
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
CARBON DISULFIDE
Compound
Carbon disulfide
Chemical name
Carbon disulfide
Synonym
Carbon bisulfide
Empirical formula
CS2
Structural formula
S = C = S
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): liquid
Boiling-point: 46°C
Odour: sweetish when pure; impurities such as hydrogen sulfide give
characteristic unpleasant odours
Lowest concentration in air which is detectable by odour: undetectable
by odour at toxic concentrations
Flash point: 20°C (open cup)
Flammability limits in air: 1.25 to 44% by volume
Solubility:
Water: 0.22 g/100 ml
Organic solvents: infinitely soluble in alcohol, ether and benzine
Specific gravity (liquid): 1.27
Specific gravity (gas): 2.64
Uses
Carbon disulfide is sometimes used as a fumigant for grain,
stored honeycombs and potatoes. It is often mixed with carbon
tetrachloride or trichlorethylene.
Residues
See below.
Effect of fumigant on treated crop
Although the vapours of carbon disulfide have commonly been
observed to disappear rapidly upon aeration of a fumigated foodstuff,
there are no precise data on the rate of disappearance. Carbon
disulfide is soluble in the lipid constituents and essential oils
which may be present in some foodstuffs.
BIOLOGICAL DATA
Biochemical aspects
Carbon disulfide has been recommended for use in tropical
countries, inter alia, as an insecticidal fumigant for various
vegetable foods, in particular legumes. It may persist in the form of
residues because of its solubility in the lipid constituents and the
essential oils. In this connexion it should be noted that a certain
number of observations show that carbon disulfide may be absorbed
through the digestive tract giving rise, if the doses are sufficiently
high, to symptoms of poisoning of the same type as those caused by
inhalation of the solvent (Zangger, 1930; Madlo and Zangger, 1930).
Furthermore the possibility must be taken into consideration of
the reaction of carbon disulfide with certain constituents of foods of
vegetable origin. In this connexion its reactivity should be stressed
with peptide and protein amino groups, as described in numerous papers
(Chervenka and Wilcox, 1956; Leonis, 1948; Levy, 1950; Soucek and
Madlo, 1953, 1954, 1955; Zahradnik, 1954, 1955).
Dithiocarbamino-carboxylic derivatives are produced, and tend to form
cyclic derivatives of 2-thio-5-thiazolidone:
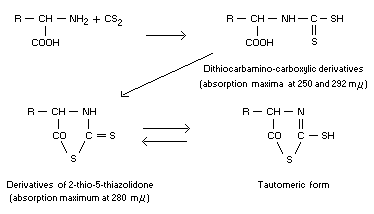 The existence of these reactions has been proved by
chromatography of the amino acids and peptides in blood brought into
contact, in vitro, with carbon disulfide labelled with 35S (Soucek
et al., 1956).
Various findings and, in particular, the increase in the thiol
groups in the blood serum of animals or human beings exposed to carbon
disulfide and especially chromatographic identification (Soucek and
Madlo, 1955) and spectrophotometric measurements (Cohen et al., 1958;
Cohen et al., 1959) show that these reactions occur in vivo.
Their toxicological implications are important from various
points of view:
(1) They may occur over and above the solubility of the poison in
lipid constituents and essential oils and thus affect the residues in
plants, in certain cases.
(2) The dithiocarbamino-carboxylic and thiazolidone derivatives (in
their tautomeric form) have the power of forming complexes, thanks to
their thiol groups, with polyvalent cations of great physiological
importance, such as zinc and copper. Thus in rabbits subjected to
poisoning with carbon disulfide, either by the cutaneous route (Cohen
et al., 1958) or by inhalation (Cohen et al., 1959), increased
excretion of zinc in the urine and faeces has been found to occur, as
well as a corresponding fall in the blood level (serum and corpuscles)
of this element. As concerns copper, a distinct fall in the content of
the cerebral cortex and spinal cord was noted. The lack of zinc, and
particularly the lack of copper in the central nervous system, may
easily have serious pathological consequences, as revealed, moreover,
by the degenerative lesions found at the histological level. The
formation by fixation of carbon disulfide on peptides and proteins, of
derivatives giving chelates with physiological polyvalent cations is
one of the fundamental biochemical mechanisms in the toxicity of
carbon disulfide (Cohen et al., 1959; Stokinger, 1963).
(3) Dithiocarbamino-carboxylic derivatives can be decomposed by the
cysteine desulfhydrylase in the liver of mammals, with liberation of
hydrogen sulfide (Madlo and Soucek, 1957). This explains why carbon
disulfide retained in the organism can subsequently be gradually
excreted in the urine after partial metabolization into mineral
sulfates, as has been demonstrated by the work of Strittmatter, Peters
and McKee (Strittmatter et al., 1950), inter alia, on the mouse and
the guinea-pig using carbon disulfide labelled with 35S.
It must be carefully stressed, nevertheless, that these data
concern the metabolism of carbon disultide in man and the higher
mammals and that no research in this direction seems to have been
carried out in the case of plants.
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rabbit Subcutaneous 300 Lewin (1879)
Rabbit Inhalation (exposure 16 mg/litre Negherbon (1959)
for six hours) dead in seven
days
Cat Inhalation (exposure 23 mg/litre Negherbon (1959)
for three hours)
Carbon disulfide emits highly toxic vapour, inhalation of which
by laboratory animals and by man causes multiple symptoms showing
attack on numerous phsyiological receptors, and especially on the
central nervous system and the liver.
Exposure of man to a concentration of 15 mg/litre during 50
minutes to an hour may cause death; exposure during the same period to
a concentration of the order of 3.5 mg/litre causes serious nervous
disturbances (Flury and Zernik, 1931; Flury and Lehmann, 1938).
Carbon disulfide is a cumulative poison. The maximum tolerable
concentration in the air adopted by the United States Association of
Government Hygienists is 20 ppm, or 60 mg/m3 (Anon, 1964). Some
workers feel that this limit should be decreased to 10 ppm or 30
mg/m3.
Short-term studies
Although much research has been carried out on the effects of
repeated inhalation of carbon disulfide and although the results
obtained, combined with observations on human subjects occupationally
exposed to inhalation of the product, have made it possible to assess
both qualitatively and quantitatively the toxicity of carbon disulfide
when absorbed through the lungs and to suggest permissible limits
under industrial conditions (10 to 20 ppm for repeated exposure over
long periods), the same does not apply as concerns absorption per
os. The few experiments recorded have been carried out on individual
animals.
Dog. A dog weighing 13 kg was given daily for five months
200 to 300 ml of water containing 0.5 g of carbon disulfide without
any apparent symptoms (Sapelier, 1894).
Long-term studies
There is no information in regard to absorption per os.
Comments on the experimental studies reported
There are extremely few data on the short-term effects of carbon
disulfide absorbed per os while data on long-term effects are
completely lacking.
Evaluation
The very scanty data available do not make it possible to
evaluate an acceptable daily intake for man.
Further work required
If the use of carbon disulfide for the fumigation of certain
types of food proved to be essential, then research would have to be
carried out:
(1) on the nature and quantity of the residues present in the treated
food;
(2) on the long-term effects in at least two animal species of carbon
disulfide and the products to which it may give rise by reaction with
the protein constituents of food.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Cohen, A. E., Paulus, H. J., Keenan, R. G. & Scheel, L. D. (1958)
Arch. industr. Hlth, 17, 164
Cohen, A. E., Scheel, L. D., Kopp, J. F., Stockell, F. R., Keenan, R.
G., Mountain, J. T. & Paulus, H. J. (1959) Amer. industr. Hyg. Ass.
Quart., 20, 303
Chervenka, H. & Wilcox, P. E. (1956) J. biol. Chem., 222, 21
Flury, F. & Lehmann, K. B. (1938) Toxicologie und Hygiene der
technischen Lösungsmitte, Springer, Berlin
Flury, F. & Zernik, F. (1931) Schädliche gase, Springer, Berlin
Leonis, J. (1948) C.R. Trav. Lab. Carlsberg fer. Chim., 26, 315
Levy, A. L. (1950) J. chem. Soc., 404
Lewin, -. (1897) Arch. Path. anat., 78, 113
Madlo, Z. & Soucek, B. (1957) Bull. Soc. Chim. biol. (Paris), 39,
989
Madlo, Z. & Zangger, H. (1930) Schweiz med. Wschr., 60, 193
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Sapelier, E. (1894) Etude sur le sulfure de carbone (Thčse pour le
Doctorat en médecine, Paris), cited by Lehman, K. B. (1894) Arch.
Hyg. (Berl.), 20, 26
Soucek, B. & Madlo, Z. (1953) Pracov. Lék., 5, 309
Soucek, B. & Madlo, Z. (1954) Pracov. Lék., 6, 11
Soucek, B. & Madlo, Z. (1955) Bull. Soc. Chim. biol. (Paris), 37,
1373
Soucek, B., Jensovsky, L., Pavelkova, E. & Zahradnik, R. (1956)
Chemické listy, 50, 1651
Soucek, B. & Madlo, Z. (1956) Arch. Gewerbepath. Gewerbehyg., 14,
511
Stokinger, H. E. (1963) Pharmacodynamie biochemical and toxicologic
methods as bases for air quality standards, Report presented at the
second symposium on the tolerable limits for toxic substances in
industry
Strittmatter, C. F., Peters, Th. & McKee, R. W. (1950) Arch.
industr. Hyg., 1, 54
Zahradnik, R. (1954) Chemické listy, 48, 11
Zahradnik, R. (1955) Chemické listy, 49, 1002
Zangger, H. (1930) Arch. Gewerbepath. Gewerbehyg., 1, 77
The existence of these reactions has been proved by
chromatography of the amino acids and peptides in blood brought into
contact, in vitro, with carbon disulfide labelled with 35S (Soucek
et al., 1956).
Various findings and, in particular, the increase in the thiol
groups in the blood serum of animals or human beings exposed to carbon
disulfide and especially chromatographic identification (Soucek and
Madlo, 1955) and spectrophotometric measurements (Cohen et al., 1958;
Cohen et al., 1959) show that these reactions occur in vivo.
Their toxicological implications are important from various
points of view:
(1) They may occur over and above the solubility of the poison in
lipid constituents and essential oils and thus affect the residues in
plants, in certain cases.
(2) The dithiocarbamino-carboxylic and thiazolidone derivatives (in
their tautomeric form) have the power of forming complexes, thanks to
their thiol groups, with polyvalent cations of great physiological
importance, such as zinc and copper. Thus in rabbits subjected to
poisoning with carbon disulfide, either by the cutaneous route (Cohen
et al., 1958) or by inhalation (Cohen et al., 1959), increased
excretion of zinc in the urine and faeces has been found to occur, as
well as a corresponding fall in the blood level (serum and corpuscles)
of this element. As concerns copper, a distinct fall in the content of
the cerebral cortex and spinal cord was noted. The lack of zinc, and
particularly the lack of copper in the central nervous system, may
easily have serious pathological consequences, as revealed, moreover,
by the degenerative lesions found at the histological level. The
formation by fixation of carbon disulfide on peptides and proteins, of
derivatives giving chelates with physiological polyvalent cations is
one of the fundamental biochemical mechanisms in the toxicity of
carbon disulfide (Cohen et al., 1959; Stokinger, 1963).
(3) Dithiocarbamino-carboxylic derivatives can be decomposed by the
cysteine desulfhydrylase in the liver of mammals, with liberation of
hydrogen sulfide (Madlo and Soucek, 1957). This explains why carbon
disulfide retained in the organism can subsequently be gradually
excreted in the urine after partial metabolization into mineral
sulfates, as has been demonstrated by the work of Strittmatter, Peters
and McKee (Strittmatter et al., 1950), inter alia, on the mouse and
the guinea-pig using carbon disulfide labelled with 35S.
It must be carefully stressed, nevertheless, that these data
concern the metabolism of carbon disultide in man and the higher
mammals and that no research in this direction seems to have been
carried out in the case of plants.
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rabbit Subcutaneous 300 Lewin (1879)
Rabbit Inhalation (exposure 16 mg/litre Negherbon (1959)
for six hours) dead in seven
days
Cat Inhalation (exposure 23 mg/litre Negherbon (1959)
for three hours)
Carbon disulfide emits highly toxic vapour, inhalation of which
by laboratory animals and by man causes multiple symptoms showing
attack on numerous phsyiological receptors, and especially on the
central nervous system and the liver.
Exposure of man to a concentration of 15 mg/litre during 50
minutes to an hour may cause death; exposure during the same period to
a concentration of the order of 3.5 mg/litre causes serious nervous
disturbances (Flury and Zernik, 1931; Flury and Lehmann, 1938).
Carbon disulfide is a cumulative poison. The maximum tolerable
concentration in the air adopted by the United States Association of
Government Hygienists is 20 ppm, or 60 mg/m3 (Anon, 1964). Some
workers feel that this limit should be decreased to 10 ppm or 30
mg/m3.
Short-term studies
Although much research has been carried out on the effects of
repeated inhalation of carbon disulfide and although the results
obtained, combined with observations on human subjects occupationally
exposed to inhalation of the product, have made it possible to assess
both qualitatively and quantitatively the toxicity of carbon disulfide
when absorbed through the lungs and to suggest permissible limits
under industrial conditions (10 to 20 ppm for repeated exposure over
long periods), the same does not apply as concerns absorption per
os. The few experiments recorded have been carried out on individual
animals.
Dog. A dog weighing 13 kg was given daily for five months
200 to 300 ml of water containing 0.5 g of carbon disulfide without
any apparent symptoms (Sapelier, 1894).
Long-term studies
There is no information in regard to absorption per os.
Comments on the experimental studies reported
There are extremely few data on the short-term effects of carbon
disulfide absorbed per os while data on long-term effects are
completely lacking.
Evaluation
The very scanty data available do not make it possible to
evaluate an acceptable daily intake for man.
Further work required
If the use of carbon disulfide for the fumigation of certain
types of food proved to be essential, then research would have to be
carried out:
(1) on the nature and quantity of the residues present in the treated
food;
(2) on the long-term effects in at least two animal species of carbon
disulfide and the products to which it may give rise by reaction with
the protein constituents of food.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Cohen, A. E., Paulus, H. J., Keenan, R. G. & Scheel, L. D. (1958)
Arch. industr. Hlth, 17, 164
Cohen, A. E., Scheel, L. D., Kopp, J. F., Stockell, F. R., Keenan, R.
G., Mountain, J. T. & Paulus, H. J. (1959) Amer. industr. Hyg. Ass.
Quart., 20, 303
Chervenka, H. & Wilcox, P. E. (1956) J. biol. Chem., 222, 21
Flury, F. & Lehmann, K. B. (1938) Toxicologie und Hygiene der
technischen Lösungsmitte, Springer, Berlin
Flury, F. & Zernik, F. (1931) Schädliche gase, Springer, Berlin
Leonis, J. (1948) C.R. Trav. Lab. Carlsberg fer. Chim., 26, 315
Levy, A. L. (1950) J. chem. Soc., 404
Lewin, -. (1897) Arch. Path. anat., 78, 113
Madlo, Z. & Soucek, B. (1957) Bull. Soc. Chim. biol. (Paris), 39,
989
Madlo, Z. & Zangger, H. (1930) Schweiz med. Wschr., 60, 193
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Sapelier, E. (1894) Etude sur le sulfure de carbone (Thčse pour le
Doctorat en médecine, Paris), cited by Lehman, K. B. (1894) Arch.
Hyg. (Berl.), 20, 26
Soucek, B. & Madlo, Z. (1953) Pracov. Lék., 5, 309
Soucek, B. & Madlo, Z. (1954) Pracov. Lék., 6, 11
Soucek, B. & Madlo, Z. (1955) Bull. Soc. Chim. biol. (Paris), 37,
1373
Soucek, B., Jensovsky, L., Pavelkova, E. & Zahradnik, R. (1956)
Chemické listy, 50, 1651
Soucek, B. & Madlo, Z. (1956) Arch. Gewerbepath. Gewerbehyg., 14,
511
Stokinger, H. E. (1963) Pharmacodynamie biochemical and toxicologic
methods as bases for air quality standards, Report presented at the
second symposium on the tolerable limits for toxic substances in
industry
Strittmatter, C. F., Peters, Th. & McKee, R. W. (1950) Arch.
industr. Hyg., 1, 54
Zahradnik, R. (1954) Chemické listy, 48, 11
Zahradnik, R. (1955) Chemické listy, 49, 1002
Zangger, H. (1930) Arch. Gewerbepath. Gewerbehyg., 1, 77
 The existence of these reactions has been proved by
chromatography of the amino acids and peptides in blood brought into
contact, in vitro, with carbon disulfide labelled with 35S (Soucek
et al., 1956).
Various findings and, in particular, the increase in the thiol
groups in the blood serum of animals or human beings exposed to carbon
disulfide and especially chromatographic identification (Soucek and
Madlo, 1955) and spectrophotometric measurements (Cohen et al., 1958;
Cohen et al., 1959) show that these reactions occur in vivo.
Their toxicological implications are important from various
points of view:
(1) They may occur over and above the solubility of the poison in
lipid constituents and essential oils and thus affect the residues in
plants, in certain cases.
(2) The dithiocarbamino-carboxylic and thiazolidone derivatives (in
their tautomeric form) have the power of forming complexes, thanks to
their thiol groups, with polyvalent cations of great physiological
importance, such as zinc and copper. Thus in rabbits subjected to
poisoning with carbon disulfide, either by the cutaneous route (Cohen
et al., 1958) or by inhalation (Cohen et al., 1959), increased
excretion of zinc in the urine and faeces has been found to occur, as
well as a corresponding fall in the blood level (serum and corpuscles)
of this element. As concerns copper, a distinct fall in the content of
the cerebral cortex and spinal cord was noted. The lack of zinc, and
particularly the lack of copper in the central nervous system, may
easily have serious pathological consequences, as revealed, moreover,
by the degenerative lesions found at the histological level. The
formation by fixation of carbon disulfide on peptides and proteins, of
derivatives giving chelates with physiological polyvalent cations is
one of the fundamental biochemical mechanisms in the toxicity of
carbon disulfide (Cohen et al., 1959; Stokinger, 1963).
(3) Dithiocarbamino-carboxylic derivatives can be decomposed by the
cysteine desulfhydrylase in the liver of mammals, with liberation of
hydrogen sulfide (Madlo and Soucek, 1957). This explains why carbon
disulfide retained in the organism can subsequently be gradually
excreted in the urine after partial metabolization into mineral
sulfates, as has been demonstrated by the work of Strittmatter, Peters
and McKee (Strittmatter et al., 1950), inter alia, on the mouse and
the guinea-pig using carbon disulfide labelled with 35S.
It must be carefully stressed, nevertheless, that these data
concern the metabolism of carbon disultide in man and the higher
mammals and that no research in this direction seems to have been
carried out in the case of plants.
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rabbit Subcutaneous 300 Lewin (1879)
Rabbit Inhalation (exposure 16 mg/litre Negherbon (1959)
for six hours) dead in seven
days
Cat Inhalation (exposure 23 mg/litre Negherbon (1959)
for three hours)
Carbon disulfide emits highly toxic vapour, inhalation of which
by laboratory animals and by man causes multiple symptoms showing
attack on numerous phsyiological receptors, and especially on the
central nervous system and the liver.
Exposure of man to a concentration of 15 mg/litre during 50
minutes to an hour may cause death; exposure during the same period to
a concentration of the order of 3.5 mg/litre causes serious nervous
disturbances (Flury and Zernik, 1931; Flury and Lehmann, 1938).
Carbon disulfide is a cumulative poison. The maximum tolerable
concentration in the air adopted by the United States Association of
Government Hygienists is 20 ppm, or 60 mg/m3 (Anon, 1964). Some
workers feel that this limit should be decreased to 10 ppm or 30
mg/m3.
Short-term studies
Although much research has been carried out on the effects of
repeated inhalation of carbon disulfide and although the results
obtained, combined with observations on human subjects occupationally
exposed to inhalation of the product, have made it possible to assess
both qualitatively and quantitatively the toxicity of carbon disulfide
when absorbed through the lungs and to suggest permissible limits
under industrial conditions (10 to 20 ppm for repeated exposure over
long periods), the same does not apply as concerns absorption per
os. The few experiments recorded have been carried out on individual
animals.
Dog. A dog weighing 13 kg was given daily for five months
200 to 300 ml of water containing 0.5 g of carbon disulfide without
any apparent symptoms (Sapelier, 1894).
Long-term studies
There is no information in regard to absorption per os.
Comments on the experimental studies reported
There are extremely few data on the short-term effects of carbon
disulfide absorbed per os while data on long-term effects are
completely lacking.
Evaluation
The very scanty data available do not make it possible to
evaluate an acceptable daily intake for man.
Further work required
If the use of carbon disulfide for the fumigation of certain
types of food proved to be essential, then research would have to be
carried out:
(1) on the nature and quantity of the residues present in the treated
food;
(2) on the long-term effects in at least two animal species of carbon
disulfide and the products to which it may give rise by reaction with
the protein constituents of food.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Cohen, A. E., Paulus, H. J., Keenan, R. G. & Scheel, L. D. (1958)
Arch. industr. Hlth, 17, 164
Cohen, A. E., Scheel, L. D., Kopp, J. F., Stockell, F. R., Keenan, R.
G., Mountain, J. T. & Paulus, H. J. (1959) Amer. industr. Hyg. Ass.
Quart., 20, 303
Chervenka, H. & Wilcox, P. E. (1956) J. biol. Chem., 222, 21
Flury, F. & Lehmann, K. B. (1938) Toxicologie und Hygiene der
technischen Lösungsmitte, Springer, Berlin
Flury, F. & Zernik, F. (1931) Schädliche gase, Springer, Berlin
Leonis, J. (1948) C.R. Trav. Lab. Carlsberg fer. Chim., 26, 315
Levy, A. L. (1950) J. chem. Soc., 404
Lewin, -. (1897) Arch. Path. anat., 78, 113
Madlo, Z. & Soucek, B. (1957) Bull. Soc. Chim. biol. (Paris), 39,
989
Madlo, Z. & Zangger, H. (1930) Schweiz med. Wschr., 60, 193
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Sapelier, E. (1894) Etude sur le sulfure de carbone (Thčse pour le
Doctorat en médecine, Paris), cited by Lehman, K. B. (1894) Arch.
Hyg. (Berl.), 20, 26
Soucek, B. & Madlo, Z. (1953) Pracov. Lék., 5, 309
Soucek, B. & Madlo, Z. (1954) Pracov. Lék., 6, 11
Soucek, B. & Madlo, Z. (1955) Bull. Soc. Chim. biol. (Paris), 37,
1373
Soucek, B., Jensovsky, L., Pavelkova, E. & Zahradnik, R. (1956)
Chemické listy, 50, 1651
Soucek, B. & Madlo, Z. (1956) Arch. Gewerbepath. Gewerbehyg., 14,
511
Stokinger, H. E. (1963) Pharmacodynamie biochemical and toxicologic
methods as bases for air quality standards, Report presented at the
second symposium on the tolerable limits for toxic substances in
industry
Strittmatter, C. F., Peters, Th. & McKee, R. W. (1950) Arch.
industr. Hyg., 1, 54
Zahradnik, R. (1954) Chemické listy, 48, 11
Zahradnik, R. (1955) Chemické listy, 49, 1002
Zangger, H. (1930) Arch. Gewerbepath. Gewerbehyg., 1, 77
The existence of these reactions has been proved by
chromatography of the amino acids and peptides in blood brought into
contact, in vitro, with carbon disulfide labelled with 35S (Soucek
et al., 1956).
Various findings and, in particular, the increase in the thiol
groups in the blood serum of animals or human beings exposed to carbon
disulfide and especially chromatographic identification (Soucek and
Madlo, 1955) and spectrophotometric measurements (Cohen et al., 1958;
Cohen et al., 1959) show that these reactions occur in vivo.
Their toxicological implications are important from various
points of view:
(1) They may occur over and above the solubility of the poison in
lipid constituents and essential oils and thus affect the residues in
plants, in certain cases.
(2) The dithiocarbamino-carboxylic and thiazolidone derivatives (in
their tautomeric form) have the power of forming complexes, thanks to
their thiol groups, with polyvalent cations of great physiological
importance, such as zinc and copper. Thus in rabbits subjected to
poisoning with carbon disulfide, either by the cutaneous route (Cohen
et al., 1958) or by inhalation (Cohen et al., 1959), increased
excretion of zinc in the urine and faeces has been found to occur, as
well as a corresponding fall in the blood level (serum and corpuscles)
of this element. As concerns copper, a distinct fall in the content of
the cerebral cortex and spinal cord was noted. The lack of zinc, and
particularly the lack of copper in the central nervous system, may
easily have serious pathological consequences, as revealed, moreover,
by the degenerative lesions found at the histological level. The
formation by fixation of carbon disulfide on peptides and proteins, of
derivatives giving chelates with physiological polyvalent cations is
one of the fundamental biochemical mechanisms in the toxicity of
carbon disulfide (Cohen et al., 1959; Stokinger, 1963).
(3) Dithiocarbamino-carboxylic derivatives can be decomposed by the
cysteine desulfhydrylase in the liver of mammals, with liberation of
hydrogen sulfide (Madlo and Soucek, 1957). This explains why carbon
disulfide retained in the organism can subsequently be gradually
excreted in the urine after partial metabolization into mineral
sulfates, as has been demonstrated by the work of Strittmatter, Peters
and McKee (Strittmatter et al., 1950), inter alia, on the mouse and
the guinea-pig using carbon disulfide labelled with 35S.
It must be carefully stressed, nevertheless, that these data
concern the metabolism of carbon disultide in man and the higher
mammals and that no research in this direction seems to have been
carried out in the case of plants.
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rabbit Subcutaneous 300 Lewin (1879)
Rabbit Inhalation (exposure 16 mg/litre Negherbon (1959)
for six hours) dead in seven
days
Cat Inhalation (exposure 23 mg/litre Negherbon (1959)
for three hours)
Carbon disulfide emits highly toxic vapour, inhalation of which
by laboratory animals and by man causes multiple symptoms showing
attack on numerous phsyiological receptors, and especially on the
central nervous system and the liver.
Exposure of man to a concentration of 15 mg/litre during 50
minutes to an hour may cause death; exposure during the same period to
a concentration of the order of 3.5 mg/litre causes serious nervous
disturbances (Flury and Zernik, 1931; Flury and Lehmann, 1938).
Carbon disulfide is a cumulative poison. The maximum tolerable
concentration in the air adopted by the United States Association of
Government Hygienists is 20 ppm, or 60 mg/m3 (Anon, 1964). Some
workers feel that this limit should be decreased to 10 ppm or 30
mg/m3.
Short-term studies
Although much research has been carried out on the effects of
repeated inhalation of carbon disulfide and although the results
obtained, combined with observations on human subjects occupationally
exposed to inhalation of the product, have made it possible to assess
both qualitatively and quantitatively the toxicity of carbon disulfide
when absorbed through the lungs and to suggest permissible limits
under industrial conditions (10 to 20 ppm for repeated exposure over
long periods), the same does not apply as concerns absorption per
os. The few experiments recorded have been carried out on individual
animals.
Dog. A dog weighing 13 kg was given daily for five months
200 to 300 ml of water containing 0.5 g of carbon disulfide without
any apparent symptoms (Sapelier, 1894).
Long-term studies
There is no information in regard to absorption per os.
Comments on the experimental studies reported
There are extremely few data on the short-term effects of carbon
disulfide absorbed per os while data on long-term effects are
completely lacking.
Evaluation
The very scanty data available do not make it possible to
evaluate an acceptable daily intake for man.
Further work required
If the use of carbon disulfide for the fumigation of certain
types of food proved to be essential, then research would have to be
carried out:
(1) on the nature and quantity of the residues present in the treated
food;
(2) on the long-term effects in at least two animal species of carbon
disulfide and the products to which it may give rise by reaction with
the protein constituents of food.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Cohen, A. E., Paulus, H. J., Keenan, R. G. & Scheel, L. D. (1958)
Arch. industr. Hlth, 17, 164
Cohen, A. E., Scheel, L. D., Kopp, J. F., Stockell, F. R., Keenan, R.
G., Mountain, J. T. & Paulus, H. J. (1959) Amer. industr. Hyg. Ass.
Quart., 20, 303
Chervenka, H. & Wilcox, P. E. (1956) J. biol. Chem., 222, 21
Flury, F. & Lehmann, K. B. (1938) Toxicologie und Hygiene der
technischen Lösungsmitte, Springer, Berlin
Flury, F. & Zernik, F. (1931) Schädliche gase, Springer, Berlin
Leonis, J. (1948) C.R. Trav. Lab. Carlsberg fer. Chim., 26, 315
Levy, A. L. (1950) J. chem. Soc., 404
Lewin, -. (1897) Arch. Path. anat., 78, 113
Madlo, Z. & Soucek, B. (1957) Bull. Soc. Chim. biol. (Paris), 39,
989
Madlo, Z. & Zangger, H. (1930) Schweiz med. Wschr., 60, 193
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Sapelier, E. (1894) Etude sur le sulfure de carbone (Thčse pour le
Doctorat en médecine, Paris), cited by Lehman, K. B. (1894) Arch.
Hyg. (Berl.), 20, 26
Soucek, B. & Madlo, Z. (1953) Pracov. Lék., 5, 309
Soucek, B. & Madlo, Z. (1954) Pracov. Lék., 6, 11
Soucek, B. & Madlo, Z. (1955) Bull. Soc. Chim. biol. (Paris), 37,
1373
Soucek, B., Jensovsky, L., Pavelkova, E. & Zahradnik, R. (1956)
Chemické listy, 50, 1651
Soucek, B. & Madlo, Z. (1956) Arch. Gewerbepath. Gewerbehyg., 14,
511
Stokinger, H. E. (1963) Pharmacodynamie biochemical and toxicologic
methods as bases for air quality standards, Report presented at the
second symposium on the tolerable limits for toxic substances in
industry
Strittmatter, C. F., Peters, Th. & McKee, R. W. (1950) Arch.
industr. Hyg., 1, 54
Zahradnik, R. (1954) Chemické listy, 48, 11
Zahradnik, R. (1955) Chemické listy, 49, 1002
Zangger, H. (1930) Arch. Gewerbepath. Gewerbehyg., 1, 77
