
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
METALAXYL
IDENTITY
Chemical names
IUPAC - (±) methyl N-(2-methoxyacetyl)-N-2,6-xylyl-alaninate
CAS - N-(2,6-dimethylphenyl)-N-methoxyacetyl-D,L-alanine-methylester
CIBA-GEIGY - methyl D,L-N-(2,6-dimethylphenyl)-N-(2'-methoxyacetyl)-
alaninate
Synonyms
CGA 48 988, RIDOMIL (R), APRON(R)
Empirical formula
C15H21NO4
Structural formula
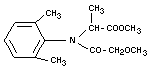 Information on identity and properties (pure a.i.)
Molecular weight 279.34
Description white crystals
Density 1.21 g/cm3 at 20°C
Melting point 71.8 - 72.3°C
Vapour pressure 2.2 × 10-6 mm Hg at 20°C
Volatility 3.4 × 10-2 mg/m3 saturated vapour
concentration at 20°C
Solubility 0.71% in water at 20°C, soluble in
methanol,methylene chloride, benzene,
isopropanol and slightly soluble in hexane
Partition coefficient
n/octanol/water log P 1.65
Hydrolysis half-lives at 20°C in buffer solutions were
>200 days at pH 1, 115 days at pH 9, 12 days
at pH 10
Thermal stability stable up to 300°C
Purity of technical active
ingredient >90%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of 14C-metalaxyl was determined in rats by testing
excreta and selected tissue for activity following oral
administration. Male and female rats were dosed with 14C-metalaxyl
at 0.5 or 25 mg/kg. The excreta and expired CO2 were collected for
analysis at 24-hour intervals. After six days, the rats were
sacrificed and the radioactivity in various tissues was measured.
After single oral doses, more than 60% of the administered
radioactivity was excreted within 24 hours, and by the sixth day
almost all of the radioactivity was excreted in the urine (37-63%) and
faeces (35-66%) for both levels. The amount of 14CO2 expired was
insignificant (<0.02%). Total recoveries were between 100% and 104%.
The excretion pattern was influenced more by the sex of the animals
than by the dose level. The females excreted somewhat higher
proportions in the urine while the males showed preferential excretion
via the faeces. Pooled urine (0-24 hours) for males and females was
analysed by thin-layer chromatography (TLC) and numerous metabolites
(primarily polar) were found in urine. No parent metalaxyl was
detected in these samples. The residual radioactivity, determined in
liver, kidney, muscle, blood, fat, brain, heart, carcase, lungs,
spleen, ovary and testes, was very low except in the liver and blood,
which contained from 0.002-0.004 ppm for 0.5 mg/kg dose rate and
0.146-0.255 ppm in liver, fat and blood for a 25 mg/kg dose rate
(Hambock 1977).
Metabolism
Sixteen female rats were dosed orally with 27.9 mg/kg of
14C-metalaxyl and the urine and faeces were collected for 48 hours.
The urine contained 63.5% of the administered dose and the faeces
32.8%. The radioactivity in urine was fractionated, and in 0-48 hour
urine 62.1% of the metabolites were in the form of glucuronic acid
conjugates as shown by aglycon released via p-glucuronidase
incubation. At least ten aglycons were released by enzyme treatment.
Many of the metabolites excreted were in their free forms as well as
their glucuronide conjugates. Findings from this study suggest that
the degradation of metalaxyl in the rat proceeds primarily via (1)
methyl ester hydrolysis, (2) N-dealkylation, (3) methyl ether cleavage
and (4) benzylic methyl oxidation with subsequent formation of
conjugates with glucuronic acid (Hambock 1978).
Further evaluation in female rats using similar oral doses of
14C-metalaxyl (27.8 mg/kg) resulted in 58% of the radioactivity being
excreted in the urine and 32% in the faeces within 48 hours.
Fractionation, TLC and/or spectroscopy demonstrated that degradation
of metalaxyl in the rat proceeds through at least four independent
pathways, as follows: (1) hydrolysis of the methyl ester and methyl
ether groups, (2) oxidation of the 2-(6)-methyl group, (3) oxidation
of the phenyl ring and (4) N-dealkylation and subsequent formation of
glucuronic acid conjugates (Hambock 1981).
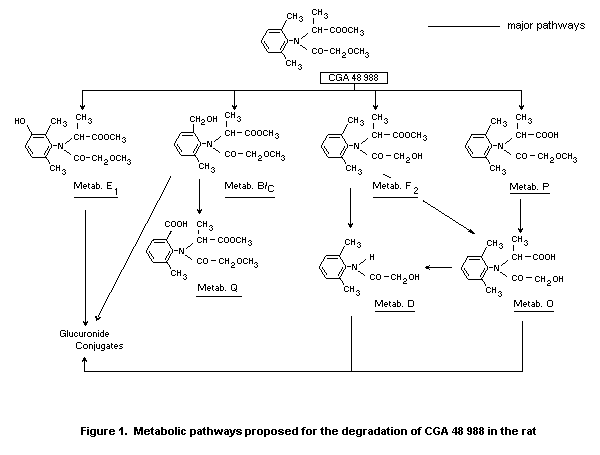
A proposed metabolic pathway for the rat is shown in Figure 1.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of 50 rats (25 of each sex/group) were fed metalaxyl in
the diet at concentrations of 0, 50, 250 and 1 250 ppm for 91 days,
after which they were mated to initiate a 2-litter per generation,
3-generation reproduction study. The first litters of each generation
were weaned at 21 days, examined and discarded. Twenty five male and
25 female rates of the F1b litter were mated to produce the F2a and
F2b litters. Twelve male and 12 female rats from the F2b litter were
reared but not mated and sacrificed after 90 days for organ weight
analysis. An additional 12 male and 24 female rats of the F2b were
mated to produce the F3a and F3b litters. The F3b pups (10 males and
10 females) were sacrificed after 21 days post-partum and used in
histopathological evaluations of control and high-dose animals.
Selected F0 (15 each) and F1b (10 each) dams were killed at day 20
of gestation, pups delivered by caesarean section and examined for
effects on late embryonic/foetal development (including visceral and
skeletal evaluations).
Information on identity and properties (pure a.i.)
Molecular weight 279.34
Description white crystals
Density 1.21 g/cm3 at 20°C
Melting point 71.8 - 72.3°C
Vapour pressure 2.2 × 10-6 mm Hg at 20°C
Volatility 3.4 × 10-2 mg/m3 saturated vapour
concentration at 20°C
Solubility 0.71% in water at 20°C, soluble in
methanol,methylene chloride, benzene,
isopropanol and slightly soluble in hexane
Partition coefficient
n/octanol/water log P 1.65
Hydrolysis half-lives at 20°C in buffer solutions were
>200 days at pH 1, 115 days at pH 9, 12 days
at pH 10
Thermal stability stable up to 300°C
Purity of technical active
ingredient >90%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of 14C-metalaxyl was determined in rats by testing
excreta and selected tissue for activity following oral
administration. Male and female rats were dosed with 14C-metalaxyl
at 0.5 or 25 mg/kg. The excreta and expired CO2 were collected for
analysis at 24-hour intervals. After six days, the rats were
sacrificed and the radioactivity in various tissues was measured.
After single oral doses, more than 60% of the administered
radioactivity was excreted within 24 hours, and by the sixth day
almost all of the radioactivity was excreted in the urine (37-63%) and
faeces (35-66%) for both levels. The amount of 14CO2 expired was
insignificant (<0.02%). Total recoveries were between 100% and 104%.
The excretion pattern was influenced more by the sex of the animals
than by the dose level. The females excreted somewhat higher
proportions in the urine while the males showed preferential excretion
via the faeces. Pooled urine (0-24 hours) for males and females was
analysed by thin-layer chromatography (TLC) and numerous metabolites
(primarily polar) were found in urine. No parent metalaxyl was
detected in these samples. The residual radioactivity, determined in
liver, kidney, muscle, blood, fat, brain, heart, carcase, lungs,
spleen, ovary and testes, was very low except in the liver and blood,
which contained from 0.002-0.004 ppm for 0.5 mg/kg dose rate and
0.146-0.255 ppm in liver, fat and blood for a 25 mg/kg dose rate
(Hambock 1977).
Metabolism
Sixteen female rats were dosed orally with 27.9 mg/kg of
14C-metalaxyl and the urine and faeces were collected for 48 hours.
The urine contained 63.5% of the administered dose and the faeces
32.8%. The radioactivity in urine was fractionated, and in 0-48 hour
urine 62.1% of the metabolites were in the form of glucuronic acid
conjugates as shown by aglycon released via p-glucuronidase
incubation. At least ten aglycons were released by enzyme treatment.
Many of the metabolites excreted were in their free forms as well as
their glucuronide conjugates. Findings from this study suggest that
the degradation of metalaxyl in the rat proceeds primarily via (1)
methyl ester hydrolysis, (2) N-dealkylation, (3) methyl ether cleavage
and (4) benzylic methyl oxidation with subsequent formation of
conjugates with glucuronic acid (Hambock 1978).
Further evaluation in female rats using similar oral doses of
14C-metalaxyl (27.8 mg/kg) resulted in 58% of the radioactivity being
excreted in the urine and 32% in the faeces within 48 hours.
Fractionation, TLC and/or spectroscopy demonstrated that degradation
of metalaxyl in the rat proceeds through at least four independent
pathways, as follows: (1) hydrolysis of the methyl ester and methyl
ether groups, (2) oxidation of the 2-(6)-methyl group, (3) oxidation
of the phenyl ring and (4) N-dealkylation and subsequent formation of
glucuronic acid conjugates (Hambock 1981).
A proposed metabolic pathway for the rat is shown in Figure 1.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of 50 rats (25 of each sex/group) were fed metalaxyl in
the diet at concentrations of 0, 50, 250 and 1 250 ppm for 91 days,
after which they were mated to initiate a 2-litter per generation,
3-generation reproduction study. The first litters of each generation
were weaned at 21 days, examined and discarded. Twenty five male and
25 female rates of the F1b litter were mated to produce the F2a and
F2b litters. Twelve male and 12 female rats from the F2b litter were
reared but not mated and sacrificed after 90 days for organ weight
analysis. An additional 12 male and 24 female rats of the F2b were
mated to produce the F3a and F3b litters. The F3b pups (10 males and
10 females) were sacrificed after 21 days post-partum and used in
histopathological evaluations of control and high-dose animals.
Selected F0 (15 each) and F1b (10 each) dams were killed at day 20
of gestation, pups delivered by caesarean section and examined for
effects on late embryonic/foetal development (including visceral and
skeletal evaluations).
 Reproduction indices, including mating, fecundity, male and
female fertility, gestation, lactation, pup mortality, litter weights
and mean pup weights, were determined and compared with control
values. All pups were examined for physical abnormalities and
viability.
There were no consistent dose-related effects on adult animals
with regard to mortality, food/water consumption, body/weight gain,
mating performance, pregnancy rate or duration of gestation. The
litter size and litter weight of control group rats in the Fo 2nd
mating were significantly reduced from treatment groups (birth through
day 21 post-partum). Mean pup weights were normal. Litter size and
mean pup weights were reduced at birth for the F1b 1st mating, group
2 animals. Mean pup weight remained significantly reduced through day
21 for these group 2 litters. These effects were not noticed in other
dose groups or other generations and are therefore not considered
compound-related.
There were no dose-related or compound-related effects on organ
weight, organ/body weight ratio or histopathology. The selected
visceral and skeletal examinations of foetuses for assessing
teratogenic effects were within normal variation for the strain/
species studied. There were no differences with respect or any of the
indices observed, and it is considered that metalaxyl, at dietary
dosage levels up to and including 1 250 ppm, caused no adverse
reproduction effects in rats (Cozens et al 1980).
Special Studies on Teratogenicity
Rat
Groups of 25 pregnants rats were administered, via intubation,
dose levels of 0, 20, 60 and 120 mg metalaxyl/kg bw from days 6
through 15 of gestation. On day 21 of gestation, all pregant animals
were killed by cervical dislocation and foetuses delivered by
caesarean section.
Survival was not affected by treatment. The ratios of
implantations and resorptions were comparable among all treatment and
control groups. Sex ratio and number of live young were not affected
by metalaxyl. No malformations were found in the foetuses nor were the
average foetal weights of the treatment groups affected. Delayed
ossification was marginally affected at the high dose within normal
variation based on historical control data. There were no indications
of embryotoxic or teratogenic effects in the rat under the conditions
of the study (Fritz 1978b).
Rabbit
Groups of pregnant Chinchilla rabbits (20 rabbits/group) were
given metalaxyl orally by intubation at dose levels of 0, 5, 10 and
20 mg/kg during days 6 through 18 of gestation. On day 28 of
gestation, all rabbits were sacrificed and examined and the foetuses
delivered by caesarean section. Foetuses were subjected to external
examination followed by inspection for Visceral and skeletal
malformations, anomalies or variants.
Reduced food consumption was observed in the 10 and 20 mg/kg
groups accompanied by slightly reduced body weight gain in dams at the
high dose. The mean number of corpora lutea and/or implantations were
comparable for all groups. Incidences of renal hypoplasia and agenesis
were not dose-related and within the spontaneous rate of historical
untreated animals of the same rabbit population. Metalaxyl did not
adversely influence embryonic or foetal development in Chinchilla
rabbits at levels up to and including 20 mg/kg (Fritz et al 1978).
Special Studies on Mutagenicity
Yeast culture evaluations
The test compound, CGA 48 988, was tested for mutagenic effects
on the D-7 strain of Saccharomyces cerevisiae at five dose levels:
400, 2 000, 4 000, 8 000 and 10 000 µg/ml, with and without microsomal
activation. The test system permitted the detection of mitotic
crossover, mitotic gene conversion and reverse mutation in the yeast
cells D-7 after 2 or 6 hours of treatment with the test compound. The
positive controls were treated with 4-nitroquinoline-N-oxide (without
S-9 mix) and cyclophosphamide (with S-9 mix) in concentrations of
0.25 µg/ml and 50-250 µg/ml, respectively.
In none of the three mutation systems was the incidence of
mutants increased, in comparison with the negative controls, as a
result of treatment of the cells with metalaxyl with or without
microsomal activation. It is concluded that under the conditions of
these experiments no evidence of mutagenic effects was obtained with
metalaxyl (Arni and Muller 1982).
DNA repair
The primary rat hepatocytes prepared from the liver of a male rat
were used for the UDS assay. A series of compartments in pertri dishes
containing gelatinized cover-slips were seeded with 2 × 10-5 cells
per compartment. The cells were allowed to attach to the coverslips
during an attachment period of 1.5-2 hours. The cell cultures attached
to the coverslips were then washed and refed with WME containing the
test compound (16, 80, 400 and 2 000 µg/ml) and 3H-thymidine
(4 µ Ci/ml). Negative controls and positive controls (4NQO and DMN)
were run concurrently with the test compound.
Comparison of the mean number of silver grains per nucleus in the
negative controls and after treatment with metalaxyl revealed no
evidence of induction of DNA damage (Puri and Muller 1982).
Mouse lymphoma assay
Metalaxyl was tested for mutagenic effects on L5178Y/TK+/- mouse
lymphoma cells in vitro. The investigations were performed with
microsomal activation at concentrations of 0.0625, 0.125, 0.25 and
0.50 mg/ml and without microsomal activation at concentrations of
0.125, 0.25, 0.50 and 1.0 mg/ml. The results were expressed in terms
of the number of induced TK-/- mutants/106 surviving cells. The
test substance is considered mutagenic in this system if the colony
count exceeds that of the solvent control by a factor of more than
2.5 at any concentration.
It was concluded that, under the given experimental conditions,
no evidence of mutagenic effects of metalaxyl was observed in this
mammalian forward mutation system (Strasser and Muller 1982).
Dominant lethal studies
Two groups of adult male albino mice, 20 per group, were
administered orally by intubation single doses of 65 and 196 mg/kg of
metalaxyl dissolved in carboxymethyl cellulose. Each male mouse was
mated with two untreated virgin female mice for eight consecutive
weeks to cover the total spermatogenic cycle. Fourteen days from the
mid-week of mating, the females were killed and examined for
pregnancies. Three parameters were used to determine the dominant
lethal effect of the test compound: mating ratio, number of
implantations and embryonic deaths. No evidence of dominant lethal
effect was obtained in the progeny of male mice treated with metalaxyl
(Fritz 1978a).
Special Studies for Carcinogenicity
Mouse
Metalaxyl was administered in the diet to groups of 60 male and
60 female mice (ICI Alderley Park, Swiss) at concentrations of 0, 50,
250 and 1 250 ppm for 104 weeks. Mice were approximately 5-6 weeks of
age when dosing commenced. The percent survival, food/water
consumption and general thriftiness of animals were unaffected by
treatment. A slight decrease in food conversion efficiency,
accompanied by decreased body weight gain, was noticed in high dose
males during weeks 11 through 30. Bone marrow and blood, sampled
throughout the study, were unaffected by treatment.
All animals that died or were sacrificed in extremis were
submitted to gross necropsy and histopathological evaluation for both
non-neoplastic and neoplastic lesions. The lesions observed were
similar among all groups and not related to the administration of
metalaxyl. Metalaxyl was not found to be a carcinogen in this strain
of mouse (McSheehy et al 1980).
Special Study on Skin Sensitization
Guinea pig
Groups of 10 male and 10 female guinea pigs (Pirbright, albino)
were subjected to a series of 0.1 ml intracutaneous injections of
metalaxyl as a 0.1% suspension in polyethylene glycol and saline,
following the optimization test of Maurer et al (1975). DNCB served
as a positive control. Metalaxyl was not sensitizing under the
conditions of the test (Sachsse and Ullmann 1976h).
Special Study on Eye Irritation
Rabbit
Approximately 100 mg of metalaxyl was placed in the conjunctival
sac of 3 male and 3 female rabbits. The eyes of 3 of the 6 rabbits
were rinsed approximately 30 seconds after instillation of the test
material. There was no irritation present in any of the rinsed eyes.
Unwashed eyes presented irritation of the conjunctiva and cornea for 3
days and were clear by day 4. While the degree of corneal involvement
appears minimal, it could not be subjectively evaluated since only
scores were presented. Rinsing the eyes prevented any irritation
effects, suggesting that the irritation in unwashed eyes may have been
more related to the physical nature of the particles than to the
chemical itself (Sachsse and Ullman 1976g).
Special Study on Dermal Irritation
Rabbit
The primary irritation was determined following the application
of 0.5g of metalaxyl to both abraded and intact skin sites on the
shaved back of 3 male and 3 female rabbits. There was no irritation on
any of the intact skin sites, while minimum erythema was observed on
the abraded skin sites of 2 rabbits 24 hours after application. The
primary irritation index was reportedly 0.1/8 (Sachsse and Ullmann
1976f).
Acute Toxicity
In acute toxicity studies carries out in animals (Table 1), toxic
signs of poisoning occurred within 2 hours after treatment and most
deaths within the first 24 hours. Symptoms observed were non-specific
in the various mammalian species and consisted of sedation, dyspnoea,
exophthalmos, tonic-clonic muscle spasms, curved or ventral position
and ruffled fur.
Metalaxyl was not toxic by the dermal route, as both rats and
rabbits tolerated doses from 3 100 to 6 000 mg/kg without visible
symptoms or effects. No mortalities were reported in either species.
Table 1. Acute Toxicity of Metalaxyl in Animals
Species Sex Route LD50 Reference
Rat m + f oral 1 669 Sachsse and Bathe, 1976a
(515-868)
Mouse m + f oral 1 788 Sachsse and Bathe, 1976b
(626-991)
Rabbit m + f oral 1 697 Sachsse and Ullman,1976e
(506-961)
Hamster m + f oral 7120 Thomann and Pericin,1977
(5250-9660)
Rat m + f dermal 1 >3 100 Sachsse and Bathe, 1976c
Rabbit m + f dermal 2 >6 000 Sachsse and Ullmann, 1978
Rat m + f i.p. 1 312 Sachsse and Bathe, 1976d
(282-345)
1 Carboxy methyl cellulose was the vehicle;
2 Polyethylene glycol was the vehicle.
Short-Term Studies
Rabbit-dermal
Metalaxyl was administered to the occluded intact and abraded
skin of albino rabbits (10 males and 10 females/group) at doses of 0,
10, 100 and 1 000 mg/kg. Test substance was applied once each day, 5
days/week for 3 consecutive weeks. Information was obtained on body
weight, food consumption, haematology, clinical chemistry, absolute
and relative organ weights and local dermal effects on the skin. All
animals were subjected to gross necropsy and histopathological
examination. There were no adverse effects related to compound
administration for any of the parameters evaluated. Several lesions
reported in both treatment and control groups were related to either
bacterial Staphylococcus aureus and epidermitis) or parasitic
(encephalitozoon, Eimeria stiedae) infections, which obscured
interpretation of results (Calkins et al 1980).
Rat - dietary
Daily oral doses of metalaxyl were administered by gavage to
groups of rats (10 males and 10 females/group) initially at levels of
0, 10, 30 and 100 mg/kg for the first 14 days. Dosing was increased to
0, 30, 100 and 300 mg/kg, respectively, for days 15-21 of treatment.
Once again, on day 22, dose levels were increased to 0, 60, 200 and
600 mg/kg, respectively, for the duration of the study, which was
terminated on day 28. No deaths occurred and food consumption, body
weight gain and mean food consumption were comparable among treated
and control groups. Haematology, blood chemistry and urinalysis were
unremarkable. Male rats in the high dose group demonstrated a
significant increase in absolute testes weight as well as a dose-
related increase in relative liver weight, for which the trend was
significant (p<0.01). The absolute and relative adrenal weights were
significantly increased (p<0.05) for females in the high dose group.
Female rats in all treatment groups administered metalaxyl had
significantly increased absolute and relative liver weights (p<0.05),
which were dose-related and for which the positive trend was similarly
significant (p<0.01) (Sachsse et al 1979).
In a 3-month dietary study using Sprague-Dawley rats, 180 rats
were allocated to two groups of 50 animals each (25 males and 25
females) and two groups of 40 animals each (20 males and 20 females).
Dose levels were 0, 50, 250 and 1 250 ppm in the diet. Five males and
five females from the control and high dose level groups were retained
for an additional 28 days without treatment for evaluation of recovery
and reversibility of effects. Minimal cellular hypertrophy of hepatic
parenchymal cells was observed in five females receiving the highest
dose. This trend was not noticeable in the recovery animals,
representing a possible reversibility for this effect. No other
changes attributed to treatment were observed and dose levels of
250 ppm or less are considered to be without adverse effects under the
conditions of this study (Drake 1977).
In a follow-up 90-day study, Gfeller et al (1980) evaluated the
effects of 0, 10, 50, 250 and 1 250 ppm of metalaxyl incorporated in
the diet of rats (RAIF, SPF). There were 20 males and 20 females in
each experimental group. Mortality and other cageside observations
were conducted daily, body weight and food consumption determined
weekly and clinical chemistries, urinalysis and haematology performed
periodically throughout the study. At the conclusion of the study, all
test and control animals were subjected to gross and histopathological
examination and selected organs were weighed.
A dose-related decrease in total leucocyte count was evident in
high dose males, as well as increased absolute and relative adrenal
weights for male rats in the 50, 250 and 1 250 ppm groups. In females,
the relative adrenal weights were increased in the high dose group
only. There was a significant increase in relative liver weights for
females in the 250 and 1-250 ppm dose groups. However, the absolute
liver weights for high dose females were not significantly different
from controls and, therefore, the difference reported for the relative
liver weights is considered as being related to the decrease in the
body weight of the high dose compared to control females, rather than
as an increase in organ weight, per se. Gross necropsy and
histopathological examinations revealed no differences between treated
and control animals. There were no structural alterations in the
adrenals reflective of the increase in both absolute and relative
weights for this organ. The level of 10 ppm of metalaxyl in the diet
of rats is considered to be without adverse effects (Gfeller
et al 1980).
Dog - dietary
Four groups of beagle dogs, 6-9 months of age, were administered
metalaxyl in their diets at levels of 0, 50, 250 and 1 250 ppm for
91 days. Control and high dose groups contained 4 males and 4 females
each, with the low and intermediate dose groups containing 3 males and
3 females each. No mortality or changes in the behaviour of the
animals were observed. Urinalysis, ophthalmoscopy, body weight, food
consumption and haematology indicated no treatment-related findings.
Blood chemistry parameters were normal, except for a significant
increase in serum alkaline phosphatase levels in high-dose males and
females, which was also time-related. Determination of SAP in two
recovery animals from the high dose group demonstrated a reversal to
normal within 4 weeks post-treatment. Macroscopic and microscopic
examinations revealed no compound-related findings. The no-effect
level was estimated to be 250 ppm (Finn et al 1977).
Technical metalaxyl was administered in the diet to groups of
beagle dogs at levels of 0, 50, 250 and 1 000 ppm for a period lasting
six months. There were 8 males and 8 females in both the control and
high dose groups, with 6 males and 6 females in each of the other two
groups. Pups were 6-8 months old at the beginning of study. Feed
consumption, body weights and urinalysis, performed periodically
throughout the study, were normal. Ophthalmic examinations did not
reveal any effects related to treatment. Clinical chemistry parameters
were all within normal variation, except for alkaline phosphatase
levels for 1 000 ppm dose-level males and females, which were
significantly increased (p<0.05) compared to control animals. A
recovery to normal was observed after dosing was stopped. Haemoglobin,
erythrocyte count and hematocrit, while significantly lower (p<0.05)
for high dose males when compared to control males in this study, were
all within normal variation when compared to historical controls.
Anaemia was not evident and bone marrow samples reportedly were
normal: however, reticulocytes and m/e ratios were not determined. The
liver/brain ratios of females in the high dose group were
significantly increased (p <0.05) compared with controls. Similarly,
a positive trend for both increased absolute and relative liver
weights was apparent in male and female dogs receiving an increased
dosage. Alkaline phosphatase is produced in many organs, with the
liver being a primary supplier. The possibility of a dose response for
alkaline phosphatase relative to the liver is supported by the trend
toward increasing liver weights with increased dosage. No such
trends, however, were seen in other enzymes also produced in the liver
(e.g. SGPT, SGOT), and no gross pathological or histopathological
changes were observed in the liver of any dogs fed metalaxyl. There
were no other effects related to treatment and a no-effect level of
250 ppm was determined. Mean daily feed consumption data indicate this
dietary level is approximately equivalent to 7.4 mg/kg/day, with
1 000 ppm corresponding to 31 and 33 mg/kg/day for males and females,
respectively (de Ward et al 1981).
Long-Term Study
Mice
(see Special Study for Carcinogenicity)
Rat
Groups of rats (80 male and 80 female Sprague-Dawley albino
rats/group) were administered metalaxyl in the diet for 104 weeks at
dosage levels of 0, 50, 250 and 1 250 ppm. Growth, as observed by body
weight changes and food consumption data, was recorded weekly for the
first 13 weeks and monthly thereafter. Rats were inspected twice daily
for behavioural changes, general health, mortality and tumour
development. At periodic intervals over the course of the study,
haematologic, clinical chemistry and urinalysis examinations were
performed.
All animals, including those dying or sacrificed, were subjected
to both gross necropsy and microscopic examinations after 55 and 105
weeks of treatment.
No effects considered to be treatment-related were noted with
respect to toxic signs, mortality, body weight, food consumption,
urinalysis, ophthalmologic examination and haematology. Survival was
greater than 50% for both sexes of all groups at 18 months. Activities
of SGPT and SGOT decreased in the 250 and 1 250 ppm females during the
first year, but recovered to normal values, comparable to the control
group, through the second year to termination of the study.
At gross necropsy, organ weight determinations demonstrated
significantly increased (p <0.01) relative liver weights for females
in the 1 250 ppm group after both 55 and 105 weeks of treatment.
Relative liver weights for males were significantly increased
(p <0.01 and <0.001) in both the 250 and 1 250 ppm groups,
respectively, after 105 weeks. These organ to body weight differences
were not accompanied by any pathological findings in the treated
animals other than slight periacinar hepatocytic vacuolation and
chronic inflammation in 1 250 ppm males.
There was a reported pathological finding of testicular atophy
(p <0.05) occurring primarily in the first 53 weeks at all treatment
levels, but the respective absolute and relative testicular weights
were comparable to those of the control group. Since there was no
positive trend with dose for this response and no impairment of
reproductive performance (Cozens et al 1980), this effect is not
considered attributable to metalaxyl. The findings of parafollicular
cell adenomas of the thyroid was significantly increased for the
250 ppm females. However, there was no dose-response relationship and
the incidence was not significantly different from historical control
data for this pathological finding at the same laboratory.
Metalaxyl is considered to be without adverse effects on rats at
levels up to and including 50 ppm in the diet. There was no evidence
of carcinogenic potential at any level tested (Ashby et al 1980).
COMMENTS
Metalaxyl, when fed to rats, is rapidly metabolized and excreted
in the urine and faeces. The metabolism has been relatively well
defined in rats and no specific organ accumulation appears to be
found. The excretion pattern is not influenced by the dose level, but
by the sex of the animals, as females demonstrate preferential
excretion via urine, while males excrete higher proportions in the
faeces.
The acute toxicity in various animals suggests a moderate acute
hazard via the oral route, but little dermal absorption occurs, as
demonstrated in both acute and subchronic dermal studies. Metalaxyl is
mildly irritating to the skin and eyes but did not elicit contact
allergenicity in a skin-sensitization study using guinea pigs.
Metalaxyl did not produce any adverse effects on reproductive
performance or pup viability at levels up to and including 1 250 ppm
in the diet. There were no indications of embryotoxic or teratogenic
effects in either rats or rabbits at levels of 120 or 20 mg/kg bw,
respectively.
Metalaxyl was evaluated in a battery of mutagenicity tests
including yeast culture, DNA repair, mouse lymphoma and dominant
lethal tests. No evidence of mutagenic potential was determined in any
of these tests.
In both short- and long-term studies, the only significant
findings were increased relative liver and adrenal weights, sometimes
accompanied by increased enzyme activities (i.e. SAP, SGPT), but for
which there was no corresponding histopathology. The adrenal weight
increase noted in the rat short-term study was not apparent in the rat
long-term study at comparable dietary levels nor in the short-term dog
study or mouse carcinogenicity study. Metalaxyl was not demonstrated
to be carcinogenic in either rats or mice. Based upon all available
data, no-effect levels in mammalian species can be determined and an
ADI for man allocated for metalaxyl.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mice : 1 250 ppm in the diet, equivalent to 187.5 mg/kg bw
Rat : 50 ppm in the diet, equivalent to 2.5 mg/kg bw
Dog : 250 ppm in the diet, equivalent to 7.4 mg/kg bw
Estimate of Acceptable Daily Intake for Man
0 - 0.03 mg/kg body weight
FURTHER WORK OR INFORMATION
Desirable
1. Metabolic studies in a non-rodent species.
2. Observations and information in humans.
REFERENCES
Arni, P. and Muller, D. Saccharomyces cerevisiae D-7/mammalian-
1982 microsome mutagenicity test in vitro with CGA 48988 (test
for mutagenic properties in yeast cells). Report from Ciba-
Geigy Ltd., Basle, Switzerland, submitted to the World
Health Organization by Ciba-Geigy Ltd. (Unpublished)
Ashby, R., Bhatt, A., Chapman, E., Hepworth, P.L. and Whitney, J.C.
1980 CGA 48988: Toxicity and oncogenicity in dietary
administration to rats for two years. Final report no.
80/CIA009/315 from Life Science Research, Stock, Essex CM4
0PE, England, submitted from Ciba-Geigy, Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Calkins, J.E., Morgan, J.M., Casey, H.W. and Page, J.G. A 21-day
1980 subacute dermal toxicity study in albino rabbits with CGA
48988. Report from Toxigenics Inc. Decator, Ill. 62526, USA,
submitted by Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Cozens, D.D., Allen, P.A., Clark, R., Offer, J.M., Gregson, R.L. and
1980 Gibson, W.A. Effect of CGA 48988 on reproductive function of
multiple generations in the rat. Report from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England,
submitted from Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Drake, J.C. 3-month dietary study in rats with compound CGA 48988.
1977 Report from Geigy Pharmaceuticals, Stamford Lodge, Wilmslow,
Chershire, U.K. submitted from Ciba-Geigy Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Finn, J.P., Briggs, K., Close, J.E., Noble, D.N., Barrett, R. and
1977 Rider, L. CGA 48988, 91-day dietary toxicity study in beagle
dogs. Report from Hazleton Laboratories Europe Ltd.,
Harrogate, HGe 1PY, England, submitted from Ciba-Geigy Ltd.,
Basle, Switzerland, to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Fritz, H. Dominant lethal study-CGA 48988 techn. mouse (Test for
1978a cytotoxic or mutagenic effects on male germinal cells).
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
1978b Reproduction study-CGA 48988 tech. Rat Seg. II (Test for
teratogenic or embryotoxic effects). Report from Ciba-Geigy
Ltd., Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Fritz, H., Becker, H. and Hess, R. Reproduction study - rabbit- CGA
1978 48988 tech. Seg. II (Test for teratogenic or embryotoxic
effects). Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Gfeller, W., Basler, W., Zak, F., Grieve, A.P. and Hess, R. CGA 48988
1980 techn. 3-month toxicity study on rats (Project No. 79 18 11)
Final Report. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Hambock, H. Distribution, degradation and excretion of CGA 48988 in
1977 the rat. Project Report 18/77 from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Metabolism of CGA 48988 in the rat. Project report 26/78
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1981 Metabolic pathways of CGA 48988 in the rat. Project Report
31/81 from Ciba-Geigy Ltd., Basle, Switzerland, submitted to
the World Health Organization by Ciba-Geigy, Ltd.
(Unpublished)
Maurer, T., Thomann, P., Weirich, E.G. and Hess, R. The optimization
1975 test in the guinea pig. A method for the predictive
evaluation of the contact allergenicity of chemicals. Agents
and Actions 5 (2): 174-179.
McSheehy, T.W., Macrae, S.M. and Whitney, J.C. CGA 48988 oncogenicity
1980 in dietary administration to mice for two years. Final
Report No. 80/CIA 008/442 from Life Science Research, Stock,
Essex CM4 9PE, England, submitted from Ciba-Geigy Ltd.
Basle, Switzerland to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Puri, E. and Muller, D. Audioradiographic DNA repair test on rat
1982 hepatocytes CGA 48988 (in vitro test for DNA-damaging
properties). Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy, Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute oral LD50 in the rat of technical CGA
1976a 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute oral LD50 in the mouse of technical CGA 48988. Report from Ciba
1976b Geigy Ltd., Basle, Switzerland submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute dermal LD50 in the rat of technical
1976c CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute intraperitoneal LD50 in the rat of technical CGA 48988. Report
1976d from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Ullmann, L. Acute oral LD50 in the rabbit of technical
1976e CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organziation by Ciba-Geigy
Ltd. (Unpublished)
1976f Skin irritation in the rabbit after single application of
technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1976g Eye irritation in the rabbit of technical CGA 48988. Report
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1976h Skin sensitizing (contact allergenic) effect in guinea pigs
of technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Acute dermal LD50 in the rabbit of technical CGA 48988.
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
Sachsse, K., Suter, P. and Luetkemeier, H. CGA 48988 techn., 28 days
1979 toxicity study on rats. Final report from Ciba-Geigy Ltd.,
Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Strasser, F.F. and Muller, D. L 5178 Y/TK+/- mouse lymphoma
1982 mutagenicity test CGA 48988. (in vitro test for mutagenic
properties of chemical substances in mammalian cells. Report
from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Thomann, P. and Pericin, C. Acute oral LD50 in the Chinese hamster of
1977 CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
de Ward, J., Beck, L. St., Kitchen, D.N. and Hepler, D.I. Six-month
1981 chronic oral toxicity study with CGA 48988 technical in
beagle dogs. Project No. 1545. Report from ELARS Bioresearch
Laboratories, Fort Collins, Colorado, USA, submitted from
Ciba-Geigy Ltd., Basle, Switzerland to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
METALAXYL
Reproduction indices, including mating, fecundity, male and
female fertility, gestation, lactation, pup mortality, litter weights
and mean pup weights, were determined and compared with control
values. All pups were examined for physical abnormalities and
viability.
There were no consistent dose-related effects on adult animals
with regard to mortality, food/water consumption, body/weight gain,
mating performance, pregnancy rate or duration of gestation. The
litter size and litter weight of control group rats in the Fo 2nd
mating were significantly reduced from treatment groups (birth through
day 21 post-partum). Mean pup weights were normal. Litter size and
mean pup weights were reduced at birth for the F1b 1st mating, group
2 animals. Mean pup weight remained significantly reduced through day
21 for these group 2 litters. These effects were not noticed in other
dose groups or other generations and are therefore not considered
compound-related.
There were no dose-related or compound-related effects on organ
weight, organ/body weight ratio or histopathology. The selected
visceral and skeletal examinations of foetuses for assessing
teratogenic effects were within normal variation for the strain/
species studied. There were no differences with respect or any of the
indices observed, and it is considered that metalaxyl, at dietary
dosage levels up to and including 1 250 ppm, caused no adverse
reproduction effects in rats (Cozens et al 1980).
Special Studies on Teratogenicity
Rat
Groups of 25 pregnants rats were administered, via intubation,
dose levels of 0, 20, 60 and 120 mg metalaxyl/kg bw from days 6
through 15 of gestation. On day 21 of gestation, all pregant animals
were killed by cervical dislocation and foetuses delivered by
caesarean section.
Survival was not affected by treatment. The ratios of
implantations and resorptions were comparable among all treatment and
control groups. Sex ratio and number of live young were not affected
by metalaxyl. No malformations were found in the foetuses nor were the
average foetal weights of the treatment groups affected. Delayed
ossification was marginally affected at the high dose within normal
variation based on historical control data. There were no indications
of embryotoxic or teratogenic effects in the rat under the conditions
of the study (Fritz 1978b).
Rabbit
Groups of pregnant Chinchilla rabbits (20 rabbits/group) were
given metalaxyl orally by intubation at dose levels of 0, 5, 10 and
20 mg/kg during days 6 through 18 of gestation. On day 28 of
gestation, all rabbits were sacrificed and examined and the foetuses
delivered by caesarean section. Foetuses were subjected to external
examination followed by inspection for Visceral and skeletal
malformations, anomalies or variants.
Reduced food consumption was observed in the 10 and 20 mg/kg
groups accompanied by slightly reduced body weight gain in dams at the
high dose. The mean number of corpora lutea and/or implantations were
comparable for all groups. Incidences of renal hypoplasia and agenesis
were not dose-related and within the spontaneous rate of historical
untreated animals of the same rabbit population. Metalaxyl did not
adversely influence embryonic or foetal development in Chinchilla
rabbits at levels up to and including 20 mg/kg (Fritz et al 1978).
Special Studies on Mutagenicity
Yeast culture evaluations
The test compound, CGA 48 988, was tested for mutagenic effects
on the D-7 strain of Saccharomyces cerevisiae at five dose levels:
400, 2 000, 4 000, 8 000 and 10 000 µg/ml, with and without microsomal
activation. The test system permitted the detection of mitotic
crossover, mitotic gene conversion and reverse mutation in the yeast
cells D-7 after 2 or 6 hours of treatment with the test compound. The
positive controls were treated with 4-nitroquinoline-N-oxide (without
S-9 mix) and cyclophosphamide (with S-9 mix) in concentrations of
0.25 µg/ml and 50-250 µg/ml, respectively.
In none of the three mutation systems was the incidence of
mutants increased, in comparison with the negative controls, as a
result of treatment of the cells with metalaxyl with or without
microsomal activation. It is concluded that under the conditions of
these experiments no evidence of mutagenic effects was obtained with
metalaxyl (Arni and Muller 1982).
DNA repair
The primary rat hepatocytes prepared from the liver of a male rat
were used for the UDS assay. A series of compartments in pertri dishes
containing gelatinized cover-slips were seeded with 2 × 10-5 cells
per compartment. The cells were allowed to attach to the coverslips
during an attachment period of 1.5-2 hours. The cell cultures attached
to the coverslips were then washed and refed with WME containing the
test compound (16, 80, 400 and 2 000 µg/ml) and 3H-thymidine
(4 µ Ci/ml). Negative controls and positive controls (4NQO and DMN)
were run concurrently with the test compound.
Comparison of the mean number of silver grains per nucleus in the
negative controls and after treatment with metalaxyl revealed no
evidence of induction of DNA damage (Puri and Muller 1982).
Mouse lymphoma assay
Metalaxyl was tested for mutagenic effects on L5178Y/TK+/- mouse
lymphoma cells in vitro. The investigations were performed with
microsomal activation at concentrations of 0.0625, 0.125, 0.25 and
0.50 mg/ml and without microsomal activation at concentrations of
0.125, 0.25, 0.50 and 1.0 mg/ml. The results were expressed in terms
of the number of induced TK-/- mutants/106 surviving cells. The
test substance is considered mutagenic in this system if the colony
count exceeds that of the solvent control by a factor of more than
2.5 at any concentration.
It was concluded that, under the given experimental conditions,
no evidence of mutagenic effects of metalaxyl was observed in this
mammalian forward mutation system (Strasser and Muller 1982).
Dominant lethal studies
Two groups of adult male albino mice, 20 per group, were
administered orally by intubation single doses of 65 and 196 mg/kg of
metalaxyl dissolved in carboxymethyl cellulose. Each male mouse was
mated with two untreated virgin female mice for eight consecutive
weeks to cover the total spermatogenic cycle. Fourteen days from the
mid-week of mating, the females were killed and examined for
pregnancies. Three parameters were used to determine the dominant
lethal effect of the test compound: mating ratio, number of
implantations and embryonic deaths. No evidence of dominant lethal
effect was obtained in the progeny of male mice treated with metalaxyl
(Fritz 1978a).
Special Studies for Carcinogenicity
Mouse
Metalaxyl was administered in the diet to groups of 60 male and
60 female mice (ICI Alderley Park, Swiss) at concentrations of 0, 50,
250 and 1 250 ppm for 104 weeks. Mice were approximately 5-6 weeks of
age when dosing commenced. The percent survival, food/water
consumption and general thriftiness of animals were unaffected by
treatment. A slight decrease in food conversion efficiency,
accompanied by decreased body weight gain, was noticed in high dose
males during weeks 11 through 30. Bone marrow and blood, sampled
throughout the study, were unaffected by treatment.
All animals that died or were sacrificed in extremis were
submitted to gross necropsy and histopathological evaluation for both
non-neoplastic and neoplastic lesions. The lesions observed were
similar among all groups and not related to the administration of
metalaxyl. Metalaxyl was not found to be a carcinogen in this strain
of mouse (McSheehy et al 1980).
Special Study on Skin Sensitization
Guinea pig
Groups of 10 male and 10 female guinea pigs (Pirbright, albino)
were subjected to a series of 0.1 ml intracutaneous injections of
metalaxyl as a 0.1% suspension in polyethylene glycol and saline,
following the optimization test of Maurer et al (1975). DNCB served
as a positive control. Metalaxyl was not sensitizing under the
conditions of the test (Sachsse and Ullmann 1976h).
Special Study on Eye Irritation
Rabbit
Approximately 100 mg of metalaxyl was placed in the conjunctival
sac of 3 male and 3 female rabbits. The eyes of 3 of the 6 rabbits
were rinsed approximately 30 seconds after instillation of the test
material. There was no irritation present in any of the rinsed eyes.
Unwashed eyes presented irritation of the conjunctiva and cornea for 3
days and were clear by day 4. While the degree of corneal involvement
appears minimal, it could not be subjectively evaluated since only
scores were presented. Rinsing the eyes prevented any irritation
effects, suggesting that the irritation in unwashed eyes may have been
more related to the physical nature of the particles than to the
chemical itself (Sachsse and Ullman 1976g).
Special Study on Dermal Irritation
Rabbit
The primary irritation was determined following the application
of 0.5g of metalaxyl to both abraded and intact skin sites on the
shaved back of 3 male and 3 female rabbits. There was no irritation on
any of the intact skin sites, while minimum erythema was observed on
the abraded skin sites of 2 rabbits 24 hours after application. The
primary irritation index was reportedly 0.1/8 (Sachsse and Ullmann
1976f).
Acute Toxicity
In acute toxicity studies carries out in animals (Table 1), toxic
signs of poisoning occurred within 2 hours after treatment and most
deaths within the first 24 hours. Symptoms observed were non-specific
in the various mammalian species and consisted of sedation, dyspnoea,
exophthalmos, tonic-clonic muscle spasms, curved or ventral position
and ruffled fur.
Metalaxyl was not toxic by the dermal route, as both rats and
rabbits tolerated doses from 3 100 to 6 000 mg/kg without visible
symptoms or effects. No mortalities were reported in either species.
Table 1. Acute Toxicity of Metalaxyl in Animals
Species Sex Route LD50 Reference
Rat m + f oral 1 669 Sachsse and Bathe, 1976a
(515-868)
Mouse m + f oral 1 788 Sachsse and Bathe, 1976b
(626-991)
Rabbit m + f oral 1 697 Sachsse and Ullman,1976e
(506-961)
Hamster m + f oral 7120 Thomann and Pericin,1977
(5250-9660)
Rat m + f dermal 1 >3 100 Sachsse and Bathe, 1976c
Rabbit m + f dermal 2 >6 000 Sachsse and Ullmann, 1978
Rat m + f i.p. 1 312 Sachsse and Bathe, 1976d
(282-345)
1 Carboxy methyl cellulose was the vehicle;
2 Polyethylene glycol was the vehicle.
Short-Term Studies
Rabbit-dermal
Metalaxyl was administered to the occluded intact and abraded
skin of albino rabbits (10 males and 10 females/group) at doses of 0,
10, 100 and 1 000 mg/kg. Test substance was applied once each day, 5
days/week for 3 consecutive weeks. Information was obtained on body
weight, food consumption, haematology, clinical chemistry, absolute
and relative organ weights and local dermal effects on the skin. All
animals were subjected to gross necropsy and histopathological
examination. There were no adverse effects related to compound
administration for any of the parameters evaluated. Several lesions
reported in both treatment and control groups were related to either
bacterial Staphylococcus aureus and epidermitis) or parasitic
(encephalitozoon, Eimeria stiedae) infections, which obscured
interpretation of results (Calkins et al 1980).
Rat - dietary
Daily oral doses of metalaxyl were administered by gavage to
groups of rats (10 males and 10 females/group) initially at levels of
0, 10, 30 and 100 mg/kg for the first 14 days. Dosing was increased to
0, 30, 100 and 300 mg/kg, respectively, for days 15-21 of treatment.
Once again, on day 22, dose levels were increased to 0, 60, 200 and
600 mg/kg, respectively, for the duration of the study, which was
terminated on day 28. No deaths occurred and food consumption, body
weight gain and mean food consumption were comparable among treated
and control groups. Haematology, blood chemistry and urinalysis were
unremarkable. Male rats in the high dose group demonstrated a
significant increase in absolute testes weight as well as a dose-
related increase in relative liver weight, for which the trend was
significant (p<0.01). The absolute and relative adrenal weights were
significantly increased (p<0.05) for females in the high dose group.
Female rats in all treatment groups administered metalaxyl had
significantly increased absolute and relative liver weights (p<0.05),
which were dose-related and for which the positive trend was similarly
significant (p<0.01) (Sachsse et al 1979).
In a 3-month dietary study using Sprague-Dawley rats, 180 rats
were allocated to two groups of 50 animals each (25 males and 25
females) and two groups of 40 animals each (20 males and 20 females).
Dose levels were 0, 50, 250 and 1 250 ppm in the diet. Five males and
five females from the control and high dose level groups were retained
for an additional 28 days without treatment for evaluation of recovery
and reversibility of effects. Minimal cellular hypertrophy of hepatic
parenchymal cells was observed in five females receiving the highest
dose. This trend was not noticeable in the recovery animals,
representing a possible reversibility for this effect. No other
changes attributed to treatment were observed and dose levels of
250 ppm or less are considered to be without adverse effects under the
conditions of this study (Drake 1977).
In a follow-up 90-day study, Gfeller et al (1980) evaluated the
effects of 0, 10, 50, 250 and 1 250 ppm of metalaxyl incorporated in
the diet of rats (RAIF, SPF). There were 20 males and 20 females in
each experimental group. Mortality and other cageside observations
were conducted daily, body weight and food consumption determined
weekly and clinical chemistries, urinalysis and haematology performed
periodically throughout the study. At the conclusion of the study, all
test and control animals were subjected to gross and histopathological
examination and selected organs were weighed.
A dose-related decrease in total leucocyte count was evident in
high dose males, as well as increased absolute and relative adrenal
weights for male rats in the 50, 250 and 1 250 ppm groups. In females,
the relative adrenal weights were increased in the high dose group
only. There was a significant increase in relative liver weights for
females in the 250 and 1-250 ppm dose groups. However, the absolute
liver weights for high dose females were not significantly different
from controls and, therefore, the difference reported for the relative
liver weights is considered as being related to the decrease in the
body weight of the high dose compared to control females, rather than
as an increase in organ weight, per se. Gross necropsy and
histopathological examinations revealed no differences between treated
and control animals. There were no structural alterations in the
adrenals reflective of the increase in both absolute and relative
weights for this organ. The level of 10 ppm of metalaxyl in the diet
of rats is considered to be without adverse effects (Gfeller
et al 1980).
Dog - dietary
Four groups of beagle dogs, 6-9 months of age, were administered
metalaxyl in their diets at levels of 0, 50, 250 and 1 250 ppm for
91 days. Control and high dose groups contained 4 males and 4 females
each, with the low and intermediate dose groups containing 3 males and
3 females each. No mortality or changes in the behaviour of the
animals were observed. Urinalysis, ophthalmoscopy, body weight, food
consumption and haematology indicated no treatment-related findings.
Blood chemistry parameters were normal, except for a significant
increase in serum alkaline phosphatase levels in high-dose males and
females, which was also time-related. Determination of SAP in two
recovery animals from the high dose group demonstrated a reversal to
normal within 4 weeks post-treatment. Macroscopic and microscopic
examinations revealed no compound-related findings. The no-effect
level was estimated to be 250 ppm (Finn et al 1977).
Technical metalaxyl was administered in the diet to groups of
beagle dogs at levels of 0, 50, 250 and 1 000 ppm for a period lasting
six months. There were 8 males and 8 females in both the control and
high dose groups, with 6 males and 6 females in each of the other two
groups. Pups were 6-8 months old at the beginning of study. Feed
consumption, body weights and urinalysis, performed periodically
throughout the study, were normal. Ophthalmic examinations did not
reveal any effects related to treatment. Clinical chemistry parameters
were all within normal variation, except for alkaline phosphatase
levels for 1 000 ppm dose-level males and females, which were
significantly increased (p<0.05) compared to control animals. A
recovery to normal was observed after dosing was stopped. Haemoglobin,
erythrocyte count and hematocrit, while significantly lower (p<0.05)
for high dose males when compared to control males in this study, were
all within normal variation when compared to historical controls.
Anaemia was not evident and bone marrow samples reportedly were
normal: however, reticulocytes and m/e ratios were not determined. The
liver/brain ratios of females in the high dose group were
significantly increased (p <0.05) compared with controls. Similarly,
a positive trend for both increased absolute and relative liver
weights was apparent in male and female dogs receiving an increased
dosage. Alkaline phosphatase is produced in many organs, with the
liver being a primary supplier. The possibility of a dose response for
alkaline phosphatase relative to the liver is supported by the trend
toward increasing liver weights with increased dosage. No such
trends, however, were seen in other enzymes also produced in the liver
(e.g. SGPT, SGOT), and no gross pathological or histopathological
changes were observed in the liver of any dogs fed metalaxyl. There
were no other effects related to treatment and a no-effect level of
250 ppm was determined. Mean daily feed consumption data indicate this
dietary level is approximately equivalent to 7.4 mg/kg/day, with
1 000 ppm corresponding to 31 and 33 mg/kg/day for males and females,
respectively (de Ward et al 1981).
Long-Term Study
Mice
(see Special Study for Carcinogenicity)
Rat
Groups of rats (80 male and 80 female Sprague-Dawley albino
rats/group) were administered metalaxyl in the diet for 104 weeks at
dosage levels of 0, 50, 250 and 1 250 ppm. Growth, as observed by body
weight changes and food consumption data, was recorded weekly for the
first 13 weeks and monthly thereafter. Rats were inspected twice daily
for behavioural changes, general health, mortality and tumour
development. At periodic intervals over the course of the study,
haematologic, clinical chemistry and urinalysis examinations were
performed.
All animals, including those dying or sacrificed, were subjected
to both gross necropsy and microscopic examinations after 55 and 105
weeks of treatment.
No effects considered to be treatment-related were noted with
respect to toxic signs, mortality, body weight, food consumption,
urinalysis, ophthalmologic examination and haematology. Survival was
greater than 50% for both sexes of all groups at 18 months. Activities
of SGPT and SGOT decreased in the 250 and 1 250 ppm females during the
first year, but recovered to normal values, comparable to the control
group, through the second year to termination of the study.
At gross necropsy, organ weight determinations demonstrated
significantly increased (p <0.01) relative liver weights for females
in the 1 250 ppm group after both 55 and 105 weeks of treatment.
Relative liver weights for males were significantly increased
(p <0.01 and <0.001) in both the 250 and 1 250 ppm groups,
respectively, after 105 weeks. These organ to body weight differences
were not accompanied by any pathological findings in the treated
animals other than slight periacinar hepatocytic vacuolation and
chronic inflammation in 1 250 ppm males.
There was a reported pathological finding of testicular atophy
(p <0.05) occurring primarily in the first 53 weeks at all treatment
levels, but the respective absolute and relative testicular weights
were comparable to those of the control group. Since there was no
positive trend with dose for this response and no impairment of
reproductive performance (Cozens et al 1980), this effect is not
considered attributable to metalaxyl. The findings of parafollicular
cell adenomas of the thyroid was significantly increased for the
250 ppm females. However, there was no dose-response relationship and
the incidence was not significantly different from historical control
data for this pathological finding at the same laboratory.
Metalaxyl is considered to be without adverse effects on rats at
levels up to and including 50 ppm in the diet. There was no evidence
of carcinogenic potential at any level tested (Ashby et al 1980).
COMMENTS
Metalaxyl, when fed to rats, is rapidly metabolized and excreted
in the urine and faeces. The metabolism has been relatively well
defined in rats and no specific organ accumulation appears to be
found. The excretion pattern is not influenced by the dose level, but
by the sex of the animals, as females demonstrate preferential
excretion via urine, while males excrete higher proportions in the
faeces.
The acute toxicity in various animals suggests a moderate acute
hazard via the oral route, but little dermal absorption occurs, as
demonstrated in both acute and subchronic dermal studies. Metalaxyl is
mildly irritating to the skin and eyes but did not elicit contact
allergenicity in a skin-sensitization study using guinea pigs.
Metalaxyl did not produce any adverse effects on reproductive
performance or pup viability at levels up to and including 1 250 ppm
in the diet. There were no indications of embryotoxic or teratogenic
effects in either rats or rabbits at levels of 120 or 20 mg/kg bw,
respectively.
Metalaxyl was evaluated in a battery of mutagenicity tests
including yeast culture, DNA repair, mouse lymphoma and dominant
lethal tests. No evidence of mutagenic potential was determined in any
of these tests.
In both short- and long-term studies, the only significant
findings were increased relative liver and adrenal weights, sometimes
accompanied by increased enzyme activities (i.e. SAP, SGPT), but for
which there was no corresponding histopathology. The adrenal weight
increase noted in the rat short-term study was not apparent in the rat
long-term study at comparable dietary levels nor in the short-term dog
study or mouse carcinogenicity study. Metalaxyl was not demonstrated
to be carcinogenic in either rats or mice. Based upon all available
data, no-effect levels in mammalian species can be determined and an
ADI for man allocated for metalaxyl.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mice : 1 250 ppm in the diet, equivalent to 187.5 mg/kg bw
Rat : 50 ppm in the diet, equivalent to 2.5 mg/kg bw
Dog : 250 ppm in the diet, equivalent to 7.4 mg/kg bw
Estimate of Acceptable Daily Intake for Man
0 - 0.03 mg/kg body weight
FURTHER WORK OR INFORMATION
Desirable
1. Metabolic studies in a non-rodent species.
2. Observations and information in humans.
REFERENCES
Arni, P. and Muller, D. Saccharomyces cerevisiae D-7/mammalian-
1982 microsome mutagenicity test in vitro with CGA 48988 (test
for mutagenic properties in yeast cells). Report from Ciba-
Geigy Ltd., Basle, Switzerland, submitted to the World
Health Organization by Ciba-Geigy Ltd. (Unpublished)
Ashby, R., Bhatt, A., Chapman, E., Hepworth, P.L. and Whitney, J.C.
1980 CGA 48988: Toxicity and oncogenicity in dietary
administration to rats for two years. Final report no.
80/CIA009/315 from Life Science Research, Stock, Essex CM4
0PE, England, submitted from Ciba-Geigy, Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Calkins, J.E., Morgan, J.M., Casey, H.W. and Page, J.G. A 21-day
1980 subacute dermal toxicity study in albino rabbits with CGA
48988. Report from Toxigenics Inc. Decator, Ill. 62526, USA,
submitted by Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Cozens, D.D., Allen, P.A., Clark, R., Offer, J.M., Gregson, R.L. and
1980 Gibson, W.A. Effect of CGA 48988 on reproductive function of
multiple generations in the rat. Report from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England,
submitted from Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Drake, J.C. 3-month dietary study in rats with compound CGA 48988.
1977 Report from Geigy Pharmaceuticals, Stamford Lodge, Wilmslow,
Chershire, U.K. submitted from Ciba-Geigy Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Finn, J.P., Briggs, K., Close, J.E., Noble, D.N., Barrett, R. and
1977 Rider, L. CGA 48988, 91-day dietary toxicity study in beagle
dogs. Report from Hazleton Laboratories Europe Ltd.,
Harrogate, HGe 1PY, England, submitted from Ciba-Geigy Ltd.,
Basle, Switzerland, to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Fritz, H. Dominant lethal study-CGA 48988 techn. mouse (Test for
1978a cytotoxic or mutagenic effects on male germinal cells).
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
1978b Reproduction study-CGA 48988 tech. Rat Seg. II (Test for
teratogenic or embryotoxic effects). Report from Ciba-Geigy
Ltd., Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Fritz, H., Becker, H. and Hess, R. Reproduction study - rabbit- CGA
1978 48988 tech. Seg. II (Test for teratogenic or embryotoxic
effects). Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Gfeller, W., Basler, W., Zak, F., Grieve, A.P. and Hess, R. CGA 48988
1980 techn. 3-month toxicity study on rats (Project No. 79 18 11)
Final Report. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Hambock, H. Distribution, degradation and excretion of CGA 48988 in
1977 the rat. Project Report 18/77 from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Metabolism of CGA 48988 in the rat. Project report 26/78
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1981 Metabolic pathways of CGA 48988 in the rat. Project Report
31/81 from Ciba-Geigy Ltd., Basle, Switzerland, submitted to
the World Health Organization by Ciba-Geigy, Ltd.
(Unpublished)
Maurer, T., Thomann, P., Weirich, E.G. and Hess, R. The optimization
1975 test in the guinea pig. A method for the predictive
evaluation of the contact allergenicity of chemicals. Agents
and Actions 5 (2): 174-179.
McSheehy, T.W., Macrae, S.M. and Whitney, J.C. CGA 48988 oncogenicity
1980 in dietary administration to mice for two years. Final
Report No. 80/CIA 008/442 from Life Science Research, Stock,
Essex CM4 9PE, England, submitted from Ciba-Geigy Ltd.
Basle, Switzerland to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Puri, E. and Muller, D. Audioradiographic DNA repair test on rat
1982 hepatocytes CGA 48988 (in vitro test for DNA-damaging
properties). Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy, Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute oral LD50 in the rat of technical CGA
1976a 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute oral LD50 in the mouse of technical CGA 48988. Report from Ciba
1976b Geigy Ltd., Basle, Switzerland submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute dermal LD50 in the rat of technical
1976c CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute intraperitoneal LD50 in the rat of technical CGA 48988. Report
1976d from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Ullmann, L. Acute oral LD50 in the rabbit of technical
1976e CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organziation by Ciba-Geigy
Ltd. (Unpublished)
1976f Skin irritation in the rabbit after single application of
technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1976g Eye irritation in the rabbit of technical CGA 48988. Report
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1976h Skin sensitizing (contact allergenic) effect in guinea pigs
of technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Acute dermal LD50 in the rabbit of technical CGA 48988.
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
Sachsse, K., Suter, P. and Luetkemeier, H. CGA 48988 techn., 28 days
1979 toxicity study on rats. Final report from Ciba-Geigy Ltd.,
Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Strasser, F.F. and Muller, D. L 5178 Y/TK+/- mouse lymphoma
1982 mutagenicity test CGA 48988. (in vitro test for mutagenic
properties of chemical substances in mammalian cells. Report
from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Thomann, P. and Pericin, C. Acute oral LD50 in the Chinese hamster of
1977 CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
de Ward, J., Beck, L. St., Kitchen, D.N. and Hepler, D.I. Six-month
1981 chronic oral toxicity study with CGA 48988 technical in
beagle dogs. Project No. 1545. Report from ELARS Bioresearch
Laboratories, Fort Collins, Colorado, USA, submitted from
Ciba-Geigy Ltd., Basle, Switzerland to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
METALAXYL
 RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales, which cause late blight, downy
mildews, damping-off and root, stem and fruit rots of many plants. The
compound is taken up by the roots, leaves, green stems and shoots and
transported acropetally within the plant (Urech et al 1977).
Commercial products first introduced as foliar sprays contained
only metalaxyl as the active ingredient or were mixtures of metalaxyl
with low rates of residual protective fungicides, such as copper or
folpet. Experience has now shown that strains of fungi resistant to
metalaxyl may develop, and in order to reduce the likelihood of this
happening and to broaden the spectrum of activity, commercial products
now contain a higher content of the residual fungicides mancozeb,
zineb, copper, folpet or carbendazim. A further measure to hinder the
development of resistance has been the limitation of the number of
sprays applied to the crop. This has generally been restricted to 3 to
4 applications, which are made during the active vegetative stage of
the crops. Commercial products containing metalaxyl only are intended
for use as seed dressing or for soil applications.
Metalaxyl is registered for use on a wide range of crops and in
several countries in temperate, subtropical and tropical regions.
Preharvest treatments
Metalaxyl is used for foliar sprays, seed dressing and soil
treatments. The main fields of application are in potatoes, grapevines
and vegetables. It is also used against diseases of tobacco and in
some non-edible crops such as ornamentals and turf.
Postharvest treatments
Metalaxyl, incorporated in wax, is applied as a pack-house spray
to citrus fruit for the control of postharvest rot. The recommended
application rates for the crops on which the compound is currently
used are given in Table 1.
Table 1. Recommended Application Rates of Metalaxyl
Crop Rate
(a.i.)
Foliar/Fruit sprays
Potato 150 - 250 g/ha
Onion 200 - 250 g/ha
Leafy vegetables 20 - 25 g/100 1
Brassicae 200 - 250 g/ha
Cucurbits 20 - 25 g/100 1
Tomato 20 - 40 g/100 1
Grape 20 - 30 g/100 1
Pineapple 25 g/100 1
Cocoa 50 - 312 g/100 1
Hops 20 - 30 g/100 1
Seed treatments
Sugarbeet 245 g/100 kg seed
Vegetables 26 - 53 g/100 kg seed
Peas 70 g/100 kg seed
Maize 175 g/100 kg seed
Millet 263 g/100 kg seed
Sorghum 175 g/100 kg seed
Sunflower 210 g/100 kg seed
Soil treatments
Red and green pepper (Capsicum annuum) 2 kg/ha
Black pepper (Piper nigrum) 1.25 - 2 g/m2
Citrus 1.25 - 2 g/m2
Avocado 2- 5 g/m2
Hops 0.2- 4 g/plant
Postharvest treatment
(pack-house spray)
Citrus 2 000 ppm a.i. in wax
1 3-4 applications.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Metalaxyl residue data have been obtained from numerous trials
carried out on a world-wide basis on main crops. Treatments were
generally made using WP or FW formulations with metalaxyl alone or
with mixtures containing residual fungicides. The dosages used in the
residue trials cover the recommendations as summarized in Table 1.
Analysis was carried out using methods determining metalaxyl
alone and/or metalaxyl and its metabolites containing the
2,6-dimethylaniline moiety.
Potatoes
Field trials were conducted in 14 countries during six years. Two
to eight applications of dosages ranging from 0.15 to 0.42 kg a.i./ha
were made at intervals of 1-3 weeks. The results of these trials are
reported in Table 2 (Ciba-Geigy undated; Finland undated). Generally,
no residues either of the parent compound or metabolites containing
the 2,6-dimethylaniline moiety were detectable in potato tubers.
Residues (0.02-0.08 mg/kg) were found in only eight of 102 samples.
The limit of determination ranged from 0.02-0.05 mg/kg.
Onions
Field trials were carried out in nine countries over four years.
Two to eight applications at dosages ranging from 0.22-0.6 kg a.i./ha
were made at intervals of 1-3 weeks. The results are shown in
Table 3 (Ciba-Geigy undated; Finland undated). Initial residues of
0.05-0.59 mg/kg generally dissipated below the limits of determination
(0.02 and 0.04 mg/kg) within 14 days. Dissipation of metalaxyl
metabolites containing the dimethylaniline moiety did not differ
significantly from that of the parent compound alone. Two weeks after
the last application, residues of metalaxyl and its metabolites ranged
between <0.05-0.09 mg/kg. The major part (73-100%) of the total
residue consisted of the parent compound.
Leafy Vegetables
Spinach
Field trials were carried out in Italy, The Netherlands,
Switzerland and the U.S. One to four applications (to run off) at
dosages ranging from 0.22-0.55 kg a.i./ha or at 0.043% were made at
intervals of 1-2 weeks. The results are reported in Table 4
(Ciba-Geigy undated). Residues of the parent compound rapidly
disappeared being at or below the limit of determination (0.05 mg/kg)
two weeks after the last application. Residues measured as the sum of
metalaxyl and its metabolites containing the dimethylaniline moiety
amounted to 1.1-7.3 mg/kg one week after the last application and
decreased to 0.27-5.0 mg/kg during the following two weeks.
Supervised trials were carried out on lettuce at six sites in the
U.K. The plants were treated at planting and after an interval of two
weeks with a formulation containing 48% mancozeb and 10% metalaxyl at
rates of 71-150 g metalaxyl/ha. The residues were below 0.1 mg/kg, the
limit of determination, in 19 samples taken after 13 to 58 days. In
the other samples, the residues were 0.55, 1.2 and 1.3 at day 21, and
0.25 mg/kg at day 35 (U.K.undated).
Table 2. Residues of Metalaxyl Following Supervised Trials in Potatoes (1976-1981)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Argentina 3 0.2-0.25 1-2 0.05
Austria 4 0.2 3 <0.03 <0.03 <0.03 <0.03 <0.03
Canada 4 0.3 2 <0.05
5 0.3 2 <0.05
6 0.25 1-2 <0.05
6 0.15 1-2 <0.05
3 0.3 2 <0.02
3 0.25 2 <0.02
4 0.25 2 <0.02 0.02
6 0.25 1-2 <0.02
6 0.3 1-2 <0.02
3 0.3 3 <0.02 0.02
2 0.3 3 <0.02
2 0.25 3 <0.02
8 0.16 1 <0.02 <0.02 <0.02
4 0.16 2 <0.02 <0.02
4 0.2 2 <0.02 <0.02
6 0.2 1-2 <0.02 <0.02 0.02 0.03
Fed. Rep. Germany 3 0.2-0.3 4 <0.05
3 0.2-0.3 4 <0.05
8 0.2 1 <0.05
8 0.2 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.04
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
Finland 2 0.25 3 <0.04
France 3 0.3 3 <0.04 1
4 0.2 2 <0.04 1
3 0.3 3 <0.02 1
U.K. 6 0.2 1-2 <0.05
5 0.3 3 <0.05 0.05 <0.05 <0.05
4 0.3 3 <0.05
6 0.3 2-3 <0.05
5 0.3 2-3 <0.05
5 0.3 2-3 <0.05
India 4 0.24 2 <0.02
Israel 5 0.25 1-2 <0.02
0.02
South Africa 5 0.2 2-3 0.02 <0.02
Spain 5 0.3 1-2 <0.05
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Sweden 5 0.15 1-2 0.05 <0.05 <0.05
4 0.2 2 <0.02
4 0.2 2-3 <0.02
5 0.15 1-2 <0.02
3 0.25 3 <0.02
Switzerland 5 0.25 2 <0.05 <0.05 <0.05
<0.06
5 0.25 2 <0.05 <0.05 <0.05
3 0.2-0.3 3 <0.05
8 0.25 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
U.S. 3 0.275 2 <0.05 2
6 0.275 2 <0.05
6 0.275 2 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 2 <0.05
6 0.412 1 <0.05
6 0.42 2 0.06 2
0.08 2
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
4 0.42 2 <0.05
5 0.42 2 <0.05
6 0.275 2 <0.05 2
<0.05 2
<0.05 2
6 0.275 2 <0.05 2 <0.05 2
<0.05 2 <0.05 2
<0.05 2 <0.05 2
6 0.22 2 <0.05 2
6 0.22 2 <0.05 2
1 Peel and pulp were analysed separately, no sample contained measurable residues.
2 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 3 Residues of Metalaxyl Following Supervised Trials in Onions (1978-1981)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
Australia 6 0.4 2 <0.04 <0.04
4 0.2 1 0.30 0.12
4 0.3 1 0.59 0.12
Canada 3 0.25 2 <0.02
3 0.24 1-2 0.05 <0.02
4 0.24 1-2 0.31 0.06
3 0.24 1-2 <0.02
5 0.24 2 0.02
4 0.24 2 <0.02
4 0.25 1-2 0.05 0.04
3 0.25 1-2 0.03
Fed.Rep. Germany 3 0.25 2 0.06 <0.02 <0.02
3 0.25 2 0.05 0.02 <0.02
Finland 2 0.25 5 <0.04
4 0.25 1 <0.04
France 3 0.225 3 0.04
U.K. 2 0.5 2 <0.02
Italy 5 0.6 2 0.02
Switzerland 8 0.3 2 <0.05
Table 3 (con't)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
U.S. 1 5 0.275 1-2 0.15
5 0.55 1-2 0.16
5 0.275 1-2 <0.05 <0.05
5 0.55 1-2 0.06 <0.05
5 0.275 1-2 <0.05
5 0.55 1-2 <0.05
5 0.22 2 0.1 0.08
5 0.22 2 0.09 0.08
3 0.275 1-2 0.26
5 0.275 1-2 <0.05 <0.05 <0.05
5 0.55 1-2 <0.05 <0.05 <0.05
5 0.22 2 <0.05 <0.05
6 0.22 2 <0.05 <0.05 <0.05
6 0.22 2 0.08 <0.05 <0.05
5 0.22 2 0.20 0.09 <0.05
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 4 Residues of Metalaxyl Following Supervised Trials in Spinach
Applications Residues (mg/kg) at intervals after
Interval between last application (days)
Country Rate (a.i. applications
No. kg/ha or %) (weeks) 3 6-8 13-15 20-22 27-31
Italy 2 0.043 2 0.05
Netherlands 1 0.25 0.53 0.06 0.03 <0.03
Switzerland 2 0.25 2 0.53 <0.05 <0.05 <0.05 <0.05
U.S. 3 0.275 2 0.06 <0.05
2.9 1 2.2 1 1.0 1
3 0.55 2 0.67 <0.05
4.9 1 3.9 1 2.4 1
3 0.275 2 1.5 1 1.4 1 1.7 1
0.55 2 7.3 1 4.1 1 5.0 1
3 0.275 1 1.2 1 0.25 1 0.27 1
0.55 1 1.1 1 0.79 1 0.86 1
4 0.22 1-2 1.1 1
4 0.22 2 1.8 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Brassicas
Field trials were carried out in broccoli, Brussels sprouts,
cabbage and cauliflower in several countries over a period of five
years. Two to five applications at dosages from 0.2 to 0.55 kg a.i./ha
were made at intervals of 1-3 weeks. The results are reported in
Table 5 (Ciba-Geigy undated). Dissipation of residues was similar
within the various varieties. Initial residues of 0.17-2.0 mg/kg
rapidly decreased and were generally below 0.1 mg/kg after two weeks.
Residues measured as metalaxyl and its metabolites containing the
dimethylaniline moiety were somewhat higher and dissipated more
slowly. The parent compound amounted to 55% of the residue containing
the dimethylaniline moiety. Two weeks after last application of the
recommended dosage of 0.25 kg a.i./ha, residues were <0.05-0.2 mg/kg.
Fruiting vegetables
Field trials were carried out with cucumbers, gherkins, melons,
squash and watermelons in different countries. Three to eight
applications at dosages of 0.2-0.55 kg a.i./ha or 0.02-0.4% were made
at intervals of 1-2 weeks. The results are reported in Table 6
(Ciba-Geigy undated). Residue levels in the different commodities were
in the same range. Initial residues were 0.06-0.4 mg/kg, depending on
the dosage rate used. They decreased slowly and were 0.03-0.23 mg/kg
after two weeks. There was no difference between residue levels
determined as the parent compound or as the total of metalaxyl plus
metabolites.
Tomatoes
Field trials were carried out in seven countries over a period of
six years. Two to nine applications at dosages of 0.125-0.8 kg a.i./ha
or 0.02-0.08% were made at intervals from one to 22 days. The results
are listed in Table 7 (Ciba-Geigy, undated). In most samples, initial
residues were below 0.1 mg/kg. Residue levels of metalaxyl and its
metabolites were at the same magnitude as the parent compound alone.
Grapes
Numerous field trials were conducted over a period of six years
in nine countries representing important vine growing areas of the
world. Due to the different climatic areas, a wide range of
experimental conditions has been considered. One to nine applications
at dosages of 0.01-0.04% a.i. or 0.15-0.5 kg a.i./ha were made at
intervals of 1 to 4 weeks. The results of these trials are reports in
Table 8 (Ciba-Geigy undated).
Table 5. Residues of Metalaxyl Following Supervlsed Trials in Brassicas (1977-1981)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
Canada
(broccoli) 3 0.24 1-2 2.0 0.09 <0.02
3 0.25 1-2 0.56 0.12 0.05
2 0.25 3 0.10
(Brussels
sprouts) 5 0.25 2 0.39 0.12 0.05
(cabbage) 2 0.25 2 0.29 0.08
(cauliflower) 4 0.25 2 0.17 0.03
U.K.
(cauliflower) 2 0.2-0.3 2 0.09 0.02 0.01 0.02
2 0.2-0.3 2 0.09
2 0.2-0.3 2 0.47
South Africa
(brassicas) 5 0.2 1 0.16 <0.05
5 0.4 1 0.90 0.09
U.S.
(broccoli) 5 0.275 2 0.17
0.35 1 0.20 1
5 0.55 2 0.21
0.46 1 0.48 1
Table 5. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
5 0.275 2 0.06 0.06
0.16 1 0.32 1
5 0.55 2 0.17 0.2
0.62 1 0.50 1
0.22 1 0.09 1
5 0.275 2 0.19-0.26
(cabbage) 0.41-0.52 1
5 0.55 2 0.1
0.31 1
5 0.275 2 0.15 1 0.19 1
5 0.55 2 0.35 1 0.26 1
5 0.275 2 0.20 1 0.07 1
5 0.55 2 0.33 1 0.17 1
5 0.275 2 0.12 1 0.10 1
5 0.55 2 0.13 1 0.12 1
5 0.275 2 0.07 0.06
0.22 1 0.09 1
5 0.55 2 0.22 1 0.16 1
5 0.22 2 0.09 1 <0.05 1
5 0.22 2 0.30 1 0.39 1
5 0.22 2 0.15 1 0.14 1
(cauliflower) 5 0.275 2 0.08 1 <0.05 1
5 0.55 2 0.14 1 0.10 1
5 0.275 2 0.17 1 0.36 1
5 0.55 2 0.56 1 0.38 1
5 0.22 2 <0.05 1 <0.05 1
1 The sum of metalaxyl and its metabolites containing the, common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 6. Residues of Metalaxyl Following Supervised Trials in Fruiting Vegetables
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Australia
(gherkins) 7 0.2 1 0.06 <0.04
7 0.4 1 0.12 <0.04
3 0.4 2 0.12 <0.04
(cucumber) 8 0.2 1 0.14 0.09 0.13
8 0.4 1 0.40 0.27 0.24
5 0.2 2 0.14 0.05
Israel
(cucumber) 8 0.25 2 0.25 0.14
(melon) 4 0.25 1 <0.05
South Africa
(cucumber) 4 0.04 2 0.30 0.25 0.22
4 0.02 2 0.26 0.19 0.19
Switzerland
(cucumber) 3 0.25 2 0.09 0.05
3 0.25 2 0.04 0.03
3 0.25 2 0.06 0.03
U.S.
(melon) 8 0.275 1 0.11 0.08
0.17 1 0.24 1
8 0.55 1 0.24 0.25
Table 6. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
(cucumber) 8 0.275 1 0.15 1 0.17 1
8 0.55 1 0.30 1 0.27 1
8 0.22 1 0.13 1
8 0.22 1 0.12 1
(squash) 8 0.22 2 0.07 1
8 0.22 2 0.06
0.07
8 0.22 1 0.06
0.05 1
(watermelon) 8 0.275 1 0.09
0.12 1
8 0.55 1 0.13
0.16 1
8 0.275 1 0.05 1
8 0.55 1 0.05 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6 dimethylaniline,
expressed at metalaxyl equivalents.
Table 7. Residues of Metalaxyl Following Supervised Trials in Tomatoes (1976-1981)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Brazil 7 0.2 0.2 0.5 <0.02
7 0.2 0.024 0.5 <0.02
France 4 0.03 2 <0.04
4 0.045 2 <0.04
2 0.125 0.5 <0.04 <0.04
4 0.5 0.5 2 0.09 0.04 <0.04
4 0.2 2 0.05 <0.04 <0.04 (0.04
Israel 3 0.25 0.04 2 <0.02
South Africa 7 0.4 0.04 1-2 0.04 0.07 <0.03 <0.03
7 0.8 0.08 1-2 0.30 0.10 0.04 <0.03
5 0.08% to 1 <0.03 <0.03 <0.03 <0.03
5 run-off 0.04 1 0.07 0.05 <0.05
Spain 3 0.1% to 2 0.48
run-off (1 day)
Switzerland 9 0.03% to 1-2 0.12 <0.04
run-off
Table 7. (con't)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
U.S. 1 6 0.42 1 <0.05
6 0.42 1 0.18
8 0.42 1 <0.05
6 0.42 1 0.22
6 0.42 1 <0.05
6 0.42 1 0.10
5 0.42 1 0.22
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety
2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 8. Residues of Metalaxyl Following Supervised Trials in Grapes and Wine (1976-1981)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Australia 5 0.02 2 1.2 0.73 0.34
7 0.02 2-4 0.23 0.15 0.14
7 0.03 2-4 0.61 0.37 0.34 0.31
7 0.02 1-4 0.35 0.37 0.43
7 0.03 1-4 0.60 0.49 0.26
Canada 5 0.15 1-2 0.09 0.05
Fed.Rep. 6 0.3 1-2 2.1 3.4 2.2 1.3 1.3
Germany 6 0.4 1-2 2.4 1.4 1.9 1.1 1.4
8 0.2-0.4 1-3 8.0 6.8 4.4 4.2 5.0
8 0.2-0.4 1-3 2.4 2.9 1.9 2.4 1.2
8 0.15-0.4 2 2.1 1.3 1.7 1.7 0.46
6 0.08-0.16 2 0.55 0.4 0.36 0.45 0.15
7 0.12-0.32 2 0.47 0.47 0.49 0.50
7 0.12-0.32 1-2 0.67 0.62 0.51 0.48 0.36 0.37
6 0.24-0.4 2 0.79 0.84 0.53 0.51 0.18
6 0.13-0.54 2-3 2.1 3.5 4.1 2.6 1.2
6 0.3-0.4 1-2 2.9 1.8 2.6 0.13 0.38
8 0.16-0.24 2 0.51 0.34 0.28 0.50
3 0.14-0.3 2 0.2 0.18 0.03
3 0.2 2 0.2 <0.02 <0.02
3 0.2 2 0.15 0.08 0.02
3 0.12-0.36 2-3 0.39 0.23 0.05
3 0.36 1-2 0.02 <0.02
3 0.2-0.3 2 0.13 0.06 0.04
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Switzerland 8 0.02-0.04 2 1.2 0.75 0.04 0.03 0.03 0.02
7 0.01-0.02 2 0.73 0.31 0.74 0.86 0.42 0.09
6 0.45-0.6 2-3 3.2 2.1 1.6 1.6
5 0.02-0.03 3-4 0.84 1.0 1.6 0.58
5 0.02-0.03 3-4 0.16 0.11 0.1 <0.05
7 0.45 2 0.77 0.77 0.70 0.60 0.61 0.26
5 0.4 1-2 2.8 1.6 2.5 2.2 2.6 1.8 2.1 0.3
5 0.4 1-2 2.7 2.7 1.3 1.7 1.6 1.4 0.3
8 0.2-0.4 2 2.9 0.94 0.84 0.53 0.8 0.8
5 0.3 1-4 1.0 1.1 0.59 0.95 0.71 0.35 0.4 0.19
5 0.3 1-4 2.2 1.4 1.6 1.1 0.79 0.69 0.86 0.74 0.19
8 0.3 1-4 1.2 1.0 0.7 0.53 0.45 0.5 0.46 0.20
6 0.4-0.63 2-3 3.3 1.9 1.8 0.91 1.1 0.49
6 0.3 2 0.23 0.21 0.24 0.11
France 1 0.3 1.4 0.9 0.65 0.4 0.1 0.04
1 0.3 1.5 0.44 0.68 0.23 0.06
9 0.07-0.45 2 <0.04 <0.02
7 0.07-0.32 2 0.26 0.10
6 0.37-0.5 2-3 0.06
7 0.03-0.21 2 0.08 0.06
Israel 1 0.25 0.14
1 0.23 0.14
Italy 8 0.03% 1-2 1.3 1.2
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
South Africa 8 0.03% to run off 2 1.2 0.92 0.96
9 0.03% " 2 0.72 1.1 0.49 0.55 0.14
8 0.02% " 2 0.64 0.42 0.31 0.37 0.1
8 0.02-0.03 " 2 5.7 5.5 3.4 2.0
1 0.03% " 5.7 3.5 3.3 2.4 2.0 0.6
1 0.03% " 4.9 2.7 2.8 1.6 1.6 0.41
Spain 6 0.2 2-3 0.14
6 0.2 2-3 0.66 0.14
6 0.2 2-3 1.25 0.11
6 0.2 2-3 0.11
7 0.03 1-3 0.30 0.07
7 0.03 1-3 0.24 0.03
7 0.03 1-3 0.21 0.04
5 0.3 3 0.22
5 0.23 3 0.20
4 0.24 2-4 0.13
4 0.24 3-4 0.06
Initial residues were 0.23-5.7 mg/kg. After two, four and six
weeks, the residues ranged between 0.14-8.0 mg/kg, 0.06-4.4 mg/kg and
0.03-2.6 mg/kg, respectively. The wide range of residue levels is
considered to be a consequence of the various climatic conditions.
Generally, residues are lower in dry and warm areas than in temperate
and more humid areas. Three to four weeks after the last applications,
residues of 0.06-1.3 mg/ kg were observed in warm areas, such as
Australia, Spain and Italy, whereas in Germany 0.34-.6.8 mg/kg were
found after the same interval. No obvious difference in residues were
observed among the various grape varieties. Residues still present in
grapes at harvest were further degraded during the wine-making
process, with residues of <0.02 to 1.2 mg/kg being detected in wine.
Most samples contained less than 0.5 mg/kg.
Pineapple
Two field trials were made in Australia. In the first one,
2 500 l/ha of 0.06% a.i. were applied once as a foliar drench. One
week later, no residues (<0.04 mg/kg) were observed in the flesh
whereas 0.39 mg/kg were detected in the skin. In the second experiment
2 500 l/ha of 0.08% a.i. were applied on the soil six times at
intervals of about four weeks. Ten months later, no residues
(<0.04 mg/kg) were detected either in the flesh or in the skin
(Ciba-Geigy undated).
Cocoa Beans
Field trials were carried out in Papua New Guinea and Cameroon.
The dosages were 0.05-0.5% a.i. (50-500 g a.i./100 l). One to five
applications at intervals of 3 to 4 weeks were made. Details and
results of the trials are reported in Table 9 (Ciba-Geigy undated). In
the raw beans, no measurable residue (<0.02 mg/kg) was observed.
Residues measured in dry and fermented beans ranged from <0.02 to
0.19 mg/kg.
Seed treatment
Residue trials with treated seed were conducted in nine
countries. The dosage ranged from 40 to 620 g a.i./100 kg seed,
depending on the various commodities tested, which were barley, maize,
peas, soybeans, sugarbeet, sunflower and wheat. No residues of either
metalaxyl (<0.02 or <0.04 mg/kg) or the parent compound plus
metabolites (<0.05 mg/kg) were detected in the edible parts of the
crops at harvest.
Soil treatment
Supervised trials were carried out after soil treatment in
avocados, citrus and pepper. Details and results of these residue
trials are reported in Table 10 (Ciba-Geigy undated).
Table 9. Residues of Metalaxyl Following Supervised Trials in Cocoa Beans
Applications Residues (mg/kg) at intervals
Country after last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) Sample 1-3 6-8 13-15 20-22
Papua New Guinea 1 0.075 dry <0.04 <0.04 0.12 <0.04
5 0.5 4 fermented and dry 0.19
Cameroon 1 0.05 raw <0.02 1
2 0.075 3 raw <0.02
1 The peel of the pods contained 0.44-2.1 mg/kg, depending on the state of maturity.
Table 10. Residues of Metalaxyl Following Supevised Trials in Avocado, Citrus and Pepper after Soil Treatment
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Avocado (whole fruit)
(Australia) 1 5.0 <0.05
1 5.0 <0.05
1 5.0 <0.05
(South Africa) 4 2.5 4 <0.05 <0.05
3 2.5 8 <0.05
(U.S.) 1 3 2.5 12 0.53 0.85-1.5
3 5.0 12 2.6 1.2
3 2.5 12 0.18 0.09-0.13 0.11-0.13
3 5.0 12 0.17 0.11
3 2.5 12 0.34 0.63 0.75
3 5.0 12 0.50 0.53
Citrus
(South Africa) 2 2.0 10 0.02 2 <0.02 2
0.19 3 0.21 3
2 2.0 14 <0.02 2 <0.02 2
0.05 3 0.04 3
2.0 10 <0.02 2 <0.02 2
0.14 3 0.16 3
Pepper (Piper nigrum)
(Brazil) 2 1.25 4 0.04
2.5 4 0.66
Table 10. (con't)
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Pepper (Capsicum annuum)
(Bulgaria) 6 1-1.5 3 0.20
6 1.5 3 0.03
4 2.5 3 <0.02
(Italy) 3 1-2 5 0.07
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalent. The concentrations of the parent compound was below 0.05 mg/kg, the limit of determination in each
case.
2 Pulp.
3 Peel.
No residues of metalaxyl (<0.05 mg/kg) were detectable in
avocado fruits one to six weeks after the last application. Residues,
however, were detected with the method used for analysing the parent
compound and the metabolites containing the dimethylaniline moiety.
These residues ranged from 0.11 to 2.6 mg/kg.
After two soil applications (at 2 g a.i./m2), residues
in Valencia oranges were at or below the limit of detection
(<0.02 mg/kg) in the pulp and 0.04-0.21 mg/kg in the peel. The
portion of the peel was 30% of the whole fruit.
In Brazil, residues up to 0.66 mg/kg were detected in
Piper nigrum five weeks after a second soil treatment at
2.5 kg a.i./ha (i.e. 1.5 g/m2).
Residues from <0.02 to 0.2 mg/kg resulted from soil treatments
of Capsicum annuum in Bulgaria and Italy.
Hops
In field trials carried out in the Federal Republic of Germany,
U.S., U.K. and Yugoslavia, metalaxyl was applied to the soil at
0.2-4 g a.i./plant or 0.55-1.1 kg a.i./ha and/or sprayed to run-off as
foliar treatment at 20-30 g a.i./100 l(from -0.24 to 1 kg a.i./ha).
Details and results of the trials are reported in Tables 11 and 12
(Ciba-Geigy undated).
After soil applications residues of metalaxyl were <0.1 to
0.9 mg/kg in dried hops at harvest. After foliar applications residues
of 5-30 mg/kg were found in green hops immediately after treatment.
These residues decreased rapidly and, except in one case, were between
2 and 6 mg/kg in dried hops sampled 7 days after treatment. In beer,
the levels of metalaxyl were below 0.1 mg/kg. No measurable residues
were found in beer when hops had received soil applications only.
Postharvest treatment
Citrus
For controlling postharvest rot in citrus, the fruits are sprayed
or dipped in formulations with or without wax at concentrations of
0.1-0.2% metalaxyl. Supervised residue trials were conducted under
laboratory conditions and in pack-houses. After treatment, the fruits
were stored at about 12°C. Details and results of the supervised
trials are reported in Table 13 (Ciba-Geigy undated; Israel 1980;
Sweden 1980). Analysis of peel and pulp showed that the latter is
largely free of residues (3 samples in 10 trials showed residues
ranging from 0.07-0.2 mg/kg). Residues in the peel generally ranged
Table 11. Residues of Metalaxyl Following Supervised Trials in Hops and Beer After Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application (months)
Rate (a.i. Interval 1-3 3-4 4-5
No. kg/ha or G/plant) (weeks) green dry green dry green dry BEER
Fed.Rep. Germany 7 0.1-0.25 2 0.71
1 0.2 <0.2 <0.02 <0.005
1 0.2 <0.1 <0.1 <0.005
1 0.2 0.18
2 0.2-0.4 8 0.44 0.33
2 0.2-0.4 8 <0.1 <0.1 <0.005
2 0.2-0.4 8 0.12 0.13
2 0.3 8 <0.1 0.25 <0.005
2 0.3 8 <0.1 0.40 <0.005
2 0.3 8 0.23 0.11
1 4.0 0.25 0.18 <0.01
1 4.0 <0.1 0.22 <0.01
1 4.0 0.54 0.93 <0.01
U.S. 1 0.55 <0.05
0.45 1
1 0.55 0.18
1.1 1
1 0.55 0.18 1 1.2 1
1 1.1 0.20 1 3.4 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalents.
Table 12 Residues of Metalaxyl Following Supervised Trials in Hops and Beer after Foliar and Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application(days)
Soil Foliar Interval 0 5 7 14-16 20 28-36
No. (g a.i./plant) (kg a.i./ha) (weeks) green green dried dried dried dried BEER
Fed.Rep. Germany 12 0.55-2.0 1-2 22 6.3 6.3 0.06
12 0.55-2.0 1-2 6.2 5 2.1 <0.05
12 0.55-2.0 1-2 38 5.6 2.1 0.06
12 0.24-0.9 1-2 17.2 3.5 1.8 <0.05
12 0.24-0.9 1-2 5.4 1.5 0.5 <0.05
12 0.24-0.9 1-2 1.6 2.7 1.7 0.05
12 0.24-0.9 1-2 14 1.7 3.4 0.03
12 0.24-0.9 1-2 29 6.8 12 0.08
12 0.24-0.9 1-2 22 4.4 2.2 <0.01
1+12 0.2 and 0.3-1.0 1-2 10 0.5 2.5 0.02
1+12 0.2 and 0.3-1.0 1-2 31 9.1 6.0 0.04
1+12 0.2 and 0.3-1.0 1-2 25.2 3.9 2.7 0.04
1+12 0.2 and 0.3-1.0 1-2 2 3.1 1.8 0.02
U.K. 8 0.15-0.33 2 1.5
8 0.1-0.2 2 0.9
8 0.03% 1-2 1.4
8 0.03% 2 0.3
Yugoslavia 1 0.03% 2.2
2 0.03% 1 1/2 2.5
Table 13. Residues of Metalaxyl Following Supervised Trials in Citrus after Postharvest Treatment
Commodity Application Residues (mg/kg) at
Rate storage intervals (days)
(a.i. Fruit
Method in %) part 0-3 5-7 14-15 28-29 38-44 51-64
Grapefruit Dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.94 0.94 0.96 0.96 0.42
spray 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.30 0.36 0.21 0.20 0.11
spray 1 0.1 pulp 0.12 0.07 0.09 0.15
peel 4.7 0.26 3.9 3.2
spray 1 0.2 pulp ND/ND/ND2
peel 5.4/3.6/
peel 2.4
Orange dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.33 0.27 0.32 0.22 0.16
spray 0.1 pulp <0.05 0.05 <0.05 <0.05 <0.05
peel 0.24 0.31 0.35 0.26 0.17
dip 1 0.2 pulp 0.09
peel 3.5
? 0.2 pulp <0.04
peel 6.9
Tangerine spray 1 0.2 pulp 0.07 0.04
peel 3.9 3.3/4.9
spray 1 0.2 pulp ND/ND/0.2
peel 11.0/12.0/
23.3
spray 1 0.2 whole 2.4
fruit 2.8
3.4
1 With wax.
2 ND = not detectable.
from 0.2 to about 6.9 mg/kg. In one trial, samples of the peel
contained 12 and 23.5 mg/kg. There was no obvious decrease in the
residues of the peel, and therefore no diffusion of the residues from
peel to pulp occurred during storage.
FATE OF RESIDUES
General Observations
The fate of metalaxyl was studied in plants (potato, grapevine,
lettuce) and in animals (rat, goat) using randomly ring 14C-labelled
fungicide. These studies showed the matching primary points of
metabolic attack as follows:
Cleavage of the carboxyester bond to the free acid;
Cleavage of the methylether bond to the free alcohol;
Oxidation of one phenylmethyl group to benzyl alcohol derivative;
Hydroxylation of the phenyl ring to phenolic derivatives.
Reactions subsequent to these primary transformations were found
to be of the following three types:
N-dealkylation to acetanilide derivatives;
Oxidation of the benzyl alcohol derivatives to benzoic acid
derivative;
Conjugation of all types of metabolites to a different extent, in
plants with glucose, in animals with glucuronic acid.
The metabolites identified by chromatography and/or spectroscopy
in plant tissues, urine and faeces are shown in Figures 1 and 2.
Chemical names of metabolites are as follows:
II.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine methyl ester
III.N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-alanine
IV.N-(2-hydroxymethylene-6-methylphenyl)-N-(methoxyacetyl)-alanine
methyl ester
V.N-(2,6-dimethyle-5-hydroxyphenyl)-N-(methoxyacetyl)-alanine methyl
ester
VI.N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)-alanine methyl ester
VII.N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)-alanine
VIII.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine
IX.N-hydroxyacetyl-2,6-dimethyl-aniline
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales, which cause late blight, downy
mildews, damping-off and root, stem and fruit rots of many plants. The
compound is taken up by the roots, leaves, green stems and shoots and
transported acropetally within the plant (Urech et al 1977).
Commercial products first introduced as foliar sprays contained
only metalaxyl as the active ingredient or were mixtures of metalaxyl
with low rates of residual protective fungicides, such as copper or
folpet. Experience has now shown that strains of fungi resistant to
metalaxyl may develop, and in order to reduce the likelihood of this
happening and to broaden the spectrum of activity, commercial products
now contain a higher content of the residual fungicides mancozeb,
zineb, copper, folpet or carbendazim. A further measure to hinder the
development of resistance has been the limitation of the number of
sprays applied to the crop. This has generally been restricted to 3 to
4 applications, which are made during the active vegetative stage of
the crops. Commercial products containing metalaxyl only are intended
for use as seed dressing or for soil applications.
Metalaxyl is registered for use on a wide range of crops and in
several countries in temperate, subtropical and tropical regions.
Preharvest treatments
Metalaxyl is used for foliar sprays, seed dressing and soil
treatments. The main fields of application are in potatoes, grapevines
and vegetables. It is also used against diseases of tobacco and in
some non-edible crops such as ornamentals and turf.
Postharvest treatments
Metalaxyl, incorporated in wax, is applied as a pack-house spray
to citrus fruit for the control of postharvest rot. The recommended
application rates for the crops on which the compound is currently
used are given in Table 1.
Table 1. Recommended Application Rates of Metalaxyl
Crop Rate
(a.i.)
Foliar/Fruit sprays
Potato 150 - 250 g/ha
Onion 200 - 250 g/ha
Leafy vegetables 20 - 25 g/100 1
Brassicae 200 - 250 g/ha
Cucurbits 20 - 25 g/100 1
Tomato 20 - 40 g/100 1
Grape 20 - 30 g/100 1
Pineapple 25 g/100 1
Cocoa 50 - 312 g/100 1
Hops 20 - 30 g/100 1
Seed treatments
Sugarbeet 245 g/100 kg seed
Vegetables 26 - 53 g/100 kg seed
Peas 70 g/100 kg seed
Maize 175 g/100 kg seed
Millet 263 g/100 kg seed
Sorghum 175 g/100 kg seed
Sunflower 210 g/100 kg seed
Soil treatments
Red and green pepper (Capsicum annuum) 2 kg/ha
Black pepper (Piper nigrum) 1.25 - 2 g/m2
Citrus 1.25 - 2 g/m2
Avocado 2- 5 g/m2
Hops 0.2- 4 g/plant
Postharvest treatment
(pack-house spray)
Citrus 2 000 ppm a.i. in wax
1 3-4 applications.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Metalaxyl residue data have been obtained from numerous trials
carried out on a world-wide basis on main crops. Treatments were
generally made using WP or FW formulations with metalaxyl alone or
with mixtures containing residual fungicides. The dosages used in the
residue trials cover the recommendations as summarized in Table 1.
Analysis was carried out using methods determining metalaxyl
alone and/or metalaxyl and its metabolites containing the
2,6-dimethylaniline moiety.
Potatoes
Field trials were conducted in 14 countries during six years. Two
to eight applications of dosages ranging from 0.15 to 0.42 kg a.i./ha
were made at intervals of 1-3 weeks. The results of these trials are
reported in Table 2 (Ciba-Geigy undated; Finland undated). Generally,
no residues either of the parent compound or metabolites containing
the 2,6-dimethylaniline moiety were detectable in potato tubers.
Residues (0.02-0.08 mg/kg) were found in only eight of 102 samples.
The limit of determination ranged from 0.02-0.05 mg/kg.
Onions
Field trials were carried out in nine countries over four years.
Two to eight applications at dosages ranging from 0.22-0.6 kg a.i./ha
were made at intervals of 1-3 weeks. The results are shown in
Table 3 (Ciba-Geigy undated; Finland undated). Initial residues of
0.05-0.59 mg/kg generally dissipated below the limits of determination
(0.02 and 0.04 mg/kg) within 14 days. Dissipation of metalaxyl
metabolites containing the dimethylaniline moiety did not differ
significantly from that of the parent compound alone. Two weeks after
the last application, residues of metalaxyl and its metabolites ranged
between <0.05-0.09 mg/kg. The major part (73-100%) of the total
residue consisted of the parent compound.
Leafy Vegetables
Spinach
Field trials were carried out in Italy, The Netherlands,
Switzerland and the U.S. One to four applications (to run off) at
dosages ranging from 0.22-0.55 kg a.i./ha or at 0.043% were made at
intervals of 1-2 weeks. The results are reported in Table 4
(Ciba-Geigy undated). Residues of the parent compound rapidly
disappeared being at or below the limit of determination (0.05 mg/kg)
two weeks after the last application. Residues measured as the sum of
metalaxyl and its metabolites containing the dimethylaniline moiety
amounted to 1.1-7.3 mg/kg one week after the last application and
decreased to 0.27-5.0 mg/kg during the following two weeks.
Supervised trials were carried out on lettuce at six sites in the
U.K. The plants were treated at planting and after an interval of two
weeks with a formulation containing 48% mancozeb and 10% metalaxyl at
rates of 71-150 g metalaxyl/ha. The residues were below 0.1 mg/kg, the
limit of determination, in 19 samples taken after 13 to 58 days. In
the other samples, the residues were 0.55, 1.2 and 1.3 at day 21, and
0.25 mg/kg at day 35 (U.K.undated).
Table 2. Residues of Metalaxyl Following Supervised Trials in Potatoes (1976-1981)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Argentina 3 0.2-0.25 1-2 0.05
Austria 4 0.2 3 <0.03 <0.03 <0.03 <0.03 <0.03
Canada 4 0.3 2 <0.05
5 0.3 2 <0.05
6 0.25 1-2 <0.05
6 0.15 1-2 <0.05
3 0.3 2 <0.02
3 0.25 2 <0.02
4 0.25 2 <0.02 0.02
6 0.25 1-2 <0.02
6 0.3 1-2 <0.02
3 0.3 3 <0.02 0.02
2 0.3 3 <0.02
2 0.25 3 <0.02
8 0.16 1 <0.02 <0.02 <0.02
4 0.16 2 <0.02 <0.02
4 0.2 2 <0.02 <0.02
6 0.2 1-2 <0.02 <0.02 0.02 0.03
Fed. Rep. Germany 3 0.2-0.3 4 <0.05
3 0.2-0.3 4 <0.05
8 0.2 1 <0.05
8 0.2 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.04
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
Finland 2 0.25 3 <0.04
France 3 0.3 3 <0.04 1
4 0.2 2 <0.04 1
3 0.3 3 <0.02 1
U.K. 6 0.2 1-2 <0.05
5 0.3 3 <0.05 0.05 <0.05 <0.05
4 0.3 3 <0.05
6 0.3 2-3 <0.05
5 0.3 2-3 <0.05
5 0.3 2-3 <0.05
India 4 0.24 2 <0.02
Israel 5 0.25 1-2 <0.02
0.02
South Africa 5 0.2 2-3 0.02 <0.02
Spain 5 0.3 1-2 <0.05
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Sweden 5 0.15 1-2 0.05 <0.05 <0.05
4 0.2 2 <0.02
4 0.2 2-3 <0.02
5 0.15 1-2 <0.02
3 0.25 3 <0.02
Switzerland 5 0.25 2 <0.05 <0.05 <0.05
<0.06
5 0.25 2 <0.05 <0.05 <0.05
3 0.2-0.3 3 <0.05
8 0.25 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
U.S. 3 0.275 2 <0.05 2
6 0.275 2 <0.05
6 0.275 2 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 2 <0.05
6 0.412 1 <0.05
6 0.42 2 0.06 2
0.08 2
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
4 0.42 2 <0.05
5 0.42 2 <0.05
6 0.275 2 <0.05 2
<0.05 2
<0.05 2
6 0.275 2 <0.05 2 <0.05 2
<0.05 2 <0.05 2
<0.05 2 <0.05 2
6 0.22 2 <0.05 2
6 0.22 2 <0.05 2
1 Peel and pulp were analysed separately, no sample contained measurable residues.
2 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 3 Residues of Metalaxyl Following Supervised Trials in Onions (1978-1981)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
Australia 6 0.4 2 <0.04 <0.04
4 0.2 1 0.30 0.12
4 0.3 1 0.59 0.12
Canada 3 0.25 2 <0.02
3 0.24 1-2 0.05 <0.02
4 0.24 1-2 0.31 0.06
3 0.24 1-2 <0.02
5 0.24 2 0.02
4 0.24 2 <0.02
4 0.25 1-2 0.05 0.04
3 0.25 1-2 0.03
Fed.Rep. Germany 3 0.25 2 0.06 <0.02 <0.02
3 0.25 2 0.05 0.02 <0.02
Finland 2 0.25 5 <0.04
4 0.25 1 <0.04
France 3 0.225 3 0.04
U.K. 2 0.5 2 <0.02
Italy 5 0.6 2 0.02
Switzerland 8 0.3 2 <0.05
Table 3 (con't)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
U.S. 1 5 0.275 1-2 0.15
5 0.55 1-2 0.16
5 0.275 1-2 <0.05 <0.05
5 0.55 1-2 0.06 <0.05
5 0.275 1-2 <0.05
5 0.55 1-2 <0.05
5 0.22 2 0.1 0.08
5 0.22 2 0.09 0.08
3 0.275 1-2 0.26
5 0.275 1-2 <0.05 <0.05 <0.05
5 0.55 1-2 <0.05 <0.05 <0.05
5 0.22 2 <0.05 <0.05
6 0.22 2 <0.05 <0.05 <0.05
6 0.22 2 0.08 <0.05 <0.05
5 0.22 2 0.20 0.09 <0.05
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 4 Residues of Metalaxyl Following Supervised Trials in Spinach
Applications Residues (mg/kg) at intervals after
Interval between last application (days)
Country Rate (a.i. applications
No. kg/ha or %) (weeks) 3 6-8 13-15 20-22 27-31
Italy 2 0.043 2 0.05
Netherlands 1 0.25 0.53 0.06 0.03 <0.03
Switzerland 2 0.25 2 0.53 <0.05 <0.05 <0.05 <0.05
U.S. 3 0.275 2 0.06 <0.05
2.9 1 2.2 1 1.0 1
3 0.55 2 0.67 <0.05
4.9 1 3.9 1 2.4 1
3 0.275 2 1.5 1 1.4 1 1.7 1
0.55 2 7.3 1 4.1 1 5.0 1
3 0.275 1 1.2 1 0.25 1 0.27 1
0.55 1 1.1 1 0.79 1 0.86 1
4 0.22 1-2 1.1 1
4 0.22 2 1.8 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Brassicas
Field trials were carried out in broccoli, Brussels sprouts,
cabbage and cauliflower in several countries over a period of five
years. Two to five applications at dosages from 0.2 to 0.55 kg a.i./ha
were made at intervals of 1-3 weeks. The results are reported in
Table 5 (Ciba-Geigy undated). Dissipation of residues was similar
within the various varieties. Initial residues of 0.17-2.0 mg/kg
rapidly decreased and were generally below 0.1 mg/kg after two weeks.
Residues measured as metalaxyl and its metabolites containing the
dimethylaniline moiety were somewhat higher and dissipated more
slowly. The parent compound amounted to 55% of the residue containing
the dimethylaniline moiety. Two weeks after last application of the
recommended dosage of 0.25 kg a.i./ha, residues were <0.05-0.2 mg/kg.
Fruiting vegetables
Field trials were carried out with cucumbers, gherkins, melons,
squash and watermelons in different countries. Three to eight
applications at dosages of 0.2-0.55 kg a.i./ha or 0.02-0.4% were made
at intervals of 1-2 weeks. The results are reported in Table 6
(Ciba-Geigy undated). Residue levels in the different commodities were
in the same range. Initial residues were 0.06-0.4 mg/kg, depending on
the dosage rate used. They decreased slowly and were 0.03-0.23 mg/kg
after two weeks. There was no difference between residue levels
determined as the parent compound or as the total of metalaxyl plus
metabolites.
Tomatoes
Field trials were carried out in seven countries over a period of
six years. Two to nine applications at dosages of 0.125-0.8 kg a.i./ha
or 0.02-0.08% were made at intervals from one to 22 days. The results
are listed in Table 7 (Ciba-Geigy, undated). In most samples, initial
residues were below 0.1 mg/kg. Residue levels of metalaxyl and its
metabolites were at the same magnitude as the parent compound alone.
Grapes
Numerous field trials were conducted over a period of six years
in nine countries representing important vine growing areas of the
world. Due to the different climatic areas, a wide range of
experimental conditions has been considered. One to nine applications
at dosages of 0.01-0.04% a.i. or 0.15-0.5 kg a.i./ha were made at
intervals of 1 to 4 weeks. The results of these trials are reports in
Table 8 (Ciba-Geigy undated).
Table 5. Residues of Metalaxyl Following Supervlsed Trials in Brassicas (1977-1981)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
Canada
(broccoli) 3 0.24 1-2 2.0 0.09 <0.02
3 0.25 1-2 0.56 0.12 0.05
2 0.25 3 0.10
(Brussels
sprouts) 5 0.25 2 0.39 0.12 0.05
(cabbage) 2 0.25 2 0.29 0.08
(cauliflower) 4 0.25 2 0.17 0.03
U.K.
(cauliflower) 2 0.2-0.3 2 0.09 0.02 0.01 0.02
2 0.2-0.3 2 0.09
2 0.2-0.3 2 0.47
South Africa
(brassicas) 5 0.2 1 0.16 <0.05
5 0.4 1 0.90 0.09
U.S.
(broccoli) 5 0.275 2 0.17
0.35 1 0.20 1
5 0.55 2 0.21
0.46 1 0.48 1
Table 5. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
5 0.275 2 0.06 0.06
0.16 1 0.32 1
5 0.55 2 0.17 0.2
0.62 1 0.50 1
0.22 1 0.09 1
5 0.275 2 0.19-0.26
(cabbage) 0.41-0.52 1
5 0.55 2 0.1
0.31 1
5 0.275 2 0.15 1 0.19 1
5 0.55 2 0.35 1 0.26 1
5 0.275 2 0.20 1 0.07 1
5 0.55 2 0.33 1 0.17 1
5 0.275 2 0.12 1 0.10 1
5 0.55 2 0.13 1 0.12 1
5 0.275 2 0.07 0.06
0.22 1 0.09 1
5 0.55 2 0.22 1 0.16 1
5 0.22 2 0.09 1 <0.05 1
5 0.22 2 0.30 1 0.39 1
5 0.22 2 0.15 1 0.14 1
(cauliflower) 5 0.275 2 0.08 1 <0.05 1
5 0.55 2 0.14 1 0.10 1
5 0.275 2 0.17 1 0.36 1
5 0.55 2 0.56 1 0.38 1
5 0.22 2 <0.05 1 <0.05 1
1 The sum of metalaxyl and its metabolites containing the, common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 6. Residues of Metalaxyl Following Supervised Trials in Fruiting Vegetables
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Australia
(gherkins) 7 0.2 1 0.06 <0.04
7 0.4 1 0.12 <0.04
3 0.4 2 0.12 <0.04
(cucumber) 8 0.2 1 0.14 0.09 0.13
8 0.4 1 0.40 0.27 0.24
5 0.2 2 0.14 0.05
Israel
(cucumber) 8 0.25 2 0.25 0.14
(melon) 4 0.25 1 <0.05
South Africa
(cucumber) 4 0.04 2 0.30 0.25 0.22
4 0.02 2 0.26 0.19 0.19
Switzerland
(cucumber) 3 0.25 2 0.09 0.05
3 0.25 2 0.04 0.03
3 0.25 2 0.06 0.03
U.S.
(melon) 8 0.275 1 0.11 0.08
0.17 1 0.24 1
8 0.55 1 0.24 0.25
Table 6. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
(cucumber) 8 0.275 1 0.15 1 0.17 1
8 0.55 1 0.30 1 0.27 1
8 0.22 1 0.13 1
8 0.22 1 0.12 1
(squash) 8 0.22 2 0.07 1
8 0.22 2 0.06
0.07
8 0.22 1 0.06
0.05 1
(watermelon) 8 0.275 1 0.09
0.12 1
8 0.55 1 0.13
0.16 1
8 0.275 1 0.05 1
8 0.55 1 0.05 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6 dimethylaniline,
expressed at metalaxyl equivalents.
Table 7. Residues of Metalaxyl Following Supervised Trials in Tomatoes (1976-1981)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Brazil 7 0.2 0.2 0.5 <0.02
7 0.2 0.024 0.5 <0.02
France 4 0.03 2 <0.04
4 0.045 2 <0.04
2 0.125 0.5 <0.04 <0.04
4 0.5 0.5 2 0.09 0.04 <0.04
4 0.2 2 0.05 <0.04 <0.04 (0.04
Israel 3 0.25 0.04 2 <0.02
South Africa 7 0.4 0.04 1-2 0.04 0.07 <0.03 <0.03
7 0.8 0.08 1-2 0.30 0.10 0.04 <0.03
5 0.08% to 1 <0.03 <0.03 <0.03 <0.03
5 run-off 0.04 1 0.07 0.05 <0.05
Spain 3 0.1% to 2 0.48
run-off (1 day)
Switzerland 9 0.03% to 1-2 0.12 <0.04
run-off
Table 7. (con't)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
U.S. 1 6 0.42 1 <0.05
6 0.42 1 0.18
8 0.42 1 <0.05
6 0.42 1 0.22
6 0.42 1 <0.05
6 0.42 1 0.10
5 0.42 1 0.22
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety
2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 8. Residues of Metalaxyl Following Supervised Trials in Grapes and Wine (1976-1981)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Australia 5 0.02 2 1.2 0.73 0.34
7 0.02 2-4 0.23 0.15 0.14
7 0.03 2-4 0.61 0.37 0.34 0.31
7 0.02 1-4 0.35 0.37 0.43
7 0.03 1-4 0.60 0.49 0.26
Canada 5 0.15 1-2 0.09 0.05
Fed.Rep. 6 0.3 1-2 2.1 3.4 2.2 1.3 1.3
Germany 6 0.4 1-2 2.4 1.4 1.9 1.1 1.4
8 0.2-0.4 1-3 8.0 6.8 4.4 4.2 5.0
8 0.2-0.4 1-3 2.4 2.9 1.9 2.4 1.2
8 0.15-0.4 2 2.1 1.3 1.7 1.7 0.46
6 0.08-0.16 2 0.55 0.4 0.36 0.45 0.15
7 0.12-0.32 2 0.47 0.47 0.49 0.50
7 0.12-0.32 1-2 0.67 0.62 0.51 0.48 0.36 0.37
6 0.24-0.4 2 0.79 0.84 0.53 0.51 0.18
6 0.13-0.54 2-3 2.1 3.5 4.1 2.6 1.2
6 0.3-0.4 1-2 2.9 1.8 2.6 0.13 0.38
8 0.16-0.24 2 0.51 0.34 0.28 0.50
3 0.14-0.3 2 0.2 0.18 0.03
3 0.2 2 0.2 <0.02 <0.02
3 0.2 2 0.15 0.08 0.02
3 0.12-0.36 2-3 0.39 0.23 0.05
3 0.36 1-2 0.02 <0.02
3 0.2-0.3 2 0.13 0.06 0.04
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Switzerland 8 0.02-0.04 2 1.2 0.75 0.04 0.03 0.03 0.02
7 0.01-0.02 2 0.73 0.31 0.74 0.86 0.42 0.09
6 0.45-0.6 2-3 3.2 2.1 1.6 1.6
5 0.02-0.03 3-4 0.84 1.0 1.6 0.58
5 0.02-0.03 3-4 0.16 0.11 0.1 <0.05
7 0.45 2 0.77 0.77 0.70 0.60 0.61 0.26
5 0.4 1-2 2.8 1.6 2.5 2.2 2.6 1.8 2.1 0.3
5 0.4 1-2 2.7 2.7 1.3 1.7 1.6 1.4 0.3
8 0.2-0.4 2 2.9 0.94 0.84 0.53 0.8 0.8
5 0.3 1-4 1.0 1.1 0.59 0.95 0.71 0.35 0.4 0.19
5 0.3 1-4 2.2 1.4 1.6 1.1 0.79 0.69 0.86 0.74 0.19
8 0.3 1-4 1.2 1.0 0.7 0.53 0.45 0.5 0.46 0.20
6 0.4-0.63 2-3 3.3 1.9 1.8 0.91 1.1 0.49
6 0.3 2 0.23 0.21 0.24 0.11
France 1 0.3 1.4 0.9 0.65 0.4 0.1 0.04
1 0.3 1.5 0.44 0.68 0.23 0.06
9 0.07-0.45 2 <0.04 <0.02
7 0.07-0.32 2 0.26 0.10
6 0.37-0.5 2-3 0.06
7 0.03-0.21 2 0.08 0.06
Israel 1 0.25 0.14
1 0.23 0.14
Italy 8 0.03% 1-2 1.3 1.2
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
South Africa 8 0.03% to run off 2 1.2 0.92 0.96
9 0.03% " 2 0.72 1.1 0.49 0.55 0.14
8 0.02% " 2 0.64 0.42 0.31 0.37 0.1
8 0.02-0.03 " 2 5.7 5.5 3.4 2.0
1 0.03% " 5.7 3.5 3.3 2.4 2.0 0.6
1 0.03% " 4.9 2.7 2.8 1.6 1.6 0.41
Spain 6 0.2 2-3 0.14
6 0.2 2-3 0.66 0.14
6 0.2 2-3 1.25 0.11
6 0.2 2-3 0.11
7 0.03 1-3 0.30 0.07
7 0.03 1-3 0.24 0.03
7 0.03 1-3 0.21 0.04
5 0.3 3 0.22
5 0.23 3 0.20
4 0.24 2-4 0.13
4 0.24 3-4 0.06
Initial residues were 0.23-5.7 mg/kg. After two, four and six
weeks, the residues ranged between 0.14-8.0 mg/kg, 0.06-4.4 mg/kg and
0.03-2.6 mg/kg, respectively. The wide range of residue levels is
considered to be a consequence of the various climatic conditions.
Generally, residues are lower in dry and warm areas than in temperate
and more humid areas. Three to four weeks after the last applications,
residues of 0.06-1.3 mg/ kg were observed in warm areas, such as
Australia, Spain and Italy, whereas in Germany 0.34-.6.8 mg/kg were
found after the same interval. No obvious difference in residues were
observed among the various grape varieties. Residues still present in
grapes at harvest were further degraded during the wine-making
process, with residues of <0.02 to 1.2 mg/kg being detected in wine.
Most samples contained less than 0.5 mg/kg.
Pineapple
Two field trials were made in Australia. In the first one,
2 500 l/ha of 0.06% a.i. were applied once as a foliar drench. One
week later, no residues (<0.04 mg/kg) were observed in the flesh
whereas 0.39 mg/kg were detected in the skin. In the second experiment
2 500 l/ha of 0.08% a.i. were applied on the soil six times at
intervals of about four weeks. Ten months later, no residues
(<0.04 mg/kg) were detected either in the flesh or in the skin
(Ciba-Geigy undated).
Cocoa Beans
Field trials were carried out in Papua New Guinea and Cameroon.
The dosages were 0.05-0.5% a.i. (50-500 g a.i./100 l). One to five
applications at intervals of 3 to 4 weeks were made. Details and
results of the trials are reported in Table 9 (Ciba-Geigy undated). In
the raw beans, no measurable residue (<0.02 mg/kg) was observed.
Residues measured in dry and fermented beans ranged from <0.02 to
0.19 mg/kg.
Seed treatment
Residue trials with treated seed were conducted in nine
countries. The dosage ranged from 40 to 620 g a.i./100 kg seed,
depending on the various commodities tested, which were barley, maize,
peas, soybeans, sugarbeet, sunflower and wheat. No residues of either
metalaxyl (<0.02 or <0.04 mg/kg) or the parent compound plus
metabolites (<0.05 mg/kg) were detected in the edible parts of the
crops at harvest.
Soil treatment
Supervised trials were carried out after soil treatment in
avocados, citrus and pepper. Details and results of these residue
trials are reported in Table 10 (Ciba-Geigy undated).
Table 9. Residues of Metalaxyl Following Supervised Trials in Cocoa Beans
Applications Residues (mg/kg) at intervals
Country after last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) Sample 1-3 6-8 13-15 20-22
Papua New Guinea 1 0.075 dry <0.04 <0.04 0.12 <0.04
5 0.5 4 fermented and dry 0.19
Cameroon 1 0.05 raw <0.02 1
2 0.075 3 raw <0.02
1 The peel of the pods contained 0.44-2.1 mg/kg, depending on the state of maturity.
Table 10. Residues of Metalaxyl Following Supevised Trials in Avocado, Citrus and Pepper after Soil Treatment
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Avocado (whole fruit)
(Australia) 1 5.0 <0.05
1 5.0 <0.05
1 5.0 <0.05
(South Africa) 4 2.5 4 <0.05 <0.05
3 2.5 8 <0.05
(U.S.) 1 3 2.5 12 0.53 0.85-1.5
3 5.0 12 2.6 1.2
3 2.5 12 0.18 0.09-0.13 0.11-0.13
3 5.0 12 0.17 0.11
3 2.5 12 0.34 0.63 0.75
3 5.0 12 0.50 0.53
Citrus
(South Africa) 2 2.0 10 0.02 2 <0.02 2
0.19 3 0.21 3
2 2.0 14 <0.02 2 <0.02 2
0.05 3 0.04 3
2.0 10 <0.02 2 <0.02 2
0.14 3 0.16 3
Pepper (Piper nigrum)
(Brazil) 2 1.25 4 0.04
2.5 4 0.66
Table 10. (con't)
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Pepper (Capsicum annuum)
(Bulgaria) 6 1-1.5 3 0.20
6 1.5 3 0.03
4 2.5 3 <0.02
(Italy) 3 1-2 5 0.07
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalent. The concentrations of the parent compound was below 0.05 mg/kg, the limit of determination in each
case.
2 Pulp.
3 Peel.
No residues of metalaxyl (<0.05 mg/kg) were detectable in
avocado fruits one to six weeks after the last application. Residues,
however, were detected with the method used for analysing the parent
compound and the metabolites containing the dimethylaniline moiety.
These residues ranged from 0.11 to 2.6 mg/kg.
After two soil applications (at 2 g a.i./m2), residues
in Valencia oranges were at or below the limit of detection
(<0.02 mg/kg) in the pulp and 0.04-0.21 mg/kg in the peel. The
portion of the peel was 30% of the whole fruit.
In Brazil, residues up to 0.66 mg/kg were detected in
Piper nigrum five weeks after a second soil treatment at
2.5 kg a.i./ha (i.e. 1.5 g/m2).
Residues from <0.02 to 0.2 mg/kg resulted from soil treatments
of Capsicum annuum in Bulgaria and Italy.
Hops
In field trials carried out in the Federal Republic of Germany,
U.S., U.K. and Yugoslavia, metalaxyl was applied to the soil at
0.2-4 g a.i./plant or 0.55-1.1 kg a.i./ha and/or sprayed to run-off as
foliar treatment at 20-30 g a.i./100 l(from -0.24 to 1 kg a.i./ha).
Details and results of the trials are reported in Tables 11 and 12
(Ciba-Geigy undated).
After soil applications residues of metalaxyl were <0.1 to
0.9 mg/kg in dried hops at harvest. After foliar applications residues
of 5-30 mg/kg were found in green hops immediately after treatment.
These residues decreased rapidly and, except in one case, were between
2 and 6 mg/kg in dried hops sampled 7 days after treatment. In beer,
the levels of metalaxyl were below 0.1 mg/kg. No measurable residues
were found in beer when hops had received soil applications only.
Postharvest treatment
Citrus
For controlling postharvest rot in citrus, the fruits are sprayed
or dipped in formulations with or without wax at concentrations of
0.1-0.2% metalaxyl. Supervised residue trials were conducted under
laboratory conditions and in pack-houses. After treatment, the fruits
were stored at about 12°C. Details and results of the supervised
trials are reported in Table 13 (Ciba-Geigy undated; Israel 1980;
Sweden 1980). Analysis of peel and pulp showed that the latter is
largely free of residues (3 samples in 10 trials showed residues
ranging from 0.07-0.2 mg/kg). Residues in the peel generally ranged
Table 11. Residues of Metalaxyl Following Supervised Trials in Hops and Beer After Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application (months)
Rate (a.i. Interval 1-3 3-4 4-5
No. kg/ha or G/plant) (weeks) green dry green dry green dry BEER
Fed.Rep. Germany 7 0.1-0.25 2 0.71
1 0.2 <0.2 <0.02 <0.005
1 0.2 <0.1 <0.1 <0.005
1 0.2 0.18
2 0.2-0.4 8 0.44 0.33
2 0.2-0.4 8 <0.1 <0.1 <0.005
2 0.2-0.4 8 0.12 0.13
2 0.3 8 <0.1 0.25 <0.005
2 0.3 8 <0.1 0.40 <0.005
2 0.3 8 0.23 0.11
1 4.0 0.25 0.18 <0.01
1 4.0 <0.1 0.22 <0.01
1 4.0 0.54 0.93 <0.01
U.S. 1 0.55 <0.05
0.45 1
1 0.55 0.18
1.1 1
1 0.55 0.18 1 1.2 1
1 1.1 0.20 1 3.4 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalents.
Table 12 Residues of Metalaxyl Following Supervised Trials in Hops and Beer after Foliar and Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application(days)
Soil Foliar Interval 0 5 7 14-16 20 28-36
No. (g a.i./plant) (kg a.i./ha) (weeks) green green dried dried dried dried BEER
Fed.Rep. Germany 12 0.55-2.0 1-2 22 6.3 6.3 0.06
12 0.55-2.0 1-2 6.2 5 2.1 <0.05
12 0.55-2.0 1-2 38 5.6 2.1 0.06
12 0.24-0.9 1-2 17.2 3.5 1.8 <0.05
12 0.24-0.9 1-2 5.4 1.5 0.5 <0.05
12 0.24-0.9 1-2 1.6 2.7 1.7 0.05
12 0.24-0.9 1-2 14 1.7 3.4 0.03
12 0.24-0.9 1-2 29 6.8 12 0.08
12 0.24-0.9 1-2 22 4.4 2.2 <0.01
1+12 0.2 and 0.3-1.0 1-2 10 0.5 2.5 0.02
1+12 0.2 and 0.3-1.0 1-2 31 9.1 6.0 0.04
1+12 0.2 and 0.3-1.0 1-2 25.2 3.9 2.7 0.04
1+12 0.2 and 0.3-1.0 1-2 2 3.1 1.8 0.02
U.K. 8 0.15-0.33 2 1.5
8 0.1-0.2 2 0.9
8 0.03% 1-2 1.4
8 0.03% 2 0.3
Yugoslavia 1 0.03% 2.2
2 0.03% 1 1/2 2.5
Table 13. Residues of Metalaxyl Following Supervised Trials in Citrus after Postharvest Treatment
Commodity Application Residues (mg/kg) at
Rate storage intervals (days)
(a.i. Fruit
Method in %) part 0-3 5-7 14-15 28-29 38-44 51-64
Grapefruit Dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.94 0.94 0.96 0.96 0.42
spray 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.30 0.36 0.21 0.20 0.11
spray 1 0.1 pulp 0.12 0.07 0.09 0.15
peel 4.7 0.26 3.9 3.2
spray 1 0.2 pulp ND/ND/ND2
peel 5.4/3.6/
peel 2.4
Orange dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.33 0.27 0.32 0.22 0.16
spray 0.1 pulp <0.05 0.05 <0.05 <0.05 <0.05
peel 0.24 0.31 0.35 0.26 0.17
dip 1 0.2 pulp 0.09
peel 3.5
? 0.2 pulp <0.04
peel 6.9
Tangerine spray 1 0.2 pulp 0.07 0.04
peel 3.9 3.3/4.9
spray 1 0.2 pulp ND/ND/0.2
peel 11.0/12.0/
23.3
spray 1 0.2 whole 2.4
fruit 2.8
3.4
1 With wax.
2 ND = not detectable.
from 0.2 to about 6.9 mg/kg. In one trial, samples of the peel
contained 12 and 23.5 mg/kg. There was no obvious decrease in the
residues of the peel, and therefore no diffusion of the residues from
peel to pulp occurred during storage.
FATE OF RESIDUES
General Observations
The fate of metalaxyl was studied in plants (potato, grapevine,
lettuce) and in animals (rat, goat) using randomly ring 14C-labelled
fungicide. These studies showed the matching primary points of
metabolic attack as follows:
Cleavage of the carboxyester bond to the free acid;
Cleavage of the methylether bond to the free alcohol;
Oxidation of one phenylmethyl group to benzyl alcohol derivative;
Hydroxylation of the phenyl ring to phenolic derivatives.
Reactions subsequent to these primary transformations were found
to be of the following three types:
N-dealkylation to acetanilide derivatives;
Oxidation of the benzyl alcohol derivatives to benzoic acid
derivative;
Conjugation of all types of metabolites to a different extent, in
plants with glucose, in animals with glucuronic acid.
The metabolites identified by chromatography and/or spectroscopy
in plant tissues, urine and faeces are shown in Figures 1 and 2.
Chemical names of metabolites are as follows:
II.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine methyl ester
III.N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-alanine
IV.N-(2-hydroxymethylene-6-methylphenyl)-N-(methoxyacetyl)-alanine
methyl ester
V.N-(2,6-dimethyle-5-hydroxyphenyl)-N-(methoxyacetyl)-alanine methyl
ester
VI.N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)-alanine methyl ester
VII.N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)-alanine
VIII.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine
IX.N-hydroxyacetyl-2,6-dimethyl-aniline
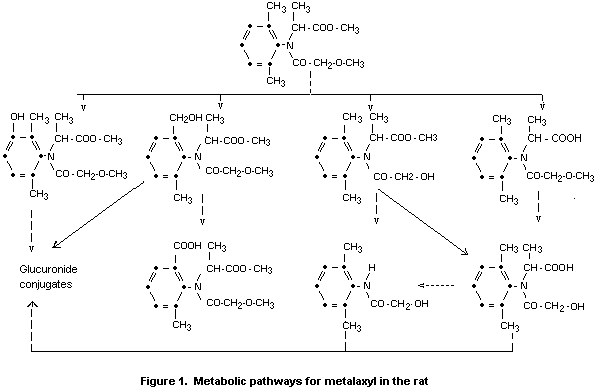
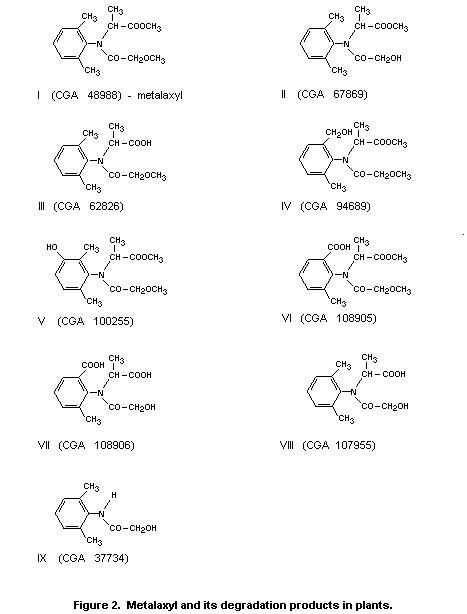 Metabolic pathways are similar in plant and in animals. No
evidence for the presence of 2,6-dimethylaniline in free or conjugated
form was found in either.
In Plants
Potato
Potato plants eight weeks old were sprayed with 14C-ring
labelled metalaxyl until run-off at a rate corresponding to
0.2 kg a.i.(500 l ha.). The treatment was repeated four times at
10-day intervals. In the mature plant, only about 1.5% of the
radioactivity was found, with more than 90% in the shoots. The tubers
contained 0.02 mg/kg metalaxyl equivalents, which indicates some
basipetal translocation.
No parent compound could be found in tubers. Radioactivity
consisted exclusively of polar metabolites. Due to the very low
amount, exact identification was not possible. In the shoots,
compounds II, IV, VI and VII (Figure 2) were identified as the main
metabolites, which is evidence for two independent degradation
pathways utilizing ring methyl oxidation and hydrolysis of the
methylester bond (Gross 1979a). Neither metalaxyl nor its degradation
products were taken up by the tubers directly from soil containing
approximately 0.5 mg/kg metalaxyl equivalents (Gross 1977).
Grape
Grapevines were sprayed six times at 14-day intervals until run-
off with 14C-ring labelled metalaxyl equivalent to 30 g a.i./100 l
water. At the time of harvest, a total of 1.4 mg/kg metalaxyl
equivalents was found in the grapes. The portion of unchanged
metalaxyl was 0.83 mg/kg (60%) (Gross 1979b), In another experiment
with an overdosage (7 treatments, 50 g a.i./100 l) application, the
total radioactivity in the mature grapes corresponded to 3.06 mg/kg
metalaxyl equivalents 52 days after the last application; the portion
of unchanged metalaxyl was 1.96 mg/kg (64%) (Gross 1978). Metabolites
III, IV, V and VIII, were identified by chromatography and
spectroscopy in both grapes and foliage. The presence of these
metabolites is evidence for independent degradation pathways that
utilize ring methyl oxidation, ring hydroxylation and hydrolysis of
the methylester and methylether bonds. All metabolites formed were
found partially conjugated with sugar (Gross 1979b). The non-
extractable radioactivity amounted to 9.4 and 4.2% of total
radioactivity in grapes and leaves, respectively.
Lettuce
Lettuce plants grown in a greenhouse were treated twice with
14C-ring labelled metalaxyl at two-week intervals at a rate
corresponding to 0.25 kg a.i./ha. The radioactivity in the green parts
was analysed two weeks after the second treatment. Less than 20% of
the total radioactivity applied was found in the aerial parts,
primarily in the first leaves directly sprayed with the fungicide;
21.5% of the total radioactivity was characterized as unchanged
metalaxyl, the rest consisted of several polar products (Gross:
1979c). Metabolites isolated from the leaves were identified as III,
IV (5.1%), V (1.6%), VI, VIII and IX. These metabolites were found
partially conjugated with glucose. A significant portion (27.7%) of
the total radioactivity was non-extractable (Gross 1980).
In Animals
A lactating goat was administered ring 14C-labelled metalaxyl by
capsule at a level of 7.00 ppm in the feed for ten consecutive days.
Urine, faeces, milk, volatiles and CO2 were collected daily. The goat
was sacrificed 24 hours after the last dosage. Analytical data on
urine and faeces indicate metalaxyl was metabolized rapidly and the
total amount administered was excreted within approximately 24 hours.
The recovery of the radioactivity applied was 107.22%, with most being
excreted in the urine (93.92%) and faeces (11.60%). A small amount of
radioactivity was found in milk (0.003 mg/kg), blood (0.06%) and
tissues (0.87%). The level of radioactivity expressed in metalaxyl
equivalents was less than 0.06 mg/kg in all tissues; milk, kidney
and liver contained 0.003 mg/kg, 0.019 mg/kg and 0.057 mg/kg,
respectively. The level of radioactivity in the tissues analysed was
<0.06. Neither metalaxyl nor its metabolites accumulated in tissues
of goats.
Two-dimensional TLC comparisons of the metalaxyl metabolites in
goat urine and rat urine revealed the same pattern of products
(conjugated highly polar acidic compounds) and suggest that the same
metabolic pathways described for the rat also take place in the goat
(Fischer et al 1978).
In Processing
Grapes originating from metabolism studies carried out under
field conditions, and containing 1.4 or 3.06 mg/kg 14C-metalaxyl
equivalents, were shredded in a food cutter and the juice was pressed
off. The total residue in the juice was 0.9 or 1.04 mg/kg metalaxyl
equivalents, with the portion of unchanged metalaxyl being ca.
0.5 mg/kg. Most of the radioactivity (approximately 90%) remained in
the presscake (Gross 1978, 1979b). The elimination of metalaxyl
residues during processing from grapes to wine under practical
conditions is shown also in Table 8. These data demonstrate that, on
average, the level of metalaxyl residues in wine is one third of the
original concentration in the grapes at harvest.
Orange fruit treated postharvest with metalaxyl was subjected to
juice extraction by the Brown technique under industrial conditions.
This technique is based on a procedure in which the fruit is sliced in
half the the juice squeezed from the two halves by rotating reamers.
Using this technique, oranges containing 3.5 mg/kg metalaxyl in the
peel and 0.09 mg/kg in the pulp (Ciba-Geigy undated) produced juice
with 0.07 mg/kg metalaxyl.
As shown in Tables 12 and 13, residues present in dried hops
result in negligible residues in beer (generally below 0.05 mg/kg).
These results were to be expected, as residues in dried hops average
2 to 6 mg/kg and the maximum amount of hops used in beer brewing is
400 g/100 l beer.
In Soil
Degradation in soil was studied under laboratory and field
conditions. In laboratory studies using 14C-ring labelled metalaxyl,
a rapid and extensive degradation was observed. In aerobically
incubated soil, the half-life was found to be ca. 40 days. Less than
2% of metalaxyl was present in the soil after one year. Degradation
proceeded mainly via cleavage of the methylester bond yielding the
corresponding acid (compound III, Figure 2) as the transient
degradation product. A pronounced evolution of 14CO2 was observed
(25% within 12 months) indicating mineralization of the fungicide.
Degradation was slower under anaerobic conditions. No degradation was
observed in autoclaved soil, indicating the importance of
microorganisms in the degradation of metalaxyl (Ellgehausen 1978).
In order to follow the dissipation of metalaxyl and its main
metabolite, the free acid (compound III) in soil under field
conditions, the fungicide was applied in a single treatment at
2 kg a.i./ha on plant-free silty and sandy soils in Switzerland
(Büttler 1977a, 1979a). Residues of metalaxyl were also determined
after repeated applications to potatoes at 0.15-0.2 kg a.i./ha. In all
trials sampling was carried out at several intervals after the last
application. Soil samples were taken from different soil layers. The
results are summarized in Table 14 (Büttler 1977a; Ciba-Geigy
undated). Most of the residue is limited to the upper 10 cm soil
layer. Metalaxyl residue alone could be detected in soil only at a
depth of 0-10 cm 180 days after spraying the soil at a rate of
2 kg a.i./ha. Neither the parent compound nor metabolite III were
detectable in samples taken to a depth of 90 cm 314 and 406 days after
application (Büttler 1977b). The metalaxyl residue did not leach
deeper than 40 cm. The main metabolite (III) was observed only in the
0-20 cm soil layer and always in smaller concentrations than the
parent compound.
Table 14. Dissipation of Metalaxyl (M) and Its Metabolite (III) in Soil
Soil Application Soil depth Residues (mg/kg) at interval (days) after
rate (cm) Compound last application
Type (kg a.i./ha) 0-7 14-21 18-31 39-44 50-60 90-122
Sandy loam 2 to plant-free soil 0-10 M 1.8 0.66 0.23
III 0.34 0.25 <0.05
10-20 M 0.21 0.09 0.12
III 0.09 <0.05 <0.05
20-30 M 0.08 <0.05 <0.05
III <0.05 <0.05 <0.05
30-40 M 0.1 <0.05
III <0.05 <0.05
40-60 M <0.05 <0.05
III <0.05 <0.05
Silt loam 8×0.16 to potato M 0.18 0.09
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.21 0.05
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.13 0.09
plants III <0.05 <0.05
Unspecified 5×0.15 to potato 0-20 0.02 0.02
plants
Unspecified 5×0.25 to potato 0-10 M 0.21
plants 10-60 1 M <0.05
1 Residue was measured individually in each 10-cm soil layer.
In Storage
Experiments have shown that samples may be deep-frozen for long
periods without appreciable loss of residues. Residue levels were
determined in lettuce samples fortified with metalaxyl at 5 mg/kg and
field treated samples stored at -20°C for 81-337 days. During this
period 96-114% of the fortified amount and 121-145% of the initial
residue of the field samples (corrected for recoveries) were recovered
(Büttler 1979b). It is presumed that the higher values obtained after
storage are due to loss of water. Studies have also been carried out
on the stability in storage at -15°c using the method for the
determination of the sum of metalaxyl and its metabolites containing
the 2,6-dimethyl-aniline moiety. Cured tobacco and potato samples
fortified with metalaxyl were used. No loss of residues occurred
during a storage period of 18 months. The recoveries averaged 89 + 9%
for tobacco and 101+12% for potatoes (Ross 1980).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
The result of a selective survey on residues in lettuce samples
at commercial outlets in the United Kingdom during 1980/81, was
provided to the Meeting and is summarized below (U.K. undated):
Residue range No. of samples in
(mg/kg) the range
<0.1 90
0.1-0.5 83
0.51-10 18
1.1-2.0 5
>2
METHODS OF RESIDUE ANALYSIS
Several residue methods, all based on gas chromatography, have
been developed for the analysis of agricultural commodities. The
limits of determination are usually 0.02 mg/kg for metalaxyl and
0.05 mg/kg for the sum of metalaxyl and its metabolites containing the
dimethylaniline moiety.
For analysis of the parent compound in vegetables, cereals and
fruits, the sample is extracted with methanol in a high speed
homogenizer. The methanol extract is diluted with water and cleaned up
by water-methanol/dichlormethane partitioning. The dichloromethane
phase is evaporated and the residues of the organic phase is cleaned
up by alumina column chromatography. The appropriate fraction is then
examined for metalaxyl by gas chromatography on 3% Carbowax
20M/GasChrom Q packing at 185°C, using either an alkali flame
ionization detector (AFID) or a Coulson nitrogen specific detector
(Ramsteiner 1976a).
For hops and avocados a more elaborate extraction and clean-up
procedure is necessary. The sample is extracted with acetone, which is
evaporated to dryness. The oily residue is dissolved in methanol and
the interferring co-extractives are precipitated with Celite (R) and
FeCl3/CuSO4 solution. After filtration, the active ingredient is
partitioned into toluene. The toluene phase is evaporated, and the
residue is cleaned up by alumina column chromatography [Ramsteiner
1976B). Final determination is made by gas chromatography using either
AFID or a Hall electrolytic conductivity detector (HECD). The limit of
determination of this method is 0.1 mg/kg except for hops when
detected with AFID, where the limit of determination is 0.2 mg/kg.
The analytical procedure was simplified for wine and beer. The
sample is cleaned up by partition chromatography with n-hexane on an
EXTRELUT(R) column. The eluate is further cleaned up by passage
through an alumina column before the final determination of the active
ingredient by gas chromatography, using a nitrogen-phosphorus flame
ionization detector. The limit of determination of this method in beer
and wine is 0.005 mg/kg (Büttler 1980).
Recovery experiments have shown that the standard method
(Ramsteiner 1976a) recovers 98 ± 12% of the active ingredient at
fortification levels of 0.05 and 0.5 mg/kg. With the method developed
for hops and avocados, recoveries were 83 ± 15% at fortification
levels of 0.2 and 1 mg/kg (Ramsteiner 1976b). In wine and beer
recoveries were 102 ± 8% at fortification levels ranging from
0.01-0.4 mg/kg [Büttler 1980).
Metalaxyl residues can be determined in grapes and vegetables by
applying the method of Ambrus et al (1981). The samples are
extracted with acetone, the extract is diluted with water containing
2% Na2SO4 and partitioned into dichloromethane. Potato and tomato
can be analysed directly using a nitrogen specific detector while
other extracts have to be cleaned up on neutral alumina column
(V. activity grade). Metalaxyl is eluted in the second fraction. The
limit of determination is 0.01-0.02 mg/kg (Hungary 1982).
The method for analysis of metalaxyl and its metabolites
is based on the hydrolysis of all derivatives containing the
2,6-dimethylaniline moiety (2,6-DMA) and final determination of
2,6-DMA. The residues are extracted by blending with 20% water/
methanol. The sample extract is evaporated and refluxed with
phosphoric acid in the presence of cobalt chloride. The solution is
then made basic and the 2,6-dimethylaniline formed is steam distilled.
The product is derivatized with trichloroacetyl chloride to minimize
problems of volatility of 2,6-dimethylaniline. The derivative is
cleaned up by alumina column chromatography and analysed by gas
chromatography, using a nitrogen specific alkali flame ionization
detector. The limit of determination for the method is 0.05 mg/kg
expressed in metalaxyl equivalents. The percent of recovery of
metalaxyl from potato tubers fortified at 0.05 to 1 mg/kg was
72 ± 10%, from cucurbits fortified at 0.05 to 0.4 mg/kg 72 ± 11% and
from cole crops fortified with 0.05 to 0.5 mg/kg 59 ± 11%
(Balasubramanian 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs and preharvest intervals reported in Table 15
have been established on the basis of local conditions.
Table 15. National Maximum Residue Limits Reported to the Meeting
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Australia Grapes 1.0 7
Custard apples, figs,
mangoes, passionfruit 0.5 7
Leafy vegetables 0.3 4
Cucurbits 0.2 4
Pineapples 0.1 28
Vegetables (other than
leafy veg. and cucurbits) 0.1 7
Allium 0.1
Avocados 0.05 7
Austria Hops 2.5 14
Grapes 0.1 21
Czechoslovakia Potatoes 21
Fed. Rep.Germany Potatoes 7
Hops 10
Grapes 35
France Grapes 0.6 15
Hungary Grapes, hops, soya 0.2 21
Italy Grapes 1.0 28
Onion, pepper, spinach,
tomatoes 1.0 10
Potato 1.0
Strawberry 0.5 40
Table 15. (con't)
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Israel Grapes 2.0 21
Citrus 2.0
Cruciferae, tomato 0.3 14
Industrial tomato 0.3 3
Netherlands Citrus 5.0
Cole crops, potato 0.05 28
Lettuce 0.05 28
South Africa Grapes 1.5 21
Potato 0.2 14
Tomatoes 0.1 7
Avocados, cruciferae, citrus 0.05 30
1
Switzerland Grapes 2.0
Wine 0.6
Potato 0.1 14
Onion 0.05 14
U.K. Potato, hops 14
Yugoslavia Hops 7
Grapes 28
1 Last treatment at end of August.
APPRAISAL
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales. It is formulated in combination
with various fungicides, such as mancozeb, zineb, copper, folpet or
carbendazim. In the active ingredient of the commercial products the
weight ratio of metalaxyl varies approximately in the range of 0.4 to
0.1. Metalaxyl is used for foliar sprays, seed dressing and soil
treatment at dosage rates of 150-200 g a.i./ha, 0.26-2.63 g a.i./kg
seed and 1.25-5 g/m2 or 2 kg a.i./ha in soil, respectively.
Residues in crops following foliar and soil treatment are
generally under 1 mg/kg one to three days after last application and
decline below 0.2-0.5 mg/kg one or two weeks thereafter. Lettuce
treated at the recommended rate contained residues up to 2 mg/kg 21
days after last application. The foliar treatments of grapes and hops
result in residues up to 5 and 10 mg/kg, respectively, at 14 days
after application. Crops harvested after seed dressing do not contain
residues above the limit of determination (0.02 mg/kg). Residues in
citrus fruits treated postharvest amounted to ca. 4 mg/kg and were
concentrated mainly in the peel. The residue in the pulp of different
varieties was below 0.2 mg/kg. The data on Brussels sprouts, cocoa
beans, pepper and pineapple were not sufficient to make
recommendations.
Metalaxyl is partly taken up by the roots, leaves, green stems
and shoots and transported acropetally within the plant. The following
reactions were found to be involved in the metabolic transformations:
cleavage of the carboxyester and methylether bonds, oxidation of the
phenylmethyl group and the consequent benzyl alcohol derivative,
hydroxylation of the phenyl ring, N-dealkylation, conjugation with
glucose in plants and with glucuronic acid in animals.
Most of the residue was detected in the leaves and shoots,
in which N-(2,6, dimethyl-phenyl)-N-(metoxyacetyl)-alanine,
N-(2-hydroxymethylene-6-methylphenyl)-N(methxyacetyl)-alanine methyl
ester, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methyl-phenyl)-N-methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)alanine
were identified as the main metabolites.
The parent compound amounted to a minimum of 55% and 60% of the
total residue containing dimethyl-aniline moiety in brassicas and
grapes, respectively, while there was no practical difference between
residue levels determined as parent compound or as the sum of
metalaxyl and its metabolites in fruiting vegetables.
Most of the total residue in spinach consisted of metabolites
containing the 2,6-dimethylanalinine moiety. Total residue levels
ranged from 1.1 mg/kg to 7.3 mg/kg, while the concentration of parent
compound ranged from 0.03 to 0.67 mg/kg in samples taken 6-8 days
after the last application. Based on experimental data, the estimated
maximum residue level, expressed as the total residue or as the parent
compound alone, would be 10 mg/kg or 1 mg/kg, respectively.
A lactating goat kept on a diet containing 7 ppm metalaxyl for
ten consecutive days excreted most of the radioactivity in the urine
and faeces within approximately 24 hours after administration. The
blood and tissues contained 0.06 and 0.87% of the total radioactivity
and 0.003 mg/kg of metalaxyl equivalents was detected in the milk,
Neither metalaxyl nor its metabolites accumulated in the tissues.
No evidence for the presence of 2,6-dimethylaniline in free or
conjugated form was found either in plants or animals. The metabolic
pathways are similar in plants and in animals.
Processing of grapes, oranges and hops treated with metalaxyl
at recommended rates results in detectable residues in the final
products. The wine contains approximately one third of the residue
found in grapes at harvest. The residue in orange pulp (less than 5%
of the residue in the whole fruit) is transferred to the juice, while
beer contains negligible residue (<0.1 mg/kg) compared to the level
in hops. The residue level remains constant in crops stored at or
below -15°C for long periods.
The degradation of metalaxyl in soil is rapid under aerobic
conditions while it is slower under anaerobic conditions and no
degradation was observed in sterilized soil. No residue was detectable
(<0.02-0.05 mg/kg) one year after application under field conditions.
The majority of residues was in the upper 10-cm soil layer. The main
metabolite (N-(2,6-dimethylphenyl)-N-(methoxyacetyl-alanine) was only
observed in the 0.20 cm soil layer and at a distinctly smaller
concentration than the parent compound.
Analytical methods based on methanol or acetone extraction,
water/methanol or dichlormethane partition, cleanup on an alumina
column and detection with nitrogen specific detectors are available
for the determination of the parent compound in various samples. These
methods are suitable for regulatory purposes. Multi-residue methods
based on similar processes seem to be applicable as well for the
determination of metalaxyl. The total residue containing the
2,6-dimethylaniline moiety may be determined after hydrolysis to
dimethylaniline and derivatization.
RECOMMENDATIONS
The Meeting concluded that the levels listed below are suitable
for establishing MRLs. The limits refer to the parent compound.
FURTHER WORK OR INFORMATION
Desirable
Additional residue data derived from supervised trials on
Brussels sprouts, cocoa beans, peppers and pineapple.
Preharvest intervals on
Estimated maximum which recommendations
Commodity residue levels (mg/kg) are based (days)
Hops (dried) 10 14
Citrus fruit 5 postharvest application
Grapes 5 14
Lettuce 2 21
Spinach 1 7
Broccoli, cabbage,cauliflower 0.5 7
Onion 0.05 1 21
Cucumber, gherkins,tomato 0.5 3
Melon, squash, watermelon 0.2 7
Potato 0.1 7
Avocado 0.1 1 7
Cereal grains 0.05 1
Sugarbeet 0.05 1 seed treatment
Sunflower seed 0.05 1
Peas 0.05 1
1 Level at or about the limit of determination.
REFERENCES
Ambrus, A., Lantos, J., Visi, E., Csatlos, I. and Sarvari, L. General
1981 method for the determination of pesticide residues in
samples of plant origin, soil and water. I. Extraction and
cleanup, Journal Assoc. Off. Anal. Chem. 64:733-768.
Balasubramanian, K. Analytical method for the determination of total
undated residues of metalaxyl in crops as 2,6-dimethylaniline.
Report AG-348 from Ciba-Geigy, U.S. (Unpublished).
Büttler, B. CGA 48988 and metabolites in soil. Residue reports RVA
1977a 252/77 A and 253/77 A from Ciba-Geigy, Basle. (Unpublished)
1977b CGA 48988 in soil. Residue report RVA 218/77 from CG, Basle.
(Unpublished)
1979a CGA 48988 and CGA 62826 in soil. Residue reports RVA 273 and
274/79A from Ciba-Geigy, Basle. (Unpublished)
1979b Stability of CGA 48988 in lettuce during frozen storage at
-20°C. Report SPR 17/79 from Ciba-Geigy, Basle.
(Unpublished)
1980 CGA 48988: Gas chromatographic determination of residues in
wine and beer. Report No. REM 1/80 from Ciba-Geigy, Basle.
(Unpublished)
Ciba-Geigy. 299 residue reports. [Unpublished)
undated
Ellgehausen, H. Degradation of CGA 48988 (RIDOMILR) in soil under
1978 aerobic, aerobic/anaerobic and sterile/aerobic conditions.
Report PR 08/78 from Ciba-Geigy, Basle. (Unpublished)
Finland Information on pesticides included in the JMPR priority list.
undated
Fischer, et al. Balance and metabolism of 0-14C-CGA-48988 in a
1978 lactating goat. Report No. 78046 from Ciba-Geigy, U.S.
(Unpublished)
Gross, D. Metabolism of CGA 48988 in field grown potato plants. Report
1977 PR 30/77 from Ciba-Geigy, Basle. (Unpublished)
1978 Metabolism of CGA 48988 in grapevine Report PR 11/78 from
Ciba-Geigy, Basle. (Unpublished)
1979a Identification of metabolites of CGA 48988 (RIDOMILR) in
field grown potato plants Report PR 39/79 from Ciba-Geigy,
Basle. (Unpublished)
1979b Identification of metabolites of CGA 48988 (RIDOMILR) in
grapevine. Report PR 06/79 from Ciba-Geigy, Basle.
(Unpublished)
1979c Fate of CGA 48988 in lettuce. Report PR 38/79 from Ciba-
Geigy, Basle. (Unpublished)
1980 Identification of degradation products of CGA 48988
(RIDOMILR) in lettuce. Report PR 38/80 from Ciba-Geigy,
Basle. (Unpublished)
Hungary Information on pesticides included in the JMPR priority
1982 list.
Israel Residues of Ridomil in citrus fruit. Ministry of
1980 Agriculture, Jerusalem. (Unpublished)
Ramsteiner, K. CGA 48988: Gas chromatographic determination of
1976a residues in soil, vegetables and grapes. Report REM 16/76
from Ciba-Geigy, Basle. (Unpublished)
1976b CGA 48988: Gas chromatographic determination of residues in
hops and tobacco. Report from Ciba-Geigy, Basle.
(Unpublished)
Ross, J.A. Stability of residues of metalaxyl and its metabolites
1980 under freezer storage conditions. Report ABR-80028 from
Ciba-Geigy, U.S. (Unpublished)
Sweden Results of analysis of Ridomil in postharvest treated
1980 oranges, 1980-06-27. Statens Lantbrukskemiska, Upsala.
(Unpublished)
Urech, P.A., Schwinn, F. and Staub, T. CGA 48988, a novel fungicide
1977 for the control of late blight, downy mildews and related
soil-borne diseases. Proceedings of the 1977 British Crop
Protection Conference, p, 623-631.
U.K. Submission to JMPR on metalaxyl (with mancozeb) residues
undated in protected lettuce (prepared by J.W. Edmunds).
Metabolic pathways are similar in plant and in animals. No
evidence for the presence of 2,6-dimethylaniline in free or conjugated
form was found in either.
In Plants
Potato
Potato plants eight weeks old were sprayed with 14C-ring
labelled metalaxyl until run-off at a rate corresponding to
0.2 kg a.i.(500 l ha.). The treatment was repeated four times at
10-day intervals. In the mature plant, only about 1.5% of the
radioactivity was found, with more than 90% in the shoots. The tubers
contained 0.02 mg/kg metalaxyl equivalents, which indicates some
basipetal translocation.
No parent compound could be found in tubers. Radioactivity
consisted exclusively of polar metabolites. Due to the very low
amount, exact identification was not possible. In the shoots,
compounds II, IV, VI and VII (Figure 2) were identified as the main
metabolites, which is evidence for two independent degradation
pathways utilizing ring methyl oxidation and hydrolysis of the
methylester bond (Gross 1979a). Neither metalaxyl nor its degradation
products were taken up by the tubers directly from soil containing
approximately 0.5 mg/kg metalaxyl equivalents (Gross 1977).
Grape
Grapevines were sprayed six times at 14-day intervals until run-
off with 14C-ring labelled metalaxyl equivalent to 30 g a.i./100 l
water. At the time of harvest, a total of 1.4 mg/kg metalaxyl
equivalents was found in the grapes. The portion of unchanged
metalaxyl was 0.83 mg/kg (60%) (Gross 1979b), In another experiment
with an overdosage (7 treatments, 50 g a.i./100 l) application, the
total radioactivity in the mature grapes corresponded to 3.06 mg/kg
metalaxyl equivalents 52 days after the last application; the portion
of unchanged metalaxyl was 1.96 mg/kg (64%) (Gross 1978). Metabolites
III, IV, V and VIII, were identified by chromatography and
spectroscopy in both grapes and foliage. The presence of these
metabolites is evidence for independent degradation pathways that
utilize ring methyl oxidation, ring hydroxylation and hydrolysis of
the methylester and methylether bonds. All metabolites formed were
found partially conjugated with sugar (Gross 1979b). The non-
extractable radioactivity amounted to 9.4 and 4.2% of total
radioactivity in grapes and leaves, respectively.
Lettuce
Lettuce plants grown in a greenhouse were treated twice with
14C-ring labelled metalaxyl at two-week intervals at a rate
corresponding to 0.25 kg a.i./ha. The radioactivity in the green parts
was analysed two weeks after the second treatment. Less than 20% of
the total radioactivity applied was found in the aerial parts,
primarily in the first leaves directly sprayed with the fungicide;
21.5% of the total radioactivity was characterized as unchanged
metalaxyl, the rest consisted of several polar products (Gross:
1979c). Metabolites isolated from the leaves were identified as III,
IV (5.1%), V (1.6%), VI, VIII and IX. These metabolites were found
partially conjugated with glucose. A significant portion (27.7%) of
the total radioactivity was non-extractable (Gross 1980).
In Animals
A lactating goat was administered ring 14C-labelled metalaxyl by
capsule at a level of 7.00 ppm in the feed for ten consecutive days.
Urine, faeces, milk, volatiles and CO2 were collected daily. The goat
was sacrificed 24 hours after the last dosage. Analytical data on
urine and faeces indicate metalaxyl was metabolized rapidly and the
total amount administered was excreted within approximately 24 hours.
The recovery of the radioactivity applied was 107.22%, with most being
excreted in the urine (93.92%) and faeces (11.60%). A small amount of
radioactivity was found in milk (0.003 mg/kg), blood (0.06%) and
tissues (0.87%). The level of radioactivity expressed in metalaxyl
equivalents was less than 0.06 mg/kg in all tissues; milk, kidney
and liver contained 0.003 mg/kg, 0.019 mg/kg and 0.057 mg/kg,
respectively. The level of radioactivity in the tissues analysed was
<0.06. Neither metalaxyl nor its metabolites accumulated in tissues
of goats.
Two-dimensional TLC comparisons of the metalaxyl metabolites in
goat urine and rat urine revealed the same pattern of products
(conjugated highly polar acidic compounds) and suggest that the same
metabolic pathways described for the rat also take place in the goat
(Fischer et al 1978).
In Processing
Grapes originating from metabolism studies carried out under
field conditions, and containing 1.4 or 3.06 mg/kg 14C-metalaxyl
equivalents, were shredded in a food cutter and the juice was pressed
off. The total residue in the juice was 0.9 or 1.04 mg/kg metalaxyl
equivalents, with the portion of unchanged metalaxyl being ca.
0.5 mg/kg. Most of the radioactivity (approximately 90%) remained in
the presscake (Gross 1978, 1979b). The elimination of metalaxyl
residues during processing from grapes to wine under practical
conditions is shown also in Table 8. These data demonstrate that, on
average, the level of metalaxyl residues in wine is one third of the
original concentration in the grapes at harvest.
Orange fruit treated postharvest with metalaxyl was subjected to
juice extraction by the Brown technique under industrial conditions.
This technique is based on a procedure in which the fruit is sliced in
half the the juice squeezed from the two halves by rotating reamers.
Using this technique, oranges containing 3.5 mg/kg metalaxyl in the
peel and 0.09 mg/kg in the pulp (Ciba-Geigy undated) produced juice
with 0.07 mg/kg metalaxyl.
As shown in Tables 12 and 13, residues present in dried hops
result in negligible residues in beer (generally below 0.05 mg/kg).
These results were to be expected, as residues in dried hops average
2 to 6 mg/kg and the maximum amount of hops used in beer brewing is
400 g/100 l beer.
In Soil
Degradation in soil was studied under laboratory and field
conditions. In laboratory studies using 14C-ring labelled metalaxyl,
a rapid and extensive degradation was observed. In aerobically
incubated soil, the half-life was found to be ca. 40 days. Less than
2% of metalaxyl was present in the soil after one year. Degradation
proceeded mainly via cleavage of the methylester bond yielding the
corresponding acid (compound III, Figure 2) as the transient
degradation product. A pronounced evolution of 14CO2 was observed
(25% within 12 months) indicating mineralization of the fungicide.
Degradation was slower under anaerobic conditions. No degradation was
observed in autoclaved soil, indicating the importance of
microorganisms in the degradation of metalaxyl (Ellgehausen 1978).
In order to follow the dissipation of metalaxyl and its main
metabolite, the free acid (compound III) in soil under field
conditions, the fungicide was applied in a single treatment at
2 kg a.i./ha on plant-free silty and sandy soils in Switzerland
(Büttler 1977a, 1979a). Residues of metalaxyl were also determined
after repeated applications to potatoes at 0.15-0.2 kg a.i./ha. In all
trials sampling was carried out at several intervals after the last
application. Soil samples were taken from different soil layers. The
results are summarized in Table 14 (Büttler 1977a; Ciba-Geigy
undated). Most of the residue is limited to the upper 10 cm soil
layer. Metalaxyl residue alone could be detected in soil only at a
depth of 0-10 cm 180 days after spraying the soil at a rate of
2 kg a.i./ha. Neither the parent compound nor metabolite III were
detectable in samples taken to a depth of 90 cm 314 and 406 days after
application (Büttler 1977b). The metalaxyl residue did not leach
deeper than 40 cm. The main metabolite (III) was observed only in the
0-20 cm soil layer and always in smaller concentrations than the
parent compound.
Table 14. Dissipation of Metalaxyl (M) and Its Metabolite (III) in Soil
Soil Application Soil depth Residues (mg/kg) at interval (days) after
rate (cm) Compound last application
Type (kg a.i./ha) 0-7 14-21 18-31 39-44 50-60 90-122
Sandy loam 2 to plant-free soil 0-10 M 1.8 0.66 0.23
III 0.34 0.25 <0.05
10-20 M 0.21 0.09 0.12
III 0.09 <0.05 <0.05
20-30 M 0.08 <0.05 <0.05
III <0.05 <0.05 <0.05
30-40 M 0.1 <0.05
III <0.05 <0.05
40-60 M <0.05 <0.05
III <0.05 <0.05
Silt loam 8×0.16 to potato M 0.18 0.09
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.21 0.05
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.13 0.09
plants III <0.05 <0.05
Unspecified 5×0.15 to potato 0-20 0.02 0.02
plants
Unspecified 5×0.25 to potato 0-10 M 0.21
plants 10-60 1 M <0.05
1 Residue was measured individually in each 10-cm soil layer.
In Storage
Experiments have shown that samples may be deep-frozen for long
periods without appreciable loss of residues. Residue levels were
determined in lettuce samples fortified with metalaxyl at 5 mg/kg and
field treated samples stored at -20°C for 81-337 days. During this
period 96-114% of the fortified amount and 121-145% of the initial
residue of the field samples (corrected for recoveries) were recovered
(Büttler 1979b). It is presumed that the higher values obtained after
storage are due to loss of water. Studies have also been carried out
on the stability in storage at -15°c using the method for the
determination of the sum of metalaxyl and its metabolites containing
the 2,6-dimethyl-aniline moiety. Cured tobacco and potato samples
fortified with metalaxyl were used. No loss of residues occurred
during a storage period of 18 months. The recoveries averaged 89 + 9%
for tobacco and 101+12% for potatoes (Ross 1980).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
The result of a selective survey on residues in lettuce samples
at commercial outlets in the United Kingdom during 1980/81, was
provided to the Meeting and is summarized below (U.K. undated):
Residue range No. of samples in
(mg/kg) the range
<0.1 90
0.1-0.5 83
0.51-10 18
1.1-2.0 5
>2
METHODS OF RESIDUE ANALYSIS
Several residue methods, all based on gas chromatography, have
been developed for the analysis of agricultural commodities. The
limits of determination are usually 0.02 mg/kg for metalaxyl and
0.05 mg/kg for the sum of metalaxyl and its metabolites containing the
dimethylaniline moiety.
For analysis of the parent compound in vegetables, cereals and
fruits, the sample is extracted with methanol in a high speed
homogenizer. The methanol extract is diluted with water and cleaned up
by water-methanol/dichlormethane partitioning. The dichloromethane
phase is evaporated and the residues of the organic phase is cleaned
up by alumina column chromatography. The appropriate fraction is then
examined for metalaxyl by gas chromatography on 3% Carbowax
20M/GasChrom Q packing at 185°C, using either an alkali flame
ionization detector (AFID) or a Coulson nitrogen specific detector
(Ramsteiner 1976a).
For hops and avocados a more elaborate extraction and clean-up
procedure is necessary. The sample is extracted with acetone, which is
evaporated to dryness. The oily residue is dissolved in methanol and
the interferring co-extractives are precipitated with Celite (R) and
FeCl3/CuSO4 solution. After filtration, the active ingredient is
partitioned into toluene. The toluene phase is evaporated, and the
residue is cleaned up by alumina column chromatography [Ramsteiner
1976B). Final determination is made by gas chromatography using either
AFID or a Hall electrolytic conductivity detector (HECD). The limit of
determination of this method is 0.1 mg/kg except for hops when
detected with AFID, where the limit of determination is 0.2 mg/kg.
The analytical procedure was simplified for wine and beer. The
sample is cleaned up by partition chromatography with n-hexane on an
EXTRELUT(R) column. The eluate is further cleaned up by passage
through an alumina column before the final determination of the active
ingredient by gas chromatography, using a nitrogen-phosphorus flame
ionization detector. The limit of determination of this method in beer
and wine is 0.005 mg/kg (Büttler 1980).
Recovery experiments have shown that the standard method
(Ramsteiner 1976a) recovers 98 ± 12% of the active ingredient at
fortification levels of 0.05 and 0.5 mg/kg. With the method developed
for hops and avocados, recoveries were 83 ± 15% at fortification
levels of 0.2 and 1 mg/kg (Ramsteiner 1976b). In wine and beer
recoveries were 102 ± 8% at fortification levels ranging from
0.01-0.4 mg/kg [Büttler 1980).
Metalaxyl residues can be determined in grapes and vegetables by
applying the method of Ambrus et al (1981). The samples are
extracted with acetone, the extract is diluted with water containing
2% Na2SO4 and partitioned into dichloromethane. Potato and tomato
can be analysed directly using a nitrogen specific detector while
other extracts have to be cleaned up on neutral alumina column
(V. activity grade). Metalaxyl is eluted in the second fraction. The
limit of determination is 0.01-0.02 mg/kg (Hungary 1982).
The method for analysis of metalaxyl and its metabolites
is based on the hydrolysis of all derivatives containing the
2,6-dimethylaniline moiety (2,6-DMA) and final determination of
2,6-DMA. The residues are extracted by blending with 20% water/
methanol. The sample extract is evaporated and refluxed with
phosphoric acid in the presence of cobalt chloride. The solution is
then made basic and the 2,6-dimethylaniline formed is steam distilled.
The product is derivatized with trichloroacetyl chloride to minimize
problems of volatility of 2,6-dimethylaniline. The derivative is
cleaned up by alumina column chromatography and analysed by gas
chromatography, using a nitrogen specific alkali flame ionization
detector. The limit of determination for the method is 0.05 mg/kg
expressed in metalaxyl equivalents. The percent of recovery of
metalaxyl from potato tubers fortified at 0.05 to 1 mg/kg was
72 ± 10%, from cucurbits fortified at 0.05 to 0.4 mg/kg 72 ± 11% and
from cole crops fortified with 0.05 to 0.5 mg/kg 59 ± 11%
(Balasubramanian 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs and preharvest intervals reported in Table 15
have been established on the basis of local conditions.
Table 15. National Maximum Residue Limits Reported to the Meeting
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Australia Grapes 1.0 7
Custard apples, figs,
mangoes, passionfruit 0.5 7
Leafy vegetables 0.3 4
Cucurbits 0.2 4
Pineapples 0.1 28
Vegetables (other than
leafy veg. and cucurbits) 0.1 7
Allium 0.1
Avocados 0.05 7
Austria Hops 2.5 14
Grapes 0.1 21
Czechoslovakia Potatoes 21
Fed. Rep.Germany Potatoes 7
Hops 10
Grapes 35
France Grapes 0.6 15
Hungary Grapes, hops, soya 0.2 21
Italy Grapes 1.0 28
Onion, pepper, spinach,
tomatoes 1.0 10
Potato 1.0
Strawberry 0.5 40
Table 15. (con't)
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Israel Grapes 2.0 21
Citrus 2.0
Cruciferae, tomato 0.3 14
Industrial tomato 0.3 3
Netherlands Citrus 5.0
Cole crops, potato 0.05 28
Lettuce 0.05 28
South Africa Grapes 1.5 21
Potato 0.2 14
Tomatoes 0.1 7
Avocados, cruciferae, citrus 0.05 30
1
Switzerland Grapes 2.0
Wine 0.6
Potato 0.1 14
Onion 0.05 14
U.K. Potato, hops 14
Yugoslavia Hops 7
Grapes 28
1 Last treatment at end of August.
APPRAISAL
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales. It is formulated in combination
with various fungicides, such as mancozeb, zineb, copper, folpet or
carbendazim. In the active ingredient of the commercial products the
weight ratio of metalaxyl varies approximately in the range of 0.4 to
0.1. Metalaxyl is used for foliar sprays, seed dressing and soil
treatment at dosage rates of 150-200 g a.i./ha, 0.26-2.63 g a.i./kg
seed and 1.25-5 g/m2 or 2 kg a.i./ha in soil, respectively.
Residues in crops following foliar and soil treatment are
generally under 1 mg/kg one to three days after last application and
decline below 0.2-0.5 mg/kg one or two weeks thereafter. Lettuce
treated at the recommended rate contained residues up to 2 mg/kg 21
days after last application. The foliar treatments of grapes and hops
result in residues up to 5 and 10 mg/kg, respectively, at 14 days
after application. Crops harvested after seed dressing do not contain
residues above the limit of determination (0.02 mg/kg). Residues in
citrus fruits treated postharvest amounted to ca. 4 mg/kg and were
concentrated mainly in the peel. The residue in the pulp of different
varieties was below 0.2 mg/kg. The data on Brussels sprouts, cocoa
beans, pepper and pineapple were not sufficient to make
recommendations.
Metalaxyl is partly taken up by the roots, leaves, green stems
and shoots and transported acropetally within the plant. The following
reactions were found to be involved in the metabolic transformations:
cleavage of the carboxyester and methylether bonds, oxidation of the
phenylmethyl group and the consequent benzyl alcohol derivative,
hydroxylation of the phenyl ring, N-dealkylation, conjugation with
glucose in plants and with glucuronic acid in animals.
Most of the residue was detected in the leaves and shoots,
in which N-(2,6, dimethyl-phenyl)-N-(metoxyacetyl)-alanine,
N-(2-hydroxymethylene-6-methylphenyl)-N(methxyacetyl)-alanine methyl
ester, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methyl-phenyl)-N-methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)alanine
were identified as the main metabolites.
The parent compound amounted to a minimum of 55% and 60% of the
total residue containing dimethyl-aniline moiety in brassicas and
grapes, respectively, while there was no practical difference between
residue levels determined as parent compound or as the sum of
metalaxyl and its metabolites in fruiting vegetables.
Most of the total residue in spinach consisted of metabolites
containing the 2,6-dimethylanalinine moiety. Total residue levels
ranged from 1.1 mg/kg to 7.3 mg/kg, while the concentration of parent
compound ranged from 0.03 to 0.67 mg/kg in samples taken 6-8 days
after the last application. Based on experimental data, the estimated
maximum residue level, expressed as the total residue or as the parent
compound alone, would be 10 mg/kg or 1 mg/kg, respectively.
A lactating goat kept on a diet containing 7 ppm metalaxyl for
ten consecutive days excreted most of the radioactivity in the urine
and faeces within approximately 24 hours after administration. The
blood and tissues contained 0.06 and 0.87% of the total radioactivity
and 0.003 mg/kg of metalaxyl equivalents was detected in the milk,
Neither metalaxyl nor its metabolites accumulated in the tissues.
No evidence for the presence of 2,6-dimethylaniline in free or
conjugated form was found either in plants or animals. The metabolic
pathways are similar in plants and in animals.
Processing of grapes, oranges and hops treated with metalaxyl
at recommended rates results in detectable residues in the final
products. The wine contains approximately one third of the residue
found in grapes at harvest. The residue in orange pulp (less than 5%
of the residue in the whole fruit) is transferred to the juice, while
beer contains negligible residue (<0.1 mg/kg) compared to the level
in hops. The residue level remains constant in crops stored at or
below -15°C for long periods.
The degradation of metalaxyl in soil is rapid under aerobic
conditions while it is slower under anaerobic conditions and no
degradation was observed in sterilized soil. No residue was detectable
(<0.02-0.05 mg/kg) one year after application under field conditions.
The majority of residues was in the upper 10-cm soil layer. The main
metabolite (N-(2,6-dimethylphenyl)-N-(methoxyacetyl-alanine) was only
observed in the 0.20 cm soil layer and at a distinctly smaller
concentration than the parent compound.
Analytical methods based on methanol or acetone extraction,
water/methanol or dichlormethane partition, cleanup on an alumina
column and detection with nitrogen specific detectors are available
for the determination of the parent compound in various samples. These
methods are suitable for regulatory purposes. Multi-residue methods
based on similar processes seem to be applicable as well for the
determination of metalaxyl. The total residue containing the
2,6-dimethylaniline moiety may be determined after hydrolysis to
dimethylaniline and derivatization.
RECOMMENDATIONS
The Meeting concluded that the levels listed below are suitable
for establishing MRLs. The limits refer to the parent compound.
FURTHER WORK OR INFORMATION
Desirable
Additional residue data derived from supervised trials on
Brussels sprouts, cocoa beans, peppers and pineapple.
Preharvest intervals on
Estimated maximum which recommendations
Commodity residue levels (mg/kg) are based (days)
Hops (dried) 10 14
Citrus fruit 5 postharvest application
Grapes 5 14
Lettuce 2 21
Spinach 1 7
Broccoli, cabbage,cauliflower 0.5 7
Onion 0.05 1 21
Cucumber, gherkins,tomato 0.5 3
Melon, squash, watermelon 0.2 7
Potato 0.1 7
Avocado 0.1 1 7
Cereal grains 0.05 1
Sugarbeet 0.05 1 seed treatment
Sunflower seed 0.05 1
Peas 0.05 1
1 Level at or about the limit of determination.
REFERENCES
Ambrus, A., Lantos, J., Visi, E., Csatlos, I. and Sarvari, L. General
1981 method for the determination of pesticide residues in
samples of plant origin, soil and water. I. Extraction and
cleanup, Journal Assoc. Off. Anal. Chem. 64:733-768.
Balasubramanian, K. Analytical method for the determination of total
undated residues of metalaxyl in crops as 2,6-dimethylaniline.
Report AG-348 from Ciba-Geigy, U.S. (Unpublished).
Büttler, B. CGA 48988 and metabolites in soil. Residue reports RVA
1977a 252/77 A and 253/77 A from Ciba-Geigy, Basle. (Unpublished)
1977b CGA 48988 in soil. Residue report RVA 218/77 from CG, Basle.
(Unpublished)
1979a CGA 48988 and CGA 62826 in soil. Residue reports RVA 273 and
274/79A from Ciba-Geigy, Basle. (Unpublished)
1979b Stability of CGA 48988 in lettuce during frozen storage at
-20°C. Report SPR 17/79 from Ciba-Geigy, Basle.
(Unpublished)
1980 CGA 48988: Gas chromatographic determination of residues in
wine and beer. Report No. REM 1/80 from Ciba-Geigy, Basle.
(Unpublished)
Ciba-Geigy. 299 residue reports. [Unpublished)
undated
Ellgehausen, H. Degradation of CGA 48988 (RIDOMILR) in soil under
1978 aerobic, aerobic/anaerobic and sterile/aerobic conditions.
Report PR 08/78 from Ciba-Geigy, Basle. (Unpublished)
Finland Information on pesticides included in the JMPR priority list.
undated
Fischer, et al. Balance and metabolism of 0-14C-CGA-48988 in a
1978 lactating goat. Report No. 78046 from Ciba-Geigy, U.S.
(Unpublished)
Gross, D. Metabolism of CGA 48988 in field grown potato plants. Report
1977 PR 30/77 from Ciba-Geigy, Basle. (Unpublished)
1978 Metabolism of CGA 48988 in grapevine Report PR 11/78 from
Ciba-Geigy, Basle. (Unpublished)
1979a Identification of metabolites of CGA 48988 (RIDOMILR) in
field grown potato plants Report PR 39/79 from Ciba-Geigy,
Basle. (Unpublished)
1979b Identification of metabolites of CGA 48988 (RIDOMILR) in
grapevine. Report PR 06/79 from Ciba-Geigy, Basle.
(Unpublished)
1979c Fate of CGA 48988 in lettuce. Report PR 38/79 from Ciba-
Geigy, Basle. (Unpublished)
1980 Identification of degradation products of CGA 48988
(RIDOMILR) in lettuce. Report PR 38/80 from Ciba-Geigy,
Basle. (Unpublished)
Hungary Information on pesticides included in the JMPR priority
1982 list.
Israel Residues of Ridomil in citrus fruit. Ministry of
1980 Agriculture, Jerusalem. (Unpublished)
Ramsteiner, K. CGA 48988: Gas chromatographic determination of
1976a residues in soil, vegetables and grapes. Report REM 16/76
from Ciba-Geigy, Basle. (Unpublished)
1976b CGA 48988: Gas chromatographic determination of residues in
hops and tobacco. Report from Ciba-Geigy, Basle.
(Unpublished)
Ross, J.A. Stability of residues of metalaxyl and its metabolites
1980 under freezer storage conditions. Report ABR-80028 from
Ciba-Geigy, U.S. (Unpublished)
Sweden Results of analysis of Ridomil in postharvest treated
1980 oranges, 1980-06-27. Statens Lantbrukskemiska, Upsala.
(Unpublished)
Urech, P.A., Schwinn, F. and Staub, T. CGA 48988, a novel fungicide
1977 for the control of late blight, downy mildews and related
soil-borne diseases. Proceedings of the 1977 British Crop
Protection Conference, p, 623-631.
U.K. Submission to JMPR on metalaxyl (with mancozeb) residues
undated in protected lettuce (prepared by J.W. Edmunds).
 Information on identity and properties (pure a.i.)
Molecular weight 279.34
Description white crystals
Density 1.21 g/cm3 at 20°C
Melting point 71.8 - 72.3°C
Vapour pressure 2.2 × 10-6 mm Hg at 20°C
Volatility 3.4 × 10-2 mg/m3 saturated vapour
concentration at 20°C
Solubility 0.71% in water at 20°C, soluble in
methanol,methylene chloride, benzene,
isopropanol and slightly soluble in hexane
Partition coefficient
n/octanol/water log P 1.65
Hydrolysis half-lives at 20°C in buffer solutions were
>200 days at pH 1, 115 days at pH 9, 12 days
at pH 10
Thermal stability stable up to 300°C
Purity of technical active
ingredient >90%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of 14C-metalaxyl was determined in rats by testing
excreta and selected tissue for activity following oral
administration. Male and female rats were dosed with 14C-metalaxyl
at 0.5 or 25 mg/kg. The excreta and expired CO2 were collected for
analysis at 24-hour intervals. After six days, the rats were
sacrificed and the radioactivity in various tissues was measured.
After single oral doses, more than 60% of the administered
radioactivity was excreted within 24 hours, and by the sixth day
almost all of the radioactivity was excreted in the urine (37-63%) and
faeces (35-66%) for both levels. The amount of 14CO2 expired was
insignificant (<0.02%). Total recoveries were between 100% and 104%.
The excretion pattern was influenced more by the sex of the animals
than by the dose level. The females excreted somewhat higher
proportions in the urine while the males showed preferential excretion
via the faeces. Pooled urine (0-24 hours) for males and females was
analysed by thin-layer chromatography (TLC) and numerous metabolites
(primarily polar) were found in urine. No parent metalaxyl was
detected in these samples. The residual radioactivity, determined in
liver, kidney, muscle, blood, fat, brain, heart, carcase, lungs,
spleen, ovary and testes, was very low except in the liver and blood,
which contained from 0.002-0.004 ppm for 0.5 mg/kg dose rate and
0.146-0.255 ppm in liver, fat and blood for a 25 mg/kg dose rate
(Hambock 1977).
Metabolism
Sixteen female rats were dosed orally with 27.9 mg/kg of
14C-metalaxyl and the urine and faeces were collected for 48 hours.
The urine contained 63.5% of the administered dose and the faeces
32.8%. The radioactivity in urine was fractionated, and in 0-48 hour
urine 62.1% of the metabolites were in the form of glucuronic acid
conjugates as shown by aglycon released via p-glucuronidase
incubation. At least ten aglycons were released by enzyme treatment.
Many of the metabolites excreted were in their free forms as well as
their glucuronide conjugates. Findings from this study suggest that
the degradation of metalaxyl in the rat proceeds primarily via (1)
methyl ester hydrolysis, (2) N-dealkylation, (3) methyl ether cleavage
and (4) benzylic methyl oxidation with subsequent formation of
conjugates with glucuronic acid (Hambock 1978).
Further evaluation in female rats using similar oral doses of
14C-metalaxyl (27.8 mg/kg) resulted in 58% of the radioactivity being
excreted in the urine and 32% in the faeces within 48 hours.
Fractionation, TLC and/or spectroscopy demonstrated that degradation
of metalaxyl in the rat proceeds through at least four independent
pathways, as follows: (1) hydrolysis of the methyl ester and methyl
ether groups, (2) oxidation of the 2-(6)-methyl group, (3) oxidation
of the phenyl ring and (4) N-dealkylation and subsequent formation of
glucuronic acid conjugates (Hambock 1981).
A proposed metabolic pathway for the rat is shown in Figure 1.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of 50 rats (25 of each sex/group) were fed metalaxyl in
the diet at concentrations of 0, 50, 250 and 1 250 ppm for 91 days,
after which they were mated to initiate a 2-litter per generation,
3-generation reproduction study. The first litters of each generation
were weaned at 21 days, examined and discarded. Twenty five male and
25 female rates of the F1b litter were mated to produce the F2a and
F2b litters. Twelve male and 12 female rats from the F2b litter were
reared but not mated and sacrificed after 90 days for organ weight
analysis. An additional 12 male and 24 female rats of the F2b were
mated to produce the F3a and F3b litters. The F3b pups (10 males and
10 females) were sacrificed after 21 days post-partum and used in
histopathological evaluations of control and high-dose animals.
Selected F0 (15 each) and F1b (10 each) dams were killed at day 20
of gestation, pups delivered by caesarean section and examined for
effects on late embryonic/foetal development (including visceral and
skeletal evaluations).
Information on identity and properties (pure a.i.)
Molecular weight 279.34
Description white crystals
Density 1.21 g/cm3 at 20°C
Melting point 71.8 - 72.3°C
Vapour pressure 2.2 × 10-6 mm Hg at 20°C
Volatility 3.4 × 10-2 mg/m3 saturated vapour
concentration at 20°C
Solubility 0.71% in water at 20°C, soluble in
methanol,methylene chloride, benzene,
isopropanol and slightly soluble in hexane
Partition coefficient
n/octanol/water log P 1.65
Hydrolysis half-lives at 20°C in buffer solutions were
>200 days at pH 1, 115 days at pH 9, 12 days
at pH 10
Thermal stability stable up to 300°C
Purity of technical active
ingredient >90%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The fate of 14C-metalaxyl was determined in rats by testing
excreta and selected tissue for activity following oral
administration. Male and female rats were dosed with 14C-metalaxyl
at 0.5 or 25 mg/kg. The excreta and expired CO2 were collected for
analysis at 24-hour intervals. After six days, the rats were
sacrificed and the radioactivity in various tissues was measured.
After single oral doses, more than 60% of the administered
radioactivity was excreted within 24 hours, and by the sixth day
almost all of the radioactivity was excreted in the urine (37-63%) and
faeces (35-66%) for both levels. The amount of 14CO2 expired was
insignificant (<0.02%). Total recoveries were between 100% and 104%.
The excretion pattern was influenced more by the sex of the animals
than by the dose level. The females excreted somewhat higher
proportions in the urine while the males showed preferential excretion
via the faeces. Pooled urine (0-24 hours) for males and females was
analysed by thin-layer chromatography (TLC) and numerous metabolites
(primarily polar) were found in urine. No parent metalaxyl was
detected in these samples. The residual radioactivity, determined in
liver, kidney, muscle, blood, fat, brain, heart, carcase, lungs,
spleen, ovary and testes, was very low except in the liver and blood,
which contained from 0.002-0.004 ppm for 0.5 mg/kg dose rate and
0.146-0.255 ppm in liver, fat and blood for a 25 mg/kg dose rate
(Hambock 1977).
Metabolism
Sixteen female rats were dosed orally with 27.9 mg/kg of
14C-metalaxyl and the urine and faeces were collected for 48 hours.
The urine contained 63.5% of the administered dose and the faeces
32.8%. The radioactivity in urine was fractionated, and in 0-48 hour
urine 62.1% of the metabolites were in the form of glucuronic acid
conjugates as shown by aglycon released via p-glucuronidase
incubation. At least ten aglycons were released by enzyme treatment.
Many of the metabolites excreted were in their free forms as well as
their glucuronide conjugates. Findings from this study suggest that
the degradation of metalaxyl in the rat proceeds primarily via (1)
methyl ester hydrolysis, (2) N-dealkylation, (3) methyl ether cleavage
and (4) benzylic methyl oxidation with subsequent formation of
conjugates with glucuronic acid (Hambock 1978).
Further evaluation in female rats using similar oral doses of
14C-metalaxyl (27.8 mg/kg) resulted in 58% of the radioactivity being
excreted in the urine and 32% in the faeces within 48 hours.
Fractionation, TLC and/or spectroscopy demonstrated that degradation
of metalaxyl in the rat proceeds through at least four independent
pathways, as follows: (1) hydrolysis of the methyl ester and methyl
ether groups, (2) oxidation of the 2-(6)-methyl group, (3) oxidation
of the phenyl ring and (4) N-dealkylation and subsequent formation of
glucuronic acid conjugates (Hambock 1981).
A proposed metabolic pathway for the rat is shown in Figure 1.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
Rat
Groups of 50 rats (25 of each sex/group) were fed metalaxyl in
the diet at concentrations of 0, 50, 250 and 1 250 ppm for 91 days,
after which they were mated to initiate a 2-litter per generation,
3-generation reproduction study. The first litters of each generation
were weaned at 21 days, examined and discarded. Twenty five male and
25 female rates of the F1b litter were mated to produce the F2a and
F2b litters. Twelve male and 12 female rats from the F2b litter were
reared but not mated and sacrificed after 90 days for organ weight
analysis. An additional 12 male and 24 female rats of the F2b were
mated to produce the F3a and F3b litters. The F3b pups (10 males and
10 females) were sacrificed after 21 days post-partum and used in
histopathological evaluations of control and high-dose animals.
Selected F0 (15 each) and F1b (10 each) dams were killed at day 20
of gestation, pups delivered by caesarean section and examined for
effects on late embryonic/foetal development (including visceral and
skeletal evaluations).
 Reproduction indices, including mating, fecundity, male and
female fertility, gestation, lactation, pup mortality, litter weights
and mean pup weights, were determined and compared with control
values. All pups were examined for physical abnormalities and
viability.
There were no consistent dose-related effects on adult animals
with regard to mortality, food/water consumption, body/weight gain,
mating performance, pregnancy rate or duration of gestation. The
litter size and litter weight of control group rats in the Fo 2nd
mating were significantly reduced from treatment groups (birth through
day 21 post-partum). Mean pup weights were normal. Litter size and
mean pup weights were reduced at birth for the F1b 1st mating, group
2 animals. Mean pup weight remained significantly reduced through day
21 for these group 2 litters. These effects were not noticed in other
dose groups or other generations and are therefore not considered
compound-related.
There were no dose-related or compound-related effects on organ
weight, organ/body weight ratio or histopathology. The selected
visceral and skeletal examinations of foetuses for assessing
teratogenic effects were within normal variation for the strain/
species studied. There were no differences with respect or any of the
indices observed, and it is considered that metalaxyl, at dietary
dosage levels up to and including 1 250 ppm, caused no adverse
reproduction effects in rats (Cozens et al 1980).
Special Studies on Teratogenicity
Rat
Groups of 25 pregnants rats were administered, via intubation,
dose levels of 0, 20, 60 and 120 mg metalaxyl/kg bw from days 6
through 15 of gestation. On day 21 of gestation, all pregant animals
were killed by cervical dislocation and foetuses delivered by
caesarean section.
Survival was not affected by treatment. The ratios of
implantations and resorptions were comparable among all treatment and
control groups. Sex ratio and number of live young were not affected
by metalaxyl. No malformations were found in the foetuses nor were the
average foetal weights of the treatment groups affected. Delayed
ossification was marginally affected at the high dose within normal
variation based on historical control data. There were no indications
of embryotoxic or teratogenic effects in the rat under the conditions
of the study (Fritz 1978b).
Rabbit
Groups of pregnant Chinchilla rabbits (20 rabbits/group) were
given metalaxyl orally by intubation at dose levels of 0, 5, 10 and
20 mg/kg during days 6 through 18 of gestation. On day 28 of
gestation, all rabbits were sacrificed and examined and the foetuses
delivered by caesarean section. Foetuses were subjected to external
examination followed by inspection for Visceral and skeletal
malformations, anomalies or variants.
Reduced food consumption was observed in the 10 and 20 mg/kg
groups accompanied by slightly reduced body weight gain in dams at the
high dose. The mean number of corpora lutea and/or implantations were
comparable for all groups. Incidences of renal hypoplasia and agenesis
were not dose-related and within the spontaneous rate of historical
untreated animals of the same rabbit population. Metalaxyl did not
adversely influence embryonic or foetal development in Chinchilla
rabbits at levels up to and including 20 mg/kg (Fritz et al 1978).
Special Studies on Mutagenicity
Yeast culture evaluations
The test compound, CGA 48 988, was tested for mutagenic effects
on the D-7 strain of Saccharomyces cerevisiae at five dose levels:
400, 2 000, 4 000, 8 000 and 10 000 µg/ml, with and without microsomal
activation. The test system permitted the detection of mitotic
crossover, mitotic gene conversion and reverse mutation in the yeast
cells D-7 after 2 or 6 hours of treatment with the test compound. The
positive controls were treated with 4-nitroquinoline-N-oxide (without
S-9 mix) and cyclophosphamide (with S-9 mix) in concentrations of
0.25 µg/ml and 50-250 µg/ml, respectively.
In none of the three mutation systems was the incidence of
mutants increased, in comparison with the negative controls, as a
result of treatment of the cells with metalaxyl with or without
microsomal activation. It is concluded that under the conditions of
these experiments no evidence of mutagenic effects was obtained with
metalaxyl (Arni and Muller 1982).
DNA repair
The primary rat hepatocytes prepared from the liver of a male rat
were used for the UDS assay. A series of compartments in pertri dishes
containing gelatinized cover-slips were seeded with 2 × 10-5 cells
per compartment. The cells were allowed to attach to the coverslips
during an attachment period of 1.5-2 hours. The cell cultures attached
to the coverslips were then washed and refed with WME containing the
test compound (16, 80, 400 and 2 000 µg/ml) and 3H-thymidine
(4 µ Ci/ml). Negative controls and positive controls (4NQO and DMN)
were run concurrently with the test compound.
Comparison of the mean number of silver grains per nucleus in the
negative controls and after treatment with metalaxyl revealed no
evidence of induction of DNA damage (Puri and Muller 1982).
Mouse lymphoma assay
Metalaxyl was tested for mutagenic effects on L5178Y/TK+/- mouse
lymphoma cells in vitro. The investigations were performed with
microsomal activation at concentrations of 0.0625, 0.125, 0.25 and
0.50 mg/ml and without microsomal activation at concentrations of
0.125, 0.25, 0.50 and 1.0 mg/ml. The results were expressed in terms
of the number of induced TK-/- mutants/106 surviving cells. The
test substance is considered mutagenic in this system if the colony
count exceeds that of the solvent control by a factor of more than
2.5 at any concentration.
It was concluded that, under the given experimental conditions,
no evidence of mutagenic effects of metalaxyl was observed in this
mammalian forward mutation system (Strasser and Muller 1982).
Dominant lethal studies
Two groups of adult male albino mice, 20 per group, were
administered orally by intubation single doses of 65 and 196 mg/kg of
metalaxyl dissolved in carboxymethyl cellulose. Each male mouse was
mated with two untreated virgin female mice for eight consecutive
weeks to cover the total spermatogenic cycle. Fourteen days from the
mid-week of mating, the females were killed and examined for
pregnancies. Three parameters were used to determine the dominant
lethal effect of the test compound: mating ratio, number of
implantations and embryonic deaths. No evidence of dominant lethal
effect was obtained in the progeny of male mice treated with metalaxyl
(Fritz 1978a).
Special Studies for Carcinogenicity
Mouse
Metalaxyl was administered in the diet to groups of 60 male and
60 female mice (ICI Alderley Park, Swiss) at concentrations of 0, 50,
250 and 1 250 ppm for 104 weeks. Mice were approximately 5-6 weeks of
age when dosing commenced. The percent survival, food/water
consumption and general thriftiness of animals were unaffected by
treatment. A slight decrease in food conversion efficiency,
accompanied by decreased body weight gain, was noticed in high dose
males during weeks 11 through 30. Bone marrow and blood, sampled
throughout the study, were unaffected by treatment.
All animals that died or were sacrificed in extremis were
submitted to gross necropsy and histopathological evaluation for both
non-neoplastic and neoplastic lesions. The lesions observed were
similar among all groups and not related to the administration of
metalaxyl. Metalaxyl was not found to be a carcinogen in this strain
of mouse (McSheehy et al 1980).
Special Study on Skin Sensitization
Guinea pig
Groups of 10 male and 10 female guinea pigs (Pirbright, albino)
were subjected to a series of 0.1 ml intracutaneous injections of
metalaxyl as a 0.1% suspension in polyethylene glycol and saline,
following the optimization test of Maurer et al (1975). DNCB served
as a positive control. Metalaxyl was not sensitizing under the
conditions of the test (Sachsse and Ullmann 1976h).
Special Study on Eye Irritation
Rabbit
Approximately 100 mg of metalaxyl was placed in the conjunctival
sac of 3 male and 3 female rabbits. The eyes of 3 of the 6 rabbits
were rinsed approximately 30 seconds after instillation of the test
material. There was no irritation present in any of the rinsed eyes.
Unwashed eyes presented irritation of the conjunctiva and cornea for 3
days and were clear by day 4. While the degree of corneal involvement
appears minimal, it could not be subjectively evaluated since only
scores were presented. Rinsing the eyes prevented any irritation
effects, suggesting that the irritation in unwashed eyes may have been
more related to the physical nature of the particles than to the
chemical itself (Sachsse and Ullman 1976g).
Special Study on Dermal Irritation
Rabbit
The primary irritation was determined following the application
of 0.5g of metalaxyl to both abraded and intact skin sites on the
shaved back of 3 male and 3 female rabbits. There was no irritation on
any of the intact skin sites, while minimum erythema was observed on
the abraded skin sites of 2 rabbits 24 hours after application. The
primary irritation index was reportedly 0.1/8 (Sachsse and Ullmann
1976f).
Acute Toxicity
In acute toxicity studies carries out in animals (Table 1), toxic
signs of poisoning occurred within 2 hours after treatment and most
deaths within the first 24 hours. Symptoms observed were non-specific
in the various mammalian species and consisted of sedation, dyspnoea,
exophthalmos, tonic-clonic muscle spasms, curved or ventral position
and ruffled fur.
Metalaxyl was not toxic by the dermal route, as both rats and
rabbits tolerated doses from 3 100 to 6 000 mg/kg without visible
symptoms or effects. No mortalities were reported in either species.
Table 1. Acute Toxicity of Metalaxyl in Animals
Species Sex Route LD50 Reference
Rat m + f oral 1 669 Sachsse and Bathe, 1976a
(515-868)
Mouse m + f oral 1 788 Sachsse and Bathe, 1976b
(626-991)
Rabbit m + f oral 1 697 Sachsse and Ullman,1976e
(506-961)
Hamster m + f oral 7120 Thomann and Pericin,1977
(5250-9660)
Rat m + f dermal 1 >3 100 Sachsse and Bathe, 1976c
Rabbit m + f dermal 2 >6 000 Sachsse and Ullmann, 1978
Rat m + f i.p. 1 312 Sachsse and Bathe, 1976d
(282-345)
1 Carboxy methyl cellulose was the vehicle;
2 Polyethylene glycol was the vehicle.
Short-Term Studies
Rabbit-dermal
Metalaxyl was administered to the occluded intact and abraded
skin of albino rabbits (10 males and 10 females/group) at doses of 0,
10, 100 and 1 000 mg/kg. Test substance was applied once each day, 5
days/week for 3 consecutive weeks. Information was obtained on body
weight, food consumption, haematology, clinical chemistry, absolute
and relative organ weights and local dermal effects on the skin. All
animals were subjected to gross necropsy and histopathological
examination. There were no adverse effects related to compound
administration for any of the parameters evaluated. Several lesions
reported in both treatment and control groups were related to either
bacterial Staphylococcus aureus and epidermitis) or parasitic
(encephalitozoon, Eimeria stiedae) infections, which obscured
interpretation of results (Calkins et al 1980).
Rat - dietary
Daily oral doses of metalaxyl were administered by gavage to
groups of rats (10 males and 10 females/group) initially at levels of
0, 10, 30 and 100 mg/kg for the first 14 days. Dosing was increased to
0, 30, 100 and 300 mg/kg, respectively, for days 15-21 of treatment.
Once again, on day 22, dose levels were increased to 0, 60, 200 and
600 mg/kg, respectively, for the duration of the study, which was
terminated on day 28. No deaths occurred and food consumption, body
weight gain and mean food consumption were comparable among treated
and control groups. Haematology, blood chemistry and urinalysis were
unremarkable. Male rats in the high dose group demonstrated a
significant increase in absolute testes weight as well as a dose-
related increase in relative liver weight, for which the trend was
significant (p<0.01). The absolute and relative adrenal weights were
significantly increased (p<0.05) for females in the high dose group.
Female rats in all treatment groups administered metalaxyl had
significantly increased absolute and relative liver weights (p<0.05),
which were dose-related and for which the positive trend was similarly
significant (p<0.01) (Sachsse et al 1979).
In a 3-month dietary study using Sprague-Dawley rats, 180 rats
were allocated to two groups of 50 animals each (25 males and 25
females) and two groups of 40 animals each (20 males and 20 females).
Dose levels were 0, 50, 250 and 1 250 ppm in the diet. Five males and
five females from the control and high dose level groups were retained
for an additional 28 days without treatment for evaluation of recovery
and reversibility of effects. Minimal cellular hypertrophy of hepatic
parenchymal cells was observed in five females receiving the highest
dose. This trend was not noticeable in the recovery animals,
representing a possible reversibility for this effect. No other
changes attributed to treatment were observed and dose levels of
250 ppm or less are considered to be without adverse effects under the
conditions of this study (Drake 1977).
In a follow-up 90-day study, Gfeller et al (1980) evaluated the
effects of 0, 10, 50, 250 and 1 250 ppm of metalaxyl incorporated in
the diet of rats (RAIF, SPF). There were 20 males and 20 females in
each experimental group. Mortality and other cageside observations
were conducted daily, body weight and food consumption determined
weekly and clinical chemistries, urinalysis and haematology performed
periodically throughout the study. At the conclusion of the study, all
test and control animals were subjected to gross and histopathological
examination and selected organs were weighed.
A dose-related decrease in total leucocyte count was evident in
high dose males, as well as increased absolute and relative adrenal
weights for male rats in the 50, 250 and 1 250 ppm groups. In females,
the relative adrenal weights were increased in the high dose group
only. There was a significant increase in relative liver weights for
females in the 250 and 1-250 ppm dose groups. However, the absolute
liver weights for high dose females were not significantly different
from controls and, therefore, the difference reported for the relative
liver weights is considered as being related to the decrease in the
body weight of the high dose compared to control females, rather than
as an increase in organ weight, per se. Gross necropsy and
histopathological examinations revealed no differences between treated
and control animals. There were no structural alterations in the
adrenals reflective of the increase in both absolute and relative
weights for this organ. The level of 10 ppm of metalaxyl in the diet
of rats is considered to be without adverse effects (Gfeller
et al 1980).
Dog - dietary
Four groups of beagle dogs, 6-9 months of age, were administered
metalaxyl in their diets at levels of 0, 50, 250 and 1 250 ppm for
91 days. Control and high dose groups contained 4 males and 4 females
each, with the low and intermediate dose groups containing 3 males and
3 females each. No mortality or changes in the behaviour of the
animals were observed. Urinalysis, ophthalmoscopy, body weight, food
consumption and haematology indicated no treatment-related findings.
Blood chemistry parameters were normal, except for a significant
increase in serum alkaline phosphatase levels in high-dose males and
females, which was also time-related. Determination of SAP in two
recovery animals from the high dose group demonstrated a reversal to
normal within 4 weeks post-treatment. Macroscopic and microscopic
examinations revealed no compound-related findings. The no-effect
level was estimated to be 250 ppm (Finn et al 1977).
Technical metalaxyl was administered in the diet to groups of
beagle dogs at levels of 0, 50, 250 and 1 000 ppm for a period lasting
six months. There were 8 males and 8 females in both the control and
high dose groups, with 6 males and 6 females in each of the other two
groups. Pups were 6-8 months old at the beginning of study. Feed
consumption, body weights and urinalysis, performed periodically
throughout the study, were normal. Ophthalmic examinations did not
reveal any effects related to treatment. Clinical chemistry parameters
were all within normal variation, except for alkaline phosphatase
levels for 1 000 ppm dose-level males and females, which were
significantly increased (p<0.05) compared to control animals. A
recovery to normal was observed after dosing was stopped. Haemoglobin,
erythrocyte count and hematocrit, while significantly lower (p<0.05)
for high dose males when compared to control males in this study, were
all within normal variation when compared to historical controls.
Anaemia was not evident and bone marrow samples reportedly were
normal: however, reticulocytes and m/e ratios were not determined. The
liver/brain ratios of females in the high dose group were
significantly increased (p <0.05) compared with controls. Similarly,
a positive trend for both increased absolute and relative liver
weights was apparent in male and female dogs receiving an increased
dosage. Alkaline phosphatase is produced in many organs, with the
liver being a primary supplier. The possibility of a dose response for
alkaline phosphatase relative to the liver is supported by the trend
toward increasing liver weights with increased dosage. No such
trends, however, were seen in other enzymes also produced in the liver
(e.g. SGPT, SGOT), and no gross pathological or histopathological
changes were observed in the liver of any dogs fed metalaxyl. There
were no other effects related to treatment and a no-effect level of
250 ppm was determined. Mean daily feed consumption data indicate this
dietary level is approximately equivalent to 7.4 mg/kg/day, with
1 000 ppm corresponding to 31 and 33 mg/kg/day for males and females,
respectively (de Ward et al 1981).
Long-Term Study
Mice
(see Special Study for Carcinogenicity)
Rat
Groups of rats (80 male and 80 female Sprague-Dawley albino
rats/group) were administered metalaxyl in the diet for 104 weeks at
dosage levels of 0, 50, 250 and 1 250 ppm. Growth, as observed by body
weight changes and food consumption data, was recorded weekly for the
first 13 weeks and monthly thereafter. Rats were inspected twice daily
for behavioural changes, general health, mortality and tumour
development. At periodic intervals over the course of the study,
haematologic, clinical chemistry and urinalysis examinations were
performed.
All animals, including those dying or sacrificed, were subjected
to both gross necropsy and microscopic examinations after 55 and 105
weeks of treatment.
No effects considered to be treatment-related were noted with
respect to toxic signs, mortality, body weight, food consumption,
urinalysis, ophthalmologic examination and haematology. Survival was
greater than 50% for both sexes of all groups at 18 months. Activities
of SGPT and SGOT decreased in the 250 and 1 250 ppm females during the
first year, but recovered to normal values, comparable to the control
group, through the second year to termination of the study.
At gross necropsy, organ weight determinations demonstrated
significantly increased (p <0.01) relative liver weights for females
in the 1 250 ppm group after both 55 and 105 weeks of treatment.
Relative liver weights for males were significantly increased
(p <0.01 and <0.001) in both the 250 and 1 250 ppm groups,
respectively, after 105 weeks. These organ to body weight differences
were not accompanied by any pathological findings in the treated
animals other than slight periacinar hepatocytic vacuolation and
chronic inflammation in 1 250 ppm males.
There was a reported pathological finding of testicular atophy
(p <0.05) occurring primarily in the first 53 weeks at all treatment
levels, but the respective absolute and relative testicular weights
were comparable to those of the control group. Since there was no
positive trend with dose for this response and no impairment of
reproductive performance (Cozens et al 1980), this effect is not
considered attributable to metalaxyl. The findings of parafollicular
cell adenomas of the thyroid was significantly increased for the
250 ppm females. However, there was no dose-response relationship and
the incidence was not significantly different from historical control
data for this pathological finding at the same laboratory.
Metalaxyl is considered to be without adverse effects on rats at
levels up to and including 50 ppm in the diet. There was no evidence
of carcinogenic potential at any level tested (Ashby et al 1980).
COMMENTS
Metalaxyl, when fed to rats, is rapidly metabolized and excreted
in the urine and faeces. The metabolism has been relatively well
defined in rats and no specific organ accumulation appears to be
found. The excretion pattern is not influenced by the dose level, but
by the sex of the animals, as females demonstrate preferential
excretion via urine, while males excrete higher proportions in the
faeces.
The acute toxicity in various animals suggests a moderate acute
hazard via the oral route, but little dermal absorption occurs, as
demonstrated in both acute and subchronic dermal studies. Metalaxyl is
mildly irritating to the skin and eyes but did not elicit contact
allergenicity in a skin-sensitization study using guinea pigs.
Metalaxyl did not produce any adverse effects on reproductive
performance or pup viability at levels up to and including 1 250 ppm
in the diet. There were no indications of embryotoxic or teratogenic
effects in either rats or rabbits at levels of 120 or 20 mg/kg bw,
respectively.
Metalaxyl was evaluated in a battery of mutagenicity tests
including yeast culture, DNA repair, mouse lymphoma and dominant
lethal tests. No evidence of mutagenic potential was determined in any
of these tests.
In both short- and long-term studies, the only significant
findings were increased relative liver and adrenal weights, sometimes
accompanied by increased enzyme activities (i.e. SAP, SGPT), but for
which there was no corresponding histopathology. The adrenal weight
increase noted in the rat short-term study was not apparent in the rat
long-term study at comparable dietary levels nor in the short-term dog
study or mouse carcinogenicity study. Metalaxyl was not demonstrated
to be carcinogenic in either rats or mice. Based upon all available
data, no-effect levels in mammalian species can be determined and an
ADI for man allocated for metalaxyl.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mice : 1 250 ppm in the diet, equivalent to 187.5 mg/kg bw
Rat : 50 ppm in the diet, equivalent to 2.5 mg/kg bw
Dog : 250 ppm in the diet, equivalent to 7.4 mg/kg bw
Estimate of Acceptable Daily Intake for Man
0 - 0.03 mg/kg body weight
FURTHER WORK OR INFORMATION
Desirable
1. Metabolic studies in a non-rodent species.
2. Observations and information in humans.
REFERENCES
Arni, P. and Muller, D. Saccharomyces cerevisiae D-7/mammalian-
1982 microsome mutagenicity test in vitro with CGA 48988 (test
for mutagenic properties in yeast cells). Report from Ciba-
Geigy Ltd., Basle, Switzerland, submitted to the World
Health Organization by Ciba-Geigy Ltd. (Unpublished)
Ashby, R., Bhatt, A., Chapman, E., Hepworth, P.L. and Whitney, J.C.
1980 CGA 48988: Toxicity and oncogenicity in dietary
administration to rats for two years. Final report no.
80/CIA009/315 from Life Science Research, Stock, Essex CM4
0PE, England, submitted from Ciba-Geigy, Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Calkins, J.E., Morgan, J.M., Casey, H.W. and Page, J.G. A 21-day
1980 subacute dermal toxicity study in albino rabbits with CGA
48988. Report from Toxigenics Inc. Decator, Ill. 62526, USA,
submitted by Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Cozens, D.D., Allen, P.A., Clark, R., Offer, J.M., Gregson, R.L. and
1980 Gibson, W.A. Effect of CGA 48988 on reproductive function of
multiple generations in the rat. Report from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England,
submitted from Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Drake, J.C. 3-month dietary study in rats with compound CGA 48988.
1977 Report from Geigy Pharmaceuticals, Stamford Lodge, Wilmslow,
Chershire, U.K. submitted from Ciba-Geigy Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Finn, J.P., Briggs, K., Close, J.E., Noble, D.N., Barrett, R. and
1977 Rider, L. CGA 48988, 91-day dietary toxicity study in beagle
dogs. Report from Hazleton Laboratories Europe Ltd.,
Harrogate, HGe 1PY, England, submitted from Ciba-Geigy Ltd.,
Basle, Switzerland, to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Fritz, H. Dominant lethal study-CGA 48988 techn. mouse (Test for
1978a cytotoxic or mutagenic effects on male germinal cells).
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
1978b Reproduction study-CGA 48988 tech. Rat Seg. II (Test for
teratogenic or embryotoxic effects). Report from Ciba-Geigy
Ltd., Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Fritz, H., Becker, H. and Hess, R. Reproduction study - rabbit- CGA
1978 48988 tech. Seg. II (Test for teratogenic or embryotoxic
effects). Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Gfeller, W., Basler, W., Zak, F., Grieve, A.P. and Hess, R. CGA 48988
1980 techn. 3-month toxicity study on rats (Project No. 79 18 11)
Final Report. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Hambock, H. Distribution, degradation and excretion of CGA 48988 in
1977 the rat. Project Report 18/77 from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Metabolism of CGA 48988 in the rat. Project report 26/78
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1981 Metabolic pathways of CGA 48988 in the rat. Project Report
31/81 from Ciba-Geigy Ltd., Basle, Switzerland, submitted to
the World Health Organization by Ciba-Geigy, Ltd.
(Unpublished)
Maurer, T., Thomann, P., Weirich, E.G. and Hess, R. The optimization
1975 test in the guinea pig. A method for the predictive
evaluation of the contact allergenicity of chemicals. Agents
and Actions 5 (2): 174-179.
McSheehy, T.W., Macrae, S.M. and Whitney, J.C. CGA 48988 oncogenicity
1980 in dietary administration to mice for two years. Final
Report No. 80/CIA 008/442 from Life Science Research, Stock,
Essex CM4 9PE, England, submitted from Ciba-Geigy Ltd.
Basle, Switzerland to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Puri, E. and Muller, D. Audioradiographic DNA repair test on rat
1982 hepatocytes CGA 48988 (in vitro test for DNA-damaging
properties). Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy, Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute oral LD50 in the rat of technical CGA
1976a 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute oral LD50 in the mouse of technical CGA 48988. Report from Ciba
1976b Geigy Ltd., Basle, Switzerland submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute dermal LD50 in the rat of technical
1976c CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute intraperitoneal LD50 in the rat of technical CGA 48988. Report
1976d from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Ullmann, L. Acute oral LD50 in the rabbit of technical
1976e CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organziation by Ciba-Geigy
Ltd. (Unpublished)
1976f Skin irritation in the rabbit after single application of
technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1976g Eye irritation in the rabbit of technical CGA 48988. Report
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1976h Skin sensitizing (contact allergenic) effect in guinea pigs
of technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Acute dermal LD50 in the rabbit of technical CGA 48988.
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
Sachsse, K., Suter, P. and Luetkemeier, H. CGA 48988 techn., 28 days
1979 toxicity study on rats. Final report from Ciba-Geigy Ltd.,
Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Strasser, F.F. and Muller, D. L 5178 Y/TK+/- mouse lymphoma
1982 mutagenicity test CGA 48988. (in vitro test for mutagenic
properties of chemical substances in mammalian cells. Report
from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Thomann, P. and Pericin, C. Acute oral LD50 in the Chinese hamster of
1977 CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
de Ward, J., Beck, L. St., Kitchen, D.N. and Hepler, D.I. Six-month
1981 chronic oral toxicity study with CGA 48988 technical in
beagle dogs. Project No. 1545. Report from ELARS Bioresearch
Laboratories, Fort Collins, Colorado, USA, submitted from
Ciba-Geigy Ltd., Basle, Switzerland to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
METALAXYL
Reproduction indices, including mating, fecundity, male and
female fertility, gestation, lactation, pup mortality, litter weights
and mean pup weights, were determined and compared with control
values. All pups were examined for physical abnormalities and
viability.
There were no consistent dose-related effects on adult animals
with regard to mortality, food/water consumption, body/weight gain,
mating performance, pregnancy rate or duration of gestation. The
litter size and litter weight of control group rats in the Fo 2nd
mating were significantly reduced from treatment groups (birth through
day 21 post-partum). Mean pup weights were normal. Litter size and
mean pup weights were reduced at birth for the F1b 1st mating, group
2 animals. Mean pup weight remained significantly reduced through day
21 for these group 2 litters. These effects were not noticed in other
dose groups or other generations and are therefore not considered
compound-related.
There were no dose-related or compound-related effects on organ
weight, organ/body weight ratio or histopathology. The selected
visceral and skeletal examinations of foetuses for assessing
teratogenic effects were within normal variation for the strain/
species studied. There were no differences with respect or any of the
indices observed, and it is considered that metalaxyl, at dietary
dosage levels up to and including 1 250 ppm, caused no adverse
reproduction effects in rats (Cozens et al 1980).
Special Studies on Teratogenicity
Rat
Groups of 25 pregnants rats were administered, via intubation,
dose levels of 0, 20, 60 and 120 mg metalaxyl/kg bw from days 6
through 15 of gestation. On day 21 of gestation, all pregant animals
were killed by cervical dislocation and foetuses delivered by
caesarean section.
Survival was not affected by treatment. The ratios of
implantations and resorptions were comparable among all treatment and
control groups. Sex ratio and number of live young were not affected
by metalaxyl. No malformations were found in the foetuses nor were the
average foetal weights of the treatment groups affected. Delayed
ossification was marginally affected at the high dose within normal
variation based on historical control data. There were no indications
of embryotoxic or teratogenic effects in the rat under the conditions
of the study (Fritz 1978b).
Rabbit
Groups of pregnant Chinchilla rabbits (20 rabbits/group) were
given metalaxyl orally by intubation at dose levels of 0, 5, 10 and
20 mg/kg during days 6 through 18 of gestation. On day 28 of
gestation, all rabbits were sacrificed and examined and the foetuses
delivered by caesarean section. Foetuses were subjected to external
examination followed by inspection for Visceral and skeletal
malformations, anomalies or variants.
Reduced food consumption was observed in the 10 and 20 mg/kg
groups accompanied by slightly reduced body weight gain in dams at the
high dose. The mean number of corpora lutea and/or implantations were
comparable for all groups. Incidences of renal hypoplasia and agenesis
were not dose-related and within the spontaneous rate of historical
untreated animals of the same rabbit population. Metalaxyl did not
adversely influence embryonic or foetal development in Chinchilla
rabbits at levels up to and including 20 mg/kg (Fritz et al 1978).
Special Studies on Mutagenicity
Yeast culture evaluations
The test compound, CGA 48 988, was tested for mutagenic effects
on the D-7 strain of Saccharomyces cerevisiae at five dose levels:
400, 2 000, 4 000, 8 000 and 10 000 µg/ml, with and without microsomal
activation. The test system permitted the detection of mitotic
crossover, mitotic gene conversion and reverse mutation in the yeast
cells D-7 after 2 or 6 hours of treatment with the test compound. The
positive controls were treated with 4-nitroquinoline-N-oxide (without
S-9 mix) and cyclophosphamide (with S-9 mix) in concentrations of
0.25 µg/ml and 50-250 µg/ml, respectively.
In none of the three mutation systems was the incidence of
mutants increased, in comparison with the negative controls, as a
result of treatment of the cells with metalaxyl with or without
microsomal activation. It is concluded that under the conditions of
these experiments no evidence of mutagenic effects was obtained with
metalaxyl (Arni and Muller 1982).
DNA repair
The primary rat hepatocytes prepared from the liver of a male rat
were used for the UDS assay. A series of compartments in pertri dishes
containing gelatinized cover-slips were seeded with 2 × 10-5 cells
per compartment. The cells were allowed to attach to the coverslips
during an attachment period of 1.5-2 hours. The cell cultures attached
to the coverslips were then washed and refed with WME containing the
test compound (16, 80, 400 and 2 000 µg/ml) and 3H-thymidine
(4 µ Ci/ml). Negative controls and positive controls (4NQO and DMN)
were run concurrently with the test compound.
Comparison of the mean number of silver grains per nucleus in the
negative controls and after treatment with metalaxyl revealed no
evidence of induction of DNA damage (Puri and Muller 1982).
Mouse lymphoma assay
Metalaxyl was tested for mutagenic effects on L5178Y/TK+/- mouse
lymphoma cells in vitro. The investigations were performed with
microsomal activation at concentrations of 0.0625, 0.125, 0.25 and
0.50 mg/ml and without microsomal activation at concentrations of
0.125, 0.25, 0.50 and 1.0 mg/ml. The results were expressed in terms
of the number of induced TK-/- mutants/106 surviving cells. The
test substance is considered mutagenic in this system if the colony
count exceeds that of the solvent control by a factor of more than
2.5 at any concentration.
It was concluded that, under the given experimental conditions,
no evidence of mutagenic effects of metalaxyl was observed in this
mammalian forward mutation system (Strasser and Muller 1982).
Dominant lethal studies
Two groups of adult male albino mice, 20 per group, were
administered orally by intubation single doses of 65 and 196 mg/kg of
metalaxyl dissolved in carboxymethyl cellulose. Each male mouse was
mated with two untreated virgin female mice for eight consecutive
weeks to cover the total spermatogenic cycle. Fourteen days from the
mid-week of mating, the females were killed and examined for
pregnancies. Three parameters were used to determine the dominant
lethal effect of the test compound: mating ratio, number of
implantations and embryonic deaths. No evidence of dominant lethal
effect was obtained in the progeny of male mice treated with metalaxyl
(Fritz 1978a).
Special Studies for Carcinogenicity
Mouse
Metalaxyl was administered in the diet to groups of 60 male and
60 female mice (ICI Alderley Park, Swiss) at concentrations of 0, 50,
250 and 1 250 ppm for 104 weeks. Mice were approximately 5-6 weeks of
age when dosing commenced. The percent survival, food/water
consumption and general thriftiness of animals were unaffected by
treatment. A slight decrease in food conversion efficiency,
accompanied by decreased body weight gain, was noticed in high dose
males during weeks 11 through 30. Bone marrow and blood, sampled
throughout the study, were unaffected by treatment.
All animals that died or were sacrificed in extremis were
submitted to gross necropsy and histopathological evaluation for both
non-neoplastic and neoplastic lesions. The lesions observed were
similar among all groups and not related to the administration of
metalaxyl. Metalaxyl was not found to be a carcinogen in this strain
of mouse (McSheehy et al 1980).
Special Study on Skin Sensitization
Guinea pig
Groups of 10 male and 10 female guinea pigs (Pirbright, albino)
were subjected to a series of 0.1 ml intracutaneous injections of
metalaxyl as a 0.1% suspension in polyethylene glycol and saline,
following the optimization test of Maurer et al (1975). DNCB served
as a positive control. Metalaxyl was not sensitizing under the
conditions of the test (Sachsse and Ullmann 1976h).
Special Study on Eye Irritation
Rabbit
Approximately 100 mg of metalaxyl was placed in the conjunctival
sac of 3 male and 3 female rabbits. The eyes of 3 of the 6 rabbits
were rinsed approximately 30 seconds after instillation of the test
material. There was no irritation present in any of the rinsed eyes.
Unwashed eyes presented irritation of the conjunctiva and cornea for 3
days and were clear by day 4. While the degree of corneal involvement
appears minimal, it could not be subjectively evaluated since only
scores were presented. Rinsing the eyes prevented any irritation
effects, suggesting that the irritation in unwashed eyes may have been
more related to the physical nature of the particles than to the
chemical itself (Sachsse and Ullman 1976g).
Special Study on Dermal Irritation
Rabbit
The primary irritation was determined following the application
of 0.5g of metalaxyl to both abraded and intact skin sites on the
shaved back of 3 male and 3 female rabbits. There was no irritation on
any of the intact skin sites, while minimum erythema was observed on
the abraded skin sites of 2 rabbits 24 hours after application. The
primary irritation index was reportedly 0.1/8 (Sachsse and Ullmann
1976f).
Acute Toxicity
In acute toxicity studies carries out in animals (Table 1), toxic
signs of poisoning occurred within 2 hours after treatment and most
deaths within the first 24 hours. Symptoms observed were non-specific
in the various mammalian species and consisted of sedation, dyspnoea,
exophthalmos, tonic-clonic muscle spasms, curved or ventral position
and ruffled fur.
Metalaxyl was not toxic by the dermal route, as both rats and
rabbits tolerated doses from 3 100 to 6 000 mg/kg without visible
symptoms or effects. No mortalities were reported in either species.
Table 1. Acute Toxicity of Metalaxyl in Animals
Species Sex Route LD50 Reference
Rat m + f oral 1 669 Sachsse and Bathe, 1976a
(515-868)
Mouse m + f oral 1 788 Sachsse and Bathe, 1976b
(626-991)
Rabbit m + f oral 1 697 Sachsse and Ullman,1976e
(506-961)
Hamster m + f oral 7120 Thomann and Pericin,1977
(5250-9660)
Rat m + f dermal 1 >3 100 Sachsse and Bathe, 1976c
Rabbit m + f dermal 2 >6 000 Sachsse and Ullmann, 1978
Rat m + f i.p. 1 312 Sachsse and Bathe, 1976d
(282-345)
1 Carboxy methyl cellulose was the vehicle;
2 Polyethylene glycol was the vehicle.
Short-Term Studies
Rabbit-dermal
Metalaxyl was administered to the occluded intact and abraded
skin of albino rabbits (10 males and 10 females/group) at doses of 0,
10, 100 and 1 000 mg/kg. Test substance was applied once each day, 5
days/week for 3 consecutive weeks. Information was obtained on body
weight, food consumption, haematology, clinical chemistry, absolute
and relative organ weights and local dermal effects on the skin. All
animals were subjected to gross necropsy and histopathological
examination. There were no adverse effects related to compound
administration for any of the parameters evaluated. Several lesions
reported in both treatment and control groups were related to either
bacterial Staphylococcus aureus and epidermitis) or parasitic
(encephalitozoon, Eimeria stiedae) infections, which obscured
interpretation of results (Calkins et al 1980).
Rat - dietary
Daily oral doses of metalaxyl were administered by gavage to
groups of rats (10 males and 10 females/group) initially at levels of
0, 10, 30 and 100 mg/kg for the first 14 days. Dosing was increased to
0, 30, 100 and 300 mg/kg, respectively, for days 15-21 of treatment.
Once again, on day 22, dose levels were increased to 0, 60, 200 and
600 mg/kg, respectively, for the duration of the study, which was
terminated on day 28. No deaths occurred and food consumption, body
weight gain and mean food consumption were comparable among treated
and control groups. Haematology, blood chemistry and urinalysis were
unremarkable. Male rats in the high dose group demonstrated a
significant increase in absolute testes weight as well as a dose-
related increase in relative liver weight, for which the trend was
significant (p<0.01). The absolute and relative adrenal weights were
significantly increased (p<0.05) for females in the high dose group.
Female rats in all treatment groups administered metalaxyl had
significantly increased absolute and relative liver weights (p<0.05),
which were dose-related and for which the positive trend was similarly
significant (p<0.01) (Sachsse et al 1979).
In a 3-month dietary study using Sprague-Dawley rats, 180 rats
were allocated to two groups of 50 animals each (25 males and 25
females) and two groups of 40 animals each (20 males and 20 females).
Dose levels were 0, 50, 250 and 1 250 ppm in the diet. Five males and
five females from the control and high dose level groups were retained
for an additional 28 days without treatment for evaluation of recovery
and reversibility of effects. Minimal cellular hypertrophy of hepatic
parenchymal cells was observed in five females receiving the highest
dose. This trend was not noticeable in the recovery animals,
representing a possible reversibility for this effect. No other
changes attributed to treatment were observed and dose levels of
250 ppm or less are considered to be without adverse effects under the
conditions of this study (Drake 1977).
In a follow-up 90-day study, Gfeller et al (1980) evaluated the
effects of 0, 10, 50, 250 and 1 250 ppm of metalaxyl incorporated in
the diet of rats (RAIF, SPF). There were 20 males and 20 females in
each experimental group. Mortality and other cageside observations
were conducted daily, body weight and food consumption determined
weekly and clinical chemistries, urinalysis and haematology performed
periodically throughout the study. At the conclusion of the study, all
test and control animals were subjected to gross and histopathological
examination and selected organs were weighed.
A dose-related decrease in total leucocyte count was evident in
high dose males, as well as increased absolute and relative adrenal
weights for male rats in the 50, 250 and 1 250 ppm groups. In females,
the relative adrenal weights were increased in the high dose group
only. There was a significant increase in relative liver weights for
females in the 250 and 1-250 ppm dose groups. However, the absolute
liver weights for high dose females were not significantly different
from controls and, therefore, the difference reported for the relative
liver weights is considered as being related to the decrease in the
body weight of the high dose compared to control females, rather than
as an increase in organ weight, per se. Gross necropsy and
histopathological examinations revealed no differences between treated
and control animals. There were no structural alterations in the
adrenals reflective of the increase in both absolute and relative
weights for this organ. The level of 10 ppm of metalaxyl in the diet
of rats is considered to be without adverse effects (Gfeller
et al 1980).
Dog - dietary
Four groups of beagle dogs, 6-9 months of age, were administered
metalaxyl in their diets at levels of 0, 50, 250 and 1 250 ppm for
91 days. Control and high dose groups contained 4 males and 4 females
each, with the low and intermediate dose groups containing 3 males and
3 females each. No mortality or changes in the behaviour of the
animals were observed. Urinalysis, ophthalmoscopy, body weight, food
consumption and haematology indicated no treatment-related findings.
Blood chemistry parameters were normal, except for a significant
increase in serum alkaline phosphatase levels in high-dose males and
females, which was also time-related. Determination of SAP in two
recovery animals from the high dose group demonstrated a reversal to
normal within 4 weeks post-treatment. Macroscopic and microscopic
examinations revealed no compound-related findings. The no-effect
level was estimated to be 250 ppm (Finn et al 1977).
Technical metalaxyl was administered in the diet to groups of
beagle dogs at levels of 0, 50, 250 and 1 000 ppm for a period lasting
six months. There were 8 males and 8 females in both the control and
high dose groups, with 6 males and 6 females in each of the other two
groups. Pups were 6-8 months old at the beginning of study. Feed
consumption, body weights and urinalysis, performed periodically
throughout the study, were normal. Ophthalmic examinations did not
reveal any effects related to treatment. Clinical chemistry parameters
were all within normal variation, except for alkaline phosphatase
levels for 1 000 ppm dose-level males and females, which were
significantly increased (p<0.05) compared to control animals. A
recovery to normal was observed after dosing was stopped. Haemoglobin,
erythrocyte count and hematocrit, while significantly lower (p<0.05)
for high dose males when compared to control males in this study, were
all within normal variation when compared to historical controls.
Anaemia was not evident and bone marrow samples reportedly were
normal: however, reticulocytes and m/e ratios were not determined. The
liver/brain ratios of females in the high dose group were
significantly increased (p <0.05) compared with controls. Similarly,
a positive trend for both increased absolute and relative liver
weights was apparent in male and female dogs receiving an increased
dosage. Alkaline phosphatase is produced in many organs, with the
liver being a primary supplier. The possibility of a dose response for
alkaline phosphatase relative to the liver is supported by the trend
toward increasing liver weights with increased dosage. No such
trends, however, were seen in other enzymes also produced in the liver
(e.g. SGPT, SGOT), and no gross pathological or histopathological
changes were observed in the liver of any dogs fed metalaxyl. There
were no other effects related to treatment and a no-effect level of
250 ppm was determined. Mean daily feed consumption data indicate this
dietary level is approximately equivalent to 7.4 mg/kg/day, with
1 000 ppm corresponding to 31 and 33 mg/kg/day for males and females,
respectively (de Ward et al 1981).
Long-Term Study
Mice
(see Special Study for Carcinogenicity)
Rat
Groups of rats (80 male and 80 female Sprague-Dawley albino
rats/group) were administered metalaxyl in the diet for 104 weeks at
dosage levels of 0, 50, 250 and 1 250 ppm. Growth, as observed by body
weight changes and food consumption data, was recorded weekly for the
first 13 weeks and monthly thereafter. Rats were inspected twice daily
for behavioural changes, general health, mortality and tumour
development. At periodic intervals over the course of the study,
haematologic, clinical chemistry and urinalysis examinations were
performed.
All animals, including those dying or sacrificed, were subjected
to both gross necropsy and microscopic examinations after 55 and 105
weeks of treatment.
No effects considered to be treatment-related were noted with
respect to toxic signs, mortality, body weight, food consumption,
urinalysis, ophthalmologic examination and haematology. Survival was
greater than 50% for both sexes of all groups at 18 months. Activities
of SGPT and SGOT decreased in the 250 and 1 250 ppm females during the
first year, but recovered to normal values, comparable to the control
group, through the second year to termination of the study.
At gross necropsy, organ weight determinations demonstrated
significantly increased (p <0.01) relative liver weights for females
in the 1 250 ppm group after both 55 and 105 weeks of treatment.
Relative liver weights for males were significantly increased
(p <0.01 and <0.001) in both the 250 and 1 250 ppm groups,
respectively, after 105 weeks. These organ to body weight differences
were not accompanied by any pathological findings in the treated
animals other than slight periacinar hepatocytic vacuolation and
chronic inflammation in 1 250 ppm males.
There was a reported pathological finding of testicular atophy
(p <0.05) occurring primarily in the first 53 weeks at all treatment
levels, but the respective absolute and relative testicular weights
were comparable to those of the control group. Since there was no
positive trend with dose for this response and no impairment of
reproductive performance (Cozens et al 1980), this effect is not
considered attributable to metalaxyl. The findings of parafollicular
cell adenomas of the thyroid was significantly increased for the
250 ppm females. However, there was no dose-response relationship and
the incidence was not significantly different from historical control
data for this pathological finding at the same laboratory.
Metalaxyl is considered to be without adverse effects on rats at
levels up to and including 50 ppm in the diet. There was no evidence
of carcinogenic potential at any level tested (Ashby et al 1980).
COMMENTS
Metalaxyl, when fed to rats, is rapidly metabolized and excreted
in the urine and faeces. The metabolism has been relatively well
defined in rats and no specific organ accumulation appears to be
found. The excretion pattern is not influenced by the dose level, but
by the sex of the animals, as females demonstrate preferential
excretion via urine, while males excrete higher proportions in the
faeces.
The acute toxicity in various animals suggests a moderate acute
hazard via the oral route, but little dermal absorption occurs, as
demonstrated in both acute and subchronic dermal studies. Metalaxyl is
mildly irritating to the skin and eyes but did not elicit contact
allergenicity in a skin-sensitization study using guinea pigs.
Metalaxyl did not produce any adverse effects on reproductive
performance or pup viability at levels up to and including 1 250 ppm
in the diet. There were no indications of embryotoxic or teratogenic
effects in either rats or rabbits at levels of 120 or 20 mg/kg bw,
respectively.
Metalaxyl was evaluated in a battery of mutagenicity tests
including yeast culture, DNA repair, mouse lymphoma and dominant
lethal tests. No evidence of mutagenic potential was determined in any
of these tests.
In both short- and long-term studies, the only significant
findings were increased relative liver and adrenal weights, sometimes
accompanied by increased enzyme activities (i.e. SAP, SGPT), but for
which there was no corresponding histopathology. The adrenal weight
increase noted in the rat short-term study was not apparent in the rat
long-term study at comparable dietary levels nor in the short-term dog
study or mouse carcinogenicity study. Metalaxyl was not demonstrated
to be carcinogenic in either rats or mice. Based upon all available
data, no-effect levels in mammalian species can be determined and an
ADI for man allocated for metalaxyl.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mice : 1 250 ppm in the diet, equivalent to 187.5 mg/kg bw
Rat : 50 ppm in the diet, equivalent to 2.5 mg/kg bw
Dog : 250 ppm in the diet, equivalent to 7.4 mg/kg bw
Estimate of Acceptable Daily Intake for Man
0 - 0.03 mg/kg body weight
FURTHER WORK OR INFORMATION
Desirable
1. Metabolic studies in a non-rodent species.
2. Observations and information in humans.
REFERENCES
Arni, P. and Muller, D. Saccharomyces cerevisiae D-7/mammalian-
1982 microsome mutagenicity test in vitro with CGA 48988 (test
for mutagenic properties in yeast cells). Report from Ciba-
Geigy Ltd., Basle, Switzerland, submitted to the World
Health Organization by Ciba-Geigy Ltd. (Unpublished)
Ashby, R., Bhatt, A., Chapman, E., Hepworth, P.L. and Whitney, J.C.
1980 CGA 48988: Toxicity and oncogenicity in dietary
administration to rats for two years. Final report no.
80/CIA009/315 from Life Science Research, Stock, Essex CM4
0PE, England, submitted from Ciba-Geigy, Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Calkins, J.E., Morgan, J.M., Casey, H.W. and Page, J.G. A 21-day
1980 subacute dermal toxicity study in albino rabbits with CGA
48988. Report from Toxigenics Inc. Decator, Ill. 62526, USA,
submitted by Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Cozens, D.D., Allen, P.A., Clark, R., Offer, J.M., Gregson, R.L. and
1980 Gibson, W.A. Effect of CGA 48988 on reproductive function of
multiple generations in the rat. Report from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England,
submitted from Ciba-Geigy Ltd., Basle, Switzerland, to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Drake, J.C. 3-month dietary study in rats with compound CGA 48988.
1977 Report from Geigy Pharmaceuticals, Stamford Lodge, Wilmslow,
Chershire, U.K. submitted from Ciba-Geigy Ltd., Basle,
Switzerland, to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Finn, J.P., Briggs, K., Close, J.E., Noble, D.N., Barrett, R. and
1977 Rider, L. CGA 48988, 91-day dietary toxicity study in beagle
dogs. Report from Hazleton Laboratories Europe Ltd.,
Harrogate, HGe 1PY, England, submitted from Ciba-Geigy Ltd.,
Basle, Switzerland, to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Fritz, H. Dominant lethal study-CGA 48988 techn. mouse (Test for
1978a cytotoxic or mutagenic effects on male germinal cells).
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
1978b Reproduction study-CGA 48988 tech. Rat Seg. II (Test for
teratogenic or embryotoxic effects). Report from Ciba-Geigy
Ltd., Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Fritz, H., Becker, H. and Hess, R. Reproduction study - rabbit- CGA
1978 48988 tech. Seg. II (Test for teratogenic or embryotoxic
effects). Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Gfeller, W., Basler, W., Zak, F., Grieve, A.P. and Hess, R. CGA 48988
1980 techn. 3-month toxicity study on rats (Project No. 79 18 11)
Final Report. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Hambock, H. Distribution, degradation and excretion of CGA 48988 in
1977 the rat. Project Report 18/77 from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Metabolism of CGA 48988 in the rat. Project report 26/78
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1981 Metabolic pathways of CGA 48988 in the rat. Project Report
31/81 from Ciba-Geigy Ltd., Basle, Switzerland, submitted to
the World Health Organization by Ciba-Geigy, Ltd.
(Unpublished)
Maurer, T., Thomann, P., Weirich, E.G. and Hess, R. The optimization
1975 test in the guinea pig. A method for the predictive
evaluation of the contact allergenicity of chemicals. Agents
and Actions 5 (2): 174-179.
McSheehy, T.W., Macrae, S.M. and Whitney, J.C. CGA 48988 oncogenicity
1980 in dietary administration to mice for two years. Final
Report No. 80/CIA 008/442 from Life Science Research, Stock,
Essex CM4 9PE, England, submitted from Ciba-Geigy Ltd.
Basle, Switzerland to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
Puri, E. and Muller, D. Audioradiographic DNA repair test on rat
1982 hepatocytes CGA 48988 (in vitro test for DNA-damaging
properties). Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy, Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute oral LD50 in the rat of technical CGA
1976a 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute oral LD50 in the mouse of technical CGA 48988. Report from Ciba
1976b Geigy Ltd., Basle, Switzerland submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Bathe, R. Acute dermal LD50 in the rat of technical
1976c CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
Acute intraperitoneal LD50 in the rat of technical CGA 48988. Report
1976d from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Sachsse, K. and Ullmann, L. Acute oral LD50 in the rabbit of technical
1976e CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organziation by Ciba-Geigy
Ltd. (Unpublished)
1976f Skin irritation in the rabbit after single application of
technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1976g Eye irritation in the rabbit of technical CGA 48988. Report
from Ciba Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
1976h Skin sensitizing (contact allergenic) effect in guinea pigs
of technical CGA 48988. Report from Ciba-Geigy Ltd., Basle,
Switzerland, submitted to the World Health Organization by
Ciba-Geigy Ltd. (Unpublished)
1978 Acute dermal LD50 in the rabbit of technical CGA 48988.
Report from Ciba-Geigy Ltd., Basle, Switzerland, submitted
to the World Health Organization by Ciba-Geigy Ltd.
(Unpublished)
Sachsse, K., Suter, P. and Luetkemeier, H. CGA 48988 techn., 28 days
1979 toxicity study on rats. Final report from Ciba-Geigy Ltd.,
Basle, Switzerland, submitted to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
Strasser, F.F. and Muller, D. L 5178 Y/TK+/- mouse lymphoma
1982 mutagenicity test CGA 48988. (in vitro test for mutagenic
properties of chemical substances in mammalian cells. Report
from Ciba-Geigy Ltd., Basle, Switzerland, submitted to the
World Health Organization by Ciba-Geigy Ltd. (Unpublished)
Thomann, P. and Pericin, C. Acute oral LD50 in the Chinese hamster of
1977 CGA 48988. Report from Ciba-Geigy Ltd., Basle, Switzerland,
submitted to the World Health Organization by Ciba-Geigy
Ltd. (Unpublished)
de Ward, J., Beck, L. St., Kitchen, D.N. and Hepler, D.I. Six-month
1981 chronic oral toxicity study with CGA 48988 technical in
beagle dogs. Project No. 1545. Report from ELARS Bioresearch
Laboratories, Fort Collins, Colorado, USA, submitted from
Ciba-Geigy Ltd., Basle, Switzerland to the World Health
Organization by Ciba-Geigy Ltd. (Unpublished)
METALAXYL
 RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales, which cause late blight, downy
mildews, damping-off and root, stem and fruit rots of many plants. The
compound is taken up by the roots, leaves, green stems and shoots and
transported acropetally within the plant (Urech et al 1977).
Commercial products first introduced as foliar sprays contained
only metalaxyl as the active ingredient or were mixtures of metalaxyl
with low rates of residual protective fungicides, such as copper or
folpet. Experience has now shown that strains of fungi resistant to
metalaxyl may develop, and in order to reduce the likelihood of this
happening and to broaden the spectrum of activity, commercial products
now contain a higher content of the residual fungicides mancozeb,
zineb, copper, folpet or carbendazim. A further measure to hinder the
development of resistance has been the limitation of the number of
sprays applied to the crop. This has generally been restricted to 3 to
4 applications, which are made during the active vegetative stage of
the crops. Commercial products containing metalaxyl only are intended
for use as seed dressing or for soil applications.
Metalaxyl is registered for use on a wide range of crops and in
several countries in temperate, subtropical and tropical regions.
Preharvest treatments
Metalaxyl is used for foliar sprays, seed dressing and soil
treatments. The main fields of application are in potatoes, grapevines
and vegetables. It is also used against diseases of tobacco and in
some non-edible crops such as ornamentals and turf.
Postharvest treatments
Metalaxyl, incorporated in wax, is applied as a pack-house spray
to citrus fruit for the control of postharvest rot. The recommended
application rates for the crops on which the compound is currently
used are given in Table 1.
Table 1. Recommended Application Rates of Metalaxyl
Crop Rate
(a.i.)
Foliar/Fruit sprays
Potato 150 - 250 g/ha
Onion 200 - 250 g/ha
Leafy vegetables 20 - 25 g/100 1
Brassicae 200 - 250 g/ha
Cucurbits 20 - 25 g/100 1
Tomato 20 - 40 g/100 1
Grape 20 - 30 g/100 1
Pineapple 25 g/100 1
Cocoa 50 - 312 g/100 1
Hops 20 - 30 g/100 1
Seed treatments
Sugarbeet 245 g/100 kg seed
Vegetables 26 - 53 g/100 kg seed
Peas 70 g/100 kg seed
Maize 175 g/100 kg seed
Millet 263 g/100 kg seed
Sorghum 175 g/100 kg seed
Sunflower 210 g/100 kg seed
Soil treatments
Red and green pepper (Capsicum annuum) 2 kg/ha
Black pepper (Piper nigrum) 1.25 - 2 g/m2
Citrus 1.25 - 2 g/m2
Avocado 2- 5 g/m2
Hops 0.2- 4 g/plant
Postharvest treatment
(pack-house spray)
Citrus 2 000 ppm a.i. in wax
1 3-4 applications.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Metalaxyl residue data have been obtained from numerous trials
carried out on a world-wide basis on main crops. Treatments were
generally made using WP or FW formulations with metalaxyl alone or
with mixtures containing residual fungicides. The dosages used in the
residue trials cover the recommendations as summarized in Table 1.
Analysis was carried out using methods determining metalaxyl
alone and/or metalaxyl and its metabolites containing the
2,6-dimethylaniline moiety.
Potatoes
Field trials were conducted in 14 countries during six years. Two
to eight applications of dosages ranging from 0.15 to 0.42 kg a.i./ha
were made at intervals of 1-3 weeks. The results of these trials are
reported in Table 2 (Ciba-Geigy undated; Finland undated). Generally,
no residues either of the parent compound or metabolites containing
the 2,6-dimethylaniline moiety were detectable in potato tubers.
Residues (0.02-0.08 mg/kg) were found in only eight of 102 samples.
The limit of determination ranged from 0.02-0.05 mg/kg.
Onions
Field trials were carried out in nine countries over four years.
Two to eight applications at dosages ranging from 0.22-0.6 kg a.i./ha
were made at intervals of 1-3 weeks. The results are shown in
Table 3 (Ciba-Geigy undated; Finland undated). Initial residues of
0.05-0.59 mg/kg generally dissipated below the limits of determination
(0.02 and 0.04 mg/kg) within 14 days. Dissipation of metalaxyl
metabolites containing the dimethylaniline moiety did not differ
significantly from that of the parent compound alone. Two weeks after
the last application, residues of metalaxyl and its metabolites ranged
between <0.05-0.09 mg/kg. The major part (73-100%) of the total
residue consisted of the parent compound.
Leafy Vegetables
Spinach
Field trials were carried out in Italy, The Netherlands,
Switzerland and the U.S. One to four applications (to run off) at
dosages ranging from 0.22-0.55 kg a.i./ha or at 0.043% were made at
intervals of 1-2 weeks. The results are reported in Table 4
(Ciba-Geigy undated). Residues of the parent compound rapidly
disappeared being at or below the limit of determination (0.05 mg/kg)
two weeks after the last application. Residues measured as the sum of
metalaxyl and its metabolites containing the dimethylaniline moiety
amounted to 1.1-7.3 mg/kg one week after the last application and
decreased to 0.27-5.0 mg/kg during the following two weeks.
Supervised trials were carried out on lettuce at six sites in the
U.K. The plants were treated at planting and after an interval of two
weeks with a formulation containing 48% mancozeb and 10% metalaxyl at
rates of 71-150 g metalaxyl/ha. The residues were below 0.1 mg/kg, the
limit of determination, in 19 samples taken after 13 to 58 days. In
the other samples, the residues were 0.55, 1.2 and 1.3 at day 21, and
0.25 mg/kg at day 35 (U.K.undated).
Table 2. Residues of Metalaxyl Following Supervised Trials in Potatoes (1976-1981)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Argentina 3 0.2-0.25 1-2 0.05
Austria 4 0.2 3 <0.03 <0.03 <0.03 <0.03 <0.03
Canada 4 0.3 2 <0.05
5 0.3 2 <0.05
6 0.25 1-2 <0.05
6 0.15 1-2 <0.05
3 0.3 2 <0.02
3 0.25 2 <0.02
4 0.25 2 <0.02 0.02
6 0.25 1-2 <0.02
6 0.3 1-2 <0.02
3 0.3 3 <0.02 0.02
2 0.3 3 <0.02
2 0.25 3 <0.02
8 0.16 1 <0.02 <0.02 <0.02
4 0.16 2 <0.02 <0.02
4 0.2 2 <0.02 <0.02
6 0.2 1-2 <0.02 <0.02 0.02 0.03
Fed. Rep. Germany 3 0.2-0.3 4 <0.05
3 0.2-0.3 4 <0.05
8 0.2 1 <0.05
8 0.2 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.04
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
Finland 2 0.25 3 <0.04
France 3 0.3 3 <0.04 1
4 0.2 2 <0.04 1
3 0.3 3 <0.02 1
U.K. 6 0.2 1-2 <0.05
5 0.3 3 <0.05 0.05 <0.05 <0.05
4 0.3 3 <0.05
6 0.3 2-3 <0.05
5 0.3 2-3 <0.05
5 0.3 2-3 <0.05
India 4 0.24 2 <0.02
Israel 5 0.25 1-2 <0.02
0.02
South Africa 5 0.2 2-3 0.02 <0.02
Spain 5 0.3 1-2 <0.05
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Sweden 5 0.15 1-2 0.05 <0.05 <0.05
4 0.2 2 <0.02
4 0.2 2-3 <0.02
5 0.15 1-2 <0.02
3 0.25 3 <0.02
Switzerland 5 0.25 2 <0.05 <0.05 <0.05
<0.06
5 0.25 2 <0.05 <0.05 <0.05
3 0.2-0.3 3 <0.05
8 0.25 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
U.S. 3 0.275 2 <0.05 2
6 0.275 2 <0.05
6 0.275 2 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 2 <0.05
6 0.412 1 <0.05
6 0.42 2 0.06 2
0.08 2
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
4 0.42 2 <0.05
5 0.42 2 <0.05
6 0.275 2 <0.05 2
<0.05 2
<0.05 2
6 0.275 2 <0.05 2 <0.05 2
<0.05 2 <0.05 2
<0.05 2 <0.05 2
6 0.22 2 <0.05 2
6 0.22 2 <0.05 2
1 Peel and pulp were analysed separately, no sample contained measurable residues.
2 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 3 Residues of Metalaxyl Following Supervised Trials in Onions (1978-1981)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
Australia 6 0.4 2 <0.04 <0.04
4 0.2 1 0.30 0.12
4 0.3 1 0.59 0.12
Canada 3 0.25 2 <0.02
3 0.24 1-2 0.05 <0.02
4 0.24 1-2 0.31 0.06
3 0.24 1-2 <0.02
5 0.24 2 0.02
4 0.24 2 <0.02
4 0.25 1-2 0.05 0.04
3 0.25 1-2 0.03
Fed.Rep. Germany 3 0.25 2 0.06 <0.02 <0.02
3 0.25 2 0.05 0.02 <0.02
Finland 2 0.25 5 <0.04
4 0.25 1 <0.04
France 3 0.225 3 0.04
U.K. 2 0.5 2 <0.02
Italy 5 0.6 2 0.02
Switzerland 8 0.3 2 <0.05
Table 3 (con't)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
U.S. 1 5 0.275 1-2 0.15
5 0.55 1-2 0.16
5 0.275 1-2 <0.05 <0.05
5 0.55 1-2 0.06 <0.05
5 0.275 1-2 <0.05
5 0.55 1-2 <0.05
5 0.22 2 0.1 0.08
5 0.22 2 0.09 0.08
3 0.275 1-2 0.26
5 0.275 1-2 <0.05 <0.05 <0.05
5 0.55 1-2 <0.05 <0.05 <0.05
5 0.22 2 <0.05 <0.05
6 0.22 2 <0.05 <0.05 <0.05
6 0.22 2 0.08 <0.05 <0.05
5 0.22 2 0.20 0.09 <0.05
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 4 Residues of Metalaxyl Following Supervised Trials in Spinach
Applications Residues (mg/kg) at intervals after
Interval between last application (days)
Country Rate (a.i. applications
No. kg/ha or %) (weeks) 3 6-8 13-15 20-22 27-31
Italy 2 0.043 2 0.05
Netherlands 1 0.25 0.53 0.06 0.03 <0.03
Switzerland 2 0.25 2 0.53 <0.05 <0.05 <0.05 <0.05
U.S. 3 0.275 2 0.06 <0.05
2.9 1 2.2 1 1.0 1
3 0.55 2 0.67 <0.05
4.9 1 3.9 1 2.4 1
3 0.275 2 1.5 1 1.4 1 1.7 1
0.55 2 7.3 1 4.1 1 5.0 1
3 0.275 1 1.2 1 0.25 1 0.27 1
0.55 1 1.1 1 0.79 1 0.86 1
4 0.22 1-2 1.1 1
4 0.22 2 1.8 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Brassicas
Field trials were carried out in broccoli, Brussels sprouts,
cabbage and cauliflower in several countries over a period of five
years. Two to five applications at dosages from 0.2 to 0.55 kg a.i./ha
were made at intervals of 1-3 weeks. The results are reported in
Table 5 (Ciba-Geigy undated). Dissipation of residues was similar
within the various varieties. Initial residues of 0.17-2.0 mg/kg
rapidly decreased and were generally below 0.1 mg/kg after two weeks.
Residues measured as metalaxyl and its metabolites containing the
dimethylaniline moiety were somewhat higher and dissipated more
slowly. The parent compound amounted to 55% of the residue containing
the dimethylaniline moiety. Two weeks after last application of the
recommended dosage of 0.25 kg a.i./ha, residues were <0.05-0.2 mg/kg.
Fruiting vegetables
Field trials were carried out with cucumbers, gherkins, melons,
squash and watermelons in different countries. Three to eight
applications at dosages of 0.2-0.55 kg a.i./ha or 0.02-0.4% were made
at intervals of 1-2 weeks. The results are reported in Table 6
(Ciba-Geigy undated). Residue levels in the different commodities were
in the same range. Initial residues were 0.06-0.4 mg/kg, depending on
the dosage rate used. They decreased slowly and were 0.03-0.23 mg/kg
after two weeks. There was no difference between residue levels
determined as the parent compound or as the total of metalaxyl plus
metabolites.
Tomatoes
Field trials were carried out in seven countries over a period of
six years. Two to nine applications at dosages of 0.125-0.8 kg a.i./ha
or 0.02-0.08% were made at intervals from one to 22 days. The results
are listed in Table 7 (Ciba-Geigy, undated). In most samples, initial
residues were below 0.1 mg/kg. Residue levels of metalaxyl and its
metabolites were at the same magnitude as the parent compound alone.
Grapes
Numerous field trials were conducted over a period of six years
in nine countries representing important vine growing areas of the
world. Due to the different climatic areas, a wide range of
experimental conditions has been considered. One to nine applications
at dosages of 0.01-0.04% a.i. or 0.15-0.5 kg a.i./ha were made at
intervals of 1 to 4 weeks. The results of these trials are reports in
Table 8 (Ciba-Geigy undated).
Table 5. Residues of Metalaxyl Following Supervlsed Trials in Brassicas (1977-1981)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
Canada
(broccoli) 3 0.24 1-2 2.0 0.09 <0.02
3 0.25 1-2 0.56 0.12 0.05
2 0.25 3 0.10
(Brussels
sprouts) 5 0.25 2 0.39 0.12 0.05
(cabbage) 2 0.25 2 0.29 0.08
(cauliflower) 4 0.25 2 0.17 0.03
U.K.
(cauliflower) 2 0.2-0.3 2 0.09 0.02 0.01 0.02
2 0.2-0.3 2 0.09
2 0.2-0.3 2 0.47
South Africa
(brassicas) 5 0.2 1 0.16 <0.05
5 0.4 1 0.90 0.09
U.S.
(broccoli) 5 0.275 2 0.17
0.35 1 0.20 1
5 0.55 2 0.21
0.46 1 0.48 1
Table 5. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
5 0.275 2 0.06 0.06
0.16 1 0.32 1
5 0.55 2 0.17 0.2
0.62 1 0.50 1
0.22 1 0.09 1
5 0.275 2 0.19-0.26
(cabbage) 0.41-0.52 1
5 0.55 2 0.1
0.31 1
5 0.275 2 0.15 1 0.19 1
5 0.55 2 0.35 1 0.26 1
5 0.275 2 0.20 1 0.07 1
5 0.55 2 0.33 1 0.17 1
5 0.275 2 0.12 1 0.10 1
5 0.55 2 0.13 1 0.12 1
5 0.275 2 0.07 0.06
0.22 1 0.09 1
5 0.55 2 0.22 1 0.16 1
5 0.22 2 0.09 1 <0.05 1
5 0.22 2 0.30 1 0.39 1
5 0.22 2 0.15 1 0.14 1
(cauliflower) 5 0.275 2 0.08 1 <0.05 1
5 0.55 2 0.14 1 0.10 1
5 0.275 2 0.17 1 0.36 1
5 0.55 2 0.56 1 0.38 1
5 0.22 2 <0.05 1 <0.05 1
1 The sum of metalaxyl and its metabolites containing the, common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 6. Residues of Metalaxyl Following Supervised Trials in Fruiting Vegetables
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Australia
(gherkins) 7 0.2 1 0.06 <0.04
7 0.4 1 0.12 <0.04
3 0.4 2 0.12 <0.04
(cucumber) 8 0.2 1 0.14 0.09 0.13
8 0.4 1 0.40 0.27 0.24
5 0.2 2 0.14 0.05
Israel
(cucumber) 8 0.25 2 0.25 0.14
(melon) 4 0.25 1 <0.05
South Africa
(cucumber) 4 0.04 2 0.30 0.25 0.22
4 0.02 2 0.26 0.19 0.19
Switzerland
(cucumber) 3 0.25 2 0.09 0.05
3 0.25 2 0.04 0.03
3 0.25 2 0.06 0.03
U.S.
(melon) 8 0.275 1 0.11 0.08
0.17 1 0.24 1
8 0.55 1 0.24 0.25
Table 6. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
(cucumber) 8 0.275 1 0.15 1 0.17 1
8 0.55 1 0.30 1 0.27 1
8 0.22 1 0.13 1
8 0.22 1 0.12 1
(squash) 8 0.22 2 0.07 1
8 0.22 2 0.06
0.07
8 0.22 1 0.06
0.05 1
(watermelon) 8 0.275 1 0.09
0.12 1
8 0.55 1 0.13
0.16 1
8 0.275 1 0.05 1
8 0.55 1 0.05 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6 dimethylaniline,
expressed at metalaxyl equivalents.
Table 7. Residues of Metalaxyl Following Supervised Trials in Tomatoes (1976-1981)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Brazil 7 0.2 0.2 0.5 <0.02
7 0.2 0.024 0.5 <0.02
France 4 0.03 2 <0.04
4 0.045 2 <0.04
2 0.125 0.5 <0.04 <0.04
4 0.5 0.5 2 0.09 0.04 <0.04
4 0.2 2 0.05 <0.04 <0.04 (0.04
Israel 3 0.25 0.04 2 <0.02
South Africa 7 0.4 0.04 1-2 0.04 0.07 <0.03 <0.03
7 0.8 0.08 1-2 0.30 0.10 0.04 <0.03
5 0.08% to 1 <0.03 <0.03 <0.03 <0.03
5 run-off 0.04 1 0.07 0.05 <0.05
Spain 3 0.1% to 2 0.48
run-off (1 day)
Switzerland 9 0.03% to 1-2 0.12 <0.04
run-off
Table 7. (con't)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
U.S. 1 6 0.42 1 <0.05
6 0.42 1 0.18
8 0.42 1 <0.05
6 0.42 1 0.22
6 0.42 1 <0.05
6 0.42 1 0.10
5 0.42 1 0.22
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety
2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 8. Residues of Metalaxyl Following Supervised Trials in Grapes and Wine (1976-1981)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Australia 5 0.02 2 1.2 0.73 0.34
7 0.02 2-4 0.23 0.15 0.14
7 0.03 2-4 0.61 0.37 0.34 0.31
7 0.02 1-4 0.35 0.37 0.43
7 0.03 1-4 0.60 0.49 0.26
Canada 5 0.15 1-2 0.09 0.05
Fed.Rep. 6 0.3 1-2 2.1 3.4 2.2 1.3 1.3
Germany 6 0.4 1-2 2.4 1.4 1.9 1.1 1.4
8 0.2-0.4 1-3 8.0 6.8 4.4 4.2 5.0
8 0.2-0.4 1-3 2.4 2.9 1.9 2.4 1.2
8 0.15-0.4 2 2.1 1.3 1.7 1.7 0.46
6 0.08-0.16 2 0.55 0.4 0.36 0.45 0.15
7 0.12-0.32 2 0.47 0.47 0.49 0.50
7 0.12-0.32 1-2 0.67 0.62 0.51 0.48 0.36 0.37
6 0.24-0.4 2 0.79 0.84 0.53 0.51 0.18
6 0.13-0.54 2-3 2.1 3.5 4.1 2.6 1.2
6 0.3-0.4 1-2 2.9 1.8 2.6 0.13 0.38
8 0.16-0.24 2 0.51 0.34 0.28 0.50
3 0.14-0.3 2 0.2 0.18 0.03
3 0.2 2 0.2 <0.02 <0.02
3 0.2 2 0.15 0.08 0.02
3 0.12-0.36 2-3 0.39 0.23 0.05
3 0.36 1-2 0.02 <0.02
3 0.2-0.3 2 0.13 0.06 0.04
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Switzerland 8 0.02-0.04 2 1.2 0.75 0.04 0.03 0.03 0.02
7 0.01-0.02 2 0.73 0.31 0.74 0.86 0.42 0.09
6 0.45-0.6 2-3 3.2 2.1 1.6 1.6
5 0.02-0.03 3-4 0.84 1.0 1.6 0.58
5 0.02-0.03 3-4 0.16 0.11 0.1 <0.05
7 0.45 2 0.77 0.77 0.70 0.60 0.61 0.26
5 0.4 1-2 2.8 1.6 2.5 2.2 2.6 1.8 2.1 0.3
5 0.4 1-2 2.7 2.7 1.3 1.7 1.6 1.4 0.3
8 0.2-0.4 2 2.9 0.94 0.84 0.53 0.8 0.8
5 0.3 1-4 1.0 1.1 0.59 0.95 0.71 0.35 0.4 0.19
5 0.3 1-4 2.2 1.4 1.6 1.1 0.79 0.69 0.86 0.74 0.19
8 0.3 1-4 1.2 1.0 0.7 0.53 0.45 0.5 0.46 0.20
6 0.4-0.63 2-3 3.3 1.9 1.8 0.91 1.1 0.49
6 0.3 2 0.23 0.21 0.24 0.11
France 1 0.3 1.4 0.9 0.65 0.4 0.1 0.04
1 0.3 1.5 0.44 0.68 0.23 0.06
9 0.07-0.45 2 <0.04 <0.02
7 0.07-0.32 2 0.26 0.10
6 0.37-0.5 2-3 0.06
7 0.03-0.21 2 0.08 0.06
Israel 1 0.25 0.14
1 0.23 0.14
Italy 8 0.03% 1-2 1.3 1.2
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
South Africa 8 0.03% to run off 2 1.2 0.92 0.96
9 0.03% " 2 0.72 1.1 0.49 0.55 0.14
8 0.02% " 2 0.64 0.42 0.31 0.37 0.1
8 0.02-0.03 " 2 5.7 5.5 3.4 2.0
1 0.03% " 5.7 3.5 3.3 2.4 2.0 0.6
1 0.03% " 4.9 2.7 2.8 1.6 1.6 0.41
Spain 6 0.2 2-3 0.14
6 0.2 2-3 0.66 0.14
6 0.2 2-3 1.25 0.11
6 0.2 2-3 0.11
7 0.03 1-3 0.30 0.07
7 0.03 1-3 0.24 0.03
7 0.03 1-3 0.21 0.04
5 0.3 3 0.22
5 0.23 3 0.20
4 0.24 2-4 0.13
4 0.24 3-4 0.06
Initial residues were 0.23-5.7 mg/kg. After two, four and six
weeks, the residues ranged between 0.14-8.0 mg/kg, 0.06-4.4 mg/kg and
0.03-2.6 mg/kg, respectively. The wide range of residue levels is
considered to be a consequence of the various climatic conditions.
Generally, residues are lower in dry and warm areas than in temperate
and more humid areas. Three to four weeks after the last applications,
residues of 0.06-1.3 mg/ kg were observed in warm areas, such as
Australia, Spain and Italy, whereas in Germany 0.34-.6.8 mg/kg were
found after the same interval. No obvious difference in residues were
observed among the various grape varieties. Residues still present in
grapes at harvest were further degraded during the wine-making
process, with residues of <0.02 to 1.2 mg/kg being detected in wine.
Most samples contained less than 0.5 mg/kg.
Pineapple
Two field trials were made in Australia. In the first one,
2 500 l/ha of 0.06% a.i. were applied once as a foliar drench. One
week later, no residues (<0.04 mg/kg) were observed in the flesh
whereas 0.39 mg/kg were detected in the skin. In the second experiment
2 500 l/ha of 0.08% a.i. were applied on the soil six times at
intervals of about four weeks. Ten months later, no residues
(<0.04 mg/kg) were detected either in the flesh or in the skin
(Ciba-Geigy undated).
Cocoa Beans
Field trials were carried out in Papua New Guinea and Cameroon.
The dosages were 0.05-0.5% a.i. (50-500 g a.i./100 l). One to five
applications at intervals of 3 to 4 weeks were made. Details and
results of the trials are reported in Table 9 (Ciba-Geigy undated). In
the raw beans, no measurable residue (<0.02 mg/kg) was observed.
Residues measured in dry and fermented beans ranged from <0.02 to
0.19 mg/kg.
Seed treatment
Residue trials with treated seed were conducted in nine
countries. The dosage ranged from 40 to 620 g a.i./100 kg seed,
depending on the various commodities tested, which were barley, maize,
peas, soybeans, sugarbeet, sunflower and wheat. No residues of either
metalaxyl (<0.02 or <0.04 mg/kg) or the parent compound plus
metabolites (<0.05 mg/kg) were detected in the edible parts of the
crops at harvest.
Soil treatment
Supervised trials were carried out after soil treatment in
avocados, citrus and pepper. Details and results of these residue
trials are reported in Table 10 (Ciba-Geigy undated).
Table 9. Residues of Metalaxyl Following Supervised Trials in Cocoa Beans
Applications Residues (mg/kg) at intervals
Country after last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) Sample 1-3 6-8 13-15 20-22
Papua New Guinea 1 0.075 dry <0.04 <0.04 0.12 <0.04
5 0.5 4 fermented and dry 0.19
Cameroon 1 0.05 raw <0.02 1
2 0.075 3 raw <0.02
1 The peel of the pods contained 0.44-2.1 mg/kg, depending on the state of maturity.
Table 10. Residues of Metalaxyl Following Supevised Trials in Avocado, Citrus and Pepper after Soil Treatment
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Avocado (whole fruit)
(Australia) 1 5.0 <0.05
1 5.0 <0.05
1 5.0 <0.05
(South Africa) 4 2.5 4 <0.05 <0.05
3 2.5 8 <0.05
(U.S.) 1 3 2.5 12 0.53 0.85-1.5
3 5.0 12 2.6 1.2
3 2.5 12 0.18 0.09-0.13 0.11-0.13
3 5.0 12 0.17 0.11
3 2.5 12 0.34 0.63 0.75
3 5.0 12 0.50 0.53
Citrus
(South Africa) 2 2.0 10 0.02 2 <0.02 2
0.19 3 0.21 3
2 2.0 14 <0.02 2 <0.02 2
0.05 3 0.04 3
2.0 10 <0.02 2 <0.02 2
0.14 3 0.16 3
Pepper (Piper nigrum)
(Brazil) 2 1.25 4 0.04
2.5 4 0.66
Table 10. (con't)
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Pepper (Capsicum annuum)
(Bulgaria) 6 1-1.5 3 0.20
6 1.5 3 0.03
4 2.5 3 <0.02
(Italy) 3 1-2 5 0.07
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalent. The concentrations of the parent compound was below 0.05 mg/kg, the limit of determination in each
case.
2 Pulp.
3 Peel.
No residues of metalaxyl (<0.05 mg/kg) were detectable in
avocado fruits one to six weeks after the last application. Residues,
however, were detected with the method used for analysing the parent
compound and the metabolites containing the dimethylaniline moiety.
These residues ranged from 0.11 to 2.6 mg/kg.
After two soil applications (at 2 g a.i./m2), residues
in Valencia oranges were at or below the limit of detection
(<0.02 mg/kg) in the pulp and 0.04-0.21 mg/kg in the peel. The
portion of the peel was 30% of the whole fruit.
In Brazil, residues up to 0.66 mg/kg were detected in
Piper nigrum five weeks after a second soil treatment at
2.5 kg a.i./ha (i.e. 1.5 g/m2).
Residues from <0.02 to 0.2 mg/kg resulted from soil treatments
of Capsicum annuum in Bulgaria and Italy.
Hops
In field trials carried out in the Federal Republic of Germany,
U.S., U.K. and Yugoslavia, metalaxyl was applied to the soil at
0.2-4 g a.i./plant or 0.55-1.1 kg a.i./ha and/or sprayed to run-off as
foliar treatment at 20-30 g a.i./100 l(from -0.24 to 1 kg a.i./ha).
Details and results of the trials are reported in Tables 11 and 12
(Ciba-Geigy undated).
After soil applications residues of metalaxyl were <0.1 to
0.9 mg/kg in dried hops at harvest. After foliar applications residues
of 5-30 mg/kg were found in green hops immediately after treatment.
These residues decreased rapidly and, except in one case, were between
2 and 6 mg/kg in dried hops sampled 7 days after treatment. In beer,
the levels of metalaxyl were below 0.1 mg/kg. No measurable residues
were found in beer when hops had received soil applications only.
Postharvest treatment
Citrus
For controlling postharvest rot in citrus, the fruits are sprayed
or dipped in formulations with or without wax at concentrations of
0.1-0.2% metalaxyl. Supervised residue trials were conducted under
laboratory conditions and in pack-houses. After treatment, the fruits
were stored at about 12°C. Details and results of the supervised
trials are reported in Table 13 (Ciba-Geigy undated; Israel 1980;
Sweden 1980). Analysis of peel and pulp showed that the latter is
largely free of residues (3 samples in 10 trials showed residues
ranging from 0.07-0.2 mg/kg). Residues in the peel generally ranged
Table 11. Residues of Metalaxyl Following Supervised Trials in Hops and Beer After Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application (months)
Rate (a.i. Interval 1-3 3-4 4-5
No. kg/ha or G/plant) (weeks) green dry green dry green dry BEER
Fed.Rep. Germany 7 0.1-0.25 2 0.71
1 0.2 <0.2 <0.02 <0.005
1 0.2 <0.1 <0.1 <0.005
1 0.2 0.18
2 0.2-0.4 8 0.44 0.33
2 0.2-0.4 8 <0.1 <0.1 <0.005
2 0.2-0.4 8 0.12 0.13
2 0.3 8 <0.1 0.25 <0.005
2 0.3 8 <0.1 0.40 <0.005
2 0.3 8 0.23 0.11
1 4.0 0.25 0.18 <0.01
1 4.0 <0.1 0.22 <0.01
1 4.0 0.54 0.93 <0.01
U.S. 1 0.55 <0.05
0.45 1
1 0.55 0.18
1.1 1
1 0.55 0.18 1 1.2 1
1 1.1 0.20 1 3.4 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalents.
Table 12 Residues of Metalaxyl Following Supervised Trials in Hops and Beer after Foliar and Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application(days)
Soil Foliar Interval 0 5 7 14-16 20 28-36
No. (g a.i./plant) (kg a.i./ha) (weeks) green green dried dried dried dried BEER
Fed.Rep. Germany 12 0.55-2.0 1-2 22 6.3 6.3 0.06
12 0.55-2.0 1-2 6.2 5 2.1 <0.05
12 0.55-2.0 1-2 38 5.6 2.1 0.06
12 0.24-0.9 1-2 17.2 3.5 1.8 <0.05
12 0.24-0.9 1-2 5.4 1.5 0.5 <0.05
12 0.24-0.9 1-2 1.6 2.7 1.7 0.05
12 0.24-0.9 1-2 14 1.7 3.4 0.03
12 0.24-0.9 1-2 29 6.8 12 0.08
12 0.24-0.9 1-2 22 4.4 2.2 <0.01
1+12 0.2 and 0.3-1.0 1-2 10 0.5 2.5 0.02
1+12 0.2 and 0.3-1.0 1-2 31 9.1 6.0 0.04
1+12 0.2 and 0.3-1.0 1-2 25.2 3.9 2.7 0.04
1+12 0.2 and 0.3-1.0 1-2 2 3.1 1.8 0.02
U.K. 8 0.15-0.33 2 1.5
8 0.1-0.2 2 0.9
8 0.03% 1-2 1.4
8 0.03% 2 0.3
Yugoslavia 1 0.03% 2.2
2 0.03% 1 1/2 2.5
Table 13. Residues of Metalaxyl Following Supervised Trials in Citrus after Postharvest Treatment
Commodity Application Residues (mg/kg) at
Rate storage intervals (days)
(a.i. Fruit
Method in %) part 0-3 5-7 14-15 28-29 38-44 51-64
Grapefruit Dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.94 0.94 0.96 0.96 0.42
spray 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.30 0.36 0.21 0.20 0.11
spray 1 0.1 pulp 0.12 0.07 0.09 0.15
peel 4.7 0.26 3.9 3.2
spray 1 0.2 pulp ND/ND/ND2
peel 5.4/3.6/
peel 2.4
Orange dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.33 0.27 0.32 0.22 0.16
spray 0.1 pulp <0.05 0.05 <0.05 <0.05 <0.05
peel 0.24 0.31 0.35 0.26 0.17
dip 1 0.2 pulp 0.09
peel 3.5
? 0.2 pulp <0.04
peel 6.9
Tangerine spray 1 0.2 pulp 0.07 0.04
peel 3.9 3.3/4.9
spray 1 0.2 pulp ND/ND/0.2
peel 11.0/12.0/
23.3
spray 1 0.2 whole 2.4
fruit 2.8
3.4
1 With wax.
2 ND = not detectable.
from 0.2 to about 6.9 mg/kg. In one trial, samples of the peel
contained 12 and 23.5 mg/kg. There was no obvious decrease in the
residues of the peel, and therefore no diffusion of the residues from
peel to pulp occurred during storage.
FATE OF RESIDUES
General Observations
The fate of metalaxyl was studied in plants (potato, grapevine,
lettuce) and in animals (rat, goat) using randomly ring 14C-labelled
fungicide. These studies showed the matching primary points of
metabolic attack as follows:
Cleavage of the carboxyester bond to the free acid;
Cleavage of the methylether bond to the free alcohol;
Oxidation of one phenylmethyl group to benzyl alcohol derivative;
Hydroxylation of the phenyl ring to phenolic derivatives.
Reactions subsequent to these primary transformations were found
to be of the following three types:
N-dealkylation to acetanilide derivatives;
Oxidation of the benzyl alcohol derivatives to benzoic acid
derivative;
Conjugation of all types of metabolites to a different extent, in
plants with glucose, in animals with glucuronic acid.
The metabolites identified by chromatography and/or spectroscopy
in plant tissues, urine and faeces are shown in Figures 1 and 2.
Chemical names of metabolites are as follows:
II.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine methyl ester
III.N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-alanine
IV.N-(2-hydroxymethylene-6-methylphenyl)-N-(methoxyacetyl)-alanine
methyl ester
V.N-(2,6-dimethyle-5-hydroxyphenyl)-N-(methoxyacetyl)-alanine methyl
ester
VI.N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)-alanine methyl ester
VII.N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)-alanine
VIII.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine
IX.N-hydroxyacetyl-2,6-dimethyl-aniline
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales, which cause late blight, downy
mildews, damping-off and root, stem and fruit rots of many plants. The
compound is taken up by the roots, leaves, green stems and shoots and
transported acropetally within the plant (Urech et al 1977).
Commercial products first introduced as foliar sprays contained
only metalaxyl as the active ingredient or were mixtures of metalaxyl
with low rates of residual protective fungicides, such as copper or
folpet. Experience has now shown that strains of fungi resistant to
metalaxyl may develop, and in order to reduce the likelihood of this
happening and to broaden the spectrum of activity, commercial products
now contain a higher content of the residual fungicides mancozeb,
zineb, copper, folpet or carbendazim. A further measure to hinder the
development of resistance has been the limitation of the number of
sprays applied to the crop. This has generally been restricted to 3 to
4 applications, which are made during the active vegetative stage of
the crops. Commercial products containing metalaxyl only are intended
for use as seed dressing or for soil applications.
Metalaxyl is registered for use on a wide range of crops and in
several countries in temperate, subtropical and tropical regions.
Preharvest treatments
Metalaxyl is used for foliar sprays, seed dressing and soil
treatments. The main fields of application are in potatoes, grapevines
and vegetables. It is also used against diseases of tobacco and in
some non-edible crops such as ornamentals and turf.
Postharvest treatments
Metalaxyl, incorporated in wax, is applied as a pack-house spray
to citrus fruit for the control of postharvest rot. The recommended
application rates for the crops on which the compound is currently
used are given in Table 1.
Table 1. Recommended Application Rates of Metalaxyl
Crop Rate
(a.i.)
Foliar/Fruit sprays
Potato 150 - 250 g/ha
Onion 200 - 250 g/ha
Leafy vegetables 20 - 25 g/100 1
Brassicae 200 - 250 g/ha
Cucurbits 20 - 25 g/100 1
Tomato 20 - 40 g/100 1
Grape 20 - 30 g/100 1
Pineapple 25 g/100 1
Cocoa 50 - 312 g/100 1
Hops 20 - 30 g/100 1
Seed treatments
Sugarbeet 245 g/100 kg seed
Vegetables 26 - 53 g/100 kg seed
Peas 70 g/100 kg seed
Maize 175 g/100 kg seed
Millet 263 g/100 kg seed
Sorghum 175 g/100 kg seed
Sunflower 210 g/100 kg seed
Soil treatments
Red and green pepper (Capsicum annuum) 2 kg/ha
Black pepper (Piper nigrum) 1.25 - 2 g/m2
Citrus 1.25 - 2 g/m2
Avocado 2- 5 g/m2
Hops 0.2- 4 g/plant
Postharvest treatment
(pack-house spray)
Citrus 2 000 ppm a.i. in wax
1 3-4 applications.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Metalaxyl residue data have been obtained from numerous trials
carried out on a world-wide basis on main crops. Treatments were
generally made using WP or FW formulations with metalaxyl alone or
with mixtures containing residual fungicides. The dosages used in the
residue trials cover the recommendations as summarized in Table 1.
Analysis was carried out using methods determining metalaxyl
alone and/or metalaxyl and its metabolites containing the
2,6-dimethylaniline moiety.
Potatoes
Field trials were conducted in 14 countries during six years. Two
to eight applications of dosages ranging from 0.15 to 0.42 kg a.i./ha
were made at intervals of 1-3 weeks. The results of these trials are
reported in Table 2 (Ciba-Geigy undated; Finland undated). Generally,
no residues either of the parent compound or metabolites containing
the 2,6-dimethylaniline moiety were detectable in potato tubers.
Residues (0.02-0.08 mg/kg) were found in only eight of 102 samples.
The limit of determination ranged from 0.02-0.05 mg/kg.
Onions
Field trials were carried out in nine countries over four years.
Two to eight applications at dosages ranging from 0.22-0.6 kg a.i./ha
were made at intervals of 1-3 weeks. The results are shown in
Table 3 (Ciba-Geigy undated; Finland undated). Initial residues of
0.05-0.59 mg/kg generally dissipated below the limits of determination
(0.02 and 0.04 mg/kg) within 14 days. Dissipation of metalaxyl
metabolites containing the dimethylaniline moiety did not differ
significantly from that of the parent compound alone. Two weeks after
the last application, residues of metalaxyl and its metabolites ranged
between <0.05-0.09 mg/kg. The major part (73-100%) of the total
residue consisted of the parent compound.
Leafy Vegetables
Spinach
Field trials were carried out in Italy, The Netherlands,
Switzerland and the U.S. One to four applications (to run off) at
dosages ranging from 0.22-0.55 kg a.i./ha or at 0.043% were made at
intervals of 1-2 weeks. The results are reported in Table 4
(Ciba-Geigy undated). Residues of the parent compound rapidly
disappeared being at or below the limit of determination (0.05 mg/kg)
two weeks after the last application. Residues measured as the sum of
metalaxyl and its metabolites containing the dimethylaniline moiety
amounted to 1.1-7.3 mg/kg one week after the last application and
decreased to 0.27-5.0 mg/kg during the following two weeks.
Supervised trials were carried out on lettuce at six sites in the
U.K. The plants were treated at planting and after an interval of two
weeks with a formulation containing 48% mancozeb and 10% metalaxyl at
rates of 71-150 g metalaxyl/ha. The residues were below 0.1 mg/kg, the
limit of determination, in 19 samples taken after 13 to 58 days. In
the other samples, the residues were 0.55, 1.2 and 1.3 at day 21, and
0.25 mg/kg at day 35 (U.K.undated).
Table 2. Residues of Metalaxyl Following Supervised Trials in Potatoes (1976-1981)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Argentina 3 0.2-0.25 1-2 0.05
Austria 4 0.2 3 <0.03 <0.03 <0.03 <0.03 <0.03
Canada 4 0.3 2 <0.05
5 0.3 2 <0.05
6 0.25 1-2 <0.05
6 0.15 1-2 <0.05
3 0.3 2 <0.02
3 0.25 2 <0.02
4 0.25 2 <0.02 0.02
6 0.25 1-2 <0.02
6 0.3 1-2 <0.02
3 0.3 3 <0.02 0.02
2 0.3 3 <0.02
2 0.25 3 <0.02
8 0.16 1 <0.02 <0.02 <0.02
4 0.16 2 <0.02 <0.02
4 0.2 2 <0.02 <0.02
6 0.2 1-2 <0.02 <0.02 0.02 0.03
Fed. Rep. Germany 3 0.2-0.3 4 <0.05
3 0.2-0.3 4 <0.05
8 0.2 1 <0.05
8 0.2 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.04
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
0.02
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
Finland 2 0.25 3 <0.04
France 3 0.3 3 <0.04 1
4 0.2 2 <0.04 1
3 0.3 3 <0.02 1
U.K. 6 0.2 1-2 <0.05
5 0.3 3 <0.05 0.05 <0.05 <0.05
4 0.3 3 <0.05
6 0.3 2-3 <0.05
5 0.3 2-3 <0.05
5 0.3 2-3 <0.05
India 4 0.24 2 <0.02
Israel 5 0.25 1-2 <0.02
0.02
South Africa 5 0.2 2-3 0.02 <0.02
Spain 5 0.3 1-2 <0.05
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
Sweden 5 0.15 1-2 0.05 <0.05 <0.05
4 0.2 2 <0.02
4 0.2 2-3 <0.02
5 0.15 1-2 <0.02
3 0.25 3 <0.02
Switzerland 5 0.25 2 <0.05 <0.05 <0.05
<0.06
5 0.25 2 <0.05 <0.05 <0.05
3 0.2-0.3 3 <0.05
8 0.25 1 <0.05
8 0.2 1-2 <0.02
8 0.2 1-2 <0.02
U.S. 3 0.275 2 <0.05 2
6 0.275 2 <0.05
6 0.275 2 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 1 <0.05
6 0.275 2 <0.05
6 0.412 1 <0.05
6 0.42 2 0.06 2
0.08 2
Table 2. (con't)
Residues (mg/kg)
Country at interval after last application
Rate [a.i./ Interval (days)
No. kg/ha or %) (weeks) 1-3 6-8 11-15 20-22 27-29 32-36 41-43 55-75
4 0.42 2 <0.05
5 0.42 2 <0.05
6 0.275 2 <0.05 2
<0.05 2
<0.05 2
6 0.275 2 <0.05 2 <0.05 2
<0.05 2 <0.05 2
<0.05 2 <0.05 2
6 0.22 2 <0.05 2
6 0.22 2 <0.05 2
1 Peel and pulp were analysed separately, no sample contained measurable residues.
2 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 3 Residues of Metalaxyl Following Supervised Trials in Onions (1978-1981)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
Australia 6 0.4 2 <0.04 <0.04
4 0.2 1 0.30 0.12
4 0.3 1 0.59 0.12
Canada 3 0.25 2 <0.02
3 0.24 1-2 0.05 <0.02
4 0.24 1-2 0.31 0.06
3 0.24 1-2 <0.02
5 0.24 2 0.02
4 0.24 2 <0.02
4 0.25 1-2 0.05 0.04
3 0.25 1-2 0.03
Fed.Rep. Germany 3 0.25 2 0.06 <0.02 <0.02
3 0.25 2 0.05 0.02 <0.02
Finland 2 0.25 5 <0.04
4 0.25 1 <0.04
France 3 0.225 3 0.04
U.K. 2 0.5 2 <0.02
Italy 5 0.6 2 0.02
Switzerland 8 0.3 2 <0.05
Table 3 (con't)
Application Residues (mg/kg) at intervals
after last application (days)
Country Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 6-8 13-15 20-22 27-29 34-36
U.S. 1 5 0.275 1-2 0.15
5 0.55 1-2 0.16
5 0.275 1-2 <0.05 <0.05
5 0.55 1-2 0.06 <0.05
5 0.275 1-2 <0.05
5 0.55 1-2 <0.05
5 0.22 2 0.1 0.08
5 0.22 2 0.09 0.08
3 0.275 1-2 0.26
5 0.275 1-2 <0.05 <0.05 <0.05
5 0.55 1-2 <0.05 <0.05 <0.05
5 0.22 2 <0.05 <0.05
6 0.22 2 <0.05 <0.05 <0.05
6 0.22 2 0.08 <0.05 <0.05
5 0.22 2 0.20 0.09 <0.05
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 4 Residues of Metalaxyl Following Supervised Trials in Spinach
Applications Residues (mg/kg) at intervals after
Interval between last application (days)
Country Rate (a.i. applications
No. kg/ha or %) (weeks) 3 6-8 13-15 20-22 27-31
Italy 2 0.043 2 0.05
Netherlands 1 0.25 0.53 0.06 0.03 <0.03
Switzerland 2 0.25 2 0.53 <0.05 <0.05 <0.05 <0.05
U.S. 3 0.275 2 0.06 <0.05
2.9 1 2.2 1 1.0 1
3 0.55 2 0.67 <0.05
4.9 1 3.9 1 2.4 1
3 0.275 2 1.5 1 1.4 1 1.7 1
0.55 2 7.3 1 4.1 1 5.0 1
3 0.275 1 1.2 1 0.25 1 0.27 1
0.55 1 1.1 1 0.79 1 0.86 1
4 0.22 1-2 1.1 1
4 0.22 2 1.8 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Brassicas
Field trials were carried out in broccoli, Brussels sprouts,
cabbage and cauliflower in several countries over a period of five
years. Two to five applications at dosages from 0.2 to 0.55 kg a.i./ha
were made at intervals of 1-3 weeks. The results are reported in
Table 5 (Ciba-Geigy undated). Dissipation of residues was similar
within the various varieties. Initial residues of 0.17-2.0 mg/kg
rapidly decreased and were generally below 0.1 mg/kg after two weeks.
Residues measured as metalaxyl and its metabolites containing the
dimethylaniline moiety were somewhat higher and dissipated more
slowly. The parent compound amounted to 55% of the residue containing
the dimethylaniline moiety. Two weeks after last application of the
recommended dosage of 0.25 kg a.i./ha, residues were <0.05-0.2 mg/kg.
Fruiting vegetables
Field trials were carried out with cucumbers, gherkins, melons,
squash and watermelons in different countries. Three to eight
applications at dosages of 0.2-0.55 kg a.i./ha or 0.02-0.4% were made
at intervals of 1-2 weeks. The results are reported in Table 6
(Ciba-Geigy undated). Residue levels in the different commodities were
in the same range. Initial residues were 0.06-0.4 mg/kg, depending on
the dosage rate used. They decreased slowly and were 0.03-0.23 mg/kg
after two weeks. There was no difference between residue levels
determined as the parent compound or as the total of metalaxyl plus
metabolites.
Tomatoes
Field trials were carried out in seven countries over a period of
six years. Two to nine applications at dosages of 0.125-0.8 kg a.i./ha
or 0.02-0.08% were made at intervals from one to 22 days. The results
are listed in Table 7 (Ciba-Geigy, undated). In most samples, initial
residues were below 0.1 mg/kg. Residue levels of metalaxyl and its
metabolites were at the same magnitude as the parent compound alone.
Grapes
Numerous field trials were conducted over a period of six years
in nine countries representing important vine growing areas of the
world. Due to the different climatic areas, a wide range of
experimental conditions has been considered. One to nine applications
at dosages of 0.01-0.04% a.i. or 0.15-0.5 kg a.i./ha were made at
intervals of 1 to 4 weeks. The results of these trials are reports in
Table 8 (Ciba-Geigy undated).
Table 5. Residues of Metalaxyl Following Supervlsed Trials in Brassicas (1977-1981)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
Canada
(broccoli) 3 0.24 1-2 2.0 0.09 <0.02
3 0.25 1-2 0.56 0.12 0.05
2 0.25 3 0.10
(Brussels
sprouts) 5 0.25 2 0.39 0.12 0.05
(cabbage) 2 0.25 2 0.29 0.08
(cauliflower) 4 0.25 2 0.17 0.03
U.K.
(cauliflower) 2 0.2-0.3 2 0.09 0.02 0.01 0.02
2 0.2-0.3 2 0.09
2 0.2-0.3 2 0.47
South Africa
(brassicas) 5 0.2 1 0.16 <0.05
5 0.4 1 0.90 0.09
U.S.
(broccoli) 5 0.275 2 0.17
0.35 1 0.20 1
5 0.55 2 0.21
0.46 1 0.48 1
Table 5. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
(commodity) Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22 27-29 34-36
5 0.275 2 0.06 0.06
0.16 1 0.32 1
5 0.55 2 0.17 0.2
0.62 1 0.50 1
0.22 1 0.09 1
5 0.275 2 0.19-0.26
(cabbage) 0.41-0.52 1
5 0.55 2 0.1
0.31 1
5 0.275 2 0.15 1 0.19 1
5 0.55 2 0.35 1 0.26 1
5 0.275 2 0.20 1 0.07 1
5 0.55 2 0.33 1 0.17 1
5 0.275 2 0.12 1 0.10 1
5 0.55 2 0.13 1 0.12 1
5 0.275 2 0.07 0.06
0.22 1 0.09 1
5 0.55 2 0.22 1 0.16 1
5 0.22 2 0.09 1 <0.05 1
5 0.22 2 0.30 1 0.39 1
5 0.22 2 0.15 1 0.14 1
(cauliflower) 5 0.275 2 0.08 1 <0.05 1
5 0.55 2 0.14 1 0.10 1
5 0.275 2 0.17 1 0.36 1
5 0.55 2 0.56 1 0.38 1
5 0.22 2 <0.05 1 <0.05 1
1 The sum of metalaxyl and its metabolites containing the, common moiety 2,6-dimethylaniline,
expressed as metalaxyl equivalents.
Table 6. Residues of Metalaxyl Following Supervised Trials in Fruiting Vegetables
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Australia
(gherkins) 7 0.2 1 0.06 <0.04
7 0.4 1 0.12 <0.04
3 0.4 2 0.12 <0.04
(cucumber) 8 0.2 1 0.14 0.09 0.13
8 0.4 1 0.40 0.27 0.24
5 0.2 2 0.14 0.05
Israel
(cucumber) 8 0.25 2 0.25 0.14
(melon) 4 0.25 1 <0.05
South Africa
(cucumber) 4 0.04 2 0.30 0.25 0.22
4 0.02 2 0.26 0.19 0.19
Switzerland
(cucumber) 3 0.25 2 0.09 0.05
3 0.25 2 0.04 0.03
3 0.25 2 0.06 0.03
U.S.
(melon) 8 0.275 1 0.11 0.08
0.17 1 0.24 1
8 0.55 1 0.24 0.25
Table 6. (con't)
Applications Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
(cucumber) 8 0.275 1 0.15 1 0.17 1
8 0.55 1 0.30 1 0.27 1
8 0.22 1 0.13 1
8 0.22 1 0.12 1
(squash) 8 0.22 2 0.07 1
8 0.22 2 0.06
0.07
8 0.22 1 0.06
0.05 1
(watermelon) 8 0.275 1 0.09
0.12 1
8 0.55 1 0.13
0.16 1
8 0.275 1 0.05 1
8 0.55 1 0.05 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6 dimethylaniline,
expressed at metalaxyl equivalents.
Table 7. Residues of Metalaxyl Following Supervised Trials in Tomatoes (1976-1981)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
Brazil 7 0.2 0.2 0.5 <0.02
7 0.2 0.024 0.5 <0.02
France 4 0.03 2 <0.04
4 0.045 2 <0.04
2 0.125 0.5 <0.04 <0.04
4 0.5 0.5 2 0.09 0.04 <0.04
4 0.2 2 0.05 <0.04 <0.04 (0.04
Israel 3 0.25 0.04 2 <0.02
South Africa 7 0.4 0.04 1-2 0.04 0.07 <0.03 <0.03
7 0.8 0.08 1-2 0.30 0.10 0.04 <0.03
5 0.08% to 1 <0.03 <0.03 <0.03 <0.03
5 run-off 0.04 1 0.07 0.05 <0.05
Spain 3 0.1% to 2 0.48
run-off (1 day)
Switzerland 9 0.03% to 1-2 0.12 <0.04
run-off
Table 7. (con't)
Application Residues (mg/kg) at intervals after
last application (days)
Country Rate (a.i., Interval
No. kg/ha or %) (weeks) 1-3 5-8 13-15 20-22
U.S. 1 6 0.42 1 <0.05
6 0.42 1 0.18
8 0.42 1 <0.05
6 0.42 1 0.22
6 0.42 1 <0.05
6 0.42 1 0.10
5 0.42 1 0.22
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety
2,6-dimethylaniline, expressed as metalaxyl equivalents.
Table 8. Residues of Metalaxyl Following Supervised Trials in Grapes and Wine (1976-1981)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Australia 5 0.02 2 1.2 0.73 0.34
7 0.02 2-4 0.23 0.15 0.14
7 0.03 2-4 0.61 0.37 0.34 0.31
7 0.02 1-4 0.35 0.37 0.43
7 0.03 1-4 0.60 0.49 0.26
Canada 5 0.15 1-2 0.09 0.05
Fed.Rep. 6 0.3 1-2 2.1 3.4 2.2 1.3 1.3
Germany 6 0.4 1-2 2.4 1.4 1.9 1.1 1.4
8 0.2-0.4 1-3 8.0 6.8 4.4 4.2 5.0
8 0.2-0.4 1-3 2.4 2.9 1.9 2.4 1.2
8 0.15-0.4 2 2.1 1.3 1.7 1.7 0.46
6 0.08-0.16 2 0.55 0.4 0.36 0.45 0.15
7 0.12-0.32 2 0.47 0.47 0.49 0.50
7 0.12-0.32 1-2 0.67 0.62 0.51 0.48 0.36 0.37
6 0.24-0.4 2 0.79 0.84 0.53 0.51 0.18
6 0.13-0.54 2-3 2.1 3.5 4.1 2.6 1.2
6 0.3-0.4 1-2 2.9 1.8 2.6 0.13 0.38
8 0.16-0.24 2 0.51 0.34 0.28 0.50
3 0.14-0.3 2 0.2 0.18 0.03
3 0.2 2 0.2 <0.02 <0.02
3 0.2 2 0.15 0.08 0.02
3 0.12-0.36 2-3 0.39 0.23 0.05
3 0.36 1-2 0.02 <0.02
3 0.2-0.3 2 0.13 0.06 0.04
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
Switzerland 8 0.02-0.04 2 1.2 0.75 0.04 0.03 0.03 0.02
7 0.01-0.02 2 0.73 0.31 0.74 0.86 0.42 0.09
6 0.45-0.6 2-3 3.2 2.1 1.6 1.6
5 0.02-0.03 3-4 0.84 1.0 1.6 0.58
5 0.02-0.03 3-4 0.16 0.11 0.1 <0.05
7 0.45 2 0.77 0.77 0.70 0.60 0.61 0.26
5 0.4 1-2 2.8 1.6 2.5 2.2 2.6 1.8 2.1 0.3
5 0.4 1-2 2.7 2.7 1.3 1.7 1.6 1.4 0.3
8 0.2-0.4 2 2.9 0.94 0.84 0.53 0.8 0.8
5 0.3 1-4 1.0 1.1 0.59 0.95 0.71 0.35 0.4 0.19
5 0.3 1-4 2.2 1.4 1.6 1.1 0.79 0.69 0.86 0.74 0.19
8 0.3 1-4 1.2 1.0 0.7 0.53 0.45 0.5 0.46 0.20
6 0.4-0.63 2-3 3.3 1.9 1.8 0.91 1.1 0.49
6 0.3 2 0.23 0.21 0.24 0.11
France 1 0.3 1.4 0.9 0.65 0.4 0.1 0.04
1 0.3 1.5 0.44 0.68 0.23 0.06
9 0.07-0.45 2 <0.04 <0.02
7 0.07-0.32 2 0.26 0.10
6 0.37-0.5 2-3 0.06
7 0.03-0.21 2 0.08 0.06
Israel 1 0.25 0.14
1 0.23 0.14
Italy 8 0.03% 1-2 1.3 1.2
Table 8. (con't)
Application Residues (mg/kg) at intervals after
Country last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) 1-2 6-8 13-15 18-23 27-30 34-36 41-43 55-75 WINE
South Africa 8 0.03% to run off 2 1.2 0.92 0.96
9 0.03% " 2 0.72 1.1 0.49 0.55 0.14
8 0.02% " 2 0.64 0.42 0.31 0.37 0.1
8 0.02-0.03 " 2 5.7 5.5 3.4 2.0
1 0.03% " 5.7 3.5 3.3 2.4 2.0 0.6
1 0.03% " 4.9 2.7 2.8 1.6 1.6 0.41
Spain 6 0.2 2-3 0.14
6 0.2 2-3 0.66 0.14
6 0.2 2-3 1.25 0.11
6 0.2 2-3 0.11
7 0.03 1-3 0.30 0.07
7 0.03 1-3 0.24 0.03
7 0.03 1-3 0.21 0.04
5 0.3 3 0.22
5 0.23 3 0.20
4 0.24 2-4 0.13
4 0.24 3-4 0.06
Initial residues were 0.23-5.7 mg/kg. After two, four and six
weeks, the residues ranged between 0.14-8.0 mg/kg, 0.06-4.4 mg/kg and
0.03-2.6 mg/kg, respectively. The wide range of residue levels is
considered to be a consequence of the various climatic conditions.
Generally, residues are lower in dry and warm areas than in temperate
and more humid areas. Three to four weeks after the last applications,
residues of 0.06-1.3 mg/ kg were observed in warm areas, such as
Australia, Spain and Italy, whereas in Germany 0.34-.6.8 mg/kg were
found after the same interval. No obvious difference in residues were
observed among the various grape varieties. Residues still present in
grapes at harvest were further degraded during the wine-making
process, with residues of <0.02 to 1.2 mg/kg being detected in wine.
Most samples contained less than 0.5 mg/kg.
Pineapple
Two field trials were made in Australia. In the first one,
2 500 l/ha of 0.06% a.i. were applied once as a foliar drench. One
week later, no residues (<0.04 mg/kg) were observed in the flesh
whereas 0.39 mg/kg were detected in the skin. In the second experiment
2 500 l/ha of 0.08% a.i. were applied on the soil six times at
intervals of about four weeks. Ten months later, no residues
(<0.04 mg/kg) were detected either in the flesh or in the skin
(Ciba-Geigy undated).
Cocoa Beans
Field trials were carried out in Papua New Guinea and Cameroon.
The dosages were 0.05-0.5% a.i. (50-500 g a.i./100 l). One to five
applications at intervals of 3 to 4 weeks were made. Details and
results of the trials are reported in Table 9 (Ciba-Geigy undated). In
the raw beans, no measurable residue (<0.02 mg/kg) was observed.
Residues measured in dry and fermented beans ranged from <0.02 to
0.19 mg/kg.
Seed treatment
Residue trials with treated seed were conducted in nine
countries. The dosage ranged from 40 to 620 g a.i./100 kg seed,
depending on the various commodities tested, which were barley, maize,
peas, soybeans, sugarbeet, sunflower and wheat. No residues of either
metalaxyl (<0.02 or <0.04 mg/kg) or the parent compound plus
metabolites (<0.05 mg/kg) were detected in the edible parts of the
crops at harvest.
Soil treatment
Supervised trials were carried out after soil treatment in
avocados, citrus and pepper. Details and results of these residue
trials are reported in Table 10 (Ciba-Geigy undated).
Table 9. Residues of Metalaxyl Following Supervised Trials in Cocoa Beans
Applications Residues (mg/kg) at intervals
Country after last application (days)
Rate (a.i. Interval
No. kg/ha or % (weeks) Sample 1-3 6-8 13-15 20-22
Papua New Guinea 1 0.075 dry <0.04 <0.04 0.12 <0.04
5 0.5 4 fermented and dry 0.19
Cameroon 1 0.05 raw <0.02 1
2 0.075 3 raw <0.02
1 The peel of the pods contained 0.44-2.1 mg/kg, depending on the state of maturity.
Table 10. Residues of Metalaxyl Following Supevised Trials in Avocado, Citrus and Pepper after Soil Treatment
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Avocado (whole fruit)
(Australia) 1 5.0 <0.05
1 5.0 <0.05
1 5.0 <0.05
(South Africa) 4 2.5 4 <0.05 <0.05
3 2.5 8 <0.05
(U.S.) 1 3 2.5 12 0.53 0.85-1.5
3 5.0 12 2.6 1.2
3 2.5 12 0.18 0.09-0.13 0.11-0.13
3 5.0 12 0.17 0.11
3 2.5 12 0.34 0.63 0.75
3 5.0 12 0.50 0.53
Citrus
(South Africa) 2 2.0 10 0.02 2 <0.02 2
0.19 3 0.21 3
2 2.0 14 <0.02 2 <0.02 2
0.05 3 0.04 3
2.0 10 <0.02 2 <0.02 2
0.14 3 0.16 3
Pepper (Piper nigrum)
(Brazil) 2 1.25 4 0.04
2.5 4 0.66
Table 10. (con't)
Application Residues (mg/kg) at intervals after
Commodity/ last application (days)
Country Rate (a. i. Interval
No. kg/ha or g/m2 (weeks) 1-3 6-8 13-15 20-22 26-29 34-37 41-43 55-75
Pepper (Capsicum annuum)
(Bulgaria) 6 1-1.5 3 0.20
6 1.5 3 0.03
4 2.5 3 <0.02
(Italy) 3 1-2 5 0.07
1 All residues are the sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalent. The concentrations of the parent compound was below 0.05 mg/kg, the limit of determination in each
case.
2 Pulp.
3 Peel.
No residues of metalaxyl (<0.05 mg/kg) were detectable in
avocado fruits one to six weeks after the last application. Residues,
however, were detected with the method used for analysing the parent
compound and the metabolites containing the dimethylaniline moiety.
These residues ranged from 0.11 to 2.6 mg/kg.
After two soil applications (at 2 g a.i./m2), residues
in Valencia oranges were at or below the limit of detection
(<0.02 mg/kg) in the pulp and 0.04-0.21 mg/kg in the peel. The
portion of the peel was 30% of the whole fruit.
In Brazil, residues up to 0.66 mg/kg were detected in
Piper nigrum five weeks after a second soil treatment at
2.5 kg a.i./ha (i.e. 1.5 g/m2).
Residues from <0.02 to 0.2 mg/kg resulted from soil treatments
of Capsicum annuum in Bulgaria and Italy.
Hops
In field trials carried out in the Federal Republic of Germany,
U.S., U.K. and Yugoslavia, metalaxyl was applied to the soil at
0.2-4 g a.i./plant or 0.55-1.1 kg a.i./ha and/or sprayed to run-off as
foliar treatment at 20-30 g a.i./100 l(from -0.24 to 1 kg a.i./ha).
Details and results of the trials are reported in Tables 11 and 12
(Ciba-Geigy undated).
After soil applications residues of metalaxyl were <0.1 to
0.9 mg/kg in dried hops at harvest. After foliar applications residues
of 5-30 mg/kg were found in green hops immediately after treatment.
These residues decreased rapidly and, except in one case, were between
2 and 6 mg/kg in dried hops sampled 7 days after treatment. In beer,
the levels of metalaxyl were below 0.1 mg/kg. No measurable residues
were found in beer when hops had received soil applications only.
Postharvest treatment
Citrus
For controlling postharvest rot in citrus, the fruits are sprayed
or dipped in formulations with or without wax at concentrations of
0.1-0.2% metalaxyl. Supervised residue trials were conducted under
laboratory conditions and in pack-houses. After treatment, the fruits
were stored at about 12°C. Details and results of the supervised
trials are reported in Table 13 (Ciba-Geigy undated; Israel 1980;
Sweden 1980). Analysis of peel and pulp showed that the latter is
largely free of residues (3 samples in 10 trials showed residues
ranging from 0.07-0.2 mg/kg). Residues in the peel generally ranged
Table 11. Residues of Metalaxyl Following Supervised Trials in Hops and Beer After Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application (months)
Rate (a.i. Interval 1-3 3-4 4-5
No. kg/ha or G/plant) (weeks) green dry green dry green dry BEER
Fed.Rep. Germany 7 0.1-0.25 2 0.71
1 0.2 <0.2 <0.02 <0.005
1 0.2 <0.1 <0.1 <0.005
1 0.2 0.18
2 0.2-0.4 8 0.44 0.33
2 0.2-0.4 8 <0.1 <0.1 <0.005
2 0.2-0.4 8 0.12 0.13
2 0.3 8 <0.1 0.25 <0.005
2 0.3 8 <0.1 0.40 <0.005
2 0.3 8 0.23 0.11
1 4.0 0.25 0.18 <0.01
1 4.0 <0.1 0.22 <0.01
1 4.0 0.54 0.93 <0.01
U.S. 1 0.55 <0.05
0.45 1
1 0.55 0.18
1.1 1
1 0.55 0.18 1 1.2 1
1 1.1 0.20 1 3.4 1
1 The sum of metalaxyl and its metabolites containing the common moiety 2,6-dimethylaniline, expressed as
metalaxyl equivalents.
Table 12 Residues of Metalaxyl Following Supervised Trials in Hops and Beer after Foliar and Soil Treatment
Application Residues (mg/kg) at intervals
Country after last application(days)
Soil Foliar Interval 0 5 7 14-16 20 28-36
No. (g a.i./plant) (kg a.i./ha) (weeks) green green dried dried dried dried BEER
Fed.Rep. Germany 12 0.55-2.0 1-2 22 6.3 6.3 0.06
12 0.55-2.0 1-2 6.2 5 2.1 <0.05
12 0.55-2.0 1-2 38 5.6 2.1 0.06
12 0.24-0.9 1-2 17.2 3.5 1.8 <0.05
12 0.24-0.9 1-2 5.4 1.5 0.5 <0.05
12 0.24-0.9 1-2 1.6 2.7 1.7 0.05
12 0.24-0.9 1-2 14 1.7 3.4 0.03
12 0.24-0.9 1-2 29 6.8 12 0.08
12 0.24-0.9 1-2 22 4.4 2.2 <0.01
1+12 0.2 and 0.3-1.0 1-2 10 0.5 2.5 0.02
1+12 0.2 and 0.3-1.0 1-2 31 9.1 6.0 0.04
1+12 0.2 and 0.3-1.0 1-2 25.2 3.9 2.7 0.04
1+12 0.2 and 0.3-1.0 1-2 2 3.1 1.8 0.02
U.K. 8 0.15-0.33 2 1.5
8 0.1-0.2 2 0.9
8 0.03% 1-2 1.4
8 0.03% 2 0.3
Yugoslavia 1 0.03% 2.2
2 0.03% 1 1/2 2.5
Table 13. Residues of Metalaxyl Following Supervised Trials in Citrus after Postharvest Treatment
Commodity Application Residues (mg/kg) at
Rate storage intervals (days)
(a.i. Fruit
Method in %) part 0-3 5-7 14-15 28-29 38-44 51-64
Grapefruit Dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.94 0.94 0.96 0.96 0.42
spray 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.30 0.36 0.21 0.20 0.11
spray 1 0.1 pulp 0.12 0.07 0.09 0.15
peel 4.7 0.26 3.9 3.2
spray 1 0.2 pulp ND/ND/ND2
peel 5.4/3.6/
peel 2.4
Orange dip 0.1 pulp <0.05 <0.05 <0.05 <0.05 <0.05
peel 0.33 0.27 0.32 0.22 0.16
spray 0.1 pulp <0.05 0.05 <0.05 <0.05 <0.05
peel 0.24 0.31 0.35 0.26 0.17
dip 1 0.2 pulp 0.09
peel 3.5
? 0.2 pulp <0.04
peel 6.9
Tangerine spray 1 0.2 pulp 0.07 0.04
peel 3.9 3.3/4.9
spray 1 0.2 pulp ND/ND/0.2
peel 11.0/12.0/
23.3
spray 1 0.2 whole 2.4
fruit 2.8
3.4
1 With wax.
2 ND = not detectable.
from 0.2 to about 6.9 mg/kg. In one trial, samples of the peel
contained 12 and 23.5 mg/kg. There was no obvious decrease in the
residues of the peel, and therefore no diffusion of the residues from
peel to pulp occurred during storage.
FATE OF RESIDUES
General Observations
The fate of metalaxyl was studied in plants (potato, grapevine,
lettuce) and in animals (rat, goat) using randomly ring 14C-labelled
fungicide. These studies showed the matching primary points of
metabolic attack as follows:
Cleavage of the carboxyester bond to the free acid;
Cleavage of the methylether bond to the free alcohol;
Oxidation of one phenylmethyl group to benzyl alcohol derivative;
Hydroxylation of the phenyl ring to phenolic derivatives.
Reactions subsequent to these primary transformations were found
to be of the following three types:
N-dealkylation to acetanilide derivatives;
Oxidation of the benzyl alcohol derivatives to benzoic acid
derivative;
Conjugation of all types of metabolites to a different extent, in
plants with glucose, in animals with glucuronic acid.
The metabolites identified by chromatography and/or spectroscopy
in plant tissues, urine and faeces are shown in Figures 1 and 2.
Chemical names of metabolites are as follows:
II.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine methyl ester
III.N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-alanine
IV.N-(2-hydroxymethylene-6-methylphenyl)-N-(methoxyacetyl)-alanine
methyl ester
V.N-(2,6-dimethyle-5-hydroxyphenyl)-N-(methoxyacetyl)-alanine methyl
ester
VI.N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)-alanine methyl ester
VII.N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)-alanine
VIII.N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)-alanine
IX.N-hydroxyacetyl-2,6-dimethyl-aniline

 Metabolic pathways are similar in plant and in animals. No
evidence for the presence of 2,6-dimethylaniline in free or conjugated
form was found in either.
In Plants
Potato
Potato plants eight weeks old were sprayed with 14C-ring
labelled metalaxyl until run-off at a rate corresponding to
0.2 kg a.i.(500 l ha.). The treatment was repeated four times at
10-day intervals. In the mature plant, only about 1.5% of the
radioactivity was found, with more than 90% in the shoots. The tubers
contained 0.02 mg/kg metalaxyl equivalents, which indicates some
basipetal translocation.
No parent compound could be found in tubers. Radioactivity
consisted exclusively of polar metabolites. Due to the very low
amount, exact identification was not possible. In the shoots,
compounds II, IV, VI and VII (Figure 2) were identified as the main
metabolites, which is evidence for two independent degradation
pathways utilizing ring methyl oxidation and hydrolysis of the
methylester bond (Gross 1979a). Neither metalaxyl nor its degradation
products were taken up by the tubers directly from soil containing
approximately 0.5 mg/kg metalaxyl equivalents (Gross 1977).
Grape
Grapevines were sprayed six times at 14-day intervals until run-
off with 14C-ring labelled metalaxyl equivalent to 30 g a.i./100 l
water. At the time of harvest, a total of 1.4 mg/kg metalaxyl
equivalents was found in the grapes. The portion of unchanged
metalaxyl was 0.83 mg/kg (60%) (Gross 1979b), In another experiment
with an overdosage (7 treatments, 50 g a.i./100 l) application, the
total radioactivity in the mature grapes corresponded to 3.06 mg/kg
metalaxyl equivalents 52 days after the last application; the portion
of unchanged metalaxyl was 1.96 mg/kg (64%) (Gross 1978). Metabolites
III, IV, V and VIII, were identified by chromatography and
spectroscopy in both grapes and foliage. The presence of these
metabolites is evidence for independent degradation pathways that
utilize ring methyl oxidation, ring hydroxylation and hydrolysis of
the methylester and methylether bonds. All metabolites formed were
found partially conjugated with sugar (Gross 1979b). The non-
extractable radioactivity amounted to 9.4 and 4.2% of total
radioactivity in grapes and leaves, respectively.
Lettuce
Lettuce plants grown in a greenhouse were treated twice with
14C-ring labelled metalaxyl at two-week intervals at a rate
corresponding to 0.25 kg a.i./ha. The radioactivity in the green parts
was analysed two weeks after the second treatment. Less than 20% of
the total radioactivity applied was found in the aerial parts,
primarily in the first leaves directly sprayed with the fungicide;
21.5% of the total radioactivity was characterized as unchanged
metalaxyl, the rest consisted of several polar products (Gross:
1979c). Metabolites isolated from the leaves were identified as III,
IV (5.1%), V (1.6%), VI, VIII and IX. These metabolites were found
partially conjugated with glucose. A significant portion (27.7%) of
the total radioactivity was non-extractable (Gross 1980).
In Animals
A lactating goat was administered ring 14C-labelled metalaxyl by
capsule at a level of 7.00 ppm in the feed for ten consecutive days.
Urine, faeces, milk, volatiles and CO2 were collected daily. The goat
was sacrificed 24 hours after the last dosage. Analytical data on
urine and faeces indicate metalaxyl was metabolized rapidly and the
total amount administered was excreted within approximately 24 hours.
The recovery of the radioactivity applied was 107.22%, with most being
excreted in the urine (93.92%) and faeces (11.60%). A small amount of
radioactivity was found in milk (0.003 mg/kg), blood (0.06%) and
tissues (0.87%). The level of radioactivity expressed in metalaxyl
equivalents was less than 0.06 mg/kg in all tissues; milk, kidney
and liver contained 0.003 mg/kg, 0.019 mg/kg and 0.057 mg/kg,
respectively. The level of radioactivity in the tissues analysed was
<0.06. Neither metalaxyl nor its metabolites accumulated in tissues
of goats.
Two-dimensional TLC comparisons of the metalaxyl metabolites in
goat urine and rat urine revealed the same pattern of products
(conjugated highly polar acidic compounds) and suggest that the same
metabolic pathways described for the rat also take place in the goat
(Fischer et al 1978).
In Processing
Grapes originating from metabolism studies carried out under
field conditions, and containing 1.4 or 3.06 mg/kg 14C-metalaxyl
equivalents, were shredded in a food cutter and the juice was pressed
off. The total residue in the juice was 0.9 or 1.04 mg/kg metalaxyl
equivalents, with the portion of unchanged metalaxyl being ca.
0.5 mg/kg. Most of the radioactivity (approximately 90%) remained in
the presscake (Gross 1978, 1979b). The elimination of metalaxyl
residues during processing from grapes to wine under practical
conditions is shown also in Table 8. These data demonstrate that, on
average, the level of metalaxyl residues in wine is one third of the
original concentration in the grapes at harvest.
Orange fruit treated postharvest with metalaxyl was subjected to
juice extraction by the Brown technique under industrial conditions.
This technique is based on a procedure in which the fruit is sliced in
half the the juice squeezed from the two halves by rotating reamers.
Using this technique, oranges containing 3.5 mg/kg metalaxyl in the
peel and 0.09 mg/kg in the pulp (Ciba-Geigy undated) produced juice
with 0.07 mg/kg metalaxyl.
As shown in Tables 12 and 13, residues present in dried hops
result in negligible residues in beer (generally below 0.05 mg/kg).
These results were to be expected, as residues in dried hops average
2 to 6 mg/kg and the maximum amount of hops used in beer brewing is
400 g/100 l beer.
In Soil
Degradation in soil was studied under laboratory and field
conditions. In laboratory studies using 14C-ring labelled metalaxyl,
a rapid and extensive degradation was observed. In aerobically
incubated soil, the half-life was found to be ca. 40 days. Less than
2% of metalaxyl was present in the soil after one year. Degradation
proceeded mainly via cleavage of the methylester bond yielding the
corresponding acid (compound III, Figure 2) as the transient
degradation product. A pronounced evolution of 14CO2 was observed
(25% within 12 months) indicating mineralization of the fungicide.
Degradation was slower under anaerobic conditions. No degradation was
observed in autoclaved soil, indicating the importance of
microorganisms in the degradation of metalaxyl (Ellgehausen 1978).
In order to follow the dissipation of metalaxyl and its main
metabolite, the free acid (compound III) in soil under field
conditions, the fungicide was applied in a single treatment at
2 kg a.i./ha on plant-free silty and sandy soils in Switzerland
(Büttler 1977a, 1979a). Residues of metalaxyl were also determined
after repeated applications to potatoes at 0.15-0.2 kg a.i./ha. In all
trials sampling was carried out at several intervals after the last
application. Soil samples were taken from different soil layers. The
results are summarized in Table 14 (Büttler 1977a; Ciba-Geigy
undated). Most of the residue is limited to the upper 10 cm soil
layer. Metalaxyl residue alone could be detected in soil only at a
depth of 0-10 cm 180 days after spraying the soil at a rate of
2 kg a.i./ha. Neither the parent compound nor metabolite III were
detectable in samples taken to a depth of 90 cm 314 and 406 days after
application (Büttler 1977b). The metalaxyl residue did not leach
deeper than 40 cm. The main metabolite (III) was observed only in the
0-20 cm soil layer and always in smaller concentrations than the
parent compound.
Table 14. Dissipation of Metalaxyl (M) and Its Metabolite (III) in Soil
Soil Application Soil depth Residues (mg/kg) at interval (days) after
rate (cm) Compound last application
Type (kg a.i./ha) 0-7 14-21 18-31 39-44 50-60 90-122
Sandy loam 2 to plant-free soil 0-10 M 1.8 0.66 0.23
III 0.34 0.25 <0.05
10-20 M 0.21 0.09 0.12
III 0.09 <0.05 <0.05
20-30 M 0.08 <0.05 <0.05
III <0.05 <0.05 <0.05
30-40 M 0.1 <0.05
III <0.05 <0.05
40-60 M <0.05 <0.05
III <0.05 <0.05
Silt loam 8×0.16 to potato M 0.18 0.09
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.21 0.05
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.13 0.09
plants III <0.05 <0.05
Unspecified 5×0.15 to potato 0-20 0.02 0.02
plants
Unspecified 5×0.25 to potato 0-10 M 0.21
plants 10-60 1 M <0.05
1 Residue was measured individually in each 10-cm soil layer.
In Storage
Experiments have shown that samples may be deep-frozen for long
periods without appreciable loss of residues. Residue levels were
determined in lettuce samples fortified with metalaxyl at 5 mg/kg and
field treated samples stored at -20°C for 81-337 days. During this
period 96-114% of the fortified amount and 121-145% of the initial
residue of the field samples (corrected for recoveries) were recovered
(Büttler 1979b). It is presumed that the higher values obtained after
storage are due to loss of water. Studies have also been carried out
on the stability in storage at -15°c using the method for the
determination of the sum of metalaxyl and its metabolites containing
the 2,6-dimethyl-aniline moiety. Cured tobacco and potato samples
fortified with metalaxyl were used. No loss of residues occurred
during a storage period of 18 months. The recoveries averaged 89 + 9%
for tobacco and 101+12% for potatoes (Ross 1980).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
The result of a selective survey on residues in lettuce samples
at commercial outlets in the United Kingdom during 1980/81, was
provided to the Meeting and is summarized below (U.K. undated):
Residue range No. of samples in
(mg/kg) the range
<0.1 90
0.1-0.5 83
0.51-10 18
1.1-2.0 5
>2
METHODS OF RESIDUE ANALYSIS
Several residue methods, all based on gas chromatography, have
been developed for the analysis of agricultural commodities. The
limits of determination are usually 0.02 mg/kg for metalaxyl and
0.05 mg/kg for the sum of metalaxyl and its metabolites containing the
dimethylaniline moiety.
For analysis of the parent compound in vegetables, cereals and
fruits, the sample is extracted with methanol in a high speed
homogenizer. The methanol extract is diluted with water and cleaned up
by water-methanol/dichlormethane partitioning. The dichloromethane
phase is evaporated and the residues of the organic phase is cleaned
up by alumina column chromatography. The appropriate fraction is then
examined for metalaxyl by gas chromatography on 3% Carbowax
20M/GasChrom Q packing at 185°C, using either an alkali flame
ionization detector (AFID) or a Coulson nitrogen specific detector
(Ramsteiner 1976a).
For hops and avocados a more elaborate extraction and clean-up
procedure is necessary. The sample is extracted with acetone, which is
evaporated to dryness. The oily residue is dissolved in methanol and
the interferring co-extractives are precipitated with Celite (R) and
FeCl3/CuSO4 solution. After filtration, the active ingredient is
partitioned into toluene. The toluene phase is evaporated, and the
residue is cleaned up by alumina column chromatography [Ramsteiner
1976B). Final determination is made by gas chromatography using either
AFID or a Hall electrolytic conductivity detector (HECD). The limit of
determination of this method is 0.1 mg/kg except for hops when
detected with AFID, where the limit of determination is 0.2 mg/kg.
The analytical procedure was simplified for wine and beer. The
sample is cleaned up by partition chromatography with n-hexane on an
EXTRELUT(R) column. The eluate is further cleaned up by passage
through an alumina column before the final determination of the active
ingredient by gas chromatography, using a nitrogen-phosphorus flame
ionization detector. The limit of determination of this method in beer
and wine is 0.005 mg/kg (Büttler 1980).
Recovery experiments have shown that the standard method
(Ramsteiner 1976a) recovers 98 ± 12% of the active ingredient at
fortification levels of 0.05 and 0.5 mg/kg. With the method developed
for hops and avocados, recoveries were 83 ± 15% at fortification
levels of 0.2 and 1 mg/kg (Ramsteiner 1976b). In wine and beer
recoveries were 102 ± 8% at fortification levels ranging from
0.01-0.4 mg/kg [Büttler 1980).
Metalaxyl residues can be determined in grapes and vegetables by
applying the method of Ambrus et al (1981). The samples are
extracted with acetone, the extract is diluted with water containing
2% Na2SO4 and partitioned into dichloromethane. Potato and tomato
can be analysed directly using a nitrogen specific detector while
other extracts have to be cleaned up on neutral alumina column
(V. activity grade). Metalaxyl is eluted in the second fraction. The
limit of determination is 0.01-0.02 mg/kg (Hungary 1982).
The method for analysis of metalaxyl and its metabolites
is based on the hydrolysis of all derivatives containing the
2,6-dimethylaniline moiety (2,6-DMA) and final determination of
2,6-DMA. The residues are extracted by blending with 20% water/
methanol. The sample extract is evaporated and refluxed with
phosphoric acid in the presence of cobalt chloride. The solution is
then made basic and the 2,6-dimethylaniline formed is steam distilled.
The product is derivatized with trichloroacetyl chloride to minimize
problems of volatility of 2,6-dimethylaniline. The derivative is
cleaned up by alumina column chromatography and analysed by gas
chromatography, using a nitrogen specific alkali flame ionization
detector. The limit of determination for the method is 0.05 mg/kg
expressed in metalaxyl equivalents. The percent of recovery of
metalaxyl from potato tubers fortified at 0.05 to 1 mg/kg was
72 ± 10%, from cucurbits fortified at 0.05 to 0.4 mg/kg 72 ± 11% and
from cole crops fortified with 0.05 to 0.5 mg/kg 59 ± 11%
(Balasubramanian 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs and preharvest intervals reported in Table 15
have been established on the basis of local conditions.
Table 15. National Maximum Residue Limits Reported to the Meeting
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Australia Grapes 1.0 7
Custard apples, figs,
mangoes, passionfruit 0.5 7
Leafy vegetables 0.3 4
Cucurbits 0.2 4
Pineapples 0.1 28
Vegetables (other than
leafy veg. and cucurbits) 0.1 7
Allium 0.1
Avocados 0.05 7
Austria Hops 2.5 14
Grapes 0.1 21
Czechoslovakia Potatoes 21
Fed. Rep.Germany Potatoes 7
Hops 10
Grapes 35
France Grapes 0.6 15
Hungary Grapes, hops, soya 0.2 21
Italy Grapes 1.0 28
Onion, pepper, spinach,
tomatoes 1.0 10
Potato 1.0
Strawberry 0.5 40
Table 15. (con't)
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Israel Grapes 2.0 21
Citrus 2.0
Cruciferae, tomato 0.3 14
Industrial tomato 0.3 3
Netherlands Citrus 5.0
Cole crops, potato 0.05 28
Lettuce 0.05 28
South Africa Grapes 1.5 21
Potato 0.2 14
Tomatoes 0.1 7
Avocados, cruciferae, citrus 0.05 30
1
Switzerland Grapes 2.0
Wine 0.6
Potato 0.1 14
Onion 0.05 14
U.K. Potato, hops 14
Yugoslavia Hops 7
Grapes 28
1 Last treatment at end of August.
APPRAISAL
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales. It is formulated in combination
with various fungicides, such as mancozeb, zineb, copper, folpet or
carbendazim. In the active ingredient of the commercial products the
weight ratio of metalaxyl varies approximately in the range of 0.4 to
0.1. Metalaxyl is used for foliar sprays, seed dressing and soil
treatment at dosage rates of 150-200 g a.i./ha, 0.26-2.63 g a.i./kg
seed and 1.25-5 g/m2 or 2 kg a.i./ha in soil, respectively.
Residues in crops following foliar and soil treatment are
generally under 1 mg/kg one to three days after last application and
decline below 0.2-0.5 mg/kg one or two weeks thereafter. Lettuce
treated at the recommended rate contained residues up to 2 mg/kg 21
days after last application. The foliar treatments of grapes and hops
result in residues up to 5 and 10 mg/kg, respectively, at 14 days
after application. Crops harvested after seed dressing do not contain
residues above the limit of determination (0.02 mg/kg). Residues in
citrus fruits treated postharvest amounted to ca. 4 mg/kg and were
concentrated mainly in the peel. The residue in the pulp of different
varieties was below 0.2 mg/kg. The data on Brussels sprouts, cocoa
beans, pepper and pineapple were not sufficient to make
recommendations.
Metalaxyl is partly taken up by the roots, leaves, green stems
and shoots and transported acropetally within the plant. The following
reactions were found to be involved in the metabolic transformations:
cleavage of the carboxyester and methylether bonds, oxidation of the
phenylmethyl group and the consequent benzyl alcohol derivative,
hydroxylation of the phenyl ring, N-dealkylation, conjugation with
glucose in plants and with glucuronic acid in animals.
Most of the residue was detected in the leaves and shoots,
in which N-(2,6, dimethyl-phenyl)-N-(metoxyacetyl)-alanine,
N-(2-hydroxymethylene-6-methylphenyl)-N(methxyacetyl)-alanine methyl
ester, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methyl-phenyl)-N-methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)alanine
were identified as the main metabolites.
The parent compound amounted to a minimum of 55% and 60% of the
total residue containing dimethyl-aniline moiety in brassicas and
grapes, respectively, while there was no practical difference between
residue levels determined as parent compound or as the sum of
metalaxyl and its metabolites in fruiting vegetables.
Most of the total residue in spinach consisted of metabolites
containing the 2,6-dimethylanalinine moiety. Total residue levels
ranged from 1.1 mg/kg to 7.3 mg/kg, while the concentration of parent
compound ranged from 0.03 to 0.67 mg/kg in samples taken 6-8 days
after the last application. Based on experimental data, the estimated
maximum residue level, expressed as the total residue or as the parent
compound alone, would be 10 mg/kg or 1 mg/kg, respectively.
A lactating goat kept on a diet containing 7 ppm metalaxyl for
ten consecutive days excreted most of the radioactivity in the urine
and faeces within approximately 24 hours after administration. The
blood and tissues contained 0.06 and 0.87% of the total radioactivity
and 0.003 mg/kg of metalaxyl equivalents was detected in the milk,
Neither metalaxyl nor its metabolites accumulated in the tissues.
No evidence for the presence of 2,6-dimethylaniline in free or
conjugated form was found either in plants or animals. The metabolic
pathways are similar in plants and in animals.
Processing of grapes, oranges and hops treated with metalaxyl
at recommended rates results in detectable residues in the final
products. The wine contains approximately one third of the residue
found in grapes at harvest. The residue in orange pulp (less than 5%
of the residue in the whole fruit) is transferred to the juice, while
beer contains negligible residue (<0.1 mg/kg) compared to the level
in hops. The residue level remains constant in crops stored at or
below -15°C for long periods.
The degradation of metalaxyl in soil is rapid under aerobic
conditions while it is slower under anaerobic conditions and no
degradation was observed in sterilized soil. No residue was detectable
(<0.02-0.05 mg/kg) one year after application under field conditions.
The majority of residues was in the upper 10-cm soil layer. The main
metabolite (N-(2,6-dimethylphenyl)-N-(methoxyacetyl-alanine) was only
observed in the 0.20 cm soil layer and at a distinctly smaller
concentration than the parent compound.
Analytical methods based on methanol or acetone extraction,
water/methanol or dichlormethane partition, cleanup on an alumina
column and detection with nitrogen specific detectors are available
for the determination of the parent compound in various samples. These
methods are suitable for regulatory purposes. Multi-residue methods
based on similar processes seem to be applicable as well for the
determination of metalaxyl. The total residue containing the
2,6-dimethylaniline moiety may be determined after hydrolysis to
dimethylaniline and derivatization.
RECOMMENDATIONS
The Meeting concluded that the levels listed below are suitable
for establishing MRLs. The limits refer to the parent compound.
FURTHER WORK OR INFORMATION
Desirable
Additional residue data derived from supervised trials on
Brussels sprouts, cocoa beans, peppers and pineapple.
Preharvest intervals on
Estimated maximum which recommendations
Commodity residue levels (mg/kg) are based (days)
Hops (dried) 10 14
Citrus fruit 5 postharvest application
Grapes 5 14
Lettuce 2 21
Spinach 1 7
Broccoli, cabbage,cauliflower 0.5 7
Onion 0.05 1 21
Cucumber, gherkins,tomato 0.5 3
Melon, squash, watermelon 0.2 7
Potato 0.1 7
Avocado 0.1 1 7
Cereal grains 0.05 1
Sugarbeet 0.05 1 seed treatment
Sunflower seed 0.05 1
Peas 0.05 1
1 Level at or about the limit of determination.
REFERENCES
Ambrus, A., Lantos, J., Visi, E., Csatlos, I. and Sarvari, L. General
1981 method for the determination of pesticide residues in
samples of plant origin, soil and water. I. Extraction and
cleanup, Journal Assoc. Off. Anal. Chem. 64:733-768.
Balasubramanian, K. Analytical method for the determination of total
undated residues of metalaxyl in crops as 2,6-dimethylaniline.
Report AG-348 from Ciba-Geigy, U.S. (Unpublished).
Büttler, B. CGA 48988 and metabolites in soil. Residue reports RVA
1977a 252/77 A and 253/77 A from Ciba-Geigy, Basle. (Unpublished)
1977b CGA 48988 in soil. Residue report RVA 218/77 from CG, Basle.
(Unpublished)
1979a CGA 48988 and CGA 62826 in soil. Residue reports RVA 273 and
274/79A from Ciba-Geigy, Basle. (Unpublished)
1979b Stability of CGA 48988 in lettuce during frozen storage at
-20°C. Report SPR 17/79 from Ciba-Geigy, Basle.
(Unpublished)
1980 CGA 48988: Gas chromatographic determination of residues in
wine and beer. Report No. REM 1/80 from Ciba-Geigy, Basle.
(Unpublished)
Ciba-Geigy. 299 residue reports. [Unpublished)
undated
Ellgehausen, H. Degradation of CGA 48988 (RIDOMILR) in soil under
1978 aerobic, aerobic/anaerobic and sterile/aerobic conditions.
Report PR 08/78 from Ciba-Geigy, Basle. (Unpublished)
Finland Information on pesticides included in the JMPR priority list.
undated
Fischer, et al. Balance and metabolism of 0-14C-CGA-48988 in a
1978 lactating goat. Report No. 78046 from Ciba-Geigy, U.S.
(Unpublished)
Gross, D. Metabolism of CGA 48988 in field grown potato plants. Report
1977 PR 30/77 from Ciba-Geigy, Basle. (Unpublished)
1978 Metabolism of CGA 48988 in grapevine Report PR 11/78 from
Ciba-Geigy, Basle. (Unpublished)
1979a Identification of metabolites of CGA 48988 (RIDOMILR) in
field grown potato plants Report PR 39/79 from Ciba-Geigy,
Basle. (Unpublished)
1979b Identification of metabolites of CGA 48988 (RIDOMILR) in
grapevine. Report PR 06/79 from Ciba-Geigy, Basle.
(Unpublished)
1979c Fate of CGA 48988 in lettuce. Report PR 38/79 from Ciba-
Geigy, Basle. (Unpublished)
1980 Identification of degradation products of CGA 48988
(RIDOMILR) in lettuce. Report PR 38/80 from Ciba-Geigy,
Basle. (Unpublished)
Hungary Information on pesticides included in the JMPR priority
1982 list.
Israel Residues of Ridomil in citrus fruit. Ministry of
1980 Agriculture, Jerusalem. (Unpublished)
Ramsteiner, K. CGA 48988: Gas chromatographic determination of
1976a residues in soil, vegetables and grapes. Report REM 16/76
from Ciba-Geigy, Basle. (Unpublished)
1976b CGA 48988: Gas chromatographic determination of residues in
hops and tobacco. Report from Ciba-Geigy, Basle.
(Unpublished)
Ross, J.A. Stability of residues of metalaxyl and its metabolites
1980 under freezer storage conditions. Report ABR-80028 from
Ciba-Geigy, U.S. (Unpublished)
Sweden Results of analysis of Ridomil in postharvest treated
1980 oranges, 1980-06-27. Statens Lantbrukskemiska, Upsala.
(Unpublished)
Urech, P.A., Schwinn, F. and Staub, T. CGA 48988, a novel fungicide
1977 for the control of late blight, downy mildews and related
soil-borne diseases. Proceedings of the 1977 British Crop
Protection Conference, p, 623-631.
U.K. Submission to JMPR on metalaxyl (with mancozeb) residues
undated in protected lettuce (prepared by J.W. Edmunds).
Metabolic pathways are similar in plant and in animals. No
evidence for the presence of 2,6-dimethylaniline in free or conjugated
form was found in either.
In Plants
Potato
Potato plants eight weeks old were sprayed with 14C-ring
labelled metalaxyl until run-off at a rate corresponding to
0.2 kg a.i.(500 l ha.). The treatment was repeated four times at
10-day intervals. In the mature plant, only about 1.5% of the
radioactivity was found, with more than 90% in the shoots. The tubers
contained 0.02 mg/kg metalaxyl equivalents, which indicates some
basipetal translocation.
No parent compound could be found in tubers. Radioactivity
consisted exclusively of polar metabolites. Due to the very low
amount, exact identification was not possible. In the shoots,
compounds II, IV, VI and VII (Figure 2) were identified as the main
metabolites, which is evidence for two independent degradation
pathways utilizing ring methyl oxidation and hydrolysis of the
methylester bond (Gross 1979a). Neither metalaxyl nor its degradation
products were taken up by the tubers directly from soil containing
approximately 0.5 mg/kg metalaxyl equivalents (Gross 1977).
Grape
Grapevines were sprayed six times at 14-day intervals until run-
off with 14C-ring labelled metalaxyl equivalent to 30 g a.i./100 l
water. At the time of harvest, a total of 1.4 mg/kg metalaxyl
equivalents was found in the grapes. The portion of unchanged
metalaxyl was 0.83 mg/kg (60%) (Gross 1979b), In another experiment
with an overdosage (7 treatments, 50 g a.i./100 l) application, the
total radioactivity in the mature grapes corresponded to 3.06 mg/kg
metalaxyl equivalents 52 days after the last application; the portion
of unchanged metalaxyl was 1.96 mg/kg (64%) (Gross 1978). Metabolites
III, IV, V and VIII, were identified by chromatography and
spectroscopy in both grapes and foliage. The presence of these
metabolites is evidence for independent degradation pathways that
utilize ring methyl oxidation, ring hydroxylation and hydrolysis of
the methylester and methylether bonds. All metabolites formed were
found partially conjugated with sugar (Gross 1979b). The non-
extractable radioactivity amounted to 9.4 and 4.2% of total
radioactivity in grapes and leaves, respectively.
Lettuce
Lettuce plants grown in a greenhouse were treated twice with
14C-ring labelled metalaxyl at two-week intervals at a rate
corresponding to 0.25 kg a.i./ha. The radioactivity in the green parts
was analysed two weeks after the second treatment. Less than 20% of
the total radioactivity applied was found in the aerial parts,
primarily in the first leaves directly sprayed with the fungicide;
21.5% of the total radioactivity was characterized as unchanged
metalaxyl, the rest consisted of several polar products (Gross:
1979c). Metabolites isolated from the leaves were identified as III,
IV (5.1%), V (1.6%), VI, VIII and IX. These metabolites were found
partially conjugated with glucose. A significant portion (27.7%) of
the total radioactivity was non-extractable (Gross 1980).
In Animals
A lactating goat was administered ring 14C-labelled metalaxyl by
capsule at a level of 7.00 ppm in the feed for ten consecutive days.
Urine, faeces, milk, volatiles and CO2 were collected daily. The goat
was sacrificed 24 hours after the last dosage. Analytical data on
urine and faeces indicate metalaxyl was metabolized rapidly and the
total amount administered was excreted within approximately 24 hours.
The recovery of the radioactivity applied was 107.22%, with most being
excreted in the urine (93.92%) and faeces (11.60%). A small amount of
radioactivity was found in milk (0.003 mg/kg), blood (0.06%) and
tissues (0.87%). The level of radioactivity expressed in metalaxyl
equivalents was less than 0.06 mg/kg in all tissues; milk, kidney
and liver contained 0.003 mg/kg, 0.019 mg/kg and 0.057 mg/kg,
respectively. The level of radioactivity in the tissues analysed was
<0.06. Neither metalaxyl nor its metabolites accumulated in tissues
of goats.
Two-dimensional TLC comparisons of the metalaxyl metabolites in
goat urine and rat urine revealed the same pattern of products
(conjugated highly polar acidic compounds) and suggest that the same
metabolic pathways described for the rat also take place in the goat
(Fischer et al 1978).
In Processing
Grapes originating from metabolism studies carried out under
field conditions, and containing 1.4 or 3.06 mg/kg 14C-metalaxyl
equivalents, were shredded in a food cutter and the juice was pressed
off. The total residue in the juice was 0.9 or 1.04 mg/kg metalaxyl
equivalents, with the portion of unchanged metalaxyl being ca.
0.5 mg/kg. Most of the radioactivity (approximately 90%) remained in
the presscake (Gross 1978, 1979b). The elimination of metalaxyl
residues during processing from grapes to wine under practical
conditions is shown also in Table 8. These data demonstrate that, on
average, the level of metalaxyl residues in wine is one third of the
original concentration in the grapes at harvest.
Orange fruit treated postharvest with metalaxyl was subjected to
juice extraction by the Brown technique under industrial conditions.
This technique is based on a procedure in which the fruit is sliced in
half the the juice squeezed from the two halves by rotating reamers.
Using this technique, oranges containing 3.5 mg/kg metalaxyl in the
peel and 0.09 mg/kg in the pulp (Ciba-Geigy undated) produced juice
with 0.07 mg/kg metalaxyl.
As shown in Tables 12 and 13, residues present in dried hops
result in negligible residues in beer (generally below 0.05 mg/kg).
These results were to be expected, as residues in dried hops average
2 to 6 mg/kg and the maximum amount of hops used in beer brewing is
400 g/100 l beer.
In Soil
Degradation in soil was studied under laboratory and field
conditions. In laboratory studies using 14C-ring labelled metalaxyl,
a rapid and extensive degradation was observed. In aerobically
incubated soil, the half-life was found to be ca. 40 days. Less than
2% of metalaxyl was present in the soil after one year. Degradation
proceeded mainly via cleavage of the methylester bond yielding the
corresponding acid (compound III, Figure 2) as the transient
degradation product. A pronounced evolution of 14CO2 was observed
(25% within 12 months) indicating mineralization of the fungicide.
Degradation was slower under anaerobic conditions. No degradation was
observed in autoclaved soil, indicating the importance of
microorganisms in the degradation of metalaxyl (Ellgehausen 1978).
In order to follow the dissipation of metalaxyl and its main
metabolite, the free acid (compound III) in soil under field
conditions, the fungicide was applied in a single treatment at
2 kg a.i./ha on plant-free silty and sandy soils in Switzerland
(Büttler 1977a, 1979a). Residues of metalaxyl were also determined
after repeated applications to potatoes at 0.15-0.2 kg a.i./ha. In all
trials sampling was carried out at several intervals after the last
application. Soil samples were taken from different soil layers. The
results are summarized in Table 14 (Büttler 1977a; Ciba-Geigy
undated). Most of the residue is limited to the upper 10 cm soil
layer. Metalaxyl residue alone could be detected in soil only at a
depth of 0-10 cm 180 days after spraying the soil at a rate of
2 kg a.i./ha. Neither the parent compound nor metabolite III were
detectable in samples taken to a depth of 90 cm 314 and 406 days after
application (Büttler 1977b). The metalaxyl residue did not leach
deeper than 40 cm. The main metabolite (III) was observed only in the
0-20 cm soil layer and always in smaller concentrations than the
parent compound.
Table 14. Dissipation of Metalaxyl (M) and Its Metabolite (III) in Soil
Soil Application Soil depth Residues (mg/kg) at interval (days) after
rate (cm) Compound last application
Type (kg a.i./ha) 0-7 14-21 18-31 39-44 50-60 90-122
Sandy loam 2 to plant-free soil 0-10 M 1.8 0.66 0.23
III 0.34 0.25 <0.05
10-20 M 0.21 0.09 0.12
III 0.09 <0.05 <0.05
20-30 M 0.08 <0.05 <0.05
III <0.05 <0.05 <0.05
30-40 M 0.1 <0.05
III <0.05 <0.05
40-60 M <0.05 <0.05
III <0.05 <0.05
Silt loam 8×0.16 to potato M 0.18 0.09
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.21 0.05
plants III <0.05 <0.05
Silt loam 4×0.16 to potato M 0.13 0.09
plants III <0.05 <0.05
Unspecified 5×0.15 to potato 0-20 0.02 0.02
plants
Unspecified 5×0.25 to potato 0-10 M 0.21
plants 10-60 1 M <0.05
1 Residue was measured individually in each 10-cm soil layer.
In Storage
Experiments have shown that samples may be deep-frozen for long
periods without appreciable loss of residues. Residue levels were
determined in lettuce samples fortified with metalaxyl at 5 mg/kg and
field treated samples stored at -20°C for 81-337 days. During this
period 96-114% of the fortified amount and 121-145% of the initial
residue of the field samples (corrected for recoveries) were recovered
(Büttler 1979b). It is presumed that the higher values obtained after
storage are due to loss of water. Studies have also been carried out
on the stability in storage at -15°c using the method for the
determination of the sum of metalaxyl and its metabolites containing
the 2,6-dimethyl-aniline moiety. Cured tobacco and potato samples
fortified with metalaxyl were used. No loss of residues occurred
during a storage period of 18 months. The recoveries averaged 89 + 9%
for tobacco and 101+12% for potatoes (Ross 1980).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
The result of a selective survey on residues in lettuce samples
at commercial outlets in the United Kingdom during 1980/81, was
provided to the Meeting and is summarized below (U.K. undated):
Residue range No. of samples in
(mg/kg) the range
<0.1 90
0.1-0.5 83
0.51-10 18
1.1-2.0 5
>2
METHODS OF RESIDUE ANALYSIS
Several residue methods, all based on gas chromatography, have
been developed for the analysis of agricultural commodities. The
limits of determination are usually 0.02 mg/kg for metalaxyl and
0.05 mg/kg for the sum of metalaxyl and its metabolites containing the
dimethylaniline moiety.
For analysis of the parent compound in vegetables, cereals and
fruits, the sample is extracted with methanol in a high speed
homogenizer. The methanol extract is diluted with water and cleaned up
by water-methanol/dichlormethane partitioning. The dichloromethane
phase is evaporated and the residues of the organic phase is cleaned
up by alumina column chromatography. The appropriate fraction is then
examined for metalaxyl by gas chromatography on 3% Carbowax
20M/GasChrom Q packing at 185°C, using either an alkali flame
ionization detector (AFID) or a Coulson nitrogen specific detector
(Ramsteiner 1976a).
For hops and avocados a more elaborate extraction and clean-up
procedure is necessary. The sample is extracted with acetone, which is
evaporated to dryness. The oily residue is dissolved in methanol and
the interferring co-extractives are precipitated with Celite (R) and
FeCl3/CuSO4 solution. After filtration, the active ingredient is
partitioned into toluene. The toluene phase is evaporated, and the
residue is cleaned up by alumina column chromatography [Ramsteiner
1976B). Final determination is made by gas chromatography using either
AFID or a Hall electrolytic conductivity detector (HECD). The limit of
determination of this method is 0.1 mg/kg except for hops when
detected with AFID, where the limit of determination is 0.2 mg/kg.
The analytical procedure was simplified for wine and beer. The
sample is cleaned up by partition chromatography with n-hexane on an
EXTRELUT(R) column. The eluate is further cleaned up by passage
through an alumina column before the final determination of the active
ingredient by gas chromatography, using a nitrogen-phosphorus flame
ionization detector. The limit of determination of this method in beer
and wine is 0.005 mg/kg (Büttler 1980).
Recovery experiments have shown that the standard method
(Ramsteiner 1976a) recovers 98 ± 12% of the active ingredient at
fortification levels of 0.05 and 0.5 mg/kg. With the method developed
for hops and avocados, recoveries were 83 ± 15% at fortification
levels of 0.2 and 1 mg/kg (Ramsteiner 1976b). In wine and beer
recoveries were 102 ± 8% at fortification levels ranging from
0.01-0.4 mg/kg [Büttler 1980).
Metalaxyl residues can be determined in grapes and vegetables by
applying the method of Ambrus et al (1981). The samples are
extracted with acetone, the extract is diluted with water containing
2% Na2SO4 and partitioned into dichloromethane. Potato and tomato
can be analysed directly using a nitrogen specific detector while
other extracts have to be cleaned up on neutral alumina column
(V. activity grade). Metalaxyl is eluted in the second fraction. The
limit of determination is 0.01-0.02 mg/kg (Hungary 1982).
The method for analysis of metalaxyl and its metabolites
is based on the hydrolysis of all derivatives containing the
2,6-dimethylaniline moiety (2,6-DMA) and final determination of
2,6-DMA. The residues are extracted by blending with 20% water/
methanol. The sample extract is evaporated and refluxed with
phosphoric acid in the presence of cobalt chloride. The solution is
then made basic and the 2,6-dimethylaniline formed is steam distilled.
The product is derivatized with trichloroacetyl chloride to minimize
problems of volatility of 2,6-dimethylaniline. The derivative is
cleaned up by alumina column chromatography and analysed by gas
chromatography, using a nitrogen specific alkali flame ionization
detector. The limit of determination for the method is 0.05 mg/kg
expressed in metalaxyl equivalents. The percent of recovery of
metalaxyl from potato tubers fortified at 0.05 to 1 mg/kg was
72 ± 10%, from cucurbits fortified at 0.05 to 0.4 mg/kg 72 ± 11% and
from cole crops fortified with 0.05 to 0.5 mg/kg 59 ± 11%
(Balasubramanian 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs and preharvest intervals reported in Table 15
have been established on the basis of local conditions.
Table 15. National Maximum Residue Limits Reported to the Meeting
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Australia Grapes 1.0 7
Custard apples, figs,
mangoes, passionfruit 0.5 7
Leafy vegetables 0.3 4
Cucurbits 0.2 4
Pineapples 0.1 28
Vegetables (other than
leafy veg. and cucurbits) 0.1 7
Allium 0.1
Avocados 0.05 7
Austria Hops 2.5 14
Grapes 0.1 21
Czechoslovakia Potatoes 21
Fed. Rep.Germany Potatoes 7
Hops 10
Grapes 35
France Grapes 0.6 15
Hungary Grapes, hops, soya 0.2 21
Italy Grapes 1.0 28
Onion, pepper, spinach,
tomatoes 1.0 10
Potato 1.0
Strawberry 0.5 40
Table 15. (con't)
Preharvest
MRL interval
Country Commodity (mg/kg) (days)
Israel Grapes 2.0 21
Citrus 2.0
Cruciferae, tomato 0.3 14
Industrial tomato 0.3 3
Netherlands Citrus 5.0
Cole crops, potato 0.05 28
Lettuce 0.05 28
South Africa Grapes 1.5 21
Potato 0.2 14
Tomatoes 0.1 7
Avocados, cruciferae, citrus 0.05 30
1
Switzerland Grapes 2.0
Wine 0.6
Potato 0.1 14
Onion 0.05 14
U.K. Potato, hops 14
Yugoslavia Hops 7
Grapes 28
1 Last treatment at end of August.
APPRAISAL
Metalaxyl is a fungicide with high activity against fungal
pathogens of the Order Peronosporales. It is formulated in combination
with various fungicides, such as mancozeb, zineb, copper, folpet or
carbendazim. In the active ingredient of the commercial products the
weight ratio of metalaxyl varies approximately in the range of 0.4 to
0.1. Metalaxyl is used for foliar sprays, seed dressing and soil
treatment at dosage rates of 150-200 g a.i./ha, 0.26-2.63 g a.i./kg
seed and 1.25-5 g/m2 or 2 kg a.i./ha in soil, respectively.
Residues in crops following foliar and soil treatment are
generally under 1 mg/kg one to three days after last application and
decline below 0.2-0.5 mg/kg one or two weeks thereafter. Lettuce
treated at the recommended rate contained residues up to 2 mg/kg 21
days after last application. The foliar treatments of grapes and hops
result in residues up to 5 and 10 mg/kg, respectively, at 14 days
after application. Crops harvested after seed dressing do not contain
residues above the limit of determination (0.02 mg/kg). Residues in
citrus fruits treated postharvest amounted to ca. 4 mg/kg and were
concentrated mainly in the peel. The residue in the pulp of different
varieties was below 0.2 mg/kg. The data on Brussels sprouts, cocoa
beans, pepper and pineapple were not sufficient to make
recommendations.
Metalaxyl is partly taken up by the roots, leaves, green stems
and shoots and transported acropetally within the plant. The following
reactions were found to be involved in the metabolic transformations:
cleavage of the carboxyester and methylether bonds, oxidation of the
phenylmethyl group and the consequent benzyl alcohol derivative,
hydroxylation of the phenyl ring, N-dealkylation, conjugation with
glucose in plants and with glucuronic acid in animals.
Most of the residue was detected in the leaves and shoots,
in which N-(2,6, dimethyl-phenyl)-N-(metoxyacetyl)-alanine,
N-(2-hydroxymethylene-6-methylphenyl)-N(methxyacetyl)-alanine methyl
ester, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methyl-phenyl)-N-methoxyacetyl)alanine
methyl ester, N-(2-carboxy-6-methylphenyl)-N-(hydroxyacetyl)alanine
were identified as the main metabolites.
The parent compound amounted to a minimum of 55% and 60% of the
total residue containing dimethyl-aniline moiety in brassicas and
grapes, respectively, while there was no practical difference between
residue levels determined as parent compound or as the sum of
metalaxyl and its metabolites in fruiting vegetables.
Most of the total residue in spinach consisted of metabolites
containing the 2,6-dimethylanalinine moiety. Total residue levels
ranged from 1.1 mg/kg to 7.3 mg/kg, while the concentration of parent
compound ranged from 0.03 to 0.67 mg/kg in samples taken 6-8 days
after the last application. Based on experimental data, the estimated
maximum residue level, expressed as the total residue or as the parent
compound alone, would be 10 mg/kg or 1 mg/kg, respectively.
A lactating goat kept on a diet containing 7 ppm metalaxyl for
ten consecutive days excreted most of the radioactivity in the urine
and faeces within approximately 24 hours after administration. The
blood and tissues contained 0.06 and 0.87% of the total radioactivity
and 0.003 mg/kg of metalaxyl equivalents was detected in the milk,
Neither metalaxyl nor its metabolites accumulated in the tissues.
No evidence for the presence of 2,6-dimethylaniline in free or
conjugated form was found either in plants or animals. The metabolic
pathways are similar in plants and in animals.
Processing of grapes, oranges and hops treated with metalaxyl
at recommended rates results in detectable residues in the final
products. The wine contains approximately one third of the residue
found in grapes at harvest. The residue in orange pulp (less than 5%
of the residue in the whole fruit) is transferred to the juice, while
beer contains negligible residue (<0.1 mg/kg) compared to the level
in hops. The residue level remains constant in crops stored at or
below -15°C for long periods.
The degradation of metalaxyl in soil is rapid under aerobic
conditions while it is slower under anaerobic conditions and no
degradation was observed in sterilized soil. No residue was detectable
(<0.02-0.05 mg/kg) one year after application under field conditions.
The majority of residues was in the upper 10-cm soil layer. The main
metabolite (N-(2,6-dimethylphenyl)-N-(methoxyacetyl-alanine) was only
observed in the 0.20 cm soil layer and at a distinctly smaller
concentration than the parent compound.
Analytical methods based on methanol or acetone extraction,
water/methanol or dichlormethane partition, cleanup on an alumina
column and detection with nitrogen specific detectors are available
for the determination of the parent compound in various samples. These
methods are suitable for regulatory purposes. Multi-residue methods
based on similar processes seem to be applicable as well for the
determination of metalaxyl. The total residue containing the
2,6-dimethylaniline moiety may be determined after hydrolysis to
dimethylaniline and derivatization.
RECOMMENDATIONS
The Meeting concluded that the levels listed below are suitable
for establishing MRLs. The limits refer to the parent compound.
FURTHER WORK OR INFORMATION
Desirable
Additional residue data derived from supervised trials on
Brussels sprouts, cocoa beans, peppers and pineapple.
Preharvest intervals on
Estimated maximum which recommendations
Commodity residue levels (mg/kg) are based (days)
Hops (dried) 10 14
Citrus fruit 5 postharvest application
Grapes 5 14
Lettuce 2 21
Spinach 1 7
Broccoli, cabbage,cauliflower 0.5 7
Onion 0.05 1 21
Cucumber, gherkins,tomato 0.5 3
Melon, squash, watermelon 0.2 7
Potato 0.1 7
Avocado 0.1 1 7
Cereal grains 0.05 1
Sugarbeet 0.05 1 seed treatment
Sunflower seed 0.05 1
Peas 0.05 1
1 Level at or about the limit of determination.
REFERENCES
Ambrus, A., Lantos, J., Visi, E., Csatlos, I. and Sarvari, L. General
1981 method for the determination of pesticide residues in
samples of plant origin, soil and water. I. Extraction and
cleanup, Journal Assoc. Off. Anal. Chem. 64:733-768.
Balasubramanian, K. Analytical method for the determination of total
undated residues of metalaxyl in crops as 2,6-dimethylaniline.
Report AG-348 from Ciba-Geigy, U.S. (Unpublished).
Büttler, B. CGA 48988 and metabolites in soil. Residue reports RVA
1977a 252/77 A and 253/77 A from Ciba-Geigy, Basle. (Unpublished)
1977b CGA 48988 in soil. Residue report RVA 218/77 from CG, Basle.
(Unpublished)
1979a CGA 48988 and CGA 62826 in soil. Residue reports RVA 273 and
274/79A from Ciba-Geigy, Basle. (Unpublished)
1979b Stability of CGA 48988 in lettuce during frozen storage at
-20°C. Report SPR 17/79 from Ciba-Geigy, Basle.
(Unpublished)
1980 CGA 48988: Gas chromatographic determination of residues in
wine and beer. Report No. REM 1/80 from Ciba-Geigy, Basle.
(Unpublished)
Ciba-Geigy. 299 residue reports. [Unpublished)
undated
Ellgehausen, H. Degradation of CGA 48988 (RIDOMILR) in soil under
1978 aerobic, aerobic/anaerobic and sterile/aerobic conditions.
Report PR 08/78 from Ciba-Geigy, Basle. (Unpublished)
Finland Information on pesticides included in the JMPR priority list.
undated
Fischer, et al. Balance and metabolism of 0-14C-CGA-48988 in a
1978 lactating goat. Report No. 78046 from Ciba-Geigy, U.S.
(Unpublished)
Gross, D. Metabolism of CGA 48988 in field grown potato plants. Report
1977 PR 30/77 from Ciba-Geigy, Basle. (Unpublished)
1978 Metabolism of CGA 48988 in grapevine Report PR 11/78 from
Ciba-Geigy, Basle. (Unpublished)
1979a Identification of metabolites of CGA 48988 (RIDOMILR) in
field grown potato plants Report PR 39/79 from Ciba-Geigy,
Basle. (Unpublished)
1979b Identification of metabolites of CGA 48988 (RIDOMILR) in
grapevine. Report PR 06/79 from Ciba-Geigy, Basle.
(Unpublished)
1979c Fate of CGA 48988 in lettuce. Report PR 38/79 from Ciba-
Geigy, Basle. (Unpublished)
1980 Identification of degradation products of CGA 48988
(RIDOMILR) in lettuce. Report PR 38/80 from Ciba-Geigy,
Basle. (Unpublished)
Hungary Information on pesticides included in the JMPR priority
1982 list.
Israel Residues of Ridomil in citrus fruit. Ministry of
1980 Agriculture, Jerusalem. (Unpublished)
Ramsteiner, K. CGA 48988: Gas chromatographic determination of
1976a residues in soil, vegetables and grapes. Report REM 16/76
from Ciba-Geigy, Basle. (Unpublished)
1976b CGA 48988: Gas chromatographic determination of residues in
hops and tobacco. Report from Ciba-Geigy, Basle.
(Unpublished)
Ross, J.A. Stability of residues of metalaxyl and its metabolites
1980 under freezer storage conditions. Report ABR-80028 from
Ciba-Geigy, U.S. (Unpublished)
Sweden Results of analysis of Ridomil in postharvest treated
1980 oranges, 1980-06-27. Statens Lantbrukskemiska, Upsala.
(Unpublished)
Urech, P.A., Schwinn, F. and Staub, T. CGA 48988, a novel fungicide
1977 for the control of late blight, downy mildews and related
soil-borne diseases. Proceedings of the 1977 British Crop
Protection Conference, p, 623-631.
U.K. Submission to JMPR on metalaxyl (with mancozeb) residues
undated in protected lettuce (prepared by J.W. Edmunds).
