
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
PROPAMOCARB
Identity
Chemical: (IUPAC and C.A.): Propyl 3 -
(dimethylaminopropyl)
carbamate hydrochloride
Synonyms: PrevicurRN, Prevex, Filex, Banol, Dynone N,
Schering Code No. SN 66752 and ZK 66752
Structural formula: CH3 O
\ "
N - (CH2)3 - NH - C - O - C3H7 HCL
/
CH3
Molecular formula: C9H21 Cl N2 O2
Other information on identity and properties
Molecular weight: 224.7
State: A colourless crystalline solid (technical
propamocarb hydrochloride) with faint
aromatic odour and strong hygroscopic
properties
Melting point: 45 - 55°C
Vapour pressure: 6 × 10-6 Torr at 25°C
Dissociation
constant (pKa): 9.1
Octanol/water
partition coefficient: 2.5 × 10-3
Solubility (at 25°C): >700 g/l in water; >500 g/l in methanol;
>430 g/l in methylene chloride; >300 g/l in
isopropanol; 23 g/l in ethyl acetate;
<0.1 g/l in toluene and hexane
Stability: Propamocarb is very stable to hydrolysis.
Even at pH 14 the half-life is five days. At
pH 9, the half-life is calculated to be
1.3 × 103 years, increasing at pH 5 to
1.3 × 107 years. The product is stable at
55°C for over two years, and to light, even
on the addition of acetone.
Specification of The technical material has a purity in excess
technical material: of 95 percent with the main impurities being
N, N - dimethyl - 1 - 3 - diaminopropane
dihydrochloride (max. 5 percent), N, N, -
bis - (3 - dimethylaminopropyl) urea
dihydrochloride (max. 2%) and solvents
(max. 1%).
Note: Unless otherwise indicated, the studies reviewed in this
monograph were conducted with Previcur N, an aqueous
formulation of technical propamocarb hydrochloride
containing approximately 65 - 70% of the active ingredient
(a.i.). For the purpose of identification a 70% aqueous
formulation of propamocarb hydrochloride as used in the text
of the monograph is synonymous with Previcur N. Unless
otherwise specified, the dosages used in the studies are
expressed in terms of the formulation.
Effects on Enzyme and Other Biochemical Parameters
In vitro
Plasma specimens from dogs and rats were separately incubated
in vitro with technical propamocarb hydrochloride or a 70% aqueous
formulation of propamocarb hydrochloride at concentrations, expressed
as the technical material, ranging from 0.925 to 74 mg/ml plasma.
Significant dose-related inhibition (22 - 60%) of both rat and dog
plasma cholinesterase was noted following incubation with either
technical or formulated propamocarb hydrochloride at plasma
concentrations of 37 mg propamocarb hydrochloride/ml and above. This
concentration corresponded to approximately 2.2 g/kg b.w. in vivo
provided that the total amount of active ingredient would be available
in the plasma and that the plasma volume is about 6% of the body
weight (Kopp, et al.,1981b).
In Vivo
Rat
Groups of 10 male and 10 female rats were intubated with
propamocarb hydrochloride as a 70% aqueous formulation at 0 or 3000 mg
a.i/kg b.w./day for 11 days. Compound-related mortality occurred in
three males and seven females of the treated group on days 7 and 8,
and in one treated female on day 9 of treatment. Assay of whole blood
cholinesterase on day 7 at 30 minutes, 1 hour, 2 hours and 3 hours
after dosing, and of brain cholinesterase on day 11 at 30 minutes
post-treatment indicated no significant compound-related inhibition of
either tissue. (Hunter, et al., 1978).
Dog
Groups of one male and two female beagles were given, by gavage,
a single dose of a 70% aqueous formulation of propamocarb
hydrochloride at 0 or 674 mg a.i./kg b.w. One male and one female of
the treated groups died 42 minutes and 35 minutes after treatment,
respectively. Mean activity of erythrocyte cholinesterase in the
treated group was inhibited (>20%) 22-36 minutes after dosing, as
compared to pre-treatment value or to concurrent control level.
Complete recovery of the enzyme activity seemed to be evident at 34-56
minutes post treatment. Plasma cholinesterase was not inhibited at
either of the two post-dosing sampling periods. Details of the study,
such as the assay method for tissue cholinesterase and the exact time
of sampling of blood from individual treated animals, were not
available. (Kopp, et al., 1981b).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Metabolism
Rat
After a single oral dose of 0.5 mg/kg b.w. of 14C-propamocarb
hydrochloride*, female rats excreted approximately 90% of the
administered radioactivity within 24 hours of dosing via the urine
(87%), faeces (2.5%) and expired air (<0.3%). Similar rapid
elimination of 14C-propamocarb hydrochloride by female rats was also
observed following oral administration of 0.5 mg 14C-propamocarb
hydrochloride/kg b.w./day for 21 days. During the 21-day dosing
period, an average of 83% and 3.2% of the daily administered dose was
recovered respectively in the urine and the faeces. At sacrifice,
24 hours after acute or subacute oral dosing, total 14C residues
detected in the 19 tissues analyzed including, among others liver,
kidney, lung, spleen, heart, adipose tissue, skin and skeletal muscle
(but excluding the alimentary tract) accounted for 1.7% of the single
dose and approximately 0.3% of the total dose given over a 21 day
dosing period (equivalent to approximately 7% of the last daily dose).
Female rats with "Acute biliary - and urethra fistula" treated with a
single intraduodenal dose of 0.5 mg/kg b.w. of 14C-propamocarb
hydrochloride eliminated 81% of the administered radio-activity in the
urine as early as six hours after dosing. By 24 hours post-
administration, a total of 92% of the 14C was recovered in the urine
as compared to only 1.8% in the bile (Kopp et al. 1979).
* 14C-propamocarb hydrochloride labelled at the 1-propyl position
was used in all of the available rat metabolism studies.
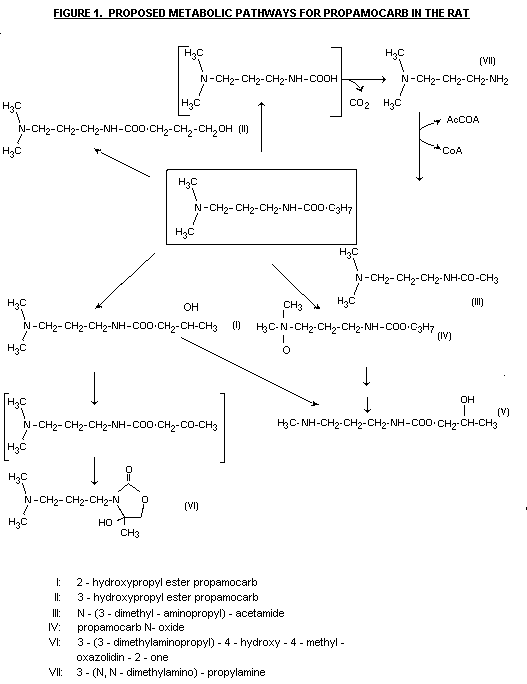
In female rats treated with a single oral dose of 10 or 100 mg
14C-propamocarb hydrochloride/kg b.w. ten to 19 urinary metabolites
were detected. The major metabolites identified were 2-hydroxypropyl
ester propamocarb (15-32% of the radioactivity recovered in the urine)
and N - (3 -dimethylaminopropyl) -acetamide (12 - 21%). Small amounts
(approx 4%) of 3-hydroxypropyl ester propamocarb were also
identifiable but only in rats given 100 mg/kg b.w. The unchanged
parent compound accounted for 3-16% of the C14 detected in the urine.
The data suggested that the relative amounts of major and minor
metabolites found and the presence or absence of certain minor
metabolites are related to dosage (Brühl & Celorio, 1982). A further
study by the same authors in female rats orally treated with 50 mg
14C-propamocarb hydrochloride/kg b.w./day for ten days indicated the
presence of at least 30 urinary metabolites. 2-Hydroxypropyl ester
propamocarb (26% of the 14C recovered in the urine),3-3-
dimethylaminopropyl)-4-hydroxy-e-methyl-oxazolidin - 2 - one (14%) and
propamocarb N-oxide (13%) were the major urinary metabolites
identified. Other compounds found in amounts not exceeding 5% each
included the parent compound, 3-hydroxypropyl ester propamocarb,
mono-demethyl 2-hydroxypropyl ester propamocarb and 3-(N,
N-dimethylamino) -propylamine. All of the identified components
together accounted for 66% of the radioactivity present in the urine.
N - (3-dimethylaminopropyl)-acetamide, identified previously as a
urinary metabolite in the rat (Brühl and Celorio 1982), was not found
in the present study. (Brühl & Celorio 1984). Fig.1 presents the
proposed metabolic pathways for propamocarb in the rat (Brühl &
Celorio 1982; Brühl & Celorio 1984).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of 25 male and 25 female (Wistar derived, SPF) were fed a
70% aqueous formulation of propamocarb hydrochloride in the diet at 0,
40, 200 or 1000 ppm a.i. for 100 days prior to mating to initiate a
three generation (two litters/generation) reproduction study.
Weanlings from the second litters were selected to become parents of
the next generation. After the second mating trial in each generation,
the dams in each dosage group were divided into four subgroups of ten
and fifteen (F0 and F1 generations or of approximately equal size
(F2 generation). One subgroup was allowed to litter and rear their
young until 21 days post partum. The other subgroup was sacrificed on
day 19 of gestation for examination of uterine contents. Foetuses were
evaluated for external, skeletal and visceral abnormalities.
In the parental generations, no compound-related mortalities or
overt signs of toxicity were observed. Frequently, a slight decrease
(<10%), not dose-related, in food consumption occurred in the treated
groups during the premating period of each generation. Parental body
weight, pregnancy rate, median pre-coital time, mating performance and
duration of gestation period were comparable to control values.
 Over the three generations, there were no treatment-related
effects on litter size on days 1, 4, 12 and 21; pup weight on days 1
and 4; sex ratio of pups and histopathological findings of F3b
weanlings. Incidence of dams with total litter loss was increased at
200 ppm only in F1b litters. Pup weight at 1000 ppm was decreased on
day 21 in F1a litters and on day 12 in F1b litters. An increase in
spleen weight was note in F3b weanlings at 1000 ppm. Examination of
pre-weaning pups from each generation for rate of physical development
(hair growth, incisor eruption, opening of the auditory canal and the
eye) and of selected F1b and F2b animals for pupillary reflex and
startle response at about 28 days of age indicated no abnormal
findings attributable to the compound.
Teratological investigation of F1b, F2b, and F3b litters
revealed no significant differences between control and treated groups
with respect to number of implantations or corpora lutea, litter size,
late resorptions of foetal weight. F2b litters at 1000 ppm exhibited
an increase in early resorptions and pre-implantation loss. Increased
renal pelvic cavitation, unilaterally or bilaterally, was found in
2/67 foetuses from 2/12 dams at 1000 ppm of the F3b litters, as
compared to an incidence of zero in 37 concurrent control foetuses
from a total of 9 dams examined for visceral abnormalities. The study
demonstrated a dietary level of 200 ppm to be without adverse effect
on the reproductive parameters examined. There was no clear evidence
for teratogenic potential at any dose tested. (Huntingdon Research
Centre, 1983).
Special Studies on Teratogenicity (Also see under "Special Studies
on Reproduction")
Rat
Groups of 25 mated female rats (Wistar - Han-Schering, SPF) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.1, 0.3, 1.0 or 3.0 ml/kg b.w./days from days 6
through day 19 of pregnancy. The dams were sacrificed on day 20
post-coitum and foetuses were removed for gross external, visceral and
skeletal examination. Five dams at 3 ml/kg b.w. and one dam at
1.0 ml/kg b.w. died, but with the exception of 1/5 dams of the top
dosage group showing severe pulmonary oedema, no abnormal findings
were reportedly seen at autopsy. In the top dosage group, a majority
of the dams exhibited toxic signs. Additionally, maternal weight gain
between days 16 and 20 post-coitum was depressed and uterine weight
was decreased. Also at the same dosage level, adverse effects were
observed on the mean number of early resorptions or live foetuses,
mean total litter size, incidence of pregnant dams with resorptions of
entire litters, post-implantation loss and foetal weight. There were
no significant differences between control and treated groups in mean
number of corpora lutea or implantations, pre-implantation loss and
sex ratio. Foetuses at both 1 and 3 ml/kg b.w. showed an increase
(dose-related) in the incidence of retarded ossification. An elevated
frequency of foetuses with 14 ribs was seen at 0.3 ml/kg b.w. and
above, but a dose-response pattern was not evident.
Based on the data, the compound appeared to retard foetal
development at and above 1 ml/kg b.w. (approximately equivalent to
700 mg a.i./kg b.w.). A dosage level of 3 ml/kg b.w. (approximately
equivalent to 2100 mg a.i./kg b.w.) was toxic to both mothers and
foetuses. There was however no clear evidence of teratogenic activity
at any level under the conditions of the experiment (Poggel, et al.,
1981).
Rabbit
Groups of 15 to 20 mated female rabbits (New Zealand White) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.2, 0.4 or 0.8 ml/kg b.w./day from days 6 through 18
of gestation. The does were sacrificed on day 28 of pregnancy and
uterine contents were examined. Live young were evaluated for external
malformation and incubated for 24 hours prior to sacrifice for
examination for skeletal and visceral abnormalities. Mortality
occurred in control and treated groups, but only two deaths at
0.8 ml/kg b.w. appeared to be treatment related. The total number of
does showing evidence of pregnancy (including those showing abortions
or total resorption) and surviving at termination was 12, 12, 11, 10
respectively at 0, 0.2, 0.4, 0.8 ml/kg b.w. Does at and above
0.4 ml/kg b.w. showed weight loss on gestation day 16 and growth
depression on day 28. Pregnancy rate, mean litter size, mean number of
early resorptions, incidence of pregnant does with early resorptions,
incidence of pregnant does with entire litters resorbed, post-
implantation loss and foetal survival rate after six- and 24-hour
incubation were all adversely affected in the top dosage group Foetal
weight was depressed at both 0.4 and 0.8 ml/kg b.w. Cleft palate, not
observed in any of the 71 foetuses from ten concurrent control litters
or in any of a combined total of 133 foetuses from 18 litters of the
two lower dosage groups, was observed in 1/21 foetuses from five
litters examined at 0.8 ml/kg b.w. Historical control data included in
the report on the incidence of cleft palate in foetuses of New Zealand
White rabbits were of limited value as a basis of comparison due to
the absence of the data in question using litter as the sampling unit
and lack of information on the time frame over which the data were
obtained. There were no significant differences between control and
treated groups in the other monitored parameters including the number
of implantations or corpora lutea, pre-implantation loss and visceral
or skeletal malformations of pups. The study appeared to indicate the
teratogenic no-effect level to be at least 0.4 ml/kg b.w. (equivalent
approximately to 280 mg/ai/kg b.w.). Maternal and foetal toxicity were
evident at this level but not at 0.2 ml/kg b.w. (Huntingdon Research
Centre, 1981).
Groups of 18 to 21 mated female rabbits (New Zealand White) were
treated orally with propamocarb hydrochloride as a 70% aqueous
formulation in water at 0, 0.02, 0.06, 0.2, 0.4 or 0.8 ml/kg b.w./day
from day 6 to day 18 of gestation. All does were sacrificed on day
28 post coitum and uterine contents were examined. Foetuses were
evaluated for external, skeletal and visceral abnormalities. Mortality
was not affected by treatment. The number of pregnant does with live
foetuses surviving on day 28 was 11, 14, 10, 11, 7, 13 respectively at
0, 0.02, 0.06, 0.2, 0.4, 0.8 ml/kg b.w. Pregnant does at 0.8 ml/kg
b.w. showed weight loss on day 18 and a decrease in weight gain on day
28. The mean number of dead foetuses per pregnant doe and the
incidence of pregnant does with dead foetuses were increased at
0.4 ml/kg b.w. only. The mean number of early resorptions was elevated
and the mean foetal weight was reduced at the top dosage level. Post-
implantation loss was increased at both 0.4 and 0.8 ml/kg b.w. Other
measured parameters, including number of corpora lutea or
implantations, litter size, pre-implantation loss and gross, skeletal
and visceral findings of foetuses, were not significantly different
from controls. Cleft palate, observed in a single foetus of the top
dosage group (0.8 ml/kg b.w.) in a previous rabbit study (Anon.,
1981), was not seen in any of the foetuses in the present study. The
teratogenic no-effect level was demonstrated at 0.8 ml/kg b.w.
(equivalent approximately to 560 mg a.i/kg b.w.) (Schering AG, 1981).
Special Studies on Mutagenicity
Mutagenicity studies for propamocarb hydrochloride as a 70%
aqueous formulation are summarized in Table 1.
Food consumption was marginally increased in males at 500 ppm
between weeks 79 and 104. Body weight, general appearance and
behaviour were unaffected. No gross pathological changes attributable
to ingestion of the compound were evident. Histopathological
examination of a wide range of tissues from all animals of control and
top dosage groups, plus any tissue showing macroscopic abnormality
suggestive of neoplasia revealed no treatment-related findings. There
was no significant difference between control and treated groups in
incidence of any particular type of tumour. The most frequently
observed tumours were liver tumours in males, lung tumours in both
sexes and lymphoreticular tumours in females. Over 65% of the
concurrent controls (both sexes) had spontaneous tumours. Under the
condition of the experiment, the test material was not carcinogenic
(Hunter, et al., 1983a).
Special Studies on Eye and Skin Irritation
In primary eye irritation studies with New Zealand White rabbits,
a 70% aqueous formulation of propamocarb hydrochloride was found to be
slightly irritating to the eye (Schöbel 1977; Ullmann & Suter 1983a).
Propamocarb hydrochloride, as a 70% aqueous formulation, was
shown to be practically non-irritating to the skin when applied to the
intact skin of New Zealand White rabbits (Ullmann & Suter 1983b).
In New Zealand White rabbits with skin sites exposed to a 70%
aqueous formulation of propamocarb hydrochloride (0.25 ml/site) five
hours/day for 28 consecutive days, signs of local irritation including
reddening, scabbing and thickening of the pithelium were noted at both
intact and abraded sites. Additionally, the abraded sites showed
reactions such as swelling and induration. Upon microscopic
examination, acanthosis, hyperkeratosis and necrosis were noted in the
abraded skin (Schöbel & Schuppler 1976).
Special Study on Skin Sensitization
A 70% aqueous formulation of propamocarb hydrochloride was not a
skin sensitizer in guinea pigs (Schöbel & Schuppler 1977).
Special Studies on Pharmacology
In mice treated with single intraveneous doses of propamocarb
hydrochloride as a 70% aqueous formulation, 30 mg/kg b.w. caused
restlessness and increased motor activity. After 100 mg/kg b.w.,
additional symptoms such as irregular breathing and exophthalmos
appeared. Death occurred at 175 mg/kg b.w. and above consequent to the
development of clonic convulsions. No clinical signs of toxicity or
abnormalities in normal reflexes and autonomic function were seen at
10 mg/kg b.w. or below. (Hara, et al., 1984).
Special Studies on Carcinogenicity (See also under "Long Term
Studies")
Mouse
Groups of 60 male and 60 female CD-1 mice, about 35 days old,
were fed dietary levels of propamocarb hydrochloride as a 70% aqueous
formulation at 0, 20, 100 or 500 ppm a.i. for two years. Mortality was
not affected by treatment. At termination, about 17 - 28% males and
42 - 52% females of control and treated groups survived. (Over 53%
males and at least 75% females per control and treated group lived at
least 78 weeks).
Single intravenous doses ranging from 3 to 100 mg/kg b.w. of the
70% aqueous formulation in mice did not produce analgesia or effects
on any of the following parameters: convulsions induced by
electroshock, barbital-induced anesthesia, pinnal and corneal reflexes
and muscular relaxation or coordination. No changes in rectal
temperature were detected in rabbits given 10 or 100 mg/kg b.w. of the
test formulation intravenously. Transitory alterations in spontaneous
EEG patterns were observed in rabbits treated intravenously at 100
mg/kg b.w. but not at 10 mg/kg b.w. and below. (Hara, et al., 1984).
Neuromuscular transmission in isolated rat diaphragm was slightly
enhanced initially but then slightly inhibited by the formulated
product at a concentration of 10-3 g/ml. Neuromuscular contraction in
rat gastrocnemius was unaffected in situ after an intravenous dose
of 100 mg/kg b.w. The test formulation, at concentrations of 10-5/ml
and above, inhibited acetylcholine- or histamine-induced contraction
of isolated guinea pig ileum and also acetylcholine-induced
contraction of isolated rabbit trachea; and enhanced nor-epinephrine-
induced contraction of isolated rat vas deferens. This concentration
(10-5 g/ml) did not modify the spontaneous contraction of atria
isolated from guinea-pig. The serotonin-induced contraction of
isolated rat fundus was depressed only at concentrations of 10-4 g/ml
and above. The oxytocin-induced contraction of isolated uterus in
oestrous rat was unaffected even at concentrations up to 10-3 g/ml.
(Hara, et al., 1984).
In anesthetized rabbits, intravenous doses of the formulation at
10 mg/kg b.w. and above produced bradycardia and antihypertensive
activity which were not affected by pretreatment with an intravenous
dose of 5 mg/kg b.w. of atropine. Tachycardia induced by 0.1 mg/kg
b.w. of isoproterenol given intraveneously was not antagonized by
intravenous administration of 30 mg/kg b.w. of the propamocarb
formulation. Blood coagulation in rats was not significantly affected
by an intravenous dose of 100 mg/kg b.w. of the aqueous formulation.
In vitro clotting time of blood from the rabbit was prolonged by the
formulation at a concentration of 10-2 g/ml but not below (Hara,
et al., 1984).
Acute Toxicity
Acute toxicity of propamocarb hydrochloride to rats, mice,
rabbits and dogs is summarized in Table 2. High doses produced
hypokinesis, convulsion, staggering gait, ataxia and piloerection
within 24 hours post-dosing.
Short Term Studies
Oral/Dietary
Rat
Groups of ten male and ten female rats were fed the free base of
propamocarb hydrochloride in their diet at 0, 20, 50 or 1000 ppm for
90 days. An additional group of the same size was given 500 ppm for
6 weeks and 1000 ppm for the remaining 7 weeks. There was no mortality
or abnormal behaviour. None of the parameters monitored, that is
weekly body weight, food consumption, haematology, urinalysis,
terminal blood chemistry, activity of plasma, erythrocyte, and brain
cholinesterase, were adversely affected. Gross pathological changes
and organ weights were not significantly different from those in the
controls. Histopathological examination of a number of selected
tissues from animals of control and top-dosage groups failed to reveal
any treatment-related findings. The no-effect level was demonstrated
at 500 ppm (de Rijke Köter & Leegwater, 1976).
Groups of 30 male and 30 female rats were fed diets containing
propamocarb hydrochloride, in the form of a 70% aqueous formulation,
at 0, 200, 1000 or 5000 ppm a.i. for 13 weeks. No mortality or
clinical signs were observed. Ophthalmoscopic findings, clinical
chemistry and haematology were not influenced by treatment. Activity
of plasma and erythrocyte cholinesterase at weeks 7 and 13 or of
terminal brain cholinesterase, determined in 24-hour fasted animals,
was not inhibited. In females at 5000 ppm, growth was slightly
decreased (< 10%) frequently during the study, and water consumption
at week 1 was reduced. Feed efficiency was decreased intermittently
over the course of the study in males at 5000 ppm and in females at
both 1000 and 5000 ppm. Proteinuria occurred in both sexes of all
groups including the control at weeks 7 and 13 with the males showing
greater degree of severity. At terminal sacrifice a decrease of
absolute weight of liver and spleen and an increase in organ/body
weight ratio of kidney in both sexes were evident in the high dose
only. There were no significant gross pathological changes between
control and treated groups. Histopathological evaluation of a number of
selected tissues including the bone marrow plus all grossly abnormal
tissues from each animal indicated an increase in incidence of
granulomatous inflammation of the liver and of hyperplasia of the
lymph node in females at 5000 ppm. The study showed the no-effect
level to be at least 200 ppm. (Kojima & Enomoto 1982).
Dog
Groups of pure-bred beagle dogs (four males and four females/
group) were fed the free base of propamocarb hydrochloride in the diet
at 0, 50, 100, 500 or 1000 ppm for 90 days with the top dosage level
being raised to 2000 ppm at week 7. No compound-related effects were
observed on survival, general appearance and behaviour, growth or food
consumption, haematology, blood chemistry and urinalysis as well as
liver and kidney function tests. Assay of plasma, erythrocyte and
brain cholinesterase revealed no significant inhibition.
At terminal sacrifice, organ weights and gross pathological
changes were not altered by the compound. Microscopic evaluation
indicated no compound-related findings. The no-effect level was at
least 1000 ppm (Reuzel & Til (1977).
Dermal
Rabbit
Groups of New Zealand White rabbits (three animals sex/group,
with skin abraded) were exposed dermally to propamocarb hydrochloride
as a 70% aqueous formulation at 0, 0.2, 0.7 or 2.0 ml/kg b.w. under
occlusive patch for a period of six-hours/day, five days/week for a
total of 15 applications in 21 days. Treated sites were washed at the
end of each exposure period. There were no compound-induced systemic
effects as judged by mortality, clinical signs, haematology, blood
chemistry, organ weight, gross pathology and histopathology.
Mild to moderate erythema and oedema were seen in animals of all
treated groups and increased with dosage (Field 1980).
Table 1. Special Studies on mutagenicity of Propamocarb Hydrochloride
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
In Vitro
Ames' test S.typhimurium 3.5- 70% negative Hastwell, et al.,
(with and strains TA 1535, 5,000 ug/plate aq with or without 1977;
without TA100,TA1537 solv. metabolic Kojima &
metabolic TA1538,TA98 activation Nakajima 1981a
activation) E.Coli WP2uvrA
Rec-assay Bacillus+subtilis 500- 70% negative Kojima &
H17 (rec±) and 10,000 ug/disk aq without Nakajima 1981b
M45 (rec) solv. metabolic
activation
Saccharomyces cerevisae
Mitotic strain D4 ) 1,000- 70% negative Jagannath & Brusick,
gene mutation ) 10,000 ug/plate aq. with or without Hoorn, 1980
) solv. metabolic
Reverse strains S138 and ) activation
mutation S211C )
)
Mitotic strain D5 )
recombination )
In Vivo
Micronucleus Mouse 1,250 to 5,000 70% negative Richold & Richardson,
test mg/kg b.w. aq. Hossack,1980
orally solv.
Table 1. (continued)
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
Dominant Mouse 424 to 1,237 70% negative Jorgenson & Jones,
lethal assay mg/kg b.w./day aq. Rushbrook,1979
for 8 weeks in solv.
drinking water;
prior to mating
weekly for 2
consecutive weeks
Table 2. Acute Toxicity of Propamocarb Hydrochloride to Animals
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Mouse M + F oral 1600 to >2858 Schöbel & Etreby,
1976a & 1976b;
Rushbrook, et al.,
1982a,Kojima, et al.,
1982a
M s.c. 1710 Kojima, et al.,
F 1870 1982b
M i.p. 457 Kojima, et al.,
F 435 1982c
M + F dermal >3000 Kojima, et al.,
1982d
Rat M + F oral 2000 to Kojima, et al.,
1982e
8600 Schöbel & Siegmund,
1976a
M s.c. 5220 Kojima,et al.,1982f
F 3230
Rat M + F i.p. 437-763 Schöbel & Schuppler,
1977; Kojima, et al.,
1982g
Rat M + F dermal >3920 Schöbel & Siegmund,
(duration of 1976g
exposure not
specified)
Rat M + F dermal >3000 Kojima, et al., 1982h
(24-hour
exposure)
Rat M + F inhalation >3960 mg/m3* Robkamp &
(4-hour Schuppler, 1976
"nose only"
exposure
Table 2. (continued)
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Rabbit M + F dermal >3920 Schöbel & Siegmund
(duration of 1976c
exposure not
specified)
Dog** M + F oral <674 Kopp, et al.,
1981a & 1981b
* 4-hour LC50
** 674, 1000 and 2000 mg/kg produced 3/6 mortalities with emesis of doses > 1000 mg/kg.
Long-term Studies
Rat
Groups of 50 male and 50 female Sprague-Dawley CD rats were fed
diets containing propamocarb hydrochloride as a 70% aqueous
formulation at 0, 40, 200 or 1000 ppm a.i. for 104 weeks. Additional
animals (ten males and ten females per group) were fed the same
dietary levels for the same duration and used primarily for routine
laboratory investigations (satellite group). Two additional groups of
ten males and ten females were fed 0 or 1000 ppm of the test diet with
one-half of the animals in each group sacrificed after 52 weeks and
the remainder at the end of a four-week withdrawal period. These
animals were subjected to macroscopic examination and organ weight
analysis.
Mortality was not influenced by treatment. Forty-six to 58% of
the males and 30-44% of the females of all groups including the
control survived at the end of two years. Over 50% of the animals
(both sexes) of control and treated groups lived at least 91 weeks.
Compound-related clinical signs were not apparent. No consistent dose-
related changes were seen on body weight, food consumption and water
intake. Haematological, clinical chemistry and urinalysis values as
well as ophthalmoscopic findings, monitored at several intervals over
the course of the study, were not significantly different between
control and treated groups. Activity of tissue cholinesterase was not
measured at any time during the study. Gross pathological changes in
treated animals dying or sacrificed in extremis during the study and
in those sacrificed at 52 weeks, after the four-week withdrawal
period, or terminally were comparable to those in the control group.
Organ weight determinations revealed no significant difference between
control and treated groups. Histopathological evaluation of non-
neoplastic findings was unremarkable except for an increased incidence
of degenerative hepatocytes/necrosis in high-dose males.
Evaluation of the neoplastic changes demonstrated an increase in
occurrence in males of subcutaneous fibrosarcoma in the treated
groups. Incidence of the tumour was 0/58 in controls, 5/56 (8.9%) at
40 ppm, 2/58 (3.5%) at 200 ppm and 7/55 (12.7%) at 1000 ppm (the
denominator denoted the number of survivors from both main and
satellite groups at each dosage level at the end of 51 weeks when the
first subcutaneous fibrosarcoma was detected in the males). The
difference from concurrent controls was statistically significant at
40 ppm and 1000 ppm. Data on background incidence of subcutaneous
fibrosarcoma in the particular strain of rats employed in the study
were not available in the report.
Over 80% of the concurrent controls (both sexes) had tumours,
with pituitary adenoma (both sexes), mammary tumours (females) and
subcutaneous fibroma and lipoma (males), the most prevalent tumours.
There were no other neoplastic differences between treated and control
groups. The no-effect level for non-neoplastic effects was equal to
8.3 mg a.i/kg b.w./day, based on food consumption and body weight data
(Hunter, et al., 1983b).
Comments
Propamocarb hydrochloride was evaluated for the first time by the
JMPR at this session.
The compound, a carbamate fungicide, is rapidly absorbed,
degraded and readily excreted primarily in the urine of (female) rats.
Oxidation of the parent molecule appears to be the major metabolic
pathway. There is no evidence of significant tissue accumulation of
propamocarb hydrochloride and/or its metabolites. Metabolism data in
mammalian species other than the rat are not available.
Propamocarb hydrochloride has a relatively low order of acute
oral toxicity in rodents, with LD5 values of over 1500 mg/kg b.w. in
both mice and rats. Limited acute oral data in dogs appeared to
indicate that they are more sensitive than rodents to toxicity of the
compound.
A three-generation reproduction study, including teratological
investigation in rats, showed a no-effect level of 200 ppm on
reproduction and teratogenicity. A teratology study in rats showed
retardation of foetal development at doses which were maternally
toxic. Teratology studies in rabbits were negative. Mutagenicity
studies were also negative. A carcinogenicity study in mice was
negative. A long-term study in rats showed some evidence of increased
incidence of sub-cutaneous fibrosarcoma. Therefore, background data on
incidence of this malignancy in the particular strain of rats are
required.
Ninety-day feeding studies in rats and dogs established no-effect
levels of at least 200 ppm and 1000 ppm respectively. Inhibition of
brain and erythrocyte cholinesterases was not observed in these
studies.
In view of the concerns with respect to the elevated incidence of
subcutaneous fibrosarcoma in male rats of the long-term study and the
non-availability of a dog study longer than three months, only a
temporary ADI was granted.
Level causing no toxicological effect
Rat: 200 ppm in the diet, equivalent to 8.3 mg/kg b.w.
Dog: 1,000 ppm in the diet, equivalent to 25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans
0 - 0.2 mg/kg b.w.
Further work or information
Required (by 1986):
Historical data on the incidence of subcutaneous fibrosarcoma in the
CD strain of rats used in the long-term study.
Complete data on the 24-month dog study known to have been completed.
Metabolism and pharmacokinetic studies in mice and rats using multiple
dosage levels.
Desirable:
Observations in humans.
Complete data on the 4-5 week feeding study in rats.
REFERENCES
Brühl, R. & Celorio, J. Propamocarb hydrochloride: Metabolism in rats.
1982 Submitted by Schering AG to WHO. (Unpublished).
Brühl, R. & Celorio, J. Metabolic pathway of propamocarb hydrochloride
1984 in the rat. Submitted by Schering AG to WHO. (Unpublished).
de Rijke, D., Köter, H.B.W.M. & Leegwater, D.C. Subchronic (90-day)
1976 toxicity study with ZK17.296 in albino rats (ZK 17296 =
SN39744 = Propamocarb). Centraal Instituut Voor
Voedingsonderzoek, The Netherlands. Submitted by Schering AG
to WHO. (Unpublished).
Field, W.E. Twenty-one day dermal toxicity study in rabbits treated
1980 with Previcur N. (Final report). CDC Research, Inc. U.S.A.
Submitted by Schering AG to WHO. (UNPUBLISHED).
Hara, K., Ikoma, Y. Arita, S., Tokiyori, Y., Nakata, Y., Yamano, K.,
1984 Mizuno, M. & Oshino, N. General pharmacological activities
of Previcur N. Nikon Schering K.K. Japan. Submitted by
Schering AG to WHO. (Unpublished).
Hastwell, R., McGregor, D.B. & Smith, I. Mutagenicity testing of
1977 SN 66.752. Inveresk Research International, Scotland.
Submitted by Schering AG to WHO. (Unpublished).
Hoorn, A.J.W., Jagannath, D.R. & Brusick, D.J. Mutagenic evaluation of
1980 Previcur N. Batch No. 271001B00000 in the mitotic gene
conversion, reverse mutation & mitotic recombination assays
in yeast. Litton Bionetics, Inc. Submitted by Schering AG to
WHO. (Unpublished).
Hossack, D.J.N., Richold, M. & Richardson, J.C. Micronucleus test on
1980 CP604 (SN66752, Previcur N). Huntingdon Research Centre,
England. Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E. & Gibson, W.A.
1978 Previcur N (SN66752). Assessment of whole blood & brain
cholinesterase activities in rats after 11 days of treatment
by oral gavage. Huntington Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Watson, M., Read, R.M., Prentice, D.E., Woodhouse, R.N.,
1983a Cherry, C.P. & Gibson, W.A. Previcur N (SN 66752): Potential
tumorigenicity to mice in dietary administration for 104
weeks (final report). Huntingdon Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E., Jolly, D.W.,
1983b Offer, J.M. & Gibson, W.A. Previcur N (SN66752): Toxicity &
potential tumorigenicity in dietary administration to rats
for 104 weeks. (final amended report). Huntingdon Research
Centre, England. Submitted by Schering AG to WHO.
(Unpublished).
Huntingdon Research Centre. Previcur N teratology study: effect of
1981 administration during organogensis on foetuses of the
rabbit. Reprotox, Deutschland. Submitted by Schering AG to
WHO. (Unpublished).
Huntingdon Research Centre. Effect of Previcur N on reproductive
1983 function of multiple generations in the rat. Reprotox,
Deutschland. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. Mutagenicity test in bacteria with Previcur
1981a N. Biosafety Research Centre, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. "REC" assay to detect mutagenic activity of
1981b Previcur N. Biosafety Research Centre, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K. & Enomoto, M. Previcur N: Three-month subchronic oral
1982 toxicity study in rats. Biosafety Research Centre, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojama, K. Seki, S. & Okamura, T. Previcur N: Acute oral toxicity in
1982a mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. Seki, S. & Okamura, T. Acute subcutaneous toxicity study in
1982b mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute intraperitoneal
1982c toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute percutaneous
1982d toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute oral toxicity study in rats.
1982e Biosafety Research Center, Foods, Drugs, & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute subcutaneous toxicity study
1982f in rats. Biosafety Research Center, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute intraperitonial toxicity
1982g study in rats. Biosafety Research Center, Foods, Drugs and
pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. and Okamura, T. Acute percutaneous toxicity study
1982h in rats. Unpublished report from Biosafety Research Center,
Foods, Drugs and Pesticides, Japan. Submitted by Schering AG
to WHO. (Unpublished).
Kopp, R., Hümpel, M., Kühne, G. Aner, B., Klawa, D. & Rzadkiewicz, M.,
1979 Pharmacokinetics of Propamocarb hydrochloride on single &
repeated oral administration of 0.5 mg/kg in rats. Schering
AG. Submitted by Schering AG to WHO. (Unpublished).
Kopp, R., Günzel, P., RoBkamp, G., Schöbel, C. & Siegmund, F. Previcur
1981a N. Systemic tolerance test (LD50) ZK66752(CP604) in the dog
following single oral administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished)
Kopp, R., Günzel, P., Bhargava, A.S., Schöbel, C. & Slabke, P.
1981b Influence of SN66.752 upon the acetylcholinesterase activity
in vitro (dog & rat) and in vivo (dog) after single oral
administration. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Poggel, H.A., Kopp, R. & Günzel, P. Previcur N. (CP604).
1981 Embryotoxicity including teratogenicity study in rats after
daily intragastrical administration from day 6 to day 19 of
gestation. Schering AG. Submitted by Schering AG to WHO.
(Unpublished).
Reuzel, P.G.J. & Til, H.P. Subchronic (90-day) feeding study with
1977 ZK17.296 (SN39744 Propamocarb) in dogs (final report).
Centraal Instituut Voor Voedingsonderzoek, The Netherlands.
Submitted by Schering AG to WHO. (Unpublished).
RoBkamp, G. & Schuppler, J. ZK66752 Acute Inhalation toxicity to the
1976 rats (M + F) of aqueous ZK66.752 following single four-hour
continuous exposure (LC50). Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Rushbrook, C.J., Jorgenson, T.A. & Jones, D.C.L. Dominant lethal study
1979 of Previcur N. SRI International, USA. Submitted by Schering
AG to WHO. (Unpublished).
Schering A.G. Previcur N (CP604): embryotoxicity including
1981 teratogenicity study in rabbits after daily intragastrical
administration from day 6 to day 18 of gestation. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C. SN66752. Eye irritation, rabbits: single application of
1977 CP604 to the rabbit eye. Schering AG. Submitted by Schering
AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752: Acute oral (i.g.) toxicity
1976a mice (male) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752. Acute oral (i.g.) toxicity
1976b mice (female) single administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C.J. and Schuppler, J. SN6675: Dermal toxicity study of CP604
1976 on the intact and scarified skin of the rabbit following
daily application for 28 days. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Schuppler, J. ZK66752. Acute i.p. toxicity rats (M + F)
1977 single application. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752: acute oral (i.g.) toxicity rats
1976a (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK 66752: acute dermal toxicity rats
1976b (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752. Acute dermal toxicity rabbits
1976c (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary eye irritation after single
1983a application with Previcur N in the rabbit. Research &
Consulting Co., AG, Switzerland. Submitted by Schering AG to
WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary skin irritation following a single
1983b four-hour occlusive application with Previcur N in the
rabbit. Research & Consulting Co., AG., Switzerland.
Submitted by Schering AG to WHO. (Unpublished).
Over the three generations, there were no treatment-related
effects on litter size on days 1, 4, 12 and 21; pup weight on days 1
and 4; sex ratio of pups and histopathological findings of F3b
weanlings. Incidence of dams with total litter loss was increased at
200 ppm only in F1b litters. Pup weight at 1000 ppm was decreased on
day 21 in F1a litters and on day 12 in F1b litters. An increase in
spleen weight was note in F3b weanlings at 1000 ppm. Examination of
pre-weaning pups from each generation for rate of physical development
(hair growth, incisor eruption, opening of the auditory canal and the
eye) and of selected F1b and F2b animals for pupillary reflex and
startle response at about 28 days of age indicated no abnormal
findings attributable to the compound.
Teratological investigation of F1b, F2b, and F3b litters
revealed no significant differences between control and treated groups
with respect to number of implantations or corpora lutea, litter size,
late resorptions of foetal weight. F2b litters at 1000 ppm exhibited
an increase in early resorptions and pre-implantation loss. Increased
renal pelvic cavitation, unilaterally or bilaterally, was found in
2/67 foetuses from 2/12 dams at 1000 ppm of the F3b litters, as
compared to an incidence of zero in 37 concurrent control foetuses
from a total of 9 dams examined for visceral abnormalities. The study
demonstrated a dietary level of 200 ppm to be without adverse effect
on the reproductive parameters examined. There was no clear evidence
for teratogenic potential at any dose tested. (Huntingdon Research
Centre, 1983).
Special Studies on Teratogenicity (Also see under "Special Studies
on Reproduction")
Rat
Groups of 25 mated female rats (Wistar - Han-Schering, SPF) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.1, 0.3, 1.0 or 3.0 ml/kg b.w./days from days 6
through day 19 of pregnancy. The dams were sacrificed on day 20
post-coitum and foetuses were removed for gross external, visceral and
skeletal examination. Five dams at 3 ml/kg b.w. and one dam at
1.0 ml/kg b.w. died, but with the exception of 1/5 dams of the top
dosage group showing severe pulmonary oedema, no abnormal findings
were reportedly seen at autopsy. In the top dosage group, a majority
of the dams exhibited toxic signs. Additionally, maternal weight gain
between days 16 and 20 post-coitum was depressed and uterine weight
was decreased. Also at the same dosage level, adverse effects were
observed on the mean number of early resorptions or live foetuses,
mean total litter size, incidence of pregnant dams with resorptions of
entire litters, post-implantation loss and foetal weight. There were
no significant differences between control and treated groups in mean
number of corpora lutea or implantations, pre-implantation loss and
sex ratio. Foetuses at both 1 and 3 ml/kg b.w. showed an increase
(dose-related) in the incidence of retarded ossification. An elevated
frequency of foetuses with 14 ribs was seen at 0.3 ml/kg b.w. and
above, but a dose-response pattern was not evident.
Based on the data, the compound appeared to retard foetal
development at and above 1 ml/kg b.w. (approximately equivalent to
700 mg a.i./kg b.w.). A dosage level of 3 ml/kg b.w. (approximately
equivalent to 2100 mg a.i./kg b.w.) was toxic to both mothers and
foetuses. There was however no clear evidence of teratogenic activity
at any level under the conditions of the experiment (Poggel, et al.,
1981).
Rabbit
Groups of 15 to 20 mated female rabbits (New Zealand White) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.2, 0.4 or 0.8 ml/kg b.w./day from days 6 through 18
of gestation. The does were sacrificed on day 28 of pregnancy and
uterine contents were examined. Live young were evaluated for external
malformation and incubated for 24 hours prior to sacrifice for
examination for skeletal and visceral abnormalities. Mortality
occurred in control and treated groups, but only two deaths at
0.8 ml/kg b.w. appeared to be treatment related. The total number of
does showing evidence of pregnancy (including those showing abortions
or total resorption) and surviving at termination was 12, 12, 11, 10
respectively at 0, 0.2, 0.4, 0.8 ml/kg b.w. Does at and above
0.4 ml/kg b.w. showed weight loss on gestation day 16 and growth
depression on day 28. Pregnancy rate, mean litter size, mean number of
early resorptions, incidence of pregnant does with early resorptions,
incidence of pregnant does with entire litters resorbed, post-
implantation loss and foetal survival rate after six- and 24-hour
incubation were all adversely affected in the top dosage group Foetal
weight was depressed at both 0.4 and 0.8 ml/kg b.w. Cleft palate, not
observed in any of the 71 foetuses from ten concurrent control litters
or in any of a combined total of 133 foetuses from 18 litters of the
two lower dosage groups, was observed in 1/21 foetuses from five
litters examined at 0.8 ml/kg b.w. Historical control data included in
the report on the incidence of cleft palate in foetuses of New Zealand
White rabbits were of limited value as a basis of comparison due to
the absence of the data in question using litter as the sampling unit
and lack of information on the time frame over which the data were
obtained. There were no significant differences between control and
treated groups in the other monitored parameters including the number
of implantations or corpora lutea, pre-implantation loss and visceral
or skeletal malformations of pups. The study appeared to indicate the
teratogenic no-effect level to be at least 0.4 ml/kg b.w. (equivalent
approximately to 280 mg/ai/kg b.w.). Maternal and foetal toxicity were
evident at this level but not at 0.2 ml/kg b.w. (Huntingdon Research
Centre, 1981).
Groups of 18 to 21 mated female rabbits (New Zealand White) were
treated orally with propamocarb hydrochloride as a 70% aqueous
formulation in water at 0, 0.02, 0.06, 0.2, 0.4 or 0.8 ml/kg b.w./day
from day 6 to day 18 of gestation. All does were sacrificed on day
28 post coitum and uterine contents were examined. Foetuses were
evaluated for external, skeletal and visceral abnormalities. Mortality
was not affected by treatment. The number of pregnant does with live
foetuses surviving on day 28 was 11, 14, 10, 11, 7, 13 respectively at
0, 0.02, 0.06, 0.2, 0.4, 0.8 ml/kg b.w. Pregnant does at 0.8 ml/kg
b.w. showed weight loss on day 18 and a decrease in weight gain on day
28. The mean number of dead foetuses per pregnant doe and the
incidence of pregnant does with dead foetuses were increased at
0.4 ml/kg b.w. only. The mean number of early resorptions was elevated
and the mean foetal weight was reduced at the top dosage level. Post-
implantation loss was increased at both 0.4 and 0.8 ml/kg b.w. Other
measured parameters, including number of corpora lutea or
implantations, litter size, pre-implantation loss and gross, skeletal
and visceral findings of foetuses, were not significantly different
from controls. Cleft palate, observed in a single foetus of the top
dosage group (0.8 ml/kg b.w.) in a previous rabbit study (Anon.,
1981), was not seen in any of the foetuses in the present study. The
teratogenic no-effect level was demonstrated at 0.8 ml/kg b.w.
(equivalent approximately to 560 mg a.i/kg b.w.) (Schering AG, 1981).
Special Studies on Mutagenicity
Mutagenicity studies for propamocarb hydrochloride as a 70%
aqueous formulation are summarized in Table 1.
Food consumption was marginally increased in males at 500 ppm
between weeks 79 and 104. Body weight, general appearance and
behaviour were unaffected. No gross pathological changes attributable
to ingestion of the compound were evident. Histopathological
examination of a wide range of tissues from all animals of control and
top dosage groups, plus any tissue showing macroscopic abnormality
suggestive of neoplasia revealed no treatment-related findings. There
was no significant difference between control and treated groups in
incidence of any particular type of tumour. The most frequently
observed tumours were liver tumours in males, lung tumours in both
sexes and lymphoreticular tumours in females. Over 65% of the
concurrent controls (both sexes) had spontaneous tumours. Under the
condition of the experiment, the test material was not carcinogenic
(Hunter, et al., 1983a).
Special Studies on Eye and Skin Irritation
In primary eye irritation studies with New Zealand White rabbits,
a 70% aqueous formulation of propamocarb hydrochloride was found to be
slightly irritating to the eye (Schöbel 1977; Ullmann & Suter 1983a).
Propamocarb hydrochloride, as a 70% aqueous formulation, was
shown to be practically non-irritating to the skin when applied to the
intact skin of New Zealand White rabbits (Ullmann & Suter 1983b).
In New Zealand White rabbits with skin sites exposed to a 70%
aqueous formulation of propamocarb hydrochloride (0.25 ml/site) five
hours/day for 28 consecutive days, signs of local irritation including
reddening, scabbing and thickening of the pithelium were noted at both
intact and abraded sites. Additionally, the abraded sites showed
reactions such as swelling and induration. Upon microscopic
examination, acanthosis, hyperkeratosis and necrosis were noted in the
abraded skin (Schöbel & Schuppler 1976).
Special Study on Skin Sensitization
A 70% aqueous formulation of propamocarb hydrochloride was not a
skin sensitizer in guinea pigs (Schöbel & Schuppler 1977).
Special Studies on Pharmacology
In mice treated with single intraveneous doses of propamocarb
hydrochloride as a 70% aqueous formulation, 30 mg/kg b.w. caused
restlessness and increased motor activity. After 100 mg/kg b.w.,
additional symptoms such as irregular breathing and exophthalmos
appeared. Death occurred at 175 mg/kg b.w. and above consequent to the
development of clonic convulsions. No clinical signs of toxicity or
abnormalities in normal reflexes and autonomic function were seen at
10 mg/kg b.w. or below. (Hara, et al., 1984).
Special Studies on Carcinogenicity (See also under "Long Term
Studies")
Mouse
Groups of 60 male and 60 female CD-1 mice, about 35 days old,
were fed dietary levels of propamocarb hydrochloride as a 70% aqueous
formulation at 0, 20, 100 or 500 ppm a.i. for two years. Mortality was
not affected by treatment. At termination, about 17 - 28% males and
42 - 52% females of control and treated groups survived. (Over 53%
males and at least 75% females per control and treated group lived at
least 78 weeks).
Single intravenous doses ranging from 3 to 100 mg/kg b.w. of the
70% aqueous formulation in mice did not produce analgesia or effects
on any of the following parameters: convulsions induced by
electroshock, barbital-induced anesthesia, pinnal and corneal reflexes
and muscular relaxation or coordination. No changes in rectal
temperature were detected in rabbits given 10 or 100 mg/kg b.w. of the
test formulation intravenously. Transitory alterations in spontaneous
EEG patterns were observed in rabbits treated intravenously at 100
mg/kg b.w. but not at 10 mg/kg b.w. and below. (Hara, et al., 1984).
Neuromuscular transmission in isolated rat diaphragm was slightly
enhanced initially but then slightly inhibited by the formulated
product at a concentration of 10-3 g/ml. Neuromuscular contraction in
rat gastrocnemius was unaffected in situ after an intravenous dose
of 100 mg/kg b.w. The test formulation, at concentrations of 10-5/ml
and above, inhibited acetylcholine- or histamine-induced contraction
of isolated guinea pig ileum and also acetylcholine-induced
contraction of isolated rabbit trachea; and enhanced nor-epinephrine-
induced contraction of isolated rat vas deferens. This concentration
(10-5 g/ml) did not modify the spontaneous contraction of atria
isolated from guinea-pig. The serotonin-induced contraction of
isolated rat fundus was depressed only at concentrations of 10-4 g/ml
and above. The oxytocin-induced contraction of isolated uterus in
oestrous rat was unaffected even at concentrations up to 10-3 g/ml.
(Hara, et al., 1984).
In anesthetized rabbits, intravenous doses of the formulation at
10 mg/kg b.w. and above produced bradycardia and antihypertensive
activity which were not affected by pretreatment with an intravenous
dose of 5 mg/kg b.w. of atropine. Tachycardia induced by 0.1 mg/kg
b.w. of isoproterenol given intraveneously was not antagonized by
intravenous administration of 30 mg/kg b.w. of the propamocarb
formulation. Blood coagulation in rats was not significantly affected
by an intravenous dose of 100 mg/kg b.w. of the aqueous formulation.
In vitro clotting time of blood from the rabbit was prolonged by the
formulation at a concentration of 10-2 g/ml but not below (Hara,
et al., 1984).
Acute Toxicity
Acute toxicity of propamocarb hydrochloride to rats, mice,
rabbits and dogs is summarized in Table 2. High doses produced
hypokinesis, convulsion, staggering gait, ataxia and piloerection
within 24 hours post-dosing.
Short Term Studies
Oral/Dietary
Rat
Groups of ten male and ten female rats were fed the free base of
propamocarb hydrochloride in their diet at 0, 20, 50 or 1000 ppm for
90 days. An additional group of the same size was given 500 ppm for
6 weeks and 1000 ppm for the remaining 7 weeks. There was no mortality
or abnormal behaviour. None of the parameters monitored, that is
weekly body weight, food consumption, haematology, urinalysis,
terminal blood chemistry, activity of plasma, erythrocyte, and brain
cholinesterase, were adversely affected. Gross pathological changes
and organ weights were not significantly different from those in the
controls. Histopathological examination of a number of selected
tissues from animals of control and top-dosage groups failed to reveal
any treatment-related findings. The no-effect level was demonstrated
at 500 ppm (de Rijke Köter & Leegwater, 1976).
Groups of 30 male and 30 female rats were fed diets containing
propamocarb hydrochloride, in the form of a 70% aqueous formulation,
at 0, 200, 1000 or 5000 ppm a.i. for 13 weeks. No mortality or
clinical signs were observed. Ophthalmoscopic findings, clinical
chemistry and haematology were not influenced by treatment. Activity
of plasma and erythrocyte cholinesterase at weeks 7 and 13 or of
terminal brain cholinesterase, determined in 24-hour fasted animals,
was not inhibited. In females at 5000 ppm, growth was slightly
decreased (< 10%) frequently during the study, and water consumption
at week 1 was reduced. Feed efficiency was decreased intermittently
over the course of the study in males at 5000 ppm and in females at
both 1000 and 5000 ppm. Proteinuria occurred in both sexes of all
groups including the control at weeks 7 and 13 with the males showing
greater degree of severity. At terminal sacrifice a decrease of
absolute weight of liver and spleen and an increase in organ/body
weight ratio of kidney in both sexes were evident in the high dose
only. There were no significant gross pathological changes between
control and treated groups. Histopathological evaluation of a number of
selected tissues including the bone marrow plus all grossly abnormal
tissues from each animal indicated an increase in incidence of
granulomatous inflammation of the liver and of hyperplasia of the
lymph node in females at 5000 ppm. The study showed the no-effect
level to be at least 200 ppm. (Kojima & Enomoto 1982).
Dog
Groups of pure-bred beagle dogs (four males and four females/
group) were fed the free base of propamocarb hydrochloride in the diet
at 0, 50, 100, 500 or 1000 ppm for 90 days with the top dosage level
being raised to 2000 ppm at week 7. No compound-related effects were
observed on survival, general appearance and behaviour, growth or food
consumption, haematology, blood chemistry and urinalysis as well as
liver and kidney function tests. Assay of plasma, erythrocyte and
brain cholinesterase revealed no significant inhibition.
At terminal sacrifice, organ weights and gross pathological
changes were not altered by the compound. Microscopic evaluation
indicated no compound-related findings. The no-effect level was at
least 1000 ppm (Reuzel & Til (1977).
Dermal
Rabbit
Groups of New Zealand White rabbits (three animals sex/group,
with skin abraded) were exposed dermally to propamocarb hydrochloride
as a 70% aqueous formulation at 0, 0.2, 0.7 or 2.0 ml/kg b.w. under
occlusive patch for a period of six-hours/day, five days/week for a
total of 15 applications in 21 days. Treated sites were washed at the
end of each exposure period. There were no compound-induced systemic
effects as judged by mortality, clinical signs, haematology, blood
chemistry, organ weight, gross pathology and histopathology.
Mild to moderate erythema and oedema were seen in animals of all
treated groups and increased with dosage (Field 1980).
Table 1. Special Studies on mutagenicity of Propamocarb Hydrochloride
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
In Vitro
Ames' test S.typhimurium 3.5- 70% negative Hastwell, et al.,
(with and strains TA 1535, 5,000 ug/plate aq with or without 1977;
without TA100,TA1537 solv. metabolic Kojima &
metabolic TA1538,TA98 activation Nakajima 1981a
activation) E.Coli WP2uvrA
Rec-assay Bacillus+subtilis 500- 70% negative Kojima &
H17 (rec±) and 10,000 ug/disk aq without Nakajima 1981b
M45 (rec) solv. metabolic
activation
Saccharomyces cerevisae
Mitotic strain D4 ) 1,000- 70% negative Jagannath & Brusick,
gene mutation ) 10,000 ug/plate aq. with or without Hoorn, 1980
) solv. metabolic
Reverse strains S138 and ) activation
mutation S211C )
)
Mitotic strain D5 )
recombination )
In Vivo
Micronucleus Mouse 1,250 to 5,000 70% negative Richold & Richardson,
test mg/kg b.w. aq. Hossack,1980
orally solv.
Table 1. (continued)
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
Dominant Mouse 424 to 1,237 70% negative Jorgenson & Jones,
lethal assay mg/kg b.w./day aq. Rushbrook,1979
for 8 weeks in solv.
drinking water;
prior to mating
weekly for 2
consecutive weeks
Table 2. Acute Toxicity of Propamocarb Hydrochloride to Animals
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Mouse M + F oral 1600 to >2858 Schöbel & Etreby,
1976a & 1976b;
Rushbrook, et al.,
1982a,Kojima, et al.,
1982a
M s.c. 1710 Kojima, et al.,
F 1870 1982b
M i.p. 457 Kojima, et al.,
F 435 1982c
M + F dermal >3000 Kojima, et al.,
1982d
Rat M + F oral 2000 to Kojima, et al.,
1982e
8600 Schöbel & Siegmund,
1976a
M s.c. 5220 Kojima,et al.,1982f
F 3230
Rat M + F i.p. 437-763 Schöbel & Schuppler,
1977; Kojima, et al.,
1982g
Rat M + F dermal >3920 Schöbel & Siegmund,
(duration of 1976g
exposure not
specified)
Rat M + F dermal >3000 Kojima, et al., 1982h
(24-hour
exposure)
Rat M + F inhalation >3960 mg/m3* Robkamp &
(4-hour Schuppler, 1976
"nose only"
exposure
Table 2. (continued)
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Rabbit M + F dermal >3920 Schöbel & Siegmund
(duration of 1976c
exposure not
specified)
Dog** M + F oral <674 Kopp, et al.,
1981a & 1981b
* 4-hour LC50
** 674, 1000 and 2000 mg/kg produced 3/6 mortalities with emesis of doses > 1000 mg/kg.
Long-term Studies
Rat
Groups of 50 male and 50 female Sprague-Dawley CD rats were fed
diets containing propamocarb hydrochloride as a 70% aqueous
formulation at 0, 40, 200 or 1000 ppm a.i. for 104 weeks. Additional
animals (ten males and ten females per group) were fed the same
dietary levels for the same duration and used primarily for routine
laboratory investigations (satellite group). Two additional groups of
ten males and ten females were fed 0 or 1000 ppm of the test diet with
one-half of the animals in each group sacrificed after 52 weeks and
the remainder at the end of a four-week withdrawal period. These
animals were subjected to macroscopic examination and organ weight
analysis.
Mortality was not influenced by treatment. Forty-six to 58% of
the males and 30-44% of the females of all groups including the
control survived at the end of two years. Over 50% of the animals
(both sexes) of control and treated groups lived at least 91 weeks.
Compound-related clinical signs were not apparent. No consistent dose-
related changes were seen on body weight, food consumption and water
intake. Haematological, clinical chemistry and urinalysis values as
well as ophthalmoscopic findings, monitored at several intervals over
the course of the study, were not significantly different between
control and treated groups. Activity of tissue cholinesterase was not
measured at any time during the study. Gross pathological changes in
treated animals dying or sacrificed in extremis during the study and
in those sacrificed at 52 weeks, after the four-week withdrawal
period, or terminally were comparable to those in the control group.
Organ weight determinations revealed no significant difference between
control and treated groups. Histopathological evaluation of non-
neoplastic findings was unremarkable except for an increased incidence
of degenerative hepatocytes/necrosis in high-dose males.
Evaluation of the neoplastic changes demonstrated an increase in
occurrence in males of subcutaneous fibrosarcoma in the treated
groups. Incidence of the tumour was 0/58 in controls, 5/56 (8.9%) at
40 ppm, 2/58 (3.5%) at 200 ppm and 7/55 (12.7%) at 1000 ppm (the
denominator denoted the number of survivors from both main and
satellite groups at each dosage level at the end of 51 weeks when the
first subcutaneous fibrosarcoma was detected in the males). The
difference from concurrent controls was statistically significant at
40 ppm and 1000 ppm. Data on background incidence of subcutaneous
fibrosarcoma in the particular strain of rats employed in the study
were not available in the report.
Over 80% of the concurrent controls (both sexes) had tumours,
with pituitary adenoma (both sexes), mammary tumours (females) and
subcutaneous fibroma and lipoma (males), the most prevalent tumours.
There were no other neoplastic differences between treated and control
groups. The no-effect level for non-neoplastic effects was equal to
8.3 mg a.i/kg b.w./day, based on food consumption and body weight data
(Hunter, et al., 1983b).
Comments
Propamocarb hydrochloride was evaluated for the first time by the
JMPR at this session.
The compound, a carbamate fungicide, is rapidly absorbed,
degraded and readily excreted primarily in the urine of (female) rats.
Oxidation of the parent molecule appears to be the major metabolic
pathway. There is no evidence of significant tissue accumulation of
propamocarb hydrochloride and/or its metabolites. Metabolism data in
mammalian species other than the rat are not available.
Propamocarb hydrochloride has a relatively low order of acute
oral toxicity in rodents, with LD5 values of over 1500 mg/kg b.w. in
both mice and rats. Limited acute oral data in dogs appeared to
indicate that they are more sensitive than rodents to toxicity of the
compound.
A three-generation reproduction study, including teratological
investigation in rats, showed a no-effect level of 200 ppm on
reproduction and teratogenicity. A teratology study in rats showed
retardation of foetal development at doses which were maternally
toxic. Teratology studies in rabbits were negative. Mutagenicity
studies were also negative. A carcinogenicity study in mice was
negative. A long-term study in rats showed some evidence of increased
incidence of sub-cutaneous fibrosarcoma. Therefore, background data on
incidence of this malignancy in the particular strain of rats are
required.
Ninety-day feeding studies in rats and dogs established no-effect
levels of at least 200 ppm and 1000 ppm respectively. Inhibition of
brain and erythrocyte cholinesterases was not observed in these
studies.
In view of the concerns with respect to the elevated incidence of
subcutaneous fibrosarcoma in male rats of the long-term study and the
non-availability of a dog study longer than three months, only a
temporary ADI was granted.
Level causing no toxicological effect
Rat: 200 ppm in the diet, equivalent to 8.3 mg/kg b.w.
Dog: 1,000 ppm in the diet, equivalent to 25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans
0 - 0.2 mg/kg b.w.
Further work or information
Required (by 1986):
Historical data on the incidence of subcutaneous fibrosarcoma in the
CD strain of rats used in the long-term study.
Complete data on the 24-month dog study known to have been completed.
Metabolism and pharmacokinetic studies in mice and rats using multiple
dosage levels.
Desirable:
Observations in humans.
Complete data on the 4-5 week feeding study in rats.
REFERENCES
Brühl, R. & Celorio, J. Propamocarb hydrochloride: Metabolism in rats.
1982 Submitted by Schering AG to WHO. (Unpublished).
Brühl, R. & Celorio, J. Metabolic pathway of propamocarb hydrochloride
1984 in the rat. Submitted by Schering AG to WHO. (Unpublished).
de Rijke, D., Köter, H.B.W.M. & Leegwater, D.C. Subchronic (90-day)
1976 toxicity study with ZK17.296 in albino rats (ZK 17296 =
SN39744 = Propamocarb). Centraal Instituut Voor
Voedingsonderzoek, The Netherlands. Submitted by Schering AG
to WHO. (Unpublished).
Field, W.E. Twenty-one day dermal toxicity study in rabbits treated
1980 with Previcur N. (Final report). CDC Research, Inc. U.S.A.
Submitted by Schering AG to WHO. (UNPUBLISHED).
Hara, K., Ikoma, Y. Arita, S., Tokiyori, Y., Nakata, Y., Yamano, K.,
1984 Mizuno, M. & Oshino, N. General pharmacological activities
of Previcur N. Nikon Schering K.K. Japan. Submitted by
Schering AG to WHO. (Unpublished).
Hastwell, R., McGregor, D.B. & Smith, I. Mutagenicity testing of
1977 SN 66.752. Inveresk Research International, Scotland.
Submitted by Schering AG to WHO. (Unpublished).
Hoorn, A.J.W., Jagannath, D.R. & Brusick, D.J. Mutagenic evaluation of
1980 Previcur N. Batch No. 271001B00000 in the mitotic gene
conversion, reverse mutation & mitotic recombination assays
in yeast. Litton Bionetics, Inc. Submitted by Schering AG to
WHO. (Unpublished).
Hossack, D.J.N., Richold, M. & Richardson, J.C. Micronucleus test on
1980 CP604 (SN66752, Previcur N). Huntingdon Research Centre,
England. Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E. & Gibson, W.A.
1978 Previcur N (SN66752). Assessment of whole blood & brain
cholinesterase activities in rats after 11 days of treatment
by oral gavage. Huntington Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Watson, M., Read, R.M., Prentice, D.E., Woodhouse, R.N.,
1983a Cherry, C.P. & Gibson, W.A. Previcur N (SN 66752): Potential
tumorigenicity to mice in dietary administration for 104
weeks (final report). Huntingdon Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E., Jolly, D.W.,
1983b Offer, J.M. & Gibson, W.A. Previcur N (SN66752): Toxicity &
potential tumorigenicity in dietary administration to rats
for 104 weeks. (final amended report). Huntingdon Research
Centre, England. Submitted by Schering AG to WHO.
(Unpublished).
Huntingdon Research Centre. Previcur N teratology study: effect of
1981 administration during organogensis on foetuses of the
rabbit. Reprotox, Deutschland. Submitted by Schering AG to
WHO. (Unpublished).
Huntingdon Research Centre. Effect of Previcur N on reproductive
1983 function of multiple generations in the rat. Reprotox,
Deutschland. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. Mutagenicity test in bacteria with Previcur
1981a N. Biosafety Research Centre, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. "REC" assay to detect mutagenic activity of
1981b Previcur N. Biosafety Research Centre, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K. & Enomoto, M. Previcur N: Three-month subchronic oral
1982 toxicity study in rats. Biosafety Research Centre, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojama, K. Seki, S. & Okamura, T. Previcur N: Acute oral toxicity in
1982a mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. Seki, S. & Okamura, T. Acute subcutaneous toxicity study in
1982b mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute intraperitoneal
1982c toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute percutaneous
1982d toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute oral toxicity study in rats.
1982e Biosafety Research Center, Foods, Drugs, & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute subcutaneous toxicity study
1982f in rats. Biosafety Research Center, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute intraperitonial toxicity
1982g study in rats. Biosafety Research Center, Foods, Drugs and
pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. and Okamura, T. Acute percutaneous toxicity study
1982h in rats. Unpublished report from Biosafety Research Center,
Foods, Drugs and Pesticides, Japan. Submitted by Schering AG
to WHO. (Unpublished).
Kopp, R., Hümpel, M., Kühne, G. Aner, B., Klawa, D. & Rzadkiewicz, M.,
1979 Pharmacokinetics of Propamocarb hydrochloride on single &
repeated oral administration of 0.5 mg/kg in rats. Schering
AG. Submitted by Schering AG to WHO. (Unpublished).
Kopp, R., Günzel, P., RoBkamp, G., Schöbel, C. & Siegmund, F. Previcur
1981a N. Systemic tolerance test (LD50) ZK66752(CP604) in the dog
following single oral administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished)
Kopp, R., Günzel, P., Bhargava, A.S., Schöbel, C. & Slabke, P.
1981b Influence of SN66.752 upon the acetylcholinesterase activity
in vitro (dog & rat) and in vivo (dog) after single oral
administration. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Poggel, H.A., Kopp, R. & Günzel, P. Previcur N. (CP604).
1981 Embryotoxicity including teratogenicity study in rats after
daily intragastrical administration from day 6 to day 19 of
gestation. Schering AG. Submitted by Schering AG to WHO.
(Unpublished).
Reuzel, P.G.J. & Til, H.P. Subchronic (90-day) feeding study with
1977 ZK17.296 (SN39744 Propamocarb) in dogs (final report).
Centraal Instituut Voor Voedingsonderzoek, The Netherlands.
Submitted by Schering AG to WHO. (Unpublished).
RoBkamp, G. & Schuppler, J. ZK66752 Acute Inhalation toxicity to the
1976 rats (M + F) of aqueous ZK66.752 following single four-hour
continuous exposure (LC50). Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Rushbrook, C.J., Jorgenson, T.A. & Jones, D.C.L. Dominant lethal study
1979 of Previcur N. SRI International, USA. Submitted by Schering
AG to WHO. (Unpublished).
Schering A.G. Previcur N (CP604): embryotoxicity including
1981 teratogenicity study in rabbits after daily intragastrical
administration from day 6 to day 18 of gestation. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C. SN66752. Eye irritation, rabbits: single application of
1977 CP604 to the rabbit eye. Schering AG. Submitted by Schering
AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752: Acute oral (i.g.) toxicity
1976a mice (male) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752. Acute oral (i.g.) toxicity
1976b mice (female) single administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C.J. and Schuppler, J. SN6675: Dermal toxicity study of CP604
1976 on the intact and scarified skin of the rabbit following
daily application for 28 days. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Schuppler, J. ZK66752. Acute i.p. toxicity rats (M + F)
1977 single application. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752: acute oral (i.g.) toxicity rats
1976a (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK 66752: acute dermal toxicity rats
1976b (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752. Acute dermal toxicity rabbits
1976c (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary eye irritation after single
1983a application with Previcur N in the rabbit. Research &
Consulting Co., AG, Switzerland. Submitted by Schering AG to
WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary skin irritation following a single
1983b four-hour occlusive application with Previcur N in the
rabbit. Research & Consulting Co., AG., Switzerland.
Submitted by Schering AG to WHO. (Unpublished).
 Over the three generations, there were no treatment-related
effects on litter size on days 1, 4, 12 and 21; pup weight on days 1
and 4; sex ratio of pups and histopathological findings of F3b
weanlings. Incidence of dams with total litter loss was increased at
200 ppm only in F1b litters. Pup weight at 1000 ppm was decreased on
day 21 in F1a litters and on day 12 in F1b litters. An increase in
spleen weight was note in F3b weanlings at 1000 ppm. Examination of
pre-weaning pups from each generation for rate of physical development
(hair growth, incisor eruption, opening of the auditory canal and the
eye) and of selected F1b and F2b animals for pupillary reflex and
startle response at about 28 days of age indicated no abnormal
findings attributable to the compound.
Teratological investigation of F1b, F2b, and F3b litters
revealed no significant differences between control and treated groups
with respect to number of implantations or corpora lutea, litter size,
late resorptions of foetal weight. F2b litters at 1000 ppm exhibited
an increase in early resorptions and pre-implantation loss. Increased
renal pelvic cavitation, unilaterally or bilaterally, was found in
2/67 foetuses from 2/12 dams at 1000 ppm of the F3b litters, as
compared to an incidence of zero in 37 concurrent control foetuses
from a total of 9 dams examined for visceral abnormalities. The study
demonstrated a dietary level of 200 ppm to be without adverse effect
on the reproductive parameters examined. There was no clear evidence
for teratogenic potential at any dose tested. (Huntingdon Research
Centre, 1983).
Special Studies on Teratogenicity (Also see under "Special Studies
on Reproduction")
Rat
Groups of 25 mated female rats (Wistar - Han-Schering, SPF) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.1, 0.3, 1.0 or 3.0 ml/kg b.w./days from days 6
through day 19 of pregnancy. The dams were sacrificed on day 20
post-coitum and foetuses were removed for gross external, visceral and
skeletal examination. Five dams at 3 ml/kg b.w. and one dam at
1.0 ml/kg b.w. died, but with the exception of 1/5 dams of the top
dosage group showing severe pulmonary oedema, no abnormal findings
were reportedly seen at autopsy. In the top dosage group, a majority
of the dams exhibited toxic signs. Additionally, maternal weight gain
between days 16 and 20 post-coitum was depressed and uterine weight
was decreased. Also at the same dosage level, adverse effects were
observed on the mean number of early resorptions or live foetuses,
mean total litter size, incidence of pregnant dams with resorptions of
entire litters, post-implantation loss and foetal weight. There were
no significant differences between control and treated groups in mean
number of corpora lutea or implantations, pre-implantation loss and
sex ratio. Foetuses at both 1 and 3 ml/kg b.w. showed an increase
(dose-related) in the incidence of retarded ossification. An elevated
frequency of foetuses with 14 ribs was seen at 0.3 ml/kg b.w. and
above, but a dose-response pattern was not evident.
Based on the data, the compound appeared to retard foetal
development at and above 1 ml/kg b.w. (approximately equivalent to
700 mg a.i./kg b.w.). A dosage level of 3 ml/kg b.w. (approximately
equivalent to 2100 mg a.i./kg b.w.) was toxic to both mothers and
foetuses. There was however no clear evidence of teratogenic activity
at any level under the conditions of the experiment (Poggel, et al.,
1981).
Rabbit
Groups of 15 to 20 mated female rabbits (New Zealand White) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.2, 0.4 or 0.8 ml/kg b.w./day from days 6 through 18
of gestation. The does were sacrificed on day 28 of pregnancy and
uterine contents were examined. Live young were evaluated for external
malformation and incubated for 24 hours prior to sacrifice for
examination for skeletal and visceral abnormalities. Mortality
occurred in control and treated groups, but only two deaths at
0.8 ml/kg b.w. appeared to be treatment related. The total number of
does showing evidence of pregnancy (including those showing abortions
or total resorption) and surviving at termination was 12, 12, 11, 10
respectively at 0, 0.2, 0.4, 0.8 ml/kg b.w. Does at and above
0.4 ml/kg b.w. showed weight loss on gestation day 16 and growth
depression on day 28. Pregnancy rate, mean litter size, mean number of
early resorptions, incidence of pregnant does with early resorptions,
incidence of pregnant does with entire litters resorbed, post-
implantation loss and foetal survival rate after six- and 24-hour
incubation were all adversely affected in the top dosage group Foetal
weight was depressed at both 0.4 and 0.8 ml/kg b.w. Cleft palate, not
observed in any of the 71 foetuses from ten concurrent control litters
or in any of a combined total of 133 foetuses from 18 litters of the
two lower dosage groups, was observed in 1/21 foetuses from five
litters examined at 0.8 ml/kg b.w. Historical control data included in
the report on the incidence of cleft palate in foetuses of New Zealand
White rabbits were of limited value as a basis of comparison due to
the absence of the data in question using litter as the sampling unit
and lack of information on the time frame over which the data were
obtained. There were no significant differences between control and
treated groups in the other monitored parameters including the number
of implantations or corpora lutea, pre-implantation loss and visceral
or skeletal malformations of pups. The study appeared to indicate the
teratogenic no-effect level to be at least 0.4 ml/kg b.w. (equivalent
approximately to 280 mg/ai/kg b.w.). Maternal and foetal toxicity were
evident at this level but not at 0.2 ml/kg b.w. (Huntingdon Research
Centre, 1981).
Groups of 18 to 21 mated female rabbits (New Zealand White) were
treated orally with propamocarb hydrochloride as a 70% aqueous
formulation in water at 0, 0.02, 0.06, 0.2, 0.4 or 0.8 ml/kg b.w./day
from day 6 to day 18 of gestation. All does were sacrificed on day
28 post coitum and uterine contents were examined. Foetuses were
evaluated for external, skeletal and visceral abnormalities. Mortality
was not affected by treatment. The number of pregnant does with live
foetuses surviving on day 28 was 11, 14, 10, 11, 7, 13 respectively at
0, 0.02, 0.06, 0.2, 0.4, 0.8 ml/kg b.w. Pregnant does at 0.8 ml/kg
b.w. showed weight loss on day 18 and a decrease in weight gain on day
28. The mean number of dead foetuses per pregnant doe and the
incidence of pregnant does with dead foetuses were increased at
0.4 ml/kg b.w. only. The mean number of early resorptions was elevated
and the mean foetal weight was reduced at the top dosage level. Post-
implantation loss was increased at both 0.4 and 0.8 ml/kg b.w. Other
measured parameters, including number of corpora lutea or
implantations, litter size, pre-implantation loss and gross, skeletal
and visceral findings of foetuses, were not significantly different
from controls. Cleft palate, observed in a single foetus of the top
dosage group (0.8 ml/kg b.w.) in a previous rabbit study (Anon.,
1981), was not seen in any of the foetuses in the present study. The
teratogenic no-effect level was demonstrated at 0.8 ml/kg b.w.
(equivalent approximately to 560 mg a.i/kg b.w.) (Schering AG, 1981).
Special Studies on Mutagenicity
Mutagenicity studies for propamocarb hydrochloride as a 70%
aqueous formulation are summarized in Table 1.
Food consumption was marginally increased in males at 500 ppm
between weeks 79 and 104. Body weight, general appearance and
behaviour were unaffected. No gross pathological changes attributable
to ingestion of the compound were evident. Histopathological
examination of a wide range of tissues from all animals of control and
top dosage groups, plus any tissue showing macroscopic abnormality
suggestive of neoplasia revealed no treatment-related findings. There
was no significant difference between control and treated groups in
incidence of any particular type of tumour. The most frequently
observed tumours were liver tumours in males, lung tumours in both
sexes and lymphoreticular tumours in females. Over 65% of the
concurrent controls (both sexes) had spontaneous tumours. Under the
condition of the experiment, the test material was not carcinogenic
(Hunter, et al., 1983a).
Special Studies on Eye and Skin Irritation
In primary eye irritation studies with New Zealand White rabbits,
a 70% aqueous formulation of propamocarb hydrochloride was found to be
slightly irritating to the eye (Schöbel 1977; Ullmann & Suter 1983a).
Propamocarb hydrochloride, as a 70% aqueous formulation, was
shown to be practically non-irritating to the skin when applied to the
intact skin of New Zealand White rabbits (Ullmann & Suter 1983b).
In New Zealand White rabbits with skin sites exposed to a 70%
aqueous formulation of propamocarb hydrochloride (0.25 ml/site) five
hours/day for 28 consecutive days, signs of local irritation including
reddening, scabbing and thickening of the pithelium were noted at both
intact and abraded sites. Additionally, the abraded sites showed
reactions such as swelling and induration. Upon microscopic
examination, acanthosis, hyperkeratosis and necrosis were noted in the
abraded skin (Schöbel & Schuppler 1976).
Special Study on Skin Sensitization
A 70% aqueous formulation of propamocarb hydrochloride was not a
skin sensitizer in guinea pigs (Schöbel & Schuppler 1977).
Special Studies on Pharmacology
In mice treated with single intraveneous doses of propamocarb
hydrochloride as a 70% aqueous formulation, 30 mg/kg b.w. caused
restlessness and increased motor activity. After 100 mg/kg b.w.,
additional symptoms such as irregular breathing and exophthalmos
appeared. Death occurred at 175 mg/kg b.w. and above consequent to the
development of clonic convulsions. No clinical signs of toxicity or
abnormalities in normal reflexes and autonomic function were seen at
10 mg/kg b.w. or below. (Hara, et al., 1984).
Special Studies on Carcinogenicity (See also under "Long Term
Studies")
Mouse
Groups of 60 male and 60 female CD-1 mice, about 35 days old,
were fed dietary levels of propamocarb hydrochloride as a 70% aqueous
formulation at 0, 20, 100 or 500 ppm a.i. for two years. Mortality was
not affected by treatment. At termination, about 17 - 28% males and
42 - 52% females of control and treated groups survived. (Over 53%
males and at least 75% females per control and treated group lived at
least 78 weeks).
Single intravenous doses ranging from 3 to 100 mg/kg b.w. of the
70% aqueous formulation in mice did not produce analgesia or effects
on any of the following parameters: convulsions induced by
electroshock, barbital-induced anesthesia, pinnal and corneal reflexes
and muscular relaxation or coordination. No changes in rectal
temperature were detected in rabbits given 10 or 100 mg/kg b.w. of the
test formulation intravenously. Transitory alterations in spontaneous
EEG patterns were observed in rabbits treated intravenously at 100
mg/kg b.w. but not at 10 mg/kg b.w. and below. (Hara, et al., 1984).
Neuromuscular transmission in isolated rat diaphragm was slightly
enhanced initially but then slightly inhibited by the formulated
product at a concentration of 10-3 g/ml. Neuromuscular contraction in
rat gastrocnemius was unaffected in situ after an intravenous dose
of 100 mg/kg b.w. The test formulation, at concentrations of 10-5/ml
and above, inhibited acetylcholine- or histamine-induced contraction
of isolated guinea pig ileum and also acetylcholine-induced
contraction of isolated rabbit trachea; and enhanced nor-epinephrine-
induced contraction of isolated rat vas deferens. This concentration
(10-5 g/ml) did not modify the spontaneous contraction of atria
isolated from guinea-pig. The serotonin-induced contraction of
isolated rat fundus was depressed only at concentrations of 10-4 g/ml
and above. The oxytocin-induced contraction of isolated uterus in
oestrous rat was unaffected even at concentrations up to 10-3 g/ml.
(Hara, et al., 1984).
In anesthetized rabbits, intravenous doses of the formulation at
10 mg/kg b.w. and above produced bradycardia and antihypertensive
activity which were not affected by pretreatment with an intravenous
dose of 5 mg/kg b.w. of atropine. Tachycardia induced by 0.1 mg/kg
b.w. of isoproterenol given intraveneously was not antagonized by
intravenous administration of 30 mg/kg b.w. of the propamocarb
formulation. Blood coagulation in rats was not significantly affected
by an intravenous dose of 100 mg/kg b.w. of the aqueous formulation.
In vitro clotting time of blood from the rabbit was prolonged by the
formulation at a concentration of 10-2 g/ml but not below (Hara,
et al., 1984).
Acute Toxicity
Acute toxicity of propamocarb hydrochloride to rats, mice,
rabbits and dogs is summarized in Table 2. High doses produced
hypokinesis, convulsion, staggering gait, ataxia and piloerection
within 24 hours post-dosing.
Short Term Studies
Oral/Dietary
Rat
Groups of ten male and ten female rats were fed the free base of
propamocarb hydrochloride in their diet at 0, 20, 50 or 1000 ppm for
90 days. An additional group of the same size was given 500 ppm for
6 weeks and 1000 ppm for the remaining 7 weeks. There was no mortality
or abnormal behaviour. None of the parameters monitored, that is
weekly body weight, food consumption, haematology, urinalysis,
terminal blood chemistry, activity of plasma, erythrocyte, and brain
cholinesterase, were adversely affected. Gross pathological changes
and organ weights were not significantly different from those in the
controls. Histopathological examination of a number of selected
tissues from animals of control and top-dosage groups failed to reveal
any treatment-related findings. The no-effect level was demonstrated
at 500 ppm (de Rijke Köter & Leegwater, 1976).
Groups of 30 male and 30 female rats were fed diets containing
propamocarb hydrochloride, in the form of a 70% aqueous formulation,
at 0, 200, 1000 or 5000 ppm a.i. for 13 weeks. No mortality or
clinical signs were observed. Ophthalmoscopic findings, clinical
chemistry and haematology were not influenced by treatment. Activity
of plasma and erythrocyte cholinesterase at weeks 7 and 13 or of
terminal brain cholinesterase, determined in 24-hour fasted animals,
was not inhibited. In females at 5000 ppm, growth was slightly
decreased (< 10%) frequently during the study, and water consumption
at week 1 was reduced. Feed efficiency was decreased intermittently
over the course of the study in males at 5000 ppm and in females at
both 1000 and 5000 ppm. Proteinuria occurred in both sexes of all
groups including the control at weeks 7 and 13 with the males showing
greater degree of severity. At terminal sacrifice a decrease of
absolute weight of liver and spleen and an increase in organ/body
weight ratio of kidney in both sexes were evident in the high dose
only. There were no significant gross pathological changes between
control and treated groups. Histopathological evaluation of a number of
selected tissues including the bone marrow plus all grossly abnormal
tissues from each animal indicated an increase in incidence of
granulomatous inflammation of the liver and of hyperplasia of the
lymph node in females at 5000 ppm. The study showed the no-effect
level to be at least 200 ppm. (Kojima & Enomoto 1982).
Dog
Groups of pure-bred beagle dogs (four males and four females/
group) were fed the free base of propamocarb hydrochloride in the diet
at 0, 50, 100, 500 or 1000 ppm for 90 days with the top dosage level
being raised to 2000 ppm at week 7. No compound-related effects were
observed on survival, general appearance and behaviour, growth or food
consumption, haematology, blood chemistry and urinalysis as well as
liver and kidney function tests. Assay of plasma, erythrocyte and
brain cholinesterase revealed no significant inhibition.
At terminal sacrifice, organ weights and gross pathological
changes were not altered by the compound. Microscopic evaluation
indicated no compound-related findings. The no-effect level was at
least 1000 ppm (Reuzel & Til (1977).
Dermal
Rabbit
Groups of New Zealand White rabbits (three animals sex/group,
with skin abraded) were exposed dermally to propamocarb hydrochloride
as a 70% aqueous formulation at 0, 0.2, 0.7 or 2.0 ml/kg b.w. under
occlusive patch for a period of six-hours/day, five days/week for a
total of 15 applications in 21 days. Treated sites were washed at the
end of each exposure period. There were no compound-induced systemic
effects as judged by mortality, clinical signs, haematology, blood
chemistry, organ weight, gross pathology and histopathology.
Mild to moderate erythema and oedema were seen in animals of all
treated groups and increased with dosage (Field 1980).
Table 1. Special Studies on mutagenicity of Propamocarb Hydrochloride
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
In Vitro
Ames' test S.typhimurium 3.5- 70% negative Hastwell, et al.,
(with and strains TA 1535, 5,000 ug/plate aq with or without 1977;
without TA100,TA1537 solv. metabolic Kojima &
metabolic TA1538,TA98 activation Nakajima 1981a
activation) E.Coli WP2uvrA
Rec-assay Bacillus+subtilis 500- 70% negative Kojima &
H17 (rec±) and 10,000 ug/disk aq without Nakajima 1981b
M45 (rec) solv. metabolic
activation
Saccharomyces cerevisae
Mitotic strain D4 ) 1,000- 70% negative Jagannath & Brusick,
gene mutation ) 10,000 ug/plate aq. with or without Hoorn, 1980
) solv. metabolic
Reverse strains S138 and ) activation
mutation S211C )
)
Mitotic strain D5 )
recombination )
In Vivo
Micronucleus Mouse 1,250 to 5,000 70% negative Richold & Richardson,
test mg/kg b.w. aq. Hossack,1980
orally solv.
Table 1. (continued)
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
Dominant Mouse 424 to 1,237 70% negative Jorgenson & Jones,
lethal assay mg/kg b.w./day aq. Rushbrook,1979
for 8 weeks in solv.
drinking water;
prior to mating
weekly for 2
consecutive weeks
Table 2. Acute Toxicity of Propamocarb Hydrochloride to Animals
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Mouse M + F oral 1600 to >2858 Schöbel & Etreby,
1976a & 1976b;
Rushbrook, et al.,
1982a,Kojima, et al.,
1982a
M s.c. 1710 Kojima, et al.,
F 1870 1982b
M i.p. 457 Kojima, et al.,
F 435 1982c
M + F dermal >3000 Kojima, et al.,
1982d
Rat M + F oral 2000 to Kojima, et al.,
1982e
8600 Schöbel & Siegmund,
1976a
M s.c. 5220 Kojima,et al.,1982f
F 3230
Rat M + F i.p. 437-763 Schöbel & Schuppler,
1977; Kojima, et al.,
1982g
Rat M + F dermal >3920 Schöbel & Siegmund,
(duration of 1976g
exposure not
specified)
Rat M + F dermal >3000 Kojima, et al., 1982h
(24-hour
exposure)
Rat M + F inhalation >3960 mg/m3* Robkamp &
(4-hour Schuppler, 1976
"nose only"
exposure
Table 2. (continued)
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Rabbit M + F dermal >3920 Schöbel & Siegmund
(duration of 1976c
exposure not
specified)
Dog** M + F oral <674 Kopp, et al.,
1981a & 1981b
* 4-hour LC50
** 674, 1000 and 2000 mg/kg produced 3/6 mortalities with emesis of doses > 1000 mg/kg.
Long-term Studies
Rat
Groups of 50 male and 50 female Sprague-Dawley CD rats were fed
diets containing propamocarb hydrochloride as a 70% aqueous
formulation at 0, 40, 200 or 1000 ppm a.i. for 104 weeks. Additional
animals (ten males and ten females per group) were fed the same
dietary levels for the same duration and used primarily for routine
laboratory investigations (satellite group). Two additional groups of
ten males and ten females were fed 0 or 1000 ppm of the test diet with
one-half of the animals in each group sacrificed after 52 weeks and
the remainder at the end of a four-week withdrawal period. These
animals were subjected to macroscopic examination and organ weight
analysis.
Mortality was not influenced by treatment. Forty-six to 58% of
the males and 30-44% of the females of all groups including the
control survived at the end of two years. Over 50% of the animals
(both sexes) of control and treated groups lived at least 91 weeks.
Compound-related clinical signs were not apparent. No consistent dose-
related changes were seen on body weight, food consumption and water
intake. Haematological, clinical chemistry and urinalysis values as
well as ophthalmoscopic findings, monitored at several intervals over
the course of the study, were not significantly different between
control and treated groups. Activity of tissue cholinesterase was not
measured at any time during the study. Gross pathological changes in
treated animals dying or sacrificed in extremis during the study and
in those sacrificed at 52 weeks, after the four-week withdrawal
period, or terminally were comparable to those in the control group.
Organ weight determinations revealed no significant difference between
control and treated groups. Histopathological evaluation of non-
neoplastic findings was unremarkable except for an increased incidence
of degenerative hepatocytes/necrosis in high-dose males.
Evaluation of the neoplastic changes demonstrated an increase in
occurrence in males of subcutaneous fibrosarcoma in the treated
groups. Incidence of the tumour was 0/58 in controls, 5/56 (8.9%) at
40 ppm, 2/58 (3.5%) at 200 ppm and 7/55 (12.7%) at 1000 ppm (the
denominator denoted the number of survivors from both main and
satellite groups at each dosage level at the end of 51 weeks when the
first subcutaneous fibrosarcoma was detected in the males). The
difference from concurrent controls was statistically significant at
40 ppm and 1000 ppm. Data on background incidence of subcutaneous
fibrosarcoma in the particular strain of rats employed in the study
were not available in the report.
Over 80% of the concurrent controls (both sexes) had tumours,
with pituitary adenoma (both sexes), mammary tumours (females) and
subcutaneous fibroma and lipoma (males), the most prevalent tumours.
There were no other neoplastic differences between treated and control
groups. The no-effect level for non-neoplastic effects was equal to
8.3 mg a.i/kg b.w./day, based on food consumption and body weight data
(Hunter, et al., 1983b).
Comments
Propamocarb hydrochloride was evaluated for the first time by the
JMPR at this session.
The compound, a carbamate fungicide, is rapidly absorbed,
degraded and readily excreted primarily in the urine of (female) rats.
Oxidation of the parent molecule appears to be the major metabolic
pathway. There is no evidence of significant tissue accumulation of
propamocarb hydrochloride and/or its metabolites. Metabolism data in
mammalian species other than the rat are not available.
Propamocarb hydrochloride has a relatively low order of acute
oral toxicity in rodents, with LD5 values of over 1500 mg/kg b.w. in
both mice and rats. Limited acute oral data in dogs appeared to
indicate that they are more sensitive than rodents to toxicity of the
compound.
A three-generation reproduction study, including teratological
investigation in rats, showed a no-effect level of 200 ppm on
reproduction and teratogenicity. A teratology study in rats showed
retardation of foetal development at doses which were maternally
toxic. Teratology studies in rabbits were negative. Mutagenicity
studies were also negative. A carcinogenicity study in mice was
negative. A long-term study in rats showed some evidence of increased
incidence of sub-cutaneous fibrosarcoma. Therefore, background data on
incidence of this malignancy in the particular strain of rats are
required.
Ninety-day feeding studies in rats and dogs established no-effect
levels of at least 200 ppm and 1000 ppm respectively. Inhibition of
brain and erythrocyte cholinesterases was not observed in these
studies.
In view of the concerns with respect to the elevated incidence of
subcutaneous fibrosarcoma in male rats of the long-term study and the
non-availability of a dog study longer than three months, only a
temporary ADI was granted.
Level causing no toxicological effect
Rat: 200 ppm in the diet, equivalent to 8.3 mg/kg b.w.
Dog: 1,000 ppm in the diet, equivalent to 25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans
0 - 0.2 mg/kg b.w.
Further work or information
Required (by 1986):
Historical data on the incidence of subcutaneous fibrosarcoma in the
CD strain of rats used in the long-term study.
Complete data on the 24-month dog study known to have been completed.
Metabolism and pharmacokinetic studies in mice and rats using multiple
dosage levels.
Desirable:
Observations in humans.
Complete data on the 4-5 week feeding study in rats.
REFERENCES
Brühl, R. & Celorio, J. Propamocarb hydrochloride: Metabolism in rats.
1982 Submitted by Schering AG to WHO. (Unpublished).
Brühl, R. & Celorio, J. Metabolic pathway of propamocarb hydrochloride
1984 in the rat. Submitted by Schering AG to WHO. (Unpublished).
de Rijke, D., Köter, H.B.W.M. & Leegwater, D.C. Subchronic (90-day)
1976 toxicity study with ZK17.296 in albino rats (ZK 17296 =
SN39744 = Propamocarb). Centraal Instituut Voor
Voedingsonderzoek, The Netherlands. Submitted by Schering AG
to WHO. (Unpublished).
Field, W.E. Twenty-one day dermal toxicity study in rabbits treated
1980 with Previcur N. (Final report). CDC Research, Inc. U.S.A.
Submitted by Schering AG to WHO. (UNPUBLISHED).
Hara, K., Ikoma, Y. Arita, S., Tokiyori, Y., Nakata, Y., Yamano, K.,
1984 Mizuno, M. & Oshino, N. General pharmacological activities
of Previcur N. Nikon Schering K.K. Japan. Submitted by
Schering AG to WHO. (Unpublished).
Hastwell, R., McGregor, D.B. & Smith, I. Mutagenicity testing of
1977 SN 66.752. Inveresk Research International, Scotland.
Submitted by Schering AG to WHO. (Unpublished).
Hoorn, A.J.W., Jagannath, D.R. & Brusick, D.J. Mutagenic evaluation of
1980 Previcur N. Batch No. 271001B00000 in the mitotic gene
conversion, reverse mutation & mitotic recombination assays
in yeast. Litton Bionetics, Inc. Submitted by Schering AG to
WHO. (Unpublished).
Hossack, D.J.N., Richold, M. & Richardson, J.C. Micronucleus test on
1980 CP604 (SN66752, Previcur N). Huntingdon Research Centre,
England. Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E. & Gibson, W.A.
1978 Previcur N (SN66752). Assessment of whole blood & brain
cholinesterase activities in rats after 11 days of treatment
by oral gavage. Huntington Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Watson, M., Read, R.M., Prentice, D.E., Woodhouse, R.N.,
1983a Cherry, C.P. & Gibson, W.A. Previcur N (SN 66752): Potential
tumorigenicity to mice in dietary administration for 104
weeks (final report). Huntingdon Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E., Jolly, D.W.,
1983b Offer, J.M. & Gibson, W.A. Previcur N (SN66752): Toxicity &
potential tumorigenicity in dietary administration to rats
for 104 weeks. (final amended report). Huntingdon Research
Centre, England. Submitted by Schering AG to WHO.
(Unpublished).
Huntingdon Research Centre. Previcur N teratology study: effect of
1981 administration during organogensis on foetuses of the
rabbit. Reprotox, Deutschland. Submitted by Schering AG to
WHO. (Unpublished).
Huntingdon Research Centre. Effect of Previcur N on reproductive
1983 function of multiple generations in the rat. Reprotox,
Deutschland. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. Mutagenicity test in bacteria with Previcur
1981a N. Biosafety Research Centre, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. "REC" assay to detect mutagenic activity of
1981b Previcur N. Biosafety Research Centre, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K. & Enomoto, M. Previcur N: Three-month subchronic oral
1982 toxicity study in rats. Biosafety Research Centre, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojama, K. Seki, S. & Okamura, T. Previcur N: Acute oral toxicity in
1982a mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. Seki, S. & Okamura, T. Acute subcutaneous toxicity study in
1982b mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute intraperitoneal
1982c toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute percutaneous
1982d toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute oral toxicity study in rats.
1982e Biosafety Research Center, Foods, Drugs, & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute subcutaneous toxicity study
1982f in rats. Biosafety Research Center, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute intraperitonial toxicity
1982g study in rats. Biosafety Research Center, Foods, Drugs and
pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. and Okamura, T. Acute percutaneous toxicity study
1982h in rats. Unpublished report from Biosafety Research Center,
Foods, Drugs and Pesticides, Japan. Submitted by Schering AG
to WHO. (Unpublished).
Kopp, R., Hümpel, M., Kühne, G. Aner, B., Klawa, D. & Rzadkiewicz, M.,
1979 Pharmacokinetics of Propamocarb hydrochloride on single &
repeated oral administration of 0.5 mg/kg in rats. Schering
AG. Submitted by Schering AG to WHO. (Unpublished).
Kopp, R., Günzel, P., RoBkamp, G., Schöbel, C. & Siegmund, F. Previcur
1981a N. Systemic tolerance test (LD50) ZK66752(CP604) in the dog
following single oral administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished)
Kopp, R., Günzel, P., Bhargava, A.S., Schöbel, C. & Slabke, P.
1981b Influence of SN66.752 upon the acetylcholinesterase activity
in vitro (dog & rat) and in vivo (dog) after single oral
administration. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Poggel, H.A., Kopp, R. & Günzel, P. Previcur N. (CP604).
1981 Embryotoxicity including teratogenicity study in rats after
daily intragastrical administration from day 6 to day 19 of
gestation. Schering AG. Submitted by Schering AG to WHO.
(Unpublished).
Reuzel, P.G.J. & Til, H.P. Subchronic (90-day) feeding study with
1977 ZK17.296 (SN39744 Propamocarb) in dogs (final report).
Centraal Instituut Voor Voedingsonderzoek, The Netherlands.
Submitted by Schering AG to WHO. (Unpublished).
RoBkamp, G. & Schuppler, J. ZK66752 Acute Inhalation toxicity to the
1976 rats (M + F) of aqueous ZK66.752 following single four-hour
continuous exposure (LC50). Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Rushbrook, C.J., Jorgenson, T.A. & Jones, D.C.L. Dominant lethal study
1979 of Previcur N. SRI International, USA. Submitted by Schering
AG to WHO. (Unpublished).
Schering A.G. Previcur N (CP604): embryotoxicity including
1981 teratogenicity study in rabbits after daily intragastrical
administration from day 6 to day 18 of gestation. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C. SN66752. Eye irritation, rabbits: single application of
1977 CP604 to the rabbit eye. Schering AG. Submitted by Schering
AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752: Acute oral (i.g.) toxicity
1976a mice (male) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752. Acute oral (i.g.) toxicity
1976b mice (female) single administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C.J. and Schuppler, J. SN6675: Dermal toxicity study of CP604
1976 on the intact and scarified skin of the rabbit following
daily application for 28 days. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Schuppler, J. ZK66752. Acute i.p. toxicity rats (M + F)
1977 single application. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752: acute oral (i.g.) toxicity rats
1976a (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK 66752: acute dermal toxicity rats
1976b (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752. Acute dermal toxicity rabbits
1976c (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary eye irritation after single
1983a application with Previcur N in the rabbit. Research &
Consulting Co., AG, Switzerland. Submitted by Schering AG to
WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary skin irritation following a single
1983b four-hour occlusive application with Previcur N in the
rabbit. Research & Consulting Co., AG., Switzerland.
Submitted by Schering AG to WHO. (Unpublished).
Over the three generations, there were no treatment-related
effects on litter size on days 1, 4, 12 and 21; pup weight on days 1
and 4; sex ratio of pups and histopathological findings of F3b
weanlings. Incidence of dams with total litter loss was increased at
200 ppm only in F1b litters. Pup weight at 1000 ppm was decreased on
day 21 in F1a litters and on day 12 in F1b litters. An increase in
spleen weight was note in F3b weanlings at 1000 ppm. Examination of
pre-weaning pups from each generation for rate of physical development
(hair growth, incisor eruption, opening of the auditory canal and the
eye) and of selected F1b and F2b animals for pupillary reflex and
startle response at about 28 days of age indicated no abnormal
findings attributable to the compound.
Teratological investigation of F1b, F2b, and F3b litters
revealed no significant differences between control and treated groups
with respect to number of implantations or corpora lutea, litter size,
late resorptions of foetal weight. F2b litters at 1000 ppm exhibited
an increase in early resorptions and pre-implantation loss. Increased
renal pelvic cavitation, unilaterally or bilaterally, was found in
2/67 foetuses from 2/12 dams at 1000 ppm of the F3b litters, as
compared to an incidence of zero in 37 concurrent control foetuses
from a total of 9 dams examined for visceral abnormalities. The study
demonstrated a dietary level of 200 ppm to be without adverse effect
on the reproductive parameters examined. There was no clear evidence
for teratogenic potential at any dose tested. (Huntingdon Research
Centre, 1983).
Special Studies on Teratogenicity (Also see under "Special Studies
on Reproduction")
Rat
Groups of 25 mated female rats (Wistar - Han-Schering, SPF) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.1, 0.3, 1.0 or 3.0 ml/kg b.w./days from days 6
through day 19 of pregnancy. The dams were sacrificed on day 20
post-coitum and foetuses were removed for gross external, visceral and
skeletal examination. Five dams at 3 ml/kg b.w. and one dam at
1.0 ml/kg b.w. died, but with the exception of 1/5 dams of the top
dosage group showing severe pulmonary oedema, no abnormal findings
were reportedly seen at autopsy. In the top dosage group, a majority
of the dams exhibited toxic signs. Additionally, maternal weight gain
between days 16 and 20 post-coitum was depressed and uterine weight
was decreased. Also at the same dosage level, adverse effects were
observed on the mean number of early resorptions or live foetuses,
mean total litter size, incidence of pregnant dams with resorptions of
entire litters, post-implantation loss and foetal weight. There were
no significant differences between control and treated groups in mean
number of corpora lutea or implantations, pre-implantation loss and
sex ratio. Foetuses at both 1 and 3 ml/kg b.w. showed an increase
(dose-related) in the incidence of retarded ossification. An elevated
frequency of foetuses with 14 ribs was seen at 0.3 ml/kg b.w. and
above, but a dose-response pattern was not evident.
Based on the data, the compound appeared to retard foetal
development at and above 1 ml/kg b.w. (approximately equivalent to
700 mg a.i./kg b.w.). A dosage level of 3 ml/kg b.w. (approximately
equivalent to 2100 mg a.i./kg b.w.) was toxic to both mothers and
foetuses. There was however no clear evidence of teratogenic activity
at any level under the conditions of the experiment (Poggel, et al.,
1981).
Rabbit
Groups of 15 to 20 mated female rabbits (New Zealand White) were
intubated with a 70% aqueous formulation of propamocarb hydrochloride
in water at 0, 0.2, 0.4 or 0.8 ml/kg b.w./day from days 6 through 18
of gestation. The does were sacrificed on day 28 of pregnancy and
uterine contents were examined. Live young were evaluated for external
malformation and incubated for 24 hours prior to sacrifice for
examination for skeletal and visceral abnormalities. Mortality
occurred in control and treated groups, but only two deaths at
0.8 ml/kg b.w. appeared to be treatment related. The total number of
does showing evidence of pregnancy (including those showing abortions
or total resorption) and surviving at termination was 12, 12, 11, 10
respectively at 0, 0.2, 0.4, 0.8 ml/kg b.w. Does at and above
0.4 ml/kg b.w. showed weight loss on gestation day 16 and growth
depression on day 28. Pregnancy rate, mean litter size, mean number of
early resorptions, incidence of pregnant does with early resorptions,
incidence of pregnant does with entire litters resorbed, post-
implantation loss and foetal survival rate after six- and 24-hour
incubation were all adversely affected in the top dosage group Foetal
weight was depressed at both 0.4 and 0.8 ml/kg b.w. Cleft palate, not
observed in any of the 71 foetuses from ten concurrent control litters
or in any of a combined total of 133 foetuses from 18 litters of the
two lower dosage groups, was observed in 1/21 foetuses from five
litters examined at 0.8 ml/kg b.w. Historical control data included in
the report on the incidence of cleft palate in foetuses of New Zealand
White rabbits were of limited value as a basis of comparison due to
the absence of the data in question using litter as the sampling unit
and lack of information on the time frame over which the data were
obtained. There were no significant differences between control and
treated groups in the other monitored parameters including the number
of implantations or corpora lutea, pre-implantation loss and visceral
or skeletal malformations of pups. The study appeared to indicate the
teratogenic no-effect level to be at least 0.4 ml/kg b.w. (equivalent
approximately to 280 mg/ai/kg b.w.). Maternal and foetal toxicity were
evident at this level but not at 0.2 ml/kg b.w. (Huntingdon Research
Centre, 1981).
Groups of 18 to 21 mated female rabbits (New Zealand White) were
treated orally with propamocarb hydrochloride as a 70% aqueous
formulation in water at 0, 0.02, 0.06, 0.2, 0.4 or 0.8 ml/kg b.w./day
from day 6 to day 18 of gestation. All does were sacrificed on day
28 post coitum and uterine contents were examined. Foetuses were
evaluated for external, skeletal and visceral abnormalities. Mortality
was not affected by treatment. The number of pregnant does with live
foetuses surviving on day 28 was 11, 14, 10, 11, 7, 13 respectively at
0, 0.02, 0.06, 0.2, 0.4, 0.8 ml/kg b.w. Pregnant does at 0.8 ml/kg
b.w. showed weight loss on day 18 and a decrease in weight gain on day
28. The mean number of dead foetuses per pregnant doe and the
incidence of pregnant does with dead foetuses were increased at
0.4 ml/kg b.w. only. The mean number of early resorptions was elevated
and the mean foetal weight was reduced at the top dosage level. Post-
implantation loss was increased at both 0.4 and 0.8 ml/kg b.w. Other
measured parameters, including number of corpora lutea or
implantations, litter size, pre-implantation loss and gross, skeletal
and visceral findings of foetuses, were not significantly different
from controls. Cleft palate, observed in a single foetus of the top
dosage group (0.8 ml/kg b.w.) in a previous rabbit study (Anon.,
1981), was not seen in any of the foetuses in the present study. The
teratogenic no-effect level was demonstrated at 0.8 ml/kg b.w.
(equivalent approximately to 560 mg a.i/kg b.w.) (Schering AG, 1981).
Special Studies on Mutagenicity
Mutagenicity studies for propamocarb hydrochloride as a 70%
aqueous formulation are summarized in Table 1.
Food consumption was marginally increased in males at 500 ppm
between weeks 79 and 104. Body weight, general appearance and
behaviour were unaffected. No gross pathological changes attributable
to ingestion of the compound were evident. Histopathological
examination of a wide range of tissues from all animals of control and
top dosage groups, plus any tissue showing macroscopic abnormality
suggestive of neoplasia revealed no treatment-related findings. There
was no significant difference between control and treated groups in
incidence of any particular type of tumour. The most frequently
observed tumours were liver tumours in males, lung tumours in both
sexes and lymphoreticular tumours in females. Over 65% of the
concurrent controls (both sexes) had spontaneous tumours. Under the
condition of the experiment, the test material was not carcinogenic
(Hunter, et al., 1983a).
Special Studies on Eye and Skin Irritation
In primary eye irritation studies with New Zealand White rabbits,
a 70% aqueous formulation of propamocarb hydrochloride was found to be
slightly irritating to the eye (Schöbel 1977; Ullmann & Suter 1983a).
Propamocarb hydrochloride, as a 70% aqueous formulation, was
shown to be practically non-irritating to the skin when applied to the
intact skin of New Zealand White rabbits (Ullmann & Suter 1983b).
In New Zealand White rabbits with skin sites exposed to a 70%
aqueous formulation of propamocarb hydrochloride (0.25 ml/site) five
hours/day for 28 consecutive days, signs of local irritation including
reddening, scabbing and thickening of the pithelium were noted at both
intact and abraded sites. Additionally, the abraded sites showed
reactions such as swelling and induration. Upon microscopic
examination, acanthosis, hyperkeratosis and necrosis were noted in the
abraded skin (Schöbel & Schuppler 1976).
Special Study on Skin Sensitization
A 70% aqueous formulation of propamocarb hydrochloride was not a
skin sensitizer in guinea pigs (Schöbel & Schuppler 1977).
Special Studies on Pharmacology
In mice treated with single intraveneous doses of propamocarb
hydrochloride as a 70% aqueous formulation, 30 mg/kg b.w. caused
restlessness and increased motor activity. After 100 mg/kg b.w.,
additional symptoms such as irregular breathing and exophthalmos
appeared. Death occurred at 175 mg/kg b.w. and above consequent to the
development of clonic convulsions. No clinical signs of toxicity or
abnormalities in normal reflexes and autonomic function were seen at
10 mg/kg b.w. or below. (Hara, et al., 1984).
Special Studies on Carcinogenicity (See also under "Long Term
Studies")
Mouse
Groups of 60 male and 60 female CD-1 mice, about 35 days old,
were fed dietary levels of propamocarb hydrochloride as a 70% aqueous
formulation at 0, 20, 100 or 500 ppm a.i. for two years. Mortality was
not affected by treatment. At termination, about 17 - 28% males and
42 - 52% females of control and treated groups survived. (Over 53%
males and at least 75% females per control and treated group lived at
least 78 weeks).
Single intravenous doses ranging from 3 to 100 mg/kg b.w. of the
70% aqueous formulation in mice did not produce analgesia or effects
on any of the following parameters: convulsions induced by
electroshock, barbital-induced anesthesia, pinnal and corneal reflexes
and muscular relaxation or coordination. No changes in rectal
temperature were detected in rabbits given 10 or 100 mg/kg b.w. of the
test formulation intravenously. Transitory alterations in spontaneous
EEG patterns were observed in rabbits treated intravenously at 100
mg/kg b.w. but not at 10 mg/kg b.w. and below. (Hara, et al., 1984).
Neuromuscular transmission in isolated rat diaphragm was slightly
enhanced initially but then slightly inhibited by the formulated
product at a concentration of 10-3 g/ml. Neuromuscular contraction in
rat gastrocnemius was unaffected in situ after an intravenous dose
of 100 mg/kg b.w. The test formulation, at concentrations of 10-5/ml
and above, inhibited acetylcholine- or histamine-induced contraction
of isolated guinea pig ileum and also acetylcholine-induced
contraction of isolated rabbit trachea; and enhanced nor-epinephrine-
induced contraction of isolated rat vas deferens. This concentration
(10-5 g/ml) did not modify the spontaneous contraction of atria
isolated from guinea-pig. The serotonin-induced contraction of
isolated rat fundus was depressed only at concentrations of 10-4 g/ml
and above. The oxytocin-induced contraction of isolated uterus in
oestrous rat was unaffected even at concentrations up to 10-3 g/ml.
(Hara, et al., 1984).
In anesthetized rabbits, intravenous doses of the formulation at
10 mg/kg b.w. and above produced bradycardia and antihypertensive
activity which were not affected by pretreatment with an intravenous
dose of 5 mg/kg b.w. of atropine. Tachycardia induced by 0.1 mg/kg
b.w. of isoproterenol given intraveneously was not antagonized by
intravenous administration of 30 mg/kg b.w. of the propamocarb
formulation. Blood coagulation in rats was not significantly affected
by an intravenous dose of 100 mg/kg b.w. of the aqueous formulation.
In vitro clotting time of blood from the rabbit was prolonged by the
formulation at a concentration of 10-2 g/ml but not below (Hara,
et al., 1984).
Acute Toxicity
Acute toxicity of propamocarb hydrochloride to rats, mice,
rabbits and dogs is summarized in Table 2. High doses produced
hypokinesis, convulsion, staggering gait, ataxia and piloerection
within 24 hours post-dosing.
Short Term Studies
Oral/Dietary
Rat
Groups of ten male and ten female rats were fed the free base of
propamocarb hydrochloride in their diet at 0, 20, 50 or 1000 ppm for
90 days. An additional group of the same size was given 500 ppm for
6 weeks and 1000 ppm for the remaining 7 weeks. There was no mortality
or abnormal behaviour. None of the parameters monitored, that is
weekly body weight, food consumption, haematology, urinalysis,
terminal blood chemistry, activity of plasma, erythrocyte, and brain
cholinesterase, were adversely affected. Gross pathological changes
and organ weights were not significantly different from those in the
controls. Histopathological examination of a number of selected
tissues from animals of control and top-dosage groups failed to reveal
any treatment-related findings. The no-effect level was demonstrated
at 500 ppm (de Rijke Köter & Leegwater, 1976).
Groups of 30 male and 30 female rats were fed diets containing
propamocarb hydrochloride, in the form of a 70% aqueous formulation,
at 0, 200, 1000 or 5000 ppm a.i. for 13 weeks. No mortality or
clinical signs were observed. Ophthalmoscopic findings, clinical
chemistry and haematology were not influenced by treatment. Activity
of plasma and erythrocyte cholinesterase at weeks 7 and 13 or of
terminal brain cholinesterase, determined in 24-hour fasted animals,
was not inhibited. In females at 5000 ppm, growth was slightly
decreased (< 10%) frequently during the study, and water consumption
at week 1 was reduced. Feed efficiency was decreased intermittently
over the course of the study in males at 5000 ppm and in females at
both 1000 and 5000 ppm. Proteinuria occurred in both sexes of all
groups including the control at weeks 7 and 13 with the males showing
greater degree of severity. At terminal sacrifice a decrease of
absolute weight of liver and spleen and an increase in organ/body
weight ratio of kidney in both sexes were evident in the high dose
only. There were no significant gross pathological changes between
control and treated groups. Histopathological evaluation of a number of
selected tissues including the bone marrow plus all grossly abnormal
tissues from each animal indicated an increase in incidence of
granulomatous inflammation of the liver and of hyperplasia of the
lymph node in females at 5000 ppm. The study showed the no-effect
level to be at least 200 ppm. (Kojima & Enomoto 1982).
Dog
Groups of pure-bred beagle dogs (four males and four females/
group) were fed the free base of propamocarb hydrochloride in the diet
at 0, 50, 100, 500 or 1000 ppm for 90 days with the top dosage level
being raised to 2000 ppm at week 7. No compound-related effects were
observed on survival, general appearance and behaviour, growth or food
consumption, haematology, blood chemistry and urinalysis as well as
liver and kidney function tests. Assay of plasma, erythrocyte and
brain cholinesterase revealed no significant inhibition.
At terminal sacrifice, organ weights and gross pathological
changes were not altered by the compound. Microscopic evaluation
indicated no compound-related findings. The no-effect level was at
least 1000 ppm (Reuzel & Til (1977).
Dermal
Rabbit
Groups of New Zealand White rabbits (three animals sex/group,
with skin abraded) were exposed dermally to propamocarb hydrochloride
as a 70% aqueous formulation at 0, 0.2, 0.7 or 2.0 ml/kg b.w. under
occlusive patch for a period of six-hours/day, five days/week for a
total of 15 applications in 21 days. Treated sites were washed at the
end of each exposure period. There were no compound-induced systemic
effects as judged by mortality, clinical signs, haematology, blood
chemistry, organ weight, gross pathology and histopathology.
Mild to moderate erythema and oedema were seen in animals of all
treated groups and increased with dosage (Field 1980).
Table 1. Special Studies on mutagenicity of Propamocarb Hydrochloride
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
In Vitro
Ames' test S.typhimurium 3.5- 70% negative Hastwell, et al.,
(with and strains TA 1535, 5,000 ug/plate aq with or without 1977;
without TA100,TA1537 solv. metabolic Kojima &
metabolic TA1538,TA98 activation Nakajima 1981a
activation) E.Coli WP2uvrA
Rec-assay Bacillus+subtilis 500- 70% negative Kojima &
H17 (rec±) and 10,000 ug/disk aq without Nakajima 1981b
M45 (rec) solv. metabolic
activation
Saccharomyces cerevisae
Mitotic strain D4 ) 1,000- 70% negative Jagannath & Brusick,
gene mutation ) 10,000 ug/plate aq. with or without Hoorn, 1980
) solv. metabolic
Reverse strains S138 and ) activation
mutation S211C )
)
Mitotic strain D5 )
recombination )
In Vivo
Micronucleus Mouse 1,250 to 5,000 70% negative Richold & Richardson,
test mg/kg b.w. aq. Hossack,1980
orally solv.
Table 1. (continued)
Test Test object Concentrations of Purity Results Reference
System propamocarb HCL used
Dominant Mouse 424 to 1,237 70% negative Jorgenson & Jones,
lethal assay mg/kg b.w./day aq. Rushbrook,1979
for 8 weeks in solv.
drinking water;
prior to mating
weekly for 2
consecutive weeks
Table 2. Acute Toxicity of Propamocarb Hydrochloride to Animals
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Mouse M + F oral 1600 to >2858 Schöbel & Etreby,
1976a & 1976b;
Rushbrook, et al.,
1982a,Kojima, et al.,
1982a
M s.c. 1710 Kojima, et al.,
F 1870 1982b
M i.p. 457 Kojima, et al.,
F 435 1982c
M + F dermal >3000 Kojima, et al.,
1982d
Rat M + F oral 2000 to Kojima, et al.,
1982e
8600 Schöbel & Siegmund,
1976a
M s.c. 5220 Kojima,et al.,1982f
F 3230
Rat M + F i.p. 437-763 Schöbel & Schuppler,
1977; Kojima, et al.,
1982g
Rat M + F dermal >3920 Schöbel & Siegmund,
(duration of 1976g
exposure not
specified)
Rat M + F dermal >3000 Kojima, et al., 1982h
(24-hour
exposure)
Rat M + F inhalation >3960 mg/m3* Robkamp &
(4-hour Schuppler, 1976
"nose only"
exposure
Table 2. (continued)
LD50
Species Sex Route (mg a.i/kg b.w.) Reference
Rabbit M + F dermal >3920 Schöbel & Siegmund
(duration of 1976c
exposure not
specified)
Dog** M + F oral <674 Kopp, et al.,
1981a & 1981b
* 4-hour LC50
** 674, 1000 and 2000 mg/kg produced 3/6 mortalities with emesis of doses > 1000 mg/kg.
Long-term Studies
Rat
Groups of 50 male and 50 female Sprague-Dawley CD rats were fed
diets containing propamocarb hydrochloride as a 70% aqueous
formulation at 0, 40, 200 or 1000 ppm a.i. for 104 weeks. Additional
animals (ten males and ten females per group) were fed the same
dietary levels for the same duration and used primarily for routine
laboratory investigations (satellite group). Two additional groups of
ten males and ten females were fed 0 or 1000 ppm of the test diet with
one-half of the animals in each group sacrificed after 52 weeks and
the remainder at the end of a four-week withdrawal period. These
animals were subjected to macroscopic examination and organ weight
analysis.
Mortality was not influenced by treatment. Forty-six to 58% of
the males and 30-44% of the females of all groups including the
control survived at the end of two years. Over 50% of the animals
(both sexes) of control and treated groups lived at least 91 weeks.
Compound-related clinical signs were not apparent. No consistent dose-
related changes were seen on body weight, food consumption and water
intake. Haematological, clinical chemistry and urinalysis values as
well as ophthalmoscopic findings, monitored at several intervals over
the course of the study, were not significantly different between
control and treated groups. Activity of tissue cholinesterase was not
measured at any time during the study. Gross pathological changes in
treated animals dying or sacrificed in extremis during the study and
in those sacrificed at 52 weeks, after the four-week withdrawal
period, or terminally were comparable to those in the control group.
Organ weight determinations revealed no significant difference between
control and treated groups. Histopathological evaluation of non-
neoplastic findings was unremarkable except for an increased incidence
of degenerative hepatocytes/necrosis in high-dose males.
Evaluation of the neoplastic changes demonstrated an increase in
occurrence in males of subcutaneous fibrosarcoma in the treated
groups. Incidence of the tumour was 0/58 in controls, 5/56 (8.9%) at
40 ppm, 2/58 (3.5%) at 200 ppm and 7/55 (12.7%) at 1000 ppm (the
denominator denoted the number of survivors from both main and
satellite groups at each dosage level at the end of 51 weeks when the
first subcutaneous fibrosarcoma was detected in the males). The
difference from concurrent controls was statistically significant at
40 ppm and 1000 ppm. Data on background incidence of subcutaneous
fibrosarcoma in the particular strain of rats employed in the study
were not available in the report.
Over 80% of the concurrent controls (both sexes) had tumours,
with pituitary adenoma (both sexes), mammary tumours (females) and
subcutaneous fibroma and lipoma (males), the most prevalent tumours.
There were no other neoplastic differences between treated and control
groups. The no-effect level for non-neoplastic effects was equal to
8.3 mg a.i/kg b.w./day, based on food consumption and body weight data
(Hunter, et al., 1983b).
Comments
Propamocarb hydrochloride was evaluated for the first time by the
JMPR at this session.
The compound, a carbamate fungicide, is rapidly absorbed,
degraded and readily excreted primarily in the urine of (female) rats.
Oxidation of the parent molecule appears to be the major metabolic
pathway. There is no evidence of significant tissue accumulation of
propamocarb hydrochloride and/or its metabolites. Metabolism data in
mammalian species other than the rat are not available.
Propamocarb hydrochloride has a relatively low order of acute
oral toxicity in rodents, with LD5 values of over 1500 mg/kg b.w. in
both mice and rats. Limited acute oral data in dogs appeared to
indicate that they are more sensitive than rodents to toxicity of the
compound.
A three-generation reproduction study, including teratological
investigation in rats, showed a no-effect level of 200 ppm on
reproduction and teratogenicity. A teratology study in rats showed
retardation of foetal development at doses which were maternally
toxic. Teratology studies in rabbits were negative. Mutagenicity
studies were also negative. A carcinogenicity study in mice was
negative. A long-term study in rats showed some evidence of increased
incidence of sub-cutaneous fibrosarcoma. Therefore, background data on
incidence of this malignancy in the particular strain of rats are
required.
Ninety-day feeding studies in rats and dogs established no-effect
levels of at least 200 ppm and 1000 ppm respectively. Inhibition of
brain and erythrocyte cholinesterases was not observed in these
studies.
In view of the concerns with respect to the elevated incidence of
subcutaneous fibrosarcoma in male rats of the long-term study and the
non-availability of a dog study longer than three months, only a
temporary ADI was granted.
Level causing no toxicological effect
Rat: 200 ppm in the diet, equivalent to 8.3 mg/kg b.w.
Dog: 1,000 ppm in the diet, equivalent to 25 mg/kg b.w.
Estimate of temporary acceptable daily intake for humans
0 - 0.2 mg/kg b.w.
Further work or information
Required (by 1986):
Historical data on the incidence of subcutaneous fibrosarcoma in the
CD strain of rats used in the long-term study.
Complete data on the 24-month dog study known to have been completed.
Metabolism and pharmacokinetic studies in mice and rats using multiple
dosage levels.
Desirable:
Observations in humans.
Complete data on the 4-5 week feeding study in rats.
REFERENCES
Brühl, R. & Celorio, J. Propamocarb hydrochloride: Metabolism in rats.
1982 Submitted by Schering AG to WHO. (Unpublished).
Brühl, R. & Celorio, J. Metabolic pathway of propamocarb hydrochloride
1984 in the rat. Submitted by Schering AG to WHO. (Unpublished).
de Rijke, D., Köter, H.B.W.M. & Leegwater, D.C. Subchronic (90-day)
1976 toxicity study with ZK17.296 in albino rats (ZK 17296 =
SN39744 = Propamocarb). Centraal Instituut Voor
Voedingsonderzoek, The Netherlands. Submitted by Schering AG
to WHO. (Unpublished).
Field, W.E. Twenty-one day dermal toxicity study in rabbits treated
1980 with Previcur N. (Final report). CDC Research, Inc. U.S.A.
Submitted by Schering AG to WHO. (UNPUBLISHED).
Hara, K., Ikoma, Y. Arita, S., Tokiyori, Y., Nakata, Y., Yamano, K.,
1984 Mizuno, M. & Oshino, N. General pharmacological activities
of Previcur N. Nikon Schering K.K. Japan. Submitted by
Schering AG to WHO. (Unpublished).
Hastwell, R., McGregor, D.B. & Smith, I. Mutagenicity testing of
1977 SN 66.752. Inveresk Research International, Scotland.
Submitted by Schering AG to WHO. (Unpublished).
Hoorn, A.J.W., Jagannath, D.R. & Brusick, D.J. Mutagenic evaluation of
1980 Previcur N. Batch No. 271001B00000 in the mitotic gene
conversion, reverse mutation & mitotic recombination assays
in yeast. Litton Bionetics, Inc. Submitted by Schering AG to
WHO. (Unpublished).
Hossack, D.J.N., Richold, M. & Richardson, J.C. Micronucleus test on
1980 CP604 (SN66752, Previcur N). Huntingdon Research Centre,
England. Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E. & Gibson, W.A.
1978 Previcur N (SN66752). Assessment of whole blood & brain
cholinesterase activities in rats after 11 days of treatment
by oral gavage. Huntington Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Watson, M., Read, R.M., Prentice, D.E., Woodhouse, R.N.,
1983a Cherry, C.P. & Gibson, W.A. Previcur N (SN 66752): Potential
tumorigenicity to mice in dietary administration for 104
weeks (final report). Huntingdon Research Centre, England.
Submitted by Schering AG to WHO. (Unpublished).
Hunter, B., Jones, D.R., Heywood, R., Street, A.E., Jolly, D.W.,
1983b Offer, J.M. & Gibson, W.A. Previcur N (SN66752): Toxicity &
potential tumorigenicity in dietary administration to rats
for 104 weeks. (final amended report). Huntingdon Research
Centre, England. Submitted by Schering AG to WHO.
(Unpublished).
Huntingdon Research Centre. Previcur N teratology study: effect of
1981 administration during organogensis on foetuses of the
rabbit. Reprotox, Deutschland. Submitted by Schering AG to
WHO. (Unpublished).
Huntingdon Research Centre. Effect of Previcur N on reproductive
1983 function of multiple generations in the rat. Reprotox,
Deutschland. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. Mutagenicity test in bacteria with Previcur
1981a N. Biosafety Research Centre, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. & Nakajima, M. "REC" assay to detect mutagenic activity of
1981b Previcur N. Biosafety Research Centre, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K. & Enomoto, M. Previcur N: Three-month subchronic oral
1982 toxicity study in rats. Biosafety Research Centre, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojama, K. Seki, S. & Okamura, T. Previcur N: Acute oral toxicity in
1982a mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K. Seki, S. & Okamura, T. Acute subcutaneous toxicity study in
1982b mice. Biosafety Research Center, Foods, Drugs & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute intraperitoneal
1982c toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Previcur N: Acute percutaneous
1982d toxicity study in mice. Biosafety Research Center, Foods,
Drugs & Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute oral toxicity study in rats.
1982e Biosafety Research Center, Foods, Drugs, & Pesticides,
Japan. Submitted by Schering AG to WHO. (Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute subcutaneous toxicity study
1982f in rats. Biosafety Research Center, Foods, Drugs &
Pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. & Okamura, T. Acute intraperitonial toxicity
1982g study in rats. Biosafety Research Center, Foods, Drugs and
pesticides, Japan. Submitted by Schering AG to WHO.
(Unpublished).
Kojima, K., Seki, S. and Okamura, T. Acute percutaneous toxicity study
1982h in rats. Unpublished report from Biosafety Research Center,
Foods, Drugs and Pesticides, Japan. Submitted by Schering AG
to WHO. (Unpublished).
Kopp, R., Hümpel, M., Kühne, G. Aner, B., Klawa, D. & Rzadkiewicz, M.,
1979 Pharmacokinetics of Propamocarb hydrochloride on single &
repeated oral administration of 0.5 mg/kg in rats. Schering
AG. Submitted by Schering AG to WHO. (Unpublished).
Kopp, R., Günzel, P., RoBkamp, G., Schöbel, C. & Siegmund, F. Previcur
1981a N. Systemic tolerance test (LD50) ZK66752(CP604) in the dog
following single oral administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished)
Kopp, R., Günzel, P., Bhargava, A.S., Schöbel, C. & Slabke, P.
1981b Influence of SN66.752 upon the acetylcholinesterase activity
in vitro (dog & rat) and in vivo (dog) after single oral
administration. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Poggel, H.A., Kopp, R. & Günzel, P. Previcur N. (CP604).
1981 Embryotoxicity including teratogenicity study in rats after
daily intragastrical administration from day 6 to day 19 of
gestation. Schering AG. Submitted by Schering AG to WHO.
(Unpublished).
Reuzel, P.G.J. & Til, H.P. Subchronic (90-day) feeding study with
1977 ZK17.296 (SN39744 Propamocarb) in dogs (final report).
Centraal Instituut Voor Voedingsonderzoek, The Netherlands.
Submitted by Schering AG to WHO. (Unpublished).
RoBkamp, G. & Schuppler, J. ZK66752 Acute Inhalation toxicity to the
1976 rats (M + F) of aqueous ZK66.752 following single four-hour
continuous exposure (LC50). Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Rushbrook, C.J., Jorgenson, T.A. & Jones, D.C.L. Dominant lethal study
1979 of Previcur N. SRI International, USA. Submitted by Schering
AG to WHO. (Unpublished).
Schering A.G. Previcur N (CP604): embryotoxicity including
1981 teratogenicity study in rabbits after daily intragastrical
administration from day 6 to day 18 of gestation. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C. SN66752. Eye irritation, rabbits: single application of
1977 CP604 to the rabbit eye. Schering AG. Submitted by Schering
AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752: Acute oral (i.g.) toxicity
1976a mice (male) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Etreby, M.F. El. ZK66752. Acute oral (i.g.) toxicity
1976b mice (female) single administration. Schering AG. Submitted
by Schering AG to WHO. (Unpublished).
Schöbel, C.J. and Schuppler, J. SN6675: Dermal toxicity study of CP604
1976 on the intact and scarified skin of the rabbit following
daily application for 28 days. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Schuppler, J. ZK66752. Acute i.p. toxicity rats (M + F)
1977 single application. Schering AG. Submitted by Schering AG to
WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752: acute oral (i.g.) toxicity rats
1976a (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK 66752: acute dermal toxicity rats
1976b (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Schöbel, C. & Siegmund, F. ZK66752. Acute dermal toxicity rabbits
1976c (M + F) single administration. Schering AG. Submitted by
Schering AG to WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary eye irritation after single
1983a application with Previcur N in the rabbit. Research &
Consulting Co., AG, Switzerland. Submitted by Schering AG to
WHO. (Unpublished).
Ullmann, L. & Suter, B. Primary skin irritation following a single
1983b four-hour occlusive application with Previcur N in the
rabbit. Research & Consulting Co., AG., Switzerland.
Submitted by Schering AG to WHO. (Unpublished).
