
METHIOCARB JMPR 1998
First draft prepared by
T.C. Marrs
Department of Health
United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Neurotoxicity
Antidotes
Interaction with other pesticides
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Methiocarb was evaluated for toxic effects by Joint Meetings in
1981, 1983, 1984, 1985, and 1987 (Annex 1, references 36, 40, 42, 44,
and 50). An ADI of 0-0.001 mg/kg bw was allocated in 1981,which was
extended at subsequent meetings. Methiocarb was evaluated by the
present Meeting within the CCPR periodic review programme. This
monograph summarizes the new data and relevant data from the previous
monographs and monograph addenda on methiocarb (Annex 1, references
37, 41, and 46).
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
[ring-1-14C]-Methiocarb (4.8 mCi/mmol; purity, > 97%) was
administered to three female rats by gavage at a dose of 20 mg/kg bw.
Similarly labelled methiocarb (14.5 mCi/mmol; purity, > 97%) was
administered to three male and three female rats by gavage at a dose
of 0.25 mg/kg bw. Organic extracts of 48-h urine samples collected
from each rat were subjected to one-dimensional or two-dimensional
thin-layer chromatography on plates pre-coated with silica gel. More
than 90% of the radiolabel administered at the higher dose was
excreted in the urine. Similar results were obtained with the lower
dose, but a somewhat smaller proportion of the radiolabel (73-86%) was
excreted over 48 h; at this dose, there was no major difference
between males and females (Stanley & Johnson, 1976).
(b) Biotransformation
In the urine samples collected by Stanley and Johnson (1976), the
major components of the chloroform extract at the higher dose were
methiocarb phenol, methiocarb sulfoxide phenol, and an unknown
component which may correspond to N-hydroxymethyl methiocarb
sulfoxide. A trace of methiocarb sulfoxide was detected. After
incubation of the aqueous phase of the urine samples with maltase,
about half of the radiolabel present was rendered organosoluble, the
major component being methiocarb sulfoxide phenol; methiocarb phenol
and methiocarb sulfone phenol were also present. The results were
similar at the lower dose, at which there was no major difference
between males and females; however, methiocarb sulfone phenol was
found in the chloroform extract.
These results differ from those of an earlier study in rats with
[carbonyl-14C]- and [methyl-3H]-methiocarb, which was not available
for evaluation. It was reported that methiocarb phenol was the primary
metabolite in the earlier study, whereas in the study of Stanley and
Johnson (1976), methiocarb sulfoxide phenol was the metabolite at
highest concentration in the urine. The report of the latter study
also cites an earlier study in dogs in which the only metabolites seen
were methiocarb sulfoxide phenol and methiocarb sulfone phenol.
In studies of the metabolism of methiocarb in vitro (Strother,
1970, 1972; Menzie, 1974) with 14C-methiocarb, human liver fractions
were slightly less metabolically active than fractions from
Sprague-Dawley rats. The major metabolite was methiocarb sulfoxide. In
a comparative study of the metabolism of methiocarb (and Zectran, a
related compound) in rat and dog liver and kidney homogenates and
various blood fractions in vitro, the metabolic pathway seemed to be
similar in the two species, sulfoxidation of the sulfur atom being the
main route. N-Methyl hydroxylation was also found to occur (Wheeler
& Strother, 1971).
Flavin adenine dinucleotide-dependent monooxygenases from pig
liver microsomes had some activity in the sulfoxidation of methiocarb
(Haijar & Hodgson, 1982). Methiocarb is reported to cross the placenta
into the fetus (Salama et al., 1993).
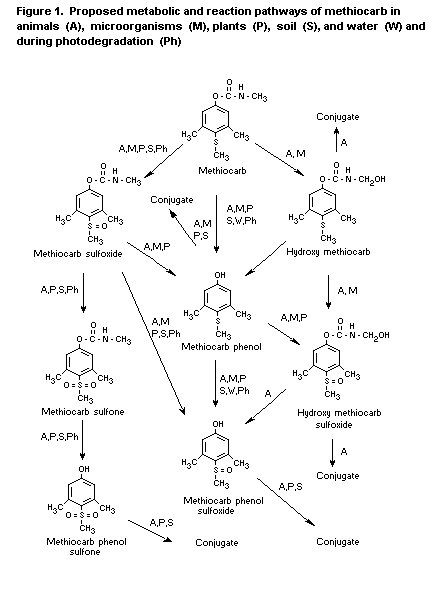
The main products of metabolism of methiocarb in plants were
conjugates of the phenol, sulfoxide phenol, and sulfone phenol;
methiocarb sulfone was also seen, while methiocarb sulfoxide occurred
in some plant products (Murphy et al., 1982).
Figure 1 shows the proposed metabolic pathways for methiocarbin
various species and media.
(c) Effects on enzymes and other biochemical parameters
Single doses of 1, 10, 25, or 50 mg/kg bw methiocarb were
administered by gavage to groups of five Wistar rats of each sex,
while five rats of each sex acted as controls. Plasma and erythrocyte
cholinesterase activity was measured after 20 min, 2 h, and 5 h, and,
in males at the highest dose, additionally at 1.5 and 3 h; brain
acetylcholinesterase activity was measured at 30-min intervals up to
5 h. Cholinergic signs, starting immediately after treatment and
abating within 2 h, were seen at > 10 mg/kg bw. Male rats receiving
the highest dose died after 2-3 h. Maximum depression of plasma
cholinesterase and erythrocyte acetylcholinesterase activity was
observed after 20 min at < 25 mg/kg bw and after 20 min to 2 h at
the highest dose. In a separate study, methiocarb was given at a dose
of 10 or 20 mg/kg bw, and brain acetylcholinesterase activity was
determined at 30 min and 1, 2, 3, and 5 h. Inhibition was maximal at
2 h.
In a four-week study, 10 rats of each sex received methiocarb at
doses of 1, 3, or 10 mg/kg bw per day by gavage. Plasma and
erythrocyte cholinesterase activity was determined in three rats of
each sex 20 min after administration of the test material on days 4,
8, 14, 21, and 28; brain acetylcholinesterase activity was determined
in five rats of each sex 2 h after the last dose. At the highest dose,
cholinergic signs were observed briefly. Plasma, erythrocyte, and
brain cholinesterase activity was depressed at 10 mg/kg bw per day.
The NOAEL for cholinesterase depression was thus 3 mg/kg bw per day
(Eben & Kimmerle, 1973).
Technical-grade methiocarb (purity, 97%) and methiocarb sulfoxide
(purity, 95.2%) were administered by gavage to groups of 15 female
Sprague-Dawley-derived rats at a dose of 0.5 or 2 mg/kg bw per day on
five days per week for four weeks, while 15 controls received the
vehicle (Carbowax). The rats were observed daily for general
appearance and, in addition, for cholinergic signs at 0.5, 1, and 4 h
after dosing for the first five days of treatment. For determination
of cholinesterase activity, each group was subdivided into three
subgroups of five. Blood was collected from the first subgroup before
and 30 min after dosing on days 0, 7, 14, 21, and 28; blood was
collected similarly from the second group but 4 h after dosing on days
4, 11, 18, and 25. The third subgroup was held in reserve in case
anaemia developed. Depression of cholinesterase activity was
calculated by comparison with activity before treatment.
Tremors were seen during the first five days of the study in some
rats receiving the sulfoxide at the higher dose; no clinical signs
were seen in other groups. A 20% depression in plasma cholinesterase
activity was found in controls 4 h after dosing on day 25, and
depressions of 1-21% were found 30 min after dosing with methiocarb at
 0.5 mg/kg bw; 4 h after dosing at 0.5 mg/kg bw, depressions of < 10%
were noted. At the higher dose of methiocarb, depressions of 19-41%
were seen 30 min after dosing and 0.2-19% 4 h after dosing. With the
sulfoxide, plasma cholinesterase activity was inhibited by 21-39% at
the low dose at 30 min but was generally not inhibited at 4 h. At the
high dose, 39-62% inhibition was seen at 0.5 h and 10-25% inhibition
at 4 h. Erythrocyte acetylcholinesterase activity was depressed by
5-12% 30 min after dosing at 0.5 mg/kg bw and by < 10% 4 h after
dosing. At the higher dose of methiocarb, erythrocyte
acetylcholinesterase activity was depressed by 15-29% 30 min after
dosing and by 1-13% 4 h after dosing. With the sulfoxide, erythrocyte
acetylcholinesterase activity was inhibited by 13-31% at the low dose
at 30 min but was generally not inhibited at 4 h. At the high dose,
32-46% inhibition was seen at 0.5 h and 10-21% at 4 h. The NOAEL for
erythrocyte acetylcholinesterase inhibition was therefore
approximately 0.5 mg/kg bw per day for methiocarb, but there was no
NOAEL for the sulfoxide (Hixson, 1981).
Technical-grade methiocarb (purity, 97%) and methiocarb sulfoxide
(purity, 95.2%) were administered orally in gelatine capsules to
groups of two adult beagle dogs and two bitches at a dose of 0.05 or
0.5 mg/kg bw per day for 29 days; two control animals of each sex
received empty capsules. The animals were observed twice daily, and
blood was taken for measurement of plasma and erythrocyte
cholinesterase activity before the first dose, 1, 3, 6, and 24 h after
the first dose, and 3 h after the third dose. During the second week,
blood was taken before the first dose, 2, 6, and 24 h later, and 2 h
after the second dose. During weeks 3 and 4, blood was taken before
the first dose, 2, 6, and 24 h later, and 2 h after the third dose. In
week 5, blood was again taken before the first dose and 2, 6, and 24 h
later; dosing was then stopped, and another blood sample was taken on
the third day.
Clinical signs of toxicity (salivation and vomiting) were
observed at the higher dose of each test material and in animals of
each sex; additionally, slight salivation was observed in one bitch
given the sulfoxide at the lower dose. Depression of cholinesterase
activity was calculated by comparison with the level measured just
before the first dose each week: inhibition was variable, but peak
inhibition occurred 0-3 h after dosing; considerable inhibition was
seen with both materials at the higher dose in animals of each sex. At
the lower doses, smaller depressions of both plasma and erythrocyte
cholinesterase activity, of up to about 20%, were sometimes seen with
both materials. Cholinesterase activity was generally normal by 6 h
(Hayes, 1981).
At equimolar concentrations, methiocarb was the least effective
of four carbamates in inhibiting bovine erythrocyte
acetycholinesterase and equine plasma cholinesterase activity
(Barthová et al., 1989).
Methiocarb was reported not affect liver function in rabbits at a
single oral dose of 25 mg/kg bw (Kimmerle, 1960).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of methiocarb and
putative metabolites of methiocarb are shown in Table 1.
No erythema or oedema was seen when technical-grade methiocarb
(purity unspecified) was applied to abraded and unabraded skin of six
New Zealand white rabbits. Application to the eyes of six rabbits did
not produce ocular irritation (Crawford & Anderson, 1970).
A study of the skin-sensitizing potential of methiocarb (purity,
97.8% pure) was conducted by the Magnusson and Kligman technique. A
group of 20 guinea-pigs (strain BOR:DHPW) each received methiocarb
intradermally at a concentration of 1% and topically at a
concentration of 25%. There were two control groups of 10 guinea-pigs.
The test animals were first challenged with methiocarb at a
concentration of 25% and then at 12.5%. Although there was a small
excess in the number of animals that reacted positively after the
second challenge, the overall result was considered to be negative
(Mihail, 1984).
In a test for dermal sensitization by the Buehler technique,
technical-grade methiocarb (purity, 99.2%) was applied to 15 Hartley
guinea-pigs once weekly for three weeks, with a challenge dose a
fortnight later. The material was applied as a single dose to five
previously unexposed animals at the time of the challenge dose.
Dinitrochlorobenzene, used as the positive control, was applied in the
same way to a further group of five guinea-pigs, weekly for three
weeks, with a challenge dose two weeks later, and to a further group
of five as a single dose at the time of the challenge dose. Methiocarb
was not considered to be a dermal sensitizer (David, 1988).
A case of allergic contact dermatitis was reported in a man who
grew carnations. He developed acute severe eczema of the hand and
showed a positive reaction in a patch test to 0.5% methiocarb (Willems
et al., 1997).
(b) Short-term studies of toxicity
Rats
Methiocarb in aqueous tragacanth suspension was given by gavage
to albino rats daily. The dose was 2 mg/kg bw per day for the first
three days and 4 mg/kg bw per day for the next 24 days. Two groups of
three animals were killed every week for determination of
cholinesterase activity. The activity had fallen to 80% of the control
values after 14 days and to 50% by the end of the study. No abnormal
clinical signs were observed; the animals gained weight normally
(Kimmerle, 1960).
Table 1. Acute toxicity of methiocarb and putative metabolites
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb
Rats Sprague-Dawley M Oral 130 Technical DuBois & Raymund (1962)
F 140
Rats Sprague-Dawley F Oral 100 Recrystallized DuBois & Raymund (1962)
Rats NR NR Oral 67 NR Kimmerle (1966a)
Rats Sprague-Dawley M Oral 30 (20-45) 99 Crawford & Anderson (1973)
F 30 (20-45)
Rats Sprague-Dawley M Oral (non-fasting) 46 (38-56) 99 Crawford & Anderson (1973)
F 47 (36-63)
Rats Sprague-Dawley M Oral 15 (9-26) Technical Lamb & Matzkanin (1976a)
F 31 (18-54)
Rats Sprague-Dawley M Oral 13 (9-17) Technical Lamb & Matzkanin (1976b)
F 32 (24-44)
Rats Sprague-Dawley M Oral 14 (12-16) Technical Lamb & Matzkanin (1977)
F 16 (13-20)
Rats Sprague-Dawley M Oral (non- 51 (45-58) Technical Lamb & Matzkanin (1977)
F 79 (65-96)
Rats NR M Oral 22 (19-25) 98.5 Flucke (1978)
F 24 (21-28)
Rats NR M Oral 22.1 (18-27) 98.3 Flucke (1980)
Rats Sprague-Dawley- M Oral 33 (22-50) 98 Nelson (1979)
derived F 47 (31-70)
Rats NR M Oral 17 (16-19) 98.6 Heimann (1983)
Rats NR M Oral 19 (16-23) 98.2 Flucke (1988)
F 26 (19-36)
Rats Albino M Oral 100 NR Kimmerle (1960)
Rats Wistar-CFN M Oral 87 NR Klimmer (1963)
Rats NR M Oral 33 (29-38) 98.4 Thyssen (1977a)
35 (29-42) 98.3
35 (30-42) 98.2
31 (26-35) 97.8 28 (24-33)97.4
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rats Sprague-Dawley M Intraperitoneal 35 Technical DuBois & Raymund (1961a)
F 30
Rats Sprague-Dawley F Intraperitoneal 25 Recrystallized DuBois & Raymund (1962)
Rats Wistar-CFN M Intraperitoneal 43 NR Klimmer (1963)
Rats Sprague-Dawley M Dermal > 200 Technical DuBois & Raymund (1961a)
Rats Sprague-Dawley F Dermal > 300 Recrystallized DuBois & Raymund (1962)
Rats Albino M Dermal > 1000 NR Kimmerle (1960)
Rats Wistar-CFN M Dermal 350-400 NR Klimmer (1963)
Rats NR M Dermal > 500 99.2 Solmecke (1969)
Rats Wistar M Dermal > 5 g 98.1 Thyssen (1977b)
F > 5
Rats Sprague-Dawley M Inhalation (1 h) 1200 mg/m3 a 98.8 Shiotsuka (1987a)
(780-1700)
F 1100 mg/m3 a
(740-1600)
Rats NR M Inhalation 540 g/L3 NR Kimmerle (1966b)
Rats Sprague-Dawley M Inhalation (1 h) > 20 000 g/L Technical Crawford & Anderson (1972a)
F > 20 000 g/L
Rats Sprague-Dawley M Inhalation (4 h) 580 mg/m3 (340-700) 98.8 Shiotsuka (1987b)
F 430 mg/m3 (290-580)
Rats Wistar M Inhalation (4 h) > 320 mg/m3 97.9 Thyssen (1982)
F > 320 mg/m3
Rats Wistar M Inhalation > 300 mg/m3 97.9 Thyssen (1982)
F (5 x 6 h) > 300 mg/m3
Mice NR M Oral 25 (21-30) NR Kohgo (1970)
Mice NR M Oral 52 98.2 Kimmerle (1972)
Mice NR M Subcutaneous 940 (760-1200) NR Kohgo (1970)
Mice Carworth Farm M Intraperitoneal 6 Technical DuBois & Raymund (1961a)
F 5.5
Mice Dierolf Farm F Intraperitoneal 16 Technical Baron et al. (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rabbits New Zealand M Dermal > 2000 99 Crawford & Anderson
white F > 2000 (1972b)
Guinea-pigs NR M Oral 40 Technical DuBois & Raymund (1961a)
Guinea-pigs NR F Oral (50-100) 99.2 Kimmerle (1969a)
Guinea-pigs Albino F Oral 14 (7.9-25) Technical Crawford & Anderson (1972a)
Guinea-pigs NR M Intraperitoneal 17 Technical DuBois & Raymund (1961a)
Dogs Beagle F Oral (10-25) 99.2 Kimmerle (1969b)
Dog Mongrel M Oral ~ 25 Technical Lamb & Matzkanin (1975)
F ~ 25
Chicken NR F Oral 175 Technical DuBois (1962)
Chicken White Leghorn F Oral 380 (300-490) 98.5 Thyssen & Schilde (1978)
Methiocarb phenol
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR M Dermal > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Methiocarb phenol sulfoxide
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Methiocarb phenol sulfone
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb sulfoxide
Rats NR NR Oral 43 (37-50) NR Solmecke (1970)
Rats Sprague-Dawley M Oral 9 (7-13) NR Lamb & Matzkanin (1976a)
F 7
Rats Sprague-Dawley M Oral 6 (5-8) NR Lamb & Matzkanin (1976a)
F 8 (6-10)
Methiocarb sulfone
Rats NR NR Oral > 1000 NR Solmecke (1970)
Hydroxymethyl methiocarb
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfone
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfoxide
Rats NR M Oral > 160 NR Nelson (1979)
F > 160
Technical, purity unstated; NR, not reported
a There was a large descrepancy between nominal and measured concentrations owing to deposition in the chamber;
these figures were measured gravimetrically.
Methiocarb was administered by gavage to groups of 25 male
Wistar-CFN rats at a dose of 2.5 or 5 mg/day on six days per week for
six months. Three deaths occurred at the higher dose due to
bronchopneumonia, but weight gain was the same in the two treated
groups and in 20 control animals; no abnormal clinical signs were seen
(Klimmer, 1963).
Groups of 12 Sprague-Dawley rats of each sex were fed diets
containing methiocarb (purity unspecified) at concentrations of 0, 5,
10, or 50 ppm for 16 weeks. Five animals of each sex from each group
were examined histopathologically, and five of each sex were used for
measurements of cholinesterase activity in blood, brain, and
submaxillary glands by a manometric method. No effect on growth rate,
food intake, or mortality rates was observed at any dose. In males at
the highest dose, serum, erythrocyte, brain, and submaxillary gland
cholinesterase activity was inhibited by 21, 14, 12, and 7%,
respectively. In females at the highest dose, serum cholinesterase
activity was reduced by 28% and erythrocyte acetylcholinesterase
activity by 15%, all by comparison with concurrent controls. Brain
acetylcholinesterase activity was reduced by 5% in females, and
inhibition of submaxillary gland enzyme was seen in all test groups. A
NOAEL could not be identified in this study (Doull et al., 1962).
Groups of 10 Wistar rats of each sex were exposed to an aerosol
of methiocarb (purity, 97.9%) of a mass median diameter of 2.1-2.5.
The groups were exposed to either air, solvent, methiocarb at 6 mg/m3
in solvent, methiocarb at 23 mg/m3 in solvent, or methiocarb at 96
mg/m3 in solvent; exposure was for 6 h/day, five days per week for
three weeks. The animals were examined daily and weighed weekly. Blood
was taken from five animals per group at the end of the study and was
used for haematological and clinical chemical tests; urinary analyses
were also carried out. Plasma and erythrocyte cholinesterase activity
was determined before treatment and after 5, 10, and 15 exposures for
all groups except the air controls. At the end of the study, the
animals were sacrificed and autopsied, selected organs were weighed
and processed for histopathological examination, and brain
acetylcholinesterase activity was measured.
No deaths were observed in any group. Animals at the highest
concentration showed clinical signs of compound-related effects
(tremor); no clinical signs were seen at lower concentrations. There
body weight of males at the highest concentration was reduced by
comparison with the air controls but not with the solvent controls. No
change in haematological or clinical chemical parameters was seen that
was attributable to the test material, with the exception of
inhibition of cholinesterases. Plasma and brain cholinesterase
activity was decreased at the highest concentration, brain
acetylcholinesterase activity being 61 and 74 % of that in concurrent
solvent controls in males and females, respectively. Some inhibition
of plasma cholinesterase activity was seen at the intermediate
concentration, and males at this concentration had an associated
decrease in brain acetylcholinesterase activity (65% of concurrent
control value). Erythrocyte acetylcholinesterase activity was less
strongly affected than plasma enzyme, but marginal inhibition was
observed in males at the highest concentration at week 1 (82% of
concurrent solvent control value). There were no toxicologically
significant alterations in organ weights, and no compound-related
findings were noted on histopathological examination. The NOAEL was
6 mg/m3 on the basis of reduced brain acetylcholinesterase activity
in males (Thyssen & Mohr, 1983).
Rabbits
Technical-grade methiocarb (purity, 99.2%) was applied to five
male and five female chinchilla rabbits, daily for two weeks, at a
dose of 500 mg; five males and five females served as controls. The
material was applied for 24 h/day, and new material was applied at the
end of each 24-h period. After the two-week application period, the
animals were observed for a further fortnight. The animals were
inspected daily, and haematological and biochemical studies were
carried out before treatment, at the end of treatment, and two weeks
later. Clinical chemistry was restricted to liver function tests and
urinary analysis; cholinesterase activity was not measured. No
abnormal clinical signs were seen, and there was no effect on
body-weight gain or any perturbation in haematological or clinical
chemical variables (Kimmerle, 1969c).
Methiocarb (purity, 99.3%) was applied at doses of 0, 60, 150, or
375 mg/kg bw per day for 6 h/day to the skin of groups of five male
and five female New Zealand white rabbits, and the site of application
was occluded. The animals were examined twice daily, and any signs of
skin irritation were scored. The rabbits were weighed twice weekly;
food consumption was measured three times weekly until the last week,
when it was measured four times. Blood was taken for haematological
and clinical chemical analysis before treatment and pre-terminally.
Plasma and erythrocyte cholinesterase activity was measured at the end
of the 6-h exposure period on days 1, 7, 14, and 21; additionally,
blood was taken 16 h after the end of exposure on these days from the
group at the high dose. Animals were sacrificed on the day after the
last treatment, at which time the brain was taken for measurement of
acetylcholinesterase activity in a homogenate of the entire left half
of the brain. Selected tissues were weighed, examined grossly,
processed, and examined histopathologically.
Two animals at the low dose did not survive to the end of the
study. No clinical signs related to the test material were seen, and
it was not irritating. There was no differences between the groups in
body weight, but food consumption appeared to be reduced in animals of
each sex at the high dose, and particularly in males. Clinical
chemical parameters did not differ between the groups, and, although
one or two differences in haematological measurements were seen, none
appeared to be related to treatment. Plasma cholinesterase activity
appeared to be reduced in males at the high dose at 14 and 21 days.
The measurements made 6 h after the end of exposure suggested that the
inhibition was not reversed overnight. No intergroup differences in
plasma cholinesterase activity were observed among females.
Erythrocyte acetylcholinesterase activity was very variable but did
not appear to be inhibited in a dose-related fashion. Intergroup
differences were not observed in brain acetylcholinesterase activity.
No gross or microscopic abnormality was observed that was attributable
to the test material. The NOAEL was 150 mg/kg bw per day on the basis
of reduced food consumption (Procter, 1988).
Methiocarb (purity, 97.5%) was applied to the skin of five male
and five female New Zealand white rabbits at a single dose of 500
mg/kg bw per day for 6 h/day under occlusion. Five controls of each
sex received saline. The animals were examined twice daily, and any
signs of skin irritation were scored. They were weighed twice weekly,
while food consumption was measured every two days. Blood was taken
for haematological and clinical chemical tests before treatment and
preterminally. Plasma and erythrocyte cholinesterase activity was
measured at the end of the 6-h exposure period on days 1, 7, 14, and
21 and, in the test animals, 16 h later. Animals were sacrificed on
the day after the last treatment, at which time the left half of the
brain was taken for measurement of cholinesterase activity. Selected
tissues were weighed, examined, processed, and examined
histopathologically.
Two test animals removed their dressings and presumably ingested
the material; these animals developed clinical signs of cholinergic
poisoning, which disappeared within a few hours. No other
compound-related clinical signs were seen, and no animal died before
completion of the study. Treated females were lighter than controls
throughout the study, and the food consumption of animals of each sex
was reduced. A reduction in serum calcium and increased activities of
alanine and aspartate aminotransferases were seen in females in
comparison with concurrent controls. Plasma cholinesterase activity
was lower than that before treatment in both males and females, but
only inconsistently in comparison with concurrent controls.
Erythrocyte acetylcholinesterase activity was reduced in males on day
1, at both 6 and 16 h, in comparison with pretreatment levels, but
inconsistently in comparison with concurrent controls; it was
concluded that compound-related inhibition of erythrocyte
acetylcholinesterase activity had not occurred in either males or
females. Brain acetylcholinesterase activity was not inhibited. No
abnormality was seen at autopsy or on histopathological examination.
The design of this study was not appropriate for identifying a NOAEL
(Procter, 1989).
Cats
Methiocarb at a dose of 5 mg/kg bw per day, given daily by gavage
to cats, was reported to have no adverse effects (Kimmerle, 1960).
Dogs
Methiocarb was incorporated into the diet of groups of two male
and two female beagles at a concentration of 0, 50, 100, or 250 ppm,
equal to 0, 1.25, 2.5, or 6.25 mg/kg bw per day. The animals were
examined daily and weighed fortnightly; plasma and erythrocyte
cholinesterase activity was measured weekly. No clinical effects were
seen, and body-weight gain was unaffected by treatment. There was no
clear difference in plasma or erythrocyte cholinesterase activity
between the test groups and concurrent controls. The small group size
renders this study inappropriate for identifying a NOAEL (Root et al.,
1963).
Chickens
Chickens (Gallus gallus Babcock 300) were fed diets containing
a 9:1 mixture of methiocarb and methiocarb sulfoxide (based on studies
of plant metabolism) at a dose of 20, 60, 120, or 360 ppm, equal to
2.5, 7.5, 15, and 45 mg/kg bw per day, over 28 days. Treatment
decreased feed consumption in a dose-related fashion, and the body
weights of birds at the two highest doses were decreased. Egg
production was unaffected. Plasma cholinesterase activity was
decreased by 40-50 % in comparison with concurrent controls at the
three highest doses but was unaffected at the lowest dose (Strankowski
& Minor, 1976).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female BOR:CFW1 mice received diets
containing methiocarb (purity, 98.5%) at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 15, 43, and 130 mg/kg bw per day in males and
0, 20, 57, and 170 mg/kg bw per day in females. Haematological and
clinical chemical tests were performed on five animals of each sex per
dose at 12 months and 10 animals of each sex per dose at 24 months,
and on satellite groups of 15 mice of each sex per dose, which were
sacrificed after one year and necropsied. Cholinesterase activity was
determined in the plasma of five animals of each sex per dose at 1 and
12 months and on 10 animals of each sex per dose (or the survivors if
fewer than 10) at the end of the study. Brain cholinesterase activity
was determined at the end of the study. Animals that died or became
moribund were autopsied, as were 10 mice of each sex per group at 12
months. All surviving animals were sacrificed at the end of the study
and autopsied, and selected organs were weighed, examined, and
processed for histopathological examination.
The appearance, behaviour, mortality rate, and food consumption
of the animals were not affected at any dose, except for a slight
decrease in body weight at the highest dose, throughout the study in
males and up to week 30 in females. Overall survival in this study was
not good: survival at 18 months was 74% of male controls, 47% males at
the low dose, 59% males at the intermediate dose, 66% males at the
high dose, 69% of female controls, 53% at the high dose, 64% at the
intermediate dose, and 69% at the high dose. Intergroup differences in
mortality rates did not appear to be compound-related, as mortality
was higher in males receiving 67 or 200 ppm than in those given the
high dose. Moreover, survival of females up to the end of the study
was poorest for controls. All treated male mice had higher mean
corpuscular haemoglobin concentrations than controls at 12 months, and
males at the highest dose had higher mean corpuscular haemoglobin
values; similar changes were not seen in females. At 24 months, the
mean corpuscular haemoglobin concentration of males at 200 and 600 ppm
was decreased and the mean corpuscular haemoglobin value at 600 ppm,
again, with no similar change in the females. Higher leukocyte counts
were observed in all treated females at 24 months, but the authors
ascribed this finding to high individual values; no perturbution of
the differential count was seen.
No clinical chemical abnormalities were found at 12 months, but
at 24 months the activity of alanine aminotransferase (ALAT) was
increased in animals at 200 and 600 ppm. At one month, males at 200
and 600 ppm had reduced plasma cholinesterase activity (by 51 and 34%
in comparison with concurrent controls), while reductions of 5% or
less were seen at 12 months and 5-11% at 24 months. In females, plasma
cholinesterase activity was inhibited by 24, 43, and 34% at the low,
intermediate, and high doses, respectively, at one month; smaller
reductions (< 12%) were observed at 12 months, and no cholinesterase
inhibition was observed at 24 months. Erythrocyte acetylcholinesterase
activity was not measured. Small reductions in brain
acetylcholinesterase activity were observed, by 5, 11, and 10% in
males at the low, intermediate, and high doses and by 3, 9, and 3% in
females at the three doses, respectively. No compound-related change
in organ weights was seen, nor was there any abnormality in gross of
histological appearance that was related to treatment. Methiocarb was
not tumorigenic. There was no NOAEL because of haematological changes
at all doses in males at 12 months and in females at all doses at 24
months (Krötlinger & Janda, 1983; Krötlinger, 1989).
Rats
Methiocarb (purity, 98.9%) was admixed with the diet of groups of
60 Wistar TNO W.74 rats for two years at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 3.3, 9.3, and 29 mg/kg bw per day for males
and 0, 5, 14, and 42 mg/kg bw per day for females. The rats were
inspected daily, and body weights were determined weekly for the first
26 weeks and then at fortnightly intervals. Haematological, clinical
chemical, and urinary measurements were made in 10 animals of each sex
per dose at 3, 6, 12, and 24 months. Cholinesterase activity in plasma
and erythrocytes was determined one and two days after the start of
the study and at 1, 2, 4, 8, 13, 26, 52, 78, and 105 weeks in 10
animals of each sex per dose. Brain acetylcholinesterase activity was
determined at the end of the experiment on 10 animals of each sex per
dose. Animals that died and those sacrificed in extremis were
examined grossly and necropsied; when possible, tissues were taken for
histopathological examination. The survivors at two years were also
examined grossly and necropsied, and tissues were taken for weighing
and histopathological examination.
None of the doses had any effect on the appearance, behaviour, or
mortality rates of rats of either sex. The mortality rates at two
years were 10-20% for males and 23-32% for females. There was no
significant intergroup difference in food consumption. Weight gain was
not depressed at 67 or 200 ppm in comparison with the controls; at 600
ppm, weight gain was slightly but consistently depressed throughout
the period of administration of methiocarb, and at termination total
body weight was reduced in the group at the high dose. At three
months, an increased leukocyte count was seen in females at the
highest dose and an increased reticulocyte count in females at the two
higher doses. At six months, the mean corpuscular haemoglobin
concentration was reduced in males at the highest dose, and some
elevation in leukocyte count was found in males at 67 and 200 ppm. Red
blood cell counts and haemoglobin and haematocrit values were
decreased in females at the two higher doses, and reticulocytosis was
observed in these groups. At 12 months, the mean corpuscular
haemoglobin concentration was decreased in males at the intermediate
dose and was increased in females at the highest dose; an increased
reticulocyte count was seen in females at the high dose. At 24 months,
no compound-related changes in haematological variables were seen.
Although a few intergroup differences were observed in
biochemical tests, the only ones that appeared to be related to
treatment were total protein concentration and cholinesterase
activity. Total protein concentrations were raised in females at the
two higher doses at six months and in males at the three highest doses
at 12 months; at 24 months, the total protein concentration was
similar in treated and control groups. ALAT activity in the blood was
elevated in all three groups of treated females at 12 months and in
females at the highest dose at two years, but at no time interval in
males. Increased urea was found in plasma from males at the highest
dose at three and 12 months and in females at 12 and 24 months. Plasma
cholinesterase activity was depressed at the high dose at one day and
from eight weeks onwards in males (except in the 52-week assay) and in
females at one day and 1, 2, 4, and 13 weeks. The measurements of
erythrocyte acetylcholinesterase activity were difficult to interpret:
a statistically significant depression was found only at the low dose
in males at 105 weeks and females at 78 weeks, and the degree of
depression was small (5 and 6%, respectively). Males at the
intermediate dose had significantly depressed activities at 8, 78, and
105 weeks, with 5, 7, and 8% depression, respectively. In females at
this dose, significant depression was seen at four and 78 weeks (8 and
13% depression, respectively). In males at the highest dose,
significant depressions were seen at 8, 13, 78, and 105 weeks (7, 7,
9, and 7%, respectively), and in females at this dose depression was
seen at two days and 4 and 78 weeks (6, 8, and 11%, respectively). The
Committee considered that none of these depressions was biologically
significant. No depression of brain acetylcholinesterase activity was
seen.
Males at the highest dose had decreased absolute weights of the
thyroid, heart, lung, liver, spleen, and adrenals, but these were
reflected only in reductions in the relative weights of the spleen and
therefore probably reflect reduced body weight. In the females, only
the absolute weight of the spleen was reduced, while the absolute
weight of the thyroid was increased. As this finding was due to a
single animal, it is unlikely to be attributable to the test material.
No gross or histopathological abnormality related to treatment was
seen at any dose. Methiocarb was not tumorigenic. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months (Krötlinger et al., 1981; Krötlinger,
1990).
Dogs
Groups of four beagle dogs and four bitches were fed methiocarb
(purity, 98.4%) in the diet at 0, 5, 60, or 240 ppm; the group given 5
ppm group had been given 15 ppm for the first 15 days. These dietary
concentrations were equivalent to daily doses of 0, 0.12, 1.5, and 6
mg/kg bw per day, ignoring the first 15 days of treatment of animals
at the lowest dose. The animals were examined daily, and food
consumption was recorded daily and body weight measured weekly.
Clinical examinations including ophthalmoscopy were undertaken, and
haematological and biochemical variables were measured in blood at
weeks 0, 14, 27, 40, 53, 66, 79, 92, and 104; urine was also analysed.
Erythrocyte and plasma cholinesterase activity was measured before the
start of treatment and at weeks 2, 3, 4, 7, 10, 13, 27, 40, 53, 66,
79, 92, and 104, before feeding and 2 h afterwards.
Acetylcholinesterase activity on the olfactory bulb of the brain was
measured at sacrifice. Animals were examined grossly post mortem,
and selected tissues were examined histologically.
One death occurred, of an animal at 5 ppm, which was considered
not to be related to treatment. The only clinical findings were mild
weakness of the hind limbs, trembling, reduced alertness, and some
vomiting at the highest dose during the first 14 weeks of the study.
The results of tests for reflexes and ophthalmic parameters were
normal. Food intake was reduced in animals of each sex at the highest
dose and in bitches at the intermediate dose, but the body weights
were not significantly affected. Haematological and biochemical
parameters were unaffected, apart from cholinesterase activity. Plasma
cholinesterase activity was depressed at doses of 15 ppm and higher,
and it was for this reason that this dose was reduced to 5 ppm.
Depression of plasma cholinesterase activity was not seen at 5 ppm but
occurred at the two higher doses. Erythrocyte and brain
acetylcholinesterase activity was not consistently inhibited at any
dose; the maximum inhibition of erythrocyte acetylcholinesterase
activity was in animals at the high dose (17% for each sex) and at the
intermediate dose (10% in dogs and 5% in bitches). Organ weights were
unaffected, and no organ-specific toxicity observed. The NOAEL was 60
ppm, equivalent to 1.5 mg/kg bw per day, on the basis of clinical
signs. The reduced food intake of bitches at the intermediate dose was
not considered relevant (Hoffman & Schilde, 1980).
(d) Genotoxicity
The results of assays for the genotoxicity of methiocarb are
shown in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 10 female FB 30 rats (Elberfield breed)
received technical-grade methiocarb (purity, 99%) admixed with the
diet at concentrations of 0, 30, 100, or 300 ppm, equivalent to 3, 10,
and 30 mg/kg bw per day. The rats were weighed weekly. The F0
generation was mated twice, first after 70 days of treatment and again
after 149 days, to produce the F1a litters, which were sacrificed,
and the F1b litters. Ten males and 10 females from each group of F1b
rats were used to produce the next generation and were again mated
twice. The resultant F2a rats were sacrificed, while 10 male and 10
female F2b rats were mated twice to produce the F3a and F3b
generations, which were sacrificed. The rats were weighed weekly, and
the body weights of the offspring were measured at birth, five days
after birth, one week after birth, and then weekly. The pups were
examined grossly for malformations immediately after birth and during
lactation. The F3a young were killed four weeks after birth and the
F3b rats at three weeks. Blood samples were taken for analysis from
the F0 generation at the end of the preliminary treatment period and
after rearing of the second little (Löser, 1969).
In the F0 generation, there was no significant difference in
weight gain between the groups, and no treatment-related changes were
seen in haematological parameters at either sampling time. Although
ALAT and aspartate aminotransferase activities were higher in animals
at the highest dose at the earlier sampling time, they were stated to
be within the normal range for the laboratory; similar increases were
not seen at the later sampling time. The gestation rate was lower at
the highest dietary concentration, but was still within the normal
range. The weight gain of F1a pups was similar in all groups, and no
malformations were seen at sacrifice. After the second mating, there
were no dose-related changes in litter size or pup weight and weight
gain. No significant differences were seen between the groups of F1b
pups. Their weight gain after weaning was similar in all groups. After
the first mating, the gestation rate was lower at the highest dietary
concentration but was still within the normal range. The weight gain
of F2a pups was similar in all groups, and no malformations were seen
at sacrifice. After the second mating, the mean weight of the group at
300 ppm was significantly lower than that of controls at the end of
the four-week lactation period; however, there were no significant
intergroup differences in body weight at sacrifice of the F2b
animals. After both matings of these rats, the gestation rate was
similar and the litter size and pup weights were comparable in all
Table 2. Results of assays for the genotoxicity of methiocarb
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 4-2500 µg/plate 98.5 Negative Herbold (1978)
TA1535, TA1537,
TA98, TA100
Reverse mutationa S. typhimurium 20-12 500 µg/plate 98.4 Negative Herbold (1986)
TA1535, TA1537,
TA98, TA100
DNA damagea E. coli pol A+ and 625-10000 µg/plate 98.6 Negative Herbold (1983)
pol A-
Gene mutation Chinese hamster 1.25-60 µg/ml 99.3 Negative Lehn (1989)
ovary cells, hprt
Unscheduled DNA Rat primary 0.1-100 µg/ml 98.8 Negative Curren (1988)
synthesis hepatocytes
Chromosomal Chinese hamster 4.92-497 µg/ml 99.4 Positive Murli (1990)
aberrationa ovary cells
Sister chromatid Chinese hamster 2-40 µg/ml 98.2 Negative Putman (1986)
exchangea ovary cells
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
Micronucleus Mice 5,10, and 20 98.5 Negative Herbold (1979a)
formation mg/kg bw
twice orally
Dominant lethal Mice 6 mg/kg bw 98.5 Negative Herbold (1979b)
mutation
a With and without metabolic activation
groups. The body weights of the F3b, but not the F3a, groups at 100
and 300 ppm during lactation were lower than those of controls. This
finding may be due to larger litter size. No abnormalities were found
at birth or sacrifice in F3a or F3b pups, and no abnormalities were
found at autopsy of the F0, F1b, and F2b generations. The NOAEL was
300 ppm, equivalent to 30 mg/kg bw per day, the highest dose tested
(Löser (1970).
(ii) Developmental toxicity
Rats
Groups of 19-20 fertilized FB 30 rats received 10 oral doses of
0, 1, 3, or 10 mg/kg bw per day methiocarb (purity, 98.9%) by gavage
on days 6-15 of gestation. The highest dose reduced weight gain, but
no other effects were observed. No effects were seen on the number of
implantations or resorptions or the weights of fetuses or placentas.
No teratogenic effects were seen, nor was fetotoxicity observed. The
NOAEL for maternal toxicity was 3 mg/kg bw per day, and that for fetal
toxicity was 10 mg/kg bw per day, the highest dose tested (Lorke,
1971; see also Renhof, 1988).
Rabbits
Methiocarb (purity, 97.3%) was administered by gavage to groups
of 17 pregnant New Zealand white rabbits at doses of 1, 3, or 10 mg/kg
bw per day on days 6-18 of gestation. A group of 19 rabbits were used
as controls. On day 29 of gestation, the rabbits were sacrificed and
examined; the uterus was weighed, and the numbers of corpora lutea,
implantation sites, and resorption sites, and the number and
distribution of live and dead fetuses were noted. The weight and sex
of the fetuses and placental weights were recorded and the fetuses
examined for abnormalities. Rabbits receiving the highest dose showed
clinical signs of cholinergic poisoning and initial marked weight
loss. Weight gain was also reduced at 3 mg/kg bw per day towards the
end of the study, but this was attributable to a single animal. Weight
gain was unaffected at the lowest dose. The litter parameters were
similar in all groups, and there was no indication of teratological
effects. The NOAEL for maternal toxicity (clinical effects and weight
loss at the highest dose) was 3 mg/kg bw per day . The NOAEL for
fetotoxicity was 10 mg/kg bw per day, the highest dose tested (Tesh et
al., 1981).
Methiocarb (purity, 99.4-99.6%) was applied under occlusion for
6 h/day to about 10% of the body area of groups of 16 mated chinchilla
rabbits at doses of 0, 10, 50, or 250 mg/kg bw per day on days 6-18
post coitum. The animals were sacrificed after 28 days and the fetuses
removed. One animal at the high dose was sacrificed because of a
broken femur. No clinical signs were observed at any dose, nor was
irritation of the skin noted. At the highest dose, reduced food
consumption was observed between 11 and 15 days post coitum and
body-weight loss between 6 and 8 days post coitum, whereas at the
lower doses,no such effect was seen. The numbers of corpora lutea,
implantations, pre- and post-implantation losses, and live fetuses
were not affected by treatment. There was a slight decrease in mean
fetal weight at the highest dose (4%), and slight retardation of
skeletal development was noted at this dose, as shown by incomplete or
no ossification of the phalangeal nuclei. No effects on the fetuses
were seen at lower doses. The NOAEL for both maternal and fetal
toxicity was 50 mg/kg bw per day (Dotti & Biedermann, 1992).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
(ii) Neurotoxicity
In a poorly described study, no persistent paralytic effects were
produced in hens (Gallus gallus) of unstated strain given a single
dose of methiocarb by gavage and observed for 14 days (DuBois, 1962).
Methiocarb (purity, 98.5%) was administered twice at a dose of
380 mg/kg bw (approximately the LD50) to 20 white Leghorn laying
hens, aged 15-20 months, after an intramuscular injection of 50 mg/kg
bw atropine sulfate. After an interval of 21 days, the 18 surviving
hens were treated again, with the same doses of atropine and
methiocarb. The 16 survivors of the second treatment were sacrificed
three weeks later, and brain, spinal cord, and portions of peripheral
nerve were excised and processed for histological examination. Five
positive controls were given tri- ortho-cresyl phosphate as a single
oral dose and sacrificed three weeks later. The hens treated with
methiocarb exhibited brief cholinergic signs and were lethargic, but
none showed ataxia or paralysis. At examination, post mortem there
were no histopathological changes indicative of polyneuropathy in
either the central or peripheral nervous system, whereas the hens
treated with tri- ortho-cresyl phosphate showed the classical signs
of delayed polyneuropathy and mild but characteristic signs of delayed
polyneuropathy on histopathological examination (Thyssen & Schilde,
1978).
(iii) Antidotes
A study was carried out in which the effect of antidotes to an
oral LD50 of methiocarb was investigated in rats. The regimens used
were atropine at 50 mg/kg bw, pralidoxime (salt unstated) at 50 mg/kg
bw, obidoxime at 20 mg/kg bw, atropine and pralidoxime at 50 mg/kg bw
each, or atropine at 50 mg/kg bw with obidoxime at 20 mg/kg bw. All
the antidotes were given intraperitoneally just before clinical signs
became evident, i.e. 1-2 min after administration of methiocarb. The
LD50 values were 67 mg/kg bw without antidotes, 470 mg/kg bw with
atropine, 190 mg/kg bw with pralidoxime, 220 mg/kg bw with obidoxime,
500 mg/kg bw with pralidoxime and atropine, and 510 mg/kg bw with
obidoxime and atropine. Thus, pralidoxime and obidoxime added little
to the antidotal effect of atropine (Kimmerle, 1966a).
In mice, 10 mg/kg bw of atropine increased the LD50 of
methiocarb by sevenfold, while pralidoxime methiodide at 100 mg/kg bw
increased the LD50 1.3 times. Administration of the two antidotes
together increased the LD50 by 21 times. Obidoxime at 50 mg/kg bw
increased the LD50 3.1 times, while obidoxime plus atropine increased
it by 9.1 times (Kohgo, 1970).
Atropine sulfate at 50 mg/kg bw administered within 10 min of
methiocarb intraperitoneally increased the LD50 of methiocarb given
orally to rats by more than sixfold, and tetraethyl ammonium chloride
given by the same route increased it by approximately fourfold;
however, the combined effect of the two antidotes was less than that
of atropine alone (Kimmerle, 1971).
In the study of Thyssen and Schilde (1978) in hens at
approximately the LD50 (see above), atropine appeared to have a
powerful antidotal effect.
(iv) Interaction with other pesticides
When an intraperitoneal dose equal to 50% of the LD50 of
methiocarb was given to female Sprague-Dawley rats in combination with
another pesticide at a dose equal to 50% of the LD50, the combined
mortality was less than 50% in all cases. The highest combined
mortality rates (> 40 to < 50%) were seen with EPN, Guthion,
mevinphos, and carbaryl. Combined mortality rates of > 20 to < 40%
were found with parathion, parathion-methyl, disulfoton, malathion,
carbophenothion, tributyl phosphorotrithioite, and ethion. The
mortality rates seen with the remaining insecticides (demeton,
dioxathion, and schradan) were 5-20% (DuBois & Raymund, 1961b). In
another study of similar design, the combined mortality rates were 55%
with trichlorfon, 50% with propoxur, 35% with coumaphos, 30% with
oxydemeton-methyl, and 20% with fenthion (DuBois & Raymund, 1961c).
3. Observations in humans
The 250 workers in two plants manufacturing methiocarb over about
20 years were subjected to annual medical examinations and assays of
cholinesterase activity in whole blood. No adverse health effects or
changes in laboratory parameters were observed (Faul, 1993).
Comments
Methiocarb appeared to have been well absorbed in a small study
of absorption, distribution, metabolism, and excretion of the
radiolabelled compound in rats. More than 70% of the administered
radiolabel was excreted within 48 h, mostly in the urine. Methiocarb
was extensively metabolized. The main route of metabolism in both
animals and plants appears to be to methiocarb phenol, methiocarb
phenol sulfoxide, and methiocarb phenol sulfone. In some studies,
methiocarb sulfoxide was also found.
In single-dose and short-term studies in rats, methiocarb
administered by gavage inhibited plasma and erythrocyte cholinesterase
activity. In the short-term study, plasma, erythrocyte, and brain
cholinesterase activities were depressed at 10 mg/kg bw per day; the
NOAEL for this effect was 3 mg/kg bw per day. In a short-term study of
the ability of methiocarb or its sulfoxide to inhibit cholinesterase
activity in rats, the NOAEL for inhibition of erythrocyte
acetylcholinesterase activity was 0.5 mg/kg bw per day for methiocarb,
but a NOAEL was not identified for the sulfoxide. In a 29-day study in
which dogs were given methiocarb or methiocarb sulfoxide in gelatine
capsules at 0.05 or 0.5 mg/kg bw per day, both plasma and erythrocyte
cholinesterase activities were inhibited by both treatments.
The acute toxicity of methiocarb given by a number of routes has
been measured in a number of species. The oral LD50 in fasting rats
ranged from 13 to 130 mg/kg bw. The oral LD50 values for methiocarb
phenol, methiocarb phenol sulfoxide, and methiocarb phenol sulfone in
rats are all greater than 1 g/kg bw, as is that of methiocarb sulfone,
but that of methiocarb sulfoxide is between 6 and 43 mg/kg bw.
WHO has classified methiocarb as moderately hazardous (WHO,
1996).
The short-term toxicity of methiocarb has been tested in rats,
rabbits, cats, dogs, and chickens. Few of these studies were
appropriate for identifying NOAEL values and, of those that were, a
study in rats was carried out by inhalation and that in rabbits by
dermal application. In the study in rats, which were exposed by
inhalation, for five days per week for three weeks, no findings
related to treatment were seen on histopathological examination but
depressed brain acetyl-cholinesterase activity was observed at the
highest dose in animals of each sex and in males at the intermediate
dose. Consequently, the NOAEL was 6 mg/m3 per day. In a 21-day study
of the dermal toxicity of methiocarb in rabbits, the substance was
applied for 6 h/day at 0, 60, 150, or 375 mg/kg bw per day. Plasma
cholinesterase activity was reduced in males at the highest dose, but
no significant differences were seen between groups in the activities
of erythrocyte and brain acetylcholinesterase. The NOAEL was 150 mg/kg
bw per day on the basis of reduced food consumption at the highest
dose.
In a long-term study of toxicity in mice, methiocarb was
administered at dietary concentrations of 0, 67, 200, or 600 ppm. A
NOAEL was not identified because haematological changes were observed
in all treated males at 12 months and in all treated females at 24
months. The LOAEL was 67 ppm, equal to 15 mg/kg bw per day, on the
basis of minor haematological changes. Methiocarb was not carcinogenic
in mice.
In a two-year study of toxicity, rats received methiocarb at
dietary concentrations of 0, 67, 200, or 600 ppm. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months. Methiocarb was not carcinogenic.
In a two-year study of toxicity, dogs were fed methiocarb in the
diet at 0, 5, 60, or 240 ppm. The NOAEL was 60 ppm, equivalent to 1.5
mg/kg bw per day, on the basis of reversible clinical signs at the
next highest dose, which were not observed after 15 weeks.
Three studies of developmental toxicity were available, one in
rats and two in rabbits. Fertilized rats received methiocarb by gavage
on days 6-15 of gestation at 0, 1, 3, or 10 mg/kg bw per day. The
NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of
reduced body-weight gain at the highest dose. As no fetotoxicity was
observed, the NOAEL for this end-point was 10 mg/kg bw per day, the
highest dose tested. In a study of developmental toxicity in rabbits,
pregnant animals received methiocarb at 0, 1, 3, or 10 mg/kg bw per
day by gavage on days 6-18 of gestation. The NOAEL was 3 mg/kg bw per
day for maternal toxicity on the basis of clinical effects and weight
loss at the highest dose. As no fetotoxicity or teratogenicity was
observed, the NOAEL for these end-points was 10 mg/kg bw per day, the
highest dose tested. In a further study, rabbits received methiocarb
by dermal application at 0, 10, 50, or 250 mg/kg bw per day for
6 h/day on days 6-18 of gestation. The NOAEL for both maternal and
fetal toxicity was 50 mg/kg bw per day on the basis of reduced
maternal food consumption and some decrease in mean fetal weight at
the highest dose; slight retardation of fetal development was also
observed. A multigeneration study of reproductive toxicity was
undertaken in rats given methiocarb at dietary concentrations of 0,
30, 100, or 300 ppm. The NOAEL was 300 ppm, equivalent to 30 mg/kg bw
per day, as no effect clearly related to treatment was observed. No
teratogenic effect was observed in any of these studies.
Methiocarb has been tested for genotoxicity in an adequate
battery of tests in vitro and in vivo. The Meeting concluded that
methiocarb is not genotoxic.
Methiocarb did not cause skin sensitization in studies conducted
by either the Magnusson and Kligman or the Beuhler technique.
In an early study of neurotoxicity in hens, methiocarb did not
cause delayed polyneuropathy of the organophosphorus type. Atropine
has consistently been shown to be an effective antidote for
methiocarb, while the effects of pyridinium oximes were somewhat
inconsistent.
The Meeting established an ADI of 0-0.02 mg/kg bw on the basis of
the NOAEL of 1.5 mg/kg bw per day in the two-year study of toxicity in
dogs and a safety factor of 100. This ADI results in a further safety
factor of 10 on the LOAEL in the long-term study of toxicity in mice.
An acute RfD was allocated on the basis of the NOAEL of 1.5 mg/kg
bw per day in the two-year study in dogs, because the signs observed
were acute, and a safety factor of 100. Of the shorter studies in
dogs, neither the 29-day nor the 12-week study was considered by the
Meeting to be adequate for the purpose of establishing a NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: No NOAEL; LOAEL: 67 ppm, equal to 15 mg/kg bw per
day (long-term study of toxicity)
Rat: 67 ppm, equal to 3.3 mg/kg bw per day (long-term
study of toxicity)
300 ppm, equivalent to 30 mg/kg bw per day
(maternal and fetal toxicity in a study of
reproductive toxicity)
3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (two-
year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would be useful for continued evaluation of the
compound
1. A modern study of absorption, distribution, metabolism, and
excretion
2. A modern multigeneration study of reproductive toxicity
3. Further observations in humans
List of end points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption No data
Dermal absorption No data
Distribution No data
Potential for accumulation Not likely to accumulate
Rate and extent of excretion 73 to > 90% within 48 h
Metabolism in animals Sulfoxidation and loss of carbamate side-chain
Toxicologically significant compounds Parent compound and methiocarb sulfoxide
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral 13-135 mg/kg bw
Rat: LD50, dermal 350 to > 5000 mg/kg bw
Rat: LC50 inhalation > 300 mg/m3
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Clinical signs
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 150 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 6 mg/m3
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Clinical signs
Lowest relevant NOAEL Dog: 1.5 mg/kg bw per day
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect No effect
Lowest relevant reproductive NOAEL Rat: 30 mg/kg bw per day
Developmental target/critical effect Maternal toxicity: reduced weight gain; no
fetotoxicity observed
Lowest relevant developmental NOAEL Rabbit: 3 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Does not cause delayed polyneuropathy
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Dog, 2 years 100
Acute reference dose 0.02 mg/kg bw Dog, 2 years 100
References
Baron, R.L., Casterline, J.L. & Fitzhugh, O.G. (1964) Specificity of
carbamate esterase inhibition in mice. Toxicol. Appl. Pharmacol., 6,
402-410.
Barthová, J., Woldemariam, M.G. & Leblová, S. (1989) The effect of
carbamates insecticides on cholinesterases of nontarget organisms.
Biologia (Bratislava), 44, 353-356.
Crawford, C.R. & Anderson, R.H. (1970) The skin and eye irritating
properties of BAY 37344 technical to rabbits. Unpublished report No.
28304 from Chemagro Corporation, Research Department, 15 October 1970.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972a) The acute oral toxicity to
guinea pigs and the acute inhalation toxicity to rats of Mesurol(R)
technical. Unpublished report No. 31987 from Chemagro Corporation,
Research Department, 24 January 1972. Submitted to WHO by Bayer AG
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972b) The dermal toxicity of
(R)Mesurol technical and Mesurol 75% wettable powder to rabbits.
Unpublished report No. 34477 from Chemagro Division of Baychem
Corporation, Research Development, 21 August 1972. Submitted to WHO by
Bayer AG Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral toxicity of
(R)Mesurol technical to rats. Unpublished report No. 34008 from
Chemagro Division of Baychem Corporation, Research and Development, 31
May 1972 and revised 8 May 1973. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 1007 from Microbiological
Associates Inc., Bethesda, Maryland and Rockville, Maryland, USA, 1
June 1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
David, R.M. (1988) EPA/OECD dermal sensitization test of Mesurol
technical in guinea pigs. Unpublished report No. 1015 from
Microbiological Associates, Inc., Bethesda, Maryland, USA, 15 April
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dotti, A. & Biedermann, K. (1992) Embryotoxicity study (including
teratogenicity) with H 321 (c.n. methiocarb) in the rabbit (dermal
application) part 1. Unpublished report No. R 5627 from RCC Research &
Consulting Co. Ltd and RCC Umweltchemie AG, Itingen, Switzerland, 6
August 1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Doull, J., Cowan, J. & Root, M. (1962) Subacute (16-week) oral
toxicity of Bayer 37344 to male and female rats. Unpublished report
No. 9456 from Department of Pharmacology, University of Chicago, USA,
12 June 1962. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. (1962) The acute oral toxicity of Bayer 37344 to
chickens. Unpublished report No. 9248 from Department of Pharmacology,
University of Chicago, USA, 16 May 1962. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K.P. (1964) The acute toxicity of some possible metabolites of
Bayer 37344. Unpublished report No. 12806 from Department of
Pharmacology, University of Chicago, USA, 22 January 1964. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961a) The acute toxicity of Bayer 37344
to mammals. Unpublished report No. 6775 from Department of
Pharmacology, University of Chicago, USA, 25 April 1961. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961b) The acute toxicity of Bayer 37344
in combination with other anticholinesterase insecticides to rats.
Unpublished report No. 7880 from Department of Pharmacology,
University of Chicago, USA, 1 November 1961. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961c) The acute toxicity of Bayer 39007
and Bayer 37344 in combination with some other anticholinesterase
insecticides in rats. Unpublished report No. 7879 from Department of
Pharmacology, University of Chicago, USA, 4 November 1961. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1962) The acute toxicity of
recrystallised Bayer 37344 and a wettable powder of Bayer 37344 to
female rats. Unpublished report No. 9424 from Department of
Pharmacology, University of Chicago, USA, 12 June 1962. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Eben, A. & Kimmerle, G. (1973) Mercaptodimethur effect of acute and
subacute oral doses on acetylcholinesterase activity in plasma,
erythrocyte and brain of rats. Unpublished report No. 4284 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 9 November
1973. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Faul, J. (1993) Mesurol active ingredient: In-company occupational
medical experience. Unpublished letter to Dr Heimann, PF-A/consulting,
Monheim, Germany, 1 April 1993. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50). Unpublished
letter to Dr Reuver, Bayer AG Institute of Toxicology, dated 27 June
1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) H 321 (mercaptodimethur). Unpublished note dated 3
November 1980. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1988) Determination of acute toxicity (LD50), Unpublished
letter to Dr Reuver, Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany, dated 1 August 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Haijar, N.P. & Hodgson, E. (1982) Sulfoxidation of
thioether-containing pesticides by the flavin-adenine
dinucleotide-dependent monooxygenase of pig liver microsomes.
Biochem. Pharmacol., 31, 745-752.
Hayes, R.H. (1981) Cholinesterase evaluation study of methiocarb
technical and methiocarb sulfoxide in dogs. Unpublished report No. 202
from Corporate Toxicology Department, Mobay Chemical Corporation,
Stillwell, Kansas, USA, 29 September 1981. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1983) Determination of acute toxicity (LD50).
Unpublished letter to Dr Reuver, PF-Zentrum, Monheim, Germany, dated
22 April 1983. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1978) H 321 (active ingredient of Mesurol)
Salmonella/microsome test for determination of point mutations.
Unpublished report No. 7978 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 6 December 1978. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B. (1979a) H 321 (active ingredient of Mesurol) micronucleus
test for mutagenic effect on mice. Unpublished report No. 8426 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 8
June 1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979b) H 321 dominant lethal study on male mouse to test
for mutagenic effects. Unpublished report No. 8395 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 23 May 1979.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) H 321 mercaptodimethur (the active ingredient of
Mesurol(R)) study of DNA damage using the E. coli pol A- test.
Unpublished report No. 11928 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 13 July 1983. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) H 321 c.n. mercaptodimethur Salmonella/microsome
test to evaluate for point mutagenic effect. Unpublished report No.
14205 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 10 January 1986. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1981) Cholinesterase no-effect level of Mesurol(R) and
Mesurol sulfoxide(R) in female rats. Unpublished report No. 199 from
Mobay Chemical Corporation, Corporate Toxicology Department, Stilwell,
Kansas, USA, 31 August 1981. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. & Schilde, B. (1980) H 321(Mesuro(R) active ingredient
-- mercaptodimethur) chronic toxicity study in dogs (2 year feeding
experiment). Unpublished report No. 9626 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 4 December 1980. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1960) Product Dr Wedemeyer H 321 (E 37 344) Production
No. 2410. Unpublished report from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 25 March 1960. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1966a) Mesurol active ingredient (Wedemeyer H 321; Ht.
No. 3657) -- antidotal effect. Unpublished letter to Crop Protection
Science, Leverkusen. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, dated 8 August 1966. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1966b) Re active ingredient Wedemeyer H 321 (Ht No
3657). Inhalation tests. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany. Unpublished letter to Crop Protection
Science, Leverkusen, Germany, dated 7 December 1966. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969a) Re Bay 37 344 (H 321) Lo No. 691. Unpublished
letter to Crop Protection Science, Leverkusen. Bayer AG, Institute for
Toxicology, Wuppertal-Elbberfeld, Germany, dates 20 May. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969b) Ref Bay 37344 (=H 321 = Methiocarb) Lot No. 691.
Unpublished letter to Crop Protection Science, Leverkusen. Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, dated 20 May.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969c) Bay 37 344 subacute dermal toxicity study on
rabbits. Unpublished report No. 1291 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 1 April 1969. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetraethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol., 27, 311-314.
Kimmerle, G. (1972) Unpublished note from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, dated 7 June 1972. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Klimmer, O.R. (1963) Toxicological testing of Bayer 37 344.
Unpublished letter from Pharmacological Institute of the University of
Bonn, Germany, to Crop Protection/Scientific Department, Leverkusen,
Germany, dated 10 December. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kohgo, L. (1970) The antagonistic effects of atropine or pyridinium
aldoxime derivatives on cholinesterase-inhibitory pesticides in male
and mice. J. Med. Soc. Toho Jpn, 17, 60-88.
Krötlinger, F. (1989) H 321 (mercaptodimethur, active ingredient of
Mesurol) chronic toxicological study on mice (feeding study over 2
year). Unpublished addendum No. 11908A to report No. 11908 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 15 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. (1990) H 321 (mercaptodimethur, the active ingredient
of Mesurol) chronic toxicity study on rats (2-year feeding
experiment). Unpublished addendum No. 10039A to report No. 10039 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 16
February 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. & Janda, B. (1983) H 321 (mercaptodimethur, the active
ingredient of Mesurol(R)) chronic toxicity study on mice (2-year
feeding experiment). Unpublished report No. 11908 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 4 July 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Löser, E. & Vogel, O. (1981) H 321 (mercaptodimethur,
the active ingredient of Mesurol) chronic toxicity study on rats
(2-year feeding experiment). Unpublished report No. 10039 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 2 July
1981. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of mesurol
technical to dogs. Unpublished note of experiment No. 450474 from
Mobay Chemical Corp., Chemagro Agricultural Division, dated 25 August
1975. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976a) The acute oral toxicities of
mesurol technical and mesurol sulfoxide. Unpublished note of
experiment No. 49541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 12 July 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976b) The acute oral toxicities of
Mesurol(R) technical and mesurol sulfoxide. Unpublished note of
experiment No. 50541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 19 August 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) A comparison of the acute oral
toxicities of Mesurol(R) technical to fasted and non-fasted rats.
Unpublished note of experiment No. 50541 from Mobay Chemical Corp.,
Chemagro Agricultural Division, dated 19 January 1977. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1989) H 321 C.N. Methiocarb mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay in
vitro. Unpublished report No. 18280 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 15 August 1989. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lorke, D. (1971) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished report No.
3133 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 30 November 1971. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1969) Bay 37 344. Blood analyses, urinalysis and clinical
chemistry examinations following oral administration rats. Unpublished
report No. 1358 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 3 March 1969. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Löser, E. (1970) Bay 37 344. Generation studies on rats. Unpublished
report No. 2208 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 10 July 1970. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Menzie, C.M. (1974) Metabolism of Pesticides: An Update (Wildlife
No. 184), Washington DC, US Department of the Interior Fish and
Wildlife Service, p. 249.
Mihail, F. (1984) H 321 (C.n. mercaptodimethur) study for
skin-sensitising effect on guinea pigs. Unpublished report No. 13125
from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany,
12 December 1984. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murli, H. (1990) Mutagenicity test on H 321 in an in vitro cytogenetic
assay measuring chromosomal abberation frequencies in Chinese hamster
ovary (CHO) cells with a confirmatory assay. Unpublished report No. R
4980 from Hazleton Laboratories America Inc., Kensingtonb Maryland,
USA, 8 March 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murphy, J.J., Timme, G. & Dräger, G. (1982) Mesurol(R) common name:
methiocarb, mercaptodimethur. Behaviour in plants. Unpublished report
from Mobay Chemical Corp., Kansas, USA, August 1982. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Nelson, D.L. (1979) Acute oral toxicity of Mesurol(R) technical and
metabolites to rats. Unpublished report No. 67815 from Mobay Chemical
Corp., Environmental Health Research, Stillwell, Kansas, USA, 27 June
1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1988) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1084 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 23 November
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1989) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1160 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 31 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1986) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells test article Mesurol technical. Unpublished report
No. 790 from Microbiological Associates Inc., Bethesda, Maryland, USA,
25 September 1986. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished addendum No.
3133A to report No. 3133 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 21 October 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Root, M., Doull, J. & Cowan, J. (1963) Determination of the safe
dietary level of Bayer 37344 for dogs. Unpublished report No. 11159
from Department of Pharmacology, University of Chicago, USA, 23 March
1963.. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Salama, A.K., Bakry, N.M. & Abou-Donia, M.B. (1993) A review article
on placental transfer of pesticides. J. Occup. Med. Toxicol., 2,
383-397.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Mesurol in rats. Unpublished report No. 940 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 31 August 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b) Acute four-hour inhalation toxicity study with
Mesurol(R) technical in rats. Unpublished report No. 870 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 18 June 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1969) Mesurol(R) active ingredient -- acute cutaneous
toxicity in rats. Unpublished letter to Bayer AG, Institute of
Toxicology, Crop Protection Science, Leverkusen, Germany, dated 11
September 1969. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1970) Oral toxicity of Mesurol metabolites to rats.
Unpublished notes addressed to Bayer AG, Institute of Toxicology, Crop
Protection Science, Leverkusen, Germany, dated 6 April 1970. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stanley, C.W. & Johnson, G.A. (1976) Metabolites of Mesurol(R) in
rat urine. Unpublished report No. MR50732 from Chemagro Agricultural
Division, Mobay Chemical Corp., USA, 16 November 1976. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Strankowski, K.J. & Minor, R.G. (1976) Effect of feeding
Mesurol(R)/Mesurol sulfoxide (9:1) to chickens for 28 days.
Unpublished report No. 49639 from Mobay Chemical Corp., Research and
Development Department, USA, 7 January 1976. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Strother, A. (1970) Comparative metabolism of N-methylcarbamates by
human and rat liver fractions. Biochem. Pharmacol., 19, 2525-2529.
Strother, A. (1972) In vitro metabolism of methylcarbamate
insecticides by human and rat liver fraction. Toxicol. Appl.
Pharmacol., 21, 112-129.
Tesh, J.M., Ross, F.W., Secker, R.C. & Wilby, O.K. (1981) H 321:
Effects of oral administration upon pregnancy in the rabbit. 2. main
study. Unpublished report No. 2173 from Life Science Research, Stock,
United Kingdom, 4 December 1981. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977a) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 15 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977b) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 30 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) H 321 (Mesurol active ingredient) acute inhalation
toxicity. Unpublished report No. 11113 from Bayer AG Institute of
Toxicology, 27 August 1982. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen, J. & Mohr, U. (1983) H 321 (Mesurol active ingredient)
subacute inhalation study with rats. Unpublished report No. 12120 from
Bayer AG Institute of Toxicology and Department of Experiment
Pathology, Hanover Medical University, Germany, 30 September 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Schilde, B. (1978) H 321 Neurotoxicity study on hens.
Unpublished report No. 7637 from Bayer Institute of Toxicology, 20
June 1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Wheeler, L. & Strother, A. (1971) In vitro metabolism of the
N-methylcarbamates, Zectran and Mesurol, by liver, kidney and blood of
dogs and rats. J. Pharm. Exp. Ther., 178, 371-382.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Willems, P.W.J.M., Geursen-Reitsma, A.M. & van Joost, T. (1997)
Allergic contact dermatitis due to methiocarb (Mesurol). Contact
Derm., 36, 270-271.
0.5 mg/kg bw; 4 h after dosing at 0.5 mg/kg bw, depressions of < 10%
were noted. At the higher dose of methiocarb, depressions of 19-41%
were seen 30 min after dosing and 0.2-19% 4 h after dosing. With the
sulfoxide, plasma cholinesterase activity was inhibited by 21-39% at
the low dose at 30 min but was generally not inhibited at 4 h. At the
high dose, 39-62% inhibition was seen at 0.5 h and 10-25% inhibition
at 4 h. Erythrocyte acetylcholinesterase activity was depressed by
5-12% 30 min after dosing at 0.5 mg/kg bw and by < 10% 4 h after
dosing. At the higher dose of methiocarb, erythrocyte
acetylcholinesterase activity was depressed by 15-29% 30 min after
dosing and by 1-13% 4 h after dosing. With the sulfoxide, erythrocyte
acetylcholinesterase activity was inhibited by 13-31% at the low dose
at 30 min but was generally not inhibited at 4 h. At the high dose,
32-46% inhibition was seen at 0.5 h and 10-21% at 4 h. The NOAEL for
erythrocyte acetylcholinesterase inhibition was therefore
approximately 0.5 mg/kg bw per day for methiocarb, but there was no
NOAEL for the sulfoxide (Hixson, 1981).
Technical-grade methiocarb (purity, 97%) and methiocarb sulfoxide
(purity, 95.2%) were administered orally in gelatine capsules to
groups of two adult beagle dogs and two bitches at a dose of 0.05 or
0.5 mg/kg bw per day for 29 days; two control animals of each sex
received empty capsules. The animals were observed twice daily, and
blood was taken for measurement of plasma and erythrocyte
cholinesterase activity before the first dose, 1, 3, 6, and 24 h after
the first dose, and 3 h after the third dose. During the second week,
blood was taken before the first dose, 2, 6, and 24 h later, and 2 h
after the second dose. During weeks 3 and 4, blood was taken before
the first dose, 2, 6, and 24 h later, and 2 h after the third dose. In
week 5, blood was again taken before the first dose and 2, 6, and 24 h
later; dosing was then stopped, and another blood sample was taken on
the third day.
Clinical signs of toxicity (salivation and vomiting) were
observed at the higher dose of each test material and in animals of
each sex; additionally, slight salivation was observed in one bitch
given the sulfoxide at the lower dose. Depression of cholinesterase
activity was calculated by comparison with the level measured just
before the first dose each week: inhibition was variable, but peak
inhibition occurred 0-3 h after dosing; considerable inhibition was
seen with both materials at the higher dose in animals of each sex. At
the lower doses, smaller depressions of both plasma and erythrocyte
cholinesterase activity, of up to about 20%, were sometimes seen with
both materials. Cholinesterase activity was generally normal by 6 h
(Hayes, 1981).
At equimolar concentrations, methiocarb was the least effective
of four carbamates in inhibiting bovine erythrocyte
acetycholinesterase and equine plasma cholinesterase activity
(Barthová et al., 1989).
Methiocarb was reported not affect liver function in rabbits at a
single oral dose of 25 mg/kg bw (Kimmerle, 1960).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of methiocarb and
putative metabolites of methiocarb are shown in Table 1.
No erythema or oedema was seen when technical-grade methiocarb
(purity unspecified) was applied to abraded and unabraded skin of six
New Zealand white rabbits. Application to the eyes of six rabbits did
not produce ocular irritation (Crawford & Anderson, 1970).
A study of the skin-sensitizing potential of methiocarb (purity,
97.8% pure) was conducted by the Magnusson and Kligman technique. A
group of 20 guinea-pigs (strain BOR:DHPW) each received methiocarb
intradermally at a concentration of 1% and topically at a
concentration of 25%. There were two control groups of 10 guinea-pigs.
The test animals were first challenged with methiocarb at a
concentration of 25% and then at 12.5%. Although there was a small
excess in the number of animals that reacted positively after the
second challenge, the overall result was considered to be negative
(Mihail, 1984).
In a test for dermal sensitization by the Buehler technique,
technical-grade methiocarb (purity, 99.2%) was applied to 15 Hartley
guinea-pigs once weekly for three weeks, with a challenge dose a
fortnight later. The material was applied as a single dose to five
previously unexposed animals at the time of the challenge dose.
Dinitrochlorobenzene, used as the positive control, was applied in the
same way to a further group of five guinea-pigs, weekly for three
weeks, with a challenge dose two weeks later, and to a further group
of five as a single dose at the time of the challenge dose. Methiocarb
was not considered to be a dermal sensitizer (David, 1988).
A case of allergic contact dermatitis was reported in a man who
grew carnations. He developed acute severe eczema of the hand and
showed a positive reaction in a patch test to 0.5% methiocarb (Willems
et al., 1997).
(b) Short-term studies of toxicity
Rats
Methiocarb in aqueous tragacanth suspension was given by gavage
to albino rats daily. The dose was 2 mg/kg bw per day for the first
three days and 4 mg/kg bw per day for the next 24 days. Two groups of
three animals were killed every week for determination of
cholinesterase activity. The activity had fallen to 80% of the control
values after 14 days and to 50% by the end of the study. No abnormal
clinical signs were observed; the animals gained weight normally
(Kimmerle, 1960).
Table 1. Acute toxicity of methiocarb and putative metabolites
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb
Rats Sprague-Dawley M Oral 130 Technical DuBois & Raymund (1962)
F 140
Rats Sprague-Dawley F Oral 100 Recrystallized DuBois & Raymund (1962)
Rats NR NR Oral 67 NR Kimmerle (1966a)
Rats Sprague-Dawley M Oral 30 (20-45) 99 Crawford & Anderson (1973)
F 30 (20-45)
Rats Sprague-Dawley M Oral (non-fasting) 46 (38-56) 99 Crawford & Anderson (1973)
F 47 (36-63)
Rats Sprague-Dawley M Oral 15 (9-26) Technical Lamb & Matzkanin (1976a)
F 31 (18-54)
Rats Sprague-Dawley M Oral 13 (9-17) Technical Lamb & Matzkanin (1976b)
F 32 (24-44)
Rats Sprague-Dawley M Oral 14 (12-16) Technical Lamb & Matzkanin (1977)
F 16 (13-20)
Rats Sprague-Dawley M Oral (non- 51 (45-58) Technical Lamb & Matzkanin (1977)
F 79 (65-96)
Rats NR M Oral 22 (19-25) 98.5 Flucke (1978)
F 24 (21-28)
Rats NR M Oral 22.1 (18-27) 98.3 Flucke (1980)
Rats Sprague-Dawley- M Oral 33 (22-50) 98 Nelson (1979)
derived F 47 (31-70)
Rats NR M Oral 17 (16-19) 98.6 Heimann (1983)
Rats NR M Oral 19 (16-23) 98.2 Flucke (1988)
F 26 (19-36)
Rats Albino M Oral 100 NR Kimmerle (1960)
Rats Wistar-CFN M Oral 87 NR Klimmer (1963)
Rats NR M Oral 33 (29-38) 98.4 Thyssen (1977a)
35 (29-42) 98.3
35 (30-42) 98.2
31 (26-35) 97.8 28 (24-33)97.4
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rats Sprague-Dawley M Intraperitoneal 35 Technical DuBois & Raymund (1961a)
F 30
Rats Sprague-Dawley F Intraperitoneal 25 Recrystallized DuBois & Raymund (1962)
Rats Wistar-CFN M Intraperitoneal 43 NR Klimmer (1963)
Rats Sprague-Dawley M Dermal > 200 Technical DuBois & Raymund (1961a)
Rats Sprague-Dawley F Dermal > 300 Recrystallized DuBois & Raymund (1962)
Rats Albino M Dermal > 1000 NR Kimmerle (1960)
Rats Wistar-CFN M Dermal 350-400 NR Klimmer (1963)
Rats NR M Dermal > 500 99.2 Solmecke (1969)
Rats Wistar M Dermal > 5 g 98.1 Thyssen (1977b)
F > 5
Rats Sprague-Dawley M Inhalation (1 h) 1200 mg/m3 a 98.8 Shiotsuka (1987a)
(780-1700)
F 1100 mg/m3 a
(740-1600)
Rats NR M Inhalation 540 g/L3 NR Kimmerle (1966b)
Rats Sprague-Dawley M Inhalation (1 h) > 20 000 g/L Technical Crawford & Anderson (1972a)
F > 20 000 g/L
Rats Sprague-Dawley M Inhalation (4 h) 580 mg/m3 (340-700) 98.8 Shiotsuka (1987b)
F 430 mg/m3 (290-580)
Rats Wistar M Inhalation (4 h) > 320 mg/m3 97.9 Thyssen (1982)
F > 320 mg/m3
Rats Wistar M Inhalation > 300 mg/m3 97.9 Thyssen (1982)
F (5 x 6 h) > 300 mg/m3
Mice NR M Oral 25 (21-30) NR Kohgo (1970)
Mice NR M Oral 52 98.2 Kimmerle (1972)
Mice NR M Subcutaneous 940 (760-1200) NR Kohgo (1970)
Mice Carworth Farm M Intraperitoneal 6 Technical DuBois & Raymund (1961a)
F 5.5
Mice Dierolf Farm F Intraperitoneal 16 Technical Baron et al. (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rabbits New Zealand M Dermal > 2000 99 Crawford & Anderson
white F > 2000 (1972b)
Guinea-pigs NR M Oral 40 Technical DuBois & Raymund (1961a)
Guinea-pigs NR F Oral (50-100) 99.2 Kimmerle (1969a)
Guinea-pigs Albino F Oral 14 (7.9-25) Technical Crawford & Anderson (1972a)
Guinea-pigs NR M Intraperitoneal 17 Technical DuBois & Raymund (1961a)
Dogs Beagle F Oral (10-25) 99.2 Kimmerle (1969b)
Dog Mongrel M Oral ~ 25 Technical Lamb & Matzkanin (1975)
F ~ 25
Chicken NR F Oral 175 Technical DuBois (1962)
Chicken White Leghorn F Oral 380 (300-490) 98.5 Thyssen & Schilde (1978)
Methiocarb phenol
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR M Dermal > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Methiocarb phenol sulfoxide
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Methiocarb phenol sulfone
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb sulfoxide
Rats NR NR Oral 43 (37-50) NR Solmecke (1970)
Rats Sprague-Dawley M Oral 9 (7-13) NR Lamb & Matzkanin (1976a)
F 7
Rats Sprague-Dawley M Oral 6 (5-8) NR Lamb & Matzkanin (1976a)
F 8 (6-10)
Methiocarb sulfone
Rats NR NR Oral > 1000 NR Solmecke (1970)
Hydroxymethyl methiocarb
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfone
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfoxide
Rats NR M Oral > 160 NR Nelson (1979)
F > 160
Technical, purity unstated; NR, not reported
a There was a large descrepancy between nominal and measured concentrations owing to deposition in the chamber;
these figures were measured gravimetrically.
Methiocarb was administered by gavage to groups of 25 male
Wistar-CFN rats at a dose of 2.5 or 5 mg/day on six days per week for
six months. Three deaths occurred at the higher dose due to
bronchopneumonia, but weight gain was the same in the two treated
groups and in 20 control animals; no abnormal clinical signs were seen
(Klimmer, 1963).
Groups of 12 Sprague-Dawley rats of each sex were fed diets
containing methiocarb (purity unspecified) at concentrations of 0, 5,
10, or 50 ppm for 16 weeks. Five animals of each sex from each group
were examined histopathologically, and five of each sex were used for
measurements of cholinesterase activity in blood, brain, and
submaxillary glands by a manometric method. No effect on growth rate,
food intake, or mortality rates was observed at any dose. In males at
the highest dose, serum, erythrocyte, brain, and submaxillary gland
cholinesterase activity was inhibited by 21, 14, 12, and 7%,
respectively. In females at the highest dose, serum cholinesterase
activity was reduced by 28% and erythrocyte acetylcholinesterase
activity by 15%, all by comparison with concurrent controls. Brain
acetylcholinesterase activity was reduced by 5% in females, and
inhibition of submaxillary gland enzyme was seen in all test groups. A
NOAEL could not be identified in this study (Doull et al., 1962).
Groups of 10 Wistar rats of each sex were exposed to an aerosol
of methiocarb (purity, 97.9%) of a mass median diameter of 2.1-2.5.
The groups were exposed to either air, solvent, methiocarb at 6 mg/m3
in solvent, methiocarb at 23 mg/m3 in solvent, or methiocarb at 96
mg/m3 in solvent; exposure was for 6 h/day, five days per week for
three weeks. The animals were examined daily and weighed weekly. Blood
was taken from five animals per group at the end of the study and was
used for haematological and clinical chemical tests; urinary analyses
were also carried out. Plasma and erythrocyte cholinesterase activity
was determined before treatment and after 5, 10, and 15 exposures for
all groups except the air controls. At the end of the study, the
animals were sacrificed and autopsied, selected organs were weighed
and processed for histopathological examination, and brain
acetylcholinesterase activity was measured.
No deaths were observed in any group. Animals at the highest
concentration showed clinical signs of compound-related effects
(tremor); no clinical signs were seen at lower concentrations. There
body weight of males at the highest concentration was reduced by
comparison with the air controls but not with the solvent controls. No
change in haematological or clinical chemical parameters was seen that
was attributable to the test material, with the exception of
inhibition of cholinesterases. Plasma and brain cholinesterase
activity was decreased at the highest concentration, brain
acetylcholinesterase activity being 61 and 74 % of that in concurrent
solvent controls in males and females, respectively. Some inhibition
of plasma cholinesterase activity was seen at the intermediate
concentration, and males at this concentration had an associated
decrease in brain acetylcholinesterase activity (65% of concurrent
control value). Erythrocyte acetylcholinesterase activity was less
strongly affected than plasma enzyme, but marginal inhibition was
observed in males at the highest concentration at week 1 (82% of
concurrent solvent control value). There were no toxicologically
significant alterations in organ weights, and no compound-related
findings were noted on histopathological examination. The NOAEL was
6 mg/m3 on the basis of reduced brain acetylcholinesterase activity
in males (Thyssen & Mohr, 1983).
Rabbits
Technical-grade methiocarb (purity, 99.2%) was applied to five
male and five female chinchilla rabbits, daily for two weeks, at a
dose of 500 mg; five males and five females served as controls. The
material was applied for 24 h/day, and new material was applied at the
end of each 24-h period. After the two-week application period, the
animals were observed for a further fortnight. The animals were
inspected daily, and haematological and biochemical studies were
carried out before treatment, at the end of treatment, and two weeks
later. Clinical chemistry was restricted to liver function tests and
urinary analysis; cholinesterase activity was not measured. No
abnormal clinical signs were seen, and there was no effect on
body-weight gain or any perturbation in haematological or clinical
chemical variables (Kimmerle, 1969c).
Methiocarb (purity, 99.3%) was applied at doses of 0, 60, 150, or
375 mg/kg bw per day for 6 h/day to the skin of groups of five male
and five female New Zealand white rabbits, and the site of application
was occluded. The animals were examined twice daily, and any signs of
skin irritation were scored. The rabbits were weighed twice weekly;
food consumption was measured three times weekly until the last week,
when it was measured four times. Blood was taken for haematological
and clinical chemical analysis before treatment and pre-terminally.
Plasma and erythrocyte cholinesterase activity was measured at the end
of the 6-h exposure period on days 1, 7, 14, and 21; additionally,
blood was taken 16 h after the end of exposure on these days from the
group at the high dose. Animals were sacrificed on the day after the
last treatment, at which time the brain was taken for measurement of
acetylcholinesterase activity in a homogenate of the entire left half
of the brain. Selected tissues were weighed, examined grossly,
processed, and examined histopathologically.
Two animals at the low dose did not survive to the end of the
study. No clinical signs related to the test material were seen, and
it was not irritating. There was no differences between the groups in
body weight, but food consumption appeared to be reduced in animals of
each sex at the high dose, and particularly in males. Clinical
chemical parameters did not differ between the groups, and, although
one or two differences in haematological measurements were seen, none
appeared to be related to treatment. Plasma cholinesterase activity
appeared to be reduced in males at the high dose at 14 and 21 days.
The measurements made 6 h after the end of exposure suggested that the
inhibition was not reversed overnight. No intergroup differences in
plasma cholinesterase activity were observed among females.
Erythrocyte acetylcholinesterase activity was very variable but did
not appear to be inhibited in a dose-related fashion. Intergroup
differences were not observed in brain acetylcholinesterase activity.
No gross or microscopic abnormality was observed that was attributable
to the test material. The NOAEL was 150 mg/kg bw per day on the basis
of reduced food consumption (Procter, 1988).
Methiocarb (purity, 97.5%) was applied to the skin of five male
and five female New Zealand white rabbits at a single dose of 500
mg/kg bw per day for 6 h/day under occlusion. Five controls of each
sex received saline. The animals were examined twice daily, and any
signs of skin irritation were scored. They were weighed twice weekly,
while food consumption was measured every two days. Blood was taken
for haematological and clinical chemical tests before treatment and
preterminally. Plasma and erythrocyte cholinesterase activity was
measured at the end of the 6-h exposure period on days 1, 7, 14, and
21 and, in the test animals, 16 h later. Animals were sacrificed on
the day after the last treatment, at which time the left half of the
brain was taken for measurement of cholinesterase activity. Selected
tissues were weighed, examined, processed, and examined
histopathologically.
Two test animals removed their dressings and presumably ingested
the material; these animals developed clinical signs of cholinergic
poisoning, which disappeared within a few hours. No other
compound-related clinical signs were seen, and no animal died before
completion of the study. Treated females were lighter than controls
throughout the study, and the food consumption of animals of each sex
was reduced. A reduction in serum calcium and increased activities of
alanine and aspartate aminotransferases were seen in females in
comparison with concurrent controls. Plasma cholinesterase activity
was lower than that before treatment in both males and females, but
only inconsistently in comparison with concurrent controls.
Erythrocyte acetylcholinesterase activity was reduced in males on day
1, at both 6 and 16 h, in comparison with pretreatment levels, but
inconsistently in comparison with concurrent controls; it was
concluded that compound-related inhibition of erythrocyte
acetylcholinesterase activity had not occurred in either males or
females. Brain acetylcholinesterase activity was not inhibited. No
abnormality was seen at autopsy or on histopathological examination.
The design of this study was not appropriate for identifying a NOAEL
(Procter, 1989).
Cats
Methiocarb at a dose of 5 mg/kg bw per day, given daily by gavage
to cats, was reported to have no adverse effects (Kimmerle, 1960).
Dogs
Methiocarb was incorporated into the diet of groups of two male
and two female beagles at a concentration of 0, 50, 100, or 250 ppm,
equal to 0, 1.25, 2.5, or 6.25 mg/kg bw per day. The animals were
examined daily and weighed fortnightly; plasma and erythrocyte
cholinesterase activity was measured weekly. No clinical effects were
seen, and body-weight gain was unaffected by treatment. There was no
clear difference in plasma or erythrocyte cholinesterase activity
between the test groups and concurrent controls. The small group size
renders this study inappropriate for identifying a NOAEL (Root et al.,
1963).
Chickens
Chickens (Gallus gallus Babcock 300) were fed diets containing
a 9:1 mixture of methiocarb and methiocarb sulfoxide (based on studies
of plant metabolism) at a dose of 20, 60, 120, or 360 ppm, equal to
2.5, 7.5, 15, and 45 mg/kg bw per day, over 28 days. Treatment
decreased feed consumption in a dose-related fashion, and the body
weights of birds at the two highest doses were decreased. Egg
production was unaffected. Plasma cholinesterase activity was
decreased by 40-50 % in comparison with concurrent controls at the
three highest doses but was unaffected at the lowest dose (Strankowski
& Minor, 1976).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female BOR:CFW1 mice received diets
containing methiocarb (purity, 98.5%) at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 15, 43, and 130 mg/kg bw per day in males and
0, 20, 57, and 170 mg/kg bw per day in females. Haematological and
clinical chemical tests were performed on five animals of each sex per
dose at 12 months and 10 animals of each sex per dose at 24 months,
and on satellite groups of 15 mice of each sex per dose, which were
sacrificed after one year and necropsied. Cholinesterase activity was
determined in the plasma of five animals of each sex per dose at 1 and
12 months and on 10 animals of each sex per dose (or the survivors if
fewer than 10) at the end of the study. Brain cholinesterase activity
was determined at the end of the study. Animals that died or became
moribund were autopsied, as were 10 mice of each sex per group at 12
months. All surviving animals were sacrificed at the end of the study
and autopsied, and selected organs were weighed, examined, and
processed for histopathological examination.
The appearance, behaviour, mortality rate, and food consumption
of the animals were not affected at any dose, except for a slight
decrease in body weight at the highest dose, throughout the study in
males and up to week 30 in females. Overall survival in this study was
not good: survival at 18 months was 74% of male controls, 47% males at
the low dose, 59% males at the intermediate dose, 66% males at the
high dose, 69% of female controls, 53% at the high dose, 64% at the
intermediate dose, and 69% at the high dose. Intergroup differences in
mortality rates did not appear to be compound-related, as mortality
was higher in males receiving 67 or 200 ppm than in those given the
high dose. Moreover, survival of females up to the end of the study
was poorest for controls. All treated male mice had higher mean
corpuscular haemoglobin concentrations than controls at 12 months, and
males at the highest dose had higher mean corpuscular haemoglobin
values; similar changes were not seen in females. At 24 months, the
mean corpuscular haemoglobin concentration of males at 200 and 600 ppm
was decreased and the mean corpuscular haemoglobin value at 600 ppm,
again, with no similar change in the females. Higher leukocyte counts
were observed in all treated females at 24 months, but the authors
ascribed this finding to high individual values; no perturbution of
the differential count was seen.
No clinical chemical abnormalities were found at 12 months, but
at 24 months the activity of alanine aminotransferase (ALAT) was
increased in animals at 200 and 600 ppm. At one month, males at 200
and 600 ppm had reduced plasma cholinesterase activity (by 51 and 34%
in comparison with concurrent controls), while reductions of 5% or
less were seen at 12 months and 5-11% at 24 months. In females, plasma
cholinesterase activity was inhibited by 24, 43, and 34% at the low,
intermediate, and high doses, respectively, at one month; smaller
reductions (< 12%) were observed at 12 months, and no cholinesterase
inhibition was observed at 24 months. Erythrocyte acetylcholinesterase
activity was not measured. Small reductions in brain
acetylcholinesterase activity were observed, by 5, 11, and 10% in
males at the low, intermediate, and high doses and by 3, 9, and 3% in
females at the three doses, respectively. No compound-related change
in organ weights was seen, nor was there any abnormality in gross of
histological appearance that was related to treatment. Methiocarb was
not tumorigenic. There was no NOAEL because of haematological changes
at all doses in males at 12 months and in females at all doses at 24
months (Krötlinger & Janda, 1983; Krötlinger, 1989).
Rats
Methiocarb (purity, 98.9%) was admixed with the diet of groups of
60 Wistar TNO W.74 rats for two years at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 3.3, 9.3, and 29 mg/kg bw per day for males
and 0, 5, 14, and 42 mg/kg bw per day for females. The rats were
inspected daily, and body weights were determined weekly for the first
26 weeks and then at fortnightly intervals. Haematological, clinical
chemical, and urinary measurements were made in 10 animals of each sex
per dose at 3, 6, 12, and 24 months. Cholinesterase activity in plasma
and erythrocytes was determined one and two days after the start of
the study and at 1, 2, 4, 8, 13, 26, 52, 78, and 105 weeks in 10
animals of each sex per dose. Brain acetylcholinesterase activity was
determined at the end of the experiment on 10 animals of each sex per
dose. Animals that died and those sacrificed in extremis were
examined grossly and necropsied; when possible, tissues were taken for
histopathological examination. The survivors at two years were also
examined grossly and necropsied, and tissues were taken for weighing
and histopathological examination.
None of the doses had any effect on the appearance, behaviour, or
mortality rates of rats of either sex. The mortality rates at two
years were 10-20% for males and 23-32% for females. There was no
significant intergroup difference in food consumption. Weight gain was
not depressed at 67 or 200 ppm in comparison with the controls; at 600
ppm, weight gain was slightly but consistently depressed throughout
the period of administration of methiocarb, and at termination total
body weight was reduced in the group at the high dose. At three
months, an increased leukocyte count was seen in females at the
highest dose and an increased reticulocyte count in females at the two
higher doses. At six months, the mean corpuscular haemoglobin
concentration was reduced in males at the highest dose, and some
elevation in leukocyte count was found in males at 67 and 200 ppm. Red
blood cell counts and haemoglobin and haematocrit values were
decreased in females at the two higher doses, and reticulocytosis was
observed in these groups. At 12 months, the mean corpuscular
haemoglobin concentration was decreased in males at the intermediate
dose and was increased in females at the highest dose; an increased
reticulocyte count was seen in females at the high dose. At 24 months,
no compound-related changes in haematological variables were seen.
Although a few intergroup differences were observed in
biochemical tests, the only ones that appeared to be related to
treatment were total protein concentration and cholinesterase
activity. Total protein concentrations were raised in females at the
two higher doses at six months and in males at the three highest doses
at 12 months; at 24 months, the total protein concentration was
similar in treated and control groups. ALAT activity in the blood was
elevated in all three groups of treated females at 12 months and in
females at the highest dose at two years, but at no time interval in
males. Increased urea was found in plasma from males at the highest
dose at three and 12 months and in females at 12 and 24 months. Plasma
cholinesterase activity was depressed at the high dose at one day and
from eight weeks onwards in males (except in the 52-week assay) and in
females at one day and 1, 2, 4, and 13 weeks. The measurements of
erythrocyte acetylcholinesterase activity were difficult to interpret:
a statistically significant depression was found only at the low dose
in males at 105 weeks and females at 78 weeks, and the degree of
depression was small (5 and 6%, respectively). Males at the
intermediate dose had significantly depressed activities at 8, 78, and
105 weeks, with 5, 7, and 8% depression, respectively. In females at
this dose, significant depression was seen at four and 78 weeks (8 and
13% depression, respectively). In males at the highest dose,
significant depressions were seen at 8, 13, 78, and 105 weeks (7, 7,
9, and 7%, respectively), and in females at this dose depression was
seen at two days and 4 and 78 weeks (6, 8, and 11%, respectively). The
Committee considered that none of these depressions was biologically
significant. No depression of brain acetylcholinesterase activity was
seen.
Males at the highest dose had decreased absolute weights of the
thyroid, heart, lung, liver, spleen, and adrenals, but these were
reflected only in reductions in the relative weights of the spleen and
therefore probably reflect reduced body weight. In the females, only
the absolute weight of the spleen was reduced, while the absolute
weight of the thyroid was increased. As this finding was due to a
single animal, it is unlikely to be attributable to the test material.
No gross or histopathological abnormality related to treatment was
seen at any dose. Methiocarb was not tumorigenic. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months (Krötlinger et al., 1981; Krötlinger,
1990).
Dogs
Groups of four beagle dogs and four bitches were fed methiocarb
(purity, 98.4%) in the diet at 0, 5, 60, or 240 ppm; the group given 5
ppm group had been given 15 ppm for the first 15 days. These dietary
concentrations were equivalent to daily doses of 0, 0.12, 1.5, and 6
mg/kg bw per day, ignoring the first 15 days of treatment of animals
at the lowest dose. The animals were examined daily, and food
consumption was recorded daily and body weight measured weekly.
Clinical examinations including ophthalmoscopy were undertaken, and
haematological and biochemical variables were measured in blood at
weeks 0, 14, 27, 40, 53, 66, 79, 92, and 104; urine was also analysed.
Erythrocyte and plasma cholinesterase activity was measured before the
start of treatment and at weeks 2, 3, 4, 7, 10, 13, 27, 40, 53, 66,
79, 92, and 104, before feeding and 2 h afterwards.
Acetylcholinesterase activity on the olfactory bulb of the brain was
measured at sacrifice. Animals were examined grossly post mortem,
and selected tissues were examined histologically.
One death occurred, of an animal at 5 ppm, which was considered
not to be related to treatment. The only clinical findings were mild
weakness of the hind limbs, trembling, reduced alertness, and some
vomiting at the highest dose during the first 14 weeks of the study.
The results of tests for reflexes and ophthalmic parameters were
normal. Food intake was reduced in animals of each sex at the highest
dose and in bitches at the intermediate dose, but the body weights
were not significantly affected. Haematological and biochemical
parameters were unaffected, apart from cholinesterase activity. Plasma
cholinesterase activity was depressed at doses of 15 ppm and higher,
and it was for this reason that this dose was reduced to 5 ppm.
Depression of plasma cholinesterase activity was not seen at 5 ppm but
occurred at the two higher doses. Erythrocyte and brain
acetylcholinesterase activity was not consistently inhibited at any
dose; the maximum inhibition of erythrocyte acetylcholinesterase
activity was in animals at the high dose (17% for each sex) and at the
intermediate dose (10% in dogs and 5% in bitches). Organ weights were
unaffected, and no organ-specific toxicity observed. The NOAEL was 60
ppm, equivalent to 1.5 mg/kg bw per day, on the basis of clinical
signs. The reduced food intake of bitches at the intermediate dose was
not considered relevant (Hoffman & Schilde, 1980).
(d) Genotoxicity
The results of assays for the genotoxicity of methiocarb are
shown in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 10 female FB 30 rats (Elberfield breed)
received technical-grade methiocarb (purity, 99%) admixed with the
diet at concentrations of 0, 30, 100, or 300 ppm, equivalent to 3, 10,
and 30 mg/kg bw per day. The rats were weighed weekly. The F0
generation was mated twice, first after 70 days of treatment and again
after 149 days, to produce the F1a litters, which were sacrificed,
and the F1b litters. Ten males and 10 females from each group of F1b
rats were used to produce the next generation and were again mated
twice. The resultant F2a rats were sacrificed, while 10 male and 10
female F2b rats were mated twice to produce the F3a and F3b
generations, which were sacrificed. The rats were weighed weekly, and
the body weights of the offspring were measured at birth, five days
after birth, one week after birth, and then weekly. The pups were
examined grossly for malformations immediately after birth and during
lactation. The F3a young were killed four weeks after birth and the
F3b rats at three weeks. Blood samples were taken for analysis from
the F0 generation at the end of the preliminary treatment period and
after rearing of the second little (Löser, 1969).
In the F0 generation, there was no significant difference in
weight gain between the groups, and no treatment-related changes were
seen in haematological parameters at either sampling time. Although
ALAT and aspartate aminotransferase activities were higher in animals
at the highest dose at the earlier sampling time, they were stated to
be within the normal range for the laboratory; similar increases were
not seen at the later sampling time. The gestation rate was lower at
the highest dietary concentration, but was still within the normal
range. The weight gain of F1a pups was similar in all groups, and no
malformations were seen at sacrifice. After the second mating, there
were no dose-related changes in litter size or pup weight and weight
gain. No significant differences were seen between the groups of F1b
pups. Their weight gain after weaning was similar in all groups. After
the first mating, the gestation rate was lower at the highest dietary
concentration but was still within the normal range. The weight gain
of F2a pups was similar in all groups, and no malformations were seen
at sacrifice. After the second mating, the mean weight of the group at
300 ppm was significantly lower than that of controls at the end of
the four-week lactation period; however, there were no significant
intergroup differences in body weight at sacrifice of the F2b
animals. After both matings of these rats, the gestation rate was
similar and the litter size and pup weights were comparable in all
Table 2. Results of assays for the genotoxicity of methiocarb
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 4-2500 µg/plate 98.5 Negative Herbold (1978)
TA1535, TA1537,
TA98, TA100
Reverse mutationa S. typhimurium 20-12 500 µg/plate 98.4 Negative Herbold (1986)
TA1535, TA1537,
TA98, TA100
DNA damagea E. coli pol A+ and 625-10000 µg/plate 98.6 Negative Herbold (1983)
pol A-
Gene mutation Chinese hamster 1.25-60 µg/ml 99.3 Negative Lehn (1989)
ovary cells, hprt
Unscheduled DNA Rat primary 0.1-100 µg/ml 98.8 Negative Curren (1988)
synthesis hepatocytes
Chromosomal Chinese hamster 4.92-497 µg/ml 99.4 Positive Murli (1990)
aberrationa ovary cells
Sister chromatid Chinese hamster 2-40 µg/ml 98.2 Negative Putman (1986)
exchangea ovary cells
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
Micronucleus Mice 5,10, and 20 98.5 Negative Herbold (1979a)
formation mg/kg bw
twice orally
Dominant lethal Mice 6 mg/kg bw 98.5 Negative Herbold (1979b)
mutation
a With and without metabolic activation
groups. The body weights of the F3b, but not the F3a, groups at 100
and 300 ppm during lactation were lower than those of controls. This
finding may be due to larger litter size. No abnormalities were found
at birth or sacrifice in F3a or F3b pups, and no abnormalities were
found at autopsy of the F0, F1b, and F2b generations. The NOAEL was
300 ppm, equivalent to 30 mg/kg bw per day, the highest dose tested
(Löser (1970).
(ii) Developmental toxicity
Rats
Groups of 19-20 fertilized FB 30 rats received 10 oral doses of
0, 1, 3, or 10 mg/kg bw per day methiocarb (purity, 98.9%) by gavage
on days 6-15 of gestation. The highest dose reduced weight gain, but
no other effects were observed. No effects were seen on the number of
implantations or resorptions or the weights of fetuses or placentas.
No teratogenic effects were seen, nor was fetotoxicity observed. The
NOAEL for maternal toxicity was 3 mg/kg bw per day, and that for fetal
toxicity was 10 mg/kg bw per day, the highest dose tested (Lorke,
1971; see also Renhof, 1988).
Rabbits
Methiocarb (purity, 97.3%) was administered by gavage to groups
of 17 pregnant New Zealand white rabbits at doses of 1, 3, or 10 mg/kg
bw per day on days 6-18 of gestation. A group of 19 rabbits were used
as controls. On day 29 of gestation, the rabbits were sacrificed and
examined; the uterus was weighed, and the numbers of corpora lutea,
implantation sites, and resorption sites, and the number and
distribution of live and dead fetuses were noted. The weight and sex
of the fetuses and placental weights were recorded and the fetuses
examined for abnormalities. Rabbits receiving the highest dose showed
clinical signs of cholinergic poisoning and initial marked weight
loss. Weight gain was also reduced at 3 mg/kg bw per day towards the
end of the study, but this was attributable to a single animal. Weight
gain was unaffected at the lowest dose. The litter parameters were
similar in all groups, and there was no indication of teratological
effects. The NOAEL for maternal toxicity (clinical effects and weight
loss at the highest dose) was 3 mg/kg bw per day . The NOAEL for
fetotoxicity was 10 mg/kg bw per day, the highest dose tested (Tesh et
al., 1981).
Methiocarb (purity, 99.4-99.6%) was applied under occlusion for
6 h/day to about 10% of the body area of groups of 16 mated chinchilla
rabbits at doses of 0, 10, 50, or 250 mg/kg bw per day on days 6-18
post coitum. The animals were sacrificed after 28 days and the fetuses
removed. One animal at the high dose was sacrificed because of a
broken femur. No clinical signs were observed at any dose, nor was
irritation of the skin noted. At the highest dose, reduced food
consumption was observed between 11 and 15 days post coitum and
body-weight loss between 6 and 8 days post coitum, whereas at the
lower doses,no such effect was seen. The numbers of corpora lutea,
implantations, pre- and post-implantation losses, and live fetuses
were not affected by treatment. There was a slight decrease in mean
fetal weight at the highest dose (4%), and slight retardation of
skeletal development was noted at this dose, as shown by incomplete or
no ossification of the phalangeal nuclei. No effects on the fetuses
were seen at lower doses. The NOAEL for both maternal and fetal
toxicity was 50 mg/kg bw per day (Dotti & Biedermann, 1992).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
(ii) Neurotoxicity
In a poorly described study, no persistent paralytic effects were
produced in hens (Gallus gallus) of unstated strain given a single
dose of methiocarb by gavage and observed for 14 days (DuBois, 1962).
Methiocarb (purity, 98.5%) was administered twice at a dose of
380 mg/kg bw (approximately the LD50) to 20 white Leghorn laying
hens, aged 15-20 months, after an intramuscular injection of 50 mg/kg
bw atropine sulfate. After an interval of 21 days, the 18 surviving
hens were treated again, with the same doses of atropine and
methiocarb. The 16 survivors of the second treatment were sacrificed
three weeks later, and brain, spinal cord, and portions of peripheral
nerve were excised and processed for histological examination. Five
positive controls were given tri- ortho-cresyl phosphate as a single
oral dose and sacrificed three weeks later. The hens treated with
methiocarb exhibited brief cholinergic signs and were lethargic, but
none showed ataxia or paralysis. At examination, post mortem there
were no histopathological changes indicative of polyneuropathy in
either the central or peripheral nervous system, whereas the hens
treated with tri- ortho-cresyl phosphate showed the classical signs
of delayed polyneuropathy and mild but characteristic signs of delayed
polyneuropathy on histopathological examination (Thyssen & Schilde,
1978).
(iii) Antidotes
A study was carried out in which the effect of antidotes to an
oral LD50 of methiocarb was investigated in rats. The regimens used
were atropine at 50 mg/kg bw, pralidoxime (salt unstated) at 50 mg/kg
bw, obidoxime at 20 mg/kg bw, atropine and pralidoxime at 50 mg/kg bw
each, or atropine at 50 mg/kg bw with obidoxime at 20 mg/kg bw. All
the antidotes were given intraperitoneally just before clinical signs
became evident, i.e. 1-2 min after administration of methiocarb. The
LD50 values were 67 mg/kg bw without antidotes, 470 mg/kg bw with
atropine, 190 mg/kg bw with pralidoxime, 220 mg/kg bw with obidoxime,
500 mg/kg bw with pralidoxime and atropine, and 510 mg/kg bw with
obidoxime and atropine. Thus, pralidoxime and obidoxime added little
to the antidotal effect of atropine (Kimmerle, 1966a).
In mice, 10 mg/kg bw of atropine increased the LD50 of
methiocarb by sevenfold, while pralidoxime methiodide at 100 mg/kg bw
increased the LD50 1.3 times. Administration of the two antidotes
together increased the LD50 by 21 times. Obidoxime at 50 mg/kg bw
increased the LD50 3.1 times, while obidoxime plus atropine increased
it by 9.1 times (Kohgo, 1970).
Atropine sulfate at 50 mg/kg bw administered within 10 min of
methiocarb intraperitoneally increased the LD50 of methiocarb given
orally to rats by more than sixfold, and tetraethyl ammonium chloride
given by the same route increased it by approximately fourfold;
however, the combined effect of the two antidotes was less than that
of atropine alone (Kimmerle, 1971).
In the study of Thyssen and Schilde (1978) in hens at
approximately the LD50 (see above), atropine appeared to have a
powerful antidotal effect.
(iv) Interaction with other pesticides
When an intraperitoneal dose equal to 50% of the LD50 of
methiocarb was given to female Sprague-Dawley rats in combination with
another pesticide at a dose equal to 50% of the LD50, the combined
mortality was less than 50% in all cases. The highest combined
mortality rates (> 40 to < 50%) were seen with EPN, Guthion,
mevinphos, and carbaryl. Combined mortality rates of > 20 to < 40%
were found with parathion, parathion-methyl, disulfoton, malathion,
carbophenothion, tributyl phosphorotrithioite, and ethion. The
mortality rates seen with the remaining insecticides (demeton,
dioxathion, and schradan) were 5-20% (DuBois & Raymund, 1961b). In
another study of similar design, the combined mortality rates were 55%
with trichlorfon, 50% with propoxur, 35% with coumaphos, 30% with
oxydemeton-methyl, and 20% with fenthion (DuBois & Raymund, 1961c).
3. Observations in humans
The 250 workers in two plants manufacturing methiocarb over about
20 years were subjected to annual medical examinations and assays of
cholinesterase activity in whole blood. No adverse health effects or
changes in laboratory parameters were observed (Faul, 1993).
Comments
Methiocarb appeared to have been well absorbed in a small study
of absorption, distribution, metabolism, and excretion of the
radiolabelled compound in rats. More than 70% of the administered
radiolabel was excreted within 48 h, mostly in the urine. Methiocarb
was extensively metabolized. The main route of metabolism in both
animals and plants appears to be to methiocarb phenol, methiocarb
phenol sulfoxide, and methiocarb phenol sulfone. In some studies,
methiocarb sulfoxide was also found.
In single-dose and short-term studies in rats, methiocarb
administered by gavage inhibited plasma and erythrocyte cholinesterase
activity. In the short-term study, plasma, erythrocyte, and brain
cholinesterase activities were depressed at 10 mg/kg bw per day; the
NOAEL for this effect was 3 mg/kg bw per day. In a short-term study of
the ability of methiocarb or its sulfoxide to inhibit cholinesterase
activity in rats, the NOAEL for inhibition of erythrocyte
acetylcholinesterase activity was 0.5 mg/kg bw per day for methiocarb,
but a NOAEL was not identified for the sulfoxide. In a 29-day study in
which dogs were given methiocarb or methiocarb sulfoxide in gelatine
capsules at 0.05 or 0.5 mg/kg bw per day, both plasma and erythrocyte
cholinesterase activities were inhibited by both treatments.
The acute toxicity of methiocarb given by a number of routes has
been measured in a number of species. The oral LD50 in fasting rats
ranged from 13 to 130 mg/kg bw. The oral LD50 values for methiocarb
phenol, methiocarb phenol sulfoxide, and methiocarb phenol sulfone in
rats are all greater than 1 g/kg bw, as is that of methiocarb sulfone,
but that of methiocarb sulfoxide is between 6 and 43 mg/kg bw.
WHO has classified methiocarb as moderately hazardous (WHO,
1996).
The short-term toxicity of methiocarb has been tested in rats,
rabbits, cats, dogs, and chickens. Few of these studies were
appropriate for identifying NOAEL values and, of those that were, a
study in rats was carried out by inhalation and that in rabbits by
dermal application. In the study in rats, which were exposed by
inhalation, for five days per week for three weeks, no findings
related to treatment were seen on histopathological examination but
depressed brain acetyl-cholinesterase activity was observed at the
highest dose in animals of each sex and in males at the intermediate
dose. Consequently, the NOAEL was 6 mg/m3 per day. In a 21-day study
of the dermal toxicity of methiocarb in rabbits, the substance was
applied for 6 h/day at 0, 60, 150, or 375 mg/kg bw per day. Plasma
cholinesterase activity was reduced in males at the highest dose, but
no significant differences were seen between groups in the activities
of erythrocyte and brain acetylcholinesterase. The NOAEL was 150 mg/kg
bw per day on the basis of reduced food consumption at the highest
dose.
In a long-term study of toxicity in mice, methiocarb was
administered at dietary concentrations of 0, 67, 200, or 600 ppm. A
NOAEL was not identified because haematological changes were observed
in all treated males at 12 months and in all treated females at 24
months. The LOAEL was 67 ppm, equal to 15 mg/kg bw per day, on the
basis of minor haematological changes. Methiocarb was not carcinogenic
in mice.
In a two-year study of toxicity, rats received methiocarb at
dietary concentrations of 0, 67, 200, or 600 ppm. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months. Methiocarb was not carcinogenic.
In a two-year study of toxicity, dogs were fed methiocarb in the
diet at 0, 5, 60, or 240 ppm. The NOAEL was 60 ppm, equivalent to 1.5
mg/kg bw per day, on the basis of reversible clinical signs at the
next highest dose, which were not observed after 15 weeks.
Three studies of developmental toxicity were available, one in
rats and two in rabbits. Fertilized rats received methiocarb by gavage
on days 6-15 of gestation at 0, 1, 3, or 10 mg/kg bw per day. The
NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of
reduced body-weight gain at the highest dose. As no fetotoxicity was
observed, the NOAEL for this end-point was 10 mg/kg bw per day, the
highest dose tested. In a study of developmental toxicity in rabbits,
pregnant animals received methiocarb at 0, 1, 3, or 10 mg/kg bw per
day by gavage on days 6-18 of gestation. The NOAEL was 3 mg/kg bw per
day for maternal toxicity on the basis of clinical effects and weight
loss at the highest dose. As no fetotoxicity or teratogenicity was
observed, the NOAEL for these end-points was 10 mg/kg bw per day, the
highest dose tested. In a further study, rabbits received methiocarb
by dermal application at 0, 10, 50, or 250 mg/kg bw per day for
6 h/day on days 6-18 of gestation. The NOAEL for both maternal and
fetal toxicity was 50 mg/kg bw per day on the basis of reduced
maternal food consumption and some decrease in mean fetal weight at
the highest dose; slight retardation of fetal development was also
observed. A multigeneration study of reproductive toxicity was
undertaken in rats given methiocarb at dietary concentrations of 0,
30, 100, or 300 ppm. The NOAEL was 300 ppm, equivalent to 30 mg/kg bw
per day, as no effect clearly related to treatment was observed. No
teratogenic effect was observed in any of these studies.
Methiocarb has been tested for genotoxicity in an adequate
battery of tests in vitro and in vivo. The Meeting concluded that
methiocarb is not genotoxic.
Methiocarb did not cause skin sensitization in studies conducted
by either the Magnusson and Kligman or the Beuhler technique.
In an early study of neurotoxicity in hens, methiocarb did not
cause delayed polyneuropathy of the organophosphorus type. Atropine
has consistently been shown to be an effective antidote for
methiocarb, while the effects of pyridinium oximes were somewhat
inconsistent.
The Meeting established an ADI of 0-0.02 mg/kg bw on the basis of
the NOAEL of 1.5 mg/kg bw per day in the two-year study of toxicity in
dogs and a safety factor of 100. This ADI results in a further safety
factor of 10 on the LOAEL in the long-term study of toxicity in mice.
An acute RfD was allocated on the basis of the NOAEL of 1.5 mg/kg
bw per day in the two-year study in dogs, because the signs observed
were acute, and a safety factor of 100. Of the shorter studies in
dogs, neither the 29-day nor the 12-week study was considered by the
Meeting to be adequate for the purpose of establishing a NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: No NOAEL; LOAEL: 67 ppm, equal to 15 mg/kg bw per
day (long-term study of toxicity)
Rat: 67 ppm, equal to 3.3 mg/kg bw per day (long-term
study of toxicity)
300 ppm, equivalent to 30 mg/kg bw per day
(maternal and fetal toxicity in a study of
reproductive toxicity)
3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (two-
year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would be useful for continued evaluation of the
compound
1. A modern study of absorption, distribution, metabolism, and
excretion
2. A modern multigeneration study of reproductive toxicity
3. Further observations in humans
List of end points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption No data
Dermal absorption No data
Distribution No data
Potential for accumulation Not likely to accumulate
Rate and extent of excretion 73 to > 90% within 48 h
Metabolism in animals Sulfoxidation and loss of carbamate side-chain
Toxicologically significant compounds Parent compound and methiocarb sulfoxide
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral 13-135 mg/kg bw
Rat: LD50, dermal 350 to > 5000 mg/kg bw
Rat: LC50 inhalation > 300 mg/m3
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Clinical signs
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 150 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 6 mg/m3
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Clinical signs
Lowest relevant NOAEL Dog: 1.5 mg/kg bw per day
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect No effect
Lowest relevant reproductive NOAEL Rat: 30 mg/kg bw per day
Developmental target/critical effect Maternal toxicity: reduced weight gain; no
fetotoxicity observed
Lowest relevant developmental NOAEL Rabbit: 3 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Does not cause delayed polyneuropathy
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Dog, 2 years 100
Acute reference dose 0.02 mg/kg bw Dog, 2 years 100
References
Baron, R.L., Casterline, J.L. & Fitzhugh, O.G. (1964) Specificity of
carbamate esterase inhibition in mice. Toxicol. Appl. Pharmacol., 6,
402-410.
Barthová, J., Woldemariam, M.G. & Leblová, S. (1989) The effect of
carbamates insecticides on cholinesterases of nontarget organisms.
Biologia (Bratislava), 44, 353-356.
Crawford, C.R. & Anderson, R.H. (1970) The skin and eye irritating
properties of BAY 37344 technical to rabbits. Unpublished report No.
28304 from Chemagro Corporation, Research Department, 15 October 1970.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972a) The acute oral toxicity to
guinea pigs and the acute inhalation toxicity to rats of Mesurol(R)
technical. Unpublished report No. 31987 from Chemagro Corporation,
Research Department, 24 January 1972. Submitted to WHO by Bayer AG
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972b) The dermal toxicity of
(R)Mesurol technical and Mesurol 75% wettable powder to rabbits.
Unpublished report No. 34477 from Chemagro Division of Baychem
Corporation, Research Development, 21 August 1972. Submitted to WHO by
Bayer AG Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral toxicity of
(R)Mesurol technical to rats. Unpublished report No. 34008 from
Chemagro Division of Baychem Corporation, Research and Development, 31
May 1972 and revised 8 May 1973. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 1007 from Microbiological
Associates Inc., Bethesda, Maryland and Rockville, Maryland, USA, 1
June 1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
David, R.M. (1988) EPA/OECD dermal sensitization test of Mesurol
technical in guinea pigs. Unpublished report No. 1015 from
Microbiological Associates, Inc., Bethesda, Maryland, USA, 15 April
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dotti, A. & Biedermann, K. (1992) Embryotoxicity study (including
teratogenicity) with H 321 (c.n. methiocarb) in the rabbit (dermal
application) part 1. Unpublished report No. R 5627 from RCC Research &
Consulting Co. Ltd and RCC Umweltchemie AG, Itingen, Switzerland, 6
August 1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Doull, J., Cowan, J. & Root, M. (1962) Subacute (16-week) oral
toxicity of Bayer 37344 to male and female rats. Unpublished report
No. 9456 from Department of Pharmacology, University of Chicago, USA,
12 June 1962. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. (1962) The acute oral toxicity of Bayer 37344 to
chickens. Unpublished report No. 9248 from Department of Pharmacology,
University of Chicago, USA, 16 May 1962. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K.P. (1964) The acute toxicity of some possible metabolites of
Bayer 37344. Unpublished report No. 12806 from Department of
Pharmacology, University of Chicago, USA, 22 January 1964. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961a) The acute toxicity of Bayer 37344
to mammals. Unpublished report No. 6775 from Department of
Pharmacology, University of Chicago, USA, 25 April 1961. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961b) The acute toxicity of Bayer 37344
in combination with other anticholinesterase insecticides to rats.
Unpublished report No. 7880 from Department of Pharmacology,
University of Chicago, USA, 1 November 1961. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961c) The acute toxicity of Bayer 39007
and Bayer 37344 in combination with some other anticholinesterase
insecticides in rats. Unpublished report No. 7879 from Department of
Pharmacology, University of Chicago, USA, 4 November 1961. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1962) The acute toxicity of
recrystallised Bayer 37344 and a wettable powder of Bayer 37344 to
female rats. Unpublished report No. 9424 from Department of
Pharmacology, University of Chicago, USA, 12 June 1962. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Eben, A. & Kimmerle, G. (1973) Mercaptodimethur effect of acute and
subacute oral doses on acetylcholinesterase activity in plasma,
erythrocyte and brain of rats. Unpublished report No. 4284 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 9 November
1973. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Faul, J. (1993) Mesurol active ingredient: In-company occupational
medical experience. Unpublished letter to Dr Heimann, PF-A/consulting,
Monheim, Germany, 1 April 1993. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50). Unpublished
letter to Dr Reuver, Bayer AG Institute of Toxicology, dated 27 June
1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) H 321 (mercaptodimethur). Unpublished note dated 3
November 1980. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1988) Determination of acute toxicity (LD50), Unpublished
letter to Dr Reuver, Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany, dated 1 August 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Haijar, N.P. & Hodgson, E. (1982) Sulfoxidation of
thioether-containing pesticides by the flavin-adenine
dinucleotide-dependent monooxygenase of pig liver microsomes.
Biochem. Pharmacol., 31, 745-752.
Hayes, R.H. (1981) Cholinesterase evaluation study of methiocarb
technical and methiocarb sulfoxide in dogs. Unpublished report No. 202
from Corporate Toxicology Department, Mobay Chemical Corporation,
Stillwell, Kansas, USA, 29 September 1981. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1983) Determination of acute toxicity (LD50).
Unpublished letter to Dr Reuver, PF-Zentrum, Monheim, Germany, dated
22 April 1983. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1978) H 321 (active ingredient of Mesurol)
Salmonella/microsome test for determination of point mutations.
Unpublished report No. 7978 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 6 December 1978. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B. (1979a) H 321 (active ingredient of Mesurol) micronucleus
test for mutagenic effect on mice. Unpublished report No. 8426 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 8
June 1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979b) H 321 dominant lethal study on male mouse to test
for mutagenic effects. Unpublished report No. 8395 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 23 May 1979.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) H 321 mercaptodimethur (the active ingredient of
Mesurol(R)) study of DNA damage using the E. coli pol A- test.
Unpublished report No. 11928 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 13 July 1983. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) H 321 c.n. mercaptodimethur Salmonella/microsome
test to evaluate for point mutagenic effect. Unpublished report No.
14205 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 10 January 1986. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1981) Cholinesterase no-effect level of Mesurol(R) and
Mesurol sulfoxide(R) in female rats. Unpublished report No. 199 from
Mobay Chemical Corporation, Corporate Toxicology Department, Stilwell,
Kansas, USA, 31 August 1981. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. & Schilde, B. (1980) H 321(Mesuro(R) active ingredient
-- mercaptodimethur) chronic toxicity study in dogs (2 year feeding
experiment). Unpublished report No. 9626 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 4 December 1980. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1960) Product Dr Wedemeyer H 321 (E 37 344) Production
No. 2410. Unpublished report from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 25 March 1960. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1966a) Mesurol active ingredient (Wedemeyer H 321; Ht.
No. 3657) -- antidotal effect. Unpublished letter to Crop Protection
Science, Leverkusen. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, dated 8 August 1966. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1966b) Re active ingredient Wedemeyer H 321 (Ht No
3657). Inhalation tests. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany. Unpublished letter to Crop Protection
Science, Leverkusen, Germany, dated 7 December 1966. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969a) Re Bay 37 344 (H 321) Lo No. 691. Unpublished
letter to Crop Protection Science, Leverkusen. Bayer AG, Institute for
Toxicology, Wuppertal-Elbberfeld, Germany, dates 20 May. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969b) Ref Bay 37344 (=H 321 = Methiocarb) Lot No. 691.
Unpublished letter to Crop Protection Science, Leverkusen. Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, dated 20 May.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969c) Bay 37 344 subacute dermal toxicity study on
rabbits. Unpublished report No. 1291 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 1 April 1969. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetraethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol., 27, 311-314.
Kimmerle, G. (1972) Unpublished note from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, dated 7 June 1972. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Klimmer, O.R. (1963) Toxicological testing of Bayer 37 344.
Unpublished letter from Pharmacological Institute of the University of
Bonn, Germany, to Crop Protection/Scientific Department, Leverkusen,
Germany, dated 10 December. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kohgo, L. (1970) The antagonistic effects of atropine or pyridinium
aldoxime derivatives on cholinesterase-inhibitory pesticides in male
and mice. J. Med. Soc. Toho Jpn, 17, 60-88.
Krötlinger, F. (1989) H 321 (mercaptodimethur, active ingredient of
Mesurol) chronic toxicological study on mice (feeding study over 2
year). Unpublished addendum No. 11908A to report No. 11908 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 15 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. (1990) H 321 (mercaptodimethur, the active ingredient
of Mesurol) chronic toxicity study on rats (2-year feeding
experiment). Unpublished addendum No. 10039A to report No. 10039 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 16
February 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. & Janda, B. (1983) H 321 (mercaptodimethur, the active
ingredient of Mesurol(R)) chronic toxicity study on mice (2-year
feeding experiment). Unpublished report No. 11908 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 4 July 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Löser, E. & Vogel, O. (1981) H 321 (mercaptodimethur,
the active ingredient of Mesurol) chronic toxicity study on rats
(2-year feeding experiment). Unpublished report No. 10039 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 2 July
1981. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of mesurol
technical to dogs. Unpublished note of experiment No. 450474 from
Mobay Chemical Corp., Chemagro Agricultural Division, dated 25 August
1975. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976a) The acute oral toxicities of
mesurol technical and mesurol sulfoxide. Unpublished note of
experiment No. 49541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 12 July 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976b) The acute oral toxicities of
Mesurol(R) technical and mesurol sulfoxide. Unpublished note of
experiment No. 50541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 19 August 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) A comparison of the acute oral
toxicities of Mesurol(R) technical to fasted and non-fasted rats.
Unpublished note of experiment No. 50541 from Mobay Chemical Corp.,
Chemagro Agricultural Division, dated 19 January 1977. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1989) H 321 C.N. Methiocarb mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay in
vitro. Unpublished report No. 18280 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 15 August 1989. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lorke, D. (1971) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished report No.
3133 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 30 November 1971. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1969) Bay 37 344. Blood analyses, urinalysis and clinical
chemistry examinations following oral administration rats. Unpublished
report No. 1358 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 3 March 1969. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Löser, E. (1970) Bay 37 344. Generation studies on rats. Unpublished
report No. 2208 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 10 July 1970. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Menzie, C.M. (1974) Metabolism of Pesticides: An Update (Wildlife
No. 184), Washington DC, US Department of the Interior Fish and
Wildlife Service, p. 249.
Mihail, F. (1984) H 321 (C.n. mercaptodimethur) study for
skin-sensitising effect on guinea pigs. Unpublished report No. 13125
from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany,
12 December 1984. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murli, H. (1990) Mutagenicity test on H 321 in an in vitro cytogenetic
assay measuring chromosomal abberation frequencies in Chinese hamster
ovary (CHO) cells with a confirmatory assay. Unpublished report No. R
4980 from Hazleton Laboratories America Inc., Kensingtonb Maryland,
USA, 8 March 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murphy, J.J., Timme, G. & Dräger, G. (1982) Mesurol(R) common name:
methiocarb, mercaptodimethur. Behaviour in plants. Unpublished report
from Mobay Chemical Corp., Kansas, USA, August 1982. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Nelson, D.L. (1979) Acute oral toxicity of Mesurol(R) technical and
metabolites to rats. Unpublished report No. 67815 from Mobay Chemical
Corp., Environmental Health Research, Stillwell, Kansas, USA, 27 June
1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1988) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1084 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 23 November
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1989) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1160 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 31 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1986) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells test article Mesurol technical. Unpublished report
No. 790 from Microbiological Associates Inc., Bethesda, Maryland, USA,
25 September 1986. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished addendum No.
3133A to report No. 3133 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 21 October 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Root, M., Doull, J. & Cowan, J. (1963) Determination of the safe
dietary level of Bayer 37344 for dogs. Unpublished report No. 11159
from Department of Pharmacology, University of Chicago, USA, 23 March
1963.. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Salama, A.K., Bakry, N.M. & Abou-Donia, M.B. (1993) A review article
on placental transfer of pesticides. J. Occup. Med. Toxicol., 2,
383-397.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Mesurol in rats. Unpublished report No. 940 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 31 August 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b) Acute four-hour inhalation toxicity study with
Mesurol(R) technical in rats. Unpublished report No. 870 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 18 June 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1969) Mesurol(R) active ingredient -- acute cutaneous
toxicity in rats. Unpublished letter to Bayer AG, Institute of
Toxicology, Crop Protection Science, Leverkusen, Germany, dated 11
September 1969. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1970) Oral toxicity of Mesurol metabolites to rats.
Unpublished notes addressed to Bayer AG, Institute of Toxicology, Crop
Protection Science, Leverkusen, Germany, dated 6 April 1970. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stanley, C.W. & Johnson, G.A. (1976) Metabolites of Mesurol(R) in
rat urine. Unpublished report No. MR50732 from Chemagro Agricultural
Division, Mobay Chemical Corp., USA, 16 November 1976. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Strankowski, K.J. & Minor, R.G. (1976) Effect of feeding
Mesurol(R)/Mesurol sulfoxide (9:1) to chickens for 28 days.
Unpublished report No. 49639 from Mobay Chemical Corp., Research and
Development Department, USA, 7 January 1976. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Strother, A. (1970) Comparative metabolism of N-methylcarbamates by
human and rat liver fractions. Biochem. Pharmacol., 19, 2525-2529.
Strother, A. (1972) In vitro metabolism of methylcarbamate
insecticides by human and rat liver fraction. Toxicol. Appl.
Pharmacol., 21, 112-129.
Tesh, J.M., Ross, F.W., Secker, R.C. & Wilby, O.K. (1981) H 321:
Effects of oral administration upon pregnancy in the rabbit. 2. main
study. Unpublished report No. 2173 from Life Science Research, Stock,
United Kingdom, 4 December 1981. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977a) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 15 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977b) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 30 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) H 321 (Mesurol active ingredient) acute inhalation
toxicity. Unpublished report No. 11113 from Bayer AG Institute of
Toxicology, 27 August 1982. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen, J. & Mohr, U. (1983) H 321 (Mesurol active ingredient)
subacute inhalation study with rats. Unpublished report No. 12120 from
Bayer AG Institute of Toxicology and Department of Experiment
Pathology, Hanover Medical University, Germany, 30 September 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Schilde, B. (1978) H 321 Neurotoxicity study on hens.
Unpublished report No. 7637 from Bayer Institute of Toxicology, 20
June 1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Wheeler, L. & Strother, A. (1971) In vitro metabolism of the
N-methylcarbamates, Zectran and Mesurol, by liver, kidney and blood of
dogs and rats. J. Pharm. Exp. Ther., 178, 371-382.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Willems, P.W.J.M., Geursen-Reitsma, A.M. & van Joost, T. (1997)
Allergic contact dermatitis due to methiocarb (Mesurol). Contact
Derm., 36, 270-271.
 0.5 mg/kg bw; 4 h after dosing at 0.5 mg/kg bw, depressions of < 10%
were noted. At the higher dose of methiocarb, depressions of 19-41%
were seen 30 min after dosing and 0.2-19% 4 h after dosing. With the
sulfoxide, plasma cholinesterase activity was inhibited by 21-39% at
the low dose at 30 min but was generally not inhibited at 4 h. At the
high dose, 39-62% inhibition was seen at 0.5 h and 10-25% inhibition
at 4 h. Erythrocyte acetylcholinesterase activity was depressed by
5-12% 30 min after dosing at 0.5 mg/kg bw and by < 10% 4 h after
dosing. At the higher dose of methiocarb, erythrocyte
acetylcholinesterase activity was depressed by 15-29% 30 min after
dosing and by 1-13% 4 h after dosing. With the sulfoxide, erythrocyte
acetylcholinesterase activity was inhibited by 13-31% at the low dose
at 30 min but was generally not inhibited at 4 h. At the high dose,
32-46% inhibition was seen at 0.5 h and 10-21% at 4 h. The NOAEL for
erythrocyte acetylcholinesterase inhibition was therefore
approximately 0.5 mg/kg bw per day for methiocarb, but there was no
NOAEL for the sulfoxide (Hixson, 1981).
Technical-grade methiocarb (purity, 97%) and methiocarb sulfoxide
(purity, 95.2%) were administered orally in gelatine capsules to
groups of two adult beagle dogs and two bitches at a dose of 0.05 or
0.5 mg/kg bw per day for 29 days; two control animals of each sex
received empty capsules. The animals were observed twice daily, and
blood was taken for measurement of plasma and erythrocyte
cholinesterase activity before the first dose, 1, 3, 6, and 24 h after
the first dose, and 3 h after the third dose. During the second week,
blood was taken before the first dose, 2, 6, and 24 h later, and 2 h
after the second dose. During weeks 3 and 4, blood was taken before
the first dose, 2, 6, and 24 h later, and 2 h after the third dose. In
week 5, blood was again taken before the first dose and 2, 6, and 24 h
later; dosing was then stopped, and another blood sample was taken on
the third day.
Clinical signs of toxicity (salivation and vomiting) were
observed at the higher dose of each test material and in animals of
each sex; additionally, slight salivation was observed in one bitch
given the sulfoxide at the lower dose. Depression of cholinesterase
activity was calculated by comparison with the level measured just
before the first dose each week: inhibition was variable, but peak
inhibition occurred 0-3 h after dosing; considerable inhibition was
seen with both materials at the higher dose in animals of each sex. At
the lower doses, smaller depressions of both plasma and erythrocyte
cholinesterase activity, of up to about 20%, were sometimes seen with
both materials. Cholinesterase activity was generally normal by 6 h
(Hayes, 1981).
At equimolar concentrations, methiocarb was the least effective
of four carbamates in inhibiting bovine erythrocyte
acetycholinesterase and equine plasma cholinesterase activity
(Barthová et al., 1989).
Methiocarb was reported not affect liver function in rabbits at a
single oral dose of 25 mg/kg bw (Kimmerle, 1960).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of methiocarb and
putative metabolites of methiocarb are shown in Table 1.
No erythema or oedema was seen when technical-grade methiocarb
(purity unspecified) was applied to abraded and unabraded skin of six
New Zealand white rabbits. Application to the eyes of six rabbits did
not produce ocular irritation (Crawford & Anderson, 1970).
A study of the skin-sensitizing potential of methiocarb (purity,
97.8% pure) was conducted by the Magnusson and Kligman technique. A
group of 20 guinea-pigs (strain BOR:DHPW) each received methiocarb
intradermally at a concentration of 1% and topically at a
concentration of 25%. There were two control groups of 10 guinea-pigs.
The test animals were first challenged with methiocarb at a
concentration of 25% and then at 12.5%. Although there was a small
excess in the number of animals that reacted positively after the
second challenge, the overall result was considered to be negative
(Mihail, 1984).
In a test for dermal sensitization by the Buehler technique,
technical-grade methiocarb (purity, 99.2%) was applied to 15 Hartley
guinea-pigs once weekly for three weeks, with a challenge dose a
fortnight later. The material was applied as a single dose to five
previously unexposed animals at the time of the challenge dose.
Dinitrochlorobenzene, used as the positive control, was applied in the
same way to a further group of five guinea-pigs, weekly for three
weeks, with a challenge dose two weeks later, and to a further group
of five as a single dose at the time of the challenge dose. Methiocarb
was not considered to be a dermal sensitizer (David, 1988).
A case of allergic contact dermatitis was reported in a man who
grew carnations. He developed acute severe eczema of the hand and
showed a positive reaction in a patch test to 0.5% methiocarb (Willems
et al., 1997).
(b) Short-term studies of toxicity
Rats
Methiocarb in aqueous tragacanth suspension was given by gavage
to albino rats daily. The dose was 2 mg/kg bw per day for the first
three days and 4 mg/kg bw per day for the next 24 days. Two groups of
three animals were killed every week for determination of
cholinesterase activity. The activity had fallen to 80% of the control
values after 14 days and to 50% by the end of the study. No abnormal
clinical signs were observed; the animals gained weight normally
(Kimmerle, 1960).
Table 1. Acute toxicity of methiocarb and putative metabolites
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb
Rats Sprague-Dawley M Oral 130 Technical DuBois & Raymund (1962)
F 140
Rats Sprague-Dawley F Oral 100 Recrystallized DuBois & Raymund (1962)
Rats NR NR Oral 67 NR Kimmerle (1966a)
Rats Sprague-Dawley M Oral 30 (20-45) 99 Crawford & Anderson (1973)
F 30 (20-45)
Rats Sprague-Dawley M Oral (non-fasting) 46 (38-56) 99 Crawford & Anderson (1973)
F 47 (36-63)
Rats Sprague-Dawley M Oral 15 (9-26) Technical Lamb & Matzkanin (1976a)
F 31 (18-54)
Rats Sprague-Dawley M Oral 13 (9-17) Technical Lamb & Matzkanin (1976b)
F 32 (24-44)
Rats Sprague-Dawley M Oral 14 (12-16) Technical Lamb & Matzkanin (1977)
F 16 (13-20)
Rats Sprague-Dawley M Oral (non- 51 (45-58) Technical Lamb & Matzkanin (1977)
F 79 (65-96)
Rats NR M Oral 22 (19-25) 98.5 Flucke (1978)
F 24 (21-28)
Rats NR M Oral 22.1 (18-27) 98.3 Flucke (1980)
Rats Sprague-Dawley- M Oral 33 (22-50) 98 Nelson (1979)
derived F 47 (31-70)
Rats NR M Oral 17 (16-19) 98.6 Heimann (1983)
Rats NR M Oral 19 (16-23) 98.2 Flucke (1988)
F 26 (19-36)
Rats Albino M Oral 100 NR Kimmerle (1960)
Rats Wistar-CFN M Oral 87 NR Klimmer (1963)
Rats NR M Oral 33 (29-38) 98.4 Thyssen (1977a)
35 (29-42) 98.3
35 (30-42) 98.2
31 (26-35) 97.8 28 (24-33)97.4
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rats Sprague-Dawley M Intraperitoneal 35 Technical DuBois & Raymund (1961a)
F 30
Rats Sprague-Dawley F Intraperitoneal 25 Recrystallized DuBois & Raymund (1962)
Rats Wistar-CFN M Intraperitoneal 43 NR Klimmer (1963)
Rats Sprague-Dawley M Dermal > 200 Technical DuBois & Raymund (1961a)
Rats Sprague-Dawley F Dermal > 300 Recrystallized DuBois & Raymund (1962)
Rats Albino M Dermal > 1000 NR Kimmerle (1960)
Rats Wistar-CFN M Dermal 350-400 NR Klimmer (1963)
Rats NR M Dermal > 500 99.2 Solmecke (1969)
Rats Wistar M Dermal > 5 g 98.1 Thyssen (1977b)
F > 5
Rats Sprague-Dawley M Inhalation (1 h) 1200 mg/m3 a 98.8 Shiotsuka (1987a)
(780-1700)
F 1100 mg/m3 a
(740-1600)
Rats NR M Inhalation 540 g/L3 NR Kimmerle (1966b)
Rats Sprague-Dawley M Inhalation (1 h) > 20 000 g/L Technical Crawford & Anderson (1972a)
F > 20 000 g/L
Rats Sprague-Dawley M Inhalation (4 h) 580 mg/m3 (340-700) 98.8 Shiotsuka (1987b)
F 430 mg/m3 (290-580)
Rats Wistar M Inhalation (4 h) > 320 mg/m3 97.9 Thyssen (1982)
F > 320 mg/m3
Rats Wistar M Inhalation > 300 mg/m3 97.9 Thyssen (1982)
F (5 x 6 h) > 300 mg/m3
Mice NR M Oral 25 (21-30) NR Kohgo (1970)
Mice NR M Oral 52 98.2 Kimmerle (1972)
Mice NR M Subcutaneous 940 (760-1200) NR Kohgo (1970)
Mice Carworth Farm M Intraperitoneal 6 Technical DuBois & Raymund (1961a)
F 5.5
Mice Dierolf Farm F Intraperitoneal 16 Technical Baron et al. (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rabbits New Zealand M Dermal > 2000 99 Crawford & Anderson
white F > 2000 (1972b)
Guinea-pigs NR M Oral 40 Technical DuBois & Raymund (1961a)
Guinea-pigs NR F Oral (50-100) 99.2 Kimmerle (1969a)
Guinea-pigs Albino F Oral 14 (7.9-25) Technical Crawford & Anderson (1972a)
Guinea-pigs NR M Intraperitoneal 17 Technical DuBois & Raymund (1961a)
Dogs Beagle F Oral (10-25) 99.2 Kimmerle (1969b)
Dog Mongrel M Oral ~ 25 Technical Lamb & Matzkanin (1975)
F ~ 25
Chicken NR F Oral 175 Technical DuBois (1962)
Chicken White Leghorn F Oral 380 (300-490) 98.5 Thyssen & Schilde (1978)
Methiocarb phenol
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR M Dermal > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Methiocarb phenol sulfoxide
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Methiocarb phenol sulfone
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb sulfoxide
Rats NR NR Oral 43 (37-50) NR Solmecke (1970)
Rats Sprague-Dawley M Oral 9 (7-13) NR Lamb & Matzkanin (1976a)
F 7
Rats Sprague-Dawley M Oral 6 (5-8) NR Lamb & Matzkanin (1976a)
F 8 (6-10)
Methiocarb sulfone
Rats NR NR Oral > 1000 NR Solmecke (1970)
Hydroxymethyl methiocarb
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfone
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfoxide
Rats NR M Oral > 160 NR Nelson (1979)
F > 160
Technical, purity unstated; NR, not reported
a There was a large descrepancy between nominal and measured concentrations owing to deposition in the chamber;
these figures were measured gravimetrically.
Methiocarb was administered by gavage to groups of 25 male
Wistar-CFN rats at a dose of 2.5 or 5 mg/day on six days per week for
six months. Three deaths occurred at the higher dose due to
bronchopneumonia, but weight gain was the same in the two treated
groups and in 20 control animals; no abnormal clinical signs were seen
(Klimmer, 1963).
Groups of 12 Sprague-Dawley rats of each sex were fed diets
containing methiocarb (purity unspecified) at concentrations of 0, 5,
10, or 50 ppm for 16 weeks. Five animals of each sex from each group
were examined histopathologically, and five of each sex were used for
measurements of cholinesterase activity in blood, brain, and
submaxillary glands by a manometric method. No effect on growth rate,
food intake, or mortality rates was observed at any dose. In males at
the highest dose, serum, erythrocyte, brain, and submaxillary gland
cholinesterase activity was inhibited by 21, 14, 12, and 7%,
respectively. In females at the highest dose, serum cholinesterase
activity was reduced by 28% and erythrocyte acetylcholinesterase
activity by 15%, all by comparison with concurrent controls. Brain
acetylcholinesterase activity was reduced by 5% in females, and
inhibition of submaxillary gland enzyme was seen in all test groups. A
NOAEL could not be identified in this study (Doull et al., 1962).
Groups of 10 Wistar rats of each sex were exposed to an aerosol
of methiocarb (purity, 97.9%) of a mass median diameter of 2.1-2.5.
The groups were exposed to either air, solvent, methiocarb at 6 mg/m3
in solvent, methiocarb at 23 mg/m3 in solvent, or methiocarb at 96
mg/m3 in solvent; exposure was for 6 h/day, five days per week for
three weeks. The animals were examined daily and weighed weekly. Blood
was taken from five animals per group at the end of the study and was
used for haematological and clinical chemical tests; urinary analyses
were also carried out. Plasma and erythrocyte cholinesterase activity
was determined before treatment and after 5, 10, and 15 exposures for
all groups except the air controls. At the end of the study, the
animals were sacrificed and autopsied, selected organs were weighed
and processed for histopathological examination, and brain
acetylcholinesterase activity was measured.
No deaths were observed in any group. Animals at the highest
concentration showed clinical signs of compound-related effects
(tremor); no clinical signs were seen at lower concentrations. There
body weight of males at the highest concentration was reduced by
comparison with the air controls but not with the solvent controls. No
change in haematological or clinical chemical parameters was seen that
was attributable to the test material, with the exception of
inhibition of cholinesterases. Plasma and brain cholinesterase
activity was decreased at the highest concentration, brain
acetylcholinesterase activity being 61 and 74 % of that in concurrent
solvent controls in males and females, respectively. Some inhibition
of plasma cholinesterase activity was seen at the intermediate
concentration, and males at this concentration had an associated
decrease in brain acetylcholinesterase activity (65% of concurrent
control value). Erythrocyte acetylcholinesterase activity was less
strongly affected than plasma enzyme, but marginal inhibition was
observed in males at the highest concentration at week 1 (82% of
concurrent solvent control value). There were no toxicologically
significant alterations in organ weights, and no compound-related
findings were noted on histopathological examination. The NOAEL was
6 mg/m3 on the basis of reduced brain acetylcholinesterase activity
in males (Thyssen & Mohr, 1983).
Rabbits
Technical-grade methiocarb (purity, 99.2%) was applied to five
male and five female chinchilla rabbits, daily for two weeks, at a
dose of 500 mg; five males and five females served as controls. The
material was applied for 24 h/day, and new material was applied at the
end of each 24-h period. After the two-week application period, the
animals were observed for a further fortnight. The animals were
inspected daily, and haematological and biochemical studies were
carried out before treatment, at the end of treatment, and two weeks
later. Clinical chemistry was restricted to liver function tests and
urinary analysis; cholinesterase activity was not measured. No
abnormal clinical signs were seen, and there was no effect on
body-weight gain or any perturbation in haematological or clinical
chemical variables (Kimmerle, 1969c).
Methiocarb (purity, 99.3%) was applied at doses of 0, 60, 150, or
375 mg/kg bw per day for 6 h/day to the skin of groups of five male
and five female New Zealand white rabbits, and the site of application
was occluded. The animals were examined twice daily, and any signs of
skin irritation were scored. The rabbits were weighed twice weekly;
food consumption was measured three times weekly until the last week,
when it was measured four times. Blood was taken for haematological
and clinical chemical analysis before treatment and pre-terminally.
Plasma and erythrocyte cholinesterase activity was measured at the end
of the 6-h exposure period on days 1, 7, 14, and 21; additionally,
blood was taken 16 h after the end of exposure on these days from the
group at the high dose. Animals were sacrificed on the day after the
last treatment, at which time the brain was taken for measurement of
acetylcholinesterase activity in a homogenate of the entire left half
of the brain. Selected tissues were weighed, examined grossly,
processed, and examined histopathologically.
Two animals at the low dose did not survive to the end of the
study. No clinical signs related to the test material were seen, and
it was not irritating. There was no differences between the groups in
body weight, but food consumption appeared to be reduced in animals of
each sex at the high dose, and particularly in males. Clinical
chemical parameters did not differ between the groups, and, although
one or two differences in haematological measurements were seen, none
appeared to be related to treatment. Plasma cholinesterase activity
appeared to be reduced in males at the high dose at 14 and 21 days.
The measurements made 6 h after the end of exposure suggested that the
inhibition was not reversed overnight. No intergroup differences in
plasma cholinesterase activity were observed among females.
Erythrocyte acetylcholinesterase activity was very variable but did
not appear to be inhibited in a dose-related fashion. Intergroup
differences were not observed in brain acetylcholinesterase activity.
No gross or microscopic abnormality was observed that was attributable
to the test material. The NOAEL was 150 mg/kg bw per day on the basis
of reduced food consumption (Procter, 1988).
Methiocarb (purity, 97.5%) was applied to the skin of five male
and five female New Zealand white rabbits at a single dose of 500
mg/kg bw per day for 6 h/day under occlusion. Five controls of each
sex received saline. The animals were examined twice daily, and any
signs of skin irritation were scored. They were weighed twice weekly,
while food consumption was measured every two days. Blood was taken
for haematological and clinical chemical tests before treatment and
preterminally. Plasma and erythrocyte cholinesterase activity was
measured at the end of the 6-h exposure period on days 1, 7, 14, and
21 and, in the test animals, 16 h later. Animals were sacrificed on
the day after the last treatment, at which time the left half of the
brain was taken for measurement of cholinesterase activity. Selected
tissues were weighed, examined, processed, and examined
histopathologically.
Two test animals removed their dressings and presumably ingested
the material; these animals developed clinical signs of cholinergic
poisoning, which disappeared within a few hours. No other
compound-related clinical signs were seen, and no animal died before
completion of the study. Treated females were lighter than controls
throughout the study, and the food consumption of animals of each sex
was reduced. A reduction in serum calcium and increased activities of
alanine and aspartate aminotransferases were seen in females in
comparison with concurrent controls. Plasma cholinesterase activity
was lower than that before treatment in both males and females, but
only inconsistently in comparison with concurrent controls.
Erythrocyte acetylcholinesterase activity was reduced in males on day
1, at both 6 and 16 h, in comparison with pretreatment levels, but
inconsistently in comparison with concurrent controls; it was
concluded that compound-related inhibition of erythrocyte
acetylcholinesterase activity had not occurred in either males or
females. Brain acetylcholinesterase activity was not inhibited. No
abnormality was seen at autopsy or on histopathological examination.
The design of this study was not appropriate for identifying a NOAEL
(Procter, 1989).
Cats
Methiocarb at a dose of 5 mg/kg bw per day, given daily by gavage
to cats, was reported to have no adverse effects (Kimmerle, 1960).
Dogs
Methiocarb was incorporated into the diet of groups of two male
and two female beagles at a concentration of 0, 50, 100, or 250 ppm,
equal to 0, 1.25, 2.5, or 6.25 mg/kg bw per day. The animals were
examined daily and weighed fortnightly; plasma and erythrocyte
cholinesterase activity was measured weekly. No clinical effects were
seen, and body-weight gain was unaffected by treatment. There was no
clear difference in plasma or erythrocyte cholinesterase activity
between the test groups and concurrent controls. The small group size
renders this study inappropriate for identifying a NOAEL (Root et al.,
1963).
Chickens
Chickens (Gallus gallus Babcock 300) were fed diets containing
a 9:1 mixture of methiocarb and methiocarb sulfoxide (based on studies
of plant metabolism) at a dose of 20, 60, 120, or 360 ppm, equal to
2.5, 7.5, 15, and 45 mg/kg bw per day, over 28 days. Treatment
decreased feed consumption in a dose-related fashion, and the body
weights of birds at the two highest doses were decreased. Egg
production was unaffected. Plasma cholinesterase activity was
decreased by 40-50 % in comparison with concurrent controls at the
three highest doses but was unaffected at the lowest dose (Strankowski
& Minor, 1976).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female BOR:CFW1 mice received diets
containing methiocarb (purity, 98.5%) at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 15, 43, and 130 mg/kg bw per day in males and
0, 20, 57, and 170 mg/kg bw per day in females. Haematological and
clinical chemical tests were performed on five animals of each sex per
dose at 12 months and 10 animals of each sex per dose at 24 months,
and on satellite groups of 15 mice of each sex per dose, which were
sacrificed after one year and necropsied. Cholinesterase activity was
determined in the plasma of five animals of each sex per dose at 1 and
12 months and on 10 animals of each sex per dose (or the survivors if
fewer than 10) at the end of the study. Brain cholinesterase activity
was determined at the end of the study. Animals that died or became
moribund were autopsied, as were 10 mice of each sex per group at 12
months. All surviving animals were sacrificed at the end of the study
and autopsied, and selected organs were weighed, examined, and
processed for histopathological examination.
The appearance, behaviour, mortality rate, and food consumption
of the animals were not affected at any dose, except for a slight
decrease in body weight at the highest dose, throughout the study in
males and up to week 30 in females. Overall survival in this study was
not good: survival at 18 months was 74% of male controls, 47% males at
the low dose, 59% males at the intermediate dose, 66% males at the
high dose, 69% of female controls, 53% at the high dose, 64% at the
intermediate dose, and 69% at the high dose. Intergroup differences in
mortality rates did not appear to be compound-related, as mortality
was higher in males receiving 67 or 200 ppm than in those given the
high dose. Moreover, survival of females up to the end of the study
was poorest for controls. All treated male mice had higher mean
corpuscular haemoglobin concentrations than controls at 12 months, and
males at the highest dose had higher mean corpuscular haemoglobin
values; similar changes were not seen in females. At 24 months, the
mean corpuscular haemoglobin concentration of males at 200 and 600 ppm
was decreased and the mean corpuscular haemoglobin value at 600 ppm,
again, with no similar change in the females. Higher leukocyte counts
were observed in all treated females at 24 months, but the authors
ascribed this finding to high individual values; no perturbution of
the differential count was seen.
No clinical chemical abnormalities were found at 12 months, but
at 24 months the activity of alanine aminotransferase (ALAT) was
increased in animals at 200 and 600 ppm. At one month, males at 200
and 600 ppm had reduced plasma cholinesterase activity (by 51 and 34%
in comparison with concurrent controls), while reductions of 5% or
less were seen at 12 months and 5-11% at 24 months. In females, plasma
cholinesterase activity was inhibited by 24, 43, and 34% at the low,
intermediate, and high doses, respectively, at one month; smaller
reductions (< 12%) were observed at 12 months, and no cholinesterase
inhibition was observed at 24 months. Erythrocyte acetylcholinesterase
activity was not measured. Small reductions in brain
acetylcholinesterase activity were observed, by 5, 11, and 10% in
males at the low, intermediate, and high doses and by 3, 9, and 3% in
females at the three doses, respectively. No compound-related change
in organ weights was seen, nor was there any abnormality in gross of
histological appearance that was related to treatment. Methiocarb was
not tumorigenic. There was no NOAEL because of haematological changes
at all doses in males at 12 months and in females at all doses at 24
months (Krötlinger & Janda, 1983; Krötlinger, 1989).
Rats
Methiocarb (purity, 98.9%) was admixed with the diet of groups of
60 Wistar TNO W.74 rats for two years at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 3.3, 9.3, and 29 mg/kg bw per day for males
and 0, 5, 14, and 42 mg/kg bw per day for females. The rats were
inspected daily, and body weights were determined weekly for the first
26 weeks and then at fortnightly intervals. Haematological, clinical
chemical, and urinary measurements were made in 10 animals of each sex
per dose at 3, 6, 12, and 24 months. Cholinesterase activity in plasma
and erythrocytes was determined one and two days after the start of
the study and at 1, 2, 4, 8, 13, 26, 52, 78, and 105 weeks in 10
animals of each sex per dose. Brain acetylcholinesterase activity was
determined at the end of the experiment on 10 animals of each sex per
dose. Animals that died and those sacrificed in extremis were
examined grossly and necropsied; when possible, tissues were taken for
histopathological examination. The survivors at two years were also
examined grossly and necropsied, and tissues were taken for weighing
and histopathological examination.
None of the doses had any effect on the appearance, behaviour, or
mortality rates of rats of either sex. The mortality rates at two
years were 10-20% for males and 23-32% for females. There was no
significant intergroup difference in food consumption. Weight gain was
not depressed at 67 or 200 ppm in comparison with the controls; at 600
ppm, weight gain was slightly but consistently depressed throughout
the period of administration of methiocarb, and at termination total
body weight was reduced in the group at the high dose. At three
months, an increased leukocyte count was seen in females at the
highest dose and an increased reticulocyte count in females at the two
higher doses. At six months, the mean corpuscular haemoglobin
concentration was reduced in males at the highest dose, and some
elevation in leukocyte count was found in males at 67 and 200 ppm. Red
blood cell counts and haemoglobin and haematocrit values were
decreased in females at the two higher doses, and reticulocytosis was
observed in these groups. At 12 months, the mean corpuscular
haemoglobin concentration was decreased in males at the intermediate
dose and was increased in females at the highest dose; an increased
reticulocyte count was seen in females at the high dose. At 24 months,
no compound-related changes in haematological variables were seen.
Although a few intergroup differences were observed in
biochemical tests, the only ones that appeared to be related to
treatment were total protein concentration and cholinesterase
activity. Total protein concentrations were raised in females at the
two higher doses at six months and in males at the three highest doses
at 12 months; at 24 months, the total protein concentration was
similar in treated and control groups. ALAT activity in the blood was
elevated in all three groups of treated females at 12 months and in
females at the highest dose at two years, but at no time interval in
males. Increased urea was found in plasma from males at the highest
dose at three and 12 months and in females at 12 and 24 months. Plasma
cholinesterase activity was depressed at the high dose at one day and
from eight weeks onwards in males (except in the 52-week assay) and in
females at one day and 1, 2, 4, and 13 weeks. The measurements of
erythrocyte acetylcholinesterase activity were difficult to interpret:
a statistically significant depression was found only at the low dose
in males at 105 weeks and females at 78 weeks, and the degree of
depression was small (5 and 6%, respectively). Males at the
intermediate dose had significantly depressed activities at 8, 78, and
105 weeks, with 5, 7, and 8% depression, respectively. In females at
this dose, significant depression was seen at four and 78 weeks (8 and
13% depression, respectively). In males at the highest dose,
significant depressions were seen at 8, 13, 78, and 105 weeks (7, 7,
9, and 7%, respectively), and in females at this dose depression was
seen at two days and 4 and 78 weeks (6, 8, and 11%, respectively). The
Committee considered that none of these depressions was biologically
significant. No depression of brain acetylcholinesterase activity was
seen.
Males at the highest dose had decreased absolute weights of the
thyroid, heart, lung, liver, spleen, and adrenals, but these were
reflected only in reductions in the relative weights of the spleen and
therefore probably reflect reduced body weight. In the females, only
the absolute weight of the spleen was reduced, while the absolute
weight of the thyroid was increased. As this finding was due to a
single animal, it is unlikely to be attributable to the test material.
No gross or histopathological abnormality related to treatment was
seen at any dose. Methiocarb was not tumorigenic. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months (Krötlinger et al., 1981; Krötlinger,
1990).
Dogs
Groups of four beagle dogs and four bitches were fed methiocarb
(purity, 98.4%) in the diet at 0, 5, 60, or 240 ppm; the group given 5
ppm group had been given 15 ppm for the first 15 days. These dietary
concentrations were equivalent to daily doses of 0, 0.12, 1.5, and 6
mg/kg bw per day, ignoring the first 15 days of treatment of animals
at the lowest dose. The animals were examined daily, and food
consumption was recorded daily and body weight measured weekly.
Clinical examinations including ophthalmoscopy were undertaken, and
haematological and biochemical variables were measured in blood at
weeks 0, 14, 27, 40, 53, 66, 79, 92, and 104; urine was also analysed.
Erythrocyte and plasma cholinesterase activity was measured before the
start of treatment and at weeks 2, 3, 4, 7, 10, 13, 27, 40, 53, 66,
79, 92, and 104, before feeding and 2 h afterwards.
Acetylcholinesterase activity on the olfactory bulb of the brain was
measured at sacrifice. Animals were examined grossly post mortem,
and selected tissues were examined histologically.
One death occurred, of an animal at 5 ppm, which was considered
not to be related to treatment. The only clinical findings were mild
weakness of the hind limbs, trembling, reduced alertness, and some
vomiting at the highest dose during the first 14 weeks of the study.
The results of tests for reflexes and ophthalmic parameters were
normal. Food intake was reduced in animals of each sex at the highest
dose and in bitches at the intermediate dose, but the body weights
were not significantly affected. Haematological and biochemical
parameters were unaffected, apart from cholinesterase activity. Plasma
cholinesterase activity was depressed at doses of 15 ppm and higher,
and it was for this reason that this dose was reduced to 5 ppm.
Depression of plasma cholinesterase activity was not seen at 5 ppm but
occurred at the two higher doses. Erythrocyte and brain
acetylcholinesterase activity was not consistently inhibited at any
dose; the maximum inhibition of erythrocyte acetylcholinesterase
activity was in animals at the high dose (17% for each sex) and at the
intermediate dose (10% in dogs and 5% in bitches). Organ weights were
unaffected, and no organ-specific toxicity observed. The NOAEL was 60
ppm, equivalent to 1.5 mg/kg bw per day, on the basis of clinical
signs. The reduced food intake of bitches at the intermediate dose was
not considered relevant (Hoffman & Schilde, 1980).
(d) Genotoxicity
The results of assays for the genotoxicity of methiocarb are
shown in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 10 female FB 30 rats (Elberfield breed)
received technical-grade methiocarb (purity, 99%) admixed with the
diet at concentrations of 0, 30, 100, or 300 ppm, equivalent to 3, 10,
and 30 mg/kg bw per day. The rats were weighed weekly. The F0
generation was mated twice, first after 70 days of treatment and again
after 149 days, to produce the F1a litters, which were sacrificed,
and the F1b litters. Ten males and 10 females from each group of F1b
rats were used to produce the next generation and were again mated
twice. The resultant F2a rats were sacrificed, while 10 male and 10
female F2b rats were mated twice to produce the F3a and F3b
generations, which were sacrificed. The rats were weighed weekly, and
the body weights of the offspring were measured at birth, five days
after birth, one week after birth, and then weekly. The pups were
examined grossly for malformations immediately after birth and during
lactation. The F3a young were killed four weeks after birth and the
F3b rats at three weeks. Blood samples were taken for analysis from
the F0 generation at the end of the preliminary treatment period and
after rearing of the second little (Löser, 1969).
In the F0 generation, there was no significant difference in
weight gain between the groups, and no treatment-related changes were
seen in haematological parameters at either sampling time. Although
ALAT and aspartate aminotransferase activities were higher in animals
at the highest dose at the earlier sampling time, they were stated to
be within the normal range for the laboratory; similar increases were
not seen at the later sampling time. The gestation rate was lower at
the highest dietary concentration, but was still within the normal
range. The weight gain of F1a pups was similar in all groups, and no
malformations were seen at sacrifice. After the second mating, there
were no dose-related changes in litter size or pup weight and weight
gain. No significant differences were seen between the groups of F1b
pups. Their weight gain after weaning was similar in all groups. After
the first mating, the gestation rate was lower at the highest dietary
concentration but was still within the normal range. The weight gain
of F2a pups was similar in all groups, and no malformations were seen
at sacrifice. After the second mating, the mean weight of the group at
300 ppm was significantly lower than that of controls at the end of
the four-week lactation period; however, there were no significant
intergroup differences in body weight at sacrifice of the F2b
animals. After both matings of these rats, the gestation rate was
similar and the litter size and pup weights were comparable in all
Table 2. Results of assays for the genotoxicity of methiocarb
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 4-2500 µg/plate 98.5 Negative Herbold (1978)
TA1535, TA1537,
TA98, TA100
Reverse mutationa S. typhimurium 20-12 500 µg/plate 98.4 Negative Herbold (1986)
TA1535, TA1537,
TA98, TA100
DNA damagea E. coli pol A+ and 625-10000 µg/plate 98.6 Negative Herbold (1983)
pol A-
Gene mutation Chinese hamster 1.25-60 µg/ml 99.3 Negative Lehn (1989)
ovary cells, hprt
Unscheduled DNA Rat primary 0.1-100 µg/ml 98.8 Negative Curren (1988)
synthesis hepatocytes
Chromosomal Chinese hamster 4.92-497 µg/ml 99.4 Positive Murli (1990)
aberrationa ovary cells
Sister chromatid Chinese hamster 2-40 µg/ml 98.2 Negative Putman (1986)
exchangea ovary cells
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
Micronucleus Mice 5,10, and 20 98.5 Negative Herbold (1979a)
formation mg/kg bw
twice orally
Dominant lethal Mice 6 mg/kg bw 98.5 Negative Herbold (1979b)
mutation
a With and without metabolic activation
groups. The body weights of the F3b, but not the F3a, groups at 100
and 300 ppm during lactation were lower than those of controls. This
finding may be due to larger litter size. No abnormalities were found
at birth or sacrifice in F3a or F3b pups, and no abnormalities were
found at autopsy of the F0, F1b, and F2b generations. The NOAEL was
300 ppm, equivalent to 30 mg/kg bw per day, the highest dose tested
(Löser (1970).
(ii) Developmental toxicity
Rats
Groups of 19-20 fertilized FB 30 rats received 10 oral doses of
0, 1, 3, or 10 mg/kg bw per day methiocarb (purity, 98.9%) by gavage
on days 6-15 of gestation. The highest dose reduced weight gain, but
no other effects were observed. No effects were seen on the number of
implantations or resorptions or the weights of fetuses or placentas.
No teratogenic effects were seen, nor was fetotoxicity observed. The
NOAEL for maternal toxicity was 3 mg/kg bw per day, and that for fetal
toxicity was 10 mg/kg bw per day, the highest dose tested (Lorke,
1971; see also Renhof, 1988).
Rabbits
Methiocarb (purity, 97.3%) was administered by gavage to groups
of 17 pregnant New Zealand white rabbits at doses of 1, 3, or 10 mg/kg
bw per day on days 6-18 of gestation. A group of 19 rabbits were used
as controls. On day 29 of gestation, the rabbits were sacrificed and
examined; the uterus was weighed, and the numbers of corpora lutea,
implantation sites, and resorption sites, and the number and
distribution of live and dead fetuses were noted. The weight and sex
of the fetuses and placental weights were recorded and the fetuses
examined for abnormalities. Rabbits receiving the highest dose showed
clinical signs of cholinergic poisoning and initial marked weight
loss. Weight gain was also reduced at 3 mg/kg bw per day towards the
end of the study, but this was attributable to a single animal. Weight
gain was unaffected at the lowest dose. The litter parameters were
similar in all groups, and there was no indication of teratological
effects. The NOAEL for maternal toxicity (clinical effects and weight
loss at the highest dose) was 3 mg/kg bw per day . The NOAEL for
fetotoxicity was 10 mg/kg bw per day, the highest dose tested (Tesh et
al., 1981).
Methiocarb (purity, 99.4-99.6%) was applied under occlusion for
6 h/day to about 10% of the body area of groups of 16 mated chinchilla
rabbits at doses of 0, 10, 50, or 250 mg/kg bw per day on days 6-18
post coitum. The animals were sacrificed after 28 days and the fetuses
removed. One animal at the high dose was sacrificed because of a
broken femur. No clinical signs were observed at any dose, nor was
irritation of the skin noted. At the highest dose, reduced food
consumption was observed between 11 and 15 days post coitum and
body-weight loss between 6 and 8 days post coitum, whereas at the
lower doses,no such effect was seen. The numbers of corpora lutea,
implantations, pre- and post-implantation losses, and live fetuses
were not affected by treatment. There was a slight decrease in mean
fetal weight at the highest dose (4%), and slight retardation of
skeletal development was noted at this dose, as shown by incomplete or
no ossification of the phalangeal nuclei. No effects on the fetuses
were seen at lower doses. The NOAEL for both maternal and fetal
toxicity was 50 mg/kg bw per day (Dotti & Biedermann, 1992).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
(ii) Neurotoxicity
In a poorly described study, no persistent paralytic effects were
produced in hens (Gallus gallus) of unstated strain given a single
dose of methiocarb by gavage and observed for 14 days (DuBois, 1962).
Methiocarb (purity, 98.5%) was administered twice at a dose of
380 mg/kg bw (approximately the LD50) to 20 white Leghorn laying
hens, aged 15-20 months, after an intramuscular injection of 50 mg/kg
bw atropine sulfate. After an interval of 21 days, the 18 surviving
hens were treated again, with the same doses of atropine and
methiocarb. The 16 survivors of the second treatment were sacrificed
three weeks later, and brain, spinal cord, and portions of peripheral
nerve were excised and processed for histological examination. Five
positive controls were given tri- ortho-cresyl phosphate as a single
oral dose and sacrificed three weeks later. The hens treated with
methiocarb exhibited brief cholinergic signs and were lethargic, but
none showed ataxia or paralysis. At examination, post mortem there
were no histopathological changes indicative of polyneuropathy in
either the central or peripheral nervous system, whereas the hens
treated with tri- ortho-cresyl phosphate showed the classical signs
of delayed polyneuropathy and mild but characteristic signs of delayed
polyneuropathy on histopathological examination (Thyssen & Schilde,
1978).
(iii) Antidotes
A study was carried out in which the effect of antidotes to an
oral LD50 of methiocarb was investigated in rats. The regimens used
were atropine at 50 mg/kg bw, pralidoxime (salt unstated) at 50 mg/kg
bw, obidoxime at 20 mg/kg bw, atropine and pralidoxime at 50 mg/kg bw
each, or atropine at 50 mg/kg bw with obidoxime at 20 mg/kg bw. All
the antidotes were given intraperitoneally just before clinical signs
became evident, i.e. 1-2 min after administration of methiocarb. The
LD50 values were 67 mg/kg bw without antidotes, 470 mg/kg bw with
atropine, 190 mg/kg bw with pralidoxime, 220 mg/kg bw with obidoxime,
500 mg/kg bw with pralidoxime and atropine, and 510 mg/kg bw with
obidoxime and atropine. Thus, pralidoxime and obidoxime added little
to the antidotal effect of atropine (Kimmerle, 1966a).
In mice, 10 mg/kg bw of atropine increased the LD50 of
methiocarb by sevenfold, while pralidoxime methiodide at 100 mg/kg bw
increased the LD50 1.3 times. Administration of the two antidotes
together increased the LD50 by 21 times. Obidoxime at 50 mg/kg bw
increased the LD50 3.1 times, while obidoxime plus atropine increased
it by 9.1 times (Kohgo, 1970).
Atropine sulfate at 50 mg/kg bw administered within 10 min of
methiocarb intraperitoneally increased the LD50 of methiocarb given
orally to rats by more than sixfold, and tetraethyl ammonium chloride
given by the same route increased it by approximately fourfold;
however, the combined effect of the two antidotes was less than that
of atropine alone (Kimmerle, 1971).
In the study of Thyssen and Schilde (1978) in hens at
approximately the LD50 (see above), atropine appeared to have a
powerful antidotal effect.
(iv) Interaction with other pesticides
When an intraperitoneal dose equal to 50% of the LD50 of
methiocarb was given to female Sprague-Dawley rats in combination with
another pesticide at a dose equal to 50% of the LD50, the combined
mortality was less than 50% in all cases. The highest combined
mortality rates (> 40 to < 50%) were seen with EPN, Guthion,
mevinphos, and carbaryl. Combined mortality rates of > 20 to < 40%
were found with parathion, parathion-methyl, disulfoton, malathion,
carbophenothion, tributyl phosphorotrithioite, and ethion. The
mortality rates seen with the remaining insecticides (demeton,
dioxathion, and schradan) were 5-20% (DuBois & Raymund, 1961b). In
another study of similar design, the combined mortality rates were 55%
with trichlorfon, 50% with propoxur, 35% with coumaphos, 30% with
oxydemeton-methyl, and 20% with fenthion (DuBois & Raymund, 1961c).
3. Observations in humans
The 250 workers in two plants manufacturing methiocarb over about
20 years were subjected to annual medical examinations and assays of
cholinesterase activity in whole blood. No adverse health effects or
changes in laboratory parameters were observed (Faul, 1993).
Comments
Methiocarb appeared to have been well absorbed in a small study
of absorption, distribution, metabolism, and excretion of the
radiolabelled compound in rats. More than 70% of the administered
radiolabel was excreted within 48 h, mostly in the urine. Methiocarb
was extensively metabolized. The main route of metabolism in both
animals and plants appears to be to methiocarb phenol, methiocarb
phenol sulfoxide, and methiocarb phenol sulfone. In some studies,
methiocarb sulfoxide was also found.
In single-dose and short-term studies in rats, methiocarb
administered by gavage inhibited plasma and erythrocyte cholinesterase
activity. In the short-term study, plasma, erythrocyte, and brain
cholinesterase activities were depressed at 10 mg/kg bw per day; the
NOAEL for this effect was 3 mg/kg bw per day. In a short-term study of
the ability of methiocarb or its sulfoxide to inhibit cholinesterase
activity in rats, the NOAEL for inhibition of erythrocyte
acetylcholinesterase activity was 0.5 mg/kg bw per day for methiocarb,
but a NOAEL was not identified for the sulfoxide. In a 29-day study in
which dogs were given methiocarb or methiocarb sulfoxide in gelatine
capsules at 0.05 or 0.5 mg/kg bw per day, both plasma and erythrocyte
cholinesterase activities were inhibited by both treatments.
The acute toxicity of methiocarb given by a number of routes has
been measured in a number of species. The oral LD50 in fasting rats
ranged from 13 to 130 mg/kg bw. The oral LD50 values for methiocarb
phenol, methiocarb phenol sulfoxide, and methiocarb phenol sulfone in
rats are all greater than 1 g/kg bw, as is that of methiocarb sulfone,
but that of methiocarb sulfoxide is between 6 and 43 mg/kg bw.
WHO has classified methiocarb as moderately hazardous (WHO,
1996).
The short-term toxicity of methiocarb has been tested in rats,
rabbits, cats, dogs, and chickens. Few of these studies were
appropriate for identifying NOAEL values and, of those that were, a
study in rats was carried out by inhalation and that in rabbits by
dermal application. In the study in rats, which were exposed by
inhalation, for five days per week for three weeks, no findings
related to treatment were seen on histopathological examination but
depressed brain acetyl-cholinesterase activity was observed at the
highest dose in animals of each sex and in males at the intermediate
dose. Consequently, the NOAEL was 6 mg/m3 per day. In a 21-day study
of the dermal toxicity of methiocarb in rabbits, the substance was
applied for 6 h/day at 0, 60, 150, or 375 mg/kg bw per day. Plasma
cholinesterase activity was reduced in males at the highest dose, but
no significant differences were seen between groups in the activities
of erythrocyte and brain acetylcholinesterase. The NOAEL was 150 mg/kg
bw per day on the basis of reduced food consumption at the highest
dose.
In a long-term study of toxicity in mice, methiocarb was
administered at dietary concentrations of 0, 67, 200, or 600 ppm. A
NOAEL was not identified because haematological changes were observed
in all treated males at 12 months and in all treated females at 24
months. The LOAEL was 67 ppm, equal to 15 mg/kg bw per day, on the
basis of minor haematological changes. Methiocarb was not carcinogenic
in mice.
In a two-year study of toxicity, rats received methiocarb at
dietary concentrations of 0, 67, 200, or 600 ppm. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months. Methiocarb was not carcinogenic.
In a two-year study of toxicity, dogs were fed methiocarb in the
diet at 0, 5, 60, or 240 ppm. The NOAEL was 60 ppm, equivalent to 1.5
mg/kg bw per day, on the basis of reversible clinical signs at the
next highest dose, which were not observed after 15 weeks.
Three studies of developmental toxicity were available, one in
rats and two in rabbits. Fertilized rats received methiocarb by gavage
on days 6-15 of gestation at 0, 1, 3, or 10 mg/kg bw per day. The
NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of
reduced body-weight gain at the highest dose. As no fetotoxicity was
observed, the NOAEL for this end-point was 10 mg/kg bw per day, the
highest dose tested. In a study of developmental toxicity in rabbits,
pregnant animals received methiocarb at 0, 1, 3, or 10 mg/kg bw per
day by gavage on days 6-18 of gestation. The NOAEL was 3 mg/kg bw per
day for maternal toxicity on the basis of clinical effects and weight
loss at the highest dose. As no fetotoxicity or teratogenicity was
observed, the NOAEL for these end-points was 10 mg/kg bw per day, the
highest dose tested. In a further study, rabbits received methiocarb
by dermal application at 0, 10, 50, or 250 mg/kg bw per day for
6 h/day on days 6-18 of gestation. The NOAEL for both maternal and
fetal toxicity was 50 mg/kg bw per day on the basis of reduced
maternal food consumption and some decrease in mean fetal weight at
the highest dose; slight retardation of fetal development was also
observed. A multigeneration study of reproductive toxicity was
undertaken in rats given methiocarb at dietary concentrations of 0,
30, 100, or 300 ppm. The NOAEL was 300 ppm, equivalent to 30 mg/kg bw
per day, as no effect clearly related to treatment was observed. No
teratogenic effect was observed in any of these studies.
Methiocarb has been tested for genotoxicity in an adequate
battery of tests in vitro and in vivo. The Meeting concluded that
methiocarb is not genotoxic.
Methiocarb did not cause skin sensitization in studies conducted
by either the Magnusson and Kligman or the Beuhler technique.
In an early study of neurotoxicity in hens, methiocarb did not
cause delayed polyneuropathy of the organophosphorus type. Atropine
has consistently been shown to be an effective antidote for
methiocarb, while the effects of pyridinium oximes were somewhat
inconsistent.
The Meeting established an ADI of 0-0.02 mg/kg bw on the basis of
the NOAEL of 1.5 mg/kg bw per day in the two-year study of toxicity in
dogs and a safety factor of 100. This ADI results in a further safety
factor of 10 on the LOAEL in the long-term study of toxicity in mice.
An acute RfD was allocated on the basis of the NOAEL of 1.5 mg/kg
bw per day in the two-year study in dogs, because the signs observed
were acute, and a safety factor of 100. Of the shorter studies in
dogs, neither the 29-day nor the 12-week study was considered by the
Meeting to be adequate for the purpose of establishing a NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: No NOAEL; LOAEL: 67 ppm, equal to 15 mg/kg bw per
day (long-term study of toxicity)
Rat: 67 ppm, equal to 3.3 mg/kg bw per day (long-term
study of toxicity)
300 ppm, equivalent to 30 mg/kg bw per day
(maternal and fetal toxicity in a study of
reproductive toxicity)
3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (two-
year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would be useful for continued evaluation of the
compound
1. A modern study of absorption, distribution, metabolism, and
excretion
2. A modern multigeneration study of reproductive toxicity
3. Further observations in humans
List of end points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption No data
Dermal absorption No data
Distribution No data
Potential for accumulation Not likely to accumulate
Rate and extent of excretion 73 to > 90% within 48 h
Metabolism in animals Sulfoxidation and loss of carbamate side-chain
Toxicologically significant compounds Parent compound and methiocarb sulfoxide
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral 13-135 mg/kg bw
Rat: LD50, dermal 350 to > 5000 mg/kg bw
Rat: LC50 inhalation > 300 mg/m3
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Clinical signs
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 150 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 6 mg/m3
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Clinical signs
Lowest relevant NOAEL Dog: 1.5 mg/kg bw per day
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect No effect
Lowest relevant reproductive NOAEL Rat: 30 mg/kg bw per day
Developmental target/critical effect Maternal toxicity: reduced weight gain; no
fetotoxicity observed
Lowest relevant developmental NOAEL Rabbit: 3 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Does not cause delayed polyneuropathy
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Dog, 2 years 100
Acute reference dose 0.02 mg/kg bw Dog, 2 years 100
References
Baron, R.L., Casterline, J.L. & Fitzhugh, O.G. (1964) Specificity of
carbamate esterase inhibition in mice. Toxicol. Appl. Pharmacol., 6,
402-410.
Barthová, J., Woldemariam, M.G. & Leblová, S. (1989) The effect of
carbamates insecticides on cholinesterases of nontarget organisms.
Biologia (Bratislava), 44, 353-356.
Crawford, C.R. & Anderson, R.H. (1970) The skin and eye irritating
properties of BAY 37344 technical to rabbits. Unpublished report No.
28304 from Chemagro Corporation, Research Department, 15 October 1970.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972a) The acute oral toxicity to
guinea pigs and the acute inhalation toxicity to rats of Mesurol(R)
technical. Unpublished report No. 31987 from Chemagro Corporation,
Research Department, 24 January 1972. Submitted to WHO by Bayer AG
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972b) The dermal toxicity of
(R)Mesurol technical and Mesurol 75% wettable powder to rabbits.
Unpublished report No. 34477 from Chemagro Division of Baychem
Corporation, Research Development, 21 August 1972. Submitted to WHO by
Bayer AG Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral toxicity of
(R)Mesurol technical to rats. Unpublished report No. 34008 from
Chemagro Division of Baychem Corporation, Research and Development, 31
May 1972 and revised 8 May 1973. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 1007 from Microbiological
Associates Inc., Bethesda, Maryland and Rockville, Maryland, USA, 1
June 1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
David, R.M. (1988) EPA/OECD dermal sensitization test of Mesurol
technical in guinea pigs. Unpublished report No. 1015 from
Microbiological Associates, Inc., Bethesda, Maryland, USA, 15 April
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dotti, A. & Biedermann, K. (1992) Embryotoxicity study (including
teratogenicity) with H 321 (c.n. methiocarb) in the rabbit (dermal
application) part 1. Unpublished report No. R 5627 from RCC Research &
Consulting Co. Ltd and RCC Umweltchemie AG, Itingen, Switzerland, 6
August 1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Doull, J., Cowan, J. & Root, M. (1962) Subacute (16-week) oral
toxicity of Bayer 37344 to male and female rats. Unpublished report
No. 9456 from Department of Pharmacology, University of Chicago, USA,
12 June 1962. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. (1962) The acute oral toxicity of Bayer 37344 to
chickens. Unpublished report No. 9248 from Department of Pharmacology,
University of Chicago, USA, 16 May 1962. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K.P. (1964) The acute toxicity of some possible metabolites of
Bayer 37344. Unpublished report No. 12806 from Department of
Pharmacology, University of Chicago, USA, 22 January 1964. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961a) The acute toxicity of Bayer 37344
to mammals. Unpublished report No. 6775 from Department of
Pharmacology, University of Chicago, USA, 25 April 1961. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961b) The acute toxicity of Bayer 37344
in combination with other anticholinesterase insecticides to rats.
Unpublished report No. 7880 from Department of Pharmacology,
University of Chicago, USA, 1 November 1961. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961c) The acute toxicity of Bayer 39007
and Bayer 37344 in combination with some other anticholinesterase
insecticides in rats. Unpublished report No. 7879 from Department of
Pharmacology, University of Chicago, USA, 4 November 1961. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1962) The acute toxicity of
recrystallised Bayer 37344 and a wettable powder of Bayer 37344 to
female rats. Unpublished report No. 9424 from Department of
Pharmacology, University of Chicago, USA, 12 June 1962. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Eben, A. & Kimmerle, G. (1973) Mercaptodimethur effect of acute and
subacute oral doses on acetylcholinesterase activity in plasma,
erythrocyte and brain of rats. Unpublished report No. 4284 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 9 November
1973. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Faul, J. (1993) Mesurol active ingredient: In-company occupational
medical experience. Unpublished letter to Dr Heimann, PF-A/consulting,
Monheim, Germany, 1 April 1993. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50). Unpublished
letter to Dr Reuver, Bayer AG Institute of Toxicology, dated 27 June
1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) H 321 (mercaptodimethur). Unpublished note dated 3
November 1980. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1988) Determination of acute toxicity (LD50), Unpublished
letter to Dr Reuver, Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany, dated 1 August 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Haijar, N.P. & Hodgson, E. (1982) Sulfoxidation of
thioether-containing pesticides by the flavin-adenine
dinucleotide-dependent monooxygenase of pig liver microsomes.
Biochem. Pharmacol., 31, 745-752.
Hayes, R.H. (1981) Cholinesterase evaluation study of methiocarb
technical and methiocarb sulfoxide in dogs. Unpublished report No. 202
from Corporate Toxicology Department, Mobay Chemical Corporation,
Stillwell, Kansas, USA, 29 September 1981. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1983) Determination of acute toxicity (LD50).
Unpublished letter to Dr Reuver, PF-Zentrum, Monheim, Germany, dated
22 April 1983. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1978) H 321 (active ingredient of Mesurol)
Salmonella/microsome test for determination of point mutations.
Unpublished report No. 7978 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 6 December 1978. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B. (1979a) H 321 (active ingredient of Mesurol) micronucleus
test for mutagenic effect on mice. Unpublished report No. 8426 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 8
June 1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979b) H 321 dominant lethal study on male mouse to test
for mutagenic effects. Unpublished report No. 8395 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 23 May 1979.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) H 321 mercaptodimethur (the active ingredient of
Mesurol(R)) study of DNA damage using the E. coli pol A- test.
Unpublished report No. 11928 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 13 July 1983. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) H 321 c.n. mercaptodimethur Salmonella/microsome
test to evaluate for point mutagenic effect. Unpublished report No.
14205 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 10 January 1986. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1981) Cholinesterase no-effect level of Mesurol(R) and
Mesurol sulfoxide(R) in female rats. Unpublished report No. 199 from
Mobay Chemical Corporation, Corporate Toxicology Department, Stilwell,
Kansas, USA, 31 August 1981. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. & Schilde, B. (1980) H 321(Mesuro(R) active ingredient
-- mercaptodimethur) chronic toxicity study in dogs (2 year feeding
experiment). Unpublished report No. 9626 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 4 December 1980. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1960) Product Dr Wedemeyer H 321 (E 37 344) Production
No. 2410. Unpublished report from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 25 March 1960. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1966a) Mesurol active ingredient (Wedemeyer H 321; Ht.
No. 3657) -- antidotal effect. Unpublished letter to Crop Protection
Science, Leverkusen. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, dated 8 August 1966. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1966b) Re active ingredient Wedemeyer H 321 (Ht No
3657). Inhalation tests. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany. Unpublished letter to Crop Protection
Science, Leverkusen, Germany, dated 7 December 1966. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969a) Re Bay 37 344 (H 321) Lo No. 691. Unpublished
letter to Crop Protection Science, Leverkusen. Bayer AG, Institute for
Toxicology, Wuppertal-Elbberfeld, Germany, dates 20 May. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969b) Ref Bay 37344 (=H 321 = Methiocarb) Lot No. 691.
Unpublished letter to Crop Protection Science, Leverkusen. Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, dated 20 May.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969c) Bay 37 344 subacute dermal toxicity study on
rabbits. Unpublished report No. 1291 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 1 April 1969. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetraethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol., 27, 311-314.
Kimmerle, G. (1972) Unpublished note from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, dated 7 June 1972. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Klimmer, O.R. (1963) Toxicological testing of Bayer 37 344.
Unpublished letter from Pharmacological Institute of the University of
Bonn, Germany, to Crop Protection/Scientific Department, Leverkusen,
Germany, dated 10 December. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kohgo, L. (1970) The antagonistic effects of atropine or pyridinium
aldoxime derivatives on cholinesterase-inhibitory pesticides in male
and mice. J. Med. Soc. Toho Jpn, 17, 60-88.
Krötlinger, F. (1989) H 321 (mercaptodimethur, active ingredient of
Mesurol) chronic toxicological study on mice (feeding study over 2
year). Unpublished addendum No. 11908A to report No. 11908 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 15 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. (1990) H 321 (mercaptodimethur, the active ingredient
of Mesurol) chronic toxicity study on rats (2-year feeding
experiment). Unpublished addendum No. 10039A to report No. 10039 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 16
February 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. & Janda, B. (1983) H 321 (mercaptodimethur, the active
ingredient of Mesurol(R)) chronic toxicity study on mice (2-year
feeding experiment). Unpublished report No. 11908 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 4 July 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Löser, E. & Vogel, O. (1981) H 321 (mercaptodimethur,
the active ingredient of Mesurol) chronic toxicity study on rats
(2-year feeding experiment). Unpublished report No. 10039 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 2 July
1981. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of mesurol
technical to dogs. Unpublished note of experiment No. 450474 from
Mobay Chemical Corp., Chemagro Agricultural Division, dated 25 August
1975. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976a) The acute oral toxicities of
mesurol technical and mesurol sulfoxide. Unpublished note of
experiment No. 49541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 12 July 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976b) The acute oral toxicities of
Mesurol(R) technical and mesurol sulfoxide. Unpublished note of
experiment No. 50541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 19 August 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) A comparison of the acute oral
toxicities of Mesurol(R) technical to fasted and non-fasted rats.
Unpublished note of experiment No. 50541 from Mobay Chemical Corp.,
Chemagro Agricultural Division, dated 19 January 1977. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1989) H 321 C.N. Methiocarb mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay in
vitro. Unpublished report No. 18280 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 15 August 1989. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lorke, D. (1971) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished report No.
3133 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 30 November 1971. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1969) Bay 37 344. Blood analyses, urinalysis and clinical
chemistry examinations following oral administration rats. Unpublished
report No. 1358 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 3 March 1969. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Löser, E. (1970) Bay 37 344. Generation studies on rats. Unpublished
report No. 2208 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 10 July 1970. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Menzie, C.M. (1974) Metabolism of Pesticides: An Update (Wildlife
No. 184), Washington DC, US Department of the Interior Fish and
Wildlife Service, p. 249.
Mihail, F. (1984) H 321 (C.n. mercaptodimethur) study for
skin-sensitising effect on guinea pigs. Unpublished report No. 13125
from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany,
12 December 1984. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murli, H. (1990) Mutagenicity test on H 321 in an in vitro cytogenetic
assay measuring chromosomal abberation frequencies in Chinese hamster
ovary (CHO) cells with a confirmatory assay. Unpublished report No. R
4980 from Hazleton Laboratories America Inc., Kensingtonb Maryland,
USA, 8 March 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murphy, J.J., Timme, G. & Dräger, G. (1982) Mesurol(R) common name:
methiocarb, mercaptodimethur. Behaviour in plants. Unpublished report
from Mobay Chemical Corp., Kansas, USA, August 1982. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Nelson, D.L. (1979) Acute oral toxicity of Mesurol(R) technical and
metabolites to rats. Unpublished report No. 67815 from Mobay Chemical
Corp., Environmental Health Research, Stillwell, Kansas, USA, 27 June
1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1988) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1084 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 23 November
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1989) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1160 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 31 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1986) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells test article Mesurol technical. Unpublished report
No. 790 from Microbiological Associates Inc., Bethesda, Maryland, USA,
25 September 1986. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished addendum No.
3133A to report No. 3133 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 21 October 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Root, M., Doull, J. & Cowan, J. (1963) Determination of the safe
dietary level of Bayer 37344 for dogs. Unpublished report No. 11159
from Department of Pharmacology, University of Chicago, USA, 23 March
1963.. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Salama, A.K., Bakry, N.M. & Abou-Donia, M.B. (1993) A review article
on placental transfer of pesticides. J. Occup. Med. Toxicol., 2,
383-397.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Mesurol in rats. Unpublished report No. 940 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 31 August 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b) Acute four-hour inhalation toxicity study with
Mesurol(R) technical in rats. Unpublished report No. 870 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 18 June 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1969) Mesurol(R) active ingredient -- acute cutaneous
toxicity in rats. Unpublished letter to Bayer AG, Institute of
Toxicology, Crop Protection Science, Leverkusen, Germany, dated 11
September 1969. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1970) Oral toxicity of Mesurol metabolites to rats.
Unpublished notes addressed to Bayer AG, Institute of Toxicology, Crop
Protection Science, Leverkusen, Germany, dated 6 April 1970. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stanley, C.W. & Johnson, G.A. (1976) Metabolites of Mesurol(R) in
rat urine. Unpublished report No. MR50732 from Chemagro Agricultural
Division, Mobay Chemical Corp., USA, 16 November 1976. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Strankowski, K.J. & Minor, R.G. (1976) Effect of feeding
Mesurol(R)/Mesurol sulfoxide (9:1) to chickens for 28 days.
Unpublished report No. 49639 from Mobay Chemical Corp., Research and
Development Department, USA, 7 January 1976. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Strother, A. (1970) Comparative metabolism of N-methylcarbamates by
human and rat liver fractions. Biochem. Pharmacol., 19, 2525-2529.
Strother, A. (1972) In vitro metabolism of methylcarbamate
insecticides by human and rat liver fraction. Toxicol. Appl.
Pharmacol., 21, 112-129.
Tesh, J.M., Ross, F.W., Secker, R.C. & Wilby, O.K. (1981) H 321:
Effects of oral administration upon pregnancy in the rabbit. 2. main
study. Unpublished report No. 2173 from Life Science Research, Stock,
United Kingdom, 4 December 1981. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977a) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 15 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977b) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 30 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) H 321 (Mesurol active ingredient) acute inhalation
toxicity. Unpublished report No. 11113 from Bayer AG Institute of
Toxicology, 27 August 1982. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen, J. & Mohr, U. (1983) H 321 (Mesurol active ingredient)
subacute inhalation study with rats. Unpublished report No. 12120 from
Bayer AG Institute of Toxicology and Department of Experiment
Pathology, Hanover Medical University, Germany, 30 September 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Schilde, B. (1978) H 321 Neurotoxicity study on hens.
Unpublished report No. 7637 from Bayer Institute of Toxicology, 20
June 1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Wheeler, L. & Strother, A. (1971) In vitro metabolism of the
N-methylcarbamates, Zectran and Mesurol, by liver, kidney and blood of
dogs and rats. J. Pharm. Exp. Ther., 178, 371-382.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Willems, P.W.J.M., Geursen-Reitsma, A.M. & van Joost, T. (1997)
Allergic contact dermatitis due to methiocarb (Mesurol). Contact
Derm., 36, 270-271.
0.5 mg/kg bw; 4 h after dosing at 0.5 mg/kg bw, depressions of < 10%
were noted. At the higher dose of methiocarb, depressions of 19-41%
were seen 30 min after dosing and 0.2-19% 4 h after dosing. With the
sulfoxide, plasma cholinesterase activity was inhibited by 21-39% at
the low dose at 30 min but was generally not inhibited at 4 h. At the
high dose, 39-62% inhibition was seen at 0.5 h and 10-25% inhibition
at 4 h. Erythrocyte acetylcholinesterase activity was depressed by
5-12% 30 min after dosing at 0.5 mg/kg bw and by < 10% 4 h after
dosing. At the higher dose of methiocarb, erythrocyte
acetylcholinesterase activity was depressed by 15-29% 30 min after
dosing and by 1-13% 4 h after dosing. With the sulfoxide, erythrocyte
acetylcholinesterase activity was inhibited by 13-31% at the low dose
at 30 min but was generally not inhibited at 4 h. At the high dose,
32-46% inhibition was seen at 0.5 h and 10-21% at 4 h. The NOAEL for
erythrocyte acetylcholinesterase inhibition was therefore
approximately 0.5 mg/kg bw per day for methiocarb, but there was no
NOAEL for the sulfoxide (Hixson, 1981).
Technical-grade methiocarb (purity, 97%) and methiocarb sulfoxide
(purity, 95.2%) were administered orally in gelatine capsules to
groups of two adult beagle dogs and two bitches at a dose of 0.05 or
0.5 mg/kg bw per day for 29 days; two control animals of each sex
received empty capsules. The animals were observed twice daily, and
blood was taken for measurement of plasma and erythrocyte
cholinesterase activity before the first dose, 1, 3, 6, and 24 h after
the first dose, and 3 h after the third dose. During the second week,
blood was taken before the first dose, 2, 6, and 24 h later, and 2 h
after the second dose. During weeks 3 and 4, blood was taken before
the first dose, 2, 6, and 24 h later, and 2 h after the third dose. In
week 5, blood was again taken before the first dose and 2, 6, and 24 h
later; dosing was then stopped, and another blood sample was taken on
the third day.
Clinical signs of toxicity (salivation and vomiting) were
observed at the higher dose of each test material and in animals of
each sex; additionally, slight salivation was observed in one bitch
given the sulfoxide at the lower dose. Depression of cholinesterase
activity was calculated by comparison with the level measured just
before the first dose each week: inhibition was variable, but peak
inhibition occurred 0-3 h after dosing; considerable inhibition was
seen with both materials at the higher dose in animals of each sex. At
the lower doses, smaller depressions of both plasma and erythrocyte
cholinesterase activity, of up to about 20%, were sometimes seen with
both materials. Cholinesterase activity was generally normal by 6 h
(Hayes, 1981).
At equimolar concentrations, methiocarb was the least effective
of four carbamates in inhibiting bovine erythrocyte
acetycholinesterase and equine plasma cholinesterase activity
(Barthová et al., 1989).
Methiocarb was reported not affect liver function in rabbits at a
single oral dose of 25 mg/kg bw (Kimmerle, 1960).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of methiocarb and
putative metabolites of methiocarb are shown in Table 1.
No erythema or oedema was seen when technical-grade methiocarb
(purity unspecified) was applied to abraded and unabraded skin of six
New Zealand white rabbits. Application to the eyes of six rabbits did
not produce ocular irritation (Crawford & Anderson, 1970).
A study of the skin-sensitizing potential of methiocarb (purity,
97.8% pure) was conducted by the Magnusson and Kligman technique. A
group of 20 guinea-pigs (strain BOR:DHPW) each received methiocarb
intradermally at a concentration of 1% and topically at a
concentration of 25%. There were two control groups of 10 guinea-pigs.
The test animals were first challenged with methiocarb at a
concentration of 25% and then at 12.5%. Although there was a small
excess in the number of animals that reacted positively after the
second challenge, the overall result was considered to be negative
(Mihail, 1984).
In a test for dermal sensitization by the Buehler technique,
technical-grade methiocarb (purity, 99.2%) was applied to 15 Hartley
guinea-pigs once weekly for three weeks, with a challenge dose a
fortnight later. The material was applied as a single dose to five
previously unexposed animals at the time of the challenge dose.
Dinitrochlorobenzene, used as the positive control, was applied in the
same way to a further group of five guinea-pigs, weekly for three
weeks, with a challenge dose two weeks later, and to a further group
of five as a single dose at the time of the challenge dose. Methiocarb
was not considered to be a dermal sensitizer (David, 1988).
A case of allergic contact dermatitis was reported in a man who
grew carnations. He developed acute severe eczema of the hand and
showed a positive reaction in a patch test to 0.5% methiocarb (Willems
et al., 1997).
(b) Short-term studies of toxicity
Rats
Methiocarb in aqueous tragacanth suspension was given by gavage
to albino rats daily. The dose was 2 mg/kg bw per day for the first
three days and 4 mg/kg bw per day for the next 24 days. Two groups of
three animals were killed every week for determination of
cholinesterase activity. The activity had fallen to 80% of the control
values after 14 days and to 50% by the end of the study. No abnormal
clinical signs were observed; the animals gained weight normally
(Kimmerle, 1960).
Table 1. Acute toxicity of methiocarb and putative metabolites
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb
Rats Sprague-Dawley M Oral 130 Technical DuBois & Raymund (1962)
F 140
Rats Sprague-Dawley F Oral 100 Recrystallized DuBois & Raymund (1962)
Rats NR NR Oral 67 NR Kimmerle (1966a)
Rats Sprague-Dawley M Oral 30 (20-45) 99 Crawford & Anderson (1973)
F 30 (20-45)
Rats Sprague-Dawley M Oral (non-fasting) 46 (38-56) 99 Crawford & Anderson (1973)
F 47 (36-63)
Rats Sprague-Dawley M Oral 15 (9-26) Technical Lamb & Matzkanin (1976a)
F 31 (18-54)
Rats Sprague-Dawley M Oral 13 (9-17) Technical Lamb & Matzkanin (1976b)
F 32 (24-44)
Rats Sprague-Dawley M Oral 14 (12-16) Technical Lamb & Matzkanin (1977)
F 16 (13-20)
Rats Sprague-Dawley M Oral (non- 51 (45-58) Technical Lamb & Matzkanin (1977)
F 79 (65-96)
Rats NR M Oral 22 (19-25) 98.5 Flucke (1978)
F 24 (21-28)
Rats NR M Oral 22.1 (18-27) 98.3 Flucke (1980)
Rats Sprague-Dawley- M Oral 33 (22-50) 98 Nelson (1979)
derived F 47 (31-70)
Rats NR M Oral 17 (16-19) 98.6 Heimann (1983)
Rats NR M Oral 19 (16-23) 98.2 Flucke (1988)
F 26 (19-36)
Rats Albino M Oral 100 NR Kimmerle (1960)
Rats Wistar-CFN M Oral 87 NR Klimmer (1963)
Rats NR M Oral 33 (29-38) 98.4 Thyssen (1977a)
35 (29-42) 98.3
35 (30-42) 98.2
31 (26-35) 97.8 28 (24-33)97.4
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rats Sprague-Dawley M Intraperitoneal 35 Technical DuBois & Raymund (1961a)
F 30
Rats Sprague-Dawley F Intraperitoneal 25 Recrystallized DuBois & Raymund (1962)
Rats Wistar-CFN M Intraperitoneal 43 NR Klimmer (1963)
Rats Sprague-Dawley M Dermal > 200 Technical DuBois & Raymund (1961a)
Rats Sprague-Dawley F Dermal > 300 Recrystallized DuBois & Raymund (1962)
Rats Albino M Dermal > 1000 NR Kimmerle (1960)
Rats Wistar-CFN M Dermal 350-400 NR Klimmer (1963)
Rats NR M Dermal > 500 99.2 Solmecke (1969)
Rats Wistar M Dermal > 5 g 98.1 Thyssen (1977b)
F > 5
Rats Sprague-Dawley M Inhalation (1 h) 1200 mg/m3 a 98.8 Shiotsuka (1987a)
(780-1700)
F 1100 mg/m3 a
(740-1600)
Rats NR M Inhalation 540 g/L3 NR Kimmerle (1966b)
Rats Sprague-Dawley M Inhalation (1 h) > 20 000 g/L Technical Crawford & Anderson (1972a)
F > 20 000 g/L
Rats Sprague-Dawley M Inhalation (4 h) 580 mg/m3 (340-700) 98.8 Shiotsuka (1987b)
F 430 mg/m3 (290-580)
Rats Wistar M Inhalation (4 h) > 320 mg/m3 97.9 Thyssen (1982)
F > 320 mg/m3
Rats Wistar M Inhalation > 300 mg/m3 97.9 Thyssen (1982)
F (5 x 6 h) > 300 mg/m3
Mice NR M Oral 25 (21-30) NR Kohgo (1970)
Mice NR M Oral 52 98.2 Kimmerle (1972)
Mice NR M Subcutaneous 940 (760-1200) NR Kohgo (1970)
Mice Carworth Farm M Intraperitoneal 6 Technical DuBois & Raymund (1961a)
F 5.5
Mice Dierolf Farm F Intraperitoneal 16 Technical Baron et al. (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Rabbits New Zealand M Dermal > 2000 99 Crawford & Anderson
white F > 2000 (1972b)
Guinea-pigs NR M Oral 40 Technical DuBois & Raymund (1961a)
Guinea-pigs NR F Oral (50-100) 99.2 Kimmerle (1969a)
Guinea-pigs Albino F Oral 14 (7.9-25) Technical Crawford & Anderson (1972a)
Guinea-pigs NR M Intraperitoneal 17 Technical DuBois & Raymund (1961a)
Dogs Beagle F Oral (10-25) 99.2 Kimmerle (1969b)
Dog Mongrel M Oral ~ 25 Technical Lamb & Matzkanin (1975)
F ~ 25
Chicken NR F Oral 175 Technical DuBois (1962)
Chicken White Leghorn F Oral 380 (300-490) 98.5 Thyssen & Schilde (1978)
Methiocarb phenol
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR M Dermal > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Methiocarb phenol sulfoxide
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Methiocarb phenol sulfone
Rats NR M Oral > 1000 NR DuBois (1964)
Rats NR NR Oral > 1000 NR Solmecke (1970)
Rats NR M Dermal > 1000 NR DuBois (1964)
Table 1. (continued)
Species Strain Sex Route LD50 (95% CI or Purity Reference
range) (mg/kg bw) (%)
Methiocarb sulfoxide
Rats NR NR Oral 43 (37-50) NR Solmecke (1970)
Rats Sprague-Dawley M Oral 9 (7-13) NR Lamb & Matzkanin (1976a)
F 7
Rats Sprague-Dawley M Oral 6 (5-8) NR Lamb & Matzkanin (1976a)
F 8 (6-10)
Methiocarb sulfone
Rats NR NR Oral > 1000 NR Solmecke (1970)
Hydroxymethyl methiocarb
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfone
Rats NR M Oral > 110 NR Nelson (1979)
F > 110
Hydroxymethyl methiocarb sulfoxide
Rats NR M Oral > 160 NR Nelson (1979)
F > 160
Technical, purity unstated; NR, not reported
a There was a large descrepancy between nominal and measured concentrations owing to deposition in the chamber;
these figures were measured gravimetrically.
Methiocarb was administered by gavage to groups of 25 male
Wistar-CFN rats at a dose of 2.5 or 5 mg/day on six days per week for
six months. Three deaths occurred at the higher dose due to
bronchopneumonia, but weight gain was the same in the two treated
groups and in 20 control animals; no abnormal clinical signs were seen
(Klimmer, 1963).
Groups of 12 Sprague-Dawley rats of each sex were fed diets
containing methiocarb (purity unspecified) at concentrations of 0, 5,
10, or 50 ppm for 16 weeks. Five animals of each sex from each group
were examined histopathologically, and five of each sex were used for
measurements of cholinesterase activity in blood, brain, and
submaxillary glands by a manometric method. No effect on growth rate,
food intake, or mortality rates was observed at any dose. In males at
the highest dose, serum, erythrocyte, brain, and submaxillary gland
cholinesterase activity was inhibited by 21, 14, 12, and 7%,
respectively. In females at the highest dose, serum cholinesterase
activity was reduced by 28% and erythrocyte acetylcholinesterase
activity by 15%, all by comparison with concurrent controls. Brain
acetylcholinesterase activity was reduced by 5% in females, and
inhibition of submaxillary gland enzyme was seen in all test groups. A
NOAEL could not be identified in this study (Doull et al., 1962).
Groups of 10 Wistar rats of each sex were exposed to an aerosol
of methiocarb (purity, 97.9%) of a mass median diameter of 2.1-2.5.
The groups were exposed to either air, solvent, methiocarb at 6 mg/m3
in solvent, methiocarb at 23 mg/m3 in solvent, or methiocarb at 96
mg/m3 in solvent; exposure was for 6 h/day, five days per week for
three weeks. The animals were examined daily and weighed weekly. Blood
was taken from five animals per group at the end of the study and was
used for haematological and clinical chemical tests; urinary analyses
were also carried out. Plasma and erythrocyte cholinesterase activity
was determined before treatment and after 5, 10, and 15 exposures for
all groups except the air controls. At the end of the study, the
animals were sacrificed and autopsied, selected organs were weighed
and processed for histopathological examination, and brain
acetylcholinesterase activity was measured.
No deaths were observed in any group. Animals at the highest
concentration showed clinical signs of compound-related effects
(tremor); no clinical signs were seen at lower concentrations. There
body weight of males at the highest concentration was reduced by
comparison with the air controls but not with the solvent controls. No
change in haematological or clinical chemical parameters was seen that
was attributable to the test material, with the exception of
inhibition of cholinesterases. Plasma and brain cholinesterase
activity was decreased at the highest concentration, brain
acetylcholinesterase activity being 61 and 74 % of that in concurrent
solvent controls in males and females, respectively. Some inhibition
of plasma cholinesterase activity was seen at the intermediate
concentration, and males at this concentration had an associated
decrease in brain acetylcholinesterase activity (65% of concurrent
control value). Erythrocyte acetylcholinesterase activity was less
strongly affected than plasma enzyme, but marginal inhibition was
observed in males at the highest concentration at week 1 (82% of
concurrent solvent control value). There were no toxicologically
significant alterations in organ weights, and no compound-related
findings were noted on histopathological examination. The NOAEL was
6 mg/m3 on the basis of reduced brain acetylcholinesterase activity
in males (Thyssen & Mohr, 1983).
Rabbits
Technical-grade methiocarb (purity, 99.2%) was applied to five
male and five female chinchilla rabbits, daily for two weeks, at a
dose of 500 mg; five males and five females served as controls. The
material was applied for 24 h/day, and new material was applied at the
end of each 24-h period. After the two-week application period, the
animals were observed for a further fortnight. The animals were
inspected daily, and haematological and biochemical studies were
carried out before treatment, at the end of treatment, and two weeks
later. Clinical chemistry was restricted to liver function tests and
urinary analysis; cholinesterase activity was not measured. No
abnormal clinical signs were seen, and there was no effect on
body-weight gain or any perturbation in haematological or clinical
chemical variables (Kimmerle, 1969c).
Methiocarb (purity, 99.3%) was applied at doses of 0, 60, 150, or
375 mg/kg bw per day for 6 h/day to the skin of groups of five male
and five female New Zealand white rabbits, and the site of application
was occluded. The animals were examined twice daily, and any signs of
skin irritation were scored. The rabbits were weighed twice weekly;
food consumption was measured three times weekly until the last week,
when it was measured four times. Blood was taken for haematological
and clinical chemical analysis before treatment and pre-terminally.
Plasma and erythrocyte cholinesterase activity was measured at the end
of the 6-h exposure period on days 1, 7, 14, and 21; additionally,
blood was taken 16 h after the end of exposure on these days from the
group at the high dose. Animals were sacrificed on the day after the
last treatment, at which time the brain was taken for measurement of
acetylcholinesterase activity in a homogenate of the entire left half
of the brain. Selected tissues were weighed, examined grossly,
processed, and examined histopathologically.
Two animals at the low dose did not survive to the end of the
study. No clinical signs related to the test material were seen, and
it was not irritating. There was no differences between the groups in
body weight, but food consumption appeared to be reduced in animals of
each sex at the high dose, and particularly in males. Clinical
chemical parameters did not differ between the groups, and, although
one or two differences in haematological measurements were seen, none
appeared to be related to treatment. Plasma cholinesterase activity
appeared to be reduced in males at the high dose at 14 and 21 days.
The measurements made 6 h after the end of exposure suggested that the
inhibition was not reversed overnight. No intergroup differences in
plasma cholinesterase activity were observed among females.
Erythrocyte acetylcholinesterase activity was very variable but did
not appear to be inhibited in a dose-related fashion. Intergroup
differences were not observed in brain acetylcholinesterase activity.
No gross or microscopic abnormality was observed that was attributable
to the test material. The NOAEL was 150 mg/kg bw per day on the basis
of reduced food consumption (Procter, 1988).
Methiocarb (purity, 97.5%) was applied to the skin of five male
and five female New Zealand white rabbits at a single dose of 500
mg/kg bw per day for 6 h/day under occlusion. Five controls of each
sex received saline. The animals were examined twice daily, and any
signs of skin irritation were scored. They were weighed twice weekly,
while food consumption was measured every two days. Blood was taken
for haematological and clinical chemical tests before treatment and
preterminally. Plasma and erythrocyte cholinesterase activity was
measured at the end of the 6-h exposure period on days 1, 7, 14, and
21 and, in the test animals, 16 h later. Animals were sacrificed on
the day after the last treatment, at which time the left half of the
brain was taken for measurement of cholinesterase activity. Selected
tissues were weighed, examined, processed, and examined
histopathologically.
Two test animals removed their dressings and presumably ingested
the material; these animals developed clinical signs of cholinergic
poisoning, which disappeared within a few hours. No other
compound-related clinical signs were seen, and no animal died before
completion of the study. Treated females were lighter than controls
throughout the study, and the food consumption of animals of each sex
was reduced. A reduction in serum calcium and increased activities of
alanine and aspartate aminotransferases were seen in females in
comparison with concurrent controls. Plasma cholinesterase activity
was lower than that before treatment in both males and females, but
only inconsistently in comparison with concurrent controls.
Erythrocyte acetylcholinesterase activity was reduced in males on day
1, at both 6 and 16 h, in comparison with pretreatment levels, but
inconsistently in comparison with concurrent controls; it was
concluded that compound-related inhibition of erythrocyte
acetylcholinesterase activity had not occurred in either males or
females. Brain acetylcholinesterase activity was not inhibited. No
abnormality was seen at autopsy or on histopathological examination.
The design of this study was not appropriate for identifying a NOAEL
(Procter, 1989).
Cats
Methiocarb at a dose of 5 mg/kg bw per day, given daily by gavage
to cats, was reported to have no adverse effects (Kimmerle, 1960).
Dogs
Methiocarb was incorporated into the diet of groups of two male
and two female beagles at a concentration of 0, 50, 100, or 250 ppm,
equal to 0, 1.25, 2.5, or 6.25 mg/kg bw per day. The animals were
examined daily and weighed fortnightly; plasma and erythrocyte
cholinesterase activity was measured weekly. No clinical effects were
seen, and body-weight gain was unaffected by treatment. There was no
clear difference in plasma or erythrocyte cholinesterase activity
between the test groups and concurrent controls. The small group size
renders this study inappropriate for identifying a NOAEL (Root et al.,
1963).
Chickens
Chickens (Gallus gallus Babcock 300) were fed diets containing
a 9:1 mixture of methiocarb and methiocarb sulfoxide (based on studies
of plant metabolism) at a dose of 20, 60, 120, or 360 ppm, equal to
2.5, 7.5, 15, and 45 mg/kg bw per day, over 28 days. Treatment
decreased feed consumption in a dose-related fashion, and the body
weights of birds at the two highest doses were decreased. Egg
production was unaffected. Plasma cholinesterase activity was
decreased by 40-50 % in comparison with concurrent controls at the
three highest doses but was unaffected at the lowest dose (Strankowski
& Minor, 1976).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female BOR:CFW1 mice received diets
containing methiocarb (purity, 98.5%) at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 15, 43, and 130 mg/kg bw per day in males and
0, 20, 57, and 170 mg/kg bw per day in females. Haematological and
clinical chemical tests were performed on five animals of each sex per
dose at 12 months and 10 animals of each sex per dose at 24 months,
and on satellite groups of 15 mice of each sex per dose, which were
sacrificed after one year and necropsied. Cholinesterase activity was
determined in the plasma of five animals of each sex per dose at 1 and
12 months and on 10 animals of each sex per dose (or the survivors if
fewer than 10) at the end of the study. Brain cholinesterase activity
was determined at the end of the study. Animals that died or became
moribund were autopsied, as were 10 mice of each sex per group at 12
months. All surviving animals were sacrificed at the end of the study
and autopsied, and selected organs were weighed, examined, and
processed for histopathological examination.
The appearance, behaviour, mortality rate, and food consumption
of the animals were not affected at any dose, except for a slight
decrease in body weight at the highest dose, throughout the study in
males and up to week 30 in females. Overall survival in this study was
not good: survival at 18 months was 74% of male controls, 47% males at
the low dose, 59% males at the intermediate dose, 66% males at the
high dose, 69% of female controls, 53% at the high dose, 64% at the
intermediate dose, and 69% at the high dose. Intergroup differences in
mortality rates did not appear to be compound-related, as mortality
was higher in males receiving 67 or 200 ppm than in those given the
high dose. Moreover, survival of females up to the end of the study
was poorest for controls. All treated male mice had higher mean
corpuscular haemoglobin concentrations than controls at 12 months, and
males at the highest dose had higher mean corpuscular haemoglobin
values; similar changes were not seen in females. At 24 months, the
mean corpuscular haemoglobin concentration of males at 200 and 600 ppm
was decreased and the mean corpuscular haemoglobin value at 600 ppm,
again, with no similar change in the females. Higher leukocyte counts
were observed in all treated females at 24 months, but the authors
ascribed this finding to high individual values; no perturbution of
the differential count was seen.
No clinical chemical abnormalities were found at 12 months, but
at 24 months the activity of alanine aminotransferase (ALAT) was
increased in animals at 200 and 600 ppm. At one month, males at 200
and 600 ppm had reduced plasma cholinesterase activity (by 51 and 34%
in comparison with concurrent controls), while reductions of 5% or
less were seen at 12 months and 5-11% at 24 months. In females, plasma
cholinesterase activity was inhibited by 24, 43, and 34% at the low,
intermediate, and high doses, respectively, at one month; smaller
reductions (< 12%) were observed at 12 months, and no cholinesterase
inhibition was observed at 24 months. Erythrocyte acetylcholinesterase
activity was not measured. Small reductions in brain
acetylcholinesterase activity were observed, by 5, 11, and 10% in
males at the low, intermediate, and high doses and by 3, 9, and 3% in
females at the three doses, respectively. No compound-related change
in organ weights was seen, nor was there any abnormality in gross of
histological appearance that was related to treatment. Methiocarb was
not tumorigenic. There was no NOAEL because of haematological changes
at all doses in males at 12 months and in females at all doses at 24
months (Krötlinger & Janda, 1983; Krötlinger, 1989).
Rats
Methiocarb (purity, 98.9%) was admixed with the diet of groups of
60 Wistar TNO W.74 rats for two years at concentrations of 0, 67, 200,
or 600 ppm, equal to 0, 3.3, 9.3, and 29 mg/kg bw per day for males
and 0, 5, 14, and 42 mg/kg bw per day for females. The rats were
inspected daily, and body weights were determined weekly for the first
26 weeks and then at fortnightly intervals. Haematological, clinical
chemical, and urinary measurements were made in 10 animals of each sex
per dose at 3, 6, 12, and 24 months. Cholinesterase activity in plasma
and erythrocytes was determined one and two days after the start of
the study and at 1, 2, 4, 8, 13, 26, 52, 78, and 105 weeks in 10
animals of each sex per dose. Brain acetylcholinesterase activity was
determined at the end of the experiment on 10 animals of each sex per
dose. Animals that died and those sacrificed in extremis were
examined grossly and necropsied; when possible, tissues were taken for
histopathological examination. The survivors at two years were also
examined grossly and necropsied, and tissues were taken for weighing
and histopathological examination.
None of the doses had any effect on the appearance, behaviour, or
mortality rates of rats of either sex. The mortality rates at two
years were 10-20% for males and 23-32% for females. There was no
significant intergroup difference in food consumption. Weight gain was
not depressed at 67 or 200 ppm in comparison with the controls; at 600
ppm, weight gain was slightly but consistently depressed throughout
the period of administration of methiocarb, and at termination total
body weight was reduced in the group at the high dose. At three
months, an increased leukocyte count was seen in females at the
highest dose and an increased reticulocyte count in females at the two
higher doses. At six months, the mean corpuscular haemoglobin
concentration was reduced in males at the highest dose, and some
elevation in leukocyte count was found in males at 67 and 200 ppm. Red
blood cell counts and haemoglobin and haematocrit values were
decreased in females at the two higher doses, and reticulocytosis was
observed in these groups. At 12 months, the mean corpuscular
haemoglobin concentration was decreased in males at the intermediate
dose and was increased in females at the highest dose; an increased
reticulocyte count was seen in females at the high dose. At 24 months,
no compound-related changes in haematological variables were seen.
Although a few intergroup differences were observed in
biochemical tests, the only ones that appeared to be related to
treatment were total protein concentration and cholinesterase
activity. Total protein concentrations were raised in females at the
two higher doses at six months and in males at the three highest doses
at 12 months; at 24 months, the total protein concentration was
similar in treated and control groups. ALAT activity in the blood was
elevated in all three groups of treated females at 12 months and in
females at the highest dose at two years, but at no time interval in
males. Increased urea was found in plasma from males at the highest
dose at three and 12 months and in females at 12 and 24 months. Plasma
cholinesterase activity was depressed at the high dose at one day and
from eight weeks onwards in males (except in the 52-week assay) and in
females at one day and 1, 2, 4, and 13 weeks. The measurements of
erythrocyte acetylcholinesterase activity were difficult to interpret:
a statistically significant depression was found only at the low dose
in males at 105 weeks and females at 78 weeks, and the degree of
depression was small (5 and 6%, respectively). Males at the
intermediate dose had significantly depressed activities at 8, 78, and
105 weeks, with 5, 7, and 8% depression, respectively. In females at
this dose, significant depression was seen at four and 78 weeks (8 and
13% depression, respectively). In males at the highest dose,
significant depressions were seen at 8, 13, 78, and 105 weeks (7, 7,
9, and 7%, respectively), and in females at this dose depression was
seen at two days and 4 and 78 weeks (6, 8, and 11%, respectively). The
Committee considered that none of these depressions was biologically
significant. No depression of brain acetylcholinesterase activity was
seen.
Males at the highest dose had decreased absolute weights of the
thyroid, heart, lung, liver, spleen, and adrenals, but these were
reflected only in reductions in the relative weights of the spleen and
therefore probably reflect reduced body weight. In the females, only
the absolute weight of the spleen was reduced, while the absolute
weight of the thyroid was increased. As this finding was due to a
single animal, it is unlikely to be attributable to the test material.
No gross or histopathological abnormality related to treatment was
seen at any dose. Methiocarb was not tumorigenic. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months (Krötlinger et al., 1981; Krötlinger,
1990).
Dogs
Groups of four beagle dogs and four bitches were fed methiocarb
(purity, 98.4%) in the diet at 0, 5, 60, or 240 ppm; the group given 5
ppm group had been given 15 ppm for the first 15 days. These dietary
concentrations were equivalent to daily doses of 0, 0.12, 1.5, and 6
mg/kg bw per day, ignoring the first 15 days of treatment of animals
at the lowest dose. The animals were examined daily, and food
consumption was recorded daily and body weight measured weekly.
Clinical examinations including ophthalmoscopy were undertaken, and
haematological and biochemical variables were measured in blood at
weeks 0, 14, 27, 40, 53, 66, 79, 92, and 104; urine was also analysed.
Erythrocyte and plasma cholinesterase activity was measured before the
start of treatment and at weeks 2, 3, 4, 7, 10, 13, 27, 40, 53, 66,
79, 92, and 104, before feeding and 2 h afterwards.
Acetylcholinesterase activity on the olfactory bulb of the brain was
measured at sacrifice. Animals were examined grossly post mortem,
and selected tissues were examined histologically.
One death occurred, of an animal at 5 ppm, which was considered
not to be related to treatment. The only clinical findings were mild
weakness of the hind limbs, trembling, reduced alertness, and some
vomiting at the highest dose during the first 14 weeks of the study.
The results of tests for reflexes and ophthalmic parameters were
normal. Food intake was reduced in animals of each sex at the highest
dose and in bitches at the intermediate dose, but the body weights
were not significantly affected. Haematological and biochemical
parameters were unaffected, apart from cholinesterase activity. Plasma
cholinesterase activity was depressed at doses of 15 ppm and higher,
and it was for this reason that this dose was reduced to 5 ppm.
Depression of plasma cholinesterase activity was not seen at 5 ppm but
occurred at the two higher doses. Erythrocyte and brain
acetylcholinesterase activity was not consistently inhibited at any
dose; the maximum inhibition of erythrocyte acetylcholinesterase
activity was in animals at the high dose (17% for each sex) and at the
intermediate dose (10% in dogs and 5% in bitches). Organ weights were
unaffected, and no organ-specific toxicity observed. The NOAEL was 60
ppm, equivalent to 1.5 mg/kg bw per day, on the basis of clinical
signs. The reduced food intake of bitches at the intermediate dose was
not considered relevant (Hoffman & Schilde, 1980).
(d) Genotoxicity
The results of assays for the genotoxicity of methiocarb are
shown in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 10 female FB 30 rats (Elberfield breed)
received technical-grade methiocarb (purity, 99%) admixed with the
diet at concentrations of 0, 30, 100, or 300 ppm, equivalent to 3, 10,
and 30 mg/kg bw per day. The rats were weighed weekly. The F0
generation was mated twice, first after 70 days of treatment and again
after 149 days, to produce the F1a litters, which were sacrificed,
and the F1b litters. Ten males and 10 females from each group of F1b
rats were used to produce the next generation and were again mated
twice. The resultant F2a rats were sacrificed, while 10 male and 10
female F2b rats were mated twice to produce the F3a and F3b
generations, which were sacrificed. The rats were weighed weekly, and
the body weights of the offspring were measured at birth, five days
after birth, one week after birth, and then weekly. The pups were
examined grossly for malformations immediately after birth and during
lactation. The F3a young were killed four weeks after birth and the
F3b rats at three weeks. Blood samples were taken for analysis from
the F0 generation at the end of the preliminary treatment period and
after rearing of the second little (Löser, 1969).
In the F0 generation, there was no significant difference in
weight gain between the groups, and no treatment-related changes were
seen in haematological parameters at either sampling time. Although
ALAT and aspartate aminotransferase activities were higher in animals
at the highest dose at the earlier sampling time, they were stated to
be within the normal range for the laboratory; similar increases were
not seen at the later sampling time. The gestation rate was lower at
the highest dietary concentration, but was still within the normal
range. The weight gain of F1a pups was similar in all groups, and no
malformations were seen at sacrifice. After the second mating, there
were no dose-related changes in litter size or pup weight and weight
gain. No significant differences were seen between the groups of F1b
pups. Their weight gain after weaning was similar in all groups. After
the first mating, the gestation rate was lower at the highest dietary
concentration but was still within the normal range. The weight gain
of F2a pups was similar in all groups, and no malformations were seen
at sacrifice. After the second mating, the mean weight of the group at
300 ppm was significantly lower than that of controls at the end of
the four-week lactation period; however, there were no significant
intergroup differences in body weight at sacrifice of the F2b
animals. After both matings of these rats, the gestation rate was
similar and the litter size and pup weights were comparable in all
Table 2. Results of assays for the genotoxicity of methiocarb
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 4-2500 µg/plate 98.5 Negative Herbold (1978)
TA1535, TA1537,
TA98, TA100
Reverse mutationa S. typhimurium 20-12 500 µg/plate 98.4 Negative Herbold (1986)
TA1535, TA1537,
TA98, TA100
DNA damagea E. coli pol A+ and 625-10000 µg/plate 98.6 Negative Herbold (1983)
pol A-
Gene mutation Chinese hamster 1.25-60 µg/ml 99.3 Negative Lehn (1989)
ovary cells, hprt
Unscheduled DNA Rat primary 0.1-100 µg/ml 98.8 Negative Curren (1988)
synthesis hepatocytes
Chromosomal Chinese hamster 4.92-497 µg/ml 99.4 Positive Murli (1990)
aberrationa ovary cells
Sister chromatid Chinese hamster 2-40 µg/ml 98.2 Negative Putman (1986)
exchangea ovary cells
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
Micronucleus Mice 5,10, and 20 98.5 Negative Herbold (1979a)
formation mg/kg bw
twice orally
Dominant lethal Mice 6 mg/kg bw 98.5 Negative Herbold (1979b)
mutation
a With and without metabolic activation
groups. The body weights of the F3b, but not the F3a, groups at 100
and 300 ppm during lactation were lower than those of controls. This
finding may be due to larger litter size. No abnormalities were found
at birth or sacrifice in F3a or F3b pups, and no abnormalities were
found at autopsy of the F0, F1b, and F2b generations. The NOAEL was
300 ppm, equivalent to 30 mg/kg bw per day, the highest dose tested
(Löser (1970).
(ii) Developmental toxicity
Rats
Groups of 19-20 fertilized FB 30 rats received 10 oral doses of
0, 1, 3, or 10 mg/kg bw per day methiocarb (purity, 98.9%) by gavage
on days 6-15 of gestation. The highest dose reduced weight gain, but
no other effects were observed. No effects were seen on the number of
implantations or resorptions or the weights of fetuses or placentas.
No teratogenic effects were seen, nor was fetotoxicity observed. The
NOAEL for maternal toxicity was 3 mg/kg bw per day, and that for fetal
toxicity was 10 mg/kg bw per day, the highest dose tested (Lorke,
1971; see also Renhof, 1988).
Rabbits
Methiocarb (purity, 97.3%) was administered by gavage to groups
of 17 pregnant New Zealand white rabbits at doses of 1, 3, or 10 mg/kg
bw per day on days 6-18 of gestation. A group of 19 rabbits were used
as controls. On day 29 of gestation, the rabbits were sacrificed and
examined; the uterus was weighed, and the numbers of corpora lutea,
implantation sites, and resorption sites, and the number and
distribution of live and dead fetuses were noted. The weight and sex
of the fetuses and placental weights were recorded and the fetuses
examined for abnormalities. Rabbits receiving the highest dose showed
clinical signs of cholinergic poisoning and initial marked weight
loss. Weight gain was also reduced at 3 mg/kg bw per day towards the
end of the study, but this was attributable to a single animal. Weight
gain was unaffected at the lowest dose. The litter parameters were
similar in all groups, and there was no indication of teratological
effects. The NOAEL for maternal toxicity (clinical effects and weight
loss at the highest dose) was 3 mg/kg bw per day . The NOAEL for
fetotoxicity was 10 mg/kg bw per day, the highest dose tested (Tesh et
al., 1981).
Methiocarb (purity, 99.4-99.6%) was applied under occlusion for
6 h/day to about 10% of the body area of groups of 16 mated chinchilla
rabbits at doses of 0, 10, 50, or 250 mg/kg bw per day on days 6-18
post coitum. The animals were sacrificed after 28 days and the fetuses
removed. One animal at the high dose was sacrificed because of a
broken femur. No clinical signs were observed at any dose, nor was
irritation of the skin noted. At the highest dose, reduced food
consumption was observed between 11 and 15 days post coitum and
body-weight loss between 6 and 8 days post coitum, whereas at the
lower doses,no such effect was seen. The numbers of corpora lutea,
implantations, pre- and post-implantation losses, and live fetuses
were not affected by treatment. There was a slight decrease in mean
fetal weight at the highest dose (4%), and slight retardation of
skeletal development was noted at this dose, as shown by incomplete or
no ossification of the phalangeal nuclei. No effects on the fetuses
were seen at lower doses. The NOAEL for both maternal and fetal
toxicity was 50 mg/kg bw per day (Dotti & Biedermann, 1992).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
(ii) Neurotoxicity
In a poorly described study, no persistent paralytic effects were
produced in hens (Gallus gallus) of unstated strain given a single
dose of methiocarb by gavage and observed for 14 days (DuBois, 1962).
Methiocarb (purity, 98.5%) was administered twice at a dose of
380 mg/kg bw (approximately the LD50) to 20 white Leghorn laying
hens, aged 15-20 months, after an intramuscular injection of 50 mg/kg
bw atropine sulfate. After an interval of 21 days, the 18 surviving
hens were treated again, with the same doses of atropine and
methiocarb. The 16 survivors of the second treatment were sacrificed
three weeks later, and brain, spinal cord, and portions of peripheral
nerve were excised and processed for histological examination. Five
positive controls were given tri- ortho-cresyl phosphate as a single
oral dose and sacrificed three weeks later. The hens treated with
methiocarb exhibited brief cholinergic signs and were lethargic, but
none showed ataxia or paralysis. At examination, post mortem there
were no histopathological changes indicative of polyneuropathy in
either the central or peripheral nervous system, whereas the hens
treated with tri- ortho-cresyl phosphate showed the classical signs
of delayed polyneuropathy and mild but characteristic signs of delayed
polyneuropathy on histopathological examination (Thyssen & Schilde,
1978).
(iii) Antidotes
A study was carried out in which the effect of antidotes to an
oral LD50 of methiocarb was investigated in rats. The regimens used
were atropine at 50 mg/kg bw, pralidoxime (salt unstated) at 50 mg/kg
bw, obidoxime at 20 mg/kg bw, atropine and pralidoxime at 50 mg/kg bw
each, or atropine at 50 mg/kg bw with obidoxime at 20 mg/kg bw. All
the antidotes were given intraperitoneally just before clinical signs
became evident, i.e. 1-2 min after administration of methiocarb. The
LD50 values were 67 mg/kg bw without antidotes, 470 mg/kg bw with
atropine, 190 mg/kg bw with pralidoxime, 220 mg/kg bw with obidoxime,
500 mg/kg bw with pralidoxime and atropine, and 510 mg/kg bw with
obidoxime and atropine. Thus, pralidoxime and obidoxime added little
to the antidotal effect of atropine (Kimmerle, 1966a).
In mice, 10 mg/kg bw of atropine increased the LD50 of
methiocarb by sevenfold, while pralidoxime methiodide at 100 mg/kg bw
increased the LD50 1.3 times. Administration of the two antidotes
together increased the LD50 by 21 times. Obidoxime at 50 mg/kg bw
increased the LD50 3.1 times, while obidoxime plus atropine increased
it by 9.1 times (Kohgo, 1970).
Atropine sulfate at 50 mg/kg bw administered within 10 min of
methiocarb intraperitoneally increased the LD50 of methiocarb given
orally to rats by more than sixfold, and tetraethyl ammonium chloride
given by the same route increased it by approximately fourfold;
however, the combined effect of the two antidotes was less than that
of atropine alone (Kimmerle, 1971).
In the study of Thyssen and Schilde (1978) in hens at
approximately the LD50 (see above), atropine appeared to have a
powerful antidotal effect.
(iv) Interaction with other pesticides
When an intraperitoneal dose equal to 50% of the LD50 of
methiocarb was given to female Sprague-Dawley rats in combination with
another pesticide at a dose equal to 50% of the LD50, the combined
mortality was less than 50% in all cases. The highest combined
mortality rates (> 40 to < 50%) were seen with EPN, Guthion,
mevinphos, and carbaryl. Combined mortality rates of > 20 to < 40%
were found with parathion, parathion-methyl, disulfoton, malathion,
carbophenothion, tributyl phosphorotrithioite, and ethion. The
mortality rates seen with the remaining insecticides (demeton,
dioxathion, and schradan) were 5-20% (DuBois & Raymund, 1961b). In
another study of similar design, the combined mortality rates were 55%
with trichlorfon, 50% with propoxur, 35% with coumaphos, 30% with
oxydemeton-methyl, and 20% with fenthion (DuBois & Raymund, 1961c).
3. Observations in humans
The 250 workers in two plants manufacturing methiocarb over about
20 years were subjected to annual medical examinations and assays of
cholinesterase activity in whole blood. No adverse health effects or
changes in laboratory parameters were observed (Faul, 1993).
Comments
Methiocarb appeared to have been well absorbed in a small study
of absorption, distribution, metabolism, and excretion of the
radiolabelled compound in rats. More than 70% of the administered
radiolabel was excreted within 48 h, mostly in the urine. Methiocarb
was extensively metabolized. The main route of metabolism in both
animals and plants appears to be to methiocarb phenol, methiocarb
phenol sulfoxide, and methiocarb phenol sulfone. In some studies,
methiocarb sulfoxide was also found.
In single-dose and short-term studies in rats, methiocarb
administered by gavage inhibited plasma and erythrocyte cholinesterase
activity. In the short-term study, plasma, erythrocyte, and brain
cholinesterase activities were depressed at 10 mg/kg bw per day; the
NOAEL for this effect was 3 mg/kg bw per day. In a short-term study of
the ability of methiocarb or its sulfoxide to inhibit cholinesterase
activity in rats, the NOAEL for inhibition of erythrocyte
acetylcholinesterase activity was 0.5 mg/kg bw per day for methiocarb,
but a NOAEL was not identified for the sulfoxide. In a 29-day study in
which dogs were given methiocarb or methiocarb sulfoxide in gelatine
capsules at 0.05 or 0.5 mg/kg bw per day, both plasma and erythrocyte
cholinesterase activities were inhibited by both treatments.
The acute toxicity of methiocarb given by a number of routes has
been measured in a number of species. The oral LD50 in fasting rats
ranged from 13 to 130 mg/kg bw. The oral LD50 values for methiocarb
phenol, methiocarb phenol sulfoxide, and methiocarb phenol sulfone in
rats are all greater than 1 g/kg bw, as is that of methiocarb sulfone,
but that of methiocarb sulfoxide is between 6 and 43 mg/kg bw.
WHO has classified methiocarb as moderately hazardous (WHO,
1996).
The short-term toxicity of methiocarb has been tested in rats,
rabbits, cats, dogs, and chickens. Few of these studies were
appropriate for identifying NOAEL values and, of those that were, a
study in rats was carried out by inhalation and that in rabbits by
dermal application. In the study in rats, which were exposed by
inhalation, for five days per week for three weeks, no findings
related to treatment were seen on histopathological examination but
depressed brain acetyl-cholinesterase activity was observed at the
highest dose in animals of each sex and in males at the intermediate
dose. Consequently, the NOAEL was 6 mg/m3 per day. In a 21-day study
of the dermal toxicity of methiocarb in rabbits, the substance was
applied for 6 h/day at 0, 60, 150, or 375 mg/kg bw per day. Plasma
cholinesterase activity was reduced in males at the highest dose, but
no significant differences were seen between groups in the activities
of erythrocyte and brain acetylcholinesterase. The NOAEL was 150 mg/kg
bw per day on the basis of reduced food consumption at the highest
dose.
In a long-term study of toxicity in mice, methiocarb was
administered at dietary concentrations of 0, 67, 200, or 600 ppm. A
NOAEL was not identified because haematological changes were observed
in all treated males at 12 months and in all treated females at 24
months. The LOAEL was 67 ppm, equal to 15 mg/kg bw per day, on the
basis of minor haematological changes. Methiocarb was not carcinogenic
in mice.
In a two-year study of toxicity, rats received methiocarb at
dietary concentrations of 0, 67, 200, or 600 ppm. The NOAEL was 67
ppm, equal to 3.3 mg/kg bw per day, on the basis of haematological
changes at 3, 6, and 12 months. Methiocarb was not carcinogenic.
In a two-year study of toxicity, dogs were fed methiocarb in the
diet at 0, 5, 60, or 240 ppm. The NOAEL was 60 ppm, equivalent to 1.5
mg/kg bw per day, on the basis of reversible clinical signs at the
next highest dose, which were not observed after 15 weeks.
Three studies of developmental toxicity were available, one in
rats and two in rabbits. Fertilized rats received methiocarb by gavage
on days 6-15 of gestation at 0, 1, 3, or 10 mg/kg bw per day. The
NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of
reduced body-weight gain at the highest dose. As no fetotoxicity was
observed, the NOAEL for this end-point was 10 mg/kg bw per day, the
highest dose tested. In a study of developmental toxicity in rabbits,
pregnant animals received methiocarb at 0, 1, 3, or 10 mg/kg bw per
day by gavage on days 6-18 of gestation. The NOAEL was 3 mg/kg bw per
day for maternal toxicity on the basis of clinical effects and weight
loss at the highest dose. As no fetotoxicity or teratogenicity was
observed, the NOAEL for these end-points was 10 mg/kg bw per day, the
highest dose tested. In a further study, rabbits received methiocarb
by dermal application at 0, 10, 50, or 250 mg/kg bw per day for
6 h/day on days 6-18 of gestation. The NOAEL for both maternal and
fetal toxicity was 50 mg/kg bw per day on the basis of reduced
maternal food consumption and some decrease in mean fetal weight at
the highest dose; slight retardation of fetal development was also
observed. A multigeneration study of reproductive toxicity was
undertaken in rats given methiocarb at dietary concentrations of 0,
30, 100, or 300 ppm. The NOAEL was 300 ppm, equivalent to 30 mg/kg bw
per day, as no effect clearly related to treatment was observed. No
teratogenic effect was observed in any of these studies.
Methiocarb has been tested for genotoxicity in an adequate
battery of tests in vitro and in vivo. The Meeting concluded that
methiocarb is not genotoxic.
Methiocarb did not cause skin sensitization in studies conducted
by either the Magnusson and Kligman or the Beuhler technique.
In an early study of neurotoxicity in hens, methiocarb did not
cause delayed polyneuropathy of the organophosphorus type. Atropine
has consistently been shown to be an effective antidote for
methiocarb, while the effects of pyridinium oximes were somewhat
inconsistent.
The Meeting established an ADI of 0-0.02 mg/kg bw on the basis of
the NOAEL of 1.5 mg/kg bw per day in the two-year study of toxicity in
dogs and a safety factor of 100. This ADI results in a further safety
factor of 10 on the LOAEL in the long-term study of toxicity in mice.
An acute RfD was allocated on the basis of the NOAEL of 1.5 mg/kg
bw per day in the two-year study in dogs, because the signs observed
were acute, and a safety factor of 100. Of the shorter studies in
dogs, neither the 29-day nor the 12-week study was considered by the
Meeting to be adequate for the purpose of establishing a NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: No NOAEL; LOAEL: 67 ppm, equal to 15 mg/kg bw per
day (long-term study of toxicity)
Rat: 67 ppm, equal to 3.3 mg/kg bw per day (long-term
study of toxicity)
300 ppm, equivalent to 30 mg/kg bw per day
(maternal and fetal toxicity in a study of
reproductive toxicity)
3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study
of developmental toxicity)
10 mg/kg bw per day (developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (two-
year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would be useful for continued evaluation of the
compound
1. A modern study of absorption, distribution, metabolism, and
excretion
2. A modern multigeneration study of reproductive toxicity
3. Further observations in humans
List of end points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption No data
Dermal absorption No data
Distribution No data
Potential for accumulation Not likely to accumulate
Rate and extent of excretion 73 to > 90% within 48 h
Metabolism in animals Sulfoxidation and loss of carbamate side-chain
Toxicologically significant compounds Parent compound and methiocarb sulfoxide
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral 13-135 mg/kg bw
Rat: LD50, dermal 350 to > 5000 mg/kg bw
Rat: LC50 inhalation > 300 mg/m3
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Clinical signs
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 150 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 6 mg/m3
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Clinical signs
Lowest relevant NOAEL Dog: 1.5 mg/kg bw per day
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect No effect
Lowest relevant reproductive NOAEL Rat: 30 mg/kg bw per day
Developmental target/critical effect Maternal toxicity: reduced weight gain; no
fetotoxicity observed
Lowest relevant developmental NOAEL Rabbit: 3 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Does not cause delayed polyneuropathy
Other toxicological studies No data
Medical data No data
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Dog, 2 years 100
Acute reference dose 0.02 mg/kg bw Dog, 2 years 100
References
Baron, R.L., Casterline, J.L. & Fitzhugh, O.G. (1964) Specificity of
carbamate esterase inhibition in mice. Toxicol. Appl. Pharmacol., 6,
402-410.
Barthová, J., Woldemariam, M.G. & Leblová, S. (1989) The effect of
carbamates insecticides on cholinesterases of nontarget organisms.
Biologia (Bratislava), 44, 353-356.
Crawford, C.R. & Anderson, R.H. (1970) The skin and eye irritating
properties of BAY 37344 technical to rabbits. Unpublished report No.
28304 from Chemagro Corporation, Research Department, 15 October 1970.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972a) The acute oral toxicity to
guinea pigs and the acute inhalation toxicity to rats of Mesurol(R)
technical. Unpublished report No. 31987 from Chemagro Corporation,
Research Department, 24 January 1972. Submitted to WHO by Bayer AG
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1972b) The dermal toxicity of
(R)Mesurol technical and Mesurol 75% wettable powder to rabbits.
Unpublished report No. 34477 from Chemagro Division of Baychem
Corporation, Research Development, 21 August 1972. Submitted to WHO by
Bayer AG Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) The acute oral toxicity of
(R)Mesurol technical to rats. Unpublished report No. 34008 from
Chemagro Division of Baychem Corporation, Research and Development, 31
May 1972 and revised 8 May 1973. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 1007 from Microbiological
Associates Inc., Bethesda, Maryland and Rockville, Maryland, USA, 1
June 1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
David, R.M. (1988) EPA/OECD dermal sensitization test of Mesurol
technical in guinea pigs. Unpublished report No. 1015 from
Microbiological Associates, Inc., Bethesda, Maryland, USA, 15 April
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dotti, A. & Biedermann, K. (1992) Embryotoxicity study (including
teratogenicity) with H 321 (c.n. methiocarb) in the rabbit (dermal
application) part 1. Unpublished report No. R 5627 from RCC Research &
Consulting Co. Ltd and RCC Umweltchemie AG, Itingen, Switzerland, 6
August 1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Doull, J., Cowan, J. & Root, M. (1962) Subacute (16-week) oral
toxicity of Bayer 37344 to male and female rats. Unpublished report
No. 9456 from Department of Pharmacology, University of Chicago, USA,
12 June 1962. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. (1962) The acute oral toxicity of Bayer 37344 to
chickens. Unpublished report No. 9248 from Department of Pharmacology,
University of Chicago, USA, 16 May 1962. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K.P. (1964) The acute toxicity of some possible metabolites of
Bayer 37344. Unpublished report No. 12806 from Department of
Pharmacology, University of Chicago, USA, 22 January 1964. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961a) The acute toxicity of Bayer 37344
to mammals. Unpublished report No. 6775 from Department of
Pharmacology, University of Chicago, USA, 25 April 1961. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961b) The acute toxicity of Bayer 37344
in combination with other anticholinesterase insecticides to rats.
Unpublished report No. 7880 from Department of Pharmacology,
University of Chicago, USA, 1 November 1961. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1961c) The acute toxicity of Bayer 39007
and Bayer 37344 in combination with some other anticholinesterase
insecticides in rats. Unpublished report No. 7879 from Department of
Pharmacology, University of Chicago, USA, 4 November 1961. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K.P. & Raymund, A.B. (1962) The acute toxicity of
recrystallised Bayer 37344 and a wettable powder of Bayer 37344 to
female rats. Unpublished report No. 9424 from Department of
Pharmacology, University of Chicago, USA, 12 June 1962. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Eben, A. & Kimmerle, G. (1973) Mercaptodimethur effect of acute and
subacute oral doses on acetylcholinesterase activity in plasma,
erythrocyte and brain of rats. Unpublished report No. 4284 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 9 November
1973. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Faul, J. (1993) Mesurol active ingredient: In-company occupational
medical experience. Unpublished letter to Dr Heimann, PF-A/consulting,
Monheim, Germany, 1 April 1993. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50). Unpublished
letter to Dr Reuver, Bayer AG Institute of Toxicology, dated 27 June
1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) H 321 (mercaptodimethur). Unpublished note dated 3
November 1980. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1988) Determination of acute toxicity (LD50), Unpublished
letter to Dr Reuver, Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany, dated 1 August 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Haijar, N.P. & Hodgson, E. (1982) Sulfoxidation of
thioether-containing pesticides by the flavin-adenine
dinucleotide-dependent monooxygenase of pig liver microsomes.
Biochem. Pharmacol., 31, 745-752.
Hayes, R.H. (1981) Cholinesterase evaluation study of methiocarb
technical and methiocarb sulfoxide in dogs. Unpublished report No. 202
from Corporate Toxicology Department, Mobay Chemical Corporation,
Stillwell, Kansas, USA, 29 September 1981. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1983) Determination of acute toxicity (LD50).
Unpublished letter to Dr Reuver, PF-Zentrum, Monheim, Germany, dated
22 April 1983. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1978) H 321 (active ingredient of Mesurol)
Salmonella/microsome test for determination of point mutations.
Unpublished report No. 7978 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 6 December 1978. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B. (1979a) H 321 (active ingredient of Mesurol) micronucleus
test for mutagenic effect on mice. Unpublished report No. 8426 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 8
June 1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979b) H 321 dominant lethal study on male mouse to test
for mutagenic effects. Unpublished report No. 8395 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 23 May 1979.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) H 321 mercaptodimethur (the active ingredient of
Mesurol(R)) study of DNA damage using the E. coli pol A- test.
Unpublished report No. 11928 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 13 July 1983. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) H 321 c.n. mercaptodimethur Salmonella/microsome
test to evaluate for point mutagenic effect. Unpublished report No.
14205 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 10 January 1986. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1981) Cholinesterase no-effect level of Mesurol(R) and
Mesurol sulfoxide(R) in female rats. Unpublished report No. 199 from
Mobay Chemical Corporation, Corporate Toxicology Department, Stilwell,
Kansas, USA, 31 August 1981. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. & Schilde, B. (1980) H 321(Mesuro(R) active ingredient
-- mercaptodimethur) chronic toxicity study in dogs (2 year feeding
experiment). Unpublished report No. 9626 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 4 December 1980. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1960) Product Dr Wedemeyer H 321 (E 37 344) Production
No. 2410. Unpublished report from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 25 March 1960. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kimmerle, G. (1966a) Mesurol active ingredient (Wedemeyer H 321; Ht.
No. 3657) -- antidotal effect. Unpublished letter to Crop Protection
Science, Leverkusen. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, dated 8 August 1966. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1966b) Re active ingredient Wedemeyer H 321 (Ht No
3657). Inhalation tests. Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany. Unpublished letter to Crop Protection
Science, Leverkusen, Germany, dated 7 December 1966. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969a) Re Bay 37 344 (H 321) Lo No. 691. Unpublished
letter to Crop Protection Science, Leverkusen. Bayer AG, Institute for
Toxicology, Wuppertal-Elbberfeld, Germany, dates 20 May. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969b) Ref Bay 37344 (=H 321 = Methiocarb) Lot No. 691.
Unpublished letter to Crop Protection Science, Leverkusen. Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, dated 20 May.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1969c) Bay 37 344 subacute dermal toxicity study on
rabbits. Unpublished report No. 1291 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 1 April 1969. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1971) Comparison of the antidotal actions of
tetraethylammonium chloride and atropine in acute poisoning of
carbamate insecticides in rats. Arch. Toxicol., 27, 311-314.
Kimmerle, G. (1972) Unpublished note from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, dated 7 June 1972. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Klimmer, O.R. (1963) Toxicological testing of Bayer 37 344.
Unpublished letter from Pharmacological Institute of the University of
Bonn, Germany, to Crop Protection/Scientific Department, Leverkusen,
Germany, dated 10 December. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kohgo, L. (1970) The antagonistic effects of atropine or pyridinium
aldoxime derivatives on cholinesterase-inhibitory pesticides in male
and mice. J. Med. Soc. Toho Jpn, 17, 60-88.
Krötlinger, F. (1989) H 321 (mercaptodimethur, active ingredient of
Mesurol) chronic toxicological study on mice (feeding study over 2
year). Unpublished addendum No. 11908A to report No. 11908 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 15 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. (1990) H 321 (mercaptodimethur, the active ingredient
of Mesurol) chronic toxicity study on rats (2-year feeding
experiment). Unpublished addendum No. 10039A to report No. 10039 from
Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 16
February 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. & Janda, B. (1983) H 321 (mercaptodimethur, the active
ingredient of Mesurol(R)) chronic toxicity study on mice (2-year
feeding experiment). Unpublished report No. 11908 from Bayer AG,
Institute for Toxicology, Wuppertal-Elberfeld, Germany, 4 July 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Löser, E. & Vogel, O. (1981) H 321 (mercaptodimethur,
the active ingredient of Mesurol) chronic toxicity study on rats
(2-year feeding experiment). Unpublished report No. 10039 from Bayer
AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany, 2 July
1981. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of mesurol
technical to dogs. Unpublished note of experiment No. 450474 from
Mobay Chemical Corp., Chemagro Agricultural Division, dated 25 August
1975. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976a) The acute oral toxicities of
mesurol technical and mesurol sulfoxide. Unpublished note of
experiment No. 49541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 12 July 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1976b) The acute oral toxicities of
Mesurol(R) technical and mesurol sulfoxide. Unpublished note of
experiment No. 50541 from Mobay Chemical Corp., Chemagro Agricultural
Division, dated 19 August 1976. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) A comparison of the acute oral
toxicities of Mesurol(R) technical to fasted and non-fasted rats.
Unpublished note of experiment No. 50541 from Mobay Chemical Corp.,
Chemagro Agricultural Division, dated 19 January 1977. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1989) H 321 C.N. Methiocarb mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay in
vitro. Unpublished report No. 18280 from Bayer AG, Institute for
Toxicology, Wuppertal-Elberfeld, Germany, 15 August 1989. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Lorke, D. (1971) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished report No.
3133 from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld,
Germany, 30 November 1971. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1969) Bay 37 344. Blood analyses, urinalysis and clinical
chemistry examinations following oral administration rats. Unpublished
report No. 1358 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 3 March 1969. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Löser, E. (1970) Bay 37 344. Generation studies on rats. Unpublished
report No. 2208 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 10 July 1970. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Menzie, C.M. (1974) Metabolism of Pesticides: An Update (Wildlife
No. 184), Washington DC, US Department of the Interior Fish and
Wildlife Service, p. 249.
Mihail, F. (1984) H 321 (C.n. mercaptodimethur) study for
skin-sensitising effect on guinea pigs. Unpublished report No. 13125
from Bayer AG, Institute for Toxicology, Wuppertal-Elberfeld, Germany,
12 December 1984. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murli, H. (1990) Mutagenicity test on H 321 in an in vitro cytogenetic
assay measuring chromosomal abberation frequencies in Chinese hamster
ovary (CHO) cells with a confirmatory assay. Unpublished report No. R
4980 from Hazleton Laboratories America Inc., Kensingtonb Maryland,
USA, 8 March 1990. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Murphy, J.J., Timme, G. & Dräger, G. (1982) Mesurol(R) common name:
methiocarb, mercaptodimethur. Behaviour in plants. Unpublished report
from Mobay Chemical Corp., Kansas, USA, August 1982. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Nelson, D.L. (1979) Acute oral toxicity of Mesurol(R) technical and
metabolites to rats. Unpublished report No. 67815 from Mobay Chemical
Corp., Environmental Health Research, Stillwell, Kansas, USA, 27 June
1979. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1988) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1084 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 23 November
1988. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Procter, B.G. (1989) A 21-day dermal toxicity study of Mesurol
technical in albino rabbits. Unpublished report No. 1160 from
Bio-Research Laboratories Ltd, Senneville, Québec, Canada, 31 August
1989. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1986) Sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells test article Mesurol technical. Unpublished report
No. 790 from Microbiological Associates Inc., Bethesda, Maryland, USA,
25 September 1986. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988) Mesurol active ingredient (Bay 37 344). Studies on
rats for embryotoxic and teratogenic effects. Unpublished addendum No.
3133A to report No. 3133 from Bayer AG, Institute for Toxicology,
Wuppertal-Elberfeld, Germany, 21 October 1988. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Root, M., Doull, J. & Cowan, J. (1963) Determination of the safe
dietary level of Bayer 37344 for dogs. Unpublished report No. 11159
from Department of Pharmacology, University of Chicago, USA, 23 March
1963.. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Salama, A.K., Bakry, N.M. & Abou-Donia, M.B. (1993) A review article
on placental transfer of pesticides. J. Occup. Med. Toxicol., 2,
383-397.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Mesurol in rats. Unpublished report No. 940 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 31 August 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b) Acute four-hour inhalation toxicity study with
Mesurol(R) technical in rats. Unpublished report No. 870 from Mobay
Chemical Corp., Corporate Toxicology Department, USA, 18 June 1987.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1969) Mesurol(R) active ingredient -- acute cutaneous
toxicity in rats. Unpublished letter to Bayer AG, Institute of
Toxicology, Crop Protection Science, Leverkusen, Germany, dated 11
September 1969. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Solmecke, B. (1970) Oral toxicity of Mesurol metabolites to rats.
Unpublished notes addressed to Bayer AG, Institute of Toxicology, Crop
Protection Science, Leverkusen, Germany, dated 6 April 1970. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stanley, C.W. & Johnson, G.A. (1976) Metabolites of Mesurol(R) in
rat urine. Unpublished report No. MR50732 from Chemagro Agricultural
Division, Mobay Chemical Corp., USA, 16 November 1976. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Strankowski, K.J. & Minor, R.G. (1976) Effect of feeding
Mesurol(R)/Mesurol sulfoxide (9:1) to chickens for 28 days.
Unpublished report No. 49639 from Mobay Chemical Corp., Research and
Development Department, USA, 7 January 1976. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Strother, A. (1970) Comparative metabolism of N-methylcarbamates by
human and rat liver fractions. Biochem. Pharmacol., 19, 2525-2529.
Strother, A. (1972) In vitro metabolism of methylcarbamate
insecticides by human and rat liver fraction. Toxicol. Appl.
Pharmacol., 21, 112-129.
Tesh, J.M., Ross, F.W., Secker, R.C. & Wilby, O.K. (1981) H 321:
Effects of oral administration upon pregnancy in the rabbit. 2. main
study. Unpublished report No. 2173 from Life Science Research, Stock,
United Kingdom, 4 December 1981. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977a) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 15 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977b) Determination of acute toxicity LD50. Unpublished
note to Bayer AG Institute of Toxicology, dated 30 November 1977.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) H 321 (Mesurol active ingredient) acute inhalation
toxicity. Unpublished report No. 11113 from Bayer AG Institute of
Toxicology, 27 August 1982. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen, J. & Mohr, U. (1983) H 321 (Mesurol active ingredient)
subacute inhalation study with rats. Unpublished report No. 12120 from
Bayer AG Institute of Toxicology and Department of Experiment
Pathology, Hanover Medical University, Germany, 30 September 1983.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Schilde, B. (1978) H 321 Neurotoxicity study on hens.
Unpublished report No. 7637 from Bayer Institute of Toxicology, 20
June 1978. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Wheeler, L. & Strother, A. (1971) In vitro metabolism of the
N-methylcarbamates, Zectran and Mesurol, by liver, kidney and blood of
dogs and rats. J. Pharm. Exp. Ther., 178, 371-382.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Willems, P.W.J.M., Geursen-Reitsma, A.M. & van Joost, T. (1997)
Allergic contact dermatitis due to methiocarb (Mesurol). Contact
Derm., 36, 270-271.
