
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
PHENYLMERCURY ACETATE (and other ORGANOMERCURY COMPOUNDS)
IDENTITY
Synonym
PMA
Formula
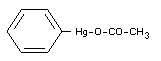 Explanatory Note
Phenylmercury acetate is an important example of twenty or more
organomercury compounds used as fungicides in agriculture. They have
the general structure R-Hg-X where R is an alkyl, alkoxyalkyl or aryl
organic radical and where the bond with the X group has the character
of a salt with a substance (acid, amide, phenol, etc.) having a
dissociating hydrogen ion.
Some other important compounds, listed in accordance with their
organic radicals, are as follows:-
a) methylmercury compounds Synonym
methylmercury dicyandiamide MMD Panogen(R)
methylmercury iodide MMI
b) ethylmercury compounds
ethylmercury chloride EMC
ethylmercury urea EMU
ethylmercury phosphate EMP
ethylmercury p-toluene sulphonamide EMTS
ethylmercury sulphate EMS
c) alkoxyethylmercury compounds
ethoxyethylmercury chloride
methoxyethylmercury chloride MMC
methoxyethylmercury acetate
d) arylmercury compounds
phenylmercury chloride PMC
phenylmercury iodide PMI
phenylmercury urea PMU
phenylmercury
methanodinaphtho-disulphonate Murfixtan,(R) PMF
phenylmercury salicylate
phenylmercury
NN-dimethyldithiocarbamate Phelam(R)
tolylmercury chloride TMC
N-tolylmercury
p-toluenesulfonanilide TMTS
Physical properties
The alkyl compounds are generally the more volatile and the aryl the
least volatile. The solubility in water of the aryl compounds also is
lower than the corresponding alkyl ones. The stability and
particularly the sensitivity to reducing agents with the eventual
production of metallic mercury, varies from one compound to another.
(R) = proprietary name.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastrointestinal tract (Fitzhugh et al., 1950, Prickett et al., 1950).
The major route of elimination of mercury after oral, intramuscular or
intravenous administration of PMA is by way of the bile and excretion
into the alimentary tract (Berlin & Ullberg, 1963; Prickett et al.,
1950).
PMA given orally and intramuscularly to 87 chicks, intramuscularly to
12 rats, and intravenously to 4 dogs, was absorbed apparently
unchanged. After about 96 hours no PMA could be detected in the
tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960).
The influence of 2,3-dimercaprol (BAL), 0.4 mg/kg, on the body
distribution of mercury was studied in mice. When
phenyl-203Hg-acetate was given as a single intravenous dose (0.5
mg/kg as Hg) or as daily injections (1 and 3 mg/kg as Hg for 16 days),
it was shown that BAL effected a redistribution of the body mercury
load. A persistent increase in the concentration of mercury was noted
in the brain, liver and muscle, while a decrease was found in the
kidney (Berlin & Rylander, 1964).
In comparison with methylmercurydicyanamide (MMD), a steady state of
excretion was reached much faster when rats were given PMA;
furthermore, a much lower accumulation of Hg was found in the brain of
rats after the administration of PMA than after MMD (Gage, 1964).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al.,1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at dosages of
1-2.5 mg/kg body-weight every other day for 4 weeks. The animals
showed gradually increasing apathy, loss of weight, and finally
neurological signs - (ataxia and paresis), especially at the higher
dosages. Histopathological examination revealed damage to the granular
layer and the Purkinje cells of the cerebellum, and to the spinal cord
(Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130 days,
the total amount of mercury consumed during the experimental period
being 770 mg. The animal showed marked growth depression and died
after 130 days. Chemical analysis revealed large amounts of mercury in
the organs - 29 mg/kg organ-weight in the kidney, 0.52 mg/kg in the
liver and 5.18 mg/kg in the gastro-intestinal tract - whereas a
control rabbit showed only 0.06 mg/kg in the kidney and traces in the
liver. Another rabbit fed a diet containing PMA for 100 days received
a total amount of 6.9 mg of mercury. There was no abnormality in
appearance or growth. The contents of mercury in the organs were 0.455
mg/kg in the kidney and 0.042 mg/kg in the liver (Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670 days
and consumed a total of 20.4 mg during the whole experimental period.
No ill-effects were observed in general appearance or growth. The
mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments
It is clear from the biochemical studies that PMA may give rise to
mercury accumulation in the tissues and the long-term study in the rat
failed to demonstrate a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per day,
produced a slight effect in the rat. Even if this figure were to be
adopted as a maximum no-effect level and the customary safety factor
applied this would give an acceptable daily intake for man of 0.00005
mg/kg body-weight. This is tantamount to zero. It is undesirable that
for the general population there should be any increase in the natural
intake of mercury.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) General properties
The suitability of a particular organomercury compound for a
particular purpose is partly dependent upon its physical properties.
Phenyl mercury acetate and other aryl compounds, which have a low
volatility, are more suitable where persistence is required. Under
certain conditions however (e.g. in enclosed seed dressing machinery)
their higher volatility enables the alkyl compounds to be applied more
effectively. Phillips, Dixon & Lidzey (1959) list the vapour pressures
of many of these compounds and compare their volatilities under
different conditions.
(b) Pre-harvest treatments
Organomercury compounds are used in seed dressings, orchard sprays,
foliar dusts and as glass house aerosols for the control of fungal
diseases. Since analysis seldom distinguishes between organically
bound and inorganic mercury, it is worth noting also that inorganic
mercury compounds are used for soil and root treatment. Powdered and
liquid preparations containing both alkyl and aryl mercury compounds
are used as cereal seed dressing. Ethylmercury phosphate is fairly
specifically used on beet seed and this and other seed dressings are
likely to lead to residue problems. Phenylmercury foliar dusts are
used to control rice blast disease.
(c) Post-harvest treatments
Organomercurials are not used post-harvest other than as bulb, tuber
and cereal seed dressings. It is possible that small residues may be
picked up e.g. by fruits, from paper wrappings or other containers
which have been treated with organomercurial fungicides.
(d) Other uses
Organomercury compounds, particularly phenylmercury salts, are used
widely as fungicides in industry, e.g. on wood pulp, paper and various
building materials.
Tolerances
Existing tolerances all refer to residues found and expressed as
mercury (Hg) without distinction between the forms in which the metal
may actually be present in food. Uses of organomercurials which could
lead to a detectable residue in food produce are not allowed in the
United States.
Residues resulting from supervised trials
(a) Pre-harvest treatments
A number of workers have measured residues in apples (summarized by
Miller, 1957) and tomatoes, (e.g. Beidas & Higgins 1957, 1959; Ross
& Stewart, 1960; Stone, 1962). Foliar applications of organomercurials
in general lead to translocation of the mercury. Pickard & Martin
(1963) studied the uptake of mercury by roots, leaves and fruits after
applications at commercial rates. The roots of lettuce and dwarf
bean plants accumulate mercury from nutrient solution containing
phenylmercuric acetate, but little translocation to the foliage
occurs. Root treatment of cauliflower seedlings with calomel or
mercuric chloride before planting lead to absorption by the roots but
the curds are uncontaminated. Carrots grown in soil treated with
mercuric chloride contained up to 0.05 ppm and roots from
calomel-treated soil showed 0.02 ppm Hg when seed was sown immediately
after soil treatment; delay in seeding eliminated contamination.
Lettuces, dwarf beans, carrots, potatoes and turnips from field
experiments using calomel and mercuric oxide soil treatments showed
mercury residues not exceeding 0.03 ppm. Apple leaves absorbed
mercury deposited as phenylmercuric acetate, within a few days.
Mercury was found in young coffee and citrus lime leaves that
emerged after spraying with phenylmercuric acetate, indicating
translocation. Broad bean plants sprayed with phenylmercuric
acetate at early flowering later showed 0.02 ppm in the pods, 0.04 ppm
in the seeds and 0.07 ppm Hg in the roots. Application of
phenylmercuric acetate to the leaves of potato plants led to
residues in the tubers (0.1 ppm in peel, 0.18 ppm Hg in flesh) and
roots (1.2 ppm Hg). Apples from commercially sprayed orchards
contained 0.05 ppm Hg distributed between the peel, flesh and core.
Five applications of phenylmercuric acetate under experimental
conditions, gave 0.24 ppm on whole fruits; one third of the mercury
was located in the flesh. Mercury deposited on the surface of the
fruits was largely held in the cuticle; much of the mercury in the
flesh arose from translocation from the leaves. Mercury was detected
in the fruitlets (0.4 ppm) and young leaves (0.07 ppm) of trees
sprayed the previous year. The bark of trees treated for six
consecutive years contained 4 ppm Hg. Naturally-occurring mercury in
soils varied between 0.05 and 0.12 ppm. Soil from beneath sprayed
apple trees contained 0.2 ppm Hg. Soils treated with inorganic
mercurials showed up to 2 ppm Hg (untreated 0.05 ppm).
In Japan the stage of plant growth affected the amount of residual Hg
in rice. Unpolished rice harvested from the rice plant sprayed with
PMA before the plant comes to ear contained 0.12 ppm of Hg, whereas,
the grain harvested from the plant treated with PMA after the plant
comes to ear contained 0.23 ppm of Hg. Epps (1966) examined rice and
various milling by-products, from rice treated in field tests with
phenylmercury acetate; residues ranged from 1.3 ppm Hg in straw and
0.8 ppm in bran to 0.1 - 0.2 ppm in the endosperm.
Smart (1963, 1964) measured residues in the eggs, flesh, etc., of hens
fed wheat treated with methylmercury dicyandiamide and in potatoes
following foliar spray application with phenylmercury chloride. Only
about 10 per cent of an organomercury compound on dressed grain was
removed by washing. Translocation of residues from potato foliage to
tubers has been demonstrated by Ross & Stewart (1964). Egan & Lidzey
(1960) and Bland & Egan (1963) showed that successive treatments of
tomatoes with phenylmercury salicylate under commercial greenhouse
conditions resulted in residues of the order of 0.01 (n + 1) ppm where
"n" is the number of treatments up to the eighth; and falls thereafter
by about 0.01 ppm for each subsequent treatment.
Mercury residues measured in Japan (direct communications, 1965) were
found to be 0.098 + 0.008 ppm Hg in peel and 0.025 + 0.008 ppm
in flesh of mandarin orange. Untreated fruit contained 0.03 - 0.05 ppm
Hg in peel and 0.01 - 0.02 ppm Hg in flesh. The residues in mandarin
oranges sprayed with water-soluble mercurial fungicides (PMA or PMF)
were generally greater than those in fruit sprayed with less
water-soluble fungicides (PMI or PMO). After five foliar applications
of MMC (12.5 ppm as Hg) to peach trees, amounts of Hg were in the
range of 0.21 - 0.25 ppm in peel and 0.02 - 0.04 ppm in flesh. Similar
treatments of a mixture of PMPS, EMP, and EMU (12.5 ppm as Hg)
resulted in an Hg residue in the range of 0.27 - 0.28 ppm in peel and
0.06 - 0.07 ppm in flesh.
Residues in food moving in commerce
Since 1961 surveys have been undertaken in Sweden on the occurrence of
mercury in eggs, game, fish and various other foodstuffs. This has
been associated with enquiries into possible effects on wildlife from
the use of mercury fungicides in industry and agriculture. The
residues found in human food have ranged from a mean of 29 parts per
thousand million in eggs marketed in Sweden, to over 20 ppm in the
livers of some 10 per cent of the game birds examined. Wheat, thought
to be untreated, from various parts of the United States of America,
examined by atomic absorption spectroscopy by Pappas and Rosenberg
(1966) showed 0.013 to 0.13 ppm Hg and 1.5 ppm Hg in commercially
treated samples. The same authors found an average of 0.02 ppm in
haddock fillets while eggs were virtually mercury-free (less than
0.005 ppm). In the examination of various foods in Japan (direct
communication) residues found were: polished rice 0.04 - 0.07;
unpolished rice 0.07 - 0.14; wheat flour 0.05; fruits and vegetables
0.02 - 0.06; meats 0.03 - 0.16; fish 0.00 - 0.10, (tuna 0.3 - 0.55);
milk 0.01 and hen eggs 0.08. Small amounts of mercury are very
widespread in nature. The extent of general environmental
contamination has been reviewed by Goldwater (1964) who quotes results
of residue analysis of food including bananas (0.31 ppm) canned
chicken (0.15 ppm) butter (0.14 ppm) Cheddar Cheese (0.082 ppm)
white bread (0.08 ppm) beer (0.04 ppm) and rice (0.02 ppm).
In each of these instances the figures are quoted as ppm Hg. Figures
for "organic" and "inorganic" mercury have rarely been obtained, there
appear to be none for residues as specified organomercury compounds.
Residues at the time or consumption
The meeting had no data on the effects of storage, cooking and other
processing. Reductions no doubt occur from volatilisation during
storage and from washing of crops and trimming (e.g. of outside parts
of fruits and vegetables) prior to cooking. Some losses during
cooking, also are likely due to the volatility of these compounds, but
figures for such losses were not available.
Methods of residue analysis
There is an extensive literature on mercury residue analysis, the
greater part of which is concerned only with total mercury and not
with intact organomercury residues as such. There is a wide
divergence between the toxicities of the various mercury compounds and
often a lack of information concerning the chemical nature of the
residues (e.g. in edible animal material) after the use of a given
compound.
(a) Total mercury (inorganic and organic)
Relatively simple methods, such as the Reinsch test, have long been
used to detect traces of metals including mercury and, with
supplementary confirmatory tests, can be used to distinguish mercury
from other residues and approximately to measure them. Very many of
the modern total mercury methods are based on extraction of the metal
by organic complexing agents, principally diphenyl thiocarbazone
(dithizone) into chloroform solution (e.g. Kanazawa & Sato, 1959).
Examples are the IUPAC method of Ljunggren and Westermark (1960) for
the determination of mercury foodstuffs and the method of the
Association of Official Analytical Chemists (1965), both based on the
method of Klein (1952). Conditions can be adjusted so that mercury is
selectively extracted. There are a number of variations of the method
of measuring the extracted mercury complex. These are normally based
on the intensity of colour of the complex solution, or of the
uncomplexed dithizone remaining when a measured excess is used;
alternatively a combined titrimetric-colorimetric procedure is used in
which small measured amounts of standardized dithizone are added
successively to the prepared sample extract until no reagent colour
change occurs. Such methods, which are sensitive to 0.01 ppm of Hg or
less in favourable cases, have been applied to a wide range of
biological tissue including many foods.
A source of difficulty, which arises also in the more modern
radioactivation method of analysis (q.v.), lies in the relatively high
volatility of mercury and its compounds. Biological tissue is normally
first oxidized, e.g. by warming with nitric acid. If too much heat is
applied or is allowed to generate, some of the mercury may be lost
from the sample as in some of the earlier work of Klein, in which the
condensate was trapped in a closed (reflux) oxidation system the main
digest thus becoming more concentrated with respect to acid with a
consequent elevation in boiling point. Gorsuch (1950) has made a
thorough study of losses in wet ashing, using radiochemical methods;
he has in this way shown the need to condense digestion vapours and
return them to the main digest. Gutenmann & Lisk (1960) and Gouverneur
& Hoedman (1964) used a modified Schoniger combustion method in place
of wet digestion. Conditions which emphasize cool oxidation were
described by Abbott and Johnson (1957), and examined collaboratively
for apples and tomatoes in Britain by the Joint Mercury Residues Panel
(1961). A further collaborative study of a method for amounts of
mercury down to 0.5 micrograms in organic matter has been described by
the Society in Analytical Chemistry (1965). Truhaut and Boudene (1963)
have used a simple flask in which oxidation, complex extraction and
colorimetric measurement can be completed without transfer.
Radioactivation methods of residue analysis are sensitive to at least
0.01 ppm Hg and avoid the need for oxidation and extraction steps.
However, preliminary removal of water from biological tissue may be
necessary and this again poses the problem of volatility of mercury
and its compound. Erwall & Westermark (1964) described the application
of this technique to foods. An atomic pile is necessary for the
activation stage and where this is available the technique can be
widely applied provided the dehydration stage is satisfactory with a
chemical separation the sensitivity level is 0.005 ppm on a 0.2 gram
sample. This sensitivity could also be obtained with a gamma counter
without spectrometry. If the irradiated sample is counted direct
without chemical separation, a gamma spectrometer would have to be
used. The sensitivity would then depend on the other short-lived
activities produced (e.g. sodium-24) and 0.5 ppm on a 0.2 gram sample
appears to be reasonable. A typical conventional dithizone method has
a sensitivity of 0.02 ppm on a 10 gram sample. Neutron activation
analysis using Hg-203 (half-life 47 days) would be less sensitive than
using Hg-197 by a factor of 20 and a longer irradiation time would be
needed. In the method of Ljunggren & Westermark (1960) biological
material is sealed into quartz tubes, activated and 77-keV gamma
radiation emission from Hg-197 measured after a cooling period of two
days. This method is sensitive to five nanograms of Hg.
Schachter (1966) has described the use of atomic absorption
spectroscopy to mercury vapour in the cold state. High sensitivity is
achieved using the whole of the ultraviolet spectrum of a quartz
mercury vapour lamp. Thus one gram of ground wheat is combusted in a
Schoniger flask and the mercury vapour collected in dil. HCl,
converted to sulfide, pyrolysed in a train of nitrogen in a small
furnace at 650°C and the mercury vapour cooled and measured. Hamilton
& Ruthven (1966) have described a field apparatus for the detection
and estimation of organomercury dusts and vapours in the atmosphere; a
similar official method is published in Britain.
(b) Organically bound mercury
Limited work has been done on the separation and measurement of
unchanged organomercury compounds. Miller, Lissis & Csonka (1958)
extract phenylmercury compounds from animal's tissue with chloroform
and determine the phenylmercury adsorptiometrically as the dithizone
complex. Kimura & Miller (1964) use different extraction solvents for
(a) phenyl and alkyl mercurials and (b) diphenyl dialkyl mercurials
and (c) ionic mercury. The reaction of dithizone with organomercury
compounds has been reviewed by Irving & Cox (1961). It should be
possible to devise extraction procedures in which intact radicals such
as phenyl-mercury can be isolated (e.g. as the chloride) and then
separated by paper. Electrophoretic separations have also been studied
or thin layer chromatographic procedures and identified and measured.
RECOMMENDATION FOR TOLERANCES
Since no acceptable daily intake level can be given for mercury it is
not possible to recommend a tolerance or a temporary tolerance. Small
natural concentrations of mercury appear to be widespread but the
levels vary from area to area; it is also difficult therefore, to
suggest a practical residue limit for mercury. By way of guidance,
however, practical residue limits of from 0.02 ppm of mercury to 0.05
ppm, according to local conditions, are suggested.
Further work or information
Sensitive methods of analysis specifically for alkyl, alkoxy and aryl
mercurials should be developed and used to study the occurrence of
these forms of mercury in foodstuffs from different sources: these
should include food stuffs known to have been treated with specified
compounds. Such analytical methods should also be used to study the
possible conversion of aryl mercury compounds to more toxic ones.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Rylander, R. (1964) J. Pharmacol. exp. Ther., 146,
236
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G., Nelson, A. A., Laug, E. P. & King, F. M. (1950)
A.M.A. Arch. industr. Hlth., 2, 433
Gage, J. C. (1964) Brit. J. industr. Med., 21, 197
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek. A. (1938) Z. Untersuch.
Lebensmitt., 16, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Prickett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. S., and Johnson, E. I. (1957) The determination of traces
of mercury in apples Analyst, 82: 206-208
Association of Official Agricultural Chemists (1965) Methods of
Analysis, 10th ed.,: 375-377
Beidas, A. S., and Higgons, D. J. (1957) Mercury residues in sprayed
crops. I. Tomatoes. J. Sci. Fd. Agric., 8: 597-600
Beidas, A. S., and Higgons, D. J. (1959) Mercury residues in sprayed
crops. II. Apples. J. Sci. Fd. Agric., 10: 527-529
Bland, M. A., and Egan, H. (1963) Phenylmercury salicylate residues in
commercial glasshouse tomato crops. Plant Pathol, 12 (2): 59-62
Egan, H., and Lidzey, R. G. (1960) Phenylmercury salicylate residues
in commercial glasshouse tomato crops. Plant Pathol, 9 (3): 58-91
Epps, E. A. (1966) Colorimetric determination of mercury residues on
rice. J. Assoc. Offic. Anal. Chemists, 49 (4): 793-795
Erwell, L. G., and Westermark, T. (1965) Determination of mercury by
activation analysis. Isotope Techniques Laboratory, Sweden.
Sjöstrand, B. (1964) Simultaneous determination of mercury and arsenic
in biological and organic materials by activation analysis. Anal.
Chem., 36, 814-819
Food and Agriculture Organization of the United Nations. (1964)
Evaluation of the toxicity of pesticide residues in food. Meeting
report. PL/1963/13
Goldwater, L. J. (1964) Absorption and excretion of mercury in man.
VII. Significance of mercury in blood Arch. environm. Hlth., 9;
735-741
Goldwater, L. J. (1965) Mercury in our environment: address to the
Third Annual Scientific meeting of the British Industrial Biological
Research Association
Gersuch, T. T. (1959) Radiochemical investigations on the recovery for
analysis of trace elements in organic and biological materials.
Analyst, 84: 135-173
Gouverneur, P., and Hoedeman, W. (1964) Determination of mercury in
organic compounds. Anal. Chim. Acta, 30 (6): 519-583
Gutenmann, W. H., and Lisk, D. J. (1960) Rapid determination of
mercury in apples by modified Schöniger combustion. J. Agric. Fd.
Chem., 8 : 306-308
Hamilton, G. A., and Ruthven, A. D. (1966) An apparatus for the
detection and estimation of organo-mercury dusts and vapours in the
atmosphere. Lab. Pract., 15: 995-997
Irving, H., and Cox, J. J. (1961) Studies with dithizone. Part VIII.
Reactions with organometallic compounds. J. Chem, Soc.,: 1470-1479.
Joint Mercury Residues Panel. (1961) The determination of mercury
residues in apples and tomatoes, Analyst, 86: 608
Kanazawa, J., and Sato, R. (1959) Determination of mercury in organic
mercury fungicides by the dithizone method Bunseti Kagaku (Japan
Analyst), 8: 440-444
Kim, C. K., and Silverman, J. (1965) Determination of mercury in wheat
and tobacco leaf by neutron activation analysis using simple exchange
separation. Anal. Chem., 37: 1616-1617
Kimura, Y., and Miller, V. L. (1964) The degradation of organomercury
fungicides in soil. J. Agric. Fd. Chem., 12 (3): 253-257
Klein, A. K. (1952) Report on mercury. J. Assoc. Off. Agric.
Chemists., 35: 537
Ljunggren, K., and Westermark, T. (1960) A method for the detection of
mercury by radioactivation analysis. Pure appl. Chem., 1: 127-133
Miller, B. J. (1957) A note on mercury spray residues in apples.
Plant Pathol., 5: 119-121
Pappas, E. G., and Rosenberg, L. A. (1966) Determination of
submicrogram quantities of mercury (in fish and eggs) by cold vapour
atomic absorption photometry. J. Assoc. Offic. Anal. Chemists, 49 (4):
782-793
Phillips, G. F., Dixon, B. E., and Lidzey, R. G. (1959) The volatility
of organomercury compounds. J. Sci. Food Agric., 11: 604-610
Pickard, J. A. and Martin, J. T., (1960) Determination of mercury in
plant material. J. Sci. Fd. Agric., 11: 374-377
Ross, R. G., and Stewart, D. K. R. (1960) Mercury residues on apple
fruit and foliage. Can. J. Plant Sci., 40: 117-122. (Also see
Movement and accumulation of mercury in apple trees and soil, (1962),
Can. J. Plant Sci., 42: 280-285. Note on mercury residues in
potatoes, (1962), Can. J. Plant Sci., 42; 370 and Mercury residues
in potatoes in relation to foliar sprays of phenyl mercury chloride,
(1964) Can. J. Plant Sci., 44: 123-125)
Schachter, M. M. (1966) Apparatus for cold vapour atomic absorption of
mercury. J. Assoc. Off. Anal. Chemists, 49 (4): 778-782
Smart, N. A., and Lloyd, M. K. (1963) Mercury residues on eggs, Flesh
and livers of hens fed on wheat treated with methylmercury
dicyandiamide. J. Sci. Food Agric., 14: 734-740
Smart, N. A. (1963) Retention of organomercury compounds in dressed
grain after washing. Nature, 199: 1206-1207
Smart, N. A. (1964) Mercury residues in potatoes following application
of foliar spray containing phenylmercury chloride. J. Sci. Food
Agric., 15: 102-107
Society or Analytical Chemistry, Analytical Methods Committee. (1965)
The determination of small amounts of mercury in organic matter.
Analyst, 90: 515-530
Stone, H. M. (1964) Mercury content of apples treated with
phenylmercury dimethyl-dithiocarbamate. New Zealand J. Agr. Res.,
7: 439
Swensson, H., and Ulfarson, U. (1963) Toxicology of organic mercury
compounds used as fungicides. Occup. Hlth Rev., 15 (3): 5
Truhaut, R. and Boudene, C. (1963) Microdosage du mercure dans les
dendtrees alimentaires. Ann. Falsif. Expert. Chim., 56: 225-242
Explanatory Note
Phenylmercury acetate is an important example of twenty or more
organomercury compounds used as fungicides in agriculture. They have
the general structure R-Hg-X where R is an alkyl, alkoxyalkyl or aryl
organic radical and where the bond with the X group has the character
of a salt with a substance (acid, amide, phenol, etc.) having a
dissociating hydrogen ion.
Some other important compounds, listed in accordance with their
organic radicals, are as follows:-
a) methylmercury compounds Synonym
methylmercury dicyandiamide MMD Panogen(R)
methylmercury iodide MMI
b) ethylmercury compounds
ethylmercury chloride EMC
ethylmercury urea EMU
ethylmercury phosphate EMP
ethylmercury p-toluene sulphonamide EMTS
ethylmercury sulphate EMS
c) alkoxyethylmercury compounds
ethoxyethylmercury chloride
methoxyethylmercury chloride MMC
methoxyethylmercury acetate
d) arylmercury compounds
phenylmercury chloride PMC
phenylmercury iodide PMI
phenylmercury urea PMU
phenylmercury
methanodinaphtho-disulphonate Murfixtan,(R) PMF
phenylmercury salicylate
phenylmercury
NN-dimethyldithiocarbamate Phelam(R)
tolylmercury chloride TMC
N-tolylmercury
p-toluenesulfonanilide TMTS
Physical properties
The alkyl compounds are generally the more volatile and the aryl the
least volatile. The solubility in water of the aryl compounds also is
lower than the corresponding alkyl ones. The stability and
particularly the sensitivity to reducing agents with the eventual
production of metallic mercury, varies from one compound to another.
(R) = proprietary name.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastrointestinal tract (Fitzhugh et al., 1950, Prickett et al., 1950).
The major route of elimination of mercury after oral, intramuscular or
intravenous administration of PMA is by way of the bile and excretion
into the alimentary tract (Berlin & Ullberg, 1963; Prickett et al.,
1950).
PMA given orally and intramuscularly to 87 chicks, intramuscularly to
12 rats, and intravenously to 4 dogs, was absorbed apparently
unchanged. After about 96 hours no PMA could be detected in the
tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960).
The influence of 2,3-dimercaprol (BAL), 0.4 mg/kg, on the body
distribution of mercury was studied in mice. When
phenyl-203Hg-acetate was given as a single intravenous dose (0.5
mg/kg as Hg) or as daily injections (1 and 3 mg/kg as Hg for 16 days),
it was shown that BAL effected a redistribution of the body mercury
load. A persistent increase in the concentration of mercury was noted
in the brain, liver and muscle, while a decrease was found in the
kidney (Berlin & Rylander, 1964).
In comparison with methylmercurydicyanamide (MMD), a steady state of
excretion was reached much faster when rats were given PMA;
furthermore, a much lower accumulation of Hg was found in the brain of
rats after the administration of PMA than after MMD (Gage, 1964).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al.,1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at dosages of
1-2.5 mg/kg body-weight every other day for 4 weeks. The animals
showed gradually increasing apathy, loss of weight, and finally
neurological signs - (ataxia and paresis), especially at the higher
dosages. Histopathological examination revealed damage to the granular
layer and the Purkinje cells of the cerebellum, and to the spinal cord
(Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130 days,
the total amount of mercury consumed during the experimental period
being 770 mg. The animal showed marked growth depression and died
after 130 days. Chemical analysis revealed large amounts of mercury in
the organs - 29 mg/kg organ-weight in the kidney, 0.52 mg/kg in the
liver and 5.18 mg/kg in the gastro-intestinal tract - whereas a
control rabbit showed only 0.06 mg/kg in the kidney and traces in the
liver. Another rabbit fed a diet containing PMA for 100 days received
a total amount of 6.9 mg of mercury. There was no abnormality in
appearance or growth. The contents of mercury in the organs were 0.455
mg/kg in the kidney and 0.042 mg/kg in the liver (Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670 days
and consumed a total of 20.4 mg during the whole experimental period.
No ill-effects were observed in general appearance or growth. The
mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments
It is clear from the biochemical studies that PMA may give rise to
mercury accumulation in the tissues and the long-term study in the rat
failed to demonstrate a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per day,
produced a slight effect in the rat. Even if this figure were to be
adopted as a maximum no-effect level and the customary safety factor
applied this would give an acceptable daily intake for man of 0.00005
mg/kg body-weight. This is tantamount to zero. It is undesirable that
for the general population there should be any increase in the natural
intake of mercury.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) General properties
The suitability of a particular organomercury compound for a
particular purpose is partly dependent upon its physical properties.
Phenyl mercury acetate and other aryl compounds, which have a low
volatility, are more suitable where persistence is required. Under
certain conditions however (e.g. in enclosed seed dressing machinery)
their higher volatility enables the alkyl compounds to be applied more
effectively. Phillips, Dixon & Lidzey (1959) list the vapour pressures
of many of these compounds and compare their volatilities under
different conditions.
(b) Pre-harvest treatments
Organomercury compounds are used in seed dressings, orchard sprays,
foliar dusts and as glass house aerosols for the control of fungal
diseases. Since analysis seldom distinguishes between organically
bound and inorganic mercury, it is worth noting also that inorganic
mercury compounds are used for soil and root treatment. Powdered and
liquid preparations containing both alkyl and aryl mercury compounds
are used as cereal seed dressing. Ethylmercury phosphate is fairly
specifically used on beet seed and this and other seed dressings are
likely to lead to residue problems. Phenylmercury foliar dusts are
used to control rice blast disease.
(c) Post-harvest treatments
Organomercurials are not used post-harvest other than as bulb, tuber
and cereal seed dressings. It is possible that small residues may be
picked up e.g. by fruits, from paper wrappings or other containers
which have been treated with organomercurial fungicides.
(d) Other uses
Organomercury compounds, particularly phenylmercury salts, are used
widely as fungicides in industry, e.g. on wood pulp, paper and various
building materials.
Tolerances
Existing tolerances all refer to residues found and expressed as
mercury (Hg) without distinction between the forms in which the metal
may actually be present in food. Uses of organomercurials which could
lead to a detectable residue in food produce are not allowed in the
United States.
Residues resulting from supervised trials
(a) Pre-harvest treatments
A number of workers have measured residues in apples (summarized by
Miller, 1957) and tomatoes, (e.g. Beidas & Higgins 1957, 1959; Ross
& Stewart, 1960; Stone, 1962). Foliar applications of organomercurials
in general lead to translocation of the mercury. Pickard & Martin
(1963) studied the uptake of mercury by roots, leaves and fruits after
applications at commercial rates. The roots of lettuce and dwarf
bean plants accumulate mercury from nutrient solution containing
phenylmercuric acetate, but little translocation to the foliage
occurs. Root treatment of cauliflower seedlings with calomel or
mercuric chloride before planting lead to absorption by the roots but
the curds are uncontaminated. Carrots grown in soil treated with
mercuric chloride contained up to 0.05 ppm and roots from
calomel-treated soil showed 0.02 ppm Hg when seed was sown immediately
after soil treatment; delay in seeding eliminated contamination.
Lettuces, dwarf beans, carrots, potatoes and turnips from field
experiments using calomel and mercuric oxide soil treatments showed
mercury residues not exceeding 0.03 ppm. Apple leaves absorbed
mercury deposited as phenylmercuric acetate, within a few days.
Mercury was found in young coffee and citrus lime leaves that
emerged after spraying with phenylmercuric acetate, indicating
translocation. Broad bean plants sprayed with phenylmercuric
acetate at early flowering later showed 0.02 ppm in the pods, 0.04 ppm
in the seeds and 0.07 ppm Hg in the roots. Application of
phenylmercuric acetate to the leaves of potato plants led to
residues in the tubers (0.1 ppm in peel, 0.18 ppm Hg in flesh) and
roots (1.2 ppm Hg). Apples from commercially sprayed orchards
contained 0.05 ppm Hg distributed between the peel, flesh and core.
Five applications of phenylmercuric acetate under experimental
conditions, gave 0.24 ppm on whole fruits; one third of the mercury
was located in the flesh. Mercury deposited on the surface of the
fruits was largely held in the cuticle; much of the mercury in the
flesh arose from translocation from the leaves. Mercury was detected
in the fruitlets (0.4 ppm) and young leaves (0.07 ppm) of trees
sprayed the previous year. The bark of trees treated for six
consecutive years contained 4 ppm Hg. Naturally-occurring mercury in
soils varied between 0.05 and 0.12 ppm. Soil from beneath sprayed
apple trees contained 0.2 ppm Hg. Soils treated with inorganic
mercurials showed up to 2 ppm Hg (untreated 0.05 ppm).
In Japan the stage of plant growth affected the amount of residual Hg
in rice. Unpolished rice harvested from the rice plant sprayed with
PMA before the plant comes to ear contained 0.12 ppm of Hg, whereas,
the grain harvested from the plant treated with PMA after the plant
comes to ear contained 0.23 ppm of Hg. Epps (1966) examined rice and
various milling by-products, from rice treated in field tests with
phenylmercury acetate; residues ranged from 1.3 ppm Hg in straw and
0.8 ppm in bran to 0.1 - 0.2 ppm in the endosperm.
Smart (1963, 1964) measured residues in the eggs, flesh, etc., of hens
fed wheat treated with methylmercury dicyandiamide and in potatoes
following foliar spray application with phenylmercury chloride. Only
about 10 per cent of an organomercury compound on dressed grain was
removed by washing. Translocation of residues from potato foliage to
tubers has been demonstrated by Ross & Stewart (1964). Egan & Lidzey
(1960) and Bland & Egan (1963) showed that successive treatments of
tomatoes with phenylmercury salicylate under commercial greenhouse
conditions resulted in residues of the order of 0.01 (n + 1) ppm where
"n" is the number of treatments up to the eighth; and falls thereafter
by about 0.01 ppm for each subsequent treatment.
Mercury residues measured in Japan (direct communications, 1965) were
found to be 0.098 + 0.008 ppm Hg in peel and 0.025 + 0.008 ppm
in flesh of mandarin orange. Untreated fruit contained 0.03 - 0.05 ppm
Hg in peel and 0.01 - 0.02 ppm Hg in flesh. The residues in mandarin
oranges sprayed with water-soluble mercurial fungicides (PMA or PMF)
were generally greater than those in fruit sprayed with less
water-soluble fungicides (PMI or PMO). After five foliar applications
of MMC (12.5 ppm as Hg) to peach trees, amounts of Hg were in the
range of 0.21 - 0.25 ppm in peel and 0.02 - 0.04 ppm in flesh. Similar
treatments of a mixture of PMPS, EMP, and EMU (12.5 ppm as Hg)
resulted in an Hg residue in the range of 0.27 - 0.28 ppm in peel and
0.06 - 0.07 ppm in flesh.
Residues in food moving in commerce
Since 1961 surveys have been undertaken in Sweden on the occurrence of
mercury in eggs, game, fish and various other foodstuffs. This has
been associated with enquiries into possible effects on wildlife from
the use of mercury fungicides in industry and agriculture. The
residues found in human food have ranged from a mean of 29 parts per
thousand million in eggs marketed in Sweden, to over 20 ppm in the
livers of some 10 per cent of the game birds examined. Wheat, thought
to be untreated, from various parts of the United States of America,
examined by atomic absorption spectroscopy by Pappas and Rosenberg
(1966) showed 0.013 to 0.13 ppm Hg and 1.5 ppm Hg in commercially
treated samples. The same authors found an average of 0.02 ppm in
haddock fillets while eggs were virtually mercury-free (less than
0.005 ppm). In the examination of various foods in Japan (direct
communication) residues found were: polished rice 0.04 - 0.07;
unpolished rice 0.07 - 0.14; wheat flour 0.05; fruits and vegetables
0.02 - 0.06; meats 0.03 - 0.16; fish 0.00 - 0.10, (tuna 0.3 - 0.55);
milk 0.01 and hen eggs 0.08. Small amounts of mercury are very
widespread in nature. The extent of general environmental
contamination has been reviewed by Goldwater (1964) who quotes results
of residue analysis of food including bananas (0.31 ppm) canned
chicken (0.15 ppm) butter (0.14 ppm) Cheddar Cheese (0.082 ppm)
white bread (0.08 ppm) beer (0.04 ppm) and rice (0.02 ppm).
In each of these instances the figures are quoted as ppm Hg. Figures
for "organic" and "inorganic" mercury have rarely been obtained, there
appear to be none for residues as specified organomercury compounds.
Residues at the time or consumption
The meeting had no data on the effects of storage, cooking and other
processing. Reductions no doubt occur from volatilisation during
storage and from washing of crops and trimming (e.g. of outside parts
of fruits and vegetables) prior to cooking. Some losses during
cooking, also are likely due to the volatility of these compounds, but
figures for such losses were not available.
Methods of residue analysis
There is an extensive literature on mercury residue analysis, the
greater part of which is concerned only with total mercury and not
with intact organomercury residues as such. There is a wide
divergence between the toxicities of the various mercury compounds and
often a lack of information concerning the chemical nature of the
residues (e.g. in edible animal material) after the use of a given
compound.
(a) Total mercury (inorganic and organic)
Relatively simple methods, such as the Reinsch test, have long been
used to detect traces of metals including mercury and, with
supplementary confirmatory tests, can be used to distinguish mercury
from other residues and approximately to measure them. Very many of
the modern total mercury methods are based on extraction of the metal
by organic complexing agents, principally diphenyl thiocarbazone
(dithizone) into chloroform solution (e.g. Kanazawa & Sato, 1959).
Examples are the IUPAC method of Ljunggren and Westermark (1960) for
the determination of mercury foodstuffs and the method of the
Association of Official Analytical Chemists (1965), both based on the
method of Klein (1952). Conditions can be adjusted so that mercury is
selectively extracted. There are a number of variations of the method
of measuring the extracted mercury complex. These are normally based
on the intensity of colour of the complex solution, or of the
uncomplexed dithizone remaining when a measured excess is used;
alternatively a combined titrimetric-colorimetric procedure is used in
which small measured amounts of standardized dithizone are added
successively to the prepared sample extract until no reagent colour
change occurs. Such methods, which are sensitive to 0.01 ppm of Hg or
less in favourable cases, have been applied to a wide range of
biological tissue including many foods.
A source of difficulty, which arises also in the more modern
radioactivation method of analysis (q.v.), lies in the relatively high
volatility of mercury and its compounds. Biological tissue is normally
first oxidized, e.g. by warming with nitric acid. If too much heat is
applied or is allowed to generate, some of the mercury may be lost
from the sample as in some of the earlier work of Klein, in which the
condensate was trapped in a closed (reflux) oxidation system the main
digest thus becoming more concentrated with respect to acid with a
consequent elevation in boiling point. Gorsuch (1950) has made a
thorough study of losses in wet ashing, using radiochemical methods;
he has in this way shown the need to condense digestion vapours and
return them to the main digest. Gutenmann & Lisk (1960) and Gouverneur
& Hoedman (1964) used a modified Schoniger combustion method in place
of wet digestion. Conditions which emphasize cool oxidation were
described by Abbott and Johnson (1957), and examined collaboratively
for apples and tomatoes in Britain by the Joint Mercury Residues Panel
(1961). A further collaborative study of a method for amounts of
mercury down to 0.5 micrograms in organic matter has been described by
the Society in Analytical Chemistry (1965). Truhaut and Boudene (1963)
have used a simple flask in which oxidation, complex extraction and
colorimetric measurement can be completed without transfer.
Radioactivation methods of residue analysis are sensitive to at least
0.01 ppm Hg and avoid the need for oxidation and extraction steps.
However, preliminary removal of water from biological tissue may be
necessary and this again poses the problem of volatility of mercury
and its compound. Erwall & Westermark (1964) described the application
of this technique to foods. An atomic pile is necessary for the
activation stage and where this is available the technique can be
widely applied provided the dehydration stage is satisfactory with a
chemical separation the sensitivity level is 0.005 ppm on a 0.2 gram
sample. This sensitivity could also be obtained with a gamma counter
without spectrometry. If the irradiated sample is counted direct
without chemical separation, a gamma spectrometer would have to be
used. The sensitivity would then depend on the other short-lived
activities produced (e.g. sodium-24) and 0.5 ppm on a 0.2 gram sample
appears to be reasonable. A typical conventional dithizone method has
a sensitivity of 0.02 ppm on a 10 gram sample. Neutron activation
analysis using Hg-203 (half-life 47 days) would be less sensitive than
using Hg-197 by a factor of 20 and a longer irradiation time would be
needed. In the method of Ljunggren & Westermark (1960) biological
material is sealed into quartz tubes, activated and 77-keV gamma
radiation emission from Hg-197 measured after a cooling period of two
days. This method is sensitive to five nanograms of Hg.
Schachter (1966) has described the use of atomic absorption
spectroscopy to mercury vapour in the cold state. High sensitivity is
achieved using the whole of the ultraviolet spectrum of a quartz
mercury vapour lamp. Thus one gram of ground wheat is combusted in a
Schoniger flask and the mercury vapour collected in dil. HCl,
converted to sulfide, pyrolysed in a train of nitrogen in a small
furnace at 650°C and the mercury vapour cooled and measured. Hamilton
& Ruthven (1966) have described a field apparatus for the detection
and estimation of organomercury dusts and vapours in the atmosphere; a
similar official method is published in Britain.
(b) Organically bound mercury
Limited work has been done on the separation and measurement of
unchanged organomercury compounds. Miller, Lissis & Csonka (1958)
extract phenylmercury compounds from animal's tissue with chloroform
and determine the phenylmercury adsorptiometrically as the dithizone
complex. Kimura & Miller (1964) use different extraction solvents for
(a) phenyl and alkyl mercurials and (b) diphenyl dialkyl mercurials
and (c) ionic mercury. The reaction of dithizone with organomercury
compounds has been reviewed by Irving & Cox (1961). It should be
possible to devise extraction procedures in which intact radicals such
as phenyl-mercury can be isolated (e.g. as the chloride) and then
separated by paper. Electrophoretic separations have also been studied
or thin layer chromatographic procedures and identified and measured.
RECOMMENDATION FOR TOLERANCES
Since no acceptable daily intake level can be given for mercury it is
not possible to recommend a tolerance or a temporary tolerance. Small
natural concentrations of mercury appear to be widespread but the
levels vary from area to area; it is also difficult therefore, to
suggest a practical residue limit for mercury. By way of guidance,
however, practical residue limits of from 0.02 ppm of mercury to 0.05
ppm, according to local conditions, are suggested.
Further work or information
Sensitive methods of analysis specifically for alkyl, alkoxy and aryl
mercurials should be developed and used to study the occurrence of
these forms of mercury in foodstuffs from different sources: these
should include food stuffs known to have been treated with specified
compounds. Such analytical methods should also be used to study the
possible conversion of aryl mercury compounds to more toxic ones.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Rylander, R. (1964) J. Pharmacol. exp. Ther., 146,
236
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G., Nelson, A. A., Laug, E. P. & King, F. M. (1950)
A.M.A. Arch. industr. Hlth., 2, 433
Gage, J. C. (1964) Brit. J. industr. Med., 21, 197
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek. A. (1938) Z. Untersuch.
Lebensmitt., 16, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Prickett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. S., and Johnson, E. I. (1957) The determination of traces
of mercury in apples Analyst, 82: 206-208
Association of Official Agricultural Chemists (1965) Methods of
Analysis, 10th ed.,: 375-377
Beidas, A. S., and Higgons, D. J. (1957) Mercury residues in sprayed
crops. I. Tomatoes. J. Sci. Fd. Agric., 8: 597-600
Beidas, A. S., and Higgons, D. J. (1959) Mercury residues in sprayed
crops. II. Apples. J. Sci. Fd. Agric., 10: 527-529
Bland, M. A., and Egan, H. (1963) Phenylmercury salicylate residues in
commercial glasshouse tomato crops. Plant Pathol, 12 (2): 59-62
Egan, H., and Lidzey, R. G. (1960) Phenylmercury salicylate residues
in commercial glasshouse tomato crops. Plant Pathol, 9 (3): 58-91
Epps, E. A. (1966) Colorimetric determination of mercury residues on
rice. J. Assoc. Offic. Anal. Chemists, 49 (4): 793-795
Erwell, L. G., and Westermark, T. (1965) Determination of mercury by
activation analysis. Isotope Techniques Laboratory, Sweden.
Sjöstrand, B. (1964) Simultaneous determination of mercury and arsenic
in biological and organic materials by activation analysis. Anal.
Chem., 36, 814-819
Food and Agriculture Organization of the United Nations. (1964)
Evaluation of the toxicity of pesticide residues in food. Meeting
report. PL/1963/13
Goldwater, L. J. (1964) Absorption and excretion of mercury in man.
VII. Significance of mercury in blood Arch. environm. Hlth., 9;
735-741
Goldwater, L. J. (1965) Mercury in our environment: address to the
Third Annual Scientific meeting of the British Industrial Biological
Research Association
Gersuch, T. T. (1959) Radiochemical investigations on the recovery for
analysis of trace elements in organic and biological materials.
Analyst, 84: 135-173
Gouverneur, P., and Hoedeman, W. (1964) Determination of mercury in
organic compounds. Anal. Chim. Acta, 30 (6): 519-583
Gutenmann, W. H., and Lisk, D. J. (1960) Rapid determination of
mercury in apples by modified Schöniger combustion. J. Agric. Fd.
Chem., 8 : 306-308
Hamilton, G. A., and Ruthven, A. D. (1966) An apparatus for the
detection and estimation of organo-mercury dusts and vapours in the
atmosphere. Lab. Pract., 15: 995-997
Irving, H., and Cox, J. J. (1961) Studies with dithizone. Part VIII.
Reactions with organometallic compounds. J. Chem, Soc.,: 1470-1479.
Joint Mercury Residues Panel. (1961) The determination of mercury
residues in apples and tomatoes, Analyst, 86: 608
Kanazawa, J., and Sato, R. (1959) Determination of mercury in organic
mercury fungicides by the dithizone method Bunseti Kagaku (Japan
Analyst), 8: 440-444
Kim, C. K., and Silverman, J. (1965) Determination of mercury in wheat
and tobacco leaf by neutron activation analysis using simple exchange
separation. Anal. Chem., 37: 1616-1617
Kimura, Y., and Miller, V. L. (1964) The degradation of organomercury
fungicides in soil. J. Agric. Fd. Chem., 12 (3): 253-257
Klein, A. K. (1952) Report on mercury. J. Assoc. Off. Agric.
Chemists., 35: 537
Ljunggren, K., and Westermark, T. (1960) A method for the detection of
mercury by radioactivation analysis. Pure appl. Chem., 1: 127-133
Miller, B. J. (1957) A note on mercury spray residues in apples.
Plant Pathol., 5: 119-121
Pappas, E. G., and Rosenberg, L. A. (1966) Determination of
submicrogram quantities of mercury (in fish and eggs) by cold vapour
atomic absorption photometry. J. Assoc. Offic. Anal. Chemists, 49 (4):
782-793
Phillips, G. F., Dixon, B. E., and Lidzey, R. G. (1959) The volatility
of organomercury compounds. J. Sci. Food Agric., 11: 604-610
Pickard, J. A. and Martin, J. T., (1960) Determination of mercury in
plant material. J. Sci. Fd. Agric., 11: 374-377
Ross, R. G., and Stewart, D. K. R. (1960) Mercury residues on apple
fruit and foliage. Can. J. Plant Sci., 40: 117-122. (Also see
Movement and accumulation of mercury in apple trees and soil, (1962),
Can. J. Plant Sci., 42: 280-285. Note on mercury residues in
potatoes, (1962), Can. J. Plant Sci., 42; 370 and Mercury residues
in potatoes in relation to foliar sprays of phenyl mercury chloride,
(1964) Can. J. Plant Sci., 44: 123-125)
Schachter, M. M. (1966) Apparatus for cold vapour atomic absorption of
mercury. J. Assoc. Off. Anal. Chemists, 49 (4): 778-782
Smart, N. A., and Lloyd, M. K. (1963) Mercury residues on eggs, Flesh
and livers of hens fed on wheat treated with methylmercury
dicyandiamide. J. Sci. Food Agric., 14: 734-740
Smart, N. A. (1963) Retention of organomercury compounds in dressed
grain after washing. Nature, 199: 1206-1207
Smart, N. A. (1964) Mercury residues in potatoes following application
of foliar spray containing phenylmercury chloride. J. Sci. Food
Agric., 15: 102-107
Society or Analytical Chemistry, Analytical Methods Committee. (1965)
The determination of small amounts of mercury in organic matter.
Analyst, 90: 515-530
Stone, H. M. (1964) Mercury content of apples treated with
phenylmercury dimethyl-dithiocarbamate. New Zealand J. Agr. Res.,
7: 439
Swensson, H., and Ulfarson, U. (1963) Toxicology of organic mercury
compounds used as fungicides. Occup. Hlth Rev., 15 (3): 5
Truhaut, R. and Boudene, C. (1963) Microdosage du mercure dans les
dendtrees alimentaires. Ann. Falsif. Expert. Chim., 56: 225-242
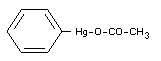 Explanatory Note
Phenylmercury acetate is an important example of twenty or more
organomercury compounds used as fungicides in agriculture. They have
the general structure R-Hg-X where R is an alkyl, alkoxyalkyl or aryl
organic radical and where the bond with the X group has the character
of a salt with a substance (acid, amide, phenol, etc.) having a
dissociating hydrogen ion.
Some other important compounds, listed in accordance with their
organic radicals, are as follows:-
a) methylmercury compounds Synonym
methylmercury dicyandiamide MMD Panogen(R)
methylmercury iodide MMI
b) ethylmercury compounds
ethylmercury chloride EMC
ethylmercury urea EMU
ethylmercury phosphate EMP
ethylmercury p-toluene sulphonamide EMTS
ethylmercury sulphate EMS
c) alkoxyethylmercury compounds
ethoxyethylmercury chloride
methoxyethylmercury chloride MMC
methoxyethylmercury acetate
d) arylmercury compounds
phenylmercury chloride PMC
phenylmercury iodide PMI
phenylmercury urea PMU
phenylmercury
methanodinaphtho-disulphonate Murfixtan,(R) PMF
phenylmercury salicylate
phenylmercury
NN-dimethyldithiocarbamate Phelam(R)
tolylmercury chloride TMC
N-tolylmercury
p-toluenesulfonanilide TMTS
Physical properties
The alkyl compounds are generally the more volatile and the aryl the
least volatile. The solubility in water of the aryl compounds also is
lower than the corresponding alkyl ones. The stability and
particularly the sensitivity to reducing agents with the eventual
production of metallic mercury, varies from one compound to another.
(R) = proprietary name.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastrointestinal tract (Fitzhugh et al., 1950, Prickett et al., 1950).
The major route of elimination of mercury after oral, intramuscular or
intravenous administration of PMA is by way of the bile and excretion
into the alimentary tract (Berlin & Ullberg, 1963; Prickett et al.,
1950).
PMA given orally and intramuscularly to 87 chicks, intramuscularly to
12 rats, and intravenously to 4 dogs, was absorbed apparently
unchanged. After about 96 hours no PMA could be detected in the
tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960).
The influence of 2,3-dimercaprol (BAL), 0.4 mg/kg, on the body
distribution of mercury was studied in mice. When
phenyl-203Hg-acetate was given as a single intravenous dose (0.5
mg/kg as Hg) or as daily injections (1 and 3 mg/kg as Hg for 16 days),
it was shown that BAL effected a redistribution of the body mercury
load. A persistent increase in the concentration of mercury was noted
in the brain, liver and muscle, while a decrease was found in the
kidney (Berlin & Rylander, 1964).
In comparison with methylmercurydicyanamide (MMD), a steady state of
excretion was reached much faster when rats were given PMA;
furthermore, a much lower accumulation of Hg was found in the brain of
rats after the administration of PMA than after MMD (Gage, 1964).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al.,1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at dosages of
1-2.5 mg/kg body-weight every other day for 4 weeks. The animals
showed gradually increasing apathy, loss of weight, and finally
neurological signs - (ataxia and paresis), especially at the higher
dosages. Histopathological examination revealed damage to the granular
layer and the Purkinje cells of the cerebellum, and to the spinal cord
(Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130 days,
the total amount of mercury consumed during the experimental period
being 770 mg. The animal showed marked growth depression and died
after 130 days. Chemical analysis revealed large amounts of mercury in
the organs - 29 mg/kg organ-weight in the kidney, 0.52 mg/kg in the
liver and 5.18 mg/kg in the gastro-intestinal tract - whereas a
control rabbit showed only 0.06 mg/kg in the kidney and traces in the
liver. Another rabbit fed a diet containing PMA for 100 days received
a total amount of 6.9 mg of mercury. There was no abnormality in
appearance or growth. The contents of mercury in the organs were 0.455
mg/kg in the kidney and 0.042 mg/kg in the liver (Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670 days
and consumed a total of 20.4 mg during the whole experimental period.
No ill-effects were observed in general appearance or growth. The
mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments
It is clear from the biochemical studies that PMA may give rise to
mercury accumulation in the tissues and the long-term study in the rat
failed to demonstrate a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per day,
produced a slight effect in the rat. Even if this figure were to be
adopted as a maximum no-effect level and the customary safety factor
applied this would give an acceptable daily intake for man of 0.00005
mg/kg body-weight. This is tantamount to zero. It is undesirable that
for the general population there should be any increase in the natural
intake of mercury.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) General properties
The suitability of a particular organomercury compound for a
particular purpose is partly dependent upon its physical properties.
Phenyl mercury acetate and other aryl compounds, which have a low
volatility, are more suitable where persistence is required. Under
certain conditions however (e.g. in enclosed seed dressing machinery)
their higher volatility enables the alkyl compounds to be applied more
effectively. Phillips, Dixon & Lidzey (1959) list the vapour pressures
of many of these compounds and compare their volatilities under
different conditions.
(b) Pre-harvest treatments
Organomercury compounds are used in seed dressings, orchard sprays,
foliar dusts and as glass house aerosols for the control of fungal
diseases. Since analysis seldom distinguishes between organically
bound and inorganic mercury, it is worth noting also that inorganic
mercury compounds are used for soil and root treatment. Powdered and
liquid preparations containing both alkyl and aryl mercury compounds
are used as cereal seed dressing. Ethylmercury phosphate is fairly
specifically used on beet seed and this and other seed dressings are
likely to lead to residue problems. Phenylmercury foliar dusts are
used to control rice blast disease.
(c) Post-harvest treatments
Organomercurials are not used post-harvest other than as bulb, tuber
and cereal seed dressings. It is possible that small residues may be
picked up e.g. by fruits, from paper wrappings or other containers
which have been treated with organomercurial fungicides.
(d) Other uses
Organomercury compounds, particularly phenylmercury salts, are used
widely as fungicides in industry, e.g. on wood pulp, paper and various
building materials.
Tolerances
Existing tolerances all refer to residues found and expressed as
mercury (Hg) without distinction between the forms in which the metal
may actually be present in food. Uses of organomercurials which could
lead to a detectable residue in food produce are not allowed in the
United States.
Residues resulting from supervised trials
(a) Pre-harvest treatments
A number of workers have measured residues in apples (summarized by
Miller, 1957) and tomatoes, (e.g. Beidas & Higgins 1957, 1959; Ross
& Stewart, 1960; Stone, 1962). Foliar applications of organomercurials
in general lead to translocation of the mercury. Pickard & Martin
(1963) studied the uptake of mercury by roots, leaves and fruits after
applications at commercial rates. The roots of lettuce and dwarf
bean plants accumulate mercury from nutrient solution containing
phenylmercuric acetate, but little translocation to the foliage
occurs. Root treatment of cauliflower seedlings with calomel or
mercuric chloride before planting lead to absorption by the roots but
the curds are uncontaminated. Carrots grown in soil treated with
mercuric chloride contained up to 0.05 ppm and roots from
calomel-treated soil showed 0.02 ppm Hg when seed was sown immediately
after soil treatment; delay in seeding eliminated contamination.
Lettuces, dwarf beans, carrots, potatoes and turnips from field
experiments using calomel and mercuric oxide soil treatments showed
mercury residues not exceeding 0.03 ppm. Apple leaves absorbed
mercury deposited as phenylmercuric acetate, within a few days.
Mercury was found in young coffee and citrus lime leaves that
emerged after spraying with phenylmercuric acetate, indicating
translocation. Broad bean plants sprayed with phenylmercuric
acetate at early flowering later showed 0.02 ppm in the pods, 0.04 ppm
in the seeds and 0.07 ppm Hg in the roots. Application of
phenylmercuric acetate to the leaves of potato plants led to
residues in the tubers (0.1 ppm in peel, 0.18 ppm Hg in flesh) and
roots (1.2 ppm Hg). Apples from commercially sprayed orchards
contained 0.05 ppm Hg distributed between the peel, flesh and core.
Five applications of phenylmercuric acetate under experimental
conditions, gave 0.24 ppm on whole fruits; one third of the mercury
was located in the flesh. Mercury deposited on the surface of the
fruits was largely held in the cuticle; much of the mercury in the
flesh arose from translocation from the leaves. Mercury was detected
in the fruitlets (0.4 ppm) and young leaves (0.07 ppm) of trees
sprayed the previous year. The bark of trees treated for six
consecutive years contained 4 ppm Hg. Naturally-occurring mercury in
soils varied between 0.05 and 0.12 ppm. Soil from beneath sprayed
apple trees contained 0.2 ppm Hg. Soils treated with inorganic
mercurials showed up to 2 ppm Hg (untreated 0.05 ppm).
In Japan the stage of plant growth affected the amount of residual Hg
in rice. Unpolished rice harvested from the rice plant sprayed with
PMA before the plant comes to ear contained 0.12 ppm of Hg, whereas,
the grain harvested from the plant treated with PMA after the plant
comes to ear contained 0.23 ppm of Hg. Epps (1966) examined rice and
various milling by-products, from rice treated in field tests with
phenylmercury acetate; residues ranged from 1.3 ppm Hg in straw and
0.8 ppm in bran to 0.1 - 0.2 ppm in the endosperm.
Smart (1963, 1964) measured residues in the eggs, flesh, etc., of hens
fed wheat treated with methylmercury dicyandiamide and in potatoes
following foliar spray application with phenylmercury chloride. Only
about 10 per cent of an organomercury compound on dressed grain was
removed by washing. Translocation of residues from potato foliage to
tubers has been demonstrated by Ross & Stewart (1964). Egan & Lidzey
(1960) and Bland & Egan (1963) showed that successive treatments of
tomatoes with phenylmercury salicylate under commercial greenhouse
conditions resulted in residues of the order of 0.01 (n + 1) ppm where
"n" is the number of treatments up to the eighth; and falls thereafter
by about 0.01 ppm for each subsequent treatment.
Mercury residues measured in Japan (direct communications, 1965) were
found to be 0.098 + 0.008 ppm Hg in peel and 0.025 + 0.008 ppm
in flesh of mandarin orange. Untreated fruit contained 0.03 - 0.05 ppm
Hg in peel and 0.01 - 0.02 ppm Hg in flesh. The residues in mandarin
oranges sprayed with water-soluble mercurial fungicides (PMA or PMF)
were generally greater than those in fruit sprayed with less
water-soluble fungicides (PMI or PMO). After five foliar applications
of MMC (12.5 ppm as Hg) to peach trees, amounts of Hg were in the
range of 0.21 - 0.25 ppm in peel and 0.02 - 0.04 ppm in flesh. Similar
treatments of a mixture of PMPS, EMP, and EMU (12.5 ppm as Hg)
resulted in an Hg residue in the range of 0.27 - 0.28 ppm in peel and
0.06 - 0.07 ppm in flesh.
Residues in food moving in commerce
Since 1961 surveys have been undertaken in Sweden on the occurrence of
mercury in eggs, game, fish and various other foodstuffs. This has
been associated with enquiries into possible effects on wildlife from
the use of mercury fungicides in industry and agriculture. The
residues found in human food have ranged from a mean of 29 parts per
thousand million in eggs marketed in Sweden, to over 20 ppm in the
livers of some 10 per cent of the game birds examined. Wheat, thought
to be untreated, from various parts of the United States of America,
examined by atomic absorption spectroscopy by Pappas and Rosenberg
(1966) showed 0.013 to 0.13 ppm Hg and 1.5 ppm Hg in commercially
treated samples. The same authors found an average of 0.02 ppm in
haddock fillets while eggs were virtually mercury-free (less than
0.005 ppm). In the examination of various foods in Japan (direct
communication) residues found were: polished rice 0.04 - 0.07;
unpolished rice 0.07 - 0.14; wheat flour 0.05; fruits and vegetables
0.02 - 0.06; meats 0.03 - 0.16; fish 0.00 - 0.10, (tuna 0.3 - 0.55);
milk 0.01 and hen eggs 0.08. Small amounts of mercury are very
widespread in nature. The extent of general environmental
contamination has been reviewed by Goldwater (1964) who quotes results
of residue analysis of food including bananas (0.31 ppm) canned
chicken (0.15 ppm) butter (0.14 ppm) Cheddar Cheese (0.082 ppm)
white bread (0.08 ppm) beer (0.04 ppm) and rice (0.02 ppm).
In each of these instances the figures are quoted as ppm Hg. Figures
for "organic" and "inorganic" mercury have rarely been obtained, there
appear to be none for residues as specified organomercury compounds.
Residues at the time or consumption
The meeting had no data on the effects of storage, cooking and other
processing. Reductions no doubt occur from volatilisation during
storage and from washing of crops and trimming (e.g. of outside parts
of fruits and vegetables) prior to cooking. Some losses during
cooking, also are likely due to the volatility of these compounds, but
figures for such losses were not available.
Methods of residue analysis
There is an extensive literature on mercury residue analysis, the
greater part of which is concerned only with total mercury and not
with intact organomercury residues as such. There is a wide
divergence between the toxicities of the various mercury compounds and
often a lack of information concerning the chemical nature of the
residues (e.g. in edible animal material) after the use of a given
compound.
(a) Total mercury (inorganic and organic)
Relatively simple methods, such as the Reinsch test, have long been
used to detect traces of metals including mercury and, with
supplementary confirmatory tests, can be used to distinguish mercury
from other residues and approximately to measure them. Very many of
the modern total mercury methods are based on extraction of the metal
by organic complexing agents, principally diphenyl thiocarbazone
(dithizone) into chloroform solution (e.g. Kanazawa & Sato, 1959).
Examples are the IUPAC method of Ljunggren and Westermark (1960) for
the determination of mercury foodstuffs and the method of the
Association of Official Analytical Chemists (1965), both based on the
method of Klein (1952). Conditions can be adjusted so that mercury is
selectively extracted. There are a number of variations of the method
of measuring the extracted mercury complex. These are normally based
on the intensity of colour of the complex solution, or of the
uncomplexed dithizone remaining when a measured excess is used;
alternatively a combined titrimetric-colorimetric procedure is used in
which small measured amounts of standardized dithizone are added
successively to the prepared sample extract until no reagent colour
change occurs. Such methods, which are sensitive to 0.01 ppm of Hg or
less in favourable cases, have been applied to a wide range of
biological tissue including many foods.
A source of difficulty, which arises also in the more modern
radioactivation method of analysis (q.v.), lies in the relatively high
volatility of mercury and its compounds. Biological tissue is normally
first oxidized, e.g. by warming with nitric acid. If too much heat is
applied or is allowed to generate, some of the mercury may be lost
from the sample as in some of the earlier work of Klein, in which the
condensate was trapped in a closed (reflux) oxidation system the main
digest thus becoming more concentrated with respect to acid with a
consequent elevation in boiling point. Gorsuch (1950) has made a
thorough study of losses in wet ashing, using radiochemical methods;
he has in this way shown the need to condense digestion vapours and
return them to the main digest. Gutenmann & Lisk (1960) and Gouverneur
& Hoedman (1964) used a modified Schoniger combustion method in place
of wet digestion. Conditions which emphasize cool oxidation were
described by Abbott and Johnson (1957), and examined collaboratively
for apples and tomatoes in Britain by the Joint Mercury Residues Panel
(1961). A further collaborative study of a method for amounts of
mercury down to 0.5 micrograms in organic matter has been described by
the Society in Analytical Chemistry (1965). Truhaut and Boudene (1963)
have used a simple flask in which oxidation, complex extraction and
colorimetric measurement can be completed without transfer.
Radioactivation methods of residue analysis are sensitive to at least
0.01 ppm Hg and avoid the need for oxidation and extraction steps.
However, preliminary removal of water from biological tissue may be
necessary and this again poses the problem of volatility of mercury
and its compound. Erwall & Westermark (1964) described the application
of this technique to foods. An atomic pile is necessary for the
activation stage and where this is available the technique can be
widely applied provided the dehydration stage is satisfactory with a
chemical separation the sensitivity level is 0.005 ppm on a 0.2 gram
sample. This sensitivity could also be obtained with a gamma counter
without spectrometry. If the irradiated sample is counted direct
without chemical separation, a gamma spectrometer would have to be
used. The sensitivity would then depend on the other short-lived
activities produced (e.g. sodium-24) and 0.5 ppm on a 0.2 gram sample
appears to be reasonable. A typical conventional dithizone method has
a sensitivity of 0.02 ppm on a 10 gram sample. Neutron activation
analysis using Hg-203 (half-life 47 days) would be less sensitive than
using Hg-197 by a factor of 20 and a longer irradiation time would be
needed. In the method of Ljunggren & Westermark (1960) biological
material is sealed into quartz tubes, activated and 77-keV gamma
radiation emission from Hg-197 measured after a cooling period of two
days. This method is sensitive to five nanograms of Hg.
Schachter (1966) has described the use of atomic absorption
spectroscopy to mercury vapour in the cold state. High sensitivity is
achieved using the whole of the ultraviolet spectrum of a quartz
mercury vapour lamp. Thus one gram of ground wheat is combusted in a
Schoniger flask and the mercury vapour collected in dil. HCl,
converted to sulfide, pyrolysed in a train of nitrogen in a small
furnace at 650°C and the mercury vapour cooled and measured. Hamilton
& Ruthven (1966) have described a field apparatus for the detection
and estimation of organomercury dusts and vapours in the atmosphere; a
similar official method is published in Britain.
(b) Organically bound mercury
Limited work has been done on the separation and measurement of
unchanged organomercury compounds. Miller, Lissis & Csonka (1958)
extract phenylmercury compounds from animal's tissue with chloroform
and determine the phenylmercury adsorptiometrically as the dithizone
complex. Kimura & Miller (1964) use different extraction solvents for
(a) phenyl and alkyl mercurials and (b) diphenyl dialkyl mercurials
and (c) ionic mercury. The reaction of dithizone with organomercury
compounds has been reviewed by Irving & Cox (1961). It should be
possible to devise extraction procedures in which intact radicals such
as phenyl-mercury can be isolated (e.g. as the chloride) and then
separated by paper. Electrophoretic separations have also been studied
or thin layer chromatographic procedures and identified and measured.
RECOMMENDATION FOR TOLERANCES
Since no acceptable daily intake level can be given for mercury it is
not possible to recommend a tolerance or a temporary tolerance. Small
natural concentrations of mercury appear to be widespread but the
levels vary from area to area; it is also difficult therefore, to
suggest a practical residue limit for mercury. By way of guidance,
however, practical residue limits of from 0.02 ppm of mercury to 0.05
ppm, according to local conditions, are suggested.
Further work or information
Sensitive methods of analysis specifically for alkyl, alkoxy and aryl
mercurials should be developed and used to study the occurrence of
these forms of mercury in foodstuffs from different sources: these
should include food stuffs known to have been treated with specified
compounds. Such analytical methods should also be used to study the
possible conversion of aryl mercury compounds to more toxic ones.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Rylander, R. (1964) J. Pharmacol. exp. Ther., 146,
236
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G., Nelson, A. A., Laug, E. P. & King, F. M. (1950)
A.M.A. Arch. industr. Hlth., 2, 433
Gage, J. C. (1964) Brit. J. industr. Med., 21, 197
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek. A. (1938) Z. Untersuch.
Lebensmitt., 16, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Prickett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. S., and Johnson, E. I. (1957) The determination of traces
of mercury in apples Analyst, 82: 206-208
Association of Official Agricultural Chemists (1965) Methods of
Analysis, 10th ed.,: 375-377
Beidas, A. S., and Higgons, D. J. (1957) Mercury residues in sprayed
crops. I. Tomatoes. J. Sci. Fd. Agric., 8: 597-600
Beidas, A. S., and Higgons, D. J. (1959) Mercury residues in sprayed
crops. II. Apples. J. Sci. Fd. Agric., 10: 527-529
Bland, M. A., and Egan, H. (1963) Phenylmercury salicylate residues in
commercial glasshouse tomato crops. Plant Pathol, 12 (2): 59-62
Egan, H., and Lidzey, R. G. (1960) Phenylmercury salicylate residues
in commercial glasshouse tomato crops. Plant Pathol, 9 (3): 58-91
Epps, E. A. (1966) Colorimetric determination of mercury residues on
rice. J. Assoc. Offic. Anal. Chemists, 49 (4): 793-795
Erwell, L. G., and Westermark, T. (1965) Determination of mercury by
activation analysis. Isotope Techniques Laboratory, Sweden.
Sjöstrand, B. (1964) Simultaneous determination of mercury and arsenic
in biological and organic materials by activation analysis. Anal.
Chem., 36, 814-819
Food and Agriculture Organization of the United Nations. (1964)
Evaluation of the toxicity of pesticide residues in food. Meeting
report. PL/1963/13
Goldwater, L. J. (1964) Absorption and excretion of mercury in man.
VII. Significance of mercury in blood Arch. environm. Hlth., 9;
735-741
Goldwater, L. J. (1965) Mercury in our environment: address to the
Third Annual Scientific meeting of the British Industrial Biological
Research Association
Gersuch, T. T. (1959) Radiochemical investigations on the recovery for
analysis of trace elements in organic and biological materials.
Analyst, 84: 135-173
Gouverneur, P., and Hoedeman, W. (1964) Determination of mercury in
organic compounds. Anal. Chim. Acta, 30 (6): 519-583
Gutenmann, W. H., and Lisk, D. J. (1960) Rapid determination of
mercury in apples by modified Schöniger combustion. J. Agric. Fd.
Chem., 8 : 306-308
Hamilton, G. A., and Ruthven, A. D. (1966) An apparatus for the
detection and estimation of organo-mercury dusts and vapours in the
atmosphere. Lab. Pract., 15: 995-997
Irving, H., and Cox, J. J. (1961) Studies with dithizone. Part VIII.
Reactions with organometallic compounds. J. Chem, Soc.,: 1470-1479.
Joint Mercury Residues Panel. (1961) The determination of mercury
residues in apples and tomatoes, Analyst, 86: 608
Kanazawa, J., and Sato, R. (1959) Determination of mercury in organic
mercury fungicides by the dithizone method Bunseti Kagaku (Japan
Analyst), 8: 440-444
Kim, C. K., and Silverman, J. (1965) Determination of mercury in wheat
and tobacco leaf by neutron activation analysis using simple exchange
separation. Anal. Chem., 37: 1616-1617
Kimura, Y., and Miller, V. L. (1964) The degradation of organomercury
fungicides in soil. J. Agric. Fd. Chem., 12 (3): 253-257
Klein, A. K. (1952) Report on mercury. J. Assoc. Off. Agric.
Chemists., 35: 537
Ljunggren, K., and Westermark, T. (1960) A method for the detection of
mercury by radioactivation analysis. Pure appl. Chem., 1: 127-133
Miller, B. J. (1957) A note on mercury spray residues in apples.
Plant Pathol., 5: 119-121
Pappas, E. G., and Rosenberg, L. A. (1966) Determination of
submicrogram quantities of mercury (in fish and eggs) by cold vapour
atomic absorption photometry. J. Assoc. Offic. Anal. Chemists, 49 (4):
782-793
Phillips, G. F., Dixon, B. E., and Lidzey, R. G. (1959) The volatility
of organomercury compounds. J. Sci. Food Agric., 11: 604-610
Pickard, J. A. and Martin, J. T., (1960) Determination of mercury in
plant material. J. Sci. Fd. Agric., 11: 374-377
Ross, R. G., and Stewart, D. K. R. (1960) Mercury residues on apple
fruit and foliage. Can. J. Plant Sci., 40: 117-122. (Also see
Movement and accumulation of mercury in apple trees and soil, (1962),
Can. J. Plant Sci., 42: 280-285. Note on mercury residues in
potatoes, (1962), Can. J. Plant Sci., 42; 370 and Mercury residues
in potatoes in relation to foliar sprays of phenyl mercury chloride,
(1964) Can. J. Plant Sci., 44: 123-125)
Schachter, M. M. (1966) Apparatus for cold vapour atomic absorption of
mercury. J. Assoc. Off. Anal. Chemists, 49 (4): 778-782
Smart, N. A., and Lloyd, M. K. (1963) Mercury residues on eggs, Flesh
and livers of hens fed on wheat treated with methylmercury
dicyandiamide. J. Sci. Food Agric., 14: 734-740
Smart, N. A. (1963) Retention of organomercury compounds in dressed
grain after washing. Nature, 199: 1206-1207
Smart, N. A. (1964) Mercury residues in potatoes following application
of foliar spray containing phenylmercury chloride. J. Sci. Food
Agric., 15: 102-107
Society or Analytical Chemistry, Analytical Methods Committee. (1965)
The determination of small amounts of mercury in organic matter.
Analyst, 90: 515-530
Stone, H. M. (1964) Mercury content of apples treated with
phenylmercury dimethyl-dithiocarbamate. New Zealand J. Agr. Res.,
7: 439
Swensson, H., and Ulfarson, U. (1963) Toxicology of organic mercury
compounds used as fungicides. Occup. Hlth Rev., 15 (3): 5
Truhaut, R. and Boudene, C. (1963) Microdosage du mercure dans les
dendtrees alimentaires. Ann. Falsif. Expert. Chim., 56: 225-242
Explanatory Note
Phenylmercury acetate is an important example of twenty or more
organomercury compounds used as fungicides in agriculture. They have
the general structure R-Hg-X where R is an alkyl, alkoxyalkyl or aryl
organic radical and where the bond with the X group has the character
of a salt with a substance (acid, amide, phenol, etc.) having a
dissociating hydrogen ion.
Some other important compounds, listed in accordance with their
organic radicals, are as follows:-
a) methylmercury compounds Synonym
methylmercury dicyandiamide MMD Panogen(R)
methylmercury iodide MMI
b) ethylmercury compounds
ethylmercury chloride EMC
ethylmercury urea EMU
ethylmercury phosphate EMP
ethylmercury p-toluene sulphonamide EMTS
ethylmercury sulphate EMS
c) alkoxyethylmercury compounds
ethoxyethylmercury chloride
methoxyethylmercury chloride MMC
methoxyethylmercury acetate
d) arylmercury compounds
phenylmercury chloride PMC
phenylmercury iodide PMI
phenylmercury urea PMU
phenylmercury
methanodinaphtho-disulphonate Murfixtan,(R) PMF
phenylmercury salicylate
phenylmercury
NN-dimethyldithiocarbamate Phelam(R)
tolylmercury chloride TMC
N-tolylmercury
p-toluenesulfonanilide TMTS
Physical properties
The alkyl compounds are generally the more volatile and the aryl the
least volatile. The solubility in water of the aryl compounds also is
lower than the corresponding alkyl ones. The stability and
particularly the sensitivity to reducing agents with the eventual
production of metallic mercury, varies from one compound to another.
(R) = proprietary name.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastrointestinal tract (Fitzhugh et al., 1950, Prickett et al., 1950).
The major route of elimination of mercury after oral, intramuscular or
intravenous administration of PMA is by way of the bile and excretion
into the alimentary tract (Berlin & Ullberg, 1963; Prickett et al.,
1950).
PMA given orally and intramuscularly to 87 chicks, intramuscularly to
12 rats, and intravenously to 4 dogs, was absorbed apparently
unchanged. After about 96 hours no PMA could be detected in the
tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960).
The influence of 2,3-dimercaprol (BAL), 0.4 mg/kg, on the body
distribution of mercury was studied in mice. When
phenyl-203Hg-acetate was given as a single intravenous dose (0.5
mg/kg as Hg) or as daily injections (1 and 3 mg/kg as Hg for 16 days),
it was shown that BAL effected a redistribution of the body mercury
load. A persistent increase in the concentration of mercury was noted
in the brain, liver and muscle, while a decrease was found in the
kidney (Berlin & Rylander, 1964).
In comparison with methylmercurydicyanamide (MMD), a steady state of
excretion was reached much faster when rats were given PMA;
furthermore, a much lower accumulation of Hg was found in the brain of
rats after the administration of PMA than after MMD (Gage, 1964).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al.,1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at dosages of
1-2.5 mg/kg body-weight every other day for 4 weeks. The animals
showed gradually increasing apathy, loss of weight, and finally
neurological signs - (ataxia and paresis), especially at the higher
dosages. Histopathological examination revealed damage to the granular
layer and the Purkinje cells of the cerebellum, and to the spinal cord
(Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130 days,
the total amount of mercury consumed during the experimental period
being 770 mg. The animal showed marked growth depression and died
after 130 days. Chemical analysis revealed large amounts of mercury in
the organs - 29 mg/kg organ-weight in the kidney, 0.52 mg/kg in the
liver and 5.18 mg/kg in the gastro-intestinal tract - whereas a
control rabbit showed only 0.06 mg/kg in the kidney and traces in the
liver. Another rabbit fed a diet containing PMA for 100 days received
a total amount of 6.9 mg of mercury. There was no abnormality in
appearance or growth. The contents of mercury in the organs were 0.455
mg/kg in the kidney and 0.042 mg/kg in the liver (Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670 days
and consumed a total of 20.4 mg during the whole experimental period.
No ill-effects were observed in general appearance or growth. The
mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments
It is clear from the biochemical studies that PMA may give rise to
mercury accumulation in the tissues and the long-term study in the rat
failed to demonstrate a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per day,
produced a slight effect in the rat. Even if this figure were to be
adopted as a maximum no-effect level and the customary safety factor
applied this would give an acceptable daily intake for man of 0.00005
mg/kg body-weight. This is tantamount to zero. It is undesirable that
for the general population there should be any increase in the natural
intake of mercury.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) General properties
The suitability of a particular organomercury compound for a
particular purpose is partly dependent upon its physical properties.
Phenyl mercury acetate and other aryl compounds, which have a low
volatility, are more suitable where persistence is required. Under
certain conditions however (e.g. in enclosed seed dressing machinery)
their higher volatility enables the alkyl compounds to be applied more
effectively. Phillips, Dixon & Lidzey (1959) list the vapour pressures
of many of these compounds and compare their volatilities under
different conditions.
(b) Pre-harvest treatments
Organomercury compounds are used in seed dressings, orchard sprays,
foliar dusts and as glass house aerosols for the control of fungal
diseases. Since analysis seldom distinguishes between organically
bound and inorganic mercury, it is worth noting also that inorganic
mercury compounds are used for soil and root treatment. Powdered and
liquid preparations containing both alkyl and aryl mercury compounds
are used as cereal seed dressing. Ethylmercury phosphate is fairly
specifically used on beet seed and this and other seed dressings are
likely to lead to residue problems. Phenylmercury foliar dusts are
used to control rice blast disease.
(c) Post-harvest treatments
Organomercurials are not used post-harvest other than as bulb, tuber
and cereal seed dressings. It is possible that small residues may be
picked up e.g. by fruits, from paper wrappings or other containers
which have been treated with organomercurial fungicides.
(d) Other uses
Organomercury compounds, particularly phenylmercury salts, are used
widely as fungicides in industry, e.g. on wood pulp, paper and various
building materials.
Tolerances
Existing tolerances all refer to residues found and expressed as
mercury (Hg) without distinction between the forms in which the metal
may actually be present in food. Uses of organomercurials which could
lead to a detectable residue in food produce are not allowed in the
United States.
Residues resulting from supervised trials
(a) Pre-harvest treatments
A number of workers have measured residues in apples (summarized by
Miller, 1957) and tomatoes, (e.g. Beidas & Higgins 1957, 1959; Ross
& Stewart, 1960; Stone, 1962). Foliar applications of organomercurials
in general lead to translocation of the mercury. Pickard & Martin
(1963) studied the uptake of mercury by roots, leaves and fruits after
applications at commercial rates. The roots of lettuce and dwarf
bean plants accumulate mercury from nutrient solution containing
phenylmercuric acetate, but little translocation to the foliage
occurs. Root treatment of cauliflower seedlings with calomel or
mercuric chloride before planting lead to absorption by the roots but
the curds are uncontaminated. Carrots grown in soil treated with
mercuric chloride contained up to 0.05 ppm and roots from
calomel-treated soil showed 0.02 ppm Hg when seed was sown immediately
after soil treatment; delay in seeding eliminated contamination.
Lettuces, dwarf beans, carrots, potatoes and turnips from field
experiments using calomel and mercuric oxide soil treatments showed
mercury residues not exceeding 0.03 ppm. Apple leaves absorbed
mercury deposited as phenylmercuric acetate, within a few days.
Mercury was found in young coffee and citrus lime leaves that
emerged after spraying with phenylmercuric acetate, indicating
translocation. Broad bean plants sprayed with phenylmercuric
acetate at early flowering later showed 0.02 ppm in the pods, 0.04 ppm
in the seeds and 0.07 ppm Hg in the roots. Application of
phenylmercuric acetate to the leaves of potato plants led to
residues in the tubers (0.1 ppm in peel, 0.18 ppm Hg in flesh) and
roots (1.2 ppm Hg). Apples from commercially sprayed orchards
contained 0.05 ppm Hg distributed between the peel, flesh and core.
Five applications of phenylmercuric acetate under experimental
conditions, gave 0.24 ppm on whole fruits; one third of the mercury
was located in the flesh. Mercury deposited on the surface of the
fruits was largely held in the cuticle; much of the mercury in the
flesh arose from translocation from the leaves. Mercury was detected
in the fruitlets (0.4 ppm) and young leaves (0.07 ppm) of trees
sprayed the previous year. The bark of trees treated for six
consecutive years contained 4 ppm Hg. Naturally-occurring mercury in
soils varied between 0.05 and 0.12 ppm. Soil from beneath sprayed
apple trees contained 0.2 ppm Hg. Soils treated with inorganic
mercurials showed up to 2 ppm Hg (untreated 0.05 ppm).
In Japan the stage of plant growth affected the amount of residual Hg
in rice. Unpolished rice harvested from the rice plant sprayed with
PMA before the plant comes to ear contained 0.12 ppm of Hg, whereas,
the grain harvested from the plant treated with PMA after the plant
comes to ear contained 0.23 ppm of Hg. Epps (1966) examined rice and
various milling by-products, from rice treated in field tests with
phenylmercury acetate; residues ranged from 1.3 ppm Hg in straw and
0.8 ppm in bran to 0.1 - 0.2 ppm in the endosperm.
Smart (1963, 1964) measured residues in the eggs, flesh, etc., of hens
fed wheat treated with methylmercury dicyandiamide and in potatoes
following foliar spray application with phenylmercury chloride. Only
about 10 per cent of an organomercury compound on dressed grain was
removed by washing. Translocation of residues from potato foliage to
tubers has been demonstrated by Ross & Stewart (1964). Egan & Lidzey
(1960) and Bland & Egan (1963) showed that successive treatments of
tomatoes with phenylmercury salicylate under commercial greenhouse
conditions resulted in residues of the order of 0.01 (n + 1) ppm where
"n" is the number of treatments up to the eighth; and falls thereafter
by about 0.01 ppm for each subsequent treatment.
Mercury residues measured in Japan (direct communications, 1965) were
found to be 0.098 + 0.008 ppm Hg in peel and 0.025 + 0.008 ppm
in flesh of mandarin orange. Untreated fruit contained 0.03 - 0.05 ppm
Hg in peel and 0.01 - 0.02 ppm Hg in flesh. The residues in mandarin
oranges sprayed with water-soluble mercurial fungicides (PMA or PMF)
were generally greater than those in fruit sprayed with less
water-soluble fungicides (PMI or PMO). After five foliar applications
of MMC (12.5 ppm as Hg) to peach trees, amounts of Hg were in the
range of 0.21 - 0.25 ppm in peel and 0.02 - 0.04 ppm in flesh. Similar
treatments of a mixture of PMPS, EMP, and EMU (12.5 ppm as Hg)
resulted in an Hg residue in the range of 0.27 - 0.28 ppm in peel and
0.06 - 0.07 ppm in flesh.
Residues in food moving in commerce
Since 1961 surveys have been undertaken in Sweden on the occurrence of
mercury in eggs, game, fish and various other foodstuffs. This has
been associated with enquiries into possible effects on wildlife from
the use of mercury fungicides in industry and agriculture. The
residues found in human food have ranged from a mean of 29 parts per
thousand million in eggs marketed in Sweden, to over 20 ppm in the
livers of some 10 per cent of the game birds examined. Wheat, thought
to be untreated, from various parts of the United States of America,
examined by atomic absorption spectroscopy by Pappas and Rosenberg
(1966) showed 0.013 to 0.13 ppm Hg and 1.5 ppm Hg in commercially
treated samples. The same authors found an average of 0.02 ppm in
haddock fillets while eggs were virtually mercury-free (less than
0.005 ppm). In the examination of various foods in Japan (direct
communication) residues found were: polished rice 0.04 - 0.07;
unpolished rice 0.07 - 0.14; wheat flour 0.05; fruits and vegetables
0.02 - 0.06; meats 0.03 - 0.16; fish 0.00 - 0.10, (tuna 0.3 - 0.55);
milk 0.01 and hen eggs 0.08. Small amounts of mercury are very
widespread in nature. The extent of general environmental
contamination has been reviewed by Goldwater (1964) who quotes results
of residue analysis of food including bananas (0.31 ppm) canned
chicken (0.15 ppm) butter (0.14 ppm) Cheddar Cheese (0.082 ppm)
white bread (0.08 ppm) beer (0.04 ppm) and rice (0.02 ppm).
In each of these instances the figures are quoted as ppm Hg. Figures
for "organic" and "inorganic" mercury have rarely been obtained, there
appear to be none for residues as specified organomercury compounds.
Residues at the time or consumption
The meeting had no data on the effects of storage, cooking and other
processing. Reductions no doubt occur from volatilisation during
storage and from washing of crops and trimming (e.g. of outside parts
of fruits and vegetables) prior to cooking. Some losses during
cooking, also are likely due to the volatility of these compounds, but
figures for such losses were not available.
Methods of residue analysis
There is an extensive literature on mercury residue analysis, the
greater part of which is concerned only with total mercury and not
with intact organomercury residues as such. There is a wide
divergence between the toxicities of the various mercury compounds and
often a lack of information concerning the chemical nature of the
residues (e.g. in edible animal material) after the use of a given
compound.
(a) Total mercury (inorganic and organic)
Relatively simple methods, such as the Reinsch test, have long been
used to detect traces of metals including mercury and, with
supplementary confirmatory tests, can be used to distinguish mercury
from other residues and approximately to measure them. Very many of
the modern total mercury methods are based on extraction of the metal
by organic complexing agents, principally diphenyl thiocarbazone
(dithizone) into chloroform solution (e.g. Kanazawa & Sato, 1959).
Examples are the IUPAC method of Ljunggren and Westermark (1960) for
the determination of mercury foodstuffs and the method of the
Association of Official Analytical Chemists (1965), both based on the
method of Klein (1952). Conditions can be adjusted so that mercury is
selectively extracted. There are a number of variations of the method
of measuring the extracted mercury complex. These are normally based
on the intensity of colour of the complex solution, or of the
uncomplexed dithizone remaining when a measured excess is used;
alternatively a combined titrimetric-colorimetric procedure is used in
which small measured amounts of standardized dithizone are added
successively to the prepared sample extract until no reagent colour
change occurs. Such methods, which are sensitive to 0.01 ppm of Hg or
less in favourable cases, have been applied to a wide range of
biological tissue including many foods.
A source of difficulty, which arises also in the more modern
radioactivation method of analysis (q.v.), lies in the relatively high
volatility of mercury and its compounds. Biological tissue is normally
first oxidized, e.g. by warming with nitric acid. If too much heat is
applied or is allowed to generate, some of the mercury may be lost
from the sample as in some of the earlier work of Klein, in which the
condensate was trapped in a closed (reflux) oxidation system the main
digest thus becoming more concentrated with respect to acid with a
consequent elevation in boiling point. Gorsuch (1950) has made a
thorough study of losses in wet ashing, using radiochemical methods;
he has in this way shown the need to condense digestion vapours and
return them to the main digest. Gutenmann & Lisk (1960) and Gouverneur
& Hoedman (1964) used a modified Schoniger combustion method in place
of wet digestion. Conditions which emphasize cool oxidation were
described by Abbott and Johnson (1957), and examined collaboratively
for apples and tomatoes in Britain by the Joint Mercury Residues Panel
(1961). A further collaborative study of a method for amounts of
mercury down to 0.5 micrograms in organic matter has been described by
the Society in Analytical Chemistry (1965). Truhaut and Boudene (1963)
have used a simple flask in which oxidation, complex extraction and
colorimetric measurement can be completed without transfer.
Radioactivation methods of residue analysis are sensitive to at least
0.01 ppm Hg and avoid the need for oxidation and extraction steps.
However, preliminary removal of water from biological tissue may be
necessary and this again poses the problem of volatility of mercury
and its compound. Erwall & Westermark (1964) described the application
of this technique to foods. An atomic pile is necessary for the
activation stage and where this is available the technique can be
widely applied provided the dehydration stage is satisfactory with a
chemical separation the sensitivity level is 0.005 ppm on a 0.2 gram
sample. This sensitivity could also be obtained with a gamma counter
without spectrometry. If the irradiated sample is counted direct
without chemical separation, a gamma spectrometer would have to be
used. The sensitivity would then depend on the other short-lived
activities produced (e.g. sodium-24) and 0.5 ppm on a 0.2 gram sample
appears to be reasonable. A typical conventional dithizone method has
a sensitivity of 0.02 ppm on a 10 gram sample. Neutron activation
analysis using Hg-203 (half-life 47 days) would be less sensitive than
using Hg-197 by a factor of 20 and a longer irradiation time would be
needed. In the method of Ljunggren & Westermark (1960) biological
material is sealed into quartz tubes, activated and 77-keV gamma
radiation emission from Hg-197 measured after a cooling period of two
days. This method is sensitive to five nanograms of Hg.
Schachter (1966) has described the use of atomic absorption
spectroscopy to mercury vapour in the cold state. High sensitivity is
achieved using the whole of the ultraviolet spectrum of a quartz
mercury vapour lamp. Thus one gram of ground wheat is combusted in a
Schoniger flask and the mercury vapour collected in dil. HCl,
converted to sulfide, pyrolysed in a train of nitrogen in a small
furnace at 650°C and the mercury vapour cooled and measured. Hamilton
& Ruthven (1966) have described a field apparatus for the detection
and estimation of organomercury dusts and vapours in the atmosphere; a
similar official method is published in Britain.
(b) Organically bound mercury
Limited work has been done on the separation and measurement of
unchanged organomercury compounds. Miller, Lissis & Csonka (1958)
extract phenylmercury compounds from animal's tissue with chloroform
and determine the phenylmercury adsorptiometrically as the dithizone
complex. Kimura & Miller (1964) use different extraction solvents for
(a) phenyl and alkyl mercurials and (b) diphenyl dialkyl mercurials
and (c) ionic mercury. The reaction of dithizone with organomercury
compounds has been reviewed by Irving & Cox (1961). It should be
possible to devise extraction procedures in which intact radicals such
as phenyl-mercury can be isolated (e.g. as the chloride) and then
separated by paper. Electrophoretic separations have also been studied
or thin layer chromatographic procedures and identified and measured.
RECOMMENDATION FOR TOLERANCES
Since no acceptable daily intake level can be given for mercury it is
not possible to recommend a tolerance or a temporary tolerance. Small
natural concentrations of mercury appear to be widespread but the
levels vary from area to area; it is also difficult therefore, to
suggest a practical residue limit for mercury. By way of guidance,
however, practical residue limits of from 0.02 ppm of mercury to 0.05
ppm, according to local conditions, are suggested.
Further work or information
Sensitive methods of analysis specifically for alkyl, alkoxy and aryl
mercurials should be developed and used to study the occurrence of
these forms of mercury in foodstuffs from different sources: these
should include food stuffs known to have been treated with specified
compounds. Such analytical methods should also be used to study the
possible conversion of aryl mercury compounds to more toxic ones.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Rylander, R. (1964) J. Pharmacol. exp. Ther., 146,
236
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G., Nelson, A. A., Laug, E. P. & King, F. M. (1950)
A.M.A. Arch. industr. Hlth., 2, 433
Gage, J. C. (1964) Brit. J. industr. Med., 21, 197
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek. A. (1938) Z. Untersuch.
Lebensmitt., 16, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Prickett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. S., and Johnson, E. I. (1957) The determination of traces
of mercury in apples Analyst, 82: 206-208
Association of Official Agricultural Chemists (1965) Methods of
Analysis, 10th ed.,: 375-377
Beidas, A. S., and Higgons, D. J. (1957) Mercury residues in sprayed
crops. I. Tomatoes. J. Sci. Fd. Agric., 8: 597-600
Beidas, A. S., and Higgons, D. J. (1959) Mercury residues in sprayed
crops. II. Apples. J. Sci. Fd. Agric., 10: 527-529
Bland, M. A., and Egan, H. (1963) Phenylmercury salicylate residues in
commercial glasshouse tomato crops. Plant Pathol, 12 (2): 59-62
Egan, H., and Lidzey, R. G. (1960) Phenylmercury salicylate residues
in commercial glasshouse tomato crops. Plant Pathol, 9 (3): 58-91
Epps, E. A. (1966) Colorimetric determination of mercury residues on
rice. J. Assoc. Offic. Anal. Chemists, 49 (4): 793-795
Erwell, L. G., and Westermark, T. (1965) Determination of mercury by
activation analysis. Isotope Techniques Laboratory, Sweden.
Sjöstrand, B. (1964) Simultaneous determination of mercury and arsenic
in biological and organic materials by activation analysis. Anal.
Chem., 36, 814-819
Food and Agriculture Organization of the United Nations. (1964)
Evaluation of the toxicity of pesticide residues in food. Meeting
report. PL/1963/13
Goldwater, L. J. (1964) Absorption and excretion of mercury in man.
VII. Significance of mercury in blood Arch. environm. Hlth., 9;
735-741
Goldwater, L. J. (1965) Mercury in our environment: address to the
Third Annual Scientific meeting of the British Industrial Biological
Research Association
Gersuch, T. T. (1959) Radiochemical investigations on the recovery for
analysis of trace elements in organic and biological materials.
Analyst, 84: 135-173
Gouverneur, P., and Hoedeman, W. (1964) Determination of mercury in
organic compounds. Anal. Chim. Acta, 30 (6): 519-583
Gutenmann, W. H., and Lisk, D. J. (1960) Rapid determination of
mercury in apples by modified Schöniger combustion. J. Agric. Fd.
Chem., 8 : 306-308
Hamilton, G. A., and Ruthven, A. D. (1966) An apparatus for the
detection and estimation of organo-mercury dusts and vapours in the
atmosphere. Lab. Pract., 15: 995-997
Irving, H., and Cox, J. J. (1961) Studies with dithizone. Part VIII.
Reactions with organometallic compounds. J. Chem, Soc.,: 1470-1479.
Joint Mercury Residues Panel. (1961) The determination of mercury
residues in apples and tomatoes, Analyst, 86: 608
Kanazawa, J., and Sato, R. (1959) Determination of mercury in organic
mercury fungicides by the dithizone method Bunseti Kagaku (Japan
Analyst), 8: 440-444
Kim, C. K., and Silverman, J. (1965) Determination of mercury in wheat
and tobacco leaf by neutron activation analysis using simple exchange
separation. Anal. Chem., 37: 1616-1617
Kimura, Y., and Miller, V. L. (1964) The degradation of organomercury
fungicides in soil. J. Agric. Fd. Chem., 12 (3): 253-257
Klein, A. K. (1952) Report on mercury. J. Assoc. Off. Agric.
Chemists., 35: 537
Ljunggren, K., and Westermark, T. (1960) A method for the detection of
mercury by radioactivation analysis. Pure appl. Chem., 1: 127-133
Miller, B. J. (1957) A note on mercury spray residues in apples.
Plant Pathol., 5: 119-121
Pappas, E. G., and Rosenberg, L. A. (1966) Determination of
submicrogram quantities of mercury (in fish and eggs) by cold vapour
atomic absorption photometry. J. Assoc. Offic. Anal. Chemists, 49 (4):
782-793
Phillips, G. F., Dixon, B. E., and Lidzey, R. G. (1959) The volatility
of organomercury compounds. J. Sci. Food Agric., 11: 604-610
Pickard, J. A. and Martin, J. T., (1960) Determination of mercury in
plant material. J. Sci. Fd. Agric., 11: 374-377
Ross, R. G., and Stewart, D. K. R. (1960) Mercury residues on apple
fruit and foliage. Can. J. Plant Sci., 40: 117-122. (Also see
Movement and accumulation of mercury in apple trees and soil, (1962),
Can. J. Plant Sci., 42: 280-285. Note on mercury residues in
potatoes, (1962), Can. J. Plant Sci., 42; 370 and Mercury residues
in potatoes in relation to foliar sprays of phenyl mercury chloride,
(1964) Can. J. Plant Sci., 44: 123-125)
Schachter, M. M. (1966) Apparatus for cold vapour atomic absorption of
mercury. J. Assoc. Off. Anal. Chemists, 49 (4): 778-782
Smart, N. A., and Lloyd, M. K. (1963) Mercury residues on eggs, Flesh
and livers of hens fed on wheat treated with methylmercury
dicyandiamide. J. Sci. Food Agric., 14: 734-740
Smart, N. A. (1963) Retention of organomercury compounds in dressed
grain after washing. Nature, 199: 1206-1207
Smart, N. A. (1964) Mercury residues in potatoes following application
of foliar spray containing phenylmercury chloride. J. Sci. Food
Agric., 15: 102-107
Society or Analytical Chemistry, Analytical Methods Committee. (1965)
The determination of small amounts of mercury in organic matter.
Analyst, 90: 515-530
Stone, H. M. (1964) Mercury content of apples treated with
phenylmercury dimethyl-dithiocarbamate. New Zealand J. Agr. Res.,
7: 439
Swensson, H., and Ulfarson, U. (1963) Toxicology of organic mercury
compounds used as fungicides. Occup. Hlth Rev., 15 (3): 5
Truhaut, R. and Boudene, C. (1963) Microdosage du mercure dans les
dendtrees alimentaires. Ann. Falsif. Expert. Chim., 56: 225-242
