
BENTAZONE (addendum) JMPR 1998
First draft prepared by
A. Protzel
US Environmental Protection Agency
Washington DC, United States
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Genotoxicity
Developmental toxicity
Studies of metabolites
Acute toxicity
Short-term studies of toxicity
Genotoxicity
Developmental toxicity
Comments
Toxicological evaluation
References
Explanation
Bentazone was first evaluated by the Joint Meeting in 1991 (Annex
1, reference 62), when an ADI of 0-0.1 mg/kg bw was allocated on the
basis of a NOAEL of 9 mg/kg bw per day in a long-term study of
toxicity in rats and a safety factor of 100. Further observations in
humans, a 90-day feeding study in rats with 6-hydroxybentazone, and
studies of genotoxicity with 6-hydroxybentazone were identified as
valuable in the continued evaluation of the compound.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Male and female CD rats were given a single intravenous dose of
4 mg/kg bw of [phenyl-U-14C] bentazone as the sodium salt, a single
oral dose of 4 or 200 mg/kg bw free acid, or a single oral dose of
4 mg/kg bw free acid after a 14-day pretreatment with unlabelled
bentazone at approximately 4 mg/kg bw per day. Recovery of radiolabel
in urine after oral dosing indicated extensive absorption from the
gastrointestinal tract. By 24 h after the single oral dose, 83-94% of
the radiolabel appeared in urine. Total recovery of radiolabel 120 h
after dosing accounted for 90-97% of the dose in the animals treated
orally and 90-95% of the dose in the animals treated intravenously.
After oral administration, 88-96% of the dose was eliminated in urine
and 0.8-2.3% in faeces over the 120-h collection period, most being
eliminated within the first 24 h. No difference between the sexes or
among dose groups was seen. Experiments with bile-duct-cannulated rats
indicated that only 0.24-1.3% of the dose of 4 mg/kg bw and 0.3-1.8%
of the dose of 200 mg/kg bw was eliminated in the bile over a 48-h
collection period.
In a parallel series of studies, CD rats of each sex were given a
single dose of 4 mg/kg bw [phenyl-U-14C]-bentazone (sodium salt)
intravenously or an oral dose of 4 (free acid or sodium salt) or
200 mg/kg bw (free acid). The area under the curve (AUC) for radioabel
in plasma per unit dose for animals that received the high dose was
nearly double that of rats given the low dose, suggesting that a non-
linear region in disposition was reached at the high dose.
Additionally, the AUC values for females given the low dose were
nearly one-half those of females dosed intravenously; the
corresponding values for males were not significantly different. The
biological significance of the difference in bioavailability in
females is unclear in view of the similar, extensive absorption of the
compound (about 90%) in both males and females, the limited metabolism
of the compound, and the apparently similar rates of excretion in the
two sexes. At sacrifice at 120 h, the total radiolabel in carcasses
represented less than 0.69% of the dose in all groups. Whole-body
autoradiography indicated steady disappearance of the la°il with time.
Signed and dated statements of compliance with good laboratory
practice (GLP) (40 CFR 160.35) and quality assurance were provided
with the study. This study satisfies the FIFRA Subdivision F Guideline
requirement for an 85-1 study of general metabolism (Hawkins et al.,
1987).
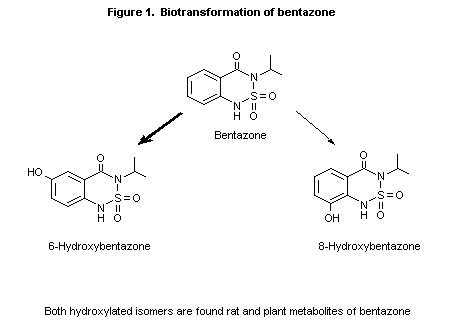
(b) Biotransformation
In the study of Hawkins et al. (1987), described above, parent
bentazone was the major excretion product in the urine of treated rats
during the 24-h collection period, representing 81-91% of the dose in
males and 77-89% in females. 6-Hydroxybentazone represented up to 6.3%
of the dose, and the isomeric 8-hydroxybentazone was present in trace
amounts, 0-0.23% of the dose (Figure 1). Although slightly less parent
bentazone was excreted in the urine of females and less
6-hydroxybentazone was excreted by rats treated intravenously (0.98%
of the dose versus 2.4-6.3% in rats given the low oral dose), there
were no major dose-dependent differences among the groups. Little or
no glucuronide or sulfate conjugation was seen.
In a previous study, adult male CD rats received 14C-bentazone,
presumably [phenyl-U-14C]-labelled, as a single oral dose of 4 mg/kg
bw of the sodium salt (expressed as the free acid), and metabolites in
the urine were analysed by thin-layer chromatography. Parent bentazone
represented 65, 15, and 3.2% of the dose in urine collected 0-6, 6-12,
and 12-24 h after dosing, respectively, for a total of 83% of the
dose; 6-hydroxybentazone represented 1.7, 0.4, and 0.1% of the dose at
 those times. 8-Hydroxybentazone was not detected. Polar radioactive
compounds at the origin represented 2.1% of the dose in the 0-24-h
period (Hawkins et al., 1986).
2. Toxicological studies
(a) Acute toxicity
Bentazone is more acutely toxic to rats by the oral route than
its 6-hydroxy or 8-hydroxy metabolites. The LD50 values for
technical-grade bentazone (purity unspecified) suspended in aqueous
carboxymethyl cellulose in Wistar rats were 1800 mg/kg bw for males,
1500 mg/kg bw for females, and 1600 mg/kg bw for males and females
combined. The LD50 value for males was estimated from deaths at four
doses, with no statistical analysis; the values for females and for
males and females combined were determined by probit analysis.
Dyspnoea and apathy were noted at doses of 825 mg/kg bw and higher,
and staggering occurred at the highest dose, 2610 mg/kg bw per day.
Animals at this dose that died had bloody ulcerations in the stomach
and intestinal contents mixed with blood (Hildebrand & Kirsch, 1982).
(b) Short-term studies of toxicity
Rats
Groups of 10 Wistar KFM-Han, outbred SPF quality rats of each sex
received technical-grade bentazone (purity, 97.8%) at a dietary level
of 0, 400, 1200, or 3600 ppm for 13 weeks, equal to doses of 0, 25,
78, and 240 mg/kg bw per day in males and 0, 29, 86, and 260 mg/kg bw
per day in females. Twenty additional rats were used for a 28-day
recovery experiment. Ten rats received 3600 ppm bentazone for 13 weeks
and were then given regular diet and observed for an additional 28
days; 10 rats served as untreated controls in the recovery experiment.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmic, urinary, haematological, and clinical
chemical parameters; organ weights; and gross and histopathological
alterations.
One male and two females at the high dose died, one of the
females under anesthesia; no signs of toxicity were reported. The body
weights of females at this dose were statistically significantly lower
than those of controls from week 10 onwards; there were no effects on
the body weights of males, and no statistically significant
differences in body weights were seen during the recovery period. The
food consumption of males at the high dose was slightly increased and
became statistically significantly greater than that of controls from
week 7 onwards; the food consumption of females was generally not
affected. No ophthalmological effects were reported. Statistically
significant increases in prothrombin time and partial thromboplastin
time were seen in males at the high dose, whereas females at all doses
had statistically significantly depressed prothrombin time and the
partial thromboplastin time was not affected. The values for certain
clinical chemical parameters were statistically significantly
different from those of controls in males at the high dose, but they
were generally within the historical control values and returned to
normal during the recovery period; the values for females were within
the historical control range.
Bentazone had a diuretic effect in animals of each sex. In both
males and females, the urine volume was increased in a dose-related
manner, becoming significantly different from that of controls at
3600 ppm. The specific gravity was decreased in animals of each sex;
the decrease was dose-related in males and was statistically
significantly different from that of controls at the high dose; in
females, the decrease was significantly different from that of
controls at all doses but did not decrease monotonically. The mean
absolute and relative kidney weights were statistically significantly
greater than those of controls in males at 3600 ppm; in females,
although both the mean absolute and relative kidney weights were
greater than those of controls, the values reached statistical
significance only for absolute weights. The only change in liver
weights was a statistically significantly increase in relative liver
weight in females at 3600 ppm. Gross examination revealed lung thrombi
in 1/10 controls and 3/10 females at the high dose and dilated uterine
horns in 1/10 controls and 3/9 females at the high dose. No
statistically significant histopathological findings were made. The
NOAEL for systemic toxicity was 1200 ppm, equal to 78 mg/kg bw per
day, and the LOAEL was 3600 ppm, equalto 240 mg/kg bw per day, on the
basis of statistically significant decreased body weights of females
throughout the latter part of treatment, increased prothrombin time
and partial thromboplastin time in males, increased urinary output
with decreased specific gravity in animals of each sex, and some
degree of kidney hypertrophy in both males and females (Tennekes et
al., 1987).
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of bentazone (purity, 97.6%) in
Tylose CB in 0.5% aqueous suspension under semiocclusion for 6 h once
a day for 21 days at a dose of 0, 250, 500, or 1000 mg/kg bw per day.
No deaths or clinical signs were seen in animals of either sex at any
dose, and no adverse effects were seen on treated skin. The NOAEL was
1000 mg/kg bw per day, the highest dose tested (Kirsch, 1993).
(c) Genotoxicity
Bentazone was considered not to be genotoxic in vitro or
in vivo in the previous evaluation. It has been tested only as a
compound of 96.7% purity for reverse mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, at
concentrations of 20-5000 µg per plate, in the presence and absence of
an exogenous metabolic activation system. Negative results were
obtained (Gelbke & Engelhardt, 1983).
(d) Developmental toxicity
Rats
In a study of developmental toxicity, pregnant Wistar/HAN rats
(Kfm: WIST, outbred, SPF quality) received technical-grade bentazone
(purity, 97.8%) mixed with 4% carboxymethyl cellulose in distilled
water by gavage at a dose of 0, 40, 100, or 250 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for mortality, clinical
signs, body weight, and food consumption; post mortem, the uterus
was removed, weighed, and opened for internal examination. The fetuses
were examined for sex, weight, and gross external abnormalities, and
underwent visceral (slice technique) and skeletal examinations. No
signs or symptoms of compound-related toxicity were reported, and
there were no effects on body weight, body-weight gain, or food
consumption. The litter incidence of fetal resorptions was increased
in dams at 250 mg/kg bw per day as compared with controls, and the
difference in total number was statistically significant (0 in
controls and 44 at the high dose). There was a small but statistically
significant decrease in mean fetal weight at the high dose (4.8 g in
controls versus 4.3 g). The rate of ossification in the phalangeal
nuclei of fore- and hindlimb digits, the fifth sternebra, and cervical
vertebrae was decreased, and the litter incidence of phalangeal nuclei
with delayed ossification was statistically significant at the high
dose (chi2, p < 0.05). The NOAEL for maternal toxicity was
250 mg/kg bw per day, the highest dose tested, and that for
developmental toxicity was 100 mg/kg bw per day on the basis of
statistically significantly decreased mean fetal weights and delays in
tissue ossification at the high dose (Becker at al., 1986).
3. Studies of metabolites
(a) Acute toxicity
The acute oral LD50 values for 6-hydroxy- and 8-hydroxybentazone
suspended in aqueous carboxymethyl cellulose in male and female Wistar
rats were > 5000 mg/kg bw. The 6-hydroxy compound was > 98%, and
the 8-hydroxy compound was > 98.5% pure. Two males that received 5000
mg/kg bw 8-hydroxybentazone died, but no deaths occurred among females
at any dose (2150, 3830, or 5000 mg/kg bw) or among any of the animals
given the 6-hydroxy metabolite. Necropsy of the rats that died after
intake of 5000 mg/kg bw 8-hydroxybentazone showed general congestion
(Kirsch & Kieczka, 1987). No lesions were found at necropsy in animals
given 5000 mg/kg bw 6-hydroxy-bentazone (Kirsch & Kieczka, 1986).
(b) Short-term studies of toxicity
Groups of 10 Wistar rats of each sex received 8-hydroxybentazone
(99.9% active ingredient) in the diet at 0, 400, 1200, or 3600 ppm for
three months, equal to doses of 0, 28, 85, and 260 mg/kg bw for males
and 0, 34, 100, and 300 for females. No compound-related effects were
seen on mortality, clinical signs, body weight, food consumption,
haematological, clinical chemical, or urinary parameters, organ
weights, or gross or histopathological appearance. In particular,
there was no significant effect on thromboplastin time at 45 or 94
days. The NOAEL was 3600 ppm, equal to 260 mg/kg bw per day, the
highest dose tested (Mellert et al., 1993)
(c) Genotoxicity
Studies on the genotoxicity of metabolites of bentazone are
summarized in Table 1. The 6- and 8-hydroxy isomers of bentazone also
gave negative results in assays for reverse mutation, with and without
microsomal activation (Gelbke & Engelhardt, 1987a,b).
8-Hydroxybentazone gave negative results in an assay for gene mutation
at the hprt locus in Chinese hamster V79 cells, with and without
metabolic activation (Mullerschon, 1992) and in an assay for
micronucleus formation in mice treated in vivo (Gelbke, 1993).
(d) Developmental toxicity
In a range-finding study for developmental toxicity, groups of
nine or 10 pregnant Wistar (Chbb:THOM SPF) rats received
8-hydroxybentazone (purity unspecified) in 0.5% aqueous carboxymethyl
cellulose (Tylose CB 30 000) at a dose of 0 or 300 mg/kg bw per day on
days 6-15 of gestation. Maternal toxicity was observed, consisting of
significant decreases in body-weight gain (89%) and food consumption
during days 6-8 of gestation. The frequency of post-implantation loss
(12.6%) was greater than that in concurrent controls (4.6%), but this
finding was within the range for historical controls (4.5-15.7%)
(BASF, 1992).
In a study of developmental toxicity, groups of 25 pregnant
Wistar (Chbb:THOM SPF) rats received 8-hydroxybentazone (purity,
99.9%) in 0.5% aqueous carboxymethyl cellulose (Tylose CB 30,000) at a
dose of 0, 40, 100, or 250 mg/kg bw per day on days 6-15 of gestation.
The dams were observed for deaths, clinical signs, body weight, and
food consumption; post mortem, the uterus was removed, weighed, and
opened for internal examination. The fetuses were observed for sex,
weight, and gross external abnormalities, and underwent visceral
(slice technique) and skeletal examinations. There was no maternal or
developmental toxicity. The NOAEL for both maternal and developmental
toxicity was 250 mg/kg bw per day, the highest dose tested (Hellwig &
Hildebrand, 1993).
Comments
After oral administration to rats, [phenyl-U-14C]-bentazone was
extensively absorbed and rapidly excreted in the urine. In rats given
a single dose, 83-94% appeared in the urine by 24 h and 90-97% by
120 h after dosing, with less than 0.7% in the residual carcase.
Biliary excretion of the compound amounted to less than 2% of the
dose. Bentazone undergoes very limited biotransformation in rats.
Bentazone was the major compound identified in urine, representing
81-91% of the dose in males and 77-89% in females. 6-Hydroxybentazone
was present in amounts up to 6.3% of the dose, and isomeric
Table 1. Results of assays for the genotoxicity of the 6-hydroxy and 8-hydroxy metabolites of bentazone
End-point Test object Concentration Purity Results Reference
(%)
6-Hydroxybentazone
Reverse mutation S. typhimurium TA98, 20-5000 µg/plate > 98 Negative Gelbke & Engelhardt
TA100, TA1535, TA1537 ± S9 (1987a)
8-Hydroxybentazone
Reverse mutation S. typhimurium 20-5000 µg/plate > 98.5 Negative Gelbke & Engelhardt
TA98,TA100, TA1535, ± S9 (1987b)
TA1537
Gene mutation Chinese hamster Trial 1: 99.9 Negative Mullerschon (1992)
V79 cells, hprt locus 55-2002 µg/ml -S9
495-5005 µg/ml +S9
Trial 2:
300-3000 µg/ml -S9
500-5000 µg/ml +S9
Micronucleus formation NMRI mouse in vivo 625, 1250, 2500 mg/kg bw 99.9 Negative Gelbke (1993)
8-hydroxybentazone was present in trace amounts (0-0.23% of the dose).
There were no major differences among the groups. Glucuronide or
sulfate conjugation was either negligible or nonexistent; 6- and
8-hydroxybentazone are also metabolites of bentazone in plants.
Bentazone is more acutely toxic to rats than are its two
hydroxylated metabolites when given by the oral route. The acute oral
LD50 of technical-grade bentazone was estimated to be 1800 mg/kg bw
in males and 1500 mg/kg bw in females. The acute oral LD50 value for
6- and 8-hydroxybentazone was 5000 mg/kg bw.
WHO has classified bentazone as slightly hazardous (WHO, 1996).
The two studies described below indicate that 8-hydroxybentazone
does not have the anticoagulant and diuretic effects of bentazone at
the doses tested and has less systemic toxicity than the parent
compound under the test conditions. No data were available on the
short-term toxicity of 6-hydroxybentazone.
Rats received technical-grade bentazone in the diet at
concentrations of 0, 400, 1200, or 3600 ppm for 13 weeks. The body
weights of females were decreased and were statistically significantly
different from those of controls at 3600 ppm from week 10 onward.
Examination of haematological parameters indicated statistically
significant increases in prothrombin time and partial thromboplastin
time in males at 3600 ppm in comparison with controls. Bentazone had a
diuretic effect in animals of each sex, reaching statistical
significance at 3600 ppm. The NOAEL for systemic toxicity was 1200 ppm
(equal to 78 mg/kg bw per day) on the basis of statistically
significant decreased body weights in females throughout the latter
part of the treatment, increased prothrombin time and partial
thromboplastin time in males, increased output of urine with decreased
specific gravity in animals of each sex, and some degree of kidney
hypertrophy in both males and females at 3600 ppm, equal to 240 mg/kg
bw per day.
Rats received 8-hydroxybentazone in the diet at concentrations of
0, 400, 1200, or 3600 ppm for three months. No compound-related
effects were observed on body weights, clinical signs, food
consumption, haematological, clinical chemical, or urinary parameters,
clotting time, organ weights, or gross or histopathological
appearance. The NOAEL was 3600 ppm (equal to 260 mg/kg bw per day),
the highest dose tested.
The following two studies of developmental toxicity indicate that
bentazone has effects at doses below a maternally toxic dose, whereas
8-hydroxybentazone had no developmental or maternal toxicity at any of
the doses tested.
Pregnant rats received technical-grade bentazone by gavage at 0,
40, 100, or 250 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 250 mg/kg bw per day, the highest dose
tested. The NOAEL for developmental toxicity was 100 mg/kg bw per day
on the basis of significantly decreased mean fetal weights and delays
in tissue ossification, which reached statistical significance on a
litter basis at the highest dose.
No developmental toxicity was observed in pregnant rats that
received 8-hydroxybentazone by gavage at 0, 40, 100, or 250 mg/kg bw
per day on days 6-15 of gestation. The NOAEL for developmental
toxicity was 250 mg/kg bw per day, the highest dose tested.
Bentazone, 6-hydroxybentazone, and 8-hydroxybentazone did not
induce reverse mutation in bacteria, and 8-hydroxybentazone did not
induce gene mutation in mammalian cells or micronucleus formation in
mice in vivo. The Meeting concluded that neither bentazone nor its
metabolites are genotoxic.
8-Hydroxybentazone was less toxic than the parent compound, and,
on the basis of the structural similarities between the 6- and
8-hydroxy isomers, the Meeting concluded that the 6-hydroxy isomer is
also less toxic than the parent. Therefore, the Meeting maintained the
ADI of 0-0.1 mg/kg bw for bentazone.
Because this was a limited review, data were not evaluated that
would permit the establish-ment of an acute reference dose.
Toxicological Evaluation
Levels that cause no toxic effect
Bentazone
Mouse: 100 ppm, equal to 12 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
1200 ppm, equal to 78 mg/kg bw per day (13-week study
of toxicity)
250 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (developmental toxicity)
Dog: 400 ppm, equal to 13 mg/kg bw per day (one-year study
of toxicity)
8-Hydroxybentazone
Rat: 3600 ppm, equal to 260 mg/kg bw per day (three-month
study of toxicity)
250 mg/kg bw per day (maternal and developmental
toxicity in study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Not considered
List of end-points relevant for comparing the toxicities of bentazone, 6-hydroxybentazone
and 8-hydroxybentazone
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 83-94% rapidly absorbed (bentazone)
Dermal absorption No data
Distribution Extensive
Potential for accumulation Little or none for bentazone; no data on metabolites
Rate and extent of excretion Rapid excretion: 83-94% of a dose excreted in urine
within 24 h (bentazone)
Metabolism in animals Very little biotransformation: 81-91%of a dose
excreted untransformed. Metabolites are
6-hydroxybentazone (6.3% of dose) and
8-hydroxybentazone (0-0.23% of dose)
Toxicologically significant compounds Bentazone
(animals, plants and environment)
Acute toxicity
Rat LD50 oral Bentazone: 1500 mg/kg bw
6-Hydroxybentazone: > 5000 mg/kg bw
8-Hydroxybentazone: > 5000 mg/kg bw
Short-term toxicity
Target/critical effect Bentazone: decreased body weights in females,
increased clotting times (prothrombin time and
partial thromboplastin time) and increased output
of urine with decreased specific gravity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no effect up to highest dose
tested
Lowest relevant oral NOAEL Rat: Bentazone: 90 days, 78 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 260 mg/kg bw per day,
highest dose tested
Lowest relevant dermal NOAEL Bentazone: 1000 mg/kg bw per day (highest dose
tested)
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Lowest relevant inhalation NOAEL No data
Genotoxicity Bentazone and its metabolites are not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect No data
Lowest relevant NOAEL No data
Carcinogenicity Bentazone: no carcinogenicity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Reproductive toxicity
Reproduction target/critical effect No data
Lowest relevant reproductive NOAEL No data
Developmental target/critical effect Bentazone: developmental effects (decreased fetal
weights and delayed ossification) below maternally
toxic dose
6-Hydroxybentazone: no data
8-Hydroxybentazone: no developmental toxicity at highest dose tested
Lowest relevant developmental NOAEL Rat: Bentazone: 100 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 250 mg/kg bw per day
Neurotoxicity / Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw Long-term toxicity, rats 100
Acute reference dose Not considered
References
BASF (1992) Results of a range-finding prenatal toxicity study with
8OH-bentazon in rats after oral administration (gavage). Unpublished
study dated January/February 1992. Project No. 10R0437/91061 from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Becker, H., Frei, D., Vogel, W. & Terrier, C. (1986) Embryotoxicity
(including teratogenicity) study with bentazone technical in the rat.
Unpublished report, BASF RZ-Report No. 86/421 dated 30 December 1986
from RCC Research and Consulting Company AG, Itingen, Switzerland, RCC
Report No. 064530. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. (1993) Cytogenetic study in vivo of 8-OH-bentazon in mice
micronucleus test single oral administration. Unpublished document No.
BASF 93/10424 dated 6 May 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project
No.26M0437/914319. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of Reg. No.
51 929 (bentazone) (ZNT test substance No. 83/3) in the Ames test
(standard plate test with Salmonella typhimurium). Unpublished
document No. BASF 83/222 dated 10 June 1983 (original German report)
and 26 October 1983 (English translation) from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Gelbke, H.P. & Engelhardt, G. (1987a) Report on the study of
6-hydroxy-bentazon (ZNT test substance No. 86/244) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/023 dated 15 January
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0244/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1987b) Report on the study of
8-hydroxy-bentazon (ZNT test substance No. 86/391) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/0168 dated 14 May
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0391/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hawkins, D.R., Elsom, L.F. & Girkin, R. (1986) Report on the urinary
metabolites of bentazone in the rat. Unpublished report, document No.
BASF 86/090 dated 31 January 1986 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigs-hafen, Germany.
Hawkins, D.R., Mayo, B.C., Pollard, A.D., Green, S.L., Biggs, S.R. &
Whitby, B.R. (1987) The biokinetics and metabolism of 14C-bentazon in
rats. Unpublished report, document No. BASF 87/0429 dated 16 September
1987 from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hellwig, J. & Hildebrand, B. (1993) Study of the prenatal toxicity of
8-OH-bentazon in rats after oral administration (gavage). Unpublished
report, document No. BASF 93/10572 dated 9 June 1993 from BASF
Aktiengesellschaft, Abteilung Toxicologie, Ludwigshafen/Rhein,
Germany, report No. 30R0437/91081. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany
Hildebrand, B. & Kirsch, P. (1982) Report on the study of the acute
oral toxicity in rats of Reg. No. 51 929 -- bentazone. Unpublished
document No. BASF 83/114 dated 30 November 1982 (original in German);
English translation dated 21 June 1983 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. (1993) Study of the dermal toxicity of Reg. No. 51 929
(bentazone) in white rabbits: Application to the intact skin over 3
weeks. Unpublished report, document No. BASF 93/10760 dated 9 June
1993 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No. 41H0243/91042. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1986) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/002 dated 29 December 1986 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1987) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/030 dated 21 January 1987 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Mellert, W., Deckardt, K., Kuttler, K. & Hildebrand, B. (1993)
Subchronic oral toxicity study with 8-OH-bentazon in Wistar rats.
Administration in the diet for 3 months. Unpublished report No. BASF
93/11011 dated 13 September 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project No.
31S0437/91060. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Mullerschon (1992) Gene mutation assay in Chinese hamster V79 cells in
vitro with 8-OH bentazone. Unpublished report No. BASF 92/11329 dated
20 October 1992 from CCR Cytotest Cell Research GmbH & Co. KG,
Germany. BASF Project No. 50M0437/919018. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
Tennekes, H., Horst, K., Luetkemeier, H., Wilson, J., Vogel, W. &
Terrier, C. (1987) Report on the 13-week oral toxicity (feeding) study
with bentazone technical in the rat. Unpublished report, document No.
BASF 87/0173 dated 12 May 1987 from RCC Research and Consulting
Company AG, Itingen, Switzerland. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
those times. 8-Hydroxybentazone was not detected. Polar radioactive
compounds at the origin represented 2.1% of the dose in the 0-24-h
period (Hawkins et al., 1986).
2. Toxicological studies
(a) Acute toxicity
Bentazone is more acutely toxic to rats by the oral route than
its 6-hydroxy or 8-hydroxy metabolites. The LD50 values for
technical-grade bentazone (purity unspecified) suspended in aqueous
carboxymethyl cellulose in Wistar rats were 1800 mg/kg bw for males,
1500 mg/kg bw for females, and 1600 mg/kg bw for males and females
combined. The LD50 value for males was estimated from deaths at four
doses, with no statistical analysis; the values for females and for
males and females combined were determined by probit analysis.
Dyspnoea and apathy were noted at doses of 825 mg/kg bw and higher,
and staggering occurred at the highest dose, 2610 mg/kg bw per day.
Animals at this dose that died had bloody ulcerations in the stomach
and intestinal contents mixed with blood (Hildebrand & Kirsch, 1982).
(b) Short-term studies of toxicity
Rats
Groups of 10 Wistar KFM-Han, outbred SPF quality rats of each sex
received technical-grade bentazone (purity, 97.8%) at a dietary level
of 0, 400, 1200, or 3600 ppm for 13 weeks, equal to doses of 0, 25,
78, and 240 mg/kg bw per day in males and 0, 29, 86, and 260 mg/kg bw
per day in females. Twenty additional rats were used for a 28-day
recovery experiment. Ten rats received 3600 ppm bentazone for 13 weeks
and were then given regular diet and observed for an additional 28
days; 10 rats served as untreated controls in the recovery experiment.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmic, urinary, haematological, and clinical
chemical parameters; organ weights; and gross and histopathological
alterations.
One male and two females at the high dose died, one of the
females under anesthesia; no signs of toxicity were reported. The body
weights of females at this dose were statistically significantly lower
than those of controls from week 10 onwards; there were no effects on
the body weights of males, and no statistically significant
differences in body weights were seen during the recovery period. The
food consumption of males at the high dose was slightly increased and
became statistically significantly greater than that of controls from
week 7 onwards; the food consumption of females was generally not
affected. No ophthalmological effects were reported. Statistically
significant increases in prothrombin time and partial thromboplastin
time were seen in males at the high dose, whereas females at all doses
had statistically significantly depressed prothrombin time and the
partial thromboplastin time was not affected. The values for certain
clinical chemical parameters were statistically significantly
different from those of controls in males at the high dose, but they
were generally within the historical control values and returned to
normal during the recovery period; the values for females were within
the historical control range.
Bentazone had a diuretic effect in animals of each sex. In both
males and females, the urine volume was increased in a dose-related
manner, becoming significantly different from that of controls at
3600 ppm. The specific gravity was decreased in animals of each sex;
the decrease was dose-related in males and was statistically
significantly different from that of controls at the high dose; in
females, the decrease was significantly different from that of
controls at all doses but did not decrease monotonically. The mean
absolute and relative kidney weights were statistically significantly
greater than those of controls in males at 3600 ppm; in females,
although both the mean absolute and relative kidney weights were
greater than those of controls, the values reached statistical
significance only for absolute weights. The only change in liver
weights was a statistically significantly increase in relative liver
weight in females at 3600 ppm. Gross examination revealed lung thrombi
in 1/10 controls and 3/10 females at the high dose and dilated uterine
horns in 1/10 controls and 3/9 females at the high dose. No
statistically significant histopathological findings were made. The
NOAEL for systemic toxicity was 1200 ppm, equal to 78 mg/kg bw per
day, and the LOAEL was 3600 ppm, equalto 240 mg/kg bw per day, on the
basis of statistically significant decreased body weights of females
throughout the latter part of treatment, increased prothrombin time
and partial thromboplastin time in males, increased urinary output
with decreased specific gravity in animals of each sex, and some
degree of kidney hypertrophy in both males and females (Tennekes et
al., 1987).
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of bentazone (purity, 97.6%) in
Tylose CB in 0.5% aqueous suspension under semiocclusion for 6 h once
a day for 21 days at a dose of 0, 250, 500, or 1000 mg/kg bw per day.
No deaths or clinical signs were seen in animals of either sex at any
dose, and no adverse effects were seen on treated skin. The NOAEL was
1000 mg/kg bw per day, the highest dose tested (Kirsch, 1993).
(c) Genotoxicity
Bentazone was considered not to be genotoxic in vitro or
in vivo in the previous evaluation. It has been tested only as a
compound of 96.7% purity for reverse mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, at
concentrations of 20-5000 µg per plate, in the presence and absence of
an exogenous metabolic activation system. Negative results were
obtained (Gelbke & Engelhardt, 1983).
(d) Developmental toxicity
Rats
In a study of developmental toxicity, pregnant Wistar/HAN rats
(Kfm: WIST, outbred, SPF quality) received technical-grade bentazone
(purity, 97.8%) mixed with 4% carboxymethyl cellulose in distilled
water by gavage at a dose of 0, 40, 100, or 250 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for mortality, clinical
signs, body weight, and food consumption; post mortem, the uterus
was removed, weighed, and opened for internal examination. The fetuses
were examined for sex, weight, and gross external abnormalities, and
underwent visceral (slice technique) and skeletal examinations. No
signs or symptoms of compound-related toxicity were reported, and
there were no effects on body weight, body-weight gain, or food
consumption. The litter incidence of fetal resorptions was increased
in dams at 250 mg/kg bw per day as compared with controls, and the
difference in total number was statistically significant (0 in
controls and 44 at the high dose). There was a small but statistically
significant decrease in mean fetal weight at the high dose (4.8 g in
controls versus 4.3 g). The rate of ossification in the phalangeal
nuclei of fore- and hindlimb digits, the fifth sternebra, and cervical
vertebrae was decreased, and the litter incidence of phalangeal nuclei
with delayed ossification was statistically significant at the high
dose (chi2, p < 0.05). The NOAEL for maternal toxicity was
250 mg/kg bw per day, the highest dose tested, and that for
developmental toxicity was 100 mg/kg bw per day on the basis of
statistically significantly decreased mean fetal weights and delays in
tissue ossification at the high dose (Becker at al., 1986).
3. Studies of metabolites
(a) Acute toxicity
The acute oral LD50 values for 6-hydroxy- and 8-hydroxybentazone
suspended in aqueous carboxymethyl cellulose in male and female Wistar
rats were > 5000 mg/kg bw. The 6-hydroxy compound was > 98%, and
the 8-hydroxy compound was > 98.5% pure. Two males that received 5000
mg/kg bw 8-hydroxybentazone died, but no deaths occurred among females
at any dose (2150, 3830, or 5000 mg/kg bw) or among any of the animals
given the 6-hydroxy metabolite. Necropsy of the rats that died after
intake of 5000 mg/kg bw 8-hydroxybentazone showed general congestion
(Kirsch & Kieczka, 1987). No lesions were found at necropsy in animals
given 5000 mg/kg bw 6-hydroxy-bentazone (Kirsch & Kieczka, 1986).
(b) Short-term studies of toxicity
Groups of 10 Wistar rats of each sex received 8-hydroxybentazone
(99.9% active ingredient) in the diet at 0, 400, 1200, or 3600 ppm for
three months, equal to doses of 0, 28, 85, and 260 mg/kg bw for males
and 0, 34, 100, and 300 for females. No compound-related effects were
seen on mortality, clinical signs, body weight, food consumption,
haematological, clinical chemical, or urinary parameters, organ
weights, or gross or histopathological appearance. In particular,
there was no significant effect on thromboplastin time at 45 or 94
days. The NOAEL was 3600 ppm, equal to 260 mg/kg bw per day, the
highest dose tested (Mellert et al., 1993)
(c) Genotoxicity
Studies on the genotoxicity of metabolites of bentazone are
summarized in Table 1. The 6- and 8-hydroxy isomers of bentazone also
gave negative results in assays for reverse mutation, with and without
microsomal activation (Gelbke & Engelhardt, 1987a,b).
8-Hydroxybentazone gave negative results in an assay for gene mutation
at the hprt locus in Chinese hamster V79 cells, with and without
metabolic activation (Mullerschon, 1992) and in an assay for
micronucleus formation in mice treated in vivo (Gelbke, 1993).
(d) Developmental toxicity
In a range-finding study for developmental toxicity, groups of
nine or 10 pregnant Wistar (Chbb:THOM SPF) rats received
8-hydroxybentazone (purity unspecified) in 0.5% aqueous carboxymethyl
cellulose (Tylose CB 30 000) at a dose of 0 or 300 mg/kg bw per day on
days 6-15 of gestation. Maternal toxicity was observed, consisting of
significant decreases in body-weight gain (89%) and food consumption
during days 6-8 of gestation. The frequency of post-implantation loss
(12.6%) was greater than that in concurrent controls (4.6%), but this
finding was within the range for historical controls (4.5-15.7%)
(BASF, 1992).
In a study of developmental toxicity, groups of 25 pregnant
Wistar (Chbb:THOM SPF) rats received 8-hydroxybentazone (purity,
99.9%) in 0.5% aqueous carboxymethyl cellulose (Tylose CB 30,000) at a
dose of 0, 40, 100, or 250 mg/kg bw per day on days 6-15 of gestation.
The dams were observed for deaths, clinical signs, body weight, and
food consumption; post mortem, the uterus was removed, weighed, and
opened for internal examination. The fetuses were observed for sex,
weight, and gross external abnormalities, and underwent visceral
(slice technique) and skeletal examinations. There was no maternal or
developmental toxicity. The NOAEL for both maternal and developmental
toxicity was 250 mg/kg bw per day, the highest dose tested (Hellwig &
Hildebrand, 1993).
Comments
After oral administration to rats, [phenyl-U-14C]-bentazone was
extensively absorbed and rapidly excreted in the urine. In rats given
a single dose, 83-94% appeared in the urine by 24 h and 90-97% by
120 h after dosing, with less than 0.7% in the residual carcase.
Biliary excretion of the compound amounted to less than 2% of the
dose. Bentazone undergoes very limited biotransformation in rats.
Bentazone was the major compound identified in urine, representing
81-91% of the dose in males and 77-89% in females. 6-Hydroxybentazone
was present in amounts up to 6.3% of the dose, and isomeric
Table 1. Results of assays for the genotoxicity of the 6-hydroxy and 8-hydroxy metabolites of bentazone
End-point Test object Concentration Purity Results Reference
(%)
6-Hydroxybentazone
Reverse mutation S. typhimurium TA98, 20-5000 µg/plate > 98 Negative Gelbke & Engelhardt
TA100, TA1535, TA1537 ± S9 (1987a)
8-Hydroxybentazone
Reverse mutation S. typhimurium 20-5000 µg/plate > 98.5 Negative Gelbke & Engelhardt
TA98,TA100, TA1535, ± S9 (1987b)
TA1537
Gene mutation Chinese hamster Trial 1: 99.9 Negative Mullerschon (1992)
V79 cells, hprt locus 55-2002 µg/ml -S9
495-5005 µg/ml +S9
Trial 2:
300-3000 µg/ml -S9
500-5000 µg/ml +S9
Micronucleus formation NMRI mouse in vivo 625, 1250, 2500 mg/kg bw 99.9 Negative Gelbke (1993)
8-hydroxybentazone was present in trace amounts (0-0.23% of the dose).
There were no major differences among the groups. Glucuronide or
sulfate conjugation was either negligible or nonexistent; 6- and
8-hydroxybentazone are also metabolites of bentazone in plants.
Bentazone is more acutely toxic to rats than are its two
hydroxylated metabolites when given by the oral route. The acute oral
LD50 of technical-grade bentazone was estimated to be 1800 mg/kg bw
in males and 1500 mg/kg bw in females. The acute oral LD50 value for
6- and 8-hydroxybentazone was 5000 mg/kg bw.
WHO has classified bentazone as slightly hazardous (WHO, 1996).
The two studies described below indicate that 8-hydroxybentazone
does not have the anticoagulant and diuretic effects of bentazone at
the doses tested and has less systemic toxicity than the parent
compound under the test conditions. No data were available on the
short-term toxicity of 6-hydroxybentazone.
Rats received technical-grade bentazone in the diet at
concentrations of 0, 400, 1200, or 3600 ppm for 13 weeks. The body
weights of females were decreased and were statistically significantly
different from those of controls at 3600 ppm from week 10 onward.
Examination of haematological parameters indicated statistically
significant increases in prothrombin time and partial thromboplastin
time in males at 3600 ppm in comparison with controls. Bentazone had a
diuretic effect in animals of each sex, reaching statistical
significance at 3600 ppm. The NOAEL for systemic toxicity was 1200 ppm
(equal to 78 mg/kg bw per day) on the basis of statistically
significant decreased body weights in females throughout the latter
part of the treatment, increased prothrombin time and partial
thromboplastin time in males, increased output of urine with decreased
specific gravity in animals of each sex, and some degree of kidney
hypertrophy in both males and females at 3600 ppm, equal to 240 mg/kg
bw per day.
Rats received 8-hydroxybentazone in the diet at concentrations of
0, 400, 1200, or 3600 ppm for three months. No compound-related
effects were observed on body weights, clinical signs, food
consumption, haematological, clinical chemical, or urinary parameters,
clotting time, organ weights, or gross or histopathological
appearance. The NOAEL was 3600 ppm (equal to 260 mg/kg bw per day),
the highest dose tested.
The following two studies of developmental toxicity indicate that
bentazone has effects at doses below a maternally toxic dose, whereas
8-hydroxybentazone had no developmental or maternal toxicity at any of
the doses tested.
Pregnant rats received technical-grade bentazone by gavage at 0,
40, 100, or 250 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 250 mg/kg bw per day, the highest dose
tested. The NOAEL for developmental toxicity was 100 mg/kg bw per day
on the basis of significantly decreased mean fetal weights and delays
in tissue ossification, which reached statistical significance on a
litter basis at the highest dose.
No developmental toxicity was observed in pregnant rats that
received 8-hydroxybentazone by gavage at 0, 40, 100, or 250 mg/kg bw
per day on days 6-15 of gestation. The NOAEL for developmental
toxicity was 250 mg/kg bw per day, the highest dose tested.
Bentazone, 6-hydroxybentazone, and 8-hydroxybentazone did not
induce reverse mutation in bacteria, and 8-hydroxybentazone did not
induce gene mutation in mammalian cells or micronucleus formation in
mice in vivo. The Meeting concluded that neither bentazone nor its
metabolites are genotoxic.
8-Hydroxybentazone was less toxic than the parent compound, and,
on the basis of the structural similarities between the 6- and
8-hydroxy isomers, the Meeting concluded that the 6-hydroxy isomer is
also less toxic than the parent. Therefore, the Meeting maintained the
ADI of 0-0.1 mg/kg bw for bentazone.
Because this was a limited review, data were not evaluated that
would permit the establish-ment of an acute reference dose.
Toxicological Evaluation
Levels that cause no toxic effect
Bentazone
Mouse: 100 ppm, equal to 12 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
1200 ppm, equal to 78 mg/kg bw per day (13-week study
of toxicity)
250 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (developmental toxicity)
Dog: 400 ppm, equal to 13 mg/kg bw per day (one-year study
of toxicity)
8-Hydroxybentazone
Rat: 3600 ppm, equal to 260 mg/kg bw per day (three-month
study of toxicity)
250 mg/kg bw per day (maternal and developmental
toxicity in study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Not considered
List of end-points relevant for comparing the toxicities of bentazone, 6-hydroxybentazone
and 8-hydroxybentazone
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 83-94% rapidly absorbed (bentazone)
Dermal absorption No data
Distribution Extensive
Potential for accumulation Little or none for bentazone; no data on metabolites
Rate and extent of excretion Rapid excretion: 83-94% of a dose excreted in urine
within 24 h (bentazone)
Metabolism in animals Very little biotransformation: 81-91%of a dose
excreted untransformed. Metabolites are
6-hydroxybentazone (6.3% of dose) and
8-hydroxybentazone (0-0.23% of dose)
Toxicologically significant compounds Bentazone
(animals, plants and environment)
Acute toxicity
Rat LD50 oral Bentazone: 1500 mg/kg bw
6-Hydroxybentazone: > 5000 mg/kg bw
8-Hydroxybentazone: > 5000 mg/kg bw
Short-term toxicity
Target/critical effect Bentazone: decreased body weights in females,
increased clotting times (prothrombin time and
partial thromboplastin time) and increased output
of urine with decreased specific gravity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no effect up to highest dose
tested
Lowest relevant oral NOAEL Rat: Bentazone: 90 days, 78 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 260 mg/kg bw per day,
highest dose tested
Lowest relevant dermal NOAEL Bentazone: 1000 mg/kg bw per day (highest dose
tested)
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Lowest relevant inhalation NOAEL No data
Genotoxicity Bentazone and its metabolites are not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect No data
Lowest relevant NOAEL No data
Carcinogenicity Bentazone: no carcinogenicity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Reproductive toxicity
Reproduction target/critical effect No data
Lowest relevant reproductive NOAEL No data
Developmental target/critical effect Bentazone: developmental effects (decreased fetal
weights and delayed ossification) below maternally
toxic dose
6-Hydroxybentazone: no data
8-Hydroxybentazone: no developmental toxicity at highest dose tested
Lowest relevant developmental NOAEL Rat: Bentazone: 100 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 250 mg/kg bw per day
Neurotoxicity / Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw Long-term toxicity, rats 100
Acute reference dose Not considered
References
BASF (1992) Results of a range-finding prenatal toxicity study with
8OH-bentazon in rats after oral administration (gavage). Unpublished
study dated January/February 1992. Project No. 10R0437/91061 from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Becker, H., Frei, D., Vogel, W. & Terrier, C. (1986) Embryotoxicity
(including teratogenicity) study with bentazone technical in the rat.
Unpublished report, BASF RZ-Report No. 86/421 dated 30 December 1986
from RCC Research and Consulting Company AG, Itingen, Switzerland, RCC
Report No. 064530. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. (1993) Cytogenetic study in vivo of 8-OH-bentazon in mice
micronucleus test single oral administration. Unpublished document No.
BASF 93/10424 dated 6 May 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project
No.26M0437/914319. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of Reg. No.
51 929 (bentazone) (ZNT test substance No. 83/3) in the Ames test
(standard plate test with Salmonella typhimurium). Unpublished
document No. BASF 83/222 dated 10 June 1983 (original German report)
and 26 October 1983 (English translation) from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Gelbke, H.P. & Engelhardt, G. (1987a) Report on the study of
6-hydroxy-bentazon (ZNT test substance No. 86/244) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/023 dated 15 January
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0244/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1987b) Report on the study of
8-hydroxy-bentazon (ZNT test substance No. 86/391) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/0168 dated 14 May
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0391/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hawkins, D.R., Elsom, L.F. & Girkin, R. (1986) Report on the urinary
metabolites of bentazone in the rat. Unpublished report, document No.
BASF 86/090 dated 31 January 1986 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigs-hafen, Germany.
Hawkins, D.R., Mayo, B.C., Pollard, A.D., Green, S.L., Biggs, S.R. &
Whitby, B.R. (1987) The biokinetics and metabolism of 14C-bentazon in
rats. Unpublished report, document No. BASF 87/0429 dated 16 September
1987 from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hellwig, J. & Hildebrand, B. (1993) Study of the prenatal toxicity of
8-OH-bentazon in rats after oral administration (gavage). Unpublished
report, document No. BASF 93/10572 dated 9 June 1993 from BASF
Aktiengesellschaft, Abteilung Toxicologie, Ludwigshafen/Rhein,
Germany, report No. 30R0437/91081. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany
Hildebrand, B. & Kirsch, P. (1982) Report on the study of the acute
oral toxicity in rats of Reg. No. 51 929 -- bentazone. Unpublished
document No. BASF 83/114 dated 30 November 1982 (original in German);
English translation dated 21 June 1983 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. (1993) Study of the dermal toxicity of Reg. No. 51 929
(bentazone) in white rabbits: Application to the intact skin over 3
weeks. Unpublished report, document No. BASF 93/10760 dated 9 June
1993 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No. 41H0243/91042. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1986) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/002 dated 29 December 1986 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1987) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/030 dated 21 January 1987 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Mellert, W., Deckardt, K., Kuttler, K. & Hildebrand, B. (1993)
Subchronic oral toxicity study with 8-OH-bentazon in Wistar rats.
Administration in the diet for 3 months. Unpublished report No. BASF
93/11011 dated 13 September 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project No.
31S0437/91060. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Mullerschon (1992) Gene mutation assay in Chinese hamster V79 cells in
vitro with 8-OH bentazone. Unpublished report No. BASF 92/11329 dated
20 October 1992 from CCR Cytotest Cell Research GmbH & Co. KG,
Germany. BASF Project No. 50M0437/919018. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
Tennekes, H., Horst, K., Luetkemeier, H., Wilson, J., Vogel, W. &
Terrier, C. (1987) Report on the 13-week oral toxicity (feeding) study
with bentazone technical in the rat. Unpublished report, document No.
BASF 87/0173 dated 12 May 1987 from RCC Research and Consulting
Company AG, Itingen, Switzerland. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
 those times. 8-Hydroxybentazone was not detected. Polar radioactive
compounds at the origin represented 2.1% of the dose in the 0-24-h
period (Hawkins et al., 1986).
2. Toxicological studies
(a) Acute toxicity
Bentazone is more acutely toxic to rats by the oral route than
its 6-hydroxy or 8-hydroxy metabolites. The LD50 values for
technical-grade bentazone (purity unspecified) suspended in aqueous
carboxymethyl cellulose in Wistar rats were 1800 mg/kg bw for males,
1500 mg/kg bw for females, and 1600 mg/kg bw for males and females
combined. The LD50 value for males was estimated from deaths at four
doses, with no statistical analysis; the values for females and for
males and females combined were determined by probit analysis.
Dyspnoea and apathy were noted at doses of 825 mg/kg bw and higher,
and staggering occurred at the highest dose, 2610 mg/kg bw per day.
Animals at this dose that died had bloody ulcerations in the stomach
and intestinal contents mixed with blood (Hildebrand & Kirsch, 1982).
(b) Short-term studies of toxicity
Rats
Groups of 10 Wistar KFM-Han, outbred SPF quality rats of each sex
received technical-grade bentazone (purity, 97.8%) at a dietary level
of 0, 400, 1200, or 3600 ppm for 13 weeks, equal to doses of 0, 25,
78, and 240 mg/kg bw per day in males and 0, 29, 86, and 260 mg/kg bw
per day in females. Twenty additional rats were used for a 28-day
recovery experiment. Ten rats received 3600 ppm bentazone for 13 weeks
and were then given regular diet and observed for an additional 28
days; 10 rats served as untreated controls in the recovery experiment.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmic, urinary, haematological, and clinical
chemical parameters; organ weights; and gross and histopathological
alterations.
One male and two females at the high dose died, one of the
females under anesthesia; no signs of toxicity were reported. The body
weights of females at this dose were statistically significantly lower
than those of controls from week 10 onwards; there were no effects on
the body weights of males, and no statistically significant
differences in body weights were seen during the recovery period. The
food consumption of males at the high dose was slightly increased and
became statistically significantly greater than that of controls from
week 7 onwards; the food consumption of females was generally not
affected. No ophthalmological effects were reported. Statistically
significant increases in prothrombin time and partial thromboplastin
time were seen in males at the high dose, whereas females at all doses
had statistically significantly depressed prothrombin time and the
partial thromboplastin time was not affected. The values for certain
clinical chemical parameters were statistically significantly
different from those of controls in males at the high dose, but they
were generally within the historical control values and returned to
normal during the recovery period; the values for females were within
the historical control range.
Bentazone had a diuretic effect in animals of each sex. In both
males and females, the urine volume was increased in a dose-related
manner, becoming significantly different from that of controls at
3600 ppm. The specific gravity was decreased in animals of each sex;
the decrease was dose-related in males and was statistically
significantly different from that of controls at the high dose; in
females, the decrease was significantly different from that of
controls at all doses but did not decrease monotonically. The mean
absolute and relative kidney weights were statistically significantly
greater than those of controls in males at 3600 ppm; in females,
although both the mean absolute and relative kidney weights were
greater than those of controls, the values reached statistical
significance only for absolute weights. The only change in liver
weights was a statistically significantly increase in relative liver
weight in females at 3600 ppm. Gross examination revealed lung thrombi
in 1/10 controls and 3/10 females at the high dose and dilated uterine
horns in 1/10 controls and 3/9 females at the high dose. No
statistically significant histopathological findings were made. The
NOAEL for systemic toxicity was 1200 ppm, equal to 78 mg/kg bw per
day, and the LOAEL was 3600 ppm, equalto 240 mg/kg bw per day, on the
basis of statistically significant decreased body weights of females
throughout the latter part of treatment, increased prothrombin time
and partial thromboplastin time in males, increased urinary output
with decreased specific gravity in animals of each sex, and some
degree of kidney hypertrophy in both males and females (Tennekes et
al., 1987).
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of bentazone (purity, 97.6%) in
Tylose CB in 0.5% aqueous suspension under semiocclusion for 6 h once
a day for 21 days at a dose of 0, 250, 500, or 1000 mg/kg bw per day.
No deaths or clinical signs were seen in animals of either sex at any
dose, and no adverse effects were seen on treated skin. The NOAEL was
1000 mg/kg bw per day, the highest dose tested (Kirsch, 1993).
(c) Genotoxicity
Bentazone was considered not to be genotoxic in vitro or
in vivo in the previous evaluation. It has been tested only as a
compound of 96.7% purity for reverse mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, at
concentrations of 20-5000 µg per plate, in the presence and absence of
an exogenous metabolic activation system. Negative results were
obtained (Gelbke & Engelhardt, 1983).
(d) Developmental toxicity
Rats
In a study of developmental toxicity, pregnant Wistar/HAN rats
(Kfm: WIST, outbred, SPF quality) received technical-grade bentazone
(purity, 97.8%) mixed with 4% carboxymethyl cellulose in distilled
water by gavage at a dose of 0, 40, 100, or 250 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for mortality, clinical
signs, body weight, and food consumption; post mortem, the uterus
was removed, weighed, and opened for internal examination. The fetuses
were examined for sex, weight, and gross external abnormalities, and
underwent visceral (slice technique) and skeletal examinations. No
signs or symptoms of compound-related toxicity were reported, and
there were no effects on body weight, body-weight gain, or food
consumption. The litter incidence of fetal resorptions was increased
in dams at 250 mg/kg bw per day as compared with controls, and the
difference in total number was statistically significant (0 in
controls and 44 at the high dose). There was a small but statistically
significant decrease in mean fetal weight at the high dose (4.8 g in
controls versus 4.3 g). The rate of ossification in the phalangeal
nuclei of fore- and hindlimb digits, the fifth sternebra, and cervical
vertebrae was decreased, and the litter incidence of phalangeal nuclei
with delayed ossification was statistically significant at the high
dose (chi2, p < 0.05). The NOAEL for maternal toxicity was
250 mg/kg bw per day, the highest dose tested, and that for
developmental toxicity was 100 mg/kg bw per day on the basis of
statistically significantly decreased mean fetal weights and delays in
tissue ossification at the high dose (Becker at al., 1986).
3. Studies of metabolites
(a) Acute toxicity
The acute oral LD50 values for 6-hydroxy- and 8-hydroxybentazone
suspended in aqueous carboxymethyl cellulose in male and female Wistar
rats were > 5000 mg/kg bw. The 6-hydroxy compound was > 98%, and
the 8-hydroxy compound was > 98.5% pure. Two males that received 5000
mg/kg bw 8-hydroxybentazone died, but no deaths occurred among females
at any dose (2150, 3830, or 5000 mg/kg bw) or among any of the animals
given the 6-hydroxy metabolite. Necropsy of the rats that died after
intake of 5000 mg/kg bw 8-hydroxybentazone showed general congestion
(Kirsch & Kieczka, 1987). No lesions were found at necropsy in animals
given 5000 mg/kg bw 6-hydroxy-bentazone (Kirsch & Kieczka, 1986).
(b) Short-term studies of toxicity
Groups of 10 Wistar rats of each sex received 8-hydroxybentazone
(99.9% active ingredient) in the diet at 0, 400, 1200, or 3600 ppm for
three months, equal to doses of 0, 28, 85, and 260 mg/kg bw for males
and 0, 34, 100, and 300 for females. No compound-related effects were
seen on mortality, clinical signs, body weight, food consumption,
haematological, clinical chemical, or urinary parameters, organ
weights, or gross or histopathological appearance. In particular,
there was no significant effect on thromboplastin time at 45 or 94
days. The NOAEL was 3600 ppm, equal to 260 mg/kg bw per day, the
highest dose tested (Mellert et al., 1993)
(c) Genotoxicity
Studies on the genotoxicity of metabolites of bentazone are
summarized in Table 1. The 6- and 8-hydroxy isomers of bentazone also
gave negative results in assays for reverse mutation, with and without
microsomal activation (Gelbke & Engelhardt, 1987a,b).
8-Hydroxybentazone gave negative results in an assay for gene mutation
at the hprt locus in Chinese hamster V79 cells, with and without
metabolic activation (Mullerschon, 1992) and in an assay for
micronucleus formation in mice treated in vivo (Gelbke, 1993).
(d) Developmental toxicity
In a range-finding study for developmental toxicity, groups of
nine or 10 pregnant Wistar (Chbb:THOM SPF) rats received
8-hydroxybentazone (purity unspecified) in 0.5% aqueous carboxymethyl
cellulose (Tylose CB 30 000) at a dose of 0 or 300 mg/kg bw per day on
days 6-15 of gestation. Maternal toxicity was observed, consisting of
significant decreases in body-weight gain (89%) and food consumption
during days 6-8 of gestation. The frequency of post-implantation loss
(12.6%) was greater than that in concurrent controls (4.6%), but this
finding was within the range for historical controls (4.5-15.7%)
(BASF, 1992).
In a study of developmental toxicity, groups of 25 pregnant
Wistar (Chbb:THOM SPF) rats received 8-hydroxybentazone (purity,
99.9%) in 0.5% aqueous carboxymethyl cellulose (Tylose CB 30,000) at a
dose of 0, 40, 100, or 250 mg/kg bw per day on days 6-15 of gestation.
The dams were observed for deaths, clinical signs, body weight, and
food consumption; post mortem, the uterus was removed, weighed, and
opened for internal examination. The fetuses were observed for sex,
weight, and gross external abnormalities, and underwent visceral
(slice technique) and skeletal examinations. There was no maternal or
developmental toxicity. The NOAEL for both maternal and developmental
toxicity was 250 mg/kg bw per day, the highest dose tested (Hellwig &
Hildebrand, 1993).
Comments
After oral administration to rats, [phenyl-U-14C]-bentazone was
extensively absorbed and rapidly excreted in the urine. In rats given
a single dose, 83-94% appeared in the urine by 24 h and 90-97% by
120 h after dosing, with less than 0.7% in the residual carcase.
Biliary excretion of the compound amounted to less than 2% of the
dose. Bentazone undergoes very limited biotransformation in rats.
Bentazone was the major compound identified in urine, representing
81-91% of the dose in males and 77-89% in females. 6-Hydroxybentazone
was present in amounts up to 6.3% of the dose, and isomeric
Table 1. Results of assays for the genotoxicity of the 6-hydroxy and 8-hydroxy metabolites of bentazone
End-point Test object Concentration Purity Results Reference
(%)
6-Hydroxybentazone
Reverse mutation S. typhimurium TA98, 20-5000 µg/plate > 98 Negative Gelbke & Engelhardt
TA100, TA1535, TA1537 ± S9 (1987a)
8-Hydroxybentazone
Reverse mutation S. typhimurium 20-5000 µg/plate > 98.5 Negative Gelbke & Engelhardt
TA98,TA100, TA1535, ± S9 (1987b)
TA1537
Gene mutation Chinese hamster Trial 1: 99.9 Negative Mullerschon (1992)
V79 cells, hprt locus 55-2002 µg/ml -S9
495-5005 µg/ml +S9
Trial 2:
300-3000 µg/ml -S9
500-5000 µg/ml +S9
Micronucleus formation NMRI mouse in vivo 625, 1250, 2500 mg/kg bw 99.9 Negative Gelbke (1993)
8-hydroxybentazone was present in trace amounts (0-0.23% of the dose).
There were no major differences among the groups. Glucuronide or
sulfate conjugation was either negligible or nonexistent; 6- and
8-hydroxybentazone are also metabolites of bentazone in plants.
Bentazone is more acutely toxic to rats than are its two
hydroxylated metabolites when given by the oral route. The acute oral
LD50 of technical-grade bentazone was estimated to be 1800 mg/kg bw
in males and 1500 mg/kg bw in females. The acute oral LD50 value for
6- and 8-hydroxybentazone was 5000 mg/kg bw.
WHO has classified bentazone as slightly hazardous (WHO, 1996).
The two studies described below indicate that 8-hydroxybentazone
does not have the anticoagulant and diuretic effects of bentazone at
the doses tested and has less systemic toxicity than the parent
compound under the test conditions. No data were available on the
short-term toxicity of 6-hydroxybentazone.
Rats received technical-grade bentazone in the diet at
concentrations of 0, 400, 1200, or 3600 ppm for 13 weeks. The body
weights of females were decreased and were statistically significantly
different from those of controls at 3600 ppm from week 10 onward.
Examination of haematological parameters indicated statistically
significant increases in prothrombin time and partial thromboplastin
time in males at 3600 ppm in comparison with controls. Bentazone had a
diuretic effect in animals of each sex, reaching statistical
significance at 3600 ppm. The NOAEL for systemic toxicity was 1200 ppm
(equal to 78 mg/kg bw per day) on the basis of statistically
significant decreased body weights in females throughout the latter
part of the treatment, increased prothrombin time and partial
thromboplastin time in males, increased output of urine with decreased
specific gravity in animals of each sex, and some degree of kidney
hypertrophy in both males and females at 3600 ppm, equal to 240 mg/kg
bw per day.
Rats received 8-hydroxybentazone in the diet at concentrations of
0, 400, 1200, or 3600 ppm for three months. No compound-related
effects were observed on body weights, clinical signs, food
consumption, haematological, clinical chemical, or urinary parameters,
clotting time, organ weights, or gross or histopathological
appearance. The NOAEL was 3600 ppm (equal to 260 mg/kg bw per day),
the highest dose tested.
The following two studies of developmental toxicity indicate that
bentazone has effects at doses below a maternally toxic dose, whereas
8-hydroxybentazone had no developmental or maternal toxicity at any of
the doses tested.
Pregnant rats received technical-grade bentazone by gavage at 0,
40, 100, or 250 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 250 mg/kg bw per day, the highest dose
tested. The NOAEL for developmental toxicity was 100 mg/kg bw per day
on the basis of significantly decreased mean fetal weights and delays
in tissue ossification, which reached statistical significance on a
litter basis at the highest dose.
No developmental toxicity was observed in pregnant rats that
received 8-hydroxybentazone by gavage at 0, 40, 100, or 250 mg/kg bw
per day on days 6-15 of gestation. The NOAEL for developmental
toxicity was 250 mg/kg bw per day, the highest dose tested.
Bentazone, 6-hydroxybentazone, and 8-hydroxybentazone did not
induce reverse mutation in bacteria, and 8-hydroxybentazone did not
induce gene mutation in mammalian cells or micronucleus formation in
mice in vivo. The Meeting concluded that neither bentazone nor its
metabolites are genotoxic.
8-Hydroxybentazone was less toxic than the parent compound, and,
on the basis of the structural similarities between the 6- and
8-hydroxy isomers, the Meeting concluded that the 6-hydroxy isomer is
also less toxic than the parent. Therefore, the Meeting maintained the
ADI of 0-0.1 mg/kg bw for bentazone.
Because this was a limited review, data were not evaluated that
would permit the establish-ment of an acute reference dose.
Toxicological Evaluation
Levels that cause no toxic effect
Bentazone
Mouse: 100 ppm, equal to 12 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
1200 ppm, equal to 78 mg/kg bw per day (13-week study
of toxicity)
250 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (developmental toxicity)
Dog: 400 ppm, equal to 13 mg/kg bw per day (one-year study
of toxicity)
8-Hydroxybentazone
Rat: 3600 ppm, equal to 260 mg/kg bw per day (three-month
study of toxicity)
250 mg/kg bw per day (maternal and developmental
toxicity in study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Not considered
List of end-points relevant for comparing the toxicities of bentazone, 6-hydroxybentazone
and 8-hydroxybentazone
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 83-94% rapidly absorbed (bentazone)
Dermal absorption No data
Distribution Extensive
Potential for accumulation Little or none for bentazone; no data on metabolites
Rate and extent of excretion Rapid excretion: 83-94% of a dose excreted in urine
within 24 h (bentazone)
Metabolism in animals Very little biotransformation: 81-91%of a dose
excreted untransformed. Metabolites are
6-hydroxybentazone (6.3% of dose) and
8-hydroxybentazone (0-0.23% of dose)
Toxicologically significant compounds Bentazone
(animals, plants and environment)
Acute toxicity
Rat LD50 oral Bentazone: 1500 mg/kg bw
6-Hydroxybentazone: > 5000 mg/kg bw
8-Hydroxybentazone: > 5000 mg/kg bw
Short-term toxicity
Target/critical effect Bentazone: decreased body weights in females,
increased clotting times (prothrombin time and
partial thromboplastin time) and increased output
of urine with decreased specific gravity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no effect up to highest dose
tested
Lowest relevant oral NOAEL Rat: Bentazone: 90 days, 78 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 260 mg/kg bw per day,
highest dose tested
Lowest relevant dermal NOAEL Bentazone: 1000 mg/kg bw per day (highest dose
tested)
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Lowest relevant inhalation NOAEL No data
Genotoxicity Bentazone and its metabolites are not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect No data
Lowest relevant NOAEL No data
Carcinogenicity Bentazone: no carcinogenicity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Reproductive toxicity
Reproduction target/critical effect No data
Lowest relevant reproductive NOAEL No data
Developmental target/critical effect Bentazone: developmental effects (decreased fetal
weights and delayed ossification) below maternally
toxic dose
6-Hydroxybentazone: no data
8-Hydroxybentazone: no developmental toxicity at highest dose tested
Lowest relevant developmental NOAEL Rat: Bentazone: 100 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 250 mg/kg bw per day
Neurotoxicity / Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw Long-term toxicity, rats 100
Acute reference dose Not considered
References
BASF (1992) Results of a range-finding prenatal toxicity study with
8OH-bentazon in rats after oral administration (gavage). Unpublished
study dated January/February 1992. Project No. 10R0437/91061 from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Becker, H., Frei, D., Vogel, W. & Terrier, C. (1986) Embryotoxicity
(including teratogenicity) study with bentazone technical in the rat.
Unpublished report, BASF RZ-Report No. 86/421 dated 30 December 1986
from RCC Research and Consulting Company AG, Itingen, Switzerland, RCC
Report No. 064530. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. (1993) Cytogenetic study in vivo of 8-OH-bentazon in mice
micronucleus test single oral administration. Unpublished document No.
BASF 93/10424 dated 6 May 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project
No.26M0437/914319. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of Reg. No.
51 929 (bentazone) (ZNT test substance No. 83/3) in the Ames test
(standard plate test with Salmonella typhimurium). Unpublished
document No. BASF 83/222 dated 10 June 1983 (original German report)
and 26 October 1983 (English translation) from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Gelbke, H.P. & Engelhardt, G. (1987a) Report on the study of
6-hydroxy-bentazon (ZNT test substance No. 86/244) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/023 dated 15 January
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0244/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1987b) Report on the study of
8-hydroxy-bentazon (ZNT test substance No. 86/391) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/0168 dated 14 May
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0391/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hawkins, D.R., Elsom, L.F. & Girkin, R. (1986) Report on the urinary
metabolites of bentazone in the rat. Unpublished report, document No.
BASF 86/090 dated 31 January 1986 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigs-hafen, Germany.
Hawkins, D.R., Mayo, B.C., Pollard, A.D., Green, S.L., Biggs, S.R. &
Whitby, B.R. (1987) The biokinetics and metabolism of 14C-bentazon in
rats. Unpublished report, document No. BASF 87/0429 dated 16 September
1987 from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hellwig, J. & Hildebrand, B. (1993) Study of the prenatal toxicity of
8-OH-bentazon in rats after oral administration (gavage). Unpublished
report, document No. BASF 93/10572 dated 9 June 1993 from BASF
Aktiengesellschaft, Abteilung Toxicologie, Ludwigshafen/Rhein,
Germany, report No. 30R0437/91081. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany
Hildebrand, B. & Kirsch, P. (1982) Report on the study of the acute
oral toxicity in rats of Reg. No. 51 929 -- bentazone. Unpublished
document No. BASF 83/114 dated 30 November 1982 (original in German);
English translation dated 21 June 1983 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. (1993) Study of the dermal toxicity of Reg. No. 51 929
(bentazone) in white rabbits: Application to the intact skin over 3
weeks. Unpublished report, document No. BASF 93/10760 dated 9 June
1993 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No. 41H0243/91042. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1986) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/002 dated 29 December 1986 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1987) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/030 dated 21 January 1987 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Mellert, W., Deckardt, K., Kuttler, K. & Hildebrand, B. (1993)
Subchronic oral toxicity study with 8-OH-bentazon in Wistar rats.
Administration in the diet for 3 months. Unpublished report No. BASF
93/11011 dated 13 September 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project No.
31S0437/91060. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Mullerschon (1992) Gene mutation assay in Chinese hamster V79 cells in
vitro with 8-OH bentazone. Unpublished report No. BASF 92/11329 dated
20 October 1992 from CCR Cytotest Cell Research GmbH & Co. KG,
Germany. BASF Project No. 50M0437/919018. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
Tennekes, H., Horst, K., Luetkemeier, H., Wilson, J., Vogel, W. &
Terrier, C. (1987) Report on the 13-week oral toxicity (feeding) study
with bentazone technical in the rat. Unpublished report, document No.
BASF 87/0173 dated 12 May 1987 from RCC Research and Consulting
Company AG, Itingen, Switzerland. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
those times. 8-Hydroxybentazone was not detected. Polar radioactive
compounds at the origin represented 2.1% of the dose in the 0-24-h
period (Hawkins et al., 1986).
2. Toxicological studies
(a) Acute toxicity
Bentazone is more acutely toxic to rats by the oral route than
its 6-hydroxy or 8-hydroxy metabolites. The LD50 values for
technical-grade bentazone (purity unspecified) suspended in aqueous
carboxymethyl cellulose in Wistar rats were 1800 mg/kg bw for males,
1500 mg/kg bw for females, and 1600 mg/kg bw for males and females
combined. The LD50 value for males was estimated from deaths at four
doses, with no statistical analysis; the values for females and for
males and females combined were determined by probit analysis.
Dyspnoea and apathy were noted at doses of 825 mg/kg bw and higher,
and staggering occurred at the highest dose, 2610 mg/kg bw per day.
Animals at this dose that died had bloody ulcerations in the stomach
and intestinal contents mixed with blood (Hildebrand & Kirsch, 1982).
(b) Short-term studies of toxicity
Rats
Groups of 10 Wistar KFM-Han, outbred SPF quality rats of each sex
received technical-grade bentazone (purity, 97.8%) at a dietary level
of 0, 400, 1200, or 3600 ppm for 13 weeks, equal to doses of 0, 25,
78, and 240 mg/kg bw per day in males and 0, 29, 86, and 260 mg/kg bw
per day in females. Twenty additional rats were used for a 28-day
recovery experiment. Ten rats received 3600 ppm bentazone for 13 weeks
and were then given regular diet and observed for an additional 28
days; 10 rats served as untreated controls in the recovery experiment.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmic, urinary, haematological, and clinical
chemical parameters; organ weights; and gross and histopathological
alterations.
One male and two females at the high dose died, one of the
females under anesthesia; no signs of toxicity were reported. The body
weights of females at this dose were statistically significantly lower
than those of controls from week 10 onwards; there were no effects on
the body weights of males, and no statistically significant
differences in body weights were seen during the recovery period. The
food consumption of males at the high dose was slightly increased and
became statistically significantly greater than that of controls from
week 7 onwards; the food consumption of females was generally not
affected. No ophthalmological effects were reported. Statistically
significant increases in prothrombin time and partial thromboplastin
time were seen in males at the high dose, whereas females at all doses
had statistically significantly depressed prothrombin time and the
partial thromboplastin time was not affected. The values for certain
clinical chemical parameters were statistically significantly
different from those of controls in males at the high dose, but they
were generally within the historical control values and returned to
normal during the recovery period; the values for females were within
the historical control range.
Bentazone had a diuretic effect in animals of each sex. In both
males and females, the urine volume was increased in a dose-related
manner, becoming significantly different from that of controls at
3600 ppm. The specific gravity was decreased in animals of each sex;
the decrease was dose-related in males and was statistically
significantly different from that of controls at the high dose; in
females, the decrease was significantly different from that of
controls at all doses but did not decrease monotonically. The mean
absolute and relative kidney weights were statistically significantly
greater than those of controls in males at 3600 ppm; in females,
although both the mean absolute and relative kidney weights were
greater than those of controls, the values reached statistical
significance only for absolute weights. The only change in liver
weights was a statistically significantly increase in relative liver
weight in females at 3600 ppm. Gross examination revealed lung thrombi
in 1/10 controls and 3/10 females at the high dose and dilated uterine
horns in 1/10 controls and 3/9 females at the high dose. No
statistically significant histopathological findings were made. The
NOAEL for systemic toxicity was 1200 ppm, equal to 78 mg/kg bw per
day, and the LOAEL was 3600 ppm, equalto 240 mg/kg bw per day, on the
basis of statistically significant decreased body weights of females
throughout the latter part of treatment, increased prothrombin time
and partial thromboplastin time in males, increased urinary output
with decreased specific gravity in animals of each sex, and some
degree of kidney hypertrophy in both males and females (Tennekes et
al., 1987).
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of bentazone (purity, 97.6%) in
Tylose CB in 0.5% aqueous suspension under semiocclusion for 6 h once
a day for 21 days at a dose of 0, 250, 500, or 1000 mg/kg bw per day.
No deaths or clinical signs were seen in animals of either sex at any
dose, and no adverse effects were seen on treated skin. The NOAEL was
1000 mg/kg bw per day, the highest dose tested (Kirsch, 1993).
(c) Genotoxicity
Bentazone was considered not to be genotoxic in vitro or
in vivo in the previous evaluation. It has been tested only as a
compound of 96.7% purity for reverse mutation in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, at
concentrations of 20-5000 µg per plate, in the presence and absence of
an exogenous metabolic activation system. Negative results were
obtained (Gelbke & Engelhardt, 1983).
(d) Developmental toxicity
Rats
In a study of developmental toxicity, pregnant Wistar/HAN rats
(Kfm: WIST, outbred, SPF quality) received technical-grade bentazone
(purity, 97.8%) mixed with 4% carboxymethyl cellulose in distilled
water by gavage at a dose of 0, 40, 100, or 250 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for mortality, clinical
signs, body weight, and food consumption; post mortem, the uterus
was removed, weighed, and opened for internal examination. The fetuses
were examined for sex, weight, and gross external abnormalities, and
underwent visceral (slice technique) and skeletal examinations. No
signs or symptoms of compound-related toxicity were reported, and
there were no effects on body weight, body-weight gain, or food
consumption. The litter incidence of fetal resorptions was increased
in dams at 250 mg/kg bw per day as compared with controls, and the
difference in total number was statistically significant (0 in
controls and 44 at the high dose). There was a small but statistically
significant decrease in mean fetal weight at the high dose (4.8 g in
controls versus 4.3 g). The rate of ossification in the phalangeal
nuclei of fore- and hindlimb digits, the fifth sternebra, and cervical
vertebrae was decreased, and the litter incidence of phalangeal nuclei
with delayed ossification was statistically significant at the high
dose (chi2, p < 0.05). The NOAEL for maternal toxicity was
250 mg/kg bw per day, the highest dose tested, and that for
developmental toxicity was 100 mg/kg bw per day on the basis of
statistically significantly decreased mean fetal weights and delays in
tissue ossification at the high dose (Becker at al., 1986).
3. Studies of metabolites
(a) Acute toxicity
The acute oral LD50 values for 6-hydroxy- and 8-hydroxybentazone
suspended in aqueous carboxymethyl cellulose in male and female Wistar
rats were > 5000 mg/kg bw. The 6-hydroxy compound was > 98%, and
the 8-hydroxy compound was > 98.5% pure. Two males that received 5000
mg/kg bw 8-hydroxybentazone died, but no deaths occurred among females
at any dose (2150, 3830, or 5000 mg/kg bw) or among any of the animals
given the 6-hydroxy metabolite. Necropsy of the rats that died after
intake of 5000 mg/kg bw 8-hydroxybentazone showed general congestion
(Kirsch & Kieczka, 1987). No lesions were found at necropsy in animals
given 5000 mg/kg bw 6-hydroxy-bentazone (Kirsch & Kieczka, 1986).
(b) Short-term studies of toxicity
Groups of 10 Wistar rats of each sex received 8-hydroxybentazone
(99.9% active ingredient) in the diet at 0, 400, 1200, or 3600 ppm for
three months, equal to doses of 0, 28, 85, and 260 mg/kg bw for males
and 0, 34, 100, and 300 for females. No compound-related effects were
seen on mortality, clinical signs, body weight, food consumption,
haematological, clinical chemical, or urinary parameters, organ
weights, or gross or histopathological appearance. In particular,
there was no significant effect on thromboplastin time at 45 or 94
days. The NOAEL was 3600 ppm, equal to 260 mg/kg bw per day, the
highest dose tested (Mellert et al., 1993)
(c) Genotoxicity
Studies on the genotoxicity of metabolites of bentazone are
summarized in Table 1. The 6- and 8-hydroxy isomers of bentazone also
gave negative results in assays for reverse mutation, with and without
microsomal activation (Gelbke & Engelhardt, 1987a,b).
8-Hydroxybentazone gave negative results in an assay for gene mutation
at the hprt locus in Chinese hamster V79 cells, with and without
metabolic activation (Mullerschon, 1992) and in an assay for
micronucleus formation in mice treated in vivo (Gelbke, 1993).
(d) Developmental toxicity
In a range-finding study for developmental toxicity, groups of
nine or 10 pregnant Wistar (Chbb:THOM SPF) rats received
8-hydroxybentazone (purity unspecified) in 0.5% aqueous carboxymethyl
cellulose (Tylose CB 30 000) at a dose of 0 or 300 mg/kg bw per day on
days 6-15 of gestation. Maternal toxicity was observed, consisting of
significant decreases in body-weight gain (89%) and food consumption
during days 6-8 of gestation. The frequency of post-implantation loss
(12.6%) was greater than that in concurrent controls (4.6%), but this
finding was within the range for historical controls (4.5-15.7%)
(BASF, 1992).
In a study of developmental toxicity, groups of 25 pregnant
Wistar (Chbb:THOM SPF) rats received 8-hydroxybentazone (purity,
99.9%) in 0.5% aqueous carboxymethyl cellulose (Tylose CB 30,000) at a
dose of 0, 40, 100, or 250 mg/kg bw per day on days 6-15 of gestation.
The dams were observed for deaths, clinical signs, body weight, and
food consumption; post mortem, the uterus was removed, weighed, and
opened for internal examination. The fetuses were observed for sex,
weight, and gross external abnormalities, and underwent visceral
(slice technique) and skeletal examinations. There was no maternal or
developmental toxicity. The NOAEL for both maternal and developmental
toxicity was 250 mg/kg bw per day, the highest dose tested (Hellwig &
Hildebrand, 1993).
Comments
After oral administration to rats, [phenyl-U-14C]-bentazone was
extensively absorbed and rapidly excreted in the urine. In rats given
a single dose, 83-94% appeared in the urine by 24 h and 90-97% by
120 h after dosing, with less than 0.7% in the residual carcase.
Biliary excretion of the compound amounted to less than 2% of the
dose. Bentazone undergoes very limited biotransformation in rats.
Bentazone was the major compound identified in urine, representing
81-91% of the dose in males and 77-89% in females. 6-Hydroxybentazone
was present in amounts up to 6.3% of the dose, and isomeric
Table 1. Results of assays for the genotoxicity of the 6-hydroxy and 8-hydroxy metabolites of bentazone
End-point Test object Concentration Purity Results Reference
(%)
6-Hydroxybentazone
Reverse mutation S. typhimurium TA98, 20-5000 µg/plate > 98 Negative Gelbke & Engelhardt
TA100, TA1535, TA1537 ± S9 (1987a)
8-Hydroxybentazone
Reverse mutation S. typhimurium 20-5000 µg/plate > 98.5 Negative Gelbke & Engelhardt
TA98,TA100, TA1535, ± S9 (1987b)
TA1537
Gene mutation Chinese hamster Trial 1: 99.9 Negative Mullerschon (1992)
V79 cells, hprt locus 55-2002 µg/ml -S9
495-5005 µg/ml +S9
Trial 2:
300-3000 µg/ml -S9
500-5000 µg/ml +S9
Micronucleus formation NMRI mouse in vivo 625, 1250, 2500 mg/kg bw 99.9 Negative Gelbke (1993)
8-hydroxybentazone was present in trace amounts (0-0.23% of the dose).
There were no major differences among the groups. Glucuronide or
sulfate conjugation was either negligible or nonexistent; 6- and
8-hydroxybentazone are also metabolites of bentazone in plants.
Bentazone is more acutely toxic to rats than are its two
hydroxylated metabolites when given by the oral route. The acute oral
LD50 of technical-grade bentazone was estimated to be 1800 mg/kg bw
in males and 1500 mg/kg bw in females. The acute oral LD50 value for
6- and 8-hydroxybentazone was 5000 mg/kg bw.
WHO has classified bentazone as slightly hazardous (WHO, 1996).
The two studies described below indicate that 8-hydroxybentazone
does not have the anticoagulant and diuretic effects of bentazone at
the doses tested and has less systemic toxicity than the parent
compound under the test conditions. No data were available on the
short-term toxicity of 6-hydroxybentazone.
Rats received technical-grade bentazone in the diet at
concentrations of 0, 400, 1200, or 3600 ppm for 13 weeks. The body
weights of females were decreased and were statistically significantly
different from those of controls at 3600 ppm from week 10 onward.
Examination of haematological parameters indicated statistically
significant increases in prothrombin time and partial thromboplastin
time in males at 3600 ppm in comparison with controls. Bentazone had a
diuretic effect in animals of each sex, reaching statistical
significance at 3600 ppm. The NOAEL for systemic toxicity was 1200 ppm
(equal to 78 mg/kg bw per day) on the basis of statistically
significant decreased body weights in females throughout the latter
part of the treatment, increased prothrombin time and partial
thromboplastin time in males, increased output of urine with decreased
specific gravity in animals of each sex, and some degree of kidney
hypertrophy in both males and females at 3600 ppm, equal to 240 mg/kg
bw per day.
Rats received 8-hydroxybentazone in the diet at concentrations of
0, 400, 1200, or 3600 ppm for three months. No compound-related
effects were observed on body weights, clinical signs, food
consumption, haematological, clinical chemical, or urinary parameters,
clotting time, organ weights, or gross or histopathological
appearance. The NOAEL was 3600 ppm (equal to 260 mg/kg bw per day),
the highest dose tested.
The following two studies of developmental toxicity indicate that
bentazone has effects at doses below a maternally toxic dose, whereas
8-hydroxybentazone had no developmental or maternal toxicity at any of
the doses tested.
Pregnant rats received technical-grade bentazone by gavage at 0,
40, 100, or 250 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 250 mg/kg bw per day, the highest dose
tested. The NOAEL for developmental toxicity was 100 mg/kg bw per day
on the basis of significantly decreased mean fetal weights and delays
in tissue ossification, which reached statistical significance on a
litter basis at the highest dose.
No developmental toxicity was observed in pregnant rats that
received 8-hydroxybentazone by gavage at 0, 40, 100, or 250 mg/kg bw
per day on days 6-15 of gestation. The NOAEL for developmental
toxicity was 250 mg/kg bw per day, the highest dose tested.
Bentazone, 6-hydroxybentazone, and 8-hydroxybentazone did not
induce reverse mutation in bacteria, and 8-hydroxybentazone did not
induce gene mutation in mammalian cells or micronucleus formation in
mice in vivo. The Meeting concluded that neither bentazone nor its
metabolites are genotoxic.
8-Hydroxybentazone was less toxic than the parent compound, and,
on the basis of the structural similarities between the 6- and
8-hydroxy isomers, the Meeting concluded that the 6-hydroxy isomer is
also less toxic than the parent. Therefore, the Meeting maintained the
ADI of 0-0.1 mg/kg bw for bentazone.
Because this was a limited review, data were not evaluated that
would permit the establish-ment of an acute reference dose.
Toxicological Evaluation
Levels that cause no toxic effect
Bentazone
Mouse: 100 ppm, equal to 12 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
1200 ppm, equal to 78 mg/kg bw per day (13-week study
of toxicity)
250 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (developmental toxicity)
Dog: 400 ppm, equal to 13 mg/kg bw per day (one-year study
of toxicity)
8-Hydroxybentazone
Rat: 3600 ppm, equal to 260 mg/kg bw per day (three-month
study of toxicity)
250 mg/kg bw per day (maternal and developmental
toxicity in study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Not considered
List of end-points relevant for comparing the toxicities of bentazone, 6-hydroxybentazone
and 8-hydroxybentazone
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 83-94% rapidly absorbed (bentazone)
Dermal absorption No data
Distribution Extensive
Potential for accumulation Little or none for bentazone; no data on metabolites
Rate and extent of excretion Rapid excretion: 83-94% of a dose excreted in urine
within 24 h (bentazone)
Metabolism in animals Very little biotransformation: 81-91%of a dose
excreted untransformed. Metabolites are
6-hydroxybentazone (6.3% of dose) and
8-hydroxybentazone (0-0.23% of dose)
Toxicologically significant compounds Bentazone
(animals, plants and environment)
Acute toxicity
Rat LD50 oral Bentazone: 1500 mg/kg bw
6-Hydroxybentazone: > 5000 mg/kg bw
8-Hydroxybentazone: > 5000 mg/kg bw
Short-term toxicity
Target/critical effect Bentazone: decreased body weights in females,
increased clotting times (prothrombin time and
partial thromboplastin time) and increased output
of urine with decreased specific gravity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no effect up to highest dose
tested
Lowest relevant oral NOAEL Rat: Bentazone: 90 days, 78 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 260 mg/kg bw per day,
highest dose tested
Lowest relevant dermal NOAEL Bentazone: 1000 mg/kg bw per day (highest dose
tested)
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Lowest relevant inhalation NOAEL No data
Genotoxicity Bentazone and its metabolites are not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect No data
Lowest relevant NOAEL No data
Carcinogenicity Bentazone: no carcinogenicity
6-Hydroxybentazone: no data
8-Hydroxybentazone: no data
Reproductive toxicity
Reproduction target/critical effect No data
Lowest relevant reproductive NOAEL No data
Developmental target/critical effect Bentazone: developmental effects (decreased fetal
weights and delayed ossification) below maternally
toxic dose
6-Hydroxybentazone: no data
8-Hydroxybentazone: no developmental toxicity at highest dose tested
Lowest relevant developmental NOAEL Rat: Bentazone: 100 mg/kg bw per day
6-Hydroxybentazone: no data
Rat: 8-Hydroxybentazone: 250 mg/kg bw per day
Neurotoxicity / Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw Long-term toxicity, rats 100
Acute reference dose Not considered
References
BASF (1992) Results of a range-finding prenatal toxicity study with
8OH-bentazon in rats after oral administration (gavage). Unpublished
study dated January/February 1992. Project No. 10R0437/91061 from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Becker, H., Frei, D., Vogel, W. & Terrier, C. (1986) Embryotoxicity
(including teratogenicity) study with bentazone technical in the rat.
Unpublished report, BASF RZ-Report No. 86/421 dated 30 December 1986
from RCC Research and Consulting Company AG, Itingen, Switzerland, RCC
Report No. 064530. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. (1993) Cytogenetic study in vivo of 8-OH-bentazon in mice
micronucleus test single oral administration. Unpublished document No.
BASF 93/10424 dated 6 May 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project
No.26M0437/914319. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of Reg. No.
51 929 (bentazone) (ZNT test substance No. 83/3) in the Ames test
(standard plate test with Salmonella typhimurium). Unpublished
document No. BASF 83/222 dated 10 June 1983 (original German report)
and 26 October 1983 (English translation) from BASF
Aktiengesellschaft, Department of Toxicology, Ludwigshafen/Rhein,
Germany. Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen,
Germany.
Gelbke, H.P. & Engelhardt, G. (1987a) Report on the study of
6-hydroxy-bentazon (ZNT test substance No. 86/244) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/023 dated 15 January
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0244/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1987b) Report on the study of
8-hydroxy-bentazon (ZNT test substance No. 86/391) in the Ames test
(standard plate test and preincubation test with Salmonella
typhimurium). Unpublished document No. BASF 87/0168 dated 14 May
1987 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No.40/1M0391/86. Submitted to WHO
by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hawkins, D.R., Elsom, L.F. & Girkin, R. (1986) Report on the urinary
metabolites of bentazone in the rat. Unpublished report, document No.
BASF 86/090 dated 31 January 1986 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigs-hafen, Germany.
Hawkins, D.R., Mayo, B.C., Pollard, A.D., Green, S.L., Biggs, S.R. &
Whitby, B.R. (1987) The biokinetics and metabolism of 14C-bentazon in
rats. Unpublished report, document No. BASF 87/0429 dated 16 September
1987 from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Hellwig, J. & Hildebrand, B. (1993) Study of the prenatal toxicity of
8-OH-bentazon in rats after oral administration (gavage). Unpublished
report, document No. BASF 93/10572 dated 9 June 1993 from BASF
Aktiengesellschaft, Abteilung Toxicologie, Ludwigshafen/Rhein,
Germany, report No. 30R0437/91081. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany
Hildebrand, B. & Kirsch, P. (1982) Report on the study of the acute
oral toxicity in rats of Reg. No. 51 929 -- bentazone. Unpublished
document No. BASF 83/114 dated 30 November 1982 (original in German);
English translation dated 21 June 1983 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. (1993) Study of the dermal toxicity of Reg. No. 51 929
(bentazone) in white rabbits: Application to the intact skin over 3
weeks. Unpublished report, document No. BASF 93/10760 dated 9 June
1993 from BASF Aktiengesellschaft, Department of Toxicology,
Ludwigshafen/Rhein, Germany, Project No. 41H0243/91042. Submitted to
WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1986) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/002 dated 29 December 1986 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Kirsch, P. & Kieczka (1987) Report on the study of acute oral toxicity
on the rat based on OECD and EPA (FIFRA). Unpublished report, document
No. BASF 87/030 dated 21 January 1987 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project.
Submitted to WHO by BASF Aktiengesellschaft, Ludwigshafen, Germany.
Mellert, W., Deckardt, K., Kuttler, K. & Hildebrand, B. (1993)
Subchronic oral toxicity study with 8-OH-bentazon in Wistar rats.
Administration in the diet for 3 months. Unpublished report No. BASF
93/11011 dated 13 September 1993 from BASF Aktiengesellschaft,
Department of Toxicology, Ludwigshafen/Rhein, Germany, Project No.
31S0437/91060. Submitted to WHO by BASF Aktiengesellschaft,
Ludwigshafen, Germany.
Mullerschon (1992) Gene mutation assay in Chinese hamster V79 cells in
vitro with 8-OH bentazone. Unpublished report No. BASF 92/11329 dated
20 October 1992 from CCR Cytotest Cell Research GmbH & Co. KG,
Germany. BASF Project No. 50M0437/919018. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
Tennekes, H., Horst, K., Luetkemeier, H., Wilson, J., Vogel, W. &
Terrier, C. (1987) Report on the 13-week oral toxicity (feeding) study
with bentazone technical in the rat. Unpublished report, document No.
BASF 87/0173 dated 12 May 1987 from RCC Research and Consulting
Company AG, Itingen, Switzerland. Submitted to WHO by BASF
Aktiengesellschaft, Ludwigshafen, Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
