
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
CHLOROPICRIN
Compound
Chloropicrin
Chemical name
Chloropicrin
Synonym
Nitrochloroform, trichloronitromethane
Empirical formula
CCl3NO2
Structural formula
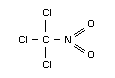 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 112°C
Odour: strongly irritating tear-gas
Lowest concentration in air which is detectable by odour: because of
irritant effect, a toxic concentration cannot be endured for more than
a few seconds
Flash point: non-flammable
Solubility:
Water: 0.227 g/100 ml
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 1.65
Specific gravity (gas): 5.7
Uses
Chloropicrin is used as an insecticide and sterilizing agent;
however, cereal grains are the only foodstuffs usually treated. Under
carefully controlled conditions it has been employed successfully for
control of insects and micro-organisms in certain seeds (Kennedy,
1961; Hsin et al., 1959). It is widely used for fumigation of soil to
control nematodes, micro-organisms, and weed seeds, but the possible
effect on food crops grown in treated soil has not been considered in
the present evaluation.
Chloropicrin has strong lachrymatory effects and is sometimes
added in small amounts to other comparatively odourless fumigants to
act as a warning agent.
A usual treatment for bulk grain is 2 to 2.5 lb per 1000 bushels.
Residues
There appear to be no published quantitative data on residues of
chloropicrin or its chemical decomposition products in foodstuffs
subsequent to fumigation.
Effect of fumigant on treated crop
If fumigated grain, or flour made from it, still contains
residual chloropicrin, milling and baking qualities may be affected
(Cotton, 1956). However, if the material is properly aerated there are
no adverse effects.
BIOLOGICAL DATA
Biochemical aspects
In this connexion, the ability of chloropicrin to react with free
thiol(-SH) groups, which become oxidized to disulfide groups, should
be stressed (Bacq, 1942a and b; Fischer, 1944; Desreux et al., 1946).
This blocking of the thiol groups causes, inter alia, a change in
the proteins (Bacq and Desreux, 1942; Bacq and Fischer, 1943) and
inhibition of enzyme systems whose activity depends on the presence of
free-SH groups, including urease (Fischer, 1946), papain (Desreux et
al., 1946) and succinodehydrogenase (Massart and Peeters, 1941).
From one study, it was concluded that the reaction of
chloropicrin with mercaptides at ordinary temperature is
3 RSH + NO2CCl3 --> (RS)3 - CNO2 + 3 HCl
but in another it was shown that chloropicrin acts as an oxidizing
agent with the simplest mercaptans, like ethylmercaptan and
thiophenol, giving the corresponding disulfides. With mercaptides,
there is always a considerable evolution of gas consisting of carbon
dioxide, considerable amounts of nitrogen and approximately equal
amounts of carbon monoxide and nitric oxide. The main reaction is
represented by the following equation:
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO2 + N2
and is accompanied to a smaller extent by the reaction
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO + 2 NO (Jackson, 1934).
The possibility of formation of residual nitrites and even
nitrosamines by action of chloropicrin on food cannot be excluded,
particularly when it is remembered that chloropicrin can undergo
photochemical decomposition in accordance with the equation:
CCl3 - NO2 --> COCl2 + NOCl
In the presence of water, nitrosyl chloride (NOCl) gives hydrochloric
acid and nitrous acid:
NOCl + H2O --> HNO2 + HCl
Acute toxicity
Animal Route LD mg/kg References
body-weight
Rabbit Intravenous 10 Gildemeister &
(aqueous emulsion (minimal Heubner, 1921
in the presence lethal dose)
of lecithin)
Cat Subcutaneous About 10 Negherbon, 1959
(alcoholic
solution)
Dog Inhalation (30 0.8 mg/litre Negherbon, 1959
minute exposure (120 ppm) (LC50)
Chloropicrin emits very irritating vapours which are lacrimatory,
and cause coughing and suffocation, as well as vomiting and
methaemoglobinaemia.
In man, a concentration of 2.4 g per m3 can cause death from
acute pulmonary oedema in a minute (Hanslian, 1921).
The maximal tolerable concentration of chloropicrin in air has
been fixed at 0.1 ppm.
Short-term studies
Cat. During two weeks a cat received a diet containing
approximately 1175 ppm of chloropicrin. A decrease in appetite was
noted, but no toxic symptoms were found (Gildemeister and Heubner,
1921).
Dog. A dog weighing 21.8 kg was fed on meat containing a total
amount of chloropicrin in excess of 1.5 g. The animal was reluctant to
eat the meat, but no symptoms of poisoning were seen (Gildemeister and
Heubner, 1921).
Long-term studies
No information.
Comments on the experimental studies reported
The short-term stidies are extremely limited (one cat, one dog),
while long-term ones are completely lacking. Moreover, the fate of
chloropicrin residues, if any, in treated food has not yet been
determined.
Evaluation
The very scanty data available do not make it possible to
estimate any acceptable daily dose for man.
Further work required
If it proves essential to continue using chloropicrin for the
fumigation of certain foods, it will be necessary to carry out
research:
(1) to define the nature and the amount of the residues present in
treated foods;
(2) to determine the long-term effects, in at least two animal
species, of chloropicrin and the degradation or reaction products
to which it may give rise in foods submitted to its action.
REFERENCES
Bacq, Z. M. (1942a) Acta biol. belg., 2, 430
Bacq, Z. M. (1942b) Bull. Acad. roy. Méd. Belg., 7, 500
Bacq, Z. M. & Desreux, V. (1942) Acta biol. belg., 3, 369
Bacq, Z. M. & Fischer, P. (1943) Bull. Soc. roy. Sci. Liège, 575 and
623
Cotton, R. T. (1956) Pest of stored grain and grain products, (rev.
ed.) Burgess Publ., Minneapolis
Desreux, V., Frèdèricq, E. & Fischer, P. (1946) Bull. Soc. Chim.
biol. (Paris), 28, 493
Fischer, P. (1944) Compt. Rend. Soc. biol (Paris), 138, 870
Fischer, P. (1946) Bull. Soc. Chim. biol. (Paris), 28, 240
Gildemeister, M. & Heubner, W. (1921) Z. ges. exp. Med., 13, 291
Hanslian, R. (1921) Ber. dtsch. pharm. gesell., 31, 222
Hsin, K. L., Hsueh, C. F., Liang, L. J., Dai, Z. S. & Chen, L. C.
(1959) Chem. Abstr., 54, 3831 c and d
Jackson, K. E. (1934) Chem. Rev., 14, 251
Kennedy, J. (1961) J. Sci. Food Agri., 12, 96
Massart, L. & Peeters, G. (1941) Acta biol. Belg., 1, 42
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 112°C
Odour: strongly irritating tear-gas
Lowest concentration in air which is detectable by odour: because of
irritant effect, a toxic concentration cannot be endured for more than
a few seconds
Flash point: non-flammable
Solubility:
Water: 0.227 g/100 ml
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 1.65
Specific gravity (gas): 5.7
Uses
Chloropicrin is used as an insecticide and sterilizing agent;
however, cereal grains are the only foodstuffs usually treated. Under
carefully controlled conditions it has been employed successfully for
control of insects and micro-organisms in certain seeds (Kennedy,
1961; Hsin et al., 1959). It is widely used for fumigation of soil to
control nematodes, micro-organisms, and weed seeds, but the possible
effect on food crops grown in treated soil has not been considered in
the present evaluation.
Chloropicrin has strong lachrymatory effects and is sometimes
added in small amounts to other comparatively odourless fumigants to
act as a warning agent.
A usual treatment for bulk grain is 2 to 2.5 lb per 1000 bushels.
Residues
There appear to be no published quantitative data on residues of
chloropicrin or its chemical decomposition products in foodstuffs
subsequent to fumigation.
Effect of fumigant on treated crop
If fumigated grain, or flour made from it, still contains
residual chloropicrin, milling and baking qualities may be affected
(Cotton, 1956). However, if the material is properly aerated there are
no adverse effects.
BIOLOGICAL DATA
Biochemical aspects
In this connexion, the ability of chloropicrin to react with free
thiol(-SH) groups, which become oxidized to disulfide groups, should
be stressed (Bacq, 1942a and b; Fischer, 1944; Desreux et al., 1946).
This blocking of the thiol groups causes, inter alia, a change in
the proteins (Bacq and Desreux, 1942; Bacq and Fischer, 1943) and
inhibition of enzyme systems whose activity depends on the presence of
free-SH groups, including urease (Fischer, 1946), papain (Desreux et
al., 1946) and succinodehydrogenase (Massart and Peeters, 1941).
From one study, it was concluded that the reaction of
chloropicrin with mercaptides at ordinary temperature is
3 RSH + NO2CCl3 --> (RS)3 - CNO2 + 3 HCl
but in another it was shown that chloropicrin acts as an oxidizing
agent with the simplest mercaptans, like ethylmercaptan and
thiophenol, giving the corresponding disulfides. With mercaptides,
there is always a considerable evolution of gas consisting of carbon
dioxide, considerable amounts of nitrogen and approximately equal
amounts of carbon monoxide and nitric oxide. The main reaction is
represented by the following equation:
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO2 + N2
and is accompanied to a smaller extent by the reaction
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO + 2 NO (Jackson, 1934).
The possibility of formation of residual nitrites and even
nitrosamines by action of chloropicrin on food cannot be excluded,
particularly when it is remembered that chloropicrin can undergo
photochemical decomposition in accordance with the equation:
CCl3 - NO2 --> COCl2 + NOCl
In the presence of water, nitrosyl chloride (NOCl) gives hydrochloric
acid and nitrous acid:
NOCl + H2O --> HNO2 + HCl
Acute toxicity
Animal Route LD mg/kg References
body-weight
Rabbit Intravenous 10 Gildemeister &
(aqueous emulsion (minimal Heubner, 1921
in the presence lethal dose)
of lecithin)
Cat Subcutaneous About 10 Negherbon, 1959
(alcoholic
solution)
Dog Inhalation (30 0.8 mg/litre Negherbon, 1959
minute exposure (120 ppm) (LC50)
Chloropicrin emits very irritating vapours which are lacrimatory,
and cause coughing and suffocation, as well as vomiting and
methaemoglobinaemia.
In man, a concentration of 2.4 g per m3 can cause death from
acute pulmonary oedema in a minute (Hanslian, 1921).
The maximal tolerable concentration of chloropicrin in air has
been fixed at 0.1 ppm.
Short-term studies
Cat. During two weeks a cat received a diet containing
approximately 1175 ppm of chloropicrin. A decrease in appetite was
noted, but no toxic symptoms were found (Gildemeister and Heubner,
1921).
Dog. A dog weighing 21.8 kg was fed on meat containing a total
amount of chloropicrin in excess of 1.5 g. The animal was reluctant to
eat the meat, but no symptoms of poisoning were seen (Gildemeister and
Heubner, 1921).
Long-term studies
No information.
Comments on the experimental studies reported
The short-term stidies are extremely limited (one cat, one dog),
while long-term ones are completely lacking. Moreover, the fate of
chloropicrin residues, if any, in treated food has not yet been
determined.
Evaluation
The very scanty data available do not make it possible to
estimate any acceptable daily dose for man.
Further work required
If it proves essential to continue using chloropicrin for the
fumigation of certain foods, it will be necessary to carry out
research:
(1) to define the nature and the amount of the residues present in
treated foods;
(2) to determine the long-term effects, in at least two animal
species, of chloropicrin and the degradation or reaction products
to which it may give rise in foods submitted to its action.
REFERENCES
Bacq, Z. M. (1942a) Acta biol. belg., 2, 430
Bacq, Z. M. (1942b) Bull. Acad. roy. Méd. Belg., 7, 500
Bacq, Z. M. & Desreux, V. (1942) Acta biol. belg., 3, 369
Bacq, Z. M. & Fischer, P. (1943) Bull. Soc. roy. Sci. Liège, 575 and
623
Cotton, R. T. (1956) Pest of stored grain and grain products, (rev.
ed.) Burgess Publ., Minneapolis
Desreux, V., Frèdèricq, E. & Fischer, P. (1946) Bull. Soc. Chim.
biol. (Paris), 28, 493
Fischer, P. (1944) Compt. Rend. Soc. biol (Paris), 138, 870
Fischer, P. (1946) Bull. Soc. Chim. biol. (Paris), 28, 240
Gildemeister, M. & Heubner, W. (1921) Z. ges. exp. Med., 13, 291
Hanslian, R. (1921) Ber. dtsch. pharm. gesell., 31, 222
Hsin, K. L., Hsueh, C. F., Liang, L. J., Dai, Z. S. & Chen, L. C.
(1959) Chem. Abstr., 54, 3831 c and d
Jackson, K. E. (1934) Chem. Rev., 14, 251
Kennedy, J. (1961) J. Sci. Food Agri., 12, 96
Massart, L. & Peeters, G. (1941) Acta biol. Belg., 1, 42
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
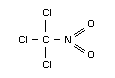 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 112°C
Odour: strongly irritating tear-gas
Lowest concentration in air which is detectable by odour: because of
irritant effect, a toxic concentration cannot be endured for more than
a few seconds
Flash point: non-flammable
Solubility:
Water: 0.227 g/100 ml
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 1.65
Specific gravity (gas): 5.7
Uses
Chloropicrin is used as an insecticide and sterilizing agent;
however, cereal grains are the only foodstuffs usually treated. Under
carefully controlled conditions it has been employed successfully for
control of insects and micro-organisms in certain seeds (Kennedy,
1961; Hsin et al., 1959). It is widely used for fumigation of soil to
control nematodes, micro-organisms, and weed seeds, but the possible
effect on food crops grown in treated soil has not been considered in
the present evaluation.
Chloropicrin has strong lachrymatory effects and is sometimes
added in small amounts to other comparatively odourless fumigants to
act as a warning agent.
A usual treatment for bulk grain is 2 to 2.5 lb per 1000 bushels.
Residues
There appear to be no published quantitative data on residues of
chloropicrin or its chemical decomposition products in foodstuffs
subsequent to fumigation.
Effect of fumigant on treated crop
If fumigated grain, or flour made from it, still contains
residual chloropicrin, milling and baking qualities may be affected
(Cotton, 1956). However, if the material is properly aerated there are
no adverse effects.
BIOLOGICAL DATA
Biochemical aspects
In this connexion, the ability of chloropicrin to react with free
thiol(-SH) groups, which become oxidized to disulfide groups, should
be stressed (Bacq, 1942a and b; Fischer, 1944; Desreux et al., 1946).
This blocking of the thiol groups causes, inter alia, a change in
the proteins (Bacq and Desreux, 1942; Bacq and Fischer, 1943) and
inhibition of enzyme systems whose activity depends on the presence of
free-SH groups, including urease (Fischer, 1946), papain (Desreux et
al., 1946) and succinodehydrogenase (Massart and Peeters, 1941).
From one study, it was concluded that the reaction of
chloropicrin with mercaptides at ordinary temperature is
3 RSH + NO2CCl3 --> (RS)3 - CNO2 + 3 HCl
but in another it was shown that chloropicrin acts as an oxidizing
agent with the simplest mercaptans, like ethylmercaptan and
thiophenol, giving the corresponding disulfides. With mercaptides,
there is always a considerable evolution of gas consisting of carbon
dioxide, considerable amounts of nitrogen and approximately equal
amounts of carbon monoxide and nitric oxide. The main reaction is
represented by the following equation:
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO2 + N2
and is accompanied to a smaller extent by the reaction
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO + 2 NO (Jackson, 1934).
The possibility of formation of residual nitrites and even
nitrosamines by action of chloropicrin on food cannot be excluded,
particularly when it is remembered that chloropicrin can undergo
photochemical decomposition in accordance with the equation:
CCl3 - NO2 --> COCl2 + NOCl
In the presence of water, nitrosyl chloride (NOCl) gives hydrochloric
acid and nitrous acid:
NOCl + H2O --> HNO2 + HCl
Acute toxicity
Animal Route LD mg/kg References
body-weight
Rabbit Intravenous 10 Gildemeister &
(aqueous emulsion (minimal Heubner, 1921
in the presence lethal dose)
of lecithin)
Cat Subcutaneous About 10 Negherbon, 1959
(alcoholic
solution)
Dog Inhalation (30 0.8 mg/litre Negherbon, 1959
minute exposure (120 ppm) (LC50)
Chloropicrin emits very irritating vapours which are lacrimatory,
and cause coughing and suffocation, as well as vomiting and
methaemoglobinaemia.
In man, a concentration of 2.4 g per m3 can cause death from
acute pulmonary oedema in a minute (Hanslian, 1921).
The maximal tolerable concentration of chloropicrin in air has
been fixed at 0.1 ppm.
Short-term studies
Cat. During two weeks a cat received a diet containing
approximately 1175 ppm of chloropicrin. A decrease in appetite was
noted, but no toxic symptoms were found (Gildemeister and Heubner,
1921).
Dog. A dog weighing 21.8 kg was fed on meat containing a total
amount of chloropicrin in excess of 1.5 g. The animal was reluctant to
eat the meat, but no symptoms of poisoning were seen (Gildemeister and
Heubner, 1921).
Long-term studies
No information.
Comments on the experimental studies reported
The short-term stidies are extremely limited (one cat, one dog),
while long-term ones are completely lacking. Moreover, the fate of
chloropicrin residues, if any, in treated food has not yet been
determined.
Evaluation
The very scanty data available do not make it possible to
estimate any acceptable daily dose for man.
Further work required
If it proves essential to continue using chloropicrin for the
fumigation of certain foods, it will be necessary to carry out
research:
(1) to define the nature and the amount of the residues present in
treated foods;
(2) to determine the long-term effects, in at least two animal
species, of chloropicrin and the degradation or reaction products
to which it may give rise in foods submitted to its action.
REFERENCES
Bacq, Z. M. (1942a) Acta biol. belg., 2, 430
Bacq, Z. M. (1942b) Bull. Acad. roy. Méd. Belg., 7, 500
Bacq, Z. M. & Desreux, V. (1942) Acta biol. belg., 3, 369
Bacq, Z. M. & Fischer, P. (1943) Bull. Soc. roy. Sci. Liège, 575 and
623
Cotton, R. T. (1956) Pest of stored grain and grain products, (rev.
ed.) Burgess Publ., Minneapolis
Desreux, V., Frèdèricq, E. & Fischer, P. (1946) Bull. Soc. Chim.
biol. (Paris), 28, 493
Fischer, P. (1944) Compt. Rend. Soc. biol (Paris), 138, 870
Fischer, P. (1946) Bull. Soc. Chim. biol. (Paris), 28, 240
Gildemeister, M. & Heubner, W. (1921) Z. ges. exp. Med., 13, 291
Hanslian, R. (1921) Ber. dtsch. pharm. gesell., 31, 222
Hsin, K. L., Hsueh, C. F., Liang, L. J., Dai, Z. S. & Chen, L. C.
(1959) Chem. Abstr., 54, 3831 c and d
Jackson, K. E. (1934) Chem. Rev., 14, 251
Kennedy, J. (1961) J. Sci. Food Agri., 12, 96
Massart, L. & Peeters, G. (1941) Acta biol. Belg., 1, 42
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 112°C
Odour: strongly irritating tear-gas
Lowest concentration in air which is detectable by odour: because of
irritant effect, a toxic concentration cannot be endured for more than
a few seconds
Flash point: non-flammable
Solubility:
Water: 0.227 g/100 ml
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 1.65
Specific gravity (gas): 5.7
Uses
Chloropicrin is used as an insecticide and sterilizing agent;
however, cereal grains are the only foodstuffs usually treated. Under
carefully controlled conditions it has been employed successfully for
control of insects and micro-organisms in certain seeds (Kennedy,
1961; Hsin et al., 1959). It is widely used for fumigation of soil to
control nematodes, micro-organisms, and weed seeds, but the possible
effect on food crops grown in treated soil has not been considered in
the present evaluation.
Chloropicrin has strong lachrymatory effects and is sometimes
added in small amounts to other comparatively odourless fumigants to
act as a warning agent.
A usual treatment for bulk grain is 2 to 2.5 lb per 1000 bushels.
Residues
There appear to be no published quantitative data on residues of
chloropicrin or its chemical decomposition products in foodstuffs
subsequent to fumigation.
Effect of fumigant on treated crop
If fumigated grain, or flour made from it, still contains
residual chloropicrin, milling and baking qualities may be affected
(Cotton, 1956). However, if the material is properly aerated there are
no adverse effects.
BIOLOGICAL DATA
Biochemical aspects
In this connexion, the ability of chloropicrin to react with free
thiol(-SH) groups, which become oxidized to disulfide groups, should
be stressed (Bacq, 1942a and b; Fischer, 1944; Desreux et al., 1946).
This blocking of the thiol groups causes, inter alia, a change in
the proteins (Bacq and Desreux, 1942; Bacq and Fischer, 1943) and
inhibition of enzyme systems whose activity depends on the presence of
free-SH groups, including urease (Fischer, 1946), papain (Desreux et
al., 1946) and succinodehydrogenase (Massart and Peeters, 1941).
From one study, it was concluded that the reaction of
chloropicrin with mercaptides at ordinary temperature is
3 RSH + NO2CCl3 --> (RS)3 - CNO2 + 3 HCl
but in another it was shown that chloropicrin acts as an oxidizing
agent with the simplest mercaptans, like ethylmercaptan and
thiophenol, giving the corresponding disulfides. With mercaptides,
there is always a considerable evolution of gas consisting of carbon
dioxide, considerable amounts of nitrogen and approximately equal
amounts of carbon monoxide and nitric oxide. The main reaction is
represented by the following equation:
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO2 + N2
and is accompanied to a smaller extent by the reaction
2(RS)3 - CNO2 --> 3 R-S-S-R + 2 CO + 2 NO (Jackson, 1934).
The possibility of formation of residual nitrites and even
nitrosamines by action of chloropicrin on food cannot be excluded,
particularly when it is remembered that chloropicrin can undergo
photochemical decomposition in accordance with the equation:
CCl3 - NO2 --> COCl2 + NOCl
In the presence of water, nitrosyl chloride (NOCl) gives hydrochloric
acid and nitrous acid:
NOCl + H2O --> HNO2 + HCl
Acute toxicity
Animal Route LD mg/kg References
body-weight
Rabbit Intravenous 10 Gildemeister &
(aqueous emulsion (minimal Heubner, 1921
in the presence lethal dose)
of lecithin)
Cat Subcutaneous About 10 Negherbon, 1959
(alcoholic
solution)
Dog Inhalation (30 0.8 mg/litre Negherbon, 1959
minute exposure (120 ppm) (LC50)
Chloropicrin emits very irritating vapours which are lacrimatory,
and cause coughing and suffocation, as well as vomiting and
methaemoglobinaemia.
In man, a concentration of 2.4 g per m3 can cause death from
acute pulmonary oedema in a minute (Hanslian, 1921).
The maximal tolerable concentration of chloropicrin in air has
been fixed at 0.1 ppm.
Short-term studies
Cat. During two weeks a cat received a diet containing
approximately 1175 ppm of chloropicrin. A decrease in appetite was
noted, but no toxic symptoms were found (Gildemeister and Heubner,
1921).
Dog. A dog weighing 21.8 kg was fed on meat containing a total
amount of chloropicrin in excess of 1.5 g. The animal was reluctant to
eat the meat, but no symptoms of poisoning were seen (Gildemeister and
Heubner, 1921).
Long-term studies
No information.
Comments on the experimental studies reported
The short-term stidies are extremely limited (one cat, one dog),
while long-term ones are completely lacking. Moreover, the fate of
chloropicrin residues, if any, in treated food has not yet been
determined.
Evaluation
The very scanty data available do not make it possible to
estimate any acceptable daily dose for man.
Further work required
If it proves essential to continue using chloropicrin for the
fumigation of certain foods, it will be necessary to carry out
research:
(1) to define the nature and the amount of the residues present in
treated foods;
(2) to determine the long-term effects, in at least two animal
species, of chloropicrin and the degradation or reaction products
to which it may give rise in foods submitted to its action.
REFERENCES
Bacq, Z. M. (1942a) Acta biol. belg., 2, 430
Bacq, Z. M. (1942b) Bull. Acad. roy. Méd. Belg., 7, 500
Bacq, Z. M. & Desreux, V. (1942) Acta biol. belg., 3, 369
Bacq, Z. M. & Fischer, P. (1943) Bull. Soc. roy. Sci. Liège, 575 and
623
Cotton, R. T. (1956) Pest of stored grain and grain products, (rev.
ed.) Burgess Publ., Minneapolis
Desreux, V., Frèdèricq, E. & Fischer, P. (1946) Bull. Soc. Chim.
biol. (Paris), 28, 493
Fischer, P. (1944) Compt. Rend. Soc. biol (Paris), 138, 870
Fischer, P. (1946) Bull. Soc. Chim. biol. (Paris), 28, 240
Gildemeister, M. & Heubner, W. (1921) Z. ges. exp. Med., 13, 291
Hanslian, R. (1921) Ber. dtsch. pharm. gesell., 31, 222
Hsin, K. L., Hsueh, C. F., Liang, L. J., Dai, Z. S. & Chen, L. C.
(1959) Chem. Abstr., 54, 3831 c and d
Jackson, K. E. (1934) Chem. Rev., 14, 251
Kennedy, J. (1961) J. Sci. Food Agri., 12, 96
Massart, L. & Peeters, G. (1941) Acta biol. Belg., 1, 42
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
