
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
PHOXIM
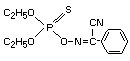 IDENTITY
Chemical Names
OO-diethyl alpha-cyanobenzylidene-amino-oxyphosphonothioate
alpha - (diethoxyphosphinothioyloxyimino) phenyl-acetonitrile
alpha - / / (diethoxyphosphinothioyl)oxy/imino/benzeneacetonitrile
O,O-diethyl phenylglyoxylonitrile oxime phosphorothioate
Synonyms
VolatonR
BaythionR
SRA 7502
BAYER 77488
Structural formula
IDENTITY
Chemical Names
OO-diethyl alpha-cyanobenzylidene-amino-oxyphosphonothioate
alpha - (diethoxyphosphinothioyloxyimino) phenyl-acetonitrile
alpha - / / (diethoxyphosphinothioyl)oxy/imino/benzeneacetonitrile
O,O-diethyl phenylglyoxylonitrile oxime phosphorothioate
Synonyms
VolatonR
BaythionR
SRA 7502
BAYER 77488
Structural formula
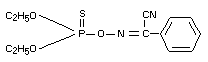 Other Information on Identity and Properties
Empirical formula C12H15N2O3PS
Molecular weight: 298.3
Appearance light yellow oily liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20°/4°C (pure active ingredient)
Vapour pressure approx. 10-4 mmHg at 20°C
Solubility (in g/100 ml at 20°C)
in water 0.7
in cyclohexane >60
in isopropyl alcohol >60
in methylene chloride >60
in toluene >60
Minimum degree of (pre-solution for reasons of stability in
purity 9-11% butanol)
82.0%
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
Male Swiss mice were given a single oral dose of 32p-phoxim in
olive oil at levels of 10.5, 114, and 955 mg/kg bw. At all three
dosage levels, the recovery of administered radioactivity in urine and
faeces was in the range of 73-82%, within 140 hours after dosing.
However, the radioactivity appeared in the urine and faeces at a much
lower than expected rate in the light of the low mammalian toxicity of
phoxim. At the three dosage levels, respectively, only 43 22 and 15%
of the administered radioactivity was excreted in the urine within 24
hours after treatment. An autopsy was performed on a mouse treated
with 114 mg/kg bw after 48 hours to determine the internal fate of the
administered dose. At this time, approximately 43% of the dose had
been excreted in the urine. The results indicate that virtually all of
the internal radioactivity was found in urinary bladder (88.4%), gut
(8.8%) and liver (1.7%). The amounts of radioactivity found in other
organs (brain, thymus gland, hind leg muscle, heart, kidney) and the
amount of organic soluble material (which could include the strong
anticholinesterase P=0 phoxim) were essentially insignificant.
The autopsy data indicate that nearly all of the internal dose of
phoxim remaining 48 hours after treatment was metabolized to water
soluble compounds (97.6%),
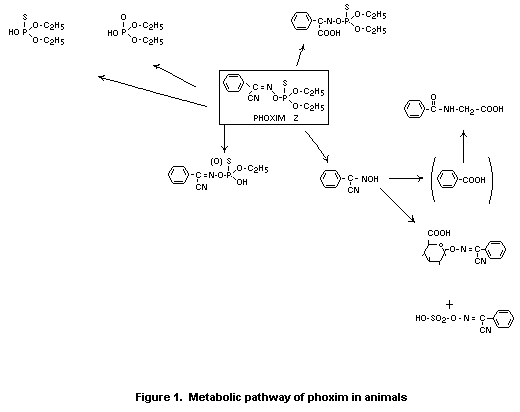
Five major metabolites were identified in the urine 0 to 24 hours
after treatment with 114 mg/kg bw: (1) diethyl phosphoric acid, 58.9%;
(2) phoxim, 1.1%; (3) phoxim carboxylic acid, 2.8%; (4) O,O-diethyl
phosphorothioic acid, 20.0%; (5) either monodesethyl phoxim or
monodesethyl P=O phoxim, 6.2%. The most relevant changes in the
relative amounts of metabolites upon increasing the dose from 114 to
955 mg/kg bw occurred with phoxim carboxylic acid (2.8% to 23.6%) and
diethyl phosphoric acid (58.9% to 43.1%) (Vinopal and Fukuto 1970).
Fig. 1 presents the proposed metabolic pathway for phoxim in
mice.
Rat
Male and female rats (Charles River CD strain) were intubated
with 14C-phoxim (labelled in the benzene moiety) at a dose of
10 mg/kg bw. A group of male rats was also intubated at a dose of
1 mg/kg bw. The compound was readily absorbed from the
gastrointestinal tract of male rats with average maximal plasma levels
equivalent to 0.35 and 2.44 µg phoxim being reached within 30 minutes
after dosing at 1 and 10 mg/kg respectively. A secondary peak,
equivalent to 1.87 µg phoxim/ml, was observed at 4 hours in animals
dosed at 10 mg/kg. A value of 0.04 µg phoxim/ml was measured at 24
hours in the plasma of rats receiving 10 mg/kg bw.
There was a rapid uptake of radioactivity into the major organs
and tissues following the administration of 10 mg/kg to male rats, the
kinetics of which was similar to that for plasma. Slightly higher
initial values were observed for kidney and liver, but levels of 0.06
to 0.6 µg equivalents of phoxim/g were reached in all tissues examined
within 24 hours. Thus, there was no evidence for the selective
retention of either phoxim or its presumed metabolites in any tissue
and accumulation in the tissues is unlikely as a result of repeated
administration.
Other Information on Identity and Properties
Empirical formula C12H15N2O3PS
Molecular weight: 298.3
Appearance light yellow oily liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20°/4°C (pure active ingredient)
Vapour pressure approx. 10-4 mmHg at 20°C
Solubility (in g/100 ml at 20°C)
in water 0.7
in cyclohexane >60
in isopropyl alcohol >60
in methylene chloride >60
in toluene >60
Minimum degree of (pre-solution for reasons of stability in
purity 9-11% butanol)
82.0%
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
Male Swiss mice were given a single oral dose of 32p-phoxim in
olive oil at levels of 10.5, 114, and 955 mg/kg bw. At all three
dosage levels, the recovery of administered radioactivity in urine and
faeces was in the range of 73-82%, within 140 hours after dosing.
However, the radioactivity appeared in the urine and faeces at a much
lower than expected rate in the light of the low mammalian toxicity of
phoxim. At the three dosage levels, respectively, only 43 22 and 15%
of the administered radioactivity was excreted in the urine within 24
hours after treatment. An autopsy was performed on a mouse treated
with 114 mg/kg bw after 48 hours to determine the internal fate of the
administered dose. At this time, approximately 43% of the dose had
been excreted in the urine. The results indicate that virtually all of
the internal radioactivity was found in urinary bladder (88.4%), gut
(8.8%) and liver (1.7%). The amounts of radioactivity found in other
organs (brain, thymus gland, hind leg muscle, heart, kidney) and the
amount of organic soluble material (which could include the strong
anticholinesterase P=0 phoxim) were essentially insignificant.
The autopsy data indicate that nearly all of the internal dose of
phoxim remaining 48 hours after treatment was metabolized to water
soluble compounds (97.6%),
Five major metabolites were identified in the urine 0 to 24 hours
after treatment with 114 mg/kg bw: (1) diethyl phosphoric acid, 58.9%;
(2) phoxim, 1.1%; (3) phoxim carboxylic acid, 2.8%; (4) O,O-diethyl
phosphorothioic acid, 20.0%; (5) either monodesethyl phoxim or
monodesethyl P=O phoxim, 6.2%. The most relevant changes in the
relative amounts of metabolites upon increasing the dose from 114 to
955 mg/kg bw occurred with phoxim carboxylic acid (2.8% to 23.6%) and
diethyl phosphoric acid (58.9% to 43.1%) (Vinopal and Fukuto 1970).
Fig. 1 presents the proposed metabolic pathway for phoxim in
mice.
Rat
Male and female rats (Charles River CD strain) were intubated
with 14C-phoxim (labelled in the benzene moiety) at a dose of
10 mg/kg bw. A group of male rats was also intubated at a dose of
1 mg/kg bw. The compound was readily absorbed from the
gastrointestinal tract of male rats with average maximal plasma levels
equivalent to 0.35 and 2.44 µg phoxim being reached within 30 minutes
after dosing at 1 and 10 mg/kg respectively. A secondary peak,
equivalent to 1.87 µg phoxim/ml, was observed at 4 hours in animals
dosed at 10 mg/kg. A value of 0.04 µg phoxim/ml was measured at 24
hours in the plasma of rats receiving 10 mg/kg bw.
There was a rapid uptake of radioactivity into the major organs
and tissues following the administration of 10 mg/kg to male rats, the
kinetics of which was similar to that for plasma. Slightly higher
initial values were observed for kidney and liver, but levels of 0.06
to 0.6 µg equivalents of phoxim/g were reached in all tissues examined
within 24 hours. Thus, there was no evidence for the selective
retention of either phoxim or its presumed metabolites in any tissue
and accumulation in the tissues is unlikely as a result of repeated
administration.
 In the 10 mg/kg treatment, male rats excreted an average of 92.9%
of the radioactivity in the urine and 4.9% in the faeces in ten days,
while females excreted 86.1% of the dose in the urine and 6.9% in the
faeces in the same period. Male rats intubated at a dose of 1 mg/kg
excreted 82% of the radioactivity in the urine and 7.9% in the faeces
in ten days. However, most of the radioactivity (80-90%) was
eliminated in 24 hours and excretion was virtually complete within 2
days. No evidence was obtained for the presence of 14CO2 in the
expired air during the initial 24 hours.
Radiochemical analysis 10 days after dosing of the major organs
and tissues from animals dosed at both 1 and 10 mg/kg indicated the
presence of only low levels of radioactivity, which corresponded to
less than 0.1 µg phoxim/g tissue. An average of 0.80% and 3.3% of the
radioactivity was excreted within 0-6 and 6-24 hours in the bile of
cannulated male rats intubated at the 10 mg/kg dose level (Daniel
et al 1978a).
The biotransformation of phoxim was investigated in adult male
rats (Charles River CD strain) following administration (by gavage) of
a dose of 10 mg/kg bw.
The major metabolites in the 24-hour urine was identified as
hippuric acid (approx. 6%) and glucuronic and sulphuric acid
conjugates (approx. 76%) of alpha-cianobenzaldoxime. Evidence was also
obtained for the presence of both syn- and anti-forms of the oxime.
Chromatographic analysis of the plasma obtained one hour after
dosing revealed the presence of four radioactive components,
two of which were characerized as mono-desethyl-phoxim (80%) and
mono-desethyl PO-phoxim (12%). No evidence was obtained for the
presence of PO-phoxim (Daniel et al 1978b).
Effects on Enzymes and Other Biochemical Parameters
In the rat, in vitro cholinesterase inhibition amounted to (I50)
4.19×10-4M for serum, 4.46×10-5M for erythrocytes and 2.51×10-6M for
brain (Kimmerle 1968).
The effect of the organophosphorus pesticide phoxim on the
activity of liver succinate dehydrogenase (SDH) and cytochrome oxidase
(CO) was studied in randomly bred albino rats. Animals received a
single p.o. administration of phoxim at 0.5 LD50 (310 mg/kg) and were
sacrificed 1 hour, 1 day and 5 days later. In chronic experiments,
rats received phoxim at 31 mg/kg/day for 1, 3 and 6 months. Single
administration of phoxim decreased CO activity in liver homogenate
1 hour after administration (5.3 IU, compared with 7.61 IU in
controls), while the decrease in SDH activity was detected only on day
1 after administration (2.9 IU, compared with 7.55 IU in controls). In
the supernatant, activities of CO and SDH showed an increase only on
day 5 after administration (0.62 IU and 0.45 IU, respectively,
compared with 0.42 IU and 0.086 IU in controls). Chronic exposure to
phoxim did not change CO activity in the liver homogenate but resulted
in an increase in CO activity in the supernatant (after 3 months
exposure, CO activity was 0.80 IU compared with 0.42 IU in controls).
Activity of SDH in the homogenate increased after exposure of 1 month
(8.49 IU, compared with 7.55 IU in controls), while activity of SDH
in the supernatant was increased only after 3 and 6 months of
administration (0.35 IU and 0.59 IU, respectively, compared with
0.09 IU in controls) (Kuz'minskaia and Veremenko 1978).
The effect of phoxim on the NADPH-dependent oxidation in the
liver endoplasmic reticulum was studied in randomly bred albino rats,
Phoxim was given p.o. for 3 days at 124 mg/kg/day (LD50 is
620 mg/kg). On days, 1, 5 and 15 after the last administration,
animals were sacrificed and activity of liver demethylase (DM),
hydroxylase (HL), uridine diphosphate glucuronyl transferase (UDPGT)
and the level of ascorbic acid excretion were assessed. Phoxim
administration was found to stimulate the process of demethylation.
DM activity began increasing on day 5 after administration and
remained at a high level up to day 15 (0.957 IU and 0.842 IU,
respectively, compared with 0.602 IU in controls). HL activity was
decreased on day 1 (0.714 IU, compared with 0.304 IU in controls),
followed by a slight increase on day 5 (0.380 IU) and normalization on
day 15. Activity of UDPGT was significantly increased only on day 1
after administration (5.48 IU, compared with 3.28 IU in controls). The
level of urinary excretion of ascorbic acid showed a progressive
increase up to 538 µg/ml on day 1, 194.8 µg/ml on day 5 and
174.8 µg/ml on day 15 (compared with 118.5 µg/ml in controls (Iakusko
1978).
The effects of phoxim on the glucose-6-phosphatase (G6P),
hexokinase (HK), total cholinesterase (ChE) and ChE isoenzyme
activities were studied in the blood of male albino rats weighing
200-250 g. Group 1 served as a control, group 2 was treated (p.o.)
with a single 310 mg/kg dose of phoxim (0.5 LD50), group 3 received
31 mg/kg/day for 1-6 months and treatment in group 4 was 3.1 mg/kg/day
for 1-6 months. In group 4 the G6P and HK activities were increased
and the ChE activity was reduced 1 hour after treatment. The HK
activity became normal 1 hour later, the G6P activity remained
elevated and the ChE activity declined further. All enzyme activities
were normal 5 days after treatment. In group 3, the G6P and G6P
dehydrogenase (G6PD) activities were increased by 45% and 70%,
respectively, and the total ChE activity was decreased by 45% at the
end of the first month. The ChE fractions 3, 4 and 5 were fully
inhibited; fraction 2 was inhibited by 78%, and the activity of
fraction 1 was increased by 61%. After treatment for 3 months the G6P
activity was increased by 89%, the G6PD activity by 42% and the total
ChE activity was inhibited by 26%, while the isoenzyme spectrum did
not differ from that in the controls. After poisoning for 6 months,
the G6P and G6PD activities were inhibited by 30%, and the total ChE
by 24%. Fraction 1 was inhibited by 45% and the activity of fraction 2
increased by 35%, while the fractions 3, 4 and 5 remained normal. The
changes seen in group 4 were similar to but less marked than those in
group 3 (Kuz'minskaia et al 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of rats (10 male and 20 female, Long Evans FB30
strain/group) were fed diets containing phoxim (technical product,
85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm (calculated as
pure a.i.) and subjected to a standard 2-litter per generation,
3-generation reproduction study. The rats were treated with the test
compound throughout the study, including mating, gestation and
lactation. At the start of the experiment, the rats were about 47 days
old. The rats selected for the study were housed singly until they
were sexually mature (up to an age of ca. 100 days). Then they were
mated to initiate the study. During the mating period, two female rats
were housed together with one male rat for 21 days. The male rats were
rotated so that each female was paired with three different males for
a period longer than one oestrous cycle. Immediately after the pups
were delivered, their number and weights were recorded. Litters
containing more than 10 pups were reduced on day 5 after delivery to
10 pups each, whereupon the weights of these litters were again
measured. The pups were nourished for up to 4 weeks, weighed weekly,
and then the offspring of each first mating (F1a, F2a, F3a) were
sacrificed.
The offspring of each second mating, as with those delivered
after the first mating, were nourished for up to 4 weeks, then weaned
and placed in separate sex groups. After week 8, 10 male and 20 female
rats were again selected from each dose group for further matings.
Upon reaching an average age of 100 days, the rats were mated as
described above. After the dams of the F0, F1b and F2b generations
had successfully nursed offspring twice, they were sacrificed.
Necropsy was performed on rats that died during the study. Gross and
histopathological examinations were performed on major organs and
tissues of one male and one female 2-week old pup of the F3b
generation from each of ten mothers in each group.
There were no significant differences between the control and
treated groups with respect to physical appearance, behavioural
patterns, body weight curves of weaned males and females of the F0,
F1b and F3b generations; some animals in both the control and treated
groups died of pneumonitis.
There were no significant differences between the control and
treated groups with respect to pregnancy rate, litter size, 5-day
survival rate (viability index), pups weights at birth and during
lactation at all dose levels tested. Only the 375 ppm dietary
concentration had a slight adverse effect on the lactation index in
F3b. The inspections of the pups immediately after birth and during
the lactation period did not reveal any signs of malformations. Gross
and histopathological examinations did not provide any evidence of
treatment-related alterations.
The no-effect level in this multigeneration reproduction study
was 75 ppm (Löser 1979).
Special Studies on Mutagenicity
The mutagenicity of phoxim (technical product, 83.8% a.i.) was
studied by rec-assay and reversion test (Salmonella/microsome).
The rec-assay was performed using two strains of Bacillus
subtilis, H 17 and M 45. Volumes of 0.2 µl to 20 µ1 of phoxim per
disc were tested. Kanamycin was used as a negative control and
mitomycin C as a positive control. Phoxim did not inhibit the growth
of H 17 and M 45 strains of B. subtilis at all the tested doses. On
the other hand, with mitomycin C used as positive control more
remarkable growth inhibition was identified in M 45 strain than in
H 17 strain, and the growth of both strains was inhibited at the same
degree with Kanamycin as the negative control.
The reversion test was performed using two strains of
Salmonella typhimurium TA 98 and TA 100, according to the Ames
procedure, both with and without S-9 Mix derived from liver of SD
strain rats treated with a single intraperitoneal injection of
Arochlor 1254 (500 mg/kg bw). Concentrations of 10 - 5000 µg/plate
were tested. 2-amino-anthracene (2-AA) and 2-(2-furyl)-3-(5-nitro-2-
furyl) acrylamide (AF-2) were used as positive controls.
A markedly increased number of revertant colonies was observed
for AF-2 on the plates without S-9 Mix and for 2-AA on the plates with
S-9 Mix. Phoxim did not increase the number of revertant colonies,
either in the presence or absence of S-9 Mix.
Under the conditions of the experiments, phoxim provided no
evidence of mutagenic activity at concentrations up to 0.02 ml/disc of
undiluted phoxim and 5 000 µg/plate, respectively, in a rec-assay and
in a reversion test (Shirasu et al 1978).
In another Ames test, phoxim (technical grade, a.i. 82.9% -
83.7%) was tested for mutagenicity with S. typhimurium TA 100, TA
1537 and TA 98 according to the Ames procedure, both in the presence
and in the absence of S-9 Mix derived from male Sprague-Dawley rats
treated with a single intraperitoneal injection of Arochlor 1254
(500 mg/kg bw). Seven concentrations (3.15-3150 nl/plate) were tested
with S-9 Mix and five concentrations (31.5-3150 nl/plate) without it.
At doses greater than 1 000 nl, however, part of the test compound
separated as droplets from the top agar. In the experiments for direct
mutagenicity N-methyl-N'-nitro-N-nitrosoguanidine and benzo(a)pyrene-
4,5-oxide were used as positive controls, whereas in the experiment
with an activating system 3-methyl-cholantrene, benzo(a)pyrene and
2-amino-anthracene were used. Negative controls in the form of
sterility controls and solvent blanks were run as well.
For all three tested strains, there were no significant
differences in the number of revertant colonies between the negative
controls and the phoxim-treated plates, both with and without S-9 Mix.
Positive controls displayed the expected increases, indicating the
activity of the metabolizing system and the mutability of the
bacteria. No bacteriotoxic effects were observed.
Phoxim did not show a mutagenic effect in the Salmonella/
microsome test at concentrations as high as 3 150 nl/plate (Oesch
1977).
Mouse - dominant lethal test on males
Groups of mice (20 male NMRI mice/group) were given by gavage a
single dose of phoxim (technical product stabilized with 10%
n-butanol, 86.5% a.i.) corresponding to 0 and 500 mg/kg bw, the
vehicle being a 0.5% Cremophor emulsion. The mouse strain used
displayed a sensitive response to known chemical mutagens, such as
cyclophosphamide. The dose was chosen on the basis of the results of a
preliminary test conducted on male mice dosed orally with acute doses
of 500, 750 and 1 000 mg/kg bw, respectively. At dose levels of 750
and 1 000 mg/kg bw the compound had a toxic effect and induced
symptoms, but no mortalities occurred. Following dosing, each male
mouse was caged with three untreated virgin female mice for 7 days.
The procedure was repeated weekly with groups of three new untreated
virgin females for a total of 8 weeks, in order to obtain and examine
a complete sample of the successive germ cell stages of the males. The
uteri of the females (466-480 per test group) were examined on
gestation day 14. Counts were made of the corpora lutea, total
implantations, viable implants and dead implants (sum of the
deciduomata, resorptions and dead embryos and foetuses).
There were no significant differences between the control and
treated groups with respect to fertility quota, total implantations,
viable implants, dead implants, ratio of dead implants to total
implants and pre-implantation loss (estimated both directly from the
difference between the number of corpora lutea and the number of
implantations and indirectly through the comparison of the average
number of implantations per fertilized female in the treated group
with that in the control group).
Thus, there was no indication for a mutagenic potential of
phoxim in the dominant lethal test on the male mouse at an oral
dose of 500 mg/kg bw (Machemer 1974).
Micronucleus test
Groups of mice (5 male and 5 female, NMRI (SPF) Han strain/group)
were given (by gavage) two single doses of phoxim (technical product
84.3% a.i.) in aqueous 0.5% Cremophor emulsion at levels of 0, 250,
500 mg/kg bw. The interval between applications was 24 hours.
Concurrently, a positive control group of mice received 2 × 100 mg/kg
bw of cyclophosphamide. The doses were chosen on the basis of the
results of a preliminary test in which groups of 5 mice were orally
dosed with phoxim at 2 × 500 mg/kg bw and 2 × 1 000 mg/kg bw,
respectively; in that test, the 2 × 500 mg/kg treatment was tolerated
with induction of only weak symptoms. Six hours after the second
application, the mice were sacrificed and femur bone marrow smears
were prepared. Erythrocytes, 1 000 per mouse, were counted and the
incidence of cells with a micronucleus was determined, as well as the
ratio of polychromatic erythrocytes to normochromatic erythrocytes.
The incidence of micronucleated polychromatic erythrocytes was
2.3/1 000 in the negative control group, and 1.8/1 000 and 1.0/1 000,
respectively, in the phoxim-treated groups. There were no relevant
differences between the negative control and phoxim-treated group with
respect to the ratio of polychromatic to normochromatic erythrocytes.
The incidence of micronucleated cells in cyclophosphamide-treated
group was 68.2/1 000 and was thus biologically significantly higher
than in the negative control group. Cyclophosphamide also exhibited
a bone marrow depression, with the ratio of polychromatic to
normochromatic erythrocytes showing a biologically relevant alteration
(1 000 : 2 424.2 vs 1 000: 838.3 in the negative control group).
The results provided no indication for a mutagenic potential of
phoxim in the micronucleus test on mouse at the tested doses of
2 × 250 and 2 × 500 mg/kg bw per os. Treatments with phoxim also did
not induce any depression of erythropoiesis (Herbold 1981).
Special Studies on Embryotoxicity and Teratogenicity
Groups of fertilized rats (20 Long Evans FB30 strain/group)
received daily doses of phoxim (technical product stabilized with
10% n-butanol, 86.5% a.i.), administered by gavage in a 0.5% aqueous
Cremophor emulsion, at levels of 0, 30, 100, 300 mg/kg bw from
gestation day 6 through 15 (total of 10 doses). On gestation day 20,
the dams were sacrificed and the foetuses removed by caesarean
section. The foetuses were examined for external, internal and
skeletal malformations. No dam died in any group and no adverse
effects on behaviour patterns and general appearance were observed.
The 300 mg/kg bw dose level had a maternal-toxic effect, resulting in
significantly less weight gain during the treatment period as compared
with the control animals. All animals in the test group showed
comparable average weight gains throughout the period of gestation.
There were no significant differences between the control and
treated groups with respect to the measured parameters: fertilization
quotas, pregnancy quotas, average numbers of implantations, foetuses
and resorptions, average foetus weight, average placenta weight,
average number of stunted foetuses (weighing less than 3 g) and type,
frequency and localization of slight alterations in bone development.
Sex distribution of foetuses was unaffected. Occasional malformations
were seen, these being most frequent in the untreated control group.
They were considered to be spontaneous malformations.
The data provided no indication for embryotoxic or teratogenic
activity in rats at an oral dose of 300 mg/kg bw and below (Machemer
1975).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Neurotoxicity Studies
Hen
Female White Leghorn hens, 2-5/group, about 14 to 18 months
old, each received a single phoxim dose (without antidote)
administered orally at levels of up to and including 50 mg/kg bw or
intraperitoneally at levels of up to and including 37.5 mg/kg bw. The
hens were kept under observations for 6 weeks. The determined LD50
was 37.5 mg/kg bw for both oral and intraperitoneal administration.
In another study, hens 3-14/group, were each given as an antidote
an intraperitoneal injection of 100 mg PAM/kg + 50 mg atropine
sulphate/kg. They then received an oral or intraperitoneal application
of phoxim at dose levels equal to the LD50 and higher. The survivors
were kept under observation for 6 weeks. No histopathological
examinations were carried out. The protection afforded by PAM +
atropine to hens was more evident with intraperitoneal injection of
phoxim than with the oral route. No positive controls were included.
In both tests, without and with antidotal protection, no clinical
signs of neurotoxic damage were seen (Kimmerle 1972).
Groups of hens (10 White Leghorn, 12 to 14 months old/group) were
fed diets containing phoxim from technical product stabilized with 10%
n-butanol, 86.1% a.i. at concentrations of 0, 5 and 10 ppm for 28
days.
During the feeding experiment, the behavioural pattern of all the
hens was within the normal range. In particular, they did not display
any symptoms of neurotoxicity. Treated and untreated controls hens
were not significantly different with respect to food consumption and
body weight gains. Blood acetylcholinesterase activity was not
significantly depressed in the 5 ppm group. In the 10 ppm group, a
marked (30%) depression of blood acetylcholinesterase activity was
measured between day 14 and day 28 of the feeding experiment. The
no-effect level was 5 ppm, equal to 0.32 mg/kg bw/day (Thyssen and
Kimmerle 1973).
Special Studies on Skin and Eye Irritation
A dermal irritation study in rabbits of both sexes ("not of pure
breed") showed that phoxim had only a very slight irritating effect on
both intact and abraded skin (Kimmerle and Solmecke 1970).
In an eye irritation test in rabbits, phoxim caused no eye
irritation (Kimmerle and Solmecke 1970).
Special Studies on Antidotes
Male rats were given single oral doses of phoxim (technical
product, 88.7% a.i.) to determine LD50 values with and without
antidotes. Before the onset of severe poisoning symptoms (5 to
90 minutes), atropine sulphate (50 mg/kg bw), 2-PAM (50 mg/kg bw) or
toxogonin (20) mg/kg bw) was given singly or in combination (atropine
plus PAM-atropine plus toxogonin) by intraperitoneal injection. The
results indicated that, among the tested antidotes, only combinations
of 2-PAM or toxigonin with atropine sulphate had significant antidotal
activity (Solmecke 1971).
Special Studies on Potentiation
Acute oral LD50 values in male Wistar II albino rats were
determined experimentally for phoxim, phenamiphos and their equitoxic
mixture. The observed LD50 value for the equitoxic mixture was
compared with the expected value (calculated assuming an additive
effect). The result was that simultaneous administration of phoxim and
phenamiphos produced a less than additive effect (Thyssen 1976a).
Special Studies on the Recovery of Cholinesterase Activity
Groups of rats (15 male and 15 female, Wistar II, SBF
albinos/group) were given daily doses of phoxim (technical product),
administered by gavage in aqueous Cremophor EL emulsion, at levels of
0, 5, 50 mg/kg bw for 21 days. One-third of the rats were sacrificed
upon termination of treatment, another third were kept under post-
treatment observations for 14 days and then sacrificed and the
remaining third were kept under post-treatment observation for 4
weeks. Determinations of acetylcholinesterase activities in
erythrocytes and plasma were carried out before beginning treatment
and thereafter at weekly intervals until 4 weeks after the end of
administration. Brain acetylcholinesterase activity was measured at
the end of treatment and 2 weeks after termination of treatment.
During the 21-day treatment period and the 4-week post-treatment
observation period, none of the treated rats in any dose group
differed from the control rats with respect to behavioural patterns,
physical appearance and body weight gain. No mortalities were
observed. The following results were obtained in male rats: at
5 mg/kg bw, plasma and erythrocyte cholinesterase activities were
not significantly different from the control group during both the
treatment period and the post-treatment observation period; at
50 mg/kg, plasma and erythrocyte cholinesterase activities were about
65% and 56%, respectively, of the control after 1 week of treatment
and remained similarly depressed during the treatment period; however,
one week after the end of treatment, cholinesterase activity levels
were again found not significantly different from the control.
The following results were obtained in female rats; at 5 mg/kg
bw, both plasma and erythrocyte cholinesterase activities were about
70% of the control after 1 week of treatment, but one week after the
termination of treatment both were in the physiological range; at
50 mg/kg bw, both plasma and erythrocyte cholinesterase activities
were about 37% of the control after 1 week of treatment; one week
after termination of treatment plasma cholinesterase was again normal,
while erythrocyte cholinesterase was 75% of the control and returned
to normal at the end of the 4-week post-treatment observation period.
Brain cholinesterase activity at the end of the treatment period
was 82% and 67% of the control, respectively, for male and female rats
of the 50 mg/kg bw group. However, the values measured 2 weeks after
the end of treatment were not significantly different.
Thus, female rats were more sensitive than males to the
depression of plasma, erythrocyte and brain cholinesterase activities.
The plasma, erythrocyte and brain cholinesterase activity depression
was reversible; plasma cholinesterase returned to normal within
1 week, while erythrocyte and brain cholinesterase were normal in 2-4
weeks.
The acute oral LD50 determinations prior to and after the 21-day
treatment gave comparable values for both male and female rats
(Thyssen 1976b).
Special Studies on the Toxicity of Metabolites
Acute toxicity studies were conducted with alpha-
cyanobenzaldoxime (cyanoxime). The metabolite (and starting material
in the synthesis of phoxim) has a very slight acute toxicity to rats:
LD50 (mg/kg)
Rat, male oral 4 520
Rat, female oral 4 063
Rat, male,female dermal (24 hours) >5 000
From the symptoms of poisoning observed (behavioural disorders,
sedation, breathing disorders) cyanoxime seemingly acts on the central
nervous system.
In acute inhalational toxicity experiments, in which rats were
exposed to dust for 1 hour and 4 hours, respectively, and rats and
mice were exposed to vapour for 6 hours, no toxic effects were
observed.
In tests for skin and eye irritation, the metabolite did not
cause any irritation to the skin of rabbits. In the eye of rabbits,
however, it caused moderate to severe irritation on the conjunctiva
and superficial corrosion on the cornea (Thyssen and Kimmerle 1976).
The glucoside of alpha cyanobenzaldoxime did not cause any toxic
effects when administered as an acute oral dose to female rats; a dose
of 2 500 mg/kg bw was tolerated without inducing any symptoms (Mihail
1979).
PO phoxim, formed as an intermediate product of metabolism, has
an oral LD50 to the mouse of 1 000 mg/kg bw (Vinopal and Fukuto
1970).
Special Studies on Cholinesterase Inhibition by P=O Phoxim
The following 150 values were determined in vitro for the P=O
analogue of phoxim: erythrocyte (bovine), 2.2 × 10-7M; brain (mouse),
6.0 × 10-8M (Vinopal and Fukuto 1970).
Special Studies on Tolerability of Phoxim by Sheep
Groups of sheep (25 freshly shorn Merinos/group) were given
phoxim once, administered by intubation as an aqueous emulsion, at
levels of 0, 25 and 50 mg/kg bw. Each of the treated groups was
divided into two and one half was treated early in the morning and the
other half late in the afternoon. This was done to vary the times of
exposure to sunlight after treatment. The sheep were examined daily
for 10 days after treatment and any abnormalities were recorded.
The sheep treated in the morning remained exposed to strong
sunlight for only 3 hours after treatment. Cloudy conditions prevailed
for the remainder of the observation period, which reduced the
possibility of determining any phototoxic effect.
No signs of systemic toxicity or dermal inflammation were seen in
any of the test animals up to day 10 after treatment (Baldock and
Hopkins 1976).
Acute Toxicity
The results of acute toxicity studies are summarized in Table 1.
Signs of poisoning in mammals after oral administration usually
developed within 5 minutes to 2 hours after dosing in mice, rats,
rabbits, cats and dogs and lasted for 1 to 7 days in survivors. Deaths
occurred after 1 to 3 days. It was only at the highest dose levels
that these symptoms took the form of acute cholinesterase depression
(cramps, trembling, diarrhoea and red tears in rats). The only
symptoms noted in the lower dose levels were lowering of the general
condition and sedative effects. The toxicity signs did not differ in
the tested species. The autopsies of the animals receiving high doses
of the active ingredient showed no changes in the internal organs
(Kimmerle and Solmecke 1970).
Following intraperitoneal injection, the symptoms of poisoning
did not appear more quickly than after oral application. As with oral
treatment, the typical cholinergic symptoms were observed only at very
high doses (Kimmerle 1968a).
Hens are considerably more sensitive to phoxim than mammals
(Kimmerle 1972; Thyssen and Kimmerle 1973).
Table 1. Acute Toxicity of Phoxim
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 2.53-3.38 Flucke 1978 a
ml/kg b.w.
F oral none 2.50-3.50 Flucke 1978 b
ml/kg b.w.
F oral none 2.62-2.77 Flucke 1979 a
ml/kg b.w.
F oral none 3.24-3.60 Flucke 1979 b
ml/kg b.w.
F oral none 2.50-3.18 Flucke 1980
ml/kg b.w.
F oral none 2.96-3.40 Heinmann 1982
ml/kg b.w.
M oral none 2 440 Kimmerle 1968
µl/kg b.w.
F 3 240
µl/kg b.w. "
M inhalation alcohol + >2.06 "
(4-h exp.) Lutrol (1:1) mg/l air
F i.v. none 950
µl/kg b.w. "
M oral none 1 645 Kimmerle and
µl/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 1 990 Kimmerle and
µl/kg b.w. Solmecke 1970
M inhalation alcohol + >3.10
(4-h exp.) Lutrol (1:1) mg/l air "
F i.v. physiological 481
solution & mg/kg b.w. "
Cremophor EL
F oral none 3.02-3.48 Mihail 1981
ml/kg b.w.
F oral none 2.98-3.49 Mihail 1982
mg/kg b.w.
Rat M oral none 7 060 Kimmerle 1968
µl/kg b.w.
F 5 800
µl/kg b.w.
M i.p. none 1 775
µl/kg b.w. "
F 1 725
µl/kg b.w.
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M dermal none > 1 000
(7-day exp.) µl/kg b.w.
M inhalation alcohol + >1.99
(4-h exp) Lutrol (1:1) mg/l air
M inhalation alcohol + >2.23 Kimmerle and
(5x4-h exp.) Lutrol (1:1) mg/l air Solmecke 1968
F inhalation >2.22
(4-h, exp. ) mg/l air
M dermal none >1 000 Kimmerle and
(7-day exp.) µl/kg b.w. Solmecke 1970
M inhalation alcohol + >2.55
(4-h. exp.) Lutrol (1:1) mg/l air
inhalation > 0.55
(5x4-h exp.) mg/l air
F inhalation >2.78
(4-h exp.) mg/l air
inhalation > 0. 55
(5x4-h exp.) mg/l air
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M oral none 1 845
µl/kg b.w.
F 1 680
µl/kg b.w.
M i. p. none 1 645
µl/kg b.w.
F 1 635
µl/kg b.w.
M oral 2 650
mg/kg b.w Solmecke 1971
M oral water & Cremophor EL 2 825 Thyssen 1976 a
mg/kg b.w.
Guinea pig F oral none 350-500 Kimmerle, 1968
µl/kg b.w.
F oral water & Cremophor EL 660 Kimmerle and
mg/kg b.w. Solmecke 1970
Rabbit M&F oral none 250-500 Kimmerle 1968
µl/kg b .w.
F oral water & Cremophor EL 250-375 Kimmerle and
mg/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Cat M&F oral none > 1 000 Kimmerle - 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
Dog M&F oral none > 1 000 Kimmerle 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
oral] approx. 37.5 Kimmerle 1972
Chickens i.p.] mg/kg b.w.
Hen oral water emulsion 19.6 Thyssen and
mg/kg b.w. Kimmerle 1973
Short-Term Studies
Rat - dietary
Oral feeding tests were conducted to determine the safety of
using Baythion (phoxim) in place of Cythion (malathion) in the
treatment of stored grain to control insect pests. Baythion and
Cythion were fed to rats at concentrations of 1, 2, 4, 6, 10 or 0 ppm
in the diet. Adult rats and their offspring were fed for a period of
up to 5 months. No adverse effects were noted as a result of these
treatments. It is concluded that 4 ppm Baythion mixed with stored
grains could be used as a substitute for Cythion, with no adverse
effects expected in humans (Lin 1974).
Groups of rats (15 male and 15 female, Wistar strain SPF/group)
were fed diets containing phoxim (technical product stabilized with
10% n-butanol, 87.2% a.i.), at concentrations of 0, 5, 15, 50, 150 and
500 ppm for 3 months. The control group comprised 30 male and 30
female rats. Haematological, clinical chemical and urinalysis
parameters were determined on 5 male and 5 female rats of each dose
group after 4 weeks and 3 months of feeding. The cholinesterase
activity in plasma and in erythrocytes was determined at 1, 4, 8 and
13 weeks after the start of the experiment in 5 male and 5 female rats
of each group. The animals dying during the experiment were subjected
to autopsy. At the end of the study, all the animals were sacrificed
and gross and histopathological examinations were performed.
There were no significant differences between control and treated
animals with respect to behaviour, food and water consumption and body
weight gain. Cholinergic symptoms were sometimes observed in the
500 ppm group only, particularly during the first half of the
experiment. Only the male rats in the 500 ppm group showed
significantly lower body weights. No compound-related mortality
occurred.
The treated animals of all dosage groups did not significantly
differ from the control animals throughout the experiment with respect
to haematological, clinical chemical and urinalysis parameters.
There was an increasing dose-dependent inactivation of both
plasma and erythrocyte cholinesterase in male rats at 50 ppm and
above. In female rats, a dose-dependent depression was noted at 15 ppm
and above in the plasma and at 50 ppm and above in the erythrocytes.
The autopsy of all rats at the end of the feeding experiment
showed no changes of the internal organs attributable to the
inclusion of phoxim in the diet. Male rats in the 500 ppm group had
significantly higher thyroid and liver weights, as compared to
controls. There were no dose-related or significant differences
between any of the dose groups up to 50 ppm and the control animals
with respect to relative organ weights. Higher relative liver weights
in males and females of the 150 ppm and 500 ppm groups were observed.
These enlargements are indicative of an influence on the liver,
although the liver function tests were all normal. Higher relative
kidney weights were observed in the males of the 500 ppm groups and in
the females of the 150 ppm and 500 ppm groups. Heart and adrenal in
the males of the 500 ppm group and thyroid and lung in the females of
500 ppm group had significantly higher relative weights, as compared
to the control animals.
The no-effect level with respect to plasma and erythrocyte
cholinesterase was 15 and 5 ppm, respectively, for male and female
rats, equal to approximately 1.45 and 0.56 mg/kg bw/day (Löser 1970a).
No histopathological change was seen in the tissues examined that
was considered to be compound-related (Vince and Spicer 1971).
Dog - dietary
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 2, 5 and 10 ppm for
3 months. The control group comprised 3 male and 3 female dogs.
Inclusion of the active ingredient in the diet at dose levels up to
10 ppm did not affect the physical appearance, behaviour, food
consumption, growth and mortality rate of male and female dogs.
Cholinesterase activity in the plasma was depressed in both males and
females at the dose level of 2 ppm after 1 month, but not at the end
of the study. The results of the haematological, clinical chemical and
urinalysis determinations showed no significant differences between
the control and treated groups. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1970b).
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 50, 200 and 1 000 ppm
for 3 months. The control group comprised 3 male and 3 female dogs.
The dogs in the 1 000 ppm group showed cholinergic symptoms, but no
dogs died in any of the dose groups during the experiment. The
dose level of 1 000 ppm caused weight loss in the female dogs.
Haematological determinations made after 6 weeks and at the end of the
study did not reveal any pathological changes in the dogs of any
groups. Clinical chemical parameters were normal in the 50 and 200 ppm
groups. Increased activities of alkaline phosphatase (ALP) and lactate
dehydrogenase (LDH) were determined in the animals of the 1 000 group.
However, the activities of liver-specific enzymes ornithine carbamyl
transferase (OCT), GPT and sorbitol dehydrogenase (SDH) did not
change. Urine examinations and the kidney function test (urea and
creatinine in serum) were normal in all groups, as were blood sugar
and cholesterol levels. Plasma cholinesterase activity was depressed
at 50 ppm and above, while erythrocyte cholinesterase was first
affected at the 200 ppm level. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1971).
Groups of beagle dogs (4 males and 4 females/group) were fed
diets containing phoxim (91.1% a.i.) at concentrations of 0, 0.3, 1
and 2 ppm for 3 months. No deaths occurred during the study. There
were no significant differences between the control and treated dogs
with respect to behavioural pattern, physical appearance, food
consumption, body weight gain, reflex, eye examinations, haematology,
clinical chemistry, thromboplastin time and urinalysis, as determined
at 0, 5 and 12 weeks.
The no-effect level for plasma cholinesterase was 0.3 ppm for
both males and females. Erythrocyte cholinesterase activity was not
affected in any of the treated groups. Autopsy and histopathological
examinations performed on all the animals provided no indication of
treatment-related alterations (Mürmann and Luchaus 1973).
Groups of beagle dogs (4 males and 4 females/group) were fed
phoxim in the diet (technical product, stabilized with 10% n-butanol,
86.5 a.i.) at the following levels for 2 years: control group - 0 ppm;
group I - males, 0.3 ppm and females 0.1 ppm from week 83; group II -
15 ppm; group III- 750 ppm. Daily examinations were made for physical
appearance and behavioural pattern. Intake of food and water was also
checked and noted daily. Body weights were recorded weekly in the
first year and thereafter at 14-day intervals. At periodical intervals
(14, 26, 39, 52, 64, 78, 92 and 104 weeks) reflex testing,
ophthalmoscopy, haematology, clinical-chemistry and urinalyses were
performed. Plasma and erythrocyte cholinesterase determinations at
4, 7, 10, 15, 27, 39, 51, 64, 77, 91 and 103 weeks and brain
cholinesterase at the conclusion of the study were performed. At the
end of the 104 weeks of dietary administration, all the dogs were
sacrificed and gross and histopathological examinations of tissues and
organs were performed.
There was no mortality over the course of the study in any group.
Treatment did not affect the behavioural patterns of any of the dogs.
The female dogs of each treated group and the male dogs of group I did
not differ in physical appearance from the controls. Male dogs in
group II and group III had a poor state of nutrition. The male dogs of
group III had a dull and ungroomed coat in the second treatment year.
Food consumption levels were not affected by treatment. The time taken
for the food ration to be consumed by the dogs was slightly longer in
group II and considerably longer in group III. Average body weight
curves, especially for the males, were lower than those for the
control group and were dose related. However, only the body weights of
group III rats were statistically significantly lower than those of
control rats. A marked decrease of body weight was evident in male
dogs of group III after 72 weeks. The reflexes were normal in all dogs
at all times tested. The ophthalmoscopic examinations of the eyes
provided no indication of any treatment-related variations from the
physiological norm in either the transparent media or on the fundus
oculi. The average total intake of phoxim per dog was 0.068 g, 3.60 g
and 181.08 g, respectively for group I, group II and group III. Data
from haematological tests and urinalyses were normal. Plasma and
erythrocyte cholinesterase activities were not affected in males of
group I, i.e. at 0.3 ppm. In females of group I, plasma cholinesterase
activity was seen to be depressed to a level 26% below the control
value in week 77. As this result was confirmed by repeated
measurements, the concentration of phoxim administered to the female
dogs of group I was reduced from 0.3 to 0.1 ppm from week 83. The
later determinations in week 91 and 103 showed that plasma
cholinesterase activities in group I females were again equal to the
control values. At the 15 ppm level and above, plasma and erythrocyte
cholinesterase activities were markedly depressed, and were dose
related, from the first determinations and remained relatively
constant over the entire study. Brain cholinesterase activity was not
depressed at dietary concentrations of up to and including 15 ppm,
being depressed 35-40% at 750 ppm level. Clinical chemistry values
(glucose, urea, creatinine, total protein, GOT, bilirubin) showed no
differences between treated dogs and controls. Statistically
significant values, increasingly higher than controls, were observed
for GPT (from week 52) and ALP (from week 14) in the dogs of group III
(less marked in the females). In the group II dogs and in the males of
group I, ALP activity values were statistically significantly higher
than in the controls; they were at the same level throughout the
second half of the experiment. In the controls, the physiological
age-related reduction of ALP activity was observed during the course
of the study. In the group III dogs the average serum cholesterol
level was lower than that measured in the control dogs at every
investigation time until the end of the feeding experiment.
At gross pathological examination, the livers of 4 female dogs
and one male dog of group III had a darker (dark brown to grey) colour
than those of the control dogs and the dogs of the other two treated
groups. Furthermore, the livers of some dogs in group III were seen to
have a marked lobular pattern. There were no noteworthy differences
between the treated dogs of all groups and the control dogs with
respect to absolute and relative organ weights, with the exception of
liver and thyroid. The absolute and relative weights of the liver in
the male and female dogs of group III were statistically significantly
higher than the weight of the liver in the control dogs. There was
also a slight increase in the relative weight of the liver in the male
dogs of group II, but this difference was not statistically
significant. The absolute and relative weights of thyroid in the
female dogs of group III were greater than those in the control dogs,
but this difference was not statistically significant. Hepatocyte
alterations were seen at histopathological examination in all the dogs
of group III. The hepatocytes were dilated, the plasma had a light
glassy appearance and was less structured than in the control dogs or
the dogs of the other two dietary concentration groups.
The increase in the absolute and relative thyroid weights in the
female dogs of group III is not associated with histopathological
alterations, so that it is doubtful whether this increase was compound
related. Some other alterations of varying degree were observed in
other tissues of both the control group and the treated groups, so
they were not considered treatment related.
The study indicated 0.3 ppm in the diet, equal to 0.068 mg/kg
bw/day, and 0.1 ppm, respectively, for male and female dogs, as no-
effect levels with respect to plasma and erythrocyte cholinesterase
(Hoffmann and Gröning 1977).
Monkey - dietary
Rhesus monkeys (Macaca mulatta) (20 males and 20 females/group)
were administered by gavage daily doses of phoxim (technical product
stabilized with 10% n-butanol, 83.8% a.i.) dissolved in maize oil at
levels of 0, 0.2, 0.65 and 2.00 mg/kg, 6 days per week for 6 months.
Throughout the study the monkeys tolerated the administration of
phoxim well and remained in good health. There was no evidence of any
cholinergic effects despite the observed reduction of plasma
cholinesterase activity. Average body weights and urinalyses were not
significantly different between the control and treated groups, There
were some differences between the control and treated groups with
respect to some clinical chemistry values (blood urea nitrogen,
glucose, total bilirubin, GOT, calcium) and some haematology values
(packed cell volume, erythrocyte count). However, these fluctuations
were, for the most part, within the normal limits for the colony of
monkeys, and in no case were dose related.
Erythrocyte cholinesterase activity was slightly reduced at
2.0 mg/kg, but plasma cholinesterase activity was reduced at all three
dose levels tested. Expressed as percent of the control groups, the
remaining activity after six months was, respectively, for each dosage
level, 62%, 43% and 34%. A no-effect level was not determined. The
microscopic examination of liver biopsy material obtained prior to the
administration of phoxim and after six months of administration
provided no evidence of an effect of phoxim on liver morphology
(Coulston et al 1978).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females/
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to phoxim (technical product stabilized with 101
n-butanol, 83.8% a.i.; as an emulsion in water and Cremophor EL) at
dose levels of 0, 0.5 and 15 mg/kg bw/day, 7 hours per day, 5 days per
week for a total of 15 applications in a 21-day period.
The treated sites (5 × 5 cm) on the flank and back of the
animals, not covered with bandages, were washed with water and soap at
the end of each daily exposure period.
Behaviour and general aspect were normal for all animals. No
treatment-related deaths occurred. There were no adverse effects on
the pre- and post-treatment values of body weight or on terminal
haematological, clinical chemical and urinalysis parameters, Erythema
was not observed in any animals with intact skin. A slight compound-
related increase in time necessary for erythema to disappear was noted
in treated animals with abraded skin. Skin-fold thickness was
comparable between the control and treated groups. There were no
significant differences between control and treated groups with
respect to both absolute and relative weights of major organs.
Histopathological examinations were performed on heart, lung, liver,
spleen, kidney, adrenals, testis, epididymis, ovary, uterus and
thyroid from control and 15 mg/kg bw groups. Some alterations occurred
in both control and treated animals and were not considered compound
related. Slight increases of degree of cell infiltrations and of
incidence of epithelium thickening were observed on the treated skin
of animals at 15 mg/kg bw with abraded skin, as compared to control
animals. The same changes were not observed in animals with intact
skin. These findings were considered the result of repeated mechanical
stimuli. Plasma and erythrocyte cholinesterase activities measured
after exposures 8 and 15 were significantly depressed (ca. 60%) at
15 mg/kg bw/day. Brain cholinesterase activity was slightly (23%)
depressed only in male rabbits at 15 mg/kg bw/day (Flucke and Schilde
1978).
Long-Term Studies
Rat
Groups of rats (50 males and 50 females, SPF Wistar strain/group)
were fed a diet containing phoxim (technical product stabilized with
10% n-butanol, 85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm
(calculated as pure active ingredient) for 24 months. The control
group included 100 males and 100 females. In addition, 5 male and
5 female rats were used in each group for clinical laboratory
examinations, which were carried out at 3, 6 and 12 months. At the end
of the study, 10 males and 10 females were used for these
examinations.
There were no differences in appearance and behaviour between the
treated and control animals during the study. In the 375 ppm group
male rats had a lower mean daily food intake and females showed
significantly less weight increase than control animals, mainly in the
second half of the study. Mortality after 1 and 2 years was not
increased in any treated groups. The overall mortality rate for
treated and control groups at the end of the study was in the range of
24-26% for male rats and 14-21% for female rats. There were no
significant differences between the control and treated groups with
respect to haematological examinations at 3, 6, 12 and 24 months.
There were no dose-related differences between control and treated
groups with respect to alkaline phosphatase, GOT, GPT, glutamic
dehydrogenase (GLDH, determined only at the end of the study), serum
bilirubin and total serum protein, as determined at 3, 6, 12 and 24
months. Urinalyses, serum urea and creatinine, and urine protein
showed no relevant differences between the control and treated groups
after 3, 6, 12 and 24 months. Urine protein was sporadically increased
in the 75 ppm group. Mean values for blood sugar and cholesterol
showed some variations between the control and treated groups that
were not dose related. Cholinesterase activity in erythrocytes and
plasma was not significantly inhibited (less than 20%) in the male and
female rats in the 15 ppm group. In the 75 ppm and 375 ppm groups, a
marked and dose-related inhibition of the enzyme in plasma (16-42% in
males, 41-64% in females) and in the erythrocytes (24-54% in males,
25-49% in females) throughout the study was observed. Brain
cholinesterase activity (measured at the end of the trial) was
slightly inhibited (18% in males, 23% in females) only in the 375 ppm
group. The autopsies on rats dying during the study (106) and those
sacrificed at the end of the treatment did not reveal any treatment-
related damage. There was a significant, but not dose related,
increase in the relative liver weight of male rats of all dose groups
as compared to controls. There was also an increase in relative
weights of spleen, lungs and heart of female rats of the 375 ppm
group.
Histopathological examinations performed on some 31 tissues of
all the rats surviving the 2-year treatment did not reveal any
treatment-related alterations. The differences in relative organ
weights found were regarded as random, as they were not dose related,
and there was no histopathological correlation. The analysis of tumour
data according to the site, type and incidence of both benign and
malignant tumours provided no indications suggestive of carcinogenic
activity of phoxim in the rat.
The no-effect level for both plasma and erythrocyte
cholinesterase was 15 ppm (Bombhard and Löser 1977).
COMMENTS
Phoxim has been evaluated for the first time by the JMPR. It has
a mild acute toxicity, as confirmed following the various routes of
application. No sex-dependent differences were observed. In the
longer-term experiments, however, females tended to display higher
sensitivity. The acute experiments revealed a species-dependent
toxicity. There was no available information on specification of the
technical product. Phoxim is readily and almost completely absorbed.
It is rapidly excreted in the rat, and there is no evidence of
bio-accumulation. The metabolic pathway in mammals follows typical
steps, such as hydrolysis, desulphuration and conjugation. Possible
metabolites have a slight to moderate acute oral toxicity.
A delayed neurotoxicity test was negative but the test was
considered to be unacceptable. No-effect levels were determined with
respect to reproduction and teratogenicity. Mutagenicity and
carcinogenicity studies were negative.
In a two-year dog study, plasma and erythrocyte cholinesterase
were more sensitive than brain cholinesterase. A 90-day dog study
provided a no-effect level bridging the large differences between
effect and no-effect levels in the 2-year dog study.
An increase in relative liver weight, not dose related, was also
observed in the 2-year rat study at all dosage levels, without
histopathological correlation. No observations in humans were
available. The available data permitted the determination of no-effect
levels in two mammalian species. Because of the unavailability of an
acceptable delayed neurotoxicity study, only a temporary ADI was
allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.56 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.005 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
An appropriate neurotoxicity study in hens.
Desirable
1. Observations in humans (particularly effects on cholinesterases),
2. Type and content of impurities in the technical product.
REFERENCES
Baldock, C. and Hopkins, T. Bay 9053. Safety in sheep. Report from
1976 Bayer, Australia, submitted to the World Health Organization
by Bayer, Australia, (Unpublished)
Bombhard, E. and Loser, E. SRA 7502. Chronic toxicity studies in rats
1977 (feeding experiments over 2 years). Report (No. 7042) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Coulston, F., Griffin, T., Talley, W. and Iatropoulos, M.J. A safety
1978 evaluation study of phoxim in Rhesus monkeys. Final Report
from Institute of Comparative and Human Toxicology, Albany
Medical College, Albany, New York, and International Center
of Environmental Safety, Albany Medical College, Holloman,
New Mexico, submitted to the World Health Organization by
Bayer F.R.G. (Unpublished)
Daniel, J.W., Swanson, S. and McLean, J. Phoxim: pharmacokinetics and
1978a biotransformation in the rat. Report (No. 78/BAG 5/194) from
Life Science Research, Stock, Essex, England, submitted to
the World Health Organization by Bayer, F.R.G.(Unpublished)
Daniel, J.W., McLean, J. and Pringuer, M. Phoxim- biotransformation in
1978b the rat. Report (No. 78/BAG 6/317) from Life Science
Research, Stock, Essex, England, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502: Bestimmung der Akuten toxzität (LD50). Report
1978a from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1978b SRA 7502 VL: bestimmung der akuten toxzitat (LD50) Report
from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. and Schilde, B. Volaton VL. Subakuter kutaner toxizitat-
1978 versuch an kaninchen. Report No. 8034 from Institut für
toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502 VL: bestimmung der akuten toxizitat (LD50).
1979a Report from Institut fur toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1979b SRA 7502 VL (volaton): bestimmung der akuten toxizitat
(LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Flucke, W. SRA 7502 Volaton VL: bestimmung der akuten toxizitat
1980 (LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Heinmann, K.G. Volaton VL: bestimmung der akuten toxizitat (LD50).
1982 Report from Institut für toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
Herbold, B. SRA 7502 (phoxim). Micronucleu test on mouse to evaluate
1981 SRA 7502 for mutagenic potential. Report (No. 9891) from
Institut für toxicologie, Bayer, F.R.G. submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Hoffman, K. and Gröning, P. SRA 7502. Chronic toxicity study on dogs.
1977 two-year feeding experiment. Report (No. 6865) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Iakushko, V.E. Effect of Valexon on NADPH-dependent oxidation in
1978 endoplasmic reticulum of rat liver. IKR Biokim. Zh., 50
(6):727-720 (in Russian). Pest. Abstracts. 12(5): 292
(1979). Abstract No. 79.1193).
Kimmerle, G. BAY 77488. Toxicological studies. Report (No. 1067) from
1968 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Kimmerle, G. and Solmecke, B. BAY 77488. Toxicological studies. Report
1970 (no. 2235) from Institute für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
1972 Phoxim: neurotoxicity tests on hens. Report from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (unpublished)
Kuz'minskaia, V.A. and Veremenko, L.M. Effect of Valexon on succinate
1978 dehydrogenase and cytochrome oxidase activities in rat
liver. UKr. Biokhim. Zh., 50(6):711-713 (in Russian),
Pest. Abstracts 12(5): 292 (1979) (Abstract No. 79-1192).
Kuz'minskaia, V.A., Alekhina, S.M. and Bersan, L.V. Dynamic changes in
1979 the blood enzyme activities in albino rats following the
administration of Valexon. Gig. Sanit. 44(7):78-79 (in
Russian), Pest. Abstract, 13:31 (1980), (Abstract No.
80-0159).
Lin, T. Toxicological study of baythion and cythion on rat feeding
1974 test. Nung Yeh Yen Chin (J. Taiwan Agric. Res.), 23(2):
149-154, Pest. Abstracts 9:427 (1976), (Abstract No.
76.1543).
Löser, E. BAY 77488. Subchronic toxicological studies on rats. Three
1970a month feeding experiment. Report (No. 2389) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization, by Bayer, F.R.G. (Unpublished)
1970b BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiments. Report (No. 2418) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1971 BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiment. Report (No. 2579) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1979 SRA 7502. Multigeneration reproduction study on rats. Report
(No. 8447) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G. (with
appended Report on the histopathological examinations by
J.E. Close and J.W. May, Hazleton Laboratories, Harrogate,
U.K.).(Unpublished)
Machemer, L. SRA 7502 (phoxim). Dominant lethal test on male mouse to
1974 evaluate SRA 7502 for mutagenic potential. Report (No. 4943)
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer F.R.G. (Unpublished)
1975 SRA 7502 (phoxim). Studies for embryotoxic and teratogenic
effects on rats following oral administration. Report (No.
5331) from Institut für toxicologie, Bayer, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Mihail, F. Cyanoximsäureglucosid, Ratte p.o. Report from Institut für
1979 toxicologie, Bayer, submitted to the World Health
Organization by Bayer F.R.G. (Unpublished)
1981 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1982 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Mürmann, P. and Luckhaus, G. SRA 7502. Subchronic toxicity study on
1973 dogs. Report (No. 4136) from Institut für toxicologie,
Bayer, submitted to the World Health Organization by Bayer,
F.R.G. (Unpublished)
Oesch, F. Ames test for Volaton (phoxim). Report from
1977 pharmakologisches Institut der Universität, Mainz, F.R.G.
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, T. Phoxim. Mutagenicity test on
1978 bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Solmecke, B. BAY 77488 (SRA 7502) antidotes study. Report from
1971 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. and Kimmerle, G. SRA 7502. Toxicological studies on hens.
1973 Report No. 4236 from Institut für toxicologie, Bayer,
submitted to the World Health Organization by Bayer F.R.G.
(Unpublished)
1976 Cyanoxim (hydroxyiminophenyl-acetonitril)
Geverbetoxicologische Untersuchungen. Report (No. 6392) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976a oral toxicity when administered simultaneously with
fensulfothion, isofenphos or phoxim. Report (No. 5958) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1976b SRA 7502. Subacute oral cumulation study on rats. Report
(No. 5954) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Vince, A.A. and Spicer, E.J.F. Pathology report on BAY 77488,
1971 Subchronic toxicological studies in rats. 3-month feeding
experiment Report (No. 4257/71/415). (Addendum to the Report
No. 2389/Bayer) from Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by
Bayer, F.R.G. (Unpublished)
Vinopal, J.H. and Fukuto, T.R. Selective toxicity of phoxim
1970 (phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate).
Pest. Biochem. Physiol. 1:44-60.
In the 10 mg/kg treatment, male rats excreted an average of 92.9%
of the radioactivity in the urine and 4.9% in the faeces in ten days,
while females excreted 86.1% of the dose in the urine and 6.9% in the
faeces in the same period. Male rats intubated at a dose of 1 mg/kg
excreted 82% of the radioactivity in the urine and 7.9% in the faeces
in ten days. However, most of the radioactivity (80-90%) was
eliminated in 24 hours and excretion was virtually complete within 2
days. No evidence was obtained for the presence of 14CO2 in the
expired air during the initial 24 hours.
Radiochemical analysis 10 days after dosing of the major organs
and tissues from animals dosed at both 1 and 10 mg/kg indicated the
presence of only low levels of radioactivity, which corresponded to
less than 0.1 µg phoxim/g tissue. An average of 0.80% and 3.3% of the
radioactivity was excreted within 0-6 and 6-24 hours in the bile of
cannulated male rats intubated at the 10 mg/kg dose level (Daniel
et al 1978a).
The biotransformation of phoxim was investigated in adult male
rats (Charles River CD strain) following administration (by gavage) of
a dose of 10 mg/kg bw.
The major metabolites in the 24-hour urine was identified as
hippuric acid (approx. 6%) and glucuronic and sulphuric acid
conjugates (approx. 76%) of alpha-cianobenzaldoxime. Evidence was also
obtained for the presence of both syn- and anti-forms of the oxime.
Chromatographic analysis of the plasma obtained one hour after
dosing revealed the presence of four radioactive components,
two of which were characerized as mono-desethyl-phoxim (80%) and
mono-desethyl PO-phoxim (12%). No evidence was obtained for the
presence of PO-phoxim (Daniel et al 1978b).
Effects on Enzymes and Other Biochemical Parameters
In the rat, in vitro cholinesterase inhibition amounted to (I50)
4.19×10-4M for serum, 4.46×10-5M for erythrocytes and 2.51×10-6M for
brain (Kimmerle 1968).
The effect of the organophosphorus pesticide phoxim on the
activity of liver succinate dehydrogenase (SDH) and cytochrome oxidase
(CO) was studied in randomly bred albino rats. Animals received a
single p.o. administration of phoxim at 0.5 LD50 (310 mg/kg) and were
sacrificed 1 hour, 1 day and 5 days later. In chronic experiments,
rats received phoxim at 31 mg/kg/day for 1, 3 and 6 months. Single
administration of phoxim decreased CO activity in liver homogenate
1 hour after administration (5.3 IU, compared with 7.61 IU in
controls), while the decrease in SDH activity was detected only on day
1 after administration (2.9 IU, compared with 7.55 IU in controls). In
the supernatant, activities of CO and SDH showed an increase only on
day 5 after administration (0.62 IU and 0.45 IU, respectively,
compared with 0.42 IU and 0.086 IU in controls). Chronic exposure to
phoxim did not change CO activity in the liver homogenate but resulted
in an increase in CO activity in the supernatant (after 3 months
exposure, CO activity was 0.80 IU compared with 0.42 IU in controls).
Activity of SDH in the homogenate increased after exposure of 1 month
(8.49 IU, compared with 7.55 IU in controls), while activity of SDH
in the supernatant was increased only after 3 and 6 months of
administration (0.35 IU and 0.59 IU, respectively, compared with
0.09 IU in controls) (Kuz'minskaia and Veremenko 1978).
The effect of phoxim on the NADPH-dependent oxidation in the
liver endoplasmic reticulum was studied in randomly bred albino rats,
Phoxim was given p.o. for 3 days at 124 mg/kg/day (LD50 is
620 mg/kg). On days, 1, 5 and 15 after the last administration,
animals were sacrificed and activity of liver demethylase (DM),
hydroxylase (HL), uridine diphosphate glucuronyl transferase (UDPGT)
and the level of ascorbic acid excretion were assessed. Phoxim
administration was found to stimulate the process of demethylation.
DM activity began increasing on day 5 after administration and
remained at a high level up to day 15 (0.957 IU and 0.842 IU,
respectively, compared with 0.602 IU in controls). HL activity was
decreased on day 1 (0.714 IU, compared with 0.304 IU in controls),
followed by a slight increase on day 5 (0.380 IU) and normalization on
day 15. Activity of UDPGT was significantly increased only on day 1
after administration (5.48 IU, compared with 3.28 IU in controls). The
level of urinary excretion of ascorbic acid showed a progressive
increase up to 538 µg/ml on day 1, 194.8 µg/ml on day 5 and
174.8 µg/ml on day 15 (compared with 118.5 µg/ml in controls (Iakusko
1978).
The effects of phoxim on the glucose-6-phosphatase (G6P),
hexokinase (HK), total cholinesterase (ChE) and ChE isoenzyme
activities were studied in the blood of male albino rats weighing
200-250 g. Group 1 served as a control, group 2 was treated (p.o.)
with a single 310 mg/kg dose of phoxim (0.5 LD50), group 3 received
31 mg/kg/day for 1-6 months and treatment in group 4 was 3.1 mg/kg/day
for 1-6 months. In group 4 the G6P and HK activities were increased
and the ChE activity was reduced 1 hour after treatment. The HK
activity became normal 1 hour later, the G6P activity remained
elevated and the ChE activity declined further. All enzyme activities
were normal 5 days after treatment. In group 3, the G6P and G6P
dehydrogenase (G6PD) activities were increased by 45% and 70%,
respectively, and the total ChE activity was decreased by 45% at the
end of the first month. The ChE fractions 3, 4 and 5 were fully
inhibited; fraction 2 was inhibited by 78%, and the activity of
fraction 1 was increased by 61%. After treatment for 3 months the G6P
activity was increased by 89%, the G6PD activity by 42% and the total
ChE activity was inhibited by 26%, while the isoenzyme spectrum did
not differ from that in the controls. After poisoning for 6 months,
the G6P and G6PD activities were inhibited by 30%, and the total ChE
by 24%. Fraction 1 was inhibited by 45% and the activity of fraction 2
increased by 35%, while the fractions 3, 4 and 5 remained normal. The
changes seen in group 4 were similar to but less marked than those in
group 3 (Kuz'minskaia et al 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of rats (10 male and 20 female, Long Evans FB30
strain/group) were fed diets containing phoxim (technical product,
85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm (calculated as
pure a.i.) and subjected to a standard 2-litter per generation,
3-generation reproduction study. The rats were treated with the test
compound throughout the study, including mating, gestation and
lactation. At the start of the experiment, the rats were about 47 days
old. The rats selected for the study were housed singly until they
were sexually mature (up to an age of ca. 100 days). Then they were
mated to initiate the study. During the mating period, two female rats
were housed together with one male rat for 21 days. The male rats were
rotated so that each female was paired with three different males for
a period longer than one oestrous cycle. Immediately after the pups
were delivered, their number and weights were recorded. Litters
containing more than 10 pups were reduced on day 5 after delivery to
10 pups each, whereupon the weights of these litters were again
measured. The pups were nourished for up to 4 weeks, weighed weekly,
and then the offspring of each first mating (F1a, F2a, F3a) were
sacrificed.
The offspring of each second mating, as with those delivered
after the first mating, were nourished for up to 4 weeks, then weaned
and placed in separate sex groups. After week 8, 10 male and 20 female
rats were again selected from each dose group for further matings.
Upon reaching an average age of 100 days, the rats were mated as
described above. After the dams of the F0, F1b and F2b generations
had successfully nursed offspring twice, they were sacrificed.
Necropsy was performed on rats that died during the study. Gross and
histopathological examinations were performed on major organs and
tissues of one male and one female 2-week old pup of the F3b
generation from each of ten mothers in each group.
There were no significant differences between the control and
treated groups with respect to physical appearance, behavioural
patterns, body weight curves of weaned males and females of the F0,
F1b and F3b generations; some animals in both the control and treated
groups died of pneumonitis.
There were no significant differences between the control and
treated groups with respect to pregnancy rate, litter size, 5-day
survival rate (viability index), pups weights at birth and during
lactation at all dose levels tested. Only the 375 ppm dietary
concentration had a slight adverse effect on the lactation index in
F3b. The inspections of the pups immediately after birth and during
the lactation period did not reveal any signs of malformations. Gross
and histopathological examinations did not provide any evidence of
treatment-related alterations.
The no-effect level in this multigeneration reproduction study
was 75 ppm (Löser 1979).
Special Studies on Mutagenicity
The mutagenicity of phoxim (technical product, 83.8% a.i.) was
studied by rec-assay and reversion test (Salmonella/microsome).
The rec-assay was performed using two strains of Bacillus
subtilis, H 17 and M 45. Volumes of 0.2 µl to 20 µ1 of phoxim per
disc were tested. Kanamycin was used as a negative control and
mitomycin C as a positive control. Phoxim did not inhibit the growth
of H 17 and M 45 strains of B. subtilis at all the tested doses. On
the other hand, with mitomycin C used as positive control more
remarkable growth inhibition was identified in M 45 strain than in
H 17 strain, and the growth of both strains was inhibited at the same
degree with Kanamycin as the negative control.
The reversion test was performed using two strains of
Salmonella typhimurium TA 98 and TA 100, according to the Ames
procedure, both with and without S-9 Mix derived from liver of SD
strain rats treated with a single intraperitoneal injection of
Arochlor 1254 (500 mg/kg bw). Concentrations of 10 - 5000 µg/plate
were tested. 2-amino-anthracene (2-AA) and 2-(2-furyl)-3-(5-nitro-2-
furyl) acrylamide (AF-2) were used as positive controls.
A markedly increased number of revertant colonies was observed
for AF-2 on the plates without S-9 Mix and for 2-AA on the plates with
S-9 Mix. Phoxim did not increase the number of revertant colonies,
either in the presence or absence of S-9 Mix.
Under the conditions of the experiments, phoxim provided no
evidence of mutagenic activity at concentrations up to 0.02 ml/disc of
undiluted phoxim and 5 000 µg/plate, respectively, in a rec-assay and
in a reversion test (Shirasu et al 1978).
In another Ames test, phoxim (technical grade, a.i. 82.9% -
83.7%) was tested for mutagenicity with S. typhimurium TA 100, TA
1537 and TA 98 according to the Ames procedure, both in the presence
and in the absence of S-9 Mix derived from male Sprague-Dawley rats
treated with a single intraperitoneal injection of Arochlor 1254
(500 mg/kg bw). Seven concentrations (3.15-3150 nl/plate) were tested
with S-9 Mix and five concentrations (31.5-3150 nl/plate) without it.
At doses greater than 1 000 nl, however, part of the test compound
separated as droplets from the top agar. In the experiments for direct
mutagenicity N-methyl-N'-nitro-N-nitrosoguanidine and benzo(a)pyrene-
4,5-oxide were used as positive controls, whereas in the experiment
with an activating system 3-methyl-cholantrene, benzo(a)pyrene and
2-amino-anthracene were used. Negative controls in the form of
sterility controls and solvent blanks were run as well.
For all three tested strains, there were no significant
differences in the number of revertant colonies between the negative
controls and the phoxim-treated plates, both with and without S-9 Mix.
Positive controls displayed the expected increases, indicating the
activity of the metabolizing system and the mutability of the
bacteria. No bacteriotoxic effects were observed.
Phoxim did not show a mutagenic effect in the Salmonella/
microsome test at concentrations as high as 3 150 nl/plate (Oesch
1977).
Mouse - dominant lethal test on males
Groups of mice (20 male NMRI mice/group) were given by gavage a
single dose of phoxim (technical product stabilized with 10%
n-butanol, 86.5% a.i.) corresponding to 0 and 500 mg/kg bw, the
vehicle being a 0.5% Cremophor emulsion. The mouse strain used
displayed a sensitive response to known chemical mutagens, such as
cyclophosphamide. The dose was chosen on the basis of the results of a
preliminary test conducted on male mice dosed orally with acute doses
of 500, 750 and 1 000 mg/kg bw, respectively. At dose levels of 750
and 1 000 mg/kg bw the compound had a toxic effect and induced
symptoms, but no mortalities occurred. Following dosing, each male
mouse was caged with three untreated virgin female mice for 7 days.
The procedure was repeated weekly with groups of three new untreated
virgin females for a total of 8 weeks, in order to obtain and examine
a complete sample of the successive germ cell stages of the males. The
uteri of the females (466-480 per test group) were examined on
gestation day 14. Counts were made of the corpora lutea, total
implantations, viable implants and dead implants (sum of the
deciduomata, resorptions and dead embryos and foetuses).
There were no significant differences between the control and
treated groups with respect to fertility quota, total implantations,
viable implants, dead implants, ratio of dead implants to total
implants and pre-implantation loss (estimated both directly from the
difference between the number of corpora lutea and the number of
implantations and indirectly through the comparison of the average
number of implantations per fertilized female in the treated group
with that in the control group).
Thus, there was no indication for a mutagenic potential of
phoxim in the dominant lethal test on the male mouse at an oral
dose of 500 mg/kg bw (Machemer 1974).
Micronucleus test
Groups of mice (5 male and 5 female, NMRI (SPF) Han strain/group)
were given (by gavage) two single doses of phoxim (technical product
84.3% a.i.) in aqueous 0.5% Cremophor emulsion at levels of 0, 250,
500 mg/kg bw. The interval between applications was 24 hours.
Concurrently, a positive control group of mice received 2 × 100 mg/kg
bw of cyclophosphamide. The doses were chosen on the basis of the
results of a preliminary test in which groups of 5 mice were orally
dosed with phoxim at 2 × 500 mg/kg bw and 2 × 1 000 mg/kg bw,
respectively; in that test, the 2 × 500 mg/kg treatment was tolerated
with induction of only weak symptoms. Six hours after the second
application, the mice were sacrificed and femur bone marrow smears
were prepared. Erythrocytes, 1 000 per mouse, were counted and the
incidence of cells with a micronucleus was determined, as well as the
ratio of polychromatic erythrocytes to normochromatic erythrocytes.
The incidence of micronucleated polychromatic erythrocytes was
2.3/1 000 in the negative control group, and 1.8/1 000 and 1.0/1 000,
respectively, in the phoxim-treated groups. There were no relevant
differences between the negative control and phoxim-treated group with
respect to the ratio of polychromatic to normochromatic erythrocytes.
The incidence of micronucleated cells in cyclophosphamide-treated
group was 68.2/1 000 and was thus biologically significantly higher
than in the negative control group. Cyclophosphamide also exhibited
a bone marrow depression, with the ratio of polychromatic to
normochromatic erythrocytes showing a biologically relevant alteration
(1 000 : 2 424.2 vs 1 000: 838.3 in the negative control group).
The results provided no indication for a mutagenic potential of
phoxim in the micronucleus test on mouse at the tested doses of
2 × 250 and 2 × 500 mg/kg bw per os. Treatments with phoxim also did
not induce any depression of erythropoiesis (Herbold 1981).
Special Studies on Embryotoxicity and Teratogenicity
Groups of fertilized rats (20 Long Evans FB30 strain/group)
received daily doses of phoxim (technical product stabilized with
10% n-butanol, 86.5% a.i.), administered by gavage in a 0.5% aqueous
Cremophor emulsion, at levels of 0, 30, 100, 300 mg/kg bw from
gestation day 6 through 15 (total of 10 doses). On gestation day 20,
the dams were sacrificed and the foetuses removed by caesarean
section. The foetuses were examined for external, internal and
skeletal malformations. No dam died in any group and no adverse
effects on behaviour patterns and general appearance were observed.
The 300 mg/kg bw dose level had a maternal-toxic effect, resulting in
significantly less weight gain during the treatment period as compared
with the control animals. All animals in the test group showed
comparable average weight gains throughout the period of gestation.
There were no significant differences between the control and
treated groups with respect to the measured parameters: fertilization
quotas, pregnancy quotas, average numbers of implantations, foetuses
and resorptions, average foetus weight, average placenta weight,
average number of stunted foetuses (weighing less than 3 g) and type,
frequency and localization of slight alterations in bone development.
Sex distribution of foetuses was unaffected. Occasional malformations
were seen, these being most frequent in the untreated control group.
They were considered to be spontaneous malformations.
The data provided no indication for embryotoxic or teratogenic
activity in rats at an oral dose of 300 mg/kg bw and below (Machemer
1975).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Neurotoxicity Studies
Hen
Female White Leghorn hens, 2-5/group, about 14 to 18 months
old, each received a single phoxim dose (without antidote)
administered orally at levels of up to and including 50 mg/kg bw or
intraperitoneally at levels of up to and including 37.5 mg/kg bw. The
hens were kept under observations for 6 weeks. The determined LD50
was 37.5 mg/kg bw for both oral and intraperitoneal administration.
In another study, hens 3-14/group, were each given as an antidote
an intraperitoneal injection of 100 mg PAM/kg + 50 mg atropine
sulphate/kg. They then received an oral or intraperitoneal application
of phoxim at dose levels equal to the LD50 and higher. The survivors
were kept under observation for 6 weeks. No histopathological
examinations were carried out. The protection afforded by PAM +
atropine to hens was more evident with intraperitoneal injection of
phoxim than with the oral route. No positive controls were included.
In both tests, without and with antidotal protection, no clinical
signs of neurotoxic damage were seen (Kimmerle 1972).
Groups of hens (10 White Leghorn, 12 to 14 months old/group) were
fed diets containing phoxim from technical product stabilized with 10%
n-butanol, 86.1% a.i. at concentrations of 0, 5 and 10 ppm for 28
days.
During the feeding experiment, the behavioural pattern of all the
hens was within the normal range. In particular, they did not display
any symptoms of neurotoxicity. Treated and untreated controls hens
were not significantly different with respect to food consumption and
body weight gains. Blood acetylcholinesterase activity was not
significantly depressed in the 5 ppm group. In the 10 ppm group, a
marked (30%) depression of blood acetylcholinesterase activity was
measured between day 14 and day 28 of the feeding experiment. The
no-effect level was 5 ppm, equal to 0.32 mg/kg bw/day (Thyssen and
Kimmerle 1973).
Special Studies on Skin and Eye Irritation
A dermal irritation study in rabbits of both sexes ("not of pure
breed") showed that phoxim had only a very slight irritating effect on
both intact and abraded skin (Kimmerle and Solmecke 1970).
In an eye irritation test in rabbits, phoxim caused no eye
irritation (Kimmerle and Solmecke 1970).
Special Studies on Antidotes
Male rats were given single oral doses of phoxim (technical
product, 88.7% a.i.) to determine LD50 values with and without
antidotes. Before the onset of severe poisoning symptoms (5 to
90 minutes), atropine sulphate (50 mg/kg bw), 2-PAM (50 mg/kg bw) or
toxogonin (20) mg/kg bw) was given singly or in combination (atropine
plus PAM-atropine plus toxogonin) by intraperitoneal injection. The
results indicated that, among the tested antidotes, only combinations
of 2-PAM or toxigonin with atropine sulphate had significant antidotal
activity (Solmecke 1971).
Special Studies on Potentiation
Acute oral LD50 values in male Wistar II albino rats were
determined experimentally for phoxim, phenamiphos and their equitoxic
mixture. The observed LD50 value for the equitoxic mixture was
compared with the expected value (calculated assuming an additive
effect). The result was that simultaneous administration of phoxim and
phenamiphos produced a less than additive effect (Thyssen 1976a).
Special Studies on the Recovery of Cholinesterase Activity
Groups of rats (15 male and 15 female, Wistar II, SBF
albinos/group) were given daily doses of phoxim (technical product),
administered by gavage in aqueous Cremophor EL emulsion, at levels of
0, 5, 50 mg/kg bw for 21 days. One-third of the rats were sacrificed
upon termination of treatment, another third were kept under post-
treatment observations for 14 days and then sacrificed and the
remaining third were kept under post-treatment observation for 4
weeks. Determinations of acetylcholinesterase activities in
erythrocytes and plasma were carried out before beginning treatment
and thereafter at weekly intervals until 4 weeks after the end of
administration. Brain acetylcholinesterase activity was measured at
the end of treatment and 2 weeks after termination of treatment.
During the 21-day treatment period and the 4-week post-treatment
observation period, none of the treated rats in any dose group
differed from the control rats with respect to behavioural patterns,
physical appearance and body weight gain. No mortalities were
observed. The following results were obtained in male rats: at
5 mg/kg bw, plasma and erythrocyte cholinesterase activities were
not significantly different from the control group during both the
treatment period and the post-treatment observation period; at
50 mg/kg, plasma and erythrocyte cholinesterase activities were about
65% and 56%, respectively, of the control after 1 week of treatment
and remained similarly depressed during the treatment period; however,
one week after the end of treatment, cholinesterase activity levels
were again found not significantly different from the control.
The following results were obtained in female rats; at 5 mg/kg
bw, both plasma and erythrocyte cholinesterase activities were about
70% of the control after 1 week of treatment, but one week after the
termination of treatment both were in the physiological range; at
50 mg/kg bw, both plasma and erythrocyte cholinesterase activities
were about 37% of the control after 1 week of treatment; one week
after termination of treatment plasma cholinesterase was again normal,
while erythrocyte cholinesterase was 75% of the control and returned
to normal at the end of the 4-week post-treatment observation period.
Brain cholinesterase activity at the end of the treatment period
was 82% and 67% of the control, respectively, for male and female rats
of the 50 mg/kg bw group. However, the values measured 2 weeks after
the end of treatment were not significantly different.
Thus, female rats were more sensitive than males to the
depression of plasma, erythrocyte and brain cholinesterase activities.
The plasma, erythrocyte and brain cholinesterase activity depression
was reversible; plasma cholinesterase returned to normal within
1 week, while erythrocyte and brain cholinesterase were normal in 2-4
weeks.
The acute oral LD50 determinations prior to and after the 21-day
treatment gave comparable values for both male and female rats
(Thyssen 1976b).
Special Studies on the Toxicity of Metabolites
Acute toxicity studies were conducted with alpha-
cyanobenzaldoxime (cyanoxime). The metabolite (and starting material
in the synthesis of phoxim) has a very slight acute toxicity to rats:
LD50 (mg/kg)
Rat, male oral 4 520
Rat, female oral 4 063
Rat, male,female dermal (24 hours) >5 000
From the symptoms of poisoning observed (behavioural disorders,
sedation, breathing disorders) cyanoxime seemingly acts on the central
nervous system.
In acute inhalational toxicity experiments, in which rats were
exposed to dust for 1 hour and 4 hours, respectively, and rats and
mice were exposed to vapour for 6 hours, no toxic effects were
observed.
In tests for skin and eye irritation, the metabolite did not
cause any irritation to the skin of rabbits. In the eye of rabbits,
however, it caused moderate to severe irritation on the conjunctiva
and superficial corrosion on the cornea (Thyssen and Kimmerle 1976).
The glucoside of alpha cyanobenzaldoxime did not cause any toxic
effects when administered as an acute oral dose to female rats; a dose
of 2 500 mg/kg bw was tolerated without inducing any symptoms (Mihail
1979).
PO phoxim, formed as an intermediate product of metabolism, has
an oral LD50 to the mouse of 1 000 mg/kg bw (Vinopal and Fukuto
1970).
Special Studies on Cholinesterase Inhibition by P=O Phoxim
The following 150 values were determined in vitro for the P=O
analogue of phoxim: erythrocyte (bovine), 2.2 × 10-7M; brain (mouse),
6.0 × 10-8M (Vinopal and Fukuto 1970).
Special Studies on Tolerability of Phoxim by Sheep
Groups of sheep (25 freshly shorn Merinos/group) were given
phoxim once, administered by intubation as an aqueous emulsion, at
levels of 0, 25 and 50 mg/kg bw. Each of the treated groups was
divided into two and one half was treated early in the morning and the
other half late in the afternoon. This was done to vary the times of
exposure to sunlight after treatment. The sheep were examined daily
for 10 days after treatment and any abnormalities were recorded.
The sheep treated in the morning remained exposed to strong
sunlight for only 3 hours after treatment. Cloudy conditions prevailed
for the remainder of the observation period, which reduced the
possibility of determining any phototoxic effect.
No signs of systemic toxicity or dermal inflammation were seen in
any of the test animals up to day 10 after treatment (Baldock and
Hopkins 1976).
Acute Toxicity
The results of acute toxicity studies are summarized in Table 1.
Signs of poisoning in mammals after oral administration usually
developed within 5 minutes to 2 hours after dosing in mice, rats,
rabbits, cats and dogs and lasted for 1 to 7 days in survivors. Deaths
occurred after 1 to 3 days. It was only at the highest dose levels
that these symptoms took the form of acute cholinesterase depression
(cramps, trembling, diarrhoea and red tears in rats). The only
symptoms noted in the lower dose levels were lowering of the general
condition and sedative effects. The toxicity signs did not differ in
the tested species. The autopsies of the animals receiving high doses
of the active ingredient showed no changes in the internal organs
(Kimmerle and Solmecke 1970).
Following intraperitoneal injection, the symptoms of poisoning
did not appear more quickly than after oral application. As with oral
treatment, the typical cholinergic symptoms were observed only at very
high doses (Kimmerle 1968a).
Hens are considerably more sensitive to phoxim than mammals
(Kimmerle 1972; Thyssen and Kimmerle 1973).
Table 1. Acute Toxicity of Phoxim
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 2.53-3.38 Flucke 1978 a
ml/kg b.w.
F oral none 2.50-3.50 Flucke 1978 b
ml/kg b.w.
F oral none 2.62-2.77 Flucke 1979 a
ml/kg b.w.
F oral none 3.24-3.60 Flucke 1979 b
ml/kg b.w.
F oral none 2.50-3.18 Flucke 1980
ml/kg b.w.
F oral none 2.96-3.40 Heinmann 1982
ml/kg b.w.
M oral none 2 440 Kimmerle 1968
µl/kg b.w.
F 3 240
µl/kg b.w. "
M inhalation alcohol + >2.06 "
(4-h exp.) Lutrol (1:1) mg/l air
F i.v. none 950
µl/kg b.w. "
M oral none 1 645 Kimmerle and
µl/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 1 990 Kimmerle and
µl/kg b.w. Solmecke 1970
M inhalation alcohol + >3.10
(4-h exp.) Lutrol (1:1) mg/l air "
F i.v. physiological 481
solution & mg/kg b.w. "
Cremophor EL
F oral none 3.02-3.48 Mihail 1981
ml/kg b.w.
F oral none 2.98-3.49 Mihail 1982
mg/kg b.w.
Rat M oral none 7 060 Kimmerle 1968
µl/kg b.w.
F 5 800
µl/kg b.w.
M i.p. none 1 775
µl/kg b.w. "
F 1 725
µl/kg b.w.
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M dermal none > 1 000
(7-day exp.) µl/kg b.w.
M inhalation alcohol + >1.99
(4-h exp) Lutrol (1:1) mg/l air
M inhalation alcohol + >2.23 Kimmerle and
(5x4-h exp.) Lutrol (1:1) mg/l air Solmecke 1968
F inhalation >2.22
(4-h, exp. ) mg/l air
M dermal none >1 000 Kimmerle and
(7-day exp.) µl/kg b.w. Solmecke 1970
M inhalation alcohol + >2.55
(4-h. exp.) Lutrol (1:1) mg/l air
inhalation > 0.55
(5x4-h exp.) mg/l air
F inhalation >2.78
(4-h exp.) mg/l air
inhalation > 0. 55
(5x4-h exp.) mg/l air
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M oral none 1 845
µl/kg b.w.
F 1 680
µl/kg b.w.
M i. p. none 1 645
µl/kg b.w.
F 1 635
µl/kg b.w.
M oral 2 650
mg/kg b.w Solmecke 1971
M oral water & Cremophor EL 2 825 Thyssen 1976 a
mg/kg b.w.
Guinea pig F oral none 350-500 Kimmerle, 1968
µl/kg b.w.
F oral water & Cremophor EL 660 Kimmerle and
mg/kg b.w. Solmecke 1970
Rabbit M&F oral none 250-500 Kimmerle 1968
µl/kg b .w.
F oral water & Cremophor EL 250-375 Kimmerle and
mg/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Cat M&F oral none > 1 000 Kimmerle - 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
Dog M&F oral none > 1 000 Kimmerle 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
oral] approx. 37.5 Kimmerle 1972
Chickens i.p.] mg/kg b.w.
Hen oral water emulsion 19.6 Thyssen and
mg/kg b.w. Kimmerle 1973
Short-Term Studies
Rat - dietary
Oral feeding tests were conducted to determine the safety of
using Baythion (phoxim) in place of Cythion (malathion) in the
treatment of stored grain to control insect pests. Baythion and
Cythion were fed to rats at concentrations of 1, 2, 4, 6, 10 or 0 ppm
in the diet. Adult rats and their offspring were fed for a period of
up to 5 months. No adverse effects were noted as a result of these
treatments. It is concluded that 4 ppm Baythion mixed with stored
grains could be used as a substitute for Cythion, with no adverse
effects expected in humans (Lin 1974).
Groups of rats (15 male and 15 female, Wistar strain SPF/group)
were fed diets containing phoxim (technical product stabilized with
10% n-butanol, 87.2% a.i.), at concentrations of 0, 5, 15, 50, 150 and
500 ppm for 3 months. The control group comprised 30 male and 30
female rats. Haematological, clinical chemical and urinalysis
parameters were determined on 5 male and 5 female rats of each dose
group after 4 weeks and 3 months of feeding. The cholinesterase
activity in plasma and in erythrocytes was determined at 1, 4, 8 and
13 weeks after the start of the experiment in 5 male and 5 female rats
of each group. The animals dying during the experiment were subjected
to autopsy. At the end of the study, all the animals were sacrificed
and gross and histopathological examinations were performed.
There were no significant differences between control and treated
animals with respect to behaviour, food and water consumption and body
weight gain. Cholinergic symptoms were sometimes observed in the
500 ppm group only, particularly during the first half of the
experiment. Only the male rats in the 500 ppm group showed
significantly lower body weights. No compound-related mortality
occurred.
The treated animals of all dosage groups did not significantly
differ from the control animals throughout the experiment with respect
to haematological, clinical chemical and urinalysis parameters.
There was an increasing dose-dependent inactivation of both
plasma and erythrocyte cholinesterase in male rats at 50 ppm and
above. In female rats, a dose-dependent depression was noted at 15 ppm
and above in the plasma and at 50 ppm and above in the erythrocytes.
The autopsy of all rats at the end of the feeding experiment
showed no changes of the internal organs attributable to the
inclusion of phoxim in the diet. Male rats in the 500 ppm group had
significantly higher thyroid and liver weights, as compared to
controls. There were no dose-related or significant differences
between any of the dose groups up to 50 ppm and the control animals
with respect to relative organ weights. Higher relative liver weights
in males and females of the 150 ppm and 500 ppm groups were observed.
These enlargements are indicative of an influence on the liver,
although the liver function tests were all normal. Higher relative
kidney weights were observed in the males of the 500 ppm groups and in
the females of the 150 ppm and 500 ppm groups. Heart and adrenal in
the males of the 500 ppm group and thyroid and lung in the females of
500 ppm group had significantly higher relative weights, as compared
to the control animals.
The no-effect level with respect to plasma and erythrocyte
cholinesterase was 15 and 5 ppm, respectively, for male and female
rats, equal to approximately 1.45 and 0.56 mg/kg bw/day (Löser 1970a).
No histopathological change was seen in the tissues examined that
was considered to be compound-related (Vince and Spicer 1971).
Dog - dietary
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 2, 5 and 10 ppm for
3 months. The control group comprised 3 male and 3 female dogs.
Inclusion of the active ingredient in the diet at dose levels up to
10 ppm did not affect the physical appearance, behaviour, food
consumption, growth and mortality rate of male and female dogs.
Cholinesterase activity in the plasma was depressed in both males and
females at the dose level of 2 ppm after 1 month, but not at the end
of the study. The results of the haematological, clinical chemical and
urinalysis determinations showed no significant differences between
the control and treated groups. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1970b).
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 50, 200 and 1 000 ppm
for 3 months. The control group comprised 3 male and 3 female dogs.
The dogs in the 1 000 ppm group showed cholinergic symptoms, but no
dogs died in any of the dose groups during the experiment. The
dose level of 1 000 ppm caused weight loss in the female dogs.
Haematological determinations made after 6 weeks and at the end of the
study did not reveal any pathological changes in the dogs of any
groups. Clinical chemical parameters were normal in the 50 and 200 ppm
groups. Increased activities of alkaline phosphatase (ALP) and lactate
dehydrogenase (LDH) were determined in the animals of the 1 000 group.
However, the activities of liver-specific enzymes ornithine carbamyl
transferase (OCT), GPT and sorbitol dehydrogenase (SDH) did not
change. Urine examinations and the kidney function test (urea and
creatinine in serum) were normal in all groups, as were blood sugar
and cholesterol levels. Plasma cholinesterase activity was depressed
at 50 ppm and above, while erythrocyte cholinesterase was first
affected at the 200 ppm level. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1971).
Groups of beagle dogs (4 males and 4 females/group) were fed
diets containing phoxim (91.1% a.i.) at concentrations of 0, 0.3, 1
and 2 ppm for 3 months. No deaths occurred during the study. There
were no significant differences between the control and treated dogs
with respect to behavioural pattern, physical appearance, food
consumption, body weight gain, reflex, eye examinations, haematology,
clinical chemistry, thromboplastin time and urinalysis, as determined
at 0, 5 and 12 weeks.
The no-effect level for plasma cholinesterase was 0.3 ppm for
both males and females. Erythrocyte cholinesterase activity was not
affected in any of the treated groups. Autopsy and histopathological
examinations performed on all the animals provided no indication of
treatment-related alterations (Mürmann and Luchaus 1973).
Groups of beagle dogs (4 males and 4 females/group) were fed
phoxim in the diet (technical product, stabilized with 10% n-butanol,
86.5 a.i.) at the following levels for 2 years: control group - 0 ppm;
group I - males, 0.3 ppm and females 0.1 ppm from week 83; group II -
15 ppm; group III- 750 ppm. Daily examinations were made for physical
appearance and behavioural pattern. Intake of food and water was also
checked and noted daily. Body weights were recorded weekly in the
first year and thereafter at 14-day intervals. At periodical intervals
(14, 26, 39, 52, 64, 78, 92 and 104 weeks) reflex testing,
ophthalmoscopy, haematology, clinical-chemistry and urinalyses were
performed. Plasma and erythrocyte cholinesterase determinations at
4, 7, 10, 15, 27, 39, 51, 64, 77, 91 and 103 weeks and brain
cholinesterase at the conclusion of the study were performed. At the
end of the 104 weeks of dietary administration, all the dogs were
sacrificed and gross and histopathological examinations of tissues and
organs were performed.
There was no mortality over the course of the study in any group.
Treatment did not affect the behavioural patterns of any of the dogs.
The female dogs of each treated group and the male dogs of group I did
not differ in physical appearance from the controls. Male dogs in
group II and group III had a poor state of nutrition. The male dogs of
group III had a dull and ungroomed coat in the second treatment year.
Food consumption levels were not affected by treatment. The time taken
for the food ration to be consumed by the dogs was slightly longer in
group II and considerably longer in group III. Average body weight
curves, especially for the males, were lower than those for the
control group and were dose related. However, only the body weights of
group III rats were statistically significantly lower than those of
control rats. A marked decrease of body weight was evident in male
dogs of group III after 72 weeks. The reflexes were normal in all dogs
at all times tested. The ophthalmoscopic examinations of the eyes
provided no indication of any treatment-related variations from the
physiological norm in either the transparent media or on the fundus
oculi. The average total intake of phoxim per dog was 0.068 g, 3.60 g
and 181.08 g, respectively for group I, group II and group III. Data
from haematological tests and urinalyses were normal. Plasma and
erythrocyte cholinesterase activities were not affected in males of
group I, i.e. at 0.3 ppm. In females of group I, plasma cholinesterase
activity was seen to be depressed to a level 26% below the control
value in week 77. As this result was confirmed by repeated
measurements, the concentration of phoxim administered to the female
dogs of group I was reduced from 0.3 to 0.1 ppm from week 83. The
later determinations in week 91 and 103 showed that plasma
cholinesterase activities in group I females were again equal to the
control values. At the 15 ppm level and above, plasma and erythrocyte
cholinesterase activities were markedly depressed, and were dose
related, from the first determinations and remained relatively
constant over the entire study. Brain cholinesterase activity was not
depressed at dietary concentrations of up to and including 15 ppm,
being depressed 35-40% at 750 ppm level. Clinical chemistry values
(glucose, urea, creatinine, total protein, GOT, bilirubin) showed no
differences between treated dogs and controls. Statistically
significant values, increasingly higher than controls, were observed
for GPT (from week 52) and ALP (from week 14) in the dogs of group III
(less marked in the females). In the group II dogs and in the males of
group I, ALP activity values were statistically significantly higher
than in the controls; they were at the same level throughout the
second half of the experiment. In the controls, the physiological
age-related reduction of ALP activity was observed during the course
of the study. In the group III dogs the average serum cholesterol
level was lower than that measured in the control dogs at every
investigation time until the end of the feeding experiment.
At gross pathological examination, the livers of 4 female dogs
and one male dog of group III had a darker (dark brown to grey) colour
than those of the control dogs and the dogs of the other two treated
groups. Furthermore, the livers of some dogs in group III were seen to
have a marked lobular pattern. There were no noteworthy differences
between the treated dogs of all groups and the control dogs with
respect to absolute and relative organ weights, with the exception of
liver and thyroid. The absolute and relative weights of the liver in
the male and female dogs of group III were statistically significantly
higher than the weight of the liver in the control dogs. There was
also a slight increase in the relative weight of the liver in the male
dogs of group II, but this difference was not statistically
significant. The absolute and relative weights of thyroid in the
female dogs of group III were greater than those in the control dogs,
but this difference was not statistically significant. Hepatocyte
alterations were seen at histopathological examination in all the dogs
of group III. The hepatocytes were dilated, the plasma had a light
glassy appearance and was less structured than in the control dogs or
the dogs of the other two dietary concentration groups.
The increase in the absolute and relative thyroid weights in the
female dogs of group III is not associated with histopathological
alterations, so that it is doubtful whether this increase was compound
related. Some other alterations of varying degree were observed in
other tissues of both the control group and the treated groups, so
they were not considered treatment related.
The study indicated 0.3 ppm in the diet, equal to 0.068 mg/kg
bw/day, and 0.1 ppm, respectively, for male and female dogs, as no-
effect levels with respect to plasma and erythrocyte cholinesterase
(Hoffmann and Gröning 1977).
Monkey - dietary
Rhesus monkeys (Macaca mulatta) (20 males and 20 females/group)
were administered by gavage daily doses of phoxim (technical product
stabilized with 10% n-butanol, 83.8% a.i.) dissolved in maize oil at
levels of 0, 0.2, 0.65 and 2.00 mg/kg, 6 days per week for 6 months.
Throughout the study the monkeys tolerated the administration of
phoxim well and remained in good health. There was no evidence of any
cholinergic effects despite the observed reduction of plasma
cholinesterase activity. Average body weights and urinalyses were not
significantly different between the control and treated groups, There
were some differences between the control and treated groups with
respect to some clinical chemistry values (blood urea nitrogen,
glucose, total bilirubin, GOT, calcium) and some haematology values
(packed cell volume, erythrocyte count). However, these fluctuations
were, for the most part, within the normal limits for the colony of
monkeys, and in no case were dose related.
Erythrocyte cholinesterase activity was slightly reduced at
2.0 mg/kg, but plasma cholinesterase activity was reduced at all three
dose levels tested. Expressed as percent of the control groups, the
remaining activity after six months was, respectively, for each dosage
level, 62%, 43% and 34%. A no-effect level was not determined. The
microscopic examination of liver biopsy material obtained prior to the
administration of phoxim and after six months of administration
provided no evidence of an effect of phoxim on liver morphology
(Coulston et al 1978).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females/
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to phoxim (technical product stabilized with 101
n-butanol, 83.8% a.i.; as an emulsion in water and Cremophor EL) at
dose levels of 0, 0.5 and 15 mg/kg bw/day, 7 hours per day, 5 days per
week for a total of 15 applications in a 21-day period.
The treated sites (5 × 5 cm) on the flank and back of the
animals, not covered with bandages, were washed with water and soap at
the end of each daily exposure period.
Behaviour and general aspect were normal for all animals. No
treatment-related deaths occurred. There were no adverse effects on
the pre- and post-treatment values of body weight or on terminal
haematological, clinical chemical and urinalysis parameters, Erythema
was not observed in any animals with intact skin. A slight compound-
related increase in time necessary for erythema to disappear was noted
in treated animals with abraded skin. Skin-fold thickness was
comparable between the control and treated groups. There were no
significant differences between control and treated groups with
respect to both absolute and relative weights of major organs.
Histopathological examinations were performed on heart, lung, liver,
spleen, kidney, adrenals, testis, epididymis, ovary, uterus and
thyroid from control and 15 mg/kg bw groups. Some alterations occurred
in both control and treated animals and were not considered compound
related. Slight increases of degree of cell infiltrations and of
incidence of epithelium thickening were observed on the treated skin
of animals at 15 mg/kg bw with abraded skin, as compared to control
animals. The same changes were not observed in animals with intact
skin. These findings were considered the result of repeated mechanical
stimuli. Plasma and erythrocyte cholinesterase activities measured
after exposures 8 and 15 were significantly depressed (ca. 60%) at
15 mg/kg bw/day. Brain cholinesterase activity was slightly (23%)
depressed only in male rabbits at 15 mg/kg bw/day (Flucke and Schilde
1978).
Long-Term Studies
Rat
Groups of rats (50 males and 50 females, SPF Wistar strain/group)
were fed a diet containing phoxim (technical product stabilized with
10% n-butanol, 85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm
(calculated as pure active ingredient) for 24 months. The control
group included 100 males and 100 females. In addition, 5 male and
5 female rats were used in each group for clinical laboratory
examinations, which were carried out at 3, 6 and 12 months. At the end
of the study, 10 males and 10 females were used for these
examinations.
There were no differences in appearance and behaviour between the
treated and control animals during the study. In the 375 ppm group
male rats had a lower mean daily food intake and females showed
significantly less weight increase than control animals, mainly in the
second half of the study. Mortality after 1 and 2 years was not
increased in any treated groups. The overall mortality rate for
treated and control groups at the end of the study was in the range of
24-26% for male rats and 14-21% for female rats. There were no
significant differences between the control and treated groups with
respect to haematological examinations at 3, 6, 12 and 24 months.
There were no dose-related differences between control and treated
groups with respect to alkaline phosphatase, GOT, GPT, glutamic
dehydrogenase (GLDH, determined only at the end of the study), serum
bilirubin and total serum protein, as determined at 3, 6, 12 and 24
months. Urinalyses, serum urea and creatinine, and urine protein
showed no relevant differences between the control and treated groups
after 3, 6, 12 and 24 months. Urine protein was sporadically increased
in the 75 ppm group. Mean values for blood sugar and cholesterol
showed some variations between the control and treated groups that
were not dose related. Cholinesterase activity in erythrocytes and
plasma was not significantly inhibited (less than 20%) in the male and
female rats in the 15 ppm group. In the 75 ppm and 375 ppm groups, a
marked and dose-related inhibition of the enzyme in plasma (16-42% in
males, 41-64% in females) and in the erythrocytes (24-54% in males,
25-49% in females) throughout the study was observed. Brain
cholinesterase activity (measured at the end of the trial) was
slightly inhibited (18% in males, 23% in females) only in the 375 ppm
group. The autopsies on rats dying during the study (106) and those
sacrificed at the end of the treatment did not reveal any treatment-
related damage. There was a significant, but not dose related,
increase in the relative liver weight of male rats of all dose groups
as compared to controls. There was also an increase in relative
weights of spleen, lungs and heart of female rats of the 375 ppm
group.
Histopathological examinations performed on some 31 tissues of
all the rats surviving the 2-year treatment did not reveal any
treatment-related alterations. The differences in relative organ
weights found were regarded as random, as they were not dose related,
and there was no histopathological correlation. The analysis of tumour
data according to the site, type and incidence of both benign and
malignant tumours provided no indications suggestive of carcinogenic
activity of phoxim in the rat.
The no-effect level for both plasma and erythrocyte
cholinesterase was 15 ppm (Bombhard and Löser 1977).
COMMENTS
Phoxim has been evaluated for the first time by the JMPR. It has
a mild acute toxicity, as confirmed following the various routes of
application. No sex-dependent differences were observed. In the
longer-term experiments, however, females tended to display higher
sensitivity. The acute experiments revealed a species-dependent
toxicity. There was no available information on specification of the
technical product. Phoxim is readily and almost completely absorbed.
It is rapidly excreted in the rat, and there is no evidence of
bio-accumulation. The metabolic pathway in mammals follows typical
steps, such as hydrolysis, desulphuration and conjugation. Possible
metabolites have a slight to moderate acute oral toxicity.
A delayed neurotoxicity test was negative but the test was
considered to be unacceptable. No-effect levels were determined with
respect to reproduction and teratogenicity. Mutagenicity and
carcinogenicity studies were negative.
In a two-year dog study, plasma and erythrocyte cholinesterase
were more sensitive than brain cholinesterase. A 90-day dog study
provided a no-effect level bridging the large differences between
effect and no-effect levels in the 2-year dog study.
An increase in relative liver weight, not dose related, was also
observed in the 2-year rat study at all dosage levels, without
histopathological correlation. No observations in humans were
available. The available data permitted the determination of no-effect
levels in two mammalian species. Because of the unavailability of an
acceptable delayed neurotoxicity study, only a temporary ADI was
allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.56 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.005 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
An appropriate neurotoxicity study in hens.
Desirable
1. Observations in humans (particularly effects on cholinesterases),
2. Type and content of impurities in the technical product.
REFERENCES
Baldock, C. and Hopkins, T. Bay 9053. Safety in sheep. Report from
1976 Bayer, Australia, submitted to the World Health Organization
by Bayer, Australia, (Unpublished)
Bombhard, E. and Loser, E. SRA 7502. Chronic toxicity studies in rats
1977 (feeding experiments over 2 years). Report (No. 7042) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Coulston, F., Griffin, T., Talley, W. and Iatropoulos, M.J. A safety
1978 evaluation study of phoxim in Rhesus monkeys. Final Report
from Institute of Comparative and Human Toxicology, Albany
Medical College, Albany, New York, and International Center
of Environmental Safety, Albany Medical College, Holloman,
New Mexico, submitted to the World Health Organization by
Bayer F.R.G. (Unpublished)
Daniel, J.W., Swanson, S. and McLean, J. Phoxim: pharmacokinetics and
1978a biotransformation in the rat. Report (No. 78/BAG 5/194) from
Life Science Research, Stock, Essex, England, submitted to
the World Health Organization by Bayer, F.R.G.(Unpublished)
Daniel, J.W., McLean, J. and Pringuer, M. Phoxim- biotransformation in
1978b the rat. Report (No. 78/BAG 6/317) from Life Science
Research, Stock, Essex, England, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502: Bestimmung der Akuten toxzität (LD50). Report
1978a from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1978b SRA 7502 VL: bestimmung der akuten toxzitat (LD50) Report
from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. and Schilde, B. Volaton VL. Subakuter kutaner toxizitat-
1978 versuch an kaninchen. Report No. 8034 from Institut für
toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502 VL: bestimmung der akuten toxizitat (LD50).
1979a Report from Institut fur toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1979b SRA 7502 VL (volaton): bestimmung der akuten toxizitat
(LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Flucke, W. SRA 7502 Volaton VL: bestimmung der akuten toxizitat
1980 (LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Heinmann, K.G. Volaton VL: bestimmung der akuten toxizitat (LD50).
1982 Report from Institut für toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
Herbold, B. SRA 7502 (phoxim). Micronucleu test on mouse to evaluate
1981 SRA 7502 for mutagenic potential. Report (No. 9891) from
Institut für toxicologie, Bayer, F.R.G. submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Hoffman, K. and Gröning, P. SRA 7502. Chronic toxicity study on dogs.
1977 two-year feeding experiment. Report (No. 6865) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Iakushko, V.E. Effect of Valexon on NADPH-dependent oxidation in
1978 endoplasmic reticulum of rat liver. IKR Biokim. Zh., 50
(6):727-720 (in Russian). Pest. Abstracts. 12(5): 292
(1979). Abstract No. 79.1193).
Kimmerle, G. BAY 77488. Toxicological studies. Report (No. 1067) from
1968 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Kimmerle, G. and Solmecke, B. BAY 77488. Toxicological studies. Report
1970 (no. 2235) from Institute für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
1972 Phoxim: neurotoxicity tests on hens. Report from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (unpublished)
Kuz'minskaia, V.A. and Veremenko, L.M. Effect of Valexon on succinate
1978 dehydrogenase and cytochrome oxidase activities in rat
liver. UKr. Biokhim. Zh., 50(6):711-713 (in Russian),
Pest. Abstracts 12(5): 292 (1979) (Abstract No. 79-1192).
Kuz'minskaia, V.A., Alekhina, S.M. and Bersan, L.V. Dynamic changes in
1979 the blood enzyme activities in albino rats following the
administration of Valexon. Gig. Sanit. 44(7):78-79 (in
Russian), Pest. Abstract, 13:31 (1980), (Abstract No.
80-0159).
Lin, T. Toxicological study of baythion and cythion on rat feeding
1974 test. Nung Yeh Yen Chin (J. Taiwan Agric. Res.), 23(2):
149-154, Pest. Abstracts 9:427 (1976), (Abstract No.
76.1543).
Löser, E. BAY 77488. Subchronic toxicological studies on rats. Three
1970a month feeding experiment. Report (No. 2389) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization, by Bayer, F.R.G. (Unpublished)
1970b BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiments. Report (No. 2418) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1971 BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiment. Report (No. 2579) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1979 SRA 7502. Multigeneration reproduction study on rats. Report
(No. 8447) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G. (with
appended Report on the histopathological examinations by
J.E. Close and J.W. May, Hazleton Laboratories, Harrogate,
U.K.).(Unpublished)
Machemer, L. SRA 7502 (phoxim). Dominant lethal test on male mouse to
1974 evaluate SRA 7502 for mutagenic potential. Report (No. 4943)
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer F.R.G. (Unpublished)
1975 SRA 7502 (phoxim). Studies for embryotoxic and teratogenic
effects on rats following oral administration. Report (No.
5331) from Institut für toxicologie, Bayer, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Mihail, F. Cyanoximsäureglucosid, Ratte p.o. Report from Institut für
1979 toxicologie, Bayer, submitted to the World Health
Organization by Bayer F.R.G. (Unpublished)
1981 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1982 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Mürmann, P. and Luckhaus, G. SRA 7502. Subchronic toxicity study on
1973 dogs. Report (No. 4136) from Institut für toxicologie,
Bayer, submitted to the World Health Organization by Bayer,
F.R.G. (Unpublished)
Oesch, F. Ames test for Volaton (phoxim). Report from
1977 pharmakologisches Institut der Universität, Mainz, F.R.G.
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, T. Phoxim. Mutagenicity test on
1978 bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Solmecke, B. BAY 77488 (SRA 7502) antidotes study. Report from
1971 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. and Kimmerle, G. SRA 7502. Toxicological studies on hens.
1973 Report No. 4236 from Institut für toxicologie, Bayer,
submitted to the World Health Organization by Bayer F.R.G.
(Unpublished)
1976 Cyanoxim (hydroxyiminophenyl-acetonitril)
Geverbetoxicologische Untersuchungen. Report (No. 6392) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976a oral toxicity when administered simultaneously with
fensulfothion, isofenphos or phoxim. Report (No. 5958) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1976b SRA 7502. Subacute oral cumulation study on rats. Report
(No. 5954) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Vince, A.A. and Spicer, E.J.F. Pathology report on BAY 77488,
1971 Subchronic toxicological studies in rats. 3-month feeding
experiment Report (No. 4257/71/415). (Addendum to the Report
No. 2389/Bayer) from Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by
Bayer, F.R.G. (Unpublished)
Vinopal, J.H. and Fukuto, T.R. Selective toxicity of phoxim
1970 (phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate).
Pest. Biochem. Physiol. 1:44-60.
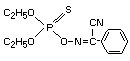 IDENTITY
Chemical Names
OO-diethyl alpha-cyanobenzylidene-amino-oxyphosphonothioate
alpha - (diethoxyphosphinothioyloxyimino) phenyl-acetonitrile
alpha - / / (diethoxyphosphinothioyl)oxy/imino/benzeneacetonitrile
O,O-diethyl phenylglyoxylonitrile oxime phosphorothioate
Synonyms
VolatonR
BaythionR
SRA 7502
BAYER 77488
Structural formula
IDENTITY
Chemical Names
OO-diethyl alpha-cyanobenzylidene-amino-oxyphosphonothioate
alpha - (diethoxyphosphinothioyloxyimino) phenyl-acetonitrile
alpha - / / (diethoxyphosphinothioyl)oxy/imino/benzeneacetonitrile
O,O-diethyl phenylglyoxylonitrile oxime phosphorothioate
Synonyms
VolatonR
BaythionR
SRA 7502
BAYER 77488
Structural formula
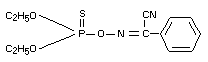 Other Information on Identity and Properties
Empirical formula C12H15N2O3PS
Molecular weight: 298.3
Appearance light yellow oily liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20°/4°C (pure active ingredient)
Vapour pressure approx. 10-4 mmHg at 20°C
Solubility (in g/100 ml at 20°C)
in water 0.7
in cyclohexane >60
in isopropyl alcohol >60
in methylene chloride >60
in toluene >60
Minimum degree of (pre-solution for reasons of stability in
purity 9-11% butanol)
82.0%
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
Male Swiss mice were given a single oral dose of 32p-phoxim in
olive oil at levels of 10.5, 114, and 955 mg/kg bw. At all three
dosage levels, the recovery of administered radioactivity in urine and
faeces was in the range of 73-82%, within 140 hours after dosing.
However, the radioactivity appeared in the urine and faeces at a much
lower than expected rate in the light of the low mammalian toxicity of
phoxim. At the three dosage levels, respectively, only 43 22 and 15%
of the administered radioactivity was excreted in the urine within 24
hours after treatment. An autopsy was performed on a mouse treated
with 114 mg/kg bw after 48 hours to determine the internal fate of the
administered dose. At this time, approximately 43% of the dose had
been excreted in the urine. The results indicate that virtually all of
the internal radioactivity was found in urinary bladder (88.4%), gut
(8.8%) and liver (1.7%). The amounts of radioactivity found in other
organs (brain, thymus gland, hind leg muscle, heart, kidney) and the
amount of organic soluble material (which could include the strong
anticholinesterase P=0 phoxim) were essentially insignificant.
The autopsy data indicate that nearly all of the internal dose of
phoxim remaining 48 hours after treatment was metabolized to water
soluble compounds (97.6%),
Five major metabolites were identified in the urine 0 to 24 hours
after treatment with 114 mg/kg bw: (1) diethyl phosphoric acid, 58.9%;
(2) phoxim, 1.1%; (3) phoxim carboxylic acid, 2.8%; (4) O,O-diethyl
phosphorothioic acid, 20.0%; (5) either monodesethyl phoxim or
monodesethyl P=O phoxim, 6.2%. The most relevant changes in the
relative amounts of metabolites upon increasing the dose from 114 to
955 mg/kg bw occurred with phoxim carboxylic acid (2.8% to 23.6%) and
diethyl phosphoric acid (58.9% to 43.1%) (Vinopal and Fukuto 1970).
Fig. 1 presents the proposed metabolic pathway for phoxim in
mice.
Rat
Male and female rats (Charles River CD strain) were intubated
with 14C-phoxim (labelled in the benzene moiety) at a dose of
10 mg/kg bw. A group of male rats was also intubated at a dose of
1 mg/kg bw. The compound was readily absorbed from the
gastrointestinal tract of male rats with average maximal plasma levels
equivalent to 0.35 and 2.44 µg phoxim being reached within 30 minutes
after dosing at 1 and 10 mg/kg respectively. A secondary peak,
equivalent to 1.87 µg phoxim/ml, was observed at 4 hours in animals
dosed at 10 mg/kg. A value of 0.04 µg phoxim/ml was measured at 24
hours in the plasma of rats receiving 10 mg/kg bw.
There was a rapid uptake of radioactivity into the major organs
and tissues following the administration of 10 mg/kg to male rats, the
kinetics of which was similar to that for plasma. Slightly higher
initial values were observed for kidney and liver, but levels of 0.06
to 0.6 µg equivalents of phoxim/g were reached in all tissues examined
within 24 hours. Thus, there was no evidence for the selective
retention of either phoxim or its presumed metabolites in any tissue
and accumulation in the tissues is unlikely as a result of repeated
administration.
Other Information on Identity and Properties
Empirical formula C12H15N2O3PS
Molecular weight: 298.3
Appearance light yellow oily liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20°/4°C (pure active ingredient)
Vapour pressure approx. 10-4 mmHg at 20°C
Solubility (in g/100 ml at 20°C)
in water 0.7
in cyclohexane >60
in isopropyl alcohol >60
in methylene chloride >60
in toluene >60
Minimum degree of (pre-solution for reasons of stability in
purity 9-11% butanol)
82.0%
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Biotransformation
Mouse
Male Swiss mice were given a single oral dose of 32p-phoxim in
olive oil at levels of 10.5, 114, and 955 mg/kg bw. At all three
dosage levels, the recovery of administered radioactivity in urine and
faeces was in the range of 73-82%, within 140 hours after dosing.
However, the radioactivity appeared in the urine and faeces at a much
lower than expected rate in the light of the low mammalian toxicity of
phoxim. At the three dosage levels, respectively, only 43 22 and 15%
of the administered radioactivity was excreted in the urine within 24
hours after treatment. An autopsy was performed on a mouse treated
with 114 mg/kg bw after 48 hours to determine the internal fate of the
administered dose. At this time, approximately 43% of the dose had
been excreted in the urine. The results indicate that virtually all of
the internal radioactivity was found in urinary bladder (88.4%), gut
(8.8%) and liver (1.7%). The amounts of radioactivity found in other
organs (brain, thymus gland, hind leg muscle, heart, kidney) and the
amount of organic soluble material (which could include the strong
anticholinesterase P=0 phoxim) were essentially insignificant.
The autopsy data indicate that nearly all of the internal dose of
phoxim remaining 48 hours after treatment was metabolized to water
soluble compounds (97.6%),
Five major metabolites were identified in the urine 0 to 24 hours
after treatment with 114 mg/kg bw: (1) diethyl phosphoric acid, 58.9%;
(2) phoxim, 1.1%; (3) phoxim carboxylic acid, 2.8%; (4) O,O-diethyl
phosphorothioic acid, 20.0%; (5) either monodesethyl phoxim or
monodesethyl P=O phoxim, 6.2%. The most relevant changes in the
relative amounts of metabolites upon increasing the dose from 114 to
955 mg/kg bw occurred with phoxim carboxylic acid (2.8% to 23.6%) and
diethyl phosphoric acid (58.9% to 43.1%) (Vinopal and Fukuto 1970).
Fig. 1 presents the proposed metabolic pathway for phoxim in
mice.
Rat
Male and female rats (Charles River CD strain) were intubated
with 14C-phoxim (labelled in the benzene moiety) at a dose of
10 mg/kg bw. A group of male rats was also intubated at a dose of
1 mg/kg bw. The compound was readily absorbed from the
gastrointestinal tract of male rats with average maximal plasma levels
equivalent to 0.35 and 2.44 µg phoxim being reached within 30 minutes
after dosing at 1 and 10 mg/kg respectively. A secondary peak,
equivalent to 1.87 µg phoxim/ml, was observed at 4 hours in animals
dosed at 10 mg/kg. A value of 0.04 µg phoxim/ml was measured at 24
hours in the plasma of rats receiving 10 mg/kg bw.
There was a rapid uptake of radioactivity into the major organs
and tissues following the administration of 10 mg/kg to male rats, the
kinetics of which was similar to that for plasma. Slightly higher
initial values were observed for kidney and liver, but levels of 0.06
to 0.6 µg equivalents of phoxim/g were reached in all tissues examined
within 24 hours. Thus, there was no evidence for the selective
retention of either phoxim or its presumed metabolites in any tissue
and accumulation in the tissues is unlikely as a result of repeated
administration.
 In the 10 mg/kg treatment, male rats excreted an average of 92.9%
of the radioactivity in the urine and 4.9% in the faeces in ten days,
while females excreted 86.1% of the dose in the urine and 6.9% in the
faeces in the same period. Male rats intubated at a dose of 1 mg/kg
excreted 82% of the radioactivity in the urine and 7.9% in the faeces
in ten days. However, most of the radioactivity (80-90%) was
eliminated in 24 hours and excretion was virtually complete within 2
days. No evidence was obtained for the presence of 14CO2 in the
expired air during the initial 24 hours.
Radiochemical analysis 10 days after dosing of the major organs
and tissues from animals dosed at both 1 and 10 mg/kg indicated the
presence of only low levels of radioactivity, which corresponded to
less than 0.1 µg phoxim/g tissue. An average of 0.80% and 3.3% of the
radioactivity was excreted within 0-6 and 6-24 hours in the bile of
cannulated male rats intubated at the 10 mg/kg dose level (Daniel
et al 1978a).
The biotransformation of phoxim was investigated in adult male
rats (Charles River CD strain) following administration (by gavage) of
a dose of 10 mg/kg bw.
The major metabolites in the 24-hour urine was identified as
hippuric acid (approx. 6%) and glucuronic and sulphuric acid
conjugates (approx. 76%) of alpha-cianobenzaldoxime. Evidence was also
obtained for the presence of both syn- and anti-forms of the oxime.
Chromatographic analysis of the plasma obtained one hour after
dosing revealed the presence of four radioactive components,
two of which were characerized as mono-desethyl-phoxim (80%) and
mono-desethyl PO-phoxim (12%). No evidence was obtained for the
presence of PO-phoxim (Daniel et al 1978b).
Effects on Enzymes and Other Biochemical Parameters
In the rat, in vitro cholinesterase inhibition amounted to (I50)
4.19×10-4M for serum, 4.46×10-5M for erythrocytes and 2.51×10-6M for
brain (Kimmerle 1968).
The effect of the organophosphorus pesticide phoxim on the
activity of liver succinate dehydrogenase (SDH) and cytochrome oxidase
(CO) was studied in randomly bred albino rats. Animals received a
single p.o. administration of phoxim at 0.5 LD50 (310 mg/kg) and were
sacrificed 1 hour, 1 day and 5 days later. In chronic experiments,
rats received phoxim at 31 mg/kg/day for 1, 3 and 6 months. Single
administration of phoxim decreased CO activity in liver homogenate
1 hour after administration (5.3 IU, compared with 7.61 IU in
controls), while the decrease in SDH activity was detected only on day
1 after administration (2.9 IU, compared with 7.55 IU in controls). In
the supernatant, activities of CO and SDH showed an increase only on
day 5 after administration (0.62 IU and 0.45 IU, respectively,
compared with 0.42 IU and 0.086 IU in controls). Chronic exposure to
phoxim did not change CO activity in the liver homogenate but resulted
in an increase in CO activity in the supernatant (after 3 months
exposure, CO activity was 0.80 IU compared with 0.42 IU in controls).
Activity of SDH in the homogenate increased after exposure of 1 month
(8.49 IU, compared with 7.55 IU in controls), while activity of SDH
in the supernatant was increased only after 3 and 6 months of
administration (0.35 IU and 0.59 IU, respectively, compared with
0.09 IU in controls) (Kuz'minskaia and Veremenko 1978).
The effect of phoxim on the NADPH-dependent oxidation in the
liver endoplasmic reticulum was studied in randomly bred albino rats,
Phoxim was given p.o. for 3 days at 124 mg/kg/day (LD50 is
620 mg/kg). On days, 1, 5 and 15 after the last administration,
animals were sacrificed and activity of liver demethylase (DM),
hydroxylase (HL), uridine diphosphate glucuronyl transferase (UDPGT)
and the level of ascorbic acid excretion were assessed. Phoxim
administration was found to stimulate the process of demethylation.
DM activity began increasing on day 5 after administration and
remained at a high level up to day 15 (0.957 IU and 0.842 IU,
respectively, compared with 0.602 IU in controls). HL activity was
decreased on day 1 (0.714 IU, compared with 0.304 IU in controls),
followed by a slight increase on day 5 (0.380 IU) and normalization on
day 15. Activity of UDPGT was significantly increased only on day 1
after administration (5.48 IU, compared with 3.28 IU in controls). The
level of urinary excretion of ascorbic acid showed a progressive
increase up to 538 µg/ml on day 1, 194.8 µg/ml on day 5 and
174.8 µg/ml on day 15 (compared with 118.5 µg/ml in controls (Iakusko
1978).
The effects of phoxim on the glucose-6-phosphatase (G6P),
hexokinase (HK), total cholinesterase (ChE) and ChE isoenzyme
activities were studied in the blood of male albino rats weighing
200-250 g. Group 1 served as a control, group 2 was treated (p.o.)
with a single 310 mg/kg dose of phoxim (0.5 LD50), group 3 received
31 mg/kg/day for 1-6 months and treatment in group 4 was 3.1 mg/kg/day
for 1-6 months. In group 4 the G6P and HK activities were increased
and the ChE activity was reduced 1 hour after treatment. The HK
activity became normal 1 hour later, the G6P activity remained
elevated and the ChE activity declined further. All enzyme activities
were normal 5 days after treatment. In group 3, the G6P and G6P
dehydrogenase (G6PD) activities were increased by 45% and 70%,
respectively, and the total ChE activity was decreased by 45% at the
end of the first month. The ChE fractions 3, 4 and 5 were fully
inhibited; fraction 2 was inhibited by 78%, and the activity of
fraction 1 was increased by 61%. After treatment for 3 months the G6P
activity was increased by 89%, the G6PD activity by 42% and the total
ChE activity was inhibited by 26%, while the isoenzyme spectrum did
not differ from that in the controls. After poisoning for 6 months,
the G6P and G6PD activities were inhibited by 30%, and the total ChE
by 24%. Fraction 1 was inhibited by 45% and the activity of fraction 2
increased by 35%, while the fractions 3, 4 and 5 remained normal. The
changes seen in group 4 were similar to but less marked than those in
group 3 (Kuz'minskaia et al 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of rats (10 male and 20 female, Long Evans FB30
strain/group) were fed diets containing phoxim (technical product,
85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm (calculated as
pure a.i.) and subjected to a standard 2-litter per generation,
3-generation reproduction study. The rats were treated with the test
compound throughout the study, including mating, gestation and
lactation. At the start of the experiment, the rats were about 47 days
old. The rats selected for the study were housed singly until they
were sexually mature (up to an age of ca. 100 days). Then they were
mated to initiate the study. During the mating period, two female rats
were housed together with one male rat for 21 days. The male rats were
rotated so that each female was paired with three different males for
a period longer than one oestrous cycle. Immediately after the pups
were delivered, their number and weights were recorded. Litters
containing more than 10 pups were reduced on day 5 after delivery to
10 pups each, whereupon the weights of these litters were again
measured. The pups were nourished for up to 4 weeks, weighed weekly,
and then the offspring of each first mating (F1a, F2a, F3a) were
sacrificed.
The offspring of each second mating, as with those delivered
after the first mating, were nourished for up to 4 weeks, then weaned
and placed in separate sex groups. After week 8, 10 male and 20 female
rats were again selected from each dose group for further matings.
Upon reaching an average age of 100 days, the rats were mated as
described above. After the dams of the F0, F1b and F2b generations
had successfully nursed offspring twice, they were sacrificed.
Necropsy was performed on rats that died during the study. Gross and
histopathological examinations were performed on major organs and
tissues of one male and one female 2-week old pup of the F3b
generation from each of ten mothers in each group.
There were no significant differences between the control and
treated groups with respect to physical appearance, behavioural
patterns, body weight curves of weaned males and females of the F0,
F1b and F3b generations; some animals in both the control and treated
groups died of pneumonitis.
There were no significant differences between the control and
treated groups with respect to pregnancy rate, litter size, 5-day
survival rate (viability index), pups weights at birth and during
lactation at all dose levels tested. Only the 375 ppm dietary
concentration had a slight adverse effect on the lactation index in
F3b. The inspections of the pups immediately after birth and during
the lactation period did not reveal any signs of malformations. Gross
and histopathological examinations did not provide any evidence of
treatment-related alterations.
The no-effect level in this multigeneration reproduction study
was 75 ppm (Löser 1979).
Special Studies on Mutagenicity
The mutagenicity of phoxim (technical product, 83.8% a.i.) was
studied by rec-assay and reversion test (Salmonella/microsome).
The rec-assay was performed using two strains of Bacillus
subtilis, H 17 and M 45. Volumes of 0.2 µl to 20 µ1 of phoxim per
disc were tested. Kanamycin was used as a negative control and
mitomycin C as a positive control. Phoxim did not inhibit the growth
of H 17 and M 45 strains of B. subtilis at all the tested doses. On
the other hand, with mitomycin C used as positive control more
remarkable growth inhibition was identified in M 45 strain than in
H 17 strain, and the growth of both strains was inhibited at the same
degree with Kanamycin as the negative control.
The reversion test was performed using two strains of
Salmonella typhimurium TA 98 and TA 100, according to the Ames
procedure, both with and without S-9 Mix derived from liver of SD
strain rats treated with a single intraperitoneal injection of
Arochlor 1254 (500 mg/kg bw). Concentrations of 10 - 5000 µg/plate
were tested. 2-amino-anthracene (2-AA) and 2-(2-furyl)-3-(5-nitro-2-
furyl) acrylamide (AF-2) were used as positive controls.
A markedly increased number of revertant colonies was observed
for AF-2 on the plates without S-9 Mix and for 2-AA on the plates with
S-9 Mix. Phoxim did not increase the number of revertant colonies,
either in the presence or absence of S-9 Mix.
Under the conditions of the experiments, phoxim provided no
evidence of mutagenic activity at concentrations up to 0.02 ml/disc of
undiluted phoxim and 5 000 µg/plate, respectively, in a rec-assay and
in a reversion test (Shirasu et al 1978).
In another Ames test, phoxim (technical grade, a.i. 82.9% -
83.7%) was tested for mutagenicity with S. typhimurium TA 100, TA
1537 and TA 98 according to the Ames procedure, both in the presence
and in the absence of S-9 Mix derived from male Sprague-Dawley rats
treated with a single intraperitoneal injection of Arochlor 1254
(500 mg/kg bw). Seven concentrations (3.15-3150 nl/plate) were tested
with S-9 Mix and five concentrations (31.5-3150 nl/plate) without it.
At doses greater than 1 000 nl, however, part of the test compound
separated as droplets from the top agar. In the experiments for direct
mutagenicity N-methyl-N'-nitro-N-nitrosoguanidine and benzo(a)pyrene-
4,5-oxide were used as positive controls, whereas in the experiment
with an activating system 3-methyl-cholantrene, benzo(a)pyrene and
2-amino-anthracene were used. Negative controls in the form of
sterility controls and solvent blanks were run as well.
For all three tested strains, there were no significant
differences in the number of revertant colonies between the negative
controls and the phoxim-treated plates, both with and without S-9 Mix.
Positive controls displayed the expected increases, indicating the
activity of the metabolizing system and the mutability of the
bacteria. No bacteriotoxic effects were observed.
Phoxim did not show a mutagenic effect in the Salmonella/
microsome test at concentrations as high as 3 150 nl/plate (Oesch
1977).
Mouse - dominant lethal test on males
Groups of mice (20 male NMRI mice/group) were given by gavage a
single dose of phoxim (technical product stabilized with 10%
n-butanol, 86.5% a.i.) corresponding to 0 and 500 mg/kg bw, the
vehicle being a 0.5% Cremophor emulsion. The mouse strain used
displayed a sensitive response to known chemical mutagens, such as
cyclophosphamide. The dose was chosen on the basis of the results of a
preliminary test conducted on male mice dosed orally with acute doses
of 500, 750 and 1 000 mg/kg bw, respectively. At dose levels of 750
and 1 000 mg/kg bw the compound had a toxic effect and induced
symptoms, but no mortalities occurred. Following dosing, each male
mouse was caged with three untreated virgin female mice for 7 days.
The procedure was repeated weekly with groups of three new untreated
virgin females for a total of 8 weeks, in order to obtain and examine
a complete sample of the successive germ cell stages of the males. The
uteri of the females (466-480 per test group) were examined on
gestation day 14. Counts were made of the corpora lutea, total
implantations, viable implants and dead implants (sum of the
deciduomata, resorptions and dead embryos and foetuses).
There were no significant differences between the control and
treated groups with respect to fertility quota, total implantations,
viable implants, dead implants, ratio of dead implants to total
implants and pre-implantation loss (estimated both directly from the
difference between the number of corpora lutea and the number of
implantations and indirectly through the comparison of the average
number of implantations per fertilized female in the treated group
with that in the control group).
Thus, there was no indication for a mutagenic potential of
phoxim in the dominant lethal test on the male mouse at an oral
dose of 500 mg/kg bw (Machemer 1974).
Micronucleus test
Groups of mice (5 male and 5 female, NMRI (SPF) Han strain/group)
were given (by gavage) two single doses of phoxim (technical product
84.3% a.i.) in aqueous 0.5% Cremophor emulsion at levels of 0, 250,
500 mg/kg bw. The interval between applications was 24 hours.
Concurrently, a positive control group of mice received 2 × 100 mg/kg
bw of cyclophosphamide. The doses were chosen on the basis of the
results of a preliminary test in which groups of 5 mice were orally
dosed with phoxim at 2 × 500 mg/kg bw and 2 × 1 000 mg/kg bw,
respectively; in that test, the 2 × 500 mg/kg treatment was tolerated
with induction of only weak symptoms. Six hours after the second
application, the mice were sacrificed and femur bone marrow smears
were prepared. Erythrocytes, 1 000 per mouse, were counted and the
incidence of cells with a micronucleus was determined, as well as the
ratio of polychromatic erythrocytes to normochromatic erythrocytes.
The incidence of micronucleated polychromatic erythrocytes was
2.3/1 000 in the negative control group, and 1.8/1 000 and 1.0/1 000,
respectively, in the phoxim-treated groups. There were no relevant
differences between the negative control and phoxim-treated group with
respect to the ratio of polychromatic to normochromatic erythrocytes.
The incidence of micronucleated cells in cyclophosphamide-treated
group was 68.2/1 000 and was thus biologically significantly higher
than in the negative control group. Cyclophosphamide also exhibited
a bone marrow depression, with the ratio of polychromatic to
normochromatic erythrocytes showing a biologically relevant alteration
(1 000 : 2 424.2 vs 1 000: 838.3 in the negative control group).
The results provided no indication for a mutagenic potential of
phoxim in the micronucleus test on mouse at the tested doses of
2 × 250 and 2 × 500 mg/kg bw per os. Treatments with phoxim also did
not induce any depression of erythropoiesis (Herbold 1981).
Special Studies on Embryotoxicity and Teratogenicity
Groups of fertilized rats (20 Long Evans FB30 strain/group)
received daily doses of phoxim (technical product stabilized with
10% n-butanol, 86.5% a.i.), administered by gavage in a 0.5% aqueous
Cremophor emulsion, at levels of 0, 30, 100, 300 mg/kg bw from
gestation day 6 through 15 (total of 10 doses). On gestation day 20,
the dams were sacrificed and the foetuses removed by caesarean
section. The foetuses were examined for external, internal and
skeletal malformations. No dam died in any group and no adverse
effects on behaviour patterns and general appearance were observed.
The 300 mg/kg bw dose level had a maternal-toxic effect, resulting in
significantly less weight gain during the treatment period as compared
with the control animals. All animals in the test group showed
comparable average weight gains throughout the period of gestation.
There were no significant differences between the control and
treated groups with respect to the measured parameters: fertilization
quotas, pregnancy quotas, average numbers of implantations, foetuses
and resorptions, average foetus weight, average placenta weight,
average number of stunted foetuses (weighing less than 3 g) and type,
frequency and localization of slight alterations in bone development.
Sex distribution of foetuses was unaffected. Occasional malformations
were seen, these being most frequent in the untreated control group.
They were considered to be spontaneous malformations.
The data provided no indication for embryotoxic or teratogenic
activity in rats at an oral dose of 300 mg/kg bw and below (Machemer
1975).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Neurotoxicity Studies
Hen
Female White Leghorn hens, 2-5/group, about 14 to 18 months
old, each received a single phoxim dose (without antidote)
administered orally at levels of up to and including 50 mg/kg bw or
intraperitoneally at levels of up to and including 37.5 mg/kg bw. The
hens were kept under observations for 6 weeks. The determined LD50
was 37.5 mg/kg bw for both oral and intraperitoneal administration.
In another study, hens 3-14/group, were each given as an antidote
an intraperitoneal injection of 100 mg PAM/kg + 50 mg atropine
sulphate/kg. They then received an oral or intraperitoneal application
of phoxim at dose levels equal to the LD50 and higher. The survivors
were kept under observation for 6 weeks. No histopathological
examinations were carried out. The protection afforded by PAM +
atropine to hens was more evident with intraperitoneal injection of
phoxim than with the oral route. No positive controls were included.
In both tests, without and with antidotal protection, no clinical
signs of neurotoxic damage were seen (Kimmerle 1972).
Groups of hens (10 White Leghorn, 12 to 14 months old/group) were
fed diets containing phoxim from technical product stabilized with 10%
n-butanol, 86.1% a.i. at concentrations of 0, 5 and 10 ppm for 28
days.
During the feeding experiment, the behavioural pattern of all the
hens was within the normal range. In particular, they did not display
any symptoms of neurotoxicity. Treated and untreated controls hens
were not significantly different with respect to food consumption and
body weight gains. Blood acetylcholinesterase activity was not
significantly depressed in the 5 ppm group. In the 10 ppm group, a
marked (30%) depression of blood acetylcholinesterase activity was
measured between day 14 and day 28 of the feeding experiment. The
no-effect level was 5 ppm, equal to 0.32 mg/kg bw/day (Thyssen and
Kimmerle 1973).
Special Studies on Skin and Eye Irritation
A dermal irritation study in rabbits of both sexes ("not of pure
breed") showed that phoxim had only a very slight irritating effect on
both intact and abraded skin (Kimmerle and Solmecke 1970).
In an eye irritation test in rabbits, phoxim caused no eye
irritation (Kimmerle and Solmecke 1970).
Special Studies on Antidotes
Male rats were given single oral doses of phoxim (technical
product, 88.7% a.i.) to determine LD50 values with and without
antidotes. Before the onset of severe poisoning symptoms (5 to
90 minutes), atropine sulphate (50 mg/kg bw), 2-PAM (50 mg/kg bw) or
toxogonin (20) mg/kg bw) was given singly or in combination (atropine
plus PAM-atropine plus toxogonin) by intraperitoneal injection. The
results indicated that, among the tested antidotes, only combinations
of 2-PAM or toxigonin with atropine sulphate had significant antidotal
activity (Solmecke 1971).
Special Studies on Potentiation
Acute oral LD50 values in male Wistar II albino rats were
determined experimentally for phoxim, phenamiphos and their equitoxic
mixture. The observed LD50 value for the equitoxic mixture was
compared with the expected value (calculated assuming an additive
effect). The result was that simultaneous administration of phoxim and
phenamiphos produced a less than additive effect (Thyssen 1976a).
Special Studies on the Recovery of Cholinesterase Activity
Groups of rats (15 male and 15 female, Wistar II, SBF
albinos/group) were given daily doses of phoxim (technical product),
administered by gavage in aqueous Cremophor EL emulsion, at levels of
0, 5, 50 mg/kg bw for 21 days. One-third of the rats were sacrificed
upon termination of treatment, another third were kept under post-
treatment observations for 14 days and then sacrificed and the
remaining third were kept under post-treatment observation for 4
weeks. Determinations of acetylcholinesterase activities in
erythrocytes and plasma were carried out before beginning treatment
and thereafter at weekly intervals until 4 weeks after the end of
administration. Brain acetylcholinesterase activity was measured at
the end of treatment and 2 weeks after termination of treatment.
During the 21-day treatment period and the 4-week post-treatment
observation period, none of the treated rats in any dose group
differed from the control rats with respect to behavioural patterns,
physical appearance and body weight gain. No mortalities were
observed. The following results were obtained in male rats: at
5 mg/kg bw, plasma and erythrocyte cholinesterase activities were
not significantly different from the control group during both the
treatment period and the post-treatment observation period; at
50 mg/kg, plasma and erythrocyte cholinesterase activities were about
65% and 56%, respectively, of the control after 1 week of treatment
and remained similarly depressed during the treatment period; however,
one week after the end of treatment, cholinesterase activity levels
were again found not significantly different from the control.
The following results were obtained in female rats; at 5 mg/kg
bw, both plasma and erythrocyte cholinesterase activities were about
70% of the control after 1 week of treatment, but one week after the
termination of treatment both were in the physiological range; at
50 mg/kg bw, both plasma and erythrocyte cholinesterase activities
were about 37% of the control after 1 week of treatment; one week
after termination of treatment plasma cholinesterase was again normal,
while erythrocyte cholinesterase was 75% of the control and returned
to normal at the end of the 4-week post-treatment observation period.
Brain cholinesterase activity at the end of the treatment period
was 82% and 67% of the control, respectively, for male and female rats
of the 50 mg/kg bw group. However, the values measured 2 weeks after
the end of treatment were not significantly different.
Thus, female rats were more sensitive than males to the
depression of plasma, erythrocyte and brain cholinesterase activities.
The plasma, erythrocyte and brain cholinesterase activity depression
was reversible; plasma cholinesterase returned to normal within
1 week, while erythrocyte and brain cholinesterase were normal in 2-4
weeks.
The acute oral LD50 determinations prior to and after the 21-day
treatment gave comparable values for both male and female rats
(Thyssen 1976b).
Special Studies on the Toxicity of Metabolites
Acute toxicity studies were conducted with alpha-
cyanobenzaldoxime (cyanoxime). The metabolite (and starting material
in the synthesis of phoxim) has a very slight acute toxicity to rats:
LD50 (mg/kg)
Rat, male oral 4 520
Rat, female oral 4 063
Rat, male,female dermal (24 hours) >5 000
From the symptoms of poisoning observed (behavioural disorders,
sedation, breathing disorders) cyanoxime seemingly acts on the central
nervous system.
In acute inhalational toxicity experiments, in which rats were
exposed to dust for 1 hour and 4 hours, respectively, and rats and
mice were exposed to vapour for 6 hours, no toxic effects were
observed.
In tests for skin and eye irritation, the metabolite did not
cause any irritation to the skin of rabbits. In the eye of rabbits,
however, it caused moderate to severe irritation on the conjunctiva
and superficial corrosion on the cornea (Thyssen and Kimmerle 1976).
The glucoside of alpha cyanobenzaldoxime did not cause any toxic
effects when administered as an acute oral dose to female rats; a dose
of 2 500 mg/kg bw was tolerated without inducing any symptoms (Mihail
1979).
PO phoxim, formed as an intermediate product of metabolism, has
an oral LD50 to the mouse of 1 000 mg/kg bw (Vinopal and Fukuto
1970).
Special Studies on Cholinesterase Inhibition by P=O Phoxim
The following 150 values were determined in vitro for the P=O
analogue of phoxim: erythrocyte (bovine), 2.2 × 10-7M; brain (mouse),
6.0 × 10-8M (Vinopal and Fukuto 1970).
Special Studies on Tolerability of Phoxim by Sheep
Groups of sheep (25 freshly shorn Merinos/group) were given
phoxim once, administered by intubation as an aqueous emulsion, at
levels of 0, 25 and 50 mg/kg bw. Each of the treated groups was
divided into two and one half was treated early in the morning and the
other half late in the afternoon. This was done to vary the times of
exposure to sunlight after treatment. The sheep were examined daily
for 10 days after treatment and any abnormalities were recorded.
The sheep treated in the morning remained exposed to strong
sunlight for only 3 hours after treatment. Cloudy conditions prevailed
for the remainder of the observation period, which reduced the
possibility of determining any phototoxic effect.
No signs of systemic toxicity or dermal inflammation were seen in
any of the test animals up to day 10 after treatment (Baldock and
Hopkins 1976).
Acute Toxicity
The results of acute toxicity studies are summarized in Table 1.
Signs of poisoning in mammals after oral administration usually
developed within 5 minutes to 2 hours after dosing in mice, rats,
rabbits, cats and dogs and lasted for 1 to 7 days in survivors. Deaths
occurred after 1 to 3 days. It was only at the highest dose levels
that these symptoms took the form of acute cholinesterase depression
(cramps, trembling, diarrhoea and red tears in rats). The only
symptoms noted in the lower dose levels were lowering of the general
condition and sedative effects. The toxicity signs did not differ in
the tested species. The autopsies of the animals receiving high doses
of the active ingredient showed no changes in the internal organs
(Kimmerle and Solmecke 1970).
Following intraperitoneal injection, the symptoms of poisoning
did not appear more quickly than after oral application. As with oral
treatment, the typical cholinergic symptoms were observed only at very
high doses (Kimmerle 1968a).
Hens are considerably more sensitive to phoxim than mammals
(Kimmerle 1972; Thyssen and Kimmerle 1973).
Table 1. Acute Toxicity of Phoxim
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 2.53-3.38 Flucke 1978 a
ml/kg b.w.
F oral none 2.50-3.50 Flucke 1978 b
ml/kg b.w.
F oral none 2.62-2.77 Flucke 1979 a
ml/kg b.w.
F oral none 3.24-3.60 Flucke 1979 b
ml/kg b.w.
F oral none 2.50-3.18 Flucke 1980
ml/kg b.w.
F oral none 2.96-3.40 Heinmann 1982
ml/kg b.w.
M oral none 2 440 Kimmerle 1968
µl/kg b.w.
F 3 240
µl/kg b.w. "
M inhalation alcohol + >2.06 "
(4-h exp.) Lutrol (1:1) mg/l air
F i.v. none 950
µl/kg b.w. "
M oral none 1 645 Kimmerle and
µl/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 1 990 Kimmerle and
µl/kg b.w. Solmecke 1970
M inhalation alcohol + >3.10
(4-h exp.) Lutrol (1:1) mg/l air "
F i.v. physiological 481
solution & mg/kg b.w. "
Cremophor EL
F oral none 3.02-3.48 Mihail 1981
ml/kg b.w.
F oral none 2.98-3.49 Mihail 1982
mg/kg b.w.
Rat M oral none 7 060 Kimmerle 1968
µl/kg b.w.
F 5 800
µl/kg b.w.
M i.p. none 1 775
µl/kg b.w. "
F 1 725
µl/kg b.w.
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M dermal none > 1 000
(7-day exp.) µl/kg b.w.
M inhalation alcohol + >1.99
(4-h exp) Lutrol (1:1) mg/l air
M inhalation alcohol + >2.23 Kimmerle and
(5x4-h exp.) Lutrol (1:1) mg/l air Solmecke 1968
F inhalation >2.22
(4-h, exp. ) mg/l air
M dermal none >1 000 Kimmerle and
(7-day exp.) µl/kg b.w. Solmecke 1970
M inhalation alcohol + >2.55
(4-h. exp.) Lutrol (1:1) mg/l air
inhalation > 0.55
(5x4-h exp.) mg/l air
F inhalation >2.78
(4-h exp.) mg/l air
inhalation > 0. 55
(5x4-h exp.) mg/l air
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M oral none 1 845
µl/kg b.w.
F 1 680
µl/kg b.w.
M i. p. none 1 645
µl/kg b.w.
F 1 635
µl/kg b.w.
M oral 2 650
mg/kg b.w Solmecke 1971
M oral water & Cremophor EL 2 825 Thyssen 1976 a
mg/kg b.w.
Guinea pig F oral none 350-500 Kimmerle, 1968
µl/kg b.w.
F oral water & Cremophor EL 660 Kimmerle and
mg/kg b.w. Solmecke 1970
Rabbit M&F oral none 250-500 Kimmerle 1968
µl/kg b .w.
F oral water & Cremophor EL 250-375 Kimmerle and
mg/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Cat M&F oral none > 1 000 Kimmerle - 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
Dog M&F oral none > 1 000 Kimmerle 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
oral] approx. 37.5 Kimmerle 1972
Chickens i.p.] mg/kg b.w.
Hen oral water emulsion 19.6 Thyssen and
mg/kg b.w. Kimmerle 1973
Short-Term Studies
Rat - dietary
Oral feeding tests were conducted to determine the safety of
using Baythion (phoxim) in place of Cythion (malathion) in the
treatment of stored grain to control insect pests. Baythion and
Cythion were fed to rats at concentrations of 1, 2, 4, 6, 10 or 0 ppm
in the diet. Adult rats and their offspring were fed for a period of
up to 5 months. No adverse effects were noted as a result of these
treatments. It is concluded that 4 ppm Baythion mixed with stored
grains could be used as a substitute for Cythion, with no adverse
effects expected in humans (Lin 1974).
Groups of rats (15 male and 15 female, Wistar strain SPF/group)
were fed diets containing phoxim (technical product stabilized with
10% n-butanol, 87.2% a.i.), at concentrations of 0, 5, 15, 50, 150 and
500 ppm for 3 months. The control group comprised 30 male and 30
female rats. Haematological, clinical chemical and urinalysis
parameters were determined on 5 male and 5 female rats of each dose
group after 4 weeks and 3 months of feeding. The cholinesterase
activity in plasma and in erythrocytes was determined at 1, 4, 8 and
13 weeks after the start of the experiment in 5 male and 5 female rats
of each group. The animals dying during the experiment were subjected
to autopsy. At the end of the study, all the animals were sacrificed
and gross and histopathological examinations were performed.
There were no significant differences between control and treated
animals with respect to behaviour, food and water consumption and body
weight gain. Cholinergic symptoms were sometimes observed in the
500 ppm group only, particularly during the first half of the
experiment. Only the male rats in the 500 ppm group showed
significantly lower body weights. No compound-related mortality
occurred.
The treated animals of all dosage groups did not significantly
differ from the control animals throughout the experiment with respect
to haematological, clinical chemical and urinalysis parameters.
There was an increasing dose-dependent inactivation of both
plasma and erythrocyte cholinesterase in male rats at 50 ppm and
above. In female rats, a dose-dependent depression was noted at 15 ppm
and above in the plasma and at 50 ppm and above in the erythrocytes.
The autopsy of all rats at the end of the feeding experiment
showed no changes of the internal organs attributable to the
inclusion of phoxim in the diet. Male rats in the 500 ppm group had
significantly higher thyroid and liver weights, as compared to
controls. There were no dose-related or significant differences
between any of the dose groups up to 50 ppm and the control animals
with respect to relative organ weights. Higher relative liver weights
in males and females of the 150 ppm and 500 ppm groups were observed.
These enlargements are indicative of an influence on the liver,
although the liver function tests were all normal. Higher relative
kidney weights were observed in the males of the 500 ppm groups and in
the females of the 150 ppm and 500 ppm groups. Heart and adrenal in
the males of the 500 ppm group and thyroid and lung in the females of
500 ppm group had significantly higher relative weights, as compared
to the control animals.
The no-effect level with respect to plasma and erythrocyte
cholinesterase was 15 and 5 ppm, respectively, for male and female
rats, equal to approximately 1.45 and 0.56 mg/kg bw/day (Löser 1970a).
No histopathological change was seen in the tissues examined that
was considered to be compound-related (Vince and Spicer 1971).
Dog - dietary
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 2, 5 and 10 ppm for
3 months. The control group comprised 3 male and 3 female dogs.
Inclusion of the active ingredient in the diet at dose levels up to
10 ppm did not affect the physical appearance, behaviour, food
consumption, growth and mortality rate of male and female dogs.
Cholinesterase activity in the plasma was depressed in both males and
females at the dose level of 2 ppm after 1 month, but not at the end
of the study. The results of the haematological, clinical chemical and
urinalysis determinations showed no significant differences between
the control and treated groups. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1970b).
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 50, 200 and 1 000 ppm
for 3 months. The control group comprised 3 male and 3 female dogs.
The dogs in the 1 000 ppm group showed cholinergic symptoms, but no
dogs died in any of the dose groups during the experiment. The
dose level of 1 000 ppm caused weight loss in the female dogs.
Haematological determinations made after 6 weeks and at the end of the
study did not reveal any pathological changes in the dogs of any
groups. Clinical chemical parameters were normal in the 50 and 200 ppm
groups. Increased activities of alkaline phosphatase (ALP) and lactate
dehydrogenase (LDH) were determined in the animals of the 1 000 group.
However, the activities of liver-specific enzymes ornithine carbamyl
transferase (OCT), GPT and sorbitol dehydrogenase (SDH) did not
change. Urine examinations and the kidney function test (urea and
creatinine in serum) were normal in all groups, as were blood sugar
and cholesterol levels. Plasma cholinesterase activity was depressed
at 50 ppm and above, while erythrocyte cholinesterase was first
affected at the 200 ppm level. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1971).
Groups of beagle dogs (4 males and 4 females/group) were fed
diets containing phoxim (91.1% a.i.) at concentrations of 0, 0.3, 1
and 2 ppm for 3 months. No deaths occurred during the study. There
were no significant differences between the control and treated dogs
with respect to behavioural pattern, physical appearance, food
consumption, body weight gain, reflex, eye examinations, haematology,
clinical chemistry, thromboplastin time and urinalysis, as determined
at 0, 5 and 12 weeks.
The no-effect level for plasma cholinesterase was 0.3 ppm for
both males and females. Erythrocyte cholinesterase activity was not
affected in any of the treated groups. Autopsy and histopathological
examinations performed on all the animals provided no indication of
treatment-related alterations (Mürmann and Luchaus 1973).
Groups of beagle dogs (4 males and 4 females/group) were fed
phoxim in the diet (technical product, stabilized with 10% n-butanol,
86.5 a.i.) at the following levels for 2 years: control group - 0 ppm;
group I - males, 0.3 ppm and females 0.1 ppm from week 83; group II -
15 ppm; group III- 750 ppm. Daily examinations were made for physical
appearance and behavioural pattern. Intake of food and water was also
checked and noted daily. Body weights were recorded weekly in the
first year and thereafter at 14-day intervals. At periodical intervals
(14, 26, 39, 52, 64, 78, 92 and 104 weeks) reflex testing,
ophthalmoscopy, haematology, clinical-chemistry and urinalyses were
performed. Plasma and erythrocyte cholinesterase determinations at
4, 7, 10, 15, 27, 39, 51, 64, 77, 91 and 103 weeks and brain
cholinesterase at the conclusion of the study were performed. At the
end of the 104 weeks of dietary administration, all the dogs were
sacrificed and gross and histopathological examinations of tissues and
organs were performed.
There was no mortality over the course of the study in any group.
Treatment did not affect the behavioural patterns of any of the dogs.
The female dogs of each treated group and the male dogs of group I did
not differ in physical appearance from the controls. Male dogs in
group II and group III had a poor state of nutrition. The male dogs of
group III had a dull and ungroomed coat in the second treatment year.
Food consumption levels were not affected by treatment. The time taken
for the food ration to be consumed by the dogs was slightly longer in
group II and considerably longer in group III. Average body weight
curves, especially for the males, were lower than those for the
control group and were dose related. However, only the body weights of
group III rats were statistically significantly lower than those of
control rats. A marked decrease of body weight was evident in male
dogs of group III after 72 weeks. The reflexes were normal in all dogs
at all times tested. The ophthalmoscopic examinations of the eyes
provided no indication of any treatment-related variations from the
physiological norm in either the transparent media or on the fundus
oculi. The average total intake of phoxim per dog was 0.068 g, 3.60 g
and 181.08 g, respectively for group I, group II and group III. Data
from haematological tests and urinalyses were normal. Plasma and
erythrocyte cholinesterase activities were not affected in males of
group I, i.e. at 0.3 ppm. In females of group I, plasma cholinesterase
activity was seen to be depressed to a level 26% below the control
value in week 77. As this result was confirmed by repeated
measurements, the concentration of phoxim administered to the female
dogs of group I was reduced from 0.3 to 0.1 ppm from week 83. The
later determinations in week 91 and 103 showed that plasma
cholinesterase activities in group I females were again equal to the
control values. At the 15 ppm level and above, plasma and erythrocyte
cholinesterase activities were markedly depressed, and were dose
related, from the first determinations and remained relatively
constant over the entire study. Brain cholinesterase activity was not
depressed at dietary concentrations of up to and including 15 ppm,
being depressed 35-40% at 750 ppm level. Clinical chemistry values
(glucose, urea, creatinine, total protein, GOT, bilirubin) showed no
differences between treated dogs and controls. Statistically
significant values, increasingly higher than controls, were observed
for GPT (from week 52) and ALP (from week 14) in the dogs of group III
(less marked in the females). In the group II dogs and in the males of
group I, ALP activity values were statistically significantly higher
than in the controls; they were at the same level throughout the
second half of the experiment. In the controls, the physiological
age-related reduction of ALP activity was observed during the course
of the study. In the group III dogs the average serum cholesterol
level was lower than that measured in the control dogs at every
investigation time until the end of the feeding experiment.
At gross pathological examination, the livers of 4 female dogs
and one male dog of group III had a darker (dark brown to grey) colour
than those of the control dogs and the dogs of the other two treated
groups. Furthermore, the livers of some dogs in group III were seen to
have a marked lobular pattern. There were no noteworthy differences
between the treated dogs of all groups and the control dogs with
respect to absolute and relative organ weights, with the exception of
liver and thyroid. The absolute and relative weights of the liver in
the male and female dogs of group III were statistically significantly
higher than the weight of the liver in the control dogs. There was
also a slight increase in the relative weight of the liver in the male
dogs of group II, but this difference was not statistically
significant. The absolute and relative weights of thyroid in the
female dogs of group III were greater than those in the control dogs,
but this difference was not statistically significant. Hepatocyte
alterations were seen at histopathological examination in all the dogs
of group III. The hepatocytes were dilated, the plasma had a light
glassy appearance and was less structured than in the control dogs or
the dogs of the other two dietary concentration groups.
The increase in the absolute and relative thyroid weights in the
female dogs of group III is not associated with histopathological
alterations, so that it is doubtful whether this increase was compound
related. Some other alterations of varying degree were observed in
other tissues of both the control group and the treated groups, so
they were not considered treatment related.
The study indicated 0.3 ppm in the diet, equal to 0.068 mg/kg
bw/day, and 0.1 ppm, respectively, for male and female dogs, as no-
effect levels with respect to plasma and erythrocyte cholinesterase
(Hoffmann and Gröning 1977).
Monkey - dietary
Rhesus monkeys (Macaca mulatta) (20 males and 20 females/group)
were administered by gavage daily doses of phoxim (technical product
stabilized with 10% n-butanol, 83.8% a.i.) dissolved in maize oil at
levels of 0, 0.2, 0.65 and 2.00 mg/kg, 6 days per week for 6 months.
Throughout the study the monkeys tolerated the administration of
phoxim well and remained in good health. There was no evidence of any
cholinergic effects despite the observed reduction of plasma
cholinesterase activity. Average body weights and urinalyses were not
significantly different between the control and treated groups, There
were some differences between the control and treated groups with
respect to some clinical chemistry values (blood urea nitrogen,
glucose, total bilirubin, GOT, calcium) and some haematology values
(packed cell volume, erythrocyte count). However, these fluctuations
were, for the most part, within the normal limits for the colony of
monkeys, and in no case were dose related.
Erythrocyte cholinesterase activity was slightly reduced at
2.0 mg/kg, but plasma cholinesterase activity was reduced at all three
dose levels tested. Expressed as percent of the control groups, the
remaining activity after six months was, respectively, for each dosage
level, 62%, 43% and 34%. A no-effect level was not determined. The
microscopic examination of liver biopsy material obtained prior to the
administration of phoxim and after six months of administration
provided no evidence of an effect of phoxim on liver morphology
(Coulston et al 1978).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females/
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to phoxim (technical product stabilized with 101
n-butanol, 83.8% a.i.; as an emulsion in water and Cremophor EL) at
dose levels of 0, 0.5 and 15 mg/kg bw/day, 7 hours per day, 5 days per
week for a total of 15 applications in a 21-day period.
The treated sites (5 × 5 cm) on the flank and back of the
animals, not covered with bandages, were washed with water and soap at
the end of each daily exposure period.
Behaviour and general aspect were normal for all animals. No
treatment-related deaths occurred. There were no adverse effects on
the pre- and post-treatment values of body weight or on terminal
haematological, clinical chemical and urinalysis parameters, Erythema
was not observed in any animals with intact skin. A slight compound-
related increase in time necessary for erythema to disappear was noted
in treated animals with abraded skin. Skin-fold thickness was
comparable between the control and treated groups. There were no
significant differences between control and treated groups with
respect to both absolute and relative weights of major organs.
Histopathological examinations were performed on heart, lung, liver,
spleen, kidney, adrenals, testis, epididymis, ovary, uterus and
thyroid from control and 15 mg/kg bw groups. Some alterations occurred
in both control and treated animals and were not considered compound
related. Slight increases of degree of cell infiltrations and of
incidence of epithelium thickening were observed on the treated skin
of animals at 15 mg/kg bw with abraded skin, as compared to control
animals. The same changes were not observed in animals with intact
skin. These findings were considered the result of repeated mechanical
stimuli. Plasma and erythrocyte cholinesterase activities measured
after exposures 8 and 15 were significantly depressed (ca. 60%) at
15 mg/kg bw/day. Brain cholinesterase activity was slightly (23%)
depressed only in male rabbits at 15 mg/kg bw/day (Flucke and Schilde
1978).
Long-Term Studies
Rat
Groups of rats (50 males and 50 females, SPF Wistar strain/group)
were fed a diet containing phoxim (technical product stabilized with
10% n-butanol, 85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm
(calculated as pure active ingredient) for 24 months. The control
group included 100 males and 100 females. In addition, 5 male and
5 female rats were used in each group for clinical laboratory
examinations, which were carried out at 3, 6 and 12 months. At the end
of the study, 10 males and 10 females were used for these
examinations.
There were no differences in appearance and behaviour between the
treated and control animals during the study. In the 375 ppm group
male rats had a lower mean daily food intake and females showed
significantly less weight increase than control animals, mainly in the
second half of the study. Mortality after 1 and 2 years was not
increased in any treated groups. The overall mortality rate for
treated and control groups at the end of the study was in the range of
24-26% for male rats and 14-21% for female rats. There were no
significant differences between the control and treated groups with
respect to haematological examinations at 3, 6, 12 and 24 months.
There were no dose-related differences between control and treated
groups with respect to alkaline phosphatase, GOT, GPT, glutamic
dehydrogenase (GLDH, determined only at the end of the study), serum
bilirubin and total serum protein, as determined at 3, 6, 12 and 24
months. Urinalyses, serum urea and creatinine, and urine protein
showed no relevant differences between the control and treated groups
after 3, 6, 12 and 24 months. Urine protein was sporadically increased
in the 75 ppm group. Mean values for blood sugar and cholesterol
showed some variations between the control and treated groups that
were not dose related. Cholinesterase activity in erythrocytes and
plasma was not significantly inhibited (less than 20%) in the male and
female rats in the 15 ppm group. In the 75 ppm and 375 ppm groups, a
marked and dose-related inhibition of the enzyme in plasma (16-42% in
males, 41-64% in females) and in the erythrocytes (24-54% in males,
25-49% in females) throughout the study was observed. Brain
cholinesterase activity (measured at the end of the trial) was
slightly inhibited (18% in males, 23% in females) only in the 375 ppm
group. The autopsies on rats dying during the study (106) and those
sacrificed at the end of the treatment did not reveal any treatment-
related damage. There was a significant, but not dose related,
increase in the relative liver weight of male rats of all dose groups
as compared to controls. There was also an increase in relative
weights of spleen, lungs and heart of female rats of the 375 ppm
group.
Histopathological examinations performed on some 31 tissues of
all the rats surviving the 2-year treatment did not reveal any
treatment-related alterations. The differences in relative organ
weights found were regarded as random, as they were not dose related,
and there was no histopathological correlation. The analysis of tumour
data according to the site, type and incidence of both benign and
malignant tumours provided no indications suggestive of carcinogenic
activity of phoxim in the rat.
The no-effect level for both plasma and erythrocyte
cholinesterase was 15 ppm (Bombhard and Löser 1977).
COMMENTS
Phoxim has been evaluated for the first time by the JMPR. It has
a mild acute toxicity, as confirmed following the various routes of
application. No sex-dependent differences were observed. In the
longer-term experiments, however, females tended to display higher
sensitivity. The acute experiments revealed a species-dependent
toxicity. There was no available information on specification of the
technical product. Phoxim is readily and almost completely absorbed.
It is rapidly excreted in the rat, and there is no evidence of
bio-accumulation. The metabolic pathway in mammals follows typical
steps, such as hydrolysis, desulphuration and conjugation. Possible
metabolites have a slight to moderate acute oral toxicity.
A delayed neurotoxicity test was negative but the test was
considered to be unacceptable. No-effect levels were determined with
respect to reproduction and teratogenicity. Mutagenicity and
carcinogenicity studies were negative.
In a two-year dog study, plasma and erythrocyte cholinesterase
were more sensitive than brain cholinesterase. A 90-day dog study
provided a no-effect level bridging the large differences between
effect and no-effect levels in the 2-year dog study.
An increase in relative liver weight, not dose related, was also
observed in the 2-year rat study at all dosage levels, without
histopathological correlation. No observations in humans were
available. The available data permitted the determination of no-effect
levels in two mammalian species. Because of the unavailability of an
acceptable delayed neurotoxicity study, only a temporary ADI was
allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.56 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.005 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
An appropriate neurotoxicity study in hens.
Desirable
1. Observations in humans (particularly effects on cholinesterases),
2. Type and content of impurities in the technical product.
REFERENCES
Baldock, C. and Hopkins, T. Bay 9053. Safety in sheep. Report from
1976 Bayer, Australia, submitted to the World Health Organization
by Bayer, Australia, (Unpublished)
Bombhard, E. and Loser, E. SRA 7502. Chronic toxicity studies in rats
1977 (feeding experiments over 2 years). Report (No. 7042) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Coulston, F., Griffin, T., Talley, W. and Iatropoulos, M.J. A safety
1978 evaluation study of phoxim in Rhesus monkeys. Final Report
from Institute of Comparative and Human Toxicology, Albany
Medical College, Albany, New York, and International Center
of Environmental Safety, Albany Medical College, Holloman,
New Mexico, submitted to the World Health Organization by
Bayer F.R.G. (Unpublished)
Daniel, J.W., Swanson, S. and McLean, J. Phoxim: pharmacokinetics and
1978a biotransformation in the rat. Report (No. 78/BAG 5/194) from
Life Science Research, Stock, Essex, England, submitted to
the World Health Organization by Bayer, F.R.G.(Unpublished)
Daniel, J.W., McLean, J. and Pringuer, M. Phoxim- biotransformation in
1978b the rat. Report (No. 78/BAG 6/317) from Life Science
Research, Stock, Essex, England, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502: Bestimmung der Akuten toxzität (LD50). Report
1978a from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1978b SRA 7502 VL: bestimmung der akuten toxzitat (LD50) Report
from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. and Schilde, B. Volaton VL. Subakuter kutaner toxizitat-
1978 versuch an kaninchen. Report No. 8034 from Institut für
toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502 VL: bestimmung der akuten toxizitat (LD50).
1979a Report from Institut fur toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1979b SRA 7502 VL (volaton): bestimmung der akuten toxizitat
(LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Flucke, W. SRA 7502 Volaton VL: bestimmung der akuten toxizitat
1980 (LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Heinmann, K.G. Volaton VL: bestimmung der akuten toxizitat (LD50).
1982 Report from Institut für toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
Herbold, B. SRA 7502 (phoxim). Micronucleu test on mouse to evaluate
1981 SRA 7502 for mutagenic potential. Report (No. 9891) from
Institut für toxicologie, Bayer, F.R.G. submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Hoffman, K. and Gröning, P. SRA 7502. Chronic toxicity study on dogs.
1977 two-year feeding experiment. Report (No. 6865) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Iakushko, V.E. Effect of Valexon on NADPH-dependent oxidation in
1978 endoplasmic reticulum of rat liver. IKR Biokim. Zh., 50
(6):727-720 (in Russian). Pest. Abstracts. 12(5): 292
(1979). Abstract No. 79.1193).
Kimmerle, G. BAY 77488. Toxicological studies. Report (No. 1067) from
1968 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Kimmerle, G. and Solmecke, B. BAY 77488. Toxicological studies. Report
1970 (no. 2235) from Institute für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
1972 Phoxim: neurotoxicity tests on hens. Report from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (unpublished)
Kuz'minskaia, V.A. and Veremenko, L.M. Effect of Valexon on succinate
1978 dehydrogenase and cytochrome oxidase activities in rat
liver. UKr. Biokhim. Zh., 50(6):711-713 (in Russian),
Pest. Abstracts 12(5): 292 (1979) (Abstract No. 79-1192).
Kuz'minskaia, V.A., Alekhina, S.M. and Bersan, L.V. Dynamic changes in
1979 the blood enzyme activities in albino rats following the
administration of Valexon. Gig. Sanit. 44(7):78-79 (in
Russian), Pest. Abstract, 13:31 (1980), (Abstract No.
80-0159).
Lin, T. Toxicological study of baythion and cythion on rat feeding
1974 test. Nung Yeh Yen Chin (J. Taiwan Agric. Res.), 23(2):
149-154, Pest. Abstracts 9:427 (1976), (Abstract No.
76.1543).
Löser, E. BAY 77488. Subchronic toxicological studies on rats. Three
1970a month feeding experiment. Report (No. 2389) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization, by Bayer, F.R.G. (Unpublished)
1970b BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiments. Report (No. 2418) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1971 BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiment. Report (No. 2579) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1979 SRA 7502. Multigeneration reproduction study on rats. Report
(No. 8447) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G. (with
appended Report on the histopathological examinations by
J.E. Close and J.W. May, Hazleton Laboratories, Harrogate,
U.K.).(Unpublished)
Machemer, L. SRA 7502 (phoxim). Dominant lethal test on male mouse to
1974 evaluate SRA 7502 for mutagenic potential. Report (No. 4943)
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer F.R.G. (Unpublished)
1975 SRA 7502 (phoxim). Studies for embryotoxic and teratogenic
effects on rats following oral administration. Report (No.
5331) from Institut für toxicologie, Bayer, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Mihail, F. Cyanoximsäureglucosid, Ratte p.o. Report from Institut für
1979 toxicologie, Bayer, submitted to the World Health
Organization by Bayer F.R.G. (Unpublished)
1981 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1982 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Mürmann, P. and Luckhaus, G. SRA 7502. Subchronic toxicity study on
1973 dogs. Report (No. 4136) from Institut für toxicologie,
Bayer, submitted to the World Health Organization by Bayer,
F.R.G. (Unpublished)
Oesch, F. Ames test for Volaton (phoxim). Report from
1977 pharmakologisches Institut der Universität, Mainz, F.R.G.
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, T. Phoxim. Mutagenicity test on
1978 bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Solmecke, B. BAY 77488 (SRA 7502) antidotes study. Report from
1971 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. and Kimmerle, G. SRA 7502. Toxicological studies on hens.
1973 Report No. 4236 from Institut für toxicologie, Bayer,
submitted to the World Health Organization by Bayer F.R.G.
(Unpublished)
1976 Cyanoxim (hydroxyiminophenyl-acetonitril)
Geverbetoxicologische Untersuchungen. Report (No. 6392) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976a oral toxicity when administered simultaneously with
fensulfothion, isofenphos or phoxim. Report (No. 5958) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1976b SRA 7502. Subacute oral cumulation study on rats. Report
(No. 5954) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Vince, A.A. and Spicer, E.J.F. Pathology report on BAY 77488,
1971 Subchronic toxicological studies in rats. 3-month feeding
experiment Report (No. 4257/71/415). (Addendum to the Report
No. 2389/Bayer) from Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by
Bayer, F.R.G. (Unpublished)
Vinopal, J.H. and Fukuto, T.R. Selective toxicity of phoxim
1970 (phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate).
Pest. Biochem. Physiol. 1:44-60.
In the 10 mg/kg treatment, male rats excreted an average of 92.9%
of the radioactivity in the urine and 4.9% in the faeces in ten days,
while females excreted 86.1% of the dose in the urine and 6.9% in the
faeces in the same period. Male rats intubated at a dose of 1 mg/kg
excreted 82% of the radioactivity in the urine and 7.9% in the faeces
in ten days. However, most of the radioactivity (80-90%) was
eliminated in 24 hours and excretion was virtually complete within 2
days. No evidence was obtained for the presence of 14CO2 in the
expired air during the initial 24 hours.
Radiochemical analysis 10 days after dosing of the major organs
and tissues from animals dosed at both 1 and 10 mg/kg indicated the
presence of only low levels of radioactivity, which corresponded to
less than 0.1 µg phoxim/g tissue. An average of 0.80% and 3.3% of the
radioactivity was excreted within 0-6 and 6-24 hours in the bile of
cannulated male rats intubated at the 10 mg/kg dose level (Daniel
et al 1978a).
The biotransformation of phoxim was investigated in adult male
rats (Charles River CD strain) following administration (by gavage) of
a dose of 10 mg/kg bw.
The major metabolites in the 24-hour urine was identified as
hippuric acid (approx. 6%) and glucuronic and sulphuric acid
conjugates (approx. 76%) of alpha-cianobenzaldoxime. Evidence was also
obtained for the presence of both syn- and anti-forms of the oxime.
Chromatographic analysis of the plasma obtained one hour after
dosing revealed the presence of four radioactive components,
two of which were characerized as mono-desethyl-phoxim (80%) and
mono-desethyl PO-phoxim (12%). No evidence was obtained for the
presence of PO-phoxim (Daniel et al 1978b).
Effects on Enzymes and Other Biochemical Parameters
In the rat, in vitro cholinesterase inhibition amounted to (I50)
4.19×10-4M for serum, 4.46×10-5M for erythrocytes and 2.51×10-6M for
brain (Kimmerle 1968).
The effect of the organophosphorus pesticide phoxim on the
activity of liver succinate dehydrogenase (SDH) and cytochrome oxidase
(CO) was studied in randomly bred albino rats. Animals received a
single p.o. administration of phoxim at 0.5 LD50 (310 mg/kg) and were
sacrificed 1 hour, 1 day and 5 days later. In chronic experiments,
rats received phoxim at 31 mg/kg/day for 1, 3 and 6 months. Single
administration of phoxim decreased CO activity in liver homogenate
1 hour after administration (5.3 IU, compared with 7.61 IU in
controls), while the decrease in SDH activity was detected only on day
1 after administration (2.9 IU, compared with 7.55 IU in controls). In
the supernatant, activities of CO and SDH showed an increase only on
day 5 after administration (0.62 IU and 0.45 IU, respectively,
compared with 0.42 IU and 0.086 IU in controls). Chronic exposure to
phoxim did not change CO activity in the liver homogenate but resulted
in an increase in CO activity in the supernatant (after 3 months
exposure, CO activity was 0.80 IU compared with 0.42 IU in controls).
Activity of SDH in the homogenate increased after exposure of 1 month
(8.49 IU, compared with 7.55 IU in controls), while activity of SDH
in the supernatant was increased only after 3 and 6 months of
administration (0.35 IU and 0.59 IU, respectively, compared with
0.09 IU in controls) (Kuz'minskaia and Veremenko 1978).
The effect of phoxim on the NADPH-dependent oxidation in the
liver endoplasmic reticulum was studied in randomly bred albino rats,
Phoxim was given p.o. for 3 days at 124 mg/kg/day (LD50 is
620 mg/kg). On days, 1, 5 and 15 after the last administration,
animals were sacrificed and activity of liver demethylase (DM),
hydroxylase (HL), uridine diphosphate glucuronyl transferase (UDPGT)
and the level of ascorbic acid excretion were assessed. Phoxim
administration was found to stimulate the process of demethylation.
DM activity began increasing on day 5 after administration and
remained at a high level up to day 15 (0.957 IU and 0.842 IU,
respectively, compared with 0.602 IU in controls). HL activity was
decreased on day 1 (0.714 IU, compared with 0.304 IU in controls),
followed by a slight increase on day 5 (0.380 IU) and normalization on
day 15. Activity of UDPGT was significantly increased only on day 1
after administration (5.48 IU, compared with 3.28 IU in controls). The
level of urinary excretion of ascorbic acid showed a progressive
increase up to 538 µg/ml on day 1, 194.8 µg/ml on day 5 and
174.8 µg/ml on day 15 (compared with 118.5 µg/ml in controls (Iakusko
1978).
The effects of phoxim on the glucose-6-phosphatase (G6P),
hexokinase (HK), total cholinesterase (ChE) and ChE isoenzyme
activities were studied in the blood of male albino rats weighing
200-250 g. Group 1 served as a control, group 2 was treated (p.o.)
with a single 310 mg/kg dose of phoxim (0.5 LD50), group 3 received
31 mg/kg/day for 1-6 months and treatment in group 4 was 3.1 mg/kg/day
for 1-6 months. In group 4 the G6P and HK activities were increased
and the ChE activity was reduced 1 hour after treatment. The HK
activity became normal 1 hour later, the G6P activity remained
elevated and the ChE activity declined further. All enzyme activities
were normal 5 days after treatment. In group 3, the G6P and G6P
dehydrogenase (G6PD) activities were increased by 45% and 70%,
respectively, and the total ChE activity was decreased by 45% at the
end of the first month. The ChE fractions 3, 4 and 5 were fully
inhibited; fraction 2 was inhibited by 78%, and the activity of
fraction 1 was increased by 61%. After treatment for 3 months the G6P
activity was increased by 89%, the G6PD activity by 42% and the total
ChE activity was inhibited by 26%, while the isoenzyme spectrum did
not differ from that in the controls. After poisoning for 6 months,
the G6P and G6PD activities were inhibited by 30%, and the total ChE
by 24%. Fraction 1 was inhibited by 45% and the activity of fraction 2
increased by 35%, while the fractions 3, 4 and 5 remained normal. The
changes seen in group 4 were similar to but less marked than those in
group 3 (Kuz'minskaia et al 1979).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Groups of rats (10 male and 20 female, Long Evans FB30
strain/group) were fed diets containing phoxim (technical product,
85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm (calculated as
pure a.i.) and subjected to a standard 2-litter per generation,
3-generation reproduction study. The rats were treated with the test
compound throughout the study, including mating, gestation and
lactation. At the start of the experiment, the rats were about 47 days
old. The rats selected for the study were housed singly until they
were sexually mature (up to an age of ca. 100 days). Then they were
mated to initiate the study. During the mating period, two female rats
were housed together with one male rat for 21 days. The male rats were
rotated so that each female was paired with three different males for
a period longer than one oestrous cycle. Immediately after the pups
were delivered, their number and weights were recorded. Litters
containing more than 10 pups were reduced on day 5 after delivery to
10 pups each, whereupon the weights of these litters were again
measured. The pups were nourished for up to 4 weeks, weighed weekly,
and then the offspring of each first mating (F1a, F2a, F3a) were
sacrificed.
The offspring of each second mating, as with those delivered
after the first mating, were nourished for up to 4 weeks, then weaned
and placed in separate sex groups. After week 8, 10 male and 20 female
rats were again selected from each dose group for further matings.
Upon reaching an average age of 100 days, the rats were mated as
described above. After the dams of the F0, F1b and F2b generations
had successfully nursed offspring twice, they were sacrificed.
Necropsy was performed on rats that died during the study. Gross and
histopathological examinations were performed on major organs and
tissues of one male and one female 2-week old pup of the F3b
generation from each of ten mothers in each group.
There were no significant differences between the control and
treated groups with respect to physical appearance, behavioural
patterns, body weight curves of weaned males and females of the F0,
F1b and F3b generations; some animals in both the control and treated
groups died of pneumonitis.
There were no significant differences between the control and
treated groups with respect to pregnancy rate, litter size, 5-day
survival rate (viability index), pups weights at birth and during
lactation at all dose levels tested. Only the 375 ppm dietary
concentration had a slight adverse effect on the lactation index in
F3b. The inspections of the pups immediately after birth and during
the lactation period did not reveal any signs of malformations. Gross
and histopathological examinations did not provide any evidence of
treatment-related alterations.
The no-effect level in this multigeneration reproduction study
was 75 ppm (Löser 1979).
Special Studies on Mutagenicity
The mutagenicity of phoxim (technical product, 83.8% a.i.) was
studied by rec-assay and reversion test (Salmonella/microsome).
The rec-assay was performed using two strains of Bacillus
subtilis, H 17 and M 45. Volumes of 0.2 µl to 20 µ1 of phoxim per
disc were tested. Kanamycin was used as a negative control and
mitomycin C as a positive control. Phoxim did not inhibit the growth
of H 17 and M 45 strains of B. subtilis at all the tested doses. On
the other hand, with mitomycin C used as positive control more
remarkable growth inhibition was identified in M 45 strain than in
H 17 strain, and the growth of both strains was inhibited at the same
degree with Kanamycin as the negative control.
The reversion test was performed using two strains of
Salmonella typhimurium TA 98 and TA 100, according to the Ames
procedure, both with and without S-9 Mix derived from liver of SD
strain rats treated with a single intraperitoneal injection of
Arochlor 1254 (500 mg/kg bw). Concentrations of 10 - 5000 µg/plate
were tested. 2-amino-anthracene (2-AA) and 2-(2-furyl)-3-(5-nitro-2-
furyl) acrylamide (AF-2) were used as positive controls.
A markedly increased number of revertant colonies was observed
for AF-2 on the plates without S-9 Mix and for 2-AA on the plates with
S-9 Mix. Phoxim did not increase the number of revertant colonies,
either in the presence or absence of S-9 Mix.
Under the conditions of the experiments, phoxim provided no
evidence of mutagenic activity at concentrations up to 0.02 ml/disc of
undiluted phoxim and 5 000 µg/plate, respectively, in a rec-assay and
in a reversion test (Shirasu et al 1978).
In another Ames test, phoxim (technical grade, a.i. 82.9% -
83.7%) was tested for mutagenicity with S. typhimurium TA 100, TA
1537 and TA 98 according to the Ames procedure, both in the presence
and in the absence of S-9 Mix derived from male Sprague-Dawley rats
treated with a single intraperitoneal injection of Arochlor 1254
(500 mg/kg bw). Seven concentrations (3.15-3150 nl/plate) were tested
with S-9 Mix and five concentrations (31.5-3150 nl/plate) without it.
At doses greater than 1 000 nl, however, part of the test compound
separated as droplets from the top agar. In the experiments for direct
mutagenicity N-methyl-N'-nitro-N-nitrosoguanidine and benzo(a)pyrene-
4,5-oxide were used as positive controls, whereas in the experiment
with an activating system 3-methyl-cholantrene, benzo(a)pyrene and
2-amino-anthracene were used. Negative controls in the form of
sterility controls and solvent blanks were run as well.
For all three tested strains, there were no significant
differences in the number of revertant colonies between the negative
controls and the phoxim-treated plates, both with and without S-9 Mix.
Positive controls displayed the expected increases, indicating the
activity of the metabolizing system and the mutability of the
bacteria. No bacteriotoxic effects were observed.
Phoxim did not show a mutagenic effect in the Salmonella/
microsome test at concentrations as high as 3 150 nl/plate (Oesch
1977).
Mouse - dominant lethal test on males
Groups of mice (20 male NMRI mice/group) were given by gavage a
single dose of phoxim (technical product stabilized with 10%
n-butanol, 86.5% a.i.) corresponding to 0 and 500 mg/kg bw, the
vehicle being a 0.5% Cremophor emulsion. The mouse strain used
displayed a sensitive response to known chemical mutagens, such as
cyclophosphamide. The dose was chosen on the basis of the results of a
preliminary test conducted on male mice dosed orally with acute doses
of 500, 750 and 1 000 mg/kg bw, respectively. At dose levels of 750
and 1 000 mg/kg bw the compound had a toxic effect and induced
symptoms, but no mortalities occurred. Following dosing, each male
mouse was caged with three untreated virgin female mice for 7 days.
The procedure was repeated weekly with groups of three new untreated
virgin females for a total of 8 weeks, in order to obtain and examine
a complete sample of the successive germ cell stages of the males. The
uteri of the females (466-480 per test group) were examined on
gestation day 14. Counts were made of the corpora lutea, total
implantations, viable implants and dead implants (sum of the
deciduomata, resorptions and dead embryos and foetuses).
There were no significant differences between the control and
treated groups with respect to fertility quota, total implantations,
viable implants, dead implants, ratio of dead implants to total
implants and pre-implantation loss (estimated both directly from the
difference between the number of corpora lutea and the number of
implantations and indirectly through the comparison of the average
number of implantations per fertilized female in the treated group
with that in the control group).
Thus, there was no indication for a mutagenic potential of
phoxim in the dominant lethal test on the male mouse at an oral
dose of 500 mg/kg bw (Machemer 1974).
Micronucleus test
Groups of mice (5 male and 5 female, NMRI (SPF) Han strain/group)
were given (by gavage) two single doses of phoxim (technical product
84.3% a.i.) in aqueous 0.5% Cremophor emulsion at levels of 0, 250,
500 mg/kg bw. The interval between applications was 24 hours.
Concurrently, a positive control group of mice received 2 × 100 mg/kg
bw of cyclophosphamide. The doses were chosen on the basis of the
results of a preliminary test in which groups of 5 mice were orally
dosed with phoxim at 2 × 500 mg/kg bw and 2 × 1 000 mg/kg bw,
respectively; in that test, the 2 × 500 mg/kg treatment was tolerated
with induction of only weak symptoms. Six hours after the second
application, the mice were sacrificed and femur bone marrow smears
were prepared. Erythrocytes, 1 000 per mouse, were counted and the
incidence of cells with a micronucleus was determined, as well as the
ratio of polychromatic erythrocytes to normochromatic erythrocytes.
The incidence of micronucleated polychromatic erythrocytes was
2.3/1 000 in the negative control group, and 1.8/1 000 and 1.0/1 000,
respectively, in the phoxim-treated groups. There were no relevant
differences between the negative control and phoxim-treated group with
respect to the ratio of polychromatic to normochromatic erythrocytes.
The incidence of micronucleated cells in cyclophosphamide-treated
group was 68.2/1 000 and was thus biologically significantly higher
than in the negative control group. Cyclophosphamide also exhibited
a bone marrow depression, with the ratio of polychromatic to
normochromatic erythrocytes showing a biologically relevant alteration
(1 000 : 2 424.2 vs 1 000: 838.3 in the negative control group).
The results provided no indication for a mutagenic potential of
phoxim in the micronucleus test on mouse at the tested doses of
2 × 250 and 2 × 500 mg/kg bw per os. Treatments with phoxim also did
not induce any depression of erythropoiesis (Herbold 1981).
Special Studies on Embryotoxicity and Teratogenicity
Groups of fertilized rats (20 Long Evans FB30 strain/group)
received daily doses of phoxim (technical product stabilized with
10% n-butanol, 86.5% a.i.), administered by gavage in a 0.5% aqueous
Cremophor emulsion, at levels of 0, 30, 100, 300 mg/kg bw from
gestation day 6 through 15 (total of 10 doses). On gestation day 20,
the dams were sacrificed and the foetuses removed by caesarean
section. The foetuses were examined for external, internal and
skeletal malformations. No dam died in any group and no adverse
effects on behaviour patterns and general appearance were observed.
The 300 mg/kg bw dose level had a maternal-toxic effect, resulting in
significantly less weight gain during the treatment period as compared
with the control animals. All animals in the test group showed
comparable average weight gains throughout the period of gestation.
There were no significant differences between the control and
treated groups with respect to the measured parameters: fertilization
quotas, pregnancy quotas, average numbers of implantations, foetuses
and resorptions, average foetus weight, average placenta weight,
average number of stunted foetuses (weighing less than 3 g) and type,
frequency and localization of slight alterations in bone development.
Sex distribution of foetuses was unaffected. Occasional malformations
were seen, these being most frequent in the untreated control group.
They were considered to be spontaneous malformations.
The data provided no indication for embryotoxic or teratogenic
activity in rats at an oral dose of 300 mg/kg bw and below (Machemer
1975).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Neurotoxicity Studies
Hen
Female White Leghorn hens, 2-5/group, about 14 to 18 months
old, each received a single phoxim dose (without antidote)
administered orally at levels of up to and including 50 mg/kg bw or
intraperitoneally at levels of up to and including 37.5 mg/kg bw. The
hens were kept under observations for 6 weeks. The determined LD50
was 37.5 mg/kg bw for both oral and intraperitoneal administration.
In another study, hens 3-14/group, were each given as an antidote
an intraperitoneal injection of 100 mg PAM/kg + 50 mg atropine
sulphate/kg. They then received an oral or intraperitoneal application
of phoxim at dose levels equal to the LD50 and higher. The survivors
were kept under observation for 6 weeks. No histopathological
examinations were carried out. The protection afforded by PAM +
atropine to hens was more evident with intraperitoneal injection of
phoxim than with the oral route. No positive controls were included.
In both tests, without and with antidotal protection, no clinical
signs of neurotoxic damage were seen (Kimmerle 1972).
Groups of hens (10 White Leghorn, 12 to 14 months old/group) were
fed diets containing phoxim from technical product stabilized with 10%
n-butanol, 86.1% a.i. at concentrations of 0, 5 and 10 ppm for 28
days.
During the feeding experiment, the behavioural pattern of all the
hens was within the normal range. In particular, they did not display
any symptoms of neurotoxicity. Treated and untreated controls hens
were not significantly different with respect to food consumption and
body weight gains. Blood acetylcholinesterase activity was not
significantly depressed in the 5 ppm group. In the 10 ppm group, a
marked (30%) depression of blood acetylcholinesterase activity was
measured between day 14 and day 28 of the feeding experiment. The
no-effect level was 5 ppm, equal to 0.32 mg/kg bw/day (Thyssen and
Kimmerle 1973).
Special Studies on Skin and Eye Irritation
A dermal irritation study in rabbits of both sexes ("not of pure
breed") showed that phoxim had only a very slight irritating effect on
both intact and abraded skin (Kimmerle and Solmecke 1970).
In an eye irritation test in rabbits, phoxim caused no eye
irritation (Kimmerle and Solmecke 1970).
Special Studies on Antidotes
Male rats were given single oral doses of phoxim (technical
product, 88.7% a.i.) to determine LD50 values with and without
antidotes. Before the onset of severe poisoning symptoms (5 to
90 minutes), atropine sulphate (50 mg/kg bw), 2-PAM (50 mg/kg bw) or
toxogonin (20) mg/kg bw) was given singly or in combination (atropine
plus PAM-atropine plus toxogonin) by intraperitoneal injection. The
results indicated that, among the tested antidotes, only combinations
of 2-PAM or toxigonin with atropine sulphate had significant antidotal
activity (Solmecke 1971).
Special Studies on Potentiation
Acute oral LD50 values in male Wistar II albino rats were
determined experimentally for phoxim, phenamiphos and their equitoxic
mixture. The observed LD50 value for the equitoxic mixture was
compared with the expected value (calculated assuming an additive
effect). The result was that simultaneous administration of phoxim and
phenamiphos produced a less than additive effect (Thyssen 1976a).
Special Studies on the Recovery of Cholinesterase Activity
Groups of rats (15 male and 15 female, Wistar II, SBF
albinos/group) were given daily doses of phoxim (technical product),
administered by gavage in aqueous Cremophor EL emulsion, at levels of
0, 5, 50 mg/kg bw for 21 days. One-third of the rats were sacrificed
upon termination of treatment, another third were kept under post-
treatment observations for 14 days and then sacrificed and the
remaining third were kept under post-treatment observation for 4
weeks. Determinations of acetylcholinesterase activities in
erythrocytes and plasma were carried out before beginning treatment
and thereafter at weekly intervals until 4 weeks after the end of
administration. Brain acetylcholinesterase activity was measured at
the end of treatment and 2 weeks after termination of treatment.
During the 21-day treatment period and the 4-week post-treatment
observation period, none of the treated rats in any dose group
differed from the control rats with respect to behavioural patterns,
physical appearance and body weight gain. No mortalities were
observed. The following results were obtained in male rats: at
5 mg/kg bw, plasma and erythrocyte cholinesterase activities were
not significantly different from the control group during both the
treatment period and the post-treatment observation period; at
50 mg/kg, plasma and erythrocyte cholinesterase activities were about
65% and 56%, respectively, of the control after 1 week of treatment
and remained similarly depressed during the treatment period; however,
one week after the end of treatment, cholinesterase activity levels
were again found not significantly different from the control.
The following results were obtained in female rats; at 5 mg/kg
bw, both plasma and erythrocyte cholinesterase activities were about
70% of the control after 1 week of treatment, but one week after the
termination of treatment both were in the physiological range; at
50 mg/kg bw, both plasma and erythrocyte cholinesterase activities
were about 37% of the control after 1 week of treatment; one week
after termination of treatment plasma cholinesterase was again normal,
while erythrocyte cholinesterase was 75% of the control and returned
to normal at the end of the 4-week post-treatment observation period.
Brain cholinesterase activity at the end of the treatment period
was 82% and 67% of the control, respectively, for male and female rats
of the 50 mg/kg bw group. However, the values measured 2 weeks after
the end of treatment were not significantly different.
Thus, female rats were more sensitive than males to the
depression of plasma, erythrocyte and brain cholinesterase activities.
The plasma, erythrocyte and brain cholinesterase activity depression
was reversible; plasma cholinesterase returned to normal within
1 week, while erythrocyte and brain cholinesterase were normal in 2-4
weeks.
The acute oral LD50 determinations prior to and after the 21-day
treatment gave comparable values for both male and female rats
(Thyssen 1976b).
Special Studies on the Toxicity of Metabolites
Acute toxicity studies were conducted with alpha-
cyanobenzaldoxime (cyanoxime). The metabolite (and starting material
in the synthesis of phoxim) has a very slight acute toxicity to rats:
LD50 (mg/kg)
Rat, male oral 4 520
Rat, female oral 4 063
Rat, male,female dermal (24 hours) >5 000
From the symptoms of poisoning observed (behavioural disorders,
sedation, breathing disorders) cyanoxime seemingly acts on the central
nervous system.
In acute inhalational toxicity experiments, in which rats were
exposed to dust for 1 hour and 4 hours, respectively, and rats and
mice were exposed to vapour for 6 hours, no toxic effects were
observed.
In tests for skin and eye irritation, the metabolite did not
cause any irritation to the skin of rabbits. In the eye of rabbits,
however, it caused moderate to severe irritation on the conjunctiva
and superficial corrosion on the cornea (Thyssen and Kimmerle 1976).
The glucoside of alpha cyanobenzaldoxime did not cause any toxic
effects when administered as an acute oral dose to female rats; a dose
of 2 500 mg/kg bw was tolerated without inducing any symptoms (Mihail
1979).
PO phoxim, formed as an intermediate product of metabolism, has
an oral LD50 to the mouse of 1 000 mg/kg bw (Vinopal and Fukuto
1970).
Special Studies on Cholinesterase Inhibition by P=O Phoxim
The following 150 values were determined in vitro for the P=O
analogue of phoxim: erythrocyte (bovine), 2.2 × 10-7M; brain (mouse),
6.0 × 10-8M (Vinopal and Fukuto 1970).
Special Studies on Tolerability of Phoxim by Sheep
Groups of sheep (25 freshly shorn Merinos/group) were given
phoxim once, administered by intubation as an aqueous emulsion, at
levels of 0, 25 and 50 mg/kg bw. Each of the treated groups was
divided into two and one half was treated early in the morning and the
other half late in the afternoon. This was done to vary the times of
exposure to sunlight after treatment. The sheep were examined daily
for 10 days after treatment and any abnormalities were recorded.
The sheep treated in the morning remained exposed to strong
sunlight for only 3 hours after treatment. Cloudy conditions prevailed
for the remainder of the observation period, which reduced the
possibility of determining any phototoxic effect.
No signs of systemic toxicity or dermal inflammation were seen in
any of the test animals up to day 10 after treatment (Baldock and
Hopkins 1976).
Acute Toxicity
The results of acute toxicity studies are summarized in Table 1.
Signs of poisoning in mammals after oral administration usually
developed within 5 minutes to 2 hours after dosing in mice, rats,
rabbits, cats and dogs and lasted for 1 to 7 days in survivors. Deaths
occurred after 1 to 3 days. It was only at the highest dose levels
that these symptoms took the form of acute cholinesterase depression
(cramps, trembling, diarrhoea and red tears in rats). The only
symptoms noted in the lower dose levels were lowering of the general
condition and sedative effects. The toxicity signs did not differ in
the tested species. The autopsies of the animals receiving high doses
of the active ingredient showed no changes in the internal organs
(Kimmerle and Solmecke 1970).
Following intraperitoneal injection, the symptoms of poisoning
did not appear more quickly than after oral application. As with oral
treatment, the typical cholinergic symptoms were observed only at very
high doses (Kimmerle 1968a).
Hens are considerably more sensitive to phoxim than mammals
(Kimmerle 1972; Thyssen and Kimmerle 1973).
Table 1. Acute Toxicity of Phoxim
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 2.53-3.38 Flucke 1978 a
ml/kg b.w.
F oral none 2.50-3.50 Flucke 1978 b
ml/kg b.w.
F oral none 2.62-2.77 Flucke 1979 a
ml/kg b.w.
F oral none 3.24-3.60 Flucke 1979 b
ml/kg b.w.
F oral none 2.50-3.18 Flucke 1980
ml/kg b.w.
F oral none 2.96-3.40 Heinmann 1982
ml/kg b.w.
M oral none 2 440 Kimmerle 1968
µl/kg b.w.
F 3 240
µl/kg b.w. "
M inhalation alcohol + >2.06 "
(4-h exp.) Lutrol (1:1) mg/l air
F i.v. none 950
µl/kg b.w. "
M oral none 1 645 Kimmerle and
µl/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Mouse F oral none 1 990 Kimmerle and
µl/kg b.w. Solmecke 1970
M inhalation alcohol + >3.10
(4-h exp.) Lutrol (1:1) mg/l air "
F i.v. physiological 481
solution & mg/kg b.w. "
Cremophor EL
F oral none 3.02-3.48 Mihail 1981
ml/kg b.w.
F oral none 2.98-3.49 Mihail 1982
mg/kg b.w.
Rat M oral none 7 060 Kimmerle 1968
µl/kg b.w.
F 5 800
µl/kg b.w.
M i.p. none 1 775
µl/kg b.w. "
F 1 725
µl/kg b.w.
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M dermal none > 1 000
(7-day exp.) µl/kg b.w.
M inhalation alcohol + >1.99
(4-h exp) Lutrol (1:1) mg/l air
M inhalation alcohol + >2.23 Kimmerle and
(5x4-h exp.) Lutrol (1:1) mg/l air Solmecke 1968
F inhalation >2.22
(4-h, exp. ) mg/l air
M dermal none >1 000 Kimmerle and
(7-day exp.) µl/kg b.w. Solmecke 1970
M inhalation alcohol + >2.55
(4-h. exp.) Lutrol (1:1) mg/l air
inhalation > 0.55
(5x4-h exp.) mg/l air
F inhalation >2.78
(4-h exp.) mg/l air
inhalation > 0. 55
(5x4-h exp.) mg/l air
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Rat (con't) M oral none 1 845
µl/kg b.w.
F 1 680
µl/kg b.w.
M i. p. none 1 645
µl/kg b.w.
F 1 635
µl/kg b.w.
M oral 2 650
mg/kg b.w Solmecke 1971
M oral water & Cremophor EL 2 825 Thyssen 1976 a
mg/kg b.w.
Guinea pig F oral none 350-500 Kimmerle, 1968
µl/kg b.w.
F oral water & Cremophor EL 660 Kimmerle and
mg/kg b.w. Solmecke 1970
Rabbit M&F oral none 250-500 Kimmerle 1968
µl/kg b .w.
F oral water & Cremophor EL 250-375 Kimmerle and
mg/kg b.w. Solmecke 1970
Table 1. (con't)
Species Sex Route Vehicle LD50 Reference
Cat M&F oral none > 1 000 Kimmerle - 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
Dog M&F oral none > 1 000 Kimmerle 1968
µl/kg b.w.
F oral water & Cremophor EL 250-500 Kimmerle and
mg/kg b.w. Solmecke 1970
oral] approx. 37.5 Kimmerle 1972
Chickens i.p.] mg/kg b.w.
Hen oral water emulsion 19.6 Thyssen and
mg/kg b.w. Kimmerle 1973
Short-Term Studies
Rat - dietary
Oral feeding tests were conducted to determine the safety of
using Baythion (phoxim) in place of Cythion (malathion) in the
treatment of stored grain to control insect pests. Baythion and
Cythion were fed to rats at concentrations of 1, 2, 4, 6, 10 or 0 ppm
in the diet. Adult rats and their offspring were fed for a period of
up to 5 months. No adverse effects were noted as a result of these
treatments. It is concluded that 4 ppm Baythion mixed with stored
grains could be used as a substitute for Cythion, with no adverse
effects expected in humans (Lin 1974).
Groups of rats (15 male and 15 female, Wistar strain SPF/group)
were fed diets containing phoxim (technical product stabilized with
10% n-butanol, 87.2% a.i.), at concentrations of 0, 5, 15, 50, 150 and
500 ppm for 3 months. The control group comprised 30 male and 30
female rats. Haematological, clinical chemical and urinalysis
parameters were determined on 5 male and 5 female rats of each dose
group after 4 weeks and 3 months of feeding. The cholinesterase
activity in plasma and in erythrocytes was determined at 1, 4, 8 and
13 weeks after the start of the experiment in 5 male and 5 female rats
of each group. The animals dying during the experiment were subjected
to autopsy. At the end of the study, all the animals were sacrificed
and gross and histopathological examinations were performed.
There were no significant differences between control and treated
animals with respect to behaviour, food and water consumption and body
weight gain. Cholinergic symptoms were sometimes observed in the
500 ppm group only, particularly during the first half of the
experiment. Only the male rats in the 500 ppm group showed
significantly lower body weights. No compound-related mortality
occurred.
The treated animals of all dosage groups did not significantly
differ from the control animals throughout the experiment with respect
to haematological, clinical chemical and urinalysis parameters.
There was an increasing dose-dependent inactivation of both
plasma and erythrocyte cholinesterase in male rats at 50 ppm and
above. In female rats, a dose-dependent depression was noted at 15 ppm
and above in the plasma and at 50 ppm and above in the erythrocytes.
The autopsy of all rats at the end of the feeding experiment
showed no changes of the internal organs attributable to the
inclusion of phoxim in the diet. Male rats in the 500 ppm group had
significantly higher thyroid and liver weights, as compared to
controls. There were no dose-related or significant differences
between any of the dose groups up to 50 ppm and the control animals
with respect to relative organ weights. Higher relative liver weights
in males and females of the 150 ppm and 500 ppm groups were observed.
These enlargements are indicative of an influence on the liver,
although the liver function tests were all normal. Higher relative
kidney weights were observed in the males of the 500 ppm groups and in
the females of the 150 ppm and 500 ppm groups. Heart and adrenal in
the males of the 500 ppm group and thyroid and lung in the females of
500 ppm group had significantly higher relative weights, as compared
to the control animals.
The no-effect level with respect to plasma and erythrocyte
cholinesterase was 15 and 5 ppm, respectively, for male and female
rats, equal to approximately 1.45 and 0.56 mg/kg bw/day (Löser 1970a).
No histopathological change was seen in the tissues examined that
was considered to be compound-related (Vince and Spicer 1971).
Dog - dietary
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 2, 5 and 10 ppm for
3 months. The control group comprised 3 male and 3 female dogs.
Inclusion of the active ingredient in the diet at dose levels up to
10 ppm did not affect the physical appearance, behaviour, food
consumption, growth and mortality rate of male and female dogs.
Cholinesterase activity in the plasma was depressed in both males and
females at the dose level of 2 ppm after 1 month, but not at the end
of the study. The results of the haematological, clinical chemical and
urinalysis determinations showed no significant differences between
the control and treated groups. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1970b).
Groups of beagle dogs (2 males and 2 females/group) were fed
diets containing phoxim (technical product stabilized with 10%
n-butanol, 87.2% a.i.) at concentrations of 0, 50, 200 and 1 000 ppm
for 3 months. The control group comprised 3 male and 3 female dogs.
The dogs in the 1 000 ppm group showed cholinergic symptoms, but no
dogs died in any of the dose groups during the experiment. The
dose level of 1 000 ppm caused weight loss in the female dogs.
Haematological determinations made after 6 weeks and at the end of the
study did not reveal any pathological changes in the dogs of any
groups. Clinical chemical parameters were normal in the 50 and 200 ppm
groups. Increased activities of alkaline phosphatase (ALP) and lactate
dehydrogenase (LDH) were determined in the animals of the 1 000 group.
However, the activities of liver-specific enzymes ornithine carbamyl
transferase (OCT), GPT and sorbitol dehydrogenase (SDH) did not
change. Urine examinations and the kidney function test (urea and
creatinine in serum) were normal in all groups, as were blood sugar
and cholesterol levels. Plasma cholinesterase activity was depressed
at 50 ppm and above, while erythrocyte cholinesterase was first
affected at the 200 ppm level. The organ weights were unaffected by
the administration of phoxim. Macroscopic examination of internal
organs of the treated animals showed no pathological changes
attributable to the inclusion of phoxim in the diet. A no-effect level
was not observed (Löser 1971).
Groups of beagle dogs (4 males and 4 females/group) were fed
diets containing phoxim (91.1% a.i.) at concentrations of 0, 0.3, 1
and 2 ppm for 3 months. No deaths occurred during the study. There
were no significant differences between the control and treated dogs
with respect to behavioural pattern, physical appearance, food
consumption, body weight gain, reflex, eye examinations, haematology,
clinical chemistry, thromboplastin time and urinalysis, as determined
at 0, 5 and 12 weeks.
The no-effect level for plasma cholinesterase was 0.3 ppm for
both males and females. Erythrocyte cholinesterase activity was not
affected in any of the treated groups. Autopsy and histopathological
examinations performed on all the animals provided no indication of
treatment-related alterations (Mürmann and Luchaus 1973).
Groups of beagle dogs (4 males and 4 females/group) were fed
phoxim in the diet (technical product, stabilized with 10% n-butanol,
86.5 a.i.) at the following levels for 2 years: control group - 0 ppm;
group I - males, 0.3 ppm and females 0.1 ppm from week 83; group II -
15 ppm; group III- 750 ppm. Daily examinations were made for physical
appearance and behavioural pattern. Intake of food and water was also
checked and noted daily. Body weights were recorded weekly in the
first year and thereafter at 14-day intervals. At periodical intervals
(14, 26, 39, 52, 64, 78, 92 and 104 weeks) reflex testing,
ophthalmoscopy, haematology, clinical-chemistry and urinalyses were
performed. Plasma and erythrocyte cholinesterase determinations at
4, 7, 10, 15, 27, 39, 51, 64, 77, 91 and 103 weeks and brain
cholinesterase at the conclusion of the study were performed. At the
end of the 104 weeks of dietary administration, all the dogs were
sacrificed and gross and histopathological examinations of tissues and
organs were performed.
There was no mortality over the course of the study in any group.
Treatment did not affect the behavioural patterns of any of the dogs.
The female dogs of each treated group and the male dogs of group I did
not differ in physical appearance from the controls. Male dogs in
group II and group III had a poor state of nutrition. The male dogs of
group III had a dull and ungroomed coat in the second treatment year.
Food consumption levels were not affected by treatment. The time taken
for the food ration to be consumed by the dogs was slightly longer in
group II and considerably longer in group III. Average body weight
curves, especially for the males, were lower than those for the
control group and were dose related. However, only the body weights of
group III rats were statistically significantly lower than those of
control rats. A marked decrease of body weight was evident in male
dogs of group III after 72 weeks. The reflexes were normal in all dogs
at all times tested. The ophthalmoscopic examinations of the eyes
provided no indication of any treatment-related variations from the
physiological norm in either the transparent media or on the fundus
oculi. The average total intake of phoxim per dog was 0.068 g, 3.60 g
and 181.08 g, respectively for group I, group II and group III. Data
from haematological tests and urinalyses were normal. Plasma and
erythrocyte cholinesterase activities were not affected in males of
group I, i.e. at 0.3 ppm. In females of group I, plasma cholinesterase
activity was seen to be depressed to a level 26% below the control
value in week 77. As this result was confirmed by repeated
measurements, the concentration of phoxim administered to the female
dogs of group I was reduced from 0.3 to 0.1 ppm from week 83. The
later determinations in week 91 and 103 showed that plasma
cholinesterase activities in group I females were again equal to the
control values. At the 15 ppm level and above, plasma and erythrocyte
cholinesterase activities were markedly depressed, and were dose
related, from the first determinations and remained relatively
constant over the entire study. Brain cholinesterase activity was not
depressed at dietary concentrations of up to and including 15 ppm,
being depressed 35-40% at 750 ppm level. Clinical chemistry values
(glucose, urea, creatinine, total protein, GOT, bilirubin) showed no
differences between treated dogs and controls. Statistically
significant values, increasingly higher than controls, were observed
for GPT (from week 52) and ALP (from week 14) in the dogs of group III
(less marked in the females). In the group II dogs and in the males of
group I, ALP activity values were statistically significantly higher
than in the controls; they were at the same level throughout the
second half of the experiment. In the controls, the physiological
age-related reduction of ALP activity was observed during the course
of the study. In the group III dogs the average serum cholesterol
level was lower than that measured in the control dogs at every
investigation time until the end of the feeding experiment.
At gross pathological examination, the livers of 4 female dogs
and one male dog of group III had a darker (dark brown to grey) colour
than those of the control dogs and the dogs of the other two treated
groups. Furthermore, the livers of some dogs in group III were seen to
have a marked lobular pattern. There were no noteworthy differences
between the treated dogs of all groups and the control dogs with
respect to absolute and relative organ weights, with the exception of
liver and thyroid. The absolute and relative weights of the liver in
the male and female dogs of group III were statistically significantly
higher than the weight of the liver in the control dogs. There was
also a slight increase in the relative weight of the liver in the male
dogs of group II, but this difference was not statistically
significant. The absolute and relative weights of thyroid in the
female dogs of group III were greater than those in the control dogs,
but this difference was not statistically significant. Hepatocyte
alterations were seen at histopathological examination in all the dogs
of group III. The hepatocytes were dilated, the plasma had a light
glassy appearance and was less structured than in the control dogs or
the dogs of the other two dietary concentration groups.
The increase in the absolute and relative thyroid weights in the
female dogs of group III is not associated with histopathological
alterations, so that it is doubtful whether this increase was compound
related. Some other alterations of varying degree were observed in
other tissues of both the control group and the treated groups, so
they were not considered treatment related.
The study indicated 0.3 ppm in the diet, equal to 0.068 mg/kg
bw/day, and 0.1 ppm, respectively, for male and female dogs, as no-
effect levels with respect to plasma and erythrocyte cholinesterase
(Hoffmann and Gröning 1977).
Monkey - dietary
Rhesus monkeys (Macaca mulatta) (20 males and 20 females/group)
were administered by gavage daily doses of phoxim (technical product
stabilized with 10% n-butanol, 83.8% a.i.) dissolved in maize oil at
levels of 0, 0.2, 0.65 and 2.00 mg/kg, 6 days per week for 6 months.
Throughout the study the monkeys tolerated the administration of
phoxim well and remained in good health. There was no evidence of any
cholinergic effects despite the observed reduction of plasma
cholinesterase activity. Average body weights and urinalyses were not
significantly different between the control and treated groups, There
were some differences between the control and treated groups with
respect to some clinical chemistry values (blood urea nitrogen,
glucose, total bilirubin, GOT, calcium) and some haematology values
(packed cell volume, erythrocyte count). However, these fluctuations
were, for the most part, within the normal limits for the colony of
monkeys, and in no case were dose related.
Erythrocyte cholinesterase activity was slightly reduced at
2.0 mg/kg, but plasma cholinesterase activity was reduced at all three
dose levels tested. Expressed as percent of the control groups, the
remaining activity after six months was, respectively, for each dosage
level, 62%, 43% and 34%. A no-effect level was not determined. The
microscopic examination of liver biopsy material obtained prior to the
administration of phoxim and after six months of administration
provided no evidence of an effect of phoxim on liver morphology
(Coulston et al 1978).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females/
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to phoxim (technical product stabilized with 101
n-butanol, 83.8% a.i.; as an emulsion in water and Cremophor EL) at
dose levels of 0, 0.5 and 15 mg/kg bw/day, 7 hours per day, 5 days per
week for a total of 15 applications in a 21-day period.
The treated sites (5 × 5 cm) on the flank and back of the
animals, not covered with bandages, were washed with water and soap at
the end of each daily exposure period.
Behaviour and general aspect were normal for all animals. No
treatment-related deaths occurred. There were no adverse effects on
the pre- and post-treatment values of body weight or on terminal
haematological, clinical chemical and urinalysis parameters, Erythema
was not observed in any animals with intact skin. A slight compound-
related increase in time necessary for erythema to disappear was noted
in treated animals with abraded skin. Skin-fold thickness was
comparable between the control and treated groups. There were no
significant differences between control and treated groups with
respect to both absolute and relative weights of major organs.
Histopathological examinations were performed on heart, lung, liver,
spleen, kidney, adrenals, testis, epididymis, ovary, uterus and
thyroid from control and 15 mg/kg bw groups. Some alterations occurred
in both control and treated animals and were not considered compound
related. Slight increases of degree of cell infiltrations and of
incidence of epithelium thickening were observed on the treated skin
of animals at 15 mg/kg bw with abraded skin, as compared to control
animals. The same changes were not observed in animals with intact
skin. These findings were considered the result of repeated mechanical
stimuli. Plasma and erythrocyte cholinesterase activities measured
after exposures 8 and 15 were significantly depressed (ca. 60%) at
15 mg/kg bw/day. Brain cholinesterase activity was slightly (23%)
depressed only in male rabbits at 15 mg/kg bw/day (Flucke and Schilde
1978).
Long-Term Studies
Rat
Groups of rats (50 males and 50 females, SPF Wistar strain/group)
were fed a diet containing phoxim (technical product stabilized with
10% n-butanol, 85.7% a.i.) at concentrations of 0, 15, 75 and 375 ppm
(calculated as pure active ingredient) for 24 months. The control
group included 100 males and 100 females. In addition, 5 male and
5 female rats were used in each group for clinical laboratory
examinations, which were carried out at 3, 6 and 12 months. At the end
of the study, 10 males and 10 females were used for these
examinations.
There were no differences in appearance and behaviour between the
treated and control animals during the study. In the 375 ppm group
male rats had a lower mean daily food intake and females showed
significantly less weight increase than control animals, mainly in the
second half of the study. Mortality after 1 and 2 years was not
increased in any treated groups. The overall mortality rate for
treated and control groups at the end of the study was in the range of
24-26% for male rats and 14-21% for female rats. There were no
significant differences between the control and treated groups with
respect to haematological examinations at 3, 6, 12 and 24 months.
There were no dose-related differences between control and treated
groups with respect to alkaline phosphatase, GOT, GPT, glutamic
dehydrogenase (GLDH, determined only at the end of the study), serum
bilirubin and total serum protein, as determined at 3, 6, 12 and 24
months. Urinalyses, serum urea and creatinine, and urine protein
showed no relevant differences between the control and treated groups
after 3, 6, 12 and 24 months. Urine protein was sporadically increased
in the 75 ppm group. Mean values for blood sugar and cholesterol
showed some variations between the control and treated groups that
were not dose related. Cholinesterase activity in erythrocytes and
plasma was not significantly inhibited (less than 20%) in the male and
female rats in the 15 ppm group. In the 75 ppm and 375 ppm groups, a
marked and dose-related inhibition of the enzyme in plasma (16-42% in
males, 41-64% in females) and in the erythrocytes (24-54% in males,
25-49% in females) throughout the study was observed. Brain
cholinesterase activity (measured at the end of the trial) was
slightly inhibited (18% in males, 23% in females) only in the 375 ppm
group. The autopsies on rats dying during the study (106) and those
sacrificed at the end of the treatment did not reveal any treatment-
related damage. There was a significant, but not dose related,
increase in the relative liver weight of male rats of all dose groups
as compared to controls. There was also an increase in relative
weights of spleen, lungs and heart of female rats of the 375 ppm
group.
Histopathological examinations performed on some 31 tissues of
all the rats surviving the 2-year treatment did not reveal any
treatment-related alterations. The differences in relative organ
weights found were regarded as random, as they were not dose related,
and there was no histopathological correlation. The analysis of tumour
data according to the site, type and incidence of both benign and
malignant tumours provided no indications suggestive of carcinogenic
activity of phoxim in the rat.
The no-effect level for both plasma and erythrocyte
cholinesterase was 15 ppm (Bombhard and Löser 1977).
COMMENTS
Phoxim has been evaluated for the first time by the JMPR. It has
a mild acute toxicity, as confirmed following the various routes of
application. No sex-dependent differences were observed. In the
longer-term experiments, however, females tended to display higher
sensitivity. The acute experiments revealed a species-dependent
toxicity. There was no available information on specification of the
technical product. Phoxim is readily and almost completely absorbed.
It is rapidly excreted in the rat, and there is no evidence of
bio-accumulation. The metabolic pathway in mammals follows typical
steps, such as hydrolysis, desulphuration and conjugation. Possible
metabolites have a slight to moderate acute oral toxicity.
A delayed neurotoxicity test was negative but the test was
considered to be unacceptable. No-effect levels were determined with
respect to reproduction and teratogenicity. Mutagenicity and
carcinogenicity studies were negative.
In a two-year dog study, plasma and erythrocyte cholinesterase
were more sensitive than brain cholinesterase. A 90-day dog study
provided a no-effect level bridging the large differences between
effect and no-effect levels in the 2-year dog study.
An increase in relative liver weight, not dose related, was also
observed in the 2-year rat study at all dosage levels, without
histopathological correlation. No observations in humans were
available. The available data permitted the determination of no-effect
levels in two mammalian species. Because of the unavailability of an
acceptable delayed neurotoxicity study, only a temporary ADI was
allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.56 mg/kg bw
Dog : 2 ppm in the diet, equivalent to 0.05 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.005 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
An appropriate neurotoxicity study in hens.
Desirable
1. Observations in humans (particularly effects on cholinesterases),
2. Type and content of impurities in the technical product.
REFERENCES
Baldock, C. and Hopkins, T. Bay 9053. Safety in sheep. Report from
1976 Bayer, Australia, submitted to the World Health Organization
by Bayer, Australia, (Unpublished)
Bombhard, E. and Loser, E. SRA 7502. Chronic toxicity studies in rats
1977 (feeding experiments over 2 years). Report (No. 7042) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Coulston, F., Griffin, T., Talley, W. and Iatropoulos, M.J. A safety
1978 evaluation study of phoxim in Rhesus monkeys. Final Report
from Institute of Comparative and Human Toxicology, Albany
Medical College, Albany, New York, and International Center
of Environmental Safety, Albany Medical College, Holloman,
New Mexico, submitted to the World Health Organization by
Bayer F.R.G. (Unpublished)
Daniel, J.W., Swanson, S. and McLean, J. Phoxim: pharmacokinetics and
1978a biotransformation in the rat. Report (No. 78/BAG 5/194) from
Life Science Research, Stock, Essex, England, submitted to
the World Health Organization by Bayer, F.R.G.(Unpublished)
Daniel, J.W., McLean, J. and Pringuer, M. Phoxim- biotransformation in
1978b the rat. Report (No. 78/BAG 6/317) from Life Science
Research, Stock, Essex, England, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502: Bestimmung der Akuten toxzität (LD50). Report
1978a from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1978b SRA 7502 VL: bestimmung der akuten toxzitat (LD50) Report
from institut fur toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. and Schilde, B. Volaton VL. Subakuter kutaner toxizitat-
1978 versuch an kaninchen. Report No. 8034 from Institut für
toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Flucke, W. SRA 7502 VL: bestimmung der akuten toxizitat (LD50).
1979a Report from Institut fur toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1979b SRA 7502 VL (volaton): bestimmung der akuten toxizitat
(LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Flucke, W. SRA 7502 Volaton VL: bestimmung der akuten toxizitat
1980 (LD50). Report from Institut fur toxicologie, Bayer,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Heinmann, K.G. Volaton VL: bestimmung der akuten toxizitat (LD50).
1982 Report from Institut für toxicologie, Bayer, submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
Herbold, B. SRA 7502 (phoxim). Micronucleu test on mouse to evaluate
1981 SRA 7502 for mutagenic potential. Report (No. 9891) from
Institut für toxicologie, Bayer, F.R.G. submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Hoffman, K. and Gröning, P. SRA 7502. Chronic toxicity study on dogs.
1977 two-year feeding experiment. Report (No. 6865) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Iakushko, V.E. Effect of Valexon on NADPH-dependent oxidation in
1978 endoplasmic reticulum of rat liver. IKR Biokim. Zh., 50
(6):727-720 (in Russian). Pest. Abstracts. 12(5): 292
(1979). Abstract No. 79.1193).
Kimmerle, G. BAY 77488. Toxicological studies. Report (No. 1067) from
1968 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Kimmerle, G. and Solmecke, B. BAY 77488. Toxicological studies. Report
1970 (no. 2235) from Institute für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
1972 Phoxim: neurotoxicity tests on hens. Report from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (unpublished)
Kuz'minskaia, V.A. and Veremenko, L.M. Effect of Valexon on succinate
1978 dehydrogenase and cytochrome oxidase activities in rat
liver. UKr. Biokhim. Zh., 50(6):711-713 (in Russian),
Pest. Abstracts 12(5): 292 (1979) (Abstract No. 79-1192).
Kuz'minskaia, V.A., Alekhina, S.M. and Bersan, L.V. Dynamic changes in
1979 the blood enzyme activities in albino rats following the
administration of Valexon. Gig. Sanit. 44(7):78-79 (in
Russian), Pest. Abstract, 13:31 (1980), (Abstract No.
80-0159).
Lin, T. Toxicological study of baythion and cythion on rat feeding
1974 test. Nung Yeh Yen Chin (J. Taiwan Agric. Res.), 23(2):
149-154, Pest. Abstracts 9:427 (1976), (Abstract No.
76.1543).
Löser, E. BAY 77488. Subchronic toxicological studies on rats. Three
1970a month feeding experiment. Report (No. 2389) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization, by Bayer, F.R.G. (Unpublished)
1970b BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiments. Report (No. 2418) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1971 BAY 77488. Subchronic toxicological studies on dogs. Three-
month feeding experiment. Report (No. 2579) from Institut
für toxicologie, Bayer, submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
1979 SRA 7502. Multigeneration reproduction study on rats. Report
(No. 8447) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G. (with
appended Report on the histopathological examinations by
J.E. Close and J.W. May, Hazleton Laboratories, Harrogate,
U.K.).(Unpublished)
Machemer, L. SRA 7502 (phoxim). Dominant lethal test on male mouse to
1974 evaluate SRA 7502 for mutagenic potential. Report (No. 4943)
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer F.R.G. (Unpublished)
1975 SRA 7502 (phoxim). Studies for embryotoxic and teratogenic
effects on rats following oral administration. Report (No.
5331) from Institut für toxicologie, Bayer, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Mihail, F. Cyanoximsäureglucosid, Ratte p.o. Report from Institut für
1979 toxicologie, Bayer, submitted to the World Health
Organization by Bayer F.R.G. (Unpublished)
1981 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1982 Volaton VL. Bestimmung der akuten toxizitat (LD50). Report
from Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Mürmann, P. and Luckhaus, G. SRA 7502. Subchronic toxicity study on
1973 dogs. Report (No. 4136) from Institut für toxicologie,
Bayer, submitted to the World Health Organization by Bayer,
F.R.G. (Unpublished)
Oesch, F. Ames test for Volaton (phoxim). Report from
1977 pharmakologisches Institut der Universität, Mainz, F.R.G.
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, T. Phoxim. Mutagenicity test on
1978 bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Solmecke, B. BAY 77488 (SRA 7502) antidotes study. Report from
1971 Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. and Kimmerle, G. SRA 7502. Toxicological studies on hens.
1973 Report No. 4236 from Institut für toxicologie, Bayer,
submitted to the World Health Organization by Bayer F.R.G.
(Unpublished)
1976 Cyanoxim (hydroxyiminophenyl-acetonitril)
Geverbetoxicologische Untersuchungen. Report (No. 6392) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976a oral toxicity when administered simultaneously with
fensulfothion, isofenphos or phoxim. Report (No. 5958) from
Institut für toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
1976b SRA 7502. Subacute oral cumulation study on rats. Report
(No. 5954) from Institut für toxicologie, Bayer, submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Vince, A.A. and Spicer, E.J.F. Pathology report on BAY 77488,
1971 Subchronic toxicological studies in rats. 3-month feeding
experiment Report (No. 4257/71/415). (Addendum to the Report
No. 2389/Bayer) from Huntingdon Research Centre, Huntingdon,
England, submitted to the World Health Organization by
Bayer, F.R.G. (Unpublished)
Vinopal, J.H. and Fukuto, T.R. Selective toxicity of phoxim
1970 (phenylglyoxylonitrile oxime O,O-diethyl phosphorothioate).
Pest. Biochem. Physiol. 1:44-60.
