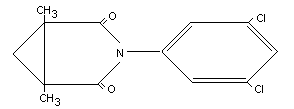
 Other information on identity and properties
Molecular weight 284.13
State White to light brownish crystalline solid
(technical material)
Specific gravity d25
25 1.42 - 1.46
Vapour pressure 1.32 × 10-4 mm Hg at 25°C
Solubility Acetone 18.0, acetonitrile 10.1,
(% w/w at 25°C) cyclohexanone 14.8, ethyl acetate 11.5,
methanol 1.6, xylene 4.3
Solubility in water 4.5 mg/l at 25°C
n-octanol/water
partition co-efficient 1.3834 × 103 at pH 7.26
Stability Stable in solvents, unstable in alkaline
media. No significant breakdown after 20
weeks storage at 60°C.
Formulation Currently available as 50% wettable powder
and 265 and 500 g/l emulsifiable concentrate
Purity of technical
product Contains normally 95-99% of procymidone.
Detailed information on the impurities in
technical procymidone ranging from 0.1-1% was
reported to the Meeting.
RESIDUES IN FOOD
USE PATTERN
Procymidone is a recently introduced moderately systemic
fungicide with a rather selective action. It is especially effective
in the control of Botrytis and Sclerotinia species on various
vegetables, fruits, flower crops and ornamentals. It is also used on
stone fruits against Monilia sp.
There are some indications that the fungicide may affect mitosis
in susceptible fungi but the actual site of action has not been
elucidated.
Procymidone is effective against Botrytis strains that have
acquired resistance to benzimidazole compounds; the latter also have
an antimitotic action in fungi owing to interference with the
functioning and assembly of microtubules, the elements of the spindle.
It is assumed that the site of action of procymidone in the mitotic
process is different from that of the benzimidazole compounds.
Procymidone is applied, often repeatedly, on the aerial parts of
fruits and vegetables and sometimes until shortly before harvest to
avoid fruit- and vegetable-rot during transport and distribution. It
is also used on young plants and/or the top layer of soil against
soil-borne diseases (Sclerotinia, Botrytis).
Products containing procymidone are registered and/or approved in
many countries, in Asia, South America and Europe as well as in
Australia and New Zealand. They are mainly used against grey mould
(mainly Botrytis cinerea), onion grey mould (Botrytis squamosa),
onion neck-rot (Botrytis aclada allii), Sclerotinia spp. and
Monilia sp. (brown rot on peaches etc., blossom blight and twig wilt
on cherries, etc.).
The current suggested uses are summarized in Tables 4 and 5.
Recommended pre-harvest intervals known to the Meeting are listed
under national maximum residue limits.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Many supervised residue trials have been carried out on a wide
variety of fruits and vegetables and also on rice and oilseeds. See
Tables 6 and 7 for details.
Fruits
Pome fruits
Residue data were obtained only on apples from Japan. Procymidone
levels were less than 3 mg/kg 21 days after the last of 3 to 5
treatments, but had risen to about 3 mg/kg 35 days after the last of 5
treatments.
Stone fruits
Residue data on cherries were available from Australia, Hungary
and Japan. Procymidone levels were generally less than 5 mg/kg one day
or more after the last treatment, except one erratic sample in
Australia which contained 6.2 mg/kg.
Residue levels in peaches were generally less than 2 mg/kg three
or more days after the last application of 0.2 to 3 kg/ha (recommended
application rates are 0.75 to 1.5 kg/ha.
In a trial in New Zealand, nectarines sprayed 7 times at
0.75 kg/ha contained procymidone at or below 0.21 mg/kg.
In a trial in France, the residue on plums after a single
application of procymidone at 0.75 kg a.i./ha was 2.4 mg/kg at day 0
(4 h after spraying) and 1.3 mg/kg 8 days later.
Berry fruits
Residue data on grapes were obtained from several countries in
Europe, from New Zealand and South Africa, where 1 to 5 treatments
were carried out at rates of 0.5 to 1.25 kg/ha. About 14 days after
the last treatment, procymidone residues were in the range of 0.4 to
9.3 mg/kg and declined to 0.6 to 5.1 mg/kg after 35 to 36 days.
Residue data were obtained on strawberries grown outdoors and in
glasshouses or plastic tunnels. The procymidone residues were 0.8 to
14.3 mg/kg 0 to 1 day after the last spraying and declined to 0.8 to
6.5 mg/kg after 6 to 8 days. The residue levels after 14 to 15 days
were generally 2 mg/kg or less.
TABLE 4. Use patterns of procymidone on fruits, registered and/or approved uses
Country
Fed.Rep.
of
Crop France Germany Luxembourg Spain Switzerland UK Bulgaria Czechoslovakia Hungary Romania Yugoslavia
Black currant X
Grape X X X X X X X X X X X
(incl. table grapes)
Peach X
Raspberry X X
Stone fruits X
Strawberry X X X X
Table 4 (continued)
Country Range of application
South New Target rates in various countries
Crop Greece Turkey Africa Chile Uruguay Japan Lebanon Australia Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Black currant 0.75
Grape X X X X X X X Bc 0.5 - 1.0 25 - 75
(incl. table grapes)
Peach X M 0.5 - 0.75 33 - 50
Raspberry Bc 0.5 - 0.75
Stone fruits X X X Bc M 0.6 - 1.0 25 - 75
Strawberry X X X X Bc 0.4 - 0.75 25 - 100
1 Bc = grey mould Botrytis sp., mainly Botrytis cinerea;
M = Monilia, including Monilinia fructigena and M. fructicola (brown rot, blossom blight and twig blight).
TABLE 5. Use patterns of procymidone on vegetables and oil seeds; registered and/or approved uses
Countries
Europe Asia Range of application
New Target rates in various countries
Crop France UK Hungary Greece Japan Korea Lebanon Taiwan Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Beans (not specified) X X X Bc Sc 0.5-0.75 25-50
Azuki bean X Bc 50
Kidney bean X 25-50
Soybean X Sc 25-50
Celery X Sc 25-50
Cucumber X Bc Sc 25-50
Eggplant X Bc Sc 25-50
Lettuce X X X X X Bc Sc 0.4-0.75 25-50
Melon X Bc Sc 0.75
Onion X Bsq Baa 50
Pepper (green) X Bc Sc 25-50
Potato X Sc 33-50
Tomato X X X Bc Sc 0.5 25-50
Vegetables (general) X X X 0.5 25-50
TABLE 5. (con't)
Countries
Europe Asia Range of application
New Target rates in various countries
Crop France UK Hungary Greece Japan Korea Lebanon Taiwan Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Rape seed X Sc 50
Sunflower X Bc 0.5
1 Bc = grey mould, mainly Botrytis cinerea;
Sc = Sclerotinia spp.;
Bsq = Botrytis squamosa;
Baa = Grey mould neck rot = Botrytis aclada allii.
TABLE 6. Residues of procymidone resulting from supervised trials in fruits and vegetables
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 01/1 3/4 6/8 14/16 20/22 26/29 35/36
(kg (%)
a.i./ha)
Grape France 1977/80 1 0.75 wp 50% 0.53
1 0.75 EC500g/l 0.48
4 0.75 wp 50% 5.0 4.8 4.7 4.5 4.8 4.9 3.4 3.6
Table
grapes 1980 4 0.75 wp 50% 3.4 3.1 1.7 1.4 1.3 1.5 0.9 1.0 0.9 0.8
(") 1980 4 2.0 2.0 1.8 1.6 2.5 2.3 1.6 1.4 1.0 1.1
1977 1 0.75 wp 50% 2.46
(2.09-5.10)
1 1.0 wp 50% 3.89
(1.76-5.42)
1977 >1 0.75 wp 50% 4.9 4.4 3.2 3.3
1977 >1 0.75 wp 50% 6.4 3.1 2.2 2.8
Fed.Rep.
of Germany 1976/77 5 1.25 wp 50% 11.2 8.4 2.4 2.1
" 5 1.25 wp 50% 9.3 10.1 6.6 5.1
" 5 1.25 wp 50% 7.2 5.7 5.9 5.2 2.4
" 4 1.25 wp 50% 8.0 5.6 4.2 3.7 0.6
" 4 1.25 wp 50% 4.2 3.7 3.2 2.9
" 4 1.25 wp 50% 5.0 3.7 2.4 2.0 1.9
" 5 0.9 wp 50% 3.7 2.5 1.9 1.6 1.2
" 5 0.9 wp 50% 6.1 3.0 2.5 2.4 1.4
" 5 0.9 wp 50% 5.1 3.4 2.8 2.1 1.7
Italy 1976/77 4 0.85 wp 50% 1.9 0.9
" 4 1.28 wp 50% 4.1 1.6
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 01/1 3/4 6/9 14/16 20/22 26/29 35/36
(kg (%)
a.i./ha)
Grape New
Zealand 1979 1 0.7 wp 50% 1.7 1.7 1.0 1.6
1.05 wp 50% 2.6 4.3 2.7 2.5
Spain 1976 3 7.5 g/
100 1 wp 50% 4.4 4.0 3.0 2.7
12.5 g/
100 l wp 50% 6.8 6.8 5.3 4.0
Table South
grapes Africa 1978 1 0.7 dust 0.7 0.7 0.3 0.4 0.5 0.5 0.4 0.4
1x4 0.75 + wp 50 +
0.7 dust 3% 0.6 0.6 0.5 0.6 0.5 0.5 0.4 0.4
UK 1978 1 0.75 wp 50% 7.9
0/1 3/4 7/9 14 21 28 35
Apple Japan 1979 3 3 wp 50% ng 2.0 2.1 2.3
5 3 wp 50% 1.2 1.1 1.2 1.3
Black
currant UK 1979 3 0.75 wp 50% 5.3 4.2
1 0.5 wp 50% 6.0 7.0 3.3 3.5 2.1
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 7/9 14 21 28 35
(kg (%)
a.i./ha)
Cherry Australia 1977 5 19 g/
100 l wp 50% 3.3 2.4
525 g/
100 l wp 50% 2.0 2.1
5 37.5 g/
100 l wp 50% 6.2 2.9
Japan 1977 1 3.5 wp 50% 1.9 1.0 1.7
3 3.5 wp 50% 3.6 2.3 1.7
3 3.5 wp 50% 1.1 1.0 0.6
5 3.5 wp 50% 1.3 1.2 1.1
Peach Australia 1978 2-9 19 g/
100 l wp 50% 2.6
(2.4-5.1)
25 g/
100 l wp 50% 4.1
(1.6-7.6)
37.5 g
/100 l wp 50% 6.3
(2.3-11.7)
Japan 1977 4 3 wp 50%
peel 73.9 72.8 43.2
pulp 1.1 1.1 0.8
1977 6 3 wp 50%
peel 51.2 44.5 29.0
pulp 1.2 1.1 1.0
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 6/9 14 20/21 28 35
(kg (%)
a.i./ha)
New
Zealand 1980 7 0.75 wp 50% 0.42 0.4 0.4
7 0.75 wp 50% 0.6 0.8 0.5 0.3
5 1.5 wp 50% 0.3 0.4 0.9 0.6
210 g/
100 l wp 50% 0.4 0.7 0.4 0.3
2 20g/
100 l wp 50% 1.7 1.1 1.3 1.1
230 g/
100 l wp 50% 2.4 1.1 1.2 0.3
Nectarines New
Zealand 1980 7 0.75 wp 50% 0.1 0.2 0.1 0.1
Plum France 1979 1 0.75 wp 50% 2.4 1.3
Raspberry Poland 1980 2 1.25 wp 50% 1.7 0.25
(1.6-1.9) (0.2-0.3)
UK 1978 1 0.75 wp 50% 0.3
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 6/9 14 20/21 28 35
(kg (%)
a.i./ha)
Kiwifruit New
Zealand 1980/81 6 1.1 wp 50% 4.4 2.6 5.9 2.8 2.0
(flesh only) 0.5 0.4 0.25
1980/81 6 1.1 wp 50% 6.3 6.7 11.4 5.4 3.3
(flesh only) 1.8 2.1 0.8
6100 g/
100 l wp 50% 5.3 4.7 8.7 4.1 2.6
450 g/
100 l wp 50% 2.9 3.0 1.9
0/2 3/4 5/6 7/8 9/10 13/15 16/19
Strawberry France 1979 1 0.75 wp 50% 0.4 0.3
outdoors 1979 2 0.75 wp 50% 1.0 0.1
1980 3 0.75 wp 50% 4.9 5.2 2.1 2.4 1.9 2.0 0.7 0.5
1980 3 0.75 wp 50% 3.8 3.6 2.1 1.9 1.3 1.4 0.4 0.4
1980 3 0.75 wp 50% 2.9 2.7 2.3 2.3 1.1 1.1 0.2 0.3
1980 3 0.75 wp 50% 3.8 3.6 2.3 2.2 1.3 1.2 0.3 0.3
1978 3 0.5 wp 50% 2.1 1.5 0.9
1978 3 0.75 wp 50% 3.0 1.3 0.8
1978 4 0.75 wp 50% 3.6 1.5
1978 3 0.5 wp 50% 0.93
1978 3 o.75 wp 50% 1.83
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/2 3/4 5/6 7/8 9/10 13/15 16/19
(kg (%)
a.i./ha)
Fed.Rep.
of
Germany 1976 3 1.5 wp 50% 1.1 0.9 0.8 0.6
1976 3 0.95 wp 50% 1.7 1.4 1.1 0.9
Italy 1977 3 0.26 wp 50% 0.7
3 9.39 wp 50% 0.7
3 9.59 wp 50% 1.2
3 0.78 wp 50% 2.7
Strawberry Netherlands 1977 1 0.75 wp 50% 1.1 0.4
outdoor (0.3-2.7) (0.2-0.6)
1 0.75 wp 50% 2.1 2.0 1.2
(0.8-3.1) (1.2-2.9) (0.6-2.1)
Poland 1980 2 1.25 wp 50% 6.9 5.0 3.6 1.1 0.8
(6.5-5.1) (4.9-5.1) (3.3-4.0) (1.0-1.2) (0.7-0.9)
UK 1980 1 0.75 wp 50% 2.5
(1.9-3.1)
1 1.5 wp 50% 1.8
(1.6-1.9)
1980 1 0.75 wp 50% 4.5 2.4
(4.1-4.8) (2.0-2.7)
1.5 wp 50% 3.9 3.2
(3.5-4.4) (3.0-3.5)
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/2 3/4 5/6 7/8 9/10 13/15 16/19
(kg (%) 1)23 2/35
a.i./ha)
glasshouse Japan 1975 3 0.6 wp 50% 6.2 7.8 4.0 4.4 2.4 2.6
6 0.6 wp 50% 12.3 14.3 6.6 8.0 ~. 7 6.5
3 0.65 wp 50% 0.7 0.8 1.1 1.8 0.6 0.8
6 0.65 wp 50% 1.2 1.4 1.1 1.2 0.8 0.8
1975 3 0.65 wp 50% 1.6 1.6 0.8 0.8 0.4 0.4
6 0.65 wp 50% 0.9 1.0 0.9 1.0 0.3 0.3
1976 3 0.5 wp 50% 1.2 1.0 0.5
6 0.5 wp 50% 1.8 1.4 0.9
plastic
tunnels UK 3 0.75 wp 50% 1.84
2 0.75 wp 50% 0.65
Days
1 4/5 8/10 12/14 15/16 19/21 27/28
Beans
kidney
bean France 1978 1 0.75 wp 50% 0.2
1 1.0 wp 50% 0.92
1 1.0 wp 50% 0.4
2 0.75 wp 50% 0.2
2 1.0 wp 50% 0.4
1 0.75 wp 50% 0.8
1 1.0 wp 50% 2.2
1 1.0 wp 50% 0.7
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation Days
(kg (%) 1 4/5 8/10 12/14 15/16 19/21 27/28
a.i./ha)
1 0.75 wp 50% 0.1
1 1.0 wp 50% 0.3
1 1.0 wp 50% 0.5
1977 2 0.75 wp 50% 0.2
2 0.75 wp 50% 0.4
2 0.75 wp 50% 0.5
Netherlands 2 0.5 wp 50% 0.21(0.10-0.21)
2 0.5 wp 50% 0.11(0.09-0.15)
New
Zealand 2 0.5 wp 50% 0.8 0.7 0.6 0.6
2 1.0 wp 50% 1.8 1.7 1.5 1.3
1 3 7 12/14 19/21 28/31
Soybean Japan 1975 3 0.5 wp 50%
pods 2.8 1.2
beans 1.7
4 0.5 wp 50%
pods 2.5
beans 1.5
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 1 3 7 12/14 19/21 28/31
(kg (%)
a.i./ha)
3 0.5 wp 50%
pods 4.3 0.6
beans 1.1
beans 4 0.5 wp 50% 1.1
Onions
bulb onions Japan 1977 4 0.75 wp 50% 0.04 0.04 0.01
8 0.75 wp 50% 0.06 0.06 0.05
4 1.0 wp 50% 0.01 0.01 0.04
8 0.5-1.0 wp 50% 0.04 0.05 0.05
4 0.5 wp 50% 0.02 0.02 0.02
8 0.5 wp 50% 0.04 0.03 0.04
Netherlands 1978 2 0.25 wp 50% 0.12
(0.10-0.13)
2 0.25 wp 50% 0.12
(0.09-0.14)
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 01 19 86
a.i./ha)
Chicory
witloof France 1978 1 on top
of roots
at forcing 0.01
(leaves) 2 g m2 <0.01
4 g m2 0.01
1 dipping of
roots
2 g/m2 0.01
in roots
after forcing 2 g/m2 0.7
4 g/m2 2.0
(days)
0/1 3/4 6/7 13/14 21/23 26/28
Lettuce
glasshouse France 1980 1 0.75 wp 50% 0.6
1980 1 0.75 EC 500
g/l 1.2 1.1
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (days)
(kg (%) 0/1 3/4 6/7 13/14 21/23 26/28
a.i./ha)
1978 3 0.5 wp 50% 29.1 9.5 0.7
3 0.75 wp 50% 47.1 12.4 0.7
3 1.0 wp 50% 77.1 17.2 1.9
1978 1 0.75 wp 50% 3.4
1978 5 0.75 wp 50% 1.6
1978 0.75 wp 50% 3.1
Outdoors Fed.Rep.
of
Germany 1978 3 0.225 wp 50% 5.1 5.3 2.3 2.5 0.7 1.1 0.1 0.2 <0.1
1978 3 0.225 wp 50% 2.9 3.2 1.7 2.3 1.1 0.9 0.6 0.4 <0.1
Japan 1977 3 1.1 wp 50% 1.3 0.8 0.2 0.4
5 wp 50% 1.0 0.8 0.4 0.3
1977 3 0.75-1.0 wp 50% 0.02 0.01 <0.01 <0.01
6 wp 50% 0.3 0.02 0.02 0.01
glasshouse UK 1979 4 0.25 wp 50% 0.76
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg 42 47/51 56 66 88 104
a.i./ha) (%)
France 1978 4 0.5 wp 50% 0.2
0.75 wp 50% 0.5
1 wp 50% 0.7
1978 4 0.5 wp 50% 1.0
0.75 wp 50% 2.7
1.0 wp 50% 3.8
1976 3 0.5 wp 50% 1.7
0.75 wp 50% 3.2
1.0 wp 50% 4.7
1978 4 0.5 wp 50% 0.8
0.75 wp 50% 1.4
1.0 wp 50% 1.8
1978 3 0.75 wp 50% 0.6
(0.4-0.8)
Glasshouse
winter Netherlands 1978 1 1 wp 50% 0.06
1.5 wp 50% 0.08
1 wp 50% 0.05
1.5 wp 50% 0.08
1 wp 50% 0.03
1.5 wp 50% 0.04
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Cucumber Japan 1976 3 1.25 wp 50% 0.34 0.12 0.09
glasshouse 6 1.25 wp 50% 0.33 0.16 0.11
1976 3 1.0-1.25 wp 50% 1.2 0.67 0.28
6 0.63-1.25 wp 50% 1.2 0.82 0.32
Gherkins Netherlands 1978 3 0.3 wp 50% 0.17
(0.16-0.18)
1978 3 0.3 wp 50% 0.32
(0.22-0.55)
1978 3 0.3 wp 50% 0.26
(0.13-0.33)
Melon
(white fruit France 1979 1 0.75 wp 50% 0.17 0.19 0.13 0.23
(edible pulp 0.06 0.12 0.08 0.12
(edible pulp 1989 2 0.75 wp 50% 0.19 0.21 0.19 0.19
(peel 1.6 1.3 1.4 1.2
(edible pulp 1980 2 0.75 wp 50% 0.28 0.32 0.28 0.29
(peel 1.7 2.0 1.4 0.9
Pepper Japan 1975 3 1.5 3.3 1.7 1.3 0.3
(green) 6 1.5 3.0 2.5 1.5 0.3
glasshouse 1976 3 1.2-1.5 3.5 2.4 1.4 0.8
6 0.7-1.5 3.8 2.7 1.6 1.0
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Tomato
outdoor France 1979 6 1.5 wp 50% 0.30 0.32
7 1.5 wp 50% 0.28 0.55
1979 1 0.75 wp 50% 0.19 0.22 0.07
1980 3 0.75 wp 50% 2.7 1.7 1.5 1.4 0.7
1980 3 0.75 wp 50% 2.0 1.7 1.4 1.3 0.6
glasshouse Japan 1977 3 1.0-1.25 wp 50% 2.0 0.9 0.9 1.0
6 1.0-1.25 wp 50% 3.1 2.3 2.8 1.4
1977 3 1.5 wp 50% 2.5 2.1 2.2 2.3
6 1.5 wp 50% 4.7 3.7 2.6 2.3
1977 2 1.0-1.25 wp 50% 1.6 0.9 1.0 0.8
4 0.75-1.25 wp 50% 0.7 1.3 1.1 0.8
1977 2 1.5 wp 50% 0.4 0.3 0.3 0.2
4 1.5 wp 50% 0.8 0.5 0.6 0.2
Outdoor New
Zealand 1978 3 0.5 wp 50% 0.1 0.1 0.1 0.04
1.0 wp 50% 0.2 0.1 0.3 0.2
glasshouse UK 1977 5 0.28 wp 50% 0.8 0.6 0.4 0.7
0.39 wp 50% 0.8 0.7 0.4 0.5
0.56 wp 50% 1.1 0.3 0.6 0.6
0.78 wp 50% 0.4 0.5 0.5 0.6
1976 3 0.22 wp 50% 0.4
0.39 wp 50% 0.7
0.78 wp 50% 0.9
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Eggplant France 1978 1 1 wp 50% 1.5
1 Reference - Sumitomo 1981;
2 day 2;
3 21 days;
4 23 days;
5 35 days.
TABLE 7. Residues of procymidone resulting from supervised trials in field crops and oil seeds1
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate (kg
a.i./ha Formulation 3 6 9/10 14/15 19/22 28/31
Potato Japan 1977 4
whole 0.08 0.05
peeled 0.03 0.03
peel 0.19 0.23
1977 4
whole 0.02 0.03
peeled 0.01 0.01
peel 0.11 0.19
Rice Japan 1976 3 0.75
unpolished 1.5 1.4
polished 0.4 0.7
straw 8.7 3.8(3.4-4.1)
(8.5-9.0)
1976 5 0.75
unpolished 2.0
polished 0.7
straw 13.0
(12.9-13.1)
1976 3 0.75
unpolished 2.1 1.5
polished 0.4
straw 8.1
(7.8-8.5) 3.0
1976 5 0.75
unpolished 2.3
polished 0.5
straw 8.7(8.6-8.7)
TABLE 7. (con't)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate (kg
a.i./ha Formulation 0/1 3 8/9 14/15 20/22 24 37/41
Sunflower Hungary 1977 3 1 wp 50%
seed <0.1
seed Hungary 1977 3 1 wp 50% 3.3(2.4-3.8)
seed 1978 3 1.5 wp 50% 0.1 0.1
seed 1978 3 1 wp 50% 0.4 0.01
(0.37-0.44)
( seed 1979 1 0.5 wp 50% 0.02
( head 1.1 0.38 0.39 0.03 0.02
seed 1979 3 0.5 wp 50% <0.2
seed 1979 1 1 wp 50% 0.05
1 Reference - Sumitomo 1981.
Residue trials on raspberries were carried out in Poland and the
UK; the residues were less than 2 mg/kg on the day of the last
spraying.
Black currant
Residues in black-currants were in the range 5.3 to 6.9 mg/kg 1
to 3 days after the last of three applications at normal rates and
declined to 3.0 to 4.2 mg/kg after 7 days.
Vegetables
Lettuce
Supervised residue trials were carried out in Japan, France, the
Federal Republic of Germany and The Netherlands in 1976 to 1980 on
lettuce grown outdoors and in glasshouses in different seasons. The
residues were generally less than 3 mg/kg 3 to 4 days after the last
of 3 to 6 treatments, except in the trials carried out in France on
glasshouse lettuce in which comparatively high residues were found.
This may have been partly because (1) the whole crop was analysed
without any trimming of the outer leaves that normally occurs and (2)
the average weight of the lettuce heads sampled was relatively low.
The residue data from France in 1977 and 1978 suggests that the
dilution effects caused by growth of the plants accounts for the major
part of the decrease in the residue on indoor lettuce.
Legume vegetables
Residue data were available on kidney beans (French beans) from
France, The Netherlands, and New Zealand and on soybeans from Japan.
Residues in kidney beans were generally less than 2 mg/kg, 1 day or
more after the last of 2 to 6 applications of 0.5 to 1.0 kg
procymidone/ha.
Fruiting vegetables
Residue data on cucumbers, eggplants, gherkins, melons, green
peppers and tomatoes were available from France, Japan, The
Netherlands, New Zealand and the United Kingdom. Most of the data were
obtained on glasshouse crops.
Procymidone levels in cucumbers were less than 2 mg/kg one day
after treatment. In a trial in France in 1977 on eggplants at 1 kg
a.i./ha, twice the normally recommended rate, the residue level was
1.5 mg/kg 14 days after spraying. In green peppers grown under glass,
procymidone residues were less than 4 mg/kg one day after application
and generally below 1 mg/kg after 14 days.
Procymidone levels in tomatoes were generally below 5 mg/kg
immediately after application. The maximum residue was 4.9 mg/kg one
day after treatment at 1.5 kg/ha (twice the normally recommended
dosage).
Root and tuber vegetables
Residue trials on potatoes were carried out in Japan; the
residues were less than 0.1 mg/kg 19 days after the last of 4
applications at 0.5 kg/ha.
Bulb vegetables
Residue data on onions were obtained from Japan and The
Netherlands. When procymidone was applied at rates up to 1 kg/ha, the
residues in the bulbs did not exceed 1 mg/kg, regardless of the
interval between the last application and harvest.
Rice
Residue data on rice were obtained from Japan. Procymidone levels
in unhusked rice were in the range 1.4 to 2.3 mg/kg 21 to 28 days
after treatment. Polishing reduced the residues to less than 1 mg/kg.
Oil seeds
Residue trials were conducted in Hungary on sunflower and the
residues in sunflower seeds were generally less than 1 mg/kg, with one
exception where a maximum residue of 3.8 mg/kg was found 20 days after
the last of three applications at 1 kg/ha. In the latter case,
paraquat was applied as a haulm killer shortly before harvest.
FATE OF RESIDUES
In processing
The residue in wine made from grapes treated with procymidone was
30 to 50% of the residue on the grapes at harvest. (See Table 8).
Residues were further reduced on distilling wine. When wine containing
procymidone residues at 0.8 mg/kg was used to produce brandy, the
procymidone residues in the distillate were 0.07 to 0.35 mg/kg,
average 0.18 mg/kg (9 samples).
In plants
The dissipation and metabolism of 14C-procymidone, labelled at
the carbonyl group, was studied in bean and cucumber plants grown
under glass. After foliar application to beans at the rate of 250 µg
per leaf, procymidone disappeared from the plants with a half-life of
approximately 20 days. After 30 days, 40.2% of the applied
radioactivity was recovered. It was shown by TLC analysis that 92% of
TABLE 8. Residue decrease during wine processing
Residue (mg/kg), mean and range
Number
Country Year of On grapes
trials at harvest In must In wine
Fed.Rep. of 1976/77 9 2.9(1.2-6.6) 1.7 (0.7-2.6) 0.9 (0.7-1.0)
Germany
Italy 1975/76 2 0.93 1.94 1.25
2 1.56 4.12 2.68
In an experiment carried out in Spain in 1976 the decrease in the
residue during fermentation of the must was evaluated and samples were
taken 15 and 40 days after grape pressing. Results are reports in
Table 9.
TABLE 9. Residue of procymidone(mg/kg)mean residue and range
On grape Grape juice on In must at intervals (days)
at harvest day of pressing after pressing
15 40
3.5 (2.7-4.4) 3.6 (2.8-4.5) 3.0 (2.5-3.6) 2.7 (2.3-3.2)
5.7 (4.0-6.8) 5.5 (4.0-6.8) 4.3 (3.8-4.8) 3.8 (3.6-4.0)
the 14C recovered after 30 days was the parent compound. Two minor
metabolites were detected, procymidone-NH-COOH and procymidone-CH2OH,
which together accounted for about 1% of the 14C applied. These
metabolites are also found in animals after the oral administration of
procymidone. Only small amounts of the applied radiocarbon was
translocated to other parts of the plant: the 14C residues in pods
and seeds were 13 µg/kg and 6 µg/kg respectively after 30 days. The
residue of procymidone remains mainly on the outside of the treated
leaves and can be rinsed off with acetone. Thirty days after
application, the distribution of the 14C, expressed as the percentage
of the applied radioactivity, was as follows (Sumitomo 1981):
Total recovered 40.2
On and in treated leaves 39.1
In surface wash (procymidone 28.4
(rinsed 3 times
with acetone) 30.2 (procymidone-NH-COOH 1.3
(others, unidentified 0.2
(procymidone 7.7
(procymidone-NH-COOH 0.1
Extracted from treated (
leaves 8.1 (procymidone CH2OH 0.1
(4-OH-procymidone 0.1
(others, not identified 0.2
Not extractable 0.8
Total in shoots 1.0
In pods and seeds <0.1
In roots <0.1
After application of a liquid formulation of 14C-procymidone to
mature cucumber leaves adjacent to fruits at a rate of 250 µg
procymidone per leaf, the 14C was hardly translocated to either
leaves or fruits. After 23 days, approximately 64% of the applied
radioactivity was recovered from the treated leaves. Unchanged
procymidone (62%) and three metabolites, procymidone-NH-COOH,
procymidone-CH2OH and 4' OH-procymidone, were found in the treated
leaves, the metabolites accounting together for 0.4% of the applied
14C (Sumitomo 1978).
Procymidone added to the nutrient solution in which cucumber
plants were growing was fairly rapidly taken up by the plant. The
highest peak of radioactivity was reached 8 days after treatment, with
a residue level of 3.4 mg/kg fresh weight, whereas the concentration
in the shoots was much higher (20 to 30 mg/kg procymidone
equivalents). The distribution of procymidone and its metabolites
expressed as a percentage of the radioactivity applied is given in
Table 10.
In soil
The degradation of 14C-procymidone was studied in four different
soils under aerobic, anaerobic and sterilized conditions. After 12
weeks of incubation, about 57% and 72% of the applied 14C was
recovered as unchanged procymidone under aerobic and anaerobic
conditions, respectively, whereas about 70 to 77% was recovered as
unchanged procymidone from the sterilized soils. The major degradation
products were procymidone-NH-COOH(2-(3,5-dichlorophenyl-carbamoyl)-
1,2-dimethyl(cyclopropanecarboxylic acid) and CO2 (Sumitomo 1976).
TABLE 10. Distribution and characterization of radioactivity after treatment of cucumbers with
radioactive procymidone
Days after Radioactivity (% of applied)
treatment procymidone procymidone procymidone procymidone procymidone
NH-COOH CH2OH 4'OH 3' Cl
2 nutrient 86.1 4.3 1.0 <0.1 0.4
solution
roots 3.5 <0.1 - - <0.1
shoots 2.4 <0.1 - <0.1 -
fruits <0.1 - - - <0.1
8 nutrient 26.1 5.0 2.8 0.5 0.5
solution
roots 4.2 0.2 - - <0.1
shoots 37.9 0.2 - 0.2 -
fruits 2.0 - - - <0.1
24 nutrient 15.6 1.5 <0.1 <0.1 <0.1
solution
roots 3.0 0.1 - - <0.1
shoots 55.0 0.6 - 0.4 -
fruits 1.3 - - - <0.1
In another experiment three types of soil were treated with
10.2 mg/kg 14C-procymidone, labelled in the carbonoyl groups and
held under glass under aerobic and submerged conditions for 15 months.
The half-life of procymidone was about 4 months under submerged
conditions, depending on the soil moisture content. The rate of
decline under 60% soil moisture content was more rapid than under 40%.
After 15 months the levels of procymidone parent compound in Azuchi,
Kodaira and Takarazuka soil were 1.93-3.16 mg/kg, 2.21-3.28mg/kg and
2.49-3.67 mg/kg respectively. The volatile 14C gradually increased
with time, amounting after 15 months of incubation to 16.9 to 39.9% of
applied 14C in Azuchi, 11.9 to 19.9% in Kodaira and 21.3 to 33% in
Takarazuka soil. The bound 14C also gradually increased with time to
12.2 to 24.5%, 42.1 to 48.6% and 15.0 to 18.2% of the applied 14C in
Azuchi, Kodaira and Takarazuka soils respectively after 9 to 12 months
of incubation, but decreased thereafter. The differences in the
proportion of bound residue are related to the organic matter content,
which is much higher in the Kodaira soil (15.3%) than in the two other
soils (2.5 to 2.7%). More than seven degradation products were
detected. None of these exceeded 5% of the applied radioactivity in
the soils held under aerobic conditions. Procymidone is hydroxylated
at the methyl group and at the 4-phenyl position in soil. Also
dichlorination and cleavage of one or both bonds of the cyclic imide
linkage takes place, ultimately yielding CO2 (Sumitomo 1980).
The leaching behaviour of procymidone was studied using 25 cm
soil columns packed with different soil types. Air-dry soil, 30 g, was
mixed with 10 mg/kg 14C-procymidone and placed on top of the column
immediately after mixing or after an incubation period of 4 weeks. The
soil columns were leached with 550 ml water at a rate of 3 ml/h for 7
weeks. The radiocarbon penetrated below 0 to 15 cm layers in the
Kodairi, Azuchi and Takarazuka soils, all of which had an organic
matter content of 2.5% or slightly higher. The eluate contained less
than 3% of the applied 14C. However, in a very light Gifu soil with
an organic matter content below 1%, the radiocarbon in the eluate
amounted to 75.4% of the applied 14C.
Incubation of the treated soil for 4 weeks resulted in a lower
proportion of the 14C leaching through the soil columns. The
radiocarbon in the leachate of a Gifu soil column was decreased to
17.3% of that applied (Sumitomo 1980).
Adsorption and desorption of 14C-procymidone was studied in
aqueous soil suspension systems. Among 11 soil characteristics
measured, the organic matter content was found to be the only factor
that significantly affected procymidone adsorption. A good linear
relationship in logarithmic expression was observed between
procymidone adsorption and organic matter content in soils. The
adsorption behaviour of procymidone is well described by the so-called
Freundlich isotherms: the Freundlich k value varied from 1.1 to 6.0
and the l/n value was close to one. The desorption isotherms exhibited
hysteresis, apparent irreversibility, in comparison with adsorption
isotherms (Sumitomo 1980).
Residue uptake in plants from treated soils
Bean seedlings were grown on two soils, incubated with 10 mg/kg
of 14C-procymidone for two weeks and 5 months respectively for 42
days. After 42 days, pods, seeds, shoots and roots were analysed for
14C. The highest 14C levels in beans grown in Kodaira and Takarazuka
soils incubated for two weeks were in the shoots 12.3 mg/kg and
15.3 mg/kg procymidone equivalents respectively, whereas the 14C
residue levels in the edible portions were 0.42 and 0.66 mg/kg.
When beans were transplanted into soils incubated for 5 months
with procymidone, the 14C residues in the shoots, pods and seeds were
lower than in the beans grown after the shorter incubation period
mentioned above. After 42 days the residue levels in the shoots were
3.92 and 4.46 mg/kg and in the pods and seeds 0.12 and 0.25 mg/kg (the
higher figures were in beans from Takarazuka soil) (Sumitomo 1981).
Photodegradation
Photodegradation of 14C-procymidone was studied in various
solutions exposed to sunlight under glasshouse conditions. The half-
lives of procymidone in distilled water, natural river-water and sea-
water were 10, 1.0 and 0.6 days respectively. The degradation of
procymidone in water exposed to sunlight seemed to be largely due to
hydrolytic reactions, with sunlight showing a slight but definite
effect on the breakdown. Procymidone was degraded via cleavage of the
cyclic imide and further cleavage of the resulting amide linkage
(Sumitomo 1980).
Hydrolysis
The hydrolysis of 14C-procymidone was studied in buffer
solutions with pH ranges from 2 to 10 and in natural river and sea
water at 15°, 30° and 45° C. Procymidone is easily hydrolysed at pH
6.3 and above at 45°, at pH 7.1 and above at 30° and at pH 8 and above
at 15°. Half-life periods ranging from 30 min. to 8.1 days were found
under these conditions. On the other hand, the compound is fairly
stable at pH 2 and half-lives of 34 days (at 45°C) to 62 days
(at 15°C) were found. The hydrolysis of procymidone proceeded
predominantly through neutral (PH-independent) and base-catalysed
processes in the regions below pH 5 and above pH 8 respectively, while
both reactions occurred in the range between pH 5 and 8. In alkaline
solutions, the degradation product formed by the cleavage of the
cyclic imide was predominant, whereas the resulting amide was cleaved
further to yield the main product under aerobic conditions (Sumitomo
1980).
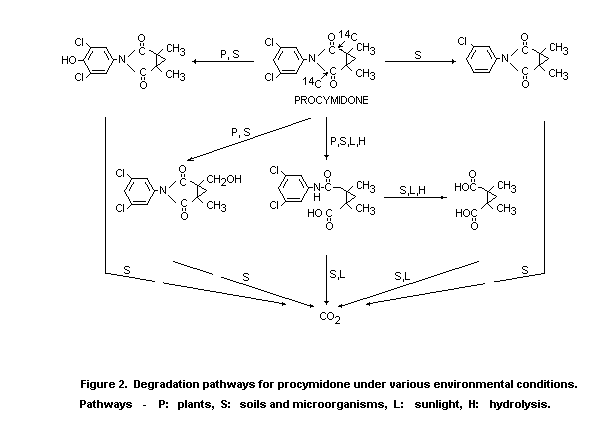
In the light of the above data, the degradation pathways for
procymidone in plants, water, soil and micro-organisms shown in Figure
2 can be proposed.
METHODS OF RESIDUE ANALYSIS
The residue determination of procymidone parent compound is
almost exclusively carried out by gas chromatography with electron
capture detection (GC-ECD). The limit of determination in most plant
material is as low as 0.2 ng or even less (Sumitomo 1976, 1978), which
enables a limit of determination of 0.04 to 0.02 mg/kg in most cases
(Sumitomo 1976,1978). The GC-ECD method is suitable or can be adapted
for regulatory purposes.
Capillary gas chromatography with a flame ionization detector
also provided high sensitivity; the limit of determination was about
0.05 ng, which enabled a limit of determination of procymidone as low
as 0.01 mg/kg (Sumitomo 1978).
Although the alkali flame ionization detector (AFID) in the
nitrogen mode may also be used for residue analysis of procymidone, it
appears that its response to procymidone was about one tenth of that
of the ECD (Sumitomo 1980).
The identity of procymidone residues can be confirmed by combined
gas chromatography-mass spectrometry (GC-MS), using selected ion
monitoring, giving 0.2 ng as a minimum detectable amount when m/e 283
and m/e 285 are monitored (Sumitomo 1980).
High-performance liquid chromatography (HPLC) with UV detection
at 254 nm permits a limit of determination of 4 ng, which corresponds
to about 0.05-0.01 mg/kg; however, the method has so far been used
very little in residue analysis for procymidone.
Extraction and clean-up procedures
Procymidone was successfully extracted from substances with a
relatively high moisture content (fruits, leafy vegetables and root
crops) by chopping and blending with polar solvents such as acetone
(Sumitomo 1978; Cooke et al 1979), acetonitrile (Sumitomo 1978), or
methanol-acetonitrile (Sumitomo 1976) or with non polar solvents, such
as trimethylpentane (Sumitomo 1976) or hexane. Procymidone was
preferably extracted from substrates with a low moisture content, such
as rice or soybeans, by pulverising and macerating with acetonitrile
(Sumitomo 1976, 1979).
Clean-up procedures for extracts from oil-containing commodities,
such as soybeans and rice, may be needed prior to further
chromatographic separations, including partition between acetonitrile
and n-hexane. Combined partition-Florisil column chromatography gave
Other information on identity and properties
Molecular weight 284.13
State White to light brownish crystalline solid
(technical material)
Specific gravity d25
25 1.42 - 1.46
Vapour pressure 1.32 × 10-4 mm Hg at 25°C
Solubility Acetone 18.0, acetonitrile 10.1,
(% w/w at 25°C) cyclohexanone 14.8, ethyl acetate 11.5,
methanol 1.6, xylene 4.3
Solubility in water 4.5 mg/l at 25°C
n-octanol/water
partition co-efficient 1.3834 × 103 at pH 7.26
Stability Stable in solvents, unstable in alkaline
media. No significant breakdown after 20
weeks storage at 60°C.
Formulation Currently available as 50% wettable powder
and 265 and 500 g/l emulsifiable concentrate
Purity of technical
product Contains normally 95-99% of procymidone.
Detailed information on the impurities in
technical procymidone ranging from 0.1-1% was
reported to the Meeting.
RESIDUES IN FOOD
USE PATTERN
Procymidone is a recently introduced moderately systemic
fungicide with a rather selective action. It is especially effective
in the control of Botrytis and Sclerotinia species on various
vegetables, fruits, flower crops and ornamentals. It is also used on
stone fruits against Monilia sp.
There are some indications that the fungicide may affect mitosis
in susceptible fungi but the actual site of action has not been
elucidated.
Procymidone is effective against Botrytis strains that have
acquired resistance to benzimidazole compounds; the latter also have
an antimitotic action in fungi owing to interference with the
functioning and assembly of microtubules, the elements of the spindle.
It is assumed that the site of action of procymidone in the mitotic
process is different from that of the benzimidazole compounds.
Procymidone is applied, often repeatedly, on the aerial parts of
fruits and vegetables and sometimes until shortly before harvest to
avoid fruit- and vegetable-rot during transport and distribution. It
is also used on young plants and/or the top layer of soil against
soil-borne diseases (Sclerotinia, Botrytis).
Products containing procymidone are registered and/or approved in
many countries, in Asia, South America and Europe as well as in
Australia and New Zealand. They are mainly used against grey mould
(mainly Botrytis cinerea), onion grey mould (Botrytis squamosa),
onion neck-rot (Botrytis aclada allii), Sclerotinia spp. and
Monilia sp. (brown rot on peaches etc., blossom blight and twig wilt
on cherries, etc.).
The current suggested uses are summarized in Tables 4 and 5.
Recommended pre-harvest intervals known to the Meeting are listed
under national maximum residue limits.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Many supervised residue trials have been carried out on a wide
variety of fruits and vegetables and also on rice and oilseeds. See
Tables 6 and 7 for details.
Fruits
Pome fruits
Residue data were obtained only on apples from Japan. Procymidone
levels were less than 3 mg/kg 21 days after the last of 3 to 5
treatments, but had risen to about 3 mg/kg 35 days after the last of 5
treatments.
Stone fruits
Residue data on cherries were available from Australia, Hungary
and Japan. Procymidone levels were generally less than 5 mg/kg one day
or more after the last treatment, except one erratic sample in
Australia which contained 6.2 mg/kg.
Residue levels in peaches were generally less than 2 mg/kg three
or more days after the last application of 0.2 to 3 kg/ha (recommended
application rates are 0.75 to 1.5 kg/ha.
In a trial in New Zealand, nectarines sprayed 7 times at
0.75 kg/ha contained procymidone at or below 0.21 mg/kg.
In a trial in France, the residue on plums after a single
application of procymidone at 0.75 kg a.i./ha was 2.4 mg/kg at day 0
(4 h after spraying) and 1.3 mg/kg 8 days later.
Berry fruits
Residue data on grapes were obtained from several countries in
Europe, from New Zealand and South Africa, where 1 to 5 treatments
were carried out at rates of 0.5 to 1.25 kg/ha. About 14 days after
the last treatment, procymidone residues were in the range of 0.4 to
9.3 mg/kg and declined to 0.6 to 5.1 mg/kg after 35 to 36 days.
Residue data were obtained on strawberries grown outdoors and in
glasshouses or plastic tunnels. The procymidone residues were 0.8 to
14.3 mg/kg 0 to 1 day after the last spraying and declined to 0.8 to
6.5 mg/kg after 6 to 8 days. The residue levels after 14 to 15 days
were generally 2 mg/kg or less.
TABLE 4. Use patterns of procymidone on fruits, registered and/or approved uses
Country
Fed.Rep.
of
Crop France Germany Luxembourg Spain Switzerland UK Bulgaria Czechoslovakia Hungary Romania Yugoslavia
Black currant X
Grape X X X X X X X X X X X
(incl. table grapes)
Peach X
Raspberry X X
Stone fruits X
Strawberry X X X X
Table 4 (continued)
Country Range of application
South New Target rates in various countries
Crop Greece Turkey Africa Chile Uruguay Japan Lebanon Australia Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Black currant 0.75
Grape X X X X X X X Bc 0.5 - 1.0 25 - 75
(incl. table grapes)
Peach X M 0.5 - 0.75 33 - 50
Raspberry Bc 0.5 - 0.75
Stone fruits X X X Bc M 0.6 - 1.0 25 - 75
Strawberry X X X X Bc 0.4 - 0.75 25 - 100
1 Bc = grey mould Botrytis sp., mainly Botrytis cinerea;
M = Monilia, including Monilinia fructigena and M. fructicola (brown rot, blossom blight and twig blight).
TABLE 5. Use patterns of procymidone on vegetables and oil seeds; registered and/or approved uses
Countries
Europe Asia Range of application
New Target rates in various countries
Crop France UK Hungary Greece Japan Korea Lebanon Taiwan Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Beans (not specified) X X X Bc Sc 0.5-0.75 25-50
Azuki bean X Bc 50
Kidney bean X 25-50
Soybean X Sc 25-50
Celery X Sc 25-50
Cucumber X Bc Sc 25-50
Eggplant X Bc Sc 25-50
Lettuce X X X X X Bc Sc 0.4-0.75 25-50
Melon X Bc Sc 0.75
Onion X Bsq Baa 50
Pepper (green) X Bc Sc 25-50
Potato X Sc 33-50
Tomato X X X Bc Sc 0.5 25-50
Vegetables (general) X X X 0.5 25-50
TABLE 5. (con't)
Countries
Europe Asia Range of application
New Target rates in various countries
Crop France UK Hungary Greece Japan Korea Lebanon Taiwan Zealand fungi1 (kg a.i./ha (g a.i./100 l)
Rape seed X Sc 50
Sunflower X Bc 0.5
1 Bc = grey mould, mainly Botrytis cinerea;
Sc = Sclerotinia spp.;
Bsq = Botrytis squamosa;
Baa = Grey mould neck rot = Botrytis aclada allii.
TABLE 6. Residues of procymidone resulting from supervised trials in fruits and vegetables
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 01/1 3/4 6/8 14/16 20/22 26/29 35/36
(kg (%)
a.i./ha)
Grape France 1977/80 1 0.75 wp 50% 0.53
1 0.75 EC500g/l 0.48
4 0.75 wp 50% 5.0 4.8 4.7 4.5 4.8 4.9 3.4 3.6
Table
grapes 1980 4 0.75 wp 50% 3.4 3.1 1.7 1.4 1.3 1.5 0.9 1.0 0.9 0.8
(") 1980 4 2.0 2.0 1.8 1.6 2.5 2.3 1.6 1.4 1.0 1.1
1977 1 0.75 wp 50% 2.46
(2.09-5.10)
1 1.0 wp 50% 3.89
(1.76-5.42)
1977 >1 0.75 wp 50% 4.9 4.4 3.2 3.3
1977 >1 0.75 wp 50% 6.4 3.1 2.2 2.8
Fed.Rep.
of Germany 1976/77 5 1.25 wp 50% 11.2 8.4 2.4 2.1
" 5 1.25 wp 50% 9.3 10.1 6.6 5.1
" 5 1.25 wp 50% 7.2 5.7 5.9 5.2 2.4
" 4 1.25 wp 50% 8.0 5.6 4.2 3.7 0.6
" 4 1.25 wp 50% 4.2 3.7 3.2 2.9
" 4 1.25 wp 50% 5.0 3.7 2.4 2.0 1.9
" 5 0.9 wp 50% 3.7 2.5 1.9 1.6 1.2
" 5 0.9 wp 50% 6.1 3.0 2.5 2.4 1.4
" 5 0.9 wp 50% 5.1 3.4 2.8 2.1 1.7
Italy 1976/77 4 0.85 wp 50% 1.9 0.9
" 4 1.28 wp 50% 4.1 1.6
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 01/1 3/4 6/9 14/16 20/22 26/29 35/36
(kg (%)
a.i./ha)
Grape New
Zealand 1979 1 0.7 wp 50% 1.7 1.7 1.0 1.6
1.05 wp 50% 2.6 4.3 2.7 2.5
Spain 1976 3 7.5 g/
100 1 wp 50% 4.4 4.0 3.0 2.7
12.5 g/
100 l wp 50% 6.8 6.8 5.3 4.0
Table South
grapes Africa 1978 1 0.7 dust 0.7 0.7 0.3 0.4 0.5 0.5 0.4 0.4
1x4 0.75 + wp 50 +
0.7 dust 3% 0.6 0.6 0.5 0.6 0.5 0.5 0.4 0.4
UK 1978 1 0.75 wp 50% 7.9
0/1 3/4 7/9 14 21 28 35
Apple Japan 1979 3 3 wp 50% ng 2.0 2.1 2.3
5 3 wp 50% 1.2 1.1 1.2 1.3
Black
currant UK 1979 3 0.75 wp 50% 5.3 4.2
1 0.5 wp 50% 6.0 7.0 3.3 3.5 2.1
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 7/9 14 21 28 35
(kg (%)
a.i./ha)
Cherry Australia 1977 5 19 g/
100 l wp 50% 3.3 2.4
525 g/
100 l wp 50% 2.0 2.1
5 37.5 g/
100 l wp 50% 6.2 2.9
Japan 1977 1 3.5 wp 50% 1.9 1.0 1.7
3 3.5 wp 50% 3.6 2.3 1.7
3 3.5 wp 50% 1.1 1.0 0.6
5 3.5 wp 50% 1.3 1.2 1.1
Peach Australia 1978 2-9 19 g/
100 l wp 50% 2.6
(2.4-5.1)
25 g/
100 l wp 50% 4.1
(1.6-7.6)
37.5 g
/100 l wp 50% 6.3
(2.3-11.7)
Japan 1977 4 3 wp 50%
peel 73.9 72.8 43.2
pulp 1.1 1.1 0.8
1977 6 3 wp 50%
peel 51.2 44.5 29.0
pulp 1.2 1.1 1.0
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 6/9 14 20/21 28 35
(kg (%)
a.i./ha)
New
Zealand 1980 7 0.75 wp 50% 0.42 0.4 0.4
7 0.75 wp 50% 0.6 0.8 0.5 0.3
5 1.5 wp 50% 0.3 0.4 0.9 0.6
210 g/
100 l wp 50% 0.4 0.7 0.4 0.3
2 20g/
100 l wp 50% 1.7 1.1 1.3 1.1
230 g/
100 l wp 50% 2.4 1.1 1.2 0.3
Nectarines New
Zealand 1980 7 0.75 wp 50% 0.1 0.2 0.1 0.1
Plum France 1979 1 0.75 wp 50% 2.4 1.3
Raspberry Poland 1980 2 1.25 wp 50% 1.7 0.25
(1.6-1.9) (0.2-0.3)
UK 1978 1 0.75 wp 50% 0.3
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/1 3/4 6/9 14 20/21 28 35
(kg (%)
a.i./ha)
Kiwifruit New
Zealand 1980/81 6 1.1 wp 50% 4.4 2.6 5.9 2.8 2.0
(flesh only) 0.5 0.4 0.25
1980/81 6 1.1 wp 50% 6.3 6.7 11.4 5.4 3.3
(flesh only) 1.8 2.1 0.8
6100 g/
100 l wp 50% 5.3 4.7 8.7 4.1 2.6
450 g/
100 l wp 50% 2.9 3.0 1.9
0/2 3/4 5/6 7/8 9/10 13/15 16/19
Strawberry France 1979 1 0.75 wp 50% 0.4 0.3
outdoors 1979 2 0.75 wp 50% 1.0 0.1
1980 3 0.75 wp 50% 4.9 5.2 2.1 2.4 1.9 2.0 0.7 0.5
1980 3 0.75 wp 50% 3.8 3.6 2.1 1.9 1.3 1.4 0.4 0.4
1980 3 0.75 wp 50% 2.9 2.7 2.3 2.3 1.1 1.1 0.2 0.3
1980 3 0.75 wp 50% 3.8 3.6 2.3 2.2 1.3 1.2 0.3 0.3
1978 3 0.5 wp 50% 2.1 1.5 0.9
1978 3 0.75 wp 50% 3.0 1.3 0.8
1978 4 0.75 wp 50% 3.6 1.5
1978 3 0.5 wp 50% 0.93
1978 3 o.75 wp 50% 1.83
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/2 3/4 5/6 7/8 9/10 13/15 16/19
(kg (%)
a.i./ha)
Fed.Rep.
of
Germany 1976 3 1.5 wp 50% 1.1 0.9 0.8 0.6
1976 3 0.95 wp 50% 1.7 1.4 1.1 0.9
Italy 1977 3 0.26 wp 50% 0.7
3 9.39 wp 50% 0.7
3 9.59 wp 50% 1.2
3 0.78 wp 50% 2.7
Strawberry Netherlands 1977 1 0.75 wp 50% 1.1 0.4
outdoor (0.3-2.7) (0.2-0.6)
1 0.75 wp 50% 2.1 2.0 1.2
(0.8-3.1) (1.2-2.9) (0.6-2.1)
Poland 1980 2 1.25 wp 50% 6.9 5.0 3.6 1.1 0.8
(6.5-5.1) (4.9-5.1) (3.3-4.0) (1.0-1.2) (0.7-0.9)
UK 1980 1 0.75 wp 50% 2.5
(1.9-3.1)
1 1.5 wp 50% 1.8
(1.6-1.9)
1980 1 0.75 wp 50% 4.5 2.4
(4.1-4.8) (2.0-2.7)
1.5 wp 50% 3.9 3.2
(3.5-4.4) (3.0-3.5)
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 0/2 3/4 5/6 7/8 9/10 13/15 16/19
(kg (%) 1)23 2/35
a.i./ha)
glasshouse Japan 1975 3 0.6 wp 50% 6.2 7.8 4.0 4.4 2.4 2.6
6 0.6 wp 50% 12.3 14.3 6.6 8.0 ~. 7 6.5
3 0.65 wp 50% 0.7 0.8 1.1 1.8 0.6 0.8
6 0.65 wp 50% 1.2 1.4 1.1 1.2 0.8 0.8
1975 3 0.65 wp 50% 1.6 1.6 0.8 0.8 0.4 0.4
6 0.65 wp 50% 0.9 1.0 0.9 1.0 0.3 0.3
1976 3 0.5 wp 50% 1.2 1.0 0.5
6 0.5 wp 50% 1.8 1.4 0.9
plastic
tunnels UK 3 0.75 wp 50% 1.84
2 0.75 wp 50% 0.65
Days
1 4/5 8/10 12/14 15/16 19/21 27/28
Beans
kidney
bean France 1978 1 0.75 wp 50% 0.2
1 1.0 wp 50% 0.92
1 1.0 wp 50% 0.4
2 0.75 wp 50% 0.2
2 1.0 wp 50% 0.4
1 0.75 wp 50% 0.8
1 1.0 wp 50% 2.2
1 1.0 wp 50% 0.7
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation Days
(kg (%) 1 4/5 8/10 12/14 15/16 19/21 27/28
a.i./ha)
1 0.75 wp 50% 0.1
1 1.0 wp 50% 0.3
1 1.0 wp 50% 0.5
1977 2 0.75 wp 50% 0.2
2 0.75 wp 50% 0.4
2 0.75 wp 50% 0.5
Netherlands 2 0.5 wp 50% 0.21(0.10-0.21)
2 0.5 wp 50% 0.11(0.09-0.15)
New
Zealand 2 0.5 wp 50% 0.8 0.7 0.6 0.6
2 1.0 wp 50% 1.8 1.7 1.5 1.3
1 3 7 12/14 19/21 28/31
Soybean Japan 1975 3 0.5 wp 50%
pods 2.8 1.2
beans 1.7
4 0.5 wp 50%
pods 2.5
beans 1.5
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation 1 3 7 12/14 19/21 28/31
(kg (%)
a.i./ha)
3 0.5 wp 50%
pods 4.3 0.6
beans 1.1
beans 4 0.5 wp 50% 1.1
Onions
bulb onions Japan 1977 4 0.75 wp 50% 0.04 0.04 0.01
8 0.75 wp 50% 0.06 0.06 0.05
4 1.0 wp 50% 0.01 0.01 0.04
8 0.5-1.0 wp 50% 0.04 0.05 0.05
4 0.5 wp 50% 0.02 0.02 0.02
8 0.5 wp 50% 0.04 0.03 0.04
Netherlands 1978 2 0.25 wp 50% 0.12
(0.10-0.13)
2 0.25 wp 50% 0.12
(0.09-0.14)
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 01 19 86
a.i./ha)
Chicory
witloof France 1978 1 on top
of roots
at forcing 0.01
(leaves) 2 g m2 <0.01
4 g m2 0.01
1 dipping of
roots
2 g/m2 0.01
in roots
after forcing 2 g/m2 0.7
4 g/m2 2.0
(days)
0/1 3/4 6/7 13/14 21/23 26/28
Lettuce
glasshouse France 1980 1 0.75 wp 50% 0.6
1980 1 0.75 EC 500
g/l 1.2 1.1
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (days)
(kg (%) 0/1 3/4 6/7 13/14 21/23 26/28
a.i./ha)
1978 3 0.5 wp 50% 29.1 9.5 0.7
3 0.75 wp 50% 47.1 12.4 0.7
3 1.0 wp 50% 77.1 17.2 1.9
1978 1 0.75 wp 50% 3.4
1978 5 0.75 wp 50% 1.6
1978 0.75 wp 50% 3.1
Outdoors Fed.Rep.
of
Germany 1978 3 0.225 wp 50% 5.1 5.3 2.3 2.5 0.7 1.1 0.1 0.2 <0.1
1978 3 0.225 wp 50% 2.9 3.2 1.7 2.3 1.1 0.9 0.6 0.4 <0.1
Japan 1977 3 1.1 wp 50% 1.3 0.8 0.2 0.4
5 wp 50% 1.0 0.8 0.4 0.3
1977 3 0.75-1.0 wp 50% 0.02 0.01 <0.01 <0.01
6 wp 50% 0.3 0.02 0.02 0.01
glasshouse UK 1979 4 0.25 wp 50% 0.76
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg 42 47/51 56 66 88 104
a.i./ha) (%)
France 1978 4 0.5 wp 50% 0.2
0.75 wp 50% 0.5
1 wp 50% 0.7
1978 4 0.5 wp 50% 1.0
0.75 wp 50% 2.7
1.0 wp 50% 3.8
1976 3 0.5 wp 50% 1.7
0.75 wp 50% 3.2
1.0 wp 50% 4.7
1978 4 0.5 wp 50% 0.8
0.75 wp 50% 1.4
1.0 wp 50% 1.8
1978 3 0.75 wp 50% 0.6
(0.4-0.8)
Glasshouse
winter Netherlands 1978 1 1 wp 50% 0.06
1.5 wp 50% 0.08
1 wp 50% 0.05
1.5 wp 50% 0.08
1 wp 50% 0.03
1.5 wp 50% 0.04
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Cucumber Japan 1976 3 1.25 wp 50% 0.34 0.12 0.09
glasshouse 6 1.25 wp 50% 0.33 0.16 0.11
1976 3 1.0-1.25 wp 50% 1.2 0.67 0.28
6 0.63-1.25 wp 50% 1.2 0.82 0.32
Gherkins Netherlands 1978 3 0.3 wp 50% 0.17
(0.16-0.18)
1978 3 0.3 wp 50% 0.32
(0.22-0.55)
1978 3 0.3 wp 50% 0.26
(0.13-0.33)
Melon
(white fruit France 1979 1 0.75 wp 50% 0.17 0.19 0.13 0.23
(edible pulp 0.06 0.12 0.08 0.12
(edible pulp 1989 2 0.75 wp 50% 0.19 0.21 0.19 0.19
(peel 1.6 1.3 1.4 1.2
(edible pulp 1980 2 0.75 wp 50% 0.28 0.32 0.28 0.29
(peel 1.7 2.0 1.4 0.9
Pepper Japan 1975 3 1.5 3.3 1.7 1.3 0.3
(green) 6 1.5 3.0 2.5 1.5 0.3
glasshouse 1976 3 1.2-1.5 3.5 2.4 1.4 0.8
6 0.7-1.5 3.8 2.7 1.6 1.0
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Tomato
outdoor France 1979 6 1.5 wp 50% 0.30 0.32
7 1.5 wp 50% 0.28 0.55
1979 1 0.75 wp 50% 0.19 0.22 0.07
1980 3 0.75 wp 50% 2.7 1.7 1.5 1.4 0.7
1980 3 0.75 wp 50% 2.0 1.7 1.4 1.3 0.6
glasshouse Japan 1977 3 1.0-1.25 wp 50% 2.0 0.9 0.9 1.0
6 1.0-1.25 wp 50% 3.1 2.3 2.8 1.4
1977 3 1.5 wp 50% 2.5 2.1 2.2 2.3
6 1.5 wp 50% 4.7 3.7 2.6 2.3
1977 2 1.0-1.25 wp 50% 1.6 0.9 1.0 0.8
4 0.75-1.25 wp 50% 0.7 1.3 1.1 0.8
1977 2 1.5 wp 50% 0.4 0.3 0.3 0.2
4 1.5 wp 50% 0.8 0.5 0.6 0.2
Outdoor New
Zealand 1978 3 0.5 wp 50% 0.1 0.1 0.1 0.04
1.0 wp 50% 0.2 0.1 0.3 0.2
glasshouse UK 1977 5 0.28 wp 50% 0.8 0.6 0.4 0.7
0.39 wp 50% 0.8 0.7 0.4 0.5
0.56 wp 50% 1.1 0.3 0.6 0.6
0.78 wp 50% 0.4 0.5 0.5 0.6
1976 3 0.22 wp 50% 0.4
0.39 wp 50% 0.7
0.78 wp 50% 0.9
TABLE 6. (con't)
Application Residues (mg/kg)at intervals(days) after application
Crop Country Year No. Rate Formulation (Days)
(kg (%) 0/1 3/4 7/9 10/11 13/14 21
Eggplant France 1978 1 1 wp 50% 1.5
1 Reference - Sumitomo 1981;
2 day 2;
3 21 days;
4 23 days;
5 35 days.
TABLE 7. Residues of procymidone resulting from supervised trials in field crops and oil seeds1
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate (kg
a.i./ha Formulation 3 6 9/10 14/15 19/22 28/31
Potato Japan 1977 4
whole 0.08 0.05
peeled 0.03 0.03
peel 0.19 0.23
1977 4
whole 0.02 0.03
peeled 0.01 0.01
peel 0.11 0.19
Rice Japan 1976 3 0.75
unpolished 1.5 1.4
polished 0.4 0.7
straw 8.7 3.8(3.4-4.1)
(8.5-9.0)
1976 5 0.75
unpolished 2.0
polished 0.7
straw 13.0
(12.9-13.1)
1976 3 0.75
unpolished 2.1 1.5
polished 0.4
straw 8.1
(7.8-8.5) 3.0
1976 5 0.75
unpolished 2.3
polished 0.5
straw 8.7(8.6-8.7)
TABLE 7. (con't)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate (kg
a.i./ha Formulation 0/1 3 8/9 14/15 20/22 24 37/41
Sunflower Hungary 1977 3 1 wp 50%
seed <0.1
seed Hungary 1977 3 1 wp 50% 3.3(2.4-3.8)
seed 1978 3 1.5 wp 50% 0.1 0.1
seed 1978 3 1 wp 50% 0.4 0.01
(0.37-0.44)
( seed 1979 1 0.5 wp 50% 0.02
( head 1.1 0.38 0.39 0.03 0.02
seed 1979 3 0.5 wp 50% <0.2
seed 1979 1 1 wp 50% 0.05
1 Reference - Sumitomo 1981.
Residue trials on raspberries were carried out in Poland and the
UK; the residues were less than 2 mg/kg on the day of the last
spraying.
Black currant
Residues in black-currants were in the range 5.3 to 6.9 mg/kg 1
to 3 days after the last of three applications at normal rates and
declined to 3.0 to 4.2 mg/kg after 7 days.
Vegetables
Lettuce
Supervised residue trials were carried out in Japan, France, the
Federal Republic of Germany and The Netherlands in 1976 to 1980 on
lettuce grown outdoors and in glasshouses in different seasons. The
residues were generally less than 3 mg/kg 3 to 4 days after the last
of 3 to 6 treatments, except in the trials carried out in France on
glasshouse lettuce in which comparatively high residues were found.
This may have been partly because (1) the whole crop was analysed
without any trimming of the outer leaves that normally occurs and (2)
the average weight of the lettuce heads sampled was relatively low.
The residue data from France in 1977 and 1978 suggests that the
dilution effects caused by growth of the plants accounts for the major
part of the decrease in the residue on indoor lettuce.
Legume vegetables
Residue data were available on kidney beans (French beans) from
France, The Netherlands, and New Zealand and on soybeans from Japan.
Residues in kidney beans were generally less than 2 mg/kg, 1 day or
more after the last of 2 to 6 applications of 0.5 to 1.0 kg
procymidone/ha.
Fruiting vegetables
Residue data on cucumbers, eggplants, gherkins, melons, green
peppers and tomatoes were available from France, Japan, The
Netherlands, New Zealand and the United Kingdom. Most of the data were
obtained on glasshouse crops.
Procymidone levels in cucumbers were less than 2 mg/kg one day
after treatment. In a trial in France in 1977 on eggplants at 1 kg
a.i./ha, twice the normally recommended rate, the residue level was
1.5 mg/kg 14 days after spraying. In green peppers grown under glass,
procymidone residues were less than 4 mg/kg one day after application
and generally below 1 mg/kg after 14 days.
Procymidone levels in tomatoes were generally below 5 mg/kg
immediately after application. The maximum residue was 4.9 mg/kg one
day after treatment at 1.5 kg/ha (twice the normally recommended
dosage).
Root and tuber vegetables
Residue trials on potatoes were carried out in Japan; the
residues were less than 0.1 mg/kg 19 days after the last of 4
applications at 0.5 kg/ha.
Bulb vegetables
Residue data on onions were obtained from Japan and The
Netherlands. When procymidone was applied at rates up to 1 kg/ha, the
residues in the bulbs did not exceed 1 mg/kg, regardless of the
interval between the last application and harvest.
Rice
Residue data on rice were obtained from Japan. Procymidone levels
in unhusked rice were in the range 1.4 to 2.3 mg/kg 21 to 28 days
after treatment. Polishing reduced the residues to less than 1 mg/kg.
Oil seeds
Residue trials were conducted in Hungary on sunflower and the
residues in sunflower seeds were generally less than 1 mg/kg, with one
exception where a maximum residue of 3.8 mg/kg was found 20 days after
the last of three applications at 1 kg/ha. In the latter case,
paraquat was applied as a haulm killer shortly before harvest.
FATE OF RESIDUES
In processing
The residue in wine made from grapes treated with procymidone was
30 to 50% of the residue on the grapes at harvest. (See Table 8).
Residues were further reduced on distilling wine. When wine containing
procymidone residues at 0.8 mg/kg was used to produce brandy, the
procymidone residues in the distillate were 0.07 to 0.35 mg/kg,
average 0.18 mg/kg (9 samples).
In plants
The dissipation and metabolism of 14C-procymidone, labelled at
the carbonyl group, was studied in bean and cucumber plants grown
under glass. After foliar application to beans at the rate of 250 µg
per leaf, procymidone disappeared from the plants with a half-life of
approximately 20 days. After 30 days, 40.2% of the applied
radioactivity was recovered. It was shown by TLC analysis that 92% of
TABLE 8. Residue decrease during wine processing
Residue (mg/kg), mean and range
Number
Country Year of On grapes
trials at harvest In must In wine
Fed.Rep. of 1976/77 9 2.9(1.2-6.6) 1.7 (0.7-2.6) 0.9 (0.7-1.0)
Germany
Italy 1975/76 2 0.93 1.94 1.25
2 1.56 4.12 2.68
In an experiment carried out in Spain in 1976 the decrease in the
residue during fermentation of the must was evaluated and samples were
taken 15 and 40 days after grape pressing. Results are reports in
Table 9.
TABLE 9. Residue of procymidone(mg/kg)mean residue and range
On grape Grape juice on In must at intervals (days)
at harvest day of pressing after pressing
15 40
3.5 (2.7-4.4) 3.6 (2.8-4.5) 3.0 (2.5-3.6) 2.7 (2.3-3.2)
5.7 (4.0-6.8) 5.5 (4.0-6.8) 4.3 (3.8-4.8) 3.8 (3.6-4.0)
the 14C recovered after 30 days was the parent compound. Two minor
metabolites were detected, procymidone-NH-COOH and procymidone-CH2OH,
which together accounted for about 1% of the 14C applied. These
metabolites are also found in animals after the oral administration of
procymidone. Only small amounts of the applied radiocarbon was
translocated to other parts of the plant: the 14C residues in pods
and seeds were 13 µg/kg and 6 µg/kg respectively after 30 days. The
residue of procymidone remains mainly on the outside of the treated
leaves and can be rinsed off with acetone. Thirty days after
application, the distribution of the 14C, expressed as the percentage
of the applied radioactivity, was as follows (Sumitomo 1981):
Total recovered 40.2
On and in treated leaves 39.1
In surface wash (procymidone 28.4
(rinsed 3 times
with acetone) 30.2 (procymidone-NH-COOH 1.3
(others, unidentified 0.2
(procymidone 7.7
(procymidone-NH-COOH 0.1
Extracted from treated (
leaves 8.1 (procymidone CH2OH 0.1
(4-OH-procymidone 0.1
(others, not identified 0.2
Not extractable 0.8
Total in shoots 1.0
In pods and seeds <0.1
In roots <0.1
After application of a liquid formulation of 14C-procymidone to
mature cucumber leaves adjacent to fruits at a rate of 250 µg
procymidone per leaf, the 14C was hardly translocated to either
leaves or fruits. After 23 days, approximately 64% of the applied
radioactivity was recovered from the treated leaves. Unchanged
procymidone (62%) and three metabolites, procymidone-NH-COOH,
procymidone-CH2OH and 4' OH-procymidone, were found in the treated
leaves, the metabolites accounting together for 0.4% of the applied
14C (Sumitomo 1978).
Procymidone added to the nutrient solution in which cucumber
plants were growing was fairly rapidly taken up by the plant. The
highest peak of radioactivity was reached 8 days after treatment, with
a residue level of 3.4 mg/kg fresh weight, whereas the concentration
in the shoots was much higher (20 to 30 mg/kg procymidone
equivalents). The distribution of procymidone and its metabolites
expressed as a percentage of the radioactivity applied is given in
Table 10.
In soil
The degradation of 14C-procymidone was studied in four different
soils under aerobic, anaerobic and sterilized conditions. After 12
weeks of incubation, about 57% and 72% of the applied 14C was
recovered as unchanged procymidone under aerobic and anaerobic
conditions, respectively, whereas about 70 to 77% was recovered as
unchanged procymidone from the sterilized soils. The major degradation
products were procymidone-NH-COOH(2-(3,5-dichlorophenyl-carbamoyl)-
1,2-dimethyl(cyclopropanecarboxylic acid) and CO2 (Sumitomo 1976).
TABLE 10. Distribution and characterization of radioactivity after treatment of cucumbers with
radioactive procymidone
Days after Radioactivity (% of applied)
treatment procymidone procymidone procymidone procymidone procymidone
NH-COOH CH2OH 4'OH 3' Cl
2 nutrient 86.1 4.3 1.0 <0.1 0.4
solution
roots 3.5 <0.1 - - <0.1
shoots 2.4 <0.1 - <0.1 -
fruits <0.1 - - - <0.1
8 nutrient 26.1 5.0 2.8 0.5 0.5
solution
roots 4.2 0.2 - - <0.1
shoots 37.9 0.2 - 0.2 -
fruits 2.0 - - - <0.1
24 nutrient 15.6 1.5 <0.1 <0.1 <0.1
solution
roots 3.0 0.1 - - <0.1
shoots 55.0 0.6 - 0.4 -
fruits 1.3 - - - <0.1
In another experiment three types of soil were treated with
10.2 mg/kg 14C-procymidone, labelled in the carbonoyl groups and
held under glass under aerobic and submerged conditions for 15 months.
The half-life of procymidone was about 4 months under submerged
conditions, depending on the soil moisture content. The rate of
decline under 60% soil moisture content was more rapid than under 40%.
After 15 months the levels of procymidone parent compound in Azuchi,
Kodaira and Takarazuka soil were 1.93-3.16 mg/kg, 2.21-3.28mg/kg and
2.49-3.67 mg/kg respectively. The volatile 14C gradually increased
with time, amounting after 15 months of incubation to 16.9 to 39.9% of
applied 14C in Azuchi, 11.9 to 19.9% in Kodaira and 21.3 to 33% in
Takarazuka soil. The bound 14C also gradually increased with time to
12.2 to 24.5%, 42.1 to 48.6% and 15.0 to 18.2% of the applied 14C in
Azuchi, Kodaira and Takarazuka soils respectively after 9 to 12 months
of incubation, but decreased thereafter. The differences in the
proportion of bound residue are related to the organic matter content,
which is much higher in the Kodaira soil (15.3%) than in the two other
soils (2.5 to 2.7%). More than seven degradation products were
detected. None of these exceeded 5% of the applied radioactivity in
the soils held under aerobic conditions. Procymidone is hydroxylated
at the methyl group and at the 4-phenyl position in soil. Also
dichlorination and cleavage of one or both bonds of the cyclic imide
linkage takes place, ultimately yielding CO2 (Sumitomo 1980).
The leaching behaviour of procymidone was studied using 25 cm
soil columns packed with different soil types. Air-dry soil, 30 g, was
mixed with 10 mg/kg 14C-procymidone and placed on top of the column
immediately after mixing or after an incubation period of 4 weeks. The
soil columns were leached with 550 ml water at a rate of 3 ml/h for 7
weeks. The radiocarbon penetrated below 0 to 15 cm layers in the
Kodairi, Azuchi and Takarazuka soils, all of which had an organic
matter content of 2.5% or slightly higher. The eluate contained less
than 3% of the applied 14C. However, in a very light Gifu soil with
an organic matter content below 1%, the radiocarbon in the eluate
amounted to 75.4% of the applied 14C.
Incubation of the treated soil for 4 weeks resulted in a lower
proportion of the 14C leaching through the soil columns. The
radiocarbon in the leachate of a Gifu soil column was decreased to
17.3% of that applied (Sumitomo 1980).
Adsorption and desorption of 14C-procymidone was studied in
aqueous soil suspension systems. Among 11 soil characteristics
measured, the organic matter content was found to be the only factor
that significantly affected procymidone adsorption. A good linear
relationship in logarithmic expression was observed between
procymidone adsorption and organic matter content in soils. The
adsorption behaviour of procymidone is well described by the so-called
Freundlich isotherms: the Freundlich k value varied from 1.1 to 6.0
and the l/n value was close to one. The desorption isotherms exhibited
hysteresis, apparent irreversibility, in comparison with adsorption
isotherms (Sumitomo 1980).
Residue uptake in plants from treated soils
Bean seedlings were grown on two soils, incubated with 10 mg/kg
of 14C-procymidone for two weeks and 5 months respectively for 42
days. After 42 days, pods, seeds, shoots and roots were analysed for
14C. The highest 14C levels in beans grown in Kodaira and Takarazuka
soils incubated for two weeks were in the shoots 12.3 mg/kg and
15.3 mg/kg procymidone equivalents respectively, whereas the 14C
residue levels in the edible portions were 0.42 and 0.66 mg/kg.
When beans were transplanted into soils incubated for 5 months
with procymidone, the 14C residues in the shoots, pods and seeds were
lower than in the beans grown after the shorter incubation period
mentioned above. After 42 days the residue levels in the shoots were
3.92 and 4.46 mg/kg and in the pods and seeds 0.12 and 0.25 mg/kg (the
higher figures were in beans from Takarazuka soil) (Sumitomo 1981).
Photodegradation
Photodegradation of 14C-procymidone was studied in various
solutions exposed to sunlight under glasshouse conditions. The half-
lives of procymidone in distilled water, natural river-water and sea-
water were 10, 1.0 and 0.6 days respectively. The degradation of
procymidone in water exposed to sunlight seemed to be largely due to
hydrolytic reactions, with sunlight showing a slight but definite
effect on the breakdown. Procymidone was degraded via cleavage of the
cyclic imide and further cleavage of the resulting amide linkage
(Sumitomo 1980).
Hydrolysis
The hydrolysis of 14C-procymidone was studied in buffer
solutions with pH ranges from 2 to 10 and in natural river and sea
water at 15°, 30° and 45° C. Procymidone is easily hydrolysed at pH
6.3 and above at 45°, at pH 7.1 and above at 30° and at pH 8 and above
at 15°. Half-life periods ranging from 30 min. to 8.1 days were found
under these conditions. On the other hand, the compound is fairly
stable at pH 2 and half-lives of 34 days (at 45°C) to 62 days
(at 15°C) were found. The hydrolysis of procymidone proceeded
predominantly through neutral (PH-independent) and base-catalysed
processes in the regions below pH 5 and above pH 8 respectively, while
both reactions occurred in the range between pH 5 and 8. In alkaline
solutions, the degradation product formed by the cleavage of the
cyclic imide was predominant, whereas the resulting amide was cleaved
further to yield the main product under aerobic conditions (Sumitomo
1980).
In the light of the above data, the degradation pathways for
procymidone in plants, water, soil and micro-organisms shown in Figure
2 can be proposed.
METHODS OF RESIDUE ANALYSIS
The residue determination of procymidone parent compound is
almost exclusively carried out by gas chromatography with electron
capture detection (GC-ECD). The limit of determination in most plant
material is as low as 0.2 ng or even less (Sumitomo 1976, 1978), which
enables a limit of determination of 0.04 to 0.02 mg/kg in most cases
(Sumitomo 1976,1978). The GC-ECD method is suitable or can be adapted
for regulatory purposes.
Capillary gas chromatography with a flame ionization detector
also provided high sensitivity; the limit of determination was about
0.05 ng, which enabled a limit of determination of procymidone as low
as 0.01 mg/kg (Sumitomo 1978).
Although the alkali flame ionization detector (AFID) in the
nitrogen mode may also be used for residue analysis of procymidone, it
appears that its response to procymidone was about one tenth of that
of the ECD (Sumitomo 1980).
The identity of procymidone residues can be confirmed by combined
gas chromatography-mass spectrometry (GC-MS), using selected ion
monitoring, giving 0.2 ng as a minimum detectable amount when m/e 283
and m/e 285 are monitored (Sumitomo 1980).
High-performance liquid chromatography (HPLC) with UV detection
at 254 nm permits a limit of determination of 4 ng, which corresponds
to about 0.05-0.01 mg/kg; however, the method has so far been used
very little in residue analysis for procymidone.
Extraction and clean-up procedures
Procymidone was successfully extracted from substances with a
relatively high moisture content (fruits, leafy vegetables and root
crops) by chopping and blending with polar solvents such as acetone
(Sumitomo 1978; Cooke et al 1979), acetonitrile (Sumitomo 1978), or
methanol-acetonitrile (Sumitomo 1976) or with non polar solvents, such
as trimethylpentane (Sumitomo 1976) or hexane. Procymidone was
preferably extracted from substrates with a low moisture content, such
as rice or soybeans, by pulverising and macerating with acetonitrile
(Sumitomo 1976, 1979).
Clean-up procedures for extracts from oil-containing commodities,
such as soybeans and rice, may be needed prior to further
chromatographic separations, including partition between acetonitrile
and n-hexane. Combined partition-Florisil column chromatography gave
 recoveries of 83 to 87% for rice and soybeans spiked at 0.2 mg/kg of
procymidone (Sumitomo 1976).
For non-oily crops, clean-up was by chromatography on Florisil,
alumina or mixed adsorbents containing active carbon, MgO and
diatomaceous earth. Recoveries of 80% and above were achieved at
fortification levels of 0.02 to 10 mg/kg for strawberries (Sumitomo
1976, 1978), tomatoes (Sumitomo 1976), peaches (Sumitomo 1978),
cucumber (Sumitomo 1976), grapes (Sumitomo 1976), lettuce (Sumitomo
1978) and beans (Sumitomo 1976).
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits established or under
consideration by May 1981 and the recommended pre-harvest intervals
are given in Table 11.
EVALUATION
The data base for toxicological evaluation of this compound
included several critical studies performed by Industrial Biotest
Laboratories. In the absence of complete reports of validations for
these studies that were essential to the estimation of an ADI, the
Meeting was unable to accept the studies for evaluation and agreed to
defer consideration of the toxicology this compound to a future
meeting.
Procymidone is a fungicide with a locally systemic action when
applied to the aerial parts of plants. It shows, however, a marked
systemic effect following applications to roots or the soil. It is
effective for the control of some species of Ascomycetes and a few
Deuteromycetes, especially Botrytis spp., in fruits, vegetables,
some field crops and oil seeds. It is marketed as a 50% wettable
powder and a 500 g/l emulsifiable concentrate.
On various crops, e.g. stone fruits (cherries, peaches) and berry
fruits (grapes, strawberries and similar berries), procymidone is used
until shortly before harvest to avoid fungal decay during transport
and distribution. Residue data from supervised trials were available
on 25 crops and from 12 countries (Japan, Australia, New Zealand and
Europe), representing a wide range of climatic conditions and
agricultural practices. Extensive information was available on the
fate of procymidone in plants, in soil (including metabolism in micro-
organisms), in water, under UV irradiation, etc.
In most cases, the residue on or in plants consists mainly of the
parent compound, often 90 to 95% or more. The main degradation pathway
is hydroxylation at the methyl group and the 4 position of the phenyl
group, followed by cleavage of the cyclic imide. The two major
metabolites are procymidone-CH2OH and procymidone-NH.COOH, but these
TABLE 11. National maximum residue limits reported to the Meeting
Country Commodity MRL (mg/kg) Pre-harvest intervals (days)
Australia stone fruits 10 1
Czechosslovakia grapes 28
France grapes 5
beans, chicory leaves, 5
lettuce
Fred. Rep.of
Germany grapes 28
Hungary berries, cherries
(sour) 3 14
grapes 3 14
sunflower seed 3 21
wine 3
Japan fruits 3
peaches 14
strawberries 3
vegetables (except potatoes) 2
beans (kidney),celery 14
beans (azuki, soy) 21
lettuce 7
onion 1
cucumber, eggplant 1
sweet pepper 7
tomato 3
potato 0.2 21
TABLE 11. (con't)
Country Commodity MRL (mg/kg) Pre-harvest intervals (days)
Netherlands strawberries 3 14
beans, endive, lettuce 1 14
vegetables (cucumber, courgette,
eggplant, gherkin,melon,sweet
pepper, tomato) 1 3
onions, shallots 0.2 14
New Zealand grapes 5 1
stone fruits 3 1
kiwi fruit 7 3
strawberries 0.5 1
beans 2 3
lettuce, tomato 1 3
South Africa grapes (table grapes) 1 7
Yugoslavia grapes 28
usually amount to only a low percentage of the total plant residue.
They are also found in animals after oral administration.
Several analytical methods are available for the residue in
crops, soil, water and animal tissues. Gas chromatography with
electron capture detection is the method of choice and is suitable or
can be adapted for regulatory purposes. The lower limit of
determination for most samples is in the range of 0.004 to 0.02 mg/kg.
RECOMMENDATIONS OF RESIDUE LIMITS
From the data available the meeting estimated the maximum residue
levels that were likely to occur on various food commodities after the
use of procymidone in accordance with present good agricultural
practice. These levels were considered suitable for establishing
guideline levels. The data presented on residues in chicory (witloof)
were not sufficient to allow guideline levels to be proposed. The
levels refer to procymidone only.
Crop GL (mg/kg) Pre-harvest interval (days)
Apples 5 7
Cherries 5 1-3
Currants (black, red,white) 10 7
Grapes 5 21
Kiwi fruit 7 7
Nectarines 10 1-7
Peaches 10 1-7
Raspberries 5 7
Strawberries 10 7 outdoors
14 glasshouse
Beans, kidney 2 14
Lettuce 5 21
Onions 0.2 21
Potatoes 0.1 21
Cucumbers 2 3
Eggplant 2 3
Gherkins 2 3
Melons 1 3
Peppers 5 3
Tomatoes 5 3
Rice husked 3 21
polished 1 -
Sunflower seed 2 21
FURTHER WORK OR INFORMATION
Desirable:
1. Information on residues from supervised trials on additional
crops on which use is recommended, e.g. soybean, plum, chicory
(witloof).
2. Information on residues in products of animal origin arising from
feeding wastes of treated crops, e.g. sunflower cake, potatoes,
pea and bean vines, feed potatoes, etc.
REFERENCES
Altman, P.L. and Dittmer, D.S. (Eds.) Biology Data Book Second
1974 Edition, Volume 3, Federation of American Societies for
experimental Biology, Bethesda, USA, p.272.
Arai, M. Hasegawa, R. and Ito, N. Three-month sub-acute toxicity study
1980a of S7131 (SumilexR, SumisclexR) in mice. Report from
Department of Pathology, Medical Technology and Nursing,
Fujita Gakuen University, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
1980b Six-month subacute toxicity study of S7131 (SumilexR,
SumisclexR) in mice. Report from Department of Pathology,
School of Medical Technology and Nursing, Fujita Gakuen
University, Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Arai, M., Tatematsu, M., Hagiwara, A., Hirose, M., Muraskai, G.,
1981a Murakami, M. Ito, S. and Ito, N. Eighteen-month chronic
toxicity and carcinogenicity study of S7131 (SumilexR,
SumisclexR) in Swiss white mice. Report from Department
of Pathology, School of Medical Technology and Nursing,
Fujita Gakuen University, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Arai, N., Tatematsu, M., Nakaniski, K., Hirose, M., Miyata, S.,
1981b Hiromori, T., Okuno, Y. and Ito, N. Two-year chronic
toxicity study of S7131 in rats. Report from Department of
Pathology, School of Medical Technology and Nursing, Fujita
Gakuen University, Japan, submitted by Sumitomo Chemical
Co. Ltd. (Unpublished)
Carlson, D.S., Burtner, B.R., Kennedy, G.L., Kinoshita, F.D. and
1977 Keplinger, M.L. 90-day sub-acute oral toxicity study with
S-7131 technical in beagle dogs. IBT No. 9531-09232. Report
from Industrial Bio-Test Laboratories, submitted by
Sumitomo Chemical Co. Ltd. (Unpublished)
Cooke, B.K, Pappas, A.C., Jordan, R.W.L. and Western, N.M.
1979 Translocation of benomyl, Prodyloraz and procymidone in
relation to control of Botrytis cinerea in strawberries.
Pesticide Science, 10:467-472.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
undated 12-week subacute oral toxicity study with S7131 in albino
mice. IBT No. 651-06728. Report from Industrial Bio-Test
Laboratories, submitted by Sumitomo Chemical Co. Ltd.,
Japan. (Unpublished)
Hara, M., Suzuki, H. and Ohkawa, H. Cytogenetic test of procymidone in
1980a mouse bone marrow. Report from Research Department,
Pesticide Division, Sumitomo Chemical Co. Ltd. submitted by
Sumitomo Chemical Co. Ltd., Japan. (Unpublished)
Hara, S., Suzuki, T. and Miyamoto, J. Skin sensitization test of S7131
1980a 50% water dispersible powder in male guinea pigs. Report
from Research Department, Pesticide Division, Sumitomo
Chemical Co., Ltd., submitted by Sumitomo Chemical Co.
Ltd., Japan. (Unpublished)
Hishada, Y., Maeda, K., Kawase, Y. and Miyamoto J. Uptake and
1976 translocation of a systemic fungicide, S7131 in cucumber
plants. Journal of Pesticide Science, 1:201-206.
Hishada, Y., Maeda, K., Tottori, N., and Kawase, Y. Plant
1976 disease control by N-(3',5'-dichlorophenyl)-
1.2-dimethylcyclopropane-1,2-dicarboximide. Journal of
Pesticide Science, 1:145-149.
Kadota, K. and Miyamoto, J. Eye and skin irritation study with S7131
1976 technical material in albino rabbits. Report from Institute
for Biological Science, Hyogo, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Kadota, T., Kohda, H. and Miyamoto, J. Acute toxicity studies with
1976 S7131 technicam material in mice and rats. Routes of
administration - oral, subcutaneous, intraperitoneal and
dermal. Report from Institute for Biological Science,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
Kato, T., Fujita, T. and Fukuda, T. Toxicity of S7131 technical
1976 material to rats in prolonged dietary administration over
nine months. Report from Research and Development Center,
Hyogo, Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Kohda, H. and Kadota, T. Acute oral and dermal toxicity studies with
1976a S7131 (Sumilex) 50% wettable powder in mice and rats.
Report from Institute for Biological Science, Hyogo, Japan,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
1976b Acute inhalation toxicity of Sumilex 50% wettable powder in
rats, Report from Institute for Biological Science, Hyogo,
Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Kohda, H., Nakamura, M., Kadota, T. and Miyamoto, J. Acute oral and
1980 subcutaneous toxicity studies of three metabolites of
procymidone. Report from Research Department, Pesticide
Division, Sumitomo Chemical Co. Ltd. submitted by Sumitomo
Chemical Co. Ltd., Japan.(Unpublished)
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.Jr., Kinoshita, F.K.
1976 and Keplinger, M.L. Teratogenic study with S7131 in albino
rabbits. IBT No. 651-06730. Report from Industrial Bio-Test
Laboratories, Inc., submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Matsubara, T., Hara, S., Suzuki, T. and Kadota, T. Primary eye and
1979 skin irritation tests of S7131 50% water dispersible powder
in rabbits. Report from Research Department Pesticides
Division, Sumitomo Chemical Co. Ltd., submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Mikami, N., Satogami, H. and Miyamoto, J. Metabolism of procymidone in
1979 rats. Journal of Pesticide Science, 4:165-174.
Moriya, M. Kato, K. and Shirasu, Y. Mutagenicity of S7131 in bacterial
1977 test systems. Report from Research Department, Pesticide
Division, Sumitomo Chemical Co. Ltd., submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Okuno, Y., Kadota, T. and Miyamoto, J. Skin sensitization study with
1975 S7131 in guinea pigs. Report from Research Department,
Pesticides Division, Sumitomo Chemical Co. Ltd., submitted
by Sumitomo Chemical Co. Ltd. (Unpublished)
Pence, D.H., Hoberman, A.M,, Durloo, R.S., Andrews, J.P. and Monsburg,
1980 P.A. Teratology study in rats S7131(technical). Report from
Hazleton Laboratories Inc., USA, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished).
Principe, P., Monaco, M. and Nunziata, A. Report on mutagenicity
1980 experiment on the substance Sumisclex (procymidone) of the
firm Sumitomo Chemical Co. Ltd. of Hyogo (Japan). Report
from Centro Ricerca Farmaceutica, S.p.A. Italy, submitted
by Sumitomo Chemical Co. Ltd. (Unpublished)
Salamon, C., Smith, S., Arnold, D.W. and Mannear, J.H. Three
1978 generation reproduction study with S7131 in albino rats.
IBT No. 623-06729. Report from Industrial Bio-Test
Laboratories Inc., submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Segawa, T. Acute intraperitoneal toxicity of S7131 50% water
1979 dispersible powder in mice. Report from Department of
Pharmacology, Institute of Pharmaceutical Science,
Hiroshima University School of Medicine, submitted by
Sumitomo Chemical Co. Ltd. (Unpublished)
Segawa, T. Acute oral, subcutaneous, intraperitoneal and dermal
1981 toxicities of S7131 in rats and mice. Report from
Department of Pharmacology, Institute of Pharmaceutical
Science, Hiroshima University, School of Medicine,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
Sumitomo. Reports submitted to FAO by Sumitomo Chemical Co. Ltd.,
1976,1978 Osaka, Japan. (Unpublished)
1980,1981
Suzuki, H. and Miyamoto, J. Studies on mutagenicity of S7131 with
1976 bacterial systems. Report from Institute for Biological
Sciences, Hyogo, Japan, submitted by Sumitomo Chemical Co.
Ltd, (Unpublished)
Suzuki, H. and Ohkawa, H. Effects of procymidone on sister chromatid
1980 exchanges (SCE) in cultured mouse embryo cells. Report from
Research Department, Pesticide Division, Sumitomo Chemical
Co. Ltd., Hyogo, Japan. (Unpublished)
Tatematsu, M., Tatatsuka, M. and Arai, M. Two-year toxicity study of
1981 SumilexR (S7131) in beagle dogs. Report from First
Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan, submitted by Sumitomo Chemical Co.
Ltd. (Unpublished)
recoveries of 83 to 87% for rice and soybeans spiked at 0.2 mg/kg of
procymidone (Sumitomo 1976).
For non-oily crops, clean-up was by chromatography on Florisil,
alumina or mixed adsorbents containing active carbon, MgO and
diatomaceous earth. Recoveries of 80% and above were achieved at
fortification levels of 0.02 to 10 mg/kg for strawberries (Sumitomo
1976, 1978), tomatoes (Sumitomo 1976), peaches (Sumitomo 1978),
cucumber (Sumitomo 1976), grapes (Sumitomo 1976), lettuce (Sumitomo
1978) and beans (Sumitomo 1976).
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits established or under
consideration by May 1981 and the recommended pre-harvest intervals
are given in Table 11.
EVALUATION
The data base for toxicological evaluation of this compound
included several critical studies performed by Industrial Biotest
Laboratories. In the absence of complete reports of validations for
these studies that were essential to the estimation of an ADI, the
Meeting was unable to accept the studies for evaluation and agreed to
defer consideration of the toxicology this compound to a future
meeting.
Procymidone is a fungicide with a locally systemic action when
applied to the aerial parts of plants. It shows, however, a marked
systemic effect following applications to roots or the soil. It is
effective for the control of some species of Ascomycetes and a few
Deuteromycetes, especially Botrytis spp., in fruits, vegetables,
some field crops and oil seeds. It is marketed as a 50% wettable
powder and a 500 g/l emulsifiable concentrate.
On various crops, e.g. stone fruits (cherries, peaches) and berry
fruits (grapes, strawberries and similar berries), procymidone is used
until shortly before harvest to avoid fungal decay during transport
and distribution. Residue data from supervised trials were available
on 25 crops and from 12 countries (Japan, Australia, New Zealand and
Europe), representing a wide range of climatic conditions and
agricultural practices. Extensive information was available on the
fate of procymidone in plants, in soil (including metabolism in micro-
organisms), in water, under UV irradiation, etc.
In most cases, the residue on or in plants consists mainly of the
parent compound, often 90 to 95% or more. The main degradation pathway
is hydroxylation at the methyl group and the 4 position of the phenyl
group, followed by cleavage of the cyclic imide. The two major
metabolites are procymidone-CH2OH and procymidone-NH.COOH, but these
TABLE 11. National maximum residue limits reported to the Meeting
Country Commodity MRL (mg/kg) Pre-harvest intervals (days)
Australia stone fruits 10 1
Czechosslovakia grapes 28
France grapes 5
beans, chicory leaves, 5
lettuce
Fred. Rep.of
Germany grapes 28
Hungary berries, cherries
(sour) 3 14
grapes 3 14
sunflower seed 3 21
wine 3
Japan fruits 3
peaches 14
strawberries 3
vegetables (except potatoes) 2
beans (kidney),celery 14
beans (azuki, soy) 21
lettuce 7
onion 1
cucumber, eggplant 1
sweet pepper 7
tomato 3
potato 0.2 21
TABLE 11. (con't)
Country Commodity MRL (mg/kg) Pre-harvest intervals (days)
Netherlands strawberries 3 14
beans, endive, lettuce 1 14
vegetables (cucumber, courgette,
eggplant, gherkin,melon,sweet
pepper, tomato) 1 3
onions, shallots 0.2 14
New Zealand grapes 5 1
stone fruits 3 1
kiwi fruit 7 3
strawberries 0.5 1
beans 2 3
lettuce, tomato 1 3
South Africa grapes (table grapes) 1 7
Yugoslavia grapes 28
usually amount to only a low percentage of the total plant residue.
They are also found in animals after oral administration.
Several analytical methods are available for the residue in
crops, soil, water and animal tissues. Gas chromatography with
electron capture detection is the method of choice and is suitable or
can be adapted for regulatory purposes. The lower limit of
determination for most samples is in the range of 0.004 to 0.02 mg/kg.
RECOMMENDATIONS OF RESIDUE LIMITS
From the data available the meeting estimated the maximum residue
levels that were likely to occur on various food commodities after the
use of procymidone in accordance with present good agricultural
practice. These levels were considered suitable for establishing
guideline levels. The data presented on residues in chicory (witloof)
were not sufficient to allow guideline levels to be proposed. The
levels refer to procymidone only.
Crop GL (mg/kg) Pre-harvest interval (days)
Apples 5 7
Cherries 5 1-3
Currants (black, red,white) 10 7
Grapes 5 21
Kiwi fruit 7 7
Nectarines 10 1-7
Peaches 10 1-7
Raspberries 5 7
Strawberries 10 7 outdoors
14 glasshouse
Beans, kidney 2 14
Lettuce 5 21
Onions 0.2 21
Potatoes 0.1 21
Cucumbers 2 3
Eggplant 2 3
Gherkins 2 3
Melons 1 3
Peppers 5 3
Tomatoes 5 3
Rice husked 3 21
polished 1 -
Sunflower seed 2 21
FURTHER WORK OR INFORMATION
Desirable:
1. Information on residues from supervised trials on additional
crops on which use is recommended, e.g. soybean, plum, chicory
(witloof).
2. Information on residues in products of animal origin arising from
feeding wastes of treated crops, e.g. sunflower cake, potatoes,
pea and bean vines, feed potatoes, etc.
REFERENCES
Altman, P.L. and Dittmer, D.S. (Eds.) Biology Data Book Second
1974 Edition, Volume 3, Federation of American Societies for
experimental Biology, Bethesda, USA, p.272.
Arai, M. Hasegawa, R. and Ito, N. Three-month sub-acute toxicity study
1980a of S7131 (SumilexR, SumisclexR) in mice. Report from
Department of Pathology, Medical Technology and Nursing,
Fujita Gakuen University, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
1980b Six-month subacute toxicity study of S7131 (SumilexR,
SumisclexR) in mice. Report from Department of Pathology,
School of Medical Technology and Nursing, Fujita Gakuen
University, Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Arai, M., Tatematsu, M., Hagiwara, A., Hirose, M., Muraskai, G.,
1981a Murakami, M. Ito, S. and Ito, N. Eighteen-month chronic
toxicity and carcinogenicity study of S7131 (SumilexR,
SumisclexR) in Swiss white mice. Report from Department
of Pathology, School of Medical Technology and Nursing,
Fujita Gakuen University, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Arai, N., Tatematsu, M., Nakaniski, K., Hirose, M., Miyata, S.,
1981b Hiromori, T., Okuno, Y. and Ito, N. Two-year chronic
toxicity study of S7131 in rats. Report from Department of
Pathology, School of Medical Technology and Nursing, Fujita
Gakuen University, Japan, submitted by Sumitomo Chemical
Co. Ltd. (Unpublished)
Carlson, D.S., Burtner, B.R., Kennedy, G.L., Kinoshita, F.D. and
1977 Keplinger, M.L. 90-day sub-acute oral toxicity study with
S-7131 technical in beagle dogs. IBT No. 9531-09232. Report
from Industrial Bio-Test Laboratories, submitted by
Sumitomo Chemical Co. Ltd. (Unpublished)
Cooke, B.K, Pappas, A.C., Jordan, R.W.L. and Western, N.M.
1979 Translocation of benomyl, Prodyloraz and procymidone in
relation to control of Botrytis cinerea in strawberries.
Pesticide Science, 10:467-472.
Fletcher, D., Jenkins, D.H., Kinoshita, F.K. and Keplinger, M.L.
undated 12-week subacute oral toxicity study with S7131 in albino
mice. IBT No. 651-06728. Report from Industrial Bio-Test
Laboratories, submitted by Sumitomo Chemical Co. Ltd.,
Japan. (Unpublished)
Hara, M., Suzuki, H. and Ohkawa, H. Cytogenetic test of procymidone in
1980a mouse bone marrow. Report from Research Department,
Pesticide Division, Sumitomo Chemical Co. Ltd. submitted by
Sumitomo Chemical Co. Ltd., Japan. (Unpublished)
Hara, S., Suzuki, T. and Miyamoto, J. Skin sensitization test of S7131
1980a 50% water dispersible powder in male guinea pigs. Report
from Research Department, Pesticide Division, Sumitomo
Chemical Co., Ltd., submitted by Sumitomo Chemical Co.
Ltd., Japan. (Unpublished)
Hishada, Y., Maeda, K., Kawase, Y. and Miyamoto J. Uptake and
1976 translocation of a systemic fungicide, S7131 in cucumber
plants. Journal of Pesticide Science, 1:201-206.
Hishada, Y., Maeda, K., Tottori, N., and Kawase, Y. Plant
1976 disease control by N-(3',5'-dichlorophenyl)-
1.2-dimethylcyclopropane-1,2-dicarboximide. Journal of
Pesticide Science, 1:145-149.
Kadota, K. and Miyamoto, J. Eye and skin irritation study with S7131
1976 technical material in albino rabbits. Report from Institute
for Biological Science, Hyogo, Japan, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Kadota, T., Kohda, H. and Miyamoto, J. Acute toxicity studies with
1976 S7131 technicam material in mice and rats. Routes of
administration - oral, subcutaneous, intraperitoneal and
dermal. Report from Institute for Biological Science,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
Kato, T., Fujita, T. and Fukuda, T. Toxicity of S7131 technical
1976 material to rats in prolonged dietary administration over
nine months. Report from Research and Development Center,
Hyogo, Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Kohda, H. and Kadota, T. Acute oral and dermal toxicity studies with
1976a S7131 (Sumilex) 50% wettable powder in mice and rats.
Report from Institute for Biological Science, Hyogo, Japan,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
1976b Acute inhalation toxicity of Sumilex 50% wettable powder in
rats, Report from Institute for Biological Science, Hyogo,
Japan, submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Kohda, H., Nakamura, M., Kadota, T. and Miyamoto, J. Acute oral and
1980 subcutaneous toxicity studies of three metabolites of
procymidone. Report from Research Department, Pesticide
Division, Sumitomo Chemical Co. Ltd. submitted by Sumitomo
Chemical Co. Ltd., Japan.(Unpublished)
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.Jr., Kinoshita, F.K.
1976 and Keplinger, M.L. Teratogenic study with S7131 in albino
rabbits. IBT No. 651-06730. Report from Industrial Bio-Test
Laboratories, Inc., submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Matsubara, T., Hara, S., Suzuki, T. and Kadota, T. Primary eye and
1979 skin irritation tests of S7131 50% water dispersible powder
in rabbits. Report from Research Department Pesticides
Division, Sumitomo Chemical Co. Ltd., submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Mikami, N., Satogami, H. and Miyamoto, J. Metabolism of procymidone in
1979 rats. Journal of Pesticide Science, 4:165-174.
Moriya, M. Kato, K. and Shirasu, Y. Mutagenicity of S7131 in bacterial
1977 test systems. Report from Research Department, Pesticide
Division, Sumitomo Chemical Co. Ltd., submitted by Sumitomo
Chemical Co. Ltd. (Unpublished)
Okuno, Y., Kadota, T. and Miyamoto, J. Skin sensitization study with
1975 S7131 in guinea pigs. Report from Research Department,
Pesticides Division, Sumitomo Chemical Co. Ltd., submitted
by Sumitomo Chemical Co. Ltd. (Unpublished)
Pence, D.H., Hoberman, A.M,, Durloo, R.S., Andrews, J.P. and Monsburg,
1980 P.A. Teratology study in rats S7131(technical). Report from
Hazleton Laboratories Inc., USA, submitted by Sumitomo
Chemical Co. Ltd. (Unpublished).
Principe, P., Monaco, M. and Nunziata, A. Report on mutagenicity
1980 experiment on the substance Sumisclex (procymidone) of the
firm Sumitomo Chemical Co. Ltd. of Hyogo (Japan). Report
from Centro Ricerca Farmaceutica, S.p.A. Italy, submitted
by Sumitomo Chemical Co. Ltd. (Unpublished)
Salamon, C., Smith, S., Arnold, D.W. and Mannear, J.H. Three
1978 generation reproduction study with S7131 in albino rats.
IBT No. 623-06729. Report from Industrial Bio-Test
Laboratories Inc., submitted by Sumitomo Chemical Co. Ltd.
(Unpublished)
Segawa, T. Acute intraperitoneal toxicity of S7131 50% water
1979 dispersible powder in mice. Report from Department of
Pharmacology, Institute of Pharmaceutical Science,
Hiroshima University School of Medicine, submitted by
Sumitomo Chemical Co. Ltd. (Unpublished)
Segawa, T. Acute oral, subcutaneous, intraperitoneal and dermal
1981 toxicities of S7131 in rats and mice. Report from
Department of Pharmacology, Institute of Pharmaceutical
Science, Hiroshima University, School of Medicine,
submitted by Sumitomo Chemical Co. Ltd. (Unpublished)
Sumitomo. Reports submitted to FAO by Sumitomo Chemical Co. Ltd.,
1976,1978 Osaka, Japan. (Unpublished)
1980,1981
Suzuki, H. and Miyamoto, J. Studies on mutagenicity of S7131 with
1976 bacterial systems. Report from Institute for Biological
Sciences, Hyogo, Japan, submitted by Sumitomo Chemical Co.
Ltd, (Unpublished)
Suzuki, H. and Ohkawa, H. Effects of procymidone on sister chromatid
1980 exchanges (SCE) in cultured mouse embryo cells. Report from
Research Department, Pesticide Division, Sumitomo Chemical
Co. Ltd., Hyogo, Japan. (Unpublished)
Tatematsu, M., Tatatsuka, M. and Arai, M. Two-year toxicity study of
1981 SumilexR (S7131) in beagle dogs. Report from First
Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan, submitted by Sumitomo Chemical Co.
Ltd. (Unpublished)
