
HEXACONAZOLE
EXPLANATION
First draft prepared by
Dr E.M. den Tonkelaar and Dr J.E.M. v. Koten-Vermeulen
National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Hexaconazole is a broad spectrum triazole fungicide that is used
against powdery mildew, scab and rust of apples and powdery mildew and
blackrot of grapes. Hexaconazole is considered for the first time by
the present meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Alpk/AP rats were given single oral doses of 1 or 200 mg
14C-phenyl hexaconazole (purity 99.3%)/kg b.w. Radioactivity was
determined in urine and faeces daily and whole body autoradiograms
were made at termination. About 90% of the administered radioactive
dose was excreted in the urine and faeces within 72 hours after
dosing; exhaled radioactivity after 48 hours was negligible. After
7 days radioactivity recovered in the urine was 43% and 66.4% and in
the faeces 53% and 29% of the administered dose for males and females,
respectively. A total of 96% of the radioactivity was recovered after
7 days with less than 1% of the administered dose present in the
tissues and carcass. Although there were no marked differences in the
relative proportions of dose excreted via urine and faeces over seven
days, the rate of excretion was more rapid at the 1 mg/kg bw level.
Autoradiography showed highest tissue residues after 24 hours in the
liver, intestinal tract and adrenal cortex. After 72 hours the
radioactivity in the liver and adrenal cortex had declined. Male
tissues generally showed higher residues than female tissues (Jones
et al., 1984).
In a similar experiment rats were given single oral doses of 200
mg 14C-phenyl ring-labelled hexaconazole (purity 92.3%)/kg b.w. Over
a period of 72 hours or 7 days male rats excreted 37% and 42% in the
urine and 40% and 52% in the faeces, respectively. The female rats
excreted most of the dose within 72 hours (63% via the urine and 30%
via the faeces). After 24 hours highest tissue residues were found in
the liver, kidneys, and pancreas. After seven days 95.6% and 98.5% of
the radioactivity had been recovered for males and females,
respectively, and tissue levels were 0.7% of the administered dose
(Trivedi et al., 1986).
Tissue distribution was measured in groups of rats over a period
up to 96 hours following single oral doses of 1 or 200 mg
14C-hexaconazole (phenyl ring labelled; purity 99.3%)/kg bw). At the
1 mg/kg bw dose peak tissue concentrations of radioactivity did not
markedly differ between the sexes. The highest concentration was
found 6 to 10 hours after dosing in the adrenal glands of male and
female rats, respectively, although the liver contained the highest
proportion of the dose (7.1% and 5.4%, respectively). The elimination
of radioactivity for all tissues was fairly rapid with elimination
half-lives of 10 to 14 hours in males and 7 to 16 hours in females.
Peak tissue concentrations of radioactivity were higher in male rats
than in females at the 200 mg/kg bw dose. Highest levels were found
in the liver in both males (3.6%) and females (2.4%) after 6 and 3
hours, respectively. Elimination half lives ranged from 10-19 hours
in males and from 10-27 hours in females. Ninety-six hours after
dosing (both at 1 and 200 mg/kg bw) all tissue residues were either
very low or were below the limit of detection (Jones, 1989a, 1989b).
Four male and four female rats were given 14 consecutive daily
oral doses of 1 mg/kg bw unlabelled hexaconazole followed by a single
oral dose of 1 mg 14C-hexaconazole (phenyl-labelled). Males excreted
41% via urine and 55% via faeces over a period of 7 days, whereas
females eliminated 63% via the urine and 35% via the faeces.
Excretion was fairly rapid with 88.7 and 93.1% of the dose excreted
within three days by males and females, respectively. Highest tissue
concentrations were found in the liver of male rats (0.12% of the
dose). In female rats, only 0.02% of the dose was found in the liver
and all other tissue residues were lower. No differences were found
in metabolic profiles in urine and methanol extracts of faeces
collected from rats given either repeated doses or a single oral dose
of hexaconazole (Jones, 1988a).
Fourteen consecutive daily oral doses of 1 mg/kg 14C-
hexaconazole (phenyl labelled) were administered to 2 male and 2
female rats. Tissue distribution was measured at 24 (1/sex) and 48
hours (1/sex) by autoradiography. In both sexes at 24 hours after
dosing, highest concentrations were observed in the adrenal gland and
a much lower radioactivity was measured in the liver, kidneys and
lungs. Forty-eight hours after dosing radioactivity was markedly
reduced or was negligible (Jones, 1988b).
Biotransformation
The metabolism of hexaconazole in male and female rats was
established following the administration of 1, 100 or 200 mg/kg bw
14C-hexaconazole (phenyl- and/or triazole-labelled). Biliary
elimination was characterized at a dose of 200 mg/kg bw
14C-phenyl-labelled hexaconazole and the fate of the triazole
component of the molecule was established using 100 or 200 mg/kg bw
14C- triazole-labelled hexaconazole. Urinary, faecal and biliary
metabolites were identified and quantified.
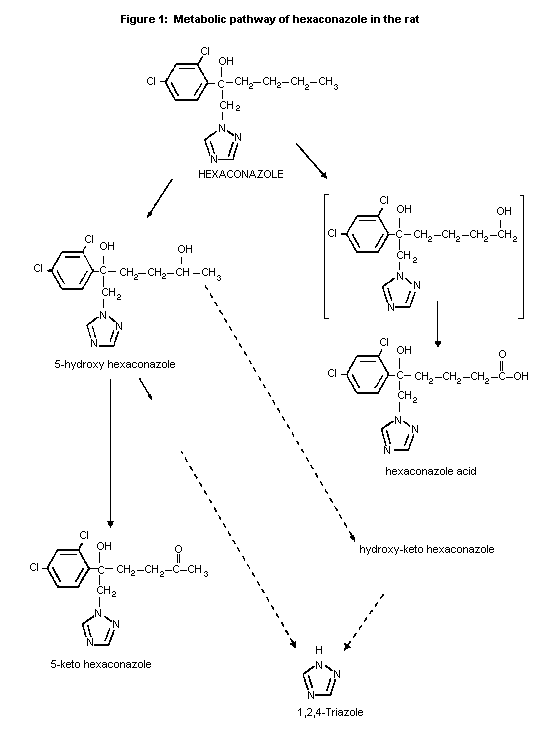
In both male and female rats hexaconazole was extensively
metabolized via two pathways involving oxidation of the n-butyl chain
and some cleavage of the triazole substituent. The major pathway
involved the conversion of hexaconazole to 5-hydroxy-hexaconazole and
5-keto-hexaconazole; the minor pathway was a two-stage oxidation of
the methyl group to form hexaconazole acid via 6-hydroxyhexaconazole.
The biliary route of elimination was important in both sexes (80% in
males and 40% in females). In both sexes half of the radioactivity
eliminated in the bile was reabsorbed and half was excreted via the
faeces as the biliary conjugates or their aglycones. The sex
difference in the proportions excreted in urine and faeces was due to
quantitative differences in biliary elimination of hexaconazole
metabolites. Metabolites in the urine were derived from initial
metabolism or following reabsorption of biliary metabolites. Biliary
metabolites in males were predominantly glucuronide conjugates such as
5-hydroxy hexaconazole (24% of the dose), hydroxy-keto hexaconazole
(22%), 5-keto hexaconazole (8%) and hexaconazole (4%) and to a lesser
extent some unidentified metabolites including 14C-phenyl labelled
products after triazole cleavage. In female rats the same metabolites
were detected in the same relative proportions. Male urinary
metabolites included triazole (18% of a 100 mg/kg bw dose, in females
13%), hexaconazole acid (7-9%), hydroxy-keto hexaconazole (6-7%), an
unidentified conjugate of 5-hydroxyconazole (5%, in females 27-34%)
and hexaconazole. Less than 5% of unchanged hexaconazole was detected
in the urine. Only trace amounts of triazole or related metabolites
were detected in faeces. The proposed metabolic pathway is described
in Figure 1 (Jones, 1989c).
Toxicological studies
Acute toxicity
The acute toxicity of hexaconazole to rats and mice is presented
in Table 1. The most common signs of toxicity (less marked after
dermal administration) were piloerection, upward curvature of the
spine, side pinched-in, hypothermia, decreased activity, urinary
incontinence, dehydration, comatosis, reduced righting reflex and
decreased respiration rate.
Table 1. Acute toxicity of hexaconazole
Species Sex Route LD50 LD50 Reference
(mg/kg b.w.) (mg/1)
Mouse M&F oral >557 Southwood, 1984a
M oral 612 Leah, 1989
F oral 918 Leah, 1989
Rat M oral 2189 Southwood, 1984a
F oral 6071 Southwood, 1984a
M oral 4013 Davison, 1988
M&F dermal >2000 Southwood, 1984a
M&F inhal >5.9* Hext, 1987
*4-hr aerosol exposure
 Short-term studies
Mice
Groups of C57BL/10JfCD-1/Alpk mice were fed diets containing 0,
25, 100, 500 or 1500 ppm hexaconazole (purity not given) in the diet
for 29 days. At 500 and 1500 ppm pronounced effects were observed on
body weight, food consumption, food efficiency and liver weight. A
slight to moderate microcytosis with a compensatory increase in red
cell numbers to maintain haemoglobin levels was observed in males at
1500 ppm and in females at 500 and 1500 ppm. Red cell counts were
also increased in males at 500 and 100 ppm. At histopathology a
dose-related liver hypertrophy and increased hepatocyte lipid
accumulation with associated cytoplasmic vacuolation were observed. A
lack of corpora lutea in the ovaries and a reduction in the size of
the uterus were observed in females receiving doses of 100 ppm
hexaconazole and above. At 1500 ppm and possibly at 500 ppm there was
some evidence of an effect on the male reproductive system with
increased abnormal precursor cells in testicular tubules and
epididymis and reduced seminal vesicular secretion. Cortical
enlargement of the adrenal glands was observed at 500 and 1500 ppm.
The NOAEL in this study was 25 ppm hexaconazole (equivalent to 3.5
mg/kg bw/day) (Forbes, 1988).
Rats
Groups of Wistar (Alpk/AP) rats (20/sex/group) were fed diets
containing 0, 50, 500 or 5000 ppm hexaconazole (purity 92.3%) for 90
days. Observations included clinical examinations, mortality, food
consumption and food efficiency, body weight, haematology, clinical
chemistry, hepatic aminopyrine-N-demethylase activity (APDM) and
urinalysis, ophthalmoscopy, macroscopy, organ weight and
histopathology.
An increased incidence of staining of the pelt around the nose
was observed in rats at 5000 ppm. Body weight gain was significantly
reduced in both male and female rats at 5000 ppm (accompanied by
reduced food consumption), in males at 500 ppm (first 3 weeks of the
study) and in females at 500 and 50 ppm (at several timepoints over
the treatment period). Final body weight was significantly reduced in
high dose rats. In males at 5000 ppm Hb (also at 500 ppm), Ht and RBC
counts were significantly decreased and WBC count was significantly
increased. Prothrombin-time was significantly reduced in males at 5000
and 500 ppm. Inconsistent changes in blood biochemistry (ALAT, ASAT,
albumin, protein, glucose and triglycerides and cholesterol) were
observed at 5000 and in some parameters also at 500 ppm. Relative
liver weight was significantly increased in both sexes at 5000 and 500
ppm. Relative weight of spleen, adrenal and testes were significantly
decreased in males and kidney weight was increased in females at 5000
ppm. Hepatic APDM activity was significantly increased at all dose
levels in both male and females. At the highest dose enlarged and
discoloured pale livers were observed in 16/20 males and 6/20 females.
At histopathology hepatocellular swelling and fatty change were seen
in males (also at 500 ppm) and females at 5000 ppm. Cortical
parenchymal vacuolation in the adrenal glands was observed in both
sexes at 5000 ppm and in males also at 500 and 50 ppm. Haemorrhages
in the thymus were observed in both sexes at 5000 ppm. The LOAEL in
this study was 50 ppm (equivalent to 2.5 mg/kg bw/day) (Kinsey
et al., 1984).
Dogs
Groups of beagle dogs (4/sex/group) received 0, 5, 25 or 125 mg
hexaconazole (purity 92.3%)/kg bw daily by capsule for 90 days. After
7 days the high dose group was terminated because of severe toxic
effects (body weight loss, vomiting, abnormalities in gait and
behaviour and one death). An additional group was then started
(4/sex) at 75 mg/kg bw/day. After 10 days this dose, showing similar
signs of toxicity, was reduced to 50 mg/kg bw/day. After the initial
effects at 75 mg/kg bw/day no dose-related effects were observed on
mortality, clinical signs, ophthalmoscopy, body weight and food
consumption at 50 mg/kg bw/day. Platelet count was significantly
increased in both sexes at 50 mg/kg bw/day. Urea, albumin,
triglycerides and cholesterol in plasma were significantly reduced and
ALAT and SAP were increased in males and in females at 50 and 25 mg/kg
bw Relative kidney weight and relative liver weight were
significantly increased in both sexes at 50 mg/kg bw/day and there
was a tendency to increased liver weight at 25 mg/kg bw.
Weights of ovaries and testes were slightly decreased at the
highest dose. At macroscopy enlargement and pallor of the liver was
observed at both the mid- and the high-dose level. These findings
were accompanied by microscopic evidence of an increase in hepatocyte
lipid accumulation. The NOAEL in this study was 5 mg/kg bw/day
(Stonard, 1989).
Groups of beagle dogs (4/sex/group) received 0, 2, 10 or 50 mg
hexaconazole (purity 90.0%)/kg bw daily by capsule for one year. Two
dogs died during the study, 1 male dog at 50 mg/kg bw/day on day 5
(the dog was replaced by another male dog) and another dog (female)
given 2 mg/kg bw/day in week 36. Neither death was considered to be
treatment-related. There were no effects on clinical condition,
ophthalmoscopy, food consumption and urinalysis. At the highest dose
body weight gain was significantly reduced during the first 5 weeks of
the study. At 50 mg/kg bw/day platelet count was significantly
increased in both sexes throughout the study and in females at 10
mg/kg bw at weeks 13 and 26. At the high dose level reductions in
plasma total protein and albumin, urea, cholesterol and triglycerides
were observed in both sexes. SAP and ALAT were increased in both male
and female dogs at 50 mg/kg bw/day (significantly) and at 10 mg/kg
bw/day. Relative liver weight at 50 and 10 mg/kg bw/day and relative
kidney weight at 50 mg/kg bw/day were significantly increased. At
macroscopy pallor, enlargement and accentuation of the lobular pattern
of the liver was observed in high dose dogs. Fatty changes of the
liver were observed in both male and female dogs at 50 mg/kg bw and to
a lesser extent in males at 10 mg/kg bw/day. The NOAEL in this study
was 2 mg/kg bw/day (Stonard, 1988).
Long-term/carcinogenicity studies
Mice
Groups of male and female C57/BL/10JfCD-1/Alpk mice
(50/sex/group) were fed diets containing 0, 0', 5, 40 or 200 ppm
hexaconazole (purity 90%) for 2 years. Two concurrent control groups
of 50 male and 50 female mice were kept. Observations included
clinical signs, body weight, food consumption, haematology,
macroscopy, liver and testes weight, and histopathology.
A slightly increased incidence of the loss of coat color (black
to grey) was observed in male mice at 200 ppm during the second year
of the study. At the highest dose, body weight gain and food
efficiency were significantly reduced in males and food consumption
was significantly reduced in females. At termination Hb, Ht and RBC
values were significantly increased in both sexes and MCV, platelet
count and WBC count in females only at the highest dose. Relative
liver weight was significantly increased in high dose males and
females accompanied by an increased incidence of minimal to moderate
centrilobular fatty changes in the liver. Tumour incidences were not
increased. The NOAEL in this study was 40 ppm (equal to 4.7 mg/kg
bw/day for male mice and 5.9 mg/kg bw/day for females) (Pigott, 1988).
Rats
Groups of male and female ALpk:APfSD rats (64/sex/group) were fed
diets containing 0, 10, 100 or 1000 ppm hexaconazole (purity 89.8%).
Twelve rats/sex from each group were used for interim sacrifice after
52 weeks and the remaining 52 rats/sex/group were continued to
terminal sacrifice after 105 weeks. Observations included clinical
signs, body weight, food consumption, ophthalmoscopy, haematology,
clinical chemistry, APDM-activity in liver, macroscopy, organ weights
(gonads, adrenals, kidneys, liver and brain), and histopathology. In
order to provide information on adrenocortical function, blood samples
of 12 male and 12 female rats of each group were taken at week 52/53
and week 78/79 and analyzed for corticosterone. Urinary sodium and
potassium levels (as an indirect method of assessing aldosterone
levels) were determined in 13/rats/sex/group at week 52.
No treatment-related effects were seen on mortality, haematology,
corticosterone, urinary sodium and potassium levels or ophthalmoscopy.
A dose-related decrease in body weight gain was seen in females at 100
ppm and in both sexes at 1000 ppm. Food consumption was lower for
high dose males and females. At 1000 ppm plasma triglyceride levels
were reduced in males and in females. Cholesterol levels were
significantly increased in high dose female rats and urea levels
decreased during the first year. Increases in ALAT and ASAT
activities were observed in male rats at 1000 ppm. Urinary protein
excretion was significantly increased in high dose males up to week 25
which was reflected in lower pH values. There was a marked increase
in hepatic amino-pyrine-N-demethylase activity in both males and
females receiving 1000 ppm; a smaller but still significant increase
was observed in males at 100 ppm. Relative liver weight was
significantly increased at the highest dose (at interim sacrifice
females at 100 ppm also showed an increased relative liver weight).
Relative adrenal and kidney weights were increased in females at 100
and 1000 ppm after 52 weeks only.
Livers of high dose rats showed an accentuation of lobular
pattern, with or without swollen or enlarged lobes and pale spots at
the interim sacrifice as well as at termination. Microscopy revealed
a dose-related increased incidence of fatty changes, primarily
centrilobular in pattern in the liver of males at 1000 and 100 ppm and
in females at 1000 ppm. High dose rats also showed an increase in the
incidence of hepatocyte hypertrophy, with a slight dose-related
increase in microcystic degeneration of the liver in males at 1000 ppm
and a slight increase at 100 ppm.
An increased incidence of cortical vacuolation was observed in
the adrenal glands of mid and high dose males. A slight increase was
seen in cortical cysts in females at 1000 ppm. In the testes of rats
at 1000 and 100 ppm, a treatment-related increased incidence was seen
in benign Leydig cell tumours. The incidence was 2/52, 2/52, 4/52,
8/52 at 0, 10, 100 and 1000 ppm, respectively. Historical control
values for this finding are 0-14.4%. The NOAEL in this study was 10
ppm (equal to 0.47 mg/kg bw/day in males and 0.61 mg/kg bw/day in
females) (Hext, 1988a; 1988b).
Reproduction study
Rats
Groups of 15 male and 15 female ALpk:APfSD rats received
hexaconazole (purity 90.0%) in the diet at 0, 20, 100 or 1000 ppm.
After 12 weeks of treatment animals were mated to start a 2-generation
(2 litters/generation) study. F1 parents selected from F1a offspring
were mated after 11 weeks. At 1000 ppm body weight gain and food
consumption were decreased in F0 and F1 parents. A trend for a
decreased body weight gain was observed in F0 males at 100 ppm. At
the highest dose birth weight and weight gain to day 36 of F1a litter
offspring were significantly reduced. In both F2a and F2b litters
total litter weight was markedly reduced and F2b pup weight was also
reduced. Absolute as well as relative liver weights were increased at
1000 ppm for F0 and F1 parents and F1a, F2a and F2b pups.
Histopathology revealed evidence of fatty changes in the liver
with or without hepatocyte hypertrophy in male and female parents and
pups at 1000 ppm. At 100 ppm there was evidence of a similar but less
marked effect on the histopathology of the liver in both parents and
offspring. Cortical cell vacuolation of the adrenal gland was
observed in both male and female parents and offspring at 1000 ppm and
to a much lesser extent this was also found at 100 ppm. No adverse
effects were observed on reproduction parameters such as fertility
indices, length of gestation, pre-coital interval, litter size and
number of live and dead fetuses. The NOAEL in this study was 20 ppm
(equivalent to 1 mg/kg bw/day) (Middleton, 1988).
Special studies on embryo/fetotoxicity
Rats
Groups of 24 pregnant Wistar Alpk/AP rats were orally dosed by
gavage at 0, 2.5, 25 or 250 mg/kg bw/day hexaconazole (purity 92.3%)
in corn oil from day 7 to day 16 of gestation. Clinical signs,
mortality, body weight and food consumption were recorded. At day 22
of gestation animals were sacrificed and the fetuses were delivered by
cesarean section. The number and positions of implantations and
corpora lutea were determined. The fetuses were counted, sexed and
weighed and examined for external, visceral and skeletal
malformations. At 250 mg/kg bw/day, maternal body weight gain and
food consumption were significantly decreased. Post-implantation loss
was significantly increased. The mean number of live fetuses was
slightly reduced and pup weight was significantly lower at the highest
dose. The number of fetuses with minor defects only was significantly
increased at 25 and 250 mg/kg bw/day. The incidence of fetuses with
extra 14th thoracic ribs was significantly increased at 25 and 250
mg/kg bw/day. At 250 mg/kg bw/day the proportion of fetuses with
unossified calcanea and partially ossified 5th sternebrae was
significantly increased and the mean manus and pes scores were
also significantly increased in this group. In this study fetotoxic
effects were observed at 250 and to a lesser extant at 25 mg/kg
bw/day, but there were no indications for structural malformations
being associated with compound administration. The NOAEL for
fetotoxicity in this study was 2.5 mg/kg bw/day (Killick et al.,
1984a).
Rabbits
Groups of 18 pregnant New Zealand white rabbits were orally dosed
by gavage with 0, 2.5, 12.5 or 50 mg hexaconazole (purity 92.3%)/kg bw
in corn oil from days 7-19 of gestation. At day 30 of gestation
animals were sacrificed and the fetuses were delivered by Cesarean
section. No dose-related maternal toxicity was observed. The number,
growth, and survival of the fetuses were not affected by treatment.
After examination of the fetuses for external, visceral and skeletal
malformations, a slight increase (not significant) in partially
ossified 5th sternebrae was seen at 50 mg/kg bw only. The NOAEL in
this study was 12.5 mg/kg bw/day (Killick et al., 1984b).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
hexaconazole. The results are summarized in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on pharmacological effects
In vivo studies
In a rat behavioural study, a pull-up test for evaluating muscle
relaxation and a Halothane sleeping time test, central nervous system
(CNS) depression was observed at high doses (>500 mg/kg bw)
hexaconazole (purity 89.8%). The NOAEL for CNS depression in these
studies was 250 mg/kg bw/day.
Hexaconazole had no effect on the cardiovascular system as
determined by blood pressure measurements, heart rate or respiration
rate and gastrointestinal motility (Allen, 1988).
In vitro studies
At high concentrations a non-competitive antagonism of both
acetylcholine and histamine in guinea-pig ileum was observed.
Complete haemolysis of rabbit erythrocytes was induced by 0.03%
and 0.1% hexaconazole. No effects were observed on (alpha)1,
(alpha)2 or œ-adrenoceptors, nicotinic acetylcholine receptors or on
smooth muscle (Allen, 1988).
Special studies on skin and eye irritation and sensitization
A dose of 500 mg hexaconazole (purity not given) moistened with
0.5 ml of deionised water, was applied under occlusive conditions to
the shaven intact back skin of 6 male New Zealand white albino rabbits
for 4 hours. No skin irritation up to 72 hours after application
occurred (Southwood, 1984b).
Table 2. Results of in vitro genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Ames test1 S. typhimurium 1.6-5000 µg/ plate, 92.3% negative Callander, 1984
TA98, TA100, 2 tests
TA1535, TA1537,
TA1538
Ames test1 E. coli WP2 uvrA 1.6-5000 µg/pla 89.8% negative Callander, 1988
pKM101 2 tests2
Cytogenetics assay human lymphocytes 15-250 µg/ml, 92.3% negative Sheldon, et al., 1984a
>200 toxic3
20-250 µg/ml4
Lymphoma forward mouse L5178Y cells 7.8-125 µg/ml 92.3% negative Cross, 1986
mutation assay >70 µg:toxic5
Unscheduled DNA rat hepatocytes 10-4, 10-5, 90.0% negative Trueman, 1988
synthesis test 10-6 or 10-7 M6
1 Both with and without rat liver S9 fraction.
2 2-Aminoanthracene and N-methyl-N1-nitro-N-nitrosoguanidine were used as positive controls.
3 Without metabolic activation, mitomycin C was used as positive control.
4 With metabolic activation, cyclophosphamide was used as a positive control.
5 Dimethylsulfoxide was used as negative control and benzo (alpha)pyrene and
ethylmethanesulfonate were used as positive controls with and without metabolic activation, respectively.
6 6-p-dimethylaminophenylazobenzthiazole was used as a positive control.
Table 3. Results of in vivo genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Micronucleus test C57/BL/6J mice 75 and 120 mg/kg 92.3% negative Sheldon, et al.,
(m+F) bone i.p.* 1984b
marrow cells
Dominant lethal test CD-1 male mice 10, 30 or 100 92.3% negative Wickramaratne,
mg/kg bw for 5 et al., 1984
cons. days*
* Cyclophosphamide was used as a positive control.
Nine New Zealand white albino rabbits were given doses of 100 mg
of undiluted hexaconazole (purity not stated) into the conjunctival
sac of the left eye. The eyes of 3/9 rabbits were washed. All
animals showed conjunctival redness, chemosis and discharge at 1 hour
after application reversible within 3 days except for slight discharge
that was still seen in 1/6 rabbits (unwashed) after 7 days (Southwood,
1984b).
Hexaconazole was tested for skin sensitization in 20 Dunkin
Hartley guinea pigs in a Magnusson Kligman test. The intradermal and
topical induction concentrations were a 0.5% solution of hexaconazole
(purity not given) in 4.5% dimethylformamide/corn oil and a 75%
suspension in dimethylformamide, respectively. After a challenge with
a 10% solution, 1/20 animals showed scattered mild redness; following
challenge with a 25% solution 12/20 animals showed scattered mild
redness. Following a re-challenge with a 25% solution 6/20 guinea
pigs gave a positive response (Southwood, 1984c).
Special study on steroid metabolism
Isolated Leydig cells, prepared from the testes of adult
Alpk:APfSD rats, were incubated with varying concentrations of either
hexaconazole (0.1-30µm) or ketoconazole (0.l-10µm) for a period up to
24 hours. After the incubation period testosterone, progesterone and
17-OH progesterone were analyzed using specific radio immunoassays.
The experiments were repeated in the presence of a maximally
stimulating dose of HCG (human chorionic gonadotropin). A dose-
related decrease in testosterone production, accompanied by an
increase in the production of progesterone and 17-OH progesterone was
observed after treatment with hexaconazole and hezaconazole in the
presence of hCG. Incubation with ketoconazole showed the same
effects, but ketaconazole is about 70-100 times more active than
hexaconazole (Foster, 1990).
The inhibition of testosterone production by ketoconazole was
also reported in several in vivo as well as in vitro studies in
humans, mice and rats (English et al., 1986; Pont et al., 1982;
Santen et al., 1983; Lambert et al., 1986)
Observations in humans
No information was available.
COMMENTS
Following oral administration to rats, hexaconazole was rapidly
and almost completely excreted via the urine and the faeces. The
total radioactivity excreted was equal for both males and females, but
females excreted a higher proportion of the dose in urine than in
faeces. The highest tissue residues were found in the liver,
intestinal contents and the adrenal cortex. Biliary excretion was
extensive, accounting for about 80% and 40% of the total radioactivity
in males and females respectively. About 50% of the radioactivity
excreted in the bile was reabsorbed by enterohepatic circulation.
Hexaconazole was metabolized via oxidation of the n-butyl chain
by two pathways. The more important pathway was conversion of
hexaconazole to 5-hydroxy-hexaconazole and 5-keto-hexaconazole; a
minor pathway involved two-stage oxidation of the terminal methyl
group to "hexaconazole acid" via 6-hydroxy-hexaconazole. Free
triazole was also formed by cleavage of hexaconazole or its
metabolites.
The compound showed slight to moderate acute oral toxicity in
rats and mice. WHO has classified hexaconazole as slightly hazardous
based on acute toxicity and has concluded that it is "unlikely to
present acute hazard in normal use" (WHO, 1990).
From acute as well as from short-term studies it appeared that
male rats were more sensitive than female rats. Short-term studies
with mice, rats and dogs indicated that the liver is the primary
target organ. Lipid accumulation in hepatic parenchymal cells was
observed, with associated disturbances in lipid metabolism and blood
chemistry. Elevated levels of aminopyrine-N-demethylase (rat)
suggest an adaptive response in the liver. In a one-year study in
dogs (capsule administration) the NOAEL was 2 mg/kg bw/day. However,
a 90-day dog study (capsule administration) indicated a NOAEL of 5
mg/kg bw/day. Since the next highest dose in the one-year dog study
was 10 mg/kg bw/day, the Meeting concluded that the appropriate NOAEL
for dogs was probably 5 mg/kg bw/day.
In the rat, cortical parenchymal vacuolation in the adrenal
glands was observed. In a short-term feeding study in mice, effects
on the male and female reproductive organs and on the adrenals were
observed. From in vitro studies it can be concluded that
hexaconazole inhibits testosterone production.
In a long-term feeding study in mice (highest dose level 200 ppm)
observed effects were reduced body weight gain, increased erythrocyte,
haemoglobin and haematocrit values and, in the liver, increased weight
and centrilobular fatty changes. The tumour incidence was not
enhanced. The NOAEL in this study was 40 ppm, equal to 4.7 mg/kg
bw/day for males and 5.9 mg/kg bw/day for females.
In a long-term feeding study in rats, the same effects as in the
short-term study were observed. In the testes, the incidence of
benign Leydig cell tumours was slightly increased. The NOAEL in this
study was 10 ppm, equal to 0.47 mg/kg bw/day in males and 0.6l mg/kg
bw/day in females.
After reviewing the available in vitro and in vivo short-term
genotoxicity tests, it was concluded that there was no evidence of
genotoxicity.
Embryotoxicity/fetotoxicity was observed in a teratogenicity
study in rats. No effects were observed at 2.5 mg/kg bw/day. Delayed
ossification was observed in a rabbit teratogenicity study at the
highest dose of 50 mg/kg bw/day; the NOAEL was 12.5 mg/kg bw/day.
In a two-generation reproduction study in rats, reproductive
performance was not affected. Parental body weight gain, food
consumption and pup weight were decreased. Histopathological
examination revealed fatty changes in the liver, either with or
without hepatocytic hypertrophy, and cortical vacuolation of the
adrenal gland in both parents and pups. The NOAEL in this study was
2.5 mg/kg bw/day.
An ADI was allocated based upon the NOAEL from the rat long-term
study (0.5 mg/kg bw/day), using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 40 ppm in the diet, equal to 5.0 mg/kg bw/day
Rat: 10 ppm in the diet, equal to 0.5 mg/kg bw/day
Dog: 5 mg/kg bw/day
Estimate of acceptable daily intake
0-0.005 mg/kg bw
Studies which will provide information valuable in
the continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, S.A. (1988) Hexaconazole: pharmacological evaluation.
Unpublished report no.: CTL/P/1970 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1984) PP523: An evaluation in the salmonella
mutagenicity assay. Unpublished report no.: CTL/P/977 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1988) Hexaconazole - an evaluation of mutagenic
potential using E.coli. Unpublished report no.: CTL/P/2143 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Cross, M.F. (1986) PP523: Assessment of mutagenic potential using
L5178Y Mouse Lymphoma Cells. Unpublished report no.: CTL/P/1298 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Davison, V.M. (1988) Hexaconazole: Acute oral toxicity to the rat.
Unpublished report no.: CTL/P/2231 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
English, H.F., Santner, S.J., Levine, H.B. and Santen, R.J. (1986)
Inhibition of testosterone production with ketoconazole alone and in
combination with a gonadotropin releasing hormone analogue in the rat.
Cancer Res. 46, 38-42.
Forbes, D. (1988) Hexaconazole: 29-day feeding study in mice.
Unpublished report no. CTL/P/2204 from ICI Central Toxicology
Laboratory. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Foster, P.M.D. (1990) Effects of hexaconazole (ICIA523) on the
steroidogenic function of isolated rat Leydig cells, Unpublished
report no.: CTL/R/1035 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1987) PP523: 4-hour acute inhalation toxicity study in the
rat. Unpublished report no.: CTL/P/1731 from ICI PLC, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Hext, P.M. (1988a) Hexaconazole: two year feeding study in rats.
Unpublished report no.: CTL/P/1920 from ICI Central Toxicology
Laboratory, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1988b) Second supplement to hexaconazole: two year feeding
study in rats measurement of plasma corticosterone levels. Unpublished
report no.: CTL/P/1920 from ICI Central Toxicology Laboratory,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K., Galvin, G. Rhodes, S.J. and Soames, A.R. (1984) PP523:
Excretion and tissue retention of a single dose (1 mg/kg) in the rat.
Unpublished report no.: CTL/P/1148 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988a) Hexaconazole: repeat dose study (1mg/kg) in the
rat. Unpublished report no.: CTL/P/2089 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988b) Hexaconazole: whole body autoradiography study in
the rat following 14 daily oral doses of 1 mg 14C-labelled
hexaconazole/kg. Unpublished report no.: CTL/P/2052 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1989a) Hexaconazole: tissue distribution and elimination
following a single oral dose (1 mg/kg) in the rat. Unpublished report
no.: CTL/P/2389 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K. (1989b) Hexaconazole: tissue distribution and elimination
following a single oral dose (200 mg/kg) in the rat. Unpublished
report no.: CTL/P/2390 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Jones, B.K. (1989c) Hexaconazole: biotranformation in the rat with
first amendment. Unpublished report no.: CTL/P/1848 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984a) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1127 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984b) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1131 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Kinsey, D.L., Hollis, K.J., Chart, I.S., Gore, C.W., Godley, M.J.
Pigott, G.H., Stonard, M.D. and Chalmers, D.T. (1984) PP523: 90-day
feeding study in rats. Unpublished report no.: CTL/P/1073 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Lambert, A., Mitchell, R., and Robertson, W.R. (1986) The effect of
ketoconazole on adrenal and testicular steroidogenesis in vitro.
Biochem. Pharmacol., 25(22), 3999-4004.
Leah, A.M. (1989) Hexaconazole: acute oral toxicity to the mouse,
including the first amendment to the report. Unpublished report no.
CTL/P/2234 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Middleton, M.C. (1988) Hexaconazole: two generation reproduction study
in the rat. Unpublished report no.: CTL/P/1598 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Pigott, G.H. (1988) Hexaconazole: two year feed study in mice.
Unpublished report no. CTL/P/1929 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK
Pont, A., Williams, P.L., Azhar, S., Reitz, R.E., Bochra, C., Smith,
E.R. and Stevens, D.A. (1982) Ketoconazole blocks testosterone
synthesis. Arch. Intern. Med. 142, 2137-2140.
Santen, R.J., Van den Bossche, H., Symoens, J., Brugmans, J. and
DeCoster, R. (1983) Site of action of low dose ketoconazole on
androgen biosynthesis in men. J. Clinical Endocrin. Metab. 57(4), 732-
736.
Sheldon, T., Howard, A.C. and Richardson, C.R. (1984a) PP523: A
cytogenetic study in human lymphocytes in vitro. Unpublished report
no. CTL/P/1186 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J. and Barber, G. (1984b) An
evaluation of PP523 in the mouse micronucleus test. Unpublished report
no. CTL/P/1136 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Southwood, J. (1984a) PP523: Acute oral and acute dermal toxicity
studies. Unpublished report no. CTL/P/1113 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984b) PP523: Skin and eye irritation studies.
Unpublished report no. CTL/P/1043 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984c) PP523: Skin sensitisation study. Unpublished
report no. CTL/P/1049 from ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Stonard, M.D. (1989) PP523: 90-day oral dosing study in dogs and first
amendment. Unpublished report no. CTL/P/1137 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Stonard, M.D. (1988) Hexaconazole: 1 year oral dosing study in dogs.
Unpublished report no. CTL/P/1942 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trivedi, S., Jones, B.K. and Soames, A.R. (1986) PP523: Excretion and
tissue retention of a single oral dose (200 mg/kg) in the rat.
Unpublished report no. CTL/P/1431 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trueman, R.W. (1988) Hexaconazole: Assessment for the induction of
unscheduled DNA synthesis in primary rat hepatocyte cultures.
Unpublished report no. CTL/P/1887 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Wickramaratne, G.A., Bramley, J.R., Banham, P.B. and Godley, M.J.
(1984) PP523: Dominant lethal study in the mouse. Unpublished report
no. CTL/P/1110 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Short-term studies
Mice
Groups of C57BL/10JfCD-1/Alpk mice were fed diets containing 0,
25, 100, 500 or 1500 ppm hexaconazole (purity not given) in the diet
for 29 days. At 500 and 1500 ppm pronounced effects were observed on
body weight, food consumption, food efficiency and liver weight. A
slight to moderate microcytosis with a compensatory increase in red
cell numbers to maintain haemoglobin levels was observed in males at
1500 ppm and in females at 500 and 1500 ppm. Red cell counts were
also increased in males at 500 and 100 ppm. At histopathology a
dose-related liver hypertrophy and increased hepatocyte lipid
accumulation with associated cytoplasmic vacuolation were observed. A
lack of corpora lutea in the ovaries and a reduction in the size of
the uterus were observed in females receiving doses of 100 ppm
hexaconazole and above. At 1500 ppm and possibly at 500 ppm there was
some evidence of an effect on the male reproductive system with
increased abnormal precursor cells in testicular tubules and
epididymis and reduced seminal vesicular secretion. Cortical
enlargement of the adrenal glands was observed at 500 and 1500 ppm.
The NOAEL in this study was 25 ppm hexaconazole (equivalent to 3.5
mg/kg bw/day) (Forbes, 1988).
Rats
Groups of Wistar (Alpk/AP) rats (20/sex/group) were fed diets
containing 0, 50, 500 or 5000 ppm hexaconazole (purity 92.3%) for 90
days. Observations included clinical examinations, mortality, food
consumption and food efficiency, body weight, haematology, clinical
chemistry, hepatic aminopyrine-N-demethylase activity (APDM) and
urinalysis, ophthalmoscopy, macroscopy, organ weight and
histopathology.
An increased incidence of staining of the pelt around the nose
was observed in rats at 5000 ppm. Body weight gain was significantly
reduced in both male and female rats at 5000 ppm (accompanied by
reduced food consumption), in males at 500 ppm (first 3 weeks of the
study) and in females at 500 and 50 ppm (at several timepoints over
the treatment period). Final body weight was significantly reduced in
high dose rats. In males at 5000 ppm Hb (also at 500 ppm), Ht and RBC
counts were significantly decreased and WBC count was significantly
increased. Prothrombin-time was significantly reduced in males at 5000
and 500 ppm. Inconsistent changes in blood biochemistry (ALAT, ASAT,
albumin, protein, glucose and triglycerides and cholesterol) were
observed at 5000 and in some parameters also at 500 ppm. Relative
liver weight was significantly increased in both sexes at 5000 and 500
ppm. Relative weight of spleen, adrenal and testes were significantly
decreased in males and kidney weight was increased in females at 5000
ppm. Hepatic APDM activity was significantly increased at all dose
levels in both male and females. At the highest dose enlarged and
discoloured pale livers were observed in 16/20 males and 6/20 females.
At histopathology hepatocellular swelling and fatty change were seen
in males (also at 500 ppm) and females at 5000 ppm. Cortical
parenchymal vacuolation in the adrenal glands was observed in both
sexes at 5000 ppm and in males also at 500 and 50 ppm. Haemorrhages
in the thymus were observed in both sexes at 5000 ppm. The LOAEL in
this study was 50 ppm (equivalent to 2.5 mg/kg bw/day) (Kinsey
et al., 1984).
Dogs
Groups of beagle dogs (4/sex/group) received 0, 5, 25 or 125 mg
hexaconazole (purity 92.3%)/kg bw daily by capsule for 90 days. After
7 days the high dose group was terminated because of severe toxic
effects (body weight loss, vomiting, abnormalities in gait and
behaviour and one death). An additional group was then started
(4/sex) at 75 mg/kg bw/day. After 10 days this dose, showing similar
signs of toxicity, was reduced to 50 mg/kg bw/day. After the initial
effects at 75 mg/kg bw/day no dose-related effects were observed on
mortality, clinical signs, ophthalmoscopy, body weight and food
consumption at 50 mg/kg bw/day. Platelet count was significantly
increased in both sexes at 50 mg/kg bw/day. Urea, albumin,
triglycerides and cholesterol in plasma were significantly reduced and
ALAT and SAP were increased in males and in females at 50 and 25 mg/kg
bw Relative kidney weight and relative liver weight were
significantly increased in both sexes at 50 mg/kg bw/day and there
was a tendency to increased liver weight at 25 mg/kg bw.
Weights of ovaries and testes were slightly decreased at the
highest dose. At macroscopy enlargement and pallor of the liver was
observed at both the mid- and the high-dose level. These findings
were accompanied by microscopic evidence of an increase in hepatocyte
lipid accumulation. The NOAEL in this study was 5 mg/kg bw/day
(Stonard, 1989).
Groups of beagle dogs (4/sex/group) received 0, 2, 10 or 50 mg
hexaconazole (purity 90.0%)/kg bw daily by capsule for one year. Two
dogs died during the study, 1 male dog at 50 mg/kg bw/day on day 5
(the dog was replaced by another male dog) and another dog (female)
given 2 mg/kg bw/day in week 36. Neither death was considered to be
treatment-related. There were no effects on clinical condition,
ophthalmoscopy, food consumption and urinalysis. At the highest dose
body weight gain was significantly reduced during the first 5 weeks of
the study. At 50 mg/kg bw/day platelet count was significantly
increased in both sexes throughout the study and in females at 10
mg/kg bw at weeks 13 and 26. At the high dose level reductions in
plasma total protein and albumin, urea, cholesterol and triglycerides
were observed in both sexes. SAP and ALAT were increased in both male
and female dogs at 50 mg/kg bw/day (significantly) and at 10 mg/kg
bw/day. Relative liver weight at 50 and 10 mg/kg bw/day and relative
kidney weight at 50 mg/kg bw/day were significantly increased. At
macroscopy pallor, enlargement and accentuation of the lobular pattern
of the liver was observed in high dose dogs. Fatty changes of the
liver were observed in both male and female dogs at 50 mg/kg bw and to
a lesser extent in males at 10 mg/kg bw/day. The NOAEL in this study
was 2 mg/kg bw/day (Stonard, 1988).
Long-term/carcinogenicity studies
Mice
Groups of male and female C57/BL/10JfCD-1/Alpk mice
(50/sex/group) were fed diets containing 0, 0', 5, 40 or 200 ppm
hexaconazole (purity 90%) for 2 years. Two concurrent control groups
of 50 male and 50 female mice were kept. Observations included
clinical signs, body weight, food consumption, haematology,
macroscopy, liver and testes weight, and histopathology.
A slightly increased incidence of the loss of coat color (black
to grey) was observed in male mice at 200 ppm during the second year
of the study. At the highest dose, body weight gain and food
efficiency were significantly reduced in males and food consumption
was significantly reduced in females. At termination Hb, Ht and RBC
values were significantly increased in both sexes and MCV, platelet
count and WBC count in females only at the highest dose. Relative
liver weight was significantly increased in high dose males and
females accompanied by an increased incidence of minimal to moderate
centrilobular fatty changes in the liver. Tumour incidences were not
increased. The NOAEL in this study was 40 ppm (equal to 4.7 mg/kg
bw/day for male mice and 5.9 mg/kg bw/day for females) (Pigott, 1988).
Rats
Groups of male and female ALpk:APfSD rats (64/sex/group) were fed
diets containing 0, 10, 100 or 1000 ppm hexaconazole (purity 89.8%).
Twelve rats/sex from each group were used for interim sacrifice after
52 weeks and the remaining 52 rats/sex/group were continued to
terminal sacrifice after 105 weeks. Observations included clinical
signs, body weight, food consumption, ophthalmoscopy, haematology,
clinical chemistry, APDM-activity in liver, macroscopy, organ weights
(gonads, adrenals, kidneys, liver and brain), and histopathology. In
order to provide information on adrenocortical function, blood samples
of 12 male and 12 female rats of each group were taken at week 52/53
and week 78/79 and analyzed for corticosterone. Urinary sodium and
potassium levels (as an indirect method of assessing aldosterone
levels) were determined in 13/rats/sex/group at week 52.
No treatment-related effects were seen on mortality, haematology,
corticosterone, urinary sodium and potassium levels or ophthalmoscopy.
A dose-related decrease in body weight gain was seen in females at 100
ppm and in both sexes at 1000 ppm. Food consumption was lower for
high dose males and females. At 1000 ppm plasma triglyceride levels
were reduced in males and in females. Cholesterol levels were
significantly increased in high dose female rats and urea levels
decreased during the first year. Increases in ALAT and ASAT
activities were observed in male rats at 1000 ppm. Urinary protein
excretion was significantly increased in high dose males up to week 25
which was reflected in lower pH values. There was a marked increase
in hepatic amino-pyrine-N-demethylase activity in both males and
females receiving 1000 ppm; a smaller but still significant increase
was observed in males at 100 ppm. Relative liver weight was
significantly increased at the highest dose (at interim sacrifice
females at 100 ppm also showed an increased relative liver weight).
Relative adrenal and kidney weights were increased in females at 100
and 1000 ppm after 52 weeks only.
Livers of high dose rats showed an accentuation of lobular
pattern, with or without swollen or enlarged lobes and pale spots at
the interim sacrifice as well as at termination. Microscopy revealed
a dose-related increased incidence of fatty changes, primarily
centrilobular in pattern in the liver of males at 1000 and 100 ppm and
in females at 1000 ppm. High dose rats also showed an increase in the
incidence of hepatocyte hypertrophy, with a slight dose-related
increase in microcystic degeneration of the liver in males at 1000 ppm
and a slight increase at 100 ppm.
An increased incidence of cortical vacuolation was observed in
the adrenal glands of mid and high dose males. A slight increase was
seen in cortical cysts in females at 1000 ppm. In the testes of rats
at 1000 and 100 ppm, a treatment-related increased incidence was seen
in benign Leydig cell tumours. The incidence was 2/52, 2/52, 4/52,
8/52 at 0, 10, 100 and 1000 ppm, respectively. Historical control
values for this finding are 0-14.4%. The NOAEL in this study was 10
ppm (equal to 0.47 mg/kg bw/day in males and 0.61 mg/kg bw/day in
females) (Hext, 1988a; 1988b).
Reproduction study
Rats
Groups of 15 male and 15 female ALpk:APfSD rats received
hexaconazole (purity 90.0%) in the diet at 0, 20, 100 or 1000 ppm.
After 12 weeks of treatment animals were mated to start a 2-generation
(2 litters/generation) study. F1 parents selected from F1a offspring
were mated after 11 weeks. At 1000 ppm body weight gain and food
consumption were decreased in F0 and F1 parents. A trend for a
decreased body weight gain was observed in F0 males at 100 ppm. At
the highest dose birth weight and weight gain to day 36 of F1a litter
offspring were significantly reduced. In both F2a and F2b litters
total litter weight was markedly reduced and F2b pup weight was also
reduced. Absolute as well as relative liver weights were increased at
1000 ppm for F0 and F1 parents and F1a, F2a and F2b pups.
Histopathology revealed evidence of fatty changes in the liver
with or without hepatocyte hypertrophy in male and female parents and
pups at 1000 ppm. At 100 ppm there was evidence of a similar but less
marked effect on the histopathology of the liver in both parents and
offspring. Cortical cell vacuolation of the adrenal gland was
observed in both male and female parents and offspring at 1000 ppm and
to a much lesser extent this was also found at 100 ppm. No adverse
effects were observed on reproduction parameters such as fertility
indices, length of gestation, pre-coital interval, litter size and
number of live and dead fetuses. The NOAEL in this study was 20 ppm
(equivalent to 1 mg/kg bw/day) (Middleton, 1988).
Special studies on embryo/fetotoxicity
Rats
Groups of 24 pregnant Wistar Alpk/AP rats were orally dosed by
gavage at 0, 2.5, 25 or 250 mg/kg bw/day hexaconazole (purity 92.3%)
in corn oil from day 7 to day 16 of gestation. Clinical signs,
mortality, body weight and food consumption were recorded. At day 22
of gestation animals were sacrificed and the fetuses were delivered by
cesarean section. The number and positions of implantations and
corpora lutea were determined. The fetuses were counted, sexed and
weighed and examined for external, visceral and skeletal
malformations. At 250 mg/kg bw/day, maternal body weight gain and
food consumption were significantly decreased. Post-implantation loss
was significantly increased. The mean number of live fetuses was
slightly reduced and pup weight was significantly lower at the highest
dose. The number of fetuses with minor defects only was significantly
increased at 25 and 250 mg/kg bw/day. The incidence of fetuses with
extra 14th thoracic ribs was significantly increased at 25 and 250
mg/kg bw/day. At 250 mg/kg bw/day the proportion of fetuses with
unossified calcanea and partially ossified 5th sternebrae was
significantly increased and the mean manus and pes scores were
also significantly increased in this group. In this study fetotoxic
effects were observed at 250 and to a lesser extant at 25 mg/kg
bw/day, but there were no indications for structural malformations
being associated with compound administration. The NOAEL for
fetotoxicity in this study was 2.5 mg/kg bw/day (Killick et al.,
1984a).
Rabbits
Groups of 18 pregnant New Zealand white rabbits were orally dosed
by gavage with 0, 2.5, 12.5 or 50 mg hexaconazole (purity 92.3%)/kg bw
in corn oil from days 7-19 of gestation. At day 30 of gestation
animals were sacrificed and the fetuses were delivered by Cesarean
section. No dose-related maternal toxicity was observed. The number,
growth, and survival of the fetuses were not affected by treatment.
After examination of the fetuses for external, visceral and skeletal
malformations, a slight increase (not significant) in partially
ossified 5th sternebrae was seen at 50 mg/kg bw only. The NOAEL in
this study was 12.5 mg/kg bw/day (Killick et al., 1984b).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
hexaconazole. The results are summarized in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on pharmacological effects
In vivo studies
In a rat behavioural study, a pull-up test for evaluating muscle
relaxation and a Halothane sleeping time test, central nervous system
(CNS) depression was observed at high doses (>500 mg/kg bw)
hexaconazole (purity 89.8%). The NOAEL for CNS depression in these
studies was 250 mg/kg bw/day.
Hexaconazole had no effect on the cardiovascular system as
determined by blood pressure measurements, heart rate or respiration
rate and gastrointestinal motility (Allen, 1988).
In vitro studies
At high concentrations a non-competitive antagonism of both
acetylcholine and histamine in guinea-pig ileum was observed.
Complete haemolysis of rabbit erythrocytes was induced by 0.03%
and 0.1% hexaconazole. No effects were observed on (alpha)1,
(alpha)2 or œ-adrenoceptors, nicotinic acetylcholine receptors or on
smooth muscle (Allen, 1988).
Special studies on skin and eye irritation and sensitization
A dose of 500 mg hexaconazole (purity not given) moistened with
0.5 ml of deionised water, was applied under occlusive conditions to
the shaven intact back skin of 6 male New Zealand white albino rabbits
for 4 hours. No skin irritation up to 72 hours after application
occurred (Southwood, 1984b).
Table 2. Results of in vitro genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Ames test1 S. typhimurium 1.6-5000 µg/ plate, 92.3% negative Callander, 1984
TA98, TA100, 2 tests
TA1535, TA1537,
TA1538
Ames test1 E. coli WP2 uvrA 1.6-5000 µg/pla 89.8% negative Callander, 1988
pKM101 2 tests2
Cytogenetics assay human lymphocytes 15-250 µg/ml, 92.3% negative Sheldon, et al., 1984a
>200 toxic3
20-250 µg/ml4
Lymphoma forward mouse L5178Y cells 7.8-125 µg/ml 92.3% negative Cross, 1986
mutation assay >70 µg:toxic5
Unscheduled DNA rat hepatocytes 10-4, 10-5, 90.0% negative Trueman, 1988
synthesis test 10-6 or 10-7 M6
1 Both with and without rat liver S9 fraction.
2 2-Aminoanthracene and N-methyl-N1-nitro-N-nitrosoguanidine were used as positive controls.
3 Without metabolic activation, mitomycin C was used as positive control.
4 With metabolic activation, cyclophosphamide was used as a positive control.
5 Dimethylsulfoxide was used as negative control and benzo (alpha)pyrene and
ethylmethanesulfonate were used as positive controls with and without metabolic activation, respectively.
6 6-p-dimethylaminophenylazobenzthiazole was used as a positive control.
Table 3. Results of in vivo genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Micronucleus test C57/BL/6J mice 75 and 120 mg/kg 92.3% negative Sheldon, et al.,
(m+F) bone i.p.* 1984b
marrow cells
Dominant lethal test CD-1 male mice 10, 30 or 100 92.3% negative Wickramaratne,
mg/kg bw for 5 et al., 1984
cons. days*
* Cyclophosphamide was used as a positive control.
Nine New Zealand white albino rabbits were given doses of 100 mg
of undiluted hexaconazole (purity not stated) into the conjunctival
sac of the left eye. The eyes of 3/9 rabbits were washed. All
animals showed conjunctival redness, chemosis and discharge at 1 hour
after application reversible within 3 days except for slight discharge
that was still seen in 1/6 rabbits (unwashed) after 7 days (Southwood,
1984b).
Hexaconazole was tested for skin sensitization in 20 Dunkin
Hartley guinea pigs in a Magnusson Kligman test. The intradermal and
topical induction concentrations were a 0.5% solution of hexaconazole
(purity not given) in 4.5% dimethylformamide/corn oil and a 75%
suspension in dimethylformamide, respectively. After a challenge with
a 10% solution, 1/20 animals showed scattered mild redness; following
challenge with a 25% solution 12/20 animals showed scattered mild
redness. Following a re-challenge with a 25% solution 6/20 guinea
pigs gave a positive response (Southwood, 1984c).
Special study on steroid metabolism
Isolated Leydig cells, prepared from the testes of adult
Alpk:APfSD rats, were incubated with varying concentrations of either
hexaconazole (0.1-30µm) or ketoconazole (0.l-10µm) for a period up to
24 hours. After the incubation period testosterone, progesterone and
17-OH progesterone were analyzed using specific radio immunoassays.
The experiments were repeated in the presence of a maximally
stimulating dose of HCG (human chorionic gonadotropin). A dose-
related decrease in testosterone production, accompanied by an
increase in the production of progesterone and 17-OH progesterone was
observed after treatment with hexaconazole and hezaconazole in the
presence of hCG. Incubation with ketoconazole showed the same
effects, but ketaconazole is about 70-100 times more active than
hexaconazole (Foster, 1990).
The inhibition of testosterone production by ketoconazole was
also reported in several in vivo as well as in vitro studies in
humans, mice and rats (English et al., 1986; Pont et al., 1982;
Santen et al., 1983; Lambert et al., 1986)
Observations in humans
No information was available.
COMMENTS
Following oral administration to rats, hexaconazole was rapidly
and almost completely excreted via the urine and the faeces. The
total radioactivity excreted was equal for both males and females, but
females excreted a higher proportion of the dose in urine than in
faeces. The highest tissue residues were found in the liver,
intestinal contents and the adrenal cortex. Biliary excretion was
extensive, accounting for about 80% and 40% of the total radioactivity
in males and females respectively. About 50% of the radioactivity
excreted in the bile was reabsorbed by enterohepatic circulation.
Hexaconazole was metabolized via oxidation of the n-butyl chain
by two pathways. The more important pathway was conversion of
hexaconazole to 5-hydroxy-hexaconazole and 5-keto-hexaconazole; a
minor pathway involved two-stage oxidation of the terminal methyl
group to "hexaconazole acid" via 6-hydroxy-hexaconazole. Free
triazole was also formed by cleavage of hexaconazole or its
metabolites.
The compound showed slight to moderate acute oral toxicity in
rats and mice. WHO has classified hexaconazole as slightly hazardous
based on acute toxicity and has concluded that it is "unlikely to
present acute hazard in normal use" (WHO, 1990).
From acute as well as from short-term studies it appeared that
male rats were more sensitive than female rats. Short-term studies
with mice, rats and dogs indicated that the liver is the primary
target organ. Lipid accumulation in hepatic parenchymal cells was
observed, with associated disturbances in lipid metabolism and blood
chemistry. Elevated levels of aminopyrine-N-demethylase (rat)
suggest an adaptive response in the liver. In a one-year study in
dogs (capsule administration) the NOAEL was 2 mg/kg bw/day. However,
a 90-day dog study (capsule administration) indicated a NOAEL of 5
mg/kg bw/day. Since the next highest dose in the one-year dog study
was 10 mg/kg bw/day, the Meeting concluded that the appropriate NOAEL
for dogs was probably 5 mg/kg bw/day.
In the rat, cortical parenchymal vacuolation in the adrenal
glands was observed. In a short-term feeding study in mice, effects
on the male and female reproductive organs and on the adrenals were
observed. From in vitro studies it can be concluded that
hexaconazole inhibits testosterone production.
In a long-term feeding study in mice (highest dose level 200 ppm)
observed effects were reduced body weight gain, increased erythrocyte,
haemoglobin and haematocrit values and, in the liver, increased weight
and centrilobular fatty changes. The tumour incidence was not
enhanced. The NOAEL in this study was 40 ppm, equal to 4.7 mg/kg
bw/day for males and 5.9 mg/kg bw/day for females.
In a long-term feeding study in rats, the same effects as in the
short-term study were observed. In the testes, the incidence of
benign Leydig cell tumours was slightly increased. The NOAEL in this
study was 10 ppm, equal to 0.47 mg/kg bw/day in males and 0.6l mg/kg
bw/day in females.
After reviewing the available in vitro and in vivo short-term
genotoxicity tests, it was concluded that there was no evidence of
genotoxicity.
Embryotoxicity/fetotoxicity was observed in a teratogenicity
study in rats. No effects were observed at 2.5 mg/kg bw/day. Delayed
ossification was observed in a rabbit teratogenicity study at the
highest dose of 50 mg/kg bw/day; the NOAEL was 12.5 mg/kg bw/day.
In a two-generation reproduction study in rats, reproductive
performance was not affected. Parental body weight gain, food
consumption and pup weight were decreased. Histopathological
examination revealed fatty changes in the liver, either with or
without hepatocytic hypertrophy, and cortical vacuolation of the
adrenal gland in both parents and pups. The NOAEL in this study was
2.5 mg/kg bw/day.
An ADI was allocated based upon the NOAEL from the rat long-term
study (0.5 mg/kg bw/day), using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 40 ppm in the diet, equal to 5.0 mg/kg bw/day
Rat: 10 ppm in the diet, equal to 0.5 mg/kg bw/day
Dog: 5 mg/kg bw/day
Estimate of acceptable daily intake
0-0.005 mg/kg bw
Studies which will provide information valuable in
the continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, S.A. (1988) Hexaconazole: pharmacological evaluation.
Unpublished report no.: CTL/P/1970 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1984) PP523: An evaluation in the salmonella
mutagenicity assay. Unpublished report no.: CTL/P/977 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1988) Hexaconazole - an evaluation of mutagenic
potential using E.coli. Unpublished report no.: CTL/P/2143 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Cross, M.F. (1986) PP523: Assessment of mutagenic potential using
L5178Y Mouse Lymphoma Cells. Unpublished report no.: CTL/P/1298 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Davison, V.M. (1988) Hexaconazole: Acute oral toxicity to the rat.
Unpublished report no.: CTL/P/2231 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
English, H.F., Santner, S.J., Levine, H.B. and Santen, R.J. (1986)
Inhibition of testosterone production with ketoconazole alone and in
combination with a gonadotropin releasing hormone analogue in the rat.
Cancer Res. 46, 38-42.
Forbes, D. (1988) Hexaconazole: 29-day feeding study in mice.
Unpublished report no. CTL/P/2204 from ICI Central Toxicology
Laboratory. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Foster, P.M.D. (1990) Effects of hexaconazole (ICIA523) on the
steroidogenic function of isolated rat Leydig cells, Unpublished
report no.: CTL/R/1035 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1987) PP523: 4-hour acute inhalation toxicity study in the
rat. Unpublished report no.: CTL/P/1731 from ICI PLC, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Hext, P.M. (1988a) Hexaconazole: two year feeding study in rats.
Unpublished report no.: CTL/P/1920 from ICI Central Toxicology
Laboratory, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1988b) Second supplement to hexaconazole: two year feeding
study in rats measurement of plasma corticosterone levels. Unpublished
report no.: CTL/P/1920 from ICI Central Toxicology Laboratory,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K., Galvin, G. Rhodes, S.J. and Soames, A.R. (1984) PP523:
Excretion and tissue retention of a single dose (1 mg/kg) in the rat.
Unpublished report no.: CTL/P/1148 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988a) Hexaconazole: repeat dose study (1mg/kg) in the
rat. Unpublished report no.: CTL/P/2089 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988b) Hexaconazole: whole body autoradiography study in
the rat following 14 daily oral doses of 1 mg 14C-labelled
hexaconazole/kg. Unpublished report no.: CTL/P/2052 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1989a) Hexaconazole: tissue distribution and elimination
following a single oral dose (1 mg/kg) in the rat. Unpublished report
no.: CTL/P/2389 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K. (1989b) Hexaconazole: tissue distribution and elimination
following a single oral dose (200 mg/kg) in the rat. Unpublished
report no.: CTL/P/2390 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Jones, B.K. (1989c) Hexaconazole: biotranformation in the rat with
first amendment. Unpublished report no.: CTL/P/1848 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984a) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1127 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984b) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1131 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Kinsey, D.L., Hollis, K.J., Chart, I.S., Gore, C.W., Godley, M.J.
Pigott, G.H., Stonard, M.D. and Chalmers, D.T. (1984) PP523: 90-day
feeding study in rats. Unpublished report no.: CTL/P/1073 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Lambert, A., Mitchell, R., and Robertson, W.R. (1986) The effect of
ketoconazole on adrenal and testicular steroidogenesis in vitro.
Biochem. Pharmacol., 25(22), 3999-4004.
Leah, A.M. (1989) Hexaconazole: acute oral toxicity to the mouse,
including the first amendment to the report. Unpublished report no.
CTL/P/2234 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Middleton, M.C. (1988) Hexaconazole: two generation reproduction study
in the rat. Unpublished report no.: CTL/P/1598 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Pigott, G.H. (1988) Hexaconazole: two year feed study in mice.
Unpublished report no. CTL/P/1929 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK
Pont, A., Williams, P.L., Azhar, S., Reitz, R.E., Bochra, C., Smith,
E.R. and Stevens, D.A. (1982) Ketoconazole blocks testosterone
synthesis. Arch. Intern. Med. 142, 2137-2140.
Santen, R.J., Van den Bossche, H., Symoens, J., Brugmans, J. and
DeCoster, R. (1983) Site of action of low dose ketoconazole on
androgen biosynthesis in men. J. Clinical Endocrin. Metab. 57(4), 732-
736.
Sheldon, T., Howard, A.C. and Richardson, C.R. (1984a) PP523: A
cytogenetic study in human lymphocytes in vitro. Unpublished report
no. CTL/P/1186 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J. and Barber, G. (1984b) An
evaluation of PP523 in the mouse micronucleus test. Unpublished report
no. CTL/P/1136 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Southwood, J. (1984a) PP523: Acute oral and acute dermal toxicity
studies. Unpublished report no. CTL/P/1113 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984b) PP523: Skin and eye irritation studies.
Unpublished report no. CTL/P/1043 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984c) PP523: Skin sensitisation study. Unpublished
report no. CTL/P/1049 from ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Stonard, M.D. (1989) PP523: 90-day oral dosing study in dogs and first
amendment. Unpublished report no. CTL/P/1137 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Stonard, M.D. (1988) Hexaconazole: 1 year oral dosing study in dogs.
Unpublished report no. CTL/P/1942 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trivedi, S., Jones, B.K. and Soames, A.R. (1986) PP523: Excretion and
tissue retention of a single oral dose (200 mg/kg) in the rat.
Unpublished report no. CTL/P/1431 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trueman, R.W. (1988) Hexaconazole: Assessment for the induction of
unscheduled DNA synthesis in primary rat hepatocyte cultures.
Unpublished report no. CTL/P/1887 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Wickramaratne, G.A., Bramley, J.R., Banham, P.B. and Godley, M.J.
(1984) PP523: Dominant lethal study in the mouse. Unpublished report
no. CTL/P/1110 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Short-term studies
Mice
Groups of C57BL/10JfCD-1/Alpk mice were fed diets containing 0,
25, 100, 500 or 1500 ppm hexaconazole (purity not given) in the diet
for 29 days. At 500 and 1500 ppm pronounced effects were observed on
body weight, food consumption, food efficiency and liver weight. A
slight to moderate microcytosis with a compensatory increase in red
cell numbers to maintain haemoglobin levels was observed in males at
1500 ppm and in females at 500 and 1500 ppm. Red cell counts were
also increased in males at 500 and 100 ppm. At histopathology a
dose-related liver hypertrophy and increased hepatocyte lipid
accumulation with associated cytoplasmic vacuolation were observed. A
lack of corpora lutea in the ovaries and a reduction in the size of
the uterus were observed in females receiving doses of 100 ppm
hexaconazole and above. At 1500 ppm and possibly at 500 ppm there was
some evidence of an effect on the male reproductive system with
increased abnormal precursor cells in testicular tubules and
epididymis and reduced seminal vesicular secretion. Cortical
enlargement of the adrenal glands was observed at 500 and 1500 ppm.
The NOAEL in this study was 25 ppm hexaconazole (equivalent to 3.5
mg/kg bw/day) (Forbes, 1988).
Rats
Groups of Wistar (Alpk/AP) rats (20/sex/group) were fed diets
containing 0, 50, 500 or 5000 ppm hexaconazole (purity 92.3%) for 90
days. Observations included clinical examinations, mortality, food
consumption and food efficiency, body weight, haematology, clinical
chemistry, hepatic aminopyrine-N-demethylase activity (APDM) and
urinalysis, ophthalmoscopy, macroscopy, organ weight and
histopathology.
An increased incidence of staining of the pelt around the nose
was observed in rats at 5000 ppm. Body weight gain was significantly
reduced in both male and female rats at 5000 ppm (accompanied by
reduced food consumption), in males at 500 ppm (first 3 weeks of the
study) and in females at 500 and 50 ppm (at several timepoints over
the treatment period). Final body weight was significantly reduced in
high dose rats. In males at 5000 ppm Hb (also at 500 ppm), Ht and RBC
counts were significantly decreased and WBC count was significantly
increased. Prothrombin-time was significantly reduced in males at 5000
and 500 ppm. Inconsistent changes in blood biochemistry (ALAT, ASAT,
albumin, protein, glucose and triglycerides and cholesterol) were
observed at 5000 and in some parameters also at 500 ppm. Relative
liver weight was significantly increased in both sexes at 5000 and 500
ppm. Relative weight of spleen, adrenal and testes were significantly
decreased in males and kidney weight was increased in females at 5000
ppm. Hepatic APDM activity was significantly increased at all dose
levels in both male and females. At the highest dose enlarged and
discoloured pale livers were observed in 16/20 males and 6/20 females.
At histopathology hepatocellular swelling and fatty change were seen
in males (also at 500 ppm) and females at 5000 ppm. Cortical
parenchymal vacuolation in the adrenal glands was observed in both
sexes at 5000 ppm and in males also at 500 and 50 ppm. Haemorrhages
in the thymus were observed in both sexes at 5000 ppm. The LOAEL in
this study was 50 ppm (equivalent to 2.5 mg/kg bw/day) (Kinsey
et al., 1984).
Dogs
Groups of beagle dogs (4/sex/group) received 0, 5, 25 or 125 mg
hexaconazole (purity 92.3%)/kg bw daily by capsule for 90 days. After
7 days the high dose group was terminated because of severe toxic
effects (body weight loss, vomiting, abnormalities in gait and
behaviour and one death). An additional group was then started
(4/sex) at 75 mg/kg bw/day. After 10 days this dose, showing similar
signs of toxicity, was reduced to 50 mg/kg bw/day. After the initial
effects at 75 mg/kg bw/day no dose-related effects were observed on
mortality, clinical signs, ophthalmoscopy, body weight and food
consumption at 50 mg/kg bw/day. Platelet count was significantly
increased in both sexes at 50 mg/kg bw/day. Urea, albumin,
triglycerides and cholesterol in plasma were significantly reduced and
ALAT and SAP were increased in males and in females at 50 and 25 mg/kg
bw Relative kidney weight and relative liver weight were
significantly increased in both sexes at 50 mg/kg bw/day and there
was a tendency to increased liver weight at 25 mg/kg bw.
Weights of ovaries and testes were slightly decreased at the
highest dose. At macroscopy enlargement and pallor of the liver was
observed at both the mid- and the high-dose level. These findings
were accompanied by microscopic evidence of an increase in hepatocyte
lipid accumulation. The NOAEL in this study was 5 mg/kg bw/day
(Stonard, 1989).
Groups of beagle dogs (4/sex/group) received 0, 2, 10 or 50 mg
hexaconazole (purity 90.0%)/kg bw daily by capsule for one year. Two
dogs died during the study, 1 male dog at 50 mg/kg bw/day on day 5
(the dog was replaced by another male dog) and another dog (female)
given 2 mg/kg bw/day in week 36. Neither death was considered to be
treatment-related. There were no effects on clinical condition,
ophthalmoscopy, food consumption and urinalysis. At the highest dose
body weight gain was significantly reduced during the first 5 weeks of
the study. At 50 mg/kg bw/day platelet count was significantly
increased in both sexes throughout the study and in females at 10
mg/kg bw at weeks 13 and 26. At the high dose level reductions in
plasma total protein and albumin, urea, cholesterol and triglycerides
were observed in both sexes. SAP and ALAT were increased in both male
and female dogs at 50 mg/kg bw/day (significantly) and at 10 mg/kg
bw/day. Relative liver weight at 50 and 10 mg/kg bw/day and relative
kidney weight at 50 mg/kg bw/day were significantly increased. At
macroscopy pallor, enlargement and accentuation of the lobular pattern
of the liver was observed in high dose dogs. Fatty changes of the
liver were observed in both male and female dogs at 50 mg/kg bw and to
a lesser extent in males at 10 mg/kg bw/day. The NOAEL in this study
was 2 mg/kg bw/day (Stonard, 1988).
Long-term/carcinogenicity studies
Mice
Groups of male and female C57/BL/10JfCD-1/Alpk mice
(50/sex/group) were fed diets containing 0, 0', 5, 40 or 200 ppm
hexaconazole (purity 90%) for 2 years. Two concurrent control groups
of 50 male and 50 female mice were kept. Observations included
clinical signs, body weight, food consumption, haematology,
macroscopy, liver and testes weight, and histopathology.
A slightly increased incidence of the loss of coat color (black
to grey) was observed in male mice at 200 ppm during the second year
of the study. At the highest dose, body weight gain and food
efficiency were significantly reduced in males and food consumption
was significantly reduced in females. At termination Hb, Ht and RBC
values were significantly increased in both sexes and MCV, platelet
count and WBC count in females only at the highest dose. Relative
liver weight was significantly increased in high dose males and
females accompanied by an increased incidence of minimal to moderate
centrilobular fatty changes in the liver. Tumour incidences were not
increased. The NOAEL in this study was 40 ppm (equal to 4.7 mg/kg
bw/day for male mice and 5.9 mg/kg bw/day for females) (Pigott, 1988).
Rats
Groups of male and female ALpk:APfSD rats (64/sex/group) were fed
diets containing 0, 10, 100 or 1000 ppm hexaconazole (purity 89.8%).
Twelve rats/sex from each group were used for interim sacrifice after
52 weeks and the remaining 52 rats/sex/group were continued to
terminal sacrifice after 105 weeks. Observations included clinical
signs, body weight, food consumption, ophthalmoscopy, haematology,
clinical chemistry, APDM-activity in liver, macroscopy, organ weights
(gonads, adrenals, kidneys, liver and brain), and histopathology. In
order to provide information on adrenocortical function, blood samples
of 12 male and 12 female rats of each group were taken at week 52/53
and week 78/79 and analyzed for corticosterone. Urinary sodium and
potassium levels (as an indirect method of assessing aldosterone
levels) were determined in 13/rats/sex/group at week 52.
No treatment-related effects were seen on mortality, haematology,
corticosterone, urinary sodium and potassium levels or ophthalmoscopy.
A dose-related decrease in body weight gain was seen in females at 100
ppm and in both sexes at 1000 ppm. Food consumption was lower for
high dose males and females. At 1000 ppm plasma triglyceride levels
were reduced in males and in females. Cholesterol levels were
significantly increased in high dose female rats and urea levels
decreased during the first year. Increases in ALAT and ASAT
activities were observed in male rats at 1000 ppm. Urinary protein
excretion was significantly increased in high dose males up to week 25
which was reflected in lower pH values. There was a marked increase
in hepatic amino-pyrine-N-demethylase activity in both males and
females receiving 1000 ppm; a smaller but still significant increase
was observed in males at 100 ppm. Relative liver weight was
significantly increased at the highest dose (at interim sacrifice
females at 100 ppm also showed an increased relative liver weight).
Relative adrenal and kidney weights were increased in females at 100
and 1000 ppm after 52 weeks only.
Livers of high dose rats showed an accentuation of lobular
pattern, with or without swollen or enlarged lobes and pale spots at
the interim sacrifice as well as at termination. Microscopy revealed
a dose-related increased incidence of fatty changes, primarily
centrilobular in pattern in the liver of males at 1000 and 100 ppm and
in females at 1000 ppm. High dose rats also showed an increase in the
incidence of hepatocyte hypertrophy, with a slight dose-related
increase in microcystic degeneration of the liver in males at 1000 ppm
and a slight increase at 100 ppm.
An increased incidence of cortical vacuolation was observed in
the adrenal glands of mid and high dose males. A slight increase was
seen in cortical cysts in females at 1000 ppm. In the testes of rats
at 1000 and 100 ppm, a treatment-related increased incidence was seen
in benign Leydig cell tumours. The incidence was 2/52, 2/52, 4/52,
8/52 at 0, 10, 100 and 1000 ppm, respectively. Historical control
values for this finding are 0-14.4%. The NOAEL in this study was 10
ppm (equal to 0.47 mg/kg bw/day in males and 0.61 mg/kg bw/day in
females) (Hext, 1988a; 1988b).
Reproduction study
Rats
Groups of 15 male and 15 female ALpk:APfSD rats received
hexaconazole (purity 90.0%) in the diet at 0, 20, 100 or 1000 ppm.
After 12 weeks of treatment animals were mated to start a 2-generation
(2 litters/generation) study. F1 parents selected from F1a offspring
were mated after 11 weeks. At 1000 ppm body weight gain and food
consumption were decreased in F0 and F1 parents. A trend for a
decreased body weight gain was observed in F0 males at 100 ppm. At
the highest dose birth weight and weight gain to day 36 of F1a litter
offspring were significantly reduced. In both F2a and F2b litters
total litter weight was markedly reduced and F2b pup weight was also
reduced. Absolute as well as relative liver weights were increased at
1000 ppm for F0 and F1 parents and F1a, F2a and F2b pups.
Histopathology revealed evidence of fatty changes in the liver
with or without hepatocyte hypertrophy in male and female parents and
pups at 1000 ppm. At 100 ppm there was evidence of a similar but less
marked effect on the histopathology of the liver in both parents and
offspring. Cortical cell vacuolation of the adrenal gland was
observed in both male and female parents and offspring at 1000 ppm and
to a much lesser extent this was also found at 100 ppm. No adverse
effects were observed on reproduction parameters such as fertility
indices, length of gestation, pre-coital interval, litter size and
number of live and dead fetuses. The NOAEL in this study was 20 ppm
(equivalent to 1 mg/kg bw/day) (Middleton, 1988).
Special studies on embryo/fetotoxicity
Rats
Groups of 24 pregnant Wistar Alpk/AP rats were orally dosed by
gavage at 0, 2.5, 25 or 250 mg/kg bw/day hexaconazole (purity 92.3%)
in corn oil from day 7 to day 16 of gestation. Clinical signs,
mortality, body weight and food consumption were recorded. At day 22
of gestation animals were sacrificed and the fetuses were delivered by
cesarean section. The number and positions of implantations and
corpora lutea were determined. The fetuses were counted, sexed and
weighed and examined for external, visceral and skeletal
malformations. At 250 mg/kg bw/day, maternal body weight gain and
food consumption were significantly decreased. Post-implantation loss
was significantly increased. The mean number of live fetuses was
slightly reduced and pup weight was significantly lower at the highest
dose. The number of fetuses with minor defects only was significantly
increased at 25 and 250 mg/kg bw/day. The incidence of fetuses with
extra 14th thoracic ribs was significantly increased at 25 and 250
mg/kg bw/day. At 250 mg/kg bw/day the proportion of fetuses with
unossified calcanea and partially ossified 5th sternebrae was
significantly increased and the mean manus and pes scores were
also significantly increased in this group. In this study fetotoxic
effects were observed at 250 and to a lesser extant at 25 mg/kg
bw/day, but there were no indications for structural malformations
being associated with compound administration. The NOAEL for
fetotoxicity in this study was 2.5 mg/kg bw/day (Killick et al.,
1984a).
Rabbits
Groups of 18 pregnant New Zealand white rabbits were orally dosed
by gavage with 0, 2.5, 12.5 or 50 mg hexaconazole (purity 92.3%)/kg bw
in corn oil from days 7-19 of gestation. At day 30 of gestation
animals were sacrificed and the fetuses were delivered by Cesarean
section. No dose-related maternal toxicity was observed. The number,
growth, and survival of the fetuses were not affected by treatment.
After examination of the fetuses for external, visceral and skeletal
malformations, a slight increase (not significant) in partially
ossified 5th sternebrae was seen at 50 mg/kg bw only. The NOAEL in
this study was 12.5 mg/kg bw/day (Killick et al., 1984b).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
hexaconazole. The results are summarized in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on pharmacological effects
In vivo studies
In a rat behavioural study, a pull-up test for evaluating muscle
relaxation and a Halothane sleeping time test, central nervous system
(CNS) depression was observed at high doses (>500 mg/kg bw)
hexaconazole (purity 89.8%). The NOAEL for CNS depression in these
studies was 250 mg/kg bw/day.
Hexaconazole had no effect on the cardiovascular system as
determined by blood pressure measurements, heart rate or respiration
rate and gastrointestinal motility (Allen, 1988).
In vitro studies
At high concentrations a non-competitive antagonism of both
acetylcholine and histamine in guinea-pig ileum was observed.
Complete haemolysis of rabbit erythrocytes was induced by 0.03%
and 0.1% hexaconazole. No effects were observed on (alpha)1,
(alpha)2 or œ-adrenoceptors, nicotinic acetylcholine receptors or on
smooth muscle (Allen, 1988).
Special studies on skin and eye irritation and sensitization
A dose of 500 mg hexaconazole (purity not given) moistened with
0.5 ml of deionised water, was applied under occlusive conditions to
the shaven intact back skin of 6 male New Zealand white albino rabbits
for 4 hours. No skin irritation up to 72 hours after application
occurred (Southwood, 1984b).
Table 2. Results of in vitro genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Ames test1 S. typhimurium 1.6-5000 µg/ plate, 92.3% negative Callander, 1984
TA98, TA100, 2 tests
TA1535, TA1537,
TA1538
Ames test1 E. coli WP2 uvrA 1.6-5000 µg/pla 89.8% negative Callander, 1988
pKM101 2 tests2
Cytogenetics assay human lymphocytes 15-250 µg/ml, 92.3% negative Sheldon, et al., 1984a
>200 toxic3
20-250 µg/ml4
Lymphoma forward mouse L5178Y cells 7.8-125 µg/ml 92.3% negative Cross, 1986
mutation assay >70 µg:toxic5
Unscheduled DNA rat hepatocytes 10-4, 10-5, 90.0% negative Trueman, 1988
synthesis test 10-6 or 10-7 M6
1 Both with and without rat liver S9 fraction.
2 2-Aminoanthracene and N-methyl-N1-nitro-N-nitrosoguanidine were used as positive controls.
3 Without metabolic activation, mitomycin C was used as positive control.
4 With metabolic activation, cyclophosphamide was used as a positive control.
5 Dimethylsulfoxide was used as negative control and benzo (alpha)pyrene and
ethylmethanesulfonate were used as positive controls with and without metabolic activation, respectively.
6 6-p-dimethylaminophenylazobenzthiazole was used as a positive control.
Table 3. Results of in vivo genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Micronucleus test C57/BL/6J mice 75 and 120 mg/kg 92.3% negative Sheldon, et al.,
(m+F) bone i.p.* 1984b
marrow cells
Dominant lethal test CD-1 male mice 10, 30 or 100 92.3% negative Wickramaratne,
mg/kg bw for 5 et al., 1984
cons. days*
* Cyclophosphamide was used as a positive control.
Nine New Zealand white albino rabbits were given doses of 100 mg
of undiluted hexaconazole (purity not stated) into the conjunctival
sac of the left eye. The eyes of 3/9 rabbits were washed. All
animals showed conjunctival redness, chemosis and discharge at 1 hour
after application reversible within 3 days except for slight discharge
that was still seen in 1/6 rabbits (unwashed) after 7 days (Southwood,
1984b).
Hexaconazole was tested for skin sensitization in 20 Dunkin
Hartley guinea pigs in a Magnusson Kligman test. The intradermal and
topical induction concentrations were a 0.5% solution of hexaconazole
(purity not given) in 4.5% dimethylformamide/corn oil and a 75%
suspension in dimethylformamide, respectively. After a challenge with
a 10% solution, 1/20 animals showed scattered mild redness; following
challenge with a 25% solution 12/20 animals showed scattered mild
redness. Following a re-challenge with a 25% solution 6/20 guinea
pigs gave a positive response (Southwood, 1984c).
Special study on steroid metabolism
Isolated Leydig cells, prepared from the testes of adult
Alpk:APfSD rats, were incubated with varying concentrations of either
hexaconazole (0.1-30µm) or ketoconazole (0.l-10µm) for a period up to
24 hours. After the incubation period testosterone, progesterone and
17-OH progesterone were analyzed using specific radio immunoassays.
The experiments were repeated in the presence of a maximally
stimulating dose of HCG (human chorionic gonadotropin). A dose-
related decrease in testosterone production, accompanied by an
increase in the production of progesterone and 17-OH progesterone was
observed after treatment with hexaconazole and hezaconazole in the
presence of hCG. Incubation with ketoconazole showed the same
effects, but ketaconazole is about 70-100 times more active than
hexaconazole (Foster, 1990).
The inhibition of testosterone production by ketoconazole was
also reported in several in vivo as well as in vitro studies in
humans, mice and rats (English et al., 1986; Pont et al., 1982;
Santen et al., 1983; Lambert et al., 1986)
Observations in humans
No information was available.
COMMENTS
Following oral administration to rats, hexaconazole was rapidly
and almost completely excreted via the urine and the faeces. The
total radioactivity excreted was equal for both males and females, but
females excreted a higher proportion of the dose in urine than in
faeces. The highest tissue residues were found in the liver,
intestinal contents and the adrenal cortex. Biliary excretion was
extensive, accounting for about 80% and 40% of the total radioactivity
in males and females respectively. About 50% of the radioactivity
excreted in the bile was reabsorbed by enterohepatic circulation.
Hexaconazole was metabolized via oxidation of the n-butyl chain
by two pathways. The more important pathway was conversion of
hexaconazole to 5-hydroxy-hexaconazole and 5-keto-hexaconazole; a
minor pathway involved two-stage oxidation of the terminal methyl
group to "hexaconazole acid" via 6-hydroxy-hexaconazole. Free
triazole was also formed by cleavage of hexaconazole or its
metabolites.
The compound showed slight to moderate acute oral toxicity in
rats and mice. WHO has classified hexaconazole as slightly hazardous
based on acute toxicity and has concluded that it is "unlikely to
present acute hazard in normal use" (WHO, 1990).
From acute as well as from short-term studies it appeared that
male rats were more sensitive than female rats. Short-term studies
with mice, rats and dogs indicated that the liver is the primary
target organ. Lipid accumulation in hepatic parenchymal cells was
observed, with associated disturbances in lipid metabolism and blood
chemistry. Elevated levels of aminopyrine-N-demethylase (rat)
suggest an adaptive response in the liver. In a one-year study in
dogs (capsule administration) the NOAEL was 2 mg/kg bw/day. However,
a 90-day dog study (capsule administration) indicated a NOAEL of 5
mg/kg bw/day. Since the next highest dose in the one-year dog study
was 10 mg/kg bw/day, the Meeting concluded that the appropriate NOAEL
for dogs was probably 5 mg/kg bw/day.
In the rat, cortical parenchymal vacuolation in the adrenal
glands was observed. In a short-term feeding study in mice, effects
on the male and female reproductive organs and on the adrenals were
observed. From in vitro studies it can be concluded that
hexaconazole inhibits testosterone production.
In a long-term feeding study in mice (highest dose level 200 ppm)
observed effects were reduced body weight gain, increased erythrocyte,
haemoglobin and haematocrit values and, in the liver, increased weight
and centrilobular fatty changes. The tumour incidence was not
enhanced. The NOAEL in this study was 40 ppm, equal to 4.7 mg/kg
bw/day for males and 5.9 mg/kg bw/day for females.
In a long-term feeding study in rats, the same effects as in the
short-term study were observed. In the testes, the incidence of
benign Leydig cell tumours was slightly increased. The NOAEL in this
study was 10 ppm, equal to 0.47 mg/kg bw/day in males and 0.6l mg/kg
bw/day in females.
After reviewing the available in vitro and in vivo short-term
genotoxicity tests, it was concluded that there was no evidence of
genotoxicity.
Embryotoxicity/fetotoxicity was observed in a teratogenicity
study in rats. No effects were observed at 2.5 mg/kg bw/day. Delayed
ossification was observed in a rabbit teratogenicity study at the
highest dose of 50 mg/kg bw/day; the NOAEL was 12.5 mg/kg bw/day.
In a two-generation reproduction study in rats, reproductive
performance was not affected. Parental body weight gain, food
consumption and pup weight were decreased. Histopathological
examination revealed fatty changes in the liver, either with or
without hepatocytic hypertrophy, and cortical vacuolation of the
adrenal gland in both parents and pups. The NOAEL in this study was
2.5 mg/kg bw/day.
An ADI was allocated based upon the NOAEL from the rat long-term
study (0.5 mg/kg bw/day), using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 40 ppm in the diet, equal to 5.0 mg/kg bw/day
Rat: 10 ppm in the diet, equal to 0.5 mg/kg bw/day
Dog: 5 mg/kg bw/day
Estimate of acceptable daily intake
0-0.005 mg/kg bw
Studies which will provide information valuable in
the continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, S.A. (1988) Hexaconazole: pharmacological evaluation.
Unpublished report no.: CTL/P/1970 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1984) PP523: An evaluation in the salmonella
mutagenicity assay. Unpublished report no.: CTL/P/977 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1988) Hexaconazole - an evaluation of mutagenic
potential using E.coli. Unpublished report no.: CTL/P/2143 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Cross, M.F. (1986) PP523: Assessment of mutagenic potential using
L5178Y Mouse Lymphoma Cells. Unpublished report no.: CTL/P/1298 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Davison, V.M. (1988) Hexaconazole: Acute oral toxicity to the rat.
Unpublished report no.: CTL/P/2231 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
English, H.F., Santner, S.J., Levine, H.B. and Santen, R.J. (1986)
Inhibition of testosterone production with ketoconazole alone and in
combination with a gonadotropin releasing hormone analogue in the rat.
Cancer Res. 46, 38-42.
Forbes, D. (1988) Hexaconazole: 29-day feeding study in mice.
Unpublished report no. CTL/P/2204 from ICI Central Toxicology
Laboratory. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Foster, P.M.D. (1990) Effects of hexaconazole (ICIA523) on the
steroidogenic function of isolated rat Leydig cells, Unpublished
report no.: CTL/R/1035 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1987) PP523: 4-hour acute inhalation toxicity study in the
rat. Unpublished report no.: CTL/P/1731 from ICI PLC, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Hext, P.M. (1988a) Hexaconazole: two year feeding study in rats.
Unpublished report no.: CTL/P/1920 from ICI Central Toxicology
Laboratory, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1988b) Second supplement to hexaconazole: two year feeding
study in rats measurement of plasma corticosterone levels. Unpublished
report no.: CTL/P/1920 from ICI Central Toxicology Laboratory,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K., Galvin, G. Rhodes, S.J. and Soames, A.R. (1984) PP523:
Excretion and tissue retention of a single dose (1 mg/kg) in the rat.
Unpublished report no.: CTL/P/1148 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988a) Hexaconazole: repeat dose study (1mg/kg) in the
rat. Unpublished report no.: CTL/P/2089 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988b) Hexaconazole: whole body autoradiography study in
the rat following 14 daily oral doses of 1 mg 14C-labelled
hexaconazole/kg. Unpublished report no.: CTL/P/2052 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1989a) Hexaconazole: tissue distribution and elimination
following a single oral dose (1 mg/kg) in the rat. Unpublished report
no.: CTL/P/2389 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K. (1989b) Hexaconazole: tissue distribution and elimination
following a single oral dose (200 mg/kg) in the rat. Unpublished
report no.: CTL/P/2390 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Jones, B.K. (1989c) Hexaconazole: biotranformation in the rat with
first amendment. Unpublished report no.: CTL/P/1848 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984a) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1127 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984b) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1131 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Kinsey, D.L., Hollis, K.J., Chart, I.S., Gore, C.W., Godley, M.J.
Pigott, G.H., Stonard, M.D. and Chalmers, D.T. (1984) PP523: 90-day
feeding study in rats. Unpublished report no.: CTL/P/1073 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Lambert, A., Mitchell, R., and Robertson, W.R. (1986) The effect of
ketoconazole on adrenal and testicular steroidogenesis in vitro.
Biochem. Pharmacol., 25(22), 3999-4004.
Leah, A.M. (1989) Hexaconazole: acute oral toxicity to the mouse,
including the first amendment to the report. Unpublished report no.
CTL/P/2234 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Middleton, M.C. (1988) Hexaconazole: two generation reproduction study
in the rat. Unpublished report no.: CTL/P/1598 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Pigott, G.H. (1988) Hexaconazole: two year feed study in mice.
Unpublished report no. CTL/P/1929 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK
Pont, A., Williams, P.L., Azhar, S., Reitz, R.E., Bochra, C., Smith,
E.R. and Stevens, D.A. (1982) Ketoconazole blocks testosterone
synthesis. Arch. Intern. Med. 142, 2137-2140.
Santen, R.J., Van den Bossche, H., Symoens, J., Brugmans, J. and
DeCoster, R. (1983) Site of action of low dose ketoconazole on
androgen biosynthesis in men. J. Clinical Endocrin. Metab. 57(4), 732-
736.
Sheldon, T., Howard, A.C. and Richardson, C.R. (1984a) PP523: A
cytogenetic study in human lymphocytes in vitro. Unpublished report
no. CTL/P/1186 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J. and Barber, G. (1984b) An
evaluation of PP523 in the mouse micronucleus test. Unpublished report
no. CTL/P/1136 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Southwood, J. (1984a) PP523: Acute oral and acute dermal toxicity
studies. Unpublished report no. CTL/P/1113 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984b) PP523: Skin and eye irritation studies.
Unpublished report no. CTL/P/1043 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984c) PP523: Skin sensitisation study. Unpublished
report no. CTL/P/1049 from ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Stonard, M.D. (1989) PP523: 90-day oral dosing study in dogs and first
amendment. Unpublished report no. CTL/P/1137 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Stonard, M.D. (1988) Hexaconazole: 1 year oral dosing study in dogs.
Unpublished report no. CTL/P/1942 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trivedi, S., Jones, B.K. and Soames, A.R. (1986) PP523: Excretion and
tissue retention of a single oral dose (200 mg/kg) in the rat.
Unpublished report no. CTL/P/1431 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trueman, R.W. (1988) Hexaconazole: Assessment for the induction of
unscheduled DNA synthesis in primary rat hepatocyte cultures.
Unpublished report no. CTL/P/1887 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Wickramaratne, G.A., Bramley, J.R., Banham, P.B. and Godley, M.J.
(1984) PP523: Dominant lethal study in the mouse. Unpublished report
no. CTL/P/1110 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Short-term studies
Mice
Groups of C57BL/10JfCD-1/Alpk mice were fed diets containing 0,
25, 100, 500 or 1500 ppm hexaconazole (purity not given) in the diet
for 29 days. At 500 and 1500 ppm pronounced effects were observed on
body weight, food consumption, food efficiency and liver weight. A
slight to moderate microcytosis with a compensatory increase in red
cell numbers to maintain haemoglobin levels was observed in males at
1500 ppm and in females at 500 and 1500 ppm. Red cell counts were
also increased in males at 500 and 100 ppm. At histopathology a
dose-related liver hypertrophy and increased hepatocyte lipid
accumulation with associated cytoplasmic vacuolation were observed. A
lack of corpora lutea in the ovaries and a reduction in the size of
the uterus were observed in females receiving doses of 100 ppm
hexaconazole and above. At 1500 ppm and possibly at 500 ppm there was
some evidence of an effect on the male reproductive system with
increased abnormal precursor cells in testicular tubules and
epididymis and reduced seminal vesicular secretion. Cortical
enlargement of the adrenal glands was observed at 500 and 1500 ppm.
The NOAEL in this study was 25 ppm hexaconazole (equivalent to 3.5
mg/kg bw/day) (Forbes, 1988).
Rats
Groups of Wistar (Alpk/AP) rats (20/sex/group) were fed diets
containing 0, 50, 500 or 5000 ppm hexaconazole (purity 92.3%) for 90
days. Observations included clinical examinations, mortality, food
consumption and food efficiency, body weight, haematology, clinical
chemistry, hepatic aminopyrine-N-demethylase activity (APDM) and
urinalysis, ophthalmoscopy, macroscopy, organ weight and
histopathology.
An increased incidence of staining of the pelt around the nose
was observed in rats at 5000 ppm. Body weight gain was significantly
reduced in both male and female rats at 5000 ppm (accompanied by
reduced food consumption), in males at 500 ppm (first 3 weeks of the
study) and in females at 500 and 50 ppm (at several timepoints over
the treatment period). Final body weight was significantly reduced in
high dose rats. In males at 5000 ppm Hb (also at 500 ppm), Ht and RBC
counts were significantly decreased and WBC count was significantly
increased. Prothrombin-time was significantly reduced in males at 5000
and 500 ppm. Inconsistent changes in blood biochemistry (ALAT, ASAT,
albumin, protein, glucose and triglycerides and cholesterol) were
observed at 5000 and in some parameters also at 500 ppm. Relative
liver weight was significantly increased in both sexes at 5000 and 500
ppm. Relative weight of spleen, adrenal and testes were significantly
decreased in males and kidney weight was increased in females at 5000
ppm. Hepatic APDM activity was significantly increased at all dose
levels in both male and females. At the highest dose enlarged and
discoloured pale livers were observed in 16/20 males and 6/20 females.
At histopathology hepatocellular swelling and fatty change were seen
in males (also at 500 ppm) and females at 5000 ppm. Cortical
parenchymal vacuolation in the adrenal glands was observed in both
sexes at 5000 ppm and in males also at 500 and 50 ppm. Haemorrhages
in the thymus were observed in both sexes at 5000 ppm. The LOAEL in
this study was 50 ppm (equivalent to 2.5 mg/kg bw/day) (Kinsey
et al., 1984).
Dogs
Groups of beagle dogs (4/sex/group) received 0, 5, 25 or 125 mg
hexaconazole (purity 92.3%)/kg bw daily by capsule for 90 days. After
7 days the high dose group was terminated because of severe toxic
effects (body weight loss, vomiting, abnormalities in gait and
behaviour and one death). An additional group was then started
(4/sex) at 75 mg/kg bw/day. After 10 days this dose, showing similar
signs of toxicity, was reduced to 50 mg/kg bw/day. After the initial
effects at 75 mg/kg bw/day no dose-related effects were observed on
mortality, clinical signs, ophthalmoscopy, body weight and food
consumption at 50 mg/kg bw/day. Platelet count was significantly
increased in both sexes at 50 mg/kg bw/day. Urea, albumin,
triglycerides and cholesterol in plasma were significantly reduced and
ALAT and SAP were increased in males and in females at 50 and 25 mg/kg
bw Relative kidney weight and relative liver weight were
significantly increased in both sexes at 50 mg/kg bw/day and there
was a tendency to increased liver weight at 25 mg/kg bw.
Weights of ovaries and testes were slightly decreased at the
highest dose. At macroscopy enlargement and pallor of the liver was
observed at both the mid- and the high-dose level. These findings
were accompanied by microscopic evidence of an increase in hepatocyte
lipid accumulation. The NOAEL in this study was 5 mg/kg bw/day
(Stonard, 1989).
Groups of beagle dogs (4/sex/group) received 0, 2, 10 or 50 mg
hexaconazole (purity 90.0%)/kg bw daily by capsule for one year. Two
dogs died during the study, 1 male dog at 50 mg/kg bw/day on day 5
(the dog was replaced by another male dog) and another dog (female)
given 2 mg/kg bw/day in week 36. Neither death was considered to be
treatment-related. There were no effects on clinical condition,
ophthalmoscopy, food consumption and urinalysis. At the highest dose
body weight gain was significantly reduced during the first 5 weeks of
the study. At 50 mg/kg bw/day platelet count was significantly
increased in both sexes throughout the study and in females at 10
mg/kg bw at weeks 13 and 26. At the high dose level reductions in
plasma total protein and albumin, urea, cholesterol and triglycerides
were observed in both sexes. SAP and ALAT were increased in both male
and female dogs at 50 mg/kg bw/day (significantly) and at 10 mg/kg
bw/day. Relative liver weight at 50 and 10 mg/kg bw/day and relative
kidney weight at 50 mg/kg bw/day were significantly increased. At
macroscopy pallor, enlargement and accentuation of the lobular pattern
of the liver was observed in high dose dogs. Fatty changes of the
liver were observed in both male and female dogs at 50 mg/kg bw and to
a lesser extent in males at 10 mg/kg bw/day. The NOAEL in this study
was 2 mg/kg bw/day (Stonard, 1988).
Long-term/carcinogenicity studies
Mice
Groups of male and female C57/BL/10JfCD-1/Alpk mice
(50/sex/group) were fed diets containing 0, 0', 5, 40 or 200 ppm
hexaconazole (purity 90%) for 2 years. Two concurrent control groups
of 50 male and 50 female mice were kept. Observations included
clinical signs, body weight, food consumption, haematology,
macroscopy, liver and testes weight, and histopathology.
A slightly increased incidence of the loss of coat color (black
to grey) was observed in male mice at 200 ppm during the second year
of the study. At the highest dose, body weight gain and food
efficiency were significantly reduced in males and food consumption
was significantly reduced in females. At termination Hb, Ht and RBC
values were significantly increased in both sexes and MCV, platelet
count and WBC count in females only at the highest dose. Relative
liver weight was significantly increased in high dose males and
females accompanied by an increased incidence of minimal to moderate
centrilobular fatty changes in the liver. Tumour incidences were not
increased. The NOAEL in this study was 40 ppm (equal to 4.7 mg/kg
bw/day for male mice and 5.9 mg/kg bw/day for females) (Pigott, 1988).
Rats
Groups of male and female ALpk:APfSD rats (64/sex/group) were fed
diets containing 0, 10, 100 or 1000 ppm hexaconazole (purity 89.8%).
Twelve rats/sex from each group were used for interim sacrifice after
52 weeks and the remaining 52 rats/sex/group were continued to
terminal sacrifice after 105 weeks. Observations included clinical
signs, body weight, food consumption, ophthalmoscopy, haematology,
clinical chemistry, APDM-activity in liver, macroscopy, organ weights
(gonads, adrenals, kidneys, liver and brain), and histopathology. In
order to provide information on adrenocortical function, blood samples
of 12 male and 12 female rats of each group were taken at week 52/53
and week 78/79 and analyzed for corticosterone. Urinary sodium and
potassium levels (as an indirect method of assessing aldosterone
levels) were determined in 13/rats/sex/group at week 52.
No treatment-related effects were seen on mortality, haematology,
corticosterone, urinary sodium and potassium levels or ophthalmoscopy.
A dose-related decrease in body weight gain was seen in females at 100
ppm and in both sexes at 1000 ppm. Food consumption was lower for
high dose males and females. At 1000 ppm plasma triglyceride levels
were reduced in males and in females. Cholesterol levels were
significantly increased in high dose female rats and urea levels
decreased during the first year. Increases in ALAT and ASAT
activities were observed in male rats at 1000 ppm. Urinary protein
excretion was significantly increased in high dose males up to week 25
which was reflected in lower pH values. There was a marked increase
in hepatic amino-pyrine-N-demethylase activity in both males and
females receiving 1000 ppm; a smaller but still significant increase
was observed in males at 100 ppm. Relative liver weight was
significantly increased at the highest dose (at interim sacrifice
females at 100 ppm also showed an increased relative liver weight).
Relative adrenal and kidney weights were increased in females at 100
and 1000 ppm after 52 weeks only.
Livers of high dose rats showed an accentuation of lobular
pattern, with or without swollen or enlarged lobes and pale spots at
the interim sacrifice as well as at termination. Microscopy revealed
a dose-related increased incidence of fatty changes, primarily
centrilobular in pattern in the liver of males at 1000 and 100 ppm and
in females at 1000 ppm. High dose rats also showed an increase in the
incidence of hepatocyte hypertrophy, with a slight dose-related
increase in microcystic degeneration of the liver in males at 1000 ppm
and a slight increase at 100 ppm.
An increased incidence of cortical vacuolation was observed in
the adrenal glands of mid and high dose males. A slight increase was
seen in cortical cysts in females at 1000 ppm. In the testes of rats
at 1000 and 100 ppm, a treatment-related increased incidence was seen
in benign Leydig cell tumours. The incidence was 2/52, 2/52, 4/52,
8/52 at 0, 10, 100 and 1000 ppm, respectively. Historical control
values for this finding are 0-14.4%. The NOAEL in this study was 10
ppm (equal to 0.47 mg/kg bw/day in males and 0.61 mg/kg bw/day in
females) (Hext, 1988a; 1988b).
Reproduction study
Rats
Groups of 15 male and 15 female ALpk:APfSD rats received
hexaconazole (purity 90.0%) in the diet at 0, 20, 100 or 1000 ppm.
After 12 weeks of treatment animals were mated to start a 2-generation
(2 litters/generation) study. F1 parents selected from F1a offspring
were mated after 11 weeks. At 1000 ppm body weight gain and food
consumption were decreased in F0 and F1 parents. A trend for a
decreased body weight gain was observed in F0 males at 100 ppm. At
the highest dose birth weight and weight gain to day 36 of F1a litter
offspring were significantly reduced. In both F2a and F2b litters
total litter weight was markedly reduced and F2b pup weight was also
reduced. Absolute as well as relative liver weights were increased at
1000 ppm for F0 and F1 parents and F1a, F2a and F2b pups.
Histopathology revealed evidence of fatty changes in the liver
with or without hepatocyte hypertrophy in male and female parents and
pups at 1000 ppm. At 100 ppm there was evidence of a similar but less
marked effect on the histopathology of the liver in both parents and
offspring. Cortical cell vacuolation of the adrenal gland was
observed in both male and female parents and offspring at 1000 ppm and
to a much lesser extent this was also found at 100 ppm. No adverse
effects were observed on reproduction parameters such as fertility
indices, length of gestation, pre-coital interval, litter size and
number of live and dead fetuses. The NOAEL in this study was 20 ppm
(equivalent to 1 mg/kg bw/day) (Middleton, 1988).
Special studies on embryo/fetotoxicity
Rats
Groups of 24 pregnant Wistar Alpk/AP rats were orally dosed by
gavage at 0, 2.5, 25 or 250 mg/kg bw/day hexaconazole (purity 92.3%)
in corn oil from day 7 to day 16 of gestation. Clinical signs,
mortality, body weight and food consumption were recorded. At day 22
of gestation animals were sacrificed and the fetuses were delivered by
cesarean section. The number and positions of implantations and
corpora lutea were determined. The fetuses were counted, sexed and
weighed and examined for external, visceral and skeletal
malformations. At 250 mg/kg bw/day, maternal body weight gain and
food consumption were significantly decreased. Post-implantation loss
was significantly increased. The mean number of live fetuses was
slightly reduced and pup weight was significantly lower at the highest
dose. The number of fetuses with minor defects only was significantly
increased at 25 and 250 mg/kg bw/day. The incidence of fetuses with
extra 14th thoracic ribs was significantly increased at 25 and 250
mg/kg bw/day. At 250 mg/kg bw/day the proportion of fetuses with
unossified calcanea and partially ossified 5th sternebrae was
significantly increased and the mean manus and pes scores were
also significantly increased in this group. In this study fetotoxic
effects were observed at 250 and to a lesser extant at 25 mg/kg
bw/day, but there were no indications for structural malformations
being associated with compound administration. The NOAEL for
fetotoxicity in this study was 2.5 mg/kg bw/day (Killick et al.,
1984a).
Rabbits
Groups of 18 pregnant New Zealand white rabbits were orally dosed
by gavage with 0, 2.5, 12.5 or 50 mg hexaconazole (purity 92.3%)/kg bw
in corn oil from days 7-19 of gestation. At day 30 of gestation
animals were sacrificed and the fetuses were delivered by Cesarean
section. No dose-related maternal toxicity was observed. The number,
growth, and survival of the fetuses were not affected by treatment.
After examination of the fetuses for external, visceral and skeletal
malformations, a slight increase (not significant) in partially
ossified 5th sternebrae was seen at 50 mg/kg bw only. The NOAEL in
this study was 12.5 mg/kg bw/day (Killick et al., 1984b).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
hexaconazole. The results are summarized in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on pharmacological effects
In vivo studies
In a rat behavioural study, a pull-up test for evaluating muscle
relaxation and a Halothane sleeping time test, central nervous system
(CNS) depression was observed at high doses (>500 mg/kg bw)
hexaconazole (purity 89.8%). The NOAEL for CNS depression in these
studies was 250 mg/kg bw/day.
Hexaconazole had no effect on the cardiovascular system as
determined by blood pressure measurements, heart rate or respiration
rate and gastrointestinal motility (Allen, 1988).
In vitro studies
At high concentrations a non-competitive antagonism of both
acetylcholine and histamine in guinea-pig ileum was observed.
Complete haemolysis of rabbit erythrocytes was induced by 0.03%
and 0.1% hexaconazole. No effects were observed on (alpha)1,
(alpha)2 or œ-adrenoceptors, nicotinic acetylcholine receptors or on
smooth muscle (Allen, 1988).
Special studies on skin and eye irritation and sensitization
A dose of 500 mg hexaconazole (purity not given) moistened with
0.5 ml of deionised water, was applied under occlusive conditions to
the shaven intact back skin of 6 male New Zealand white albino rabbits
for 4 hours. No skin irritation up to 72 hours after application
occurred (Southwood, 1984b).
Table 2. Results of in vitro genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Ames test1 S. typhimurium 1.6-5000 µg/ plate, 92.3% negative Callander, 1984
TA98, TA100, 2 tests
TA1535, TA1537,
TA1538
Ames test1 E. coli WP2 uvrA 1.6-5000 µg/pla 89.8% negative Callander, 1988
pKM101 2 tests2
Cytogenetics assay human lymphocytes 15-250 µg/ml, 92.3% negative Sheldon, et al., 1984a
>200 toxic3
20-250 µg/ml4
Lymphoma forward mouse L5178Y cells 7.8-125 µg/ml 92.3% negative Cross, 1986
mutation assay >70 µg:toxic5
Unscheduled DNA rat hepatocytes 10-4, 10-5, 90.0% negative Trueman, 1988
synthesis test 10-6 or 10-7 M6
1 Both with and without rat liver S9 fraction.
2 2-Aminoanthracene and N-methyl-N1-nitro-N-nitrosoguanidine were used as positive controls.
3 Without metabolic activation, mitomycin C was used as positive control.
4 With metabolic activation, cyclophosphamide was used as a positive control.
5 Dimethylsulfoxide was used as negative control and benzo (alpha)pyrene and
ethylmethanesulfonate were used as positive controls with and without metabolic activation, respectively.
6 6-p-dimethylaminophenylazobenzthiazole was used as a positive control.
Table 3. Results of in vivo genotoxicity assays on hexaconazole
Test system Test object Concentration Purity Results Reference
Micronucleus test C57/BL/6J mice 75 and 120 mg/kg 92.3% negative Sheldon, et al.,
(m+F) bone i.p.* 1984b
marrow cells
Dominant lethal test CD-1 male mice 10, 30 or 100 92.3% negative Wickramaratne,
mg/kg bw for 5 et al., 1984
cons. days*
* Cyclophosphamide was used as a positive control.
Nine New Zealand white albino rabbits were given doses of 100 mg
of undiluted hexaconazole (purity not stated) into the conjunctival
sac of the left eye. The eyes of 3/9 rabbits were washed. All
animals showed conjunctival redness, chemosis and discharge at 1 hour
after application reversible within 3 days except for slight discharge
that was still seen in 1/6 rabbits (unwashed) after 7 days (Southwood,
1984b).
Hexaconazole was tested for skin sensitization in 20 Dunkin
Hartley guinea pigs in a Magnusson Kligman test. The intradermal and
topical induction concentrations were a 0.5% solution of hexaconazole
(purity not given) in 4.5% dimethylformamide/corn oil and a 75%
suspension in dimethylformamide, respectively. After a challenge with
a 10% solution, 1/20 animals showed scattered mild redness; following
challenge with a 25% solution 12/20 animals showed scattered mild
redness. Following a re-challenge with a 25% solution 6/20 guinea
pigs gave a positive response (Southwood, 1984c).
Special study on steroid metabolism
Isolated Leydig cells, prepared from the testes of adult
Alpk:APfSD rats, were incubated with varying concentrations of either
hexaconazole (0.1-30µm) or ketoconazole (0.l-10µm) for a period up to
24 hours. After the incubation period testosterone, progesterone and
17-OH progesterone were analyzed using specific radio immunoassays.
The experiments were repeated in the presence of a maximally
stimulating dose of HCG (human chorionic gonadotropin). A dose-
related decrease in testosterone production, accompanied by an
increase in the production of progesterone and 17-OH progesterone was
observed after treatment with hexaconazole and hezaconazole in the
presence of hCG. Incubation with ketoconazole showed the same
effects, but ketaconazole is about 70-100 times more active than
hexaconazole (Foster, 1990).
The inhibition of testosterone production by ketoconazole was
also reported in several in vivo as well as in vitro studies in
humans, mice and rats (English et al., 1986; Pont et al., 1982;
Santen et al., 1983; Lambert et al., 1986)
Observations in humans
No information was available.
COMMENTS
Following oral administration to rats, hexaconazole was rapidly
and almost completely excreted via the urine and the faeces. The
total radioactivity excreted was equal for both males and females, but
females excreted a higher proportion of the dose in urine than in
faeces. The highest tissue residues were found in the liver,
intestinal contents and the adrenal cortex. Biliary excretion was
extensive, accounting for about 80% and 40% of the total radioactivity
in males and females respectively. About 50% of the radioactivity
excreted in the bile was reabsorbed by enterohepatic circulation.
Hexaconazole was metabolized via oxidation of the n-butyl chain
by two pathways. The more important pathway was conversion of
hexaconazole to 5-hydroxy-hexaconazole and 5-keto-hexaconazole; a
minor pathway involved two-stage oxidation of the terminal methyl
group to "hexaconazole acid" via 6-hydroxy-hexaconazole. Free
triazole was also formed by cleavage of hexaconazole or its
metabolites.
The compound showed slight to moderate acute oral toxicity in
rats and mice. WHO has classified hexaconazole as slightly hazardous
based on acute toxicity and has concluded that it is "unlikely to
present acute hazard in normal use" (WHO, 1990).
From acute as well as from short-term studies it appeared that
male rats were more sensitive than female rats. Short-term studies
with mice, rats and dogs indicated that the liver is the primary
target organ. Lipid accumulation in hepatic parenchymal cells was
observed, with associated disturbances in lipid metabolism and blood
chemistry. Elevated levels of aminopyrine-N-demethylase (rat)
suggest an adaptive response in the liver. In a one-year study in
dogs (capsule administration) the NOAEL was 2 mg/kg bw/day. However,
a 90-day dog study (capsule administration) indicated a NOAEL of 5
mg/kg bw/day. Since the next highest dose in the one-year dog study
was 10 mg/kg bw/day, the Meeting concluded that the appropriate NOAEL
for dogs was probably 5 mg/kg bw/day.
In the rat, cortical parenchymal vacuolation in the adrenal
glands was observed. In a short-term feeding study in mice, effects
on the male and female reproductive organs and on the adrenals were
observed. From in vitro studies it can be concluded that
hexaconazole inhibits testosterone production.
In a long-term feeding study in mice (highest dose level 200 ppm)
observed effects were reduced body weight gain, increased erythrocyte,
haemoglobin and haematocrit values and, in the liver, increased weight
and centrilobular fatty changes. The tumour incidence was not
enhanced. The NOAEL in this study was 40 ppm, equal to 4.7 mg/kg
bw/day for males and 5.9 mg/kg bw/day for females.
In a long-term feeding study in rats, the same effects as in the
short-term study were observed. In the testes, the incidence of
benign Leydig cell tumours was slightly increased. The NOAEL in this
study was 10 ppm, equal to 0.47 mg/kg bw/day in males and 0.6l mg/kg
bw/day in females.
After reviewing the available in vitro and in vivo short-term
genotoxicity tests, it was concluded that there was no evidence of
genotoxicity.
Embryotoxicity/fetotoxicity was observed in a teratogenicity
study in rats. No effects were observed at 2.5 mg/kg bw/day. Delayed
ossification was observed in a rabbit teratogenicity study at the
highest dose of 50 mg/kg bw/day; the NOAEL was 12.5 mg/kg bw/day.
In a two-generation reproduction study in rats, reproductive
performance was not affected. Parental body weight gain, food
consumption and pup weight were decreased. Histopathological
examination revealed fatty changes in the liver, either with or
without hepatocytic hypertrophy, and cortical vacuolation of the
adrenal gland in both parents and pups. The NOAEL in this study was
2.5 mg/kg bw/day.
An ADI was allocated based upon the NOAEL from the rat long-term
study (0.5 mg/kg bw/day), using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 40 ppm in the diet, equal to 5.0 mg/kg bw/day
Rat: 10 ppm in the diet, equal to 0.5 mg/kg bw/day
Dog: 5 mg/kg bw/day
Estimate of acceptable daily intake
0-0.005 mg/kg bw
Studies which will provide information valuable in
the continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, S.A. (1988) Hexaconazole: pharmacological evaluation.
Unpublished report no.: CTL/P/1970 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1984) PP523: An evaluation in the salmonella
mutagenicity assay. Unpublished report no.: CTL/P/977 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Callander, R.D. (1988) Hexaconazole - an evaluation of mutagenic
potential using E.coli. Unpublished report no.: CTL/P/2143 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Cross, M.F. (1986) PP523: Assessment of mutagenic potential using
L5178Y Mouse Lymphoma Cells. Unpublished report no.: CTL/P/1298 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Davison, V.M. (1988) Hexaconazole: Acute oral toxicity to the rat.
Unpublished report no.: CTL/P/2231 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO
by ICI Agrochemicals, Surrey, UK.
English, H.F., Santner, S.J., Levine, H.B. and Santen, R.J. (1986)
Inhibition of testosterone production with ketoconazole alone and in
combination with a gonadotropin releasing hormone analogue in the rat.
Cancer Res. 46, 38-42.
Forbes, D. (1988) Hexaconazole: 29-day feeding study in mice.
Unpublished report no. CTL/P/2204 from ICI Central Toxicology
Laboratory. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Foster, P.M.D. (1990) Effects of hexaconazole (ICIA523) on the
steroidogenic function of isolated rat Leydig cells, Unpublished
report no.: CTL/R/1035 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1987) PP523: 4-hour acute inhalation toxicity study in the
rat. Unpublished report no.: CTL/P/1731 from ICI PLC, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Hext, P.M. (1988a) Hexaconazole: two year feeding study in rats.
Unpublished report no.: CTL/P/1920 from ICI Central Toxicology
Laboratory, Macclesfield, Cheshire UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Hext, P.M. (1988b) Second supplement to hexaconazole: two year feeding
study in rats measurement of plasma corticosterone levels. Unpublished
report no.: CTL/P/1920 from ICI Central Toxicology Laboratory,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K., Galvin, G. Rhodes, S.J. and Soames, A.R. (1984) PP523:
Excretion and tissue retention of a single dose (1 mg/kg) in the rat.
Unpublished report no.: CTL/P/1148 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988a) Hexaconazole: repeat dose study (1mg/kg) in the
rat. Unpublished report no.: CTL/P/2089 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1988b) Hexaconazole: whole body autoradiography study in
the rat following 14 daily oral doses of 1 mg 14C-labelled
hexaconazole/kg. Unpublished report no.: CTL/P/2052 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Jones, B.K. (1989a) Hexaconazole: tissue distribution and elimination
following a single oral dose (1 mg/kg) in the rat. Unpublished report
no.: CTL/P/2389 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Jones, B.K. (1989b) Hexaconazole: tissue distribution and elimination
following a single oral dose (200 mg/kg) in the rat. Unpublished
report no.: CTL/P/2390 from ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Jones, B.K. (1989c) Hexaconazole: biotranformation in the rat with
first amendment. Unpublished report no.: CTL/P/1848 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984a) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1127 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Killick, M.E., Wickramaratne, G.A., Banham, P.B. and Thomas, M.R.
(1984b) PP523: Teratogenicity study in the rat. Unpublished report
no.: CTL/P/1131 from ICI Central Toxicology Laboratory, Alderley park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Kinsey, D.L., Hollis, K.J., Chart, I.S., Gore, C.W., Godley, M.J.
Pigott, G.H., Stonard, M.D. and Chalmers, D.T. (1984) PP523: 90-day
feeding study in rats. Unpublished report no.: CTL/P/1073 from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
UK. Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Lambert, A., Mitchell, R., and Robertson, W.R. (1986) The effect of
ketoconazole on adrenal and testicular steroidogenesis in vitro.
Biochem. Pharmacol., 25(22), 3999-4004.
Leah, A.M. (1989) Hexaconazole: acute oral toxicity to the mouse,
including the first amendment to the report. Unpublished report no.
CTL/P/2234 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Middleton, M.C. (1988) Hexaconazole: two generation reproduction study
in the rat. Unpublished report no.: CTL/P/1598 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Pigott, G.H. (1988) Hexaconazole: two year feed study in mice.
Unpublished report no. CTL/P/1929 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK
Pont, A., Williams, P.L., Azhar, S., Reitz, R.E., Bochra, C., Smith,
E.R. and Stevens, D.A. (1982) Ketoconazole blocks testosterone
synthesis. Arch. Intern. Med. 142, 2137-2140.
Santen, R.J., Van den Bossche, H., Symoens, J., Brugmans, J. and
DeCoster, R. (1983) Site of action of low dose ketoconazole on
androgen biosynthesis in men. J. Clinical Endocrin. Metab. 57(4), 732-
736.
Sheldon, T., Howard, A.C. and Richardson, C.R. (1984a) PP523: A
cytogenetic study in human lymphocytes in vitro. Unpublished report
no. CTL/P/1186 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J. and Barber, G. (1984b) An
evaluation of PP523 in the mouse micronucleus test. Unpublished report
no. CTL/P/1136 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
Southwood, J. (1984a) PP523: Acute oral and acute dermal toxicity
studies. Unpublished report no. CTL/P/1113 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984b) PP523: Skin and eye irritation studies.
Unpublished report no. CTL/P/1043 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Southwood, J. (1984c) PP523: Skin sensitisation study. Unpublished
report no. CTL/P/1049 from ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, UK. Submitted to WHO by ICI
Agrochemicals, Surrey, UK.
Stonard, M.D. (1989) PP523: 90-day oral dosing study in dogs and first
amendment. Unpublished report no. CTL/P/1137 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
Submitted to WHO by ICI Agrochemicals, Surrey, UK.
Stonard, M.D. (1988) Hexaconazole: 1 year oral dosing study in dogs.
Unpublished report no. CTL/P/1942 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trivedi, S., Jones, B.K. and Soames, A.R. (1986) PP523: Excretion and
tissue retention of a single oral dose (200 mg/kg) in the rat.
Unpublished report no. CTL/P/1431 from ICI Central Toxicology
Laboratory, Alderley park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Trueman, R.W. (1988) Hexaconazole: Assessment for the induction of
unscheduled DNA synthesis in primary rat hepatocyte cultures.
Unpublished report no. CTL/P/1887 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Submitted to
WHO by ICI Agrochemicals, Surrey, UK.
Wickramaratne, G.A., Bramley, J.R., Banham, P.B. and Godley, M.J.
(1984) PP523: Dominant lethal study in the mouse. Unpublished report
no. CTL/P/1110 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, UK. Submitted to WHO by ICI Agrochemicals,
Surrey, UK.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
