
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
AZOCYCLOTIN/CYHEXATIN
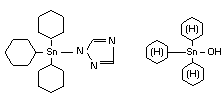 Explanation
Cyhexatin was evaluated in 1970, 1973, 1974, 1978 and 1981
(FAO/WHO 1971, 1974, 1975, 1979 and 1982)1/. A temporary ADI was
recommended in 1978 and converted to an ADI in 1981. Azocyclotin was
evaluated in 1979 and 1981 (FAO/WHO 1980, 1982). An ADI was
recommended in 1981. The 1979 evaluation of azocyclotin resulted in
some recommended temporary MRL's, which were at variance (lower) with
those already proposed for cyhexatin for the same commodities. The
1979 Meeting noted that residues from these two compounds cannot be
distinguished shortly after application, and therefore required
additional information on use patterns and residue data to resolve
this problem and harmonize residue limits.
Information was received on analytical methodology and on the
results of a joint residue study by the manufacturers.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Parallel trials were carried out by Dow in England (Dow 1982) and
by Bayer in the Federal Republic of Germany (Bayer 1982) in which
apples (James Grieve in England and Golden Delicious in FRG) were
treated with Plictran 25W and Peropal WP, each at the rate of 375 g
a.i./ha in a spray volume of 1 500 litres of water. Similar mist
blower applications were used in both countries. Samples from each
trial were collected at 0, 7, 14, 28 and 35 days after the last
application and divided into two sets. One set was exchanged between
labs in the respective countries. One set from each country was
analysed for total organotin residues, using the colorimetric method
by Dow in England, and the other two sets were analysed by Bayer,
using their gas chromatographic method, which determines azocyclotin,
cyhexatin and dicyclohexyl tin oxide. The results of the Dow analyses
are summarized in Table 1 and the results of the Bayer analyses are
summarized in Table 2.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Residues in Apples Determined by Colorimetric Analysis
Residues (mg/kg) at interval post-treatment (days)1/
Location 0 7 14 28 35
of Product mean mean me an mean mean
treatment used (range) (range) (range) (range) (range)
UK 1 Plictran 25W 0.46 0.90 0.31 0.12 0.08
(0.33-0.63) (0.71-1.03) (0.21-0.44) (0.11-0.13) (0.05-0.12)
Peropal WP 0.49 0.73 0.28 0.11 0.04
(0.42-0.58) (0.54-1.10) (0.19-0.37) (0.09-0.16) (0.02-0.05)
UK 2 Plictran 25W 1.04 0.46 0.39 0.14 0.08
(0.69-1.18) (0.27-0.54) (0.31-0.48) (0.10-0.16) (0.07-0.09)
Peropal WP 0.90 0.39 0-29 0.14 0.14
(0.66-0.99) (0.28-0.53) (0.18-0.42) (0.09-0.23) (0.09-0.19)
FRG 1 Plictran 25W 0.39 0.15 0.14 0.07 0.13
(0.37-0.41) (0.13-0.18) (1.10-0.19) (0.06-0.08) (0.07-0.19)
Peropal WP 0.54 0.37 0.31 0.09 0.14
(0.53-0.54) (0.26-0.48) (0.31-0.32) (0.05-0.12) (0.12-0.16)
FRG 2 Plictran 25W 0.50 0.59 0.53 0.28 0.29
(0.49-0.52) (0.58-0.60) (0.51-0.56) (0.25-0.31) (0.29-0.30)
Peropal WP 0.32 0.49 0.31 0.19 0.14
(0.24-0.40) (0.45-0.52) (0.28-0.34) (0.11-0.26) (0.10-0.18)
1/ Residues not corrected for recovery or control, but corrected for reagent blanks. Average
recovery = 93.7%.
Table 2. Residues in Apples Determined by Gas Chromatographic
Analysis
Location Residues (mg/kg) at interval
of Product post-treatment (days)1/
treatment used 0 7 14 28 35
UK 1 Plictran 25W 0.35 0.72 0.25 0.18 0.10
Peropal WP 0.44 0.54 0.31 0.16 0.06
UK 2 Plictran 25W 1.20 0.51 0.51 0.21 0.21
Peropal WP 1.06 0.33 0.58 0.24 0.23
FRG 1 Plictran 25W 0.97 1.06 0.68 0.37 0.40
Peropal WP 0.42 0.60 0.53 0.37 0.34
FRG 2 Plictran 0.50 0.23 0.30 0.11 0.15
Peropal WP 0.50 0.25 0.23 0.13 0.14
1/ Plictran values reported as the sum of cyhexatin and
dicyclohexyltin oxide. Peropal values reported as the sum of
azocyclotin + cyhexatin and dicyclohexyltin oxide.
It is evident from a comparison of the values both within the
tables and between the tables, that there is no significant difference
in residues on apples from the use of either azocyclotin or cyhexatin.
Therefore, the discrepancies noted by the 1979 Joint Meeting are
mainly due to the use of different application methods and not to any
differences in the analytical methods or in the degradation chemistry.
METHODS OF RESIDUE ANALYSIS
Definition of residue
In order to better conform to current analytical practice, the
Meeting proposes that the definitions of the residues of azocyclotin
and cyhexatin be changed to read as follows:
azocyclotin - sum of azocyclotin, cyhexatin and dicyclohexyltin
oxide, expressed as cyhexatin.
cyhexatin - the sum of cyhexatin and dicyclohexyltin oxide,
expressed as cyhexatin.
These redefinitions have no effect on recommended maximum residue
limits for either product.
APPRAISAL
The 1979 evaluation of azocyclotin resulted in a discrepancy in
certain recommended maximum residue limits for commodities for which
limits had already been recommended, based on data from the use of
cyhexatin, which is also a degradation product of azocyclotin. In
response to this problem, the manufacturers conducted a joint project
on apples in England and the Federal Republic of Germany. The results
of these tests clearly indicate that the discrepancy was due to
different application technologies and that no significant differences
in residues would result under identical treatment, climate and
analytical conditions. Inasmuch as these new data support the existing
recommendations for apples and pears associated with cyhexatin, no
changes in any of the cyhexatin maximum residue levels are needed.
Residues resulting from the use of either of these products
cannot be distinguished analytically because they are principally
surface residues and the degradation products are determined by
environmental rather than metabolic processes. In addition, the
analytical method converts both azocyclotin and cyhexatin to
tricyclohexylmethyl tin. Therefore, it is necessary to revise the
recommended limit for azocyclotin on apples, beans and strawberries to
the same limits recommended for cyhexatin on those commodities. In the
case of grapes and eggplant for which supervised trial data are
available only for azocyclotin, the recommended limits for azocyclotin
remain unchanged.
New definitions of the residues are proposed for both products to
conform more with current analytical practice.
RECOMMENDATIONS
Cyhexatin: Recommended maximum residue limits, all commodities -
no change. Residues to which the limits apply are the
sum of cyhexatin and dicyclohexyltin oxide expressed as
cyhexatin.
Azocyclotin: The previously recommended maximum residue limits are
increased to the values listed below. The limits for
azocyclotin represent the sum of azocyclotin, cyhexatin
and dicyclohexyltin oxide, expressed as cyhexatin.
Commodity Limit (mg/kg)
Apples 2 (increased from 0.1)
Beans 0.5 (increased from 0.2)
Strawberries 2 (increased from 0.1)
REFERENCES
Bayer AG Residue trials on apples with Peropal and Plictran.
1982 RA-643181B (Plus covering letter). (Unpublished)
Dow Ltd. Organotin residues in apples following treatments with
1982 Plictran 25W and Peropal WP acaricides in England and the
Federal Republic of Germany - 1981. (Plus covering letter)
(Unpublished)
Explanation
Cyhexatin was evaluated in 1970, 1973, 1974, 1978 and 1981
(FAO/WHO 1971, 1974, 1975, 1979 and 1982)1/. A temporary ADI was
recommended in 1978 and converted to an ADI in 1981. Azocyclotin was
evaluated in 1979 and 1981 (FAO/WHO 1980, 1982). An ADI was
recommended in 1981. The 1979 evaluation of azocyclotin resulted in
some recommended temporary MRL's, which were at variance (lower) with
those already proposed for cyhexatin for the same commodities. The
1979 Meeting noted that residues from these two compounds cannot be
distinguished shortly after application, and therefore required
additional information on use patterns and residue data to resolve
this problem and harmonize residue limits.
Information was received on analytical methodology and on the
results of a joint residue study by the manufacturers.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Parallel trials were carried out by Dow in England (Dow 1982) and
by Bayer in the Federal Republic of Germany (Bayer 1982) in which
apples (James Grieve in England and Golden Delicious in FRG) were
treated with Plictran 25W and Peropal WP, each at the rate of 375 g
a.i./ha in a spray volume of 1 500 litres of water. Similar mist
blower applications were used in both countries. Samples from each
trial were collected at 0, 7, 14, 28 and 35 days after the last
application and divided into two sets. One set was exchanged between
labs in the respective countries. One set from each country was
analysed for total organotin residues, using the colorimetric method
by Dow in England, and the other two sets were analysed by Bayer,
using their gas chromatographic method, which determines azocyclotin,
cyhexatin and dicyclohexyl tin oxide. The results of the Dow analyses
are summarized in Table 1 and the results of the Bayer analyses are
summarized in Table 2.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Residues in Apples Determined by Colorimetric Analysis
Residues (mg/kg) at interval post-treatment (days)1/
Location 0 7 14 28 35
of Product mean mean me an mean mean
treatment used (range) (range) (range) (range) (range)
UK 1 Plictran 25W 0.46 0.90 0.31 0.12 0.08
(0.33-0.63) (0.71-1.03) (0.21-0.44) (0.11-0.13) (0.05-0.12)
Peropal WP 0.49 0.73 0.28 0.11 0.04
(0.42-0.58) (0.54-1.10) (0.19-0.37) (0.09-0.16) (0.02-0.05)
UK 2 Plictran 25W 1.04 0.46 0.39 0.14 0.08
(0.69-1.18) (0.27-0.54) (0.31-0.48) (0.10-0.16) (0.07-0.09)
Peropal WP 0.90 0.39 0-29 0.14 0.14
(0.66-0.99) (0.28-0.53) (0.18-0.42) (0.09-0.23) (0.09-0.19)
FRG 1 Plictran 25W 0.39 0.15 0.14 0.07 0.13
(0.37-0.41) (0.13-0.18) (1.10-0.19) (0.06-0.08) (0.07-0.19)
Peropal WP 0.54 0.37 0.31 0.09 0.14
(0.53-0.54) (0.26-0.48) (0.31-0.32) (0.05-0.12) (0.12-0.16)
FRG 2 Plictran 25W 0.50 0.59 0.53 0.28 0.29
(0.49-0.52) (0.58-0.60) (0.51-0.56) (0.25-0.31) (0.29-0.30)
Peropal WP 0.32 0.49 0.31 0.19 0.14
(0.24-0.40) (0.45-0.52) (0.28-0.34) (0.11-0.26) (0.10-0.18)
1/ Residues not corrected for recovery or control, but corrected for reagent blanks. Average
recovery = 93.7%.
Table 2. Residues in Apples Determined by Gas Chromatographic
Analysis
Location Residues (mg/kg) at interval
of Product post-treatment (days)1/
treatment used 0 7 14 28 35
UK 1 Plictran 25W 0.35 0.72 0.25 0.18 0.10
Peropal WP 0.44 0.54 0.31 0.16 0.06
UK 2 Plictran 25W 1.20 0.51 0.51 0.21 0.21
Peropal WP 1.06 0.33 0.58 0.24 0.23
FRG 1 Plictran 25W 0.97 1.06 0.68 0.37 0.40
Peropal WP 0.42 0.60 0.53 0.37 0.34
FRG 2 Plictran 0.50 0.23 0.30 0.11 0.15
Peropal WP 0.50 0.25 0.23 0.13 0.14
1/ Plictran values reported as the sum of cyhexatin and
dicyclohexyltin oxide. Peropal values reported as the sum of
azocyclotin + cyhexatin and dicyclohexyltin oxide.
It is evident from a comparison of the values both within the
tables and between the tables, that there is no significant difference
in residues on apples from the use of either azocyclotin or cyhexatin.
Therefore, the discrepancies noted by the 1979 Joint Meeting are
mainly due to the use of different application methods and not to any
differences in the analytical methods or in the degradation chemistry.
METHODS OF RESIDUE ANALYSIS
Definition of residue
In order to better conform to current analytical practice, the
Meeting proposes that the definitions of the residues of azocyclotin
and cyhexatin be changed to read as follows:
azocyclotin - sum of azocyclotin, cyhexatin and dicyclohexyltin
oxide, expressed as cyhexatin.
cyhexatin - the sum of cyhexatin and dicyclohexyltin oxide,
expressed as cyhexatin.
These redefinitions have no effect on recommended maximum residue
limits for either product.
APPRAISAL
The 1979 evaluation of azocyclotin resulted in a discrepancy in
certain recommended maximum residue limits for commodities for which
limits had already been recommended, based on data from the use of
cyhexatin, which is also a degradation product of azocyclotin. In
response to this problem, the manufacturers conducted a joint project
on apples in England and the Federal Republic of Germany. The results
of these tests clearly indicate that the discrepancy was due to
different application technologies and that no significant differences
in residues would result under identical treatment, climate and
analytical conditions. Inasmuch as these new data support the existing
recommendations for apples and pears associated with cyhexatin, no
changes in any of the cyhexatin maximum residue levels are needed.
Residues resulting from the use of either of these products
cannot be distinguished analytically because they are principally
surface residues and the degradation products are determined by
environmental rather than metabolic processes. In addition, the
analytical method converts both azocyclotin and cyhexatin to
tricyclohexylmethyl tin. Therefore, it is necessary to revise the
recommended limit for azocyclotin on apples, beans and strawberries to
the same limits recommended for cyhexatin on those commodities. In the
case of grapes and eggplant for which supervised trial data are
available only for azocyclotin, the recommended limits for azocyclotin
remain unchanged.
New definitions of the residues are proposed for both products to
conform more with current analytical practice.
RECOMMENDATIONS
Cyhexatin: Recommended maximum residue limits, all commodities -
no change. Residues to which the limits apply are the
sum of cyhexatin and dicyclohexyltin oxide expressed as
cyhexatin.
Azocyclotin: The previously recommended maximum residue limits are
increased to the values listed below. The limits for
azocyclotin represent the sum of azocyclotin, cyhexatin
and dicyclohexyltin oxide, expressed as cyhexatin.
Commodity Limit (mg/kg)
Apples 2 (increased from 0.1)
Beans 0.5 (increased from 0.2)
Strawberries 2 (increased from 0.1)
REFERENCES
Bayer AG Residue trials on apples with Peropal and Plictran.
1982 RA-643181B (Plus covering letter). (Unpublished)
Dow Ltd. Organotin residues in apples following treatments with
1982 Plictran 25W and Peropal WP acaricides in England and the
Federal Republic of Germany - 1981. (Plus covering letter)
(Unpublished)
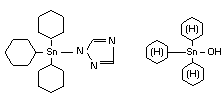 Explanation
Cyhexatin was evaluated in 1970, 1973, 1974, 1978 and 1981
(FAO/WHO 1971, 1974, 1975, 1979 and 1982)1/. A temporary ADI was
recommended in 1978 and converted to an ADI in 1981. Azocyclotin was
evaluated in 1979 and 1981 (FAO/WHO 1980, 1982). An ADI was
recommended in 1981. The 1979 evaluation of azocyclotin resulted in
some recommended temporary MRL's, which were at variance (lower) with
those already proposed for cyhexatin for the same commodities. The
1979 Meeting noted that residues from these two compounds cannot be
distinguished shortly after application, and therefore required
additional information on use patterns and residue data to resolve
this problem and harmonize residue limits.
Information was received on analytical methodology and on the
results of a joint residue study by the manufacturers.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Parallel trials were carried out by Dow in England (Dow 1982) and
by Bayer in the Federal Republic of Germany (Bayer 1982) in which
apples (James Grieve in England and Golden Delicious in FRG) were
treated with Plictran 25W and Peropal WP, each at the rate of 375 g
a.i./ha in a spray volume of 1 500 litres of water. Similar mist
blower applications were used in both countries. Samples from each
trial were collected at 0, 7, 14, 28 and 35 days after the last
application and divided into two sets. One set was exchanged between
labs in the respective countries. One set from each country was
analysed for total organotin residues, using the colorimetric method
by Dow in England, and the other two sets were analysed by Bayer,
using their gas chromatographic method, which determines azocyclotin,
cyhexatin and dicyclohexyl tin oxide. The results of the Dow analyses
are summarized in Table 1 and the results of the Bayer analyses are
summarized in Table 2.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Residues in Apples Determined by Colorimetric Analysis
Residues (mg/kg) at interval post-treatment (days)1/
Location 0 7 14 28 35
of Product mean mean me an mean mean
treatment used (range) (range) (range) (range) (range)
UK 1 Plictran 25W 0.46 0.90 0.31 0.12 0.08
(0.33-0.63) (0.71-1.03) (0.21-0.44) (0.11-0.13) (0.05-0.12)
Peropal WP 0.49 0.73 0.28 0.11 0.04
(0.42-0.58) (0.54-1.10) (0.19-0.37) (0.09-0.16) (0.02-0.05)
UK 2 Plictran 25W 1.04 0.46 0.39 0.14 0.08
(0.69-1.18) (0.27-0.54) (0.31-0.48) (0.10-0.16) (0.07-0.09)
Peropal WP 0.90 0.39 0-29 0.14 0.14
(0.66-0.99) (0.28-0.53) (0.18-0.42) (0.09-0.23) (0.09-0.19)
FRG 1 Plictran 25W 0.39 0.15 0.14 0.07 0.13
(0.37-0.41) (0.13-0.18) (1.10-0.19) (0.06-0.08) (0.07-0.19)
Peropal WP 0.54 0.37 0.31 0.09 0.14
(0.53-0.54) (0.26-0.48) (0.31-0.32) (0.05-0.12) (0.12-0.16)
FRG 2 Plictran 25W 0.50 0.59 0.53 0.28 0.29
(0.49-0.52) (0.58-0.60) (0.51-0.56) (0.25-0.31) (0.29-0.30)
Peropal WP 0.32 0.49 0.31 0.19 0.14
(0.24-0.40) (0.45-0.52) (0.28-0.34) (0.11-0.26) (0.10-0.18)
1/ Residues not corrected for recovery or control, but corrected for reagent blanks. Average
recovery = 93.7%.
Table 2. Residues in Apples Determined by Gas Chromatographic
Analysis
Location Residues (mg/kg) at interval
of Product post-treatment (days)1/
treatment used 0 7 14 28 35
UK 1 Plictran 25W 0.35 0.72 0.25 0.18 0.10
Peropal WP 0.44 0.54 0.31 0.16 0.06
UK 2 Plictran 25W 1.20 0.51 0.51 0.21 0.21
Peropal WP 1.06 0.33 0.58 0.24 0.23
FRG 1 Plictran 25W 0.97 1.06 0.68 0.37 0.40
Peropal WP 0.42 0.60 0.53 0.37 0.34
FRG 2 Plictran 0.50 0.23 0.30 0.11 0.15
Peropal WP 0.50 0.25 0.23 0.13 0.14
1/ Plictran values reported as the sum of cyhexatin and
dicyclohexyltin oxide. Peropal values reported as the sum of
azocyclotin + cyhexatin and dicyclohexyltin oxide.
It is evident from a comparison of the values both within the
tables and between the tables, that there is no significant difference
in residues on apples from the use of either azocyclotin or cyhexatin.
Therefore, the discrepancies noted by the 1979 Joint Meeting are
mainly due to the use of different application methods and not to any
differences in the analytical methods or in the degradation chemistry.
METHODS OF RESIDUE ANALYSIS
Definition of residue
In order to better conform to current analytical practice, the
Meeting proposes that the definitions of the residues of azocyclotin
and cyhexatin be changed to read as follows:
azocyclotin - sum of azocyclotin, cyhexatin and dicyclohexyltin
oxide, expressed as cyhexatin.
cyhexatin - the sum of cyhexatin and dicyclohexyltin oxide,
expressed as cyhexatin.
These redefinitions have no effect on recommended maximum residue
limits for either product.
APPRAISAL
The 1979 evaluation of azocyclotin resulted in a discrepancy in
certain recommended maximum residue limits for commodities for which
limits had already been recommended, based on data from the use of
cyhexatin, which is also a degradation product of azocyclotin. In
response to this problem, the manufacturers conducted a joint project
on apples in England and the Federal Republic of Germany. The results
of these tests clearly indicate that the discrepancy was due to
different application technologies and that no significant differences
in residues would result under identical treatment, climate and
analytical conditions. Inasmuch as these new data support the existing
recommendations for apples and pears associated with cyhexatin, no
changes in any of the cyhexatin maximum residue levels are needed.
Residues resulting from the use of either of these products
cannot be distinguished analytically because they are principally
surface residues and the degradation products are determined by
environmental rather than metabolic processes. In addition, the
analytical method converts both azocyclotin and cyhexatin to
tricyclohexylmethyl tin. Therefore, it is necessary to revise the
recommended limit for azocyclotin on apples, beans and strawberries to
the same limits recommended for cyhexatin on those commodities. In the
case of grapes and eggplant for which supervised trial data are
available only for azocyclotin, the recommended limits for azocyclotin
remain unchanged.
New definitions of the residues are proposed for both products to
conform more with current analytical practice.
RECOMMENDATIONS
Cyhexatin: Recommended maximum residue limits, all commodities -
no change. Residues to which the limits apply are the
sum of cyhexatin and dicyclohexyltin oxide expressed as
cyhexatin.
Azocyclotin: The previously recommended maximum residue limits are
increased to the values listed below. The limits for
azocyclotin represent the sum of azocyclotin, cyhexatin
and dicyclohexyltin oxide, expressed as cyhexatin.
Commodity Limit (mg/kg)
Apples 2 (increased from 0.1)
Beans 0.5 (increased from 0.2)
Strawberries 2 (increased from 0.1)
REFERENCES
Bayer AG Residue trials on apples with Peropal and Plictran.
1982 RA-643181B (Plus covering letter). (Unpublished)
Dow Ltd. Organotin residues in apples following treatments with
1982 Plictran 25W and Peropal WP acaricides in England and the
Federal Republic of Germany - 1981. (Plus covering letter)
(Unpublished)
Explanation
Cyhexatin was evaluated in 1970, 1973, 1974, 1978 and 1981
(FAO/WHO 1971, 1974, 1975, 1979 and 1982)1/. A temporary ADI was
recommended in 1978 and converted to an ADI in 1981. Azocyclotin was
evaluated in 1979 and 1981 (FAO/WHO 1980, 1982). An ADI was
recommended in 1981. The 1979 evaluation of azocyclotin resulted in
some recommended temporary MRL's, which were at variance (lower) with
those already proposed for cyhexatin for the same commodities. The
1979 Meeting noted that residues from these two compounds cannot be
distinguished shortly after application, and therefore required
additional information on use patterns and residue data to resolve
this problem and harmonize residue limits.
Information was received on analytical methodology and on the
results of a joint residue study by the manufacturers.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Parallel trials were carried out by Dow in England (Dow 1982) and
by Bayer in the Federal Republic of Germany (Bayer 1982) in which
apples (James Grieve in England and Golden Delicious in FRG) were
treated with Plictran 25W and Peropal WP, each at the rate of 375 g
a.i./ha in a spray volume of 1 500 litres of water. Similar mist
blower applications were used in both countries. Samples from each
trial were collected at 0, 7, 14, 28 and 35 days after the last
application and divided into two sets. One set was exchanged between
labs in the respective countries. One set from each country was
analysed for total organotin residues, using the colorimetric method
by Dow in England, and the other two sets were analysed by Bayer,
using their gas chromatographic method, which determines azocyclotin,
cyhexatin and dicyclohexyl tin oxide. The results of the Dow analyses
are summarized in Table 1 and the results of the Bayer analyses are
summarized in Table 2.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Residues in Apples Determined by Colorimetric Analysis
Residues (mg/kg) at interval post-treatment (days)1/
Location 0 7 14 28 35
of Product mean mean me an mean mean
treatment used (range) (range) (range) (range) (range)
UK 1 Plictran 25W 0.46 0.90 0.31 0.12 0.08
(0.33-0.63) (0.71-1.03) (0.21-0.44) (0.11-0.13) (0.05-0.12)
Peropal WP 0.49 0.73 0.28 0.11 0.04
(0.42-0.58) (0.54-1.10) (0.19-0.37) (0.09-0.16) (0.02-0.05)
UK 2 Plictran 25W 1.04 0.46 0.39 0.14 0.08
(0.69-1.18) (0.27-0.54) (0.31-0.48) (0.10-0.16) (0.07-0.09)
Peropal WP 0.90 0.39 0-29 0.14 0.14
(0.66-0.99) (0.28-0.53) (0.18-0.42) (0.09-0.23) (0.09-0.19)
FRG 1 Plictran 25W 0.39 0.15 0.14 0.07 0.13
(0.37-0.41) (0.13-0.18) (1.10-0.19) (0.06-0.08) (0.07-0.19)
Peropal WP 0.54 0.37 0.31 0.09 0.14
(0.53-0.54) (0.26-0.48) (0.31-0.32) (0.05-0.12) (0.12-0.16)
FRG 2 Plictran 25W 0.50 0.59 0.53 0.28 0.29
(0.49-0.52) (0.58-0.60) (0.51-0.56) (0.25-0.31) (0.29-0.30)
Peropal WP 0.32 0.49 0.31 0.19 0.14
(0.24-0.40) (0.45-0.52) (0.28-0.34) (0.11-0.26) (0.10-0.18)
1/ Residues not corrected for recovery or control, but corrected for reagent blanks. Average
recovery = 93.7%.
Table 2. Residues in Apples Determined by Gas Chromatographic
Analysis
Location Residues (mg/kg) at interval
of Product post-treatment (days)1/
treatment used 0 7 14 28 35
UK 1 Plictran 25W 0.35 0.72 0.25 0.18 0.10
Peropal WP 0.44 0.54 0.31 0.16 0.06
UK 2 Plictran 25W 1.20 0.51 0.51 0.21 0.21
Peropal WP 1.06 0.33 0.58 0.24 0.23
FRG 1 Plictran 25W 0.97 1.06 0.68 0.37 0.40
Peropal WP 0.42 0.60 0.53 0.37 0.34
FRG 2 Plictran 0.50 0.23 0.30 0.11 0.15
Peropal WP 0.50 0.25 0.23 0.13 0.14
1/ Plictran values reported as the sum of cyhexatin and
dicyclohexyltin oxide. Peropal values reported as the sum of
azocyclotin + cyhexatin and dicyclohexyltin oxide.
It is evident from a comparison of the values both within the
tables and between the tables, that there is no significant difference
in residues on apples from the use of either azocyclotin or cyhexatin.
Therefore, the discrepancies noted by the 1979 Joint Meeting are
mainly due to the use of different application methods and not to any
differences in the analytical methods or in the degradation chemistry.
METHODS OF RESIDUE ANALYSIS
Definition of residue
In order to better conform to current analytical practice, the
Meeting proposes that the definitions of the residues of azocyclotin
and cyhexatin be changed to read as follows:
azocyclotin - sum of azocyclotin, cyhexatin and dicyclohexyltin
oxide, expressed as cyhexatin.
cyhexatin - the sum of cyhexatin and dicyclohexyltin oxide,
expressed as cyhexatin.
These redefinitions have no effect on recommended maximum residue
limits for either product.
APPRAISAL
The 1979 evaluation of azocyclotin resulted in a discrepancy in
certain recommended maximum residue limits for commodities for which
limits had already been recommended, based on data from the use of
cyhexatin, which is also a degradation product of azocyclotin. In
response to this problem, the manufacturers conducted a joint project
on apples in England and the Federal Republic of Germany. The results
of these tests clearly indicate that the discrepancy was due to
different application technologies and that no significant differences
in residues would result under identical treatment, climate and
analytical conditions. Inasmuch as these new data support the existing
recommendations for apples and pears associated with cyhexatin, no
changes in any of the cyhexatin maximum residue levels are needed.
Residues resulting from the use of either of these products
cannot be distinguished analytically because they are principally
surface residues and the degradation products are determined by
environmental rather than metabolic processes. In addition, the
analytical method converts both azocyclotin and cyhexatin to
tricyclohexylmethyl tin. Therefore, it is necessary to revise the
recommended limit for azocyclotin on apples, beans and strawberries to
the same limits recommended for cyhexatin on those commodities. In the
case of grapes and eggplant for which supervised trial data are
available only for azocyclotin, the recommended limits for azocyclotin
remain unchanged.
New definitions of the residues are proposed for both products to
conform more with current analytical practice.
RECOMMENDATIONS
Cyhexatin: Recommended maximum residue limits, all commodities -
no change. Residues to which the limits apply are the
sum of cyhexatin and dicyclohexyltin oxide expressed as
cyhexatin.
Azocyclotin: The previously recommended maximum residue limits are
increased to the values listed below. The limits for
azocyclotin represent the sum of azocyclotin, cyhexatin
and dicyclohexyltin oxide, expressed as cyhexatin.
Commodity Limit (mg/kg)
Apples 2 (increased from 0.1)
Beans 0.5 (increased from 0.2)
Strawberries 2 (increased from 0.1)
REFERENCES
Bayer AG Residue trials on apples with Peropal and Plictran.
1982 RA-643181B (Plus covering letter). (Unpublished)
Dow Ltd. Organotin residues in apples following treatments with
1982 Plictran 25W and Peropal WP acaricides in England and the
Federal Republic of Germany - 1981. (Plus covering letter)
(Unpublished)
