
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
ETHYLENE DICHLORIDE
Compound
Ethylene dichloride
Chemical name
Ethylene chloride
Synonyms
1,2-dichloroethane; ethylene dichloride; glycoldichloride
Emperical formula
C2H4Cl2
Structural formula
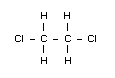 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 83.5°C
Odour: chloroform-like odour
Lowest concentration in air which is detectable by odour: 50 ppm
Flash point: ca 12°C (open cup)
Flammability limits in air: 6.2 to 15.9% by volume
Solubility:
Water: insoluble
Organic solvents: soluble in most common organic solvents
Specific gravity (liquid): 1.26
Specific gravity (gas): 3.42
Uses
Ethylene dichloride, being flammable, is rarely used alone. When
mixed with carbon tetrachloride it is extensively used as a liquid
grain fumigant for bulk storage in bins or on floors. The mixture
generally used is in the proportion of 3:1 by volume of ethylene
dichloride and carbon tetrachloride, this being the smallest
proportion of the latter which renders the mixture non-flammable. A
small proportion of ethylene dibromide (e.g. 2.5 to 5% by volume) is
occaaionaliy added to these or to other mixtures of ethylene
dichloride and carbon tetrachloride to improve surface treatment.
Rates of dosage of these mixtures for grain are usually between 1
gallon for 5 tons and 1 gallon for 10 tons in a 7-day exposure period.
The 3:1 mixture is also used for fumigating a variety of
commodities in bags including grain, pulses and animal feeding stuffs
usually on a fairly small scale in gas-tight chambers or other types
of sealed containers but in some tropical countries large stacks of
bagged grain have been treated under a covering of gasproof sheets.
Residues
During fumigation of cereal grains with ethylene dichloride or
its mixtures with other halogenated hydrocarbons, relatively heavy and
continuous sorption of the fumigant takes place (Winteringham, 1944).
The amount sorbed is higher at lower temperatures. The adsorbed
fumigant airs off slowly from whole grains over a period of months.
During handling, cleaning or milling processes the amount of adsorbed
fumigant is progressively reduced (Lynn and Vorkes, 1957). After
milling, a greater proportion of ethylene dichloride is found in the
bran than in the whole grain before milling (Conroy et al., 1957).
When treated at 9 gallons/1000 bushels with 3:1 ethylene
dichloride carbon tetrachloride mixture, triple the dose recommended
by the United States Department of Agriculture, showed a maximum of
140 ppm ethylene dichloride three days after application of fumigant
(ibid). Loss of fumigant during tempering and cleaning processes was
up to 70% and the maximum residue found in flour made from this batch
was 5 ppm.
When amounts of ethylene dichloride up to 10 times the residue
found in the flour from the previous study were added to flour which
was then made into loaves, no unchanged fumigant was found in the
baked bread (Munsey et al., 1957). (Sensitivity of method 2 ppm.)
When added to grain fed to cows, at levels up to 1000 ppm an
average of less than 0.25 ppm ethylene dichloride was found in the
milk. There appears to be no direct correlation between amounts of
ethylene dichloride added to the grain and that found in the milk
(Sykes and Klein, 1957).
When a 140-lb bag of 85% extraction wheat flour was fumigated for
48 hours in a 17.5 ft3 chamber at 25°C with 300 g of ethylene
dichloride applied as vapour (equivalent to 11.1 lb per ton of flour)
the following amounts of residual ethylene dichloride in ppm were
found after different periods of airing of the bag in still air (Pest
Infestation Laboratory, 1943).
1 hour 2 days 7 days
Surface of bag 1060 210 22
Centre of bag 1030 350 46
Baking tests carried out after seven days of airing showed no
detectable damage to baking quality and no taint of the fumigant was
left in the finished bread.
Effect of fumigant on treated crop
There appears to be no reaction between ethylene dichloride and
the constituents of grain. Ethylene dichloride added to grain can be
recovered intact by steam distillation with a solvent extraction
procedure (Heuser, 1964, personal communication).
BIOLOGICAL DATA
Biochemical aspects
In rabbits ethylene dichloride is mainly exhaled unchanged and no
metabolites have been described (Williams, 1959).
Toxicological studies
1. The fumigant
Maximum permissible concentration in the atmosphere recommended
for industrial hygiene in the United States is 50 ppm (200 mg/m3)
(Anon, 1964). Other maximum allowable concentrations are 3.0 mg/m3
(Ryazonov, 1959) and 4.0 mg/m3 (Borisova, 1960).
In experimental animals corneal lesions have been produced by
both subcutaneous and oral routes of administration (Hubbs and
Prusmack, 1955).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse oral 910 Smyth et al., 1936
Rat " 680 McCollister et al., 1956
Rat " 770 Smyth et al., 1936
Rabbit " 910 Smyth et al., 1936
Animals fatally poisoned by ethylene dichloride (inhalation)
showed hyperaemia and oedema of the lungs, degenerative kidney
changes, damage to liver and adrenals (von Oettingen, 1955).
Short- and long-term studies
Groups of 4-6 female rats were exposed to single doses of varying
concentrations of ethylene dichloride vapour for different lengths
of time and an estimate of single doses having no observable adverse
effects was made. For 12 000 ppm, 3000 ppm, 1000 ppm, 300 ppm and 200
ppm the maximum time was 0.1, 0.3, 1.5, 3.0 and 7.0 hours
respectively.
Intermittent exposure of animals to various concentrations of
ethylene dichloride vapour for 7 hours showed the following
no-effect levels (full histopathological examination and clinical
biochemical tests were reported): rat (15 male, 15 female) 151
exposures in 212 days, 200 ppm; guinea-pigs (8 male) 121 exposures in
170 days, 1000 ppm; guinea-pigs (8 female) 162 exposures in 226 days,
1000 ppm; rabbits (2 male) 165 exposures in 232 days, 400 ppm and
monkeys (2 male) 140 exposures in 212 days, 100 ppm (Spencer et al.,
1951).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 29.2% (by
weight) of ethylene dichloride appeared unaffected. The level of
ethylene dichloride in the grain was not stated and no
histopathological examinations were made (Rowe et al., 1956).
Comments on the experimental studies reported
1. Quite high levels of ethylene dichloride may remain in whole grain
after fumigation.
2. These high residues fall markedly when the grain is processed and
flour contains measurable but very low residues.
3. There is no evidence that ethylene dichloride combines chemically
with food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for ethylene dichloride. It
should be used as a fumigant under conditions which will result in the
lowest possible residues in the foodstuffs as consumed.
Further work required
1. Further investigation of the amount of the residual ethylene
dichloride remaining in the food after treatment and the effect
on this of processing and cooking.
2. Feeding studies should be carried out on two mammalian species to
determine the effect of the long-term feeding of ethylene
dichloride with particular reference to reproduction.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Borisova, M. K. (1960) Predel' no Dopustimye Kontsentratsii
Atmosfern. Zagryazneii, 4, 61
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Assoc. Off.
Agric. Chem., 40, 163
Hubbs, R. A. & Prusmack, J. J. (1955) J. Amer. med. Ass., 159, 673
Lynn, G. E. & Vorkes, F. A. (1957) J. Assoc. Off. Agric. Chem.,
40, 163
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Assoc. Off.
Agric. Chem., 40, 201
von Oettingen, W. F. (1955) The halogenated aliphatic, olefinic,
cyclic, aromatic and aliphatic-aromatic hydrocarbons, including the
halogenated insecticides, their toxicity and potential dangers,
Public Health Service Publ. No. 414
Pest Infestation Laboratory (1943) Pest Infestation Research Report,
87 (Unpublished document)
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Ryazonov, V. A. (1959) Proc. Int. Clean Air Conf., Nat. Soc. for
clean air, London
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Spencer, H. C. et al. (1951) Arch. industr. Hyg., 4, 482
Sykes, J. F. & Klein, A. K. (1957) J. Assoc. Off. Agric. Chem.,
40, 203
Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall Ltd
Winteringham, F. P. W. (1944) J. Soc. chem. Ind. (Lond.), 63, 144
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 83.5°C
Odour: chloroform-like odour
Lowest concentration in air which is detectable by odour: 50 ppm
Flash point: ca 12°C (open cup)
Flammability limits in air: 6.2 to 15.9% by volume
Solubility:
Water: insoluble
Organic solvents: soluble in most common organic solvents
Specific gravity (liquid): 1.26
Specific gravity (gas): 3.42
Uses
Ethylene dichloride, being flammable, is rarely used alone. When
mixed with carbon tetrachloride it is extensively used as a liquid
grain fumigant for bulk storage in bins or on floors. The mixture
generally used is in the proportion of 3:1 by volume of ethylene
dichloride and carbon tetrachloride, this being the smallest
proportion of the latter which renders the mixture non-flammable. A
small proportion of ethylene dibromide (e.g. 2.5 to 5% by volume) is
occaaionaliy added to these or to other mixtures of ethylene
dichloride and carbon tetrachloride to improve surface treatment.
Rates of dosage of these mixtures for grain are usually between 1
gallon for 5 tons and 1 gallon for 10 tons in a 7-day exposure period.
The 3:1 mixture is also used for fumigating a variety of
commodities in bags including grain, pulses and animal feeding stuffs
usually on a fairly small scale in gas-tight chambers or other types
of sealed containers but in some tropical countries large stacks of
bagged grain have been treated under a covering of gasproof sheets.
Residues
During fumigation of cereal grains with ethylene dichloride or
its mixtures with other halogenated hydrocarbons, relatively heavy and
continuous sorption of the fumigant takes place (Winteringham, 1944).
The amount sorbed is higher at lower temperatures. The adsorbed
fumigant airs off slowly from whole grains over a period of months.
During handling, cleaning or milling processes the amount of adsorbed
fumigant is progressively reduced (Lynn and Vorkes, 1957). After
milling, a greater proportion of ethylene dichloride is found in the
bran than in the whole grain before milling (Conroy et al., 1957).
When treated at 9 gallons/1000 bushels with 3:1 ethylene
dichloride carbon tetrachloride mixture, triple the dose recommended
by the United States Department of Agriculture, showed a maximum of
140 ppm ethylene dichloride three days after application of fumigant
(ibid). Loss of fumigant during tempering and cleaning processes was
up to 70% and the maximum residue found in flour made from this batch
was 5 ppm.
When amounts of ethylene dichloride up to 10 times the residue
found in the flour from the previous study were added to flour which
was then made into loaves, no unchanged fumigant was found in the
baked bread (Munsey et al., 1957). (Sensitivity of method 2 ppm.)
When added to grain fed to cows, at levels up to 1000 ppm an
average of less than 0.25 ppm ethylene dichloride was found in the
milk. There appears to be no direct correlation between amounts of
ethylene dichloride added to the grain and that found in the milk
(Sykes and Klein, 1957).
When a 140-lb bag of 85% extraction wheat flour was fumigated for
48 hours in a 17.5 ft3 chamber at 25°C with 300 g of ethylene
dichloride applied as vapour (equivalent to 11.1 lb per ton of flour)
the following amounts of residual ethylene dichloride in ppm were
found after different periods of airing of the bag in still air (Pest
Infestation Laboratory, 1943).
1 hour 2 days 7 days
Surface of bag 1060 210 22
Centre of bag 1030 350 46
Baking tests carried out after seven days of airing showed no
detectable damage to baking quality and no taint of the fumigant was
left in the finished bread.
Effect of fumigant on treated crop
There appears to be no reaction between ethylene dichloride and
the constituents of grain. Ethylene dichloride added to grain can be
recovered intact by steam distillation with a solvent extraction
procedure (Heuser, 1964, personal communication).
BIOLOGICAL DATA
Biochemical aspects
In rabbits ethylene dichloride is mainly exhaled unchanged and no
metabolites have been described (Williams, 1959).
Toxicological studies
1. The fumigant
Maximum permissible concentration in the atmosphere recommended
for industrial hygiene in the United States is 50 ppm (200 mg/m3)
(Anon, 1964). Other maximum allowable concentrations are 3.0 mg/m3
(Ryazonov, 1959) and 4.0 mg/m3 (Borisova, 1960).
In experimental animals corneal lesions have been produced by
both subcutaneous and oral routes of administration (Hubbs and
Prusmack, 1955).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse oral 910 Smyth et al., 1936
Rat " 680 McCollister et al., 1956
Rat " 770 Smyth et al., 1936
Rabbit " 910 Smyth et al., 1936
Animals fatally poisoned by ethylene dichloride (inhalation)
showed hyperaemia and oedema of the lungs, degenerative kidney
changes, damage to liver and adrenals (von Oettingen, 1955).
Short- and long-term studies
Groups of 4-6 female rats were exposed to single doses of varying
concentrations of ethylene dichloride vapour for different lengths
of time and an estimate of single doses having no observable adverse
effects was made. For 12 000 ppm, 3000 ppm, 1000 ppm, 300 ppm and 200
ppm the maximum time was 0.1, 0.3, 1.5, 3.0 and 7.0 hours
respectively.
Intermittent exposure of animals to various concentrations of
ethylene dichloride vapour for 7 hours showed the following
no-effect levels (full histopathological examination and clinical
biochemical tests were reported): rat (15 male, 15 female) 151
exposures in 212 days, 200 ppm; guinea-pigs (8 male) 121 exposures in
170 days, 1000 ppm; guinea-pigs (8 female) 162 exposures in 226 days,
1000 ppm; rabbits (2 male) 165 exposures in 232 days, 400 ppm and
monkeys (2 male) 140 exposures in 212 days, 100 ppm (Spencer et al.,
1951).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 29.2% (by
weight) of ethylene dichloride appeared unaffected. The level of
ethylene dichloride in the grain was not stated and no
histopathological examinations were made (Rowe et al., 1956).
Comments on the experimental studies reported
1. Quite high levels of ethylene dichloride may remain in whole grain
after fumigation.
2. These high residues fall markedly when the grain is processed and
flour contains measurable but very low residues.
3. There is no evidence that ethylene dichloride combines chemically
with food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for ethylene dichloride. It
should be used as a fumigant under conditions which will result in the
lowest possible residues in the foodstuffs as consumed.
Further work required
1. Further investigation of the amount of the residual ethylene
dichloride remaining in the food after treatment and the effect
on this of processing and cooking.
2. Feeding studies should be carried out on two mammalian species to
determine the effect of the long-term feeding of ethylene
dichloride with particular reference to reproduction.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Borisova, M. K. (1960) Predel' no Dopustimye Kontsentratsii
Atmosfern. Zagryazneii, 4, 61
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Assoc. Off.
Agric. Chem., 40, 163
Hubbs, R. A. & Prusmack, J. J. (1955) J. Amer. med. Ass., 159, 673
Lynn, G. E. & Vorkes, F. A. (1957) J. Assoc. Off. Agric. Chem.,
40, 163
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Assoc. Off.
Agric. Chem., 40, 201
von Oettingen, W. F. (1955) The halogenated aliphatic, olefinic,
cyclic, aromatic and aliphatic-aromatic hydrocarbons, including the
halogenated insecticides, their toxicity and potential dangers,
Public Health Service Publ. No. 414
Pest Infestation Laboratory (1943) Pest Infestation Research Report,
87 (Unpublished document)
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Ryazonov, V. A. (1959) Proc. Int. Clean Air Conf., Nat. Soc. for
clean air, London
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Spencer, H. C. et al. (1951) Arch. industr. Hyg., 4, 482
Sykes, J. F. & Klein, A. K. (1957) J. Assoc. Off. Agric. Chem.,
40, 203
Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall Ltd
Winteringham, F. P. W. (1944) J. Soc. chem. Ind. (Lond.), 63, 144
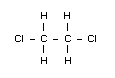 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 83.5°C
Odour: chloroform-like odour
Lowest concentration in air which is detectable by odour: 50 ppm
Flash point: ca 12°C (open cup)
Flammability limits in air: 6.2 to 15.9% by volume
Solubility:
Water: insoluble
Organic solvents: soluble in most common organic solvents
Specific gravity (liquid): 1.26
Specific gravity (gas): 3.42
Uses
Ethylene dichloride, being flammable, is rarely used alone. When
mixed with carbon tetrachloride it is extensively used as a liquid
grain fumigant for bulk storage in bins or on floors. The mixture
generally used is in the proportion of 3:1 by volume of ethylene
dichloride and carbon tetrachloride, this being the smallest
proportion of the latter which renders the mixture non-flammable. A
small proportion of ethylene dibromide (e.g. 2.5 to 5% by volume) is
occaaionaliy added to these or to other mixtures of ethylene
dichloride and carbon tetrachloride to improve surface treatment.
Rates of dosage of these mixtures for grain are usually between 1
gallon for 5 tons and 1 gallon for 10 tons in a 7-day exposure period.
The 3:1 mixture is also used for fumigating a variety of
commodities in bags including grain, pulses and animal feeding stuffs
usually on a fairly small scale in gas-tight chambers or other types
of sealed containers but in some tropical countries large stacks of
bagged grain have been treated under a covering of gasproof sheets.
Residues
During fumigation of cereal grains with ethylene dichloride or
its mixtures with other halogenated hydrocarbons, relatively heavy and
continuous sorption of the fumigant takes place (Winteringham, 1944).
The amount sorbed is higher at lower temperatures. The adsorbed
fumigant airs off slowly from whole grains over a period of months.
During handling, cleaning or milling processes the amount of adsorbed
fumigant is progressively reduced (Lynn and Vorkes, 1957). After
milling, a greater proportion of ethylene dichloride is found in the
bran than in the whole grain before milling (Conroy et al., 1957).
When treated at 9 gallons/1000 bushels with 3:1 ethylene
dichloride carbon tetrachloride mixture, triple the dose recommended
by the United States Department of Agriculture, showed a maximum of
140 ppm ethylene dichloride three days after application of fumigant
(ibid). Loss of fumigant during tempering and cleaning processes was
up to 70% and the maximum residue found in flour made from this batch
was 5 ppm.
When amounts of ethylene dichloride up to 10 times the residue
found in the flour from the previous study were added to flour which
was then made into loaves, no unchanged fumigant was found in the
baked bread (Munsey et al., 1957). (Sensitivity of method 2 ppm.)
When added to grain fed to cows, at levels up to 1000 ppm an
average of less than 0.25 ppm ethylene dichloride was found in the
milk. There appears to be no direct correlation between amounts of
ethylene dichloride added to the grain and that found in the milk
(Sykes and Klein, 1957).
When a 140-lb bag of 85% extraction wheat flour was fumigated for
48 hours in a 17.5 ft3 chamber at 25°C with 300 g of ethylene
dichloride applied as vapour (equivalent to 11.1 lb per ton of flour)
the following amounts of residual ethylene dichloride in ppm were
found after different periods of airing of the bag in still air (Pest
Infestation Laboratory, 1943).
1 hour 2 days 7 days
Surface of bag 1060 210 22
Centre of bag 1030 350 46
Baking tests carried out after seven days of airing showed no
detectable damage to baking quality and no taint of the fumigant was
left in the finished bread.
Effect of fumigant on treated crop
There appears to be no reaction between ethylene dichloride and
the constituents of grain. Ethylene dichloride added to grain can be
recovered intact by steam distillation with a solvent extraction
procedure (Heuser, 1964, personal communication).
BIOLOGICAL DATA
Biochemical aspects
In rabbits ethylene dichloride is mainly exhaled unchanged and no
metabolites have been described (Williams, 1959).
Toxicological studies
1. The fumigant
Maximum permissible concentration in the atmosphere recommended
for industrial hygiene in the United States is 50 ppm (200 mg/m3)
(Anon, 1964). Other maximum allowable concentrations are 3.0 mg/m3
(Ryazonov, 1959) and 4.0 mg/m3 (Borisova, 1960).
In experimental animals corneal lesions have been produced by
both subcutaneous and oral routes of administration (Hubbs and
Prusmack, 1955).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse oral 910 Smyth et al., 1936
Rat " 680 McCollister et al., 1956
Rat " 770 Smyth et al., 1936
Rabbit " 910 Smyth et al., 1936
Animals fatally poisoned by ethylene dichloride (inhalation)
showed hyperaemia and oedema of the lungs, degenerative kidney
changes, damage to liver and adrenals (von Oettingen, 1955).
Short- and long-term studies
Groups of 4-6 female rats were exposed to single doses of varying
concentrations of ethylene dichloride vapour for different lengths
of time and an estimate of single doses having no observable adverse
effects was made. For 12 000 ppm, 3000 ppm, 1000 ppm, 300 ppm and 200
ppm the maximum time was 0.1, 0.3, 1.5, 3.0 and 7.0 hours
respectively.
Intermittent exposure of animals to various concentrations of
ethylene dichloride vapour for 7 hours showed the following
no-effect levels (full histopathological examination and clinical
biochemical tests were reported): rat (15 male, 15 female) 151
exposures in 212 days, 200 ppm; guinea-pigs (8 male) 121 exposures in
170 days, 1000 ppm; guinea-pigs (8 female) 162 exposures in 226 days,
1000 ppm; rabbits (2 male) 165 exposures in 232 days, 400 ppm and
monkeys (2 male) 140 exposures in 212 days, 100 ppm (Spencer et al.,
1951).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 29.2% (by
weight) of ethylene dichloride appeared unaffected. The level of
ethylene dichloride in the grain was not stated and no
histopathological examinations were made (Rowe et al., 1956).
Comments on the experimental studies reported
1. Quite high levels of ethylene dichloride may remain in whole grain
after fumigation.
2. These high residues fall markedly when the grain is processed and
flour contains measurable but very low residues.
3. There is no evidence that ethylene dichloride combines chemically
with food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for ethylene dichloride. It
should be used as a fumigant under conditions which will result in the
lowest possible residues in the foodstuffs as consumed.
Further work required
1. Further investigation of the amount of the residual ethylene
dichloride remaining in the food after treatment and the effect
on this of processing and cooking.
2. Feeding studies should be carried out on two mammalian species to
determine the effect of the long-term feeding of ethylene
dichloride with particular reference to reproduction.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Borisova, M. K. (1960) Predel' no Dopustimye Kontsentratsii
Atmosfern. Zagryazneii, 4, 61
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Assoc. Off.
Agric. Chem., 40, 163
Hubbs, R. A. & Prusmack, J. J. (1955) J. Amer. med. Ass., 159, 673
Lynn, G. E. & Vorkes, F. A. (1957) J. Assoc. Off. Agric. Chem.,
40, 163
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Assoc. Off.
Agric. Chem., 40, 201
von Oettingen, W. F. (1955) The halogenated aliphatic, olefinic,
cyclic, aromatic and aliphatic-aromatic hydrocarbons, including the
halogenated insecticides, their toxicity and potential dangers,
Public Health Service Publ. No. 414
Pest Infestation Laboratory (1943) Pest Infestation Research Report,
87 (Unpublished document)
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Ryazonov, V. A. (1959) Proc. Int. Clean Air Conf., Nat. Soc. for
clean air, London
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Spencer, H. C. et al. (1951) Arch. industr. Hyg., 4, 482
Sykes, J. F. & Klein, A. K. (1957) J. Assoc. Off. Agric. Chem.,
40, 203
Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall Ltd
Winteringham, F. P. W. (1944) J. Soc. chem. Ind. (Lond.), 63, 144
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 83.5°C
Odour: chloroform-like odour
Lowest concentration in air which is detectable by odour: 50 ppm
Flash point: ca 12°C (open cup)
Flammability limits in air: 6.2 to 15.9% by volume
Solubility:
Water: insoluble
Organic solvents: soluble in most common organic solvents
Specific gravity (liquid): 1.26
Specific gravity (gas): 3.42
Uses
Ethylene dichloride, being flammable, is rarely used alone. When
mixed with carbon tetrachloride it is extensively used as a liquid
grain fumigant for bulk storage in bins or on floors. The mixture
generally used is in the proportion of 3:1 by volume of ethylene
dichloride and carbon tetrachloride, this being the smallest
proportion of the latter which renders the mixture non-flammable. A
small proportion of ethylene dibromide (e.g. 2.5 to 5% by volume) is
occaaionaliy added to these or to other mixtures of ethylene
dichloride and carbon tetrachloride to improve surface treatment.
Rates of dosage of these mixtures for grain are usually between 1
gallon for 5 tons and 1 gallon for 10 tons in a 7-day exposure period.
The 3:1 mixture is also used for fumigating a variety of
commodities in bags including grain, pulses and animal feeding stuffs
usually on a fairly small scale in gas-tight chambers or other types
of sealed containers but in some tropical countries large stacks of
bagged grain have been treated under a covering of gasproof sheets.
Residues
During fumigation of cereal grains with ethylene dichloride or
its mixtures with other halogenated hydrocarbons, relatively heavy and
continuous sorption of the fumigant takes place (Winteringham, 1944).
The amount sorbed is higher at lower temperatures. The adsorbed
fumigant airs off slowly from whole grains over a period of months.
During handling, cleaning or milling processes the amount of adsorbed
fumigant is progressively reduced (Lynn and Vorkes, 1957). After
milling, a greater proportion of ethylene dichloride is found in the
bran than in the whole grain before milling (Conroy et al., 1957).
When treated at 9 gallons/1000 bushels with 3:1 ethylene
dichloride carbon tetrachloride mixture, triple the dose recommended
by the United States Department of Agriculture, showed a maximum of
140 ppm ethylene dichloride three days after application of fumigant
(ibid). Loss of fumigant during tempering and cleaning processes was
up to 70% and the maximum residue found in flour made from this batch
was 5 ppm.
When amounts of ethylene dichloride up to 10 times the residue
found in the flour from the previous study were added to flour which
was then made into loaves, no unchanged fumigant was found in the
baked bread (Munsey et al., 1957). (Sensitivity of method 2 ppm.)
When added to grain fed to cows, at levels up to 1000 ppm an
average of less than 0.25 ppm ethylene dichloride was found in the
milk. There appears to be no direct correlation between amounts of
ethylene dichloride added to the grain and that found in the milk
(Sykes and Klein, 1957).
When a 140-lb bag of 85% extraction wheat flour was fumigated for
48 hours in a 17.5 ft3 chamber at 25°C with 300 g of ethylene
dichloride applied as vapour (equivalent to 11.1 lb per ton of flour)
the following amounts of residual ethylene dichloride in ppm were
found after different periods of airing of the bag in still air (Pest
Infestation Laboratory, 1943).
1 hour 2 days 7 days
Surface of bag 1060 210 22
Centre of bag 1030 350 46
Baking tests carried out after seven days of airing showed no
detectable damage to baking quality and no taint of the fumigant was
left in the finished bread.
Effect of fumigant on treated crop
There appears to be no reaction between ethylene dichloride and
the constituents of grain. Ethylene dichloride added to grain can be
recovered intact by steam distillation with a solvent extraction
procedure (Heuser, 1964, personal communication).
BIOLOGICAL DATA
Biochemical aspects
In rabbits ethylene dichloride is mainly exhaled unchanged and no
metabolites have been described (Williams, 1959).
Toxicological studies
1. The fumigant
Maximum permissible concentration in the atmosphere recommended
for industrial hygiene in the United States is 50 ppm (200 mg/m3)
(Anon, 1964). Other maximum allowable concentrations are 3.0 mg/m3
(Ryazonov, 1959) and 4.0 mg/m3 (Borisova, 1960).
In experimental animals corneal lesions have been produced by
both subcutaneous and oral routes of administration (Hubbs and
Prusmack, 1955).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse oral 910 Smyth et al., 1936
Rat " 680 McCollister et al., 1956
Rat " 770 Smyth et al., 1936
Rabbit " 910 Smyth et al., 1936
Animals fatally poisoned by ethylene dichloride (inhalation)
showed hyperaemia and oedema of the lungs, degenerative kidney
changes, damage to liver and adrenals (von Oettingen, 1955).
Short- and long-term studies
Groups of 4-6 female rats were exposed to single doses of varying
concentrations of ethylene dichloride vapour for different lengths
of time and an estimate of single doses having no observable adverse
effects was made. For 12 000 ppm, 3000 ppm, 1000 ppm, 300 ppm and 200
ppm the maximum time was 0.1, 0.3, 1.5, 3.0 and 7.0 hours
respectively.
Intermittent exposure of animals to various concentrations of
ethylene dichloride vapour for 7 hours showed the following
no-effect levels (full histopathological examination and clinical
biochemical tests were reported): rat (15 male, 15 female) 151
exposures in 212 days, 200 ppm; guinea-pigs (8 male) 121 exposures in
170 days, 1000 ppm; guinea-pigs (8 female) 162 exposures in 226 days,
1000 ppm; rabbits (2 male) 165 exposures in 232 days, 400 ppm and
monkeys (2 male) 140 exposures in 212 days, 100 ppm (Spencer et al.,
1951).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 29.2% (by
weight) of ethylene dichloride appeared unaffected. The level of
ethylene dichloride in the grain was not stated and no
histopathological examinations were made (Rowe et al., 1956).
Comments on the experimental studies reported
1. Quite high levels of ethylene dichloride may remain in whole grain
after fumigation.
2. These high residues fall markedly when the grain is processed and
flour contains measurable but very low residues.
3. There is no evidence that ethylene dichloride combines chemically
with food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for ethylene dichloride. It
should be used as a fumigant under conditions which will result in the
lowest possible residues in the foodstuffs as consumed.
Further work required
1. Further investigation of the amount of the residual ethylene
dichloride remaining in the food after treatment and the effect
on this of processing and cooking.
2. Feeding studies should be carried out on two mammalian species to
determine the effect of the long-term feeding of ethylene
dichloride with particular reference to reproduction.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth, 9, 545
Borisova, M. K. (1960) Predel' no Dopustimye Kontsentratsii
Atmosfern. Zagryazneii, 4, 61
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Assoc. Off.
Agric. Chem., 40, 163
Hubbs, R. A. & Prusmack, J. J. (1955) J. Amer. med. Ass., 159, 673
Lynn, G. E. & Vorkes, F. A. (1957) J. Assoc. Off. Agric. Chem.,
40, 163
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Assoc. Off.
Agric. Chem., 40, 201
von Oettingen, W. F. (1955) The halogenated aliphatic, olefinic,
cyclic, aromatic and aliphatic-aromatic hydrocarbons, including the
halogenated insecticides, their toxicity and potential dangers,
Public Health Service Publ. No. 414
Pest Infestation Laboratory (1943) Pest Infestation Research Report,
87 (Unpublished document)
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Ryazonov, V. A. (1959) Proc. Int. Clean Air Conf., Nat. Soc. for
clean air, London
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Spencer, H. C. et al. (1951) Arch. industr. Hyg., 4, 482
Sykes, J. F. & Klein, A. K. (1957) J. Assoc. Off. Agric. Chem.,
40, 203
Williams, R. T. (1959) Detoxication mechanisms, London, Chapman &
Hall Ltd
Winteringham, F. P. W. (1944) J. Soc. chem. Ind. (Lond.), 63, 144
