
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
gamma-BHC
IDENTITY
Synonyms
gamma HCH, lindane; gammexane (R)
Explanation
Unless it is specifically stated to the contrary, the data here
reviewed refer to lindane, which is defined by the International
Standards Organization as a product of specified purity, not less than
99 per cent gamma-BHC.
Chemical name
gamma-1,2,3,4,5,6-hexachlorocyclohexane
Formula
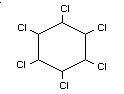 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
gamma-BHC is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, gamma-BHC is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of
gamma-BHC in growing animals is, however, much lower than that of
beta-BHC or of DDT. In rats ingesting repeated small doses of
gamma-BHC, the concentration in the fat depots is in a state of
equilibrium with the concentration of the diet (Lehman, 1953). The
data on gamma-BHC metabolism are very incomplete. According to certain
in vitro tests it would seem that part is broken down in the liver.
The unchanged compound is eliminated in the faeces and urine and is
found in the milk (Ely et al., 1953; Van Asperen, 1957; Ware &
Gilmore, 1959). Very little information is available about its effects
on basic enzyme systems. Growing animals are particularly susceptible
to its toxic action (Kastli, 1955).
A single dose of 60 mg/kg in rats has been shown to accelerate
detoxication of several drugs processed by the liver microsomes,
including paraoxon (Ghazal at al., 1964).
The solubilities of the isomers of BBC in rat fat are: alpha, 4.05 per
cent; beta, 0.66 per cent; gamma, 12-55 per cent; delta, 19.10 per
cent. However, when each isomer is administered separately, the in
vivo storage depends on the metabolism, the solubility being only a
limiting factor (Sedlak, 1965).
In an analysis of 282 samples of human fat obtained from the general
population in the United States of America, an average content of 0.57
ppm was found (Hoffmann at al., 1964). A study in the United Kingdom
on 20 human fat samples showed a mean gamma-BHC concentration of 0.015
ppm (Robinson et al., 1965). Single spraying of cattle with 0.03 per
cent, or hogs with 0.06 per cent gamma-BHC and dipping of sheep and
goats in a 0.025 per cent suspension, caused the appearance of
gamma-BHC in the fat at levels ranging from 0.47 to 4.22 ppm (Claborn
et al., 1960). gamma-BHC has been found in 30 samples of unprocessed
cows' milk at a mean level of 0.01 ppm (Paccagnella et al., 1966).
Acute toxicity
From the viewpoint of acute toxicity, gamma-BHC is the most toxic of
all the isomers present in the technical hexachlorocyclohexanes. It is
a neurotropic poison causing excitation of the central nervous system
with convulsions, followed - if the dose is sufficiently high - by
depressive phenomena leading to death from respiratory collapse.
The fatal dose for man is estimated to be 150 mg/kg body-weight, i.e.,
of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Plasma taken from a three-year-old boy approximately 6 hours after he
swallowed gamma-BHC, and 3 hours after the last convulsion, contained
0.29 ppm gamma-BHC. Seven days later the concentration was 0.02 ppm
and the patient was asymptomatic (Dale et al., 1966).
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 86 Woodard & Hagan, 1947
Rat Oral 125-200 Dallemagne & Philippot, 1948
Lehman, 1948
Lehman, 1951
Rat Oral 177-230 Woodard & Hagan, 1947
Klosa, 1950
Rat Intraperitoneal 35-85 Coper et al., 1951
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Rabbit Oral 60-200 Cameron, 1945
Dog Oral 40 (lethal) Barke, 1950
100-200 (lethal) Landle & Schneider, 1950
Dog Intravenous 7.5 (lethal) Barke, 1950
Short-term studies
Rat. When rats were fed a diet containing 400 ppm gamma-BHC for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given gamma-BHC by
stomach tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous signs (trembling, spasms), fatty degeneration of the liver and
of the epithelium of the renal tubules, vacuolization of the cerebral
cells and a marked increase in mortality rate were observed. With 10
mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950, 1951; Kewitz, 1952; Kewitz & Reinert, 1952),; when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of gamma-BHC the general behaviour of the animals, their
body-weight and post-mortem gross and histological examinations showed
no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to daily intramuscular
administration at doses of 40 mg gamma-BHC for 19 days, 80 mg for 9
days and 160 mg for three days. The animals died and showed among
other things, hepatic degeneration (Dallemagne & Philippot, 1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed hepatic
lesions (Woodard & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10 per cent
solution of gamma-BHC in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of gamma-BHC brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied gamma-BHC during an
antimalaria campaign, about 46 per cent became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal applications
of 0.5 per cent gamma-BHC in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3 per cent of gamma-BHC and were observed for 10 months. No
abnormalities were found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given diets
containing 5 to 1600 ppm of the alpha-, beta-, and gamma-isomers of
BHC for two years. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of gamma-BHC, the lowest concentration causing significant
liver changes was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of 20
young rats (10 males and 10 females) were fed diets containing 25, 50
and 100 ppm of gamma-BHC throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration, 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets containing 50
and 100 ppm of gamma-BHC; histological lesions were found in the liver
parenchyma (Ortega et al., 1957).
Twenty-five young rats were given gamma-BHC daily by stomach-tube at a
dosage of 10 µg/kg body-weight (corresponding to about 0.1 and 0.15
ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments
In all these animal species, gamma-BHC has proved to be a cumulative
poison, causing hepatic and renal lesions and disturbances of the
central nervous system. The dog seems particularly susceptible to
neurological effects.
Evidence of stimulatory activity on liver microsomal metabolism such
as that observed for other chlorinated hydrocarbons has been presented
for gamma-BHC, long-term studies have been performed only in rats and
did not include reproductive aspects.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in the rat
25 ppm of gamma-BHC in the diet, equivalent to 1.25 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0125 mg/kg/body-weight.
Further work required
Elucidation of the significance of the finding that gamma-BHC is one
of the compounds which affect liver cellular metabolism (p. 3).
The chemical nature of the residue occurring in plants, which has not
been fully investigated.
This information should be provided not later than five years after
the publication of this report, when a re-evaluation of this compound
will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Soon after the introduction of gamma-BHC and prior to about 1950 its
uses included applications to fruits, vegetables and other edible
crops. These have almost ceased in Europe and N. America, but not in
various other parts of the world, where it is known to be used for
foliage application (e.g. to control cocoa insects). (However the
meeting had very little information on the scale of use in different
countries.)
In the production of cereals, gamma-BHC is used either by application
to plants, to the soil or to seeds. The dosage for foliar application
usually is less than 2 kg of gamma-BHC per hectare possibly repeated
two or three times: for soil insect control a dosage up to 1.8 kg per
hectare may be used. In many countries the recommended interval
between last application and harvest is 30 days.
It was the opinion of the meeting that uses on stone, pome and citrus
fruits and on berries have almost ceased. (Member countries having
requirements for retaining these uses should supply supporting data.)
Applications to soil used in the production of vegetables range from 4
oz to 24 oz per acre: foliar applications from 1 to 16 oz.
Aerosols, smokes and vapours of gamma-BHC are sometimes used on
vegetable and fruit crops in glasshouses.
Restrictions in various countries normally include measures against
tainting and against excessive residues and minimum periods between
treatments of soil (e.g. one to three years) and the planting of
edible crops are specified.
Gamma-BHC has had important uses against biting flies, lice, fleas,
ticks and in the control of mange, scab or scabies caused by mites on
livestock. Careful limitations in use are required, since in some
instances repeated applications are required to obtain effective
control. Use on meat animals is usually restricted (e.g. to 21 days
before slaughter for cattle, sheep and goats, and seven days for
hogs). Use on lactating animals or in milk rooms is not usually
permitted.
(b) Post-harvest uses
Gamma-BHC has been widely used for protecting harvested cereals,
pulses and nuts and for treating premises in which foods are stored.
In one or two countries gamma-BHC is mixed at about 3 ppm with cereals
in store. In some tropical countries (e.g. Ghana) maize, millets,
guinea corn and rice paddy have been treated at doses up to 11 ppm.
Gamma-BHC has also been used in holds of ships and has uses against
some insects of public health importance.
Residues resulting from supervised trials
Fruit and vegetables
Data on residues from supervised trials are not comprehensive. Many
were obtained around 1950 and call for confirmation with the use of
more modern analytical methods. The figures show rapid losses from
leafy vegetables and a greater persistence on fruit; but after
applications to soil significant residues remain for three years or
more. Brett & Bowery (1958) reported residues below 0.1 ppm on
tomatoes after three days, on snap beans after four days and on
collards after about 13 days. To obtain data on the maximum possible
residues, Lilly & Fahey (1956) used dosages as high as 50 times those
required to produce effective insect control. The maximum residues
found over a four-year period on soybeans were 0.74, 0.89, 0.27 and
1.21 ppm (averages for each of the four years), on field corn over a
three-year period they were up to 0.44 ppm. In the same series of
trials sweet corn showed variations from 0.02 to 0.41 ppm, popcorn
zero to 0.13 ppm. Other work reported by Lilly & Fahey (1956) on
sunflowers, grain sorghums and a variety of garden beans over a
four-year period showed no residues in excess of 0.6 ppm. Lichtenstein
(1959) also cites residues in crops resulting from excessive
applications of gamma-BHC to three soil types. Following normal
agricultural practices San Antonio (1959) found that soil containing 5
ppm resulted in 3 ppm in the edible parts of carrots, even though as
high as 25 ppm was found in the fibrous root hairs. (He also found
that the 3 ppm residue was greatly reduced by scraping the peel.) In
more recent studies, using gas/liquid chromatographic analysis,
Maier-Bode (1964) found not more than 0.5 ppm in beet treated 143 days
previously at dosages of 1.5 to 3 kg/hectare. Other residues after
treatments at the same dosage were 0.08 and 0.1 ppm in potatoes, and
0.03, 0.06 ppm, 0.05 and 0.09 ppm in cabbages. Using twice the dosage
employed in practice, Maier-Bode (1963) also found 0.5 to 1.0 ppm in
beets. Beran (1961) reported 0.2 ppm in radishes and 0.11 ppm in
beans.
Tolerances
Country Product Tolerance Comments
ppm
Canada Corn, beans and peas 10.0
Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Cereals No tolerance
USA Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Netherlands General 2.0
USSR General 2.0 Temporary
Federal Vegetables, pulses and 2.0 Proposed for 1968
German fruit
Republic Cereals 0.1 " " "
Milling products from 0.03 " " "
Cereals
Comecon General 5.0
(Eastern
Europe)
Hungary General 2.5
Brazil General 10.0 (Not specified)
France Wheat 5.0 (On condition that
flour does not contain
more than 0.1 ppm)
(continued)
Country Product Tolerance Comments
ppm
India Wheat 3.0 Under consideration
Italy Wheat 2.5 Cleaning and washing
required before milling
Kenya Wheat 1.0
Britain Cereals 2.5 Practical (not legal)
limit
In largely unpublished compilations of information submitted to USFDA
hearings in 1950, residues of less than 0.6 ppm were found on peaches,
30 days after application of 6.0 per cent sprays and up to 5.1 ppm on
apples after 21 days.
Animal feed
Gamma-BHC has been used for both soil treatments and foliar
applications on a variety of forage crops used as animal feed. To
reduce sources of contamination of milk and meat it is necessary to
examine these practices. Data on the occurrence of residues and on
their fate during curing and storage of hay or silage, etc. are not
extensive. On alfalfa, residues ranging from 0.05 ppm after 35 days
(Penn. State, 1963) to 0.4 ppm (France et al., 1961) have been
reported. Maier-Bode (1964) found 0.19 ppm in grass 28 days after
application. Applications within 14 days of cutting resulted in up to
1.1 ppm in hay baled and held up to eight months (Treece & Ware,
1965); at 24 and 31 days before harvest, 0.7 and 0.1 ppm (Fahey et
al., 1960). Hardee et al. (1963) demonstrated differences in the rate
of disappearance from alfalfa, clover and trefoil; which indicates a
need for caution in the extrapolation of results from one species to
another.
No substantial information seems to be available on residues in forage
resulting from soil treatments. The current data suggest, however,
that the residues resulting from direct foliar application on forage
crops may not be acceptable on animal food crops. The FAO Working
Party would therefore like to see more information of recent origin,
before making specific recommendations on this aspect. Information
from feeding trials with animals at the very low levels of residues
that might be expected from soil or seed treatments would also be
valuable.
Use on animals
Gamma-BHC has been popular for ectoparasite control in poultry houses,
by direct application to hens and their roosts. Fairchild & Dahm
(1955) applied one per cent dust to litter for lice control. Residues
in successive fat and liver samples dissipated rapidly. Skin samples
taken two weeks after dusting contained 0.44, 0.84 and 7.5 ppm from
0.06, 0.25 and one per cent dusts. Ivey et al. (1961) confined
chickens for 16 weeks in treated poultry houses. Residues in fat after
four weeks were 27.7 to 58.8 ppm, in eggs 4 ppm. Even light treatments
of similar poultry houses resulted in 6.6 ppm in fat after two weeks
and 1.0 after 20 weeks. Eggs from these hens contained 0.81 ppm after
two weeks and 0.08 ppm after 16 weeks. Lower concentrations were used
by Ware & Naber (1961) to determine the effect of low level
contamination of food. 0.01 ppm in feed resulted in detectable
residues in eggs and chicken tissues. After 60 days' feeding, residues
in egg yolks were 0.26, 0.46 and 4.96 ppm respectively from hens
receiving 0.1, 1.0 and 10 ppm in their diets. After transfer to clean
food, residues were down to a trace after eight days for the first
group, to 0.09 ppm after 24 days for the second group and to 0.08
after 53 days for the last group of birds.
Ware & Naber (1962) also reported that direct application to poultry
and to houses resulted in an initial residue of 5.57 ppm decreasing to
1.03 ppm after 14 days. Treating roosts only, on dipping legs,
resulted in lower residues. Deema, Naber & Ware (1965) evaluated
residues resulting from confining hens above cartridges producing
gamma-BHC vapour and found 0.13 ppm, 0.24 and 0.33 ppm after 80 days
at three dosage rates. Harrison et al. (1963) after feeding poultry
with 4, 16 and 64 ppm for 27 days found 19.1, 56.4 and 156.0 ppm in
the body fat. Stadelman et al. (1964) after feeding hens at 0.15 for
14 days, measured no detectable residues in eggs and less than 0.3 ppm
in fat, 15 ppm for five days resulted in 0.7 ppm in fat. One week
after discontinuing the pesticide, eggs contained 0.4 ppm and none was
detected in fat or flesh 11 weeks later.
Studies in New Zealand, USA and Britain suggest that the condition of
fleece is an important factor in determining the residues of gamma-BHC
and their persistence in the fat and meat of sheep following dipping.
Jackson et al. (1959), by biopsy two weeks after dipping in 0.025 per
cent solution, found 4.22 ppm in sheep fat and 2.67 ppm in goats' fat.
Twelve weeks were required for these residues to reduce to zero.
Dipping shorn sheep at the same strength, Egan (1965) found 1.9 ppm
and 0.2 ppm in kidney fat and meat respectively after one week and 0.1
ppm in each after six weeks, in unshorn sheep however the residues
were 8.9 and 1.0 ppm after one week, 3.8 and 0.4 ppm after six weeks,
2.2 and 0.1 after 12 weeks, and 40 weeks passed before the levels were
both down to 0.1 ppm.
Collett & Harrison (1963) using a dip containing 0.0125 per cent
emulsion also found higher residues in unshorn sheep. Fat of shorn
sheep two weeks after dipping contained 2.5 ppm and unshorn sheep 5.0
ppm (average figures) and residues; in fat of shorn sheep were
negligible in eight weeks and in unshorn sheep in 12 weeks. From these
results it seems that fleece is a reservoir for continued absorption.
Swine appear to store gamma-BHC less than sheep. Fat of animals
sprayed with normal dosages after one week contained 0.03 and 0.05
ppm, and those after twice the dosage 0.5 and 0.06 ppm (USDA, 1965).
Fat samples taken by biopsy showed 0.1 to 0.3 after 28 days (USDA,
1965a).
Gyrisko et al. (1959) reviewed the effects on flavour and residues in
milk of feeding low residues on hay to dairy cattle. In agreement with
earlier work they found that as much as 10 ppm in feed did not cause
off-flavour in milk. Feed which contained 0.6 ppm at harvest had
reduced to non-detectable levels when feeding was initiated.
Unfortunately, no recent information is available on the feeding to
lactating animals of the very low levels of residues which may be
present on cull root crops, sugar beets and forage. Most countries do
not register or recommend the use of gamma-BHC in buildings in which
dairy animals or dairy products are kept and processed, or on
lactating animals.
Residues in food moving in commerce
Few figures are available for individual food commodities. Total diet
studies in the USA (Williams, 1964; Duggan et al., 1966; Cummings,
1966) and Canadian restaurant meal surveys indicate very minor
incidence in diets of these countries. The USA five-year survey using
three different methods of analysis, rarely recorded residues in
excess of 0.01 ppm.
By contrast, British investigations of residues in home-killed mutton
in 1962 (Egan, 1965) indicated a constant low level of residue in fat
of meat in 61 samples. Thirty-seven samples contained not more than
0.1 ppm, 48 not more than 0.3 ppm and three samples between 1.1 and
2.0 ppm. The Reports of the Government Chemist (UK) for 1964 and 1965
contain further useful information on the amount of total BHC isomers
in food of origin from different countries:
1964 1965
Food Country Number of Range Arithmetic Number of Range Arithmetic
samples ppm mean ppm samples ppm mean ppm
Butter Australian 45 0.0-0.40 0.02 45 0.0-0.11 0.02
Danish 30 0.0-0.20 0.05 24 0.0-0.08 0.04
New Zealand 45 0.0-0.02 <0.01 40 0.0-0.04 0.01
UK 18 0.0-0.20 0.05 18 0.0-0.13 0.04
Milk UK 60 0.00-0.18 0.003 85 0.0-0.022 0.0045
Mutton Argentine 17 0.00-15.5 1.1 13 0.0-1.6 0.25
Kidney Australian 15 0.00-0.05 0.01 6 <0.05 -
Fat New Zealand 12 0.00-0.25 0.05 10 <0.05 -
UK 107 0.00-5.1 0.43 128 0.0-10.6 0.46
Beef Argentine 13 0.0-1.1 0.15 22 0.0-0.80 0.13
Kidney UK 66 0.0-0.3 0.05 59 0.0-0.42 0.04
Fat
Corned
beef Argentine 20 0.0-0.1 0.03 26 0.0-0.1 0.02
Hardon (1960) reported that beans sampled in commerce contained 0.1
ppm gamma-BHC, and canned tomatoes 0.13 ppm (Alessandrini at al.,
1959).
Fate of residues
(a) In soil
Gamma-BHC is relatively volatile and moderately soluble in water, for
which reasons it is one of the less persistent organochlorine
pesticides. It is generally considered that three years should elapse
after heavy applications and before root crops are planted in treated
soils; but soil type has an important influence on the rate of
disappearance from soil (Harris, 1966; Duffy, 1966; Lichtenstein &
Schulz, 1965; Lichtenstein et al., 1963, 1964; Harris & Lichtenstein,
1961, etc.). Some of the early work may have been misleading due to
inadequacies in the analytical method and this has been recently
reviewed (Yule, Chiba & Morley, 1967). Enzymatic processes have been
responsible for the detoxification of insects (e.g. Bradbury, 1957;
Clark et al., 1966; Ishida & Dahm, 1965) and in the rat (Grover &
Sims, 1965). Gamma-pentachlorocyclohexane has been identified as a
decomposition product in soil. It is 103 less toxic to insects than
gamma-BHC. Soil microorganisms and alkaline soils are involved in this
degradation process (Yule, Chiba & Morley, 1967). Further work is
required to determine whether the disappearance of gamma-BHC from soil
can be achieved in a reasonable period by land and crop management
practices.
(b) In plants
It has been demonstrated that gamma-BHC can be absorbed by roots and
translocated to leaves (Bradbury & Whitaker, 1956) where it can kill
insects (Howe, 1950; Starnes, 1950; Shapiro, 1951; Ehrenhardt, 1954).
Jameson (1959) concluded that (when used as a dressing) the
insecticide penetrates into seeds and is absorbed by the cotyledons.
Way (1959) compared the action of gamma-BHC with other organochlorine
seed dressings and Bradbury (1963) the action on cabbage and wheat, in
both of which the gamma-BHC fell off rapidly.
There appears to be very little information on metabolism and
degradation in plants. Such information is necessary because
toxicological evaluation has been based solely on feeding the parent
compound.
(c) In storage and processing
Because of the volatility and solubility of gamma-BHC, storage and
processing, including cooking, would be expected to result in losses.
The literature contains divergencies on this subject probably due to
the use of non-specific analytical methods in the early work and
because the losses were closely dependent upon the temperatures,
degrees of exposure to free air and other conditions pertaining in the
particular test.
From his own studies Van den Driessche (1958) suggests that in the
canning and cooking process 23 per cent of residues of BHC (presumably
13 per cent gamma) in treated vegetables would be lost in cooking
water, and in the canning of beans and peas 63 per cent of the residue
might be dissipated.
Information on losses of gamma-BHC during the storage of cereals and
on its carry over into flour and bread were reviewed in some detail at
the second session of the FAO Working Party on Pesticide Residues
(PL/1965/12). Losses during storage depend upon the physical
conditions (e.g. Bridges, 1958) being high in the tropics and in well
ventilated stores (e.g. Giles, 1964) and low under cooler closed
conditions (e.g. Gunther, Lindgren & Blinn, 1958; Schesser, Priddle &
Farrell, 1958). During the milling of flour, the losses depend on the
particular processes used. Preliminary cleaning, brushing and washing
have been shown to remove insecticide and several workers have found
appreciably lower ppm residues in the flour (i.e. up to 50 per cent)
than in the original grain, although the residues in certain
by-products (e.g. bran for feeding to animals) may contain a higher
ppm (Bridges, 1958; Feuersinger, 1960, 1962; Schesser et al., 1958;
Sellke, 1952; Zeumer & Neuhaus, 1953). There are few data on losses of
residues during bread making. Bridges (1958a) found that flour
containing 2.5 ppm contained 40 per cent of this as unchanged residue
in the bread with the remainder probably as tri-, di-, and mono-
chlorbenzenes.
Loss from Cheddar and Stilton cheese was slow (Bridges, 1958), about
40 per cent remaining after 44 weeks. Penetration into both types was
slow but no chemical changes were found in either raw or cooked
cheese. Johnson (1958) reported the results of processing on residues
in cucumbers used for pickles. It was concluded that final residues in
pickles would be less than 0.5 ppm from field treated cucumbers, but a
concentration of 10 ppm could result if harvested cucumbers were
sprayed directly.
Method of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of gamma-BHC, together with residues of a
number of other compounds, including other BHC isomers. An example is
the AOAC system (1966) in which acetonitrile partition and Florisil
column clean-up are identified and measured by gas chromatography
coupled with thin layer or paper chromatography. Alternative clean-up
systems; e.g that of de Faubert Maunder et al. (1964) using
dimethylformamide, and other methods of confirmation of identity,
e.g., using infra-red spectrophotometry, are also available. The
methods are in general sensitive to 0.002 ppm of gamma-BHC in milk and
0.02 ppm of gamma-BHC in most other foods, though under favourable
conditions greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The residue data reviewed at the meeting, for the most part, were
produced between 1948 and 1959 before improved specific methods of
analysis were available. These results can only be used as a guide,
subject to review, when better data are available. When considering
information on residues of this pesticide, the meeting found it
necessary to rely on the expert opinion of its members, rather than
conclusions that could be derived exclusively from obsolete or
incomplete data.
The properties of this pesticide are such that it was assumed that
substantial losses of residue would occur in storage, processing and
cooking of foods such as some vegetables, meat and cereals, but only
minor losses in the case of small fruits and milk products.
Tolerances recommended for residues of gamma-BHC based on these guide
lines are:
Small fruits 3.0 ppm )
)
Vegetables 3.0 ppm )
) Temporary tolerance
Cereals 0.5 ppm )
)
Milk products 0.1 ppm (fat basis) )
Meat and poultry 0.7 ppm (fat basis) ) Practical
) residue
Fluid milk 0.004 ppm ) level
These should be reviewed in the light of additional data, but in
any event not later than within three years.
Further work
1. Guidance is required from countries on uses still of value in
food production or protection in storage and transit.
2. Data are needed on the disappearance of residues during storage
and processing of food, and information on the chemical nature of
terminal residues on food as consumed.
3. Residue data, using specific methods of analysis which will
identify metabolites and gamma-BHC, after supervised trials and
on residues in food moving in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Barke, A. (1950) Tierärzliche Umschau, 5, 611
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff,
R. D. (1960) J. Agr. Food. Chem., 8, 439
Coper, F., Herken, H. & Klempau, I. (1951) Arch. exp. path.
Pharmakol., 212, 463
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life sciences, 5,
47
Dallemagne, M. J. & Philippot, E. (1948) Arch. int. pharm. Ther.,
76, 274
Doisy, E. A. & Bocklage, B. C. (1949) Proc. Soc. exp. Biol. (N.Y.),
71, 490
Ely, R. E., Underwood, P. C., Moore, L. A., Mann, F. D. & Carter, R.
H. (1953) J. Amer. vet. med. Ass., 123, 448
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Pharmacol.
exp. Ther., 100, 59
Ghazal, A., Koransky, W., Portig, J., Vohland, H. W. & Klempau, I.
(1964) Arch. exp. path. Pharmakol., 249, 1
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel Forsch., 1, 356
Hoffman, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth., 9, 387
Kastli, P. (1955) Féd. Int. des Laiteries Réunies, Bruxelles,
Report No. 3
Kewitz, H. (1952) Arch. exp. path. Pharmakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. path. Pharmakol., 227, 183
Klosa, J. (1950) Die Pharmazie, 5, 615
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A.J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A.J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Lendle, L. & Schneider, H. H. (1950) Arch. exp. path. Pharmakol.,
210, 119
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F., jr (1957) Arch. Path.,
64, 614
Paccagnella, B., Brati, L. & Gavazzini, C. (1966) Unpublished report
of the Institute of Hygiene of Ferrara, Italy
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Sedlak, V. A. (1965) Toxicol. appl. Pharmacol., 7, 79
Truhaut, R. (1954) Communication au symposium intern. de la prévention
du cancer, Sao Paulo
Van Asperen, K. (1957) C.R. 4é Congrčs Intern. de Lutte control les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wasserman, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu, G.
& Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 386
REFERENCES PERTINENT TO AGRICULTURAL DATA
Alessandri, M. E., Doretti, M. & Lanforti, G. F. (1959) Insecticide
residues in samples of peeled, canned tomatoes coming from experiments
carried out in Italy in 1958. Presented to 17th IUPAC Congress,
Munich, reported by Maier-Bode, H., Gesunde Pflanzen, 11, 225-229
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49, 222-30
Beran, F. (1961) Das Problem der Pflanzenschutzmittelruckstande in
europaischer Sicht. - Pflanzenschutzberichte (Wien) 27, 11-50
Bradbury, F. R. (1957) Absorption and metabolism of BHC in susceptible
and resistant houseflies. J. Sci. Food Agric., 8, 90-96
Bradbury, F. R. (1963) The systemic action of benzene hexachloride
seed dressings. Ann. appl. Biol., 52, 361-370
Bradbury, F. R. & Whitaker, W. O. (1956) The systemic action of
benzene hexachloride in plants; quantitative measurements. J. Sci Food
Agric., 4, 248
Brett, C. H. & Bowery, T. G. (1958) Insecticide Residues on
Vegetables. J. Econ. Ent., 51, 818-821
Bridges, R. G. (1958) Fate of labelled insecticide residues in food
products VI. Retention of gamma-Benzene hexachloride by wheat and
cheese. J. Sci. Food Agric., 9, 431-439
Bridges, R. G. (1958a) Fate of labelled insecticide residues in food
products VII. The fate of gamma-Benzene hexachloride residues in flour
during baking. J. Sci. Food Agric., 9, 439-448
Clark, A. G., Hitchcock, M., Smith, J. N. (1966) Metabolism of
gammexane in flies, ticks and locusts. Nature, 209, 103
Collett, J. N. & Harrison, D. L. (1963) Lindane residues in sheep
following dipping. N.Z. J. Agric. Res., 6, 39-42
Cummings, J. G. (1966) Pesticides in the total diet. Residue Reviews,
16, 30-45
Deema, P., Naber, E. D. & Ware, G. W. (1965) Residues in hen eggs from
vaporizing insecticide tablets. J. Econ. Ent., 58, 904-906
Duffy, J. R. & Wong, N. (1966) Insecticide Residues and their
metabolites in Atlantic Soils. In press.
Duggan, R. E., Barry, H. C. & Johnson, L. Y. (1966) Pesticide Residues
in total diet samples. Science, 151, 101
Egan, H. (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Food Agric., 16,
489-498
Ehrenhardt, H. (1954) Uber die Werkung des Hexachlorocyclohexans als
systemisches Insektizid. Anz. Schadlingsk., 27, 1
Fahey, J. E., Wilson, M. C. & Rusk, H. W. (1960) Persistence of BHC,
lindane, and thiodan residues when applied to alfalfa to control the
meadow spittlebug. J. Econ. Ent., 53, 960-1
Fairchild, H. E. & Dahm, Paul A. (1955) Lice control on chickens with
chlorinated hydrocarbon insecticides. J. Econ. Ent., 48, 141-146
de Faubert Maunder, M. J., Egan, H. & Godly, R. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Feuersenger, M. (1960) Uber die Bestimmung von
Schadlingsbekamfungsmitteln in Lebensmitteln. Bundesgesundheitsblatt,
NR10, 149-152
Feuersenger, M. (1962) Ruckstandfragen bei der Anwendug von
Kontaktininsektizieden in Getreideschutz Nachrichtenbl. dstch.
Pflanzenschutzdienstes (Braunschiveig), 14, 189
France, H. L., Treece, R. E. & Ware, W. G., (1961) Chemical and
biological assays of lindane residues on alfalfa. J. Econ. Ent., 54,
642-3
Giles, P. H., (1964) Lindane contamination in stored sorghum and
millet in N. Nigeria. Trop. Sci., 6, 113-121
Grover, P. L. & Sims, P. (1965) The metabolism of gamma-2,3,4,5,6
pentachlorocyclohex-1-ene and gamma-hexachlorocyclohexane in rats.
Biochem J., 96, 521-525
Gunther, F. A., Lindgren, D. L. & Blinn, R. C. (1958) Biological
effectiveness and persistence of malathion and lindane used for
protection of stored wheat. J. Econ. Ent., 510 843-4
Gyrisco, G. G., Norton, L. B., Trimberger, G. W., Holland, R. F.,
McEnerney, P. J. & Muka, A. A. (1959) Effects of feeding low levels of
insecticide residues on hay to dairy cattle on flavor and residues in
milk. Agric. & Food Chem., 7, 707-711
Hardee, D. D., Huddleston, E. W. & Gyrisco, G. G. (1963) Initial
deposit and disappearance rates of various insecticides as affected by
forage crop species. J. Econ. Ent., 56, 98-101
Hardon, H. J. (1960) Pestizidruckstande in Marktmustern von Gemuse und
Abst. - Verhandl. IV. Inter. Congress of Plant Protection, Hamburg,
Band 2. (Braunschweig, 1960) 1671-1674
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in southwestern Ontario. Agric. & Food Chem., 14,
398-403
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent., 54,
1038-1045
Harrison, D. L., Poole, W. S. H. & Mol, J. C. M. (1963) Observations
on feeding lindane-fortified mash to chickens. N.Z. Veterinary
Journal, 11, 137-140
Howe, W. L. (1950) Reactions of squash vine borer to certain
insecticidal soil treatments. J. Econ. Ent., 43, 549
Ishida, M. & Dahm, P. A. (1965) Metabolism of Benzene Hexachloride
Isomers and Related Compounds in Vitro. I. Properties and Distribution
of the Enzyme. J. Econ. Ent., 58, 383-392
Ivey, M. C., Roberts, R. H., Mann, H. D. & Claborn, H. V. (1961)
Lindane residues in chickens and eggs following poultry house sprays.
J. Econ. Ent., 54, 487-488
Jackson, J. B., Ivey, M. C., Roberts, R. H. & Radeleff, R. D. (1959)
Residue studies in sheep and goats dipped in 0.025% lindane. J. Econ.
Ent., 52, 1031-1032
Jameson, H. R. (1959) The mechanism of the control of turnip flea
beetle with benzene hexachloride dressings of Brassicae seed. J. Sci.
Food Agric., 9, 590
Johnson, M. R. (1958) Residue changes during the fermentation and
processing of pickles. Food Technology June, 1958
Lichtenstein, E. P. (1959) Absorption of some chlorinated hydrocarbon
insecticides from soils into various crops. Agric. and Food Chem., 7,
430-433
Lichtenstein, E. P. & Schulz, K. R. (1959) Persistence of some
chlorinated hydrocarbon insecticides as influenced by soil types, rate
of application and temperature. J. Econ. Ent., 52, 289-293
Lichtenstein, E. P., Schulz, K. R. & Cowley, G. T. (1963) Inhibition
of the conversion of aldrin to dieldrin in soils with
methylenedioxyphenyl synergists. J. Econ. Ent., 56, 485-489
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1964) Effect of
formulation and mode of application of aldrin on the loss of aldrin
and its epoxide from soils and their translocation into carrots. J.
Econ. Ent., 57, 133
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13, 57-62
Lilly, J. H. & Fahey, J. E. (1956) Translocation of BHC from
high-dosage soil treatments applied before planting. J. Econ. Ent.,
49, 815-818
Maier-Bode, H. (1963) Vergiftungen durch
Pflanzenschutzmittel-Ruckstande-Pflanzenschutzberichte (Wien) 30,
49-77
Maier-Bode, H. (1964) Pflanzenschutzmittel-Ruckstande. Insektizide,
Verlag Eugen Ulmer, Stuttgart. 455 pp.
Penn. State Univ. Agric. Exp. Sta. (1963) Pesticide residue
investigations on raw agricultural commodities. Penn. State Univ.
Bulletin 703
San Antonio, J. P. (1959) Demonstration of lindane and a lindane
metabolite in plants by paper chromatography. Agric. & Food Chem., 7.
322-325
Schesser, J. H., Priddle, W. E. & Farrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion, and lindane. J. Econ. Ent., 51, 516-518
Sellke, K. (1952) Kornkofereinstaubmittel und Ihr Verhalten in
behandelten Getreide Nachrichtenbl. dstch. Pflanzenschutzdienst
(Berlin) N.F.6, 121-127
Shapiro, I. D. (1951) On the toxic action of hexachlorane on insects
through the plant. Dokl. Akad. Nauk. SSSR(NS) 80, 481
Stadelman, W. J., Liska, B. J. & Langlois, G. C., Moster, T. G. &
Stemp, A. R. (1964) Persistence of chlorinated hydrocarbon residues in
chicken tissues and eggs. Poultry Science, 43, Sept. 1964
Starnes, O. (1950) Absorption and translocation of insecticides
through the root system of plants. J. Econ. Ent., 43, 338
Treece, R. E. & Ware, G. W. (1965) Lindane Residues on Alfalfa and in
Milk. J. Econ. Ent., 58, 218-219
USDA (1965) Distribution of Lindane Residues in Swine. Agr. Res. Serv.
Ent. Res. Div. Pesticide Chemicals Research Branch Spec. Report
PC-B-65-10
USDA (1965a) Lindane Residues in Swine. Agr. Res. Serv. Ent. Res. Div.
Pesticide Chemicals Research Branch Spec. Report PC-B-65-17
Van den Driessche, S. (1958) Influence of sterilization on
hexachloroxyclohexane (HCH) residues in preserved foods. Rev.
Conserve 13 (1), 27
Ware, G. W. & Naber. E. C. (1961) Lindane in eggs and chicken tissues.
J. Econ. Ent., 54, 675-677
Ware, G. W. & Naber, E. C. (1962) Lindane and BHC in egg yolks
following recommended uses for louse and mite control. J. Econ. Ent.,
55, 568-570
Way, M. J. (1959) Experiments on the mode of action of insecticidal
seed-dressings, especially against leptohylemyia coarctata fall.,
muscidae, the wheat bulb fly. Ann. appl. Biol., 47. 783-801
Williams, S. (1964) Pesticide residues in total diet samples.
J.A.O.A.C., 47, 815-821
Yule, W. N., Chiba, M. & Morley, H. V. (1967) Fate of Insecticide
Residues - The Decomposition of lindane in soil. J. Agr. Food Chem.
(In press)
Zeumer, H. & Neuhaus, K. (1953) Die Bestimmung von
Kontaktinsektizieden, Getreide und Mehl Heft 8; Beilage 2.
Wochenschrift Die Muhle, Detmold
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
gamma-BHC is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, gamma-BHC is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of
gamma-BHC in growing animals is, however, much lower than that of
beta-BHC or of DDT. In rats ingesting repeated small doses of
gamma-BHC, the concentration in the fat depots is in a state of
equilibrium with the concentration of the diet (Lehman, 1953). The
data on gamma-BHC metabolism are very incomplete. According to certain
in vitro tests it would seem that part is broken down in the liver.
The unchanged compound is eliminated in the faeces and urine and is
found in the milk (Ely et al., 1953; Van Asperen, 1957; Ware &
Gilmore, 1959). Very little information is available about its effects
on basic enzyme systems. Growing animals are particularly susceptible
to its toxic action (Kastli, 1955).
A single dose of 60 mg/kg in rats has been shown to accelerate
detoxication of several drugs processed by the liver microsomes,
including paraoxon (Ghazal at al., 1964).
The solubilities of the isomers of BBC in rat fat are: alpha, 4.05 per
cent; beta, 0.66 per cent; gamma, 12-55 per cent; delta, 19.10 per
cent. However, when each isomer is administered separately, the in
vivo storage depends on the metabolism, the solubility being only a
limiting factor (Sedlak, 1965).
In an analysis of 282 samples of human fat obtained from the general
population in the United States of America, an average content of 0.57
ppm was found (Hoffmann at al., 1964). A study in the United Kingdom
on 20 human fat samples showed a mean gamma-BHC concentration of 0.015
ppm (Robinson et al., 1965). Single spraying of cattle with 0.03 per
cent, or hogs with 0.06 per cent gamma-BHC and dipping of sheep and
goats in a 0.025 per cent suspension, caused the appearance of
gamma-BHC in the fat at levels ranging from 0.47 to 4.22 ppm (Claborn
et al., 1960). gamma-BHC has been found in 30 samples of unprocessed
cows' milk at a mean level of 0.01 ppm (Paccagnella et al., 1966).
Acute toxicity
From the viewpoint of acute toxicity, gamma-BHC is the most toxic of
all the isomers present in the technical hexachlorocyclohexanes. It is
a neurotropic poison causing excitation of the central nervous system
with convulsions, followed - if the dose is sufficiently high - by
depressive phenomena leading to death from respiratory collapse.
The fatal dose for man is estimated to be 150 mg/kg body-weight, i.e.,
of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Plasma taken from a three-year-old boy approximately 6 hours after he
swallowed gamma-BHC, and 3 hours after the last convulsion, contained
0.29 ppm gamma-BHC. Seven days later the concentration was 0.02 ppm
and the patient was asymptomatic (Dale et al., 1966).
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 86 Woodard & Hagan, 1947
Rat Oral 125-200 Dallemagne & Philippot, 1948
Lehman, 1948
Lehman, 1951
Rat Oral 177-230 Woodard & Hagan, 1947
Klosa, 1950
Rat Intraperitoneal 35-85 Coper et al., 1951
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Rabbit Oral 60-200 Cameron, 1945
Dog Oral 40 (lethal) Barke, 1950
100-200 (lethal) Landle & Schneider, 1950
Dog Intravenous 7.5 (lethal) Barke, 1950
Short-term studies
Rat. When rats were fed a diet containing 400 ppm gamma-BHC for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given gamma-BHC by
stomach tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous signs (trembling, spasms), fatty degeneration of the liver and
of the epithelium of the renal tubules, vacuolization of the cerebral
cells and a marked increase in mortality rate were observed. With 10
mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950, 1951; Kewitz, 1952; Kewitz & Reinert, 1952),; when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of gamma-BHC the general behaviour of the animals, their
body-weight and post-mortem gross and histological examinations showed
no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to daily intramuscular
administration at doses of 40 mg gamma-BHC for 19 days, 80 mg for 9
days and 160 mg for three days. The animals died and showed among
other things, hepatic degeneration (Dallemagne & Philippot, 1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed hepatic
lesions (Woodard & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10 per cent
solution of gamma-BHC in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of gamma-BHC brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied gamma-BHC during an
antimalaria campaign, about 46 per cent became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal applications
of 0.5 per cent gamma-BHC in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3 per cent of gamma-BHC and were observed for 10 months. No
abnormalities were found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given diets
containing 5 to 1600 ppm of the alpha-, beta-, and gamma-isomers of
BHC for two years. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of gamma-BHC, the lowest concentration causing significant
liver changes was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of 20
young rats (10 males and 10 females) were fed diets containing 25, 50
and 100 ppm of gamma-BHC throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration, 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets containing 50
and 100 ppm of gamma-BHC; histological lesions were found in the liver
parenchyma (Ortega et al., 1957).
Twenty-five young rats were given gamma-BHC daily by stomach-tube at a
dosage of 10 µg/kg body-weight (corresponding to about 0.1 and 0.15
ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments
In all these animal species, gamma-BHC has proved to be a cumulative
poison, causing hepatic and renal lesions and disturbances of the
central nervous system. The dog seems particularly susceptible to
neurological effects.
Evidence of stimulatory activity on liver microsomal metabolism such
as that observed for other chlorinated hydrocarbons has been presented
for gamma-BHC, long-term studies have been performed only in rats and
did not include reproductive aspects.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in the rat
25 ppm of gamma-BHC in the diet, equivalent to 1.25 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0125 mg/kg/body-weight.
Further work required
Elucidation of the significance of the finding that gamma-BHC is one
of the compounds which affect liver cellular metabolism (p. 3).
The chemical nature of the residue occurring in plants, which has not
been fully investigated.
This information should be provided not later than five years after
the publication of this report, when a re-evaluation of this compound
will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Soon after the introduction of gamma-BHC and prior to about 1950 its
uses included applications to fruits, vegetables and other edible
crops. These have almost ceased in Europe and N. America, but not in
various other parts of the world, where it is known to be used for
foliage application (e.g. to control cocoa insects). (However the
meeting had very little information on the scale of use in different
countries.)
In the production of cereals, gamma-BHC is used either by application
to plants, to the soil or to seeds. The dosage for foliar application
usually is less than 2 kg of gamma-BHC per hectare possibly repeated
two or three times: for soil insect control a dosage up to 1.8 kg per
hectare may be used. In many countries the recommended interval
between last application and harvest is 30 days.
It was the opinion of the meeting that uses on stone, pome and citrus
fruits and on berries have almost ceased. (Member countries having
requirements for retaining these uses should supply supporting data.)
Applications to soil used in the production of vegetables range from 4
oz to 24 oz per acre: foliar applications from 1 to 16 oz.
Aerosols, smokes and vapours of gamma-BHC are sometimes used on
vegetable and fruit crops in glasshouses.
Restrictions in various countries normally include measures against
tainting and against excessive residues and minimum periods between
treatments of soil (e.g. one to three years) and the planting of
edible crops are specified.
Gamma-BHC has had important uses against biting flies, lice, fleas,
ticks and in the control of mange, scab or scabies caused by mites on
livestock. Careful limitations in use are required, since in some
instances repeated applications are required to obtain effective
control. Use on meat animals is usually restricted (e.g. to 21 days
before slaughter for cattle, sheep and goats, and seven days for
hogs). Use on lactating animals or in milk rooms is not usually
permitted.
(b) Post-harvest uses
Gamma-BHC has been widely used for protecting harvested cereals,
pulses and nuts and for treating premises in which foods are stored.
In one or two countries gamma-BHC is mixed at about 3 ppm with cereals
in store. In some tropical countries (e.g. Ghana) maize, millets,
guinea corn and rice paddy have been treated at doses up to 11 ppm.
Gamma-BHC has also been used in holds of ships and has uses against
some insects of public health importance.
Residues resulting from supervised trials
Fruit and vegetables
Data on residues from supervised trials are not comprehensive. Many
were obtained around 1950 and call for confirmation with the use of
more modern analytical methods. The figures show rapid losses from
leafy vegetables and a greater persistence on fruit; but after
applications to soil significant residues remain for three years or
more. Brett & Bowery (1958) reported residues below 0.1 ppm on
tomatoes after three days, on snap beans after four days and on
collards after about 13 days. To obtain data on the maximum possible
residues, Lilly & Fahey (1956) used dosages as high as 50 times those
required to produce effective insect control. The maximum residues
found over a four-year period on soybeans were 0.74, 0.89, 0.27 and
1.21 ppm (averages for each of the four years), on field corn over a
three-year period they were up to 0.44 ppm. In the same series of
trials sweet corn showed variations from 0.02 to 0.41 ppm, popcorn
zero to 0.13 ppm. Other work reported by Lilly & Fahey (1956) on
sunflowers, grain sorghums and a variety of garden beans over a
four-year period showed no residues in excess of 0.6 ppm. Lichtenstein
(1959) also cites residues in crops resulting from excessive
applications of gamma-BHC to three soil types. Following normal
agricultural practices San Antonio (1959) found that soil containing 5
ppm resulted in 3 ppm in the edible parts of carrots, even though as
high as 25 ppm was found in the fibrous root hairs. (He also found
that the 3 ppm residue was greatly reduced by scraping the peel.) In
more recent studies, using gas/liquid chromatographic analysis,
Maier-Bode (1964) found not more than 0.5 ppm in beet treated 143 days
previously at dosages of 1.5 to 3 kg/hectare. Other residues after
treatments at the same dosage were 0.08 and 0.1 ppm in potatoes, and
0.03, 0.06 ppm, 0.05 and 0.09 ppm in cabbages. Using twice the dosage
employed in practice, Maier-Bode (1963) also found 0.5 to 1.0 ppm in
beets. Beran (1961) reported 0.2 ppm in radishes and 0.11 ppm in
beans.
Tolerances
Country Product Tolerance Comments
ppm
Canada Corn, beans and peas 10.0
Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Cereals No tolerance
USA Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Netherlands General 2.0
USSR General 2.0 Temporary
Federal Vegetables, pulses and 2.0 Proposed for 1968
German fruit
Republic Cereals 0.1 " " "
Milling products from 0.03 " " "
Cereals
Comecon General 5.0
(Eastern
Europe)
Hungary General 2.5
Brazil General 10.0 (Not specified)
France Wheat 5.0 (On condition that
flour does not contain
more than 0.1 ppm)
(continued)
Country Product Tolerance Comments
ppm
India Wheat 3.0 Under consideration
Italy Wheat 2.5 Cleaning and washing
required before milling
Kenya Wheat 1.0
Britain Cereals 2.5 Practical (not legal)
limit
In largely unpublished compilations of information submitted to USFDA
hearings in 1950, residues of less than 0.6 ppm were found on peaches,
30 days after application of 6.0 per cent sprays and up to 5.1 ppm on
apples after 21 days.
Animal feed
Gamma-BHC has been used for both soil treatments and foliar
applications on a variety of forage crops used as animal feed. To
reduce sources of contamination of milk and meat it is necessary to
examine these practices. Data on the occurrence of residues and on
their fate during curing and storage of hay or silage, etc. are not
extensive. On alfalfa, residues ranging from 0.05 ppm after 35 days
(Penn. State, 1963) to 0.4 ppm (France et al., 1961) have been
reported. Maier-Bode (1964) found 0.19 ppm in grass 28 days after
application. Applications within 14 days of cutting resulted in up to
1.1 ppm in hay baled and held up to eight months (Treece & Ware,
1965); at 24 and 31 days before harvest, 0.7 and 0.1 ppm (Fahey et
al., 1960). Hardee et al. (1963) demonstrated differences in the rate
of disappearance from alfalfa, clover and trefoil; which indicates a
need for caution in the extrapolation of results from one species to
another.
No substantial information seems to be available on residues in forage
resulting from soil treatments. The current data suggest, however,
that the residues resulting from direct foliar application on forage
crops may not be acceptable on animal food crops. The FAO Working
Party would therefore like to see more information of recent origin,
before making specific recommendations on this aspect. Information
from feeding trials with animals at the very low levels of residues
that might be expected from soil or seed treatments would also be
valuable.
Use on animals
Gamma-BHC has been popular for ectoparasite control in poultry houses,
by direct application to hens and their roosts. Fairchild & Dahm
(1955) applied one per cent dust to litter for lice control. Residues
in successive fat and liver samples dissipated rapidly. Skin samples
taken two weeks after dusting contained 0.44, 0.84 and 7.5 ppm from
0.06, 0.25 and one per cent dusts. Ivey et al. (1961) confined
chickens for 16 weeks in treated poultry houses. Residues in fat after
four weeks were 27.7 to 58.8 ppm, in eggs 4 ppm. Even light treatments
of similar poultry houses resulted in 6.6 ppm in fat after two weeks
and 1.0 after 20 weeks. Eggs from these hens contained 0.81 ppm after
two weeks and 0.08 ppm after 16 weeks. Lower concentrations were used
by Ware & Naber (1961) to determine the effect of low level
contamination of food. 0.01 ppm in feed resulted in detectable
residues in eggs and chicken tissues. After 60 days' feeding, residues
in egg yolks were 0.26, 0.46 and 4.96 ppm respectively from hens
receiving 0.1, 1.0 and 10 ppm in their diets. After transfer to clean
food, residues were down to a trace after eight days for the first
group, to 0.09 ppm after 24 days for the second group and to 0.08
after 53 days for the last group of birds.
Ware & Naber (1962) also reported that direct application to poultry
and to houses resulted in an initial residue of 5.57 ppm decreasing to
1.03 ppm after 14 days. Treating roosts only, on dipping legs,
resulted in lower residues. Deema, Naber & Ware (1965) evaluated
residues resulting from confining hens above cartridges producing
gamma-BHC vapour and found 0.13 ppm, 0.24 and 0.33 ppm after 80 days
at three dosage rates. Harrison et al. (1963) after feeding poultry
with 4, 16 and 64 ppm for 27 days found 19.1, 56.4 and 156.0 ppm in
the body fat. Stadelman et al. (1964) after feeding hens at 0.15 for
14 days, measured no detectable residues in eggs and less than 0.3 ppm
in fat, 15 ppm for five days resulted in 0.7 ppm in fat. One week
after discontinuing the pesticide, eggs contained 0.4 ppm and none was
detected in fat or flesh 11 weeks later.
Studies in New Zealand, USA and Britain suggest that the condition of
fleece is an important factor in determining the residues of gamma-BHC
and their persistence in the fat and meat of sheep following dipping.
Jackson et al. (1959), by biopsy two weeks after dipping in 0.025 per
cent solution, found 4.22 ppm in sheep fat and 2.67 ppm in goats' fat.
Twelve weeks were required for these residues to reduce to zero.
Dipping shorn sheep at the same strength, Egan (1965) found 1.9 ppm
and 0.2 ppm in kidney fat and meat respectively after one week and 0.1
ppm in each after six weeks, in unshorn sheep however the residues
were 8.9 and 1.0 ppm after one week, 3.8 and 0.4 ppm after six weeks,
2.2 and 0.1 after 12 weeks, and 40 weeks passed before the levels were
both down to 0.1 ppm.
Collett & Harrison (1963) using a dip containing 0.0125 per cent
emulsion also found higher residues in unshorn sheep. Fat of shorn
sheep two weeks after dipping contained 2.5 ppm and unshorn sheep 5.0
ppm (average figures) and residues; in fat of shorn sheep were
negligible in eight weeks and in unshorn sheep in 12 weeks. From these
results it seems that fleece is a reservoir for continued absorption.
Swine appear to store gamma-BHC less than sheep. Fat of animals
sprayed with normal dosages after one week contained 0.03 and 0.05
ppm, and those after twice the dosage 0.5 and 0.06 ppm (USDA, 1965).
Fat samples taken by biopsy showed 0.1 to 0.3 after 28 days (USDA,
1965a).
Gyrisko et al. (1959) reviewed the effects on flavour and residues in
milk of feeding low residues on hay to dairy cattle. In agreement with
earlier work they found that as much as 10 ppm in feed did not cause
off-flavour in milk. Feed which contained 0.6 ppm at harvest had
reduced to non-detectable levels when feeding was initiated.
Unfortunately, no recent information is available on the feeding to
lactating animals of the very low levels of residues which may be
present on cull root crops, sugar beets and forage. Most countries do
not register or recommend the use of gamma-BHC in buildings in which
dairy animals or dairy products are kept and processed, or on
lactating animals.
Residues in food moving in commerce
Few figures are available for individual food commodities. Total diet
studies in the USA (Williams, 1964; Duggan et al., 1966; Cummings,
1966) and Canadian restaurant meal surveys indicate very minor
incidence in diets of these countries. The USA five-year survey using
three different methods of analysis, rarely recorded residues in
excess of 0.01 ppm.
By contrast, British investigations of residues in home-killed mutton
in 1962 (Egan, 1965) indicated a constant low level of residue in fat
of meat in 61 samples. Thirty-seven samples contained not more than
0.1 ppm, 48 not more than 0.3 ppm and three samples between 1.1 and
2.0 ppm. The Reports of the Government Chemist (UK) for 1964 and 1965
contain further useful information on the amount of total BHC isomers
in food of origin from different countries:
1964 1965
Food Country Number of Range Arithmetic Number of Range Arithmetic
samples ppm mean ppm samples ppm mean ppm
Butter Australian 45 0.0-0.40 0.02 45 0.0-0.11 0.02
Danish 30 0.0-0.20 0.05 24 0.0-0.08 0.04
New Zealand 45 0.0-0.02 <0.01 40 0.0-0.04 0.01
UK 18 0.0-0.20 0.05 18 0.0-0.13 0.04
Milk UK 60 0.00-0.18 0.003 85 0.0-0.022 0.0045
Mutton Argentine 17 0.00-15.5 1.1 13 0.0-1.6 0.25
Kidney Australian 15 0.00-0.05 0.01 6 <0.05 -
Fat New Zealand 12 0.00-0.25 0.05 10 <0.05 -
UK 107 0.00-5.1 0.43 128 0.0-10.6 0.46
Beef Argentine 13 0.0-1.1 0.15 22 0.0-0.80 0.13
Kidney UK 66 0.0-0.3 0.05 59 0.0-0.42 0.04
Fat
Corned
beef Argentine 20 0.0-0.1 0.03 26 0.0-0.1 0.02
Hardon (1960) reported that beans sampled in commerce contained 0.1
ppm gamma-BHC, and canned tomatoes 0.13 ppm (Alessandrini at al.,
1959).
Fate of residues
(a) In soil
Gamma-BHC is relatively volatile and moderately soluble in water, for
which reasons it is one of the less persistent organochlorine
pesticides. It is generally considered that three years should elapse
after heavy applications and before root crops are planted in treated
soils; but soil type has an important influence on the rate of
disappearance from soil (Harris, 1966; Duffy, 1966; Lichtenstein &
Schulz, 1965; Lichtenstein et al., 1963, 1964; Harris & Lichtenstein,
1961, etc.). Some of the early work may have been misleading due to
inadequacies in the analytical method and this has been recently
reviewed (Yule, Chiba & Morley, 1967). Enzymatic processes have been
responsible for the detoxification of insects (e.g. Bradbury, 1957;
Clark et al., 1966; Ishida & Dahm, 1965) and in the rat (Grover &
Sims, 1965). Gamma-pentachlorocyclohexane has been identified as a
decomposition product in soil. It is 103 less toxic to insects than
gamma-BHC. Soil microorganisms and alkaline soils are involved in this
degradation process (Yule, Chiba & Morley, 1967). Further work is
required to determine whether the disappearance of gamma-BHC from soil
can be achieved in a reasonable period by land and crop management
practices.
(b) In plants
It has been demonstrated that gamma-BHC can be absorbed by roots and
translocated to leaves (Bradbury & Whitaker, 1956) where it can kill
insects (Howe, 1950; Starnes, 1950; Shapiro, 1951; Ehrenhardt, 1954).
Jameson (1959) concluded that (when used as a dressing) the
insecticide penetrates into seeds and is absorbed by the cotyledons.
Way (1959) compared the action of gamma-BHC with other organochlorine
seed dressings and Bradbury (1963) the action on cabbage and wheat, in
both of which the gamma-BHC fell off rapidly.
There appears to be very little information on metabolism and
degradation in plants. Such information is necessary because
toxicological evaluation has been based solely on feeding the parent
compound.
(c) In storage and processing
Because of the volatility and solubility of gamma-BHC, storage and
processing, including cooking, would be expected to result in losses.
The literature contains divergencies on this subject probably due to
the use of non-specific analytical methods in the early work and
because the losses were closely dependent upon the temperatures,
degrees of exposure to free air and other conditions pertaining in the
particular test.
From his own studies Van den Driessche (1958) suggests that in the
canning and cooking process 23 per cent of residues of BHC (presumably
13 per cent gamma) in treated vegetables would be lost in cooking
water, and in the canning of beans and peas 63 per cent of the residue
might be dissipated.
Information on losses of gamma-BHC during the storage of cereals and
on its carry over into flour and bread were reviewed in some detail at
the second session of the FAO Working Party on Pesticide Residues
(PL/1965/12). Losses during storage depend upon the physical
conditions (e.g. Bridges, 1958) being high in the tropics and in well
ventilated stores (e.g. Giles, 1964) and low under cooler closed
conditions (e.g. Gunther, Lindgren & Blinn, 1958; Schesser, Priddle &
Farrell, 1958). During the milling of flour, the losses depend on the
particular processes used. Preliminary cleaning, brushing and washing
have been shown to remove insecticide and several workers have found
appreciably lower ppm residues in the flour (i.e. up to 50 per cent)
than in the original grain, although the residues in certain
by-products (e.g. bran for feeding to animals) may contain a higher
ppm (Bridges, 1958; Feuersinger, 1960, 1962; Schesser et al., 1958;
Sellke, 1952; Zeumer & Neuhaus, 1953). There are few data on losses of
residues during bread making. Bridges (1958a) found that flour
containing 2.5 ppm contained 40 per cent of this as unchanged residue
in the bread with the remainder probably as tri-, di-, and mono-
chlorbenzenes.
Loss from Cheddar and Stilton cheese was slow (Bridges, 1958), about
40 per cent remaining after 44 weeks. Penetration into both types was
slow but no chemical changes were found in either raw or cooked
cheese. Johnson (1958) reported the results of processing on residues
in cucumbers used for pickles. It was concluded that final residues in
pickles would be less than 0.5 ppm from field treated cucumbers, but a
concentration of 10 ppm could result if harvested cucumbers were
sprayed directly.
Method of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of gamma-BHC, together with residues of a
number of other compounds, including other BHC isomers. An example is
the AOAC system (1966) in which acetonitrile partition and Florisil
column clean-up are identified and measured by gas chromatography
coupled with thin layer or paper chromatography. Alternative clean-up
systems; e.g that of de Faubert Maunder et al. (1964) using
dimethylformamide, and other methods of confirmation of identity,
e.g., using infra-red spectrophotometry, are also available. The
methods are in general sensitive to 0.002 ppm of gamma-BHC in milk and
0.02 ppm of gamma-BHC in most other foods, though under favourable
conditions greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The residue data reviewed at the meeting, for the most part, were
produced between 1948 and 1959 before improved specific methods of
analysis were available. These results can only be used as a guide,
subject to review, when better data are available. When considering
information on residues of this pesticide, the meeting found it
necessary to rely on the expert opinion of its members, rather than
conclusions that could be derived exclusively from obsolete or
incomplete data.
The properties of this pesticide are such that it was assumed that
substantial losses of residue would occur in storage, processing and
cooking of foods such as some vegetables, meat and cereals, but only
minor losses in the case of small fruits and milk products.
Tolerances recommended for residues of gamma-BHC based on these guide
lines are:
Small fruits 3.0 ppm )
)
Vegetables 3.0 ppm )
) Temporary tolerance
Cereals 0.5 ppm )
)
Milk products 0.1 ppm (fat basis) )
Meat and poultry 0.7 ppm (fat basis) ) Practical
) residue
Fluid milk 0.004 ppm ) level
These should be reviewed in the light of additional data, but in
any event not later than within three years.
Further work
1. Guidance is required from countries on uses still of value in
food production or protection in storage and transit.
2. Data are needed on the disappearance of residues during storage
and processing of food, and information on the chemical nature of
terminal residues on food as consumed.
3. Residue data, using specific methods of analysis which will
identify metabolites and gamma-BHC, after supervised trials and
on residues in food moving in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Barke, A. (1950) Tierärzliche Umschau, 5, 611
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff,
R. D. (1960) J. Agr. Food. Chem., 8, 439
Coper, F., Herken, H. & Klempau, I. (1951) Arch. exp. path.
Pharmakol., 212, 463
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life sciences, 5,
47
Dallemagne, M. J. & Philippot, E. (1948) Arch. int. pharm. Ther.,
76, 274
Doisy, E. A. & Bocklage, B. C. (1949) Proc. Soc. exp. Biol. (N.Y.),
71, 490
Ely, R. E., Underwood, P. C., Moore, L. A., Mann, F. D. & Carter, R.
H. (1953) J. Amer. vet. med. Ass., 123, 448
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Pharmacol.
exp. Ther., 100, 59
Ghazal, A., Koransky, W., Portig, J., Vohland, H. W. & Klempau, I.
(1964) Arch. exp. path. Pharmakol., 249, 1
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel Forsch., 1, 356
Hoffman, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth., 9, 387
Kastli, P. (1955) Féd. Int. des Laiteries Réunies, Bruxelles,
Report No. 3
Kewitz, H. (1952) Arch. exp. path. Pharmakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. path. Pharmakol., 227, 183
Klosa, J. (1950) Die Pharmazie, 5, 615
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A.J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A.J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Lendle, L. & Schneider, H. H. (1950) Arch. exp. path. Pharmakol.,
210, 119
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F., jr (1957) Arch. Path.,
64, 614
Paccagnella, B., Brati, L. & Gavazzini, C. (1966) Unpublished report
of the Institute of Hygiene of Ferrara, Italy
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Sedlak, V. A. (1965) Toxicol. appl. Pharmacol., 7, 79
Truhaut, R. (1954) Communication au symposium intern. de la prévention
du cancer, Sao Paulo
Van Asperen, K. (1957) C.R. 4é Congrčs Intern. de Lutte control les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wasserman, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu, G.
& Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 386
REFERENCES PERTINENT TO AGRICULTURAL DATA
Alessandri, M. E., Doretti, M. & Lanforti, G. F. (1959) Insecticide
residues in samples of peeled, canned tomatoes coming from experiments
carried out in Italy in 1958. Presented to 17th IUPAC Congress,
Munich, reported by Maier-Bode, H., Gesunde Pflanzen, 11, 225-229
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49, 222-30
Beran, F. (1961) Das Problem der Pflanzenschutzmittelruckstande in
europaischer Sicht. - Pflanzenschutzberichte (Wien) 27, 11-50
Bradbury, F. R. (1957) Absorption and metabolism of BHC in susceptible
and resistant houseflies. J. Sci. Food Agric., 8, 90-96
Bradbury, F. R. (1963) The systemic action of benzene hexachloride
seed dressings. Ann. appl. Biol., 52, 361-370
Bradbury, F. R. & Whitaker, W. O. (1956) The systemic action of
benzene hexachloride in plants; quantitative measurements. J. Sci Food
Agric., 4, 248
Brett, C. H. & Bowery, T. G. (1958) Insecticide Residues on
Vegetables. J. Econ. Ent., 51, 818-821
Bridges, R. G. (1958) Fate of labelled insecticide residues in food
products VI. Retention of gamma-Benzene hexachloride by wheat and
cheese. J. Sci. Food Agric., 9, 431-439
Bridges, R. G. (1958a) Fate of labelled insecticide residues in food
products VII. The fate of gamma-Benzene hexachloride residues in flour
during baking. J. Sci. Food Agric., 9, 439-448
Clark, A. G., Hitchcock, M., Smith, J. N. (1966) Metabolism of
gammexane in flies, ticks and locusts. Nature, 209, 103
Collett, J. N. & Harrison, D. L. (1963) Lindane residues in sheep
following dipping. N.Z. J. Agric. Res., 6, 39-42
Cummings, J. G. (1966) Pesticides in the total diet. Residue Reviews,
16, 30-45
Deema, P., Naber, E. D. & Ware, G. W. (1965) Residues in hen eggs from
vaporizing insecticide tablets. J. Econ. Ent., 58, 904-906
Duffy, J. R. & Wong, N. (1966) Insecticide Residues and their
metabolites in Atlantic Soils. In press.
Duggan, R. E., Barry, H. C. & Johnson, L. Y. (1966) Pesticide Residues
in total diet samples. Science, 151, 101
Egan, H. (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Food Agric., 16,
489-498
Ehrenhardt, H. (1954) Uber die Werkung des Hexachlorocyclohexans als
systemisches Insektizid. Anz. Schadlingsk., 27, 1
Fahey, J. E., Wilson, M. C. & Rusk, H. W. (1960) Persistence of BHC,
lindane, and thiodan residues when applied to alfalfa to control the
meadow spittlebug. J. Econ. Ent., 53, 960-1
Fairchild, H. E. & Dahm, Paul A. (1955) Lice control on chickens with
chlorinated hydrocarbon insecticides. J. Econ. Ent., 48, 141-146
de Faubert Maunder, M. J., Egan, H. & Godly, R. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Feuersenger, M. (1960) Uber die Bestimmung von
Schadlingsbekamfungsmitteln in Lebensmitteln. Bundesgesundheitsblatt,
NR10, 149-152
Feuersenger, M. (1962) Ruckstandfragen bei der Anwendug von
Kontaktininsektizieden in Getreideschutz Nachrichtenbl. dstch.
Pflanzenschutzdienstes (Braunschiveig), 14, 189
France, H. L., Treece, R. E. & Ware, W. G., (1961) Chemical and
biological assays of lindane residues on alfalfa. J. Econ. Ent., 54,
642-3
Giles, P. H., (1964) Lindane contamination in stored sorghum and
millet in N. Nigeria. Trop. Sci., 6, 113-121
Grover, P. L. & Sims, P. (1965) The metabolism of gamma-2,3,4,5,6
pentachlorocyclohex-1-ene and gamma-hexachlorocyclohexane in rats.
Biochem J., 96, 521-525
Gunther, F. A., Lindgren, D. L. & Blinn, R. C. (1958) Biological
effectiveness and persistence of malathion and lindane used for
protection of stored wheat. J. Econ. Ent., 510 843-4
Gyrisco, G. G., Norton, L. B., Trimberger, G. W., Holland, R. F.,
McEnerney, P. J. & Muka, A. A. (1959) Effects of feeding low levels of
insecticide residues on hay to dairy cattle on flavor and residues in
milk. Agric. & Food Chem., 7, 707-711
Hardee, D. D., Huddleston, E. W. & Gyrisco, G. G. (1963) Initial
deposit and disappearance rates of various insecticides as affected by
forage crop species. J. Econ. Ent., 56, 98-101
Hardon, H. J. (1960) Pestizidruckstande in Marktmustern von Gemuse und
Abst. - Verhandl. IV. Inter. Congress of Plant Protection, Hamburg,
Band 2. (Braunschweig, 1960) 1671-1674
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in southwestern Ontario. Agric. & Food Chem., 14,
398-403
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent., 54,
1038-1045
Harrison, D. L., Poole, W. S. H. & Mol, J. C. M. (1963) Observations
on feeding lindane-fortified mash to chickens. N.Z. Veterinary
Journal, 11, 137-140
Howe, W. L. (1950) Reactions of squash vine borer to certain
insecticidal soil treatments. J. Econ. Ent., 43, 549
Ishida, M. & Dahm, P. A. (1965) Metabolism of Benzene Hexachloride
Isomers and Related Compounds in Vitro. I. Properties and Distribution
of the Enzyme. J. Econ. Ent., 58, 383-392
Ivey, M. C., Roberts, R. H., Mann, H. D. & Claborn, H. V. (1961)
Lindane residues in chickens and eggs following poultry house sprays.
J. Econ. Ent., 54, 487-488
Jackson, J. B., Ivey, M. C., Roberts, R. H. & Radeleff, R. D. (1959)
Residue studies in sheep and goats dipped in 0.025% lindane. J. Econ.
Ent., 52, 1031-1032
Jameson, H. R. (1959) The mechanism of the control of turnip flea
beetle with benzene hexachloride dressings of Brassicae seed. J. Sci.
Food Agric., 9, 590
Johnson, M. R. (1958) Residue changes during the fermentation and
processing of pickles. Food Technology June, 1958
Lichtenstein, E. P. (1959) Absorption of some chlorinated hydrocarbon
insecticides from soils into various crops. Agric. and Food Chem., 7,
430-433
Lichtenstein, E. P. & Schulz, K. R. (1959) Persistence of some
chlorinated hydrocarbon insecticides as influenced by soil types, rate
of application and temperature. J. Econ. Ent., 52, 289-293
Lichtenstein, E. P., Schulz, K. R. & Cowley, G. T. (1963) Inhibition
of the conversion of aldrin to dieldrin in soils with
methylenedioxyphenyl synergists. J. Econ. Ent., 56, 485-489
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1964) Effect of
formulation and mode of application of aldrin on the loss of aldrin
and its epoxide from soils and their translocation into carrots. J.
Econ. Ent., 57, 133
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13, 57-62
Lilly, J. H. & Fahey, J. E. (1956) Translocation of BHC from
high-dosage soil treatments applied before planting. J. Econ. Ent.,
49, 815-818
Maier-Bode, H. (1963) Vergiftungen durch
Pflanzenschutzmittel-Ruckstande-Pflanzenschutzberichte (Wien) 30,
49-77
Maier-Bode, H. (1964) Pflanzenschutzmittel-Ruckstande. Insektizide,
Verlag Eugen Ulmer, Stuttgart. 455 pp.
Penn. State Univ. Agric. Exp. Sta. (1963) Pesticide residue
investigations on raw agricultural commodities. Penn. State Univ.
Bulletin 703
San Antonio, J. P. (1959) Demonstration of lindane and a lindane
metabolite in plants by paper chromatography. Agric. & Food Chem., 7.
322-325
Schesser, J. H., Priddle, W. E. & Farrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion, and lindane. J. Econ. Ent., 51, 516-518
Sellke, K. (1952) Kornkofereinstaubmittel und Ihr Verhalten in
behandelten Getreide Nachrichtenbl. dstch. Pflanzenschutzdienst
(Berlin) N.F.6, 121-127
Shapiro, I. D. (1951) On the toxic action of hexachlorane on insects
through the plant. Dokl. Akad. Nauk. SSSR(NS) 80, 481
Stadelman, W. J., Liska, B. J. & Langlois, G. C., Moster, T. G. &
Stemp, A. R. (1964) Persistence of chlorinated hydrocarbon residues in
chicken tissues and eggs. Poultry Science, 43, Sept. 1964
Starnes, O. (1950) Absorption and translocation of insecticides
through the root system of plants. J. Econ. Ent., 43, 338
Treece, R. E. & Ware, G. W. (1965) Lindane Residues on Alfalfa and in
Milk. J. Econ. Ent., 58, 218-219
USDA (1965) Distribution of Lindane Residues in Swine. Agr. Res. Serv.
Ent. Res. Div. Pesticide Chemicals Research Branch Spec. Report
PC-B-65-10
USDA (1965a) Lindane Residues in Swine. Agr. Res. Serv. Ent. Res. Div.
Pesticide Chemicals Research Branch Spec. Report PC-B-65-17
Van den Driessche, S. (1958) Influence of sterilization on
hexachloroxyclohexane (HCH) residues in preserved foods. Rev.
Conserve 13 (1), 27
Ware, G. W. & Naber. E. C. (1961) Lindane in eggs and chicken tissues.
J. Econ. Ent., 54, 675-677
Ware, G. W. & Naber, E. C. (1962) Lindane and BHC in egg yolks
following recommended uses for louse and mite control. J. Econ. Ent.,
55, 568-570
Way, M. J. (1959) Experiments on the mode of action of insecticidal
seed-dressings, especially against leptohylemyia coarctata fall.,
muscidae, the wheat bulb fly. Ann. appl. Biol., 47. 783-801
Williams, S. (1964) Pesticide residues in total diet samples.
J.A.O.A.C., 47, 815-821
Yule, W. N., Chiba, M. & Morley, H. V. (1967) Fate of Insecticide
Residues - The Decomposition of lindane in soil. J. Agr. Food Chem.
(In press)
Zeumer, H. & Neuhaus, K. (1953) Die Bestimmung von
Kontaktinsektizieden, Getreide und Mehl Heft 8; Beilage 2.
Wochenschrift Die Muhle, Detmold
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
gamma-BHC is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, gamma-BHC is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of
gamma-BHC in growing animals is, however, much lower than that of
beta-BHC or of DDT. In rats ingesting repeated small doses of
gamma-BHC, the concentration in the fat depots is in a state of
equilibrium with the concentration of the diet (Lehman, 1953). The
data on gamma-BHC metabolism are very incomplete. According to certain
in vitro tests it would seem that part is broken down in the liver.
The unchanged compound is eliminated in the faeces and urine and is
found in the milk (Ely et al., 1953; Van Asperen, 1957; Ware &
Gilmore, 1959). Very little information is available about its effects
on basic enzyme systems. Growing animals are particularly susceptible
to its toxic action (Kastli, 1955).
A single dose of 60 mg/kg in rats has been shown to accelerate
detoxication of several drugs processed by the liver microsomes,
including paraoxon (Ghazal at al., 1964).
The solubilities of the isomers of BBC in rat fat are: alpha, 4.05 per
cent; beta, 0.66 per cent; gamma, 12-55 per cent; delta, 19.10 per
cent. However, when each isomer is administered separately, the in
vivo storage depends on the metabolism, the solubility being only a
limiting factor (Sedlak, 1965).
In an analysis of 282 samples of human fat obtained from the general
population in the United States of America, an average content of 0.57
ppm was found (Hoffmann at al., 1964). A study in the United Kingdom
on 20 human fat samples showed a mean gamma-BHC concentration of 0.015
ppm (Robinson et al., 1965). Single spraying of cattle with 0.03 per
cent, or hogs with 0.06 per cent gamma-BHC and dipping of sheep and
goats in a 0.025 per cent suspension, caused the appearance of
gamma-BHC in the fat at levels ranging from 0.47 to 4.22 ppm (Claborn
et al., 1960). gamma-BHC has been found in 30 samples of unprocessed
cows' milk at a mean level of 0.01 ppm (Paccagnella et al., 1966).
Acute toxicity
From the viewpoint of acute toxicity, gamma-BHC is the most toxic of
all the isomers present in the technical hexachlorocyclohexanes. It is
a neurotropic poison causing excitation of the central nervous system
with convulsions, followed - if the dose is sufficiently high - by
depressive phenomena leading to death from respiratory collapse.
The fatal dose for man is estimated to be 150 mg/kg body-weight, i.e.,
of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Plasma taken from a three-year-old boy approximately 6 hours after he
swallowed gamma-BHC, and 3 hours after the last convulsion, contained
0.29 ppm gamma-BHC. Seven days later the concentration was 0.02 ppm
and the patient was asymptomatic (Dale et al., 1966).
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 86 Woodard & Hagan, 1947
Rat Oral 125-200 Dallemagne & Philippot, 1948
Lehman, 1948
Lehman, 1951
Rat Oral 177-230 Woodard & Hagan, 1947
Klosa, 1950
Rat Intraperitoneal 35-85 Coper et al., 1951
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Rabbit Oral 60-200 Cameron, 1945
Dog Oral 40 (lethal) Barke, 1950
100-200 (lethal) Landle & Schneider, 1950
Dog Intravenous 7.5 (lethal) Barke, 1950
Short-term studies
Rat. When rats were fed a diet containing 400 ppm gamma-BHC for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given gamma-BHC by
stomach tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous signs (trembling, spasms), fatty degeneration of the liver and
of the epithelium of the renal tubules, vacuolization of the cerebral
cells and a marked increase in mortality rate were observed. With 10
mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950, 1951; Kewitz, 1952; Kewitz & Reinert, 1952),; when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of gamma-BHC the general behaviour of the animals, their
body-weight and post-mortem gross and histological examinations showed
no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to daily intramuscular
administration at doses of 40 mg gamma-BHC for 19 days, 80 mg for 9
days and 160 mg for three days. The animals died and showed among
other things, hepatic degeneration (Dallemagne & Philippot, 1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed hepatic
lesions (Woodard & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10 per cent
solution of gamma-BHC in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of gamma-BHC brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied gamma-BHC during an
antimalaria campaign, about 46 per cent became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal applications
of 0.5 per cent gamma-BHC in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3 per cent of gamma-BHC and were observed for 10 months. No
abnormalities were found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given diets
containing 5 to 1600 ppm of the alpha-, beta-, and gamma-isomers of
BHC for two years. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of gamma-BHC, the lowest concentration causing significant
liver changes was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of 20
young rats (10 males and 10 females) were fed diets containing 25, 50
and 100 ppm of gamma-BHC throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration, 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets containing 50
and 100 ppm of gamma-BHC; histological lesions were found in the liver
parenchyma (Ortega et al., 1957).
Twenty-five young rats were given gamma-BHC daily by stomach-tube at a
dosage of 10 µg/kg body-weight (corresponding to about 0.1 and 0.15
ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments
In all these animal species, gamma-BHC has proved to be a cumulative
poison, causing hepatic and renal lesions and disturbances of the
central nervous system. The dog seems particularly susceptible to
neurological effects.
Evidence of stimulatory activity on liver microsomal metabolism such
as that observed for other chlorinated hydrocarbons has been presented
for gamma-BHC, long-term studies have been performed only in rats and
did not include reproductive aspects.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in the rat
25 ppm of gamma-BHC in the diet, equivalent to 1.25 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0125 mg/kg/body-weight.
Further work required
Elucidation of the significance of the finding that gamma-BHC is one
of the compounds which affect liver cellular metabolism (p. 3).
The chemical nature of the residue occurring in plants, which has not
been fully investigated.
This information should be provided not later than five years after
the publication of this report, when a re-evaluation of this compound
will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Soon after the introduction of gamma-BHC and prior to about 1950 its
uses included applications to fruits, vegetables and other edible
crops. These have almost ceased in Europe and N. America, but not in
various other parts of the world, where it is known to be used for
foliage application (e.g. to control cocoa insects). (However the
meeting had very little information on the scale of use in different
countries.)
In the production of cereals, gamma-BHC is used either by application
to plants, to the soil or to seeds. The dosage for foliar application
usually is less than 2 kg of gamma-BHC per hectare possibly repeated
two or three times: for soil insect control a dosage up to 1.8 kg per
hectare may be used. In many countries the recommended interval
between last application and harvest is 30 days.
It was the opinion of the meeting that uses on stone, pome and citrus
fruits and on berries have almost ceased. (Member countries having
requirements for retaining these uses should supply supporting data.)
Applications to soil used in the production of vegetables range from 4
oz to 24 oz per acre: foliar applications from 1 to 16 oz.
Aerosols, smokes and vapours of gamma-BHC are sometimes used on
vegetable and fruit crops in glasshouses.
Restrictions in various countries normally include measures against
tainting and against excessive residues and minimum periods between
treatments of soil (e.g. one to three years) and the planting of
edible crops are specified.
Gamma-BHC has had important uses against biting flies, lice, fleas,
ticks and in the control of mange, scab or scabies caused by mites on
livestock. Careful limitations in use are required, since in some
instances repeated applications are required to obtain effective
control. Use on meat animals is usually restricted (e.g. to 21 days
before slaughter for cattle, sheep and goats, and seven days for
hogs). Use on lactating animals or in milk rooms is not usually
permitted.
(b) Post-harvest uses
Gamma-BHC has been widely used for protecting harvested cereals,
pulses and nuts and for treating premises in which foods are stored.
In one or two countries gamma-BHC is mixed at about 3 ppm with cereals
in store. In some tropical countries (e.g. Ghana) maize, millets,
guinea corn and rice paddy have been treated at doses up to 11 ppm.
Gamma-BHC has also been used in holds of ships and has uses against
some insects of public health importance.
Residues resulting from supervised trials
Fruit and vegetables
Data on residues from supervised trials are not comprehensive. Many
were obtained around 1950 and call for confirmation with the use of
more modern analytical methods. The figures show rapid losses from
leafy vegetables and a greater persistence on fruit; but after
applications to soil significant residues remain for three years or
more. Brett & Bowery (1958) reported residues below 0.1 ppm on
tomatoes after three days, on snap beans after four days and on
collards after about 13 days. To obtain data on the maximum possible
residues, Lilly & Fahey (1956) used dosages as high as 50 times those
required to produce effective insect control. The maximum residues
found over a four-year period on soybeans were 0.74, 0.89, 0.27 and
1.21 ppm (averages for each of the four years), on field corn over a
three-year period they were up to 0.44 ppm. In the same series of
trials sweet corn showed variations from 0.02 to 0.41 ppm, popcorn
zero to 0.13 ppm. Other work reported by Lilly & Fahey (1956) on
sunflowers, grain sorghums and a variety of garden beans over a
four-year period showed no residues in excess of 0.6 ppm. Lichtenstein
(1959) also cites residues in crops resulting from excessive
applications of gamma-BHC to three soil types. Following normal
agricultural practices San Antonio (1959) found that soil containing 5
ppm resulted in 3 ppm in the edible parts of carrots, even though as
high as 25 ppm was found in the fibrous root hairs. (He also found
that the 3 ppm residue was greatly reduced by scraping the peel.) In
more recent studies, using gas/liquid chromatographic analysis,
Maier-Bode (1964) found not more than 0.5 ppm in beet treated 143 days
previously at dosages of 1.5 to 3 kg/hectare. Other residues after
treatments at the same dosage were 0.08 and 0.1 ppm in potatoes, and
0.03, 0.06 ppm, 0.05 and 0.09 ppm in cabbages. Using twice the dosage
employed in practice, Maier-Bode (1963) also found 0.5 to 1.0 ppm in
beets. Beran (1961) reported 0.2 ppm in radishes and 0.11 ppm in
beans.
Tolerances
Country Product Tolerance Comments
ppm
Canada Corn, beans and peas 10.0
Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Cereals No tolerance
USA Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Netherlands General 2.0
USSR General 2.0 Temporary
Federal Vegetables, pulses and 2.0 Proposed for 1968
German fruit
Republic Cereals 0.1 " " "
Milling products from 0.03 " " "
Cereals
Comecon General 5.0
(Eastern
Europe)
Hungary General 2.5
Brazil General 10.0 (Not specified)
France Wheat 5.0 (On condition that
flour does not contain
more than 0.1 ppm)
(continued)
Country Product Tolerance Comments
ppm
India Wheat 3.0 Under consideration
Italy Wheat 2.5 Cleaning and washing
required before milling
Kenya Wheat 1.0
Britain Cereals 2.5 Practical (not legal)
limit
In largely unpublished compilations of information submitted to USFDA
hearings in 1950, residues of less than 0.6 ppm were found on peaches,
30 days after application of 6.0 per cent sprays and up to 5.1 ppm on
apples after 21 days.
Animal feed
Gamma-BHC has been used for both soil treatments and foliar
applications on a variety of forage crops used as animal feed. To
reduce sources of contamination of milk and meat it is necessary to
examine these practices. Data on the occurrence of residues and on
their fate during curing and storage of hay or silage, etc. are not
extensive. On alfalfa, residues ranging from 0.05 ppm after 35 days
(Penn. State, 1963) to 0.4 ppm (France et al., 1961) have been
reported. Maier-Bode (1964) found 0.19 ppm in grass 28 days after
application. Applications within 14 days of cutting resulted in up to
1.1 ppm in hay baled and held up to eight months (Treece & Ware,
1965); at 24 and 31 days before harvest, 0.7 and 0.1 ppm (Fahey et
al., 1960). Hardee et al. (1963) demonstrated differences in the rate
of disappearance from alfalfa, clover and trefoil; which indicates a
need for caution in the extrapolation of results from one species to
another.
No substantial information seems to be available on residues in forage
resulting from soil treatments. The current data suggest, however,
that the residues resulting from direct foliar application on forage
crops may not be acceptable on animal food crops. The FAO Working
Party would therefore like to see more information of recent origin,
before making specific recommendations on this aspect. Information
from feeding trials with animals at the very low levels of residues
that might be expected from soil or seed treatments would also be
valuable.
Use on animals
Gamma-BHC has been popular for ectoparasite control in poultry houses,
by direct application to hens and their roosts. Fairchild & Dahm
(1955) applied one per cent dust to litter for lice control. Residues
in successive fat and liver samples dissipated rapidly. Skin samples
taken two weeks after dusting contained 0.44, 0.84 and 7.5 ppm from
0.06, 0.25 and one per cent dusts. Ivey et al. (1961) confined
chickens for 16 weeks in treated poultry houses. Residues in fat after
four weeks were 27.7 to 58.8 ppm, in eggs 4 ppm. Even light treatments
of similar poultry houses resulted in 6.6 ppm in fat after two weeks
and 1.0 after 20 weeks. Eggs from these hens contained 0.81 ppm after
two weeks and 0.08 ppm after 16 weeks. Lower concentrations were used
by Ware & Naber (1961) to determine the effect of low level
contamination of food. 0.01 ppm in feed resulted in detectable
residues in eggs and chicken tissues. After 60 days' feeding, residues
in egg yolks were 0.26, 0.46 and 4.96 ppm respectively from hens
receiving 0.1, 1.0 and 10 ppm in their diets. After transfer to clean
food, residues were down to a trace after eight days for the first
group, to 0.09 ppm after 24 days for the second group and to 0.08
after 53 days for the last group of birds.
Ware & Naber (1962) also reported that direct application to poultry
and to houses resulted in an initial residue of 5.57 ppm decreasing to
1.03 ppm after 14 days. Treating roosts only, on dipping legs,
resulted in lower residues. Deema, Naber & Ware (1965) evaluated
residues resulting from confining hens above cartridges producing
gamma-BHC vapour and found 0.13 ppm, 0.24 and 0.33 ppm after 80 days
at three dosage rates. Harrison et al. (1963) after feeding poultry
with 4, 16 and 64 ppm for 27 days found 19.1, 56.4 and 156.0 ppm in
the body fat. Stadelman et al. (1964) after feeding hens at 0.15 for
14 days, measured no detectable residues in eggs and less than 0.3 ppm
in fat, 15 ppm for five days resulted in 0.7 ppm in fat. One week
after discontinuing the pesticide, eggs contained 0.4 ppm and none was
detected in fat or flesh 11 weeks later.
Studies in New Zealand, USA and Britain suggest that the condition of
fleece is an important factor in determining the residues of gamma-BHC
and their persistence in the fat and meat of sheep following dipping.
Jackson et al. (1959), by biopsy two weeks after dipping in 0.025 per
cent solution, found 4.22 ppm in sheep fat and 2.67 ppm in goats' fat.
Twelve weeks were required for these residues to reduce to zero.
Dipping shorn sheep at the same strength, Egan (1965) found 1.9 ppm
and 0.2 ppm in kidney fat and meat respectively after one week and 0.1
ppm in each after six weeks, in unshorn sheep however the residues
were 8.9 and 1.0 ppm after one week, 3.8 and 0.4 ppm after six weeks,
2.2 and 0.1 after 12 weeks, and 40 weeks passed before the levels were
both down to 0.1 ppm.
Collett & Harrison (1963) using a dip containing 0.0125 per cent
emulsion also found higher residues in unshorn sheep. Fat of shorn
sheep two weeks after dipping contained 2.5 ppm and unshorn sheep 5.0
ppm (average figures) and residues; in fat of shorn sheep were
negligible in eight weeks and in unshorn sheep in 12 weeks. From these
results it seems that fleece is a reservoir for continued absorption.
Swine appear to store gamma-BHC less than sheep. Fat of animals
sprayed with normal dosages after one week contained 0.03 and 0.05
ppm, and those after twice the dosage 0.5 and 0.06 ppm (USDA, 1965).
Fat samples taken by biopsy showed 0.1 to 0.3 after 28 days (USDA,
1965a).
Gyrisko et al. (1959) reviewed the effects on flavour and residues in
milk of feeding low residues on hay to dairy cattle. In agreement with
earlier work they found that as much as 10 ppm in feed did not cause
off-flavour in milk. Feed which contained 0.6 ppm at harvest had
reduced to non-detectable levels when feeding was initiated.
Unfortunately, no recent information is available on the feeding to
lactating animals of the very low levels of residues which may be
present on cull root crops, sugar beets and forage. Most countries do
not register or recommend the use of gamma-BHC in buildings in which
dairy animals or dairy products are kept and processed, or on
lactating animals.
Residues in food moving in commerce
Few figures are available for individual food commodities. Total diet
studies in the USA (Williams, 1964; Duggan et al., 1966; Cummings,
1966) and Canadian restaurant meal surveys indicate very minor
incidence in diets of these countries. The USA five-year survey using
three different methods of analysis, rarely recorded residues in
excess of 0.01 ppm.
By contrast, British investigations of residues in home-killed mutton
in 1962 (Egan, 1965) indicated a constant low level of residue in fat
of meat in 61 samples. Thirty-seven samples contained not more than
0.1 ppm, 48 not more than 0.3 ppm and three samples between 1.1 and
2.0 ppm. The Reports of the Government Chemist (UK) for 1964 and 1965
contain further useful information on the amount of total BHC isomers
in food of origin from different countries:
1964 1965
Food Country Number of Range Arithmetic Number of Range Arithmetic
samples ppm mean ppm samples ppm mean ppm
Butter Australian 45 0.0-0.40 0.02 45 0.0-0.11 0.02
Danish 30 0.0-0.20 0.05 24 0.0-0.08 0.04
New Zealand 45 0.0-0.02 <0.01 40 0.0-0.04 0.01
UK 18 0.0-0.20 0.05 18 0.0-0.13 0.04
Milk UK 60 0.00-0.18 0.003 85 0.0-0.022 0.0045
Mutton Argentine 17 0.00-15.5 1.1 13 0.0-1.6 0.25
Kidney Australian 15 0.00-0.05 0.01 6 <0.05 -
Fat New Zealand 12 0.00-0.25 0.05 10 <0.05 -
UK 107 0.00-5.1 0.43 128 0.0-10.6 0.46
Beef Argentine 13 0.0-1.1 0.15 22 0.0-0.80 0.13
Kidney UK 66 0.0-0.3 0.05 59 0.0-0.42 0.04
Fat
Corned
beef Argentine 20 0.0-0.1 0.03 26 0.0-0.1 0.02
Hardon (1960) reported that beans sampled in commerce contained 0.1
ppm gamma-BHC, and canned tomatoes 0.13 ppm (Alessandrini at al.,
1959).
Fate of residues
(a) In soil
Gamma-BHC is relatively volatile and moderately soluble in water, for
which reasons it is one of the less persistent organochlorine
pesticides. It is generally considered that three years should elapse
after heavy applications and before root crops are planted in treated
soils; but soil type has an important influence on the rate of
disappearance from soil (Harris, 1966; Duffy, 1966; Lichtenstein &
Schulz, 1965; Lichtenstein et al., 1963, 1964; Harris & Lichtenstein,
1961, etc.). Some of the early work may have been misleading due to
inadequacies in the analytical method and this has been recently
reviewed (Yule, Chiba & Morley, 1967). Enzymatic processes have been
responsible for the detoxification of insects (e.g. Bradbury, 1957;
Clark et al., 1966; Ishida & Dahm, 1965) and in the rat (Grover &
Sims, 1965). Gamma-pentachlorocyclohexane has been identified as a
decomposition product in soil. It is 103 less toxic to insects than
gamma-BHC. Soil microorganisms and alkaline soils are involved in this
degradation process (Yule, Chiba & Morley, 1967). Further work is
required to determine whether the disappearance of gamma-BHC from soil
can be achieved in a reasonable period by land and crop management
practices.
(b) In plants
It has been demonstrated that gamma-BHC can be absorbed by roots and
translocated to leaves (Bradbury & Whitaker, 1956) where it can kill
insects (Howe, 1950; Starnes, 1950; Shapiro, 1951; Ehrenhardt, 1954).
Jameson (1959) concluded that (when used as a dressing) the
insecticide penetrates into seeds and is absorbed by the cotyledons.
Way (1959) compared the action of gamma-BHC with other organochlorine
seed dressings and Bradbury (1963) the action on cabbage and wheat, in
both of which the gamma-BHC fell off rapidly.
There appears to be very little information on metabolism and
degradation in plants. Such information is necessary because
toxicological evaluation has been based solely on feeding the parent
compound.
(c) In storage and processing
Because of the volatility and solubility of gamma-BHC, storage and
processing, including cooking, would be expected to result in losses.
The literature contains divergencies on this subject probably due to
the use of non-specific analytical methods in the early work and
because the losses were closely dependent upon the temperatures,
degrees of exposure to free air and other conditions pertaining in the
particular test.
From his own studies Van den Driessche (1958) suggests that in the
canning and cooking process 23 per cent of residues of BHC (presumably
13 per cent gamma) in treated vegetables would be lost in cooking
water, and in the canning of beans and peas 63 per cent of the residue
might be dissipated.
Information on losses of gamma-BHC during the storage of cereals and
on its carry over into flour and bread were reviewed in some detail at
the second session of the FAO Working Party on Pesticide Residues
(PL/1965/12). Losses during storage depend upon the physical
conditions (e.g. Bridges, 1958) being high in the tropics and in well
ventilated stores (e.g. Giles, 1964) and low under cooler closed
conditions (e.g. Gunther, Lindgren & Blinn, 1958; Schesser, Priddle &
Farrell, 1958). During the milling of flour, the losses depend on the
particular processes used. Preliminary cleaning, brushing and washing
have been shown to remove insecticide and several workers have found
appreciably lower ppm residues in the flour (i.e. up to 50 per cent)
than in the original grain, although the residues in certain
by-products (e.g. bran for feeding to animals) may contain a higher
ppm (Bridges, 1958; Feuersinger, 1960, 1962; Schesser et al., 1958;
Sellke, 1952; Zeumer & Neuhaus, 1953). There are few data on losses of
residues during bread making. Bridges (1958a) found that flour
containing 2.5 ppm contained 40 per cent of this as unchanged residue
in the bread with the remainder probably as tri-, di-, and mono-
chlorbenzenes.
Loss from Cheddar and Stilton cheese was slow (Bridges, 1958), about
40 per cent remaining after 44 weeks. Penetration into both types was
slow but no chemical changes were found in either raw or cooked
cheese. Johnson (1958) reported the results of processing on residues
in cucumbers used for pickles. It was concluded that final residues in
pickles would be less than 0.5 ppm from field treated cucumbers, but a
concentration of 10 ppm could result if harvested cucumbers were
sprayed directly.
Method of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of gamma-BHC, together with residues of a
number of other compounds, including other BHC isomers. An example is
the AOAC system (1966) in which acetonitrile partition and Florisil
column clean-up are identified and measured by gas chromatography
coupled with thin layer or paper chromatography. Alternative clean-up
systems; e.g that of de Faubert Maunder et al. (1964) using
dimethylformamide, and other methods of confirmation of identity,
e.g., using infra-red spectrophotometry, are also available. The
methods are in general sensitive to 0.002 ppm of gamma-BHC in milk and
0.02 ppm of gamma-BHC in most other foods, though under favourable
conditions greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The residue data reviewed at the meeting, for the most part, were
produced between 1948 and 1959 before improved specific methods of
analysis were available. These results can only be used as a guide,
subject to review, when better data are available. When considering
information on residues of this pesticide, the meeting found it
necessary to rely on the expert opinion of its members, rather than
conclusions that could be derived exclusively from obsolete or
incomplete data.
The properties of this pesticide are such that it was assumed that
substantial losses of residue would occur in storage, processing and
cooking of foods such as some vegetables, meat and cereals, but only
minor losses in the case of small fruits and milk products.
Tolerances recommended for residues of gamma-BHC based on these guide
lines are:
Small fruits 3.0 ppm )
)
Vegetables 3.0 ppm )
) Temporary tolerance
Cereals 0.5 ppm )
)
Milk products 0.1 ppm (fat basis) )
Meat and poultry 0.7 ppm (fat basis) ) Practical
) residue
Fluid milk 0.004 ppm ) level
These should be reviewed in the light of additional data, but in
any event not later than within three years.
Further work
1. Guidance is required from countries on uses still of value in
food production or protection in storage and transit.
2. Data are needed on the disappearance of residues during storage
and processing of food, and information on the chemical nature of
terminal residues on food as consumed.
3. Residue data, using specific methods of analysis which will
identify metabolites and gamma-BHC, after supervised trials and
on residues in food moving in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Barke, A. (1950) Tierärzliche Umschau, 5, 611
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff,
R. D. (1960) J. Agr. Food. Chem., 8, 439
Coper, F., Herken, H. & Klempau, I. (1951) Arch. exp. path.
Pharmakol., 212, 463
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life sciences, 5,
47
Dallemagne, M. J. & Philippot, E. (1948) Arch. int. pharm. Ther.,
76, 274
Doisy, E. A. & Bocklage, B. C. (1949) Proc. Soc. exp. Biol. (N.Y.),
71, 490
Ely, R. E., Underwood, P. C., Moore, L. A., Mann, F. D. & Carter, R.
H. (1953) J. Amer. vet. med. Ass., 123, 448
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Pharmacol.
exp. Ther., 100, 59
Ghazal, A., Koransky, W., Portig, J., Vohland, H. W. & Klempau, I.
(1964) Arch. exp. path. Pharmakol., 249, 1
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel Forsch., 1, 356
Hoffman, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth., 9, 387
Kastli, P. (1955) Féd. Int. des Laiteries Réunies, Bruxelles,
Report No. 3
Kewitz, H. (1952) Arch. exp. path. Pharmakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. path. Pharmakol., 227, 183
Klosa, J. (1950) Die Pharmazie, 5, 615
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A.J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A.J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Lendle, L. & Schneider, H. H. (1950) Arch. exp. path. Pharmakol.,
210, 119
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F., jr (1957) Arch. Path.,
64, 614
Paccagnella, B., Brati, L. & Gavazzini, C. (1966) Unpublished report
of the Institute of Hygiene of Ferrara, Italy
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Sedlak, V. A. (1965) Toxicol. appl. Pharmacol., 7, 79
Truhaut, R. (1954) Communication au symposium intern. de la prévention
du cancer, Sao Paulo
Van Asperen, K. (1957) C.R. 4é Congrčs Intern. de Lutte control les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wasserman, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu, G.
& Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 386
REFERENCES PERTINENT TO AGRICULTURAL DATA
Alessandri, M. E., Doretti, M. & Lanforti, G. F. (1959) Insecticide
residues in samples of peeled, canned tomatoes coming from experiments
carried out in Italy in 1958. Presented to 17th IUPAC Congress,
Munich, reported by Maier-Bode, H., Gesunde Pflanzen, 11, 225-229
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49, 222-30
Beran, F. (1961) Das Problem der Pflanzenschutzmittelruckstande in
europaischer Sicht. - Pflanzenschutzberichte (Wien) 27, 11-50
Bradbury, F. R. (1957) Absorption and metabolism of BHC in susceptible
and resistant houseflies. J. Sci. Food Agric., 8, 90-96
Bradbury, F. R. (1963) The systemic action of benzene hexachloride
seed dressings. Ann. appl. Biol., 52, 361-370
Bradbury, F. R. & Whitaker, W. O. (1956) The systemic action of
benzene hexachloride in plants; quantitative measurements. J. Sci Food
Agric., 4, 248
Brett, C. H. & Bowery, T. G. (1958) Insecticide Residues on
Vegetables. J. Econ. Ent., 51, 818-821
Bridges, R. G. (1958) Fate of labelled insecticide residues in food
products VI. Retention of gamma-Benzene hexachloride by wheat and
cheese. J. Sci. Food Agric., 9, 431-439
Bridges, R. G. (1958a) Fate of labelled insecticide residues in food
products VII. The fate of gamma-Benzene hexachloride residues in flour
during baking. J. Sci. Food Agric., 9, 439-448
Clark, A. G., Hitchcock, M., Smith, J. N. (1966) Metabolism of
gammexane in flies, ticks and locusts. Nature, 209, 103
Collett, J. N. & Harrison, D. L. (1963) Lindane residues in sheep
following dipping. N.Z. J. Agric. Res., 6, 39-42
Cummings, J. G. (1966) Pesticides in the total diet. Residue Reviews,
16, 30-45
Deema, P., Naber, E. D. & Ware, G. W. (1965) Residues in hen eggs from
vaporizing insecticide tablets. J. Econ. Ent., 58, 904-906
Duffy, J. R. & Wong, N. (1966) Insecticide Residues and their
metabolites in Atlantic Soils. In press.
Duggan, R. E., Barry, H. C. & Johnson, L. Y. (1966) Pesticide Residues
in total diet samples. Science, 151, 101
Egan, H. (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Food Agric., 16,
489-498
Ehrenhardt, H. (1954) Uber die Werkung des Hexachlorocyclohexans als
systemisches Insektizid. Anz. Schadlingsk., 27, 1
Fahey, J. E., Wilson, M. C. & Rusk, H. W. (1960) Persistence of BHC,
lindane, and thiodan residues when applied to alfalfa to control the
meadow spittlebug. J. Econ. Ent., 53, 960-1
Fairchild, H. E. & Dahm, Paul A. (1955) Lice control on chickens with
chlorinated hydrocarbon insecticides. J. Econ. Ent., 48, 141-146
de Faubert Maunder, M. J., Egan, H. & Godly, R. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Feuersenger, M. (1960) Uber die Bestimmung von
Schadlingsbekamfungsmitteln in Lebensmitteln. Bundesgesundheitsblatt,
NR10, 149-152
Feuersenger, M. (1962) Ruckstandfragen bei der Anwendug von
Kontaktininsektizieden in Getreideschutz Nachrichtenbl. dstch.
Pflanzenschutzdienstes (Braunschiveig), 14, 189
France, H. L., Treece, R. E. & Ware, W. G., (1961) Chemical and
biological assays of lindane residues on alfalfa. J. Econ. Ent., 54,
642-3
Giles, P. H., (1964) Lindane contamination in stored sorghum and
millet in N. Nigeria. Trop. Sci., 6, 113-121
Grover, P. L. & Sims, P. (1965) The metabolism of gamma-2,3,4,5,6
pentachlorocyclohex-1-ene and gamma-hexachlorocyclohexane in rats.
Biochem J., 96, 521-525
Gunther, F. A., Lindgren, D. L. & Blinn, R. C. (1958) Biological
effectiveness and persistence of malathion and lindane used for
protection of stored wheat. J. Econ. Ent., 510 843-4
Gyrisco, G. G., Norton, L. B., Trimberger, G. W., Holland, R. F.,
McEnerney, P. J. & Muka, A. A. (1959) Effects of feeding low levels of
insecticide residues on hay to dairy cattle on flavor and residues in
milk. Agric. & Food Chem., 7, 707-711
Hardee, D. D., Huddleston, E. W. & Gyrisco, G. G. (1963) Initial
deposit and disappearance rates of various insecticides as affected by
forage crop species. J. Econ. Ent., 56, 98-101
Hardon, H. J. (1960) Pestizidruckstande in Marktmustern von Gemuse und
Abst. - Verhandl. IV. Inter. Congress of Plant Protection, Hamburg,
Band 2. (Braunschweig, 1960) 1671-1674
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in southwestern Ontario. Agric. & Food Chem., 14,
398-403
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent., 54,
1038-1045
Harrison, D. L., Poole, W. S. H. & Mol, J. C. M. (1963) Observations
on feeding lindane-fortified mash to chickens. N.Z. Veterinary
Journal, 11, 137-140
Howe, W. L. (1950) Reactions of squash vine borer to certain
insecticidal soil treatments. J. Econ. Ent., 43, 549
Ishida, M. & Dahm, P. A. (1965) Metabolism of Benzene Hexachloride
Isomers and Related Compounds in Vitro. I. Properties and Distribution
of the Enzyme. J. Econ. Ent., 58, 383-392
Ivey, M. C., Roberts, R. H., Mann, H. D. & Claborn, H. V. (1961)
Lindane residues in chickens and eggs following poultry house sprays.
J. Econ. Ent., 54, 487-488
Jackson, J. B., Ivey, M. C., Roberts, R. H. & Radeleff, R. D. (1959)
Residue studies in sheep and goats dipped in 0.025% lindane. J. Econ.
Ent., 52, 1031-1032
Jameson, H. R. (1959) The mechanism of the control of turnip flea
beetle with benzene hexachloride dressings of Brassicae seed. J. Sci.
Food Agric., 9, 590
Johnson, M. R. (1958) Residue changes during the fermentation and
processing of pickles. Food Technology June, 1958
Lichtenstein, E. P. (1959) Absorption of some chlorinated hydrocarbon
insecticides from soils into various crops. Agric. and Food Chem., 7,
430-433
Lichtenstein, E. P. & Schulz, K. R. (1959) Persistence of some
chlorinated hydrocarbon insecticides as influenced by soil types, rate
of application and temperature. J. Econ. Ent., 52, 289-293
Lichtenstein, E. P., Schulz, K. R. & Cowley, G. T. (1963) Inhibition
of the conversion of aldrin to dieldrin in soils with
methylenedioxyphenyl synergists. J. Econ. Ent., 56, 485-489
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1964) Effect of
formulation and mode of application of aldrin on the loss of aldrin
and its epoxide from soils and their translocation into carrots. J.
Econ. Ent., 57, 133
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13, 57-62
Lilly, J. H. & Fahey, J. E. (1956) Translocation of BHC from
high-dosage soil treatments applied before planting. J. Econ. Ent.,
49, 815-818
Maier-Bode, H. (1963) Vergiftungen durch
Pflanzenschutzmittel-Ruckstande-Pflanzenschutzberichte (Wien) 30,
49-77
Maier-Bode, H. (1964) Pflanzenschutzmittel-Ruckstande. Insektizide,
Verlag Eugen Ulmer, Stuttgart. 455 pp.
Penn. State Univ. Agric. Exp. Sta. (1963) Pesticide residue
investigations on raw agricultural commodities. Penn. State Univ.
Bulletin 703
San Antonio, J. P. (1959) Demonstration of lindane and a lindane
metabolite in plants by paper chromatography. Agric. & Food Chem., 7.
322-325
Schesser, J. H., Priddle, W. E. & Farrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion, and lindane. J. Econ. Ent., 51, 516-518
Sellke, K. (1952) Kornkofereinstaubmittel und Ihr Verhalten in
behandelten Getreide Nachrichtenbl. dstch. Pflanzenschutzdienst
(Berlin) N.F.6, 121-127
Shapiro, I. D. (1951) On the toxic action of hexachlorane on insects
through the plant. Dokl. Akad. Nauk. SSSR(NS) 80, 481
Stadelman, W. J., Liska, B. J. & Langlois, G. C., Moster, T. G. &
Stemp, A. R. (1964) Persistence of chlorinated hydrocarbon residues in
chicken tissues and eggs. Poultry Science, 43, Sept. 1964
Starnes, O. (1950) Absorption and translocation of insecticides
through the root system of plants. J. Econ. Ent., 43, 338
Treece, R. E. & Ware, G. W. (1965) Lindane Residues on Alfalfa and in
Milk. J. Econ. Ent., 58, 218-219
USDA (1965) Distribution of Lindane Residues in Swine. Agr. Res. Serv.
Ent. Res. Div. Pesticide Chemicals Research Branch Spec. Report
PC-B-65-10
USDA (1965a) Lindane Residues in Swine. Agr. Res. Serv. Ent. Res. Div.
Pesticide Chemicals Research Branch Spec. Report PC-B-65-17
Van den Driessche, S. (1958) Influence of sterilization on
hexachloroxyclohexane (HCH) residues in preserved foods. Rev.
Conserve 13 (1), 27
Ware, G. W. & Naber. E. C. (1961) Lindane in eggs and chicken tissues.
J. Econ. Ent., 54, 675-677
Ware, G. W. & Naber, E. C. (1962) Lindane and BHC in egg yolks
following recommended uses for louse and mite control. J. Econ. Ent.,
55, 568-570
Way, M. J. (1959) Experiments on the mode of action of insecticidal
seed-dressings, especially against leptohylemyia coarctata fall.,
muscidae, the wheat bulb fly. Ann. appl. Biol., 47. 783-801
Williams, S. (1964) Pesticide residues in total diet samples.
J.A.O.A.C., 47, 815-821
Yule, W. N., Chiba, M. & Morley, H. V. (1967) Fate of Insecticide
Residues - The Decomposition of lindane in soil. J. Agr. Food Chem.
(In press)
Zeumer, H. & Neuhaus, K. (1953) Die Bestimmung von
Kontaktinsektizieden, Getreide und Mehl Heft 8; Beilage 2.
Wochenschrift Die Muhle, Detmold
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
gamma-BHC is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, gamma-BHC is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of
gamma-BHC in growing animals is, however, much lower than that of
beta-BHC or of DDT. In rats ingesting repeated small doses of
gamma-BHC, the concentration in the fat depots is in a state of
equilibrium with the concentration of the diet (Lehman, 1953). The
data on gamma-BHC metabolism are very incomplete. According to certain
in vitro tests it would seem that part is broken down in the liver.
The unchanged compound is eliminated in the faeces and urine and is
found in the milk (Ely et al., 1953; Van Asperen, 1957; Ware &
Gilmore, 1959). Very little information is available about its effects
on basic enzyme systems. Growing animals are particularly susceptible
to its toxic action (Kastli, 1955).
A single dose of 60 mg/kg in rats has been shown to accelerate
detoxication of several drugs processed by the liver microsomes,
including paraoxon (Ghazal at al., 1964).
The solubilities of the isomers of BBC in rat fat are: alpha, 4.05 per
cent; beta, 0.66 per cent; gamma, 12-55 per cent; delta, 19.10 per
cent. However, when each isomer is administered separately, the in
vivo storage depends on the metabolism, the solubility being only a
limiting factor (Sedlak, 1965).
In an analysis of 282 samples of human fat obtained from the general
population in the United States of America, an average content of 0.57
ppm was found (Hoffmann at al., 1964). A study in the United Kingdom
on 20 human fat samples showed a mean gamma-BHC concentration of 0.015
ppm (Robinson et al., 1965). Single spraying of cattle with 0.03 per
cent, or hogs with 0.06 per cent gamma-BHC and dipping of sheep and
goats in a 0.025 per cent suspension, caused the appearance of
gamma-BHC in the fat at levels ranging from 0.47 to 4.22 ppm (Claborn
et al., 1960). gamma-BHC has been found in 30 samples of unprocessed
cows' milk at a mean level of 0.01 ppm (Paccagnella et al., 1966).
Acute toxicity
From the viewpoint of acute toxicity, gamma-BHC is the most toxic of
all the isomers present in the technical hexachlorocyclohexanes. It is
a neurotropic poison causing excitation of the central nervous system
with convulsions, followed - if the dose is sufficiently high - by
depressive phenomena leading to death from respiratory collapse.
The fatal dose for man is estimated to be 150 mg/kg body-weight, i.e.,
of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Plasma taken from a three-year-old boy approximately 6 hours after he
swallowed gamma-BHC, and 3 hours after the last convulsion, contained
0.29 ppm gamma-BHC. Seven days later the concentration was 0.02 ppm
and the patient was asymptomatic (Dale et al., 1966).
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 86 Woodard & Hagan, 1947
Rat Oral 125-200 Dallemagne & Philippot, 1948
Lehman, 1948
Lehman, 1951
Rat Oral 177-230 Woodard & Hagan, 1947
Klosa, 1950
Rat Intraperitoneal 35-85 Coper et al., 1951
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Rabbit Oral 60-200 Cameron, 1945
Dog Oral 40 (lethal) Barke, 1950
100-200 (lethal) Landle & Schneider, 1950
Dog Intravenous 7.5 (lethal) Barke, 1950
Short-term studies
Rat. When rats were fed a diet containing 400 ppm gamma-BHC for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given gamma-BHC by
stomach tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous signs (trembling, spasms), fatty degeneration of the liver and
of the epithelium of the renal tubules, vacuolization of the cerebral
cells and a marked increase in mortality rate were observed. With 10
mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950, 1951; Kewitz, 1952; Kewitz & Reinert, 1952),; when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of gamma-BHC the general behaviour of the animals, their
body-weight and post-mortem gross and histological examinations showed
no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to daily intramuscular
administration at doses of 40 mg gamma-BHC for 19 days, 80 mg for 9
days and 160 mg for three days. The animals died and showed among
other things, hepatic degeneration (Dallemagne & Philippot, 1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed hepatic
lesions (Woodard & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10 per cent
solution of gamma-BHC in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of gamma-BHC brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied gamma-BHC during an
antimalaria campaign, about 46 per cent became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal applications
of 0.5 per cent gamma-BHC in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3 per cent of gamma-BHC and were observed for 10 months. No
abnormalities were found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given diets
containing 5 to 1600 ppm of the alpha-, beta-, and gamma-isomers of
BHC for two years. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of gamma-BHC, the lowest concentration causing significant
liver changes was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of 20
young rats (10 males and 10 females) were fed diets containing 25, 50
and 100 ppm of gamma-BHC throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration, 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets containing 50
and 100 ppm of gamma-BHC; histological lesions were found in the liver
parenchyma (Ortega et al., 1957).
Twenty-five young rats were given gamma-BHC daily by stomach-tube at a
dosage of 10 µg/kg body-weight (corresponding to about 0.1 and 0.15
ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments
In all these animal species, gamma-BHC has proved to be a cumulative
poison, causing hepatic and renal lesions and disturbances of the
central nervous system. The dog seems particularly susceptible to
neurological effects.
Evidence of stimulatory activity on liver microsomal metabolism such
as that observed for other chlorinated hydrocarbons has been presented
for gamma-BHC, long-term studies have been performed only in rats and
did not include reproductive aspects.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in the rat
25 ppm of gamma-BHC in the diet, equivalent to 1.25 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0125 mg/kg/body-weight.
Further work required
Elucidation of the significance of the finding that gamma-BHC is one
of the compounds which affect liver cellular metabolism (p. 3).
The chemical nature of the residue occurring in plants, which has not
been fully investigated.
This information should be provided not later than five years after
the publication of this report, when a re-evaluation of this compound
will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Soon after the introduction of gamma-BHC and prior to about 1950 its
uses included applications to fruits, vegetables and other edible
crops. These have almost ceased in Europe and N. America, but not in
various other parts of the world, where it is known to be used for
foliage application (e.g. to control cocoa insects). (However the
meeting had very little information on the scale of use in different
countries.)
In the production of cereals, gamma-BHC is used either by application
to plants, to the soil or to seeds. The dosage for foliar application
usually is less than 2 kg of gamma-BHC per hectare possibly repeated
two or three times: for soil insect control a dosage up to 1.8 kg per
hectare may be used. In many countries the recommended interval
between last application and harvest is 30 days.
It was the opinion of the meeting that uses on stone, pome and citrus
fruits and on berries have almost ceased. (Member countries having
requirements for retaining these uses should supply supporting data.)
Applications to soil used in the production of vegetables range from 4
oz to 24 oz per acre: foliar applications from 1 to 16 oz.
Aerosols, smokes and vapours of gamma-BHC are sometimes used on
vegetable and fruit crops in glasshouses.
Restrictions in various countries normally include measures against
tainting and against excessive residues and minimum periods between
treatments of soil (e.g. one to three years) and the planting of
edible crops are specified.
Gamma-BHC has had important uses against biting flies, lice, fleas,
ticks and in the control of mange, scab or scabies caused by mites on
livestock. Careful limitations in use are required, since in some
instances repeated applications are required to obtain effective
control. Use on meat animals is usually restricted (e.g. to 21 days
before slaughter for cattle, sheep and goats, and seven days for
hogs). Use on lactating animals or in milk rooms is not usually
permitted.
(b) Post-harvest uses
Gamma-BHC has been widely used for protecting harvested cereals,
pulses and nuts and for treating premises in which foods are stored.
In one or two countries gamma-BHC is mixed at about 3 ppm with cereals
in store. In some tropical countries (e.g. Ghana) maize, millets,
guinea corn and rice paddy have been treated at doses up to 11 ppm.
Gamma-BHC has also been used in holds of ships and has uses against
some insects of public health importance.
Residues resulting from supervised trials
Fruit and vegetables
Data on residues from supervised trials are not comprehensive. Many
were obtained around 1950 and call for confirmation with the use of
more modern analytical methods. The figures show rapid losses from
leafy vegetables and a greater persistence on fruit; but after
applications to soil significant residues remain for three years or
more. Brett & Bowery (1958) reported residues below 0.1 ppm on
tomatoes after three days, on snap beans after four days and on
collards after about 13 days. To obtain data on the maximum possible
residues, Lilly & Fahey (1956) used dosages as high as 50 times those
required to produce effective insect control. The maximum residues
found over a four-year period on soybeans were 0.74, 0.89, 0.27 and
1.21 ppm (averages for each of the four years), on field corn over a
three-year period they were up to 0.44 ppm. In the same series of
trials sweet corn showed variations from 0.02 to 0.41 ppm, popcorn
zero to 0.13 ppm. Other work reported by Lilly & Fahey (1956) on
sunflowers, grain sorghums and a variety of garden beans over a
four-year period showed no residues in excess of 0.6 ppm. Lichtenstein
(1959) also cites residues in crops resulting from excessive
applications of gamma-BHC to three soil types. Following normal
agricultural practices San Antonio (1959) found that soil containing 5
ppm resulted in 3 ppm in the edible parts of carrots, even though as
high as 25 ppm was found in the fibrous root hairs. (He also found
that the 3 ppm residue was greatly reduced by scraping the peel.) In
more recent studies, using gas/liquid chromatographic analysis,
Maier-Bode (1964) found not more than 0.5 ppm in beet treated 143 days
previously at dosages of 1.5 to 3 kg/hectare. Other residues after
treatments at the same dosage were 0.08 and 0.1 ppm in potatoes, and
0.03, 0.06 ppm, 0.05 and 0.09 ppm in cabbages. Using twice the dosage
employed in practice, Maier-Bode (1963) also found 0.5 to 1.0 ppm in
beets. Beran (1961) reported 0.2 ppm in radishes and 0.11 ppm in
beans.
Tolerances
Country Product Tolerance Comments
ppm
Canada Corn, beans and peas 10.0
Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Cereals No tolerance
USA Fruits 10.0
Vegetables 10.0
Fat of cattle, goats 7.0
and sheep
Fat of hogs 4.0
Netherlands General 2.0
USSR General 2.0 Temporary
Federal Vegetables, pulses and 2.0 Proposed for 1968
German fruit
Republic Cereals 0.1 " " "
Milling products from 0.03 " " "
Cereals
Comecon General 5.0
(Eastern
Europe)
Hungary General 2.5
Brazil General 10.0 (Not specified)
France Wheat 5.0 (On condition that
flour does not contain
more than 0.1 ppm)
(continued)
Country Product Tolerance Comments
ppm
India Wheat 3.0 Under consideration
Italy Wheat 2.5 Cleaning and washing
required before milling
Kenya Wheat 1.0
Britain Cereals 2.5 Practical (not legal)
limit
In largely unpublished compilations of information submitted to USFDA
hearings in 1950, residues of less than 0.6 ppm were found on peaches,
30 days after application of 6.0 per cent sprays and up to 5.1 ppm on
apples after 21 days.
Animal feed
Gamma-BHC has been used for both soil treatments and foliar
applications on a variety of forage crops used as animal feed. To
reduce sources of contamination of milk and meat it is necessary to
examine these practices. Data on the occurrence of residues and on
their fate during curing and storage of hay or silage, etc. are not
extensive. On alfalfa, residues ranging from 0.05 ppm after 35 days
(Penn. State, 1963) to 0.4 ppm (France et al., 1961) have been
reported. Maier-Bode (1964) found 0.19 ppm in grass 28 days after
application. Applications within 14 days of cutting resulted in up to
1.1 ppm in hay baled and held up to eight months (Treece & Ware,
1965); at 24 and 31 days before harvest, 0.7 and 0.1 ppm (Fahey et
al., 1960). Hardee et al. (1963) demonstrated differences in the rate
of disappearance from alfalfa, clover and trefoil; which indicates a
need for caution in the extrapolation of results from one species to
another.
No substantial information seems to be available on residues in forage
resulting from soil treatments. The current data suggest, however,
that the residues resulting from direct foliar application on forage
crops may not be acceptable on animal food crops. The FAO Working
Party would therefore like to see more information of recent origin,
before making specific recommendations on this aspect. Information
from feeding trials with animals at the very low levels of residues
that might be expected from soil or seed treatments would also be
valuable.
Use on animals
Gamma-BHC has been popular for ectoparasite control in poultry houses,
by direct application to hens and their roosts. Fairchild & Dahm
(1955) applied one per cent dust to litter for lice control. Residues
in successive fat and liver samples dissipated rapidly. Skin samples
taken two weeks after dusting contained 0.44, 0.84 and 7.5 ppm from
0.06, 0.25 and one per cent dusts. Ivey et al. (1961) confined
chickens for 16 weeks in treated poultry houses. Residues in fat after
four weeks were 27.7 to 58.8 ppm, in eggs 4 ppm. Even light treatments
of similar poultry houses resulted in 6.6 ppm in fat after two weeks
and 1.0 after 20 weeks. Eggs from these hens contained 0.81 ppm after
two weeks and 0.08 ppm after 16 weeks. Lower concentrations were used
by Ware & Naber (1961) to determine the effect of low level
contamination of food. 0.01 ppm in feed resulted in detectable
residues in eggs and chicken tissues. After 60 days' feeding, residues
in egg yolks were 0.26, 0.46 and 4.96 ppm respectively from hens
receiving 0.1, 1.0 and 10 ppm in their diets. After transfer to clean
food, residues were down to a trace after eight days for the first
group, to 0.09 ppm after 24 days for the second group and to 0.08
after 53 days for the last group of birds.
Ware & Naber (1962) also reported that direct application to poultry
and to houses resulted in an initial residue of 5.57 ppm decreasing to
1.03 ppm after 14 days. Treating roosts only, on dipping legs,
resulted in lower residues. Deema, Naber & Ware (1965) evaluated
residues resulting from confining hens above cartridges producing
gamma-BHC vapour and found 0.13 ppm, 0.24 and 0.33 ppm after 80 days
at three dosage rates. Harrison et al. (1963) after feeding poultry
with 4, 16 and 64 ppm for 27 days found 19.1, 56.4 and 156.0 ppm in
the body fat. Stadelman et al. (1964) after feeding hens at 0.15 for
14 days, measured no detectable residues in eggs and less than 0.3 ppm
in fat, 15 ppm for five days resulted in 0.7 ppm in fat. One week
after discontinuing the pesticide, eggs contained 0.4 ppm and none was
detected in fat or flesh 11 weeks later.
Studies in New Zealand, USA and Britain suggest that the condition of
fleece is an important factor in determining the residues of gamma-BHC
and their persistence in the fat and meat of sheep following dipping.
Jackson et al. (1959), by biopsy two weeks after dipping in 0.025 per
cent solution, found 4.22 ppm in sheep fat and 2.67 ppm in goats' fat.
Twelve weeks were required for these residues to reduce to zero.
Dipping shorn sheep at the same strength, Egan (1965) found 1.9 ppm
and 0.2 ppm in kidney fat and meat respectively after one week and 0.1
ppm in each after six weeks, in unshorn sheep however the residues
were 8.9 and 1.0 ppm after one week, 3.8 and 0.4 ppm after six weeks,
2.2 and 0.1 after 12 weeks, and 40 weeks passed before the levels were
both down to 0.1 ppm.
Collett & Harrison (1963) using a dip containing 0.0125 per cent
emulsion also found higher residues in unshorn sheep. Fat of shorn
sheep two weeks after dipping contained 2.5 ppm and unshorn sheep 5.0
ppm (average figures) and residues; in fat of shorn sheep were
negligible in eight weeks and in unshorn sheep in 12 weeks. From these
results it seems that fleece is a reservoir for continued absorption.
Swine appear to store gamma-BHC less than sheep. Fat of animals
sprayed with normal dosages after one week contained 0.03 and 0.05
ppm, and those after twice the dosage 0.5 and 0.06 ppm (USDA, 1965).
Fat samples taken by biopsy showed 0.1 to 0.3 after 28 days (USDA,
1965a).
Gyrisko et al. (1959) reviewed the effects on flavour and residues in
milk of feeding low residues on hay to dairy cattle. In agreement with
earlier work they found that as much as 10 ppm in feed did not cause
off-flavour in milk. Feed which contained 0.6 ppm at harvest had
reduced to non-detectable levels when feeding was initiated.
Unfortunately, no recent information is available on the feeding to
lactating animals of the very low levels of residues which may be
present on cull root crops, sugar beets and forage. Most countries do
not register or recommend the use of gamma-BHC in buildings in which
dairy animals or dairy products are kept and processed, or on
lactating animals.
Residues in food moving in commerce
Few figures are available for individual food commodities. Total diet
studies in the USA (Williams, 1964; Duggan et al., 1966; Cummings,
1966) and Canadian restaurant meal surveys indicate very minor
incidence in diets of these countries. The USA five-year survey using
three different methods of analysis, rarely recorded residues in
excess of 0.01 ppm.
By contrast, British investigations of residues in home-killed mutton
in 1962 (Egan, 1965) indicated a constant low level of residue in fat
of meat in 61 samples. Thirty-seven samples contained not more than
0.1 ppm, 48 not more than 0.3 ppm and three samples between 1.1 and
2.0 ppm. The Reports of the Government Chemist (UK) for 1964 and 1965
contain further useful information on the amount of total BHC isomers
in food of origin from different countries:
1964 1965
Food Country Number of Range Arithmetic Number of Range Arithmetic
samples ppm mean ppm samples ppm mean ppm
Butter Australian 45 0.0-0.40 0.02 45 0.0-0.11 0.02
Danish 30 0.0-0.20 0.05 24 0.0-0.08 0.04
New Zealand 45 0.0-0.02 <0.01 40 0.0-0.04 0.01
UK 18 0.0-0.20 0.05 18 0.0-0.13 0.04
Milk UK 60 0.00-0.18 0.003 85 0.0-0.022 0.0045
Mutton Argentine 17 0.00-15.5 1.1 13 0.0-1.6 0.25
Kidney Australian 15 0.00-0.05 0.01 6 <0.05 -
Fat New Zealand 12 0.00-0.25 0.05 10 <0.05 -
UK 107 0.00-5.1 0.43 128 0.0-10.6 0.46
Beef Argentine 13 0.0-1.1 0.15 22 0.0-0.80 0.13
Kidney UK 66 0.0-0.3 0.05 59 0.0-0.42 0.04
Fat
Corned
beef Argentine 20 0.0-0.1 0.03 26 0.0-0.1 0.02
Hardon (1960) reported that beans sampled in commerce contained 0.1
ppm gamma-BHC, and canned tomatoes 0.13 ppm (Alessandrini at al.,
1959).
Fate of residues
(a) In soil
Gamma-BHC is relatively volatile and moderately soluble in water, for
which reasons it is one of the less persistent organochlorine
pesticides. It is generally considered that three years should elapse
after heavy applications and before root crops are planted in treated
soils; but soil type has an important influence on the rate of
disappearance from soil (Harris, 1966; Duffy, 1966; Lichtenstein &
Schulz, 1965; Lichtenstein et al., 1963, 1964; Harris & Lichtenstein,
1961, etc.). Some of the early work may have been misleading due to
inadequacies in the analytical method and this has been recently
reviewed (Yule, Chiba & Morley, 1967). Enzymatic processes have been
responsible for the detoxification of insects (e.g. Bradbury, 1957;
Clark et al., 1966; Ishida & Dahm, 1965) and in the rat (Grover &
Sims, 1965). Gamma-pentachlorocyclohexane has been identified as a
decomposition product in soil. It is 103 less toxic to insects than
gamma-BHC. Soil microorganisms and alkaline soils are involved in this
degradation process (Yule, Chiba & Morley, 1967). Further work is
required to determine whether the disappearance of gamma-BHC from soil
can be achieved in a reasonable period by land and crop management
practices.
(b) In plants
It has been demonstrated that gamma-BHC can be absorbed by roots and
translocated to leaves (Bradbury & Whitaker, 1956) where it can kill
insects (Howe, 1950; Starnes, 1950; Shapiro, 1951; Ehrenhardt, 1954).
Jameson (1959) concluded that (when used as a dressing) the
insecticide penetrates into seeds and is absorbed by the cotyledons.
Way (1959) compared the action of gamma-BHC with other organochlorine
seed dressings and Bradbury (1963) the action on cabbage and wheat, in
both of which the gamma-BHC fell off rapidly.
There appears to be very little information on metabolism and
degradation in plants. Such information is necessary because
toxicological evaluation has been based solely on feeding the parent
compound.
(c) In storage and processing
Because of the volatility and solubility of gamma-BHC, storage and
processing, including cooking, would be expected to result in losses.
The literature contains divergencies on this subject probably due to
the use of non-specific analytical methods in the early work and
because the losses were closely dependent upon the temperatures,
degrees of exposure to free air and other conditions pertaining in the
particular test.
From his own studies Van den Driessche (1958) suggests that in the
canning and cooking process 23 per cent of residues of BHC (presumably
13 per cent gamma) in treated vegetables would be lost in cooking
water, and in the canning of beans and peas 63 per cent of the residue
might be dissipated.
Information on losses of gamma-BHC during the storage of cereals and
on its carry over into flour and bread were reviewed in some detail at
the second session of the FAO Working Party on Pesticide Residues
(PL/1965/12). Losses during storage depend upon the physical
conditions (e.g. Bridges, 1958) being high in the tropics and in well
ventilated stores (e.g. Giles, 1964) and low under cooler closed
conditions (e.g. Gunther, Lindgren & Blinn, 1958; Schesser, Priddle &
Farrell, 1958). During the milling of flour, the losses depend on the
particular processes used. Preliminary cleaning, brushing and washing
have been shown to remove insecticide and several workers have found
appreciably lower ppm residues in the flour (i.e. up to 50 per cent)
than in the original grain, although the residues in certain
by-products (e.g. bran for feeding to animals) may contain a higher
ppm (Bridges, 1958; Feuersinger, 1960, 1962; Schesser et al., 1958;
Sellke, 1952; Zeumer & Neuhaus, 1953). There are few data on losses of
residues during bread making. Bridges (1958a) found that flour
containing 2.5 ppm contained 40 per cent of this as unchanged residue
in the bread with the remainder probably as tri-, di-, and mono-
chlorbenzenes.
Loss from Cheddar and Stilton cheese was slow (Bridges, 1958), about
40 per cent remaining after 44 weeks. Penetration into both types was
slow but no chemical changes were found in either raw or cooked
cheese. Johnson (1958) reported the results of processing on residues
in cucumbers used for pickles. It was concluded that final residues in
pickles would be less than 0.5 ppm from field treated cucumbers, but a
concentration of 10 ppm could result if harvested cucumbers were
sprayed directly.
Method of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of gamma-BHC, together with residues of a
number of other compounds, including other BHC isomers. An example is
the AOAC system (1966) in which acetonitrile partition and Florisil
column clean-up are identified and measured by gas chromatography
coupled with thin layer or paper chromatography. Alternative clean-up
systems; e.g that of de Faubert Maunder et al. (1964) using
dimethylformamide, and other methods of confirmation of identity,
e.g., using infra-red spectrophotometry, are also available. The
methods are in general sensitive to 0.002 ppm of gamma-BHC in milk and
0.02 ppm of gamma-BHC in most other foods, though under favourable
conditions greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The residue data reviewed at the meeting, for the most part, were
produced between 1948 and 1959 before improved specific methods of
analysis were available. These results can only be used as a guide,
subject to review, when better data are available. When considering
information on residues of this pesticide, the meeting found it
necessary to rely on the expert opinion of its members, rather than
conclusions that could be derived exclusively from obsolete or
incomplete data.
The properties of this pesticide are such that it was assumed that
substantial losses of residue would occur in storage, processing and
cooking of foods such as some vegetables, meat and cereals, but only
minor losses in the case of small fruits and milk products.
Tolerances recommended for residues of gamma-BHC based on these guide
lines are:
Small fruits 3.0 ppm )
)
Vegetables 3.0 ppm )
) Temporary tolerance
Cereals 0.5 ppm )
)
Milk products 0.1 ppm (fat basis) )
Meat and poultry 0.7 ppm (fat basis) ) Practical
) residue
Fluid milk 0.004 ppm ) level
These should be reviewed in the light of additional data, but in
any event not later than within three years.
Further work
1. Guidance is required from countries on uses still of value in
food production or protection in storage and transit.
2. Data are needed on the disappearance of residues during storage
and processing of food, and information on the chemical nature of
terminal residues on food as consumed.
3. Residue data, using specific methods of analysis which will
identify metabolites and gamma-BHC, after supervised trials and
on residues in food moving in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Barke, A. (1950) Tierärzliche Umschau, 5, 611
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff,
R. D. (1960) J. Agr. Food. Chem., 8, 439
Coper, F., Herken, H. & Klempau, I. (1951) Arch. exp. path.
Pharmakol., 212, 463
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life sciences, 5,
47
Dallemagne, M. J. & Philippot, E. (1948) Arch. int. pharm. Ther.,
76, 274
Doisy, E. A. & Bocklage, B. C. (1949) Proc. Soc. exp. Biol. (N.Y.),
71, 490
Ely, R. E., Underwood, P. C., Moore, L. A., Mann, F. D. & Carter, R.
H. (1953) J. Amer. vet. med. Ass., 123, 448
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Pharmacol.
exp. Ther., 100, 59
Ghazal, A., Koransky, W., Portig, J., Vohland, H. W. & Klempau, I.
(1964) Arch. exp. path. Pharmakol., 249, 1
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel Forsch., 1, 356
Hoffman, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth., 9, 387
Kastli, P. (1955) Féd. Int. des Laiteries Réunies, Bruxelles,
Report No. 3
Kewitz, H. (1952) Arch. exp. path. Pharmakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. path. Pharmakol., 227, 183
Klosa, J. (1950) Die Pharmazie, 5, 615
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A.J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A.J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Lendle, L. & Schneider, H. H. (1950) Arch. exp. path. Pharmakol.,
210, 119
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F., jr (1957) Arch. Path.,
64, 614
Paccagnella, B., Brati, L. & Gavazzini, C. (1966) Unpublished report
of the Institute of Hygiene of Ferrara, Italy
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Sedlak, V. A. (1965) Toxicol. appl. Pharmacol., 7, 79
Truhaut, R. (1954) Communication au symposium intern. de la prévention
du cancer, Sao Paulo
Van Asperen, K. (1957) C.R. 4é Congrčs Intern. de Lutte control les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wasserman, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu, G.
& Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 386
REFERENCES PERTINENT TO AGRICULTURAL DATA
Alessandri, M. E., Doretti, M. & Lanforti, G. F. (1959) Insecticide
residues in samples of peeled, canned tomatoes coming from experiments
carried out in Italy in 1958. Presented to 17th IUPAC Congress,
Munich, reported by Maier-Bode, H., Gesunde Pflanzen, 11, 225-229
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49, 222-30
Beran, F. (1961) Das Problem der Pflanzenschutzmittelruckstande in
europaischer Sicht. - Pflanzenschutzberichte (Wien) 27, 11-50
Bradbury, F. R. (1957) Absorption and metabolism of BHC in susceptible
and resistant houseflies. J. Sci. Food Agric., 8, 90-96
Bradbury, F. R. (1963) The systemic action of benzene hexachloride
seed dressings. Ann. appl. Biol., 52, 361-370
Bradbury, F. R. & Whitaker, W. O. (1956) The systemic action of
benzene hexachloride in plants; quantitative measurements. J. Sci Food
Agric., 4, 248
Brett, C. H. & Bowery, T. G. (1958) Insecticide Residues on
Vegetables. J. Econ. Ent., 51, 818-821
Bridges, R. G. (1958) Fate of labelled insecticide residues in food
products VI. Retention of gamma-Benzene hexachloride by wheat and
cheese. J. Sci. Food Agric., 9, 431-439
Bridges, R. G. (1958a) Fate of labelled insecticide residues in food
products VII. The fate of gamma-Benzene hexachloride residues in flour
during baking. J. Sci. Food Agric., 9, 439-448
Clark, A. G., Hitchcock, M., Smith, J. N. (1966) Metabolism of
gammexane in flies, ticks and locusts. Nature, 209, 103
Collett, J. N. & Harrison, D. L. (1963) Lindane residues in sheep
following dipping. N.Z. J. Agric. Res., 6, 39-42
Cummings, J. G. (1966) Pesticides in the total diet. Residue Reviews,
16, 30-45
Deema, P., Naber, E. D. & Ware, G. W. (1965) Residues in hen eggs from
vaporizing insecticide tablets. J. Econ. Ent., 58, 904-906
Duffy, J. R. & Wong, N. (1966) Insecticide Residues and their
metabolites in Atlantic Soils. In press.
Duggan, R. E., Barry, H. C. & Johnson, L. Y. (1966) Pesticide Residues
in total diet samples. Science, 151, 101
Egan, H. (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Food Agric., 16,
489-498
Ehrenhardt, H. (1954) Uber die Werkung des Hexachlorocyclohexans als
systemisches Insektizid. Anz. Schadlingsk., 27, 1
Fahey, J. E., Wilson, M. C. & Rusk, H. W. (1960) Persistence of BHC,
lindane, and thiodan residues when applied to alfalfa to control the
meadow spittlebug. J. Econ. Ent., 53, 960-1
Fairchild, H. E. & Dahm, Paul A. (1955) Lice control on chickens with
chlorinated hydrocarbon insecticides. J. Econ. Ent., 48, 141-146
de Faubert Maunder, M. J., Egan, H. & Godly, R. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Feuersenger, M. (1960) Uber die Bestimmung von
Schadlingsbekamfungsmitteln in Lebensmitteln. Bundesgesundheitsblatt,
NR10, 149-152
Feuersenger, M. (1962) Ruckstandfragen bei der Anwendug von
Kontaktininsektizieden in Getreideschutz Nachrichtenbl. dstch.
Pflanzenschutzdienstes (Braunschiveig), 14, 189
France, H. L., Treece, R. E. & Ware, W. G., (1961) Chemical and
biological assays of lindane residues on alfalfa. J. Econ. Ent., 54,
642-3
Giles, P. H., (1964) Lindane contamination in stored sorghum and
millet in N. Nigeria. Trop. Sci., 6, 113-121
Grover, P. L. & Sims, P. (1965) The metabolism of gamma-2,3,4,5,6
pentachlorocyclohex-1-ene and gamma-hexachlorocyclohexane in rats.
Biochem J., 96, 521-525
Gunther, F. A., Lindgren, D. L. & Blinn, R. C. (1958) Biological
effectiveness and persistence of malathion and lindane used for
protection of stored wheat. J. Econ. Ent., 510 843-4
Gyrisco, G. G., Norton, L. B., Trimberger, G. W., Holland, R. F.,
McEnerney, P. J. & Muka, A. A. (1959) Effects of feeding low levels of
insecticide residues on hay to dairy cattle on flavor and residues in
milk. Agric. & Food Chem., 7, 707-711
Hardee, D. D., Huddleston, E. W. & Gyrisco, G. G. (1963) Initial
deposit and disappearance rates of various insecticides as affected by
forage crop species. J. Econ. Ent., 56, 98-101
Hardon, H. J. (1960) Pestizidruckstande in Marktmustern von Gemuse und
Abst. - Verhandl. IV. Inter. Congress of Plant Protection, Hamburg,
Band 2. (Braunschweig, 1960) 1671-1674
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in southwestern Ontario. Agric. & Food Chem., 14,
398-403
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent., 54,
1038-1045
Harrison, D. L., Poole, W. S. H. & Mol, J. C. M. (1963) Observations
on feeding lindane-fortified mash to chickens. N.Z. Veterinary
Journal, 11, 137-140
Howe, W. L. (1950) Reactions of squash vine borer to certain
insecticidal soil treatments. J. Econ. Ent., 43, 549
Ishida, M. & Dahm, P. A. (1965) Metabolism of Benzene Hexachloride
Isomers and Related Compounds in Vitro. I. Properties and Distribution
of the Enzyme. J. Econ. Ent., 58, 383-392
Ivey, M. C., Roberts, R. H., Mann, H. D. & Claborn, H. V. (1961)
Lindane residues in chickens and eggs following poultry house sprays.
J. Econ. Ent., 54, 487-488
Jackson, J. B., Ivey, M. C., Roberts, R. H. & Radeleff, R. D. (1959)
Residue studies in sheep and goats dipped in 0.025% lindane. J. Econ.
Ent., 52, 1031-1032
Jameson, H. R. (1959) The mechanism of the control of turnip flea
beetle with benzene hexachloride dressings of Brassicae seed. J. Sci.
Food Agric., 9, 590
Johnson, M. R. (1958) Residue changes during the fermentation and
processing of pickles. Food Technology June, 1958
Lichtenstein, E. P. (1959) Absorption of some chlorinated hydrocarbon
insecticides from soils into various crops. Agric. and Food Chem., 7,
430-433
Lichtenstein, E. P. & Schulz, K. R. (1959) Persistence of some
chlorinated hydrocarbon insecticides as influenced by soil types, rate
of application and temperature. J. Econ. Ent., 52, 289-293
Lichtenstein, E. P., Schulz, K. R. & Cowley, G. T. (1963) Inhibition
of the conversion of aldrin to dieldrin in soils with
methylenedioxyphenyl synergists. J. Econ. Ent., 56, 485-489
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1964) Effect of
formulation and mode of application of aldrin on the loss of aldrin
and its epoxide from soils and their translocation into carrots. J.
Econ. Ent., 57, 133
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13, 57-62
Lilly, J. H. & Fahey, J. E. (1956) Translocation of BHC from
high-dosage soil treatments applied before planting. J. Econ. Ent.,
49, 815-818
Maier-Bode, H. (1963) Vergiftungen durch
Pflanzenschutzmittel-Ruckstande-Pflanzenschutzberichte (Wien) 30,
49-77
Maier-Bode, H. (1964) Pflanzenschutzmittel-Ruckstande. Insektizide,
Verlag Eugen Ulmer, Stuttgart. 455 pp.
Penn. State Univ. Agric. Exp. Sta. (1963) Pesticide residue
investigations on raw agricultural commodities. Penn. State Univ.
Bulletin 703
San Antonio, J. P. (1959) Demonstration of lindane and a lindane
metabolite in plants by paper chromatography. Agric. & Food Chem., 7.
322-325
Schesser, J. H., Priddle, W. E. & Farrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion, and lindane. J. Econ. Ent., 51, 516-518
Sellke, K. (1952) Kornkofereinstaubmittel und Ihr Verhalten in
behandelten Getreide Nachrichtenbl. dstch. Pflanzenschutzdienst
(Berlin) N.F.6, 121-127
Shapiro, I. D. (1951) On the toxic action of hexachlorane on insects
through the plant. Dokl. Akad. Nauk. SSSR(NS) 80, 481
Stadelman, W. J., Liska, B. J. & Langlois, G. C., Moster, T. G. &
Stemp, A. R. (1964) Persistence of chlorinated hydrocarbon residues in
chicken tissues and eggs. Poultry Science, 43, Sept. 1964
Starnes, O. (1950) Absorption and translocation of insecticides
through the root system of plants. J. Econ. Ent., 43, 338
Treece, R. E. & Ware, G. W. (1965) Lindane Residues on Alfalfa and in
Milk. J. Econ. Ent., 58, 218-219
USDA (1965) Distribution of Lindane Residues in Swine. Agr. Res. Serv.
Ent. Res. Div. Pesticide Chemicals Research Branch Spec. Report
PC-B-65-10
USDA (1965a) Lindane Residues in Swine. Agr. Res. Serv. Ent. Res. Div.
Pesticide Chemicals Research Branch Spec. Report PC-B-65-17
Van den Driessche, S. (1958) Influence of sterilization on
hexachloroxyclohexane (HCH) residues in preserved foods. Rev.
Conserve 13 (1), 27
Ware, G. W. & Naber. E. C. (1961) Lindane in eggs and chicken tissues.
J. Econ. Ent., 54, 675-677
Ware, G. W. & Naber, E. C. (1962) Lindane and BHC in egg yolks
following recommended uses for louse and mite control. J. Econ. Ent.,
55, 568-570
Way, M. J. (1959) Experiments on the mode of action of insecticidal
seed-dressings, especially against leptohylemyia coarctata fall.,
muscidae, the wheat bulb fly. Ann. appl. Biol., 47. 783-801
Williams, S. (1964) Pesticide residues in total diet samples.
J.A.O.A.C., 47, 815-821
Yule, W. N., Chiba, M. & Morley, H. V. (1967) Fate of Insecticide
Residues - The Decomposition of lindane in soil. J. Agr. Food Chem.
(In press)
Zeumer, H. & Neuhaus, K. (1953) Die Bestimmung von
Kontaktinsektizieden, Getreide und Mehl Heft 8; Beilage 2.
Wochenschrift Die Muhle, Detmold
