
DIPHENYLAMINE (addendum) JMPR 1998
First draft prepared by
A. Protzel
Environmental Protection Agency
Washington DC, United States
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reroductive toxicity
Developmental toxicity
Special studies
Cystic kidney disease
Renal papillary necrosis
Comments
Toxicological evaluation
References
Explanation
Diphenylamine was first evaluated by the JMPR in 1969 (Annex 1,
reference 12), when an ADI of 0.025 mg/kg bw was established on the
basis of a NOAEL of 2.5 mg/kg per day in a two-year study in dogs.
Diphenylamine was re-evaluated in 1976 (Annex 1, reference 26), when
an ADI of 0-0.02 mg/kg was allocated on the basis of a NOAEL of 1.5
mg/kg per day for Heinz-body formation reported in a six-month study
in mice and a safety factor of 100. The 1982 JMPR considered
impurities in commercial-grade diphenylamine and concluded that
additional data on this aspect were desirable (Annex 1, reference 38);
the ADI was made temporary, and the Meeting required additional data
on teratogenicity, haematological effects, and mutagenicity. The 1984
JMPR established an ADI of 0-0.02 mg/kg bw for diphenylamine of 99.9%
purity on the basis of a NOAEL of 1.5 mg/kg bw per day in mice (Annex
1, reference3 42).
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Uniformly ring-labelled 14C-diphenylamine was administered to
groups of five male and five female Sprague-Dawley rats orally in corn
oil as a single oral dose of 5 mg/kg bw, as a single oral dose of 5
mg/kg bw preceded by 5 mg/kg bw per day of non-radioactive
diphenylamine for 14 days, or as a single oral dose of 750 mg/kg bw.
Urine, faeces, and cage washes were collected 4, 8, 12, and 24 h after
dosing and at 24-h intervals up to 168 h thereafter. The recovery of
radiolabel in urine after 168 h, representing 68-89% of the dose,
indicated extensive absorption of the compound. Total recovery of
radiolabel 168 h after dosing accounted for 94-105% of the dose. After
the single dose of 5 mg/kg bw, the mean percent of radiolabel
recovered was 81% in urine, 9.1% in faeces, and 9.2% in cage washes
for males, and 72% in urine, 16% in faeces, and 11% in cage washes for
females. When this dose was preceded by the 14-day pretreatment, 89%
of the radiolabel was recovered in urine, 7.6% in faeces, and 7.7% in
cage washes for males, and 68% in urine, 21% in faeces, and 12% in
cage washes for females. After the high single dose, 75% of the
radiolabel was found in urine, 15% in faeces, and 4% in cage washes
for males, and 73% in urine, 8.8% in faeces, and 11% in cage washes
for females. The mean percent of the dose in residual carcass plus
tissues was 0.41% in males and 0.28% in females at the high dose and
0.14- 0.28% of the dose at the other dosages (Wu, 1993).
Uniformly ring-labelled 14C-diphenylamine was administered in
capsules with corn meal to two female Toggenburg goats at a dose of 50
mg/kg bw per day for seven days. The doses were based on feed
consumption and were targeted to yield a dose equivalent to the
consumption of feed containing diphenylamine at 50 ppm. An additional
goat received the vehicle alone. Urine, faeces, and milk were
collected twice daily, covering 0-8 h after dosing and 8-24 h after
dosing, and cages were washed once daily. The goats were sacrificed
24-26 h after the last dose, and the liver, kidneys, omental and back
fat, loin muscle, and leg muscle were analysed for residues and
metabolites. Urine was the major route of elimination, the two goats
eliminating 85-91% of the daily dose in urine, 3.4-8.6% in faeces, and
0.52-0.78% in milk; the cage washes contained 1-3.8% of the dose. A
total of 92-96% of the dose was recovered. The cumulative percent of
the dose that was excreted (96.5% for both goats) was very similar to
the values for percent of the daily dose excreted, indicating that
each dose of the test material was largely excreted within 24 h. The
concentrations of residues in milk, expressed as ppm
14C-diphenylamine equivalents, plateaued on the first day and were
0.77-0.91 ppm for goat 2 and 0.53-0.66 ppm for goat 3 after the 8-h
collection period and 0.22-0.43 ppm for both goats after the 16-h
periods. The total amounts of radiolabelled residues in tissues were
0.1-0.11 ppm in liver, 0.07-0.12 ppm in kidney, 0.006-0.007 ppm in leg
muscle, 0.006-0.008 ppm in loin muscle, 0.021-0.026 ppm in back fat,
and 0.02 ppm in omental fat (Kim-Kang, 1994a).
Uniformly ring-labelled 14C-diphenylamine was administered in
capsules with corn meal to 20 laying hens (Gallus domesticus, Hyline
6-36) at a concentration equivalent to administration of diphenylamine
in the diet at 50 ppm, for seven days. Five additional hens received
the vehicle only. Eggs were collected twice a day during treatment;
excreta were collected daily. The hens were sacrificed 22-24 h after
the last dose, and liver, kidneys, skin (with fat), and thigh and
breast muscles were analysed for residues and metabolites. On days
2-7, 84-98% of the daily dose was recovered. Cumulative recovery of
radiolabel in the excreta was 91% of the dose. The concentrations of
residues in egg yolk, expressed in ppm as 14C-diphenylamine
equivalents, did not plateau during treatment and increased from less
than 0.01 ppm on day 1 to 0.31 ppm on day 7; no radiolabel was
detected in egg white. The total concentrations of radiolabelled
residues in tissues were 0.15 ppm in liver, 0.21 ppm in kidney,
< 0.01 ppm in thigh muscle, < 0.01 ppm in breast muscle, and 0.04
ppm in fat and skin (Kim-Kang, 1994b).
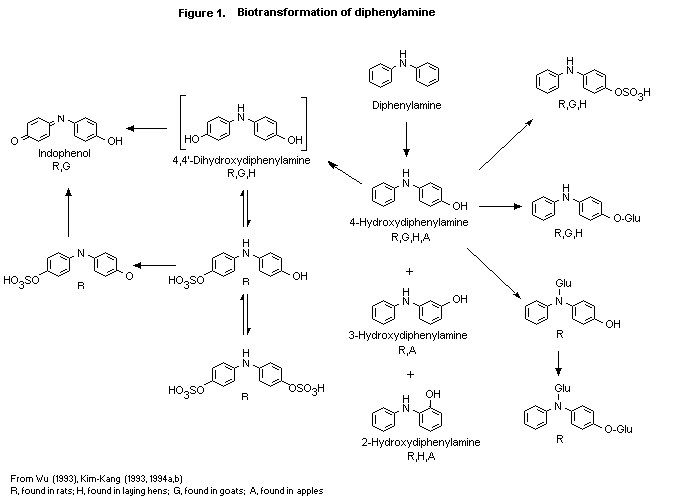
(b) Biotransformation
The biotransformation of diphenylamine was studied in rats,
treated as described above (Wu, 1993). Diphenylamine underwent
extensive biotransformation, as no more than 2.7% of the dose was
found as untransformed diphenylamine in any group. The structures of
the metabolites were elucidated by co-chromatography (high-performance
liquid or thin-layer chromatography) or mass spectral techniques. The
following 12 metabolites were identified at all doses:
4,4'-dihydroxydiphenylamine (unconjugated and as the O-sulfate and
the O, O-disulfate), 4-hydroxy-diphenylamine (unconjugated and as
the O-glucuronide, N-glucuronide, O-sulfate, and
O, N-diglucuronide), indophenol (unconjugated and as the
O-sulfate), 3-hydroxydiphenylamine and 2-hydroxydiphenylamine. These
metabolites plus parent accounted for 82-92% of the dose in excreta
and were found mainly as their sulfate and glucuronide conjugates.
Some quantitative sex- and dose-related differences in the metabolite
patterns were seen. The proposed metabolic pathway for the
biotransformation of diphenylamine in rats is shown in Figure 1.
Diphenylamine undergoes biotransformation involving hydroxylation at
various positions of the phenyl ring, primarily in the para
position, followed by sulfation and/or glucuronidation and excretion.
No cleavage of the diphenylamine structure was observed.
In the study of Kim-Kang (1994a), described above, no metabolites
were identified in the urine and faeces of the two lactating goats. Of
the total 23% radiolabelled residue found in the liver, 5.9% was
identified as diphenylamine, 1.7% as 4-hydroxydiphenylamine, 2.3% as
4.4'-dihydroxy-diphenylamine, 2.9% as 4-hydroxydiphenylamine
glucuronide, 8.3% as 4-hydroxydiphenylamine sulfate, and 2.1% as
indophenol. Of the total 73% radiolabelled residue in kidney, 36% was
identified as diphenylamine, 12% as 4-hydroxydiphenylamine
 glucuronide, 24% as 4-hydroxy-diphenylamine sulfate, and 1.3% as
indophenol. Of the total 94% radiolabelled residue in milk, 7.4% was
identified as diphenylamine, 39% as 4-hydroxydiphenylamine
glucuronide, and 47% as 4-hydroxydiphenylamine sulfate. Of the total
40% radiolabelled residue in omental fat, 36% was identified as
diphenylamine and 3.6% as 4-hydroxydiphenylamine.
In the study in hens (Kim-Kang, 1994b), no metabolites were
identified in urine or faeces. Of the total 85% radioactive residue in
egg yolks, 17% was identified as diphenylamine, 4.8% as
4-hydroxydiphenylamine, 0.6% as 4.4'-dihydroxydiphenylamine, 3.2% as
4-hydroxydiphenylamine glucuronide, 57% as 4-hydroxydiphenylamine
sulfate, and 1.9% as a polar oligomer conjugate of
4-hydroxydiphenylamine. Of the total 31% radiolabelled residue in
liver, 7.9% was identified as diphenylamine, 4.5% as
2-hydroxydiphenylamine, 3% as 4.4'-di-hydroxydiphenylamine, 1.4% as
4-hydroxydiphenylamine glucuronide, 8.6% as 4-hydroxydiphenylamine
sulfate, 4.7% as a polar oligomer conjugate of 4-hydroxydiphenylamine,
and 1.3% as indophenol. Of the total 39% radiolabelled residue in
kidney, 1.1% was identified as diphenylamine, 0.3% as
2-hydroxy-diphenylamine, 0.2% as 4-hydroxydiphenylamine sulfate, and
38% as a polar oligomer conjugate of 4-hydroxydiphenylamine. Of the
total 58% radiolabelled residue in skin and fat, 35% was identified as
diphenylamine and 23% as 4-hydroxydiphenylamine sulfate.
The biotransformation of 14C-diphenylamine was also studied in
Red Delicious apples. Residues of a number of plant metabolites were
identified in apple peel and pulp, and untransformed diphenylamine was
the major contributor to the total residue 40 weeks after application.
The major metabolite was 4-hydroxydiphenylamine, present as the
glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxydiphenylamine, and
dihydroxydiphenylamine (possibly the 2,4-isomer). These compounds were
present free or as conjugates with mono- or oligosaccharides
(Kim-Kang, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of diphenylamine are
summarized in Table 1. After acute oral administration to rats in one
study, diphenylamine (purity, 99.9%) was slightly toxic, with an LD50
of 3000 mg/kg bw in males and 2700 mg/kg bw in females (Spanjers &
Til, 1982). In another study, diphenylamine (purity, 99-100.1%) was
generally not toxic, the LD50 being > 15 000 mg/kg bw for animals of
each sex (Majnarich, 1991a).
Table 1. Acute toxicity of diphenylamine in rats
Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Male Oral 99.9 3000 Spanjers & Til (1982)
Female 2700
Male and female Oral 99.9-100.1 > 15 000 Majnarich (1991a)
Not reported Dermal (24 h) 99.9-100.1 > 5000 Majnarich (1991b)
The acute dermal LD50 after a 24-h exposure to diphenylamine
(purity, 99.9-100.1%) was > 2 g/kg bw in New Zealand white rabbits of
each sex. No clinical signs were noted (Majnarich, 1991b).
Diphenylamine (purity, 99.9-100.1%) applied to the eyes of one
rabbit for seven days without rinsing was corrosive and induced
corneal opacity (Kreuzmann, 1991a). The same preparation was not
irritating to the skin of rabbits (Kreuzmann, 1991b). Diphenylamine
(purity, 99.9%) did not produce dermal sensitization in guinea-pigs
(Kiplinger, 1995).
(b) Short-term studies of toxicity
Mice
Groups of 15 male and 15 female Swiss-derived CD-1 mice received
technical-grade diphenylamine in the diet at 0, 10, 520, 260, or 5200
ppm for 90 days, equal to doses of 1.7, 94, 440, and 920 mg/kg bw per
day in males and 2.1, 110, 560, and 1100 mg/kg bw per day in females.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmological and haematological examinations
were carried out, organs were weighed, and the animals were examined
grossly and histopathologically. The hair of animals at the
intermediate and high doses had a greenish tint, which may have been
due to staining with diphenylamine or a metabolite. Three deaths
occurred among controls and among males at the high dose; two of the
latter had enlarged spleens, and one also had cystitis, probably
related to treatment. There were no treatment-related effects on body
weight, food consumption, or ophthalmic parameters. Haematology
indicated dose-related decreases in erythrocyte counts and haematocrit
in animals at the two higher doses that were statistically
significantly different from controls. The values for mean corpuscular
haemoglobin, mean corpuscular volume, and mean corpuscular haemoglobin
content increased with dose and were statistically significantly
different from those of controls in animals at the two higher doses;
the mean corpuscular haemoglobin content was also statistically
significantly increased in males at 525 ppm. The reticulocyte counts
increased with dose and were statistically significantly different
from those of controls at the high dose. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly different from those of controls at the
two higher doses; the relative weights of the kidney and heart were
statistically significantly different from those of controls in mice
at the high dose. In females, the absolute and relative weights of the
spleen increased with dose and were statistically significantly
different from those of the controls in animals at the two higher
doses; the absolute and relative weights of the liver and the relative
weights of the kidney were statistically significantly different from
those of controls in females at the high dose. Necropsy of females
revealed dark, enlarged spleens at the three higher doses, dark livers
at the two higher doses, and dark kidneys at the highest dose. In
males, necropsy showed dark, enlarged spleens and dark livers at the
two higher doses. Histopathological examination of the liver showed
increased pigment deposition and slight haematopoiesis in animals of
each sex at the two higher doses. The spleen showed haemosiderosis and
congestion at the three higher doses, reaching incidences of 14/15 or
more at the two higher doses; the severity of spleen haematopoiesis
was also increased at the three higher doses. The kidneys showed
pigment deposition at the two higher doses. Cystitis was observed in
9/15 males at the high dose and in 2/15 females at 2625 ppm and 8/14
females at 5250 ppm. The cellularity of the bone marrow was increased
at the two higher doses. The NOAEL was 10 ppm, equal to 1.7 mg/kg bw
per day, on the basis of changes in haematological parameters and
findings at necropsy (Botta, 1992).
Rats
Groups of 10 male and 10 female Sprague Dawley rats received
technical-grade diphenylamine in the diet at 0, 150, 1500, 7500, or 15
000 ppm for 90 days, equal to doses of 0, 9.6, 96, 550, and 1200 mg/kg
bw per day in males and 0, 12, 110, 650, and 1300 mg/kg bw per day in
females. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmic, urinary, haematological, and
clinical chemical end-points, organ weights, and gross and
histopathological appearance. Greenish hair was first seen in females
at 1500 ppm, later in 60% of males and 100% of females at 7500 ppm,
and then in 70% of males and 100% of females at 15 000 ppm. Pale skin
was seen in 100% of females at 7500 and 15 000 ppm and in 40% of males
at 15 000 ppm. Two males at 15 000 ppm were found dead on day 6 of
dosing, apparently due to gastroenteritis; there were no other deaths.
The body weights and body-weight gains of animals of each sex at 7500
and 15 000 ppm were consistently and statistically significantly below
those of controls; although these values were generally lower than
those of controls in animals at 1500 ppm, they were not statistically
significantly different. Food consumption was not affected at any
dose. The frequency of darkening of the urine increased with dose,
starting with one female at 1500 ppm and 100% of rats at 15 000 ppm.
Haematological measures indicated decreased erythrocyte counts and
haemoglobin values, which were statistically significantly different
from those of controls in animals at 7500 and 15 000 ppm at
termination. The haematocrits were statistically significantly lower
than those of controls in females at the three highest doses. Small,
statistically significant increases in alkaline phosphatase activity,
albumin content, and albumin:globulin ratio in males and glucose and
albumin content and albumin:globulin ratio in females were observed at
7500 and 15 000 ppm. The cholesterol concentration increased with dose
in females and was statistically significantly different from that of
controls at the three higher doses. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly raised at 7500 and 15 000 ppm; the
relative weights of the kidney and gonad also increased with dose and
were also statistically significant at the two higher doses. In
females, the absolute and relative weights of the liver increased with
dose, and the change in relative weights was statistically significant
at doses > 1500 ppm. The kidneys were dark in animals of each sex
at 7500 and 15 000 ppm, and about 60% of the females at the high dose
had dark and/or enlarged livers. The spleens of both males and females
at the two higher doses were congested. Histopathological examination
revealed an increased incidence of haematopoiesis and pigment in the
liver, haematopoiesis, haemosiderosis, and congestion in the spleen,
and pigmented kidneys in animals of each sex at 7500 and 15 000 ppm.
The spleens of all females at 1500 ppm also showed an increase from
minimal to slight haematopoiesis and haemosiderosis. The NOAEL was 150
ppm, equal to 12 mg/kg bw per day, on the basis of increased clinical
signs of toxicity, clinical chemical changes, organ weights, and gross
and histopathological appearance (Krohmer, 1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of technical-grade diphenylamine
dissolved in distilled water at doses of 100, 500, or 1000 mg/kg bw
per day. The material was applied daily for 6 h to an area of clipped
skin corresponding to about 10% of the body surface and kept under
occlusion for 21 consecutive days, with terminal sacrifice on day 22.
Two additional groups of five rabbits of each sex served as vehicle
controls. The animals were observed for clinical signs, deaths,
ophthalmoscopic parameters, erythema, oedema, desquamation, and other
adverse skin reactions, body weight, food consumption, urinary,
haematological, and clinical chemical end-points, organ weights, and
gross and histopathological appearance, the latter limited to the
liver, kidneys, spleen, treated and untreated skin, and gross lesions.
There were no deaths or treatment-related effects on clinical signs,
body weights, food consumption, or haematological end-points. The only
possible treatment-related effects on clinical chemistry were on
sodium and potassium concentrations; females at all three doses had
depressed sodium values, and those at the intermediate and high doses
and males at the high dose had depressed potassium values with respect
to controls. Gross necropsy, revealed dark-red foci in the stomachs of
rabbits of each sex at the intermediate and high doses, which
increased in frequency with dose: the incidences were 1/5 in males at
the intermediate dose, 4/5 in those at the high dose, 1/5 in females
at the intermediate dose, and 2/5 in females at the high dose. No
dark-red foci were seen in the stomachs of controls or rabbits at the
low dose. The NOAEL for systemic toxicity was 100 mg/kg bw per day on
the basis of the presence of dark-red foci in the stomachs of males
and females. The NOAEL for dermal effects was 1000 mg/kg bw per day,
the highest dose tested (Siglin, 1992).
Dogs
Groups of four pure-bred beagle dogs of each sex received
technical-grade diphenylamine (purity, > 99%) in gelatin capsules at
doses of 0, 10, 25, or 50 mg/kg bw per day for 90 days. They were
observed for deaths, clinical signs, body weight, food consumption,
ophthalmological, haematological, clinical chemical, and urinary
parameters, organ weights, and gross and histopathological appearance.
There were no deaths, and no treatment-related changes were seen in
any of the above parameters. Statistically significant increases were
seen, however, in some clinical chemical parameters including albumin
content, the albumin:globulin ratio in males, and bilirubin content in
females at the high dose. These effects may have been incidental. The
NOAEL was 50 mg/kg bw/day, the highest dose tested (Krohmer, 1992b).
(c ) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 CD-1 mice of each sex received diets containing
technical-grade diphenylamine (purity, > 99%) at concentrations of 0,
520, 2600, or 5200 ppm for up to 78 weeks, equal to 0, 73, 370, and
760 mg/kg bw per day for males and 0, 90, 460, and 940 mg/kg bw per
day for females. Ten mice of each sex per dose were sacrificed at 52
weeks. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmological and haematological
end-points, organ weights, and gross and histopathological appearance.
An increase in the incidence of greenish staining of the fur,
especially around the anogenital area, with dose was seen as the study
progressed. By week 26, most of the mice at 5250 ppm were affected,
and by the end of the study some mice at 525 ppm group showed
staining. The incidence of penile prolapse increased with dose,
affecting seven males at 2625 ppm and 17 at 5250 ppm by 78 weeks. The
frequency of unkempt appearance also increased with dose, with a
higher incidence among males. The mortality rate increased with dose,
becoming statistically significantly different from controls for males
at 2625 and 5250 ppm by 52 weeks. The deaths were attributed mainly to
cystitis among males and amyloidosis in females. The mean body-weight
gains were 87, 86, and 91% of control values for males at 5250 ppm and
104, 93, and 93% of control values for females at 5250 ppm at 13, 52,
and 78 weeks, respectively. The body-weight gains of males at 5250 ppm
and occasionally animals at 2625 ppm were statistically significantly
decreased throughout the study (mainly through week 58). The
body-weight gain of females at 5250 ppm was significantly decreased
during the first three weeks and then occasionally for the remainder
of the study. Mean food consumption was statistically significantly
decreased during the first week of treatment in males at the high dose
and remained increased throughout treatment for males at the two
higher doses, with occasional statistically significant differences
from controls. The food consumption of females at any dose showed
little or no statistically significant difference from that of
controls.
At the interim haematological evaluation at 52 weeks, males
showed dose-related decreases in haematocrit and erythrocyte counts
that reached statistical significance at 2625 and 5250 ppm; and mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin content increased with dose and reached statistical
significance in males at these doses. Females showed dose-related
decreases in haematocrit that reached statistical significance at
> 525 ppm; their erythrocyte counts decreased in a dose-related
fashion and reached statistical significance at 2625 and 5250 ppm.
Mean corpuscular volume, mean corpuscular haemoglobin, and mean
corpuscular haemoglobin content increased with dose, the latter two
reaching statistical significance at 2625 and 5250 ppm and the mean
corpuscular volume at 5250 ppm. At termination at 78 weeks, males and
females showed dose-related decreases in haematocrit and erythrocyte
counts that reached statistical significance at 2625 and 5250 ppm;
reticulocyte counts, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin content increased with
dose and reached statistical significance in males at these doses.
At the interim necropsy, darkened spleens were seen in most mice
at 2625 ppm and in all those at 5250 ppm. Darkened livers were seen in
most mice at 5250 ppm and pale kidneys in many. At terminal necropsy,
the livers of some mice at 2625 ppm and most at 5250 ppm were dark.
The spleens of most treated mice were dark and often enlarged.
Dose-related increases in the absolute and relative weights of the
spleen, liver, and heart were seen in animals of each sex at the
interim and final sacrifices. At interim sacrifice, the absolute
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly different from those of controls, while
the increases in the relative liver weights reached statistical
significance only at the highest dose. In females, the absolute and
relative weights of the spleen were statistically significantly
increased only at the highest dose. The absolute and relative weights
of the heart were statistically significantly increased in females at
5250 ppm; the increases in males were not statistically different from
those in controls. At final sacrifice, the absolute and relative
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly increased. In females, the absolute and
relative weights of the spleen were significantly increased only at
5250 ppm,; the differences in relative liver weight reached
statistical significance at 2625 and 5250 ppm. The absolute and
relative weights of the heart were statistically significantly
increased in animals of each sex at 5250 ppm. Changes in the absolute
and relative weights of the pituitary and thyroid were not
consistently dose-related and were thus considered not to be of
toxicological significance.
Histopathological examination revealed treatment-related effects
in the kidney, liver, spleen, bone marrow, urinary bladder, and penis.
The incidences of haematopoiesis and pigment deposition in the liver
were increased at the intermediate and high doses. Of mice at 2625 ppm
that were found dead or moribund or were sacrificed at termination,
19/51 males and 24/51 females showed liver haematopoiesis and 15/51
males and 37/51 females showed liver pigmentation; higher incidences
of these effects were seen at the high dose. The incidences of spleen
congestion and haemosiderosis were increased in all treated mice. Of
the mice at the low dose found dead or moribund or sacrificed at
termination, 11/50 males and 8/50 females showed spleen congestion and
8/50 males and 35/50 females showed spleen haemosiderosis; higher
incidences of these effects were seen at the higher doses. The
frequency of pigment deposition was increased in males at the high
dose and in females at the intermediate and high doses. Among females
found dead or moribund or sacrificed at termination, pigment was found
in 5/51 at the intermediate dose and 7/51 at the high dose. Among
males found dead or moribund or sacrificed at termination, 5/51 at the
intermediate dose and 7/51 at the high dose showed pigment
accumulation; additionally, 5/54 males had pyelonephritis. Although
the incidence of spleen haematopoiesis did not increase with dose, the
severity scores increased from minimal or slight in controls to mixed
scores including moderate or marked at the high dose. The severity of
haematopoiesis in the spleen increased from minimal to slight at 525
ppm in contrast to the largely minimal level in controls.
Additionally, bone-marrow cellularity changed from predominantly
moderate in controls to marked at the higher doses. The incidences of
urinary bladder cystitis and dilatation increased with dose, reaching
statistical significance at the intermediate and high doses. Among
mice at the intermediate dose that were found dead or moribund or were
sacrificed at termination, 24/51 males and 13/48 females had cystitis
and 18/51 males and 13/48 females had dilatation; higher values were
seen at the high dose. The incidence of balanoposthitis apparently
increased with dose; however, no statistical analysis was available.
In females at 5250 ppm, the incidence of amyloidosis in the thyroid,
adrenals, kidneys, stomach, small intestine, ovaries, and uterus was
increased. The incidence of tumours was comparable in the treated and
control groups. The NOAEL for toxicity was 525 ppm, equal to 73 mg/kg
bw per day, on the basis of decreased body-weight gain, decreased
survival, and significant haematological and gross and microscopic
pathological alterations (Botta, 1994a).
Rats
Groups of 60 male and 60 female Sprague-Dawley rats received
diets containing technical-grade diphenylamine (purity, > 99%) at
concentrations of 0, 200, 750, 3750, or 7500 ppm for males and 0, 150,
500, 2500, or 5000 ppm for females for up to two years, equal to 0,
8.1, 29, 150, and 300 mg/kg bw per day for males and 0, 7.5, 25, 140,
and 290 mg/kg bw per day for females. Groups of 10 rats of each sex
per group were killed at a one-year interim sacrifice. The animals
were observed for clinical signs, deaths, body weight, food
consumption, ophthalmological, haematological, clinical chemical, and
urinary end-points, organ weights, and gross and histopathological
appearance.
The only treatment-related clinical finding was greenish
colouration of the fur in the urogenital or ventral cervical area in
animals of each sex at the two higher doses. The effect was attributed
to the presence of a metabolite in urine or faeces. No treatment-
related effects on mortality rates were observed; however, the study
was terminated at 102 weeks because of increased mortality rates in
controls and animals at the low dose: survival among males was 22% at
0 and 200 ppm and 55% at 7500 ppm. Survival thus seemed to increase
with dose, and an analysis of the survival data indicated a
statistically significant negative trend for mortality as the dose
increased in animals of each sex; the mortality rates at the two
higher doses were statistically significantly lower than those for the
control group. A dose-related decrease in body weight and body-weight
gain was seen throughout most of the study, which reached statistical
significance at the two higher doses. The body-weight gains of males
at the two higher doses were depressed to 95 and 87% of the control
values at 78 weeks and equal to those of controls at 102 weeks; in
females, the corresponding values were 78 and 56% of controls at 78
weeks and 80 and 61% at 102 weeks. There was no decrease in food
consumption, except during the first week; at other times, food
consumption appeared to have increased, possibly due to food wastage.
There were no treatment related effects on ophthalmological
parameters.
Haematological examination revealed dose-related decreases in
erythrocyte counts, haemoglobin, and haematocrit in animals at the two
higher doses throughout treatment. The decreases reached statistical
significance for erythrocyte count and haemoglobin in males at 3750
and 7500 ppm in week 26 and at termination and for erythrocyte counts,
haemoglobin, and haematocrit in females at 2500 and 5000 ppm through
most of the treatment and at termination. Although the erythrocyte
counts, haemoglobin, and haematocrit were decreased in males at 750
ppm and in females at 500 ppm, the decreases reached statistical
significance only sporadically during treatment. The mean corpuscular
volume and mean corpuscular haemoglobin content were significantly
different from those of controls in males at the three higher doses
and in females at the two higher doses.
Dose-related, statistically significant increases in the absolute
and relative weights of the spleen were seen in females at the two
higher doses at interim sacrifice and at termination. A similar effect
on spleen weights was observed in males, except that the changes in
rats at 3750 ppm were not statistically significant. The relative
weight of the liver was statistically significantly increased in
females at 5000 ppm at termination and at 3750 and 5000 ppm at interim
sacrifice; no increase in liver weights was observed in males. The
increases in spleen and liver weights are consistent with the
haematological effects of the compound. Findings of dark and/or
enlarged spleens at necropsy in males at > 750 ppm and in females
at 500 ppm are probably related to the haematological effects.
Microscopic examination revealed treatment-related effects in the
kidney, liver, spleen, and bone marrow. The incidence of pigment
deposition in the kidney increased in a dose-related fashion and
reached 44/50 in males and 44/52 in females at the high dose that were
found dead or moribund or were sacrificed at termination. The
incidences of haematopoiesis and pigment deposition in the liver
increased in a dose-related fashion and reached 21/50 and 27/50 in
males and 41/52 and 45/52 in females at the high dose that were found
dead or moribund or sacrificed at termination. Erythroid hyperplasia
was seen at the higher dose but not in controls or at the low dose.
The incidence of congestion of the spleen increased in a dose-related
fashion and reached incidences of 50/50 in males and 47/52 in females
at the high dose that were found dead or moribund or sacrificed at
termination. These findings are all related to the observed
haematological effects. No treatment-related increase in tumour
incidence was observed. The NOAEL for toxicity was 150-200 ppm, equal
to 7.5 mg/kg bw per day, on the basis of changes in haematological
parameters and in the histopathological appearance of the spleen,
kidney, and liver (Botta, 1994b).
Dogs
Four beagles of each sex received diphenylamine (purity, > 99%)
by gelatin capsule at a dose of 0, 10, 25, or 100 mg/kg bw per day for
52 weeks and were observed for clinical signs, body weight, food
consumption, ophthalmological, haematological, clinical chaemical, and
urinary end-points, organ weights, and gross and histopathological
appearance. No treatment-related clinical signs were seen at
termination. One dog at the intermediate dose and two at the high dose
had greenish hair. There were no deaths or treatment-related effects
on body weight, food consumption, or ophthalmological parameters.
Haematological examination revealed decreased mean erythrocyte counts
(by 11% in comparison with controls), haemoglobin (9.3%), and
haematocrit (8.7%) in males at the high dose; smaller decreases in
these parameters were found in females. The platelet count increased
with dose in males at the 13-, 26-, 39-, and 52-week evaluation
periods, becoming statistically significant at the intermediate and
high doses. There was a dose-related increase in mean total bilirubin
concentration, which was statistically significant for animals at the
the intermediate and high doses throughout the study, in animals at
the low dose at week 26, and in females only at week 39. The mean
cholesterol concentration appeared to increase with dose at all
evaluation periods but was statistically significantly increased only
in males at the high dose at week 13 (by 68%) and in females at the
high dose at week 39 (by 37%). The blood urea nitrogen concentration
was decreased in females at the intermediate (by 16%) and high doses
(by 20%) at week 52. The mean absolute and relative weights of the
liver and thyroid appeared to increase with dose in males, but only
the mean absolute liver weight of males at the high dose was
statistically significantly increased. The mean absolute and relative
weights of the thyroid decreased with dose in females but did not
reached statistical significance at any dose. There were no
treatment-related gross or histopathological changes. The NOAEL for
toxicity was 10 mg/kg bw per day on the basis of haematological and
clinical chemical changes (Botta, 1994c).
(d) Genotoxicity
As summarized in Table 2, negative results were obtained for
mutation in bacteria (Lawlor, 1992) and for induction of micronuclei
in mouse bone marrow in vivo (Murli 1992 a,b). A weakly positive
response was observed for mutation in mouse lymphoma cells in vitro
at a dose range that was toxic, and the mutant frequency did not
increase with dose (Cifone, 1992). The Meeting concluded that although
diphenylamine has some genotoxic potential it is unlikely to be a
human genotoxic hazard.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, groups of 28
Sprague-Dawley rats received diphenylamine (purity, 99.8%) in the diet
at concentrations of 0, 500, 1500, or 5000 ppm, equal to 0, 40, 120,
and 400 mg/kg bw per day for F0 males and 0, 46, 130, and 450 mg/kg
bw per day for F0 females for 70 days before mating. After weaning,
28 F1 rats of each sex per group were also given the test diets for
up to 70 days before mating and were selected as parents for the F2
litters. The parent animals were observed for deaths, clinical signs,
body weight, food consumption, mating and fertility indices and other
measures of reproductive performance, organ weights, and gross and
histopathological appearance. The offspring were observed at birth and
through lactation for clinical signs, deaths, weights, and sex ratio;
all underwent necropsy.
Toxicity was dose-related and was observed in animals of each sex
and generations at all doses. In general, females were more affected
than males, and F1 animals were more affected than F0 animals. In
the F0 generation, the incidences of bluish coats and a bluish fluid
in the cages were increased in males and females at 5000 ppm, and
similar findings were made in the F1 generation. Swelling of the
mammary glands were seen in females, and palpable masses were noted in
lateral or ventral regions, especially in females. These masses
appeared to be transient but were not examined grossly or
microscopically. Body weight and body-weight gain were significantly
decreased at various times in animals at 1500 and 5000 ppm. At 5000
ppm, there was a decrease in body weight compared with controls, of
6-9% for F0 males, 5-8% for F0 females, 22-28% for F1 males, and
11-23% for F1 females. At 1500 ppm, there was a 5-8% decrease in body
weight for F0 females, 7-9% for F1 males, and 5% for F1 females.
The relative weights of the kidney, liver, and spleen of F0 and F1
males were significantly increased at 5000 ppm, and the relative
Table 2. Results of assays for the genotoxicity of diphenylamine
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Forward mutation S. typhimurium 6.67-333 µg/platea 99.9 Negative Lawlor (1992)
TA98, TA100, TA1535, 10-667 µg/plateb
TA1537, TA1538
Gene mutation L5178Y tk+/- mouse 5-80 µg/ml > 93 Weakly Cifone (1992)
lymphoma cells positiveb
Negativea
In vivo
Micronucleus Mouse (ICR strain) 250-1000 mg/kg bw (males) 99.9 Negative Murli (1992a,b)
formation 375-1500 mg/kg bw (females)
weights of the liver and spleen of F0 and F1 females were
significantly increased at 1500 ppm. The relative weights of the liver
were significantly increased at 1500 and 5000 ppm in F0 females but
only at 5000 ppm in F1 females.
Gross observations at necropsy included enlarged spleens in
animals of each sex and generation at 1500 and 5000 ppm and in F1
females at 500 ppm, in addition to blackish-purple spleens in animals
of each sex and generation at 500, 1500, and 5000 ppm. The incidences
of blackish-purple spleen in F1 rats at 500 ppm were 9/28 males and
6/28 females; incidences of 27/28 or higher were seen at higher doses.
Histopathological examination revealed a brown pigment in the proximal
tubules of the kidney in animals of each sex and generation at 5000
ppm; and 3/28 F1 females at 1500 ppm also had brown pigment.
Congestion and haemosiderosis of the spleen were seen in animals of
each sex and generation at 500, 1500, and 5000 ppm. Increased spleen
erythropoiesis was seen in 4/28 males at 5000 ppm. Hepatocyte
hypertrophy was seen in males of both generations at 1500 and 5000
ppm; in females, it was seen at all doses in the F0 generation but
only at 5000 ppm in the F1 generation. Kupffer cells with brown
pigment containing iron were seen in animals of each generation, at
5000 ppm in males and at 1500 and 5000 ppm in females.
Reproductive toxicity was seen in the form of decreased mean pup
weight at various times in each generation and reduced litter size at
the high dose; no other parameters were affected by treatment. F1
pups at 5000 ppm had statistically significantly decreased mean body
weight (11% less than controls at birth and 14-25% less than controls
during lactation). F2 pups showed statistically significantly
decreased mean body weight at 1500 ppm (10-12% less than controls
during late lactation) and at 5000 ppm (10-19% less than controls
throughout lactation). The mean body weight of pups in the F2 litters
at birth was about 4.8% lower than that of controls, but the
difference was not statistically significant. The mean litter size
decreased with dose in both generations, by 21% at 5000 ppm in the F1
generation, and was statistically significantly different from control
values. Although the mean litter size in the F0 generation at 5000
ppm was decreased by 10%, this value was not statistically
significant. The decrease in litter sizes with dose correlated with
the number of uterine implantation scars observed at necropsy, and the
mean uterine implantation scar count decreased with dose in both
generations. The mean scar count at 5000 ppm was decreased by 16% and
was statistically significant; although the mean scar count in the F0
generation was decreased by 7.3% at 5000 ppm, the value was not
statistically significant.
A NOAEL was not identified for parental systemic toxicity.
Although haemosiderin and congestion in the spleen and hepatocyte
hypertrophy were seen at all doses, the incidence and intensity of
these effects were appreciably smaller at the lower dose. For example,
only 4/28 females at the low dose had spleen congestion, rated as
minimal, whereas 22/28 females at the intermediate dose had this
effect and its intensity was nearly equally divided between minimal
and mild. These observations suggest that that the lower dose in this
study is close to the NOAEL for parental systemic toxicity. The NOAEL
for reproductive toxicity was 1500 ppm, equivalent to 120mg/kg bw per
day for F0 animals, on the basis of decreased mean litter size at the
high dose. The NOAEL for developmental toxicity was 500 ppm,
equivalent to 46 mg/kg bw per day in maternal animals, on the basis of
the statistically significantly decreased mean body weights of F2
pups (Rodwell, 1993).
(ii) Developmental toxicity
Rats
In a range-finding study for developmental toxicity, groups of
six pregnant Sprague-Dawley Crl:CDTM BR VAF/Plus rats received
diphenylamine (purity, 99.9%) in corn oil by gavage at doses of 0, 10,
50, 100, 200, 300, or 400 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity was seen at doses of 100 mg/kg bw per day and
higher. Two deaths occurred at 400 mg/kg bw per day, and there were
dose-related decreases in food consumption, body weight, and body-
weight gain starting at a dose of 100 mg/kg bw per day. Necropsy
revealed a purplish-black spleen in one dam at 100 mg/kg bw per day
and in all surviving dams at higher doses. The increased incidence of
early resorptions was apparent dose-related and was accompanied by
decreases in mean fetal weight and gravid uterine weight at doses of
200 and 300 mg/kg bw per day. There were no treatment-related external
malformations or developmental variations. On the basis of the results
of this study, 0, 10, 50, and 100 mg/kg bw per day were selected for
use in the definitive study (Rodwell, 1992a).
In the definitive study, groups of 25 pregnant Sprague-Dawley
Crl:CDTM BR VAF/Plus rats received diphenylamine (purity, 99.9%) in
corn oil by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for deaths, clinical
signs, body weight, and food consumption. All rats were sacrificed on
day 20 of gestation and were necropsied grossly. The spleens were
weighed, and the uterus was removed, examined externally, weighed, and
opened for internal examination. The fetuses were observed externally,
and their viscera and skeleton were examination. There were no deaths
or treatment-related effects on clinical signs, body weights, or food
consumption. At necropsy, 5/25 dams at the high dose had enlarged,
blackish-purple spleens, and the mean weights of the spleens were
significantly greater than those of controls. No treatment-related
effects were seen on gross necropsy, in the appearance or weight of
the uterus, or in the numbers of viable fetuses, early and late
resorptions, and corpora lutea, or on external, visceral, or skeletal
malformations or variations in the fetuses. The NOAEL for maternal
toxicity was 50 mg/kg bw per day on the basis of effects on the spleen
in dams at the high dose. The NOAEL for developmental toxicity was 100
mg/kg bw per day, the highest dose tested, in the absence of
developmental effects (Rodwell, 1992b).
Rabbits
In a study of developmental toxicity, groups of 16-18 pregnant
New Zealand white rabbits received diphenylamine (purity, 99.9%) in 1%
methyl cellulose by gavage at a dose of 0, 33, 100, or 300 mg/kg bw
per day on days 7-19 of gestation. The dams were observed for deaths,
clinical signs, body weight, and food consumption. All rats were
sacrificed on day 29 of gestation and subjected to gross necropsy; the
ovaries and uterus of each animal were removed and examined to
determine the number of corpora lutea and the type, distribution, and
number of implantation sites. The fetuses were examined externally,
and their viscera and skeleton were analysed. No deaths occurred
during the study. The mean food consumption of dams at 300 mg/kg bw
per day was reduced on days 7-29 of gestation, and a slight decrease
in body weight was noted. Green discolouration of the urine was seen
in all treated animals. No treatment-related effects were seen at
necropsy or on embryonic or fetal growth or development. The NOAEL for
maternal toxicity was 100 mg/kg bw per day on the basis of decreased
body-weight gain and food consumption early during treatment. The
NOAEL for developmental toxicity was 300 mg/kg bw per day, the highest
dose tested (Edwards et al., 1983).
(f) Special studies
(i) Renal cystic disease
The issue of tubular cyst formation in diphenylamine-treated rats
was reviewed previously (Annex 1, references 13, 27, 39, and 43).
Additional observations on the induction of tubular cysts in the
kidneys of mice and rats treated with diphenylamine in the diet are
summarized below.
Renal histopathology and function were studied in male
Sprague-Dawley rats fed pelleted diets containing 1% w/w (i.e. 10 000
ppm) diphenylamine (purity unspecified) for 5-20 months. Control
animals received identical diets in which methyl cellulose was
incorporated instead of diphenylamine. The animals were observed for
renal tubular diameter and renal functional parameters including
intratubular hydrostatic pressure, single-nephron glomerular
filtration rate, and transit times through the loop of Henle; light
and scanning electron microscopy was performed. The luminal diameters
of 22 dilated nephrons from diphenylamine-treated rats were 34-110 µm,
and those of 10 undilated nephrons were 21-450 µm; the luminal
diameters in six nephrons of two control rats were 26-34 µm. The
intraluminal hydrostatic pressure was significantly higher in dilated
tubules than in undilated tubules from treated rats; there was no
difference in intraluminal hydrostatic pressures among control rats.
The single-nephron glomerular filtration rate was also similar in
dilated and undilated tubules, consistent with an intrinsic change in
glomerular function. The transit times through the loop of Henle were
three to four times faster in dilated than in undilated nephrons of
treated and control rats. Microscopic examination indicated that
dilatation along the proximal and collecting tubules was segmental,
not diffuse, some dilated tubules communicated with cysts deep in the
renal substance, the dilated collecting tubules occasionally contained
debris, tubules adjacent to cysts appeared to be compressed, and
diphenylamine-exposed kidneys contained proximal convoluted tubules
with segments of apparent narrowing. The authors concluded that cyst
formation results from initial obstruction and a subsequent increase
in intraluminal pressure arising from sustained glomerular filtration
and unaltered water reabsorption (Gardner et al., 1976).
The time-course of development of lesions in the renal collecting
tubules was studied in male Sprague-Dawley rats fed pelleted diets
containing 1% w/w (i.e. 10 000 ppm) diphenylamine (purity unspecified)
for up to 76 weeks. Control animals received standard diet. The
animals were observed for renal concentrating ability (urine
osmolality) at 2, 4, 5, and 20 weeks of treatment; light and
transmission and scanning electron microscopy was performed at 2, 5,
10, 15, 20, 25, 52, and 78 weeks of treatment; and autoradiography
with tritiated thymidine was conducted to determine whether
hypertrophy was the mechanism of cyst formation. Decreased urine
osmolality was seen in treated rats, which was statistically
significantly different from control values at 6 and 20 weeks.
Structural changes first appeared after five weeks of treatment. Light
microscopy of the medullary collecting tubules at five weeks revealed
focal areas of apparent thickening along the walls, resulting from
layering of cells. By 10 weeks, some tubules were dilated and
contained focal areas of cellular necrosis. By 15-20 weeks, many more
ducts were dilated and contained cast material. By 24 weeks, frank
cysts were visible in the cortex and medulla, and large collecting
tubular cysts were seen; some proximal tubules and renal corpuscles
were dilated. Over time, cysts were found in every segment of the
nephron and collecting tubules. Transmission electron microscopy at
five weeks revealed changes in the medullary collecting tubules. The
tubular cells appeared to be enriched in mitochondria, and their
apical border was studded with long microvilli. At 10 weeks, necrotic
cells were seen along the collecting tubules. Scanning electron
microscopy showed that the collecting duct cysts were lined with
irregular cells which no longer resembled collecting tubule cells. The
labelling index for the collecting tubules reached a plateau at weeks
5-15 and had decreased to background levels by week 52. Because the
number of nuclei counted on cross-sections of the collecting tubules
increased gradually with time, the authors concluded that the increase
in labelling index was the result of hyperplasia of the tubular cells
and not of a degeneration or regeneration phenomenon. The authors
concluded that one of the initial events in the pathogenesis of cystic
renal disease is a hyperplastic response of the collecting tubule
cells (Evan et al., 1978).
The effect of diphenylamine on the composition of the native
renal basement membrane was studied in male C57Bl/6 mice fed pelleted
diets containing 2% w/w (i.e. 20 000 ppm) diphenylamine (purity
unspecified) for up to 10 months. Control animals received chow
without diphenylamine. After 10 months of treatment, the mice were
sacrificed and their kidneys processed for light microscopy and
immunohistology of bamin, a glycoprotein associated with the matrix of
the glomerular basement membrane. Microscopy revealed cysts in the
cortical and medullary portions of the kidney. The cysts were lined
with flattened or somewhat cuboidal cells and contained a brownish
coagulum that appeared to be constituted of necrotic cells. Brownish
discolouration was also seen in the cytoplasm of neighbouring
non-cystic cells and in tubular cells of animals sacrificed before the
formation of cysts. The authors speculated that the brownish debris
preceded the formation of cysts. When immunohistology was performed
with antibodies to bamin, the antibodies bound to the glomeruli of
control mice but not to those of treated animals.This result was
obtained in the three-week experiment. In another series of
experiments, male C57Bl/6 mice were injected with EHS tumour cells and
five days later received a diet containing diphenylamine (at an
unspecified level). After three weeks, the animals were killed, and
tumours were removed for analysis of the proteins of the basement
membrane. Analysis by sodium dodecylate sulfate-polyacrylamide gel
electrophoresis and by immunoblot revealed the presence of a 75-80-kDa
protein in controls but little or none in the diphenylamine-treated
mice. The authors were unclear how the loss of bamin, a molecule
associated with the glomerular but not the tubular basement membrane,
would be related to the formation of tubular cysts. They speculated
that material from an abnormal glomerular filtrate might damage the
tubular epithelial cells and result in the formation of tubular cysts
(Rohrbach et al., 1993).
(iv) Renal papillary necrosis
A series of studies has been reported on the induction of renal
papillary necrosis by diphenylamine in hamsters, rats, and Mongolian
gerbils. The development of animal models for this lesion is of
interest because it is the initial stage in human analgesic-induced
nephropathy.
Groups of 10 male Syrian hamsters, 40 Sprague-Dawley rats, and 40
Mongolian gerbils received diphenylamine (purity unspecified) at doses
of 0, 400, 600, or 800 mg/kg bw per day in peanut oil by gavage for
three days. Animals that became moribund were sacrificed and
necropsied; survivors were sacrificed 24 h after the third dose of
diphenylamine. All animals were observed for death and were examined
for gross and microscopic lesions. Forty percent of hamsters at the
low dose and 100% of those at the two higher doses died or were
sacrificed in extremis during treatment; there were no deaths among
the rats or gerbils. At necropsy, brown kidneys and yellow-brown
papillae were seen in 50% or more of hamsters at the intermediate and
high doses and in none of those at the low dose. The only gross lesion
in hamsters at the low dose was splenomegaly (90%), which was not seen
at higher doses. Microscopic examination revealed total renal
papillary necrosis, i.e. necrosis of all elements of the renal
papillae, including collecting tubules, at all doses in 4/10 hamsters
at the low, 7/10 at the intermediate, and 4/10 at the high dose. Only
two rats at the high dose had renal lesions, which consisted of
necrosis of the medullary interstitial cells and vasa recta, limited
to the apex, and degeneration of the renal interstitial matrix. As
noted by the authors, the limited incidence and extent of the lesions
in the Sprague-Dawley rats contrasted with the more extensive effects
observed in Wistar rats by Powell et al. (1985). No effects were
observed in Mongolian gerbils (Lenz & Carlton, 1990).
A total of 27 male Syrian hamsters were given diphenylamine by
gavage (purity unspecified) dissolved in peanut oil at a dose of 600
mg/kg bw. At intervals of 0.5, 1, 2, 4, 8, 16, and 24 h after dosing,
three hamsters were perfused in situ with fixative and their kidneys
were removed for examination by transmission electron microscopy.
Starting 1 h after dosing, the basal plasma membrane of the
endothelial cells of the ascending vasa recta in the proximal portion
of the renal papilla became separated from the basal lamina, forming
large subendothelial vacuoles. These alterations persisted during the
first 8 h after dosing. By 16 h, the endothelial cell nuclear
membranes and the luminal plasma membrane had become convoluted, and
by 24 h platelets were seen to adhere to the basal lamina, which had
become exposed. By 2 h after dosing, the adjacent interstitial cells
started undergoing structural alterations, and by 24 h there was
necrosis. Ultrastructural alterations became visible in the thin limb
of the loop of Henle by 24 h and in collecting tubule epithelial cells
by 14-16 h. The authors speculated that the selective effect on the
endothelial cells of the ascending vasa recta were attributable to
generation of a toxic metabolite by the endothelial cell or to
interference with the metabolism of the endothelial cells by a
metabolite of diphenylamine (Lenz et al., 1995).
The toxicity of diphenylamine dissolved in dimethyl sulfoxide to
renal papilla was studied in Syrian hamsters, rats, and Mongolian
gerbils. Male Syrian hamsters and Sprague-Dawley rats were given
diphenylamine (purity unspecified) by gavage at doses of 0, 400, 600,
or 800 mg/kg bw per day for up to nine days. Only one of 30 hamsters
at 400 mg/kg bw per day showed renal papillary necrosis. This result
contrasted with previous results (Lenz & Carlton, 1990) in which oral
administration of diphenylamine dissolved in peanut oil produced
extensive necrosis in male Syrian hamsters at the same doses;
furthermore, pretreatment of the hamsters with dimethyl sulfoxide
protected the animals against the renal papillary necrosis induced by
administration of diphenylamine. Renal papillary necrosis occurred in
4/30 rats at the high dose; none was seen in Mongolian gerbils. The
incidence in rats and gerbils did not differ from that observed when
peanut oil was used as the vehicle in a previous study (Lenz &
Carlton, 1990). The mechanism by which dimethyl sulfoxide exerts its
protective effect in Syrian hamsters was not studied (Lenz & Carlton,
1991).
The effect of diphenylamine on renal glutathione concentrations
was studied in groups of eight male Syrian hamsters given
diphenylamine (purity unspecified) by gavage at a dose of 0, 200, 400,
or 600 mg/kg bw 1 and 4 h after dosing. The concentrations in the
cortical area decreased with dose, reaching statistical significance
at the low dose at 1 h and at the intermediate dose at 4 h; the
concentrations at 1 h were 41% of the control value at 200 mg/kg bw,
31% at 400 mg/kg bw, and 29% at 600 mg/kg bw. The glutathione
concentrations in the outer medulla and the renal papilla were not
statistically significant decreased.
In order to study the effect of glutathione reduction on toxicity
to renal papilla, groups of eight male Syrian hamsters were given
buthionine sulfoxime, an inhibitor of glutathione biosynthesis, at 500
mg/kg bw intrapeitoneally, diphenylamine in peanut oil at 400 mg/kg bw
by gavage, the same doses of buthionine sulfoxime plus diphenylamine,
or the peanut oil vehicle alone. The animals were sacrificed 24 h
after administration of diphenylamine for gross and microscopic
examination of the kidney. No gross or microscopic lesions indicative
of papillary necrosis were observed in any group. Because a dose of
diphenylamine that is toxic to the papilla did not deplete glutathione
in that region and because depletion of glutathione (to 71% of the
control value) by buthionine sulfoxime did not enhance the toxicity of
diphenylamine to the papilla, the author concluded that this toxic
effect of diphenylamine is mediated by mechanisms independent of
oxidative injury (Lenz, 1996).
Comments
After oral administration to rats, goats, or hens,
14C-diphenylamine was extensively absorbed and rapidly excreted. In
rats given a single dose, 45-72% appeared in the urine within 24 h and
68-89% by 168 h after dosing, with less than 0.4% of the dose in the
residual carcass and less than 0.05% in any individual tissue. In
goats treated with single daily doses for seven days, 85-91% of the
daily dose was excreted in urine and 0.5-0.8% in milk; the
concentrations of residues in milk plateaued after 24 h. When laying
hens were treated with single daily oral doses for seven days, 84-98%
of the daily dose was recovered in the excreta; the concentrations in
egg yolk reached 0.31 mg/kg on day 7, but no residues were found in
egg whites. Diphenylamine underwent extensive biotransformation in
rats, goats, and hens, with ring hydroxylation and formation of
glucuronide and sulfate conjugates. In addition to untransformed
diphenylamine (< 3% of the dose), the following 12 metabolites were
identified at all doses: 4,4'-dihydroxydiphenylamine (unconjugated and
as the O-sulfate and the O, O-disulfate), 4-hydroxydiphenylamine
(unconjugated and as the O-glucuronide, N-glucuronide,
O-sulfate, and O, N-diglucuronide), indophenol (unconjugated and
as the O-sulfate), 3-hydroxydiphenylamine, and
2-hydroxydiphenylamine. These metabolites accounted for about 80-90%
of the dose and were excreted largely as their sulfate and glucuronide
conjugates. There was no cleavage of the diphenylamine structure.
Except for a polar oligomer of 4-hydroxydiphenylamine found only in
the eggs and tissues of hens, all of the metabolites reported in hens
and goats were detected in rats. Residues of a number of plant
metabolites were identified in apple peel and pulp, but untransformed
diphenylamine was the major contributor to the total residue 40 weeks
after application. The major metabolite was 4-hydroxydiphenylamine, as
the glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxy-diphenylamine, and
dihydroxydiphenylamine (possibly the 2,4 isomer). These compounds were
either free or conjugated with mono- or oligosaccharides. Although all
of the hydroxylated metabolites (aglycones) identified in plants were
seen in rats, the conjugating species were generally different.
After acute oral administration to rats, diphenylamine (99.9%
pure) was slightly toxic (LD50 about 3000 mg/kg bw) in one study,
whereas in another study super-refined diphenylamine (purity,
99.0-100.1%) was essentially nontoxic (LD50 > 15 000 mg/kg bw).
WHO has not classified diphenylamine for acute toxicity.
In mice given diphenylamine at dietary concentrations of 0, 10,
520, 2600, or 5200 ppm for 90 days, dose-related changes in
haematological parameters (decreased erythrocyte counts and packed
cell volumes and increased reticulocyte counts) were observed. The
mean corpuscular haemoglobin count was significantly increased at
dietary levels of 520 ppm and above. Necropsy revealed dark, enlarged
spleens with haemosiderosis and congestion at dietary levels of 520
ppm and above. Spleen haematopoiesis was increased in animals of each
sex at 520 ppm. The NOAEL was 10 ppm (equal to 1.7 mg/kg bw per day)
on the basis of changes in haematological parameters and findings at
necropsy in animals at 520 ppm.
In rats that received diphenylamine in the diet at concentrations
of 0, 150, 1500, 7500, or 15 000 ppm for 90 days, body weights and
body-weight changes, although generally lower than the control values
at 1500 ppm, were not significantly different from those of controls
at doses below 7500 ppm. The cholesterol concentration increased with
dose in females and was significantly different from that of controls
at 1500 ppm. In females, the absolute and relative weights of the
liver increased with dose, and the relative liver weights were
significantly different from those of controls in animals at 1500 ppm
and above. Histopathological examination revealed increased
haematopoiesis and pigment in the liver, haematopoiesis,
haemosiderosis, and congestion in the spleen, and pigmented kidneys in
animals of each sex at 7500 and 15 000 ppm. Additionally, the spleens
of all females at 1500 ppm showed minimal or slight haematopoiesis and
haemosiderosis. The NOAEL was 150 ppm (equal to 12 mg/kg bw per day)
on the basis of changes in clinical chemical parameters, increased
organ weights, and gross and histological changes in female rats at
1500 ppm.
Groups of rabbits were exposed dermally to diphenylamine in
distilled water at doses of 0, 100, 500, or 1000 mg/kg bw per day for
6 h per day. No effects were observed. Gross necropsy revealed
dark-red foci in the stomachs of rabbits at the intermediate and high
doses, which increased in number with dose. The NOAEL for systemic
effects was 100 mg/kg bw per day on the basis of effects on the
stomach at 500 mg/kg bw per day in animals of each sex.
Groups of dogs received diphenylamine by gelatin capsule at doses
of 0, 10, 25, or 50 mg/kg bw per day for 90 days. There were no
treatment-related effects. The NOAEL was 50 mg/kg bw per day, the
highest dose tested.
In a study of carcinogenicity, mice received diets containing
diphenylamine at concentrations of 0, 520, 2625, or 5200 ppm for 78
weeks. At 520 ppm and above, decreased packed cell volumes were seen
in females and spleen congestion and haemosiderosis in animals of each
sex. At 2600 ppm and above, clear haematological effects, consistent
with regenerative anaemia, were observed. The incidences of tumours
were not increased when compared with those in controls. The NOAEL for
toxicity was 520 ppm (equal to 73 mg/kg bw per day) on the basis of
decreased body-weight gain, reduced survival, and significant
alterations in haematological and gross and microscopic pathological
parameters at higher levels. Examination of the incidence and severity
of some haematological effects at 520 ppm suggested that this dose is
close to the NOAEL/LOAEL threshold. There was no evidence of
carcinogenicity.
In a combined study of toxicity and carcinogenicity, rats
received diets containing diphenylamine at concentrations of 0, 200,
750, 3750, or 7500 ppm (males) and 0, 150, 500, 2500, or 5000 ppm
(females). At 500 ppm and above, decreased erythrocyte count,
haemoglobin, and packed cell volumes were observed in animals of each
sex; these decreases reached statistical significance only
sporadically during treatment in males at 750 ppm and in females at
500 ppm. Haematopoiesis was increased in the liver and spleen of males
at 750 ppm and above. At 2625 ppm and above, clear haematological
effects consistent with regenerative anaemia were observed. There was
no significant increase in the incidence of tumours when compared with
that in controls. The NOAEL was 150-200 ppm (equal to 7.5 mg/kg bw per
day) based on haematological and histological effects at dietary
levels equal to or greater than 500 ppm. This appeared to be close to
the threshold dose, since body-weight gain was not depressed and only
sporadic haematological changes were observed at 500-750 ppm. There
was no evidence of carcinogenicity.
Dogs received diphenylamine by gelatin capsule at doses of 0, 10,
25, or 100 mg/kg bw per day for one year. Platelet counts in males and
total bilirubin concentrations in animals of each sex were
statistically significantly higher than those of controls. The NOAEL
for toxicity was 10 mg/kg bw per day, and the LOAEL was 25 mg/kg bw
per day, both based on haematological and clinical chemical changes.
In a two-generation study of reproductive toxicity, rats received
diphenylamine in the diet at concentrations of 0, 500, 1500, or 5000
ppm during premating. A NOAEL for parental toxicity was not observed;
the LOAEL was 500 ppm (equal to 40 mg/kg bw per day) on the basis of
enlarged spleens in F1 females, increased spleen congestion and
haemosiderosis in animals of each sex in all generations, and
hepatocyte hypertrophy in F0 females. The NOAEL for developmental
toxicity was 500 ppm (equal to 46 mg/kg bw per day) on the basis of
statistically significantly decreased mean body weight in F2 pups at
1500 ppm and above. The NOAEL for reproductive toxicity was 1500 ppm
(equal to 120 mg/kg bw per day). Although haemosiderin, congestion of
the spleen, and hepatocyte hypertrophy were observed in parental
animals at all doses, they occurred at appreciably lower incidence and
intensity at the lower dose than at the higher doses, which suggested
that the lower dose was close to the NOAEL/LOAEL threshold for
parental toxicity.
In a study of developmental toxicity, rats received diphenylamine
by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on days 6-15
of gestation. The NOAEL for maternal toxicity was 50 mg/kg bw per day
on the basis of enlarged, blackish, heavier spleens at 100 mg/kg bw
per day. The NOAEL for developmental toxicity was 100 mg/kg bw per
day, the highest dose tested.
In a study of developmental toxicity, rabbits received
diphenylamine by gavage at doses of 0, 33, 100, or 300 mg/kg bw per
day on days 7-19 of gestation. The NOAEL for maternal toxicity was 100
mg/kg bw per day on the basis of decreased body-weight gain and food
consumption at 300 mg/kg bw per day. The NOAEL for developmental
toxicity was 300 mg/kg bw per day, the highest dose tested.
Diphenylamine has been tested for genotoxicity in three assays.
Negative results were obtained for mutation in bacteria and for
induction of micronuclei in mouse bone marrow in vivo. A weakly
positive response was observed only for mutation in mouse lymphoma
cells in vitro at a dose range in which the toxicity was relatively
high and the mutant frequency did not increase with dose. The Meeting
concluded that, although diphenylamine has some genotoxic potential,
it is unlikely to present a human genotoxic hazard.
An ADI of 0-0.08 mg/kg bw was established on the basis of the
NOAEL of 150 ppm, equal to 7.5 mg/kg bw per day, in the two-year study
of toxicity and carcinogenicity in rats and a 100-fold safety factor.
An acute RfD was not allocated because diphenylamine is of low
acute toxicity. The Meeting concluded that the acute intake of
residues is unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 520 ppm, equal to 73 mg/kg bw per day (78-week study of
carcinogenicity)
Rat: 150-200 ppm, equal to 7.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equal to 46 mg/kg bw per day (reproductive
toxicity in a two-generation study of reproductive
toxicity)
Rat 50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
300 mg/kg bw per day (highest dose in a study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.08 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid absorption: at least 68-89% of a dose
Dermal absorption No data
Distribution Extensive
Potential for accumulation Very little
Rate and extent of excretion At 24 h, 45-72% of a dose found in urine
Metabolism in animals Extensively metabolized. Parent < 3%. Approximately
80-90% of a dose appeared as 12 metabolites: indophenol
and various isomers of mono- and
di-hydroxydiphenylamine excreted in urine as sulfate
and glucuronide conjugates
Toxicologically significant compounds Parent. Indophenol might undergo electrophilic
(animals, plants, and environment) interactions
Acute toxicity
Rat: LD50 oral 3000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
LC50 inhalation No data
Skin irritation None to slight
Eye irritation Slight to corrosive with corneal opacity
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Erythrocytes/anaemia
Lowest relevant oral NOAEL Mouse: 1.7 mg/kg bw per day, 90-day study
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity Unlikely to be a human genotoxic hazard
Long-term toxicity and carcinogenicity
Target: critical effect Erythrocytes/haemolytic anaemia
Lowest relevant NOAEL Rat: 7.5 mg/kg bw per day
Carcinogenicity No carcinogenicity
Reproductive toxicity
Reproduction target /critical effect Decreased mean litter size in both generations and
decreased mean body weights of F2 pups at maternally
toxic doses
Lowest relevant reproductive NOAEL Rat: 46 mg/kg bw per day
Developmental target/critical effect Rat: No developmental toxicity
Lowest relevant developmental NOAEL Rat: 100 mg/kg bw per day, highest dose tested
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.08 mg/kg bw Long-term toxicity 100
and carcinogenicity,
rats
Acute reference dose Not allocated
(unnecessary)
References
Botta, J.A., Jr (1992) 90 Day subchronic toxicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426E-001-034-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994a) 18 Month oncogenicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426H-002-646-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994b) 24 Month combined oncogenicity/toxicity
evaluation of diphenylamine in rats. Unpublished report No.
426D-102-048-91 from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Botta, J.A., Jr (1994c) One year chronic study of diphenylamine in
dogs. Unpublished report No. 426B-502-044-91 from T.P.S., Inc., Mt
Vernon, Indiana, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Cifone, M. (1992) Mutagenicity test on diphenylamine in the L5178Y
TK+/- mouse lymphoma forward mutation assay. Unpublished report No.
14902-0-431 from Hazleton Washington, Inc., Rockville, Maryland, and
Vienna, Virginia, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Edwards, J.A., Leemings, N.M., Clark, R. & Offer, J.M. (1983) Effect
of diphenylamine on pregnancy of the New Zealand white rabbit.
Unpublished study No. PWT 1/2/83409 prepared by Huntington Research
Centre, Huntington, Cambridgeshire, United Kingdom.
Evan, A.P., Hong, S.K., Gardner, K., Jr, Park, Y.S. & Itagaki, R.
(1978) Evolution of the collecting tubular lesion in
diphenylamine-induced renal disease. Lab. Invest., 38, 244-252.
Gardner, K.D., Jr, Solomon, S., Fitzgerrel, W.W. & Evan, A.P. (1976)
Function and structure in the diphenylamine-exposed kidney. J. Clin.
Invest., 57, 796-806.
Kim-Kang, H. (1993) Metabolism of [14C]diphenylamine in stored
apples: Nature of the residue in plants. Unpublished study No. XBL
91071, report No. RPT00124 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Abstract of study submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Kim-Kang, H. (1994a) Metabolism of [14C]diphenylamine in lactating
goats. Unpublished study No. XBL 92089, report No. RPT00150 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kim-Kang H (1994b) Metabolism of [14C]diphenylamine in the laying
hen. Unpublished study No. XBL 93041, report No. RPT00161 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA
Kiplinger, G. (1995) Skin sensitization study of diphenylamine
technical in albino guinea pigs. Final report: Lab project No.
WIL-256001. Unpublished study prepared by WIL Research Labs, Inc.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Kreuzman, J. (1991a) Primary eye irritation study in rabbits without
rinsing with diphenylamine super-refined. Lab project No. 91-8052-21
(B). Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kreuzman, J. (1991b) Primary skin irritation study in rabbits with
diphenylamine super-refined. Lab project No. 91-8052-21 (A).
Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to WHO
by the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Krohmer, R.W. (1992a) 90 Day evaluation of diphenylamine in the dog.
Unpublished study No. 426C-501-034-91 from T.P.S., Inc., Mt Vernon,
Indiana, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Krohmer, R.W. (1992b) 90 Day subchronic toxicity evaluation of
diphenylamine in rats. Unpublished study No. 426C-10-034-91 from
T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the DPA
Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA
Lawlor, T. (1992) Mutagenicity test on diphenylamine in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test).
Unpublished report No.14902-0-401 from Hazleton Washington, Inc.,
Rockville, Maryland, and Vienna, Virginia, USA. Submitted to WHO by
the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Lenz, S.D. (1996) Investigation of regional glutathione levels in a
model of chemically-induced renal papillary necrosis. Food Chem.
Toxicol., 34, 489-494.
Lenz, S.D. & Carlton, W.W. (1990) Diphenylamine-induced renal
papillary necrosis and necrosis of the pars recta in laboratory
rodents. Vet. Pathol., 27, 171-178.
Lenz, S.D. & Carlton, W.W. (1991) Decreased incidence of
diphenylamine-induced renal papillary necrosis in Syrian hamsters
given dimethylsulphoxide. Food Chem. Toxicol., 29, 409-418.
Lenz, S.D., Turek, J.J. & Carlton, W.W. (1995) Early ultrastuctural
lesions of diphenylamine-induced renal papillary necrosis in Syrian
hamsters. Exp. Toxicol. Pathol., 47, 447-452.
Majnarich, J. (1991a) Diphenylamine: Super refined: Acute oral
toxicity, LD50 (rat). Unpublished report No. 022-91 from
Bioconsultants, Inc. Submitted to WHO by the DPA Task Force, John Wise
& Associates Ltd, Liberty, Missouri, USA.
Majnarich, J. (1991b) Diphenylamine: Super refined: Dermal LD50.
Unpublished report No. 024-91 from Bioconsultants, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Murli, H. (1992a) Dose range-finding study on diphenylamine for in
vivo murine micronucleus assay. Unpublished report No. 14902-0-459PO
from Hazleton Washington, Inc., Rockville, Maryland, and Vienna,
Virginia, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Murli, H. (1992b) Mutagenicity test on diphenylamine for in vivo
mammalian micronucleus assay. Unpublished report No. 14902-0-455 from
Hazleton Washington, Inc., Rockville, Maryland, and Vienna, Virginia,
USA. Submitted to WHO by the DPA Task Force, John Wise & Associates
Ltd, Liberty, Missouri, USA.
Powell, C.J., Adams, T., Hall, D.E., Bach, P.H. & Bridges, J.W. (1985)
The comparative subacute nephrotoxicity of diphenylamine and
N-phenylanthranilic acid. In: Bach, P.H. & Bridges, J.W., eds,
Renal Heterogeneity and Target Cell Toxicity, Chichester, John Wiley
& Sons, pp. 199-202.
Rodwell, D.E. (1992a) Range finding teratology study in rats with
diphenylamine (DPA). Unpublished study No. 3255.2 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rodwell, D.E. (1992b) Teratology study in rats with diphenylamine
(DPA). Unpublished study No. 3255.3 prepared by Springborn
Laboratories, Inc., Life Sciences Division, Spencerville, Ohio, USA.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Rodwell, D.E. (1993) Two-generation reproduction study in rats with
diphenylamine (DPA). Unpublished study No. 3255.4 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rohrbach, D.H., Robinson, L.K. & Murrah, V.A. (1993) Loss of the
basement membrane matrix molecule, bamin, in diphenylamine-treated
mice. Matrix, 13, 341-350.
Siglin, J.C. (1991) Repeated dose dermal toxicity: 21 Day study.
Unpublished study No. 3255.1 prepared by Springborn Laboratories,
Inc., Life Sciences Division, Spencerville, Ohio, USA. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Wu, D. (1993) Diphenylamine: Rat metabolism study. Unpublished study
XBL No. 92081, report No. RPT00131 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Submitted to WHO by the DPA Task Force,
John Wise & Associates Ltd, Liberty, Missouri, USA.
glucuronide, 24% as 4-hydroxy-diphenylamine sulfate, and 1.3% as
indophenol. Of the total 94% radiolabelled residue in milk, 7.4% was
identified as diphenylamine, 39% as 4-hydroxydiphenylamine
glucuronide, and 47% as 4-hydroxydiphenylamine sulfate. Of the total
40% radiolabelled residue in omental fat, 36% was identified as
diphenylamine and 3.6% as 4-hydroxydiphenylamine.
In the study in hens (Kim-Kang, 1994b), no metabolites were
identified in urine or faeces. Of the total 85% radioactive residue in
egg yolks, 17% was identified as diphenylamine, 4.8% as
4-hydroxydiphenylamine, 0.6% as 4.4'-dihydroxydiphenylamine, 3.2% as
4-hydroxydiphenylamine glucuronide, 57% as 4-hydroxydiphenylamine
sulfate, and 1.9% as a polar oligomer conjugate of
4-hydroxydiphenylamine. Of the total 31% radiolabelled residue in
liver, 7.9% was identified as diphenylamine, 4.5% as
2-hydroxydiphenylamine, 3% as 4.4'-di-hydroxydiphenylamine, 1.4% as
4-hydroxydiphenylamine glucuronide, 8.6% as 4-hydroxydiphenylamine
sulfate, 4.7% as a polar oligomer conjugate of 4-hydroxydiphenylamine,
and 1.3% as indophenol. Of the total 39% radiolabelled residue in
kidney, 1.1% was identified as diphenylamine, 0.3% as
2-hydroxy-diphenylamine, 0.2% as 4-hydroxydiphenylamine sulfate, and
38% as a polar oligomer conjugate of 4-hydroxydiphenylamine. Of the
total 58% radiolabelled residue in skin and fat, 35% was identified as
diphenylamine and 23% as 4-hydroxydiphenylamine sulfate.
The biotransformation of 14C-diphenylamine was also studied in
Red Delicious apples. Residues of a number of plant metabolites were
identified in apple peel and pulp, and untransformed diphenylamine was
the major contributor to the total residue 40 weeks after application.
The major metabolite was 4-hydroxydiphenylamine, present as the
glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxydiphenylamine, and
dihydroxydiphenylamine (possibly the 2,4-isomer). These compounds were
present free or as conjugates with mono- or oligosaccharides
(Kim-Kang, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of diphenylamine are
summarized in Table 1. After acute oral administration to rats in one
study, diphenylamine (purity, 99.9%) was slightly toxic, with an LD50
of 3000 mg/kg bw in males and 2700 mg/kg bw in females (Spanjers &
Til, 1982). In another study, diphenylamine (purity, 99-100.1%) was
generally not toxic, the LD50 being > 15 000 mg/kg bw for animals of
each sex (Majnarich, 1991a).
Table 1. Acute toxicity of diphenylamine in rats
Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Male Oral 99.9 3000 Spanjers & Til (1982)
Female 2700
Male and female Oral 99.9-100.1 > 15 000 Majnarich (1991a)
Not reported Dermal (24 h) 99.9-100.1 > 5000 Majnarich (1991b)
The acute dermal LD50 after a 24-h exposure to diphenylamine
(purity, 99.9-100.1%) was > 2 g/kg bw in New Zealand white rabbits of
each sex. No clinical signs were noted (Majnarich, 1991b).
Diphenylamine (purity, 99.9-100.1%) applied to the eyes of one
rabbit for seven days without rinsing was corrosive and induced
corneal opacity (Kreuzmann, 1991a). The same preparation was not
irritating to the skin of rabbits (Kreuzmann, 1991b). Diphenylamine
(purity, 99.9%) did not produce dermal sensitization in guinea-pigs
(Kiplinger, 1995).
(b) Short-term studies of toxicity
Mice
Groups of 15 male and 15 female Swiss-derived CD-1 mice received
technical-grade diphenylamine in the diet at 0, 10, 520, 260, or 5200
ppm for 90 days, equal to doses of 1.7, 94, 440, and 920 mg/kg bw per
day in males and 2.1, 110, 560, and 1100 mg/kg bw per day in females.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmological and haematological examinations
were carried out, organs were weighed, and the animals were examined
grossly and histopathologically. The hair of animals at the
intermediate and high doses had a greenish tint, which may have been
due to staining with diphenylamine or a metabolite. Three deaths
occurred among controls and among males at the high dose; two of the
latter had enlarged spleens, and one also had cystitis, probably
related to treatment. There were no treatment-related effects on body
weight, food consumption, or ophthalmic parameters. Haematology
indicated dose-related decreases in erythrocyte counts and haematocrit
in animals at the two higher doses that were statistically
significantly different from controls. The values for mean corpuscular
haemoglobin, mean corpuscular volume, and mean corpuscular haemoglobin
content increased with dose and were statistically significantly
different from those of controls in animals at the two higher doses;
the mean corpuscular haemoglobin content was also statistically
significantly increased in males at 525 ppm. The reticulocyte counts
increased with dose and were statistically significantly different
from those of controls at the high dose. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly different from those of controls at the
two higher doses; the relative weights of the kidney and heart were
statistically significantly different from those of controls in mice
at the high dose. In females, the absolute and relative weights of the
spleen increased with dose and were statistically significantly
different from those of the controls in animals at the two higher
doses; the absolute and relative weights of the liver and the relative
weights of the kidney were statistically significantly different from
those of controls in females at the high dose. Necropsy of females
revealed dark, enlarged spleens at the three higher doses, dark livers
at the two higher doses, and dark kidneys at the highest dose. In
males, necropsy showed dark, enlarged spleens and dark livers at the
two higher doses. Histopathological examination of the liver showed
increased pigment deposition and slight haematopoiesis in animals of
each sex at the two higher doses. The spleen showed haemosiderosis and
congestion at the three higher doses, reaching incidences of 14/15 or
more at the two higher doses; the severity of spleen haematopoiesis
was also increased at the three higher doses. The kidneys showed
pigment deposition at the two higher doses. Cystitis was observed in
9/15 males at the high dose and in 2/15 females at 2625 ppm and 8/14
females at 5250 ppm. The cellularity of the bone marrow was increased
at the two higher doses. The NOAEL was 10 ppm, equal to 1.7 mg/kg bw
per day, on the basis of changes in haematological parameters and
findings at necropsy (Botta, 1992).
Rats
Groups of 10 male and 10 female Sprague Dawley rats received
technical-grade diphenylamine in the diet at 0, 150, 1500, 7500, or 15
000 ppm for 90 days, equal to doses of 0, 9.6, 96, 550, and 1200 mg/kg
bw per day in males and 0, 12, 110, 650, and 1300 mg/kg bw per day in
females. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmic, urinary, haematological, and
clinical chemical end-points, organ weights, and gross and
histopathological appearance. Greenish hair was first seen in females
at 1500 ppm, later in 60% of males and 100% of females at 7500 ppm,
and then in 70% of males and 100% of females at 15 000 ppm. Pale skin
was seen in 100% of females at 7500 and 15 000 ppm and in 40% of males
at 15 000 ppm. Two males at 15 000 ppm were found dead on day 6 of
dosing, apparently due to gastroenteritis; there were no other deaths.
The body weights and body-weight gains of animals of each sex at 7500
and 15 000 ppm were consistently and statistically significantly below
those of controls; although these values were generally lower than
those of controls in animals at 1500 ppm, they were not statistically
significantly different. Food consumption was not affected at any
dose. The frequency of darkening of the urine increased with dose,
starting with one female at 1500 ppm and 100% of rats at 15 000 ppm.
Haematological measures indicated decreased erythrocyte counts and
haemoglobin values, which were statistically significantly different
from those of controls in animals at 7500 and 15 000 ppm at
termination. The haematocrits were statistically significantly lower
than those of controls in females at the three highest doses. Small,
statistically significant increases in alkaline phosphatase activity,
albumin content, and albumin:globulin ratio in males and glucose and
albumin content and albumin:globulin ratio in females were observed at
7500 and 15 000 ppm. The cholesterol concentration increased with dose
in females and was statistically significantly different from that of
controls at the three higher doses. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly raised at 7500 and 15 000 ppm; the
relative weights of the kidney and gonad also increased with dose and
were also statistically significant at the two higher doses. In
females, the absolute and relative weights of the liver increased with
dose, and the change in relative weights was statistically significant
at doses > 1500 ppm. The kidneys were dark in animals of each sex
at 7500 and 15 000 ppm, and about 60% of the females at the high dose
had dark and/or enlarged livers. The spleens of both males and females
at the two higher doses were congested. Histopathological examination
revealed an increased incidence of haematopoiesis and pigment in the
liver, haematopoiesis, haemosiderosis, and congestion in the spleen,
and pigmented kidneys in animals of each sex at 7500 and 15 000 ppm.
The spleens of all females at 1500 ppm also showed an increase from
minimal to slight haematopoiesis and haemosiderosis. The NOAEL was 150
ppm, equal to 12 mg/kg bw per day, on the basis of increased clinical
signs of toxicity, clinical chemical changes, organ weights, and gross
and histopathological appearance (Krohmer, 1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of technical-grade diphenylamine
dissolved in distilled water at doses of 100, 500, or 1000 mg/kg bw
per day. The material was applied daily for 6 h to an area of clipped
skin corresponding to about 10% of the body surface and kept under
occlusion for 21 consecutive days, with terminal sacrifice on day 22.
Two additional groups of five rabbits of each sex served as vehicle
controls. The animals were observed for clinical signs, deaths,
ophthalmoscopic parameters, erythema, oedema, desquamation, and other
adverse skin reactions, body weight, food consumption, urinary,
haematological, and clinical chemical end-points, organ weights, and
gross and histopathological appearance, the latter limited to the
liver, kidneys, spleen, treated and untreated skin, and gross lesions.
There were no deaths or treatment-related effects on clinical signs,
body weights, food consumption, or haematological end-points. The only
possible treatment-related effects on clinical chemistry were on
sodium and potassium concentrations; females at all three doses had
depressed sodium values, and those at the intermediate and high doses
and males at the high dose had depressed potassium values with respect
to controls. Gross necropsy, revealed dark-red foci in the stomachs of
rabbits of each sex at the intermediate and high doses, which
increased in frequency with dose: the incidences were 1/5 in males at
the intermediate dose, 4/5 in those at the high dose, 1/5 in females
at the intermediate dose, and 2/5 in females at the high dose. No
dark-red foci were seen in the stomachs of controls or rabbits at the
low dose. The NOAEL for systemic toxicity was 100 mg/kg bw per day on
the basis of the presence of dark-red foci in the stomachs of males
and females. The NOAEL for dermal effects was 1000 mg/kg bw per day,
the highest dose tested (Siglin, 1992).
Dogs
Groups of four pure-bred beagle dogs of each sex received
technical-grade diphenylamine (purity, > 99%) in gelatin capsules at
doses of 0, 10, 25, or 50 mg/kg bw per day for 90 days. They were
observed for deaths, clinical signs, body weight, food consumption,
ophthalmological, haematological, clinical chemical, and urinary
parameters, organ weights, and gross and histopathological appearance.
There were no deaths, and no treatment-related changes were seen in
any of the above parameters. Statistically significant increases were
seen, however, in some clinical chemical parameters including albumin
content, the albumin:globulin ratio in males, and bilirubin content in
females at the high dose. These effects may have been incidental. The
NOAEL was 50 mg/kg bw/day, the highest dose tested (Krohmer, 1992b).
(c ) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 CD-1 mice of each sex received diets containing
technical-grade diphenylamine (purity, > 99%) at concentrations of 0,
520, 2600, or 5200 ppm for up to 78 weeks, equal to 0, 73, 370, and
760 mg/kg bw per day for males and 0, 90, 460, and 940 mg/kg bw per
day for females. Ten mice of each sex per dose were sacrificed at 52
weeks. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmological and haematological
end-points, organ weights, and gross and histopathological appearance.
An increase in the incidence of greenish staining of the fur,
especially around the anogenital area, with dose was seen as the study
progressed. By week 26, most of the mice at 5250 ppm were affected,
and by the end of the study some mice at 525 ppm group showed
staining. The incidence of penile prolapse increased with dose,
affecting seven males at 2625 ppm and 17 at 5250 ppm by 78 weeks. The
frequency of unkempt appearance also increased with dose, with a
higher incidence among males. The mortality rate increased with dose,
becoming statistically significantly different from controls for males
at 2625 and 5250 ppm by 52 weeks. The deaths were attributed mainly to
cystitis among males and amyloidosis in females. The mean body-weight
gains were 87, 86, and 91% of control values for males at 5250 ppm and
104, 93, and 93% of control values for females at 5250 ppm at 13, 52,
and 78 weeks, respectively. The body-weight gains of males at 5250 ppm
and occasionally animals at 2625 ppm were statistically significantly
decreased throughout the study (mainly through week 58). The
body-weight gain of females at 5250 ppm was significantly decreased
during the first three weeks and then occasionally for the remainder
of the study. Mean food consumption was statistically significantly
decreased during the first week of treatment in males at the high dose
and remained increased throughout treatment for males at the two
higher doses, with occasional statistically significant differences
from controls. The food consumption of females at any dose showed
little or no statistically significant difference from that of
controls.
At the interim haematological evaluation at 52 weeks, males
showed dose-related decreases in haematocrit and erythrocyte counts
that reached statistical significance at 2625 and 5250 ppm; and mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin content increased with dose and reached statistical
significance in males at these doses. Females showed dose-related
decreases in haematocrit that reached statistical significance at
> 525 ppm; their erythrocyte counts decreased in a dose-related
fashion and reached statistical significance at 2625 and 5250 ppm.
Mean corpuscular volume, mean corpuscular haemoglobin, and mean
corpuscular haemoglobin content increased with dose, the latter two
reaching statistical significance at 2625 and 5250 ppm and the mean
corpuscular volume at 5250 ppm. At termination at 78 weeks, males and
females showed dose-related decreases in haematocrit and erythrocyte
counts that reached statistical significance at 2625 and 5250 ppm;
reticulocyte counts, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin content increased with
dose and reached statistical significance in males at these doses.
At the interim necropsy, darkened spleens were seen in most mice
at 2625 ppm and in all those at 5250 ppm. Darkened livers were seen in
most mice at 5250 ppm and pale kidneys in many. At terminal necropsy,
the livers of some mice at 2625 ppm and most at 5250 ppm were dark.
The spleens of most treated mice were dark and often enlarged.
Dose-related increases in the absolute and relative weights of the
spleen, liver, and heart were seen in animals of each sex at the
interim and final sacrifices. At interim sacrifice, the absolute
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly different from those of controls, while
the increases in the relative liver weights reached statistical
significance only at the highest dose. In females, the absolute and
relative weights of the spleen were statistically significantly
increased only at the highest dose. The absolute and relative weights
of the heart were statistically significantly increased in females at
5250 ppm; the increases in males were not statistically different from
those in controls. At final sacrifice, the absolute and relative
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly increased. In females, the absolute and
relative weights of the spleen were significantly increased only at
5250 ppm,; the differences in relative liver weight reached
statistical significance at 2625 and 5250 ppm. The absolute and
relative weights of the heart were statistically significantly
increased in animals of each sex at 5250 ppm. Changes in the absolute
and relative weights of the pituitary and thyroid were not
consistently dose-related and were thus considered not to be of
toxicological significance.
Histopathological examination revealed treatment-related effects
in the kidney, liver, spleen, bone marrow, urinary bladder, and penis.
The incidences of haematopoiesis and pigment deposition in the liver
were increased at the intermediate and high doses. Of mice at 2625 ppm
that were found dead or moribund or were sacrificed at termination,
19/51 males and 24/51 females showed liver haematopoiesis and 15/51
males and 37/51 females showed liver pigmentation; higher incidences
of these effects were seen at the high dose. The incidences of spleen
congestion and haemosiderosis were increased in all treated mice. Of
the mice at the low dose found dead or moribund or sacrificed at
termination, 11/50 males and 8/50 females showed spleen congestion and
8/50 males and 35/50 females showed spleen haemosiderosis; higher
incidences of these effects were seen at the higher doses. The
frequency of pigment deposition was increased in males at the high
dose and in females at the intermediate and high doses. Among females
found dead or moribund or sacrificed at termination, pigment was found
in 5/51 at the intermediate dose and 7/51 at the high dose. Among
males found dead or moribund or sacrificed at termination, 5/51 at the
intermediate dose and 7/51 at the high dose showed pigment
accumulation; additionally, 5/54 males had pyelonephritis. Although
the incidence of spleen haematopoiesis did not increase with dose, the
severity scores increased from minimal or slight in controls to mixed
scores including moderate or marked at the high dose. The severity of
haematopoiesis in the spleen increased from minimal to slight at 525
ppm in contrast to the largely minimal level in controls.
Additionally, bone-marrow cellularity changed from predominantly
moderate in controls to marked at the higher doses. The incidences of
urinary bladder cystitis and dilatation increased with dose, reaching
statistical significance at the intermediate and high doses. Among
mice at the intermediate dose that were found dead or moribund or were
sacrificed at termination, 24/51 males and 13/48 females had cystitis
and 18/51 males and 13/48 females had dilatation; higher values were
seen at the high dose. The incidence of balanoposthitis apparently
increased with dose; however, no statistical analysis was available.
In females at 5250 ppm, the incidence of amyloidosis in the thyroid,
adrenals, kidneys, stomach, small intestine, ovaries, and uterus was
increased. The incidence of tumours was comparable in the treated and
control groups. The NOAEL for toxicity was 525 ppm, equal to 73 mg/kg
bw per day, on the basis of decreased body-weight gain, decreased
survival, and significant haematological and gross and microscopic
pathological alterations (Botta, 1994a).
Rats
Groups of 60 male and 60 female Sprague-Dawley rats received
diets containing technical-grade diphenylamine (purity, > 99%) at
concentrations of 0, 200, 750, 3750, or 7500 ppm for males and 0, 150,
500, 2500, or 5000 ppm for females for up to two years, equal to 0,
8.1, 29, 150, and 300 mg/kg bw per day for males and 0, 7.5, 25, 140,
and 290 mg/kg bw per day for females. Groups of 10 rats of each sex
per group were killed at a one-year interim sacrifice. The animals
were observed for clinical signs, deaths, body weight, food
consumption, ophthalmological, haematological, clinical chemical, and
urinary end-points, organ weights, and gross and histopathological
appearance.
The only treatment-related clinical finding was greenish
colouration of the fur in the urogenital or ventral cervical area in
animals of each sex at the two higher doses. The effect was attributed
to the presence of a metabolite in urine or faeces. No treatment-
related effects on mortality rates were observed; however, the study
was terminated at 102 weeks because of increased mortality rates in
controls and animals at the low dose: survival among males was 22% at
0 and 200 ppm and 55% at 7500 ppm. Survival thus seemed to increase
with dose, and an analysis of the survival data indicated a
statistically significant negative trend for mortality as the dose
increased in animals of each sex; the mortality rates at the two
higher doses were statistically significantly lower than those for the
control group. A dose-related decrease in body weight and body-weight
gain was seen throughout most of the study, which reached statistical
significance at the two higher doses. The body-weight gains of males
at the two higher doses were depressed to 95 and 87% of the control
values at 78 weeks and equal to those of controls at 102 weeks; in
females, the corresponding values were 78 and 56% of controls at 78
weeks and 80 and 61% at 102 weeks. There was no decrease in food
consumption, except during the first week; at other times, food
consumption appeared to have increased, possibly due to food wastage.
There were no treatment related effects on ophthalmological
parameters.
Haematological examination revealed dose-related decreases in
erythrocyte counts, haemoglobin, and haematocrit in animals at the two
higher doses throughout treatment. The decreases reached statistical
significance for erythrocyte count and haemoglobin in males at 3750
and 7500 ppm in week 26 and at termination and for erythrocyte counts,
haemoglobin, and haematocrit in females at 2500 and 5000 ppm through
most of the treatment and at termination. Although the erythrocyte
counts, haemoglobin, and haematocrit were decreased in males at 750
ppm and in females at 500 ppm, the decreases reached statistical
significance only sporadically during treatment. The mean corpuscular
volume and mean corpuscular haemoglobin content were significantly
different from those of controls in males at the three higher doses
and in females at the two higher doses.
Dose-related, statistically significant increases in the absolute
and relative weights of the spleen were seen in females at the two
higher doses at interim sacrifice and at termination. A similar effect
on spleen weights was observed in males, except that the changes in
rats at 3750 ppm were not statistically significant. The relative
weight of the liver was statistically significantly increased in
females at 5000 ppm at termination and at 3750 and 5000 ppm at interim
sacrifice; no increase in liver weights was observed in males. The
increases in spleen and liver weights are consistent with the
haematological effects of the compound. Findings of dark and/or
enlarged spleens at necropsy in males at > 750 ppm and in females
at 500 ppm are probably related to the haematological effects.
Microscopic examination revealed treatment-related effects in the
kidney, liver, spleen, and bone marrow. The incidence of pigment
deposition in the kidney increased in a dose-related fashion and
reached 44/50 in males and 44/52 in females at the high dose that were
found dead or moribund or were sacrificed at termination. The
incidences of haematopoiesis and pigment deposition in the liver
increased in a dose-related fashion and reached 21/50 and 27/50 in
males and 41/52 and 45/52 in females at the high dose that were found
dead or moribund or sacrificed at termination. Erythroid hyperplasia
was seen at the higher dose but not in controls or at the low dose.
The incidence of congestion of the spleen increased in a dose-related
fashion and reached incidences of 50/50 in males and 47/52 in females
at the high dose that were found dead or moribund or sacrificed at
termination. These findings are all related to the observed
haematological effects. No treatment-related increase in tumour
incidence was observed. The NOAEL for toxicity was 150-200 ppm, equal
to 7.5 mg/kg bw per day, on the basis of changes in haematological
parameters and in the histopathological appearance of the spleen,
kidney, and liver (Botta, 1994b).
Dogs
Four beagles of each sex received diphenylamine (purity, > 99%)
by gelatin capsule at a dose of 0, 10, 25, or 100 mg/kg bw per day for
52 weeks and were observed for clinical signs, body weight, food
consumption, ophthalmological, haematological, clinical chaemical, and
urinary end-points, organ weights, and gross and histopathological
appearance. No treatment-related clinical signs were seen at
termination. One dog at the intermediate dose and two at the high dose
had greenish hair. There were no deaths or treatment-related effects
on body weight, food consumption, or ophthalmological parameters.
Haematological examination revealed decreased mean erythrocyte counts
(by 11% in comparison with controls), haemoglobin (9.3%), and
haematocrit (8.7%) in males at the high dose; smaller decreases in
these parameters were found in females. The platelet count increased
with dose in males at the 13-, 26-, 39-, and 52-week evaluation
periods, becoming statistically significant at the intermediate and
high doses. There was a dose-related increase in mean total bilirubin
concentration, which was statistically significant for animals at the
the intermediate and high doses throughout the study, in animals at
the low dose at week 26, and in females only at week 39. The mean
cholesterol concentration appeared to increase with dose at all
evaluation periods but was statistically significantly increased only
in males at the high dose at week 13 (by 68%) and in females at the
high dose at week 39 (by 37%). The blood urea nitrogen concentration
was decreased in females at the intermediate (by 16%) and high doses
(by 20%) at week 52. The mean absolute and relative weights of the
liver and thyroid appeared to increase with dose in males, but only
the mean absolute liver weight of males at the high dose was
statistically significantly increased. The mean absolute and relative
weights of the thyroid decreased with dose in females but did not
reached statistical significance at any dose. There were no
treatment-related gross or histopathological changes. The NOAEL for
toxicity was 10 mg/kg bw per day on the basis of haematological and
clinical chemical changes (Botta, 1994c).
(d) Genotoxicity
As summarized in Table 2, negative results were obtained for
mutation in bacteria (Lawlor, 1992) and for induction of micronuclei
in mouse bone marrow in vivo (Murli 1992 a,b). A weakly positive
response was observed for mutation in mouse lymphoma cells in vitro
at a dose range that was toxic, and the mutant frequency did not
increase with dose (Cifone, 1992). The Meeting concluded that although
diphenylamine has some genotoxic potential it is unlikely to be a
human genotoxic hazard.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, groups of 28
Sprague-Dawley rats received diphenylamine (purity, 99.8%) in the diet
at concentrations of 0, 500, 1500, or 5000 ppm, equal to 0, 40, 120,
and 400 mg/kg bw per day for F0 males and 0, 46, 130, and 450 mg/kg
bw per day for F0 females for 70 days before mating. After weaning,
28 F1 rats of each sex per group were also given the test diets for
up to 70 days before mating and were selected as parents for the F2
litters. The parent animals were observed for deaths, clinical signs,
body weight, food consumption, mating and fertility indices and other
measures of reproductive performance, organ weights, and gross and
histopathological appearance. The offspring were observed at birth and
through lactation for clinical signs, deaths, weights, and sex ratio;
all underwent necropsy.
Toxicity was dose-related and was observed in animals of each sex
and generations at all doses. In general, females were more affected
than males, and F1 animals were more affected than F0 animals. In
the F0 generation, the incidences of bluish coats and a bluish fluid
in the cages were increased in males and females at 5000 ppm, and
similar findings were made in the F1 generation. Swelling of the
mammary glands were seen in females, and palpable masses were noted in
lateral or ventral regions, especially in females. These masses
appeared to be transient but were not examined grossly or
microscopically. Body weight and body-weight gain were significantly
decreased at various times in animals at 1500 and 5000 ppm. At 5000
ppm, there was a decrease in body weight compared with controls, of
6-9% for F0 males, 5-8% for F0 females, 22-28% for F1 males, and
11-23% for F1 females. At 1500 ppm, there was a 5-8% decrease in body
weight for F0 females, 7-9% for F1 males, and 5% for F1 females.
The relative weights of the kidney, liver, and spleen of F0 and F1
males were significantly increased at 5000 ppm, and the relative
Table 2. Results of assays for the genotoxicity of diphenylamine
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Forward mutation S. typhimurium 6.67-333 µg/platea 99.9 Negative Lawlor (1992)
TA98, TA100, TA1535, 10-667 µg/plateb
TA1537, TA1538
Gene mutation L5178Y tk+/- mouse 5-80 µg/ml > 93 Weakly Cifone (1992)
lymphoma cells positiveb
Negativea
In vivo
Micronucleus Mouse (ICR strain) 250-1000 mg/kg bw (males) 99.9 Negative Murli (1992a,b)
formation 375-1500 mg/kg bw (females)
weights of the liver and spleen of F0 and F1 females were
significantly increased at 1500 ppm. The relative weights of the liver
were significantly increased at 1500 and 5000 ppm in F0 females but
only at 5000 ppm in F1 females.
Gross observations at necropsy included enlarged spleens in
animals of each sex and generation at 1500 and 5000 ppm and in F1
females at 500 ppm, in addition to blackish-purple spleens in animals
of each sex and generation at 500, 1500, and 5000 ppm. The incidences
of blackish-purple spleen in F1 rats at 500 ppm were 9/28 males and
6/28 females; incidences of 27/28 or higher were seen at higher doses.
Histopathological examination revealed a brown pigment in the proximal
tubules of the kidney in animals of each sex and generation at 5000
ppm; and 3/28 F1 females at 1500 ppm also had brown pigment.
Congestion and haemosiderosis of the spleen were seen in animals of
each sex and generation at 500, 1500, and 5000 ppm. Increased spleen
erythropoiesis was seen in 4/28 males at 5000 ppm. Hepatocyte
hypertrophy was seen in males of both generations at 1500 and 5000
ppm; in females, it was seen at all doses in the F0 generation but
only at 5000 ppm in the F1 generation. Kupffer cells with brown
pigment containing iron were seen in animals of each generation, at
5000 ppm in males and at 1500 and 5000 ppm in females.
Reproductive toxicity was seen in the form of decreased mean pup
weight at various times in each generation and reduced litter size at
the high dose; no other parameters were affected by treatment. F1
pups at 5000 ppm had statistically significantly decreased mean body
weight (11% less than controls at birth and 14-25% less than controls
during lactation). F2 pups showed statistically significantly
decreased mean body weight at 1500 ppm (10-12% less than controls
during late lactation) and at 5000 ppm (10-19% less than controls
throughout lactation). The mean body weight of pups in the F2 litters
at birth was about 4.8% lower than that of controls, but the
difference was not statistically significant. The mean litter size
decreased with dose in both generations, by 21% at 5000 ppm in the F1
generation, and was statistically significantly different from control
values. Although the mean litter size in the F0 generation at 5000
ppm was decreased by 10%, this value was not statistically
significant. The decrease in litter sizes with dose correlated with
the number of uterine implantation scars observed at necropsy, and the
mean uterine implantation scar count decreased with dose in both
generations. The mean scar count at 5000 ppm was decreased by 16% and
was statistically significant; although the mean scar count in the F0
generation was decreased by 7.3% at 5000 ppm, the value was not
statistically significant.
A NOAEL was not identified for parental systemic toxicity.
Although haemosiderin and congestion in the spleen and hepatocyte
hypertrophy were seen at all doses, the incidence and intensity of
these effects were appreciably smaller at the lower dose. For example,
only 4/28 females at the low dose had spleen congestion, rated as
minimal, whereas 22/28 females at the intermediate dose had this
effect and its intensity was nearly equally divided between minimal
and mild. These observations suggest that that the lower dose in this
study is close to the NOAEL for parental systemic toxicity. The NOAEL
for reproductive toxicity was 1500 ppm, equivalent to 120mg/kg bw per
day for F0 animals, on the basis of decreased mean litter size at the
high dose. The NOAEL for developmental toxicity was 500 ppm,
equivalent to 46 mg/kg bw per day in maternal animals, on the basis of
the statistically significantly decreased mean body weights of F2
pups (Rodwell, 1993).
(ii) Developmental toxicity
Rats
In a range-finding study for developmental toxicity, groups of
six pregnant Sprague-Dawley Crl:CDTM BR VAF/Plus rats received
diphenylamine (purity, 99.9%) in corn oil by gavage at doses of 0, 10,
50, 100, 200, 300, or 400 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity was seen at doses of 100 mg/kg bw per day and
higher. Two deaths occurred at 400 mg/kg bw per day, and there were
dose-related decreases in food consumption, body weight, and body-
weight gain starting at a dose of 100 mg/kg bw per day. Necropsy
revealed a purplish-black spleen in one dam at 100 mg/kg bw per day
and in all surviving dams at higher doses. The increased incidence of
early resorptions was apparent dose-related and was accompanied by
decreases in mean fetal weight and gravid uterine weight at doses of
200 and 300 mg/kg bw per day. There were no treatment-related external
malformations or developmental variations. On the basis of the results
of this study, 0, 10, 50, and 100 mg/kg bw per day were selected for
use in the definitive study (Rodwell, 1992a).
In the definitive study, groups of 25 pregnant Sprague-Dawley
Crl:CDTM BR VAF/Plus rats received diphenylamine (purity, 99.9%) in
corn oil by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for deaths, clinical
signs, body weight, and food consumption. All rats were sacrificed on
day 20 of gestation and were necropsied grossly. The spleens were
weighed, and the uterus was removed, examined externally, weighed, and
opened for internal examination. The fetuses were observed externally,
and their viscera and skeleton were examination. There were no deaths
or treatment-related effects on clinical signs, body weights, or food
consumption. At necropsy, 5/25 dams at the high dose had enlarged,
blackish-purple spleens, and the mean weights of the spleens were
significantly greater than those of controls. No treatment-related
effects were seen on gross necropsy, in the appearance or weight of
the uterus, or in the numbers of viable fetuses, early and late
resorptions, and corpora lutea, or on external, visceral, or skeletal
malformations or variations in the fetuses. The NOAEL for maternal
toxicity was 50 mg/kg bw per day on the basis of effects on the spleen
in dams at the high dose. The NOAEL for developmental toxicity was 100
mg/kg bw per day, the highest dose tested, in the absence of
developmental effects (Rodwell, 1992b).
Rabbits
In a study of developmental toxicity, groups of 16-18 pregnant
New Zealand white rabbits received diphenylamine (purity, 99.9%) in 1%
methyl cellulose by gavage at a dose of 0, 33, 100, or 300 mg/kg bw
per day on days 7-19 of gestation. The dams were observed for deaths,
clinical signs, body weight, and food consumption. All rats were
sacrificed on day 29 of gestation and subjected to gross necropsy; the
ovaries and uterus of each animal were removed and examined to
determine the number of corpora lutea and the type, distribution, and
number of implantation sites. The fetuses were examined externally,
and their viscera and skeleton were analysed. No deaths occurred
during the study. The mean food consumption of dams at 300 mg/kg bw
per day was reduced on days 7-29 of gestation, and a slight decrease
in body weight was noted. Green discolouration of the urine was seen
in all treated animals. No treatment-related effects were seen at
necropsy or on embryonic or fetal growth or development. The NOAEL for
maternal toxicity was 100 mg/kg bw per day on the basis of decreased
body-weight gain and food consumption early during treatment. The
NOAEL for developmental toxicity was 300 mg/kg bw per day, the highest
dose tested (Edwards et al., 1983).
(f) Special studies
(i) Renal cystic disease
The issue of tubular cyst formation in diphenylamine-treated rats
was reviewed previously (Annex 1, references 13, 27, 39, and 43).
Additional observations on the induction of tubular cysts in the
kidneys of mice and rats treated with diphenylamine in the diet are
summarized below.
Renal histopathology and function were studied in male
Sprague-Dawley rats fed pelleted diets containing 1% w/w (i.e. 10 000
ppm) diphenylamine (purity unspecified) for 5-20 months. Control
animals received identical diets in which methyl cellulose was
incorporated instead of diphenylamine. The animals were observed for
renal tubular diameter and renal functional parameters including
intratubular hydrostatic pressure, single-nephron glomerular
filtration rate, and transit times through the loop of Henle; light
and scanning electron microscopy was performed. The luminal diameters
of 22 dilated nephrons from diphenylamine-treated rats were 34-110 µm,
and those of 10 undilated nephrons were 21-450 µm; the luminal
diameters in six nephrons of two control rats were 26-34 µm. The
intraluminal hydrostatic pressure was significantly higher in dilated
tubules than in undilated tubules from treated rats; there was no
difference in intraluminal hydrostatic pressures among control rats.
The single-nephron glomerular filtration rate was also similar in
dilated and undilated tubules, consistent with an intrinsic change in
glomerular function. The transit times through the loop of Henle were
three to four times faster in dilated than in undilated nephrons of
treated and control rats. Microscopic examination indicated that
dilatation along the proximal and collecting tubules was segmental,
not diffuse, some dilated tubules communicated with cysts deep in the
renal substance, the dilated collecting tubules occasionally contained
debris, tubules adjacent to cysts appeared to be compressed, and
diphenylamine-exposed kidneys contained proximal convoluted tubules
with segments of apparent narrowing. The authors concluded that cyst
formation results from initial obstruction and a subsequent increase
in intraluminal pressure arising from sustained glomerular filtration
and unaltered water reabsorption (Gardner et al., 1976).
The time-course of development of lesions in the renal collecting
tubules was studied in male Sprague-Dawley rats fed pelleted diets
containing 1% w/w (i.e. 10 000 ppm) diphenylamine (purity unspecified)
for up to 76 weeks. Control animals received standard diet. The
animals were observed for renal concentrating ability (urine
osmolality) at 2, 4, 5, and 20 weeks of treatment; light and
transmission and scanning electron microscopy was performed at 2, 5,
10, 15, 20, 25, 52, and 78 weeks of treatment; and autoradiography
with tritiated thymidine was conducted to determine whether
hypertrophy was the mechanism of cyst formation. Decreased urine
osmolality was seen in treated rats, which was statistically
significantly different from control values at 6 and 20 weeks.
Structural changes first appeared after five weeks of treatment. Light
microscopy of the medullary collecting tubules at five weeks revealed
focal areas of apparent thickening along the walls, resulting from
layering of cells. By 10 weeks, some tubules were dilated and
contained focal areas of cellular necrosis. By 15-20 weeks, many more
ducts were dilated and contained cast material. By 24 weeks, frank
cysts were visible in the cortex and medulla, and large collecting
tubular cysts were seen; some proximal tubules and renal corpuscles
were dilated. Over time, cysts were found in every segment of the
nephron and collecting tubules. Transmission electron microscopy at
five weeks revealed changes in the medullary collecting tubules. The
tubular cells appeared to be enriched in mitochondria, and their
apical border was studded with long microvilli. At 10 weeks, necrotic
cells were seen along the collecting tubules. Scanning electron
microscopy showed that the collecting duct cysts were lined with
irregular cells which no longer resembled collecting tubule cells. The
labelling index for the collecting tubules reached a plateau at weeks
5-15 and had decreased to background levels by week 52. Because the
number of nuclei counted on cross-sections of the collecting tubules
increased gradually with time, the authors concluded that the increase
in labelling index was the result of hyperplasia of the tubular cells
and not of a degeneration or regeneration phenomenon. The authors
concluded that one of the initial events in the pathogenesis of cystic
renal disease is a hyperplastic response of the collecting tubule
cells (Evan et al., 1978).
The effect of diphenylamine on the composition of the native
renal basement membrane was studied in male C57Bl/6 mice fed pelleted
diets containing 2% w/w (i.e. 20 000 ppm) diphenylamine (purity
unspecified) for up to 10 months. Control animals received chow
without diphenylamine. After 10 months of treatment, the mice were
sacrificed and their kidneys processed for light microscopy and
immunohistology of bamin, a glycoprotein associated with the matrix of
the glomerular basement membrane. Microscopy revealed cysts in the
cortical and medullary portions of the kidney. The cysts were lined
with flattened or somewhat cuboidal cells and contained a brownish
coagulum that appeared to be constituted of necrotic cells. Brownish
discolouration was also seen in the cytoplasm of neighbouring
non-cystic cells and in tubular cells of animals sacrificed before the
formation of cysts. The authors speculated that the brownish debris
preceded the formation of cysts. When immunohistology was performed
with antibodies to bamin, the antibodies bound to the glomeruli of
control mice but not to those of treated animals.This result was
obtained in the three-week experiment. In another series of
experiments, male C57Bl/6 mice were injected with EHS tumour cells and
five days later received a diet containing diphenylamine (at an
unspecified level). After three weeks, the animals were killed, and
tumours were removed for analysis of the proteins of the basement
membrane. Analysis by sodium dodecylate sulfate-polyacrylamide gel
electrophoresis and by immunoblot revealed the presence of a 75-80-kDa
protein in controls but little or none in the diphenylamine-treated
mice. The authors were unclear how the loss of bamin, a molecule
associated with the glomerular but not the tubular basement membrane,
would be related to the formation of tubular cysts. They speculated
that material from an abnormal glomerular filtrate might damage the
tubular epithelial cells and result in the formation of tubular cysts
(Rohrbach et al., 1993).
(iv) Renal papillary necrosis
A series of studies has been reported on the induction of renal
papillary necrosis by diphenylamine in hamsters, rats, and Mongolian
gerbils. The development of animal models for this lesion is of
interest because it is the initial stage in human analgesic-induced
nephropathy.
Groups of 10 male Syrian hamsters, 40 Sprague-Dawley rats, and 40
Mongolian gerbils received diphenylamine (purity unspecified) at doses
of 0, 400, 600, or 800 mg/kg bw per day in peanut oil by gavage for
three days. Animals that became moribund were sacrificed and
necropsied; survivors were sacrificed 24 h after the third dose of
diphenylamine. All animals were observed for death and were examined
for gross and microscopic lesions. Forty percent of hamsters at the
low dose and 100% of those at the two higher doses died or were
sacrificed in extremis during treatment; there were no deaths among
the rats or gerbils. At necropsy, brown kidneys and yellow-brown
papillae were seen in 50% or more of hamsters at the intermediate and
high doses and in none of those at the low dose. The only gross lesion
in hamsters at the low dose was splenomegaly (90%), which was not seen
at higher doses. Microscopic examination revealed total renal
papillary necrosis, i.e. necrosis of all elements of the renal
papillae, including collecting tubules, at all doses in 4/10 hamsters
at the low, 7/10 at the intermediate, and 4/10 at the high dose. Only
two rats at the high dose had renal lesions, which consisted of
necrosis of the medullary interstitial cells and vasa recta, limited
to the apex, and degeneration of the renal interstitial matrix. As
noted by the authors, the limited incidence and extent of the lesions
in the Sprague-Dawley rats contrasted with the more extensive effects
observed in Wistar rats by Powell et al. (1985). No effects were
observed in Mongolian gerbils (Lenz & Carlton, 1990).
A total of 27 male Syrian hamsters were given diphenylamine by
gavage (purity unspecified) dissolved in peanut oil at a dose of 600
mg/kg bw. At intervals of 0.5, 1, 2, 4, 8, 16, and 24 h after dosing,
three hamsters were perfused in situ with fixative and their kidneys
were removed for examination by transmission electron microscopy.
Starting 1 h after dosing, the basal plasma membrane of the
endothelial cells of the ascending vasa recta in the proximal portion
of the renal papilla became separated from the basal lamina, forming
large subendothelial vacuoles. These alterations persisted during the
first 8 h after dosing. By 16 h, the endothelial cell nuclear
membranes and the luminal plasma membrane had become convoluted, and
by 24 h platelets were seen to adhere to the basal lamina, which had
become exposed. By 2 h after dosing, the adjacent interstitial cells
started undergoing structural alterations, and by 24 h there was
necrosis. Ultrastructural alterations became visible in the thin limb
of the loop of Henle by 24 h and in collecting tubule epithelial cells
by 14-16 h. The authors speculated that the selective effect on the
endothelial cells of the ascending vasa recta were attributable to
generation of a toxic metabolite by the endothelial cell or to
interference with the metabolism of the endothelial cells by a
metabolite of diphenylamine (Lenz et al., 1995).
The toxicity of diphenylamine dissolved in dimethyl sulfoxide to
renal papilla was studied in Syrian hamsters, rats, and Mongolian
gerbils. Male Syrian hamsters and Sprague-Dawley rats were given
diphenylamine (purity unspecified) by gavage at doses of 0, 400, 600,
or 800 mg/kg bw per day for up to nine days. Only one of 30 hamsters
at 400 mg/kg bw per day showed renal papillary necrosis. This result
contrasted with previous results (Lenz & Carlton, 1990) in which oral
administration of diphenylamine dissolved in peanut oil produced
extensive necrosis in male Syrian hamsters at the same doses;
furthermore, pretreatment of the hamsters with dimethyl sulfoxide
protected the animals against the renal papillary necrosis induced by
administration of diphenylamine. Renal papillary necrosis occurred in
4/30 rats at the high dose; none was seen in Mongolian gerbils. The
incidence in rats and gerbils did not differ from that observed when
peanut oil was used as the vehicle in a previous study (Lenz &
Carlton, 1990). The mechanism by which dimethyl sulfoxide exerts its
protective effect in Syrian hamsters was not studied (Lenz & Carlton,
1991).
The effect of diphenylamine on renal glutathione concentrations
was studied in groups of eight male Syrian hamsters given
diphenylamine (purity unspecified) by gavage at a dose of 0, 200, 400,
or 600 mg/kg bw 1 and 4 h after dosing. The concentrations in the
cortical area decreased with dose, reaching statistical significance
at the low dose at 1 h and at the intermediate dose at 4 h; the
concentrations at 1 h were 41% of the control value at 200 mg/kg bw,
31% at 400 mg/kg bw, and 29% at 600 mg/kg bw. The glutathione
concentrations in the outer medulla and the renal papilla were not
statistically significant decreased.
In order to study the effect of glutathione reduction on toxicity
to renal papilla, groups of eight male Syrian hamsters were given
buthionine sulfoxime, an inhibitor of glutathione biosynthesis, at 500
mg/kg bw intrapeitoneally, diphenylamine in peanut oil at 400 mg/kg bw
by gavage, the same doses of buthionine sulfoxime plus diphenylamine,
or the peanut oil vehicle alone. The animals were sacrificed 24 h
after administration of diphenylamine for gross and microscopic
examination of the kidney. No gross or microscopic lesions indicative
of papillary necrosis were observed in any group. Because a dose of
diphenylamine that is toxic to the papilla did not deplete glutathione
in that region and because depletion of glutathione (to 71% of the
control value) by buthionine sulfoxime did not enhance the toxicity of
diphenylamine to the papilla, the author concluded that this toxic
effect of diphenylamine is mediated by mechanisms independent of
oxidative injury (Lenz, 1996).
Comments
After oral administration to rats, goats, or hens,
14C-diphenylamine was extensively absorbed and rapidly excreted. In
rats given a single dose, 45-72% appeared in the urine within 24 h and
68-89% by 168 h after dosing, with less than 0.4% of the dose in the
residual carcass and less than 0.05% in any individual tissue. In
goats treated with single daily doses for seven days, 85-91% of the
daily dose was excreted in urine and 0.5-0.8% in milk; the
concentrations of residues in milk plateaued after 24 h. When laying
hens were treated with single daily oral doses for seven days, 84-98%
of the daily dose was recovered in the excreta; the concentrations in
egg yolk reached 0.31 mg/kg on day 7, but no residues were found in
egg whites. Diphenylamine underwent extensive biotransformation in
rats, goats, and hens, with ring hydroxylation and formation of
glucuronide and sulfate conjugates. In addition to untransformed
diphenylamine (< 3% of the dose), the following 12 metabolites were
identified at all doses: 4,4'-dihydroxydiphenylamine (unconjugated and
as the O-sulfate and the O, O-disulfate), 4-hydroxydiphenylamine
(unconjugated and as the O-glucuronide, N-glucuronide,
O-sulfate, and O, N-diglucuronide), indophenol (unconjugated and
as the O-sulfate), 3-hydroxydiphenylamine, and
2-hydroxydiphenylamine. These metabolites accounted for about 80-90%
of the dose and were excreted largely as their sulfate and glucuronide
conjugates. There was no cleavage of the diphenylamine structure.
Except for a polar oligomer of 4-hydroxydiphenylamine found only in
the eggs and tissues of hens, all of the metabolites reported in hens
and goats were detected in rats. Residues of a number of plant
metabolites were identified in apple peel and pulp, but untransformed
diphenylamine was the major contributor to the total residue 40 weeks
after application. The major metabolite was 4-hydroxydiphenylamine, as
the glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxy-diphenylamine, and
dihydroxydiphenylamine (possibly the 2,4 isomer). These compounds were
either free or conjugated with mono- or oligosaccharides. Although all
of the hydroxylated metabolites (aglycones) identified in plants were
seen in rats, the conjugating species were generally different.
After acute oral administration to rats, diphenylamine (99.9%
pure) was slightly toxic (LD50 about 3000 mg/kg bw) in one study,
whereas in another study super-refined diphenylamine (purity,
99.0-100.1%) was essentially nontoxic (LD50 > 15 000 mg/kg bw).
WHO has not classified diphenylamine for acute toxicity.
In mice given diphenylamine at dietary concentrations of 0, 10,
520, 2600, or 5200 ppm for 90 days, dose-related changes in
haematological parameters (decreased erythrocyte counts and packed
cell volumes and increased reticulocyte counts) were observed. The
mean corpuscular haemoglobin count was significantly increased at
dietary levels of 520 ppm and above. Necropsy revealed dark, enlarged
spleens with haemosiderosis and congestion at dietary levels of 520
ppm and above. Spleen haematopoiesis was increased in animals of each
sex at 520 ppm. The NOAEL was 10 ppm (equal to 1.7 mg/kg bw per day)
on the basis of changes in haematological parameters and findings at
necropsy in animals at 520 ppm.
In rats that received diphenylamine in the diet at concentrations
of 0, 150, 1500, 7500, or 15 000 ppm for 90 days, body weights and
body-weight changes, although generally lower than the control values
at 1500 ppm, were not significantly different from those of controls
at doses below 7500 ppm. The cholesterol concentration increased with
dose in females and was significantly different from that of controls
at 1500 ppm. In females, the absolute and relative weights of the
liver increased with dose, and the relative liver weights were
significantly different from those of controls in animals at 1500 ppm
and above. Histopathological examination revealed increased
haematopoiesis and pigment in the liver, haematopoiesis,
haemosiderosis, and congestion in the spleen, and pigmented kidneys in
animals of each sex at 7500 and 15 000 ppm. Additionally, the spleens
of all females at 1500 ppm showed minimal or slight haematopoiesis and
haemosiderosis. The NOAEL was 150 ppm (equal to 12 mg/kg bw per day)
on the basis of changes in clinical chemical parameters, increased
organ weights, and gross and histological changes in female rats at
1500 ppm.
Groups of rabbits were exposed dermally to diphenylamine in
distilled water at doses of 0, 100, 500, or 1000 mg/kg bw per day for
6 h per day. No effects were observed. Gross necropsy revealed
dark-red foci in the stomachs of rabbits at the intermediate and high
doses, which increased in number with dose. The NOAEL for systemic
effects was 100 mg/kg bw per day on the basis of effects on the
stomach at 500 mg/kg bw per day in animals of each sex.
Groups of dogs received diphenylamine by gelatin capsule at doses
of 0, 10, 25, or 50 mg/kg bw per day for 90 days. There were no
treatment-related effects. The NOAEL was 50 mg/kg bw per day, the
highest dose tested.
In a study of carcinogenicity, mice received diets containing
diphenylamine at concentrations of 0, 520, 2625, or 5200 ppm for 78
weeks. At 520 ppm and above, decreased packed cell volumes were seen
in females and spleen congestion and haemosiderosis in animals of each
sex. At 2600 ppm and above, clear haematological effects, consistent
with regenerative anaemia, were observed. The incidences of tumours
were not increased when compared with those in controls. The NOAEL for
toxicity was 520 ppm (equal to 73 mg/kg bw per day) on the basis of
decreased body-weight gain, reduced survival, and significant
alterations in haematological and gross and microscopic pathological
parameters at higher levels. Examination of the incidence and severity
of some haematological effects at 520 ppm suggested that this dose is
close to the NOAEL/LOAEL threshold. There was no evidence of
carcinogenicity.
In a combined study of toxicity and carcinogenicity, rats
received diets containing diphenylamine at concentrations of 0, 200,
750, 3750, or 7500 ppm (males) and 0, 150, 500, 2500, or 5000 ppm
(females). At 500 ppm and above, decreased erythrocyte count,
haemoglobin, and packed cell volumes were observed in animals of each
sex; these decreases reached statistical significance only
sporadically during treatment in males at 750 ppm and in females at
500 ppm. Haematopoiesis was increased in the liver and spleen of males
at 750 ppm and above. At 2625 ppm and above, clear haematological
effects consistent with regenerative anaemia were observed. There was
no significant increase in the incidence of tumours when compared with
that in controls. The NOAEL was 150-200 ppm (equal to 7.5 mg/kg bw per
day) based on haematological and histological effects at dietary
levels equal to or greater than 500 ppm. This appeared to be close to
the threshold dose, since body-weight gain was not depressed and only
sporadic haematological changes were observed at 500-750 ppm. There
was no evidence of carcinogenicity.
Dogs received diphenylamine by gelatin capsule at doses of 0, 10,
25, or 100 mg/kg bw per day for one year. Platelet counts in males and
total bilirubin concentrations in animals of each sex were
statistically significantly higher than those of controls. The NOAEL
for toxicity was 10 mg/kg bw per day, and the LOAEL was 25 mg/kg bw
per day, both based on haematological and clinical chemical changes.
In a two-generation study of reproductive toxicity, rats received
diphenylamine in the diet at concentrations of 0, 500, 1500, or 5000
ppm during premating. A NOAEL for parental toxicity was not observed;
the LOAEL was 500 ppm (equal to 40 mg/kg bw per day) on the basis of
enlarged spleens in F1 females, increased spleen congestion and
haemosiderosis in animals of each sex in all generations, and
hepatocyte hypertrophy in F0 females. The NOAEL for developmental
toxicity was 500 ppm (equal to 46 mg/kg bw per day) on the basis of
statistically significantly decreased mean body weight in F2 pups at
1500 ppm and above. The NOAEL for reproductive toxicity was 1500 ppm
(equal to 120 mg/kg bw per day). Although haemosiderin, congestion of
the spleen, and hepatocyte hypertrophy were observed in parental
animals at all doses, they occurred at appreciably lower incidence and
intensity at the lower dose than at the higher doses, which suggested
that the lower dose was close to the NOAEL/LOAEL threshold for
parental toxicity.
In a study of developmental toxicity, rats received diphenylamine
by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on days 6-15
of gestation. The NOAEL for maternal toxicity was 50 mg/kg bw per day
on the basis of enlarged, blackish, heavier spleens at 100 mg/kg bw
per day. The NOAEL for developmental toxicity was 100 mg/kg bw per
day, the highest dose tested.
In a study of developmental toxicity, rabbits received
diphenylamine by gavage at doses of 0, 33, 100, or 300 mg/kg bw per
day on days 7-19 of gestation. The NOAEL for maternal toxicity was 100
mg/kg bw per day on the basis of decreased body-weight gain and food
consumption at 300 mg/kg bw per day. The NOAEL for developmental
toxicity was 300 mg/kg bw per day, the highest dose tested.
Diphenylamine has been tested for genotoxicity in three assays.
Negative results were obtained for mutation in bacteria and for
induction of micronuclei in mouse bone marrow in vivo. A weakly
positive response was observed only for mutation in mouse lymphoma
cells in vitro at a dose range in which the toxicity was relatively
high and the mutant frequency did not increase with dose. The Meeting
concluded that, although diphenylamine has some genotoxic potential,
it is unlikely to present a human genotoxic hazard.
An ADI of 0-0.08 mg/kg bw was established on the basis of the
NOAEL of 150 ppm, equal to 7.5 mg/kg bw per day, in the two-year study
of toxicity and carcinogenicity in rats and a 100-fold safety factor.
An acute RfD was not allocated because diphenylamine is of low
acute toxicity. The Meeting concluded that the acute intake of
residues is unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 520 ppm, equal to 73 mg/kg bw per day (78-week study of
carcinogenicity)
Rat: 150-200 ppm, equal to 7.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equal to 46 mg/kg bw per day (reproductive
toxicity in a two-generation study of reproductive
toxicity)
Rat 50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
300 mg/kg bw per day (highest dose in a study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.08 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid absorption: at least 68-89% of a dose
Dermal absorption No data
Distribution Extensive
Potential for accumulation Very little
Rate and extent of excretion At 24 h, 45-72% of a dose found in urine
Metabolism in animals Extensively metabolized. Parent < 3%. Approximately
80-90% of a dose appeared as 12 metabolites: indophenol
and various isomers of mono- and
di-hydroxydiphenylamine excreted in urine as sulfate
and glucuronide conjugates
Toxicologically significant compounds Parent. Indophenol might undergo electrophilic
(animals, plants, and environment) interactions
Acute toxicity
Rat: LD50 oral 3000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
LC50 inhalation No data
Skin irritation None to slight
Eye irritation Slight to corrosive with corneal opacity
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Erythrocytes/anaemia
Lowest relevant oral NOAEL Mouse: 1.7 mg/kg bw per day, 90-day study
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity Unlikely to be a human genotoxic hazard
Long-term toxicity and carcinogenicity
Target: critical effect Erythrocytes/haemolytic anaemia
Lowest relevant NOAEL Rat: 7.5 mg/kg bw per day
Carcinogenicity No carcinogenicity
Reproductive toxicity
Reproduction target /critical effect Decreased mean litter size in both generations and
decreased mean body weights of F2 pups at maternally
toxic doses
Lowest relevant reproductive NOAEL Rat: 46 mg/kg bw per day
Developmental target/critical effect Rat: No developmental toxicity
Lowest relevant developmental NOAEL Rat: 100 mg/kg bw per day, highest dose tested
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.08 mg/kg bw Long-term toxicity 100
and carcinogenicity,
rats
Acute reference dose Not allocated
(unnecessary)
References
Botta, J.A., Jr (1992) 90 Day subchronic toxicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426E-001-034-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994a) 18 Month oncogenicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426H-002-646-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994b) 24 Month combined oncogenicity/toxicity
evaluation of diphenylamine in rats. Unpublished report No.
426D-102-048-91 from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Botta, J.A., Jr (1994c) One year chronic study of diphenylamine in
dogs. Unpublished report No. 426B-502-044-91 from T.P.S., Inc., Mt
Vernon, Indiana, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Cifone, M. (1992) Mutagenicity test on diphenylamine in the L5178Y
TK+/- mouse lymphoma forward mutation assay. Unpublished report No.
14902-0-431 from Hazleton Washington, Inc., Rockville, Maryland, and
Vienna, Virginia, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Edwards, J.A., Leemings, N.M., Clark, R. & Offer, J.M. (1983) Effect
of diphenylamine on pregnancy of the New Zealand white rabbit.
Unpublished study No. PWT 1/2/83409 prepared by Huntington Research
Centre, Huntington, Cambridgeshire, United Kingdom.
Evan, A.P., Hong, S.K., Gardner, K., Jr, Park, Y.S. & Itagaki, R.
(1978) Evolution of the collecting tubular lesion in
diphenylamine-induced renal disease. Lab. Invest., 38, 244-252.
Gardner, K.D., Jr, Solomon, S., Fitzgerrel, W.W. & Evan, A.P. (1976)
Function and structure in the diphenylamine-exposed kidney. J. Clin.
Invest., 57, 796-806.
Kim-Kang, H. (1993) Metabolism of [14C]diphenylamine in stored
apples: Nature of the residue in plants. Unpublished study No. XBL
91071, report No. RPT00124 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Abstract of study submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Kim-Kang, H. (1994a) Metabolism of [14C]diphenylamine in lactating
goats. Unpublished study No. XBL 92089, report No. RPT00150 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kim-Kang H (1994b) Metabolism of [14C]diphenylamine in the laying
hen. Unpublished study No. XBL 93041, report No. RPT00161 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA
Kiplinger, G. (1995) Skin sensitization study of diphenylamine
technical in albino guinea pigs. Final report: Lab project No.
WIL-256001. Unpublished study prepared by WIL Research Labs, Inc.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Kreuzman, J. (1991a) Primary eye irritation study in rabbits without
rinsing with diphenylamine super-refined. Lab project No. 91-8052-21
(B). Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kreuzman, J. (1991b) Primary skin irritation study in rabbits with
diphenylamine super-refined. Lab project No. 91-8052-21 (A).
Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to WHO
by the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Krohmer, R.W. (1992a) 90 Day evaluation of diphenylamine in the dog.
Unpublished study No. 426C-501-034-91 from T.P.S., Inc., Mt Vernon,
Indiana, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Krohmer, R.W. (1992b) 90 Day subchronic toxicity evaluation of
diphenylamine in rats. Unpublished study No. 426C-10-034-91 from
T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the DPA
Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA
Lawlor, T. (1992) Mutagenicity test on diphenylamine in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test).
Unpublished report No.14902-0-401 from Hazleton Washington, Inc.,
Rockville, Maryland, and Vienna, Virginia, USA. Submitted to WHO by
the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Lenz, S.D. (1996) Investigation of regional glutathione levels in a
model of chemically-induced renal papillary necrosis. Food Chem.
Toxicol., 34, 489-494.
Lenz, S.D. & Carlton, W.W. (1990) Diphenylamine-induced renal
papillary necrosis and necrosis of the pars recta in laboratory
rodents. Vet. Pathol., 27, 171-178.
Lenz, S.D. & Carlton, W.W. (1991) Decreased incidence of
diphenylamine-induced renal papillary necrosis in Syrian hamsters
given dimethylsulphoxide. Food Chem. Toxicol., 29, 409-418.
Lenz, S.D., Turek, J.J. & Carlton, W.W. (1995) Early ultrastuctural
lesions of diphenylamine-induced renal papillary necrosis in Syrian
hamsters. Exp. Toxicol. Pathol., 47, 447-452.
Majnarich, J. (1991a) Diphenylamine: Super refined: Acute oral
toxicity, LD50 (rat). Unpublished report No. 022-91 from
Bioconsultants, Inc. Submitted to WHO by the DPA Task Force, John Wise
& Associates Ltd, Liberty, Missouri, USA.
Majnarich, J. (1991b) Diphenylamine: Super refined: Dermal LD50.
Unpublished report No. 024-91 from Bioconsultants, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Murli, H. (1992a) Dose range-finding study on diphenylamine for in
vivo murine micronucleus assay. Unpublished report No. 14902-0-459PO
from Hazleton Washington, Inc., Rockville, Maryland, and Vienna,
Virginia, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Murli, H. (1992b) Mutagenicity test on diphenylamine for in vivo
mammalian micronucleus assay. Unpublished report No. 14902-0-455 from
Hazleton Washington, Inc., Rockville, Maryland, and Vienna, Virginia,
USA. Submitted to WHO by the DPA Task Force, John Wise & Associates
Ltd, Liberty, Missouri, USA.
Powell, C.J., Adams, T., Hall, D.E., Bach, P.H. & Bridges, J.W. (1985)
The comparative subacute nephrotoxicity of diphenylamine and
N-phenylanthranilic acid. In: Bach, P.H. & Bridges, J.W., eds,
Renal Heterogeneity and Target Cell Toxicity, Chichester, John Wiley
& Sons, pp. 199-202.
Rodwell, D.E. (1992a) Range finding teratology study in rats with
diphenylamine (DPA). Unpublished study No. 3255.2 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rodwell, D.E. (1992b) Teratology study in rats with diphenylamine
(DPA). Unpublished study No. 3255.3 prepared by Springborn
Laboratories, Inc., Life Sciences Division, Spencerville, Ohio, USA.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Rodwell, D.E. (1993) Two-generation reproduction study in rats with
diphenylamine (DPA). Unpublished study No. 3255.4 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rohrbach, D.H., Robinson, L.K. & Murrah, V.A. (1993) Loss of the
basement membrane matrix molecule, bamin, in diphenylamine-treated
mice. Matrix, 13, 341-350.
Siglin, J.C. (1991) Repeated dose dermal toxicity: 21 Day study.
Unpublished study No. 3255.1 prepared by Springborn Laboratories,
Inc., Life Sciences Division, Spencerville, Ohio, USA. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Wu, D. (1993) Diphenylamine: Rat metabolism study. Unpublished study
XBL No. 92081, report No. RPT00131 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Submitted to WHO by the DPA Task Force,
John Wise & Associates Ltd, Liberty, Missouri, USA.
 glucuronide, 24% as 4-hydroxy-diphenylamine sulfate, and 1.3% as
indophenol. Of the total 94% radiolabelled residue in milk, 7.4% was
identified as diphenylamine, 39% as 4-hydroxydiphenylamine
glucuronide, and 47% as 4-hydroxydiphenylamine sulfate. Of the total
40% radiolabelled residue in omental fat, 36% was identified as
diphenylamine and 3.6% as 4-hydroxydiphenylamine.
In the study in hens (Kim-Kang, 1994b), no metabolites were
identified in urine or faeces. Of the total 85% radioactive residue in
egg yolks, 17% was identified as diphenylamine, 4.8% as
4-hydroxydiphenylamine, 0.6% as 4.4'-dihydroxydiphenylamine, 3.2% as
4-hydroxydiphenylamine glucuronide, 57% as 4-hydroxydiphenylamine
sulfate, and 1.9% as a polar oligomer conjugate of
4-hydroxydiphenylamine. Of the total 31% radiolabelled residue in
liver, 7.9% was identified as diphenylamine, 4.5% as
2-hydroxydiphenylamine, 3% as 4.4'-di-hydroxydiphenylamine, 1.4% as
4-hydroxydiphenylamine glucuronide, 8.6% as 4-hydroxydiphenylamine
sulfate, 4.7% as a polar oligomer conjugate of 4-hydroxydiphenylamine,
and 1.3% as indophenol. Of the total 39% radiolabelled residue in
kidney, 1.1% was identified as diphenylamine, 0.3% as
2-hydroxy-diphenylamine, 0.2% as 4-hydroxydiphenylamine sulfate, and
38% as a polar oligomer conjugate of 4-hydroxydiphenylamine. Of the
total 58% radiolabelled residue in skin and fat, 35% was identified as
diphenylamine and 23% as 4-hydroxydiphenylamine sulfate.
The biotransformation of 14C-diphenylamine was also studied in
Red Delicious apples. Residues of a number of plant metabolites were
identified in apple peel and pulp, and untransformed diphenylamine was
the major contributor to the total residue 40 weeks after application.
The major metabolite was 4-hydroxydiphenylamine, present as the
glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxydiphenylamine, and
dihydroxydiphenylamine (possibly the 2,4-isomer). These compounds were
present free or as conjugates with mono- or oligosaccharides
(Kim-Kang, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of diphenylamine are
summarized in Table 1. After acute oral administration to rats in one
study, diphenylamine (purity, 99.9%) was slightly toxic, with an LD50
of 3000 mg/kg bw in males and 2700 mg/kg bw in females (Spanjers &
Til, 1982). In another study, diphenylamine (purity, 99-100.1%) was
generally not toxic, the LD50 being > 15 000 mg/kg bw for animals of
each sex (Majnarich, 1991a).
Table 1. Acute toxicity of diphenylamine in rats
Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Male Oral 99.9 3000 Spanjers & Til (1982)
Female 2700
Male and female Oral 99.9-100.1 > 15 000 Majnarich (1991a)
Not reported Dermal (24 h) 99.9-100.1 > 5000 Majnarich (1991b)
The acute dermal LD50 after a 24-h exposure to diphenylamine
(purity, 99.9-100.1%) was > 2 g/kg bw in New Zealand white rabbits of
each sex. No clinical signs were noted (Majnarich, 1991b).
Diphenylamine (purity, 99.9-100.1%) applied to the eyes of one
rabbit for seven days without rinsing was corrosive and induced
corneal opacity (Kreuzmann, 1991a). The same preparation was not
irritating to the skin of rabbits (Kreuzmann, 1991b). Diphenylamine
(purity, 99.9%) did not produce dermal sensitization in guinea-pigs
(Kiplinger, 1995).
(b) Short-term studies of toxicity
Mice
Groups of 15 male and 15 female Swiss-derived CD-1 mice received
technical-grade diphenylamine in the diet at 0, 10, 520, 260, or 5200
ppm for 90 days, equal to doses of 1.7, 94, 440, and 920 mg/kg bw per
day in males and 2.1, 110, 560, and 1100 mg/kg bw per day in females.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmological and haematological examinations
were carried out, organs were weighed, and the animals were examined
grossly and histopathologically. The hair of animals at the
intermediate and high doses had a greenish tint, which may have been
due to staining with diphenylamine or a metabolite. Three deaths
occurred among controls and among males at the high dose; two of the
latter had enlarged spleens, and one also had cystitis, probably
related to treatment. There were no treatment-related effects on body
weight, food consumption, or ophthalmic parameters. Haematology
indicated dose-related decreases in erythrocyte counts and haematocrit
in animals at the two higher doses that were statistically
significantly different from controls. The values for mean corpuscular
haemoglobin, mean corpuscular volume, and mean corpuscular haemoglobin
content increased with dose and were statistically significantly
different from those of controls in animals at the two higher doses;
the mean corpuscular haemoglobin content was also statistically
significantly increased in males at 525 ppm. The reticulocyte counts
increased with dose and were statistically significantly different
from those of controls at the high dose. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly different from those of controls at the
two higher doses; the relative weights of the kidney and heart were
statistically significantly different from those of controls in mice
at the high dose. In females, the absolute and relative weights of the
spleen increased with dose and were statistically significantly
different from those of the controls in animals at the two higher
doses; the absolute and relative weights of the liver and the relative
weights of the kidney were statistically significantly different from
those of controls in females at the high dose. Necropsy of females
revealed dark, enlarged spleens at the three higher doses, dark livers
at the two higher doses, and dark kidneys at the highest dose. In
males, necropsy showed dark, enlarged spleens and dark livers at the
two higher doses. Histopathological examination of the liver showed
increased pigment deposition and slight haematopoiesis in animals of
each sex at the two higher doses. The spleen showed haemosiderosis and
congestion at the three higher doses, reaching incidences of 14/15 or
more at the two higher doses; the severity of spleen haematopoiesis
was also increased at the three higher doses. The kidneys showed
pigment deposition at the two higher doses. Cystitis was observed in
9/15 males at the high dose and in 2/15 females at 2625 ppm and 8/14
females at 5250 ppm. The cellularity of the bone marrow was increased
at the two higher doses. The NOAEL was 10 ppm, equal to 1.7 mg/kg bw
per day, on the basis of changes in haematological parameters and
findings at necropsy (Botta, 1992).
Rats
Groups of 10 male and 10 female Sprague Dawley rats received
technical-grade diphenylamine in the diet at 0, 150, 1500, 7500, or 15
000 ppm for 90 days, equal to doses of 0, 9.6, 96, 550, and 1200 mg/kg
bw per day in males and 0, 12, 110, 650, and 1300 mg/kg bw per day in
females. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmic, urinary, haematological, and
clinical chemical end-points, organ weights, and gross and
histopathological appearance. Greenish hair was first seen in females
at 1500 ppm, later in 60% of males and 100% of females at 7500 ppm,
and then in 70% of males and 100% of females at 15 000 ppm. Pale skin
was seen in 100% of females at 7500 and 15 000 ppm and in 40% of males
at 15 000 ppm. Two males at 15 000 ppm were found dead on day 6 of
dosing, apparently due to gastroenteritis; there were no other deaths.
The body weights and body-weight gains of animals of each sex at 7500
and 15 000 ppm were consistently and statistically significantly below
those of controls; although these values were generally lower than
those of controls in animals at 1500 ppm, they were not statistically
significantly different. Food consumption was not affected at any
dose. The frequency of darkening of the urine increased with dose,
starting with one female at 1500 ppm and 100% of rats at 15 000 ppm.
Haematological measures indicated decreased erythrocyte counts and
haemoglobin values, which were statistically significantly different
from those of controls in animals at 7500 and 15 000 ppm at
termination. The haematocrits were statistically significantly lower
than those of controls in females at the three highest doses. Small,
statistically significant increases in alkaline phosphatase activity,
albumin content, and albumin:globulin ratio in males and glucose and
albumin content and albumin:globulin ratio in females were observed at
7500 and 15 000 ppm. The cholesterol concentration increased with dose
in females and was statistically significantly different from that of
controls at the three higher doses. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly raised at 7500 and 15 000 ppm; the
relative weights of the kidney and gonad also increased with dose and
were also statistically significant at the two higher doses. In
females, the absolute and relative weights of the liver increased with
dose, and the change in relative weights was statistically significant
at doses > 1500 ppm. The kidneys were dark in animals of each sex
at 7500 and 15 000 ppm, and about 60% of the females at the high dose
had dark and/or enlarged livers. The spleens of both males and females
at the two higher doses were congested. Histopathological examination
revealed an increased incidence of haematopoiesis and pigment in the
liver, haematopoiesis, haemosiderosis, and congestion in the spleen,
and pigmented kidneys in animals of each sex at 7500 and 15 000 ppm.
The spleens of all females at 1500 ppm also showed an increase from
minimal to slight haematopoiesis and haemosiderosis. The NOAEL was 150
ppm, equal to 12 mg/kg bw per day, on the basis of increased clinical
signs of toxicity, clinical chemical changes, organ weights, and gross
and histopathological appearance (Krohmer, 1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of technical-grade diphenylamine
dissolved in distilled water at doses of 100, 500, or 1000 mg/kg bw
per day. The material was applied daily for 6 h to an area of clipped
skin corresponding to about 10% of the body surface and kept under
occlusion for 21 consecutive days, with terminal sacrifice on day 22.
Two additional groups of five rabbits of each sex served as vehicle
controls. The animals were observed for clinical signs, deaths,
ophthalmoscopic parameters, erythema, oedema, desquamation, and other
adverse skin reactions, body weight, food consumption, urinary,
haematological, and clinical chemical end-points, organ weights, and
gross and histopathological appearance, the latter limited to the
liver, kidneys, spleen, treated and untreated skin, and gross lesions.
There were no deaths or treatment-related effects on clinical signs,
body weights, food consumption, or haematological end-points. The only
possible treatment-related effects on clinical chemistry were on
sodium and potassium concentrations; females at all three doses had
depressed sodium values, and those at the intermediate and high doses
and males at the high dose had depressed potassium values with respect
to controls. Gross necropsy, revealed dark-red foci in the stomachs of
rabbits of each sex at the intermediate and high doses, which
increased in frequency with dose: the incidences were 1/5 in males at
the intermediate dose, 4/5 in those at the high dose, 1/5 in females
at the intermediate dose, and 2/5 in females at the high dose. No
dark-red foci were seen in the stomachs of controls or rabbits at the
low dose. The NOAEL for systemic toxicity was 100 mg/kg bw per day on
the basis of the presence of dark-red foci in the stomachs of males
and females. The NOAEL for dermal effects was 1000 mg/kg bw per day,
the highest dose tested (Siglin, 1992).
Dogs
Groups of four pure-bred beagle dogs of each sex received
technical-grade diphenylamine (purity, > 99%) in gelatin capsules at
doses of 0, 10, 25, or 50 mg/kg bw per day for 90 days. They were
observed for deaths, clinical signs, body weight, food consumption,
ophthalmological, haematological, clinical chemical, and urinary
parameters, organ weights, and gross and histopathological appearance.
There were no deaths, and no treatment-related changes were seen in
any of the above parameters. Statistically significant increases were
seen, however, in some clinical chemical parameters including albumin
content, the albumin:globulin ratio in males, and bilirubin content in
females at the high dose. These effects may have been incidental. The
NOAEL was 50 mg/kg bw/day, the highest dose tested (Krohmer, 1992b).
(c ) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 CD-1 mice of each sex received diets containing
technical-grade diphenylamine (purity, > 99%) at concentrations of 0,
520, 2600, or 5200 ppm for up to 78 weeks, equal to 0, 73, 370, and
760 mg/kg bw per day for males and 0, 90, 460, and 940 mg/kg bw per
day for females. Ten mice of each sex per dose were sacrificed at 52
weeks. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmological and haematological
end-points, organ weights, and gross and histopathological appearance.
An increase in the incidence of greenish staining of the fur,
especially around the anogenital area, with dose was seen as the study
progressed. By week 26, most of the mice at 5250 ppm were affected,
and by the end of the study some mice at 525 ppm group showed
staining. The incidence of penile prolapse increased with dose,
affecting seven males at 2625 ppm and 17 at 5250 ppm by 78 weeks. The
frequency of unkempt appearance also increased with dose, with a
higher incidence among males. The mortality rate increased with dose,
becoming statistically significantly different from controls for males
at 2625 and 5250 ppm by 52 weeks. The deaths were attributed mainly to
cystitis among males and amyloidosis in females. The mean body-weight
gains were 87, 86, and 91% of control values for males at 5250 ppm and
104, 93, and 93% of control values for females at 5250 ppm at 13, 52,
and 78 weeks, respectively. The body-weight gains of males at 5250 ppm
and occasionally animals at 2625 ppm were statistically significantly
decreased throughout the study (mainly through week 58). The
body-weight gain of females at 5250 ppm was significantly decreased
during the first three weeks and then occasionally for the remainder
of the study. Mean food consumption was statistically significantly
decreased during the first week of treatment in males at the high dose
and remained increased throughout treatment for males at the two
higher doses, with occasional statistically significant differences
from controls. The food consumption of females at any dose showed
little or no statistically significant difference from that of
controls.
At the interim haematological evaluation at 52 weeks, males
showed dose-related decreases in haematocrit and erythrocyte counts
that reached statistical significance at 2625 and 5250 ppm; and mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin content increased with dose and reached statistical
significance in males at these doses. Females showed dose-related
decreases in haematocrit that reached statistical significance at
> 525 ppm; their erythrocyte counts decreased in a dose-related
fashion and reached statistical significance at 2625 and 5250 ppm.
Mean corpuscular volume, mean corpuscular haemoglobin, and mean
corpuscular haemoglobin content increased with dose, the latter two
reaching statistical significance at 2625 and 5250 ppm and the mean
corpuscular volume at 5250 ppm. At termination at 78 weeks, males and
females showed dose-related decreases in haematocrit and erythrocyte
counts that reached statistical significance at 2625 and 5250 ppm;
reticulocyte counts, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin content increased with
dose and reached statistical significance in males at these doses.
At the interim necropsy, darkened spleens were seen in most mice
at 2625 ppm and in all those at 5250 ppm. Darkened livers were seen in
most mice at 5250 ppm and pale kidneys in many. At terminal necropsy,
the livers of some mice at 2625 ppm and most at 5250 ppm were dark.
The spleens of most treated mice were dark and often enlarged.
Dose-related increases in the absolute and relative weights of the
spleen, liver, and heart were seen in animals of each sex at the
interim and final sacrifices. At interim sacrifice, the absolute
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly different from those of controls, while
the increases in the relative liver weights reached statistical
significance only at the highest dose. In females, the absolute and
relative weights of the spleen were statistically significantly
increased only at the highest dose. The absolute and relative weights
of the heart were statistically significantly increased in females at
5250 ppm; the increases in males were not statistically different from
those in controls. At final sacrifice, the absolute and relative
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly increased. In females, the absolute and
relative weights of the spleen were significantly increased only at
5250 ppm,; the differences in relative liver weight reached
statistical significance at 2625 and 5250 ppm. The absolute and
relative weights of the heart were statistically significantly
increased in animals of each sex at 5250 ppm. Changes in the absolute
and relative weights of the pituitary and thyroid were not
consistently dose-related and were thus considered not to be of
toxicological significance.
Histopathological examination revealed treatment-related effects
in the kidney, liver, spleen, bone marrow, urinary bladder, and penis.
The incidences of haematopoiesis and pigment deposition in the liver
were increased at the intermediate and high doses. Of mice at 2625 ppm
that were found dead or moribund or were sacrificed at termination,
19/51 males and 24/51 females showed liver haematopoiesis and 15/51
males and 37/51 females showed liver pigmentation; higher incidences
of these effects were seen at the high dose. The incidences of spleen
congestion and haemosiderosis were increased in all treated mice. Of
the mice at the low dose found dead or moribund or sacrificed at
termination, 11/50 males and 8/50 females showed spleen congestion and
8/50 males and 35/50 females showed spleen haemosiderosis; higher
incidences of these effects were seen at the higher doses. The
frequency of pigment deposition was increased in males at the high
dose and in females at the intermediate and high doses. Among females
found dead or moribund or sacrificed at termination, pigment was found
in 5/51 at the intermediate dose and 7/51 at the high dose. Among
males found dead or moribund or sacrificed at termination, 5/51 at the
intermediate dose and 7/51 at the high dose showed pigment
accumulation; additionally, 5/54 males had pyelonephritis. Although
the incidence of spleen haematopoiesis did not increase with dose, the
severity scores increased from minimal or slight in controls to mixed
scores including moderate or marked at the high dose. The severity of
haematopoiesis in the spleen increased from minimal to slight at 525
ppm in contrast to the largely minimal level in controls.
Additionally, bone-marrow cellularity changed from predominantly
moderate in controls to marked at the higher doses. The incidences of
urinary bladder cystitis and dilatation increased with dose, reaching
statistical significance at the intermediate and high doses. Among
mice at the intermediate dose that were found dead or moribund or were
sacrificed at termination, 24/51 males and 13/48 females had cystitis
and 18/51 males and 13/48 females had dilatation; higher values were
seen at the high dose. The incidence of balanoposthitis apparently
increased with dose; however, no statistical analysis was available.
In females at 5250 ppm, the incidence of amyloidosis in the thyroid,
adrenals, kidneys, stomach, small intestine, ovaries, and uterus was
increased. The incidence of tumours was comparable in the treated and
control groups. The NOAEL for toxicity was 525 ppm, equal to 73 mg/kg
bw per day, on the basis of decreased body-weight gain, decreased
survival, and significant haematological and gross and microscopic
pathological alterations (Botta, 1994a).
Rats
Groups of 60 male and 60 female Sprague-Dawley rats received
diets containing technical-grade diphenylamine (purity, > 99%) at
concentrations of 0, 200, 750, 3750, or 7500 ppm for males and 0, 150,
500, 2500, or 5000 ppm for females for up to two years, equal to 0,
8.1, 29, 150, and 300 mg/kg bw per day for males and 0, 7.5, 25, 140,
and 290 mg/kg bw per day for females. Groups of 10 rats of each sex
per group were killed at a one-year interim sacrifice. The animals
were observed for clinical signs, deaths, body weight, food
consumption, ophthalmological, haematological, clinical chemical, and
urinary end-points, organ weights, and gross and histopathological
appearance.
The only treatment-related clinical finding was greenish
colouration of the fur in the urogenital or ventral cervical area in
animals of each sex at the two higher doses. The effect was attributed
to the presence of a metabolite in urine or faeces. No treatment-
related effects on mortality rates were observed; however, the study
was terminated at 102 weeks because of increased mortality rates in
controls and animals at the low dose: survival among males was 22% at
0 and 200 ppm and 55% at 7500 ppm. Survival thus seemed to increase
with dose, and an analysis of the survival data indicated a
statistically significant negative trend for mortality as the dose
increased in animals of each sex; the mortality rates at the two
higher doses were statistically significantly lower than those for the
control group. A dose-related decrease in body weight and body-weight
gain was seen throughout most of the study, which reached statistical
significance at the two higher doses. The body-weight gains of males
at the two higher doses were depressed to 95 and 87% of the control
values at 78 weeks and equal to those of controls at 102 weeks; in
females, the corresponding values were 78 and 56% of controls at 78
weeks and 80 and 61% at 102 weeks. There was no decrease in food
consumption, except during the first week; at other times, food
consumption appeared to have increased, possibly due to food wastage.
There were no treatment related effects on ophthalmological
parameters.
Haematological examination revealed dose-related decreases in
erythrocyte counts, haemoglobin, and haematocrit in animals at the two
higher doses throughout treatment. The decreases reached statistical
significance for erythrocyte count and haemoglobin in males at 3750
and 7500 ppm in week 26 and at termination and for erythrocyte counts,
haemoglobin, and haematocrit in females at 2500 and 5000 ppm through
most of the treatment and at termination. Although the erythrocyte
counts, haemoglobin, and haematocrit were decreased in males at 750
ppm and in females at 500 ppm, the decreases reached statistical
significance only sporadically during treatment. The mean corpuscular
volume and mean corpuscular haemoglobin content were significantly
different from those of controls in males at the three higher doses
and in females at the two higher doses.
Dose-related, statistically significant increases in the absolute
and relative weights of the spleen were seen in females at the two
higher doses at interim sacrifice and at termination. A similar effect
on spleen weights was observed in males, except that the changes in
rats at 3750 ppm were not statistically significant. The relative
weight of the liver was statistically significantly increased in
females at 5000 ppm at termination and at 3750 and 5000 ppm at interim
sacrifice; no increase in liver weights was observed in males. The
increases in spleen and liver weights are consistent with the
haematological effects of the compound. Findings of dark and/or
enlarged spleens at necropsy in males at > 750 ppm and in females
at 500 ppm are probably related to the haematological effects.
Microscopic examination revealed treatment-related effects in the
kidney, liver, spleen, and bone marrow. The incidence of pigment
deposition in the kidney increased in a dose-related fashion and
reached 44/50 in males and 44/52 in females at the high dose that were
found dead or moribund or were sacrificed at termination. The
incidences of haematopoiesis and pigment deposition in the liver
increased in a dose-related fashion and reached 21/50 and 27/50 in
males and 41/52 and 45/52 in females at the high dose that were found
dead or moribund or sacrificed at termination. Erythroid hyperplasia
was seen at the higher dose but not in controls or at the low dose.
The incidence of congestion of the spleen increased in a dose-related
fashion and reached incidences of 50/50 in males and 47/52 in females
at the high dose that were found dead or moribund or sacrificed at
termination. These findings are all related to the observed
haematological effects. No treatment-related increase in tumour
incidence was observed. The NOAEL for toxicity was 150-200 ppm, equal
to 7.5 mg/kg bw per day, on the basis of changes in haematological
parameters and in the histopathological appearance of the spleen,
kidney, and liver (Botta, 1994b).
Dogs
Four beagles of each sex received diphenylamine (purity, > 99%)
by gelatin capsule at a dose of 0, 10, 25, or 100 mg/kg bw per day for
52 weeks and were observed for clinical signs, body weight, food
consumption, ophthalmological, haematological, clinical chaemical, and
urinary end-points, organ weights, and gross and histopathological
appearance. No treatment-related clinical signs were seen at
termination. One dog at the intermediate dose and two at the high dose
had greenish hair. There were no deaths or treatment-related effects
on body weight, food consumption, or ophthalmological parameters.
Haematological examination revealed decreased mean erythrocyte counts
(by 11% in comparison with controls), haemoglobin (9.3%), and
haematocrit (8.7%) in males at the high dose; smaller decreases in
these parameters were found in females. The platelet count increased
with dose in males at the 13-, 26-, 39-, and 52-week evaluation
periods, becoming statistically significant at the intermediate and
high doses. There was a dose-related increase in mean total bilirubin
concentration, which was statistically significant for animals at the
the intermediate and high doses throughout the study, in animals at
the low dose at week 26, and in females only at week 39. The mean
cholesterol concentration appeared to increase with dose at all
evaluation periods but was statistically significantly increased only
in males at the high dose at week 13 (by 68%) and in females at the
high dose at week 39 (by 37%). The blood urea nitrogen concentration
was decreased in females at the intermediate (by 16%) and high doses
(by 20%) at week 52. The mean absolute and relative weights of the
liver and thyroid appeared to increase with dose in males, but only
the mean absolute liver weight of males at the high dose was
statistically significantly increased. The mean absolute and relative
weights of the thyroid decreased with dose in females but did not
reached statistical significance at any dose. There were no
treatment-related gross or histopathological changes. The NOAEL for
toxicity was 10 mg/kg bw per day on the basis of haematological and
clinical chemical changes (Botta, 1994c).
(d) Genotoxicity
As summarized in Table 2, negative results were obtained for
mutation in bacteria (Lawlor, 1992) and for induction of micronuclei
in mouse bone marrow in vivo (Murli 1992 a,b). A weakly positive
response was observed for mutation in mouse lymphoma cells in vitro
at a dose range that was toxic, and the mutant frequency did not
increase with dose (Cifone, 1992). The Meeting concluded that although
diphenylamine has some genotoxic potential it is unlikely to be a
human genotoxic hazard.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, groups of 28
Sprague-Dawley rats received diphenylamine (purity, 99.8%) in the diet
at concentrations of 0, 500, 1500, or 5000 ppm, equal to 0, 40, 120,
and 400 mg/kg bw per day for F0 males and 0, 46, 130, and 450 mg/kg
bw per day for F0 females for 70 days before mating. After weaning,
28 F1 rats of each sex per group were also given the test diets for
up to 70 days before mating and were selected as parents for the F2
litters. The parent animals were observed for deaths, clinical signs,
body weight, food consumption, mating and fertility indices and other
measures of reproductive performance, organ weights, and gross and
histopathological appearance. The offspring were observed at birth and
through lactation for clinical signs, deaths, weights, and sex ratio;
all underwent necropsy.
Toxicity was dose-related and was observed in animals of each sex
and generations at all doses. In general, females were more affected
than males, and F1 animals were more affected than F0 animals. In
the F0 generation, the incidences of bluish coats and a bluish fluid
in the cages were increased in males and females at 5000 ppm, and
similar findings were made in the F1 generation. Swelling of the
mammary glands were seen in females, and palpable masses were noted in
lateral or ventral regions, especially in females. These masses
appeared to be transient but were not examined grossly or
microscopically. Body weight and body-weight gain were significantly
decreased at various times in animals at 1500 and 5000 ppm. At 5000
ppm, there was a decrease in body weight compared with controls, of
6-9% for F0 males, 5-8% for F0 females, 22-28% for F1 males, and
11-23% for F1 females. At 1500 ppm, there was a 5-8% decrease in body
weight for F0 females, 7-9% for F1 males, and 5% for F1 females.
The relative weights of the kidney, liver, and spleen of F0 and F1
males were significantly increased at 5000 ppm, and the relative
Table 2. Results of assays for the genotoxicity of diphenylamine
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Forward mutation S. typhimurium 6.67-333 µg/platea 99.9 Negative Lawlor (1992)
TA98, TA100, TA1535, 10-667 µg/plateb
TA1537, TA1538
Gene mutation L5178Y tk+/- mouse 5-80 µg/ml > 93 Weakly Cifone (1992)
lymphoma cells positiveb
Negativea
In vivo
Micronucleus Mouse (ICR strain) 250-1000 mg/kg bw (males) 99.9 Negative Murli (1992a,b)
formation 375-1500 mg/kg bw (females)
weights of the liver and spleen of F0 and F1 females were
significantly increased at 1500 ppm. The relative weights of the liver
were significantly increased at 1500 and 5000 ppm in F0 females but
only at 5000 ppm in F1 females.
Gross observations at necropsy included enlarged spleens in
animals of each sex and generation at 1500 and 5000 ppm and in F1
females at 500 ppm, in addition to blackish-purple spleens in animals
of each sex and generation at 500, 1500, and 5000 ppm. The incidences
of blackish-purple spleen in F1 rats at 500 ppm were 9/28 males and
6/28 females; incidences of 27/28 or higher were seen at higher doses.
Histopathological examination revealed a brown pigment in the proximal
tubules of the kidney in animals of each sex and generation at 5000
ppm; and 3/28 F1 females at 1500 ppm also had brown pigment.
Congestion and haemosiderosis of the spleen were seen in animals of
each sex and generation at 500, 1500, and 5000 ppm. Increased spleen
erythropoiesis was seen in 4/28 males at 5000 ppm. Hepatocyte
hypertrophy was seen in males of both generations at 1500 and 5000
ppm; in females, it was seen at all doses in the F0 generation but
only at 5000 ppm in the F1 generation. Kupffer cells with brown
pigment containing iron were seen in animals of each generation, at
5000 ppm in males and at 1500 and 5000 ppm in females.
Reproductive toxicity was seen in the form of decreased mean pup
weight at various times in each generation and reduced litter size at
the high dose; no other parameters were affected by treatment. F1
pups at 5000 ppm had statistically significantly decreased mean body
weight (11% less than controls at birth and 14-25% less than controls
during lactation). F2 pups showed statistically significantly
decreased mean body weight at 1500 ppm (10-12% less than controls
during late lactation) and at 5000 ppm (10-19% less than controls
throughout lactation). The mean body weight of pups in the F2 litters
at birth was about 4.8% lower than that of controls, but the
difference was not statistically significant. The mean litter size
decreased with dose in both generations, by 21% at 5000 ppm in the F1
generation, and was statistically significantly different from control
values. Although the mean litter size in the F0 generation at 5000
ppm was decreased by 10%, this value was not statistically
significant. The decrease in litter sizes with dose correlated with
the number of uterine implantation scars observed at necropsy, and the
mean uterine implantation scar count decreased with dose in both
generations. The mean scar count at 5000 ppm was decreased by 16% and
was statistically significant; although the mean scar count in the F0
generation was decreased by 7.3% at 5000 ppm, the value was not
statistically significant.
A NOAEL was not identified for parental systemic toxicity.
Although haemosiderin and congestion in the spleen and hepatocyte
hypertrophy were seen at all doses, the incidence and intensity of
these effects were appreciably smaller at the lower dose. For example,
only 4/28 females at the low dose had spleen congestion, rated as
minimal, whereas 22/28 females at the intermediate dose had this
effect and its intensity was nearly equally divided between minimal
and mild. These observations suggest that that the lower dose in this
study is close to the NOAEL for parental systemic toxicity. The NOAEL
for reproductive toxicity was 1500 ppm, equivalent to 120mg/kg bw per
day for F0 animals, on the basis of decreased mean litter size at the
high dose. The NOAEL for developmental toxicity was 500 ppm,
equivalent to 46 mg/kg bw per day in maternal animals, on the basis of
the statistically significantly decreased mean body weights of F2
pups (Rodwell, 1993).
(ii) Developmental toxicity
Rats
In a range-finding study for developmental toxicity, groups of
six pregnant Sprague-Dawley Crl:CDTM BR VAF/Plus rats received
diphenylamine (purity, 99.9%) in corn oil by gavage at doses of 0, 10,
50, 100, 200, 300, or 400 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity was seen at doses of 100 mg/kg bw per day and
higher. Two deaths occurred at 400 mg/kg bw per day, and there were
dose-related decreases in food consumption, body weight, and body-
weight gain starting at a dose of 100 mg/kg bw per day. Necropsy
revealed a purplish-black spleen in one dam at 100 mg/kg bw per day
and in all surviving dams at higher doses. The increased incidence of
early resorptions was apparent dose-related and was accompanied by
decreases in mean fetal weight and gravid uterine weight at doses of
200 and 300 mg/kg bw per day. There were no treatment-related external
malformations or developmental variations. On the basis of the results
of this study, 0, 10, 50, and 100 mg/kg bw per day were selected for
use in the definitive study (Rodwell, 1992a).
In the definitive study, groups of 25 pregnant Sprague-Dawley
Crl:CDTM BR VAF/Plus rats received diphenylamine (purity, 99.9%) in
corn oil by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for deaths, clinical
signs, body weight, and food consumption. All rats were sacrificed on
day 20 of gestation and were necropsied grossly. The spleens were
weighed, and the uterus was removed, examined externally, weighed, and
opened for internal examination. The fetuses were observed externally,
and their viscera and skeleton were examination. There were no deaths
or treatment-related effects on clinical signs, body weights, or food
consumption. At necropsy, 5/25 dams at the high dose had enlarged,
blackish-purple spleens, and the mean weights of the spleens were
significantly greater than those of controls. No treatment-related
effects were seen on gross necropsy, in the appearance or weight of
the uterus, or in the numbers of viable fetuses, early and late
resorptions, and corpora lutea, or on external, visceral, or skeletal
malformations or variations in the fetuses. The NOAEL for maternal
toxicity was 50 mg/kg bw per day on the basis of effects on the spleen
in dams at the high dose. The NOAEL for developmental toxicity was 100
mg/kg bw per day, the highest dose tested, in the absence of
developmental effects (Rodwell, 1992b).
Rabbits
In a study of developmental toxicity, groups of 16-18 pregnant
New Zealand white rabbits received diphenylamine (purity, 99.9%) in 1%
methyl cellulose by gavage at a dose of 0, 33, 100, or 300 mg/kg bw
per day on days 7-19 of gestation. The dams were observed for deaths,
clinical signs, body weight, and food consumption. All rats were
sacrificed on day 29 of gestation and subjected to gross necropsy; the
ovaries and uterus of each animal were removed and examined to
determine the number of corpora lutea and the type, distribution, and
number of implantation sites. The fetuses were examined externally,
and their viscera and skeleton were analysed. No deaths occurred
during the study. The mean food consumption of dams at 300 mg/kg bw
per day was reduced on days 7-29 of gestation, and a slight decrease
in body weight was noted. Green discolouration of the urine was seen
in all treated animals. No treatment-related effects were seen at
necropsy or on embryonic or fetal growth or development. The NOAEL for
maternal toxicity was 100 mg/kg bw per day on the basis of decreased
body-weight gain and food consumption early during treatment. The
NOAEL for developmental toxicity was 300 mg/kg bw per day, the highest
dose tested (Edwards et al., 1983).
(f) Special studies
(i) Renal cystic disease
The issue of tubular cyst formation in diphenylamine-treated rats
was reviewed previously (Annex 1, references 13, 27, 39, and 43).
Additional observations on the induction of tubular cysts in the
kidneys of mice and rats treated with diphenylamine in the diet are
summarized below.
Renal histopathology and function were studied in male
Sprague-Dawley rats fed pelleted diets containing 1% w/w (i.e. 10 000
ppm) diphenylamine (purity unspecified) for 5-20 months. Control
animals received identical diets in which methyl cellulose was
incorporated instead of diphenylamine. The animals were observed for
renal tubular diameter and renal functional parameters including
intratubular hydrostatic pressure, single-nephron glomerular
filtration rate, and transit times through the loop of Henle; light
and scanning electron microscopy was performed. The luminal diameters
of 22 dilated nephrons from diphenylamine-treated rats were 34-110 µm,
and those of 10 undilated nephrons were 21-450 µm; the luminal
diameters in six nephrons of two control rats were 26-34 µm. The
intraluminal hydrostatic pressure was significantly higher in dilated
tubules than in undilated tubules from treated rats; there was no
difference in intraluminal hydrostatic pressures among control rats.
The single-nephron glomerular filtration rate was also similar in
dilated and undilated tubules, consistent with an intrinsic change in
glomerular function. The transit times through the loop of Henle were
three to four times faster in dilated than in undilated nephrons of
treated and control rats. Microscopic examination indicated that
dilatation along the proximal and collecting tubules was segmental,
not diffuse, some dilated tubules communicated with cysts deep in the
renal substance, the dilated collecting tubules occasionally contained
debris, tubules adjacent to cysts appeared to be compressed, and
diphenylamine-exposed kidneys contained proximal convoluted tubules
with segments of apparent narrowing. The authors concluded that cyst
formation results from initial obstruction and a subsequent increase
in intraluminal pressure arising from sustained glomerular filtration
and unaltered water reabsorption (Gardner et al., 1976).
The time-course of development of lesions in the renal collecting
tubules was studied in male Sprague-Dawley rats fed pelleted diets
containing 1% w/w (i.e. 10 000 ppm) diphenylamine (purity unspecified)
for up to 76 weeks. Control animals received standard diet. The
animals were observed for renal concentrating ability (urine
osmolality) at 2, 4, 5, and 20 weeks of treatment; light and
transmission and scanning electron microscopy was performed at 2, 5,
10, 15, 20, 25, 52, and 78 weeks of treatment; and autoradiography
with tritiated thymidine was conducted to determine whether
hypertrophy was the mechanism of cyst formation. Decreased urine
osmolality was seen in treated rats, which was statistically
significantly different from control values at 6 and 20 weeks.
Structural changes first appeared after five weeks of treatment. Light
microscopy of the medullary collecting tubules at five weeks revealed
focal areas of apparent thickening along the walls, resulting from
layering of cells. By 10 weeks, some tubules were dilated and
contained focal areas of cellular necrosis. By 15-20 weeks, many more
ducts were dilated and contained cast material. By 24 weeks, frank
cysts were visible in the cortex and medulla, and large collecting
tubular cysts were seen; some proximal tubules and renal corpuscles
were dilated. Over time, cysts were found in every segment of the
nephron and collecting tubules. Transmission electron microscopy at
five weeks revealed changes in the medullary collecting tubules. The
tubular cells appeared to be enriched in mitochondria, and their
apical border was studded with long microvilli. At 10 weeks, necrotic
cells were seen along the collecting tubules. Scanning electron
microscopy showed that the collecting duct cysts were lined with
irregular cells which no longer resembled collecting tubule cells. The
labelling index for the collecting tubules reached a plateau at weeks
5-15 and had decreased to background levels by week 52. Because the
number of nuclei counted on cross-sections of the collecting tubules
increased gradually with time, the authors concluded that the increase
in labelling index was the result of hyperplasia of the tubular cells
and not of a degeneration or regeneration phenomenon. The authors
concluded that one of the initial events in the pathogenesis of cystic
renal disease is a hyperplastic response of the collecting tubule
cells (Evan et al., 1978).
The effect of diphenylamine on the composition of the native
renal basement membrane was studied in male C57Bl/6 mice fed pelleted
diets containing 2% w/w (i.e. 20 000 ppm) diphenylamine (purity
unspecified) for up to 10 months. Control animals received chow
without diphenylamine. After 10 months of treatment, the mice were
sacrificed and their kidneys processed for light microscopy and
immunohistology of bamin, a glycoprotein associated with the matrix of
the glomerular basement membrane. Microscopy revealed cysts in the
cortical and medullary portions of the kidney. The cysts were lined
with flattened or somewhat cuboidal cells and contained a brownish
coagulum that appeared to be constituted of necrotic cells. Brownish
discolouration was also seen in the cytoplasm of neighbouring
non-cystic cells and in tubular cells of animals sacrificed before the
formation of cysts. The authors speculated that the brownish debris
preceded the formation of cysts. When immunohistology was performed
with antibodies to bamin, the antibodies bound to the glomeruli of
control mice but not to those of treated animals.This result was
obtained in the three-week experiment. In another series of
experiments, male C57Bl/6 mice were injected with EHS tumour cells and
five days later received a diet containing diphenylamine (at an
unspecified level). After three weeks, the animals were killed, and
tumours were removed for analysis of the proteins of the basement
membrane. Analysis by sodium dodecylate sulfate-polyacrylamide gel
electrophoresis and by immunoblot revealed the presence of a 75-80-kDa
protein in controls but little or none in the diphenylamine-treated
mice. The authors were unclear how the loss of bamin, a molecule
associated with the glomerular but not the tubular basement membrane,
would be related to the formation of tubular cysts. They speculated
that material from an abnormal glomerular filtrate might damage the
tubular epithelial cells and result in the formation of tubular cysts
(Rohrbach et al., 1993).
(iv) Renal papillary necrosis
A series of studies has been reported on the induction of renal
papillary necrosis by diphenylamine in hamsters, rats, and Mongolian
gerbils. The development of animal models for this lesion is of
interest because it is the initial stage in human analgesic-induced
nephropathy.
Groups of 10 male Syrian hamsters, 40 Sprague-Dawley rats, and 40
Mongolian gerbils received diphenylamine (purity unspecified) at doses
of 0, 400, 600, or 800 mg/kg bw per day in peanut oil by gavage for
three days. Animals that became moribund were sacrificed and
necropsied; survivors were sacrificed 24 h after the third dose of
diphenylamine. All animals were observed for death and were examined
for gross and microscopic lesions. Forty percent of hamsters at the
low dose and 100% of those at the two higher doses died or were
sacrificed in extremis during treatment; there were no deaths among
the rats or gerbils. At necropsy, brown kidneys and yellow-brown
papillae were seen in 50% or more of hamsters at the intermediate and
high doses and in none of those at the low dose. The only gross lesion
in hamsters at the low dose was splenomegaly (90%), which was not seen
at higher doses. Microscopic examination revealed total renal
papillary necrosis, i.e. necrosis of all elements of the renal
papillae, including collecting tubules, at all doses in 4/10 hamsters
at the low, 7/10 at the intermediate, and 4/10 at the high dose. Only
two rats at the high dose had renal lesions, which consisted of
necrosis of the medullary interstitial cells and vasa recta, limited
to the apex, and degeneration of the renal interstitial matrix. As
noted by the authors, the limited incidence and extent of the lesions
in the Sprague-Dawley rats contrasted with the more extensive effects
observed in Wistar rats by Powell et al. (1985). No effects were
observed in Mongolian gerbils (Lenz & Carlton, 1990).
A total of 27 male Syrian hamsters were given diphenylamine by
gavage (purity unspecified) dissolved in peanut oil at a dose of 600
mg/kg bw. At intervals of 0.5, 1, 2, 4, 8, 16, and 24 h after dosing,
three hamsters were perfused in situ with fixative and their kidneys
were removed for examination by transmission electron microscopy.
Starting 1 h after dosing, the basal plasma membrane of the
endothelial cells of the ascending vasa recta in the proximal portion
of the renal papilla became separated from the basal lamina, forming
large subendothelial vacuoles. These alterations persisted during the
first 8 h after dosing. By 16 h, the endothelial cell nuclear
membranes and the luminal plasma membrane had become convoluted, and
by 24 h platelets were seen to adhere to the basal lamina, which had
become exposed. By 2 h after dosing, the adjacent interstitial cells
started undergoing structural alterations, and by 24 h there was
necrosis. Ultrastructural alterations became visible in the thin limb
of the loop of Henle by 24 h and in collecting tubule epithelial cells
by 14-16 h. The authors speculated that the selective effect on the
endothelial cells of the ascending vasa recta were attributable to
generation of a toxic metabolite by the endothelial cell or to
interference with the metabolism of the endothelial cells by a
metabolite of diphenylamine (Lenz et al., 1995).
The toxicity of diphenylamine dissolved in dimethyl sulfoxide to
renal papilla was studied in Syrian hamsters, rats, and Mongolian
gerbils. Male Syrian hamsters and Sprague-Dawley rats were given
diphenylamine (purity unspecified) by gavage at doses of 0, 400, 600,
or 800 mg/kg bw per day for up to nine days. Only one of 30 hamsters
at 400 mg/kg bw per day showed renal papillary necrosis. This result
contrasted with previous results (Lenz & Carlton, 1990) in which oral
administration of diphenylamine dissolved in peanut oil produced
extensive necrosis in male Syrian hamsters at the same doses;
furthermore, pretreatment of the hamsters with dimethyl sulfoxide
protected the animals against the renal papillary necrosis induced by
administration of diphenylamine. Renal papillary necrosis occurred in
4/30 rats at the high dose; none was seen in Mongolian gerbils. The
incidence in rats and gerbils did not differ from that observed when
peanut oil was used as the vehicle in a previous study (Lenz &
Carlton, 1990). The mechanism by which dimethyl sulfoxide exerts its
protective effect in Syrian hamsters was not studied (Lenz & Carlton,
1991).
The effect of diphenylamine on renal glutathione concentrations
was studied in groups of eight male Syrian hamsters given
diphenylamine (purity unspecified) by gavage at a dose of 0, 200, 400,
or 600 mg/kg bw 1 and 4 h after dosing. The concentrations in the
cortical area decreased with dose, reaching statistical significance
at the low dose at 1 h and at the intermediate dose at 4 h; the
concentrations at 1 h were 41% of the control value at 200 mg/kg bw,
31% at 400 mg/kg bw, and 29% at 600 mg/kg bw. The glutathione
concentrations in the outer medulla and the renal papilla were not
statistically significant decreased.
In order to study the effect of glutathione reduction on toxicity
to renal papilla, groups of eight male Syrian hamsters were given
buthionine sulfoxime, an inhibitor of glutathione biosynthesis, at 500
mg/kg bw intrapeitoneally, diphenylamine in peanut oil at 400 mg/kg bw
by gavage, the same doses of buthionine sulfoxime plus diphenylamine,
or the peanut oil vehicle alone. The animals were sacrificed 24 h
after administration of diphenylamine for gross and microscopic
examination of the kidney. No gross or microscopic lesions indicative
of papillary necrosis were observed in any group. Because a dose of
diphenylamine that is toxic to the papilla did not deplete glutathione
in that region and because depletion of glutathione (to 71% of the
control value) by buthionine sulfoxime did not enhance the toxicity of
diphenylamine to the papilla, the author concluded that this toxic
effect of diphenylamine is mediated by mechanisms independent of
oxidative injury (Lenz, 1996).
Comments
After oral administration to rats, goats, or hens,
14C-diphenylamine was extensively absorbed and rapidly excreted. In
rats given a single dose, 45-72% appeared in the urine within 24 h and
68-89% by 168 h after dosing, with less than 0.4% of the dose in the
residual carcass and less than 0.05% in any individual tissue. In
goats treated with single daily doses for seven days, 85-91% of the
daily dose was excreted in urine and 0.5-0.8% in milk; the
concentrations of residues in milk plateaued after 24 h. When laying
hens were treated with single daily oral doses for seven days, 84-98%
of the daily dose was recovered in the excreta; the concentrations in
egg yolk reached 0.31 mg/kg on day 7, but no residues were found in
egg whites. Diphenylamine underwent extensive biotransformation in
rats, goats, and hens, with ring hydroxylation and formation of
glucuronide and sulfate conjugates. In addition to untransformed
diphenylamine (< 3% of the dose), the following 12 metabolites were
identified at all doses: 4,4'-dihydroxydiphenylamine (unconjugated and
as the O-sulfate and the O, O-disulfate), 4-hydroxydiphenylamine
(unconjugated and as the O-glucuronide, N-glucuronide,
O-sulfate, and O, N-diglucuronide), indophenol (unconjugated and
as the O-sulfate), 3-hydroxydiphenylamine, and
2-hydroxydiphenylamine. These metabolites accounted for about 80-90%
of the dose and were excreted largely as their sulfate and glucuronide
conjugates. There was no cleavage of the diphenylamine structure.
Except for a polar oligomer of 4-hydroxydiphenylamine found only in
the eggs and tissues of hens, all of the metabolites reported in hens
and goats were detected in rats. Residues of a number of plant
metabolites were identified in apple peel and pulp, but untransformed
diphenylamine was the major contributor to the total residue 40 weeks
after application. The major metabolite was 4-hydroxydiphenylamine, as
the glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxy-diphenylamine, and
dihydroxydiphenylamine (possibly the 2,4 isomer). These compounds were
either free or conjugated with mono- or oligosaccharides. Although all
of the hydroxylated metabolites (aglycones) identified in plants were
seen in rats, the conjugating species were generally different.
After acute oral administration to rats, diphenylamine (99.9%
pure) was slightly toxic (LD50 about 3000 mg/kg bw) in one study,
whereas in another study super-refined diphenylamine (purity,
99.0-100.1%) was essentially nontoxic (LD50 > 15 000 mg/kg bw).
WHO has not classified diphenylamine for acute toxicity.
In mice given diphenylamine at dietary concentrations of 0, 10,
520, 2600, or 5200 ppm for 90 days, dose-related changes in
haematological parameters (decreased erythrocyte counts and packed
cell volumes and increased reticulocyte counts) were observed. The
mean corpuscular haemoglobin count was significantly increased at
dietary levels of 520 ppm and above. Necropsy revealed dark, enlarged
spleens with haemosiderosis and congestion at dietary levels of 520
ppm and above. Spleen haematopoiesis was increased in animals of each
sex at 520 ppm. The NOAEL was 10 ppm (equal to 1.7 mg/kg bw per day)
on the basis of changes in haematological parameters and findings at
necropsy in animals at 520 ppm.
In rats that received diphenylamine in the diet at concentrations
of 0, 150, 1500, 7500, or 15 000 ppm for 90 days, body weights and
body-weight changes, although generally lower than the control values
at 1500 ppm, were not significantly different from those of controls
at doses below 7500 ppm. The cholesterol concentration increased with
dose in females and was significantly different from that of controls
at 1500 ppm. In females, the absolute and relative weights of the
liver increased with dose, and the relative liver weights were
significantly different from those of controls in animals at 1500 ppm
and above. Histopathological examination revealed increased
haematopoiesis and pigment in the liver, haematopoiesis,
haemosiderosis, and congestion in the spleen, and pigmented kidneys in
animals of each sex at 7500 and 15 000 ppm. Additionally, the spleens
of all females at 1500 ppm showed minimal or slight haematopoiesis and
haemosiderosis. The NOAEL was 150 ppm (equal to 12 mg/kg bw per day)
on the basis of changes in clinical chemical parameters, increased
organ weights, and gross and histological changes in female rats at
1500 ppm.
Groups of rabbits were exposed dermally to diphenylamine in
distilled water at doses of 0, 100, 500, or 1000 mg/kg bw per day for
6 h per day. No effects were observed. Gross necropsy revealed
dark-red foci in the stomachs of rabbits at the intermediate and high
doses, which increased in number with dose. The NOAEL for systemic
effects was 100 mg/kg bw per day on the basis of effects on the
stomach at 500 mg/kg bw per day in animals of each sex.
Groups of dogs received diphenylamine by gelatin capsule at doses
of 0, 10, 25, or 50 mg/kg bw per day for 90 days. There were no
treatment-related effects. The NOAEL was 50 mg/kg bw per day, the
highest dose tested.
In a study of carcinogenicity, mice received diets containing
diphenylamine at concentrations of 0, 520, 2625, or 5200 ppm for 78
weeks. At 520 ppm and above, decreased packed cell volumes were seen
in females and spleen congestion and haemosiderosis in animals of each
sex. At 2600 ppm and above, clear haematological effects, consistent
with regenerative anaemia, were observed. The incidences of tumours
were not increased when compared with those in controls. The NOAEL for
toxicity was 520 ppm (equal to 73 mg/kg bw per day) on the basis of
decreased body-weight gain, reduced survival, and significant
alterations in haematological and gross and microscopic pathological
parameters at higher levels. Examination of the incidence and severity
of some haematological effects at 520 ppm suggested that this dose is
close to the NOAEL/LOAEL threshold. There was no evidence of
carcinogenicity.
In a combined study of toxicity and carcinogenicity, rats
received diets containing diphenylamine at concentrations of 0, 200,
750, 3750, or 7500 ppm (males) and 0, 150, 500, 2500, or 5000 ppm
(females). At 500 ppm and above, decreased erythrocyte count,
haemoglobin, and packed cell volumes were observed in animals of each
sex; these decreases reached statistical significance only
sporadically during treatment in males at 750 ppm and in females at
500 ppm. Haematopoiesis was increased in the liver and spleen of males
at 750 ppm and above. At 2625 ppm and above, clear haematological
effects consistent with regenerative anaemia were observed. There was
no significant increase in the incidence of tumours when compared with
that in controls. The NOAEL was 150-200 ppm (equal to 7.5 mg/kg bw per
day) based on haematological and histological effects at dietary
levels equal to or greater than 500 ppm. This appeared to be close to
the threshold dose, since body-weight gain was not depressed and only
sporadic haematological changes were observed at 500-750 ppm. There
was no evidence of carcinogenicity.
Dogs received diphenylamine by gelatin capsule at doses of 0, 10,
25, or 100 mg/kg bw per day for one year. Platelet counts in males and
total bilirubin concentrations in animals of each sex were
statistically significantly higher than those of controls. The NOAEL
for toxicity was 10 mg/kg bw per day, and the LOAEL was 25 mg/kg bw
per day, both based on haematological and clinical chemical changes.
In a two-generation study of reproductive toxicity, rats received
diphenylamine in the diet at concentrations of 0, 500, 1500, or 5000
ppm during premating. A NOAEL for parental toxicity was not observed;
the LOAEL was 500 ppm (equal to 40 mg/kg bw per day) on the basis of
enlarged spleens in F1 females, increased spleen congestion and
haemosiderosis in animals of each sex in all generations, and
hepatocyte hypertrophy in F0 females. The NOAEL for developmental
toxicity was 500 ppm (equal to 46 mg/kg bw per day) on the basis of
statistically significantly decreased mean body weight in F2 pups at
1500 ppm and above. The NOAEL for reproductive toxicity was 1500 ppm
(equal to 120 mg/kg bw per day). Although haemosiderin, congestion of
the spleen, and hepatocyte hypertrophy were observed in parental
animals at all doses, they occurred at appreciably lower incidence and
intensity at the lower dose than at the higher doses, which suggested
that the lower dose was close to the NOAEL/LOAEL threshold for
parental toxicity.
In a study of developmental toxicity, rats received diphenylamine
by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on days 6-15
of gestation. The NOAEL for maternal toxicity was 50 mg/kg bw per day
on the basis of enlarged, blackish, heavier spleens at 100 mg/kg bw
per day. The NOAEL for developmental toxicity was 100 mg/kg bw per
day, the highest dose tested.
In a study of developmental toxicity, rabbits received
diphenylamine by gavage at doses of 0, 33, 100, or 300 mg/kg bw per
day on days 7-19 of gestation. The NOAEL for maternal toxicity was 100
mg/kg bw per day on the basis of decreased body-weight gain and food
consumption at 300 mg/kg bw per day. The NOAEL for developmental
toxicity was 300 mg/kg bw per day, the highest dose tested.
Diphenylamine has been tested for genotoxicity in three assays.
Negative results were obtained for mutation in bacteria and for
induction of micronuclei in mouse bone marrow in vivo. A weakly
positive response was observed only for mutation in mouse lymphoma
cells in vitro at a dose range in which the toxicity was relatively
high and the mutant frequency did not increase with dose. The Meeting
concluded that, although diphenylamine has some genotoxic potential,
it is unlikely to present a human genotoxic hazard.
An ADI of 0-0.08 mg/kg bw was established on the basis of the
NOAEL of 150 ppm, equal to 7.5 mg/kg bw per day, in the two-year study
of toxicity and carcinogenicity in rats and a 100-fold safety factor.
An acute RfD was not allocated because diphenylamine is of low
acute toxicity. The Meeting concluded that the acute intake of
residues is unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 520 ppm, equal to 73 mg/kg bw per day (78-week study of
carcinogenicity)
Rat: 150-200 ppm, equal to 7.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equal to 46 mg/kg bw per day (reproductive
toxicity in a two-generation study of reproductive
toxicity)
Rat 50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
300 mg/kg bw per day (highest dose in a study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.08 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid absorption: at least 68-89% of a dose
Dermal absorption No data
Distribution Extensive
Potential for accumulation Very little
Rate and extent of excretion At 24 h, 45-72% of a dose found in urine
Metabolism in animals Extensively metabolized. Parent < 3%. Approximately
80-90% of a dose appeared as 12 metabolites: indophenol
and various isomers of mono- and
di-hydroxydiphenylamine excreted in urine as sulfate
and glucuronide conjugates
Toxicologically significant compounds Parent. Indophenol might undergo electrophilic
(animals, plants, and environment) interactions
Acute toxicity
Rat: LD50 oral 3000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
LC50 inhalation No data
Skin irritation None to slight
Eye irritation Slight to corrosive with corneal opacity
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Erythrocytes/anaemia
Lowest relevant oral NOAEL Mouse: 1.7 mg/kg bw per day, 90-day study
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity Unlikely to be a human genotoxic hazard
Long-term toxicity and carcinogenicity
Target: critical effect Erythrocytes/haemolytic anaemia
Lowest relevant NOAEL Rat: 7.5 mg/kg bw per day
Carcinogenicity No carcinogenicity
Reproductive toxicity
Reproduction target /critical effect Decreased mean litter size in both generations and
decreased mean body weights of F2 pups at maternally
toxic doses
Lowest relevant reproductive NOAEL Rat: 46 mg/kg bw per day
Developmental target/critical effect Rat: No developmental toxicity
Lowest relevant developmental NOAEL Rat: 100 mg/kg bw per day, highest dose tested
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.08 mg/kg bw Long-term toxicity 100
and carcinogenicity,
rats
Acute reference dose Not allocated
(unnecessary)
References
Botta, J.A., Jr (1992) 90 Day subchronic toxicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426E-001-034-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994a) 18 Month oncogenicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426H-002-646-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994b) 24 Month combined oncogenicity/toxicity
evaluation of diphenylamine in rats. Unpublished report No.
426D-102-048-91 from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Botta, J.A., Jr (1994c) One year chronic study of diphenylamine in
dogs. Unpublished report No. 426B-502-044-91 from T.P.S., Inc., Mt
Vernon, Indiana, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Cifone, M. (1992) Mutagenicity test on diphenylamine in the L5178Y
TK+/- mouse lymphoma forward mutation assay. Unpublished report No.
14902-0-431 from Hazleton Washington, Inc., Rockville, Maryland, and
Vienna, Virginia, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Edwards, J.A., Leemings, N.M., Clark, R. & Offer, J.M. (1983) Effect
of diphenylamine on pregnancy of the New Zealand white rabbit.
Unpublished study No. PWT 1/2/83409 prepared by Huntington Research
Centre, Huntington, Cambridgeshire, United Kingdom.
Evan, A.P., Hong, S.K., Gardner, K., Jr, Park, Y.S. & Itagaki, R.
(1978) Evolution of the collecting tubular lesion in
diphenylamine-induced renal disease. Lab. Invest., 38, 244-252.
Gardner, K.D., Jr, Solomon, S., Fitzgerrel, W.W. & Evan, A.P. (1976)
Function and structure in the diphenylamine-exposed kidney. J. Clin.
Invest., 57, 796-806.
Kim-Kang, H. (1993) Metabolism of [14C]diphenylamine in stored
apples: Nature of the residue in plants. Unpublished study No. XBL
91071, report No. RPT00124 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Abstract of study submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Kim-Kang, H. (1994a) Metabolism of [14C]diphenylamine in lactating
goats. Unpublished study No. XBL 92089, report No. RPT00150 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kim-Kang H (1994b) Metabolism of [14C]diphenylamine in the laying
hen. Unpublished study No. XBL 93041, report No. RPT00161 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA
Kiplinger, G. (1995) Skin sensitization study of diphenylamine
technical in albino guinea pigs. Final report: Lab project No.
WIL-256001. Unpublished study prepared by WIL Research Labs, Inc.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Kreuzman, J. (1991a) Primary eye irritation study in rabbits without
rinsing with diphenylamine super-refined. Lab project No. 91-8052-21
(B). Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kreuzman, J. (1991b) Primary skin irritation study in rabbits with
diphenylamine super-refined. Lab project No. 91-8052-21 (A).
Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to WHO
by the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Krohmer, R.W. (1992a) 90 Day evaluation of diphenylamine in the dog.
Unpublished study No. 426C-501-034-91 from T.P.S., Inc., Mt Vernon,
Indiana, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Krohmer, R.W. (1992b) 90 Day subchronic toxicity evaluation of
diphenylamine in rats. Unpublished study No. 426C-10-034-91 from
T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the DPA
Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA
Lawlor, T. (1992) Mutagenicity test on diphenylamine in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test).
Unpublished report No.14902-0-401 from Hazleton Washington, Inc.,
Rockville, Maryland, and Vienna, Virginia, USA. Submitted to WHO by
the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Lenz, S.D. (1996) Investigation of regional glutathione levels in a
model of chemically-induced renal papillary necrosis. Food Chem.
Toxicol., 34, 489-494.
Lenz, S.D. & Carlton, W.W. (1990) Diphenylamine-induced renal
papillary necrosis and necrosis of the pars recta in laboratory
rodents. Vet. Pathol., 27, 171-178.
Lenz, S.D. & Carlton, W.W. (1991) Decreased incidence of
diphenylamine-induced renal papillary necrosis in Syrian hamsters
given dimethylsulphoxide. Food Chem. Toxicol., 29, 409-418.
Lenz, S.D., Turek, J.J. & Carlton, W.W. (1995) Early ultrastuctural
lesions of diphenylamine-induced renal papillary necrosis in Syrian
hamsters. Exp. Toxicol. Pathol., 47, 447-452.
Majnarich, J. (1991a) Diphenylamine: Super refined: Acute oral
toxicity, LD50 (rat). Unpublished report No. 022-91 from
Bioconsultants, Inc. Submitted to WHO by the DPA Task Force, John Wise
& Associates Ltd, Liberty, Missouri, USA.
Majnarich, J. (1991b) Diphenylamine: Super refined: Dermal LD50.
Unpublished report No. 024-91 from Bioconsultants, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Murli, H. (1992a) Dose range-finding study on diphenylamine for in
vivo murine micronucleus assay. Unpublished report No. 14902-0-459PO
from Hazleton Washington, Inc., Rockville, Maryland, and Vienna,
Virginia, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Murli, H. (1992b) Mutagenicity test on diphenylamine for in vivo
mammalian micronucleus assay. Unpublished report No. 14902-0-455 from
Hazleton Washington, Inc., Rockville, Maryland, and Vienna, Virginia,
USA. Submitted to WHO by the DPA Task Force, John Wise & Associates
Ltd, Liberty, Missouri, USA.
Powell, C.J., Adams, T., Hall, D.E., Bach, P.H. & Bridges, J.W. (1985)
The comparative subacute nephrotoxicity of diphenylamine and
N-phenylanthranilic acid. In: Bach, P.H. & Bridges, J.W., eds,
Renal Heterogeneity and Target Cell Toxicity, Chichester, John Wiley
& Sons, pp. 199-202.
Rodwell, D.E. (1992a) Range finding teratology study in rats with
diphenylamine (DPA). Unpublished study No. 3255.2 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rodwell, D.E. (1992b) Teratology study in rats with diphenylamine
(DPA). Unpublished study No. 3255.3 prepared by Springborn
Laboratories, Inc., Life Sciences Division, Spencerville, Ohio, USA.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Rodwell, D.E. (1993) Two-generation reproduction study in rats with
diphenylamine (DPA). Unpublished study No. 3255.4 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rohrbach, D.H., Robinson, L.K. & Murrah, V.A. (1993) Loss of the
basement membrane matrix molecule, bamin, in diphenylamine-treated
mice. Matrix, 13, 341-350.
Siglin, J.C. (1991) Repeated dose dermal toxicity: 21 Day study.
Unpublished study No. 3255.1 prepared by Springborn Laboratories,
Inc., Life Sciences Division, Spencerville, Ohio, USA. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Wu, D. (1993) Diphenylamine: Rat metabolism study. Unpublished study
XBL No. 92081, report No. RPT00131 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Submitted to WHO by the DPA Task Force,
John Wise & Associates Ltd, Liberty, Missouri, USA.
glucuronide, 24% as 4-hydroxy-diphenylamine sulfate, and 1.3% as
indophenol. Of the total 94% radiolabelled residue in milk, 7.4% was
identified as diphenylamine, 39% as 4-hydroxydiphenylamine
glucuronide, and 47% as 4-hydroxydiphenylamine sulfate. Of the total
40% radiolabelled residue in omental fat, 36% was identified as
diphenylamine and 3.6% as 4-hydroxydiphenylamine.
In the study in hens (Kim-Kang, 1994b), no metabolites were
identified in urine or faeces. Of the total 85% radioactive residue in
egg yolks, 17% was identified as diphenylamine, 4.8% as
4-hydroxydiphenylamine, 0.6% as 4.4'-dihydroxydiphenylamine, 3.2% as
4-hydroxydiphenylamine glucuronide, 57% as 4-hydroxydiphenylamine
sulfate, and 1.9% as a polar oligomer conjugate of
4-hydroxydiphenylamine. Of the total 31% radiolabelled residue in
liver, 7.9% was identified as diphenylamine, 4.5% as
2-hydroxydiphenylamine, 3% as 4.4'-di-hydroxydiphenylamine, 1.4% as
4-hydroxydiphenylamine glucuronide, 8.6% as 4-hydroxydiphenylamine
sulfate, 4.7% as a polar oligomer conjugate of 4-hydroxydiphenylamine,
and 1.3% as indophenol. Of the total 39% radiolabelled residue in
kidney, 1.1% was identified as diphenylamine, 0.3% as
2-hydroxy-diphenylamine, 0.2% as 4-hydroxydiphenylamine sulfate, and
38% as a polar oligomer conjugate of 4-hydroxydiphenylamine. Of the
total 58% radiolabelled residue in skin and fat, 35% was identified as
diphenylamine and 23% as 4-hydroxydiphenylamine sulfate.
The biotransformation of 14C-diphenylamine was also studied in
Red Delicious apples. Residues of a number of plant metabolites were
identified in apple peel and pulp, and untransformed diphenylamine was
the major contributor to the total residue 40 weeks after application.
The major metabolite was 4-hydroxydiphenylamine, present as the
glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxydiphenylamine, and
dihydroxydiphenylamine (possibly the 2,4-isomer). These compounds were
present free or as conjugates with mono- or oligosaccharides
(Kim-Kang, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of diphenylamine are
summarized in Table 1. After acute oral administration to rats in one
study, diphenylamine (purity, 99.9%) was slightly toxic, with an LD50
of 3000 mg/kg bw in males and 2700 mg/kg bw in females (Spanjers &
Til, 1982). In another study, diphenylamine (purity, 99-100.1%) was
generally not toxic, the LD50 being > 15 000 mg/kg bw for animals of
each sex (Majnarich, 1991a).
Table 1. Acute toxicity of diphenylamine in rats
Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Male Oral 99.9 3000 Spanjers & Til (1982)
Female 2700
Male and female Oral 99.9-100.1 > 15 000 Majnarich (1991a)
Not reported Dermal (24 h) 99.9-100.1 > 5000 Majnarich (1991b)
The acute dermal LD50 after a 24-h exposure to diphenylamine
(purity, 99.9-100.1%) was > 2 g/kg bw in New Zealand white rabbits of
each sex. No clinical signs were noted (Majnarich, 1991b).
Diphenylamine (purity, 99.9-100.1%) applied to the eyes of one
rabbit for seven days without rinsing was corrosive and induced
corneal opacity (Kreuzmann, 1991a). The same preparation was not
irritating to the skin of rabbits (Kreuzmann, 1991b). Diphenylamine
(purity, 99.9%) did not produce dermal sensitization in guinea-pigs
(Kiplinger, 1995).
(b) Short-term studies of toxicity
Mice
Groups of 15 male and 15 female Swiss-derived CD-1 mice received
technical-grade diphenylamine in the diet at 0, 10, 520, 260, or 5200
ppm for 90 days, equal to doses of 1.7, 94, 440, and 920 mg/kg bw per
day in males and 2.1, 110, 560, and 1100 mg/kg bw per day in females.
The animals were observed for clinical signs, deaths, body weight, and
food consumption; ophthalmological and haematological examinations
were carried out, organs were weighed, and the animals were examined
grossly and histopathologically. The hair of animals at the
intermediate and high doses had a greenish tint, which may have been
due to staining with diphenylamine or a metabolite. Three deaths
occurred among controls and among males at the high dose; two of the
latter had enlarged spleens, and one also had cystitis, probably
related to treatment. There were no treatment-related effects on body
weight, food consumption, or ophthalmic parameters. Haematology
indicated dose-related decreases in erythrocyte counts and haematocrit
in animals at the two higher doses that were statistically
significantly different from controls. The values for mean corpuscular
haemoglobin, mean corpuscular volume, and mean corpuscular haemoglobin
content increased with dose and were statistically significantly
different from those of controls in animals at the two higher doses;
the mean corpuscular haemoglobin content was also statistically
significantly increased in males at 525 ppm. The reticulocyte counts
increased with dose and were statistically significantly different
from those of controls at the high dose. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly different from those of controls at the
two higher doses; the relative weights of the kidney and heart were
statistically significantly different from those of controls in mice
at the high dose. In females, the absolute and relative weights of the
spleen increased with dose and were statistically significantly
different from those of the controls in animals at the two higher
doses; the absolute and relative weights of the liver and the relative
weights of the kidney were statistically significantly different from
those of controls in females at the high dose. Necropsy of females
revealed dark, enlarged spleens at the three higher doses, dark livers
at the two higher doses, and dark kidneys at the highest dose. In
males, necropsy showed dark, enlarged spleens and dark livers at the
two higher doses. Histopathological examination of the liver showed
increased pigment deposition and slight haematopoiesis in animals of
each sex at the two higher doses. The spleen showed haemosiderosis and
congestion at the three higher doses, reaching incidences of 14/15 or
more at the two higher doses; the severity of spleen haematopoiesis
was also increased at the three higher doses. The kidneys showed
pigment deposition at the two higher doses. Cystitis was observed in
9/15 males at the high dose and in 2/15 females at 2625 ppm and 8/14
females at 5250 ppm. The cellularity of the bone marrow was increased
at the two higher doses. The NOAEL was 10 ppm, equal to 1.7 mg/kg bw
per day, on the basis of changes in haematological parameters and
findings at necropsy (Botta, 1992).
Rats
Groups of 10 male and 10 female Sprague Dawley rats received
technical-grade diphenylamine in the diet at 0, 150, 1500, 7500, or 15
000 ppm for 90 days, equal to doses of 0, 9.6, 96, 550, and 1200 mg/kg
bw per day in males and 0, 12, 110, 650, and 1300 mg/kg bw per day in
females. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmic, urinary, haematological, and
clinical chemical end-points, organ weights, and gross and
histopathological appearance. Greenish hair was first seen in females
at 1500 ppm, later in 60% of males and 100% of females at 7500 ppm,
and then in 70% of males and 100% of females at 15 000 ppm. Pale skin
was seen in 100% of females at 7500 and 15 000 ppm and in 40% of males
at 15 000 ppm. Two males at 15 000 ppm were found dead on day 6 of
dosing, apparently due to gastroenteritis; there were no other deaths.
The body weights and body-weight gains of animals of each sex at 7500
and 15 000 ppm were consistently and statistically significantly below
those of controls; although these values were generally lower than
those of controls in animals at 1500 ppm, they were not statistically
significantly different. Food consumption was not affected at any
dose. The frequency of darkening of the urine increased with dose,
starting with one female at 1500 ppm and 100% of rats at 15 000 ppm.
Haematological measures indicated decreased erythrocyte counts and
haemoglobin values, which were statistically significantly different
from those of controls in animals at 7500 and 15 000 ppm at
termination. The haematocrits were statistically significantly lower
than those of controls in females at the three highest doses. Small,
statistically significant increases in alkaline phosphatase activity,
albumin content, and albumin:globulin ratio in males and glucose and
albumin content and albumin:globulin ratio in females were observed at
7500 and 15 000 ppm. The cholesterol concentration increased with dose
in females and was statistically significantly different from that of
controls at the three higher doses. In males, the absolute and
relative weights of the liver and spleen increased with dose and were
statistically significantly raised at 7500 and 15 000 ppm; the
relative weights of the kidney and gonad also increased with dose and
were also statistically significant at the two higher doses. In
females, the absolute and relative weights of the liver increased with
dose, and the change in relative weights was statistically significant
at doses > 1500 ppm. The kidneys were dark in animals of each sex
at 7500 and 15 000 ppm, and about 60% of the females at the high dose
had dark and/or enlarged livers. The spleens of both males and females
at the two higher doses were congested. Histopathological examination
revealed an increased incidence of haematopoiesis and pigment in the
liver, haematopoiesis, haemosiderosis, and congestion in the spleen,
and pigmented kidneys in animals of each sex at 7500 and 15 000 ppm.
The spleens of all females at 1500 ppm also showed an increase from
minimal to slight haematopoiesis and haemosiderosis. The NOAEL was 150
ppm, equal to 12 mg/kg bw per day, on the basis of increased clinical
signs of toxicity, clinical chemical changes, organ weights, and gross
and histopathological appearance (Krohmer, 1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received repeated dermal applications of technical-grade diphenylamine
dissolved in distilled water at doses of 100, 500, or 1000 mg/kg bw
per day. The material was applied daily for 6 h to an area of clipped
skin corresponding to about 10% of the body surface and kept under
occlusion for 21 consecutive days, with terminal sacrifice on day 22.
Two additional groups of five rabbits of each sex served as vehicle
controls. The animals were observed for clinical signs, deaths,
ophthalmoscopic parameters, erythema, oedema, desquamation, and other
adverse skin reactions, body weight, food consumption, urinary,
haematological, and clinical chemical end-points, organ weights, and
gross and histopathological appearance, the latter limited to the
liver, kidneys, spleen, treated and untreated skin, and gross lesions.
There were no deaths or treatment-related effects on clinical signs,
body weights, food consumption, or haematological end-points. The only
possible treatment-related effects on clinical chemistry were on
sodium and potassium concentrations; females at all three doses had
depressed sodium values, and those at the intermediate and high doses
and males at the high dose had depressed potassium values with respect
to controls. Gross necropsy, revealed dark-red foci in the stomachs of
rabbits of each sex at the intermediate and high doses, which
increased in frequency with dose: the incidences were 1/5 in males at
the intermediate dose, 4/5 in those at the high dose, 1/5 in females
at the intermediate dose, and 2/5 in females at the high dose. No
dark-red foci were seen in the stomachs of controls or rabbits at the
low dose. The NOAEL for systemic toxicity was 100 mg/kg bw per day on
the basis of the presence of dark-red foci in the stomachs of males
and females. The NOAEL for dermal effects was 1000 mg/kg bw per day,
the highest dose tested (Siglin, 1992).
Dogs
Groups of four pure-bred beagle dogs of each sex received
technical-grade diphenylamine (purity, > 99%) in gelatin capsules at
doses of 0, 10, 25, or 50 mg/kg bw per day for 90 days. They were
observed for deaths, clinical signs, body weight, food consumption,
ophthalmological, haematological, clinical chemical, and urinary
parameters, organ weights, and gross and histopathological appearance.
There were no deaths, and no treatment-related changes were seen in
any of the above parameters. Statistically significant increases were
seen, however, in some clinical chemical parameters including albumin
content, the albumin:globulin ratio in males, and bilirubin content in
females at the high dose. These effects may have been incidental. The
NOAEL was 50 mg/kg bw/day, the highest dose tested (Krohmer, 1992b).
(c ) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 CD-1 mice of each sex received diets containing
technical-grade diphenylamine (purity, > 99%) at concentrations of 0,
520, 2600, or 5200 ppm for up to 78 weeks, equal to 0, 73, 370, and
760 mg/kg bw per day for males and 0, 90, 460, and 940 mg/kg bw per
day for females. Ten mice of each sex per dose were sacrificed at 52
weeks. The animals were observed for clinical signs, deaths, body
weight, food consumption, ophthalmological and haematological
end-points, organ weights, and gross and histopathological appearance.
An increase in the incidence of greenish staining of the fur,
especially around the anogenital area, with dose was seen as the study
progressed. By week 26, most of the mice at 5250 ppm were affected,
and by the end of the study some mice at 525 ppm group showed
staining. The incidence of penile prolapse increased with dose,
affecting seven males at 2625 ppm and 17 at 5250 ppm by 78 weeks. The
frequency of unkempt appearance also increased with dose, with a
higher incidence among males. The mortality rate increased with dose,
becoming statistically significantly different from controls for males
at 2625 and 5250 ppm by 52 weeks. The deaths were attributed mainly to
cystitis among males and amyloidosis in females. The mean body-weight
gains were 87, 86, and 91% of control values for males at 5250 ppm and
104, 93, and 93% of control values for females at 5250 ppm at 13, 52,
and 78 weeks, respectively. The body-weight gains of males at 5250 ppm
and occasionally animals at 2625 ppm were statistically significantly
decreased throughout the study (mainly through week 58). The
body-weight gain of females at 5250 ppm was significantly decreased
during the first three weeks and then occasionally for the remainder
of the study. Mean food consumption was statistically significantly
decreased during the first week of treatment in males at the high dose
and remained increased throughout treatment for males at the two
higher doses, with occasional statistically significant differences
from controls. The food consumption of females at any dose showed
little or no statistically significant difference from that of
controls.
At the interim haematological evaluation at 52 weeks, males
showed dose-related decreases in haematocrit and erythrocyte counts
that reached statistical significance at 2625 and 5250 ppm; and mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin content increased with dose and reached statistical
significance in males at these doses. Females showed dose-related
decreases in haematocrit that reached statistical significance at
> 525 ppm; their erythrocyte counts decreased in a dose-related
fashion and reached statistical significance at 2625 and 5250 ppm.
Mean corpuscular volume, mean corpuscular haemoglobin, and mean
corpuscular haemoglobin content increased with dose, the latter two
reaching statistical significance at 2625 and 5250 ppm and the mean
corpuscular volume at 5250 ppm. At termination at 78 weeks, males and
females showed dose-related decreases in haematocrit and erythrocyte
counts that reached statistical significance at 2625 and 5250 ppm;
reticulocyte counts, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin content increased with
dose and reached statistical significance in males at these doses.
At the interim necropsy, darkened spleens were seen in most mice
at 2625 ppm and in all those at 5250 ppm. Darkened livers were seen in
most mice at 5250 ppm and pale kidneys in many. At terminal necropsy,
the livers of some mice at 2625 ppm and most at 5250 ppm were dark.
The spleens of most treated mice were dark and often enlarged.
Dose-related increases in the absolute and relative weights of the
spleen, liver, and heart were seen in animals of each sex at the
interim and final sacrifices. At interim sacrifice, the absolute
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly different from those of controls, while
the increases in the relative liver weights reached statistical
significance only at the highest dose. In females, the absolute and
relative weights of the spleen were statistically significantly
increased only at the highest dose. The absolute and relative weights
of the heart were statistically significantly increased in females at
5250 ppm; the increases in males were not statistically different from
those in controls. At final sacrifice, the absolute and relative
weights of the spleen and liver of males at 2625 and 5250 ppm were
statistically significantly increased. In females, the absolute and
relative weights of the spleen were significantly increased only at
5250 ppm,; the differences in relative liver weight reached
statistical significance at 2625 and 5250 ppm. The absolute and
relative weights of the heart were statistically significantly
increased in animals of each sex at 5250 ppm. Changes in the absolute
and relative weights of the pituitary and thyroid were not
consistently dose-related and were thus considered not to be of
toxicological significance.
Histopathological examination revealed treatment-related effects
in the kidney, liver, spleen, bone marrow, urinary bladder, and penis.
The incidences of haematopoiesis and pigment deposition in the liver
were increased at the intermediate and high doses. Of mice at 2625 ppm
that were found dead or moribund or were sacrificed at termination,
19/51 males and 24/51 females showed liver haematopoiesis and 15/51
males and 37/51 females showed liver pigmentation; higher incidences
of these effects were seen at the high dose. The incidences of spleen
congestion and haemosiderosis were increased in all treated mice. Of
the mice at the low dose found dead or moribund or sacrificed at
termination, 11/50 males and 8/50 females showed spleen congestion and
8/50 males and 35/50 females showed spleen haemosiderosis; higher
incidences of these effects were seen at the higher doses. The
frequency of pigment deposition was increased in males at the high
dose and in females at the intermediate and high doses. Among females
found dead or moribund or sacrificed at termination, pigment was found
in 5/51 at the intermediate dose and 7/51 at the high dose. Among
males found dead or moribund or sacrificed at termination, 5/51 at the
intermediate dose and 7/51 at the high dose showed pigment
accumulation; additionally, 5/54 males had pyelonephritis. Although
the incidence of spleen haematopoiesis did not increase with dose, the
severity scores increased from minimal or slight in controls to mixed
scores including moderate or marked at the high dose. The severity of
haematopoiesis in the spleen increased from minimal to slight at 525
ppm in contrast to the largely minimal level in controls.
Additionally, bone-marrow cellularity changed from predominantly
moderate in controls to marked at the higher doses. The incidences of
urinary bladder cystitis and dilatation increased with dose, reaching
statistical significance at the intermediate and high doses. Among
mice at the intermediate dose that were found dead or moribund or were
sacrificed at termination, 24/51 males and 13/48 females had cystitis
and 18/51 males and 13/48 females had dilatation; higher values were
seen at the high dose. The incidence of balanoposthitis apparently
increased with dose; however, no statistical analysis was available.
In females at 5250 ppm, the incidence of amyloidosis in the thyroid,
adrenals, kidneys, stomach, small intestine, ovaries, and uterus was
increased. The incidence of tumours was comparable in the treated and
control groups. The NOAEL for toxicity was 525 ppm, equal to 73 mg/kg
bw per day, on the basis of decreased body-weight gain, decreased
survival, and significant haematological and gross and microscopic
pathological alterations (Botta, 1994a).
Rats
Groups of 60 male and 60 female Sprague-Dawley rats received
diets containing technical-grade diphenylamine (purity, > 99%) at
concentrations of 0, 200, 750, 3750, or 7500 ppm for males and 0, 150,
500, 2500, or 5000 ppm for females for up to two years, equal to 0,
8.1, 29, 150, and 300 mg/kg bw per day for males and 0, 7.5, 25, 140,
and 290 mg/kg bw per day for females. Groups of 10 rats of each sex
per group were killed at a one-year interim sacrifice. The animals
were observed for clinical signs, deaths, body weight, food
consumption, ophthalmological, haematological, clinical chemical, and
urinary end-points, organ weights, and gross and histopathological
appearance.
The only treatment-related clinical finding was greenish
colouration of the fur in the urogenital or ventral cervical area in
animals of each sex at the two higher doses. The effect was attributed
to the presence of a metabolite in urine or faeces. No treatment-
related effects on mortality rates were observed; however, the study
was terminated at 102 weeks because of increased mortality rates in
controls and animals at the low dose: survival among males was 22% at
0 and 200 ppm and 55% at 7500 ppm. Survival thus seemed to increase
with dose, and an analysis of the survival data indicated a
statistically significant negative trend for mortality as the dose
increased in animals of each sex; the mortality rates at the two
higher doses were statistically significantly lower than those for the
control group. A dose-related decrease in body weight and body-weight
gain was seen throughout most of the study, which reached statistical
significance at the two higher doses. The body-weight gains of males
at the two higher doses were depressed to 95 and 87% of the control
values at 78 weeks and equal to those of controls at 102 weeks; in
females, the corresponding values were 78 and 56% of controls at 78
weeks and 80 and 61% at 102 weeks. There was no decrease in food
consumption, except during the first week; at other times, food
consumption appeared to have increased, possibly due to food wastage.
There were no treatment related effects on ophthalmological
parameters.
Haematological examination revealed dose-related decreases in
erythrocyte counts, haemoglobin, and haematocrit in animals at the two
higher doses throughout treatment. The decreases reached statistical
significance for erythrocyte count and haemoglobin in males at 3750
and 7500 ppm in week 26 and at termination and for erythrocyte counts,
haemoglobin, and haematocrit in females at 2500 and 5000 ppm through
most of the treatment and at termination. Although the erythrocyte
counts, haemoglobin, and haematocrit were decreased in males at 750
ppm and in females at 500 ppm, the decreases reached statistical
significance only sporadically during treatment. The mean corpuscular
volume and mean corpuscular haemoglobin content were significantly
different from those of controls in males at the three higher doses
and in females at the two higher doses.
Dose-related, statistically significant increases in the absolute
and relative weights of the spleen were seen in females at the two
higher doses at interim sacrifice and at termination. A similar effect
on spleen weights was observed in males, except that the changes in
rats at 3750 ppm were not statistically significant. The relative
weight of the liver was statistically significantly increased in
females at 5000 ppm at termination and at 3750 and 5000 ppm at interim
sacrifice; no increase in liver weights was observed in males. The
increases in spleen and liver weights are consistent with the
haematological effects of the compound. Findings of dark and/or
enlarged spleens at necropsy in males at > 750 ppm and in females
at 500 ppm are probably related to the haematological effects.
Microscopic examination revealed treatment-related effects in the
kidney, liver, spleen, and bone marrow. The incidence of pigment
deposition in the kidney increased in a dose-related fashion and
reached 44/50 in males and 44/52 in females at the high dose that were
found dead or moribund or were sacrificed at termination. The
incidences of haematopoiesis and pigment deposition in the liver
increased in a dose-related fashion and reached 21/50 and 27/50 in
males and 41/52 and 45/52 in females at the high dose that were found
dead or moribund or sacrificed at termination. Erythroid hyperplasia
was seen at the higher dose but not in controls or at the low dose.
The incidence of congestion of the spleen increased in a dose-related
fashion and reached incidences of 50/50 in males and 47/52 in females
at the high dose that were found dead or moribund or sacrificed at
termination. These findings are all related to the observed
haematological effects. No treatment-related increase in tumour
incidence was observed. The NOAEL for toxicity was 150-200 ppm, equal
to 7.5 mg/kg bw per day, on the basis of changes in haematological
parameters and in the histopathological appearance of the spleen,
kidney, and liver (Botta, 1994b).
Dogs
Four beagles of each sex received diphenylamine (purity, > 99%)
by gelatin capsule at a dose of 0, 10, 25, or 100 mg/kg bw per day for
52 weeks and were observed for clinical signs, body weight, food
consumption, ophthalmological, haematological, clinical chaemical, and
urinary end-points, organ weights, and gross and histopathological
appearance. No treatment-related clinical signs were seen at
termination. One dog at the intermediate dose and two at the high dose
had greenish hair. There were no deaths or treatment-related effects
on body weight, food consumption, or ophthalmological parameters.
Haematological examination revealed decreased mean erythrocyte counts
(by 11% in comparison with controls), haemoglobin (9.3%), and
haematocrit (8.7%) in males at the high dose; smaller decreases in
these parameters were found in females. The platelet count increased
with dose in males at the 13-, 26-, 39-, and 52-week evaluation
periods, becoming statistically significant at the intermediate and
high doses. There was a dose-related increase in mean total bilirubin
concentration, which was statistically significant for animals at the
the intermediate and high doses throughout the study, in animals at
the low dose at week 26, and in females only at week 39. The mean
cholesterol concentration appeared to increase with dose at all
evaluation periods but was statistically significantly increased only
in males at the high dose at week 13 (by 68%) and in females at the
high dose at week 39 (by 37%). The blood urea nitrogen concentration
was decreased in females at the intermediate (by 16%) and high doses
(by 20%) at week 52. The mean absolute and relative weights of the
liver and thyroid appeared to increase with dose in males, but only
the mean absolute liver weight of males at the high dose was
statistically significantly increased. The mean absolute and relative
weights of the thyroid decreased with dose in females but did not
reached statistical significance at any dose. There were no
treatment-related gross or histopathological changes. The NOAEL for
toxicity was 10 mg/kg bw per day on the basis of haematological and
clinical chemical changes (Botta, 1994c).
(d) Genotoxicity
As summarized in Table 2, negative results were obtained for
mutation in bacteria (Lawlor, 1992) and for induction of micronuclei
in mouse bone marrow in vivo (Murli 1992 a,b). A weakly positive
response was observed for mutation in mouse lymphoma cells in vitro
at a dose range that was toxic, and the mutant frequency did not
increase with dose (Cifone, 1992). The Meeting concluded that although
diphenylamine has some genotoxic potential it is unlikely to be a
human genotoxic hazard.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, groups of 28
Sprague-Dawley rats received diphenylamine (purity, 99.8%) in the diet
at concentrations of 0, 500, 1500, or 5000 ppm, equal to 0, 40, 120,
and 400 mg/kg bw per day for F0 males and 0, 46, 130, and 450 mg/kg
bw per day for F0 females for 70 days before mating. After weaning,
28 F1 rats of each sex per group were also given the test diets for
up to 70 days before mating and were selected as parents for the F2
litters. The parent animals were observed for deaths, clinical signs,
body weight, food consumption, mating and fertility indices and other
measures of reproductive performance, organ weights, and gross and
histopathological appearance. The offspring were observed at birth and
through lactation for clinical signs, deaths, weights, and sex ratio;
all underwent necropsy.
Toxicity was dose-related and was observed in animals of each sex
and generations at all doses. In general, females were more affected
than males, and F1 animals were more affected than F0 animals. In
the F0 generation, the incidences of bluish coats and a bluish fluid
in the cages were increased in males and females at 5000 ppm, and
similar findings were made in the F1 generation. Swelling of the
mammary glands were seen in females, and palpable masses were noted in
lateral or ventral regions, especially in females. These masses
appeared to be transient but were not examined grossly or
microscopically. Body weight and body-weight gain were significantly
decreased at various times in animals at 1500 and 5000 ppm. At 5000
ppm, there was a decrease in body weight compared with controls, of
6-9% for F0 males, 5-8% for F0 females, 22-28% for F1 males, and
11-23% for F1 females. At 1500 ppm, there was a 5-8% decrease in body
weight for F0 females, 7-9% for F1 males, and 5% for F1 females.
The relative weights of the kidney, liver, and spleen of F0 and F1
males were significantly increased at 5000 ppm, and the relative
Table 2. Results of assays for the genotoxicity of diphenylamine
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Forward mutation S. typhimurium 6.67-333 µg/platea 99.9 Negative Lawlor (1992)
TA98, TA100, TA1535, 10-667 µg/plateb
TA1537, TA1538
Gene mutation L5178Y tk+/- mouse 5-80 µg/ml > 93 Weakly Cifone (1992)
lymphoma cells positiveb
Negativea
In vivo
Micronucleus Mouse (ICR strain) 250-1000 mg/kg bw (males) 99.9 Negative Murli (1992a,b)
formation 375-1500 mg/kg bw (females)
weights of the liver and spleen of F0 and F1 females were
significantly increased at 1500 ppm. The relative weights of the liver
were significantly increased at 1500 and 5000 ppm in F0 females but
only at 5000 ppm in F1 females.
Gross observations at necropsy included enlarged spleens in
animals of each sex and generation at 1500 and 5000 ppm and in F1
females at 500 ppm, in addition to blackish-purple spleens in animals
of each sex and generation at 500, 1500, and 5000 ppm. The incidences
of blackish-purple spleen in F1 rats at 500 ppm were 9/28 males and
6/28 females; incidences of 27/28 or higher were seen at higher doses.
Histopathological examination revealed a brown pigment in the proximal
tubules of the kidney in animals of each sex and generation at 5000
ppm; and 3/28 F1 females at 1500 ppm also had brown pigment.
Congestion and haemosiderosis of the spleen were seen in animals of
each sex and generation at 500, 1500, and 5000 ppm. Increased spleen
erythropoiesis was seen in 4/28 males at 5000 ppm. Hepatocyte
hypertrophy was seen in males of both generations at 1500 and 5000
ppm; in females, it was seen at all doses in the F0 generation but
only at 5000 ppm in the F1 generation. Kupffer cells with brown
pigment containing iron were seen in animals of each generation, at
5000 ppm in males and at 1500 and 5000 ppm in females.
Reproductive toxicity was seen in the form of decreased mean pup
weight at various times in each generation and reduced litter size at
the high dose; no other parameters were affected by treatment. F1
pups at 5000 ppm had statistically significantly decreased mean body
weight (11% less than controls at birth and 14-25% less than controls
during lactation). F2 pups showed statistically significantly
decreased mean body weight at 1500 ppm (10-12% less than controls
during late lactation) and at 5000 ppm (10-19% less than controls
throughout lactation). The mean body weight of pups in the F2 litters
at birth was about 4.8% lower than that of controls, but the
difference was not statistically significant. The mean litter size
decreased with dose in both generations, by 21% at 5000 ppm in the F1
generation, and was statistically significantly different from control
values. Although the mean litter size in the F0 generation at 5000
ppm was decreased by 10%, this value was not statistically
significant. The decrease in litter sizes with dose correlated with
the number of uterine implantation scars observed at necropsy, and the
mean uterine implantation scar count decreased with dose in both
generations. The mean scar count at 5000 ppm was decreased by 16% and
was statistically significant; although the mean scar count in the F0
generation was decreased by 7.3% at 5000 ppm, the value was not
statistically significant.
A NOAEL was not identified for parental systemic toxicity.
Although haemosiderin and congestion in the spleen and hepatocyte
hypertrophy were seen at all doses, the incidence and intensity of
these effects were appreciably smaller at the lower dose. For example,
only 4/28 females at the low dose had spleen congestion, rated as
minimal, whereas 22/28 females at the intermediate dose had this
effect and its intensity was nearly equally divided between minimal
and mild. These observations suggest that that the lower dose in this
study is close to the NOAEL for parental systemic toxicity. The NOAEL
for reproductive toxicity was 1500 ppm, equivalent to 120mg/kg bw per
day for F0 animals, on the basis of decreased mean litter size at the
high dose. The NOAEL for developmental toxicity was 500 ppm,
equivalent to 46 mg/kg bw per day in maternal animals, on the basis of
the statistically significantly decreased mean body weights of F2
pups (Rodwell, 1993).
(ii) Developmental toxicity
Rats
In a range-finding study for developmental toxicity, groups of
six pregnant Sprague-Dawley Crl:CDTM BR VAF/Plus rats received
diphenylamine (purity, 99.9%) in corn oil by gavage at doses of 0, 10,
50, 100, 200, 300, or 400 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity was seen at doses of 100 mg/kg bw per day and
higher. Two deaths occurred at 400 mg/kg bw per day, and there were
dose-related decreases in food consumption, body weight, and body-
weight gain starting at a dose of 100 mg/kg bw per day. Necropsy
revealed a purplish-black spleen in one dam at 100 mg/kg bw per day
and in all surviving dams at higher doses. The increased incidence of
early resorptions was apparent dose-related and was accompanied by
decreases in mean fetal weight and gravid uterine weight at doses of
200 and 300 mg/kg bw per day. There were no treatment-related external
malformations or developmental variations. On the basis of the results
of this study, 0, 10, 50, and 100 mg/kg bw per day were selected for
use in the definitive study (Rodwell, 1992a).
In the definitive study, groups of 25 pregnant Sprague-Dawley
Crl:CDTM BR VAF/Plus rats received diphenylamine (purity, 99.9%) in
corn oil by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on
days 6-15 of gestation. The dams were observed for deaths, clinical
signs, body weight, and food consumption. All rats were sacrificed on
day 20 of gestation and were necropsied grossly. The spleens were
weighed, and the uterus was removed, examined externally, weighed, and
opened for internal examination. The fetuses were observed externally,
and their viscera and skeleton were examination. There were no deaths
or treatment-related effects on clinical signs, body weights, or food
consumption. At necropsy, 5/25 dams at the high dose had enlarged,
blackish-purple spleens, and the mean weights of the spleens were
significantly greater than those of controls. No treatment-related
effects were seen on gross necropsy, in the appearance or weight of
the uterus, or in the numbers of viable fetuses, early and late
resorptions, and corpora lutea, or on external, visceral, or skeletal
malformations or variations in the fetuses. The NOAEL for maternal
toxicity was 50 mg/kg bw per day on the basis of effects on the spleen
in dams at the high dose. The NOAEL for developmental toxicity was 100
mg/kg bw per day, the highest dose tested, in the absence of
developmental effects (Rodwell, 1992b).
Rabbits
In a study of developmental toxicity, groups of 16-18 pregnant
New Zealand white rabbits received diphenylamine (purity, 99.9%) in 1%
methyl cellulose by gavage at a dose of 0, 33, 100, or 300 mg/kg bw
per day on days 7-19 of gestation. The dams were observed for deaths,
clinical signs, body weight, and food consumption. All rats were
sacrificed on day 29 of gestation and subjected to gross necropsy; the
ovaries and uterus of each animal were removed and examined to
determine the number of corpora lutea and the type, distribution, and
number of implantation sites. The fetuses were examined externally,
and their viscera and skeleton were analysed. No deaths occurred
during the study. The mean food consumption of dams at 300 mg/kg bw
per day was reduced on days 7-29 of gestation, and a slight decrease
in body weight was noted. Green discolouration of the urine was seen
in all treated animals. No treatment-related effects were seen at
necropsy or on embryonic or fetal growth or development. The NOAEL for
maternal toxicity was 100 mg/kg bw per day on the basis of decreased
body-weight gain and food consumption early during treatment. The
NOAEL for developmental toxicity was 300 mg/kg bw per day, the highest
dose tested (Edwards et al., 1983).
(f) Special studies
(i) Renal cystic disease
The issue of tubular cyst formation in diphenylamine-treated rats
was reviewed previously (Annex 1, references 13, 27, 39, and 43).
Additional observations on the induction of tubular cysts in the
kidneys of mice and rats treated with diphenylamine in the diet are
summarized below.
Renal histopathology and function were studied in male
Sprague-Dawley rats fed pelleted diets containing 1% w/w (i.e. 10 000
ppm) diphenylamine (purity unspecified) for 5-20 months. Control
animals received identical diets in which methyl cellulose was
incorporated instead of diphenylamine. The animals were observed for
renal tubular diameter and renal functional parameters including
intratubular hydrostatic pressure, single-nephron glomerular
filtration rate, and transit times through the loop of Henle; light
and scanning electron microscopy was performed. The luminal diameters
of 22 dilated nephrons from diphenylamine-treated rats were 34-110 µm,
and those of 10 undilated nephrons were 21-450 µm; the luminal
diameters in six nephrons of two control rats were 26-34 µm. The
intraluminal hydrostatic pressure was significantly higher in dilated
tubules than in undilated tubules from treated rats; there was no
difference in intraluminal hydrostatic pressures among control rats.
The single-nephron glomerular filtration rate was also similar in
dilated and undilated tubules, consistent with an intrinsic change in
glomerular function. The transit times through the loop of Henle were
three to four times faster in dilated than in undilated nephrons of
treated and control rats. Microscopic examination indicated that
dilatation along the proximal and collecting tubules was segmental,
not diffuse, some dilated tubules communicated with cysts deep in the
renal substance, the dilated collecting tubules occasionally contained
debris, tubules adjacent to cysts appeared to be compressed, and
diphenylamine-exposed kidneys contained proximal convoluted tubules
with segments of apparent narrowing. The authors concluded that cyst
formation results from initial obstruction and a subsequent increase
in intraluminal pressure arising from sustained glomerular filtration
and unaltered water reabsorption (Gardner et al., 1976).
The time-course of development of lesions in the renal collecting
tubules was studied in male Sprague-Dawley rats fed pelleted diets
containing 1% w/w (i.e. 10 000 ppm) diphenylamine (purity unspecified)
for up to 76 weeks. Control animals received standard diet. The
animals were observed for renal concentrating ability (urine
osmolality) at 2, 4, 5, and 20 weeks of treatment; light and
transmission and scanning electron microscopy was performed at 2, 5,
10, 15, 20, 25, 52, and 78 weeks of treatment; and autoradiography
with tritiated thymidine was conducted to determine whether
hypertrophy was the mechanism of cyst formation. Decreased urine
osmolality was seen in treated rats, which was statistically
significantly different from control values at 6 and 20 weeks.
Structural changes first appeared after five weeks of treatment. Light
microscopy of the medullary collecting tubules at five weeks revealed
focal areas of apparent thickening along the walls, resulting from
layering of cells. By 10 weeks, some tubules were dilated and
contained focal areas of cellular necrosis. By 15-20 weeks, many more
ducts were dilated and contained cast material. By 24 weeks, frank
cysts were visible in the cortex and medulla, and large collecting
tubular cysts were seen; some proximal tubules and renal corpuscles
were dilated. Over time, cysts were found in every segment of the
nephron and collecting tubules. Transmission electron microscopy at
five weeks revealed changes in the medullary collecting tubules. The
tubular cells appeared to be enriched in mitochondria, and their
apical border was studded with long microvilli. At 10 weeks, necrotic
cells were seen along the collecting tubules. Scanning electron
microscopy showed that the collecting duct cysts were lined with
irregular cells which no longer resembled collecting tubule cells. The
labelling index for the collecting tubules reached a plateau at weeks
5-15 and had decreased to background levels by week 52. Because the
number of nuclei counted on cross-sections of the collecting tubules
increased gradually with time, the authors concluded that the increase
in labelling index was the result of hyperplasia of the tubular cells
and not of a degeneration or regeneration phenomenon. The authors
concluded that one of the initial events in the pathogenesis of cystic
renal disease is a hyperplastic response of the collecting tubule
cells (Evan et al., 1978).
The effect of diphenylamine on the composition of the native
renal basement membrane was studied in male C57Bl/6 mice fed pelleted
diets containing 2% w/w (i.e. 20 000 ppm) diphenylamine (purity
unspecified) for up to 10 months. Control animals received chow
without diphenylamine. After 10 months of treatment, the mice were
sacrificed and their kidneys processed for light microscopy and
immunohistology of bamin, a glycoprotein associated with the matrix of
the glomerular basement membrane. Microscopy revealed cysts in the
cortical and medullary portions of the kidney. The cysts were lined
with flattened or somewhat cuboidal cells and contained a brownish
coagulum that appeared to be constituted of necrotic cells. Brownish
discolouration was also seen in the cytoplasm of neighbouring
non-cystic cells and in tubular cells of animals sacrificed before the
formation of cysts. The authors speculated that the brownish debris
preceded the formation of cysts. When immunohistology was performed
with antibodies to bamin, the antibodies bound to the glomeruli of
control mice but not to those of treated animals.This result was
obtained in the three-week experiment. In another series of
experiments, male C57Bl/6 mice were injected with EHS tumour cells and
five days later received a diet containing diphenylamine (at an
unspecified level). After three weeks, the animals were killed, and
tumours were removed for analysis of the proteins of the basement
membrane. Analysis by sodium dodecylate sulfate-polyacrylamide gel
electrophoresis and by immunoblot revealed the presence of a 75-80-kDa
protein in controls but little or none in the diphenylamine-treated
mice. The authors were unclear how the loss of bamin, a molecule
associated with the glomerular but not the tubular basement membrane,
would be related to the formation of tubular cysts. They speculated
that material from an abnormal glomerular filtrate might damage the
tubular epithelial cells and result in the formation of tubular cysts
(Rohrbach et al., 1993).
(iv) Renal papillary necrosis
A series of studies has been reported on the induction of renal
papillary necrosis by diphenylamine in hamsters, rats, and Mongolian
gerbils. The development of animal models for this lesion is of
interest because it is the initial stage in human analgesic-induced
nephropathy.
Groups of 10 male Syrian hamsters, 40 Sprague-Dawley rats, and 40
Mongolian gerbils received diphenylamine (purity unspecified) at doses
of 0, 400, 600, or 800 mg/kg bw per day in peanut oil by gavage for
three days. Animals that became moribund were sacrificed and
necropsied; survivors were sacrificed 24 h after the third dose of
diphenylamine. All animals were observed for death and were examined
for gross and microscopic lesions. Forty percent of hamsters at the
low dose and 100% of those at the two higher doses died or were
sacrificed in extremis during treatment; there were no deaths among
the rats or gerbils. At necropsy, brown kidneys and yellow-brown
papillae were seen in 50% or more of hamsters at the intermediate and
high doses and in none of those at the low dose. The only gross lesion
in hamsters at the low dose was splenomegaly (90%), which was not seen
at higher doses. Microscopic examination revealed total renal
papillary necrosis, i.e. necrosis of all elements of the renal
papillae, including collecting tubules, at all doses in 4/10 hamsters
at the low, 7/10 at the intermediate, and 4/10 at the high dose. Only
two rats at the high dose had renal lesions, which consisted of
necrosis of the medullary interstitial cells and vasa recta, limited
to the apex, and degeneration of the renal interstitial matrix. As
noted by the authors, the limited incidence and extent of the lesions
in the Sprague-Dawley rats contrasted with the more extensive effects
observed in Wistar rats by Powell et al. (1985). No effects were
observed in Mongolian gerbils (Lenz & Carlton, 1990).
A total of 27 male Syrian hamsters were given diphenylamine by
gavage (purity unspecified) dissolved in peanut oil at a dose of 600
mg/kg bw. At intervals of 0.5, 1, 2, 4, 8, 16, and 24 h after dosing,
three hamsters were perfused in situ with fixative and their kidneys
were removed for examination by transmission electron microscopy.
Starting 1 h after dosing, the basal plasma membrane of the
endothelial cells of the ascending vasa recta in the proximal portion
of the renal papilla became separated from the basal lamina, forming
large subendothelial vacuoles. These alterations persisted during the
first 8 h after dosing. By 16 h, the endothelial cell nuclear
membranes and the luminal plasma membrane had become convoluted, and
by 24 h platelets were seen to adhere to the basal lamina, which had
become exposed. By 2 h after dosing, the adjacent interstitial cells
started undergoing structural alterations, and by 24 h there was
necrosis. Ultrastructural alterations became visible in the thin limb
of the loop of Henle by 24 h and in collecting tubule epithelial cells
by 14-16 h. The authors speculated that the selective effect on the
endothelial cells of the ascending vasa recta were attributable to
generation of a toxic metabolite by the endothelial cell or to
interference with the metabolism of the endothelial cells by a
metabolite of diphenylamine (Lenz et al., 1995).
The toxicity of diphenylamine dissolved in dimethyl sulfoxide to
renal papilla was studied in Syrian hamsters, rats, and Mongolian
gerbils. Male Syrian hamsters and Sprague-Dawley rats were given
diphenylamine (purity unspecified) by gavage at doses of 0, 400, 600,
or 800 mg/kg bw per day for up to nine days. Only one of 30 hamsters
at 400 mg/kg bw per day showed renal papillary necrosis. This result
contrasted with previous results (Lenz & Carlton, 1990) in which oral
administration of diphenylamine dissolved in peanut oil produced
extensive necrosis in male Syrian hamsters at the same doses;
furthermore, pretreatment of the hamsters with dimethyl sulfoxide
protected the animals against the renal papillary necrosis induced by
administration of diphenylamine. Renal papillary necrosis occurred in
4/30 rats at the high dose; none was seen in Mongolian gerbils. The
incidence in rats and gerbils did not differ from that observed when
peanut oil was used as the vehicle in a previous study (Lenz &
Carlton, 1990). The mechanism by which dimethyl sulfoxide exerts its
protective effect in Syrian hamsters was not studied (Lenz & Carlton,
1991).
The effect of diphenylamine on renal glutathione concentrations
was studied in groups of eight male Syrian hamsters given
diphenylamine (purity unspecified) by gavage at a dose of 0, 200, 400,
or 600 mg/kg bw 1 and 4 h after dosing. The concentrations in the
cortical area decreased with dose, reaching statistical significance
at the low dose at 1 h and at the intermediate dose at 4 h; the
concentrations at 1 h were 41% of the control value at 200 mg/kg bw,
31% at 400 mg/kg bw, and 29% at 600 mg/kg bw. The glutathione
concentrations in the outer medulla and the renal papilla were not
statistically significant decreased.
In order to study the effect of glutathione reduction on toxicity
to renal papilla, groups of eight male Syrian hamsters were given
buthionine sulfoxime, an inhibitor of glutathione biosynthesis, at 500
mg/kg bw intrapeitoneally, diphenylamine in peanut oil at 400 mg/kg bw
by gavage, the same doses of buthionine sulfoxime plus diphenylamine,
or the peanut oil vehicle alone. The animals were sacrificed 24 h
after administration of diphenylamine for gross and microscopic
examination of the kidney. No gross or microscopic lesions indicative
of papillary necrosis were observed in any group. Because a dose of
diphenylamine that is toxic to the papilla did not deplete glutathione
in that region and because depletion of glutathione (to 71% of the
control value) by buthionine sulfoxime did not enhance the toxicity of
diphenylamine to the papilla, the author concluded that this toxic
effect of diphenylamine is mediated by mechanisms independent of
oxidative injury (Lenz, 1996).
Comments
After oral administration to rats, goats, or hens,
14C-diphenylamine was extensively absorbed and rapidly excreted. In
rats given a single dose, 45-72% appeared in the urine within 24 h and
68-89% by 168 h after dosing, with less than 0.4% of the dose in the
residual carcass and less than 0.05% in any individual tissue. In
goats treated with single daily doses for seven days, 85-91% of the
daily dose was excreted in urine and 0.5-0.8% in milk; the
concentrations of residues in milk plateaued after 24 h. When laying
hens were treated with single daily oral doses for seven days, 84-98%
of the daily dose was recovered in the excreta; the concentrations in
egg yolk reached 0.31 mg/kg on day 7, but no residues were found in
egg whites. Diphenylamine underwent extensive biotransformation in
rats, goats, and hens, with ring hydroxylation and formation of
glucuronide and sulfate conjugates. In addition to untransformed
diphenylamine (< 3% of the dose), the following 12 metabolites were
identified at all doses: 4,4'-dihydroxydiphenylamine (unconjugated and
as the O-sulfate and the O, O-disulfate), 4-hydroxydiphenylamine
(unconjugated and as the O-glucuronide, N-glucuronide,
O-sulfate, and O, N-diglucuronide), indophenol (unconjugated and
as the O-sulfate), 3-hydroxydiphenylamine, and
2-hydroxydiphenylamine. These metabolites accounted for about 80-90%
of the dose and were excreted largely as their sulfate and glucuronide
conjugates. There was no cleavage of the diphenylamine structure.
Except for a polar oligomer of 4-hydroxydiphenylamine found only in
the eggs and tissues of hens, all of the metabolites reported in hens
and goats were detected in rats. Residues of a number of plant
metabolites were identified in apple peel and pulp, but untransformed
diphenylamine was the major contributor to the total residue 40 weeks
after application. The major metabolite was 4-hydroxydiphenylamine, as
the glucose conjugate. Other metabolites identified were
2-hydroxydiphenylamine, 3-hydroxy-diphenylamine, and
dihydroxydiphenylamine (possibly the 2,4 isomer). These compounds were
either free or conjugated with mono- or oligosaccharides. Although all
of the hydroxylated metabolites (aglycones) identified in plants were
seen in rats, the conjugating species were generally different.
After acute oral administration to rats, diphenylamine (99.9%
pure) was slightly toxic (LD50 about 3000 mg/kg bw) in one study,
whereas in another study super-refined diphenylamine (purity,
99.0-100.1%) was essentially nontoxic (LD50 > 15 000 mg/kg bw).
WHO has not classified diphenylamine for acute toxicity.
In mice given diphenylamine at dietary concentrations of 0, 10,
520, 2600, or 5200 ppm for 90 days, dose-related changes in
haematological parameters (decreased erythrocyte counts and packed
cell volumes and increased reticulocyte counts) were observed. The
mean corpuscular haemoglobin count was significantly increased at
dietary levels of 520 ppm and above. Necropsy revealed dark, enlarged
spleens with haemosiderosis and congestion at dietary levels of 520
ppm and above. Spleen haematopoiesis was increased in animals of each
sex at 520 ppm. The NOAEL was 10 ppm (equal to 1.7 mg/kg bw per day)
on the basis of changes in haematological parameters and findings at
necropsy in animals at 520 ppm.
In rats that received diphenylamine in the diet at concentrations
of 0, 150, 1500, 7500, or 15 000 ppm for 90 days, body weights and
body-weight changes, although generally lower than the control values
at 1500 ppm, were not significantly different from those of controls
at doses below 7500 ppm. The cholesterol concentration increased with
dose in females and was significantly different from that of controls
at 1500 ppm. In females, the absolute and relative weights of the
liver increased with dose, and the relative liver weights were
significantly different from those of controls in animals at 1500 ppm
and above. Histopathological examination revealed increased
haematopoiesis and pigment in the liver, haematopoiesis,
haemosiderosis, and congestion in the spleen, and pigmented kidneys in
animals of each sex at 7500 and 15 000 ppm. Additionally, the spleens
of all females at 1500 ppm showed minimal or slight haematopoiesis and
haemosiderosis. The NOAEL was 150 ppm (equal to 12 mg/kg bw per day)
on the basis of changes in clinical chemical parameters, increased
organ weights, and gross and histological changes in female rats at
1500 ppm.
Groups of rabbits were exposed dermally to diphenylamine in
distilled water at doses of 0, 100, 500, or 1000 mg/kg bw per day for
6 h per day. No effects were observed. Gross necropsy revealed
dark-red foci in the stomachs of rabbits at the intermediate and high
doses, which increased in number with dose. The NOAEL for systemic
effects was 100 mg/kg bw per day on the basis of effects on the
stomach at 500 mg/kg bw per day in animals of each sex.
Groups of dogs received diphenylamine by gelatin capsule at doses
of 0, 10, 25, or 50 mg/kg bw per day for 90 days. There were no
treatment-related effects. The NOAEL was 50 mg/kg bw per day, the
highest dose tested.
In a study of carcinogenicity, mice received diets containing
diphenylamine at concentrations of 0, 520, 2625, or 5200 ppm for 78
weeks. At 520 ppm and above, decreased packed cell volumes were seen
in females and spleen congestion and haemosiderosis in animals of each
sex. At 2600 ppm and above, clear haematological effects, consistent
with regenerative anaemia, were observed. The incidences of tumours
were not increased when compared with those in controls. The NOAEL for
toxicity was 520 ppm (equal to 73 mg/kg bw per day) on the basis of
decreased body-weight gain, reduced survival, and significant
alterations in haematological and gross and microscopic pathological
parameters at higher levels. Examination of the incidence and severity
of some haematological effects at 520 ppm suggested that this dose is
close to the NOAEL/LOAEL threshold. There was no evidence of
carcinogenicity.
In a combined study of toxicity and carcinogenicity, rats
received diets containing diphenylamine at concentrations of 0, 200,
750, 3750, or 7500 ppm (males) and 0, 150, 500, 2500, or 5000 ppm
(females). At 500 ppm and above, decreased erythrocyte count,
haemoglobin, and packed cell volumes were observed in animals of each
sex; these decreases reached statistical significance only
sporadically during treatment in males at 750 ppm and in females at
500 ppm. Haematopoiesis was increased in the liver and spleen of males
at 750 ppm and above. At 2625 ppm and above, clear haematological
effects consistent with regenerative anaemia were observed. There was
no significant increase in the incidence of tumours when compared with
that in controls. The NOAEL was 150-200 ppm (equal to 7.5 mg/kg bw per
day) based on haematological and histological effects at dietary
levels equal to or greater than 500 ppm. This appeared to be close to
the threshold dose, since body-weight gain was not depressed and only
sporadic haematological changes were observed at 500-750 ppm. There
was no evidence of carcinogenicity.
Dogs received diphenylamine by gelatin capsule at doses of 0, 10,
25, or 100 mg/kg bw per day for one year. Platelet counts in males and
total bilirubin concentrations in animals of each sex were
statistically significantly higher than those of controls. The NOAEL
for toxicity was 10 mg/kg bw per day, and the LOAEL was 25 mg/kg bw
per day, both based on haematological and clinical chemical changes.
In a two-generation study of reproductive toxicity, rats received
diphenylamine in the diet at concentrations of 0, 500, 1500, or 5000
ppm during premating. A NOAEL for parental toxicity was not observed;
the LOAEL was 500 ppm (equal to 40 mg/kg bw per day) on the basis of
enlarged spleens in F1 females, increased spleen congestion and
haemosiderosis in animals of each sex in all generations, and
hepatocyte hypertrophy in F0 females. The NOAEL for developmental
toxicity was 500 ppm (equal to 46 mg/kg bw per day) on the basis of
statistically significantly decreased mean body weight in F2 pups at
1500 ppm and above. The NOAEL for reproductive toxicity was 1500 ppm
(equal to 120 mg/kg bw per day). Although haemosiderin, congestion of
the spleen, and hepatocyte hypertrophy were observed in parental
animals at all doses, they occurred at appreciably lower incidence and
intensity at the lower dose than at the higher doses, which suggested
that the lower dose was close to the NOAEL/LOAEL threshold for
parental toxicity.
In a study of developmental toxicity, rats received diphenylamine
by gavage at doses of 0, 10, 50, or 100 mg/kg bw per day on days 6-15
of gestation. The NOAEL for maternal toxicity was 50 mg/kg bw per day
on the basis of enlarged, blackish, heavier spleens at 100 mg/kg bw
per day. The NOAEL for developmental toxicity was 100 mg/kg bw per
day, the highest dose tested.
In a study of developmental toxicity, rabbits received
diphenylamine by gavage at doses of 0, 33, 100, or 300 mg/kg bw per
day on days 7-19 of gestation. The NOAEL for maternal toxicity was 100
mg/kg bw per day on the basis of decreased body-weight gain and food
consumption at 300 mg/kg bw per day. The NOAEL for developmental
toxicity was 300 mg/kg bw per day, the highest dose tested.
Diphenylamine has been tested for genotoxicity in three assays.
Negative results were obtained for mutation in bacteria and for
induction of micronuclei in mouse bone marrow in vivo. A weakly
positive response was observed only for mutation in mouse lymphoma
cells in vitro at a dose range in which the toxicity was relatively
high and the mutant frequency did not increase with dose. The Meeting
concluded that, although diphenylamine has some genotoxic potential,
it is unlikely to present a human genotoxic hazard.
An ADI of 0-0.08 mg/kg bw was established on the basis of the
NOAEL of 150 ppm, equal to 7.5 mg/kg bw per day, in the two-year study
of toxicity and carcinogenicity in rats and a 100-fold safety factor.
An acute RfD was not allocated because diphenylamine is of low
acute toxicity. The Meeting concluded that the acute intake of
residues is unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 520 ppm, equal to 73 mg/kg bw per day (78-week study of
carcinogenicity)
Rat: 150-200 ppm, equal to 7.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
500 ppm, equal to 46 mg/kg bw per day (reproductive
toxicity in a two-generation study of reproductive
toxicity)
Rat 50 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
100 mg/kg bw per day (study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
300 mg/kg bw per day (highest dose in a study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.08 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid absorption: at least 68-89% of a dose
Dermal absorption No data
Distribution Extensive
Potential for accumulation Very little
Rate and extent of excretion At 24 h, 45-72% of a dose found in urine
Metabolism in animals Extensively metabolized. Parent < 3%. Approximately
80-90% of a dose appeared as 12 metabolites: indophenol
and various isomers of mono- and
di-hydroxydiphenylamine excreted in urine as sulfate
and glucuronide conjugates
Toxicologically significant compounds Parent. Indophenol might undergo electrophilic
(animals, plants, and environment) interactions
Acute toxicity
Rat: LD50 oral 3000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
LC50 inhalation No data
Skin irritation None to slight
Eye irritation Slight to corrosive with corneal opacity
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Erythrocytes/anaemia
Lowest relevant oral NOAEL Mouse: 1.7 mg/kg bw per day, 90-day study
Lowest relevant dermal NOAEL No data
Lowest relevant inhalation NOAEL No data
Genotoxicity Unlikely to be a human genotoxic hazard
Long-term toxicity and carcinogenicity
Target: critical effect Erythrocytes/haemolytic anaemia
Lowest relevant NOAEL Rat: 7.5 mg/kg bw per day
Carcinogenicity No carcinogenicity
Reproductive toxicity
Reproduction target /critical effect Decreased mean litter size in both generations and
decreased mean body weights of F2 pups at maternally
toxic doses
Lowest relevant reproductive NOAEL Rat: 46 mg/kg bw per day
Developmental target/critical effect Rat: No developmental toxicity
Lowest relevant developmental NOAEL Rat: 100 mg/kg bw per day, highest dose tested
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.08 mg/kg bw Long-term toxicity 100
and carcinogenicity,
rats
Acute reference dose Not allocated
(unnecessary)
References
Botta, J.A., Jr (1992) 90 Day subchronic toxicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426E-001-034-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994a) 18 Month oncogenicity evaluation of
diphenylamine in the mouse. Unpublished report No. 426H-002-646-91
from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Botta, J.A., Jr (1994b) 24 Month combined oncogenicity/toxicity
evaluation of diphenylamine in rats. Unpublished report No.
426D-102-048-91 from T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Botta, J.A., Jr (1994c) One year chronic study of diphenylamine in
dogs. Unpublished report No. 426B-502-044-91 from T.P.S., Inc., Mt
Vernon, Indiana, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Cifone, M. (1992) Mutagenicity test on diphenylamine in the L5178Y
TK+/- mouse lymphoma forward mutation assay. Unpublished report No.
14902-0-431 from Hazleton Washington, Inc., Rockville, Maryland, and
Vienna, Virginia, USA. Submitted to WHO by the DPA Task Force, John
Wise & Associates Ltd, Liberty, Missouri, USA.
Edwards, J.A., Leemings, N.M., Clark, R. & Offer, J.M. (1983) Effect
of diphenylamine on pregnancy of the New Zealand white rabbit.
Unpublished study No. PWT 1/2/83409 prepared by Huntington Research
Centre, Huntington, Cambridgeshire, United Kingdom.
Evan, A.P., Hong, S.K., Gardner, K., Jr, Park, Y.S. & Itagaki, R.
(1978) Evolution of the collecting tubular lesion in
diphenylamine-induced renal disease. Lab. Invest., 38, 244-252.
Gardner, K.D., Jr, Solomon, S., Fitzgerrel, W.W. & Evan, A.P. (1976)
Function and structure in the diphenylamine-exposed kidney. J. Clin.
Invest., 57, 796-806.
Kim-Kang, H. (1993) Metabolism of [14C]diphenylamine in stored
apples: Nature of the residue in plants. Unpublished study No. XBL
91071, report No. RPT00124 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Abstract of study submitted to WHO by the
DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA.
Kim-Kang, H. (1994a) Metabolism of [14C]diphenylamine in lactating
goats. Unpublished study No. XBL 92089, report No. RPT00150 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kim-Kang H (1994b) Metabolism of [14C]diphenylamine in the laying
hen. Unpublished study No. XBL 93041, report No. RPT00161 from
Xenobiotic Laboratories, Inc., Plainsboro, New Jersey, USA. Submitted
to WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA
Kiplinger, G. (1995) Skin sensitization study of diphenylamine
technical in albino guinea pigs. Final report: Lab project No.
WIL-256001. Unpublished study prepared by WIL Research Labs, Inc.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Kreuzman, J. (1991a) Primary eye irritation study in rabbits without
rinsing with diphenylamine super-refined. Lab project No. 91-8052-21
(B). Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Kreuzman, J. (1991b) Primary skin irritation study in rabbits with
diphenylamine super-refined. Lab project No. 91-8052-21 (A).
Unpublished study prepared by Hill Top Biolabs, Inc. Submitted to WHO
by the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Krohmer, R.W. (1992a) 90 Day evaluation of diphenylamine in the dog.
Unpublished study No. 426C-501-034-91 from T.P.S., Inc., Mt Vernon,
Indiana, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Krohmer, R.W. (1992b) 90 Day subchronic toxicity evaluation of
diphenylamine in rats. Unpublished study No. 426C-10-034-91 from
T.P.S., Inc., Mt Vernon, Indiana, USA. Submitted to WHO by the DPA
Task Force, John Wise & Associates Ltd, Liberty, Missouri, USA
Lawlor, T. (1992) Mutagenicity test on diphenylamine in the
Salmonella/mammalian-microsome reverse mutation assay (Ames test).
Unpublished report No.14902-0-401 from Hazleton Washington, Inc.,
Rockville, Maryland, and Vienna, Virginia, USA. Submitted to WHO by
the DPA Task Force, John Wise & Associates Ltd, Liberty, Missouri,
USA.
Lenz, S.D. (1996) Investigation of regional glutathione levels in a
model of chemically-induced renal papillary necrosis. Food Chem.
Toxicol., 34, 489-494.
Lenz, S.D. & Carlton, W.W. (1990) Diphenylamine-induced renal
papillary necrosis and necrosis of the pars recta in laboratory
rodents. Vet. Pathol., 27, 171-178.
Lenz, S.D. & Carlton, W.W. (1991) Decreased incidence of
diphenylamine-induced renal papillary necrosis in Syrian hamsters
given dimethylsulphoxide. Food Chem. Toxicol., 29, 409-418.
Lenz, S.D., Turek, J.J. & Carlton, W.W. (1995) Early ultrastuctural
lesions of diphenylamine-induced renal papillary necrosis in Syrian
hamsters. Exp. Toxicol. Pathol., 47, 447-452.
Majnarich, J. (1991a) Diphenylamine: Super refined: Acute oral
toxicity, LD50 (rat). Unpublished report No. 022-91 from
Bioconsultants, Inc. Submitted to WHO by the DPA Task Force, John Wise
& Associates Ltd, Liberty, Missouri, USA.
Majnarich, J. (1991b) Diphenylamine: Super refined: Dermal LD50.
Unpublished report No. 024-91 from Bioconsultants, Inc. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Murli, H. (1992a) Dose range-finding study on diphenylamine for in
vivo murine micronucleus assay. Unpublished report No. 14902-0-459PO
from Hazleton Washington, Inc., Rockville, Maryland, and Vienna,
Virginia, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA
Murli, H. (1992b) Mutagenicity test on diphenylamine for in vivo
mammalian micronucleus assay. Unpublished report No. 14902-0-455 from
Hazleton Washington, Inc., Rockville, Maryland, and Vienna, Virginia,
USA. Submitted to WHO by the DPA Task Force, John Wise & Associates
Ltd, Liberty, Missouri, USA.
Powell, C.J., Adams, T., Hall, D.E., Bach, P.H. & Bridges, J.W. (1985)
The comparative subacute nephrotoxicity of diphenylamine and
N-phenylanthranilic acid. In: Bach, P.H. & Bridges, J.W., eds,
Renal Heterogeneity and Target Cell Toxicity, Chichester, John Wiley
& Sons, pp. 199-202.
Rodwell, D.E. (1992a) Range finding teratology study in rats with
diphenylamine (DPA). Unpublished study No. 3255.2 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rodwell, D.E. (1992b) Teratology study in rats with diphenylamine
(DPA). Unpublished study No. 3255.3 prepared by Springborn
Laboratories, Inc., Life Sciences Division, Spencerville, Ohio, USA.
Submitted to WHO by the DPA Task Force, John Wise & Associates Ltd,
Liberty, Missouri, USA.
Rodwell, D.E. (1993) Two-generation reproduction study in rats with
diphenylamine (DPA). Unpublished study No. 3255.4 prepared by
Springborn Laboratories, Inc., Life Sciences Division, Spencerville,
Ohio, USA. Submitted to WHO by the DPA Task Force, John Wise &
Associates Ltd, Liberty, Missouri, USA.
Rohrbach, D.H., Robinson, L.K. & Murrah, V.A. (1993) Loss of the
basement membrane matrix molecule, bamin, in diphenylamine-treated
mice. Matrix, 13, 341-350.
Siglin, J.C. (1991) Repeated dose dermal toxicity: 21 Day study.
Unpublished study No. 3255.1 prepared by Springborn Laboratories,
Inc., Life Sciences Division, Spencerville, Ohio, USA. Submitted to
WHO by the DPA Task Force, John Wise & Associates Ltd, Liberty,
Missouri, USA.
Wu, D. (1993) Diphenylamine: Rat metabolism study. Unpublished study
XBL No. 92081, report No. RPT00131 from Xenobiotic Laboratories, Inc.,
Plainsboro, New Jersey, USA. Submitted to WHO by the DPA Task Force,
John Wise & Associates Ltd, Liberty, Missouri, USA.
