
TOLYLFLUANID
EXPLANATION
Tolylfluanid, a broad spectrum fungicide, is considered for the
first time by the present Meeting.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Single doses of tolylfluanid, 14C-labelled at the
fluorodichloromethylsulfenyl group, were orally administered to rats.
In the dose range of 0.1-20 mg/kg, 50-60% and 20-30% of the
administered activity was eliminated by the urine and the faeces,
respectively, within 48 hours from administration. Urinary elimination
took place with a half-life of 2-3 hours during the first 24 hours and
then more slowly with a half-life of about 40 hours from the third
day.
Experiments in rats with biliary fistula showed that, after
intraduodenal administration of 0.5 mg/kg, about 6% of total
radioactivity was eliminated via the bile. However, when 5 mg/kg bw of
tolylfluanid were administered, approximately 16% of the activity was
exhaled in 48 hours. After oral administration of 5 mg/kg bw, maximal
plasma concentration (about 1.5 ppm) was attained after two hours.
While plasma half-life was 2-3 hours during the first 6-8 hours,
elimination slowed to about 40 hours after 3 days. Of the administered
activity, 6%, 2%, 1% and 0.5% was retained (excluding gastrointestinal
tract) 8 hours, 48 hours, 6 and 12 days after administration,
respectively. The highest concentrations were found in the thyroid
gland: 5 ppm and 1 ppm, 1 and 10 days after the administration of
5 mg/kg, respectively. Whole-body autoradiography (after i.v.
injection of 10 mg/kg bw of tolylfluanid) confirmed this observation
(Weber et al., 1977).
In another study, doses of 2 or 20 mg/kg bw of 14C-ring-
labelled tolylfluanid were orally administered to male and female
Wistar rats. A group of rats of both sexes were also pretreated
for 14 days with daily doses of 2 mg/kg bw of non-radioactive
tolylfluanid before receiving the radioactive compound. Tolylfluanid
was quickly and almost completely absorbed (>95%). The peak plasma
concentration was reached within 1.5 hours. Within 48 hours, 75-80% of
radioactivity was excreted in urine (half-life of 4-8 hours), 14-25%
with faeces, and 0.06% in expired air. An experiment in animals with
biliary fistulae showed that biliary secretion accounted for about 14%
of total elimination. Total residual radioactivity was 0.07-0.20% of
the total administered dose. Higher concentrations of radioactivity
were found in liver and kidney (3 to 7 times the mean body
concentration). Lesser concentrations were found in perirenal fat,
brain, gonads and thyroid (3 to 9 times less than the mean body
concentration). Renal clearance was 3.1-4.0 ml/min, which correspond
to normal renal plasma flow in rats. No influence of dose was observed
on pharmacokinetics (Abbink & Weber, 1988).
An autoradiographic study showed a difference between i.v. and
oral administration. Intravenous injection is characterized by a great
uniformity of distribution with relatively high concentration in
almost all body tissues (including highly lipophilic tissues). After
oral administration, radioactivity in lipophilic tissues was very low.
It may therefore be assumed that after oral administration
tolylfluanid is rapidly metabolized prior to or during the absorption
and distribution process (Weber, 1988).
Biotransformation
A single dose of fluorodichloromethyl-14C-labelled tolylfluanid
was administered orally or intravenously to rats (5 or 10 mg/kg bw).
The main radioactive metabolite in the urine of treated rats was found
to be thiazolidine-2-thione-4-carbonic acid (TTC). TTC accounted for
74% of radioactivity contained in the urine 8 hours after intravenous
administration and for 50-63% after oral administration. Tolylfluanid
was not detected in urine (Ecker, 1978).
A single oral dose of 20 mg/kg bw of C14-benzene ring-
tolylfluanid was administered to male Wistar rats. 90% of urine
radioactivity and 70% of faecal radioactivity was due to one
metabolite which was found to be 4-dimethylamino-sulphonylamino-
benzoic acid. A minor metabolite (6% of total radioactivity) could not
be identified (Ecker & Brauner, 1987).
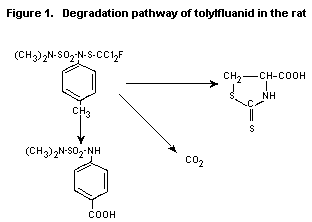
The degradation pathway of tolylfluanid in the rat is described
in Figure 1.
 Toxicological Studies
Special studies on carcinogenicity
Mice
Groups of NMRI mice (50/sex/group) were given tolylfluanid
technical (99.1% purity in a 90% pre-mix) in the diet (0, 200, 1000,
5000 ppm) for 104 weeks. In relation to body weight, the males were
given 32.6, 161.8 or 768.9 mg/kg bw and the females 45.6, 202.4 or
952.3 mg/kg bw on average. Animals were observed twice a day for
mortality or adverse physical/behavioural signs. Body weights were
recorded weekly and food consumption of each animal was determined
twice a week. Routine haematological and clinical chemistry
examinations were performed before the start of the study and after 12
and 24 months. All animals, including those which died or were
sacrificed before the end of the study, were dissected, their organs
weighed and tissues prepared for microscopic examination. For
technical reasons the treated groups started 4 months after the
control group. This discrepancy in time was considered by the author
to be the cause of the higher body weight gain (+55% and +10/12% of
the starting weight in controls and treated males, respectively; +64%
and +18/24% in control and treated females, respectively) and food
consumption (about 30% higher) found in control animals and of the
differences between control and study groups in the haematological and
clinical laboratory examinations. No dose-related difference in any of
these parameters was, however, found in animals of the treated groups.
The mortality rate was higher in female groups but a dose-related
effect was not evident.
At necropsy, hepatocellular adenomas and pulmonary adenomas were
found in all control and treated groups. The incidence of liver
rumours was higher in male groups, but no treatment-related effect was
evident (10/51, 7/50, 13/50 and 9/50 in control, 200, 1000 and
5000 ppm groups, respectively). The incidence of lung adenomas was
17/51, 17/50, 20/50 and 20/50 in males and 9/49, 6/50, 14/50 and 7/51
in females of control, 200, 1000 and 5000 ppm groups, respectively.
Incidence, latency, variety and organ distribution of rumours did not
indicate any dose-related effect of tolylfluanid (Mohr, 1982).
Rats
(See "Long-term studies".)
Special studies on embryotoxicity and teratogenicity
Groups (n=22-24) of fertilized female rats (75-95 day-old Long
Evans FB 30) were given oral daily doses (0, 100, 300, 1000 mg/kg bw)
of tolylfluanid (99.9% purity) from day 6 through day 15 of gestation.
Animals were observed routinely for physical appearance, behaviour
and body weight gain. At day 20 of gestation pups were delivered
by caesarean section. Foetuses were weighed, sexed, inspected for
external abnormalities and examined for visceral and bone
malformations.
No alteration of physical appearance and behaviour was observed
in any group. Two dams of the mid-dose group died of effects unrelated
to treatment. Body weight gains were reduced in a dose-dependent
manner in the mid- and high-dose groups. However, dams compensated
after end of treatment. The average foetal weight was reduced by
treatment of the dams with 300 or 1000 mg/kg bw. The resorption ratio
in the high-dose group was slightly, not statistically significantly,
higher than in the control group, because of complete loss of embryos
by one female. There was no evidence of teratogenicity at any of the
doses used. The dose of 100 mg/kg bw had no toxic effect to dams and
foetuses (Machemer, 1976).
Acute toxicity of metabolites
4-dimethylamino-sulftoluidine, a metabolite of tolylfluanid, was
tested for acute toxicity. After single administration, LD50s were
as follows (Kimmerle & Solmecke, 1971b).
Species Route mg/kg bw
Rat (male) p.o. >2500
Mouse (male) p.o. >1000
Rabbit (male) p.o. >1000
Rat (male) i.p. 551
Special studies on mutagenicity
Tolylfluanid was variably active in a range of in vitro
assays. In vivo, however, tolylfluanid was inactive. Results are
summarized in Table 1.
Table 1. Results of Mutagenicity assays on tolylfluanid
Test system Test Object Concentration of tolylfluanid Purity Results References
Ames test (with and S. typhimurium 3.15-1000 µg/plate 100% weakly Oesch, 1977
without activation) TA-98,100,1537 dissolved in DMSO positive 1/
Ames test (with and S. typhimurium 4-2500 µg/plate 100% weakly Herbold, 1979
without activation) TA-98,100,1535, dissolved in DMSO positive 2/
1537
Reverse mutation Saccharomyces Nonactivated: 1-200 µg/ml 99.1% negative Hoorn, 1984
in vitro cervisiae Activated: 1-100 µg/ml
(S138, S211alfa)
Gene mutation Somatic Nonactivated: 4-40 µg/ml 98.5% negative 3/ Heidemann,1987
(HGPRT-test) mammalian cells Activated: 300-3000 µg/ml
in vitro (V79)
Gene mutation Chinese hamster Nonactivated: 0.5-6 µg/ml 98.5% negative 4/ den Boer, 1987
(HGPRT-test) ovary cells Activated: 3-30 µg/ml
in vitro
Forward mutation Mouse lymphoma Nonactivated: 25-300 µg/ml 99.1% positive Hoorn, 1985
in vitro cells (L5178Y) Activated: 500-7500 µg/ml
Cytogenetic assay Human lymphocytes 0.1-10 µg/ml (activated 99.2% positive 5/ Herbold, 1984b
in vitro and nonactivated)
Micronucleus test Mice (NMRI strain) 2x250 and 2x500 mg/kg 98.8% negative 6/ Herbold, 1980
(p.o. 24 h apart)
Table 1. (cont'd)
Test system Test object Concentration of tolylfluanid Purity Results References
Dominant lethal test Mice (NMRI strain) 4000 mg/kg p.o.(mortality:5/50) 98.8% negative Herbold, 1986
8000 mg/kg p.o.(mortality:12/50)
Cytogenetic assay Chinese hamster 4000 mg/kg p.o. 99.7% negative 6/ Herbold, 1983
in vivo bone marrow
Cytogenetic assay Chinese hamster 2x250 and 2x500 mg/kg 93.1% inconclusive Herbold, 1984a
in vivo spermatogonia (p.o. 24 h apart) 7/
Cytogenetic assay Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 8/ Voelkner,1988b
in vivo germ cells
Sister chromatide Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 7/ Voelkner,1988a
exchange bone marrow
Mammalian Mice C5781/6JxT 1750-7000 mg/kg bw p.o. 98.4% negative 9/ Herbold, 1988
Spot test
1/ With S-9 mix only; positive controls (benzo(a)pyrene 4,5-oxide, N-methyl-N'-nitro-N-nitrosoguanidine,
3,methylcholanthrene, 2-aminoanthracene) yielded expected positive results.
2/ TA-100 only; positive controls (endoxan, trypaflavin) yielded expected positive results.
3/ Positive controls (ethyl-methane sulfonate (EMS), 9,10-dimethyl-1,2-benzanthracene (DMBA) yielded expected positive
results.
4/ Positive controls (5-bromodeoxyuridine and 3-methylcholanthrene) yielded expected positive results.
5/ At cytotoxic concentrations; positive controls (mitomycin c, endoxan) yielded expected positive results.
6/ Positive controls (endoxan) yielded expected positive results.
7/ Positive controls (doxorubicin) yielded expected positive results.
8/ Positive controls (cyclophosphamide) yielded expected positive results.
9/ Positive controls (1-ethyl-1-nitrosourea) yielded expected positive results.
Special study on potentiating effects
Equitoxic doses of tolylfluanid (purity not reported) and
triadimefon (89.5% purity) were given in combination to rats. No
potentiating effect was noted (Flucke & Kimmerle, 1978).
Special study on reproduction
Groups of Long Evans FB30 rats (30 animals/dose, 10 males and 20
females) were fed diets containing tolylfluanid (technical grade,
98.8% purity in a 90% pre-mix) at levels of of 0, 300, 1500 or
7500 ppm. After 70 days of treatment F0 animals were mated on a
2-female-to-1-male basis. After delivery (F1a) and a 4-week
lactation and a 2-week waiting period, F0 rats were mated again.
After delivery (F1b) lactation lasted for 4 weeks and then F0
animals were sacrificed. F1b animals (20 females-10 males/dose) were
then mated and F1a and F1b generations were obtained. The study
was ended when F1b generation reached 4 weeks of age.
Rats were weighed weekly and pups were weighed at birth, 5 and 7
days after birth, and weekly thereafter. Pups were also observed
immediately after birth and during lactation.
F0 generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in a
dose-related manner in male rats treated with 1500 or 7500 ppm; only
the dose of 7500 ppm was associated with reduced body weight in female
rats. Fertility indices and litter sizes of first and second matings
were not reduced by any of the treatments. Survival rate was slightly
reduced in F1b generation of the high-dose group. Lactation index (%
of pups surviving 4 weeks) was reduced in the F1a generation of the
7500 ppm group, but in the F1b generation of the same group was
significantly higher than controls. Body weight of pups at birth and
body weight gain were significantly lower in both F1a and F1b
generations of the 7500 ppm group. None of the pups showed
malformations.
F1b generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in the
7500 ppm group. Fertility indices and litter sizes of first and second
matings were not reduced by any of the treatments. Survival rate was
reduced in F2b generation of the high-dose group. Lactation index
(% of pups surviving 4 weeks) was reduced in F2a and F2b
generations of the 7500 ppm group and in the F2b generation of the
1500 ppm group. In the latter group, however, the number of pups
surviving four weeks was the same as in the control group and the
lower lactation index was due to a higher number of pups at 5 days
when all litters were reduced to a maximum of 10 pups rearing. Body
weight of pups at birth and body weight gain was lower in both F2a
and F2b generations of the 7500 ppm group. None of the pups showed
malformations.
In summary, this study shows that tolylfluanid at doses up to
1500 ppm did not affect the reproduction of rats. The NOAEL in this
study was found to be 300 ppm (Loser, 1980).
Special study on skin and eye irritation
A 90% pre-mix with 99.2% active ingredient was applied to rabbit
skin for 24 hours under semi-occlusive conditions. Tolylfluanid did
not show skin irritant properties. The test compound dust (0.1 g),
however, when placed on the conjuntival sac of rabbit eyes, displayed
a strong irritant potential (redness and swelling of the conjuntivae).
The effect was reversible within 14 days (Helmann & Pauluhn, 1983).
A further study was conducted on irritant and corrosive effects
on the skin and eyes. Tolylfluanid (99.1% purity) did not show
irritant effect on the skin (4 hours' exposure). However, 70 mg of
a.i. placed in the conjuntival sac produced redness and swelling of
the conjuntivae which were completely reversible within 21 days. No
cornea or iris lesions were found at any time (Pauluhn, 1984).
Special study on subacute cutaneous toxicity in rabbits
Rabbits (5 males and 5 females) received daily dermal doses of
500 mg/kg bw of tolylfluanid (98.9% purity) for fourteen days. Each
exposure lasted 24 hours. Before and immediately after the end of
treatment, and 2 weeks later, clinical-chemical tests were performed,
including blood tests (erythrocyte, leucocyte and thrombocyte count,
haemoglobin determination, etc.), liver function tests, serum
transaminases, sorbitol dehydrogenase, bilirubin and albumin) and
kidney function tests (urinanalysis and serum urea concentration). No
variation was found between control and treated animals (Kimmerle &
Solmecke, 1971a).
Acute toxicity
Table 2 reports the LD50 values for tolylfluanid and
metabolites as determined in different studies and animal species.
In rats, signs of toxicity observed after oral administration
were altered behaviour, disturbed motility and dyspnoea. In mice,
guinea pigs, rabbits and cats tolylfluanid caused deterioration of
general conditions and (in cats only) vomiting. In sheep,
tolylfluanid, orally administered, caused anorexia, weakness of
extremities and loose faeces.
Intraperitoneal injection to rats caused disturbed motility,
staggering, spastic gait, distention of abdomen, narrowed eyelids, a
specific disturbed behaviour. Irritation of abdominal organs was found
at autopsy.
The dermal application of tolylfluanid at doses up to
5000 mg/kg bw was tolerated without signs of toxicity or skin
alterations.
The skin-sensitising potential of tolylfluanid was assessed by
the Magnusson and Klingman's maximization test on guinea pigs (n=15).
The concentration of tolylfluanid (99.2% purity in a 90% pre-mix) used
were 1% for the intradermal induction, 0.6% for the topical induction,
and 0.3% for the topical challenge. The results indicate that
tolylfluanid has a skin-sensitising potential (Heimann, 1983).
Short-term studies
Rats
0, 150, 500, 1500 or 4500 ppm of tolylfluanid (99% purity) were
given to rats (15 males and 15 females) in the diet for 3 months. All
dose-regimens were tolerated without alteration of behaviour, weight,
mortality or clinical chemistry tests. Morphological examination of
organs and tissues did not reveal any treatment-induced alterations
(Bomhard and Schilde, 1976).
Dogs
Beagle dogs (4 males and 4 females/group) were administered
0, 330, 1000 or 3000 ppm of tolylfluanid (99.7% purity) in diet for 3
months. Animals of the high-dose group showed poor general condition
and 6/8 animals had a reduced food intake and weight gain. In this
group an increased serum alkaline phosphatase activity and a
retardation of its age-related decrease as compared to controls was
found. Increased liver relative weight and increased PAS histological
reaction were also found in these animals. Doses up to 1000 ppm did
not induce any detectable adverse effect (Hoffmann & Mirea, 1974).
Table 2. Acute toxicity of tolylfluanid (mg/kg bw)
Species Sex Route LD50 LC50 Reference
Mouse M oral >1000 1/ - Kimmerle, 1968
Rat M oral >2500 2/ - Kimmerle & Lorke, 1967.
Rat M inhalation* - 265 2/ Kimmerle & Lorke, 1967
(4-hr exp.)
Rat M i.p. 20.2 1/ - Kimmerle, 1968
Rat F i.p. 25.6 1/ - Kimmerle, 1968
Rat F oral >5000 3/ - Heimann & Pauluhn, 1983
Rat M i.p. 14.7 3/ - "
Rat F i.p. 15.9 3/ - "
Rat M&F dermal >5000 3/ - "
(24-hr exp.)
Guinea pig F oral 250-500 1/ - Kimmerle, 1968
Rabbit M oral 250-500 1/ - Kimmerle, 1968
Cat M&F oral >500 1/ - Kimmerle, 1968
Sheep M&F oral 625-1250 3/ - Hoffmann, 1983
1/ 99.6% purity
2/ 98.7% purity
3/ 99.2% purity in a 90% pre-mix
* Dynamic spraying with Lutrol/ethanol. In static spraying experiments, mice, guinea
pigs and rabbits were found to be more sensitive than rats to tolylfluanid.
In another study, groups (4 male/4 female) of beagle dogs were
orally treated for 12 months with tolylfluanid (99.2% purity in a 90%
pre-mix) 0, 2.5, 12.5 or 62.5 (let to 33rd week) and 125 (34th to 52nd
week) mg/kg bw. The test compound was administered in gelatin
capsules. All animals survived the study and did not show any
treatment-related effect on appearance or behaviour. Body weight
development in male control animals (+4.4 kg in 12 months) was above
the average of the historical controls (+2.8 kg in 12 months), while
it was significantly reduced in the animals of the high-dose group.
The females in the high-dose group also had lower body weight gain.
Animals of the high-dose group vomited quite frequently after
administration of the compound. They also had a significant increase
of serum alanine aminotransferase (ALT) and glutamate dehydrogenase
(GLDH), and a retarding of the physiological age-related decrease of
the alkaline phosphatase activity. No liver alteration was found,
however, at histology.
An effect on renal function was also evident in the high-dose
group. Urea and creatinine concentrations were significantly increased
and in two dogs glycosuria and proteinuria were found. Histology
revealed slight alterations of cortical tubules (dilation, epithelial
flattening, focal hypertrophy and/or desquamation of the epithelia) in
all dogs of the high-dose group. Slightly lower serum potassium
concentrations were found in the high-dose group animals as compared
to control values, possibly as a result of the frequent vomiting.
No treatment-related effect was evident on blood, blood
coagulation and transparent media and fundus of the eye.
Oral doses of tolylfluanid up to 12.5 mg/kg bw were therefore
tolerated by dogs for 12 months without detectable adverse effects
(von Keutz & Nash, 1986).
Long-term studies
Rats
Wistar rats (50 males and 50 females/group; 100 male and 100
females, controls) were fed for 2 years with tolylfluanid (98.9%
purity in 90% pre-mix) at concentrations of 0, 300, 1500, 7500 ppm.
Toxicity following chronic administration and carcinogenic potential
of tolylfluanid were evaluated.
The animals in the high-dose group had a reduced food consumption
(-10%) and a reduced (about 10%) body weight gain; appearance,
behaviour and mortality were not affected by the treatment. Clinical
laboratory tests showed random alteration of some blood parameters
(mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte
count, polymorphonuclear cells and lymphocytes). Plasma alkaline
phosphatase (AP), transaminases (ALT and AST), bilirubin and total
protein were measured as indices of liver function. No dose-related
alteration of these parameters was found. Gross pathological and
histological examinations did not detect any indication of liver
damage. Urinanalysis did not indicate any dose-related kidney
alteration. Blood sugar and cholesterol were within normal range in
all groups. On autopsy, bone alterations (diffuse hyperostosis of the
rib bones, hardened cranial bones, focal hyperostosis of the skull
caps) in both sexes, and a faster growth of the incisors of the upper
jaw in males only, were found in the 1500 and 7500 ppm groups. These
effects were presumably due to an increased fluorine intake from the
active ingredient.
Tumours of endocrine and reproductive system accounted for about
80% of the rumours seen in the study. Only malignant uterine tumours
appeared higher in all treated groups as compared to controls. The
incidence was 3/50, 8/50, 12/50 and 13/50 in the 0, 300, 1500 and
7500 ppm groups, respectively. An unusually low incidence of these
fairly con, non tumours was found in control animals. Therefore, a
second, concurrent group of 50 controls was studied in which the
incidence of malignant uterine rumours was similar (9/50) to that of
the treated groups. The test, therefore, did not provide indications
of carcinogenic potential for tolylfluanid.
The concentration of tolylfluanid without detectable adverse
effect was 300 ppm (equivalent to 15 mg/kg bw) (Krötlinger &
Loser, 1982).
Observations in humans
Small cottonwool pads on which an unknown concentration of
tolylfluanid was applied, were placed on the forearm of 10 male
volunteers for 24 hours. Subjects were observed for 10 days; no
skin-irritant effects were noted (Kimmerle, 1968).
COMMENTS
Following oral administration to rats, tolylfluanid was rapidly
absorbed, metabolized and eliminated in urine and faeces. When using
14C-ring-labelled tolylfluanid (2 or 20 mg/kg bw), less than 0.2% of
the administered radioactivity was detectable in the body 2 days after
administration. The highest concentrations were found in liver and
kidney.
When using 14C-tolylfluanid (0.1-20 mg/kg bw) labelled at the
fluorodichloronenthyl group instead, accumulation of radioactivity was
evident in the thyroid. The major urinary metabolites were thiazolidine-
2-thione-4-carboxylic acid and 4-dimethylaminosulphonyl-amino-benzoic
acid.
The oral LD50 is greater than 5000 mg/kg bw in rats and
250-500 mg/kg bw in guinea pigs and rabbits. The oral LD50 of a
major plant metabolite, DMST (N,N-dimethyl-N'-p-tolylsulphamide),
exceeds 2500 mg/kg in rats and 1000 mg/kg in mice and rabbits.
In one study in rats, tolylfluanid was found to be maternally
toxic and embryotoxic at doses above 100 mg/kg bw/day but not
teratogenic at doses up to and including 1000 mg/kg bw/day. In a
two-generation two-litter-per-generation reproduction study, dietary
levels up to and including 1500 ppm did not affect rat reproduction.
No malformations were observed at dietary levels up to and including
7500 ppm.
In a short-term toxicity study with beagle dogs, tolylfluanid did
not cause adverse effects for 12 months at doses up to and including
12.5 mg/kg bw/day.
Tolylfluanid was mutagenic in prokaryotes and eukaryotes in four
of seven in vitro assays. In vivo tests, however, did not indicate
a mutagenic potential.
In a long-term study in Wistar rats, a higher incidence of
malignant uterine tumours was found in the treated groups as compared
to controls, but no statistically significant dose-related pattern was
evident. Moreover in a second, concurrent, group of controls the
incidence was comparable to that in treated groups. In animals treated
with 1500 or 7500 ppm tolylfluanid, bone alterations were also found
which were suggested to be due to the fluorine constituent of
tolylfluanid. No alterations were found in the thyroid or in other
organs.
The Meeting noted that in the NMRI mouse study, the control
animals were four months older than the treated groups, thus
compromising the design of the study. Lung and liver adenomas occurred
at similar frequency in all treated groups, but because of their known
occurrence in mice they were not considered to be biologically
relevant. The Meeting therefore concluded that the results of this
study did not indicate an oncogenic potential.
The Meeting did not request a special study on thyroid function
because the dichlorofluorometylthio group is common to dichlofluanid,
for which a NOAEL for the thyroid has been demonstrated to be greater
than 1500 ppm.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 300 ppm in the diet, equal to 15 mg/kg bw/day
Dog: 12.5 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Observations in man.
REFERENCES
Abbink, J. & Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: investigation
of the biokinetic behaviour in the rat. Institute for Metabolism
Research, Bayer AG. Unpublished report No. 181081-9. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Bomhard, E. & Schilde, B. 1976. KUE 1318T / Subchronic Toxicological
Experiments on Rats (feeding experiment over 3 months). Institute of
Toxicology, Bayer AG. Unpublished report No. 5929. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
den Boer, W.C. 1987. Mutagenicity test on KUE 13183b in the CHO/HGPRT
forward mutation assay. Hazleton Biotechnologies, The Netherlands.
Unpublished report No. R4204. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Ecker, W. 1978. Biotransformation of (14C) tolylfluanid in the rat.
Institute for Pharmakocinetics. Bayer AG. Unpublished report No. 1281.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Ecker, W. & Brauner, A. 1987. Biotransformation of <ring-U-14C>
tolylfluanid by the rat following oral administration. Institute for
Pharmacokinetics, Bayer AG. Unpublished report No. 2826. Submitted to
WHO by Bayer AG, Bayerwerk, FRG.
Flucke, W. & Kimmerle, G. 1978. Triadimefon (MEB 6447) and
Tolylfluanid (KUE 13183b) Study for Acute Combination Toxicity.
Institute of Toxicology, Bayer AG. Unpublished report No. 7304.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heidemann, A. 1987. KUE 13183b / Detection of gene mutations in
somatic mammalian cells in culture: HGPRT-Test with V79 cells.
Laboratory for Mutagenicity Testing, Darmstadt. Unpublished report
No. R 4103. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. 1983. KUE 13183b / Study for sensitising effect on
guinea pigs. Institute of Toxicology, Bayer AG. Unpublished report
No. 11492. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. & Pauluhn, J. 1983. 13183b / Study for Acute Toxicity
Institute of Toxicology, Bayer AG. Unpublished report No. 11383.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1979. 13183b / Salmonella-microsome test for point
mutagenic effects. Institute of Toxicology, Bayer AG. Unpublished
report No. 8265. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1980. KUE 13183b / Micronucleus test on the mouse to
evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 9149. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1983. KUE 13183b / Cytogenetic study of the Chinese
hamster's bone marrow in vivo to evaluate for mutagenic effect.
Institute of Toxicology, Bayer AG. Unpublished report No. 11792.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984a. KUE 13183b / Cytogenicity study of the
spermatogonia of the Chinese hamster in vivo to evaluate for
mutagenic effect. Institute of Toxicology, Bayer AG. Unpublished
report No. 12739. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984b. KUE 13183b / Cytogenetic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Institute of Toxicology, Bayer AG. Unpublished report
No. 12836. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1986. KUE 13183b / Dominant lethal test on the male mouse
to evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 15017. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1988. Spot test on cross-bred C57B1/6J × T stock mouse
fetuses to evaluate for induced somatic changes in the genes of the
coat pigment cells. Institute of Toxicology, Bayer AG. Unpublished
report No. 16752. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. 1983. KUE 13183b / Acute toxicity to the sheep after oral
administration. Institute of Toxicology, Bayer AG. Unpublished report
No. 11975. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. & Mirea, D. 1974. KUE 13183b / Subchronic toxicity study
on dogs (13-week feeding experiment). Institute of Toxicology,
Bayer AG. Unpublished report No. 4957. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Hoorn, A.J.W. 1984. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the reverse mutation induction assay with saccharomyces cerevisiae
strains S138 and S211a. Litton Bionetics, The Netherlands. Unpublished
report No. 3060. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoorn, A.J.W. 1985. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the mouse lymphoma forward mutation assay. Litton Bionetics, The
Netherlands. Unpublished report No. R 3192. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
von Keutz, E. & Nash, G. 1986. KUE 13183b / Chronic toxicity to dogs
after oral administration (12-month capsule study). Institute of
Toxicology, Bayer AG. Unpublished report No. 12999. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. 1968. BAY 49854 / Toxicological studies. Institute of
Toxicology, Bayer AG. Unpublished report No. 832. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Lorke, D. 1967. Toxicological studies on the active
ingredient BAY 49854. Institute of Toxicology, Bayer AG. Unpublished
report No. 323. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971a. Methyl-Euparen / Subacute cutaneous
application to rabbits. Institute of Toxicology, Bayer AG. Unpublished
report No. 2619. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971b. DMST / Toxicological Study.
Institute of Toxicology, Bayer AG. Unpublished report No. 2681.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Krötlinger, F. & Loser, E. 1982. KUE 13183b / Chronic toxicological
study in rats (feeding for 2 years). Institute of Toxicology, Bayer
AG. Unpublished report No. 10978. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Loser, E. 1980. KUE 13183b / Generation study with rats. Institute of
Toxicology, Bayer AG. Unpublished report No. 9419. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Machemer, L. 1976. KUE 13183b / Studies on embryotoxic and teratogenic
effects on rats after oral administration. Institute of Toxicology,
Bayer AG. Unpublished report No. 5888. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Mohr, U. 1982. KUE 13183b / Study for cancerogenic effect on NMRI-mice
(feeding study for 104 weeks). Appendix: Histopathological Individual
Findings. Medizinische Hochshule, Hannover. Unpublished report
No. R 2225. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Oesch, F. 1977. Kue 13183b / Ames Test for guparen M. Institute of
Biochemical Pharmacology, University of Mainz. Unpublished report from
November 10. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Pauluhn, J. 1984. KUE 13183b / Study for irritant/corrosive effect on
skin and eye (rabbit). Institute of Toxicology, Bayer AG. Unpublished
report No. 12362. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Voelkner, W. 1988a. Sister chromatide exchange assay in bone marrow
cells of the mouse. Cytotest Cell Research GmbH, Darmstadt, FRG.
Unpublished report No. 4422. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Voelkner, W. 1988b. Mouse germ-cell cytogenetic assay. Cytotest Cell
Research GmbH, Darmstadt, FRG. Unpublished report No. 4485. Submitted
to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. et al. 1977. KUE 13183b / Biokinetic Investigations of
Rats. Institute for Pharmacokinetics, Bayer AG. Unpublished report
No. 1165. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: whole body
autoradiographic distribution of the radioactivity in the rat.
Institute for Metabolism Research, Bayer AG. Unpublished report
No. 181080-8. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Toxicological Studies
Special studies on carcinogenicity
Mice
Groups of NMRI mice (50/sex/group) were given tolylfluanid
technical (99.1% purity in a 90% pre-mix) in the diet (0, 200, 1000,
5000 ppm) for 104 weeks. In relation to body weight, the males were
given 32.6, 161.8 or 768.9 mg/kg bw and the females 45.6, 202.4 or
952.3 mg/kg bw on average. Animals were observed twice a day for
mortality or adverse physical/behavioural signs. Body weights were
recorded weekly and food consumption of each animal was determined
twice a week. Routine haematological and clinical chemistry
examinations were performed before the start of the study and after 12
and 24 months. All animals, including those which died or were
sacrificed before the end of the study, were dissected, their organs
weighed and tissues prepared for microscopic examination. For
technical reasons the treated groups started 4 months after the
control group. This discrepancy in time was considered by the author
to be the cause of the higher body weight gain (+55% and +10/12% of
the starting weight in controls and treated males, respectively; +64%
and +18/24% in control and treated females, respectively) and food
consumption (about 30% higher) found in control animals and of the
differences between control and study groups in the haematological and
clinical laboratory examinations. No dose-related difference in any of
these parameters was, however, found in animals of the treated groups.
The mortality rate was higher in female groups but a dose-related
effect was not evident.
At necropsy, hepatocellular adenomas and pulmonary adenomas were
found in all control and treated groups. The incidence of liver
rumours was higher in male groups, but no treatment-related effect was
evident (10/51, 7/50, 13/50 and 9/50 in control, 200, 1000 and
5000 ppm groups, respectively). The incidence of lung adenomas was
17/51, 17/50, 20/50 and 20/50 in males and 9/49, 6/50, 14/50 and 7/51
in females of control, 200, 1000 and 5000 ppm groups, respectively.
Incidence, latency, variety and organ distribution of rumours did not
indicate any dose-related effect of tolylfluanid (Mohr, 1982).
Rats
(See "Long-term studies".)
Special studies on embryotoxicity and teratogenicity
Groups (n=22-24) of fertilized female rats (75-95 day-old Long
Evans FB 30) were given oral daily doses (0, 100, 300, 1000 mg/kg bw)
of tolylfluanid (99.9% purity) from day 6 through day 15 of gestation.
Animals were observed routinely for physical appearance, behaviour
and body weight gain. At day 20 of gestation pups were delivered
by caesarean section. Foetuses were weighed, sexed, inspected for
external abnormalities and examined for visceral and bone
malformations.
No alteration of physical appearance and behaviour was observed
in any group. Two dams of the mid-dose group died of effects unrelated
to treatment. Body weight gains were reduced in a dose-dependent
manner in the mid- and high-dose groups. However, dams compensated
after end of treatment. The average foetal weight was reduced by
treatment of the dams with 300 or 1000 mg/kg bw. The resorption ratio
in the high-dose group was slightly, not statistically significantly,
higher than in the control group, because of complete loss of embryos
by one female. There was no evidence of teratogenicity at any of the
doses used. The dose of 100 mg/kg bw had no toxic effect to dams and
foetuses (Machemer, 1976).
Acute toxicity of metabolites
4-dimethylamino-sulftoluidine, a metabolite of tolylfluanid, was
tested for acute toxicity. After single administration, LD50s were
as follows (Kimmerle & Solmecke, 1971b).
Species Route mg/kg bw
Rat (male) p.o. >2500
Mouse (male) p.o. >1000
Rabbit (male) p.o. >1000
Rat (male) i.p. 551
Special studies on mutagenicity
Tolylfluanid was variably active in a range of in vitro
assays. In vivo, however, tolylfluanid was inactive. Results are
summarized in Table 1.
Table 1. Results of Mutagenicity assays on tolylfluanid
Test system Test Object Concentration of tolylfluanid Purity Results References
Ames test (with and S. typhimurium 3.15-1000 µg/plate 100% weakly Oesch, 1977
without activation) TA-98,100,1537 dissolved in DMSO positive 1/
Ames test (with and S. typhimurium 4-2500 µg/plate 100% weakly Herbold, 1979
without activation) TA-98,100,1535, dissolved in DMSO positive 2/
1537
Reverse mutation Saccharomyces Nonactivated: 1-200 µg/ml 99.1% negative Hoorn, 1984
in vitro cervisiae Activated: 1-100 µg/ml
(S138, S211alfa)
Gene mutation Somatic Nonactivated: 4-40 µg/ml 98.5% negative 3/ Heidemann,1987
(HGPRT-test) mammalian cells Activated: 300-3000 µg/ml
in vitro (V79)
Gene mutation Chinese hamster Nonactivated: 0.5-6 µg/ml 98.5% negative 4/ den Boer, 1987
(HGPRT-test) ovary cells Activated: 3-30 µg/ml
in vitro
Forward mutation Mouse lymphoma Nonactivated: 25-300 µg/ml 99.1% positive Hoorn, 1985
in vitro cells (L5178Y) Activated: 500-7500 µg/ml
Cytogenetic assay Human lymphocytes 0.1-10 µg/ml (activated 99.2% positive 5/ Herbold, 1984b
in vitro and nonactivated)
Micronucleus test Mice (NMRI strain) 2x250 and 2x500 mg/kg 98.8% negative 6/ Herbold, 1980
(p.o. 24 h apart)
Table 1. (cont'd)
Test system Test object Concentration of tolylfluanid Purity Results References
Dominant lethal test Mice (NMRI strain) 4000 mg/kg p.o.(mortality:5/50) 98.8% negative Herbold, 1986
8000 mg/kg p.o.(mortality:12/50)
Cytogenetic assay Chinese hamster 4000 mg/kg p.o. 99.7% negative 6/ Herbold, 1983
in vivo bone marrow
Cytogenetic assay Chinese hamster 2x250 and 2x500 mg/kg 93.1% inconclusive Herbold, 1984a
in vivo spermatogonia (p.o. 24 h apart) 7/
Cytogenetic assay Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 8/ Voelkner,1988b
in vivo germ cells
Sister chromatide Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 7/ Voelkner,1988a
exchange bone marrow
Mammalian Mice C5781/6JxT 1750-7000 mg/kg bw p.o. 98.4% negative 9/ Herbold, 1988
Spot test
1/ With S-9 mix only; positive controls (benzo(a)pyrene 4,5-oxide, N-methyl-N'-nitro-N-nitrosoguanidine,
3,methylcholanthrene, 2-aminoanthracene) yielded expected positive results.
2/ TA-100 only; positive controls (endoxan, trypaflavin) yielded expected positive results.
3/ Positive controls (ethyl-methane sulfonate (EMS), 9,10-dimethyl-1,2-benzanthracene (DMBA) yielded expected positive
results.
4/ Positive controls (5-bromodeoxyuridine and 3-methylcholanthrene) yielded expected positive results.
5/ At cytotoxic concentrations; positive controls (mitomycin c, endoxan) yielded expected positive results.
6/ Positive controls (endoxan) yielded expected positive results.
7/ Positive controls (doxorubicin) yielded expected positive results.
8/ Positive controls (cyclophosphamide) yielded expected positive results.
9/ Positive controls (1-ethyl-1-nitrosourea) yielded expected positive results.
Special study on potentiating effects
Equitoxic doses of tolylfluanid (purity not reported) and
triadimefon (89.5% purity) were given in combination to rats. No
potentiating effect was noted (Flucke & Kimmerle, 1978).
Special study on reproduction
Groups of Long Evans FB30 rats (30 animals/dose, 10 males and 20
females) were fed diets containing tolylfluanid (technical grade,
98.8% purity in a 90% pre-mix) at levels of of 0, 300, 1500 or
7500 ppm. After 70 days of treatment F0 animals were mated on a
2-female-to-1-male basis. After delivery (F1a) and a 4-week
lactation and a 2-week waiting period, F0 rats were mated again.
After delivery (F1b) lactation lasted for 4 weeks and then F0
animals were sacrificed. F1b animals (20 females-10 males/dose) were
then mated and F1a and F1b generations were obtained. The study
was ended when F1b generation reached 4 weeks of age.
Rats were weighed weekly and pups were weighed at birth, 5 and 7
days after birth, and weekly thereafter. Pups were also observed
immediately after birth and during lactation.
F0 generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in a
dose-related manner in male rats treated with 1500 or 7500 ppm; only
the dose of 7500 ppm was associated with reduced body weight in female
rats. Fertility indices and litter sizes of first and second matings
were not reduced by any of the treatments. Survival rate was slightly
reduced in F1b generation of the high-dose group. Lactation index (%
of pups surviving 4 weeks) was reduced in the F1a generation of the
7500 ppm group, but in the F1b generation of the same group was
significantly higher than controls. Body weight of pups at birth and
body weight gain were significantly lower in both F1a and F1b
generations of the 7500 ppm group. None of the pups showed
malformations.
F1b generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in the
7500 ppm group. Fertility indices and litter sizes of first and second
matings were not reduced by any of the treatments. Survival rate was
reduced in F2b generation of the high-dose group. Lactation index
(% of pups surviving 4 weeks) was reduced in F2a and F2b
generations of the 7500 ppm group and in the F2b generation of the
1500 ppm group. In the latter group, however, the number of pups
surviving four weeks was the same as in the control group and the
lower lactation index was due to a higher number of pups at 5 days
when all litters were reduced to a maximum of 10 pups rearing. Body
weight of pups at birth and body weight gain was lower in both F2a
and F2b generations of the 7500 ppm group. None of the pups showed
malformations.
In summary, this study shows that tolylfluanid at doses up to
1500 ppm did not affect the reproduction of rats. The NOAEL in this
study was found to be 300 ppm (Loser, 1980).
Special study on skin and eye irritation
A 90% pre-mix with 99.2% active ingredient was applied to rabbit
skin for 24 hours under semi-occlusive conditions. Tolylfluanid did
not show skin irritant properties. The test compound dust (0.1 g),
however, when placed on the conjuntival sac of rabbit eyes, displayed
a strong irritant potential (redness and swelling of the conjuntivae).
The effect was reversible within 14 days (Helmann & Pauluhn, 1983).
A further study was conducted on irritant and corrosive effects
on the skin and eyes. Tolylfluanid (99.1% purity) did not show
irritant effect on the skin (4 hours' exposure). However, 70 mg of
a.i. placed in the conjuntival sac produced redness and swelling of
the conjuntivae which were completely reversible within 21 days. No
cornea or iris lesions were found at any time (Pauluhn, 1984).
Special study on subacute cutaneous toxicity in rabbits
Rabbits (5 males and 5 females) received daily dermal doses of
500 mg/kg bw of tolylfluanid (98.9% purity) for fourteen days. Each
exposure lasted 24 hours. Before and immediately after the end of
treatment, and 2 weeks later, clinical-chemical tests were performed,
including blood tests (erythrocyte, leucocyte and thrombocyte count,
haemoglobin determination, etc.), liver function tests, serum
transaminases, sorbitol dehydrogenase, bilirubin and albumin) and
kidney function tests (urinanalysis and serum urea concentration). No
variation was found between control and treated animals (Kimmerle &
Solmecke, 1971a).
Acute toxicity
Table 2 reports the LD50 values for tolylfluanid and
metabolites as determined in different studies and animal species.
In rats, signs of toxicity observed after oral administration
were altered behaviour, disturbed motility and dyspnoea. In mice,
guinea pigs, rabbits and cats tolylfluanid caused deterioration of
general conditions and (in cats only) vomiting. In sheep,
tolylfluanid, orally administered, caused anorexia, weakness of
extremities and loose faeces.
Intraperitoneal injection to rats caused disturbed motility,
staggering, spastic gait, distention of abdomen, narrowed eyelids, a
specific disturbed behaviour. Irritation of abdominal organs was found
at autopsy.
The dermal application of tolylfluanid at doses up to
5000 mg/kg bw was tolerated without signs of toxicity or skin
alterations.
The skin-sensitising potential of tolylfluanid was assessed by
the Magnusson and Klingman's maximization test on guinea pigs (n=15).
The concentration of tolylfluanid (99.2% purity in a 90% pre-mix) used
were 1% for the intradermal induction, 0.6% for the topical induction,
and 0.3% for the topical challenge. The results indicate that
tolylfluanid has a skin-sensitising potential (Heimann, 1983).
Short-term studies
Rats
0, 150, 500, 1500 or 4500 ppm of tolylfluanid (99% purity) were
given to rats (15 males and 15 females) in the diet for 3 months. All
dose-regimens were tolerated without alteration of behaviour, weight,
mortality or clinical chemistry tests. Morphological examination of
organs and tissues did not reveal any treatment-induced alterations
(Bomhard and Schilde, 1976).
Dogs
Beagle dogs (4 males and 4 females/group) were administered
0, 330, 1000 or 3000 ppm of tolylfluanid (99.7% purity) in diet for 3
months. Animals of the high-dose group showed poor general condition
and 6/8 animals had a reduced food intake and weight gain. In this
group an increased serum alkaline phosphatase activity and a
retardation of its age-related decrease as compared to controls was
found. Increased liver relative weight and increased PAS histological
reaction were also found in these animals. Doses up to 1000 ppm did
not induce any detectable adverse effect (Hoffmann & Mirea, 1974).
Table 2. Acute toxicity of tolylfluanid (mg/kg bw)
Species Sex Route LD50 LC50 Reference
Mouse M oral >1000 1/ - Kimmerle, 1968
Rat M oral >2500 2/ - Kimmerle & Lorke, 1967.
Rat M inhalation* - 265 2/ Kimmerle & Lorke, 1967
(4-hr exp.)
Rat M i.p. 20.2 1/ - Kimmerle, 1968
Rat F i.p. 25.6 1/ - Kimmerle, 1968
Rat F oral >5000 3/ - Heimann & Pauluhn, 1983
Rat M i.p. 14.7 3/ - "
Rat F i.p. 15.9 3/ - "
Rat M&F dermal >5000 3/ - "
(24-hr exp.)
Guinea pig F oral 250-500 1/ - Kimmerle, 1968
Rabbit M oral 250-500 1/ - Kimmerle, 1968
Cat M&F oral >500 1/ - Kimmerle, 1968
Sheep M&F oral 625-1250 3/ - Hoffmann, 1983
1/ 99.6% purity
2/ 98.7% purity
3/ 99.2% purity in a 90% pre-mix
* Dynamic spraying with Lutrol/ethanol. In static spraying experiments, mice, guinea
pigs and rabbits were found to be more sensitive than rats to tolylfluanid.
In another study, groups (4 male/4 female) of beagle dogs were
orally treated for 12 months with tolylfluanid (99.2% purity in a 90%
pre-mix) 0, 2.5, 12.5 or 62.5 (let to 33rd week) and 125 (34th to 52nd
week) mg/kg bw. The test compound was administered in gelatin
capsules. All animals survived the study and did not show any
treatment-related effect on appearance or behaviour. Body weight
development in male control animals (+4.4 kg in 12 months) was above
the average of the historical controls (+2.8 kg in 12 months), while
it was significantly reduced in the animals of the high-dose group.
The females in the high-dose group also had lower body weight gain.
Animals of the high-dose group vomited quite frequently after
administration of the compound. They also had a significant increase
of serum alanine aminotransferase (ALT) and glutamate dehydrogenase
(GLDH), and a retarding of the physiological age-related decrease of
the alkaline phosphatase activity. No liver alteration was found,
however, at histology.
An effect on renal function was also evident in the high-dose
group. Urea and creatinine concentrations were significantly increased
and in two dogs glycosuria and proteinuria were found. Histology
revealed slight alterations of cortical tubules (dilation, epithelial
flattening, focal hypertrophy and/or desquamation of the epithelia) in
all dogs of the high-dose group. Slightly lower serum potassium
concentrations were found in the high-dose group animals as compared
to control values, possibly as a result of the frequent vomiting.
No treatment-related effect was evident on blood, blood
coagulation and transparent media and fundus of the eye.
Oral doses of tolylfluanid up to 12.5 mg/kg bw were therefore
tolerated by dogs for 12 months without detectable adverse effects
(von Keutz & Nash, 1986).
Long-term studies
Rats
Wistar rats (50 males and 50 females/group; 100 male and 100
females, controls) were fed for 2 years with tolylfluanid (98.9%
purity in 90% pre-mix) at concentrations of 0, 300, 1500, 7500 ppm.
Toxicity following chronic administration and carcinogenic potential
of tolylfluanid were evaluated.
The animals in the high-dose group had a reduced food consumption
(-10%) and a reduced (about 10%) body weight gain; appearance,
behaviour and mortality were not affected by the treatment. Clinical
laboratory tests showed random alteration of some blood parameters
(mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte
count, polymorphonuclear cells and lymphocytes). Plasma alkaline
phosphatase (AP), transaminases (ALT and AST), bilirubin and total
protein were measured as indices of liver function. No dose-related
alteration of these parameters was found. Gross pathological and
histological examinations did not detect any indication of liver
damage. Urinanalysis did not indicate any dose-related kidney
alteration. Blood sugar and cholesterol were within normal range in
all groups. On autopsy, bone alterations (diffuse hyperostosis of the
rib bones, hardened cranial bones, focal hyperostosis of the skull
caps) in both sexes, and a faster growth of the incisors of the upper
jaw in males only, were found in the 1500 and 7500 ppm groups. These
effects were presumably due to an increased fluorine intake from the
active ingredient.
Tumours of endocrine and reproductive system accounted for about
80% of the rumours seen in the study. Only malignant uterine tumours
appeared higher in all treated groups as compared to controls. The
incidence was 3/50, 8/50, 12/50 and 13/50 in the 0, 300, 1500 and
7500 ppm groups, respectively. An unusually low incidence of these
fairly con, non tumours was found in control animals. Therefore, a
second, concurrent group of 50 controls was studied in which the
incidence of malignant uterine rumours was similar (9/50) to that of
the treated groups. The test, therefore, did not provide indications
of carcinogenic potential for tolylfluanid.
The concentration of tolylfluanid without detectable adverse
effect was 300 ppm (equivalent to 15 mg/kg bw) (Krötlinger &
Loser, 1982).
Observations in humans
Small cottonwool pads on which an unknown concentration of
tolylfluanid was applied, were placed on the forearm of 10 male
volunteers for 24 hours. Subjects were observed for 10 days; no
skin-irritant effects were noted (Kimmerle, 1968).
COMMENTS
Following oral administration to rats, tolylfluanid was rapidly
absorbed, metabolized and eliminated in urine and faeces. When using
14C-ring-labelled tolylfluanid (2 or 20 mg/kg bw), less than 0.2% of
the administered radioactivity was detectable in the body 2 days after
administration. The highest concentrations were found in liver and
kidney.
When using 14C-tolylfluanid (0.1-20 mg/kg bw) labelled at the
fluorodichloronenthyl group instead, accumulation of radioactivity was
evident in the thyroid. The major urinary metabolites were thiazolidine-
2-thione-4-carboxylic acid and 4-dimethylaminosulphonyl-amino-benzoic
acid.
The oral LD50 is greater than 5000 mg/kg bw in rats and
250-500 mg/kg bw in guinea pigs and rabbits. The oral LD50 of a
major plant metabolite, DMST (N,N-dimethyl-N'-p-tolylsulphamide),
exceeds 2500 mg/kg in rats and 1000 mg/kg in mice and rabbits.
In one study in rats, tolylfluanid was found to be maternally
toxic and embryotoxic at doses above 100 mg/kg bw/day but not
teratogenic at doses up to and including 1000 mg/kg bw/day. In a
two-generation two-litter-per-generation reproduction study, dietary
levels up to and including 1500 ppm did not affect rat reproduction.
No malformations were observed at dietary levels up to and including
7500 ppm.
In a short-term toxicity study with beagle dogs, tolylfluanid did
not cause adverse effects for 12 months at doses up to and including
12.5 mg/kg bw/day.
Tolylfluanid was mutagenic in prokaryotes and eukaryotes in four
of seven in vitro assays. In vivo tests, however, did not indicate
a mutagenic potential.
In a long-term study in Wistar rats, a higher incidence of
malignant uterine tumours was found in the treated groups as compared
to controls, but no statistically significant dose-related pattern was
evident. Moreover in a second, concurrent, group of controls the
incidence was comparable to that in treated groups. In animals treated
with 1500 or 7500 ppm tolylfluanid, bone alterations were also found
which were suggested to be due to the fluorine constituent of
tolylfluanid. No alterations were found in the thyroid or in other
organs.
The Meeting noted that in the NMRI mouse study, the control
animals were four months older than the treated groups, thus
compromising the design of the study. Lung and liver adenomas occurred
at similar frequency in all treated groups, but because of their known
occurrence in mice they were not considered to be biologically
relevant. The Meeting therefore concluded that the results of this
study did not indicate an oncogenic potential.
The Meeting did not request a special study on thyroid function
because the dichlorofluorometylthio group is common to dichlofluanid,
for which a NOAEL for the thyroid has been demonstrated to be greater
than 1500 ppm.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 300 ppm in the diet, equal to 15 mg/kg bw/day
Dog: 12.5 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Observations in man.
REFERENCES
Abbink, J. & Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: investigation
of the biokinetic behaviour in the rat. Institute for Metabolism
Research, Bayer AG. Unpublished report No. 181081-9. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Bomhard, E. & Schilde, B. 1976. KUE 1318T / Subchronic Toxicological
Experiments on Rats (feeding experiment over 3 months). Institute of
Toxicology, Bayer AG. Unpublished report No. 5929. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
den Boer, W.C. 1987. Mutagenicity test on KUE 13183b in the CHO/HGPRT
forward mutation assay. Hazleton Biotechnologies, The Netherlands.
Unpublished report No. R4204. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Ecker, W. 1978. Biotransformation of (14C) tolylfluanid in the rat.
Institute for Pharmakocinetics. Bayer AG. Unpublished report No. 1281.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Ecker, W. & Brauner, A. 1987. Biotransformation of <ring-U-14C>
tolylfluanid by the rat following oral administration. Institute for
Pharmacokinetics, Bayer AG. Unpublished report No. 2826. Submitted to
WHO by Bayer AG, Bayerwerk, FRG.
Flucke, W. & Kimmerle, G. 1978. Triadimefon (MEB 6447) and
Tolylfluanid (KUE 13183b) Study for Acute Combination Toxicity.
Institute of Toxicology, Bayer AG. Unpublished report No. 7304.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heidemann, A. 1987. KUE 13183b / Detection of gene mutations in
somatic mammalian cells in culture: HGPRT-Test with V79 cells.
Laboratory for Mutagenicity Testing, Darmstadt. Unpublished report
No. R 4103. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. 1983. KUE 13183b / Study for sensitising effect on
guinea pigs. Institute of Toxicology, Bayer AG. Unpublished report
No. 11492. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. & Pauluhn, J. 1983. 13183b / Study for Acute Toxicity
Institute of Toxicology, Bayer AG. Unpublished report No. 11383.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1979. 13183b / Salmonella-microsome test for point
mutagenic effects. Institute of Toxicology, Bayer AG. Unpublished
report No. 8265. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1980. KUE 13183b / Micronucleus test on the mouse to
evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 9149. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1983. KUE 13183b / Cytogenetic study of the Chinese
hamster's bone marrow in vivo to evaluate for mutagenic effect.
Institute of Toxicology, Bayer AG. Unpublished report No. 11792.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984a. KUE 13183b / Cytogenicity study of the
spermatogonia of the Chinese hamster in vivo to evaluate for
mutagenic effect. Institute of Toxicology, Bayer AG. Unpublished
report No. 12739. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984b. KUE 13183b / Cytogenetic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Institute of Toxicology, Bayer AG. Unpublished report
No. 12836. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1986. KUE 13183b / Dominant lethal test on the male mouse
to evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 15017. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1988. Spot test on cross-bred C57B1/6J × T stock mouse
fetuses to evaluate for induced somatic changes in the genes of the
coat pigment cells. Institute of Toxicology, Bayer AG. Unpublished
report No. 16752. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. 1983. KUE 13183b / Acute toxicity to the sheep after oral
administration. Institute of Toxicology, Bayer AG. Unpublished report
No. 11975. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. & Mirea, D. 1974. KUE 13183b / Subchronic toxicity study
on dogs (13-week feeding experiment). Institute of Toxicology,
Bayer AG. Unpublished report No. 4957. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Hoorn, A.J.W. 1984. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the reverse mutation induction assay with saccharomyces cerevisiae
strains S138 and S211a. Litton Bionetics, The Netherlands. Unpublished
report No. 3060. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoorn, A.J.W. 1985. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the mouse lymphoma forward mutation assay. Litton Bionetics, The
Netherlands. Unpublished report No. R 3192. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
von Keutz, E. & Nash, G. 1986. KUE 13183b / Chronic toxicity to dogs
after oral administration (12-month capsule study). Institute of
Toxicology, Bayer AG. Unpublished report No. 12999. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. 1968. BAY 49854 / Toxicological studies. Institute of
Toxicology, Bayer AG. Unpublished report No. 832. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Lorke, D. 1967. Toxicological studies on the active
ingredient BAY 49854. Institute of Toxicology, Bayer AG. Unpublished
report No. 323. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971a. Methyl-Euparen / Subacute cutaneous
application to rabbits. Institute of Toxicology, Bayer AG. Unpublished
report No. 2619. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971b. DMST / Toxicological Study.
Institute of Toxicology, Bayer AG. Unpublished report No. 2681.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Krötlinger, F. & Loser, E. 1982. KUE 13183b / Chronic toxicological
study in rats (feeding for 2 years). Institute of Toxicology, Bayer
AG. Unpublished report No. 10978. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Loser, E. 1980. KUE 13183b / Generation study with rats. Institute of
Toxicology, Bayer AG. Unpublished report No. 9419. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Machemer, L. 1976. KUE 13183b / Studies on embryotoxic and teratogenic
effects on rats after oral administration. Institute of Toxicology,
Bayer AG. Unpublished report No. 5888. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Mohr, U. 1982. KUE 13183b / Study for cancerogenic effect on NMRI-mice
(feeding study for 104 weeks). Appendix: Histopathological Individual
Findings. Medizinische Hochshule, Hannover. Unpublished report
No. R 2225. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Oesch, F. 1977. Kue 13183b / Ames Test for guparen M. Institute of
Biochemical Pharmacology, University of Mainz. Unpublished report from
November 10. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Pauluhn, J. 1984. KUE 13183b / Study for irritant/corrosive effect on
skin and eye (rabbit). Institute of Toxicology, Bayer AG. Unpublished
report No. 12362. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Voelkner, W. 1988a. Sister chromatide exchange assay in bone marrow
cells of the mouse. Cytotest Cell Research GmbH, Darmstadt, FRG.
Unpublished report No. 4422. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Voelkner, W. 1988b. Mouse germ-cell cytogenetic assay. Cytotest Cell
Research GmbH, Darmstadt, FRG. Unpublished report No. 4485. Submitted
to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. et al. 1977. KUE 13183b / Biokinetic Investigations of
Rats. Institute for Pharmacokinetics, Bayer AG. Unpublished report
No. 1165. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: whole body
autoradiographic distribution of the radioactivity in the rat.
Institute for Metabolism Research, Bayer AG. Unpublished report
No. 181080-8. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
 Toxicological Studies
Special studies on carcinogenicity
Mice
Groups of NMRI mice (50/sex/group) were given tolylfluanid
technical (99.1% purity in a 90% pre-mix) in the diet (0, 200, 1000,
5000 ppm) for 104 weeks. In relation to body weight, the males were
given 32.6, 161.8 or 768.9 mg/kg bw and the females 45.6, 202.4 or
952.3 mg/kg bw on average. Animals were observed twice a day for
mortality or adverse physical/behavioural signs. Body weights were
recorded weekly and food consumption of each animal was determined
twice a week. Routine haematological and clinical chemistry
examinations were performed before the start of the study and after 12
and 24 months. All animals, including those which died or were
sacrificed before the end of the study, were dissected, their organs
weighed and tissues prepared for microscopic examination. For
technical reasons the treated groups started 4 months after the
control group. This discrepancy in time was considered by the author
to be the cause of the higher body weight gain (+55% and +10/12% of
the starting weight in controls and treated males, respectively; +64%
and +18/24% in control and treated females, respectively) and food
consumption (about 30% higher) found in control animals and of the
differences between control and study groups in the haematological and
clinical laboratory examinations. No dose-related difference in any of
these parameters was, however, found in animals of the treated groups.
The mortality rate was higher in female groups but a dose-related
effect was not evident.
At necropsy, hepatocellular adenomas and pulmonary adenomas were
found in all control and treated groups. The incidence of liver
rumours was higher in male groups, but no treatment-related effect was
evident (10/51, 7/50, 13/50 and 9/50 in control, 200, 1000 and
5000 ppm groups, respectively). The incidence of lung adenomas was
17/51, 17/50, 20/50 and 20/50 in males and 9/49, 6/50, 14/50 and 7/51
in females of control, 200, 1000 and 5000 ppm groups, respectively.
Incidence, latency, variety and organ distribution of rumours did not
indicate any dose-related effect of tolylfluanid (Mohr, 1982).
Rats
(See "Long-term studies".)
Special studies on embryotoxicity and teratogenicity
Groups (n=22-24) of fertilized female rats (75-95 day-old Long
Evans FB 30) were given oral daily doses (0, 100, 300, 1000 mg/kg bw)
of tolylfluanid (99.9% purity) from day 6 through day 15 of gestation.
Animals were observed routinely for physical appearance, behaviour
and body weight gain. At day 20 of gestation pups were delivered
by caesarean section. Foetuses were weighed, sexed, inspected for
external abnormalities and examined for visceral and bone
malformations.
No alteration of physical appearance and behaviour was observed
in any group. Two dams of the mid-dose group died of effects unrelated
to treatment. Body weight gains were reduced in a dose-dependent
manner in the mid- and high-dose groups. However, dams compensated
after end of treatment. The average foetal weight was reduced by
treatment of the dams with 300 or 1000 mg/kg bw. The resorption ratio
in the high-dose group was slightly, not statistically significantly,
higher than in the control group, because of complete loss of embryos
by one female. There was no evidence of teratogenicity at any of the
doses used. The dose of 100 mg/kg bw had no toxic effect to dams and
foetuses (Machemer, 1976).
Acute toxicity of metabolites
4-dimethylamino-sulftoluidine, a metabolite of tolylfluanid, was
tested for acute toxicity. After single administration, LD50s were
as follows (Kimmerle & Solmecke, 1971b).
Species Route mg/kg bw
Rat (male) p.o. >2500
Mouse (male) p.o. >1000
Rabbit (male) p.o. >1000
Rat (male) i.p. 551
Special studies on mutagenicity
Tolylfluanid was variably active in a range of in vitro
assays. In vivo, however, tolylfluanid was inactive. Results are
summarized in Table 1.
Table 1. Results of Mutagenicity assays on tolylfluanid
Test system Test Object Concentration of tolylfluanid Purity Results References
Ames test (with and S. typhimurium 3.15-1000 µg/plate 100% weakly Oesch, 1977
without activation) TA-98,100,1537 dissolved in DMSO positive 1/
Ames test (with and S. typhimurium 4-2500 µg/plate 100% weakly Herbold, 1979
without activation) TA-98,100,1535, dissolved in DMSO positive 2/
1537
Reverse mutation Saccharomyces Nonactivated: 1-200 µg/ml 99.1% negative Hoorn, 1984
in vitro cervisiae Activated: 1-100 µg/ml
(S138, S211alfa)
Gene mutation Somatic Nonactivated: 4-40 µg/ml 98.5% negative 3/ Heidemann,1987
(HGPRT-test) mammalian cells Activated: 300-3000 µg/ml
in vitro (V79)
Gene mutation Chinese hamster Nonactivated: 0.5-6 µg/ml 98.5% negative 4/ den Boer, 1987
(HGPRT-test) ovary cells Activated: 3-30 µg/ml
in vitro
Forward mutation Mouse lymphoma Nonactivated: 25-300 µg/ml 99.1% positive Hoorn, 1985
in vitro cells (L5178Y) Activated: 500-7500 µg/ml
Cytogenetic assay Human lymphocytes 0.1-10 µg/ml (activated 99.2% positive 5/ Herbold, 1984b
in vitro and nonactivated)
Micronucleus test Mice (NMRI strain) 2x250 and 2x500 mg/kg 98.8% negative 6/ Herbold, 1980
(p.o. 24 h apart)
Table 1. (cont'd)
Test system Test object Concentration of tolylfluanid Purity Results References
Dominant lethal test Mice (NMRI strain) 4000 mg/kg p.o.(mortality:5/50) 98.8% negative Herbold, 1986
8000 mg/kg p.o.(mortality:12/50)
Cytogenetic assay Chinese hamster 4000 mg/kg p.o. 99.7% negative 6/ Herbold, 1983
in vivo bone marrow
Cytogenetic assay Chinese hamster 2x250 and 2x500 mg/kg 93.1% inconclusive Herbold, 1984a
in vivo spermatogonia (p.o. 24 h apart) 7/
Cytogenetic assay Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 8/ Voelkner,1988b
in vivo germ cells
Sister chromatide Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 7/ Voelkner,1988a
exchange bone marrow
Mammalian Mice C5781/6JxT 1750-7000 mg/kg bw p.o. 98.4% negative 9/ Herbold, 1988
Spot test
1/ With S-9 mix only; positive controls (benzo(a)pyrene 4,5-oxide, N-methyl-N'-nitro-N-nitrosoguanidine,
3,methylcholanthrene, 2-aminoanthracene) yielded expected positive results.
2/ TA-100 only; positive controls (endoxan, trypaflavin) yielded expected positive results.
3/ Positive controls (ethyl-methane sulfonate (EMS), 9,10-dimethyl-1,2-benzanthracene (DMBA) yielded expected positive
results.
4/ Positive controls (5-bromodeoxyuridine and 3-methylcholanthrene) yielded expected positive results.
5/ At cytotoxic concentrations; positive controls (mitomycin c, endoxan) yielded expected positive results.
6/ Positive controls (endoxan) yielded expected positive results.
7/ Positive controls (doxorubicin) yielded expected positive results.
8/ Positive controls (cyclophosphamide) yielded expected positive results.
9/ Positive controls (1-ethyl-1-nitrosourea) yielded expected positive results.
Special study on potentiating effects
Equitoxic doses of tolylfluanid (purity not reported) and
triadimefon (89.5% purity) were given in combination to rats. No
potentiating effect was noted (Flucke & Kimmerle, 1978).
Special study on reproduction
Groups of Long Evans FB30 rats (30 animals/dose, 10 males and 20
females) were fed diets containing tolylfluanid (technical grade,
98.8% purity in a 90% pre-mix) at levels of of 0, 300, 1500 or
7500 ppm. After 70 days of treatment F0 animals were mated on a
2-female-to-1-male basis. After delivery (F1a) and a 4-week
lactation and a 2-week waiting period, F0 rats were mated again.
After delivery (F1b) lactation lasted for 4 weeks and then F0
animals were sacrificed. F1b animals (20 females-10 males/dose) were
then mated and F1a and F1b generations were obtained. The study
was ended when F1b generation reached 4 weeks of age.
Rats were weighed weekly and pups were weighed at birth, 5 and 7
days after birth, and weekly thereafter. Pups were also observed
immediately after birth and during lactation.
F0 generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in a
dose-related manner in male rats treated with 1500 or 7500 ppm; only
the dose of 7500 ppm was associated with reduced body weight in female
rats. Fertility indices and litter sizes of first and second matings
were not reduced by any of the treatments. Survival rate was slightly
reduced in F1b generation of the high-dose group. Lactation index (%
of pups surviving 4 weeks) was reduced in the F1a generation of the
7500 ppm group, but in the F1b generation of the same group was
significantly higher than controls. Body weight of pups at birth and
body weight gain were significantly lower in both F1a and F1b
generations of the 7500 ppm group. None of the pups showed
malformations.
F1b generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in the
7500 ppm group. Fertility indices and litter sizes of first and second
matings were not reduced by any of the treatments. Survival rate was
reduced in F2b generation of the high-dose group. Lactation index
(% of pups surviving 4 weeks) was reduced in F2a and F2b
generations of the 7500 ppm group and in the F2b generation of the
1500 ppm group. In the latter group, however, the number of pups
surviving four weeks was the same as in the control group and the
lower lactation index was due to a higher number of pups at 5 days
when all litters were reduced to a maximum of 10 pups rearing. Body
weight of pups at birth and body weight gain was lower in both F2a
and F2b generations of the 7500 ppm group. None of the pups showed
malformations.
In summary, this study shows that tolylfluanid at doses up to
1500 ppm did not affect the reproduction of rats. The NOAEL in this
study was found to be 300 ppm (Loser, 1980).
Special study on skin and eye irritation
A 90% pre-mix with 99.2% active ingredient was applied to rabbit
skin for 24 hours under semi-occlusive conditions. Tolylfluanid did
not show skin irritant properties. The test compound dust (0.1 g),
however, when placed on the conjuntival sac of rabbit eyes, displayed
a strong irritant potential (redness and swelling of the conjuntivae).
The effect was reversible within 14 days (Helmann & Pauluhn, 1983).
A further study was conducted on irritant and corrosive effects
on the skin and eyes. Tolylfluanid (99.1% purity) did not show
irritant effect on the skin (4 hours' exposure). However, 70 mg of
a.i. placed in the conjuntival sac produced redness and swelling of
the conjuntivae which were completely reversible within 21 days. No
cornea or iris lesions were found at any time (Pauluhn, 1984).
Special study on subacute cutaneous toxicity in rabbits
Rabbits (5 males and 5 females) received daily dermal doses of
500 mg/kg bw of tolylfluanid (98.9% purity) for fourteen days. Each
exposure lasted 24 hours. Before and immediately after the end of
treatment, and 2 weeks later, clinical-chemical tests were performed,
including blood tests (erythrocyte, leucocyte and thrombocyte count,
haemoglobin determination, etc.), liver function tests, serum
transaminases, sorbitol dehydrogenase, bilirubin and albumin) and
kidney function tests (urinanalysis and serum urea concentration). No
variation was found between control and treated animals (Kimmerle &
Solmecke, 1971a).
Acute toxicity
Table 2 reports the LD50 values for tolylfluanid and
metabolites as determined in different studies and animal species.
In rats, signs of toxicity observed after oral administration
were altered behaviour, disturbed motility and dyspnoea. In mice,
guinea pigs, rabbits and cats tolylfluanid caused deterioration of
general conditions and (in cats only) vomiting. In sheep,
tolylfluanid, orally administered, caused anorexia, weakness of
extremities and loose faeces.
Intraperitoneal injection to rats caused disturbed motility,
staggering, spastic gait, distention of abdomen, narrowed eyelids, a
specific disturbed behaviour. Irritation of abdominal organs was found
at autopsy.
The dermal application of tolylfluanid at doses up to
5000 mg/kg bw was tolerated without signs of toxicity or skin
alterations.
The skin-sensitising potential of tolylfluanid was assessed by
the Magnusson and Klingman's maximization test on guinea pigs (n=15).
The concentration of tolylfluanid (99.2% purity in a 90% pre-mix) used
were 1% for the intradermal induction, 0.6% for the topical induction,
and 0.3% for the topical challenge. The results indicate that
tolylfluanid has a skin-sensitising potential (Heimann, 1983).
Short-term studies
Rats
0, 150, 500, 1500 or 4500 ppm of tolylfluanid (99% purity) were
given to rats (15 males and 15 females) in the diet for 3 months. All
dose-regimens were tolerated without alteration of behaviour, weight,
mortality or clinical chemistry tests. Morphological examination of
organs and tissues did not reveal any treatment-induced alterations
(Bomhard and Schilde, 1976).
Dogs
Beagle dogs (4 males and 4 females/group) were administered
0, 330, 1000 or 3000 ppm of tolylfluanid (99.7% purity) in diet for 3
months. Animals of the high-dose group showed poor general condition
and 6/8 animals had a reduced food intake and weight gain. In this
group an increased serum alkaline phosphatase activity and a
retardation of its age-related decrease as compared to controls was
found. Increased liver relative weight and increased PAS histological
reaction were also found in these animals. Doses up to 1000 ppm did
not induce any detectable adverse effect (Hoffmann & Mirea, 1974).
Table 2. Acute toxicity of tolylfluanid (mg/kg bw)
Species Sex Route LD50 LC50 Reference
Mouse M oral >1000 1/ - Kimmerle, 1968
Rat M oral >2500 2/ - Kimmerle & Lorke, 1967.
Rat M inhalation* - 265 2/ Kimmerle & Lorke, 1967
(4-hr exp.)
Rat M i.p. 20.2 1/ - Kimmerle, 1968
Rat F i.p. 25.6 1/ - Kimmerle, 1968
Rat F oral >5000 3/ - Heimann & Pauluhn, 1983
Rat M i.p. 14.7 3/ - "
Rat F i.p. 15.9 3/ - "
Rat M&F dermal >5000 3/ - "
(24-hr exp.)
Guinea pig F oral 250-500 1/ - Kimmerle, 1968
Rabbit M oral 250-500 1/ - Kimmerle, 1968
Cat M&F oral >500 1/ - Kimmerle, 1968
Sheep M&F oral 625-1250 3/ - Hoffmann, 1983
1/ 99.6% purity
2/ 98.7% purity
3/ 99.2% purity in a 90% pre-mix
* Dynamic spraying with Lutrol/ethanol. In static spraying experiments, mice, guinea
pigs and rabbits were found to be more sensitive than rats to tolylfluanid.
In another study, groups (4 male/4 female) of beagle dogs were
orally treated for 12 months with tolylfluanid (99.2% purity in a 90%
pre-mix) 0, 2.5, 12.5 or 62.5 (let to 33rd week) and 125 (34th to 52nd
week) mg/kg bw. The test compound was administered in gelatin
capsules. All animals survived the study and did not show any
treatment-related effect on appearance or behaviour. Body weight
development in male control animals (+4.4 kg in 12 months) was above
the average of the historical controls (+2.8 kg in 12 months), while
it was significantly reduced in the animals of the high-dose group.
The females in the high-dose group also had lower body weight gain.
Animals of the high-dose group vomited quite frequently after
administration of the compound. They also had a significant increase
of serum alanine aminotransferase (ALT) and glutamate dehydrogenase
(GLDH), and a retarding of the physiological age-related decrease of
the alkaline phosphatase activity. No liver alteration was found,
however, at histology.
An effect on renal function was also evident in the high-dose
group. Urea and creatinine concentrations were significantly increased
and in two dogs glycosuria and proteinuria were found. Histology
revealed slight alterations of cortical tubules (dilation, epithelial
flattening, focal hypertrophy and/or desquamation of the epithelia) in
all dogs of the high-dose group. Slightly lower serum potassium
concentrations were found in the high-dose group animals as compared
to control values, possibly as a result of the frequent vomiting.
No treatment-related effect was evident on blood, blood
coagulation and transparent media and fundus of the eye.
Oral doses of tolylfluanid up to 12.5 mg/kg bw were therefore
tolerated by dogs for 12 months without detectable adverse effects
(von Keutz & Nash, 1986).
Long-term studies
Rats
Wistar rats (50 males and 50 females/group; 100 male and 100
females, controls) were fed for 2 years with tolylfluanid (98.9%
purity in 90% pre-mix) at concentrations of 0, 300, 1500, 7500 ppm.
Toxicity following chronic administration and carcinogenic potential
of tolylfluanid were evaluated.
The animals in the high-dose group had a reduced food consumption
(-10%) and a reduced (about 10%) body weight gain; appearance,
behaviour and mortality were not affected by the treatment. Clinical
laboratory tests showed random alteration of some blood parameters
(mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte
count, polymorphonuclear cells and lymphocytes). Plasma alkaline
phosphatase (AP), transaminases (ALT and AST), bilirubin and total
protein were measured as indices of liver function. No dose-related
alteration of these parameters was found. Gross pathological and
histological examinations did not detect any indication of liver
damage. Urinanalysis did not indicate any dose-related kidney
alteration. Blood sugar and cholesterol were within normal range in
all groups. On autopsy, bone alterations (diffuse hyperostosis of the
rib bones, hardened cranial bones, focal hyperostosis of the skull
caps) in both sexes, and a faster growth of the incisors of the upper
jaw in males only, were found in the 1500 and 7500 ppm groups. These
effects were presumably due to an increased fluorine intake from the
active ingredient.
Tumours of endocrine and reproductive system accounted for about
80% of the rumours seen in the study. Only malignant uterine tumours
appeared higher in all treated groups as compared to controls. The
incidence was 3/50, 8/50, 12/50 and 13/50 in the 0, 300, 1500 and
7500 ppm groups, respectively. An unusually low incidence of these
fairly con, non tumours was found in control animals. Therefore, a
second, concurrent group of 50 controls was studied in which the
incidence of malignant uterine rumours was similar (9/50) to that of
the treated groups. The test, therefore, did not provide indications
of carcinogenic potential for tolylfluanid.
The concentration of tolylfluanid without detectable adverse
effect was 300 ppm (equivalent to 15 mg/kg bw) (Krötlinger &
Loser, 1982).
Observations in humans
Small cottonwool pads on which an unknown concentration of
tolylfluanid was applied, were placed on the forearm of 10 male
volunteers for 24 hours. Subjects were observed for 10 days; no
skin-irritant effects were noted (Kimmerle, 1968).
COMMENTS
Following oral administration to rats, tolylfluanid was rapidly
absorbed, metabolized and eliminated in urine and faeces. When using
14C-ring-labelled tolylfluanid (2 or 20 mg/kg bw), less than 0.2% of
the administered radioactivity was detectable in the body 2 days after
administration. The highest concentrations were found in liver and
kidney.
When using 14C-tolylfluanid (0.1-20 mg/kg bw) labelled at the
fluorodichloronenthyl group instead, accumulation of radioactivity was
evident in the thyroid. The major urinary metabolites were thiazolidine-
2-thione-4-carboxylic acid and 4-dimethylaminosulphonyl-amino-benzoic
acid.
The oral LD50 is greater than 5000 mg/kg bw in rats and
250-500 mg/kg bw in guinea pigs and rabbits. The oral LD50 of a
major plant metabolite, DMST (N,N-dimethyl-N'-p-tolylsulphamide),
exceeds 2500 mg/kg in rats and 1000 mg/kg in mice and rabbits.
In one study in rats, tolylfluanid was found to be maternally
toxic and embryotoxic at doses above 100 mg/kg bw/day but not
teratogenic at doses up to and including 1000 mg/kg bw/day. In a
two-generation two-litter-per-generation reproduction study, dietary
levels up to and including 1500 ppm did not affect rat reproduction.
No malformations were observed at dietary levels up to and including
7500 ppm.
In a short-term toxicity study with beagle dogs, tolylfluanid did
not cause adverse effects for 12 months at doses up to and including
12.5 mg/kg bw/day.
Tolylfluanid was mutagenic in prokaryotes and eukaryotes in four
of seven in vitro assays. In vivo tests, however, did not indicate
a mutagenic potential.
In a long-term study in Wistar rats, a higher incidence of
malignant uterine tumours was found in the treated groups as compared
to controls, but no statistically significant dose-related pattern was
evident. Moreover in a second, concurrent, group of controls the
incidence was comparable to that in treated groups. In animals treated
with 1500 or 7500 ppm tolylfluanid, bone alterations were also found
which were suggested to be due to the fluorine constituent of
tolylfluanid. No alterations were found in the thyroid or in other
organs.
The Meeting noted that in the NMRI mouse study, the control
animals were four months older than the treated groups, thus
compromising the design of the study. Lung and liver adenomas occurred
at similar frequency in all treated groups, but because of their known
occurrence in mice they were not considered to be biologically
relevant. The Meeting therefore concluded that the results of this
study did not indicate an oncogenic potential.
The Meeting did not request a special study on thyroid function
because the dichlorofluorometylthio group is common to dichlofluanid,
for which a NOAEL for the thyroid has been demonstrated to be greater
than 1500 ppm.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 300 ppm in the diet, equal to 15 mg/kg bw/day
Dog: 12.5 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Observations in man.
REFERENCES
Abbink, J. & Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: investigation
of the biokinetic behaviour in the rat. Institute for Metabolism
Research, Bayer AG. Unpublished report No. 181081-9. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Bomhard, E. & Schilde, B. 1976. KUE 1318T / Subchronic Toxicological
Experiments on Rats (feeding experiment over 3 months). Institute of
Toxicology, Bayer AG. Unpublished report No. 5929. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
den Boer, W.C. 1987. Mutagenicity test on KUE 13183b in the CHO/HGPRT
forward mutation assay. Hazleton Biotechnologies, The Netherlands.
Unpublished report No. R4204. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Ecker, W. 1978. Biotransformation of (14C) tolylfluanid in the rat.
Institute for Pharmakocinetics. Bayer AG. Unpublished report No. 1281.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Ecker, W. & Brauner, A. 1987. Biotransformation of <ring-U-14C>
tolylfluanid by the rat following oral administration. Institute for
Pharmacokinetics, Bayer AG. Unpublished report No. 2826. Submitted to
WHO by Bayer AG, Bayerwerk, FRG.
Flucke, W. & Kimmerle, G. 1978. Triadimefon (MEB 6447) and
Tolylfluanid (KUE 13183b) Study for Acute Combination Toxicity.
Institute of Toxicology, Bayer AG. Unpublished report No. 7304.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heidemann, A. 1987. KUE 13183b / Detection of gene mutations in
somatic mammalian cells in culture: HGPRT-Test with V79 cells.
Laboratory for Mutagenicity Testing, Darmstadt. Unpublished report
No. R 4103. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. 1983. KUE 13183b / Study for sensitising effect on
guinea pigs. Institute of Toxicology, Bayer AG. Unpublished report
No. 11492. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. & Pauluhn, J. 1983. 13183b / Study for Acute Toxicity
Institute of Toxicology, Bayer AG. Unpublished report No. 11383.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1979. 13183b / Salmonella-microsome test for point
mutagenic effects. Institute of Toxicology, Bayer AG. Unpublished
report No. 8265. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1980. KUE 13183b / Micronucleus test on the mouse to
evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 9149. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1983. KUE 13183b / Cytogenetic study of the Chinese
hamster's bone marrow in vivo to evaluate for mutagenic effect.
Institute of Toxicology, Bayer AG. Unpublished report No. 11792.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984a. KUE 13183b / Cytogenicity study of the
spermatogonia of the Chinese hamster in vivo to evaluate for
mutagenic effect. Institute of Toxicology, Bayer AG. Unpublished
report No. 12739. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984b. KUE 13183b / Cytogenetic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Institute of Toxicology, Bayer AG. Unpublished report
No. 12836. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1986. KUE 13183b / Dominant lethal test on the male mouse
to evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 15017. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1988. Spot test on cross-bred C57B1/6J × T stock mouse
fetuses to evaluate for induced somatic changes in the genes of the
coat pigment cells. Institute of Toxicology, Bayer AG. Unpublished
report No. 16752. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. 1983. KUE 13183b / Acute toxicity to the sheep after oral
administration. Institute of Toxicology, Bayer AG. Unpublished report
No. 11975. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. & Mirea, D. 1974. KUE 13183b / Subchronic toxicity study
on dogs (13-week feeding experiment). Institute of Toxicology,
Bayer AG. Unpublished report No. 4957. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Hoorn, A.J.W. 1984. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the reverse mutation induction assay with saccharomyces cerevisiae
strains S138 and S211a. Litton Bionetics, The Netherlands. Unpublished
report No. 3060. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoorn, A.J.W. 1985. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the mouse lymphoma forward mutation assay. Litton Bionetics, The
Netherlands. Unpublished report No. R 3192. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
von Keutz, E. & Nash, G. 1986. KUE 13183b / Chronic toxicity to dogs
after oral administration (12-month capsule study). Institute of
Toxicology, Bayer AG. Unpublished report No. 12999. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. 1968. BAY 49854 / Toxicological studies. Institute of
Toxicology, Bayer AG. Unpublished report No. 832. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Lorke, D. 1967. Toxicological studies on the active
ingredient BAY 49854. Institute of Toxicology, Bayer AG. Unpublished
report No. 323. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971a. Methyl-Euparen / Subacute cutaneous
application to rabbits. Institute of Toxicology, Bayer AG. Unpublished
report No. 2619. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971b. DMST / Toxicological Study.
Institute of Toxicology, Bayer AG. Unpublished report No. 2681.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Krötlinger, F. & Loser, E. 1982. KUE 13183b / Chronic toxicological
study in rats (feeding for 2 years). Institute of Toxicology, Bayer
AG. Unpublished report No. 10978. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Loser, E. 1980. KUE 13183b / Generation study with rats. Institute of
Toxicology, Bayer AG. Unpublished report No. 9419. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Machemer, L. 1976. KUE 13183b / Studies on embryotoxic and teratogenic
effects on rats after oral administration. Institute of Toxicology,
Bayer AG. Unpublished report No. 5888. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Mohr, U. 1982. KUE 13183b / Study for cancerogenic effect on NMRI-mice
(feeding study for 104 weeks). Appendix: Histopathological Individual
Findings. Medizinische Hochshule, Hannover. Unpublished report
No. R 2225. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Oesch, F. 1977. Kue 13183b / Ames Test for guparen M. Institute of
Biochemical Pharmacology, University of Mainz. Unpublished report from
November 10. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Pauluhn, J. 1984. KUE 13183b / Study for irritant/corrosive effect on
skin and eye (rabbit). Institute of Toxicology, Bayer AG. Unpublished
report No. 12362. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Voelkner, W. 1988a. Sister chromatide exchange assay in bone marrow
cells of the mouse. Cytotest Cell Research GmbH, Darmstadt, FRG.
Unpublished report No. 4422. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Voelkner, W. 1988b. Mouse germ-cell cytogenetic assay. Cytotest Cell
Research GmbH, Darmstadt, FRG. Unpublished report No. 4485. Submitted
to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. et al. 1977. KUE 13183b / Biokinetic Investigations of
Rats. Institute for Pharmacokinetics, Bayer AG. Unpublished report
No. 1165. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: whole body
autoradiographic distribution of the radioactivity in the rat.
Institute for Metabolism Research, Bayer AG. Unpublished report
No. 181080-8. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Toxicological Studies
Special studies on carcinogenicity
Mice
Groups of NMRI mice (50/sex/group) were given tolylfluanid
technical (99.1% purity in a 90% pre-mix) in the diet (0, 200, 1000,
5000 ppm) for 104 weeks. In relation to body weight, the males were
given 32.6, 161.8 or 768.9 mg/kg bw and the females 45.6, 202.4 or
952.3 mg/kg bw on average. Animals were observed twice a day for
mortality or adverse physical/behavioural signs. Body weights were
recorded weekly and food consumption of each animal was determined
twice a week. Routine haematological and clinical chemistry
examinations were performed before the start of the study and after 12
and 24 months. All animals, including those which died or were
sacrificed before the end of the study, were dissected, their organs
weighed and tissues prepared for microscopic examination. For
technical reasons the treated groups started 4 months after the
control group. This discrepancy in time was considered by the author
to be the cause of the higher body weight gain (+55% and +10/12% of
the starting weight in controls and treated males, respectively; +64%
and +18/24% in control and treated females, respectively) and food
consumption (about 30% higher) found in control animals and of the
differences between control and study groups in the haematological and
clinical laboratory examinations. No dose-related difference in any of
these parameters was, however, found in animals of the treated groups.
The mortality rate was higher in female groups but a dose-related
effect was not evident.
At necropsy, hepatocellular adenomas and pulmonary adenomas were
found in all control and treated groups. The incidence of liver
rumours was higher in male groups, but no treatment-related effect was
evident (10/51, 7/50, 13/50 and 9/50 in control, 200, 1000 and
5000 ppm groups, respectively). The incidence of lung adenomas was
17/51, 17/50, 20/50 and 20/50 in males and 9/49, 6/50, 14/50 and 7/51
in females of control, 200, 1000 and 5000 ppm groups, respectively.
Incidence, latency, variety and organ distribution of rumours did not
indicate any dose-related effect of tolylfluanid (Mohr, 1982).
Rats
(See "Long-term studies".)
Special studies on embryotoxicity and teratogenicity
Groups (n=22-24) of fertilized female rats (75-95 day-old Long
Evans FB 30) were given oral daily doses (0, 100, 300, 1000 mg/kg bw)
of tolylfluanid (99.9% purity) from day 6 through day 15 of gestation.
Animals were observed routinely for physical appearance, behaviour
and body weight gain. At day 20 of gestation pups were delivered
by caesarean section. Foetuses were weighed, sexed, inspected for
external abnormalities and examined for visceral and bone
malformations.
No alteration of physical appearance and behaviour was observed
in any group. Two dams of the mid-dose group died of effects unrelated
to treatment. Body weight gains were reduced in a dose-dependent
manner in the mid- and high-dose groups. However, dams compensated
after end of treatment. The average foetal weight was reduced by
treatment of the dams with 300 or 1000 mg/kg bw. The resorption ratio
in the high-dose group was slightly, not statistically significantly,
higher than in the control group, because of complete loss of embryos
by one female. There was no evidence of teratogenicity at any of the
doses used. The dose of 100 mg/kg bw had no toxic effect to dams and
foetuses (Machemer, 1976).
Acute toxicity of metabolites
4-dimethylamino-sulftoluidine, a metabolite of tolylfluanid, was
tested for acute toxicity. After single administration, LD50s were
as follows (Kimmerle & Solmecke, 1971b).
Species Route mg/kg bw
Rat (male) p.o. >2500
Mouse (male) p.o. >1000
Rabbit (male) p.o. >1000
Rat (male) i.p. 551
Special studies on mutagenicity
Tolylfluanid was variably active in a range of in vitro
assays. In vivo, however, tolylfluanid was inactive. Results are
summarized in Table 1.
Table 1. Results of Mutagenicity assays on tolylfluanid
Test system Test Object Concentration of tolylfluanid Purity Results References
Ames test (with and S. typhimurium 3.15-1000 µg/plate 100% weakly Oesch, 1977
without activation) TA-98,100,1537 dissolved in DMSO positive 1/
Ames test (with and S. typhimurium 4-2500 µg/plate 100% weakly Herbold, 1979
without activation) TA-98,100,1535, dissolved in DMSO positive 2/
1537
Reverse mutation Saccharomyces Nonactivated: 1-200 µg/ml 99.1% negative Hoorn, 1984
in vitro cervisiae Activated: 1-100 µg/ml
(S138, S211alfa)
Gene mutation Somatic Nonactivated: 4-40 µg/ml 98.5% negative 3/ Heidemann,1987
(HGPRT-test) mammalian cells Activated: 300-3000 µg/ml
in vitro (V79)
Gene mutation Chinese hamster Nonactivated: 0.5-6 µg/ml 98.5% negative 4/ den Boer, 1987
(HGPRT-test) ovary cells Activated: 3-30 µg/ml
in vitro
Forward mutation Mouse lymphoma Nonactivated: 25-300 µg/ml 99.1% positive Hoorn, 1985
in vitro cells (L5178Y) Activated: 500-7500 µg/ml
Cytogenetic assay Human lymphocytes 0.1-10 µg/ml (activated 99.2% positive 5/ Herbold, 1984b
in vitro and nonactivated)
Micronucleus test Mice (NMRI strain) 2x250 and 2x500 mg/kg 98.8% negative 6/ Herbold, 1980
(p.o. 24 h apart)
Table 1. (cont'd)
Test system Test object Concentration of tolylfluanid Purity Results References
Dominant lethal test Mice (NMRI strain) 4000 mg/kg p.o.(mortality:5/50) 98.8% negative Herbold, 1986
8000 mg/kg p.o.(mortality:12/50)
Cytogenetic assay Chinese hamster 4000 mg/kg p.o. 99.7% negative 6/ Herbold, 1983
in vivo bone marrow
Cytogenetic assay Chinese hamster 2x250 and 2x500 mg/kg 93.1% inconclusive Herbold, 1984a
in vivo spermatogonia (p.o. 24 h apart) 7/
Cytogenetic assay Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 8/ Voelkner,1988b
in vivo germ cells
Sister chromatide Mice (NMRI strain) 500-5000 mg/kg bw p.o. 97.9% negative 7/ Voelkner,1988a
exchange bone marrow
Mammalian Mice C5781/6JxT 1750-7000 mg/kg bw p.o. 98.4% negative 9/ Herbold, 1988
Spot test
1/ With S-9 mix only; positive controls (benzo(a)pyrene 4,5-oxide, N-methyl-N'-nitro-N-nitrosoguanidine,
3,methylcholanthrene, 2-aminoanthracene) yielded expected positive results.
2/ TA-100 only; positive controls (endoxan, trypaflavin) yielded expected positive results.
3/ Positive controls (ethyl-methane sulfonate (EMS), 9,10-dimethyl-1,2-benzanthracene (DMBA) yielded expected positive
results.
4/ Positive controls (5-bromodeoxyuridine and 3-methylcholanthrene) yielded expected positive results.
5/ At cytotoxic concentrations; positive controls (mitomycin c, endoxan) yielded expected positive results.
6/ Positive controls (endoxan) yielded expected positive results.
7/ Positive controls (doxorubicin) yielded expected positive results.
8/ Positive controls (cyclophosphamide) yielded expected positive results.
9/ Positive controls (1-ethyl-1-nitrosourea) yielded expected positive results.
Special study on potentiating effects
Equitoxic doses of tolylfluanid (purity not reported) and
triadimefon (89.5% purity) were given in combination to rats. No
potentiating effect was noted (Flucke & Kimmerle, 1978).
Special study on reproduction
Groups of Long Evans FB30 rats (30 animals/dose, 10 males and 20
females) were fed diets containing tolylfluanid (technical grade,
98.8% purity in a 90% pre-mix) at levels of of 0, 300, 1500 or
7500 ppm. After 70 days of treatment F0 animals were mated on a
2-female-to-1-male basis. After delivery (F1a) and a 4-week
lactation and a 2-week waiting period, F0 rats were mated again.
After delivery (F1b) lactation lasted for 4 weeks and then F0
animals were sacrificed. F1b animals (20 females-10 males/dose) were
then mated and F1a and F1b generations were obtained. The study
was ended when F1b generation reached 4 weeks of age.
Rats were weighed weekly and pups were weighed at birth, 5 and 7
days after birth, and weekly thereafter. Pups were also observed
immediately after birth and during lactation.
F0 generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in a
dose-related manner in male rats treated with 1500 or 7500 ppm; only
the dose of 7500 ppm was associated with reduced body weight in female
rats. Fertility indices and litter sizes of first and second matings
were not reduced by any of the treatments. Survival rate was slightly
reduced in F1b generation of the high-dose group. Lactation index (%
of pups surviving 4 weeks) was reduced in the F1a generation of the
7500 ppm group, but in the F1b generation of the same group was
significantly higher than controls. Body weight of pups at birth and
body weight gain were significantly lower in both F1a and F1b
generations of the 7500 ppm group. None of the pups showed
malformations.
F1b generation:
Appearance and behaviour of all treated animals did not differ
from controls throughout the study. Body weight was reduced in the
7500 ppm group. Fertility indices and litter sizes of first and second
matings were not reduced by any of the treatments. Survival rate was
reduced in F2b generation of the high-dose group. Lactation index
(% of pups surviving 4 weeks) was reduced in F2a and F2b
generations of the 7500 ppm group and in the F2b generation of the
1500 ppm group. In the latter group, however, the number of pups
surviving four weeks was the same as in the control group and the
lower lactation index was due to a higher number of pups at 5 days
when all litters were reduced to a maximum of 10 pups rearing. Body
weight of pups at birth and body weight gain was lower in both F2a
and F2b generations of the 7500 ppm group. None of the pups showed
malformations.
In summary, this study shows that tolylfluanid at doses up to
1500 ppm did not affect the reproduction of rats. The NOAEL in this
study was found to be 300 ppm (Loser, 1980).
Special study on skin and eye irritation
A 90% pre-mix with 99.2% active ingredient was applied to rabbit
skin for 24 hours under semi-occlusive conditions. Tolylfluanid did
not show skin irritant properties. The test compound dust (0.1 g),
however, when placed on the conjuntival sac of rabbit eyes, displayed
a strong irritant potential (redness and swelling of the conjuntivae).
The effect was reversible within 14 days (Helmann & Pauluhn, 1983).
A further study was conducted on irritant and corrosive effects
on the skin and eyes. Tolylfluanid (99.1% purity) did not show
irritant effect on the skin (4 hours' exposure). However, 70 mg of
a.i. placed in the conjuntival sac produced redness and swelling of
the conjuntivae which were completely reversible within 21 days. No
cornea or iris lesions were found at any time (Pauluhn, 1984).
Special study on subacute cutaneous toxicity in rabbits
Rabbits (5 males and 5 females) received daily dermal doses of
500 mg/kg bw of tolylfluanid (98.9% purity) for fourteen days. Each
exposure lasted 24 hours. Before and immediately after the end of
treatment, and 2 weeks later, clinical-chemical tests were performed,
including blood tests (erythrocyte, leucocyte and thrombocyte count,
haemoglobin determination, etc.), liver function tests, serum
transaminases, sorbitol dehydrogenase, bilirubin and albumin) and
kidney function tests (urinanalysis and serum urea concentration). No
variation was found between control and treated animals (Kimmerle &
Solmecke, 1971a).
Acute toxicity
Table 2 reports the LD50 values for tolylfluanid and
metabolites as determined in different studies and animal species.
In rats, signs of toxicity observed after oral administration
were altered behaviour, disturbed motility and dyspnoea. In mice,
guinea pigs, rabbits and cats tolylfluanid caused deterioration of
general conditions and (in cats only) vomiting. In sheep,
tolylfluanid, orally administered, caused anorexia, weakness of
extremities and loose faeces.
Intraperitoneal injection to rats caused disturbed motility,
staggering, spastic gait, distention of abdomen, narrowed eyelids, a
specific disturbed behaviour. Irritation of abdominal organs was found
at autopsy.
The dermal application of tolylfluanid at doses up to
5000 mg/kg bw was tolerated without signs of toxicity or skin
alterations.
The skin-sensitising potential of tolylfluanid was assessed by
the Magnusson and Klingman's maximization test on guinea pigs (n=15).
The concentration of tolylfluanid (99.2% purity in a 90% pre-mix) used
were 1% for the intradermal induction, 0.6% for the topical induction,
and 0.3% for the topical challenge. The results indicate that
tolylfluanid has a skin-sensitising potential (Heimann, 1983).
Short-term studies
Rats
0, 150, 500, 1500 or 4500 ppm of tolylfluanid (99% purity) were
given to rats (15 males and 15 females) in the diet for 3 months. All
dose-regimens were tolerated without alteration of behaviour, weight,
mortality or clinical chemistry tests. Morphological examination of
organs and tissues did not reveal any treatment-induced alterations
(Bomhard and Schilde, 1976).
Dogs
Beagle dogs (4 males and 4 females/group) were administered
0, 330, 1000 or 3000 ppm of tolylfluanid (99.7% purity) in diet for 3
months. Animals of the high-dose group showed poor general condition
and 6/8 animals had a reduced food intake and weight gain. In this
group an increased serum alkaline phosphatase activity and a
retardation of its age-related decrease as compared to controls was
found. Increased liver relative weight and increased PAS histological
reaction were also found in these animals. Doses up to 1000 ppm did
not induce any detectable adverse effect (Hoffmann & Mirea, 1974).
Table 2. Acute toxicity of tolylfluanid (mg/kg bw)
Species Sex Route LD50 LC50 Reference
Mouse M oral >1000 1/ - Kimmerle, 1968
Rat M oral >2500 2/ - Kimmerle & Lorke, 1967.
Rat M inhalation* - 265 2/ Kimmerle & Lorke, 1967
(4-hr exp.)
Rat M i.p. 20.2 1/ - Kimmerle, 1968
Rat F i.p. 25.6 1/ - Kimmerle, 1968
Rat F oral >5000 3/ - Heimann & Pauluhn, 1983
Rat M i.p. 14.7 3/ - "
Rat F i.p. 15.9 3/ - "
Rat M&F dermal >5000 3/ - "
(24-hr exp.)
Guinea pig F oral 250-500 1/ - Kimmerle, 1968
Rabbit M oral 250-500 1/ - Kimmerle, 1968
Cat M&F oral >500 1/ - Kimmerle, 1968
Sheep M&F oral 625-1250 3/ - Hoffmann, 1983
1/ 99.6% purity
2/ 98.7% purity
3/ 99.2% purity in a 90% pre-mix
* Dynamic spraying with Lutrol/ethanol. In static spraying experiments, mice, guinea
pigs and rabbits were found to be more sensitive than rats to tolylfluanid.
In another study, groups (4 male/4 female) of beagle dogs were
orally treated for 12 months with tolylfluanid (99.2% purity in a 90%
pre-mix) 0, 2.5, 12.5 or 62.5 (let to 33rd week) and 125 (34th to 52nd
week) mg/kg bw. The test compound was administered in gelatin
capsules. All animals survived the study and did not show any
treatment-related effect on appearance or behaviour. Body weight
development in male control animals (+4.4 kg in 12 months) was above
the average of the historical controls (+2.8 kg in 12 months), while
it was significantly reduced in the animals of the high-dose group.
The females in the high-dose group also had lower body weight gain.
Animals of the high-dose group vomited quite frequently after
administration of the compound. They also had a significant increase
of serum alanine aminotransferase (ALT) and glutamate dehydrogenase
(GLDH), and a retarding of the physiological age-related decrease of
the alkaline phosphatase activity. No liver alteration was found,
however, at histology.
An effect on renal function was also evident in the high-dose
group. Urea and creatinine concentrations were significantly increased
and in two dogs glycosuria and proteinuria were found. Histology
revealed slight alterations of cortical tubules (dilation, epithelial
flattening, focal hypertrophy and/or desquamation of the epithelia) in
all dogs of the high-dose group. Slightly lower serum potassium
concentrations were found in the high-dose group animals as compared
to control values, possibly as a result of the frequent vomiting.
No treatment-related effect was evident on blood, blood
coagulation and transparent media and fundus of the eye.
Oral doses of tolylfluanid up to 12.5 mg/kg bw were therefore
tolerated by dogs for 12 months without detectable adverse effects
(von Keutz & Nash, 1986).
Long-term studies
Rats
Wistar rats (50 males and 50 females/group; 100 male and 100
females, controls) were fed for 2 years with tolylfluanid (98.9%
purity in 90% pre-mix) at concentrations of 0, 300, 1500, 7500 ppm.
Toxicity following chronic administration and carcinogenic potential
of tolylfluanid were evaluated.
The animals in the high-dose group had a reduced food consumption
(-10%) and a reduced (about 10%) body weight gain; appearance,
behaviour and mortality were not affected by the treatment. Clinical
laboratory tests showed random alteration of some blood parameters
(mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte
count, polymorphonuclear cells and lymphocytes). Plasma alkaline
phosphatase (AP), transaminases (ALT and AST), bilirubin and total
protein were measured as indices of liver function. No dose-related
alteration of these parameters was found. Gross pathological and
histological examinations did not detect any indication of liver
damage. Urinanalysis did not indicate any dose-related kidney
alteration. Blood sugar and cholesterol were within normal range in
all groups. On autopsy, bone alterations (diffuse hyperostosis of the
rib bones, hardened cranial bones, focal hyperostosis of the skull
caps) in both sexes, and a faster growth of the incisors of the upper
jaw in males only, were found in the 1500 and 7500 ppm groups. These
effects were presumably due to an increased fluorine intake from the
active ingredient.
Tumours of endocrine and reproductive system accounted for about
80% of the rumours seen in the study. Only malignant uterine tumours
appeared higher in all treated groups as compared to controls. The
incidence was 3/50, 8/50, 12/50 and 13/50 in the 0, 300, 1500 and
7500 ppm groups, respectively. An unusually low incidence of these
fairly con, non tumours was found in control animals. Therefore, a
second, concurrent group of 50 controls was studied in which the
incidence of malignant uterine rumours was similar (9/50) to that of
the treated groups. The test, therefore, did not provide indications
of carcinogenic potential for tolylfluanid.
The concentration of tolylfluanid without detectable adverse
effect was 300 ppm (equivalent to 15 mg/kg bw) (Krötlinger &
Loser, 1982).
Observations in humans
Small cottonwool pads on which an unknown concentration of
tolylfluanid was applied, were placed on the forearm of 10 male
volunteers for 24 hours. Subjects were observed for 10 days; no
skin-irritant effects were noted (Kimmerle, 1968).
COMMENTS
Following oral administration to rats, tolylfluanid was rapidly
absorbed, metabolized and eliminated in urine and faeces. When using
14C-ring-labelled tolylfluanid (2 or 20 mg/kg bw), less than 0.2% of
the administered radioactivity was detectable in the body 2 days after
administration. The highest concentrations were found in liver and
kidney.
When using 14C-tolylfluanid (0.1-20 mg/kg bw) labelled at the
fluorodichloronenthyl group instead, accumulation of radioactivity was
evident in the thyroid. The major urinary metabolites were thiazolidine-
2-thione-4-carboxylic acid and 4-dimethylaminosulphonyl-amino-benzoic
acid.
The oral LD50 is greater than 5000 mg/kg bw in rats and
250-500 mg/kg bw in guinea pigs and rabbits. The oral LD50 of a
major plant metabolite, DMST (N,N-dimethyl-N'-p-tolylsulphamide),
exceeds 2500 mg/kg in rats and 1000 mg/kg in mice and rabbits.
In one study in rats, tolylfluanid was found to be maternally
toxic and embryotoxic at doses above 100 mg/kg bw/day but not
teratogenic at doses up to and including 1000 mg/kg bw/day. In a
two-generation two-litter-per-generation reproduction study, dietary
levels up to and including 1500 ppm did not affect rat reproduction.
No malformations were observed at dietary levels up to and including
7500 ppm.
In a short-term toxicity study with beagle dogs, tolylfluanid did
not cause adverse effects for 12 months at doses up to and including
12.5 mg/kg bw/day.
Tolylfluanid was mutagenic in prokaryotes and eukaryotes in four
of seven in vitro assays. In vivo tests, however, did not indicate
a mutagenic potential.
In a long-term study in Wistar rats, a higher incidence of
malignant uterine tumours was found in the treated groups as compared
to controls, but no statistically significant dose-related pattern was
evident. Moreover in a second, concurrent, group of controls the
incidence was comparable to that in treated groups. In animals treated
with 1500 or 7500 ppm tolylfluanid, bone alterations were also found
which were suggested to be due to the fluorine constituent of
tolylfluanid. No alterations were found in the thyroid or in other
organs.
The Meeting noted that in the NMRI mouse study, the control
animals were four months older than the treated groups, thus
compromising the design of the study. Lung and liver adenomas occurred
at similar frequency in all treated groups, but because of their known
occurrence in mice they were not considered to be biologically
relevant. The Meeting therefore concluded that the results of this
study did not indicate an oncogenic potential.
The Meeting did not request a special study on thyroid function
because the dichlorofluorometylthio group is common to dichlofluanid,
for which a NOAEL for the thyroid has been demonstrated to be greater
than 1500 ppm.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 300 ppm in the diet, equal to 15 mg/kg bw/day
Dog: 12.5 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.1 mg/kg bw
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Observations in man.
REFERENCES
Abbink, J. & Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: investigation
of the biokinetic behaviour in the rat. Institute for Metabolism
Research, Bayer AG. Unpublished report No. 181081-9. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Bomhard, E. & Schilde, B. 1976. KUE 1318T / Subchronic Toxicological
Experiments on Rats (feeding experiment over 3 months). Institute of
Toxicology, Bayer AG. Unpublished report No. 5929. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
den Boer, W.C. 1987. Mutagenicity test on KUE 13183b in the CHO/HGPRT
forward mutation assay. Hazleton Biotechnologies, The Netherlands.
Unpublished report No. R4204. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Ecker, W. 1978. Biotransformation of (14C) tolylfluanid in the rat.
Institute for Pharmakocinetics. Bayer AG. Unpublished report No. 1281.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Ecker, W. & Brauner, A. 1987. Biotransformation of <ring-U-14C>
tolylfluanid by the rat following oral administration. Institute for
Pharmacokinetics, Bayer AG. Unpublished report No. 2826. Submitted to
WHO by Bayer AG, Bayerwerk, FRG.
Flucke, W. & Kimmerle, G. 1978. Triadimefon (MEB 6447) and
Tolylfluanid (KUE 13183b) Study for Acute Combination Toxicity.
Institute of Toxicology, Bayer AG. Unpublished report No. 7304.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heidemann, A. 1987. KUE 13183b / Detection of gene mutations in
somatic mammalian cells in culture: HGPRT-Test with V79 cells.
Laboratory for Mutagenicity Testing, Darmstadt. Unpublished report
No. R 4103. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. 1983. KUE 13183b / Study for sensitising effect on
guinea pigs. Institute of Toxicology, Bayer AG. Unpublished report
No. 11492. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Heimann, K.G. & Pauluhn, J. 1983. 13183b / Study for Acute Toxicity
Institute of Toxicology, Bayer AG. Unpublished report No. 11383.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1979. 13183b / Salmonella-microsome test for point
mutagenic effects. Institute of Toxicology, Bayer AG. Unpublished
report No. 8265. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1980. KUE 13183b / Micronucleus test on the mouse to
evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 9149. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1983. KUE 13183b / Cytogenetic study of the Chinese
hamster's bone marrow in vivo to evaluate for mutagenic effect.
Institute of Toxicology, Bayer AG. Unpublished report No. 11792.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984a. KUE 13183b / Cytogenicity study of the
spermatogonia of the Chinese hamster in vivo to evaluate for
mutagenic effect. Institute of Toxicology, Bayer AG. Unpublished
report No. 12739. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1984b. KUE 13183b / Cytogenetic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Institute of Toxicology, Bayer AG. Unpublished report
No. 12836. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. 1986. KUE 13183b / Dominant lethal test on the male mouse
to evaluate for mutagenic effect. Institute of Toxicology, Bayer AG.
Unpublished report No. 15017. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Herbold, B. 1988. Spot test on cross-bred C57B1/6J × T stock mouse
fetuses to evaluate for induced somatic changes in the genes of the
coat pigment cells. Institute of Toxicology, Bayer AG. Unpublished
report No. 16752. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. 1983. KUE 13183b / Acute toxicity to the sheep after oral
administration. Institute of Toxicology, Bayer AG. Unpublished report
No. 11975. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoffmann, K. & Mirea, D. 1974. KUE 13183b / Subchronic toxicity study
on dogs (13-week feeding experiment). Institute of Toxicology,
Bayer AG. Unpublished report No. 4957. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Hoorn, A.J.W. 1984. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the reverse mutation induction assay with saccharomyces cerevisiae
strains S138 and S211a. Litton Bionetics, The Netherlands. Unpublished
report No. 3060. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Hoorn, A.J.W. 1985. KUE 13183b / Mutagenicity evaluation of KUE 13183b
in the mouse lymphoma forward mutation assay. Litton Bionetics, The
Netherlands. Unpublished report No. R 3192. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
von Keutz, E. & Nash, G. 1986. KUE 13183b / Chronic toxicity to dogs
after oral administration (12-month capsule study). Institute of
Toxicology, Bayer AG. Unpublished report No. 12999. Submitted to WHO
by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. 1968. BAY 49854 / Toxicological studies. Institute of
Toxicology, Bayer AG. Unpublished report No. 832. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Lorke, D. 1967. Toxicological studies on the active
ingredient BAY 49854. Institute of Toxicology, Bayer AG. Unpublished
report No. 323. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971a. Methyl-Euparen / Subacute cutaneous
application to rabbits. Institute of Toxicology, Bayer AG. Unpublished
report No. 2619. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Kimmerle, G. & Solmecke, B. 1971b. DMST / Toxicological Study.
Institute of Toxicology, Bayer AG. Unpublished report No. 2681.
Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Krötlinger, F. & Loser, E. 1982. KUE 13183b / Chronic toxicological
study in rats (feeding for 2 years). Institute of Toxicology, Bayer
AG. Unpublished report No. 10978. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Loser, E. 1980. KUE 13183b / Generation study with rats. Institute of
Toxicology, Bayer AG. Unpublished report No. 9419. Submitted to WHO by
Bayer AG, Bayerwerk, FRG.
Machemer, L. 1976. KUE 13183b / Studies on embryotoxic and teratogenic
effects on rats after oral administration. Institute of Toxicology,
Bayer AG. Unpublished report No. 5888. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Mohr, U. 1982. KUE 13183b / Study for cancerogenic effect on NMRI-mice
(feeding study for 104 weeks). Appendix: Histopathological Individual
Findings. Medizinische Hochshule, Hannover. Unpublished report
No. R 2225. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Oesch, F. 1977. Kue 13183b / Ames Test for guparen M. Institute of
Biochemical Pharmacology, University of Mainz. Unpublished report from
November 10. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Pauluhn, J. 1984. KUE 13183b / Study for irritant/corrosive effect on
skin and eye (rabbit). Institute of Toxicology, Bayer AG. Unpublished
report No. 12362. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Voelkner, W. 1988a. Sister chromatide exchange assay in bone marrow
cells of the mouse. Cytotest Cell Research GmbH, Darmstadt, FRG.
Unpublished report No. 4422. Submitted to WHO by Bayer AG,
Bayerwerk, FRG.
Voelkner, W. 1988b. Mouse germ-cell cytogenetic assay. Cytotest Cell
Research GmbH, Darmstadt, FRG. Unpublished report No. 4485. Submitted
to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. et al. 1977. KUE 13183b / Biokinetic Investigations of
Rats. Institute for Pharmacokinetics, Bayer AG. Unpublished report
No. 1165. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Weber, H. 1988. Phenyl-UL-14C-tolylfluanid: whole body
autoradiographic distribution of the radioactivity in the rat.
Institute for Metabolism Research, Bayer AG. Unpublished report
No. 181080-8. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
