montedison S.p.A.
Unpublished Report No. 88, (1979b).
Iwata, Y. et al. Behaviour of Phenthoate (Cidial) Deposits and
Residues on and in Grapefruits, Lemons, Lemon Leaves, Oranges and
Orange Leaves and in the Soil Beneath Orange Trees. J. Agric. Food
Chem. 25 (2): 382-388.
Iwata, Y., Ithig, M. and Gunther, F.A. Degradation of
O,O-Dimethyl-S-alpha-(carboethoxy)-benzyl phosphorodithioate
(Phenthoate), in Soil. Arch. Environm. Contam. Toxicol. 6: 1-12.
Iwata, Y. et al. Phenthoate Applied to California Citrus Trees: Worker
Environment Research and Residue Levels on and In Fruit. Report of
Department of Entomology, University of California. Montedison S.p.A.
Unpublished Report No. 72b, (1979).
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., Kinashita, F.K.
and Keplinger, M.L. Teratogenic Study with Cidial in Albino Rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
reviewed and validated by Bob West Associates, Inc. (Nov. 21, 1978),
submitted to the World Health Organization by Montedison.
Lee, S.G.K. and Fukuto, T.R. Effect of Impurities in Technical
Phenthoate (Cidial(R)) on Mammalian Carboxyesterases. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #35. Division of Pesticide Chemistry, American
Chemical Society.
Mallipudi, N.M., Badaway, S.M.A. and Fukuto, T.R. Inhibition of
Mammalian Esterases by O,O,S-Trimethyl Phosphorothioate. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #37, Division of Pesticide Chemistry,
American Chemical Society.
Mallipudi N.M. and Fukuto, T.R. Characterization of Bound Phenthoate
Residues in Citrus. Submitted to Archives of Environ. Contam.
Toxicol., 1980.
Marshall B.L. Cidial Fish Residue Study. Marine Research
Institute/Massachusetts. Montedison S.p.A. Unpublished Report No. 86,
(1979).
Moye et al. Residue Analysis of Phenthoate (Cidial(R)) and Phenthoate
Oxon in Citrus, Processed citrus fractions and soil. Unpublished
Report of University of Florida for Montedison USA.
Nelson, R.F., Kennedy, G.L. and Keplinger, M.L. Two Year Chronic Oral
Toxicity with Phenthoate (Cidial) Technical in Beagle Dogs. (1972)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
C-8884), reviewed and validated by BoB West Associates, Inc. (Nov. 13,
1978), submitted to the World Health Organization by Montedison.
Nigg, H.N. and Stamper, J.H. Persistence of Phenthoate (Cidial) and
Phenthoate Oxon on Fruit, Leaf and Soil Surfaces and in Air in Florida
Citrus. Submitted for publication to Florida Agricultural Experiment
Stations Journal, 1980.
Noakes, D.N. Summary of Toxicological Data on "Cidial". (1963)
Unpublished report from Medical Department, Fisons Pest Control
Limited, submitted to the World Health Organization by Montedison.
Oscarson, E.T., Marias, A.J., Kennedy G.L., Kinoshita, F.K. and
Keplinger, M.L. Eighteen Month Carcinogenicity Study with Elsan
(Phenthoate) in Albino Mice. (1976) Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 622-04624), reviewed and validated by
Bob West Associates, Inc. (Dec, 10, 1979), submitted to the World
Health Organization by Montedison.
Paa, H., Mastri, C.W. and Keplinger, M.L. Twenty-one-day Subacute
Dermal Toxicity Study with Cidial E4 in Albino Rabbits. (1974)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
601-03802), reviewed and validated by Bob West Associates Inc. (Jan.
11, 1974), submitted to the World Health Organization by Montedison.
Pellegrini, G. and Santi, R. Potentiation of Toxicity of
Organophosphorous Compounds Containing Carboxylic Acid Ester Functions
Towards Warm Blooded Animals by Some Organophosphorous Impurities. J.
Agr. Food. Chem. 20: 944-50.
Pfeifer, W.D., Smith, S., Kennedy, G.L., Kinoshita, F.K. and
Keplinger, M.L. Three-generation Reproduction Study with Elsan in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03513), reviewed and validated by Bob West
Associates, Inc. (Jan. 23, 1980), submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Eye Irritating Properties of Technical Cidial
and Cidial 50L. (1975a) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Intraperitoneal
Toxicity of Technical Cidial. (1975b) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Rats. (1978a) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Mice. (1978b) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity of Technical Cidial in
Albino Rats. (1978c) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity Study of Technical
Cidial in Albino Rabbits. (1978d) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Primary Skin Irritation Test of Technical
Cidial in Albino Rabbits. (1978e) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Salvaneschi, S. Acute Toxicity of the Commercially produced technical
Cidial and its Formulations to Laboratory Warm Blooded Animals. (1968)
Unpublished report from Agricultural Research Institute,
Montecatini-Edison (No. 45/1968), submitted to the World Health
Organization by Montedison.
Santi, R., Radice, M. and Martinotti, P. Degradation, metabolism and
residues of 32P-labelled CidialR in apples, pears, olives and olive
oil. Montedison S.p.A. Unpublished report no. 66, (1967).
Serota, D.G., Fezio, W.L. and Voelker, R.W. Three-week Dermal Toxicity
Study in Rabbits. (1979) Unpublished report from Hazleton Laboratories
America, Inc. (No. 2063-100), submitted to the World Health
Organization by Montedison.
Shirasu, Y., Moriya, M. and Kato, K. Microbial Mutagenic Study on
Elsan (Phenthoate). (1976) Unpublished report from Institute of
Environmental Toxicology, Nissan Chemical Industries, Inc., submitted
to the World Health Organization by Montedison.
Sikka H.C. Effect of Phenthoate on Microbial Functions. Syracuse
Research Corporation. (1979) Unpublished Report No. SRC L 1363-07.
Takade, D.Y. et al. Metabolism of O,O-Dimethyl
S-alpha-(carboetoxy)-benzyl phosphorodithioate (Phenthoate) in the
White Mouse and House Flies. Pesticide Biochemistry and Physiology 6:
367-376.
Takade, D.Y., Seo, M.S. and Fukuto, T.R. Alteration O,O-dimethyl
S-alpha-(carboetoxy)benzyl Phosphorodithioate (Phenthoate) in Citrus,
Water and upon Exposure to Air and Sunlight. Arch. Environ. Contam.
Toxicol. 5: 63.
Thompson, W., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Two-year
Chronic Oral Toxicity Study with Phenthoate (Cidial) in Albino Rats.
(1973) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(No. B-8882), reviewed and validated by Bob West Associates, Inc. (MaY
3, 1973), submitted to the World Health Organization by Montedison.
Toyoshima, S., Sasaki, H., Sato, R., Sato, S., Kato, M., and Nizeki,
H. Acute Toxicity Test Elsan(R) with Rats and Mice by Intraperitoneal
and Subcutaneous. (1971) Unpublished report from the Japan
Experimental Medical Research Institute, Co., Ltd., submitted to the
World Health Organization by Montedison.
Toyoshima, S., Sato, R., Sato, S., Kajima, M. and Nizeki, H.
Three-month Dietary Feeding Study of Elsan (Phenthoate) in Rats and
Mice. (1973) Unpublished report from The Japan Experimental Medical
Research Institute, Co., Ltd., submitted to the World Health
Organization by Montedison.
Trabucchi, E. Cidial Toxicology. (1965) Unpublished report from The
Instituto di Pharmacologia e di Terapia, Milan, submitted to the World
Health Organization by Montedison.
Umetsu, N., Hammond, P.S., Mallipudi, N.M., Toia, R.F. and Fukuto,
T.R. Toxicological Properties of Phosphorothioate and Dithioate
Impurities Present in Technical Organophosphorus Insecticides. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #36. Division of Pesticide Chemistry,
American Chemical Society.
Wargo, J.P. Cidial bovine metabolism and residue study. Research
Report Phase 1-6. (Analytical Development Corporation) Montedison
S.p.A. Unpublished Report No.64.
Wargo, J.P., Jr. Cidial Bovine Metabolism and Residue Study. (1975)
Unpublished report from Analytical Development Corp. (No.210),
submitted to the World Health Organization by Montedison.
Wright, G., Smith, S., Kennedy, G.L., Kinoshita, F.K. and Keplinger,
M.L. Three-generation Reproduction Study with Cidial Technical in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03803), reviewed and validated by Bob West
Associates, Inc. (Nov. 15, 1978), submitted to the World Health
Organization by Montedison.
The metabolism of phenthoate in the two varieties of pears showed a
slightly different pattern. Phenthoate acid,
O,O-dimethyl-thio-phosphoric acid, O,O-dimethyl dithio phosphoric
acid, dimethyl phosphoric acid and phosphoric acid were identified and
four other unidentified compounds were found in the "water-soluble"
extract. The quantity of any of them did not reach 0.1 mg/kg
phenthoate equivalent. Intact phenthoate in varying amounts, traces
of P=O derivative and unidentified compounds were found on the surface
of olive at different intervals after treatment. Phenthoate
penetrated into drupes with subsequent hydrolysis and oxidation
process and formation of the same metabolites as found in other
fruits. The processed oil contained only intact phenthoate (0.14
mg/kg), while in olive husks 0.23 mg/kg phenthoate and 3 mg/kg of
other 32P containing compounds were detected.
A 0.05% emulsified solution of 14C-labelled phenthoate was sprayed on
cabbage seedlings and brushed over Hime apple fruits and also
strawberries. Phenthoate remained mostly on the surface and only
3.3-6.7% of the total amount penetrated into the plants. The residue
decreased rapidly both on the surface and in the plants. Eight days
after treatment the residue on the surface of cabbage consisted of 86%
intact phenthoate, 8% bis-alpha-carbethoxy-benzyl disulphide, 1.9%
ethyl mandelate and 1.3% unidentified compound. Phenthoate oxon was
detectable in traces (0.4-0.7%) during the first day after treatment.
Within the cabbage seedlings 8 days after treatment intact phenthoate
was present in an amount of 10.2% and the metabolites identified were
mandelic acid (46.9%), bis-alpha-carbethoxy-benzyl disulphide (6.5%),
phenthoate acid (4.8%), desmethyl phenthoate (3.2%), phenthoate oxon
(1.8%). Six unidentified compounds were separated (total amount
18.8%) of which two accounted for 14.3%. The degradation of
phenthoate in/on apple took place more slowly than in/on cabbage, or
strawberry, while the metabolite patterns were rather similar in case
of apple and strawberry to that found in/on cabbage seedlings (Hirose
et al, 1971).
In soil
The degradation of ring-labelled 14C-phenthoate in a moist (50% of
capacity) loam and silty clay loam soil was studied (Iwata, 1977b).
Phenthoate was rapidly degraded by heat-labile soil enzymes which
converted it to phenthoate acid under both aerobic and anaerobic
conditions, even with a soil treatment of 100 mg/kg. Under aerobic
conditions and in low concentration the phenthoate acid underwent
extensive microbiological degradation to CO2 (up to 50% of the
theoretical value) and polar products. At 100 mg/kg level or under
anaerobic conditions it degraded by first order kinetics, presumably
by simple hydrolysis.
In a loam soil phenthoate at 1.25 and 2.5 mg/kg had no effects on the
rates of degradation of 14C-cellulose or 14C-starch by soil microbes
over a 25-day period as measured by the evolution of 14C. The rate
of nitrification in soil amended with (NH4)2SO4 was likewise
unaffected over a 28 days period (Sikka, 1979).
Phenthoate appears to be relatively persistent under dry soil
conditions (Iwata, 1977a) and in dry dust adhering to plant surfaces
(Iwata, 1975). In two sets of experiments 95% and 62% of the initial
doses remained unchanged after 59 and 75 days respectively.
The results indicate that conditions favouring microbial activity,
such as adequate moisture and warm temperatures, make soil type of
secondary importance for phenthoate degradation.
The fate of residues was also studied under field condition (Moye et
al, undated). Soil samples were taken from the drip-line and middle
at 0-6 and 6-12 cm depths under the orange trees treated with Cidial 4
E solution of 0.03% ai 1, 3, 7, 14 and 28 days after treatment. Four
samples were taken from both depths for each time. The maximum of
phenthoate concentration was observed at the drip-line on the third
day (1 mg/kg, in 0-6 cm and 0.68 mg/kg in 6-12 cm).
The mean residue at 0-6 cm was 0.56 mg/kg which decreased to 0.12
mg/kg 28 days after treatment.
Phenthoate oxon was not detectable in any of the experiments. The
mobility of phenthoate and its soil metabolites were studied by
leaching experiments using sandy soil (organic matter 1.6%), silt loam
(o.m. 6%), sandy loam (o.m. 3.3%), clay loam (o.m. 5.9%) (Anonymous,
1978a). The columns were filled to 25.4 cm with the untreated soils
after prewetting the column with water. The fortified soil (5 cm) was
layered over the untreated soil. For studying the mobility of
metabolites the fortified soils were aged for 30 days under greenhouse
conditions. Water equivalent to 500 mm rain for parent compound and
to 380 mm for metabolise was allowed to pass through the column and
analyzed. The columns were sectioned into four equal lengths for
analyses by the measurement of radioactivity. The upper 15 cm of the
sandy soil contained 72.3% of phenthoate and 85.7% of the metabolites
while the other soils retained 87.3-88.8% and 89.4-90.9% respectively.
The water effluent from the light sandy soil contained 8.32% and 6.62%
of the original activity. From the other soils the 14C activity in
the water effluent amounted to 2.9-6.44% and 3.39-4.21%.
In water
14C phenthoate was added to 0.01 M sodium phosphate buffers (pH 6.0,
7.0 or 8.0) to give a final concentration of 1.5 × 10 -5 M. Aliquots
were taken 3, 7, 10, 14, 21 and 28 days after addition of the 14C
material to the buffer and analyzed an TLC plates subsequent to
appropriate preparation.
32P-phenthoate was added to the buffer solutions in a 1 × 10-4 M
final concentration and was treated similarly to the 14C compound.
All solutions were held at 24.5 ± 1°C in a constant temperature room.
The pH of buffered solutions, containing radiolabelled phenthoate
under similar conditions, was monitored over the experimental period
and no pH change was detectable.
Evidently, phenthoate is fairly stable in water at the indicated pH
values. 45%, 21%, 22% of its initial amount were present after 28
days at pH 6, 7, 8 respectively. Its half life is about 12 days at pH
8.0. Phenthoate acid was observed in greatest amount (30-55%)
irrespective of pH. Hydrolysis of carbonyletoxy moiety appeared to be
fastest at pH 8.0. On the other hand the rate of formation of
desmethyl phenthoate, the second most prominent hydrolysis product,
was fastest at pH 6. Phenthoate oxon was not detected at any time.
Other products indicated in Figure 2 were observed in small amounts
(Takade, 1977).
Photodegradation
The photodegradation of 14C-phenthoate was studied in distilled water
(pH = 7.0±0.5) at concentrations of 8 mg/kg and 5 mg/kg (Anonymous,
1978).
A medium pressure mercury lamp, fitted with a Pyrex 7740 filter that
excludes light of wavelength less than 280 nm, was immersed in the
solution to be irradiated. After 5 and 8 hours of irradiation,
aliquots of the photolysed solutions were taken and analyzed applying
HPLC and TLC techniques. Approximately 50% of phenthoate had degraded
after 6-8 hours of irradiation, while there was no loss in another
phenthoate solution kept in the dark for the same time. Ethyl
mandelate was found to be the major degradation product. In addition
three unknown products were detected but each of them accounted for
less than 10% of the parent compound.
An acetone solution of radiolabelled phenthoate (32P or 14C) was
applied to glass plates, which were exposed to sunlight daily for 7
hours (Takade, 1977).
Air temperature during the exposure ranged from 7.2 to 23.3°C. Under
these conditions approximately 90% of the applied phenthoate was lost
after 40 hrs of exposure by volatilisation.
The unchanged phenthoate gradually decreased in proportion to the
other alteration product during the exposure (see Figure 2).
Phenthoate oxon was identified as the major product. Other products
isolated were desmethyl phenthoate, mandelic acid, bis-[alpha-carboxy)
benzyl]-disulphide, bis[alpha-/carbethoxy/benzyl]disulphide and
O,O-dimethyl phosphorothioic acid. Results of other experiments
indicated that elevated temperatures may speed up the phenthoate oxon
formation and its disappearance. The latter is also facilitated by
rainfall (Nigg and Stamper, 1980).
The degradation products formed upon exposure to sunlight and air on
glass plates or found on citrus leaves and fruits are essentially the
same.
In processing
The effect of processing was studied on orange, lemon and grapefruit
treated in the field twice with Cidial 4 E in solutions of 0.03% or
0.06% ai. The samples were taken 50, 19 and 18 days after last
treatment respectively and were processed with a FMC processor. The
residue ranges found in 4 samples taken at each stage are summarised
in Table 13 (Moye et al, undated).
Little, if any, residue decrease occurred from washing of oranges and
lemons, which is in good agreement with the findings of Iwata et al,
who carried out laboratory washing to simulate packing house
treatment. On the other hand laboratory washing of the grapefruit
removed about 50% of the residue found 3 days after treatment and from
about 25% to less than 10% thereafter.
Phenthoate seems to be concentrated in the waxes and oils of the rind.
Fruit juice contained negligible residues (<0.01 mg/kg). Residues
found in the dried rind, which is used as a cattle feed, show an
approximate 44-58% loss of residue during processing and drying.
The majority of the residue found in/on olives remained in the olive
husk and the processed oil contained only intact phenthoate (4.1% of
total residue) (Santi, 1976).
METHODS OF RESIDUE ANALYSIS
Methods that have been developed for the determination of phenthoate
in crops and animal tissues have been reviewed. Bazzi (undated)
described a method which is based on acetone extraction, liquid-liquid
partition clean up on a Plorex column and gas chromatographic
determination using 3.8% UCCW 982 on Gas Chrom Q packing and a flame
photometric detector. The limit of determination is 0.04 mg/kg or
less depending on the type of sample. The method is suitable for the
determination of phenthoate and its oxon in citrus and their processed
by-products in maize, cattle meat and soil.
Moye et al (undated) found acetone: acetonitrile 1:1 mixture most
efficient for the extraction of phenthoate from soil, fruits and
by-products of citrus processing. OV-101, OV-17 (Iwata et al, 1979)
and OV-225 (Moye et al, undated) liquid phases were used to separate
phenthoate, phenthoate oxon from other organophosphate pesticides also
applied in citrus orchards.
Another method for the determination of residues in animal products
includes extraction by acetonitrile from muscle, kidney and liver, by
acetone and benzene from milk and by heptane from fat; partition with
benzene, hexane and acetonitrile; column chromatography on Floricil;
and GLC determination using 3% OV-101 packing with FPD. Limit of
determination: 0.01 mg/kg for milk, 0.05 mg/kg for tissues. The
recovery at 0.05-0.1 mg/kg level is about 92% for all samples.
The basic method described by Bazzi (undated) in combination with the
other liquid phases and slight modifications proposed by the other
authors is suitable for regulatory purposes.
NATIONAL MRL'S REPORTED TO THE MEETING
Phenthoate is registered in 36 countries all around the world. Some
of the maximum residue limits and pre-harvest intervals were reported
to the meeting.
Pre-harvest
intervals MRL
(days) (mg/kg)
Hungary apple, pear,
sugar-beet 21 0.5
Italy apple, pear, walnut,
vegetables, rice 20 0.3
citrus, olive 60 0.3
Israel oranges, grapefruit 20-30
Japan pear, peach 7 0.1
pumpkin 1 0.1
mandarine, orange 14 0.1
rice (unpolished) 7 0.05
japanese pear 30 0.1
Netherlands citrus fruit 0.5
New Zealand brassica crops 0.7
South Africa citrus 21 1.0
brassica 3 1.0
onion,potato 7 0.1
Sweden citrus 1.0
USA citrus 2.0
TABLE 5. Residues of Phenthoate in oranges
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia USA 1973 1 8.5 50 L 0.8 0.5 0.4 0.3 0.2 0.1 0.1 Iwata, 1977a
(Florida) 1 4.3 50 L 0.5 0.3 0.2 0.1 0.09 0.09 0.07
Valencia USA 1979 1 4.2 4 EC 2.9 2.3 1.5 0.58 0.23 Iwata, 1979
(peel) (Florida) 1 8.4 4 EC 4.0 3.0 2.1 0.85 0.35
1 8.41 4 EC 6.3 4.2 3.0 1.4 0.38
Valencia USA
(peel) (Florida) 1979 2 0.03%3 4 EC 1.65 0.67 0.29 0.38 Moye et al
(pulp) 0.06% 0.03 0.04 0.01 0.01 (undated)
Hamlin Brazil 1979 1 0.1% 50 L 1.38 0.79 0.5 0.33 0.22 0.22-0.12 Camargo,1980
(peel)
Valencia Italy 1965 1 0.06%1,2 50 L 4.9 1.6 1 1.1 0.8 0.6 Anonym, 1965
Moro (peel) Italy 1963 1 0.05%1,2 50 L 3.2 1.8 1.2
Valencia Israel 1973 1 7.5+0.5% oil 50 L 1.66 1.08 0.5 0.2 Greenberg
1 10 50 L 0.71 0.58 0.04 0.09 0.02 et al, 1974
1 20 50 L 2.1 1.33 0.75 0.37 0.12
Shamouti Israel 1973 1 7.5 50 L 1.02 0.98 0.45 0.21
1 7.5+0.5% oil 50 L 0.98 1.08 0.4 0.36
1 15 50 L 1.68 1.41 0.82 0.4
Orange Japan 3 0.05% 50 EC 0.01 0.008 0.004 Anonym, 1980
(pulp) 5 0.05% 0.01 0.009 0.009
3 0.05% 0.02 0.005 0.008
5 0.05% 0.015 0.006 0.005
TABLE 5. Continued...
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia S. Africa 1970 1 0.213% 50 L 3.7 2.05 1.1
1971 1 0.1% 50 L 1.21 0.56 0.32 0.23 Anonym,
1970
Valencia S. Africa 0.37 0.3 0.12
(pulp)
1 Low volume application with a spraying volume of 940 l/ha
2 Colorimetric method was used for the determination
3 Three months elapsed between applications; the maximum residue values found in four replicates are given
TABLE 6. Residues of Phenthoate in Grapefruit and Lemon
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-4 8-11 18-21 33-35 38-46 60
Grapefruit USA 1978 1 3.4 4 EC 0.94 0.77 0.48 Iwata, 1979
(peel) 1 6.7 4 EC 2.1 1.6 0.87
1 6.71 4 EC 3.9 2.5 1.4
1979 1 0.03%2 4 EC 0.9 0.5 0.31
0.06%2 1.8 0.77 0.59
(pulp) USA 1979 2 0.03+0.063,4 4 EC 0.007 0.008 0.011 0.002 <0.002 Moye et al, undated
Whole fruit Israel 1973 1 0.075% 50 L 0.97 0.47 0.23 1.131 Greenberg et al,
1 0-0.75%4 50 L 1.4 0.63 0.39 0.14 1974
1 0.075%+0.5% oil 50 L 0.84 0.79 0.93 0.1
2 0.075%+0.5% oil4 50 L 1.0 1.0 0.74 0.16
1 0.15% 50 L 1.25 1.5 0.41 2.7
1 0.75%4 2.0 2.0 0.57 0.42
Lemon USA 1974 1 7 50 L 1.2 0.8 0.6 0.4 0.3 0.3 Iwata, 1979a
(peel) 1974 1 1.8 50 L 0.5 0.3 0.2 0.2 0.1 0.1
1978 1 4.2 4 EC 1.5 0.9 0.47
1 8.2 4 EC 2.8 1.7 0.78
1 8.21 4 EC 3.8 3.3 2.2
1979 1 5 4 EC 3.7 2.7 1.1
10 4 EC 7 4.5 1.9 Iwata, 1979
10 4 EC 14 11 5.7
Whole fruit S.Africa 1973 1 0.04% 50 L 0.4 0.26 0.08 0.09 Anonym, 1970
0.1% 50 L 1.4 0.96 0.47 0.17
1 Low volume application with a spraying volume of 940 l/ha
2 Sprays were applied to achieve full coverage
3 17 days elapsed between applications
4 The maximum residue values found in four replicates are given.
TABLE 7. Residues of Phenthoate in Apple and Pear
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0 1 4-7 9 14-17 21-26 29-34 39-46
Apple Italy 19621 1 0.04% 50 L 2.8 1.19 0.47 0.22 0.16 0.11 <0.1 Anonym, 1972
1962 1 0.03% 50 L 1.57 0.78 0.34 0.18 0.13 0.1
19621 1 0.04% 50 L 1.6 0.93 0.36 0.1 <0.1
1962 4 0.04% 50 L 1.92 1.21 0.48 0.12 <0.1
Pear Italy 19631 1 0.04% 50 L 1.45 1.1 0.6 0.4 0.25 Anonym, 1973
1963 1 0.04% 50 L 1.9 1.7 0.54 0.3 0.1 <0.1
1963 6 0.05% 50 L 0.64 0.35 0.18
Japanese Japan 3 0.05% 0.3 0.11 0.05 Anonym, 1980
pear 5 0 05% 0.44 0.16 0.09
3 0.05% 0.48 0.16 0.03
4 0.05% 0.58 0.1 0.08
1 Colourimetric method was used for the determination.
TABLE 8. Residues of phenthoate in other crope resulting from supervised trials
Application Residues in mg/kg, at intervals (days) Reference
after application
Crop Country Year No. rate % formulation 0 1 6-7 13-14 20-21 21-26 34 70
volume (1)
Cabbage Italy 1965 2 0.05 50 L <0. 1 <0.1 Anonym.,
6 3.18 0.11 0.09 1965
Olives Italy 1963 1 0.05/3000 50 L 10.9 8.1 0.49 0.49 0.2 Anonym.,
1963
Onion Japan 2 0.05/1500 0.01 Anonym.,
4 0.01 1980
Radish 2 0.05/2000 0.005
4 0.05/1500-2000 0.005 0.005 0.005 0.005
3 0.05/1800
Spinach Japan 2 0 05/1500 0.015
3 0:05/1500 0.018
2 0 05/1500 0.005 0.015
3 0:05/1500 0.007 0.005
Tea/leaf Japan 2 0.05/2000 0.008
3 0.05/2000 0.04
2 0.05/3000 0.04
3 0.05/3000 0.04
Lettuce Japan 2 0.05/2000 0.08 0.08 0.02
4 O.05/2000 0.06 0.05 0.09
TABLE 9. Carbon 14 residues in bovine tissue after feeding at a
20 mg/kg level in the diet
mg/kg in tissues after feeding for
Tissue 8 days 18 days 26 days 26 days
7 days
withdrawal
Muscle 0.013 <0.033 <0.033 <0.033
Fat 0.016 0.056 0.045 <0.045
Kidney 0.324 0.408 0.502 0.116
Liver 0.238 0.228 0.297 0.140
Heart 0.028 0.061 0.087 0.052
Brain 0.036 <0.042 0.054 <0.042
TABLE 10. The penetration of 14C and 32P phenthoate into orange
fruit, and the distribution of radioactivity in the surface wash,
acetone extract and solid residue.
Percentage of the applied radioactivity
recovered at indicated time (days)
0 3 7 10 14
14C-labelled phenthoate
External surface wash 72.5 66.1 9.8 8.1 4.6
Internal acetone extract1 - 5.4 15.6 12.8 7.8
Solid residue1 27.5 21.7 21.6 34.7 28.3
Total recovered 100.0 93.2 47.0 55.6 40.7
32P-labelled phenthoate
External surface wash 96.4 63.4 17.4 13.6 11.6
Internal acetone extract1 - 5.9 22.0 24.2 18.2
Solid residue1 3.6 19.4 13.9 16.3 13.2
Total recovered 100.0 88.7 53.3 54.1 43.0
1 Includes peel only. Radioactivity in pulp was less than 1% of
the applied.
TABLE 11. Amount of 14C metabolites found in the surface wash and
acetone extract of orange leaves after indicated time intervals
Compound found in Percent of recovered radioactivity
indicated material after indicated time (days)
3 7 10 14
Phenthoate
Surface wash 84.4 45.5 35.4 41.6
Acetone extract 4.6 46.1 37.7 17.0
Phenthoate
Surface wash 0 0 2.8 5.1
Acetone extract 0.2 0 7.2 12.4
TABLE 12. 32P Phenthoate equivalents (mg/kg) in treated apples, pears and olives
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Apples: var. Stark Delicious (0.02% solution)
2 hrs. 1.00 0.21 0.17 0.04 - - 1.17
1 day 0.26 traces 0.31 0.06 - - 0.57
15 days 0.02 - 0.08 0.02 0.95 0.80 0.10
29 days 0.00 - 0.00 - 0.96 0.69 0.00
Pears: var. Trionfo di Vienna (0.04% solution)
2 hrs. 1.35 traces 0.19 0.02 - - 1.44
1 day 1.19 traces 0.40 0.05 - - 1.59
4 days 0.33 - 0.26 0.09 - - 0.59
9 days 0.19 - 0.12 0.07 0.43 0.18 0.31
31 days1 0.006 - 0.01 0.02 0.16 0.09 0.016
Pears: var. Passacrassana (0.045% solution)
2 hrs. 1.23 traces 0.03 traces - - 1.26
1 day 1.01 traces 0.07 traces - - 1.08
5 days 0.55 - 0.05 - 0.04 0.02 0.60
25 days1 0.28 - 0.16 - 0.39 0.07 0.44
40 days2 0.20 traces 0.10 traces 0.34 0.14 0.30
TABLE 12. Continued...
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Olives /oils-yielding/: var. Leccino (0.05% solution)
2 hrs. 11.16 traces 0.15 0.01 traces - 11.31
1 day 5.92 traces 1.50 0.36 0.10 - 7.42
9 days 0.36 0.18 0.53 0.38 3.60 0.84 0.89
30 days 0.19 0.03 0.06 0.35 2.58 0.86 0.25
70 days 0.05 - 0.10 0.05 3.23 0.59 0.15
1 Fruit picking
2 Pears of this winter variety were analysed after picking from trees
and stored for 15 days at 19-20°C.
TABLE 13. Phenthoate residue (mg/kg) detected in processed citrus by-products
ORANGE LEMON GRAPEFRUIT
Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon
Unwashed peel 0.24-1.00 0.06-0.09 0.08-0.28 0.06-0.12 0.031-0.74 <0.01
Washed peel 0.23-0.73 0.036-0.069 0.15-0.32 <0.01 0.23-0.51 <0.01-0.09
Unwashed pulp <0.002-0.031 0.01 0.003-0.024 <0.01 0.007-0.031 <0.01
Washed pulp 0.005-0.011 0.01 0.002-0.004 <0.01 0.02-0.09 <0.01
Chopped peel 0.34-0.67 0.01-0.03 0.08-0.26 0.03 0.26-0.49 0.002-0.051
Peel frits 0.32-0.52 0.05-0.15 0.25-0.74 0.05-0.15 1.14-2.45 0.015-0.031
Finisher pulp 0.008-0.015 <0.01 0.008-0.023 <0.01 0.003-0.008 <0.01
Dried rind 0.13-0.43 0.01-0.036 2.2-2.7 0.01 0.72-1.51 0.06-0.21
Press liquor 0.08-0.14 <0.01-0.01 0.008-0.032 0.009-0.017 0.13-0.19 0.01-0.013
Emulsion water 0.004-0.07 <0.004-0.1 0.003-0.009 0.04-0.06 0.006-0.021 <0.01
Fruit juice 0.002-0.003 <0.01 0.004-0.009 <0.01 <0.002 <0.01
After-water rinse <0.002-0.004 <0.01 <0.002-0.005 <0.01 <0.002 <0.01
Pre-water rinse <0.002 <0.01 0.002 <0.01 <0.002 <0.01
Molasses 0.047-0.068 <0.01 0.01-0.03 <0.01 0.037-0.071 0.01
Oil 3.29-14.05 1.18-2.48 7.25-17.25 1.65-2.5 5.4-8.5 0.45-1.1
EVALUATION
COMMENTS AND APPRAISAL
Phenthoate is a wide-spectrum organophosphorus insecticide, mainly
used on citrus at rates of 0.6 to 5 kg ai/ha.
The technical material contains a minimum of 92% of phenthoate.
Detailed analyses of the typical technical material, including the
identity of the minor components, was provided. It is generally
soluble in most polar and non-polar organic solvents and also slightly
soluble in water.
Phenthoate is a cholinergic compound of moderately acute toxicity and
is rapidly absorbed, metabolized and excreted in mammals. The pattern
of metabolism is similar in both plants and animals and is similar to
that noted with other organophosphorus esters. The rate of metabolism
is shown to be significantly affected (with potentiation of toxicity)
by organophosphorus esters commonly found as impurities in the
technical product. The acute toxicity of phenthoate is dependant on
the purity of the technical product, a feature which raised
significant questions about occupational exposure. It was suggested
that a more purified product should be manufactured and used to
alleviate this problem. Formulations that are stored also have the
potential to become more toxic under certain conditions. Although
these factors do not directly affect phenthoate residues in food they
should not be ignored.
Phenthoate is moderately toxic to mammals. The acute LD50 was noted
to vary from 78 to over 4500 mg/kg bw, depending possibly on the
impurities and the species. Antidotal studies are not available.
Phenthoate does not induce a delayed neurotoxic reaction in hens, is
not mutagenic or teratogenic, nor does it interfere with reproduction,
as evidenced by a standardized 3-generation study in rats. In a
series of short-term toxicity studies, cholinesterase depression was
the most significant measure of effect of exposure. In dermal and
ophthalmological studies, phenthoate applied as a formulated product
was irritating. On the basis of available short-term studies, it is
concluded that phenthoate is a potent cholinesterase inhibitor.
A two-year study in the dog showing red blood cell cholinesterase
depression served as the primary basis to evaluate a no-effect level.
Phenthoate is not a carcinogen in mice. A two.year study in rats was
considered invalid and not included in the evaluation.
On the basis of available data, no-effect levels in mammalian species
were determined and a temporary ADI for man was allocated. This
evaluation was made on the assumption that the manufacture of
phenthoate would be by a process that would assure that a product of
at least 92% purity would be marketed. As this was the product on
which toxicological evaluations were made and as impurities have a
substantial toxicological significance this purity restriction was
important.
Phenthoate residues gradually disappear from treated plants within 4
to 10 weeks, most being lost by volatilization. Decomposition of
surface deposits is mainly due to photodegradation, resulting in
phenthoate oxon as the main metabolite. On cabbage, the surface
residue 8 days after application consisted of 86% phenthoate but no
oxon was detected; on orange leaves, 41.6% of the total residue was
phenthoate and 5.1% was the oxon at 14 days after treatment.
Phenthoate is not systemic but does enter the peel of fruit, where it
undergoes hydrolytic cleavage and oxidation. Virtually all of the
residue remains in the peel of treated citrus fruit, the pulp
containing less than 1% of the total residue. Phenthoate oxon has
been detected only in the peel.
The main metabolites in plants, phenthoate oxon, dimethyl phenthoate,
mandelic acid, bis-2-(carbethoxy) benzyl disulphide, O,O-dimethyl
phosphorodithioic acid, O,O-dimethyl phosphorothioic acid and
phenthoate acid, are similar to those secreted by animals. Their
amounts and relative proportions depend on the crop and the time
elapsed since application but, in practice, the parent phenthoate
should be considered as the only residue of importance.
Repeated treatments of citrus fruits or apples according to
recommended usage do not lead to increased residues or changed
metabolite patterns. The residue is contained mostly in the waxes and
oils of the citrus rind and is not decreased significantly by washing;
the fruit juice contains negligible residues. Dried rind as used for
cattle feed can contain 40 to 60% of the residue originally present in
the peel.
Laying hens fed for 30 days with feed containing 1.8 to 2.6 mg/kg of
phenthoate showed no reduction of egg production or any abnormalities.
Residues in the muscle and in eggs were low and disappeared after
withdrawal of the compound from the diet; in kidney and liver levels
reached a plateau between the third and seventh days of treatment.
Neither phenthoate nor its metabolites accumulated in fish kept in
water containing phenthoate at the 10-4 to 10-6 mg/l levels.
Lactating cows given 14C-phenthoate at levels of 1.5 and 20 mg/kg in
the feed excreted the material mainly in the urine and faeces. The
main metabolite was phenthoate acid, which accounted for 45% of the
14C activity of the urine. Residues in milk reached a plateau 2 to 3
days after administration began. The organosoluble fraction of the
residue in milk, possibly phenthoate, phenthoate oxon and unconjugated
metabolites, was very low (0.0028 mg/kg) even at the higher dosage
rate. Meat from the cattle contained traces of residues at the higher
dosage rate only, the muscle tissue and the fat containing the
residues in roughly equal proportions. Residues in various tissues
declined rapidly when the pesticide was withdrawn from the diet; even
the bound (unextractable) metabolites were eliminated from kidney and
liver. Neither phenthoate nor its oxon could be detected in tissue or
milk samples by conventional analytical methods at limits of
determination of 0.05 and 0.01 mg/kg respectively. It was concluded
that feeding cattle with citrus by-products prepared from
phenthoate-treated fruit would not result in detectable residues in
meat or milk.
In soil, phenthoate is rapidly converted to phenthoate acid under both
aerobic and anaerobic conditions, even when as much as 100 mg/kg is
added to the soil. Under conditions favourable for microbiological
activity, such as adequate moisture and warm temperature, the soil
type has no influence on the degradation of phenthoate. On the other
hand, phenthoate persists for a relatively long time under dry soil
conditions. Phenthoate and its metabolites show moderate mobility in
soil, the majority of the residue being contained in the upper 15 cm.
The likelihood of contamination of lower soil layers or underground
waters is very low in view of the high degradability and slow leaching
properties of the residues.
Analytical methods, based on GLC separation on columns of different
polarity with flame photometric detection are available and suitable
for regulatory purposes.
Level-causing no toxicological effect in:
Dog: 10 mg/kg in the diet equivalent to 0.29 mg/kg bw/day.
Mouse: 30 mg/kg in the diet equivalent to 4.5 mg/kg bw/day.
Rat: 10 mg/kg in the diet equivalent to 1.0 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that available information was adequate for the
estimation of maximum residue levels for phenthoate on citrus fruits
only. The data on other crops were not considered adequate for
estimating maximum residue levels. The levels apply to the parent
phenthoate excluding all metabolites.
Commodity Temporary maximum Interval between last
residue level (mg/kg) application and harvest
on which levels are
based
Citrus fruit 1 21
Carcass meat of cattle 0.051
Milk 0.011
Egg 0.051
1 At the limit of determination.
FURTHER WORK OR INFORMATION
Required (by June, 1984)
A chronic toxicity study in an acceptable mammalian species (rodent).
Desirable
1. Further information on impurities in the technical products in
order to evaluate the degree to which these impurities potentiate the
toxicity of phenthoate.
2. Data from supervised trials on other commodities in accordance
with present uses of the compound.
3. Residue data from crops, known to have been treated under
practical conditions, moving in commerce.
REFERENCES
Anonymous. Cidial Residues in Apples. Montedison S.p.A. Unpublished
report no. 77, (1962).
Anonymous. Cidial Residues in Pears, Olives. Montedison S.p.A,
Unpublished report nos.78, 79, (1963).
Anonymous. Cidial Residues in Oranges. Montedison S.p.A., Unpublished
report no 74/b, (1964).
Anonymous. Cidial Residues on Oranges, Cabbages. Montedison S.p.A.
Unpublished reports Nos. 76/b, 80, (1965).
Anonymous. Cidial Residues on Citrus Form Chem. Report Montedison
S.p.A. Unpublished report no. 74/a, (1970).
Anonymous. Determination of Cidial [Ethyl
alpha(/Dimethoxy-phosphinothioyl/thio) benzene acetate] in muscle,
milk, kidney, liver and fat. Thompson-Hayward Chemical Co., Montedison
S.p.A. Unpublished report no. 62, (1975).
Anonymous. Cidial Leaching Study on Parent compound and on its Soil
Degradation Products. Carried out by Thompson-Hayward Chemical
Company. Montedison S.p.A. Unpublished Report no 91, (1978a).
Anonymous. Photodegradation of Phenthoate in Aqueous Solution.
Submitted by Syracuse Research Corporation. Montedison S.p.A.
Unpublished Report no. 93, (1978b).
Anonymous. Interpretation and discussion on the metabolism of Cidial
in the dairy cow. Unpublished Report of Analytical Development
Corporation. Montedison S.p.A. Report No. 65, (1979).
Anonymous. Summary of Residue Data in Japan. Nissan Chemical
Industries Ltd. Unpublished reports, (1980).
Arnold, D., Kennedy, G.L., Kinoshita, F.K. and Keplinger, M.L.
Mutagenic Study with Cidial in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (No. 622-05876), reviewed and
validated by Bob West Associates, Inc. (Nov. 6, 1978), submitted to
the World Health Organization by Montedison.
Arnold, D., Kennedy, G.L. and Keplinger, M.L. Mutagenic Study with
Elsan (Phenthoate) in Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 632-05147), reviewed and validated by
Bob West Associates, Inc. (Feb, 8, 1980), submitted to the World
Health Organization by Montedison.
Bazzi, B. Phenthoate in Zweg G., ed. Analytical Methods for Pesticides
and Plant Growth Regulators. Academic Press, New York, Vol. VIII, p.
159.
Booth G.M. Bioaccumulation Study of Cidial in Channel Catfish.
Montedison S.p.A. Unpublished report no. 87, (1978).
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04413), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04524), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Camargo de., J.L.G. Residues de carbophenothione Phenthoate em cascas
e polpas de laranjos hamlin determinados por cromatografia em fase
gasosa. Dissertatio submitted to Luiz de Queiroz University, Sao
Paulo, 1980.
de Carneri, I. Microbiological Study of Mutagenesis on CRA 133 (GMA
267/78) Genetic Mutation Test in Salmonella Typhimurium (Ames). (1979)
Unpublished report from Farmitalia Carlo Erba, Research and
Development, submitted to the World Health Organization by Montedison.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
Neurotoxicity Study with Elsan in Chickens. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (no. 8580-80635), reviewed and
validated by Bob West Associates, Inc. (Jan. 25, 1980), submitted to
the World Health Organization by Montedison.
Good, J., Cooper, D. and Terrell, Y. Acute Delayed Neurotoxicity Study
of Cidial Technical 96% Batch 104 in Female Chickens. (1979)
Unpublished report from Cannon Laboratories, Inc. (No. 8E1191),
submitted to the World Health Organization by Montedison.
Greenberg, R. et al. Residues and Disappearance of Cidial in Valencia
Oranges. Montedison S.p.a. Unpublished Report No. 73/a, (1974).
Hammond, P.S., Talcott, R.E. and Fukuto, T.R. Metabolism and Mode of
Action of O,O,S-Trimethyl Phosphorothioate in the Rat. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #38, Division of Pesticide Chemistry, American
Chemical Society.
Hathaway, D., Keplinger, M.L. and Fletcher, D.B. Acute Inhalation
Toxicity Study with Phenthoate (Cidial) Technical in Albino Rats.
(1971) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(no. N-9324), reviewed and validated by Bob West Associates, Inc.
(Nov. 6, 1978), submitted to the World Health Organization by
Montedison.
Hirose, M. et al. Residue Degradation and Metabolism of 14C Labelled
Elsan(R) (O,O-Dimethyl-S-alpha-carboetoxy-benzyl phosphorodithioate)
in Cabbage, Hime Apples and Strawberries. (In Japanese). Scientific
Pest. Control 36: 43-51.
Huhtanen, K. Poultry Tissue and Egg Study with Cidial. Cammon
Laboratories Inc. Montedison S.p.A. Unpublished Report Nos. 84, 85,
(1979a).
Huhtanen, K. Accumulation of 14C Cidial in Bluegill Sunfish Exposed
in Dynamic Flow System. Cammon Laboratories, Inc. Montedison S.p.A.
Unpublished Report No. 88, (1979b).
Iwata, Y. et al. Behaviour of Phenthoate (Cidial) Deposits and
Residues on and in Grapefruits, Lemons, Lemon Leaves, Oranges and
Orange Leaves and in the Soil Beneath Orange Trees. J. Agric. Food
Chem. 25 (2): 382-388.
Iwata, Y., Ithig, M. and Gunther, F.A. Degradation of
O,O-Dimethyl-S-alpha-(carboethoxy)-benzyl phosphorodithioate
(Phenthoate), in Soil. Arch. Environm. Contam. Toxicol. 6: 1-12.
Iwata, Y. et al. Phenthoate Applied to California Citrus Trees: Worker
Environment Research and Residue Levels on and In Fruit. Report of
Department of Entomology, University of California. Montedison S.p.A.
Unpublished Report No. 72b, (1979).
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., Kinashita, F.K.
and Keplinger, M.L. Teratogenic Study with Cidial in Albino Rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
reviewed and validated by Bob West Associates, Inc. (Nov. 21, 1978),
submitted to the World Health Organization by Montedison.
Lee, S.G.K. and Fukuto, T.R. Effect of Impurities in Technical
Phenthoate (Cidial(R)) on Mammalian Carboxyesterases. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #35. Division of Pesticide Chemistry, American
Chemical Society.
Mallipudi, N.M., Badaway, S.M.A. and Fukuto, T.R. Inhibition of
Mammalian Esterases by O,O,S-Trimethyl Phosphorothioate. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #37, Division of Pesticide Chemistry,
American Chemical Society.
Mallipudi N.M. and Fukuto, T.R. Characterization of Bound Phenthoate
Residues in Citrus. Submitted to Archives of Environ. Contam.
Toxicol., 1980.
Marshall B.L. Cidial Fish Residue Study. Marine Research
Institute/Massachusetts. Montedison S.p.A. Unpublished Report No. 86,
(1979).
Moye et al. Residue Analysis of Phenthoate (Cidial(R)) and Phenthoate
Oxon in Citrus, Processed citrus fractions and soil. Unpublished
Report of University of Florida for Montedison USA.
Nelson, R.F., Kennedy, G.L. and Keplinger, M.L. Two Year Chronic Oral
Toxicity with Phenthoate (Cidial) Technical in Beagle Dogs. (1972)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
C-8884), reviewed and validated by BoB West Associates, Inc. (Nov. 13,
1978), submitted to the World Health Organization by Montedison.
Nigg, H.N. and Stamper, J.H. Persistence of Phenthoate (Cidial) and
Phenthoate Oxon on Fruit, Leaf and Soil Surfaces and in Air in Florida
Citrus. Submitted for publication to Florida Agricultural Experiment
Stations Journal, 1980.
Noakes, D.N. Summary of Toxicological Data on "Cidial". (1963)
Unpublished report from Medical Department, Fisons Pest Control
Limited, submitted to the World Health Organization by Montedison.
Oscarson, E.T., Marias, A.J., Kennedy G.L., Kinoshita, F.K. and
Keplinger, M.L. Eighteen Month Carcinogenicity Study with Elsan
(Phenthoate) in Albino Mice. (1976) Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 622-04624), reviewed and validated by
Bob West Associates, Inc. (Dec, 10, 1979), submitted to the World
Health Organization by Montedison.
Paa, H., Mastri, C.W. and Keplinger, M.L. Twenty-one-day Subacute
Dermal Toxicity Study with Cidial E4 in Albino Rabbits. (1974)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
601-03802), reviewed and validated by Bob West Associates Inc. (Jan.
11, 1974), submitted to the World Health Organization by Montedison.
Pellegrini, G. and Santi, R. Potentiation of Toxicity of
Organophosphorous Compounds Containing Carboxylic Acid Ester Functions
Towards Warm Blooded Animals by Some Organophosphorous Impurities. J.
Agr. Food. Chem. 20: 944-50.
Pfeifer, W.D., Smith, S., Kennedy, G.L., Kinoshita, F.K. and
Keplinger, M.L. Three-generation Reproduction Study with Elsan in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03513), reviewed and validated by Bob West
Associates, Inc. (Jan. 23, 1980), submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Eye Irritating Properties of Technical Cidial
and Cidial 50L. (1975a) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Intraperitoneal
Toxicity of Technical Cidial. (1975b) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Rats. (1978a) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Mice. (1978b) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity of Technical Cidial in
Albino Rats. (1978c) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity Study of Technical
Cidial in Albino Rabbits. (1978d) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Primary Skin Irritation Test of Technical
Cidial in Albino Rabbits. (1978e) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Salvaneschi, S. Acute Toxicity of the Commercially produced technical
Cidial and its Formulations to Laboratory Warm Blooded Animals. (1968)
Unpublished report from Agricultural Research Institute,
Montecatini-Edison (No. 45/1968), submitted to the World Health
Organization by Montedison.
Santi, R., Radice, M. and Martinotti, P. Degradation, metabolism and
residues of 32P-labelled CidialR in apples, pears, olives and olive
oil. Montedison S.p.A. Unpublished report no. 66, (1967).
Serota, D.G., Fezio, W.L. and Voelker, R.W. Three-week Dermal Toxicity
Study in Rabbits. (1979) Unpublished report from Hazleton Laboratories
America, Inc. (No. 2063-100), submitted to the World Health
Organization by Montedison.
Shirasu, Y., Moriya, M. and Kato, K. Microbial Mutagenic Study on
Elsan (Phenthoate). (1976) Unpublished report from Institute of
Environmental Toxicology, Nissan Chemical Industries, Inc., submitted
to the World Health Organization by Montedison.
Sikka H.C. Effect of Phenthoate on Microbial Functions. Syracuse
Research Corporation. (1979) Unpublished Report No. SRC L 1363-07.
Takade, D.Y. et al. Metabolism of O,O-Dimethyl
S-alpha-(carboetoxy)-benzyl phosphorodithioate (Phenthoate) in the
White Mouse and House Flies. Pesticide Biochemistry and Physiology 6:
367-376.
Takade, D.Y., Seo, M.S. and Fukuto, T.R. Alteration O,O-dimethyl
S-alpha-(carboetoxy)benzyl Phosphorodithioate (Phenthoate) in Citrus,
Water and upon Exposure to Air and Sunlight. Arch. Environ. Contam.
Toxicol. 5: 63.
Thompson, W., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Two-year
Chronic Oral Toxicity Study with Phenthoate (Cidial) in Albino Rats.
(1973) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(No. B-8882), reviewed and validated by Bob West Associates, Inc. (MaY
3, 1973), submitted to the World Health Organization by Montedison.
Toyoshima, S., Sasaki, H., Sato, R., Sato, S., Kato, M., and Nizeki,
H. Acute Toxicity Test Elsan(R) with Rats and Mice by Intraperitoneal
and Subcutaneous. (1971) Unpublished report from the Japan
Experimental Medical Research Institute, Co., Ltd., submitted to the
World Health Organization by Montedison.
Toyoshima, S., Sato, R., Sato, S., Kajima, M. and Nizeki, H.
Three-month Dietary Feeding Study of Elsan (Phenthoate) in Rats and
Mice. (1973) Unpublished report from The Japan Experimental Medical
Research Institute, Co., Ltd., submitted to the World Health
Organization by Montedison.
Trabucchi, E. Cidial Toxicology. (1965) Unpublished report from The
Instituto di Pharmacologia e di Terapia, Milan, submitted to the World
Health Organization by Montedison.
Umetsu, N., Hammond, P.S., Mallipudi, N.M., Toia, R.F. and Fukuto,
T.R. Toxicological Properties of Phosphorothioate and Dithioate
Impurities Present in Technical Organophosphorus Insecticides. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #36. Division of Pesticide Chemistry,
American Chemical Society.
Wargo, J.P. Cidial bovine metabolism and residue study. Research
Report Phase 1-6. (Analytical Development Corporation) Montedison
S.p.A. Unpublished Report No.64.
Wargo, J.P., Jr. Cidial Bovine Metabolism and Residue Study. (1975)
Unpublished report from Analytical Development Corp. (No.210),
submitted to the World Health Organization by Montedison.
Wright, G., Smith, S., Kennedy, G.L., Kinoshita, F.K. and Keplinger,
M.L. Three-generation Reproduction Study with Cidial Technical in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03803), reviewed and validated by Bob West
Associates, Inc. (Nov. 15, 1978), submitted to the World Health
Organization by Montedison.
 Molecular formula: C12H17O4PS2
Molecular weight: 320.4
Colour: reddish yellow
Melting point: 17.5 ± 0.5°C
Boiling point: the compound decomposes at normal
pressure before reaching the boiling
point.
Vapour pressure: 4 × 10-5 torr at 40°C
20
Density d 1.226
4
20
Refractive index: d /1.552 approx.
D
Flash point (/Cleveland): 165°C approx.
Partition coefficient: Octanol//water log P = 3.69, 3.82 at
two concentrations
Solubility of pure compound: in water at 24°C approx 10 mg/l.
Solubility of technical grade material: miscible in all proportion
with acetone, benzene, carbon disulphide, carbon-tetrachloride,
cyclohexane, cyclohexanone, dioxane, ethanol, ethyl ether,
methanol, methyl cellosolve; soluble at 2O°C 200 mg/l in water, 120
g/l in n-hexane, 17% in ligroin, above 20% in diethylene glycol and
above 100 g/l in petroleum solvent.
Stability of technical material: after one year of storage at room
temperature in the original sealed containers, the decrease of the
active ingredient content is about 1-2% of the original value. The
active ingredient content decreases by 1-4% after one month at
50°C. In water-ethanol 1:1 solutions buffered to pH 3.9, 5.8 and
7.8 the degradation of phenthoate is relatively slight after about
20 days. At pH 9.7 the degradation is approximately 25% after 20
days.
Typical composition of the technical material: 92% ai minimum
Formulations: Cidial E-4, Cidial 50L, Cidial 50% ES, Cidial AS,
Cidial ULV, Elsan 50 EC, Elsan dust (2%), Elsan 40WP
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phenthoate is rapidly absorbed, distributed, and metabolites
excreted following oral administration to female mice (3-4 months
of age). Within 24 hours following administration using either 14C
or 32P-radiolabelled phenthoate, at dosage levels ranging from 17
to 161 mg/kg, all administered radioactivity was recovered,
predominantly in urine (over 90%) and faeces. The major residues
found in urine were water-soluble degradation products (Takade et
al, 1976).
Biotransformation
Phenthoate is metabolised rapidly in the mouse with the major
portion of the molecule degraded and the products appearing in
urine within 24 hours of administration. The metabolic sequence
appears to follow that noted with a variety of other
organophosphorous compounds and includes both oxidative and
hydrolytic degradation (Takade et al, 1976).
The metabolic fate of phenthoate was examined in a cow administered
a daily dose of 20 mg/kg (relative to its dietary intake) for eight
days. Urinary metabolites, isolated over the course of the study,
were identified as phenthoate acid and desmethyl phenthoate acid.
No other metabolic products were identified (Wargo, 1975).
A proposed metabolic pathway for mammalian species is shown in
Figure 1.
Molecular formula: C12H17O4PS2
Molecular weight: 320.4
Colour: reddish yellow
Melting point: 17.5 ± 0.5°C
Boiling point: the compound decomposes at normal
pressure before reaching the boiling
point.
Vapour pressure: 4 × 10-5 torr at 40°C
20
Density d 1.226
4
20
Refractive index: d /1.552 approx.
D
Flash point (/Cleveland): 165°C approx.
Partition coefficient: Octanol//water log P = 3.69, 3.82 at
two concentrations
Solubility of pure compound: in water at 24°C approx 10 mg/l.
Solubility of technical grade material: miscible in all proportion
with acetone, benzene, carbon disulphide, carbon-tetrachloride,
cyclohexane, cyclohexanone, dioxane, ethanol, ethyl ether,
methanol, methyl cellosolve; soluble at 2O°C 200 mg/l in water, 120
g/l in n-hexane, 17% in ligroin, above 20% in diethylene glycol and
above 100 g/l in petroleum solvent.
Stability of technical material: after one year of storage at room
temperature in the original sealed containers, the decrease of the
active ingredient content is about 1-2% of the original value. The
active ingredient content decreases by 1-4% after one month at
50°C. In water-ethanol 1:1 solutions buffered to pH 3.9, 5.8 and
7.8 the degradation of phenthoate is relatively slight after about
20 days. At pH 9.7 the degradation is approximately 25% after 20
days.
Typical composition of the technical material: 92% ai minimum
Formulations: Cidial E-4, Cidial 50L, Cidial 50% ES, Cidial AS,
Cidial ULV, Elsan 50 EC, Elsan dust (2%), Elsan 40WP
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phenthoate is rapidly absorbed, distributed, and metabolites
excreted following oral administration to female mice (3-4 months
of age). Within 24 hours following administration using either 14C
or 32P-radiolabelled phenthoate, at dosage levels ranging from 17
to 161 mg/kg, all administered radioactivity was recovered,
predominantly in urine (over 90%) and faeces. The major residues
found in urine were water-soluble degradation products (Takade et
al, 1976).
Biotransformation
Phenthoate is metabolised rapidly in the mouse with the major
portion of the molecule degraded and the products appearing in
urine within 24 hours of administration. The metabolic sequence
appears to follow that noted with a variety of other
organophosphorous compounds and includes both oxidative and
hydrolytic degradation (Takade et al, 1976).
The metabolic fate of phenthoate was examined in a cow administered
a daily dose of 20 mg/kg (relative to its dietary intake) for eight
days. Urinary metabolites, isolated over the course of the study,
were identified as phenthoate acid and desmethyl phenthoate acid.
No other metabolic products were identified (Wargo, 1975).
A proposed metabolic pathway for mammalian species is shown in
Figure 1.
 Effects on enzymes and other biochemical parameters
Groups of rats (15 males and females/group) were administered
phenthoate orally for 90 days at daily dosage levels of 0, 2.5,
5.0, 12.5 or 25 mg/kg bw. Plasma, erythrocyte, and brain
cholinesterase were the most sensitive, being inhibited about 50%
of normal at the highest dose (plasma was slightly less inhibited).
No significant inhibition was observed at 5 mg/kg (Trabucchi,
1965). Also see Special Studies on Potentiation for studies on the
effects of organophosphate esters on serum and liver
carboxyesterases, enzymes directly involved in the degradation of
phenthoate.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rats
Two almost identical reproduction studies were performed. In both
studies, groups of rats (8 male and 16 female rats/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg
and subjected to a standard 2-litter per generation, 3 generation
reproduction study. Animals were fed phenthoate from 21 days of
age until 100 days of age when they were mated to initiate the
study. The first litters were weaned at 21 days, examined, and
discarded. The parental animals were again mated to provide a
second litter, which became the parental animals for the second
generation. Eight males and 16 females from the second litters of
each generation were selected for the succeeding generation. Gross
pathology examinations were performed on the second litters of each
generation. In one of the studies, gross and microscopic
examinations of selected tissues and organs were conducted on males
and females in the control and high-level group of each parental
generation and on the second litter of the third generation
animals. Additionally, in one of the studies, cholinesterase
activity was measured in male and female animals following weaning
of the second litter. This was conducted on both the parental and
weanling animals with respect to plasma, erythrocyte and brain
cholinesterase activity. Although these two latter parameters were
not evaluated in one reproduction study, gross pathological
examinations were made.
Reproduction indices, including mating, fecundity, male and female
fertility, gestation, and lactation, were calculated and compared
with control values. All pups were examined for physical
abnormalities and viability.
With the exception of a slightly depressed erythrocyte
cholinesterase activity observed at 100 mg/kg in all generations of
both parents and weanlings, there were no significant effects of
phenthoate on any reproduction parameter measured in the study. In
both studies, there were no differences with respect to any of the
indices observed, and it was considered that phenthoate, at dietary
dosage levels up to and including 100 mg/kg displays no reproduction
hazard to rats (Pfeifer et al, 1975; Wright et al, 1975).
Special study on teratogenicity
Groups of rabbits (17 pregnant New Zealand albino rabbits/group)
were administered phenthoate orally at dosages of 0, 3 or 10 mg/kg
body weight from days 6 through 18 of gestation in a standard
teratology bioassay. A positive control with thalidomide (50 mg/kg
bw), administered during gestation, was included in the study. On
day 29 of gestation, all pregnant animals were sacrificed and
foetuses delivered by caesarean section.
There was no mortality attributable to phenthoate. The data
related to reproduction (implantation sites, resorption sites, and
live young) were not affected by phenthoate at the highest dose
level studies. There were no external or internal abnormalities
(teratogenic events) associated with phenthoate treatment of
pregnant rabbits. Growth of the foetuses was unaffected as was
survival of young for 24 hours after delivery. Internal
development, including somatic and skeletal development, was
normal. There were no teratogenic effects noted on the
administration of phenthoate to pregnant rabbits during the
critical period of organogenesis.
In contrast to the lack of teratogenic response with phenthoate,
thalidomide reduced the number of live young, increased the number
of resorption sites, and reduced foetal viability. Several of the
foetuses exhibited external somatic and skeletal abnormalities
suggesting that the strain of rabbit used in the study was
susceptible to eliciting a chemically-induced teratogenic response.
Treatment of rabbits with phenthoate during gestation (foetal
organogenesis) did not induce a teratogenic response (Ladd. et
al, 1975).
Special studies on mutagenicity
Mammalian tests
In two identical studies, groups of male mice (12 mice/group) were
administered phenthoate as a single intraperitoneal injection at
dosage levels of 0, 150 or 300 mg/kg bw. Each animal of each group
was mated with groups of three untreated, virgin females for a
period of one week after which the females were removed and
replaced by another group of females. This procedure continued for
six consecutive weeks, the period required for maturation of the
male germ cell. Females were sacrificed approximately one week
after breeding and the number of implantation sites, resorption
sites, and embryos were recorded. Data were collected with respect
to early and late deaths.
There was no mortality in male mice attributable to the phenthoate
treatment; the high-dose group was slightly hypoactive for 3-4
hours after treatment. Pregnancy rates of females mated to males
receiving 300 mg/kg was slightly lower than the control values for
weeks 4 and 6. The numbers of implantation sites, resorption
sites, and embryos from females mated with treated males were the
same as those from control matings. There were essentially no
differences between treatment groups and controls with respect to
mutation rates indicating that, in this in vivo mutagenicity
study, phenthoate is not a mutagen affecting male germinal cells
(Arnold et al, 1974; 1975).
Microbiological tests
An evaluation of the mutagenicity potential of phenthoate was
performed using the standard Ames assay and other microbial test
systems. Strains of Salmonella typhimurium (TA1535, TA1538,
TA98 and TA100), with and without a metabolic activation system S-9
derived from rat liver-induced with Aroclor 1254, were tested at
concentrations up to and including 2 mg/plate in an effort to
evaluate genetic mutations. Standard positive control chemicals
were used (2-AAF, 2-AA, and B(a)P) in the presence of the metabolic
activation system and (MMNG, 2NF, and 9-AA) in the absence of the
metabolic activation system.
Under the conditions of the experimental assay, phenthoate showed
no mutagenic activity towards these strains of microorganisms
either in the presence or in the absence of an enzymatic metabolic
system. (Carneri, 1979).
A similar study with Salmonella typhimurium was reported
where phenthoate was tested at concentrations up to and including
5 mg/plate, in the presence and absence of a male rat liver
metabolic activation system. In addition, two strains of E.
coli WP-2 were tested for mutagenicity at the same
concentrations. Again, there was no evidence of mutagenicity,
either in the presence or absence of the metabolic activation
system utilised, with either species tested (Shirasu, et al,
1976).
The recombination-capacity of B. subtilis (H-17 and M-45) was
tested following exposure to phenthoate. Phenthoate did not
prohibit growth in this assay, again demonstrating no mutagenic
potential (Shirasu et al, 1976).
A standard, host-mediated assay was performed with mice, utilising
S. typhimurium as an indicator strain. Phenthoate was
administered to groups of 6 mice at dosage levels of O, 100 or 300
mg/kg body weight by oral intubation for two consecutive days.
After the second dosing, the indicator strain was administered to
the peritoneal cavity, recovered and grown and the mutagenic
potential of phenthoate evaluated. The number of reverted colonies
did not increase as a result of in vivo phenthoate treatment
suggesting that, under the conditions of this microbial assay,
phenthoate is not mutagenic (Shirasu et al, 1976).
Special studies on neurotoxicity
Chickens
Groups of adult hens, fasted for 16 hours, were administered
phenthoate orally at dosage levels of 0 or 2,990 mg/kg bw (a
previously observed LD50 value). A positive control (TOCP, 0.5
mg/kg) was also used. Ten animals were employed in each test
group. With the exception of the TOCP treatment, the single dose
was repeated following a 21-day observation period. At the
conclusion of the study (42 days), all surviving birds were
sacrificed and subjected to gross and microscopic examinations for
axon and myelin disruption. These included the brain, sciatic
nerve, and spinal cord.
There were no signs of delayed neurotoxicity observed with
phenthoate following both treatments although half of the animals
died over the course of the study. The positive control animals
(TOCP) were observed to lose weight and on microscopic examination
were found to display myelin and axon disruption in the spinal cord
and sciatic nerve. Axon degeneration and myelin disruption were
not seen in the brain of the TOCP-treated animals. Based on the
results of this study, it was concluded that phenthoate does not
induce a delayed neurotoxic reaction similar to that seen with TOCP
in adult chickens (Fletcher et al, 1976).
Groups of hens (10 adult hens/group) were administered phenthoate
orally at dosage levels of 0, 10, 50, 100 or 750 mg/kg/bw to assess
the delayed-neurotoxic potential of phenthoate. Animals
administered the highest dose level were also administered atropine
and 2-PAM to control cholinergic signs of poisoning. A positive
control of TOCP (1,000 mg/kg) and an untreated negative control
were included in the study.
When mortality was observed at the highest dose level, a second
group of hens was introduced using this dose level. These, too,
were protected from the acute cholinergic signs of poisoning. In
all phenthoate and negative control animals, there were no clinical
or histological signs of delayed neurotoxicity, although acute
signs of cholinergic stimulation were evident with phenthoate. The
positive control animals, administered TOCP, exhibited ataxia,
paralysis, and standard signs of delayed neurotoxicity.
Histological examination of the TOCP animals revealed a significant
number of animals with histological defects in the nervous tissue.
Based upon this bioassay, phenthoate does not induce a
delayed-neurotoxic reaction in hens (Good et al, 1979).
Special studies on potentiation
Pellegrini and Santi (1972) have reported on the potentiating
effect of a series of common organophosphorous compounds found as
impurities in technical phenthoate (as well as other methyl
organophosphate esters). Several of the trimethylphosphate
impurities substantially potentiate the acute toxicity of
phenthoate, probably through interaction at the carboxylic acid
portion of the molecule. Similar results (with respect to increased
acute toxicity when combinations of OP's were tested) were obtained
with malathion, providing evidence for the inhibition of
carboxyesterase activity as a primary mode of action in this
potentiation.
The acute LD50 of phenthoate was seen to change substantially as
the presence of impurities in technical mixture increased. A
highly purified phenthoate technical sample (98.5%) had an oral
LD50 in rats of 4,728 mg/kg body weight. As the content of
impurities increased, the rat oral LD50 value decreased (90.50% =
LD50 of 242 mg/kg; 78.7% = 118 mg/kg; 61.2% = 77.7 mg/kg). Two
impurities, O,S,S-trimethyl phosphorodithioate and O,O,S-trimethyl
phosphorothioate, were found to be extremely active in potentiating
the oral LD50 in rats. Fukuto and his coworkers (Stevens and
Fukuto, 1980; Umetsu et al, 1980; Mallipudi et al, 1980;
Hammond et al, 1980) have recently confirmed this potentiation
and its probable mode of action. In in vitro studies,
hydrolysis of phenthoate by rat liver and serum carboxyesterases
was examined in the presence and absence of these two impurities.
Phenthoate acid was the exclusive degradation product obtained with
the carboxyesterases. In the presence of impurities, the rate of
hydrolysis of phenthoate to phenthoate acid was substantially
diminished, supporting the conclusion that inhibition of
carboxyesterases is the primary cause of potentiation of phenthoate
toxicity.
Special study for carcinogenicity
Mice
Groups of mice (50 male and 50 female (Charles River) mice/group)
were fed phenthoate in the diet at dosage levels of 0, 500 or 1000
mg/kg for 18 months. The animals were examined daily for mortality
and adverse behaviourial reactions. At the conclusion of the
study, growth and microscopic examination of tissues and organs was
performed. Microscopic analysis of 10 animals of each sex surviving
the 18-month treatment was performed, as were microscopic studies
on animals that were sacrificed or had died during the course of
the study. All neoplasms and tissues with suspected neoplasms were
examined histologically. Complete pathological examinations were
made specifically to identify neoplastic lesions.
There were no unusual behaviourial changes or increases in
mortality over the course of the study attributable to the presence
of phenthoate. At the conclusion of the study, histological
examination revealed no treatment-related lesions among any of the
animals. The lesions that did occur were considered to be
naturally occurring, not attributable to the presence of phenthoate
and not unusual for the strain of mice. Although a substantial
number of animals that died were not examined histologically
TABLE 1. Acute Toxicity
Species Route Sex LD50 mg/kg bw Reference
Rat Oral2 M+F 245-440 Salvaneschi, 1968; He & Gera,
1978a; Toyoshima et al, 1978;
Trabucchi, 1965; Pellegrini &
Santi, 1972.
M 270 (231-316) Toyoshima et al, 1978.
F 255 (216-301) Toyoshima et al, 1978.
IP M+F 720 (672-77) Re and Gera, 1975b
M 720 (634-868) Toyoshima et al, 1971.
F 745 (615-901) Toyoshima et al, 1971.
SC M+F >2000 Toyoshima et al, 1971.
Dermal M+F 2100 Re and Gera, 1978c
(1522-2898)
M+F >5000 Toyoshima et al, 1968.
Mouse Oral M+F 360-840 Salvaneschi, 1968; Re and Gera,
1978b; Trabucchi, 1965;
Pellegrini & Santi, 1972.
IP M+F 420-430 Toyoshima et al, 1971.
IV M+F >250 Salvaneschi, 1968.
SC M+F >2000 Toyoshima, et al, 1971.
Dog Oral >500 Salvaneschi, 1968.
ca 500 Trabucchi, 1965.
Guinea pig Oral 377 Salvaneschi, 1968; Pellegrini
& Santi, 1977.
ca 400 Trabucchi, 1965.
Rabbit Oral ca 210 Salvaneschi, 1968; Pellegrini
& Santi, 1972.
Dermal 1830 Re and Gera, 1978d.
(unabraded skin) (1194-1595)
Abraded skin 2220 Re and Gera, 1978d.
(1872-2697)
Hare M 72 Pellegrini & Santi, 1971.
Chicken Oral ca 2551 Salvaneschi, 1968; Pellegrini
& Santi 1972.
2990 Fletcher et al, 1976
1 This value was obtained with technical product of 90.5% purity. A purified
technical product had an LD50 of 2800 mg/kg. (See the section on potentiation
for an explanation).
2 The acute oral toxicity of phenthoate oxon is 63 mg/kg bw (Pellegrini and
Santi, 1972).
because of autolysis, a sufficient number of survivors were
available to evaluate the carcinogenic potential. In this study,
phenthoate was not a carcinogen in mice (Oscarson et al, 1976).
Toxic signs of poisoning were similar to those noted with other
cholinergic organophosphate esters. Mortality occurred within 1-10
hours following acute intoxication. Signs of poisoning included
salivation, lacrimation, slowed or laboured respiration, tachycardia,
exophthalmus, tremors and convulsions followed by death.
No antidotal studies were reported, although it is probable that
atropine and 2-PAM will be therapeutic in the event of acute
overexposure.
Phenthoate (technical product) applied to the skin of rabbits was
found to be non-irritating, inducing a slight erythema 24 hours after
treatment (Re and Gera, 1978e). Formulated phenthoate is extremely
irritating (see short-term dermal studies in rabbits).
As with dermal studies, technical phenthoate, administered to the
conjunctival sac of rabbits, was found to be a non-irritant. However,
a formulation of phenthoate was irritating, increasing in its
irritability properties for 2-3 days after administration and
producing lasting effects for up to 14 days after treatment. While
phenthoate is not an irritant as a technical product, the formulated
products are irritants (Re and Gera, 1975a).
Acute toxicity studies examining the stability of formulated
phenthoate during storage have shown an increased toxicity when the
product was stored at 40°C for 12 months. The acute oral LD50 value
decreased from 258 mg/kg at the inception of the study to 130 mg/kg at
one year. The rate of change of acute toxicity was as shown in Table
2.
TABLE 2. Change of acute toxicity with time
Storage Time LD50
(months) (mg/kg)
0 258 (217-307)
1 282 (185-430)
3 200 (163-244)
6 119
12 130 (100-170)
(Noakes, 1963)
All samples produced similar signs of poisoning but those stored for 6
months or longer had a more rapid onset of toxic signs. The increased
toxicity may be as a result of formation of the trimethylthiophosphate
impurities known to potentiate the acute toxicity of phenthoate (see
Special studies on potentiation).
Short-term studies
Rabbit - dermal
In a series of three short-term dermal studies, a phenthoate
formulation was administered to rabbits and its dermal toxicity
evaluated. Groups of young adult rabbits (5 male and 5 female (3 of
each sex were tested at the two lowest doses) New Zealand albino
rabbits/group) were administered phenthoate (Cidial(R) E-4) dermally,
five days per week for three weeks to intact or abraded skin at dosage
rates of 2, 20, 75, 200, or 400 mg/kg/bw. Negative controls were run
with each test. Animals were observed daily for mortality and growth
was evaluated by body weight changes at weekly intervals. At the
beginning and at the end of the study, haematologic, clinical
chemistry, and urine analyses ware performed. Cholinesterase activity
was measured only in those rabbits treated at dosage levels below 200
mg/kg. Growth and microscopic examinations were performed on a
variety of tissues and organs in all animals. Over the course of the
studies, mortality was reported, primarily in the 400 mg/kg dosage
group. Hyperactivity was noted in all treated animals. It was
observed that at all dosage levels, the phenthoate formulation was
severely irritating to the skin and probably contributed to the
hyperactivity. Muscular fibrillations were observed in all animals at
the highest dose level. Significant body weight loss was observed in
all treated animals at the highest dose level. There were no unusual
effects noted in the haematologic, clinical chemistry, or urinalysis
parameters.
Significant erythrocyte cholinesterase was observed at 75 mg/kg in
both abraded and non-abraded groups. Plasma cholinesterase was only
slightly affected, predominantly toward the end of the treatment
period. The effect of phenthoate on blood cholinesterase appeared to
be cumulative, reaching maximal levels towards the end of the
treatment interval. After the dosing ended, plasma cholinesterase
recovered rapidly while erythrocyte cholinesterase, depressed at the
conclusion of the study, was still approaching normal 21 days after
the last treatment. No inhibition of cholinesterase was observed at
20 mg/kg.
There were no significant gross or microscopic changes noted, with the
exception of dermal changes characterised by pale red erythema, slight
to moderate edema, moderate desquamation and superficial escharosis.
Microscopic examination also revealed dermal changes in the epidermis,
predominantly at the high dose level. There were no other gross or
microscopic changes attributable to the administration of phenthoate
(Brett et al, 1974a; 1974b; Paa et al, 1974).
Groups of rabbits (2 male and 2 female rabbits/group) were
administered phenthoate dermally, to the intact or abraded skin, 5
days/week for 3 weeks at dosage levels of 0, 10, 50 or 100 mg/kg bw.
Growth, physical appearance and behaviour, food consumption, clinical
chemistry, haematology, and urine analyses were performed. At the
conclusion of the study, absolute and relative organ weights were
obtained on gross examination. Microscopic examinations of tissues
and organs were also performed.
Mortality was observed, as 1 male and 1 female administered 100 mg/kg
(intact skin) died during the course of the study. These animals had
shown severe signs of poisoning and mortality was attributable to the
phenthoate treatments.
An apparent anaemia (normocytic) was observed predominantly in the
survivors of the high-dose group. It was characterized by reduced
haematocrit, haemoglobin and red blood cell concentrations. A
dose-related reduction of cholinesterase activity was also noted. Red
blood cell, plasma and brain, cholinesterase activity were depressed
at all dose levels. No other significant effects were noted with
respect to clinical chemistry, haematology, and urinalyses. Organ
weight data as well as gross and microscopic pathology were not
unusual. Other than severe dermatitis, there were no pathological
effects noted in the study (Serota et al, 1979).
Rat - dietary
Groups of rats (10 male and 10 female, Donryu rats/group) were fed
phenthoate in the diet at dosage levels of 0, 5, 10, 30, 100, 300 or
1000 mg/kg for 3 months. Animals were observed daily for behaviourial
changes and growth was recorded at weekly intervals. At the
conclusion of the study, haematological, blood chemistry, and urine
analyses were performed. Animals were sacrificed at 3 months and
gross and microscopic examinations of the tissues and organs were
performed.
While there were no gross toxic signs of poisoning, growth, especially
in male rats fed 1000 mg/kg was reduced. Females receiving 1000 mg/kg
in the diet displayed a slight depression of body weight during the
course of the study. Food consumption data were normal. At 1000
mg/kg, there was a significant change in total and differential white
blood cell counts in both males and females. Urinalysis data, with
the exception of slight changes in sodium excretion values in both
males and females (males increased, females decreased) at 1000 mg/kg,
did not show changes attributable to the presence of phenthoate in the
diet. Slight blood chemistry changes were also observed at 1000 mg/kg
with respect to several parameters (SGOT, BUN, A/G ratios, and
cholinesterase activity). With the exception of cholinesterase
depression, the other clinical chemistry parameters were not
substantially different from control values.
Erythrocyte cholinesterase was significantly depressed in both males
and females at 30 mg/kg and above. At 10 mg/kg, a slight reduction of
activity was reported (less than 25%). Significant inhibition of
plasma cholinesterase was noted only in male rats fed 1000 mg/kg.
There was no significant inhibition of brain cholinesterase at 1000
mg/kg.
Gross and microscopic examinations of tissues and organs showed no
significant effects attributable to the presence of phenthoate at
dosage levels below 1000 mg/kg. At 1000 mg/kg several major organs in
both males and females showed significant weight depression: thymus,
heart, adrenal glands, testes, and ovaries. In males, the weight of
liver, kidney, spleen, and seminal vesicles was decreased while in
females, the weight of the heart, lung, and uterus was decreased. The
gross changes observed at the conclusion of the study were not
reflected in abnormalities noted on microscopic histopathologic
examination.
Based on these studies, a no-effect level is 10 mg/kg (Toyoshima et
al, 1973).
Mice
Groups of mice (10 male and 10 female ICR- mice/group) were
administered phenthoate in the diet at dosage levels of 0, 5, 10, 30,
100, 300 or 1000 mg/kg for 3 months. Growth, as observed by body
weight changes, was recorded on a weekly basis. Food and water
consumption data were recorded and at the conclusion of the study,
animals were sacrificed for haematological, blood chemistry, and urine
analyses. Gross and microscopic examinations of tissues and organs
were performed at the conclusion of the study.
There was no mortality observed over the 3-month interval. Growth was
depressed at 1000 mg/kg, primarily during the initial parts of the
study. After the first month, growth was normal in all test groups.
Food and water consumption were not affected by the presence of
phenthoate. Haematologic values in both males and females showed a
trend towards a reduction in the total and differential leucocyte
count in both males and females. This trend was not significantly
different from control values at dosage levels below 1000 mg/kg.
Urinalyses were normal as were all blood chemistry values with the
exception of cholinesterase activity. Significant plasma
cholinesterase depression was observed only at 1000 mg/kg. At 100
mg/kg and above, erythrocyte cholinesterase was inhibited. Brain
cholinesterase depression was not observed at any dose level.
On gross examination at the conclusion of the study, organ weight
changes were observed at 1000 mg/kg. Male mice showed a significant
depression of the thymus and adrenal glands, while female mice showed
significant depression of the weight of heart, lungs, thymus, liver,
kidneys, spleen, uterus, and ovaries. On histological examination,
the tissues of treated animals did not display pathological changes
attributable to the presence of phenthoate, suggesting that the gross
changes were physiological adaptation to the high dosage of
phenthoate. A no-effect level in the study, based upon cholinesterase
depression in both males and females, is 30 mg/kg in the diet
(Toyoshima et al, 1973).
Dogs
Groups of dogs (4 female and 4 male, beagle dogs/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg for
2 years. Daily examinations were made for clinical signs of poisoning
or adverse behaviour. Growth, as evidenced by body weight, was
recorded weekly as were food consumption data. At periodic intervals
(1, 3, 6, 9, 12, 18, and 24 months), haematology, blood chemistry
(including cholinesterase) and urine analyses were performed. Plasma
and erythrocyte cholinesterase determinations were performed at 2, 4,
6, 9, 13, 26, 78, and 104 weeks and brain cholinesterase analyses were
conducted at the conclusion of the study. At the conclusion of 104
weeks of dietary administration, each dog was sacrificed and gross and
microscopic examinations of tissues and organs were performed.
There was no mortality over the course of the study at any dosage
level. Growth and food consumption were normal. With the exception
of cholinesterase depression, data from blood clinical chemistry
tests, haematological tests, and urinalyses were normal. Erythrocyte
cholinesterase inhibition was observed at 30 mg/kg and above. This
effect was first noted 2 weeks after the initiation of the study and
was relatively constant over the entire study. Plasma and brain
cholinesterase activity were unaffected and considered normal at all
dietary levels. Gross and microscopic examinations of tissues and
organs at the conclusion of the study did not show any significant
effect of the inclusion of phenthoate in the diet at dose levels up to
and including 100 mg/kg. A no-effect level in this study, based on
cholinesterase depression, was 10 mg/kg in the diet equivalent to 0.29
mg/kg bw, a value derived from the average food consumption values
over the 2-year period (Nelson et al, 1972).
Long-term study
Mice
(See special study on carcinogenicity).
Rats
No data available.
RESIDUES IN FOOD
USE PATTERN
Phenthoate is a wide spectrum insecticide for agricultural and
domestic use. It is active against both chewing and sucking insects,
in particular against lepidopterous larvae, soft scales and larvae and
adults of some species of mosquitoes. The pesticide should be applied
in enough water to insure complete coverage of the foliage, branches
and fruit when applied as a ground application.
The registered use patterns reported from Italy, Israel, Japan and
South Africa are summarised in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
The trials were performed in Brazil, Florida, Italy and South Africa
with both the recommended and approximately double dose rates. The
results are summarised in Tables 5 and 6. Treatments carried out with
low volume spraying (about 940 l/ha) or with 0.5% oil additive
resulted in somewhat higher residue than the dilute application, when
the spray was applied until run off to achieve full coverage.
The repeated treatments carried out following the recommended use
pattern did not increase the residue in the fruit significantly. The
colometric method used in 1963-65 experiments showed 3-4 times higher
residues than those obtained with GLC determination of samples
deriving from similarly treated plots at later trials.
The phenthoate residues remain entirely in the peel of fruits. The
pulp did not contain detectable residues (limit of determination
0.02-0-03 mg/kg) at any time after treatment with the exception of two
sets of experiments. In one case about 10-25% of the residue found in
the peel was detected in the pulp at the day of treatment and after 98
days, respectively. However in the other experiment the residue in
the pulp was only about 2.55% of the residue found in the peel and
decreased with time.
The phenthoate oxon was only detected in the peel. Limit of
determination in the pulp was 0.01 mg/kg (Moye). The oxon derivative
amounted to a maximum of 0.12 mg/kg (43%) on lemon, 0.08 mg/kg (15%)
on grapefruit and 0.62 mg/kg (38%) on orange. The values in
parentheses refer to the ratio of oxon to the parent compound. The
results of other trials (Iwata, 1979) indicate similar residue ranges.
The residue, on a whole fruit basis, can be calculated from the
residue measured in the peel taking into account the peel/pulp weight
ratio which is fairly constant for different varieties (Iwata, 1977).
For instance, in the case of valencia orange 1/5 of the peel residue
can be considered as the residue on whole fruit basis, while for the
variety Hamlin, the ratio is 1/4.
Other Crops
A limited number of supervised trials were carried out on apple, pear,
cabbage, lettuce, olives, onions, radish, spinach, sweet potato and
sugarbeet in Italy and Japan in 1962-65. The results of these
experiments were provided to the Joint Meeting for evaluation (Tables
7 and 8). However they were not considered to represent the current
use of phenthoate or to be sufficient for evaluation, thus no
TABLE 3. Registered Uses of Phenthoate
Crop Pest ai [g/100R] Formulation No. of treatments Amount of water l/ha
Citrus Scale insects ) 50 - 100 EC 1 - 2 1000-10000
Aphids ) 40 WP (generally)
Orange leaf miner )
Leaf rollers )
Apple Codling moth 40 - 50 EC 5 - 10 1000-2500
WP 1 - 6 5000
Brassica Large white butterfly 50 - 1000 EC 1 or more up to 1000
Japanese pears Leaf rollers 40 - 50 WP 1 - 6 3000
Fruit moths
Mealy bugs
Nuts Codling moth 70 EC 3 - 4 2000-10000
Olives Black scale 60 - 75 EC 3 - 4 2000-10000
Olive thrips
Onions Thrips 75 EC 1 or more up to 1000
Pears Codling moth 40 - 50 EC 3 - 7 1000-2000
Pear psylla 200 250 200 - 500
Potatoes Potato tuber moth 100 EC 1 or more up to 1000
Rice (paddy) Rice stem borers ) 50 - 100 EC 1 - 4 800 - 1500
Leaf and plant )
hoppers ) 1.65 - 3.3 kg EC 1 30-40 aerial
Rice bugs ) 0.6-0.8/kg/ha. Dust
Rice leaf beetle )
Rice leaf miner )
recommendation was made by the Meeting for the maximum residue levels
in/on these commodities.
FATE OF RESIDUES
In animals
Lactating dairy cattle were administered 14C-labelled phenthoate at
levels of 1, 5 and 20 mg/kg on feed basis (Wargo, Anonym. 1979).
Samples of urine, faeces and milk were collected every day during the
administration for 8, 18, 26 days and withdrawal (7 days).
The animals were slaughtered the following day and the tissue samples
were taken. The total 14C residue measured in various tissues and
calculated as parent compound at a dose rate of 20 mg/kg is given in
Table 9. Muscle and fat did not contain detectable residues at rates
of 1 and 5 mg/kg. The residue in the other tissues also decreased at
the lower rates.
Total 14C extractable residues in liver and kidney were 29% and 51%
respectively, and consisted of organosoluble and watersoluble
metabolites.
The metabolites were separated with various chromatographic methods.
In the liver at least eleven compounds were found, of which the major
component represented 8% of the total 14C residues in liver. One of
the remaining ten was present at a higher concentration than 3%. The
unextractable residue was quantitatively released by acidic
hydrolysis. At each feeding level the cows with a seven-day
withdrawal period demonstrated a significant decrease in residues
compared to the cows sacrificed 24 hours after the last 14C
phenthoate dose. Even the "bound" metabolites were eliminated from
the tissues of kidney and liver.
The 14C residues in milk reached the plateau on the second or third
day of treatment. During the feeding period the total residue
plateaued at the levels given below:
Level in diet Residue in milk, mg/kg
1 mg/kg 0.001-0.003
5 mg/kg 0.008-0.026
20 mg/kg 0.015-0.04
The residue declined after withdrawal of the pesticide from the diet
and approached the limit of determination after 3-6 days.
The 0.04 mg/kg total 14C residue in milk consisted of 7% (0.0028
mg/kg) organosoluble, 16% (O.006 mg/kg) water-soluble compounds and
52% (0.019 mg/kg) milk solids.
The residue level in each fraction was too low for further
investigation.
Neither phenthoate nor its oxon could be detected by conventional
analytical methods based on GLC determination in any of the tissue or
milk samples (limit of determination 0.01 mg/kg for fat and 0.05 mg/kg
for the other samples).
White leghorn laying hens were given feed containing 14C phenthoate
at 1.8, 5.9 or 26 mg/kg for 30 days. The birds were sacrificed on
days 1, 3, 7, 14, 21 and 30 during the treatment and on days 7 and 15
during the withdrawal period (Huhtanen, 1979a). The maximum residue
contents of tissues analyzed are given in table 4.
TABLE 4. Residues of phenthoate in tissues of chickens fed phenthoate
in the diet at various levels for 30 days
Tissue Concentration of phenthoate in diet
1.8 mg/kg 5.9 mg/kg 26 mg/kg
breast muscle 0.333 0.072 0.073
leg muscle 0.018 0.035 0.13
liver 0.024 0.04 0.48
kidney 0.094 0.53 1.3
skin 0.039 0.16 0.38
fat 0.019 0.07 0.16
eggs 0.014 0.058 0.35
limits of detection
for non-fatty tissues 0.0077 0.027 0.066
for fatty tissues 0.016 0.053 0.13
A plateau of the residue was reached in liver and kidney between the
third and seventh day of treatment. In the muscles detectable
residues were found from 21st day of treatment but no residue was
detected during the withdrawal period either in the muscles or in the
eggs. Less than 10% of the extracted material, which accounted for 70
of total radioactivity, was of non-polar character in the kidney. A
large percentage (50%) of the more polar compounds were acidic.
No abnormalities were observed in the organs of the hens and the
production of eggs was not reduced during the treatment period.
Fish residue studies were carried out with channel catfish and
bluegill sunfish in order to determine the biomagnification behaviour
of phenthoate.
In water containing 14C-phenthoate at 10-6 mg/l level the average
concentration ratios from catfish tissue/mud and catfish tissue/water
were 0.17 (0.09-0.33) and 5.76 (2.47-9.97) respectively after 33 days.
Residue levels in contaminated fishes showed a gradual reduction if
the fishes were placed in uncontaminated water (Marshall, 1979).
The uptake and bioaccumulation of 14C-phenthoate residues from soil
aged for 21 days in the aquaria into channel catfish were studied at
fortified soil levels of 0.01 and 1 mg/kg. The residues in the
catfish "meat" were 0.003 mg/kg 28 days after placing them into the
contaminated water (Booth, 1978).
Bluegill sunfish were exposed to 14C-phenthoate at 2.1 × 10-5 mg/l or
at 2 × 10-4 mg/1 in the water of a simulated dynamic water system for
30 days. The average bioaccumulation ratio of 14C was 816 and 901
respectively, while for the edible tissue 240 and 248 were the
calculated values. The residues found in tissues of 14-day withdrawal
samples taken from the higher level tank was only 3% of the level
found after 30 days of exposure (Huhtanen, 1979b).
In plants
Selected leaves and fruit of a Valencia orange tree were treated by
brush application with a solution of 30 mg phenthoate [32P or 14C] in
50 cm of water containing 0.06% emulsifier (Takade, 1977). Samples of
leaves and fruits were taken 3, 7, 10 and 14 days after treatment and
rinsed 5 times with acetone to remove the surface material. After
rinsing with acetone, the fruit was carefully peeled. The leaves,
peel and pulp were cut into small pieces and placed in a vacuum oven
at 40°C for approximately 10 hours. No loss in radioactivity was
detected for the drying process. The dried samples were extracted
with acetone. The metabolites were separated on thin layer plates
using four solvent systems. The total radioactivity of solid
materials was then determined by combustion analysis.
The major portion of phenthoate applied to leaves was lost by
volatilisation. The concentration and distribution of residue in
orange fruit are given in Table 10. Significant loss in total
recovery wan observed in fruit. Less than 1% of the total activity
applied was detected in the orange pulp. The majority of the residue
both in fruit and in leaves was intact phenthoate. The ratio of
phenthoate and its phenthoate oxon is shown in Table 11. Other
metabolites, desmethyl phenthoate, mandelic acid, bis-alpha-ethoxy
carbonylbenzyl disulphide, O,O-dimethyl phosphorodithioic acid, O,O-
dimethyl-phosphorothioic acid and two unidentified compounds amounted
to 0-5.2% individually. Glucosidase, hydrochloric acid and sodium
hydroxide together dissolved about 92-98% of the bound residues in the
peel. The major conjugated metabolites were, in order of importance,
ethyl mandelate, mandelic acid, desmethyl phenthoate and phenthoate
acid (Mallipudi and Fukuto, 1980).
Apple, pear and olive trees were treated with 32P-labelled phenthoate
according to the recommended use pattern (Santi, 1967). The surface
of the fruits was washed with methanol to remove the original active
substance or any other derivatives containing methanol-soluble 32P
still present on the peel (methanol extract).
After washing, the various fruits were homogenised in a blender with
chloroform (chloroform extract).
The plant filter cake was further extracted with water to separate
eventual water-soluble compounds (water extract). The 32P content of
various extracts and of residual plant was determined radiometrically.
The phenthoate equivalents of the radioactive residues found at
certain times after treatments are listed in Table 12.
Column chromatography, thin-layer chromatography and paper
chromatography were used for the separation and identification of the
compounds in the various extracts.
The probable metabolic pathways are shown in Fig. 2.
The repeated treatments of apple trees with a solution containing
0.02% ai (half the dosage usually applied) resulted in qualitatively
similar picture regardless the number of treatments. The phenthoate,
although not systemic, penetrates slightly into the fruits and
undergoes oxidation and hydrolysis; 15-29 days after treatment 95% of
water-soluble residue was phosphoric acid while monomethyl and
dimethyl phosphoric acids were detected in traces. Phenthoate oxon
was detected in traces only 1 day after treatment.
Effects on enzymes and other biochemical parameters
Groups of rats (15 males and females/group) were administered
phenthoate orally for 90 days at daily dosage levels of 0, 2.5,
5.0, 12.5 or 25 mg/kg bw. Plasma, erythrocyte, and brain
cholinesterase were the most sensitive, being inhibited about 50%
of normal at the highest dose (plasma was slightly less inhibited).
No significant inhibition was observed at 5 mg/kg (Trabucchi,
1965). Also see Special Studies on Potentiation for studies on the
effects of organophosphate esters on serum and liver
carboxyesterases, enzymes directly involved in the degradation of
phenthoate.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rats
Two almost identical reproduction studies were performed. In both
studies, groups of rats (8 male and 16 female rats/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg
and subjected to a standard 2-litter per generation, 3 generation
reproduction study. Animals were fed phenthoate from 21 days of
age until 100 days of age when they were mated to initiate the
study. The first litters were weaned at 21 days, examined, and
discarded. The parental animals were again mated to provide a
second litter, which became the parental animals for the second
generation. Eight males and 16 females from the second litters of
each generation were selected for the succeeding generation. Gross
pathology examinations were performed on the second litters of each
generation. In one of the studies, gross and microscopic
examinations of selected tissues and organs were conducted on males
and females in the control and high-level group of each parental
generation and on the second litter of the third generation
animals. Additionally, in one of the studies, cholinesterase
activity was measured in male and female animals following weaning
of the second litter. This was conducted on both the parental and
weanling animals with respect to plasma, erythrocyte and brain
cholinesterase activity. Although these two latter parameters were
not evaluated in one reproduction study, gross pathological
examinations were made.
Reproduction indices, including mating, fecundity, male and female
fertility, gestation, and lactation, were calculated and compared
with control values. All pups were examined for physical
abnormalities and viability.
With the exception of a slightly depressed erythrocyte
cholinesterase activity observed at 100 mg/kg in all generations of
both parents and weanlings, there were no significant effects of
phenthoate on any reproduction parameter measured in the study. In
both studies, there were no differences with respect to any of the
indices observed, and it was considered that phenthoate, at dietary
dosage levels up to and including 100 mg/kg displays no reproduction
hazard to rats (Pfeifer et al, 1975; Wright et al, 1975).
Special study on teratogenicity
Groups of rabbits (17 pregnant New Zealand albino rabbits/group)
were administered phenthoate orally at dosages of 0, 3 or 10 mg/kg
body weight from days 6 through 18 of gestation in a standard
teratology bioassay. A positive control with thalidomide (50 mg/kg
bw), administered during gestation, was included in the study. On
day 29 of gestation, all pregnant animals were sacrificed and
foetuses delivered by caesarean section.
There was no mortality attributable to phenthoate. The data
related to reproduction (implantation sites, resorption sites, and
live young) were not affected by phenthoate at the highest dose
level studies. There were no external or internal abnormalities
(teratogenic events) associated with phenthoate treatment of
pregnant rabbits. Growth of the foetuses was unaffected as was
survival of young for 24 hours after delivery. Internal
development, including somatic and skeletal development, was
normal. There were no teratogenic effects noted on the
administration of phenthoate to pregnant rabbits during the
critical period of organogenesis.
In contrast to the lack of teratogenic response with phenthoate,
thalidomide reduced the number of live young, increased the number
of resorption sites, and reduced foetal viability. Several of the
foetuses exhibited external somatic and skeletal abnormalities
suggesting that the strain of rabbit used in the study was
susceptible to eliciting a chemically-induced teratogenic response.
Treatment of rabbits with phenthoate during gestation (foetal
organogenesis) did not induce a teratogenic response (Ladd. et
al, 1975).
Special studies on mutagenicity
Mammalian tests
In two identical studies, groups of male mice (12 mice/group) were
administered phenthoate as a single intraperitoneal injection at
dosage levels of 0, 150 or 300 mg/kg bw. Each animal of each group
was mated with groups of three untreated, virgin females for a
period of one week after which the females were removed and
replaced by another group of females. This procedure continued for
six consecutive weeks, the period required for maturation of the
male germ cell. Females were sacrificed approximately one week
after breeding and the number of implantation sites, resorption
sites, and embryos were recorded. Data were collected with respect
to early and late deaths.
There was no mortality in male mice attributable to the phenthoate
treatment; the high-dose group was slightly hypoactive for 3-4
hours after treatment. Pregnancy rates of females mated to males
receiving 300 mg/kg was slightly lower than the control values for
weeks 4 and 6. The numbers of implantation sites, resorption
sites, and embryos from females mated with treated males were the
same as those from control matings. There were essentially no
differences between treatment groups and controls with respect to
mutation rates indicating that, in this in vivo mutagenicity
study, phenthoate is not a mutagen affecting male germinal cells
(Arnold et al, 1974; 1975).
Microbiological tests
An evaluation of the mutagenicity potential of phenthoate was
performed using the standard Ames assay and other microbial test
systems. Strains of Salmonella typhimurium (TA1535, TA1538,
TA98 and TA100), with and without a metabolic activation system S-9
derived from rat liver-induced with Aroclor 1254, were tested at
concentrations up to and including 2 mg/plate in an effort to
evaluate genetic mutations. Standard positive control chemicals
were used (2-AAF, 2-AA, and B(a)P) in the presence of the metabolic
activation system and (MMNG, 2NF, and 9-AA) in the absence of the
metabolic activation system.
Under the conditions of the experimental assay, phenthoate showed
no mutagenic activity towards these strains of microorganisms
either in the presence or in the absence of an enzymatic metabolic
system. (Carneri, 1979).
A similar study with Salmonella typhimurium was reported
where phenthoate was tested at concentrations up to and including
5 mg/plate, in the presence and absence of a male rat liver
metabolic activation system. In addition, two strains of E.
coli WP-2 were tested for mutagenicity at the same
concentrations. Again, there was no evidence of mutagenicity,
either in the presence or absence of the metabolic activation
system utilised, with either species tested (Shirasu, et al,
1976).
The recombination-capacity of B. subtilis (H-17 and M-45) was
tested following exposure to phenthoate. Phenthoate did not
prohibit growth in this assay, again demonstrating no mutagenic
potential (Shirasu et al, 1976).
A standard, host-mediated assay was performed with mice, utilising
S. typhimurium as an indicator strain. Phenthoate was
administered to groups of 6 mice at dosage levels of O, 100 or 300
mg/kg body weight by oral intubation for two consecutive days.
After the second dosing, the indicator strain was administered to
the peritoneal cavity, recovered and grown and the mutagenic
potential of phenthoate evaluated. The number of reverted colonies
did not increase as a result of in vivo phenthoate treatment
suggesting that, under the conditions of this microbial assay,
phenthoate is not mutagenic (Shirasu et al, 1976).
Special studies on neurotoxicity
Chickens
Groups of adult hens, fasted for 16 hours, were administered
phenthoate orally at dosage levels of 0 or 2,990 mg/kg bw (a
previously observed LD50 value). A positive control (TOCP, 0.5
mg/kg) was also used. Ten animals were employed in each test
group. With the exception of the TOCP treatment, the single dose
was repeated following a 21-day observation period. At the
conclusion of the study (42 days), all surviving birds were
sacrificed and subjected to gross and microscopic examinations for
axon and myelin disruption. These included the brain, sciatic
nerve, and spinal cord.
There were no signs of delayed neurotoxicity observed with
phenthoate following both treatments although half of the animals
died over the course of the study. The positive control animals
(TOCP) were observed to lose weight and on microscopic examination
were found to display myelin and axon disruption in the spinal cord
and sciatic nerve. Axon degeneration and myelin disruption were
not seen in the brain of the TOCP-treated animals. Based on the
results of this study, it was concluded that phenthoate does not
induce a delayed neurotoxic reaction similar to that seen with TOCP
in adult chickens (Fletcher et al, 1976).
Groups of hens (10 adult hens/group) were administered phenthoate
orally at dosage levels of 0, 10, 50, 100 or 750 mg/kg/bw to assess
the delayed-neurotoxic potential of phenthoate. Animals
administered the highest dose level were also administered atropine
and 2-PAM to control cholinergic signs of poisoning. A positive
control of TOCP (1,000 mg/kg) and an untreated negative control
were included in the study.
When mortality was observed at the highest dose level, a second
group of hens was introduced using this dose level. These, too,
were protected from the acute cholinergic signs of poisoning. In
all phenthoate and negative control animals, there were no clinical
or histological signs of delayed neurotoxicity, although acute
signs of cholinergic stimulation were evident with phenthoate. The
positive control animals, administered TOCP, exhibited ataxia,
paralysis, and standard signs of delayed neurotoxicity.
Histological examination of the TOCP animals revealed a significant
number of animals with histological defects in the nervous tissue.
Based upon this bioassay, phenthoate does not induce a
delayed-neurotoxic reaction in hens (Good et al, 1979).
Special studies on potentiation
Pellegrini and Santi (1972) have reported on the potentiating
effect of a series of common organophosphorous compounds found as
impurities in technical phenthoate (as well as other methyl
organophosphate esters). Several of the trimethylphosphate
impurities substantially potentiate the acute toxicity of
phenthoate, probably through interaction at the carboxylic acid
portion of the molecule. Similar results (with respect to increased
acute toxicity when combinations of OP's were tested) were obtained
with malathion, providing evidence for the inhibition of
carboxyesterase activity as a primary mode of action in this
potentiation.
The acute LD50 of phenthoate was seen to change substantially as
the presence of impurities in technical mixture increased. A
highly purified phenthoate technical sample (98.5%) had an oral
LD50 in rats of 4,728 mg/kg body weight. As the content of
impurities increased, the rat oral LD50 value decreased (90.50% =
LD50 of 242 mg/kg; 78.7% = 118 mg/kg; 61.2% = 77.7 mg/kg). Two
impurities, O,S,S-trimethyl phosphorodithioate and O,O,S-trimethyl
phosphorothioate, were found to be extremely active in potentiating
the oral LD50 in rats. Fukuto and his coworkers (Stevens and
Fukuto, 1980; Umetsu et al, 1980; Mallipudi et al, 1980;
Hammond et al, 1980) have recently confirmed this potentiation
and its probable mode of action. In in vitro studies,
hydrolysis of phenthoate by rat liver and serum carboxyesterases
was examined in the presence and absence of these two impurities.
Phenthoate acid was the exclusive degradation product obtained with
the carboxyesterases. In the presence of impurities, the rate of
hydrolysis of phenthoate to phenthoate acid was substantially
diminished, supporting the conclusion that inhibition of
carboxyesterases is the primary cause of potentiation of phenthoate
toxicity.
Special study for carcinogenicity
Mice
Groups of mice (50 male and 50 female (Charles River) mice/group)
were fed phenthoate in the diet at dosage levels of 0, 500 or 1000
mg/kg for 18 months. The animals were examined daily for mortality
and adverse behaviourial reactions. At the conclusion of the
study, growth and microscopic examination of tissues and organs was
performed. Microscopic analysis of 10 animals of each sex surviving
the 18-month treatment was performed, as were microscopic studies
on animals that were sacrificed or had died during the course of
the study. All neoplasms and tissues with suspected neoplasms were
examined histologically. Complete pathological examinations were
made specifically to identify neoplastic lesions.
There were no unusual behaviourial changes or increases in
mortality over the course of the study attributable to the presence
of phenthoate. At the conclusion of the study, histological
examination revealed no treatment-related lesions among any of the
animals. The lesions that did occur were considered to be
naturally occurring, not attributable to the presence of phenthoate
and not unusual for the strain of mice. Although a substantial
number of animals that died were not examined histologically
TABLE 1. Acute Toxicity
Species Route Sex LD50 mg/kg bw Reference
Rat Oral2 M+F 245-440 Salvaneschi, 1968; He & Gera,
1978a; Toyoshima et al, 1978;
Trabucchi, 1965; Pellegrini &
Santi, 1972.
M 270 (231-316) Toyoshima et al, 1978.
F 255 (216-301) Toyoshima et al, 1978.
IP M+F 720 (672-77) Re and Gera, 1975b
M 720 (634-868) Toyoshima et al, 1971.
F 745 (615-901) Toyoshima et al, 1971.
SC M+F >2000 Toyoshima et al, 1971.
Dermal M+F 2100 Re and Gera, 1978c
(1522-2898)
M+F >5000 Toyoshima et al, 1968.
Mouse Oral M+F 360-840 Salvaneschi, 1968; Re and Gera,
1978b; Trabucchi, 1965;
Pellegrini & Santi, 1972.
IP M+F 420-430 Toyoshima et al, 1971.
IV M+F >250 Salvaneschi, 1968.
SC M+F >2000 Toyoshima, et al, 1971.
Dog Oral >500 Salvaneschi, 1968.
ca 500 Trabucchi, 1965.
Guinea pig Oral 377 Salvaneschi, 1968; Pellegrini
& Santi, 1977.
ca 400 Trabucchi, 1965.
Rabbit Oral ca 210 Salvaneschi, 1968; Pellegrini
& Santi, 1972.
Dermal 1830 Re and Gera, 1978d.
(unabraded skin) (1194-1595)
Abraded skin 2220 Re and Gera, 1978d.
(1872-2697)
Hare M 72 Pellegrini & Santi, 1971.
Chicken Oral ca 2551 Salvaneschi, 1968; Pellegrini
& Santi 1972.
2990 Fletcher et al, 1976
1 This value was obtained with technical product of 90.5% purity. A purified
technical product had an LD50 of 2800 mg/kg. (See the section on potentiation
for an explanation).
2 The acute oral toxicity of phenthoate oxon is 63 mg/kg bw (Pellegrini and
Santi, 1972).
because of autolysis, a sufficient number of survivors were
available to evaluate the carcinogenic potential. In this study,
phenthoate was not a carcinogen in mice (Oscarson et al, 1976).
Toxic signs of poisoning were similar to those noted with other
cholinergic organophosphate esters. Mortality occurred within 1-10
hours following acute intoxication. Signs of poisoning included
salivation, lacrimation, slowed or laboured respiration, tachycardia,
exophthalmus, tremors and convulsions followed by death.
No antidotal studies were reported, although it is probable that
atropine and 2-PAM will be therapeutic in the event of acute
overexposure.
Phenthoate (technical product) applied to the skin of rabbits was
found to be non-irritating, inducing a slight erythema 24 hours after
treatment (Re and Gera, 1978e). Formulated phenthoate is extremely
irritating (see short-term dermal studies in rabbits).
As with dermal studies, technical phenthoate, administered to the
conjunctival sac of rabbits, was found to be a non-irritant. However,
a formulation of phenthoate was irritating, increasing in its
irritability properties for 2-3 days after administration and
producing lasting effects for up to 14 days after treatment. While
phenthoate is not an irritant as a technical product, the formulated
products are irritants (Re and Gera, 1975a).
Acute toxicity studies examining the stability of formulated
phenthoate during storage have shown an increased toxicity when the
product was stored at 40°C for 12 months. The acute oral LD50 value
decreased from 258 mg/kg at the inception of the study to 130 mg/kg at
one year. The rate of change of acute toxicity was as shown in Table
2.
TABLE 2. Change of acute toxicity with time
Storage Time LD50
(months) (mg/kg)
0 258 (217-307)
1 282 (185-430)
3 200 (163-244)
6 119
12 130 (100-170)
(Noakes, 1963)
All samples produced similar signs of poisoning but those stored for 6
months or longer had a more rapid onset of toxic signs. The increased
toxicity may be as a result of formation of the trimethylthiophosphate
impurities known to potentiate the acute toxicity of phenthoate (see
Special studies on potentiation).
Short-term studies
Rabbit - dermal
In a series of three short-term dermal studies, a phenthoate
formulation was administered to rabbits and its dermal toxicity
evaluated. Groups of young adult rabbits (5 male and 5 female (3 of
each sex were tested at the two lowest doses) New Zealand albino
rabbits/group) were administered phenthoate (Cidial(R) E-4) dermally,
five days per week for three weeks to intact or abraded skin at dosage
rates of 2, 20, 75, 200, or 400 mg/kg/bw. Negative controls were run
with each test. Animals were observed daily for mortality and growth
was evaluated by body weight changes at weekly intervals. At the
beginning and at the end of the study, haematologic, clinical
chemistry, and urine analyses ware performed. Cholinesterase activity
was measured only in those rabbits treated at dosage levels below 200
mg/kg. Growth and microscopic examinations were performed on a
variety of tissues and organs in all animals. Over the course of the
studies, mortality was reported, primarily in the 400 mg/kg dosage
group. Hyperactivity was noted in all treated animals. It was
observed that at all dosage levels, the phenthoate formulation was
severely irritating to the skin and probably contributed to the
hyperactivity. Muscular fibrillations were observed in all animals at
the highest dose level. Significant body weight loss was observed in
all treated animals at the highest dose level. There were no unusual
effects noted in the haematologic, clinical chemistry, or urinalysis
parameters.
Significant erythrocyte cholinesterase was observed at 75 mg/kg in
both abraded and non-abraded groups. Plasma cholinesterase was only
slightly affected, predominantly toward the end of the treatment
period. The effect of phenthoate on blood cholinesterase appeared to
be cumulative, reaching maximal levels towards the end of the
treatment interval. After the dosing ended, plasma cholinesterase
recovered rapidly while erythrocyte cholinesterase, depressed at the
conclusion of the study, was still approaching normal 21 days after
the last treatment. No inhibition of cholinesterase was observed at
20 mg/kg.
There were no significant gross or microscopic changes noted, with the
exception of dermal changes characterised by pale red erythema, slight
to moderate edema, moderate desquamation and superficial escharosis.
Microscopic examination also revealed dermal changes in the epidermis,
predominantly at the high dose level. There were no other gross or
microscopic changes attributable to the administration of phenthoate
(Brett et al, 1974a; 1974b; Paa et al, 1974).
Groups of rabbits (2 male and 2 female rabbits/group) were
administered phenthoate dermally, to the intact or abraded skin, 5
days/week for 3 weeks at dosage levels of 0, 10, 50 or 100 mg/kg bw.
Growth, physical appearance and behaviour, food consumption, clinical
chemistry, haematology, and urine analyses were performed. At the
conclusion of the study, absolute and relative organ weights were
obtained on gross examination. Microscopic examinations of tissues
and organs were also performed.
Mortality was observed, as 1 male and 1 female administered 100 mg/kg
(intact skin) died during the course of the study. These animals had
shown severe signs of poisoning and mortality was attributable to the
phenthoate treatments.
An apparent anaemia (normocytic) was observed predominantly in the
survivors of the high-dose group. It was characterized by reduced
haematocrit, haemoglobin and red blood cell concentrations. A
dose-related reduction of cholinesterase activity was also noted. Red
blood cell, plasma and brain, cholinesterase activity were depressed
at all dose levels. No other significant effects were noted with
respect to clinical chemistry, haematology, and urinalyses. Organ
weight data as well as gross and microscopic pathology were not
unusual. Other than severe dermatitis, there were no pathological
effects noted in the study (Serota et al, 1979).
Rat - dietary
Groups of rats (10 male and 10 female, Donryu rats/group) were fed
phenthoate in the diet at dosage levels of 0, 5, 10, 30, 100, 300 or
1000 mg/kg for 3 months. Animals were observed daily for behaviourial
changes and growth was recorded at weekly intervals. At the
conclusion of the study, haematological, blood chemistry, and urine
analyses were performed. Animals were sacrificed at 3 months and
gross and microscopic examinations of the tissues and organs were
performed.
While there were no gross toxic signs of poisoning, growth, especially
in male rats fed 1000 mg/kg was reduced. Females receiving 1000 mg/kg
in the diet displayed a slight depression of body weight during the
course of the study. Food consumption data were normal. At 1000
mg/kg, there was a significant change in total and differential white
blood cell counts in both males and females. Urinalysis data, with
the exception of slight changes in sodium excretion values in both
males and females (males increased, females decreased) at 1000 mg/kg,
did not show changes attributable to the presence of phenthoate in the
diet. Slight blood chemistry changes were also observed at 1000 mg/kg
with respect to several parameters (SGOT, BUN, A/G ratios, and
cholinesterase activity). With the exception of cholinesterase
depression, the other clinical chemistry parameters were not
substantially different from control values.
Erythrocyte cholinesterase was significantly depressed in both males
and females at 30 mg/kg and above. At 10 mg/kg, a slight reduction of
activity was reported (less than 25%). Significant inhibition of
plasma cholinesterase was noted only in male rats fed 1000 mg/kg.
There was no significant inhibition of brain cholinesterase at 1000
mg/kg.
Gross and microscopic examinations of tissues and organs showed no
significant effects attributable to the presence of phenthoate at
dosage levels below 1000 mg/kg. At 1000 mg/kg several major organs in
both males and females showed significant weight depression: thymus,
heart, adrenal glands, testes, and ovaries. In males, the weight of
liver, kidney, spleen, and seminal vesicles was decreased while in
females, the weight of the heart, lung, and uterus was decreased. The
gross changes observed at the conclusion of the study were not
reflected in abnormalities noted on microscopic histopathologic
examination.
Based on these studies, a no-effect level is 10 mg/kg (Toyoshima et
al, 1973).
Mice
Groups of mice (10 male and 10 female ICR- mice/group) were
administered phenthoate in the diet at dosage levels of 0, 5, 10, 30,
100, 300 or 1000 mg/kg for 3 months. Growth, as observed by body
weight changes, was recorded on a weekly basis. Food and water
consumption data were recorded and at the conclusion of the study,
animals were sacrificed for haematological, blood chemistry, and urine
analyses. Gross and microscopic examinations of tissues and organs
were performed at the conclusion of the study.
There was no mortality observed over the 3-month interval. Growth was
depressed at 1000 mg/kg, primarily during the initial parts of the
study. After the first month, growth was normal in all test groups.
Food and water consumption were not affected by the presence of
phenthoate. Haematologic values in both males and females showed a
trend towards a reduction in the total and differential leucocyte
count in both males and females. This trend was not significantly
different from control values at dosage levels below 1000 mg/kg.
Urinalyses were normal as were all blood chemistry values with the
exception of cholinesterase activity. Significant plasma
cholinesterase depression was observed only at 1000 mg/kg. At 100
mg/kg and above, erythrocyte cholinesterase was inhibited. Brain
cholinesterase depression was not observed at any dose level.
On gross examination at the conclusion of the study, organ weight
changes were observed at 1000 mg/kg. Male mice showed a significant
depression of the thymus and adrenal glands, while female mice showed
significant depression of the weight of heart, lungs, thymus, liver,
kidneys, spleen, uterus, and ovaries. On histological examination,
the tissues of treated animals did not display pathological changes
attributable to the presence of phenthoate, suggesting that the gross
changes were physiological adaptation to the high dosage of
phenthoate. A no-effect level in the study, based upon cholinesterase
depression in both males and females, is 30 mg/kg in the diet
(Toyoshima et al, 1973).
Dogs
Groups of dogs (4 female and 4 male, beagle dogs/group) were fed
phenthoate in the diet at dosage levels of 0, 10, 30, or 100 mg/kg for
2 years. Daily examinations were made for clinical signs of poisoning
or adverse behaviour. Growth, as evidenced by body weight, was
recorded weekly as were food consumption data. At periodic intervals
(1, 3, 6, 9, 12, 18, and 24 months), haematology, blood chemistry
(including cholinesterase) and urine analyses were performed. Plasma
and erythrocyte cholinesterase determinations were performed at 2, 4,
6, 9, 13, 26, 78, and 104 weeks and brain cholinesterase analyses were
conducted at the conclusion of the study. At the conclusion of 104
weeks of dietary administration, each dog was sacrificed and gross and
microscopic examinations of tissues and organs were performed.
There was no mortality over the course of the study at any dosage
level. Growth and food consumption were normal. With the exception
of cholinesterase depression, data from blood clinical chemistry
tests, haematological tests, and urinalyses were normal. Erythrocyte
cholinesterase inhibition was observed at 30 mg/kg and above. This
effect was first noted 2 weeks after the initiation of the study and
was relatively constant over the entire study. Plasma and brain
cholinesterase activity were unaffected and considered normal at all
dietary levels. Gross and microscopic examinations of tissues and
organs at the conclusion of the study did not show any significant
effect of the inclusion of phenthoate in the diet at dose levels up to
and including 100 mg/kg. A no-effect level in this study, based on
cholinesterase depression, was 10 mg/kg in the diet equivalent to 0.29
mg/kg bw, a value derived from the average food consumption values
over the 2-year period (Nelson et al, 1972).
Long-term study
Mice
(See special study on carcinogenicity).
Rats
No data available.
RESIDUES IN FOOD
USE PATTERN
Phenthoate is a wide spectrum insecticide for agricultural and
domestic use. It is active against both chewing and sucking insects,
in particular against lepidopterous larvae, soft scales and larvae and
adults of some species of mosquitoes. The pesticide should be applied
in enough water to insure complete coverage of the foliage, branches
and fruit when applied as a ground application.
The registered use patterns reported from Italy, Israel, Japan and
South Africa are summarised in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Citrus fruit
The trials were performed in Brazil, Florida, Italy and South Africa
with both the recommended and approximately double dose rates. The
results are summarised in Tables 5 and 6. Treatments carried out with
low volume spraying (about 940 l/ha) or with 0.5% oil additive
resulted in somewhat higher residue than the dilute application, when
the spray was applied until run off to achieve full coverage.
The repeated treatments carried out following the recommended use
pattern did not increase the residue in the fruit significantly. The
colometric method used in 1963-65 experiments showed 3-4 times higher
residues than those obtained with GLC determination of samples
deriving from similarly treated plots at later trials.
The phenthoate residues remain entirely in the peel of fruits. The
pulp did not contain detectable residues (limit of determination
0.02-0-03 mg/kg) at any time after treatment with the exception of two
sets of experiments. In one case about 10-25% of the residue found in
the peel was detected in the pulp at the day of treatment and after 98
days, respectively. However in the other experiment the residue in
the pulp was only about 2.55% of the residue found in the peel and
decreased with time.
The phenthoate oxon was only detected in the peel. Limit of
determination in the pulp was 0.01 mg/kg (Moye). The oxon derivative
amounted to a maximum of 0.12 mg/kg (43%) on lemon, 0.08 mg/kg (15%)
on grapefruit and 0.62 mg/kg (38%) on orange. The values in
parentheses refer to the ratio of oxon to the parent compound. The
results of other trials (Iwata, 1979) indicate similar residue ranges.
The residue, on a whole fruit basis, can be calculated from the
residue measured in the peel taking into account the peel/pulp weight
ratio which is fairly constant for different varieties (Iwata, 1977).
For instance, in the case of valencia orange 1/5 of the peel residue
can be considered as the residue on whole fruit basis, while for the
variety Hamlin, the ratio is 1/4.
Other Crops
A limited number of supervised trials were carried out on apple, pear,
cabbage, lettuce, olives, onions, radish, spinach, sweet potato and
sugarbeet in Italy and Japan in 1962-65. The results of these
experiments were provided to the Joint Meeting for evaluation (Tables
7 and 8). However they were not considered to represent the current
use of phenthoate or to be sufficient for evaluation, thus no
TABLE 3. Registered Uses of Phenthoate
Crop Pest ai [g/100R] Formulation No. of treatments Amount of water l/ha
Citrus Scale insects ) 50 - 100 EC 1 - 2 1000-10000
Aphids ) 40 WP (generally)
Orange leaf miner )
Leaf rollers )
Apple Codling moth 40 - 50 EC 5 - 10 1000-2500
WP 1 - 6 5000
Brassica Large white butterfly 50 - 1000 EC 1 or more up to 1000
Japanese pears Leaf rollers 40 - 50 WP 1 - 6 3000
Fruit moths
Mealy bugs
Nuts Codling moth 70 EC 3 - 4 2000-10000
Olives Black scale 60 - 75 EC 3 - 4 2000-10000
Olive thrips
Onions Thrips 75 EC 1 or more up to 1000
Pears Codling moth 40 - 50 EC 3 - 7 1000-2000
Pear psylla 200 250 200 - 500
Potatoes Potato tuber moth 100 EC 1 or more up to 1000
Rice (paddy) Rice stem borers ) 50 - 100 EC 1 - 4 800 - 1500
Leaf and plant )
hoppers ) 1.65 - 3.3 kg EC 1 30-40 aerial
Rice bugs ) 0.6-0.8/kg/ha. Dust
Rice leaf beetle )
Rice leaf miner )
recommendation was made by the Meeting for the maximum residue levels
in/on these commodities.
FATE OF RESIDUES
In animals
Lactating dairy cattle were administered 14C-labelled phenthoate at
levels of 1, 5 and 20 mg/kg on feed basis (Wargo, Anonym. 1979).
Samples of urine, faeces and milk were collected every day during the
administration for 8, 18, 26 days and withdrawal (7 days).
The animals were slaughtered the following day and the tissue samples
were taken. The total 14C residue measured in various tissues and
calculated as parent compound at a dose rate of 20 mg/kg is given in
Table 9. Muscle and fat did not contain detectable residues at rates
of 1 and 5 mg/kg. The residue in the other tissues also decreased at
the lower rates.
Total 14C extractable residues in liver and kidney were 29% and 51%
respectively, and consisted of organosoluble and watersoluble
metabolites.
The metabolites were separated with various chromatographic methods.
In the liver at least eleven compounds were found, of which the major
component represented 8% of the total 14C residues in liver. One of
the remaining ten was present at a higher concentration than 3%. The
unextractable residue was quantitatively released by acidic
hydrolysis. At each feeding level the cows with a seven-day
withdrawal period demonstrated a significant decrease in residues
compared to the cows sacrificed 24 hours after the last 14C
phenthoate dose. Even the "bound" metabolites were eliminated from
the tissues of kidney and liver.
The 14C residues in milk reached the plateau on the second or third
day of treatment. During the feeding period the total residue
plateaued at the levels given below:
Level in diet Residue in milk, mg/kg
1 mg/kg 0.001-0.003
5 mg/kg 0.008-0.026
20 mg/kg 0.015-0.04
The residue declined after withdrawal of the pesticide from the diet
and approached the limit of determination after 3-6 days.
The 0.04 mg/kg total 14C residue in milk consisted of 7% (0.0028
mg/kg) organosoluble, 16% (O.006 mg/kg) water-soluble compounds and
52% (0.019 mg/kg) milk solids.
The residue level in each fraction was too low for further
investigation.
Neither phenthoate nor its oxon could be detected by conventional
analytical methods based on GLC determination in any of the tissue or
milk samples (limit of determination 0.01 mg/kg for fat and 0.05 mg/kg
for the other samples).
White leghorn laying hens were given feed containing 14C phenthoate
at 1.8, 5.9 or 26 mg/kg for 30 days. The birds were sacrificed on
days 1, 3, 7, 14, 21 and 30 during the treatment and on days 7 and 15
during the withdrawal period (Huhtanen, 1979a). The maximum residue
contents of tissues analyzed are given in table 4.
TABLE 4. Residues of phenthoate in tissues of chickens fed phenthoate
in the diet at various levels for 30 days
Tissue Concentration of phenthoate in diet
1.8 mg/kg 5.9 mg/kg 26 mg/kg
breast muscle 0.333 0.072 0.073
leg muscle 0.018 0.035 0.13
liver 0.024 0.04 0.48
kidney 0.094 0.53 1.3
skin 0.039 0.16 0.38
fat 0.019 0.07 0.16
eggs 0.014 0.058 0.35
limits of detection
for non-fatty tissues 0.0077 0.027 0.066
for fatty tissues 0.016 0.053 0.13
A plateau of the residue was reached in liver and kidney between the
third and seventh day of treatment. In the muscles detectable
residues were found from 21st day of treatment but no residue was
detected during the withdrawal period either in the muscles or in the
eggs. Less than 10% of the extracted material, which accounted for 70
of total radioactivity, was of non-polar character in the kidney. A
large percentage (50%) of the more polar compounds were acidic.
No abnormalities were observed in the organs of the hens and the
production of eggs was not reduced during the treatment period.
Fish residue studies were carried out with channel catfish and
bluegill sunfish in order to determine the biomagnification behaviour
of phenthoate.
In water containing 14C-phenthoate at 10-6 mg/l level the average
concentration ratios from catfish tissue/mud and catfish tissue/water
were 0.17 (0.09-0.33) and 5.76 (2.47-9.97) respectively after 33 days.
Residue levels in contaminated fishes showed a gradual reduction if
the fishes were placed in uncontaminated water (Marshall, 1979).
The uptake and bioaccumulation of 14C-phenthoate residues from soil
aged for 21 days in the aquaria into channel catfish were studied at
fortified soil levels of 0.01 and 1 mg/kg. The residues in the
catfish "meat" were 0.003 mg/kg 28 days after placing them into the
contaminated water (Booth, 1978).
Bluegill sunfish were exposed to 14C-phenthoate at 2.1 × 10-5 mg/l or
at 2 × 10-4 mg/1 in the water of a simulated dynamic water system for
30 days. The average bioaccumulation ratio of 14C was 816 and 901
respectively, while for the edible tissue 240 and 248 were the
calculated values. The residues found in tissues of 14-day withdrawal
samples taken from the higher level tank was only 3% of the level
found after 30 days of exposure (Huhtanen, 1979b).
In plants
Selected leaves and fruit of a Valencia orange tree were treated by
brush application with a solution of 30 mg phenthoate [32P or 14C] in
50 cm of water containing 0.06% emulsifier (Takade, 1977). Samples of
leaves and fruits were taken 3, 7, 10 and 14 days after treatment and
rinsed 5 times with acetone to remove the surface material. After
rinsing with acetone, the fruit was carefully peeled. The leaves,
peel and pulp were cut into small pieces and placed in a vacuum oven
at 40°C for approximately 10 hours. No loss in radioactivity was
detected for the drying process. The dried samples were extracted
with acetone. The metabolites were separated on thin layer plates
using four solvent systems. The total radioactivity of solid
materials was then determined by combustion analysis.
The major portion of phenthoate applied to leaves was lost by
volatilisation. The concentration and distribution of residue in
orange fruit are given in Table 10. Significant loss in total
recovery wan observed in fruit. Less than 1% of the total activity
applied was detected in the orange pulp. The majority of the residue
both in fruit and in leaves was intact phenthoate. The ratio of
phenthoate and its phenthoate oxon is shown in Table 11. Other
metabolites, desmethyl phenthoate, mandelic acid, bis-alpha-ethoxy
carbonylbenzyl disulphide, O,O-dimethyl phosphorodithioic acid, O,O-
dimethyl-phosphorothioic acid and two unidentified compounds amounted
to 0-5.2% individually. Glucosidase, hydrochloric acid and sodium
hydroxide together dissolved about 92-98% of the bound residues in the
peel. The major conjugated metabolites were, in order of importance,
ethyl mandelate, mandelic acid, desmethyl phenthoate and phenthoate
acid (Mallipudi and Fukuto, 1980).
Apple, pear and olive trees were treated with 32P-labelled phenthoate
according to the recommended use pattern (Santi, 1967). The surface
of the fruits was washed with methanol to remove the original active
substance or any other derivatives containing methanol-soluble 32P
still present on the peel (methanol extract).
After washing, the various fruits were homogenised in a blender with
chloroform (chloroform extract).
The plant filter cake was further extracted with water to separate
eventual water-soluble compounds (water extract). The 32P content of
various extracts and of residual plant was determined radiometrically.
The phenthoate equivalents of the radioactive residues found at
certain times after treatments are listed in Table 12.
Column chromatography, thin-layer chromatography and paper
chromatography were used for the separation and identification of the
compounds in the various extracts.
The probable metabolic pathways are shown in Fig. 2.
The repeated treatments of apple trees with a solution containing
0.02% ai (half the dosage usually applied) resulted in qualitatively
similar picture regardless the number of treatments. The phenthoate,
although not systemic, penetrates slightly into the fruits and
undergoes oxidation and hydrolysis; 15-29 days after treatment 95% of
water-soluble residue was phosphoric acid while monomethyl and
dimethyl phosphoric acids were detected in traces. Phenthoate oxon
was detected in traces only 1 day after treatment.
 The metabolism of phenthoate in the two varieties of pears showed a
slightly different pattern. Phenthoate acid,
O,O-dimethyl-thio-phosphoric acid, O,O-dimethyl dithio phosphoric
acid, dimethyl phosphoric acid and phosphoric acid were identified and
four other unidentified compounds were found in the "water-soluble"
extract. The quantity of any of them did not reach 0.1 mg/kg
phenthoate equivalent. Intact phenthoate in varying amounts, traces
of P=O derivative and unidentified compounds were found on the surface
of olive at different intervals after treatment. Phenthoate
penetrated into drupes with subsequent hydrolysis and oxidation
process and formation of the same metabolites as found in other
fruits. The processed oil contained only intact phenthoate (0.14
mg/kg), while in olive husks 0.23 mg/kg phenthoate and 3 mg/kg of
other 32P containing compounds were detected.
A 0.05% emulsified solution of 14C-labelled phenthoate was sprayed on
cabbage seedlings and brushed over Hime apple fruits and also
strawberries. Phenthoate remained mostly on the surface and only
3.3-6.7% of the total amount penetrated into the plants. The residue
decreased rapidly both on the surface and in the plants. Eight days
after treatment the residue on the surface of cabbage consisted of 86%
intact phenthoate, 8% bis-alpha-carbethoxy-benzyl disulphide, 1.9%
ethyl mandelate and 1.3% unidentified compound. Phenthoate oxon was
detectable in traces (0.4-0.7%) during the first day after treatment.
Within the cabbage seedlings 8 days after treatment intact phenthoate
was present in an amount of 10.2% and the metabolites identified were
mandelic acid (46.9%), bis-alpha-carbethoxy-benzyl disulphide (6.5%),
phenthoate acid (4.8%), desmethyl phenthoate (3.2%), phenthoate oxon
(1.8%). Six unidentified compounds were separated (total amount
18.8%) of which two accounted for 14.3%. The degradation of
phenthoate in/on apple took place more slowly than in/on cabbage, or
strawberry, while the metabolite patterns were rather similar in case
of apple and strawberry to that found in/on cabbage seedlings (Hirose
et al, 1971).
In soil
The degradation of ring-labelled 14C-phenthoate in a moist (50% of
capacity) loam and silty clay loam soil was studied (Iwata, 1977b).
Phenthoate was rapidly degraded by heat-labile soil enzymes which
converted it to phenthoate acid under both aerobic and anaerobic
conditions, even with a soil treatment of 100 mg/kg. Under aerobic
conditions and in low concentration the phenthoate acid underwent
extensive microbiological degradation to CO2 (up to 50% of the
theoretical value) and polar products. At 100 mg/kg level or under
anaerobic conditions it degraded by first order kinetics, presumably
by simple hydrolysis.
In a loam soil phenthoate at 1.25 and 2.5 mg/kg had no effects on the
rates of degradation of 14C-cellulose or 14C-starch by soil microbes
over a 25-day period as measured by the evolution of 14C. The rate
of nitrification in soil amended with (NH4)2SO4 was likewise
unaffected over a 28 days period (Sikka, 1979).
Phenthoate appears to be relatively persistent under dry soil
conditions (Iwata, 1977a) and in dry dust adhering to plant surfaces
(Iwata, 1975). In two sets of experiments 95% and 62% of the initial
doses remained unchanged after 59 and 75 days respectively.
The results indicate that conditions favouring microbial activity,
such as adequate moisture and warm temperatures, make soil type of
secondary importance for phenthoate degradation.
The fate of residues was also studied under field condition (Moye et
al, undated). Soil samples were taken from the drip-line and middle
at 0-6 and 6-12 cm depths under the orange trees treated with Cidial 4
E solution of 0.03% ai 1, 3, 7, 14 and 28 days after treatment. Four
samples were taken from both depths for each time. The maximum of
phenthoate concentration was observed at the drip-line on the third
day (1 mg/kg, in 0-6 cm and 0.68 mg/kg in 6-12 cm).
The mean residue at 0-6 cm was 0.56 mg/kg which decreased to 0.12
mg/kg 28 days after treatment.
Phenthoate oxon was not detectable in any of the experiments. The
mobility of phenthoate and its soil metabolites were studied by
leaching experiments using sandy soil (organic matter 1.6%), silt loam
(o.m. 6%), sandy loam (o.m. 3.3%), clay loam (o.m. 5.9%) (Anonymous,
1978a). The columns were filled to 25.4 cm with the untreated soils
after prewetting the column with water. The fortified soil (5 cm) was
layered over the untreated soil. For studying the mobility of
metabolites the fortified soils were aged for 30 days under greenhouse
conditions. Water equivalent to 500 mm rain for parent compound and
to 380 mm for metabolise was allowed to pass through the column and
analyzed. The columns were sectioned into four equal lengths for
analyses by the measurement of radioactivity. The upper 15 cm of the
sandy soil contained 72.3% of phenthoate and 85.7% of the metabolites
while the other soils retained 87.3-88.8% and 89.4-90.9% respectively.
The water effluent from the light sandy soil contained 8.32% and 6.62%
of the original activity. From the other soils the 14C activity in
the water effluent amounted to 2.9-6.44% and 3.39-4.21%.
In water
14C phenthoate was added to 0.01 M sodium phosphate buffers (pH 6.0,
7.0 or 8.0) to give a final concentration of 1.5 × 10 -5 M. Aliquots
were taken 3, 7, 10, 14, 21 and 28 days after addition of the 14C
material to the buffer and analyzed an TLC plates subsequent to
appropriate preparation.
32P-phenthoate was added to the buffer solutions in a 1 × 10-4 M
final concentration and was treated similarly to the 14C compound.
All solutions were held at 24.5 ± 1°C in a constant temperature room.
The pH of buffered solutions, containing radiolabelled phenthoate
under similar conditions, was monitored over the experimental period
and no pH change was detectable.
Evidently, phenthoate is fairly stable in water at the indicated pH
values. 45%, 21%, 22% of its initial amount were present after 28
days at pH 6, 7, 8 respectively. Its half life is about 12 days at pH
8.0. Phenthoate acid was observed in greatest amount (30-55%)
irrespective of pH. Hydrolysis of carbonyletoxy moiety appeared to be
fastest at pH 8.0. On the other hand the rate of formation of
desmethyl phenthoate, the second most prominent hydrolysis product,
was fastest at pH 6. Phenthoate oxon was not detected at any time.
Other products indicated in Figure 2 were observed in small amounts
(Takade, 1977).
Photodegradation
The photodegradation of 14C-phenthoate was studied in distilled water
(pH = 7.0±0.5) at concentrations of 8 mg/kg and 5 mg/kg (Anonymous,
1978).
A medium pressure mercury lamp, fitted with a Pyrex 7740 filter that
excludes light of wavelength less than 280 nm, was immersed in the
solution to be irradiated. After 5 and 8 hours of irradiation,
aliquots of the photolysed solutions were taken and analyzed applying
HPLC and TLC techniques. Approximately 50% of phenthoate had degraded
after 6-8 hours of irradiation, while there was no loss in another
phenthoate solution kept in the dark for the same time. Ethyl
mandelate was found to be the major degradation product. In addition
three unknown products were detected but each of them accounted for
less than 10% of the parent compound.
An acetone solution of radiolabelled phenthoate (32P or 14C) was
applied to glass plates, which were exposed to sunlight daily for 7
hours (Takade, 1977).
Air temperature during the exposure ranged from 7.2 to 23.3°C. Under
these conditions approximately 90% of the applied phenthoate was lost
after 40 hrs of exposure by volatilisation.
The unchanged phenthoate gradually decreased in proportion to the
other alteration product during the exposure (see Figure 2).
Phenthoate oxon was identified as the major product. Other products
isolated were desmethyl phenthoate, mandelic acid, bis-[alpha-carboxy)
benzyl]-disulphide, bis[alpha-/carbethoxy/benzyl]disulphide and
O,O-dimethyl phosphorothioic acid. Results of other experiments
indicated that elevated temperatures may speed up the phenthoate oxon
formation and its disappearance. The latter is also facilitated by
rainfall (Nigg and Stamper, 1980).
The degradation products formed upon exposure to sunlight and air on
glass plates or found on citrus leaves and fruits are essentially the
same.
In processing
The effect of processing was studied on orange, lemon and grapefruit
treated in the field twice with Cidial 4 E in solutions of 0.03% or
0.06% ai. The samples were taken 50, 19 and 18 days after last
treatment respectively and were processed with a FMC processor. The
residue ranges found in 4 samples taken at each stage are summarised
in Table 13 (Moye et al, undated).
Little, if any, residue decrease occurred from washing of oranges and
lemons, which is in good agreement with the findings of Iwata et al,
who carried out laboratory washing to simulate packing house
treatment. On the other hand laboratory washing of the grapefruit
removed about 50% of the residue found 3 days after treatment and from
about 25% to less than 10% thereafter.
Phenthoate seems to be concentrated in the waxes and oils of the rind.
Fruit juice contained negligible residues (<0.01 mg/kg). Residues
found in the dried rind, which is used as a cattle feed, show an
approximate 44-58% loss of residue during processing and drying.
The majority of the residue found in/on olives remained in the olive
husk and the processed oil contained only intact phenthoate (4.1% of
total residue) (Santi, 1976).
METHODS OF RESIDUE ANALYSIS
Methods that have been developed for the determination of phenthoate
in crops and animal tissues have been reviewed. Bazzi (undated)
described a method which is based on acetone extraction, liquid-liquid
partition clean up on a Plorex column and gas chromatographic
determination using 3.8% UCCW 982 on Gas Chrom Q packing and a flame
photometric detector. The limit of determination is 0.04 mg/kg or
less depending on the type of sample. The method is suitable for the
determination of phenthoate and its oxon in citrus and their processed
by-products in maize, cattle meat and soil.
Moye et al (undated) found acetone: acetonitrile 1:1 mixture most
efficient for the extraction of phenthoate from soil, fruits and
by-products of citrus processing. OV-101, OV-17 (Iwata et al, 1979)
and OV-225 (Moye et al, undated) liquid phases were used to separate
phenthoate, phenthoate oxon from other organophosphate pesticides also
applied in citrus orchards.
Another method for the determination of residues in animal products
includes extraction by acetonitrile from muscle, kidney and liver, by
acetone and benzene from milk and by heptane from fat; partition with
benzene, hexane and acetonitrile; column chromatography on Floricil;
and GLC determination using 3% OV-101 packing with FPD. Limit of
determination: 0.01 mg/kg for milk, 0.05 mg/kg for tissues. The
recovery at 0.05-0.1 mg/kg level is about 92% for all samples.
The basic method described by Bazzi (undated) in combination with the
other liquid phases and slight modifications proposed by the other
authors is suitable for regulatory purposes.
NATIONAL MRL'S REPORTED TO THE MEETING
Phenthoate is registered in 36 countries all around the world. Some
of the maximum residue limits and pre-harvest intervals were reported
to the meeting.
Pre-harvest
intervals MRL
(days) (mg/kg)
Hungary apple, pear,
sugar-beet 21 0.5
Italy apple, pear, walnut,
vegetables, rice 20 0.3
citrus, olive 60 0.3
Israel oranges, grapefruit 20-30
Japan pear, peach 7 0.1
pumpkin 1 0.1
mandarine, orange 14 0.1
rice (unpolished) 7 0.05
japanese pear 30 0.1
Netherlands citrus fruit 0.5
New Zealand brassica crops 0.7
South Africa citrus 21 1.0
brassica 3 1.0
onion,potato 7 0.1
Sweden citrus 1.0
USA citrus 2.0
TABLE 5. Residues of Phenthoate in oranges
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia USA 1973 1 8.5 50 L 0.8 0.5 0.4 0.3 0.2 0.1 0.1 Iwata, 1977a
(Florida) 1 4.3 50 L 0.5 0.3 0.2 0.1 0.09 0.09 0.07
Valencia USA 1979 1 4.2 4 EC 2.9 2.3 1.5 0.58 0.23 Iwata, 1979
(peel) (Florida) 1 8.4 4 EC 4.0 3.0 2.1 0.85 0.35
1 8.41 4 EC 6.3 4.2 3.0 1.4 0.38
Valencia USA
(peel) (Florida) 1979 2 0.03%3 4 EC 1.65 0.67 0.29 0.38 Moye et al
(pulp) 0.06% 0.03 0.04 0.01 0.01 (undated)
Hamlin Brazil 1979 1 0.1% 50 L 1.38 0.79 0.5 0.33 0.22 0.22-0.12 Camargo,1980
(peel)
Valencia Italy 1965 1 0.06%1,2 50 L 4.9 1.6 1 1.1 0.8 0.6 Anonym, 1965
Moro (peel) Italy 1963 1 0.05%1,2 50 L 3.2 1.8 1.2
Valencia Israel 1973 1 7.5+0.5% oil 50 L 1.66 1.08 0.5 0.2 Greenberg
1 10 50 L 0.71 0.58 0.04 0.09 0.02 et al, 1974
1 20 50 L 2.1 1.33 0.75 0.37 0.12
Shamouti Israel 1973 1 7.5 50 L 1.02 0.98 0.45 0.21
1 7.5+0.5% oil 50 L 0.98 1.08 0.4 0.36
1 15 50 L 1.68 1.41 0.82 0.4
Orange Japan 3 0.05% 50 EC 0.01 0.008 0.004 Anonym, 1980
(pulp) 5 0.05% 0.01 0.009 0.009
3 0.05% 0.02 0.005 0.008
5 0.05% 0.015 0.006 0.005
TABLE 5. Continued...
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia S. Africa 1970 1 0.213% 50 L 3.7 2.05 1.1
1971 1 0.1% 50 L 1.21 0.56 0.32 0.23 Anonym,
1970
Valencia S. Africa 0.37 0.3 0.12
(pulp)
1 Low volume application with a spraying volume of 940 l/ha
2 Colorimetric method was used for the determination
3 Three months elapsed between applications; the maximum residue values found in four replicates are given
TABLE 6. Residues of Phenthoate in Grapefruit and Lemon
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-4 8-11 18-21 33-35 38-46 60
Grapefruit USA 1978 1 3.4 4 EC 0.94 0.77 0.48 Iwata, 1979
(peel) 1 6.7 4 EC 2.1 1.6 0.87
1 6.71 4 EC 3.9 2.5 1.4
1979 1 0.03%2 4 EC 0.9 0.5 0.31
0.06%2 1.8 0.77 0.59
(pulp) USA 1979 2 0.03+0.063,4 4 EC 0.007 0.008 0.011 0.002 <0.002 Moye et al, undated
Whole fruit Israel 1973 1 0.075% 50 L 0.97 0.47 0.23 1.131 Greenberg et al,
1 0-0.75%4 50 L 1.4 0.63 0.39 0.14 1974
1 0.075%+0.5% oil 50 L 0.84 0.79 0.93 0.1
2 0.075%+0.5% oil4 50 L 1.0 1.0 0.74 0.16
1 0.15% 50 L 1.25 1.5 0.41 2.7
1 0.75%4 2.0 2.0 0.57 0.42
Lemon USA 1974 1 7 50 L 1.2 0.8 0.6 0.4 0.3 0.3 Iwata, 1979a
(peel) 1974 1 1.8 50 L 0.5 0.3 0.2 0.2 0.1 0.1
1978 1 4.2 4 EC 1.5 0.9 0.47
1 8.2 4 EC 2.8 1.7 0.78
1 8.21 4 EC 3.8 3.3 2.2
1979 1 5 4 EC 3.7 2.7 1.1
10 4 EC 7 4.5 1.9 Iwata, 1979
10 4 EC 14 11 5.7
Whole fruit S.Africa 1973 1 0.04% 50 L 0.4 0.26 0.08 0.09 Anonym, 1970
0.1% 50 L 1.4 0.96 0.47 0.17
1 Low volume application with a spraying volume of 940 l/ha
2 Sprays were applied to achieve full coverage
3 17 days elapsed between applications
4 The maximum residue values found in four replicates are given.
TABLE 7. Residues of Phenthoate in Apple and Pear
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0 1 4-7 9 14-17 21-26 29-34 39-46
Apple Italy 19621 1 0.04% 50 L 2.8 1.19 0.47 0.22 0.16 0.11 <0.1 Anonym, 1972
1962 1 0.03% 50 L 1.57 0.78 0.34 0.18 0.13 0.1
19621 1 0.04% 50 L 1.6 0.93 0.36 0.1 <0.1
1962 4 0.04% 50 L 1.92 1.21 0.48 0.12 <0.1
Pear Italy 19631 1 0.04% 50 L 1.45 1.1 0.6 0.4 0.25 Anonym, 1973
1963 1 0.04% 50 L 1.9 1.7 0.54 0.3 0.1 <0.1
1963 6 0.05% 50 L 0.64 0.35 0.18
Japanese Japan 3 0.05% 0.3 0.11 0.05 Anonym, 1980
pear 5 0 05% 0.44 0.16 0.09
3 0.05% 0.48 0.16 0.03
4 0.05% 0.58 0.1 0.08
1 Colourimetric method was used for the determination.
TABLE 8. Residues of phenthoate in other crope resulting from supervised trials
Application Residues in mg/kg, at intervals (days) Reference
after application
Crop Country Year No. rate % formulation 0 1 6-7 13-14 20-21 21-26 34 70
volume (1)
Cabbage Italy 1965 2 0.05 50 L <0. 1 <0.1 Anonym.,
6 3.18 0.11 0.09 1965
Olives Italy 1963 1 0.05/3000 50 L 10.9 8.1 0.49 0.49 0.2 Anonym.,
1963
Onion Japan 2 0.05/1500 0.01 Anonym.,
4 0.01 1980
Radish 2 0.05/2000 0.005
4 0.05/1500-2000 0.005 0.005 0.005 0.005
3 0.05/1800
Spinach Japan 2 0 05/1500 0.015
3 0:05/1500 0.018
2 0 05/1500 0.005 0.015
3 0:05/1500 0.007 0.005
Tea/leaf Japan 2 0.05/2000 0.008
3 0.05/2000 0.04
2 0.05/3000 0.04
3 0.05/3000 0.04
Lettuce Japan 2 0.05/2000 0.08 0.08 0.02
4 O.05/2000 0.06 0.05 0.09
TABLE 9. Carbon 14 residues in bovine tissue after feeding at a
20 mg/kg level in the diet
mg/kg in tissues after feeding for
Tissue 8 days 18 days 26 days 26 days
7 days
withdrawal
Muscle 0.013 <0.033 <0.033 <0.033
Fat 0.016 0.056 0.045 <0.045
Kidney 0.324 0.408 0.502 0.116
Liver 0.238 0.228 0.297 0.140
Heart 0.028 0.061 0.087 0.052
Brain 0.036 <0.042 0.054 <0.042
TABLE 10. The penetration of 14C and 32P phenthoate into orange
fruit, and the distribution of radioactivity in the surface wash,
acetone extract and solid residue.
Percentage of the applied radioactivity
recovered at indicated time (days)
0 3 7 10 14
14C-labelled phenthoate
External surface wash 72.5 66.1 9.8 8.1 4.6
Internal acetone extract1 - 5.4 15.6 12.8 7.8
Solid residue1 27.5 21.7 21.6 34.7 28.3
Total recovered 100.0 93.2 47.0 55.6 40.7
32P-labelled phenthoate
External surface wash 96.4 63.4 17.4 13.6 11.6
Internal acetone extract1 - 5.9 22.0 24.2 18.2
Solid residue1 3.6 19.4 13.9 16.3 13.2
Total recovered 100.0 88.7 53.3 54.1 43.0
1 Includes peel only. Radioactivity in pulp was less than 1% of
the applied.
TABLE 11. Amount of 14C metabolites found in the surface wash and
acetone extract of orange leaves after indicated time intervals
Compound found in Percent of recovered radioactivity
indicated material after indicated time (days)
3 7 10 14
Phenthoate
Surface wash 84.4 45.5 35.4 41.6
Acetone extract 4.6 46.1 37.7 17.0
Phenthoate
Surface wash 0 0 2.8 5.1
Acetone extract 0.2 0 7.2 12.4
TABLE 12. 32P Phenthoate equivalents (mg/kg) in treated apples, pears and olives
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Apples: var. Stark Delicious (0.02% solution)
2 hrs. 1.00 0.21 0.17 0.04 - - 1.17
1 day 0.26 traces 0.31 0.06 - - 0.57
15 days 0.02 - 0.08 0.02 0.95 0.80 0.10
29 days 0.00 - 0.00 - 0.96 0.69 0.00
Pears: var. Trionfo di Vienna (0.04% solution)
2 hrs. 1.35 traces 0.19 0.02 - - 1.44
1 day 1.19 traces 0.40 0.05 - - 1.59
4 days 0.33 - 0.26 0.09 - - 0.59
9 days 0.19 - 0.12 0.07 0.43 0.18 0.31
31 days1 0.006 - 0.01 0.02 0.16 0.09 0.016
Pears: var. Passacrassana (0.045% solution)
2 hrs. 1.23 traces 0.03 traces - - 1.26
1 day 1.01 traces 0.07 traces - - 1.08
5 days 0.55 - 0.05 - 0.04 0.02 0.60
25 days1 0.28 - 0.16 - 0.39 0.07 0.44
40 days2 0.20 traces 0.10 traces 0.34 0.14 0.30
TABLE 12. Continued...
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Olives /oils-yielding/: var. Leccino (0.05% solution)
2 hrs. 11.16 traces 0.15 0.01 traces - 11.31
1 day 5.92 traces 1.50 0.36 0.10 - 7.42
9 days 0.36 0.18 0.53 0.38 3.60 0.84 0.89
30 days 0.19 0.03 0.06 0.35 2.58 0.86 0.25
70 days 0.05 - 0.10 0.05 3.23 0.59 0.15
1 Fruit picking
2 Pears of this winter variety were analysed after picking from trees
and stored for 15 days at 19-20°C.
TABLE 13. Phenthoate residue (mg/kg) detected in processed citrus by-products
ORANGE LEMON GRAPEFRUIT
Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon
Unwashed peel 0.24-1.00 0.06-0.09 0.08-0.28 0.06-0.12 0.031-0.74 <0.01
Washed peel 0.23-0.73 0.036-0.069 0.15-0.32 <0.01 0.23-0.51 <0.01-0.09
Unwashed pulp <0.002-0.031 0.01 0.003-0.024 <0.01 0.007-0.031 <0.01
Washed pulp 0.005-0.011 0.01 0.002-0.004 <0.01 0.02-0.09 <0.01
Chopped peel 0.34-0.67 0.01-0.03 0.08-0.26 0.03 0.26-0.49 0.002-0.051
Peel frits 0.32-0.52 0.05-0.15 0.25-0.74 0.05-0.15 1.14-2.45 0.015-0.031
Finisher pulp 0.008-0.015 <0.01 0.008-0.023 <0.01 0.003-0.008 <0.01
Dried rind 0.13-0.43 0.01-0.036 2.2-2.7 0.01 0.72-1.51 0.06-0.21
Press liquor 0.08-0.14 <0.01-0.01 0.008-0.032 0.009-0.017 0.13-0.19 0.01-0.013
Emulsion water 0.004-0.07 <0.004-0.1 0.003-0.009 0.04-0.06 0.006-0.021 <0.01
Fruit juice 0.002-0.003 <0.01 0.004-0.009 <0.01 <0.002 <0.01
After-water rinse <0.002-0.004 <0.01 <0.002-0.005 <0.01 <0.002 <0.01
Pre-water rinse <0.002 <0.01 0.002 <0.01 <0.002 <0.01
Molasses 0.047-0.068 <0.01 0.01-0.03 <0.01 0.037-0.071 0.01
Oil 3.29-14.05 1.18-2.48 7.25-17.25 1.65-2.5 5.4-8.5 0.45-1.1
EVALUATION
COMMENTS AND APPRAISAL
Phenthoate is a wide-spectrum organophosphorus insecticide, mainly
used on citrus at rates of 0.6 to 5 kg ai/ha.
The technical material contains a minimum of 92% of phenthoate.
Detailed analyses of the typical technical material, including the
identity of the minor components, was provided. It is generally
soluble in most polar and non-polar organic solvents and also slightly
soluble in water.
Phenthoate is a cholinergic compound of moderately acute toxicity and
is rapidly absorbed, metabolized and excreted in mammals. The pattern
of metabolism is similar in both plants and animals and is similar to
that noted with other organophosphorus esters. The rate of metabolism
is shown to be significantly affected (with potentiation of toxicity)
by organophosphorus esters commonly found as impurities in the
technical product. The acute toxicity of phenthoate is dependant on
the purity of the technical product, a feature which raised
significant questions about occupational exposure. It was suggested
that a more purified product should be manufactured and used to
alleviate this problem. Formulations that are stored also have the
potential to become more toxic under certain conditions. Although
these factors do not directly affect phenthoate residues in food they
should not be ignored.
Phenthoate is moderately toxic to mammals. The acute LD50 was noted
to vary from 78 to over 4500 mg/kg bw, depending possibly on the
impurities and the species. Antidotal studies are not available.
Phenthoate does not induce a delayed neurotoxic reaction in hens, is
not mutagenic or teratogenic, nor does it interfere with reproduction,
as evidenced by a standardized 3-generation study in rats. In a
series of short-term toxicity studies, cholinesterase depression was
the most significant measure of effect of exposure. In dermal and
ophthalmological studies, phenthoate applied as a formulated product
was irritating. On the basis of available short-term studies, it is
concluded that phenthoate is a potent cholinesterase inhibitor.
A two-year study in the dog showing red blood cell cholinesterase
depression served as the primary basis to evaluate a no-effect level.
Phenthoate is not a carcinogen in mice. A two.year study in rats was
considered invalid and not included in the evaluation.
On the basis of available data, no-effect levels in mammalian species
were determined and a temporary ADI for man was allocated. This
evaluation was made on the assumption that the manufacture of
phenthoate would be by a process that would assure that a product of
at least 92% purity would be marketed. As this was the product on
which toxicological evaluations were made and as impurities have a
substantial toxicological significance this purity restriction was
important.
Phenthoate residues gradually disappear from treated plants within 4
to 10 weeks, most being lost by volatilization. Decomposition of
surface deposits is mainly due to photodegradation, resulting in
phenthoate oxon as the main metabolite. On cabbage, the surface
residue 8 days after application consisted of 86% phenthoate but no
oxon was detected; on orange leaves, 41.6% of the total residue was
phenthoate and 5.1% was the oxon at 14 days after treatment.
Phenthoate is not systemic but does enter the peel of fruit, where it
undergoes hydrolytic cleavage and oxidation. Virtually all of the
residue remains in the peel of treated citrus fruit, the pulp
containing less than 1% of the total residue. Phenthoate oxon has
been detected only in the peel.
The main metabolites in plants, phenthoate oxon, dimethyl phenthoate,
mandelic acid, bis-2-(carbethoxy) benzyl disulphide, O,O-dimethyl
phosphorodithioic acid, O,O-dimethyl phosphorothioic acid and
phenthoate acid, are similar to those secreted by animals. Their
amounts and relative proportions depend on the crop and the time
elapsed since application but, in practice, the parent phenthoate
should be considered as the only residue of importance.
Repeated treatments of citrus fruits or apples according to
recommended usage do not lead to increased residues or changed
metabolite patterns. The residue is contained mostly in the waxes and
oils of the citrus rind and is not decreased significantly by washing;
the fruit juice contains negligible residues. Dried rind as used for
cattle feed can contain 40 to 60% of the residue originally present in
the peel.
Laying hens fed for 30 days with feed containing 1.8 to 2.6 mg/kg of
phenthoate showed no reduction of egg production or any abnormalities.
Residues in the muscle and in eggs were low and disappeared after
withdrawal of the compound from the diet; in kidney and liver levels
reached a plateau between the third and seventh days of treatment.
Neither phenthoate nor its metabolites accumulated in fish kept in
water containing phenthoate at the 10-4 to 10-6 mg/l levels.
Lactating cows given 14C-phenthoate at levels of 1.5 and 20 mg/kg in
the feed excreted the material mainly in the urine and faeces. The
main metabolite was phenthoate acid, which accounted for 45% of the
14C activity of the urine. Residues in milk reached a plateau 2 to 3
days after administration began. The organosoluble fraction of the
residue in milk, possibly phenthoate, phenthoate oxon and unconjugated
metabolites, was very low (0.0028 mg/kg) even at the higher dosage
rate. Meat from the cattle contained traces of residues at the higher
dosage rate only, the muscle tissue and the fat containing the
residues in roughly equal proportions. Residues in various tissues
declined rapidly when the pesticide was withdrawn from the diet; even
the bound (unextractable) metabolites were eliminated from kidney and
liver. Neither phenthoate nor its oxon could be detected in tissue or
milk samples by conventional analytical methods at limits of
determination of 0.05 and 0.01 mg/kg respectively. It was concluded
that feeding cattle with citrus by-products prepared from
phenthoate-treated fruit would not result in detectable residues in
meat or milk.
In soil, phenthoate is rapidly converted to phenthoate acid under both
aerobic and anaerobic conditions, even when as much as 100 mg/kg is
added to the soil. Under conditions favourable for microbiological
activity, such as adequate moisture and warm temperature, the soil
type has no influence on the degradation of phenthoate. On the other
hand, phenthoate persists for a relatively long time under dry soil
conditions. Phenthoate and its metabolites show moderate mobility in
soil, the majority of the residue being contained in the upper 15 cm.
The likelihood of contamination of lower soil layers or underground
waters is very low in view of the high degradability and slow leaching
properties of the residues.
Analytical methods, based on GLC separation on columns of different
polarity with flame photometric detection are available and suitable
for regulatory purposes.
Level-causing no toxicological effect in:
Dog: 10 mg/kg in the diet equivalent to 0.29 mg/kg bw/day.
Mouse: 30 mg/kg in the diet equivalent to 4.5 mg/kg bw/day.
Rat: 10 mg/kg in the diet equivalent to 1.0 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that available information was adequate for the
estimation of maximum residue levels for phenthoate on citrus fruits
only. The data on other crops were not considered adequate for
estimating maximum residue levels. The levels apply to the parent
phenthoate excluding all metabolites.
Commodity Temporary maximum Interval between last
residue level (mg/kg) application and harvest
on which levels are
based
Citrus fruit 1 21
Carcass meat of cattle 0.051
Milk 0.011
Egg 0.051
1 At the limit of determination.
FURTHER WORK OR INFORMATION
Required (by June, 1984)
A chronic toxicity study in an acceptable mammalian species (rodent).
Desirable
1. Further information on impurities in the technical products in
order to evaluate the degree to which these impurities potentiate the
toxicity of phenthoate.
2. Data from supervised trials on other commodities in accordance
with present uses of the compound.
3. Residue data from crops, known to have been treated under
practical conditions, moving in commerce.
REFERENCES
Anonymous. Cidial Residues in Apples. Montedison S.p.A. Unpublished
report no. 77, (1962).
Anonymous. Cidial Residues in Pears, Olives. Montedison S.p.A,
Unpublished report nos.78, 79, (1963).
Anonymous. Cidial Residues in Oranges. Montedison S.p.A., Unpublished
report no 74/b, (1964).
Anonymous. Cidial Residues on Oranges, Cabbages. Montedison S.p.A.
Unpublished reports Nos. 76/b, 80, (1965).
Anonymous. Cidial Residues on Citrus Form Chem. Report Montedison
S.p.A. Unpublished report no. 74/a, (1970).
Anonymous. Determination of Cidial [Ethyl
alpha(/Dimethoxy-phosphinothioyl/thio) benzene acetate] in muscle,
milk, kidney, liver and fat. Thompson-Hayward Chemical Co., Montedison
S.p.A. Unpublished report no. 62, (1975).
Anonymous. Cidial Leaching Study on Parent compound and on its Soil
Degradation Products. Carried out by Thompson-Hayward Chemical
Company. Montedison S.p.A. Unpublished Report no 91, (1978a).
Anonymous. Photodegradation of Phenthoate in Aqueous Solution.
Submitted by Syracuse Research Corporation. Montedison S.p.A.
Unpublished Report no. 93, (1978b).
Anonymous. Interpretation and discussion on the metabolism of Cidial
in the dairy cow. Unpublished Report of Analytical Development
Corporation. Montedison S.p.A. Report No. 65, (1979).
Anonymous. Summary of Residue Data in Japan. Nissan Chemical
Industries Ltd. Unpublished reports, (1980).
Arnold, D., Kennedy, G.L., Kinoshita, F.K. and Keplinger, M.L.
Mutagenic Study with Cidial in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (No. 622-05876), reviewed and
validated by Bob West Associates, Inc. (Nov. 6, 1978), submitted to
the World Health Organization by Montedison.
Arnold, D., Kennedy, G.L. and Keplinger, M.L. Mutagenic Study with
Elsan (Phenthoate) in Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 632-05147), reviewed and validated by
Bob West Associates, Inc. (Feb, 8, 1980), submitted to the World
Health Organization by Montedison.
Bazzi, B. Phenthoate in Zweg G., ed. Analytical Methods for Pesticides
and Plant Growth Regulators. Academic Press, New York, Vol. VIII, p.
159.
Booth G.M. Bioaccumulation Study of Cidial in Channel Catfish.
Montedison S.p.A. Unpublished report no. 87, (1978).
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04413), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04524), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Camargo de., J.L.G. Residues de carbophenothione Phenthoate em cascas
e polpas de laranjos hamlin determinados por cromatografia em fase
gasosa. Dissertatio submitted to Luiz de Queiroz University, Sao
Paulo, 1980.
de Carneri, I. Microbiological Study of Mutagenesis on CRA 133 (GMA
267/78) Genetic Mutation Test in Salmonella Typhimurium (Ames). (1979)
Unpublished report from Farmitalia Carlo Erba, Research and
Development, submitted to the World Health Organization by Montedison.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
Neurotoxicity Study with Elsan in Chickens. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (no. 8580-80635), reviewed and
validated by Bob West Associates, Inc. (Jan. 25, 1980), submitted to
the World Health Organization by Montedison.
Good, J., Cooper, D. and Terrell, Y. Acute Delayed Neurotoxicity Study
of Cidial Technical 96% Batch 104 in Female Chickens. (1979)
Unpublished report from Cannon Laboratories, Inc. (No. 8E1191),
submitted to the World Health Organization by Montedison.
Greenberg, R. et al. Residues and Disappearance of Cidial in Valencia
Oranges. Montedison S.p.a. Unpublished Report No. 73/a, (1974).
Hammond, P.S., Talcott, R.E. and Fukuto, T.R. Metabolism and Mode of
Action of O,O,S-Trimethyl Phosphorothioate in the Rat. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #38, Division of Pesticide Chemistry, American
Chemical Society.
Hathaway, D., Keplinger, M.L. and Fletcher, D.B. Acute Inhalation
Toxicity Study with Phenthoate (Cidial) Technical in Albino Rats.
(1971) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(no. N-9324), reviewed and validated by Bob West Associates, Inc.
(Nov. 6, 1978), submitted to the World Health Organization by
Montedison.
Hirose, M. et al. Residue Degradation and Metabolism of 14C Labelled
Elsan(R) (O,O-Dimethyl-S-alpha-carboetoxy-benzyl phosphorodithioate)
in Cabbage, Hime Apples and Strawberries. (In Japanese). Scientific
Pest. Control 36: 43-51.
Huhtanen, K. Poultry Tissue and Egg Study with Cidial. Cammon
Laboratories Inc. Montedison S.p.A. Unpublished Report Nos. 84, 85,
(1979a).
Huhtanen, K. Accumulation of 14C Cidial in Bluegill Sunfish Exposed
in Dynamic Flow System. Cammon Laboratories, Inc. Montedison S.p.A.
Unpublished Report No. 88, (1979b).
Iwata, Y. et al. Behaviour of Phenthoate (Cidial) Deposits and
Residues on and in Grapefruits, Lemons, Lemon Leaves, Oranges and
Orange Leaves and in the Soil Beneath Orange Trees. J. Agric. Food
Chem. 25 (2): 382-388.
Iwata, Y., Ithig, M. and Gunther, F.A. Degradation of
O,O-Dimethyl-S-alpha-(carboethoxy)-benzyl phosphorodithioate
(Phenthoate), in Soil. Arch. Environm. Contam. Toxicol. 6: 1-12.
Iwata, Y. et al. Phenthoate Applied to California Citrus Trees: Worker
Environment Research and Residue Levels on and In Fruit. Report of
Department of Entomology, University of California. Montedison S.p.A.
Unpublished Report No. 72b, (1979).
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., Kinashita, F.K.
and Keplinger, M.L. Teratogenic Study with Cidial in Albino Rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
reviewed and validated by Bob West Associates, Inc. (Nov. 21, 1978),
submitted to the World Health Organization by Montedison.
Lee, S.G.K. and Fukuto, T.R. Effect of Impurities in Technical
Phenthoate (Cidial(R)) on Mammalian Carboxyesterases. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #35. Division of Pesticide Chemistry, American
Chemical Society.
Mallipudi, N.M., Badaway, S.M.A. and Fukuto, T.R. Inhibition of
Mammalian Esterases by O,O,S-Trimethyl Phosphorothioate. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #37, Division of Pesticide Chemistry,
American Chemical Society.
Mallipudi N.M. and Fukuto, T.R. Characterization of Bound Phenthoate
Residues in Citrus. Submitted to Archives of Environ. Contam.
Toxicol., 1980.
Marshall B.L. Cidial Fish Residue Study. Marine Research
Institute/Massachusetts. Montedison S.p.A. Unpublished Report No. 86,
(1979).
Moye et al. Residue Analysis of Phenthoate (Cidial(R)) and Phenthoate
Oxon in Citrus, Processed citrus fractions and soil. Unpublished
Report of University of Florida for Montedison USA.
Nelson, R.F., Kennedy, G.L. and Keplinger, M.L. Two Year Chronic Oral
Toxicity with Phenthoate (Cidial) Technical in Beagle Dogs. (1972)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
C-8884), reviewed and validated by BoB West Associates, Inc. (Nov. 13,
1978), submitted to the World Health Organization by Montedison.
Nigg, H.N. and Stamper, J.H. Persistence of Phenthoate (Cidial) and
Phenthoate Oxon on Fruit, Leaf and Soil Surfaces and in Air in Florida
Citrus. Submitted for publication to Florida Agricultural Experiment
Stations Journal, 1980.
Noakes, D.N. Summary of Toxicological Data on "Cidial". (1963)
Unpublished report from Medical Department, Fisons Pest Control
Limited, submitted to the World Health Organization by Montedison.
Oscarson, E.T., Marias, A.J., Kennedy G.L., Kinoshita, F.K. and
Keplinger, M.L. Eighteen Month Carcinogenicity Study with Elsan
(Phenthoate) in Albino Mice. (1976) Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 622-04624), reviewed and validated by
Bob West Associates, Inc. (Dec, 10, 1979), submitted to the World
Health Organization by Montedison.
Paa, H., Mastri, C.W. and Keplinger, M.L. Twenty-one-day Subacute
Dermal Toxicity Study with Cidial E4 in Albino Rabbits. (1974)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
601-03802), reviewed and validated by Bob West Associates Inc. (Jan.
11, 1974), submitted to the World Health Organization by Montedison.
Pellegrini, G. and Santi, R. Potentiation of Toxicity of
Organophosphorous Compounds Containing Carboxylic Acid Ester Functions
Towards Warm Blooded Animals by Some Organophosphorous Impurities. J.
Agr. Food. Chem. 20: 944-50.
Pfeifer, W.D., Smith, S., Kennedy, G.L., Kinoshita, F.K. and
Keplinger, M.L. Three-generation Reproduction Study with Elsan in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03513), reviewed and validated by Bob West
Associates, Inc. (Jan. 23, 1980), submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Eye Irritating Properties of Technical Cidial
and Cidial 50L. (1975a) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Intraperitoneal
Toxicity of Technical Cidial. (1975b) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Rats. (1978a) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Mice. (1978b) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity of Technical Cidial in
Albino Rats. (1978c) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity Study of Technical
Cidial in Albino Rabbits. (1978d) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Primary Skin Irritation Test of Technical
Cidial in Albino Rabbits. (1978e) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Salvaneschi, S. Acute Toxicity of the Commercially produced technical
Cidial and its Formulations to Laboratory Warm Blooded Animals. (1968)
Unpublished report from Agricultural Research Institute,
Montecatini-Edison (No. 45/1968), submitted to the World Health
Organization by Montedison.
Santi, R., Radice, M. and Martinotti, P. Degradation, metabolism and
residues of 32P-labelled CidialR in apples, pears, olives and olive
oil. Montedison S.p.A. Unpublished report no. 66, (1967).
Serota, D.G., Fezio, W.L. and Voelker, R.W. Three-week Dermal Toxicity
Study in Rabbits. (1979) Unpublished report from Hazleton Laboratories
America, Inc. (No. 2063-100), submitted to the World Health
Organization by Montedison.
Shirasu, Y., Moriya, M. and Kato, K. Microbial Mutagenic Study on
Elsan (Phenthoate). (1976) Unpublished report from Institute of
Environmental Toxicology, Nissan Chemical Industries, Inc., submitted
to the World Health Organization by Montedison.
Sikka H.C. Effect of Phenthoate on Microbial Functions. Syracuse
Research Corporation. (1979) Unpublished Report No. SRC L 1363-07.
Takade, D.Y. et al. Metabolism of O,O-Dimethyl
S-alpha-(carboetoxy)-benzyl phosphorodithioate (Phenthoate) in the
White Mouse and House Flies. Pesticide Biochemistry and Physiology 6:
367-376.
Takade, D.Y., Seo, M.S. and Fukuto, T.R. Alteration O,O-dimethyl
S-alpha-(carboetoxy)benzyl Phosphorodithioate (Phenthoate) in Citrus,
Water and upon Exposure to Air and Sunlight. Arch. Environ. Contam.
Toxicol. 5: 63.
Thompson, W., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Two-year
Chronic Oral Toxicity Study with Phenthoate (Cidial) in Albino Rats.
(1973) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(No. B-8882), reviewed and validated by Bob West Associates, Inc. (MaY
3, 1973), submitted to the World Health Organization by Montedison.
Toyoshima, S., Sasaki, H., Sato, R., Sato, S., Kato, M., and Nizeki,
H. Acute Toxicity Test Elsan(R) with Rats and Mice by Intraperitoneal
and Subcutaneous. (1971) Unpublished report from the Japan
Experimental Medical Research Institute, Co., Ltd., submitted to the
World Health Organization by Montedison.
Toyoshima, S., Sato, R., Sato, S., Kajima, M. and Nizeki, H.
Three-month Dietary Feeding Study of Elsan (Phenthoate) in Rats and
Mice. (1973) Unpublished report from The Japan Experimental Medical
Research Institute, Co., Ltd., submitted to the World Health
Organization by Montedison.
Trabucchi, E. Cidial Toxicology. (1965) Unpublished report from The
Instituto di Pharmacologia e di Terapia, Milan, submitted to the World
Health Organization by Montedison.
Umetsu, N., Hammond, P.S., Mallipudi, N.M., Toia, R.F. and Fukuto,
T.R. Toxicological Properties of Phosphorothioate and Dithioate
Impurities Present in Technical Organophosphorus Insecticides. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #36. Division of Pesticide Chemistry,
American Chemical Society.
Wargo, J.P. Cidial bovine metabolism and residue study. Research
Report Phase 1-6. (Analytical Development Corporation) Montedison
S.p.A. Unpublished Report No.64.
Wargo, J.P., Jr. Cidial Bovine Metabolism and Residue Study. (1975)
Unpublished report from Analytical Development Corp. (No.210),
submitted to the World Health Organization by Montedison.
Wright, G., Smith, S., Kennedy, G.L., Kinoshita, F.K. and Keplinger,
M.L. Three-generation Reproduction Study with Cidial Technical in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03803), reviewed and validated by Bob West
Associates, Inc. (Nov. 15, 1978), submitted to the World Health
Organization by Montedison.
The metabolism of phenthoate in the two varieties of pears showed a
slightly different pattern. Phenthoate acid,
O,O-dimethyl-thio-phosphoric acid, O,O-dimethyl dithio phosphoric
acid, dimethyl phosphoric acid and phosphoric acid were identified and
four other unidentified compounds were found in the "water-soluble"
extract. The quantity of any of them did not reach 0.1 mg/kg
phenthoate equivalent. Intact phenthoate in varying amounts, traces
of P=O derivative and unidentified compounds were found on the surface
of olive at different intervals after treatment. Phenthoate
penetrated into drupes with subsequent hydrolysis and oxidation
process and formation of the same metabolites as found in other
fruits. The processed oil contained only intact phenthoate (0.14
mg/kg), while in olive husks 0.23 mg/kg phenthoate and 3 mg/kg of
other 32P containing compounds were detected.
A 0.05% emulsified solution of 14C-labelled phenthoate was sprayed on
cabbage seedlings and brushed over Hime apple fruits and also
strawberries. Phenthoate remained mostly on the surface and only
3.3-6.7% of the total amount penetrated into the plants. The residue
decreased rapidly both on the surface and in the plants. Eight days
after treatment the residue on the surface of cabbage consisted of 86%
intact phenthoate, 8% bis-alpha-carbethoxy-benzyl disulphide, 1.9%
ethyl mandelate and 1.3% unidentified compound. Phenthoate oxon was
detectable in traces (0.4-0.7%) during the first day after treatment.
Within the cabbage seedlings 8 days after treatment intact phenthoate
was present in an amount of 10.2% and the metabolites identified were
mandelic acid (46.9%), bis-alpha-carbethoxy-benzyl disulphide (6.5%),
phenthoate acid (4.8%), desmethyl phenthoate (3.2%), phenthoate oxon
(1.8%). Six unidentified compounds were separated (total amount
18.8%) of which two accounted for 14.3%. The degradation of
phenthoate in/on apple took place more slowly than in/on cabbage, or
strawberry, while the metabolite patterns were rather similar in case
of apple and strawberry to that found in/on cabbage seedlings (Hirose
et al, 1971).
In soil
The degradation of ring-labelled 14C-phenthoate in a moist (50% of
capacity) loam and silty clay loam soil was studied (Iwata, 1977b).
Phenthoate was rapidly degraded by heat-labile soil enzymes which
converted it to phenthoate acid under both aerobic and anaerobic
conditions, even with a soil treatment of 100 mg/kg. Under aerobic
conditions and in low concentration the phenthoate acid underwent
extensive microbiological degradation to CO2 (up to 50% of the
theoretical value) and polar products. At 100 mg/kg level or under
anaerobic conditions it degraded by first order kinetics, presumably
by simple hydrolysis.
In a loam soil phenthoate at 1.25 and 2.5 mg/kg had no effects on the
rates of degradation of 14C-cellulose or 14C-starch by soil microbes
over a 25-day period as measured by the evolution of 14C. The rate
of nitrification in soil amended with (NH4)2SO4 was likewise
unaffected over a 28 days period (Sikka, 1979).
Phenthoate appears to be relatively persistent under dry soil
conditions (Iwata, 1977a) and in dry dust adhering to plant surfaces
(Iwata, 1975). In two sets of experiments 95% and 62% of the initial
doses remained unchanged after 59 and 75 days respectively.
The results indicate that conditions favouring microbial activity,
such as adequate moisture and warm temperatures, make soil type of
secondary importance for phenthoate degradation.
The fate of residues was also studied under field condition (Moye et
al, undated). Soil samples were taken from the drip-line and middle
at 0-6 and 6-12 cm depths under the orange trees treated with Cidial 4
E solution of 0.03% ai 1, 3, 7, 14 and 28 days after treatment. Four
samples were taken from both depths for each time. The maximum of
phenthoate concentration was observed at the drip-line on the third
day (1 mg/kg, in 0-6 cm and 0.68 mg/kg in 6-12 cm).
The mean residue at 0-6 cm was 0.56 mg/kg which decreased to 0.12
mg/kg 28 days after treatment.
Phenthoate oxon was not detectable in any of the experiments. The
mobility of phenthoate and its soil metabolites were studied by
leaching experiments using sandy soil (organic matter 1.6%), silt loam
(o.m. 6%), sandy loam (o.m. 3.3%), clay loam (o.m. 5.9%) (Anonymous,
1978a). The columns were filled to 25.4 cm with the untreated soils
after prewetting the column with water. The fortified soil (5 cm) was
layered over the untreated soil. For studying the mobility of
metabolites the fortified soils were aged for 30 days under greenhouse
conditions. Water equivalent to 500 mm rain for parent compound and
to 380 mm for metabolise was allowed to pass through the column and
analyzed. The columns were sectioned into four equal lengths for
analyses by the measurement of radioactivity. The upper 15 cm of the
sandy soil contained 72.3% of phenthoate and 85.7% of the metabolites
while the other soils retained 87.3-88.8% and 89.4-90.9% respectively.
The water effluent from the light sandy soil contained 8.32% and 6.62%
of the original activity. From the other soils the 14C activity in
the water effluent amounted to 2.9-6.44% and 3.39-4.21%.
In water
14C phenthoate was added to 0.01 M sodium phosphate buffers (pH 6.0,
7.0 or 8.0) to give a final concentration of 1.5 × 10 -5 M. Aliquots
were taken 3, 7, 10, 14, 21 and 28 days after addition of the 14C
material to the buffer and analyzed an TLC plates subsequent to
appropriate preparation.
32P-phenthoate was added to the buffer solutions in a 1 × 10-4 M
final concentration and was treated similarly to the 14C compound.
All solutions were held at 24.5 ± 1°C in a constant temperature room.
The pH of buffered solutions, containing radiolabelled phenthoate
under similar conditions, was monitored over the experimental period
and no pH change was detectable.
Evidently, phenthoate is fairly stable in water at the indicated pH
values. 45%, 21%, 22% of its initial amount were present after 28
days at pH 6, 7, 8 respectively. Its half life is about 12 days at pH
8.0. Phenthoate acid was observed in greatest amount (30-55%)
irrespective of pH. Hydrolysis of carbonyletoxy moiety appeared to be
fastest at pH 8.0. On the other hand the rate of formation of
desmethyl phenthoate, the second most prominent hydrolysis product,
was fastest at pH 6. Phenthoate oxon was not detected at any time.
Other products indicated in Figure 2 were observed in small amounts
(Takade, 1977).
Photodegradation
The photodegradation of 14C-phenthoate was studied in distilled water
(pH = 7.0±0.5) at concentrations of 8 mg/kg and 5 mg/kg (Anonymous,
1978).
A medium pressure mercury lamp, fitted with a Pyrex 7740 filter that
excludes light of wavelength less than 280 nm, was immersed in the
solution to be irradiated. After 5 and 8 hours of irradiation,
aliquots of the photolysed solutions were taken and analyzed applying
HPLC and TLC techniques. Approximately 50% of phenthoate had degraded
after 6-8 hours of irradiation, while there was no loss in another
phenthoate solution kept in the dark for the same time. Ethyl
mandelate was found to be the major degradation product. In addition
three unknown products were detected but each of them accounted for
less than 10% of the parent compound.
An acetone solution of radiolabelled phenthoate (32P or 14C) was
applied to glass plates, which were exposed to sunlight daily for 7
hours (Takade, 1977).
Air temperature during the exposure ranged from 7.2 to 23.3°C. Under
these conditions approximately 90% of the applied phenthoate was lost
after 40 hrs of exposure by volatilisation.
The unchanged phenthoate gradually decreased in proportion to the
other alteration product during the exposure (see Figure 2).
Phenthoate oxon was identified as the major product. Other products
isolated were desmethyl phenthoate, mandelic acid, bis-[alpha-carboxy)
benzyl]-disulphide, bis[alpha-/carbethoxy/benzyl]disulphide and
O,O-dimethyl phosphorothioic acid. Results of other experiments
indicated that elevated temperatures may speed up the phenthoate oxon
formation and its disappearance. The latter is also facilitated by
rainfall (Nigg and Stamper, 1980).
The degradation products formed upon exposure to sunlight and air on
glass plates or found on citrus leaves and fruits are essentially the
same.
In processing
The effect of processing was studied on orange, lemon and grapefruit
treated in the field twice with Cidial 4 E in solutions of 0.03% or
0.06% ai. The samples were taken 50, 19 and 18 days after last
treatment respectively and were processed with a FMC processor. The
residue ranges found in 4 samples taken at each stage are summarised
in Table 13 (Moye et al, undated).
Little, if any, residue decrease occurred from washing of oranges and
lemons, which is in good agreement with the findings of Iwata et al,
who carried out laboratory washing to simulate packing house
treatment. On the other hand laboratory washing of the grapefruit
removed about 50% of the residue found 3 days after treatment and from
about 25% to less than 10% thereafter.
Phenthoate seems to be concentrated in the waxes and oils of the rind.
Fruit juice contained negligible residues (<0.01 mg/kg). Residues
found in the dried rind, which is used as a cattle feed, show an
approximate 44-58% loss of residue during processing and drying.
The majority of the residue found in/on olives remained in the olive
husk and the processed oil contained only intact phenthoate (4.1% of
total residue) (Santi, 1976).
METHODS OF RESIDUE ANALYSIS
Methods that have been developed for the determination of phenthoate
in crops and animal tissues have been reviewed. Bazzi (undated)
described a method which is based on acetone extraction, liquid-liquid
partition clean up on a Plorex column and gas chromatographic
determination using 3.8% UCCW 982 on Gas Chrom Q packing and a flame
photometric detector. The limit of determination is 0.04 mg/kg or
less depending on the type of sample. The method is suitable for the
determination of phenthoate and its oxon in citrus and their processed
by-products in maize, cattle meat and soil.
Moye et al (undated) found acetone: acetonitrile 1:1 mixture most
efficient for the extraction of phenthoate from soil, fruits and
by-products of citrus processing. OV-101, OV-17 (Iwata et al, 1979)
and OV-225 (Moye et al, undated) liquid phases were used to separate
phenthoate, phenthoate oxon from other organophosphate pesticides also
applied in citrus orchards.
Another method for the determination of residues in animal products
includes extraction by acetonitrile from muscle, kidney and liver, by
acetone and benzene from milk and by heptane from fat; partition with
benzene, hexane and acetonitrile; column chromatography on Floricil;
and GLC determination using 3% OV-101 packing with FPD. Limit of
determination: 0.01 mg/kg for milk, 0.05 mg/kg for tissues. The
recovery at 0.05-0.1 mg/kg level is about 92% for all samples.
The basic method described by Bazzi (undated) in combination with the
other liquid phases and slight modifications proposed by the other
authors is suitable for regulatory purposes.
NATIONAL MRL'S REPORTED TO THE MEETING
Phenthoate is registered in 36 countries all around the world. Some
of the maximum residue limits and pre-harvest intervals were reported
to the meeting.
Pre-harvest
intervals MRL
(days) (mg/kg)
Hungary apple, pear,
sugar-beet 21 0.5
Italy apple, pear, walnut,
vegetables, rice 20 0.3
citrus, olive 60 0.3
Israel oranges, grapefruit 20-30
Japan pear, peach 7 0.1
pumpkin 1 0.1
mandarine, orange 14 0.1
rice (unpolished) 7 0.05
japanese pear 30 0.1
Netherlands citrus fruit 0.5
New Zealand brassica crops 0.7
South Africa citrus 21 1.0
brassica 3 1.0
onion,potato 7 0.1
Sweden citrus 1.0
USA citrus 2.0
TABLE 5. Residues of Phenthoate in oranges
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia USA 1973 1 8.5 50 L 0.8 0.5 0.4 0.3 0.2 0.1 0.1 Iwata, 1977a
(Florida) 1 4.3 50 L 0.5 0.3 0.2 0.1 0.09 0.09 0.07
Valencia USA 1979 1 4.2 4 EC 2.9 2.3 1.5 0.58 0.23 Iwata, 1979
(peel) (Florida) 1 8.4 4 EC 4.0 3.0 2.1 0.85 0.35
1 8.41 4 EC 6.3 4.2 3.0 1.4 0.38
Valencia USA
(peel) (Florida) 1979 2 0.03%3 4 EC 1.65 0.67 0.29 0.38 Moye et al
(pulp) 0.06% 0.03 0.04 0.01 0.01 (undated)
Hamlin Brazil 1979 1 0.1% 50 L 1.38 0.79 0.5 0.33 0.22 0.22-0.12 Camargo,1980
(peel)
Valencia Italy 1965 1 0.06%1,2 50 L 4.9 1.6 1 1.1 0.8 0.6 Anonym, 1965
Moro (peel) Italy 1963 1 0.05%1,2 50 L 3.2 1.8 1.2
Valencia Israel 1973 1 7.5+0.5% oil 50 L 1.66 1.08 0.5 0.2 Greenberg
1 10 50 L 0.71 0.58 0.04 0.09 0.02 et al, 1974
1 20 50 L 2.1 1.33 0.75 0.37 0.12
Shamouti Israel 1973 1 7.5 50 L 1.02 0.98 0.45 0.21
1 7.5+0.5% oil 50 L 0.98 1.08 0.4 0.36
1 15 50 L 1.68 1.41 0.82 0.4
Orange Japan 3 0.05% 50 EC 0.01 0.008 0.004 Anonym, 1980
(pulp) 5 0.05% 0.01 0.009 0.009
3 0.05% 0.02 0.005 0.008
5 0.05% 0.015 0.006 0.005
TABLE 5. Continued...
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-3 7-10 14-19 24-25 31-32 39-45 56
Valencia S. Africa 1970 1 0.213% 50 L 3.7 2.05 1.1
1971 1 0.1% 50 L 1.21 0.56 0.32 0.23 Anonym,
1970
Valencia S. Africa 0.37 0.3 0.12
(pulp)
1 Low volume application with a spraying volume of 940 l/ha
2 Colorimetric method was used for the determination
3 Three months elapsed between applications; the maximum residue values found in four replicates are given
TABLE 6. Residues of Phenthoate in Grapefruit and Lemon
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0-4 8-11 18-21 33-35 38-46 60
Grapefruit USA 1978 1 3.4 4 EC 0.94 0.77 0.48 Iwata, 1979
(peel) 1 6.7 4 EC 2.1 1.6 0.87
1 6.71 4 EC 3.9 2.5 1.4
1979 1 0.03%2 4 EC 0.9 0.5 0.31
0.06%2 1.8 0.77 0.59
(pulp) USA 1979 2 0.03+0.063,4 4 EC 0.007 0.008 0.011 0.002 <0.002 Moye et al, undated
Whole fruit Israel 1973 1 0.075% 50 L 0.97 0.47 0.23 1.131 Greenberg et al,
1 0-0.75%4 50 L 1.4 0.63 0.39 0.14 1974
1 0.075%+0.5% oil 50 L 0.84 0.79 0.93 0.1
2 0.075%+0.5% oil4 50 L 1.0 1.0 0.74 0.16
1 0.15% 50 L 1.25 1.5 0.41 2.7
1 0.75%4 2.0 2.0 0.57 0.42
Lemon USA 1974 1 7 50 L 1.2 0.8 0.6 0.4 0.3 0.3 Iwata, 1979a
(peel) 1974 1 1.8 50 L 0.5 0.3 0.2 0.2 0.1 0.1
1978 1 4.2 4 EC 1.5 0.9 0.47
1 8.2 4 EC 2.8 1.7 0.78
1 8.21 4 EC 3.8 3.3 2.2
1979 1 5 4 EC 3.7 2.7 1.1
10 4 EC 7 4.5 1.9 Iwata, 1979
10 4 EC 14 11 5.7
Whole fruit S.Africa 1973 1 0.04% 50 L 0.4 0.26 0.08 0.09 Anonym, 1970
0.1% 50 L 1.4 0.96 0.47 0.17
1 Low volume application with a spraying volume of 940 l/ha
2 Sprays were applied to achieve full coverage
3 17 days elapsed between applications
4 The maximum residue values found in four replicates are given.
TABLE 7. Residues of Phenthoate in Apple and Pear
Residues in mg/kg, at intervals/days/
Variety Application after application Reference
(and part Country Year No. kg ai/ha formulation
of fruit) or % 0 1 4-7 9 14-17 21-26 29-34 39-46
Apple Italy 19621 1 0.04% 50 L 2.8 1.19 0.47 0.22 0.16 0.11 <0.1 Anonym, 1972
1962 1 0.03% 50 L 1.57 0.78 0.34 0.18 0.13 0.1
19621 1 0.04% 50 L 1.6 0.93 0.36 0.1 <0.1
1962 4 0.04% 50 L 1.92 1.21 0.48 0.12 <0.1
Pear Italy 19631 1 0.04% 50 L 1.45 1.1 0.6 0.4 0.25 Anonym, 1973
1963 1 0.04% 50 L 1.9 1.7 0.54 0.3 0.1 <0.1
1963 6 0.05% 50 L 0.64 0.35 0.18
Japanese Japan 3 0.05% 0.3 0.11 0.05 Anonym, 1980
pear 5 0 05% 0.44 0.16 0.09
3 0.05% 0.48 0.16 0.03
4 0.05% 0.58 0.1 0.08
1 Colourimetric method was used for the determination.
TABLE 8. Residues of phenthoate in other crope resulting from supervised trials
Application Residues in mg/kg, at intervals (days) Reference
after application
Crop Country Year No. rate % formulation 0 1 6-7 13-14 20-21 21-26 34 70
volume (1)
Cabbage Italy 1965 2 0.05 50 L <0. 1 <0.1 Anonym.,
6 3.18 0.11 0.09 1965
Olives Italy 1963 1 0.05/3000 50 L 10.9 8.1 0.49 0.49 0.2 Anonym.,
1963
Onion Japan 2 0.05/1500 0.01 Anonym.,
4 0.01 1980
Radish 2 0.05/2000 0.005
4 0.05/1500-2000 0.005 0.005 0.005 0.005
3 0.05/1800
Spinach Japan 2 0 05/1500 0.015
3 0:05/1500 0.018
2 0 05/1500 0.005 0.015
3 0:05/1500 0.007 0.005
Tea/leaf Japan 2 0.05/2000 0.008
3 0.05/2000 0.04
2 0.05/3000 0.04
3 0.05/3000 0.04
Lettuce Japan 2 0.05/2000 0.08 0.08 0.02
4 O.05/2000 0.06 0.05 0.09
TABLE 9. Carbon 14 residues in bovine tissue after feeding at a
20 mg/kg level in the diet
mg/kg in tissues after feeding for
Tissue 8 days 18 days 26 days 26 days
7 days
withdrawal
Muscle 0.013 <0.033 <0.033 <0.033
Fat 0.016 0.056 0.045 <0.045
Kidney 0.324 0.408 0.502 0.116
Liver 0.238 0.228 0.297 0.140
Heart 0.028 0.061 0.087 0.052
Brain 0.036 <0.042 0.054 <0.042
TABLE 10. The penetration of 14C and 32P phenthoate into orange
fruit, and the distribution of radioactivity in the surface wash,
acetone extract and solid residue.
Percentage of the applied radioactivity
recovered at indicated time (days)
0 3 7 10 14
14C-labelled phenthoate
External surface wash 72.5 66.1 9.8 8.1 4.6
Internal acetone extract1 - 5.4 15.6 12.8 7.8
Solid residue1 27.5 21.7 21.6 34.7 28.3
Total recovered 100.0 93.2 47.0 55.6 40.7
32P-labelled phenthoate
External surface wash 96.4 63.4 17.4 13.6 11.6
Internal acetone extract1 - 5.9 22.0 24.2 18.2
Solid residue1 3.6 19.4 13.9 16.3 13.2
Total recovered 100.0 88.7 53.3 54.1 43.0
1 Includes peel only. Radioactivity in pulp was less than 1% of
the applied.
TABLE 11. Amount of 14C metabolites found in the surface wash and
acetone extract of orange leaves after indicated time intervals
Compound found in Percent of recovered radioactivity
indicated material after indicated time (days)
3 7 10 14
Phenthoate
Surface wash 84.4 45.5 35.4 41.6
Acetone extract 4.6 46.1 37.7 17.0
Phenthoate
Surface wash 0 0 2.8 5.1
Acetone extract 0.2 0 7.2 12.4
TABLE 12. 32P Phenthoate equivalents (mg/kg) in treated apples, pears and olives
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Apples: var. Stark Delicious (0.02% solution)
2 hrs. 1.00 0.21 0.17 0.04 - - 1.17
1 day 0.26 traces 0.31 0.06 - - 0.57
15 days 0.02 - 0.08 0.02 0.95 0.80 0.10
29 days 0.00 - 0.00 - 0.96 0.69 0.00
Pears: var. Trionfo di Vienna (0.04% solution)
2 hrs. 1.35 traces 0.19 0.02 - - 1.44
1 day 1.19 traces 0.40 0.05 - - 1.59
4 days 0.33 - 0.26 0.09 - - 0.59
9 days 0.19 - 0.12 0.07 0.43 0.18 0.31
31 days1 0.006 - 0.01 0.02 0.16 0.09 0.016
Pears: var. Passacrassana (0.045% solution)
2 hrs. 1.23 traces 0.03 traces - - 1.26
1 day 1.01 traces 0.07 traces - - 1.08
5 days 0.55 - 0.05 - 0.04 0.02 0.60
25 days1 0.28 - 0.16 - 0.39 0.07 0.44
40 days2 0.20 traces 0.10 traces 0.34 0.14 0.30
TABLE 12. Continued...
Time interval External substances Internal substances Internal Internal Total
soluble in methanol soluble in chloroform substances substances internal +
soluble insoluble external
Phenthoate Other P32 Phenthoate Other P32 in water in chloroform phenthoate
containing containing and water residue
substances substances
Olives /oils-yielding/: var. Leccino (0.05% solution)
2 hrs. 11.16 traces 0.15 0.01 traces - 11.31
1 day 5.92 traces 1.50 0.36 0.10 - 7.42
9 days 0.36 0.18 0.53 0.38 3.60 0.84 0.89
30 days 0.19 0.03 0.06 0.35 2.58 0.86 0.25
70 days 0.05 - 0.10 0.05 3.23 0.59 0.15
1 Fruit picking
2 Pears of this winter variety were analysed after picking from trees
and stored for 15 days at 19-20°C.
TABLE 13. Phenthoate residue (mg/kg) detected in processed citrus by-products
ORANGE LEMON GRAPEFRUIT
Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon Phenthoate Phenthoateoxon
Unwashed peel 0.24-1.00 0.06-0.09 0.08-0.28 0.06-0.12 0.031-0.74 <0.01
Washed peel 0.23-0.73 0.036-0.069 0.15-0.32 <0.01 0.23-0.51 <0.01-0.09
Unwashed pulp <0.002-0.031 0.01 0.003-0.024 <0.01 0.007-0.031 <0.01
Washed pulp 0.005-0.011 0.01 0.002-0.004 <0.01 0.02-0.09 <0.01
Chopped peel 0.34-0.67 0.01-0.03 0.08-0.26 0.03 0.26-0.49 0.002-0.051
Peel frits 0.32-0.52 0.05-0.15 0.25-0.74 0.05-0.15 1.14-2.45 0.015-0.031
Finisher pulp 0.008-0.015 <0.01 0.008-0.023 <0.01 0.003-0.008 <0.01
Dried rind 0.13-0.43 0.01-0.036 2.2-2.7 0.01 0.72-1.51 0.06-0.21
Press liquor 0.08-0.14 <0.01-0.01 0.008-0.032 0.009-0.017 0.13-0.19 0.01-0.013
Emulsion water 0.004-0.07 <0.004-0.1 0.003-0.009 0.04-0.06 0.006-0.021 <0.01
Fruit juice 0.002-0.003 <0.01 0.004-0.009 <0.01 <0.002 <0.01
After-water rinse <0.002-0.004 <0.01 <0.002-0.005 <0.01 <0.002 <0.01
Pre-water rinse <0.002 <0.01 0.002 <0.01 <0.002 <0.01
Molasses 0.047-0.068 <0.01 0.01-0.03 <0.01 0.037-0.071 0.01
Oil 3.29-14.05 1.18-2.48 7.25-17.25 1.65-2.5 5.4-8.5 0.45-1.1
EVALUATION
COMMENTS AND APPRAISAL
Phenthoate is a wide-spectrum organophosphorus insecticide, mainly
used on citrus at rates of 0.6 to 5 kg ai/ha.
The technical material contains a minimum of 92% of phenthoate.
Detailed analyses of the typical technical material, including the
identity of the minor components, was provided. It is generally
soluble in most polar and non-polar organic solvents and also slightly
soluble in water.
Phenthoate is a cholinergic compound of moderately acute toxicity and
is rapidly absorbed, metabolized and excreted in mammals. The pattern
of metabolism is similar in both plants and animals and is similar to
that noted with other organophosphorus esters. The rate of metabolism
is shown to be significantly affected (with potentiation of toxicity)
by organophosphorus esters commonly found as impurities in the
technical product. The acute toxicity of phenthoate is dependant on
the purity of the technical product, a feature which raised
significant questions about occupational exposure. It was suggested
that a more purified product should be manufactured and used to
alleviate this problem. Formulations that are stored also have the
potential to become more toxic under certain conditions. Although
these factors do not directly affect phenthoate residues in food they
should not be ignored.
Phenthoate is moderately toxic to mammals. The acute LD50 was noted
to vary from 78 to over 4500 mg/kg bw, depending possibly on the
impurities and the species. Antidotal studies are not available.
Phenthoate does not induce a delayed neurotoxic reaction in hens, is
not mutagenic or teratogenic, nor does it interfere with reproduction,
as evidenced by a standardized 3-generation study in rats. In a
series of short-term toxicity studies, cholinesterase depression was
the most significant measure of effect of exposure. In dermal and
ophthalmological studies, phenthoate applied as a formulated product
was irritating. On the basis of available short-term studies, it is
concluded that phenthoate is a potent cholinesterase inhibitor.
A two-year study in the dog showing red blood cell cholinesterase
depression served as the primary basis to evaluate a no-effect level.
Phenthoate is not a carcinogen in mice. A two.year study in rats was
considered invalid and not included in the evaluation.
On the basis of available data, no-effect levels in mammalian species
were determined and a temporary ADI for man was allocated. This
evaluation was made on the assumption that the manufacture of
phenthoate would be by a process that would assure that a product of
at least 92% purity would be marketed. As this was the product on
which toxicological evaluations were made and as impurities have a
substantial toxicological significance this purity restriction was
important.
Phenthoate residues gradually disappear from treated plants within 4
to 10 weeks, most being lost by volatilization. Decomposition of
surface deposits is mainly due to photodegradation, resulting in
phenthoate oxon as the main metabolite. On cabbage, the surface
residue 8 days after application consisted of 86% phenthoate but no
oxon was detected; on orange leaves, 41.6% of the total residue was
phenthoate and 5.1% was the oxon at 14 days after treatment.
Phenthoate is not systemic but does enter the peel of fruit, where it
undergoes hydrolytic cleavage and oxidation. Virtually all of the
residue remains in the peel of treated citrus fruit, the pulp
containing less than 1% of the total residue. Phenthoate oxon has
been detected only in the peel.
The main metabolites in plants, phenthoate oxon, dimethyl phenthoate,
mandelic acid, bis-2-(carbethoxy) benzyl disulphide, O,O-dimethyl
phosphorodithioic acid, O,O-dimethyl phosphorothioic acid and
phenthoate acid, are similar to those secreted by animals. Their
amounts and relative proportions depend on the crop and the time
elapsed since application but, in practice, the parent phenthoate
should be considered as the only residue of importance.
Repeated treatments of citrus fruits or apples according to
recommended usage do not lead to increased residues or changed
metabolite patterns. The residue is contained mostly in the waxes and
oils of the citrus rind and is not decreased significantly by washing;
the fruit juice contains negligible residues. Dried rind as used for
cattle feed can contain 40 to 60% of the residue originally present in
the peel.
Laying hens fed for 30 days with feed containing 1.8 to 2.6 mg/kg of
phenthoate showed no reduction of egg production or any abnormalities.
Residues in the muscle and in eggs were low and disappeared after
withdrawal of the compound from the diet; in kidney and liver levels
reached a plateau between the third and seventh days of treatment.
Neither phenthoate nor its metabolites accumulated in fish kept in
water containing phenthoate at the 10-4 to 10-6 mg/l levels.
Lactating cows given 14C-phenthoate at levels of 1.5 and 20 mg/kg in
the feed excreted the material mainly in the urine and faeces. The
main metabolite was phenthoate acid, which accounted for 45% of the
14C activity of the urine. Residues in milk reached a plateau 2 to 3
days after administration began. The organosoluble fraction of the
residue in milk, possibly phenthoate, phenthoate oxon and unconjugated
metabolites, was very low (0.0028 mg/kg) even at the higher dosage
rate. Meat from the cattle contained traces of residues at the higher
dosage rate only, the muscle tissue and the fat containing the
residues in roughly equal proportions. Residues in various tissues
declined rapidly when the pesticide was withdrawn from the diet; even
the bound (unextractable) metabolites were eliminated from kidney and
liver. Neither phenthoate nor its oxon could be detected in tissue or
milk samples by conventional analytical methods at limits of
determination of 0.05 and 0.01 mg/kg respectively. It was concluded
that feeding cattle with citrus by-products prepared from
phenthoate-treated fruit would not result in detectable residues in
meat or milk.
In soil, phenthoate is rapidly converted to phenthoate acid under both
aerobic and anaerobic conditions, even when as much as 100 mg/kg is
added to the soil. Under conditions favourable for microbiological
activity, such as adequate moisture and warm temperature, the soil
type has no influence on the degradation of phenthoate. On the other
hand, phenthoate persists for a relatively long time under dry soil
conditions. Phenthoate and its metabolites show moderate mobility in
soil, the majority of the residue being contained in the upper 15 cm.
The likelihood of contamination of lower soil layers or underground
waters is very low in view of the high degradability and slow leaching
properties of the residues.
Analytical methods, based on GLC separation on columns of different
polarity with flame photometric detection are available and suitable
for regulatory purposes.
Level-causing no toxicological effect in:
Dog: 10 mg/kg in the diet equivalent to 0.29 mg/kg bw/day.
Mouse: 30 mg/kg in the diet equivalent to 4.5 mg/kg bw/day.
Rat: 10 mg/kg in the diet equivalent to 1.0 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concluded that available information was adequate for the
estimation of maximum residue levels for phenthoate on citrus fruits
only. The data on other crops were not considered adequate for
estimating maximum residue levels. The levels apply to the parent
phenthoate excluding all metabolites.
Commodity Temporary maximum Interval between last
residue level (mg/kg) application and harvest
on which levels are
based
Citrus fruit 1 21
Carcass meat of cattle 0.051
Milk 0.011
Egg 0.051
1 At the limit of determination.
FURTHER WORK OR INFORMATION
Required (by June, 1984)
A chronic toxicity study in an acceptable mammalian species (rodent).
Desirable
1. Further information on impurities in the technical products in
order to evaluate the degree to which these impurities potentiate the
toxicity of phenthoate.
2. Data from supervised trials on other commodities in accordance
with present uses of the compound.
3. Residue data from crops, known to have been treated under
practical conditions, moving in commerce.
REFERENCES
Anonymous. Cidial Residues in Apples. Montedison S.p.A. Unpublished
report no. 77, (1962).
Anonymous. Cidial Residues in Pears, Olives. Montedison S.p.A,
Unpublished report nos.78, 79, (1963).
Anonymous. Cidial Residues in Oranges. Montedison S.p.A., Unpublished
report no 74/b, (1964).
Anonymous. Cidial Residues on Oranges, Cabbages. Montedison S.p.A.
Unpublished reports Nos. 76/b, 80, (1965).
Anonymous. Cidial Residues on Citrus Form Chem. Report Montedison
S.p.A. Unpublished report no. 74/a, (1970).
Anonymous. Determination of Cidial [Ethyl
alpha(/Dimethoxy-phosphinothioyl/thio) benzene acetate] in muscle,
milk, kidney, liver and fat. Thompson-Hayward Chemical Co., Montedison
S.p.A. Unpublished report no. 62, (1975).
Anonymous. Cidial Leaching Study on Parent compound and on its Soil
Degradation Products. Carried out by Thompson-Hayward Chemical
Company. Montedison S.p.A. Unpublished Report no 91, (1978a).
Anonymous. Photodegradation of Phenthoate in Aqueous Solution.
Submitted by Syracuse Research Corporation. Montedison S.p.A.
Unpublished Report no. 93, (1978b).
Anonymous. Interpretation and discussion on the metabolism of Cidial
in the dairy cow. Unpublished Report of Analytical Development
Corporation. Montedison S.p.A. Report No. 65, (1979).
Anonymous. Summary of Residue Data in Japan. Nissan Chemical
Industries Ltd. Unpublished reports, (1980).
Arnold, D., Kennedy, G.L., Kinoshita, F.K. and Keplinger, M.L.
Mutagenic Study with Cidial in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (No. 622-05876), reviewed and
validated by Bob West Associates, Inc. (Nov. 6, 1978), submitted to
the World Health Organization by Montedison.
Arnold, D., Kennedy, G.L. and Keplinger, M.L. Mutagenic Study with
Elsan (Phenthoate) in Albino Mice. Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 632-05147), reviewed and validated by
Bob West Associates, Inc. (Feb, 8, 1980), submitted to the World
Health Organization by Montedison.
Bazzi, B. Phenthoate in Zweg G., ed. Analytical Methods for Pesticides
and Plant Growth Regulators. Academic Press, New York, Vol. VIII, p.
159.
Booth G.M. Bioaccumulation Study of Cidial in Channel Catfish.
Montedison S.p.A. Unpublished report no. 87, (1978).
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04413), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Brett, B., Paa, H., Mastri, C.W. and Keplinger, M.L. Subacute Dermal
Toxicity Study with Cidial E4 in Albino Rabbits. Unpublished report
from Industrial Bio-Test Laboratories, Inc. (No. 601-04524), reviewed
and validated by Bob West Associates, Inc. (Jan. 28, 1974), submitted
to the World Health Organization by Montedison.
Camargo de., J.L.G. Residues de carbophenothione Phenthoate em cascas
e polpas de laranjos hamlin determinados por cromatografia em fase
gasosa. Dissertatio submitted to Luiz de Queiroz University, Sao
Paulo, 1980.
de Carneri, I. Microbiological Study of Mutagenesis on CRA 133 (GMA
267/78) Genetic Mutation Test in Salmonella Typhimurium (Ames). (1979)
Unpublished report from Farmitalia Carlo Erba, Research and
Development, submitted to the World Health Organization by Montedison.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
Neurotoxicity Study with Elsan in Chickens. Unpublished report from
Industrial Bio-Test Laboratories, Inc. (no. 8580-80635), reviewed and
validated by Bob West Associates, Inc. (Jan. 25, 1980), submitted to
the World Health Organization by Montedison.
Good, J., Cooper, D. and Terrell, Y. Acute Delayed Neurotoxicity Study
of Cidial Technical 96% Batch 104 in Female Chickens. (1979)
Unpublished report from Cannon Laboratories, Inc. (No. 8E1191),
submitted to the World Health Organization by Montedison.
Greenberg, R. et al. Residues and Disappearance of Cidial in Valencia
Oranges. Montedison S.p.a. Unpublished Report No. 73/a, (1974).
Hammond, P.S., Talcott, R.E. and Fukuto, T.R. Metabolism and Mode of
Action of O,O,S-Trimethyl Phosphorothioate in the Rat. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #38, Division of Pesticide Chemistry, American
Chemical Society.
Hathaway, D., Keplinger, M.L. and Fletcher, D.B. Acute Inhalation
Toxicity Study with Phenthoate (Cidial) Technical in Albino Rats.
(1971) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(no. N-9324), reviewed and validated by Bob West Associates, Inc.
(Nov. 6, 1978), submitted to the World Health Organization by
Montedison.
Hirose, M. et al. Residue Degradation and Metabolism of 14C Labelled
Elsan(R) (O,O-Dimethyl-S-alpha-carboetoxy-benzyl phosphorodithioate)
in Cabbage, Hime Apples and Strawberries. (In Japanese). Scientific
Pest. Control 36: 43-51.
Huhtanen, K. Poultry Tissue and Egg Study with Cidial. Cammon
Laboratories Inc. Montedison S.p.A. Unpublished Report Nos. 84, 85,
(1979a).
Huhtanen, K. Accumulation of 14C Cidial in Bluegill Sunfish Exposed
in Dynamic Flow System. Cammon Laboratories, Inc. Montedison S.p.A.
Unpublished Report No. 88, (1979b).
Iwata, Y. et al. Behaviour of Phenthoate (Cidial) Deposits and
Residues on and in Grapefruits, Lemons, Lemon Leaves, Oranges and
Orange Leaves and in the Soil Beneath Orange Trees. J. Agric. Food
Chem. 25 (2): 382-388.
Iwata, Y., Ithig, M. and Gunther, F.A. Degradation of
O,O-Dimethyl-S-alpha-(carboethoxy)-benzyl phosphorodithioate
(Phenthoate), in Soil. Arch. Environm. Contam. Toxicol. 6: 1-12.
Iwata, Y. et al. Phenthoate Applied to California Citrus Trees: Worker
Environment Research and Residue Levels on and In Fruit. Report of
Department of Entomology, University of California. Montedison S.p.A.
Unpublished Report No. 72b, (1979).
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., Kinashita, F.K.
and Keplinger, M.L. Teratogenic Study with Cidial in Albino Rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
reviewed and validated by Bob West Associates, Inc. (Nov. 21, 1978),
submitted to the World Health Organization by Montedison.
Lee, S.G.K. and Fukuto, T.R. Effect of Impurities in Technical
Phenthoate (Cidial(R)) on Mammalian Carboxyesterases. Second Chemical
Congress of the North American Continent, Las Vegas, Nevada, August
25-29, 1980. Abstract #35. Division of Pesticide Chemistry, American
Chemical Society.
Mallipudi, N.M., Badaway, S.M.A. and Fukuto, T.R. Inhibition of
Mammalian Esterases by O,O,S-Trimethyl Phosphorothioate. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #37, Division of Pesticide Chemistry,
American Chemical Society.
Mallipudi N.M. and Fukuto, T.R. Characterization of Bound Phenthoate
Residues in Citrus. Submitted to Archives of Environ. Contam.
Toxicol., 1980.
Marshall B.L. Cidial Fish Residue Study. Marine Research
Institute/Massachusetts. Montedison S.p.A. Unpublished Report No. 86,
(1979).
Moye et al. Residue Analysis of Phenthoate (Cidial(R)) and Phenthoate
Oxon in Citrus, Processed citrus fractions and soil. Unpublished
Report of University of Florida for Montedison USA.
Nelson, R.F., Kennedy, G.L. and Keplinger, M.L. Two Year Chronic Oral
Toxicity with Phenthoate (Cidial) Technical in Beagle Dogs. (1972)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
C-8884), reviewed and validated by BoB West Associates, Inc. (Nov. 13,
1978), submitted to the World Health Organization by Montedison.
Nigg, H.N. and Stamper, J.H. Persistence of Phenthoate (Cidial) and
Phenthoate Oxon on Fruit, Leaf and Soil Surfaces and in Air in Florida
Citrus. Submitted for publication to Florida Agricultural Experiment
Stations Journal, 1980.
Noakes, D.N. Summary of Toxicological Data on "Cidial". (1963)
Unpublished report from Medical Department, Fisons Pest Control
Limited, submitted to the World Health Organization by Montedison.
Oscarson, E.T., Marias, A.J., Kennedy G.L., Kinoshita, F.K. and
Keplinger, M.L. Eighteen Month Carcinogenicity Study with Elsan
(Phenthoate) in Albino Mice. (1976) Unpublished report from Industrial
Bio-Test Laboratories, Inc. (No. 622-04624), reviewed and validated by
Bob West Associates, Inc. (Dec, 10, 1979), submitted to the World
Health Organization by Montedison.
Paa, H., Mastri, C.W. and Keplinger, M.L. Twenty-one-day Subacute
Dermal Toxicity Study with Cidial E4 in Albino Rabbits. (1974)
Unpublished report from Industrial Bio-Test Laboratories, Inc. (No.
601-03802), reviewed and validated by Bob West Associates Inc. (Jan.
11, 1974), submitted to the World Health Organization by Montedison.
Pellegrini, G. and Santi, R. Potentiation of Toxicity of
Organophosphorous Compounds Containing Carboxylic Acid Ester Functions
Towards Warm Blooded Animals by Some Organophosphorous Impurities. J.
Agr. Food. Chem. 20: 944-50.
Pfeifer, W.D., Smith, S., Kennedy, G.L., Kinoshita, F.K. and
Keplinger, M.L. Three-generation Reproduction Study with Elsan in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03513), reviewed and validated by Bob West
Associates, Inc. (Jan. 23, 1980), submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Eye Irritating Properties of Technical Cidial
and Cidial 50L. (1975a) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Intraperitoneal
Toxicity of Technical Cidial. (1975b) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Rats. (1978a) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Determination of the Acute Oral Toxicity of
Technical Cidial in Albino Mice. (1978b) Unpublished report from
Centro Ricerche Antiparassitari, Montedison, submitted to the World
Health Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity of Technical Cidial in
Albino Rats. (1978c) Unpublished report from Centro Ricerche
Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Acute Dermal Toxicity Study of Technical
Cidial in Albino Rabbits. (1978d) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Re, U.D. and Gera, F.V. Primary Skin Irritation Test of Technical
Cidial in Albino Rabbits. (1978e) Unpublished report from Centro
Ricerche Antiparassitari, Montedison, submitted to the World Health
Organization by Montedison.
Salvaneschi, S. Acute Toxicity of the Commercially produced technical
Cidial and its Formulations to Laboratory Warm Blooded Animals. (1968)
Unpublished report from Agricultural Research Institute,
Montecatini-Edison (No. 45/1968), submitted to the World Health
Organization by Montedison.
Santi, R., Radice, M. and Martinotti, P. Degradation, metabolism and
residues of 32P-labelled CidialR in apples, pears, olives and olive
oil. Montedison S.p.A. Unpublished report no. 66, (1967).
Serota, D.G., Fezio, W.L. and Voelker, R.W. Three-week Dermal Toxicity
Study in Rabbits. (1979) Unpublished report from Hazleton Laboratories
America, Inc. (No. 2063-100), submitted to the World Health
Organization by Montedison.
Shirasu, Y., Moriya, M. and Kato, K. Microbial Mutagenic Study on
Elsan (Phenthoate). (1976) Unpublished report from Institute of
Environmental Toxicology, Nissan Chemical Industries, Inc., submitted
to the World Health Organization by Montedison.
Sikka H.C. Effect of Phenthoate on Microbial Functions. Syracuse
Research Corporation. (1979) Unpublished Report No. SRC L 1363-07.
Takade, D.Y. et al. Metabolism of O,O-Dimethyl
S-alpha-(carboetoxy)-benzyl phosphorodithioate (Phenthoate) in the
White Mouse and House Flies. Pesticide Biochemistry and Physiology 6:
367-376.
Takade, D.Y., Seo, M.S. and Fukuto, T.R. Alteration O,O-dimethyl
S-alpha-(carboetoxy)benzyl Phosphorodithioate (Phenthoate) in Citrus,
Water and upon Exposure to Air and Sunlight. Arch. Environ. Contam.
Toxicol. 5: 63.
Thompson, W., Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Two-year
Chronic Oral Toxicity Study with Phenthoate (Cidial) in Albino Rats.
(1973) Unpublished report from Industrial Bio-Test Laboratories, Inc.
(No. B-8882), reviewed and validated by Bob West Associates, Inc. (MaY
3, 1973), submitted to the World Health Organization by Montedison.
Toyoshima, S., Sasaki, H., Sato, R., Sato, S., Kato, M., and Nizeki,
H. Acute Toxicity Test Elsan(R) with Rats and Mice by Intraperitoneal
and Subcutaneous. (1971) Unpublished report from the Japan
Experimental Medical Research Institute, Co., Ltd., submitted to the
World Health Organization by Montedison.
Toyoshima, S., Sato, R., Sato, S., Kajima, M. and Nizeki, H.
Three-month Dietary Feeding Study of Elsan (Phenthoate) in Rats and
Mice. (1973) Unpublished report from The Japan Experimental Medical
Research Institute, Co., Ltd., submitted to the World Health
Organization by Montedison.
Trabucchi, E. Cidial Toxicology. (1965) Unpublished report from The
Instituto di Pharmacologia e di Terapia, Milan, submitted to the World
Health Organization by Montedison.
Umetsu, N., Hammond, P.S., Mallipudi, N.M., Toia, R.F. and Fukuto,
T.R. Toxicological Properties of Phosphorothioate and Dithioate
Impurities Present in Technical Organophosphorus Insecticides. Second
Chemical Congress of the North American Continent, Las Vegas, Nevada,
August 25-29, 1980. Abstract #36. Division of Pesticide Chemistry,
American Chemical Society.
Wargo, J.P. Cidial bovine metabolism and residue study. Research
Report Phase 1-6. (Analytical Development Corporation) Montedison
S.p.A. Unpublished Report No.64.
Wargo, J.P., Jr. Cidial Bovine Metabolism and Residue Study. (1975)
Unpublished report from Analytical Development Corp. (No.210),
submitted to the World Health Organization by Montedison.
Wright, G., Smith, S., Kennedy, G.L., Kinoshita, F.K. and Keplinger,
M.L. Three-generation Reproduction Study with Cidial Technical in
Albino Rats. (1975) Unpublished report from Industrial Bio-Test
Laboratories, Inc. (No. 623-03803), reviewed and validated by Bob West
Associates, Inc. (Nov. 15, 1978), submitted to the World Health
Organization by Montedison.
