
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
CHINOMETHIONAT
Explanation
Under the name of oxythioquinox, chinomethionat was first
evaluated by the JMPR in 1968.* At that time, no ADI was established
as a no-effect level could not be deduced from the long-term studies
performed in rats.
Animals at the lowest level of treatment (10 ppm) (in the 1974
monograph this dose was misleadingly stated to be 10 mg/kg) showed
liver hypertrophy.
On the basis of additional information reviewed by the JMPR in
1974, a temporary ADI of 0.003 mg/kg bw was allocated (no-effect
level in a long-term study in rats: 12 ppm). At that meeting, the
submission, by 1977, of the results of studies to identify and
evaluate the toxicity of metabolites was requested. Furthermore, the
presentation of the following information was considered desirable:
1. Results of studies on the relationship between observed liver
enlargement and reduced microsomal enzyme activity.
2. Results of studies on metabolism in non-rodent species.
3. Observations in humans.
For the 1977 JMPR, only some new metabolic data were submitted.
The temporary ADI, established in 1974, was extended and the
submissions before July 1981, of the results of the following
investigations was requested:
1. studies on the identity and relative toxicity of metabolites.
2. An additional carcinogenicity study in another species, in view
of the hepatic toxicity observed in rodents.
As in 1974, observations on humans were declared desirable.
The requested studies were not available for evaluation by the
1981 JMPR, but the Meeting was informed that a long-term study in mice
was in progress and should be available by 1985. A 12-month dog study
is also in progress.
Chinomethionat was evaluated by the 1968, 1974 and 1977 Joint
Meetings and a number of temporary maximum residue limits were
recommended.
* See Annex II for FAO and WHO documentation.
Some additional data on residues in treated and rotational crops
on the fate of residues in animals and on environmental behaviour have
been provided to the Joint Meeting for evaluation. The results of
experiments are discussed in the present evaluation.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special studies on mutagenicity in bacteria
Chinomethionat was tested with Bacillus subtilis (strains M45 and
H17) over the range of 20 to 2 000 µg/plate in the rec-assay, as well
as with Escherichia coli (strain WP2 hcr) and Salmonella
typhimurium (strains TA 1535, 100, 1537, 1538 and 98) over the range
of 1 to 5 000 µg/plate for reverse mutations. The latter studies
were performed both in the presence and absence of appropriate
activation systems derived from rats following induction by Arochlor
1254.
In none of these assays were signs of mutagenicity detected. In
one study performed with strains TA 100, 1537 and 98 (3.15 to
315 µg/plate), it was noted that the test substance precipitated in
the culture medium. It is therefore questionable whether the negative
results of these tests can be interpreted in a dose-related manner.
The lack of mutagenicity in bacteria appears to be proven only for the
dissolved fraction of chinomethionat and for its soluble impurities.
Special studies on embryotoxicity and teratogenicity
Three groups of 15 rabbits (Himalayan strain) each received 13
daily oral doses of 10, 20 or 100 mg/kg of chinomethionat between days
6 and 18 of gestation. A group of equal size, given the vehicle only
without the test substance, served as control.
On day 29 of gestation the dams were sacrificed and the foetuses
removed by caesarean section. Examination of the offspring by standard
methods and the evaluation of the results gave no indication of
teratogenicity.
On the other hand, in the group treated with 100 mg/kg of
chinomethionat, a significant decrease in both the number and size of
foetuses was found. In this group, pronounced signs of maternal
toxicity (e.g. diarrhoea, reduced feed intake, loss of weight) were
also observed,
No difference could, however, be detected between the two lower
dose groups (10 and 30 mg/kg) and the controls.
It is concluded that embryotoxicity at the 100 mg/kg dose level
is only secondary and merely the consequence of the toxicity of
chinomethionat to the dams.
Eye and dermal irritancy of chinomethionat
The left eye of 9 New Zealand white rabbits was treated with
100 mg chinomethionat per animal. After forty-five seconds, the
treated eyes of 3 rabbits were rinsed with 200 ml lukewarm water each.
No ocular changes were detected in these animals. The eyes of the
remaining 6 rabbits were not washed. As appraised by the method of
Draize, 2 of the 6 animals showed symptoms of severe eye irritation.
Four shaven test sites (on the size and the back), 2 of which
were abraded per animal, were exposed for 24 h to 0.5 mg of
chinomethional (moistened with physiological saline). Appraisal 24 h
and 72 h following the application of the test substance revealed no
signs of dermal irritation.
RESIDUES IN FOOD
USE PATTERN
In addition to the use patterns given in previous evaluations,
the registered use on cucumber in Japan was provided. Chinomethionat
is used up to 0.0125% against mildew and 0.025% against spider mites
on cucumber, which is equivalent to 0.25 mg a.i./ha and 0.5 mg a.i./ha
respectively.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Almond
Supervised trials were carried out in California by applying
chinomethionat as a foliar spray to almond trees at a rate of
1.7 kg/ha. In one experiment the samples were taken 57 days
post-treatment. Kernel, shells and hulls were analysed, and they
contained residues of <0.02 mg/kg, <0.02 mg/kg and 0.21-0.28 mg/kg,
respectively. In the other study hulls taken from various positions on
the trees at day 0 contained 2.7 mg/kg, 3.8 mg/kg and 8 mg/kg
chinomethionat in the top, middle and bottom portions, respectively.
Cucumber
Supervised trials were carried out on different varieties of
cucumber in the Federal Republic of Germany (Bayer 1975) and in Japan
(Nikon Tokushu Noyaku Seizo KK 1971). Chinomethionat was applied at a
rate of 0.075 to 0.75 kg/ha 1 to 10 times, most frequently at
intervals of 5 to 7 days. The results are summarized in Table 1.
TABLE 1. Residues of chinomethionat in cucumber
Application Residues (mg/kg) at intervals (days) after
Country Year No. Rate last application
(kg a.i./ha) Formulation
0 1 3 4 to 5 7 10
Federal 1976 8 0.075 25 WP <0.02 <0.02
Republic 1976 8 0.075 25 WP <0.02 <0.02
of Germany 1975 8 2x0.075+2x
0.125+4x 25 WP 0.07 0.05 <0.02
0.25
3 to 5 days apart
1975 8 4x0.075+0.125
+3x0.25 25 WP 0.025 0.011 0.012 0.007
3 to 5 days apart
1976 8 0.125
6 to 7 days apart 25 WP 0.07 0.04 0.08 0.15
1976 8 0.15 1 25 WF 0.02 0.06 0.04
5 to 8 days apart
Japan 1971 5 0.23-0.46 25 WP 0.07 0.04 <0.02
5 days apart
10 0.23-0.46 25 WP 0.1 0.03 <0.02
5 days apart
1 0.38 25 WP 0.063
2 0.38)5 days 25 WP 0.046
3 0.38)apart 25 WP 0.064 0.036 0.015
1 0.5 25 WP 0.11
2 0.5)5 days 25 WP 0.13
3 0.5)apart 25 WP 0.18 0.1 0.02
1 0.75 25 WP 0.48
2 0.75)5 days 25 WP 0.37
3 0.75) apart 25 WP 0.61 0.34 0.11
5 0.25 7 days apart 25 WP 0.17 0.09 0.04 0.02
1 The seventh treatment was 12 days before the last application.
Currant
Chinomethionat was applied two times, 13 days apart, at a rate of
0.15 kg/ha. Samples were taken 7, 14, 21 and 28 days post-treatment;
the residue content was found to be 0.03, 0.015, 0.008 and
0.005 mg/kg, respectively (Bayer 1974).
Melon
Supervised trials were carried out at various locations in the
USA and in Japan and Mexico on different varieties.
Chinomethionat was applied at a dose rate of 0.15 to 0.5 kg/ha 4
to 10 times at intervals of 5 to 10 days. Samples were taken at 0 to 8
days post-treatment. The pulp and the rind or peel were analysed
separately. The weight of different portions was measured at the time
of analysis and the residue was calculated on a whole fruit basis. The
results are summarized in Table 2 (Mobay 1979; Nikon Tokushu Noyaku
Seizo KK 1976).
The residue in the pulp was generally lower than the limit of
determination (< 0.005 to 0.01 mg/kg); however, in few cases, 0.02
and on one occasion 0.03 mg/kg were detected, while the rind contained
< 0.01 - 0.06 mg/kg chinomethionat. In one sample 0.9 mg/kg was
measured at day 0.
Kaki persimmon
Chinomethionat was applied at a rate of 0.5 - 0.75 kg/ha to kaki
persimmon 3 to 5 times in four experiments. The last application
followed the previous ones 15 or more days later. Eight samples were
taken 12 to 57 days after the last application. The residue ranged
from 0.005 to 0.036 mg/kg at the 12 to 39-day interval, while it was
undetectable (<0.005 mg/kg) later (Nikon Tokushu Noyaku Seizo KK
1973).
Orange
Supervised trials were carried out in California in 1977-1978 and
in Japan in 1974 (Mobay 1978; Nikon Tokushu Noyaku Seizo KK 1974).
Chinomethionat was applied 1 to 3 times at intervals of 1 month
or longer at a dose rate of 0.15 to 7 kg/ha.
In California the same orchards were treated in spring, when
samples were taken from 0 to 6 days post-treatment, and in autumn,
when fields were sampled 14 to 35 days after the last application.
Peel and pulp were analysed separately and the residue was calculated
on a whole fruit basis. The results are summarized in Table 3.
TABLE 2. Residues of dhinomethionat in melons
Application Residues in whole fruit (mg/kg) at
Crop Country Year No. Rate intervals (days) after application
(kg a.i./ha) Formulation
0 1 2 7-8
Cantaloupe Mexico 1979 6 0.15 5x7 27 25 WP 0.03
days apart
Honeydew Mexico 1979 9 0.5 7 to 9 days 25 WP <0.01 <0.01
apart
USA
Arizona 1979 6 0.42 8 to 10 25 WP 0.43 0.35 0.11
days apart
Missouri 1977 5 0.42 6 to 7 25 WP 0.01 <0.01 0.01
days apart
Florida 1977 6 0.42 9 to 10 25 WP 0.43 0.01 0.01
days apart
Texas 1976 6 0.42 9 to 10 25 WP 0.09 0.02 0.01
days apart
Muskmelon Arizona 1977 6 0.42 7 days 25 WP 0.01 <0.01 0.01
apart
Florida 1977 6 0.42 10 days 25 WP 0.01 0.03 0.01
apart
Japan 1976 4) 0.25 to 0.63 25 WP <0.0051 <0.0051 <0.0051
8) 6 days apart 25 WP <0.0051 <0.0051 <0.0051
Japan 1976 5) 0.37 25 WP <0.0051 <0.0051 <0.0051
10) 5 to 6 days
apart 25 WP <0.0051 <0.0051 <0.0051
TABLE 2 (con't)
Application Residues in whole fruit (mg/kg) at
Crop Country Year No. Rate intervals (days) after application
(kg a.i./ha) Formulation
0 1 2 7-8
USA
Watermelon Texas 1976 6 0.42 t0 to 12 25 WP <0.01 <0.01
days apart
Florida 1977 6 0.42 10 days 25 WP 0.04 0.02 0.01
apart
Arizona 1977 6 0.42 2x10+13+
5+4 days apart 25 WP 0.03 0.02 0.02
1 Pulp only.
TABLE 3. Residues resulting from supervised trials on orange
Application Residues (mg/kg) at intervals (days) after last application
Country Year No. Rate Portion
(kg a.i./ha) Formulation analysed 0 3 6 14 21 28 35 >46
USA
California 1977 1 1.4 25 WP whole 0.15 0.23 0.05
1978 pulp 0.06 0.1 0.02
1 1.4 25 WP whole 0.12 0.08 0.08 0.05
pulp 0.03 0.02 0.01 0.01
1 1.4 25 WP whole 0.13 0.09 0.18
pulp 0.03 0.03 0.05
1 1.4 25 WP whole 0.05 0.03 0.03 0.03
pulp 0.02 0.01 0.01 0.01
1 1.4 25 WP whole 0.12 0.14 0.08
pulp 0.04 0.07 0.03
1 1.4 25 WP whole 0.05 0.02 0.03 0.02
pulp 0.02 0.01 0.01 0.01
1 7 25 WP whole 0.14 0.07 0.09
pulp 0.03 0.01 0.03
1 7 25 WP whole 0.21 0.06 0.07 0.05
pulp 0.09 0.01 0.01 <0.01
1 7 25 WP whole 0.13 0.11 0.13
pulp 0.07 0.02 0.03
TABLE 3 (con't)
Application Residues (mg/kg) at intervals (days) after last application
Country Year No. Rate Portion
(kg a.i./ha) Formulation analysed 0 3 6 14 21 28 35 >46
1 7 25 WP whole 0.07 0.02 0.04 0.03
pulp 0.01 0.01 0.01 <0.01
2 7 3 months 25 WP whole 0.08 0.15 0.08
apart pulp 0.03 0.05 0.03
1 7 25 WP whole 0.14 0.03 0.06 0.06
pulp 0.04 0.01 0.02 0.01
1 4.2 25 WP whole 0.1 0.09 0.03
pulp 0.05 0.04 0.01
1 4.2 25 WP whole 0.06 0.02 0.02 <0.01
pulp 0.05 0.01 0.01 <0.01
1 0.15 25 WP whole 0.12 0.02 0.07
pulp 0.02 0.01 0.02
1 0.15 25 WP whole 0.03 0.02 0.03 0.03
pulp 0.02 0.01 0.02 0.02
1 0.15 25 WP whole 0.05 0.13 0.04
pulp 0.02 0.02 0.01
whole 0.04 0.02 0.03 0.02
pulp 0.01 0.01 0.01 0.01
TABLE 3 (con't)
Application Residues (mg/kg) at intervals (days) after last application
Country Year No. Rate Portion
(kg a.i./ha) Formulation analysed 0 3 6 14 21 28 35 \>46
Japan 1 0.88 25 WP whole1 <0.02
3 0.88 1 month 25 WP pulp 1 <0.02 <0.02
apart
1 Two sets of experiments.
Residue in the pulp was always below 0.1 mg/kg and <0.05 mg/kg
6 days after last application, independently of the dosage. The peel
contained residue in the range of 0.15 to 0.51 mg/kg at 0 to 6 day
intervals and the residue decreased in the peel. The residue was
always below 0.2 mg/kg on whole fruit basis.
Strawberry
In England strawberries were treated two times at a 44 day-
interval. Samples taken from the two plots 14 days after the last
application contained 0.01 and <0.01 mg/kg residue (Bayer 1969).
In Japan, the plots were treated 2 or 4 times at 5-day intervals
at a rate of 0.4 kg/ha. The fruits were analysed 1, 5 and 10 days
after last application, and contained residues of 0.31, 0.14 and
0.08 mg/kg after 2 treatments and 0.3, 0.21 and 0.08 mg/kg
respectively after 4 treatments (Nikon Tokushu Noyaku Seizo KK 1978).
Tomato
Tomatoes grown in a greenhouse were treated 1 to 3 times at 5-day
intervals with chinomethionat at a rate of 0.5 kg/ha (Nikon Tokushu
Noyaku Seizo KK 1978). Samples taken 1 to 7 days after last
application were analysed. The residues detected are summarized in
Table 4.
TABLE 4. Residues resulting from supervised trials on tomato
Residue (mg/kg) after application (days)
No. of treatments 1 3 7
1 0.05
0.24
2 0.07
0.26
3 0.12 0.1 0.12
0.38 0.26 0.18
FATE OF RESIDUES
In animals
Two dairy cows were fed a ration containing 20% chinomethionat-
treated almond hulls, which were from almonds treated 56 days prior to
harvest with Morestan 25 WP at a rate of 1.7 kg/ha, or on which
(2,3-14C)-chinomethionat was applied individually in a quantity
approximating the average chinomethionat residue detected on the non-
radioactive chinomethionat-treated almonds. The almond hull feed
contained approximately 0.08 mg/kg chinomethionat equivalent.
Radioactive residues in milk reached a maximum 10 days after the
feeding study began, but never exceeded 0.008 mg/kg. Radioactive
residues (mg/kg) in the tissues were: liver 0.03; kidney 0.03; heart
< 0.02; muscle < 0.02; brain < 0.02; and fat 0.02 (Murphy et al
1977).
The results of this experiment support the conclusions drawn from
studies on other commodities and animals that indicate low biological
availability of plant residues.
Bluegill fish, Leponis macrochirus, were continuously exposed
to (2,3-14C)chinomethionat for 14 days at 1 µg/kg concentration in a
continuous flow system (Lamb 1975). Fish were collected from aquaria
on day 0 (15 min, 1, 2, 4 and 8 h) on days 1, 2, 4, 7 and 14 of the
exposure period, and on day 1, 2, 4, 7, 14, 21, 28 and 51 of the
withdrawal period. During the first 7 days, 14C residues in the fish
tissue constantly increased to a maximum level of approximately 1 100
times the level in the water. No further accumulation was found after
14 days, and it appears that an accumulation factor plateau of 700 to
1,100 was established between 4 and 7 days of exposure.
On the seventh day of exposure, the nonedible portion (head,
viscera and scales) accounted for 45% of the body weight and contained
93% of the extractable residues. Only 10% of the whole body 14C
residue was extractable in hexane, while 90% was extracted into the
polar solvent (acetonitrile).
Following transfer of fish to uncontaminated water, whole body
14C residues declined at a slow rate. After 51 days of withdrawal,
approximately 10% of the accumulated residues remained in the fish
tissue.
In plants
Uptake of chinomethionat soil residues by rotational crops was
studied under greenhouse conditions. (13C, 14C) chinomethionat, as
25 WP, was added to the soil in a water solution at a rate equivalent
to 0.42 kg a.i./ha. The applications were made 6 times at 10-day
intervals.
Lettuce, red beets and oats were chosen to represent the leafy
vegetable, root and grain rotational crops respectively. The plants
were planted 30 days, 120 days and 1 year after the last of the 6
applications and they were harvested when their maturity was estimated
to be 1/4, 1/2, 3/4 and complete. The total radioactive residue in all
plants was less than 0.1 mg/kg at any time, with the exception of
lettuce harvested at 1/4 maturity from soil treated 1 year before and
of oat foliage, in which 0.2 mg/kg and 0.12-0.26 mg/kg were found,
respectively. The soil residue ranged from 0.72 to 1.05 mg/kg dry soil
during the study (Stoner 1980). The total radioactive residue found in
various portions of plants at harvest are summarized in Table 5.
TABLE 5. Residues in plants grown in soil treated with chinomethionat
before planting
Portion of plant Residue (mg/kg)
30 days 120 days 365 days
Lettuce 0.015 0.007 0.011
Red beet
root 0.047 0.019 0.012
tops 0.03 0.016 0.015
Oats
foliage 0.077 0.261 0.122
grain 0.017 0.061 0.056
In soil
The metabolism of chinomethionat was studied in sandy loam and
silt loam under aerobic, anaerobic and sterile conditions after
application of 14C-chinomethionat, formulated as a 25% wettable
powder, at 2.46 kg a.i./ha (Shaw II 1980).
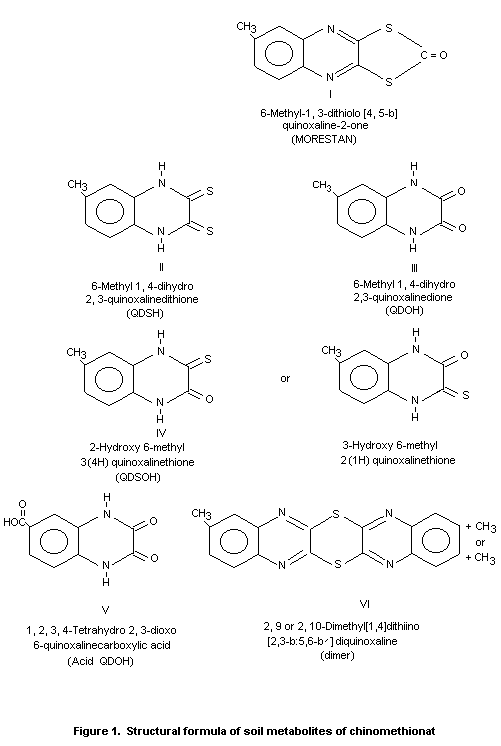
The major metabolite identified in these soils was 6-methyl
1,4-dihydro 2,3-quinoxalinedione (QDOH). During a year of soil
incubation, QDOH reached a maximum content of 57% of applied
radioactivity in sandy loam at 180 days and 42% in silt loam by 90
days. It then decreased to 48% in sandy loam and 30% in silt loam at
one year post-treatment.
Minor metabolites identified and their maximum proportion, found
under aerobic condition respectively in sandy loam and silt loam, were
2-hydroxy or 3-hydroxy 6-methyl 2(1H) quinoxalinethione (QDSOH) 16%
and 15%; 6-methyl 1,4-dihydro 2,3-quinoxalinedithione (QDSOH) 4% and
10%; 1, 2, 3, 4-tetrahydro 2,3-dioxo 6-quinoxalinecarboxylic acid
(acid QDOH) 4% and 4%; and 2,9 or 2,10 dimethyl (1,4 dithiino-)2,3-b:
5,6-b')-diquinoxaline (dimer) 1%. The structural formulas of these
metabolites are shown in Figure 1.
The aerobic and anaerobic conditions resulted in similar
metabolites and rate of metabolite formation, except possibly in the
formation of acid QDOH. Soil microorganisms, however, did appear to be
involved in the degradation of chinomethionat, as its half life under
sterile conditions was 52 days in sandy loam and 18 days in silt loam,
in contrast to the 4 day and < 1 day half lives under nonsterile,
aerobic conditions. Hydrolysis, carried out primarily by soil
microbes, was the main mechanism for degradation of chinomethionat.
Other than radioactive carbon dioxide (< 1%) no volatile
radioactivity was detected. During the one-year study, 85% to 97% of
the applied radioactivity in the sandy loam and 77% to 90% in the silt
loam were identified or characterized.
Runoff characteristics of chinomethionat were studied on apple
trees treated once with spray containing 0.08% a.i. or four times with
0.03% a.i. spray. Simulated and natural rainfall were measured and the
runoff was collected in buckets from a ditch dug just outside the
periphery of the foliage. Runoff on the soil surface was tested on
three soil types, by collecting runoff water from the area treated at
3.36 kg a.i./ha after the water had traversed 1.5, 3 and 6 m of
untreated soil.
The runoff water under the apple tree collected after 3.8 mm rain
on the day of application contained less than 1% of the applied
material. Following 2mm of rain 2 days post-treatment, the runoff
water from silt loam soil on an 8.5% slope contained 0.185, 0.079 and
0.031% of applied chinomethionat at the end of test soil plots of 1.5,
3 and 6 m length respectively (Flint and Shaw 1970).
The low mobility of chinomethionat was also indicated by
adsorption and soil column leaching experiments. Adsorption
coefficients of chinomethionat (kd) in soil/water systems at 0.05,
0.2 and 1 mg/kg concentration in water were 52.5 g/ml in sandy loam,
45 g/ml in silt loam and 90 in high organic silt loam (Flint and Shaw
1970). Shaw (1979) found the adsorption coefficients (Ka) determined
by the Freudlich equation to be 37 for sandy loam and 41 for silt loam
over the concentration range of 0.5 to 12.1 mg/kg. After leaching in a
soil column, the chinomethionat remained in the upper layer of soil
(Flint and Shaw 1970). Column leaching of chinomethionat on three
different soils (organic carbon content 0.51%, 0.69% and 2.89%)
resulted in non-detectable residue in the leachates (Bayer 1976).
 Leaching characteristics of aged chinomethionat soil residues
were studied in two sets of experiments. Sandy loam soil was fortified
at 7.05 mg/kg with (13C, 14C)-chinomethionat, followed by incubation
at room temperature for 30 days. Of the original applied activity, 12%
was lost during the ageing process, probably due to volatilization. In
three replicates, the aged soil was placed on top of sandy loam soil
columns, which were 30 cm high and 4.3 cm in internal diameter.
Leaching was effected by passing water, equivalent to 1.25 cm of
rainfall daily, through the columns for 45 days. Only 4% of the
applied radioactivity was found in the water leaching gradually over
the 45-day period, with no apparent peak, while nearly 90% of the
activity was retained in the upper 1.25 cm of the column.
The residue in soil contained primarily QDOH (39%), QDSH (13%),
QDSOH (6%) and chinomethionat (4%). About 1.5+1.1% of applied activity
was associated with the insoluble humin fraction of soil, and the
remainder of the activity distributed into the fluvic (98 ± 1.1%) and
humic (4.3%) acid fractions (Obrist 1979). The residues in the
leachate were identified in the other experiments carried out with
standard and modified soil columns type 2.1 (Vogeler 1981). The water
collected under the modified soil column contained 6.8 and 6.9% of
applied activity. Two metabolites, identified in water, were QDOH and
a small amount of QDSH.
In water
Technical chinomethionat was dissolved in phosphate buffers of
pH 5 and pH 7, with the final concentration being approximately
0.05 mg/kg. The solutions were placed in capped amber bottles and
maintained in constant temperature water baths at 30 and 50°C. The
concentration of chinomethionat decreased to half of the initial value
within 109 hours at 30°C and 27 hours at 50°C. An increase of pH from
5 to 7 decreased the water stability of chinomethionat three and five
fold, respectively.
In the outdoor experiment, conducted in a mini-pond containing
5 cm silt and 25 cm of surface water of pH 7, 100 mg/kg of
chinomethionat decomposed rapidly, showing a half life of 3.4 h in a
7-hour experiment during which the temperature of water increased from
27°C to 37°C (Flint and Shaw 1970).
Photodegradation
In a laboratory experiment, 14C-chinomethionat applied to a thin
layer of soil on a glass plate was irradiated with light from a high
intensity, mercury vapour lamp. Soil samples were analysed after 1, 2,
2.2, 3.5, 5, 7, 11 and 12 days exposure. Several minor products were
detected. Chinomethionat was the major residue component at each
sampling time, although several minor photolysis products were
detected. The "dimer" and QDOH were tentatively identified.
Disappearance of chinomethionat was influenced by several
factors. It decreased by approximately 50% during the first day but
declined more slowly subsequently. After 12 days, about 25% of the
initial concentration was still detectable on the plates (Mulkey
et al 1980).
EVALUATION
COMMENTS AND APPRAISAL
Additional data evaluated by this Meeting further extend the
basis for the toxicological characterization of chinomethionat. The
outcome of studies on mutagenicity in bacteria and in mice, as well as
on embryotoxicity and teratogenicity in rabbits, supports earlier
evaluations. It was noted that a long-term study performed on an
additional species is still outstanding. However, the Meeting was
informed that a long-term study in mice and a 1-year study in dogs
were in progress. On this basis, the Meeting decided to extend the
temporary ADI of 0.003 mg/kg until 1984.
Chinomethionat was last evaluated by the 1977 Joint Meeting.
Since then, some additional data have been made available for
evaluation of residues. Results of supervised trials on almond and
currant showed a residue pattern similar to that of the previous
experiments, while in orange the residue was lower and did not exceed
0.2 mg/kg on a whole product basis.
The residue in orange pulp was always below 0.1 mg/kg and
<0.05 mg/kg at 6 days after last application. The peel contained
residue in the range of 0.15 to 0.51 mg/kg at 0 to 6 days interval.
The residue in cucumber, treated at the recommended rates up to
0.5 mg a.i./ha, did not exceed 0.1 mg/kg, which is the present TMRL.
The results of these supervised trials support the maximum residue
levels recommended by the 1974 Joint Meeting.
Residues in melon, kaki persimmon, strawberry and tomato
were evaluated for the first time. In melon and kaki persimmon,
chinomethionat residue was low, i.e. less than 0.1 and 0.05 mg/kg at
the time of harvest, and concentrated in the peel or rind.
The residues in strawberry were less than 0.5 mg/kg at day 1
post-treatment even after 4 applications and declined to under
0.1 mg/kg 9 days later. Tomatoes treated in a greenhouse contained
residue in the range of 0.05 to 0.38 mg/kg at 1 to 3 days after last
application. A feeding study was carried out on dairy cows kept on a
diet consisting of 20% chinomethionat-treated almond hulls containing
0.08 mg/kg chinomethionat equivalent. Radioactive residues in milk
reached a maximum 10 days after the feeding study began, but never
exceeded 0.008 mg/kg chinomethionat equivalent. Radioactive residues
were 0.03 mg/kg in liver and kidney and <0.02 mg/kg in other tissues.
These results confirmed the low biological availability of plant
residues found in previous experiments.
A plateau of the accumulation factor in bluegill fish was
observed in the range of 700 to 1 100 after 4 to 7 days of exposure.
Approximately 10% of the accumulated residue remained in the fish 51
days after withdrawal.
The metabolism of chinomethionat was studied in sandy loam and
silt loam under aerobic, anaerobic and sterile conditions. The major
metabolite identified in these soils was 6-methyl 1,4-dihydro
2,3-quinoxalinedione (QDOH). Its maximum concentration ranged between
42 and 57% of applied radioactivity. Minor metabolites identified and
their maximum concentrations found under aerobic conditions were
2-hydroxy or 3-hydroxy 6-methyl 2 (1H) quinoxalinethione (QDSOH) 15 to
16%; 6-methyl 1,4-dihydro-2,3-quinoxalinedithione (QDSH) 4 to 10%;
1, 2, 3, 4-tetrahydro 2,3-dioxo 6-quinoxaline carboxylic acid (acid
QDOH) 4%, and 2, 9-or 2,10-dimethyl (1,4)-dithiino (2,3-b:5,6-b'-
diquinoxaline (dimer) 1%. Aerobic and anaerobic conditions resulted in
similar metabolites. Microbial degradation is of primary importance,
as the hydrolysis carried out by soil microbes appeared to be the main
mechanism for degradation of chinomethionat. The concentration of
chinomethionat declined rapidly in soils and was about 6 to 10% of the
applied dose 30 days after soil treatment.
At harvest, the total radioactive residue in lettuce, red beet
and oats planted 30 to 65 days after soil treatment with radioactive
chinomethionat were in the range of 0.03 to 0.06 mg/kg. Although
rotational crops can take up soil residues, owing to the rapid
decomposition of chinomethionat, no intact parent compound can be
expected in detectable amounts in mature crops planted 30 days or
later post-treatment.
Studies on the runoff characteristics of chinomethionat from
treated trees or from a soil surface revealed that less than 1% of
the applied dose could be washed off by rain, even on the day of
application. The low mobility of chinomethionat and its soil
metabolites was also indicated by adsorption, desorption and soil
column leaching experiments. The concentration of chinomethionat
decreased to half of initial value within 109 hours at 30°C in
phosphate buffer of pH 5. At pH 7 the rate of degradation increased
three fold.
Level causing no toxicological effect
Rat : 12 ppm in the diet equivalent to 0.6 mg/kg bw/day,
Estimate of temporary acceptable daily intake for man
0 to 0.003 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of new data, the Meeting recommends the following
additional residue limits:
PHI on which
Commodity Limits recommendations are
based (days)
Melon 0.1 3
Watermelon 0.02 3
Strawberry 0.2 7
Kaki persimmon 0.05 14
FURTHER WORK OR INFORMATION
Required (by 1984)
1. The submission of the studies known to be in progress.
Desirable
1. Information on use pattern on tomato and additional residue data
deriving from supervised trials.
REFERENCES
Bayer Residue Reports, Nos. 322-323/69.
1969
1974 Residue Report, No. 8400/74 (black currant).
1975 Residue Reports, Nos. 8400-8405/75 (cucumbers),
1976 Leaching studies. Reports Nos. 8400-8402/76.
Flint, D.R. and Shaw, H.R. Soil runoff, leaching, adsorption and water
1970 stability studies with MORESTAN. Mobay Report No. 28 365.
Lamb, D.W., Roney, D.J. and Jones, R.E. Accumulation and persistence
1975 of residues in bluegill fish exposed to MORESTAN-14C. Mobay
Report No. 44 329.
Mobay Residue Reports, Nos. 613-66621. 1978
1979 Residue Reports, Nos. 51125, 51126, 51384, 51706, 51707,
53050, 53051, 53082, 67200-67203.
Mulkey, N.S., Herrera, R., Breault, G.O. and Wargo Jr., J.P.
1980 Photodegradation of 14C-MORESTAN on soil surface. Mobay
Report No. 68 881.
Murphy, J.J. et al. 14C MORESTAN - Almond hull - dairy cow feeding
1977 study. Mobay Report No. 53 619.
Nikon Tokushu Noyaku Seizo KK. Residue Reports, Nos. 98, 268, 622,
1971 623, 795 (cucumbers).
1973 Residue Reports, Nos. 242, 243.
1974 Residue Reports, Nos. 95, 96.
1976 Residue Reports, Nos. 431, 432
1978 Residue Reports, Nos. 100, 620, 621.
Obrist, J.J. Leaching Characteristics of Aged MORESTAN Soil Residues.
1979 Mobay Report No. 67 821.
Shaw II, H.R. Adsorption and desorption of (13,14C)-MORESTAN by Soils.
1979 Mobay Report No. 67 484.
1980 Metabolism of MORESTAN in soil. Mobay Report No. 67 820.
Stoner, J.H. MORESTAN 14C. A rotational crop study. Mobay Report No.
1980 69 199.
Vogeler, K. Untersuchungen zum leachingverhalten von Chinomethionat.
1981 Bayer AG, Pflanzenschutz-Anwendungstechnik, Report RA - 194.
(Unpublished)
Leaching characteristics of aged chinomethionat soil residues
were studied in two sets of experiments. Sandy loam soil was fortified
at 7.05 mg/kg with (13C, 14C)-chinomethionat, followed by incubation
at room temperature for 30 days. Of the original applied activity, 12%
was lost during the ageing process, probably due to volatilization. In
three replicates, the aged soil was placed on top of sandy loam soil
columns, which were 30 cm high and 4.3 cm in internal diameter.
Leaching was effected by passing water, equivalent to 1.25 cm of
rainfall daily, through the columns for 45 days. Only 4% of the
applied radioactivity was found in the water leaching gradually over
the 45-day period, with no apparent peak, while nearly 90% of the
activity was retained in the upper 1.25 cm of the column.
The residue in soil contained primarily QDOH (39%), QDSH (13%),
QDSOH (6%) and chinomethionat (4%). About 1.5+1.1% of applied activity
was associated with the insoluble humin fraction of soil, and the
remainder of the activity distributed into the fluvic (98 ± 1.1%) and
humic (4.3%) acid fractions (Obrist 1979). The residues in the
leachate were identified in the other experiments carried out with
standard and modified soil columns type 2.1 (Vogeler 1981). The water
collected under the modified soil column contained 6.8 and 6.9% of
applied activity. Two metabolites, identified in water, were QDOH and
a small amount of QDSH.
In water
Technical chinomethionat was dissolved in phosphate buffers of
pH 5 and pH 7, with the final concentration being approximately
0.05 mg/kg. The solutions were placed in capped amber bottles and
maintained in constant temperature water baths at 30 and 50°C. The
concentration of chinomethionat decreased to half of the initial value
within 109 hours at 30°C and 27 hours at 50°C. An increase of pH from
5 to 7 decreased the water stability of chinomethionat three and five
fold, respectively.
In the outdoor experiment, conducted in a mini-pond containing
5 cm silt and 25 cm of surface water of pH 7, 100 mg/kg of
chinomethionat decomposed rapidly, showing a half life of 3.4 h in a
7-hour experiment during which the temperature of water increased from
27°C to 37°C (Flint and Shaw 1970).
Photodegradation
In a laboratory experiment, 14C-chinomethionat applied to a thin
layer of soil on a glass plate was irradiated with light from a high
intensity, mercury vapour lamp. Soil samples were analysed after 1, 2,
2.2, 3.5, 5, 7, 11 and 12 days exposure. Several minor products were
detected. Chinomethionat was the major residue component at each
sampling time, although several minor photolysis products were
detected. The "dimer" and QDOH were tentatively identified.
Disappearance of chinomethionat was influenced by several
factors. It decreased by approximately 50% during the first day but
declined more slowly subsequently. After 12 days, about 25% of the
initial concentration was still detectable on the plates (Mulkey
et al 1980).
EVALUATION
COMMENTS AND APPRAISAL
Additional data evaluated by this Meeting further extend the
basis for the toxicological characterization of chinomethionat. The
outcome of studies on mutagenicity in bacteria and in mice, as well as
on embryotoxicity and teratogenicity in rabbits, supports earlier
evaluations. It was noted that a long-term study performed on an
additional species is still outstanding. However, the Meeting was
informed that a long-term study in mice and a 1-year study in dogs
were in progress. On this basis, the Meeting decided to extend the
temporary ADI of 0.003 mg/kg until 1984.
Chinomethionat was last evaluated by the 1977 Joint Meeting.
Since then, some additional data have been made available for
evaluation of residues. Results of supervised trials on almond and
currant showed a residue pattern similar to that of the previous
experiments, while in orange the residue was lower and did not exceed
0.2 mg/kg on a whole product basis.
The residue in orange pulp was always below 0.1 mg/kg and
<0.05 mg/kg at 6 days after last application. The peel contained
residue in the range of 0.15 to 0.51 mg/kg at 0 to 6 days interval.
The residue in cucumber, treated at the recommended rates up to
0.5 mg a.i./ha, did not exceed 0.1 mg/kg, which is the present TMRL.
The results of these supervised trials support the maximum residue
levels recommended by the 1974 Joint Meeting.
Residues in melon, kaki persimmon, strawberry and tomato
were evaluated for the first time. In melon and kaki persimmon,
chinomethionat residue was low, i.e. less than 0.1 and 0.05 mg/kg at
the time of harvest, and concentrated in the peel or rind.
The residues in strawberry were less than 0.5 mg/kg at day 1
post-treatment even after 4 applications and declined to under
0.1 mg/kg 9 days later. Tomatoes treated in a greenhouse contained
residue in the range of 0.05 to 0.38 mg/kg at 1 to 3 days after last
application. A feeding study was carried out on dairy cows kept on a
diet consisting of 20% chinomethionat-treated almond hulls containing
0.08 mg/kg chinomethionat equivalent. Radioactive residues in milk
reached a maximum 10 days after the feeding study began, but never
exceeded 0.008 mg/kg chinomethionat equivalent. Radioactive residues
were 0.03 mg/kg in liver and kidney and <0.02 mg/kg in other tissues.
These results confirmed the low biological availability of plant
residues found in previous experiments.
A plateau of the accumulation factor in bluegill fish was
observed in the range of 700 to 1 100 after 4 to 7 days of exposure.
Approximately 10% of the accumulated residue remained in the fish 51
days after withdrawal.
The metabolism of chinomethionat was studied in sandy loam and
silt loam under aerobic, anaerobic and sterile conditions. The major
metabolite identified in these soils was 6-methyl 1,4-dihydro
2,3-quinoxalinedione (QDOH). Its maximum concentration ranged between
42 and 57% of applied radioactivity. Minor metabolites identified and
their maximum concentrations found under aerobic conditions were
2-hydroxy or 3-hydroxy 6-methyl 2 (1H) quinoxalinethione (QDSOH) 15 to
16%; 6-methyl 1,4-dihydro-2,3-quinoxalinedithione (QDSH) 4 to 10%;
1, 2, 3, 4-tetrahydro 2,3-dioxo 6-quinoxaline carboxylic acid (acid
QDOH) 4%, and 2, 9-or 2,10-dimethyl (1,4)-dithiino (2,3-b:5,6-b'-
diquinoxaline (dimer) 1%. Aerobic and anaerobic conditions resulted in
similar metabolites. Microbial degradation is of primary importance,
as the hydrolysis carried out by soil microbes appeared to be the main
mechanism for degradation of chinomethionat. The concentration of
chinomethionat declined rapidly in soils and was about 6 to 10% of the
applied dose 30 days after soil treatment.
At harvest, the total radioactive residue in lettuce, red beet
and oats planted 30 to 65 days after soil treatment with radioactive
chinomethionat were in the range of 0.03 to 0.06 mg/kg. Although
rotational crops can take up soil residues, owing to the rapid
decomposition of chinomethionat, no intact parent compound can be
expected in detectable amounts in mature crops planted 30 days or
later post-treatment.
Studies on the runoff characteristics of chinomethionat from
treated trees or from a soil surface revealed that less than 1% of
the applied dose could be washed off by rain, even on the day of
application. The low mobility of chinomethionat and its soil
metabolites was also indicated by adsorption, desorption and soil
column leaching experiments. The concentration of chinomethionat
decreased to half of initial value within 109 hours at 30°C in
phosphate buffer of pH 5. At pH 7 the rate of degradation increased
three fold.
Level causing no toxicological effect
Rat : 12 ppm in the diet equivalent to 0.6 mg/kg bw/day,
Estimate of temporary acceptable daily intake for man
0 to 0.003 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of new data, the Meeting recommends the following
additional residue limits:
PHI on which
Commodity Limits recommendations are
based (days)
Melon 0.1 3
Watermelon 0.02 3
Strawberry 0.2 7
Kaki persimmon 0.05 14
FURTHER WORK OR INFORMATION
Required (by 1984)
1. The submission of the studies known to be in progress.
Desirable
1. Information on use pattern on tomato and additional residue data
deriving from supervised trials.
REFERENCES
Bayer Residue Reports, Nos. 322-323/69.
1969
1974 Residue Report, No. 8400/74 (black currant).
1975 Residue Reports, Nos. 8400-8405/75 (cucumbers),
1976 Leaching studies. Reports Nos. 8400-8402/76.
Flint, D.R. and Shaw, H.R. Soil runoff, leaching, adsorption and water
1970 stability studies with MORESTAN. Mobay Report No. 28 365.
Lamb, D.W., Roney, D.J. and Jones, R.E. Accumulation and persistence
1975 of residues in bluegill fish exposed to MORESTAN-14C. Mobay
Report No. 44 329.
Mobay Residue Reports, Nos. 613-66621. 1978
1979 Residue Reports, Nos. 51125, 51126, 51384, 51706, 51707,
53050, 53051, 53082, 67200-67203.
Mulkey, N.S., Herrera, R., Breault, G.O. and Wargo Jr., J.P.
1980 Photodegradation of 14C-MORESTAN on soil surface. Mobay
Report No. 68 881.
Murphy, J.J. et al. 14C MORESTAN - Almond hull - dairy cow feeding
1977 study. Mobay Report No. 53 619.
Nikon Tokushu Noyaku Seizo KK. Residue Reports, Nos. 98, 268, 622,
1971 623, 795 (cucumbers).
1973 Residue Reports, Nos. 242, 243.
1974 Residue Reports, Nos. 95, 96.
1976 Residue Reports, Nos. 431, 432
1978 Residue Reports, Nos. 100, 620, 621.
Obrist, J.J. Leaching Characteristics of Aged MORESTAN Soil Residues.
1979 Mobay Report No. 67 821.
Shaw II, H.R. Adsorption and desorption of (13,14C)-MORESTAN by Soils.
1979 Mobay Report No. 67 484.
1980 Metabolism of MORESTAN in soil. Mobay Report No. 67 820.
Stoner, J.H. MORESTAN 14C. A rotational crop study. Mobay Report No.
1980 69 199.
Vogeler, K. Untersuchungen zum leachingverhalten von Chinomethionat.
1981 Bayer AG, Pflanzenschutz-Anwendungstechnik, Report RA - 194.
(Unpublished)
 Leaching characteristics of aged chinomethionat soil residues
were studied in two sets of experiments. Sandy loam soil was fortified
at 7.05 mg/kg with (13C, 14C)-chinomethionat, followed by incubation
at room temperature for 30 days. Of the original applied activity, 12%
was lost during the ageing process, probably due to volatilization. In
three replicates, the aged soil was placed on top of sandy loam soil
columns, which were 30 cm high and 4.3 cm in internal diameter.
Leaching was effected by passing water, equivalent to 1.25 cm of
rainfall daily, through the columns for 45 days. Only 4% of the
applied radioactivity was found in the water leaching gradually over
the 45-day period, with no apparent peak, while nearly 90% of the
activity was retained in the upper 1.25 cm of the column.
The residue in soil contained primarily QDOH (39%), QDSH (13%),
QDSOH (6%) and chinomethionat (4%). About 1.5+1.1% of applied activity
was associated with the insoluble humin fraction of soil, and the
remainder of the activity distributed into the fluvic (98 ± 1.1%) and
humic (4.3%) acid fractions (Obrist 1979). The residues in the
leachate were identified in the other experiments carried out with
standard and modified soil columns type 2.1 (Vogeler 1981). The water
collected under the modified soil column contained 6.8 and 6.9% of
applied activity. Two metabolites, identified in water, were QDOH and
a small amount of QDSH.
In water
Technical chinomethionat was dissolved in phosphate buffers of
pH 5 and pH 7, with the final concentration being approximately
0.05 mg/kg. The solutions were placed in capped amber bottles and
maintained in constant temperature water baths at 30 and 50°C. The
concentration of chinomethionat decreased to half of the initial value
within 109 hours at 30°C and 27 hours at 50°C. An increase of pH from
5 to 7 decreased the water stability of chinomethionat three and five
fold, respectively.
In the outdoor experiment, conducted in a mini-pond containing
5 cm silt and 25 cm of surface water of pH 7, 100 mg/kg of
chinomethionat decomposed rapidly, showing a half life of 3.4 h in a
7-hour experiment during which the temperature of water increased from
27°C to 37°C (Flint and Shaw 1970).
Photodegradation
In a laboratory experiment, 14C-chinomethionat applied to a thin
layer of soil on a glass plate was irradiated with light from a high
intensity, mercury vapour lamp. Soil samples were analysed after 1, 2,
2.2, 3.5, 5, 7, 11 and 12 days exposure. Several minor products were
detected. Chinomethionat was the major residue component at each
sampling time, although several minor photolysis products were
detected. The "dimer" and QDOH were tentatively identified.
Disappearance of chinomethionat was influenced by several
factors. It decreased by approximately 50% during the first day but
declined more slowly subsequently. After 12 days, about 25% of the
initial concentration was still detectable on the plates (Mulkey
et al 1980).
EVALUATION
COMMENTS AND APPRAISAL
Additional data evaluated by this Meeting further extend the
basis for the toxicological characterization of chinomethionat. The
outcome of studies on mutagenicity in bacteria and in mice, as well as
on embryotoxicity and teratogenicity in rabbits, supports earlier
evaluations. It was noted that a long-term study performed on an
additional species is still outstanding. However, the Meeting was
informed that a long-term study in mice and a 1-year study in dogs
were in progress. On this basis, the Meeting decided to extend the
temporary ADI of 0.003 mg/kg until 1984.
Chinomethionat was last evaluated by the 1977 Joint Meeting.
Since then, some additional data have been made available for
evaluation of residues. Results of supervised trials on almond and
currant showed a residue pattern similar to that of the previous
experiments, while in orange the residue was lower and did not exceed
0.2 mg/kg on a whole product basis.
The residue in orange pulp was always below 0.1 mg/kg and
<0.05 mg/kg at 6 days after last application. The peel contained
residue in the range of 0.15 to 0.51 mg/kg at 0 to 6 days interval.
The residue in cucumber, treated at the recommended rates up to
0.5 mg a.i./ha, did not exceed 0.1 mg/kg, which is the present TMRL.
The results of these supervised trials support the maximum residue
levels recommended by the 1974 Joint Meeting.
Residues in melon, kaki persimmon, strawberry and tomato
were evaluated for the first time. In melon and kaki persimmon,
chinomethionat residue was low, i.e. less than 0.1 and 0.05 mg/kg at
the time of harvest, and concentrated in the peel or rind.
The residues in strawberry were less than 0.5 mg/kg at day 1
post-treatment even after 4 applications and declined to under
0.1 mg/kg 9 days later. Tomatoes treated in a greenhouse contained
residue in the range of 0.05 to 0.38 mg/kg at 1 to 3 days after last
application. A feeding study was carried out on dairy cows kept on a
diet consisting of 20% chinomethionat-treated almond hulls containing
0.08 mg/kg chinomethionat equivalent. Radioactive residues in milk
reached a maximum 10 days after the feeding study began, but never
exceeded 0.008 mg/kg chinomethionat equivalent. Radioactive residues
were 0.03 mg/kg in liver and kidney and <0.02 mg/kg in other tissues.
These results confirmed the low biological availability of plant
residues found in previous experiments.
A plateau of the accumulation factor in bluegill fish was
observed in the range of 700 to 1 100 after 4 to 7 days of exposure.
Approximately 10% of the accumulated residue remained in the fish 51
days after withdrawal.
The metabolism of chinomethionat was studied in sandy loam and
silt loam under aerobic, anaerobic and sterile conditions. The major
metabolite identified in these soils was 6-methyl 1,4-dihydro
2,3-quinoxalinedione (QDOH). Its maximum concentration ranged between
42 and 57% of applied radioactivity. Minor metabolites identified and
their maximum concentrations found under aerobic conditions were
2-hydroxy or 3-hydroxy 6-methyl 2 (1H) quinoxalinethione (QDSOH) 15 to
16%; 6-methyl 1,4-dihydro-2,3-quinoxalinedithione (QDSH) 4 to 10%;
1, 2, 3, 4-tetrahydro 2,3-dioxo 6-quinoxaline carboxylic acid (acid
QDOH) 4%, and 2, 9-or 2,10-dimethyl (1,4)-dithiino (2,3-b:5,6-b'-
diquinoxaline (dimer) 1%. Aerobic and anaerobic conditions resulted in
similar metabolites. Microbial degradation is of primary importance,
as the hydrolysis carried out by soil microbes appeared to be the main
mechanism for degradation of chinomethionat. The concentration of
chinomethionat declined rapidly in soils and was about 6 to 10% of the
applied dose 30 days after soil treatment.
At harvest, the total radioactive residue in lettuce, red beet
and oats planted 30 to 65 days after soil treatment with radioactive
chinomethionat were in the range of 0.03 to 0.06 mg/kg. Although
rotational crops can take up soil residues, owing to the rapid
decomposition of chinomethionat, no intact parent compound can be
expected in detectable amounts in mature crops planted 30 days or
later post-treatment.
Studies on the runoff characteristics of chinomethionat from
treated trees or from a soil surface revealed that less than 1% of
the applied dose could be washed off by rain, even on the day of
application. The low mobility of chinomethionat and its soil
metabolites was also indicated by adsorption, desorption and soil
column leaching experiments. The concentration of chinomethionat
decreased to half of initial value within 109 hours at 30°C in
phosphate buffer of pH 5. At pH 7 the rate of degradation increased
three fold.
Level causing no toxicological effect
Rat : 12 ppm in the diet equivalent to 0.6 mg/kg bw/day,
Estimate of temporary acceptable daily intake for man
0 to 0.003 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of new data, the Meeting recommends the following
additional residue limits:
PHI on which
Commodity Limits recommendations are
based (days)
Melon 0.1 3
Watermelon 0.02 3
Strawberry 0.2 7
Kaki persimmon 0.05 14
FURTHER WORK OR INFORMATION
Required (by 1984)
1. The submission of the studies known to be in progress.
Desirable
1. Information on use pattern on tomato and additional residue data
deriving from supervised trials.
REFERENCES
Bayer Residue Reports, Nos. 322-323/69.
1969
1974 Residue Report, No. 8400/74 (black currant).
1975 Residue Reports, Nos. 8400-8405/75 (cucumbers),
1976 Leaching studies. Reports Nos. 8400-8402/76.
Flint, D.R. and Shaw, H.R. Soil runoff, leaching, adsorption and water
1970 stability studies with MORESTAN. Mobay Report No. 28 365.
Lamb, D.W., Roney, D.J. and Jones, R.E. Accumulation and persistence
1975 of residues in bluegill fish exposed to MORESTAN-14C. Mobay
Report No. 44 329.
Mobay Residue Reports, Nos. 613-66621. 1978
1979 Residue Reports, Nos. 51125, 51126, 51384, 51706, 51707,
53050, 53051, 53082, 67200-67203.
Mulkey, N.S., Herrera, R., Breault, G.O. and Wargo Jr., J.P.
1980 Photodegradation of 14C-MORESTAN on soil surface. Mobay
Report No. 68 881.
Murphy, J.J. et al. 14C MORESTAN - Almond hull - dairy cow feeding
1977 study. Mobay Report No. 53 619.
Nikon Tokushu Noyaku Seizo KK. Residue Reports, Nos. 98, 268, 622,
1971 623, 795 (cucumbers).
1973 Residue Reports, Nos. 242, 243.
1974 Residue Reports, Nos. 95, 96.
1976 Residue Reports, Nos. 431, 432
1978 Residue Reports, Nos. 100, 620, 621.
Obrist, J.J. Leaching Characteristics of Aged MORESTAN Soil Residues.
1979 Mobay Report No. 67 821.
Shaw II, H.R. Adsorption and desorption of (13,14C)-MORESTAN by Soils.
1979 Mobay Report No. 67 484.
1980 Metabolism of MORESTAN in soil. Mobay Report No. 67 820.
Stoner, J.H. MORESTAN 14C. A rotational crop study. Mobay Report No.
1980 69 199.
Vogeler, K. Untersuchungen zum leachingverhalten von Chinomethionat.
1981 Bayer AG, Pflanzenschutz-Anwendungstechnik, Report RA - 194.
(Unpublished)
Leaching characteristics of aged chinomethionat soil residues
were studied in two sets of experiments. Sandy loam soil was fortified
at 7.05 mg/kg with (13C, 14C)-chinomethionat, followed by incubation
at room temperature for 30 days. Of the original applied activity, 12%
was lost during the ageing process, probably due to volatilization. In
three replicates, the aged soil was placed on top of sandy loam soil
columns, which were 30 cm high and 4.3 cm in internal diameter.
Leaching was effected by passing water, equivalent to 1.25 cm of
rainfall daily, through the columns for 45 days. Only 4% of the
applied radioactivity was found in the water leaching gradually over
the 45-day period, with no apparent peak, while nearly 90% of the
activity was retained in the upper 1.25 cm of the column.
The residue in soil contained primarily QDOH (39%), QDSH (13%),
QDSOH (6%) and chinomethionat (4%). About 1.5+1.1% of applied activity
was associated with the insoluble humin fraction of soil, and the
remainder of the activity distributed into the fluvic (98 ± 1.1%) and
humic (4.3%) acid fractions (Obrist 1979). The residues in the
leachate were identified in the other experiments carried out with
standard and modified soil columns type 2.1 (Vogeler 1981). The water
collected under the modified soil column contained 6.8 and 6.9% of
applied activity. Two metabolites, identified in water, were QDOH and
a small amount of QDSH.
In water
Technical chinomethionat was dissolved in phosphate buffers of
pH 5 and pH 7, with the final concentration being approximately
0.05 mg/kg. The solutions were placed in capped amber bottles and
maintained in constant temperature water baths at 30 and 50°C. The
concentration of chinomethionat decreased to half of the initial value
within 109 hours at 30°C and 27 hours at 50°C. An increase of pH from
5 to 7 decreased the water stability of chinomethionat three and five
fold, respectively.
In the outdoor experiment, conducted in a mini-pond containing
5 cm silt and 25 cm of surface water of pH 7, 100 mg/kg of
chinomethionat decomposed rapidly, showing a half life of 3.4 h in a
7-hour experiment during which the temperature of water increased from
27°C to 37°C (Flint and Shaw 1970).
Photodegradation
In a laboratory experiment, 14C-chinomethionat applied to a thin
layer of soil on a glass plate was irradiated with light from a high
intensity, mercury vapour lamp. Soil samples were analysed after 1, 2,
2.2, 3.5, 5, 7, 11 and 12 days exposure. Several minor products were
detected. Chinomethionat was the major residue component at each
sampling time, although several minor photolysis products were
detected. The "dimer" and QDOH were tentatively identified.
Disappearance of chinomethionat was influenced by several
factors. It decreased by approximately 50% during the first day but
declined more slowly subsequently. After 12 days, about 25% of the
initial concentration was still detectable on the plates (Mulkey
et al 1980).
EVALUATION
COMMENTS AND APPRAISAL
Additional data evaluated by this Meeting further extend the
basis for the toxicological characterization of chinomethionat. The
outcome of studies on mutagenicity in bacteria and in mice, as well as
on embryotoxicity and teratogenicity in rabbits, supports earlier
evaluations. It was noted that a long-term study performed on an
additional species is still outstanding. However, the Meeting was
informed that a long-term study in mice and a 1-year study in dogs
were in progress. On this basis, the Meeting decided to extend the
temporary ADI of 0.003 mg/kg until 1984.
Chinomethionat was last evaluated by the 1977 Joint Meeting.
Since then, some additional data have been made available for
evaluation of residues. Results of supervised trials on almond and
currant showed a residue pattern similar to that of the previous
experiments, while in orange the residue was lower and did not exceed
0.2 mg/kg on a whole product basis.
The residue in orange pulp was always below 0.1 mg/kg and
<0.05 mg/kg at 6 days after last application. The peel contained
residue in the range of 0.15 to 0.51 mg/kg at 0 to 6 days interval.
The residue in cucumber, treated at the recommended rates up to
0.5 mg a.i./ha, did not exceed 0.1 mg/kg, which is the present TMRL.
The results of these supervised trials support the maximum residue
levels recommended by the 1974 Joint Meeting.
Residues in melon, kaki persimmon, strawberry and tomato
were evaluated for the first time. In melon and kaki persimmon,
chinomethionat residue was low, i.e. less than 0.1 and 0.05 mg/kg at
the time of harvest, and concentrated in the peel or rind.
The residues in strawberry were less than 0.5 mg/kg at day 1
post-treatment even after 4 applications and declined to under
0.1 mg/kg 9 days later. Tomatoes treated in a greenhouse contained
residue in the range of 0.05 to 0.38 mg/kg at 1 to 3 days after last
application. A feeding study was carried out on dairy cows kept on a
diet consisting of 20% chinomethionat-treated almond hulls containing
0.08 mg/kg chinomethionat equivalent. Radioactive residues in milk
reached a maximum 10 days after the feeding study began, but never
exceeded 0.008 mg/kg chinomethionat equivalent. Radioactive residues
were 0.03 mg/kg in liver and kidney and <0.02 mg/kg in other tissues.
These results confirmed the low biological availability of plant
residues found in previous experiments.
A plateau of the accumulation factor in bluegill fish was
observed in the range of 700 to 1 100 after 4 to 7 days of exposure.
Approximately 10% of the accumulated residue remained in the fish 51
days after withdrawal.
The metabolism of chinomethionat was studied in sandy loam and
silt loam under aerobic, anaerobic and sterile conditions. The major
metabolite identified in these soils was 6-methyl 1,4-dihydro
2,3-quinoxalinedione (QDOH). Its maximum concentration ranged between
42 and 57% of applied radioactivity. Minor metabolites identified and
their maximum concentrations found under aerobic conditions were
2-hydroxy or 3-hydroxy 6-methyl 2 (1H) quinoxalinethione (QDSOH) 15 to
16%; 6-methyl 1,4-dihydro-2,3-quinoxalinedithione (QDSH) 4 to 10%;
1, 2, 3, 4-tetrahydro 2,3-dioxo 6-quinoxaline carboxylic acid (acid
QDOH) 4%, and 2, 9-or 2,10-dimethyl (1,4)-dithiino (2,3-b:5,6-b'-
diquinoxaline (dimer) 1%. Aerobic and anaerobic conditions resulted in
similar metabolites. Microbial degradation is of primary importance,
as the hydrolysis carried out by soil microbes appeared to be the main
mechanism for degradation of chinomethionat. The concentration of
chinomethionat declined rapidly in soils and was about 6 to 10% of the
applied dose 30 days after soil treatment.
At harvest, the total radioactive residue in lettuce, red beet
and oats planted 30 to 65 days after soil treatment with radioactive
chinomethionat were in the range of 0.03 to 0.06 mg/kg. Although
rotational crops can take up soil residues, owing to the rapid
decomposition of chinomethionat, no intact parent compound can be
expected in detectable amounts in mature crops planted 30 days or
later post-treatment.
Studies on the runoff characteristics of chinomethionat from
treated trees or from a soil surface revealed that less than 1% of
the applied dose could be washed off by rain, even on the day of
application. The low mobility of chinomethionat and its soil
metabolites was also indicated by adsorption, desorption and soil
column leaching experiments. The concentration of chinomethionat
decreased to half of initial value within 109 hours at 30°C in
phosphate buffer of pH 5. At pH 7 the rate of degradation increased
three fold.
Level causing no toxicological effect
Rat : 12 ppm in the diet equivalent to 0.6 mg/kg bw/day,
Estimate of temporary acceptable daily intake for man
0 to 0.003 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
On the basis of new data, the Meeting recommends the following
additional residue limits:
PHI on which
Commodity Limits recommendations are
based (days)
Melon 0.1 3
Watermelon 0.02 3
Strawberry 0.2 7
Kaki persimmon 0.05 14
FURTHER WORK OR INFORMATION
Required (by 1984)
1. The submission of the studies known to be in progress.
Desirable
1. Information on use pattern on tomato and additional residue data
deriving from supervised trials.
REFERENCES
Bayer Residue Reports, Nos. 322-323/69.
1969
1974 Residue Report, No. 8400/74 (black currant).
1975 Residue Reports, Nos. 8400-8405/75 (cucumbers),
1976 Leaching studies. Reports Nos. 8400-8402/76.
Flint, D.R. and Shaw, H.R. Soil runoff, leaching, adsorption and water
1970 stability studies with MORESTAN. Mobay Report No. 28 365.
Lamb, D.W., Roney, D.J. and Jones, R.E. Accumulation and persistence
1975 of residues in bluegill fish exposed to MORESTAN-14C. Mobay
Report No. 44 329.
Mobay Residue Reports, Nos. 613-66621. 1978
1979 Residue Reports, Nos. 51125, 51126, 51384, 51706, 51707,
53050, 53051, 53082, 67200-67203.
Mulkey, N.S., Herrera, R., Breault, G.O. and Wargo Jr., J.P.
1980 Photodegradation of 14C-MORESTAN on soil surface. Mobay
Report No. 68 881.
Murphy, J.J. et al. 14C MORESTAN - Almond hull - dairy cow feeding
1977 study. Mobay Report No. 53 619.
Nikon Tokushu Noyaku Seizo KK. Residue Reports, Nos. 98, 268, 622,
1971 623, 795 (cucumbers).
1973 Residue Reports, Nos. 242, 243.
1974 Residue Reports, Nos. 95, 96.
1976 Residue Reports, Nos. 431, 432
1978 Residue Reports, Nos. 100, 620, 621.
Obrist, J.J. Leaching Characteristics of Aged MORESTAN Soil Residues.
1979 Mobay Report No. 67 821.
Shaw II, H.R. Adsorption and desorption of (13,14C)-MORESTAN by Soils.
1979 Mobay Report No. 67 484.
1980 Metabolism of MORESTAN in soil. Mobay Report No. 67 820.
Stoner, J.H. MORESTAN 14C. A rotational crop study. Mobay Report No.
1980 69 199.
Vogeler, K. Untersuchungen zum leachingverhalten von Chinomethionat.
1981 Bayer AG, Pflanzenschutz-Anwendungstechnik, Report RA - 194.
(Unpublished)
