
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
FENBUCONAZOLE
First draft prepared by
M. Watson
Ricerca Inc., Cleveland, Ohio, USA
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Dermal and ocular irritation and dermal
sensitization
Effects on thyroid function and the liver
Comments
Toxicological evaluation
References
Explanation
Fenbuconazole is the common name for 4-(4-chlorophenyl)-2-phenyl-
2-(1 H-1,2,4-triazol-1-ylmethyl)butyronitrile. It is a triazole
fungicide intended for use as an agricultural and horticultural
fungicide spray for the control of leaf spot, yellow and brown rust,
powdery mildew, and net blotch on wheat and barley and apple scab,
pear scab, and apple powdery mildew on apples and pears. Fenbuconazole
was considered for the first time at the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Groups of four Crl:CD-BR rats were given uniformly
phenyl-ring-labelled 14C-fenbuconazole (radiochemical purity, > 99%)
by gavage in a 0.5% suspension of methyl cellulose at 100 mg/kg bw and
were killed seven days later. Whole-blood samples were taken from the
four males in the first group for liquid scintillation counting 0.25,
1, 3, 6, 24, 48, 72, 96, and 168 h after treatment. In the second
group of four males, radiolabel was measured in urine and faeces
collected 0, 6, 24, 48, 72, 96, and 168 h after treatment, in expired
carbon dioxide, and in selected tissues and organs. Urine and faeces
were collected at the same intervals from four males and four females
but were frozen over liquid nitrogen for analysis of metabolites.
The average total recovery was 67% of the administered dose,
predominantly in faeces (62%) with about 4% in urine; radiolabel in
expired carbon dioxide accounted for only 0.05% of the administered
dose. Most of the excretion had occurred by 48 h. Peak blood and
plasma radiolabel levels were detected at 6 h. A biphasic elimination
pattern was seen, with a rapid alpha-phase (half-life, 7 h) followed
by a slower ß-phase (half-life, about 50 h for plasma and about 187 h
for whole blood). After seven days, < 0.5% of the administered dose
was detected in tissues, the levels in liver being the highest: 2.5
ppm (0.13% of the dose). Of the average total 0.53% of the radiolabel
found in the bodies after seven days, 0.24% occurred in the carcass
(Anderson et al., 1988).
Groups of four male and four female Crl:CD-BR rats were given
uniformly phenyl-ring-labelled 14C-fenbuconazole (radiochemical
purity, 99.4%) by gavage in a 0.5% suspension of methyl cellulose or
intravenously in dimethyl sulfoxide. Groups received an oral or an
intravenous dose of 1 mg/kg bw, an oral dose of 100 mg/kg bw, or an
oral (pulse) dose of 1 mg/kg bw after 14 daily doses of 10 ppm
unlabelled compound in the diet; samples of excreta were taken for
analysis at 0, 24, 48, 72, and 96 h, when the animals were killed for
analyses of radiolabel in various tissues. Two further groups received
oral doses of 1 or 100 mg/kg bw, and blood samples were taken for
analysis at 0.5, 1, 3, 6, 24, 48, 72, and 96 h, when the animals were
killed. The final group received single oral doses of 100 mg/kg bw,
and three animals each were killed at 1, 6, 24, and 48 h for analysis
of radiolabel in tissues.
After intravenous treatment, 88% of the dose was detected in
females and 98% in males; 64-79% was detected in faeces within 24 h,
and the percentage continued to decrease up to 96 h. Urinary and
faecal excretion in these animals over 96 h was 7 and 91% in males and
10 and 77% in females, respectively. Similar profiles and rates of
excretion were seen in animals treated orally at 1 mg/kg bw (both
single and pulse treatments), with 6-10% in urine and 79-84% in
faeces. Total excretion after the 100 mg/kg bw dose was similar to
that at the low doses -- 5-13% in urine and 76-77% in faeces -- but
excretion was slightly slower, proportionately more being seen at
24-48 h and females showed a slight trend to increased urinary
excretion.
At the dose of 1 mg/kg bw, radiolabel was detected only between 1
and 6 h (not at 0.5 or > 24 h), while at 100 mg/kg bw various
levels of radiolabel were detected at 0.5-96 h. On the basis of the
limited data for the lower dose and more information for the higher
dose, the maximum level in blood and plasma was reached at 3-6 h. The
maximum levels detected after the high dose were 8.99-9.99 g
equivalents per g blood and 13.1-13.5 g equivalents per g plasma; the
half-life for elimination of radiolabel at this dose was 10-20 h for
both male and female rats.
Radiolabel was generally undetectable in most tissues after the
low oral dose, apart from the liver and kidneys, in which levels
< 0.1 g equivalents per g were found. Similar levels were seen
after intravenous treatment. At the higher dose, radiolabel was
detected in most tissues at 96 h, the highest levels occurring in the
liver in animals of each sex (3.6-5.0 g equivalents per g), in the
adrenals (0.6-2.1 g equivalents per g) in females, and in the kidneys
(0.7-1.2 g equivalents per g) in males. By 96 h, < 1% of the dose
remained in the carcass. In the animals at 100 mg/kg bw that were
killed serially, the tissue levels increased after 1 h to maximum
levels at 6 h of 75-95 g equivalents per g in the liver, followed by
the adrenals and fat. The levels in all tissues then continued to
decline for 24-48 h, with no evidence of retention (LeVan, 1990).
Groups of four male Crl:CD-BR rats received 14C-fenbuconazole
(specific activity, 20.8 mCi/g and radiopurity, 95.7%) topically after
a preliminary study in which groups of four rats received topical
applications of 0.1% or undiluted material to determine application
and washing techniques. About 100 µl of an aqueous suspension was
applied in plastic skin enclosures to shaven areas (2.5 × 5 cm) on the
back and covered with a nonocclusive dressing. Four animals were given
the 0.1% dilution at 2 g/cm2, equivalent to 0.1 mg/kg bw; the site
was washed with soap after 10 h, and excreta were collected until the
rats were killed seven days later. Concentrations of 0.1 or 1%
fenbuconazole in acetone and carboxymethyl cellulose or 250 mg/ml in a
'2F' formulation were applied to provide doses equal to 0.1, 1.5, or
110 mg/kg bw, respectively. Four animals in each group were killed
after 0.5, 1, 2, 4, 10, and 24 h for analysis of radiolabel in
residual urine in the urinary bladder, blood, plasma, faeces, skin
(after washing), and carcass by liquid scintillation counting; two
further animals received the formulation without radiolabel and were
killed after 24 h for determination of the background levels of
radioactivity.
The average recovery of radiolabel was > 98%. No clinical
symptoms of toxicity were seen during the study. In the preliminary
study, in which animals were killed 4 h after treatment, 80-85% of the
dose (diluted and undiluted material, respectively) was found in the
skin wash, < 0.1% in excreta, 0.15-1.5% in the skin of animals washed
before death, and 1.0-13% in animals washed after death. Skin was
therefore subsequently washed before killing. After 168 h, the mean
recovery was 73%, with 69% in the skin wash, 3.2% in faeces, 0.19% in
urine, and none detected in the carcass. In animals killed after 24 h,
the total recoveries ranged from 92% at 0.1 mg/kg bw to 105% at 111
mg/kg bw. There was significant variation in the total recovery and in
the recovery in skin washes among individuals in both groups treated
at the lowest dose, which was attributed by the author to difficulty
in maintaining a homogeneous suspension. By the end of the study,
77-110% of the dose had been detected in the skin washes and 0.13-12%
was determined to have been absorbed, since it was detected in faeces,
urine, carcass, and skin. The proportion of the dose absorbed from the
acetone, carboxymethyl cellulose and '2F' formulations after 10 h was
4.3% at 0.1 mg/kg bw, 2.1% at 1.5 mg/kg bw, and 0.5% at 110 mg/kg bw;
by 24 h, 12, 5.3, and 1.6% had been absorbed, respectively. These
figures compare well with the absorption calculated by subtracting the
totals in skin washes from the total dose: 13, 6.4, and 2%,
respectively (Cheng, 1990).
In a further study, groups of male and female Crl:CD-BR rats
received pretreatment with 10 ppm fenbuconazole in the diet and then
radiolabelled material at 1 or 100 mg/kg bw. In addition, rats with
bile-duct cannulae received 1 mg/kg bw, and bile and excreta were
collected over three days. The results were generally similar to those
seen in the two previous studies. Overall, 88-91% of the oral dose of
1 mg/kg bw was absorbed systemically. In the rats with bile-duct
cannulae, 79-87% of the dose was detected in bile (DiDonato &
Hazelton, 1993).
(b) Biotransformation
In the studies conducted by Anderson et al. and DiDonato &
Hazleton, frozen samples of urine and faeces were investigated for
metabolites. Conjugates were investigated after acid hydrolysis, and
sulfate conjugates were determined directly. A total of 19 metabolites
were either identified or predicted, including compounds in the
following 10 classes: lactones, iminolactone alpha-alcohol, phenols,
phenol lactones, keto acid, sulfates, triazole, and triazole cleavage
product. The parent compound, 12 metabolites, and conjugates were
identified. The major metabolites resulted from enzymic oxidation of
the first carbon a atom to the chlorophenol ring or to the 3- or
4-position of the phenol ring. Further cyclization of the alcohol with
the adjacent nitrile group, followed by hydrolysis, led to
iminolactones and further hydrolysis to lactones. Iminolactones and
lactones were isolated in both A and B diasteromeric forms. Cleavage
to the triazole was identified as a minor pathway. The metabolic
profiles in males and females were similar, although some quantitative
diiferences were seen. Conjugation of the hydroxyl groups was also
identified, as sulfate or, predominantly, the glucuronide. A proposed
scheme of the metabolism of fenbuconazole in rats is shown in Figure
1.
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of fenbuconazole are
summarized in Table 1. The clinical signs of toxicity after treatment
with fenbuconazole were generally nonspecific. Oral administration of
2 or 5 mg/kg bw produced signs which indicated effects on the central
nervous system (passivity, ataxia, tremors, prostration, and arched
back), on the autonomic or peripheral nervous system (lachrymation and
salivation), on the respiratory system, and on the gastrointestinal
tract. Necropsy of decedents and survivors revealed no remarkable
changes.
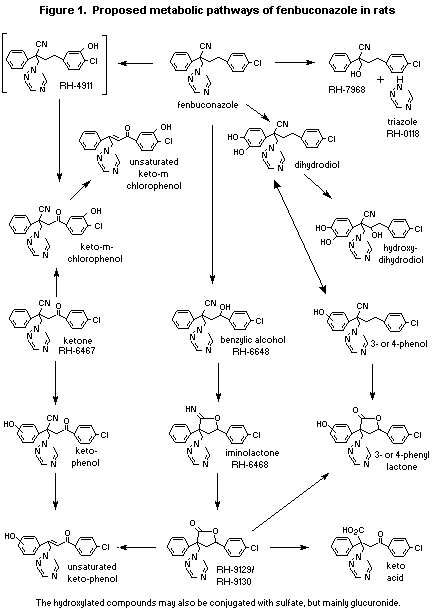 Table 1. Acute toxicity of fenbuconazole
Species, strain Sex Route and vehicle LD50/LC50 Purity Reference
(mg/kg bw (%)
or mg/L air)
Rat, Crl:CD(SD)BR M Oral; aqueous methocel > 2000 96.4 Lampé et al. (1987a)
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 2000 96.7 Krajewski et al. (1988a)
< 5000
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 5000 97.1 Lutz & Parno (1994)
Rat, Crl:CD(SD)BR M Dermal, 0.85% saline > 5000 96.4 Lampé et al. (1987b)
Rat, Crl:CD(SD)BR M,F Dermal, 0.85% saline > 5000 96.7 Krajewski et al. (1988b)
Rat, Crl:CD(SD)BR M,F Inhalation > 2.1 96.7 Duchosal & Thevenaz (1989)
(b) Short-term toxicity
Mice
In a two-week range-finding study, groups of five male and five
female Crl:CD-1(ICR)BR mice were given diets containing fenbuconazole
(purity, 98%) at doses of 0, 100, 250, 500, or 1000 ppm. There were no
deaths, no clinical signs of reaction to treatment, and no
treatment-related effect on weight gain or food intake. The weights of
the livers of animals treated with doses of > 250 ppm were
increased, and there was histopatholgical evidence of hepatotoxicity
at the two highest doses. The NOAEL was 100 ppm, equal to 20 mg/kg bw
per day (Morrison & Hazleton, 1986a).
Groups of Crl:CD-1(ICR)BR mice were given diets containing
fenbuconazole (purity, 96.4%) at 0, 20, 60, 180, or 540 ppm for three
months. The only treatment-related effect observed was evidence of
hepatotoxicity at the two higher doses. At 180 ppm, relatively mild
effects were seen, including slightly increased liver weight in males,
hepatocellular hypertrophy in animals of each sex, single-cell
necrosis in one male, and increased aspartate aminotransferase
activity in males. At 540 ppm, these effects were more pronounced and
were seen in both males and females, in addition to hepatocyte
vacuolation and increased alanine aminotransferase activity. The NOAEL
was 60 ppm, equal to 11-18 mg/kg bw per day (Harris & Hazelton, 1988).
Groups of 10 male and 10 female Crl:CD-1(ICR)BR mice were given
fenbuconazole (purity, 96.7%) in the diet at 0, 540, 1000, 3000, or 10
000 ppm for three months. Treatment-related effects were observed in
all treated animals. At 540 ppm, the effects were similar to those
observed in the preceding study, i.e. increased liver weights,
hepatocellular hypertrophy, hepatocellular vacuolation, and
single-cell or focal necrosis, in animals of each sex. The effects on
the liver were more pronounced at higher doses, and the incidence
and/or severity was greater in males than in females. At the higher
doses, decreased renal weights were seen in males and decreased
body-weight gain and food intake and changes in clinical chemical
parameters in animals of each sex. At 10 000 ppm, 80-100% of the
animals died within three weeks (Wolfe, 1989).
Rats
In a two-week range-finding study, groups of five male and five
female Crl:CD-BR rats were given diets containing fenbuconazole
(purity, 98%) at 0, 100, 300, 1000, or 3000 ppm. There were no deaths
and no clinical signs of reaction to treatment. The rats receiving
1000 or 3000 ppm had lower weight gain and food intake than controls.
The liver weights of animals treated with doses > 300 ppm and of
males at 100 ppm were increased; histopathological evidence of
hepatotoxicity was seen at 1000 and 3000 ppm, and treatment-related
changes in hepatic mixed-function oxidase activity were seen at all
doses. There was no NOAEL (Morrison & Hazleton, 1986b).
Groups of 10 male and 10 female CRL:CD-BR rats were given
fenbuconazole (purity, 96.4%) in the diet at 0, 20, 80, 400, or 1600
ppm for three months. No deaths or clinical symptoms of toxicity were
seen during the study. Both food consumption and body-weight gain were
significantly reduced in animals of each sex at 1600 ppm, although the
magnitude of these effects declined towards the end of the study, with
comparable or increased food consumption from week 9 onwards. No
treatment-related ophthalmoscopic effects were seen, and no effects
were seen in urinalyses. There were no treatment-related effects on
either erythrocyte parameters or differential leukocyte counts. Serum
g-glutamyl transferase activity was increased in females at 1600 ppm,
and decreased triglycerides and increased cholesterol were seen in
animals of each sex at this dose. Gross pathological examination
revealed increased lobulization of the liver in animals of each sex at
1600 ppm and dark-brown discolouration of the liver in four females at
this dose. Adrenal weights were increased in animals of each sex
(relative weight only in females) at 1600 ppm, but no histopatholgical
correlate was seen in either sex. Increased ovarian weights seen at
1600 ppm were of questionable toxicological significant, given that no
histopathological effects occurred. Other increases in relative organ
weights at this dose were considered to be related to decreased
terminal body weight. A dose-related increase in the incidence and
magnitude of the hepatic histopathological effects was seen.
Hepatocellular hypertrophy was seen in one male at 80 ppm and most
rats at doses > 400 ppm; it was mainly centrilobular with
eosinophilic cells, some of which had basophilic nuclei. Mid-zonal
vacuolation was seen in none of the controls, two rats at 80 ppm, four
at 400 ppm, and six at 1600 ppm, while mid-zonal and periportal or
perilobular vacuolation were seen in females at doses > 400 ppm.
Centrilobular necrosis was seen in two males at 400 ppm. Thyroid
follicular epithelial hyperplasia was seen in nine males and two
females at 400 ppm and in eight males and 10 females at 1600 ppm. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of a
slight increase in the incidence of hepatic effects at 80 ppm (Bemacki
& Hazelton, 1988).
Groups of six male and six female Crl:CD-BR rats received
technical-grade fenbuconazole (purity, 97.1%) moistened with saline
topically on clipped areas of the back at doses of 0 or 1000 mg/kg bw
per day or a water-dispersible formulation of fenbuconazole at 62.5,
250, or 1000 mg/kg bw per day. Exposure was maintained with a patch of
absorbent gauze under an occlusive dressing for 6 h per day, five days
per week for 21-22 days; due to logistical constraints, half the
animals of each sex at each dose were killed 24 h apart.
No deaths or treatment-related clinical symptoms of toxicity were
seen, and food consumption and body-weight gain were unaffected by
treatment. No irritation was seen with the active ingredient alone.
None-to-moderate erythema was seen in animals of each sex at the
highest dose of the formulation, from day 19 in males and day 12 in
females; females receiving the formulation control also showed
erythema from day 11. Desiccation, reddened areas, and scabs were seen
in these animals at necropsy. There were no treatment-related effects
on clinical chemical, haematological, or urinary parameters. No gross
or histopathological effects were seen in association with
treatment-related systemic toxicity. The only apparent effect on organ
weight was an increase in relative liver weights at 1000 mg/kg bw per
day of the active ingredient in formulation in females and with the
technical-grade material in males, which is of questionable
toxicological significance and possibly related to the slightly
lowered terminal body weights. Increased incidences of acanthosis,
parakeratosis, eschar, or superficial exudate and necrosis of the
epidermis were seen both with the formulation and formulation blank.
The NOAEL for systemic toxicity was > 1000 mg/kg bw per day. Repeated
dermal application of relatively high doses of the fenbuconazole
co-formulants produced evidence of dermal irritation (Lampé et al.,
1991).
Dogs
In a four-week range-finding study, groups of one or two beagle
dogs of each sex were fed diets containing 0, 200, 400, 800, 1600, or
3200 ppm fenbuconazole (purity, 96.4%). The only effect seen at 800
ppm was increased serum alkaline phosphatase activity. Adverse effects
seen at 1600 ppm included decreased food intake and weight gain,
increased alanine aminotransferase activity, and decreased cholesterol
level. At 3200 ppm, the effects on weight gain and food intake were
incompatible with survival, and treatment was limited to two weeks.
The NOAEL was 400 ppm, equivalent to 10 mg/kg bw per day (O'Hara et
al., 1987).
In a second four-week range-finding study, two male and two
female beagles were given fenbuconazole (purity, 96.7%) in the diet at
levels of 0, 100, 1600, or 3200 ppm. No deaths occurred, and there
were no treatment-related clinical signs of toxicity. Decreased body
weight was seen at 1600 and 3200 ppm, especially during the first two
weeks of treatment, correlated with decreased food consumption at 1600
and especially 3200 ppm. Gross examination showed no treatment-related
effects. The NOAEL in this limited study was 100 ppm, equal to 3.6
mg/kg bw per day, on the basis of effects on food consumption and body
weight at 1600 ppm (Richards, 1991).
Groups of four male and four female beagles were given
fenbuconazole (purity, 96.4%) in the diet at levels of 0, 30, 100,
400, or 1600 ppm for three months. No deaths or clinical symptoms of
toxicity were seen. Body weights were decreased in animals of each sex
at 1600 ppm up to two weeks of the study, correlated with a
significant reduction in food consumption (23-41%). Body-weight gain
was comparable at 2-13 weeks, although the cumulative weight gain at
2-8 and 8-13 weeks was lower at 1600 ppm, especially in females. Food
consumption was generally slightly lower in animals of each sex at
1600 ppm, but the decrease was statistically significant only at 0-2
weeks. By three months, a statistically significant reduction in
erythrocyte count and decreased haematocrit and haemoglobin levels
were seen in females at 1600 ppm, whereas the platelet counts were
increased at both one and three months. Mean haemoglobin and cell
volume were increased in animals of each sex at 1600 ppm. These
effects were only slight, not related to dose, and of questionable
toxicological significance in males at 100 and 400 ppm.
Clinical chemical tests showed significant increases in alkaline
phosphatase activity in animals of each sex at 1600 ppm and in females
at 400 ppm at one and three months. Females at the highest dose also
showed raised serum alanine aminotransferase and g-glutamyltransferase
activity at these intervals. Cholesterol levels were slightly raised
in females at 400 ppm but were lower at 1600 ppm at one and three
months in females at 1600 ppm. The other effects were unremarkable and
not clearly related to treatment. No effects were seen in urinalyses
or ophthalmoscopic investigations. A dose-related trend to increased
liver weight (absolute and relative) was noted at doses > 400 ppm
in animals of each sex at three months. Hepatocytic hypertrophy was
seen, with eosinophilia at 400 and 1600 ppm and minimal-to-slight
multifocal vacuolation in animals of each sex at 1600 ppm. The NOAEL
was 100 ppm, equal to 3.3 mg/kg bw per day, on the basis of hepatic
hypertrophy with clinical chemical effects in animals of each sex at
higher doses (Hazelton & Shade, 1988).
Fenbuconazole (purity, 96.7%) was administered in the diet to
four male and four female beagles at concentrations of 0, 15, 150, or
1200 ppm for one year. No treatment-related effects on survival were
seen, nor were any clinical symptoms of toxicity or ophthalmoscopic
effects. Body-weight gain was reduced consistently in females at 1200
ppm and also at 150 ppm, predominantly during weeks 41-52; no clear
treatment-related effects were seen in males. Food consumption was
unaffected by treatment, except during the first few weeks at the
highest dose. No treatment-related haematological effects were seen at
12 or 26 weeks; however, at 52 weeks two males at 1200 ppm had
creneated erythrocytes, and one also had Burr cells. At 1200 ppm,
alkaline phosphatase activity was consistently increased in animals of
each sex at 13, 26, and 52 weeks; alanine aminotransferase activity
was increased consistently in one female at all three times and in
males at 52 weeks. No treatment-related effects were seen in
urinalyses. Total protein was reduced in females at 1200 ppm at 26
weeks and slightly reduced in males at this dose at 13, 26, and 52
weeks. These males also had reduced albumin levels at 26 and 52 weeks.
Triglyceride levels were consistently increased in animals of each sex
at 1200 ppm at all three times. Cholesterol levels were often slightly
lower at this dose, mainly in males, but with no dose-response
relationship; the only statistically significant result was seen in
females at 26 weeks. The level of total bilirubin was raised in two of
four males at 1200 ppm. The absolute and relative weights of the liver
and adrenals and the relative renal weight were increased in animals
of each sex at 1200 ppm. Histopathological examination showed
eosinophilic hypertrophic hepatocytes (mainly mid-zonal) in all
animals at 1200 ppm but not in other animals. Hepatocyte pigmentation
reported to be consistent with lipofuscin (slight to moderate) was
also seen in these animals. The NOAEL was 150 ppm, equal to 5.2 mg/kg
bw per day, as the effects on body weight in females at 150 ppm were
seen only towards the end of the study, with no evidence of systemic
toxicity. Consistent reductions in body, weight, increased liver
weight with histopathological changes, and possibly associated changes
in clinical chemistry were, however, seen at 1200 ppm (Morgan, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female CD-1 mice were given
fenbuconazole (purity, 96.7%) in the diet for 78 weeks. On the basis
of calculations of the maximum tolerated dose in previous studies,
males were treated at 0, 10, 200, or 650 ppm and females at 0, 10,
650, or 1300 ppm. Ten mice of each sex at each dose were killed at 52
weeks. No treatment-related effects on survival were seen, the rate
being > 70% at the end of the study. The body-weight gain of males
was reduced and was 13% less than that of controls at the end of the
study. No treatment-related effects on food consumption were seen. No
remarkable effects were seen on differential leukocyte counts, cell
morphology, nucleated erythrocyte counts, or myeloid:erythroid ratio.
Gross pathological examination showed an increase incidence of
enlarged livers in both surviving and dead animals of each sex at the
highest dose. The relative and absolute weights of the livers were
increased at 52 weeks in males and females at 650 ppm and in females
at 1300 ppm. Similar effects were seen at the time of the terminal
kill, and increased weights were also seen in males at 200 ppm.
Histopathological examination showed centrilobular to mid-zonal
hepatocyte hypertrophy and vacuolation with some hyperplasia. The
incidence of hepatocellular adenomas and carcinomas combined (8.3%)
was significantly increased in females at 1300 ppm, although the
control value was low (0); the incidence of hepatic tumours was very
slightly greater than the range in historical controls (0-6.1%). The
incidence of carcinomas in males at 200-650 ppm showed a
nonsignificant trend when considered in isolation. The effects of
fenbuconazole on the liver in this study are summarized in Table 2. No
other treatment-related pathological effects were seen. The NOAEL was
10 ppm, equal to 1.3 mg/kg bw per day, on the basis of hepatic effects
at higher doses. Only equivocal evidence of hepatocellular
tumorigenicity was seen, which was statistically significant only in
females at a dose equivalent to 209 mg/kg bw per day (Wolfe, 1991a).
Rats
Groups of 70 male and 70 female Sprague-Dawley rats were fed
diets containing fenbuconazole (purity, 96.7%) at concentrations of 0,
8, 80, or 800 ppm for two years; lower levels (4, 40, or 400 ppm) had
been fed up to week 2, then 6, 60, and 600 ppm up to week 4. Ten rats
of each sex in each group were killed at 52 weeks. No
treatment-related effects on survival were seen and no clinical
symptoms of toxicity or ophthalmoscopic effects. Body-weight gain was
consistently, significantly reduced in females at 800 ppm.
Haematological analyses showed no consistent, dose-related effects in
erythrocyte or leukocyte parameters. Females at 800 ppm had raised
cholesterol levels, but the other effects were transient and not
Table 2. Incidences of histopathological changes in the livers of CD-1 mice fed fenbuconazole for 52 or 78 weeks
Dose (ppm)
Males Females
0 10 200 650 0 10 650 1300
No. examined 60 59 60 60 58 60 57 60
Hepatocellular effect
Hypertrophy 4 4 22 55 1 1 34 49
Vacuolation 2 1 11 31 4 1 20 31
Hyperplasia 3 0 1 7 0 1 0 3
Adenoma 8 1 8 6 0 0 0 4
Carcinoma 1 1 3 5 0 1 0 1
Adenoma and/or carcinoma 9 2 10 10 0 1 0 5
related to dose. The results of urinalyses were similarly
unremarkable. No treatment-related gross pathological effects were
seen; however, at 800 ppm, significantly increased liver weights
(absolute and relative in males and relative in females) were seen at
the time of the interim sacrifice, and both absolute and relative
weights were increased in animals of each sex at the terminal kill.
Thyroid/parathyroid weights were also increased at the terminal kill
in animals at 800 ppm (absolute and relative in males and relative in
females). The only treatment-related histopathological effects seen in
mice that died during the study and at the interim kill were
slight-to-moderate centrilobular to mid-zonal hepatocyte hypertrophy
and vacuolization in animals of each sex; similar effects were seen at
the terminal kill in animals at this dose. The incidence of focal
cystic thyroid hyperplasia was increased in males at 800 ppm (12/70;
1/70 in controls), and the incidence of thyroid follicular adenoma and
carcinoma was also increased in these animals (6/70 and 4/70 in eight
rats; one adenoma in control males). No other treatment-related
effects or tumorigenicity were seen. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day, on the basis of effects on body weight, effects
associated with hepatic hypertrophy (weight change, cholesterol
levels, and histopathological effects), and histopathological effects
in the thyroid at 800 ppm. The NOAEL for thyroid tumorigenicity (seen
only in males) was also 80 ppm (Wolfe, 1990).
Groups of 60 male Sprague-Dawley rats were given fenbuconazole
(purity, 96.7%) in the diet for two years in order to ensure that the
male rats in the previous study had been treated at the maximum
tolerated dose. The dietary concentrations were altered to accommodate
increasing body weights and food consumption at weeks 2 and 4, so that
the animals received 0, (400, 600) then 800 ppm to term or (800, 1200)
then 1600 ppm to term. Ten rats in each group were killed at 52 weeks.
No treatment-related effects on survival were seen, nor were there any
clinical symptoms of toxicity or ophthalmoscopic effects. Body-weight
gain was increasingly reduced during the study in rats at 1600 ppm. No
consistent effects on the results of haematology or clinical chemistry
were seen, and no gross pathological effects were seen. At the interim
kill, the liver weights (absolute and relative) were increased in
animals at 1600 ppm, and the relative liver weights were increased at
800 ppm. At the terminal kill, only the relative liver weights were
higher (with lower body weights) at 1600 ppm. The absolute and
relative thyroid and parathyroid weights were also increased at 800
and 1600 ppm at the interim kill. These effects correlated with
histopathological findings, with slight-to-moderate centrilobular to
mid-zonal vacuolization in 7/10 animals at 800 ppm and all animals at
1600 ppm at the interim kill. In 8/10 animals at 1600 ppm,
minimal-to-slight thyroid follicular-cell hypertrophy was also seen.
Dose-related effects on the degree of hypertrophy of livers were seen,
with occasional vacuolation in animals at doses > 800 ppm at the
terminal kill. These effects were seen in all surviving animals and in
most of those that died during the study. A trend in the severity of
the effects on the liver was seen, from minimal to moderate effects at
800 ppm to moderate to moderately severe hypertrophy at 1600 ppm.
Slight thyroid follicular hypertrophy occurred in 12/27 animals at
1600 ppm. An apparent dose-response relationship in the incidence of
thyroid follicular adenomas (2/60, 5/60, and 9/60) was seen at doses
of 0, 800, and 1600 ppm, respectively; two carcinomas were seen at 0
and 1600 ppm. No NOAEL was identified (Wolfe, 1991b).
(d) Genotoxicity
The results of tests for the genotoxicity of fenbuconazole are
summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, 25 male and 25 female Crl:CD-BR rats
(21 of each sex of the second parental group at 800 ppm) were given
fenbuconazole (purity, 96.7%) in the diet at 0, 8, 80, or 800 ppm. The
parental animals were fed for a minimum of 10 weeks before mating then
throughout mating, gestation, and lactation. Only one litter per
generation was produced. When the youngest litter reached 25 days, one
animal (F1) per sex per litter was randomly selected to serve as the
parents to produce the F2 litter; however, F0 males at 0 and 800 ppm
were subsequently mated with untreated females. Males were killed and
necropsied after the lactation period. Four days post partum, the
litters were culled randomly to eight (four of each sex when
possible), and dams that had not delivered were killed. No deaths or
symptoms of toxicity were seen during treatment, but F0 and F1 dams
at 800 ppm showed increased mortality during delivery, with 13/25 and
5/21 surviving, respectively. Reduced body-weight gain was seen in F0
dams and F1 parental males and females at 800 ppm before mating, and
these reductions were maintained in the females during gestation and
lactation. The effects correlated with reduced food consumption in
these animals. On necropsy, increased liver weights were seen in F0
and F1 parental males and females at 800 ppm. A slight, equivocal
increase in liver weight was seen at 80 ppm only in F1 dams, with no
evidence of associated histopathological changes. Thyroid weights were
increased F0 and F1 parental males; relative adrenal weights were
increased in 15-19 F0 and F1 dams at 800 ppm and in only about seven
at 0-80 ppm. Histopathological examination confirmed the hypertrophic
effects in these three organs at 800 ppm, with centrilobular to
mid-zonal hepatocyte hypertrophy and vacuolation, follicular-cell
hypertrophy, and hypertrophy of the zona glomerulosa, respectively.
Three F0 and four F1 dams at 800 ppm also had centrilobular
hepatocellular necrosis No treatment-related effects on fertility were
seen in males at doses up to 800 ppm; however, the numbers of F0 and
F1 dams at this dose that delivered live young was reduced to 10/25
and 4/21, respectively, and the number of stillborn pups was
increased, so that the total and mean numbers of pups were reduced.
The viability index (survival on day 4) was also reduced, from about
97% in the other groups to 85% at 800 ppm, and pup weight was
Table 2. Results of assays for genotoxicity with fenbuconazole
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium < 7500 mg/plate Reportedly Negativea Chism (1984)
TA98, TA100, 100
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.4 Negativea Sames & Frank (1987)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.7 Negativea Sames & Frank (1988)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 2000 mg/plate 98 Negativea Sames & Ella (1993)
TA98, TA100,
TA1535, TA1537
Gene mutation Chinese hamster < 60 mg/ml 96.7 Negativea Thilagar (1988a)
ovary cells, hprt
locus
Chromosomal Chinese hamster < 30 mg/ml 96.7 Negativea Thilagar (1990)
aberration ovary cells
Unscheduled Rat hepatocytes < 15 mg/ml 96.7 Negative Thilagar (1988b)
DNA synthesis
DNA repair B. subtilis < 2000 mg/disc 97.1 Negative Sarwar & Suzuki
(1994)
M45, H17
In vivo
Chromosomal Rat bone marrow < 1 × 2500 96.7 Negative Thilagar (1988c)
aberration mg/kg bw
a With and without metabolic activation
consistently lower. No treatment-related gross pathological effects
were seen in the offspring. The NOAEL was 80 ppm, equivalent to 4
mg/kg bw per day, on the basis of liver hypertrophy and maternal
toxicity and fetotoxicity at 800 ppm (Solomon & Kulwich, 1990).
(ii) Developmental toxicity
Rats
Groups of 12 mated Sprague-Dawley rats received fenbuconazole
(purity, 96.7%) at doses of 0, 50, 100, or 150 mg/kg bw per day by
gavage on days 6-15 post coitum. Maternal and fetal toxicity
occurred at the highest dose. The incidences of individual visceral
and skeletal alterations at this dose were similar to those in
controls, except that the total number of affected litters was higher
(Solomon & Lutz, 1987).
Fenbuconazole (purity, 96.4%) in an aqueous suspension of 0.5%
methylcellulose was administered at 0, 30, 75, or 150 mg/kg bw per day
to groups of 25 mated Sprague-Dawley rats by gavage on days 6-15
post coitum. The animals were killed on day 20. No treatment-related
mortality was seen, although one animal at 150 mg/kg bw per day died,
reportedly due to an intubation error. Animals at doses > 75 mg/kg
bw per day had alopecia and few faeces, and the mean body weights were
reduced during days 6-8, such that overall body-weight gain was lower
by the end of the study. Food consumption was apparently not recorded.
The numbers of animals that did not become pregnant (1, 3, 2, and 2 at
the four doses, respectively) was acceptable. At 150 mg/kg bw per day,
increased early, late, and total (one animal) resorptions were seen,
with a corresponding reduction in the average number of fetuses per
litter; average fetal weight was also reduced. No treatment-related
fetal malformations were seen. A dose-related trend in the number of
litters in which fetuses had partially or unossified sternebrae was
seen at doses > 75 mg/kg bw per day, with a corresponding increase
in the number of fetuses affected. In addition, an increased incidence
of rudimentary 14th ribs and partially or unossified pubic bones
occurred at 150 mg/kg bw per day, resulting in an overall increase in
the number of fetuses per litter with developmental effects. The NOAEL
for maternal toxicity was 30 mg/kg bw per day on the basis of reduced
body-weight gain and clinical symptoms at doses > 75 mg/kg bw per
day. The NOAEL for fetotoxicity was also 30 mg/kg bw per day on the
basis of an increased incidence of partially or unossified sternebrae
at the next dose. No evidence of teratogenicity was seen (Solomon &
Lutz, 1988).
Rabbits
Fenbuconazole (purity, 96.7%) in an aqueous suspension of
methylcellulose was administered to groups of 21 mated New Zealand
white rabbits at 0, 10, 30, or 60 mg/kg bw per day by gavage on days
7-20 post coitum. The animals were killed on day 29. One animal at
60 mg/kg bw per day died and one was killed in extremis after an
early abortion; a further animal died due to an intubation error.
Reduced food consumption and soft or few faeces were seen at 30-60
mg/kg bw per day. At 60 mg/kg bw per day, only 1/19 pregnant does
produced a viable litter, with 10 total resorptions and a total of six
abortions. At this dose, only eight fetuses were available for
examination. No treatment-related effects were seen in reproductive
parameters at doses of 10-30 mg/kg bw per day. The incidences of
retarded development and malformation were not increased in animals at
these doses. The NOAEL for maternal toxicity was 10 mg/kg bw per day
on the basis of clinical symptoms of toxicity at higher doses. The
NOAEL for fetotoxicity was 30 mg/kg bw per day, as postimplantation
losses and abortion were seen at 60 mg/kg bw per day, with no evidence
of teratogenicity (Soloman & Lutz, 1989).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six New Zealand white rabbits received 0.5 g fenbuconazole
(purity, 96.4%) topically as a paste with 1 ml of saline onto clipped
areas of the back. Exposure was maintained for 4 h under a
semi-occlusive dressing. No erythema or oedema was observed in any of
the animals after 24, 48, or 72 h (Lampé et al., 1987c).
Nine male New Zealand white rabbits received 0.1 g fenbuconazole
(purity, 96.4%) into one conjunctival sac. The eyes of six animals
were left unirrigated, while those of an additional three animals were
rinsed for 1 min about 30 s after administration. No corneal, iridial,
or conjunctival reactions were seen at 24, 48, or 72 h (Lampé et al.,
1987d).
Fenbuconazole (purity, 96.7%) was administered topically to 10
male and 10 female Hartley guinea-pigs, and their reactions were
compared with those of five positive controls of each sex treated with
1-chloro-2,4-dinitrobenzene at 1600 ppm in 80% aqueous ethanol and of
five negative controls of each sex. Fenbuconazole was applied for 6 h
once a week for three weeks as a 25% w/v solution in acetone that had
been shown to cause only slight erythema in a range-finding study.
Exposure to 0.4 ml of the test materials on clipped areas of the flank
was maintained under an occlusive dressing; negative controls were
shaved and received the dressing. A topical challenge exposure was
given two weeks after the third induction, in which negative controls
and fenbuconazole-treated animals received 0.4 ml of a 20% w/v
solution in acetone, and positive controls received 0.4 ml of an
800-ppm solution in acetone. The animals' backs were depilated 19-22 h
later with hair remover, and any erythema scored 24 h after removal of
the challenge patch. One negative control animal had erythema, but
none reacted to the challenge with fenbuconazole. All positive
controls reacted to challenge with 1-chloro-2,4-dinitrobenzene.
Fenbuconazole thus showed no potential for skin sensitization in this
Buehler test (Bonin et al., 1988).
The ability of fenbuconazole to produce delayed contact
hypersensitivity in guinea-pigs was tested with the maximization
technique of Magnusson and Kligman. After initial screening tests, the
animals were induced by intradermal injection of a 10% formulation of
fenbuconazole in acetone and by topical application of a 25%
formulation in the same solvent; they were challenged with a 10%
formulation in acetone. A sensitization rate of 10% was observed after
induction with fenbuconazole, but a 40% sensitization rate was
elicited in the controls receiving acetone alone. After a second
challenge with fenbuconazole as a 10% formulation in diethyl
phthalate, the sensitization rate was 16% with fenbuconazole and 10%
with the vehicle. The positive control substance, 85%
hexylcinnamaldehyde, induced 100% sensitization. The results indicate
that fenbuconazole has weak sensitizing potential (Morris, 1994).
(ii) Effects on thyroid function and the liver
Thyroid function and hepatic clearance of tetraiodothyronine
(thyroxine; T4) were investigated in male Crl:CD-BR rats given
fenbuconazole (purity, 97.1%) at concentrations of 8 or 800 ppm (10
rats) or 0, 1600, or 3200 ppm (20 rats) for four or 13 weeks; a
further two groups were treated with 1600 or 3200 ppm for four weeks
before receiving control diet for nine weeks. Serum thyroid hormone
levels were investigated in 10 rats per group at four and 13 weeks,
and liver microsomal UDP-glucuronsyltransferase and biliary excretion
of 125I-L-T4 were investigated in animals at 0 and 3200 ppm at four
and 13 weeks and at four weeks plus recovery.
No treatment-related deaths were seen. The only clinical symptom
apparently associated with treatment was squinting in animals at 3200
ppm for 13 weeks. The body weights of animals fed 1600 or 3200 ppm for
13 weeks were reduced but not those of rats fed identical levels of
treated diet followed by control diet. Corresponding effects on food
consumption was seen, being reduced in rats fed 1600 or 3200 ppm for
13 weeks and increased in the group allowed to recover. At 1600 and
3200 ppm, there were no treatment-related effects on serum aspartate
or alanine aminotransferase activities. Gross pathological examination
revealed only increased weights of the liver and thyroid. After four
weeks, the relative and absolute thyroid weights were increased by
about 35% at 1600 ppm and the relative weights by about 50% at 3200
ppm. Dose-related increases in relative and absolute liver weights
were seen after four weeks, by about 20 and 30% at both 800 and 1600
ppm and by about 85 and 40% at 3200 ppm, respectively. After 13 weeks
of treatment, the absolute and relative thyroid weights were increased
by 30-31% at 800 ppm, 41-47% at 1600 ppm, and 34-67% at 3200 ppm; the
absolute and relative liver weights were increased by 21% at 800 ppm,
45-51% at 1600 ppm, and 53-92% at 3200 ppm. After four weeks'
treatment at 1600 or 3200 ppm and nine weeks' recovery, the weights of
both the liver and thyroid had recovered and were comparable to those
of controls.
Histopathological examination of the thyroid after four weeks
showed dose-related increases in the incidence and severity of diffuse
follicular hypertrophy and hyperplasia in 4/10 animals at 800 ppm,
9/10 at 1600 ppm, and 10/10 at 3200 ppm, with focal hyperplasia in one
animal each at 1600 and 3200 ppm. Diffuse hypertrophy and hyperplasia
were also seen at dose-related severity after 13 weeks in 9/10 rats at
800 ppm and all rats at 1600 or 3200 ppm, with focal hyperplasia in
one rat at 3200 ppm. These hypertrophic effects were reversible after
nine weeks, the effects being of comparable severity in 6/10 controls,
6/10 at 1600 ppm, and 8/10 at 3200 ppm. Focal hyperplasia was still
present in one animal at 3200 ppm.
After four weeks, the average thyroid-stimulating hormone (TSH)
concentrations were increased in a dose-related trend, by about 79% at
800 ppm, 83% at 1600 ppm, and 105% at 3200 ppm. The L-T4 and reverse
L-triiodothyronine (rT3) levels were reduced to about 50% of the
control levels at 3200 ppm, while the T3 levels were unaffected. After
13 weeks, statistically significantly increased TSH levels were seen
at 3200 ppm (63%) and reduced L-T4 levels at 1600 or 3200 ppm (by 53
and 105%, respectively); however dose-related increases in TSH levels
were seen at 800-3200 ppm (13, 58, and 63%, respectively), and rT3
levels were reduced by about 49% at 3200 ppm. In the animals that were
allowed to recover, the hormone levels were comparable with those in
control animals, with the exception of rT3 in rats at 3200 ppm
(Hazelton et al., 1991).
Biliary excretion of L-T4 was investigated over 4 h in
bile-cannulated rats at 0 or 3200 ppm after four weeks and 13 weeks
and in the group allowed to recover after receiving 3200 ppm after
intravenous injection of 125I-L-T4. In treated rats, the biliary
clearance rate was increased by 165-281% at four weeks and 220-336% at
13 weeks; 14.7-14.9% of the administered radiolabel was excreted by
these animals in comparison to 7.2-7.5% by controls. About 70 and 85%
of the increased biliary excretion correlated to increased excretion
of L-T4-glucuronide at four and 13 weeks, respectively. By 13 weeks,
these parameters were lower in animals allowed to recover and were
comparable to those in control animals. In addition, a corresponding
increase in the activity of UDP-glucuronosyltransferase was seen in
these animals at four weeks, by 54% when expressed per mg microsomal
protein, 187% per g liver, and 337% per liver; at 13 weeks, the
respective activities were increased by 25, 144, and 300%, while the
activity in animals allowed to recover was comparable to that of
controls. Therefore, in rats at relatively high dietary doses, hepatic
metabolism and biliary excretion of T4 were increased, and TSH levels
were correspondingly increased, with hypertrophy and hyperplasia of
this gland. The NOAEL for these effects was 8 ppm, equal to 1 mg/kg bw
per day over 13 weeks (Hazelton et al., 1991).
Six groups of female CD-1 mice received fenbuconazole in the diet
at 0, 20, 60, 180, or 1300 ppm or 1000 ppm phenobarbital for one and
four weeks. Groups of male CD rats received diets containing 1600 ppm
fenbuconazole or 1000 ppm phenobarbital for four weeks. In addition,
three groups of mice and rats received 1300 or 3200 ppm fenbuconazole
or 1000 ppm phenobarbital for four weeks, followed by control diet for
nine weeks.
No treatment-related effects on the liver were seen in mice at
< 60 ppm. At 180 ppm, the effects on the liver included increased
activities of cytochrome P450, cytochrome b5, and 7-pentoxyresorufin
O-deethylase. The increase in cytochrome P450 was due to an increase
in the phenobarbital-inducible form of the enzyme. At 1300 ppm, the
effects were more pronounced and included hepatic enlargement,
hepatocellular hypertrophy, and hepatocellular proliferation, as
determined by bromodeoxyuridine immunohistochemistry. In rats,
fenbuconazole increased liver weights and induced hepatocellular
hypertrophy and cytochrome P450 activity, again due to an increase in
the phenobarbital-inducible form of the enzyme. In both species, the
effects on the liver were similar to phenobarbital-induced toxicity at
1000 ppm and were reversible after cessation of treatment with
fenbuconazole or phenobarbital (Hazelton et al., 1995).
The synthesis of T4 and T3 in the thyroid depends on a dietary
supply of iodine, which is taken up in the thyroid follicular cells,
oxidized by thyroid peroxidase to iodine, and bound at the apical
membrane to tyrosyl residues on thyroglobulin, either as
monoiodotyrosine or diiodotyrosine, which are coupled to produce T3
and T4, which remain part of thyroglobulin. This protein is secreted
into the follicular lumen and taken up by the follicular cells by
pinocytosis, where monoiodotyrosine, diiodotyrosine, T3, and T4 are
released. While T3 and T4 pass into the circulation, mono- and
diiodotyrosine remain in the follicular cells, where they are
deiodinated; the iodine is used to produce more hormone.
The level of circulating T4 is monitored by thyrotrophs in the
anterior pituitary, which are responsible for the production of TSH,
the major thyrotrophic hormone. In the thyrotrophs, T4 is deiodinized
in the outer ring by 5'-deiodinase II, to give T3, which then binds to
nuclear receptors in the cell. A decrease in occupancy of T3 receptors
results in increased synthesis of TSH. Greater control is exercised by
the hypothalamus, by the secretion of throtrophin-releasing hormone,
which also stimulates the release of TSH from thyrotrophs. Thus, any
reduction in the level of circulating T4 will result in an increased
level of TSH. In humans, thyroid-binding globulin is the main plasma
protein that binds thyroid hormones, with a greater affinity for T4
than T3. In rats, thyroid hormones are bound mainly and with lower
affinity to albumin, and only low levels of a protein that has 70%
homology with human thyroid-binding globulin are present (Imamura et
al., 1991).
Circulating T4 is taken up by various organs, but most is
metabolized in the liver by 5'-deiodinase I. This enzyme can catalyse
deiodination of both the outer ring to give T3, the more active
thyroid hormone, and the inner ring to give rT3, which has no known
function. Deiodination is the main route of catabolism in humans,
finally resulting in thyronine production. Thyroid hormones are also
sulfated and glucuronidated; the latter pathway is of little
importance in humans but the major route in rats.
Broadly speaking, five categories of xenobiotic influence thyroid
hormone homeostasis:
- directly acting substances that inhibit either iodine uptake
by the thyroid or thyroid peroxidase activity; e.g.
aminosalicylic acid, propylthiourea, and resorcinol;
- substances that stimulate T4 clearance, predominantly
through effects on the liver, by inducing microsomal enzymes
(resulting in increased biliary clearance; e.g.
phenobarbital) or by affecting hepatic transport of thyroid
hormones;
- substances that influence deiodination, either by
stimulating 5'-deiodinase I (e.g. phenobarbital) or by
inhibiting 5'-deiodinase II (e.g. iopanoic acid);
- substances that affect plasma binding of thyroid hormones
(e.g. salicylates); and
- substances that interact with receptors of neurotransmitters
such as dopamine that have been implicated in the control of
TSH output by thyrotrophin-releasing hormone (e.g.
clomiphene).
Thus, studies of the effects of fenbuconazole on the liver in
male mice and rats show that the microsomal enzymes cytochrome b5 and
7-pentoxyresorufin- O-deethylase (a marker of the cytochrome P450 2B
subfamily) are induced in both species (Hazelton et al., 1995). More
significantly, UDP-glucuronosyltransferase activity and biliary
clearance of 125I-thyroxine metabolites, including T4-glucuronide,
were increased after dietary administration, although the only dose
tested was 3200 ppm (Hazelton et al., 1991). In studies by dietary
administration to rats for up to 13 weeks, the plasma levels of TSH
were increased at doses > 800 ppm, while those of T4 and rT3 were
decreased and those of T3 unaltered; these changes correlated to
thyroid follicular hypertrophy and hyperplasia and increased thyroid
weight (Hazelton et al., 1991). None of these effects was seen at 8
ppm (Wolfe, 1990). A dietary concentration of 800 ppm was the lowest
at which an increased incidence of adenomas and carcinomas of thyroid
follicular cells was seen in rats treated for up to two years (Wolfe,
1991b). Thus, the basis for the possible carcinogenicity of
fenbuconazole is chronic stimulation of the thyroid by TSH with
reduced plasma T4 concentrations. The reason for the reduced T4 levels
at all relevant doses of fenbuconazole has not been found, but the
observation of increased biliary clearance of thyroid hormone
metabolites at a high dose (the only one tested) indicates another
component of the mechanism of carcinogenesis.
Comments
Fenbuconazole is rapidly absorbed and eliminated, mainly in the
faeces through significant biliary excretion; there was no evidence of
significant retention in tissues. The compound was also extensively
metabolized by phase-I oxidation or hydroxylation at a number of sites
in the molecule, followed by phase-II sulfate and glucuronide
conjugation (predominantly glucuronidation). Dermal absorption of
fenbuconazole (technical material and a formulation) constituted 2-13%
of an administered dose over 24 h, the absorption over 10 h being
< 5%.
Fenbuconazole was of low acute toxicity when administered orally
(LD50 > 2000 mg/kg bw), dermally (LD50 > 5000 mg/kg bw), or by
inhalation (LC50 > 2.1 mg/L air). It was not irritating to the skin
or eyes and was not a sensitizer in a Buehler test, but was a weak
sensitizer in a maximization test. WHO has not yet classified
fenbuconazole for acute toxicity.
After dietary administration, hepatomegaly with associated
effects on clinical chemistry, such as changes in cholesterol and
triglyceride levels and increases in the serum activity of hepatic
enzymes, were seen in mice, rats, and dogs. In a 13-week study of
toxicity in mice with dietary levels of 0, 20, 60, 180, or 540 ppm the
NOAEL was 60 ppm (equal to 11 mg/kg bw per day) on the basis of
hepatic effects at higher doses. In a three-month study of toxicity in
rats with dietary levels of 0, 20, 80, 400, or 1600 ppm, the NOAEL was
20 ppm (equal to 1.3 mg/kg bw per day) on the basis of hepatic effects
and hypertrophy of thyroid gland follicular cells at higher doses. In
a 13-week study of toxicity in dogs with dietary levels of 0, 30, 100,
400, or 1600 ppm, the NOAEL was 100 ppm (equal to 3.3 mg/kg bw per
day). In a one-year study in dogs with dietary levels of 0, 15, 150,
or 1200 ppm, the NOAEL was 150 ppm (equal to 5.2 mg/kg bw per day).
The NOAELs in the studies in dogs were based on decreased body-weight
gain and increased incidences of hepatic hypertrophy with associated
effects on clinical chemistry at higher doses.
In a 78-week study of toxicity and carcinogenicity in mice, with
dietary levels of 0, 10, 200, or 650 ppm in males and 0, 10, 650, or
1300 ppm in females, there was clear evidence of treatment-related
hepatomegaly, with dose-related hepatocytic hypertrophy and
vacuolation, and limited evidence of treatment-related hyperplasia and
tumorigenicity in the liver at the highest dose. The NOAEL was 10 ppm
(equal to 1.3 mg/kg bw per day). In a two-year study in rats with
dietary levels of 0, 8, 80, or 800 ppm, the predominant effects were
hepatocytic hypertrophy, thyroid follicular-cell hypertrophy, and an
increase in thyroid follicular-cell adenomas; in addition, thyroid
carcinomas were seen at the high dose. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day.
The etiology of the hepatic and thyroidal effects in rats was
further investigated in a 4-13-week study which illustrated the
biological feedback mechanism in rats: hepatomegaly leading to
increased metabolism and excretion of thyroxine, increased levels of
thyroid stimulating hormone, and thyroid hypertrophy/hyperplasia. The
effects seen after four weeks in this study were reversible. In
studies designed to investigate the hepatotoxicity of fenbuconazole,
hepatic effects were seen in rats and mice that were similar to those
induced by phenobarbital. Increased cytochrome P450 activity (CYP2B
form) was observed, with hepatocellular hypertrophy and proliferation.
The NOAEL in mice after treatment for 13 weeks was 60 ppm (equal to 14
mg/kg bw per day).
Fenbuconazole was adequately tested for genotoxicity in vitro
and in vivo. The Meeting concluded that it is not genotoxic.
Fenbuconazole was not teratogenic in either rats (at doses of 0,
30, 75, or 150 mg/kg bw per day) or rabbits (at doses of 0, 10, 30, or
60 mg/kg bw per day), but fetotoxicity was seen in both species, with
an NOAEL of 30 mg/kg bw per day. The NOAELs for maternal toxicity were
30 mg/kg bw per day in rats and 10 mg/kg bw per day in rabbits. No
effects on reproductive parameters were seen in a multigeneration
study in rats at dietary levels of 0, 8, 80, or 800 ppm, but
fetotoxicity was again seen at high doses, with maternal toxicity. The
NOAEL was 80 ppm, equal to 5.8 mg/kg bw per day.
An ADI of 0-0.03 mg/kg bw was allocated, on the basis of the
NOAEL of 3 mg/kg bw per day in the two-year study in rats and a safety
factor of 100. The Meeting noted that the NOAEL in the 13-week study
in rats and in the 78-week study in mice was 1.3 mg/kg bw per day, but
it concluded that this figure should not be used to derive the ADI.
The NOAEL from the 13-week study in rats was not considered to be
relevant in the light of the results of the larger, two-year study.
The Meeting concluded that the overall NOAEL in mice was 14 mg/kg bw
per day. This figure was taken from the 13-week study, which included
detailed investigations of hepatotoxicity. Hepatotoxicity was the
critical effect in the long-term study in mice, and the NOAEL in the
13-week study was lower than the lowest dose that was hepatotoxic in
the long-term study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 60 ppm, equal to 14 mg/kg bw per day (13-week study of
hepatotoxicity)
10 ppm, equal to 1.3 mg/kg bw per day (78-week study of
toxicity)
Rat: 20 ppm, equal to 1.3 mg/kg bw per day (13-week study of
toxicity)
80 ppm, equal to 3.0 mg/kg bw per day (two-year study
of toxicity)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenbuconazole
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral toxicity, rat LD50 > 2000 mg/kg bw
(1-7 days) Dermal toxicity, rat LD50 > 5000 mg/kg bw
Inhalation toxicity, rat LC50 > 2.1 mg/L
Dermal irritation, rabbit Not irritating
Ocular irritation, rabbit Not irritating
Dermal sensitization, guinea pig Not sensitizing in Buehler test, weakly
sensitizing in maximization test
Medium-term Repeated oral, 1-year, toxicity, dog NOAEL = 5.2 mg/kg bw per day: hepatic effects
(1-26 weeks) Repeated dermal, 4 weeks, toxicity, rat NOAEL = 1000 mg/kg bw per day (highest dose
tested)
Repeated oral, reproductive toxicity, rat NOAEL = 5.8 mg/kg bw per day: maternal and
fetal toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day: maternal
toxicity
Long term Repeated oral, 2 years, toxicity and NOAEL = 3 mg/kg bw per day: hepatic and
(> 1 year) carcinogenicity, rat thyroid effects
80 ppm, equal to 5.8 mg/kg bw per day (two-generation
study of reproductive toxicity)
30 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 150 ppm, equal to 5.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Anderson, D.M., DiDonato, L.J. & Longacre, S.L. (1988) 14C-RH-7592:
Range-finding kinetic and metabolite identification study in rats.
Unpublished study from Rohm & Haas Co., Report No. 87R-059 (European
Region reference No. 16.1).Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Bemacki, H.J. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-103 (European Region reference No. 8.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Bonin, R., Shade, W.D. & Hazelton, G.A. (1988) RH-7592 technical:
Delayed contact hypersensitivity study in guinea pigs. Unpublished
study from Rohm & Haas Co., Report No. 88R-027 (European Region
reference No. 4.3). Submitted to WHO by Rohm & Haas Co., Philadelphia,
PA, USA.
Cheng, T. (1990) RH-7592: Dermal absorption in male rats (preliminary
and definitive phases). Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 89RC-291 (European Region
reference No. 19.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Chism, E.M. (1984) RH-7592: Microbial mutagen test. Unpublished study
from Rohm & Haas Co., Report No. 84R-069 (European Region reference
No. 20.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
DiDonato, L.J. & Hazelton, G.A. (1993) 14-C-RH-7592: Disposition and
elimination study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 92R-060 (European Region reference No. 43.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Duchosal, F. & Thevenaz, P. (1989) 4-Hour, acute inhalation toxicity
study with RH-7592 technical in rats. Unpublished study from Research
and Consulting Co. AG. Rohm & Haas Co. report No. 89RC-023 (European
Region reference No. 16.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Harris, J.C. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in mice. Unpublished study from Rohm & Haas Co., Report
No. 87R-090 (European Region reference No. 5.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Shade, W.D. (1988) RH-7592: Three-month dietary
toxicity study in dogs. Unpublished study from Rohm & Haas Co., Report
No. 87R-127 (European Region reference No. 6.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J., Quinn, D.L., Shade, W.D. & Frantz,
J.D. (1991) RH-7592: Thyroid function and hepatic clearance of
thyroxine in male rats. Unpublished study from Rohm & Haas Co., Report
No. 90R-071 (European Region reference No. 23.6). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J. & Lomax, L.G. (1995) RH-7592: Cell
proliferation and enzyme induction in the liver of female mice.
Unpublished study from Rohm & Haas Co., Report No. 95R-035. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Imamura, S., Mori, Y., Yamamori, I., Miura, Y., Oiso, Y., Seo, H.,
Matsui, N. & Refetoff, S. (1991) Molecular cloning and primary
structure of rat thyroxine-binding globulin. Biochemistry, 30,
5406-5411.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-7592: Acute
oral toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-001 (European Region reference No.
1.20). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-7592: Acute
dermal toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-002 (European Region reference No.
1.17). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) RH-7592: Acute
oral toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-098 (European Region reference No. 1.12). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) RH-7592: Acute
dermal toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-099 (European Region reference No. 1.11). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987c) RH-7592: Rabbit
skin irritation study. Unpublished study from Rohm & Haas Co., Report
No. 87R-100/87R-100A (European Region reference No. 1.9). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987d) RH-7592: Eye
irritation study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 87R-101/87R-101A (European Region reference No. 1.10).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Kulwich, B.A. & Baldwin, R.C. (1991) RH-7592 2F and
technical fungicides four-week dermal toxicity study in rats.
Unpublished study from Rohm & Haas Co., Report No. 90R-084/90R-084A
(European Region references No. 25.2 and 25.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
LeVan, L.W. (1990) 14C-RH-7592: Pharmacokinetic study in rats.
Unpublished study from Rohm & Haas Co., Report No. 88RC-071 (European
Region reference No. 14.9). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1994) RH-7592 technical: Acute oral toxicity
study in male and female rats. Unpublished study from Rohm & Haas Co.,
Report No. 94R-107 (European Region reference No. 47.4). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morgan, C. (1990) RH-7592: 52-Week oral (dietary administration)
toxicity study in the beagle. Unpublished study from Hazleton UK,
report No. 6464-616/5. Rohm & Haas Co. report No. 88RC-115 (European
Region reference No. 19.3). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Morris, T.D. (1994) RH-7592 technical: Delayed contact
hypersensitivity study in guinea pigs (maximization technique).
Unpublished study from Hill Top Biolabs, Inc. Rohm & Haas Co. report
No. 94RC-063. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Morrison, R.D. & Hazelton, G.A. (1986a) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in mice. Unpublished study from Rohm
& Haas Co., Report No. 86R-131 (European Region reference No. 5.5).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hazelton, G.A. (1986b) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in rats. Unpublished study from Rohm
& Haas Co., Report No. 86R-130 (European Region reference No. 8.1).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
O'Hara, G.P., Frantz, J.D. & Poorman, K.B. (1987) RH-7592: 4-Week
range finding study in male and female dogs. Unpublished study from
Rohm & Haas Co., Report No. 87R-075 (see Rohm & Haas Co. report No.
87R-127, Appendix 13). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1991) RH-7592: 4-Week (dietary administration)
toxicity study in the beagle. Unpublished study from Hazelton, United
Kingdom. Rohm & Haas Co. report No. 88RC-097 (European Region
reference No. 30.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-7592: Salmonella typhimurium gene
mutation assay (Ames). Unpublished study from Rohm & Haas Co., Report
No. 92R-195 (European Region reference No. 42.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1987) RH-7592: Microbial mutagenicity
assay. Unpublished study from Rohm & Haas Co., Report No. 87R-044
(European Region reference No. 1.24). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1988) RH-7592: Salmonella typhimurium
gene mutation assay. Unpublished study from Rohm & Haas Co., Report
No. 88R-009 (European Region reference No. 1.23). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sarwar, G. & Suzuki, K. (1994) DNA repair test of H-7592 technical.
Unpublished study from Nippon Experimental Medical Research Institute
Co. Rohm & Haas Co. report No. 94RC-114 (European Region reference No.
48.7). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Kulwich, B.A. (1990) RH-7592: Two-generation
reproduction study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 88R-241 (European Region reference No. 15.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1987) RH-7592: Oral (gavage) developmental
toxicity screen in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-014 (see Rohm & Haas Co. report No. 87R-065, Appendix
L, p. 197). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Solomon, H.M. & Lutz, M.F. (1988) RH-7592: Oral (gavage) developmental
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-014 (European Region reference No. 1.22). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1989) RH-7592: Oral (gavage) developmental
toxicity study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 88R-195 (European Region reference No. 12.1). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988a) RH-7592 technical: Test for chemical induction of
gene mutation at the HGRPT locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Unpublished study
from Sitek Research Laboratories. Rohm & Haas Co. report No. 88RC-062
(European Region reference No. 7.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Thilagar, A. (1988b) RH-7592 technical: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished study from Sitek Research Laboratories.
Rohm & Haas Co. report No. 88RC-061 (European Region reference No.
7.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988c) RH-7592 technical: Acute test for chemical
induction of chromosome aberration in rat bone marrow cells in vivo.
Unpublished study from Sitek Research Laboratories. Rohm & Haas Co.
report No. 88RC-063 (European Region reference No. 7.3). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990) Test for chemical induction of chromosome
aberration using monolayer cultures of Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished study from
Sitek Research Laboratories. Rohm & Haas Co. report No. 89RC-090
(European Region reference No. 14.8). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1989) 13-Week dietary toxicity range-finding (RF) study
in mice with RH-7592. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-112 (European Region
reference No. 13.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1990) RH-7592 technical: 24-Month dietary chronic
toxicity-oncogenicity study in rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-098
(European Region reference No. 18.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1991a) RH-7592 technical: 78-Week dietary oncogenicity
toxicity study in mice. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-107 (European Region
reference No. 29.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1991b) RH-7592 technical: 104-Week chronic oncogenicity
toxicity study in male rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-116
(European Region reference No. 32.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Table 1. Acute toxicity of fenbuconazole
Species, strain Sex Route and vehicle LD50/LC50 Purity Reference
(mg/kg bw (%)
or mg/L air)
Rat, Crl:CD(SD)BR M Oral; aqueous methocel > 2000 96.4 Lampé et al. (1987a)
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 2000 96.7 Krajewski et al. (1988a)
< 5000
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 5000 97.1 Lutz & Parno (1994)
Rat, Crl:CD(SD)BR M Dermal, 0.85% saline > 5000 96.4 Lampé et al. (1987b)
Rat, Crl:CD(SD)BR M,F Dermal, 0.85% saline > 5000 96.7 Krajewski et al. (1988b)
Rat, Crl:CD(SD)BR M,F Inhalation > 2.1 96.7 Duchosal & Thevenaz (1989)
(b) Short-term toxicity
Mice
In a two-week range-finding study, groups of five male and five
female Crl:CD-1(ICR)BR mice were given diets containing fenbuconazole
(purity, 98%) at doses of 0, 100, 250, 500, or 1000 ppm. There were no
deaths, no clinical signs of reaction to treatment, and no
treatment-related effect on weight gain or food intake. The weights of
the livers of animals treated with doses of > 250 ppm were
increased, and there was histopatholgical evidence of hepatotoxicity
at the two highest doses. The NOAEL was 100 ppm, equal to 20 mg/kg bw
per day (Morrison & Hazleton, 1986a).
Groups of Crl:CD-1(ICR)BR mice were given diets containing
fenbuconazole (purity, 96.4%) at 0, 20, 60, 180, or 540 ppm for three
months. The only treatment-related effect observed was evidence of
hepatotoxicity at the two higher doses. At 180 ppm, relatively mild
effects were seen, including slightly increased liver weight in males,
hepatocellular hypertrophy in animals of each sex, single-cell
necrosis in one male, and increased aspartate aminotransferase
activity in males. At 540 ppm, these effects were more pronounced and
were seen in both males and females, in addition to hepatocyte
vacuolation and increased alanine aminotransferase activity. The NOAEL
was 60 ppm, equal to 11-18 mg/kg bw per day (Harris & Hazelton, 1988).
Groups of 10 male and 10 female Crl:CD-1(ICR)BR mice were given
fenbuconazole (purity, 96.7%) in the diet at 0, 540, 1000, 3000, or 10
000 ppm for three months. Treatment-related effects were observed in
all treated animals. At 540 ppm, the effects were similar to those
observed in the preceding study, i.e. increased liver weights,
hepatocellular hypertrophy, hepatocellular vacuolation, and
single-cell or focal necrosis, in animals of each sex. The effects on
the liver were more pronounced at higher doses, and the incidence
and/or severity was greater in males than in females. At the higher
doses, decreased renal weights were seen in males and decreased
body-weight gain and food intake and changes in clinical chemical
parameters in animals of each sex. At 10 000 ppm, 80-100% of the
animals died within three weeks (Wolfe, 1989).
Rats
In a two-week range-finding study, groups of five male and five
female Crl:CD-BR rats were given diets containing fenbuconazole
(purity, 98%) at 0, 100, 300, 1000, or 3000 ppm. There were no deaths
and no clinical signs of reaction to treatment. The rats receiving
1000 or 3000 ppm had lower weight gain and food intake than controls.
The liver weights of animals treated with doses > 300 ppm and of
males at 100 ppm were increased; histopathological evidence of
hepatotoxicity was seen at 1000 and 3000 ppm, and treatment-related
changes in hepatic mixed-function oxidase activity were seen at all
doses. There was no NOAEL (Morrison & Hazleton, 1986b).
Groups of 10 male and 10 female CRL:CD-BR rats were given
fenbuconazole (purity, 96.4%) in the diet at 0, 20, 80, 400, or 1600
ppm for three months. No deaths or clinical symptoms of toxicity were
seen during the study. Both food consumption and body-weight gain were
significantly reduced in animals of each sex at 1600 ppm, although the
magnitude of these effects declined towards the end of the study, with
comparable or increased food consumption from week 9 onwards. No
treatment-related ophthalmoscopic effects were seen, and no effects
were seen in urinalyses. There were no treatment-related effects on
either erythrocyte parameters or differential leukocyte counts. Serum
g-glutamyl transferase activity was increased in females at 1600 ppm,
and decreased triglycerides and increased cholesterol were seen in
animals of each sex at this dose. Gross pathological examination
revealed increased lobulization of the liver in animals of each sex at
1600 ppm and dark-brown discolouration of the liver in four females at
this dose. Adrenal weights were increased in animals of each sex
(relative weight only in females) at 1600 ppm, but no histopatholgical
correlate was seen in either sex. Increased ovarian weights seen at
1600 ppm were of questionable toxicological significant, given that no
histopathological effects occurred. Other increases in relative organ
weights at this dose were considered to be related to decreased
terminal body weight. A dose-related increase in the incidence and
magnitude of the hepatic histopathological effects was seen.
Hepatocellular hypertrophy was seen in one male at 80 ppm and most
rats at doses > 400 ppm; it was mainly centrilobular with
eosinophilic cells, some of which had basophilic nuclei. Mid-zonal
vacuolation was seen in none of the controls, two rats at 80 ppm, four
at 400 ppm, and six at 1600 ppm, while mid-zonal and periportal or
perilobular vacuolation were seen in females at doses > 400 ppm.
Centrilobular necrosis was seen in two males at 400 ppm. Thyroid
follicular epithelial hyperplasia was seen in nine males and two
females at 400 ppm and in eight males and 10 females at 1600 ppm. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of a
slight increase in the incidence of hepatic effects at 80 ppm (Bemacki
& Hazelton, 1988).
Groups of six male and six female Crl:CD-BR rats received
technical-grade fenbuconazole (purity, 97.1%) moistened with saline
topically on clipped areas of the back at doses of 0 or 1000 mg/kg bw
per day or a water-dispersible formulation of fenbuconazole at 62.5,
250, or 1000 mg/kg bw per day. Exposure was maintained with a patch of
absorbent gauze under an occlusive dressing for 6 h per day, five days
per week for 21-22 days; due to logistical constraints, half the
animals of each sex at each dose were killed 24 h apart.
No deaths or treatment-related clinical symptoms of toxicity were
seen, and food consumption and body-weight gain were unaffected by
treatment. No irritation was seen with the active ingredient alone.
None-to-moderate erythema was seen in animals of each sex at the
highest dose of the formulation, from day 19 in males and day 12 in
females; females receiving the formulation control also showed
erythema from day 11. Desiccation, reddened areas, and scabs were seen
in these animals at necropsy. There were no treatment-related effects
on clinical chemical, haematological, or urinary parameters. No gross
or histopathological effects were seen in association with
treatment-related systemic toxicity. The only apparent effect on organ
weight was an increase in relative liver weights at 1000 mg/kg bw per
day of the active ingredient in formulation in females and with the
technical-grade material in males, which is of questionable
toxicological significance and possibly related to the slightly
lowered terminal body weights. Increased incidences of acanthosis,
parakeratosis, eschar, or superficial exudate and necrosis of the
epidermis were seen both with the formulation and formulation blank.
The NOAEL for systemic toxicity was > 1000 mg/kg bw per day. Repeated
dermal application of relatively high doses of the fenbuconazole
co-formulants produced evidence of dermal irritation (Lampé et al.,
1991).
Dogs
In a four-week range-finding study, groups of one or two beagle
dogs of each sex were fed diets containing 0, 200, 400, 800, 1600, or
3200 ppm fenbuconazole (purity, 96.4%). The only effect seen at 800
ppm was increased serum alkaline phosphatase activity. Adverse effects
seen at 1600 ppm included decreased food intake and weight gain,
increased alanine aminotransferase activity, and decreased cholesterol
level. At 3200 ppm, the effects on weight gain and food intake were
incompatible with survival, and treatment was limited to two weeks.
The NOAEL was 400 ppm, equivalent to 10 mg/kg bw per day (O'Hara et
al., 1987).
In a second four-week range-finding study, two male and two
female beagles were given fenbuconazole (purity, 96.7%) in the diet at
levels of 0, 100, 1600, or 3200 ppm. No deaths occurred, and there
were no treatment-related clinical signs of toxicity. Decreased body
weight was seen at 1600 and 3200 ppm, especially during the first two
weeks of treatment, correlated with decreased food consumption at 1600
and especially 3200 ppm. Gross examination showed no treatment-related
effects. The NOAEL in this limited study was 100 ppm, equal to 3.6
mg/kg bw per day, on the basis of effects on food consumption and body
weight at 1600 ppm (Richards, 1991).
Groups of four male and four female beagles were given
fenbuconazole (purity, 96.4%) in the diet at levels of 0, 30, 100,
400, or 1600 ppm for three months. No deaths or clinical symptoms of
toxicity were seen. Body weights were decreased in animals of each sex
at 1600 ppm up to two weeks of the study, correlated with a
significant reduction in food consumption (23-41%). Body-weight gain
was comparable at 2-13 weeks, although the cumulative weight gain at
2-8 and 8-13 weeks was lower at 1600 ppm, especially in females. Food
consumption was generally slightly lower in animals of each sex at
1600 ppm, but the decrease was statistically significant only at 0-2
weeks. By three months, a statistically significant reduction in
erythrocyte count and decreased haematocrit and haemoglobin levels
were seen in females at 1600 ppm, whereas the platelet counts were
increased at both one and three months. Mean haemoglobin and cell
volume were increased in animals of each sex at 1600 ppm. These
effects were only slight, not related to dose, and of questionable
toxicological significance in males at 100 and 400 ppm.
Clinical chemical tests showed significant increases in alkaline
phosphatase activity in animals of each sex at 1600 ppm and in females
at 400 ppm at one and three months. Females at the highest dose also
showed raised serum alanine aminotransferase and g-glutamyltransferase
activity at these intervals. Cholesterol levels were slightly raised
in females at 400 ppm but were lower at 1600 ppm at one and three
months in females at 1600 ppm. The other effects were unremarkable and
not clearly related to treatment. No effects were seen in urinalyses
or ophthalmoscopic investigations. A dose-related trend to increased
liver weight (absolute and relative) was noted at doses > 400 ppm
in animals of each sex at three months. Hepatocytic hypertrophy was
seen, with eosinophilia at 400 and 1600 ppm and minimal-to-slight
multifocal vacuolation in animals of each sex at 1600 ppm. The NOAEL
was 100 ppm, equal to 3.3 mg/kg bw per day, on the basis of hepatic
hypertrophy with clinical chemical effects in animals of each sex at
higher doses (Hazelton & Shade, 1988).
Fenbuconazole (purity, 96.7%) was administered in the diet to
four male and four female beagles at concentrations of 0, 15, 150, or
1200 ppm for one year. No treatment-related effects on survival were
seen, nor were any clinical symptoms of toxicity or ophthalmoscopic
effects. Body-weight gain was reduced consistently in females at 1200
ppm and also at 150 ppm, predominantly during weeks 41-52; no clear
treatment-related effects were seen in males. Food consumption was
unaffected by treatment, except during the first few weeks at the
highest dose. No treatment-related haematological effects were seen at
12 or 26 weeks; however, at 52 weeks two males at 1200 ppm had
creneated erythrocytes, and one also had Burr cells. At 1200 ppm,
alkaline phosphatase activity was consistently increased in animals of
each sex at 13, 26, and 52 weeks; alanine aminotransferase activity
was increased consistently in one female at all three times and in
males at 52 weeks. No treatment-related effects were seen in
urinalyses. Total protein was reduced in females at 1200 ppm at 26
weeks and slightly reduced in males at this dose at 13, 26, and 52
weeks. These males also had reduced albumin levels at 26 and 52 weeks.
Triglyceride levels were consistently increased in animals of each sex
at 1200 ppm at all three times. Cholesterol levels were often slightly
lower at this dose, mainly in males, but with no dose-response
relationship; the only statistically significant result was seen in
females at 26 weeks. The level of total bilirubin was raised in two of
four males at 1200 ppm. The absolute and relative weights of the liver
and adrenals and the relative renal weight were increased in animals
of each sex at 1200 ppm. Histopathological examination showed
eosinophilic hypertrophic hepatocytes (mainly mid-zonal) in all
animals at 1200 ppm but not in other animals. Hepatocyte pigmentation
reported to be consistent with lipofuscin (slight to moderate) was
also seen in these animals. The NOAEL was 150 ppm, equal to 5.2 mg/kg
bw per day, as the effects on body weight in females at 150 ppm were
seen only towards the end of the study, with no evidence of systemic
toxicity. Consistent reductions in body, weight, increased liver
weight with histopathological changes, and possibly associated changes
in clinical chemistry were, however, seen at 1200 ppm (Morgan, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female CD-1 mice were given
fenbuconazole (purity, 96.7%) in the diet for 78 weeks. On the basis
of calculations of the maximum tolerated dose in previous studies,
males were treated at 0, 10, 200, or 650 ppm and females at 0, 10,
650, or 1300 ppm. Ten mice of each sex at each dose were killed at 52
weeks. No treatment-related effects on survival were seen, the rate
being > 70% at the end of the study. The body-weight gain of males
was reduced and was 13% less than that of controls at the end of the
study. No treatment-related effects on food consumption were seen. No
remarkable effects were seen on differential leukocyte counts, cell
morphology, nucleated erythrocyte counts, or myeloid:erythroid ratio.
Gross pathological examination showed an increase incidence of
enlarged livers in both surviving and dead animals of each sex at the
highest dose. The relative and absolute weights of the livers were
increased at 52 weeks in males and females at 650 ppm and in females
at 1300 ppm. Similar effects were seen at the time of the terminal
kill, and increased weights were also seen in males at 200 ppm.
Histopathological examination showed centrilobular to mid-zonal
hepatocyte hypertrophy and vacuolation with some hyperplasia. The
incidence of hepatocellular adenomas and carcinomas combined (8.3%)
was significantly increased in females at 1300 ppm, although the
control value was low (0); the incidence of hepatic tumours was very
slightly greater than the range in historical controls (0-6.1%). The
incidence of carcinomas in males at 200-650 ppm showed a
nonsignificant trend when considered in isolation. The effects of
fenbuconazole on the liver in this study are summarized in Table 2. No
other treatment-related pathological effects were seen. The NOAEL was
10 ppm, equal to 1.3 mg/kg bw per day, on the basis of hepatic effects
at higher doses. Only equivocal evidence of hepatocellular
tumorigenicity was seen, which was statistically significant only in
females at a dose equivalent to 209 mg/kg bw per day (Wolfe, 1991a).
Rats
Groups of 70 male and 70 female Sprague-Dawley rats were fed
diets containing fenbuconazole (purity, 96.7%) at concentrations of 0,
8, 80, or 800 ppm for two years; lower levels (4, 40, or 400 ppm) had
been fed up to week 2, then 6, 60, and 600 ppm up to week 4. Ten rats
of each sex in each group were killed at 52 weeks. No
treatment-related effects on survival were seen and no clinical
symptoms of toxicity or ophthalmoscopic effects. Body-weight gain was
consistently, significantly reduced in females at 800 ppm.
Haematological analyses showed no consistent, dose-related effects in
erythrocyte or leukocyte parameters. Females at 800 ppm had raised
cholesterol levels, but the other effects were transient and not
Table 2. Incidences of histopathological changes in the livers of CD-1 mice fed fenbuconazole for 52 or 78 weeks
Dose (ppm)
Males Females
0 10 200 650 0 10 650 1300
No. examined 60 59 60 60 58 60 57 60
Hepatocellular effect
Hypertrophy 4 4 22 55 1 1 34 49
Vacuolation 2 1 11 31 4 1 20 31
Hyperplasia 3 0 1 7 0 1 0 3
Adenoma 8 1 8 6 0 0 0 4
Carcinoma 1 1 3 5 0 1 0 1
Adenoma and/or carcinoma 9 2 10 10 0 1 0 5
related to dose. The results of urinalyses were similarly
unremarkable. No treatment-related gross pathological effects were
seen; however, at 800 ppm, significantly increased liver weights
(absolute and relative in males and relative in females) were seen at
the time of the interim sacrifice, and both absolute and relative
weights were increased in animals of each sex at the terminal kill.
Thyroid/parathyroid weights were also increased at the terminal kill
in animals at 800 ppm (absolute and relative in males and relative in
females). The only treatment-related histopathological effects seen in
mice that died during the study and at the interim kill were
slight-to-moderate centrilobular to mid-zonal hepatocyte hypertrophy
and vacuolization in animals of each sex; similar effects were seen at
the terminal kill in animals at this dose. The incidence of focal
cystic thyroid hyperplasia was increased in males at 800 ppm (12/70;
1/70 in controls), and the incidence of thyroid follicular adenoma and
carcinoma was also increased in these animals (6/70 and 4/70 in eight
rats; one adenoma in control males). No other treatment-related
effects or tumorigenicity were seen. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day, on the basis of effects on body weight, effects
associated with hepatic hypertrophy (weight change, cholesterol
levels, and histopathological effects), and histopathological effects
in the thyroid at 800 ppm. The NOAEL for thyroid tumorigenicity (seen
only in males) was also 80 ppm (Wolfe, 1990).
Groups of 60 male Sprague-Dawley rats were given fenbuconazole
(purity, 96.7%) in the diet for two years in order to ensure that the
male rats in the previous study had been treated at the maximum
tolerated dose. The dietary concentrations were altered to accommodate
increasing body weights and food consumption at weeks 2 and 4, so that
the animals received 0, (400, 600) then 800 ppm to term or (800, 1200)
then 1600 ppm to term. Ten rats in each group were killed at 52 weeks.
No treatment-related effects on survival were seen, nor were there any
clinical symptoms of toxicity or ophthalmoscopic effects. Body-weight
gain was increasingly reduced during the study in rats at 1600 ppm. No
consistent effects on the results of haematology or clinical chemistry
were seen, and no gross pathological effects were seen. At the interim
kill, the liver weights (absolute and relative) were increased in
animals at 1600 ppm, and the relative liver weights were increased at
800 ppm. At the terminal kill, only the relative liver weights were
higher (with lower body weights) at 1600 ppm. The absolute and
relative thyroid and parathyroid weights were also increased at 800
and 1600 ppm at the interim kill. These effects correlated with
histopathological findings, with slight-to-moderate centrilobular to
mid-zonal vacuolization in 7/10 animals at 800 ppm and all animals at
1600 ppm at the interim kill. In 8/10 animals at 1600 ppm,
minimal-to-slight thyroid follicular-cell hypertrophy was also seen.
Dose-related effects on the degree of hypertrophy of livers were seen,
with occasional vacuolation in animals at doses > 800 ppm at the
terminal kill. These effects were seen in all surviving animals and in
most of those that died during the study. A trend in the severity of
the effects on the liver was seen, from minimal to moderate effects at
800 ppm to moderate to moderately severe hypertrophy at 1600 ppm.
Slight thyroid follicular hypertrophy occurred in 12/27 animals at
1600 ppm. An apparent dose-response relationship in the incidence of
thyroid follicular adenomas (2/60, 5/60, and 9/60) was seen at doses
of 0, 800, and 1600 ppm, respectively; two carcinomas were seen at 0
and 1600 ppm. No NOAEL was identified (Wolfe, 1991b).
(d) Genotoxicity
The results of tests for the genotoxicity of fenbuconazole are
summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, 25 male and 25 female Crl:CD-BR rats
(21 of each sex of the second parental group at 800 ppm) were given
fenbuconazole (purity, 96.7%) in the diet at 0, 8, 80, or 800 ppm. The
parental animals were fed for a minimum of 10 weeks before mating then
throughout mating, gestation, and lactation. Only one litter per
generation was produced. When the youngest litter reached 25 days, one
animal (F1) per sex per litter was randomly selected to serve as the
parents to produce the F2 litter; however, F0 males at 0 and 800 ppm
were subsequently mated with untreated females. Males were killed and
necropsied after the lactation period. Four days post partum, the
litters were culled randomly to eight (four of each sex when
possible), and dams that had not delivered were killed. No deaths or
symptoms of toxicity were seen during treatment, but F0 and F1 dams
at 800 ppm showed increased mortality during delivery, with 13/25 and
5/21 surviving, respectively. Reduced body-weight gain was seen in F0
dams and F1 parental males and females at 800 ppm before mating, and
these reductions were maintained in the females during gestation and
lactation. The effects correlated with reduced food consumption in
these animals. On necropsy, increased liver weights were seen in F0
and F1 parental males and females at 800 ppm. A slight, equivocal
increase in liver weight was seen at 80 ppm only in F1 dams, with no
evidence of associated histopathological changes. Thyroid weights were
increased F0 and F1 parental males; relative adrenal weights were
increased in 15-19 F0 and F1 dams at 800 ppm and in only about seven
at 0-80 ppm. Histopathological examination confirmed the hypertrophic
effects in these three organs at 800 ppm, with centrilobular to
mid-zonal hepatocyte hypertrophy and vacuolation, follicular-cell
hypertrophy, and hypertrophy of the zona glomerulosa, respectively.
Three F0 and four F1 dams at 800 ppm also had centrilobular
hepatocellular necrosis No treatment-related effects on fertility were
seen in males at doses up to 800 ppm; however, the numbers of F0 and
F1 dams at this dose that delivered live young was reduced to 10/25
and 4/21, respectively, and the number of stillborn pups was
increased, so that the total and mean numbers of pups were reduced.
The viability index (survival on day 4) was also reduced, from about
97% in the other groups to 85% at 800 ppm, and pup weight was
Table 2. Results of assays for genotoxicity with fenbuconazole
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium < 7500 mg/plate Reportedly Negativea Chism (1984)
TA98, TA100, 100
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.4 Negativea Sames & Frank (1987)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.7 Negativea Sames & Frank (1988)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 2000 mg/plate 98 Negativea Sames & Ella (1993)
TA98, TA100,
TA1535, TA1537
Gene mutation Chinese hamster < 60 mg/ml 96.7 Negativea Thilagar (1988a)
ovary cells, hprt
locus
Chromosomal Chinese hamster < 30 mg/ml 96.7 Negativea Thilagar (1990)
aberration ovary cells
Unscheduled Rat hepatocytes < 15 mg/ml 96.7 Negative Thilagar (1988b)
DNA synthesis
DNA repair B. subtilis < 2000 mg/disc 97.1 Negative Sarwar & Suzuki
(1994)
M45, H17
In vivo
Chromosomal Rat bone marrow < 1 × 2500 96.7 Negative Thilagar (1988c)
aberration mg/kg bw
a With and without metabolic activation
consistently lower. No treatment-related gross pathological effects
were seen in the offspring. The NOAEL was 80 ppm, equivalent to 4
mg/kg bw per day, on the basis of liver hypertrophy and maternal
toxicity and fetotoxicity at 800 ppm (Solomon & Kulwich, 1990).
(ii) Developmental toxicity
Rats
Groups of 12 mated Sprague-Dawley rats received fenbuconazole
(purity, 96.7%) at doses of 0, 50, 100, or 150 mg/kg bw per day by
gavage on days 6-15 post coitum. Maternal and fetal toxicity
occurred at the highest dose. The incidences of individual visceral
and skeletal alterations at this dose were similar to those in
controls, except that the total number of affected litters was higher
(Solomon & Lutz, 1987).
Fenbuconazole (purity, 96.4%) in an aqueous suspension of 0.5%
methylcellulose was administered at 0, 30, 75, or 150 mg/kg bw per day
to groups of 25 mated Sprague-Dawley rats by gavage on days 6-15
post coitum. The animals were killed on day 20. No treatment-related
mortality was seen, although one animal at 150 mg/kg bw per day died,
reportedly due to an intubation error. Animals at doses > 75 mg/kg
bw per day had alopecia and few faeces, and the mean body weights were
reduced during days 6-8, such that overall body-weight gain was lower
by the end of the study. Food consumption was apparently not recorded.
The numbers of animals that did not become pregnant (1, 3, 2, and 2 at
the four doses, respectively) was acceptable. At 150 mg/kg bw per day,
increased early, late, and total (one animal) resorptions were seen,
with a corresponding reduction in the average number of fetuses per
litter; average fetal weight was also reduced. No treatment-related
fetal malformations were seen. A dose-related trend in the number of
litters in which fetuses had partially or unossified sternebrae was
seen at doses > 75 mg/kg bw per day, with a corresponding increase
in the number of fetuses affected. In addition, an increased incidence
of rudimentary 14th ribs and partially or unossified pubic bones
occurred at 150 mg/kg bw per day, resulting in an overall increase in
the number of fetuses per litter with developmental effects. The NOAEL
for maternal toxicity was 30 mg/kg bw per day on the basis of reduced
body-weight gain and clinical symptoms at doses > 75 mg/kg bw per
day. The NOAEL for fetotoxicity was also 30 mg/kg bw per day on the
basis of an increased incidence of partially or unossified sternebrae
at the next dose. No evidence of teratogenicity was seen (Solomon &
Lutz, 1988).
Rabbits
Fenbuconazole (purity, 96.7%) in an aqueous suspension of
methylcellulose was administered to groups of 21 mated New Zealand
white rabbits at 0, 10, 30, or 60 mg/kg bw per day by gavage on days
7-20 post coitum. The animals were killed on day 29. One animal at
60 mg/kg bw per day died and one was killed in extremis after an
early abortion; a further animal died due to an intubation error.
Reduced food consumption and soft or few faeces were seen at 30-60
mg/kg bw per day. At 60 mg/kg bw per day, only 1/19 pregnant does
produced a viable litter, with 10 total resorptions and a total of six
abortions. At this dose, only eight fetuses were available for
examination. No treatment-related effects were seen in reproductive
parameters at doses of 10-30 mg/kg bw per day. The incidences of
retarded development and malformation were not increased in animals at
these doses. The NOAEL for maternal toxicity was 10 mg/kg bw per day
on the basis of clinical symptoms of toxicity at higher doses. The
NOAEL for fetotoxicity was 30 mg/kg bw per day, as postimplantation
losses and abortion were seen at 60 mg/kg bw per day, with no evidence
of teratogenicity (Soloman & Lutz, 1989).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six New Zealand white rabbits received 0.5 g fenbuconazole
(purity, 96.4%) topically as a paste with 1 ml of saline onto clipped
areas of the back. Exposure was maintained for 4 h under a
semi-occlusive dressing. No erythema or oedema was observed in any of
the animals after 24, 48, or 72 h (Lampé et al., 1987c).
Nine male New Zealand white rabbits received 0.1 g fenbuconazole
(purity, 96.4%) into one conjunctival sac. The eyes of six animals
were left unirrigated, while those of an additional three animals were
rinsed for 1 min about 30 s after administration. No corneal, iridial,
or conjunctival reactions were seen at 24, 48, or 72 h (Lampé et al.,
1987d).
Fenbuconazole (purity, 96.7%) was administered topically to 10
male and 10 female Hartley guinea-pigs, and their reactions were
compared with those of five positive controls of each sex treated with
1-chloro-2,4-dinitrobenzene at 1600 ppm in 80% aqueous ethanol and of
five negative controls of each sex. Fenbuconazole was applied for 6 h
once a week for three weeks as a 25% w/v solution in acetone that had
been shown to cause only slight erythema in a range-finding study.
Exposure to 0.4 ml of the test materials on clipped areas of the flank
was maintained under an occlusive dressing; negative controls were
shaved and received the dressing. A topical challenge exposure was
given two weeks after the third induction, in which negative controls
and fenbuconazole-treated animals received 0.4 ml of a 20% w/v
solution in acetone, and positive controls received 0.4 ml of an
800-ppm solution in acetone. The animals' backs were depilated 19-22 h
later with hair remover, and any erythema scored 24 h after removal of
the challenge patch. One negative control animal had erythema, but
none reacted to the challenge with fenbuconazole. All positive
controls reacted to challenge with 1-chloro-2,4-dinitrobenzene.
Fenbuconazole thus showed no potential for skin sensitization in this
Buehler test (Bonin et al., 1988).
The ability of fenbuconazole to produce delayed contact
hypersensitivity in guinea-pigs was tested with the maximization
technique of Magnusson and Kligman. After initial screening tests, the
animals were induced by intradermal injection of a 10% formulation of
fenbuconazole in acetone and by topical application of a 25%
formulation in the same solvent; they were challenged with a 10%
formulation in acetone. A sensitization rate of 10% was observed after
induction with fenbuconazole, but a 40% sensitization rate was
elicited in the controls receiving acetone alone. After a second
challenge with fenbuconazole as a 10% formulation in diethyl
phthalate, the sensitization rate was 16% with fenbuconazole and 10%
with the vehicle. The positive control substance, 85%
hexylcinnamaldehyde, induced 100% sensitization. The results indicate
that fenbuconazole has weak sensitizing potential (Morris, 1994).
(ii) Effects on thyroid function and the liver
Thyroid function and hepatic clearance of tetraiodothyronine
(thyroxine; T4) were investigated in male Crl:CD-BR rats given
fenbuconazole (purity, 97.1%) at concentrations of 8 or 800 ppm (10
rats) or 0, 1600, or 3200 ppm (20 rats) for four or 13 weeks; a
further two groups were treated with 1600 or 3200 ppm for four weeks
before receiving control diet for nine weeks. Serum thyroid hormone
levels were investigated in 10 rats per group at four and 13 weeks,
and liver microsomal UDP-glucuronsyltransferase and biliary excretion
of 125I-L-T4 were investigated in animals at 0 and 3200 ppm at four
and 13 weeks and at four weeks plus recovery.
No treatment-related deaths were seen. The only clinical symptom
apparently associated with treatment was squinting in animals at 3200
ppm for 13 weeks. The body weights of animals fed 1600 or 3200 ppm for
13 weeks were reduced but not those of rats fed identical levels of
treated diet followed by control diet. Corresponding effects on food
consumption was seen, being reduced in rats fed 1600 or 3200 ppm for
13 weeks and increased in the group allowed to recover. At 1600 and
3200 ppm, there were no treatment-related effects on serum aspartate
or alanine aminotransferase activities. Gross pathological examination
revealed only increased weights of the liver and thyroid. After four
weeks, the relative and absolute thyroid weights were increased by
about 35% at 1600 ppm and the relative weights by about 50% at 3200
ppm. Dose-related increases in relative and absolute liver weights
were seen after four weeks, by about 20 and 30% at both 800 and 1600
ppm and by about 85 and 40% at 3200 ppm, respectively. After 13 weeks
of treatment, the absolute and relative thyroid weights were increased
by 30-31% at 800 ppm, 41-47% at 1600 ppm, and 34-67% at 3200 ppm; the
absolute and relative liver weights were increased by 21% at 800 ppm,
45-51% at 1600 ppm, and 53-92% at 3200 ppm. After four weeks'
treatment at 1600 or 3200 ppm and nine weeks' recovery, the weights of
both the liver and thyroid had recovered and were comparable to those
of controls.
Histopathological examination of the thyroid after four weeks
showed dose-related increases in the incidence and severity of diffuse
follicular hypertrophy and hyperplasia in 4/10 animals at 800 ppm,
9/10 at 1600 ppm, and 10/10 at 3200 ppm, with focal hyperplasia in one
animal each at 1600 and 3200 ppm. Diffuse hypertrophy and hyperplasia
were also seen at dose-related severity after 13 weeks in 9/10 rats at
800 ppm and all rats at 1600 or 3200 ppm, with focal hyperplasia in
one rat at 3200 ppm. These hypertrophic effects were reversible after
nine weeks, the effects being of comparable severity in 6/10 controls,
6/10 at 1600 ppm, and 8/10 at 3200 ppm. Focal hyperplasia was still
present in one animal at 3200 ppm.
After four weeks, the average thyroid-stimulating hormone (TSH)
concentrations were increased in a dose-related trend, by about 79% at
800 ppm, 83% at 1600 ppm, and 105% at 3200 ppm. The L-T4 and reverse
L-triiodothyronine (rT3) levels were reduced to about 50% of the
control levels at 3200 ppm, while the T3 levels were unaffected. After
13 weeks, statistically significantly increased TSH levels were seen
at 3200 ppm (63%) and reduced L-T4 levels at 1600 or 3200 ppm (by 53
and 105%, respectively); however dose-related increases in TSH levels
were seen at 800-3200 ppm (13, 58, and 63%, respectively), and rT3
levels were reduced by about 49% at 3200 ppm. In the animals that were
allowed to recover, the hormone levels were comparable with those in
control animals, with the exception of rT3 in rats at 3200 ppm
(Hazelton et al., 1991).
Biliary excretion of L-T4 was investigated over 4 h in
bile-cannulated rats at 0 or 3200 ppm after four weeks and 13 weeks
and in the group allowed to recover after receiving 3200 ppm after
intravenous injection of 125I-L-T4. In treated rats, the biliary
clearance rate was increased by 165-281% at four weeks and 220-336% at
13 weeks; 14.7-14.9% of the administered radiolabel was excreted by
these animals in comparison to 7.2-7.5% by controls. About 70 and 85%
of the increased biliary excretion correlated to increased excretion
of L-T4-glucuronide at four and 13 weeks, respectively. By 13 weeks,
these parameters were lower in animals allowed to recover and were
comparable to those in control animals. In addition, a corresponding
increase in the activity of UDP-glucuronosyltransferase was seen in
these animals at four weeks, by 54% when expressed per mg microsomal
protein, 187% per g liver, and 337% per liver; at 13 weeks, the
respective activities were increased by 25, 144, and 300%, while the
activity in animals allowed to recover was comparable to that of
controls. Therefore, in rats at relatively high dietary doses, hepatic
metabolism and biliary excretion of T4 were increased, and TSH levels
were correspondingly increased, with hypertrophy and hyperplasia of
this gland. The NOAEL for these effects was 8 ppm, equal to 1 mg/kg bw
per day over 13 weeks (Hazelton et al., 1991).
Six groups of female CD-1 mice received fenbuconazole in the diet
at 0, 20, 60, 180, or 1300 ppm or 1000 ppm phenobarbital for one and
four weeks. Groups of male CD rats received diets containing 1600 ppm
fenbuconazole or 1000 ppm phenobarbital for four weeks. In addition,
three groups of mice and rats received 1300 or 3200 ppm fenbuconazole
or 1000 ppm phenobarbital for four weeks, followed by control diet for
nine weeks.
No treatment-related effects on the liver were seen in mice at
< 60 ppm. At 180 ppm, the effects on the liver included increased
activities of cytochrome P450, cytochrome b5, and 7-pentoxyresorufin
O-deethylase. The increase in cytochrome P450 was due to an increase
in the phenobarbital-inducible form of the enzyme. At 1300 ppm, the
effects were more pronounced and included hepatic enlargement,
hepatocellular hypertrophy, and hepatocellular proliferation, as
determined by bromodeoxyuridine immunohistochemistry. In rats,
fenbuconazole increased liver weights and induced hepatocellular
hypertrophy and cytochrome P450 activity, again due to an increase in
the phenobarbital-inducible form of the enzyme. In both species, the
effects on the liver were similar to phenobarbital-induced toxicity at
1000 ppm and were reversible after cessation of treatment with
fenbuconazole or phenobarbital (Hazelton et al., 1995).
The synthesis of T4 and T3 in the thyroid depends on a dietary
supply of iodine, which is taken up in the thyroid follicular cells,
oxidized by thyroid peroxidase to iodine, and bound at the apical
membrane to tyrosyl residues on thyroglobulin, either as
monoiodotyrosine or diiodotyrosine, which are coupled to produce T3
and T4, which remain part of thyroglobulin. This protein is secreted
into the follicular lumen and taken up by the follicular cells by
pinocytosis, where monoiodotyrosine, diiodotyrosine, T3, and T4 are
released. While T3 and T4 pass into the circulation, mono- and
diiodotyrosine remain in the follicular cells, where they are
deiodinated; the iodine is used to produce more hormone.
The level of circulating T4 is monitored by thyrotrophs in the
anterior pituitary, which are responsible for the production of TSH,
the major thyrotrophic hormone. In the thyrotrophs, T4 is deiodinized
in the outer ring by 5'-deiodinase II, to give T3, which then binds to
nuclear receptors in the cell. A decrease in occupancy of T3 receptors
results in increased synthesis of TSH. Greater control is exercised by
the hypothalamus, by the secretion of throtrophin-releasing hormone,
which also stimulates the release of TSH from thyrotrophs. Thus, any
reduction in the level of circulating T4 will result in an increased
level of TSH. In humans, thyroid-binding globulin is the main plasma
protein that binds thyroid hormones, with a greater affinity for T4
than T3. In rats, thyroid hormones are bound mainly and with lower
affinity to albumin, and only low levels of a protein that has 70%
homology with human thyroid-binding globulin are present (Imamura et
al., 1991).
Circulating T4 is taken up by various organs, but most is
metabolized in the liver by 5'-deiodinase I. This enzyme can catalyse
deiodination of both the outer ring to give T3, the more active
thyroid hormone, and the inner ring to give rT3, which has no known
function. Deiodination is the main route of catabolism in humans,
finally resulting in thyronine production. Thyroid hormones are also
sulfated and glucuronidated; the latter pathway is of little
importance in humans but the major route in rats.
Broadly speaking, five categories of xenobiotic influence thyroid
hormone homeostasis:
- directly acting substances that inhibit either iodine uptake
by the thyroid or thyroid peroxidase activity; e.g.
aminosalicylic acid, propylthiourea, and resorcinol;
- substances that stimulate T4 clearance, predominantly
through effects on the liver, by inducing microsomal enzymes
(resulting in increased biliary clearance; e.g.
phenobarbital) or by affecting hepatic transport of thyroid
hormones;
- substances that influence deiodination, either by
stimulating 5'-deiodinase I (e.g. phenobarbital) or by
inhibiting 5'-deiodinase II (e.g. iopanoic acid);
- substances that affect plasma binding of thyroid hormones
(e.g. salicylates); and
- substances that interact with receptors of neurotransmitters
such as dopamine that have been implicated in the control of
TSH output by thyrotrophin-releasing hormone (e.g.
clomiphene).
Thus, studies of the effects of fenbuconazole on the liver in
male mice and rats show that the microsomal enzymes cytochrome b5 and
7-pentoxyresorufin- O-deethylase (a marker of the cytochrome P450 2B
subfamily) are induced in both species (Hazelton et al., 1995). More
significantly, UDP-glucuronosyltransferase activity and biliary
clearance of 125I-thyroxine metabolites, including T4-glucuronide,
were increased after dietary administration, although the only dose
tested was 3200 ppm (Hazelton et al., 1991). In studies by dietary
administration to rats for up to 13 weeks, the plasma levels of TSH
were increased at doses > 800 ppm, while those of T4 and rT3 were
decreased and those of T3 unaltered; these changes correlated to
thyroid follicular hypertrophy and hyperplasia and increased thyroid
weight (Hazelton et al., 1991). None of these effects was seen at 8
ppm (Wolfe, 1990). A dietary concentration of 800 ppm was the lowest
at which an increased incidence of adenomas and carcinomas of thyroid
follicular cells was seen in rats treated for up to two years (Wolfe,
1991b). Thus, the basis for the possible carcinogenicity of
fenbuconazole is chronic stimulation of the thyroid by TSH with
reduced plasma T4 concentrations. The reason for the reduced T4 levels
at all relevant doses of fenbuconazole has not been found, but the
observation of increased biliary clearance of thyroid hormone
metabolites at a high dose (the only one tested) indicates another
component of the mechanism of carcinogenesis.
Comments
Fenbuconazole is rapidly absorbed and eliminated, mainly in the
faeces through significant biliary excretion; there was no evidence of
significant retention in tissues. The compound was also extensively
metabolized by phase-I oxidation or hydroxylation at a number of sites
in the molecule, followed by phase-II sulfate and glucuronide
conjugation (predominantly glucuronidation). Dermal absorption of
fenbuconazole (technical material and a formulation) constituted 2-13%
of an administered dose over 24 h, the absorption over 10 h being
< 5%.
Fenbuconazole was of low acute toxicity when administered orally
(LD50 > 2000 mg/kg bw), dermally (LD50 > 5000 mg/kg bw), or by
inhalation (LC50 > 2.1 mg/L air). It was not irritating to the skin
or eyes and was not a sensitizer in a Buehler test, but was a weak
sensitizer in a maximization test. WHO has not yet classified
fenbuconazole for acute toxicity.
After dietary administration, hepatomegaly with associated
effects on clinical chemistry, such as changes in cholesterol and
triglyceride levels and increases in the serum activity of hepatic
enzymes, were seen in mice, rats, and dogs. In a 13-week study of
toxicity in mice with dietary levels of 0, 20, 60, 180, or 540 ppm the
NOAEL was 60 ppm (equal to 11 mg/kg bw per day) on the basis of
hepatic effects at higher doses. In a three-month study of toxicity in
rats with dietary levels of 0, 20, 80, 400, or 1600 ppm, the NOAEL was
20 ppm (equal to 1.3 mg/kg bw per day) on the basis of hepatic effects
and hypertrophy of thyroid gland follicular cells at higher doses. In
a 13-week study of toxicity in dogs with dietary levels of 0, 30, 100,
400, or 1600 ppm, the NOAEL was 100 ppm (equal to 3.3 mg/kg bw per
day). In a one-year study in dogs with dietary levels of 0, 15, 150,
or 1200 ppm, the NOAEL was 150 ppm (equal to 5.2 mg/kg bw per day).
The NOAELs in the studies in dogs were based on decreased body-weight
gain and increased incidences of hepatic hypertrophy with associated
effects on clinical chemistry at higher doses.
In a 78-week study of toxicity and carcinogenicity in mice, with
dietary levels of 0, 10, 200, or 650 ppm in males and 0, 10, 650, or
1300 ppm in females, there was clear evidence of treatment-related
hepatomegaly, with dose-related hepatocytic hypertrophy and
vacuolation, and limited evidence of treatment-related hyperplasia and
tumorigenicity in the liver at the highest dose. The NOAEL was 10 ppm
(equal to 1.3 mg/kg bw per day). In a two-year study in rats with
dietary levels of 0, 8, 80, or 800 ppm, the predominant effects were
hepatocytic hypertrophy, thyroid follicular-cell hypertrophy, and an
increase in thyroid follicular-cell adenomas; in addition, thyroid
carcinomas were seen at the high dose. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day.
The etiology of the hepatic and thyroidal effects in rats was
further investigated in a 4-13-week study which illustrated the
biological feedback mechanism in rats: hepatomegaly leading to
increased metabolism and excretion of thyroxine, increased levels of
thyroid stimulating hormone, and thyroid hypertrophy/hyperplasia. The
effects seen after four weeks in this study were reversible. In
studies designed to investigate the hepatotoxicity of fenbuconazole,
hepatic effects were seen in rats and mice that were similar to those
induced by phenobarbital. Increased cytochrome P450 activity (CYP2B
form) was observed, with hepatocellular hypertrophy and proliferation.
The NOAEL in mice after treatment for 13 weeks was 60 ppm (equal to 14
mg/kg bw per day).
Fenbuconazole was adequately tested for genotoxicity in vitro
and in vivo. The Meeting concluded that it is not genotoxic.
Fenbuconazole was not teratogenic in either rats (at doses of 0,
30, 75, or 150 mg/kg bw per day) or rabbits (at doses of 0, 10, 30, or
60 mg/kg bw per day), but fetotoxicity was seen in both species, with
an NOAEL of 30 mg/kg bw per day. The NOAELs for maternal toxicity were
30 mg/kg bw per day in rats and 10 mg/kg bw per day in rabbits. No
effects on reproductive parameters were seen in a multigeneration
study in rats at dietary levels of 0, 8, 80, or 800 ppm, but
fetotoxicity was again seen at high doses, with maternal toxicity. The
NOAEL was 80 ppm, equal to 5.8 mg/kg bw per day.
An ADI of 0-0.03 mg/kg bw was allocated, on the basis of the
NOAEL of 3 mg/kg bw per day in the two-year study in rats and a safety
factor of 100. The Meeting noted that the NOAEL in the 13-week study
in rats and in the 78-week study in mice was 1.3 mg/kg bw per day, but
it concluded that this figure should not be used to derive the ADI.
The NOAEL from the 13-week study in rats was not considered to be
relevant in the light of the results of the larger, two-year study.
The Meeting concluded that the overall NOAEL in mice was 14 mg/kg bw
per day. This figure was taken from the 13-week study, which included
detailed investigations of hepatotoxicity. Hepatotoxicity was the
critical effect in the long-term study in mice, and the NOAEL in the
13-week study was lower than the lowest dose that was hepatotoxic in
the long-term study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 60 ppm, equal to 14 mg/kg bw per day (13-week study of
hepatotoxicity)
10 ppm, equal to 1.3 mg/kg bw per day (78-week study of
toxicity)
Rat: 20 ppm, equal to 1.3 mg/kg bw per day (13-week study of
toxicity)
80 ppm, equal to 3.0 mg/kg bw per day (two-year study
of toxicity)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenbuconazole
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral toxicity, rat LD50 > 2000 mg/kg bw
(1-7 days) Dermal toxicity, rat LD50 > 5000 mg/kg bw
Inhalation toxicity, rat LC50 > 2.1 mg/L
Dermal irritation, rabbit Not irritating
Ocular irritation, rabbit Not irritating
Dermal sensitization, guinea pig Not sensitizing in Buehler test, weakly
sensitizing in maximization test
Medium-term Repeated oral, 1-year, toxicity, dog NOAEL = 5.2 mg/kg bw per day: hepatic effects
(1-26 weeks) Repeated dermal, 4 weeks, toxicity, rat NOAEL = 1000 mg/kg bw per day (highest dose
tested)
Repeated oral, reproductive toxicity, rat NOAEL = 5.8 mg/kg bw per day: maternal and
fetal toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day: maternal
toxicity
Long term Repeated oral, 2 years, toxicity and NOAEL = 3 mg/kg bw per day: hepatic and
(> 1 year) carcinogenicity, rat thyroid effects
80 ppm, equal to 5.8 mg/kg bw per day (two-generation
study of reproductive toxicity)
30 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 150 ppm, equal to 5.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Anderson, D.M., DiDonato, L.J. & Longacre, S.L. (1988) 14C-RH-7592:
Range-finding kinetic and metabolite identification study in rats.
Unpublished study from Rohm & Haas Co., Report No. 87R-059 (European
Region reference No. 16.1).Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Bemacki, H.J. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-103 (European Region reference No. 8.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Bonin, R., Shade, W.D. & Hazelton, G.A. (1988) RH-7592 technical:
Delayed contact hypersensitivity study in guinea pigs. Unpublished
study from Rohm & Haas Co., Report No. 88R-027 (European Region
reference No. 4.3). Submitted to WHO by Rohm & Haas Co., Philadelphia,
PA, USA.
Cheng, T. (1990) RH-7592: Dermal absorption in male rats (preliminary
and definitive phases). Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 89RC-291 (European Region
reference No. 19.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Chism, E.M. (1984) RH-7592: Microbial mutagen test. Unpublished study
from Rohm & Haas Co., Report No. 84R-069 (European Region reference
No. 20.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
DiDonato, L.J. & Hazelton, G.A. (1993) 14-C-RH-7592: Disposition and
elimination study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 92R-060 (European Region reference No. 43.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Duchosal, F. & Thevenaz, P. (1989) 4-Hour, acute inhalation toxicity
study with RH-7592 technical in rats. Unpublished study from Research
and Consulting Co. AG. Rohm & Haas Co. report No. 89RC-023 (European
Region reference No. 16.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Harris, J.C. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in mice. Unpublished study from Rohm & Haas Co., Report
No. 87R-090 (European Region reference No. 5.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Shade, W.D. (1988) RH-7592: Three-month dietary
toxicity study in dogs. Unpublished study from Rohm & Haas Co., Report
No. 87R-127 (European Region reference No. 6.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J., Quinn, D.L., Shade, W.D. & Frantz,
J.D. (1991) RH-7592: Thyroid function and hepatic clearance of
thyroxine in male rats. Unpublished study from Rohm & Haas Co., Report
No. 90R-071 (European Region reference No. 23.6). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J. & Lomax, L.G. (1995) RH-7592: Cell
proliferation and enzyme induction in the liver of female mice.
Unpublished study from Rohm & Haas Co., Report No. 95R-035. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Imamura, S., Mori, Y., Yamamori, I., Miura, Y., Oiso, Y., Seo, H.,
Matsui, N. & Refetoff, S. (1991) Molecular cloning and primary
structure of rat thyroxine-binding globulin. Biochemistry, 30,
5406-5411.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-7592: Acute
oral toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-001 (European Region reference No.
1.20). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-7592: Acute
dermal toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-002 (European Region reference No.
1.17). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) RH-7592: Acute
oral toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-098 (European Region reference No. 1.12). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) RH-7592: Acute
dermal toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-099 (European Region reference No. 1.11). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987c) RH-7592: Rabbit
skin irritation study. Unpublished study from Rohm & Haas Co., Report
No. 87R-100/87R-100A (European Region reference No. 1.9). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987d) RH-7592: Eye
irritation study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 87R-101/87R-101A (European Region reference No. 1.10).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Kulwich, B.A. & Baldwin, R.C. (1991) RH-7592 2F and
technical fungicides four-week dermal toxicity study in rats.
Unpublished study from Rohm & Haas Co., Report No. 90R-084/90R-084A
(European Region references No. 25.2 and 25.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
LeVan, L.W. (1990) 14C-RH-7592: Pharmacokinetic study in rats.
Unpublished study from Rohm & Haas Co., Report No. 88RC-071 (European
Region reference No. 14.9). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1994) RH-7592 technical: Acute oral toxicity
study in male and female rats. Unpublished study from Rohm & Haas Co.,
Report No. 94R-107 (European Region reference No. 47.4). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morgan, C. (1990) RH-7592: 52-Week oral (dietary administration)
toxicity study in the beagle. Unpublished study from Hazleton UK,
report No. 6464-616/5. Rohm & Haas Co. report No. 88RC-115 (European
Region reference No. 19.3). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Morris, T.D. (1994) RH-7592 technical: Delayed contact
hypersensitivity study in guinea pigs (maximization technique).
Unpublished study from Hill Top Biolabs, Inc. Rohm & Haas Co. report
No. 94RC-063. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Morrison, R.D. & Hazelton, G.A. (1986a) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in mice. Unpublished study from Rohm
& Haas Co., Report No. 86R-131 (European Region reference No. 5.5).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hazelton, G.A. (1986b) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in rats. Unpublished study from Rohm
& Haas Co., Report No. 86R-130 (European Region reference No. 8.1).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
O'Hara, G.P., Frantz, J.D. & Poorman, K.B. (1987) RH-7592: 4-Week
range finding study in male and female dogs. Unpublished study from
Rohm & Haas Co., Report No. 87R-075 (see Rohm & Haas Co. report No.
87R-127, Appendix 13). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1991) RH-7592: 4-Week (dietary administration)
toxicity study in the beagle. Unpublished study from Hazelton, United
Kingdom. Rohm & Haas Co. report No. 88RC-097 (European Region
reference No. 30.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-7592: Salmonella typhimurium gene
mutation assay (Ames). Unpublished study from Rohm & Haas Co., Report
No. 92R-195 (European Region reference No. 42.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1987) RH-7592: Microbial mutagenicity
assay. Unpublished study from Rohm & Haas Co., Report No. 87R-044
(European Region reference No. 1.24). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1988) RH-7592: Salmonella typhimurium
gene mutation assay. Unpublished study from Rohm & Haas Co., Report
No. 88R-009 (European Region reference No. 1.23). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sarwar, G. & Suzuki, K. (1994) DNA repair test of H-7592 technical.
Unpublished study from Nippon Experimental Medical Research Institute
Co. Rohm & Haas Co. report No. 94RC-114 (European Region reference No.
48.7). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Kulwich, B.A. (1990) RH-7592: Two-generation
reproduction study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 88R-241 (European Region reference No. 15.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1987) RH-7592: Oral (gavage) developmental
toxicity screen in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-014 (see Rohm & Haas Co. report No. 87R-065, Appendix
L, p. 197). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Solomon, H.M. & Lutz, M.F. (1988) RH-7592: Oral (gavage) developmental
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-014 (European Region reference No. 1.22). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1989) RH-7592: Oral (gavage) developmental
toxicity study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 88R-195 (European Region reference No. 12.1). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988a) RH-7592 technical: Test for chemical induction of
gene mutation at the HGRPT locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Unpublished study
from Sitek Research Laboratories. Rohm & Haas Co. report No. 88RC-062
(European Region reference No. 7.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Thilagar, A. (1988b) RH-7592 technical: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished study from Sitek Research Laboratories.
Rohm & Haas Co. report No. 88RC-061 (European Region reference No.
7.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988c) RH-7592 technical: Acute test for chemical
induction of chromosome aberration in rat bone marrow cells in vivo.
Unpublished study from Sitek Research Laboratories. Rohm & Haas Co.
report No. 88RC-063 (European Region reference No. 7.3). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990) Test for chemical induction of chromosome
aberration using monolayer cultures of Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished study from
Sitek Research Laboratories. Rohm & Haas Co. report No. 89RC-090
(European Region reference No. 14.8). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1989) 13-Week dietary toxicity range-finding (RF) study
in mice with RH-7592. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-112 (European Region
reference No. 13.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1990) RH-7592 technical: 24-Month dietary chronic
toxicity-oncogenicity study in rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-098
(European Region reference No. 18.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1991a) RH-7592 technical: 78-Week dietary oncogenicity
toxicity study in mice. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-107 (European Region
reference No. 29.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1991b) RH-7592 technical: 104-Week chronic oncogenicity
toxicity study in male rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-116
(European Region reference No. 32.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
 Table 1. Acute toxicity of fenbuconazole
Species, strain Sex Route and vehicle LD50/LC50 Purity Reference
(mg/kg bw (%)
or mg/L air)
Rat, Crl:CD(SD)BR M Oral; aqueous methocel > 2000 96.4 Lampé et al. (1987a)
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 2000 96.7 Krajewski et al. (1988a)
< 5000
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 5000 97.1 Lutz & Parno (1994)
Rat, Crl:CD(SD)BR M Dermal, 0.85% saline > 5000 96.4 Lampé et al. (1987b)
Rat, Crl:CD(SD)BR M,F Dermal, 0.85% saline > 5000 96.7 Krajewski et al. (1988b)
Rat, Crl:CD(SD)BR M,F Inhalation > 2.1 96.7 Duchosal & Thevenaz (1989)
(b) Short-term toxicity
Mice
In a two-week range-finding study, groups of five male and five
female Crl:CD-1(ICR)BR mice were given diets containing fenbuconazole
(purity, 98%) at doses of 0, 100, 250, 500, or 1000 ppm. There were no
deaths, no clinical signs of reaction to treatment, and no
treatment-related effect on weight gain or food intake. The weights of
the livers of animals treated with doses of > 250 ppm were
increased, and there was histopatholgical evidence of hepatotoxicity
at the two highest doses. The NOAEL was 100 ppm, equal to 20 mg/kg bw
per day (Morrison & Hazleton, 1986a).
Groups of Crl:CD-1(ICR)BR mice were given diets containing
fenbuconazole (purity, 96.4%) at 0, 20, 60, 180, or 540 ppm for three
months. The only treatment-related effect observed was evidence of
hepatotoxicity at the two higher doses. At 180 ppm, relatively mild
effects were seen, including slightly increased liver weight in males,
hepatocellular hypertrophy in animals of each sex, single-cell
necrosis in one male, and increased aspartate aminotransferase
activity in males. At 540 ppm, these effects were more pronounced and
were seen in both males and females, in addition to hepatocyte
vacuolation and increased alanine aminotransferase activity. The NOAEL
was 60 ppm, equal to 11-18 mg/kg bw per day (Harris & Hazelton, 1988).
Groups of 10 male and 10 female Crl:CD-1(ICR)BR mice were given
fenbuconazole (purity, 96.7%) in the diet at 0, 540, 1000, 3000, or 10
000 ppm for three months. Treatment-related effects were observed in
all treated animals. At 540 ppm, the effects were similar to those
observed in the preceding study, i.e. increased liver weights,
hepatocellular hypertrophy, hepatocellular vacuolation, and
single-cell or focal necrosis, in animals of each sex. The effects on
the liver were more pronounced at higher doses, and the incidence
and/or severity was greater in males than in females. At the higher
doses, decreased renal weights were seen in males and decreased
body-weight gain and food intake and changes in clinical chemical
parameters in animals of each sex. At 10 000 ppm, 80-100% of the
animals died within three weeks (Wolfe, 1989).
Rats
In a two-week range-finding study, groups of five male and five
female Crl:CD-BR rats were given diets containing fenbuconazole
(purity, 98%) at 0, 100, 300, 1000, or 3000 ppm. There were no deaths
and no clinical signs of reaction to treatment. The rats receiving
1000 or 3000 ppm had lower weight gain and food intake than controls.
The liver weights of animals treated with doses > 300 ppm and of
males at 100 ppm were increased; histopathological evidence of
hepatotoxicity was seen at 1000 and 3000 ppm, and treatment-related
changes in hepatic mixed-function oxidase activity were seen at all
doses. There was no NOAEL (Morrison & Hazleton, 1986b).
Groups of 10 male and 10 female CRL:CD-BR rats were given
fenbuconazole (purity, 96.4%) in the diet at 0, 20, 80, 400, or 1600
ppm for three months. No deaths or clinical symptoms of toxicity were
seen during the study. Both food consumption and body-weight gain were
significantly reduced in animals of each sex at 1600 ppm, although the
magnitude of these effects declined towards the end of the study, with
comparable or increased food consumption from week 9 onwards. No
treatment-related ophthalmoscopic effects were seen, and no effects
were seen in urinalyses. There were no treatment-related effects on
either erythrocyte parameters or differential leukocyte counts. Serum
g-glutamyl transferase activity was increased in females at 1600 ppm,
and decreased triglycerides and increased cholesterol were seen in
animals of each sex at this dose. Gross pathological examination
revealed increased lobulization of the liver in animals of each sex at
1600 ppm and dark-brown discolouration of the liver in four females at
this dose. Adrenal weights were increased in animals of each sex
(relative weight only in females) at 1600 ppm, but no histopatholgical
correlate was seen in either sex. Increased ovarian weights seen at
1600 ppm were of questionable toxicological significant, given that no
histopathological effects occurred. Other increases in relative organ
weights at this dose were considered to be related to decreased
terminal body weight. A dose-related increase in the incidence and
magnitude of the hepatic histopathological effects was seen.
Hepatocellular hypertrophy was seen in one male at 80 ppm and most
rats at doses > 400 ppm; it was mainly centrilobular with
eosinophilic cells, some of which had basophilic nuclei. Mid-zonal
vacuolation was seen in none of the controls, two rats at 80 ppm, four
at 400 ppm, and six at 1600 ppm, while mid-zonal and periportal or
perilobular vacuolation were seen in females at doses > 400 ppm.
Centrilobular necrosis was seen in two males at 400 ppm. Thyroid
follicular epithelial hyperplasia was seen in nine males and two
females at 400 ppm and in eight males and 10 females at 1600 ppm. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of a
slight increase in the incidence of hepatic effects at 80 ppm (Bemacki
& Hazelton, 1988).
Groups of six male and six female Crl:CD-BR rats received
technical-grade fenbuconazole (purity, 97.1%) moistened with saline
topically on clipped areas of the back at doses of 0 or 1000 mg/kg bw
per day or a water-dispersible formulation of fenbuconazole at 62.5,
250, or 1000 mg/kg bw per day. Exposure was maintained with a patch of
absorbent gauze under an occlusive dressing for 6 h per day, five days
per week for 21-22 days; due to logistical constraints, half the
animals of each sex at each dose were killed 24 h apart.
No deaths or treatment-related clinical symptoms of toxicity were
seen, and food consumption and body-weight gain were unaffected by
treatment. No irritation was seen with the active ingredient alone.
None-to-moderate erythema was seen in animals of each sex at the
highest dose of the formulation, from day 19 in males and day 12 in
females; females receiving the formulation control also showed
erythema from day 11. Desiccation, reddened areas, and scabs were seen
in these animals at necropsy. There were no treatment-related effects
on clinical chemical, haematological, or urinary parameters. No gross
or histopathological effects were seen in association with
treatment-related systemic toxicity. The only apparent effect on organ
weight was an increase in relative liver weights at 1000 mg/kg bw per
day of the active ingredient in formulation in females and with the
technical-grade material in males, which is of questionable
toxicological significance and possibly related to the slightly
lowered terminal body weights. Increased incidences of acanthosis,
parakeratosis, eschar, or superficial exudate and necrosis of the
epidermis were seen both with the formulation and formulation blank.
The NOAEL for systemic toxicity was > 1000 mg/kg bw per day. Repeated
dermal application of relatively high doses of the fenbuconazole
co-formulants produced evidence of dermal irritation (Lampé et al.,
1991).
Dogs
In a four-week range-finding study, groups of one or two beagle
dogs of each sex were fed diets containing 0, 200, 400, 800, 1600, or
3200 ppm fenbuconazole (purity, 96.4%). The only effect seen at 800
ppm was increased serum alkaline phosphatase activity. Adverse effects
seen at 1600 ppm included decreased food intake and weight gain,
increased alanine aminotransferase activity, and decreased cholesterol
level. At 3200 ppm, the effects on weight gain and food intake were
incompatible with survival, and treatment was limited to two weeks.
The NOAEL was 400 ppm, equivalent to 10 mg/kg bw per day (O'Hara et
al., 1987).
In a second four-week range-finding study, two male and two
female beagles were given fenbuconazole (purity, 96.7%) in the diet at
levels of 0, 100, 1600, or 3200 ppm. No deaths occurred, and there
were no treatment-related clinical signs of toxicity. Decreased body
weight was seen at 1600 and 3200 ppm, especially during the first two
weeks of treatment, correlated with decreased food consumption at 1600
and especially 3200 ppm. Gross examination showed no treatment-related
effects. The NOAEL in this limited study was 100 ppm, equal to 3.6
mg/kg bw per day, on the basis of effects on food consumption and body
weight at 1600 ppm (Richards, 1991).
Groups of four male and four female beagles were given
fenbuconazole (purity, 96.4%) in the diet at levels of 0, 30, 100,
400, or 1600 ppm for three months. No deaths or clinical symptoms of
toxicity were seen. Body weights were decreased in animals of each sex
at 1600 ppm up to two weeks of the study, correlated with a
significant reduction in food consumption (23-41%). Body-weight gain
was comparable at 2-13 weeks, although the cumulative weight gain at
2-8 and 8-13 weeks was lower at 1600 ppm, especially in females. Food
consumption was generally slightly lower in animals of each sex at
1600 ppm, but the decrease was statistically significant only at 0-2
weeks. By three months, a statistically significant reduction in
erythrocyte count and decreased haematocrit and haemoglobin levels
were seen in females at 1600 ppm, whereas the platelet counts were
increased at both one and three months. Mean haemoglobin and cell
volume were increased in animals of each sex at 1600 ppm. These
effects were only slight, not related to dose, and of questionable
toxicological significance in males at 100 and 400 ppm.
Clinical chemical tests showed significant increases in alkaline
phosphatase activity in animals of each sex at 1600 ppm and in females
at 400 ppm at one and three months. Females at the highest dose also
showed raised serum alanine aminotransferase and g-glutamyltransferase
activity at these intervals. Cholesterol levels were slightly raised
in females at 400 ppm but were lower at 1600 ppm at one and three
months in females at 1600 ppm. The other effects were unremarkable and
not clearly related to treatment. No effects were seen in urinalyses
or ophthalmoscopic investigations. A dose-related trend to increased
liver weight (absolute and relative) was noted at doses > 400 ppm
in animals of each sex at three months. Hepatocytic hypertrophy was
seen, with eosinophilia at 400 and 1600 ppm and minimal-to-slight
multifocal vacuolation in animals of each sex at 1600 ppm. The NOAEL
was 100 ppm, equal to 3.3 mg/kg bw per day, on the basis of hepatic
hypertrophy with clinical chemical effects in animals of each sex at
higher doses (Hazelton & Shade, 1988).
Fenbuconazole (purity, 96.7%) was administered in the diet to
four male and four female beagles at concentrations of 0, 15, 150, or
1200 ppm for one year. No treatment-related effects on survival were
seen, nor were any clinical symptoms of toxicity or ophthalmoscopic
effects. Body-weight gain was reduced consistently in females at 1200
ppm and also at 150 ppm, predominantly during weeks 41-52; no clear
treatment-related effects were seen in males. Food consumption was
unaffected by treatment, except during the first few weeks at the
highest dose. No treatment-related haematological effects were seen at
12 or 26 weeks; however, at 52 weeks two males at 1200 ppm had
creneated erythrocytes, and one also had Burr cells. At 1200 ppm,
alkaline phosphatase activity was consistently increased in animals of
each sex at 13, 26, and 52 weeks; alanine aminotransferase activity
was increased consistently in one female at all three times and in
males at 52 weeks. No treatment-related effects were seen in
urinalyses. Total protein was reduced in females at 1200 ppm at 26
weeks and slightly reduced in males at this dose at 13, 26, and 52
weeks. These males also had reduced albumin levels at 26 and 52 weeks.
Triglyceride levels were consistently increased in animals of each sex
at 1200 ppm at all three times. Cholesterol levels were often slightly
lower at this dose, mainly in males, but with no dose-response
relationship; the only statistically significant result was seen in
females at 26 weeks. The level of total bilirubin was raised in two of
four males at 1200 ppm. The absolute and relative weights of the liver
and adrenals and the relative renal weight were increased in animals
of each sex at 1200 ppm. Histopathological examination showed
eosinophilic hypertrophic hepatocytes (mainly mid-zonal) in all
animals at 1200 ppm but not in other animals. Hepatocyte pigmentation
reported to be consistent with lipofuscin (slight to moderate) was
also seen in these animals. The NOAEL was 150 ppm, equal to 5.2 mg/kg
bw per day, as the effects on body weight in females at 150 ppm were
seen only towards the end of the study, with no evidence of systemic
toxicity. Consistent reductions in body, weight, increased liver
weight with histopathological changes, and possibly associated changes
in clinical chemistry were, however, seen at 1200 ppm (Morgan, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female CD-1 mice were given
fenbuconazole (purity, 96.7%) in the diet for 78 weeks. On the basis
of calculations of the maximum tolerated dose in previous studies,
males were treated at 0, 10, 200, or 650 ppm and females at 0, 10,
650, or 1300 ppm. Ten mice of each sex at each dose were killed at 52
weeks. No treatment-related effects on survival were seen, the rate
being > 70% at the end of the study. The body-weight gain of males
was reduced and was 13% less than that of controls at the end of the
study. No treatment-related effects on food consumption were seen. No
remarkable effects were seen on differential leukocyte counts, cell
morphology, nucleated erythrocyte counts, or myeloid:erythroid ratio.
Gross pathological examination showed an increase incidence of
enlarged livers in both surviving and dead animals of each sex at the
highest dose. The relative and absolute weights of the livers were
increased at 52 weeks in males and females at 650 ppm and in females
at 1300 ppm. Similar effects were seen at the time of the terminal
kill, and increased weights were also seen in males at 200 ppm.
Histopathological examination showed centrilobular to mid-zonal
hepatocyte hypertrophy and vacuolation with some hyperplasia. The
incidence of hepatocellular adenomas and carcinomas combined (8.3%)
was significantly increased in females at 1300 ppm, although the
control value was low (0); the incidence of hepatic tumours was very
slightly greater than the range in historical controls (0-6.1%). The
incidence of carcinomas in males at 200-650 ppm showed a
nonsignificant trend when considered in isolation. The effects of
fenbuconazole on the liver in this study are summarized in Table 2. No
other treatment-related pathological effects were seen. The NOAEL was
10 ppm, equal to 1.3 mg/kg bw per day, on the basis of hepatic effects
at higher doses. Only equivocal evidence of hepatocellular
tumorigenicity was seen, which was statistically significant only in
females at a dose equivalent to 209 mg/kg bw per day (Wolfe, 1991a).
Rats
Groups of 70 male and 70 female Sprague-Dawley rats were fed
diets containing fenbuconazole (purity, 96.7%) at concentrations of 0,
8, 80, or 800 ppm for two years; lower levels (4, 40, or 400 ppm) had
been fed up to week 2, then 6, 60, and 600 ppm up to week 4. Ten rats
of each sex in each group were killed at 52 weeks. No
treatment-related effects on survival were seen and no clinical
symptoms of toxicity or ophthalmoscopic effects. Body-weight gain was
consistently, significantly reduced in females at 800 ppm.
Haematological analyses showed no consistent, dose-related effects in
erythrocyte or leukocyte parameters. Females at 800 ppm had raised
cholesterol levels, but the other effects were transient and not
Table 2. Incidences of histopathological changes in the livers of CD-1 mice fed fenbuconazole for 52 or 78 weeks
Dose (ppm)
Males Females
0 10 200 650 0 10 650 1300
No. examined 60 59 60 60 58 60 57 60
Hepatocellular effect
Hypertrophy 4 4 22 55 1 1 34 49
Vacuolation 2 1 11 31 4 1 20 31
Hyperplasia 3 0 1 7 0 1 0 3
Adenoma 8 1 8 6 0 0 0 4
Carcinoma 1 1 3 5 0 1 0 1
Adenoma and/or carcinoma 9 2 10 10 0 1 0 5
related to dose. The results of urinalyses were similarly
unremarkable. No treatment-related gross pathological effects were
seen; however, at 800 ppm, significantly increased liver weights
(absolute and relative in males and relative in females) were seen at
the time of the interim sacrifice, and both absolute and relative
weights were increased in animals of each sex at the terminal kill.
Thyroid/parathyroid weights were also increased at the terminal kill
in animals at 800 ppm (absolute and relative in males and relative in
females). The only treatment-related histopathological effects seen in
mice that died during the study and at the interim kill were
slight-to-moderate centrilobular to mid-zonal hepatocyte hypertrophy
and vacuolization in animals of each sex; similar effects were seen at
the terminal kill in animals at this dose. The incidence of focal
cystic thyroid hyperplasia was increased in males at 800 ppm (12/70;
1/70 in controls), and the incidence of thyroid follicular adenoma and
carcinoma was also increased in these animals (6/70 and 4/70 in eight
rats; one adenoma in control males). No other treatment-related
effects or tumorigenicity were seen. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day, on the basis of effects on body weight, effects
associated with hepatic hypertrophy (weight change, cholesterol
levels, and histopathological effects), and histopathological effects
in the thyroid at 800 ppm. The NOAEL for thyroid tumorigenicity (seen
only in males) was also 80 ppm (Wolfe, 1990).
Groups of 60 male Sprague-Dawley rats were given fenbuconazole
(purity, 96.7%) in the diet for two years in order to ensure that the
male rats in the previous study had been treated at the maximum
tolerated dose. The dietary concentrations were altered to accommodate
increasing body weights and food consumption at weeks 2 and 4, so that
the animals received 0, (400, 600) then 800 ppm to term or (800, 1200)
then 1600 ppm to term. Ten rats in each group were killed at 52 weeks.
No treatment-related effects on survival were seen, nor were there any
clinical symptoms of toxicity or ophthalmoscopic effects. Body-weight
gain was increasingly reduced during the study in rats at 1600 ppm. No
consistent effects on the results of haematology or clinical chemistry
were seen, and no gross pathological effects were seen. At the interim
kill, the liver weights (absolute and relative) were increased in
animals at 1600 ppm, and the relative liver weights were increased at
800 ppm. At the terminal kill, only the relative liver weights were
higher (with lower body weights) at 1600 ppm. The absolute and
relative thyroid and parathyroid weights were also increased at 800
and 1600 ppm at the interim kill. These effects correlated with
histopathological findings, with slight-to-moderate centrilobular to
mid-zonal vacuolization in 7/10 animals at 800 ppm and all animals at
1600 ppm at the interim kill. In 8/10 animals at 1600 ppm,
minimal-to-slight thyroid follicular-cell hypertrophy was also seen.
Dose-related effects on the degree of hypertrophy of livers were seen,
with occasional vacuolation in animals at doses > 800 ppm at the
terminal kill. These effects were seen in all surviving animals and in
most of those that died during the study. A trend in the severity of
the effects on the liver was seen, from minimal to moderate effects at
800 ppm to moderate to moderately severe hypertrophy at 1600 ppm.
Slight thyroid follicular hypertrophy occurred in 12/27 animals at
1600 ppm. An apparent dose-response relationship in the incidence of
thyroid follicular adenomas (2/60, 5/60, and 9/60) was seen at doses
of 0, 800, and 1600 ppm, respectively; two carcinomas were seen at 0
and 1600 ppm. No NOAEL was identified (Wolfe, 1991b).
(d) Genotoxicity
The results of tests for the genotoxicity of fenbuconazole are
summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, 25 male and 25 female Crl:CD-BR rats
(21 of each sex of the second parental group at 800 ppm) were given
fenbuconazole (purity, 96.7%) in the diet at 0, 8, 80, or 800 ppm. The
parental animals were fed for a minimum of 10 weeks before mating then
throughout mating, gestation, and lactation. Only one litter per
generation was produced. When the youngest litter reached 25 days, one
animal (F1) per sex per litter was randomly selected to serve as the
parents to produce the F2 litter; however, F0 males at 0 and 800 ppm
were subsequently mated with untreated females. Males were killed and
necropsied after the lactation period. Four days post partum, the
litters were culled randomly to eight (four of each sex when
possible), and dams that had not delivered were killed. No deaths or
symptoms of toxicity were seen during treatment, but F0 and F1 dams
at 800 ppm showed increased mortality during delivery, with 13/25 and
5/21 surviving, respectively. Reduced body-weight gain was seen in F0
dams and F1 parental males and females at 800 ppm before mating, and
these reductions were maintained in the females during gestation and
lactation. The effects correlated with reduced food consumption in
these animals. On necropsy, increased liver weights were seen in F0
and F1 parental males and females at 800 ppm. A slight, equivocal
increase in liver weight was seen at 80 ppm only in F1 dams, with no
evidence of associated histopathological changes. Thyroid weights were
increased F0 and F1 parental males; relative adrenal weights were
increased in 15-19 F0 and F1 dams at 800 ppm and in only about seven
at 0-80 ppm. Histopathological examination confirmed the hypertrophic
effects in these three organs at 800 ppm, with centrilobular to
mid-zonal hepatocyte hypertrophy and vacuolation, follicular-cell
hypertrophy, and hypertrophy of the zona glomerulosa, respectively.
Three F0 and four F1 dams at 800 ppm also had centrilobular
hepatocellular necrosis No treatment-related effects on fertility were
seen in males at doses up to 800 ppm; however, the numbers of F0 and
F1 dams at this dose that delivered live young was reduced to 10/25
and 4/21, respectively, and the number of stillborn pups was
increased, so that the total and mean numbers of pups were reduced.
The viability index (survival on day 4) was also reduced, from about
97% in the other groups to 85% at 800 ppm, and pup weight was
Table 2. Results of assays for genotoxicity with fenbuconazole
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium < 7500 mg/plate Reportedly Negativea Chism (1984)
TA98, TA100, 100
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.4 Negativea Sames & Frank (1987)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.7 Negativea Sames & Frank (1988)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 2000 mg/plate 98 Negativea Sames & Ella (1993)
TA98, TA100,
TA1535, TA1537
Gene mutation Chinese hamster < 60 mg/ml 96.7 Negativea Thilagar (1988a)
ovary cells, hprt
locus
Chromosomal Chinese hamster < 30 mg/ml 96.7 Negativea Thilagar (1990)
aberration ovary cells
Unscheduled Rat hepatocytes < 15 mg/ml 96.7 Negative Thilagar (1988b)
DNA synthesis
DNA repair B. subtilis < 2000 mg/disc 97.1 Negative Sarwar & Suzuki
(1994)
M45, H17
In vivo
Chromosomal Rat bone marrow < 1 × 2500 96.7 Negative Thilagar (1988c)
aberration mg/kg bw
a With and without metabolic activation
consistently lower. No treatment-related gross pathological effects
were seen in the offspring. The NOAEL was 80 ppm, equivalent to 4
mg/kg bw per day, on the basis of liver hypertrophy and maternal
toxicity and fetotoxicity at 800 ppm (Solomon & Kulwich, 1990).
(ii) Developmental toxicity
Rats
Groups of 12 mated Sprague-Dawley rats received fenbuconazole
(purity, 96.7%) at doses of 0, 50, 100, or 150 mg/kg bw per day by
gavage on days 6-15 post coitum. Maternal and fetal toxicity
occurred at the highest dose. The incidences of individual visceral
and skeletal alterations at this dose were similar to those in
controls, except that the total number of affected litters was higher
(Solomon & Lutz, 1987).
Fenbuconazole (purity, 96.4%) in an aqueous suspension of 0.5%
methylcellulose was administered at 0, 30, 75, or 150 mg/kg bw per day
to groups of 25 mated Sprague-Dawley rats by gavage on days 6-15
post coitum. The animals were killed on day 20. No treatment-related
mortality was seen, although one animal at 150 mg/kg bw per day died,
reportedly due to an intubation error. Animals at doses > 75 mg/kg
bw per day had alopecia and few faeces, and the mean body weights were
reduced during days 6-8, such that overall body-weight gain was lower
by the end of the study. Food consumption was apparently not recorded.
The numbers of animals that did not become pregnant (1, 3, 2, and 2 at
the four doses, respectively) was acceptable. At 150 mg/kg bw per day,
increased early, late, and total (one animal) resorptions were seen,
with a corresponding reduction in the average number of fetuses per
litter; average fetal weight was also reduced. No treatment-related
fetal malformations were seen. A dose-related trend in the number of
litters in which fetuses had partially or unossified sternebrae was
seen at doses > 75 mg/kg bw per day, with a corresponding increase
in the number of fetuses affected. In addition, an increased incidence
of rudimentary 14th ribs and partially or unossified pubic bones
occurred at 150 mg/kg bw per day, resulting in an overall increase in
the number of fetuses per litter with developmental effects. The NOAEL
for maternal toxicity was 30 mg/kg bw per day on the basis of reduced
body-weight gain and clinical symptoms at doses > 75 mg/kg bw per
day. The NOAEL for fetotoxicity was also 30 mg/kg bw per day on the
basis of an increased incidence of partially or unossified sternebrae
at the next dose. No evidence of teratogenicity was seen (Solomon &
Lutz, 1988).
Rabbits
Fenbuconazole (purity, 96.7%) in an aqueous suspension of
methylcellulose was administered to groups of 21 mated New Zealand
white rabbits at 0, 10, 30, or 60 mg/kg bw per day by gavage on days
7-20 post coitum. The animals were killed on day 29. One animal at
60 mg/kg bw per day died and one was killed in extremis after an
early abortion; a further animal died due to an intubation error.
Reduced food consumption and soft or few faeces were seen at 30-60
mg/kg bw per day. At 60 mg/kg bw per day, only 1/19 pregnant does
produced a viable litter, with 10 total resorptions and a total of six
abortions. At this dose, only eight fetuses were available for
examination. No treatment-related effects were seen in reproductive
parameters at doses of 10-30 mg/kg bw per day. The incidences of
retarded development and malformation were not increased in animals at
these doses. The NOAEL for maternal toxicity was 10 mg/kg bw per day
on the basis of clinical symptoms of toxicity at higher doses. The
NOAEL for fetotoxicity was 30 mg/kg bw per day, as postimplantation
losses and abortion were seen at 60 mg/kg bw per day, with no evidence
of teratogenicity (Soloman & Lutz, 1989).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six New Zealand white rabbits received 0.5 g fenbuconazole
(purity, 96.4%) topically as a paste with 1 ml of saline onto clipped
areas of the back. Exposure was maintained for 4 h under a
semi-occlusive dressing. No erythema or oedema was observed in any of
the animals after 24, 48, or 72 h (Lampé et al., 1987c).
Nine male New Zealand white rabbits received 0.1 g fenbuconazole
(purity, 96.4%) into one conjunctival sac. The eyes of six animals
were left unirrigated, while those of an additional three animals were
rinsed for 1 min about 30 s after administration. No corneal, iridial,
or conjunctival reactions were seen at 24, 48, or 72 h (Lampé et al.,
1987d).
Fenbuconazole (purity, 96.7%) was administered topically to 10
male and 10 female Hartley guinea-pigs, and their reactions were
compared with those of five positive controls of each sex treated with
1-chloro-2,4-dinitrobenzene at 1600 ppm in 80% aqueous ethanol and of
five negative controls of each sex. Fenbuconazole was applied for 6 h
once a week for three weeks as a 25% w/v solution in acetone that had
been shown to cause only slight erythema in a range-finding study.
Exposure to 0.4 ml of the test materials on clipped areas of the flank
was maintained under an occlusive dressing; negative controls were
shaved and received the dressing. A topical challenge exposure was
given two weeks after the third induction, in which negative controls
and fenbuconazole-treated animals received 0.4 ml of a 20% w/v
solution in acetone, and positive controls received 0.4 ml of an
800-ppm solution in acetone. The animals' backs were depilated 19-22 h
later with hair remover, and any erythema scored 24 h after removal of
the challenge patch. One negative control animal had erythema, but
none reacted to the challenge with fenbuconazole. All positive
controls reacted to challenge with 1-chloro-2,4-dinitrobenzene.
Fenbuconazole thus showed no potential for skin sensitization in this
Buehler test (Bonin et al., 1988).
The ability of fenbuconazole to produce delayed contact
hypersensitivity in guinea-pigs was tested with the maximization
technique of Magnusson and Kligman. After initial screening tests, the
animals were induced by intradermal injection of a 10% formulation of
fenbuconazole in acetone and by topical application of a 25%
formulation in the same solvent; they were challenged with a 10%
formulation in acetone. A sensitization rate of 10% was observed after
induction with fenbuconazole, but a 40% sensitization rate was
elicited in the controls receiving acetone alone. After a second
challenge with fenbuconazole as a 10% formulation in diethyl
phthalate, the sensitization rate was 16% with fenbuconazole and 10%
with the vehicle. The positive control substance, 85%
hexylcinnamaldehyde, induced 100% sensitization. The results indicate
that fenbuconazole has weak sensitizing potential (Morris, 1994).
(ii) Effects on thyroid function and the liver
Thyroid function and hepatic clearance of tetraiodothyronine
(thyroxine; T4) were investigated in male Crl:CD-BR rats given
fenbuconazole (purity, 97.1%) at concentrations of 8 or 800 ppm (10
rats) or 0, 1600, or 3200 ppm (20 rats) for four or 13 weeks; a
further two groups were treated with 1600 or 3200 ppm for four weeks
before receiving control diet for nine weeks. Serum thyroid hormone
levels were investigated in 10 rats per group at four and 13 weeks,
and liver microsomal UDP-glucuronsyltransferase and biliary excretion
of 125I-L-T4 were investigated in animals at 0 and 3200 ppm at four
and 13 weeks and at four weeks plus recovery.
No treatment-related deaths were seen. The only clinical symptom
apparently associated with treatment was squinting in animals at 3200
ppm for 13 weeks. The body weights of animals fed 1600 or 3200 ppm for
13 weeks were reduced but not those of rats fed identical levels of
treated diet followed by control diet. Corresponding effects on food
consumption was seen, being reduced in rats fed 1600 or 3200 ppm for
13 weeks and increased in the group allowed to recover. At 1600 and
3200 ppm, there were no treatment-related effects on serum aspartate
or alanine aminotransferase activities. Gross pathological examination
revealed only increased weights of the liver and thyroid. After four
weeks, the relative and absolute thyroid weights were increased by
about 35% at 1600 ppm and the relative weights by about 50% at 3200
ppm. Dose-related increases in relative and absolute liver weights
were seen after four weeks, by about 20 and 30% at both 800 and 1600
ppm and by about 85 and 40% at 3200 ppm, respectively. After 13 weeks
of treatment, the absolute and relative thyroid weights were increased
by 30-31% at 800 ppm, 41-47% at 1600 ppm, and 34-67% at 3200 ppm; the
absolute and relative liver weights were increased by 21% at 800 ppm,
45-51% at 1600 ppm, and 53-92% at 3200 ppm. After four weeks'
treatment at 1600 or 3200 ppm and nine weeks' recovery, the weights of
both the liver and thyroid had recovered and were comparable to those
of controls.
Histopathological examination of the thyroid after four weeks
showed dose-related increases in the incidence and severity of diffuse
follicular hypertrophy and hyperplasia in 4/10 animals at 800 ppm,
9/10 at 1600 ppm, and 10/10 at 3200 ppm, with focal hyperplasia in one
animal each at 1600 and 3200 ppm. Diffuse hypertrophy and hyperplasia
were also seen at dose-related severity after 13 weeks in 9/10 rats at
800 ppm and all rats at 1600 or 3200 ppm, with focal hyperplasia in
one rat at 3200 ppm. These hypertrophic effects were reversible after
nine weeks, the effects being of comparable severity in 6/10 controls,
6/10 at 1600 ppm, and 8/10 at 3200 ppm. Focal hyperplasia was still
present in one animal at 3200 ppm.
After four weeks, the average thyroid-stimulating hormone (TSH)
concentrations were increased in a dose-related trend, by about 79% at
800 ppm, 83% at 1600 ppm, and 105% at 3200 ppm. The L-T4 and reverse
L-triiodothyronine (rT3) levels were reduced to about 50% of the
control levels at 3200 ppm, while the T3 levels were unaffected. After
13 weeks, statistically significantly increased TSH levels were seen
at 3200 ppm (63%) and reduced L-T4 levels at 1600 or 3200 ppm (by 53
and 105%, respectively); however dose-related increases in TSH levels
were seen at 800-3200 ppm (13, 58, and 63%, respectively), and rT3
levels were reduced by about 49% at 3200 ppm. In the animals that were
allowed to recover, the hormone levels were comparable with those in
control animals, with the exception of rT3 in rats at 3200 ppm
(Hazelton et al., 1991).
Biliary excretion of L-T4 was investigated over 4 h in
bile-cannulated rats at 0 or 3200 ppm after four weeks and 13 weeks
and in the group allowed to recover after receiving 3200 ppm after
intravenous injection of 125I-L-T4. In treated rats, the biliary
clearance rate was increased by 165-281% at four weeks and 220-336% at
13 weeks; 14.7-14.9% of the administered radiolabel was excreted by
these animals in comparison to 7.2-7.5% by controls. About 70 and 85%
of the increased biliary excretion correlated to increased excretion
of L-T4-glucuronide at four and 13 weeks, respectively. By 13 weeks,
these parameters were lower in animals allowed to recover and were
comparable to those in control animals. In addition, a corresponding
increase in the activity of UDP-glucuronosyltransferase was seen in
these animals at four weeks, by 54% when expressed per mg microsomal
protein, 187% per g liver, and 337% per liver; at 13 weeks, the
respective activities were increased by 25, 144, and 300%, while the
activity in animals allowed to recover was comparable to that of
controls. Therefore, in rats at relatively high dietary doses, hepatic
metabolism and biliary excretion of T4 were increased, and TSH levels
were correspondingly increased, with hypertrophy and hyperplasia of
this gland. The NOAEL for these effects was 8 ppm, equal to 1 mg/kg bw
per day over 13 weeks (Hazelton et al., 1991).
Six groups of female CD-1 mice received fenbuconazole in the diet
at 0, 20, 60, 180, or 1300 ppm or 1000 ppm phenobarbital for one and
four weeks. Groups of male CD rats received diets containing 1600 ppm
fenbuconazole or 1000 ppm phenobarbital for four weeks. In addition,
three groups of mice and rats received 1300 or 3200 ppm fenbuconazole
or 1000 ppm phenobarbital for four weeks, followed by control diet for
nine weeks.
No treatment-related effects on the liver were seen in mice at
< 60 ppm. At 180 ppm, the effects on the liver included increased
activities of cytochrome P450, cytochrome b5, and 7-pentoxyresorufin
O-deethylase. The increase in cytochrome P450 was due to an increase
in the phenobarbital-inducible form of the enzyme. At 1300 ppm, the
effects were more pronounced and included hepatic enlargement,
hepatocellular hypertrophy, and hepatocellular proliferation, as
determined by bromodeoxyuridine immunohistochemistry. In rats,
fenbuconazole increased liver weights and induced hepatocellular
hypertrophy and cytochrome P450 activity, again due to an increase in
the phenobarbital-inducible form of the enzyme. In both species, the
effects on the liver were similar to phenobarbital-induced toxicity at
1000 ppm and were reversible after cessation of treatment with
fenbuconazole or phenobarbital (Hazelton et al., 1995).
The synthesis of T4 and T3 in the thyroid depends on a dietary
supply of iodine, which is taken up in the thyroid follicular cells,
oxidized by thyroid peroxidase to iodine, and bound at the apical
membrane to tyrosyl residues on thyroglobulin, either as
monoiodotyrosine or diiodotyrosine, which are coupled to produce T3
and T4, which remain part of thyroglobulin. This protein is secreted
into the follicular lumen and taken up by the follicular cells by
pinocytosis, where monoiodotyrosine, diiodotyrosine, T3, and T4 are
released. While T3 and T4 pass into the circulation, mono- and
diiodotyrosine remain in the follicular cells, where they are
deiodinated; the iodine is used to produce more hormone.
The level of circulating T4 is monitored by thyrotrophs in the
anterior pituitary, which are responsible for the production of TSH,
the major thyrotrophic hormone. In the thyrotrophs, T4 is deiodinized
in the outer ring by 5'-deiodinase II, to give T3, which then binds to
nuclear receptors in the cell. A decrease in occupancy of T3 receptors
results in increased synthesis of TSH. Greater control is exercised by
the hypothalamus, by the secretion of throtrophin-releasing hormone,
which also stimulates the release of TSH from thyrotrophs. Thus, any
reduction in the level of circulating T4 will result in an increased
level of TSH. In humans, thyroid-binding globulin is the main plasma
protein that binds thyroid hormones, with a greater affinity for T4
than T3. In rats, thyroid hormones are bound mainly and with lower
affinity to albumin, and only low levels of a protein that has 70%
homology with human thyroid-binding globulin are present (Imamura et
al., 1991).
Circulating T4 is taken up by various organs, but most is
metabolized in the liver by 5'-deiodinase I. This enzyme can catalyse
deiodination of both the outer ring to give T3, the more active
thyroid hormone, and the inner ring to give rT3, which has no known
function. Deiodination is the main route of catabolism in humans,
finally resulting in thyronine production. Thyroid hormones are also
sulfated and glucuronidated; the latter pathway is of little
importance in humans but the major route in rats.
Broadly speaking, five categories of xenobiotic influence thyroid
hormone homeostasis:
- directly acting substances that inhibit either iodine uptake
by the thyroid or thyroid peroxidase activity; e.g.
aminosalicylic acid, propylthiourea, and resorcinol;
- substances that stimulate T4 clearance, predominantly
through effects on the liver, by inducing microsomal enzymes
(resulting in increased biliary clearance; e.g.
phenobarbital) or by affecting hepatic transport of thyroid
hormones;
- substances that influence deiodination, either by
stimulating 5'-deiodinase I (e.g. phenobarbital) or by
inhibiting 5'-deiodinase II (e.g. iopanoic acid);
- substances that affect plasma binding of thyroid hormones
(e.g. salicylates); and
- substances that interact with receptors of neurotransmitters
such as dopamine that have been implicated in the control of
TSH output by thyrotrophin-releasing hormone (e.g.
clomiphene).
Thus, studies of the effects of fenbuconazole on the liver in
male mice and rats show that the microsomal enzymes cytochrome b5 and
7-pentoxyresorufin- O-deethylase (a marker of the cytochrome P450 2B
subfamily) are induced in both species (Hazelton et al., 1995). More
significantly, UDP-glucuronosyltransferase activity and biliary
clearance of 125I-thyroxine metabolites, including T4-glucuronide,
were increased after dietary administration, although the only dose
tested was 3200 ppm (Hazelton et al., 1991). In studies by dietary
administration to rats for up to 13 weeks, the plasma levels of TSH
were increased at doses > 800 ppm, while those of T4 and rT3 were
decreased and those of T3 unaltered; these changes correlated to
thyroid follicular hypertrophy and hyperplasia and increased thyroid
weight (Hazelton et al., 1991). None of these effects was seen at 8
ppm (Wolfe, 1990). A dietary concentration of 800 ppm was the lowest
at which an increased incidence of adenomas and carcinomas of thyroid
follicular cells was seen in rats treated for up to two years (Wolfe,
1991b). Thus, the basis for the possible carcinogenicity of
fenbuconazole is chronic stimulation of the thyroid by TSH with
reduced plasma T4 concentrations. The reason for the reduced T4 levels
at all relevant doses of fenbuconazole has not been found, but the
observation of increased biliary clearance of thyroid hormone
metabolites at a high dose (the only one tested) indicates another
component of the mechanism of carcinogenesis.
Comments
Fenbuconazole is rapidly absorbed and eliminated, mainly in the
faeces through significant biliary excretion; there was no evidence of
significant retention in tissues. The compound was also extensively
metabolized by phase-I oxidation or hydroxylation at a number of sites
in the molecule, followed by phase-II sulfate and glucuronide
conjugation (predominantly glucuronidation). Dermal absorption of
fenbuconazole (technical material and a formulation) constituted 2-13%
of an administered dose over 24 h, the absorption over 10 h being
< 5%.
Fenbuconazole was of low acute toxicity when administered orally
(LD50 > 2000 mg/kg bw), dermally (LD50 > 5000 mg/kg bw), or by
inhalation (LC50 > 2.1 mg/L air). It was not irritating to the skin
or eyes and was not a sensitizer in a Buehler test, but was a weak
sensitizer in a maximization test. WHO has not yet classified
fenbuconazole for acute toxicity.
After dietary administration, hepatomegaly with associated
effects on clinical chemistry, such as changes in cholesterol and
triglyceride levels and increases in the serum activity of hepatic
enzymes, were seen in mice, rats, and dogs. In a 13-week study of
toxicity in mice with dietary levels of 0, 20, 60, 180, or 540 ppm the
NOAEL was 60 ppm (equal to 11 mg/kg bw per day) on the basis of
hepatic effects at higher doses. In a three-month study of toxicity in
rats with dietary levels of 0, 20, 80, 400, or 1600 ppm, the NOAEL was
20 ppm (equal to 1.3 mg/kg bw per day) on the basis of hepatic effects
and hypertrophy of thyroid gland follicular cells at higher doses. In
a 13-week study of toxicity in dogs with dietary levels of 0, 30, 100,
400, or 1600 ppm, the NOAEL was 100 ppm (equal to 3.3 mg/kg bw per
day). In a one-year study in dogs with dietary levels of 0, 15, 150,
or 1200 ppm, the NOAEL was 150 ppm (equal to 5.2 mg/kg bw per day).
The NOAELs in the studies in dogs were based on decreased body-weight
gain and increased incidences of hepatic hypertrophy with associated
effects on clinical chemistry at higher doses.
In a 78-week study of toxicity and carcinogenicity in mice, with
dietary levels of 0, 10, 200, or 650 ppm in males and 0, 10, 650, or
1300 ppm in females, there was clear evidence of treatment-related
hepatomegaly, with dose-related hepatocytic hypertrophy and
vacuolation, and limited evidence of treatment-related hyperplasia and
tumorigenicity in the liver at the highest dose. The NOAEL was 10 ppm
(equal to 1.3 mg/kg bw per day). In a two-year study in rats with
dietary levels of 0, 8, 80, or 800 ppm, the predominant effects were
hepatocytic hypertrophy, thyroid follicular-cell hypertrophy, and an
increase in thyroid follicular-cell adenomas; in addition, thyroid
carcinomas were seen at the high dose. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day.
The etiology of the hepatic and thyroidal effects in rats was
further investigated in a 4-13-week study which illustrated the
biological feedback mechanism in rats: hepatomegaly leading to
increased metabolism and excretion of thyroxine, increased levels of
thyroid stimulating hormone, and thyroid hypertrophy/hyperplasia. The
effects seen after four weeks in this study were reversible. In
studies designed to investigate the hepatotoxicity of fenbuconazole,
hepatic effects were seen in rats and mice that were similar to those
induced by phenobarbital. Increased cytochrome P450 activity (CYP2B
form) was observed, with hepatocellular hypertrophy and proliferation.
The NOAEL in mice after treatment for 13 weeks was 60 ppm (equal to 14
mg/kg bw per day).
Fenbuconazole was adequately tested for genotoxicity in vitro
and in vivo. The Meeting concluded that it is not genotoxic.
Fenbuconazole was not teratogenic in either rats (at doses of 0,
30, 75, or 150 mg/kg bw per day) or rabbits (at doses of 0, 10, 30, or
60 mg/kg bw per day), but fetotoxicity was seen in both species, with
an NOAEL of 30 mg/kg bw per day. The NOAELs for maternal toxicity were
30 mg/kg bw per day in rats and 10 mg/kg bw per day in rabbits. No
effects on reproductive parameters were seen in a multigeneration
study in rats at dietary levels of 0, 8, 80, or 800 ppm, but
fetotoxicity was again seen at high doses, with maternal toxicity. The
NOAEL was 80 ppm, equal to 5.8 mg/kg bw per day.
An ADI of 0-0.03 mg/kg bw was allocated, on the basis of the
NOAEL of 3 mg/kg bw per day in the two-year study in rats and a safety
factor of 100. The Meeting noted that the NOAEL in the 13-week study
in rats and in the 78-week study in mice was 1.3 mg/kg bw per day, but
it concluded that this figure should not be used to derive the ADI.
The NOAEL from the 13-week study in rats was not considered to be
relevant in the light of the results of the larger, two-year study.
The Meeting concluded that the overall NOAEL in mice was 14 mg/kg bw
per day. This figure was taken from the 13-week study, which included
detailed investigations of hepatotoxicity. Hepatotoxicity was the
critical effect in the long-term study in mice, and the NOAEL in the
13-week study was lower than the lowest dose that was hepatotoxic in
the long-term study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 60 ppm, equal to 14 mg/kg bw per day (13-week study of
hepatotoxicity)
10 ppm, equal to 1.3 mg/kg bw per day (78-week study of
toxicity)
Rat: 20 ppm, equal to 1.3 mg/kg bw per day (13-week study of
toxicity)
80 ppm, equal to 3.0 mg/kg bw per day (two-year study
of toxicity)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenbuconazole
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral toxicity, rat LD50 > 2000 mg/kg bw
(1-7 days) Dermal toxicity, rat LD50 > 5000 mg/kg bw
Inhalation toxicity, rat LC50 > 2.1 mg/L
Dermal irritation, rabbit Not irritating
Ocular irritation, rabbit Not irritating
Dermal sensitization, guinea pig Not sensitizing in Buehler test, weakly
sensitizing in maximization test
Medium-term Repeated oral, 1-year, toxicity, dog NOAEL = 5.2 mg/kg bw per day: hepatic effects
(1-26 weeks) Repeated dermal, 4 weeks, toxicity, rat NOAEL = 1000 mg/kg bw per day (highest dose
tested)
Repeated oral, reproductive toxicity, rat NOAEL = 5.8 mg/kg bw per day: maternal and
fetal toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day: maternal
toxicity
Long term Repeated oral, 2 years, toxicity and NOAEL = 3 mg/kg bw per day: hepatic and
(> 1 year) carcinogenicity, rat thyroid effects
80 ppm, equal to 5.8 mg/kg bw per day (two-generation
study of reproductive toxicity)
30 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 150 ppm, equal to 5.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Anderson, D.M., DiDonato, L.J. & Longacre, S.L. (1988) 14C-RH-7592:
Range-finding kinetic and metabolite identification study in rats.
Unpublished study from Rohm & Haas Co., Report No. 87R-059 (European
Region reference No. 16.1).Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Bemacki, H.J. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-103 (European Region reference No. 8.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Bonin, R., Shade, W.D. & Hazelton, G.A. (1988) RH-7592 technical:
Delayed contact hypersensitivity study in guinea pigs. Unpublished
study from Rohm & Haas Co., Report No. 88R-027 (European Region
reference No. 4.3). Submitted to WHO by Rohm & Haas Co., Philadelphia,
PA, USA.
Cheng, T. (1990) RH-7592: Dermal absorption in male rats (preliminary
and definitive phases). Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 89RC-291 (European Region
reference No. 19.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Chism, E.M. (1984) RH-7592: Microbial mutagen test. Unpublished study
from Rohm & Haas Co., Report No. 84R-069 (European Region reference
No. 20.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
DiDonato, L.J. & Hazelton, G.A. (1993) 14-C-RH-7592: Disposition and
elimination study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 92R-060 (European Region reference No. 43.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Duchosal, F. & Thevenaz, P. (1989) 4-Hour, acute inhalation toxicity
study with RH-7592 technical in rats. Unpublished study from Research
and Consulting Co. AG. Rohm & Haas Co. report No. 89RC-023 (European
Region reference No. 16.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Harris, J.C. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in mice. Unpublished study from Rohm & Haas Co., Report
No. 87R-090 (European Region reference No. 5.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Shade, W.D. (1988) RH-7592: Three-month dietary
toxicity study in dogs. Unpublished study from Rohm & Haas Co., Report
No. 87R-127 (European Region reference No. 6.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J., Quinn, D.L., Shade, W.D. & Frantz,
J.D. (1991) RH-7592: Thyroid function and hepatic clearance of
thyroxine in male rats. Unpublished study from Rohm & Haas Co., Report
No. 90R-071 (European Region reference No. 23.6). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J. & Lomax, L.G. (1995) RH-7592: Cell
proliferation and enzyme induction in the liver of female mice.
Unpublished study from Rohm & Haas Co., Report No. 95R-035. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Imamura, S., Mori, Y., Yamamori, I., Miura, Y., Oiso, Y., Seo, H.,
Matsui, N. & Refetoff, S. (1991) Molecular cloning and primary
structure of rat thyroxine-binding globulin. Biochemistry, 30,
5406-5411.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-7592: Acute
oral toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-001 (European Region reference No.
1.20). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-7592: Acute
dermal toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-002 (European Region reference No.
1.17). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) RH-7592: Acute
oral toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-098 (European Region reference No. 1.12). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) RH-7592: Acute
dermal toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-099 (European Region reference No. 1.11). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987c) RH-7592: Rabbit
skin irritation study. Unpublished study from Rohm & Haas Co., Report
No. 87R-100/87R-100A (European Region reference No. 1.9). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987d) RH-7592: Eye
irritation study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 87R-101/87R-101A (European Region reference No. 1.10).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Kulwich, B.A. & Baldwin, R.C. (1991) RH-7592 2F and
technical fungicides four-week dermal toxicity study in rats.
Unpublished study from Rohm & Haas Co., Report No. 90R-084/90R-084A
(European Region references No. 25.2 and 25.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
LeVan, L.W. (1990) 14C-RH-7592: Pharmacokinetic study in rats.
Unpublished study from Rohm & Haas Co., Report No. 88RC-071 (European
Region reference No. 14.9). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1994) RH-7592 technical: Acute oral toxicity
study in male and female rats. Unpublished study from Rohm & Haas Co.,
Report No. 94R-107 (European Region reference No. 47.4). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morgan, C. (1990) RH-7592: 52-Week oral (dietary administration)
toxicity study in the beagle. Unpublished study from Hazleton UK,
report No. 6464-616/5. Rohm & Haas Co. report No. 88RC-115 (European
Region reference No. 19.3). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Morris, T.D. (1994) RH-7592 technical: Delayed contact
hypersensitivity study in guinea pigs (maximization technique).
Unpublished study from Hill Top Biolabs, Inc. Rohm & Haas Co. report
No. 94RC-063. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Morrison, R.D. & Hazelton, G.A. (1986a) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in mice. Unpublished study from Rohm
& Haas Co., Report No. 86R-131 (European Region reference No. 5.5).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hazelton, G.A. (1986b) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in rats. Unpublished study from Rohm
& Haas Co., Report No. 86R-130 (European Region reference No. 8.1).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
O'Hara, G.P., Frantz, J.D. & Poorman, K.B. (1987) RH-7592: 4-Week
range finding study in male and female dogs. Unpublished study from
Rohm & Haas Co., Report No. 87R-075 (see Rohm & Haas Co. report No.
87R-127, Appendix 13). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1991) RH-7592: 4-Week (dietary administration)
toxicity study in the beagle. Unpublished study from Hazelton, United
Kingdom. Rohm & Haas Co. report No. 88RC-097 (European Region
reference No. 30.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-7592: Salmonella typhimurium gene
mutation assay (Ames). Unpublished study from Rohm & Haas Co., Report
No. 92R-195 (European Region reference No. 42.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1987) RH-7592: Microbial mutagenicity
assay. Unpublished study from Rohm & Haas Co., Report No. 87R-044
(European Region reference No. 1.24). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1988) RH-7592: Salmonella typhimurium
gene mutation assay. Unpublished study from Rohm & Haas Co., Report
No. 88R-009 (European Region reference No. 1.23). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sarwar, G. & Suzuki, K. (1994) DNA repair test of H-7592 technical.
Unpublished study from Nippon Experimental Medical Research Institute
Co. Rohm & Haas Co. report No. 94RC-114 (European Region reference No.
48.7). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Kulwich, B.A. (1990) RH-7592: Two-generation
reproduction study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 88R-241 (European Region reference No. 15.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1987) RH-7592: Oral (gavage) developmental
toxicity screen in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-014 (see Rohm & Haas Co. report No. 87R-065, Appendix
L, p. 197). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Solomon, H.M. & Lutz, M.F. (1988) RH-7592: Oral (gavage) developmental
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-014 (European Region reference No. 1.22). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1989) RH-7592: Oral (gavage) developmental
toxicity study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 88R-195 (European Region reference No. 12.1). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988a) RH-7592 technical: Test for chemical induction of
gene mutation at the HGRPT locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Unpublished study
from Sitek Research Laboratories. Rohm & Haas Co. report No. 88RC-062
(European Region reference No. 7.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Thilagar, A. (1988b) RH-7592 technical: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished study from Sitek Research Laboratories.
Rohm & Haas Co. report No. 88RC-061 (European Region reference No.
7.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988c) RH-7592 technical: Acute test for chemical
induction of chromosome aberration in rat bone marrow cells in vivo.
Unpublished study from Sitek Research Laboratories. Rohm & Haas Co.
report No. 88RC-063 (European Region reference No. 7.3). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990) Test for chemical induction of chromosome
aberration using monolayer cultures of Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished study from
Sitek Research Laboratories. Rohm & Haas Co. report No. 89RC-090
(European Region reference No. 14.8). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1989) 13-Week dietary toxicity range-finding (RF) study
in mice with RH-7592. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-112 (European Region
reference No. 13.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1990) RH-7592 technical: 24-Month dietary chronic
toxicity-oncogenicity study in rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-098
(European Region reference No. 18.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1991a) RH-7592 technical: 78-Week dietary oncogenicity
toxicity study in mice. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-107 (European Region
reference No. 29.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1991b) RH-7592 technical: 104-Week chronic oncogenicity
toxicity study in male rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-116
(European Region reference No. 32.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Table 1. Acute toxicity of fenbuconazole
Species, strain Sex Route and vehicle LD50/LC50 Purity Reference
(mg/kg bw (%)
or mg/L air)
Rat, Crl:CD(SD)BR M Oral; aqueous methocel > 2000 96.4 Lampé et al. (1987a)
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 2000 96.7 Krajewski et al. (1988a)
< 5000
Rat, Crl:CD(SD)BR M,F Oral; aqueous methocel > 5000 97.1 Lutz & Parno (1994)
Rat, Crl:CD(SD)BR M Dermal, 0.85% saline > 5000 96.4 Lampé et al. (1987b)
Rat, Crl:CD(SD)BR M,F Dermal, 0.85% saline > 5000 96.7 Krajewski et al. (1988b)
Rat, Crl:CD(SD)BR M,F Inhalation > 2.1 96.7 Duchosal & Thevenaz (1989)
(b) Short-term toxicity
Mice
In a two-week range-finding study, groups of five male and five
female Crl:CD-1(ICR)BR mice were given diets containing fenbuconazole
(purity, 98%) at doses of 0, 100, 250, 500, or 1000 ppm. There were no
deaths, no clinical signs of reaction to treatment, and no
treatment-related effect on weight gain or food intake. The weights of
the livers of animals treated with doses of > 250 ppm were
increased, and there was histopatholgical evidence of hepatotoxicity
at the two highest doses. The NOAEL was 100 ppm, equal to 20 mg/kg bw
per day (Morrison & Hazleton, 1986a).
Groups of Crl:CD-1(ICR)BR mice were given diets containing
fenbuconazole (purity, 96.4%) at 0, 20, 60, 180, or 540 ppm for three
months. The only treatment-related effect observed was evidence of
hepatotoxicity at the two higher doses. At 180 ppm, relatively mild
effects were seen, including slightly increased liver weight in males,
hepatocellular hypertrophy in animals of each sex, single-cell
necrosis in one male, and increased aspartate aminotransferase
activity in males. At 540 ppm, these effects were more pronounced and
were seen in both males and females, in addition to hepatocyte
vacuolation and increased alanine aminotransferase activity. The NOAEL
was 60 ppm, equal to 11-18 mg/kg bw per day (Harris & Hazelton, 1988).
Groups of 10 male and 10 female Crl:CD-1(ICR)BR mice were given
fenbuconazole (purity, 96.7%) in the diet at 0, 540, 1000, 3000, or 10
000 ppm for three months. Treatment-related effects were observed in
all treated animals. At 540 ppm, the effects were similar to those
observed in the preceding study, i.e. increased liver weights,
hepatocellular hypertrophy, hepatocellular vacuolation, and
single-cell or focal necrosis, in animals of each sex. The effects on
the liver were more pronounced at higher doses, and the incidence
and/or severity was greater in males than in females. At the higher
doses, decreased renal weights were seen in males and decreased
body-weight gain and food intake and changes in clinical chemical
parameters in animals of each sex. At 10 000 ppm, 80-100% of the
animals died within three weeks (Wolfe, 1989).
Rats
In a two-week range-finding study, groups of five male and five
female Crl:CD-BR rats were given diets containing fenbuconazole
(purity, 98%) at 0, 100, 300, 1000, or 3000 ppm. There were no deaths
and no clinical signs of reaction to treatment. The rats receiving
1000 or 3000 ppm had lower weight gain and food intake than controls.
The liver weights of animals treated with doses > 300 ppm and of
males at 100 ppm were increased; histopathological evidence of
hepatotoxicity was seen at 1000 and 3000 ppm, and treatment-related
changes in hepatic mixed-function oxidase activity were seen at all
doses. There was no NOAEL (Morrison & Hazleton, 1986b).
Groups of 10 male and 10 female CRL:CD-BR rats were given
fenbuconazole (purity, 96.4%) in the diet at 0, 20, 80, 400, or 1600
ppm for three months. No deaths or clinical symptoms of toxicity were
seen during the study. Both food consumption and body-weight gain were
significantly reduced in animals of each sex at 1600 ppm, although the
magnitude of these effects declined towards the end of the study, with
comparable or increased food consumption from week 9 onwards. No
treatment-related ophthalmoscopic effects were seen, and no effects
were seen in urinalyses. There were no treatment-related effects on
either erythrocyte parameters or differential leukocyte counts. Serum
g-glutamyl transferase activity was increased in females at 1600 ppm,
and decreased triglycerides and increased cholesterol were seen in
animals of each sex at this dose. Gross pathological examination
revealed increased lobulization of the liver in animals of each sex at
1600 ppm and dark-brown discolouration of the liver in four females at
this dose. Adrenal weights were increased in animals of each sex
(relative weight only in females) at 1600 ppm, but no histopatholgical
correlate was seen in either sex. Increased ovarian weights seen at
1600 ppm were of questionable toxicological significant, given that no
histopathological effects occurred. Other increases in relative organ
weights at this dose were considered to be related to decreased
terminal body weight. A dose-related increase in the incidence and
magnitude of the hepatic histopathological effects was seen.
Hepatocellular hypertrophy was seen in one male at 80 ppm and most
rats at doses > 400 ppm; it was mainly centrilobular with
eosinophilic cells, some of which had basophilic nuclei. Mid-zonal
vacuolation was seen in none of the controls, two rats at 80 ppm, four
at 400 ppm, and six at 1600 ppm, while mid-zonal and periportal or
perilobular vacuolation were seen in females at doses > 400 ppm.
Centrilobular necrosis was seen in two males at 400 ppm. Thyroid
follicular epithelial hyperplasia was seen in nine males and two
females at 400 ppm and in eight males and 10 females at 1600 ppm. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of a
slight increase in the incidence of hepatic effects at 80 ppm (Bemacki
& Hazelton, 1988).
Groups of six male and six female Crl:CD-BR rats received
technical-grade fenbuconazole (purity, 97.1%) moistened with saline
topically on clipped areas of the back at doses of 0 or 1000 mg/kg bw
per day or a water-dispersible formulation of fenbuconazole at 62.5,
250, or 1000 mg/kg bw per day. Exposure was maintained with a patch of
absorbent gauze under an occlusive dressing for 6 h per day, five days
per week for 21-22 days; due to logistical constraints, half the
animals of each sex at each dose were killed 24 h apart.
No deaths or treatment-related clinical symptoms of toxicity were
seen, and food consumption and body-weight gain were unaffected by
treatment. No irritation was seen with the active ingredient alone.
None-to-moderate erythema was seen in animals of each sex at the
highest dose of the formulation, from day 19 in males and day 12 in
females; females receiving the formulation control also showed
erythema from day 11. Desiccation, reddened areas, and scabs were seen
in these animals at necropsy. There were no treatment-related effects
on clinical chemical, haematological, or urinary parameters. No gross
or histopathological effects were seen in association with
treatment-related systemic toxicity. The only apparent effect on organ
weight was an increase in relative liver weights at 1000 mg/kg bw per
day of the active ingredient in formulation in females and with the
technical-grade material in males, which is of questionable
toxicological significance and possibly related to the slightly
lowered terminal body weights. Increased incidences of acanthosis,
parakeratosis, eschar, or superficial exudate and necrosis of the
epidermis were seen both with the formulation and formulation blank.
The NOAEL for systemic toxicity was > 1000 mg/kg bw per day. Repeated
dermal application of relatively high doses of the fenbuconazole
co-formulants produced evidence of dermal irritation (Lampé et al.,
1991).
Dogs
In a four-week range-finding study, groups of one or two beagle
dogs of each sex were fed diets containing 0, 200, 400, 800, 1600, or
3200 ppm fenbuconazole (purity, 96.4%). The only effect seen at 800
ppm was increased serum alkaline phosphatase activity. Adverse effects
seen at 1600 ppm included decreased food intake and weight gain,
increased alanine aminotransferase activity, and decreased cholesterol
level. At 3200 ppm, the effects on weight gain and food intake were
incompatible with survival, and treatment was limited to two weeks.
The NOAEL was 400 ppm, equivalent to 10 mg/kg bw per day (O'Hara et
al., 1987).
In a second four-week range-finding study, two male and two
female beagles were given fenbuconazole (purity, 96.7%) in the diet at
levels of 0, 100, 1600, or 3200 ppm. No deaths occurred, and there
were no treatment-related clinical signs of toxicity. Decreased body
weight was seen at 1600 and 3200 ppm, especially during the first two
weeks of treatment, correlated with decreased food consumption at 1600
and especially 3200 ppm. Gross examination showed no treatment-related
effects. The NOAEL in this limited study was 100 ppm, equal to 3.6
mg/kg bw per day, on the basis of effects on food consumption and body
weight at 1600 ppm (Richards, 1991).
Groups of four male and four female beagles were given
fenbuconazole (purity, 96.4%) in the diet at levels of 0, 30, 100,
400, or 1600 ppm for three months. No deaths or clinical symptoms of
toxicity were seen. Body weights were decreased in animals of each sex
at 1600 ppm up to two weeks of the study, correlated with a
significant reduction in food consumption (23-41%). Body-weight gain
was comparable at 2-13 weeks, although the cumulative weight gain at
2-8 and 8-13 weeks was lower at 1600 ppm, especially in females. Food
consumption was generally slightly lower in animals of each sex at
1600 ppm, but the decrease was statistically significant only at 0-2
weeks. By three months, a statistically significant reduction in
erythrocyte count and decreased haematocrit and haemoglobin levels
were seen in females at 1600 ppm, whereas the platelet counts were
increased at both one and three months. Mean haemoglobin and cell
volume were increased in animals of each sex at 1600 ppm. These
effects were only slight, not related to dose, and of questionable
toxicological significance in males at 100 and 400 ppm.
Clinical chemical tests showed significant increases in alkaline
phosphatase activity in animals of each sex at 1600 ppm and in females
at 400 ppm at one and three months. Females at the highest dose also
showed raised serum alanine aminotransferase and g-glutamyltransferase
activity at these intervals. Cholesterol levels were slightly raised
in females at 400 ppm but were lower at 1600 ppm at one and three
months in females at 1600 ppm. The other effects were unremarkable and
not clearly related to treatment. No effects were seen in urinalyses
or ophthalmoscopic investigations. A dose-related trend to increased
liver weight (absolute and relative) was noted at doses > 400 ppm
in animals of each sex at three months. Hepatocytic hypertrophy was
seen, with eosinophilia at 400 and 1600 ppm and minimal-to-slight
multifocal vacuolation in animals of each sex at 1600 ppm. The NOAEL
was 100 ppm, equal to 3.3 mg/kg bw per day, on the basis of hepatic
hypertrophy with clinical chemical effects in animals of each sex at
higher doses (Hazelton & Shade, 1988).
Fenbuconazole (purity, 96.7%) was administered in the diet to
four male and four female beagles at concentrations of 0, 15, 150, or
1200 ppm for one year. No treatment-related effects on survival were
seen, nor were any clinical symptoms of toxicity or ophthalmoscopic
effects. Body-weight gain was reduced consistently in females at 1200
ppm and also at 150 ppm, predominantly during weeks 41-52; no clear
treatment-related effects were seen in males. Food consumption was
unaffected by treatment, except during the first few weeks at the
highest dose. No treatment-related haematological effects were seen at
12 or 26 weeks; however, at 52 weeks two males at 1200 ppm had
creneated erythrocytes, and one also had Burr cells. At 1200 ppm,
alkaline phosphatase activity was consistently increased in animals of
each sex at 13, 26, and 52 weeks; alanine aminotransferase activity
was increased consistently in one female at all three times and in
males at 52 weeks. No treatment-related effects were seen in
urinalyses. Total protein was reduced in females at 1200 ppm at 26
weeks and slightly reduced in males at this dose at 13, 26, and 52
weeks. These males also had reduced albumin levels at 26 and 52 weeks.
Triglyceride levels were consistently increased in animals of each sex
at 1200 ppm at all three times. Cholesterol levels were often slightly
lower at this dose, mainly in males, but with no dose-response
relationship; the only statistically significant result was seen in
females at 26 weeks. The level of total bilirubin was raised in two of
four males at 1200 ppm. The absolute and relative weights of the liver
and adrenals and the relative renal weight were increased in animals
of each sex at 1200 ppm. Histopathological examination showed
eosinophilic hypertrophic hepatocytes (mainly mid-zonal) in all
animals at 1200 ppm but not in other animals. Hepatocyte pigmentation
reported to be consistent with lipofuscin (slight to moderate) was
also seen in these animals. The NOAEL was 150 ppm, equal to 5.2 mg/kg
bw per day, as the effects on body weight in females at 150 ppm were
seen only towards the end of the study, with no evidence of systemic
toxicity. Consistent reductions in body, weight, increased liver
weight with histopathological changes, and possibly associated changes
in clinical chemistry were, however, seen at 1200 ppm (Morgan, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female CD-1 mice were given
fenbuconazole (purity, 96.7%) in the diet for 78 weeks. On the basis
of calculations of the maximum tolerated dose in previous studies,
males were treated at 0, 10, 200, or 650 ppm and females at 0, 10,
650, or 1300 ppm. Ten mice of each sex at each dose were killed at 52
weeks. No treatment-related effects on survival were seen, the rate
being > 70% at the end of the study. The body-weight gain of males
was reduced and was 13% less than that of controls at the end of the
study. No treatment-related effects on food consumption were seen. No
remarkable effects were seen on differential leukocyte counts, cell
morphology, nucleated erythrocyte counts, or myeloid:erythroid ratio.
Gross pathological examination showed an increase incidence of
enlarged livers in both surviving and dead animals of each sex at the
highest dose. The relative and absolute weights of the livers were
increased at 52 weeks in males and females at 650 ppm and in females
at 1300 ppm. Similar effects were seen at the time of the terminal
kill, and increased weights were also seen in males at 200 ppm.
Histopathological examination showed centrilobular to mid-zonal
hepatocyte hypertrophy and vacuolation with some hyperplasia. The
incidence of hepatocellular adenomas and carcinomas combined (8.3%)
was significantly increased in females at 1300 ppm, although the
control value was low (0); the incidence of hepatic tumours was very
slightly greater than the range in historical controls (0-6.1%). The
incidence of carcinomas in males at 200-650 ppm showed a
nonsignificant trend when considered in isolation. The effects of
fenbuconazole on the liver in this study are summarized in Table 2. No
other treatment-related pathological effects were seen. The NOAEL was
10 ppm, equal to 1.3 mg/kg bw per day, on the basis of hepatic effects
at higher doses. Only equivocal evidence of hepatocellular
tumorigenicity was seen, which was statistically significant only in
females at a dose equivalent to 209 mg/kg bw per day (Wolfe, 1991a).
Rats
Groups of 70 male and 70 female Sprague-Dawley rats were fed
diets containing fenbuconazole (purity, 96.7%) at concentrations of 0,
8, 80, or 800 ppm for two years; lower levels (4, 40, or 400 ppm) had
been fed up to week 2, then 6, 60, and 600 ppm up to week 4. Ten rats
of each sex in each group were killed at 52 weeks. No
treatment-related effects on survival were seen and no clinical
symptoms of toxicity or ophthalmoscopic effects. Body-weight gain was
consistently, significantly reduced in females at 800 ppm.
Haematological analyses showed no consistent, dose-related effects in
erythrocyte or leukocyte parameters. Females at 800 ppm had raised
cholesterol levels, but the other effects were transient and not
Table 2. Incidences of histopathological changes in the livers of CD-1 mice fed fenbuconazole for 52 or 78 weeks
Dose (ppm)
Males Females
0 10 200 650 0 10 650 1300
No. examined 60 59 60 60 58 60 57 60
Hepatocellular effect
Hypertrophy 4 4 22 55 1 1 34 49
Vacuolation 2 1 11 31 4 1 20 31
Hyperplasia 3 0 1 7 0 1 0 3
Adenoma 8 1 8 6 0 0 0 4
Carcinoma 1 1 3 5 0 1 0 1
Adenoma and/or carcinoma 9 2 10 10 0 1 0 5
related to dose. The results of urinalyses were similarly
unremarkable. No treatment-related gross pathological effects were
seen; however, at 800 ppm, significantly increased liver weights
(absolute and relative in males and relative in females) were seen at
the time of the interim sacrifice, and both absolute and relative
weights were increased in animals of each sex at the terminal kill.
Thyroid/parathyroid weights were also increased at the terminal kill
in animals at 800 ppm (absolute and relative in males and relative in
females). The only treatment-related histopathological effects seen in
mice that died during the study and at the interim kill were
slight-to-moderate centrilobular to mid-zonal hepatocyte hypertrophy
and vacuolization in animals of each sex; similar effects were seen at
the terminal kill in animals at this dose. The incidence of focal
cystic thyroid hyperplasia was increased in males at 800 ppm (12/70;
1/70 in controls), and the incidence of thyroid follicular adenoma and
carcinoma was also increased in these animals (6/70 and 4/70 in eight
rats; one adenoma in control males). No other treatment-related
effects or tumorigenicity were seen. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day, on the basis of effects on body weight, effects
associated with hepatic hypertrophy (weight change, cholesterol
levels, and histopathological effects), and histopathological effects
in the thyroid at 800 ppm. The NOAEL for thyroid tumorigenicity (seen
only in males) was also 80 ppm (Wolfe, 1990).
Groups of 60 male Sprague-Dawley rats were given fenbuconazole
(purity, 96.7%) in the diet for two years in order to ensure that the
male rats in the previous study had been treated at the maximum
tolerated dose. The dietary concentrations were altered to accommodate
increasing body weights and food consumption at weeks 2 and 4, so that
the animals received 0, (400, 600) then 800 ppm to term or (800, 1200)
then 1600 ppm to term. Ten rats in each group were killed at 52 weeks.
No treatment-related effects on survival were seen, nor were there any
clinical symptoms of toxicity or ophthalmoscopic effects. Body-weight
gain was increasingly reduced during the study in rats at 1600 ppm. No
consistent effects on the results of haematology or clinical chemistry
were seen, and no gross pathological effects were seen. At the interim
kill, the liver weights (absolute and relative) were increased in
animals at 1600 ppm, and the relative liver weights were increased at
800 ppm. At the terminal kill, only the relative liver weights were
higher (with lower body weights) at 1600 ppm. The absolute and
relative thyroid and parathyroid weights were also increased at 800
and 1600 ppm at the interim kill. These effects correlated with
histopathological findings, with slight-to-moderate centrilobular to
mid-zonal vacuolization in 7/10 animals at 800 ppm and all animals at
1600 ppm at the interim kill. In 8/10 animals at 1600 ppm,
minimal-to-slight thyroid follicular-cell hypertrophy was also seen.
Dose-related effects on the degree of hypertrophy of livers were seen,
with occasional vacuolation in animals at doses > 800 ppm at the
terminal kill. These effects were seen in all surviving animals and in
most of those that died during the study. A trend in the severity of
the effects on the liver was seen, from minimal to moderate effects at
800 ppm to moderate to moderately severe hypertrophy at 1600 ppm.
Slight thyroid follicular hypertrophy occurred in 12/27 animals at
1600 ppm. An apparent dose-response relationship in the incidence of
thyroid follicular adenomas (2/60, 5/60, and 9/60) was seen at doses
of 0, 800, and 1600 ppm, respectively; two carcinomas were seen at 0
and 1600 ppm. No NOAEL was identified (Wolfe, 1991b).
(d) Genotoxicity
The results of tests for the genotoxicity of fenbuconazole are
summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, 25 male and 25 female Crl:CD-BR rats
(21 of each sex of the second parental group at 800 ppm) were given
fenbuconazole (purity, 96.7%) in the diet at 0, 8, 80, or 800 ppm. The
parental animals were fed for a minimum of 10 weeks before mating then
throughout mating, gestation, and lactation. Only one litter per
generation was produced. When the youngest litter reached 25 days, one
animal (F1) per sex per litter was randomly selected to serve as the
parents to produce the F2 litter; however, F0 males at 0 and 800 ppm
were subsequently mated with untreated females. Males were killed and
necropsied after the lactation period. Four days post partum, the
litters were culled randomly to eight (four of each sex when
possible), and dams that had not delivered were killed. No deaths or
symptoms of toxicity were seen during treatment, but F0 and F1 dams
at 800 ppm showed increased mortality during delivery, with 13/25 and
5/21 surviving, respectively. Reduced body-weight gain was seen in F0
dams and F1 parental males and females at 800 ppm before mating, and
these reductions were maintained in the females during gestation and
lactation. The effects correlated with reduced food consumption in
these animals. On necropsy, increased liver weights were seen in F0
and F1 parental males and females at 800 ppm. A slight, equivocal
increase in liver weight was seen at 80 ppm only in F1 dams, with no
evidence of associated histopathological changes. Thyroid weights were
increased F0 and F1 parental males; relative adrenal weights were
increased in 15-19 F0 and F1 dams at 800 ppm and in only about seven
at 0-80 ppm. Histopathological examination confirmed the hypertrophic
effects in these three organs at 800 ppm, with centrilobular to
mid-zonal hepatocyte hypertrophy and vacuolation, follicular-cell
hypertrophy, and hypertrophy of the zona glomerulosa, respectively.
Three F0 and four F1 dams at 800 ppm also had centrilobular
hepatocellular necrosis No treatment-related effects on fertility were
seen in males at doses up to 800 ppm; however, the numbers of F0 and
F1 dams at this dose that delivered live young was reduced to 10/25
and 4/21, respectively, and the number of stillborn pups was
increased, so that the total and mean numbers of pups were reduced.
The viability index (survival on day 4) was also reduced, from about
97% in the other groups to 85% at 800 ppm, and pup weight was
Table 2. Results of assays for genotoxicity with fenbuconazole
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium < 7500 mg/plate Reportedly Negativea Chism (1984)
TA98, TA100, 100
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.4 Negativea Sames & Frank (1987)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 5000 mg/plate 96.7 Negativea Sames & Frank (1988)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium < 2000 mg/plate 98 Negativea Sames & Ella (1993)
TA98, TA100,
TA1535, TA1537
Gene mutation Chinese hamster < 60 mg/ml 96.7 Negativea Thilagar (1988a)
ovary cells, hprt
locus
Chromosomal Chinese hamster < 30 mg/ml 96.7 Negativea Thilagar (1990)
aberration ovary cells
Unscheduled Rat hepatocytes < 15 mg/ml 96.7 Negative Thilagar (1988b)
DNA synthesis
DNA repair B. subtilis < 2000 mg/disc 97.1 Negative Sarwar & Suzuki
(1994)
M45, H17
In vivo
Chromosomal Rat bone marrow < 1 × 2500 96.7 Negative Thilagar (1988c)
aberration mg/kg bw
a With and without metabolic activation
consistently lower. No treatment-related gross pathological effects
were seen in the offspring. The NOAEL was 80 ppm, equivalent to 4
mg/kg bw per day, on the basis of liver hypertrophy and maternal
toxicity and fetotoxicity at 800 ppm (Solomon & Kulwich, 1990).
(ii) Developmental toxicity
Rats
Groups of 12 mated Sprague-Dawley rats received fenbuconazole
(purity, 96.7%) at doses of 0, 50, 100, or 150 mg/kg bw per day by
gavage on days 6-15 post coitum. Maternal and fetal toxicity
occurred at the highest dose. The incidences of individual visceral
and skeletal alterations at this dose were similar to those in
controls, except that the total number of affected litters was higher
(Solomon & Lutz, 1987).
Fenbuconazole (purity, 96.4%) in an aqueous suspension of 0.5%
methylcellulose was administered at 0, 30, 75, or 150 mg/kg bw per day
to groups of 25 mated Sprague-Dawley rats by gavage on days 6-15
post coitum. The animals were killed on day 20. No treatment-related
mortality was seen, although one animal at 150 mg/kg bw per day died,
reportedly due to an intubation error. Animals at doses > 75 mg/kg
bw per day had alopecia and few faeces, and the mean body weights were
reduced during days 6-8, such that overall body-weight gain was lower
by the end of the study. Food consumption was apparently not recorded.
The numbers of animals that did not become pregnant (1, 3, 2, and 2 at
the four doses, respectively) was acceptable. At 150 mg/kg bw per day,
increased early, late, and total (one animal) resorptions were seen,
with a corresponding reduction in the average number of fetuses per
litter; average fetal weight was also reduced. No treatment-related
fetal malformations were seen. A dose-related trend in the number of
litters in which fetuses had partially or unossified sternebrae was
seen at doses > 75 mg/kg bw per day, with a corresponding increase
in the number of fetuses affected. In addition, an increased incidence
of rudimentary 14th ribs and partially or unossified pubic bones
occurred at 150 mg/kg bw per day, resulting in an overall increase in
the number of fetuses per litter with developmental effects. The NOAEL
for maternal toxicity was 30 mg/kg bw per day on the basis of reduced
body-weight gain and clinical symptoms at doses > 75 mg/kg bw per
day. The NOAEL for fetotoxicity was also 30 mg/kg bw per day on the
basis of an increased incidence of partially or unossified sternebrae
at the next dose. No evidence of teratogenicity was seen (Solomon &
Lutz, 1988).
Rabbits
Fenbuconazole (purity, 96.7%) in an aqueous suspension of
methylcellulose was administered to groups of 21 mated New Zealand
white rabbits at 0, 10, 30, or 60 mg/kg bw per day by gavage on days
7-20 post coitum. The animals were killed on day 29. One animal at
60 mg/kg bw per day died and one was killed in extremis after an
early abortion; a further animal died due to an intubation error.
Reduced food consumption and soft or few faeces were seen at 30-60
mg/kg bw per day. At 60 mg/kg bw per day, only 1/19 pregnant does
produced a viable litter, with 10 total resorptions and a total of six
abortions. At this dose, only eight fetuses were available for
examination. No treatment-related effects were seen in reproductive
parameters at doses of 10-30 mg/kg bw per day. The incidences of
retarded development and malformation were not increased in animals at
these doses. The NOAEL for maternal toxicity was 10 mg/kg bw per day
on the basis of clinical symptoms of toxicity at higher doses. The
NOAEL for fetotoxicity was 30 mg/kg bw per day, as postimplantation
losses and abortion were seen at 60 mg/kg bw per day, with no evidence
of teratogenicity (Soloman & Lutz, 1989).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six New Zealand white rabbits received 0.5 g fenbuconazole
(purity, 96.4%) topically as a paste with 1 ml of saline onto clipped
areas of the back. Exposure was maintained for 4 h under a
semi-occlusive dressing. No erythema or oedema was observed in any of
the animals after 24, 48, or 72 h (Lampé et al., 1987c).
Nine male New Zealand white rabbits received 0.1 g fenbuconazole
(purity, 96.4%) into one conjunctival sac. The eyes of six animals
were left unirrigated, while those of an additional three animals were
rinsed for 1 min about 30 s after administration. No corneal, iridial,
or conjunctival reactions were seen at 24, 48, or 72 h (Lampé et al.,
1987d).
Fenbuconazole (purity, 96.7%) was administered topically to 10
male and 10 female Hartley guinea-pigs, and their reactions were
compared with those of five positive controls of each sex treated with
1-chloro-2,4-dinitrobenzene at 1600 ppm in 80% aqueous ethanol and of
five negative controls of each sex. Fenbuconazole was applied for 6 h
once a week for three weeks as a 25% w/v solution in acetone that had
been shown to cause only slight erythema in a range-finding study.
Exposure to 0.4 ml of the test materials on clipped areas of the flank
was maintained under an occlusive dressing; negative controls were
shaved and received the dressing. A topical challenge exposure was
given two weeks after the third induction, in which negative controls
and fenbuconazole-treated animals received 0.4 ml of a 20% w/v
solution in acetone, and positive controls received 0.4 ml of an
800-ppm solution in acetone. The animals' backs were depilated 19-22 h
later with hair remover, and any erythema scored 24 h after removal of
the challenge patch. One negative control animal had erythema, but
none reacted to the challenge with fenbuconazole. All positive
controls reacted to challenge with 1-chloro-2,4-dinitrobenzene.
Fenbuconazole thus showed no potential for skin sensitization in this
Buehler test (Bonin et al., 1988).
The ability of fenbuconazole to produce delayed contact
hypersensitivity in guinea-pigs was tested with the maximization
technique of Magnusson and Kligman. After initial screening tests, the
animals were induced by intradermal injection of a 10% formulation of
fenbuconazole in acetone and by topical application of a 25%
formulation in the same solvent; they were challenged with a 10%
formulation in acetone. A sensitization rate of 10% was observed after
induction with fenbuconazole, but a 40% sensitization rate was
elicited in the controls receiving acetone alone. After a second
challenge with fenbuconazole as a 10% formulation in diethyl
phthalate, the sensitization rate was 16% with fenbuconazole and 10%
with the vehicle. The positive control substance, 85%
hexylcinnamaldehyde, induced 100% sensitization. The results indicate
that fenbuconazole has weak sensitizing potential (Morris, 1994).
(ii) Effects on thyroid function and the liver
Thyroid function and hepatic clearance of tetraiodothyronine
(thyroxine; T4) were investigated in male Crl:CD-BR rats given
fenbuconazole (purity, 97.1%) at concentrations of 8 or 800 ppm (10
rats) or 0, 1600, or 3200 ppm (20 rats) for four or 13 weeks; a
further two groups were treated with 1600 or 3200 ppm for four weeks
before receiving control diet for nine weeks. Serum thyroid hormone
levels were investigated in 10 rats per group at four and 13 weeks,
and liver microsomal UDP-glucuronsyltransferase and biliary excretion
of 125I-L-T4 were investigated in animals at 0 and 3200 ppm at four
and 13 weeks and at four weeks plus recovery.
No treatment-related deaths were seen. The only clinical symptom
apparently associated with treatment was squinting in animals at 3200
ppm for 13 weeks. The body weights of animals fed 1600 or 3200 ppm for
13 weeks were reduced but not those of rats fed identical levels of
treated diet followed by control diet. Corresponding effects on food
consumption was seen, being reduced in rats fed 1600 or 3200 ppm for
13 weeks and increased in the group allowed to recover. At 1600 and
3200 ppm, there were no treatment-related effects on serum aspartate
or alanine aminotransferase activities. Gross pathological examination
revealed only increased weights of the liver and thyroid. After four
weeks, the relative and absolute thyroid weights were increased by
about 35% at 1600 ppm and the relative weights by about 50% at 3200
ppm. Dose-related increases in relative and absolute liver weights
were seen after four weeks, by about 20 and 30% at both 800 and 1600
ppm and by about 85 and 40% at 3200 ppm, respectively. After 13 weeks
of treatment, the absolute and relative thyroid weights were increased
by 30-31% at 800 ppm, 41-47% at 1600 ppm, and 34-67% at 3200 ppm; the
absolute and relative liver weights were increased by 21% at 800 ppm,
45-51% at 1600 ppm, and 53-92% at 3200 ppm. After four weeks'
treatment at 1600 or 3200 ppm and nine weeks' recovery, the weights of
both the liver and thyroid had recovered and were comparable to those
of controls.
Histopathological examination of the thyroid after four weeks
showed dose-related increases in the incidence and severity of diffuse
follicular hypertrophy and hyperplasia in 4/10 animals at 800 ppm,
9/10 at 1600 ppm, and 10/10 at 3200 ppm, with focal hyperplasia in one
animal each at 1600 and 3200 ppm. Diffuse hypertrophy and hyperplasia
were also seen at dose-related severity after 13 weeks in 9/10 rats at
800 ppm and all rats at 1600 or 3200 ppm, with focal hyperplasia in
one rat at 3200 ppm. These hypertrophic effects were reversible after
nine weeks, the effects being of comparable severity in 6/10 controls,
6/10 at 1600 ppm, and 8/10 at 3200 ppm. Focal hyperplasia was still
present in one animal at 3200 ppm.
After four weeks, the average thyroid-stimulating hormone (TSH)
concentrations were increased in a dose-related trend, by about 79% at
800 ppm, 83% at 1600 ppm, and 105% at 3200 ppm. The L-T4 and reverse
L-triiodothyronine (rT3) levels were reduced to about 50% of the
control levels at 3200 ppm, while the T3 levels were unaffected. After
13 weeks, statistically significantly increased TSH levels were seen
at 3200 ppm (63%) and reduced L-T4 levels at 1600 or 3200 ppm (by 53
and 105%, respectively); however dose-related increases in TSH levels
were seen at 800-3200 ppm (13, 58, and 63%, respectively), and rT3
levels were reduced by about 49% at 3200 ppm. In the animals that were
allowed to recover, the hormone levels were comparable with those in
control animals, with the exception of rT3 in rats at 3200 ppm
(Hazelton et al., 1991).
Biliary excretion of L-T4 was investigated over 4 h in
bile-cannulated rats at 0 or 3200 ppm after four weeks and 13 weeks
and in the group allowed to recover after receiving 3200 ppm after
intravenous injection of 125I-L-T4. In treated rats, the biliary
clearance rate was increased by 165-281% at four weeks and 220-336% at
13 weeks; 14.7-14.9% of the administered radiolabel was excreted by
these animals in comparison to 7.2-7.5% by controls. About 70 and 85%
of the increased biliary excretion correlated to increased excretion
of L-T4-glucuronide at four and 13 weeks, respectively. By 13 weeks,
these parameters were lower in animals allowed to recover and were
comparable to those in control animals. In addition, a corresponding
increase in the activity of UDP-glucuronosyltransferase was seen in
these animals at four weeks, by 54% when expressed per mg microsomal
protein, 187% per g liver, and 337% per liver; at 13 weeks, the
respective activities were increased by 25, 144, and 300%, while the
activity in animals allowed to recover was comparable to that of
controls. Therefore, in rats at relatively high dietary doses, hepatic
metabolism and biliary excretion of T4 were increased, and TSH levels
were correspondingly increased, with hypertrophy and hyperplasia of
this gland. The NOAEL for these effects was 8 ppm, equal to 1 mg/kg bw
per day over 13 weeks (Hazelton et al., 1991).
Six groups of female CD-1 mice received fenbuconazole in the diet
at 0, 20, 60, 180, or 1300 ppm or 1000 ppm phenobarbital for one and
four weeks. Groups of male CD rats received diets containing 1600 ppm
fenbuconazole or 1000 ppm phenobarbital for four weeks. In addition,
three groups of mice and rats received 1300 or 3200 ppm fenbuconazole
or 1000 ppm phenobarbital for four weeks, followed by control diet for
nine weeks.
No treatment-related effects on the liver were seen in mice at
< 60 ppm. At 180 ppm, the effects on the liver included increased
activities of cytochrome P450, cytochrome b5, and 7-pentoxyresorufin
O-deethylase. The increase in cytochrome P450 was due to an increase
in the phenobarbital-inducible form of the enzyme. At 1300 ppm, the
effects were more pronounced and included hepatic enlargement,
hepatocellular hypertrophy, and hepatocellular proliferation, as
determined by bromodeoxyuridine immunohistochemistry. In rats,
fenbuconazole increased liver weights and induced hepatocellular
hypertrophy and cytochrome P450 activity, again due to an increase in
the phenobarbital-inducible form of the enzyme. In both species, the
effects on the liver were similar to phenobarbital-induced toxicity at
1000 ppm and were reversible after cessation of treatment with
fenbuconazole or phenobarbital (Hazelton et al., 1995).
The synthesis of T4 and T3 in the thyroid depends on a dietary
supply of iodine, which is taken up in the thyroid follicular cells,
oxidized by thyroid peroxidase to iodine, and bound at the apical
membrane to tyrosyl residues on thyroglobulin, either as
monoiodotyrosine or diiodotyrosine, which are coupled to produce T3
and T4, which remain part of thyroglobulin. This protein is secreted
into the follicular lumen and taken up by the follicular cells by
pinocytosis, where monoiodotyrosine, diiodotyrosine, T3, and T4 are
released. While T3 and T4 pass into the circulation, mono- and
diiodotyrosine remain in the follicular cells, where they are
deiodinated; the iodine is used to produce more hormone.
The level of circulating T4 is monitored by thyrotrophs in the
anterior pituitary, which are responsible for the production of TSH,
the major thyrotrophic hormone. In the thyrotrophs, T4 is deiodinized
in the outer ring by 5'-deiodinase II, to give T3, which then binds to
nuclear receptors in the cell. A decrease in occupancy of T3 receptors
results in increased synthesis of TSH. Greater control is exercised by
the hypothalamus, by the secretion of throtrophin-releasing hormone,
which also stimulates the release of TSH from thyrotrophs. Thus, any
reduction in the level of circulating T4 will result in an increased
level of TSH. In humans, thyroid-binding globulin is the main plasma
protein that binds thyroid hormones, with a greater affinity for T4
than T3. In rats, thyroid hormones are bound mainly and with lower
affinity to albumin, and only low levels of a protein that has 70%
homology with human thyroid-binding globulin are present (Imamura et
al., 1991).
Circulating T4 is taken up by various organs, but most is
metabolized in the liver by 5'-deiodinase I. This enzyme can catalyse
deiodination of both the outer ring to give T3, the more active
thyroid hormone, and the inner ring to give rT3, which has no known
function. Deiodination is the main route of catabolism in humans,
finally resulting in thyronine production. Thyroid hormones are also
sulfated and glucuronidated; the latter pathway is of little
importance in humans but the major route in rats.
Broadly speaking, five categories of xenobiotic influence thyroid
hormone homeostasis:
- directly acting substances that inhibit either iodine uptake
by the thyroid or thyroid peroxidase activity; e.g.
aminosalicylic acid, propylthiourea, and resorcinol;
- substances that stimulate T4 clearance, predominantly
through effects on the liver, by inducing microsomal enzymes
(resulting in increased biliary clearance; e.g.
phenobarbital) or by affecting hepatic transport of thyroid
hormones;
- substances that influence deiodination, either by
stimulating 5'-deiodinase I (e.g. phenobarbital) or by
inhibiting 5'-deiodinase II (e.g. iopanoic acid);
- substances that affect plasma binding of thyroid hormones
(e.g. salicylates); and
- substances that interact with receptors of neurotransmitters
such as dopamine that have been implicated in the control of
TSH output by thyrotrophin-releasing hormone (e.g.
clomiphene).
Thus, studies of the effects of fenbuconazole on the liver in
male mice and rats show that the microsomal enzymes cytochrome b5 and
7-pentoxyresorufin- O-deethylase (a marker of the cytochrome P450 2B
subfamily) are induced in both species (Hazelton et al., 1995). More
significantly, UDP-glucuronosyltransferase activity and biliary
clearance of 125I-thyroxine metabolites, including T4-glucuronide,
were increased after dietary administration, although the only dose
tested was 3200 ppm (Hazelton et al., 1991). In studies by dietary
administration to rats for up to 13 weeks, the plasma levels of TSH
were increased at doses > 800 ppm, while those of T4 and rT3 were
decreased and those of T3 unaltered; these changes correlated to
thyroid follicular hypertrophy and hyperplasia and increased thyroid
weight (Hazelton et al., 1991). None of these effects was seen at 8
ppm (Wolfe, 1990). A dietary concentration of 800 ppm was the lowest
at which an increased incidence of adenomas and carcinomas of thyroid
follicular cells was seen in rats treated for up to two years (Wolfe,
1991b). Thus, the basis for the possible carcinogenicity of
fenbuconazole is chronic stimulation of the thyroid by TSH with
reduced plasma T4 concentrations. The reason for the reduced T4 levels
at all relevant doses of fenbuconazole has not been found, but the
observation of increased biliary clearance of thyroid hormone
metabolites at a high dose (the only one tested) indicates another
component of the mechanism of carcinogenesis.
Comments
Fenbuconazole is rapidly absorbed and eliminated, mainly in the
faeces through significant biliary excretion; there was no evidence of
significant retention in tissues. The compound was also extensively
metabolized by phase-I oxidation or hydroxylation at a number of sites
in the molecule, followed by phase-II sulfate and glucuronide
conjugation (predominantly glucuronidation). Dermal absorption of
fenbuconazole (technical material and a formulation) constituted 2-13%
of an administered dose over 24 h, the absorption over 10 h being
< 5%.
Fenbuconazole was of low acute toxicity when administered orally
(LD50 > 2000 mg/kg bw), dermally (LD50 > 5000 mg/kg bw), or by
inhalation (LC50 > 2.1 mg/L air). It was not irritating to the skin
or eyes and was not a sensitizer in a Buehler test, but was a weak
sensitizer in a maximization test. WHO has not yet classified
fenbuconazole for acute toxicity.
After dietary administration, hepatomegaly with associated
effects on clinical chemistry, such as changes in cholesterol and
triglyceride levels and increases in the serum activity of hepatic
enzymes, were seen in mice, rats, and dogs. In a 13-week study of
toxicity in mice with dietary levels of 0, 20, 60, 180, or 540 ppm the
NOAEL was 60 ppm (equal to 11 mg/kg bw per day) on the basis of
hepatic effects at higher doses. In a three-month study of toxicity in
rats with dietary levels of 0, 20, 80, 400, or 1600 ppm, the NOAEL was
20 ppm (equal to 1.3 mg/kg bw per day) on the basis of hepatic effects
and hypertrophy of thyroid gland follicular cells at higher doses. In
a 13-week study of toxicity in dogs with dietary levels of 0, 30, 100,
400, or 1600 ppm, the NOAEL was 100 ppm (equal to 3.3 mg/kg bw per
day). In a one-year study in dogs with dietary levels of 0, 15, 150,
or 1200 ppm, the NOAEL was 150 ppm (equal to 5.2 mg/kg bw per day).
The NOAELs in the studies in dogs were based on decreased body-weight
gain and increased incidences of hepatic hypertrophy with associated
effects on clinical chemistry at higher doses.
In a 78-week study of toxicity and carcinogenicity in mice, with
dietary levels of 0, 10, 200, or 650 ppm in males and 0, 10, 650, or
1300 ppm in females, there was clear evidence of treatment-related
hepatomegaly, with dose-related hepatocytic hypertrophy and
vacuolation, and limited evidence of treatment-related hyperplasia and
tumorigenicity in the liver at the highest dose. The NOAEL was 10 ppm
(equal to 1.3 mg/kg bw per day). In a two-year study in rats with
dietary levels of 0, 8, 80, or 800 ppm, the predominant effects were
hepatocytic hypertrophy, thyroid follicular-cell hypertrophy, and an
increase in thyroid follicular-cell adenomas; in addition, thyroid
carcinomas were seen at the high dose. The NOAEL was 80 ppm, equal to
3.0 mg/kg bw per day.
The etiology of the hepatic and thyroidal effects in rats was
further investigated in a 4-13-week study which illustrated the
biological feedback mechanism in rats: hepatomegaly leading to
increased metabolism and excretion of thyroxine, increased levels of
thyroid stimulating hormone, and thyroid hypertrophy/hyperplasia. The
effects seen after four weeks in this study were reversible. In
studies designed to investigate the hepatotoxicity of fenbuconazole,
hepatic effects were seen in rats and mice that were similar to those
induced by phenobarbital. Increased cytochrome P450 activity (CYP2B
form) was observed, with hepatocellular hypertrophy and proliferation.
The NOAEL in mice after treatment for 13 weeks was 60 ppm (equal to 14
mg/kg bw per day).
Fenbuconazole was adequately tested for genotoxicity in vitro
and in vivo. The Meeting concluded that it is not genotoxic.
Fenbuconazole was not teratogenic in either rats (at doses of 0,
30, 75, or 150 mg/kg bw per day) or rabbits (at doses of 0, 10, 30, or
60 mg/kg bw per day), but fetotoxicity was seen in both species, with
an NOAEL of 30 mg/kg bw per day. The NOAELs for maternal toxicity were
30 mg/kg bw per day in rats and 10 mg/kg bw per day in rabbits. No
effects on reproductive parameters were seen in a multigeneration
study in rats at dietary levels of 0, 8, 80, or 800 ppm, but
fetotoxicity was again seen at high doses, with maternal toxicity. The
NOAEL was 80 ppm, equal to 5.8 mg/kg bw per day.
An ADI of 0-0.03 mg/kg bw was allocated, on the basis of the
NOAEL of 3 mg/kg bw per day in the two-year study in rats and a safety
factor of 100. The Meeting noted that the NOAEL in the 13-week study
in rats and in the 78-week study in mice was 1.3 mg/kg bw per day, but
it concluded that this figure should not be used to derive the ADI.
The NOAEL from the 13-week study in rats was not considered to be
relevant in the light of the results of the larger, two-year study.
The Meeting concluded that the overall NOAEL in mice was 14 mg/kg bw
per day. This figure was taken from the 13-week study, which included
detailed investigations of hepatotoxicity. Hepatotoxicity was the
critical effect in the long-term study in mice, and the NOAEL in the
13-week study was lower than the lowest dose that was hepatotoxic in
the long-term study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 60 ppm, equal to 14 mg/kg bw per day (13-week study of
hepatotoxicity)
10 ppm, equal to 1.3 mg/kg bw per day (78-week study of
toxicity)
Rat: 20 ppm, equal to 1.3 mg/kg bw per day (13-week study of
toxicity)
80 ppm, equal to 3.0 mg/kg bw per day (two-year study
of toxicity)
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenbuconazole
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral toxicity, rat LD50 > 2000 mg/kg bw
(1-7 days) Dermal toxicity, rat LD50 > 5000 mg/kg bw
Inhalation toxicity, rat LC50 > 2.1 mg/L
Dermal irritation, rabbit Not irritating
Ocular irritation, rabbit Not irritating
Dermal sensitization, guinea pig Not sensitizing in Buehler test, weakly
sensitizing in maximization test
Medium-term Repeated oral, 1-year, toxicity, dog NOAEL = 5.2 mg/kg bw per day: hepatic effects
(1-26 weeks) Repeated dermal, 4 weeks, toxicity, rat NOAEL = 1000 mg/kg bw per day (highest dose
tested)
Repeated oral, reproductive toxicity, rat NOAEL = 5.8 mg/kg bw per day: maternal and
fetal toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day: maternal
toxicity
Long term Repeated oral, 2 years, toxicity and NOAEL = 3 mg/kg bw per day: hepatic and
(> 1 year) carcinogenicity, rat thyroid effects
80 ppm, equal to 5.8 mg/kg bw per day (two-generation
study of reproductive toxicity)
30 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 150 ppm, equal to 5.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Anderson, D.M., DiDonato, L.J. & Longacre, S.L. (1988) 14C-RH-7592:
Range-finding kinetic and metabolite identification study in rats.
Unpublished study from Rohm & Haas Co., Report No. 87R-059 (European
Region reference No. 16.1).Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Bemacki, H.J. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-103 (European Region reference No. 8.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Bonin, R., Shade, W.D. & Hazelton, G.A. (1988) RH-7592 technical:
Delayed contact hypersensitivity study in guinea pigs. Unpublished
study from Rohm & Haas Co., Report No. 88R-027 (European Region
reference No. 4.3). Submitted to WHO by Rohm & Haas Co., Philadelphia,
PA, USA.
Cheng, T. (1990) RH-7592: Dermal absorption in male rats (preliminary
and definitive phases). Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 89RC-291 (European Region
reference No. 19.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Chism, E.M. (1984) RH-7592: Microbial mutagen test. Unpublished study
from Rohm & Haas Co., Report No. 84R-069 (European Region reference
No. 20.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
DiDonato, L.J. & Hazelton, G.A. (1993) 14-C-RH-7592: Disposition and
elimination study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 92R-060 (European Region reference No. 43.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Duchosal, F. & Thevenaz, P. (1989) 4-Hour, acute inhalation toxicity
study with RH-7592 technical in rats. Unpublished study from Research
and Consulting Co. AG. Rohm & Haas Co. report No. 89RC-023 (European
Region reference No. 16.2). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Harris, J.C. & Hazelton, G.A. (1988) RH-7592: Three-month dietary
toxicity study in mice. Unpublished study from Rohm & Haas Co., Report
No. 87R-090 (European Region reference No. 5.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Shade, W.D. (1988) RH-7592: Three-month dietary
toxicity study in dogs. Unpublished study from Rohm & Haas Co., Report
No. 87R-127 (European Region reference No. 6.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J., Quinn, D.L., Shade, W.D. & Frantz,
J.D. (1991) RH-7592: Thyroid function and hepatic clearance of
thyroxine in male rats. Unpublished study from Rohm & Haas Co., Report
No. 90R-071 (European Region reference No. 23.6). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A., DiDonato, L.J. & Lomax, L.G. (1995) RH-7592: Cell
proliferation and enzyme induction in the liver of female mice.
Unpublished study from Rohm & Haas Co., Report No. 95R-035. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Imamura, S., Mori, Y., Yamamori, I., Miura, Y., Oiso, Y., Seo, H.,
Matsui, N. & Refetoff, S. (1991) Molecular cloning and primary
structure of rat thyroxine-binding globulin. Biochemistry, 30,
5406-5411.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-7592: Acute
oral toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-001 (European Region reference No.
1.20). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-7592: Acute
dermal toxicity study in male and female rats. Unpublished study from
Rohm & Haas Co., Report No. 88R-002 (European Region reference No.
1.17). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) RH-7592: Acute
oral toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-098 (European Region reference No. 1.12). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) RH-7592: Acute
dermal toxicity study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-099 (European Region reference No. 1.11). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987c) RH-7592: Rabbit
skin irritation study. Unpublished study from Rohm & Haas Co., Report
No. 87R-100/87R-100A (European Region reference No. 1.9). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Morrison, R.D. & Baldwin, R.C. (1987d) RH-7592: Eye
irritation study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 87R-101/87R-101A (European Region reference No. 1.10).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lampé, K.R., Kulwich, B.A. & Baldwin, R.C. (1991) RH-7592 2F and
technical fungicides four-week dermal toxicity study in rats.
Unpublished study from Rohm & Haas Co., Report No. 90R-084/90R-084A
(European Region references No. 25.2 and 25.1). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
LeVan, L.W. (1990) 14C-RH-7592: Pharmacokinetic study in rats.
Unpublished study from Rohm & Haas Co., Report No. 88RC-071 (European
Region reference No. 14.9). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1994) RH-7592 technical: Acute oral toxicity
study in male and female rats. Unpublished study from Rohm & Haas Co.,
Report No. 94R-107 (European Region reference No. 47.4). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morgan, C. (1990) RH-7592: 52-Week oral (dietary administration)
toxicity study in the beagle. Unpublished study from Hazleton UK,
report No. 6464-616/5. Rohm & Haas Co. report No. 88RC-115 (European
Region reference No. 19.3). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Morris, T.D. (1994) RH-7592 technical: Delayed contact
hypersensitivity study in guinea pigs (maximization technique).
Unpublished study from Hill Top Biolabs, Inc. Rohm & Haas Co. report
No. 94RC-063. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Morrison, R.D. & Hazelton, G.A. (1986a) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in mice. Unpublished study from Rohm
& Haas Co., Report No. 86R-131 (European Region reference No. 5.5).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hazelton, G.A. (1986b) RH-7592: 2-Week dietary
toxicity range-finding (RF) study in rats. Unpublished study from Rohm
& Haas Co., Report No. 86R-130 (European Region reference No. 8.1).
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
O'Hara, G.P., Frantz, J.D. & Poorman, K.B. (1987) RH-7592: 4-Week
range finding study in male and female dogs. Unpublished study from
Rohm & Haas Co., Report No. 87R-075 (see Rohm & Haas Co. report No.
87R-127, Appendix 13). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1991) RH-7592: 4-Week (dietary administration)
toxicity study in the beagle. Unpublished study from Hazelton, United
Kingdom. Rohm & Haas Co. report No. 88RC-097 (European Region
reference No. 30.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-7592: Salmonella typhimurium gene
mutation assay (Ames). Unpublished study from Rohm & Haas Co., Report
No. 92R-195 (European Region reference No. 42.5). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1987) RH-7592: Microbial mutagenicity
assay. Unpublished study from Rohm & Haas Co., Report No. 87R-044
(European Region reference No. 1.24). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Sames, J.L. & Frank, J.P. (1988) RH-7592: Salmonella typhimurium
gene mutation assay. Unpublished study from Rohm & Haas Co., Report
No. 88R-009 (European Region reference No. 1.23). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sarwar, G. & Suzuki, K. (1994) DNA repair test of H-7592 technical.
Unpublished study from Nippon Experimental Medical Research Institute
Co. Rohm & Haas Co. report No. 94RC-114 (European Region reference No.
48.7). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Kulwich, B.A. (1990) RH-7592: Two-generation
reproduction study in rats. Unpublished study from Rohm & Haas Co.,
Report No. 88R-241 (European Region reference No. 15.2). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1987) RH-7592: Oral (gavage) developmental
toxicity screen in rats. Unpublished study from Rohm & Haas Co.,
Report No. 87R-014 (see Rohm & Haas Co. report No. 87R-065, Appendix
L, p. 197). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Solomon, H.M. & Lutz, M.F. (1988) RH-7592: Oral (gavage) developmental
toxicity study in rats. Unpublished study from Rohm & Haas Co., Report
No. 87R-014 (European Region reference No. 1.22). Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Lutz, M.F. (1989) RH-7592: Oral (gavage) developmental
toxicity study in rabbits. Unpublished study from Rohm & Haas Co.,
Report No. 88R-195 (European Region reference No. 12.1). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988a) RH-7592 technical: Test for chemical induction of
gene mutation at the HGRPT locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Unpublished study
from Sitek Research Laboratories. Rohm & Haas Co. report No. 88RC-062
(European Region reference No. 7.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Thilagar, A. (1988b) RH-7592 technical: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished study from Sitek Research Laboratories.
Rohm & Haas Co. report No. 88RC-061 (European Region reference No.
7.5). Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1988c) RH-7592 technical: Acute test for chemical
induction of chromosome aberration in rat bone marrow cells in vivo.
Unpublished study from Sitek Research Laboratories. Rohm & Haas Co.
report No. 88RC-063 (European Region reference No. 7.3). Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990) Test for chemical induction of chromosome
aberration using monolayer cultures of Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished study from
Sitek Research Laboratories. Rohm & Haas Co. report No. 89RC-090
(European Region reference No. 14.8). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1989) 13-Week dietary toxicity range-finding (RF) study
in mice with RH-7592. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-112 (European Region
reference No. 13.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1990) RH-7592 technical: 24-Month dietary chronic
toxicity-oncogenicity study in rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-098
(European Region reference No. 18.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Wolfe, G.W. (1991a) RH-7592 technical: 78-Week dietary oncogenicity
toxicity study in mice. Unpublished study from Hazelton Laboratories
America, Inc. Rohm & Haas Co. report No. 88RC-107 (European Region
reference No. 29.1). Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Wolfe, G.W. (1991b) RH-7592 technical: 104-Week chronic oncogenicity
toxicity study in male rats. Unpublished study from Hazelton
Laboratories America, Inc. Rohm & Haas Co. report No. 88RC-116
(European Region reference No. 32.1). Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
