
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
PHOSPHINE
(As derived from aluminium phosphide)
Compound
Phosphine
Chemical name
Hydrogen phosphide
Synonym
Phosphine
Empirical formula
H3P
Structural formula
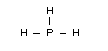 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: -87.7°C
Odour: like carbide
Flammability limits in air: 1.79% and above, by volume
Solubility:
Water: slightly soluble
Specific gravity (liquid): 0.746
Specific gravity (gas): 1.53
Uses
Phosphine is not applied directly but is evolved from aluminium
phosphide powders or tablets by the action of atmospheric moisture.
The tablets contain fire-suppressing materials such as ammonium
carbamate which gives off both ammonia gas and carbon dioxide to
prevent combustion as the phosphine is generated at the beginning of
fumigation. Phosphine is used principally as a grain fumigant. A usual
rate of application is 6 to 10 tablets, each yielding 1 g of phosphine
per ton of grain which is kept under fumigation for 72 hours at
temperatures at or above 20°C. Under very favourable conditions doses
as low as 3 tablets per ton have been used.
Residues
As far as is known at present there are three different residues
left in fumigated grains:
(a) Phosphine itself.
(b) Traces of unreacted aluminium phosphide.
(c) A fine grey powder of aluminium hydroxide as the product of the
reaction of water with aluminium phosphide.
A detailed study (Bruce et al., 1962) of residues of phosphine in
wheat fumigated under field conditions revealed a maximum residue of
0.046 ppm of phosphine in grain which had been turned. In this sample
the residue dropped to 0.006 ppm after aeration. The range of residues
in fumigated wheat was from 0.001 ppm to 0.046 ppm. These residues
were found in wheat fumigated at doses from 3 to 10 tablets per ton
with the analyses made from 1 to 14 days after fumigation following
various grain handling procedures, singly or in combination, such as
turning, aerating, cleaning or washing. No significant residue of
phosphine could be found in bread made from fumigated flour.
Most of the unreacted aluminium phosphide decomposes rapidly as
soon as the grain is moved.
Aluminium phosphide is reported as being removed during the
cleaning and washing of grain (Horak and Strosova, 1963).
Effect of fumigant on treated crop
As far as is known at present, with normal fumigation procedures,
phosphine does not react chemically with the treated materials. In
moist foodstuffs a slight increase of the total, phosphorus content
may appear (Laue, 1954).
Toxicological studies
Phosphine is a very toxic gas with a cumulative effect. There are
no data available on the acute LD50 of aluminium phosphide. The LD50
for rats of the related compound zinc phosphide is 40.5 + 2.9 mg/kg
(Johnson and Voss, 1952).
Man: 2.8 mg phosphine/l are lethal for man in a short time (Flury
and Zernik, 1931).
The threshold limit for phosphine is set at 0.4 mg/m3 (Anon, 1964).
Acute toxicity (Phosphine)
Animal Route Concentration Death occurred after the References
following time of exposure
Rat inhal. 0.68 mg/l 65-75 min. Neubert &
Hoffmeister, 1960
Rat " 1.47 mg/l 35-50 min.
Rat, rabbit " 0.2-1% 30 min. Laue, 1954
Rabbit " 10 ppm 120 min. daily, during 2 Harger &
days Spolyar, 1958
Guinea-pig " 25 ppm 4 hr daily, during 2 days "
Mouse " 50 ppm 2 hr daily, during 1 day "
Cat " 25 ppm 2, 3 to 4 hr daily, during
3 days
Short-term and long-term studies
It is reported that rats, fed with grain which had been fumigated
at excessive dosages, showed no ill effects, even when the grain was
not cleaned before consumption (Schulemann, 1953).
Evaluation
Materials treated with aluminium phosphide must be properly
cleaned and washed before being processed for food, so that no residue
of powder derived from the fumigant material reaches the consumer.
An acceptable daily intake of phosphine or aluminium phosphide
cannot be estimated until long-term toxicity data and metabolic
studies are available.
Further work required
Long-term studies on two species of animals and research on the
fate of the compound in food and its biochemical mechanism of action
in animals.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Bruce, R. B, Robbins, A. J. & Tuft, T. O. (1962) J. Agric. Food
Chem., 10, 18
Flury, F. & Zernik, F. (1931) Schädliche Gase, Berlin, Springer
Harger, R. N. & Spolyar, L. W. (1958) Arch. industr. Hlth, 18, 497
Horak, E. & Strosova, J. (1963) Nlynsko Pekarensky Prumysl Tech.
Skladovani Obili, 9, 486 (Chem. Abstr., 60, 16445c)
Johnson, H. D. & Voss, E. (1952) J. Amer. pharm. Ass., 41, 468
Laue, V. G. (1954) Nachrichtenblatt fur den deutschen
Pflanzenschutzdienst., 8, 13
Neubert, D. & Hoffmeister, I. (1960) Arch. exp. Path. u. Pharmak.,
239, 219
Schulemann (1953) Unpublished report from Degesch, Frankfurt-am-Main
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: -87.7°C
Odour: like carbide
Flammability limits in air: 1.79% and above, by volume
Solubility:
Water: slightly soluble
Specific gravity (liquid): 0.746
Specific gravity (gas): 1.53
Uses
Phosphine is not applied directly but is evolved from aluminium
phosphide powders or tablets by the action of atmospheric moisture.
The tablets contain fire-suppressing materials such as ammonium
carbamate which gives off both ammonia gas and carbon dioxide to
prevent combustion as the phosphine is generated at the beginning of
fumigation. Phosphine is used principally as a grain fumigant. A usual
rate of application is 6 to 10 tablets, each yielding 1 g of phosphine
per ton of grain which is kept under fumigation for 72 hours at
temperatures at or above 20°C. Under very favourable conditions doses
as low as 3 tablets per ton have been used.
Residues
As far as is known at present there are three different residues
left in fumigated grains:
(a) Phosphine itself.
(b) Traces of unreacted aluminium phosphide.
(c) A fine grey powder of aluminium hydroxide as the product of the
reaction of water with aluminium phosphide.
A detailed study (Bruce et al., 1962) of residues of phosphine in
wheat fumigated under field conditions revealed a maximum residue of
0.046 ppm of phosphine in grain which had been turned. In this sample
the residue dropped to 0.006 ppm after aeration. The range of residues
in fumigated wheat was from 0.001 ppm to 0.046 ppm. These residues
were found in wheat fumigated at doses from 3 to 10 tablets per ton
with the analyses made from 1 to 14 days after fumigation following
various grain handling procedures, singly or in combination, such as
turning, aerating, cleaning or washing. No significant residue of
phosphine could be found in bread made from fumigated flour.
Most of the unreacted aluminium phosphide decomposes rapidly as
soon as the grain is moved.
Aluminium phosphide is reported as being removed during the
cleaning and washing of grain (Horak and Strosova, 1963).
Effect of fumigant on treated crop
As far as is known at present, with normal fumigation procedures,
phosphine does not react chemically with the treated materials. In
moist foodstuffs a slight increase of the total, phosphorus content
may appear (Laue, 1954).
Toxicological studies
Phosphine is a very toxic gas with a cumulative effect. There are
no data available on the acute LD50 of aluminium phosphide. The LD50
for rats of the related compound zinc phosphide is 40.5 + 2.9 mg/kg
(Johnson and Voss, 1952).
Man: 2.8 mg phosphine/l are lethal for man in a short time (Flury
and Zernik, 1931).
The threshold limit for phosphine is set at 0.4 mg/m3 (Anon, 1964).
Acute toxicity (Phosphine)
Animal Route Concentration Death occurred after the References
following time of exposure
Rat inhal. 0.68 mg/l 65-75 min. Neubert &
Hoffmeister, 1960
Rat " 1.47 mg/l 35-50 min.
Rat, rabbit " 0.2-1% 30 min. Laue, 1954
Rabbit " 10 ppm 120 min. daily, during 2 Harger &
days Spolyar, 1958
Guinea-pig " 25 ppm 4 hr daily, during 2 days "
Mouse " 50 ppm 2 hr daily, during 1 day "
Cat " 25 ppm 2, 3 to 4 hr daily, during
3 days
Short-term and long-term studies
It is reported that rats, fed with grain which had been fumigated
at excessive dosages, showed no ill effects, even when the grain was
not cleaned before consumption (Schulemann, 1953).
Evaluation
Materials treated with aluminium phosphide must be properly
cleaned and washed before being processed for food, so that no residue
of powder derived from the fumigant material reaches the consumer.
An acceptable daily intake of phosphine or aluminium phosphide
cannot be estimated until long-term toxicity data and metabolic
studies are available.
Further work required
Long-term studies on two species of animals and research on the
fate of the compound in food and its biochemical mechanism of action
in animals.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Bruce, R. B, Robbins, A. J. & Tuft, T. O. (1962) J. Agric. Food
Chem., 10, 18
Flury, F. & Zernik, F. (1931) Schädliche Gase, Berlin, Springer
Harger, R. N. & Spolyar, L. W. (1958) Arch. industr. Hlth, 18, 497
Horak, E. & Strosova, J. (1963) Nlynsko Pekarensky Prumysl Tech.
Skladovani Obili, 9, 486 (Chem. Abstr., 60, 16445c)
Johnson, H. D. & Voss, E. (1952) J. Amer. pharm. Ass., 41, 468
Laue, V. G. (1954) Nachrichtenblatt fur den deutschen
Pflanzenschutzdienst., 8, 13
Neubert, D. & Hoffmeister, I. (1960) Arch. exp. Path. u. Pharmak.,
239, 219
Schulemann (1953) Unpublished report from Degesch, Frankfurt-am-Main
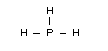 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: -87.7°C
Odour: like carbide
Flammability limits in air: 1.79% and above, by volume
Solubility:
Water: slightly soluble
Specific gravity (liquid): 0.746
Specific gravity (gas): 1.53
Uses
Phosphine is not applied directly but is evolved from aluminium
phosphide powders or tablets by the action of atmospheric moisture.
The tablets contain fire-suppressing materials such as ammonium
carbamate which gives off both ammonia gas and carbon dioxide to
prevent combustion as the phosphine is generated at the beginning of
fumigation. Phosphine is used principally as a grain fumigant. A usual
rate of application is 6 to 10 tablets, each yielding 1 g of phosphine
per ton of grain which is kept under fumigation for 72 hours at
temperatures at or above 20°C. Under very favourable conditions doses
as low as 3 tablets per ton have been used.
Residues
As far as is known at present there are three different residues
left in fumigated grains:
(a) Phosphine itself.
(b) Traces of unreacted aluminium phosphide.
(c) A fine grey powder of aluminium hydroxide as the product of the
reaction of water with aluminium phosphide.
A detailed study (Bruce et al., 1962) of residues of phosphine in
wheat fumigated under field conditions revealed a maximum residue of
0.046 ppm of phosphine in grain which had been turned. In this sample
the residue dropped to 0.006 ppm after aeration. The range of residues
in fumigated wheat was from 0.001 ppm to 0.046 ppm. These residues
were found in wheat fumigated at doses from 3 to 10 tablets per ton
with the analyses made from 1 to 14 days after fumigation following
various grain handling procedures, singly or in combination, such as
turning, aerating, cleaning or washing. No significant residue of
phosphine could be found in bread made from fumigated flour.
Most of the unreacted aluminium phosphide decomposes rapidly as
soon as the grain is moved.
Aluminium phosphide is reported as being removed during the
cleaning and washing of grain (Horak and Strosova, 1963).
Effect of fumigant on treated crop
As far as is known at present, with normal fumigation procedures,
phosphine does not react chemically with the treated materials. In
moist foodstuffs a slight increase of the total, phosphorus content
may appear (Laue, 1954).
Toxicological studies
Phosphine is a very toxic gas with a cumulative effect. There are
no data available on the acute LD50 of aluminium phosphide. The LD50
for rats of the related compound zinc phosphide is 40.5 + 2.9 mg/kg
(Johnson and Voss, 1952).
Man: 2.8 mg phosphine/l are lethal for man in a short time (Flury
and Zernik, 1931).
The threshold limit for phosphine is set at 0.4 mg/m3 (Anon, 1964).
Acute toxicity (Phosphine)
Animal Route Concentration Death occurred after the References
following time of exposure
Rat inhal. 0.68 mg/l 65-75 min. Neubert &
Hoffmeister, 1960
Rat " 1.47 mg/l 35-50 min.
Rat, rabbit " 0.2-1% 30 min. Laue, 1954
Rabbit " 10 ppm 120 min. daily, during 2 Harger &
days Spolyar, 1958
Guinea-pig " 25 ppm 4 hr daily, during 2 days "
Mouse " 50 ppm 2 hr daily, during 1 day "
Cat " 25 ppm 2, 3 to 4 hr daily, during
3 days
Short-term and long-term studies
It is reported that rats, fed with grain which had been fumigated
at excessive dosages, showed no ill effects, even when the grain was
not cleaned before consumption (Schulemann, 1953).
Evaluation
Materials treated with aluminium phosphide must be properly
cleaned and washed before being processed for food, so that no residue
of powder derived from the fumigant material reaches the consumer.
An acceptable daily intake of phosphine or aluminium phosphide
cannot be estimated until long-term toxicity data and metabolic
studies are available.
Further work required
Long-term studies on two species of animals and research on the
fate of the compound in food and its biochemical mechanism of action
in animals.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Bruce, R. B, Robbins, A. J. & Tuft, T. O. (1962) J. Agric. Food
Chem., 10, 18
Flury, F. & Zernik, F. (1931) Schädliche Gase, Berlin, Springer
Harger, R. N. & Spolyar, L. W. (1958) Arch. industr. Hlth, 18, 497
Horak, E. & Strosova, J. (1963) Nlynsko Pekarensky Prumysl Tech.
Skladovani Obili, 9, 486 (Chem. Abstr., 60, 16445c)
Johnson, H. D. & Voss, E. (1952) J. Amer. pharm. Ass., 41, 468
Laue, V. G. (1954) Nachrichtenblatt fur den deutschen
Pflanzenschutzdienst., 8, 13
Neubert, D. & Hoffmeister, I. (1960) Arch. exp. Path. u. Pharmak.,
239, 219
Schulemann (1953) Unpublished report from Degesch, Frankfurt-am-Main
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: -87.7°C
Odour: like carbide
Flammability limits in air: 1.79% and above, by volume
Solubility:
Water: slightly soluble
Specific gravity (liquid): 0.746
Specific gravity (gas): 1.53
Uses
Phosphine is not applied directly but is evolved from aluminium
phosphide powders or tablets by the action of atmospheric moisture.
The tablets contain fire-suppressing materials such as ammonium
carbamate which gives off both ammonia gas and carbon dioxide to
prevent combustion as the phosphine is generated at the beginning of
fumigation. Phosphine is used principally as a grain fumigant. A usual
rate of application is 6 to 10 tablets, each yielding 1 g of phosphine
per ton of grain which is kept under fumigation for 72 hours at
temperatures at or above 20°C. Under very favourable conditions doses
as low as 3 tablets per ton have been used.
Residues
As far as is known at present there are three different residues
left in fumigated grains:
(a) Phosphine itself.
(b) Traces of unreacted aluminium phosphide.
(c) A fine grey powder of aluminium hydroxide as the product of the
reaction of water with aluminium phosphide.
A detailed study (Bruce et al., 1962) of residues of phosphine in
wheat fumigated under field conditions revealed a maximum residue of
0.046 ppm of phosphine in grain which had been turned. In this sample
the residue dropped to 0.006 ppm after aeration. The range of residues
in fumigated wheat was from 0.001 ppm to 0.046 ppm. These residues
were found in wheat fumigated at doses from 3 to 10 tablets per ton
with the analyses made from 1 to 14 days after fumigation following
various grain handling procedures, singly or in combination, such as
turning, aerating, cleaning or washing. No significant residue of
phosphine could be found in bread made from fumigated flour.
Most of the unreacted aluminium phosphide decomposes rapidly as
soon as the grain is moved.
Aluminium phosphide is reported as being removed during the
cleaning and washing of grain (Horak and Strosova, 1963).
Effect of fumigant on treated crop
As far as is known at present, with normal fumigation procedures,
phosphine does not react chemically with the treated materials. In
moist foodstuffs a slight increase of the total, phosphorus content
may appear (Laue, 1954).
Toxicological studies
Phosphine is a very toxic gas with a cumulative effect. There are
no data available on the acute LD50 of aluminium phosphide. The LD50
for rats of the related compound zinc phosphide is 40.5 + 2.9 mg/kg
(Johnson and Voss, 1952).
Man: 2.8 mg phosphine/l are lethal for man in a short time (Flury
and Zernik, 1931).
The threshold limit for phosphine is set at 0.4 mg/m3 (Anon, 1964).
Acute toxicity (Phosphine)
Animal Route Concentration Death occurred after the References
following time of exposure
Rat inhal. 0.68 mg/l 65-75 min. Neubert &
Hoffmeister, 1960
Rat " 1.47 mg/l 35-50 min.
Rat, rabbit " 0.2-1% 30 min. Laue, 1954
Rabbit " 10 ppm 120 min. daily, during 2 Harger &
days Spolyar, 1958
Guinea-pig " 25 ppm 4 hr daily, during 2 days "
Mouse " 50 ppm 2 hr daily, during 1 day "
Cat " 25 ppm 2, 3 to 4 hr daily, during
3 days
Short-term and long-term studies
It is reported that rats, fed with grain which had been fumigated
at excessive dosages, showed no ill effects, even when the grain was
not cleaned before consumption (Schulemann, 1953).
Evaluation
Materials treated with aluminium phosphide must be properly
cleaned and washed before being processed for food, so that no residue
of powder derived from the fumigant material reaches the consumer.
An acceptable daily intake of phosphine or aluminium phosphide
cannot be estimated until long-term toxicity data and metabolic
studies are available.
Further work required
Long-term studies on two species of animals and research on the
fate of the compound in food and its biochemical mechanism of action
in animals.
REFERENCES
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Bruce, R. B, Robbins, A. J. & Tuft, T. O. (1962) J. Agric. Food
Chem., 10, 18
Flury, F. & Zernik, F. (1931) Schädliche Gase, Berlin, Springer
Harger, R. N. & Spolyar, L. W. (1958) Arch. industr. Hlth, 18, 497
Horak, E. & Strosova, J. (1963) Nlynsko Pekarensky Prumysl Tech.
Skladovani Obili, 9, 486 (Chem. Abstr., 60, 16445c)
Johnson, H. D. & Voss, E. (1952) J. Amer. pharm. Ass., 41, 468
Laue, V. G. (1954) Nachrichtenblatt fur den deutschen
Pflanzenschutzdienst., 8, 13
Neubert, D. & Hoffmeister, I. (1960) Arch. exp. Path. u. Pharmak.,
239, 219
Schulemann (1953) Unpublished report from Degesch, Frankfurt-am-Main
