
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
BENDIOCARB
IDENTITY
Chemical Name
2,3-isopropylidene-dioxyphenyl methyl carbamate
2,2-dimethyl-1,3-benzodioxol-4-yl N-methyl carbamate
2,2-dimethyl-1,3-benzodioxol-4-ol methyl carbamate
Synonyms
FicamR: GarvoR: SeedoxR: TurcamR: MultamatR: NiomilR: TattooR:
Ent-27695: OMS 1394: NC 6897.
Structural formula
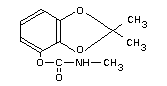 Molecular formula C11 H13 NO4
Other Information on Identity and Properties
Molecular weight 223.25
State white crystalline solid
Melting point 132°C
Vapour pressure 5.0 × 10-6mm Hg at 25°C
Solubility At 25°C 0.04 g/l in water:
0.35 g/l in hexane: 0.3 g/l in
kerosene: 10 g/l in
trichloroethylene and o-xylene:
40 g/l in benzene and ethanol:
200 g/l in acetone, chloroform,
dichloromethane and dioxane:
300 g/l in glycerol and dimethyl
sulphoxide: 640 g/l in dimethyl
formamide.
Stability In aqueous solution at 25°C the
half-life of bendiocarb is 48
days at pH5, 81 hours at pH7 and
45 minutes at pH9. At pH9
hydrolysis of bendiocarb results
in the formulation of the phenol,
NC 7312 whereas below pH5
bendiocarb slowly degrades to
pyrogallol and acetone.
Bendiocarb is stable at
temperatures of up to 100°C and
on non-absorptive surfaces and at
low humidity it resists
oxidation. In aqueous solutions
above pH6 hydrolysis rather than
photolysis is the principal
method of degradation but below
pH5 hydrolysis has little effect
and the photolysis half life is
51 hours.
Specification of technical material Current technical material has a
typical purity of about 98%, the
main impurities being 2,2-
dimethyl-1,3-benzoxdiol-4-ol(NC
7312), and to a lesser extent,
pyrogallol tris (methyl
carbamate).
Physical and Chemical Data on NC 7312, a metabolite of Bendiocarb
Chemical Names
2,2-dimethyl-1,3-benzoxodiol-4-ol
Synonyms
2,2-dimethyl-4-hydroxy-1,3-benzodioxole
2,3-isopropylidene dioxyphenol
Structural formula
Molecular formula C11 H13 NO4
Other Information on Identity and Properties
Molecular weight 223.25
State white crystalline solid
Melting point 132°C
Vapour pressure 5.0 × 10-6mm Hg at 25°C
Solubility At 25°C 0.04 g/l in water:
0.35 g/l in hexane: 0.3 g/l in
kerosene: 10 g/l in
trichloroethylene and o-xylene:
40 g/l in benzene and ethanol:
200 g/l in acetone, chloroform,
dichloromethane and dioxane:
300 g/l in glycerol and dimethyl
sulphoxide: 640 g/l in dimethyl
formamide.
Stability In aqueous solution at 25°C the
half-life of bendiocarb is 48
days at pH5, 81 hours at pH7 and
45 minutes at pH9. At pH9
hydrolysis of bendiocarb results
in the formulation of the phenol,
NC 7312 whereas below pH5
bendiocarb slowly degrades to
pyrogallol and acetone.
Bendiocarb is stable at
temperatures of up to 100°C and
on non-absorptive surfaces and at
low humidity it resists
oxidation. In aqueous solutions
above pH6 hydrolysis rather than
photolysis is the principal
method of degradation but below
pH5 hydrolysis has little effect
and the photolysis half life is
51 hours.
Specification of technical material Current technical material has a
typical purity of about 98%, the
main impurities being 2,2-
dimethyl-1,3-benzoxdiol-4-ol(NC
7312), and to a lesser extent,
pyrogallol tris (methyl
carbamate).
Physical and Chemical Data on NC 7312, a metabolite of Bendiocarb
Chemical Names
2,2-dimethyl-1,3-benzoxodiol-4-ol
Synonyms
2,2-dimethyl-4-hydroxy-1,3-benzodioxole
2,3-isopropylidene dioxyphenol
Structural formula
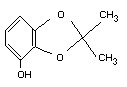 Molecular formula
C9H10O3
Other information on identity and properties
Molecular weight 166
State White crystalline solid
Melting point 91-93°C
Solubility At 25°C 20 g/l in water and
50 g/l in dichloromethane; very
soluble in methanol, ethanol and
acetone.
Vapour pressure 2.34 × 10-3mm Hg at 25°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
In groups of male mice (CFLP strain) acutely intubated with
14C-bendiocarb (14C-bendiocarb with label at the heteroclyclic ring
was used in this and all of the other available metabolism studies on
bendiocarb) at 1, 5 or 10 mg/kg body weight, over 80% of the
administered dose was excreted in 24 hours, primarily in the urine.
Less than 10% of the administered 14C was recovered in the faeces.
The proportion of metabolites formed in the urine, but not the rate
and route of elimination, was dose-dependent. Thus, the level of NC
7312 (2,2-dimethyl-1,3-benzoxodiol-4-ol) (as conjugates) recovered in
the urine was 36, 32, 17 (as % of administered radioactivity),
respectively, at 1, 5, 10 mg/kg. Only one urinary metabolite other
than conjugated NC 7312 was detected, which was believed by
the authors of the report to be probably a conjugate of
6-hydroxybendiocarb (Challis and Adcock 1977a).
Another similar mouse study with groups of males of the CD-1
strain treated orally with single doses of 14C-bendiocarb at 1, 5 or
10 mg/kg body weight indicated recovery of about 80% of the
administered radioactive dose in 24 hours, predominantly in the urine.
A significant dose-dependent relationship was not evident with respect
to rate and route of elimination or metabolic profile of bendiocarb.
About 33-56% of the administered 14C-doses was recovered in the urine
as conjugates of NC 7312. Conjugated 6-hydroxy bendiocarb was believed
by the authors of the study to be the only other major urinary
metabolite (Pearce et al 1977).
Rat
Male and female rats treated with a single oral dose of
14C-bendiocarb at approximately 10 mg/kg body weight eliminated over
95% of the administered radioactivity in urine (89%), expired air (6%)
and faeces (2%) within 2 days of dosing. There was no significant sex
different in rate and route of elimination. Following an acute oral
dose of 14C-bendiocarb at 1 mg/kg body weight, plasma levels in male
and female rats peaked at 10 minutes post treatment with plasma half-
life being calculated at 4.4 hours for both sexes. The major urinary
metabolite was the phenol (NC 7312) which, excreted as ß-glucuronide
and sulphate conjugates, accounted for more than 85% of the
administered dose recovered in the urine during the first 24 hours
after treatment. The remaining 15% eliminated over this time period
comprised a mixture of sulphate and ß-glucuronide conjugates of at
least seven minor metabolites. One of the latter appeared to be
N-hydroxy methyl bendiocarb. No unchanged parent compound was found in
the urine. In the faeces free NC 7312 was the primary metabolite with
a small amount of 14C-bendiocarb being detectable (Adcock and Challis
1976a).
Conjugated NC 7312 was found to be the primary metabolite in
urine collected during 0-24 hours after treatment withdrawal in male
and female rats (CFY strain) fed 20 ppm of 14C-bendiocarb in the diet
for 10 days. At sacrifice 6 days after termination of dietary feeding,
detectable 14C residues were found in the tissues analysed (fat,
liver, kidney, muscle and brain) with the fat tissue having the
highest residue level. According to the partition characteristics of
the 14C residue in fat into organic solvents, radioactive residue in
fat was probably not mainly due to bendiocarb or NC 7312 (Pearce and
Adcock 1978).
Hamster
Groups of 6 or 10 male hamsters were intubated with
14C-bendiocarb in glycerol formal at 1, 5 or 10 mg/kg. Four female
hamsters were similarly treated at 10 mg/kg body weight. Over 90% of
the administered 14C-dose was recovered in 24 hours in the faeces and
the urine, with the latter being the primary route of elimination. The
rate and route of excretion was not modified by sex or dosage level.
The proportion of total radioactivity recovered in the urine as
conjugates of NC 7312 was about 37% at 1 mg/kg, 16% at 5 mg/kg and 19%
at 10 mg/kg. Besides conjugated NC 7312, urine of male hamsters
contained two metabolites in approximately equal amounts, while that
of female hamsters had only one radioactive component. A conjugate of
6-hydroxy bendiocarb was believed by the authors to be the major
urinary metabolite in both males and females. The other urinary
metabolite in male hamsters had not been identified. The seeming sex
difference in metabolic fate of bendiocarb remains to be ascertained,
since the male and female hamsters used in the study were housed and
dosed at different laboratories (Challis and Adcock 1977b).
Rabbit
Groups of pregnant rabbits were intubated on day 20 of gestation
with one dose of 2.5 or 10 mg/kg body weight of 14C bendiocarb.
Within 24 hours of dosing, nearly 90% of the administered dose had
been eliminated, primarily in the urine, with only a small amount
(<2.5%) being detected in the faeces. The rate and route of
elimination and metabolic profile of bendiocarb appeared to be
independent of dosage. The major urinary metabolite was identified as
NC 7312, which accounted for 90% of the radioactivity extracted from
urine samples following enzyme (sulphatase and ß-glucuronidase)
hydrolysis (Challis 1980).
Dog
Groups of one male and one female beagles (fasted overnight and
for 8 hours following dosing) were treated once with 14C-bendiocarb
in gelatin capsules at 0.1, 1.0 or 10 mg/kg body weight. Regardless of
dosage level, the radioactive dose was almost completely excreted
during the first 24 hours post-dosing, primarily in the urine. The
amount of radioactivity found in the urine of both sexes over the
48-hour period seemingly decreased with dosage (about 90% at 0.1 mg/kg
body weight, 80% at 1.0 mg/kg body weight and 70% at 10 mg/kg body
weight). This appeared to be accompanied by a corresponding increase
in faecal recovery (about 19%, 21% and 24%, respectively, at 0.1, 1.0
and 10 mg/kg body weight). In the urine, approximately 84%, 67% and
43% of the administered radioactivity, respectively, at 0.1, 1.0 and
10 mg/kg body weight occurred as sulphate and ß-glucuronide conjugates
of NC 7312 with no recovery of any 14C-bendiocarb. Analysis of urine
samples from male dogs at 10 mg/kg body weight indicated the probable
presence of ring hydroxylated derivatives of bendiocarb as minor
metabolites. Unchanged 14C-bendiocarb was the only component
identified in the faeces and amounted to about 80% of the
radioactivity recovered via this route (Warner et al 1977).
Effects on Enzymes and Other Biochemical Parameters
Mouse
In male and female mice (Charles River CD-1 strain) fed technical
bendiocarb containing 0.09% w/w of dimethoate) in their diets at 0 or
1 250 ppm for 19 days, whole blood cholinesterase in blood sampled
between 06:00 hour and 08:00 hour, but not between 09:00 hour and 11
hours (peak feeding period stated to occur between 22:00 hour and
09:00 hour) was depressed (>20%) only in treated males in weeks 2 and
3 (Hounsell and Rush 1980).
Male CFLP mice and male CD 1 mice were fed dietary levels of
technical bendiocarb at 0, 500 or 1 000 ppm for up to 7 days. In the
CFLP strain, whole blood cholinesterase activity was reduced (>20%)
at 1 000 ppm in animals fed the diet ad libitum and in those
following a 1-hour feeding period on the 7th day after an overnight
fast. In the CD-1 mice, whole blood cholinesterase was depressed
(>20%) in the 500 and 1 000 ppm groups following an overnight fast
and exposure to the diet for 1 hour on day 7. Ad libitum feeding
resulted in decreased whole blood cholinesterase in the 500 but not in
the 1 000 ppm groups (Hounsell 1981a).
Rat
Groups of rats (sex unspecified) were treated orally with
4 mg/kg/day of technical bendiocarb in glycerol formal for three
consecutive days. Inhibition (65-85%) of cholinesterase in whole blood
and plasma peaked at 10 minutes after each dose on days 1 and 3.
Recovery of whole blood and plasma cholinesterase began to occur 30
minutes following each dose, and was practically complete three days
after treatment withdrawal. Whole blood cholinesterase appeared to be
more sensitive than plasma cholinesterase to the inhibitory effect of
bendiocarb (study cited by Kemp and Hounsell 1974).
Male rats (CFY Sprague-Dawley) were fed dietary levels of
technical bendiocarb up to 10 ppm for 14 days, followed by an
overnight fast. Whole blood cholinesterase, assayed in the morning of
the 15th day after a 1-hour feeding period, was not significantly
inhibited (<20%) (Hounsell 1981b). Another similar study with the
same strain and sex of rats fed diets containing technical bendiocarb
for up to 23 days indicated that, following overnight fasting and a
one-hour feeding period, animals exhibited a depression (20%) of whole
blood cholinesterase at 50 ppm on days 15 or 16 and 22 or 23 (Hounsell
1981c).
Dog
In 4 male beagles (fasted the day before the test) fed a diet
containing 1 000 ppm of technical bendiocarb for up to one hour
(actual intake of the test material estimated at 6 - 8.6 mg/kg body
weight), inhibition (>50%) of brain and whole blood cholinesterase
was apparent 15 minutes after the feeding period. Whereas depression
of whole blood cholinesterase peaked at 2 hours and was completely
reversible at 3 hours following the feeding period, the extent of
inhibition of brain cholinesterase remained fairly constant between
15 minutes and 3 hours (the last sampling interval) after the dosing
period (Hounsell 1974). Another experiment with 2 female beagles
(24-hour fasted) fed a dietary level of 10 ppm of technical bendiocarb
for about 10 minutes (actual intake of the test material estimated at
approximately 32.4 mg/kg) showed reduction of whole blood
cholinesterase activity of about 27% and 52% of the pretreatment level
at 1 hour and 6 hours, respectively, post dosing. Cholinegesic
symptoms were noted within 24 minutes of the initiation of feeding.
Complete recovery of whole blood cholinesterase activity and from
toxic signs occurred 24 to 25 hours after treatment (Kemp and Hounsell
1974).
Cow
A lactating dairy cow was fed technical bendiocarb in the daily
diet at 3.6 ppm for two consecutive days (ration was offered twice
daily). No reduction (<20%) in whole blood cholinesterase was
observed at any of the 8-hourly intervals following the morning
feeding on both days (Roberts 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 17 to 21 mated rats (Sprague-Dawley CFY strain) were
fed diets containing technical bendiocarb at 0, 200, 400 or 800 ppm
from 14 days post-coitum until weaning of their pups. A decrease in
maternal body weight gain was noted at 800 ppm on days 17 and 20 post-
coitum and practically throughout the 21-day lactation period. At this
dietary level, survival and weight gain of pups were depressed
throughout lactation. No treatment-related effects were observed on
other parameters monitored, i.e. mortality and clinical signs of
pregnant dams, sex ratio and gross abnormalities of offspring (Jackson
1978).
Groups of 10 males and 20 female rats (SPF, CFY strains) were fed
dietary concentrations of bendiocarb (presumably technical grade) at
0, 200, 400 or 800 ppm for one week prior to mating. The females were
maintained on test diets through day 18 of gestation and the males
until termination. There were no mortalities or toxic symptoms. Growth
was slightly depressed in males at 800 ppm and in females at 400 ppm
and above. Pregnancy rate, duration of gestation period and terminal
gross pathology were not adversely affected. Medium precoital time (as
an indication of mating performance) of dams was lengthened at both
400 and 800 ppm. In the progeny generation, except for a slight
decrease at 400 ppm and above in mean pup weight at birth, no apparent
dose- or treatment-related effects were evident on litter size at
birth, survival and weight of pups through lactation period to
weaning, sex ratio and incidence of gross abnormalities of weanlings
(Palmer and Allen 1978).
Groups of 30 males and 30 females (Charles River CD strain) were
fed dietary levels of technical bendiocarb at 0, 10, 50 or 250 ppm for
90 days prior to mating to initiate a reproduction study covering
three generations with two litters per generation. Weanlings from the
second litters were selected to become parents of the next generation.
After the second mating trial in each generation, the dams in each
group were divided into two approximately equal sub-groups. One
sub-group was allowed to deliver and rear their young until 25 days
post-partum. Another sub-group was sacrificed on day 21 of gestation
for examination of uterine contents. Foetuses were examined for
external, skeletal and soft tissue malformations.
In the parental generations, no mortality or clinical signs
attributable to the compound were evident. Dams displayed slight
growth depression during the gestation period of F1a litters at
50 ppm and above and of F2b litters at 250 ppm. Food consumption was
normal, but water intake was slightly decreased at 250 ppm in F1
females. Males of the F2 generation seemed to exhibit an increase in
incidence and severity of "geriatric nephropathy" at and above 50 ppm.
Ophthalmoscopic examination conducted on F2 adults between 31 and 35
weeks of age revealed no treatment-related findings. At terminal
sacrifice of parental animals after weaning of the second litters,
some variations from controls in absolute weights and/or organ/body
weight ratios of certain tissues such as adrenal, pituitary, thyroid,
seminal vesicles and uterus were noted at both 50 and 250 ppm, but
these observations were not consistently present through the
generations and were not accompanied by histopathological lesions of
the organs. Mating and fertility indices as well as length of
gestation period were not modified by bendiocarb. There was an
increase in frequency of F0 females being acyclic or pseudopregnant
at 250 ppm and of F2 females with irregular oestrous cycles at 50 ppm
and above, prior to the first mating trial in both cases. Pre-coital
interval was lengthened at 250 ppm in the F0 generation (first
mating).
Over the three progeny generations, the viability index was
slightly depressed in F1a litters at 250 ppm from days 10 through 25
and in F2b litters at 50 ppm and above on day 25. Pup weight was
reduced at 250 ppm in F1a and F1b litters on day 25 and in F2b
litters at birth through day 25. In F2a litters, pup weight on day 25
was decreased in all treated groups, but the difference was indicated
by the authors of the report to be statistically significant only at
10 ppm, but not above. There were no significant differences between
control and treated groups in litter size (at birth and on day 1), sex
ratio, ophthalmoscopic observations of F3b pups between 19 and 31
days of age, organ weight and histopathological findings of 25-day old
F3b pups. Examination of 25-day old pups from the second litters of
each generation for rate of physical development and sensory functions
revealed "the onset of pinna unfolding, hair growth, tooth eruption
and eye opening was marginally delayed in all treated (F1b) litters,
but the overall pattern of physical development was essentially
similar in all groups, and "the onset of pinna unfolding and hair
growth was marginally delayed (in F2b litters at both 50 and 250 ppm)
but eye opening and tooth eruption was unaffected".
Teratological investigation of F1b, F2b and F3b litters showed
no significant differences between control and treated groups with
respect to number of implantations, number of viable young, early or
late resorptions, post-implantation loss and foetal weight. A rise in
pre-implantation loss in F1b litters was noted at 250 ppm. Also at
the top dosage level, there were higher incidences of generalized
subcutaneous oedema, incomplete ossification of cranial bone,
uni/bilateral hydronephrosis and bilateral ureter in F1b litters and
of subcutaneous scapular haemorrhage in F3b litters, as compared to
concurrent controls. Based on the data, a dietary level of 10 ppm was
without significant adverse effect on the reproductive parameters
evaluated. The teratogenic "no effect" level was demonstrated to be
50 ppm (Tesh et al 1981).
Special Studies on Teratogenicity
(also see under "Special Studies on Reproduction")
Rat
Groups of 24 mated rats (Sprague-Dawley derived, CD strain) were
intubated with technical bendiocarb as a suspension in gum tragacanth
at 0, 0.25, 1 or 4 mg/kg body weight/day between gestation day 6 and
day 15 (day 0 = day of positive vaginal smear). The dams were
sacrificed on gestation day 21 and foetuses were removed by caesarean
section for external, visceral and skeletal examination. There was no
mortality. Dams at 4 mg/kg body weight/day exhibited toxic symptoms
characteristic of cholinesterase inhibition and slight growth
inhibition on gestation day 21. Two dams (out of 21) at 4 mg/kg body
weight/day and 1/22 dams at 1 mg/kg body weight/day displayed a large
number of late uterine deaths. Mean litter size was depressed at
4 mg/kg body weight/day. There were no significant differences between
control and treated groups in pregnancy rate, number of corpora lutea
or implantation sites, number of early resorptions, foetal weight and
frequency of foetal abnormalities. The study demonstrated bendiocarb
to be non-teratogenic under the conditions of the experiment (Tucker
1974).
Rabbit
Groups of 27-29 female rabbits (New Zealand White) that had been
artificially inseminated were intubated with technical bendiocarb as a
suspension in aqueous gum tragacanth at 0, 1, 2.5 or 5 mg/kg body
weight/day from day 6 to day 28 inclusive of gestation. (Day 0 = day
of insemination.) The does were sacrificed on day 29 of gestation and
uterine contents were examined. Foetuses were subjected to evaluation
for external, skeletal and internal malformations. Six to eight
females per control or treated group died 6 to 29 days after
insemination mainly due to "tracheal intubation". Clinical signs such
as increased respiratory rate and prostration were noted at and above
2.5 mg/kg body weight/day. Growth was slightly depressed at 5 mg/kg
body weight/day between gestation days 16 and 28. Whole blood
cholinesterase assayed 30 minutes post-dosing on day 28 was depressed
(31-70%), in a dose-dependent manner, in all treated groups. One doe
each at 2.5 and 5 mg/kg body weight/day aborted on day 22 and 20,
respectively. Premature parturition occurred in one doe each of 2.5
and 5 mg/kg body weight/day groups on day 28. An increase was observed
in the mean number of late resorptions per litter and the incidence of
pregnant does with late resorptions at both 2.5 and 5 mg/kg body
weight/day. Post-implantation loss (number of implantations - number
of viable foetuses/number of implantations × 100) was elevated at
2.5 mg/kg body weight/day and above.
Other parameters monitored, including number of corpora lutea,
implantations, viable young, or early resorptions, pre-implantation
loss, foetal weight and placental weight, were not adversely affected
by treatment. There was a dose-related increase in incidence of
foetuses with eye anomalies (mainly "cloudy areas in eyes") and with
absence of pubic bones at both 2.5 and 5 mg/kg body weight/day.
Incidence of litters containing foetuses showing such abnormalities
was also elevated at and above 2.5 mg/kg body weight/day. In the
absence of background data on frequency of the abnormalities in
question, 1 mg/kg body weight/day may be considered as a clearcut "no
effect" level with respect to teratogenicity (Tesh et al 1980).
Special Studies on Mutagenicity
Two separate experiments were conducted with technical bendiocarb
and NC 7312 to evaluate their genetic activity in vitro microbial
systems (plate assays), with or without the addition of a mammalian
metabolic activation preparation (S-9 mix from the liver of male rats
pre-treated with Aroclor 1254 i.p. at 500 mg/kg/day for five days).
Indicator organisms used were Salmonella typhimurium strains TA
1535, TA 100, TA 1537, TA 1538 and TA 98. Results suggest
non-mutagenicity of the test compounds to any of the tested strains at
the (non-toxic) concentrations used (3.3-1 000 µg/plate of bendiocarb
and 10-3 300 µg/plate of NC 7312) in the presence or absence of
metabolic activation and under the conditions of the assays
(McConville and McGregor 1979; McConville and Harris 1979). Negative
results were also obtained in another reversion mutation test using
Escherichia coli WP2 and five strains of the Salmonella
typhimurium TA series as indicator organisms in the presence of
absence of a liver metabolic activation system at concentrations of
technical bendiocarb ranging from 5 to 1 000 µg/plate (Moriya
et al 1981).
A rec-assay conducted with Bacillus subtilis H17 (rec+) and
M45 (rec-) in the absence of a metabolic activation preparation
indicated that technical bendiocarb, at concentrations of 20 to
5 000 µg/disc, did not induce any inhibitory zone in either tested
strain (Moriya et al 1981).
Technical bendiocarb was evaluated for its mutagenic activity in
a mouse lymphoma L5178Y specific locus mutation assay. Under the
conditions of the test, there appeared to be no consistent indications
of mutagenicity of the compound in the absence or presence of a
metabolic activation system (S-9 homogenate of liver from male adult
Fischer rats induced with Aroclor 1254) at concentrations ranging from
5 to 20 µg/ml (McGregor and Ross 1981).
In a micronucleus test, groups of male mice were treated with
technical bendiocarb i.p. at 0, 0.625, 1.25 or 2.5 mg/kg body
weight/day for two consecutive days (the two doses were given 24 hours
apart). The animals were sacrificed 6 hours after the second dose and
the femurs were removed for the preparation of bone marrow smears.
There was no significant increase in the frequency of polychromatic
erythrocytes containing micronuclei or change in polychromatic/
normochromatic ratio in any treated group (Hounsell and Walker 1982).
Groups of 20 sexually mature virgin male rats (Sprague-Dawley CD
strain) were fed diets containing technical bendiocarb at 0, 10, 50 or
250 ppm for 13 consecutive weeks and then mated with sexually mature
virgin female rats for seven days in a dominant lethal assay. (Each
group was sub-divided into sub-groups A and B with commencement of
dietary feeding of the groups being staggered by one week.) The
females were sacrificed 14 ± 1 days after mating and autopsied for the
examination of uterine contents. The treated males exhibited no
clinical signs and their body weight and food consumption were not
adversely affected by treatment. The incidence of non-fertile males
were 1/20 at 50 ppm and 2/20 each at 10 ppm and 250 ppm. With the
exception of one infertile male at 250 ppm, abnormally small testes
and epididymis were found in the other four infertile males. An
increase (not dose-related) in incidence of litters with at least one
early death was observed (in sub-group B only) at 10 ppm and 250 ppm,
but this was not accompanied by a rise in the number of early deaths
per litter. There were no significant differences between control and
treated groups with respect to pregnancy rate, number of corpora
lutea, number of total implants, number of viable implants and pre-
implantation loss. Bendiocarb did not appear to induce dominant lethal
mutation under the experimental conditions used (Kemp and Jackson
1977).
Special Studies for Carcinogenicity
Mouse
Groups of 50 males and 50 females (HaM/ICR Swiss mice CD-1; about
35 days old) were fed diets containing technical bendiocarb, found to
contain up to 0.5% dimethoate or dimethoate decomposition products, at
50, 250 or 1 250 ppm. The control group consisted of 100 males and 100
females. The experiment was originally scheduled to be terminated
at a predetermined survival level or 104 weeks at the latest. The
predetermined level was reached after 89 weeks in males and 94 weeks
in females. Mortality rate was increased in females at 1 250 ppm after
85 weeks. Less than 40% of animals in control and treated groups were
still alive at termination, but over 50% of animals of all groups,
including the control, survived at least 82 weeks. All animals that
died or were sacrificed in moribund condition during the study and
those sacrificed terminally were necropsied. A wide range of tissues
and any unusual lesions from animals of all groups, including the
control, found dead or sacrificed moribund and from all terminal
survivors of control and top dosage groups were examined
microscopically. Any unusual lesions from terminal survivors at both
50 and 250 ppm were also subject to histopathological evaluation.
Clinical signs, body weight, food and water consumption were not
significantly different from those in the controls. Ophthalmoscopic
examination conducted at four intervals during the study seemed to
suggest a probable increase at week 89 in incidence of cataract
("posterior cortical or more advanced") in males at 1 250 ppm. At
terminal sacrifice, males at 1 250 ppm showed an increase in absolute
testes weight and testes/body weight ratio and females of all treated
groups displayed a dose-related increase in absolute weight and
organ/body weight ratio of kidney. There were no treatment-related
microscopic changes in the tissues evaluated, including testes and
kidney.
Analysis of tumour data appeared to indicate no significant
difference between control and top dosage groups in the incidence of
any particular type of tumour. Under the conditions of the study,
bendiocarb was not carcinogenic to the mouse (Serota et al 1981).
Rat
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Seven adult hens (15 months old) were given a single subcutaneous
dose of bendiocarb ("laboratory grade") at 60 mg/kg body weight (the
subcutaneous LD50 of bendiocarb in hens was indicated to be about
80 mg/kg body weight). A second dose was given 3 weeks later. The
control group consisted of seven untreated hens. The birds were
observed for a total of 6 weeks from the initial dose before sacrifice
for gross pathology and histopathological examination of the
spinal cord and sciatic nerve. There were no clinical signs or
histopathological evidence suggestive of delayed neurotoxicity of the
compound in any of the treated hens (Sanderson and Hounsell 1969).
Groups of domestic hens, approximately 14-months old (20 birds in
the top dosage group and 10 birds in each other group) were acutely
intubated with technical bendiocarb in maize oil at 0, 189, 378 or
757 mg/kg body weight and observed for 21 days prior to sacrifice for
histopathological examination of the brain, spinal cord and distal
sciatic nerve. The hens were given 10 mg/kg body weight of atropine
intramuscularly immediately before the dose of bendiocarb. (Before
initiation of the neurotoxicity study, the oral LD50 of bendiocarb
was found to be 137.3 mg/kg body weight in hens not pretreated
intramuscularly with 10 mg/kg body weight of atropine and 757 mg/kg
body weight in atropine-protected hens.) One hen at 378 mg/kg body
weight and a total of 8 hens at 757 mg/kg body weight died. Except for
"doubtful signs" of ataxia noted in 1/10 hens at 189 mg/kg body weight
on days 9 and 10 only, there were no delayed neurotoxic signs in any
other treated birds during the 21-day exposure observation period.
None of the bendiocarb-treated survivors or negative controls
exhibited microscopic evidence of neurological lesions. Positive
controls, dosed orally with TOCP at 500 mg/kg body weight, displayed
typical neurotoxic signs and significant morphological changes of the
nervous tissues (Ross et al 1978).
Special Studies on Skin and Eye Irritation
Technical bendiocarb was shown to be a mild irritant when applied
to the skin of New Zealand White rabbits (Cuthbert 1978).
An eye irritant study in New Zealand White rabbits indicated
technical bendiocarb to be minimally irritating to the eye (Cuthbert
1980).
Special Studies on the Acute and Subacute Oral Toxicity of NC 7312
Rat
The oral LD50 of NC 7312 (2,2-dimethyl-1,3-benzoxodiol-4-01) a
metabolite of bendiocarb) in male and female rats (CFY strain) was
greater than 4 640 mg/kg body weight. Cholinergic symptoms were
observed at dosages above 464 mg/kg body weight (Mallyon and Sanderson
1977).
Groups of rats (CFY strain, 10 male and 10 female controls, 5
males and 5 females per treated group) were intubated with NC 7312 as
a suspension in aqueous gum tragacanth at 0, 5, 20, 80, 320 or
1 280 mg/kg body weight/day for 16 days. There were neither mortality
nor compound-related effects on clinical signs, haematological, blood
chemistry and urinalysis parameters. Growth, food conversion factor
and water intake were slightly decreased in females at 1 280 mg/kg
body weight on week 2. Terminal survivors sacrificed showed no gross
or histopathological changes associated with treatment. Absolute
weight and organ/body weight ratio of ovary were reduced at 80 mg/kg
body weight and above, but concomitant microscopic changes of the
tissue were not evident (Kemp and Brooks 1978).
Acute Toxicity
The acute toxicity of several animal species to bendiocarb is
summarized in Table 1.
Toxic signs were characteristic of anticholinesterase poisoning
and seen within a few minutes after oral, intraperitoneal or
inhalation exposure in all species of mammals tested. Deaths usually
occurred after 5 minutes to 2 hours and survivors began to recover
after 1/2 to 2 hours. Within 24 hours of dosing, survivors recovered
completely from clinical signs. After dermal exposure, toxic signs
began to appear after 2 to 21 hours with recovery being much slower
than that following acute oral doses (Sanderson 1921).
Short-Term Studies
Rat - dietary
In a 28-day study with groups of five female rats (Sprague-Dawley
CFY strain) fed diets containing 0, 400, 600 or 800 ppm of technical
bendiocarb, mortality, body weight, food consumption, oestrous cycle
(as evidenced by vaginal cytology taken from the fourth day to
termination) and terminal gross pathology were not affected by
treatment. Whole blood cholinesterase was inhibited (52-62%) at all
test levels and toxic symptoms were observed at both 600 and 800 ppm
on day 28 after the animals (fasted overnight) were given access to an
appropriate test diet for a restricted one-hour feeding period
(Jackson 1977).
Table 1. Acute Toxicity of Bendiocarb in Animals
LD50
Species Sex Route Vehicle (mg/kg body Reference
weight)
Mouse F oral glycerol 45
(CFW) formal Sanderson 1971
Rat M oral glycerol 40-64 Sanderson 1970;
(Wistar) formal Sanderson 1971,
F 34-40 Sanderson 1971
Rat M oral aqueous 108-156 Crome and Sanderson
(Charles River gum 1980
COBS CD tragacanth
Sprague-Dawley)
Rat M&F i.p. glycerol 8 Sanderson 1971
(Wistar) formal
Rat F dermal glycerol 566-800 "
(Wistar) (24-hour formal Ben-Dyke 1932
M exposure) 566
Rat (Carworth M inhalation1/ None 250 mg Sanderson and
Sprague-Dawley) (6-hour a.i./l air2/ Mallyon 1979
exposure)
Hamster F oral water 141 "
(Syrian)
Guinea pig F oral glycerol 35 Sanderson 1971
formal
Rabbit M oral glycerol 40 "
F formal 35
1/ Animals were exposed to an 80% wettable powder as a dust in a "nose only"
exposure chamber with 58% particles of size 10 microns and 18% particles
5 microns.
2/ 6-hour LD50 in terms of actual chamber concentration of the active ingredient.
Groups of 10 male and 10 female rats (Sprague-Dawley CFY strain)
were fed dietary levels of technical bendiocarb at 0, 2, 10, 50 or
250 ppm for 13 weeks. Mortality, clinical signs, body weight, food and
water consumption, ophthalmoscopic findings, clinical chemistry,
haematological and urinalysis parameters were not influenced by the
presence of the compound in the diet. Whole blood cholinesterase,
determined in animals (fasted 16 hours) shortly after a 1-hour feeding
period, was depressed (20%) at 250 ppm in both sexes during weeks 4
and 9, at both 50 and 250 ppm in males and at 250 ppm in females
during week 13. Terminal gross pathological changes and organ weights
were not significantly different from those in the controls.
Histopathological evaluation of a variety of tissues from animals of
the control and top dosage groups suggested the only notable finding
to be an increase in incidence of males at 250 ppm showing vacuolated
centrilobular hepatocytes. This liver lesion, not noted even at
200 ppm in the long-term rat study (see under Long-Term Studies), was
considered unlikely to occur in the lower dosage groups of the study,
although there were no data to confirm this supposition. It would
appear that the no-effect level was 10 ppm on the basis of
cholinesterase levels and was 50 ppm on the basis of the other tested
parameters (Hunter et al 1978).
Hamster
Groups of hamsters (20 male and 20 female controls, 10 males and
10 females per treated group) were fed technical bendiocarb in their
diet at 0, 4, 20, 100 or 500 ppm for 30 days. There were no
indications of any treatment-related effect at levels up to 500 ppm as
judged by mortality, clinical signs, body weight, food and water
intake, terminal haematology, organ weight and histopathology of
selected tissues from control and 500 ppm groups (Hounsell and Brooks
1981).
Dog
Groups of four male and four female beagles were fed dietary
levels of technical bendiocarb at 0, 20, 100 or 500 ppm for 16 weeks
with the top dosage level being raised to 1 000 ppm on day 89 (test
diets were offered to the dogs for a 1-hour period morning and
evening). Inclusion of the compound in the diet did not adversely
affect survival, clinical signs, food consumption, terminal
haematology, blood chemistry and urinalyses values. Males of the
highest dosage group exhibited actual weight loss at termination.
Assays1/ at weeks 5, 9, 14, 15 and 16 of cholinesterase in whole
1/ Whole blood was diluted 1:1 200 in the assay. No data were
available on the amount of diet actually consumed by individual
animals during the 1-hour feeding period prior to sampling for
assay of cholinesterase in blood and brain.
blood and plasma of animals (fasted overnight) following a 1-hour
feeding period indicated depression (>20%) at the top dosage level of
cholinesterase in plasma and whole blood at most sampling periods.
Whole blood cholinesterase was decreased (>20%) even at 100 ppm in
males at week 9 and in females at weeks 9 and 15. Terminal brain
cholinesterase of the top dosage group (males and females) was also
inhibited (>20%). At the conclusion of the study, organ weights and
gross pathological changes were unaffected by the compound.
Microscopic examination of about 20 tissues from each animal suggested
no treatment-related changes other than the occurrence of thyroid
lesions (focal C-cell hyperplasia and focal atrophy) in 1/4 males and
1/4 females of the top dosage group but in none of the concurrent
controls or of animals in the lower dosage groups. The study suggested
the no-effect level with respect to cholinesterase and other criteria
evaluated to be 20 ppm and 100 ppm, respectively (Litton 1974).
Groups of 8 male and 8 female purebred beagles were fed a diet
containing technical bendiocarb at 0, 20, 100 or 500 ppm for 2 years
(control or treated diet was given to each animal every morning).
Survival, clinical symptoms and growth during the study as well as
haematology, urinalysis and ophthalmoscopic parameters evaluated at
five more intervals over the course of the experiment were not
significantly affected by the presence of the test material in the
diet. Less food was consumed by animals (both sexes) of all treated
groups throughout the study. Nevertheless, the authors of the report
stated that "statistical analysis of food residue for weeks 1-3, 1-26,
1-52, 53-104 and 1-104 did not reveal any statistically significant
difference between control and test group means". Based on weekly mean
food consumption and mean body weight data at the end of each week,
the dietary levels of 20, 100 and 500 ppm were equivalent to 0.7, 3.1
and 16.3 mg/kg body weight/day (males and females combined). Water
consumption was slightly but consistently increased in both sexes at
100 ppm and above during weeks 36-39 and at 500 ppm not infrequently
thereafter. Serum cholesterol was elevated at 500 ppm (both sexes) at
several sampling intervals. A dose-related decrease in serum calcium
level was noted at both 100 and 500 ppm in weeks 14 and 25.
Measurement of whole blood cholinesterase in animals (fasted
overnight) shortly after a 60-minute feeding period at eight sampling
intervals during the study showed a tendency to frequent depression
(>20%) of the enzyme at 500 ppm. Brain cholinesterase in animals
(fasted overnight) sacrificed following a 1-hour feeding period was
depressed (>20%) at 500 ppm after 52 and 104 weeks and probably also
at 100 ppm after 104 weeks. Organ weight analysis, gross pathological
examination and histopathological evaluation of over 30 tissues from
each animal sacrificed at the end of 52 weeks (3 males and 3
females/group) or terminally (all survivors per group) showed no
significant alterations related to treatment.
The dietary level of 20 ppm appeared to be a no-effect level on
the tested criteria. It was noteworthy that the actual amount of
bendiocarb ingested by animals during the 1-hour feeding period prior
to the assay of tissue cholinesterase varied markedly, even among
animals in the same dosage group, owing to substantial individual
variation in the quantity of diet (ranging from 0 to 400 g per animal)
consumed. Also, there were incidences where individual animals of a
high dosage group, e.g. 500 ppm, actually had a lower intake of
bendiocarb than those of a lower dosage group (100 ppm) (Chesterman
et al 1980).
Rat - dermal
Groups of 6 rats (Wistar strain) were exposed dermally to an 80%
wettable powder of bendiocarb as a 40% w/v aqueous suspension at 50,
100, 200, 400 or 800 mg a.i./kg body weight under an occlusive patch
for a period of 6 hours/day, 5 days/week for a total of 15
applications in 21 days. The treated site was washed at the end of
each exposure period. There was no mortality. Toxic signs,
characteristic of anticholinesterase poisoning and dose-related in
severity, were noted at 200 mg/kg body weight and above. Whole blood
cholinesterase, determined at the end of the 6-hour exposure period,
was inhibited by more than 30% at 200 mg/kg body weight and above
after only one dose and at 50 mg/kg body weight and above after 15
exposures. No gross pathological changes or microscopic evidence of
dermal irritation attributable to treatment were apparent at terminal
sacrifice (Sanderson 1972).
Long-Term Studies
Rat
Groups of male and female rats (CFY strain, 60 male and 120
female controls, 30 male and 60 female per treated group) were fed
dietary levels of technical bendiocarb at 0, 2, 20 or 200 ppm for 7
days and then caged together (1 male and 2 females). At the end of a
20-day mating period, the females were individually housed and allowed
to litter and rear their pups to weaning. This part of the study was
known as the reproductive phase. A sufficient number of weanlings from
the control and treated groups were randomly selected to initiate
another segment of the study referred to as the main phase. The latter
comprised the main group and the satellite group. In the main group,
weanlings (50 males and 50 females per treated group, 100 male and 100
female controls) were given bendiocarb-treated diets at 0, 2, 20 or
200 ppm for 104 weeks. For the satellite group, used primarily for
laboratory investigations, animals (30 males and 30 females per
control or treated group) were fed bendiocarb at the same dietary
levels, but only for 60 weeks. The 2 ppm level was raised to 10 ppm
after 2 weeks in the main phase, and this group will be known as the
2-->10 ppm group hereafter for the purpose of identification. Dietary
administration was continued throughout both the reproduction and main
phase. All animals died or sacrificed in extremis during the 104-week
study and those sacrificed terminally were necropsied. Animals of all
groups, including the control, dying or sacrificed in extremis during
the study and terminal survivors of the control and top dosage groups
were subjected to microscopic examination of a set of about 30
selected tissues plus grossly observed nodules and tissue masses.
Additionally, all suspected tumours from terminal survivors of lower
dosage groups were evaluated histopathologically.
For the reproductive phase, mortality and behaviour of parental
animals, pregnancy rate, duration of gestation period, litter size and
pup weight at birth, sex ratio and incidence of external abnormalities
of pups were not affected by bendiocarb. There were some indications
of adverse effects related to treatment at 200 ppm on maternal body
weight gain during gestation, litter size on lactation day 4 and pup
weight from lactation day 4 through weaning. A dietary level of 20 ppm
was a no-effect level on this phase of the study.
In the 104-week study (main group) of the main phase, mortality
rate, although unaffected by the compound, was high in all groups,
including the control, Only about 16-26% of males and 24-42% of
females survived 104 weeks. Nevertheless, over 55% of males and
females lived at least 82 weeks.
A number of findings associated with treatment were noted at
200 ppm. These included an increased incidence of males exhibiting
hyperactivity in response to external stimuli, growth depression
during the first 52 or 56 weeks, depressed water consumption during
week 6-12 but not in week 25. Periodic measurements of haematological,
biochemical and urinalysis values at several intervals over the course
of the study revealed significant changes in several parameters
(e.g. total WBC, neutrophil and lymphocyte counts, levels of serum
cholesterol and total protein) to be also confined only to the top
dosage group. There was a dose-related decrease in body weight gain
associated with a depression in food consumption in males of all
treated groups during the first two weeks of the study but not
thereafter. Ophthalmoscopic examination of the surviving animals in
the main group only for a total of seven intervals between 13 and 102
weeks indicated a statistically significant increase in incidence of
lenticular opacities in males at 200 ppm at all intervals from 52
weeks onward. A statistically significant trend (p <0.01) for
dose-dependent incidence of this eye lesion was indicated to be
apparent for males at both 20 and 200 ppm from 66 weeks onward and for
males of all treated groups at 80 weeks and thereafter. It was pointed
out that the difference in frequency of lenticular opacities was not
statistically significant between males of control and low dosage
(2-->10 ppm) groups or among female groups at any time interval. The
lenticular opacities observed reportedly were "essentially atypical of
chemically induced lenticular lesions in the rat". Overall,
2-->10 ppm might probably be considered a borderline no-effect level
with respect to the particular eye lesion.
Activity of whole blood cholinesterase determined in animals
(fasted overnight) following a one-hour feeding period at several
intervals during the study (animals of the satellite group) and at
termination (animals of the main group) was frequently depressed
(>20%) at 200 ppm in both sexes. Whole blood cholinesterase activity
in animals fasted overnight without access to food immediately prior
to sampling of blood was not reduced. The brain cholinesterase level
was inhibited (>20%) at 200 ppm in animals (both sexes) sacrificed at
52 weeks but not terminally. Organ weight determination at 52- and
60-week interim sacrifice and at terminal sacrifice showed deviations
from controls of several tissues such as liver, thymus, prostate and
adrenals, but these were essentially restricted to the top dosage
level only and were not accompanied by histopathological lesions.
Gross pathological changes were not significantly different from those
in the controls. Histopathologically, an increase in incidence of
stomach lesions, characterized by "hyperplasia/hyperkeratosis/
acanthosis with or without inflammation of the non-glandular portion",
occurred in the male terminal survivors at 200 ppm. There were no
microscopic lesions observed in any of the other tissues examined that
were attributable to the compound.
An evaluation of tumour data suggested a dose-related increase of
incidence of total lymphoreticular tumours in males of all treated
groups (incidence 3, 8, 10, 12%) respectively at 0, 2-->10, 20 and
200 ppm), with the difference from concurrent controls being
statistically significant only at 200 ppm. Frequency of each type of
lymphoreticular tumour (lymphosarcoma, thymic lymphosarcoma, reticulum
cell sarcoma, lymphatic leukaemia and myeloid leukaemia), when
considered alone, was not significantly increased in any treated
group. Latency period (or time of detection of the tumour when the
animal died or was sacrificed) was also not modified by treatment.
Other than the particular malignancy just mentioned, incidence, type
and location of tumours were not significantly higher at 200 ppm than
in the controls. It may be noted that 56% of males and 92% of females
of the concurrent control groups were bearers of tumours with
pituitary adenoma (both sexes), mammary tumour (females) and
pheochromocytoma (both sexes) being the most frequently observed
tumours.
Based on the available data, 20 ppm was without any significant
effect on cholinesterase. The lowest tested level, 2-->10 ppm
(indicated to be equivalent to an average achieved intake, throughout
the study of 0.38 mg/kg body weight/day) may be acknowledged as a
marginal no-effect level on the other tested parameters (Hunter
et al 1981).
Observations in Humans
In a male volunteer given a single oral dose of 9.8 mg or
approximately 0.12 mg/kg of 14C-bendiocarb, over 99% of the
administered dose was excreted in the urine in 22 hours. The plasma
half-life and the half-life for renal elimination for total
radioactivity was estimated at 3.5 and 3.3 hours, respectively. The
major urinary metabolites were sulphate and ß-glucuronide conjugates
of NC 7312, which together amounted to 95% of the radioactivity dose
excreted. Small amounts of conjugated bendiocarb and N-hydroxy methyl
bendiocarb were the only compounds detected in the urine (Adcock and
Challis 1976b).
An area on the forearm of each of two male volunteers was exposed
for 3 hours to 14C-bendiocarb (as an aqueous solution in a wetting
agent) at an average dosage of approximately 0.0013 mg/kg body weight
under occluded conditions in one experiment and non-occluded
conditions in another. (No information was available on the time
interval between the two experiments.) Over the 48-hour period
following exposure, an average of 58% of the applied dose under
occluded conditions vs. an average of 12.5% under non-occluded
conditions was recovered in the urine, suggesting better absorption of
bendiocarb through occluded skin. The only urinary metabolite found
was NC 7312 as conjugates of sulphate and ß-glucuronide (Warner and
Adcock 1980).
In a 60-year old male volunteer given bendiocarb (as an 80%
wettable powder) orally in H2O serially at 0.004, 0.012, 0.037,
0.125, 0.187 and 0.25 mg/kg body weight (dosage in terms of w.p.) with
at least a 48-hour interval between doses, 0.125 mg/kg was found
to be a clear-cut no-effect level on symptoms and whole blood
cholinesterase. Toxic sumptoms such as mild vertigo, nausea and
vomiting together with significant inhibition (30-40%) of whole blood
cholinesterase were induced after the single dose at 0.25 mg/kg body
weight. The symptoms subsided in 30 minutes and whole blood
cholinesterase recovered completely in 4 hours post-treatment. In the
same subject, doses of the 80% w.p. at 0.125 mg/kg given orally at an
interval of 4 hours, but not less, produced no toxic signs or
inhibition of whole blood cholinesterase. A double-blind cross over
experiment involving three other male volunteers indicated a single
dose of 0.004 mg/kg body weight, the only dose administered, of the
80% w.p. resulting in no adverse effects on any of the parameters
assessed (heart rate, clinical signs, mean diastolic/systolic pressure
and whole blood cholinesterase). Following a single dose of 0.12 mg/kg
body weight of the 80% w.p. given orally to the same three volunteers
72 hours later in another similar experiment, a transient depression
(21% of whole blood cholinesterase was observed in one of the three
subjects at 30 minutes post-treatment (Drummond and Kemp 1976).
The safety of bendiocarb (80% wettable powder), when used as an
insecticide against adult mosquitoes, to operators and inhabitants was
evaluated in Iran and Indonesia. The studies were organized along the
line of the WHO expanded Stage V evaluation programme. Each trial
involved the treatment of 13 villages with a total population of over
4 000 by 15 or 16 spray operators. Results indicated that very few
operators reported any toxic signs or symptoms and the latter, when
noted, were usually mild and transient. Inhibition of whole blood
cholinesterase, slight to mild, was only infrequently observed and was
readily reversible. Analysis of urine collected from the operators
suggested absorption of only low levels of bendiocarb during spraying.
No complaints were received from the villagers (Motabar et al 1981;
Bonsall et al 1981).
COMMENTS
Bendiocarb, a carbamate insecticide, evaluated for the first time
by the Meeting, was rapidly absorbed and readily eliminated primarily
in the urine in all of the mammalian species studied, including
humans. The major metabolic pathway in these species involved cleavage
of the carbamate ester group to yield the corresponding phenol
(NC 7312). There was no evidence of tissue accumulation in mammals.
Mammalian acute oral toxicity varied between strains and species, but
not between sexes.
A 3-generation reproduction study, including teratological
evaluation, in rats demonstrated 10 ppm and 50 ppm to be the no-effect
level, respectively, on reproductive parameters and teratogenicity. A
teratogenic study in the same species failed to give any indication of
teratogenicity even at the top dosage level (4 mg/kg body weight)
tested. In a rabbit teratology study, 1 mg/kg body weight was shown to
be a clear-cut teratogenic no-effect level. The available mutagenic
studies including reversion mutation and rec assays, mouse
micronucleus test, mouse lymphoma assay and dominant lethal assay in
rats were all negative. A carcinogenicity study in mice revealed no
carcinogenic activity of the compound, under the conditions of the
experiment. In a long-term feeding study with rats, a significant
increase in incidence of total lymphoreticular tumours, but not
individual type of lymphoreticular tumours, was noted in males of the
highest dosage group (200 ppm). Latency period of these tumours was
not reduced. Although the possibility of the finding in question
regarding lymphoreticular tumours being related to bendiocarb does not
appear likely, it cannot be entirely excluded. Background data on
incidence of total lymphoreticular tumours in CFY strain of rats
maintained in the testing laboratory would be helpful to resolve the
uncertainty. It should be noted that concurrent controls in the study
had a high incidence of spontaneous tumours. Additionally, less than
25% males and 45% females survived the duration of the 104-week study,
although over 50% of animals in all groups including the control were
still alive at the end of 82 weeks. Together these factors might
compromise sensitivity of the study to detect carcinogenic activity of
the compound. This study also demonstrated an increased incidence of
lenticular opacities in male rats at the two top dietary levels.
Neurotoxicity studies in hens gave no indications suggestive of
delayed neurotoxic potential of the insecticide.
No-effect levels were determined in short-term studies in rats
(13-week) and dogs (16-week and 2-year) and a long-term study in rats.
In the dog, however, the Meeting noted the possible adverse effects of
dilution during the determination of tissue cholinesterase, and also
the practice of tissue sampling from overnight-fasted dogs following a
1-hour feeding period. Furthermore, there was some concern on the
absence of data on the levels of food ingestion during the 1-hour
feeding period in one study, and food intake was extremely variable in
the second study.
Observations in humans indicated rapid reversal of cholinesterase
inhibition and of clinical symptoms following both acute and subacute
exposures.
Because of the concerns regarding the increased incidence of
lymphoreticular tumours in male rats of the top dose group in the
long-term study, and the probability that the no-effect level
established for dogs is nominal rather than actual, only a temporary
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0-0.38 mg/kg bw/day
Dog: 0-0.07 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0-0.002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Historical data on the incidence of total lymphoreticular tumours
in the CFY strains of rats used in the long-term study.
2. Short-term (28-day) study in dogs to clarify the relationship
between dietary intake of bendiocarb and terminal erythrocyte and
brain cholinesterase activity, using an appropriate method for
cholinesterase determination.
Desirable
Further investigation on the cataractogenic activity of
bendiocarb at low dosage levels in rats.
REFERENCES
Adcock, J.W. and Challis, I.R. The metabolism of 14C-bendiocarb in
1976a the rat. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1976b The pharmacokinetics and metabolism of bendiocarb in man.
Report from Fisons Ltd. submitted to the World Health
Organization by FBC Limited. (Unpublished)
Ben-Dyke, R. The toxicology of NC 6897. Acute dermal toxicity of
1972 technical bendiocarb, Report from Fisons Limited submitted to
the World Health Organization by FBC Limited. (Unpublished)
Bonsall, J.L., Foulkes, D.M., Goose, J., Leake, C.R. and Reary,
1981 J.B. Safety studies with bendiocarb in a village-scale field
trial against mosquitoes in Indonesia, 1981. Document
(WHO/VBC/81.831), World Health Organization, Geneva.
Challis, I.R. The metabolism of bendiocarb in the pregnant rabbit,
1980 Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in the
1977a mouse. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1977b The metabolism of bendiocarb in the hamster. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Gopinath,
1980 C., Harling, S., Majeed, S. and Prentice, D.E. NC 6897
Toxicity study in beagle dogs (final report dietary intake for
104 weeks). Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Crome, S.J. and Sanderson, D.M. A comparison of the acute oral
1980 toxicities of three batches of bendiocarb CR 4971/2, CR 4799/9
and CR 4500/20 to the male rat. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Cuthbert, J.A. Technical bendiocarb EX Muskegon primary eye irritancy
1980 in rabbits. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
1978 Technical bendiocarb. Primary skin irritancy study on rabbits.
Report from Inveresk Research International, Scotland,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Drummond, P. and Kemp, A. The effects of the acute oral administration
1976 of NC 6897 80 WP to male volunteers. Report TOX/76/133-58 from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hounsell, I.A. Brain cholinesterase inhibition in dogs fed NC 6897.
1974 Report from Fisons Limited Agrochemical Division submitted to
the World Health Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Rush, K.C. The effect of dietary administration
1980 of NC 6897 at 1 250 ppm on whole blood cholinesterase activity
in mice. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Brooks, P.N. The 30-day subchronic oral toxicity
1981 of NC 6897, technical CR 4799/1 in the diet to the hamster.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A.G. The effect of dietary administration of NC 6897 at
1981a 500 and 1 000 ppm on whole blood cholinesterase activity in
male mice of the CFLP and CD1 strains. Report from FBC Limited
submitted to the World Health Organization by FBC Ltd.
(Unpublished)
1981b The effect of dietary administration of NC 6897 at 0.4, 2 and
10 ppm on whole blood cholinesterase activity in male rats.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1981c The effect of dietary administration of NC 6897 at 0.4, 2, 10
and 50 ppm on whole blood cholinesterase activity in male
rats. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Walker, A.K. A micronucleus study in mice using
1982 technical NC 6897, CR 4971/2. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Offer, J.M. and
1978 Gregson, R.L. NC 6897 Toxicity to rats when administered in
the diet for 13 weeks. Final Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Prentice, D.E.,
1981 Gopinath, C. and Gibson, W.A. NC 6897 Toxicity and
tumorigenicity to rats in long-term dietary administration.
Report from Huntingdon Research Centre, England, submitted to
the World Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. Effects on adult female rats of one month exposure to
1977 high dietary concentration of NC 6897 (technical). Report from
Fisons Limited Agrochemical Division submitted to the World
Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. A peri- and post-natal study in rats with technical NC
1978 6897, Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Hounsell, I. Evidence for the reversal of cholinesterase
1974 inhibition by NC 6897 in laboratory animals. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Kemp, A. and Jackson, C.M. A test for the induction of dominant lethal
1977 mutations in male rats by NC 6897. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Brooks, P.N. The effects of the daily oral administration
1978 of NC 7312 to male and female rats for 16 days. Report from
Fisons Limited Agrochemical Division submitted by FBC Limited.
(Unpublished)
Litton. 16-week subacute toxicity study in dogs. NC 6897 technical.
1974 Report from Litton Bionetics Inc., U.S., submitted to the
World Health Organization by FBC Ltd. (Unpublished)
Mallyon, B.A. and Sanderson, D.M. The acute oral toxicity of NC 7312
1977 to male and female rats. Report from Fisons Limited
Agrochemical Division submitted by FBC Limited. (Unpublished)
McConville, M. and McGregor, D.B. Testing the mutagenic activity of
1979 technical NC 6897, Report from Inveresk Research
International, Edinburgh, Scotland, submitted to the World
Health Organization by FBC Limited. (Unpublished)
McConville, M. and Harris, W.J. NC 7312: Ames test for mutagenic
1979 activity. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
McGregor, D.B. and Ross, C.A. NC 6897: The assessment of mutagenic
1981 potential in the mouse lymphoma mutation assay. Report from
Inveresk Research International, Scotland, submitted to the
World Health Organization by FBC Limited. (Unpublished)
Moriya, M., Ohta, T. and Shirasu, Y. KNT (bendiocarb): microbial
1981 mutagenicity study. Report from the Institute of Environmental
Toxicology, Japan, submitted to the World Health Organization
by FBC Limited. (Unpublished)
Motabar, M., Mallyon, B.A., Goose, J. and Adcock, J.W. Document
1981 (WHO/VBC/81.821) World Health Organization, Geneva.
Palmer, A.K. and Allen, P.A. Effect of NC 6897 on neonatal pup
1978 mortality, Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Pearce, J.C., Warner, P.A. and Adcock, J.W. The metabolism of
1977 bendiocarb in the mouse (strain CD 1). Report from Fisons
Limited submitted to the World Health Organization by FBC
Limited. (Unpublished)
Pearce, J.C. and Adcock, J.W. Investigation of residue accumulation
1978 in the rat following repeated administration of
14C-bendiocarb. Report from Fisons Limited submitted to the
World Health Organization by FBC Limited. (Unpublished)
Roberts, N.L. Blood cholinesterase levels in the cow following dietary
1979 inclusion of bendiocarb. Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Ross, D.B., Roberts, N.L., Cameron, D.M., Prentice, D.E. and Majeed,
1978 S.K. Examination of NC 6897 for neurotoxicity in the domestic
hen. Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Sanderson, D.M. The toxicity of NC 6897: Acute toxicity to the rat of
1970 technical grade NC 6897. Report from Fisons Limited submitted
to the World Health Organization by FBC Limited. (Unpublished)
1971 The toxicology of NC 6897: Acute toxicity of pure NC 6897.
Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1972 Toxicology of NC 6897: 15-dose cumulative dermal study with
Ficam in male rats. Report from Fisons Limited submitted to
the World Health Organization by FBC Limited (Unpublished)
Sanderson, D.M. and Hounsell, I.A. The toxicology of NC 6897. Test
for neurotoxicity of NC 6897 to the chicken. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Sanderson, D.M. and Mallyon, B. The acute oral toxicity to the
1977 hamster of technical bendiocarb. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
1979 The acute inhalational toxicity to rats of Ficam 80W, an 80%
wettable powder formulation of bendiocarb, CR 13374/27H.
Report from Fisons Agrochemical Division submitted to the
World Health Organization by FBC Limited. (Unpublished)
Serota, D.G., Voelker, R.W. and Fezio, W.L. A chronic toxicity and
1981 carcinogenicity study in mice, NC 6897. Final report from
Hazleton Laboratories America, Inc. submitted to the World
Health Organization by FBC Limited. (Unpublished)
Tesh, J.M., Bartlett, A., Tesh, S.A., Whitney, J.C. and Finn, J.P.
Technical NC 6897 (CR 4799/1): Effects of dietary
administration upon reproductive performance and teratogenic
response in rats treated continuously through three successive
generations. Report from Life Science Research, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Tesh, J.M., Ross, F.W. and Tesh, S.A. Technical NC 6897: Effects of
1980 oral administration upon pregnancy in the rabbit. Report from
Life Science Research, England, submitted to the World Health
Organization by FBC Limited. (Unpublished)
Tucker, M.L. NC 6897: Teratogenicity study in the rat. Report from
1974 Consultox Laboratories Ltd., England, submitted to the World
Health Organization by FBC Limited. (Unpublished)
Warner, P.A., Adcock, J.W. and Pearce, J.C. The metabolism of
1977 14C-bendiocarb in the dog. Report from Fisons Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Warner, P.A. and Adcock, P.A. The dermal absorption of 14C-bendiocarb
1980 in man. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
BENDIOCARB
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bendiocarb is available as a wide range of formulations,
including wettable powders (200, 500 and 800 g a.i./kg); granules
(30, 50 and 100 g a.i./kg); dust (10 g a.i./kg); oil suspension for
ULV application (250 g a.i./kg); residual sprays, paint-on and
granular baits and aerosols.
Bendiocarb is used for the controls of soil and foliar insect
pests on a wide range of crops, ectoparasites on domestic animals and
mosquitos and nuisance pests. The Meeting was informed that it is sold
for these uses in approximately 100 countries. Information was
provided to the Meeting on official nationally approves uses (good
agricultural practices, GAP) by the Netherlands. Good agricultural
practices of The Netherlands are summarized in Table 1 (Netherlands
1982).
Table 1. Bendiocarb Good Agricultural Practices, Netherlands 1982
Application Used
Crop Pests rate (kg/ha) Formulation Treatment Since
Sugarbeet Fritfly, wireworm, 0.3-0.4 3% G seed furrow 1979
spring tails, 0.4 80% WP seed furrow 1981
pygmy beetle 500 g/l
liquid
6.4 g/kg " seed dressing 1981
seed
Maize Fritfly, 0.3-0.4 3% G seed furrow 1979
wireworm 4 g/kg 80% WP seed dressing 1981
seed 500 g/l
liquid
The Meeting was provided with a tabulation of recommended
application rates, which are summarized in Table 2 (Netherlands 1982).
No information was provided on preharvest intervals. The recommended
application rates are consistent with GAP of The Netherlands for maize
and sugarbeets. Information was not provided on whether the
recommended uses other than on sugarbeet and maize are also nationally
approved and, if so, where.
Table 2. Recommended Application Rates of Bendiocarb, Netherlands 1982
Crop Major pests Formulation Rate (a.i.)
Maize Wireworm, symphlids, fritfly Granule 0.3-0.35 kg/ha
Wireworm, symphlids, fritfly Seed dressing 4 g/kg seed
Corn rootworm Granule 0.84-1.12 kg/ha
Soil pests (in furrow) Spray 0.4 kg/ha
Sugarbeet Wireworm, pygmy beetle,
springtails Granule 0.3-0.35 kg/ha
Wireworm, pygmy beetle,
springtails Seed dressing 6 g/kg seed
Soil pests (in-furrow) Spray 0.4 kg/ha
Rice Rice water weevil, stem
borer Granule 2 kg/ha
Leafhoppers, planthoppers Spray 0.5 kg/ha
Leafhoppers, planthoppers Dust 0.5-0.75 kg/ha
Oilseed Flea beetle Granule 0.14-0.28 kg/ha
rape Spray 0.1-0.2 kg/ha
Ryegrass Fritfly, wireworm,
leatherjackets Granule 0.75 kg/ha
Spray 0.5-0.75 kg/ha
Seed dressing 10 g/kg seed
Cereals Fritfly, wireworm,
leatherjackets Spray 0.75 kg/ha
Seed dressing 2-4 g/kg seed
Potatoes Colorado beetle Spray 0-125-0.25 kg/ha
tuber moth Spray 0.5 kg/ha
Brassicas Diamond-back moth Spray 0.1-0.8 g/l
Peas Pea moth, pea weevil Spray 0.2-0.5 kg/ha
Apple/Pear Codling moth, tortrix moth,
aphids Spray 0.25-0.6 kg/ha (LV)
Mushrooms Mushroom fly Wall spray 30-50 g/100 m2
Preharvest Uses
Residue trials utilizing preharvest treatments have been
conducted on a variety of plant and animal products. Results are
detailed under "Residues Resulting From Supervised Trials".
Postharvest Uses
The Meeting was informed of residue investigations in wheat grain
stored in small bins for six months after treatment of interior
surfaces, but no details were provided. Likewise, no details were
provided on studies said to have been conducted on foodstuffs treated
in food-handling premises.
Other Uses
The Meeting was informed that 80 W or ULV formulations may be
used for control of mosquitos and the ULV formulation against
warehouse pests. Studies have been conducted to determine the extent
of potential exposure. Although contamination levels were said to be
within safe limits, details of these studies were not provided.
Bendiocarb is said to be widely used for control of cockroaches
and other nuisance pests in and around the home, hotels, food-handling
establishments, aircraft etc. and as a paint for fly control in animal
houses. No details on these uses were provided.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries on a wide
range of commodities, representing a variety of treatments and
climatic conditions.
Root and Tuber Vegetables
Potatoes
Twenty five trials were conducted in three European countries
either by spray, broadcast or in-furrow treatments with either 3G or
20W formulations. Results are summarized in Table 3 (FBC 1982a; Reary
1979d; Browne and Reary 1981; Browne 1981e). Although most of the data
were from application of the 3G formulation, no information was
provided on recommended or approved uses for that formulation on
potatoes. The wettable powder spray applications were at one and two
times the recommended rate. Recoveries were generally 75% or better
for bendiocarb and 60% or better for NC 7312. The limit of
determination is said to be approximately 0.01 and 0.02 mg/kg for
bendiocarb and NC 7312, respectively.
Table 3. Bendiocarb Residues in Potatoes, from Supervised Trials
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 2 4-5 3G 1 kg/ha In-furrow 119-155 0.01-0.03 <0.02,0.26
1978 2 4-5 3G 2 kg/ha In-furrow 119-155 0.01-0.04 0.02,0.58
2 4-5 3G 3 kg/ha In-furrow 119-155 0.01,0.03 0.04,0.69
1 7 3G 1 kg/ha Broadcast 112 0.01 0.02
1 7 3G 1.5 kg/ha Broadcast 112 0.04 0.05
2 4-7 3G 2 kg/ha Broadcast 112-155 <0.01-0.03 <0.02,0.07
2 4-7 3G 3 kg/ha Broadcast 112-155 <0.01-0.04 <0.02,0.11
2 4-7 3G 4 kg/ha Broadcast 112-155 0.01-0.03 0.03,0.08
3 Untreated
controls 0.003-0.008 0.003-0.0.7
German Federal 3 3 20W 0.12 kg/ha Spray 03/ <0.01 0.014-0.12
Republic 3 3 (<0.005-0.017) (<0.01-0.14
1980 2 3 7 <0.01 <0.01-0.14
(<0.005-0.009) (<0.01-0.15)
14 <0.01 0.01-0.15
(<0.005-0.015) (<0.01-0.18)
1 3 15 <0.005) 0.014
3 2 - - Untreated
controls - 0.001-0.044/ (<0.01-0.024)
0.001-0.007
Table 3 (con't)
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 1 (3 plots) 20W 0.53 kg/ha Spray 145/ <0.01 0.03-0.05
1981 1 (3 plots) 20W 1.06 kg/ha 145/ 0.005 0.06-0.07
1 Untreated
controls <0.005 0.01
1/ Limit of determination 0.01 mg/kg for bendiocarb and 0.02 mg/kg for NC 7312.
2/ Means of replicates; range of individual replicates in parentheses.
3/ Day 0 represents final spray of a weekly 3-spray programme, each application at 0.12 kg/ha.
4/ <0.01 except the 0.04 mg/kg value.
5/ Days after final spray of 2- or 3-spray programme, commencing 7 or 14 days before the final application.
Maximum bendiocarb residues at harvest resulting from at or
before harvest treatments were 0.04 mg/kg (mean of replicates) and
0.7 mg/kg for NC 7312.
From multiple spray applications, maximum bendiocarb residues at
harvest were < 0.01 mg/kg (mean of replications) or 0.017 mg/kg for
individual replicates. Maximum residues of NC 7312 were 0.15 mg/kg
(mean of replicates) or 0.18 mg/kg for individual replicates. For both
the means of the replicates and individual replicates residues of
bendiocarb and NC 7312 were each generally comparable from 0 to 15
days after last application. Apparent residues in untreated controls
were generally < 0.01 mg/kg for bendiocarb and < 0.02 mg/kg for
NC 7312. Residues of NC 7312 at harvest were 1-20 times that of
bendiocarb per se, and averaged six times higher.
Since residues from in-furrow or broadcast applications can be
0.04 mg/kg and untreated controls can, on occasion, reach this level,
a 0.05 mg/kg for bendiocarb per se can be supported. If NC 7312 were
also included, a limit of 0.5 mg/kg would be in order for combined
residues.
Sugarbeet
Thirty one supervised residue trials were conducted in five
European countries and residue decline studies in three (Table 4)
(Browne 1982c; FBC 1982b; Reary 1974, 1975e, 1979h,i,n,q, 1980f,j).
The seed treatment applications of 80W were at 0.8-2.7 times the
recommended 6 g/kg rate, which is also consistent with GAP of The
Netherlands. In-furrow applications of a granular formulation were
representative of the 0.3-0.4 kg a.i./ha GAP of The Netherlands and
recommended application rates.
In the supervised trials, bendiocarb residues at harvest were
below 0.01 mg/kg. Some samples were also analysed for the metabolite
NC 7312 and all were below the 0.01 mg/kg limit of determination.
Similarly, in the decline studies, residues in sugarbeet roots were
less than the 0.02 mg/kg limit of determination. Residues in untreated
controls were generally < 0.01 mg/kg, except for one set of trials
where residues ranged up to 0.11 mg/kg (average 0.005 mg/kg). The same
basic analytical procedure was used in all of the trials, but modified
in some to permit analysis of NC 7312. The limit of determination for
sugarbeet generally ranged from 0.005-0.02 mg/kg for bendiocarb and
0.01-0.02 mg/kg for NC 7312. Recoveries were generally > 75% for
parent compound and > 60% for NC 7312. In general, recoveries were
less in tops than in roots and less for NC 7312 than for bendiocarb.
Table 4. Bendiocarb Residues in Sugarbeets From Supervised Trials and Residue Decline Studies
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
Denmark 1 1 80W 15 g/kg seed Pelleted seed 204 roots <0.005
1974 1 untreated controls roots <0.005
France 2 8 80W 12 g/kg seed Pelleted seed 163 roots <0.005
1974 2 untreated controls roots <0.005
German 1 1 6 g/kg seed Seed dressing 196 roots <0.005
Federal 1 1 196 tops <0.005
Republic 1 1 80W 12 g/kg seed Seed dressing 196 roots <0.005
1974 1 1 196 tops <0.005
untreated controls roots or
tops <0.005
U.K. 2 4 80W 8 g/kg seed Pelleted seed 213 roots <0.005
1974 untreated 213 tops <0.0055/
2 control roots <0.005
tops <0.0055/
France 3 3 3G 0.36 kg/ha In furrow 160-168 roots <0.005
1976 3 3 160-168 tops <0.01
3 6 3G 0.45 kg/ha In furrow 171-190 roots <0.005
3 6 171-190 tops <0.01
3 6 untreated controls tops and
roots <0.006
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
France 2 2 3G 0.46 kg/ha In furrow 180-185 roots <0.005
1977 2 2 180-185 tops <0.01
2 2 untreated controls tops and <0.006
roots
France6/ 2 3G 0.3 kg/ha In furrow 152-170 roots <0.01
1978 2 152-170 tops <0.01
2 3G 0.6 kg/ha In furrow 152-170 roots <0.01
2 2 untreated controls tops and
roots <0.005
Italy6/ 1 1 3G 0.3 kg/ha In furrow 110 roots <0.01
1978 1 1 110 tops <0.01
1 1 3G 0.6 kg/ha In furrow 110 roots <0.01
1 untreated controls tops and
roots <0.005
U.K.6/ 3 3 3G 0.3 kg/ha In furrow 154-188 roots <0.01
1978 3 3 154-188 tops <0.01
1 1 3G 0.33 kg/ha In furrow 202 roots <0.01
1 1 202 tops <0.01
1 1 3G 0.4 kg/ha In furrow 188 roots <0.01
188 tops
1 1 untreated controls tops and
roots <0.007
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K.6/ 6 1 3G 0.3 kg/ha In furrow 144-167 roots <0.01
1979 6 1 144-167 tops <0.01
6 6 untreated controls tops and
roots <0.01
Italy6/ 1 3G 0.30 kg/ha In furrow 154 roots <0.01
1981 1 154 tops <0.01
1 3G 0.32 kg/ha In furrow 183 roots <0.01
1 183 tops <0.01
2 2 Untreated roots <0.001
controls tops 0.003
German 4 12 80W 5 g/kg seed Seed dressing 71-166 roots <0.02
Federal tops <0.02
Republic 4 12 Untreated roots 0.002-0.11
1978 4 12 controls (0.005 av.)
tops 0.005-0.015
(0.008 av.)
3 8 3G 0.33 kg/ha In furrow 56-70 roots <0.01
3 9 tops <0.01
71-166 tops <0.015
5 Untreated 71-166 roots <0.01
controls tops <0.01
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K. 1 5 3G 0.3 kg/ha In furrow 25-40 roots 1.367/
1979 41-55 roots 0.127/
56-70 roots <0.01
71-166 roots <0.01
7 56-70 tops <0.01
71-166 tops <0.01
1 5 Untreated 56-70 roots <0.01
controls tops <0.01
1/ Bendiocarb only (see also 6/);
2/ Corrected for recoveries of >75% except for 59% recovery in tops. Fortification levels ranged from 0.02-0.01 mg/kg;
3/ Limit of determination in individual trials generally ranged from 0.005-0.02 mg/kg;
4/ Duplicate analyses of 0.006 and 0.003 mg/kg;
5/ Individual values up to 0.07 mg/kg but <0.005 mg/kg when repeated;
6/ Sample also analysed for primary metabolite NC 7312. Residues after 56 days were generally below the 0.01 mg/kg limit
of detection except one 0.03 mg/kg value in tops (limit of determination in that trial or a 0.02 limit of determination.
Residues in seedlings were 0.42 and 0.07 mg/kg at 28 and 41 days respectively after in-furrow application at planting;
7/ Seedlings analysed whole.
Since residues are below the claimed limits of determination, a
limit of 0.02 mg/kg bendiocarb per se would probably not be
unrealistic. However, because of high apparent residues in one
untreated control, a 0.05 mg/kg limit at or about the limit of
determination may be more suitable. Based on a 0.02 mg/kg limit of
determination for NC 7312, a limit on the order of 0.1 mg/kg would be
appropriate if both bendiocarb and free NC 7312 were included. Actual
residues were too low to determine whether residues of NC 7312 exceed
those of bendiocarb, but judging from those found in potatoes, it is
likely.
Leafy Vegetables, Except Brassicas
Sugarbeet leaves
From the residue trials and decline studies discussed previously
(Table 4), residues in sugarbeet tops at harvest from recommended
application rates were generally less than the 0.01-0.03 mg/kg limits
of determination. Only one trial had a limit of determination of
0.03 mg/kg for tops. The limit of determination was considered to be
< 0.02 mg/kg for the remaining 30 trials. Bendiocarb residues were
1.4 and 0.12 mg/kg in seedlings at 28 and 41 days, respectively, after
in furrow treatments at planting. The data support a 0.05 mg/kg at or
about limit of determination at harvest as the maximum residue level.
Brassica Leafy Vegetables
Cabbage
No information was available on specific nationally approved
uses. Supervised trials with a wettable powder formulation were
conducted in one country (Table 5) (Browne 1981a,c, 1982a,b; Browne
and Manley 1982), at application rates up to 0.25 times the maximum
recommended application rate (Table 2). No specific preharvest
interval was recommended. Maximum residues ranged from 0.18 mg/kg one
day after the last of seven applications to <0.01 mg/kg after 14
days. Untreated controls were < 0.02 mg/kg and analytical
recoveries averaged 82% from fortification levels at 0.08-0.2 mg/kg.
The limit of determination was said to be 0.01 mg/kg, but considering
untreated control analyses, a 0.05 mg/kg limit of determination may be
more practical.
Because the trials do not reflect maximum recommended application
rates, recommended usage does not specify the number of applications
or a preharvest interval and the data are minimal, a maximum residue
level for bendiocarb in cabbage cannot be estimated with confidence.
Table 5. Bendiocarb and NC 7312 in Crops from Supervised Trials
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Cabbage1/ Philippines 7 0.06 50W 1 0.1 3
1981 0.1 a.i./l 3 0.04 3
7 0.01 3
14 <0.01 3
0.12 50W 1 0.18 3
0.2 g a.i./l 3 0.05 3
7 0.02 3
14 <0.01 3
Untreated 1 <0.01 3
control 3 0.02 3
7 0.01 3
14 <0.01 3
Peas2/ U.K. 1 0.2 50SC3/ 27 <0.01 <0.01 5
flowering 1981 1 0.3 <0.01 <0.01 5
stage (MarQ) 1 0.4 <0.01 <0.01 5
1 0.05 <0.01 <0.01 5
Untreated
control <0.01 <0.01 5
4-leaf 1 0.2 27 <0.01 <0.01 5
stage 1 0.3 <0.01 <0.01 5
1 0.4 <0.01 <0.01 5
1 0.5 <0.01 <0.01 5
Untreated
control <0.01 0.01 5
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pea pods 1 0.2 50SC3/ 83 <0.01 <0.02 5
flowering 1 0.3 <0.01 <0.06 5
stage 1 0.4 <0.01 0.07 5
1 0.5 <0.01 0.03 5
Untreated
control <0.01 0.03 5
4-leaf
stage 1 0.2 83 <0.01 0.05 5
1 0.3 <0.01 0.05 5
1 0.4 <0.01 0.08 5
1 0.5 <0.01 0.06 5
Untreated <0.01 0.05 5
control
Apple
(Cox's U.K. 24/ 0.04 and 20W 57 <0.01-0.01 <0.01 6
Orange 1981 0.08% 50W,
Pippin) 50 SC
Untreated 0.006 0.005 1
14/ 0.04 and 20W, 106 <0.01-0.01 <0.01-0.01 6
0.08% 50W,
50SC 0.009 0.003 1
4/ Untreated
2 0.02, 20W, 98 <0.01-0.01 <0.01-0.01 9
0.04 and 50W,
0.08% 50SC
untreated 0.003 0.008 1
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pear 14/ 0.02% 50SC 158 0.01 <0.01 1
(Conference) 14/ 0.04% 0.02 0.01 1
14/ 0.08% untreated 0.01 0.005 1
Mushrooms The Netherlands
1980 2 5 50SC 21 0.04-0.06 0.01-0.03 4
(50 g a.i./ <0.05 av.) (<0.02 av.)
100 m2)
2 7.5 50SC 21 0.08-0.12 0.01-0.04 4
(75 g a.i./ (0.1 av.) (0.02 av.)
100 m2)
2 10 50SC 21 0.11-0.14 0.02-0.06 4
(100 g a.i./ (0.13 av.) (0.04 av.)
100 m2)
Untreated <0.01-0.02 <0.01-0.1 4
(0.01 av.) (0.01 av.)
U.K. g a.i./100 m2
1981 wall spray5/
1 52.2 50SC 69 N.D.6/ <0.02 1
untreated 69 N.D. N.D. 1
1 20 80W 33 N.D. N.D. 1
untreated 33 0.001 N.D. 1
1 48 80W 41 <0.02 0.02 1
untreated 41 0.04 0.02 1
(g a.i./100 m2
wall spray 5/)
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Mushrooms U.K. 1 20 80W 26 <0.02 <0.02 1
1981 untreated 26 N.D. N.D. 1
1 20 80W 33 <0.02 <0.02 1
untreated 33 N.D. 0.003 1
1 20 80W 52 N.D. <0.02 1
20 80W 60 0.04 0.02 1
untreated 52 N.D. N.D.
48 80W 42 0.06 0.1 1
48 80W 42 0.09 0.13 1
48 80W 42 0.08 0.10 1
1/ Residue results corrected for mean analytical recovery of 82% with fortification at 0.08-0.2 mg/kg.
2/ Results corrected for recoveries. Harvest (127 days after planting) said to be 2-3 weeks after normal harvest.
3/ SC = Suspendable concentrate.
4/ 1.5 1 spray solution per tree.
5/ Mushroom plots established the day after wall treatment.
6/ N.D. = no detectable chromatographic peaks.
Legume Vegetables
Peas
Supervised residue trials were conducted in the U.K. with single
applications at rates consistent with recommended ones. No information
was available on specific nationally approved good agricultural
practices, nor was information available on recommended numbers of
applications or preharvest intervals.
Peas were sampled at 2-3 weeks after normal harvest (127 days
after planting). At all application rates tested, residues of either
bendiocarb or NC 7312 (corrected for recovery) were <0.01 mg/kg in
peas, including untreated controls (Table 5). The same is true for
bendiocarb in pea pods, but residues of NC 7312 ranged from
0.03-0.08 mg/kg (0.05 mg/kg av.), and untreated controls were 0.02 and
0.05 mg/kg. Although bendiocarb residues were at or about the limit of
determination, the data suggest that terminal residues of NC 7312 may
be 2-10 times that of bendiocarb.
Analytical recoveries averaged 86 and 72% for bendiocarb and NC
7312, respectively, in peas from fortification levels of 0.04 and
0.2 mg/kg. Recoveries in pods averaged only 58 and 63%, respectively.
The limit of determination was said to be 0.01 mg/kg for bendiocarb
and NC 7312 in both peas and pods. However, based on untreated
controls, 0.05 mg/kg may be a more appropriate limit of determination
for NC 7312 in pea pods.
Because GAP information is unavailable and samples were not
harvested at normal harvest, a maximum residue level cannot be
estimated with confidence.
Pome Fruit
Apples and Pears
Residue data were available from supervised trials in the U.K. at
application rates of 0.02-0.08% active ingredient (1.5 1/tree),
representing several formulations. Applications were at pre- or
post-blossom and/or at codling stage, and fruit was sampled at normal
harvest of 57-158 days after last treatment. No information was
available on specific nationally approved agricultural practices.
Recommended applications rates of 0.25-0.5 kg/ha are not directly
comparable to the 0.02%-0.08% used in the field trials. No information
was provided on recommended preharvest intervals or numbers of
applications.
Of the 24 apple and pear samples treated in three locations and
in untreated controls, residues of bendiocarb and NC 7312 were
< 0.01 mg/kg, except for one 0.02 mg/kg value for pears treated
once with a 0.08% 50 SC formulation (Table 5). Mean recoveries of
bendiocarb and NC 7312 on apples and pears were 94 and 73%,
respectively, when fortified from 0.1 to 0.4 mg/kg. The limit of
determination is said to be 0.01 mg/kg for both bendiocarb and
NC 7312.
If the formulations, application rates, number of applications
and preharvest intervals of the field trials represent GAP, the data
could support a maximum residue level of 0.02 mg/kg for bendiocarb per
se or 0.05 mg/kg for combined residues of bendiocarb and NC 7312.
Cereal Grains
Barley
Fourteen supervised trials were conducted in the U.K. with
in-furrow granular, 50W spray or 80W seed dressing applications. No
nationally approved agricultural practice information was provided,
although recommended uses for cereals were reported (Table 2). No
recommended uses for the granular formulation (2 trials) were
provided. Only two of the trials were from spray treatments and the
remainder were from seed treatments. The spray applications at 0.5 and
1.0 kg a.i./ha reasonably reflect the recommended 0.75 kg a.i./ha
although the data are limited (Table 6) (Browne and Reary 1980c,d,e;
Housden and Reary 1981; Reary 1978 h,i,j,n; Reary 1979e,f,g,j,k,l,m,o,
1980a,e; Reary and Browne 1978). The seed treatments at 2-5 g a.i./kg
seed reflect the recommended rate of 2-5 g a.i./kg.
Residues of bendiocarb at normal harvest in barley grain were
<0.02 mg/kg and <0.03 mg/kg in straw. Untreated controls in
barley grain and straw were at the same level. Some samples were
also analysed for combined conjugated metabolites, NC 7312 and
N-hydroxymethylbendiocarb. In all three trials, metabolite residues
were <0.02 mg/kg. Mean analytical recoveries for bendiocarb in
barley during the supervised trials were 94 and 84%, respectively, for
grain and straw when fortified at 0.02-0.2 mg/kg. In a separate study,
bendiocarb recoveries in barley grain were 61-125% (av. 94%) after
fortifications at 0.02-0.2 mg/kg (Reary 1979b).
Table 6. Bendiocarb Residues in Cereals from Supervised Trials
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
Barley, wheat and oats
U.K. 1 1 80W 4 g/kg seed Seed dressing 148 barley grain <0.02
1977 1 1 148 barley straw <0.02
1 2 80W Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
1 2 50SC 4 g/kg seed Seed dressing 148 barley grain <0.02
1 2 148 barley straw <0.02
1 2 50SC Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
U.K. 3 3 80W 4 g/kg seed Seed dressing 113-145 barley grain <0.02
1978 2 2 113-145 barley straw <0.03
2 2 124-137 wheat grain <0.02
1 1 124-137 wheat straw <0.06
3 3 (10 rep) 134-141 oat grain <0.02
2 2 (10 rep) 134-141 oat grain <0.02
2 2 80W 8 g/kg seed Seed dressing 136-147 oat grain 0.47
3 2 136-147 oat straw 0.02-0.05
U.K.1/ 1 1 3G2/ 0.5 kg/ha In furrow 148 barley grain <0.01
1979 1 1 148 barley straw <0.01
1 1 3G2/ 1.0 kg/ha In furrow 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 50W2/ 0.5 kg/ha Spray 148 barley grain <0.01
148 barley straw <0.01
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 50W2/ 1.0 kg/ha Spray 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 80W 2 g/kg seed Seed dressing 336 wheat grain <0.01
1 1 336 wheat straw <0.01
3 3 80W 4 g/kg seed Seed dressing 119-148 barley grain <0.01
119-148 barley straw <0.01-0.02
3 3 127-336 wheat grain <0.01
2 2 127-336 wheat straw <0.01
2 2 105-125 oat grain <0.012
2 2 105-125 oat straw <0.01,<0.03
2 2 80W 5 g/kg seed Seed dressing 119-127 barley grain <0.01
2 2 119-127 barley straw <0.01,<0.02
1 1 127 wheat grain <0.01
1 1 127 wheat straw <0.01
2 2 105-125 oat grain <0.01
2 2 105-125 oat straw <0.01
Maize harvest samples
Canada 22 37 10G 0.84-2.24 band 153-189 grain <0.02
U.S. 8 8 kg/ha at 132-165 cob <0.02
1977-78 21 38 plant 132-189 stover3/ <0.03
10 28 110-134 silage <0.023
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
German 25 30 3G 0.2-0.8 in furrow 133-184 grain <0.024/
Fed. Rep. 26 36 kg/ha at plant 128-184 cobs <0.024/
5 12 128-184 plants <0.01
U.K. 10 10 146-184 foliage <0.01
France 5 5 135-164 silage <0.02
Italy 133-154 stover <0.02
1976-79
France 30 36 76 or 2-16 g/kg seed 125-189 grain <0.035/
U.K., U.S. 30 24 80W seed treatment 160-161 cob <0.026/
1975-1979 18 23 125-167 silage <0.02
11 10 160-189 stover <0.02
14 8 141-164 plants <0.056/
Rice (rough) harvest samples
Philippines7/ 1 1 5G 1.5 + 0.5 Incorporation + 53 grain <0.01
1978 surface applied straw <0.02
1 1 5G 2.0 + 0.6 Incorporation + 53 grain <0.01
surface applied straw <0.02
1 1 50W 0.5 kg/ha Spray 90 grain 0.01
straw <0.02
1 1 50W 0.6 kg/ha Spray 90 grain <0.01
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
2 2 80W 0.5 kg/ha Spray 60-90 grain <0.01
straw <0.02
2 2 80W 0.6 kg/ha Spray 60-90 grain <0.01
straw <0.02
Philippines7/ 1 1 50W 0.5 kg/ha Spray 248/ grain <0.01
1979 straw <0.01
1 1 50W 0.7 kg/ha Spray 248/ grain <0.01
straw 0.01
1 1 50W 1.0 kg/ha Spray 248/ grain 0.01
straw <0.01
1 1 50W 0.5 kg/ha Spray 459/ grain 0.01
straw -
1 1 50W 0.75 kg/ha Spray 459/ grain <0.01
straw -
1 1 50W 0.5 kg/ha Spray 4910/ grain <0.01
straw <0.01
1 1 50W 0.75 kg/ha Spray 4910/ grain 0.01
straw 0.02
South Korea7/ 1 1 50W 0.75 kg/ha Spray 10 grain 0.6, 1.111/
1979 straw <0.6
1 1 2D 0.8 kg/ha Dust 10 grain 1.5, 1.411/
straw <0.65
1 1 3GW 0.9 kg/ha Seed box 119 grain 0.06, 0.03
incorporation straw 0.02
(100 g/box)
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 5G 1.5 kg/ha Soil 100 grain <0.6
incorporation straw 0.04
Philippines
1980 1 1 50W 0.25 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.50 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.75 kg/ha Spray 6212/ grain <0.02
straw <0.01
1/ Also analysed for combined residues of conjugated NC 7312 and N-hydroxymethylbendiocarb. All metabolite residues were
< 0.02 mg/kg except for residues of 0.07 and 0.05 mg/kg from one trial in oat grain from seed dressing of spring oats
with an 80 WP formulation at 4 and 5 g a.i./kg seed. The two residues resulted from oat grain samples with bendiocarb
residues 0.012 and 0.007 mg/kg respectively. Untreated controls were 0.011 mg/kg.
2/ Seed previously treated with an 80% WP formulation
3/ Stover = leaves and stems after removal of cobs.
4/ Analysis of oil from kernels with <0.02 mg/kg residue from one site showed no residue greater than the 0.02 mg/kg limit
of determination.
5/ The 0.03 mg/kg on grain resulted from treatments at 8 and 12 g a.i/kg seed. At <4 g a.i./kg seed residues were
<0.02 mg/kg in grain.
6/ Maize (sweet corn) comprised 14 of the trials at 2-8 g a.i./kg. All plants in this group were sweet corn. The 0.05 mg/kg
residue on sweet corn plants resulted from 2-4 g a.i./kg seed.
Table 6 (con't)
7/ Also analysed for metabolite NC 7312. No residues above the limits of detection (0.01 mg/kg in grain and 0.02 mg/kg in
straw) were found, except for 0.11 and 0.17 mg/kg in grain from dust and spray treated samples at the 10-day interval and
one 5G formulation at 100 days, in which it was 0.03 mg/kg. In the latter, the control was also 0.03 mg/kg.
8/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 17, 27, 39, 52,
66 and 80 days after transplanting.
9/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 10, 25 40 and
55 days after transplanting.
10/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 7, 20 and 45
days after transplanting.
11/ Values reflect residue determination by electron capture and fluorescence, respectively.
12/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 22, 35, 51 and
68 days after transplanting.
The limit of determination was said to be 0.01 mg/kg for both
cereal grain straws and grains, although apparent residues in controls
(<0.02 and <0.03 mg/kg in barley grain and straw, respectively)
suggest 0.02 or even 0.05 mg/kg may be a more realistic estimate for
both. When considered with data from other cereal grains and straws, a
0.05 mg/kg limit for bendiocarb per se at or about the limit of
determination could be supported if recommended uses represent GAP. A
0.1 mg/kg limit would be appropriate if both bendiocarb and
metabolites are included.
Maize
Over 100 supervised trials were conducted in six countries, two
in North America and four in Europe (Table 6). Applications were made
with granular formulations at planting, either by band or in-furrow
treatment and seed was treated with wettable powder formulations.
In-furrow applications of a 3G formulation at 0.2-0.8 kg a.i./ha
corresponds to the 0.3-0.4 kg a.i./ha Netherlands GAP, although no
data were available from The Netherlands. The recommended uses on
maize provided to the Meeting did not include the method of
application for granular formulations.
Granular band treatments at planting of 0.84-2.24 kg a.i./ha did
not correspond with certainty to either The Netherlands GAP or
recommended usage for maize. The 2-16 g a.i./kg wettable powder seed
treatments more than accommodate the 2-4 g a.i. GAP of The Netherlands
or recommended application rates. Additional information on official
national GAP is needed.
Maximum residues of bendiocarb per se from all treatments were
<0.03, <0.02, <0.023, 0.05 and <0.01 mg/kg in grain, cobs,
stover, silage, plants and foliage, respectively. Analysis of oil from
one grain sample with field-incurred residues of <0.02 mg/kg also
showed residues of less than the 0.02 mg/kg limit of determination.
The 0.03 mg/kg level in grain resulted from applications at 2-3 times
the recommended rate. At recommended rates all grain residues were
<0.02 mg/kg. The 0.05 mg/kg residue on plants was on maize (sweet
corn) treated according to recommended rates.
The limit of determination was said to be 0.01-0.02 mg/kg for the
various maize fractions. Apparent residues in untreated controls were
also in this range. Analytical recoveries during the trials averaged
approximately 81, 78, 72, 80 and 78% for grain, cob, silage, stover
and plants, respectively. A 0.05 mg/kg limit at or about the limit of
determination could be supported for bendiocarb per se in maize (field
corn) grain, fodder and/or forage. Additional data would be required
for inclusion of metabolites.
In addition to the trials at harvest, 12 residue decline trials
were conducted in four countries (FBC 1982a; Browne and Reary 1979d;
Reary 1978l,m, 1979p, 1980h,i), mostly from granular in-band or
in-furrow treatments at planting ranging from 0.3-2.24 kg a.i./ha.
Residues in grain from granular treatments were <0.01 mg/kg. In
plants, residues ranged from <0.05-0.9 mg/kg at 25-40 days after
treatment (0.9 at 29 days), declining to <0.07 mg/kg after 49 days
and <0.02 after 56-70 days. Residues in stover and cobs were <0.02
and <0.02 mg/kg, respectively, after 71 days.
From the single 4 g a.i./kg seed treatment with a wettable powder
formulation, residues on plants were 12.4 mg/kg 29 days after
treatment but rapidly declined to _0.023 mg/kg after 42 days.
Oats
Residue data were available for seven supervised trials in the
U.K. from 4-8 g a.i./kg seed 80W seed dressings (Table 6). No
information was provided on specific nationally approved practices.
At application rates approximating the 4 g a.i./kg seed
recommended application rate, residues in oat grain at normal harvest
were 0.02 mg/kg and in straw were _0.02 mg/kg. One of the two grain
samples treated at 8 g a.i./kg seed had a residue of 0.47 mg/kg.
Contamination of this sample and its 0.12 mg/kg untreated control was
suspected by the manufacturer to have occurred during sampling.
Maximum residues in straw from the 8 g a.i/kg seed application rate
were 0.05 mg/kg. While this could be aberrant, as the untreated
control was 0.12 mg/kg, it is not necessarily so, since residues are
as high as 0.03 mg/kg in straw of oats and barley from recommended
application rates. Residues in untreated controls were <0.02 and
<0.04 mg/kg in grain and straw, respectively, except for 0.12 mg/kg
from a grain and straw sample with suspected contamination.
A limited amount of residue data were also available for combined
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) in some
oat samples, with residues being <0.012 mg/kg and <0.01 mg/kg in
grain and straw, respectively.
Mean recoveries of bendiocarb were 89 and 94% for oat grain and
straw, respectively. The limit of determination was said to be
0.02 mg/kg for both, but based on the 0.035 mg/kg apparent residue in
untreated controls, 0.05 mg/kg may be more appropriate, especially
when barley and wheat are also considered. A 0.05 mg/kg limit at or
about the limit of determination could be supported for bendiocarb per
se if recommended uses represent GAP. A 0.1 mg/kg limit would be
required if both bendiocarb and metabolites were included.
Rice
Residue data (corrected for mean recoveries) were available from
22 supervised trials on rough rice at normal harvest in The
Philippines and South Korea (Table 6). Applications were by granular,
spray and dust formulations. No information was available on
nationally approved agricultural practices on rice. Recommended
application rates for rice are 2 and 0.5 kg a.i./ha for granular and
spray formulations, respectively, and 0.5-0.75 kg a.i./ha for dust, as
summarized in Table 2. No information was provided on approved
intervals from last application or maximum number of applications.
Only one trial utilized a dust formulation, and the application
rate reflects the recommended use. Bendiocarb residues were 1.5 mg/kg
and 0.65 mg/kg, respectively, for grain and straw 10 days after
application as compared to 0.6-1.1 mg/kg for a spray application after
the same interval. Residues for NC 7312 were 0.17 mg/kg at the same
10-day interval. Untreated controls were <0.3 mg/kg and 0.04 mg/kg,
respectively, for grain and straw.
Spray applications of 0.25-0.75 kg a.i./ha covers the 0.5 kg/ha
recommended rate. As in the case of dust treatments, the spray
applications at 10 days before normal harvest resulted in higher
residues than at longer intervals, measuring <1.1 and <0.5 mg/kg,
respectively, for grain and straw. Similarly, residues were higher for
NC 7312 in grain, being 0.11 mg/kg. The same control as for the 10-day
interval dust treatment applies.
At longer intervals from last treatment with 50W or 80W
formulations (24-90 days) and up to 1.5 times the recommended rate,
residues were <0.02 mg/kg for both grain and straw. Untreated
controls for spray treatments, except for the 10-day interval
treatment, were <0.021 and <0.02 mg/kg, respectively, for
bendiocarb in grain and straw, except for one untreated straw sample
at 0.04 mg/kg. Residues of NC 7312 were <0.03 mg/kg and 0.02 mg/kg
for grain and straw, respectively, from spray treatments.
Four trials were conducted with either 3G or 5G formulations at
rates reflecting the recommended application rate and different
application methods. Maximum residues of bendiocarb were <0.6 mg/kg
and 0.04 mg/kg, respectively, in grain and straw. Untreated controls
were <0.07 and <0.04 mg/kg bendiocarb in grain and straw,
respectively. Residues of NC 7312 were <0.03 and <0.02 mg/kg in
grain and straw, respectively.
Analytical recoveries of bendiocarb during the trials averaged 77
and 68%, respectively, in rice grain and straw from fortifications at
0.02-0.25 mg/kg. Average recoveries were less (64 and 56%) for NC
7312. In a separate study on recoveries (Browne and Reary 1979b) at
similar fortification levels, mean recoveries were better, being 86
and 74% for bendiocarb in grain and straw, and 70 and 56%,
respectively, for NC 7312.
In the absence of GAP information, 2 mg/kg and 1 mg/kg limits
would be required for bendiocarb per se in rice grain and straw,
respectively, to accommodate the uses of the supervised trials.
Wheat
Residue data, corrected for recoveries, were available from eight
supervised trials in the U.K. from seed treatments with 80W or 50SC at
2-5 g a.i./kg seed (Table 6). No information was available on specific
nationally approved agricultural practices, although the application
rates used reflect the 2-4 g a.i./kg seed recommended rate for
cereals.
Residues for bendiocarb at harvest were <0.02 mg/kg for both
grain and straw, except for one 0.06 mg/kg value for straw. This
0.02 mg/kg level is approximately the claimed limit of determination.
Untreated controls were <0.04 mg/kg and <0.12 mg/kg,
respectively, for grain and straw. The 0.12 mg/kg for straw is from
the same study in which the high treated sample residue was found.
Contamination was suspected. Other values were <0.02 mg/kg.
Analytical recoveries from the individual studies were relatively
low, averaging only 63-65% for grain and straw at fortification levels
of 0.02-0.2 mg/kg. In a separate determination (Reary 1979b)
recoveries of bendiocarb in grains were better, averaging 84%. The
limit of determination for barley, oats and wheat is said to be
<0.02 mg/kg for grain and straw. Overall data support a 0.05 mg/kg
limit for bendiocarb per se.
Limited data were also available for combined conjugated residues
of NC 7312 and N-hydroxymethylbendiocarb. Residues were <0.01 and
<0.12 mg/kg for wheat grain and straw, respectively, although
analytical recoveries were only 62 and 67% in cereal grains and straw,
respectively.
Fodders and Straws
Fodders and straws of cereal grains have been discussed
previously. The other member of this group for which data were
provided is grass fodder, or more specifically turf and grass. Data
were available from 72 supervised trials in the U.K., U.S. and
Australia from 1975-1980, representing a variety of formulations,
single or multiple applications at several rates, intervals, and
methods of application as well as irrigated and unirrigated conditions
(Table 7) (Browne 1981d; FBC 1982c; Reary 1975d, 1978f,g, 1980b,d,e,k,
1981m; Reary and Whiteoak 1977a,b,c,d). No information was available
on specific national agricultural practices, although recommended
rates for rye grass is given in Table 1.
Granular treatments, both single and multiple broadcasts, were
mostly at application rates 6-12 times the recommended rate (for rye
grass). Three granular broadcast trials (single applications) at 1.6
times the recommended rate resulted in maximum residues ranging from
0.24 mg/kg at 43 days after treatment to 0.09 mg/kg after 55 days.
Residues were <0.1 mg/kg from three granular seed mix trials (single
application) at 0.6-1.4 times the recommended granular application
rate when analysed 196 days after treatment.
As in the case of granular treatments, single or multiple spray
treatments were mostly at rates 6-12 times the maximum recommended
application rate for rye grass. After single applications at rates
approximating recommended rates (1-1.6x), maximum residues from spray
treatments ranged from 103 mg/kg (193 mg/kg dry basis) at day of
application to 0.03 mg/kg after 141 days.
No recommended rates were available specifically for ULV aerial
applications. Residues from a single application at 0.28 kg/ha ranged
from 50 mg/kg (111 mg/kg dry basis) on day of application to 5.5 mg/kg
(12 mg/kg dry basis) after 29 days.
Seed dressing trials were conducted at treatments rates of 1-4
times the recommended rate of 10 g a.i./kg seed. Residues were
<0.2 mg/kg at all rates after 43 days.
Although many of the treatments with the various formulations
were at exaggerated application rates, these trials did permit
estimations of decline rates, the potential of residue accumulation
and effect of irrigation on residues. Residue half-lives were greater
for granular broadcast applications, for which 37-42 days was
estimated. For spray application half-lives ranged from 2-15 days,
depending on the trial. The half-lives were 5.3 and 2.4 days for seed
treatments and aerial ULV treatments, respectively. In general,
irrigation did not significantly affect decline rates from granular
broadcast or wettable powder spray treatments as compared to rates
measured under non-irrigated conditions. Residues did not accumulate
from multiple applications at 28-day intervals.
Table 7. Bendiocarb Residues in Grass and Turf from Supervised Trials
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 1 - 80W 1 kg/ha Spray Rye grass <1 130 1
1975 1-5 <109 3
6-10 <40 2
11-20 <15 2
21-50 <7 3
U.S. 1* 4.3 76W 2.24 kg/ha Spray Turf grass <1 <236 6
1976 1-5 <225 6
6-10 <115 4
11-20 <19 2
1* Thatch <1 <25 6
1-5 <26 6
6-10 <9 4
11-20 <1 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <326 6
1-5 <386 6
6-10 <190 4
11-20 <46 2
1* Thatch <1 <36 6
1-5 <97 6
6-10 <79 4
11-20 <1.6 2
1* 2-4 76W 2.24 kg/ha Spray Turf grass <1 <293 5
1-5 <201 4
6-10 <4 4
11-20 <2.8 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1* Thatch <1 <19 5
1-5 <13 4
6-10 <16 4
11-20 <21 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <607 5
1-5 <710 4
6-10 <30 4
11-20 <7.5 2
Thatch <1 <56 5
1-5 <18 4
6-10 <32 4
11-20 <26 2
1* 5G 2.24 kg/ha Broadcast Turf grass <1 <1.4 5
1-5 <2.7 4
6-10 <0.6 4
11-20 <0.3 2
1* Thatch <1 <13 5
1-5 <11 4
6-10 <18 4
11-20 <17 2
1* 5G 4.48 kg/ha Broadcast Turf grass <1 <3 5
1-5 <6 4
6-10 <8 4
11-20 <0.7 2
1* Thatch <1 <34 5
1-5 <42 4
6-10 <47 4
11-20 <27 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1977 2* 5-15* 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
21-50
51-80
81-110
111-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 5-15 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
11-20
21-50
51-80
81-110
110-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 76W Multi-spray2/ Turf grass 1-5 <16 3
6-10 <16 2
11-20 <9 2
21-50 <2 2
51-80 <0.9 2
81-110 <0.3 2
111-140 <0.1 2
>141 0.1 1
2* 76W Multi-spray3/ Turf grass 1-5 <24 2
6-10 <2 2
11-20 <3 2
21-50 <1.7 2
51-80 <1.1 2
81-110 <0.3 2
111-140 0.1 1
2* 76W Multi-spray4/ Turf grass 1-5 <37 4
6-10 <2.5 2
11-20 <3.4 2
21-50 <1 2
51-80 <0.4 2
81-110 0.1 1
2* 76W Multi-spray5/ Turf grass 1-5 <80 4
6-10 <80 4
11-20 <20 2
21-50 <3.7 2
51-80 0.5 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 37-42 5G Broadcast6/ Turf grass 1-5 <8 5
6-10 <3.3 2
11-20 <1.9 2
21-50 <3.7 2
51-80 <8.1 2
81-110 <2.6 2
111-140 <1.6 2
>141 <0.5 2
2 5G Broadcast1/ Turf grass 1-5 <7 5
6-10 <7 2
11-20 <1.4 2
21-50 <2.8 2
51-80 <0.16 2
81-110 <0.7 2
111-140 <0.55 2
>141 0.16 1
2 5G Broadcast2/ Turf grass 1-5 <9.5 4
6-10 <4.7 2
11-20 <4.4 2
21-50 <3.5 2
51-80 <0.7 2
81-110 <0.7 2
111-140 <0.7 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 5G Broadcast3/ Turf grass 1-5 <1.0 2
6-10 <9.0 2
11-20 <8.0 2
21-50 <4.0 2
51-80 <2.0 2
81-110 <0.8 2
111-140 0.8 1
2 5G Broadcast4/ Turf grass 1-5 <9.1 4
6-10 <8.2 2
11-20 <7.4 2
21-50 <7.6 2
51-80 <3.9 2
2 5G Broadcast5/ Turf grass 1-5 <7.3 4
6-10 <6.1 2
11-20 <5.7 2
21-50 <2.8 2
Australia 1 80W 1.6 g/kg seed Seed dressing Grass (pasture) 81-110 <0.02 2
1979 111-140 <0.02 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 3 80W 10 g/kg seed Seed dressing Grass (grazing) 21-50 0.09 1
1979 51-80 <0.06 2
3 80W 40 g/kg seed Seed dressing Grass " 21-50 0.17 1
51-80 <0.11 2
3 3G 1.2 kg/ha Broadcast Grass (grazing) 21-50 0.24 1
51-80 <0.09 2
3 50SC 1.2 kg/ha Spray Grass (grazing) 21-50 <0.2 3
2 50SC 10 g/kg seed Seed dressing Grass (grazing) >141 <0.01 3
1 50SC 15 g/kg seed Seed dressing " " >141 0.01 1
2 50SC 20 g/kg seed " " " " >141 <0.01 3
2 50SC 0.5 kg/ha Spray Grass (grazing) >141 <0.01 3
1 50SC 0.75 kg/ha Spray " " >141 0.03 1
2 50SC 1.0 kg/ha Spray " " >141 <0.015 3
U.S. 1 6 50SC 0.84 kg/ha Spray Wheat grass <1 103 1 193
1979 1-5 <97 2 <139
6-10 26 1 34
11-20 16 1 18
21-50 <9.3 2 <16
U.K. 2 5.3 80W 10 g/kg seed Seed Dressing Grass (grazing) 51-80 <0.02 2
1980 2 80W 15 g/kg seed Seed dressing " " 51-80 <0.03 2
2 80W 20 g/kg seed Seed dressing " " 51-80 <0.1 3
1 50SC 5×0.5 kg/ha Spray Grass (grazing) 51-80 <0.03 6
81-110 0.01 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 50SC 0.75 kg/ha Spray Grass (grazing) 51-80 <0.02 2
2 50SC 1.0 kg/ha Spray Grass " 51-80 <0.03 2
1 5G 0.75 kg/ha Seed mix Grass (grazing) 51-80 <0.08 2
1 3G 0.45 kg/ha Seed mix Rye Grass >141 <0.1 2
1 5G 0.75 kg/ha Seed mix Rye grass >141 <0.1 2
1 7G 1.05 kg/ha Seed mix Rye grass >141 <0.1 1
U.S. 1 2.4 25ULV 0.28 kg/ha Aerial Spray Rangeland grass <1 50 1 111
1980 1-5 5.36 2 <73
6-10 1.7 1 4
11-20 0.7 1 2
21-50 5.5 1 12
* Trials irrigated and non-irrigated. Residue levels maximum found.
1/ Rate of 4.48 kg/ha.
2/ Multi-application programme of 4.48 + 2.24 kg/ha, 28 days apart.
3/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha each 28 days apart.
4/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha, each 28 days apart.
5/ Multi-application programme of 4.48 + 2.24 + 2.24 + 4.48 kg/ha, each 28 days apart.
6/ Rate of 8.96 kg/ha.
Apparent bendiocarb in untreated grass controls were generally
<0.2 mg/kg, although they occasionally approached 1 mg/kg and were
1.8 mg/kg in one sample. The limit of determination was said to be
0.01-0.1 mg/kg, depending on the trial. Based on untreated controls,
1 mg/kg may be a more realistic limit of determination for grass.
Analytical recoveries during the trials ranged from 43 to 119% and the
mean for all the trials was approximately 80%.
Legume Animal Fodders
See discussion on pea pods under legume vegetables.
Oilseeds
Bendiocarb residue data on oilseed rape resulting from supervised
trials were available from one country where applications of a 5G
formulation was applied at sowing with the seed at application rates
of 0.140 and 0.28 kg a.i./ha which is the recommended rate for
granular formulations (Table 2) (Browne and Reary 1980f). No
information was available on specific nationally approved agricultural
practices.
Nine replicates were analysed for bendiocarb per se on mature
seed (114 days after application) at each rate with residues
<0.006 mg/kg. Samples were not analysed for conjugates of NC 7312
and N-hydroxymethylbendiocarb, although a metabolism study has shown
these to be the major metabolites in rape seed (see section on "Fate
of Residues in Plants"). Residues in untreated controls were also
<0.006 mg/kg with mean analytical recoveries of 84% for
fortifications at 0.05-0.5 mg/kg.
These data are inadequate for estimating a maximum residue level.
Additional data from granular applications as well as data from spray
treatments reflecting GAP are needed, as well as information on
nationally approved uses, including preharvest intervals and number of
applications.
Meat
Cattle
Residue data available from two feeding studies and two dermal
treatment studies are given in Table 8 (Browne and Reary 1978a,b;
Reary 1978a, 1979a, 1980c).
Table 8. Bendiocarb Residues in Milk and Cattle Tissues after Feeding Trials or Dermal Treatments1/
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
Milk
(non-conjugated2/
residues) <0.01 <0.01 <0.01 <0.01 <0.01 0.014 0.005 0.015 0.06 <0.02 <0.04
Milk
(conjugated3/ <0.02 <0.02 <0.02 <0.02 <0.02 0.027 0.027 0.039 0.057 <0.02 <0.02
residues)
Muscle4/ <0.02 <0.02 <0.02 <0.02 <0.02 - - - - <0.01 <0.01
Omental fat4/ <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.03
Perirenal fat4/ <0.05 <0.05 <0.05 <0.05 <0.02 <0.05 <0.04
Subcutaneous-
muscle4/ <0.01 <0.01
fat4/ <0.15 <0.12
Table 8. (con't)
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
liver4/ <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
kidney4/ <0.1 <0.1 <0.1 <0.1 <0.1 <0.05 <0.11
1/ Corrected for mean recoveries;
2/ Bendiocarb and non-conjugated NC 7312 and N-hydroxymethyl bendiocarb (fat fraction);
3/ Total conjugated NC 7312 and N-hydroxymethyl bendiocarb (aqueous or lactose fraction);
4/ Total residues of bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb;
5/ Analysed 6 hours post-treatment;
6/ 7-21 day self-treatment. Treatment rate estimated to be approximately 5 g product/cow/day.
In a feeding study (Browne and Reary 1978a), lactating dairy cows
were fed for a minimum of 28 days with a diet containing 0, 0.25, 0.75
and 2.5 mg/kg bendiocarb. A maize premix was the vehicle for adding
bendiocarb to the diet twice daily. Premises were stored at -4°C prior
to use. Each treatment group consisted of two cows. Milk samples were
pooled for both cows in each treatment group over 24 hours. Samples
were taken before treatment and after 1, 2, 4, 8, 12, 16, 22 and 28
days on the bendiocarb fortified diet. Milk extracts were separated
into a fat fraction (hexane/ether soluble) and a lactose fraction
(water soluble), the first of which was analysed for total
non-conjugated residues of bendiocarb, NC 7312 and
N-hydroxymethylbendiocarb. The lactose fraction was analysed for total
conjugated residues. Residues (bendiocarb equivalent in milk) in all
fat samples were less than the estimated 0.01 mg/kg limit of
determination, corrected for mean recoveries of 64%. Residues in the
lactose fraction were also <0.01 mg/kg, but the limit of
determination was considered 0.02 mg/kg because of chromatographic
interferences. Based on the chromatograms, 0.05-0.1 mg/kg may be a
more realistic limit of determination for the aqueous fraction when
interfering co-extractives are taken into consideration. In each case,
untreated controls gave no apparent residue above the estimated limits
of determination.
Samples of omental fat, perirenal fat, muscle, liver and kidney
were taken from each animal at sacrifice (within 18 hours of final
bendiocarb intake). Each was analysed for total residues of bendiocarb
and metabolites, conjugated and non-conjugated. No residue (corrected
for recovery) was found above the estimated limits of determination of
0.02 mg/kg for muscle and omental fat, 0.05 mg/kg for liver and
perirenal fat and 0.1 mg/kg for kidney. Muscle samples at the 0.25 and
0.75 mg/kg dietary feeding level did show apparent residues at the
0.02 mg/kg limit of determination as did one untreated control. Based
on representative chromatograms, the above estimated limits of
determination appear to be reasonable, except that a 0.2 mg/kg may be
more realistic for kidney.
Mean recoveries determined separately on unconjugated bendiocarb,
NC 7312 and N-hydroxymethylbendiocarb from cattle meat and fat tissues
were approximately 70%, with a standard deviation ranging from 10 to
22. The large standard deviations reflect rather poor recoveries,
especially of bendiocarb per se, (< approximately 50%) in a number
of muscle, kidney and liver samples. For example, recoveries of
bendiocarb averaged only 60% in muscle and kidney.
Another feeding study (Reary 1978a) was conducted in a similar
fashion but at higher dietary levels (7.5 and 25 mg/kg in the diet).
Maximum residues in milk were <0.01 mg/kg (limit of determination
for the fat soluble fractions) and <0.02 mg/kg for the aqueous
portion. As in the lower level feeding study, apparent residues of
total bendiocarb and conjugated and non-conjugated metabolites were
<0.02 mg/kg in muscle, <0.02 in omental fat and <0.05 mg/kg in
perirenal fat and liver. Residues were at the estimated 0.1 mg/kg
limit of determination for kidney (one control sample was also at this
level). This supports the view that 0.2 mg/kg is a more realistic
limit of determination for cattle kidney.
Analytical recoveries averaged approximately 70% with standard
deviations from 11-21%, reflecting rather poor recoveries. Average
recoveries were only 56% for bendiocarb in muscle and 52% for the
N-hydroxy metabolite in liver.
No information was provided on national GAP or recommended uses
for dermal applications to dairy or beef cattle. Results of
investigations for proposes uses for fly control by dermal
applications with a 1% bendiocarb dust formulation were provided. A 5g
dust treatment (50 mg a.i.) was described as a "normal" daily dose.
In one set of experiments (Reary 1979a) lactating dairy cattle
were manually treated at measured rates for safety evaluations or by
hessian bag self-treatment for efficacy evaluations. In the safety
evaluations, groups of three shorthorn cows were manually brushed on
the head, neck and shoulders with 0, 5, 15 and 25 g of the 1% dust
formulation; milk was analysed pre-treatment and six hours post-
treatment for each animal. As in the feeding studies, milk was
separated into fat and lactose fractions.
Non-conjugated residues in milk ranged from 0.003-0.015 mg/kg
(0.009 mg/kg mean) for all application rates, with no discernible
difference between application rates. Total conjugated residues ranged
from 0.019 to 0.039 mg/kg (0.028 mg/kg mean). Control means for the
safety evaluation trials were 0.006 and 0.032 mg/kg for non-conjugated
and conjugated residues, respectively. Limits of determination were
considered to be 0.02 and 0.05 mg/kg for the fat and aqueous
fractions, respectively.
In the efficacy trials, four herds were self-treated at an
estimated rate of 5 g of dust formulation/day/cow. Milk was pooled for
each herd at 7, 14 and 21 days after the beginning of treatment. For
the fat soluble non-conjugated fractions, residues were <0.017 mg/kg
at 7-21 days, except for one pooled milk sample from one herd at 7
days, for which a residue of 0.06 mg/kg was detected. Therefore, non-
conjugated residues did occur at and above the limit of determination.
Conjugated residues were <0.057 mg/kg, or just over the estimated
limit of determination. Samples used as untreated controls had mean
residues of 0.005 mg/kg and <0.028 mg/kg for fat soluble and
aqueous fractions, respectively.
In another dermal treatment investigation (Reary 1980c) groups of
three lactating dairy cows were manually treated on the head, neck and
back daily for seven successive days with a 1% dust formulation at 0,
5 or 25 g product/cow/treatment. Daily milk production from each of
two daily milkings for each cow was kept separate. Samples of muscle,
omental fat, perirenal fat, liver, kidney and subcutaneous fat were
taken within 18 hours after the final treatment.
Non-conjugated residues in milk (fat fraction) after correction
for analytical recoveries ranged from <0.01-0.04 mg/kg for 78
samples. All but six were <0.01 mg/kg. All residues >0.02 mg/kg
were from the higher treatment rate, except for one sample at
0.02 mg/kg. Comparable residues in 24 untreated controls were
<0.01 mg/kg, except for one sample at 0.02 mg/kg. All conjugated
residues (aqueous fraction) were <0.02 mg/kg and untreated controls
were <0.02 mg/kg. Mean analytical recoveries were 82% and 72% for the
milk fat and lactose fractions, respectively. The limit of
determination was considered to be 0.01 and 0.02 mg/kg for the fat and
lactose fractions, respectively.
In tissues, residues at both treatment levels were <0.01 mg/kg
in muscle, <0.03 mg/ kg in omental fat, <0.05 mg/kg in perirenal
fat, <0.01 mg/kg in subcutaneous muscle and <0.15 mg/kg (mean
0.08 mg/kg) in subcutaneous fat. Except for subcutaneous muscle, for
which there were no untreated controls, apparent residues in controls
for these tissues were _0.03 mg/kg, except for one 0.04 mg/kg sample of
subcutaneous fat. Residues from both levels were <0.05 mg/kg in
liver and <0.11 mg/kg in kidney. All residues >0.05 mg/kg in
kidney were from the higher treatment level. Apparent residues in
untreated controls were <0.03 mg/kg in liver and kidney.
An estimate of potential residues in meat, milk and meat by-
products must take into consideration all potential routes to animal
exposure, which include the dietary and dermal routes. Of the feed
crops for which residue data and good agricultural practice
information were provided, the greatest potential for residues in
cattle meat, fat and meat by-products and milk would be expected
from uses on maize (field corn). Assuming maximum residues are
<0.05 mg/kg in maize grain, forage and/or fodder, total conjugated
and non-conjugated residues of bendiocarb and metabolites would be
expected to be <0.05 mg/kg in cattle carcase meat, cattle fat and
cattle meat by-products (except kidney) and <0.01 mg/kg (claimed
limit of determination) in kidney, although a 0.2 mg/kg limit of
determination may be more realistic for kidney. Residues in milk would
not be expected to exceed the approximate limits of determination of
0.01 mg/kg and 0.02 mg/kg for non-conjugated and conjugated residues,
respectively, or a total of 0.03 mg/kg. These estimates should also be
adequate from feeding of rice.
Data from the use of bendiocarb on grasses or as a dermal
treatment on cattle suggests a possible need for higher limits than
would be required for maize grain, forage and fodder if such uses are,
or should become, good agricultural practice. Additional information
on good agricultural practices are necessary before maximum residue
estimates can be made for these uses. Any additional information
should provide the formulation, application rate, number of
applications, type of application and preharvest intervals or other
restrictions. Depending on these uses, feeding studies at higher
levels may be necessary before maximum residue levels can be
estimated.
Poultry meat, meat by-products and eggs
Laying hens in groups of ten were fed (free access) a diet
containing 0, 0.05, 0.15 and 0.5 mg/kg bendiocarb for 21 days to
permit estimation of potential residues resulting from the feeding of
maize grain, for which maximum residues of 0.05 mg/kg are assumed
(Reary 1978b). Owing to analytical difficulties, information on the
stability of residues over the feeding period was not provided.
Information was not provided on storage conditions of the fortified
pre-mix.
Eggs were individually collected and labelled; 98 were analysed
at higher feeding levels and later sampling intervals. Apparent total
residues (corrected for recovery) of bendiocarb and metabolites
(conjugated and non-conjugated) at all treatment levels were
<0.02 mg/kg 1-21 days after treatment. The limit of determination was
reported as approximately 0.02 mg/kg, supported by sample
chromatograms, although pre-treatment controls were <0.013 mg/kg.
Analytical recoveries of bendiocarb and unconjugated metabolites
ranged from 44-85% (67% av.).
Samples of breast muscle, leg muscle, liver, kidney, subcutaneous
fat and heart were taken after sacrifice on day 21 of feeding the
fortified diet, with completion of dissection the following day. The
sum of bendiocarb and its major metabolites conjugated and non-
conjugated, was determined. No hen tissue residues were found in
excess of the claimed limits of determination of 0.02 mg/kg for legs,
breast, liver and subcutaneous fat and 0.05 mg/kg for kidney and
heart. Sample chromatograms tend to confine these limits of
determination, although apparent residues near the claimed limits of
determination in untreated controls of breasts (<0.016 mg/kg),
liver (<0.014 mg/kg) and fat (<0.016 mg/kg) suggest that
0.05 mg/kg may be a more practical limit of determination for each of
the tissues.
As in cattle tissues and eggs, analytical recoveries were only
marginally acceptable, ranging from 40-98% for bendiocarb or its major
non-conjugated metabolites. Mean residues of bendiocarb and its non-
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) ranged
from only 56% in chicken legs to 79% in fat. Improved analytical
methodologies are needed for commodities of animal origin.
Mushrooms
Supervised trials were conducted in The Netherlands and the U.K.
In The Netherlands mushroom plots were presumably sprayed directly
with a bendiocarb 50SC formulation and in the U.K. the plots were
established the day following wall treatments with either a 50SC or
80W spray. No information was provided on approved agricultural
practices for these uses, including preharvest intervals, although
recommended wall spray application rates of 30-50 g/100 m2 (Table 2)
were provided.
In the first trials (Browne 1981a) a 50SC bendiocarb formulation
was sprayed twice at rates of 50, 75 and 100 g a.i./100 m2 (5, 7.5
and 10 kg a.i./ha), first after spawning and the second after casing.
Harvest was 21 days after the last treatment.
Residues of bendiocarb and non-conjugated NC 7312, corrected for
mean recoveries, are summarized in Table 5. Maximum residues were
0.013 mg/kg and 0.04 mg/kg, respectively, for bendiocarb and NC 7312
at the highest application rate. Residues increase with increased
application rate and residues of NC 7312 tend to be less than those of
bendiocarb under the test conditions.
In the series of trials in the U.K., (Browne, 1982a) mushroom
plots were established the day following hut wall treatments with
either 50SC or 80W bendiocarb formulations at application rates of
20-53 g a.i./100 m2. Harvest was 26-69 days after the treatments,
which reflected the recommended application rates. Bendiocarb residues
ranged from <0.02 to 0.09 mg/kg and NC 7312 from <0.02 to
0.13 mg/kg, both corrected for recoveries (see Table 5). In contrast
to the trials discussed above, residues of NC 7312 in these trials
were of the same order of magnitude as for bendiocarb.
The limit of determination for bendiocarb in mushrooms was
reported to be 0.01-0.02 mg/kg for bendiocarb or NC 7312, depending on
the trials. Based on apparent residues of 0.04 mg/kg of bendiocarb and
0.02 mg/kg of NC 7312 in more than one untreated control, limits of
determination of 0.05 mg/kg may be more practical. Analytical
recoveries averaged approximately 82% for bendiocarb and 73% for non-
conjugated NC 7312 from fortifications of both at 0.04-0.2 mg/kg,
although standard deviations were 14-19.
As no information was available even on recommended uses for
bendiocarb other than for wall treatments, data from wall treatments
are more pertinent to the estimation of maximum residue levels. Based
on recommended application rates, the available residue data are
consistent with a maximum residue level of 0.1 mg/kg for bendiocarb
per se and 0.2 mg/kg if NC 7312 is also included. This is based on the
assumption of minimum application to harvest intervals. Information on
nationally approved agricultural practices for bendiocarb use on
mushrooms is needed. Additional data from supervised trials are
desirable.
FATE OF RESIDUES
Metabolism studies have been carried out in a range of plants and
animals and in soil. In most plant species examined, the major
metabolites formed are glycoside conjugates of the phenol NC 7312 and
N-hydroxymethyl bendiocarb. Metabolism in animals generally involves
cleavage of the carbamate ester group to yield NC 7312, which is
excreted as sulphate and beta-glucuronide conjugates. In soil under
aerobic conditions, the only extractable radioactivity is unchanged
bendiocarb, degradation occurring to CO2, and an unidentified bound
residue. Under anaerobic conditions, bendiocarb is hydrolysed to
NC 7312.
In animals
For discussions on the metabolic fate of bendiocarb in the rat,
dog, hamster, rabbit, mouse and humans, see "Biochemical Aspects".
Residue feeding and/or dermal studies on cattle and/or poultry have
been discussed under "Residues from Supervised Trials". In addition to
the aforementioned studies, the metabolic fate of bendiocarb has been
investigated in the pig, cow and hen.
Pig
Pigs were orally dosed once by gelatin capsule at 0.2 mg/kg/bw
with 14C-bendiocarb (Adcock et al 1976b). Urine samples were
collected over a period of four days, after which the animals were
sacrificed and samples of liver, kidney, muscle, perirenal fat and
subcutaneous fat were taken.
Seventy-eight percent of the administered dose was excreted in
the urine as equal levels of sulphate and ß-glucuronide conjugates of
NC 7312 within one day and 84% within four days. No residues of
unchanged bendiocarb were detected. Corresponding residues in faeces
were 2.4 and 7.2% for a total recovery of 91.5% of administered
radioactivity recovered in urine and faeces after four days. The
residues in faeces were not identified. No residues were found in
tissues at the 0.01 mg/kg limit of sensitivity for bendiocarb
equivalents. The finding of relatively large levels of NC 7312
sulphate was of considerable interest, since phenolic sulphates are
generally minor in pigs.
The metabolism is similar to that in humans and the rat,
involving cleavage of the carbamate ester to yield NC 7312, which
becomes conjugated as sulphates and glucuronides.
Cow
A lactating dairy cow was given 14C-bendiocarb by a single
gelatin capsule at a dose of 0.2 mg/kg/bw (Adcock et al 1976a).
Urine was collected at intervals of 4-96 hours after dosing and faeces
every 24 hours. Milk was collected morning and evening until sacrifice
on the fourth day, at which time samples of liver, kidney, muscle,
heart, perirenal fat, omental fat and subcutaneous fat were taken for
analysis. Milk was separated into lactose, fat and casein fractions.
No residues of unchanged bendiocarb were found in the urine.
Seventy-nine percent of the administered radioactivity was excreted in
the urine within eight hours and 92% after two days, after which
little was excreted in urine. Corresponding residues in faeces were
5.3% at one day and 12.3% after two days. The urinary metabolites were
determined to be sulphate and glucuronide conjugates of NC 7312 at 90%
and 10% each, respectively. Residues in faeces were not identified.
Bendiocarb equivalents were found in milk the first day up to a
level of 0.002 mg/kg, but none were detectable thereafter. Over 80%
was in the lactose fraction, 2% in the hexane fraction and most of the
remainder in the casein fraction. Tissue residue levels were below the
0.01 mg/kg limit of sensitivity as bendiocarb equivalents.
Metabolism in the cow appears to be similar to that in the rat,
human and pigs.
Poultry
Three laying hens were each dosed once with 14C-bendiocarb by
oral gavage at 0.25 mg/kg/bw (equivalent to 5 mg/kg in the diet) and
excrement and eggs collected at intervals from 24-96 hours after
dosing. Liver, kidney, muscle and fat samples were taken at sacrifice
four days after treatment (Lewis and Challis 1978).
Sixty-one to 90% of administered radioactivity was excreted
within 24 hours, and 73-91% within 4 days. Recovery was inexplicably
low (73%) in two of the hens. Losses were presumed to be due to
expiration and/or losses during dosing or excrement collection, since
residues in tissues were low. However, as a percent of radioactivity
excreted in the first 24 hours, residues were 10-57% free NC 7312,
26-63% NC 7312 conjugates, 12-19% unidentified unhydrolysed water
soluble residues, 2-5% other non-polar metabolites and 1-3% other
unidentified conjugates. Conjugation of NC 7312 occurred more rapidly
in the hen in which radioactivity excretion was more rapid and
complete.
Radioactivity in egg white and yolk represented only 0.01% of the
administered dose. The maximum bendiocarb equivalent was highest in
egg white on the first day (max. 0.009 mg/kg) and on day four in yolk
(0.003 mg/kg), both above the limits of sensitivity. The maximum
bendiocarb equivalent was 0.004 mg/kg in liver and fat, 0.002 mg/kg in
kidney and below the 0.001 mg/kg limit of sensitivity in leg and
breast muscle.
In Plants
Metabolism studies have been carried out in sugarbeet, maize,
rice, barley and oilseed rape, both in the laboratory and under field
conditions. A variety of separative, determinative and conjugative
hydrolysis steps were utilized. In all these crops, the major
identified metabolite was the phenol NC 7312, with lower levels of
N-hydroxymethylbendiocarb, both primarily as glycoside conjugates. In
some of the crops studied, sampling at harvest showed small quantities
of unconjugated N-hydroxymethylbendiocarb.
Three metabolism studies were conducted in sugarbeets. In one
study, radioactive bendiocarb was applied under greenhouse conditions
to the potted soil or to the leaves by direct syringe applications
(Challis 1975). Residues were characterized in the plant tops only.
Metabolic products were qualitatively similar in both cases,
resulting in residues of free bendiocarb, bendiocarb glycoside,
free NC 7312 (foliar application only), NC 7312 glycosides,
N-hydroxymethylbendiocarb, N-hydroxymethylbendiocarb glycosides,
unidentified glycosides, unidentified water soluble residues and
unextractable unidentified fibre-bound residues.
The major metabolites from soil application were glycoside
conjugates of NC 7312 and N-hydroxymethylbendiocarb. Trace amounts of
free N-hydroxymethylbendiocarb were also found. Unidentified residues
accounted for 36 and 67% of radioactivity 8 and 34 days after
treatment, respectively, with a ratio of about 1:3 water soluble to
fibre-bound residues.
From foliar applications, over 93% of leaf-applied bendiocarb was
present on the leaf surface after 34 days. A bendiocarb glycoside was
the major metabolite. Unidentified residues accounted for 48% of the
total residue after eight days, but this was reduced to 25% after 34
days. Low levels of free NC 7312 were also present. The proposed
metabolic pathway for bendiocarb in sugarbeet tops is illustrated in
Figure 1. Except for the omission of substantial at-harvest
Molecular formula
C9H10O3
Other information on identity and properties
Molecular weight 166
State White crystalline solid
Melting point 91-93°C
Solubility At 25°C 20 g/l in water and
50 g/l in dichloromethane; very
soluble in methanol, ethanol and
acetone.
Vapour pressure 2.34 × 10-3mm Hg at 25°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
In groups of male mice (CFLP strain) acutely intubated with
14C-bendiocarb (14C-bendiocarb with label at the heteroclyclic ring
was used in this and all of the other available metabolism studies on
bendiocarb) at 1, 5 or 10 mg/kg body weight, over 80% of the
administered dose was excreted in 24 hours, primarily in the urine.
Less than 10% of the administered 14C was recovered in the faeces.
The proportion of metabolites formed in the urine, but not the rate
and route of elimination, was dose-dependent. Thus, the level of NC
7312 (2,2-dimethyl-1,3-benzoxodiol-4-ol) (as conjugates) recovered in
the urine was 36, 32, 17 (as % of administered radioactivity),
respectively, at 1, 5, 10 mg/kg. Only one urinary metabolite other
than conjugated NC 7312 was detected, which was believed by
the authors of the report to be probably a conjugate of
6-hydroxybendiocarb (Challis and Adcock 1977a).
Another similar mouse study with groups of males of the CD-1
strain treated orally with single doses of 14C-bendiocarb at 1, 5 or
10 mg/kg body weight indicated recovery of about 80% of the
administered radioactive dose in 24 hours, predominantly in the urine.
A significant dose-dependent relationship was not evident with respect
to rate and route of elimination or metabolic profile of bendiocarb.
About 33-56% of the administered 14C-doses was recovered in the urine
as conjugates of NC 7312. Conjugated 6-hydroxy bendiocarb was believed
by the authors of the study to be the only other major urinary
metabolite (Pearce et al 1977).
Rat
Male and female rats treated with a single oral dose of
14C-bendiocarb at approximately 10 mg/kg body weight eliminated over
95% of the administered radioactivity in urine (89%), expired air (6%)
and faeces (2%) within 2 days of dosing. There was no significant sex
different in rate and route of elimination. Following an acute oral
dose of 14C-bendiocarb at 1 mg/kg body weight, plasma levels in male
and female rats peaked at 10 minutes post treatment with plasma half-
life being calculated at 4.4 hours for both sexes. The major urinary
metabolite was the phenol (NC 7312) which, excreted as ß-glucuronide
and sulphate conjugates, accounted for more than 85% of the
administered dose recovered in the urine during the first 24 hours
after treatment. The remaining 15% eliminated over this time period
comprised a mixture of sulphate and ß-glucuronide conjugates of at
least seven minor metabolites. One of the latter appeared to be
N-hydroxy methyl bendiocarb. No unchanged parent compound was found in
the urine. In the faeces free NC 7312 was the primary metabolite with
a small amount of 14C-bendiocarb being detectable (Adcock and Challis
1976a).
Conjugated NC 7312 was found to be the primary metabolite in
urine collected during 0-24 hours after treatment withdrawal in male
and female rats (CFY strain) fed 20 ppm of 14C-bendiocarb in the diet
for 10 days. At sacrifice 6 days after termination of dietary feeding,
detectable 14C residues were found in the tissues analysed (fat,
liver, kidney, muscle and brain) with the fat tissue having the
highest residue level. According to the partition characteristics of
the 14C residue in fat into organic solvents, radioactive residue in
fat was probably not mainly due to bendiocarb or NC 7312 (Pearce and
Adcock 1978).
Hamster
Groups of 6 or 10 male hamsters were intubated with
14C-bendiocarb in glycerol formal at 1, 5 or 10 mg/kg. Four female
hamsters were similarly treated at 10 mg/kg body weight. Over 90% of
the administered 14C-dose was recovered in 24 hours in the faeces and
the urine, with the latter being the primary route of elimination. The
rate and route of excretion was not modified by sex or dosage level.
The proportion of total radioactivity recovered in the urine as
conjugates of NC 7312 was about 37% at 1 mg/kg, 16% at 5 mg/kg and 19%
at 10 mg/kg. Besides conjugated NC 7312, urine of male hamsters
contained two metabolites in approximately equal amounts, while that
of female hamsters had only one radioactive component. A conjugate of
6-hydroxy bendiocarb was believed by the authors to be the major
urinary metabolite in both males and females. The other urinary
metabolite in male hamsters had not been identified. The seeming sex
difference in metabolic fate of bendiocarb remains to be ascertained,
since the male and female hamsters used in the study were housed and
dosed at different laboratories (Challis and Adcock 1977b).
Rabbit
Groups of pregnant rabbits were intubated on day 20 of gestation
with one dose of 2.5 or 10 mg/kg body weight of 14C bendiocarb.
Within 24 hours of dosing, nearly 90% of the administered dose had
been eliminated, primarily in the urine, with only a small amount
(<2.5%) being detected in the faeces. The rate and route of
elimination and metabolic profile of bendiocarb appeared to be
independent of dosage. The major urinary metabolite was identified as
NC 7312, which accounted for 90% of the radioactivity extracted from
urine samples following enzyme (sulphatase and ß-glucuronidase)
hydrolysis (Challis 1980).
Dog
Groups of one male and one female beagles (fasted overnight and
for 8 hours following dosing) were treated once with 14C-bendiocarb
in gelatin capsules at 0.1, 1.0 or 10 mg/kg body weight. Regardless of
dosage level, the radioactive dose was almost completely excreted
during the first 24 hours post-dosing, primarily in the urine. The
amount of radioactivity found in the urine of both sexes over the
48-hour period seemingly decreased with dosage (about 90% at 0.1 mg/kg
body weight, 80% at 1.0 mg/kg body weight and 70% at 10 mg/kg body
weight). This appeared to be accompanied by a corresponding increase
in faecal recovery (about 19%, 21% and 24%, respectively, at 0.1, 1.0
and 10 mg/kg body weight). In the urine, approximately 84%, 67% and
43% of the administered radioactivity, respectively, at 0.1, 1.0 and
10 mg/kg body weight occurred as sulphate and ß-glucuronide conjugates
of NC 7312 with no recovery of any 14C-bendiocarb. Analysis of urine
samples from male dogs at 10 mg/kg body weight indicated the probable
presence of ring hydroxylated derivatives of bendiocarb as minor
metabolites. Unchanged 14C-bendiocarb was the only component
identified in the faeces and amounted to about 80% of the
radioactivity recovered via this route (Warner et al 1977).
Effects on Enzymes and Other Biochemical Parameters
Mouse
In male and female mice (Charles River CD-1 strain) fed technical
bendiocarb containing 0.09% w/w of dimethoate) in their diets at 0 or
1 250 ppm for 19 days, whole blood cholinesterase in blood sampled
between 06:00 hour and 08:00 hour, but not between 09:00 hour and 11
hours (peak feeding period stated to occur between 22:00 hour and
09:00 hour) was depressed (>20%) only in treated males in weeks 2 and
3 (Hounsell and Rush 1980).
Male CFLP mice and male CD 1 mice were fed dietary levels of
technical bendiocarb at 0, 500 or 1 000 ppm for up to 7 days. In the
CFLP strain, whole blood cholinesterase activity was reduced (>20%)
at 1 000 ppm in animals fed the diet ad libitum and in those
following a 1-hour feeding period on the 7th day after an overnight
fast. In the CD-1 mice, whole blood cholinesterase was depressed
(>20%) in the 500 and 1 000 ppm groups following an overnight fast
and exposure to the diet for 1 hour on day 7. Ad libitum feeding
resulted in decreased whole blood cholinesterase in the 500 but not in
the 1 000 ppm groups (Hounsell 1981a).
Rat
Groups of rats (sex unspecified) were treated orally with
4 mg/kg/day of technical bendiocarb in glycerol formal for three
consecutive days. Inhibition (65-85%) of cholinesterase in whole blood
and plasma peaked at 10 minutes after each dose on days 1 and 3.
Recovery of whole blood and plasma cholinesterase began to occur 30
minutes following each dose, and was practically complete three days
after treatment withdrawal. Whole blood cholinesterase appeared to be
more sensitive than plasma cholinesterase to the inhibitory effect of
bendiocarb (study cited by Kemp and Hounsell 1974).
Male rats (CFY Sprague-Dawley) were fed dietary levels of
technical bendiocarb up to 10 ppm for 14 days, followed by an
overnight fast. Whole blood cholinesterase, assayed in the morning of
the 15th day after a 1-hour feeding period, was not significantly
inhibited (<20%) (Hounsell 1981b). Another similar study with the
same strain and sex of rats fed diets containing technical bendiocarb
for up to 23 days indicated that, following overnight fasting and a
one-hour feeding period, animals exhibited a depression (20%) of whole
blood cholinesterase at 50 ppm on days 15 or 16 and 22 or 23 (Hounsell
1981c).
Dog
In 4 male beagles (fasted the day before the test) fed a diet
containing 1 000 ppm of technical bendiocarb for up to one hour
(actual intake of the test material estimated at 6 - 8.6 mg/kg body
weight), inhibition (>50%) of brain and whole blood cholinesterase
was apparent 15 minutes after the feeding period. Whereas depression
of whole blood cholinesterase peaked at 2 hours and was completely
reversible at 3 hours following the feeding period, the extent of
inhibition of brain cholinesterase remained fairly constant between
15 minutes and 3 hours (the last sampling interval) after the dosing
period (Hounsell 1974). Another experiment with 2 female beagles
(24-hour fasted) fed a dietary level of 10 ppm of technical bendiocarb
for about 10 minutes (actual intake of the test material estimated at
approximately 32.4 mg/kg) showed reduction of whole blood
cholinesterase activity of about 27% and 52% of the pretreatment level
at 1 hour and 6 hours, respectively, post dosing. Cholinegesic
symptoms were noted within 24 minutes of the initiation of feeding.
Complete recovery of whole blood cholinesterase activity and from
toxic signs occurred 24 to 25 hours after treatment (Kemp and Hounsell
1974).
Cow
A lactating dairy cow was fed technical bendiocarb in the daily
diet at 3.6 ppm for two consecutive days (ration was offered twice
daily). No reduction (<20%) in whole blood cholinesterase was
observed at any of the 8-hourly intervals following the morning
feeding on both days (Roberts 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 17 to 21 mated rats (Sprague-Dawley CFY strain) were
fed diets containing technical bendiocarb at 0, 200, 400 or 800 ppm
from 14 days post-coitum until weaning of their pups. A decrease in
maternal body weight gain was noted at 800 ppm on days 17 and 20 post-
coitum and practically throughout the 21-day lactation period. At this
dietary level, survival and weight gain of pups were depressed
throughout lactation. No treatment-related effects were observed on
other parameters monitored, i.e. mortality and clinical signs of
pregnant dams, sex ratio and gross abnormalities of offspring (Jackson
1978).
Groups of 10 males and 20 female rats (SPF, CFY strains) were fed
dietary concentrations of bendiocarb (presumably technical grade) at
0, 200, 400 or 800 ppm for one week prior to mating. The females were
maintained on test diets through day 18 of gestation and the males
until termination. There were no mortalities or toxic symptoms. Growth
was slightly depressed in males at 800 ppm and in females at 400 ppm
and above. Pregnancy rate, duration of gestation period and terminal
gross pathology were not adversely affected. Medium precoital time (as
an indication of mating performance) of dams was lengthened at both
400 and 800 ppm. In the progeny generation, except for a slight
decrease at 400 ppm and above in mean pup weight at birth, no apparent
dose- or treatment-related effects were evident on litter size at
birth, survival and weight of pups through lactation period to
weaning, sex ratio and incidence of gross abnormalities of weanlings
(Palmer and Allen 1978).
Groups of 30 males and 30 females (Charles River CD strain) were
fed dietary levels of technical bendiocarb at 0, 10, 50 or 250 ppm for
90 days prior to mating to initiate a reproduction study covering
three generations with two litters per generation. Weanlings from the
second litters were selected to become parents of the next generation.
After the second mating trial in each generation, the dams in each
group were divided into two approximately equal sub-groups. One
sub-group was allowed to deliver and rear their young until 25 days
post-partum. Another sub-group was sacrificed on day 21 of gestation
for examination of uterine contents. Foetuses were examined for
external, skeletal and soft tissue malformations.
In the parental generations, no mortality or clinical signs
attributable to the compound were evident. Dams displayed slight
growth depression during the gestation period of F1a litters at
50 ppm and above and of F2b litters at 250 ppm. Food consumption was
normal, but water intake was slightly decreased at 250 ppm in F1
females. Males of the F2 generation seemed to exhibit an increase in
incidence and severity of "geriatric nephropathy" at and above 50 ppm.
Ophthalmoscopic examination conducted on F2 adults between 31 and 35
weeks of age revealed no treatment-related findings. At terminal
sacrifice of parental animals after weaning of the second litters,
some variations from controls in absolute weights and/or organ/body
weight ratios of certain tissues such as adrenal, pituitary, thyroid,
seminal vesicles and uterus were noted at both 50 and 250 ppm, but
these observations were not consistently present through the
generations and were not accompanied by histopathological lesions of
the organs. Mating and fertility indices as well as length of
gestation period were not modified by bendiocarb. There was an
increase in frequency of F0 females being acyclic or pseudopregnant
at 250 ppm and of F2 females with irregular oestrous cycles at 50 ppm
and above, prior to the first mating trial in both cases. Pre-coital
interval was lengthened at 250 ppm in the F0 generation (first
mating).
Over the three progeny generations, the viability index was
slightly depressed in F1a litters at 250 ppm from days 10 through 25
and in F2b litters at 50 ppm and above on day 25. Pup weight was
reduced at 250 ppm in F1a and F1b litters on day 25 and in F2b
litters at birth through day 25. In F2a litters, pup weight on day 25
was decreased in all treated groups, but the difference was indicated
by the authors of the report to be statistically significant only at
10 ppm, but not above. There were no significant differences between
control and treated groups in litter size (at birth and on day 1), sex
ratio, ophthalmoscopic observations of F3b pups between 19 and 31
days of age, organ weight and histopathological findings of 25-day old
F3b pups. Examination of 25-day old pups from the second litters of
each generation for rate of physical development and sensory functions
revealed "the onset of pinna unfolding, hair growth, tooth eruption
and eye opening was marginally delayed in all treated (F1b) litters,
but the overall pattern of physical development was essentially
similar in all groups, and "the onset of pinna unfolding and hair
growth was marginally delayed (in F2b litters at both 50 and 250 ppm)
but eye opening and tooth eruption was unaffected".
Teratological investigation of F1b, F2b and F3b litters showed
no significant differences between control and treated groups with
respect to number of implantations, number of viable young, early or
late resorptions, post-implantation loss and foetal weight. A rise in
pre-implantation loss in F1b litters was noted at 250 ppm. Also at
the top dosage level, there were higher incidences of generalized
subcutaneous oedema, incomplete ossification of cranial bone,
uni/bilateral hydronephrosis and bilateral ureter in F1b litters and
of subcutaneous scapular haemorrhage in F3b litters, as compared to
concurrent controls. Based on the data, a dietary level of 10 ppm was
without significant adverse effect on the reproductive parameters
evaluated. The teratogenic "no effect" level was demonstrated to be
50 ppm (Tesh et al 1981).
Special Studies on Teratogenicity
(also see under "Special Studies on Reproduction")
Rat
Groups of 24 mated rats (Sprague-Dawley derived, CD strain) were
intubated with technical bendiocarb as a suspension in gum tragacanth
at 0, 0.25, 1 or 4 mg/kg body weight/day between gestation day 6 and
day 15 (day 0 = day of positive vaginal smear). The dams were
sacrificed on gestation day 21 and foetuses were removed by caesarean
section for external, visceral and skeletal examination. There was no
mortality. Dams at 4 mg/kg body weight/day exhibited toxic symptoms
characteristic of cholinesterase inhibition and slight growth
inhibition on gestation day 21. Two dams (out of 21) at 4 mg/kg body
weight/day and 1/22 dams at 1 mg/kg body weight/day displayed a large
number of late uterine deaths. Mean litter size was depressed at
4 mg/kg body weight/day. There were no significant differences between
control and treated groups in pregnancy rate, number of corpora lutea
or implantation sites, number of early resorptions, foetal weight and
frequency of foetal abnormalities. The study demonstrated bendiocarb
to be non-teratogenic under the conditions of the experiment (Tucker
1974).
Rabbit
Groups of 27-29 female rabbits (New Zealand White) that had been
artificially inseminated were intubated with technical bendiocarb as a
suspension in aqueous gum tragacanth at 0, 1, 2.5 or 5 mg/kg body
weight/day from day 6 to day 28 inclusive of gestation. (Day 0 = day
of insemination.) The does were sacrificed on day 29 of gestation and
uterine contents were examined. Foetuses were subjected to evaluation
for external, skeletal and internal malformations. Six to eight
females per control or treated group died 6 to 29 days after
insemination mainly due to "tracheal intubation". Clinical signs such
as increased respiratory rate and prostration were noted at and above
2.5 mg/kg body weight/day. Growth was slightly depressed at 5 mg/kg
body weight/day between gestation days 16 and 28. Whole blood
cholinesterase assayed 30 minutes post-dosing on day 28 was depressed
(31-70%), in a dose-dependent manner, in all treated groups. One doe
each at 2.5 and 5 mg/kg body weight/day aborted on day 22 and 20,
respectively. Premature parturition occurred in one doe each of 2.5
and 5 mg/kg body weight/day groups on day 28. An increase was observed
in the mean number of late resorptions per litter and the incidence of
pregnant does with late resorptions at both 2.5 and 5 mg/kg body
weight/day. Post-implantation loss (number of implantations - number
of viable foetuses/number of implantations × 100) was elevated at
2.5 mg/kg body weight/day and above.
Other parameters monitored, including number of corpora lutea,
implantations, viable young, or early resorptions, pre-implantation
loss, foetal weight and placental weight, were not adversely affected
by treatment. There was a dose-related increase in incidence of
foetuses with eye anomalies (mainly "cloudy areas in eyes") and with
absence of pubic bones at both 2.5 and 5 mg/kg body weight/day.
Incidence of litters containing foetuses showing such abnormalities
was also elevated at and above 2.5 mg/kg body weight/day. In the
absence of background data on frequency of the abnormalities in
question, 1 mg/kg body weight/day may be considered as a clearcut "no
effect" level with respect to teratogenicity (Tesh et al 1980).
Special Studies on Mutagenicity
Two separate experiments were conducted with technical bendiocarb
and NC 7312 to evaluate their genetic activity in vitro microbial
systems (plate assays), with or without the addition of a mammalian
metabolic activation preparation (S-9 mix from the liver of male rats
pre-treated with Aroclor 1254 i.p. at 500 mg/kg/day for five days).
Indicator organisms used were Salmonella typhimurium strains TA
1535, TA 100, TA 1537, TA 1538 and TA 98. Results suggest
non-mutagenicity of the test compounds to any of the tested strains at
the (non-toxic) concentrations used (3.3-1 000 µg/plate of bendiocarb
and 10-3 300 µg/plate of NC 7312) in the presence or absence of
metabolic activation and under the conditions of the assays
(McConville and McGregor 1979; McConville and Harris 1979). Negative
results were also obtained in another reversion mutation test using
Escherichia coli WP2 and five strains of the Salmonella
typhimurium TA series as indicator organisms in the presence of
absence of a liver metabolic activation system at concentrations of
technical bendiocarb ranging from 5 to 1 000 µg/plate (Moriya
et al 1981).
A rec-assay conducted with Bacillus subtilis H17 (rec+) and
M45 (rec-) in the absence of a metabolic activation preparation
indicated that technical bendiocarb, at concentrations of 20 to
5 000 µg/disc, did not induce any inhibitory zone in either tested
strain (Moriya et al 1981).
Technical bendiocarb was evaluated for its mutagenic activity in
a mouse lymphoma L5178Y specific locus mutation assay. Under the
conditions of the test, there appeared to be no consistent indications
of mutagenicity of the compound in the absence or presence of a
metabolic activation system (S-9 homogenate of liver from male adult
Fischer rats induced with Aroclor 1254) at concentrations ranging from
5 to 20 µg/ml (McGregor and Ross 1981).
In a micronucleus test, groups of male mice were treated with
technical bendiocarb i.p. at 0, 0.625, 1.25 or 2.5 mg/kg body
weight/day for two consecutive days (the two doses were given 24 hours
apart). The animals were sacrificed 6 hours after the second dose and
the femurs were removed for the preparation of bone marrow smears.
There was no significant increase in the frequency of polychromatic
erythrocytes containing micronuclei or change in polychromatic/
normochromatic ratio in any treated group (Hounsell and Walker 1982).
Groups of 20 sexually mature virgin male rats (Sprague-Dawley CD
strain) were fed diets containing technical bendiocarb at 0, 10, 50 or
250 ppm for 13 consecutive weeks and then mated with sexually mature
virgin female rats for seven days in a dominant lethal assay. (Each
group was sub-divided into sub-groups A and B with commencement of
dietary feeding of the groups being staggered by one week.) The
females were sacrificed 14 ± 1 days after mating and autopsied for the
examination of uterine contents. The treated males exhibited no
clinical signs and their body weight and food consumption were not
adversely affected by treatment. The incidence of non-fertile males
were 1/20 at 50 ppm and 2/20 each at 10 ppm and 250 ppm. With the
exception of one infertile male at 250 ppm, abnormally small testes
and epididymis were found in the other four infertile males. An
increase (not dose-related) in incidence of litters with at least one
early death was observed (in sub-group B only) at 10 ppm and 250 ppm,
but this was not accompanied by a rise in the number of early deaths
per litter. There were no significant differences between control and
treated groups with respect to pregnancy rate, number of corpora
lutea, number of total implants, number of viable implants and pre-
implantation loss. Bendiocarb did not appear to induce dominant lethal
mutation under the experimental conditions used (Kemp and Jackson
1977).
Special Studies for Carcinogenicity
Mouse
Groups of 50 males and 50 females (HaM/ICR Swiss mice CD-1; about
35 days old) were fed diets containing technical bendiocarb, found to
contain up to 0.5% dimethoate or dimethoate decomposition products, at
50, 250 or 1 250 ppm. The control group consisted of 100 males and 100
females. The experiment was originally scheduled to be terminated
at a predetermined survival level or 104 weeks at the latest. The
predetermined level was reached after 89 weeks in males and 94 weeks
in females. Mortality rate was increased in females at 1 250 ppm after
85 weeks. Less than 40% of animals in control and treated groups were
still alive at termination, but over 50% of animals of all groups,
including the control, survived at least 82 weeks. All animals that
died or were sacrificed in moribund condition during the study and
those sacrificed terminally were necropsied. A wide range of tissues
and any unusual lesions from animals of all groups, including the
control, found dead or sacrificed moribund and from all terminal
survivors of control and top dosage groups were examined
microscopically. Any unusual lesions from terminal survivors at both
50 and 250 ppm were also subject to histopathological evaluation.
Clinical signs, body weight, food and water consumption were not
significantly different from those in the controls. Ophthalmoscopic
examination conducted at four intervals during the study seemed to
suggest a probable increase at week 89 in incidence of cataract
("posterior cortical or more advanced") in males at 1 250 ppm. At
terminal sacrifice, males at 1 250 ppm showed an increase in absolute
testes weight and testes/body weight ratio and females of all treated
groups displayed a dose-related increase in absolute weight and
organ/body weight ratio of kidney. There were no treatment-related
microscopic changes in the tissues evaluated, including testes and
kidney.
Analysis of tumour data appeared to indicate no significant
difference between control and top dosage groups in the incidence of
any particular type of tumour. Under the conditions of the study,
bendiocarb was not carcinogenic to the mouse (Serota et al 1981).
Rat
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Seven adult hens (15 months old) were given a single subcutaneous
dose of bendiocarb ("laboratory grade") at 60 mg/kg body weight (the
subcutaneous LD50 of bendiocarb in hens was indicated to be about
80 mg/kg body weight). A second dose was given 3 weeks later. The
control group consisted of seven untreated hens. The birds were
observed for a total of 6 weeks from the initial dose before sacrifice
for gross pathology and histopathological examination of the
spinal cord and sciatic nerve. There were no clinical signs or
histopathological evidence suggestive of delayed neurotoxicity of the
compound in any of the treated hens (Sanderson and Hounsell 1969).
Groups of domestic hens, approximately 14-months old (20 birds in
the top dosage group and 10 birds in each other group) were acutely
intubated with technical bendiocarb in maize oil at 0, 189, 378 or
757 mg/kg body weight and observed for 21 days prior to sacrifice for
histopathological examination of the brain, spinal cord and distal
sciatic nerve. The hens were given 10 mg/kg body weight of atropine
intramuscularly immediately before the dose of bendiocarb. (Before
initiation of the neurotoxicity study, the oral LD50 of bendiocarb
was found to be 137.3 mg/kg body weight in hens not pretreated
intramuscularly with 10 mg/kg body weight of atropine and 757 mg/kg
body weight in atropine-protected hens.) One hen at 378 mg/kg body
weight and a total of 8 hens at 757 mg/kg body weight died. Except for
"doubtful signs" of ataxia noted in 1/10 hens at 189 mg/kg body weight
on days 9 and 10 only, there were no delayed neurotoxic signs in any
other treated birds during the 21-day exposure observation period.
None of the bendiocarb-treated survivors or negative controls
exhibited microscopic evidence of neurological lesions. Positive
controls, dosed orally with TOCP at 500 mg/kg body weight, displayed
typical neurotoxic signs and significant morphological changes of the
nervous tissues (Ross et al 1978).
Special Studies on Skin and Eye Irritation
Technical bendiocarb was shown to be a mild irritant when applied
to the skin of New Zealand White rabbits (Cuthbert 1978).
An eye irritant study in New Zealand White rabbits indicated
technical bendiocarb to be minimally irritating to the eye (Cuthbert
1980).
Special Studies on the Acute and Subacute Oral Toxicity of NC 7312
Rat
The oral LD50 of NC 7312 (2,2-dimethyl-1,3-benzoxodiol-4-01) a
metabolite of bendiocarb) in male and female rats (CFY strain) was
greater than 4 640 mg/kg body weight. Cholinergic symptoms were
observed at dosages above 464 mg/kg body weight (Mallyon and Sanderson
1977).
Groups of rats (CFY strain, 10 male and 10 female controls, 5
males and 5 females per treated group) were intubated with NC 7312 as
a suspension in aqueous gum tragacanth at 0, 5, 20, 80, 320 or
1 280 mg/kg body weight/day for 16 days. There were neither mortality
nor compound-related effects on clinical signs, haematological, blood
chemistry and urinalysis parameters. Growth, food conversion factor
and water intake were slightly decreased in females at 1 280 mg/kg
body weight on week 2. Terminal survivors sacrificed showed no gross
or histopathological changes associated with treatment. Absolute
weight and organ/body weight ratio of ovary were reduced at 80 mg/kg
body weight and above, but concomitant microscopic changes of the
tissue were not evident (Kemp and Brooks 1978).
Acute Toxicity
The acute toxicity of several animal species to bendiocarb is
summarized in Table 1.
Toxic signs were characteristic of anticholinesterase poisoning
and seen within a few minutes after oral, intraperitoneal or
inhalation exposure in all species of mammals tested. Deaths usually
occurred after 5 minutes to 2 hours and survivors began to recover
after 1/2 to 2 hours. Within 24 hours of dosing, survivors recovered
completely from clinical signs. After dermal exposure, toxic signs
began to appear after 2 to 21 hours with recovery being much slower
than that following acute oral doses (Sanderson 1921).
Short-Term Studies
Rat - dietary
In a 28-day study with groups of five female rats (Sprague-Dawley
CFY strain) fed diets containing 0, 400, 600 or 800 ppm of technical
bendiocarb, mortality, body weight, food consumption, oestrous cycle
(as evidenced by vaginal cytology taken from the fourth day to
termination) and terminal gross pathology were not affected by
treatment. Whole blood cholinesterase was inhibited (52-62%) at all
test levels and toxic symptoms were observed at both 600 and 800 ppm
on day 28 after the animals (fasted overnight) were given access to an
appropriate test diet for a restricted one-hour feeding period
(Jackson 1977).
Table 1. Acute Toxicity of Bendiocarb in Animals
LD50
Species Sex Route Vehicle (mg/kg body Reference
weight)
Mouse F oral glycerol 45
(CFW) formal Sanderson 1971
Rat M oral glycerol 40-64 Sanderson 1970;
(Wistar) formal Sanderson 1971,
F 34-40 Sanderson 1971
Rat M oral aqueous 108-156 Crome and Sanderson
(Charles River gum 1980
COBS CD tragacanth
Sprague-Dawley)
Rat M&F i.p. glycerol 8 Sanderson 1971
(Wistar) formal
Rat F dermal glycerol 566-800 "
(Wistar) (24-hour formal Ben-Dyke 1932
M exposure) 566
Rat (Carworth M inhalation1/ None 250 mg Sanderson and
Sprague-Dawley) (6-hour a.i./l air2/ Mallyon 1979
exposure)
Hamster F oral water 141 "
(Syrian)
Guinea pig F oral glycerol 35 Sanderson 1971
formal
Rabbit M oral glycerol 40 "
F formal 35
1/ Animals were exposed to an 80% wettable powder as a dust in a "nose only"
exposure chamber with 58% particles of size 10 microns and 18% particles
5 microns.
2/ 6-hour LD50 in terms of actual chamber concentration of the active ingredient.
Groups of 10 male and 10 female rats (Sprague-Dawley CFY strain)
were fed dietary levels of technical bendiocarb at 0, 2, 10, 50 or
250 ppm for 13 weeks. Mortality, clinical signs, body weight, food and
water consumption, ophthalmoscopic findings, clinical chemistry,
haematological and urinalysis parameters were not influenced by the
presence of the compound in the diet. Whole blood cholinesterase,
determined in animals (fasted 16 hours) shortly after a 1-hour feeding
period, was depressed (20%) at 250 ppm in both sexes during weeks 4
and 9, at both 50 and 250 ppm in males and at 250 ppm in females
during week 13. Terminal gross pathological changes and organ weights
were not significantly different from those in the controls.
Histopathological evaluation of a variety of tissues from animals of
the control and top dosage groups suggested the only notable finding
to be an increase in incidence of males at 250 ppm showing vacuolated
centrilobular hepatocytes. This liver lesion, not noted even at
200 ppm in the long-term rat study (see under Long-Term Studies), was
considered unlikely to occur in the lower dosage groups of the study,
although there were no data to confirm this supposition. It would
appear that the no-effect level was 10 ppm on the basis of
cholinesterase levels and was 50 ppm on the basis of the other tested
parameters (Hunter et al 1978).
Hamster
Groups of hamsters (20 male and 20 female controls, 10 males and
10 females per treated group) were fed technical bendiocarb in their
diet at 0, 4, 20, 100 or 500 ppm for 30 days. There were no
indications of any treatment-related effect at levels up to 500 ppm as
judged by mortality, clinical signs, body weight, food and water
intake, terminal haematology, organ weight and histopathology of
selected tissues from control and 500 ppm groups (Hounsell and Brooks
1981).
Dog
Groups of four male and four female beagles were fed dietary
levels of technical bendiocarb at 0, 20, 100 or 500 ppm for 16 weeks
with the top dosage level being raised to 1 000 ppm on day 89 (test
diets were offered to the dogs for a 1-hour period morning and
evening). Inclusion of the compound in the diet did not adversely
affect survival, clinical signs, food consumption, terminal
haematology, blood chemistry and urinalyses values. Males of the
highest dosage group exhibited actual weight loss at termination.
Assays1/ at weeks 5, 9, 14, 15 and 16 of cholinesterase in whole
1/ Whole blood was diluted 1:1 200 in the assay. No data were
available on the amount of diet actually consumed by individual
animals during the 1-hour feeding period prior to sampling for
assay of cholinesterase in blood and brain.
blood and plasma of animals (fasted overnight) following a 1-hour
feeding period indicated depression (>20%) at the top dosage level of
cholinesterase in plasma and whole blood at most sampling periods.
Whole blood cholinesterase was decreased (>20%) even at 100 ppm in
males at week 9 and in females at weeks 9 and 15. Terminal brain
cholinesterase of the top dosage group (males and females) was also
inhibited (>20%). At the conclusion of the study, organ weights and
gross pathological changes were unaffected by the compound.
Microscopic examination of about 20 tissues from each animal suggested
no treatment-related changes other than the occurrence of thyroid
lesions (focal C-cell hyperplasia and focal atrophy) in 1/4 males and
1/4 females of the top dosage group but in none of the concurrent
controls or of animals in the lower dosage groups. The study suggested
the no-effect level with respect to cholinesterase and other criteria
evaluated to be 20 ppm and 100 ppm, respectively (Litton 1974).
Groups of 8 male and 8 female purebred beagles were fed a diet
containing technical bendiocarb at 0, 20, 100 or 500 ppm for 2 years
(control or treated diet was given to each animal every morning).
Survival, clinical symptoms and growth during the study as well as
haematology, urinalysis and ophthalmoscopic parameters evaluated at
five more intervals over the course of the experiment were not
significantly affected by the presence of the test material in the
diet. Less food was consumed by animals (both sexes) of all treated
groups throughout the study. Nevertheless, the authors of the report
stated that "statistical analysis of food residue for weeks 1-3, 1-26,
1-52, 53-104 and 1-104 did not reveal any statistically significant
difference between control and test group means". Based on weekly mean
food consumption and mean body weight data at the end of each week,
the dietary levels of 20, 100 and 500 ppm were equivalent to 0.7, 3.1
and 16.3 mg/kg body weight/day (males and females combined). Water
consumption was slightly but consistently increased in both sexes at
100 ppm and above during weeks 36-39 and at 500 ppm not infrequently
thereafter. Serum cholesterol was elevated at 500 ppm (both sexes) at
several sampling intervals. A dose-related decrease in serum calcium
level was noted at both 100 and 500 ppm in weeks 14 and 25.
Measurement of whole blood cholinesterase in animals (fasted
overnight) shortly after a 60-minute feeding period at eight sampling
intervals during the study showed a tendency to frequent depression
(>20%) of the enzyme at 500 ppm. Brain cholinesterase in animals
(fasted overnight) sacrificed following a 1-hour feeding period was
depressed (>20%) at 500 ppm after 52 and 104 weeks and probably also
at 100 ppm after 104 weeks. Organ weight analysis, gross pathological
examination and histopathological evaluation of over 30 tissues from
each animal sacrificed at the end of 52 weeks (3 males and 3
females/group) or terminally (all survivors per group) showed no
significant alterations related to treatment.
The dietary level of 20 ppm appeared to be a no-effect level on
the tested criteria. It was noteworthy that the actual amount of
bendiocarb ingested by animals during the 1-hour feeding period prior
to the assay of tissue cholinesterase varied markedly, even among
animals in the same dosage group, owing to substantial individual
variation in the quantity of diet (ranging from 0 to 400 g per animal)
consumed. Also, there were incidences where individual animals of a
high dosage group, e.g. 500 ppm, actually had a lower intake of
bendiocarb than those of a lower dosage group (100 ppm) (Chesterman
et al 1980).
Rat - dermal
Groups of 6 rats (Wistar strain) were exposed dermally to an 80%
wettable powder of bendiocarb as a 40% w/v aqueous suspension at 50,
100, 200, 400 or 800 mg a.i./kg body weight under an occlusive patch
for a period of 6 hours/day, 5 days/week for a total of 15
applications in 21 days. The treated site was washed at the end of
each exposure period. There was no mortality. Toxic signs,
characteristic of anticholinesterase poisoning and dose-related in
severity, were noted at 200 mg/kg body weight and above. Whole blood
cholinesterase, determined at the end of the 6-hour exposure period,
was inhibited by more than 30% at 200 mg/kg body weight and above
after only one dose and at 50 mg/kg body weight and above after 15
exposures. No gross pathological changes or microscopic evidence of
dermal irritation attributable to treatment were apparent at terminal
sacrifice (Sanderson 1972).
Long-Term Studies
Rat
Groups of male and female rats (CFY strain, 60 male and 120
female controls, 30 male and 60 female per treated group) were fed
dietary levels of technical bendiocarb at 0, 2, 20 or 200 ppm for 7
days and then caged together (1 male and 2 females). At the end of a
20-day mating period, the females were individually housed and allowed
to litter and rear their pups to weaning. This part of the study was
known as the reproductive phase. A sufficient number of weanlings from
the control and treated groups were randomly selected to initiate
another segment of the study referred to as the main phase. The latter
comprised the main group and the satellite group. In the main group,
weanlings (50 males and 50 females per treated group, 100 male and 100
female controls) were given bendiocarb-treated diets at 0, 2, 20 or
200 ppm for 104 weeks. For the satellite group, used primarily for
laboratory investigations, animals (30 males and 30 females per
control or treated group) were fed bendiocarb at the same dietary
levels, but only for 60 weeks. The 2 ppm level was raised to 10 ppm
after 2 weeks in the main phase, and this group will be known as the
2-->10 ppm group hereafter for the purpose of identification. Dietary
administration was continued throughout both the reproduction and main
phase. All animals died or sacrificed in extremis during the 104-week
study and those sacrificed terminally were necropsied. Animals of all
groups, including the control, dying or sacrificed in extremis during
the study and terminal survivors of the control and top dosage groups
were subjected to microscopic examination of a set of about 30
selected tissues plus grossly observed nodules and tissue masses.
Additionally, all suspected tumours from terminal survivors of lower
dosage groups were evaluated histopathologically.
For the reproductive phase, mortality and behaviour of parental
animals, pregnancy rate, duration of gestation period, litter size and
pup weight at birth, sex ratio and incidence of external abnormalities
of pups were not affected by bendiocarb. There were some indications
of adverse effects related to treatment at 200 ppm on maternal body
weight gain during gestation, litter size on lactation day 4 and pup
weight from lactation day 4 through weaning. A dietary level of 20 ppm
was a no-effect level on this phase of the study.
In the 104-week study (main group) of the main phase, mortality
rate, although unaffected by the compound, was high in all groups,
including the control, Only about 16-26% of males and 24-42% of
females survived 104 weeks. Nevertheless, over 55% of males and
females lived at least 82 weeks.
A number of findings associated with treatment were noted at
200 ppm. These included an increased incidence of males exhibiting
hyperactivity in response to external stimuli, growth depression
during the first 52 or 56 weeks, depressed water consumption during
week 6-12 but not in week 25. Periodic measurements of haematological,
biochemical and urinalysis values at several intervals over the course
of the study revealed significant changes in several parameters
(e.g. total WBC, neutrophil and lymphocyte counts, levels of serum
cholesterol and total protein) to be also confined only to the top
dosage group. There was a dose-related decrease in body weight gain
associated with a depression in food consumption in males of all
treated groups during the first two weeks of the study but not
thereafter. Ophthalmoscopic examination of the surviving animals in
the main group only for a total of seven intervals between 13 and 102
weeks indicated a statistically significant increase in incidence of
lenticular opacities in males at 200 ppm at all intervals from 52
weeks onward. A statistically significant trend (p <0.01) for
dose-dependent incidence of this eye lesion was indicated to be
apparent for males at both 20 and 200 ppm from 66 weeks onward and for
males of all treated groups at 80 weeks and thereafter. It was pointed
out that the difference in frequency of lenticular opacities was not
statistically significant between males of control and low dosage
(2-->10 ppm) groups or among female groups at any time interval. The
lenticular opacities observed reportedly were "essentially atypical of
chemically induced lenticular lesions in the rat". Overall,
2-->10 ppm might probably be considered a borderline no-effect level
with respect to the particular eye lesion.
Activity of whole blood cholinesterase determined in animals
(fasted overnight) following a one-hour feeding period at several
intervals during the study (animals of the satellite group) and at
termination (animals of the main group) was frequently depressed
(>20%) at 200 ppm in both sexes. Whole blood cholinesterase activity
in animals fasted overnight without access to food immediately prior
to sampling of blood was not reduced. The brain cholinesterase level
was inhibited (>20%) at 200 ppm in animals (both sexes) sacrificed at
52 weeks but not terminally. Organ weight determination at 52- and
60-week interim sacrifice and at terminal sacrifice showed deviations
from controls of several tissues such as liver, thymus, prostate and
adrenals, but these were essentially restricted to the top dosage
level only and were not accompanied by histopathological lesions.
Gross pathological changes were not significantly different from those
in the controls. Histopathologically, an increase in incidence of
stomach lesions, characterized by "hyperplasia/hyperkeratosis/
acanthosis with or without inflammation of the non-glandular portion",
occurred in the male terminal survivors at 200 ppm. There were no
microscopic lesions observed in any of the other tissues examined that
were attributable to the compound.
An evaluation of tumour data suggested a dose-related increase of
incidence of total lymphoreticular tumours in males of all treated
groups (incidence 3, 8, 10, 12%) respectively at 0, 2-->10, 20 and
200 ppm), with the difference from concurrent controls being
statistically significant only at 200 ppm. Frequency of each type of
lymphoreticular tumour (lymphosarcoma, thymic lymphosarcoma, reticulum
cell sarcoma, lymphatic leukaemia and myeloid leukaemia), when
considered alone, was not significantly increased in any treated
group. Latency period (or time of detection of the tumour when the
animal died or was sacrificed) was also not modified by treatment.
Other than the particular malignancy just mentioned, incidence, type
and location of tumours were not significantly higher at 200 ppm than
in the controls. It may be noted that 56% of males and 92% of females
of the concurrent control groups were bearers of tumours with
pituitary adenoma (both sexes), mammary tumour (females) and
pheochromocytoma (both sexes) being the most frequently observed
tumours.
Based on the available data, 20 ppm was without any significant
effect on cholinesterase. The lowest tested level, 2-->10 ppm
(indicated to be equivalent to an average achieved intake, throughout
the study of 0.38 mg/kg body weight/day) may be acknowledged as a
marginal no-effect level on the other tested parameters (Hunter
et al 1981).
Observations in Humans
In a male volunteer given a single oral dose of 9.8 mg or
approximately 0.12 mg/kg of 14C-bendiocarb, over 99% of the
administered dose was excreted in the urine in 22 hours. The plasma
half-life and the half-life for renal elimination for total
radioactivity was estimated at 3.5 and 3.3 hours, respectively. The
major urinary metabolites were sulphate and ß-glucuronide conjugates
of NC 7312, which together amounted to 95% of the radioactivity dose
excreted. Small amounts of conjugated bendiocarb and N-hydroxy methyl
bendiocarb were the only compounds detected in the urine (Adcock and
Challis 1976b).
An area on the forearm of each of two male volunteers was exposed
for 3 hours to 14C-bendiocarb (as an aqueous solution in a wetting
agent) at an average dosage of approximately 0.0013 mg/kg body weight
under occluded conditions in one experiment and non-occluded
conditions in another. (No information was available on the time
interval between the two experiments.) Over the 48-hour period
following exposure, an average of 58% of the applied dose under
occluded conditions vs. an average of 12.5% under non-occluded
conditions was recovered in the urine, suggesting better absorption of
bendiocarb through occluded skin. The only urinary metabolite found
was NC 7312 as conjugates of sulphate and ß-glucuronide (Warner and
Adcock 1980).
In a 60-year old male volunteer given bendiocarb (as an 80%
wettable powder) orally in H2O serially at 0.004, 0.012, 0.037,
0.125, 0.187 and 0.25 mg/kg body weight (dosage in terms of w.p.) with
at least a 48-hour interval between doses, 0.125 mg/kg was found
to be a clear-cut no-effect level on symptoms and whole blood
cholinesterase. Toxic sumptoms such as mild vertigo, nausea and
vomiting together with significant inhibition (30-40%) of whole blood
cholinesterase were induced after the single dose at 0.25 mg/kg body
weight. The symptoms subsided in 30 minutes and whole blood
cholinesterase recovered completely in 4 hours post-treatment. In the
same subject, doses of the 80% w.p. at 0.125 mg/kg given orally at an
interval of 4 hours, but not less, produced no toxic signs or
inhibition of whole blood cholinesterase. A double-blind cross over
experiment involving three other male volunteers indicated a single
dose of 0.004 mg/kg body weight, the only dose administered, of the
80% w.p. resulting in no adverse effects on any of the parameters
assessed (heart rate, clinical signs, mean diastolic/systolic pressure
and whole blood cholinesterase). Following a single dose of 0.12 mg/kg
body weight of the 80% w.p. given orally to the same three volunteers
72 hours later in another similar experiment, a transient depression
(21% of whole blood cholinesterase was observed in one of the three
subjects at 30 minutes post-treatment (Drummond and Kemp 1976).
The safety of bendiocarb (80% wettable powder), when used as an
insecticide against adult mosquitoes, to operators and inhabitants was
evaluated in Iran and Indonesia. The studies were organized along the
line of the WHO expanded Stage V evaluation programme. Each trial
involved the treatment of 13 villages with a total population of over
4 000 by 15 or 16 spray operators. Results indicated that very few
operators reported any toxic signs or symptoms and the latter, when
noted, were usually mild and transient. Inhibition of whole blood
cholinesterase, slight to mild, was only infrequently observed and was
readily reversible. Analysis of urine collected from the operators
suggested absorption of only low levels of bendiocarb during spraying.
No complaints were received from the villagers (Motabar et al 1981;
Bonsall et al 1981).
COMMENTS
Bendiocarb, a carbamate insecticide, evaluated for the first time
by the Meeting, was rapidly absorbed and readily eliminated primarily
in the urine in all of the mammalian species studied, including
humans. The major metabolic pathway in these species involved cleavage
of the carbamate ester group to yield the corresponding phenol
(NC 7312). There was no evidence of tissue accumulation in mammals.
Mammalian acute oral toxicity varied between strains and species, but
not between sexes.
A 3-generation reproduction study, including teratological
evaluation, in rats demonstrated 10 ppm and 50 ppm to be the no-effect
level, respectively, on reproductive parameters and teratogenicity. A
teratogenic study in the same species failed to give any indication of
teratogenicity even at the top dosage level (4 mg/kg body weight)
tested. In a rabbit teratology study, 1 mg/kg body weight was shown to
be a clear-cut teratogenic no-effect level. The available mutagenic
studies including reversion mutation and rec assays, mouse
micronucleus test, mouse lymphoma assay and dominant lethal assay in
rats were all negative. A carcinogenicity study in mice revealed no
carcinogenic activity of the compound, under the conditions of the
experiment. In a long-term feeding study with rats, a significant
increase in incidence of total lymphoreticular tumours, but not
individual type of lymphoreticular tumours, was noted in males of the
highest dosage group (200 ppm). Latency period of these tumours was
not reduced. Although the possibility of the finding in question
regarding lymphoreticular tumours being related to bendiocarb does not
appear likely, it cannot be entirely excluded. Background data on
incidence of total lymphoreticular tumours in CFY strain of rats
maintained in the testing laboratory would be helpful to resolve the
uncertainty. It should be noted that concurrent controls in the study
had a high incidence of spontaneous tumours. Additionally, less than
25% males and 45% females survived the duration of the 104-week study,
although over 50% of animals in all groups including the control were
still alive at the end of 82 weeks. Together these factors might
compromise sensitivity of the study to detect carcinogenic activity of
the compound. This study also demonstrated an increased incidence of
lenticular opacities in male rats at the two top dietary levels.
Neurotoxicity studies in hens gave no indications suggestive of
delayed neurotoxic potential of the insecticide.
No-effect levels were determined in short-term studies in rats
(13-week) and dogs (16-week and 2-year) and a long-term study in rats.
In the dog, however, the Meeting noted the possible adverse effects of
dilution during the determination of tissue cholinesterase, and also
the practice of tissue sampling from overnight-fasted dogs following a
1-hour feeding period. Furthermore, there was some concern on the
absence of data on the levels of food ingestion during the 1-hour
feeding period in one study, and food intake was extremely variable in
the second study.
Observations in humans indicated rapid reversal of cholinesterase
inhibition and of clinical symptoms following both acute and subacute
exposures.
Because of the concerns regarding the increased incidence of
lymphoreticular tumours in male rats of the top dose group in the
long-term study, and the probability that the no-effect level
established for dogs is nominal rather than actual, only a temporary
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0-0.38 mg/kg bw/day
Dog: 0-0.07 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0-0.002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Historical data on the incidence of total lymphoreticular tumours
in the CFY strains of rats used in the long-term study.
2. Short-term (28-day) study in dogs to clarify the relationship
between dietary intake of bendiocarb and terminal erythrocyte and
brain cholinesterase activity, using an appropriate method for
cholinesterase determination.
Desirable
Further investigation on the cataractogenic activity of
bendiocarb at low dosage levels in rats.
REFERENCES
Adcock, J.W. and Challis, I.R. The metabolism of 14C-bendiocarb in
1976a the rat. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1976b The pharmacokinetics and metabolism of bendiocarb in man.
Report from Fisons Ltd. submitted to the World Health
Organization by FBC Limited. (Unpublished)
Ben-Dyke, R. The toxicology of NC 6897. Acute dermal toxicity of
1972 technical bendiocarb, Report from Fisons Limited submitted to
the World Health Organization by FBC Limited. (Unpublished)
Bonsall, J.L., Foulkes, D.M., Goose, J., Leake, C.R. and Reary,
1981 J.B. Safety studies with bendiocarb in a village-scale field
trial against mosquitoes in Indonesia, 1981. Document
(WHO/VBC/81.831), World Health Organization, Geneva.
Challis, I.R. The metabolism of bendiocarb in the pregnant rabbit,
1980 Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in the
1977a mouse. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1977b The metabolism of bendiocarb in the hamster. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Gopinath,
1980 C., Harling, S., Majeed, S. and Prentice, D.E. NC 6897
Toxicity study in beagle dogs (final report dietary intake for
104 weeks). Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Crome, S.J. and Sanderson, D.M. A comparison of the acute oral
1980 toxicities of three batches of bendiocarb CR 4971/2, CR 4799/9
and CR 4500/20 to the male rat. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Cuthbert, J.A. Technical bendiocarb EX Muskegon primary eye irritancy
1980 in rabbits. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
1978 Technical bendiocarb. Primary skin irritancy study on rabbits.
Report from Inveresk Research International, Scotland,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Drummond, P. and Kemp, A. The effects of the acute oral administration
1976 of NC 6897 80 WP to male volunteers. Report TOX/76/133-58 from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hounsell, I.A. Brain cholinesterase inhibition in dogs fed NC 6897.
1974 Report from Fisons Limited Agrochemical Division submitted to
the World Health Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Rush, K.C. The effect of dietary administration
1980 of NC 6897 at 1 250 ppm on whole blood cholinesterase activity
in mice. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Brooks, P.N. The 30-day subchronic oral toxicity
1981 of NC 6897, technical CR 4799/1 in the diet to the hamster.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A.G. The effect of dietary administration of NC 6897 at
1981a 500 and 1 000 ppm on whole blood cholinesterase activity in
male mice of the CFLP and CD1 strains. Report from FBC Limited
submitted to the World Health Organization by FBC Ltd.
(Unpublished)
1981b The effect of dietary administration of NC 6897 at 0.4, 2 and
10 ppm on whole blood cholinesterase activity in male rats.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1981c The effect of dietary administration of NC 6897 at 0.4, 2, 10
and 50 ppm on whole blood cholinesterase activity in male
rats. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Walker, A.K. A micronucleus study in mice using
1982 technical NC 6897, CR 4971/2. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Offer, J.M. and
1978 Gregson, R.L. NC 6897 Toxicity to rats when administered in
the diet for 13 weeks. Final Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Prentice, D.E.,
1981 Gopinath, C. and Gibson, W.A. NC 6897 Toxicity and
tumorigenicity to rats in long-term dietary administration.
Report from Huntingdon Research Centre, England, submitted to
the World Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. Effects on adult female rats of one month exposure to
1977 high dietary concentration of NC 6897 (technical). Report from
Fisons Limited Agrochemical Division submitted to the World
Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. A peri- and post-natal study in rats with technical NC
1978 6897, Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Hounsell, I. Evidence for the reversal of cholinesterase
1974 inhibition by NC 6897 in laboratory animals. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Kemp, A. and Jackson, C.M. A test for the induction of dominant lethal
1977 mutations in male rats by NC 6897. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Brooks, P.N. The effects of the daily oral administration
1978 of NC 7312 to male and female rats for 16 days. Report from
Fisons Limited Agrochemical Division submitted by FBC Limited.
(Unpublished)
Litton. 16-week subacute toxicity study in dogs. NC 6897 technical.
1974 Report from Litton Bionetics Inc., U.S., submitted to the
World Health Organization by FBC Ltd. (Unpublished)
Mallyon, B.A. and Sanderson, D.M. The acute oral toxicity of NC 7312
1977 to male and female rats. Report from Fisons Limited
Agrochemical Division submitted by FBC Limited. (Unpublished)
McConville, M. and McGregor, D.B. Testing the mutagenic activity of
1979 technical NC 6897, Report from Inveresk Research
International, Edinburgh, Scotland, submitted to the World
Health Organization by FBC Limited. (Unpublished)
McConville, M. and Harris, W.J. NC 7312: Ames test for mutagenic
1979 activity. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
McGregor, D.B. and Ross, C.A. NC 6897: The assessment of mutagenic
1981 potential in the mouse lymphoma mutation assay. Report from
Inveresk Research International, Scotland, submitted to the
World Health Organization by FBC Limited. (Unpublished)
Moriya, M., Ohta, T. and Shirasu, Y. KNT (bendiocarb): microbial
1981 mutagenicity study. Report from the Institute of Environmental
Toxicology, Japan, submitted to the World Health Organization
by FBC Limited. (Unpublished)
Motabar, M., Mallyon, B.A., Goose, J. and Adcock, J.W. Document
1981 (WHO/VBC/81.821) World Health Organization, Geneva.
Palmer, A.K. and Allen, P.A. Effect of NC 6897 on neonatal pup
1978 mortality, Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Pearce, J.C., Warner, P.A. and Adcock, J.W. The metabolism of
1977 bendiocarb in the mouse (strain CD 1). Report from Fisons
Limited submitted to the World Health Organization by FBC
Limited. (Unpublished)
Pearce, J.C. and Adcock, J.W. Investigation of residue accumulation
1978 in the rat following repeated administration of
14C-bendiocarb. Report from Fisons Limited submitted to the
World Health Organization by FBC Limited. (Unpublished)
Roberts, N.L. Blood cholinesterase levels in the cow following dietary
1979 inclusion of bendiocarb. Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Ross, D.B., Roberts, N.L., Cameron, D.M., Prentice, D.E. and Majeed,
1978 S.K. Examination of NC 6897 for neurotoxicity in the domestic
hen. Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Sanderson, D.M. The toxicity of NC 6897: Acute toxicity to the rat of
1970 technical grade NC 6897. Report from Fisons Limited submitted
to the World Health Organization by FBC Limited. (Unpublished)
1971 The toxicology of NC 6897: Acute toxicity of pure NC 6897.
Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1972 Toxicology of NC 6897: 15-dose cumulative dermal study with
Ficam in male rats. Report from Fisons Limited submitted to
the World Health Organization by FBC Limited (Unpublished)
Sanderson, D.M. and Hounsell, I.A. The toxicology of NC 6897. Test
for neurotoxicity of NC 6897 to the chicken. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Sanderson, D.M. and Mallyon, B. The acute oral toxicity to the
1977 hamster of technical bendiocarb. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
1979 The acute inhalational toxicity to rats of Ficam 80W, an 80%
wettable powder formulation of bendiocarb, CR 13374/27H.
Report from Fisons Agrochemical Division submitted to the
World Health Organization by FBC Limited. (Unpublished)
Serota, D.G., Voelker, R.W. and Fezio, W.L. A chronic toxicity and
1981 carcinogenicity study in mice, NC 6897. Final report from
Hazleton Laboratories America, Inc. submitted to the World
Health Organization by FBC Limited. (Unpublished)
Tesh, J.M., Bartlett, A., Tesh, S.A., Whitney, J.C. and Finn, J.P.
Technical NC 6897 (CR 4799/1): Effects of dietary
administration upon reproductive performance and teratogenic
response in rats treated continuously through three successive
generations. Report from Life Science Research, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Tesh, J.M., Ross, F.W. and Tesh, S.A. Technical NC 6897: Effects of
1980 oral administration upon pregnancy in the rabbit. Report from
Life Science Research, England, submitted to the World Health
Organization by FBC Limited. (Unpublished)
Tucker, M.L. NC 6897: Teratogenicity study in the rat. Report from
1974 Consultox Laboratories Ltd., England, submitted to the World
Health Organization by FBC Limited. (Unpublished)
Warner, P.A., Adcock, J.W. and Pearce, J.C. The metabolism of
1977 14C-bendiocarb in the dog. Report from Fisons Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Warner, P.A. and Adcock, P.A. The dermal absorption of 14C-bendiocarb
1980 in man. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
BENDIOCARB
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bendiocarb is available as a wide range of formulations,
including wettable powders (200, 500 and 800 g a.i./kg); granules
(30, 50 and 100 g a.i./kg); dust (10 g a.i./kg); oil suspension for
ULV application (250 g a.i./kg); residual sprays, paint-on and
granular baits and aerosols.
Bendiocarb is used for the controls of soil and foliar insect
pests on a wide range of crops, ectoparasites on domestic animals and
mosquitos and nuisance pests. The Meeting was informed that it is sold
for these uses in approximately 100 countries. Information was
provided to the Meeting on official nationally approves uses (good
agricultural practices, GAP) by the Netherlands. Good agricultural
practices of The Netherlands are summarized in Table 1 (Netherlands
1982).
Table 1. Bendiocarb Good Agricultural Practices, Netherlands 1982
Application Used
Crop Pests rate (kg/ha) Formulation Treatment Since
Sugarbeet Fritfly, wireworm, 0.3-0.4 3% G seed furrow 1979
spring tails, 0.4 80% WP seed furrow 1981
pygmy beetle 500 g/l
liquid
6.4 g/kg " seed dressing 1981
seed
Maize Fritfly, 0.3-0.4 3% G seed furrow 1979
wireworm 4 g/kg 80% WP seed dressing 1981
seed 500 g/l
liquid
The Meeting was provided with a tabulation of recommended
application rates, which are summarized in Table 2 (Netherlands 1982).
No information was provided on preharvest intervals. The recommended
application rates are consistent with GAP of The Netherlands for maize
and sugarbeets. Information was not provided on whether the
recommended uses other than on sugarbeet and maize are also nationally
approved and, if so, where.
Table 2. Recommended Application Rates of Bendiocarb, Netherlands 1982
Crop Major pests Formulation Rate (a.i.)
Maize Wireworm, symphlids, fritfly Granule 0.3-0.35 kg/ha
Wireworm, symphlids, fritfly Seed dressing 4 g/kg seed
Corn rootworm Granule 0.84-1.12 kg/ha
Soil pests (in furrow) Spray 0.4 kg/ha
Sugarbeet Wireworm, pygmy beetle,
springtails Granule 0.3-0.35 kg/ha
Wireworm, pygmy beetle,
springtails Seed dressing 6 g/kg seed
Soil pests (in-furrow) Spray 0.4 kg/ha
Rice Rice water weevil, stem
borer Granule 2 kg/ha
Leafhoppers, planthoppers Spray 0.5 kg/ha
Leafhoppers, planthoppers Dust 0.5-0.75 kg/ha
Oilseed Flea beetle Granule 0.14-0.28 kg/ha
rape Spray 0.1-0.2 kg/ha
Ryegrass Fritfly, wireworm,
leatherjackets Granule 0.75 kg/ha
Spray 0.5-0.75 kg/ha
Seed dressing 10 g/kg seed
Cereals Fritfly, wireworm,
leatherjackets Spray 0.75 kg/ha
Seed dressing 2-4 g/kg seed
Potatoes Colorado beetle Spray 0-125-0.25 kg/ha
tuber moth Spray 0.5 kg/ha
Brassicas Diamond-back moth Spray 0.1-0.8 g/l
Peas Pea moth, pea weevil Spray 0.2-0.5 kg/ha
Apple/Pear Codling moth, tortrix moth,
aphids Spray 0.25-0.6 kg/ha (LV)
Mushrooms Mushroom fly Wall spray 30-50 g/100 m2
Preharvest Uses
Residue trials utilizing preharvest treatments have been
conducted on a variety of plant and animal products. Results are
detailed under "Residues Resulting From Supervised Trials".
Postharvest Uses
The Meeting was informed of residue investigations in wheat grain
stored in small bins for six months after treatment of interior
surfaces, but no details were provided. Likewise, no details were
provided on studies said to have been conducted on foodstuffs treated
in food-handling premises.
Other Uses
The Meeting was informed that 80 W or ULV formulations may be
used for control of mosquitos and the ULV formulation against
warehouse pests. Studies have been conducted to determine the extent
of potential exposure. Although contamination levels were said to be
within safe limits, details of these studies were not provided.
Bendiocarb is said to be widely used for control of cockroaches
and other nuisance pests in and around the home, hotels, food-handling
establishments, aircraft etc. and as a paint for fly control in animal
houses. No details on these uses were provided.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries on a wide
range of commodities, representing a variety of treatments and
climatic conditions.
Root and Tuber Vegetables
Potatoes
Twenty five trials were conducted in three European countries
either by spray, broadcast or in-furrow treatments with either 3G or
20W formulations. Results are summarized in Table 3 (FBC 1982a; Reary
1979d; Browne and Reary 1981; Browne 1981e). Although most of the data
were from application of the 3G formulation, no information was
provided on recommended or approved uses for that formulation on
potatoes. The wettable powder spray applications were at one and two
times the recommended rate. Recoveries were generally 75% or better
for bendiocarb and 60% or better for NC 7312. The limit of
determination is said to be approximately 0.01 and 0.02 mg/kg for
bendiocarb and NC 7312, respectively.
Table 3. Bendiocarb Residues in Potatoes, from Supervised Trials
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 2 4-5 3G 1 kg/ha In-furrow 119-155 0.01-0.03 <0.02,0.26
1978 2 4-5 3G 2 kg/ha In-furrow 119-155 0.01-0.04 0.02,0.58
2 4-5 3G 3 kg/ha In-furrow 119-155 0.01,0.03 0.04,0.69
1 7 3G 1 kg/ha Broadcast 112 0.01 0.02
1 7 3G 1.5 kg/ha Broadcast 112 0.04 0.05
2 4-7 3G 2 kg/ha Broadcast 112-155 <0.01-0.03 <0.02,0.07
2 4-7 3G 3 kg/ha Broadcast 112-155 <0.01-0.04 <0.02,0.11
2 4-7 3G 4 kg/ha Broadcast 112-155 0.01-0.03 0.03,0.08
3 Untreated
controls 0.003-0.008 0.003-0.0.7
German Federal 3 3 20W 0.12 kg/ha Spray 03/ <0.01 0.014-0.12
Republic 3 3 (<0.005-0.017) (<0.01-0.14
1980 2 3 7 <0.01 <0.01-0.14
(<0.005-0.009) (<0.01-0.15)
14 <0.01 0.01-0.15
(<0.005-0.015) (<0.01-0.18)
1 3 15 <0.005) 0.014
3 2 - - Untreated
controls - 0.001-0.044/ (<0.01-0.024)
0.001-0.007
Table 3 (con't)
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 1 (3 plots) 20W 0.53 kg/ha Spray 145/ <0.01 0.03-0.05
1981 1 (3 plots) 20W 1.06 kg/ha 145/ 0.005 0.06-0.07
1 Untreated
controls <0.005 0.01
1/ Limit of determination 0.01 mg/kg for bendiocarb and 0.02 mg/kg for NC 7312.
2/ Means of replicates; range of individual replicates in parentheses.
3/ Day 0 represents final spray of a weekly 3-spray programme, each application at 0.12 kg/ha.
4/ <0.01 except the 0.04 mg/kg value.
5/ Days after final spray of 2- or 3-spray programme, commencing 7 or 14 days before the final application.
Maximum bendiocarb residues at harvest resulting from at or
before harvest treatments were 0.04 mg/kg (mean of replicates) and
0.7 mg/kg for NC 7312.
From multiple spray applications, maximum bendiocarb residues at
harvest were < 0.01 mg/kg (mean of replications) or 0.017 mg/kg for
individual replicates. Maximum residues of NC 7312 were 0.15 mg/kg
(mean of replicates) or 0.18 mg/kg for individual replicates. For both
the means of the replicates and individual replicates residues of
bendiocarb and NC 7312 were each generally comparable from 0 to 15
days after last application. Apparent residues in untreated controls
were generally < 0.01 mg/kg for bendiocarb and < 0.02 mg/kg for
NC 7312. Residues of NC 7312 at harvest were 1-20 times that of
bendiocarb per se, and averaged six times higher.
Since residues from in-furrow or broadcast applications can be
0.04 mg/kg and untreated controls can, on occasion, reach this level,
a 0.05 mg/kg for bendiocarb per se can be supported. If NC 7312 were
also included, a limit of 0.5 mg/kg would be in order for combined
residues.
Sugarbeet
Thirty one supervised residue trials were conducted in five
European countries and residue decline studies in three (Table 4)
(Browne 1982c; FBC 1982b; Reary 1974, 1975e, 1979h,i,n,q, 1980f,j).
The seed treatment applications of 80W were at 0.8-2.7 times the
recommended 6 g/kg rate, which is also consistent with GAP of The
Netherlands. In-furrow applications of a granular formulation were
representative of the 0.3-0.4 kg a.i./ha GAP of The Netherlands and
recommended application rates.
In the supervised trials, bendiocarb residues at harvest were
below 0.01 mg/kg. Some samples were also analysed for the metabolite
NC 7312 and all were below the 0.01 mg/kg limit of determination.
Similarly, in the decline studies, residues in sugarbeet roots were
less than the 0.02 mg/kg limit of determination. Residues in untreated
controls were generally < 0.01 mg/kg, except for one set of trials
where residues ranged up to 0.11 mg/kg (average 0.005 mg/kg). The same
basic analytical procedure was used in all of the trials, but modified
in some to permit analysis of NC 7312. The limit of determination for
sugarbeet generally ranged from 0.005-0.02 mg/kg for bendiocarb and
0.01-0.02 mg/kg for NC 7312. Recoveries were generally > 75% for
parent compound and > 60% for NC 7312. In general, recoveries were
less in tops than in roots and less for NC 7312 than for bendiocarb.
Table 4. Bendiocarb Residues in Sugarbeets From Supervised Trials and Residue Decline Studies
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
Denmark 1 1 80W 15 g/kg seed Pelleted seed 204 roots <0.005
1974 1 untreated controls roots <0.005
France 2 8 80W 12 g/kg seed Pelleted seed 163 roots <0.005
1974 2 untreated controls roots <0.005
German 1 1 6 g/kg seed Seed dressing 196 roots <0.005
Federal 1 1 196 tops <0.005
Republic 1 1 80W 12 g/kg seed Seed dressing 196 roots <0.005
1974 1 1 196 tops <0.005
untreated controls roots or
tops <0.005
U.K. 2 4 80W 8 g/kg seed Pelleted seed 213 roots <0.005
1974 untreated 213 tops <0.0055/
2 control roots <0.005
tops <0.0055/
France 3 3 3G 0.36 kg/ha In furrow 160-168 roots <0.005
1976 3 3 160-168 tops <0.01
3 6 3G 0.45 kg/ha In furrow 171-190 roots <0.005
3 6 171-190 tops <0.01
3 6 untreated controls tops and
roots <0.006
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
France 2 2 3G 0.46 kg/ha In furrow 180-185 roots <0.005
1977 2 2 180-185 tops <0.01
2 2 untreated controls tops and <0.006
roots
France6/ 2 3G 0.3 kg/ha In furrow 152-170 roots <0.01
1978 2 152-170 tops <0.01
2 3G 0.6 kg/ha In furrow 152-170 roots <0.01
2 2 untreated controls tops and
roots <0.005
Italy6/ 1 1 3G 0.3 kg/ha In furrow 110 roots <0.01
1978 1 1 110 tops <0.01
1 1 3G 0.6 kg/ha In furrow 110 roots <0.01
1 untreated controls tops and
roots <0.005
U.K.6/ 3 3 3G 0.3 kg/ha In furrow 154-188 roots <0.01
1978 3 3 154-188 tops <0.01
1 1 3G 0.33 kg/ha In furrow 202 roots <0.01
1 1 202 tops <0.01
1 1 3G 0.4 kg/ha In furrow 188 roots <0.01
188 tops
1 1 untreated controls tops and
roots <0.007
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K.6/ 6 1 3G 0.3 kg/ha In furrow 144-167 roots <0.01
1979 6 1 144-167 tops <0.01
6 6 untreated controls tops and
roots <0.01
Italy6/ 1 3G 0.30 kg/ha In furrow 154 roots <0.01
1981 1 154 tops <0.01
1 3G 0.32 kg/ha In furrow 183 roots <0.01
1 183 tops <0.01
2 2 Untreated roots <0.001
controls tops 0.003
German 4 12 80W 5 g/kg seed Seed dressing 71-166 roots <0.02
Federal tops <0.02
Republic 4 12 Untreated roots 0.002-0.11
1978 4 12 controls (0.005 av.)
tops 0.005-0.015
(0.008 av.)
3 8 3G 0.33 kg/ha In furrow 56-70 roots <0.01
3 9 tops <0.01
71-166 tops <0.015
5 Untreated 71-166 roots <0.01
controls tops <0.01
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K. 1 5 3G 0.3 kg/ha In furrow 25-40 roots 1.367/
1979 41-55 roots 0.127/
56-70 roots <0.01
71-166 roots <0.01
7 56-70 tops <0.01
71-166 tops <0.01
1 5 Untreated 56-70 roots <0.01
controls tops <0.01
1/ Bendiocarb only (see also 6/);
2/ Corrected for recoveries of >75% except for 59% recovery in tops. Fortification levels ranged from 0.02-0.01 mg/kg;
3/ Limit of determination in individual trials generally ranged from 0.005-0.02 mg/kg;
4/ Duplicate analyses of 0.006 and 0.003 mg/kg;
5/ Individual values up to 0.07 mg/kg but <0.005 mg/kg when repeated;
6/ Sample also analysed for primary metabolite NC 7312. Residues after 56 days were generally below the 0.01 mg/kg limit
of detection except one 0.03 mg/kg value in tops (limit of determination in that trial or a 0.02 limit of determination.
Residues in seedlings were 0.42 and 0.07 mg/kg at 28 and 41 days respectively after in-furrow application at planting;
7/ Seedlings analysed whole.
Since residues are below the claimed limits of determination, a
limit of 0.02 mg/kg bendiocarb per se would probably not be
unrealistic. However, because of high apparent residues in one
untreated control, a 0.05 mg/kg limit at or about the limit of
determination may be more suitable. Based on a 0.02 mg/kg limit of
determination for NC 7312, a limit on the order of 0.1 mg/kg would be
appropriate if both bendiocarb and free NC 7312 were included. Actual
residues were too low to determine whether residues of NC 7312 exceed
those of bendiocarb, but judging from those found in potatoes, it is
likely.
Leafy Vegetables, Except Brassicas
Sugarbeet leaves
From the residue trials and decline studies discussed previously
(Table 4), residues in sugarbeet tops at harvest from recommended
application rates were generally less than the 0.01-0.03 mg/kg limits
of determination. Only one trial had a limit of determination of
0.03 mg/kg for tops. The limit of determination was considered to be
< 0.02 mg/kg for the remaining 30 trials. Bendiocarb residues were
1.4 and 0.12 mg/kg in seedlings at 28 and 41 days, respectively, after
in furrow treatments at planting. The data support a 0.05 mg/kg at or
about limit of determination at harvest as the maximum residue level.
Brassica Leafy Vegetables
Cabbage
No information was available on specific nationally approved
uses. Supervised trials with a wettable powder formulation were
conducted in one country (Table 5) (Browne 1981a,c, 1982a,b; Browne
and Manley 1982), at application rates up to 0.25 times the maximum
recommended application rate (Table 2). No specific preharvest
interval was recommended. Maximum residues ranged from 0.18 mg/kg one
day after the last of seven applications to <0.01 mg/kg after 14
days. Untreated controls were < 0.02 mg/kg and analytical
recoveries averaged 82% from fortification levels at 0.08-0.2 mg/kg.
The limit of determination was said to be 0.01 mg/kg, but considering
untreated control analyses, a 0.05 mg/kg limit of determination may be
more practical.
Because the trials do not reflect maximum recommended application
rates, recommended usage does not specify the number of applications
or a preharvest interval and the data are minimal, a maximum residue
level for bendiocarb in cabbage cannot be estimated with confidence.
Table 5. Bendiocarb and NC 7312 in Crops from Supervised Trials
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Cabbage1/ Philippines 7 0.06 50W 1 0.1 3
1981 0.1 a.i./l 3 0.04 3
7 0.01 3
14 <0.01 3
0.12 50W 1 0.18 3
0.2 g a.i./l 3 0.05 3
7 0.02 3
14 <0.01 3
Untreated 1 <0.01 3
control 3 0.02 3
7 0.01 3
14 <0.01 3
Peas2/ U.K. 1 0.2 50SC3/ 27 <0.01 <0.01 5
flowering 1981 1 0.3 <0.01 <0.01 5
stage (MarQ) 1 0.4 <0.01 <0.01 5
1 0.05 <0.01 <0.01 5
Untreated
control <0.01 <0.01 5
4-leaf 1 0.2 27 <0.01 <0.01 5
stage 1 0.3 <0.01 <0.01 5
1 0.4 <0.01 <0.01 5
1 0.5 <0.01 <0.01 5
Untreated
control <0.01 0.01 5
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pea pods 1 0.2 50SC3/ 83 <0.01 <0.02 5
flowering 1 0.3 <0.01 <0.06 5
stage 1 0.4 <0.01 0.07 5
1 0.5 <0.01 0.03 5
Untreated
control <0.01 0.03 5
4-leaf
stage 1 0.2 83 <0.01 0.05 5
1 0.3 <0.01 0.05 5
1 0.4 <0.01 0.08 5
1 0.5 <0.01 0.06 5
Untreated <0.01 0.05 5
control
Apple
(Cox's U.K. 24/ 0.04 and 20W 57 <0.01-0.01 <0.01 6
Orange 1981 0.08% 50W,
Pippin) 50 SC
Untreated 0.006 0.005 1
14/ 0.04 and 20W, 106 <0.01-0.01 <0.01-0.01 6
0.08% 50W,
50SC 0.009 0.003 1
4/ Untreated
2 0.02, 20W, 98 <0.01-0.01 <0.01-0.01 9
0.04 and 50W,
0.08% 50SC
untreated 0.003 0.008 1
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pear 14/ 0.02% 50SC 158 0.01 <0.01 1
(Conference) 14/ 0.04% 0.02 0.01 1
14/ 0.08% untreated 0.01 0.005 1
Mushrooms The Netherlands
1980 2 5 50SC 21 0.04-0.06 0.01-0.03 4
(50 g a.i./ <0.05 av.) (<0.02 av.)
100 m2)
2 7.5 50SC 21 0.08-0.12 0.01-0.04 4
(75 g a.i./ (0.1 av.) (0.02 av.)
100 m2)
2 10 50SC 21 0.11-0.14 0.02-0.06 4
(100 g a.i./ (0.13 av.) (0.04 av.)
100 m2)
Untreated <0.01-0.02 <0.01-0.1 4
(0.01 av.) (0.01 av.)
U.K. g a.i./100 m2
1981 wall spray5/
1 52.2 50SC 69 N.D.6/ <0.02 1
untreated 69 N.D. N.D. 1
1 20 80W 33 N.D. N.D. 1
untreated 33 0.001 N.D. 1
1 48 80W 41 <0.02 0.02 1
untreated 41 0.04 0.02 1
(g a.i./100 m2
wall spray 5/)
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Mushrooms U.K. 1 20 80W 26 <0.02 <0.02 1
1981 untreated 26 N.D. N.D. 1
1 20 80W 33 <0.02 <0.02 1
untreated 33 N.D. 0.003 1
1 20 80W 52 N.D. <0.02 1
20 80W 60 0.04 0.02 1
untreated 52 N.D. N.D.
48 80W 42 0.06 0.1 1
48 80W 42 0.09 0.13 1
48 80W 42 0.08 0.10 1
1/ Residue results corrected for mean analytical recovery of 82% with fortification at 0.08-0.2 mg/kg.
2/ Results corrected for recoveries. Harvest (127 days after planting) said to be 2-3 weeks after normal harvest.
3/ SC = Suspendable concentrate.
4/ 1.5 1 spray solution per tree.
5/ Mushroom plots established the day after wall treatment.
6/ N.D. = no detectable chromatographic peaks.
Legume Vegetables
Peas
Supervised residue trials were conducted in the U.K. with single
applications at rates consistent with recommended ones. No information
was available on specific nationally approved good agricultural
practices, nor was information available on recommended numbers of
applications or preharvest intervals.
Peas were sampled at 2-3 weeks after normal harvest (127 days
after planting). At all application rates tested, residues of either
bendiocarb or NC 7312 (corrected for recovery) were <0.01 mg/kg in
peas, including untreated controls (Table 5). The same is true for
bendiocarb in pea pods, but residues of NC 7312 ranged from
0.03-0.08 mg/kg (0.05 mg/kg av.), and untreated controls were 0.02 and
0.05 mg/kg. Although bendiocarb residues were at or about the limit of
determination, the data suggest that terminal residues of NC 7312 may
be 2-10 times that of bendiocarb.
Analytical recoveries averaged 86 and 72% for bendiocarb and NC
7312, respectively, in peas from fortification levels of 0.04 and
0.2 mg/kg. Recoveries in pods averaged only 58 and 63%, respectively.
The limit of determination was said to be 0.01 mg/kg for bendiocarb
and NC 7312 in both peas and pods. However, based on untreated
controls, 0.05 mg/kg may be a more appropriate limit of determination
for NC 7312 in pea pods.
Because GAP information is unavailable and samples were not
harvested at normal harvest, a maximum residue level cannot be
estimated with confidence.
Pome Fruit
Apples and Pears
Residue data were available from supervised trials in the U.K. at
application rates of 0.02-0.08% active ingredient (1.5 1/tree),
representing several formulations. Applications were at pre- or
post-blossom and/or at codling stage, and fruit was sampled at normal
harvest of 57-158 days after last treatment. No information was
available on specific nationally approved agricultural practices.
Recommended applications rates of 0.25-0.5 kg/ha are not directly
comparable to the 0.02%-0.08% used in the field trials. No information
was provided on recommended preharvest intervals or numbers of
applications.
Of the 24 apple and pear samples treated in three locations and
in untreated controls, residues of bendiocarb and NC 7312 were
< 0.01 mg/kg, except for one 0.02 mg/kg value for pears treated
once with a 0.08% 50 SC formulation (Table 5). Mean recoveries of
bendiocarb and NC 7312 on apples and pears were 94 and 73%,
respectively, when fortified from 0.1 to 0.4 mg/kg. The limit of
determination is said to be 0.01 mg/kg for both bendiocarb and
NC 7312.
If the formulations, application rates, number of applications
and preharvest intervals of the field trials represent GAP, the data
could support a maximum residue level of 0.02 mg/kg for bendiocarb per
se or 0.05 mg/kg for combined residues of bendiocarb and NC 7312.
Cereal Grains
Barley
Fourteen supervised trials were conducted in the U.K. with
in-furrow granular, 50W spray or 80W seed dressing applications. No
nationally approved agricultural practice information was provided,
although recommended uses for cereals were reported (Table 2). No
recommended uses for the granular formulation (2 trials) were
provided. Only two of the trials were from spray treatments and the
remainder were from seed treatments. The spray applications at 0.5 and
1.0 kg a.i./ha reasonably reflect the recommended 0.75 kg a.i./ha
although the data are limited (Table 6) (Browne and Reary 1980c,d,e;
Housden and Reary 1981; Reary 1978 h,i,j,n; Reary 1979e,f,g,j,k,l,m,o,
1980a,e; Reary and Browne 1978). The seed treatments at 2-5 g a.i./kg
seed reflect the recommended rate of 2-5 g a.i./kg.
Residues of bendiocarb at normal harvest in barley grain were
<0.02 mg/kg and <0.03 mg/kg in straw. Untreated controls in
barley grain and straw were at the same level. Some samples were
also analysed for combined conjugated metabolites, NC 7312 and
N-hydroxymethylbendiocarb. In all three trials, metabolite residues
were <0.02 mg/kg. Mean analytical recoveries for bendiocarb in
barley during the supervised trials were 94 and 84%, respectively, for
grain and straw when fortified at 0.02-0.2 mg/kg. In a separate study,
bendiocarb recoveries in barley grain were 61-125% (av. 94%) after
fortifications at 0.02-0.2 mg/kg (Reary 1979b).
Table 6. Bendiocarb Residues in Cereals from Supervised Trials
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
Barley, wheat and oats
U.K. 1 1 80W 4 g/kg seed Seed dressing 148 barley grain <0.02
1977 1 1 148 barley straw <0.02
1 2 80W Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
1 2 50SC 4 g/kg seed Seed dressing 148 barley grain <0.02
1 2 148 barley straw <0.02
1 2 50SC Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
U.K. 3 3 80W 4 g/kg seed Seed dressing 113-145 barley grain <0.02
1978 2 2 113-145 barley straw <0.03
2 2 124-137 wheat grain <0.02
1 1 124-137 wheat straw <0.06
3 3 (10 rep) 134-141 oat grain <0.02
2 2 (10 rep) 134-141 oat grain <0.02
2 2 80W 8 g/kg seed Seed dressing 136-147 oat grain 0.47
3 2 136-147 oat straw 0.02-0.05
U.K.1/ 1 1 3G2/ 0.5 kg/ha In furrow 148 barley grain <0.01
1979 1 1 148 barley straw <0.01
1 1 3G2/ 1.0 kg/ha In furrow 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 50W2/ 0.5 kg/ha Spray 148 barley grain <0.01
148 barley straw <0.01
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 50W2/ 1.0 kg/ha Spray 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 80W 2 g/kg seed Seed dressing 336 wheat grain <0.01
1 1 336 wheat straw <0.01
3 3 80W 4 g/kg seed Seed dressing 119-148 barley grain <0.01
119-148 barley straw <0.01-0.02
3 3 127-336 wheat grain <0.01
2 2 127-336 wheat straw <0.01
2 2 105-125 oat grain <0.012
2 2 105-125 oat straw <0.01,<0.03
2 2 80W 5 g/kg seed Seed dressing 119-127 barley grain <0.01
2 2 119-127 barley straw <0.01,<0.02
1 1 127 wheat grain <0.01
1 1 127 wheat straw <0.01
2 2 105-125 oat grain <0.01
2 2 105-125 oat straw <0.01
Maize harvest samples
Canada 22 37 10G 0.84-2.24 band 153-189 grain <0.02
U.S. 8 8 kg/ha at 132-165 cob <0.02
1977-78 21 38 plant 132-189 stover3/ <0.03
10 28 110-134 silage <0.023
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
German 25 30 3G 0.2-0.8 in furrow 133-184 grain <0.024/
Fed. Rep. 26 36 kg/ha at plant 128-184 cobs <0.024/
5 12 128-184 plants <0.01
U.K. 10 10 146-184 foliage <0.01
France 5 5 135-164 silage <0.02
Italy 133-154 stover <0.02
1976-79
France 30 36 76 or 2-16 g/kg seed 125-189 grain <0.035/
U.K., U.S. 30 24 80W seed treatment 160-161 cob <0.026/
1975-1979 18 23 125-167 silage <0.02
11 10 160-189 stover <0.02
14 8 141-164 plants <0.056/
Rice (rough) harvest samples
Philippines7/ 1 1 5G 1.5 + 0.5 Incorporation + 53 grain <0.01
1978 surface applied straw <0.02
1 1 5G 2.0 + 0.6 Incorporation + 53 grain <0.01
surface applied straw <0.02
1 1 50W 0.5 kg/ha Spray 90 grain 0.01
straw <0.02
1 1 50W 0.6 kg/ha Spray 90 grain <0.01
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
2 2 80W 0.5 kg/ha Spray 60-90 grain <0.01
straw <0.02
2 2 80W 0.6 kg/ha Spray 60-90 grain <0.01
straw <0.02
Philippines7/ 1 1 50W 0.5 kg/ha Spray 248/ grain <0.01
1979 straw <0.01
1 1 50W 0.7 kg/ha Spray 248/ grain <0.01
straw 0.01
1 1 50W 1.0 kg/ha Spray 248/ grain 0.01
straw <0.01
1 1 50W 0.5 kg/ha Spray 459/ grain 0.01
straw -
1 1 50W 0.75 kg/ha Spray 459/ grain <0.01
straw -
1 1 50W 0.5 kg/ha Spray 4910/ grain <0.01
straw <0.01
1 1 50W 0.75 kg/ha Spray 4910/ grain 0.01
straw 0.02
South Korea7/ 1 1 50W 0.75 kg/ha Spray 10 grain 0.6, 1.111/
1979 straw <0.6
1 1 2D 0.8 kg/ha Dust 10 grain 1.5, 1.411/
straw <0.65
1 1 3GW 0.9 kg/ha Seed box 119 grain 0.06, 0.03
incorporation straw 0.02
(100 g/box)
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 5G 1.5 kg/ha Soil 100 grain <0.6
incorporation straw 0.04
Philippines
1980 1 1 50W 0.25 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.50 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.75 kg/ha Spray 6212/ grain <0.02
straw <0.01
1/ Also analysed for combined residues of conjugated NC 7312 and N-hydroxymethylbendiocarb. All metabolite residues were
< 0.02 mg/kg except for residues of 0.07 and 0.05 mg/kg from one trial in oat grain from seed dressing of spring oats
with an 80 WP formulation at 4 and 5 g a.i./kg seed. The two residues resulted from oat grain samples with bendiocarb
residues 0.012 and 0.007 mg/kg respectively. Untreated controls were 0.011 mg/kg.
2/ Seed previously treated with an 80% WP formulation
3/ Stover = leaves and stems after removal of cobs.
4/ Analysis of oil from kernels with <0.02 mg/kg residue from one site showed no residue greater than the 0.02 mg/kg limit
of determination.
5/ The 0.03 mg/kg on grain resulted from treatments at 8 and 12 g a.i/kg seed. At <4 g a.i./kg seed residues were
<0.02 mg/kg in grain.
6/ Maize (sweet corn) comprised 14 of the trials at 2-8 g a.i./kg. All plants in this group were sweet corn. The 0.05 mg/kg
residue on sweet corn plants resulted from 2-4 g a.i./kg seed.
Table 6 (con't)
7/ Also analysed for metabolite NC 7312. No residues above the limits of detection (0.01 mg/kg in grain and 0.02 mg/kg in
straw) were found, except for 0.11 and 0.17 mg/kg in grain from dust and spray treated samples at the 10-day interval and
one 5G formulation at 100 days, in which it was 0.03 mg/kg. In the latter, the control was also 0.03 mg/kg.
8/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 17, 27, 39, 52,
66 and 80 days after transplanting.
9/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 10, 25 40 and
55 days after transplanting.
10/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 7, 20 and 45
days after transplanting.
11/ Values reflect residue determination by electron capture and fluorescence, respectively.
12/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 22, 35, 51 and
68 days after transplanting.
The limit of determination was said to be 0.01 mg/kg for both
cereal grain straws and grains, although apparent residues in controls
(<0.02 and <0.03 mg/kg in barley grain and straw, respectively)
suggest 0.02 or even 0.05 mg/kg may be a more realistic estimate for
both. When considered with data from other cereal grains and straws, a
0.05 mg/kg limit for bendiocarb per se at or about the limit of
determination could be supported if recommended uses represent GAP. A
0.1 mg/kg limit would be appropriate if both bendiocarb and
metabolites are included.
Maize
Over 100 supervised trials were conducted in six countries, two
in North America and four in Europe (Table 6). Applications were made
with granular formulations at planting, either by band or in-furrow
treatment and seed was treated with wettable powder formulations.
In-furrow applications of a 3G formulation at 0.2-0.8 kg a.i./ha
corresponds to the 0.3-0.4 kg a.i./ha Netherlands GAP, although no
data were available from The Netherlands. The recommended uses on
maize provided to the Meeting did not include the method of
application for granular formulations.
Granular band treatments at planting of 0.84-2.24 kg a.i./ha did
not correspond with certainty to either The Netherlands GAP or
recommended usage for maize. The 2-16 g a.i./kg wettable powder seed
treatments more than accommodate the 2-4 g a.i. GAP of The Netherlands
or recommended application rates. Additional information on official
national GAP is needed.
Maximum residues of bendiocarb per se from all treatments were
<0.03, <0.02, <0.023, 0.05 and <0.01 mg/kg in grain, cobs,
stover, silage, plants and foliage, respectively. Analysis of oil from
one grain sample with field-incurred residues of <0.02 mg/kg also
showed residues of less than the 0.02 mg/kg limit of determination.
The 0.03 mg/kg level in grain resulted from applications at 2-3 times
the recommended rate. At recommended rates all grain residues were
<0.02 mg/kg. The 0.05 mg/kg residue on plants was on maize (sweet
corn) treated according to recommended rates.
The limit of determination was said to be 0.01-0.02 mg/kg for the
various maize fractions. Apparent residues in untreated controls were
also in this range. Analytical recoveries during the trials averaged
approximately 81, 78, 72, 80 and 78% for grain, cob, silage, stover
and plants, respectively. A 0.05 mg/kg limit at or about the limit of
determination could be supported for bendiocarb per se in maize (field
corn) grain, fodder and/or forage. Additional data would be required
for inclusion of metabolites.
In addition to the trials at harvest, 12 residue decline trials
were conducted in four countries (FBC 1982a; Browne and Reary 1979d;
Reary 1978l,m, 1979p, 1980h,i), mostly from granular in-band or
in-furrow treatments at planting ranging from 0.3-2.24 kg a.i./ha.
Residues in grain from granular treatments were <0.01 mg/kg. In
plants, residues ranged from <0.05-0.9 mg/kg at 25-40 days after
treatment (0.9 at 29 days), declining to <0.07 mg/kg after 49 days
and <0.02 after 56-70 days. Residues in stover and cobs were <0.02
and <0.02 mg/kg, respectively, after 71 days.
From the single 4 g a.i./kg seed treatment with a wettable powder
formulation, residues on plants were 12.4 mg/kg 29 days after
treatment but rapidly declined to _0.023 mg/kg after 42 days.
Oats
Residue data were available for seven supervised trials in the
U.K. from 4-8 g a.i./kg seed 80W seed dressings (Table 6). No
information was provided on specific nationally approved practices.
At application rates approximating the 4 g a.i./kg seed
recommended application rate, residues in oat grain at normal harvest
were 0.02 mg/kg and in straw were _0.02 mg/kg. One of the two grain
samples treated at 8 g a.i./kg seed had a residue of 0.47 mg/kg.
Contamination of this sample and its 0.12 mg/kg untreated control was
suspected by the manufacturer to have occurred during sampling.
Maximum residues in straw from the 8 g a.i/kg seed application rate
were 0.05 mg/kg. While this could be aberrant, as the untreated
control was 0.12 mg/kg, it is not necessarily so, since residues are
as high as 0.03 mg/kg in straw of oats and barley from recommended
application rates. Residues in untreated controls were <0.02 and
<0.04 mg/kg in grain and straw, respectively, except for 0.12 mg/kg
from a grain and straw sample with suspected contamination.
A limited amount of residue data were also available for combined
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) in some
oat samples, with residues being <0.012 mg/kg and <0.01 mg/kg in
grain and straw, respectively.
Mean recoveries of bendiocarb were 89 and 94% for oat grain and
straw, respectively. The limit of determination was said to be
0.02 mg/kg for both, but based on the 0.035 mg/kg apparent residue in
untreated controls, 0.05 mg/kg may be more appropriate, especially
when barley and wheat are also considered. A 0.05 mg/kg limit at or
about the limit of determination could be supported for bendiocarb per
se if recommended uses represent GAP. A 0.1 mg/kg limit would be
required if both bendiocarb and metabolites were included.
Rice
Residue data (corrected for mean recoveries) were available from
22 supervised trials on rough rice at normal harvest in The
Philippines and South Korea (Table 6). Applications were by granular,
spray and dust formulations. No information was available on
nationally approved agricultural practices on rice. Recommended
application rates for rice are 2 and 0.5 kg a.i./ha for granular and
spray formulations, respectively, and 0.5-0.75 kg a.i./ha for dust, as
summarized in Table 2. No information was provided on approved
intervals from last application or maximum number of applications.
Only one trial utilized a dust formulation, and the application
rate reflects the recommended use. Bendiocarb residues were 1.5 mg/kg
and 0.65 mg/kg, respectively, for grain and straw 10 days after
application as compared to 0.6-1.1 mg/kg for a spray application after
the same interval. Residues for NC 7312 were 0.17 mg/kg at the same
10-day interval. Untreated controls were <0.3 mg/kg and 0.04 mg/kg,
respectively, for grain and straw.
Spray applications of 0.25-0.75 kg a.i./ha covers the 0.5 kg/ha
recommended rate. As in the case of dust treatments, the spray
applications at 10 days before normal harvest resulted in higher
residues than at longer intervals, measuring <1.1 and <0.5 mg/kg,
respectively, for grain and straw. Similarly, residues were higher for
NC 7312 in grain, being 0.11 mg/kg. The same control as for the 10-day
interval dust treatment applies.
At longer intervals from last treatment with 50W or 80W
formulations (24-90 days) and up to 1.5 times the recommended rate,
residues were <0.02 mg/kg for both grain and straw. Untreated
controls for spray treatments, except for the 10-day interval
treatment, were <0.021 and <0.02 mg/kg, respectively, for
bendiocarb in grain and straw, except for one untreated straw sample
at 0.04 mg/kg. Residues of NC 7312 were <0.03 mg/kg and 0.02 mg/kg
for grain and straw, respectively, from spray treatments.
Four trials were conducted with either 3G or 5G formulations at
rates reflecting the recommended application rate and different
application methods. Maximum residues of bendiocarb were <0.6 mg/kg
and 0.04 mg/kg, respectively, in grain and straw. Untreated controls
were <0.07 and <0.04 mg/kg bendiocarb in grain and straw,
respectively. Residues of NC 7312 were <0.03 and <0.02 mg/kg in
grain and straw, respectively.
Analytical recoveries of bendiocarb during the trials averaged 77
and 68%, respectively, in rice grain and straw from fortifications at
0.02-0.25 mg/kg. Average recoveries were less (64 and 56%) for NC
7312. In a separate study on recoveries (Browne and Reary 1979b) at
similar fortification levels, mean recoveries were better, being 86
and 74% for bendiocarb in grain and straw, and 70 and 56%,
respectively, for NC 7312.
In the absence of GAP information, 2 mg/kg and 1 mg/kg limits
would be required for bendiocarb per se in rice grain and straw,
respectively, to accommodate the uses of the supervised trials.
Wheat
Residue data, corrected for recoveries, were available from eight
supervised trials in the U.K. from seed treatments with 80W or 50SC at
2-5 g a.i./kg seed (Table 6). No information was available on specific
nationally approved agricultural practices, although the application
rates used reflect the 2-4 g a.i./kg seed recommended rate for
cereals.
Residues for bendiocarb at harvest were <0.02 mg/kg for both
grain and straw, except for one 0.06 mg/kg value for straw. This
0.02 mg/kg level is approximately the claimed limit of determination.
Untreated controls were <0.04 mg/kg and <0.12 mg/kg,
respectively, for grain and straw. The 0.12 mg/kg for straw is from
the same study in which the high treated sample residue was found.
Contamination was suspected. Other values were <0.02 mg/kg.
Analytical recoveries from the individual studies were relatively
low, averaging only 63-65% for grain and straw at fortification levels
of 0.02-0.2 mg/kg. In a separate determination (Reary 1979b)
recoveries of bendiocarb in grains were better, averaging 84%. The
limit of determination for barley, oats and wheat is said to be
<0.02 mg/kg for grain and straw. Overall data support a 0.05 mg/kg
limit for bendiocarb per se.
Limited data were also available for combined conjugated residues
of NC 7312 and N-hydroxymethylbendiocarb. Residues were <0.01 and
<0.12 mg/kg for wheat grain and straw, respectively, although
analytical recoveries were only 62 and 67% in cereal grains and straw,
respectively.
Fodders and Straws
Fodders and straws of cereal grains have been discussed
previously. The other member of this group for which data were
provided is grass fodder, or more specifically turf and grass. Data
were available from 72 supervised trials in the U.K., U.S. and
Australia from 1975-1980, representing a variety of formulations,
single or multiple applications at several rates, intervals, and
methods of application as well as irrigated and unirrigated conditions
(Table 7) (Browne 1981d; FBC 1982c; Reary 1975d, 1978f,g, 1980b,d,e,k,
1981m; Reary and Whiteoak 1977a,b,c,d). No information was available
on specific national agricultural practices, although recommended
rates for rye grass is given in Table 1.
Granular treatments, both single and multiple broadcasts, were
mostly at application rates 6-12 times the recommended rate (for rye
grass). Three granular broadcast trials (single applications) at 1.6
times the recommended rate resulted in maximum residues ranging from
0.24 mg/kg at 43 days after treatment to 0.09 mg/kg after 55 days.
Residues were <0.1 mg/kg from three granular seed mix trials (single
application) at 0.6-1.4 times the recommended granular application
rate when analysed 196 days after treatment.
As in the case of granular treatments, single or multiple spray
treatments were mostly at rates 6-12 times the maximum recommended
application rate for rye grass. After single applications at rates
approximating recommended rates (1-1.6x), maximum residues from spray
treatments ranged from 103 mg/kg (193 mg/kg dry basis) at day of
application to 0.03 mg/kg after 141 days.
No recommended rates were available specifically for ULV aerial
applications. Residues from a single application at 0.28 kg/ha ranged
from 50 mg/kg (111 mg/kg dry basis) on day of application to 5.5 mg/kg
(12 mg/kg dry basis) after 29 days.
Seed dressing trials were conducted at treatments rates of 1-4
times the recommended rate of 10 g a.i./kg seed. Residues were
<0.2 mg/kg at all rates after 43 days.
Although many of the treatments with the various formulations
were at exaggerated application rates, these trials did permit
estimations of decline rates, the potential of residue accumulation
and effect of irrigation on residues. Residue half-lives were greater
for granular broadcast applications, for which 37-42 days was
estimated. For spray application half-lives ranged from 2-15 days,
depending on the trial. The half-lives were 5.3 and 2.4 days for seed
treatments and aerial ULV treatments, respectively. In general,
irrigation did not significantly affect decline rates from granular
broadcast or wettable powder spray treatments as compared to rates
measured under non-irrigated conditions. Residues did not accumulate
from multiple applications at 28-day intervals.
Table 7. Bendiocarb Residues in Grass and Turf from Supervised Trials
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 1 - 80W 1 kg/ha Spray Rye grass <1 130 1
1975 1-5 <109 3
6-10 <40 2
11-20 <15 2
21-50 <7 3
U.S. 1* 4.3 76W 2.24 kg/ha Spray Turf grass <1 <236 6
1976 1-5 <225 6
6-10 <115 4
11-20 <19 2
1* Thatch <1 <25 6
1-5 <26 6
6-10 <9 4
11-20 <1 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <326 6
1-5 <386 6
6-10 <190 4
11-20 <46 2
1* Thatch <1 <36 6
1-5 <97 6
6-10 <79 4
11-20 <1.6 2
1* 2-4 76W 2.24 kg/ha Spray Turf grass <1 <293 5
1-5 <201 4
6-10 <4 4
11-20 <2.8 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1* Thatch <1 <19 5
1-5 <13 4
6-10 <16 4
11-20 <21 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <607 5
1-5 <710 4
6-10 <30 4
11-20 <7.5 2
Thatch <1 <56 5
1-5 <18 4
6-10 <32 4
11-20 <26 2
1* 5G 2.24 kg/ha Broadcast Turf grass <1 <1.4 5
1-5 <2.7 4
6-10 <0.6 4
11-20 <0.3 2
1* Thatch <1 <13 5
1-5 <11 4
6-10 <18 4
11-20 <17 2
1* 5G 4.48 kg/ha Broadcast Turf grass <1 <3 5
1-5 <6 4
6-10 <8 4
11-20 <0.7 2
1* Thatch <1 <34 5
1-5 <42 4
6-10 <47 4
11-20 <27 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1977 2* 5-15* 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
21-50
51-80
81-110
111-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 5-15 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
11-20
21-50
51-80
81-110
110-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 76W Multi-spray2/ Turf grass 1-5 <16 3
6-10 <16 2
11-20 <9 2
21-50 <2 2
51-80 <0.9 2
81-110 <0.3 2
111-140 <0.1 2
>141 0.1 1
2* 76W Multi-spray3/ Turf grass 1-5 <24 2
6-10 <2 2
11-20 <3 2
21-50 <1.7 2
51-80 <1.1 2
81-110 <0.3 2
111-140 0.1 1
2* 76W Multi-spray4/ Turf grass 1-5 <37 4
6-10 <2.5 2
11-20 <3.4 2
21-50 <1 2
51-80 <0.4 2
81-110 0.1 1
2* 76W Multi-spray5/ Turf grass 1-5 <80 4
6-10 <80 4
11-20 <20 2
21-50 <3.7 2
51-80 0.5 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 37-42 5G Broadcast6/ Turf grass 1-5 <8 5
6-10 <3.3 2
11-20 <1.9 2
21-50 <3.7 2
51-80 <8.1 2
81-110 <2.6 2
111-140 <1.6 2
>141 <0.5 2
2 5G Broadcast1/ Turf grass 1-5 <7 5
6-10 <7 2
11-20 <1.4 2
21-50 <2.8 2
51-80 <0.16 2
81-110 <0.7 2
111-140 <0.55 2
>141 0.16 1
2 5G Broadcast2/ Turf grass 1-5 <9.5 4
6-10 <4.7 2
11-20 <4.4 2
21-50 <3.5 2
51-80 <0.7 2
81-110 <0.7 2
111-140 <0.7 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 5G Broadcast3/ Turf grass 1-5 <1.0 2
6-10 <9.0 2
11-20 <8.0 2
21-50 <4.0 2
51-80 <2.0 2
81-110 <0.8 2
111-140 0.8 1
2 5G Broadcast4/ Turf grass 1-5 <9.1 4
6-10 <8.2 2
11-20 <7.4 2
21-50 <7.6 2
51-80 <3.9 2
2 5G Broadcast5/ Turf grass 1-5 <7.3 4
6-10 <6.1 2
11-20 <5.7 2
21-50 <2.8 2
Australia 1 80W 1.6 g/kg seed Seed dressing Grass (pasture) 81-110 <0.02 2
1979 111-140 <0.02 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 3 80W 10 g/kg seed Seed dressing Grass (grazing) 21-50 0.09 1
1979 51-80 <0.06 2
3 80W 40 g/kg seed Seed dressing Grass " 21-50 0.17 1
51-80 <0.11 2
3 3G 1.2 kg/ha Broadcast Grass (grazing) 21-50 0.24 1
51-80 <0.09 2
3 50SC 1.2 kg/ha Spray Grass (grazing) 21-50 <0.2 3
2 50SC 10 g/kg seed Seed dressing Grass (grazing) >141 <0.01 3
1 50SC 15 g/kg seed Seed dressing " " >141 0.01 1
2 50SC 20 g/kg seed " " " " >141 <0.01 3
2 50SC 0.5 kg/ha Spray Grass (grazing) >141 <0.01 3
1 50SC 0.75 kg/ha Spray " " >141 0.03 1
2 50SC 1.0 kg/ha Spray " " >141 <0.015 3
U.S. 1 6 50SC 0.84 kg/ha Spray Wheat grass <1 103 1 193
1979 1-5 <97 2 <139
6-10 26 1 34
11-20 16 1 18
21-50 <9.3 2 <16
U.K. 2 5.3 80W 10 g/kg seed Seed Dressing Grass (grazing) 51-80 <0.02 2
1980 2 80W 15 g/kg seed Seed dressing " " 51-80 <0.03 2
2 80W 20 g/kg seed Seed dressing " " 51-80 <0.1 3
1 50SC 5×0.5 kg/ha Spray Grass (grazing) 51-80 <0.03 6
81-110 0.01 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 50SC 0.75 kg/ha Spray Grass (grazing) 51-80 <0.02 2
2 50SC 1.0 kg/ha Spray Grass " 51-80 <0.03 2
1 5G 0.75 kg/ha Seed mix Grass (grazing) 51-80 <0.08 2
1 3G 0.45 kg/ha Seed mix Rye Grass >141 <0.1 2
1 5G 0.75 kg/ha Seed mix Rye grass >141 <0.1 2
1 7G 1.05 kg/ha Seed mix Rye grass >141 <0.1 1
U.S. 1 2.4 25ULV 0.28 kg/ha Aerial Spray Rangeland grass <1 50 1 111
1980 1-5 5.36 2 <73
6-10 1.7 1 4
11-20 0.7 1 2
21-50 5.5 1 12
* Trials irrigated and non-irrigated. Residue levels maximum found.
1/ Rate of 4.48 kg/ha.
2/ Multi-application programme of 4.48 + 2.24 kg/ha, 28 days apart.
3/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha each 28 days apart.
4/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha, each 28 days apart.
5/ Multi-application programme of 4.48 + 2.24 + 2.24 + 4.48 kg/ha, each 28 days apart.
6/ Rate of 8.96 kg/ha.
Apparent bendiocarb in untreated grass controls were generally
<0.2 mg/kg, although they occasionally approached 1 mg/kg and were
1.8 mg/kg in one sample. The limit of determination was said to be
0.01-0.1 mg/kg, depending on the trial. Based on untreated controls,
1 mg/kg may be a more realistic limit of determination for grass.
Analytical recoveries during the trials ranged from 43 to 119% and the
mean for all the trials was approximately 80%.
Legume Animal Fodders
See discussion on pea pods under legume vegetables.
Oilseeds
Bendiocarb residue data on oilseed rape resulting from supervised
trials were available from one country where applications of a 5G
formulation was applied at sowing with the seed at application rates
of 0.140 and 0.28 kg a.i./ha which is the recommended rate for
granular formulations (Table 2) (Browne and Reary 1980f). No
information was available on specific nationally approved agricultural
practices.
Nine replicates were analysed for bendiocarb per se on mature
seed (114 days after application) at each rate with residues
<0.006 mg/kg. Samples were not analysed for conjugates of NC 7312
and N-hydroxymethylbendiocarb, although a metabolism study has shown
these to be the major metabolites in rape seed (see section on "Fate
of Residues in Plants"). Residues in untreated controls were also
<0.006 mg/kg with mean analytical recoveries of 84% for
fortifications at 0.05-0.5 mg/kg.
These data are inadequate for estimating a maximum residue level.
Additional data from granular applications as well as data from spray
treatments reflecting GAP are needed, as well as information on
nationally approved uses, including preharvest intervals and number of
applications.
Meat
Cattle
Residue data available from two feeding studies and two dermal
treatment studies are given in Table 8 (Browne and Reary 1978a,b;
Reary 1978a, 1979a, 1980c).
Table 8. Bendiocarb Residues in Milk and Cattle Tissues after Feeding Trials or Dermal Treatments1/
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
Milk
(non-conjugated2/
residues) <0.01 <0.01 <0.01 <0.01 <0.01 0.014 0.005 0.015 0.06 <0.02 <0.04
Milk
(conjugated3/ <0.02 <0.02 <0.02 <0.02 <0.02 0.027 0.027 0.039 0.057 <0.02 <0.02
residues)
Muscle4/ <0.02 <0.02 <0.02 <0.02 <0.02 - - - - <0.01 <0.01
Omental fat4/ <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.03
Perirenal fat4/ <0.05 <0.05 <0.05 <0.05 <0.02 <0.05 <0.04
Subcutaneous-
muscle4/ <0.01 <0.01
fat4/ <0.15 <0.12
Table 8. (con't)
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
liver4/ <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
kidney4/ <0.1 <0.1 <0.1 <0.1 <0.1 <0.05 <0.11
1/ Corrected for mean recoveries;
2/ Bendiocarb and non-conjugated NC 7312 and N-hydroxymethyl bendiocarb (fat fraction);
3/ Total conjugated NC 7312 and N-hydroxymethyl bendiocarb (aqueous or lactose fraction);
4/ Total residues of bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb;
5/ Analysed 6 hours post-treatment;
6/ 7-21 day self-treatment. Treatment rate estimated to be approximately 5 g product/cow/day.
In a feeding study (Browne and Reary 1978a), lactating dairy cows
were fed for a minimum of 28 days with a diet containing 0, 0.25, 0.75
and 2.5 mg/kg bendiocarb. A maize premix was the vehicle for adding
bendiocarb to the diet twice daily. Premises were stored at -4°C prior
to use. Each treatment group consisted of two cows. Milk samples were
pooled for both cows in each treatment group over 24 hours. Samples
were taken before treatment and after 1, 2, 4, 8, 12, 16, 22 and 28
days on the bendiocarb fortified diet. Milk extracts were separated
into a fat fraction (hexane/ether soluble) and a lactose fraction
(water soluble), the first of which was analysed for total
non-conjugated residues of bendiocarb, NC 7312 and
N-hydroxymethylbendiocarb. The lactose fraction was analysed for total
conjugated residues. Residues (bendiocarb equivalent in milk) in all
fat samples were less than the estimated 0.01 mg/kg limit of
determination, corrected for mean recoveries of 64%. Residues in the
lactose fraction were also <0.01 mg/kg, but the limit of
determination was considered 0.02 mg/kg because of chromatographic
interferences. Based on the chromatograms, 0.05-0.1 mg/kg may be a
more realistic limit of determination for the aqueous fraction when
interfering co-extractives are taken into consideration. In each case,
untreated controls gave no apparent residue above the estimated limits
of determination.
Samples of omental fat, perirenal fat, muscle, liver and kidney
were taken from each animal at sacrifice (within 18 hours of final
bendiocarb intake). Each was analysed for total residues of bendiocarb
and metabolites, conjugated and non-conjugated. No residue (corrected
for recovery) was found above the estimated limits of determination of
0.02 mg/kg for muscle and omental fat, 0.05 mg/kg for liver and
perirenal fat and 0.1 mg/kg for kidney. Muscle samples at the 0.25 and
0.75 mg/kg dietary feeding level did show apparent residues at the
0.02 mg/kg limit of determination as did one untreated control. Based
on representative chromatograms, the above estimated limits of
determination appear to be reasonable, except that a 0.2 mg/kg may be
more realistic for kidney.
Mean recoveries determined separately on unconjugated bendiocarb,
NC 7312 and N-hydroxymethylbendiocarb from cattle meat and fat tissues
were approximately 70%, with a standard deviation ranging from 10 to
22. The large standard deviations reflect rather poor recoveries,
especially of bendiocarb per se, (< approximately 50%) in a number
of muscle, kidney and liver samples. For example, recoveries of
bendiocarb averaged only 60% in muscle and kidney.
Another feeding study (Reary 1978a) was conducted in a similar
fashion but at higher dietary levels (7.5 and 25 mg/kg in the diet).
Maximum residues in milk were <0.01 mg/kg (limit of determination
for the fat soluble fractions) and <0.02 mg/kg for the aqueous
portion. As in the lower level feeding study, apparent residues of
total bendiocarb and conjugated and non-conjugated metabolites were
<0.02 mg/kg in muscle, <0.02 in omental fat and <0.05 mg/kg in
perirenal fat and liver. Residues were at the estimated 0.1 mg/kg
limit of determination for kidney (one control sample was also at this
level). This supports the view that 0.2 mg/kg is a more realistic
limit of determination for cattle kidney.
Analytical recoveries averaged approximately 70% with standard
deviations from 11-21%, reflecting rather poor recoveries. Average
recoveries were only 56% for bendiocarb in muscle and 52% for the
N-hydroxy metabolite in liver.
No information was provided on national GAP or recommended uses
for dermal applications to dairy or beef cattle. Results of
investigations for proposes uses for fly control by dermal
applications with a 1% bendiocarb dust formulation were provided. A 5g
dust treatment (50 mg a.i.) was described as a "normal" daily dose.
In one set of experiments (Reary 1979a) lactating dairy cattle
were manually treated at measured rates for safety evaluations or by
hessian bag self-treatment for efficacy evaluations. In the safety
evaluations, groups of three shorthorn cows were manually brushed on
the head, neck and shoulders with 0, 5, 15 and 25 g of the 1% dust
formulation; milk was analysed pre-treatment and six hours post-
treatment for each animal. As in the feeding studies, milk was
separated into fat and lactose fractions.
Non-conjugated residues in milk ranged from 0.003-0.015 mg/kg
(0.009 mg/kg mean) for all application rates, with no discernible
difference between application rates. Total conjugated residues ranged
from 0.019 to 0.039 mg/kg (0.028 mg/kg mean). Control means for the
safety evaluation trials were 0.006 and 0.032 mg/kg for non-conjugated
and conjugated residues, respectively. Limits of determination were
considered to be 0.02 and 0.05 mg/kg for the fat and aqueous
fractions, respectively.
In the efficacy trials, four herds were self-treated at an
estimated rate of 5 g of dust formulation/day/cow. Milk was pooled for
each herd at 7, 14 and 21 days after the beginning of treatment. For
the fat soluble non-conjugated fractions, residues were <0.017 mg/kg
at 7-21 days, except for one pooled milk sample from one herd at 7
days, for which a residue of 0.06 mg/kg was detected. Therefore, non-
conjugated residues did occur at and above the limit of determination.
Conjugated residues were <0.057 mg/kg, or just over the estimated
limit of determination. Samples used as untreated controls had mean
residues of 0.005 mg/kg and <0.028 mg/kg for fat soluble and
aqueous fractions, respectively.
In another dermal treatment investigation (Reary 1980c) groups of
three lactating dairy cows were manually treated on the head, neck and
back daily for seven successive days with a 1% dust formulation at 0,
5 or 25 g product/cow/treatment. Daily milk production from each of
two daily milkings for each cow was kept separate. Samples of muscle,
omental fat, perirenal fat, liver, kidney and subcutaneous fat were
taken within 18 hours after the final treatment.
Non-conjugated residues in milk (fat fraction) after correction
for analytical recoveries ranged from <0.01-0.04 mg/kg for 78
samples. All but six were <0.01 mg/kg. All residues >0.02 mg/kg
were from the higher treatment rate, except for one sample at
0.02 mg/kg. Comparable residues in 24 untreated controls were
<0.01 mg/kg, except for one sample at 0.02 mg/kg. All conjugated
residues (aqueous fraction) were <0.02 mg/kg and untreated controls
were <0.02 mg/kg. Mean analytical recoveries were 82% and 72% for the
milk fat and lactose fractions, respectively. The limit of
determination was considered to be 0.01 and 0.02 mg/kg for the fat and
lactose fractions, respectively.
In tissues, residues at both treatment levels were <0.01 mg/kg
in muscle, <0.03 mg/ kg in omental fat, <0.05 mg/kg in perirenal
fat, <0.01 mg/kg in subcutaneous muscle and <0.15 mg/kg (mean
0.08 mg/kg) in subcutaneous fat. Except for subcutaneous muscle, for
which there were no untreated controls, apparent residues in controls
for these tissues were _0.03 mg/kg, except for one 0.04 mg/kg sample of
subcutaneous fat. Residues from both levels were <0.05 mg/kg in
liver and <0.11 mg/kg in kidney. All residues >0.05 mg/kg in
kidney were from the higher treatment level. Apparent residues in
untreated controls were <0.03 mg/kg in liver and kidney.
An estimate of potential residues in meat, milk and meat by-
products must take into consideration all potential routes to animal
exposure, which include the dietary and dermal routes. Of the feed
crops for which residue data and good agricultural practice
information were provided, the greatest potential for residues in
cattle meat, fat and meat by-products and milk would be expected
from uses on maize (field corn). Assuming maximum residues are
<0.05 mg/kg in maize grain, forage and/or fodder, total conjugated
and non-conjugated residues of bendiocarb and metabolites would be
expected to be <0.05 mg/kg in cattle carcase meat, cattle fat and
cattle meat by-products (except kidney) and <0.01 mg/kg (claimed
limit of determination) in kidney, although a 0.2 mg/kg limit of
determination may be more realistic for kidney. Residues in milk would
not be expected to exceed the approximate limits of determination of
0.01 mg/kg and 0.02 mg/kg for non-conjugated and conjugated residues,
respectively, or a total of 0.03 mg/kg. These estimates should also be
adequate from feeding of rice.
Data from the use of bendiocarb on grasses or as a dermal
treatment on cattle suggests a possible need for higher limits than
would be required for maize grain, forage and fodder if such uses are,
or should become, good agricultural practice. Additional information
on good agricultural practices are necessary before maximum residue
estimates can be made for these uses. Any additional information
should provide the formulation, application rate, number of
applications, type of application and preharvest intervals or other
restrictions. Depending on these uses, feeding studies at higher
levels may be necessary before maximum residue levels can be
estimated.
Poultry meat, meat by-products and eggs
Laying hens in groups of ten were fed (free access) a diet
containing 0, 0.05, 0.15 and 0.5 mg/kg bendiocarb for 21 days to
permit estimation of potential residues resulting from the feeding of
maize grain, for which maximum residues of 0.05 mg/kg are assumed
(Reary 1978b). Owing to analytical difficulties, information on the
stability of residues over the feeding period was not provided.
Information was not provided on storage conditions of the fortified
pre-mix.
Eggs were individually collected and labelled; 98 were analysed
at higher feeding levels and later sampling intervals. Apparent total
residues (corrected for recovery) of bendiocarb and metabolites
(conjugated and non-conjugated) at all treatment levels were
<0.02 mg/kg 1-21 days after treatment. The limit of determination was
reported as approximately 0.02 mg/kg, supported by sample
chromatograms, although pre-treatment controls were <0.013 mg/kg.
Analytical recoveries of bendiocarb and unconjugated metabolites
ranged from 44-85% (67% av.).
Samples of breast muscle, leg muscle, liver, kidney, subcutaneous
fat and heart were taken after sacrifice on day 21 of feeding the
fortified diet, with completion of dissection the following day. The
sum of bendiocarb and its major metabolites conjugated and non-
conjugated, was determined. No hen tissue residues were found in
excess of the claimed limits of determination of 0.02 mg/kg for legs,
breast, liver and subcutaneous fat and 0.05 mg/kg for kidney and
heart. Sample chromatograms tend to confine these limits of
determination, although apparent residues near the claimed limits of
determination in untreated controls of breasts (<0.016 mg/kg),
liver (<0.014 mg/kg) and fat (<0.016 mg/kg) suggest that
0.05 mg/kg may be a more practical limit of determination for each of
the tissues.
As in cattle tissues and eggs, analytical recoveries were only
marginally acceptable, ranging from 40-98% for bendiocarb or its major
non-conjugated metabolites. Mean residues of bendiocarb and its non-
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) ranged
from only 56% in chicken legs to 79% in fat. Improved analytical
methodologies are needed for commodities of animal origin.
Mushrooms
Supervised trials were conducted in The Netherlands and the U.K.
In The Netherlands mushroom plots were presumably sprayed directly
with a bendiocarb 50SC formulation and in the U.K. the plots were
established the day following wall treatments with either a 50SC or
80W spray. No information was provided on approved agricultural
practices for these uses, including preharvest intervals, although
recommended wall spray application rates of 30-50 g/100 m2 (Table 2)
were provided.
In the first trials (Browne 1981a) a 50SC bendiocarb formulation
was sprayed twice at rates of 50, 75 and 100 g a.i./100 m2 (5, 7.5
and 10 kg a.i./ha), first after spawning and the second after casing.
Harvest was 21 days after the last treatment.
Residues of bendiocarb and non-conjugated NC 7312, corrected for
mean recoveries, are summarized in Table 5. Maximum residues were
0.013 mg/kg and 0.04 mg/kg, respectively, for bendiocarb and NC 7312
at the highest application rate. Residues increase with increased
application rate and residues of NC 7312 tend to be less than those of
bendiocarb under the test conditions.
In the series of trials in the U.K., (Browne, 1982a) mushroom
plots were established the day following hut wall treatments with
either 50SC or 80W bendiocarb formulations at application rates of
20-53 g a.i./100 m2. Harvest was 26-69 days after the treatments,
which reflected the recommended application rates. Bendiocarb residues
ranged from <0.02 to 0.09 mg/kg and NC 7312 from <0.02 to
0.13 mg/kg, both corrected for recoveries (see Table 5). In contrast
to the trials discussed above, residues of NC 7312 in these trials
were of the same order of magnitude as for bendiocarb.
The limit of determination for bendiocarb in mushrooms was
reported to be 0.01-0.02 mg/kg for bendiocarb or NC 7312, depending on
the trials. Based on apparent residues of 0.04 mg/kg of bendiocarb and
0.02 mg/kg of NC 7312 in more than one untreated control, limits of
determination of 0.05 mg/kg may be more practical. Analytical
recoveries averaged approximately 82% for bendiocarb and 73% for non-
conjugated NC 7312 from fortifications of both at 0.04-0.2 mg/kg,
although standard deviations were 14-19.
As no information was available even on recommended uses for
bendiocarb other than for wall treatments, data from wall treatments
are more pertinent to the estimation of maximum residue levels. Based
on recommended application rates, the available residue data are
consistent with a maximum residue level of 0.1 mg/kg for bendiocarb
per se and 0.2 mg/kg if NC 7312 is also included. This is based on the
assumption of minimum application to harvest intervals. Information on
nationally approved agricultural practices for bendiocarb use on
mushrooms is needed. Additional data from supervised trials are
desirable.
FATE OF RESIDUES
Metabolism studies have been carried out in a range of plants and
animals and in soil. In most plant species examined, the major
metabolites formed are glycoside conjugates of the phenol NC 7312 and
N-hydroxymethyl bendiocarb. Metabolism in animals generally involves
cleavage of the carbamate ester group to yield NC 7312, which is
excreted as sulphate and beta-glucuronide conjugates. In soil under
aerobic conditions, the only extractable radioactivity is unchanged
bendiocarb, degradation occurring to CO2, and an unidentified bound
residue. Under anaerobic conditions, bendiocarb is hydrolysed to
NC 7312.
In animals
For discussions on the metabolic fate of bendiocarb in the rat,
dog, hamster, rabbit, mouse and humans, see "Biochemical Aspects".
Residue feeding and/or dermal studies on cattle and/or poultry have
been discussed under "Residues from Supervised Trials". In addition to
the aforementioned studies, the metabolic fate of bendiocarb has been
investigated in the pig, cow and hen.
Pig
Pigs were orally dosed once by gelatin capsule at 0.2 mg/kg/bw
with 14C-bendiocarb (Adcock et al 1976b). Urine samples were
collected over a period of four days, after which the animals were
sacrificed and samples of liver, kidney, muscle, perirenal fat and
subcutaneous fat were taken.
Seventy-eight percent of the administered dose was excreted in
the urine as equal levels of sulphate and ß-glucuronide conjugates of
NC 7312 within one day and 84% within four days. No residues of
unchanged bendiocarb were detected. Corresponding residues in faeces
were 2.4 and 7.2% for a total recovery of 91.5% of administered
radioactivity recovered in urine and faeces after four days. The
residues in faeces were not identified. No residues were found in
tissues at the 0.01 mg/kg limit of sensitivity for bendiocarb
equivalents. The finding of relatively large levels of NC 7312
sulphate was of considerable interest, since phenolic sulphates are
generally minor in pigs.
The metabolism is similar to that in humans and the rat,
involving cleavage of the carbamate ester to yield NC 7312, which
becomes conjugated as sulphates and glucuronides.
Cow
A lactating dairy cow was given 14C-bendiocarb by a single
gelatin capsule at a dose of 0.2 mg/kg/bw (Adcock et al 1976a).
Urine was collected at intervals of 4-96 hours after dosing and faeces
every 24 hours. Milk was collected morning and evening until sacrifice
on the fourth day, at which time samples of liver, kidney, muscle,
heart, perirenal fat, omental fat and subcutaneous fat were taken for
analysis. Milk was separated into lactose, fat and casein fractions.
No residues of unchanged bendiocarb were found in the urine.
Seventy-nine percent of the administered radioactivity was excreted in
the urine within eight hours and 92% after two days, after which
little was excreted in urine. Corresponding residues in faeces were
5.3% at one day and 12.3% after two days. The urinary metabolites were
determined to be sulphate and glucuronide conjugates of NC 7312 at 90%
and 10% each, respectively. Residues in faeces were not identified.
Bendiocarb equivalents were found in milk the first day up to a
level of 0.002 mg/kg, but none were detectable thereafter. Over 80%
was in the lactose fraction, 2% in the hexane fraction and most of the
remainder in the casein fraction. Tissue residue levels were below the
0.01 mg/kg limit of sensitivity as bendiocarb equivalents.
Metabolism in the cow appears to be similar to that in the rat,
human and pigs.
Poultry
Three laying hens were each dosed once with 14C-bendiocarb by
oral gavage at 0.25 mg/kg/bw (equivalent to 5 mg/kg in the diet) and
excrement and eggs collected at intervals from 24-96 hours after
dosing. Liver, kidney, muscle and fat samples were taken at sacrifice
four days after treatment (Lewis and Challis 1978).
Sixty-one to 90% of administered radioactivity was excreted
within 24 hours, and 73-91% within 4 days. Recovery was inexplicably
low (73%) in two of the hens. Losses were presumed to be due to
expiration and/or losses during dosing or excrement collection, since
residues in tissues were low. However, as a percent of radioactivity
excreted in the first 24 hours, residues were 10-57% free NC 7312,
26-63% NC 7312 conjugates, 12-19% unidentified unhydrolysed water
soluble residues, 2-5% other non-polar metabolites and 1-3% other
unidentified conjugates. Conjugation of NC 7312 occurred more rapidly
in the hen in which radioactivity excretion was more rapid and
complete.
Radioactivity in egg white and yolk represented only 0.01% of the
administered dose. The maximum bendiocarb equivalent was highest in
egg white on the first day (max. 0.009 mg/kg) and on day four in yolk
(0.003 mg/kg), both above the limits of sensitivity. The maximum
bendiocarb equivalent was 0.004 mg/kg in liver and fat, 0.002 mg/kg in
kidney and below the 0.001 mg/kg limit of sensitivity in leg and
breast muscle.
In Plants
Metabolism studies have been carried out in sugarbeet, maize,
rice, barley and oilseed rape, both in the laboratory and under field
conditions. A variety of separative, determinative and conjugative
hydrolysis steps were utilized. In all these crops, the major
identified metabolite was the phenol NC 7312, with lower levels of
N-hydroxymethylbendiocarb, both primarily as glycoside conjugates. In
some of the crops studied, sampling at harvest showed small quantities
of unconjugated N-hydroxymethylbendiocarb.
Three metabolism studies were conducted in sugarbeets. In one
study, radioactive bendiocarb was applied under greenhouse conditions
to the potted soil or to the leaves by direct syringe applications
(Challis 1975). Residues were characterized in the plant tops only.
Metabolic products were qualitatively similar in both cases,
resulting in residues of free bendiocarb, bendiocarb glycoside,
free NC 7312 (foliar application only), NC 7312 glycosides,
N-hydroxymethylbendiocarb, N-hydroxymethylbendiocarb glycosides,
unidentified glycosides, unidentified water soluble residues and
unextractable unidentified fibre-bound residues.
The major metabolites from soil application were glycoside
conjugates of NC 7312 and N-hydroxymethylbendiocarb. Trace amounts of
free N-hydroxymethylbendiocarb were also found. Unidentified residues
accounted for 36 and 67% of radioactivity 8 and 34 days after
treatment, respectively, with a ratio of about 1:3 water soluble to
fibre-bound residues.
From foliar applications, over 93% of leaf-applied bendiocarb was
present on the leaf surface after 34 days. A bendiocarb glycoside was
the major metabolite. Unidentified residues accounted for 48% of the
total residue after eight days, but this was reduced to 25% after 34
days. Low levels of free NC 7312 were also present. The proposed
metabolic pathway for bendiocarb in sugarbeet tops is illustrated in
Figure 1. Except for the omission of substantial at-harvest
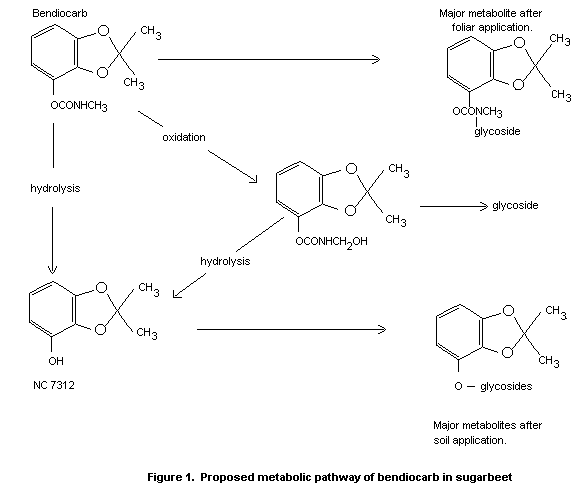 unidentified water soluble and fibre-bound residues, other metabolism
studies discussed below suggest that the pathway is similar in other
plants.
In a second sugarbeet metabolism study (Adcock 1976),
14C-bendiocarb was applied as a seed dressing for plants grown in
outdoor drums in sandy loam soils at 6.7 and 7.5 pH values at a rate
equivalent to 16 g a.i./kg seed. Leaves and/or roots were analysed for
radioactivity at intervals from 13-114 days after sowing, although the
residues were not identified. In accordance with decreased bendiocarb
stability in more alkaline soils, extractable and fibre-bound residues
were less than the 0.02 mg/kg limit of detection (bendiocarb
equivalents) in foliage and roots of the more alkaline soil after 114
days. Residues were greater at earlier intervals. For example, at 36
days residues were on the order of 0.1 mg/kg bendiocarb equivalent in
leaves or roots from either extractable or fibre-bound sources.
Consistent with pH stability, residues were higher in the more acidic
soil, being up to 0.06 mg/kg and 0.02 mg/kg extractable residues in
leaves and roots, respectively, after 114 days; they were appreciably
higher at shorter intervals. A maximum of 18% of applied radioactivity
was found in immature sugarbeet (36-72 days after sowing).
In the third sugarbeet metabolism study (Challis and Adcock
1978), the crop was grown under field conditions and treated at sowing
with 14C-bendiocarb granules at the recommended rate of 0.36 kg
a.i./ha. Plants were harvested at intervals ranging from 46-190 days
after treatment. Results were similar to those of Challis (1975).
After 90 days, radioactivity was too low to permit determination of
individual metabolite levels. In harvested beets, mean residues of
only 0.009 mg/kg bendiocarb equivalent were found after 190 days.
Extractable residues were below the limits of sensitivity.
As would be expected, residues were greater at earlier intervals
and identification was possible. For example, at 46 days residues
(bendiocarb equivalent) were 1.0, 0.22, 0.14, 0.76 and 0.45 mg/kg for
bendiocarb, NC 7312 conjugates, N-hydroxymethylbendiocarb conjugates,
unidentified water soluble residues and fibre-bound residues,
respectively. There were lesser amounts of free metabolites and other
conjugates.
Bendiocarb metabolism was investigated in maize under glasshouse
conditions by applying a solution of 14C-bendiocarb to pH 5.4 soil in
drums with newly germinated maize plants at a rate equivalent to
1.1 kg a.i./ha (Challis and Adcock 1977). Plants were analysed at
intervals from 10-125 days. Mean residues of bendiocarb per se in
plants ranged from 1.4 mg/kg after 10 days to 0.01 mg/kg after 125
days and unidentified soluble residue (bendiocarb equivalent) in
kernels and cobs were 0.005 and 0.001 mg/kg, respectively, after 125
days. As a percentage of total radioactivity, the major residues after
10, 60 or 125 days were:
Foliage Kernels Cob
10 days 60 days 125 days 125 days 125 days
Bendiocarb 38.1 24.2
N-hydroxymethylbendiocarb 8.2 5.3 8
NC 7312 conjugates 163.3 2.7 20
Water soluble 13.6 11 9.1
(non-hydrolysable)
Fibre-bound 13.6 27 61 89 97
Free NC 7312, other non-polar metabolites and other conjugates
were also present at lower levels. Seventy percent of the residues in
plants at harvest were either water non-hydrolysable of fibre-bound
unidentified residues. Although they occurred at very low levels,
fibre-bound unidentified residues constituted 89 and 97 percent of
total radioactive residues in kernels and cobs, respectively, at
harvest.
Investigations were conducted on paddy rice metabolism of
14C-bendiocarb applied as a solution with a syringe under glass to
either the foliage or flood water surrounding rice transplants (Lewis
and Challis 1979). Application rates were equivalent to 0.6 kg a.i./ha
and plant samples were taken at intervals ranging from 15-174 days
after treatments. Foliage and grain were analysed separately at 174
days. Qualitatively, residues were similar to those in sugarbeet and
maize, although N-hydromethylbendiocarb or its conjugates were not
specifically identified in rice. They could have been present under
residues described either as other non-polar metabolites or other
conjugates.
Mean free bendiocarb in rice plants ranged from 1.7 mg/kg after
15 days to 0.0022 mg/kg (total radioactivity as bendiocarb
equivalents) after 174 days from the foliar treatments. The most
abundant metabolite by far was NC 7312, mostly as a conjugate
(0.006 mg/kg bendiocarb equivalent). Residues were 0.009 mg/kg in rice
husks and flour after 174 days.
In plants growing in treated water, residues of free bendiocarb
at 15 days were approximately one tenth of that from foliar
treatments, the major residue being NC 7312 conjugates at 0.54 mg/kg.
Individual residues could not be determined after 60 days. At harvest,
total radioactivity levels are similar regardless of the type of
treatment. Quantitatively, major residues in plants as a percent of
total radioactivity at 15 or 174 days are:
15 days 174 days
Foliar Water Foliar Water
Bendiocarb 37 12
NC 7312 13 37 39 31
conjugates
Water soluble 7 10
(unhydrolysable)
Fibre-bound 33 33 61 69
All of the radioactive residue at harvest in rice husks or flour
was NC 7312 conjugates, regardless of whether application was by
foliar or water treatment. As in the case of maize foliage, residues
in rice plants were mostly unidentified fibre-bound ones at harvest.
The difference was in the grain, which contained mostly unidentified
fibre-bound residues in maize and mostly NC 7312 conjugates in rice.
Greenhouse-grown barley plants were treated with 14C-bendiocarb
at rates equivalent to 1 kg/ha. A solution was applied by pipette to
soil surrounding the plants which were analysed at intervals ranging
from 4-94 days after treatment. Samples at 94 days were separated into
foliage, husks and grain, which were analysed separately (Challis and
Swalwell 1980).
Results of this investigation were similar to that of
rice. However, in the case of barley, conjugates of
N-hydroxymethylbendiocarb were identified, but total radioactive
residues in husks and grain at 94 days (1.46 and 0.09 mg/kg bendiocarb
equivalent, respectively) were not characterized. Free bendiocarb
residues in foliage ranged from 0.09 mg/kg at 4 days, declined through
60 days and increased to <0.07 mg/kg at 94 days. Similar patterns
were observed for conjugates of NC 7312 and N-hydroxymethylbendiocarb,
the major components of the residue throughout the period. Again,
unidentified water soluble or fibre-bound residues constituted a major
part of the residue, which increased until harvest (12 and 42% of
total radioactivity, respectively).
The metabolism of 14C-bendiocarb was studied in greenhouse grown
oilseed rape treated at the seedling stage at application rates
equivalent to 140 and 500 g a.i./ha. Application was by pipette to
soil surrounding the plant (Warner 1980). Plant samples were taken at
intervals ranging from 5 to 140 days after treatment. At 140 days,
samples were separated into foliage, pods, and seed, which were
analysed separately.
As in the case of barley, the major residues in the plants were
highest at earlier intervals and, except for free bendiocarb, declined
and then increased by harvest. In both cases this was probably due to
plant growth patterns and dessication near harvest. Free bendiocarb
was the major residue at day 5 (66-69%), but declined rapidly
thereafter (2.5 mg/kg at day 5 to 0.01 mg/kg at 140 days from the
high treatment rate). Again, conjugates of NC 7312 and
N-hydroxymethylbendiocarb were the major identified metabolites and,
by harvest, unidentified water soluble materials and fibre-bound
residues formed the major portion of total radioactivity which was 26
and 43%, respectively, from the higher treatment rate. Although
residue levels were dependent on the application rate, on the basis of
percentage of total residue, results were similar at both application
rates.
Extractable residues in pods, seeds and foliage at harvest were
0.09, 0.001 and 0.16 mg/kg, respectively, at the low application rate
and 0.74, 0.011 and 0.84 mg/kg, respectively, at the high rate.
In Soil
Laboratory studies on residue decline in a pH 7.7 sandy loam soil
treated in flasks or jars with 5 mg/kg 14C-bendiocarb showed
degradation to CO2 and unidentified bound residues under aerobic
conditions, with a half-life of 5 days for the parent compound. No NC
7312 was detected. With flooding or nitrogen flushing and under
sterile anaerobic conditions, bendiocarb was hydrolysed to NC 7312,
with little or no further degradation (Adcock et al 1975a).
In a laboratory soil leaching study with the same soil type, 60%
of the radioactivity applied to a 30 cm column as 14C-bendiocarb was
eluted with 50 cm of water over 11 days, mostly as NC 7312 (Adcock
et al 1975b). Presumably, conditions were effectively anaerobic and
the rapid degradation of bendiocarb did not permit estimation of its
leachability.
In another leaching study, 14C-bendiocarb, leachability was
investigated for an agricultural sand (pH 5.3), a clay loam (pH 7.3),
a sandy loam (pH 7.8) and a silt loam (pH 7.2) using soil column and
thin-layer techniques (Adcock 1978). The 30 cm columns were percolated
over a 2-9 day period with a 20 cm column of water. The percentage of
radioactivity recovered in leachate was 75, 11.9, 7.9 and 0.8% for the
respective soils, decreasing with increased organic content.
Recovery in leachate was slightly less than for 2,4-D in
agricultural sand and clay loam, and substantially less in the other
two soils under similar conditions. Thin-layer results confirm these
observations and show that NC 7312 is somewhat less leachable than the
parent compound in all four soil types. It also shows that bendiocarb
is more leachable than trietazene, which places it in the moderately
mobile class.
When bendiocarb 80%WP, 3%G and 1% dust formulations were leached
from sand (pH 7-7.6) loamy sand (pH 6-6.5) and sand loam (pH 5.6-7.8)
in 30 cm × 5 cm columns with 20 cm water over 48 hours, leachability
was again inversely proportional to organic matter content of the soil
(Reary 1975a; Browne and Reary 1979a, 1980a). As a percent of the
amount applied, bendiocarb recovery in leachate ranged from 0 to 93%
WP, 0.6 to 84% dust and 0 to 22% granular. From the same formulations
leachate contained 0 to 25% bendiocarb and 0 to 68% NC 7312; 0 to 45%
bendiocarb, 0 to 62% NC 7312 and 0 to 4% bendiocarb, 0 to 19% NC 7312,
respectively. Therefore, leachability decreased in the order of WP,
dust and granular formulations.
Bendiocarb stability in standard soils has been shown to be
dependent on soil pH, being much slower at low pHs (Reary 1975b). The
half-life was 10 days in one soil at pH 2.2 and 58 days in another at
pH 5.2.
Soil residue decline studies have also been carried out in trials
determining bendiocarb residues in turf and maize. Results again show
that the rate of degradation of bendiocarb is pH-dependent. Repeated
applications showed no evidence of a build-up of bendiocarb residues
in soil. Dissipation rates (in days) for various formulations in
various soil types and pHs are given in Table 9 (Browne and Reary
1978c; Reary 1975b, 1978f, g, k, 1980g; Reary and Whiteoak 1977a, b,
d).
In Storage and Processing
It was noted under the section on "Residues from Supervised
Trials" and in Table 6 that oil processed from maize with field-
incurred residues of <0.02 mg/kg bendiocarb also contained residues
of <0.02 mg/kg. In another investigation, 10G was applied as a band
along the maize row at sowing at 1.1-2.2 kg a.i./ha and the maize
sampled at normal harvest. Oil was solvent-extracted from the grain,
which had residues of <0.01 mg/kg, the limit of determination.
Analysis of the oil showed no residues in excess of the 0.05 mg/kg
limit of determination (Reary 1978c).
Investigation with radio-labelled bendiocarb in granular
application trials in France, the U.K. and the U.S. showed residues in
solvent-extracted oil to be 0.008, 0.011 and 0.021 mg/kg,
respectively, from kernels with residues of 0.011, 0.013 and 0.022
mg/kg, respectively. In most kernel or oil samples residues were
<2X the reported limit of sensitivity (Lewis and Adcock 1978a, b, c).
RESIDUES ON FOOD IN COMMERCE OR AT CONSUMPTION
No information was provided on residues in commerce or at
consumption. The Meeting considered such information desirable.
Table 9. Summary of Bendiocarb Dissipation Rates in Soil
Soil Dissipation time (days)
Type pH Formulation 50% 75% 90%
Sandy loam 5.2 Technical1/ 58 ? ?
Sandy 6.5 76W 25 50 75
loam 76W2/ 12-34 24-69 37-104
Sandy loam 6.5 5G2/ 26-70 52-140 78-210
Silty clay 6.5 10G 19 38 57
Silty clay loam 6.7 76W 30 60 ?
Sand 6.8 Technical1/ 10 24 50
Loam 7.2 10G 7.5-10 15-20 22.5-30
Loam 7.7 76W 12 24 36
Sandy 7.7 76W 2.4 4.8 7.2
loam 76W2/ 2-5 3-10 5-15
Sandy loam 7.7 5G2/ 12-26 30-52 45-78
Sandy loam 7.8 3G 12 24 36
1/ Laboratory studies.
2/ Multiple applications at up to 5-monthly intervals;
dissipation times determined after each application.
METHODS OF RESIDUE ANALYSIS
Residue analysis methods for various plant substrates have been
developed for bendiocarb alone or for bendiocarb plus the non-
conjugated metabolite NC 7312, either separately or as a total
residue. In animal substrates, enzymic liberation of NC 7312 from
conjugates is more successful than in plants. Consequently, residue
methods for animal tissues, milk and eggs include determinations of
the conjugated residues.
As the carbamate functional group does not possess strong
electron capturing properties, derivatization of the bendiocarb
molecule is necessary to facilitate the measurement of low residue
concentrations. Formation of the 2,4-dinitrophenyl ether was found to
be most suitable. This results in a product with strong electron
capturing properties and a long gas chromatographic (GLC) retention,
thus ensuring excellent sensitivity and minimal co-extractive
interference.
In the standard procedure (Reary 1975c) the carbamate is
hydrolysed in an alkaline buffer containing 1-fluoro-2,
4-dinitrobenzene so that the dinitrophenyl ether is formed immediately
after hydrolysis. This method has the added advantage the phenolic
degradation product, NC 7312, forms the same product in the reaction.
Thus, the combined residue can be measured unless bendiocarb and NC
7312 are separated prior to derivatization. This separation is readily
effected by high pressure liquid chromatography (HPLC) or with
commercially available liquid chromatographic cartridges. Collection
of the appropriate fraction allows individual analysis for bendiocarb
and NC 7312 from the same extract (Brown and Reary 1979b).
Bendiocarb is extracted from soil (Reary 1978d), grass (Reary
1978e) sugarbeet (Reary 1975c), maize (Browne and Reary 1979c), cereal
grain (Reary 1979b), rice (Browne and Reary 1979b), rape seed (Browne
and Reary 1980b), potatoes (Reary 1979c), cabbage (Browne 1981b),
mushrooms (Browne 1982a), apples/pears (Browne 1982b) and peas (Browne
and Manley 1982), under reflux with dichloromethane or by shaking with
diethyl ether. Oily extracts are improved by partition between hexane
and acetonitrile. Further clean up, when required, is by silica gel
chromatography. This is easily done using commercially available
disposable cartridges, which can eliminate or separate any NC 7312
present in the extract.
A method has been developed for the residue analysis of
bendiocarb and major metabolites in milk, eggs, meat, fat and offal
(Browne and Reary 1978b). Residues are extracted under reflux with
acetonitrile. Conjugated metabolites are hydrolysed with snail
digestive juice before formation of the dinitrophenyl derivative. All
major bendiocarb related metabolites form the same derivative, which
is then analysed by the standard method as a total residue.
In general, mean analytical recoveries in most commodities were
>75% for bendiocarb and >60% for NC 7312. Analytical
sensitivities, recoveries, recoveries of fortified commodities and
apparent residue in untreated controls for individual commodities are
discussed under "Residues Resulting from Supervised Trials."
Information represents field-type conditions and is somewhat less
assuring than mean recoveries presented with the reports on each
individual analytical method. Although mean analytical recoveries
appeared acceptable, at least for bendiocarb per se, the range of
residues was generally large, resulting in standard deviations ranging
from 6 to 33. In animal tissues in particular, and to some extent in
cereal grains and fodder/forage, analytical recoveries were frequently
low. For example, in animal products analytical recoveries were
frequently only 50-60% for bendiocarb per se or the N-hydroxy
metabolite, and sometimes less.
NATIONAL MAXIMUM RESIDUE LIMITS
Information on national maximum residue limits were available
from only one country. Limits have been proposed in four other
countries (Table 10).
Table 10. National Maximum Residue Limits
Country Commodity MRL (mg/kg)
Netherlands1/ "All commodities"3/ 0 (0.05)4/
Australia2/ Poultry meat, eggs 0.05
Milk, milk by-products, meat
and other edible offal 0.1
Cereal grain 10.0
Belgium2/ Maize and sugarbeet 0.02
Switzerland2/ General foodstuffs (excl. milk) 0.2
Milk 0.005
U.S.2/ Maize (grain fodder, forage) 0.05
Maize oil 0.1
Milk, eggs, meat fat, meat
by-products (except kidneys)
of cattle, goats, pigs horses,
poultry, sheep 0.05
Kidneys of cattle, goats, pigs,
horses, poultry, sheep 0.1
1/ Established;
2/ Proposed;
3/ It is unclear whether "all commodities" include
commodities other than maize and sugarbeet, for which
good agricultural practice information was provided;
4/ 0 (0.05) mg/kg presumed to mean no residues allowed with
a 0.05 mg/kg limit of determination. Large-scale use.
APPRAISAL
The Meeting reviewed substantial information on residue levels
from supervised trials in a variety of commodities, fate of residues
and analytical methodology for bendiocarb, a carbamate pesticide used
for control of a wide range of agricultural and public health pests.
Information on official nationally approved agricultural practices and
national maximum residue limits were available for only two
commodities (maize and sugarbeet) in one country, although recommended
application rates were provided for a number of commodities. Since
information on good agricultural practices for all commodities except
maize and sugarbeet is lacking, only TMRLs are estimated for other
than these two commodities. National tolerances have been proposed in
at least four countries, mostly on maize and other cereals and animal
products.
Residue data were available from supervised trials on a variety
of commodities in several countries and using a variety of
formulations and agricultural practices. In most cases, free
bendiocarb per se was the only residue measured. In a few cases, the
major metabolite 2,2-dimethyl-1,3-benzoxodiol-4-ol (NC 7312) was
measured in its free form and in some cereals, combined residues of
conjugated NC 7312 and N-hydroxymethylbendiocarb were measured as
well. Data were inadequate for estimating a limit for oilseed rape,
cabbage and peas, although the Meeting was informed that additional
data are being compiled for the latter.
For root or tuber vegetables, data were available for potatoes
and sugarbeet. Data on potatoes show residues of free NC 7312 at
harvest to be up to 20 times that of the parent compound (average 6X).
This rate could not be determined for sugarbeet with available data.
Limited data were available in pome fruit from one country and
residues of NC 7312 were equal to or less than those of the parent
compound. The Meeting was informed that additional data are being
gathered.
Bendiocarb residue data on cereals were available for barley,
oats, wheat, maize and rice. For the latter, limited data were also
available for free NC 7312, and the first three, for combined residues
of conjugates of NC 7312 and N-hydroxymethylbendicarb. Data were
mostly from seed treatments in barley, oats and wheat. No data were
available for metabolites in maize, although metabolism studies have
shown >50% of residues in foliage to be metabolites and/or
unidentified water-soluble or bound residues; in kernels at harvest,
approximately 90% of radioactivity was unidentified fibre-bound
residues. Analytical recoveries were only marginally acceptable in
some cereal trials, especially those in wheat.
Considerable data were available for grass and turf, mostly at
higher than recommended rates. Half-lives were greater from granular
broadcast applications than from spray applications and irrigation did
not significantly affect decline rates for either.
As in maize, data for oilseed rape were for the parent compound
only, and metabolism studies showed >50% of residues at harvest to be
conjugated metabolites and (mostly) unidentified water-soluble or
fibre-bound residues.
Data were available for mushrooms from spray treatments (plot
presumed) and wall treatments (before plot establishment). Residues
increased with application rates and residues of free NC 7312 from
wall treatments were on the same order of magnitude as those of the
parent compound, but were less following direct spray treatment. No
information was provided on good agricultural practices for direct
spray treatment.
Animal feeding trials were conducted in which lactating dairy
cattle were fed diets for 28 days containing up to 25 mg/kg
bendiocarb. In contrast to field trials, in which the parent compound
was determined separately from metabolites, animal tissues were
analysed for total residues of free bendiocarb and non-conjugated
metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb.
Milk was separated into a fat fraction and lactose (aqueous) fraction.
The fat fraction was analysed for bendiocarb and non-conjugated NC
7312 and N-hydroxymethylbendiocarb and the lactose fraction for the
conjugated residues of the two metabolites.
Studies using topical treatments were also conducted, but no
information was provided on approved or recommended uses for such
treatments. Dermal treatments generally resulted in higher residues in
tissues and milk than feeding trials. In the absence of good
agricultural practice information for dermal treatments (or grass
uses) residue estimates in cattle are based on potential residues from
the feeding of maize, for which national good agricultural practice
information was available. From this potential source of exposure, no
residues are expected in products of animal origin above the limit of
determination at or about 0.05 mg/kg (0.2 mg/kg in kidney).
In animal products in general, analytical recoveries were
disappointingly low, frequently only 50-60% or less for some residues
in some tissues, and thus can be considered marginally acceptable for
enforcement purposes only, since residues are expected to be
relatively low as a result of uses considered in residue level
estimates.
Animal metabolism studies were conducted in the rat, dog,
hamster, rabbit, mouse, pig, cow and poultry and in humans. In the
pig, cow and poultry single dosing was with 14C-bendiocarb, by
gelatin capsule in pig and cow and by gavage in poultry. Approximately
80% of applied radioactivity was eliminated in the urine within one
day or less in pigs and cows and 61-90% in poultry. It consisted
mostly of the sulphate or glucuronide conjugates of NC 7312 in all
three, although in poultry significant levels of unidentified
water-soluble residues were also present, as well as low levels of
free NC 7312 and other unidentified moieties. Low levels of NC 7312
sulphate and glucuronide were also present in faeces of cows and pigs.
In pigs the NC 7312 sulphate to glucuronide ratio was unexpectedly
high.
In animal tissues and milk, residue levels were generally not far
from the limit of determination and only 0.01% of the administered
dose was found in eggs. Owing to the low levels, they were not
identified.
Bendiocarb metabolism in cows, pigs and poultry appears to be
similar to that in smaller animals. Carbamate is hydrolysed to
NC 7312, which forms glucuronide and sulphate conjugates. The
apparent major difference is the lack of conjugates of
N-hydroxymethylbendiocarb also found in plants, and 5,6, or
7-hydroxybendiocarb found or tentatively identified in the smaller
animals. However, dosing rates of 0.2-0.25 mg/kg bw in cows, pigs and
hens were substantially less than the maximum dosage rates of 10 mg/kg
bw used for the smaller animals. More importantly, cows, pigs and
poultry were sacrificed four days after dose administration, which
precludes measurement or characterization of additional radioactivity
closer to administration. This could also account for residue levels
in animal products (cows, pigs and poultry) being too low for
identification. A metabolism study conducted in both ruminants and
poultry at high levels and a much shorter sacrifice interval would
have been preferred and are desirable. Such a metabolism study should
be considered essential if higher than the current ones for animal
products are required from the feeding of grain, fodders or straw of
cereals.
Metabolism studies in sugarbeet, maize, barley, rice and oilseed
rape under laboratory or field conditions showed similar quantitative
results, although ratios of the various metabolites and degradation
products varied considerably, depending on the plant, type of
application, interval from application to harvest, part of plant and
other factors.
A major metabolic pathway for bendiocarb in some plants is
hydrolysis to NC 7312, which becomes conjugated as a glycoside.
In another pathway, bendiocarb can first be oxidized to
N-hydroxymethylbendiocarb, which may hydrolyse to NC 7312
before glycosidic conjugation or become conjugated as
N-hydroxymethylbendiocarb glycoside. Another route can be direct
glycosidic conjugation of bendiocarb per se. This was a major residue
in beet leaves following a surface application, but NC 7312 conjugates
were found following surface treatments to rice. Undoubtedly other
pathways exist, since low levels of unidentified conjugates are known
to occur. In one beet study, limited leaf penetration was detected
from surface application.
As the interval from application to harvest increased, residues
of bendiocarb per se decreased with an increase in NC 7312 and
N-hydroxymethylbendiocarb conjugates, which were frequently the major
identified metabolites. Unidentified water-soluble, non-hydrolysable
residues and unidentified fibre-bound residues have also been shown to
increase in the foliage of several plants; at harvest they constitute
26 and 60%, respectively, of the total remaining radioactivity. In
maize kernels and cobs at harvest the fibre-bound unknown was shown to
consist of >90% of the remaining low residue.
Low levels of free NC 7312 and other unidentified unconjugated
residues were found in some plants. Free NC 7312 exceeded that of the
parent compound in potatoes and probably in pea pods. In other plants,
free NC 7312 residues were either less than or equal to that of the
parent compound. Differences in method of application (for example,
foliar versus ground application) affected the ratios of the various
metabolites in some, but not all, plants tested, although residues
were qualitatively similar.
In soils, bendiocarb was degraded to CO2 and unidentified bound
residues under aerobic conditions whereas degradation was mostly to
NC 7312 under anaerobic conditions. Bendiocarb stability was dependent
on soil pH, being much more stable under acidic conditions. Half-lives
ranged from 2-70 days, depending on conditions.
Leaching studies carried out on a variety of soils, under
different conditions and with several formulations showed leaching to
be largely dependent on the organic content of the soil, decreasing
with higher levels of organic matter. Although generally considered to
be in only a moderately leachable category, significant leaching of
bendiocarb can occur in sandy soils with low organic matter content.
Leachability appears to increase somewhat from granular to dust to
wettable powder formulations. One study indicated that NC 7312 leached
slightly less than bendiocarb per se. Although build-up of soil
residues of bendiocarb per se do not appear to be indicated, any
potential residues of bendiocarb or its metabolites in ground water
would be greatest from sandy soils of low organic matter content.
Most investigations on possible residues of bendiocarb in
processed products were conducted on oil, husks or flour from cereal
grain. Although no concentration of residues was demonstrated in these
studies, residues in the grain from which the products were processed
had residues below or near the limits of determination. Therefore,
firm conclusions cannot be drawn.
Analytical methods are available for the determination of free
bendiocarb in all plant commodities examined and free NC 7312 in some.
In the basic procedure, bendiocarb is hydrolysed to its phenol under
alkaline conditions in the present of 1-fluoro-2,4-dinitrobenzene to
form the dinitrophenyl ether, which is preferably determined by
electron capture gas chromatography using an internal standard.
Bendiocarb and NC 7312 can be determined together or separately, if
they are separated by HPLC or silica gel chromatography prior to
derivitization. In most commodities of plant origin, field trials were
for the free parent compound only and free NC 7312 was also determined
separately in a few. Analytical recoveries were highly variable on
some commodities, especially cereals.
For animals, the same basic analytical approach is used, except
that a hydrolysis step is included to hydrolyse conjugated residue.
Hydrolysis has been accomplished by a variety of enzymatic and/or
acidic conditions. Enzymatic approaches are preferred and snail
gastric juice is frequently used. In contrast to plants, residues
determined for animal tissues and milk include the sum of residues of
bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and
N-hydroxymethylbendiocarb. Aqueous (lactose) and non-aqueous (fat)
fractions of milk are analysed separately. Analytical recoveries for
products of animal origin are only marginally acceptable for
enforcement purposes. Improved analytical methods are needed for any
uses requiring maximum residues levels in products of animal origin
that are higher than the current limits of determination.
RECOMMENDATIONS
The Meeting considered information on residues resulting from
good agricultural practices or recommended application rates on a
number of commodities and possible residues in animals resulting from
the feeding of grain, fodders or straw of cereals treated according to
good agricultural practice and concluded that the maximum residue
levels or temporary maximum residue levels below are suitable for
establishing maximum residue limits. Regardless of the status of the
ADI, temporary status was designated for all commodities except maize,
maize fodder and forage, sugarbeet and sugarbeet tops, pending
availability of good agricultural practice information. Except for
potatoes, some fruit, rice and rice straw, estimated residue levels
apply to treatment before planting and/or seed application only.
In the absence of good agricultural practice information, the
temporary limits are based on supervised trials data only. Residues
for products of plant origin refer to residues of unconjugated
bendiocarb. Residues in products of animal origin refer to the
sum of free and conjugated bendiocarb, NC 7312, and
N-hydroxymethylbendiocarb, expressed as bendiocarb.
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Potatoes 0.051/ 100 (granular)
0 (spray)
Sugarbeet 0.051/ 150
Sugarbeet tops 0.051/ 150
Pome fruit 0.021/ 50
Cereal Grains
Barley 0.051/ 100
Oats 0.051/ 100
Wheat 0.051/ 125
Maize (field) 0.051/ 125
Rice (in husk) 2 10
Fodders and Straws
Barley 0.051/ 125
Oats 0.051/ 100
Wheat 0.051/ 125
Maize 0.051/ 125
Rice 1 10
Mushrooms 0.12/ 50
(con't)
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Animal Products3/
Carcase meat of cattle 0.051/
Fat of cattle 0.051/
Meat by-products of cattle
(except kidney) 0.051/
Kidney of cattle 0.21/
Milk 0.051/
Poultry 0.051/
Fat of poultry 0.051/
Poultry meat by-products 0.051/
Eggs 0.051/
1/ At or about the limit of determination.
2/ Limit reflects maximum residues expected from wall treatments
prior to establishment of plots.
3/ Limits reflect maximum residues expected from the feeding of
cereal grains, fodders or straws harvested at given intervals
from application to harvest.
FURTHER WORK OR INFORMATION
Required (by 1984)
Information on nationally approved agricultural practices for the
use of bendiocarb on all commodities for which limits are estimated,
except for maize and sugarbeet.
Desirable
1. Additional metabolism studies in ruminants and poultry at
sufficiently high dosing levels and with sacrifice at a short
interval after dosing, instead of several days thereafter, to
permit a more complete characterization of residues in tissues,
milk and eggs. The analyses should include all residues
identified, or tentatively identified, in smaller animals and in
plants. These studies should be considered required before new
limits or future upward revisions of established ones on feed
items are established significantly higher than at current limits
of determination. The same is true should substantially higher
limits on tissues, eggs or milk be required for approved dermal
treatments.
2. Additional residue data, reflecting nationally approved uses
especially on, but not limited to, mushrooms and pome fruit.
3. Information on the possible occurrence of residues in food in
commerce or at consumption.
REFERENCES
Adcock, J.W. Investigation of residues in sugarbeet following the
1976 use of 14C-bendiocarb as a seed dressing. Fisons Report
METAB/76/1. (Unpublished)
1978 The leaching of 14C-bendiocarb in four soil types. Fisons
Report METAB/78/11. (Unpublished)
Adcock, J.W., Bentley, A.P., Challis, I.R. and Pearce, J.C. The
1975a decline of NC 6897 in a sandy loam soil. Fisons Report
METAB/75.2.(Unpublished)
Adcock, J.W., Challis, I.R. and Pearce, J.C. Soil leaching of NC
1975b 6897 and degradation products. Fisons Report METAB/75/3.
(Unpublished)
Adcock, J.W., Challis, I.R., and Warner, P.A. The metabolism of
1976a 14C-bendiocarb in the dairy cow. Fisons Report
METAB/76/23. (Unpublished)
Adcock, J.W., Warner, P.A. and Challis, I.R. The metabolism of
1976b 14C-bendiocarb in the pig. Fisons Report METAB/76/19.
(Unpublished)
Browne, P.M. Residues of Bendiocarb and NC 7312 in mushrooms
1981a treated with a 50SC formulation in Holland, 1980. FBC Report
RESID/8140. (Unpublished)
Browne, P.M. Analytical method for residues of bendiocarb in
1981b cabbage. FBC Report RES/81/65. (Unpublished)
1981c Residues of bendiocarb in cabbage following repeat
applications of a 50WP formulation in the Philippines,
1980-81. FBC Report RESID/81/53. (Unpublished)
1981d Residues of bendiocarb in rye grass after autumn
applications of granular and seed treatment formulations in
the U.K., 1980. FBC Report RESID/81/69. (Unpublished)
1981e Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in the U.K., 1981. FBC
Report RESID/81/74. (Unpublished)
1982a Residues of bendiocarb and NC 7312 in mushrooms following
wall spray treatment with 80WP or 50SC. FBC Report
RESID/82/20. (Unpublished)
1982b Residues of bendiocarb and NC 7312 in apples and pears
following treatment with 20W, 50W or 50SC formulations in
the U.K., 1981. FBC Report RESID/82/41. (Unpublished)
1982c Residues of bendiocarb and NC 7312 in sugarbeet following
application of a granular (3G) formulation in Italy, 1981.
FBC Report RESID/82/13. (Unpublished)
Browne, P.M. and Manley, J.D. Residues of bendiocarb and NC 7312 in
1982 peas and pea pods following treatment with a 50SC
formulation in the UK, 1981. FBC Report RESID/82/61.
(Unpublished)
Browne, P.M. and Reary, J.B. Residues in milk and tissues following
1978a a 28-day feeding study with bendiocarb in dairy cows. Fisons
Report RESID/78/58. (Unpublished)
Browne, P.M. and Reary, J.B. Analytical method for residues of
1978b bendiocarb metabolites in milk and tissues of dairy cows.
Fisons Report RESID/78/54. (Unpublished)
1978c Bendiocarb residue decline in soil after treatment of maize
plots with a 10G formulation in Illinois, 1977. Fisons
Report RESID/78/93. (Unpublished)
1979a Laboratory leaching study with a granular (3G) formulation
of bendiocarb in three standard soils from West Germany.
Fisons Report RESID/79/78. (Unpublished)
1979b Analytical method for residues of bendiocarb 7312 in rice
grain and straw. Fisons Report RESID/79/22. (Unpublished)
1979c Improved method for the analysis of bendiocarb residues in
maize grain, stover and whole silage plants. Fisons Report
RESID/79/24. (Unpublished)
1979d Residues of bendiocarb in maize at different growth stages,
after application of a 3G formulation in France, 1978.
Fisons Report RESID/79/2. (Unpublished)
1980a Laboratory leaching study with a 1% dust formulation of
bendiocarb in three standard soils from West Germany. Fisons
Report RESID/800/111. (Unpublished)
1980b Analytical method for residues of bendiocarb in rape seed.
Fisons Report RESID/80/59. (Unpublished)
1980c Residues in silage maize grown in the U.K. from seed dressed
with bendiocarb, 1979. Fisons Report RESID/80/12.
(Unpublished)
1980d Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1979. Fisons Report RESID/80/13.
(Unpublished)
1980e Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979 (2nd
Report). Fisons Report RESID/80/20. (Unpublished)
1980f Residues of bendiocarb in rape seed after application of a
granular (5G) formulation in Canada, 1979. Fisons Report
RESID/80/60. (Unpublished)
1981 Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in West Germany, 1980.
FBC Report RESID/81/9. (Unpublished)
Challis, I.R. The metabolism of 14C-bendiocarb in sugarbeet. Fisons
1975 Report METAB/75/15. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in maize
1977 plants. Fisons Report METAB/77/32. (Unpublished)
1978 The metabolism of bendiocarb in sugarbeet grown under field
conditions. Fisons Report METAB/78/19. (Unpublished)
Challis, I.R. and Swalwell, L.M. The metabolism of bendiocarb by
1980 barley plants. Fisons Report METAB/80/9. (Unpublished)
FBC Bendiocarb, submission to the Joint Meeting of the FAO
1982a Panel of Experts on Pesticide Residues in Food and the
Environment and the WHO Expert Group on Pesticide Residues,
FBC Limited, Registration Department, Saffron Walden, Essex,
England 1982.
1982b Harvest residues of bendiocarb (NC 6897) in sugarbeet after
application of a granular (3G) formulations. (Unpublished)
1982c Residues in grass following the use of bendiocarb (50SC)
(CR 15 340) as a seed dressing or spray dressing or spray
treatment in the U.K., autumn 1979. (Unpublished)
Housden, M.C. and Reary, J.B. Bendiocarb residues in rice treated
1981 with a 50WP formulation in the Philippines during the 1980
dry season. FBC Report RESID/81/16. (Unpublished)
Lewis, J.A. and Adcock, J.W. Investigation of residue levels in
1978a maize plants treated with 14C-bendiocarb in France. Fisons
Report RESID/78/50. (Unpublished)
1978b Investigation of residue levels in maize treated with
14C-bendiocarb granules in the U.K. Fisons Report
METAB/78/19. (Unpublished)
1978c Investigation of residue levels in maize plants treated with
14C-bendiocarb granules. Fisons Report METAB/78/7.
(Unpublished)
Lewis, J.A. and Challis, I.R. The metabolism of 14C-bendiocarb in
1978 the hen. Fisons Report METAB/78/28. (Unpublished)
1979 The metabolism of 14C-bendiocarb in paddy rice. Fisons
Report METAB/79/1. (Unpublished)
Netherlands. Information on pesticides included in the JMPR priority
1982 list provided by The Netherlands Government.
Reary, J.B. Harvest residues of bendiocarb in sugarbeet following
1974 its use as a seed dressing. Fisons Report RESID/74/36,
December 1974. (Unpublished)
1975a Soil percolation experiments with bendiocarb. Fisons Report
RESID/75/12. (Unpublished)
1975b Laboratory study of bendiocarb degradation in two standard
soils from West Germany. (Unpublished)
1975c Analytical method for residues of bendiocarb in sugarbeet.
FBC Report RESID/75/26. (Unpublished)
1975d Bendiocarb (NC 6897) residue decline on laboratory grown rye
grass sprayed with Ficam W. Fisons Report RESID/75/5.
(Unpublished)
1975e Harvest residues of bendiocarb (NC6897) in sugarbeet
following its use as a seed dressing in West Germany (1974).
Fisons Report RESID/75/12. (Unpublished)
1978a Residues in milk and tissues following a 28-day feeding
study with up to 25 ppm bendiocarb in the diet of dairy
cows. Fisons Report RESID/78/101.(Unpublished)
1978b Residues in eggs and tissues following a 21-day feeding
study with bendiocarb in laying hens. Fisons Report,
RESID/78/59. (Unpublished)
1978c Residues of bendiocarb in laboratory-extracted oil from
maize harvested after band application of a granular (10G)
formulation in the U.S., 1977. Fisons Report RESID/78/95.
(Unpublished)
1978d Analytical method for bendiocarb residues in soil. Fisons
Report RESID/78/37. (Unpublished)
1978e Analytical method for bendiocarb residues in grass. Fisons
Report RESID/78/61. (Unpublished)
1978f Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 76 WP formulation at
28-day intervals, in California and Massachusetts, 1977.
Fisons Report RESID/78/9. (Unpublished)
1978g Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 5G formulation at
28-day intervals in California and Massachusetts, 1977.
Fisons Report RESID/78/32. (Unpublished)
1978h Residues in maize grown in the U.K. from seed dressed with
bendiocarb 80W, 1977. Fisons Report RESID/78/86.
(Unpublished)
1978i Residues of bendiocarb in maize treated with a granular (3G)
formulation in the U.K., 1977. Fisons Report RESID/78/97.
(Unpublished)
1978j Residues in maize grown in France during 1975, 1976 and 1978
from seed dressed with bendiocarb 80W. Fisons Report
RESID/78/106. (Unpublished)
1978k Bendiocarb residue decline in soil after band treatment of
maize plots with a 10G formulation in Missouri, 1977. Fisons
Report RESID/78/50. (Unpublished)
1978l Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a 10G granular formulation
in Missouri, 1977. Fisons Report RESID/78/51. (Unpublished)
1978m Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a granular (10G)
formulation in Illinois, 1977. Fisons Report RESID/78/60.
(Unpublished)
1978n Residues of bendiocarb in the mature maize crop (grain, cobs
and stover) after band application of a granular (10G)
formulation in regions of the U.S., 1977. First Revision,
Fisons Report RESID/78/61/1. (Unpublished)
1979a Residues in milk from cows treated dermally with FICAM D in
Australia, 1979. Fisons Report RESID/79/49. (Unpublished)
1979b Analytical method for bendiocarb residues in barley, wheat,
oats and maize (corn) grain. Fisons Report RESID/79/17.
(Unpublished)
1979c Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. (Extract of
analytical method from the above report). Fisons Report
RESID/79/9. (Unpublished)
1979d Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/9. (Unpublished)
1979e Residues of bendiocarb in barley, wheat and oats grown from
treated seed in the U.K., 1977 and 1978. Fisons Report
RESID/79/3. (Unpublished)
1979f Residues of bendiocarb in silage maize treated with a
granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/12. (Unpublished)
1979g Residues in silage maize grown in the U.K. from seed dressed
with an 80W formulation of bendiocarb, 1978. Fisons Report
RESID/79/13. (Unpublished)
1979h Harvest residues of bendiocarb and NC 7312 in sugarbeet
after application of a granular formulation (3G) in the
U.K., 1978. Fisons Report RESID/79/14. (Unpublished)
1979i Residues of bendiocarb and NC 7312 in sugarbeet after
application of a granular (3G) formulation in France and
Italy, 1978. Fisons Report RESID/79/15. (Unpublished)
1979j Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1978. Fisons Report RESID/79/16.
(Unpublished)
1979k Residues of bendiocarb and NC 7312 in rice treated with a
granular (3G) or spray (50W or 80W) application in the
Philippines, 1978. Fisons Report RESID/79/23. (Unpublished)
1979l Residues of bendiocarb in maize (corn) grown in the U.S.,
from seed dressed with a 76WP formulation, 1977 and 1978.
Fisons Report RESID/79/25. (Unpublished)
1979m Residues of bendiocarb in maize (corn) after band
application of a granular (10G) formulation in Canada, 1978.
Fisons Report RESID/79/26. (Unpublished)
1979n Residue analysis of fodder beet following the use of
bendiocarb as a seed dressing in West Germany, 1978. Fisons
Report RESID/79/45. (Unpublished)
1979o Residues of bendiocarb in silage and mature (grain and
stover) maize after band application of a granular (10G)
formulation in the U.S., 1978. Fisons Report RESID/79/57.
(Unpublished)
1979p Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/76. (Unpublished)
1970q Residues of bendiocarb in sugarbeet following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/75. (Unpublished)
1980a Residue in barley, wheat and oats following seed treatment
with bendiocarb in the U.K., 1979 (80WP) batch 8A8, 50W
spray or 15 181 and 3G or 15 094. Fisons Report RESID/80/80.
(Unpublished)
1980b Bendiocarb residue decline on rangeland grass after ULV
treatment (by air) with a 25% formulation (15 15 458) in
Montana, U.S., 1980. Fisons Report RESID/80/95.
(Unpublished)
1980c Preliminary report on residues in milk and tissues from
dairy cows treated dermally with bendiocarb dust for 7 days.
Fisons Report RESID/79/55. (Unpublished)
1980d Residues of bendiocarb in grass grown from treated seed in
Australia, 1979. Fisons Report RESID/80/9. (Unpublished)
1980e Residues in grass following the use of bendiocarb as a
granular (3G), seed dressing (80W) or spray (50W) treatment
in the U.K., Spring 1979. Fisons Report RESID/80/11.
(Unpublished)
Reary, J.B. Residue decline in sugarbeet treated with bendiocarb 3G
1980f in the U.K., 1979. Fisons Report RESID/80/4. (Unpublished)
1980g Residue decline in soil after furrow application of
bendiocarb 3G granules at Shelford, U.K., 1979. Fisons
Report RESID/80/14. (Unpublished)
1980h Residue decline in maize treated with bendiocarb 3G in the
U.K., 1979. Fisons Report RESID/80/17. (Unpublished)
1980i Residue decline in maize grown from seed dressed with
bendiocarb in the U.K., 1979. Fisons Report RESID/80/18.
(Unpublished)
1980j Residues in sugarbeet from grower trials with a granular
(3G) formulation of bendiocarb in the U.K., 1979. Fisons
Report RESID/80/29. (Unpublished)
1980k Bendiocarb residue decline on rangeland grass after low
volume treatment with a 50SC formulation (CR 15 340) in
Montana, U.S., 1979. Fisons Report RESID/80/37.
(Unpublished)
1980l Residues in rice treated with bendiocarb formulations in the
Philippines and Korea 1979. Fisons Report RESID/80/46.
(Unpublished)
1981m Residues in grass following the use of bendiocarb as a seed
dressing, granular or spray treatment in the U.K., 1980. FBC
Report RESID/81/10. (Unpublished)
Reary, J.B. and Browne, P.M. Residues of bendiocarb (NC 6897) in
1978 mature maize, after application of a granular formulation in
France, 1976 and 1977. Fisons Report RESID/78/23.
(Unpublished)
Reary, J.B. and Whiteoak, R.J. Bendiocarb residue decline on soil,
1977a grass and thatch after treatment of turf with a 76WP
formulation in Mississippi, 1976. Fisons Report RESID/77/43.
(Unpublished)
1977b Bendiocarb residue decline in soil, grass and thatch after
treatment of turf with a 76WP formulation in Ohio, 1976.
Fisons Report RESID/77/52. (Unpublished)
1977c Bendiocarb residue decline on soil, grass and thatch after
treatment of turf with a granular formulation in Ohio, 1976.
Fisons Report RESID/77/61. (Unpublished)
1977d Bendiocarb residue decline on grass and soil after treatment
of turf with a 76WP formulation in California and
Massachusetts, 1977. Fisons Report, RESID/77/62.
(Unpublished)
Warner, P.A. The metabolism of 14C-bendiocarb in oilseed rape. Fisons
1980 Report METAB/80/20. (Unpublished)
unidentified water soluble and fibre-bound residues, other metabolism
studies discussed below suggest that the pathway is similar in other
plants.
In a second sugarbeet metabolism study (Adcock 1976),
14C-bendiocarb was applied as a seed dressing for plants grown in
outdoor drums in sandy loam soils at 6.7 and 7.5 pH values at a rate
equivalent to 16 g a.i./kg seed. Leaves and/or roots were analysed for
radioactivity at intervals from 13-114 days after sowing, although the
residues were not identified. In accordance with decreased bendiocarb
stability in more alkaline soils, extractable and fibre-bound residues
were less than the 0.02 mg/kg limit of detection (bendiocarb
equivalents) in foliage and roots of the more alkaline soil after 114
days. Residues were greater at earlier intervals. For example, at 36
days residues were on the order of 0.1 mg/kg bendiocarb equivalent in
leaves or roots from either extractable or fibre-bound sources.
Consistent with pH stability, residues were higher in the more acidic
soil, being up to 0.06 mg/kg and 0.02 mg/kg extractable residues in
leaves and roots, respectively, after 114 days; they were appreciably
higher at shorter intervals. A maximum of 18% of applied radioactivity
was found in immature sugarbeet (36-72 days after sowing).
In the third sugarbeet metabolism study (Challis and Adcock
1978), the crop was grown under field conditions and treated at sowing
with 14C-bendiocarb granules at the recommended rate of 0.36 kg
a.i./ha. Plants were harvested at intervals ranging from 46-190 days
after treatment. Results were similar to those of Challis (1975).
After 90 days, radioactivity was too low to permit determination of
individual metabolite levels. In harvested beets, mean residues of
only 0.009 mg/kg bendiocarb equivalent were found after 190 days.
Extractable residues were below the limits of sensitivity.
As would be expected, residues were greater at earlier intervals
and identification was possible. For example, at 46 days residues
(bendiocarb equivalent) were 1.0, 0.22, 0.14, 0.76 and 0.45 mg/kg for
bendiocarb, NC 7312 conjugates, N-hydroxymethylbendiocarb conjugates,
unidentified water soluble residues and fibre-bound residues,
respectively. There were lesser amounts of free metabolites and other
conjugates.
Bendiocarb metabolism was investigated in maize under glasshouse
conditions by applying a solution of 14C-bendiocarb to pH 5.4 soil in
drums with newly germinated maize plants at a rate equivalent to
1.1 kg a.i./ha (Challis and Adcock 1977). Plants were analysed at
intervals from 10-125 days. Mean residues of bendiocarb per se in
plants ranged from 1.4 mg/kg after 10 days to 0.01 mg/kg after 125
days and unidentified soluble residue (bendiocarb equivalent) in
kernels and cobs were 0.005 and 0.001 mg/kg, respectively, after 125
days. As a percentage of total radioactivity, the major residues after
10, 60 or 125 days were:
Foliage Kernels Cob
10 days 60 days 125 days 125 days 125 days
Bendiocarb 38.1 24.2
N-hydroxymethylbendiocarb 8.2 5.3 8
NC 7312 conjugates 163.3 2.7 20
Water soluble 13.6 11 9.1
(non-hydrolysable)
Fibre-bound 13.6 27 61 89 97
Free NC 7312, other non-polar metabolites and other conjugates
were also present at lower levels. Seventy percent of the residues in
plants at harvest were either water non-hydrolysable of fibre-bound
unidentified residues. Although they occurred at very low levels,
fibre-bound unidentified residues constituted 89 and 97 percent of
total radioactive residues in kernels and cobs, respectively, at
harvest.
Investigations were conducted on paddy rice metabolism of
14C-bendiocarb applied as a solution with a syringe under glass to
either the foliage or flood water surrounding rice transplants (Lewis
and Challis 1979). Application rates were equivalent to 0.6 kg a.i./ha
and plant samples were taken at intervals ranging from 15-174 days
after treatments. Foliage and grain were analysed separately at 174
days. Qualitatively, residues were similar to those in sugarbeet and
maize, although N-hydromethylbendiocarb or its conjugates were not
specifically identified in rice. They could have been present under
residues described either as other non-polar metabolites or other
conjugates.
Mean free bendiocarb in rice plants ranged from 1.7 mg/kg after
15 days to 0.0022 mg/kg (total radioactivity as bendiocarb
equivalents) after 174 days from the foliar treatments. The most
abundant metabolite by far was NC 7312, mostly as a conjugate
(0.006 mg/kg bendiocarb equivalent). Residues were 0.009 mg/kg in rice
husks and flour after 174 days.
In plants growing in treated water, residues of free bendiocarb
at 15 days were approximately one tenth of that from foliar
treatments, the major residue being NC 7312 conjugates at 0.54 mg/kg.
Individual residues could not be determined after 60 days. At harvest,
total radioactivity levels are similar regardless of the type of
treatment. Quantitatively, major residues in plants as a percent of
total radioactivity at 15 or 174 days are:
15 days 174 days
Foliar Water Foliar Water
Bendiocarb 37 12
NC 7312 13 37 39 31
conjugates
Water soluble 7 10
(unhydrolysable)
Fibre-bound 33 33 61 69
All of the radioactive residue at harvest in rice husks or flour
was NC 7312 conjugates, regardless of whether application was by
foliar or water treatment. As in the case of maize foliage, residues
in rice plants were mostly unidentified fibre-bound ones at harvest.
The difference was in the grain, which contained mostly unidentified
fibre-bound residues in maize and mostly NC 7312 conjugates in rice.
Greenhouse-grown barley plants were treated with 14C-bendiocarb
at rates equivalent to 1 kg/ha. A solution was applied by pipette to
soil surrounding the plants which were analysed at intervals ranging
from 4-94 days after treatment. Samples at 94 days were separated into
foliage, husks and grain, which were analysed separately (Challis and
Swalwell 1980).
Results of this investigation were similar to that of
rice. However, in the case of barley, conjugates of
N-hydroxymethylbendiocarb were identified, but total radioactive
residues in husks and grain at 94 days (1.46 and 0.09 mg/kg bendiocarb
equivalent, respectively) were not characterized. Free bendiocarb
residues in foliage ranged from 0.09 mg/kg at 4 days, declined through
60 days and increased to <0.07 mg/kg at 94 days. Similar patterns
were observed for conjugates of NC 7312 and N-hydroxymethylbendiocarb,
the major components of the residue throughout the period. Again,
unidentified water soluble or fibre-bound residues constituted a major
part of the residue, which increased until harvest (12 and 42% of
total radioactivity, respectively).
The metabolism of 14C-bendiocarb was studied in greenhouse grown
oilseed rape treated at the seedling stage at application rates
equivalent to 140 and 500 g a.i./ha. Application was by pipette to
soil surrounding the plant (Warner 1980). Plant samples were taken at
intervals ranging from 5 to 140 days after treatment. At 140 days,
samples were separated into foliage, pods, and seed, which were
analysed separately.
As in the case of barley, the major residues in the plants were
highest at earlier intervals and, except for free bendiocarb, declined
and then increased by harvest. In both cases this was probably due to
plant growth patterns and dessication near harvest. Free bendiocarb
was the major residue at day 5 (66-69%), but declined rapidly
thereafter (2.5 mg/kg at day 5 to 0.01 mg/kg at 140 days from the
high treatment rate). Again, conjugates of NC 7312 and
N-hydroxymethylbendiocarb were the major identified metabolites and,
by harvest, unidentified water soluble materials and fibre-bound
residues formed the major portion of total radioactivity which was 26
and 43%, respectively, from the higher treatment rate. Although
residue levels were dependent on the application rate, on the basis of
percentage of total residue, results were similar at both application
rates.
Extractable residues in pods, seeds and foliage at harvest were
0.09, 0.001 and 0.16 mg/kg, respectively, at the low application rate
and 0.74, 0.011 and 0.84 mg/kg, respectively, at the high rate.
In Soil
Laboratory studies on residue decline in a pH 7.7 sandy loam soil
treated in flasks or jars with 5 mg/kg 14C-bendiocarb showed
degradation to CO2 and unidentified bound residues under aerobic
conditions, with a half-life of 5 days for the parent compound. No NC
7312 was detected. With flooding or nitrogen flushing and under
sterile anaerobic conditions, bendiocarb was hydrolysed to NC 7312,
with little or no further degradation (Adcock et al 1975a).
In a laboratory soil leaching study with the same soil type, 60%
of the radioactivity applied to a 30 cm column as 14C-bendiocarb was
eluted with 50 cm of water over 11 days, mostly as NC 7312 (Adcock
et al 1975b). Presumably, conditions were effectively anaerobic and
the rapid degradation of bendiocarb did not permit estimation of its
leachability.
In another leaching study, 14C-bendiocarb, leachability was
investigated for an agricultural sand (pH 5.3), a clay loam (pH 7.3),
a sandy loam (pH 7.8) and a silt loam (pH 7.2) using soil column and
thin-layer techniques (Adcock 1978). The 30 cm columns were percolated
over a 2-9 day period with a 20 cm column of water. The percentage of
radioactivity recovered in leachate was 75, 11.9, 7.9 and 0.8% for the
respective soils, decreasing with increased organic content.
Recovery in leachate was slightly less than for 2,4-D in
agricultural sand and clay loam, and substantially less in the other
two soils under similar conditions. Thin-layer results confirm these
observations and show that NC 7312 is somewhat less leachable than the
parent compound in all four soil types. It also shows that bendiocarb
is more leachable than trietazene, which places it in the moderately
mobile class.
When bendiocarb 80%WP, 3%G and 1% dust formulations were leached
from sand (pH 7-7.6) loamy sand (pH 6-6.5) and sand loam (pH 5.6-7.8)
in 30 cm × 5 cm columns with 20 cm water over 48 hours, leachability
was again inversely proportional to organic matter content of the soil
(Reary 1975a; Browne and Reary 1979a, 1980a). As a percent of the
amount applied, bendiocarb recovery in leachate ranged from 0 to 93%
WP, 0.6 to 84% dust and 0 to 22% granular. From the same formulations
leachate contained 0 to 25% bendiocarb and 0 to 68% NC 7312; 0 to 45%
bendiocarb, 0 to 62% NC 7312 and 0 to 4% bendiocarb, 0 to 19% NC 7312,
respectively. Therefore, leachability decreased in the order of WP,
dust and granular formulations.
Bendiocarb stability in standard soils has been shown to be
dependent on soil pH, being much slower at low pHs (Reary 1975b). The
half-life was 10 days in one soil at pH 2.2 and 58 days in another at
pH 5.2.
Soil residue decline studies have also been carried out in trials
determining bendiocarb residues in turf and maize. Results again show
that the rate of degradation of bendiocarb is pH-dependent. Repeated
applications showed no evidence of a build-up of bendiocarb residues
in soil. Dissipation rates (in days) for various formulations in
various soil types and pHs are given in Table 9 (Browne and Reary
1978c; Reary 1975b, 1978f, g, k, 1980g; Reary and Whiteoak 1977a, b,
d).
In Storage and Processing
It was noted under the section on "Residues from Supervised
Trials" and in Table 6 that oil processed from maize with field-
incurred residues of <0.02 mg/kg bendiocarb also contained residues
of <0.02 mg/kg. In another investigation, 10G was applied as a band
along the maize row at sowing at 1.1-2.2 kg a.i./ha and the maize
sampled at normal harvest. Oil was solvent-extracted from the grain,
which had residues of <0.01 mg/kg, the limit of determination.
Analysis of the oil showed no residues in excess of the 0.05 mg/kg
limit of determination (Reary 1978c).
Investigation with radio-labelled bendiocarb in granular
application trials in France, the U.K. and the U.S. showed residues in
solvent-extracted oil to be 0.008, 0.011 and 0.021 mg/kg,
respectively, from kernels with residues of 0.011, 0.013 and 0.022
mg/kg, respectively. In most kernel or oil samples residues were
<2X the reported limit of sensitivity (Lewis and Adcock 1978a, b, c).
RESIDUES ON FOOD IN COMMERCE OR AT CONSUMPTION
No information was provided on residues in commerce or at
consumption. The Meeting considered such information desirable.
Table 9. Summary of Bendiocarb Dissipation Rates in Soil
Soil Dissipation time (days)
Type pH Formulation 50% 75% 90%
Sandy loam 5.2 Technical1/ 58 ? ?
Sandy 6.5 76W 25 50 75
loam 76W2/ 12-34 24-69 37-104
Sandy loam 6.5 5G2/ 26-70 52-140 78-210
Silty clay 6.5 10G 19 38 57
Silty clay loam 6.7 76W 30 60 ?
Sand 6.8 Technical1/ 10 24 50
Loam 7.2 10G 7.5-10 15-20 22.5-30
Loam 7.7 76W 12 24 36
Sandy 7.7 76W 2.4 4.8 7.2
loam 76W2/ 2-5 3-10 5-15
Sandy loam 7.7 5G2/ 12-26 30-52 45-78
Sandy loam 7.8 3G 12 24 36
1/ Laboratory studies.
2/ Multiple applications at up to 5-monthly intervals;
dissipation times determined after each application.
METHODS OF RESIDUE ANALYSIS
Residue analysis methods for various plant substrates have been
developed for bendiocarb alone or for bendiocarb plus the non-
conjugated metabolite NC 7312, either separately or as a total
residue. In animal substrates, enzymic liberation of NC 7312 from
conjugates is more successful than in plants. Consequently, residue
methods for animal tissues, milk and eggs include determinations of
the conjugated residues.
As the carbamate functional group does not possess strong
electron capturing properties, derivatization of the bendiocarb
molecule is necessary to facilitate the measurement of low residue
concentrations. Formation of the 2,4-dinitrophenyl ether was found to
be most suitable. This results in a product with strong electron
capturing properties and a long gas chromatographic (GLC) retention,
thus ensuring excellent sensitivity and minimal co-extractive
interference.
In the standard procedure (Reary 1975c) the carbamate is
hydrolysed in an alkaline buffer containing 1-fluoro-2,
4-dinitrobenzene so that the dinitrophenyl ether is formed immediately
after hydrolysis. This method has the added advantage the phenolic
degradation product, NC 7312, forms the same product in the reaction.
Thus, the combined residue can be measured unless bendiocarb and NC
7312 are separated prior to derivatization. This separation is readily
effected by high pressure liquid chromatography (HPLC) or with
commercially available liquid chromatographic cartridges. Collection
of the appropriate fraction allows individual analysis for bendiocarb
and NC 7312 from the same extract (Brown and Reary 1979b).
Bendiocarb is extracted from soil (Reary 1978d), grass (Reary
1978e) sugarbeet (Reary 1975c), maize (Browne and Reary 1979c), cereal
grain (Reary 1979b), rice (Browne and Reary 1979b), rape seed (Browne
and Reary 1980b), potatoes (Reary 1979c), cabbage (Browne 1981b),
mushrooms (Browne 1982a), apples/pears (Browne 1982b) and peas (Browne
and Manley 1982), under reflux with dichloromethane or by shaking with
diethyl ether. Oily extracts are improved by partition between hexane
and acetonitrile. Further clean up, when required, is by silica gel
chromatography. This is easily done using commercially available
disposable cartridges, which can eliminate or separate any NC 7312
present in the extract.
A method has been developed for the residue analysis of
bendiocarb and major metabolites in milk, eggs, meat, fat and offal
(Browne and Reary 1978b). Residues are extracted under reflux with
acetonitrile. Conjugated metabolites are hydrolysed with snail
digestive juice before formation of the dinitrophenyl derivative. All
major bendiocarb related metabolites form the same derivative, which
is then analysed by the standard method as a total residue.
In general, mean analytical recoveries in most commodities were
>75% for bendiocarb and >60% for NC 7312. Analytical
sensitivities, recoveries, recoveries of fortified commodities and
apparent residue in untreated controls for individual commodities are
discussed under "Residues Resulting from Supervised Trials."
Information represents field-type conditions and is somewhat less
assuring than mean recoveries presented with the reports on each
individual analytical method. Although mean analytical recoveries
appeared acceptable, at least for bendiocarb per se, the range of
residues was generally large, resulting in standard deviations ranging
from 6 to 33. In animal tissues in particular, and to some extent in
cereal grains and fodder/forage, analytical recoveries were frequently
low. For example, in animal products analytical recoveries were
frequently only 50-60% for bendiocarb per se or the N-hydroxy
metabolite, and sometimes less.
NATIONAL MAXIMUM RESIDUE LIMITS
Information on national maximum residue limits were available
from only one country. Limits have been proposed in four other
countries (Table 10).
Table 10. National Maximum Residue Limits
Country Commodity MRL (mg/kg)
Netherlands1/ "All commodities"3/ 0 (0.05)4/
Australia2/ Poultry meat, eggs 0.05
Milk, milk by-products, meat
and other edible offal 0.1
Cereal grain 10.0
Belgium2/ Maize and sugarbeet 0.02
Switzerland2/ General foodstuffs (excl. milk) 0.2
Milk 0.005
U.S.2/ Maize (grain fodder, forage) 0.05
Maize oil 0.1
Milk, eggs, meat fat, meat
by-products (except kidneys)
of cattle, goats, pigs horses,
poultry, sheep 0.05
Kidneys of cattle, goats, pigs,
horses, poultry, sheep 0.1
1/ Established;
2/ Proposed;
3/ It is unclear whether "all commodities" include
commodities other than maize and sugarbeet, for which
good agricultural practice information was provided;
4/ 0 (0.05) mg/kg presumed to mean no residues allowed with
a 0.05 mg/kg limit of determination. Large-scale use.
APPRAISAL
The Meeting reviewed substantial information on residue levels
from supervised trials in a variety of commodities, fate of residues
and analytical methodology for bendiocarb, a carbamate pesticide used
for control of a wide range of agricultural and public health pests.
Information on official nationally approved agricultural practices and
national maximum residue limits were available for only two
commodities (maize and sugarbeet) in one country, although recommended
application rates were provided for a number of commodities. Since
information on good agricultural practices for all commodities except
maize and sugarbeet is lacking, only TMRLs are estimated for other
than these two commodities. National tolerances have been proposed in
at least four countries, mostly on maize and other cereals and animal
products.
Residue data were available from supervised trials on a variety
of commodities in several countries and using a variety of
formulations and agricultural practices. In most cases, free
bendiocarb per se was the only residue measured. In a few cases, the
major metabolite 2,2-dimethyl-1,3-benzoxodiol-4-ol (NC 7312) was
measured in its free form and in some cereals, combined residues of
conjugated NC 7312 and N-hydroxymethylbendiocarb were measured as
well. Data were inadequate for estimating a limit for oilseed rape,
cabbage and peas, although the Meeting was informed that additional
data are being compiled for the latter.
For root or tuber vegetables, data were available for potatoes
and sugarbeet. Data on potatoes show residues of free NC 7312 at
harvest to be up to 20 times that of the parent compound (average 6X).
This rate could not be determined for sugarbeet with available data.
Limited data were available in pome fruit from one country and
residues of NC 7312 were equal to or less than those of the parent
compound. The Meeting was informed that additional data are being
gathered.
Bendiocarb residue data on cereals were available for barley,
oats, wheat, maize and rice. For the latter, limited data were also
available for free NC 7312, and the first three, for combined residues
of conjugates of NC 7312 and N-hydroxymethylbendicarb. Data were
mostly from seed treatments in barley, oats and wheat. No data were
available for metabolites in maize, although metabolism studies have
shown >50% of residues in foliage to be metabolites and/or
unidentified water-soluble or bound residues; in kernels at harvest,
approximately 90% of radioactivity was unidentified fibre-bound
residues. Analytical recoveries were only marginally acceptable in
some cereal trials, especially those in wheat.
Considerable data were available for grass and turf, mostly at
higher than recommended rates. Half-lives were greater from granular
broadcast applications than from spray applications and irrigation did
not significantly affect decline rates for either.
As in maize, data for oilseed rape were for the parent compound
only, and metabolism studies showed >50% of residues at harvest to be
conjugated metabolites and (mostly) unidentified water-soluble or
fibre-bound residues.
Data were available for mushrooms from spray treatments (plot
presumed) and wall treatments (before plot establishment). Residues
increased with application rates and residues of free NC 7312 from
wall treatments were on the same order of magnitude as those of the
parent compound, but were less following direct spray treatment. No
information was provided on good agricultural practices for direct
spray treatment.
Animal feeding trials were conducted in which lactating dairy
cattle were fed diets for 28 days containing up to 25 mg/kg
bendiocarb. In contrast to field trials, in which the parent compound
was determined separately from metabolites, animal tissues were
analysed for total residues of free bendiocarb and non-conjugated
metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb.
Milk was separated into a fat fraction and lactose (aqueous) fraction.
The fat fraction was analysed for bendiocarb and non-conjugated NC
7312 and N-hydroxymethylbendiocarb and the lactose fraction for the
conjugated residues of the two metabolites.
Studies using topical treatments were also conducted, but no
information was provided on approved or recommended uses for such
treatments. Dermal treatments generally resulted in higher residues in
tissues and milk than feeding trials. In the absence of good
agricultural practice information for dermal treatments (or grass
uses) residue estimates in cattle are based on potential residues from
the feeding of maize, for which national good agricultural practice
information was available. From this potential source of exposure, no
residues are expected in products of animal origin above the limit of
determination at or about 0.05 mg/kg (0.2 mg/kg in kidney).
In animal products in general, analytical recoveries were
disappointingly low, frequently only 50-60% or less for some residues
in some tissues, and thus can be considered marginally acceptable for
enforcement purposes only, since residues are expected to be
relatively low as a result of uses considered in residue level
estimates.
Animal metabolism studies were conducted in the rat, dog,
hamster, rabbit, mouse, pig, cow and poultry and in humans. In the
pig, cow and poultry single dosing was with 14C-bendiocarb, by
gelatin capsule in pig and cow and by gavage in poultry. Approximately
80% of applied radioactivity was eliminated in the urine within one
day or less in pigs and cows and 61-90% in poultry. It consisted
mostly of the sulphate or glucuronide conjugates of NC 7312 in all
three, although in poultry significant levels of unidentified
water-soluble residues were also present, as well as low levels of
free NC 7312 and other unidentified moieties. Low levels of NC 7312
sulphate and glucuronide were also present in faeces of cows and pigs.
In pigs the NC 7312 sulphate to glucuronide ratio was unexpectedly
high.
In animal tissues and milk, residue levels were generally not far
from the limit of determination and only 0.01% of the administered
dose was found in eggs. Owing to the low levels, they were not
identified.
Bendiocarb metabolism in cows, pigs and poultry appears to be
similar to that in smaller animals. Carbamate is hydrolysed to
NC 7312, which forms glucuronide and sulphate conjugates. The
apparent major difference is the lack of conjugates of
N-hydroxymethylbendiocarb also found in plants, and 5,6, or
7-hydroxybendiocarb found or tentatively identified in the smaller
animals. However, dosing rates of 0.2-0.25 mg/kg bw in cows, pigs and
hens were substantially less than the maximum dosage rates of 10 mg/kg
bw used for the smaller animals. More importantly, cows, pigs and
poultry were sacrificed four days after dose administration, which
precludes measurement or characterization of additional radioactivity
closer to administration. This could also account for residue levels
in animal products (cows, pigs and poultry) being too low for
identification. A metabolism study conducted in both ruminants and
poultry at high levels and a much shorter sacrifice interval would
have been preferred and are desirable. Such a metabolism study should
be considered essential if higher than the current ones for animal
products are required from the feeding of grain, fodders or straw of
cereals.
Metabolism studies in sugarbeet, maize, barley, rice and oilseed
rape under laboratory or field conditions showed similar quantitative
results, although ratios of the various metabolites and degradation
products varied considerably, depending on the plant, type of
application, interval from application to harvest, part of plant and
other factors.
A major metabolic pathway for bendiocarb in some plants is
hydrolysis to NC 7312, which becomes conjugated as a glycoside.
In another pathway, bendiocarb can first be oxidized to
N-hydroxymethylbendiocarb, which may hydrolyse to NC 7312
before glycosidic conjugation or become conjugated as
N-hydroxymethylbendiocarb glycoside. Another route can be direct
glycosidic conjugation of bendiocarb per se. This was a major residue
in beet leaves following a surface application, but NC 7312 conjugates
were found following surface treatments to rice. Undoubtedly other
pathways exist, since low levels of unidentified conjugates are known
to occur. In one beet study, limited leaf penetration was detected
from surface application.
As the interval from application to harvest increased, residues
of bendiocarb per se decreased with an increase in NC 7312 and
N-hydroxymethylbendiocarb conjugates, which were frequently the major
identified metabolites. Unidentified water-soluble, non-hydrolysable
residues and unidentified fibre-bound residues have also been shown to
increase in the foliage of several plants; at harvest they constitute
26 and 60%, respectively, of the total remaining radioactivity. In
maize kernels and cobs at harvest the fibre-bound unknown was shown to
consist of >90% of the remaining low residue.
Low levels of free NC 7312 and other unidentified unconjugated
residues were found in some plants. Free NC 7312 exceeded that of the
parent compound in potatoes and probably in pea pods. In other plants,
free NC 7312 residues were either less than or equal to that of the
parent compound. Differences in method of application (for example,
foliar versus ground application) affected the ratios of the various
metabolites in some, but not all, plants tested, although residues
were qualitatively similar.
In soils, bendiocarb was degraded to CO2 and unidentified bound
residues under aerobic conditions whereas degradation was mostly to
NC 7312 under anaerobic conditions. Bendiocarb stability was dependent
on soil pH, being much more stable under acidic conditions. Half-lives
ranged from 2-70 days, depending on conditions.
Leaching studies carried out on a variety of soils, under
different conditions and with several formulations showed leaching to
be largely dependent on the organic content of the soil, decreasing
with higher levels of organic matter. Although generally considered to
be in only a moderately leachable category, significant leaching of
bendiocarb can occur in sandy soils with low organic matter content.
Leachability appears to increase somewhat from granular to dust to
wettable powder formulations. One study indicated that NC 7312 leached
slightly less than bendiocarb per se. Although build-up of soil
residues of bendiocarb per se do not appear to be indicated, any
potential residues of bendiocarb or its metabolites in ground water
would be greatest from sandy soils of low organic matter content.
Most investigations on possible residues of bendiocarb in
processed products were conducted on oil, husks or flour from cereal
grain. Although no concentration of residues was demonstrated in these
studies, residues in the grain from which the products were processed
had residues below or near the limits of determination. Therefore,
firm conclusions cannot be drawn.
Analytical methods are available for the determination of free
bendiocarb in all plant commodities examined and free NC 7312 in some.
In the basic procedure, bendiocarb is hydrolysed to its phenol under
alkaline conditions in the present of 1-fluoro-2,4-dinitrobenzene to
form the dinitrophenyl ether, which is preferably determined by
electron capture gas chromatography using an internal standard.
Bendiocarb and NC 7312 can be determined together or separately, if
they are separated by HPLC or silica gel chromatography prior to
derivitization. In most commodities of plant origin, field trials were
for the free parent compound only and free NC 7312 was also determined
separately in a few. Analytical recoveries were highly variable on
some commodities, especially cereals.
For animals, the same basic analytical approach is used, except
that a hydrolysis step is included to hydrolyse conjugated residue.
Hydrolysis has been accomplished by a variety of enzymatic and/or
acidic conditions. Enzymatic approaches are preferred and snail
gastric juice is frequently used. In contrast to plants, residues
determined for animal tissues and milk include the sum of residues of
bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and
N-hydroxymethylbendiocarb. Aqueous (lactose) and non-aqueous (fat)
fractions of milk are analysed separately. Analytical recoveries for
products of animal origin are only marginally acceptable for
enforcement purposes. Improved analytical methods are needed for any
uses requiring maximum residues levels in products of animal origin
that are higher than the current limits of determination.
RECOMMENDATIONS
The Meeting considered information on residues resulting from
good agricultural practices or recommended application rates on a
number of commodities and possible residues in animals resulting from
the feeding of grain, fodders or straw of cereals treated according to
good agricultural practice and concluded that the maximum residue
levels or temporary maximum residue levels below are suitable for
establishing maximum residue limits. Regardless of the status of the
ADI, temporary status was designated for all commodities except maize,
maize fodder and forage, sugarbeet and sugarbeet tops, pending
availability of good agricultural practice information. Except for
potatoes, some fruit, rice and rice straw, estimated residue levels
apply to treatment before planting and/or seed application only.
In the absence of good agricultural practice information, the
temporary limits are based on supervised trials data only. Residues
for products of plant origin refer to residues of unconjugated
bendiocarb. Residues in products of animal origin refer to the
sum of free and conjugated bendiocarb, NC 7312, and
N-hydroxymethylbendiocarb, expressed as bendiocarb.
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Potatoes 0.051/ 100 (granular)
0 (spray)
Sugarbeet 0.051/ 150
Sugarbeet tops 0.051/ 150
Pome fruit 0.021/ 50
Cereal Grains
Barley 0.051/ 100
Oats 0.051/ 100
Wheat 0.051/ 125
Maize (field) 0.051/ 125
Rice (in husk) 2 10
Fodders and Straws
Barley 0.051/ 125
Oats 0.051/ 100
Wheat 0.051/ 125
Maize 0.051/ 125
Rice 1 10
Mushrooms 0.12/ 50
(con't)
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Animal Products3/
Carcase meat of cattle 0.051/
Fat of cattle 0.051/
Meat by-products of cattle
(except kidney) 0.051/
Kidney of cattle 0.21/
Milk 0.051/
Poultry 0.051/
Fat of poultry 0.051/
Poultry meat by-products 0.051/
Eggs 0.051/
1/ At or about the limit of determination.
2/ Limit reflects maximum residues expected from wall treatments
prior to establishment of plots.
3/ Limits reflect maximum residues expected from the feeding of
cereal grains, fodders or straws harvested at given intervals
from application to harvest.
FURTHER WORK OR INFORMATION
Required (by 1984)
Information on nationally approved agricultural practices for the
use of bendiocarb on all commodities for which limits are estimated,
except for maize and sugarbeet.
Desirable
1. Additional metabolism studies in ruminants and poultry at
sufficiently high dosing levels and with sacrifice at a short
interval after dosing, instead of several days thereafter, to
permit a more complete characterization of residues in tissues,
milk and eggs. The analyses should include all residues
identified, or tentatively identified, in smaller animals and in
plants. These studies should be considered required before new
limits or future upward revisions of established ones on feed
items are established significantly higher than at current limits
of determination. The same is true should substantially higher
limits on tissues, eggs or milk be required for approved dermal
treatments.
2. Additional residue data, reflecting nationally approved uses
especially on, but not limited to, mushrooms and pome fruit.
3. Information on the possible occurrence of residues in food in
commerce or at consumption.
REFERENCES
Adcock, J.W. Investigation of residues in sugarbeet following the
1976 use of 14C-bendiocarb as a seed dressing. Fisons Report
METAB/76/1. (Unpublished)
1978 The leaching of 14C-bendiocarb in four soil types. Fisons
Report METAB/78/11. (Unpublished)
Adcock, J.W., Bentley, A.P., Challis, I.R. and Pearce, J.C. The
1975a decline of NC 6897 in a sandy loam soil. Fisons Report
METAB/75.2.(Unpublished)
Adcock, J.W., Challis, I.R. and Pearce, J.C. Soil leaching of NC
1975b 6897 and degradation products. Fisons Report METAB/75/3.
(Unpublished)
Adcock, J.W., Challis, I.R., and Warner, P.A. The metabolism of
1976a 14C-bendiocarb in the dairy cow. Fisons Report
METAB/76/23. (Unpublished)
Adcock, J.W., Warner, P.A. and Challis, I.R. The metabolism of
1976b 14C-bendiocarb in the pig. Fisons Report METAB/76/19.
(Unpublished)
Browne, P.M. Residues of Bendiocarb and NC 7312 in mushrooms
1981a treated with a 50SC formulation in Holland, 1980. FBC Report
RESID/8140. (Unpublished)
Browne, P.M. Analytical method for residues of bendiocarb in
1981b cabbage. FBC Report RES/81/65. (Unpublished)
1981c Residues of bendiocarb in cabbage following repeat
applications of a 50WP formulation in the Philippines,
1980-81. FBC Report RESID/81/53. (Unpublished)
1981d Residues of bendiocarb in rye grass after autumn
applications of granular and seed treatment formulations in
the U.K., 1980. FBC Report RESID/81/69. (Unpublished)
1981e Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in the U.K., 1981. FBC
Report RESID/81/74. (Unpublished)
1982a Residues of bendiocarb and NC 7312 in mushrooms following
wall spray treatment with 80WP or 50SC. FBC Report
RESID/82/20. (Unpublished)
1982b Residues of bendiocarb and NC 7312 in apples and pears
following treatment with 20W, 50W or 50SC formulations in
the U.K., 1981. FBC Report RESID/82/41. (Unpublished)
1982c Residues of bendiocarb and NC 7312 in sugarbeet following
application of a granular (3G) formulation in Italy, 1981.
FBC Report RESID/82/13. (Unpublished)
Browne, P.M. and Manley, J.D. Residues of bendiocarb and NC 7312 in
1982 peas and pea pods following treatment with a 50SC
formulation in the UK, 1981. FBC Report RESID/82/61.
(Unpublished)
Browne, P.M. and Reary, J.B. Residues in milk and tissues following
1978a a 28-day feeding study with bendiocarb in dairy cows. Fisons
Report RESID/78/58. (Unpublished)
Browne, P.M. and Reary, J.B. Analytical method for residues of
1978b bendiocarb metabolites in milk and tissues of dairy cows.
Fisons Report RESID/78/54. (Unpublished)
1978c Bendiocarb residue decline in soil after treatment of maize
plots with a 10G formulation in Illinois, 1977. Fisons
Report RESID/78/93. (Unpublished)
1979a Laboratory leaching study with a granular (3G) formulation
of bendiocarb in three standard soils from West Germany.
Fisons Report RESID/79/78. (Unpublished)
1979b Analytical method for residues of bendiocarb 7312 in rice
grain and straw. Fisons Report RESID/79/22. (Unpublished)
1979c Improved method for the analysis of bendiocarb residues in
maize grain, stover and whole silage plants. Fisons Report
RESID/79/24. (Unpublished)
1979d Residues of bendiocarb in maize at different growth stages,
after application of a 3G formulation in France, 1978.
Fisons Report RESID/79/2. (Unpublished)
1980a Laboratory leaching study with a 1% dust formulation of
bendiocarb in three standard soils from West Germany. Fisons
Report RESID/800/111. (Unpublished)
1980b Analytical method for residues of bendiocarb in rape seed.
Fisons Report RESID/80/59. (Unpublished)
1980c Residues in silage maize grown in the U.K. from seed dressed
with bendiocarb, 1979. Fisons Report RESID/80/12.
(Unpublished)
1980d Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1979. Fisons Report RESID/80/13.
(Unpublished)
1980e Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979 (2nd
Report). Fisons Report RESID/80/20. (Unpublished)
1980f Residues of bendiocarb in rape seed after application of a
granular (5G) formulation in Canada, 1979. Fisons Report
RESID/80/60. (Unpublished)
1981 Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in West Germany, 1980.
FBC Report RESID/81/9. (Unpublished)
Challis, I.R. The metabolism of 14C-bendiocarb in sugarbeet. Fisons
1975 Report METAB/75/15. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in maize
1977 plants. Fisons Report METAB/77/32. (Unpublished)
1978 The metabolism of bendiocarb in sugarbeet grown under field
conditions. Fisons Report METAB/78/19. (Unpublished)
Challis, I.R. and Swalwell, L.M. The metabolism of bendiocarb by
1980 barley plants. Fisons Report METAB/80/9. (Unpublished)
FBC Bendiocarb, submission to the Joint Meeting of the FAO
1982a Panel of Experts on Pesticide Residues in Food and the
Environment and the WHO Expert Group on Pesticide Residues,
FBC Limited, Registration Department, Saffron Walden, Essex,
England 1982.
1982b Harvest residues of bendiocarb (NC 6897) in sugarbeet after
application of a granular (3G) formulations. (Unpublished)
1982c Residues in grass following the use of bendiocarb (50SC)
(CR 15 340) as a seed dressing or spray dressing or spray
treatment in the U.K., autumn 1979. (Unpublished)
Housden, M.C. and Reary, J.B. Bendiocarb residues in rice treated
1981 with a 50WP formulation in the Philippines during the 1980
dry season. FBC Report RESID/81/16. (Unpublished)
Lewis, J.A. and Adcock, J.W. Investigation of residue levels in
1978a maize plants treated with 14C-bendiocarb in France. Fisons
Report RESID/78/50. (Unpublished)
1978b Investigation of residue levels in maize treated with
14C-bendiocarb granules in the U.K. Fisons Report
METAB/78/19. (Unpublished)
1978c Investigation of residue levels in maize plants treated with
14C-bendiocarb granules. Fisons Report METAB/78/7.
(Unpublished)
Lewis, J.A. and Challis, I.R. The metabolism of 14C-bendiocarb in
1978 the hen. Fisons Report METAB/78/28. (Unpublished)
1979 The metabolism of 14C-bendiocarb in paddy rice. Fisons
Report METAB/79/1. (Unpublished)
Netherlands. Information on pesticides included in the JMPR priority
1982 list provided by The Netherlands Government.
Reary, J.B. Harvest residues of bendiocarb in sugarbeet following
1974 its use as a seed dressing. Fisons Report RESID/74/36,
December 1974. (Unpublished)
1975a Soil percolation experiments with bendiocarb. Fisons Report
RESID/75/12. (Unpublished)
1975b Laboratory study of bendiocarb degradation in two standard
soils from West Germany. (Unpublished)
1975c Analytical method for residues of bendiocarb in sugarbeet.
FBC Report RESID/75/26. (Unpublished)
1975d Bendiocarb (NC 6897) residue decline on laboratory grown rye
grass sprayed with Ficam W. Fisons Report RESID/75/5.
(Unpublished)
1975e Harvest residues of bendiocarb (NC6897) in sugarbeet
following its use as a seed dressing in West Germany (1974).
Fisons Report RESID/75/12. (Unpublished)
1978a Residues in milk and tissues following a 28-day feeding
study with up to 25 ppm bendiocarb in the diet of dairy
cows. Fisons Report RESID/78/101.(Unpublished)
1978b Residues in eggs and tissues following a 21-day feeding
study with bendiocarb in laying hens. Fisons Report,
RESID/78/59. (Unpublished)
1978c Residues of bendiocarb in laboratory-extracted oil from
maize harvested after band application of a granular (10G)
formulation in the U.S., 1977. Fisons Report RESID/78/95.
(Unpublished)
1978d Analytical method for bendiocarb residues in soil. Fisons
Report RESID/78/37. (Unpublished)
1978e Analytical method for bendiocarb residues in grass. Fisons
Report RESID/78/61. (Unpublished)
1978f Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 76 WP formulation at
28-day intervals, in California and Massachusetts, 1977.
Fisons Report RESID/78/9. (Unpublished)
1978g Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 5G formulation at
28-day intervals in California and Massachusetts, 1977.
Fisons Report RESID/78/32. (Unpublished)
1978h Residues in maize grown in the U.K. from seed dressed with
bendiocarb 80W, 1977. Fisons Report RESID/78/86.
(Unpublished)
1978i Residues of bendiocarb in maize treated with a granular (3G)
formulation in the U.K., 1977. Fisons Report RESID/78/97.
(Unpublished)
1978j Residues in maize grown in France during 1975, 1976 and 1978
from seed dressed with bendiocarb 80W. Fisons Report
RESID/78/106. (Unpublished)
1978k Bendiocarb residue decline in soil after band treatment of
maize plots with a 10G formulation in Missouri, 1977. Fisons
Report RESID/78/50. (Unpublished)
1978l Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a 10G granular formulation
in Missouri, 1977. Fisons Report RESID/78/51. (Unpublished)
1978m Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a granular (10G)
formulation in Illinois, 1977. Fisons Report RESID/78/60.
(Unpublished)
1978n Residues of bendiocarb in the mature maize crop (grain, cobs
and stover) after band application of a granular (10G)
formulation in regions of the U.S., 1977. First Revision,
Fisons Report RESID/78/61/1. (Unpublished)
1979a Residues in milk from cows treated dermally with FICAM D in
Australia, 1979. Fisons Report RESID/79/49. (Unpublished)
1979b Analytical method for bendiocarb residues in barley, wheat,
oats and maize (corn) grain. Fisons Report RESID/79/17.
(Unpublished)
1979c Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. (Extract of
analytical method from the above report). Fisons Report
RESID/79/9. (Unpublished)
1979d Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/9. (Unpublished)
1979e Residues of bendiocarb in barley, wheat and oats grown from
treated seed in the U.K., 1977 and 1978. Fisons Report
RESID/79/3. (Unpublished)
1979f Residues of bendiocarb in silage maize treated with a
granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/12. (Unpublished)
1979g Residues in silage maize grown in the U.K. from seed dressed
with an 80W formulation of bendiocarb, 1978. Fisons Report
RESID/79/13. (Unpublished)
1979h Harvest residues of bendiocarb and NC 7312 in sugarbeet
after application of a granular formulation (3G) in the
U.K., 1978. Fisons Report RESID/79/14. (Unpublished)
1979i Residues of bendiocarb and NC 7312 in sugarbeet after
application of a granular (3G) formulation in France and
Italy, 1978. Fisons Report RESID/79/15. (Unpublished)
1979j Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1978. Fisons Report RESID/79/16.
(Unpublished)
1979k Residues of bendiocarb and NC 7312 in rice treated with a
granular (3G) or spray (50W or 80W) application in the
Philippines, 1978. Fisons Report RESID/79/23. (Unpublished)
1979l Residues of bendiocarb in maize (corn) grown in the U.S.,
from seed dressed with a 76WP formulation, 1977 and 1978.
Fisons Report RESID/79/25. (Unpublished)
1979m Residues of bendiocarb in maize (corn) after band
application of a granular (10G) formulation in Canada, 1978.
Fisons Report RESID/79/26. (Unpublished)
1979n Residue analysis of fodder beet following the use of
bendiocarb as a seed dressing in West Germany, 1978. Fisons
Report RESID/79/45. (Unpublished)
1979o Residues of bendiocarb in silage and mature (grain and
stover) maize after band application of a granular (10G)
formulation in the U.S., 1978. Fisons Report RESID/79/57.
(Unpublished)
1979p Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/76. (Unpublished)
1970q Residues of bendiocarb in sugarbeet following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/75. (Unpublished)
1980a Residue in barley, wheat and oats following seed treatment
with bendiocarb in the U.K., 1979 (80WP) batch 8A8, 50W
spray or 15 181 and 3G or 15 094. Fisons Report RESID/80/80.
(Unpublished)
1980b Bendiocarb residue decline on rangeland grass after ULV
treatment (by air) with a 25% formulation (15 15 458) in
Montana, U.S., 1980. Fisons Report RESID/80/95.
(Unpublished)
1980c Preliminary report on residues in milk and tissues from
dairy cows treated dermally with bendiocarb dust for 7 days.
Fisons Report RESID/79/55. (Unpublished)
1980d Residues of bendiocarb in grass grown from treated seed in
Australia, 1979. Fisons Report RESID/80/9. (Unpublished)
1980e Residues in grass following the use of bendiocarb as a
granular (3G), seed dressing (80W) or spray (50W) treatment
in the U.K., Spring 1979. Fisons Report RESID/80/11.
(Unpublished)
Reary, J.B. Residue decline in sugarbeet treated with bendiocarb 3G
1980f in the U.K., 1979. Fisons Report RESID/80/4. (Unpublished)
1980g Residue decline in soil after furrow application of
bendiocarb 3G granules at Shelford, U.K., 1979. Fisons
Report RESID/80/14. (Unpublished)
1980h Residue decline in maize treated with bendiocarb 3G in the
U.K., 1979. Fisons Report RESID/80/17. (Unpublished)
1980i Residue decline in maize grown from seed dressed with
bendiocarb in the U.K., 1979. Fisons Report RESID/80/18.
(Unpublished)
1980j Residues in sugarbeet from grower trials with a granular
(3G) formulation of bendiocarb in the U.K., 1979. Fisons
Report RESID/80/29. (Unpublished)
1980k Bendiocarb residue decline on rangeland grass after low
volume treatment with a 50SC formulation (CR 15 340) in
Montana, U.S., 1979. Fisons Report RESID/80/37.
(Unpublished)
1980l Residues in rice treated with bendiocarb formulations in the
Philippines and Korea 1979. Fisons Report RESID/80/46.
(Unpublished)
1981m Residues in grass following the use of bendiocarb as a seed
dressing, granular or spray treatment in the U.K., 1980. FBC
Report RESID/81/10. (Unpublished)
Reary, J.B. and Browne, P.M. Residues of bendiocarb (NC 6897) in
1978 mature maize, after application of a granular formulation in
France, 1976 and 1977. Fisons Report RESID/78/23.
(Unpublished)
Reary, J.B. and Whiteoak, R.J. Bendiocarb residue decline on soil,
1977a grass and thatch after treatment of turf with a 76WP
formulation in Mississippi, 1976. Fisons Report RESID/77/43.
(Unpublished)
1977b Bendiocarb residue decline in soil, grass and thatch after
treatment of turf with a 76WP formulation in Ohio, 1976.
Fisons Report RESID/77/52. (Unpublished)
1977c Bendiocarb residue decline on soil, grass and thatch after
treatment of turf with a granular formulation in Ohio, 1976.
Fisons Report RESID/77/61. (Unpublished)
1977d Bendiocarb residue decline on grass and soil after treatment
of turf with a 76WP formulation in California and
Massachusetts, 1977. Fisons Report, RESID/77/62.
(Unpublished)
Warner, P.A. The metabolism of 14C-bendiocarb in oilseed rape. Fisons
1980 Report METAB/80/20. (Unpublished)
 Molecular formula C11 H13 NO4
Other Information on Identity and Properties
Molecular weight 223.25
State white crystalline solid
Melting point 132°C
Vapour pressure 5.0 × 10-6mm Hg at 25°C
Solubility At 25°C 0.04 g/l in water:
0.35 g/l in hexane: 0.3 g/l in
kerosene: 10 g/l in
trichloroethylene and o-xylene:
40 g/l in benzene and ethanol:
200 g/l in acetone, chloroform,
dichloromethane and dioxane:
300 g/l in glycerol and dimethyl
sulphoxide: 640 g/l in dimethyl
formamide.
Stability In aqueous solution at 25°C the
half-life of bendiocarb is 48
days at pH5, 81 hours at pH7 and
45 minutes at pH9. At pH9
hydrolysis of bendiocarb results
in the formulation of the phenol,
NC 7312 whereas below pH5
bendiocarb slowly degrades to
pyrogallol and acetone.
Bendiocarb is stable at
temperatures of up to 100°C and
on non-absorptive surfaces and at
low humidity it resists
oxidation. In aqueous solutions
above pH6 hydrolysis rather than
photolysis is the principal
method of degradation but below
pH5 hydrolysis has little effect
and the photolysis half life is
51 hours.
Specification of technical material Current technical material has a
typical purity of about 98%, the
main impurities being 2,2-
dimethyl-1,3-benzoxdiol-4-ol(NC
7312), and to a lesser extent,
pyrogallol tris (methyl
carbamate).
Physical and Chemical Data on NC 7312, a metabolite of Bendiocarb
Chemical Names
2,2-dimethyl-1,3-benzoxodiol-4-ol
Synonyms
2,2-dimethyl-4-hydroxy-1,3-benzodioxole
2,3-isopropylidene dioxyphenol
Structural formula
Molecular formula C11 H13 NO4
Other Information on Identity and Properties
Molecular weight 223.25
State white crystalline solid
Melting point 132°C
Vapour pressure 5.0 × 10-6mm Hg at 25°C
Solubility At 25°C 0.04 g/l in water:
0.35 g/l in hexane: 0.3 g/l in
kerosene: 10 g/l in
trichloroethylene and o-xylene:
40 g/l in benzene and ethanol:
200 g/l in acetone, chloroform,
dichloromethane and dioxane:
300 g/l in glycerol and dimethyl
sulphoxide: 640 g/l in dimethyl
formamide.
Stability In aqueous solution at 25°C the
half-life of bendiocarb is 48
days at pH5, 81 hours at pH7 and
45 minutes at pH9. At pH9
hydrolysis of bendiocarb results
in the formulation of the phenol,
NC 7312 whereas below pH5
bendiocarb slowly degrades to
pyrogallol and acetone.
Bendiocarb is stable at
temperatures of up to 100°C and
on non-absorptive surfaces and at
low humidity it resists
oxidation. In aqueous solutions
above pH6 hydrolysis rather than
photolysis is the principal
method of degradation but below
pH5 hydrolysis has little effect
and the photolysis half life is
51 hours.
Specification of technical material Current technical material has a
typical purity of about 98%, the
main impurities being 2,2-
dimethyl-1,3-benzoxdiol-4-ol(NC
7312), and to a lesser extent,
pyrogallol tris (methyl
carbamate).
Physical and Chemical Data on NC 7312, a metabolite of Bendiocarb
Chemical Names
2,2-dimethyl-1,3-benzoxodiol-4-ol
Synonyms
2,2-dimethyl-4-hydroxy-1,3-benzodioxole
2,3-isopropylidene dioxyphenol
Structural formula
 Molecular formula
C9H10O3
Other information on identity and properties
Molecular weight 166
State White crystalline solid
Melting point 91-93°C
Solubility At 25°C 20 g/l in water and
50 g/l in dichloromethane; very
soluble in methanol, ethanol and
acetone.
Vapour pressure 2.34 × 10-3mm Hg at 25°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
In groups of male mice (CFLP strain) acutely intubated with
14C-bendiocarb (14C-bendiocarb with label at the heteroclyclic ring
was used in this and all of the other available metabolism studies on
bendiocarb) at 1, 5 or 10 mg/kg body weight, over 80% of the
administered dose was excreted in 24 hours, primarily in the urine.
Less than 10% of the administered 14C was recovered in the faeces.
The proportion of metabolites formed in the urine, but not the rate
and route of elimination, was dose-dependent. Thus, the level of NC
7312 (2,2-dimethyl-1,3-benzoxodiol-4-ol) (as conjugates) recovered in
the urine was 36, 32, 17 (as % of administered radioactivity),
respectively, at 1, 5, 10 mg/kg. Only one urinary metabolite other
than conjugated NC 7312 was detected, which was believed by
the authors of the report to be probably a conjugate of
6-hydroxybendiocarb (Challis and Adcock 1977a).
Another similar mouse study with groups of males of the CD-1
strain treated orally with single doses of 14C-bendiocarb at 1, 5 or
10 mg/kg body weight indicated recovery of about 80% of the
administered radioactive dose in 24 hours, predominantly in the urine.
A significant dose-dependent relationship was not evident with respect
to rate and route of elimination or metabolic profile of bendiocarb.
About 33-56% of the administered 14C-doses was recovered in the urine
as conjugates of NC 7312. Conjugated 6-hydroxy bendiocarb was believed
by the authors of the study to be the only other major urinary
metabolite (Pearce et al 1977).
Rat
Male and female rats treated with a single oral dose of
14C-bendiocarb at approximately 10 mg/kg body weight eliminated over
95% of the administered radioactivity in urine (89%), expired air (6%)
and faeces (2%) within 2 days of dosing. There was no significant sex
different in rate and route of elimination. Following an acute oral
dose of 14C-bendiocarb at 1 mg/kg body weight, plasma levels in male
and female rats peaked at 10 minutes post treatment with plasma half-
life being calculated at 4.4 hours for both sexes. The major urinary
metabolite was the phenol (NC 7312) which, excreted as ß-glucuronide
and sulphate conjugates, accounted for more than 85% of the
administered dose recovered in the urine during the first 24 hours
after treatment. The remaining 15% eliminated over this time period
comprised a mixture of sulphate and ß-glucuronide conjugates of at
least seven minor metabolites. One of the latter appeared to be
N-hydroxy methyl bendiocarb. No unchanged parent compound was found in
the urine. In the faeces free NC 7312 was the primary metabolite with
a small amount of 14C-bendiocarb being detectable (Adcock and Challis
1976a).
Conjugated NC 7312 was found to be the primary metabolite in
urine collected during 0-24 hours after treatment withdrawal in male
and female rats (CFY strain) fed 20 ppm of 14C-bendiocarb in the diet
for 10 days. At sacrifice 6 days after termination of dietary feeding,
detectable 14C residues were found in the tissues analysed (fat,
liver, kidney, muscle and brain) with the fat tissue having the
highest residue level. According to the partition characteristics of
the 14C residue in fat into organic solvents, radioactive residue in
fat was probably not mainly due to bendiocarb or NC 7312 (Pearce and
Adcock 1978).
Hamster
Groups of 6 or 10 male hamsters were intubated with
14C-bendiocarb in glycerol formal at 1, 5 or 10 mg/kg. Four female
hamsters were similarly treated at 10 mg/kg body weight. Over 90% of
the administered 14C-dose was recovered in 24 hours in the faeces and
the urine, with the latter being the primary route of elimination. The
rate and route of excretion was not modified by sex or dosage level.
The proportion of total radioactivity recovered in the urine as
conjugates of NC 7312 was about 37% at 1 mg/kg, 16% at 5 mg/kg and 19%
at 10 mg/kg. Besides conjugated NC 7312, urine of male hamsters
contained two metabolites in approximately equal amounts, while that
of female hamsters had only one radioactive component. A conjugate of
6-hydroxy bendiocarb was believed by the authors to be the major
urinary metabolite in both males and females. The other urinary
metabolite in male hamsters had not been identified. The seeming sex
difference in metabolic fate of bendiocarb remains to be ascertained,
since the male and female hamsters used in the study were housed and
dosed at different laboratories (Challis and Adcock 1977b).
Rabbit
Groups of pregnant rabbits were intubated on day 20 of gestation
with one dose of 2.5 or 10 mg/kg body weight of 14C bendiocarb.
Within 24 hours of dosing, nearly 90% of the administered dose had
been eliminated, primarily in the urine, with only a small amount
(<2.5%) being detected in the faeces. The rate and route of
elimination and metabolic profile of bendiocarb appeared to be
independent of dosage. The major urinary metabolite was identified as
NC 7312, which accounted for 90% of the radioactivity extracted from
urine samples following enzyme (sulphatase and ß-glucuronidase)
hydrolysis (Challis 1980).
Dog
Groups of one male and one female beagles (fasted overnight and
for 8 hours following dosing) were treated once with 14C-bendiocarb
in gelatin capsules at 0.1, 1.0 or 10 mg/kg body weight. Regardless of
dosage level, the radioactive dose was almost completely excreted
during the first 24 hours post-dosing, primarily in the urine. The
amount of radioactivity found in the urine of both sexes over the
48-hour period seemingly decreased with dosage (about 90% at 0.1 mg/kg
body weight, 80% at 1.0 mg/kg body weight and 70% at 10 mg/kg body
weight). This appeared to be accompanied by a corresponding increase
in faecal recovery (about 19%, 21% and 24%, respectively, at 0.1, 1.0
and 10 mg/kg body weight). In the urine, approximately 84%, 67% and
43% of the administered radioactivity, respectively, at 0.1, 1.0 and
10 mg/kg body weight occurred as sulphate and ß-glucuronide conjugates
of NC 7312 with no recovery of any 14C-bendiocarb. Analysis of urine
samples from male dogs at 10 mg/kg body weight indicated the probable
presence of ring hydroxylated derivatives of bendiocarb as minor
metabolites. Unchanged 14C-bendiocarb was the only component
identified in the faeces and amounted to about 80% of the
radioactivity recovered via this route (Warner et al 1977).
Effects on Enzymes and Other Biochemical Parameters
Mouse
In male and female mice (Charles River CD-1 strain) fed technical
bendiocarb containing 0.09% w/w of dimethoate) in their diets at 0 or
1 250 ppm for 19 days, whole blood cholinesterase in blood sampled
between 06:00 hour and 08:00 hour, but not between 09:00 hour and 11
hours (peak feeding period stated to occur between 22:00 hour and
09:00 hour) was depressed (>20%) only in treated males in weeks 2 and
3 (Hounsell and Rush 1980).
Male CFLP mice and male CD 1 mice were fed dietary levels of
technical bendiocarb at 0, 500 or 1 000 ppm for up to 7 days. In the
CFLP strain, whole blood cholinesterase activity was reduced (>20%)
at 1 000 ppm in animals fed the diet ad libitum and in those
following a 1-hour feeding period on the 7th day after an overnight
fast. In the CD-1 mice, whole blood cholinesterase was depressed
(>20%) in the 500 and 1 000 ppm groups following an overnight fast
and exposure to the diet for 1 hour on day 7. Ad libitum feeding
resulted in decreased whole blood cholinesterase in the 500 but not in
the 1 000 ppm groups (Hounsell 1981a).
Rat
Groups of rats (sex unspecified) were treated orally with
4 mg/kg/day of technical bendiocarb in glycerol formal for three
consecutive days. Inhibition (65-85%) of cholinesterase in whole blood
and plasma peaked at 10 minutes after each dose on days 1 and 3.
Recovery of whole blood and plasma cholinesterase began to occur 30
minutes following each dose, and was practically complete three days
after treatment withdrawal. Whole blood cholinesterase appeared to be
more sensitive than plasma cholinesterase to the inhibitory effect of
bendiocarb (study cited by Kemp and Hounsell 1974).
Male rats (CFY Sprague-Dawley) were fed dietary levels of
technical bendiocarb up to 10 ppm for 14 days, followed by an
overnight fast. Whole blood cholinesterase, assayed in the morning of
the 15th day after a 1-hour feeding period, was not significantly
inhibited (<20%) (Hounsell 1981b). Another similar study with the
same strain and sex of rats fed diets containing technical bendiocarb
for up to 23 days indicated that, following overnight fasting and a
one-hour feeding period, animals exhibited a depression (20%) of whole
blood cholinesterase at 50 ppm on days 15 or 16 and 22 or 23 (Hounsell
1981c).
Dog
In 4 male beagles (fasted the day before the test) fed a diet
containing 1 000 ppm of technical bendiocarb for up to one hour
(actual intake of the test material estimated at 6 - 8.6 mg/kg body
weight), inhibition (>50%) of brain and whole blood cholinesterase
was apparent 15 minutes after the feeding period. Whereas depression
of whole blood cholinesterase peaked at 2 hours and was completely
reversible at 3 hours following the feeding period, the extent of
inhibition of brain cholinesterase remained fairly constant between
15 minutes and 3 hours (the last sampling interval) after the dosing
period (Hounsell 1974). Another experiment with 2 female beagles
(24-hour fasted) fed a dietary level of 10 ppm of technical bendiocarb
for about 10 minutes (actual intake of the test material estimated at
approximately 32.4 mg/kg) showed reduction of whole blood
cholinesterase activity of about 27% and 52% of the pretreatment level
at 1 hour and 6 hours, respectively, post dosing. Cholinegesic
symptoms were noted within 24 minutes of the initiation of feeding.
Complete recovery of whole blood cholinesterase activity and from
toxic signs occurred 24 to 25 hours after treatment (Kemp and Hounsell
1974).
Cow
A lactating dairy cow was fed technical bendiocarb in the daily
diet at 3.6 ppm for two consecutive days (ration was offered twice
daily). No reduction (<20%) in whole blood cholinesterase was
observed at any of the 8-hourly intervals following the morning
feeding on both days (Roberts 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 17 to 21 mated rats (Sprague-Dawley CFY strain) were
fed diets containing technical bendiocarb at 0, 200, 400 or 800 ppm
from 14 days post-coitum until weaning of their pups. A decrease in
maternal body weight gain was noted at 800 ppm on days 17 and 20 post-
coitum and practically throughout the 21-day lactation period. At this
dietary level, survival and weight gain of pups were depressed
throughout lactation. No treatment-related effects were observed on
other parameters monitored, i.e. mortality and clinical signs of
pregnant dams, sex ratio and gross abnormalities of offspring (Jackson
1978).
Groups of 10 males and 20 female rats (SPF, CFY strains) were fed
dietary concentrations of bendiocarb (presumably technical grade) at
0, 200, 400 or 800 ppm for one week prior to mating. The females were
maintained on test diets through day 18 of gestation and the males
until termination. There were no mortalities or toxic symptoms. Growth
was slightly depressed in males at 800 ppm and in females at 400 ppm
and above. Pregnancy rate, duration of gestation period and terminal
gross pathology were not adversely affected. Medium precoital time (as
an indication of mating performance) of dams was lengthened at both
400 and 800 ppm. In the progeny generation, except for a slight
decrease at 400 ppm and above in mean pup weight at birth, no apparent
dose- or treatment-related effects were evident on litter size at
birth, survival and weight of pups through lactation period to
weaning, sex ratio and incidence of gross abnormalities of weanlings
(Palmer and Allen 1978).
Groups of 30 males and 30 females (Charles River CD strain) were
fed dietary levels of technical bendiocarb at 0, 10, 50 or 250 ppm for
90 days prior to mating to initiate a reproduction study covering
three generations with two litters per generation. Weanlings from the
second litters were selected to become parents of the next generation.
After the second mating trial in each generation, the dams in each
group were divided into two approximately equal sub-groups. One
sub-group was allowed to deliver and rear their young until 25 days
post-partum. Another sub-group was sacrificed on day 21 of gestation
for examination of uterine contents. Foetuses were examined for
external, skeletal and soft tissue malformations.
In the parental generations, no mortality or clinical signs
attributable to the compound were evident. Dams displayed slight
growth depression during the gestation period of F1a litters at
50 ppm and above and of F2b litters at 250 ppm. Food consumption was
normal, but water intake was slightly decreased at 250 ppm in F1
females. Males of the F2 generation seemed to exhibit an increase in
incidence and severity of "geriatric nephropathy" at and above 50 ppm.
Ophthalmoscopic examination conducted on F2 adults between 31 and 35
weeks of age revealed no treatment-related findings. At terminal
sacrifice of parental animals after weaning of the second litters,
some variations from controls in absolute weights and/or organ/body
weight ratios of certain tissues such as adrenal, pituitary, thyroid,
seminal vesicles and uterus were noted at both 50 and 250 ppm, but
these observations were not consistently present through the
generations and were not accompanied by histopathological lesions of
the organs. Mating and fertility indices as well as length of
gestation period were not modified by bendiocarb. There was an
increase in frequency of F0 females being acyclic or pseudopregnant
at 250 ppm and of F2 females with irregular oestrous cycles at 50 ppm
and above, prior to the first mating trial in both cases. Pre-coital
interval was lengthened at 250 ppm in the F0 generation (first
mating).
Over the three progeny generations, the viability index was
slightly depressed in F1a litters at 250 ppm from days 10 through 25
and in F2b litters at 50 ppm and above on day 25. Pup weight was
reduced at 250 ppm in F1a and F1b litters on day 25 and in F2b
litters at birth through day 25. In F2a litters, pup weight on day 25
was decreased in all treated groups, but the difference was indicated
by the authors of the report to be statistically significant only at
10 ppm, but not above. There were no significant differences between
control and treated groups in litter size (at birth and on day 1), sex
ratio, ophthalmoscopic observations of F3b pups between 19 and 31
days of age, organ weight and histopathological findings of 25-day old
F3b pups. Examination of 25-day old pups from the second litters of
each generation for rate of physical development and sensory functions
revealed "the onset of pinna unfolding, hair growth, tooth eruption
and eye opening was marginally delayed in all treated (F1b) litters,
but the overall pattern of physical development was essentially
similar in all groups, and "the onset of pinna unfolding and hair
growth was marginally delayed (in F2b litters at both 50 and 250 ppm)
but eye opening and tooth eruption was unaffected".
Teratological investigation of F1b, F2b and F3b litters showed
no significant differences between control and treated groups with
respect to number of implantations, number of viable young, early or
late resorptions, post-implantation loss and foetal weight. A rise in
pre-implantation loss in F1b litters was noted at 250 ppm. Also at
the top dosage level, there were higher incidences of generalized
subcutaneous oedema, incomplete ossification of cranial bone,
uni/bilateral hydronephrosis and bilateral ureter in F1b litters and
of subcutaneous scapular haemorrhage in F3b litters, as compared to
concurrent controls. Based on the data, a dietary level of 10 ppm was
without significant adverse effect on the reproductive parameters
evaluated. The teratogenic "no effect" level was demonstrated to be
50 ppm (Tesh et al 1981).
Special Studies on Teratogenicity
(also see under "Special Studies on Reproduction")
Rat
Groups of 24 mated rats (Sprague-Dawley derived, CD strain) were
intubated with technical bendiocarb as a suspension in gum tragacanth
at 0, 0.25, 1 or 4 mg/kg body weight/day between gestation day 6 and
day 15 (day 0 = day of positive vaginal smear). The dams were
sacrificed on gestation day 21 and foetuses were removed by caesarean
section for external, visceral and skeletal examination. There was no
mortality. Dams at 4 mg/kg body weight/day exhibited toxic symptoms
characteristic of cholinesterase inhibition and slight growth
inhibition on gestation day 21. Two dams (out of 21) at 4 mg/kg body
weight/day and 1/22 dams at 1 mg/kg body weight/day displayed a large
number of late uterine deaths. Mean litter size was depressed at
4 mg/kg body weight/day. There were no significant differences between
control and treated groups in pregnancy rate, number of corpora lutea
or implantation sites, number of early resorptions, foetal weight and
frequency of foetal abnormalities. The study demonstrated bendiocarb
to be non-teratogenic under the conditions of the experiment (Tucker
1974).
Rabbit
Groups of 27-29 female rabbits (New Zealand White) that had been
artificially inseminated were intubated with technical bendiocarb as a
suspension in aqueous gum tragacanth at 0, 1, 2.5 or 5 mg/kg body
weight/day from day 6 to day 28 inclusive of gestation. (Day 0 = day
of insemination.) The does were sacrificed on day 29 of gestation and
uterine contents were examined. Foetuses were subjected to evaluation
for external, skeletal and internal malformations. Six to eight
females per control or treated group died 6 to 29 days after
insemination mainly due to "tracheal intubation". Clinical signs such
as increased respiratory rate and prostration were noted at and above
2.5 mg/kg body weight/day. Growth was slightly depressed at 5 mg/kg
body weight/day between gestation days 16 and 28. Whole blood
cholinesterase assayed 30 minutes post-dosing on day 28 was depressed
(31-70%), in a dose-dependent manner, in all treated groups. One doe
each at 2.5 and 5 mg/kg body weight/day aborted on day 22 and 20,
respectively. Premature parturition occurred in one doe each of 2.5
and 5 mg/kg body weight/day groups on day 28. An increase was observed
in the mean number of late resorptions per litter and the incidence of
pregnant does with late resorptions at both 2.5 and 5 mg/kg body
weight/day. Post-implantation loss (number of implantations - number
of viable foetuses/number of implantations × 100) was elevated at
2.5 mg/kg body weight/day and above.
Other parameters monitored, including number of corpora lutea,
implantations, viable young, or early resorptions, pre-implantation
loss, foetal weight and placental weight, were not adversely affected
by treatment. There was a dose-related increase in incidence of
foetuses with eye anomalies (mainly "cloudy areas in eyes") and with
absence of pubic bones at both 2.5 and 5 mg/kg body weight/day.
Incidence of litters containing foetuses showing such abnormalities
was also elevated at and above 2.5 mg/kg body weight/day. In the
absence of background data on frequency of the abnormalities in
question, 1 mg/kg body weight/day may be considered as a clearcut "no
effect" level with respect to teratogenicity (Tesh et al 1980).
Special Studies on Mutagenicity
Two separate experiments were conducted with technical bendiocarb
and NC 7312 to evaluate their genetic activity in vitro microbial
systems (plate assays), with or without the addition of a mammalian
metabolic activation preparation (S-9 mix from the liver of male rats
pre-treated with Aroclor 1254 i.p. at 500 mg/kg/day for five days).
Indicator organisms used were Salmonella typhimurium strains TA
1535, TA 100, TA 1537, TA 1538 and TA 98. Results suggest
non-mutagenicity of the test compounds to any of the tested strains at
the (non-toxic) concentrations used (3.3-1 000 µg/plate of bendiocarb
and 10-3 300 µg/plate of NC 7312) in the presence or absence of
metabolic activation and under the conditions of the assays
(McConville and McGregor 1979; McConville and Harris 1979). Negative
results were also obtained in another reversion mutation test using
Escherichia coli WP2 and five strains of the Salmonella
typhimurium TA series as indicator organisms in the presence of
absence of a liver metabolic activation system at concentrations of
technical bendiocarb ranging from 5 to 1 000 µg/plate (Moriya
et al 1981).
A rec-assay conducted with Bacillus subtilis H17 (rec+) and
M45 (rec-) in the absence of a metabolic activation preparation
indicated that technical bendiocarb, at concentrations of 20 to
5 000 µg/disc, did not induce any inhibitory zone in either tested
strain (Moriya et al 1981).
Technical bendiocarb was evaluated for its mutagenic activity in
a mouse lymphoma L5178Y specific locus mutation assay. Under the
conditions of the test, there appeared to be no consistent indications
of mutagenicity of the compound in the absence or presence of a
metabolic activation system (S-9 homogenate of liver from male adult
Fischer rats induced with Aroclor 1254) at concentrations ranging from
5 to 20 µg/ml (McGregor and Ross 1981).
In a micronucleus test, groups of male mice were treated with
technical bendiocarb i.p. at 0, 0.625, 1.25 or 2.5 mg/kg body
weight/day for two consecutive days (the two doses were given 24 hours
apart). The animals were sacrificed 6 hours after the second dose and
the femurs were removed for the preparation of bone marrow smears.
There was no significant increase in the frequency of polychromatic
erythrocytes containing micronuclei or change in polychromatic/
normochromatic ratio in any treated group (Hounsell and Walker 1982).
Groups of 20 sexually mature virgin male rats (Sprague-Dawley CD
strain) were fed diets containing technical bendiocarb at 0, 10, 50 or
250 ppm for 13 consecutive weeks and then mated with sexually mature
virgin female rats for seven days in a dominant lethal assay. (Each
group was sub-divided into sub-groups A and B with commencement of
dietary feeding of the groups being staggered by one week.) The
females were sacrificed 14 ± 1 days after mating and autopsied for the
examination of uterine contents. The treated males exhibited no
clinical signs and their body weight and food consumption were not
adversely affected by treatment. The incidence of non-fertile males
were 1/20 at 50 ppm and 2/20 each at 10 ppm and 250 ppm. With the
exception of one infertile male at 250 ppm, abnormally small testes
and epididymis were found in the other four infertile males. An
increase (not dose-related) in incidence of litters with at least one
early death was observed (in sub-group B only) at 10 ppm and 250 ppm,
but this was not accompanied by a rise in the number of early deaths
per litter. There were no significant differences between control and
treated groups with respect to pregnancy rate, number of corpora
lutea, number of total implants, number of viable implants and pre-
implantation loss. Bendiocarb did not appear to induce dominant lethal
mutation under the experimental conditions used (Kemp and Jackson
1977).
Special Studies for Carcinogenicity
Mouse
Groups of 50 males and 50 females (HaM/ICR Swiss mice CD-1; about
35 days old) were fed diets containing technical bendiocarb, found to
contain up to 0.5% dimethoate or dimethoate decomposition products, at
50, 250 or 1 250 ppm. The control group consisted of 100 males and 100
females. The experiment was originally scheduled to be terminated
at a predetermined survival level or 104 weeks at the latest. The
predetermined level was reached after 89 weeks in males and 94 weeks
in females. Mortality rate was increased in females at 1 250 ppm after
85 weeks. Less than 40% of animals in control and treated groups were
still alive at termination, but over 50% of animals of all groups,
including the control, survived at least 82 weeks. All animals that
died or were sacrificed in moribund condition during the study and
those sacrificed terminally were necropsied. A wide range of tissues
and any unusual lesions from animals of all groups, including the
control, found dead or sacrificed moribund and from all terminal
survivors of control and top dosage groups were examined
microscopically. Any unusual lesions from terminal survivors at both
50 and 250 ppm were also subject to histopathological evaluation.
Clinical signs, body weight, food and water consumption were not
significantly different from those in the controls. Ophthalmoscopic
examination conducted at four intervals during the study seemed to
suggest a probable increase at week 89 in incidence of cataract
("posterior cortical or more advanced") in males at 1 250 ppm. At
terminal sacrifice, males at 1 250 ppm showed an increase in absolute
testes weight and testes/body weight ratio and females of all treated
groups displayed a dose-related increase in absolute weight and
organ/body weight ratio of kidney. There were no treatment-related
microscopic changes in the tissues evaluated, including testes and
kidney.
Analysis of tumour data appeared to indicate no significant
difference between control and top dosage groups in the incidence of
any particular type of tumour. Under the conditions of the study,
bendiocarb was not carcinogenic to the mouse (Serota et al 1981).
Rat
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Seven adult hens (15 months old) were given a single subcutaneous
dose of bendiocarb ("laboratory grade") at 60 mg/kg body weight (the
subcutaneous LD50 of bendiocarb in hens was indicated to be about
80 mg/kg body weight). A second dose was given 3 weeks later. The
control group consisted of seven untreated hens. The birds were
observed for a total of 6 weeks from the initial dose before sacrifice
for gross pathology and histopathological examination of the
spinal cord and sciatic nerve. There were no clinical signs or
histopathological evidence suggestive of delayed neurotoxicity of the
compound in any of the treated hens (Sanderson and Hounsell 1969).
Groups of domestic hens, approximately 14-months old (20 birds in
the top dosage group and 10 birds in each other group) were acutely
intubated with technical bendiocarb in maize oil at 0, 189, 378 or
757 mg/kg body weight and observed for 21 days prior to sacrifice for
histopathological examination of the brain, spinal cord and distal
sciatic nerve. The hens were given 10 mg/kg body weight of atropine
intramuscularly immediately before the dose of bendiocarb. (Before
initiation of the neurotoxicity study, the oral LD50 of bendiocarb
was found to be 137.3 mg/kg body weight in hens not pretreated
intramuscularly with 10 mg/kg body weight of atropine and 757 mg/kg
body weight in atropine-protected hens.) One hen at 378 mg/kg body
weight and a total of 8 hens at 757 mg/kg body weight died. Except for
"doubtful signs" of ataxia noted in 1/10 hens at 189 mg/kg body weight
on days 9 and 10 only, there were no delayed neurotoxic signs in any
other treated birds during the 21-day exposure observation period.
None of the bendiocarb-treated survivors or negative controls
exhibited microscopic evidence of neurological lesions. Positive
controls, dosed orally with TOCP at 500 mg/kg body weight, displayed
typical neurotoxic signs and significant morphological changes of the
nervous tissues (Ross et al 1978).
Special Studies on Skin and Eye Irritation
Technical bendiocarb was shown to be a mild irritant when applied
to the skin of New Zealand White rabbits (Cuthbert 1978).
An eye irritant study in New Zealand White rabbits indicated
technical bendiocarb to be minimally irritating to the eye (Cuthbert
1980).
Special Studies on the Acute and Subacute Oral Toxicity of NC 7312
Rat
The oral LD50 of NC 7312 (2,2-dimethyl-1,3-benzoxodiol-4-01) a
metabolite of bendiocarb) in male and female rats (CFY strain) was
greater than 4 640 mg/kg body weight. Cholinergic symptoms were
observed at dosages above 464 mg/kg body weight (Mallyon and Sanderson
1977).
Groups of rats (CFY strain, 10 male and 10 female controls, 5
males and 5 females per treated group) were intubated with NC 7312 as
a suspension in aqueous gum tragacanth at 0, 5, 20, 80, 320 or
1 280 mg/kg body weight/day for 16 days. There were neither mortality
nor compound-related effects on clinical signs, haematological, blood
chemistry and urinalysis parameters. Growth, food conversion factor
and water intake were slightly decreased in females at 1 280 mg/kg
body weight on week 2. Terminal survivors sacrificed showed no gross
or histopathological changes associated with treatment. Absolute
weight and organ/body weight ratio of ovary were reduced at 80 mg/kg
body weight and above, but concomitant microscopic changes of the
tissue were not evident (Kemp and Brooks 1978).
Acute Toxicity
The acute toxicity of several animal species to bendiocarb is
summarized in Table 1.
Toxic signs were characteristic of anticholinesterase poisoning
and seen within a few minutes after oral, intraperitoneal or
inhalation exposure in all species of mammals tested. Deaths usually
occurred after 5 minutes to 2 hours and survivors began to recover
after 1/2 to 2 hours. Within 24 hours of dosing, survivors recovered
completely from clinical signs. After dermal exposure, toxic signs
began to appear after 2 to 21 hours with recovery being much slower
than that following acute oral doses (Sanderson 1921).
Short-Term Studies
Rat - dietary
In a 28-day study with groups of five female rats (Sprague-Dawley
CFY strain) fed diets containing 0, 400, 600 or 800 ppm of technical
bendiocarb, mortality, body weight, food consumption, oestrous cycle
(as evidenced by vaginal cytology taken from the fourth day to
termination) and terminal gross pathology were not affected by
treatment. Whole blood cholinesterase was inhibited (52-62%) at all
test levels and toxic symptoms were observed at both 600 and 800 ppm
on day 28 after the animals (fasted overnight) were given access to an
appropriate test diet for a restricted one-hour feeding period
(Jackson 1977).
Table 1. Acute Toxicity of Bendiocarb in Animals
LD50
Species Sex Route Vehicle (mg/kg body Reference
weight)
Mouse F oral glycerol 45
(CFW) formal Sanderson 1971
Rat M oral glycerol 40-64 Sanderson 1970;
(Wistar) formal Sanderson 1971,
F 34-40 Sanderson 1971
Rat M oral aqueous 108-156 Crome and Sanderson
(Charles River gum 1980
COBS CD tragacanth
Sprague-Dawley)
Rat M&F i.p. glycerol 8 Sanderson 1971
(Wistar) formal
Rat F dermal glycerol 566-800 "
(Wistar) (24-hour formal Ben-Dyke 1932
M exposure) 566
Rat (Carworth M inhalation1/ None 250 mg Sanderson and
Sprague-Dawley) (6-hour a.i./l air2/ Mallyon 1979
exposure)
Hamster F oral water 141 "
(Syrian)
Guinea pig F oral glycerol 35 Sanderson 1971
formal
Rabbit M oral glycerol 40 "
F formal 35
1/ Animals were exposed to an 80% wettable powder as a dust in a "nose only"
exposure chamber with 58% particles of size 10 microns and 18% particles
5 microns.
2/ 6-hour LD50 in terms of actual chamber concentration of the active ingredient.
Groups of 10 male and 10 female rats (Sprague-Dawley CFY strain)
were fed dietary levels of technical bendiocarb at 0, 2, 10, 50 or
250 ppm for 13 weeks. Mortality, clinical signs, body weight, food and
water consumption, ophthalmoscopic findings, clinical chemistry,
haematological and urinalysis parameters were not influenced by the
presence of the compound in the diet. Whole blood cholinesterase,
determined in animals (fasted 16 hours) shortly after a 1-hour feeding
period, was depressed (20%) at 250 ppm in both sexes during weeks 4
and 9, at both 50 and 250 ppm in males and at 250 ppm in females
during week 13. Terminal gross pathological changes and organ weights
were not significantly different from those in the controls.
Histopathological evaluation of a variety of tissues from animals of
the control and top dosage groups suggested the only notable finding
to be an increase in incidence of males at 250 ppm showing vacuolated
centrilobular hepatocytes. This liver lesion, not noted even at
200 ppm in the long-term rat study (see under Long-Term Studies), was
considered unlikely to occur in the lower dosage groups of the study,
although there were no data to confirm this supposition. It would
appear that the no-effect level was 10 ppm on the basis of
cholinesterase levels and was 50 ppm on the basis of the other tested
parameters (Hunter et al 1978).
Hamster
Groups of hamsters (20 male and 20 female controls, 10 males and
10 females per treated group) were fed technical bendiocarb in their
diet at 0, 4, 20, 100 or 500 ppm for 30 days. There were no
indications of any treatment-related effect at levels up to 500 ppm as
judged by mortality, clinical signs, body weight, food and water
intake, terminal haematology, organ weight and histopathology of
selected tissues from control and 500 ppm groups (Hounsell and Brooks
1981).
Dog
Groups of four male and four female beagles were fed dietary
levels of technical bendiocarb at 0, 20, 100 or 500 ppm for 16 weeks
with the top dosage level being raised to 1 000 ppm on day 89 (test
diets were offered to the dogs for a 1-hour period morning and
evening). Inclusion of the compound in the diet did not adversely
affect survival, clinical signs, food consumption, terminal
haematology, blood chemistry and urinalyses values. Males of the
highest dosage group exhibited actual weight loss at termination.
Assays1/ at weeks 5, 9, 14, 15 and 16 of cholinesterase in whole
1/ Whole blood was diluted 1:1 200 in the assay. No data were
available on the amount of diet actually consumed by individual
animals during the 1-hour feeding period prior to sampling for
assay of cholinesterase in blood and brain.
blood and plasma of animals (fasted overnight) following a 1-hour
feeding period indicated depression (>20%) at the top dosage level of
cholinesterase in plasma and whole blood at most sampling periods.
Whole blood cholinesterase was decreased (>20%) even at 100 ppm in
males at week 9 and in females at weeks 9 and 15. Terminal brain
cholinesterase of the top dosage group (males and females) was also
inhibited (>20%). At the conclusion of the study, organ weights and
gross pathological changes were unaffected by the compound.
Microscopic examination of about 20 tissues from each animal suggested
no treatment-related changes other than the occurrence of thyroid
lesions (focal C-cell hyperplasia and focal atrophy) in 1/4 males and
1/4 females of the top dosage group but in none of the concurrent
controls or of animals in the lower dosage groups. The study suggested
the no-effect level with respect to cholinesterase and other criteria
evaluated to be 20 ppm and 100 ppm, respectively (Litton 1974).
Groups of 8 male and 8 female purebred beagles were fed a diet
containing technical bendiocarb at 0, 20, 100 or 500 ppm for 2 years
(control or treated diet was given to each animal every morning).
Survival, clinical symptoms and growth during the study as well as
haematology, urinalysis and ophthalmoscopic parameters evaluated at
five more intervals over the course of the experiment were not
significantly affected by the presence of the test material in the
diet. Less food was consumed by animals (both sexes) of all treated
groups throughout the study. Nevertheless, the authors of the report
stated that "statistical analysis of food residue for weeks 1-3, 1-26,
1-52, 53-104 and 1-104 did not reveal any statistically significant
difference between control and test group means". Based on weekly mean
food consumption and mean body weight data at the end of each week,
the dietary levels of 20, 100 and 500 ppm were equivalent to 0.7, 3.1
and 16.3 mg/kg body weight/day (males and females combined). Water
consumption was slightly but consistently increased in both sexes at
100 ppm and above during weeks 36-39 and at 500 ppm not infrequently
thereafter. Serum cholesterol was elevated at 500 ppm (both sexes) at
several sampling intervals. A dose-related decrease in serum calcium
level was noted at both 100 and 500 ppm in weeks 14 and 25.
Measurement of whole blood cholinesterase in animals (fasted
overnight) shortly after a 60-minute feeding period at eight sampling
intervals during the study showed a tendency to frequent depression
(>20%) of the enzyme at 500 ppm. Brain cholinesterase in animals
(fasted overnight) sacrificed following a 1-hour feeding period was
depressed (>20%) at 500 ppm after 52 and 104 weeks and probably also
at 100 ppm after 104 weeks. Organ weight analysis, gross pathological
examination and histopathological evaluation of over 30 tissues from
each animal sacrificed at the end of 52 weeks (3 males and 3
females/group) or terminally (all survivors per group) showed no
significant alterations related to treatment.
The dietary level of 20 ppm appeared to be a no-effect level on
the tested criteria. It was noteworthy that the actual amount of
bendiocarb ingested by animals during the 1-hour feeding period prior
to the assay of tissue cholinesterase varied markedly, even among
animals in the same dosage group, owing to substantial individual
variation in the quantity of diet (ranging from 0 to 400 g per animal)
consumed. Also, there were incidences where individual animals of a
high dosage group, e.g. 500 ppm, actually had a lower intake of
bendiocarb than those of a lower dosage group (100 ppm) (Chesterman
et al 1980).
Rat - dermal
Groups of 6 rats (Wistar strain) were exposed dermally to an 80%
wettable powder of bendiocarb as a 40% w/v aqueous suspension at 50,
100, 200, 400 or 800 mg a.i./kg body weight under an occlusive patch
for a period of 6 hours/day, 5 days/week for a total of 15
applications in 21 days. The treated site was washed at the end of
each exposure period. There was no mortality. Toxic signs,
characteristic of anticholinesterase poisoning and dose-related in
severity, were noted at 200 mg/kg body weight and above. Whole blood
cholinesterase, determined at the end of the 6-hour exposure period,
was inhibited by more than 30% at 200 mg/kg body weight and above
after only one dose and at 50 mg/kg body weight and above after 15
exposures. No gross pathological changes or microscopic evidence of
dermal irritation attributable to treatment were apparent at terminal
sacrifice (Sanderson 1972).
Long-Term Studies
Rat
Groups of male and female rats (CFY strain, 60 male and 120
female controls, 30 male and 60 female per treated group) were fed
dietary levels of technical bendiocarb at 0, 2, 20 or 200 ppm for 7
days and then caged together (1 male and 2 females). At the end of a
20-day mating period, the females were individually housed and allowed
to litter and rear their pups to weaning. This part of the study was
known as the reproductive phase. A sufficient number of weanlings from
the control and treated groups were randomly selected to initiate
another segment of the study referred to as the main phase. The latter
comprised the main group and the satellite group. In the main group,
weanlings (50 males and 50 females per treated group, 100 male and 100
female controls) were given bendiocarb-treated diets at 0, 2, 20 or
200 ppm for 104 weeks. For the satellite group, used primarily for
laboratory investigations, animals (30 males and 30 females per
control or treated group) were fed bendiocarb at the same dietary
levels, but only for 60 weeks. The 2 ppm level was raised to 10 ppm
after 2 weeks in the main phase, and this group will be known as the
2-->10 ppm group hereafter for the purpose of identification. Dietary
administration was continued throughout both the reproduction and main
phase. All animals died or sacrificed in extremis during the 104-week
study and those sacrificed terminally were necropsied. Animals of all
groups, including the control, dying or sacrificed in extremis during
the study and terminal survivors of the control and top dosage groups
were subjected to microscopic examination of a set of about 30
selected tissues plus grossly observed nodules and tissue masses.
Additionally, all suspected tumours from terminal survivors of lower
dosage groups were evaluated histopathologically.
For the reproductive phase, mortality and behaviour of parental
animals, pregnancy rate, duration of gestation period, litter size and
pup weight at birth, sex ratio and incidence of external abnormalities
of pups were not affected by bendiocarb. There were some indications
of adverse effects related to treatment at 200 ppm on maternal body
weight gain during gestation, litter size on lactation day 4 and pup
weight from lactation day 4 through weaning. A dietary level of 20 ppm
was a no-effect level on this phase of the study.
In the 104-week study (main group) of the main phase, mortality
rate, although unaffected by the compound, was high in all groups,
including the control, Only about 16-26% of males and 24-42% of
females survived 104 weeks. Nevertheless, over 55% of males and
females lived at least 82 weeks.
A number of findings associated with treatment were noted at
200 ppm. These included an increased incidence of males exhibiting
hyperactivity in response to external stimuli, growth depression
during the first 52 or 56 weeks, depressed water consumption during
week 6-12 but not in week 25. Periodic measurements of haematological,
biochemical and urinalysis values at several intervals over the course
of the study revealed significant changes in several parameters
(e.g. total WBC, neutrophil and lymphocyte counts, levels of serum
cholesterol and total protein) to be also confined only to the top
dosage group. There was a dose-related decrease in body weight gain
associated with a depression in food consumption in males of all
treated groups during the first two weeks of the study but not
thereafter. Ophthalmoscopic examination of the surviving animals in
the main group only for a total of seven intervals between 13 and 102
weeks indicated a statistically significant increase in incidence of
lenticular opacities in males at 200 ppm at all intervals from 52
weeks onward. A statistically significant trend (p <0.01) for
dose-dependent incidence of this eye lesion was indicated to be
apparent for males at both 20 and 200 ppm from 66 weeks onward and for
males of all treated groups at 80 weeks and thereafter. It was pointed
out that the difference in frequency of lenticular opacities was not
statistically significant between males of control and low dosage
(2-->10 ppm) groups or among female groups at any time interval. The
lenticular opacities observed reportedly were "essentially atypical of
chemically induced lenticular lesions in the rat". Overall,
2-->10 ppm might probably be considered a borderline no-effect level
with respect to the particular eye lesion.
Activity of whole blood cholinesterase determined in animals
(fasted overnight) following a one-hour feeding period at several
intervals during the study (animals of the satellite group) and at
termination (animals of the main group) was frequently depressed
(>20%) at 200 ppm in both sexes. Whole blood cholinesterase activity
in animals fasted overnight without access to food immediately prior
to sampling of blood was not reduced. The brain cholinesterase level
was inhibited (>20%) at 200 ppm in animals (both sexes) sacrificed at
52 weeks but not terminally. Organ weight determination at 52- and
60-week interim sacrifice and at terminal sacrifice showed deviations
from controls of several tissues such as liver, thymus, prostate and
adrenals, but these were essentially restricted to the top dosage
level only and were not accompanied by histopathological lesions.
Gross pathological changes were not significantly different from those
in the controls. Histopathologically, an increase in incidence of
stomach lesions, characterized by "hyperplasia/hyperkeratosis/
acanthosis with or without inflammation of the non-glandular portion",
occurred in the male terminal survivors at 200 ppm. There were no
microscopic lesions observed in any of the other tissues examined that
were attributable to the compound.
An evaluation of tumour data suggested a dose-related increase of
incidence of total lymphoreticular tumours in males of all treated
groups (incidence 3, 8, 10, 12%) respectively at 0, 2-->10, 20 and
200 ppm), with the difference from concurrent controls being
statistically significant only at 200 ppm. Frequency of each type of
lymphoreticular tumour (lymphosarcoma, thymic lymphosarcoma, reticulum
cell sarcoma, lymphatic leukaemia and myeloid leukaemia), when
considered alone, was not significantly increased in any treated
group. Latency period (or time of detection of the tumour when the
animal died or was sacrificed) was also not modified by treatment.
Other than the particular malignancy just mentioned, incidence, type
and location of tumours were not significantly higher at 200 ppm than
in the controls. It may be noted that 56% of males and 92% of females
of the concurrent control groups were bearers of tumours with
pituitary adenoma (both sexes), mammary tumour (females) and
pheochromocytoma (both sexes) being the most frequently observed
tumours.
Based on the available data, 20 ppm was without any significant
effect on cholinesterase. The lowest tested level, 2-->10 ppm
(indicated to be equivalent to an average achieved intake, throughout
the study of 0.38 mg/kg body weight/day) may be acknowledged as a
marginal no-effect level on the other tested parameters (Hunter
et al 1981).
Observations in Humans
In a male volunteer given a single oral dose of 9.8 mg or
approximately 0.12 mg/kg of 14C-bendiocarb, over 99% of the
administered dose was excreted in the urine in 22 hours. The plasma
half-life and the half-life for renal elimination for total
radioactivity was estimated at 3.5 and 3.3 hours, respectively. The
major urinary metabolites were sulphate and ß-glucuronide conjugates
of NC 7312, which together amounted to 95% of the radioactivity dose
excreted. Small amounts of conjugated bendiocarb and N-hydroxy methyl
bendiocarb were the only compounds detected in the urine (Adcock and
Challis 1976b).
An area on the forearm of each of two male volunteers was exposed
for 3 hours to 14C-bendiocarb (as an aqueous solution in a wetting
agent) at an average dosage of approximately 0.0013 mg/kg body weight
under occluded conditions in one experiment and non-occluded
conditions in another. (No information was available on the time
interval between the two experiments.) Over the 48-hour period
following exposure, an average of 58% of the applied dose under
occluded conditions vs. an average of 12.5% under non-occluded
conditions was recovered in the urine, suggesting better absorption of
bendiocarb through occluded skin. The only urinary metabolite found
was NC 7312 as conjugates of sulphate and ß-glucuronide (Warner and
Adcock 1980).
In a 60-year old male volunteer given bendiocarb (as an 80%
wettable powder) orally in H2O serially at 0.004, 0.012, 0.037,
0.125, 0.187 and 0.25 mg/kg body weight (dosage in terms of w.p.) with
at least a 48-hour interval between doses, 0.125 mg/kg was found
to be a clear-cut no-effect level on symptoms and whole blood
cholinesterase. Toxic sumptoms such as mild vertigo, nausea and
vomiting together with significant inhibition (30-40%) of whole blood
cholinesterase were induced after the single dose at 0.25 mg/kg body
weight. The symptoms subsided in 30 minutes and whole blood
cholinesterase recovered completely in 4 hours post-treatment. In the
same subject, doses of the 80% w.p. at 0.125 mg/kg given orally at an
interval of 4 hours, but not less, produced no toxic signs or
inhibition of whole blood cholinesterase. A double-blind cross over
experiment involving three other male volunteers indicated a single
dose of 0.004 mg/kg body weight, the only dose administered, of the
80% w.p. resulting in no adverse effects on any of the parameters
assessed (heart rate, clinical signs, mean diastolic/systolic pressure
and whole blood cholinesterase). Following a single dose of 0.12 mg/kg
body weight of the 80% w.p. given orally to the same three volunteers
72 hours later in another similar experiment, a transient depression
(21% of whole blood cholinesterase was observed in one of the three
subjects at 30 minutes post-treatment (Drummond and Kemp 1976).
The safety of bendiocarb (80% wettable powder), when used as an
insecticide against adult mosquitoes, to operators and inhabitants was
evaluated in Iran and Indonesia. The studies were organized along the
line of the WHO expanded Stage V evaluation programme. Each trial
involved the treatment of 13 villages with a total population of over
4 000 by 15 or 16 spray operators. Results indicated that very few
operators reported any toxic signs or symptoms and the latter, when
noted, were usually mild and transient. Inhibition of whole blood
cholinesterase, slight to mild, was only infrequently observed and was
readily reversible. Analysis of urine collected from the operators
suggested absorption of only low levels of bendiocarb during spraying.
No complaints were received from the villagers (Motabar et al 1981;
Bonsall et al 1981).
COMMENTS
Bendiocarb, a carbamate insecticide, evaluated for the first time
by the Meeting, was rapidly absorbed and readily eliminated primarily
in the urine in all of the mammalian species studied, including
humans. The major metabolic pathway in these species involved cleavage
of the carbamate ester group to yield the corresponding phenol
(NC 7312). There was no evidence of tissue accumulation in mammals.
Mammalian acute oral toxicity varied between strains and species, but
not between sexes.
A 3-generation reproduction study, including teratological
evaluation, in rats demonstrated 10 ppm and 50 ppm to be the no-effect
level, respectively, on reproductive parameters and teratogenicity. A
teratogenic study in the same species failed to give any indication of
teratogenicity even at the top dosage level (4 mg/kg body weight)
tested. In a rabbit teratology study, 1 mg/kg body weight was shown to
be a clear-cut teratogenic no-effect level. The available mutagenic
studies including reversion mutation and rec assays, mouse
micronucleus test, mouse lymphoma assay and dominant lethal assay in
rats were all negative. A carcinogenicity study in mice revealed no
carcinogenic activity of the compound, under the conditions of the
experiment. In a long-term feeding study with rats, a significant
increase in incidence of total lymphoreticular tumours, but not
individual type of lymphoreticular tumours, was noted in males of the
highest dosage group (200 ppm). Latency period of these tumours was
not reduced. Although the possibility of the finding in question
regarding lymphoreticular tumours being related to bendiocarb does not
appear likely, it cannot be entirely excluded. Background data on
incidence of total lymphoreticular tumours in CFY strain of rats
maintained in the testing laboratory would be helpful to resolve the
uncertainty. It should be noted that concurrent controls in the study
had a high incidence of spontaneous tumours. Additionally, less than
25% males and 45% females survived the duration of the 104-week study,
although over 50% of animals in all groups including the control were
still alive at the end of 82 weeks. Together these factors might
compromise sensitivity of the study to detect carcinogenic activity of
the compound. This study also demonstrated an increased incidence of
lenticular opacities in male rats at the two top dietary levels.
Neurotoxicity studies in hens gave no indications suggestive of
delayed neurotoxic potential of the insecticide.
No-effect levels were determined in short-term studies in rats
(13-week) and dogs (16-week and 2-year) and a long-term study in rats.
In the dog, however, the Meeting noted the possible adverse effects of
dilution during the determination of tissue cholinesterase, and also
the practice of tissue sampling from overnight-fasted dogs following a
1-hour feeding period. Furthermore, there was some concern on the
absence of data on the levels of food ingestion during the 1-hour
feeding period in one study, and food intake was extremely variable in
the second study.
Observations in humans indicated rapid reversal of cholinesterase
inhibition and of clinical symptoms following both acute and subacute
exposures.
Because of the concerns regarding the increased incidence of
lymphoreticular tumours in male rats of the top dose group in the
long-term study, and the probability that the no-effect level
established for dogs is nominal rather than actual, only a temporary
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0-0.38 mg/kg bw/day
Dog: 0-0.07 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0-0.002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Historical data on the incidence of total lymphoreticular tumours
in the CFY strains of rats used in the long-term study.
2. Short-term (28-day) study in dogs to clarify the relationship
between dietary intake of bendiocarb and terminal erythrocyte and
brain cholinesterase activity, using an appropriate method for
cholinesterase determination.
Desirable
Further investigation on the cataractogenic activity of
bendiocarb at low dosage levels in rats.
REFERENCES
Adcock, J.W. and Challis, I.R. The metabolism of 14C-bendiocarb in
1976a the rat. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1976b The pharmacokinetics and metabolism of bendiocarb in man.
Report from Fisons Ltd. submitted to the World Health
Organization by FBC Limited. (Unpublished)
Ben-Dyke, R. The toxicology of NC 6897. Acute dermal toxicity of
1972 technical bendiocarb, Report from Fisons Limited submitted to
the World Health Organization by FBC Limited. (Unpublished)
Bonsall, J.L., Foulkes, D.M., Goose, J., Leake, C.R. and Reary,
1981 J.B. Safety studies with bendiocarb in a village-scale field
trial against mosquitoes in Indonesia, 1981. Document
(WHO/VBC/81.831), World Health Organization, Geneva.
Challis, I.R. The metabolism of bendiocarb in the pregnant rabbit,
1980 Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in the
1977a mouse. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1977b The metabolism of bendiocarb in the hamster. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Gopinath,
1980 C., Harling, S., Majeed, S. and Prentice, D.E. NC 6897
Toxicity study in beagle dogs (final report dietary intake for
104 weeks). Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Crome, S.J. and Sanderson, D.M. A comparison of the acute oral
1980 toxicities of three batches of bendiocarb CR 4971/2, CR 4799/9
and CR 4500/20 to the male rat. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Cuthbert, J.A. Technical bendiocarb EX Muskegon primary eye irritancy
1980 in rabbits. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
1978 Technical bendiocarb. Primary skin irritancy study on rabbits.
Report from Inveresk Research International, Scotland,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Drummond, P. and Kemp, A. The effects of the acute oral administration
1976 of NC 6897 80 WP to male volunteers. Report TOX/76/133-58 from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hounsell, I.A. Brain cholinesterase inhibition in dogs fed NC 6897.
1974 Report from Fisons Limited Agrochemical Division submitted to
the World Health Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Rush, K.C. The effect of dietary administration
1980 of NC 6897 at 1 250 ppm on whole blood cholinesterase activity
in mice. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Brooks, P.N. The 30-day subchronic oral toxicity
1981 of NC 6897, technical CR 4799/1 in the diet to the hamster.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A.G. The effect of dietary administration of NC 6897 at
1981a 500 and 1 000 ppm on whole blood cholinesterase activity in
male mice of the CFLP and CD1 strains. Report from FBC Limited
submitted to the World Health Organization by FBC Ltd.
(Unpublished)
1981b The effect of dietary administration of NC 6897 at 0.4, 2 and
10 ppm on whole blood cholinesterase activity in male rats.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1981c The effect of dietary administration of NC 6897 at 0.4, 2, 10
and 50 ppm on whole blood cholinesterase activity in male
rats. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Walker, A.K. A micronucleus study in mice using
1982 technical NC 6897, CR 4971/2. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Offer, J.M. and
1978 Gregson, R.L. NC 6897 Toxicity to rats when administered in
the diet for 13 weeks. Final Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Prentice, D.E.,
1981 Gopinath, C. and Gibson, W.A. NC 6897 Toxicity and
tumorigenicity to rats in long-term dietary administration.
Report from Huntingdon Research Centre, England, submitted to
the World Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. Effects on adult female rats of one month exposure to
1977 high dietary concentration of NC 6897 (technical). Report from
Fisons Limited Agrochemical Division submitted to the World
Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. A peri- and post-natal study in rats with technical NC
1978 6897, Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Hounsell, I. Evidence for the reversal of cholinesterase
1974 inhibition by NC 6897 in laboratory animals. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Kemp, A. and Jackson, C.M. A test for the induction of dominant lethal
1977 mutations in male rats by NC 6897. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Brooks, P.N. The effects of the daily oral administration
1978 of NC 7312 to male and female rats for 16 days. Report from
Fisons Limited Agrochemical Division submitted by FBC Limited.
(Unpublished)
Litton. 16-week subacute toxicity study in dogs. NC 6897 technical.
1974 Report from Litton Bionetics Inc., U.S., submitted to the
World Health Organization by FBC Ltd. (Unpublished)
Mallyon, B.A. and Sanderson, D.M. The acute oral toxicity of NC 7312
1977 to male and female rats. Report from Fisons Limited
Agrochemical Division submitted by FBC Limited. (Unpublished)
McConville, M. and McGregor, D.B. Testing the mutagenic activity of
1979 technical NC 6897, Report from Inveresk Research
International, Edinburgh, Scotland, submitted to the World
Health Organization by FBC Limited. (Unpublished)
McConville, M. and Harris, W.J. NC 7312: Ames test for mutagenic
1979 activity. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
McGregor, D.B. and Ross, C.A. NC 6897: The assessment of mutagenic
1981 potential in the mouse lymphoma mutation assay. Report from
Inveresk Research International, Scotland, submitted to the
World Health Organization by FBC Limited. (Unpublished)
Moriya, M., Ohta, T. and Shirasu, Y. KNT (bendiocarb): microbial
1981 mutagenicity study. Report from the Institute of Environmental
Toxicology, Japan, submitted to the World Health Organization
by FBC Limited. (Unpublished)
Motabar, M., Mallyon, B.A., Goose, J. and Adcock, J.W. Document
1981 (WHO/VBC/81.821) World Health Organization, Geneva.
Palmer, A.K. and Allen, P.A. Effect of NC 6897 on neonatal pup
1978 mortality, Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Pearce, J.C., Warner, P.A. and Adcock, J.W. The metabolism of
1977 bendiocarb in the mouse (strain CD 1). Report from Fisons
Limited submitted to the World Health Organization by FBC
Limited. (Unpublished)
Pearce, J.C. and Adcock, J.W. Investigation of residue accumulation
1978 in the rat following repeated administration of
14C-bendiocarb. Report from Fisons Limited submitted to the
World Health Organization by FBC Limited. (Unpublished)
Roberts, N.L. Blood cholinesterase levels in the cow following dietary
1979 inclusion of bendiocarb. Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Ross, D.B., Roberts, N.L., Cameron, D.M., Prentice, D.E. and Majeed,
1978 S.K. Examination of NC 6897 for neurotoxicity in the domestic
hen. Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Sanderson, D.M. The toxicity of NC 6897: Acute toxicity to the rat of
1970 technical grade NC 6897. Report from Fisons Limited submitted
to the World Health Organization by FBC Limited. (Unpublished)
1971 The toxicology of NC 6897: Acute toxicity of pure NC 6897.
Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1972 Toxicology of NC 6897: 15-dose cumulative dermal study with
Ficam in male rats. Report from Fisons Limited submitted to
the World Health Organization by FBC Limited (Unpublished)
Sanderson, D.M. and Hounsell, I.A. The toxicology of NC 6897. Test
for neurotoxicity of NC 6897 to the chicken. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Sanderson, D.M. and Mallyon, B. The acute oral toxicity to the
1977 hamster of technical bendiocarb. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
1979 The acute inhalational toxicity to rats of Ficam 80W, an 80%
wettable powder formulation of bendiocarb, CR 13374/27H.
Report from Fisons Agrochemical Division submitted to the
World Health Organization by FBC Limited. (Unpublished)
Serota, D.G., Voelker, R.W. and Fezio, W.L. A chronic toxicity and
1981 carcinogenicity study in mice, NC 6897. Final report from
Hazleton Laboratories America, Inc. submitted to the World
Health Organization by FBC Limited. (Unpublished)
Tesh, J.M., Bartlett, A., Tesh, S.A., Whitney, J.C. and Finn, J.P.
Technical NC 6897 (CR 4799/1): Effects of dietary
administration upon reproductive performance and teratogenic
response in rats treated continuously through three successive
generations. Report from Life Science Research, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Tesh, J.M., Ross, F.W. and Tesh, S.A. Technical NC 6897: Effects of
1980 oral administration upon pregnancy in the rabbit. Report from
Life Science Research, England, submitted to the World Health
Organization by FBC Limited. (Unpublished)
Tucker, M.L. NC 6897: Teratogenicity study in the rat. Report from
1974 Consultox Laboratories Ltd., England, submitted to the World
Health Organization by FBC Limited. (Unpublished)
Warner, P.A., Adcock, J.W. and Pearce, J.C. The metabolism of
1977 14C-bendiocarb in the dog. Report from Fisons Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Warner, P.A. and Adcock, P.A. The dermal absorption of 14C-bendiocarb
1980 in man. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
BENDIOCARB
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bendiocarb is available as a wide range of formulations,
including wettable powders (200, 500 and 800 g a.i./kg); granules
(30, 50 and 100 g a.i./kg); dust (10 g a.i./kg); oil suspension for
ULV application (250 g a.i./kg); residual sprays, paint-on and
granular baits and aerosols.
Bendiocarb is used for the controls of soil and foliar insect
pests on a wide range of crops, ectoparasites on domestic animals and
mosquitos and nuisance pests. The Meeting was informed that it is sold
for these uses in approximately 100 countries. Information was
provided to the Meeting on official nationally approves uses (good
agricultural practices, GAP) by the Netherlands. Good agricultural
practices of The Netherlands are summarized in Table 1 (Netherlands
1982).
Table 1. Bendiocarb Good Agricultural Practices, Netherlands 1982
Application Used
Crop Pests rate (kg/ha) Formulation Treatment Since
Sugarbeet Fritfly, wireworm, 0.3-0.4 3% G seed furrow 1979
spring tails, 0.4 80% WP seed furrow 1981
pygmy beetle 500 g/l
liquid
6.4 g/kg " seed dressing 1981
seed
Maize Fritfly, 0.3-0.4 3% G seed furrow 1979
wireworm 4 g/kg 80% WP seed dressing 1981
seed 500 g/l
liquid
The Meeting was provided with a tabulation of recommended
application rates, which are summarized in Table 2 (Netherlands 1982).
No information was provided on preharvest intervals. The recommended
application rates are consistent with GAP of The Netherlands for maize
and sugarbeets. Information was not provided on whether the
recommended uses other than on sugarbeet and maize are also nationally
approved and, if so, where.
Table 2. Recommended Application Rates of Bendiocarb, Netherlands 1982
Crop Major pests Formulation Rate (a.i.)
Maize Wireworm, symphlids, fritfly Granule 0.3-0.35 kg/ha
Wireworm, symphlids, fritfly Seed dressing 4 g/kg seed
Corn rootworm Granule 0.84-1.12 kg/ha
Soil pests (in furrow) Spray 0.4 kg/ha
Sugarbeet Wireworm, pygmy beetle,
springtails Granule 0.3-0.35 kg/ha
Wireworm, pygmy beetle,
springtails Seed dressing 6 g/kg seed
Soil pests (in-furrow) Spray 0.4 kg/ha
Rice Rice water weevil, stem
borer Granule 2 kg/ha
Leafhoppers, planthoppers Spray 0.5 kg/ha
Leafhoppers, planthoppers Dust 0.5-0.75 kg/ha
Oilseed Flea beetle Granule 0.14-0.28 kg/ha
rape Spray 0.1-0.2 kg/ha
Ryegrass Fritfly, wireworm,
leatherjackets Granule 0.75 kg/ha
Spray 0.5-0.75 kg/ha
Seed dressing 10 g/kg seed
Cereals Fritfly, wireworm,
leatherjackets Spray 0.75 kg/ha
Seed dressing 2-4 g/kg seed
Potatoes Colorado beetle Spray 0-125-0.25 kg/ha
tuber moth Spray 0.5 kg/ha
Brassicas Diamond-back moth Spray 0.1-0.8 g/l
Peas Pea moth, pea weevil Spray 0.2-0.5 kg/ha
Apple/Pear Codling moth, tortrix moth,
aphids Spray 0.25-0.6 kg/ha (LV)
Mushrooms Mushroom fly Wall spray 30-50 g/100 m2
Preharvest Uses
Residue trials utilizing preharvest treatments have been
conducted on a variety of plant and animal products. Results are
detailed under "Residues Resulting From Supervised Trials".
Postharvest Uses
The Meeting was informed of residue investigations in wheat grain
stored in small bins for six months after treatment of interior
surfaces, but no details were provided. Likewise, no details were
provided on studies said to have been conducted on foodstuffs treated
in food-handling premises.
Other Uses
The Meeting was informed that 80 W or ULV formulations may be
used for control of mosquitos and the ULV formulation against
warehouse pests. Studies have been conducted to determine the extent
of potential exposure. Although contamination levels were said to be
within safe limits, details of these studies were not provided.
Bendiocarb is said to be widely used for control of cockroaches
and other nuisance pests in and around the home, hotels, food-handling
establishments, aircraft etc. and as a paint for fly control in animal
houses. No details on these uses were provided.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries on a wide
range of commodities, representing a variety of treatments and
climatic conditions.
Root and Tuber Vegetables
Potatoes
Twenty five trials were conducted in three European countries
either by spray, broadcast or in-furrow treatments with either 3G or
20W formulations. Results are summarized in Table 3 (FBC 1982a; Reary
1979d; Browne and Reary 1981; Browne 1981e). Although most of the data
were from application of the 3G formulation, no information was
provided on recommended or approved uses for that formulation on
potatoes. The wettable powder spray applications were at one and two
times the recommended rate. Recoveries were generally 75% or better
for bendiocarb and 60% or better for NC 7312. The limit of
determination is said to be approximately 0.01 and 0.02 mg/kg for
bendiocarb and NC 7312, respectively.
Table 3. Bendiocarb Residues in Potatoes, from Supervised Trials
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 2 4-5 3G 1 kg/ha In-furrow 119-155 0.01-0.03 <0.02,0.26
1978 2 4-5 3G 2 kg/ha In-furrow 119-155 0.01-0.04 0.02,0.58
2 4-5 3G 3 kg/ha In-furrow 119-155 0.01,0.03 0.04,0.69
1 7 3G 1 kg/ha Broadcast 112 0.01 0.02
1 7 3G 1.5 kg/ha Broadcast 112 0.04 0.05
2 4-7 3G 2 kg/ha Broadcast 112-155 <0.01-0.03 <0.02,0.07
2 4-7 3G 3 kg/ha Broadcast 112-155 <0.01-0.04 <0.02,0.11
2 4-7 3G 4 kg/ha Broadcast 112-155 0.01-0.03 0.03,0.08
3 Untreated
controls 0.003-0.008 0.003-0.0.7
German Federal 3 3 20W 0.12 kg/ha Spray 03/ <0.01 0.014-0.12
Republic 3 3 (<0.005-0.017) (<0.01-0.14
1980 2 3 7 <0.01 <0.01-0.14
(<0.005-0.009) (<0.01-0.15)
14 <0.01 0.01-0.15
(<0.005-0.015) (<0.01-0.18)
1 3 15 <0.005) 0.014
3 2 - - Untreated
controls - 0.001-0.044/ (<0.01-0.024)
0.001-0.007
Table 3 (con't)
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 1 (3 plots) 20W 0.53 kg/ha Spray 145/ <0.01 0.03-0.05
1981 1 (3 plots) 20W 1.06 kg/ha 145/ 0.005 0.06-0.07
1 Untreated
controls <0.005 0.01
1/ Limit of determination 0.01 mg/kg for bendiocarb and 0.02 mg/kg for NC 7312.
2/ Means of replicates; range of individual replicates in parentheses.
3/ Day 0 represents final spray of a weekly 3-spray programme, each application at 0.12 kg/ha.
4/ <0.01 except the 0.04 mg/kg value.
5/ Days after final spray of 2- or 3-spray programme, commencing 7 or 14 days before the final application.
Maximum bendiocarb residues at harvest resulting from at or
before harvest treatments were 0.04 mg/kg (mean of replicates) and
0.7 mg/kg for NC 7312.
From multiple spray applications, maximum bendiocarb residues at
harvest were < 0.01 mg/kg (mean of replications) or 0.017 mg/kg for
individual replicates. Maximum residues of NC 7312 were 0.15 mg/kg
(mean of replicates) or 0.18 mg/kg for individual replicates. For both
the means of the replicates and individual replicates residues of
bendiocarb and NC 7312 were each generally comparable from 0 to 15
days after last application. Apparent residues in untreated controls
were generally < 0.01 mg/kg for bendiocarb and < 0.02 mg/kg for
NC 7312. Residues of NC 7312 at harvest were 1-20 times that of
bendiocarb per se, and averaged six times higher.
Since residues from in-furrow or broadcast applications can be
0.04 mg/kg and untreated controls can, on occasion, reach this level,
a 0.05 mg/kg for bendiocarb per se can be supported. If NC 7312 were
also included, a limit of 0.5 mg/kg would be in order for combined
residues.
Sugarbeet
Thirty one supervised residue trials were conducted in five
European countries and residue decline studies in three (Table 4)
(Browne 1982c; FBC 1982b; Reary 1974, 1975e, 1979h,i,n,q, 1980f,j).
The seed treatment applications of 80W were at 0.8-2.7 times the
recommended 6 g/kg rate, which is also consistent with GAP of The
Netherlands. In-furrow applications of a granular formulation were
representative of the 0.3-0.4 kg a.i./ha GAP of The Netherlands and
recommended application rates.
In the supervised trials, bendiocarb residues at harvest were
below 0.01 mg/kg. Some samples were also analysed for the metabolite
NC 7312 and all were below the 0.01 mg/kg limit of determination.
Similarly, in the decline studies, residues in sugarbeet roots were
less than the 0.02 mg/kg limit of determination. Residues in untreated
controls were generally < 0.01 mg/kg, except for one set of trials
where residues ranged up to 0.11 mg/kg (average 0.005 mg/kg). The same
basic analytical procedure was used in all of the trials, but modified
in some to permit analysis of NC 7312. The limit of determination for
sugarbeet generally ranged from 0.005-0.02 mg/kg for bendiocarb and
0.01-0.02 mg/kg for NC 7312. Recoveries were generally > 75% for
parent compound and > 60% for NC 7312. In general, recoveries were
less in tops than in roots and less for NC 7312 than for bendiocarb.
Table 4. Bendiocarb Residues in Sugarbeets From Supervised Trials and Residue Decline Studies
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
Denmark 1 1 80W 15 g/kg seed Pelleted seed 204 roots <0.005
1974 1 untreated controls roots <0.005
France 2 8 80W 12 g/kg seed Pelleted seed 163 roots <0.005
1974 2 untreated controls roots <0.005
German 1 1 6 g/kg seed Seed dressing 196 roots <0.005
Federal 1 1 196 tops <0.005
Republic 1 1 80W 12 g/kg seed Seed dressing 196 roots <0.005
1974 1 1 196 tops <0.005
untreated controls roots or
tops <0.005
U.K. 2 4 80W 8 g/kg seed Pelleted seed 213 roots <0.005
1974 untreated 213 tops <0.0055/
2 control roots <0.005
tops <0.0055/
France 3 3 3G 0.36 kg/ha In furrow 160-168 roots <0.005
1976 3 3 160-168 tops <0.01
3 6 3G 0.45 kg/ha In furrow 171-190 roots <0.005
3 6 171-190 tops <0.01
3 6 untreated controls tops and
roots <0.006
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
France 2 2 3G 0.46 kg/ha In furrow 180-185 roots <0.005
1977 2 2 180-185 tops <0.01
2 2 untreated controls tops and <0.006
roots
France6/ 2 3G 0.3 kg/ha In furrow 152-170 roots <0.01
1978 2 152-170 tops <0.01
2 3G 0.6 kg/ha In furrow 152-170 roots <0.01
2 2 untreated controls tops and
roots <0.005
Italy6/ 1 1 3G 0.3 kg/ha In furrow 110 roots <0.01
1978 1 1 110 tops <0.01
1 1 3G 0.6 kg/ha In furrow 110 roots <0.01
1 untreated controls tops and
roots <0.005
U.K.6/ 3 3 3G 0.3 kg/ha In furrow 154-188 roots <0.01
1978 3 3 154-188 tops <0.01
1 1 3G 0.33 kg/ha In furrow 202 roots <0.01
1 1 202 tops <0.01
1 1 3G 0.4 kg/ha In furrow 188 roots <0.01
188 tops
1 1 untreated controls tops and
roots <0.007
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K.6/ 6 1 3G 0.3 kg/ha In furrow 144-167 roots <0.01
1979 6 1 144-167 tops <0.01
6 6 untreated controls tops and
roots <0.01
Italy6/ 1 3G 0.30 kg/ha In furrow 154 roots <0.01
1981 1 154 tops <0.01
1 3G 0.32 kg/ha In furrow 183 roots <0.01
1 183 tops <0.01
2 2 Untreated roots <0.001
controls tops 0.003
German 4 12 80W 5 g/kg seed Seed dressing 71-166 roots <0.02
Federal tops <0.02
Republic 4 12 Untreated roots 0.002-0.11
1978 4 12 controls (0.005 av.)
tops 0.005-0.015
(0.008 av.)
3 8 3G 0.33 kg/ha In furrow 56-70 roots <0.01
3 9 tops <0.01
71-166 tops <0.015
5 Untreated 71-166 roots <0.01
controls tops <0.01
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K. 1 5 3G 0.3 kg/ha In furrow 25-40 roots 1.367/
1979 41-55 roots 0.127/
56-70 roots <0.01
71-166 roots <0.01
7 56-70 tops <0.01
71-166 tops <0.01
1 5 Untreated 56-70 roots <0.01
controls tops <0.01
1/ Bendiocarb only (see also 6/);
2/ Corrected for recoveries of >75% except for 59% recovery in tops. Fortification levels ranged from 0.02-0.01 mg/kg;
3/ Limit of determination in individual trials generally ranged from 0.005-0.02 mg/kg;
4/ Duplicate analyses of 0.006 and 0.003 mg/kg;
5/ Individual values up to 0.07 mg/kg but <0.005 mg/kg when repeated;
6/ Sample also analysed for primary metabolite NC 7312. Residues after 56 days were generally below the 0.01 mg/kg limit
of detection except one 0.03 mg/kg value in tops (limit of determination in that trial or a 0.02 limit of determination.
Residues in seedlings were 0.42 and 0.07 mg/kg at 28 and 41 days respectively after in-furrow application at planting;
7/ Seedlings analysed whole.
Since residues are below the claimed limits of determination, a
limit of 0.02 mg/kg bendiocarb per se would probably not be
unrealistic. However, because of high apparent residues in one
untreated control, a 0.05 mg/kg limit at or about the limit of
determination may be more suitable. Based on a 0.02 mg/kg limit of
determination for NC 7312, a limit on the order of 0.1 mg/kg would be
appropriate if both bendiocarb and free NC 7312 were included. Actual
residues were too low to determine whether residues of NC 7312 exceed
those of bendiocarb, but judging from those found in potatoes, it is
likely.
Leafy Vegetables, Except Brassicas
Sugarbeet leaves
From the residue trials and decline studies discussed previously
(Table 4), residues in sugarbeet tops at harvest from recommended
application rates were generally less than the 0.01-0.03 mg/kg limits
of determination. Only one trial had a limit of determination of
0.03 mg/kg for tops. The limit of determination was considered to be
< 0.02 mg/kg for the remaining 30 trials. Bendiocarb residues were
1.4 and 0.12 mg/kg in seedlings at 28 and 41 days, respectively, after
in furrow treatments at planting. The data support a 0.05 mg/kg at or
about limit of determination at harvest as the maximum residue level.
Brassica Leafy Vegetables
Cabbage
No information was available on specific nationally approved
uses. Supervised trials with a wettable powder formulation were
conducted in one country (Table 5) (Browne 1981a,c, 1982a,b; Browne
and Manley 1982), at application rates up to 0.25 times the maximum
recommended application rate (Table 2). No specific preharvest
interval was recommended. Maximum residues ranged from 0.18 mg/kg one
day after the last of seven applications to <0.01 mg/kg after 14
days. Untreated controls were < 0.02 mg/kg and analytical
recoveries averaged 82% from fortification levels at 0.08-0.2 mg/kg.
The limit of determination was said to be 0.01 mg/kg, but considering
untreated control analyses, a 0.05 mg/kg limit of determination may be
more practical.
Because the trials do not reflect maximum recommended application
rates, recommended usage does not specify the number of applications
or a preharvest interval and the data are minimal, a maximum residue
level for bendiocarb in cabbage cannot be estimated with confidence.
Table 5. Bendiocarb and NC 7312 in Crops from Supervised Trials
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Cabbage1/ Philippines 7 0.06 50W 1 0.1 3
1981 0.1 a.i./l 3 0.04 3
7 0.01 3
14 <0.01 3
0.12 50W 1 0.18 3
0.2 g a.i./l 3 0.05 3
7 0.02 3
14 <0.01 3
Untreated 1 <0.01 3
control 3 0.02 3
7 0.01 3
14 <0.01 3
Peas2/ U.K. 1 0.2 50SC3/ 27 <0.01 <0.01 5
flowering 1981 1 0.3 <0.01 <0.01 5
stage (MarQ) 1 0.4 <0.01 <0.01 5
1 0.05 <0.01 <0.01 5
Untreated
control <0.01 <0.01 5
4-leaf 1 0.2 27 <0.01 <0.01 5
stage 1 0.3 <0.01 <0.01 5
1 0.4 <0.01 <0.01 5
1 0.5 <0.01 <0.01 5
Untreated
control <0.01 0.01 5
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pea pods 1 0.2 50SC3/ 83 <0.01 <0.02 5
flowering 1 0.3 <0.01 <0.06 5
stage 1 0.4 <0.01 0.07 5
1 0.5 <0.01 0.03 5
Untreated
control <0.01 0.03 5
4-leaf
stage 1 0.2 83 <0.01 0.05 5
1 0.3 <0.01 0.05 5
1 0.4 <0.01 0.08 5
1 0.5 <0.01 0.06 5
Untreated <0.01 0.05 5
control
Apple
(Cox's U.K. 24/ 0.04 and 20W 57 <0.01-0.01 <0.01 6
Orange 1981 0.08% 50W,
Pippin) 50 SC
Untreated 0.006 0.005 1
14/ 0.04 and 20W, 106 <0.01-0.01 <0.01-0.01 6
0.08% 50W,
50SC 0.009 0.003 1
4/ Untreated
2 0.02, 20W, 98 <0.01-0.01 <0.01-0.01 9
0.04 and 50W,
0.08% 50SC
untreated 0.003 0.008 1
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pear 14/ 0.02% 50SC 158 0.01 <0.01 1
(Conference) 14/ 0.04% 0.02 0.01 1
14/ 0.08% untreated 0.01 0.005 1
Mushrooms The Netherlands
1980 2 5 50SC 21 0.04-0.06 0.01-0.03 4
(50 g a.i./ <0.05 av.) (<0.02 av.)
100 m2)
2 7.5 50SC 21 0.08-0.12 0.01-0.04 4
(75 g a.i./ (0.1 av.) (0.02 av.)
100 m2)
2 10 50SC 21 0.11-0.14 0.02-0.06 4
(100 g a.i./ (0.13 av.) (0.04 av.)
100 m2)
Untreated <0.01-0.02 <0.01-0.1 4
(0.01 av.) (0.01 av.)
U.K. g a.i./100 m2
1981 wall spray5/
1 52.2 50SC 69 N.D.6/ <0.02 1
untreated 69 N.D. N.D. 1
1 20 80W 33 N.D. N.D. 1
untreated 33 0.001 N.D. 1
1 48 80W 41 <0.02 0.02 1
untreated 41 0.04 0.02 1
(g a.i./100 m2
wall spray 5/)
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Mushrooms U.K. 1 20 80W 26 <0.02 <0.02 1
1981 untreated 26 N.D. N.D. 1
1 20 80W 33 <0.02 <0.02 1
untreated 33 N.D. 0.003 1
1 20 80W 52 N.D. <0.02 1
20 80W 60 0.04 0.02 1
untreated 52 N.D. N.D.
48 80W 42 0.06 0.1 1
48 80W 42 0.09 0.13 1
48 80W 42 0.08 0.10 1
1/ Residue results corrected for mean analytical recovery of 82% with fortification at 0.08-0.2 mg/kg.
2/ Results corrected for recoveries. Harvest (127 days after planting) said to be 2-3 weeks after normal harvest.
3/ SC = Suspendable concentrate.
4/ 1.5 1 spray solution per tree.
5/ Mushroom plots established the day after wall treatment.
6/ N.D. = no detectable chromatographic peaks.
Legume Vegetables
Peas
Supervised residue trials were conducted in the U.K. with single
applications at rates consistent with recommended ones. No information
was available on specific nationally approved good agricultural
practices, nor was information available on recommended numbers of
applications or preharvest intervals.
Peas were sampled at 2-3 weeks after normal harvest (127 days
after planting). At all application rates tested, residues of either
bendiocarb or NC 7312 (corrected for recovery) were <0.01 mg/kg in
peas, including untreated controls (Table 5). The same is true for
bendiocarb in pea pods, but residues of NC 7312 ranged from
0.03-0.08 mg/kg (0.05 mg/kg av.), and untreated controls were 0.02 and
0.05 mg/kg. Although bendiocarb residues were at or about the limit of
determination, the data suggest that terminal residues of NC 7312 may
be 2-10 times that of bendiocarb.
Analytical recoveries averaged 86 and 72% for bendiocarb and NC
7312, respectively, in peas from fortification levels of 0.04 and
0.2 mg/kg. Recoveries in pods averaged only 58 and 63%, respectively.
The limit of determination was said to be 0.01 mg/kg for bendiocarb
and NC 7312 in both peas and pods. However, based on untreated
controls, 0.05 mg/kg may be a more appropriate limit of determination
for NC 7312 in pea pods.
Because GAP information is unavailable and samples were not
harvested at normal harvest, a maximum residue level cannot be
estimated with confidence.
Pome Fruit
Apples and Pears
Residue data were available from supervised trials in the U.K. at
application rates of 0.02-0.08% active ingredient (1.5 1/tree),
representing several formulations. Applications were at pre- or
post-blossom and/or at codling stage, and fruit was sampled at normal
harvest of 57-158 days after last treatment. No information was
available on specific nationally approved agricultural practices.
Recommended applications rates of 0.25-0.5 kg/ha are not directly
comparable to the 0.02%-0.08% used in the field trials. No information
was provided on recommended preharvest intervals or numbers of
applications.
Of the 24 apple and pear samples treated in three locations and
in untreated controls, residues of bendiocarb and NC 7312 were
< 0.01 mg/kg, except for one 0.02 mg/kg value for pears treated
once with a 0.08% 50 SC formulation (Table 5). Mean recoveries of
bendiocarb and NC 7312 on apples and pears were 94 and 73%,
respectively, when fortified from 0.1 to 0.4 mg/kg. The limit of
determination is said to be 0.01 mg/kg for both bendiocarb and
NC 7312.
If the formulations, application rates, number of applications
and preharvest intervals of the field trials represent GAP, the data
could support a maximum residue level of 0.02 mg/kg for bendiocarb per
se or 0.05 mg/kg for combined residues of bendiocarb and NC 7312.
Cereal Grains
Barley
Fourteen supervised trials were conducted in the U.K. with
in-furrow granular, 50W spray or 80W seed dressing applications. No
nationally approved agricultural practice information was provided,
although recommended uses for cereals were reported (Table 2). No
recommended uses for the granular formulation (2 trials) were
provided. Only two of the trials were from spray treatments and the
remainder were from seed treatments. The spray applications at 0.5 and
1.0 kg a.i./ha reasonably reflect the recommended 0.75 kg a.i./ha
although the data are limited (Table 6) (Browne and Reary 1980c,d,e;
Housden and Reary 1981; Reary 1978 h,i,j,n; Reary 1979e,f,g,j,k,l,m,o,
1980a,e; Reary and Browne 1978). The seed treatments at 2-5 g a.i./kg
seed reflect the recommended rate of 2-5 g a.i./kg.
Residues of bendiocarb at normal harvest in barley grain were
<0.02 mg/kg and <0.03 mg/kg in straw. Untreated controls in
barley grain and straw were at the same level. Some samples were
also analysed for combined conjugated metabolites, NC 7312 and
N-hydroxymethylbendiocarb. In all three trials, metabolite residues
were <0.02 mg/kg. Mean analytical recoveries for bendiocarb in
barley during the supervised trials were 94 and 84%, respectively, for
grain and straw when fortified at 0.02-0.2 mg/kg. In a separate study,
bendiocarb recoveries in barley grain were 61-125% (av. 94%) after
fortifications at 0.02-0.2 mg/kg (Reary 1979b).
Table 6. Bendiocarb Residues in Cereals from Supervised Trials
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
Barley, wheat and oats
U.K. 1 1 80W 4 g/kg seed Seed dressing 148 barley grain <0.02
1977 1 1 148 barley straw <0.02
1 2 80W Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
1 2 50SC 4 g/kg seed Seed dressing 148 barley grain <0.02
1 2 148 barley straw <0.02
1 2 50SC Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
U.K. 3 3 80W 4 g/kg seed Seed dressing 113-145 barley grain <0.02
1978 2 2 113-145 barley straw <0.03
2 2 124-137 wheat grain <0.02
1 1 124-137 wheat straw <0.06
3 3 (10 rep) 134-141 oat grain <0.02
2 2 (10 rep) 134-141 oat grain <0.02
2 2 80W 8 g/kg seed Seed dressing 136-147 oat grain 0.47
3 2 136-147 oat straw 0.02-0.05
U.K.1/ 1 1 3G2/ 0.5 kg/ha In furrow 148 barley grain <0.01
1979 1 1 148 barley straw <0.01
1 1 3G2/ 1.0 kg/ha In furrow 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 50W2/ 0.5 kg/ha Spray 148 barley grain <0.01
148 barley straw <0.01
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 50W2/ 1.0 kg/ha Spray 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 80W 2 g/kg seed Seed dressing 336 wheat grain <0.01
1 1 336 wheat straw <0.01
3 3 80W 4 g/kg seed Seed dressing 119-148 barley grain <0.01
119-148 barley straw <0.01-0.02
3 3 127-336 wheat grain <0.01
2 2 127-336 wheat straw <0.01
2 2 105-125 oat grain <0.012
2 2 105-125 oat straw <0.01,<0.03
2 2 80W 5 g/kg seed Seed dressing 119-127 barley grain <0.01
2 2 119-127 barley straw <0.01,<0.02
1 1 127 wheat grain <0.01
1 1 127 wheat straw <0.01
2 2 105-125 oat grain <0.01
2 2 105-125 oat straw <0.01
Maize harvest samples
Canada 22 37 10G 0.84-2.24 band 153-189 grain <0.02
U.S. 8 8 kg/ha at 132-165 cob <0.02
1977-78 21 38 plant 132-189 stover3/ <0.03
10 28 110-134 silage <0.023
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
German 25 30 3G 0.2-0.8 in furrow 133-184 grain <0.024/
Fed. Rep. 26 36 kg/ha at plant 128-184 cobs <0.024/
5 12 128-184 plants <0.01
U.K. 10 10 146-184 foliage <0.01
France 5 5 135-164 silage <0.02
Italy 133-154 stover <0.02
1976-79
France 30 36 76 or 2-16 g/kg seed 125-189 grain <0.035/
U.K., U.S. 30 24 80W seed treatment 160-161 cob <0.026/
1975-1979 18 23 125-167 silage <0.02
11 10 160-189 stover <0.02
14 8 141-164 plants <0.056/
Rice (rough) harvest samples
Philippines7/ 1 1 5G 1.5 + 0.5 Incorporation + 53 grain <0.01
1978 surface applied straw <0.02
1 1 5G 2.0 + 0.6 Incorporation + 53 grain <0.01
surface applied straw <0.02
1 1 50W 0.5 kg/ha Spray 90 grain 0.01
straw <0.02
1 1 50W 0.6 kg/ha Spray 90 grain <0.01
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
2 2 80W 0.5 kg/ha Spray 60-90 grain <0.01
straw <0.02
2 2 80W 0.6 kg/ha Spray 60-90 grain <0.01
straw <0.02
Philippines7/ 1 1 50W 0.5 kg/ha Spray 248/ grain <0.01
1979 straw <0.01
1 1 50W 0.7 kg/ha Spray 248/ grain <0.01
straw 0.01
1 1 50W 1.0 kg/ha Spray 248/ grain 0.01
straw <0.01
1 1 50W 0.5 kg/ha Spray 459/ grain 0.01
straw -
1 1 50W 0.75 kg/ha Spray 459/ grain <0.01
straw -
1 1 50W 0.5 kg/ha Spray 4910/ grain <0.01
straw <0.01
1 1 50W 0.75 kg/ha Spray 4910/ grain 0.01
straw 0.02
South Korea7/ 1 1 50W 0.75 kg/ha Spray 10 grain 0.6, 1.111/
1979 straw <0.6
1 1 2D 0.8 kg/ha Dust 10 grain 1.5, 1.411/
straw <0.65
1 1 3GW 0.9 kg/ha Seed box 119 grain 0.06, 0.03
incorporation straw 0.02
(100 g/box)
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 5G 1.5 kg/ha Soil 100 grain <0.6
incorporation straw 0.04
Philippines
1980 1 1 50W 0.25 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.50 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.75 kg/ha Spray 6212/ grain <0.02
straw <0.01
1/ Also analysed for combined residues of conjugated NC 7312 and N-hydroxymethylbendiocarb. All metabolite residues were
< 0.02 mg/kg except for residues of 0.07 and 0.05 mg/kg from one trial in oat grain from seed dressing of spring oats
with an 80 WP formulation at 4 and 5 g a.i./kg seed. The two residues resulted from oat grain samples with bendiocarb
residues 0.012 and 0.007 mg/kg respectively. Untreated controls were 0.011 mg/kg.
2/ Seed previously treated with an 80% WP formulation
3/ Stover = leaves and stems after removal of cobs.
4/ Analysis of oil from kernels with <0.02 mg/kg residue from one site showed no residue greater than the 0.02 mg/kg limit
of determination.
5/ The 0.03 mg/kg on grain resulted from treatments at 8 and 12 g a.i/kg seed. At <4 g a.i./kg seed residues were
<0.02 mg/kg in grain.
6/ Maize (sweet corn) comprised 14 of the trials at 2-8 g a.i./kg. All plants in this group were sweet corn. The 0.05 mg/kg
residue on sweet corn plants resulted from 2-4 g a.i./kg seed.
Table 6 (con't)
7/ Also analysed for metabolite NC 7312. No residues above the limits of detection (0.01 mg/kg in grain and 0.02 mg/kg in
straw) were found, except for 0.11 and 0.17 mg/kg in grain from dust and spray treated samples at the 10-day interval and
one 5G formulation at 100 days, in which it was 0.03 mg/kg. In the latter, the control was also 0.03 mg/kg.
8/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 17, 27, 39, 52,
66 and 80 days after transplanting.
9/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 10, 25 40 and
55 days after transplanting.
10/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 7, 20 and 45
days after transplanting.
11/ Values reflect residue determination by electron capture and fluorescence, respectively.
12/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 22, 35, 51 and
68 days after transplanting.
The limit of determination was said to be 0.01 mg/kg for both
cereal grain straws and grains, although apparent residues in controls
(<0.02 and <0.03 mg/kg in barley grain and straw, respectively)
suggest 0.02 or even 0.05 mg/kg may be a more realistic estimate for
both. When considered with data from other cereal grains and straws, a
0.05 mg/kg limit for bendiocarb per se at or about the limit of
determination could be supported if recommended uses represent GAP. A
0.1 mg/kg limit would be appropriate if both bendiocarb and
metabolites are included.
Maize
Over 100 supervised trials were conducted in six countries, two
in North America and four in Europe (Table 6). Applications were made
with granular formulations at planting, either by band or in-furrow
treatment and seed was treated with wettable powder formulations.
In-furrow applications of a 3G formulation at 0.2-0.8 kg a.i./ha
corresponds to the 0.3-0.4 kg a.i./ha Netherlands GAP, although no
data were available from The Netherlands. The recommended uses on
maize provided to the Meeting did not include the method of
application for granular formulations.
Granular band treatments at planting of 0.84-2.24 kg a.i./ha did
not correspond with certainty to either The Netherlands GAP or
recommended usage for maize. The 2-16 g a.i./kg wettable powder seed
treatments more than accommodate the 2-4 g a.i. GAP of The Netherlands
or recommended application rates. Additional information on official
national GAP is needed.
Maximum residues of bendiocarb per se from all treatments were
<0.03, <0.02, <0.023, 0.05 and <0.01 mg/kg in grain, cobs,
stover, silage, plants and foliage, respectively. Analysis of oil from
one grain sample with field-incurred residues of <0.02 mg/kg also
showed residues of less than the 0.02 mg/kg limit of determination.
The 0.03 mg/kg level in grain resulted from applications at 2-3 times
the recommended rate. At recommended rates all grain residues were
<0.02 mg/kg. The 0.05 mg/kg residue on plants was on maize (sweet
corn) treated according to recommended rates.
The limit of determination was said to be 0.01-0.02 mg/kg for the
various maize fractions. Apparent residues in untreated controls were
also in this range. Analytical recoveries during the trials averaged
approximately 81, 78, 72, 80 and 78% for grain, cob, silage, stover
and plants, respectively. A 0.05 mg/kg limit at or about the limit of
determination could be supported for bendiocarb per se in maize (field
corn) grain, fodder and/or forage. Additional data would be required
for inclusion of metabolites.
In addition to the trials at harvest, 12 residue decline trials
were conducted in four countries (FBC 1982a; Browne and Reary 1979d;
Reary 1978l,m, 1979p, 1980h,i), mostly from granular in-band or
in-furrow treatments at planting ranging from 0.3-2.24 kg a.i./ha.
Residues in grain from granular treatments were <0.01 mg/kg. In
plants, residues ranged from <0.05-0.9 mg/kg at 25-40 days after
treatment (0.9 at 29 days), declining to <0.07 mg/kg after 49 days
and <0.02 after 56-70 days. Residues in stover and cobs were <0.02
and <0.02 mg/kg, respectively, after 71 days.
From the single 4 g a.i./kg seed treatment with a wettable powder
formulation, residues on plants were 12.4 mg/kg 29 days after
treatment but rapidly declined to _0.023 mg/kg after 42 days.
Oats
Residue data were available for seven supervised trials in the
U.K. from 4-8 g a.i./kg seed 80W seed dressings (Table 6). No
information was provided on specific nationally approved practices.
At application rates approximating the 4 g a.i./kg seed
recommended application rate, residues in oat grain at normal harvest
were 0.02 mg/kg and in straw were _0.02 mg/kg. One of the two grain
samples treated at 8 g a.i./kg seed had a residue of 0.47 mg/kg.
Contamination of this sample and its 0.12 mg/kg untreated control was
suspected by the manufacturer to have occurred during sampling.
Maximum residues in straw from the 8 g a.i/kg seed application rate
were 0.05 mg/kg. While this could be aberrant, as the untreated
control was 0.12 mg/kg, it is not necessarily so, since residues are
as high as 0.03 mg/kg in straw of oats and barley from recommended
application rates. Residues in untreated controls were <0.02 and
<0.04 mg/kg in grain and straw, respectively, except for 0.12 mg/kg
from a grain and straw sample with suspected contamination.
A limited amount of residue data were also available for combined
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) in some
oat samples, with residues being <0.012 mg/kg and <0.01 mg/kg in
grain and straw, respectively.
Mean recoveries of bendiocarb were 89 and 94% for oat grain and
straw, respectively. The limit of determination was said to be
0.02 mg/kg for both, but based on the 0.035 mg/kg apparent residue in
untreated controls, 0.05 mg/kg may be more appropriate, especially
when barley and wheat are also considered. A 0.05 mg/kg limit at or
about the limit of determination could be supported for bendiocarb per
se if recommended uses represent GAP. A 0.1 mg/kg limit would be
required if both bendiocarb and metabolites were included.
Rice
Residue data (corrected for mean recoveries) were available from
22 supervised trials on rough rice at normal harvest in The
Philippines and South Korea (Table 6). Applications were by granular,
spray and dust formulations. No information was available on
nationally approved agricultural practices on rice. Recommended
application rates for rice are 2 and 0.5 kg a.i./ha for granular and
spray formulations, respectively, and 0.5-0.75 kg a.i./ha for dust, as
summarized in Table 2. No information was provided on approved
intervals from last application or maximum number of applications.
Only one trial utilized a dust formulation, and the application
rate reflects the recommended use. Bendiocarb residues were 1.5 mg/kg
and 0.65 mg/kg, respectively, for grain and straw 10 days after
application as compared to 0.6-1.1 mg/kg for a spray application after
the same interval. Residues for NC 7312 were 0.17 mg/kg at the same
10-day interval. Untreated controls were <0.3 mg/kg and 0.04 mg/kg,
respectively, for grain and straw.
Spray applications of 0.25-0.75 kg a.i./ha covers the 0.5 kg/ha
recommended rate. As in the case of dust treatments, the spray
applications at 10 days before normal harvest resulted in higher
residues than at longer intervals, measuring <1.1 and <0.5 mg/kg,
respectively, for grain and straw. Similarly, residues were higher for
NC 7312 in grain, being 0.11 mg/kg. The same control as for the 10-day
interval dust treatment applies.
At longer intervals from last treatment with 50W or 80W
formulations (24-90 days) and up to 1.5 times the recommended rate,
residues were <0.02 mg/kg for both grain and straw. Untreated
controls for spray treatments, except for the 10-day interval
treatment, were <0.021 and <0.02 mg/kg, respectively, for
bendiocarb in grain and straw, except for one untreated straw sample
at 0.04 mg/kg. Residues of NC 7312 were <0.03 mg/kg and 0.02 mg/kg
for grain and straw, respectively, from spray treatments.
Four trials were conducted with either 3G or 5G formulations at
rates reflecting the recommended application rate and different
application methods. Maximum residues of bendiocarb were <0.6 mg/kg
and 0.04 mg/kg, respectively, in grain and straw. Untreated controls
were <0.07 and <0.04 mg/kg bendiocarb in grain and straw,
respectively. Residues of NC 7312 were <0.03 and <0.02 mg/kg in
grain and straw, respectively.
Analytical recoveries of bendiocarb during the trials averaged 77
and 68%, respectively, in rice grain and straw from fortifications at
0.02-0.25 mg/kg. Average recoveries were less (64 and 56%) for NC
7312. In a separate study on recoveries (Browne and Reary 1979b) at
similar fortification levels, mean recoveries were better, being 86
and 74% for bendiocarb in grain and straw, and 70 and 56%,
respectively, for NC 7312.
In the absence of GAP information, 2 mg/kg and 1 mg/kg limits
would be required for bendiocarb per se in rice grain and straw,
respectively, to accommodate the uses of the supervised trials.
Wheat
Residue data, corrected for recoveries, were available from eight
supervised trials in the U.K. from seed treatments with 80W or 50SC at
2-5 g a.i./kg seed (Table 6). No information was available on specific
nationally approved agricultural practices, although the application
rates used reflect the 2-4 g a.i./kg seed recommended rate for
cereals.
Residues for bendiocarb at harvest were <0.02 mg/kg for both
grain and straw, except for one 0.06 mg/kg value for straw. This
0.02 mg/kg level is approximately the claimed limit of determination.
Untreated controls were <0.04 mg/kg and <0.12 mg/kg,
respectively, for grain and straw. The 0.12 mg/kg for straw is from
the same study in which the high treated sample residue was found.
Contamination was suspected. Other values were <0.02 mg/kg.
Analytical recoveries from the individual studies were relatively
low, averaging only 63-65% for grain and straw at fortification levels
of 0.02-0.2 mg/kg. In a separate determination (Reary 1979b)
recoveries of bendiocarb in grains were better, averaging 84%. The
limit of determination for barley, oats and wheat is said to be
<0.02 mg/kg for grain and straw. Overall data support a 0.05 mg/kg
limit for bendiocarb per se.
Limited data were also available for combined conjugated residues
of NC 7312 and N-hydroxymethylbendiocarb. Residues were <0.01 and
<0.12 mg/kg for wheat grain and straw, respectively, although
analytical recoveries were only 62 and 67% in cereal grains and straw,
respectively.
Fodders and Straws
Fodders and straws of cereal grains have been discussed
previously. The other member of this group for which data were
provided is grass fodder, or more specifically turf and grass. Data
were available from 72 supervised trials in the U.K., U.S. and
Australia from 1975-1980, representing a variety of formulations,
single or multiple applications at several rates, intervals, and
methods of application as well as irrigated and unirrigated conditions
(Table 7) (Browne 1981d; FBC 1982c; Reary 1975d, 1978f,g, 1980b,d,e,k,
1981m; Reary and Whiteoak 1977a,b,c,d). No information was available
on specific national agricultural practices, although recommended
rates for rye grass is given in Table 1.
Granular treatments, both single and multiple broadcasts, were
mostly at application rates 6-12 times the recommended rate (for rye
grass). Three granular broadcast trials (single applications) at 1.6
times the recommended rate resulted in maximum residues ranging from
0.24 mg/kg at 43 days after treatment to 0.09 mg/kg after 55 days.
Residues were <0.1 mg/kg from three granular seed mix trials (single
application) at 0.6-1.4 times the recommended granular application
rate when analysed 196 days after treatment.
As in the case of granular treatments, single or multiple spray
treatments were mostly at rates 6-12 times the maximum recommended
application rate for rye grass. After single applications at rates
approximating recommended rates (1-1.6x), maximum residues from spray
treatments ranged from 103 mg/kg (193 mg/kg dry basis) at day of
application to 0.03 mg/kg after 141 days.
No recommended rates were available specifically for ULV aerial
applications. Residues from a single application at 0.28 kg/ha ranged
from 50 mg/kg (111 mg/kg dry basis) on day of application to 5.5 mg/kg
(12 mg/kg dry basis) after 29 days.
Seed dressing trials were conducted at treatments rates of 1-4
times the recommended rate of 10 g a.i./kg seed. Residues were
<0.2 mg/kg at all rates after 43 days.
Although many of the treatments with the various formulations
were at exaggerated application rates, these trials did permit
estimations of decline rates, the potential of residue accumulation
and effect of irrigation on residues. Residue half-lives were greater
for granular broadcast applications, for which 37-42 days was
estimated. For spray application half-lives ranged from 2-15 days,
depending on the trial. The half-lives were 5.3 and 2.4 days for seed
treatments and aerial ULV treatments, respectively. In general,
irrigation did not significantly affect decline rates from granular
broadcast or wettable powder spray treatments as compared to rates
measured under non-irrigated conditions. Residues did not accumulate
from multiple applications at 28-day intervals.
Table 7. Bendiocarb Residues in Grass and Turf from Supervised Trials
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 1 - 80W 1 kg/ha Spray Rye grass <1 130 1
1975 1-5 <109 3
6-10 <40 2
11-20 <15 2
21-50 <7 3
U.S. 1* 4.3 76W 2.24 kg/ha Spray Turf grass <1 <236 6
1976 1-5 <225 6
6-10 <115 4
11-20 <19 2
1* Thatch <1 <25 6
1-5 <26 6
6-10 <9 4
11-20 <1 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <326 6
1-5 <386 6
6-10 <190 4
11-20 <46 2
1* Thatch <1 <36 6
1-5 <97 6
6-10 <79 4
11-20 <1.6 2
1* 2-4 76W 2.24 kg/ha Spray Turf grass <1 <293 5
1-5 <201 4
6-10 <4 4
11-20 <2.8 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1* Thatch <1 <19 5
1-5 <13 4
6-10 <16 4
11-20 <21 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <607 5
1-5 <710 4
6-10 <30 4
11-20 <7.5 2
Thatch <1 <56 5
1-5 <18 4
6-10 <32 4
11-20 <26 2
1* 5G 2.24 kg/ha Broadcast Turf grass <1 <1.4 5
1-5 <2.7 4
6-10 <0.6 4
11-20 <0.3 2
1* Thatch <1 <13 5
1-5 <11 4
6-10 <18 4
11-20 <17 2
1* 5G 4.48 kg/ha Broadcast Turf grass <1 <3 5
1-5 <6 4
6-10 <8 4
11-20 <0.7 2
1* Thatch <1 <34 5
1-5 <42 4
6-10 <47 4
11-20 <27 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1977 2* 5-15* 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
21-50
51-80
81-110
111-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 5-15 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
11-20
21-50
51-80
81-110
110-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 76W Multi-spray2/ Turf grass 1-5 <16 3
6-10 <16 2
11-20 <9 2
21-50 <2 2
51-80 <0.9 2
81-110 <0.3 2
111-140 <0.1 2
>141 0.1 1
2* 76W Multi-spray3/ Turf grass 1-5 <24 2
6-10 <2 2
11-20 <3 2
21-50 <1.7 2
51-80 <1.1 2
81-110 <0.3 2
111-140 0.1 1
2* 76W Multi-spray4/ Turf grass 1-5 <37 4
6-10 <2.5 2
11-20 <3.4 2
21-50 <1 2
51-80 <0.4 2
81-110 0.1 1
2* 76W Multi-spray5/ Turf grass 1-5 <80 4
6-10 <80 4
11-20 <20 2
21-50 <3.7 2
51-80 0.5 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 37-42 5G Broadcast6/ Turf grass 1-5 <8 5
6-10 <3.3 2
11-20 <1.9 2
21-50 <3.7 2
51-80 <8.1 2
81-110 <2.6 2
111-140 <1.6 2
>141 <0.5 2
2 5G Broadcast1/ Turf grass 1-5 <7 5
6-10 <7 2
11-20 <1.4 2
21-50 <2.8 2
51-80 <0.16 2
81-110 <0.7 2
111-140 <0.55 2
>141 0.16 1
2 5G Broadcast2/ Turf grass 1-5 <9.5 4
6-10 <4.7 2
11-20 <4.4 2
21-50 <3.5 2
51-80 <0.7 2
81-110 <0.7 2
111-140 <0.7 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 5G Broadcast3/ Turf grass 1-5 <1.0 2
6-10 <9.0 2
11-20 <8.0 2
21-50 <4.0 2
51-80 <2.0 2
81-110 <0.8 2
111-140 0.8 1
2 5G Broadcast4/ Turf grass 1-5 <9.1 4
6-10 <8.2 2
11-20 <7.4 2
21-50 <7.6 2
51-80 <3.9 2
2 5G Broadcast5/ Turf grass 1-5 <7.3 4
6-10 <6.1 2
11-20 <5.7 2
21-50 <2.8 2
Australia 1 80W 1.6 g/kg seed Seed dressing Grass (pasture) 81-110 <0.02 2
1979 111-140 <0.02 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 3 80W 10 g/kg seed Seed dressing Grass (grazing) 21-50 0.09 1
1979 51-80 <0.06 2
3 80W 40 g/kg seed Seed dressing Grass " 21-50 0.17 1
51-80 <0.11 2
3 3G 1.2 kg/ha Broadcast Grass (grazing) 21-50 0.24 1
51-80 <0.09 2
3 50SC 1.2 kg/ha Spray Grass (grazing) 21-50 <0.2 3
2 50SC 10 g/kg seed Seed dressing Grass (grazing) >141 <0.01 3
1 50SC 15 g/kg seed Seed dressing " " >141 0.01 1
2 50SC 20 g/kg seed " " " " >141 <0.01 3
2 50SC 0.5 kg/ha Spray Grass (grazing) >141 <0.01 3
1 50SC 0.75 kg/ha Spray " " >141 0.03 1
2 50SC 1.0 kg/ha Spray " " >141 <0.015 3
U.S. 1 6 50SC 0.84 kg/ha Spray Wheat grass <1 103 1 193
1979 1-5 <97 2 <139
6-10 26 1 34
11-20 16 1 18
21-50 <9.3 2 <16
U.K. 2 5.3 80W 10 g/kg seed Seed Dressing Grass (grazing) 51-80 <0.02 2
1980 2 80W 15 g/kg seed Seed dressing " " 51-80 <0.03 2
2 80W 20 g/kg seed Seed dressing " " 51-80 <0.1 3
1 50SC 5×0.5 kg/ha Spray Grass (grazing) 51-80 <0.03 6
81-110 0.01 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 50SC 0.75 kg/ha Spray Grass (grazing) 51-80 <0.02 2
2 50SC 1.0 kg/ha Spray Grass " 51-80 <0.03 2
1 5G 0.75 kg/ha Seed mix Grass (grazing) 51-80 <0.08 2
1 3G 0.45 kg/ha Seed mix Rye Grass >141 <0.1 2
1 5G 0.75 kg/ha Seed mix Rye grass >141 <0.1 2
1 7G 1.05 kg/ha Seed mix Rye grass >141 <0.1 1
U.S. 1 2.4 25ULV 0.28 kg/ha Aerial Spray Rangeland grass <1 50 1 111
1980 1-5 5.36 2 <73
6-10 1.7 1 4
11-20 0.7 1 2
21-50 5.5 1 12
* Trials irrigated and non-irrigated. Residue levels maximum found.
1/ Rate of 4.48 kg/ha.
2/ Multi-application programme of 4.48 + 2.24 kg/ha, 28 days apart.
3/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha each 28 days apart.
4/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha, each 28 days apart.
5/ Multi-application programme of 4.48 + 2.24 + 2.24 + 4.48 kg/ha, each 28 days apart.
6/ Rate of 8.96 kg/ha.
Apparent bendiocarb in untreated grass controls were generally
<0.2 mg/kg, although they occasionally approached 1 mg/kg and were
1.8 mg/kg in one sample. The limit of determination was said to be
0.01-0.1 mg/kg, depending on the trial. Based on untreated controls,
1 mg/kg may be a more realistic limit of determination for grass.
Analytical recoveries during the trials ranged from 43 to 119% and the
mean for all the trials was approximately 80%.
Legume Animal Fodders
See discussion on pea pods under legume vegetables.
Oilseeds
Bendiocarb residue data on oilseed rape resulting from supervised
trials were available from one country where applications of a 5G
formulation was applied at sowing with the seed at application rates
of 0.140 and 0.28 kg a.i./ha which is the recommended rate for
granular formulations (Table 2) (Browne and Reary 1980f). No
information was available on specific nationally approved agricultural
practices.
Nine replicates were analysed for bendiocarb per se on mature
seed (114 days after application) at each rate with residues
<0.006 mg/kg. Samples were not analysed for conjugates of NC 7312
and N-hydroxymethylbendiocarb, although a metabolism study has shown
these to be the major metabolites in rape seed (see section on "Fate
of Residues in Plants"). Residues in untreated controls were also
<0.006 mg/kg with mean analytical recoveries of 84% for
fortifications at 0.05-0.5 mg/kg.
These data are inadequate for estimating a maximum residue level.
Additional data from granular applications as well as data from spray
treatments reflecting GAP are needed, as well as information on
nationally approved uses, including preharvest intervals and number of
applications.
Meat
Cattle
Residue data available from two feeding studies and two dermal
treatment studies are given in Table 8 (Browne and Reary 1978a,b;
Reary 1978a, 1979a, 1980c).
Table 8. Bendiocarb Residues in Milk and Cattle Tissues after Feeding Trials or Dermal Treatments1/
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
Milk
(non-conjugated2/
residues) <0.01 <0.01 <0.01 <0.01 <0.01 0.014 0.005 0.015 0.06 <0.02 <0.04
Milk
(conjugated3/ <0.02 <0.02 <0.02 <0.02 <0.02 0.027 0.027 0.039 0.057 <0.02 <0.02
residues)
Muscle4/ <0.02 <0.02 <0.02 <0.02 <0.02 - - - - <0.01 <0.01
Omental fat4/ <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.03
Perirenal fat4/ <0.05 <0.05 <0.05 <0.05 <0.02 <0.05 <0.04
Subcutaneous-
muscle4/ <0.01 <0.01
fat4/ <0.15 <0.12
Table 8. (con't)
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
liver4/ <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
kidney4/ <0.1 <0.1 <0.1 <0.1 <0.1 <0.05 <0.11
1/ Corrected for mean recoveries;
2/ Bendiocarb and non-conjugated NC 7312 and N-hydroxymethyl bendiocarb (fat fraction);
3/ Total conjugated NC 7312 and N-hydroxymethyl bendiocarb (aqueous or lactose fraction);
4/ Total residues of bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb;
5/ Analysed 6 hours post-treatment;
6/ 7-21 day self-treatment. Treatment rate estimated to be approximately 5 g product/cow/day.
In a feeding study (Browne and Reary 1978a), lactating dairy cows
were fed for a minimum of 28 days with a diet containing 0, 0.25, 0.75
and 2.5 mg/kg bendiocarb. A maize premix was the vehicle for adding
bendiocarb to the diet twice daily. Premises were stored at -4°C prior
to use. Each treatment group consisted of two cows. Milk samples were
pooled for both cows in each treatment group over 24 hours. Samples
were taken before treatment and after 1, 2, 4, 8, 12, 16, 22 and 28
days on the bendiocarb fortified diet. Milk extracts were separated
into a fat fraction (hexane/ether soluble) and a lactose fraction
(water soluble), the first of which was analysed for total
non-conjugated residues of bendiocarb, NC 7312 and
N-hydroxymethylbendiocarb. The lactose fraction was analysed for total
conjugated residues. Residues (bendiocarb equivalent in milk) in all
fat samples were less than the estimated 0.01 mg/kg limit of
determination, corrected for mean recoveries of 64%. Residues in the
lactose fraction were also <0.01 mg/kg, but the limit of
determination was considered 0.02 mg/kg because of chromatographic
interferences. Based on the chromatograms, 0.05-0.1 mg/kg may be a
more realistic limit of determination for the aqueous fraction when
interfering co-extractives are taken into consideration. In each case,
untreated controls gave no apparent residue above the estimated limits
of determination.
Samples of omental fat, perirenal fat, muscle, liver and kidney
were taken from each animal at sacrifice (within 18 hours of final
bendiocarb intake). Each was analysed for total residues of bendiocarb
and metabolites, conjugated and non-conjugated. No residue (corrected
for recovery) was found above the estimated limits of determination of
0.02 mg/kg for muscle and omental fat, 0.05 mg/kg for liver and
perirenal fat and 0.1 mg/kg for kidney. Muscle samples at the 0.25 and
0.75 mg/kg dietary feeding level did show apparent residues at the
0.02 mg/kg limit of determination as did one untreated control. Based
on representative chromatograms, the above estimated limits of
determination appear to be reasonable, except that a 0.2 mg/kg may be
more realistic for kidney.
Mean recoveries determined separately on unconjugated bendiocarb,
NC 7312 and N-hydroxymethylbendiocarb from cattle meat and fat tissues
were approximately 70%, with a standard deviation ranging from 10 to
22. The large standard deviations reflect rather poor recoveries,
especially of bendiocarb per se, (< approximately 50%) in a number
of muscle, kidney and liver samples. For example, recoveries of
bendiocarb averaged only 60% in muscle and kidney.
Another feeding study (Reary 1978a) was conducted in a similar
fashion but at higher dietary levels (7.5 and 25 mg/kg in the diet).
Maximum residues in milk were <0.01 mg/kg (limit of determination
for the fat soluble fractions) and <0.02 mg/kg for the aqueous
portion. As in the lower level feeding study, apparent residues of
total bendiocarb and conjugated and non-conjugated metabolites were
<0.02 mg/kg in muscle, <0.02 in omental fat and <0.05 mg/kg in
perirenal fat and liver. Residues were at the estimated 0.1 mg/kg
limit of determination for kidney (one control sample was also at this
level). This supports the view that 0.2 mg/kg is a more realistic
limit of determination for cattle kidney.
Analytical recoveries averaged approximately 70% with standard
deviations from 11-21%, reflecting rather poor recoveries. Average
recoveries were only 56% for bendiocarb in muscle and 52% for the
N-hydroxy metabolite in liver.
No information was provided on national GAP or recommended uses
for dermal applications to dairy or beef cattle. Results of
investigations for proposes uses for fly control by dermal
applications with a 1% bendiocarb dust formulation were provided. A 5g
dust treatment (50 mg a.i.) was described as a "normal" daily dose.
In one set of experiments (Reary 1979a) lactating dairy cattle
were manually treated at measured rates for safety evaluations or by
hessian bag self-treatment for efficacy evaluations. In the safety
evaluations, groups of three shorthorn cows were manually brushed on
the head, neck and shoulders with 0, 5, 15 and 25 g of the 1% dust
formulation; milk was analysed pre-treatment and six hours post-
treatment for each animal. As in the feeding studies, milk was
separated into fat and lactose fractions.
Non-conjugated residues in milk ranged from 0.003-0.015 mg/kg
(0.009 mg/kg mean) for all application rates, with no discernible
difference between application rates. Total conjugated residues ranged
from 0.019 to 0.039 mg/kg (0.028 mg/kg mean). Control means for the
safety evaluation trials were 0.006 and 0.032 mg/kg for non-conjugated
and conjugated residues, respectively. Limits of determination were
considered to be 0.02 and 0.05 mg/kg for the fat and aqueous
fractions, respectively.
In the efficacy trials, four herds were self-treated at an
estimated rate of 5 g of dust formulation/day/cow. Milk was pooled for
each herd at 7, 14 and 21 days after the beginning of treatment. For
the fat soluble non-conjugated fractions, residues were <0.017 mg/kg
at 7-21 days, except for one pooled milk sample from one herd at 7
days, for which a residue of 0.06 mg/kg was detected. Therefore, non-
conjugated residues did occur at and above the limit of determination.
Conjugated residues were <0.057 mg/kg, or just over the estimated
limit of determination. Samples used as untreated controls had mean
residues of 0.005 mg/kg and <0.028 mg/kg for fat soluble and
aqueous fractions, respectively.
In another dermal treatment investigation (Reary 1980c) groups of
three lactating dairy cows were manually treated on the head, neck and
back daily for seven successive days with a 1% dust formulation at 0,
5 or 25 g product/cow/treatment. Daily milk production from each of
two daily milkings for each cow was kept separate. Samples of muscle,
omental fat, perirenal fat, liver, kidney and subcutaneous fat were
taken within 18 hours after the final treatment.
Non-conjugated residues in milk (fat fraction) after correction
for analytical recoveries ranged from <0.01-0.04 mg/kg for 78
samples. All but six were <0.01 mg/kg. All residues >0.02 mg/kg
were from the higher treatment rate, except for one sample at
0.02 mg/kg. Comparable residues in 24 untreated controls were
<0.01 mg/kg, except for one sample at 0.02 mg/kg. All conjugated
residues (aqueous fraction) were <0.02 mg/kg and untreated controls
were <0.02 mg/kg. Mean analytical recoveries were 82% and 72% for the
milk fat and lactose fractions, respectively. The limit of
determination was considered to be 0.01 and 0.02 mg/kg for the fat and
lactose fractions, respectively.
In tissues, residues at both treatment levels were <0.01 mg/kg
in muscle, <0.03 mg/ kg in omental fat, <0.05 mg/kg in perirenal
fat, <0.01 mg/kg in subcutaneous muscle and <0.15 mg/kg (mean
0.08 mg/kg) in subcutaneous fat. Except for subcutaneous muscle, for
which there were no untreated controls, apparent residues in controls
for these tissues were _0.03 mg/kg, except for one 0.04 mg/kg sample of
subcutaneous fat. Residues from both levels were <0.05 mg/kg in
liver and <0.11 mg/kg in kidney. All residues >0.05 mg/kg in
kidney were from the higher treatment level. Apparent residues in
untreated controls were <0.03 mg/kg in liver and kidney.
An estimate of potential residues in meat, milk and meat by-
products must take into consideration all potential routes to animal
exposure, which include the dietary and dermal routes. Of the feed
crops for which residue data and good agricultural practice
information were provided, the greatest potential for residues in
cattle meat, fat and meat by-products and milk would be expected
from uses on maize (field corn). Assuming maximum residues are
<0.05 mg/kg in maize grain, forage and/or fodder, total conjugated
and non-conjugated residues of bendiocarb and metabolites would be
expected to be <0.05 mg/kg in cattle carcase meat, cattle fat and
cattle meat by-products (except kidney) and <0.01 mg/kg (claimed
limit of determination) in kidney, although a 0.2 mg/kg limit of
determination may be more realistic for kidney. Residues in milk would
not be expected to exceed the approximate limits of determination of
0.01 mg/kg and 0.02 mg/kg for non-conjugated and conjugated residues,
respectively, or a total of 0.03 mg/kg. These estimates should also be
adequate from feeding of rice.
Data from the use of bendiocarb on grasses or as a dermal
treatment on cattle suggests a possible need for higher limits than
would be required for maize grain, forage and fodder if such uses are,
or should become, good agricultural practice. Additional information
on good agricultural practices are necessary before maximum residue
estimates can be made for these uses. Any additional information
should provide the formulation, application rate, number of
applications, type of application and preharvest intervals or other
restrictions. Depending on these uses, feeding studies at higher
levels may be necessary before maximum residue levels can be
estimated.
Poultry meat, meat by-products and eggs
Laying hens in groups of ten were fed (free access) a diet
containing 0, 0.05, 0.15 and 0.5 mg/kg bendiocarb for 21 days to
permit estimation of potential residues resulting from the feeding of
maize grain, for which maximum residues of 0.05 mg/kg are assumed
(Reary 1978b). Owing to analytical difficulties, information on the
stability of residues over the feeding period was not provided.
Information was not provided on storage conditions of the fortified
pre-mix.
Eggs were individually collected and labelled; 98 were analysed
at higher feeding levels and later sampling intervals. Apparent total
residues (corrected for recovery) of bendiocarb and metabolites
(conjugated and non-conjugated) at all treatment levels were
<0.02 mg/kg 1-21 days after treatment. The limit of determination was
reported as approximately 0.02 mg/kg, supported by sample
chromatograms, although pre-treatment controls were <0.013 mg/kg.
Analytical recoveries of bendiocarb and unconjugated metabolites
ranged from 44-85% (67% av.).
Samples of breast muscle, leg muscle, liver, kidney, subcutaneous
fat and heart were taken after sacrifice on day 21 of feeding the
fortified diet, with completion of dissection the following day. The
sum of bendiocarb and its major metabolites conjugated and non-
conjugated, was determined. No hen tissue residues were found in
excess of the claimed limits of determination of 0.02 mg/kg for legs,
breast, liver and subcutaneous fat and 0.05 mg/kg for kidney and
heart. Sample chromatograms tend to confine these limits of
determination, although apparent residues near the claimed limits of
determination in untreated controls of breasts (<0.016 mg/kg),
liver (<0.014 mg/kg) and fat (<0.016 mg/kg) suggest that
0.05 mg/kg may be a more practical limit of determination for each of
the tissues.
As in cattle tissues and eggs, analytical recoveries were only
marginally acceptable, ranging from 40-98% for bendiocarb or its major
non-conjugated metabolites. Mean residues of bendiocarb and its non-
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) ranged
from only 56% in chicken legs to 79% in fat. Improved analytical
methodologies are needed for commodities of animal origin.
Mushrooms
Supervised trials were conducted in The Netherlands and the U.K.
In The Netherlands mushroom plots were presumably sprayed directly
with a bendiocarb 50SC formulation and in the U.K. the plots were
established the day following wall treatments with either a 50SC or
80W spray. No information was provided on approved agricultural
practices for these uses, including preharvest intervals, although
recommended wall spray application rates of 30-50 g/100 m2 (Table 2)
were provided.
In the first trials (Browne 1981a) a 50SC bendiocarb formulation
was sprayed twice at rates of 50, 75 and 100 g a.i./100 m2 (5, 7.5
and 10 kg a.i./ha), first after spawning and the second after casing.
Harvest was 21 days after the last treatment.
Residues of bendiocarb and non-conjugated NC 7312, corrected for
mean recoveries, are summarized in Table 5. Maximum residues were
0.013 mg/kg and 0.04 mg/kg, respectively, for bendiocarb and NC 7312
at the highest application rate. Residues increase with increased
application rate and residues of NC 7312 tend to be less than those of
bendiocarb under the test conditions.
In the series of trials in the U.K., (Browne, 1982a) mushroom
plots were established the day following hut wall treatments with
either 50SC or 80W bendiocarb formulations at application rates of
20-53 g a.i./100 m2. Harvest was 26-69 days after the treatments,
which reflected the recommended application rates. Bendiocarb residues
ranged from <0.02 to 0.09 mg/kg and NC 7312 from <0.02 to
0.13 mg/kg, both corrected for recoveries (see Table 5). In contrast
to the trials discussed above, residues of NC 7312 in these trials
were of the same order of magnitude as for bendiocarb.
The limit of determination for bendiocarb in mushrooms was
reported to be 0.01-0.02 mg/kg for bendiocarb or NC 7312, depending on
the trials. Based on apparent residues of 0.04 mg/kg of bendiocarb and
0.02 mg/kg of NC 7312 in more than one untreated control, limits of
determination of 0.05 mg/kg may be more practical. Analytical
recoveries averaged approximately 82% for bendiocarb and 73% for non-
conjugated NC 7312 from fortifications of both at 0.04-0.2 mg/kg,
although standard deviations were 14-19.
As no information was available even on recommended uses for
bendiocarb other than for wall treatments, data from wall treatments
are more pertinent to the estimation of maximum residue levels. Based
on recommended application rates, the available residue data are
consistent with a maximum residue level of 0.1 mg/kg for bendiocarb
per se and 0.2 mg/kg if NC 7312 is also included. This is based on the
assumption of minimum application to harvest intervals. Information on
nationally approved agricultural practices for bendiocarb use on
mushrooms is needed. Additional data from supervised trials are
desirable.
FATE OF RESIDUES
Metabolism studies have been carried out in a range of plants and
animals and in soil. In most plant species examined, the major
metabolites formed are glycoside conjugates of the phenol NC 7312 and
N-hydroxymethyl bendiocarb. Metabolism in animals generally involves
cleavage of the carbamate ester group to yield NC 7312, which is
excreted as sulphate and beta-glucuronide conjugates. In soil under
aerobic conditions, the only extractable radioactivity is unchanged
bendiocarb, degradation occurring to CO2, and an unidentified bound
residue. Under anaerobic conditions, bendiocarb is hydrolysed to
NC 7312.
In animals
For discussions on the metabolic fate of bendiocarb in the rat,
dog, hamster, rabbit, mouse and humans, see "Biochemical Aspects".
Residue feeding and/or dermal studies on cattle and/or poultry have
been discussed under "Residues from Supervised Trials". In addition to
the aforementioned studies, the metabolic fate of bendiocarb has been
investigated in the pig, cow and hen.
Pig
Pigs were orally dosed once by gelatin capsule at 0.2 mg/kg/bw
with 14C-bendiocarb (Adcock et al 1976b). Urine samples were
collected over a period of four days, after which the animals were
sacrificed and samples of liver, kidney, muscle, perirenal fat and
subcutaneous fat were taken.
Seventy-eight percent of the administered dose was excreted in
the urine as equal levels of sulphate and ß-glucuronide conjugates of
NC 7312 within one day and 84% within four days. No residues of
unchanged bendiocarb were detected. Corresponding residues in faeces
were 2.4 and 7.2% for a total recovery of 91.5% of administered
radioactivity recovered in urine and faeces after four days. The
residues in faeces were not identified. No residues were found in
tissues at the 0.01 mg/kg limit of sensitivity for bendiocarb
equivalents. The finding of relatively large levels of NC 7312
sulphate was of considerable interest, since phenolic sulphates are
generally minor in pigs.
The metabolism is similar to that in humans and the rat,
involving cleavage of the carbamate ester to yield NC 7312, which
becomes conjugated as sulphates and glucuronides.
Cow
A lactating dairy cow was given 14C-bendiocarb by a single
gelatin capsule at a dose of 0.2 mg/kg/bw (Adcock et al 1976a).
Urine was collected at intervals of 4-96 hours after dosing and faeces
every 24 hours. Milk was collected morning and evening until sacrifice
on the fourth day, at which time samples of liver, kidney, muscle,
heart, perirenal fat, omental fat and subcutaneous fat were taken for
analysis. Milk was separated into lactose, fat and casein fractions.
No residues of unchanged bendiocarb were found in the urine.
Seventy-nine percent of the administered radioactivity was excreted in
the urine within eight hours and 92% after two days, after which
little was excreted in urine. Corresponding residues in faeces were
5.3% at one day and 12.3% after two days. The urinary metabolites were
determined to be sulphate and glucuronide conjugates of NC 7312 at 90%
and 10% each, respectively. Residues in faeces were not identified.
Bendiocarb equivalents were found in milk the first day up to a
level of 0.002 mg/kg, but none were detectable thereafter. Over 80%
was in the lactose fraction, 2% in the hexane fraction and most of the
remainder in the casein fraction. Tissue residue levels were below the
0.01 mg/kg limit of sensitivity as bendiocarb equivalents.
Metabolism in the cow appears to be similar to that in the rat,
human and pigs.
Poultry
Three laying hens were each dosed once with 14C-bendiocarb by
oral gavage at 0.25 mg/kg/bw (equivalent to 5 mg/kg in the diet) and
excrement and eggs collected at intervals from 24-96 hours after
dosing. Liver, kidney, muscle and fat samples were taken at sacrifice
four days after treatment (Lewis and Challis 1978).
Sixty-one to 90% of administered radioactivity was excreted
within 24 hours, and 73-91% within 4 days. Recovery was inexplicably
low (73%) in two of the hens. Losses were presumed to be due to
expiration and/or losses during dosing or excrement collection, since
residues in tissues were low. However, as a percent of radioactivity
excreted in the first 24 hours, residues were 10-57% free NC 7312,
26-63% NC 7312 conjugates, 12-19% unidentified unhydrolysed water
soluble residues, 2-5% other non-polar metabolites and 1-3% other
unidentified conjugates. Conjugation of NC 7312 occurred more rapidly
in the hen in which radioactivity excretion was more rapid and
complete.
Radioactivity in egg white and yolk represented only 0.01% of the
administered dose. The maximum bendiocarb equivalent was highest in
egg white on the first day (max. 0.009 mg/kg) and on day four in yolk
(0.003 mg/kg), both above the limits of sensitivity. The maximum
bendiocarb equivalent was 0.004 mg/kg in liver and fat, 0.002 mg/kg in
kidney and below the 0.001 mg/kg limit of sensitivity in leg and
breast muscle.
In Plants
Metabolism studies have been carried out in sugarbeet, maize,
rice, barley and oilseed rape, both in the laboratory and under field
conditions. A variety of separative, determinative and conjugative
hydrolysis steps were utilized. In all these crops, the major
identified metabolite was the phenol NC 7312, with lower levels of
N-hydroxymethylbendiocarb, both primarily as glycoside conjugates. In
some of the crops studied, sampling at harvest showed small quantities
of unconjugated N-hydroxymethylbendiocarb.
Three metabolism studies were conducted in sugarbeets. In one
study, radioactive bendiocarb was applied under greenhouse conditions
to the potted soil or to the leaves by direct syringe applications
(Challis 1975). Residues were characterized in the plant tops only.
Metabolic products were qualitatively similar in both cases,
resulting in residues of free bendiocarb, bendiocarb glycoside,
free NC 7312 (foliar application only), NC 7312 glycosides,
N-hydroxymethylbendiocarb, N-hydroxymethylbendiocarb glycosides,
unidentified glycosides, unidentified water soluble residues and
unextractable unidentified fibre-bound residues.
The major metabolites from soil application were glycoside
conjugates of NC 7312 and N-hydroxymethylbendiocarb. Trace amounts of
free N-hydroxymethylbendiocarb were also found. Unidentified residues
accounted for 36 and 67% of radioactivity 8 and 34 days after
treatment, respectively, with a ratio of about 1:3 water soluble to
fibre-bound residues.
From foliar applications, over 93% of leaf-applied bendiocarb was
present on the leaf surface after 34 days. A bendiocarb glycoside was
the major metabolite. Unidentified residues accounted for 48% of the
total residue after eight days, but this was reduced to 25% after 34
days. Low levels of free NC 7312 were also present. The proposed
metabolic pathway for bendiocarb in sugarbeet tops is illustrated in
Figure 1. Except for the omission of substantial at-harvest
Molecular formula
C9H10O3
Other information on identity and properties
Molecular weight 166
State White crystalline solid
Melting point 91-93°C
Solubility At 25°C 20 g/l in water and
50 g/l in dichloromethane; very
soluble in methanol, ethanol and
acetone.
Vapour pressure 2.34 × 10-3mm Hg at 25°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
In groups of male mice (CFLP strain) acutely intubated with
14C-bendiocarb (14C-bendiocarb with label at the heteroclyclic ring
was used in this and all of the other available metabolism studies on
bendiocarb) at 1, 5 or 10 mg/kg body weight, over 80% of the
administered dose was excreted in 24 hours, primarily in the urine.
Less than 10% of the administered 14C was recovered in the faeces.
The proportion of metabolites formed in the urine, but not the rate
and route of elimination, was dose-dependent. Thus, the level of NC
7312 (2,2-dimethyl-1,3-benzoxodiol-4-ol) (as conjugates) recovered in
the urine was 36, 32, 17 (as % of administered radioactivity),
respectively, at 1, 5, 10 mg/kg. Only one urinary metabolite other
than conjugated NC 7312 was detected, which was believed by
the authors of the report to be probably a conjugate of
6-hydroxybendiocarb (Challis and Adcock 1977a).
Another similar mouse study with groups of males of the CD-1
strain treated orally with single doses of 14C-bendiocarb at 1, 5 or
10 mg/kg body weight indicated recovery of about 80% of the
administered radioactive dose in 24 hours, predominantly in the urine.
A significant dose-dependent relationship was not evident with respect
to rate and route of elimination or metabolic profile of bendiocarb.
About 33-56% of the administered 14C-doses was recovered in the urine
as conjugates of NC 7312. Conjugated 6-hydroxy bendiocarb was believed
by the authors of the study to be the only other major urinary
metabolite (Pearce et al 1977).
Rat
Male and female rats treated with a single oral dose of
14C-bendiocarb at approximately 10 mg/kg body weight eliminated over
95% of the administered radioactivity in urine (89%), expired air (6%)
and faeces (2%) within 2 days of dosing. There was no significant sex
different in rate and route of elimination. Following an acute oral
dose of 14C-bendiocarb at 1 mg/kg body weight, plasma levels in male
and female rats peaked at 10 minutes post treatment with plasma half-
life being calculated at 4.4 hours for both sexes. The major urinary
metabolite was the phenol (NC 7312) which, excreted as ß-glucuronide
and sulphate conjugates, accounted for more than 85% of the
administered dose recovered in the urine during the first 24 hours
after treatment. The remaining 15% eliminated over this time period
comprised a mixture of sulphate and ß-glucuronide conjugates of at
least seven minor metabolites. One of the latter appeared to be
N-hydroxy methyl bendiocarb. No unchanged parent compound was found in
the urine. In the faeces free NC 7312 was the primary metabolite with
a small amount of 14C-bendiocarb being detectable (Adcock and Challis
1976a).
Conjugated NC 7312 was found to be the primary metabolite in
urine collected during 0-24 hours after treatment withdrawal in male
and female rats (CFY strain) fed 20 ppm of 14C-bendiocarb in the diet
for 10 days. At sacrifice 6 days after termination of dietary feeding,
detectable 14C residues were found in the tissues analysed (fat,
liver, kidney, muscle and brain) with the fat tissue having the
highest residue level. According to the partition characteristics of
the 14C residue in fat into organic solvents, radioactive residue in
fat was probably not mainly due to bendiocarb or NC 7312 (Pearce and
Adcock 1978).
Hamster
Groups of 6 or 10 male hamsters were intubated with
14C-bendiocarb in glycerol formal at 1, 5 or 10 mg/kg. Four female
hamsters were similarly treated at 10 mg/kg body weight. Over 90% of
the administered 14C-dose was recovered in 24 hours in the faeces and
the urine, with the latter being the primary route of elimination. The
rate and route of excretion was not modified by sex or dosage level.
The proportion of total radioactivity recovered in the urine as
conjugates of NC 7312 was about 37% at 1 mg/kg, 16% at 5 mg/kg and 19%
at 10 mg/kg. Besides conjugated NC 7312, urine of male hamsters
contained two metabolites in approximately equal amounts, while that
of female hamsters had only one radioactive component. A conjugate of
6-hydroxy bendiocarb was believed by the authors to be the major
urinary metabolite in both males and females. The other urinary
metabolite in male hamsters had not been identified. The seeming sex
difference in metabolic fate of bendiocarb remains to be ascertained,
since the male and female hamsters used in the study were housed and
dosed at different laboratories (Challis and Adcock 1977b).
Rabbit
Groups of pregnant rabbits were intubated on day 20 of gestation
with one dose of 2.5 or 10 mg/kg body weight of 14C bendiocarb.
Within 24 hours of dosing, nearly 90% of the administered dose had
been eliminated, primarily in the urine, with only a small amount
(<2.5%) being detected in the faeces. The rate and route of
elimination and metabolic profile of bendiocarb appeared to be
independent of dosage. The major urinary metabolite was identified as
NC 7312, which accounted for 90% of the radioactivity extracted from
urine samples following enzyme (sulphatase and ß-glucuronidase)
hydrolysis (Challis 1980).
Dog
Groups of one male and one female beagles (fasted overnight and
for 8 hours following dosing) were treated once with 14C-bendiocarb
in gelatin capsules at 0.1, 1.0 or 10 mg/kg body weight. Regardless of
dosage level, the radioactive dose was almost completely excreted
during the first 24 hours post-dosing, primarily in the urine. The
amount of radioactivity found in the urine of both sexes over the
48-hour period seemingly decreased with dosage (about 90% at 0.1 mg/kg
body weight, 80% at 1.0 mg/kg body weight and 70% at 10 mg/kg body
weight). This appeared to be accompanied by a corresponding increase
in faecal recovery (about 19%, 21% and 24%, respectively, at 0.1, 1.0
and 10 mg/kg body weight). In the urine, approximately 84%, 67% and
43% of the administered radioactivity, respectively, at 0.1, 1.0 and
10 mg/kg body weight occurred as sulphate and ß-glucuronide conjugates
of NC 7312 with no recovery of any 14C-bendiocarb. Analysis of urine
samples from male dogs at 10 mg/kg body weight indicated the probable
presence of ring hydroxylated derivatives of bendiocarb as minor
metabolites. Unchanged 14C-bendiocarb was the only component
identified in the faeces and amounted to about 80% of the
radioactivity recovered via this route (Warner et al 1977).
Effects on Enzymes and Other Biochemical Parameters
Mouse
In male and female mice (Charles River CD-1 strain) fed technical
bendiocarb containing 0.09% w/w of dimethoate) in their diets at 0 or
1 250 ppm for 19 days, whole blood cholinesterase in blood sampled
between 06:00 hour and 08:00 hour, but not between 09:00 hour and 11
hours (peak feeding period stated to occur between 22:00 hour and
09:00 hour) was depressed (>20%) only in treated males in weeks 2 and
3 (Hounsell and Rush 1980).
Male CFLP mice and male CD 1 mice were fed dietary levels of
technical bendiocarb at 0, 500 or 1 000 ppm for up to 7 days. In the
CFLP strain, whole blood cholinesterase activity was reduced (>20%)
at 1 000 ppm in animals fed the diet ad libitum and in those
following a 1-hour feeding period on the 7th day after an overnight
fast. In the CD-1 mice, whole blood cholinesterase was depressed
(>20%) in the 500 and 1 000 ppm groups following an overnight fast
and exposure to the diet for 1 hour on day 7. Ad libitum feeding
resulted in decreased whole blood cholinesterase in the 500 but not in
the 1 000 ppm groups (Hounsell 1981a).
Rat
Groups of rats (sex unspecified) were treated orally with
4 mg/kg/day of technical bendiocarb in glycerol formal for three
consecutive days. Inhibition (65-85%) of cholinesterase in whole blood
and plasma peaked at 10 minutes after each dose on days 1 and 3.
Recovery of whole blood and plasma cholinesterase began to occur 30
minutes following each dose, and was practically complete three days
after treatment withdrawal. Whole blood cholinesterase appeared to be
more sensitive than plasma cholinesterase to the inhibitory effect of
bendiocarb (study cited by Kemp and Hounsell 1974).
Male rats (CFY Sprague-Dawley) were fed dietary levels of
technical bendiocarb up to 10 ppm for 14 days, followed by an
overnight fast. Whole blood cholinesterase, assayed in the morning of
the 15th day after a 1-hour feeding period, was not significantly
inhibited (<20%) (Hounsell 1981b). Another similar study with the
same strain and sex of rats fed diets containing technical bendiocarb
for up to 23 days indicated that, following overnight fasting and a
one-hour feeding period, animals exhibited a depression (20%) of whole
blood cholinesterase at 50 ppm on days 15 or 16 and 22 or 23 (Hounsell
1981c).
Dog
In 4 male beagles (fasted the day before the test) fed a diet
containing 1 000 ppm of technical bendiocarb for up to one hour
(actual intake of the test material estimated at 6 - 8.6 mg/kg body
weight), inhibition (>50%) of brain and whole blood cholinesterase
was apparent 15 minutes after the feeding period. Whereas depression
of whole blood cholinesterase peaked at 2 hours and was completely
reversible at 3 hours following the feeding period, the extent of
inhibition of brain cholinesterase remained fairly constant between
15 minutes and 3 hours (the last sampling interval) after the dosing
period (Hounsell 1974). Another experiment with 2 female beagles
(24-hour fasted) fed a dietary level of 10 ppm of technical bendiocarb
for about 10 minutes (actual intake of the test material estimated at
approximately 32.4 mg/kg) showed reduction of whole blood
cholinesterase activity of about 27% and 52% of the pretreatment level
at 1 hour and 6 hours, respectively, post dosing. Cholinegesic
symptoms were noted within 24 minutes of the initiation of feeding.
Complete recovery of whole blood cholinesterase activity and from
toxic signs occurred 24 to 25 hours after treatment (Kemp and Hounsell
1974).
Cow
A lactating dairy cow was fed technical bendiocarb in the daily
diet at 3.6 ppm for two consecutive days (ration was offered twice
daily). No reduction (<20%) in whole blood cholinesterase was
observed at any of the 8-hourly intervals following the morning
feeding on both days (Roberts 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 17 to 21 mated rats (Sprague-Dawley CFY strain) were
fed diets containing technical bendiocarb at 0, 200, 400 or 800 ppm
from 14 days post-coitum until weaning of their pups. A decrease in
maternal body weight gain was noted at 800 ppm on days 17 and 20 post-
coitum and practically throughout the 21-day lactation period. At this
dietary level, survival and weight gain of pups were depressed
throughout lactation. No treatment-related effects were observed on
other parameters monitored, i.e. mortality and clinical signs of
pregnant dams, sex ratio and gross abnormalities of offspring (Jackson
1978).
Groups of 10 males and 20 female rats (SPF, CFY strains) were fed
dietary concentrations of bendiocarb (presumably technical grade) at
0, 200, 400 or 800 ppm for one week prior to mating. The females were
maintained on test diets through day 18 of gestation and the males
until termination. There were no mortalities or toxic symptoms. Growth
was slightly depressed in males at 800 ppm and in females at 400 ppm
and above. Pregnancy rate, duration of gestation period and terminal
gross pathology were not adversely affected. Medium precoital time (as
an indication of mating performance) of dams was lengthened at both
400 and 800 ppm. In the progeny generation, except for a slight
decrease at 400 ppm and above in mean pup weight at birth, no apparent
dose- or treatment-related effects were evident on litter size at
birth, survival and weight of pups through lactation period to
weaning, sex ratio and incidence of gross abnormalities of weanlings
(Palmer and Allen 1978).
Groups of 30 males and 30 females (Charles River CD strain) were
fed dietary levels of technical bendiocarb at 0, 10, 50 or 250 ppm for
90 days prior to mating to initiate a reproduction study covering
three generations with two litters per generation. Weanlings from the
second litters were selected to become parents of the next generation.
After the second mating trial in each generation, the dams in each
group were divided into two approximately equal sub-groups. One
sub-group was allowed to deliver and rear their young until 25 days
post-partum. Another sub-group was sacrificed on day 21 of gestation
for examination of uterine contents. Foetuses were examined for
external, skeletal and soft tissue malformations.
In the parental generations, no mortality or clinical signs
attributable to the compound were evident. Dams displayed slight
growth depression during the gestation period of F1a litters at
50 ppm and above and of F2b litters at 250 ppm. Food consumption was
normal, but water intake was slightly decreased at 250 ppm in F1
females. Males of the F2 generation seemed to exhibit an increase in
incidence and severity of "geriatric nephropathy" at and above 50 ppm.
Ophthalmoscopic examination conducted on F2 adults between 31 and 35
weeks of age revealed no treatment-related findings. At terminal
sacrifice of parental animals after weaning of the second litters,
some variations from controls in absolute weights and/or organ/body
weight ratios of certain tissues such as adrenal, pituitary, thyroid,
seminal vesicles and uterus were noted at both 50 and 250 ppm, but
these observations were not consistently present through the
generations and were not accompanied by histopathological lesions of
the organs. Mating and fertility indices as well as length of
gestation period were not modified by bendiocarb. There was an
increase in frequency of F0 females being acyclic or pseudopregnant
at 250 ppm and of F2 females with irregular oestrous cycles at 50 ppm
and above, prior to the first mating trial in both cases. Pre-coital
interval was lengthened at 250 ppm in the F0 generation (first
mating).
Over the three progeny generations, the viability index was
slightly depressed in F1a litters at 250 ppm from days 10 through 25
and in F2b litters at 50 ppm and above on day 25. Pup weight was
reduced at 250 ppm in F1a and F1b litters on day 25 and in F2b
litters at birth through day 25. In F2a litters, pup weight on day 25
was decreased in all treated groups, but the difference was indicated
by the authors of the report to be statistically significant only at
10 ppm, but not above. There were no significant differences between
control and treated groups in litter size (at birth and on day 1), sex
ratio, ophthalmoscopic observations of F3b pups between 19 and 31
days of age, organ weight and histopathological findings of 25-day old
F3b pups. Examination of 25-day old pups from the second litters of
each generation for rate of physical development and sensory functions
revealed "the onset of pinna unfolding, hair growth, tooth eruption
and eye opening was marginally delayed in all treated (F1b) litters,
but the overall pattern of physical development was essentially
similar in all groups, and "the onset of pinna unfolding and hair
growth was marginally delayed (in F2b litters at both 50 and 250 ppm)
but eye opening and tooth eruption was unaffected".
Teratological investigation of F1b, F2b and F3b litters showed
no significant differences between control and treated groups with
respect to number of implantations, number of viable young, early or
late resorptions, post-implantation loss and foetal weight. A rise in
pre-implantation loss in F1b litters was noted at 250 ppm. Also at
the top dosage level, there were higher incidences of generalized
subcutaneous oedema, incomplete ossification of cranial bone,
uni/bilateral hydronephrosis and bilateral ureter in F1b litters and
of subcutaneous scapular haemorrhage in F3b litters, as compared to
concurrent controls. Based on the data, a dietary level of 10 ppm was
without significant adverse effect on the reproductive parameters
evaluated. The teratogenic "no effect" level was demonstrated to be
50 ppm (Tesh et al 1981).
Special Studies on Teratogenicity
(also see under "Special Studies on Reproduction")
Rat
Groups of 24 mated rats (Sprague-Dawley derived, CD strain) were
intubated with technical bendiocarb as a suspension in gum tragacanth
at 0, 0.25, 1 or 4 mg/kg body weight/day between gestation day 6 and
day 15 (day 0 = day of positive vaginal smear). The dams were
sacrificed on gestation day 21 and foetuses were removed by caesarean
section for external, visceral and skeletal examination. There was no
mortality. Dams at 4 mg/kg body weight/day exhibited toxic symptoms
characteristic of cholinesterase inhibition and slight growth
inhibition on gestation day 21. Two dams (out of 21) at 4 mg/kg body
weight/day and 1/22 dams at 1 mg/kg body weight/day displayed a large
number of late uterine deaths. Mean litter size was depressed at
4 mg/kg body weight/day. There were no significant differences between
control and treated groups in pregnancy rate, number of corpora lutea
or implantation sites, number of early resorptions, foetal weight and
frequency of foetal abnormalities. The study demonstrated bendiocarb
to be non-teratogenic under the conditions of the experiment (Tucker
1974).
Rabbit
Groups of 27-29 female rabbits (New Zealand White) that had been
artificially inseminated were intubated with technical bendiocarb as a
suspension in aqueous gum tragacanth at 0, 1, 2.5 or 5 mg/kg body
weight/day from day 6 to day 28 inclusive of gestation. (Day 0 = day
of insemination.) The does were sacrificed on day 29 of gestation and
uterine contents were examined. Foetuses were subjected to evaluation
for external, skeletal and internal malformations. Six to eight
females per control or treated group died 6 to 29 days after
insemination mainly due to "tracheal intubation". Clinical signs such
as increased respiratory rate and prostration were noted at and above
2.5 mg/kg body weight/day. Growth was slightly depressed at 5 mg/kg
body weight/day between gestation days 16 and 28. Whole blood
cholinesterase assayed 30 minutes post-dosing on day 28 was depressed
(31-70%), in a dose-dependent manner, in all treated groups. One doe
each at 2.5 and 5 mg/kg body weight/day aborted on day 22 and 20,
respectively. Premature parturition occurred in one doe each of 2.5
and 5 mg/kg body weight/day groups on day 28. An increase was observed
in the mean number of late resorptions per litter and the incidence of
pregnant does with late resorptions at both 2.5 and 5 mg/kg body
weight/day. Post-implantation loss (number of implantations - number
of viable foetuses/number of implantations × 100) was elevated at
2.5 mg/kg body weight/day and above.
Other parameters monitored, including number of corpora lutea,
implantations, viable young, or early resorptions, pre-implantation
loss, foetal weight and placental weight, were not adversely affected
by treatment. There was a dose-related increase in incidence of
foetuses with eye anomalies (mainly "cloudy areas in eyes") and with
absence of pubic bones at both 2.5 and 5 mg/kg body weight/day.
Incidence of litters containing foetuses showing such abnormalities
was also elevated at and above 2.5 mg/kg body weight/day. In the
absence of background data on frequency of the abnormalities in
question, 1 mg/kg body weight/day may be considered as a clearcut "no
effect" level with respect to teratogenicity (Tesh et al 1980).
Special Studies on Mutagenicity
Two separate experiments were conducted with technical bendiocarb
and NC 7312 to evaluate their genetic activity in vitro microbial
systems (plate assays), with or without the addition of a mammalian
metabolic activation preparation (S-9 mix from the liver of male rats
pre-treated with Aroclor 1254 i.p. at 500 mg/kg/day for five days).
Indicator organisms used were Salmonella typhimurium strains TA
1535, TA 100, TA 1537, TA 1538 and TA 98. Results suggest
non-mutagenicity of the test compounds to any of the tested strains at
the (non-toxic) concentrations used (3.3-1 000 µg/plate of bendiocarb
and 10-3 300 µg/plate of NC 7312) in the presence or absence of
metabolic activation and under the conditions of the assays
(McConville and McGregor 1979; McConville and Harris 1979). Negative
results were also obtained in another reversion mutation test using
Escherichia coli WP2 and five strains of the Salmonella
typhimurium TA series as indicator organisms in the presence of
absence of a liver metabolic activation system at concentrations of
technical bendiocarb ranging from 5 to 1 000 µg/plate (Moriya
et al 1981).
A rec-assay conducted with Bacillus subtilis H17 (rec+) and
M45 (rec-) in the absence of a metabolic activation preparation
indicated that technical bendiocarb, at concentrations of 20 to
5 000 µg/disc, did not induce any inhibitory zone in either tested
strain (Moriya et al 1981).
Technical bendiocarb was evaluated for its mutagenic activity in
a mouse lymphoma L5178Y specific locus mutation assay. Under the
conditions of the test, there appeared to be no consistent indications
of mutagenicity of the compound in the absence or presence of a
metabolic activation system (S-9 homogenate of liver from male adult
Fischer rats induced with Aroclor 1254) at concentrations ranging from
5 to 20 µg/ml (McGregor and Ross 1981).
In a micronucleus test, groups of male mice were treated with
technical bendiocarb i.p. at 0, 0.625, 1.25 or 2.5 mg/kg body
weight/day for two consecutive days (the two doses were given 24 hours
apart). The animals were sacrificed 6 hours after the second dose and
the femurs were removed for the preparation of bone marrow smears.
There was no significant increase in the frequency of polychromatic
erythrocytes containing micronuclei or change in polychromatic/
normochromatic ratio in any treated group (Hounsell and Walker 1982).
Groups of 20 sexually mature virgin male rats (Sprague-Dawley CD
strain) were fed diets containing technical bendiocarb at 0, 10, 50 or
250 ppm for 13 consecutive weeks and then mated with sexually mature
virgin female rats for seven days in a dominant lethal assay. (Each
group was sub-divided into sub-groups A and B with commencement of
dietary feeding of the groups being staggered by one week.) The
females were sacrificed 14 ± 1 days after mating and autopsied for the
examination of uterine contents. The treated males exhibited no
clinical signs and their body weight and food consumption were not
adversely affected by treatment. The incidence of non-fertile males
were 1/20 at 50 ppm and 2/20 each at 10 ppm and 250 ppm. With the
exception of one infertile male at 250 ppm, abnormally small testes
and epididymis were found in the other four infertile males. An
increase (not dose-related) in incidence of litters with at least one
early death was observed (in sub-group B only) at 10 ppm and 250 ppm,
but this was not accompanied by a rise in the number of early deaths
per litter. There were no significant differences between control and
treated groups with respect to pregnancy rate, number of corpora
lutea, number of total implants, number of viable implants and pre-
implantation loss. Bendiocarb did not appear to induce dominant lethal
mutation under the experimental conditions used (Kemp and Jackson
1977).
Special Studies for Carcinogenicity
Mouse
Groups of 50 males and 50 females (HaM/ICR Swiss mice CD-1; about
35 days old) were fed diets containing technical bendiocarb, found to
contain up to 0.5% dimethoate or dimethoate decomposition products, at
50, 250 or 1 250 ppm. The control group consisted of 100 males and 100
females. The experiment was originally scheduled to be terminated
at a predetermined survival level or 104 weeks at the latest. The
predetermined level was reached after 89 weeks in males and 94 weeks
in females. Mortality rate was increased in females at 1 250 ppm after
85 weeks. Less than 40% of animals in control and treated groups were
still alive at termination, but over 50% of animals of all groups,
including the control, survived at least 82 weeks. All animals that
died or were sacrificed in moribund condition during the study and
those sacrificed terminally were necropsied. A wide range of tissues
and any unusual lesions from animals of all groups, including the
control, found dead or sacrificed moribund and from all terminal
survivors of control and top dosage groups were examined
microscopically. Any unusual lesions from terminal survivors at both
50 and 250 ppm were also subject to histopathological evaluation.
Clinical signs, body weight, food and water consumption were not
significantly different from those in the controls. Ophthalmoscopic
examination conducted at four intervals during the study seemed to
suggest a probable increase at week 89 in incidence of cataract
("posterior cortical or more advanced") in males at 1 250 ppm. At
terminal sacrifice, males at 1 250 ppm showed an increase in absolute
testes weight and testes/body weight ratio and females of all treated
groups displayed a dose-related increase in absolute weight and
organ/body weight ratio of kidney. There were no treatment-related
microscopic changes in the tissues evaluated, including testes and
kidney.
Analysis of tumour data appeared to indicate no significant
difference between control and top dosage groups in the incidence of
any particular type of tumour. Under the conditions of the study,
bendiocarb was not carcinogenic to the mouse (Serota et al 1981).
Rat
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Seven adult hens (15 months old) were given a single subcutaneous
dose of bendiocarb ("laboratory grade") at 60 mg/kg body weight (the
subcutaneous LD50 of bendiocarb in hens was indicated to be about
80 mg/kg body weight). A second dose was given 3 weeks later. The
control group consisted of seven untreated hens. The birds were
observed for a total of 6 weeks from the initial dose before sacrifice
for gross pathology and histopathological examination of the
spinal cord and sciatic nerve. There were no clinical signs or
histopathological evidence suggestive of delayed neurotoxicity of the
compound in any of the treated hens (Sanderson and Hounsell 1969).
Groups of domestic hens, approximately 14-months old (20 birds in
the top dosage group and 10 birds in each other group) were acutely
intubated with technical bendiocarb in maize oil at 0, 189, 378 or
757 mg/kg body weight and observed for 21 days prior to sacrifice for
histopathological examination of the brain, spinal cord and distal
sciatic nerve. The hens were given 10 mg/kg body weight of atropine
intramuscularly immediately before the dose of bendiocarb. (Before
initiation of the neurotoxicity study, the oral LD50 of bendiocarb
was found to be 137.3 mg/kg body weight in hens not pretreated
intramuscularly with 10 mg/kg body weight of atropine and 757 mg/kg
body weight in atropine-protected hens.) One hen at 378 mg/kg body
weight and a total of 8 hens at 757 mg/kg body weight died. Except for
"doubtful signs" of ataxia noted in 1/10 hens at 189 mg/kg body weight
on days 9 and 10 only, there were no delayed neurotoxic signs in any
other treated birds during the 21-day exposure observation period.
None of the bendiocarb-treated survivors or negative controls
exhibited microscopic evidence of neurological lesions. Positive
controls, dosed orally with TOCP at 500 mg/kg body weight, displayed
typical neurotoxic signs and significant morphological changes of the
nervous tissues (Ross et al 1978).
Special Studies on Skin and Eye Irritation
Technical bendiocarb was shown to be a mild irritant when applied
to the skin of New Zealand White rabbits (Cuthbert 1978).
An eye irritant study in New Zealand White rabbits indicated
technical bendiocarb to be minimally irritating to the eye (Cuthbert
1980).
Special Studies on the Acute and Subacute Oral Toxicity of NC 7312
Rat
The oral LD50 of NC 7312 (2,2-dimethyl-1,3-benzoxodiol-4-01) a
metabolite of bendiocarb) in male and female rats (CFY strain) was
greater than 4 640 mg/kg body weight. Cholinergic symptoms were
observed at dosages above 464 mg/kg body weight (Mallyon and Sanderson
1977).
Groups of rats (CFY strain, 10 male and 10 female controls, 5
males and 5 females per treated group) were intubated with NC 7312 as
a suspension in aqueous gum tragacanth at 0, 5, 20, 80, 320 or
1 280 mg/kg body weight/day for 16 days. There were neither mortality
nor compound-related effects on clinical signs, haematological, blood
chemistry and urinalysis parameters. Growth, food conversion factor
and water intake were slightly decreased in females at 1 280 mg/kg
body weight on week 2. Terminal survivors sacrificed showed no gross
or histopathological changes associated with treatment. Absolute
weight and organ/body weight ratio of ovary were reduced at 80 mg/kg
body weight and above, but concomitant microscopic changes of the
tissue were not evident (Kemp and Brooks 1978).
Acute Toxicity
The acute toxicity of several animal species to bendiocarb is
summarized in Table 1.
Toxic signs were characteristic of anticholinesterase poisoning
and seen within a few minutes after oral, intraperitoneal or
inhalation exposure in all species of mammals tested. Deaths usually
occurred after 5 minutes to 2 hours and survivors began to recover
after 1/2 to 2 hours. Within 24 hours of dosing, survivors recovered
completely from clinical signs. After dermal exposure, toxic signs
began to appear after 2 to 21 hours with recovery being much slower
than that following acute oral doses (Sanderson 1921).
Short-Term Studies
Rat - dietary
In a 28-day study with groups of five female rats (Sprague-Dawley
CFY strain) fed diets containing 0, 400, 600 or 800 ppm of technical
bendiocarb, mortality, body weight, food consumption, oestrous cycle
(as evidenced by vaginal cytology taken from the fourth day to
termination) and terminal gross pathology were not affected by
treatment. Whole blood cholinesterase was inhibited (52-62%) at all
test levels and toxic symptoms were observed at both 600 and 800 ppm
on day 28 after the animals (fasted overnight) were given access to an
appropriate test diet for a restricted one-hour feeding period
(Jackson 1977).
Table 1. Acute Toxicity of Bendiocarb in Animals
LD50
Species Sex Route Vehicle (mg/kg body Reference
weight)
Mouse F oral glycerol 45
(CFW) formal Sanderson 1971
Rat M oral glycerol 40-64 Sanderson 1970;
(Wistar) formal Sanderson 1971,
F 34-40 Sanderson 1971
Rat M oral aqueous 108-156 Crome and Sanderson
(Charles River gum 1980
COBS CD tragacanth
Sprague-Dawley)
Rat M&F i.p. glycerol 8 Sanderson 1971
(Wistar) formal
Rat F dermal glycerol 566-800 "
(Wistar) (24-hour formal Ben-Dyke 1932
M exposure) 566
Rat (Carworth M inhalation1/ None 250 mg Sanderson and
Sprague-Dawley) (6-hour a.i./l air2/ Mallyon 1979
exposure)
Hamster F oral water 141 "
(Syrian)
Guinea pig F oral glycerol 35 Sanderson 1971
formal
Rabbit M oral glycerol 40 "
F formal 35
1/ Animals were exposed to an 80% wettable powder as a dust in a "nose only"
exposure chamber with 58% particles of size 10 microns and 18% particles
5 microns.
2/ 6-hour LD50 in terms of actual chamber concentration of the active ingredient.
Groups of 10 male and 10 female rats (Sprague-Dawley CFY strain)
were fed dietary levels of technical bendiocarb at 0, 2, 10, 50 or
250 ppm for 13 weeks. Mortality, clinical signs, body weight, food and
water consumption, ophthalmoscopic findings, clinical chemistry,
haematological and urinalysis parameters were not influenced by the
presence of the compound in the diet. Whole blood cholinesterase,
determined in animals (fasted 16 hours) shortly after a 1-hour feeding
period, was depressed (20%) at 250 ppm in both sexes during weeks 4
and 9, at both 50 and 250 ppm in males and at 250 ppm in females
during week 13. Terminal gross pathological changes and organ weights
were not significantly different from those in the controls.
Histopathological evaluation of a variety of tissues from animals of
the control and top dosage groups suggested the only notable finding
to be an increase in incidence of males at 250 ppm showing vacuolated
centrilobular hepatocytes. This liver lesion, not noted even at
200 ppm in the long-term rat study (see under Long-Term Studies), was
considered unlikely to occur in the lower dosage groups of the study,
although there were no data to confirm this supposition. It would
appear that the no-effect level was 10 ppm on the basis of
cholinesterase levels and was 50 ppm on the basis of the other tested
parameters (Hunter et al 1978).
Hamster
Groups of hamsters (20 male and 20 female controls, 10 males and
10 females per treated group) were fed technical bendiocarb in their
diet at 0, 4, 20, 100 or 500 ppm for 30 days. There were no
indications of any treatment-related effect at levels up to 500 ppm as
judged by mortality, clinical signs, body weight, food and water
intake, terminal haematology, organ weight and histopathology of
selected tissues from control and 500 ppm groups (Hounsell and Brooks
1981).
Dog
Groups of four male and four female beagles were fed dietary
levels of technical bendiocarb at 0, 20, 100 or 500 ppm for 16 weeks
with the top dosage level being raised to 1 000 ppm on day 89 (test
diets were offered to the dogs for a 1-hour period morning and
evening). Inclusion of the compound in the diet did not adversely
affect survival, clinical signs, food consumption, terminal
haematology, blood chemistry and urinalyses values. Males of the
highest dosage group exhibited actual weight loss at termination.
Assays1/ at weeks 5, 9, 14, 15 and 16 of cholinesterase in whole
1/ Whole blood was diluted 1:1 200 in the assay. No data were
available on the amount of diet actually consumed by individual
animals during the 1-hour feeding period prior to sampling for
assay of cholinesterase in blood and brain.
blood and plasma of animals (fasted overnight) following a 1-hour
feeding period indicated depression (>20%) at the top dosage level of
cholinesterase in plasma and whole blood at most sampling periods.
Whole blood cholinesterase was decreased (>20%) even at 100 ppm in
males at week 9 and in females at weeks 9 and 15. Terminal brain
cholinesterase of the top dosage group (males and females) was also
inhibited (>20%). At the conclusion of the study, organ weights and
gross pathological changes were unaffected by the compound.
Microscopic examination of about 20 tissues from each animal suggested
no treatment-related changes other than the occurrence of thyroid
lesions (focal C-cell hyperplasia and focal atrophy) in 1/4 males and
1/4 females of the top dosage group but in none of the concurrent
controls or of animals in the lower dosage groups. The study suggested
the no-effect level with respect to cholinesterase and other criteria
evaluated to be 20 ppm and 100 ppm, respectively (Litton 1974).
Groups of 8 male and 8 female purebred beagles were fed a diet
containing technical bendiocarb at 0, 20, 100 or 500 ppm for 2 years
(control or treated diet was given to each animal every morning).
Survival, clinical symptoms and growth during the study as well as
haematology, urinalysis and ophthalmoscopic parameters evaluated at
five more intervals over the course of the experiment were not
significantly affected by the presence of the test material in the
diet. Less food was consumed by animals (both sexes) of all treated
groups throughout the study. Nevertheless, the authors of the report
stated that "statistical analysis of food residue for weeks 1-3, 1-26,
1-52, 53-104 and 1-104 did not reveal any statistically significant
difference between control and test group means". Based on weekly mean
food consumption and mean body weight data at the end of each week,
the dietary levels of 20, 100 and 500 ppm were equivalent to 0.7, 3.1
and 16.3 mg/kg body weight/day (males and females combined). Water
consumption was slightly but consistently increased in both sexes at
100 ppm and above during weeks 36-39 and at 500 ppm not infrequently
thereafter. Serum cholesterol was elevated at 500 ppm (both sexes) at
several sampling intervals. A dose-related decrease in serum calcium
level was noted at both 100 and 500 ppm in weeks 14 and 25.
Measurement of whole blood cholinesterase in animals (fasted
overnight) shortly after a 60-minute feeding period at eight sampling
intervals during the study showed a tendency to frequent depression
(>20%) of the enzyme at 500 ppm. Brain cholinesterase in animals
(fasted overnight) sacrificed following a 1-hour feeding period was
depressed (>20%) at 500 ppm after 52 and 104 weeks and probably also
at 100 ppm after 104 weeks. Organ weight analysis, gross pathological
examination and histopathological evaluation of over 30 tissues from
each animal sacrificed at the end of 52 weeks (3 males and 3
females/group) or terminally (all survivors per group) showed no
significant alterations related to treatment.
The dietary level of 20 ppm appeared to be a no-effect level on
the tested criteria. It was noteworthy that the actual amount of
bendiocarb ingested by animals during the 1-hour feeding period prior
to the assay of tissue cholinesterase varied markedly, even among
animals in the same dosage group, owing to substantial individual
variation in the quantity of diet (ranging from 0 to 400 g per animal)
consumed. Also, there were incidences where individual animals of a
high dosage group, e.g. 500 ppm, actually had a lower intake of
bendiocarb than those of a lower dosage group (100 ppm) (Chesterman
et al 1980).
Rat - dermal
Groups of 6 rats (Wistar strain) were exposed dermally to an 80%
wettable powder of bendiocarb as a 40% w/v aqueous suspension at 50,
100, 200, 400 or 800 mg a.i./kg body weight under an occlusive patch
for a period of 6 hours/day, 5 days/week for a total of 15
applications in 21 days. The treated site was washed at the end of
each exposure period. There was no mortality. Toxic signs,
characteristic of anticholinesterase poisoning and dose-related in
severity, were noted at 200 mg/kg body weight and above. Whole blood
cholinesterase, determined at the end of the 6-hour exposure period,
was inhibited by more than 30% at 200 mg/kg body weight and above
after only one dose and at 50 mg/kg body weight and above after 15
exposures. No gross pathological changes or microscopic evidence of
dermal irritation attributable to treatment were apparent at terminal
sacrifice (Sanderson 1972).
Long-Term Studies
Rat
Groups of male and female rats (CFY strain, 60 male and 120
female controls, 30 male and 60 female per treated group) were fed
dietary levels of technical bendiocarb at 0, 2, 20 or 200 ppm for 7
days and then caged together (1 male and 2 females). At the end of a
20-day mating period, the females were individually housed and allowed
to litter and rear their pups to weaning. This part of the study was
known as the reproductive phase. A sufficient number of weanlings from
the control and treated groups were randomly selected to initiate
another segment of the study referred to as the main phase. The latter
comprised the main group and the satellite group. In the main group,
weanlings (50 males and 50 females per treated group, 100 male and 100
female controls) were given bendiocarb-treated diets at 0, 2, 20 or
200 ppm for 104 weeks. For the satellite group, used primarily for
laboratory investigations, animals (30 males and 30 females per
control or treated group) were fed bendiocarb at the same dietary
levels, but only for 60 weeks. The 2 ppm level was raised to 10 ppm
after 2 weeks in the main phase, and this group will be known as the
2-->10 ppm group hereafter for the purpose of identification. Dietary
administration was continued throughout both the reproduction and main
phase. All animals died or sacrificed in extremis during the 104-week
study and those sacrificed terminally were necropsied. Animals of all
groups, including the control, dying or sacrificed in extremis during
the study and terminal survivors of the control and top dosage groups
were subjected to microscopic examination of a set of about 30
selected tissues plus grossly observed nodules and tissue masses.
Additionally, all suspected tumours from terminal survivors of lower
dosage groups were evaluated histopathologically.
For the reproductive phase, mortality and behaviour of parental
animals, pregnancy rate, duration of gestation period, litter size and
pup weight at birth, sex ratio and incidence of external abnormalities
of pups were not affected by bendiocarb. There were some indications
of adverse effects related to treatment at 200 ppm on maternal body
weight gain during gestation, litter size on lactation day 4 and pup
weight from lactation day 4 through weaning. A dietary level of 20 ppm
was a no-effect level on this phase of the study.
In the 104-week study (main group) of the main phase, mortality
rate, although unaffected by the compound, was high in all groups,
including the control, Only about 16-26% of males and 24-42% of
females survived 104 weeks. Nevertheless, over 55% of males and
females lived at least 82 weeks.
A number of findings associated with treatment were noted at
200 ppm. These included an increased incidence of males exhibiting
hyperactivity in response to external stimuli, growth depression
during the first 52 or 56 weeks, depressed water consumption during
week 6-12 but not in week 25. Periodic measurements of haematological,
biochemical and urinalysis values at several intervals over the course
of the study revealed significant changes in several parameters
(e.g. total WBC, neutrophil and lymphocyte counts, levels of serum
cholesterol and total protein) to be also confined only to the top
dosage group. There was a dose-related decrease in body weight gain
associated with a depression in food consumption in males of all
treated groups during the first two weeks of the study but not
thereafter. Ophthalmoscopic examination of the surviving animals in
the main group only for a total of seven intervals between 13 and 102
weeks indicated a statistically significant increase in incidence of
lenticular opacities in males at 200 ppm at all intervals from 52
weeks onward. A statistically significant trend (p <0.01) for
dose-dependent incidence of this eye lesion was indicated to be
apparent for males at both 20 and 200 ppm from 66 weeks onward and for
males of all treated groups at 80 weeks and thereafter. It was pointed
out that the difference in frequency of lenticular opacities was not
statistically significant between males of control and low dosage
(2-->10 ppm) groups or among female groups at any time interval. The
lenticular opacities observed reportedly were "essentially atypical of
chemically induced lenticular lesions in the rat". Overall,
2-->10 ppm might probably be considered a borderline no-effect level
with respect to the particular eye lesion.
Activity of whole blood cholinesterase determined in animals
(fasted overnight) following a one-hour feeding period at several
intervals during the study (animals of the satellite group) and at
termination (animals of the main group) was frequently depressed
(>20%) at 200 ppm in both sexes. Whole blood cholinesterase activity
in animals fasted overnight without access to food immediately prior
to sampling of blood was not reduced. The brain cholinesterase level
was inhibited (>20%) at 200 ppm in animals (both sexes) sacrificed at
52 weeks but not terminally. Organ weight determination at 52- and
60-week interim sacrifice and at terminal sacrifice showed deviations
from controls of several tissues such as liver, thymus, prostate and
adrenals, but these were essentially restricted to the top dosage
level only and were not accompanied by histopathological lesions.
Gross pathological changes were not significantly different from those
in the controls. Histopathologically, an increase in incidence of
stomach lesions, characterized by "hyperplasia/hyperkeratosis/
acanthosis with or without inflammation of the non-glandular portion",
occurred in the male terminal survivors at 200 ppm. There were no
microscopic lesions observed in any of the other tissues examined that
were attributable to the compound.
An evaluation of tumour data suggested a dose-related increase of
incidence of total lymphoreticular tumours in males of all treated
groups (incidence 3, 8, 10, 12%) respectively at 0, 2-->10, 20 and
200 ppm), with the difference from concurrent controls being
statistically significant only at 200 ppm. Frequency of each type of
lymphoreticular tumour (lymphosarcoma, thymic lymphosarcoma, reticulum
cell sarcoma, lymphatic leukaemia and myeloid leukaemia), when
considered alone, was not significantly increased in any treated
group. Latency period (or time of detection of the tumour when the
animal died or was sacrificed) was also not modified by treatment.
Other than the particular malignancy just mentioned, incidence, type
and location of tumours were not significantly higher at 200 ppm than
in the controls. It may be noted that 56% of males and 92% of females
of the concurrent control groups were bearers of tumours with
pituitary adenoma (both sexes), mammary tumour (females) and
pheochromocytoma (both sexes) being the most frequently observed
tumours.
Based on the available data, 20 ppm was without any significant
effect on cholinesterase. The lowest tested level, 2-->10 ppm
(indicated to be equivalent to an average achieved intake, throughout
the study of 0.38 mg/kg body weight/day) may be acknowledged as a
marginal no-effect level on the other tested parameters (Hunter
et al 1981).
Observations in Humans
In a male volunteer given a single oral dose of 9.8 mg or
approximately 0.12 mg/kg of 14C-bendiocarb, over 99% of the
administered dose was excreted in the urine in 22 hours. The plasma
half-life and the half-life for renal elimination for total
radioactivity was estimated at 3.5 and 3.3 hours, respectively. The
major urinary metabolites were sulphate and ß-glucuronide conjugates
of NC 7312, which together amounted to 95% of the radioactivity dose
excreted. Small amounts of conjugated bendiocarb and N-hydroxy methyl
bendiocarb were the only compounds detected in the urine (Adcock and
Challis 1976b).
An area on the forearm of each of two male volunteers was exposed
for 3 hours to 14C-bendiocarb (as an aqueous solution in a wetting
agent) at an average dosage of approximately 0.0013 mg/kg body weight
under occluded conditions in one experiment and non-occluded
conditions in another. (No information was available on the time
interval between the two experiments.) Over the 48-hour period
following exposure, an average of 58% of the applied dose under
occluded conditions vs. an average of 12.5% under non-occluded
conditions was recovered in the urine, suggesting better absorption of
bendiocarb through occluded skin. The only urinary metabolite found
was NC 7312 as conjugates of sulphate and ß-glucuronide (Warner and
Adcock 1980).
In a 60-year old male volunteer given bendiocarb (as an 80%
wettable powder) orally in H2O serially at 0.004, 0.012, 0.037,
0.125, 0.187 and 0.25 mg/kg body weight (dosage in terms of w.p.) with
at least a 48-hour interval between doses, 0.125 mg/kg was found
to be a clear-cut no-effect level on symptoms and whole blood
cholinesterase. Toxic sumptoms such as mild vertigo, nausea and
vomiting together with significant inhibition (30-40%) of whole blood
cholinesterase were induced after the single dose at 0.25 mg/kg body
weight. The symptoms subsided in 30 minutes and whole blood
cholinesterase recovered completely in 4 hours post-treatment. In the
same subject, doses of the 80% w.p. at 0.125 mg/kg given orally at an
interval of 4 hours, but not less, produced no toxic signs or
inhibition of whole blood cholinesterase. A double-blind cross over
experiment involving three other male volunteers indicated a single
dose of 0.004 mg/kg body weight, the only dose administered, of the
80% w.p. resulting in no adverse effects on any of the parameters
assessed (heart rate, clinical signs, mean diastolic/systolic pressure
and whole blood cholinesterase). Following a single dose of 0.12 mg/kg
body weight of the 80% w.p. given orally to the same three volunteers
72 hours later in another similar experiment, a transient depression
(21% of whole blood cholinesterase was observed in one of the three
subjects at 30 minutes post-treatment (Drummond and Kemp 1976).
The safety of bendiocarb (80% wettable powder), when used as an
insecticide against adult mosquitoes, to operators and inhabitants was
evaluated in Iran and Indonesia. The studies were organized along the
line of the WHO expanded Stage V evaluation programme. Each trial
involved the treatment of 13 villages with a total population of over
4 000 by 15 or 16 spray operators. Results indicated that very few
operators reported any toxic signs or symptoms and the latter, when
noted, were usually mild and transient. Inhibition of whole blood
cholinesterase, slight to mild, was only infrequently observed and was
readily reversible. Analysis of urine collected from the operators
suggested absorption of only low levels of bendiocarb during spraying.
No complaints were received from the villagers (Motabar et al 1981;
Bonsall et al 1981).
COMMENTS
Bendiocarb, a carbamate insecticide, evaluated for the first time
by the Meeting, was rapidly absorbed and readily eliminated primarily
in the urine in all of the mammalian species studied, including
humans. The major metabolic pathway in these species involved cleavage
of the carbamate ester group to yield the corresponding phenol
(NC 7312). There was no evidence of tissue accumulation in mammals.
Mammalian acute oral toxicity varied between strains and species, but
not between sexes.
A 3-generation reproduction study, including teratological
evaluation, in rats demonstrated 10 ppm and 50 ppm to be the no-effect
level, respectively, on reproductive parameters and teratogenicity. A
teratogenic study in the same species failed to give any indication of
teratogenicity even at the top dosage level (4 mg/kg body weight)
tested. In a rabbit teratology study, 1 mg/kg body weight was shown to
be a clear-cut teratogenic no-effect level. The available mutagenic
studies including reversion mutation and rec assays, mouse
micronucleus test, mouse lymphoma assay and dominant lethal assay in
rats were all negative. A carcinogenicity study in mice revealed no
carcinogenic activity of the compound, under the conditions of the
experiment. In a long-term feeding study with rats, a significant
increase in incidence of total lymphoreticular tumours, but not
individual type of lymphoreticular tumours, was noted in males of the
highest dosage group (200 ppm). Latency period of these tumours was
not reduced. Although the possibility of the finding in question
regarding lymphoreticular tumours being related to bendiocarb does not
appear likely, it cannot be entirely excluded. Background data on
incidence of total lymphoreticular tumours in CFY strain of rats
maintained in the testing laboratory would be helpful to resolve the
uncertainty. It should be noted that concurrent controls in the study
had a high incidence of spontaneous tumours. Additionally, less than
25% males and 45% females survived the duration of the 104-week study,
although over 50% of animals in all groups including the control were
still alive at the end of 82 weeks. Together these factors might
compromise sensitivity of the study to detect carcinogenic activity of
the compound. This study also demonstrated an increased incidence of
lenticular opacities in male rats at the two top dietary levels.
Neurotoxicity studies in hens gave no indications suggestive of
delayed neurotoxic potential of the insecticide.
No-effect levels were determined in short-term studies in rats
(13-week) and dogs (16-week and 2-year) and a long-term study in rats.
In the dog, however, the Meeting noted the possible adverse effects of
dilution during the determination of tissue cholinesterase, and also
the practice of tissue sampling from overnight-fasted dogs following a
1-hour feeding period. Furthermore, there was some concern on the
absence of data on the levels of food ingestion during the 1-hour
feeding period in one study, and food intake was extremely variable in
the second study.
Observations in humans indicated rapid reversal of cholinesterase
inhibition and of clinical symptoms following both acute and subacute
exposures.
Because of the concerns regarding the increased incidence of
lymphoreticular tumours in male rats of the top dose group in the
long-term study, and the probability that the no-effect level
established for dogs is nominal rather than actual, only a temporary
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0-0.38 mg/kg bw/day
Dog: 0-0.07 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0-0.002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Historical data on the incidence of total lymphoreticular tumours
in the CFY strains of rats used in the long-term study.
2. Short-term (28-day) study in dogs to clarify the relationship
between dietary intake of bendiocarb and terminal erythrocyte and
brain cholinesterase activity, using an appropriate method for
cholinesterase determination.
Desirable
Further investigation on the cataractogenic activity of
bendiocarb at low dosage levels in rats.
REFERENCES
Adcock, J.W. and Challis, I.R. The metabolism of 14C-bendiocarb in
1976a the rat. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1976b The pharmacokinetics and metabolism of bendiocarb in man.
Report from Fisons Ltd. submitted to the World Health
Organization by FBC Limited. (Unpublished)
Ben-Dyke, R. The toxicology of NC 6897. Acute dermal toxicity of
1972 technical bendiocarb, Report from Fisons Limited submitted to
the World Health Organization by FBC Limited. (Unpublished)
Bonsall, J.L., Foulkes, D.M., Goose, J., Leake, C.R. and Reary,
1981 J.B. Safety studies with bendiocarb in a village-scale field
trial against mosquitoes in Indonesia, 1981. Document
(WHO/VBC/81.831), World Health Organization, Geneva.
Challis, I.R. The metabolism of bendiocarb in the pregnant rabbit,
1980 Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in the
1977a mouse. Report from Fisons Limited submitted to the World
Health Organization by FBC Limited. (Unpublished)
1977b The metabolism of bendiocarb in the hamster. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Gopinath,
1980 C., Harling, S., Majeed, S. and Prentice, D.E. NC 6897
Toxicity study in beagle dogs (final report dietary intake for
104 weeks). Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Crome, S.J. and Sanderson, D.M. A comparison of the acute oral
1980 toxicities of three batches of bendiocarb CR 4971/2, CR 4799/9
and CR 4500/20 to the male rat. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Cuthbert, J.A. Technical bendiocarb EX Muskegon primary eye irritancy
1980 in rabbits. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
1978 Technical bendiocarb. Primary skin irritancy study on rabbits.
Report from Inveresk Research International, Scotland,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Drummond, P. and Kemp, A. The effects of the acute oral administration
1976 of NC 6897 80 WP to male volunteers. Report TOX/76/133-58 from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hounsell, I.A. Brain cholinesterase inhibition in dogs fed NC 6897.
1974 Report from Fisons Limited Agrochemical Division submitted to
the World Health Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Rush, K.C. The effect of dietary administration
1980 of NC 6897 at 1 250 ppm on whole blood cholinesterase activity
in mice. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Brooks, P.N. The 30-day subchronic oral toxicity
1981 of NC 6897, technical CR 4799/1 in the diet to the hamster.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A.G. The effect of dietary administration of NC 6897 at
1981a 500 and 1 000 ppm on whole blood cholinesterase activity in
male mice of the CFLP and CD1 strains. Report from FBC Limited
submitted to the World Health Organization by FBC Ltd.
(Unpublished)
1981b The effect of dietary administration of NC 6897 at 0.4, 2 and
10 ppm on whole blood cholinesterase activity in male rats.
Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1981c The effect of dietary administration of NC 6897 at 0.4, 2, 10
and 50 ppm on whole blood cholinesterase activity in male
rats. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Hounsell, I.A. and Walker, A.K. A micronucleus study in mice using
1982 technical NC 6897, CR 4971/2. Report from FBC Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Offer, J.M. and
1978 Gregson, R.L. NC 6897 Toxicity to rats when administered in
the diet for 13 weeks. Final Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Hunter, B., Watson, M., Heywood, R., Street, A.E., Prentice, D.E.,
1981 Gopinath, C. and Gibson, W.A. NC 6897 Toxicity and
tumorigenicity to rats in long-term dietary administration.
Report from Huntingdon Research Centre, England, submitted to
the World Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. Effects on adult female rats of one month exposure to
1977 high dietary concentration of NC 6897 (technical). Report from
Fisons Limited Agrochemical Division submitted to the World
Health Organization by FBC Limited. (Unpublished)
Jackson, C.M. A peri- and post-natal study in rats with technical NC
1978 6897, Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Hounsell, I. Evidence for the reversal of cholinesterase
1974 inhibition by NC 6897 in laboratory animals. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Kemp, A. and Jackson, C.M. A test for the induction of dominant lethal
1977 mutations in male rats by NC 6897. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
Kemp, A. and Brooks, P.N. The effects of the daily oral administration
1978 of NC 7312 to male and female rats for 16 days. Report from
Fisons Limited Agrochemical Division submitted by FBC Limited.
(Unpublished)
Litton. 16-week subacute toxicity study in dogs. NC 6897 technical.
1974 Report from Litton Bionetics Inc., U.S., submitted to the
World Health Organization by FBC Ltd. (Unpublished)
Mallyon, B.A. and Sanderson, D.M. The acute oral toxicity of NC 7312
1977 to male and female rats. Report from Fisons Limited
Agrochemical Division submitted by FBC Limited. (Unpublished)
McConville, M. and McGregor, D.B. Testing the mutagenic activity of
1979 technical NC 6897, Report from Inveresk Research
International, Edinburgh, Scotland, submitted to the World
Health Organization by FBC Limited. (Unpublished)
McConville, M. and Harris, W.J. NC 7312: Ames test for mutagenic
1979 activity. Report from Inveresk Research International,
Scotland, submitted to the World Health Organization by FBC
Limited. (Unpublished)
McGregor, D.B. and Ross, C.A. NC 6897: The assessment of mutagenic
1981 potential in the mouse lymphoma mutation assay. Report from
Inveresk Research International, Scotland, submitted to the
World Health Organization by FBC Limited. (Unpublished)
Moriya, M., Ohta, T. and Shirasu, Y. KNT (bendiocarb): microbial
1981 mutagenicity study. Report from the Institute of Environmental
Toxicology, Japan, submitted to the World Health Organization
by FBC Limited. (Unpublished)
Motabar, M., Mallyon, B.A., Goose, J. and Adcock, J.W. Document
1981 (WHO/VBC/81.821) World Health Organization, Geneva.
Palmer, A.K. and Allen, P.A. Effect of NC 6897 on neonatal pup
1978 mortality, Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Pearce, J.C., Warner, P.A. and Adcock, J.W. The metabolism of
1977 bendiocarb in the mouse (strain CD 1). Report from Fisons
Limited submitted to the World Health Organization by FBC
Limited. (Unpublished)
Pearce, J.C. and Adcock, J.W. Investigation of residue accumulation
1978 in the rat following repeated administration of
14C-bendiocarb. Report from Fisons Limited submitted to the
World Health Organization by FBC Limited. (Unpublished)
Roberts, N.L. Blood cholinesterase levels in the cow following dietary
1979 inclusion of bendiocarb. Report from Huntingdon Research
Centre, England, submitted to the World Health Organization by
FBC Limited. (Unpublished)
Ross, D.B., Roberts, N.L., Cameron, D.M., Prentice, D.E. and Majeed,
1978 S.K. Examination of NC 6897 for neurotoxicity in the domestic
hen. Report from Huntingdon Research Centre, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Sanderson, D.M. The toxicity of NC 6897: Acute toxicity to the rat of
1970 technical grade NC 6897. Report from Fisons Limited submitted
to the World Health Organization by FBC Limited. (Unpublished)
1971 The toxicology of NC 6897: Acute toxicity of pure NC 6897.
Report from Fisons Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
1972 Toxicology of NC 6897: 15-dose cumulative dermal study with
Ficam in male rats. Report from Fisons Limited submitted to
the World Health Organization by FBC Limited (Unpublished)
Sanderson, D.M. and Hounsell, I.A. The toxicology of NC 6897. Test
for neurotoxicity of NC 6897 to the chicken. Report from
Fisons Limited submitted to the World Health Organization by
FBC Limited. (Unpublished)
Sanderson, D.M. and Mallyon, B. The acute oral toxicity to the
1977 hamster of technical bendiocarb. Report from Fisons Limited
Agrochemical Division submitted to the World Health
Organization by FBC Limited. (Unpublished)
1979 The acute inhalational toxicity to rats of Ficam 80W, an 80%
wettable powder formulation of bendiocarb, CR 13374/27H.
Report from Fisons Agrochemical Division submitted to the
World Health Organization by FBC Limited. (Unpublished)
Serota, D.G., Voelker, R.W. and Fezio, W.L. A chronic toxicity and
1981 carcinogenicity study in mice, NC 6897. Final report from
Hazleton Laboratories America, Inc. submitted to the World
Health Organization by FBC Limited. (Unpublished)
Tesh, J.M., Bartlett, A., Tesh, S.A., Whitney, J.C. and Finn, J.P.
Technical NC 6897 (CR 4799/1): Effects of dietary
administration upon reproductive performance and teratogenic
response in rats treated continuously through three successive
generations. Report from Life Science Research, England,
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Tesh, J.M., Ross, F.W. and Tesh, S.A. Technical NC 6897: Effects of
1980 oral administration upon pregnancy in the rabbit. Report from
Life Science Research, England, submitted to the World Health
Organization by FBC Limited. (Unpublished)
Tucker, M.L. NC 6897: Teratogenicity study in the rat. Report from
1974 Consultox Laboratories Ltd., England, submitted to the World
Health Organization by FBC Limited. (Unpublished)
Warner, P.A., Adcock, J.W. and Pearce, J.C. The metabolism of
1977 14C-bendiocarb in the dog. Report from Fisons Limited
submitted to the World Health Organization by FBC Limited.
(Unpublished)
Warner, P.A. and Adcock, P.A. The dermal absorption of 14C-bendiocarb
1980 in man. Report from FBC Limited submitted to the World Health
Organization by FBC Limited. (Unpublished)
BENDIOCARB
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bendiocarb is available as a wide range of formulations,
including wettable powders (200, 500 and 800 g a.i./kg); granules
(30, 50 and 100 g a.i./kg); dust (10 g a.i./kg); oil suspension for
ULV application (250 g a.i./kg); residual sprays, paint-on and
granular baits and aerosols.
Bendiocarb is used for the controls of soil and foliar insect
pests on a wide range of crops, ectoparasites on domestic animals and
mosquitos and nuisance pests. The Meeting was informed that it is sold
for these uses in approximately 100 countries. Information was
provided to the Meeting on official nationally approves uses (good
agricultural practices, GAP) by the Netherlands. Good agricultural
practices of The Netherlands are summarized in Table 1 (Netherlands
1982).
Table 1. Bendiocarb Good Agricultural Practices, Netherlands 1982
Application Used
Crop Pests rate (kg/ha) Formulation Treatment Since
Sugarbeet Fritfly, wireworm, 0.3-0.4 3% G seed furrow 1979
spring tails, 0.4 80% WP seed furrow 1981
pygmy beetle 500 g/l
liquid
6.4 g/kg " seed dressing 1981
seed
Maize Fritfly, 0.3-0.4 3% G seed furrow 1979
wireworm 4 g/kg 80% WP seed dressing 1981
seed 500 g/l
liquid
The Meeting was provided with a tabulation of recommended
application rates, which are summarized in Table 2 (Netherlands 1982).
No information was provided on preharvest intervals. The recommended
application rates are consistent with GAP of The Netherlands for maize
and sugarbeets. Information was not provided on whether the
recommended uses other than on sugarbeet and maize are also nationally
approved and, if so, where.
Table 2. Recommended Application Rates of Bendiocarb, Netherlands 1982
Crop Major pests Formulation Rate (a.i.)
Maize Wireworm, symphlids, fritfly Granule 0.3-0.35 kg/ha
Wireworm, symphlids, fritfly Seed dressing 4 g/kg seed
Corn rootworm Granule 0.84-1.12 kg/ha
Soil pests (in furrow) Spray 0.4 kg/ha
Sugarbeet Wireworm, pygmy beetle,
springtails Granule 0.3-0.35 kg/ha
Wireworm, pygmy beetle,
springtails Seed dressing 6 g/kg seed
Soil pests (in-furrow) Spray 0.4 kg/ha
Rice Rice water weevil, stem
borer Granule 2 kg/ha
Leafhoppers, planthoppers Spray 0.5 kg/ha
Leafhoppers, planthoppers Dust 0.5-0.75 kg/ha
Oilseed Flea beetle Granule 0.14-0.28 kg/ha
rape Spray 0.1-0.2 kg/ha
Ryegrass Fritfly, wireworm,
leatherjackets Granule 0.75 kg/ha
Spray 0.5-0.75 kg/ha
Seed dressing 10 g/kg seed
Cereals Fritfly, wireworm,
leatherjackets Spray 0.75 kg/ha
Seed dressing 2-4 g/kg seed
Potatoes Colorado beetle Spray 0-125-0.25 kg/ha
tuber moth Spray 0.5 kg/ha
Brassicas Diamond-back moth Spray 0.1-0.8 g/l
Peas Pea moth, pea weevil Spray 0.2-0.5 kg/ha
Apple/Pear Codling moth, tortrix moth,
aphids Spray 0.25-0.6 kg/ha (LV)
Mushrooms Mushroom fly Wall spray 30-50 g/100 m2
Preharvest Uses
Residue trials utilizing preharvest treatments have been
conducted on a variety of plant and animal products. Results are
detailed under "Residues Resulting From Supervised Trials".
Postharvest Uses
The Meeting was informed of residue investigations in wheat grain
stored in small bins for six months after treatment of interior
surfaces, but no details were provided. Likewise, no details were
provided on studies said to have been conducted on foodstuffs treated
in food-handling premises.
Other Uses
The Meeting was informed that 80 W or ULV formulations may be
used for control of mosquitos and the ULV formulation against
warehouse pests. Studies have been conducted to determine the extent
of potential exposure. Although contamination levels were said to be
within safe limits, details of these studies were not provided.
Bendiocarb is said to be widely used for control of cockroaches
and other nuisance pests in and around the home, hotels, food-handling
establishments, aircraft etc. and as a paint for fly control in animal
houses. No details on these uses were provided.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue trials have been conducted in many countries on a wide
range of commodities, representing a variety of treatments and
climatic conditions.
Root and Tuber Vegetables
Potatoes
Twenty five trials were conducted in three European countries
either by spray, broadcast or in-furrow treatments with either 3G or
20W formulations. Results are summarized in Table 3 (FBC 1982a; Reary
1979d; Browne and Reary 1981; Browne 1981e). Although most of the data
were from application of the 3G formulation, no information was
provided on recommended or approved uses for that formulation on
potatoes. The wettable powder spray applications were at one and two
times the recommended rate. Recoveries were generally 75% or better
for bendiocarb and 60% or better for NC 7312. The limit of
determination is said to be approximately 0.01 and 0.02 mg/kg for
bendiocarb and NC 7312, respectively.
Table 3. Bendiocarb Residues in Potatoes, from Supervised Trials
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 2 4-5 3G 1 kg/ha In-furrow 119-155 0.01-0.03 <0.02,0.26
1978 2 4-5 3G 2 kg/ha In-furrow 119-155 0.01-0.04 0.02,0.58
2 4-5 3G 3 kg/ha In-furrow 119-155 0.01,0.03 0.04,0.69
1 7 3G 1 kg/ha Broadcast 112 0.01 0.02
1 7 3G 1.5 kg/ha Broadcast 112 0.04 0.05
2 4-7 3G 2 kg/ha Broadcast 112-155 <0.01-0.03 <0.02,0.07
2 4-7 3G 3 kg/ha Broadcast 112-155 <0.01-0.04 <0.02,0.11
2 4-7 3G 4 kg/ha Broadcast 112-155 0.01-0.03 0.03,0.08
3 Untreated
controls 0.003-0.008 0.003-0.0.7
German Federal 3 3 20W 0.12 kg/ha Spray 03/ <0.01 0.014-0.12
Republic 3 3 (<0.005-0.017) (<0.01-0.14
1980 2 3 7 <0.01 <0.01-0.14
(<0.005-0.009) (<0.01-0.15)
14 <0.01 0.01-0.15
(<0.005-0.015) (<0.01-0.18)
1 3 15 <0.005) 0.014
3 2 - - Untreated
controls - 0.001-0.044/ (<0.01-0.024)
0.001-0.007
Table 3 (con't)
No. Application Interval Residues (mg/kg)1/ 2/
Country No. Trials Replicates Formulation Rate (a.i.) Method (days) Bendiocarb NC 7312
U.K. 1 (3 plots) 20W 0.53 kg/ha Spray 145/ <0.01 0.03-0.05
1981 1 (3 plots) 20W 1.06 kg/ha 145/ 0.005 0.06-0.07
1 Untreated
controls <0.005 0.01
1/ Limit of determination 0.01 mg/kg for bendiocarb and 0.02 mg/kg for NC 7312.
2/ Means of replicates; range of individual replicates in parentheses.
3/ Day 0 represents final spray of a weekly 3-spray programme, each application at 0.12 kg/ha.
4/ <0.01 except the 0.04 mg/kg value.
5/ Days after final spray of 2- or 3-spray programme, commencing 7 or 14 days before the final application.
Maximum bendiocarb residues at harvest resulting from at or
before harvest treatments were 0.04 mg/kg (mean of replicates) and
0.7 mg/kg for NC 7312.
From multiple spray applications, maximum bendiocarb residues at
harvest were < 0.01 mg/kg (mean of replications) or 0.017 mg/kg for
individual replicates. Maximum residues of NC 7312 were 0.15 mg/kg
(mean of replicates) or 0.18 mg/kg for individual replicates. For both
the means of the replicates and individual replicates residues of
bendiocarb and NC 7312 were each generally comparable from 0 to 15
days after last application. Apparent residues in untreated controls
were generally < 0.01 mg/kg for bendiocarb and < 0.02 mg/kg for
NC 7312. Residues of NC 7312 at harvest were 1-20 times that of
bendiocarb per se, and averaged six times higher.
Since residues from in-furrow or broadcast applications can be
0.04 mg/kg and untreated controls can, on occasion, reach this level,
a 0.05 mg/kg for bendiocarb per se can be supported. If NC 7312 were
also included, a limit of 0.5 mg/kg would be in order for combined
residues.
Sugarbeet
Thirty one supervised residue trials were conducted in five
European countries and residue decline studies in three (Table 4)
(Browne 1982c; FBC 1982b; Reary 1974, 1975e, 1979h,i,n,q, 1980f,j).
The seed treatment applications of 80W were at 0.8-2.7 times the
recommended 6 g/kg rate, which is also consistent with GAP of The
Netherlands. In-furrow applications of a granular formulation were
representative of the 0.3-0.4 kg a.i./ha GAP of The Netherlands and
recommended application rates.
In the supervised trials, bendiocarb residues at harvest were
below 0.01 mg/kg. Some samples were also analysed for the metabolite
NC 7312 and all were below the 0.01 mg/kg limit of determination.
Similarly, in the decline studies, residues in sugarbeet roots were
less than the 0.02 mg/kg limit of determination. Residues in untreated
controls were generally < 0.01 mg/kg, except for one set of trials
where residues ranged up to 0.11 mg/kg (average 0.005 mg/kg). The same
basic analytical procedure was used in all of the trials, but modified
in some to permit analysis of NC 7312. The limit of determination for
sugarbeet generally ranged from 0.005-0.02 mg/kg for bendiocarb and
0.01-0.02 mg/kg for NC 7312. Recoveries were generally > 75% for
parent compound and > 60% for NC 7312. In general, recoveries were
less in tops than in roots and less for NC 7312 than for bendiocarb.
Table 4. Bendiocarb Residues in Sugarbeets From Supervised Trials and Residue Decline Studies
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
Denmark 1 1 80W 15 g/kg seed Pelleted seed 204 roots <0.005
1974 1 untreated controls roots <0.005
France 2 8 80W 12 g/kg seed Pelleted seed 163 roots <0.005
1974 2 untreated controls roots <0.005
German 1 1 6 g/kg seed Seed dressing 196 roots <0.005
Federal 1 1 196 tops <0.005
Republic 1 1 80W 12 g/kg seed Seed dressing 196 roots <0.005
1974 1 1 196 tops <0.005
untreated controls roots or
tops <0.005
U.K. 2 4 80W 8 g/kg seed Pelleted seed 213 roots <0.005
1974 untreated 213 tops <0.0055/
2 control roots <0.005
tops <0.0055/
France 3 3 3G 0.36 kg/ha In furrow 160-168 roots <0.005
1976 3 3 160-168 tops <0.01
3 6 3G 0.45 kg/ha In furrow 171-190 roots <0.005
3 6 171-190 tops <0.01
3 6 untreated controls tops and
roots <0.006
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
France 2 2 3G 0.46 kg/ha In furrow 180-185 roots <0.005
1977 2 2 180-185 tops <0.01
2 2 untreated controls tops and <0.006
roots
France6/ 2 3G 0.3 kg/ha In furrow 152-170 roots <0.01
1978 2 152-170 tops <0.01
2 3G 0.6 kg/ha In furrow 152-170 roots <0.01
2 2 untreated controls tops and
roots <0.005
Italy6/ 1 1 3G 0.3 kg/ha In furrow 110 roots <0.01
1978 1 1 110 tops <0.01
1 1 3G 0.6 kg/ha In furrow 110 roots <0.01
1 untreated controls tops and
roots <0.005
U.K.6/ 3 3 3G 0.3 kg/ha In furrow 154-188 roots <0.01
1978 3 3 154-188 tops <0.01
1 1 3G 0.33 kg/ha In furrow 202 roots <0.01
1 1 202 tops <0.01
1 1 3G 0.4 kg/ha In furrow 188 roots <0.01
188 tops
1 1 untreated controls tops and
roots <0.007
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K.6/ 6 1 3G 0.3 kg/ha In furrow 144-167 roots <0.01
1979 6 1 144-167 tops <0.01
6 6 untreated controls tops and
roots <0.01
Italy6/ 1 3G 0.30 kg/ha In furrow 154 roots <0.01
1981 1 154 tops <0.01
1 3G 0.32 kg/ha In furrow 183 roots <0.01
1 183 tops <0.01
2 2 Untreated roots <0.001
controls tops 0.003
German 4 12 80W 5 g/kg seed Seed dressing 71-166 roots <0.02
Federal tops <0.02
Republic 4 12 Untreated roots 0.002-0.11
1978 4 12 controls (0.005 av.)
tops 0.005-0.015
(0.008 av.)
3 8 3G 0.33 kg/ha In furrow 56-70 roots <0.01
3 9 tops <0.01
71-166 tops <0.015
5 Untreated 71-166 roots <0.01
controls tops <0.01
Table 4. (con't)
No. No. Application Interval Residue 1/2/3/4/
Country/Year Trials Samples Formulation Rate (a.i.) Method (days) (mg/kg)
U.K. 1 5 3G 0.3 kg/ha In furrow 25-40 roots 1.367/
1979 41-55 roots 0.127/
56-70 roots <0.01
71-166 roots <0.01
7 56-70 tops <0.01
71-166 tops <0.01
1 5 Untreated 56-70 roots <0.01
controls tops <0.01
1/ Bendiocarb only (see also 6/);
2/ Corrected for recoveries of >75% except for 59% recovery in tops. Fortification levels ranged from 0.02-0.01 mg/kg;
3/ Limit of determination in individual trials generally ranged from 0.005-0.02 mg/kg;
4/ Duplicate analyses of 0.006 and 0.003 mg/kg;
5/ Individual values up to 0.07 mg/kg but <0.005 mg/kg when repeated;
6/ Sample also analysed for primary metabolite NC 7312. Residues after 56 days were generally below the 0.01 mg/kg limit
of detection except one 0.03 mg/kg value in tops (limit of determination in that trial or a 0.02 limit of determination.
Residues in seedlings were 0.42 and 0.07 mg/kg at 28 and 41 days respectively after in-furrow application at planting;
7/ Seedlings analysed whole.
Since residues are below the claimed limits of determination, a
limit of 0.02 mg/kg bendiocarb per se would probably not be
unrealistic. However, because of high apparent residues in one
untreated control, a 0.05 mg/kg limit at or about the limit of
determination may be more suitable. Based on a 0.02 mg/kg limit of
determination for NC 7312, a limit on the order of 0.1 mg/kg would be
appropriate if both bendiocarb and free NC 7312 were included. Actual
residues were too low to determine whether residues of NC 7312 exceed
those of bendiocarb, but judging from those found in potatoes, it is
likely.
Leafy Vegetables, Except Brassicas
Sugarbeet leaves
From the residue trials and decline studies discussed previously
(Table 4), residues in sugarbeet tops at harvest from recommended
application rates were generally less than the 0.01-0.03 mg/kg limits
of determination. Only one trial had a limit of determination of
0.03 mg/kg for tops. The limit of determination was considered to be
< 0.02 mg/kg for the remaining 30 trials. Bendiocarb residues were
1.4 and 0.12 mg/kg in seedlings at 28 and 41 days, respectively, after
in furrow treatments at planting. The data support a 0.05 mg/kg at or
about limit of determination at harvest as the maximum residue level.
Brassica Leafy Vegetables
Cabbage
No information was available on specific nationally approved
uses. Supervised trials with a wettable powder formulation were
conducted in one country (Table 5) (Browne 1981a,c, 1982a,b; Browne
and Manley 1982), at application rates up to 0.25 times the maximum
recommended application rate (Table 2). No specific preharvest
interval was recommended. Maximum residues ranged from 0.18 mg/kg one
day after the last of seven applications to <0.01 mg/kg after 14
days. Untreated controls were < 0.02 mg/kg and analytical
recoveries averaged 82% from fortification levels at 0.08-0.2 mg/kg.
The limit of determination was said to be 0.01 mg/kg, but considering
untreated control analyses, a 0.05 mg/kg limit of determination may be
more practical.
Because the trials do not reflect maximum recommended application
rates, recommended usage does not specify the number of applications
or a preharvest interval and the data are minimal, a maximum residue
level for bendiocarb in cabbage cannot be estimated with confidence.
Table 5. Bendiocarb and NC 7312 in Crops from Supervised Trials
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Cabbage1/ Philippines 7 0.06 50W 1 0.1 3
1981 0.1 a.i./l 3 0.04 3
7 0.01 3
14 <0.01 3
0.12 50W 1 0.18 3
0.2 g a.i./l 3 0.05 3
7 0.02 3
14 <0.01 3
Untreated 1 <0.01 3
control 3 0.02 3
7 0.01 3
14 <0.01 3
Peas2/ U.K. 1 0.2 50SC3/ 27 <0.01 <0.01 5
flowering 1981 1 0.3 <0.01 <0.01 5
stage (MarQ) 1 0.4 <0.01 <0.01 5
1 0.05 <0.01 <0.01 5
Untreated
control <0.01 <0.01 5
4-leaf 1 0.2 27 <0.01 <0.01 5
stage 1 0.3 <0.01 <0.01 5
1 0.4 <0.01 <0.01 5
1 0.5 <0.01 <0.01 5
Untreated
control <0.01 0.01 5
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pea pods 1 0.2 50SC3/ 83 <0.01 <0.02 5
flowering 1 0.3 <0.01 <0.06 5
stage 1 0.4 <0.01 0.07 5
1 0.5 <0.01 0.03 5
Untreated
control <0.01 0.03 5
4-leaf
stage 1 0.2 83 <0.01 0.05 5
1 0.3 <0.01 0.05 5
1 0.4 <0.01 0.08 5
1 0.5 <0.01 0.06 5
Untreated <0.01 0.05 5
control
Apple
(Cox's U.K. 24/ 0.04 and 20W 57 <0.01-0.01 <0.01 6
Orange 1981 0.08% 50W,
Pippin) 50 SC
Untreated 0.006 0.005 1
14/ 0.04 and 20W, 106 <0.01-0.01 <0.01-0.01 6
0.08% 50W,
50SC 0.009 0.003 1
4/ Untreated
2 0.02, 20W, 98 <0.01-0.01 <0.01-0.01 9
0.04 and 50W,
0.08% 50SC
untreated 0.003 0.008 1
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Pear 14/ 0.02% 50SC 158 0.01 <0.01 1
(Conference) 14/ 0.04% 0.02 0.01 1
14/ 0.08% untreated 0.01 0.005 1
Mushrooms The Netherlands
1980 2 5 50SC 21 0.04-0.06 0.01-0.03 4
(50 g a.i./ <0.05 av.) (<0.02 av.)
100 m2)
2 7.5 50SC 21 0.08-0.12 0.01-0.04 4
(75 g a.i./ (0.1 av.) (0.02 av.)
100 m2)
2 10 50SC 21 0.11-0.14 0.02-0.06 4
(100 g a.i./ (0.13 av.) (0.04 av.)
100 m2)
Untreated <0.01-0.02 <0.01-0.1 4
(0.01 av.) (0.01 av.)
U.K. g a.i./100 m2
1981 wall spray5/
1 52.2 50SC 69 N.D.6/ <0.02 1
untreated 69 N.D. N.D. 1
1 20 80W 33 N.D. N.D. 1
untreated 33 0.001 N.D. 1
1 48 80W 41 <0.02 0.02 1
untreated 41 0.04 0.02 1
(g a.i./100 m2
wall spray 5/)
Table 5. (con't)
Application
Country/ Rate Preharvest
Crop Year No. (kg a.i./ha Formulation interval Residues (mg/kg) No. samples No. replicates
or % a.i.) (days) Bendiocarb NC 7312
Mushrooms U.K. 1 20 80W 26 <0.02 <0.02 1
1981 untreated 26 N.D. N.D. 1
1 20 80W 33 <0.02 <0.02 1
untreated 33 N.D. 0.003 1
1 20 80W 52 N.D. <0.02 1
20 80W 60 0.04 0.02 1
untreated 52 N.D. N.D.
48 80W 42 0.06 0.1 1
48 80W 42 0.09 0.13 1
48 80W 42 0.08 0.10 1
1/ Residue results corrected for mean analytical recovery of 82% with fortification at 0.08-0.2 mg/kg.
2/ Results corrected for recoveries. Harvest (127 days after planting) said to be 2-3 weeks after normal harvest.
3/ SC = Suspendable concentrate.
4/ 1.5 1 spray solution per tree.
5/ Mushroom plots established the day after wall treatment.
6/ N.D. = no detectable chromatographic peaks.
Legume Vegetables
Peas
Supervised residue trials were conducted in the U.K. with single
applications at rates consistent with recommended ones. No information
was available on specific nationally approved good agricultural
practices, nor was information available on recommended numbers of
applications or preharvest intervals.
Peas were sampled at 2-3 weeks after normal harvest (127 days
after planting). At all application rates tested, residues of either
bendiocarb or NC 7312 (corrected for recovery) were <0.01 mg/kg in
peas, including untreated controls (Table 5). The same is true for
bendiocarb in pea pods, but residues of NC 7312 ranged from
0.03-0.08 mg/kg (0.05 mg/kg av.), and untreated controls were 0.02 and
0.05 mg/kg. Although bendiocarb residues were at or about the limit of
determination, the data suggest that terminal residues of NC 7312 may
be 2-10 times that of bendiocarb.
Analytical recoveries averaged 86 and 72% for bendiocarb and NC
7312, respectively, in peas from fortification levels of 0.04 and
0.2 mg/kg. Recoveries in pods averaged only 58 and 63%, respectively.
The limit of determination was said to be 0.01 mg/kg for bendiocarb
and NC 7312 in both peas and pods. However, based on untreated
controls, 0.05 mg/kg may be a more appropriate limit of determination
for NC 7312 in pea pods.
Because GAP information is unavailable and samples were not
harvested at normal harvest, a maximum residue level cannot be
estimated with confidence.
Pome Fruit
Apples and Pears
Residue data were available from supervised trials in the U.K. at
application rates of 0.02-0.08% active ingredient (1.5 1/tree),
representing several formulations. Applications were at pre- or
post-blossom and/or at codling stage, and fruit was sampled at normal
harvest of 57-158 days after last treatment. No information was
available on specific nationally approved agricultural practices.
Recommended applications rates of 0.25-0.5 kg/ha are not directly
comparable to the 0.02%-0.08% used in the field trials. No information
was provided on recommended preharvest intervals or numbers of
applications.
Of the 24 apple and pear samples treated in three locations and
in untreated controls, residues of bendiocarb and NC 7312 were
< 0.01 mg/kg, except for one 0.02 mg/kg value for pears treated
once with a 0.08% 50 SC formulation (Table 5). Mean recoveries of
bendiocarb and NC 7312 on apples and pears were 94 and 73%,
respectively, when fortified from 0.1 to 0.4 mg/kg. The limit of
determination is said to be 0.01 mg/kg for both bendiocarb and
NC 7312.
If the formulations, application rates, number of applications
and preharvest intervals of the field trials represent GAP, the data
could support a maximum residue level of 0.02 mg/kg for bendiocarb per
se or 0.05 mg/kg for combined residues of bendiocarb and NC 7312.
Cereal Grains
Barley
Fourteen supervised trials were conducted in the U.K. with
in-furrow granular, 50W spray or 80W seed dressing applications. No
nationally approved agricultural practice information was provided,
although recommended uses for cereals were reported (Table 2). No
recommended uses for the granular formulation (2 trials) were
provided. Only two of the trials were from spray treatments and the
remainder were from seed treatments. The spray applications at 0.5 and
1.0 kg a.i./ha reasonably reflect the recommended 0.75 kg a.i./ha
although the data are limited (Table 6) (Browne and Reary 1980c,d,e;
Housden and Reary 1981; Reary 1978 h,i,j,n; Reary 1979e,f,g,j,k,l,m,o,
1980a,e; Reary and Browne 1978). The seed treatments at 2-5 g a.i./kg
seed reflect the recommended rate of 2-5 g a.i./kg.
Residues of bendiocarb at normal harvest in barley grain were
<0.02 mg/kg and <0.03 mg/kg in straw. Untreated controls in
barley grain and straw were at the same level. Some samples were
also analysed for combined conjugated metabolites, NC 7312 and
N-hydroxymethylbendiocarb. In all three trials, metabolite residues
were <0.02 mg/kg. Mean analytical recoveries for bendiocarb in
barley during the supervised trials were 94 and 84%, respectively, for
grain and straw when fortified at 0.02-0.2 mg/kg. In a separate study,
bendiocarb recoveries in barley grain were 61-125% (av. 94%) after
fortifications at 0.02-0.2 mg/kg (Reary 1979b).
Table 6. Bendiocarb Residues in Cereals from Supervised Trials
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
Barley, wheat and oats
U.K. 1 1 80W 4 g/kg seed Seed dressing 148 barley grain <0.02
1977 1 1 148 barley straw <0.02
1 2 80W Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
1 2 50SC 4 g/kg seed Seed dressing 148 barley grain <0.02
1 2 148 barley straw <0.02
1 2 50SC Seed dressing 148 wheat grain <0.02
1 2 148 wheat straw <0.02
U.K. 3 3 80W 4 g/kg seed Seed dressing 113-145 barley grain <0.02
1978 2 2 113-145 barley straw <0.03
2 2 124-137 wheat grain <0.02
1 1 124-137 wheat straw <0.06
3 3 (10 rep) 134-141 oat grain <0.02
2 2 (10 rep) 134-141 oat grain <0.02
2 2 80W 8 g/kg seed Seed dressing 136-147 oat grain 0.47
3 2 136-147 oat straw 0.02-0.05
U.K.1/ 1 1 3G2/ 0.5 kg/ha In furrow 148 barley grain <0.01
1979 1 1 148 barley straw <0.01
1 1 3G2/ 1.0 kg/ha In furrow 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 50W2/ 0.5 kg/ha Spray 148 barley grain <0.01
148 barley straw <0.01
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 50W2/ 1.0 kg/ha Spray 148 barley grain <0.01
1 1 148 barley straw <0.01
1 1 80W 2 g/kg seed Seed dressing 336 wheat grain <0.01
1 1 336 wheat straw <0.01
3 3 80W 4 g/kg seed Seed dressing 119-148 barley grain <0.01
119-148 barley straw <0.01-0.02
3 3 127-336 wheat grain <0.01
2 2 127-336 wheat straw <0.01
2 2 105-125 oat grain <0.012
2 2 105-125 oat straw <0.01,<0.03
2 2 80W 5 g/kg seed Seed dressing 119-127 barley grain <0.01
2 2 119-127 barley straw <0.01,<0.02
1 1 127 wheat grain <0.01
1 1 127 wheat straw <0.01
2 2 105-125 oat grain <0.01
2 2 105-125 oat straw <0.01
Maize harvest samples
Canada 22 37 10G 0.84-2.24 band 153-189 grain <0.02
U.S. 8 8 kg/ha at 132-165 cob <0.02
1977-78 21 38 plant 132-189 stover3/ <0.03
10 28 110-134 silage <0.023
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
German 25 30 3G 0.2-0.8 in furrow 133-184 grain <0.024/
Fed. Rep. 26 36 kg/ha at plant 128-184 cobs <0.024/
5 12 128-184 plants <0.01
U.K. 10 10 146-184 foliage <0.01
France 5 5 135-164 silage <0.02
Italy 133-154 stover <0.02
1976-79
France 30 36 76 or 2-16 g/kg seed 125-189 grain <0.035/
U.K., U.S. 30 24 80W seed treatment 160-161 cob <0.026/
1975-1979 18 23 125-167 silage <0.02
11 10 160-189 stover <0.02
14 8 141-164 plants <0.056/
Rice (rough) harvest samples
Philippines7/ 1 1 5G 1.5 + 0.5 Incorporation + 53 grain <0.01
1978 surface applied straw <0.02
1 1 5G 2.0 + 0.6 Incorporation + 53 grain <0.01
surface applied straw <0.02
1 1 50W 0.5 kg/ha Spray 90 grain 0.01
straw <0.02
1 1 50W 0.6 kg/ha Spray 90 grain <0.01
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
2 2 80W 0.5 kg/ha Spray 60-90 grain <0.01
straw <0.02
2 2 80W 0.6 kg/ha Spray 60-90 grain <0.01
straw <0.02
Philippines7/ 1 1 50W 0.5 kg/ha Spray 248/ grain <0.01
1979 straw <0.01
1 1 50W 0.7 kg/ha Spray 248/ grain <0.01
straw 0.01
1 1 50W 1.0 kg/ha Spray 248/ grain 0.01
straw <0.01
1 1 50W 0.5 kg/ha Spray 459/ grain 0.01
straw -
1 1 50W 0.75 kg/ha Spray 459/ grain <0.01
straw -
1 1 50W 0.5 kg/ha Spray 4910/ grain <0.01
straw <0.01
1 1 50W 0.75 kg/ha Spray 4910/ grain 0.01
straw 0.02
South Korea7/ 1 1 50W 0.75 kg/ha Spray 10 grain 0.6, 1.111/
1979 straw <0.6
1 1 2D 0.8 kg/ha Dust 10 grain 1.5, 1.411/
straw <0.65
1 1 3GW 0.9 kg/ha Seed box 119 grain 0.06, 0.03
incorporation straw 0.02
(100 g/box)
straw <0.02
Table 6. (con't)
No. No. Application Interval after Residues
Country Trials Samples Formulation Rate (a.i.) Method application Sample (mg/kg)
(days)
1 1 5G 1.5 kg/ha Soil 100 grain <0.6
incorporation straw 0.04
Philippines
1980 1 1 50W 0.25 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.50 kg/ha Spray 6212/ grain <0.02
straw <0.01
1 1 50W 0.75 kg/ha Spray 6212/ grain <0.02
straw <0.01
1/ Also analysed for combined residues of conjugated NC 7312 and N-hydroxymethylbendiocarb. All metabolite residues were
< 0.02 mg/kg except for residues of 0.07 and 0.05 mg/kg from one trial in oat grain from seed dressing of spring oats
with an 80 WP formulation at 4 and 5 g a.i./kg seed. The two residues resulted from oat grain samples with bendiocarb
residues 0.012 and 0.007 mg/kg respectively. Untreated controls were 0.011 mg/kg.
2/ Seed previously treated with an 80% WP formulation
3/ Stover = leaves and stems after removal of cobs.
4/ Analysis of oil from kernels with <0.02 mg/kg residue from one site showed no residue greater than the 0.02 mg/kg limit
of determination.
5/ The 0.03 mg/kg on grain resulted from treatments at 8 and 12 g a.i/kg seed. At <4 g a.i./kg seed residues were
<0.02 mg/kg in grain.
6/ Maize (sweet corn) comprised 14 of the trials at 2-8 g a.i./kg. All plants in this group were sweet corn. The 0.05 mg/kg
residue on sweet corn plants resulted from 2-4 g a.i./kg seed.
Table 6 (con't)
7/ Also analysed for metabolite NC 7312. No residues above the limits of detection (0.01 mg/kg in grain and 0.02 mg/kg in
straw) were found, except for 0.11 and 0.17 mg/kg in grain from dust and spray treated samples at the 10-day interval and
one 5G formulation at 100 days, in which it was 0.03 mg/kg. In the latter, the control was also 0.03 mg/kg.
8/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 17, 27, 39, 52,
66 and 80 days after transplanting.
9/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 10, 25 40 and
55 days after transplanting.
10/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 7, 20 and 45
days after transplanting.
11/ Values reflect residue determination by electron capture and fluorescence, respectively.
12/ Period between final application and harvest in multi-spray programme. Sprays, at same rates, were made 22, 35, 51 and
68 days after transplanting.
The limit of determination was said to be 0.01 mg/kg for both
cereal grain straws and grains, although apparent residues in controls
(<0.02 and <0.03 mg/kg in barley grain and straw, respectively)
suggest 0.02 or even 0.05 mg/kg may be a more realistic estimate for
both. When considered with data from other cereal grains and straws, a
0.05 mg/kg limit for bendiocarb per se at or about the limit of
determination could be supported if recommended uses represent GAP. A
0.1 mg/kg limit would be appropriate if both bendiocarb and
metabolites are included.
Maize
Over 100 supervised trials were conducted in six countries, two
in North America and four in Europe (Table 6). Applications were made
with granular formulations at planting, either by band or in-furrow
treatment and seed was treated with wettable powder formulations.
In-furrow applications of a 3G formulation at 0.2-0.8 kg a.i./ha
corresponds to the 0.3-0.4 kg a.i./ha Netherlands GAP, although no
data were available from The Netherlands. The recommended uses on
maize provided to the Meeting did not include the method of
application for granular formulations.
Granular band treatments at planting of 0.84-2.24 kg a.i./ha did
not correspond with certainty to either The Netherlands GAP or
recommended usage for maize. The 2-16 g a.i./kg wettable powder seed
treatments more than accommodate the 2-4 g a.i. GAP of The Netherlands
or recommended application rates. Additional information on official
national GAP is needed.
Maximum residues of bendiocarb per se from all treatments were
<0.03, <0.02, <0.023, 0.05 and <0.01 mg/kg in grain, cobs,
stover, silage, plants and foliage, respectively. Analysis of oil from
one grain sample with field-incurred residues of <0.02 mg/kg also
showed residues of less than the 0.02 mg/kg limit of determination.
The 0.03 mg/kg level in grain resulted from applications at 2-3 times
the recommended rate. At recommended rates all grain residues were
<0.02 mg/kg. The 0.05 mg/kg residue on plants was on maize (sweet
corn) treated according to recommended rates.
The limit of determination was said to be 0.01-0.02 mg/kg for the
various maize fractions. Apparent residues in untreated controls were
also in this range. Analytical recoveries during the trials averaged
approximately 81, 78, 72, 80 and 78% for grain, cob, silage, stover
and plants, respectively. A 0.05 mg/kg limit at or about the limit of
determination could be supported for bendiocarb per se in maize (field
corn) grain, fodder and/or forage. Additional data would be required
for inclusion of metabolites.
In addition to the trials at harvest, 12 residue decline trials
were conducted in four countries (FBC 1982a; Browne and Reary 1979d;
Reary 1978l,m, 1979p, 1980h,i), mostly from granular in-band or
in-furrow treatments at planting ranging from 0.3-2.24 kg a.i./ha.
Residues in grain from granular treatments were <0.01 mg/kg. In
plants, residues ranged from <0.05-0.9 mg/kg at 25-40 days after
treatment (0.9 at 29 days), declining to <0.07 mg/kg after 49 days
and <0.02 after 56-70 days. Residues in stover and cobs were <0.02
and <0.02 mg/kg, respectively, after 71 days.
From the single 4 g a.i./kg seed treatment with a wettable powder
formulation, residues on plants were 12.4 mg/kg 29 days after
treatment but rapidly declined to _0.023 mg/kg after 42 days.
Oats
Residue data were available for seven supervised trials in the
U.K. from 4-8 g a.i./kg seed 80W seed dressings (Table 6). No
information was provided on specific nationally approved practices.
At application rates approximating the 4 g a.i./kg seed
recommended application rate, residues in oat grain at normal harvest
were 0.02 mg/kg and in straw were _0.02 mg/kg. One of the two grain
samples treated at 8 g a.i./kg seed had a residue of 0.47 mg/kg.
Contamination of this sample and its 0.12 mg/kg untreated control was
suspected by the manufacturer to have occurred during sampling.
Maximum residues in straw from the 8 g a.i/kg seed application rate
were 0.05 mg/kg. While this could be aberrant, as the untreated
control was 0.12 mg/kg, it is not necessarily so, since residues are
as high as 0.03 mg/kg in straw of oats and barley from recommended
application rates. Residues in untreated controls were <0.02 and
<0.04 mg/kg in grain and straw, respectively, except for 0.12 mg/kg
from a grain and straw sample with suspected contamination.
A limited amount of residue data were also available for combined
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) in some
oat samples, with residues being <0.012 mg/kg and <0.01 mg/kg in
grain and straw, respectively.
Mean recoveries of bendiocarb were 89 and 94% for oat grain and
straw, respectively. The limit of determination was said to be
0.02 mg/kg for both, but based on the 0.035 mg/kg apparent residue in
untreated controls, 0.05 mg/kg may be more appropriate, especially
when barley and wheat are also considered. A 0.05 mg/kg limit at or
about the limit of determination could be supported for bendiocarb per
se if recommended uses represent GAP. A 0.1 mg/kg limit would be
required if both bendiocarb and metabolites were included.
Rice
Residue data (corrected for mean recoveries) were available from
22 supervised trials on rough rice at normal harvest in The
Philippines and South Korea (Table 6). Applications were by granular,
spray and dust formulations. No information was available on
nationally approved agricultural practices on rice. Recommended
application rates for rice are 2 and 0.5 kg a.i./ha for granular and
spray formulations, respectively, and 0.5-0.75 kg a.i./ha for dust, as
summarized in Table 2. No information was provided on approved
intervals from last application or maximum number of applications.
Only one trial utilized a dust formulation, and the application
rate reflects the recommended use. Bendiocarb residues were 1.5 mg/kg
and 0.65 mg/kg, respectively, for grain and straw 10 days after
application as compared to 0.6-1.1 mg/kg for a spray application after
the same interval. Residues for NC 7312 were 0.17 mg/kg at the same
10-day interval. Untreated controls were <0.3 mg/kg and 0.04 mg/kg,
respectively, for grain and straw.
Spray applications of 0.25-0.75 kg a.i./ha covers the 0.5 kg/ha
recommended rate. As in the case of dust treatments, the spray
applications at 10 days before normal harvest resulted in higher
residues than at longer intervals, measuring <1.1 and <0.5 mg/kg,
respectively, for grain and straw. Similarly, residues were higher for
NC 7312 in grain, being 0.11 mg/kg. The same control as for the 10-day
interval dust treatment applies.
At longer intervals from last treatment with 50W or 80W
formulations (24-90 days) and up to 1.5 times the recommended rate,
residues were <0.02 mg/kg for both grain and straw. Untreated
controls for spray treatments, except for the 10-day interval
treatment, were <0.021 and <0.02 mg/kg, respectively, for
bendiocarb in grain and straw, except for one untreated straw sample
at 0.04 mg/kg. Residues of NC 7312 were <0.03 mg/kg and 0.02 mg/kg
for grain and straw, respectively, from spray treatments.
Four trials were conducted with either 3G or 5G formulations at
rates reflecting the recommended application rate and different
application methods. Maximum residues of bendiocarb were <0.6 mg/kg
and 0.04 mg/kg, respectively, in grain and straw. Untreated controls
were <0.07 and <0.04 mg/kg bendiocarb in grain and straw,
respectively. Residues of NC 7312 were <0.03 and <0.02 mg/kg in
grain and straw, respectively.
Analytical recoveries of bendiocarb during the trials averaged 77
and 68%, respectively, in rice grain and straw from fortifications at
0.02-0.25 mg/kg. Average recoveries were less (64 and 56%) for NC
7312. In a separate study on recoveries (Browne and Reary 1979b) at
similar fortification levels, mean recoveries were better, being 86
and 74% for bendiocarb in grain and straw, and 70 and 56%,
respectively, for NC 7312.
In the absence of GAP information, 2 mg/kg and 1 mg/kg limits
would be required for bendiocarb per se in rice grain and straw,
respectively, to accommodate the uses of the supervised trials.
Wheat
Residue data, corrected for recoveries, were available from eight
supervised trials in the U.K. from seed treatments with 80W or 50SC at
2-5 g a.i./kg seed (Table 6). No information was available on specific
nationally approved agricultural practices, although the application
rates used reflect the 2-4 g a.i./kg seed recommended rate for
cereals.
Residues for bendiocarb at harvest were <0.02 mg/kg for both
grain and straw, except for one 0.06 mg/kg value for straw. This
0.02 mg/kg level is approximately the claimed limit of determination.
Untreated controls were <0.04 mg/kg and <0.12 mg/kg,
respectively, for grain and straw. The 0.12 mg/kg for straw is from
the same study in which the high treated sample residue was found.
Contamination was suspected. Other values were <0.02 mg/kg.
Analytical recoveries from the individual studies were relatively
low, averaging only 63-65% for grain and straw at fortification levels
of 0.02-0.2 mg/kg. In a separate determination (Reary 1979b)
recoveries of bendiocarb in grains were better, averaging 84%. The
limit of determination for barley, oats and wheat is said to be
<0.02 mg/kg for grain and straw. Overall data support a 0.05 mg/kg
limit for bendiocarb per se.
Limited data were also available for combined conjugated residues
of NC 7312 and N-hydroxymethylbendiocarb. Residues were <0.01 and
<0.12 mg/kg for wheat grain and straw, respectively, although
analytical recoveries were only 62 and 67% in cereal grains and straw,
respectively.
Fodders and Straws
Fodders and straws of cereal grains have been discussed
previously. The other member of this group for which data were
provided is grass fodder, or more specifically turf and grass. Data
were available from 72 supervised trials in the U.K., U.S. and
Australia from 1975-1980, representing a variety of formulations,
single or multiple applications at several rates, intervals, and
methods of application as well as irrigated and unirrigated conditions
(Table 7) (Browne 1981d; FBC 1982c; Reary 1975d, 1978f,g, 1980b,d,e,k,
1981m; Reary and Whiteoak 1977a,b,c,d). No information was available
on specific national agricultural practices, although recommended
rates for rye grass is given in Table 1.
Granular treatments, both single and multiple broadcasts, were
mostly at application rates 6-12 times the recommended rate (for rye
grass). Three granular broadcast trials (single applications) at 1.6
times the recommended rate resulted in maximum residues ranging from
0.24 mg/kg at 43 days after treatment to 0.09 mg/kg after 55 days.
Residues were <0.1 mg/kg from three granular seed mix trials (single
application) at 0.6-1.4 times the recommended granular application
rate when analysed 196 days after treatment.
As in the case of granular treatments, single or multiple spray
treatments were mostly at rates 6-12 times the maximum recommended
application rate for rye grass. After single applications at rates
approximating recommended rates (1-1.6x), maximum residues from spray
treatments ranged from 103 mg/kg (193 mg/kg dry basis) at day of
application to 0.03 mg/kg after 141 days.
No recommended rates were available specifically for ULV aerial
applications. Residues from a single application at 0.28 kg/ha ranged
from 50 mg/kg (111 mg/kg dry basis) on day of application to 5.5 mg/kg
(12 mg/kg dry basis) after 29 days.
Seed dressing trials were conducted at treatments rates of 1-4
times the recommended rate of 10 g a.i./kg seed. Residues were
<0.2 mg/kg at all rates after 43 days.
Although many of the treatments with the various formulations
were at exaggerated application rates, these trials did permit
estimations of decline rates, the potential of residue accumulation
and effect of irrigation on residues. Residue half-lives were greater
for granular broadcast applications, for which 37-42 days was
estimated. For spray application half-lives ranged from 2-15 days,
depending on the trial. The half-lives were 5.3 and 2.4 days for seed
treatments and aerial ULV treatments, respectively. In general,
irrigation did not significantly affect decline rates from granular
broadcast or wettable powder spray treatments as compared to rates
measured under non-irrigated conditions. Residues did not accumulate
from multiple applications at 28-day intervals.
Table 7. Bendiocarb Residues in Grass and Turf from Supervised Trials
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 1 - 80W 1 kg/ha Spray Rye grass <1 130 1
1975 1-5 <109 3
6-10 <40 2
11-20 <15 2
21-50 <7 3
U.S. 1* 4.3 76W 2.24 kg/ha Spray Turf grass <1 <236 6
1976 1-5 <225 6
6-10 <115 4
11-20 <19 2
1* Thatch <1 <25 6
1-5 <26 6
6-10 <9 4
11-20 <1 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <326 6
1-5 <386 6
6-10 <190 4
11-20 <46 2
1* Thatch <1 <36 6
1-5 <97 6
6-10 <79 4
11-20 <1.6 2
1* 2-4 76W 2.24 kg/ha Spray Turf grass <1 <293 5
1-5 <201 4
6-10 <4 4
11-20 <2.8 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1* Thatch <1 <19 5
1-5 <13 4
6-10 <16 4
11-20 <21 2
1* 76W 4.48 kg/ha Spray Turf grass <1 <607 5
1-5 <710 4
6-10 <30 4
11-20 <7.5 2
Thatch <1 <56 5
1-5 <18 4
6-10 <32 4
11-20 <26 2
1* 5G 2.24 kg/ha Broadcast Turf grass <1 <1.4 5
1-5 <2.7 4
6-10 <0.6 4
11-20 <0.3 2
1* Thatch <1 <13 5
1-5 <11 4
6-10 <18 4
11-20 <17 2
1* 5G 4.48 kg/ha Broadcast Turf grass <1 <3 5
1-5 <6 4
6-10 <8 4
11-20 <0.7 2
1* Thatch <1 <34 5
1-5 <42 4
6-10 <47 4
11-20 <27 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
1977 2* 5-15* 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
21-50
51-80
81-110
111-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 5-15 76W 4.48 kg/ha Spray Turf grass 1-5 <30 4
6-10 <34 2
11-20 <10 2
21-50 <7 4
51-80 <3 2
81-110 <1.2 2
111-140 <0.3 3
>141 0.2 1
2* 76W 8.96 kg/ha Spray Grass 1-5 <125 5
6-10 <109 2
11-20 <19 2
21-50 <23 2
51-80 <2.5 2
81-110 <0.8 2
111-140 <0.4 3
>141 0.3 1
2* 5.6-14 76W Multi-spray1/ Turf grass 1-5
6-10
11-20
21-50
51-80
81-110
110-140
>141
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2* 76W Multi-spray2/ Turf grass 1-5 <16 3
6-10 <16 2
11-20 <9 2
21-50 <2 2
51-80 <0.9 2
81-110 <0.3 2
111-140 <0.1 2
>141 0.1 1
2* 76W Multi-spray3/ Turf grass 1-5 <24 2
6-10 <2 2
11-20 <3 2
21-50 <1.7 2
51-80 <1.1 2
81-110 <0.3 2
111-140 0.1 1
2* 76W Multi-spray4/ Turf grass 1-5 <37 4
6-10 <2.5 2
11-20 <3.4 2
21-50 <1 2
51-80 <0.4 2
81-110 0.1 1
2* 76W Multi-spray5/ Turf grass 1-5 <80 4
6-10 <80 4
11-20 <20 2
21-50 <3.7 2
51-80 0.5 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 37-42 5G Broadcast6/ Turf grass 1-5 <8 5
6-10 <3.3 2
11-20 <1.9 2
21-50 <3.7 2
51-80 <8.1 2
81-110 <2.6 2
111-140 <1.6 2
>141 <0.5 2
2 5G Broadcast1/ Turf grass 1-5 <7 5
6-10 <7 2
11-20 <1.4 2
21-50 <2.8 2
51-80 <0.16 2
81-110 <0.7 2
111-140 <0.55 2
>141 0.16 1
2 5G Broadcast2/ Turf grass 1-5 <9.5 4
6-10 <4.7 2
11-20 <4.4 2
21-50 <3.5 2
51-80 <0.7 2
81-110 <0.7 2
111-140 <0.7 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 5G Broadcast3/ Turf grass 1-5 <1.0 2
6-10 <9.0 2
11-20 <8.0 2
21-50 <4.0 2
51-80 <2.0 2
81-110 <0.8 2
111-140 0.8 1
2 5G Broadcast4/ Turf grass 1-5 <9.1 4
6-10 <8.2 2
11-20 <7.4 2
21-50 <7.6 2
51-80 <3.9 2
2 5G Broadcast5/ Turf grass 1-5 <7.3 4
6-10 <6.1 2
11-20 <5.7 2
21-50 <2.8 2
Australia 1 80W 1.6 g/kg seed Seed dressing Grass (pasture) 81-110 <0.02 2
1979 111-140 <0.02 2
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
U.K. 3 80W 10 g/kg seed Seed dressing Grass (grazing) 21-50 0.09 1
1979 51-80 <0.06 2
3 80W 40 g/kg seed Seed dressing Grass " 21-50 0.17 1
51-80 <0.11 2
3 3G 1.2 kg/ha Broadcast Grass (grazing) 21-50 0.24 1
51-80 <0.09 2
3 50SC 1.2 kg/ha Spray Grass (grazing) 21-50 <0.2 3
2 50SC 10 g/kg seed Seed dressing Grass (grazing) >141 <0.01 3
1 50SC 15 g/kg seed Seed dressing " " >141 0.01 1
2 50SC 20 g/kg seed " " " " >141 <0.01 3
2 50SC 0.5 kg/ha Spray Grass (grazing) >141 <0.01 3
1 50SC 0.75 kg/ha Spray " " >141 0.03 1
2 50SC 1.0 kg/ha Spray " " >141 <0.015 3
U.S. 1 6 50SC 0.84 kg/ha Spray Wheat grass <1 103 1 193
1979 1-5 <97 2 <139
6-10 26 1 34
11-20 16 1 18
21-50 <9.3 2 <16
U.K. 2 5.3 80W 10 g/kg seed Seed Dressing Grass (grazing) 51-80 <0.02 2
1980 2 80W 15 g/kg seed Seed dressing " " 51-80 <0.03 2
2 80W 20 g/kg seed Seed dressing " " 51-80 <0.1 3
1 50SC 5×0.5 kg/ha Spray Grass (grazing) 51-80 <0.03 6
81-110 0.01 1
Table 7. (con't)
Country/ No. Half- Application Application Sample Interval after Residues No. of Dry
Year Trials life Formulation Rate (a.i.) Method application (mg/kg) Samples weight
(days) (days) basis
2 50SC 0.75 kg/ha Spray Grass (grazing) 51-80 <0.02 2
2 50SC 1.0 kg/ha Spray Grass " 51-80 <0.03 2
1 5G 0.75 kg/ha Seed mix Grass (grazing) 51-80 <0.08 2
1 3G 0.45 kg/ha Seed mix Rye Grass >141 <0.1 2
1 5G 0.75 kg/ha Seed mix Rye grass >141 <0.1 2
1 7G 1.05 kg/ha Seed mix Rye grass >141 <0.1 1
U.S. 1 2.4 25ULV 0.28 kg/ha Aerial Spray Rangeland grass <1 50 1 111
1980 1-5 5.36 2 <73
6-10 1.7 1 4
11-20 0.7 1 2
21-50 5.5 1 12
* Trials irrigated and non-irrigated. Residue levels maximum found.
1/ Rate of 4.48 kg/ha.
2/ Multi-application programme of 4.48 + 2.24 kg/ha, 28 days apart.
3/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha each 28 days apart.
4/ Multi-application programme of 4.48 + 2.24 + 2.24 kg/ha, each 28 days apart.
5/ Multi-application programme of 4.48 + 2.24 + 2.24 + 4.48 kg/ha, each 28 days apart.
6/ Rate of 8.96 kg/ha.
Apparent bendiocarb in untreated grass controls were generally
<0.2 mg/kg, although they occasionally approached 1 mg/kg and were
1.8 mg/kg in one sample. The limit of determination was said to be
0.01-0.1 mg/kg, depending on the trial. Based on untreated controls,
1 mg/kg may be a more realistic limit of determination for grass.
Analytical recoveries during the trials ranged from 43 to 119% and the
mean for all the trials was approximately 80%.
Legume Animal Fodders
See discussion on pea pods under legume vegetables.
Oilseeds
Bendiocarb residue data on oilseed rape resulting from supervised
trials were available from one country where applications of a 5G
formulation was applied at sowing with the seed at application rates
of 0.140 and 0.28 kg a.i./ha which is the recommended rate for
granular formulations (Table 2) (Browne and Reary 1980f). No
information was available on specific nationally approved agricultural
practices.
Nine replicates were analysed for bendiocarb per se on mature
seed (114 days after application) at each rate with residues
<0.006 mg/kg. Samples were not analysed for conjugates of NC 7312
and N-hydroxymethylbendiocarb, although a metabolism study has shown
these to be the major metabolites in rape seed (see section on "Fate
of Residues in Plants"). Residues in untreated controls were also
<0.006 mg/kg with mean analytical recoveries of 84% for
fortifications at 0.05-0.5 mg/kg.
These data are inadequate for estimating a maximum residue level.
Additional data from granular applications as well as data from spray
treatments reflecting GAP are needed, as well as information on
nationally approved uses, including preharvest intervals and number of
applications.
Meat
Cattle
Residue data available from two feeding studies and two dermal
treatment studies are given in Table 8 (Browne and Reary 1978a,b;
Reary 1978a, 1979a, 1980c).
Table 8. Bendiocarb Residues in Milk and Cattle Tissues after Feeding Trials or Dermal Treatments1/
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
Milk
(non-conjugated2/
residues) <0.01 <0.01 <0.01 <0.01 <0.01 0.014 0.005 0.015 0.06 <0.02 <0.04
Milk
(conjugated3/ <0.02 <0.02 <0.02 <0.02 <0.02 0.027 0.027 0.039 0.057 <0.02 <0.02
residues)
Muscle4/ <0.02 <0.02 <0.02 <0.02 <0.02 - - - - <0.01 <0.01
Omental fat4/ <0.02 <0.02 <0.02 <0.02 <0.02 <0.02 <0.03
Perirenal fat4/ <0.05 <0.05 <0.05 <0.05 <0.02 <0.05 <0.04
Subcutaneous-
muscle4/ <0.01 <0.01
fat4/ <0.15 <0.12
Table 8. (con't)
28-Day Feeding Studies at Given 1% Dust Dermal Treatments at Given Treatment
Dietary Levels (mg/kg) Levels (g product/treatment/cow)
Single Self Manual treatment
Manual Treatment5/ treatment6/ (7 successive days)
Tissue 0.25 0.75 2.5 7.5 25 5 15 25 5 5 25
liver4/ <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
kidney4/ <0.1 <0.1 <0.1 <0.1 <0.1 <0.05 <0.11
1/ Corrected for mean recoveries;
2/ Bendiocarb and non-conjugated NC 7312 and N-hydroxymethyl bendiocarb (fat fraction);
3/ Total conjugated NC 7312 and N-hydroxymethyl bendiocarb (aqueous or lactose fraction);
4/ Total residues of bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb;
5/ Analysed 6 hours post-treatment;
6/ 7-21 day self-treatment. Treatment rate estimated to be approximately 5 g product/cow/day.
In a feeding study (Browne and Reary 1978a), lactating dairy cows
were fed for a minimum of 28 days with a diet containing 0, 0.25, 0.75
and 2.5 mg/kg bendiocarb. A maize premix was the vehicle for adding
bendiocarb to the diet twice daily. Premises were stored at -4°C prior
to use. Each treatment group consisted of two cows. Milk samples were
pooled for both cows in each treatment group over 24 hours. Samples
were taken before treatment and after 1, 2, 4, 8, 12, 16, 22 and 28
days on the bendiocarb fortified diet. Milk extracts were separated
into a fat fraction (hexane/ether soluble) and a lactose fraction
(water soluble), the first of which was analysed for total
non-conjugated residues of bendiocarb, NC 7312 and
N-hydroxymethylbendiocarb. The lactose fraction was analysed for total
conjugated residues. Residues (bendiocarb equivalent in milk) in all
fat samples were less than the estimated 0.01 mg/kg limit of
determination, corrected for mean recoveries of 64%. Residues in the
lactose fraction were also <0.01 mg/kg, but the limit of
determination was considered 0.02 mg/kg because of chromatographic
interferences. Based on the chromatograms, 0.05-0.1 mg/kg may be a
more realistic limit of determination for the aqueous fraction when
interfering co-extractives are taken into consideration. In each case,
untreated controls gave no apparent residue above the estimated limits
of determination.
Samples of omental fat, perirenal fat, muscle, liver and kidney
were taken from each animal at sacrifice (within 18 hours of final
bendiocarb intake). Each was analysed for total residues of bendiocarb
and metabolites, conjugated and non-conjugated. No residue (corrected
for recovery) was found above the estimated limits of determination of
0.02 mg/kg for muscle and omental fat, 0.05 mg/kg for liver and
perirenal fat and 0.1 mg/kg for kidney. Muscle samples at the 0.25 and
0.75 mg/kg dietary feeding level did show apparent residues at the
0.02 mg/kg limit of determination as did one untreated control. Based
on representative chromatograms, the above estimated limits of
determination appear to be reasonable, except that a 0.2 mg/kg may be
more realistic for kidney.
Mean recoveries determined separately on unconjugated bendiocarb,
NC 7312 and N-hydroxymethylbendiocarb from cattle meat and fat tissues
were approximately 70%, with a standard deviation ranging from 10 to
22. The large standard deviations reflect rather poor recoveries,
especially of bendiocarb per se, (< approximately 50%) in a number
of muscle, kidney and liver samples. For example, recoveries of
bendiocarb averaged only 60% in muscle and kidney.
Another feeding study (Reary 1978a) was conducted in a similar
fashion but at higher dietary levels (7.5 and 25 mg/kg in the diet).
Maximum residues in milk were <0.01 mg/kg (limit of determination
for the fat soluble fractions) and <0.02 mg/kg for the aqueous
portion. As in the lower level feeding study, apparent residues of
total bendiocarb and conjugated and non-conjugated metabolites were
<0.02 mg/kg in muscle, <0.02 in omental fat and <0.05 mg/kg in
perirenal fat and liver. Residues were at the estimated 0.1 mg/kg
limit of determination for kidney (one control sample was also at this
level). This supports the view that 0.2 mg/kg is a more realistic
limit of determination for cattle kidney.
Analytical recoveries averaged approximately 70% with standard
deviations from 11-21%, reflecting rather poor recoveries. Average
recoveries were only 56% for bendiocarb in muscle and 52% for the
N-hydroxy metabolite in liver.
No information was provided on national GAP or recommended uses
for dermal applications to dairy or beef cattle. Results of
investigations for proposes uses for fly control by dermal
applications with a 1% bendiocarb dust formulation were provided. A 5g
dust treatment (50 mg a.i.) was described as a "normal" daily dose.
In one set of experiments (Reary 1979a) lactating dairy cattle
were manually treated at measured rates for safety evaluations or by
hessian bag self-treatment for efficacy evaluations. In the safety
evaluations, groups of three shorthorn cows were manually brushed on
the head, neck and shoulders with 0, 5, 15 and 25 g of the 1% dust
formulation; milk was analysed pre-treatment and six hours post-
treatment for each animal. As in the feeding studies, milk was
separated into fat and lactose fractions.
Non-conjugated residues in milk ranged from 0.003-0.015 mg/kg
(0.009 mg/kg mean) for all application rates, with no discernible
difference between application rates. Total conjugated residues ranged
from 0.019 to 0.039 mg/kg (0.028 mg/kg mean). Control means for the
safety evaluation trials were 0.006 and 0.032 mg/kg for non-conjugated
and conjugated residues, respectively. Limits of determination were
considered to be 0.02 and 0.05 mg/kg for the fat and aqueous
fractions, respectively.
In the efficacy trials, four herds were self-treated at an
estimated rate of 5 g of dust formulation/day/cow. Milk was pooled for
each herd at 7, 14 and 21 days after the beginning of treatment. For
the fat soluble non-conjugated fractions, residues were <0.017 mg/kg
at 7-21 days, except for one pooled milk sample from one herd at 7
days, for which a residue of 0.06 mg/kg was detected. Therefore, non-
conjugated residues did occur at and above the limit of determination.
Conjugated residues were <0.057 mg/kg, or just over the estimated
limit of determination. Samples used as untreated controls had mean
residues of 0.005 mg/kg and <0.028 mg/kg for fat soluble and
aqueous fractions, respectively.
In another dermal treatment investigation (Reary 1980c) groups of
three lactating dairy cows were manually treated on the head, neck and
back daily for seven successive days with a 1% dust formulation at 0,
5 or 25 g product/cow/treatment. Daily milk production from each of
two daily milkings for each cow was kept separate. Samples of muscle,
omental fat, perirenal fat, liver, kidney and subcutaneous fat were
taken within 18 hours after the final treatment.
Non-conjugated residues in milk (fat fraction) after correction
for analytical recoveries ranged from <0.01-0.04 mg/kg for 78
samples. All but six were <0.01 mg/kg. All residues >0.02 mg/kg
were from the higher treatment rate, except for one sample at
0.02 mg/kg. Comparable residues in 24 untreated controls were
<0.01 mg/kg, except for one sample at 0.02 mg/kg. All conjugated
residues (aqueous fraction) were <0.02 mg/kg and untreated controls
were <0.02 mg/kg. Mean analytical recoveries were 82% and 72% for the
milk fat and lactose fractions, respectively. The limit of
determination was considered to be 0.01 and 0.02 mg/kg for the fat and
lactose fractions, respectively.
In tissues, residues at both treatment levels were <0.01 mg/kg
in muscle, <0.03 mg/ kg in omental fat, <0.05 mg/kg in perirenal
fat, <0.01 mg/kg in subcutaneous muscle and <0.15 mg/kg (mean
0.08 mg/kg) in subcutaneous fat. Except for subcutaneous muscle, for
which there were no untreated controls, apparent residues in controls
for these tissues were _0.03 mg/kg, except for one 0.04 mg/kg sample of
subcutaneous fat. Residues from both levels were <0.05 mg/kg in
liver and <0.11 mg/kg in kidney. All residues >0.05 mg/kg in
kidney were from the higher treatment level. Apparent residues in
untreated controls were <0.03 mg/kg in liver and kidney.
An estimate of potential residues in meat, milk and meat by-
products must take into consideration all potential routes to animal
exposure, which include the dietary and dermal routes. Of the feed
crops for which residue data and good agricultural practice
information were provided, the greatest potential for residues in
cattle meat, fat and meat by-products and milk would be expected
from uses on maize (field corn). Assuming maximum residues are
<0.05 mg/kg in maize grain, forage and/or fodder, total conjugated
and non-conjugated residues of bendiocarb and metabolites would be
expected to be <0.05 mg/kg in cattle carcase meat, cattle fat and
cattle meat by-products (except kidney) and <0.01 mg/kg (claimed
limit of determination) in kidney, although a 0.2 mg/kg limit of
determination may be more realistic for kidney. Residues in milk would
not be expected to exceed the approximate limits of determination of
0.01 mg/kg and 0.02 mg/kg for non-conjugated and conjugated residues,
respectively, or a total of 0.03 mg/kg. These estimates should also be
adequate from feeding of rice.
Data from the use of bendiocarb on grasses or as a dermal
treatment on cattle suggests a possible need for higher limits than
would be required for maize grain, forage and fodder if such uses are,
or should become, good agricultural practice. Additional information
on good agricultural practices are necessary before maximum residue
estimates can be made for these uses. Any additional information
should provide the formulation, application rate, number of
applications, type of application and preharvest intervals or other
restrictions. Depending on these uses, feeding studies at higher
levels may be necessary before maximum residue levels can be
estimated.
Poultry meat, meat by-products and eggs
Laying hens in groups of ten were fed (free access) a diet
containing 0, 0.05, 0.15 and 0.5 mg/kg bendiocarb for 21 days to
permit estimation of potential residues resulting from the feeding of
maize grain, for which maximum residues of 0.05 mg/kg are assumed
(Reary 1978b). Owing to analytical difficulties, information on the
stability of residues over the feeding period was not provided.
Information was not provided on storage conditions of the fortified
pre-mix.
Eggs were individually collected and labelled; 98 were analysed
at higher feeding levels and later sampling intervals. Apparent total
residues (corrected for recovery) of bendiocarb and metabolites
(conjugated and non-conjugated) at all treatment levels were
<0.02 mg/kg 1-21 days after treatment. The limit of determination was
reported as approximately 0.02 mg/kg, supported by sample
chromatograms, although pre-treatment controls were <0.013 mg/kg.
Analytical recoveries of bendiocarb and unconjugated metabolites
ranged from 44-85% (67% av.).
Samples of breast muscle, leg muscle, liver, kidney, subcutaneous
fat and heart were taken after sacrifice on day 21 of feeding the
fortified diet, with completion of dissection the following day. The
sum of bendiocarb and its major metabolites conjugated and non-
conjugated, was determined. No hen tissue residues were found in
excess of the claimed limits of determination of 0.02 mg/kg for legs,
breast, liver and subcutaneous fat and 0.05 mg/kg for kidney and
heart. Sample chromatograms tend to confine these limits of
determination, although apparent residues near the claimed limits of
determination in untreated controls of breasts (<0.016 mg/kg),
liver (<0.014 mg/kg) and fat (<0.016 mg/kg) suggest that
0.05 mg/kg may be a more practical limit of determination for each of
the tissues.
As in cattle tissues and eggs, analytical recoveries were only
marginally acceptable, ranging from 40-98% for bendiocarb or its major
non-conjugated metabolites. Mean residues of bendiocarb and its non-
conjugated metabolites (NC 7312 and N-hydroxymethylbendiocarb) ranged
from only 56% in chicken legs to 79% in fat. Improved analytical
methodologies are needed for commodities of animal origin.
Mushrooms
Supervised trials were conducted in The Netherlands and the U.K.
In The Netherlands mushroom plots were presumably sprayed directly
with a bendiocarb 50SC formulation and in the U.K. the plots were
established the day following wall treatments with either a 50SC or
80W spray. No information was provided on approved agricultural
practices for these uses, including preharvest intervals, although
recommended wall spray application rates of 30-50 g/100 m2 (Table 2)
were provided.
In the first trials (Browne 1981a) a 50SC bendiocarb formulation
was sprayed twice at rates of 50, 75 and 100 g a.i./100 m2 (5, 7.5
and 10 kg a.i./ha), first after spawning and the second after casing.
Harvest was 21 days after the last treatment.
Residues of bendiocarb and non-conjugated NC 7312, corrected for
mean recoveries, are summarized in Table 5. Maximum residues were
0.013 mg/kg and 0.04 mg/kg, respectively, for bendiocarb and NC 7312
at the highest application rate. Residues increase with increased
application rate and residues of NC 7312 tend to be less than those of
bendiocarb under the test conditions.
In the series of trials in the U.K., (Browne, 1982a) mushroom
plots were established the day following hut wall treatments with
either 50SC or 80W bendiocarb formulations at application rates of
20-53 g a.i./100 m2. Harvest was 26-69 days after the treatments,
which reflected the recommended application rates. Bendiocarb residues
ranged from <0.02 to 0.09 mg/kg and NC 7312 from <0.02 to
0.13 mg/kg, both corrected for recoveries (see Table 5). In contrast
to the trials discussed above, residues of NC 7312 in these trials
were of the same order of magnitude as for bendiocarb.
The limit of determination for bendiocarb in mushrooms was
reported to be 0.01-0.02 mg/kg for bendiocarb or NC 7312, depending on
the trials. Based on apparent residues of 0.04 mg/kg of bendiocarb and
0.02 mg/kg of NC 7312 in more than one untreated control, limits of
determination of 0.05 mg/kg may be more practical. Analytical
recoveries averaged approximately 82% for bendiocarb and 73% for non-
conjugated NC 7312 from fortifications of both at 0.04-0.2 mg/kg,
although standard deviations were 14-19.
As no information was available even on recommended uses for
bendiocarb other than for wall treatments, data from wall treatments
are more pertinent to the estimation of maximum residue levels. Based
on recommended application rates, the available residue data are
consistent with a maximum residue level of 0.1 mg/kg for bendiocarb
per se and 0.2 mg/kg if NC 7312 is also included. This is based on the
assumption of minimum application to harvest intervals. Information on
nationally approved agricultural practices for bendiocarb use on
mushrooms is needed. Additional data from supervised trials are
desirable.
FATE OF RESIDUES
Metabolism studies have been carried out in a range of plants and
animals and in soil. In most plant species examined, the major
metabolites formed are glycoside conjugates of the phenol NC 7312 and
N-hydroxymethyl bendiocarb. Metabolism in animals generally involves
cleavage of the carbamate ester group to yield NC 7312, which is
excreted as sulphate and beta-glucuronide conjugates. In soil under
aerobic conditions, the only extractable radioactivity is unchanged
bendiocarb, degradation occurring to CO2, and an unidentified bound
residue. Under anaerobic conditions, bendiocarb is hydrolysed to
NC 7312.
In animals
For discussions on the metabolic fate of bendiocarb in the rat,
dog, hamster, rabbit, mouse and humans, see "Biochemical Aspects".
Residue feeding and/or dermal studies on cattle and/or poultry have
been discussed under "Residues from Supervised Trials". In addition to
the aforementioned studies, the metabolic fate of bendiocarb has been
investigated in the pig, cow and hen.
Pig
Pigs were orally dosed once by gelatin capsule at 0.2 mg/kg/bw
with 14C-bendiocarb (Adcock et al 1976b). Urine samples were
collected over a period of four days, after which the animals were
sacrificed and samples of liver, kidney, muscle, perirenal fat and
subcutaneous fat were taken.
Seventy-eight percent of the administered dose was excreted in
the urine as equal levels of sulphate and ß-glucuronide conjugates of
NC 7312 within one day and 84% within four days. No residues of
unchanged bendiocarb were detected. Corresponding residues in faeces
were 2.4 and 7.2% for a total recovery of 91.5% of administered
radioactivity recovered in urine and faeces after four days. The
residues in faeces were not identified. No residues were found in
tissues at the 0.01 mg/kg limit of sensitivity for bendiocarb
equivalents. The finding of relatively large levels of NC 7312
sulphate was of considerable interest, since phenolic sulphates are
generally minor in pigs.
The metabolism is similar to that in humans and the rat,
involving cleavage of the carbamate ester to yield NC 7312, which
becomes conjugated as sulphates and glucuronides.
Cow
A lactating dairy cow was given 14C-bendiocarb by a single
gelatin capsule at a dose of 0.2 mg/kg/bw (Adcock et al 1976a).
Urine was collected at intervals of 4-96 hours after dosing and faeces
every 24 hours. Milk was collected morning and evening until sacrifice
on the fourth day, at which time samples of liver, kidney, muscle,
heart, perirenal fat, omental fat and subcutaneous fat were taken for
analysis. Milk was separated into lactose, fat and casein fractions.
No residues of unchanged bendiocarb were found in the urine.
Seventy-nine percent of the administered radioactivity was excreted in
the urine within eight hours and 92% after two days, after which
little was excreted in urine. Corresponding residues in faeces were
5.3% at one day and 12.3% after two days. The urinary metabolites were
determined to be sulphate and glucuronide conjugates of NC 7312 at 90%
and 10% each, respectively. Residues in faeces were not identified.
Bendiocarb equivalents were found in milk the first day up to a
level of 0.002 mg/kg, but none were detectable thereafter. Over 80%
was in the lactose fraction, 2% in the hexane fraction and most of the
remainder in the casein fraction. Tissue residue levels were below the
0.01 mg/kg limit of sensitivity as bendiocarb equivalents.
Metabolism in the cow appears to be similar to that in the rat,
human and pigs.
Poultry
Three laying hens were each dosed once with 14C-bendiocarb by
oral gavage at 0.25 mg/kg/bw (equivalent to 5 mg/kg in the diet) and
excrement and eggs collected at intervals from 24-96 hours after
dosing. Liver, kidney, muscle and fat samples were taken at sacrifice
four days after treatment (Lewis and Challis 1978).
Sixty-one to 90% of administered radioactivity was excreted
within 24 hours, and 73-91% within 4 days. Recovery was inexplicably
low (73%) in two of the hens. Losses were presumed to be due to
expiration and/or losses during dosing or excrement collection, since
residues in tissues were low. However, as a percent of radioactivity
excreted in the first 24 hours, residues were 10-57% free NC 7312,
26-63% NC 7312 conjugates, 12-19% unidentified unhydrolysed water
soluble residues, 2-5% other non-polar metabolites and 1-3% other
unidentified conjugates. Conjugation of NC 7312 occurred more rapidly
in the hen in which radioactivity excretion was more rapid and
complete.
Radioactivity in egg white and yolk represented only 0.01% of the
administered dose. The maximum bendiocarb equivalent was highest in
egg white on the first day (max. 0.009 mg/kg) and on day four in yolk
(0.003 mg/kg), both above the limits of sensitivity. The maximum
bendiocarb equivalent was 0.004 mg/kg in liver and fat, 0.002 mg/kg in
kidney and below the 0.001 mg/kg limit of sensitivity in leg and
breast muscle.
In Plants
Metabolism studies have been carried out in sugarbeet, maize,
rice, barley and oilseed rape, both in the laboratory and under field
conditions. A variety of separative, determinative and conjugative
hydrolysis steps were utilized. In all these crops, the major
identified metabolite was the phenol NC 7312, with lower levels of
N-hydroxymethylbendiocarb, both primarily as glycoside conjugates. In
some of the crops studied, sampling at harvest showed small quantities
of unconjugated N-hydroxymethylbendiocarb.
Three metabolism studies were conducted in sugarbeets. In one
study, radioactive bendiocarb was applied under greenhouse conditions
to the potted soil or to the leaves by direct syringe applications
(Challis 1975). Residues were characterized in the plant tops only.
Metabolic products were qualitatively similar in both cases,
resulting in residues of free bendiocarb, bendiocarb glycoside,
free NC 7312 (foliar application only), NC 7312 glycosides,
N-hydroxymethylbendiocarb, N-hydroxymethylbendiocarb glycosides,
unidentified glycosides, unidentified water soluble residues and
unextractable unidentified fibre-bound residues.
The major metabolites from soil application were glycoside
conjugates of NC 7312 and N-hydroxymethylbendiocarb. Trace amounts of
free N-hydroxymethylbendiocarb were also found. Unidentified residues
accounted for 36 and 67% of radioactivity 8 and 34 days after
treatment, respectively, with a ratio of about 1:3 water soluble to
fibre-bound residues.
From foliar applications, over 93% of leaf-applied bendiocarb was
present on the leaf surface after 34 days. A bendiocarb glycoside was
the major metabolite. Unidentified residues accounted for 48% of the
total residue after eight days, but this was reduced to 25% after 34
days. Low levels of free NC 7312 were also present. The proposed
metabolic pathway for bendiocarb in sugarbeet tops is illustrated in
Figure 1. Except for the omission of substantial at-harvest
 unidentified water soluble and fibre-bound residues, other metabolism
studies discussed below suggest that the pathway is similar in other
plants.
In a second sugarbeet metabolism study (Adcock 1976),
14C-bendiocarb was applied as a seed dressing for plants grown in
outdoor drums in sandy loam soils at 6.7 and 7.5 pH values at a rate
equivalent to 16 g a.i./kg seed. Leaves and/or roots were analysed for
radioactivity at intervals from 13-114 days after sowing, although the
residues were not identified. In accordance with decreased bendiocarb
stability in more alkaline soils, extractable and fibre-bound residues
were less than the 0.02 mg/kg limit of detection (bendiocarb
equivalents) in foliage and roots of the more alkaline soil after 114
days. Residues were greater at earlier intervals. For example, at 36
days residues were on the order of 0.1 mg/kg bendiocarb equivalent in
leaves or roots from either extractable or fibre-bound sources.
Consistent with pH stability, residues were higher in the more acidic
soil, being up to 0.06 mg/kg and 0.02 mg/kg extractable residues in
leaves and roots, respectively, after 114 days; they were appreciably
higher at shorter intervals. A maximum of 18% of applied radioactivity
was found in immature sugarbeet (36-72 days after sowing).
In the third sugarbeet metabolism study (Challis and Adcock
1978), the crop was grown under field conditions and treated at sowing
with 14C-bendiocarb granules at the recommended rate of 0.36 kg
a.i./ha. Plants were harvested at intervals ranging from 46-190 days
after treatment. Results were similar to those of Challis (1975).
After 90 days, radioactivity was too low to permit determination of
individual metabolite levels. In harvested beets, mean residues of
only 0.009 mg/kg bendiocarb equivalent were found after 190 days.
Extractable residues were below the limits of sensitivity.
As would be expected, residues were greater at earlier intervals
and identification was possible. For example, at 46 days residues
(bendiocarb equivalent) were 1.0, 0.22, 0.14, 0.76 and 0.45 mg/kg for
bendiocarb, NC 7312 conjugates, N-hydroxymethylbendiocarb conjugates,
unidentified water soluble residues and fibre-bound residues,
respectively. There were lesser amounts of free metabolites and other
conjugates.
Bendiocarb metabolism was investigated in maize under glasshouse
conditions by applying a solution of 14C-bendiocarb to pH 5.4 soil in
drums with newly germinated maize plants at a rate equivalent to
1.1 kg a.i./ha (Challis and Adcock 1977). Plants were analysed at
intervals from 10-125 days. Mean residues of bendiocarb per se in
plants ranged from 1.4 mg/kg after 10 days to 0.01 mg/kg after 125
days and unidentified soluble residue (bendiocarb equivalent) in
kernels and cobs were 0.005 and 0.001 mg/kg, respectively, after 125
days. As a percentage of total radioactivity, the major residues after
10, 60 or 125 days were:
Foliage Kernels Cob
10 days 60 days 125 days 125 days 125 days
Bendiocarb 38.1 24.2
N-hydroxymethylbendiocarb 8.2 5.3 8
NC 7312 conjugates 163.3 2.7 20
Water soluble 13.6 11 9.1
(non-hydrolysable)
Fibre-bound 13.6 27 61 89 97
Free NC 7312, other non-polar metabolites and other conjugates
were also present at lower levels. Seventy percent of the residues in
plants at harvest were either water non-hydrolysable of fibre-bound
unidentified residues. Although they occurred at very low levels,
fibre-bound unidentified residues constituted 89 and 97 percent of
total radioactive residues in kernels and cobs, respectively, at
harvest.
Investigations were conducted on paddy rice metabolism of
14C-bendiocarb applied as a solution with a syringe under glass to
either the foliage or flood water surrounding rice transplants (Lewis
and Challis 1979). Application rates were equivalent to 0.6 kg a.i./ha
and plant samples were taken at intervals ranging from 15-174 days
after treatments. Foliage and grain were analysed separately at 174
days. Qualitatively, residues were similar to those in sugarbeet and
maize, although N-hydromethylbendiocarb or its conjugates were not
specifically identified in rice. They could have been present under
residues described either as other non-polar metabolites or other
conjugates.
Mean free bendiocarb in rice plants ranged from 1.7 mg/kg after
15 days to 0.0022 mg/kg (total radioactivity as bendiocarb
equivalents) after 174 days from the foliar treatments. The most
abundant metabolite by far was NC 7312, mostly as a conjugate
(0.006 mg/kg bendiocarb equivalent). Residues were 0.009 mg/kg in rice
husks and flour after 174 days.
In plants growing in treated water, residues of free bendiocarb
at 15 days were approximately one tenth of that from foliar
treatments, the major residue being NC 7312 conjugates at 0.54 mg/kg.
Individual residues could not be determined after 60 days. At harvest,
total radioactivity levels are similar regardless of the type of
treatment. Quantitatively, major residues in plants as a percent of
total radioactivity at 15 or 174 days are:
15 days 174 days
Foliar Water Foliar Water
Bendiocarb 37 12
NC 7312 13 37 39 31
conjugates
Water soluble 7 10
(unhydrolysable)
Fibre-bound 33 33 61 69
All of the radioactive residue at harvest in rice husks or flour
was NC 7312 conjugates, regardless of whether application was by
foliar or water treatment. As in the case of maize foliage, residues
in rice plants were mostly unidentified fibre-bound ones at harvest.
The difference was in the grain, which contained mostly unidentified
fibre-bound residues in maize and mostly NC 7312 conjugates in rice.
Greenhouse-grown barley plants were treated with 14C-bendiocarb
at rates equivalent to 1 kg/ha. A solution was applied by pipette to
soil surrounding the plants which were analysed at intervals ranging
from 4-94 days after treatment. Samples at 94 days were separated into
foliage, husks and grain, which were analysed separately (Challis and
Swalwell 1980).
Results of this investigation were similar to that of
rice. However, in the case of barley, conjugates of
N-hydroxymethylbendiocarb were identified, but total radioactive
residues in husks and grain at 94 days (1.46 and 0.09 mg/kg bendiocarb
equivalent, respectively) were not characterized. Free bendiocarb
residues in foliage ranged from 0.09 mg/kg at 4 days, declined through
60 days and increased to <0.07 mg/kg at 94 days. Similar patterns
were observed for conjugates of NC 7312 and N-hydroxymethylbendiocarb,
the major components of the residue throughout the period. Again,
unidentified water soluble or fibre-bound residues constituted a major
part of the residue, which increased until harvest (12 and 42% of
total radioactivity, respectively).
The metabolism of 14C-bendiocarb was studied in greenhouse grown
oilseed rape treated at the seedling stage at application rates
equivalent to 140 and 500 g a.i./ha. Application was by pipette to
soil surrounding the plant (Warner 1980). Plant samples were taken at
intervals ranging from 5 to 140 days after treatment. At 140 days,
samples were separated into foliage, pods, and seed, which were
analysed separately.
As in the case of barley, the major residues in the plants were
highest at earlier intervals and, except for free bendiocarb, declined
and then increased by harvest. In both cases this was probably due to
plant growth patterns and dessication near harvest. Free bendiocarb
was the major residue at day 5 (66-69%), but declined rapidly
thereafter (2.5 mg/kg at day 5 to 0.01 mg/kg at 140 days from the
high treatment rate). Again, conjugates of NC 7312 and
N-hydroxymethylbendiocarb were the major identified metabolites and,
by harvest, unidentified water soluble materials and fibre-bound
residues formed the major portion of total radioactivity which was 26
and 43%, respectively, from the higher treatment rate. Although
residue levels were dependent on the application rate, on the basis of
percentage of total residue, results were similar at both application
rates.
Extractable residues in pods, seeds and foliage at harvest were
0.09, 0.001 and 0.16 mg/kg, respectively, at the low application rate
and 0.74, 0.011 and 0.84 mg/kg, respectively, at the high rate.
In Soil
Laboratory studies on residue decline in a pH 7.7 sandy loam soil
treated in flasks or jars with 5 mg/kg 14C-bendiocarb showed
degradation to CO2 and unidentified bound residues under aerobic
conditions, with a half-life of 5 days for the parent compound. No NC
7312 was detected. With flooding or nitrogen flushing and under
sterile anaerobic conditions, bendiocarb was hydrolysed to NC 7312,
with little or no further degradation (Adcock et al 1975a).
In a laboratory soil leaching study with the same soil type, 60%
of the radioactivity applied to a 30 cm column as 14C-bendiocarb was
eluted with 50 cm of water over 11 days, mostly as NC 7312 (Adcock
et al 1975b). Presumably, conditions were effectively anaerobic and
the rapid degradation of bendiocarb did not permit estimation of its
leachability.
In another leaching study, 14C-bendiocarb, leachability was
investigated for an agricultural sand (pH 5.3), a clay loam (pH 7.3),
a sandy loam (pH 7.8) and a silt loam (pH 7.2) using soil column and
thin-layer techniques (Adcock 1978). The 30 cm columns were percolated
over a 2-9 day period with a 20 cm column of water. The percentage of
radioactivity recovered in leachate was 75, 11.9, 7.9 and 0.8% for the
respective soils, decreasing with increased organic content.
Recovery in leachate was slightly less than for 2,4-D in
agricultural sand and clay loam, and substantially less in the other
two soils under similar conditions. Thin-layer results confirm these
observations and show that NC 7312 is somewhat less leachable than the
parent compound in all four soil types. It also shows that bendiocarb
is more leachable than trietazene, which places it in the moderately
mobile class.
When bendiocarb 80%WP, 3%G and 1% dust formulations were leached
from sand (pH 7-7.6) loamy sand (pH 6-6.5) and sand loam (pH 5.6-7.8)
in 30 cm × 5 cm columns with 20 cm water over 48 hours, leachability
was again inversely proportional to organic matter content of the soil
(Reary 1975a; Browne and Reary 1979a, 1980a). As a percent of the
amount applied, bendiocarb recovery in leachate ranged from 0 to 93%
WP, 0.6 to 84% dust and 0 to 22% granular. From the same formulations
leachate contained 0 to 25% bendiocarb and 0 to 68% NC 7312; 0 to 45%
bendiocarb, 0 to 62% NC 7312 and 0 to 4% bendiocarb, 0 to 19% NC 7312,
respectively. Therefore, leachability decreased in the order of WP,
dust and granular formulations.
Bendiocarb stability in standard soils has been shown to be
dependent on soil pH, being much slower at low pHs (Reary 1975b). The
half-life was 10 days in one soil at pH 2.2 and 58 days in another at
pH 5.2.
Soil residue decline studies have also been carried out in trials
determining bendiocarb residues in turf and maize. Results again show
that the rate of degradation of bendiocarb is pH-dependent. Repeated
applications showed no evidence of a build-up of bendiocarb residues
in soil. Dissipation rates (in days) for various formulations in
various soil types and pHs are given in Table 9 (Browne and Reary
1978c; Reary 1975b, 1978f, g, k, 1980g; Reary and Whiteoak 1977a, b,
d).
In Storage and Processing
It was noted under the section on "Residues from Supervised
Trials" and in Table 6 that oil processed from maize with field-
incurred residues of <0.02 mg/kg bendiocarb also contained residues
of <0.02 mg/kg. In another investigation, 10G was applied as a band
along the maize row at sowing at 1.1-2.2 kg a.i./ha and the maize
sampled at normal harvest. Oil was solvent-extracted from the grain,
which had residues of <0.01 mg/kg, the limit of determination.
Analysis of the oil showed no residues in excess of the 0.05 mg/kg
limit of determination (Reary 1978c).
Investigation with radio-labelled bendiocarb in granular
application trials in France, the U.K. and the U.S. showed residues in
solvent-extracted oil to be 0.008, 0.011 and 0.021 mg/kg,
respectively, from kernels with residues of 0.011, 0.013 and 0.022
mg/kg, respectively. In most kernel or oil samples residues were
<2X the reported limit of sensitivity (Lewis and Adcock 1978a, b, c).
RESIDUES ON FOOD IN COMMERCE OR AT CONSUMPTION
No information was provided on residues in commerce or at
consumption. The Meeting considered such information desirable.
Table 9. Summary of Bendiocarb Dissipation Rates in Soil
Soil Dissipation time (days)
Type pH Formulation 50% 75% 90%
Sandy loam 5.2 Technical1/ 58 ? ?
Sandy 6.5 76W 25 50 75
loam 76W2/ 12-34 24-69 37-104
Sandy loam 6.5 5G2/ 26-70 52-140 78-210
Silty clay 6.5 10G 19 38 57
Silty clay loam 6.7 76W 30 60 ?
Sand 6.8 Technical1/ 10 24 50
Loam 7.2 10G 7.5-10 15-20 22.5-30
Loam 7.7 76W 12 24 36
Sandy 7.7 76W 2.4 4.8 7.2
loam 76W2/ 2-5 3-10 5-15
Sandy loam 7.7 5G2/ 12-26 30-52 45-78
Sandy loam 7.8 3G 12 24 36
1/ Laboratory studies.
2/ Multiple applications at up to 5-monthly intervals;
dissipation times determined after each application.
METHODS OF RESIDUE ANALYSIS
Residue analysis methods for various plant substrates have been
developed for bendiocarb alone or for bendiocarb plus the non-
conjugated metabolite NC 7312, either separately or as a total
residue. In animal substrates, enzymic liberation of NC 7312 from
conjugates is more successful than in plants. Consequently, residue
methods for animal tissues, milk and eggs include determinations of
the conjugated residues.
As the carbamate functional group does not possess strong
electron capturing properties, derivatization of the bendiocarb
molecule is necessary to facilitate the measurement of low residue
concentrations. Formation of the 2,4-dinitrophenyl ether was found to
be most suitable. This results in a product with strong electron
capturing properties and a long gas chromatographic (GLC) retention,
thus ensuring excellent sensitivity and minimal co-extractive
interference.
In the standard procedure (Reary 1975c) the carbamate is
hydrolysed in an alkaline buffer containing 1-fluoro-2,
4-dinitrobenzene so that the dinitrophenyl ether is formed immediately
after hydrolysis. This method has the added advantage the phenolic
degradation product, NC 7312, forms the same product in the reaction.
Thus, the combined residue can be measured unless bendiocarb and NC
7312 are separated prior to derivatization. This separation is readily
effected by high pressure liquid chromatography (HPLC) or with
commercially available liquid chromatographic cartridges. Collection
of the appropriate fraction allows individual analysis for bendiocarb
and NC 7312 from the same extract (Brown and Reary 1979b).
Bendiocarb is extracted from soil (Reary 1978d), grass (Reary
1978e) sugarbeet (Reary 1975c), maize (Browne and Reary 1979c), cereal
grain (Reary 1979b), rice (Browne and Reary 1979b), rape seed (Browne
and Reary 1980b), potatoes (Reary 1979c), cabbage (Browne 1981b),
mushrooms (Browne 1982a), apples/pears (Browne 1982b) and peas (Browne
and Manley 1982), under reflux with dichloromethane or by shaking with
diethyl ether. Oily extracts are improved by partition between hexane
and acetonitrile. Further clean up, when required, is by silica gel
chromatography. This is easily done using commercially available
disposable cartridges, which can eliminate or separate any NC 7312
present in the extract.
A method has been developed for the residue analysis of
bendiocarb and major metabolites in milk, eggs, meat, fat and offal
(Browne and Reary 1978b). Residues are extracted under reflux with
acetonitrile. Conjugated metabolites are hydrolysed with snail
digestive juice before formation of the dinitrophenyl derivative. All
major bendiocarb related metabolites form the same derivative, which
is then analysed by the standard method as a total residue.
In general, mean analytical recoveries in most commodities were
>75% for bendiocarb and >60% for NC 7312. Analytical
sensitivities, recoveries, recoveries of fortified commodities and
apparent residue in untreated controls for individual commodities are
discussed under "Residues Resulting from Supervised Trials."
Information represents field-type conditions and is somewhat less
assuring than mean recoveries presented with the reports on each
individual analytical method. Although mean analytical recoveries
appeared acceptable, at least for bendiocarb per se, the range of
residues was generally large, resulting in standard deviations ranging
from 6 to 33. In animal tissues in particular, and to some extent in
cereal grains and fodder/forage, analytical recoveries were frequently
low. For example, in animal products analytical recoveries were
frequently only 50-60% for bendiocarb per se or the N-hydroxy
metabolite, and sometimes less.
NATIONAL MAXIMUM RESIDUE LIMITS
Information on national maximum residue limits were available
from only one country. Limits have been proposed in four other
countries (Table 10).
Table 10. National Maximum Residue Limits
Country Commodity MRL (mg/kg)
Netherlands1/ "All commodities"3/ 0 (0.05)4/
Australia2/ Poultry meat, eggs 0.05
Milk, milk by-products, meat
and other edible offal 0.1
Cereal grain 10.0
Belgium2/ Maize and sugarbeet 0.02
Switzerland2/ General foodstuffs (excl. milk) 0.2
Milk 0.005
U.S.2/ Maize (grain fodder, forage) 0.05
Maize oil 0.1
Milk, eggs, meat fat, meat
by-products (except kidneys)
of cattle, goats, pigs horses,
poultry, sheep 0.05
Kidneys of cattle, goats, pigs,
horses, poultry, sheep 0.1
1/ Established;
2/ Proposed;
3/ It is unclear whether "all commodities" include
commodities other than maize and sugarbeet, for which
good agricultural practice information was provided;
4/ 0 (0.05) mg/kg presumed to mean no residues allowed with
a 0.05 mg/kg limit of determination. Large-scale use.
APPRAISAL
The Meeting reviewed substantial information on residue levels
from supervised trials in a variety of commodities, fate of residues
and analytical methodology for bendiocarb, a carbamate pesticide used
for control of a wide range of agricultural and public health pests.
Information on official nationally approved agricultural practices and
national maximum residue limits were available for only two
commodities (maize and sugarbeet) in one country, although recommended
application rates were provided for a number of commodities. Since
information on good agricultural practices for all commodities except
maize and sugarbeet is lacking, only TMRLs are estimated for other
than these two commodities. National tolerances have been proposed in
at least four countries, mostly on maize and other cereals and animal
products.
Residue data were available from supervised trials on a variety
of commodities in several countries and using a variety of
formulations and agricultural practices. In most cases, free
bendiocarb per se was the only residue measured. In a few cases, the
major metabolite 2,2-dimethyl-1,3-benzoxodiol-4-ol (NC 7312) was
measured in its free form and in some cereals, combined residues of
conjugated NC 7312 and N-hydroxymethylbendiocarb were measured as
well. Data were inadequate for estimating a limit for oilseed rape,
cabbage and peas, although the Meeting was informed that additional
data are being compiled for the latter.
For root or tuber vegetables, data were available for potatoes
and sugarbeet. Data on potatoes show residues of free NC 7312 at
harvest to be up to 20 times that of the parent compound (average 6X).
This rate could not be determined for sugarbeet with available data.
Limited data were available in pome fruit from one country and
residues of NC 7312 were equal to or less than those of the parent
compound. The Meeting was informed that additional data are being
gathered.
Bendiocarb residue data on cereals were available for barley,
oats, wheat, maize and rice. For the latter, limited data were also
available for free NC 7312, and the first three, for combined residues
of conjugates of NC 7312 and N-hydroxymethylbendicarb. Data were
mostly from seed treatments in barley, oats and wheat. No data were
available for metabolites in maize, although metabolism studies have
shown >50% of residues in foliage to be metabolites and/or
unidentified water-soluble or bound residues; in kernels at harvest,
approximately 90% of radioactivity was unidentified fibre-bound
residues. Analytical recoveries were only marginally acceptable in
some cereal trials, especially those in wheat.
Considerable data were available for grass and turf, mostly at
higher than recommended rates. Half-lives were greater from granular
broadcast applications than from spray applications and irrigation did
not significantly affect decline rates for either.
As in maize, data for oilseed rape were for the parent compound
only, and metabolism studies showed >50% of residues at harvest to be
conjugated metabolites and (mostly) unidentified water-soluble or
fibre-bound residues.
Data were available for mushrooms from spray treatments (plot
presumed) and wall treatments (before plot establishment). Residues
increased with application rates and residues of free NC 7312 from
wall treatments were on the same order of magnitude as those of the
parent compound, but were less following direct spray treatment. No
information was provided on good agricultural practices for direct
spray treatment.
Animal feeding trials were conducted in which lactating dairy
cattle were fed diets for 28 days containing up to 25 mg/kg
bendiocarb. In contrast to field trials, in which the parent compound
was determined separately from metabolites, animal tissues were
analysed for total residues of free bendiocarb and non-conjugated
metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb.
Milk was separated into a fat fraction and lactose (aqueous) fraction.
The fat fraction was analysed for bendiocarb and non-conjugated NC
7312 and N-hydroxymethylbendiocarb and the lactose fraction for the
conjugated residues of the two metabolites.
Studies using topical treatments were also conducted, but no
information was provided on approved or recommended uses for such
treatments. Dermal treatments generally resulted in higher residues in
tissues and milk than feeding trials. In the absence of good
agricultural practice information for dermal treatments (or grass
uses) residue estimates in cattle are based on potential residues from
the feeding of maize, for which national good agricultural practice
information was available. From this potential source of exposure, no
residues are expected in products of animal origin above the limit of
determination at or about 0.05 mg/kg (0.2 mg/kg in kidney).
In animal products in general, analytical recoveries were
disappointingly low, frequently only 50-60% or less for some residues
in some tissues, and thus can be considered marginally acceptable for
enforcement purposes only, since residues are expected to be
relatively low as a result of uses considered in residue level
estimates.
Animal metabolism studies were conducted in the rat, dog,
hamster, rabbit, mouse, pig, cow and poultry and in humans. In the
pig, cow and poultry single dosing was with 14C-bendiocarb, by
gelatin capsule in pig and cow and by gavage in poultry. Approximately
80% of applied radioactivity was eliminated in the urine within one
day or less in pigs and cows and 61-90% in poultry. It consisted
mostly of the sulphate or glucuronide conjugates of NC 7312 in all
three, although in poultry significant levels of unidentified
water-soluble residues were also present, as well as low levels of
free NC 7312 and other unidentified moieties. Low levels of NC 7312
sulphate and glucuronide were also present in faeces of cows and pigs.
In pigs the NC 7312 sulphate to glucuronide ratio was unexpectedly
high.
In animal tissues and milk, residue levels were generally not far
from the limit of determination and only 0.01% of the administered
dose was found in eggs. Owing to the low levels, they were not
identified.
Bendiocarb metabolism in cows, pigs and poultry appears to be
similar to that in smaller animals. Carbamate is hydrolysed to
NC 7312, which forms glucuronide and sulphate conjugates. The
apparent major difference is the lack of conjugates of
N-hydroxymethylbendiocarb also found in plants, and 5,6, or
7-hydroxybendiocarb found or tentatively identified in the smaller
animals. However, dosing rates of 0.2-0.25 mg/kg bw in cows, pigs and
hens were substantially less than the maximum dosage rates of 10 mg/kg
bw used for the smaller animals. More importantly, cows, pigs and
poultry were sacrificed four days after dose administration, which
precludes measurement or characterization of additional radioactivity
closer to administration. This could also account for residue levels
in animal products (cows, pigs and poultry) being too low for
identification. A metabolism study conducted in both ruminants and
poultry at high levels and a much shorter sacrifice interval would
have been preferred and are desirable. Such a metabolism study should
be considered essential if higher than the current ones for animal
products are required from the feeding of grain, fodders or straw of
cereals.
Metabolism studies in sugarbeet, maize, barley, rice and oilseed
rape under laboratory or field conditions showed similar quantitative
results, although ratios of the various metabolites and degradation
products varied considerably, depending on the plant, type of
application, interval from application to harvest, part of plant and
other factors.
A major metabolic pathway for bendiocarb in some plants is
hydrolysis to NC 7312, which becomes conjugated as a glycoside.
In another pathway, bendiocarb can first be oxidized to
N-hydroxymethylbendiocarb, which may hydrolyse to NC 7312
before glycosidic conjugation or become conjugated as
N-hydroxymethylbendiocarb glycoside. Another route can be direct
glycosidic conjugation of bendiocarb per se. This was a major residue
in beet leaves following a surface application, but NC 7312 conjugates
were found following surface treatments to rice. Undoubtedly other
pathways exist, since low levels of unidentified conjugates are known
to occur. In one beet study, limited leaf penetration was detected
from surface application.
As the interval from application to harvest increased, residues
of bendiocarb per se decreased with an increase in NC 7312 and
N-hydroxymethylbendiocarb conjugates, which were frequently the major
identified metabolites. Unidentified water-soluble, non-hydrolysable
residues and unidentified fibre-bound residues have also been shown to
increase in the foliage of several plants; at harvest they constitute
26 and 60%, respectively, of the total remaining radioactivity. In
maize kernels and cobs at harvest the fibre-bound unknown was shown to
consist of >90% of the remaining low residue.
Low levels of free NC 7312 and other unidentified unconjugated
residues were found in some plants. Free NC 7312 exceeded that of the
parent compound in potatoes and probably in pea pods. In other plants,
free NC 7312 residues were either less than or equal to that of the
parent compound. Differences in method of application (for example,
foliar versus ground application) affected the ratios of the various
metabolites in some, but not all, plants tested, although residues
were qualitatively similar.
In soils, bendiocarb was degraded to CO2 and unidentified bound
residues under aerobic conditions whereas degradation was mostly to
NC 7312 under anaerobic conditions. Bendiocarb stability was dependent
on soil pH, being much more stable under acidic conditions. Half-lives
ranged from 2-70 days, depending on conditions.
Leaching studies carried out on a variety of soils, under
different conditions and with several formulations showed leaching to
be largely dependent on the organic content of the soil, decreasing
with higher levels of organic matter. Although generally considered to
be in only a moderately leachable category, significant leaching of
bendiocarb can occur in sandy soils with low organic matter content.
Leachability appears to increase somewhat from granular to dust to
wettable powder formulations. One study indicated that NC 7312 leached
slightly less than bendiocarb per se. Although build-up of soil
residues of bendiocarb per se do not appear to be indicated, any
potential residues of bendiocarb or its metabolites in ground water
would be greatest from sandy soils of low organic matter content.
Most investigations on possible residues of bendiocarb in
processed products were conducted on oil, husks or flour from cereal
grain. Although no concentration of residues was demonstrated in these
studies, residues in the grain from which the products were processed
had residues below or near the limits of determination. Therefore,
firm conclusions cannot be drawn.
Analytical methods are available for the determination of free
bendiocarb in all plant commodities examined and free NC 7312 in some.
In the basic procedure, bendiocarb is hydrolysed to its phenol under
alkaline conditions in the present of 1-fluoro-2,4-dinitrobenzene to
form the dinitrophenyl ether, which is preferably determined by
electron capture gas chromatography using an internal standard.
Bendiocarb and NC 7312 can be determined together or separately, if
they are separated by HPLC or silica gel chromatography prior to
derivitization. In most commodities of plant origin, field trials were
for the free parent compound only and free NC 7312 was also determined
separately in a few. Analytical recoveries were highly variable on
some commodities, especially cereals.
For animals, the same basic analytical approach is used, except
that a hydrolysis step is included to hydrolyse conjugated residue.
Hydrolysis has been accomplished by a variety of enzymatic and/or
acidic conditions. Enzymatic approaches are preferred and snail
gastric juice is frequently used. In contrast to plants, residues
determined for animal tissues and milk include the sum of residues of
bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and
N-hydroxymethylbendiocarb. Aqueous (lactose) and non-aqueous (fat)
fractions of milk are analysed separately. Analytical recoveries for
products of animal origin are only marginally acceptable for
enforcement purposes. Improved analytical methods are needed for any
uses requiring maximum residues levels in products of animal origin
that are higher than the current limits of determination.
RECOMMENDATIONS
The Meeting considered information on residues resulting from
good agricultural practices or recommended application rates on a
number of commodities and possible residues in animals resulting from
the feeding of grain, fodders or straw of cereals treated according to
good agricultural practice and concluded that the maximum residue
levels or temporary maximum residue levels below are suitable for
establishing maximum residue limits. Regardless of the status of the
ADI, temporary status was designated for all commodities except maize,
maize fodder and forage, sugarbeet and sugarbeet tops, pending
availability of good agricultural practice information. Except for
potatoes, some fruit, rice and rice straw, estimated residue levels
apply to treatment before planting and/or seed application only.
In the absence of good agricultural practice information, the
temporary limits are based on supervised trials data only. Residues
for products of plant origin refer to residues of unconjugated
bendiocarb. Residues in products of animal origin refer to the
sum of free and conjugated bendiocarb, NC 7312, and
N-hydroxymethylbendiocarb, expressed as bendiocarb.
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Potatoes 0.051/ 100 (granular)
0 (spray)
Sugarbeet 0.051/ 150
Sugarbeet tops 0.051/ 150
Pome fruit 0.021/ 50
Cereal Grains
Barley 0.051/ 100
Oats 0.051/ 100
Wheat 0.051/ 125
Maize (field) 0.051/ 125
Rice (in husk) 2 10
Fodders and Straws
Barley 0.051/ 125
Oats 0.051/ 100
Wheat 0.051/ 125
Maize 0.051/ 125
Rice 1 10
Mushrooms 0.12/ 50
(con't)
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Animal Products3/
Carcase meat of cattle 0.051/
Fat of cattle 0.051/
Meat by-products of cattle
(except kidney) 0.051/
Kidney of cattle 0.21/
Milk 0.051/
Poultry 0.051/
Fat of poultry 0.051/
Poultry meat by-products 0.051/
Eggs 0.051/
1/ At or about the limit of determination.
2/ Limit reflects maximum residues expected from wall treatments
prior to establishment of plots.
3/ Limits reflect maximum residues expected from the feeding of
cereal grains, fodders or straws harvested at given intervals
from application to harvest.
FURTHER WORK OR INFORMATION
Required (by 1984)
Information on nationally approved agricultural practices for the
use of bendiocarb on all commodities for which limits are estimated,
except for maize and sugarbeet.
Desirable
1. Additional metabolism studies in ruminants and poultry at
sufficiently high dosing levels and with sacrifice at a short
interval after dosing, instead of several days thereafter, to
permit a more complete characterization of residues in tissues,
milk and eggs. The analyses should include all residues
identified, or tentatively identified, in smaller animals and in
plants. These studies should be considered required before new
limits or future upward revisions of established ones on feed
items are established significantly higher than at current limits
of determination. The same is true should substantially higher
limits on tissues, eggs or milk be required for approved dermal
treatments.
2. Additional residue data, reflecting nationally approved uses
especially on, but not limited to, mushrooms and pome fruit.
3. Information on the possible occurrence of residues in food in
commerce or at consumption.
REFERENCES
Adcock, J.W. Investigation of residues in sugarbeet following the
1976 use of 14C-bendiocarb as a seed dressing. Fisons Report
METAB/76/1. (Unpublished)
1978 The leaching of 14C-bendiocarb in four soil types. Fisons
Report METAB/78/11. (Unpublished)
Adcock, J.W., Bentley, A.P., Challis, I.R. and Pearce, J.C. The
1975a decline of NC 6897 in a sandy loam soil. Fisons Report
METAB/75.2.(Unpublished)
Adcock, J.W., Challis, I.R. and Pearce, J.C. Soil leaching of NC
1975b 6897 and degradation products. Fisons Report METAB/75/3.
(Unpublished)
Adcock, J.W., Challis, I.R., and Warner, P.A. The metabolism of
1976a 14C-bendiocarb in the dairy cow. Fisons Report
METAB/76/23. (Unpublished)
Adcock, J.W., Warner, P.A. and Challis, I.R. The metabolism of
1976b 14C-bendiocarb in the pig. Fisons Report METAB/76/19.
(Unpublished)
Browne, P.M. Residues of Bendiocarb and NC 7312 in mushrooms
1981a treated with a 50SC formulation in Holland, 1980. FBC Report
RESID/8140. (Unpublished)
Browne, P.M. Analytical method for residues of bendiocarb in
1981b cabbage. FBC Report RES/81/65. (Unpublished)
1981c Residues of bendiocarb in cabbage following repeat
applications of a 50WP formulation in the Philippines,
1980-81. FBC Report RESID/81/53. (Unpublished)
1981d Residues of bendiocarb in rye grass after autumn
applications of granular and seed treatment formulations in
the U.K., 1980. FBC Report RESID/81/69. (Unpublished)
1981e Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in the U.K., 1981. FBC
Report RESID/81/74. (Unpublished)
1982a Residues of bendiocarb and NC 7312 in mushrooms following
wall spray treatment with 80WP or 50SC. FBC Report
RESID/82/20. (Unpublished)
1982b Residues of bendiocarb and NC 7312 in apples and pears
following treatment with 20W, 50W or 50SC formulations in
the U.K., 1981. FBC Report RESID/82/41. (Unpublished)
1982c Residues of bendiocarb and NC 7312 in sugarbeet following
application of a granular (3G) formulation in Italy, 1981.
FBC Report RESID/82/13. (Unpublished)
Browne, P.M. and Manley, J.D. Residues of bendiocarb and NC 7312 in
1982 peas and pea pods following treatment with a 50SC
formulation in the UK, 1981. FBC Report RESID/82/61.
(Unpublished)
Browne, P.M. and Reary, J.B. Residues in milk and tissues following
1978a a 28-day feeding study with bendiocarb in dairy cows. Fisons
Report RESID/78/58. (Unpublished)
Browne, P.M. and Reary, J.B. Analytical method for residues of
1978b bendiocarb metabolites in milk and tissues of dairy cows.
Fisons Report RESID/78/54. (Unpublished)
1978c Bendiocarb residue decline in soil after treatment of maize
plots with a 10G formulation in Illinois, 1977. Fisons
Report RESID/78/93. (Unpublished)
1979a Laboratory leaching study with a granular (3G) formulation
of bendiocarb in three standard soils from West Germany.
Fisons Report RESID/79/78. (Unpublished)
1979b Analytical method for residues of bendiocarb 7312 in rice
grain and straw. Fisons Report RESID/79/22. (Unpublished)
1979c Improved method for the analysis of bendiocarb residues in
maize grain, stover and whole silage plants. Fisons Report
RESID/79/24. (Unpublished)
1979d Residues of bendiocarb in maize at different growth stages,
after application of a 3G formulation in France, 1978.
Fisons Report RESID/79/2. (Unpublished)
1980a Laboratory leaching study with a 1% dust formulation of
bendiocarb in three standard soils from West Germany. Fisons
Report RESID/800/111. (Unpublished)
1980b Analytical method for residues of bendiocarb in rape seed.
Fisons Report RESID/80/59. (Unpublished)
1980c Residues in silage maize grown in the U.K. from seed dressed
with bendiocarb, 1979. Fisons Report RESID/80/12.
(Unpublished)
1980d Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1979. Fisons Report RESID/80/13.
(Unpublished)
1980e Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979 (2nd
Report). Fisons Report RESID/80/20. (Unpublished)
1980f Residues of bendiocarb in rape seed after application of a
granular (5G) formulation in Canada, 1979. Fisons Report
RESID/80/60. (Unpublished)
1981 Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in West Germany, 1980.
FBC Report RESID/81/9. (Unpublished)
Challis, I.R. The metabolism of 14C-bendiocarb in sugarbeet. Fisons
1975 Report METAB/75/15. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in maize
1977 plants. Fisons Report METAB/77/32. (Unpublished)
1978 The metabolism of bendiocarb in sugarbeet grown under field
conditions. Fisons Report METAB/78/19. (Unpublished)
Challis, I.R. and Swalwell, L.M. The metabolism of bendiocarb by
1980 barley plants. Fisons Report METAB/80/9. (Unpublished)
FBC Bendiocarb, submission to the Joint Meeting of the FAO
1982a Panel of Experts on Pesticide Residues in Food and the
Environment and the WHO Expert Group on Pesticide Residues,
FBC Limited, Registration Department, Saffron Walden, Essex,
England 1982.
1982b Harvest residues of bendiocarb (NC 6897) in sugarbeet after
application of a granular (3G) formulations. (Unpublished)
1982c Residues in grass following the use of bendiocarb (50SC)
(CR 15 340) as a seed dressing or spray dressing or spray
treatment in the U.K., autumn 1979. (Unpublished)
Housden, M.C. and Reary, J.B. Bendiocarb residues in rice treated
1981 with a 50WP formulation in the Philippines during the 1980
dry season. FBC Report RESID/81/16. (Unpublished)
Lewis, J.A. and Adcock, J.W. Investigation of residue levels in
1978a maize plants treated with 14C-bendiocarb in France. Fisons
Report RESID/78/50. (Unpublished)
1978b Investigation of residue levels in maize treated with
14C-bendiocarb granules in the U.K. Fisons Report
METAB/78/19. (Unpublished)
1978c Investigation of residue levels in maize plants treated with
14C-bendiocarb granules. Fisons Report METAB/78/7.
(Unpublished)
Lewis, J.A. and Challis, I.R. The metabolism of 14C-bendiocarb in
1978 the hen. Fisons Report METAB/78/28. (Unpublished)
1979 The metabolism of 14C-bendiocarb in paddy rice. Fisons
Report METAB/79/1. (Unpublished)
Netherlands. Information on pesticides included in the JMPR priority
1982 list provided by The Netherlands Government.
Reary, J.B. Harvest residues of bendiocarb in sugarbeet following
1974 its use as a seed dressing. Fisons Report RESID/74/36,
December 1974. (Unpublished)
1975a Soil percolation experiments with bendiocarb. Fisons Report
RESID/75/12. (Unpublished)
1975b Laboratory study of bendiocarb degradation in two standard
soils from West Germany. (Unpublished)
1975c Analytical method for residues of bendiocarb in sugarbeet.
FBC Report RESID/75/26. (Unpublished)
1975d Bendiocarb (NC 6897) residue decline on laboratory grown rye
grass sprayed with Ficam W. Fisons Report RESID/75/5.
(Unpublished)
1975e Harvest residues of bendiocarb (NC6897) in sugarbeet
following its use as a seed dressing in West Germany (1974).
Fisons Report RESID/75/12. (Unpublished)
1978a Residues in milk and tissues following a 28-day feeding
study with up to 25 ppm bendiocarb in the diet of dairy
cows. Fisons Report RESID/78/101.(Unpublished)
1978b Residues in eggs and tissues following a 21-day feeding
study with bendiocarb in laying hens. Fisons Report,
RESID/78/59. (Unpublished)
1978c Residues of bendiocarb in laboratory-extracted oil from
maize harvested after band application of a granular (10G)
formulation in the U.S., 1977. Fisons Report RESID/78/95.
(Unpublished)
1978d Analytical method for bendiocarb residues in soil. Fisons
Report RESID/78/37. (Unpublished)
1978e Analytical method for bendiocarb residues in grass. Fisons
Report RESID/78/61. (Unpublished)
1978f Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 76 WP formulation at
28-day intervals, in California and Massachusetts, 1977.
Fisons Report RESID/78/9. (Unpublished)
1978g Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 5G formulation at
28-day intervals in California and Massachusetts, 1977.
Fisons Report RESID/78/32. (Unpublished)
1978h Residues in maize grown in the U.K. from seed dressed with
bendiocarb 80W, 1977. Fisons Report RESID/78/86.
(Unpublished)
1978i Residues of bendiocarb in maize treated with a granular (3G)
formulation in the U.K., 1977. Fisons Report RESID/78/97.
(Unpublished)
1978j Residues in maize grown in France during 1975, 1976 and 1978
from seed dressed with bendiocarb 80W. Fisons Report
RESID/78/106. (Unpublished)
1978k Bendiocarb residue decline in soil after band treatment of
maize plots with a 10G formulation in Missouri, 1977. Fisons
Report RESID/78/50. (Unpublished)
1978l Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a 10G granular formulation
in Missouri, 1977. Fisons Report RESID/78/51. (Unpublished)
1978m Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a granular (10G)
formulation in Illinois, 1977. Fisons Report RESID/78/60.
(Unpublished)
1978n Residues of bendiocarb in the mature maize crop (grain, cobs
and stover) after band application of a granular (10G)
formulation in regions of the U.S., 1977. First Revision,
Fisons Report RESID/78/61/1. (Unpublished)
1979a Residues in milk from cows treated dermally with FICAM D in
Australia, 1979. Fisons Report RESID/79/49. (Unpublished)
1979b Analytical method for bendiocarb residues in barley, wheat,
oats and maize (corn) grain. Fisons Report RESID/79/17.
(Unpublished)
1979c Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. (Extract of
analytical method from the above report). Fisons Report
RESID/79/9. (Unpublished)
1979d Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/9. (Unpublished)
1979e Residues of bendiocarb in barley, wheat and oats grown from
treated seed in the U.K., 1977 and 1978. Fisons Report
RESID/79/3. (Unpublished)
1979f Residues of bendiocarb in silage maize treated with a
granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/12. (Unpublished)
1979g Residues in silage maize grown in the U.K. from seed dressed
with an 80W formulation of bendiocarb, 1978. Fisons Report
RESID/79/13. (Unpublished)
1979h Harvest residues of bendiocarb and NC 7312 in sugarbeet
after application of a granular formulation (3G) in the
U.K., 1978. Fisons Report RESID/79/14. (Unpublished)
1979i Residues of bendiocarb and NC 7312 in sugarbeet after
application of a granular (3G) formulation in France and
Italy, 1978. Fisons Report RESID/79/15. (Unpublished)
1979j Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1978. Fisons Report RESID/79/16.
(Unpublished)
1979k Residues of bendiocarb and NC 7312 in rice treated with a
granular (3G) or spray (50W or 80W) application in the
Philippines, 1978. Fisons Report RESID/79/23. (Unpublished)
1979l Residues of bendiocarb in maize (corn) grown in the U.S.,
from seed dressed with a 76WP formulation, 1977 and 1978.
Fisons Report RESID/79/25. (Unpublished)
1979m Residues of bendiocarb in maize (corn) after band
application of a granular (10G) formulation in Canada, 1978.
Fisons Report RESID/79/26. (Unpublished)
1979n Residue analysis of fodder beet following the use of
bendiocarb as a seed dressing in West Germany, 1978. Fisons
Report RESID/79/45. (Unpublished)
1979o Residues of bendiocarb in silage and mature (grain and
stover) maize after band application of a granular (10G)
formulation in the U.S., 1978. Fisons Report RESID/79/57.
(Unpublished)
1979p Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/76. (Unpublished)
1970q Residues of bendiocarb in sugarbeet following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/75. (Unpublished)
1980a Residue in barley, wheat and oats following seed treatment
with bendiocarb in the U.K., 1979 (80WP) batch 8A8, 50W
spray or 15 181 and 3G or 15 094. Fisons Report RESID/80/80.
(Unpublished)
1980b Bendiocarb residue decline on rangeland grass after ULV
treatment (by air) with a 25% formulation (15 15 458) in
Montana, U.S., 1980. Fisons Report RESID/80/95.
(Unpublished)
1980c Preliminary report on residues in milk and tissues from
dairy cows treated dermally with bendiocarb dust for 7 days.
Fisons Report RESID/79/55. (Unpublished)
1980d Residues of bendiocarb in grass grown from treated seed in
Australia, 1979. Fisons Report RESID/80/9. (Unpublished)
1980e Residues in grass following the use of bendiocarb as a
granular (3G), seed dressing (80W) or spray (50W) treatment
in the U.K., Spring 1979. Fisons Report RESID/80/11.
(Unpublished)
Reary, J.B. Residue decline in sugarbeet treated with bendiocarb 3G
1980f in the U.K., 1979. Fisons Report RESID/80/4. (Unpublished)
1980g Residue decline in soil after furrow application of
bendiocarb 3G granules at Shelford, U.K., 1979. Fisons
Report RESID/80/14. (Unpublished)
1980h Residue decline in maize treated with bendiocarb 3G in the
U.K., 1979. Fisons Report RESID/80/17. (Unpublished)
1980i Residue decline in maize grown from seed dressed with
bendiocarb in the U.K., 1979. Fisons Report RESID/80/18.
(Unpublished)
1980j Residues in sugarbeet from grower trials with a granular
(3G) formulation of bendiocarb in the U.K., 1979. Fisons
Report RESID/80/29. (Unpublished)
1980k Bendiocarb residue decline on rangeland grass after low
volume treatment with a 50SC formulation (CR 15 340) in
Montana, U.S., 1979. Fisons Report RESID/80/37.
(Unpublished)
1980l Residues in rice treated with bendiocarb formulations in the
Philippines and Korea 1979. Fisons Report RESID/80/46.
(Unpublished)
1981m Residues in grass following the use of bendiocarb as a seed
dressing, granular or spray treatment in the U.K., 1980. FBC
Report RESID/81/10. (Unpublished)
Reary, J.B. and Browne, P.M. Residues of bendiocarb (NC 6897) in
1978 mature maize, after application of a granular formulation in
France, 1976 and 1977. Fisons Report RESID/78/23.
(Unpublished)
Reary, J.B. and Whiteoak, R.J. Bendiocarb residue decline on soil,
1977a grass and thatch after treatment of turf with a 76WP
formulation in Mississippi, 1976. Fisons Report RESID/77/43.
(Unpublished)
1977b Bendiocarb residue decline in soil, grass and thatch after
treatment of turf with a 76WP formulation in Ohio, 1976.
Fisons Report RESID/77/52. (Unpublished)
1977c Bendiocarb residue decline on soil, grass and thatch after
treatment of turf with a granular formulation in Ohio, 1976.
Fisons Report RESID/77/61. (Unpublished)
1977d Bendiocarb residue decline on grass and soil after treatment
of turf with a 76WP formulation in California and
Massachusetts, 1977. Fisons Report, RESID/77/62.
(Unpublished)
Warner, P.A. The metabolism of 14C-bendiocarb in oilseed rape. Fisons
1980 Report METAB/80/20. (Unpublished)
unidentified water soluble and fibre-bound residues, other metabolism
studies discussed below suggest that the pathway is similar in other
plants.
In a second sugarbeet metabolism study (Adcock 1976),
14C-bendiocarb was applied as a seed dressing for plants grown in
outdoor drums in sandy loam soils at 6.7 and 7.5 pH values at a rate
equivalent to 16 g a.i./kg seed. Leaves and/or roots were analysed for
radioactivity at intervals from 13-114 days after sowing, although the
residues were not identified. In accordance with decreased bendiocarb
stability in more alkaline soils, extractable and fibre-bound residues
were less than the 0.02 mg/kg limit of detection (bendiocarb
equivalents) in foliage and roots of the more alkaline soil after 114
days. Residues were greater at earlier intervals. For example, at 36
days residues were on the order of 0.1 mg/kg bendiocarb equivalent in
leaves or roots from either extractable or fibre-bound sources.
Consistent with pH stability, residues were higher in the more acidic
soil, being up to 0.06 mg/kg and 0.02 mg/kg extractable residues in
leaves and roots, respectively, after 114 days; they were appreciably
higher at shorter intervals. A maximum of 18% of applied radioactivity
was found in immature sugarbeet (36-72 days after sowing).
In the third sugarbeet metabolism study (Challis and Adcock
1978), the crop was grown under field conditions and treated at sowing
with 14C-bendiocarb granules at the recommended rate of 0.36 kg
a.i./ha. Plants were harvested at intervals ranging from 46-190 days
after treatment. Results were similar to those of Challis (1975).
After 90 days, radioactivity was too low to permit determination of
individual metabolite levels. In harvested beets, mean residues of
only 0.009 mg/kg bendiocarb equivalent were found after 190 days.
Extractable residues were below the limits of sensitivity.
As would be expected, residues were greater at earlier intervals
and identification was possible. For example, at 46 days residues
(bendiocarb equivalent) were 1.0, 0.22, 0.14, 0.76 and 0.45 mg/kg for
bendiocarb, NC 7312 conjugates, N-hydroxymethylbendiocarb conjugates,
unidentified water soluble residues and fibre-bound residues,
respectively. There were lesser amounts of free metabolites and other
conjugates.
Bendiocarb metabolism was investigated in maize under glasshouse
conditions by applying a solution of 14C-bendiocarb to pH 5.4 soil in
drums with newly germinated maize plants at a rate equivalent to
1.1 kg a.i./ha (Challis and Adcock 1977). Plants were analysed at
intervals from 10-125 days. Mean residues of bendiocarb per se in
plants ranged from 1.4 mg/kg after 10 days to 0.01 mg/kg after 125
days and unidentified soluble residue (bendiocarb equivalent) in
kernels and cobs were 0.005 and 0.001 mg/kg, respectively, after 125
days. As a percentage of total radioactivity, the major residues after
10, 60 or 125 days were:
Foliage Kernels Cob
10 days 60 days 125 days 125 days 125 days
Bendiocarb 38.1 24.2
N-hydroxymethylbendiocarb 8.2 5.3 8
NC 7312 conjugates 163.3 2.7 20
Water soluble 13.6 11 9.1
(non-hydrolysable)
Fibre-bound 13.6 27 61 89 97
Free NC 7312, other non-polar metabolites and other conjugates
were also present at lower levels. Seventy percent of the residues in
plants at harvest were either water non-hydrolysable of fibre-bound
unidentified residues. Although they occurred at very low levels,
fibre-bound unidentified residues constituted 89 and 97 percent of
total radioactive residues in kernels and cobs, respectively, at
harvest.
Investigations were conducted on paddy rice metabolism of
14C-bendiocarb applied as a solution with a syringe under glass to
either the foliage or flood water surrounding rice transplants (Lewis
and Challis 1979). Application rates were equivalent to 0.6 kg a.i./ha
and plant samples were taken at intervals ranging from 15-174 days
after treatments. Foliage and grain were analysed separately at 174
days. Qualitatively, residues were similar to those in sugarbeet and
maize, although N-hydromethylbendiocarb or its conjugates were not
specifically identified in rice. They could have been present under
residues described either as other non-polar metabolites or other
conjugates.
Mean free bendiocarb in rice plants ranged from 1.7 mg/kg after
15 days to 0.0022 mg/kg (total radioactivity as bendiocarb
equivalents) after 174 days from the foliar treatments. The most
abundant metabolite by far was NC 7312, mostly as a conjugate
(0.006 mg/kg bendiocarb equivalent). Residues were 0.009 mg/kg in rice
husks and flour after 174 days.
In plants growing in treated water, residues of free bendiocarb
at 15 days were approximately one tenth of that from foliar
treatments, the major residue being NC 7312 conjugates at 0.54 mg/kg.
Individual residues could not be determined after 60 days. At harvest,
total radioactivity levels are similar regardless of the type of
treatment. Quantitatively, major residues in plants as a percent of
total radioactivity at 15 or 174 days are:
15 days 174 days
Foliar Water Foliar Water
Bendiocarb 37 12
NC 7312 13 37 39 31
conjugates
Water soluble 7 10
(unhydrolysable)
Fibre-bound 33 33 61 69
All of the radioactive residue at harvest in rice husks or flour
was NC 7312 conjugates, regardless of whether application was by
foliar or water treatment. As in the case of maize foliage, residues
in rice plants were mostly unidentified fibre-bound ones at harvest.
The difference was in the grain, which contained mostly unidentified
fibre-bound residues in maize and mostly NC 7312 conjugates in rice.
Greenhouse-grown barley plants were treated with 14C-bendiocarb
at rates equivalent to 1 kg/ha. A solution was applied by pipette to
soil surrounding the plants which were analysed at intervals ranging
from 4-94 days after treatment. Samples at 94 days were separated into
foliage, husks and grain, which were analysed separately (Challis and
Swalwell 1980).
Results of this investigation were similar to that of
rice. However, in the case of barley, conjugates of
N-hydroxymethylbendiocarb were identified, but total radioactive
residues in husks and grain at 94 days (1.46 and 0.09 mg/kg bendiocarb
equivalent, respectively) were not characterized. Free bendiocarb
residues in foliage ranged from 0.09 mg/kg at 4 days, declined through
60 days and increased to <0.07 mg/kg at 94 days. Similar patterns
were observed for conjugates of NC 7312 and N-hydroxymethylbendiocarb,
the major components of the residue throughout the period. Again,
unidentified water soluble or fibre-bound residues constituted a major
part of the residue, which increased until harvest (12 and 42% of
total radioactivity, respectively).
The metabolism of 14C-bendiocarb was studied in greenhouse grown
oilseed rape treated at the seedling stage at application rates
equivalent to 140 and 500 g a.i./ha. Application was by pipette to
soil surrounding the plant (Warner 1980). Plant samples were taken at
intervals ranging from 5 to 140 days after treatment. At 140 days,
samples were separated into foliage, pods, and seed, which were
analysed separately.
As in the case of barley, the major residues in the plants were
highest at earlier intervals and, except for free bendiocarb, declined
and then increased by harvest. In both cases this was probably due to
plant growth patterns and dessication near harvest. Free bendiocarb
was the major residue at day 5 (66-69%), but declined rapidly
thereafter (2.5 mg/kg at day 5 to 0.01 mg/kg at 140 days from the
high treatment rate). Again, conjugates of NC 7312 and
N-hydroxymethylbendiocarb were the major identified metabolites and,
by harvest, unidentified water soluble materials and fibre-bound
residues formed the major portion of total radioactivity which was 26
and 43%, respectively, from the higher treatment rate. Although
residue levels were dependent on the application rate, on the basis of
percentage of total residue, results were similar at both application
rates.
Extractable residues in pods, seeds and foliage at harvest were
0.09, 0.001 and 0.16 mg/kg, respectively, at the low application rate
and 0.74, 0.011 and 0.84 mg/kg, respectively, at the high rate.
In Soil
Laboratory studies on residue decline in a pH 7.7 sandy loam soil
treated in flasks or jars with 5 mg/kg 14C-bendiocarb showed
degradation to CO2 and unidentified bound residues under aerobic
conditions, with a half-life of 5 days for the parent compound. No NC
7312 was detected. With flooding or nitrogen flushing and under
sterile anaerobic conditions, bendiocarb was hydrolysed to NC 7312,
with little or no further degradation (Adcock et al 1975a).
In a laboratory soil leaching study with the same soil type, 60%
of the radioactivity applied to a 30 cm column as 14C-bendiocarb was
eluted with 50 cm of water over 11 days, mostly as NC 7312 (Adcock
et al 1975b). Presumably, conditions were effectively anaerobic and
the rapid degradation of bendiocarb did not permit estimation of its
leachability.
In another leaching study, 14C-bendiocarb, leachability was
investigated for an agricultural sand (pH 5.3), a clay loam (pH 7.3),
a sandy loam (pH 7.8) and a silt loam (pH 7.2) using soil column and
thin-layer techniques (Adcock 1978). The 30 cm columns were percolated
over a 2-9 day period with a 20 cm column of water. The percentage of
radioactivity recovered in leachate was 75, 11.9, 7.9 and 0.8% for the
respective soils, decreasing with increased organic content.
Recovery in leachate was slightly less than for 2,4-D in
agricultural sand and clay loam, and substantially less in the other
two soils under similar conditions. Thin-layer results confirm these
observations and show that NC 7312 is somewhat less leachable than the
parent compound in all four soil types. It also shows that bendiocarb
is more leachable than trietazene, which places it in the moderately
mobile class.
When bendiocarb 80%WP, 3%G and 1% dust formulations were leached
from sand (pH 7-7.6) loamy sand (pH 6-6.5) and sand loam (pH 5.6-7.8)
in 30 cm × 5 cm columns with 20 cm water over 48 hours, leachability
was again inversely proportional to organic matter content of the soil
(Reary 1975a; Browne and Reary 1979a, 1980a). As a percent of the
amount applied, bendiocarb recovery in leachate ranged from 0 to 93%
WP, 0.6 to 84% dust and 0 to 22% granular. From the same formulations
leachate contained 0 to 25% bendiocarb and 0 to 68% NC 7312; 0 to 45%
bendiocarb, 0 to 62% NC 7312 and 0 to 4% bendiocarb, 0 to 19% NC 7312,
respectively. Therefore, leachability decreased in the order of WP,
dust and granular formulations.
Bendiocarb stability in standard soils has been shown to be
dependent on soil pH, being much slower at low pHs (Reary 1975b). The
half-life was 10 days in one soil at pH 2.2 and 58 days in another at
pH 5.2.
Soil residue decline studies have also been carried out in trials
determining bendiocarb residues in turf and maize. Results again show
that the rate of degradation of bendiocarb is pH-dependent. Repeated
applications showed no evidence of a build-up of bendiocarb residues
in soil. Dissipation rates (in days) for various formulations in
various soil types and pHs are given in Table 9 (Browne and Reary
1978c; Reary 1975b, 1978f, g, k, 1980g; Reary and Whiteoak 1977a, b,
d).
In Storage and Processing
It was noted under the section on "Residues from Supervised
Trials" and in Table 6 that oil processed from maize with field-
incurred residues of <0.02 mg/kg bendiocarb also contained residues
of <0.02 mg/kg. In another investigation, 10G was applied as a band
along the maize row at sowing at 1.1-2.2 kg a.i./ha and the maize
sampled at normal harvest. Oil was solvent-extracted from the grain,
which had residues of <0.01 mg/kg, the limit of determination.
Analysis of the oil showed no residues in excess of the 0.05 mg/kg
limit of determination (Reary 1978c).
Investigation with radio-labelled bendiocarb in granular
application trials in France, the U.K. and the U.S. showed residues in
solvent-extracted oil to be 0.008, 0.011 and 0.021 mg/kg,
respectively, from kernels with residues of 0.011, 0.013 and 0.022
mg/kg, respectively. In most kernel or oil samples residues were
<2X the reported limit of sensitivity (Lewis and Adcock 1978a, b, c).
RESIDUES ON FOOD IN COMMERCE OR AT CONSUMPTION
No information was provided on residues in commerce or at
consumption. The Meeting considered such information desirable.
Table 9. Summary of Bendiocarb Dissipation Rates in Soil
Soil Dissipation time (days)
Type pH Formulation 50% 75% 90%
Sandy loam 5.2 Technical1/ 58 ? ?
Sandy 6.5 76W 25 50 75
loam 76W2/ 12-34 24-69 37-104
Sandy loam 6.5 5G2/ 26-70 52-140 78-210
Silty clay 6.5 10G 19 38 57
Silty clay loam 6.7 76W 30 60 ?
Sand 6.8 Technical1/ 10 24 50
Loam 7.2 10G 7.5-10 15-20 22.5-30
Loam 7.7 76W 12 24 36
Sandy 7.7 76W 2.4 4.8 7.2
loam 76W2/ 2-5 3-10 5-15
Sandy loam 7.7 5G2/ 12-26 30-52 45-78
Sandy loam 7.8 3G 12 24 36
1/ Laboratory studies.
2/ Multiple applications at up to 5-monthly intervals;
dissipation times determined after each application.
METHODS OF RESIDUE ANALYSIS
Residue analysis methods for various plant substrates have been
developed for bendiocarb alone or for bendiocarb plus the non-
conjugated metabolite NC 7312, either separately or as a total
residue. In animal substrates, enzymic liberation of NC 7312 from
conjugates is more successful than in plants. Consequently, residue
methods for animal tissues, milk and eggs include determinations of
the conjugated residues.
As the carbamate functional group does not possess strong
electron capturing properties, derivatization of the bendiocarb
molecule is necessary to facilitate the measurement of low residue
concentrations. Formation of the 2,4-dinitrophenyl ether was found to
be most suitable. This results in a product with strong electron
capturing properties and a long gas chromatographic (GLC) retention,
thus ensuring excellent sensitivity and minimal co-extractive
interference.
In the standard procedure (Reary 1975c) the carbamate is
hydrolysed in an alkaline buffer containing 1-fluoro-2,
4-dinitrobenzene so that the dinitrophenyl ether is formed immediately
after hydrolysis. This method has the added advantage the phenolic
degradation product, NC 7312, forms the same product in the reaction.
Thus, the combined residue can be measured unless bendiocarb and NC
7312 are separated prior to derivatization. This separation is readily
effected by high pressure liquid chromatography (HPLC) or with
commercially available liquid chromatographic cartridges. Collection
of the appropriate fraction allows individual analysis for bendiocarb
and NC 7312 from the same extract (Brown and Reary 1979b).
Bendiocarb is extracted from soil (Reary 1978d), grass (Reary
1978e) sugarbeet (Reary 1975c), maize (Browne and Reary 1979c), cereal
grain (Reary 1979b), rice (Browne and Reary 1979b), rape seed (Browne
and Reary 1980b), potatoes (Reary 1979c), cabbage (Browne 1981b),
mushrooms (Browne 1982a), apples/pears (Browne 1982b) and peas (Browne
and Manley 1982), under reflux with dichloromethane or by shaking with
diethyl ether. Oily extracts are improved by partition between hexane
and acetonitrile. Further clean up, when required, is by silica gel
chromatography. This is easily done using commercially available
disposable cartridges, which can eliminate or separate any NC 7312
present in the extract.
A method has been developed for the residue analysis of
bendiocarb and major metabolites in milk, eggs, meat, fat and offal
(Browne and Reary 1978b). Residues are extracted under reflux with
acetonitrile. Conjugated metabolites are hydrolysed with snail
digestive juice before formation of the dinitrophenyl derivative. All
major bendiocarb related metabolites form the same derivative, which
is then analysed by the standard method as a total residue.
In general, mean analytical recoveries in most commodities were
>75% for bendiocarb and >60% for NC 7312. Analytical
sensitivities, recoveries, recoveries of fortified commodities and
apparent residue in untreated controls for individual commodities are
discussed under "Residues Resulting from Supervised Trials."
Information represents field-type conditions and is somewhat less
assuring than mean recoveries presented with the reports on each
individual analytical method. Although mean analytical recoveries
appeared acceptable, at least for bendiocarb per se, the range of
residues was generally large, resulting in standard deviations ranging
from 6 to 33. In animal tissues in particular, and to some extent in
cereal grains and fodder/forage, analytical recoveries were frequently
low. For example, in animal products analytical recoveries were
frequently only 50-60% for bendiocarb per se or the N-hydroxy
metabolite, and sometimes less.
NATIONAL MAXIMUM RESIDUE LIMITS
Information on national maximum residue limits were available
from only one country. Limits have been proposed in four other
countries (Table 10).
Table 10. National Maximum Residue Limits
Country Commodity MRL (mg/kg)
Netherlands1/ "All commodities"3/ 0 (0.05)4/
Australia2/ Poultry meat, eggs 0.05
Milk, milk by-products, meat
and other edible offal 0.1
Cereal grain 10.0
Belgium2/ Maize and sugarbeet 0.02
Switzerland2/ General foodstuffs (excl. milk) 0.2
Milk 0.005
U.S.2/ Maize (grain fodder, forage) 0.05
Maize oil 0.1
Milk, eggs, meat fat, meat
by-products (except kidneys)
of cattle, goats, pigs horses,
poultry, sheep 0.05
Kidneys of cattle, goats, pigs,
horses, poultry, sheep 0.1
1/ Established;
2/ Proposed;
3/ It is unclear whether "all commodities" include
commodities other than maize and sugarbeet, for which
good agricultural practice information was provided;
4/ 0 (0.05) mg/kg presumed to mean no residues allowed with
a 0.05 mg/kg limit of determination. Large-scale use.
APPRAISAL
The Meeting reviewed substantial information on residue levels
from supervised trials in a variety of commodities, fate of residues
and analytical methodology for bendiocarb, a carbamate pesticide used
for control of a wide range of agricultural and public health pests.
Information on official nationally approved agricultural practices and
national maximum residue limits were available for only two
commodities (maize and sugarbeet) in one country, although recommended
application rates were provided for a number of commodities. Since
information on good agricultural practices for all commodities except
maize and sugarbeet is lacking, only TMRLs are estimated for other
than these two commodities. National tolerances have been proposed in
at least four countries, mostly on maize and other cereals and animal
products.
Residue data were available from supervised trials on a variety
of commodities in several countries and using a variety of
formulations and agricultural practices. In most cases, free
bendiocarb per se was the only residue measured. In a few cases, the
major metabolite 2,2-dimethyl-1,3-benzoxodiol-4-ol (NC 7312) was
measured in its free form and in some cereals, combined residues of
conjugated NC 7312 and N-hydroxymethylbendiocarb were measured as
well. Data were inadequate for estimating a limit for oilseed rape,
cabbage and peas, although the Meeting was informed that additional
data are being compiled for the latter.
For root or tuber vegetables, data were available for potatoes
and sugarbeet. Data on potatoes show residues of free NC 7312 at
harvest to be up to 20 times that of the parent compound (average 6X).
This rate could not be determined for sugarbeet with available data.
Limited data were available in pome fruit from one country and
residues of NC 7312 were equal to or less than those of the parent
compound. The Meeting was informed that additional data are being
gathered.
Bendiocarb residue data on cereals were available for barley,
oats, wheat, maize and rice. For the latter, limited data were also
available for free NC 7312, and the first three, for combined residues
of conjugates of NC 7312 and N-hydroxymethylbendicarb. Data were
mostly from seed treatments in barley, oats and wheat. No data were
available for metabolites in maize, although metabolism studies have
shown >50% of residues in foliage to be metabolites and/or
unidentified water-soluble or bound residues; in kernels at harvest,
approximately 90% of radioactivity was unidentified fibre-bound
residues. Analytical recoveries were only marginally acceptable in
some cereal trials, especially those in wheat.
Considerable data were available for grass and turf, mostly at
higher than recommended rates. Half-lives were greater from granular
broadcast applications than from spray applications and irrigation did
not significantly affect decline rates for either.
As in maize, data for oilseed rape were for the parent compound
only, and metabolism studies showed >50% of residues at harvest to be
conjugated metabolites and (mostly) unidentified water-soluble or
fibre-bound residues.
Data were available for mushrooms from spray treatments (plot
presumed) and wall treatments (before plot establishment). Residues
increased with application rates and residues of free NC 7312 from
wall treatments were on the same order of magnitude as those of the
parent compound, but were less following direct spray treatment. No
information was provided on good agricultural practices for direct
spray treatment.
Animal feeding trials were conducted in which lactating dairy
cattle were fed diets for 28 days containing up to 25 mg/kg
bendiocarb. In contrast to field trials, in which the parent compound
was determined separately from metabolites, animal tissues were
analysed for total residues of free bendiocarb and non-conjugated
metabolites plus conjugated NC 7312 and N-hydroxymethylbendiocarb.
Milk was separated into a fat fraction and lactose (aqueous) fraction.
The fat fraction was analysed for bendiocarb and non-conjugated NC
7312 and N-hydroxymethylbendiocarb and the lactose fraction for the
conjugated residues of the two metabolites.
Studies using topical treatments were also conducted, but no
information was provided on approved or recommended uses for such
treatments. Dermal treatments generally resulted in higher residues in
tissues and milk than feeding trials. In the absence of good
agricultural practice information for dermal treatments (or grass
uses) residue estimates in cattle are based on potential residues from
the feeding of maize, for which national good agricultural practice
information was available. From this potential source of exposure, no
residues are expected in products of animal origin above the limit of
determination at or about 0.05 mg/kg (0.2 mg/kg in kidney).
In animal products in general, analytical recoveries were
disappointingly low, frequently only 50-60% or less for some residues
in some tissues, and thus can be considered marginally acceptable for
enforcement purposes only, since residues are expected to be
relatively low as a result of uses considered in residue level
estimates.
Animal metabolism studies were conducted in the rat, dog,
hamster, rabbit, mouse, pig, cow and poultry and in humans. In the
pig, cow and poultry single dosing was with 14C-bendiocarb, by
gelatin capsule in pig and cow and by gavage in poultry. Approximately
80% of applied radioactivity was eliminated in the urine within one
day or less in pigs and cows and 61-90% in poultry. It consisted
mostly of the sulphate or glucuronide conjugates of NC 7312 in all
three, although in poultry significant levels of unidentified
water-soluble residues were also present, as well as low levels of
free NC 7312 and other unidentified moieties. Low levels of NC 7312
sulphate and glucuronide were also present in faeces of cows and pigs.
In pigs the NC 7312 sulphate to glucuronide ratio was unexpectedly
high.
In animal tissues and milk, residue levels were generally not far
from the limit of determination and only 0.01% of the administered
dose was found in eggs. Owing to the low levels, they were not
identified.
Bendiocarb metabolism in cows, pigs and poultry appears to be
similar to that in smaller animals. Carbamate is hydrolysed to
NC 7312, which forms glucuronide and sulphate conjugates. The
apparent major difference is the lack of conjugates of
N-hydroxymethylbendiocarb also found in plants, and 5,6, or
7-hydroxybendiocarb found or tentatively identified in the smaller
animals. However, dosing rates of 0.2-0.25 mg/kg bw in cows, pigs and
hens were substantially less than the maximum dosage rates of 10 mg/kg
bw used for the smaller animals. More importantly, cows, pigs and
poultry were sacrificed four days after dose administration, which
precludes measurement or characterization of additional radioactivity
closer to administration. This could also account for residue levels
in animal products (cows, pigs and poultry) being too low for
identification. A metabolism study conducted in both ruminants and
poultry at high levels and a much shorter sacrifice interval would
have been preferred and are desirable. Such a metabolism study should
be considered essential if higher than the current ones for animal
products are required from the feeding of grain, fodders or straw of
cereals.
Metabolism studies in sugarbeet, maize, barley, rice and oilseed
rape under laboratory or field conditions showed similar quantitative
results, although ratios of the various metabolites and degradation
products varied considerably, depending on the plant, type of
application, interval from application to harvest, part of plant and
other factors.
A major metabolic pathway for bendiocarb in some plants is
hydrolysis to NC 7312, which becomes conjugated as a glycoside.
In another pathway, bendiocarb can first be oxidized to
N-hydroxymethylbendiocarb, which may hydrolyse to NC 7312
before glycosidic conjugation or become conjugated as
N-hydroxymethylbendiocarb glycoside. Another route can be direct
glycosidic conjugation of bendiocarb per se. This was a major residue
in beet leaves following a surface application, but NC 7312 conjugates
were found following surface treatments to rice. Undoubtedly other
pathways exist, since low levels of unidentified conjugates are known
to occur. In one beet study, limited leaf penetration was detected
from surface application.
As the interval from application to harvest increased, residues
of bendiocarb per se decreased with an increase in NC 7312 and
N-hydroxymethylbendiocarb conjugates, which were frequently the major
identified metabolites. Unidentified water-soluble, non-hydrolysable
residues and unidentified fibre-bound residues have also been shown to
increase in the foliage of several plants; at harvest they constitute
26 and 60%, respectively, of the total remaining radioactivity. In
maize kernels and cobs at harvest the fibre-bound unknown was shown to
consist of >90% of the remaining low residue.
Low levels of free NC 7312 and other unidentified unconjugated
residues were found in some plants. Free NC 7312 exceeded that of the
parent compound in potatoes and probably in pea pods. In other plants,
free NC 7312 residues were either less than or equal to that of the
parent compound. Differences in method of application (for example,
foliar versus ground application) affected the ratios of the various
metabolites in some, but not all, plants tested, although residues
were qualitatively similar.
In soils, bendiocarb was degraded to CO2 and unidentified bound
residues under aerobic conditions whereas degradation was mostly to
NC 7312 under anaerobic conditions. Bendiocarb stability was dependent
on soil pH, being much more stable under acidic conditions. Half-lives
ranged from 2-70 days, depending on conditions.
Leaching studies carried out on a variety of soils, under
different conditions and with several formulations showed leaching to
be largely dependent on the organic content of the soil, decreasing
with higher levels of organic matter. Although generally considered to
be in only a moderately leachable category, significant leaching of
bendiocarb can occur in sandy soils with low organic matter content.
Leachability appears to increase somewhat from granular to dust to
wettable powder formulations. One study indicated that NC 7312 leached
slightly less than bendiocarb per se. Although build-up of soil
residues of bendiocarb per se do not appear to be indicated, any
potential residues of bendiocarb or its metabolites in ground water
would be greatest from sandy soils of low organic matter content.
Most investigations on possible residues of bendiocarb in
processed products were conducted on oil, husks or flour from cereal
grain. Although no concentration of residues was demonstrated in these
studies, residues in the grain from which the products were processed
had residues below or near the limits of determination. Therefore,
firm conclusions cannot be drawn.
Analytical methods are available for the determination of free
bendiocarb in all plant commodities examined and free NC 7312 in some.
In the basic procedure, bendiocarb is hydrolysed to its phenol under
alkaline conditions in the present of 1-fluoro-2,4-dinitrobenzene to
form the dinitrophenyl ether, which is preferably determined by
electron capture gas chromatography using an internal standard.
Bendiocarb and NC 7312 can be determined together or separately, if
they are separated by HPLC or silica gel chromatography prior to
derivitization. In most commodities of plant origin, field trials were
for the free parent compound only and free NC 7312 was also determined
separately in a few. Analytical recoveries were highly variable on
some commodities, especially cereals.
For animals, the same basic analytical approach is used, except
that a hydrolysis step is included to hydrolyse conjugated residue.
Hydrolysis has been accomplished by a variety of enzymatic and/or
acidic conditions. Enzymatic approaches are preferred and snail
gastric juice is frequently used. In contrast to plants, residues
determined for animal tissues and milk include the sum of residues of
bendiocarb and non-conjugated metabolites plus conjugated NC 7312 and
N-hydroxymethylbendiocarb. Aqueous (lactose) and non-aqueous (fat)
fractions of milk are analysed separately. Analytical recoveries for
products of animal origin are only marginally acceptable for
enforcement purposes. Improved analytical methods are needed for any
uses requiring maximum residues levels in products of animal origin
that are higher than the current limits of determination.
RECOMMENDATIONS
The Meeting considered information on residues resulting from
good agricultural practices or recommended application rates on a
number of commodities and possible residues in animals resulting from
the feeding of grain, fodders or straw of cereals treated according to
good agricultural practice and concluded that the maximum residue
levels or temporary maximum residue levels below are suitable for
establishing maximum residue limits. Regardless of the status of the
ADI, temporary status was designated for all commodities except maize,
maize fodder and forage, sugarbeet and sugarbeet tops, pending
availability of good agricultural practice information. Except for
potatoes, some fruit, rice and rice straw, estimated residue levels
apply to treatment before planting and/or seed application only.
In the absence of good agricultural practice information, the
temporary limits are based on supervised trials data only. Residues
for products of plant origin refer to residues of unconjugated
bendiocarb. Residues in products of animal origin refer to the
sum of free and conjugated bendiocarb, NC 7312, and
N-hydroxymethylbendiocarb, expressed as bendiocarb.
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Potatoes 0.051/ 100 (granular)
0 (spray)
Sugarbeet 0.051/ 150
Sugarbeet tops 0.051/ 150
Pome fruit 0.021/ 50
Cereal Grains
Barley 0.051/ 100
Oats 0.051/ 100
Wheat 0.051/ 125
Maize (field) 0.051/ 125
Rice (in husk) 2 10
Fodders and Straws
Barley 0.051/ 125
Oats 0.051/ 100
Wheat 0.051/ 125
Maize 0.051/ 125
Rice 1 10
Mushrooms 0.12/ 50
(con't)
Limitations or Interval
TMRL last application to
Commodity (mg/kg) harvest (days)
Animal Products3/
Carcase meat of cattle 0.051/
Fat of cattle 0.051/
Meat by-products of cattle
(except kidney) 0.051/
Kidney of cattle 0.21/
Milk 0.051/
Poultry 0.051/
Fat of poultry 0.051/
Poultry meat by-products 0.051/
Eggs 0.051/
1/ At or about the limit of determination.
2/ Limit reflects maximum residues expected from wall treatments
prior to establishment of plots.
3/ Limits reflect maximum residues expected from the feeding of
cereal grains, fodders or straws harvested at given intervals
from application to harvest.
FURTHER WORK OR INFORMATION
Required (by 1984)
Information on nationally approved agricultural practices for the
use of bendiocarb on all commodities for which limits are estimated,
except for maize and sugarbeet.
Desirable
1. Additional metabolism studies in ruminants and poultry at
sufficiently high dosing levels and with sacrifice at a short
interval after dosing, instead of several days thereafter, to
permit a more complete characterization of residues in tissues,
milk and eggs. The analyses should include all residues
identified, or tentatively identified, in smaller animals and in
plants. These studies should be considered required before new
limits or future upward revisions of established ones on feed
items are established significantly higher than at current limits
of determination. The same is true should substantially higher
limits on tissues, eggs or milk be required for approved dermal
treatments.
2. Additional residue data, reflecting nationally approved uses
especially on, but not limited to, mushrooms and pome fruit.
3. Information on the possible occurrence of residues in food in
commerce or at consumption.
REFERENCES
Adcock, J.W. Investigation of residues in sugarbeet following the
1976 use of 14C-bendiocarb as a seed dressing. Fisons Report
METAB/76/1. (Unpublished)
1978 The leaching of 14C-bendiocarb in four soil types. Fisons
Report METAB/78/11. (Unpublished)
Adcock, J.W., Bentley, A.P., Challis, I.R. and Pearce, J.C. The
1975a decline of NC 6897 in a sandy loam soil. Fisons Report
METAB/75.2.(Unpublished)
Adcock, J.W., Challis, I.R. and Pearce, J.C. Soil leaching of NC
1975b 6897 and degradation products. Fisons Report METAB/75/3.
(Unpublished)
Adcock, J.W., Challis, I.R., and Warner, P.A. The metabolism of
1976a 14C-bendiocarb in the dairy cow. Fisons Report
METAB/76/23. (Unpublished)
Adcock, J.W., Warner, P.A. and Challis, I.R. The metabolism of
1976b 14C-bendiocarb in the pig. Fisons Report METAB/76/19.
(Unpublished)
Browne, P.M. Residues of Bendiocarb and NC 7312 in mushrooms
1981a treated with a 50SC formulation in Holland, 1980. FBC Report
RESID/8140. (Unpublished)
Browne, P.M. Analytical method for residues of bendiocarb in
1981b cabbage. FBC Report RES/81/65. (Unpublished)
1981c Residues of bendiocarb in cabbage following repeat
applications of a 50WP formulation in the Philippines,
1980-81. FBC Report RESID/81/53. (Unpublished)
1981d Residues of bendiocarb in rye grass after autumn
applications of granular and seed treatment formulations in
the U.K., 1980. FBC Report RESID/81/69. (Unpublished)
1981e Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in the U.K., 1981. FBC
Report RESID/81/74. (Unpublished)
1982a Residues of bendiocarb and NC 7312 in mushrooms following
wall spray treatment with 80WP or 50SC. FBC Report
RESID/82/20. (Unpublished)
1982b Residues of bendiocarb and NC 7312 in apples and pears
following treatment with 20W, 50W or 50SC formulations in
the U.K., 1981. FBC Report RESID/82/41. (Unpublished)
1982c Residues of bendiocarb and NC 7312 in sugarbeet following
application of a granular (3G) formulation in Italy, 1981.
FBC Report RESID/82/13. (Unpublished)
Browne, P.M. and Manley, J.D. Residues of bendiocarb and NC 7312 in
1982 peas and pea pods following treatment with a 50SC
formulation in the UK, 1981. FBC Report RESID/82/61.
(Unpublished)
Browne, P.M. and Reary, J.B. Residues in milk and tissues following
1978a a 28-day feeding study with bendiocarb in dairy cows. Fisons
Report RESID/78/58. (Unpublished)
Browne, P.M. and Reary, J.B. Analytical method for residues of
1978b bendiocarb metabolites in milk and tissues of dairy cows.
Fisons Report RESID/78/54. (Unpublished)
1978c Bendiocarb residue decline in soil after treatment of maize
plots with a 10G formulation in Illinois, 1977. Fisons
Report RESID/78/93. (Unpublished)
1979a Laboratory leaching study with a granular (3G) formulation
of bendiocarb in three standard soils from West Germany.
Fisons Report RESID/79/78. (Unpublished)
1979b Analytical method for residues of bendiocarb 7312 in rice
grain and straw. Fisons Report RESID/79/22. (Unpublished)
1979c Improved method for the analysis of bendiocarb residues in
maize grain, stover and whole silage plants. Fisons Report
RESID/79/24. (Unpublished)
1979d Residues of bendiocarb in maize at different growth stages,
after application of a 3G formulation in France, 1978.
Fisons Report RESID/79/2. (Unpublished)
1980a Laboratory leaching study with a 1% dust formulation of
bendiocarb in three standard soils from West Germany. Fisons
Report RESID/800/111. (Unpublished)
1980b Analytical method for residues of bendiocarb in rape seed.
Fisons Report RESID/80/59. (Unpublished)
1980c Residues in silage maize grown in the U.K. from seed dressed
with bendiocarb, 1979. Fisons Report RESID/80/12.
(Unpublished)
1980d Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1979. Fisons Report RESID/80/13.
(Unpublished)
1980e Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979 (2nd
Report). Fisons Report RESID/80/20. (Unpublished)
1980f Residues of bendiocarb in rape seed after application of a
granular (5G) formulation in Canada, 1979. Fisons Report
RESID/80/60. (Unpublished)
1981 Residues of bendiocarb and NC 7312 in potatoes sprayed with
a wettable powder formulation (20W) in West Germany, 1980.
FBC Report RESID/81/9. (Unpublished)
Challis, I.R. The metabolism of 14C-bendiocarb in sugarbeet. Fisons
1975 Report METAB/75/15. (Unpublished)
Challis, I.R. and Adcock, J.W. The metabolism of bendiocarb in maize
1977 plants. Fisons Report METAB/77/32. (Unpublished)
1978 The metabolism of bendiocarb in sugarbeet grown under field
conditions. Fisons Report METAB/78/19. (Unpublished)
Challis, I.R. and Swalwell, L.M. The metabolism of bendiocarb by
1980 barley plants. Fisons Report METAB/80/9. (Unpublished)
FBC Bendiocarb, submission to the Joint Meeting of the FAO
1982a Panel of Experts on Pesticide Residues in Food and the
Environment and the WHO Expert Group on Pesticide Residues,
FBC Limited, Registration Department, Saffron Walden, Essex,
England 1982.
1982b Harvest residues of bendiocarb (NC 6897) in sugarbeet after
application of a granular (3G) formulations. (Unpublished)
1982c Residues in grass following the use of bendiocarb (50SC)
(CR 15 340) as a seed dressing or spray dressing or spray
treatment in the U.K., autumn 1979. (Unpublished)
Housden, M.C. and Reary, J.B. Bendiocarb residues in rice treated
1981 with a 50WP formulation in the Philippines during the 1980
dry season. FBC Report RESID/81/16. (Unpublished)
Lewis, J.A. and Adcock, J.W. Investigation of residue levels in
1978a maize plants treated with 14C-bendiocarb in France. Fisons
Report RESID/78/50. (Unpublished)
1978b Investigation of residue levels in maize treated with
14C-bendiocarb granules in the U.K. Fisons Report
METAB/78/19. (Unpublished)
1978c Investigation of residue levels in maize plants treated with
14C-bendiocarb granules. Fisons Report METAB/78/7.
(Unpublished)
Lewis, J.A. and Challis, I.R. The metabolism of 14C-bendiocarb in
1978 the hen. Fisons Report METAB/78/28. (Unpublished)
1979 The metabolism of 14C-bendiocarb in paddy rice. Fisons
Report METAB/79/1. (Unpublished)
Netherlands. Information on pesticides included in the JMPR priority
1982 list provided by The Netherlands Government.
Reary, J.B. Harvest residues of bendiocarb in sugarbeet following
1974 its use as a seed dressing. Fisons Report RESID/74/36,
December 1974. (Unpublished)
1975a Soil percolation experiments with bendiocarb. Fisons Report
RESID/75/12. (Unpublished)
1975b Laboratory study of bendiocarb degradation in two standard
soils from West Germany. (Unpublished)
1975c Analytical method for residues of bendiocarb in sugarbeet.
FBC Report RESID/75/26. (Unpublished)
1975d Bendiocarb (NC 6897) residue decline on laboratory grown rye
grass sprayed with Ficam W. Fisons Report RESID/75/5.
(Unpublished)
1975e Harvest residues of bendiocarb (NC6897) in sugarbeet
following its use as a seed dressing in West Germany (1974).
Fisons Report RESID/75/12. (Unpublished)
1978a Residues in milk and tissues following a 28-day feeding
study with up to 25 ppm bendiocarb in the diet of dairy
cows. Fisons Report RESID/78/101.(Unpublished)
1978b Residues in eggs and tissues following a 21-day feeding
study with bendiocarb in laying hens. Fisons Report,
RESID/78/59. (Unpublished)
1978c Residues of bendiocarb in laboratory-extracted oil from
maize harvested after band application of a granular (10G)
formulation in the U.S., 1977. Fisons Report RESID/78/95.
(Unpublished)
1978d Analytical method for bendiocarb residues in soil. Fisons
Report RESID/78/37. (Unpublished)
1978e Analytical method for bendiocarb residues in grass. Fisons
Report RESID/78/61. (Unpublished)
1978f Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 76 WP formulation at
28-day intervals, in California and Massachusetts, 1977.
Fisons Report RESID/78/9. (Unpublished)
1978g Bendiocarb residue decline on grass and soil after treatment
of turf with repeated applications of a 5G formulation at
28-day intervals in California and Massachusetts, 1977.
Fisons Report RESID/78/32. (Unpublished)
1978h Residues in maize grown in the U.K. from seed dressed with
bendiocarb 80W, 1977. Fisons Report RESID/78/86.
(Unpublished)
1978i Residues of bendiocarb in maize treated with a granular (3G)
formulation in the U.K., 1977. Fisons Report RESID/78/97.
(Unpublished)
1978j Residues in maize grown in France during 1975, 1976 and 1978
from seed dressed with bendiocarb 80W. Fisons Report
RESID/78/106. (Unpublished)
1978k Bendiocarb residue decline in soil after band treatment of
maize plots with a 10G formulation in Missouri, 1977. Fisons
Report RESID/78/50. (Unpublished)
1978l Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a 10G granular formulation
in Missouri, 1977. Fisons Report RESID/78/51. (Unpublished)
1978m Residues of bendiocarb in maize (corn) at different growth
stages, after band application of a granular (10G)
formulation in Illinois, 1977. Fisons Report RESID/78/60.
(Unpublished)
1978n Residues of bendiocarb in the mature maize crop (grain, cobs
and stover) after band application of a granular (10G)
formulation in regions of the U.S., 1977. First Revision,
Fisons Report RESID/78/61/1. (Unpublished)
1979a Residues in milk from cows treated dermally with FICAM D in
Australia, 1979. Fisons Report RESID/79/49. (Unpublished)
1979b Analytical method for bendiocarb residues in barley, wheat,
oats and maize (corn) grain. Fisons Report RESID/79/17.
(Unpublished)
1979c Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. (Extract of
analytical method from the above report). Fisons Report
RESID/79/9. (Unpublished)
1979d Residues of bendiocarb and NC 7312 in potatoes treated with
a granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/9. (Unpublished)
1979e Residues of bendiocarb in barley, wheat and oats grown from
treated seed in the U.K., 1977 and 1978. Fisons Report
RESID/79/3. (Unpublished)
1979f Residues of bendiocarb in silage maize treated with a
granular (3G) formulation in the U.K., 1978. Fisons Report
RESID/79/12. (Unpublished)
1979g Residues in silage maize grown in the U.K. from seed dressed
with an 80W formulation of bendiocarb, 1978. Fisons Report
RESID/79/13. (Unpublished)
1979h Harvest residues of bendiocarb and NC 7312 in sugarbeet
after application of a granular formulation (3G) in the
U.K., 1978. Fisons Report RESID/79/14. (Unpublished)
1979i Residues of bendiocarb and NC 7312 in sugarbeet after
application of a granular (3G) formulation in France and
Italy, 1978. Fisons Report RESID/79/15. (Unpublished)
1979j Residues of bendiocarb in maize treated with a granular (3G)
formulation in Italy, 1978. Fisons Report RESID/79/16.
(Unpublished)
1979k Residues of bendiocarb and NC 7312 in rice treated with a
granular (3G) or spray (50W or 80W) application in the
Philippines, 1978. Fisons Report RESID/79/23. (Unpublished)
1979l Residues of bendiocarb in maize (corn) grown in the U.S.,
from seed dressed with a 76WP formulation, 1977 and 1978.
Fisons Report RESID/79/25. (Unpublished)
1979m Residues of bendiocarb in maize (corn) after band
application of a granular (10G) formulation in Canada, 1978.
Fisons Report RESID/79/26. (Unpublished)
1979n Residue analysis of fodder beet following the use of
bendiocarb as a seed dressing in West Germany, 1978. Fisons
Report RESID/79/45. (Unpublished)
1979o Residues of bendiocarb in silage and mature (grain and
stover) maize after band application of a granular (10G)
formulation in the U.S., 1978. Fisons Report RESID/79/57.
(Unpublished)
1979p Residues of bendiocarb in maize following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/76. (Unpublished)
1970q Residues of bendiocarb in sugarbeet following the use of a
granular (3G) formulation in West Germany, 1979. Fisons
Report RESID/79/75. (Unpublished)
1980a Residue in barley, wheat and oats following seed treatment
with bendiocarb in the U.K., 1979 (80WP) batch 8A8, 50W
spray or 15 181 and 3G or 15 094. Fisons Report RESID/80/80.
(Unpublished)
1980b Bendiocarb residue decline on rangeland grass after ULV
treatment (by air) with a 25% formulation (15 15 458) in
Montana, U.S., 1980. Fisons Report RESID/80/95.
(Unpublished)
1980c Preliminary report on residues in milk and tissues from
dairy cows treated dermally with bendiocarb dust for 7 days.
Fisons Report RESID/79/55. (Unpublished)
1980d Residues of bendiocarb in grass grown from treated seed in
Australia, 1979. Fisons Report RESID/80/9. (Unpublished)
1980e Residues in grass following the use of bendiocarb as a
granular (3G), seed dressing (80W) or spray (50W) treatment
in the U.K., Spring 1979. Fisons Report RESID/80/11.
(Unpublished)
Reary, J.B. Residue decline in sugarbeet treated with bendiocarb 3G
1980f in the U.K., 1979. Fisons Report RESID/80/4. (Unpublished)
1980g Residue decline in soil after furrow application of
bendiocarb 3G granules at Shelford, U.K., 1979. Fisons
Report RESID/80/14. (Unpublished)
1980h Residue decline in maize treated with bendiocarb 3G in the
U.K., 1979. Fisons Report RESID/80/17. (Unpublished)
1980i Residue decline in maize grown from seed dressed with
bendiocarb in the U.K., 1979. Fisons Report RESID/80/18.
(Unpublished)
1980j Residues in sugarbeet from grower trials with a granular
(3G) formulation of bendiocarb in the U.K., 1979. Fisons
Report RESID/80/29. (Unpublished)
1980k Bendiocarb residue decline on rangeland grass after low
volume treatment with a 50SC formulation (CR 15 340) in
Montana, U.S., 1979. Fisons Report RESID/80/37.
(Unpublished)
1980l Residues in rice treated with bendiocarb formulations in the
Philippines and Korea 1979. Fisons Report RESID/80/46.
(Unpublished)
1981m Residues in grass following the use of bendiocarb as a seed
dressing, granular or spray treatment in the U.K., 1980. FBC
Report RESID/81/10. (Unpublished)
Reary, J.B. and Browne, P.M. Residues of bendiocarb (NC 6897) in
1978 mature maize, after application of a granular formulation in
France, 1976 and 1977. Fisons Report RESID/78/23.
(Unpublished)
Reary, J.B. and Whiteoak, R.J. Bendiocarb residue decline on soil,
1977a grass and thatch after treatment of turf with a 76WP
formulation in Mississippi, 1976. Fisons Report RESID/77/43.
(Unpublished)
1977b Bendiocarb residue decline in soil, grass and thatch after
treatment of turf with a 76WP formulation in Ohio, 1976.
Fisons Report RESID/77/52. (Unpublished)
1977c Bendiocarb residue decline on soil, grass and thatch after
treatment of turf with a granular formulation in Ohio, 1976.
Fisons Report RESID/77/61. (Unpublished)
1977d Bendiocarb residue decline on grass and soil after treatment
of turf with a 76WP formulation in California and
Massachusetts, 1977. Fisons Report, RESID/77/62.
(Unpublished)
Warner, P.A. The metabolism of 14C-bendiocarb in oilseed rape. Fisons
1980 Report METAB/80/20. (Unpublished)
