
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
PARATHION METHYL
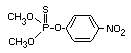 Explanation
Parathion methyl was evaluated by the Joint Meeting in 1968,
1975, 1929 and 1980 (FAO/WHO 1969, 1976, 1980 and 1981)1 and a
temporary ADI of 0 - 0,001 mg/kg bw/day was allocated. Reproduction
study in species appropriate to such a test and results of adequate
long-term study reported to be in progress then were required by the
1980 Meeting. These studies, as well as other additional toxicological
data, have been received and are reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Elimination
In male rats, treated with a single oral dose of 14C-parathion
methyl (benzene ring-labelled) at 0.1, 1.0 or 5 mg/kg bw and in female
rats given a single oral dose of 1 mg/kg bw, over 99% of the
administered dose was excreted in the urine and the faeces within
48 hours. Elimination in the faeces accounted for only 5 - 7% after
1 or 5 mg/kg bw, but amounted to about 20% after 0.1 mg/kg bw. Male
rats treated with an intravenous dose of 1 mg/kg bw excreted about 99%
of the administered radioactivity in the urine within 48 hours, and
approximately 1% of the dose in the faeces. Total radioactive residues
recovered in the 12 tissues analysed (excluding the gastrointestinal
tract) from rats given a single oral dose of 5 mg/kg bw were about 11%
of the administered dose 1 hour after treatment, declining to 0.3% at
24 hours, about 0.1% at 48 hours and to only 0.04% 6 days later. The
kidney had the highest relative activity up to 8 hours post-treatment.
The 14C activity in the plasma was initially about 5 times higher
than that in the erythrocytes. However, from day 2 to day 6 after
dosing, the 14C-activity in the erythrocytes was greater than that in
the plasma and remained constant. Following an intraduodenal dose of
1 See Annex 2 for WHO and FAO documentation.
1 mg/kg bw, male rats with fistulated bile duct excreted only 3% of
the radioactive dose in the bile with the remainder being eliminated
primarily (95%) in the urine within 24 hours (Weber et al 1979).
The elimination of parathion methyl was rapid in the dog. A
terminal half-life of 7.2 hours was estimated in dogs given an
intravenous dose of 10 mg/kg bw (Braeckman et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 10 male and 20 female rats (SPF-Wistar W74 strain, 5-6
weeks old) were fed a diet containing technical parathion methyl
(95% pure) at 0, 2, 10 or 50 ppm for 77 days and then mated to
initiate a standard 3-generation (2 litters/generation) reproduction
study. Pups of the second litters (F1b and F2b) provided the parents
of the next generation. Through the parental generations, there was no
mortality related to compound. Treatment at the top dietary level
(50 ppm) induced convulsions in F1 parents, growth retardation in
both F0 and F1 adults (both sexes) and significant adverse effects
on litter size and pup weight at birth and through lactation
practically through all progeny generations. All F2b pups at 50 ppm
died 5 days after birth, resulting in the absence of data on F3a and
F3b litters for this dosage group. Fertility rate was slightly
decreased at 10 ppm in F2 generation (both mating trials, i,e. F3a
and F3b litters). A reduction in litter size occurred at 10 ppm in
F3a litters at birth, in F1b litters after 5 days and in F2a and F2b
litters at 5 weeks. At 2 ppm, a non-dose-related decrease in litter
size at birth and a slightly depressed survival of pups to 5 days were
observed in F2b litters. These findings, occurring in a single
progeny generation, were not believed to be attributable to parathion-
methyl in the diet. Gross examination of F2b and F3b pups at birth
and during rearing revealed no malformations. Histopathological
evaluation of reproductive tract plus liver and kidney from 5 males
and 5 females per group of F2 parents and of a set of about 15
tissues including the reproductive tract from 10 male and 10 female
F3b weanlings per group showed no microscopic changes associated with
treatment. The no-effect level in the study was 2 ppm (Lösen and Eiben
1982; Glaister 1981).
Special Studies on Mutagenicity
Parathion methyl (94.3 - 94.5% pure) was tested for its mutagenic
potential in in vitro microbial assays with and without the presence
of a metabolic activation preparation (S-9 fraction from liver of rats
induced with Aroclor 1254). The indicator organisms used were
Salmonella typhimurium strains TA 1535, TA 100, TA 1537 and TA 98.
Positive mutagenic response was obtained with and without metabolic
activation in strain TA 1535 at concentrations at and above
1 000 µg/plate and in strain TA 100 at and above 500 µg/plate. There
were no indications of mutagenic activity in assays with tester
strains TA 1537 and TA 98 at concentrations of 20 to 2 500 µg/plate
with or without metabolic activation (Herbold 1982a).
Parathion methyl (96.8% pure) was shown to induce a significant
increase (dose-related) of sister chromatid exchanges and cell cycle
delay in Chinese hamster cell line V79 and in two human lymphoid cell
lines (Chen et al 1981).
In a micronucleus test, groups of 5 male and 5 female mice were
intubated with parathion methyl (95.6%) at 0, 5 or 10 mg/kg bw/day for
2 consecutive days with an interval of 24 hours between doses. The
animals were sacrificed 6 hours after the second dose and femoral bone
marrow smears were prepared. Results of examination of the smears did
not indicate any significant increase in incidence of micronucleated
polychromatic erythrocytes in the treated groups (Herbold 1982b).
Special Studies on Eye and Skin Irritation
Results of irritation studies with New Zealand White rabbits
indicated parathion methyl (96.1%/95.6%) to be slightly irritating to
the skin. The compound was not an irritant to eyes (Thyssen and Lorke
1982).
Long-Term Studies
Rat
Groups of 5 to 6 weeks old male and female rats (SPF, Wistar
TNO/W74 strain; 100 male and 100 female controls, 50 males and 50
females/treated group) were fed parathion methyl (94.8% pure) in their
diet at 0, 2, 10 or 50 ppm for 2 years. Additional groups of 10 males
and 10 females per group were included for interim sacrifice of 5
males and 5 females/group after 6 and 12 months. Mortality was
increased in females at 50 ppm during the first year but about 79-90%
males and 58 - 78% females of control and treated groups still
survived at the end of the study. Animals (both sexes) in the top
dosage group exhibited cholinergic symptoms (tremors) at times during
the study and growth retardation throughout the experiment.
Haematology, clinical chemistry and urinalysis carried out at five
intervals during the study indicated deviations from control values
essentially only at 50 ppm in a number of parameters. These included
an increase in reticulocyte counts and a decrease in hematocrit and
haemoglobin values in both sexes after 6 and/or 24 months, depressed
plasma protein levels in females at all intervals, elevated serum urea
in females at most intervals and in males after 24 months, increased
plasma alkaline phosphatase activity in females after 3 and 6 months
and a rise in urinary protein level in females after 1 and 6 months.
(Plasma protein level was also depressed in females at 10 ppm but only
after 1 month.) Cholinesterase in plasma and erythrocyte, assayed nine
times over the course of the study, was consistently inhibited
(>20%), in a dose-dependent pattern, at 50 ppm at all intervals and
at 10 ppm frequently during the study. The extent of depression was
greater with plasma cholinesterase than with erythrocyte
cholinesterase. Terminal brain cholinesterase was inhibited (>20%) in
males at both 10 and 50 ppm and in females at 50 ppm.
Gross pathological findings in animals dying or sacrificed in
moribund condition during the study and in those sacrificed at interim
or terminally were comparable to those in the controls. A reduction of
absolute weight, but not organ/body weight ratio, of several organs
was demonstrated in animals at 50 ppm. Histopathological evaluation of
a wide range of tissues from animals of control and top dosage groups
only revealed a significant increase in frequency of males at 50 ppm
(33% vs 4% in controls) displaying very slight to moderate degree of
"excessive Oil Red 0(ORO)-positive substance in hepatocytoplasm". A
no-effect level on this particular liver lesion could not be defined
due to the absence of data on microscopic findings in the liver
of treated animals below 50 ppm. No other non-neoplastic
histopathological changes attributable to treatment were evident.
Notably, histopathological data were available for only half of the
animals in the control group.
When histopathological and tumour data from only 50 male and 50
female controls were used as the basis of comparison, there did not
seem to be any significant difference between the control and top
dosage groups in the incidence of animals with tumours (benign and/or
malignant), malignant tumours, benign tumours or multiple primary
tumours. However, there appeared to be an increase in incidence at
50 ppm of thyroid adenoma in males (11/45 vs 4/43 controls) and of
uterine adenocarcinoma (8/42 vs 3/44 controls)2. The increase was not
statistically significant if incidence for the respective tumours in
43 and 44 concurrent controls were used for comparison. Whether the
control incidence for the tumours in question would be significantly
altered, if histopathological findings in all 100 male and 100 female
concurrent controls were taken into consideration, is not known. The
incidence of uterine adenocarcinoma at 50 ppm was indicated in the
2 According to "Table: Tumour hosts with descriptions of all
tumours" included in the report, 4 female controls had adenocarcinoma
of the uterus. An examination of "histopathological single finding" in
Tables 344-347 revealed the uterus of one (animal No.149) of the 4
supposedly affected control animals was not examined microscopically.
report to "remain within the variability of untreated animals of this
strain observable in other virtually parallel studies". Background
incidences of this particular malignancy and thyroid adenoma were not
given (Bomhard et al 1981).
Observations in Humans
In 15 healthy male workers (with blood cholinesterase level
<75% of presumably the mean normal levels) in a pesticide plant who
were repeatedly or chronically exposed to parathion methyl for
durations ranging from 1 week to 7 years, but with intermittent
periods of non-exposure, there was no increased frequency of
chromosome aberrations in lymphocyte cultures, as compared to 13
matched controls (having a blood cholinesterase level of 100%) not
occupationally exposed to any chemical (Stocco et al 1982),
COMMENTS
The reproduction and long-term studies required by the 1980 Joint
Meeting and other additional studies have been made available. A
no-effect level of 2 ppm on re-production capability was demonstrated
in the 3-generation rat reproduction study. Parathion methyl was
mutagenic in the in vitro microbial assays to 2 of the 4
Salmonella typhimurium tester strains and in sister-chromatid
exchanges and cell cycle delay studies with Chinese hamster cell line
V79 and with 2 human lymphoid cell lines. On the other hand, a
micronucleus test in mice was negative. Also, workers exposed to
parathion methyl occupationally for long periods exhibited no
increased frequency of chromosome aberrations in lymphocyte cultures.
In the case of the 2-year feeding study in rats, the available data
did not permit a complete evaluation of the carcinogenic activity of
the compound or the determination of a definite no-effect level in the
species. The Meeting, therefore, decided to extend the temporary ADI
and requested that additional data pertaining to the study be
provided.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Man : 0.3 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
Additional data on the 2-year feeding study in rats including:
a. Historical data on incidences of thyroid adenoma and
adenocarcinoma of the uterus in the particular strain of
rats (SPF Wistar TNO/W74) used in the study.
b. Histopathological data, not presently available, on the
remaining 50 males and 50 females in the control group or
satisfactory and acceptable explanation for the absence of
such data.
c. Results of microscopic examination of the liver from treated
males below 50 ppm.
REFERENCES
Bomhard, E., Löser, E. and Schilde, B. E605-Methyl (parathion-methyl)
1981 chronic toxicological study on rats (feeding experiment of
two years). Report from Bayer AG submitted to the World
Health Organization by Bayer AG. (Unpublished)
Braeckman, R.A., Godefront, M.G., Blondeel, G.M., Belpaire, F.M. and
1980 Williams, J.L. Kinetic analysis of the fate of methyl
parathion in the dog. Arch. Toxicol. 43: 263-271.
Chen, H.H., Haveh, J.L., Sirianni, S.R. and Huang, C.C. Induction of
1981 sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res. 88: 307-316.
Glaister, J.R. E605-methyl rat breeding study (histopathology). Report
1981 from Hazleton Laboratories Europe, Ltd., England, submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl ( Salmonella/microsome test to
1982a evaluate forepoint mutation. Report from Bayer AG submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl micronucleus test on the mouse to
1982b evaluate for mutagenic effect. Report from Bayer AG
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Löser, E. and Eiben, R. E 605-methyl multigeneration studies on rats.
1982 Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Stocco, R. de Cassia, Becak, W., Gaeta, R. and Rabello-Gay, M.N.
1982 Cytogenetic study of workers exposed to methyl-parathion.
Mut. Res. 103:71-76.
Thyssen, J. and Lorke, D. Parathion-methyl (E120) tests for irritant
1982 effects on the skin and eye. Batch No. 23010618. Report from
Bayer AG submitted to the World Health Organization by Bayer
AG. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. (14C)methyl-parathion
1979 (benzene ring-labelled compound) biokinetic studies on rats.
Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Explanation
Parathion methyl was evaluated by the Joint Meeting in 1968,
1975, 1929 and 1980 (FAO/WHO 1969, 1976, 1980 and 1981)1 and a
temporary ADI of 0 - 0,001 mg/kg bw/day was allocated. Reproduction
study in species appropriate to such a test and results of adequate
long-term study reported to be in progress then were required by the
1980 Meeting. These studies, as well as other additional toxicological
data, have been received and are reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Elimination
In male rats, treated with a single oral dose of 14C-parathion
methyl (benzene ring-labelled) at 0.1, 1.0 or 5 mg/kg bw and in female
rats given a single oral dose of 1 mg/kg bw, over 99% of the
administered dose was excreted in the urine and the faeces within
48 hours. Elimination in the faeces accounted for only 5 - 7% after
1 or 5 mg/kg bw, but amounted to about 20% after 0.1 mg/kg bw. Male
rats treated with an intravenous dose of 1 mg/kg bw excreted about 99%
of the administered radioactivity in the urine within 48 hours, and
approximately 1% of the dose in the faeces. Total radioactive residues
recovered in the 12 tissues analysed (excluding the gastrointestinal
tract) from rats given a single oral dose of 5 mg/kg bw were about 11%
of the administered dose 1 hour after treatment, declining to 0.3% at
24 hours, about 0.1% at 48 hours and to only 0.04% 6 days later. The
kidney had the highest relative activity up to 8 hours post-treatment.
The 14C activity in the plasma was initially about 5 times higher
than that in the erythrocytes. However, from day 2 to day 6 after
dosing, the 14C-activity in the erythrocytes was greater than that in
the plasma and remained constant. Following an intraduodenal dose of
1 See Annex 2 for WHO and FAO documentation.
1 mg/kg bw, male rats with fistulated bile duct excreted only 3% of
the radioactive dose in the bile with the remainder being eliminated
primarily (95%) in the urine within 24 hours (Weber et al 1979).
The elimination of parathion methyl was rapid in the dog. A
terminal half-life of 7.2 hours was estimated in dogs given an
intravenous dose of 10 mg/kg bw (Braeckman et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 10 male and 20 female rats (SPF-Wistar W74 strain, 5-6
weeks old) were fed a diet containing technical parathion methyl
(95% pure) at 0, 2, 10 or 50 ppm for 77 days and then mated to
initiate a standard 3-generation (2 litters/generation) reproduction
study. Pups of the second litters (F1b and F2b) provided the parents
of the next generation. Through the parental generations, there was no
mortality related to compound. Treatment at the top dietary level
(50 ppm) induced convulsions in F1 parents, growth retardation in
both F0 and F1 adults (both sexes) and significant adverse effects
on litter size and pup weight at birth and through lactation
practically through all progeny generations. All F2b pups at 50 ppm
died 5 days after birth, resulting in the absence of data on F3a and
F3b litters for this dosage group. Fertility rate was slightly
decreased at 10 ppm in F2 generation (both mating trials, i,e. F3a
and F3b litters). A reduction in litter size occurred at 10 ppm in
F3a litters at birth, in F1b litters after 5 days and in F2a and F2b
litters at 5 weeks. At 2 ppm, a non-dose-related decrease in litter
size at birth and a slightly depressed survival of pups to 5 days were
observed in F2b litters. These findings, occurring in a single
progeny generation, were not believed to be attributable to parathion-
methyl in the diet. Gross examination of F2b and F3b pups at birth
and during rearing revealed no malformations. Histopathological
evaluation of reproductive tract plus liver and kidney from 5 males
and 5 females per group of F2 parents and of a set of about 15
tissues including the reproductive tract from 10 male and 10 female
F3b weanlings per group showed no microscopic changes associated with
treatment. The no-effect level in the study was 2 ppm (Lösen and Eiben
1982; Glaister 1981).
Special Studies on Mutagenicity
Parathion methyl (94.3 - 94.5% pure) was tested for its mutagenic
potential in in vitro microbial assays with and without the presence
of a metabolic activation preparation (S-9 fraction from liver of rats
induced with Aroclor 1254). The indicator organisms used were
Salmonella typhimurium strains TA 1535, TA 100, TA 1537 and TA 98.
Positive mutagenic response was obtained with and without metabolic
activation in strain TA 1535 at concentrations at and above
1 000 µg/plate and in strain TA 100 at and above 500 µg/plate. There
were no indications of mutagenic activity in assays with tester
strains TA 1537 and TA 98 at concentrations of 20 to 2 500 µg/plate
with or without metabolic activation (Herbold 1982a).
Parathion methyl (96.8% pure) was shown to induce a significant
increase (dose-related) of sister chromatid exchanges and cell cycle
delay in Chinese hamster cell line V79 and in two human lymphoid cell
lines (Chen et al 1981).
In a micronucleus test, groups of 5 male and 5 female mice were
intubated with parathion methyl (95.6%) at 0, 5 or 10 mg/kg bw/day for
2 consecutive days with an interval of 24 hours between doses. The
animals were sacrificed 6 hours after the second dose and femoral bone
marrow smears were prepared. Results of examination of the smears did
not indicate any significant increase in incidence of micronucleated
polychromatic erythrocytes in the treated groups (Herbold 1982b).
Special Studies on Eye and Skin Irritation
Results of irritation studies with New Zealand White rabbits
indicated parathion methyl (96.1%/95.6%) to be slightly irritating to
the skin. The compound was not an irritant to eyes (Thyssen and Lorke
1982).
Long-Term Studies
Rat
Groups of 5 to 6 weeks old male and female rats (SPF, Wistar
TNO/W74 strain; 100 male and 100 female controls, 50 males and 50
females/treated group) were fed parathion methyl (94.8% pure) in their
diet at 0, 2, 10 or 50 ppm for 2 years. Additional groups of 10 males
and 10 females per group were included for interim sacrifice of 5
males and 5 females/group after 6 and 12 months. Mortality was
increased in females at 50 ppm during the first year but about 79-90%
males and 58 - 78% females of control and treated groups still
survived at the end of the study. Animals (both sexes) in the top
dosage group exhibited cholinergic symptoms (tremors) at times during
the study and growth retardation throughout the experiment.
Haematology, clinical chemistry and urinalysis carried out at five
intervals during the study indicated deviations from control values
essentially only at 50 ppm in a number of parameters. These included
an increase in reticulocyte counts and a decrease in hematocrit and
haemoglobin values in both sexes after 6 and/or 24 months, depressed
plasma protein levels in females at all intervals, elevated serum urea
in females at most intervals and in males after 24 months, increased
plasma alkaline phosphatase activity in females after 3 and 6 months
and a rise in urinary protein level in females after 1 and 6 months.
(Plasma protein level was also depressed in females at 10 ppm but only
after 1 month.) Cholinesterase in plasma and erythrocyte, assayed nine
times over the course of the study, was consistently inhibited
(>20%), in a dose-dependent pattern, at 50 ppm at all intervals and
at 10 ppm frequently during the study. The extent of depression was
greater with plasma cholinesterase than with erythrocyte
cholinesterase. Terminal brain cholinesterase was inhibited (>20%) in
males at both 10 and 50 ppm and in females at 50 ppm.
Gross pathological findings in animals dying or sacrificed in
moribund condition during the study and in those sacrificed at interim
or terminally were comparable to those in the controls. A reduction of
absolute weight, but not organ/body weight ratio, of several organs
was demonstrated in animals at 50 ppm. Histopathological evaluation of
a wide range of tissues from animals of control and top dosage groups
only revealed a significant increase in frequency of males at 50 ppm
(33% vs 4% in controls) displaying very slight to moderate degree of
"excessive Oil Red 0(ORO)-positive substance in hepatocytoplasm". A
no-effect level on this particular liver lesion could not be defined
due to the absence of data on microscopic findings in the liver
of treated animals below 50 ppm. No other non-neoplastic
histopathological changes attributable to treatment were evident.
Notably, histopathological data were available for only half of the
animals in the control group.
When histopathological and tumour data from only 50 male and 50
female controls were used as the basis of comparison, there did not
seem to be any significant difference between the control and top
dosage groups in the incidence of animals with tumours (benign and/or
malignant), malignant tumours, benign tumours or multiple primary
tumours. However, there appeared to be an increase in incidence at
50 ppm of thyroid adenoma in males (11/45 vs 4/43 controls) and of
uterine adenocarcinoma (8/42 vs 3/44 controls)2. The increase was not
statistically significant if incidence for the respective tumours in
43 and 44 concurrent controls were used for comparison. Whether the
control incidence for the tumours in question would be significantly
altered, if histopathological findings in all 100 male and 100 female
concurrent controls were taken into consideration, is not known. The
incidence of uterine adenocarcinoma at 50 ppm was indicated in the
2 According to "Table: Tumour hosts with descriptions of all
tumours" included in the report, 4 female controls had adenocarcinoma
of the uterus. An examination of "histopathological single finding" in
Tables 344-347 revealed the uterus of one (animal No.149) of the 4
supposedly affected control animals was not examined microscopically.
report to "remain within the variability of untreated animals of this
strain observable in other virtually parallel studies". Background
incidences of this particular malignancy and thyroid adenoma were not
given (Bomhard et al 1981).
Observations in Humans
In 15 healthy male workers (with blood cholinesterase level
<75% of presumably the mean normal levels) in a pesticide plant who
were repeatedly or chronically exposed to parathion methyl for
durations ranging from 1 week to 7 years, but with intermittent
periods of non-exposure, there was no increased frequency of
chromosome aberrations in lymphocyte cultures, as compared to 13
matched controls (having a blood cholinesterase level of 100%) not
occupationally exposed to any chemical (Stocco et al 1982),
COMMENTS
The reproduction and long-term studies required by the 1980 Joint
Meeting and other additional studies have been made available. A
no-effect level of 2 ppm on re-production capability was demonstrated
in the 3-generation rat reproduction study. Parathion methyl was
mutagenic in the in vitro microbial assays to 2 of the 4
Salmonella typhimurium tester strains and in sister-chromatid
exchanges and cell cycle delay studies with Chinese hamster cell line
V79 and with 2 human lymphoid cell lines. On the other hand, a
micronucleus test in mice was negative. Also, workers exposed to
parathion methyl occupationally for long periods exhibited no
increased frequency of chromosome aberrations in lymphocyte cultures.
In the case of the 2-year feeding study in rats, the available data
did not permit a complete evaluation of the carcinogenic activity of
the compound or the determination of a definite no-effect level in the
species. The Meeting, therefore, decided to extend the temporary ADI
and requested that additional data pertaining to the study be
provided.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Man : 0.3 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
Additional data on the 2-year feeding study in rats including:
a. Historical data on incidences of thyroid adenoma and
adenocarcinoma of the uterus in the particular strain of
rats (SPF Wistar TNO/W74) used in the study.
b. Histopathological data, not presently available, on the
remaining 50 males and 50 females in the control group or
satisfactory and acceptable explanation for the absence of
such data.
c. Results of microscopic examination of the liver from treated
males below 50 ppm.
REFERENCES
Bomhard, E., Löser, E. and Schilde, B. E605-Methyl (parathion-methyl)
1981 chronic toxicological study on rats (feeding experiment of
two years). Report from Bayer AG submitted to the World
Health Organization by Bayer AG. (Unpublished)
Braeckman, R.A., Godefront, M.G., Blondeel, G.M., Belpaire, F.M. and
1980 Williams, J.L. Kinetic analysis of the fate of methyl
parathion in the dog. Arch. Toxicol. 43: 263-271.
Chen, H.H., Haveh, J.L., Sirianni, S.R. and Huang, C.C. Induction of
1981 sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res. 88: 307-316.
Glaister, J.R. E605-methyl rat breeding study (histopathology). Report
1981 from Hazleton Laboratories Europe, Ltd., England, submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl ( Salmonella/microsome test to
1982a evaluate forepoint mutation. Report from Bayer AG submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl micronucleus test on the mouse to
1982b evaluate for mutagenic effect. Report from Bayer AG
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Löser, E. and Eiben, R. E 605-methyl multigeneration studies on rats.
1982 Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Stocco, R. de Cassia, Becak, W., Gaeta, R. and Rabello-Gay, M.N.
1982 Cytogenetic study of workers exposed to methyl-parathion.
Mut. Res. 103:71-76.
Thyssen, J. and Lorke, D. Parathion-methyl (E120) tests for irritant
1982 effects on the skin and eye. Batch No. 23010618. Report from
Bayer AG submitted to the World Health Organization by Bayer
AG. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. (14C)methyl-parathion
1979 (benzene ring-labelled compound) biokinetic studies on rats.
Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
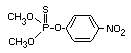 Explanation
Parathion methyl was evaluated by the Joint Meeting in 1968,
1975, 1929 and 1980 (FAO/WHO 1969, 1976, 1980 and 1981)1 and a
temporary ADI of 0 - 0,001 mg/kg bw/day was allocated. Reproduction
study in species appropriate to such a test and results of adequate
long-term study reported to be in progress then were required by the
1980 Meeting. These studies, as well as other additional toxicological
data, have been received and are reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Elimination
In male rats, treated with a single oral dose of 14C-parathion
methyl (benzene ring-labelled) at 0.1, 1.0 or 5 mg/kg bw and in female
rats given a single oral dose of 1 mg/kg bw, over 99% of the
administered dose was excreted in the urine and the faeces within
48 hours. Elimination in the faeces accounted for only 5 - 7% after
1 or 5 mg/kg bw, but amounted to about 20% after 0.1 mg/kg bw. Male
rats treated with an intravenous dose of 1 mg/kg bw excreted about 99%
of the administered radioactivity in the urine within 48 hours, and
approximately 1% of the dose in the faeces. Total radioactive residues
recovered in the 12 tissues analysed (excluding the gastrointestinal
tract) from rats given a single oral dose of 5 mg/kg bw were about 11%
of the administered dose 1 hour after treatment, declining to 0.3% at
24 hours, about 0.1% at 48 hours and to only 0.04% 6 days later. The
kidney had the highest relative activity up to 8 hours post-treatment.
The 14C activity in the plasma was initially about 5 times higher
than that in the erythrocytes. However, from day 2 to day 6 after
dosing, the 14C-activity in the erythrocytes was greater than that in
the plasma and remained constant. Following an intraduodenal dose of
1 See Annex 2 for WHO and FAO documentation.
1 mg/kg bw, male rats with fistulated bile duct excreted only 3% of
the radioactive dose in the bile with the remainder being eliminated
primarily (95%) in the urine within 24 hours (Weber et al 1979).
The elimination of parathion methyl was rapid in the dog. A
terminal half-life of 7.2 hours was estimated in dogs given an
intravenous dose of 10 mg/kg bw (Braeckman et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 10 male and 20 female rats (SPF-Wistar W74 strain, 5-6
weeks old) were fed a diet containing technical parathion methyl
(95% pure) at 0, 2, 10 or 50 ppm for 77 days and then mated to
initiate a standard 3-generation (2 litters/generation) reproduction
study. Pups of the second litters (F1b and F2b) provided the parents
of the next generation. Through the parental generations, there was no
mortality related to compound. Treatment at the top dietary level
(50 ppm) induced convulsions in F1 parents, growth retardation in
both F0 and F1 adults (both sexes) and significant adverse effects
on litter size and pup weight at birth and through lactation
practically through all progeny generations. All F2b pups at 50 ppm
died 5 days after birth, resulting in the absence of data on F3a and
F3b litters for this dosage group. Fertility rate was slightly
decreased at 10 ppm in F2 generation (both mating trials, i,e. F3a
and F3b litters). A reduction in litter size occurred at 10 ppm in
F3a litters at birth, in F1b litters after 5 days and in F2a and F2b
litters at 5 weeks. At 2 ppm, a non-dose-related decrease in litter
size at birth and a slightly depressed survival of pups to 5 days were
observed in F2b litters. These findings, occurring in a single
progeny generation, were not believed to be attributable to parathion-
methyl in the diet. Gross examination of F2b and F3b pups at birth
and during rearing revealed no malformations. Histopathological
evaluation of reproductive tract plus liver and kidney from 5 males
and 5 females per group of F2 parents and of a set of about 15
tissues including the reproductive tract from 10 male and 10 female
F3b weanlings per group showed no microscopic changes associated with
treatment. The no-effect level in the study was 2 ppm (Lösen and Eiben
1982; Glaister 1981).
Special Studies on Mutagenicity
Parathion methyl (94.3 - 94.5% pure) was tested for its mutagenic
potential in in vitro microbial assays with and without the presence
of a metabolic activation preparation (S-9 fraction from liver of rats
induced with Aroclor 1254). The indicator organisms used were
Salmonella typhimurium strains TA 1535, TA 100, TA 1537 and TA 98.
Positive mutagenic response was obtained with and without metabolic
activation in strain TA 1535 at concentrations at and above
1 000 µg/plate and in strain TA 100 at and above 500 µg/plate. There
were no indications of mutagenic activity in assays with tester
strains TA 1537 and TA 98 at concentrations of 20 to 2 500 µg/plate
with or without metabolic activation (Herbold 1982a).
Parathion methyl (96.8% pure) was shown to induce a significant
increase (dose-related) of sister chromatid exchanges and cell cycle
delay in Chinese hamster cell line V79 and in two human lymphoid cell
lines (Chen et al 1981).
In a micronucleus test, groups of 5 male and 5 female mice were
intubated with parathion methyl (95.6%) at 0, 5 or 10 mg/kg bw/day for
2 consecutive days with an interval of 24 hours between doses. The
animals were sacrificed 6 hours after the second dose and femoral bone
marrow smears were prepared. Results of examination of the smears did
not indicate any significant increase in incidence of micronucleated
polychromatic erythrocytes in the treated groups (Herbold 1982b).
Special Studies on Eye and Skin Irritation
Results of irritation studies with New Zealand White rabbits
indicated parathion methyl (96.1%/95.6%) to be slightly irritating to
the skin. The compound was not an irritant to eyes (Thyssen and Lorke
1982).
Long-Term Studies
Rat
Groups of 5 to 6 weeks old male and female rats (SPF, Wistar
TNO/W74 strain; 100 male and 100 female controls, 50 males and 50
females/treated group) were fed parathion methyl (94.8% pure) in their
diet at 0, 2, 10 or 50 ppm for 2 years. Additional groups of 10 males
and 10 females per group were included for interim sacrifice of 5
males and 5 females/group after 6 and 12 months. Mortality was
increased in females at 50 ppm during the first year but about 79-90%
males and 58 - 78% females of control and treated groups still
survived at the end of the study. Animals (both sexes) in the top
dosage group exhibited cholinergic symptoms (tremors) at times during
the study and growth retardation throughout the experiment.
Haematology, clinical chemistry and urinalysis carried out at five
intervals during the study indicated deviations from control values
essentially only at 50 ppm in a number of parameters. These included
an increase in reticulocyte counts and a decrease in hematocrit and
haemoglobin values in both sexes after 6 and/or 24 months, depressed
plasma protein levels in females at all intervals, elevated serum urea
in females at most intervals and in males after 24 months, increased
plasma alkaline phosphatase activity in females after 3 and 6 months
and a rise in urinary protein level in females after 1 and 6 months.
(Plasma protein level was also depressed in females at 10 ppm but only
after 1 month.) Cholinesterase in plasma and erythrocyte, assayed nine
times over the course of the study, was consistently inhibited
(>20%), in a dose-dependent pattern, at 50 ppm at all intervals and
at 10 ppm frequently during the study. The extent of depression was
greater with plasma cholinesterase than with erythrocyte
cholinesterase. Terminal brain cholinesterase was inhibited (>20%) in
males at both 10 and 50 ppm and in females at 50 ppm.
Gross pathological findings in animals dying or sacrificed in
moribund condition during the study and in those sacrificed at interim
or terminally were comparable to those in the controls. A reduction of
absolute weight, but not organ/body weight ratio, of several organs
was demonstrated in animals at 50 ppm. Histopathological evaluation of
a wide range of tissues from animals of control and top dosage groups
only revealed a significant increase in frequency of males at 50 ppm
(33% vs 4% in controls) displaying very slight to moderate degree of
"excessive Oil Red 0(ORO)-positive substance in hepatocytoplasm". A
no-effect level on this particular liver lesion could not be defined
due to the absence of data on microscopic findings in the liver
of treated animals below 50 ppm. No other non-neoplastic
histopathological changes attributable to treatment were evident.
Notably, histopathological data were available for only half of the
animals in the control group.
When histopathological and tumour data from only 50 male and 50
female controls were used as the basis of comparison, there did not
seem to be any significant difference between the control and top
dosage groups in the incidence of animals with tumours (benign and/or
malignant), malignant tumours, benign tumours or multiple primary
tumours. However, there appeared to be an increase in incidence at
50 ppm of thyroid adenoma in males (11/45 vs 4/43 controls) and of
uterine adenocarcinoma (8/42 vs 3/44 controls)2. The increase was not
statistically significant if incidence for the respective tumours in
43 and 44 concurrent controls were used for comparison. Whether the
control incidence for the tumours in question would be significantly
altered, if histopathological findings in all 100 male and 100 female
concurrent controls were taken into consideration, is not known. The
incidence of uterine adenocarcinoma at 50 ppm was indicated in the
2 According to "Table: Tumour hosts with descriptions of all
tumours" included in the report, 4 female controls had adenocarcinoma
of the uterus. An examination of "histopathological single finding" in
Tables 344-347 revealed the uterus of one (animal No.149) of the 4
supposedly affected control animals was not examined microscopically.
report to "remain within the variability of untreated animals of this
strain observable in other virtually parallel studies". Background
incidences of this particular malignancy and thyroid adenoma were not
given (Bomhard et al 1981).
Observations in Humans
In 15 healthy male workers (with blood cholinesterase level
<75% of presumably the mean normal levels) in a pesticide plant who
were repeatedly or chronically exposed to parathion methyl for
durations ranging from 1 week to 7 years, but with intermittent
periods of non-exposure, there was no increased frequency of
chromosome aberrations in lymphocyte cultures, as compared to 13
matched controls (having a blood cholinesterase level of 100%) not
occupationally exposed to any chemical (Stocco et al 1982),
COMMENTS
The reproduction and long-term studies required by the 1980 Joint
Meeting and other additional studies have been made available. A
no-effect level of 2 ppm on re-production capability was demonstrated
in the 3-generation rat reproduction study. Parathion methyl was
mutagenic in the in vitro microbial assays to 2 of the 4
Salmonella typhimurium tester strains and in sister-chromatid
exchanges and cell cycle delay studies with Chinese hamster cell line
V79 and with 2 human lymphoid cell lines. On the other hand, a
micronucleus test in mice was negative. Also, workers exposed to
parathion methyl occupationally for long periods exhibited no
increased frequency of chromosome aberrations in lymphocyte cultures.
In the case of the 2-year feeding study in rats, the available data
did not permit a complete evaluation of the carcinogenic activity of
the compound or the determination of a definite no-effect level in the
species. The Meeting, therefore, decided to extend the temporary ADI
and requested that additional data pertaining to the study be
provided.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Man : 0.3 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
Additional data on the 2-year feeding study in rats including:
a. Historical data on incidences of thyroid adenoma and
adenocarcinoma of the uterus in the particular strain of
rats (SPF Wistar TNO/W74) used in the study.
b. Histopathological data, not presently available, on the
remaining 50 males and 50 females in the control group or
satisfactory and acceptable explanation for the absence of
such data.
c. Results of microscopic examination of the liver from treated
males below 50 ppm.
REFERENCES
Bomhard, E., Löser, E. and Schilde, B. E605-Methyl (parathion-methyl)
1981 chronic toxicological study on rats (feeding experiment of
two years). Report from Bayer AG submitted to the World
Health Organization by Bayer AG. (Unpublished)
Braeckman, R.A., Godefront, M.G., Blondeel, G.M., Belpaire, F.M. and
1980 Williams, J.L. Kinetic analysis of the fate of methyl
parathion in the dog. Arch. Toxicol. 43: 263-271.
Chen, H.H., Haveh, J.L., Sirianni, S.R. and Huang, C.C. Induction of
1981 sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res. 88: 307-316.
Glaister, J.R. E605-methyl rat breeding study (histopathology). Report
1981 from Hazleton Laboratories Europe, Ltd., England, submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl ( Salmonella/microsome test to
1982a evaluate forepoint mutation. Report from Bayer AG submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl micronucleus test on the mouse to
1982b evaluate for mutagenic effect. Report from Bayer AG
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Löser, E. and Eiben, R. E 605-methyl multigeneration studies on rats.
1982 Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Stocco, R. de Cassia, Becak, W., Gaeta, R. and Rabello-Gay, M.N.
1982 Cytogenetic study of workers exposed to methyl-parathion.
Mut. Res. 103:71-76.
Thyssen, J. and Lorke, D. Parathion-methyl (E120) tests for irritant
1982 effects on the skin and eye. Batch No. 23010618. Report from
Bayer AG submitted to the World Health Organization by Bayer
AG. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. (14C)methyl-parathion
1979 (benzene ring-labelled compound) biokinetic studies on rats.
Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Explanation
Parathion methyl was evaluated by the Joint Meeting in 1968,
1975, 1929 and 1980 (FAO/WHO 1969, 1976, 1980 and 1981)1 and a
temporary ADI of 0 - 0,001 mg/kg bw/day was allocated. Reproduction
study in species appropriate to such a test and results of adequate
long-term study reported to be in progress then were required by the
1980 Meeting. These studies, as well as other additional toxicological
data, have been received and are reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Elimination
In male rats, treated with a single oral dose of 14C-parathion
methyl (benzene ring-labelled) at 0.1, 1.0 or 5 mg/kg bw and in female
rats given a single oral dose of 1 mg/kg bw, over 99% of the
administered dose was excreted in the urine and the faeces within
48 hours. Elimination in the faeces accounted for only 5 - 7% after
1 or 5 mg/kg bw, but amounted to about 20% after 0.1 mg/kg bw. Male
rats treated with an intravenous dose of 1 mg/kg bw excreted about 99%
of the administered radioactivity in the urine within 48 hours, and
approximately 1% of the dose in the faeces. Total radioactive residues
recovered in the 12 tissues analysed (excluding the gastrointestinal
tract) from rats given a single oral dose of 5 mg/kg bw were about 11%
of the administered dose 1 hour after treatment, declining to 0.3% at
24 hours, about 0.1% at 48 hours and to only 0.04% 6 days later. The
kidney had the highest relative activity up to 8 hours post-treatment.
The 14C activity in the plasma was initially about 5 times higher
than that in the erythrocytes. However, from day 2 to day 6 after
dosing, the 14C-activity in the erythrocytes was greater than that in
the plasma and remained constant. Following an intraduodenal dose of
1 See Annex 2 for WHO and FAO documentation.
1 mg/kg bw, male rats with fistulated bile duct excreted only 3% of
the radioactive dose in the bile with the remainder being eliminated
primarily (95%) in the urine within 24 hours (Weber et al 1979).
The elimination of parathion methyl was rapid in the dog. A
terminal half-life of 7.2 hours was estimated in dogs given an
intravenous dose of 10 mg/kg bw (Braeckman et al 1980).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 10 male and 20 female rats (SPF-Wistar W74 strain, 5-6
weeks old) were fed a diet containing technical parathion methyl
(95% pure) at 0, 2, 10 or 50 ppm for 77 days and then mated to
initiate a standard 3-generation (2 litters/generation) reproduction
study. Pups of the second litters (F1b and F2b) provided the parents
of the next generation. Through the parental generations, there was no
mortality related to compound. Treatment at the top dietary level
(50 ppm) induced convulsions in F1 parents, growth retardation in
both F0 and F1 adults (both sexes) and significant adverse effects
on litter size and pup weight at birth and through lactation
practically through all progeny generations. All F2b pups at 50 ppm
died 5 days after birth, resulting in the absence of data on F3a and
F3b litters for this dosage group. Fertility rate was slightly
decreased at 10 ppm in F2 generation (both mating trials, i,e. F3a
and F3b litters). A reduction in litter size occurred at 10 ppm in
F3a litters at birth, in F1b litters after 5 days and in F2a and F2b
litters at 5 weeks. At 2 ppm, a non-dose-related decrease in litter
size at birth and a slightly depressed survival of pups to 5 days were
observed in F2b litters. These findings, occurring in a single
progeny generation, were not believed to be attributable to parathion-
methyl in the diet. Gross examination of F2b and F3b pups at birth
and during rearing revealed no malformations. Histopathological
evaluation of reproductive tract plus liver and kidney from 5 males
and 5 females per group of F2 parents and of a set of about 15
tissues including the reproductive tract from 10 male and 10 female
F3b weanlings per group showed no microscopic changes associated with
treatment. The no-effect level in the study was 2 ppm (Lösen and Eiben
1982; Glaister 1981).
Special Studies on Mutagenicity
Parathion methyl (94.3 - 94.5% pure) was tested for its mutagenic
potential in in vitro microbial assays with and without the presence
of a metabolic activation preparation (S-9 fraction from liver of rats
induced with Aroclor 1254). The indicator organisms used were
Salmonella typhimurium strains TA 1535, TA 100, TA 1537 and TA 98.
Positive mutagenic response was obtained with and without metabolic
activation in strain TA 1535 at concentrations at and above
1 000 µg/plate and in strain TA 100 at and above 500 µg/plate. There
were no indications of mutagenic activity in assays with tester
strains TA 1537 and TA 98 at concentrations of 20 to 2 500 µg/plate
with or without metabolic activation (Herbold 1982a).
Parathion methyl (96.8% pure) was shown to induce a significant
increase (dose-related) of sister chromatid exchanges and cell cycle
delay in Chinese hamster cell line V79 and in two human lymphoid cell
lines (Chen et al 1981).
In a micronucleus test, groups of 5 male and 5 female mice were
intubated with parathion methyl (95.6%) at 0, 5 or 10 mg/kg bw/day for
2 consecutive days with an interval of 24 hours between doses. The
animals were sacrificed 6 hours after the second dose and femoral bone
marrow smears were prepared. Results of examination of the smears did
not indicate any significant increase in incidence of micronucleated
polychromatic erythrocytes in the treated groups (Herbold 1982b).
Special Studies on Eye and Skin Irritation
Results of irritation studies with New Zealand White rabbits
indicated parathion methyl (96.1%/95.6%) to be slightly irritating to
the skin. The compound was not an irritant to eyes (Thyssen and Lorke
1982).
Long-Term Studies
Rat
Groups of 5 to 6 weeks old male and female rats (SPF, Wistar
TNO/W74 strain; 100 male and 100 female controls, 50 males and 50
females/treated group) were fed parathion methyl (94.8% pure) in their
diet at 0, 2, 10 or 50 ppm for 2 years. Additional groups of 10 males
and 10 females per group were included for interim sacrifice of 5
males and 5 females/group after 6 and 12 months. Mortality was
increased in females at 50 ppm during the first year but about 79-90%
males and 58 - 78% females of control and treated groups still
survived at the end of the study. Animals (both sexes) in the top
dosage group exhibited cholinergic symptoms (tremors) at times during
the study and growth retardation throughout the experiment.
Haematology, clinical chemistry and urinalysis carried out at five
intervals during the study indicated deviations from control values
essentially only at 50 ppm in a number of parameters. These included
an increase in reticulocyte counts and a decrease in hematocrit and
haemoglobin values in both sexes after 6 and/or 24 months, depressed
plasma protein levels in females at all intervals, elevated serum urea
in females at most intervals and in males after 24 months, increased
plasma alkaline phosphatase activity in females after 3 and 6 months
and a rise in urinary protein level in females after 1 and 6 months.
(Plasma protein level was also depressed in females at 10 ppm but only
after 1 month.) Cholinesterase in plasma and erythrocyte, assayed nine
times over the course of the study, was consistently inhibited
(>20%), in a dose-dependent pattern, at 50 ppm at all intervals and
at 10 ppm frequently during the study. The extent of depression was
greater with plasma cholinesterase than with erythrocyte
cholinesterase. Terminal brain cholinesterase was inhibited (>20%) in
males at both 10 and 50 ppm and in females at 50 ppm.
Gross pathological findings in animals dying or sacrificed in
moribund condition during the study and in those sacrificed at interim
or terminally were comparable to those in the controls. A reduction of
absolute weight, but not organ/body weight ratio, of several organs
was demonstrated in animals at 50 ppm. Histopathological evaluation of
a wide range of tissues from animals of control and top dosage groups
only revealed a significant increase in frequency of males at 50 ppm
(33% vs 4% in controls) displaying very slight to moderate degree of
"excessive Oil Red 0(ORO)-positive substance in hepatocytoplasm". A
no-effect level on this particular liver lesion could not be defined
due to the absence of data on microscopic findings in the liver
of treated animals below 50 ppm. No other non-neoplastic
histopathological changes attributable to treatment were evident.
Notably, histopathological data were available for only half of the
animals in the control group.
When histopathological and tumour data from only 50 male and 50
female controls were used as the basis of comparison, there did not
seem to be any significant difference between the control and top
dosage groups in the incidence of animals with tumours (benign and/or
malignant), malignant tumours, benign tumours or multiple primary
tumours. However, there appeared to be an increase in incidence at
50 ppm of thyroid adenoma in males (11/45 vs 4/43 controls) and of
uterine adenocarcinoma (8/42 vs 3/44 controls)2. The increase was not
statistically significant if incidence for the respective tumours in
43 and 44 concurrent controls were used for comparison. Whether the
control incidence for the tumours in question would be significantly
altered, if histopathological findings in all 100 male and 100 female
concurrent controls were taken into consideration, is not known. The
incidence of uterine adenocarcinoma at 50 ppm was indicated in the
2 According to "Table: Tumour hosts with descriptions of all
tumours" included in the report, 4 female controls had adenocarcinoma
of the uterus. An examination of "histopathological single finding" in
Tables 344-347 revealed the uterus of one (animal No.149) of the 4
supposedly affected control animals was not examined microscopically.
report to "remain within the variability of untreated animals of this
strain observable in other virtually parallel studies". Background
incidences of this particular malignancy and thyroid adenoma were not
given (Bomhard et al 1981).
Observations in Humans
In 15 healthy male workers (with blood cholinesterase level
<75% of presumably the mean normal levels) in a pesticide plant who
were repeatedly or chronically exposed to parathion methyl for
durations ranging from 1 week to 7 years, but with intermittent
periods of non-exposure, there was no increased frequency of
chromosome aberrations in lymphocyte cultures, as compared to 13
matched controls (having a blood cholinesterase level of 100%) not
occupationally exposed to any chemical (Stocco et al 1982),
COMMENTS
The reproduction and long-term studies required by the 1980 Joint
Meeting and other additional studies have been made available. A
no-effect level of 2 ppm on re-production capability was demonstrated
in the 3-generation rat reproduction study. Parathion methyl was
mutagenic in the in vitro microbial assays to 2 of the 4
Salmonella typhimurium tester strains and in sister-chromatid
exchanges and cell cycle delay studies with Chinese hamster cell line
V79 and with 2 human lymphoid cell lines. On the other hand, a
micronucleus test in mice was negative. Also, workers exposed to
parathion methyl occupationally for long periods exhibited no
increased frequency of chromosome aberrations in lymphocyte cultures.
In the case of the 2-year feeding study in rats, the available data
did not permit a complete evaluation of the carcinogenic activity of
the compound or the determination of a definite no-effect level in the
species. The Meeting, therefore, decided to extend the temporary ADI
and requested that additional data pertaining to the study be
provided.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Man : 0.3 mg/kg bw/day
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
Additional data on the 2-year feeding study in rats including:
a. Historical data on incidences of thyroid adenoma and
adenocarcinoma of the uterus in the particular strain of
rats (SPF Wistar TNO/W74) used in the study.
b. Histopathological data, not presently available, on the
remaining 50 males and 50 females in the control group or
satisfactory and acceptable explanation for the absence of
such data.
c. Results of microscopic examination of the liver from treated
males below 50 ppm.
REFERENCES
Bomhard, E., Löser, E. and Schilde, B. E605-Methyl (parathion-methyl)
1981 chronic toxicological study on rats (feeding experiment of
two years). Report from Bayer AG submitted to the World
Health Organization by Bayer AG. (Unpublished)
Braeckman, R.A., Godefront, M.G., Blondeel, G.M., Belpaire, F.M. and
1980 Williams, J.L. Kinetic analysis of the fate of methyl
parathion in the dog. Arch. Toxicol. 43: 263-271.
Chen, H.H., Haveh, J.L., Sirianni, S.R. and Huang, C.C. Induction of
1981 sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res. 88: 307-316.
Glaister, J.R. E605-methyl rat breeding study (histopathology). Report
1981 from Hazleton Laboratories Europe, Ltd., England, submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl ( Salmonella/microsome test to
1982a evaluate forepoint mutation. Report from Bayer AG submitted
to the World Health Organization by Bayer AG. (Unpublished)
Herbold, B. E 120 parathion-methyl micronucleus test on the mouse to
1982b evaluate for mutagenic effect. Report from Bayer AG
submitted to the World Health Organization by Bayer AG.
(Unpublished)
Löser, E. and Eiben, R. E 605-methyl multigeneration studies on rats.
1982 Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
Stocco, R. de Cassia, Becak, W., Gaeta, R. and Rabello-Gay, M.N.
1982 Cytogenetic study of workers exposed to methyl-parathion.
Mut. Res. 103:71-76.
Thyssen, J. and Lorke, D. Parathion-methyl (E120) tests for irritant
1982 effects on the skin and eye. Batch No. 23010618. Report from
Bayer AG submitted to the World Health Organization by Bayer
AG. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. (14C)methyl-parathion
1979 (benzene ring-labelled compound) biokinetic studies on rats.
Report from Bayer AG submitted to the World Health
Organization by Bayer AG. (Unpublished)
