
FENTIN
First draft prepared by
Mrs. M.F.A. Wouters,
Mrs. J.E.M. van Koten-Vermeulen,
Mrs. Dr.E.M. den Tonkelaar,
National Institute of Public Health
and Environmental Protection,
Bilthoven, Netherlands
EXPLANATION
Fentin toxicity has been reviewed by the Joint Meetings in
1963, 1965 and 1970 (Annex I, 2, 3 and 14). Data include
biochemical studies in rats, sheep and cows, acute and short-term
tests in rats, guinea pigs and dogs, long-term studies in rats and
guinea pigs, reproduction studies in rats and immunotoxicity
examinations in rats and guinea pigs. The 1970 Joint meeting
established an ADI of 0-0.0005 mg/kg bw applicable to
triphenyltinhydroxide (TPTH), triphenyltinacetate (TPTA) and
triphenyl-tinchloride (TPTCl) and to the sum of TPTH + TPTA +
TPTC1. This ADI was based on a NOAEL of 0.1 mg/kg bw in a chronic
rat study with a safety factor of 200. New studies have become
available, which were evaluated by the present Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rat
Ten male rats received a single oral dose of 5 mg 14C-TPTH/kg
bw dissolved in 0.5 ml of ethanol. After 7 days 39.2% of applied
radioactivity was excreted in the urine and 51.5% in the faeces
(Bakke et al., 1982).
Two female rats received a single oral dose of 10 mg
14C-TPTH/kg bw. Within 72 hours 71.7% of applied radioactivity was
excreted in the faeces, 20.8% of the dose in the urine., 6.5%
remained in the carcass. Exhalation of 14CO2 was below 0.1%
(Lachmann & Siegemund, 1987).
Groups of 5 rats/sex received single oral doses of 2 or 10 mg
14C-TPTH/kg bw. Additionally repeated application was studied by
14 daily doses of 2 mg/kg and one dose of 2 mg 14C-TPTH/kg bw. The
faeces appeared to be the principal route of excretion (32-53% of
the dose). Approximately 12-24% was excreted via the urine.
Excretion was biphasic with half-lives of 9 and 62 hours. After 7
days concentrations of applied radioactivity were found in the
liver (0.14-0.16 mg/kg at 2 mg/kg, 0.17-0.21 mg/kg at repeated
treatment and 0.37-0.42 mg/kg at 10 mg/kg). In the kidneys
0.023-0.035 mg/kg was found at 2 mg/kg, 0.04-0.06 mg/kg at repeated
treatment and 0.13-0.25 mg/kg at 10 mg/kg. All other concentrations
found in tissues, blood and plasma were below 0.1 mg/kg or the
detection limit. Absorption was incomplete, with an average of 40%
of the dose. The recovery rates showed considerable variations,
ranging from one group to another between 48 and 84%. The expired
air contained less than 1% of the dose (Kellner & Eckert, 1986).
The authors explained the low and variable recovery by assuming
that a volatile metabolite (benzene) was formed and escaped from
the faeces and during processing of the analytical samples.
Two groups of rats received a single or seven daily oral
administrations of 2 mg 113Sn-TPTH/kg bw. Rats were sacrificed
after 1 and 7 days after the single dose and 1, 3, 7 and 14 days
after 7 daily doses. The radioactivity was excreted predominantly
via the faeces (96.2% to 100.8%) independent of sex and number of
doses. Excretion via the faeces was biphasic with half-lives of 9
and 53 hours. Only up to 1.4% was excreted via the urine, with a
half-life of about 45 hours. Seven days after single dosing the
amount of radioactivity in the examined tissues and organs
corresponded to about 2.8-3.2% of the administered radioactivity,
with highest concentrations in the kidneys (0.26-0.37 mg/kg), liver
(0.15-0.30 mg/kg), brain (0.09-0.11 mg/kg) and heart (0.07-0.10
mg/kg). One day after the first dose the highest concentrations
were detected in the liver (1.95 mg/kg), kidneys (1.78 mg/kg) and
walls of the g.i.t. (1.26 mg/kg). In all other organs the
concentrations were below 1 mg/kg. Radioactivity increased in the
organs during repeated dosage. Residues after multiple dosing were
up to seven times higher than those after single administration,
indicating accumulation of metabolites. Analysis of these data
showed that the cumulated residue would reach maximum values in all
organs after multiple dosing after 20-25 days. The concentrations
decreased after the last dose and had dropped to 0.67 mg/kg in
kidneys 14 days after the last administration. In the remaining
organs levels were below 0.5 mg/kg (liver, brain, skeletal
musculature, spleen, heart muscle) or 0.1 mg/kg (testes, lungs,
walls of gastro-intestinal tract) or were below the detection limit
(fat). The radioactivity in all organs/tissues accounted for 0.29%
of the administered dose at that time (Eckert et al, 1989;
Kellner & Eckert, 1989).
Three groups of 20 male rats received single dermal
applications with 0.09, 0.96 and 12.82 mg 14C-labelled TPTH/kg bw
as aqueous suspension, wrapped with a non-occlusive cover. After
periods ranging from 0.5 to 10 hours the rats were sacrificed.
Total radioactivity in excreta (urine) was less than 1% of applied
radioactivity. The amount excreted increased with increasing
application time. Measurable radioactivity levels were found in
blood of the mid and highest dose group. The highest concentration
was 0.019 mg/kg. Muscle tissue beneath the application site
contained low levels of residues, the highest concentration found
was 0.173 ppm. Total recovery in the mid and high dose group was
90.7 and 101.5%, respectively. The total amount absorbed was 34-38%
of which a very high percentage (>95%) was bound to the skin
(Resnis et al., 1986)
Three groups of 20 male rats received single dermal
applications of 0.11, 0.99 or 10.24 mg 14C-TPTH/kg bw as aqueous
suspension. After 10 hours exposure TPTH was washed from the skin
and about 45, 60 and 78% of applied radioactivity was removed from
the groups treated with 0.11, 0.99 or 10.24 mg/kg, respectively.
The remaining radioactivity is absorbed into the skin. At the
lowest dose about 2, 3.4, 26, 34 and 28% was penetrated through the
skin after 10 and 24 hours and 7, 14 and 21 days, respectively.
From these penetrated amounts 13, 27, 88, 91 and 99% was excreted
(cumulative). At higher doses the amount which penetrated skin is
lower, maximal 15 and 10%, respectively (Craine, 1987).
Rats were fed a diet containing 0, 5, 20 or 80 ppm TPTH for
104 weeks. Tissue residue levels were recorded after 52 and 104
weeks. The highest levels were observed in kidneys (up to 25 mg
total tin/kg after 52 weeks and 10.5 mg/kg after 104 weeks in the
80 ppm group) and liver (up to 9.5 mg total tin/kg after 52 weeks
and 3.1 mg/kg after 104 weeks in the 80 ppm group). The lowest
residue level was observed in blood (up to 0.11 mg total tin/kg in
the 80 ppm group) (Tennekes et al, 1989a; Dorn & Werner, 1989b).
Guinea-pig
From a single dermal application of 113Sn-TPTA 3.04% and 7.97%
was absorbed percutaneously through the skin 1 and 2 days after
application. About 16.3% and 12.4% remained in the applied area,
respectively (Nagamatsu et al., 1978)
After a single subcutaneous injection of 2 mg 113Sn-TPTA to
guinea-pigs about 21% remained in the body 20 days after
administration. The biological half-life was estimated as 9.4 days.
Radioactivity was mainly excreted in the faeces, the daily
excretion rate was highest in the early stage after injection. The
absorption of radioactivity from the injection site was rather
slow. About 18.5%, 4.3% and 0.1% of the dose were retained at the
injection site after 5, 10 and 20 days, respectively. The highest
concentration of radioactivity was noted in the liver, adrenal
glands, kidneys and brain. The parent compound, diphenyltin and
monophenyltin were identified in the faeces (Nagamatsu et al.,
1978).
Dog
After feeding a diet containing 0, 2, 6 or 18 ppm for 52 weeks
highest tin levels were found at the 18 ppm group in the liver (up
to 12 mg/kg) and kidneys (up to 3.25 mg/kg) while the lowest
concentration was found in blood (up to 0.04 mg/kg). The residue
levels were similar after 4 and 52 weeks (Sachsse et al., 1987)
(Dorn & Werner, 1989a).
Biotransformation
Triphenyltinacetate (TPTA) was rapidly and completely
hydrolyzed to triphenyltinhydroxide (TPTH) at pH 3-8 and at
23-24 °C. Therefore it is assumed that it will rapidly hydrolyze to
TPTH in vivo (Buerkle, 1985).
Groups of male and female rats received single oral doses with
2 or 10 mg 14C-TPTH/kg bw. Additionally repeated application was
studied by 14 daily administrations of 2 mg/kg were followed by one
dose of 2 mg 14C-TPTH/kg bw. The applied radioactivity was
excreted within two days via the faeces (50-70%) and urine
(10-20%). Repeated administration caused a higher rate of renal
excretion. In faeces the major radioactive substance present was
unchanged parent compound. Metabolites found included di- and
monophenyltin as well as a significant portion of non-extractable
bound residues. Also benzene has been shown to be evaporated from
the faeces. Repeated dosage lowered the proportion of TPTH whereas
the more polar degradation products were increased. The composition
of renally excreted material revealed a moderate difference between
the dosage levels. Low dosing resulted in the excretion of the
sulfate conjugates of hydroquinone, catechol and phenol whereas
high level dosing led to additional formation of resorcinol sulfate
and a medium polar metabolite. These metabolites are comparable to
those formed when benzene is given to warm-blooded animals.
Phenylmercapturic acid, which is an enzymatic degradation product
of phenyl glutathionate also occurred in trace amounts. Phenyltin
compounds were not detected in the urine. The proposed metabolic
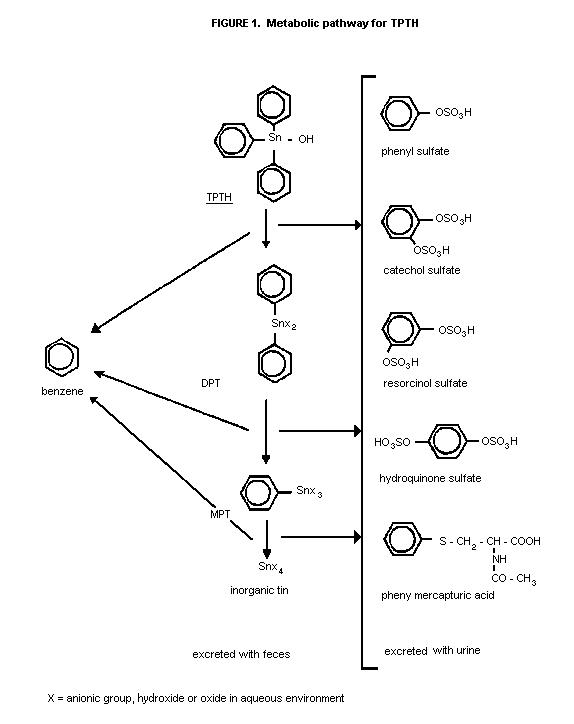
pathway is given in Figure 1 (Buerkle et al., 1986).
14C-TPTH (purity 87%) was administered orally at 2 mg/kg bw
by stomach tube to 3 male rats with a bile fistula. About 2.8% of
the applied radioactivity was excreted with bile within 30 hours
after dosing. In addition 11% of applied radioactivity was excreted
with the urine and 0.4% was detected in the cage wash. The
radioactivity excreted with the bile consisted of three extremely
to moderately polar metabolite fractions. Due to acid hydrolysis
their polarity decreased. Unchanged parent compound was not
excreted via the bile (Buerkle & Kellner, 1987).
Groups of rats received a single oral dose of 2 mg
113Sn-TPTH/kg bw. Within 30 hours after administration 6.2% (male)
and 2.8% (female) of applied radioactivity was excreted via the
bile. Urinary excretion was low and amounted up to 0.07%. After 30
hours the carcass of male rats (excluding gastrointestinal tract)
contained 21.6% of the dose and the carcass of female rats
contained 8.9%. Bile contained besides 14% parent compound, 40%
diphenyltin, 21% monophenyltin, and 25% soluble inorganic tin
(Kellner et al., 1989).
 A 576 kg milk cow received a single oral dose of 5055 g
14C-TPTH and a 74 kg ewe received a dose of 1011 g 14C-TPTH, in
capsules. In the cow after 6 days 12.9% of radioactivity was
excreted in urine and 78% in the faeces. Tissues contained 5.1%.
Maximum radioactivity levels were found in the liver (5.5 mg/kg),
kidney and adrenals (both 2.2 mg/kg). In the sheep, 32.6% of
applied radioactivity was found in urine and 47.5% in the faeces
after 8 days. Tissues contained 3.1%. The liver showed highest
level of radioactivity, 6.6 mg/kg. Triphenyltinchloride was
identified as the major faecal excretion product. Phenol,
hydroquinone, catechol and resorcinol were isolated from the urine
(Bakke et al., 1982).
Toxicological studies
Acute toxicity
The LD50 and LC50 values for TPTH and TPTA in various species
are given in Tables 1 and 2, respectively.
The observed intoxication signs following oral exposure
include anorexia, emesis, tremor and diarrhoea followed by
drowsiness and ataxia. After dermal exposure to TPTH, skin
irritation was observed.
Five applications of 50 mg TPTA (purity not stated)/kg applied
to the nuchal skin of 5 rats caused no mortality but some signs of
local irritation. Five applications of 100 mg/kg to 5 rats caused
death of one animal and local signs of irritation. Five
applications of 200 mg/kg were lethal to rats. (Summary only)
(Hollander & Weigand, 1974d).
A single dermal application of 12.5 mg TPTA/kg to 3 rabbits
was lethal for 2 rabbits and caused black coloration of the skin
and scarcely pilous sites of the body. (Summary only) (Hollander &
Weigand 1974d).
Table 1. Acute toxicity of TPTH
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/m3)
Rat M oral ? 165 Leist & Weigand (1981a)
F oral ? 156 Leist & Weigand (1981b)
F perc. ? 1600 Leist & Weigand (1981c)
M&F inhal.a 97.0% 60.3 Hollander & Weigand (1981)
Rabbit M perc. ? 127 Leist & Weigand (1981d)
Dog M&F oral ? »100 Brunk & Weigand (1981)
a indicates 4 hour exposure
Table 2. Acute toxicity of TPTA
Species Sex Route Purity LD50 LC50 Reference
% (mg/kg bw) (mg/m3)
Mouse M oral 99 81.0 Ueda & Iijima (1961)
M oral 99 93.3 Ueda & Iijima (1961)
M s.c. 99 44.0 Ueda & Iijima (1961)
M dermal 99 350 Ueda & Iijima (1961)
Rat F oral ? 298 Scholz & Weigand (1969b)
F oral ? 140 Hollander & Weigand (1974a)
M&F dermal 97 >2000 Diehl & Leist (1986b)
M i.p. 97 3.6 Diehl & Leist (1986a)
F i.p. 97 12.7 Diehl & Leist (1986a)
M inhal. 97 44a Hollander & Weigand (1986)
F inhal. 97 69a Hollander & Weigand (1986)
Guinea pig ? oral ? 25 Hollander & Weigand (1974b)
Rabbit M oral ? 80 Hollander & Weigand (1980)
a indicates 4 hour exposure, whole body exposure, dust inhalation
Short-term studies
Mice
Male and female NMRI mice (10/sex/group) were fed diets
containing 0, 4, 20 or 100 ppm TPTH technical (purity 97.2%) for
three months. No compound-related effects were observed on
mortality, clinical signs, food consumption, body weight,
macroscopy and histopathology. At 100 ppm several haematological
and biochemical parameters were affected. Erythrocyte count and Hb
were decreased and platelet count increased in males and females.
The incidence of Heinz bodies was increased in males and MCH and
MCHC were decreased in females. Mouse immunoglobulins (IgA and IgG)
were decreased, IgM levels were decreased in females only. In
female mice total protein, albumin and alpha-globulin were
increased and gammaglobulin was decreased. Liver weight was
increased in males and females and a decrease was observed in the
relative weights of ovaries, adrenals, kidneys, heart and brain in
females. The NOAEL in this study is 20 ppm, equal to 3.4 mg/kg bw
(males) and 4.1 mg/kg bw (females) (Suter & Horst, 1986a).
Rats
In a range-finding study groups of rats (Hoe: WISKf;
10/sex/group) were fed diets containing 0, 5, 10, 20, 40 or 80 ppm
TPTH (97.0%) for 30 days. No compound-related effects were observed
on clinical signs, water consumption, urinalysis, organ weight,
macroscopy nor histopathology. In male rats at 80 ppm body weight
gain was decreased. Food consumption was decreased during the first
weeks in males at 80 ppm and females at 40 and 80 ppm. An increase
in thrombocyte count was observed in female rats at 40 and 80 ppm;
at the highest dose a decrease in Hb was observed in males and a
decrease in haematocrit was observed in females. At the highest
dose bilirubin, creatinin (males only), SGPT and urea (males only)
were increased. SAP was increased in a non dose-related manner in
males at 20 ppm and higher and in high-dose females. There was no
effect on spleen weight. The NOAEL in this study is 20 ppm equal to
2.01 mg/kg bw (males) or 1.83 mg/kg bw (females) (Leist et al.,
1982).
Groups of Wistar rats (15/sex/group) received dietary
concentrations of 0, 4, 20 or 100 ppm TPTH-technical (purity 97.2%)
for 13 weeks. The treatment period was followed by a 4-week
recovery period (5 male and 5 female rats/group). Observations
included clinical signs, food consumption, body weight,
ophthalmoscopy, hearing tests, haematology, clinical chemistry,
organ weights, macroscopy and histopathology (54 tissues; only in
control and high-dose rats). After 13 weeks of treatment the
following parameters were significantly affected; mean body weight
gain and food consumption were slightly decreased in high-dose
rats. RBC was increased, MCV (in males also at 20 ppm) and MCH were
decreased. In females WBC decreased at 20 and 100 ppm. Most
biochemical parameters were significantly affected at the highest
dose. The observed increase in ASAT was also apparent at 20 ppm as
was the decrease in albumin in females (also at 4 ppm). IgG was
slightly lower. The 18-hour urine volume was increased and the
specific gravity was decreased at 100 ppm. Relative testes weight
was significantly higher in high-dose males. After the recovery
period ASAT was still increased at 100 ppm and IgG was decreased in
males and significantly in females at all dose levels. No effects
were observed on spleen and thymus weights. The NOAEL in this study
is 4 ppm equal to 0.30 mg/kg bw (males) or 0.35 mg/kg bw (females)
(Suter & Horst, 1986b).
Four different experimental groups received 21 dermal
applications of 0, 5, 10 or 20 mg TPTA (purity unknown)/kg bw/day
in a 29-day period. Dosage groups consisted of 12 rats/sex in the
0 and 20 mg/kg groups and of 6 rats/sex in the 5 and 10 mg/kg
groups. At the end of the treatment period 6 rats/sex of the 0 and
20 mg/kg groups were kept for a 14 day recovery period. Rats were
observed for clinical signs, body weight, food consumption,
haematology, blood chemistry, urinalysis, organ weights, macroscopy
and histopathology. Piloerection, mydriasis, increased muscle tones
and dyspnoea were observed in high dose rats. Dose-related increase
in erythema and scale formation was observed in all treated groups.
Dose-related cracked skin and thickening of the skin fold was
observed at 10 and 20 mg/kg groups. Crusts and slight oedema were
observed in some animals at 20 mg/kg. At 20 mg/kg 4 animals died.
Lymphocytes decreased in males and females at 20 mg/kg. Monocytes
increased in females at 20 mg/kg. Sodium level decreased in all
treated males (n.d.r.) and females. Potassium decreased in 20 mg/kg
females. Inorganic phosphorus and total protein increased in 20
mg/kg females. A/G-ratio and albumin increased in 20 mg/kg males.
A1-globulins decreased in 20 mg/kg males. Macroscopy and
microscopy showed dose-related skin lesions such as hyperkeratosis
and epidermal hyperplasia in all treated groups. All effects were
reversible. No effects on spleen weights were observed. The NOAEL
for systemic toxicity in this study is 10 mg/kg bw (Leist, 1988).
TPTH (purity 97.1%) was applied to the shaven unabraded backs
of Charles River rats (10/sex/group) at doses of 0, 5, 10 or 20
mg/kg bw for 15 applications in 3 weeks. No treatment-related
effects were observed on food consumption, haematology, clinical
chemistry, urinalysis nor organ weights. Male body weight gain was
very slightly decreased in all treated groups and significantly
decreased during the first week at the highest dose. Dermal
irritation, increasing with each application, was observed in all
treated rats during the first 2 weeks. At the third week the
irritation was less severe and severe erythema was not present any
more. At macroscopy an increased incidence in ulcerated, exfoliated
and thickened skin was observed in all treated groups. Microscopic
evaluation of the treated skin revealed acanthosis and
hyperkeratosis. Also changes such as focal ulceration and mild
dermal inflammatory changes correlated with the observed dermal
irritation were seen. No systemic toxicity was observed (Laveglia
et al., 1985).
Groups of SPF-Wistar rats (10/sex/group) were exposed to 0.05,
0.5 and 2.0 mg TPTH (purity 96.2%)/m3 for 6 hours/day, 5 days/week
for 13 weeks (nose only exposure). Analytical concentrations were:
0.014, 0.338 or 1.997 mg/m3. The MMAD was 3 µm. The reversibility
of treatment-related changes was studied after a 4-week recovery
period in the control and highest dose group with 10 additional
animals/sex. Observations were made for clinical signs, body
weight, food consumption, ophthalmoscopy, haematology and clinical
chemistry, organ weights (6), macroscopy and histopathology. In the
highest dose group 10 males and 1 female died between test weeks
2-13 compared to none in the other groups. Laboured respiration and
rales were observed only in the highest exposure group. These signs
were still noted during the first week of recovery. RBC, Hb and Hc
were decreased in high exposed females, while platelet count was
increased. WBC was decreased in females at 0.5 and 2.0 mg/m3.
Glucose and total bilirubin were decreased in females at 0.5 and
2.0 mg/m3. Creatinine, albumin, gamma-glutamyltransferase and
potassium levels were decreased in females at 2.0 mg/m3. Calcium
and phosphorus level were decreased in all exposed female groups.
Calcium level decreased also in males at 0.5 and 2.0 mg/m3. IgG
increased in females at the high exposure level and IgM increased
in males at 0.5 and 2.0 mg/m3. Relative lung weights increased in
high exposed males, but no effect on thymus or spleen weight was
observed. Macroscopic examination showed lesions in the form of
multiple red foci in the lungs of most dead rats. Histopathology
revealed in both sexes at 2.0 mg/m3 degenerative and/or
inflammatory lesions in the anterior part of the nasal cavity, in
the trachea and in the lungs. The lower air passages and in
particular the lungs were severely affected. The pathomorphologic
sequence of events in the lungs seemed to start with degeneration
and multifocal necrosis of the bronchial epithelium followed by
multifocal or lobular fibrinous bronchopneumonia. The NOAEL in this
study is 0.05 mg/m3 (Duchosal et al., 1989).
Groups of 10 rats/sex were fed diets containing TPTA (purity
not given) at a rate of 0, 37.5, 150 or 625 ppm, during 30 days. No
effects were found on haematology and urinalysis. Seven rats/sex
from the 625 ppm group died during the study. Signs in this group
were piloerection and deterioration of the general condition. At
150 and 625 ppm temporary body weight gain decreases were seen.
Histopathology showed gastrointestinal haemorrhages and hepatic
changes (effects on cell metabolism) in the 625 ppm group. The
NOAEL in this study is 37.5 ppm, equivalent to 1.9 mg/kg bw.
(Summary only) (Hollander & Weigand, 1974c).
Guinea-pigs
Groups of guinea pigs (10/sex/group) were fed a diet
containing TPTA (purity not given) at a rate of 0, 7.5, 30.0 or 125
ppm. No effects were found on haematology nor urinalysis. In the
125 ppm group 8 male and 5 female guinea pigs died during
treatment. Effects found in this group included poor general
condition, piloerection and decrease in body weight gain.
Histological evaluation revealed fatty changes in the livers of
guinea-pigs treated with the highest dose (summary only) (Hollander
& Weigand, 1974c)
Dogs
Male and female dogs (beagle, 10/sex/group) received a diet
containing 0, 2, 6 or 18 ppm TPTH (purity 97.2%) for 52 weeks.
Interim necropsy on 2 dogs/sex/group was performed after 4, 13 and
27 weeks. Observations were made for clinical signs, food
consumption, body weight, hearing, ophthalmoscopy, haematology,
clinical chemistry (including immunoglobulins) and urinalysis. The
weights of 12 organs/animal were recorded (including spleen and
thymus). Gross necropsy and histopathology (about 40
tissues/animal) were carried out. A/G-ratio decreased in males at
18 ppm after 26 and 52 weeks. In females albumin decreased at 6 and
18 ppm after 52 weeks. In females at 18 ppm relative liver weight
was increased. The NOAEL in this study is 6 ppm, equal to 0.21
mg/kg bw (Sachsse et al., 1987)
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
37.5 or 75 ppm TPTH (purity ?) for 78 weeks. There was an
additional observation period of 26 weeks for both groups. Twenty
male and 20 female mice were used as controls. Dosed feed
preparations containing 37.5 or 75 ppm TPTH were analyzed
spectrophotometrically. 88% of the theoretical amount of TPTH was
recovered immediately after preparation but after 1 week only 57.9%
was recovered. The data were not corrected for any loss which may
have been due to the instability of TPTH. No treatment-related
effects were observed on clinical signs and body weight. Survival
was decreased in female mice (survival for males was: 65%, 72% and
76% and for females 95%, 74% and 66% for 0, 37.5 and 75 ppm,
respectively). At histopathology no increase in tumour incidence
was observed (NTP, 1978)
Groups of CD1-Charles River mice (60/sex/group) were fed diets
(prepared at weekly intervals) containing 0, 7, 28 or 52 ppm
technical TPTH (purity 96.3%) for 18 months. Observations included
clinical signs, mortality, body weight, food consumption,
macroscopy and histopathology (19 tissues). Survival rate was
significantly decreased in male mice in the mid and highest dose
groups (70%, 70%, 41% and 41% at 0, 7, 28 and 52 ppm,
respectively). Survival rates for females were 55%, 71.7%, 53.3%
and 58.3% at 0, 7, 28 and 52 ppm, respectively). The frequency of
aggression, alopecia, irritation, laceration and scabs was
dose-relatedly increased in both males and females. Food
consumption was reduced during some short periods in all treated
females. At histopathology an increase in the occurrence of
endometrial hyperplasia with cyst formation of the uterus was
observed at 28 and 52 ppm. The tumour incidence was not increased
(Terrel & Reddy, 1978). Extensive histomorphologic examination of
the uterus revealed that the observed cystic endometrial
hyperplasia was considered to be a hyperplastic reaction and not
neoplastic (Brown, 1985).
Groups of 50 male and 50 female mice (KFM-Han. NMRI) were fed
diets (prepared fresh daily) containing 0, 5, 20, 80 ppm TPTH
(purity 97.2%) for 80 weeks. Observations were made for clinical
signs, body weight, food consumption, ophthalmoscopy, haematology
(differential wbc count), clinical chemistry (including
immunoglobulin classes and subclasses) and organ weights (no spleen
weight). Gross pathology and histopathology (35 tissues including
bone marrow smears) were carried out. Intercurrent mortality was
significantly increased for 80 ppm females. At 80 ppm mice showed
ruffled fur towards the end of the study as well as skin lesions.
Body weight gain was reduced for 80 ppm males and 20 and 80 ppm
females. IgG, IgA, IgM, IgG4(f), IgG2a(f), IgG2b and IgG3 were
slightly or moderately decreased at 80 ppm. The decrease in IgA was
also found at 20 and 5 ppm (n.d.r.) and in IgM at 20 and 5 ppm in
male mice. Relative liver weight was markedly increased and
relative brain weight slightly increased at 80 ppm males and
females. Relative kidney weight was decreased at 80 ppm in males
and females. Macroscopic examination showed nodules, foci and
nodular lesions in the livers of mice in 80 ppm males. Skin lesions
were observed in both sexes at 80 ppm. Microscopy showed a higher
incidence of slight to moderate, focal to multifocal, nodular
hyperplasia in the liver of 80 ppm males and females. A higher
incidence of hepatocellular adenomas was observed at 80 ppm
(incidence: 12.2%, 20%, 26% and 32% for 0, 5, 20 and 80 ppm,
respectively, for male mice, and 0% in 0, 5 and 20 ppm females and
18% in 80 ppm females; only significant at 80 ppm). An increased
incidence in hepatocellular carcinomas was observed in 80 ppm
females. The incidence was 6% compared to 0% in the 0, 5 and 20 ppm
groups. An increased incidence of chronic suppurative dermatitis
and related non-specific skin lesions including acanthosis and
hyperkeratosis was observed in 80 ppm males and females. An
increased relative number of lymphoid cells was detected in femoral
bone marrow myelograms for all treated groups. The relative numbers
of granulocytes were significantly decreased for females at 5 ppm
and both sexes at 20 and 80 ppm. The relative numbers of
erythrocyte precursors were significantly decreased for males at
20 and 80 ppm. However, it is difficult to evaluate these data in
the absence of red cell counts in peripheral blood. Although a
reduction in serum immunoglobulin concentrations was reported, the
Meeting noted that there was no corresponding effect on
haematological parameters and that the method of analysis has not
been validated in rodents. The data on serum immunoglobulin
concentrations was therefore not utilized in the determination of
the NOAEL for this study. Based on reduced body weight gain, the
NOAEL is 5 ppm, equal to 1 mg/kg bw/day (Tennekes et al., 1989a).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
prepared weekly containing 37.5 or 75 ppm TPTH (purity not stated)
for 78 weeks. There was an additional observation period of 26
weeks for both groups. Twenty male and 20 female rats were used as
controls. Feed preparations containing 37.5 or 75 ppm TPTH were
analyzed spectrophotometrically. Of the theoretical amount of
TPTH, 80% was recovered immediately after preparation but after 1
week only 57.9% was recovered. The data were not corrected for any
loss which may have been due to the instability of TPTH. No effects
were observed on clinical signs, mortality, food consumption,
macroscopy nor histopathology (31 tissues including pituitary gland
and testes). Body weight was slightly but dose dependently
decreased in male rats after week 40. Survival rates were 75%, 76%
and 84% for males and 90%, 80% and 90% for females at 0, 37.5 and
75 ppm TPTH. The tumour incidence was not enhanced (NTP, 1978).
Groups of 70 rats/sex (SPF-bred KFM-Han. Wistar) rats received
a diet containing 0, 5, 20 or 80 ppm TPTH (purity 97.2%) for 104
weeks. The diets were prepared twice monthly and frozen. A fresh
amount of diet was given daily. Observations were made for
clinical signs, food consumption, body weight, ophthalmoscopy,
haematology, clinical chemistry (including immunoglobulin classes
and subclasses), urinalysis, organ weights, macroscopy and
histopathology (about 40 tissues/animal, including bone marrow
smears). Mortality was increased (dose-related) in all treated
females. The survival was 75, 51, 36 and 23%, respectively.
Preceding mortality clinical signs were ruffled fur, reduced
activity, ataxia, stiff gait and hunched posture. Due to this
excess mortality no laboratory investigations could be made for the
80 ppm female group at 104 weeks. Group mean food intake was
reduced for males and females at 80 ppm. Body weight gain was
dose-relatedly decreased in males and females at 20 and 80 ppm.
Ophthalmoscopy showed an increased incidence of unilateral or
bilateral corneal opacity in males at 20 and 80 ppm at 104 weeks.
Hb and haematocrit were decreased in female rats at 20 and 80 ppm.
ASAT, ALAT, ALP were increased in males and females at 80 ppm. The
following changes in immunoglobulin levels were observed only after
50 weeks: IgG1 levels were reduced for all treated females and 80
ppm males, IgG2a levels were reduced for all treated females and
IgG2c levels were reduced for all treated males, IgA levels were
reduced for males at 20 and 80 ppm and IgM levels were increased
for both sexes at 20 and 80 ppm. At termination most relative organ
weights were increased in the 80 ppm groups, including spleen
weight. An increased incidence of pituitary adenomas was diagnosed
in females at 20 and 80 ppm. The recorded incidences were 64.4%,
63.2%, 76.8% and 93.1% for 0, 5, 20 and 80 ppm groups,
respectively. An increased incidence of testicular Leydig cell
tumours was diagnosed at 80 ppm. The recorded incidences were 1.7%,
8.5%, 5.0% and 16.7% for 0, 5, 20 and 80 ppm, respectively.
Non-neoplastic lesions observed included an increase in hyperplasia
of the pars intermedia of the pituitary gland in males at 80 ppm.
An increased incidence of cystoid change of the pars intermedia of
the pituitary gland was noted in males at 20 and males and females
at 80 ppm at 52 weeks. Increased incidences of testicular Leydig
cell hyperplasia and tubular atrophy were noted in rats at 80 ppm
after 104 weeks. Increased incidences of hepatic bile duct
proliferations and portal sclerosis were noted in females at 20 and
80 ppm after 52 and 104 weeks of treatment. An increased incidence
of skeletal muscle atrophy was noted in male rats at 80 ppm after
104 weeks of treatment. Because mortality was increased in females
and serum immunoglobulins were decreased at the lowest dose level
of 5 ppm (equal to 0.3 (males) 0.4 (females) mg/kg bw no NOAEL
could be established (Tennekes et al., 1989b).
Reproduction studies
In a range-finding study Wistar rats (10/sex/group) were
administered 0, 12.5, 25, 50, 100 or 200 ppm TPTH (weekly prepared,
purity not stated) in the diet for in total 90 days (from 59 day
pre-mating period up to 21 day gestation period). All animals were
killed following weaning. Observations included clinical signs,
mortality, body weight, organ weight and food consumption. Parents
were observed haematologically and histopathologically. Pups were
sexed, weighed and observed for gross abnormalities. Severe
clinical signs like rough coat, hunched back, lethargy, nasal
discharge and alopecia were observed in rats at 200 ppm. At 200 ppm
body weight gain was significantly reduced in males and food
consumption was slightly reduced in male and female rats. White
blood cell count was significantly decreased in high dose females.
A lower pregnancy rate (4/10) was observed in females at 200 ppm,
no viable offspring resulted. At 100 ppm pup survival, pupweight
and litter size (at 50 ppm not significantly) were dose-relatedly
decreased. At histopathology necrosis of the renal papilla or
medulla was observed in males at 100 and 200 ppm. One high-dose
male rat that died after six weeks showed hepatic, thymic and
testicular necrosis (Carlton & Crisp, 1982).
In a two-generation reproduction study groups of Wistar
(Crl:(WI)BR)COBS rats (30/sex/group) were given diets (prepared
every 5-7 days) containing 0, 0', 5, 18.5 or 50 ppm TPTH (purity
97.2%) during growth, mating, gestation and lactation for one
litter per generation. Observations included clinical signs, body
weight, food consumption, mating performance and reproductive
parameters. Organ weights (including spleen and thymus) of parents
and pups were recorded and the parents were examined histologically
(control group 1 and 50 ppm groups only). Pups were sexed, and
examined for gross malformations and the number of stillborn and
live pups were recorded.
Body weight gain and food consumption were occasionally
decreased in F0 parents at 50 ppm. The number of dead F1 pups
increased and mean litter size decreased at 50 ppm. Mean pup weight
was decreased at 50 ppm on lactation days 14 and 21. In F0 parents
the body weight gains and food consumption of both sexes were lower
at 50 ppm and occasionally at 18.5 ppm. Mean litter size decreased
at 18.5 and 50 ppm. Viability of F2 pups at 50 ppm, prior to
culling was slightly decreased. Mean pup weight was decreased at 50
ppm on lactation days 7, 14 and 21. At 18.5 mg/kg a slight decrease
was seen. Pups from one litter at 50 ppm appeared emaciated on
lactation day 21. Several pups in another litter in this group had
wet yellow urogenital staining on lactation day 21. At 50 ppm the
relative weights of brain, testes, ovaries, adrenals, kidneys
spleen and heart were increased at several occasions in F0 and/or
in F1 adults and/or F1 and F2 weanlings (males and females). At
18.5 ppm increases were also observed for kidneys, testes and heart
weights, whereas at 5 ppm only relative heart weight was increased
in male F1 adults. A dose-related decrease was observed in spleen
and thymus weight in F2 male and female weanlings at 50 and 18.5
ppm. In F1 weanlings the same trend was observed. The NOAEL in
this study is 5 ppm, equal to 0.4 mg/kg bw/day (Young, 1986).
Two groups of 13 male rats (29 days old) received a diet
containing 0 or 20 mg TPTA/kg bw for 25 days. Four animals from
each group were sacrificed on day 21 and the remaining animals
received the test diet for another 4 days and a control diet for an
additional 70 days at which time they were sacrificed. Observations
were made for the distribution of the eight phases of
spermatogenesis. In rats sacrificed after 21 days all 8
spermatogenic phases were seen, but there was a general paucity of
mature sperm and the distribution showed some predominance of
immature sperm. Recovery was seen after the 70 days control diet
(Snow & Hays, 1983).
Special studies on embryotoxicity and/or teratogenicity
Rat
Pregnant Sprague-Dawley rats (20/group) received by gavage 0,
1.25, 5, 8.75 or 12.5 mg/kg TPTH (purity unknown) bw/day, day 6
through 15 of gestation. The rats were sacrificed on day 20 of
gestation. Observations were made for body weight, number of
implantations, number of live and dead fetuses, number of
resorptions and number of corpora lutea. The fetuses were weighed
and examined for external, visceral and skeletal abnormalities.
Three animals each in the 8.75 and 12 mg/kg groups aborted. Only
those animals showed abnormal behavioural reaction. One female in
the 12.5 mg/kg group died. Females at 8.75 and 12.5 mg/kg showed a
lower food consumption and lower body weight gain (dose-
relatedly). In the 8.75 and 12.5 mg/kg groups an increase in
resorptions was observed. Fetuses from these groups also exhibited
a lower weight and a decrease in viable fetuses. A non dose-related
increase in hydrocephalus and hydronephrosis was seen in all
treated groups (2%, 5%, 12%, 16% and 6%, respectively) (Ravert &
Parke, 1976).
In a teratogenicity study (Segment II + III) groups of 25
pregnant Sprague-Dawley rats were given oral daily doses of 0,
0.35, 1, 2.8 or 8 mg TPTH (purity 97.1%)/kg b.w. in corn oil from
day 6 or 15 of gestation. All animals were allowed to deliver
naturally and rear the offspring to weaning on lactation day 21. At
lactation day 4, 10 pups/litter were selected and killed on the
28th day. The dams were killed on day 21 of lactation. Dams were
observed for mortality, clinical signs and body weight. Litters
were examined for gross malformations and the number of live and
dead fetuses was recorded. Fetuses were observed for bodyweight and
examined for haematology, clinical chemistry and urinalysis,
macroscopy, kidney weight and histopathology of the kidney. Dams at
8 mg/kg bw showed lethargy, yellow staining in the urogenital area,
and a more extensive hairloss than dams in the other groups. At the
same dose group maternal body weight gain was significantly
decreased (only during treatment) and gestation length was
significantly increased, and pup survival was decreased on
lactation day 0. Only LDH values in blood decreased in pups at 1,
2.8 and 8 mg TPTH/kg bw and ASAT was decreased in females pups at
all doses. Globulin levels were significantly decreased in female
pups at 2.8 and 8.0 mg/kg bw and A/G ratio was increased at the
highest dose. Relative kidney weight in pups was significantly
increased at 2.8 and 8 mg/kg bw. The dose of 1 mg/kg bw can be
regarded as a NOAEL for embryotoxicity (Rodwell, 1985a).
Twenty to 26 pregnant rats per group received by gavage 0,
1.0, 2.8 and 8 mg TPTH (purity unknown)/kg bw/day suspended in corn
oil, day 5 through 19 of gestation. On day 20 all females were
sacrificed. Uterus weight was determined and corpora lutea were
counted. Fetuses and resorption sites were noted. Fetuses were
weighed, sexed and observed for external, internal and skeletal
malformations. In the 2.8 and 8 mg/kg groups a dose-related nasal
and oral discharge were observed. In the highest group two animals
died. Body weight gain was significantly reduced at 8 mg/kg. The
number of dead or resorbed fetuses was higher at 8 mg/kg. Pup
weight was depressed in the 8 mg/kg group. The occurrence of
hydro-ureter was increased at the highest dose (Carlton & Connell,
1981; Carlton, 1985).
In order to clarify the occurrence of hydronephrosis,
hydrocephalus and hydroureter, another rat study was carried out.
Groups of 45 pregnant rats received 0, 0.35, 1, 2.8 and 8 mg TPTH
(purity 97.1%)/kg bw/day day 6 through 15 of gestation. Day 20 of
gestation all rats were sacrificed. Throughout gestation
observations were made for clinical signs, body weights and food
consumption. After sacrifice the dams were observed for number and
location of viable and nonviable fetuses, early and late
resorptions and the number of implantation sites. The corpora lutea
were counted. Fetuses were weighed, sexed and examined for
external, internal and skeletal anomalies. During the treatment
period, hair loss was more extensive in the 8 mg/kg group. A few
animals in this group were emaciated, lethargic and had yellow
staining in the urogenital area. One female in this group had an
abortion. A dose-related decrease in body weight gain and food
consumption was apparent in the 2.8 and 8 mg/kg groups. Number of
nongravid females, number of total litter resorptions and early
resorptions increased at 8 mg/kg. Number of viable fetuses and
fetal weight decreased significantly at 8 mg/kg. The incidence of
absent or delayed ossification in high dosed litters was increased.
The percentage of fetuses with hydrocephaly was 0.4, 0, 0, 0.4 and
1%, respectively, and for fetuses with omphalocele 0.2, 0.2, 0.2 0,
and 0.5%, respectively for the 0, 0.35, 1, 2.8 and 8 mg/kg groups.
The NOAEL for maternal toxicity was 1 mg/kg bw and the NOAEL for
embrytoxicity was 2.8 mg/kg bw. There was no evidence for
TPTH-induced irreversible structural effects (Rodwell, 1985b).
In a special study consisting of three tests the potential of
TPTH to induce alterations in the urogenital tract of exposed rat
fetuses was investigated. In test I pregnant Sprague Dawley rats
received 0, 4 or 8 mg TPTH (purity unknown)/kg bw by gavage during
day 7 through 20 of gestation. In test II (a and b) doses of 0 and
4 mg/kg bw were given. All females from test I and IIa were
sacrificed on day 21 of gestation. The uteri were removed, weighed,
and examined to determine the number of live, dead and resorbed
fetuses. Live fetuses were examined for gross external anomalies.
In test IIb the animals delivered normally and weaned their young
for 28 days. In these young animals renal morphology and function
were examined. At 8 mg/kg bw maternal (death and reduced
extra-uterine weight gain during pregnancy) and fetal (mortality
and decreased body weight) toxicity were observed. There was no
effect on cerebral ventricles. At 4 mg/kg bw only slightly dilated
renal pelvis was seen and at 4 and 8 mg/kg bw (n.d.r.) dilated
ureter was observed. In a replicate test (IIa) these effects were
hardly observed. In test IIb no effects at all where found for the
renal function or morphology. Only the treated pups only showed a
lower pup weight (Kavlock, 1985).
Four groups of 12 pregnant rats (Sprague Dawley) received from
day 6 through 15 of gestation 0, 5, 10 or 15 mg TPTA (purity
97.1%)/kg bw/day. Females were sacrificed on day 21 of gestation.
Dams were observed for number of resorptions, number of
implantations, post-implantation loss, viable and non viable
fetuses. Fetuses were weighed and examined for external, visceral
and skeletal anomalies. Two rats treated with 15 mg/kg died during
the study and 4 had complete resorptions. Average maternal weight
gain decreased at 5 mg/kg and significantly 10 and 15 mg/kg.
Post-implantation loss increased significantly at 15 mg/kg. A
significant reduction of some ossification centers, mainly at the
level of the metacarpus and caudal vertebrae, was seen in all
treated groups. Minor anomalies (hydroureter, vertebrae missing or
bipartite) increased significantly at 15 mg/kg (Giavini et al.,
1980).
Groups of pregnant rats (Wistar) received during day 7 through
16 of gravidity 0, 1.25, 3.2 or 8 mg TPTA/kg bw/day. TPTA (purity
97.0%) was dissolved in sesame oil. The females were sacrificed and
delivered on day 21 of gestation. The dams were observed for
clinical signs, body weight, food consumption, number of
implantations, resorptions and corpora lutea, viable and non viable
fetuses, organ weights (heart, liver, kidneys and spleen) and
macroscopy. Fetuses were weighed, and examined for length, sex,
external, internal and visceral anomalies. Ten dams at 8 mg/kg had
abortions. Dams at 8 mg/kg showed signs of clinical intolerance as
piloerection, bloody nose secretion, local hair loss accompanied by
scab formation. Body weight gain and food consumption were
decreased in this group. At 8 mg/kg early and total intrauterine
deaths increased and number of implantations, total live fetuses,
fetal body weight and crown/rump length decreased. At 8.0 mg/kg an
increase in fetuses with non ossification or only weak ossification
of the sternebrae was observed. At 8 mg/kg also an increase (5%) in
distended uni- or bilateral ureter was observed. The NOAEL in this
study is 3.2 mg TPTA/kg bw (Baeder, 1987a).
Hamsters
Four groups of 20-25 pregnant Syrian hamsters received by
gavage 0, 2.25, 5.08, or 12 mg TPTH (purity not given)/kg bw in
0.3% hydroxypropyl cellulose in saline from day 5 through 14.
Vitamin A palmitate was administered by gavage on day 8. All dams
were sacrificed on gestation day 15. The weight of the gravid
uterus was determined and corpora lutea were counted. Fetuses and
resorption sites were noted. Fetuses were weighed and observed for
external, visceral and skeletal malformations. Four animals died in
the highest-dose group and several animals showed an abnormal
behaviour. In the mid-dose group and in the lowest-dose group 2
animals died. Hamsters from the highest-dose group showed a
decreased mean body weight gain and a decreased food consumption.
Fetuses from this group showed also a decreased pup weight. The
average number of minor anomalies (such as kinky tail, haematomas
and anaemic appearance) of fetuses per litter was significantly
greater among the 12 mg/kg group. A significantly greater number of
skeletal variations (poorly ossified, missing metatarsals or
missing sternebrae) was observed among the 12 mg/kg group. Three
cases of hydrone-phrosis were seen at 5.08 mg/kg bw and 1 case of
hydrocephalus was seen at 12 mg/kg bw (Carlton & Howard, 1982;
Carlton, 1985).
Rabbits
Three groups of 22 pregnant New Zealand White rabbits received
by gavage 0, 0.1, 0.3 or 0.9 mg TPTH/kg bw/day mixed in 1% aqueous
CMC, day 6 through 18 of gestation. Dams were sacrificed day 29 of
gestation. Observations were made for clinical signs, body weight
and food consumption. After sacrifice corpora lutea, early and
late resorptions and number of implantations were counted. All
fetuses were weighed, sexed and examined for external, skeletal and
visceral anomalies and developmental variations. Two rabbits from
the 0.9 mg/kg group aborted. A dose-related decrease in mean body
weight gain and food consumption was observed in the 0.3 and 0.9
mg/kg groups. Mean fetal weight was lower in the 0.9 mg/kg group.
The NOAEL for maternal toxicity was 0.1 mg/kg bw and the NOAEL for
embryotoxicity was 0.3 mg/kg bw (Rodwell, 1987).
TPTA (purity 97.0%) was dissolved in sesame oil and
administered orally by gavage to groups of 15 pregnant rabbits
during day 6 through 18 of gestation. The dose levels were 0, 0.1,
0.32 or 1.0 mg/kg bw/day. On day 29 of gestation the dams were
sacrificed. Dams were observed for clinical signs, body weights,
food consumption, number of resorptions, number of implantations,
number of corpora lutea, viable and non viable tissues, organ
weights (heart, liver kidney, spleen) and macroscopy. Fetuses were
weighed and examined for sex, length, external, internal and
skeletal anomalies. In the 1.0 mg/kg group 1 dam died, 3 dams
aborted, 1 dam was sacrificed due to a premature delivery and 2
dams had only intra-uterine deaths. Three dams showed vaginal
haemorrhages. The water consumption and food consumption of dams at
1 mg/kg decreased. The number of implantations and of live fetuses
decreased at 1 mg/kg. Mean fetal weight, mean crown/rump length
and mean placental weight decreased in pups at 1 mg/kg. At 1 mg/kg
4 pups (15.4%) showed omphalocele with protrusion of intestinal
coils or liver tissue. Slight retardation of skeletal ossification
was detected in the 1.0 mg/kg group. There was an increase in the
number of fetuses with fewer than 13 ossified caudal vertebrae and
some fetuses showed weak ossification of the hyoid bone or non
ossification or only slight ossification of the os pubis. The
NOAEL for maternal and embryotoxicity in this study is 0.32 mg/kg
bw/day (Baeder, 1987b).
Special studies on immunotoxicity
Seinen and Penninks (1987) reviewed the available data on
immunotoxic properties of TPTH from the published literature and
confidential studies provided by the manufacturer. They concluded
that subacute treatment with TPTH resulted in lymphopenia and
lymphocyte depletion of spleen and thymus. After treatment up to 13
weeks these effects appeared to be transient. Rats fed 25 ppm TPTH
(not 5 ppm) showed cell mediated immunity as measured by slightly
suppressed DTH (delayed type hypersensitivity) to ovalbumin and
tuberculin. The humoral immunity was not affected at doses up to 25
ppm. However in mice dosed with TPTH up to 20 mg/kg bw for 14 days
DTH response to keyhole limpet haemocyanin was not affected,
whereas the humural activity was suppressed at 20 mg/kg bw. In
various other immune function tests no disturbances were observed.
The authors concluded therefore that TPT-compounds display weak
immunosuppressive potential. This immunosuppressive activity is
observed at dose levels that markedly reduce the numbers of blood
lymphocytes and the weight of the lymphoid organs (Seinen &
Penninks, 1987). The original data for certain studies in this
review were not available to the JMPR.
Mice
In a limited experiment young and mature mice were fed a diet
containing TPTA (purity >99%) at a rate of 30, 100 or 300 ppm for
4 days. Feeding resulted in a dose-related decrease in spleen,
heart and liver weight and number of blood leucocytes (Ishaaya
et al., 1976; Seinen & Penninks, 1987).
Groups of B6C3F1/CrlBR mice (18/sex/group) were administered
0, 1, 5, 25, 50 or 125 ppm TPTH (purity 96.4%) for 28 days. A
positive control group (18/sex) was fed control diet and received
a single i.p. injection of 180 mg cyclophosphamide/kg bw 48 hours
prior to the end of the treatment period. Twelve male and 12
female mice were killed at day 29 and the remaining mice were
returned to control diets and were killed on day 57. Observations
were made on survival and clinical signs, body weight, food
consumption, haematology, liver, spleen, and thymus weight,
macroscopy and histopathology. Special immunotoxicological
parameters as splenocyte counts, thymocyte counts, number of
splenic T- and B-cells and basal serum immunoglobulin levels were
recorded and the bone marrow was examined. A significantly
decreased body weight gain was observed in male and female mice at
125 ppm, from which they recovered after 28 days. Food consumption
was significantly decreased at 50 and 250 ppm. Relative liver
weight was increased at 25 (females only), 50 and 125 ppm, relative
spleen weight was clearly decreased in males at 50 and 125 ppm and
in females at 25 ppm and higher and relative thymus weight was
decreased at 125 ppm in males. At histopathology lymphoid depletion
in the thymus and spleen was observed in mice at 125 ppm. No
dose-related effects were observed on any of the bone marrow
parameters (note: only 50% of the smears could be evaluated,
because of technical problems). The main haematological effects
were a decrease in total white blood cells, neutrophils and
lymphocytes at 50 and 125 ppm in males and females. Dose-related
changes in haematological parameters were observed in males at 25,
50 and 125 ppm and in females in all dose levels (MCH and MCHC). At
the highest dose a decrease in total cells/spleen and splenic
B-cells were observed and a decrease in total cells/thymus and
splenic T-cells were seen in males. IgM levels were decreased in
females at 25 ppm and higher, but were not clearly dose-related.
All effects were reversible. Mice fed cyclophosphamide showed
decreased spleen and thymus weight accompanied by histologic
evidence of lymphoid depletion. Cyclophosphamide also altered the
% T- and % B-cells in both sexes and reduced the spleen and thymus
cellularity. The effects were not reversible. The NOAEL for
immunotoxicity is 5 ppm, equal to 1 mg/kg bw for males and 1.15 for
females (McCormick & Thomas, 1990a).
Rats
A similar study was conducted in 18 male and 18 female Wistar
rats. A positive control group was fed a diet containing 125 mg
DOTDC (dioctyltin-dichloride)/kg. No effects were observed on
mortality, clinical signs, body weight, organ weight, macroscopy.
Food consumption was decreased in female rats at 125 ppm. No
dose-related effects were seen at histopathology (note only 50% of
the bone marrow smears could be used in the histopathological
evaluation). In both males and females red blood cell parameters
were affected at the highest groups. The white blood cells and
lymphocytes were significantly decreased in males at 125 ppm and
females showed a tendency to a decrease at 50 and 125 ppm. In male
rats at 50 and 25 ppm platelet count was increased. No effect was
observed on the viability of the thymus or spleen cells nor on the
percentages of splenic T- and B-cells in either sex. IgG levels
were significantly reduced in females at 50 and 125 ppm. All
effects were reversible. The effects in the positive control group
were as expected. These effects were largely reversible following
cessation of DOTDC exposure. The NOAEL for immunotoxicity is 25 ppm
equal to 1.82 mg/kg bw for females and 1.71 mg/kg bw for males
(McCormick & Thomas 1990b).
In order to investigate the reaction of rats to infection with
Trichinella spiralis female rats were administered 2.5 mg TPTH
(purity 97.2%)/kg bw over a 10 day period. This administration had
no effect on host resistance in contrast to the positive controls
dexamethasone and cyclophosphamide. There are also no effects on
additional parameters, i.e., thymus weights, percentage of
lymphocytes in the differential blood count, and body weight gains
(Diehl & Leist, 1987b).
Special studies on genotoxicity
TPTH as well as TPTA were tested in various genotoxicity
assays. See Tables 3 and 4 respectively, for a summary of the
studies considered. In most of the assays negative results were
obtained. The positive results in the chromosome aberration assays
are probably related to the toxic effect of the compound on
T-lymphocytes and not to a genotoxic action of TPTH.
Special studies on sensitization
At concentrations which were irritant to the skin, TPTH
(purity 97.0%) showed no skin sensitization in guinea-pigs in the
Buehler test (Leist & Weigand, 1981f; Schollmeier & Leist, 1989)
nor in the maximization test (Diehl & Leist, 1987a).
TPTA gave a positive response when tested for skin
sensitization in guinea-pigs in the Buehler test (Diehl & Leist,
1986e).
Table 3. Results of genotoxicity assays on TPTH
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Ames test a,b S. typhimurium 0.31-5 µg/pl 97.0 Negative Richold et al. (1981)
TA 1535, TA100, in DMSO
TA1537, TA98,
TA1538
E. coli WP2 uvra 313-5000 µg/pl 97.0 Negative
in DMSO
Yeast forward S. pombe p1 0.05-1 µg/ml 97.2 Negative Milone & Hirsch (1985b)
mutation test in DMSOa
Mitotic gene Saccharomyces 0.1, 1, 3 or 97.2 Negativec Milone & Hirsch (1985a)
conversion assay cerevisiae D4 5 µg/mla
1, 5, 10 or
15 µg/mlb
Mouse lymphoma L5178Y mouse 10-150 ng/mla 97.2 Negative DenBoer & Hoorn (1985)
forward mutation lymphoma cells (>80 ng/ml toxic) Weakly
assay (TK +/-) 40-600 ng/mlb positive
(>300 ng/ml toxic)
Mouse lymphoma L5178Y mouse 0.01-2 µg/1a ? Positive NCI (1984)
forward mutation lymphoma cells (>0.1 toxic) Negative
assay (TK +/-) 1.7-8 µg/1b
Chromosome Human lymphocytes 31.25-1000 ng/mla Positive Kirkland (1985)
aberration assay 250-2000 ng/mlb
both in DMSO
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Chromosome Human lymphocytes 0.681-1.47 µg/mla 96.2 Negative Nunziata & Conzonni (1988)
aberration assay in DMSO Positive
0.316-1.47 µg/mlb
in DMSO
Unscheduled Rat hepatocytes 0.01-2.0 µg/ml in DMSO 97.2 Negativec Cifone & Myhr (1985)
DNA synthesis >0.25 µg/ml toxic
Survival (cell Rat embryo cells 0.019 µg/5.2 x 104 cells ? Positived Traul et al. (1981)
transformation) infected with or 0.0145 µg/5.2 x 104
assay leukaemia virus
(2FR450 cells)
Micronucleus test NMRI mouse bone 35, 70 or 140 mg/kg bw po 97.2 Negative Banduhn et al. (1985)
NMRI marrow cells
Cytogenic assay Chinese hamster bone 20, 50 or 80 mg/kg bw po 96.2 Negative Mueller (1987)
marrow cells
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Dominant lethal Sprague-Dawley rats daily ip injections 94.8 Negative Ravert et al. (1978)
assay with 3, 20, 38
or 150 mg/kg bw in
corn oil; 150 mg/kg
bw toxic (8/10
died). Concentration
of TPTA
a without metabolic activation
b with metabolic activation
c positive control yielded positive results
d only summary available
Table 4. Results of genotoxicity assays on TPTA
Test system Test object Concentration of Purity Results Reference
TPTA (%)
Ames testa,b S. typhimurium 0.032-1000 µg/plate 97.1 Negative Jung & Weigand (1986)
TA100, TA1535, in DMSO
TA1537, TA1538,
TA98
E. coli WP2 uvra
Gene conversiona,b S. cerevisiae D4 1-50 µg/ml in DMSO 97.1 Negative Milone & Hirsch (1986a)
Dominant Sprague-Dawley rats daily i.p. injections 94.8 Negative Ravert et al. (1978)
lethal assay with 3, 20, 38 or
150 mg/kg bw in corn oil;
150 mg/kg bw toxic (8/10
died)). Concentration of
TPTA
a without metabolic activation
b with metabolic activation
Special studies on skin and eye irritation
A dose of 500 mg TPTH (purity not stated) diluted in a 0.9%
NaCl solution was slightly irritant when applied under occlusive
conditions to the shaven intact and shaven abraded skin of 6 New
Zealand rabbits for 24 hours (Leist & Weigand, 1981e).
Dilutions of 1:10, 1:100 and 1:1000 prepared from 2.5 g TPTA
(purity not stated) in 0.9% NaCl were examined in the Barail
Intracutaneous test by intracutaneous injections of 0.02 ml
administered to the skin of rabbits. No irritation was seen. Five
applications of 0.5 ml of the TPTA solution to depilated and intact
rabbits skin caused slight local irritation. (Summary only)
(Hollander & Weigand, 1974e).
A dose of 500 mg TPTA (purity 97%) moistened with
physiological saline was applied under semi-occlusive conditions to
the shaven intact skin of 6 New Zealand rabbits for 54 hours. No
irritation was observed up to 72 hours after application (Diehl &
Leist, 1986c).
Application of 100 mg TPTH (purity not stated) diluted in a
0.9% NaCl-solution into the eyes of 9 New Zealand rabbits caused
severe irritation; increasing adverse effects resulted in a
discontinuation of the experiment after 72 hours (Leist & Weigand,
1981e).
Rabbits received an instillation of 0.1 ml of a solution of
2.5 g TPTA (purity not stated) in 0.9% NaCl into one eye. After 24
hours slight local irritation was observed (Summary only)
(Hollander & Weigand, 1974e).
Nine New Zealand rabbits received an instillation of 100 mg
TPTA (purity 97%) into one eye. All rabbits developed severe ocular
lesions (swollen conjunctivae, markedly injected blood vessels,
diffusely crimson red colouring, nacreous opacity and whitish and
reddish brown discharge). After 72 hours all animals were
sacrificed because the lesions were non-reversible (Diehl & Leist,
1986d).
Observations in humans
Two cases of poisoning with Brestan 60 (60% TPTA and 15%
Maneb) have been reported. In both cases (farmers of 75 and 53
years) inhalation of the powder took probably place during
preparation of the spray solution. Symptoms observed were nausea,
dizziness, transient loss of consciousness, convulsions, persistent
headache, and photophobia, as well as impaired liver functions.
Both patients were discharged in good health after 10 and 15 days
(Manzo & Richelmi, 1981).
Acute severe intoxication was reported in a 23-year old male
in an attempted suicide with a TPT-compound (molluscicidal agent,
no specifications given). The patient was suffering from abdominal
pains, diarrhoea and vomiting and also showed reversible acute or
delayed neurological symptoms. The patient's health was completely
recovered within 3 1/2 months (Wu et al., 1990).
COMMENTS
Most toxicological studies on fentin were carried out with
triphenyltin hydroxide (TPTH). Since triphenyltin acetate (TPTA) is
hydrolysed rapidly to TPTH in an aqueous medium, studies with both
compounds have been used for the evaluation of fentin.
After oral administration of 14C-labelled TPTH the
radioactivity was mainly eliminated via the faeces (50-70%), but
also via the urine (20-25%). Biliary excretion was also
demonstrated. After 7 days residual radioactivity was present with
the highest concentration in the liver and the next highest in the
kidneys. After oral administration of 113Sn-labelled TPTH,
elimination was almost exclusively via faeces. In both cases faecal
elimination was biphasic with half-lives of 9 and 50-60 hours. With
113Sn-labelled TPTH the highest concentration was found in the
kidneys. After administration of the 14C-labelled compound,
radioactivity in the faeces of the rat consisted of parent compound
and di- and monophenyltin as well as non-extractable bound residues
and tin (measured as Sn). It was reported that benzene was found in
the faeces. Sulfate conjugates of phenol, hydroquinone, catechol
and resorcinol as well as phenylmercapturic acid were present in
the urine. Phenyltin compounds were not found in the urine.
TPTH is toxic to rats after acute oral administration with
LD50 values of about 160 mg/kg bw. TPTA showed similar acute
toxicity.
In a 3-month study in mice at dietary concentrations of TPTH
of 0, 4, 20 or 100 ppm, a number of effects were observed at 100
ppm. The main effects were a decrease in haemoglobin concentration
and erythrocyte count and an increase in Heinz bodies.
Immunoglobulins were decreased and liver weight was increased. The
NOAEL was 20 ppm, equal to 3.4 and 4.1 mg/kg bw/day for males and
females, respectively.
Dietary concentrations of TPTH of 0, 4, 20 or 100 ppm were
tested in a 13-week study in rats. The NOAEL was 4 ppm, equal to
0.30 and 0.35 mg/kg bw/day for males and females respectively,
based on a decrease in white blood cells and plasma albumin and an
increase in plasma aspartate aminotransferase at 20 ppm.
In a 52-week study in dogs (dietary concentrations of TPTH of
0, 2, 6 or 18 ppm) the NOAEL was 6 ppm, equal to 0.2 mg/kg bw/day,
based on a decreased albumin level and an increase in relative
liver weight at 18 ppm.
Two long-term/carcinogenicity studies in mice and one in rats
were performed in which there was no evidence of carcinogenicity.
However, there were indications in each of these studies that the
doses received by the animals may have been lower than intended
owing to instability of the test compound in the diet.
In a third long-term/carcinogenicity study in mice at dietary
concentrations of TPTH of 0, 5, 20 or 80 ppm, increased liver and
decreased kidney weights, increased nodular hyperplasia and
hepatocellular adenoma and carcinoma occurred at 80 ppm only. The
NOAEL was 5 ppm, equal to 0.85 mg/kg bw/day for males and 1.36
mg/kg bw/day for females, based on decreased body-weight gain at 20
ppm.
In a second long-term/carcinogenicity study in rats (with
dietary concentrations of TPTH of 0, 5, 20 or 80 ppm), an increase
in mortality was observed at all dose levels. A NOAEL could not
be established at the lowest concentration of 5 ppm, equal to 0.3
and 0.4 mg/kg bw/day for males and females respectively. The
incidence of pituitary adenomas was increased in females at 20 and
80 ppm. At 80 ppm, the incidence of Leydig cell tumours was
increased. These changes were accompanied by non-neoplastic
lesions in the pituitary and the testes.
In a 2-generation reproduction study in rats with one litter
per generation (dietary concentrations of TPTH of 0, 5, 18.5 or 50
ppm) fertility was not affected. The NOAEL was 5 ppm (equal to 0.4
mg/kg bw/day) based on decreased litter size, decreased pup weight
and decreased relative spleen and thymus weight in the weanlings.
In several teratogenicity studies with rats, hamsters, and
rabbits, TPTA or TPTH caused maternal and embryo toxicity, but
irreversible structural effects were not observed. In rabbits, the
most sensitive species, the NOAEL for embryo/fetotoxicity was 0.3
mg/kg bw/day in a study that utilized doses of 0, 0.1, 0.3 or 0.9
mg/kg bw/day TPTA. In this study the NOAEL for maternal toxicity
was 0.1 mg/kg bw/day.
Most in vitro and in vivo genotoxicity tests were
negative. However, two human lymphocyte chromosomal aberration
assays and two mouse lymphoma mutation assays were positive. The
latter responses may have been caused by a variety of effects,
including chromosomal aberrations. Since two in vivo studies for
chromosomal aberrations (a micronucleus test in mice and a
cytogenetic test in Chinese hamsters) were negative, it would
appear that any genotoxic properties are of low potency. It was
therefore concluded that fentin does not present a genotoxic hazard
for man.
Effects on the immune system were observed in short- as well
as in long-term toxicity studies. In special studies with mice and
rats, TPTH showed immunosuppressive properties (lymphopenia and
lymphocyte depletion of spleen and thymus), resulting in altered
humoral and cellular immunity. The NOAEL in mice was 5 ppm, equal
to 1 mg/kg bw/day, based on a decrease in splenic weight found at
25 ppm. In rats, the NOAEL was 25 ppm, equal to 1.7 and 1.8 mg/kg
bw/day for males and females respectively, based upon a significant
decrease in immunoglobulin G and a marginal decrease in leucocyte
and lymphocyte counts at 50 ppm.
The Meeting noted that there was increased mortality at the
lowest dose tested in the most recent long-term study in rats (0.3
mg/kg bw/day). Applying a 500-fold safety factor to this LOAEL
would result in approximately the same ADI as the previously
established ADI based upon a NOAEL of 0.1 mg/kg bw/day in an
earlier long-term study in rats. The previous ADI was therefore
retained. This ADI is supported by NOAELs derived from recent
studies, including the NOAEL of 0.4 mg/kg bw/day in the
2-generation reproduction study, the NOAELs in short-term studies
in rats (0.3 mg/kg bw/day) and dogs (0.2 mg/kg bw/day) and in a
teratology study in rabbits (0.1 mg/kg bw/day for maternal
toxicity).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm in the diet, equal to 1 mg/kg bw/day
Rat: <5 ppm in the diet, equal to <0.3 mg/kg bw/day
(long-term/carcinogenicity study)
5 ppm in the diet, equal to 0.4 mg/kg bw/day
(multigeneration reproduction study)
Dog: 6 ppm, equal to 0.2 mg/kg bw/day
Rabbit: 0.1 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Ongoing studies on the endocrinological effects of fentin
2. Observations in humans.
REFERENCES
Baeder, Ch. (1987a) Fentinacetate - substance, technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Wistar rats
after oral administration. Unpublished Report 89.1478 (A42190) of
Pharma Research Toxicology and Pathology, Hoechst AG. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Baeder, Ch. (1987b) Fentin acetate - substance technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished Report 89.1559
(A42324) of Pharma Research Toxicology and Pathology, Hoechst AG.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Bakke, J.E., Feil, V.J., Aschbacher, P.W., & Price, C.E. (1982)
Metabolism of 14C-triphenyltinhydroxide by the rat, sheep and cow.
Unpublished Report (A35976) of metabolism and radiation research
laboratory Agricultural Research Service, Fargo, ND, USA.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Banduhn, N., Sachsse, K. & Terrier, C. (1985) Mouse micronucleus
test with TPTH-technical (HOE 029664 OF ZD97 0004). Unpublished
Report (A31583), project 049522 from RCC, Switzerland. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Brown, W.R. (1985) 18-Month carcinogenicity study of Technical TPTH
96.3% (Lot no. SWRAM-1K) in CD Mice. Histomorphology of the Uterus.
Unpublished Report (A32214) from Research Pathology Services. Inc.,
New Britain, CT, USA Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Brunk, Dr. & Weigand, W. (1981) Acute oral toxicity of
Fentin-Hydroxide, active ingredient (Code: HOE 29664 O F AT201) to
the male and female beagle dog. Unpublished Report 462/81 from
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Beurkle, W.L. (1985). Hoe 002782-14-C (triphenyltin acetate),
analysis of hydrolysis byproducts. Unpublished Report CM009/85
(A31534) from Analytisches Laboratorium, Hoechst. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L., Rutz, U., & Müller, A., Eckert, H.G., Kellner, H.M.
& Esinger, W. (1986) Metabolism in rats after single and repeated
oral administration at the two dosage levels 2 and 10 mg/kg body
weight. Unpublished Report CM 011/85 (A34163) of Radiochemisches
Laboratorium and Analytisches Laboratorium, Hoechst AG. Submitted
to WHO by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L. & Kellner, H.M. (1987) HOE 029664 (TPTH)-14C.
Excretion study in rats with bile fistula following oral
administration of 2 mg a.i./kg body weight. Unpublished Report
(B)97/87 (A36680) of Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. (1985) Triphenyltin hydroxide. Additional data on
Batelle rat and hamster teratology studies (31184). Unpublished
data of Batelle Columbus Laboratories, Columbus, Ohio. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Connell, M.M. (1981) Final report on evaluation of
the teratogenicity of triphenyltin hydroxide (TPTH) in the
Sprague-Dawley rat. Unpublished Report NO723-0200 (A22211) of
Batelle Columbus Laboratories, Columbus, Ohio. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Crisp, C.E. (1982) Range-finding study for the
evaluation of reproductive effects of triphenyltin Hydroxide (TPTH)
in the Wistar rat. Unpublished Report (A25091) project NO723-0400)
from Battelle, Columbus, Ohio. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. & Howard M. (1982) The evaluation of the
teratogenicity of triphenyltin hydroxide (TPTH) in the Syrian
hamster. Unpublished Report
NO723-0100 (A22884) of Battelle, Columbus Laboratories, Columbus,
Ohio. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Fentinhydroxide,
substance technical grade (Code: HOE 029664 OF ZD97 0004) in the
rat primary hepatocyte unscheduled DNA synthesis assay. Final
Report (A32084) GT85.1091 from Litton Bionetics, Inc. Maryland.
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Craine, E.M. (1987) An extended duration dermal absorption study in
rats with 14C-triphenyltin hydroxide. Unpublished Report WIL-39037
(A35945) of WIL Research Laboratories. A subsidiary of Great Lakes
Chemical Corporation, Ashland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
DenBoer, W.C. & Hoorn, A.J.W. (1985) Mutagenicity evaluation of HOE
0 29664 -Substance technical (Code: HOE 029664 OF ZD97 0004) in the
L5178Y TK +/- Mouse Lyphoma forward mutation assay. Unpublished
Final Report (A31582) from Litton Bionetics, The Netherlands.
Submitted to WHO by Hoechst AG. Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986a) Fentin acetate - active
ingredient technical. Testing for acute intraperitoneal toxicity in
the male and female rat. Unpublished Report 86.1047 (A37976) of
Hoechst Pharma Research Toxicology and Pharmacology, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986b) Fentin acetate - active
ingredient technical. Testing for acute dermal toxicity in the male
and female Wistar rat. Unpublished Report 86.0816 (A37384) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986c) Fentin acetate - active
ingredient technical. Testing for primary dermal irritation in the
rabbit. Unpublished Report 86.0619 (A37383) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986d) Fentin acetate - active
ingredient technical. Testing for primary eye irritation in the
rabbit. Unpublished Report 86.0620 (A37382) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986e) Testing for sensitizing
properties in the Pirbright-White guinea pig according to the
technique of Buehler. Unpublished Report 86.0803 (A36975) of
Hoechst Pharma Research Toxicology and Pathology. Submitted by WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987a) Fentin-hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing for
sensitizing properties in the Pirbright-White guinea pig in a
maximization test. Unpublished Report 87.0201 (A35628) from Hoechst
Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987b) Fentin hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing of
host resistance in the female Wistar rat (Immunotoxicological
screening with Trichinella spiralis) -2nd study-. Unpublished
Report 87.0955 (A36065) of Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Dorn, E. & Werner, H.J. (1989a) Triphenyltinhydroxide (Hoe 029964
OF ZD97 0004). Determination of residues in organs, tissues and
blood of dogs after chronic feeding (52 weeks). RCC Project 047024
and RCC Project 047013. Unpublished Report CR076/88 (A41963) of
Analytisches Laboratorium Hoechst AG. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Dorn, E & Werner, H.J. (1989b) Triphenyltinhydroxide (Hoe 029664 OF
ZD 97 0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished Report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG.,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Duchosal, F., Thevenaz, Ph., Luetkemeier, H., Vogel, O., Pappritz,
G., Mladenovic, P. & Terrier, Ch. (1989) Fentinhydroxid (TPTH)
technical grade. Subchronic (90-day) repeated dose inhalation
toxicity study in rats. Unpublished Report 202353 (A40323) of RCC,
Research and Consulting Company AG., Carouge-Geneva, Switzerland.
Eckert, H.G., Kellner, H.M., Beurkle, W.L., (1989) Accumulation
study; kinetics and metabolism in the rat after single and repeated
oral administration, of 2 mg/kg body weight. Unpublished Report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L,
Oekologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Giavini, E., Prati, M. & Vismara, C. (1980) Effects of triphenyltin
acetate on pregnancy in the rats. Bull. Environm. Contam. Toxicol.
24: 936-939 (A19995). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1974a) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished Report 16454
(A16454) from Hoechst Pharma Forschung Toxicologie. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Hollander H. & Weigand W. (1974b) Triphenyltin acetate. Acute oral
toxicity (LD50) in guinea pigs. Unpublished Report (A15562) of
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974c) Triphenyltin acetate.
Subchronic (30-day) oral toxicity testing in rats and guinea pigs.
Unpublished Report (A37587) of Hoechst Pharma Research Toxicology.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1974d) Triphenyltin acetate dermal
toxicity in rats and rabbits. Unpublished Data (A15561) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974e) Triphenyltin acetate skin and
eye irritation in rabbits. Unpublished Report (15564) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1980) Acute oral toxicity of fentin
acetate technical (Hoe 02782) in male rabbits. Unpublished Report
(A28616) of Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1981) Dust inhalation of HOE 29664 -
active ingredient in the male and female SPF-Wistar rat. 4 h -LC50.
Unpublished Report 335/81 (A22293) from Hoechst Pharma Forschung
Toxicologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1986) Fentin acetate - active
ingredient technical. Testing for acute dust inhalation toxicity in
the male and female SPF rat 4-hour LC50. Unpublished Report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ishaaya, I., Engel, J.L. & Casida, J.E. (1976) Dietary
triorganotins affect lymphatic tissues and blood composition of
mice. Pestic. Biochem. Physiol. 6: 270-279. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main. (A31628)
Jung, R. & Miltenburger, H.G. (1986) Fentin acetate - substance
technical (Code: Hoe 002782 OF ZD97 0002). Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with
V79 cells. Unpublished Report LMP 229 (A35212) of Hoechst
Laboratory for mutagenicity testing. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Jung, R. & Weigand, W. (1986) Fentin acetate - substance technical,
Code: Hoe 002782 OF ZD97 0002. Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames test) and Escherichia
coli. Unpublished Report 86.0042 (A33021) of Hoechst Pharma
Research Toxicology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kavlock, R.J. (1985) Triphenyltin hydroxide developmental
toxicology study. Unpublished Report of United States Environmental
Protection Agency (A31424). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1986) Kinetics in the rats following
single and repeated administration of 2 mg/kg and single
administration of 10 mg/kg body weight. Unpublished Report
01-L42-0489-86 (A34186) of Hoechst Radiochemisches Laboratorium.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1989) Addendum to report
01-L42-0565-89. Hoe 029664 (TPTH)-113Sn. Accumulation and depletion
study in rats after single and repeated oral administration of 2
mg/kg body weight. Unpublished Report 01-L42-582-90 (A43434) of
Pharma Forschung GB-L Radiochemisches Laboratorium Hoechst,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M., Eckert, H.G. & Bürkle, W.L. (1989) Hoe 029664
(TPTH)-113Sn. Absorption studies in rats with bile fistula after
a single oral dose of approx. 2 mg/kg body weight. Unpublished
Report 01-L42-0564-89 (A41409) of Hoechst Pharma Foreschung
Radiochemisches Laboratorium Productent-wicklung GB-C, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of HOE 029664 - substance technical by its effects on
cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished Report A31614 from Microtest Research Limited, UK.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Lachmann, G. & Siegemund, B. (1987) Exhalation experiment with
triphenyltinhydroxide (Hoe 029664) in rats. Unpublished Report
(A34601) of Battelle-Institute. V., Frankfurt-am-Main. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Laveglia, J., Hagen, C.A. & Nemec, M.D. (1985) 21-Day dermal
toxicity study in rats with triphenyltin hydroxide (Code: Hoe
029664 OF ZD97 0001 technical substance). Unpublished Final Report
(A31616) project no WIL-39018 from American Hoechst Company, New
Jersey. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. (1988) Fentin acetate-substance technical (Code HOE
002782 OF ZD97 0002). Repeated dose dermal toxicity study (21
applications in 29 days) with a 14 day withdrawal period.
Unpublished Report (A39231) 3104 TSR of Pharma Forschung
Toxicologie und Pathologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981a) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 29664 O F AT201) to the male rat.
Unpublished Report (A22115) no 182/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981b) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 19664 O F AT201) to the female rat.
Unpublished Report (A22114) 183/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981c) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 O F AT201) to the
female rat. Unpublished Report (A22292) 184/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981d) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished Report (A22112) 229/81 from Hoechst Pharma
Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981e) HOE 29664 - active ingredient
(Code: HOE 29664 O F AT201). Irritance to the rabbit skin and eye
mucosa. Unpublished Report (A22113) 104/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981f) Test for sensitizing properties
of Fentin hydroxide-technical (Code : HOE 29664 O F AT201) in
guinea-pig (according to Buehler). Unpublished Report (A22433)
724/81 from Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Leist, K.H., Weigand, W. & Kramer, Dr. (1982) Range-finding test
von Hoe 29664-Wirkstoff (Fentinhydroxid) (Code: Hoe 29664 O F
AT201) im 30-Tage-Fuetterungsversuch and SPF-Wistar-Ratten.
Unpublished Report (A24003) 426/82 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Loquet, C.(1985) Fentin acetate (Code: Hoe 002782 OF ZD97 0001)
Micronucleus test in mice. Unpublished Report 1592 MAS (A32196) of
Centre International de Toxicologie at Misery. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990a) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH) substance
technical (Code HOE 029664 OFZD97 0004) in mice. Unpublished Report
L08252SN01 (A44053) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990b) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH), substance
technical (Code HOE 029664 OFZD97 0004) in rats. Unpublished Report
L08252SN02 (A44054) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Manzo, L. & Richelmi, P. (1981) Poisoning by triphenyltin acetate.
Report of two cases and determination of tin in blood and urine by
neutron activation analysis. Clinical Toxicology, 18(11)
1343-1353. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
(A44030)
Milone, M.F. & Hirsch, E. (1985a). Study of the capacity of the
test article HOE 029664 - Substance technical grade - (Code No. HOE
029664 OF ZD97 0004) to induce gene conversion in saccharomyces
cerevisiae. Unpublished Report (A32085) experiment no.M 890 from
RBM, Ivrea, Italy. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1985b) Study of the capacity of the test
article HOE 029664 - Substance technical grade (Code HOE 029664 OF
ZD97 0004) to induce gene mutation in Schizosaccharomyces pombe.
Unpublished Report (A31613) from RBM Italy. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986a) Study of the capacity of the
article fentin-acetate-substance technical grade (Code No. Hoe
002782 OF ZD97 0002) to induce gene conversion in Saccharomyces
cerevisiae. Unpublished Report M1023/1-2 (A34018) of Instituto di
Ricerche Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986b) Fentin acetate substance
technical grade. Forward Mutation test in S. pombe P1.
Unpublished Report M898 (A32812) of Instituto di Ricerche
Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Mueller, W. (1987) Evaluation of HOE 029664 OF ZD97 0004 in the
in vivo cytogenetic test in bone marrow cells of the Chinese
hamster - chromosome analysis. Unpublished Report 87.0031 (A36080)
from Hoechst Pharma research Toxicology and Pathology. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Nagamatsu, K., Kido, Y., Urakubo, G., Aida, Y., Ikeda, Y. & Suzuki,
Y. (1978) Absorption, distribution and excretion of triphenyltin
acetate and stannic chloride in the guinea pig. Japanese Journal
of Hygiene, 33: 486-496.
NCI (1984) Mouse lymphoma with triphenyltin hydroxide. Unpublished
Report (A30130), National Cancer Institute. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS no. 76-87-9 (A199994). Technical report series
No.139. US Department of Health, Education, and Welfare.
Nunziata, A. & Consonni, R.F. (1988) Chromosome aberrations in
human lymphocytes cultured in vitro. Test substance: HOE 029664
Fentin hydroxide substance technical (Code: Hoe 029664 OF ZD97
0004). Unpublished Final Report 157024-M-04487 (A38456) from Life
Science Research Roma Toxicology Centre S.P.A. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ravert, J. & Parke, G.S.E. (1976) Investigation of teratogenic and
toxic potential of technical triphenyltinhydroxide (lot #SWRAM-1K).
Unpublished Report GT85.0797 (31618) of Cannon Laboratories, Inc.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Ravert, J., Parke, G.S. & Charles, S.J. (1978) The dominant lethal
assay of technical TPTH (Triphenyltin hydroxide) in Sprague-Dawley
rats. Unpublished Report (A24394) from Cannon Laboratories Inc.,
Reading. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Resnis, P., Craine, E.M. & Nemec, M.D. (1986) A dermal absorption
study in rats with 14C-triphenyltin hydroxide. Unpublished Report
(WIL-39020) (32810) of WIL Research Laboratories. A subsidiary of
Great Lakes Chemical Corporation, Ashland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Richold, M., Jones, E. & Fleming, P.M. (1981) Ames metabolic
activation test to assess the potential mutagenic effect of HOE
29664 OF AT201. Unpublished Report 450/81A (A21735) from Huntingdon
Research Centre, GBR. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1985a) One generation teratology and reproductive
study in rats with triphenyltin hydroxide (Code: HOE 029664 OF ZD97
0001 Technical substance). Unpublished Final Report project
WIL-39013 (A31617) from Wil Research Laboratories Inc., Ashland
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Rodwell, D.E. (1985b) A teratology study in rats with triphenyltin
hydroxide (Code: HOE 029664 OF ZD97 0001 Technical Substance).
Unpublished Report WIL-39011 (A31079) of WIL Research Laboratories
Inc, Ashland USA. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
Report WIL-39012 (A35220) of WIL Research Laboratories, Ashland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Sachsse, K., Frei, T., Luetkemeier, H., Vogel, W., Pappritz, G. &
Terrier, C. (1987) TPTH - Substance technical (Hoe 029664 OF ZD 97
0004) Chronic oral toxicity 52-week deeding study in beagle dogs.
Unpublished Report 047013/047024 (A35733) of RCC Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Schollmeier, U. & Leist, K.H. (1989) Triphenyltin hydroxide -
active ingredient technical (Code: Hoe 029664 OF ZD97 0004).
Testing for sensitising properties in the Pirbright-White guinea
pig according to the technique of Buehler. Unpublished Report
89.1006 (A42667) of Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Scholz & Weigand, W., (1969b) Triphenyltin acetate Study No. 2116.
Unpublished Data A37396 from Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Seinen, W. & Penninks, A.H. (1987) Immunotoxicity of triphenyltin
compounds. Department pharmacology, pharmacy and toxicity. Faculty
of Veterinary Sciences, University of Utrecht, The Netherlands.
Document A35456. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Snow, R.L. & Hays, R.L. (1983) Phasic distribution of seminiferous
tubulus in rats treated with triphenyltin compounds. Bull.
Environ. Contam. Toxicol. 31: 658-665. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986a) 13-week oral toxicity (feeding) study
with TPTH-technical (code: HOE 029664 OF ZD97 0004 in the mouse.
Unpublished Report project 046991 (A32420) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986b) 13-week oral toxicity (feeding) study
with TPTH-technical (Code : HOE 029664 OF ZD97 0004) in the rat.
Unpublished Report project 046978 (A32419) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Vogel, O.,
Armstrong, J., Ehlers, H.A., Müller, E. & Terrier, Ch. (1989a)
TPTH-technical (Code: HOE 029664 OF ZD97 0004) oncogenicity 80-week
feeding study in mice. Unpublished Report 047002 (A40467) of RCC,
Research and Consulting Company AG., Itingen, Switzerland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Schlotke, B.,
Vogel, O., Ehlers, H.A., Mueller, E. & Terrier, Ch. (1989b)
TPTH-technical (Code: HOE 029664 OF ZD97 0007) chronic
toxicity/oncogenicity 104-week feeding study in rats. Unpublished
Report 046980 (A40468) of RCC, Research and Consulting Company AG.,
Itingen, Switzerland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Terrel, Y & Reddy, A.K. (1978) Report: 18-month carcinogenicity
study of technical TPTH 96.3% lot no. SWRAM-1K in CD1 mice.
Unpublished Report project 6E-725 (A26239) from Cannon
Laboratories, Inc. Reading. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Traul, K.A., Takayama, K., Kachevsky, V., Hink, R.J. & Wolff, J.S.
(1981) A rapid in vitro assay for carcinogenicity of chemical
substances in mammalian cells utilizing an attachment-independence
endpoint. J. Appl. Toxicol. 1(3), 190-195.
Ueda, K. & Iijima, K. (1961) Acute toxicity of Brestan Technical
(Triphenyltin Acetate) for mice. Unpublished Report A15046 from
Laboratory of Hygienics, Tokyo Dental College, Japan. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Wu, R,M., Chang, Y.C. & Chiu, H.C. (1990) Acute triphenyltin
intoxication: a case report. J. Neurol. Neurosurg. Psych., 53,
356-357.
Young, D.L. (1986) A dietary two-generation reproduction study in
rats with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final Report project WIL-39022 (A35378) from
WIL research laboratories, Inc. Ashland USA. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
A 576 kg milk cow received a single oral dose of 5055 g
14C-TPTH and a 74 kg ewe received a dose of 1011 g 14C-TPTH, in
capsules. In the cow after 6 days 12.9% of radioactivity was
excreted in urine and 78% in the faeces. Tissues contained 5.1%.
Maximum radioactivity levels were found in the liver (5.5 mg/kg),
kidney and adrenals (both 2.2 mg/kg). In the sheep, 32.6% of
applied radioactivity was found in urine and 47.5% in the faeces
after 8 days. Tissues contained 3.1%. The liver showed highest
level of radioactivity, 6.6 mg/kg. Triphenyltinchloride was
identified as the major faecal excretion product. Phenol,
hydroquinone, catechol and resorcinol were isolated from the urine
(Bakke et al., 1982).
Toxicological studies
Acute toxicity
The LD50 and LC50 values for TPTH and TPTA in various species
are given in Tables 1 and 2, respectively.
The observed intoxication signs following oral exposure
include anorexia, emesis, tremor and diarrhoea followed by
drowsiness and ataxia. After dermal exposure to TPTH, skin
irritation was observed.
Five applications of 50 mg TPTA (purity not stated)/kg applied
to the nuchal skin of 5 rats caused no mortality but some signs of
local irritation. Five applications of 100 mg/kg to 5 rats caused
death of one animal and local signs of irritation. Five
applications of 200 mg/kg were lethal to rats. (Summary only)
(Hollander & Weigand, 1974d).
A single dermal application of 12.5 mg TPTA/kg to 3 rabbits
was lethal for 2 rabbits and caused black coloration of the skin
and scarcely pilous sites of the body. (Summary only) (Hollander &
Weigand 1974d).
Table 1. Acute toxicity of TPTH
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/m3)
Rat M oral ? 165 Leist & Weigand (1981a)
F oral ? 156 Leist & Weigand (1981b)
F perc. ? 1600 Leist & Weigand (1981c)
M&F inhal.a 97.0% 60.3 Hollander & Weigand (1981)
Rabbit M perc. ? 127 Leist & Weigand (1981d)
Dog M&F oral ? »100 Brunk & Weigand (1981)
a indicates 4 hour exposure
Table 2. Acute toxicity of TPTA
Species Sex Route Purity LD50 LC50 Reference
% (mg/kg bw) (mg/m3)
Mouse M oral 99 81.0 Ueda & Iijima (1961)
M oral 99 93.3 Ueda & Iijima (1961)
M s.c. 99 44.0 Ueda & Iijima (1961)
M dermal 99 350 Ueda & Iijima (1961)
Rat F oral ? 298 Scholz & Weigand (1969b)
F oral ? 140 Hollander & Weigand (1974a)
M&F dermal 97 >2000 Diehl & Leist (1986b)
M i.p. 97 3.6 Diehl & Leist (1986a)
F i.p. 97 12.7 Diehl & Leist (1986a)
M inhal. 97 44a Hollander & Weigand (1986)
F inhal. 97 69a Hollander & Weigand (1986)
Guinea pig ? oral ? 25 Hollander & Weigand (1974b)
Rabbit M oral ? 80 Hollander & Weigand (1980)
a indicates 4 hour exposure, whole body exposure, dust inhalation
Short-term studies
Mice
Male and female NMRI mice (10/sex/group) were fed diets
containing 0, 4, 20 or 100 ppm TPTH technical (purity 97.2%) for
three months. No compound-related effects were observed on
mortality, clinical signs, food consumption, body weight,
macroscopy and histopathology. At 100 ppm several haematological
and biochemical parameters were affected. Erythrocyte count and Hb
were decreased and platelet count increased in males and females.
The incidence of Heinz bodies was increased in males and MCH and
MCHC were decreased in females. Mouse immunoglobulins (IgA and IgG)
were decreased, IgM levels were decreased in females only. In
female mice total protein, albumin and alpha-globulin were
increased and gammaglobulin was decreased. Liver weight was
increased in males and females and a decrease was observed in the
relative weights of ovaries, adrenals, kidneys, heart and brain in
females. The NOAEL in this study is 20 ppm, equal to 3.4 mg/kg bw
(males) and 4.1 mg/kg bw (females) (Suter & Horst, 1986a).
Rats
In a range-finding study groups of rats (Hoe: WISKf;
10/sex/group) were fed diets containing 0, 5, 10, 20, 40 or 80 ppm
TPTH (97.0%) for 30 days. No compound-related effects were observed
on clinical signs, water consumption, urinalysis, organ weight,
macroscopy nor histopathology. In male rats at 80 ppm body weight
gain was decreased. Food consumption was decreased during the first
weeks in males at 80 ppm and females at 40 and 80 ppm. An increase
in thrombocyte count was observed in female rats at 40 and 80 ppm;
at the highest dose a decrease in Hb was observed in males and a
decrease in haematocrit was observed in females. At the highest
dose bilirubin, creatinin (males only), SGPT and urea (males only)
were increased. SAP was increased in a non dose-related manner in
males at 20 ppm and higher and in high-dose females. There was no
effect on spleen weight. The NOAEL in this study is 20 ppm equal to
2.01 mg/kg bw (males) or 1.83 mg/kg bw (females) (Leist et al.,
1982).
Groups of Wistar rats (15/sex/group) received dietary
concentrations of 0, 4, 20 or 100 ppm TPTH-technical (purity 97.2%)
for 13 weeks. The treatment period was followed by a 4-week
recovery period (5 male and 5 female rats/group). Observations
included clinical signs, food consumption, body weight,
ophthalmoscopy, hearing tests, haematology, clinical chemistry,
organ weights, macroscopy and histopathology (54 tissues; only in
control and high-dose rats). After 13 weeks of treatment the
following parameters were significantly affected; mean body weight
gain and food consumption were slightly decreased in high-dose
rats. RBC was increased, MCV (in males also at 20 ppm) and MCH were
decreased. In females WBC decreased at 20 and 100 ppm. Most
biochemical parameters were significantly affected at the highest
dose. The observed increase in ASAT was also apparent at 20 ppm as
was the decrease in albumin in females (also at 4 ppm). IgG was
slightly lower. The 18-hour urine volume was increased and the
specific gravity was decreased at 100 ppm. Relative testes weight
was significantly higher in high-dose males. After the recovery
period ASAT was still increased at 100 ppm and IgG was decreased in
males and significantly in females at all dose levels. No effects
were observed on spleen and thymus weights. The NOAEL in this study
is 4 ppm equal to 0.30 mg/kg bw (males) or 0.35 mg/kg bw (females)
(Suter & Horst, 1986b).
Four different experimental groups received 21 dermal
applications of 0, 5, 10 or 20 mg TPTA (purity unknown)/kg bw/day
in a 29-day period. Dosage groups consisted of 12 rats/sex in the
0 and 20 mg/kg groups and of 6 rats/sex in the 5 and 10 mg/kg
groups. At the end of the treatment period 6 rats/sex of the 0 and
20 mg/kg groups were kept for a 14 day recovery period. Rats were
observed for clinical signs, body weight, food consumption,
haematology, blood chemistry, urinalysis, organ weights, macroscopy
and histopathology. Piloerection, mydriasis, increased muscle tones
and dyspnoea were observed in high dose rats. Dose-related increase
in erythema and scale formation was observed in all treated groups.
Dose-related cracked skin and thickening of the skin fold was
observed at 10 and 20 mg/kg groups. Crusts and slight oedema were
observed in some animals at 20 mg/kg. At 20 mg/kg 4 animals died.
Lymphocytes decreased in males and females at 20 mg/kg. Monocytes
increased in females at 20 mg/kg. Sodium level decreased in all
treated males (n.d.r.) and females. Potassium decreased in 20 mg/kg
females. Inorganic phosphorus and total protein increased in 20
mg/kg females. A/G-ratio and albumin increased in 20 mg/kg males.
A1-globulins decreased in 20 mg/kg males. Macroscopy and
microscopy showed dose-related skin lesions such as hyperkeratosis
and epidermal hyperplasia in all treated groups. All effects were
reversible. No effects on spleen weights were observed. The NOAEL
for systemic toxicity in this study is 10 mg/kg bw (Leist, 1988).
TPTH (purity 97.1%) was applied to the shaven unabraded backs
of Charles River rats (10/sex/group) at doses of 0, 5, 10 or 20
mg/kg bw for 15 applications in 3 weeks. No treatment-related
effects were observed on food consumption, haematology, clinical
chemistry, urinalysis nor organ weights. Male body weight gain was
very slightly decreased in all treated groups and significantly
decreased during the first week at the highest dose. Dermal
irritation, increasing with each application, was observed in all
treated rats during the first 2 weeks. At the third week the
irritation was less severe and severe erythema was not present any
more. At macroscopy an increased incidence in ulcerated, exfoliated
and thickened skin was observed in all treated groups. Microscopic
evaluation of the treated skin revealed acanthosis and
hyperkeratosis. Also changes such as focal ulceration and mild
dermal inflammatory changes correlated with the observed dermal
irritation were seen. No systemic toxicity was observed (Laveglia
et al., 1985).
Groups of SPF-Wistar rats (10/sex/group) were exposed to 0.05,
0.5 and 2.0 mg TPTH (purity 96.2%)/m3 for 6 hours/day, 5 days/week
for 13 weeks (nose only exposure). Analytical concentrations were:
0.014, 0.338 or 1.997 mg/m3. The MMAD was 3 µm. The reversibility
of treatment-related changes was studied after a 4-week recovery
period in the control and highest dose group with 10 additional
animals/sex. Observations were made for clinical signs, body
weight, food consumption, ophthalmoscopy, haematology and clinical
chemistry, organ weights (6), macroscopy and histopathology. In the
highest dose group 10 males and 1 female died between test weeks
2-13 compared to none in the other groups. Laboured respiration and
rales were observed only in the highest exposure group. These signs
were still noted during the first week of recovery. RBC, Hb and Hc
were decreased in high exposed females, while platelet count was
increased. WBC was decreased in females at 0.5 and 2.0 mg/m3.
Glucose and total bilirubin were decreased in females at 0.5 and
2.0 mg/m3. Creatinine, albumin, gamma-glutamyltransferase and
potassium levels were decreased in females at 2.0 mg/m3. Calcium
and phosphorus level were decreased in all exposed female groups.
Calcium level decreased also in males at 0.5 and 2.0 mg/m3. IgG
increased in females at the high exposure level and IgM increased
in males at 0.5 and 2.0 mg/m3. Relative lung weights increased in
high exposed males, but no effect on thymus or spleen weight was
observed. Macroscopic examination showed lesions in the form of
multiple red foci in the lungs of most dead rats. Histopathology
revealed in both sexes at 2.0 mg/m3 degenerative and/or
inflammatory lesions in the anterior part of the nasal cavity, in
the trachea and in the lungs. The lower air passages and in
particular the lungs were severely affected. The pathomorphologic
sequence of events in the lungs seemed to start with degeneration
and multifocal necrosis of the bronchial epithelium followed by
multifocal or lobular fibrinous bronchopneumonia. The NOAEL in this
study is 0.05 mg/m3 (Duchosal et al., 1989).
Groups of 10 rats/sex were fed diets containing TPTA (purity
not given) at a rate of 0, 37.5, 150 or 625 ppm, during 30 days. No
effects were found on haematology and urinalysis. Seven rats/sex
from the 625 ppm group died during the study. Signs in this group
were piloerection and deterioration of the general condition. At
150 and 625 ppm temporary body weight gain decreases were seen.
Histopathology showed gastrointestinal haemorrhages and hepatic
changes (effects on cell metabolism) in the 625 ppm group. The
NOAEL in this study is 37.5 ppm, equivalent to 1.9 mg/kg bw.
(Summary only) (Hollander & Weigand, 1974c).
Guinea-pigs
Groups of guinea pigs (10/sex/group) were fed a diet
containing TPTA (purity not given) at a rate of 0, 7.5, 30.0 or 125
ppm. No effects were found on haematology nor urinalysis. In the
125 ppm group 8 male and 5 female guinea pigs died during
treatment. Effects found in this group included poor general
condition, piloerection and decrease in body weight gain.
Histological evaluation revealed fatty changes in the livers of
guinea-pigs treated with the highest dose (summary only) (Hollander
& Weigand, 1974c)
Dogs
Male and female dogs (beagle, 10/sex/group) received a diet
containing 0, 2, 6 or 18 ppm TPTH (purity 97.2%) for 52 weeks.
Interim necropsy on 2 dogs/sex/group was performed after 4, 13 and
27 weeks. Observations were made for clinical signs, food
consumption, body weight, hearing, ophthalmoscopy, haematology,
clinical chemistry (including immunoglobulins) and urinalysis. The
weights of 12 organs/animal were recorded (including spleen and
thymus). Gross necropsy and histopathology (about 40
tissues/animal) were carried out. A/G-ratio decreased in males at
18 ppm after 26 and 52 weeks. In females albumin decreased at 6 and
18 ppm after 52 weeks. In females at 18 ppm relative liver weight
was increased. The NOAEL in this study is 6 ppm, equal to 0.21
mg/kg bw (Sachsse et al., 1987)
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
37.5 or 75 ppm TPTH (purity ?) for 78 weeks. There was an
additional observation period of 26 weeks for both groups. Twenty
male and 20 female mice were used as controls. Dosed feed
preparations containing 37.5 or 75 ppm TPTH were analyzed
spectrophotometrically. 88% of the theoretical amount of TPTH was
recovered immediately after preparation but after 1 week only 57.9%
was recovered. The data were not corrected for any loss which may
have been due to the instability of TPTH. No treatment-related
effects were observed on clinical signs and body weight. Survival
was decreased in female mice (survival for males was: 65%, 72% and
76% and for females 95%, 74% and 66% for 0, 37.5 and 75 ppm,
respectively). At histopathology no increase in tumour incidence
was observed (NTP, 1978)
Groups of CD1-Charles River mice (60/sex/group) were fed diets
(prepared at weekly intervals) containing 0, 7, 28 or 52 ppm
technical TPTH (purity 96.3%) for 18 months. Observations included
clinical signs, mortality, body weight, food consumption,
macroscopy and histopathology (19 tissues). Survival rate was
significantly decreased in male mice in the mid and highest dose
groups (70%, 70%, 41% and 41% at 0, 7, 28 and 52 ppm,
respectively). Survival rates for females were 55%, 71.7%, 53.3%
and 58.3% at 0, 7, 28 and 52 ppm, respectively). The frequency of
aggression, alopecia, irritation, laceration and scabs was
dose-relatedly increased in both males and females. Food
consumption was reduced during some short periods in all treated
females. At histopathology an increase in the occurrence of
endometrial hyperplasia with cyst formation of the uterus was
observed at 28 and 52 ppm. The tumour incidence was not increased
(Terrel & Reddy, 1978). Extensive histomorphologic examination of
the uterus revealed that the observed cystic endometrial
hyperplasia was considered to be a hyperplastic reaction and not
neoplastic (Brown, 1985).
Groups of 50 male and 50 female mice (KFM-Han. NMRI) were fed
diets (prepared fresh daily) containing 0, 5, 20, 80 ppm TPTH
(purity 97.2%) for 80 weeks. Observations were made for clinical
signs, body weight, food consumption, ophthalmoscopy, haematology
(differential wbc count), clinical chemistry (including
immunoglobulin classes and subclasses) and organ weights (no spleen
weight). Gross pathology and histopathology (35 tissues including
bone marrow smears) were carried out. Intercurrent mortality was
significantly increased for 80 ppm females. At 80 ppm mice showed
ruffled fur towards the end of the study as well as skin lesions.
Body weight gain was reduced for 80 ppm males and 20 and 80 ppm
females. IgG, IgA, IgM, IgG4(f), IgG2a(f), IgG2b and IgG3 were
slightly or moderately decreased at 80 ppm. The decrease in IgA was
also found at 20 and 5 ppm (n.d.r.) and in IgM at 20 and 5 ppm in
male mice. Relative liver weight was markedly increased and
relative brain weight slightly increased at 80 ppm males and
females. Relative kidney weight was decreased at 80 ppm in males
and females. Macroscopic examination showed nodules, foci and
nodular lesions in the livers of mice in 80 ppm males. Skin lesions
were observed in both sexes at 80 ppm. Microscopy showed a higher
incidence of slight to moderate, focal to multifocal, nodular
hyperplasia in the liver of 80 ppm males and females. A higher
incidence of hepatocellular adenomas was observed at 80 ppm
(incidence: 12.2%, 20%, 26% and 32% for 0, 5, 20 and 80 ppm,
respectively, for male mice, and 0% in 0, 5 and 20 ppm females and
18% in 80 ppm females; only significant at 80 ppm). An increased
incidence in hepatocellular carcinomas was observed in 80 ppm
females. The incidence was 6% compared to 0% in the 0, 5 and 20 ppm
groups. An increased incidence of chronic suppurative dermatitis
and related non-specific skin lesions including acanthosis and
hyperkeratosis was observed in 80 ppm males and females. An
increased relative number of lymphoid cells was detected in femoral
bone marrow myelograms for all treated groups. The relative numbers
of granulocytes were significantly decreased for females at 5 ppm
and both sexes at 20 and 80 ppm. The relative numbers of
erythrocyte precursors were significantly decreased for males at
20 and 80 ppm. However, it is difficult to evaluate these data in
the absence of red cell counts in peripheral blood. Although a
reduction in serum immunoglobulin concentrations was reported, the
Meeting noted that there was no corresponding effect on
haematological parameters and that the method of analysis has not
been validated in rodents. The data on serum immunoglobulin
concentrations was therefore not utilized in the determination of
the NOAEL for this study. Based on reduced body weight gain, the
NOAEL is 5 ppm, equal to 1 mg/kg bw/day (Tennekes et al., 1989a).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
prepared weekly containing 37.5 or 75 ppm TPTH (purity not stated)
for 78 weeks. There was an additional observation period of 26
weeks for both groups. Twenty male and 20 female rats were used as
controls. Feed preparations containing 37.5 or 75 ppm TPTH were
analyzed spectrophotometrically. Of the theoretical amount of
TPTH, 80% was recovered immediately after preparation but after 1
week only 57.9% was recovered. The data were not corrected for any
loss which may have been due to the instability of TPTH. No effects
were observed on clinical signs, mortality, food consumption,
macroscopy nor histopathology (31 tissues including pituitary gland
and testes). Body weight was slightly but dose dependently
decreased in male rats after week 40. Survival rates were 75%, 76%
and 84% for males and 90%, 80% and 90% for females at 0, 37.5 and
75 ppm TPTH. The tumour incidence was not enhanced (NTP, 1978).
Groups of 70 rats/sex (SPF-bred KFM-Han. Wistar) rats received
a diet containing 0, 5, 20 or 80 ppm TPTH (purity 97.2%) for 104
weeks. The diets were prepared twice monthly and frozen. A fresh
amount of diet was given daily. Observations were made for
clinical signs, food consumption, body weight, ophthalmoscopy,
haematology, clinical chemistry (including immunoglobulin classes
and subclasses), urinalysis, organ weights, macroscopy and
histopathology (about 40 tissues/animal, including bone marrow
smears). Mortality was increased (dose-related) in all treated
females. The survival was 75, 51, 36 and 23%, respectively.
Preceding mortality clinical signs were ruffled fur, reduced
activity, ataxia, stiff gait and hunched posture. Due to this
excess mortality no laboratory investigations could be made for the
80 ppm female group at 104 weeks. Group mean food intake was
reduced for males and females at 80 ppm. Body weight gain was
dose-relatedly decreased in males and females at 20 and 80 ppm.
Ophthalmoscopy showed an increased incidence of unilateral or
bilateral corneal opacity in males at 20 and 80 ppm at 104 weeks.
Hb and haematocrit were decreased in female rats at 20 and 80 ppm.
ASAT, ALAT, ALP were increased in males and females at 80 ppm. The
following changes in immunoglobulin levels were observed only after
50 weeks: IgG1 levels were reduced for all treated females and 80
ppm males, IgG2a levels were reduced for all treated females and
IgG2c levels were reduced for all treated males, IgA levels were
reduced for males at 20 and 80 ppm and IgM levels were increased
for both sexes at 20 and 80 ppm. At termination most relative organ
weights were increased in the 80 ppm groups, including spleen
weight. An increased incidence of pituitary adenomas was diagnosed
in females at 20 and 80 ppm. The recorded incidences were 64.4%,
63.2%, 76.8% and 93.1% for 0, 5, 20 and 80 ppm groups,
respectively. An increased incidence of testicular Leydig cell
tumours was diagnosed at 80 ppm. The recorded incidences were 1.7%,
8.5%, 5.0% and 16.7% for 0, 5, 20 and 80 ppm, respectively.
Non-neoplastic lesions observed included an increase in hyperplasia
of the pars intermedia of the pituitary gland in males at 80 ppm.
An increased incidence of cystoid change of the pars intermedia of
the pituitary gland was noted in males at 20 and males and females
at 80 ppm at 52 weeks. Increased incidences of testicular Leydig
cell hyperplasia and tubular atrophy were noted in rats at 80 ppm
after 104 weeks. Increased incidences of hepatic bile duct
proliferations and portal sclerosis were noted in females at 20 and
80 ppm after 52 and 104 weeks of treatment. An increased incidence
of skeletal muscle atrophy was noted in male rats at 80 ppm after
104 weeks of treatment. Because mortality was increased in females
and serum immunoglobulins were decreased at the lowest dose level
of 5 ppm (equal to 0.3 (males) 0.4 (females) mg/kg bw no NOAEL
could be established (Tennekes et al., 1989b).
Reproduction studies
In a range-finding study Wistar rats (10/sex/group) were
administered 0, 12.5, 25, 50, 100 or 200 ppm TPTH (weekly prepared,
purity not stated) in the diet for in total 90 days (from 59 day
pre-mating period up to 21 day gestation period). All animals were
killed following weaning. Observations included clinical signs,
mortality, body weight, organ weight and food consumption. Parents
were observed haematologically and histopathologically. Pups were
sexed, weighed and observed for gross abnormalities. Severe
clinical signs like rough coat, hunched back, lethargy, nasal
discharge and alopecia were observed in rats at 200 ppm. At 200 ppm
body weight gain was significantly reduced in males and food
consumption was slightly reduced in male and female rats. White
blood cell count was significantly decreased in high dose females.
A lower pregnancy rate (4/10) was observed in females at 200 ppm,
no viable offspring resulted. At 100 ppm pup survival, pupweight
and litter size (at 50 ppm not significantly) were dose-relatedly
decreased. At histopathology necrosis of the renal papilla or
medulla was observed in males at 100 and 200 ppm. One high-dose
male rat that died after six weeks showed hepatic, thymic and
testicular necrosis (Carlton & Crisp, 1982).
In a two-generation reproduction study groups of Wistar
(Crl:(WI)BR)COBS rats (30/sex/group) were given diets (prepared
every 5-7 days) containing 0, 0', 5, 18.5 or 50 ppm TPTH (purity
97.2%) during growth, mating, gestation and lactation for one
litter per generation. Observations included clinical signs, body
weight, food consumption, mating performance and reproductive
parameters. Organ weights (including spleen and thymus) of parents
and pups were recorded and the parents were examined histologically
(control group 1 and 50 ppm groups only). Pups were sexed, and
examined for gross malformations and the number of stillborn and
live pups were recorded.
Body weight gain and food consumption were occasionally
decreased in F0 parents at 50 ppm. The number of dead F1 pups
increased and mean litter size decreased at 50 ppm. Mean pup weight
was decreased at 50 ppm on lactation days 14 and 21. In F0 parents
the body weight gains and food consumption of both sexes were lower
at 50 ppm and occasionally at 18.5 ppm. Mean litter size decreased
at 18.5 and 50 ppm. Viability of F2 pups at 50 ppm, prior to
culling was slightly decreased. Mean pup weight was decreased at 50
ppm on lactation days 7, 14 and 21. At 18.5 mg/kg a slight decrease
was seen. Pups from one litter at 50 ppm appeared emaciated on
lactation day 21. Several pups in another litter in this group had
wet yellow urogenital staining on lactation day 21. At 50 ppm the
relative weights of brain, testes, ovaries, adrenals, kidneys
spleen and heart were increased at several occasions in F0 and/or
in F1 adults and/or F1 and F2 weanlings (males and females). At
18.5 ppm increases were also observed for kidneys, testes and heart
weights, whereas at 5 ppm only relative heart weight was increased
in male F1 adults. A dose-related decrease was observed in spleen
and thymus weight in F2 male and female weanlings at 50 and 18.5
ppm. In F1 weanlings the same trend was observed. The NOAEL in
this study is 5 ppm, equal to 0.4 mg/kg bw/day (Young, 1986).
Two groups of 13 male rats (29 days old) received a diet
containing 0 or 20 mg TPTA/kg bw for 25 days. Four animals from
each group were sacrificed on day 21 and the remaining animals
received the test diet for another 4 days and a control diet for an
additional 70 days at which time they were sacrificed. Observations
were made for the distribution of the eight phases of
spermatogenesis. In rats sacrificed after 21 days all 8
spermatogenic phases were seen, but there was a general paucity of
mature sperm and the distribution showed some predominance of
immature sperm. Recovery was seen after the 70 days control diet
(Snow & Hays, 1983).
Special studies on embryotoxicity and/or teratogenicity
Rat
Pregnant Sprague-Dawley rats (20/group) received by gavage 0,
1.25, 5, 8.75 or 12.5 mg/kg TPTH (purity unknown) bw/day, day 6
through 15 of gestation. The rats were sacrificed on day 20 of
gestation. Observations were made for body weight, number of
implantations, number of live and dead fetuses, number of
resorptions and number of corpora lutea. The fetuses were weighed
and examined for external, visceral and skeletal abnormalities.
Three animals each in the 8.75 and 12 mg/kg groups aborted. Only
those animals showed abnormal behavioural reaction. One female in
the 12.5 mg/kg group died. Females at 8.75 and 12.5 mg/kg showed a
lower food consumption and lower body weight gain (dose-
relatedly). In the 8.75 and 12.5 mg/kg groups an increase in
resorptions was observed. Fetuses from these groups also exhibited
a lower weight and a decrease in viable fetuses. A non dose-related
increase in hydrocephalus and hydronephrosis was seen in all
treated groups (2%, 5%, 12%, 16% and 6%, respectively) (Ravert &
Parke, 1976).
In a teratogenicity study (Segment II + III) groups of 25
pregnant Sprague-Dawley rats were given oral daily doses of 0,
0.35, 1, 2.8 or 8 mg TPTH (purity 97.1%)/kg b.w. in corn oil from
day 6 or 15 of gestation. All animals were allowed to deliver
naturally and rear the offspring to weaning on lactation day 21. At
lactation day 4, 10 pups/litter were selected and killed on the
28th day. The dams were killed on day 21 of lactation. Dams were
observed for mortality, clinical signs and body weight. Litters
were examined for gross malformations and the number of live and
dead fetuses was recorded. Fetuses were observed for bodyweight and
examined for haematology, clinical chemistry and urinalysis,
macroscopy, kidney weight and histopathology of the kidney. Dams at
8 mg/kg bw showed lethargy, yellow staining in the urogenital area,
and a more extensive hairloss than dams in the other groups. At the
same dose group maternal body weight gain was significantly
decreased (only during treatment) and gestation length was
significantly increased, and pup survival was decreased on
lactation day 0. Only LDH values in blood decreased in pups at 1,
2.8 and 8 mg TPTH/kg bw and ASAT was decreased in females pups at
all doses. Globulin levels were significantly decreased in female
pups at 2.8 and 8.0 mg/kg bw and A/G ratio was increased at the
highest dose. Relative kidney weight in pups was significantly
increased at 2.8 and 8 mg/kg bw. The dose of 1 mg/kg bw can be
regarded as a NOAEL for embryotoxicity (Rodwell, 1985a).
Twenty to 26 pregnant rats per group received by gavage 0,
1.0, 2.8 and 8 mg TPTH (purity unknown)/kg bw/day suspended in corn
oil, day 5 through 19 of gestation. On day 20 all females were
sacrificed. Uterus weight was determined and corpora lutea were
counted. Fetuses and resorption sites were noted. Fetuses were
weighed, sexed and observed for external, internal and skeletal
malformations. In the 2.8 and 8 mg/kg groups a dose-related nasal
and oral discharge were observed. In the highest group two animals
died. Body weight gain was significantly reduced at 8 mg/kg. The
number of dead or resorbed fetuses was higher at 8 mg/kg. Pup
weight was depressed in the 8 mg/kg group. The occurrence of
hydro-ureter was increased at the highest dose (Carlton & Connell,
1981; Carlton, 1985).
In order to clarify the occurrence of hydronephrosis,
hydrocephalus and hydroureter, another rat study was carried out.
Groups of 45 pregnant rats received 0, 0.35, 1, 2.8 and 8 mg TPTH
(purity 97.1%)/kg bw/day day 6 through 15 of gestation. Day 20 of
gestation all rats were sacrificed. Throughout gestation
observations were made for clinical signs, body weights and food
consumption. After sacrifice the dams were observed for number and
location of viable and nonviable fetuses, early and late
resorptions and the number of implantation sites. The corpora lutea
were counted. Fetuses were weighed, sexed and examined for
external, internal and skeletal anomalies. During the treatment
period, hair loss was more extensive in the 8 mg/kg group. A few
animals in this group were emaciated, lethargic and had yellow
staining in the urogenital area. One female in this group had an
abortion. A dose-related decrease in body weight gain and food
consumption was apparent in the 2.8 and 8 mg/kg groups. Number of
nongravid females, number of total litter resorptions and early
resorptions increased at 8 mg/kg. Number of viable fetuses and
fetal weight decreased significantly at 8 mg/kg. The incidence of
absent or delayed ossification in high dosed litters was increased.
The percentage of fetuses with hydrocephaly was 0.4, 0, 0, 0.4 and
1%, respectively, and for fetuses with omphalocele 0.2, 0.2, 0.2 0,
and 0.5%, respectively for the 0, 0.35, 1, 2.8 and 8 mg/kg groups.
The NOAEL for maternal toxicity was 1 mg/kg bw and the NOAEL for
embrytoxicity was 2.8 mg/kg bw. There was no evidence for
TPTH-induced irreversible structural effects (Rodwell, 1985b).
In a special study consisting of three tests the potential of
TPTH to induce alterations in the urogenital tract of exposed rat
fetuses was investigated. In test I pregnant Sprague Dawley rats
received 0, 4 or 8 mg TPTH (purity unknown)/kg bw by gavage during
day 7 through 20 of gestation. In test II (a and b) doses of 0 and
4 mg/kg bw were given. All females from test I and IIa were
sacrificed on day 21 of gestation. The uteri were removed, weighed,
and examined to determine the number of live, dead and resorbed
fetuses. Live fetuses were examined for gross external anomalies.
In test IIb the animals delivered normally and weaned their young
for 28 days. In these young animals renal morphology and function
were examined. At 8 mg/kg bw maternal (death and reduced
extra-uterine weight gain during pregnancy) and fetal (mortality
and decreased body weight) toxicity were observed. There was no
effect on cerebral ventricles. At 4 mg/kg bw only slightly dilated
renal pelvis was seen and at 4 and 8 mg/kg bw (n.d.r.) dilated
ureter was observed. In a replicate test (IIa) these effects were
hardly observed. In test IIb no effects at all where found for the
renal function or morphology. Only the treated pups only showed a
lower pup weight (Kavlock, 1985).
Four groups of 12 pregnant rats (Sprague Dawley) received from
day 6 through 15 of gestation 0, 5, 10 or 15 mg TPTA (purity
97.1%)/kg bw/day. Females were sacrificed on day 21 of gestation.
Dams were observed for number of resorptions, number of
implantations, post-implantation loss, viable and non viable
fetuses. Fetuses were weighed and examined for external, visceral
and skeletal anomalies. Two rats treated with 15 mg/kg died during
the study and 4 had complete resorptions. Average maternal weight
gain decreased at 5 mg/kg and significantly 10 and 15 mg/kg.
Post-implantation loss increased significantly at 15 mg/kg. A
significant reduction of some ossification centers, mainly at the
level of the metacarpus and caudal vertebrae, was seen in all
treated groups. Minor anomalies (hydroureter, vertebrae missing or
bipartite) increased significantly at 15 mg/kg (Giavini et al.,
1980).
Groups of pregnant rats (Wistar) received during day 7 through
16 of gravidity 0, 1.25, 3.2 or 8 mg TPTA/kg bw/day. TPTA (purity
97.0%) was dissolved in sesame oil. The females were sacrificed and
delivered on day 21 of gestation. The dams were observed for
clinical signs, body weight, food consumption, number of
implantations, resorptions and corpora lutea, viable and non viable
fetuses, organ weights (heart, liver, kidneys and spleen) and
macroscopy. Fetuses were weighed, and examined for length, sex,
external, internal and visceral anomalies. Ten dams at 8 mg/kg had
abortions. Dams at 8 mg/kg showed signs of clinical intolerance as
piloerection, bloody nose secretion, local hair loss accompanied by
scab formation. Body weight gain and food consumption were
decreased in this group. At 8 mg/kg early and total intrauterine
deaths increased and number of implantations, total live fetuses,
fetal body weight and crown/rump length decreased. At 8.0 mg/kg an
increase in fetuses with non ossification or only weak ossification
of the sternebrae was observed. At 8 mg/kg also an increase (5%) in
distended uni- or bilateral ureter was observed. The NOAEL in this
study is 3.2 mg TPTA/kg bw (Baeder, 1987a).
Hamsters
Four groups of 20-25 pregnant Syrian hamsters received by
gavage 0, 2.25, 5.08, or 12 mg TPTH (purity not given)/kg bw in
0.3% hydroxypropyl cellulose in saline from day 5 through 14.
Vitamin A palmitate was administered by gavage on day 8. All dams
were sacrificed on gestation day 15. The weight of the gravid
uterus was determined and corpora lutea were counted. Fetuses and
resorption sites were noted. Fetuses were weighed and observed for
external, visceral and skeletal malformations. Four animals died in
the highest-dose group and several animals showed an abnormal
behaviour. In the mid-dose group and in the lowest-dose group 2
animals died. Hamsters from the highest-dose group showed a
decreased mean body weight gain and a decreased food consumption.
Fetuses from this group showed also a decreased pup weight. The
average number of minor anomalies (such as kinky tail, haematomas
and anaemic appearance) of fetuses per litter was significantly
greater among the 12 mg/kg group. A significantly greater number of
skeletal variations (poorly ossified, missing metatarsals or
missing sternebrae) was observed among the 12 mg/kg group. Three
cases of hydrone-phrosis were seen at 5.08 mg/kg bw and 1 case of
hydrocephalus was seen at 12 mg/kg bw (Carlton & Howard, 1982;
Carlton, 1985).
Rabbits
Three groups of 22 pregnant New Zealand White rabbits received
by gavage 0, 0.1, 0.3 or 0.9 mg TPTH/kg bw/day mixed in 1% aqueous
CMC, day 6 through 18 of gestation. Dams were sacrificed day 29 of
gestation. Observations were made for clinical signs, body weight
and food consumption. After sacrifice corpora lutea, early and
late resorptions and number of implantations were counted. All
fetuses were weighed, sexed and examined for external, skeletal and
visceral anomalies and developmental variations. Two rabbits from
the 0.9 mg/kg group aborted. A dose-related decrease in mean body
weight gain and food consumption was observed in the 0.3 and 0.9
mg/kg groups. Mean fetal weight was lower in the 0.9 mg/kg group.
The NOAEL for maternal toxicity was 0.1 mg/kg bw and the NOAEL for
embryotoxicity was 0.3 mg/kg bw (Rodwell, 1987).
TPTA (purity 97.0%) was dissolved in sesame oil and
administered orally by gavage to groups of 15 pregnant rabbits
during day 6 through 18 of gestation. The dose levels were 0, 0.1,
0.32 or 1.0 mg/kg bw/day. On day 29 of gestation the dams were
sacrificed. Dams were observed for clinical signs, body weights,
food consumption, number of resorptions, number of implantations,
number of corpora lutea, viable and non viable tissues, organ
weights (heart, liver kidney, spleen) and macroscopy. Fetuses were
weighed and examined for sex, length, external, internal and
skeletal anomalies. In the 1.0 mg/kg group 1 dam died, 3 dams
aborted, 1 dam was sacrificed due to a premature delivery and 2
dams had only intra-uterine deaths. Three dams showed vaginal
haemorrhages. The water consumption and food consumption of dams at
1 mg/kg decreased. The number of implantations and of live fetuses
decreased at 1 mg/kg. Mean fetal weight, mean crown/rump length
and mean placental weight decreased in pups at 1 mg/kg. At 1 mg/kg
4 pups (15.4%) showed omphalocele with protrusion of intestinal
coils or liver tissue. Slight retardation of skeletal ossification
was detected in the 1.0 mg/kg group. There was an increase in the
number of fetuses with fewer than 13 ossified caudal vertebrae and
some fetuses showed weak ossification of the hyoid bone or non
ossification or only slight ossification of the os pubis. The
NOAEL for maternal and embryotoxicity in this study is 0.32 mg/kg
bw/day (Baeder, 1987b).
Special studies on immunotoxicity
Seinen and Penninks (1987) reviewed the available data on
immunotoxic properties of TPTH from the published literature and
confidential studies provided by the manufacturer. They concluded
that subacute treatment with TPTH resulted in lymphopenia and
lymphocyte depletion of spleen and thymus. After treatment up to 13
weeks these effects appeared to be transient. Rats fed 25 ppm TPTH
(not 5 ppm) showed cell mediated immunity as measured by slightly
suppressed DTH (delayed type hypersensitivity) to ovalbumin and
tuberculin. The humoral immunity was not affected at doses up to 25
ppm. However in mice dosed with TPTH up to 20 mg/kg bw for 14 days
DTH response to keyhole limpet haemocyanin was not affected,
whereas the humural activity was suppressed at 20 mg/kg bw. In
various other immune function tests no disturbances were observed.
The authors concluded therefore that TPT-compounds display weak
immunosuppressive potential. This immunosuppressive activity is
observed at dose levels that markedly reduce the numbers of blood
lymphocytes and the weight of the lymphoid organs (Seinen &
Penninks, 1987). The original data for certain studies in this
review were not available to the JMPR.
Mice
In a limited experiment young and mature mice were fed a diet
containing TPTA (purity >99%) at a rate of 30, 100 or 300 ppm for
4 days. Feeding resulted in a dose-related decrease in spleen,
heart and liver weight and number of blood leucocytes (Ishaaya
et al., 1976; Seinen & Penninks, 1987).
Groups of B6C3F1/CrlBR mice (18/sex/group) were administered
0, 1, 5, 25, 50 or 125 ppm TPTH (purity 96.4%) for 28 days. A
positive control group (18/sex) was fed control diet and received
a single i.p. injection of 180 mg cyclophosphamide/kg bw 48 hours
prior to the end of the treatment period. Twelve male and 12
female mice were killed at day 29 and the remaining mice were
returned to control diets and were killed on day 57. Observations
were made on survival and clinical signs, body weight, food
consumption, haematology, liver, spleen, and thymus weight,
macroscopy and histopathology. Special immunotoxicological
parameters as splenocyte counts, thymocyte counts, number of
splenic T- and B-cells and basal serum immunoglobulin levels were
recorded and the bone marrow was examined. A significantly
decreased body weight gain was observed in male and female mice at
125 ppm, from which they recovered after 28 days. Food consumption
was significantly decreased at 50 and 250 ppm. Relative liver
weight was increased at 25 (females only), 50 and 125 ppm, relative
spleen weight was clearly decreased in males at 50 and 125 ppm and
in females at 25 ppm and higher and relative thymus weight was
decreased at 125 ppm in males. At histopathology lymphoid depletion
in the thymus and spleen was observed in mice at 125 ppm. No
dose-related effects were observed on any of the bone marrow
parameters (note: only 50% of the smears could be evaluated,
because of technical problems). The main haematological effects
were a decrease in total white blood cells, neutrophils and
lymphocytes at 50 and 125 ppm in males and females. Dose-related
changes in haematological parameters were observed in males at 25,
50 and 125 ppm and in females in all dose levels (MCH and MCHC). At
the highest dose a decrease in total cells/spleen and splenic
B-cells were observed and a decrease in total cells/thymus and
splenic T-cells were seen in males. IgM levels were decreased in
females at 25 ppm and higher, but were not clearly dose-related.
All effects were reversible. Mice fed cyclophosphamide showed
decreased spleen and thymus weight accompanied by histologic
evidence of lymphoid depletion. Cyclophosphamide also altered the
% T- and % B-cells in both sexes and reduced the spleen and thymus
cellularity. The effects were not reversible. The NOAEL for
immunotoxicity is 5 ppm, equal to 1 mg/kg bw for males and 1.15 for
females (McCormick & Thomas, 1990a).
Rats
A similar study was conducted in 18 male and 18 female Wistar
rats. A positive control group was fed a diet containing 125 mg
DOTDC (dioctyltin-dichloride)/kg. No effects were observed on
mortality, clinical signs, body weight, organ weight, macroscopy.
Food consumption was decreased in female rats at 125 ppm. No
dose-related effects were seen at histopathology (note only 50% of
the bone marrow smears could be used in the histopathological
evaluation). In both males and females red blood cell parameters
were affected at the highest groups. The white blood cells and
lymphocytes were significantly decreased in males at 125 ppm and
females showed a tendency to a decrease at 50 and 125 ppm. In male
rats at 50 and 25 ppm platelet count was increased. No effect was
observed on the viability of the thymus or spleen cells nor on the
percentages of splenic T- and B-cells in either sex. IgG levels
were significantly reduced in females at 50 and 125 ppm. All
effects were reversible. The effects in the positive control group
were as expected. These effects were largely reversible following
cessation of DOTDC exposure. The NOAEL for immunotoxicity is 25 ppm
equal to 1.82 mg/kg bw for females and 1.71 mg/kg bw for males
(McCormick & Thomas 1990b).
In order to investigate the reaction of rats to infection with
Trichinella spiralis female rats were administered 2.5 mg TPTH
(purity 97.2%)/kg bw over a 10 day period. This administration had
no effect on host resistance in contrast to the positive controls
dexamethasone and cyclophosphamide. There are also no effects on
additional parameters, i.e., thymus weights, percentage of
lymphocytes in the differential blood count, and body weight gains
(Diehl & Leist, 1987b).
Special studies on genotoxicity
TPTH as well as TPTA were tested in various genotoxicity
assays. See Tables 3 and 4 respectively, for a summary of the
studies considered. In most of the assays negative results were
obtained. The positive results in the chromosome aberration assays
are probably related to the toxic effect of the compound on
T-lymphocytes and not to a genotoxic action of TPTH.
Special studies on sensitization
At concentrations which were irritant to the skin, TPTH
(purity 97.0%) showed no skin sensitization in guinea-pigs in the
Buehler test (Leist & Weigand, 1981f; Schollmeier & Leist, 1989)
nor in the maximization test (Diehl & Leist, 1987a).
TPTA gave a positive response when tested for skin
sensitization in guinea-pigs in the Buehler test (Diehl & Leist,
1986e).
Table 3. Results of genotoxicity assays on TPTH
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Ames test a,b S. typhimurium 0.31-5 µg/pl 97.0 Negative Richold et al. (1981)
TA 1535, TA100, in DMSO
TA1537, TA98,
TA1538
E. coli WP2 uvra 313-5000 µg/pl 97.0 Negative
in DMSO
Yeast forward S. pombe p1 0.05-1 µg/ml 97.2 Negative Milone & Hirsch (1985b)
mutation test in DMSOa
Mitotic gene Saccharomyces 0.1, 1, 3 or 97.2 Negativec Milone & Hirsch (1985a)
conversion assay cerevisiae D4 5 µg/mla
1, 5, 10 or
15 µg/mlb
Mouse lymphoma L5178Y mouse 10-150 ng/mla 97.2 Negative DenBoer & Hoorn (1985)
forward mutation lymphoma cells (>80 ng/ml toxic) Weakly
assay (TK +/-) 40-600 ng/mlb positive
(>300 ng/ml toxic)
Mouse lymphoma L5178Y mouse 0.01-2 µg/1a ? Positive NCI (1984)
forward mutation lymphoma cells (>0.1 toxic) Negative
assay (TK +/-) 1.7-8 µg/1b
Chromosome Human lymphocytes 31.25-1000 ng/mla Positive Kirkland (1985)
aberration assay 250-2000 ng/mlb
both in DMSO
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Chromosome Human lymphocytes 0.681-1.47 µg/mla 96.2 Negative Nunziata & Conzonni (1988)
aberration assay in DMSO Positive
0.316-1.47 µg/mlb
in DMSO
Unscheduled Rat hepatocytes 0.01-2.0 µg/ml in DMSO 97.2 Negativec Cifone & Myhr (1985)
DNA synthesis >0.25 µg/ml toxic
Survival (cell Rat embryo cells 0.019 µg/5.2 x 104 cells ? Positived Traul et al. (1981)
transformation) infected with or 0.0145 µg/5.2 x 104
assay leukaemia virus
(2FR450 cells)
Micronucleus test NMRI mouse bone 35, 70 or 140 mg/kg bw po 97.2 Negative Banduhn et al. (1985)
NMRI marrow cells
Cytogenic assay Chinese hamster bone 20, 50 or 80 mg/kg bw po 96.2 Negative Mueller (1987)
marrow cells
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Dominant lethal Sprague-Dawley rats daily ip injections 94.8 Negative Ravert et al. (1978)
assay with 3, 20, 38
or 150 mg/kg bw in
corn oil; 150 mg/kg
bw toxic (8/10
died). Concentration
of TPTA
a without metabolic activation
b with metabolic activation
c positive control yielded positive results
d only summary available
Table 4. Results of genotoxicity assays on TPTA
Test system Test object Concentration of Purity Results Reference
TPTA (%)
Ames testa,b S. typhimurium 0.032-1000 µg/plate 97.1 Negative Jung & Weigand (1986)
TA100, TA1535, in DMSO
TA1537, TA1538,
TA98
E. coli WP2 uvra
Gene conversiona,b S. cerevisiae D4 1-50 µg/ml in DMSO 97.1 Negative Milone & Hirsch (1986a)
Dominant Sprague-Dawley rats daily i.p. injections 94.8 Negative Ravert et al. (1978)
lethal assay with 3, 20, 38 or
150 mg/kg bw in corn oil;
150 mg/kg bw toxic (8/10
died)). Concentration of
TPTA
a without metabolic activation
b with metabolic activation
Special studies on skin and eye irritation
A dose of 500 mg TPTH (purity not stated) diluted in a 0.9%
NaCl solution was slightly irritant when applied under occlusive
conditions to the shaven intact and shaven abraded skin of 6 New
Zealand rabbits for 24 hours (Leist & Weigand, 1981e).
Dilutions of 1:10, 1:100 and 1:1000 prepared from 2.5 g TPTA
(purity not stated) in 0.9% NaCl were examined in the Barail
Intracutaneous test by intracutaneous injections of 0.02 ml
administered to the skin of rabbits. No irritation was seen. Five
applications of 0.5 ml of the TPTA solution to depilated and intact
rabbits skin caused slight local irritation. (Summary only)
(Hollander & Weigand, 1974e).
A dose of 500 mg TPTA (purity 97%) moistened with
physiological saline was applied under semi-occlusive conditions to
the shaven intact skin of 6 New Zealand rabbits for 54 hours. No
irritation was observed up to 72 hours after application (Diehl &
Leist, 1986c).
Application of 100 mg TPTH (purity not stated) diluted in a
0.9% NaCl-solution into the eyes of 9 New Zealand rabbits caused
severe irritation; increasing adverse effects resulted in a
discontinuation of the experiment after 72 hours (Leist & Weigand,
1981e).
Rabbits received an instillation of 0.1 ml of a solution of
2.5 g TPTA (purity not stated) in 0.9% NaCl into one eye. After 24
hours slight local irritation was observed (Summary only)
(Hollander & Weigand, 1974e).
Nine New Zealand rabbits received an instillation of 100 mg
TPTA (purity 97%) into one eye. All rabbits developed severe ocular
lesions (swollen conjunctivae, markedly injected blood vessels,
diffusely crimson red colouring, nacreous opacity and whitish and
reddish brown discharge). After 72 hours all animals were
sacrificed because the lesions were non-reversible (Diehl & Leist,
1986d).
Observations in humans
Two cases of poisoning with Brestan 60 (60% TPTA and 15%
Maneb) have been reported. In both cases (farmers of 75 and 53
years) inhalation of the powder took probably place during
preparation of the spray solution. Symptoms observed were nausea,
dizziness, transient loss of consciousness, convulsions, persistent
headache, and photophobia, as well as impaired liver functions.
Both patients were discharged in good health after 10 and 15 days
(Manzo & Richelmi, 1981).
Acute severe intoxication was reported in a 23-year old male
in an attempted suicide with a TPT-compound (molluscicidal agent,
no specifications given). The patient was suffering from abdominal
pains, diarrhoea and vomiting and also showed reversible acute or
delayed neurological symptoms. The patient's health was completely
recovered within 3 1/2 months (Wu et al., 1990).
COMMENTS
Most toxicological studies on fentin were carried out with
triphenyltin hydroxide (TPTH). Since triphenyltin acetate (TPTA) is
hydrolysed rapidly to TPTH in an aqueous medium, studies with both
compounds have been used for the evaluation of fentin.
After oral administration of 14C-labelled TPTH the
radioactivity was mainly eliminated via the faeces (50-70%), but
also via the urine (20-25%). Biliary excretion was also
demonstrated. After 7 days residual radioactivity was present with
the highest concentration in the liver and the next highest in the
kidneys. After oral administration of 113Sn-labelled TPTH,
elimination was almost exclusively via faeces. In both cases faecal
elimination was biphasic with half-lives of 9 and 50-60 hours. With
113Sn-labelled TPTH the highest concentration was found in the
kidneys. After administration of the 14C-labelled compound,
radioactivity in the faeces of the rat consisted of parent compound
and di- and monophenyltin as well as non-extractable bound residues
and tin (measured as Sn). It was reported that benzene was found in
the faeces. Sulfate conjugates of phenol, hydroquinone, catechol
and resorcinol as well as phenylmercapturic acid were present in
the urine. Phenyltin compounds were not found in the urine.
TPTH is toxic to rats after acute oral administration with
LD50 values of about 160 mg/kg bw. TPTA showed similar acute
toxicity.
In a 3-month study in mice at dietary concentrations of TPTH
of 0, 4, 20 or 100 ppm, a number of effects were observed at 100
ppm. The main effects were a decrease in haemoglobin concentration
and erythrocyte count and an increase in Heinz bodies.
Immunoglobulins were decreased and liver weight was increased. The
NOAEL was 20 ppm, equal to 3.4 and 4.1 mg/kg bw/day for males and
females, respectively.
Dietary concentrations of TPTH of 0, 4, 20 or 100 ppm were
tested in a 13-week study in rats. The NOAEL was 4 ppm, equal to
0.30 and 0.35 mg/kg bw/day for males and females respectively,
based on a decrease in white blood cells and plasma albumin and an
increase in plasma aspartate aminotransferase at 20 ppm.
In a 52-week study in dogs (dietary concentrations of TPTH of
0, 2, 6 or 18 ppm) the NOAEL was 6 ppm, equal to 0.2 mg/kg bw/day,
based on a decreased albumin level and an increase in relative
liver weight at 18 ppm.
Two long-term/carcinogenicity studies in mice and one in rats
were performed in which there was no evidence of carcinogenicity.
However, there were indications in each of these studies that the
doses received by the animals may have been lower than intended
owing to instability of the test compound in the diet.
In a third long-term/carcinogenicity study in mice at dietary
concentrations of TPTH of 0, 5, 20 or 80 ppm, increased liver and
decreased kidney weights, increased nodular hyperplasia and
hepatocellular adenoma and carcinoma occurred at 80 ppm only. The
NOAEL was 5 ppm, equal to 0.85 mg/kg bw/day for males and 1.36
mg/kg bw/day for females, based on decreased body-weight gain at 20
ppm.
In a second long-term/carcinogenicity study in rats (with
dietary concentrations of TPTH of 0, 5, 20 or 80 ppm), an increase
in mortality was observed at all dose levels. A NOAEL could not
be established at the lowest concentration of 5 ppm, equal to 0.3
and 0.4 mg/kg bw/day for males and females respectively. The
incidence of pituitary adenomas was increased in females at 20 and
80 ppm. At 80 ppm, the incidence of Leydig cell tumours was
increased. These changes were accompanied by non-neoplastic
lesions in the pituitary and the testes.
In a 2-generation reproduction study in rats with one litter
per generation (dietary concentrations of TPTH of 0, 5, 18.5 or 50
ppm) fertility was not affected. The NOAEL was 5 ppm (equal to 0.4
mg/kg bw/day) based on decreased litter size, decreased pup weight
and decreased relative spleen and thymus weight in the weanlings.
In several teratogenicity studies with rats, hamsters, and
rabbits, TPTA or TPTH caused maternal and embryo toxicity, but
irreversible structural effects were not observed. In rabbits, the
most sensitive species, the NOAEL for embryo/fetotoxicity was 0.3
mg/kg bw/day in a study that utilized doses of 0, 0.1, 0.3 or 0.9
mg/kg bw/day TPTA. In this study the NOAEL for maternal toxicity
was 0.1 mg/kg bw/day.
Most in vitro and in vivo genotoxicity tests were
negative. However, two human lymphocyte chromosomal aberration
assays and two mouse lymphoma mutation assays were positive. The
latter responses may have been caused by a variety of effects,
including chromosomal aberrations. Since two in vivo studies for
chromosomal aberrations (a micronucleus test in mice and a
cytogenetic test in Chinese hamsters) were negative, it would
appear that any genotoxic properties are of low potency. It was
therefore concluded that fentin does not present a genotoxic hazard
for man.
Effects on the immune system were observed in short- as well
as in long-term toxicity studies. In special studies with mice and
rats, TPTH showed immunosuppressive properties (lymphopenia and
lymphocyte depletion of spleen and thymus), resulting in altered
humoral and cellular immunity. The NOAEL in mice was 5 ppm, equal
to 1 mg/kg bw/day, based on a decrease in splenic weight found at
25 ppm. In rats, the NOAEL was 25 ppm, equal to 1.7 and 1.8 mg/kg
bw/day for males and females respectively, based upon a significant
decrease in immunoglobulin G and a marginal decrease in leucocyte
and lymphocyte counts at 50 ppm.
The Meeting noted that there was increased mortality at the
lowest dose tested in the most recent long-term study in rats (0.3
mg/kg bw/day). Applying a 500-fold safety factor to this LOAEL
would result in approximately the same ADI as the previously
established ADI based upon a NOAEL of 0.1 mg/kg bw/day in an
earlier long-term study in rats. The previous ADI was therefore
retained. This ADI is supported by NOAELs derived from recent
studies, including the NOAEL of 0.4 mg/kg bw/day in the
2-generation reproduction study, the NOAELs in short-term studies
in rats (0.3 mg/kg bw/day) and dogs (0.2 mg/kg bw/day) and in a
teratology study in rabbits (0.1 mg/kg bw/day for maternal
toxicity).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm in the diet, equal to 1 mg/kg bw/day
Rat: <5 ppm in the diet, equal to <0.3 mg/kg bw/day
(long-term/carcinogenicity study)
5 ppm in the diet, equal to 0.4 mg/kg bw/day
(multigeneration reproduction study)
Dog: 6 ppm, equal to 0.2 mg/kg bw/day
Rabbit: 0.1 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Ongoing studies on the endocrinological effects of fentin
2. Observations in humans.
REFERENCES
Baeder, Ch. (1987a) Fentinacetate - substance, technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Wistar rats
after oral administration. Unpublished Report 89.1478 (A42190) of
Pharma Research Toxicology and Pathology, Hoechst AG. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Baeder, Ch. (1987b) Fentin acetate - substance technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished Report 89.1559
(A42324) of Pharma Research Toxicology and Pathology, Hoechst AG.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Bakke, J.E., Feil, V.J., Aschbacher, P.W., & Price, C.E. (1982)
Metabolism of 14C-triphenyltinhydroxide by the rat, sheep and cow.
Unpublished Report (A35976) of metabolism and radiation research
laboratory Agricultural Research Service, Fargo, ND, USA.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Banduhn, N., Sachsse, K. & Terrier, C. (1985) Mouse micronucleus
test with TPTH-technical (HOE 029664 OF ZD97 0004). Unpublished
Report (A31583), project 049522 from RCC, Switzerland. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Brown, W.R. (1985) 18-Month carcinogenicity study of Technical TPTH
96.3% (Lot no. SWRAM-1K) in CD Mice. Histomorphology of the Uterus.
Unpublished Report (A32214) from Research Pathology Services. Inc.,
New Britain, CT, USA Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Brunk, Dr. & Weigand, W. (1981) Acute oral toxicity of
Fentin-Hydroxide, active ingredient (Code: HOE 29664 O F AT201) to
the male and female beagle dog. Unpublished Report 462/81 from
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Beurkle, W.L. (1985). Hoe 002782-14-C (triphenyltin acetate),
analysis of hydrolysis byproducts. Unpublished Report CM009/85
(A31534) from Analytisches Laboratorium, Hoechst. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L., Rutz, U., & Müller, A., Eckert, H.G., Kellner, H.M.
& Esinger, W. (1986) Metabolism in rats after single and repeated
oral administration at the two dosage levels 2 and 10 mg/kg body
weight. Unpublished Report CM 011/85 (A34163) of Radiochemisches
Laboratorium and Analytisches Laboratorium, Hoechst AG. Submitted
to WHO by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L. & Kellner, H.M. (1987) HOE 029664 (TPTH)-14C.
Excretion study in rats with bile fistula following oral
administration of 2 mg a.i./kg body weight. Unpublished Report
(B)97/87 (A36680) of Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. (1985) Triphenyltin hydroxide. Additional data on
Batelle rat and hamster teratology studies (31184). Unpublished
data of Batelle Columbus Laboratories, Columbus, Ohio. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Connell, M.M. (1981) Final report on evaluation of
the teratogenicity of triphenyltin hydroxide (TPTH) in the
Sprague-Dawley rat. Unpublished Report NO723-0200 (A22211) of
Batelle Columbus Laboratories, Columbus, Ohio. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Crisp, C.E. (1982) Range-finding study for the
evaluation of reproductive effects of triphenyltin Hydroxide (TPTH)
in the Wistar rat. Unpublished Report (A25091) project NO723-0400)
from Battelle, Columbus, Ohio. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. & Howard M. (1982) The evaluation of the
teratogenicity of triphenyltin hydroxide (TPTH) in the Syrian
hamster. Unpublished Report
NO723-0100 (A22884) of Battelle, Columbus Laboratories, Columbus,
Ohio. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Fentinhydroxide,
substance technical grade (Code: HOE 029664 OF ZD97 0004) in the
rat primary hepatocyte unscheduled DNA synthesis assay. Final
Report (A32084) GT85.1091 from Litton Bionetics, Inc. Maryland.
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Craine, E.M. (1987) An extended duration dermal absorption study in
rats with 14C-triphenyltin hydroxide. Unpublished Report WIL-39037
(A35945) of WIL Research Laboratories. A subsidiary of Great Lakes
Chemical Corporation, Ashland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
DenBoer, W.C. & Hoorn, A.J.W. (1985) Mutagenicity evaluation of HOE
0 29664 -Substance technical (Code: HOE 029664 OF ZD97 0004) in the
L5178Y TK +/- Mouse Lyphoma forward mutation assay. Unpublished
Final Report (A31582) from Litton Bionetics, The Netherlands.
Submitted to WHO by Hoechst AG. Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986a) Fentin acetate - active
ingredient technical. Testing for acute intraperitoneal toxicity in
the male and female rat. Unpublished Report 86.1047 (A37976) of
Hoechst Pharma Research Toxicology and Pharmacology, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986b) Fentin acetate - active
ingredient technical. Testing for acute dermal toxicity in the male
and female Wistar rat. Unpublished Report 86.0816 (A37384) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986c) Fentin acetate - active
ingredient technical. Testing for primary dermal irritation in the
rabbit. Unpublished Report 86.0619 (A37383) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986d) Fentin acetate - active
ingredient technical. Testing for primary eye irritation in the
rabbit. Unpublished Report 86.0620 (A37382) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986e) Testing for sensitizing
properties in the Pirbright-White guinea pig according to the
technique of Buehler. Unpublished Report 86.0803 (A36975) of
Hoechst Pharma Research Toxicology and Pathology. Submitted by WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987a) Fentin-hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing for
sensitizing properties in the Pirbright-White guinea pig in a
maximization test. Unpublished Report 87.0201 (A35628) from Hoechst
Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987b) Fentin hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing of
host resistance in the female Wistar rat (Immunotoxicological
screening with Trichinella spiralis) -2nd study-. Unpublished
Report 87.0955 (A36065) of Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Dorn, E. & Werner, H.J. (1989a) Triphenyltinhydroxide (Hoe 029964
OF ZD97 0004). Determination of residues in organs, tissues and
blood of dogs after chronic feeding (52 weeks). RCC Project 047024
and RCC Project 047013. Unpublished Report CR076/88 (A41963) of
Analytisches Laboratorium Hoechst AG. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Dorn, E & Werner, H.J. (1989b) Triphenyltinhydroxide (Hoe 029664 OF
ZD 97 0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished Report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG.,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Duchosal, F., Thevenaz, Ph., Luetkemeier, H., Vogel, O., Pappritz,
G., Mladenovic, P. & Terrier, Ch. (1989) Fentinhydroxid (TPTH)
technical grade. Subchronic (90-day) repeated dose inhalation
toxicity study in rats. Unpublished Report 202353 (A40323) of RCC,
Research and Consulting Company AG., Carouge-Geneva, Switzerland.
Eckert, H.G., Kellner, H.M., Beurkle, W.L., (1989) Accumulation
study; kinetics and metabolism in the rat after single and repeated
oral administration, of 2 mg/kg body weight. Unpublished Report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L,
Oekologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Giavini, E., Prati, M. & Vismara, C. (1980) Effects of triphenyltin
acetate on pregnancy in the rats. Bull. Environm. Contam. Toxicol.
24: 936-939 (A19995). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1974a) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished Report 16454
(A16454) from Hoechst Pharma Forschung Toxicologie. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Hollander H. & Weigand W. (1974b) Triphenyltin acetate. Acute oral
toxicity (LD50) in guinea pigs. Unpublished Report (A15562) of
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974c) Triphenyltin acetate.
Subchronic (30-day) oral toxicity testing in rats and guinea pigs.
Unpublished Report (A37587) of Hoechst Pharma Research Toxicology.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1974d) Triphenyltin acetate dermal
toxicity in rats and rabbits. Unpublished Data (A15561) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974e) Triphenyltin acetate skin and
eye irritation in rabbits. Unpublished Report (15564) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1980) Acute oral toxicity of fentin
acetate technical (Hoe 02782) in male rabbits. Unpublished Report
(A28616) of Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1981) Dust inhalation of HOE 29664 -
active ingredient in the male and female SPF-Wistar rat. 4 h -LC50.
Unpublished Report 335/81 (A22293) from Hoechst Pharma Forschung
Toxicologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1986) Fentin acetate - active
ingredient technical. Testing for acute dust inhalation toxicity in
the male and female SPF rat 4-hour LC50. Unpublished Report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ishaaya, I., Engel, J.L. & Casida, J.E. (1976) Dietary
triorganotins affect lymphatic tissues and blood composition of
mice. Pestic. Biochem. Physiol. 6: 270-279. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main. (A31628)
Jung, R. & Miltenburger, H.G. (1986) Fentin acetate - substance
technical (Code: Hoe 002782 OF ZD97 0002). Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with
V79 cells. Unpublished Report LMP 229 (A35212) of Hoechst
Laboratory for mutagenicity testing. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Jung, R. & Weigand, W. (1986) Fentin acetate - substance technical,
Code: Hoe 002782 OF ZD97 0002. Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames test) and Escherichia
coli. Unpublished Report 86.0042 (A33021) of Hoechst Pharma
Research Toxicology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kavlock, R.J. (1985) Triphenyltin hydroxide developmental
toxicology study. Unpublished Report of United States Environmental
Protection Agency (A31424). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1986) Kinetics in the rats following
single and repeated administration of 2 mg/kg and single
administration of 10 mg/kg body weight. Unpublished Report
01-L42-0489-86 (A34186) of Hoechst Radiochemisches Laboratorium.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1989) Addendum to report
01-L42-0565-89. Hoe 029664 (TPTH)-113Sn. Accumulation and depletion
study in rats after single and repeated oral administration of 2
mg/kg body weight. Unpublished Report 01-L42-582-90 (A43434) of
Pharma Forschung GB-L Radiochemisches Laboratorium Hoechst,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M., Eckert, H.G. & Bürkle, W.L. (1989) Hoe 029664
(TPTH)-113Sn. Absorption studies in rats with bile fistula after
a single oral dose of approx. 2 mg/kg body weight. Unpublished
Report 01-L42-0564-89 (A41409) of Hoechst Pharma Foreschung
Radiochemisches Laboratorium Productent-wicklung GB-C, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of HOE 029664 - substance technical by its effects on
cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished Report A31614 from Microtest Research Limited, UK.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Lachmann, G. & Siegemund, B. (1987) Exhalation experiment with
triphenyltinhydroxide (Hoe 029664) in rats. Unpublished Report
(A34601) of Battelle-Institute. V., Frankfurt-am-Main. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Laveglia, J., Hagen, C.A. & Nemec, M.D. (1985) 21-Day dermal
toxicity study in rats with triphenyltin hydroxide (Code: Hoe
029664 OF ZD97 0001 technical substance). Unpublished Final Report
(A31616) project no WIL-39018 from American Hoechst Company, New
Jersey. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. (1988) Fentin acetate-substance technical (Code HOE
002782 OF ZD97 0002). Repeated dose dermal toxicity study (21
applications in 29 days) with a 14 day withdrawal period.
Unpublished Report (A39231) 3104 TSR of Pharma Forschung
Toxicologie und Pathologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981a) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 29664 O F AT201) to the male rat.
Unpublished Report (A22115) no 182/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981b) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 19664 O F AT201) to the female rat.
Unpublished Report (A22114) 183/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981c) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 O F AT201) to the
female rat. Unpublished Report (A22292) 184/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981d) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished Report (A22112) 229/81 from Hoechst Pharma
Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981e) HOE 29664 - active ingredient
(Code: HOE 29664 O F AT201). Irritance to the rabbit skin and eye
mucosa. Unpublished Report (A22113) 104/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981f) Test for sensitizing properties
of Fentin hydroxide-technical (Code : HOE 29664 O F AT201) in
guinea-pig (according to Buehler). Unpublished Report (A22433)
724/81 from Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Leist, K.H., Weigand, W. & Kramer, Dr. (1982) Range-finding test
von Hoe 29664-Wirkstoff (Fentinhydroxid) (Code: Hoe 29664 O F
AT201) im 30-Tage-Fuetterungsversuch and SPF-Wistar-Ratten.
Unpublished Report (A24003) 426/82 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Loquet, C.(1985) Fentin acetate (Code: Hoe 002782 OF ZD97 0001)
Micronucleus test in mice. Unpublished Report 1592 MAS (A32196) of
Centre International de Toxicologie at Misery. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990a) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH) substance
technical (Code HOE 029664 OFZD97 0004) in mice. Unpublished Report
L08252SN01 (A44053) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990b) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH), substance
technical (Code HOE 029664 OFZD97 0004) in rats. Unpublished Report
L08252SN02 (A44054) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Manzo, L. & Richelmi, P. (1981) Poisoning by triphenyltin acetate.
Report of two cases and determination of tin in blood and urine by
neutron activation analysis. Clinical Toxicology, 18(11)
1343-1353. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
(A44030)
Milone, M.F. & Hirsch, E. (1985a). Study of the capacity of the
test article HOE 029664 - Substance technical grade - (Code No. HOE
029664 OF ZD97 0004) to induce gene conversion in saccharomyces
cerevisiae. Unpublished Report (A32085) experiment no.M 890 from
RBM, Ivrea, Italy. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1985b) Study of the capacity of the test
article HOE 029664 - Substance technical grade (Code HOE 029664 OF
ZD97 0004) to induce gene mutation in Schizosaccharomyces pombe.
Unpublished Report (A31613) from RBM Italy. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986a) Study of the capacity of the
article fentin-acetate-substance technical grade (Code No. Hoe
002782 OF ZD97 0002) to induce gene conversion in Saccharomyces
cerevisiae. Unpublished Report M1023/1-2 (A34018) of Instituto di
Ricerche Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986b) Fentin acetate substance
technical grade. Forward Mutation test in S. pombe P1.
Unpublished Report M898 (A32812) of Instituto di Ricerche
Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Mueller, W. (1987) Evaluation of HOE 029664 OF ZD97 0004 in the
in vivo cytogenetic test in bone marrow cells of the Chinese
hamster - chromosome analysis. Unpublished Report 87.0031 (A36080)
from Hoechst Pharma research Toxicology and Pathology. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Nagamatsu, K., Kido, Y., Urakubo, G., Aida, Y., Ikeda, Y. & Suzuki,
Y. (1978) Absorption, distribution and excretion of triphenyltin
acetate and stannic chloride in the guinea pig. Japanese Journal
of Hygiene, 33: 486-496.
NCI (1984) Mouse lymphoma with triphenyltin hydroxide. Unpublished
Report (A30130), National Cancer Institute. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS no. 76-87-9 (A199994). Technical report series
No.139. US Department of Health, Education, and Welfare.
Nunziata, A. & Consonni, R.F. (1988) Chromosome aberrations in
human lymphocytes cultured in vitro. Test substance: HOE 029664
Fentin hydroxide substance technical (Code: Hoe 029664 OF ZD97
0004). Unpublished Final Report 157024-M-04487 (A38456) from Life
Science Research Roma Toxicology Centre S.P.A. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ravert, J. & Parke, G.S.E. (1976) Investigation of teratogenic and
toxic potential of technical triphenyltinhydroxide (lot #SWRAM-1K).
Unpublished Report GT85.0797 (31618) of Cannon Laboratories, Inc.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Ravert, J., Parke, G.S. & Charles, S.J. (1978) The dominant lethal
assay of technical TPTH (Triphenyltin hydroxide) in Sprague-Dawley
rats. Unpublished Report (A24394) from Cannon Laboratories Inc.,
Reading. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Resnis, P., Craine, E.M. & Nemec, M.D. (1986) A dermal absorption
study in rats with 14C-triphenyltin hydroxide. Unpublished Report
(WIL-39020) (32810) of WIL Research Laboratories. A subsidiary of
Great Lakes Chemical Corporation, Ashland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Richold, M., Jones, E. & Fleming, P.M. (1981) Ames metabolic
activation test to assess the potential mutagenic effect of HOE
29664 OF AT201. Unpublished Report 450/81A (A21735) from Huntingdon
Research Centre, GBR. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1985a) One generation teratology and reproductive
study in rats with triphenyltin hydroxide (Code: HOE 029664 OF ZD97
0001 Technical substance). Unpublished Final Report project
WIL-39013 (A31617) from Wil Research Laboratories Inc., Ashland
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Rodwell, D.E. (1985b) A teratology study in rats with triphenyltin
hydroxide (Code: HOE 029664 OF ZD97 0001 Technical Substance).
Unpublished Report WIL-39011 (A31079) of WIL Research Laboratories
Inc, Ashland USA. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
Report WIL-39012 (A35220) of WIL Research Laboratories, Ashland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Sachsse, K., Frei, T., Luetkemeier, H., Vogel, W., Pappritz, G. &
Terrier, C. (1987) TPTH - Substance technical (Hoe 029664 OF ZD 97
0004) Chronic oral toxicity 52-week deeding study in beagle dogs.
Unpublished Report 047013/047024 (A35733) of RCC Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Schollmeier, U. & Leist, K.H. (1989) Triphenyltin hydroxide -
active ingredient technical (Code: Hoe 029664 OF ZD97 0004).
Testing for sensitising properties in the Pirbright-White guinea
pig according to the technique of Buehler. Unpublished Report
89.1006 (A42667) of Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Scholz & Weigand, W., (1969b) Triphenyltin acetate Study No. 2116.
Unpublished Data A37396 from Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Seinen, W. & Penninks, A.H. (1987) Immunotoxicity of triphenyltin
compounds. Department pharmacology, pharmacy and toxicity. Faculty
of Veterinary Sciences, University of Utrecht, The Netherlands.
Document A35456. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Snow, R.L. & Hays, R.L. (1983) Phasic distribution of seminiferous
tubulus in rats treated with triphenyltin compounds. Bull.
Environ. Contam. Toxicol. 31: 658-665. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986a) 13-week oral toxicity (feeding) study
with TPTH-technical (code: HOE 029664 OF ZD97 0004 in the mouse.
Unpublished Report project 046991 (A32420) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986b) 13-week oral toxicity (feeding) study
with TPTH-technical (Code : HOE 029664 OF ZD97 0004) in the rat.
Unpublished Report project 046978 (A32419) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Vogel, O.,
Armstrong, J., Ehlers, H.A., Müller, E. & Terrier, Ch. (1989a)
TPTH-technical (Code: HOE 029664 OF ZD97 0004) oncogenicity 80-week
feeding study in mice. Unpublished Report 047002 (A40467) of RCC,
Research and Consulting Company AG., Itingen, Switzerland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Schlotke, B.,
Vogel, O., Ehlers, H.A., Mueller, E. & Terrier, Ch. (1989b)
TPTH-technical (Code: HOE 029664 OF ZD97 0007) chronic
toxicity/oncogenicity 104-week feeding study in rats. Unpublished
Report 046980 (A40468) of RCC, Research and Consulting Company AG.,
Itingen, Switzerland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Terrel, Y & Reddy, A.K. (1978) Report: 18-month carcinogenicity
study of technical TPTH 96.3% lot no. SWRAM-1K in CD1 mice.
Unpublished Report project 6E-725 (A26239) from Cannon
Laboratories, Inc. Reading. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Traul, K.A., Takayama, K., Kachevsky, V., Hink, R.J. & Wolff, J.S.
(1981) A rapid in vitro assay for carcinogenicity of chemical
substances in mammalian cells utilizing an attachment-independence
endpoint. J. Appl. Toxicol. 1(3), 190-195.
Ueda, K. & Iijima, K. (1961) Acute toxicity of Brestan Technical
(Triphenyltin Acetate) for mice. Unpublished Report A15046 from
Laboratory of Hygienics, Tokyo Dental College, Japan. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Wu, R,M., Chang, Y.C. & Chiu, H.C. (1990) Acute triphenyltin
intoxication: a case report. J. Neurol. Neurosurg. Psych., 53,
356-357.
Young, D.L. (1986) A dietary two-generation reproduction study in
rats with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final Report project WIL-39022 (A35378) from
WIL research laboratories, Inc. Ashland USA. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
 A 576 kg milk cow received a single oral dose of 5055 g
14C-TPTH and a 74 kg ewe received a dose of 1011 g 14C-TPTH, in
capsules. In the cow after 6 days 12.9% of radioactivity was
excreted in urine and 78% in the faeces. Tissues contained 5.1%.
Maximum radioactivity levels were found in the liver (5.5 mg/kg),
kidney and adrenals (both 2.2 mg/kg). In the sheep, 32.6% of
applied radioactivity was found in urine and 47.5% in the faeces
after 8 days. Tissues contained 3.1%. The liver showed highest
level of radioactivity, 6.6 mg/kg. Triphenyltinchloride was
identified as the major faecal excretion product. Phenol,
hydroquinone, catechol and resorcinol were isolated from the urine
(Bakke et al., 1982).
Toxicological studies
Acute toxicity
The LD50 and LC50 values for TPTH and TPTA in various species
are given in Tables 1 and 2, respectively.
The observed intoxication signs following oral exposure
include anorexia, emesis, tremor and diarrhoea followed by
drowsiness and ataxia. After dermal exposure to TPTH, skin
irritation was observed.
Five applications of 50 mg TPTA (purity not stated)/kg applied
to the nuchal skin of 5 rats caused no mortality but some signs of
local irritation. Five applications of 100 mg/kg to 5 rats caused
death of one animal and local signs of irritation. Five
applications of 200 mg/kg were lethal to rats. (Summary only)
(Hollander & Weigand, 1974d).
A single dermal application of 12.5 mg TPTA/kg to 3 rabbits
was lethal for 2 rabbits and caused black coloration of the skin
and scarcely pilous sites of the body. (Summary only) (Hollander &
Weigand 1974d).
Table 1. Acute toxicity of TPTH
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/m3)
Rat M oral ? 165 Leist & Weigand (1981a)
F oral ? 156 Leist & Weigand (1981b)
F perc. ? 1600 Leist & Weigand (1981c)
M&F inhal.a 97.0% 60.3 Hollander & Weigand (1981)
Rabbit M perc. ? 127 Leist & Weigand (1981d)
Dog M&F oral ? »100 Brunk & Weigand (1981)
a indicates 4 hour exposure
Table 2. Acute toxicity of TPTA
Species Sex Route Purity LD50 LC50 Reference
% (mg/kg bw) (mg/m3)
Mouse M oral 99 81.0 Ueda & Iijima (1961)
M oral 99 93.3 Ueda & Iijima (1961)
M s.c. 99 44.0 Ueda & Iijima (1961)
M dermal 99 350 Ueda & Iijima (1961)
Rat F oral ? 298 Scholz & Weigand (1969b)
F oral ? 140 Hollander & Weigand (1974a)
M&F dermal 97 >2000 Diehl & Leist (1986b)
M i.p. 97 3.6 Diehl & Leist (1986a)
F i.p. 97 12.7 Diehl & Leist (1986a)
M inhal. 97 44a Hollander & Weigand (1986)
F inhal. 97 69a Hollander & Weigand (1986)
Guinea pig ? oral ? 25 Hollander & Weigand (1974b)
Rabbit M oral ? 80 Hollander & Weigand (1980)
a indicates 4 hour exposure, whole body exposure, dust inhalation
Short-term studies
Mice
Male and female NMRI mice (10/sex/group) were fed diets
containing 0, 4, 20 or 100 ppm TPTH technical (purity 97.2%) for
three months. No compound-related effects were observed on
mortality, clinical signs, food consumption, body weight,
macroscopy and histopathology. At 100 ppm several haematological
and biochemical parameters were affected. Erythrocyte count and Hb
were decreased and platelet count increased in males and females.
The incidence of Heinz bodies was increased in males and MCH and
MCHC were decreased in females. Mouse immunoglobulins (IgA and IgG)
were decreased, IgM levels were decreased in females only. In
female mice total protein, albumin and alpha-globulin were
increased and gammaglobulin was decreased. Liver weight was
increased in males and females and a decrease was observed in the
relative weights of ovaries, adrenals, kidneys, heart and brain in
females. The NOAEL in this study is 20 ppm, equal to 3.4 mg/kg bw
(males) and 4.1 mg/kg bw (females) (Suter & Horst, 1986a).
Rats
In a range-finding study groups of rats (Hoe: WISKf;
10/sex/group) were fed diets containing 0, 5, 10, 20, 40 or 80 ppm
TPTH (97.0%) for 30 days. No compound-related effects were observed
on clinical signs, water consumption, urinalysis, organ weight,
macroscopy nor histopathology. In male rats at 80 ppm body weight
gain was decreased. Food consumption was decreased during the first
weeks in males at 80 ppm and females at 40 and 80 ppm. An increase
in thrombocyte count was observed in female rats at 40 and 80 ppm;
at the highest dose a decrease in Hb was observed in males and a
decrease in haematocrit was observed in females. At the highest
dose bilirubin, creatinin (males only), SGPT and urea (males only)
were increased. SAP was increased in a non dose-related manner in
males at 20 ppm and higher and in high-dose females. There was no
effect on spleen weight. The NOAEL in this study is 20 ppm equal to
2.01 mg/kg bw (males) or 1.83 mg/kg bw (females) (Leist et al.,
1982).
Groups of Wistar rats (15/sex/group) received dietary
concentrations of 0, 4, 20 or 100 ppm TPTH-technical (purity 97.2%)
for 13 weeks. The treatment period was followed by a 4-week
recovery period (5 male and 5 female rats/group). Observations
included clinical signs, food consumption, body weight,
ophthalmoscopy, hearing tests, haematology, clinical chemistry,
organ weights, macroscopy and histopathology (54 tissues; only in
control and high-dose rats). After 13 weeks of treatment the
following parameters were significantly affected; mean body weight
gain and food consumption were slightly decreased in high-dose
rats. RBC was increased, MCV (in males also at 20 ppm) and MCH were
decreased. In females WBC decreased at 20 and 100 ppm. Most
biochemical parameters were significantly affected at the highest
dose. The observed increase in ASAT was also apparent at 20 ppm as
was the decrease in albumin in females (also at 4 ppm). IgG was
slightly lower. The 18-hour urine volume was increased and the
specific gravity was decreased at 100 ppm. Relative testes weight
was significantly higher in high-dose males. After the recovery
period ASAT was still increased at 100 ppm and IgG was decreased in
males and significantly in females at all dose levels. No effects
were observed on spleen and thymus weights. The NOAEL in this study
is 4 ppm equal to 0.30 mg/kg bw (males) or 0.35 mg/kg bw (females)
(Suter & Horst, 1986b).
Four different experimental groups received 21 dermal
applications of 0, 5, 10 or 20 mg TPTA (purity unknown)/kg bw/day
in a 29-day period. Dosage groups consisted of 12 rats/sex in the
0 and 20 mg/kg groups and of 6 rats/sex in the 5 and 10 mg/kg
groups. At the end of the treatment period 6 rats/sex of the 0 and
20 mg/kg groups were kept for a 14 day recovery period. Rats were
observed for clinical signs, body weight, food consumption,
haematology, blood chemistry, urinalysis, organ weights, macroscopy
and histopathology. Piloerection, mydriasis, increased muscle tones
and dyspnoea were observed in high dose rats. Dose-related increase
in erythema and scale formation was observed in all treated groups.
Dose-related cracked skin and thickening of the skin fold was
observed at 10 and 20 mg/kg groups. Crusts and slight oedema were
observed in some animals at 20 mg/kg. At 20 mg/kg 4 animals died.
Lymphocytes decreased in males and females at 20 mg/kg. Monocytes
increased in females at 20 mg/kg. Sodium level decreased in all
treated males (n.d.r.) and females. Potassium decreased in 20 mg/kg
females. Inorganic phosphorus and total protein increased in 20
mg/kg females. A/G-ratio and albumin increased in 20 mg/kg males.
A1-globulins decreased in 20 mg/kg males. Macroscopy and
microscopy showed dose-related skin lesions such as hyperkeratosis
and epidermal hyperplasia in all treated groups. All effects were
reversible. No effects on spleen weights were observed. The NOAEL
for systemic toxicity in this study is 10 mg/kg bw (Leist, 1988).
TPTH (purity 97.1%) was applied to the shaven unabraded backs
of Charles River rats (10/sex/group) at doses of 0, 5, 10 or 20
mg/kg bw for 15 applications in 3 weeks. No treatment-related
effects were observed on food consumption, haematology, clinical
chemistry, urinalysis nor organ weights. Male body weight gain was
very slightly decreased in all treated groups and significantly
decreased during the first week at the highest dose. Dermal
irritation, increasing with each application, was observed in all
treated rats during the first 2 weeks. At the third week the
irritation was less severe and severe erythema was not present any
more. At macroscopy an increased incidence in ulcerated, exfoliated
and thickened skin was observed in all treated groups. Microscopic
evaluation of the treated skin revealed acanthosis and
hyperkeratosis. Also changes such as focal ulceration and mild
dermal inflammatory changes correlated with the observed dermal
irritation were seen. No systemic toxicity was observed (Laveglia
et al., 1985).
Groups of SPF-Wistar rats (10/sex/group) were exposed to 0.05,
0.5 and 2.0 mg TPTH (purity 96.2%)/m3 for 6 hours/day, 5 days/week
for 13 weeks (nose only exposure). Analytical concentrations were:
0.014, 0.338 or 1.997 mg/m3. The MMAD was 3 µm. The reversibility
of treatment-related changes was studied after a 4-week recovery
period in the control and highest dose group with 10 additional
animals/sex. Observations were made for clinical signs, body
weight, food consumption, ophthalmoscopy, haematology and clinical
chemistry, organ weights (6), macroscopy and histopathology. In the
highest dose group 10 males and 1 female died between test weeks
2-13 compared to none in the other groups. Laboured respiration and
rales were observed only in the highest exposure group. These signs
were still noted during the first week of recovery. RBC, Hb and Hc
were decreased in high exposed females, while platelet count was
increased. WBC was decreased in females at 0.5 and 2.0 mg/m3.
Glucose and total bilirubin were decreased in females at 0.5 and
2.0 mg/m3. Creatinine, albumin, gamma-glutamyltransferase and
potassium levels were decreased in females at 2.0 mg/m3. Calcium
and phosphorus level were decreased in all exposed female groups.
Calcium level decreased also in males at 0.5 and 2.0 mg/m3. IgG
increased in females at the high exposure level and IgM increased
in males at 0.5 and 2.0 mg/m3. Relative lung weights increased in
high exposed males, but no effect on thymus or spleen weight was
observed. Macroscopic examination showed lesions in the form of
multiple red foci in the lungs of most dead rats. Histopathology
revealed in both sexes at 2.0 mg/m3 degenerative and/or
inflammatory lesions in the anterior part of the nasal cavity, in
the trachea and in the lungs. The lower air passages and in
particular the lungs were severely affected. The pathomorphologic
sequence of events in the lungs seemed to start with degeneration
and multifocal necrosis of the bronchial epithelium followed by
multifocal or lobular fibrinous bronchopneumonia. The NOAEL in this
study is 0.05 mg/m3 (Duchosal et al., 1989).
Groups of 10 rats/sex were fed diets containing TPTA (purity
not given) at a rate of 0, 37.5, 150 or 625 ppm, during 30 days. No
effects were found on haematology and urinalysis. Seven rats/sex
from the 625 ppm group died during the study. Signs in this group
were piloerection and deterioration of the general condition. At
150 and 625 ppm temporary body weight gain decreases were seen.
Histopathology showed gastrointestinal haemorrhages and hepatic
changes (effects on cell metabolism) in the 625 ppm group. The
NOAEL in this study is 37.5 ppm, equivalent to 1.9 mg/kg bw.
(Summary only) (Hollander & Weigand, 1974c).
Guinea-pigs
Groups of guinea pigs (10/sex/group) were fed a diet
containing TPTA (purity not given) at a rate of 0, 7.5, 30.0 or 125
ppm. No effects were found on haematology nor urinalysis. In the
125 ppm group 8 male and 5 female guinea pigs died during
treatment. Effects found in this group included poor general
condition, piloerection and decrease in body weight gain.
Histological evaluation revealed fatty changes in the livers of
guinea-pigs treated with the highest dose (summary only) (Hollander
& Weigand, 1974c)
Dogs
Male and female dogs (beagle, 10/sex/group) received a diet
containing 0, 2, 6 or 18 ppm TPTH (purity 97.2%) for 52 weeks.
Interim necropsy on 2 dogs/sex/group was performed after 4, 13 and
27 weeks. Observations were made for clinical signs, food
consumption, body weight, hearing, ophthalmoscopy, haematology,
clinical chemistry (including immunoglobulins) and urinalysis. The
weights of 12 organs/animal were recorded (including spleen and
thymus). Gross necropsy and histopathology (about 40
tissues/animal) were carried out. A/G-ratio decreased in males at
18 ppm after 26 and 52 weeks. In females albumin decreased at 6 and
18 ppm after 52 weeks. In females at 18 ppm relative liver weight
was increased. The NOAEL in this study is 6 ppm, equal to 0.21
mg/kg bw (Sachsse et al., 1987)
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
37.5 or 75 ppm TPTH (purity ?) for 78 weeks. There was an
additional observation period of 26 weeks for both groups. Twenty
male and 20 female mice were used as controls. Dosed feed
preparations containing 37.5 or 75 ppm TPTH were analyzed
spectrophotometrically. 88% of the theoretical amount of TPTH was
recovered immediately after preparation but after 1 week only 57.9%
was recovered. The data were not corrected for any loss which may
have been due to the instability of TPTH. No treatment-related
effects were observed on clinical signs and body weight. Survival
was decreased in female mice (survival for males was: 65%, 72% and
76% and for females 95%, 74% and 66% for 0, 37.5 and 75 ppm,
respectively). At histopathology no increase in tumour incidence
was observed (NTP, 1978)
Groups of CD1-Charles River mice (60/sex/group) were fed diets
(prepared at weekly intervals) containing 0, 7, 28 or 52 ppm
technical TPTH (purity 96.3%) for 18 months. Observations included
clinical signs, mortality, body weight, food consumption,
macroscopy and histopathology (19 tissues). Survival rate was
significantly decreased in male mice in the mid and highest dose
groups (70%, 70%, 41% and 41% at 0, 7, 28 and 52 ppm,
respectively). Survival rates for females were 55%, 71.7%, 53.3%
and 58.3% at 0, 7, 28 and 52 ppm, respectively). The frequency of
aggression, alopecia, irritation, laceration and scabs was
dose-relatedly increased in both males and females. Food
consumption was reduced during some short periods in all treated
females. At histopathology an increase in the occurrence of
endometrial hyperplasia with cyst formation of the uterus was
observed at 28 and 52 ppm. The tumour incidence was not increased
(Terrel & Reddy, 1978). Extensive histomorphologic examination of
the uterus revealed that the observed cystic endometrial
hyperplasia was considered to be a hyperplastic reaction and not
neoplastic (Brown, 1985).
Groups of 50 male and 50 female mice (KFM-Han. NMRI) were fed
diets (prepared fresh daily) containing 0, 5, 20, 80 ppm TPTH
(purity 97.2%) for 80 weeks. Observations were made for clinical
signs, body weight, food consumption, ophthalmoscopy, haematology
(differential wbc count), clinical chemistry (including
immunoglobulin classes and subclasses) and organ weights (no spleen
weight). Gross pathology and histopathology (35 tissues including
bone marrow smears) were carried out. Intercurrent mortality was
significantly increased for 80 ppm females. At 80 ppm mice showed
ruffled fur towards the end of the study as well as skin lesions.
Body weight gain was reduced for 80 ppm males and 20 and 80 ppm
females. IgG, IgA, IgM, IgG4(f), IgG2a(f), IgG2b and IgG3 were
slightly or moderately decreased at 80 ppm. The decrease in IgA was
also found at 20 and 5 ppm (n.d.r.) and in IgM at 20 and 5 ppm in
male mice. Relative liver weight was markedly increased and
relative brain weight slightly increased at 80 ppm males and
females. Relative kidney weight was decreased at 80 ppm in males
and females. Macroscopic examination showed nodules, foci and
nodular lesions in the livers of mice in 80 ppm males. Skin lesions
were observed in both sexes at 80 ppm. Microscopy showed a higher
incidence of slight to moderate, focal to multifocal, nodular
hyperplasia in the liver of 80 ppm males and females. A higher
incidence of hepatocellular adenomas was observed at 80 ppm
(incidence: 12.2%, 20%, 26% and 32% for 0, 5, 20 and 80 ppm,
respectively, for male mice, and 0% in 0, 5 and 20 ppm females and
18% in 80 ppm females; only significant at 80 ppm). An increased
incidence in hepatocellular carcinomas was observed in 80 ppm
females. The incidence was 6% compared to 0% in the 0, 5 and 20 ppm
groups. An increased incidence of chronic suppurative dermatitis
and related non-specific skin lesions including acanthosis and
hyperkeratosis was observed in 80 ppm males and females. An
increased relative number of lymphoid cells was detected in femoral
bone marrow myelograms for all treated groups. The relative numbers
of granulocytes were significantly decreased for females at 5 ppm
and both sexes at 20 and 80 ppm. The relative numbers of
erythrocyte precursors were significantly decreased for males at
20 and 80 ppm. However, it is difficult to evaluate these data in
the absence of red cell counts in peripheral blood. Although a
reduction in serum immunoglobulin concentrations was reported, the
Meeting noted that there was no corresponding effect on
haematological parameters and that the method of analysis has not
been validated in rodents. The data on serum immunoglobulin
concentrations was therefore not utilized in the determination of
the NOAEL for this study. Based on reduced body weight gain, the
NOAEL is 5 ppm, equal to 1 mg/kg bw/day (Tennekes et al., 1989a).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
prepared weekly containing 37.5 or 75 ppm TPTH (purity not stated)
for 78 weeks. There was an additional observation period of 26
weeks for both groups. Twenty male and 20 female rats were used as
controls. Feed preparations containing 37.5 or 75 ppm TPTH were
analyzed spectrophotometrically. Of the theoretical amount of
TPTH, 80% was recovered immediately after preparation but after 1
week only 57.9% was recovered. The data were not corrected for any
loss which may have been due to the instability of TPTH. No effects
were observed on clinical signs, mortality, food consumption,
macroscopy nor histopathology (31 tissues including pituitary gland
and testes). Body weight was slightly but dose dependently
decreased in male rats after week 40. Survival rates were 75%, 76%
and 84% for males and 90%, 80% and 90% for females at 0, 37.5 and
75 ppm TPTH. The tumour incidence was not enhanced (NTP, 1978).
Groups of 70 rats/sex (SPF-bred KFM-Han. Wistar) rats received
a diet containing 0, 5, 20 or 80 ppm TPTH (purity 97.2%) for 104
weeks. The diets were prepared twice monthly and frozen. A fresh
amount of diet was given daily. Observations were made for
clinical signs, food consumption, body weight, ophthalmoscopy,
haematology, clinical chemistry (including immunoglobulin classes
and subclasses), urinalysis, organ weights, macroscopy and
histopathology (about 40 tissues/animal, including bone marrow
smears). Mortality was increased (dose-related) in all treated
females. The survival was 75, 51, 36 and 23%, respectively.
Preceding mortality clinical signs were ruffled fur, reduced
activity, ataxia, stiff gait and hunched posture. Due to this
excess mortality no laboratory investigations could be made for the
80 ppm female group at 104 weeks. Group mean food intake was
reduced for males and females at 80 ppm. Body weight gain was
dose-relatedly decreased in males and females at 20 and 80 ppm.
Ophthalmoscopy showed an increased incidence of unilateral or
bilateral corneal opacity in males at 20 and 80 ppm at 104 weeks.
Hb and haematocrit were decreased in female rats at 20 and 80 ppm.
ASAT, ALAT, ALP were increased in males and females at 80 ppm. The
following changes in immunoglobulin levels were observed only after
50 weeks: IgG1 levels were reduced for all treated females and 80
ppm males, IgG2a levels were reduced for all treated females and
IgG2c levels were reduced for all treated males, IgA levels were
reduced for males at 20 and 80 ppm and IgM levels were increased
for both sexes at 20 and 80 ppm. At termination most relative organ
weights were increased in the 80 ppm groups, including spleen
weight. An increased incidence of pituitary adenomas was diagnosed
in females at 20 and 80 ppm. The recorded incidences were 64.4%,
63.2%, 76.8% and 93.1% for 0, 5, 20 and 80 ppm groups,
respectively. An increased incidence of testicular Leydig cell
tumours was diagnosed at 80 ppm. The recorded incidences were 1.7%,
8.5%, 5.0% and 16.7% for 0, 5, 20 and 80 ppm, respectively.
Non-neoplastic lesions observed included an increase in hyperplasia
of the pars intermedia of the pituitary gland in males at 80 ppm.
An increased incidence of cystoid change of the pars intermedia of
the pituitary gland was noted in males at 20 and males and females
at 80 ppm at 52 weeks. Increased incidences of testicular Leydig
cell hyperplasia and tubular atrophy were noted in rats at 80 ppm
after 104 weeks. Increased incidences of hepatic bile duct
proliferations and portal sclerosis were noted in females at 20 and
80 ppm after 52 and 104 weeks of treatment. An increased incidence
of skeletal muscle atrophy was noted in male rats at 80 ppm after
104 weeks of treatment. Because mortality was increased in females
and serum immunoglobulins were decreased at the lowest dose level
of 5 ppm (equal to 0.3 (males) 0.4 (females) mg/kg bw no NOAEL
could be established (Tennekes et al., 1989b).
Reproduction studies
In a range-finding study Wistar rats (10/sex/group) were
administered 0, 12.5, 25, 50, 100 or 200 ppm TPTH (weekly prepared,
purity not stated) in the diet for in total 90 days (from 59 day
pre-mating period up to 21 day gestation period). All animals were
killed following weaning. Observations included clinical signs,
mortality, body weight, organ weight and food consumption. Parents
were observed haematologically and histopathologically. Pups were
sexed, weighed and observed for gross abnormalities. Severe
clinical signs like rough coat, hunched back, lethargy, nasal
discharge and alopecia were observed in rats at 200 ppm. At 200 ppm
body weight gain was significantly reduced in males and food
consumption was slightly reduced in male and female rats. White
blood cell count was significantly decreased in high dose females.
A lower pregnancy rate (4/10) was observed in females at 200 ppm,
no viable offspring resulted. At 100 ppm pup survival, pupweight
and litter size (at 50 ppm not significantly) were dose-relatedly
decreased. At histopathology necrosis of the renal papilla or
medulla was observed in males at 100 and 200 ppm. One high-dose
male rat that died after six weeks showed hepatic, thymic and
testicular necrosis (Carlton & Crisp, 1982).
In a two-generation reproduction study groups of Wistar
(Crl:(WI)BR)COBS rats (30/sex/group) were given diets (prepared
every 5-7 days) containing 0, 0', 5, 18.5 or 50 ppm TPTH (purity
97.2%) during growth, mating, gestation and lactation for one
litter per generation. Observations included clinical signs, body
weight, food consumption, mating performance and reproductive
parameters. Organ weights (including spleen and thymus) of parents
and pups were recorded and the parents were examined histologically
(control group 1 and 50 ppm groups only). Pups were sexed, and
examined for gross malformations and the number of stillborn and
live pups were recorded.
Body weight gain and food consumption were occasionally
decreased in F0 parents at 50 ppm. The number of dead F1 pups
increased and mean litter size decreased at 50 ppm. Mean pup weight
was decreased at 50 ppm on lactation days 14 and 21. In F0 parents
the body weight gains and food consumption of both sexes were lower
at 50 ppm and occasionally at 18.5 ppm. Mean litter size decreased
at 18.5 and 50 ppm. Viability of F2 pups at 50 ppm, prior to
culling was slightly decreased. Mean pup weight was decreased at 50
ppm on lactation days 7, 14 and 21. At 18.5 mg/kg a slight decrease
was seen. Pups from one litter at 50 ppm appeared emaciated on
lactation day 21. Several pups in another litter in this group had
wet yellow urogenital staining on lactation day 21. At 50 ppm the
relative weights of brain, testes, ovaries, adrenals, kidneys
spleen and heart were increased at several occasions in F0 and/or
in F1 adults and/or F1 and F2 weanlings (males and females). At
18.5 ppm increases were also observed for kidneys, testes and heart
weights, whereas at 5 ppm only relative heart weight was increased
in male F1 adults. A dose-related decrease was observed in spleen
and thymus weight in F2 male and female weanlings at 50 and 18.5
ppm. In F1 weanlings the same trend was observed. The NOAEL in
this study is 5 ppm, equal to 0.4 mg/kg bw/day (Young, 1986).
Two groups of 13 male rats (29 days old) received a diet
containing 0 or 20 mg TPTA/kg bw for 25 days. Four animals from
each group were sacrificed on day 21 and the remaining animals
received the test diet for another 4 days and a control diet for an
additional 70 days at which time they were sacrificed. Observations
were made for the distribution of the eight phases of
spermatogenesis. In rats sacrificed after 21 days all 8
spermatogenic phases were seen, but there was a general paucity of
mature sperm and the distribution showed some predominance of
immature sperm. Recovery was seen after the 70 days control diet
(Snow & Hays, 1983).
Special studies on embryotoxicity and/or teratogenicity
Rat
Pregnant Sprague-Dawley rats (20/group) received by gavage 0,
1.25, 5, 8.75 or 12.5 mg/kg TPTH (purity unknown) bw/day, day 6
through 15 of gestation. The rats were sacrificed on day 20 of
gestation. Observations were made for body weight, number of
implantations, number of live and dead fetuses, number of
resorptions and number of corpora lutea. The fetuses were weighed
and examined for external, visceral and skeletal abnormalities.
Three animals each in the 8.75 and 12 mg/kg groups aborted. Only
those animals showed abnormal behavioural reaction. One female in
the 12.5 mg/kg group died. Females at 8.75 and 12.5 mg/kg showed a
lower food consumption and lower body weight gain (dose-
relatedly). In the 8.75 and 12.5 mg/kg groups an increase in
resorptions was observed. Fetuses from these groups also exhibited
a lower weight and a decrease in viable fetuses. A non dose-related
increase in hydrocephalus and hydronephrosis was seen in all
treated groups (2%, 5%, 12%, 16% and 6%, respectively) (Ravert &
Parke, 1976).
In a teratogenicity study (Segment II + III) groups of 25
pregnant Sprague-Dawley rats were given oral daily doses of 0,
0.35, 1, 2.8 or 8 mg TPTH (purity 97.1%)/kg b.w. in corn oil from
day 6 or 15 of gestation. All animals were allowed to deliver
naturally and rear the offspring to weaning on lactation day 21. At
lactation day 4, 10 pups/litter were selected and killed on the
28th day. The dams were killed on day 21 of lactation. Dams were
observed for mortality, clinical signs and body weight. Litters
were examined for gross malformations and the number of live and
dead fetuses was recorded. Fetuses were observed for bodyweight and
examined for haematology, clinical chemistry and urinalysis,
macroscopy, kidney weight and histopathology of the kidney. Dams at
8 mg/kg bw showed lethargy, yellow staining in the urogenital area,
and a more extensive hairloss than dams in the other groups. At the
same dose group maternal body weight gain was significantly
decreased (only during treatment) and gestation length was
significantly increased, and pup survival was decreased on
lactation day 0. Only LDH values in blood decreased in pups at 1,
2.8 and 8 mg TPTH/kg bw and ASAT was decreased in females pups at
all doses. Globulin levels were significantly decreased in female
pups at 2.8 and 8.0 mg/kg bw and A/G ratio was increased at the
highest dose. Relative kidney weight in pups was significantly
increased at 2.8 and 8 mg/kg bw. The dose of 1 mg/kg bw can be
regarded as a NOAEL for embryotoxicity (Rodwell, 1985a).
Twenty to 26 pregnant rats per group received by gavage 0,
1.0, 2.8 and 8 mg TPTH (purity unknown)/kg bw/day suspended in corn
oil, day 5 through 19 of gestation. On day 20 all females were
sacrificed. Uterus weight was determined and corpora lutea were
counted. Fetuses and resorption sites were noted. Fetuses were
weighed, sexed and observed for external, internal and skeletal
malformations. In the 2.8 and 8 mg/kg groups a dose-related nasal
and oral discharge were observed. In the highest group two animals
died. Body weight gain was significantly reduced at 8 mg/kg. The
number of dead or resorbed fetuses was higher at 8 mg/kg. Pup
weight was depressed in the 8 mg/kg group. The occurrence of
hydro-ureter was increased at the highest dose (Carlton & Connell,
1981; Carlton, 1985).
In order to clarify the occurrence of hydronephrosis,
hydrocephalus and hydroureter, another rat study was carried out.
Groups of 45 pregnant rats received 0, 0.35, 1, 2.8 and 8 mg TPTH
(purity 97.1%)/kg bw/day day 6 through 15 of gestation. Day 20 of
gestation all rats were sacrificed. Throughout gestation
observations were made for clinical signs, body weights and food
consumption. After sacrifice the dams were observed for number and
location of viable and nonviable fetuses, early and late
resorptions and the number of implantation sites. The corpora lutea
were counted. Fetuses were weighed, sexed and examined for
external, internal and skeletal anomalies. During the treatment
period, hair loss was more extensive in the 8 mg/kg group. A few
animals in this group were emaciated, lethargic and had yellow
staining in the urogenital area. One female in this group had an
abortion. A dose-related decrease in body weight gain and food
consumption was apparent in the 2.8 and 8 mg/kg groups. Number of
nongravid females, number of total litter resorptions and early
resorptions increased at 8 mg/kg. Number of viable fetuses and
fetal weight decreased significantly at 8 mg/kg. The incidence of
absent or delayed ossification in high dosed litters was increased.
The percentage of fetuses with hydrocephaly was 0.4, 0, 0, 0.4 and
1%, respectively, and for fetuses with omphalocele 0.2, 0.2, 0.2 0,
and 0.5%, respectively for the 0, 0.35, 1, 2.8 and 8 mg/kg groups.
The NOAEL for maternal toxicity was 1 mg/kg bw and the NOAEL for
embrytoxicity was 2.8 mg/kg bw. There was no evidence for
TPTH-induced irreversible structural effects (Rodwell, 1985b).
In a special study consisting of three tests the potential of
TPTH to induce alterations in the urogenital tract of exposed rat
fetuses was investigated. In test I pregnant Sprague Dawley rats
received 0, 4 or 8 mg TPTH (purity unknown)/kg bw by gavage during
day 7 through 20 of gestation. In test II (a and b) doses of 0 and
4 mg/kg bw were given. All females from test I and IIa were
sacrificed on day 21 of gestation. The uteri were removed, weighed,
and examined to determine the number of live, dead and resorbed
fetuses. Live fetuses were examined for gross external anomalies.
In test IIb the animals delivered normally and weaned their young
for 28 days. In these young animals renal morphology and function
were examined. At 8 mg/kg bw maternal (death and reduced
extra-uterine weight gain during pregnancy) and fetal (mortality
and decreased body weight) toxicity were observed. There was no
effect on cerebral ventricles. At 4 mg/kg bw only slightly dilated
renal pelvis was seen and at 4 and 8 mg/kg bw (n.d.r.) dilated
ureter was observed. In a replicate test (IIa) these effects were
hardly observed. In test IIb no effects at all where found for the
renal function or morphology. Only the treated pups only showed a
lower pup weight (Kavlock, 1985).
Four groups of 12 pregnant rats (Sprague Dawley) received from
day 6 through 15 of gestation 0, 5, 10 or 15 mg TPTA (purity
97.1%)/kg bw/day. Females were sacrificed on day 21 of gestation.
Dams were observed for number of resorptions, number of
implantations, post-implantation loss, viable and non viable
fetuses. Fetuses were weighed and examined for external, visceral
and skeletal anomalies. Two rats treated with 15 mg/kg died during
the study and 4 had complete resorptions. Average maternal weight
gain decreased at 5 mg/kg and significantly 10 and 15 mg/kg.
Post-implantation loss increased significantly at 15 mg/kg. A
significant reduction of some ossification centers, mainly at the
level of the metacarpus and caudal vertebrae, was seen in all
treated groups. Minor anomalies (hydroureter, vertebrae missing or
bipartite) increased significantly at 15 mg/kg (Giavini et al.,
1980).
Groups of pregnant rats (Wistar) received during day 7 through
16 of gravidity 0, 1.25, 3.2 or 8 mg TPTA/kg bw/day. TPTA (purity
97.0%) was dissolved in sesame oil. The females were sacrificed and
delivered on day 21 of gestation. The dams were observed for
clinical signs, body weight, food consumption, number of
implantations, resorptions and corpora lutea, viable and non viable
fetuses, organ weights (heart, liver, kidneys and spleen) and
macroscopy. Fetuses were weighed, and examined for length, sex,
external, internal and visceral anomalies. Ten dams at 8 mg/kg had
abortions. Dams at 8 mg/kg showed signs of clinical intolerance as
piloerection, bloody nose secretion, local hair loss accompanied by
scab formation. Body weight gain and food consumption were
decreased in this group. At 8 mg/kg early and total intrauterine
deaths increased and number of implantations, total live fetuses,
fetal body weight and crown/rump length decreased. At 8.0 mg/kg an
increase in fetuses with non ossification or only weak ossification
of the sternebrae was observed. At 8 mg/kg also an increase (5%) in
distended uni- or bilateral ureter was observed. The NOAEL in this
study is 3.2 mg TPTA/kg bw (Baeder, 1987a).
Hamsters
Four groups of 20-25 pregnant Syrian hamsters received by
gavage 0, 2.25, 5.08, or 12 mg TPTH (purity not given)/kg bw in
0.3% hydroxypropyl cellulose in saline from day 5 through 14.
Vitamin A palmitate was administered by gavage on day 8. All dams
were sacrificed on gestation day 15. The weight of the gravid
uterus was determined and corpora lutea were counted. Fetuses and
resorption sites were noted. Fetuses were weighed and observed for
external, visceral and skeletal malformations. Four animals died in
the highest-dose group and several animals showed an abnormal
behaviour. In the mid-dose group and in the lowest-dose group 2
animals died. Hamsters from the highest-dose group showed a
decreased mean body weight gain and a decreased food consumption.
Fetuses from this group showed also a decreased pup weight. The
average number of minor anomalies (such as kinky tail, haematomas
and anaemic appearance) of fetuses per litter was significantly
greater among the 12 mg/kg group. A significantly greater number of
skeletal variations (poorly ossified, missing metatarsals or
missing sternebrae) was observed among the 12 mg/kg group. Three
cases of hydrone-phrosis were seen at 5.08 mg/kg bw and 1 case of
hydrocephalus was seen at 12 mg/kg bw (Carlton & Howard, 1982;
Carlton, 1985).
Rabbits
Three groups of 22 pregnant New Zealand White rabbits received
by gavage 0, 0.1, 0.3 or 0.9 mg TPTH/kg bw/day mixed in 1% aqueous
CMC, day 6 through 18 of gestation. Dams were sacrificed day 29 of
gestation. Observations were made for clinical signs, body weight
and food consumption. After sacrifice corpora lutea, early and
late resorptions and number of implantations were counted. All
fetuses were weighed, sexed and examined for external, skeletal and
visceral anomalies and developmental variations. Two rabbits from
the 0.9 mg/kg group aborted. A dose-related decrease in mean body
weight gain and food consumption was observed in the 0.3 and 0.9
mg/kg groups. Mean fetal weight was lower in the 0.9 mg/kg group.
The NOAEL for maternal toxicity was 0.1 mg/kg bw and the NOAEL for
embryotoxicity was 0.3 mg/kg bw (Rodwell, 1987).
TPTA (purity 97.0%) was dissolved in sesame oil and
administered orally by gavage to groups of 15 pregnant rabbits
during day 6 through 18 of gestation. The dose levels were 0, 0.1,
0.32 or 1.0 mg/kg bw/day. On day 29 of gestation the dams were
sacrificed. Dams were observed for clinical signs, body weights,
food consumption, number of resorptions, number of implantations,
number of corpora lutea, viable and non viable tissues, organ
weights (heart, liver kidney, spleen) and macroscopy. Fetuses were
weighed and examined for sex, length, external, internal and
skeletal anomalies. In the 1.0 mg/kg group 1 dam died, 3 dams
aborted, 1 dam was sacrificed due to a premature delivery and 2
dams had only intra-uterine deaths. Three dams showed vaginal
haemorrhages. The water consumption and food consumption of dams at
1 mg/kg decreased. The number of implantations and of live fetuses
decreased at 1 mg/kg. Mean fetal weight, mean crown/rump length
and mean placental weight decreased in pups at 1 mg/kg. At 1 mg/kg
4 pups (15.4%) showed omphalocele with protrusion of intestinal
coils or liver tissue. Slight retardation of skeletal ossification
was detected in the 1.0 mg/kg group. There was an increase in the
number of fetuses with fewer than 13 ossified caudal vertebrae and
some fetuses showed weak ossification of the hyoid bone or non
ossification or only slight ossification of the os pubis. The
NOAEL for maternal and embryotoxicity in this study is 0.32 mg/kg
bw/day (Baeder, 1987b).
Special studies on immunotoxicity
Seinen and Penninks (1987) reviewed the available data on
immunotoxic properties of TPTH from the published literature and
confidential studies provided by the manufacturer. They concluded
that subacute treatment with TPTH resulted in lymphopenia and
lymphocyte depletion of spleen and thymus. After treatment up to 13
weeks these effects appeared to be transient. Rats fed 25 ppm TPTH
(not 5 ppm) showed cell mediated immunity as measured by slightly
suppressed DTH (delayed type hypersensitivity) to ovalbumin and
tuberculin. The humoral immunity was not affected at doses up to 25
ppm. However in mice dosed with TPTH up to 20 mg/kg bw for 14 days
DTH response to keyhole limpet haemocyanin was not affected,
whereas the humural activity was suppressed at 20 mg/kg bw. In
various other immune function tests no disturbances were observed.
The authors concluded therefore that TPT-compounds display weak
immunosuppressive potential. This immunosuppressive activity is
observed at dose levels that markedly reduce the numbers of blood
lymphocytes and the weight of the lymphoid organs (Seinen &
Penninks, 1987). The original data for certain studies in this
review were not available to the JMPR.
Mice
In a limited experiment young and mature mice were fed a diet
containing TPTA (purity >99%) at a rate of 30, 100 or 300 ppm for
4 days. Feeding resulted in a dose-related decrease in spleen,
heart and liver weight and number of blood leucocytes (Ishaaya
et al., 1976; Seinen & Penninks, 1987).
Groups of B6C3F1/CrlBR mice (18/sex/group) were administered
0, 1, 5, 25, 50 or 125 ppm TPTH (purity 96.4%) for 28 days. A
positive control group (18/sex) was fed control diet and received
a single i.p. injection of 180 mg cyclophosphamide/kg bw 48 hours
prior to the end of the treatment period. Twelve male and 12
female mice were killed at day 29 and the remaining mice were
returned to control diets and were killed on day 57. Observations
were made on survival and clinical signs, body weight, food
consumption, haematology, liver, spleen, and thymus weight,
macroscopy and histopathology. Special immunotoxicological
parameters as splenocyte counts, thymocyte counts, number of
splenic T- and B-cells and basal serum immunoglobulin levels were
recorded and the bone marrow was examined. A significantly
decreased body weight gain was observed in male and female mice at
125 ppm, from which they recovered after 28 days. Food consumption
was significantly decreased at 50 and 250 ppm. Relative liver
weight was increased at 25 (females only), 50 and 125 ppm, relative
spleen weight was clearly decreased in males at 50 and 125 ppm and
in females at 25 ppm and higher and relative thymus weight was
decreased at 125 ppm in males. At histopathology lymphoid depletion
in the thymus and spleen was observed in mice at 125 ppm. No
dose-related effects were observed on any of the bone marrow
parameters (note: only 50% of the smears could be evaluated,
because of technical problems). The main haematological effects
were a decrease in total white blood cells, neutrophils and
lymphocytes at 50 and 125 ppm in males and females. Dose-related
changes in haematological parameters were observed in males at 25,
50 and 125 ppm and in females in all dose levels (MCH and MCHC). At
the highest dose a decrease in total cells/spleen and splenic
B-cells were observed and a decrease in total cells/thymus and
splenic T-cells were seen in males. IgM levels were decreased in
females at 25 ppm and higher, but were not clearly dose-related.
All effects were reversible. Mice fed cyclophosphamide showed
decreased spleen and thymus weight accompanied by histologic
evidence of lymphoid depletion. Cyclophosphamide also altered the
% T- and % B-cells in both sexes and reduced the spleen and thymus
cellularity. The effects were not reversible. The NOAEL for
immunotoxicity is 5 ppm, equal to 1 mg/kg bw for males and 1.15 for
females (McCormick & Thomas, 1990a).
Rats
A similar study was conducted in 18 male and 18 female Wistar
rats. A positive control group was fed a diet containing 125 mg
DOTDC (dioctyltin-dichloride)/kg. No effects were observed on
mortality, clinical signs, body weight, organ weight, macroscopy.
Food consumption was decreased in female rats at 125 ppm. No
dose-related effects were seen at histopathology (note only 50% of
the bone marrow smears could be used in the histopathological
evaluation). In both males and females red blood cell parameters
were affected at the highest groups. The white blood cells and
lymphocytes were significantly decreased in males at 125 ppm and
females showed a tendency to a decrease at 50 and 125 ppm. In male
rats at 50 and 25 ppm platelet count was increased. No effect was
observed on the viability of the thymus or spleen cells nor on the
percentages of splenic T- and B-cells in either sex. IgG levels
were significantly reduced in females at 50 and 125 ppm. All
effects were reversible. The effects in the positive control group
were as expected. These effects were largely reversible following
cessation of DOTDC exposure. The NOAEL for immunotoxicity is 25 ppm
equal to 1.82 mg/kg bw for females and 1.71 mg/kg bw for males
(McCormick & Thomas 1990b).
In order to investigate the reaction of rats to infection with
Trichinella spiralis female rats were administered 2.5 mg TPTH
(purity 97.2%)/kg bw over a 10 day period. This administration had
no effect on host resistance in contrast to the positive controls
dexamethasone and cyclophosphamide. There are also no effects on
additional parameters, i.e., thymus weights, percentage of
lymphocytes in the differential blood count, and body weight gains
(Diehl & Leist, 1987b).
Special studies on genotoxicity
TPTH as well as TPTA were tested in various genotoxicity
assays. See Tables 3 and 4 respectively, for a summary of the
studies considered. In most of the assays negative results were
obtained. The positive results in the chromosome aberration assays
are probably related to the toxic effect of the compound on
T-lymphocytes and not to a genotoxic action of TPTH.
Special studies on sensitization
At concentrations which were irritant to the skin, TPTH
(purity 97.0%) showed no skin sensitization in guinea-pigs in the
Buehler test (Leist & Weigand, 1981f; Schollmeier & Leist, 1989)
nor in the maximization test (Diehl & Leist, 1987a).
TPTA gave a positive response when tested for skin
sensitization in guinea-pigs in the Buehler test (Diehl & Leist,
1986e).
Table 3. Results of genotoxicity assays on TPTH
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Ames test a,b S. typhimurium 0.31-5 µg/pl 97.0 Negative Richold et al. (1981)
TA 1535, TA100, in DMSO
TA1537, TA98,
TA1538
E. coli WP2 uvra 313-5000 µg/pl 97.0 Negative
in DMSO
Yeast forward S. pombe p1 0.05-1 µg/ml 97.2 Negative Milone & Hirsch (1985b)
mutation test in DMSOa
Mitotic gene Saccharomyces 0.1, 1, 3 or 97.2 Negativec Milone & Hirsch (1985a)
conversion assay cerevisiae D4 5 µg/mla
1, 5, 10 or
15 µg/mlb
Mouse lymphoma L5178Y mouse 10-150 ng/mla 97.2 Negative DenBoer & Hoorn (1985)
forward mutation lymphoma cells (>80 ng/ml toxic) Weakly
assay (TK +/-) 40-600 ng/mlb positive
(>300 ng/ml toxic)
Mouse lymphoma L5178Y mouse 0.01-2 µg/1a ? Positive NCI (1984)
forward mutation lymphoma cells (>0.1 toxic) Negative
assay (TK +/-) 1.7-8 µg/1b
Chromosome Human lymphocytes 31.25-1000 ng/mla Positive Kirkland (1985)
aberration assay 250-2000 ng/mlb
both in DMSO
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Chromosome Human lymphocytes 0.681-1.47 µg/mla 96.2 Negative Nunziata & Conzonni (1988)
aberration assay in DMSO Positive
0.316-1.47 µg/mlb
in DMSO
Unscheduled Rat hepatocytes 0.01-2.0 µg/ml in DMSO 97.2 Negativec Cifone & Myhr (1985)
DNA synthesis >0.25 µg/ml toxic
Survival (cell Rat embryo cells 0.019 µg/5.2 x 104 cells ? Positived Traul et al. (1981)
transformation) infected with or 0.0145 µg/5.2 x 104
assay leukaemia virus
(2FR450 cells)
Micronucleus test NMRI mouse bone 35, 70 or 140 mg/kg bw po 97.2 Negative Banduhn et al. (1985)
NMRI marrow cells
Cytogenic assay Chinese hamster bone 20, 50 or 80 mg/kg bw po 96.2 Negative Mueller (1987)
marrow cells
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Dominant lethal Sprague-Dawley rats daily ip injections 94.8 Negative Ravert et al. (1978)
assay with 3, 20, 38
or 150 mg/kg bw in
corn oil; 150 mg/kg
bw toxic (8/10
died). Concentration
of TPTA
a without metabolic activation
b with metabolic activation
c positive control yielded positive results
d only summary available
Table 4. Results of genotoxicity assays on TPTA
Test system Test object Concentration of Purity Results Reference
TPTA (%)
Ames testa,b S. typhimurium 0.032-1000 µg/plate 97.1 Negative Jung & Weigand (1986)
TA100, TA1535, in DMSO
TA1537, TA1538,
TA98
E. coli WP2 uvra
Gene conversiona,b S. cerevisiae D4 1-50 µg/ml in DMSO 97.1 Negative Milone & Hirsch (1986a)
Dominant Sprague-Dawley rats daily i.p. injections 94.8 Negative Ravert et al. (1978)
lethal assay with 3, 20, 38 or
150 mg/kg bw in corn oil;
150 mg/kg bw toxic (8/10
died)). Concentration of
TPTA
a without metabolic activation
b with metabolic activation
Special studies on skin and eye irritation
A dose of 500 mg TPTH (purity not stated) diluted in a 0.9%
NaCl solution was slightly irritant when applied under occlusive
conditions to the shaven intact and shaven abraded skin of 6 New
Zealand rabbits for 24 hours (Leist & Weigand, 1981e).
Dilutions of 1:10, 1:100 and 1:1000 prepared from 2.5 g TPTA
(purity not stated) in 0.9% NaCl were examined in the Barail
Intracutaneous test by intracutaneous injections of 0.02 ml
administered to the skin of rabbits. No irritation was seen. Five
applications of 0.5 ml of the TPTA solution to depilated and intact
rabbits skin caused slight local irritation. (Summary only)
(Hollander & Weigand, 1974e).
A dose of 500 mg TPTA (purity 97%) moistened with
physiological saline was applied under semi-occlusive conditions to
the shaven intact skin of 6 New Zealand rabbits for 54 hours. No
irritation was observed up to 72 hours after application (Diehl &
Leist, 1986c).
Application of 100 mg TPTH (purity not stated) diluted in a
0.9% NaCl-solution into the eyes of 9 New Zealand rabbits caused
severe irritation; increasing adverse effects resulted in a
discontinuation of the experiment after 72 hours (Leist & Weigand,
1981e).
Rabbits received an instillation of 0.1 ml of a solution of
2.5 g TPTA (purity not stated) in 0.9% NaCl into one eye. After 24
hours slight local irritation was observed (Summary only)
(Hollander & Weigand, 1974e).
Nine New Zealand rabbits received an instillation of 100 mg
TPTA (purity 97%) into one eye. All rabbits developed severe ocular
lesions (swollen conjunctivae, markedly injected blood vessels,
diffusely crimson red colouring, nacreous opacity and whitish and
reddish brown discharge). After 72 hours all animals were
sacrificed because the lesions were non-reversible (Diehl & Leist,
1986d).
Observations in humans
Two cases of poisoning with Brestan 60 (60% TPTA and 15%
Maneb) have been reported. In both cases (farmers of 75 and 53
years) inhalation of the powder took probably place during
preparation of the spray solution. Symptoms observed were nausea,
dizziness, transient loss of consciousness, convulsions, persistent
headache, and photophobia, as well as impaired liver functions.
Both patients were discharged in good health after 10 and 15 days
(Manzo & Richelmi, 1981).
Acute severe intoxication was reported in a 23-year old male
in an attempted suicide with a TPT-compound (molluscicidal agent,
no specifications given). The patient was suffering from abdominal
pains, diarrhoea and vomiting and also showed reversible acute or
delayed neurological symptoms. The patient's health was completely
recovered within 3 1/2 months (Wu et al., 1990).
COMMENTS
Most toxicological studies on fentin were carried out with
triphenyltin hydroxide (TPTH). Since triphenyltin acetate (TPTA) is
hydrolysed rapidly to TPTH in an aqueous medium, studies with both
compounds have been used for the evaluation of fentin.
After oral administration of 14C-labelled TPTH the
radioactivity was mainly eliminated via the faeces (50-70%), but
also via the urine (20-25%). Biliary excretion was also
demonstrated. After 7 days residual radioactivity was present with
the highest concentration in the liver and the next highest in the
kidneys. After oral administration of 113Sn-labelled TPTH,
elimination was almost exclusively via faeces. In both cases faecal
elimination was biphasic with half-lives of 9 and 50-60 hours. With
113Sn-labelled TPTH the highest concentration was found in the
kidneys. After administration of the 14C-labelled compound,
radioactivity in the faeces of the rat consisted of parent compound
and di- and monophenyltin as well as non-extractable bound residues
and tin (measured as Sn). It was reported that benzene was found in
the faeces. Sulfate conjugates of phenol, hydroquinone, catechol
and resorcinol as well as phenylmercapturic acid were present in
the urine. Phenyltin compounds were not found in the urine.
TPTH is toxic to rats after acute oral administration with
LD50 values of about 160 mg/kg bw. TPTA showed similar acute
toxicity.
In a 3-month study in mice at dietary concentrations of TPTH
of 0, 4, 20 or 100 ppm, a number of effects were observed at 100
ppm. The main effects were a decrease in haemoglobin concentration
and erythrocyte count and an increase in Heinz bodies.
Immunoglobulins were decreased and liver weight was increased. The
NOAEL was 20 ppm, equal to 3.4 and 4.1 mg/kg bw/day for males and
females, respectively.
Dietary concentrations of TPTH of 0, 4, 20 or 100 ppm were
tested in a 13-week study in rats. The NOAEL was 4 ppm, equal to
0.30 and 0.35 mg/kg bw/day for males and females respectively,
based on a decrease in white blood cells and plasma albumin and an
increase in plasma aspartate aminotransferase at 20 ppm.
In a 52-week study in dogs (dietary concentrations of TPTH of
0, 2, 6 or 18 ppm) the NOAEL was 6 ppm, equal to 0.2 mg/kg bw/day,
based on a decreased albumin level and an increase in relative
liver weight at 18 ppm.
Two long-term/carcinogenicity studies in mice and one in rats
were performed in which there was no evidence of carcinogenicity.
However, there were indications in each of these studies that the
doses received by the animals may have been lower than intended
owing to instability of the test compound in the diet.
In a third long-term/carcinogenicity study in mice at dietary
concentrations of TPTH of 0, 5, 20 or 80 ppm, increased liver and
decreased kidney weights, increased nodular hyperplasia and
hepatocellular adenoma and carcinoma occurred at 80 ppm only. The
NOAEL was 5 ppm, equal to 0.85 mg/kg bw/day for males and 1.36
mg/kg bw/day for females, based on decreased body-weight gain at 20
ppm.
In a second long-term/carcinogenicity study in rats (with
dietary concentrations of TPTH of 0, 5, 20 or 80 ppm), an increase
in mortality was observed at all dose levels. A NOAEL could not
be established at the lowest concentration of 5 ppm, equal to 0.3
and 0.4 mg/kg bw/day for males and females respectively. The
incidence of pituitary adenomas was increased in females at 20 and
80 ppm. At 80 ppm, the incidence of Leydig cell tumours was
increased. These changes were accompanied by non-neoplastic
lesions in the pituitary and the testes.
In a 2-generation reproduction study in rats with one litter
per generation (dietary concentrations of TPTH of 0, 5, 18.5 or 50
ppm) fertility was not affected. The NOAEL was 5 ppm (equal to 0.4
mg/kg bw/day) based on decreased litter size, decreased pup weight
and decreased relative spleen and thymus weight in the weanlings.
In several teratogenicity studies with rats, hamsters, and
rabbits, TPTA or TPTH caused maternal and embryo toxicity, but
irreversible structural effects were not observed. In rabbits, the
most sensitive species, the NOAEL for embryo/fetotoxicity was 0.3
mg/kg bw/day in a study that utilized doses of 0, 0.1, 0.3 or 0.9
mg/kg bw/day TPTA. In this study the NOAEL for maternal toxicity
was 0.1 mg/kg bw/day.
Most in vitro and in vivo genotoxicity tests were
negative. However, two human lymphocyte chromosomal aberration
assays and two mouse lymphoma mutation assays were positive. The
latter responses may have been caused by a variety of effects,
including chromosomal aberrations. Since two in vivo studies for
chromosomal aberrations (a micronucleus test in mice and a
cytogenetic test in Chinese hamsters) were negative, it would
appear that any genotoxic properties are of low potency. It was
therefore concluded that fentin does not present a genotoxic hazard
for man.
Effects on the immune system were observed in short- as well
as in long-term toxicity studies. In special studies with mice and
rats, TPTH showed immunosuppressive properties (lymphopenia and
lymphocyte depletion of spleen and thymus), resulting in altered
humoral and cellular immunity. The NOAEL in mice was 5 ppm, equal
to 1 mg/kg bw/day, based on a decrease in splenic weight found at
25 ppm. In rats, the NOAEL was 25 ppm, equal to 1.7 and 1.8 mg/kg
bw/day for males and females respectively, based upon a significant
decrease in immunoglobulin G and a marginal decrease in leucocyte
and lymphocyte counts at 50 ppm.
The Meeting noted that there was increased mortality at the
lowest dose tested in the most recent long-term study in rats (0.3
mg/kg bw/day). Applying a 500-fold safety factor to this LOAEL
would result in approximately the same ADI as the previously
established ADI based upon a NOAEL of 0.1 mg/kg bw/day in an
earlier long-term study in rats. The previous ADI was therefore
retained. This ADI is supported by NOAELs derived from recent
studies, including the NOAEL of 0.4 mg/kg bw/day in the
2-generation reproduction study, the NOAELs in short-term studies
in rats (0.3 mg/kg bw/day) and dogs (0.2 mg/kg bw/day) and in a
teratology study in rabbits (0.1 mg/kg bw/day for maternal
toxicity).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm in the diet, equal to 1 mg/kg bw/day
Rat: <5 ppm in the diet, equal to <0.3 mg/kg bw/day
(long-term/carcinogenicity study)
5 ppm in the diet, equal to 0.4 mg/kg bw/day
(multigeneration reproduction study)
Dog: 6 ppm, equal to 0.2 mg/kg bw/day
Rabbit: 0.1 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Ongoing studies on the endocrinological effects of fentin
2. Observations in humans.
REFERENCES
Baeder, Ch. (1987a) Fentinacetate - substance, technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Wistar rats
after oral administration. Unpublished Report 89.1478 (A42190) of
Pharma Research Toxicology and Pathology, Hoechst AG. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Baeder, Ch. (1987b) Fentin acetate - substance technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished Report 89.1559
(A42324) of Pharma Research Toxicology and Pathology, Hoechst AG.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Bakke, J.E., Feil, V.J., Aschbacher, P.W., & Price, C.E. (1982)
Metabolism of 14C-triphenyltinhydroxide by the rat, sheep and cow.
Unpublished Report (A35976) of metabolism and radiation research
laboratory Agricultural Research Service, Fargo, ND, USA.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Banduhn, N., Sachsse, K. & Terrier, C. (1985) Mouse micronucleus
test with TPTH-technical (HOE 029664 OF ZD97 0004). Unpublished
Report (A31583), project 049522 from RCC, Switzerland. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Brown, W.R. (1985) 18-Month carcinogenicity study of Technical TPTH
96.3% (Lot no. SWRAM-1K) in CD Mice. Histomorphology of the Uterus.
Unpublished Report (A32214) from Research Pathology Services. Inc.,
New Britain, CT, USA Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Brunk, Dr. & Weigand, W. (1981) Acute oral toxicity of
Fentin-Hydroxide, active ingredient (Code: HOE 29664 O F AT201) to
the male and female beagle dog. Unpublished Report 462/81 from
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Beurkle, W.L. (1985). Hoe 002782-14-C (triphenyltin acetate),
analysis of hydrolysis byproducts. Unpublished Report CM009/85
(A31534) from Analytisches Laboratorium, Hoechst. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L., Rutz, U., & Müller, A., Eckert, H.G., Kellner, H.M.
& Esinger, W. (1986) Metabolism in rats after single and repeated
oral administration at the two dosage levels 2 and 10 mg/kg body
weight. Unpublished Report CM 011/85 (A34163) of Radiochemisches
Laboratorium and Analytisches Laboratorium, Hoechst AG. Submitted
to WHO by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L. & Kellner, H.M. (1987) HOE 029664 (TPTH)-14C.
Excretion study in rats with bile fistula following oral
administration of 2 mg a.i./kg body weight. Unpublished Report
(B)97/87 (A36680) of Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. (1985) Triphenyltin hydroxide. Additional data on
Batelle rat and hamster teratology studies (31184). Unpublished
data of Batelle Columbus Laboratories, Columbus, Ohio. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Connell, M.M. (1981) Final report on evaluation of
the teratogenicity of triphenyltin hydroxide (TPTH) in the
Sprague-Dawley rat. Unpublished Report NO723-0200 (A22211) of
Batelle Columbus Laboratories, Columbus, Ohio. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Crisp, C.E. (1982) Range-finding study for the
evaluation of reproductive effects of triphenyltin Hydroxide (TPTH)
in the Wistar rat. Unpublished Report (A25091) project NO723-0400)
from Battelle, Columbus, Ohio. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. & Howard M. (1982) The evaluation of the
teratogenicity of triphenyltin hydroxide (TPTH) in the Syrian
hamster. Unpublished Report
NO723-0100 (A22884) of Battelle, Columbus Laboratories, Columbus,
Ohio. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Fentinhydroxide,
substance technical grade (Code: HOE 029664 OF ZD97 0004) in the
rat primary hepatocyte unscheduled DNA synthesis assay. Final
Report (A32084) GT85.1091 from Litton Bionetics, Inc. Maryland.
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Craine, E.M. (1987) An extended duration dermal absorption study in
rats with 14C-triphenyltin hydroxide. Unpublished Report WIL-39037
(A35945) of WIL Research Laboratories. A subsidiary of Great Lakes
Chemical Corporation, Ashland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
DenBoer, W.C. & Hoorn, A.J.W. (1985) Mutagenicity evaluation of HOE
0 29664 -Substance technical (Code: HOE 029664 OF ZD97 0004) in the
L5178Y TK +/- Mouse Lyphoma forward mutation assay. Unpublished
Final Report (A31582) from Litton Bionetics, The Netherlands.
Submitted to WHO by Hoechst AG. Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986a) Fentin acetate - active
ingredient technical. Testing for acute intraperitoneal toxicity in
the male and female rat. Unpublished Report 86.1047 (A37976) of
Hoechst Pharma Research Toxicology and Pharmacology, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986b) Fentin acetate - active
ingredient technical. Testing for acute dermal toxicity in the male
and female Wistar rat. Unpublished Report 86.0816 (A37384) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986c) Fentin acetate - active
ingredient technical. Testing for primary dermal irritation in the
rabbit. Unpublished Report 86.0619 (A37383) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986d) Fentin acetate - active
ingredient technical. Testing for primary eye irritation in the
rabbit. Unpublished Report 86.0620 (A37382) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986e) Testing for sensitizing
properties in the Pirbright-White guinea pig according to the
technique of Buehler. Unpublished Report 86.0803 (A36975) of
Hoechst Pharma Research Toxicology and Pathology. Submitted by WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987a) Fentin-hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing for
sensitizing properties in the Pirbright-White guinea pig in a
maximization test. Unpublished Report 87.0201 (A35628) from Hoechst
Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987b) Fentin hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing of
host resistance in the female Wistar rat (Immunotoxicological
screening with Trichinella spiralis) -2nd study-. Unpublished
Report 87.0955 (A36065) of Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Dorn, E. & Werner, H.J. (1989a) Triphenyltinhydroxide (Hoe 029964
OF ZD97 0004). Determination of residues in organs, tissues and
blood of dogs after chronic feeding (52 weeks). RCC Project 047024
and RCC Project 047013. Unpublished Report CR076/88 (A41963) of
Analytisches Laboratorium Hoechst AG. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Dorn, E & Werner, H.J. (1989b) Triphenyltinhydroxide (Hoe 029664 OF
ZD 97 0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished Report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG.,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Duchosal, F., Thevenaz, Ph., Luetkemeier, H., Vogel, O., Pappritz,
G., Mladenovic, P. & Terrier, Ch. (1989) Fentinhydroxid (TPTH)
technical grade. Subchronic (90-day) repeated dose inhalation
toxicity study in rats. Unpublished Report 202353 (A40323) of RCC,
Research and Consulting Company AG., Carouge-Geneva, Switzerland.
Eckert, H.G., Kellner, H.M., Beurkle, W.L., (1989) Accumulation
study; kinetics and metabolism in the rat after single and repeated
oral administration, of 2 mg/kg body weight. Unpublished Report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L,
Oekologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Giavini, E., Prati, M. & Vismara, C. (1980) Effects of triphenyltin
acetate on pregnancy in the rats. Bull. Environm. Contam. Toxicol.
24: 936-939 (A19995). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1974a) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished Report 16454
(A16454) from Hoechst Pharma Forschung Toxicologie. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Hollander H. & Weigand W. (1974b) Triphenyltin acetate. Acute oral
toxicity (LD50) in guinea pigs. Unpublished Report (A15562) of
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974c) Triphenyltin acetate.
Subchronic (30-day) oral toxicity testing in rats and guinea pigs.
Unpublished Report (A37587) of Hoechst Pharma Research Toxicology.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1974d) Triphenyltin acetate dermal
toxicity in rats and rabbits. Unpublished Data (A15561) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974e) Triphenyltin acetate skin and
eye irritation in rabbits. Unpublished Report (15564) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1980) Acute oral toxicity of fentin
acetate technical (Hoe 02782) in male rabbits. Unpublished Report
(A28616) of Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1981) Dust inhalation of HOE 29664 -
active ingredient in the male and female SPF-Wistar rat. 4 h -LC50.
Unpublished Report 335/81 (A22293) from Hoechst Pharma Forschung
Toxicologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1986) Fentin acetate - active
ingredient technical. Testing for acute dust inhalation toxicity in
the male and female SPF rat 4-hour LC50. Unpublished Report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ishaaya, I., Engel, J.L. & Casida, J.E. (1976) Dietary
triorganotins affect lymphatic tissues and blood composition of
mice. Pestic. Biochem. Physiol. 6: 270-279. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main. (A31628)
Jung, R. & Miltenburger, H.G. (1986) Fentin acetate - substance
technical (Code: Hoe 002782 OF ZD97 0002). Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with
V79 cells. Unpublished Report LMP 229 (A35212) of Hoechst
Laboratory for mutagenicity testing. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Jung, R. & Weigand, W. (1986) Fentin acetate - substance technical,
Code: Hoe 002782 OF ZD97 0002. Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames test) and Escherichia
coli. Unpublished Report 86.0042 (A33021) of Hoechst Pharma
Research Toxicology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kavlock, R.J. (1985) Triphenyltin hydroxide developmental
toxicology study. Unpublished Report of United States Environmental
Protection Agency (A31424). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1986) Kinetics in the rats following
single and repeated administration of 2 mg/kg and single
administration of 10 mg/kg body weight. Unpublished Report
01-L42-0489-86 (A34186) of Hoechst Radiochemisches Laboratorium.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1989) Addendum to report
01-L42-0565-89. Hoe 029664 (TPTH)-113Sn. Accumulation and depletion
study in rats after single and repeated oral administration of 2
mg/kg body weight. Unpublished Report 01-L42-582-90 (A43434) of
Pharma Forschung GB-L Radiochemisches Laboratorium Hoechst,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M., Eckert, H.G. & Bürkle, W.L. (1989) Hoe 029664
(TPTH)-113Sn. Absorption studies in rats with bile fistula after
a single oral dose of approx. 2 mg/kg body weight. Unpublished
Report 01-L42-0564-89 (A41409) of Hoechst Pharma Foreschung
Radiochemisches Laboratorium Productent-wicklung GB-C, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of HOE 029664 - substance technical by its effects on
cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished Report A31614 from Microtest Research Limited, UK.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Lachmann, G. & Siegemund, B. (1987) Exhalation experiment with
triphenyltinhydroxide (Hoe 029664) in rats. Unpublished Report
(A34601) of Battelle-Institute. V., Frankfurt-am-Main. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Laveglia, J., Hagen, C.A. & Nemec, M.D. (1985) 21-Day dermal
toxicity study in rats with triphenyltin hydroxide (Code: Hoe
029664 OF ZD97 0001 technical substance). Unpublished Final Report
(A31616) project no WIL-39018 from American Hoechst Company, New
Jersey. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. (1988) Fentin acetate-substance technical (Code HOE
002782 OF ZD97 0002). Repeated dose dermal toxicity study (21
applications in 29 days) with a 14 day withdrawal period.
Unpublished Report (A39231) 3104 TSR of Pharma Forschung
Toxicologie und Pathologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981a) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 29664 O F AT201) to the male rat.
Unpublished Report (A22115) no 182/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981b) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 19664 O F AT201) to the female rat.
Unpublished Report (A22114) 183/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981c) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 O F AT201) to the
female rat. Unpublished Report (A22292) 184/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981d) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished Report (A22112) 229/81 from Hoechst Pharma
Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981e) HOE 29664 - active ingredient
(Code: HOE 29664 O F AT201). Irritance to the rabbit skin and eye
mucosa. Unpublished Report (A22113) 104/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981f) Test for sensitizing properties
of Fentin hydroxide-technical (Code : HOE 29664 O F AT201) in
guinea-pig (according to Buehler). Unpublished Report (A22433)
724/81 from Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Leist, K.H., Weigand, W. & Kramer, Dr. (1982) Range-finding test
von Hoe 29664-Wirkstoff (Fentinhydroxid) (Code: Hoe 29664 O F
AT201) im 30-Tage-Fuetterungsversuch and SPF-Wistar-Ratten.
Unpublished Report (A24003) 426/82 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Loquet, C.(1985) Fentin acetate (Code: Hoe 002782 OF ZD97 0001)
Micronucleus test in mice. Unpublished Report 1592 MAS (A32196) of
Centre International de Toxicologie at Misery. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990a) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH) substance
technical (Code HOE 029664 OFZD97 0004) in mice. Unpublished Report
L08252SN01 (A44053) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990b) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH), substance
technical (Code HOE 029664 OFZD97 0004) in rats. Unpublished Report
L08252SN02 (A44054) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Manzo, L. & Richelmi, P. (1981) Poisoning by triphenyltin acetate.
Report of two cases and determination of tin in blood and urine by
neutron activation analysis. Clinical Toxicology, 18(11)
1343-1353. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
(A44030)
Milone, M.F. & Hirsch, E. (1985a). Study of the capacity of the
test article HOE 029664 - Substance technical grade - (Code No. HOE
029664 OF ZD97 0004) to induce gene conversion in saccharomyces
cerevisiae. Unpublished Report (A32085) experiment no.M 890 from
RBM, Ivrea, Italy. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1985b) Study of the capacity of the test
article HOE 029664 - Substance technical grade (Code HOE 029664 OF
ZD97 0004) to induce gene mutation in Schizosaccharomyces pombe.
Unpublished Report (A31613) from RBM Italy. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986a) Study of the capacity of the
article fentin-acetate-substance technical grade (Code No. Hoe
002782 OF ZD97 0002) to induce gene conversion in Saccharomyces
cerevisiae. Unpublished Report M1023/1-2 (A34018) of Instituto di
Ricerche Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986b) Fentin acetate substance
technical grade. Forward Mutation test in S. pombe P1.
Unpublished Report M898 (A32812) of Instituto di Ricerche
Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Mueller, W. (1987) Evaluation of HOE 029664 OF ZD97 0004 in the
in vivo cytogenetic test in bone marrow cells of the Chinese
hamster - chromosome analysis. Unpublished Report 87.0031 (A36080)
from Hoechst Pharma research Toxicology and Pathology. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Nagamatsu, K., Kido, Y., Urakubo, G., Aida, Y., Ikeda, Y. & Suzuki,
Y. (1978) Absorption, distribution and excretion of triphenyltin
acetate and stannic chloride in the guinea pig. Japanese Journal
of Hygiene, 33: 486-496.
NCI (1984) Mouse lymphoma with triphenyltin hydroxide. Unpublished
Report (A30130), National Cancer Institute. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS no. 76-87-9 (A199994). Technical report series
No.139. US Department of Health, Education, and Welfare.
Nunziata, A. & Consonni, R.F. (1988) Chromosome aberrations in
human lymphocytes cultured in vitro. Test substance: HOE 029664
Fentin hydroxide substance technical (Code: Hoe 029664 OF ZD97
0004). Unpublished Final Report 157024-M-04487 (A38456) from Life
Science Research Roma Toxicology Centre S.P.A. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ravert, J. & Parke, G.S.E. (1976) Investigation of teratogenic and
toxic potential of technical triphenyltinhydroxide (lot #SWRAM-1K).
Unpublished Report GT85.0797 (31618) of Cannon Laboratories, Inc.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Ravert, J., Parke, G.S. & Charles, S.J. (1978) The dominant lethal
assay of technical TPTH (Triphenyltin hydroxide) in Sprague-Dawley
rats. Unpublished Report (A24394) from Cannon Laboratories Inc.,
Reading. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Resnis, P., Craine, E.M. & Nemec, M.D. (1986) A dermal absorption
study in rats with 14C-triphenyltin hydroxide. Unpublished Report
(WIL-39020) (32810) of WIL Research Laboratories. A subsidiary of
Great Lakes Chemical Corporation, Ashland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Richold, M., Jones, E. & Fleming, P.M. (1981) Ames metabolic
activation test to assess the potential mutagenic effect of HOE
29664 OF AT201. Unpublished Report 450/81A (A21735) from Huntingdon
Research Centre, GBR. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1985a) One generation teratology and reproductive
study in rats with triphenyltin hydroxide (Code: HOE 029664 OF ZD97
0001 Technical substance). Unpublished Final Report project
WIL-39013 (A31617) from Wil Research Laboratories Inc., Ashland
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Rodwell, D.E. (1985b) A teratology study in rats with triphenyltin
hydroxide (Code: HOE 029664 OF ZD97 0001 Technical Substance).
Unpublished Report WIL-39011 (A31079) of WIL Research Laboratories
Inc, Ashland USA. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
Report WIL-39012 (A35220) of WIL Research Laboratories, Ashland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Sachsse, K., Frei, T., Luetkemeier, H., Vogel, W., Pappritz, G. &
Terrier, C. (1987) TPTH - Substance technical (Hoe 029664 OF ZD 97
0004) Chronic oral toxicity 52-week deeding study in beagle dogs.
Unpublished Report 047013/047024 (A35733) of RCC Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Schollmeier, U. & Leist, K.H. (1989) Triphenyltin hydroxide -
active ingredient technical (Code: Hoe 029664 OF ZD97 0004).
Testing for sensitising properties in the Pirbright-White guinea
pig according to the technique of Buehler. Unpublished Report
89.1006 (A42667) of Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Scholz & Weigand, W., (1969b) Triphenyltin acetate Study No. 2116.
Unpublished Data A37396 from Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Seinen, W. & Penninks, A.H. (1987) Immunotoxicity of triphenyltin
compounds. Department pharmacology, pharmacy and toxicity. Faculty
of Veterinary Sciences, University of Utrecht, The Netherlands.
Document A35456. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Snow, R.L. & Hays, R.L. (1983) Phasic distribution of seminiferous
tubulus in rats treated with triphenyltin compounds. Bull.
Environ. Contam. Toxicol. 31: 658-665. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986a) 13-week oral toxicity (feeding) study
with TPTH-technical (code: HOE 029664 OF ZD97 0004 in the mouse.
Unpublished Report project 046991 (A32420) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986b) 13-week oral toxicity (feeding) study
with TPTH-technical (Code : HOE 029664 OF ZD97 0004) in the rat.
Unpublished Report project 046978 (A32419) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Vogel, O.,
Armstrong, J., Ehlers, H.A., Müller, E. & Terrier, Ch. (1989a)
TPTH-technical (Code: HOE 029664 OF ZD97 0004) oncogenicity 80-week
feeding study in mice. Unpublished Report 047002 (A40467) of RCC,
Research and Consulting Company AG., Itingen, Switzerland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Schlotke, B.,
Vogel, O., Ehlers, H.A., Mueller, E. & Terrier, Ch. (1989b)
TPTH-technical (Code: HOE 029664 OF ZD97 0007) chronic
toxicity/oncogenicity 104-week feeding study in rats. Unpublished
Report 046980 (A40468) of RCC, Research and Consulting Company AG.,
Itingen, Switzerland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Terrel, Y & Reddy, A.K. (1978) Report: 18-month carcinogenicity
study of technical TPTH 96.3% lot no. SWRAM-1K in CD1 mice.
Unpublished Report project 6E-725 (A26239) from Cannon
Laboratories, Inc. Reading. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Traul, K.A., Takayama, K., Kachevsky, V., Hink, R.J. & Wolff, J.S.
(1981) A rapid in vitro assay for carcinogenicity of chemical
substances in mammalian cells utilizing an attachment-independence
endpoint. J. Appl. Toxicol. 1(3), 190-195.
Ueda, K. & Iijima, K. (1961) Acute toxicity of Brestan Technical
(Triphenyltin Acetate) for mice. Unpublished Report A15046 from
Laboratory of Hygienics, Tokyo Dental College, Japan. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Wu, R,M., Chang, Y.C. & Chiu, H.C. (1990) Acute triphenyltin
intoxication: a case report. J. Neurol. Neurosurg. Psych., 53,
356-357.
Young, D.L. (1986) A dietary two-generation reproduction study in
rats with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final Report project WIL-39022 (A35378) from
WIL research laboratories, Inc. Ashland USA. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
A 576 kg milk cow received a single oral dose of 5055 g
14C-TPTH and a 74 kg ewe received a dose of 1011 g 14C-TPTH, in
capsules. In the cow after 6 days 12.9% of radioactivity was
excreted in urine and 78% in the faeces. Tissues contained 5.1%.
Maximum radioactivity levels were found in the liver (5.5 mg/kg),
kidney and adrenals (both 2.2 mg/kg). In the sheep, 32.6% of
applied radioactivity was found in urine and 47.5% in the faeces
after 8 days. Tissues contained 3.1%. The liver showed highest
level of radioactivity, 6.6 mg/kg. Triphenyltinchloride was
identified as the major faecal excretion product. Phenol,
hydroquinone, catechol and resorcinol were isolated from the urine
(Bakke et al., 1982).
Toxicological studies
Acute toxicity
The LD50 and LC50 values for TPTH and TPTA in various species
are given in Tables 1 and 2, respectively.
The observed intoxication signs following oral exposure
include anorexia, emesis, tremor and diarrhoea followed by
drowsiness and ataxia. After dermal exposure to TPTH, skin
irritation was observed.
Five applications of 50 mg TPTA (purity not stated)/kg applied
to the nuchal skin of 5 rats caused no mortality but some signs of
local irritation. Five applications of 100 mg/kg to 5 rats caused
death of one animal and local signs of irritation. Five
applications of 200 mg/kg were lethal to rats. (Summary only)
(Hollander & Weigand, 1974d).
A single dermal application of 12.5 mg TPTA/kg to 3 rabbits
was lethal for 2 rabbits and caused black coloration of the skin
and scarcely pilous sites of the body. (Summary only) (Hollander &
Weigand 1974d).
Table 1. Acute toxicity of TPTH
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/m3)
Rat M oral ? 165 Leist & Weigand (1981a)
F oral ? 156 Leist & Weigand (1981b)
F perc. ? 1600 Leist & Weigand (1981c)
M&F inhal.a 97.0% 60.3 Hollander & Weigand (1981)
Rabbit M perc. ? 127 Leist & Weigand (1981d)
Dog M&F oral ? »100 Brunk & Weigand (1981)
a indicates 4 hour exposure
Table 2. Acute toxicity of TPTA
Species Sex Route Purity LD50 LC50 Reference
% (mg/kg bw) (mg/m3)
Mouse M oral 99 81.0 Ueda & Iijima (1961)
M oral 99 93.3 Ueda & Iijima (1961)
M s.c. 99 44.0 Ueda & Iijima (1961)
M dermal 99 350 Ueda & Iijima (1961)
Rat F oral ? 298 Scholz & Weigand (1969b)
F oral ? 140 Hollander & Weigand (1974a)
M&F dermal 97 >2000 Diehl & Leist (1986b)
M i.p. 97 3.6 Diehl & Leist (1986a)
F i.p. 97 12.7 Diehl & Leist (1986a)
M inhal. 97 44a Hollander & Weigand (1986)
F inhal. 97 69a Hollander & Weigand (1986)
Guinea pig ? oral ? 25 Hollander & Weigand (1974b)
Rabbit M oral ? 80 Hollander & Weigand (1980)
a indicates 4 hour exposure, whole body exposure, dust inhalation
Short-term studies
Mice
Male and female NMRI mice (10/sex/group) were fed diets
containing 0, 4, 20 or 100 ppm TPTH technical (purity 97.2%) for
three months. No compound-related effects were observed on
mortality, clinical signs, food consumption, body weight,
macroscopy and histopathology. At 100 ppm several haematological
and biochemical parameters were affected. Erythrocyte count and Hb
were decreased and platelet count increased in males and females.
The incidence of Heinz bodies was increased in males and MCH and
MCHC were decreased in females. Mouse immunoglobulins (IgA and IgG)
were decreased, IgM levels were decreased in females only. In
female mice total protein, albumin and alpha-globulin were
increased and gammaglobulin was decreased. Liver weight was
increased in males and females and a decrease was observed in the
relative weights of ovaries, adrenals, kidneys, heart and brain in
females. The NOAEL in this study is 20 ppm, equal to 3.4 mg/kg bw
(males) and 4.1 mg/kg bw (females) (Suter & Horst, 1986a).
Rats
In a range-finding study groups of rats (Hoe: WISKf;
10/sex/group) were fed diets containing 0, 5, 10, 20, 40 or 80 ppm
TPTH (97.0%) for 30 days. No compound-related effects were observed
on clinical signs, water consumption, urinalysis, organ weight,
macroscopy nor histopathology. In male rats at 80 ppm body weight
gain was decreased. Food consumption was decreased during the first
weeks in males at 80 ppm and females at 40 and 80 ppm. An increase
in thrombocyte count was observed in female rats at 40 and 80 ppm;
at the highest dose a decrease in Hb was observed in males and a
decrease in haematocrit was observed in females. At the highest
dose bilirubin, creatinin (males only), SGPT and urea (males only)
were increased. SAP was increased in a non dose-related manner in
males at 20 ppm and higher and in high-dose females. There was no
effect on spleen weight. The NOAEL in this study is 20 ppm equal to
2.01 mg/kg bw (males) or 1.83 mg/kg bw (females) (Leist et al.,
1982).
Groups of Wistar rats (15/sex/group) received dietary
concentrations of 0, 4, 20 or 100 ppm TPTH-technical (purity 97.2%)
for 13 weeks. The treatment period was followed by a 4-week
recovery period (5 male and 5 female rats/group). Observations
included clinical signs, food consumption, body weight,
ophthalmoscopy, hearing tests, haematology, clinical chemistry,
organ weights, macroscopy and histopathology (54 tissues; only in
control and high-dose rats). After 13 weeks of treatment the
following parameters were significantly affected; mean body weight
gain and food consumption were slightly decreased in high-dose
rats. RBC was increased, MCV (in males also at 20 ppm) and MCH were
decreased. In females WBC decreased at 20 and 100 ppm. Most
biochemical parameters were significantly affected at the highest
dose. The observed increase in ASAT was also apparent at 20 ppm as
was the decrease in albumin in females (also at 4 ppm). IgG was
slightly lower. The 18-hour urine volume was increased and the
specific gravity was decreased at 100 ppm. Relative testes weight
was significantly higher in high-dose males. After the recovery
period ASAT was still increased at 100 ppm and IgG was decreased in
males and significantly in females at all dose levels. No effects
were observed on spleen and thymus weights. The NOAEL in this study
is 4 ppm equal to 0.30 mg/kg bw (males) or 0.35 mg/kg bw (females)
(Suter & Horst, 1986b).
Four different experimental groups received 21 dermal
applications of 0, 5, 10 or 20 mg TPTA (purity unknown)/kg bw/day
in a 29-day period. Dosage groups consisted of 12 rats/sex in the
0 and 20 mg/kg groups and of 6 rats/sex in the 5 and 10 mg/kg
groups. At the end of the treatment period 6 rats/sex of the 0 and
20 mg/kg groups were kept for a 14 day recovery period. Rats were
observed for clinical signs, body weight, food consumption,
haematology, blood chemistry, urinalysis, organ weights, macroscopy
and histopathology. Piloerection, mydriasis, increased muscle tones
and dyspnoea were observed in high dose rats. Dose-related increase
in erythema and scale formation was observed in all treated groups.
Dose-related cracked skin and thickening of the skin fold was
observed at 10 and 20 mg/kg groups. Crusts and slight oedema were
observed in some animals at 20 mg/kg. At 20 mg/kg 4 animals died.
Lymphocytes decreased in males and females at 20 mg/kg. Monocytes
increased in females at 20 mg/kg. Sodium level decreased in all
treated males (n.d.r.) and females. Potassium decreased in 20 mg/kg
females. Inorganic phosphorus and total protein increased in 20
mg/kg females. A/G-ratio and albumin increased in 20 mg/kg males.
A1-globulins decreased in 20 mg/kg males. Macroscopy and
microscopy showed dose-related skin lesions such as hyperkeratosis
and epidermal hyperplasia in all treated groups. All effects were
reversible. No effects on spleen weights were observed. The NOAEL
for systemic toxicity in this study is 10 mg/kg bw (Leist, 1988).
TPTH (purity 97.1%) was applied to the shaven unabraded backs
of Charles River rats (10/sex/group) at doses of 0, 5, 10 or 20
mg/kg bw for 15 applications in 3 weeks. No treatment-related
effects were observed on food consumption, haematology, clinical
chemistry, urinalysis nor organ weights. Male body weight gain was
very slightly decreased in all treated groups and significantly
decreased during the first week at the highest dose. Dermal
irritation, increasing with each application, was observed in all
treated rats during the first 2 weeks. At the third week the
irritation was less severe and severe erythema was not present any
more. At macroscopy an increased incidence in ulcerated, exfoliated
and thickened skin was observed in all treated groups. Microscopic
evaluation of the treated skin revealed acanthosis and
hyperkeratosis. Also changes such as focal ulceration and mild
dermal inflammatory changes correlated with the observed dermal
irritation were seen. No systemic toxicity was observed (Laveglia
et al., 1985).
Groups of SPF-Wistar rats (10/sex/group) were exposed to 0.05,
0.5 and 2.0 mg TPTH (purity 96.2%)/m3 for 6 hours/day, 5 days/week
for 13 weeks (nose only exposure). Analytical concentrations were:
0.014, 0.338 or 1.997 mg/m3. The MMAD was 3 µm. The reversibility
of treatment-related changes was studied after a 4-week recovery
period in the control and highest dose group with 10 additional
animals/sex. Observations were made for clinical signs, body
weight, food consumption, ophthalmoscopy, haematology and clinical
chemistry, organ weights (6), macroscopy and histopathology. In the
highest dose group 10 males and 1 female died between test weeks
2-13 compared to none in the other groups. Laboured respiration and
rales were observed only in the highest exposure group. These signs
were still noted during the first week of recovery. RBC, Hb and Hc
were decreased in high exposed females, while platelet count was
increased. WBC was decreased in females at 0.5 and 2.0 mg/m3.
Glucose and total bilirubin were decreased in females at 0.5 and
2.0 mg/m3. Creatinine, albumin, gamma-glutamyltransferase and
potassium levels were decreased in females at 2.0 mg/m3. Calcium
and phosphorus level were decreased in all exposed female groups.
Calcium level decreased also in males at 0.5 and 2.0 mg/m3. IgG
increased in females at the high exposure level and IgM increased
in males at 0.5 and 2.0 mg/m3. Relative lung weights increased in
high exposed males, but no effect on thymus or spleen weight was
observed. Macroscopic examination showed lesions in the form of
multiple red foci in the lungs of most dead rats. Histopathology
revealed in both sexes at 2.0 mg/m3 degenerative and/or
inflammatory lesions in the anterior part of the nasal cavity, in
the trachea and in the lungs. The lower air passages and in
particular the lungs were severely affected. The pathomorphologic
sequence of events in the lungs seemed to start with degeneration
and multifocal necrosis of the bronchial epithelium followed by
multifocal or lobular fibrinous bronchopneumonia. The NOAEL in this
study is 0.05 mg/m3 (Duchosal et al., 1989).
Groups of 10 rats/sex were fed diets containing TPTA (purity
not given) at a rate of 0, 37.5, 150 or 625 ppm, during 30 days. No
effects were found on haematology and urinalysis. Seven rats/sex
from the 625 ppm group died during the study. Signs in this group
were piloerection and deterioration of the general condition. At
150 and 625 ppm temporary body weight gain decreases were seen.
Histopathology showed gastrointestinal haemorrhages and hepatic
changes (effects on cell metabolism) in the 625 ppm group. The
NOAEL in this study is 37.5 ppm, equivalent to 1.9 mg/kg bw.
(Summary only) (Hollander & Weigand, 1974c).
Guinea-pigs
Groups of guinea pigs (10/sex/group) were fed a diet
containing TPTA (purity not given) at a rate of 0, 7.5, 30.0 or 125
ppm. No effects were found on haematology nor urinalysis. In the
125 ppm group 8 male and 5 female guinea pigs died during
treatment. Effects found in this group included poor general
condition, piloerection and decrease in body weight gain.
Histological evaluation revealed fatty changes in the livers of
guinea-pigs treated with the highest dose (summary only) (Hollander
& Weigand, 1974c)
Dogs
Male and female dogs (beagle, 10/sex/group) received a diet
containing 0, 2, 6 or 18 ppm TPTH (purity 97.2%) for 52 weeks.
Interim necropsy on 2 dogs/sex/group was performed after 4, 13 and
27 weeks. Observations were made for clinical signs, food
consumption, body weight, hearing, ophthalmoscopy, haematology,
clinical chemistry (including immunoglobulins) and urinalysis. The
weights of 12 organs/animal were recorded (including spleen and
thymus). Gross necropsy and histopathology (about 40
tissues/animal) were carried out. A/G-ratio decreased in males at
18 ppm after 26 and 52 weeks. In females albumin decreased at 6 and
18 ppm after 52 weeks. In females at 18 ppm relative liver weight
was increased. The NOAEL in this study is 6 ppm, equal to 0.21
mg/kg bw (Sachsse et al., 1987)
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
37.5 or 75 ppm TPTH (purity ?) for 78 weeks. There was an
additional observation period of 26 weeks for both groups. Twenty
male and 20 female mice were used as controls. Dosed feed
preparations containing 37.5 or 75 ppm TPTH were analyzed
spectrophotometrically. 88% of the theoretical amount of TPTH was
recovered immediately after preparation but after 1 week only 57.9%
was recovered. The data were not corrected for any loss which may
have been due to the instability of TPTH. No treatment-related
effects were observed on clinical signs and body weight. Survival
was decreased in female mice (survival for males was: 65%, 72% and
76% and for females 95%, 74% and 66% for 0, 37.5 and 75 ppm,
respectively). At histopathology no increase in tumour incidence
was observed (NTP, 1978)
Groups of CD1-Charles River mice (60/sex/group) were fed diets
(prepared at weekly intervals) containing 0, 7, 28 or 52 ppm
technical TPTH (purity 96.3%) for 18 months. Observations included
clinical signs, mortality, body weight, food consumption,
macroscopy and histopathology (19 tissues). Survival rate was
significantly decreased in male mice in the mid and highest dose
groups (70%, 70%, 41% and 41% at 0, 7, 28 and 52 ppm,
respectively). Survival rates for females were 55%, 71.7%, 53.3%
and 58.3% at 0, 7, 28 and 52 ppm, respectively). The frequency of
aggression, alopecia, irritation, laceration and scabs was
dose-relatedly increased in both males and females. Food
consumption was reduced during some short periods in all treated
females. At histopathology an increase in the occurrence of
endometrial hyperplasia with cyst formation of the uterus was
observed at 28 and 52 ppm. The tumour incidence was not increased
(Terrel & Reddy, 1978). Extensive histomorphologic examination of
the uterus revealed that the observed cystic endometrial
hyperplasia was considered to be a hyperplastic reaction and not
neoplastic (Brown, 1985).
Groups of 50 male and 50 female mice (KFM-Han. NMRI) were fed
diets (prepared fresh daily) containing 0, 5, 20, 80 ppm TPTH
(purity 97.2%) for 80 weeks. Observations were made for clinical
signs, body weight, food consumption, ophthalmoscopy, haematology
(differential wbc count), clinical chemistry (including
immunoglobulin classes and subclasses) and organ weights (no spleen
weight). Gross pathology and histopathology (35 tissues including
bone marrow smears) were carried out. Intercurrent mortality was
significantly increased for 80 ppm females. At 80 ppm mice showed
ruffled fur towards the end of the study as well as skin lesions.
Body weight gain was reduced for 80 ppm males and 20 and 80 ppm
females. IgG, IgA, IgM, IgG4(f), IgG2a(f), IgG2b and IgG3 were
slightly or moderately decreased at 80 ppm. The decrease in IgA was
also found at 20 and 5 ppm (n.d.r.) and in IgM at 20 and 5 ppm in
male mice. Relative liver weight was markedly increased and
relative brain weight slightly increased at 80 ppm males and
females. Relative kidney weight was decreased at 80 ppm in males
and females. Macroscopic examination showed nodules, foci and
nodular lesions in the livers of mice in 80 ppm males. Skin lesions
were observed in both sexes at 80 ppm. Microscopy showed a higher
incidence of slight to moderate, focal to multifocal, nodular
hyperplasia in the liver of 80 ppm males and females. A higher
incidence of hepatocellular adenomas was observed at 80 ppm
(incidence: 12.2%, 20%, 26% and 32% for 0, 5, 20 and 80 ppm,
respectively, for male mice, and 0% in 0, 5 and 20 ppm females and
18% in 80 ppm females; only significant at 80 ppm). An increased
incidence in hepatocellular carcinomas was observed in 80 ppm
females. The incidence was 6% compared to 0% in the 0, 5 and 20 ppm
groups. An increased incidence of chronic suppurative dermatitis
and related non-specific skin lesions including acanthosis and
hyperkeratosis was observed in 80 ppm males and females. An
increased relative number of lymphoid cells was detected in femoral
bone marrow myelograms for all treated groups. The relative numbers
of granulocytes were significantly decreased for females at 5 ppm
and both sexes at 20 and 80 ppm. The relative numbers of
erythrocyte precursors were significantly decreased for males at
20 and 80 ppm. However, it is difficult to evaluate these data in
the absence of red cell counts in peripheral blood. Although a
reduction in serum immunoglobulin concentrations was reported, the
Meeting noted that there was no corresponding effect on
haematological parameters and that the method of analysis has not
been validated in rodents. The data on serum immunoglobulin
concentrations was therefore not utilized in the determination of
the NOAEL for this study. Based on reduced body weight gain, the
NOAEL is 5 ppm, equal to 1 mg/kg bw/day (Tennekes et al., 1989a).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
prepared weekly containing 37.5 or 75 ppm TPTH (purity not stated)
for 78 weeks. There was an additional observation period of 26
weeks for both groups. Twenty male and 20 female rats were used as
controls. Feed preparations containing 37.5 or 75 ppm TPTH were
analyzed spectrophotometrically. Of the theoretical amount of
TPTH, 80% was recovered immediately after preparation but after 1
week only 57.9% was recovered. The data were not corrected for any
loss which may have been due to the instability of TPTH. No effects
were observed on clinical signs, mortality, food consumption,
macroscopy nor histopathology (31 tissues including pituitary gland
and testes). Body weight was slightly but dose dependently
decreased in male rats after week 40. Survival rates were 75%, 76%
and 84% for males and 90%, 80% and 90% for females at 0, 37.5 and
75 ppm TPTH. The tumour incidence was not enhanced (NTP, 1978).
Groups of 70 rats/sex (SPF-bred KFM-Han. Wistar) rats received
a diet containing 0, 5, 20 or 80 ppm TPTH (purity 97.2%) for 104
weeks. The diets were prepared twice monthly and frozen. A fresh
amount of diet was given daily. Observations were made for
clinical signs, food consumption, body weight, ophthalmoscopy,
haematology, clinical chemistry (including immunoglobulin classes
and subclasses), urinalysis, organ weights, macroscopy and
histopathology (about 40 tissues/animal, including bone marrow
smears). Mortality was increased (dose-related) in all treated
females. The survival was 75, 51, 36 and 23%, respectively.
Preceding mortality clinical signs were ruffled fur, reduced
activity, ataxia, stiff gait and hunched posture. Due to this
excess mortality no laboratory investigations could be made for the
80 ppm female group at 104 weeks. Group mean food intake was
reduced for males and females at 80 ppm. Body weight gain was
dose-relatedly decreased in males and females at 20 and 80 ppm.
Ophthalmoscopy showed an increased incidence of unilateral or
bilateral corneal opacity in males at 20 and 80 ppm at 104 weeks.
Hb and haematocrit were decreased in female rats at 20 and 80 ppm.
ASAT, ALAT, ALP were increased in males and females at 80 ppm. The
following changes in immunoglobulin levels were observed only after
50 weeks: IgG1 levels were reduced for all treated females and 80
ppm males, IgG2a levels were reduced for all treated females and
IgG2c levels were reduced for all treated males, IgA levels were
reduced for males at 20 and 80 ppm and IgM levels were increased
for both sexes at 20 and 80 ppm. At termination most relative organ
weights were increased in the 80 ppm groups, including spleen
weight. An increased incidence of pituitary adenomas was diagnosed
in females at 20 and 80 ppm. The recorded incidences were 64.4%,
63.2%, 76.8% and 93.1% for 0, 5, 20 and 80 ppm groups,
respectively. An increased incidence of testicular Leydig cell
tumours was diagnosed at 80 ppm. The recorded incidences were 1.7%,
8.5%, 5.0% and 16.7% for 0, 5, 20 and 80 ppm, respectively.
Non-neoplastic lesions observed included an increase in hyperplasia
of the pars intermedia of the pituitary gland in males at 80 ppm.
An increased incidence of cystoid change of the pars intermedia of
the pituitary gland was noted in males at 20 and males and females
at 80 ppm at 52 weeks. Increased incidences of testicular Leydig
cell hyperplasia and tubular atrophy were noted in rats at 80 ppm
after 104 weeks. Increased incidences of hepatic bile duct
proliferations and portal sclerosis were noted in females at 20 and
80 ppm after 52 and 104 weeks of treatment. An increased incidence
of skeletal muscle atrophy was noted in male rats at 80 ppm after
104 weeks of treatment. Because mortality was increased in females
and serum immunoglobulins were decreased at the lowest dose level
of 5 ppm (equal to 0.3 (males) 0.4 (females) mg/kg bw no NOAEL
could be established (Tennekes et al., 1989b).
Reproduction studies
In a range-finding study Wistar rats (10/sex/group) were
administered 0, 12.5, 25, 50, 100 or 200 ppm TPTH (weekly prepared,
purity not stated) in the diet for in total 90 days (from 59 day
pre-mating period up to 21 day gestation period). All animals were
killed following weaning. Observations included clinical signs,
mortality, body weight, organ weight and food consumption. Parents
were observed haematologically and histopathologically. Pups were
sexed, weighed and observed for gross abnormalities. Severe
clinical signs like rough coat, hunched back, lethargy, nasal
discharge and alopecia were observed in rats at 200 ppm. At 200 ppm
body weight gain was significantly reduced in males and food
consumption was slightly reduced in male and female rats. White
blood cell count was significantly decreased in high dose females.
A lower pregnancy rate (4/10) was observed in females at 200 ppm,
no viable offspring resulted. At 100 ppm pup survival, pupweight
and litter size (at 50 ppm not significantly) were dose-relatedly
decreased. At histopathology necrosis of the renal papilla or
medulla was observed in males at 100 and 200 ppm. One high-dose
male rat that died after six weeks showed hepatic, thymic and
testicular necrosis (Carlton & Crisp, 1982).
In a two-generation reproduction study groups of Wistar
(Crl:(WI)BR)COBS rats (30/sex/group) were given diets (prepared
every 5-7 days) containing 0, 0', 5, 18.5 or 50 ppm TPTH (purity
97.2%) during growth, mating, gestation and lactation for one
litter per generation. Observations included clinical signs, body
weight, food consumption, mating performance and reproductive
parameters. Organ weights (including spleen and thymus) of parents
and pups were recorded and the parents were examined histologically
(control group 1 and 50 ppm groups only). Pups were sexed, and
examined for gross malformations and the number of stillborn and
live pups were recorded.
Body weight gain and food consumption were occasionally
decreased in F0 parents at 50 ppm. The number of dead F1 pups
increased and mean litter size decreased at 50 ppm. Mean pup weight
was decreased at 50 ppm on lactation days 14 and 21. In F0 parents
the body weight gains and food consumption of both sexes were lower
at 50 ppm and occasionally at 18.5 ppm. Mean litter size decreased
at 18.5 and 50 ppm. Viability of F2 pups at 50 ppm, prior to
culling was slightly decreased. Mean pup weight was decreased at 50
ppm on lactation days 7, 14 and 21. At 18.5 mg/kg a slight decrease
was seen. Pups from one litter at 50 ppm appeared emaciated on
lactation day 21. Several pups in another litter in this group had
wet yellow urogenital staining on lactation day 21. At 50 ppm the
relative weights of brain, testes, ovaries, adrenals, kidneys
spleen and heart were increased at several occasions in F0 and/or
in F1 adults and/or F1 and F2 weanlings (males and females). At
18.5 ppm increases were also observed for kidneys, testes and heart
weights, whereas at 5 ppm only relative heart weight was increased
in male F1 adults. A dose-related decrease was observed in spleen
and thymus weight in F2 male and female weanlings at 50 and 18.5
ppm. In F1 weanlings the same trend was observed. The NOAEL in
this study is 5 ppm, equal to 0.4 mg/kg bw/day (Young, 1986).
Two groups of 13 male rats (29 days old) received a diet
containing 0 or 20 mg TPTA/kg bw for 25 days. Four animals from
each group were sacrificed on day 21 and the remaining animals
received the test diet for another 4 days and a control diet for an
additional 70 days at which time they were sacrificed. Observations
were made for the distribution of the eight phases of
spermatogenesis. In rats sacrificed after 21 days all 8
spermatogenic phases were seen, but there was a general paucity of
mature sperm and the distribution showed some predominance of
immature sperm. Recovery was seen after the 70 days control diet
(Snow & Hays, 1983).
Special studies on embryotoxicity and/or teratogenicity
Rat
Pregnant Sprague-Dawley rats (20/group) received by gavage 0,
1.25, 5, 8.75 or 12.5 mg/kg TPTH (purity unknown) bw/day, day 6
through 15 of gestation. The rats were sacrificed on day 20 of
gestation. Observations were made for body weight, number of
implantations, number of live and dead fetuses, number of
resorptions and number of corpora lutea. The fetuses were weighed
and examined for external, visceral and skeletal abnormalities.
Three animals each in the 8.75 and 12 mg/kg groups aborted. Only
those animals showed abnormal behavioural reaction. One female in
the 12.5 mg/kg group died. Females at 8.75 and 12.5 mg/kg showed a
lower food consumption and lower body weight gain (dose-
relatedly). In the 8.75 and 12.5 mg/kg groups an increase in
resorptions was observed. Fetuses from these groups also exhibited
a lower weight and a decrease in viable fetuses. A non dose-related
increase in hydrocephalus and hydronephrosis was seen in all
treated groups (2%, 5%, 12%, 16% and 6%, respectively) (Ravert &
Parke, 1976).
In a teratogenicity study (Segment II + III) groups of 25
pregnant Sprague-Dawley rats were given oral daily doses of 0,
0.35, 1, 2.8 or 8 mg TPTH (purity 97.1%)/kg b.w. in corn oil from
day 6 or 15 of gestation. All animals were allowed to deliver
naturally and rear the offspring to weaning on lactation day 21. At
lactation day 4, 10 pups/litter were selected and killed on the
28th day. The dams were killed on day 21 of lactation. Dams were
observed for mortality, clinical signs and body weight. Litters
were examined for gross malformations and the number of live and
dead fetuses was recorded. Fetuses were observed for bodyweight and
examined for haematology, clinical chemistry and urinalysis,
macroscopy, kidney weight and histopathology of the kidney. Dams at
8 mg/kg bw showed lethargy, yellow staining in the urogenital area,
and a more extensive hairloss than dams in the other groups. At the
same dose group maternal body weight gain was significantly
decreased (only during treatment) and gestation length was
significantly increased, and pup survival was decreased on
lactation day 0. Only LDH values in blood decreased in pups at 1,
2.8 and 8 mg TPTH/kg bw and ASAT was decreased in females pups at
all doses. Globulin levels were significantly decreased in female
pups at 2.8 and 8.0 mg/kg bw and A/G ratio was increased at the
highest dose. Relative kidney weight in pups was significantly
increased at 2.8 and 8 mg/kg bw. The dose of 1 mg/kg bw can be
regarded as a NOAEL for embryotoxicity (Rodwell, 1985a).
Twenty to 26 pregnant rats per group received by gavage 0,
1.0, 2.8 and 8 mg TPTH (purity unknown)/kg bw/day suspended in corn
oil, day 5 through 19 of gestation. On day 20 all females were
sacrificed. Uterus weight was determined and corpora lutea were
counted. Fetuses and resorption sites were noted. Fetuses were
weighed, sexed and observed for external, internal and skeletal
malformations. In the 2.8 and 8 mg/kg groups a dose-related nasal
and oral discharge were observed. In the highest group two animals
died. Body weight gain was significantly reduced at 8 mg/kg. The
number of dead or resorbed fetuses was higher at 8 mg/kg. Pup
weight was depressed in the 8 mg/kg group. The occurrence of
hydro-ureter was increased at the highest dose (Carlton & Connell,
1981; Carlton, 1985).
In order to clarify the occurrence of hydronephrosis,
hydrocephalus and hydroureter, another rat study was carried out.
Groups of 45 pregnant rats received 0, 0.35, 1, 2.8 and 8 mg TPTH
(purity 97.1%)/kg bw/day day 6 through 15 of gestation. Day 20 of
gestation all rats were sacrificed. Throughout gestation
observations were made for clinical signs, body weights and food
consumption. After sacrifice the dams were observed for number and
location of viable and nonviable fetuses, early and late
resorptions and the number of implantation sites. The corpora lutea
were counted. Fetuses were weighed, sexed and examined for
external, internal and skeletal anomalies. During the treatment
period, hair loss was more extensive in the 8 mg/kg group. A few
animals in this group were emaciated, lethargic and had yellow
staining in the urogenital area. One female in this group had an
abortion. A dose-related decrease in body weight gain and food
consumption was apparent in the 2.8 and 8 mg/kg groups. Number of
nongravid females, number of total litter resorptions and early
resorptions increased at 8 mg/kg. Number of viable fetuses and
fetal weight decreased significantly at 8 mg/kg. The incidence of
absent or delayed ossification in high dosed litters was increased.
The percentage of fetuses with hydrocephaly was 0.4, 0, 0, 0.4 and
1%, respectively, and for fetuses with omphalocele 0.2, 0.2, 0.2 0,
and 0.5%, respectively for the 0, 0.35, 1, 2.8 and 8 mg/kg groups.
The NOAEL for maternal toxicity was 1 mg/kg bw and the NOAEL for
embrytoxicity was 2.8 mg/kg bw. There was no evidence for
TPTH-induced irreversible structural effects (Rodwell, 1985b).
In a special study consisting of three tests the potential of
TPTH to induce alterations in the urogenital tract of exposed rat
fetuses was investigated. In test I pregnant Sprague Dawley rats
received 0, 4 or 8 mg TPTH (purity unknown)/kg bw by gavage during
day 7 through 20 of gestation. In test II (a and b) doses of 0 and
4 mg/kg bw were given. All females from test I and IIa were
sacrificed on day 21 of gestation. The uteri were removed, weighed,
and examined to determine the number of live, dead and resorbed
fetuses. Live fetuses were examined for gross external anomalies.
In test IIb the animals delivered normally and weaned their young
for 28 days. In these young animals renal morphology and function
were examined. At 8 mg/kg bw maternal (death and reduced
extra-uterine weight gain during pregnancy) and fetal (mortality
and decreased body weight) toxicity were observed. There was no
effect on cerebral ventricles. At 4 mg/kg bw only slightly dilated
renal pelvis was seen and at 4 and 8 mg/kg bw (n.d.r.) dilated
ureter was observed. In a replicate test (IIa) these effects were
hardly observed. In test IIb no effects at all where found for the
renal function or morphology. Only the treated pups only showed a
lower pup weight (Kavlock, 1985).
Four groups of 12 pregnant rats (Sprague Dawley) received from
day 6 through 15 of gestation 0, 5, 10 or 15 mg TPTA (purity
97.1%)/kg bw/day. Females were sacrificed on day 21 of gestation.
Dams were observed for number of resorptions, number of
implantations, post-implantation loss, viable and non viable
fetuses. Fetuses were weighed and examined for external, visceral
and skeletal anomalies. Two rats treated with 15 mg/kg died during
the study and 4 had complete resorptions. Average maternal weight
gain decreased at 5 mg/kg and significantly 10 and 15 mg/kg.
Post-implantation loss increased significantly at 15 mg/kg. A
significant reduction of some ossification centers, mainly at the
level of the metacarpus and caudal vertebrae, was seen in all
treated groups. Minor anomalies (hydroureter, vertebrae missing or
bipartite) increased significantly at 15 mg/kg (Giavini et al.,
1980).
Groups of pregnant rats (Wistar) received during day 7 through
16 of gravidity 0, 1.25, 3.2 or 8 mg TPTA/kg bw/day. TPTA (purity
97.0%) was dissolved in sesame oil. The females were sacrificed and
delivered on day 21 of gestation. The dams were observed for
clinical signs, body weight, food consumption, number of
implantations, resorptions and corpora lutea, viable and non viable
fetuses, organ weights (heart, liver, kidneys and spleen) and
macroscopy. Fetuses were weighed, and examined for length, sex,
external, internal and visceral anomalies. Ten dams at 8 mg/kg had
abortions. Dams at 8 mg/kg showed signs of clinical intolerance as
piloerection, bloody nose secretion, local hair loss accompanied by
scab formation. Body weight gain and food consumption were
decreased in this group. At 8 mg/kg early and total intrauterine
deaths increased and number of implantations, total live fetuses,
fetal body weight and crown/rump length decreased. At 8.0 mg/kg an
increase in fetuses with non ossification or only weak ossification
of the sternebrae was observed. At 8 mg/kg also an increase (5%) in
distended uni- or bilateral ureter was observed. The NOAEL in this
study is 3.2 mg TPTA/kg bw (Baeder, 1987a).
Hamsters
Four groups of 20-25 pregnant Syrian hamsters received by
gavage 0, 2.25, 5.08, or 12 mg TPTH (purity not given)/kg bw in
0.3% hydroxypropyl cellulose in saline from day 5 through 14.
Vitamin A palmitate was administered by gavage on day 8. All dams
were sacrificed on gestation day 15. The weight of the gravid
uterus was determined and corpora lutea were counted. Fetuses and
resorption sites were noted. Fetuses were weighed and observed for
external, visceral and skeletal malformations. Four animals died in
the highest-dose group and several animals showed an abnormal
behaviour. In the mid-dose group and in the lowest-dose group 2
animals died. Hamsters from the highest-dose group showed a
decreased mean body weight gain and a decreased food consumption.
Fetuses from this group showed also a decreased pup weight. The
average number of minor anomalies (such as kinky tail, haematomas
and anaemic appearance) of fetuses per litter was significantly
greater among the 12 mg/kg group. A significantly greater number of
skeletal variations (poorly ossified, missing metatarsals or
missing sternebrae) was observed among the 12 mg/kg group. Three
cases of hydrone-phrosis were seen at 5.08 mg/kg bw and 1 case of
hydrocephalus was seen at 12 mg/kg bw (Carlton & Howard, 1982;
Carlton, 1985).
Rabbits
Three groups of 22 pregnant New Zealand White rabbits received
by gavage 0, 0.1, 0.3 or 0.9 mg TPTH/kg bw/day mixed in 1% aqueous
CMC, day 6 through 18 of gestation. Dams were sacrificed day 29 of
gestation. Observations were made for clinical signs, body weight
and food consumption. After sacrifice corpora lutea, early and
late resorptions and number of implantations were counted. All
fetuses were weighed, sexed and examined for external, skeletal and
visceral anomalies and developmental variations. Two rabbits from
the 0.9 mg/kg group aborted. A dose-related decrease in mean body
weight gain and food consumption was observed in the 0.3 and 0.9
mg/kg groups. Mean fetal weight was lower in the 0.9 mg/kg group.
The NOAEL for maternal toxicity was 0.1 mg/kg bw and the NOAEL for
embryotoxicity was 0.3 mg/kg bw (Rodwell, 1987).
TPTA (purity 97.0%) was dissolved in sesame oil and
administered orally by gavage to groups of 15 pregnant rabbits
during day 6 through 18 of gestation. The dose levels were 0, 0.1,
0.32 or 1.0 mg/kg bw/day. On day 29 of gestation the dams were
sacrificed. Dams were observed for clinical signs, body weights,
food consumption, number of resorptions, number of implantations,
number of corpora lutea, viable and non viable tissues, organ
weights (heart, liver kidney, spleen) and macroscopy. Fetuses were
weighed and examined for sex, length, external, internal and
skeletal anomalies. In the 1.0 mg/kg group 1 dam died, 3 dams
aborted, 1 dam was sacrificed due to a premature delivery and 2
dams had only intra-uterine deaths. Three dams showed vaginal
haemorrhages. The water consumption and food consumption of dams at
1 mg/kg decreased. The number of implantations and of live fetuses
decreased at 1 mg/kg. Mean fetal weight, mean crown/rump length
and mean placental weight decreased in pups at 1 mg/kg. At 1 mg/kg
4 pups (15.4%) showed omphalocele with protrusion of intestinal
coils or liver tissue. Slight retardation of skeletal ossification
was detected in the 1.0 mg/kg group. There was an increase in the
number of fetuses with fewer than 13 ossified caudal vertebrae and
some fetuses showed weak ossification of the hyoid bone or non
ossification or only slight ossification of the os pubis. The
NOAEL for maternal and embryotoxicity in this study is 0.32 mg/kg
bw/day (Baeder, 1987b).
Special studies on immunotoxicity
Seinen and Penninks (1987) reviewed the available data on
immunotoxic properties of TPTH from the published literature and
confidential studies provided by the manufacturer. They concluded
that subacute treatment with TPTH resulted in lymphopenia and
lymphocyte depletion of spleen and thymus. After treatment up to 13
weeks these effects appeared to be transient. Rats fed 25 ppm TPTH
(not 5 ppm) showed cell mediated immunity as measured by slightly
suppressed DTH (delayed type hypersensitivity) to ovalbumin and
tuberculin. The humoral immunity was not affected at doses up to 25
ppm. However in mice dosed with TPTH up to 20 mg/kg bw for 14 days
DTH response to keyhole limpet haemocyanin was not affected,
whereas the humural activity was suppressed at 20 mg/kg bw. In
various other immune function tests no disturbances were observed.
The authors concluded therefore that TPT-compounds display weak
immunosuppressive potential. This immunosuppressive activity is
observed at dose levels that markedly reduce the numbers of blood
lymphocytes and the weight of the lymphoid organs (Seinen &
Penninks, 1987). The original data for certain studies in this
review were not available to the JMPR.
Mice
In a limited experiment young and mature mice were fed a diet
containing TPTA (purity >99%) at a rate of 30, 100 or 300 ppm for
4 days. Feeding resulted in a dose-related decrease in spleen,
heart and liver weight and number of blood leucocytes (Ishaaya
et al., 1976; Seinen & Penninks, 1987).
Groups of B6C3F1/CrlBR mice (18/sex/group) were administered
0, 1, 5, 25, 50 or 125 ppm TPTH (purity 96.4%) for 28 days. A
positive control group (18/sex) was fed control diet and received
a single i.p. injection of 180 mg cyclophosphamide/kg bw 48 hours
prior to the end of the treatment period. Twelve male and 12
female mice were killed at day 29 and the remaining mice were
returned to control diets and were killed on day 57. Observations
were made on survival and clinical signs, body weight, food
consumption, haematology, liver, spleen, and thymus weight,
macroscopy and histopathology. Special immunotoxicological
parameters as splenocyte counts, thymocyte counts, number of
splenic T- and B-cells and basal serum immunoglobulin levels were
recorded and the bone marrow was examined. A significantly
decreased body weight gain was observed in male and female mice at
125 ppm, from which they recovered after 28 days. Food consumption
was significantly decreased at 50 and 250 ppm. Relative liver
weight was increased at 25 (females only), 50 and 125 ppm, relative
spleen weight was clearly decreased in males at 50 and 125 ppm and
in females at 25 ppm and higher and relative thymus weight was
decreased at 125 ppm in males. At histopathology lymphoid depletion
in the thymus and spleen was observed in mice at 125 ppm. No
dose-related effects were observed on any of the bone marrow
parameters (note: only 50% of the smears could be evaluated,
because of technical problems). The main haematological effects
were a decrease in total white blood cells, neutrophils and
lymphocytes at 50 and 125 ppm in males and females. Dose-related
changes in haematological parameters were observed in males at 25,
50 and 125 ppm and in females in all dose levels (MCH and MCHC). At
the highest dose a decrease in total cells/spleen and splenic
B-cells were observed and a decrease in total cells/thymus and
splenic T-cells were seen in males. IgM levels were decreased in
females at 25 ppm and higher, but were not clearly dose-related.
All effects were reversible. Mice fed cyclophosphamide showed
decreased spleen and thymus weight accompanied by histologic
evidence of lymphoid depletion. Cyclophosphamide also altered the
% T- and % B-cells in both sexes and reduced the spleen and thymus
cellularity. The effects were not reversible. The NOAEL for
immunotoxicity is 5 ppm, equal to 1 mg/kg bw for males and 1.15 for
females (McCormick & Thomas, 1990a).
Rats
A similar study was conducted in 18 male and 18 female Wistar
rats. A positive control group was fed a diet containing 125 mg
DOTDC (dioctyltin-dichloride)/kg. No effects were observed on
mortality, clinical signs, body weight, organ weight, macroscopy.
Food consumption was decreased in female rats at 125 ppm. No
dose-related effects were seen at histopathology (note only 50% of
the bone marrow smears could be used in the histopathological
evaluation). In both males and females red blood cell parameters
were affected at the highest groups. The white blood cells and
lymphocytes were significantly decreased in males at 125 ppm and
females showed a tendency to a decrease at 50 and 125 ppm. In male
rats at 50 and 25 ppm platelet count was increased. No effect was
observed on the viability of the thymus or spleen cells nor on the
percentages of splenic T- and B-cells in either sex. IgG levels
were significantly reduced in females at 50 and 125 ppm. All
effects were reversible. The effects in the positive control group
were as expected. These effects were largely reversible following
cessation of DOTDC exposure. The NOAEL for immunotoxicity is 25 ppm
equal to 1.82 mg/kg bw for females and 1.71 mg/kg bw for males
(McCormick & Thomas 1990b).
In order to investigate the reaction of rats to infection with
Trichinella spiralis female rats were administered 2.5 mg TPTH
(purity 97.2%)/kg bw over a 10 day period. This administration had
no effect on host resistance in contrast to the positive controls
dexamethasone and cyclophosphamide. There are also no effects on
additional parameters, i.e., thymus weights, percentage of
lymphocytes in the differential blood count, and body weight gains
(Diehl & Leist, 1987b).
Special studies on genotoxicity
TPTH as well as TPTA were tested in various genotoxicity
assays. See Tables 3 and 4 respectively, for a summary of the
studies considered. In most of the assays negative results were
obtained. The positive results in the chromosome aberration assays
are probably related to the toxic effect of the compound on
T-lymphocytes and not to a genotoxic action of TPTH.
Special studies on sensitization
At concentrations which were irritant to the skin, TPTH
(purity 97.0%) showed no skin sensitization in guinea-pigs in the
Buehler test (Leist & Weigand, 1981f; Schollmeier & Leist, 1989)
nor in the maximization test (Diehl & Leist, 1987a).
TPTA gave a positive response when tested for skin
sensitization in guinea-pigs in the Buehler test (Diehl & Leist,
1986e).
Table 3. Results of genotoxicity assays on TPTH
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Ames test a,b S. typhimurium 0.31-5 µg/pl 97.0 Negative Richold et al. (1981)
TA 1535, TA100, in DMSO
TA1537, TA98,
TA1538
E. coli WP2 uvra 313-5000 µg/pl 97.0 Negative
in DMSO
Yeast forward S. pombe p1 0.05-1 µg/ml 97.2 Negative Milone & Hirsch (1985b)
mutation test in DMSOa
Mitotic gene Saccharomyces 0.1, 1, 3 or 97.2 Negativec Milone & Hirsch (1985a)
conversion assay cerevisiae D4 5 µg/mla
1, 5, 10 or
15 µg/mlb
Mouse lymphoma L5178Y mouse 10-150 ng/mla 97.2 Negative DenBoer & Hoorn (1985)
forward mutation lymphoma cells (>80 ng/ml toxic) Weakly
assay (TK +/-) 40-600 ng/mlb positive
(>300 ng/ml toxic)
Mouse lymphoma L5178Y mouse 0.01-2 µg/1a ? Positive NCI (1984)
forward mutation lymphoma cells (>0.1 toxic) Negative
assay (TK +/-) 1.7-8 µg/1b
Chromosome Human lymphocytes 31.25-1000 ng/mla Positive Kirkland (1985)
aberration assay 250-2000 ng/mlb
both in DMSO
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Chromosome Human lymphocytes 0.681-1.47 µg/mla 96.2 Negative Nunziata & Conzonni (1988)
aberration assay in DMSO Positive
0.316-1.47 µg/mlb
in DMSO
Unscheduled Rat hepatocytes 0.01-2.0 µg/ml in DMSO 97.2 Negativec Cifone & Myhr (1985)
DNA synthesis >0.25 µg/ml toxic
Survival (cell Rat embryo cells 0.019 µg/5.2 x 104 cells ? Positived Traul et al. (1981)
transformation) infected with or 0.0145 µg/5.2 x 104
assay leukaemia virus
(2FR450 cells)
Micronucleus test NMRI mouse bone 35, 70 or 140 mg/kg bw po 97.2 Negative Banduhn et al. (1985)
NMRI marrow cells
Cytogenic assay Chinese hamster bone 20, 50 or 80 mg/kg bw po 96.2 Negative Mueller (1987)
marrow cells
Table 3 (contd).
Test system Test object Concentration of Purity Results Reference
TPTH (%)
Dominant lethal Sprague-Dawley rats daily ip injections 94.8 Negative Ravert et al. (1978)
assay with 3, 20, 38
or 150 mg/kg bw in
corn oil; 150 mg/kg
bw toxic (8/10
died). Concentration
of TPTA
a without metabolic activation
b with metabolic activation
c positive control yielded positive results
d only summary available
Table 4. Results of genotoxicity assays on TPTA
Test system Test object Concentration of Purity Results Reference
TPTA (%)
Ames testa,b S. typhimurium 0.032-1000 µg/plate 97.1 Negative Jung & Weigand (1986)
TA100, TA1535, in DMSO
TA1537, TA1538,
TA98
E. coli WP2 uvra
Gene conversiona,b S. cerevisiae D4 1-50 µg/ml in DMSO 97.1 Negative Milone & Hirsch (1986a)
Dominant Sprague-Dawley rats daily i.p. injections 94.8 Negative Ravert et al. (1978)
lethal assay with 3, 20, 38 or
150 mg/kg bw in corn oil;
150 mg/kg bw toxic (8/10
died)). Concentration of
TPTA
a without metabolic activation
b with metabolic activation
Special studies on skin and eye irritation
A dose of 500 mg TPTH (purity not stated) diluted in a 0.9%
NaCl solution was slightly irritant when applied under occlusive
conditions to the shaven intact and shaven abraded skin of 6 New
Zealand rabbits for 24 hours (Leist & Weigand, 1981e).
Dilutions of 1:10, 1:100 and 1:1000 prepared from 2.5 g TPTA
(purity not stated) in 0.9% NaCl were examined in the Barail
Intracutaneous test by intracutaneous injections of 0.02 ml
administered to the skin of rabbits. No irritation was seen. Five
applications of 0.5 ml of the TPTA solution to depilated and intact
rabbits skin caused slight local irritation. (Summary only)
(Hollander & Weigand, 1974e).
A dose of 500 mg TPTA (purity 97%) moistened with
physiological saline was applied under semi-occlusive conditions to
the shaven intact skin of 6 New Zealand rabbits for 54 hours. No
irritation was observed up to 72 hours after application (Diehl &
Leist, 1986c).
Application of 100 mg TPTH (purity not stated) diluted in a
0.9% NaCl-solution into the eyes of 9 New Zealand rabbits caused
severe irritation; increasing adverse effects resulted in a
discontinuation of the experiment after 72 hours (Leist & Weigand,
1981e).
Rabbits received an instillation of 0.1 ml of a solution of
2.5 g TPTA (purity not stated) in 0.9% NaCl into one eye. After 24
hours slight local irritation was observed (Summary only)
(Hollander & Weigand, 1974e).
Nine New Zealand rabbits received an instillation of 100 mg
TPTA (purity 97%) into one eye. All rabbits developed severe ocular
lesions (swollen conjunctivae, markedly injected blood vessels,
diffusely crimson red colouring, nacreous opacity and whitish and
reddish brown discharge). After 72 hours all animals were
sacrificed because the lesions were non-reversible (Diehl & Leist,
1986d).
Observations in humans
Two cases of poisoning with Brestan 60 (60% TPTA and 15%
Maneb) have been reported. In both cases (farmers of 75 and 53
years) inhalation of the powder took probably place during
preparation of the spray solution. Symptoms observed were nausea,
dizziness, transient loss of consciousness, convulsions, persistent
headache, and photophobia, as well as impaired liver functions.
Both patients were discharged in good health after 10 and 15 days
(Manzo & Richelmi, 1981).
Acute severe intoxication was reported in a 23-year old male
in an attempted suicide with a TPT-compound (molluscicidal agent,
no specifications given). The patient was suffering from abdominal
pains, diarrhoea and vomiting and also showed reversible acute or
delayed neurological symptoms. The patient's health was completely
recovered within 3 1/2 months (Wu et al., 1990).
COMMENTS
Most toxicological studies on fentin were carried out with
triphenyltin hydroxide (TPTH). Since triphenyltin acetate (TPTA) is
hydrolysed rapidly to TPTH in an aqueous medium, studies with both
compounds have been used for the evaluation of fentin.
After oral administration of 14C-labelled TPTH the
radioactivity was mainly eliminated via the faeces (50-70%), but
also via the urine (20-25%). Biliary excretion was also
demonstrated. After 7 days residual radioactivity was present with
the highest concentration in the liver and the next highest in the
kidneys. After oral administration of 113Sn-labelled TPTH,
elimination was almost exclusively via faeces. In both cases faecal
elimination was biphasic with half-lives of 9 and 50-60 hours. With
113Sn-labelled TPTH the highest concentration was found in the
kidneys. After administration of the 14C-labelled compound,
radioactivity in the faeces of the rat consisted of parent compound
and di- and monophenyltin as well as non-extractable bound residues
and tin (measured as Sn). It was reported that benzene was found in
the faeces. Sulfate conjugates of phenol, hydroquinone, catechol
and resorcinol as well as phenylmercapturic acid were present in
the urine. Phenyltin compounds were not found in the urine.
TPTH is toxic to rats after acute oral administration with
LD50 values of about 160 mg/kg bw. TPTA showed similar acute
toxicity.
In a 3-month study in mice at dietary concentrations of TPTH
of 0, 4, 20 or 100 ppm, a number of effects were observed at 100
ppm. The main effects were a decrease in haemoglobin concentration
and erythrocyte count and an increase in Heinz bodies.
Immunoglobulins were decreased and liver weight was increased. The
NOAEL was 20 ppm, equal to 3.4 and 4.1 mg/kg bw/day for males and
females, respectively.
Dietary concentrations of TPTH of 0, 4, 20 or 100 ppm were
tested in a 13-week study in rats. The NOAEL was 4 ppm, equal to
0.30 and 0.35 mg/kg bw/day for males and females respectively,
based on a decrease in white blood cells and plasma albumin and an
increase in plasma aspartate aminotransferase at 20 ppm.
In a 52-week study in dogs (dietary concentrations of TPTH of
0, 2, 6 or 18 ppm) the NOAEL was 6 ppm, equal to 0.2 mg/kg bw/day,
based on a decreased albumin level and an increase in relative
liver weight at 18 ppm.
Two long-term/carcinogenicity studies in mice and one in rats
were performed in which there was no evidence of carcinogenicity.
However, there were indications in each of these studies that the
doses received by the animals may have been lower than intended
owing to instability of the test compound in the diet.
In a third long-term/carcinogenicity study in mice at dietary
concentrations of TPTH of 0, 5, 20 or 80 ppm, increased liver and
decreased kidney weights, increased nodular hyperplasia and
hepatocellular adenoma and carcinoma occurred at 80 ppm only. The
NOAEL was 5 ppm, equal to 0.85 mg/kg bw/day for males and 1.36
mg/kg bw/day for females, based on decreased body-weight gain at 20
ppm.
In a second long-term/carcinogenicity study in rats (with
dietary concentrations of TPTH of 0, 5, 20 or 80 ppm), an increase
in mortality was observed at all dose levels. A NOAEL could not
be established at the lowest concentration of 5 ppm, equal to 0.3
and 0.4 mg/kg bw/day for males and females respectively. The
incidence of pituitary adenomas was increased in females at 20 and
80 ppm. At 80 ppm, the incidence of Leydig cell tumours was
increased. These changes were accompanied by non-neoplastic
lesions in the pituitary and the testes.
In a 2-generation reproduction study in rats with one litter
per generation (dietary concentrations of TPTH of 0, 5, 18.5 or 50
ppm) fertility was not affected. The NOAEL was 5 ppm (equal to 0.4
mg/kg bw/day) based on decreased litter size, decreased pup weight
and decreased relative spleen and thymus weight in the weanlings.
In several teratogenicity studies with rats, hamsters, and
rabbits, TPTA or TPTH caused maternal and embryo toxicity, but
irreversible structural effects were not observed. In rabbits, the
most sensitive species, the NOAEL for embryo/fetotoxicity was 0.3
mg/kg bw/day in a study that utilized doses of 0, 0.1, 0.3 or 0.9
mg/kg bw/day TPTA. In this study the NOAEL for maternal toxicity
was 0.1 mg/kg bw/day.
Most in vitro and in vivo genotoxicity tests were
negative. However, two human lymphocyte chromosomal aberration
assays and two mouse lymphoma mutation assays were positive. The
latter responses may have been caused by a variety of effects,
including chromosomal aberrations. Since two in vivo studies for
chromosomal aberrations (a micronucleus test in mice and a
cytogenetic test in Chinese hamsters) were negative, it would
appear that any genotoxic properties are of low potency. It was
therefore concluded that fentin does not present a genotoxic hazard
for man.
Effects on the immune system were observed in short- as well
as in long-term toxicity studies. In special studies with mice and
rats, TPTH showed immunosuppressive properties (lymphopenia and
lymphocyte depletion of spleen and thymus), resulting in altered
humoral and cellular immunity. The NOAEL in mice was 5 ppm, equal
to 1 mg/kg bw/day, based on a decrease in splenic weight found at
25 ppm. In rats, the NOAEL was 25 ppm, equal to 1.7 and 1.8 mg/kg
bw/day for males and females respectively, based upon a significant
decrease in immunoglobulin G and a marginal decrease in leucocyte
and lymphocyte counts at 50 ppm.
The Meeting noted that there was increased mortality at the
lowest dose tested in the most recent long-term study in rats (0.3
mg/kg bw/day). Applying a 500-fold safety factor to this LOAEL
would result in approximately the same ADI as the previously
established ADI based upon a NOAEL of 0.1 mg/kg bw/day in an
earlier long-term study in rats. The previous ADI was therefore
retained. This ADI is supported by NOAELs derived from recent
studies, including the NOAEL of 0.4 mg/kg bw/day in the
2-generation reproduction study, the NOAELs in short-term studies
in rats (0.3 mg/kg bw/day) and dogs (0.2 mg/kg bw/day) and in a
teratology study in rabbits (0.1 mg/kg bw/day for maternal
toxicity).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm in the diet, equal to 1 mg/kg bw/day
Rat: <5 ppm in the diet, equal to <0.3 mg/kg bw/day
(long-term/carcinogenicity study)
5 ppm in the diet, equal to 0.4 mg/kg bw/day
(multigeneration reproduction study)
Dog: 6 ppm, equal to 0.2 mg/kg bw/day
Rabbit: 0.1 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Ongoing studies on the endocrinological effects of fentin
2. Observations in humans.
REFERENCES
Baeder, Ch. (1987a) Fentinacetate - substance, technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Wistar rats
after oral administration. Unpublished Report 89.1478 (A42190) of
Pharma Research Toxicology and Pathology, Hoechst AG. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Baeder, Ch. (1987b) Fentin acetate - substance technical (Code: Hoe
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished Report 89.1559
(A42324) of Pharma Research Toxicology and Pathology, Hoechst AG.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Bakke, J.E., Feil, V.J., Aschbacher, P.W., & Price, C.E. (1982)
Metabolism of 14C-triphenyltinhydroxide by the rat, sheep and cow.
Unpublished Report (A35976) of metabolism and radiation research
laboratory Agricultural Research Service, Fargo, ND, USA.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Banduhn, N., Sachsse, K. & Terrier, C. (1985) Mouse micronucleus
test with TPTH-technical (HOE 029664 OF ZD97 0004). Unpublished
Report (A31583), project 049522 from RCC, Switzerland. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Brown, W.R. (1985) 18-Month carcinogenicity study of Technical TPTH
96.3% (Lot no. SWRAM-1K) in CD Mice. Histomorphology of the Uterus.
Unpublished Report (A32214) from Research Pathology Services. Inc.,
New Britain, CT, USA Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Brunk, Dr. & Weigand, W. (1981) Acute oral toxicity of
Fentin-Hydroxide, active ingredient (Code: HOE 29664 O F AT201) to
the male and female beagle dog. Unpublished Report 462/81 from
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Beurkle, W.L. (1985). Hoe 002782-14-C (triphenyltin acetate),
analysis of hydrolysis byproducts. Unpublished Report CM009/85
(A31534) from Analytisches Laboratorium, Hoechst. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L., Rutz, U., & Müller, A., Eckert, H.G., Kellner, H.M.
& Esinger, W. (1986) Metabolism in rats after single and repeated
oral administration at the two dosage levels 2 and 10 mg/kg body
weight. Unpublished Report CM 011/85 (A34163) of Radiochemisches
Laboratorium and Analytisches Laboratorium, Hoechst AG. Submitted
to WHO by Hoechst AG., Frankfurt-am-Main.
Beurkle, W.L. & Kellner, H.M. (1987) HOE 029664 (TPTH)-14C.
Excretion study in rats with bile fistula following oral
administration of 2 mg a.i./kg body weight. Unpublished Report
(B)97/87 (A36680) of Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. (1985) Triphenyltin hydroxide. Additional data on
Batelle rat and hamster teratology studies (31184). Unpublished
data of Batelle Columbus Laboratories, Columbus, Ohio. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Connell, M.M. (1981) Final report on evaluation of
the teratogenicity of triphenyltin hydroxide (TPTH) in the
Sprague-Dawley rat. Unpublished Report NO723-0200 (A22211) of
Batelle Columbus Laboratories, Columbus, Ohio. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Carlton, B.D. & Crisp, C.E. (1982) Range-finding study for the
evaluation of reproductive effects of triphenyltin Hydroxide (TPTH)
in the Wistar rat. Unpublished Report (A25091) project NO723-0400)
from Battelle, Columbus, Ohio. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Carlton, B.D. & Howard M. (1982) The evaluation of the
teratogenicity of triphenyltin hydroxide (TPTH) in the Syrian
hamster. Unpublished Report
NO723-0100 (A22884) of Battelle, Columbus Laboratories, Columbus,
Ohio. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Fentinhydroxide,
substance technical grade (Code: HOE 029664 OF ZD97 0004) in the
rat primary hepatocyte unscheduled DNA synthesis assay. Final
Report (A32084) GT85.1091 from Litton Bionetics, Inc. Maryland.
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Craine, E.M. (1987) An extended duration dermal absorption study in
rats with 14C-triphenyltin hydroxide. Unpublished Report WIL-39037
(A35945) of WIL Research Laboratories. A subsidiary of Great Lakes
Chemical Corporation, Ashland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
DenBoer, W.C. & Hoorn, A.J.W. (1985) Mutagenicity evaluation of HOE
0 29664 -Substance technical (Code: HOE 029664 OF ZD97 0004) in the
L5178Y TK +/- Mouse Lyphoma forward mutation assay. Unpublished
Final Report (A31582) from Litton Bionetics, The Netherlands.
Submitted to WHO by Hoechst AG. Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986a) Fentin acetate - active
ingredient technical. Testing for acute intraperitoneal toxicity in
the male and female rat. Unpublished Report 86.1047 (A37976) of
Hoechst Pharma Research Toxicology and Pharmacology, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986b) Fentin acetate - active
ingredient technical. Testing for acute dermal toxicity in the male
and female Wistar rat. Unpublished Report 86.0816 (A37384) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986c) Fentin acetate - active
ingredient technical. Testing for primary dermal irritation in the
rabbit. Unpublished Report 86.0619 (A37383) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986d) Fentin acetate - active
ingredient technical. Testing for primary eye irritation in the
rabbit. Unpublished Report 86.0620 (A37382) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1986e) Testing for sensitizing
properties in the Pirbright-White guinea pig according to the
technique of Buehler. Unpublished Report 86.0803 (A36975) of
Hoechst Pharma Research Toxicology and Pathology. Submitted by WHO
by Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987a) Fentin-hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing for
sensitizing properties in the Pirbright-White guinea pig in a
maximization test. Unpublished Report 87.0201 (A35628) from Hoechst
Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Diehl, K.H. & Leist, K.H. (1987b) Fentin hydroxide - active
ingredient technical (Code: Hoe 029664 OF ZD97 0004). Testing of
host resistance in the female Wistar rat (Immunotoxicological
screening with Trichinella spiralis) -2nd study-. Unpublished
Report 87.0955 (A36065) of Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Dorn, E. & Werner, H.J. (1989a) Triphenyltinhydroxide (Hoe 029964
OF ZD97 0004). Determination of residues in organs, tissues and
blood of dogs after chronic feeding (52 weeks). RCC Project 047024
and RCC Project 047013. Unpublished Report CR076/88 (A41963) of
Analytisches Laboratorium Hoechst AG. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Dorn, E & Werner, H.J. (1989b) Triphenyltinhydroxide (Hoe 029664 OF
ZD 97 0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished Report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG.,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Duchosal, F., Thevenaz, Ph., Luetkemeier, H., Vogel, O., Pappritz,
G., Mladenovic, P. & Terrier, Ch. (1989) Fentinhydroxid (TPTH)
technical grade. Subchronic (90-day) repeated dose inhalation
toxicity study in rats. Unpublished Report 202353 (A40323) of RCC,
Research and Consulting Company AG., Carouge-Geneva, Switzerland.
Eckert, H.G., Kellner, H.M., Beurkle, W.L., (1989) Accumulation
study; kinetics and metabolism in the rat after single and repeated
oral administration, of 2 mg/kg body weight. Unpublished Report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L,
Oekologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Giavini, E., Prati, M. & Vismara, C. (1980) Effects of triphenyltin
acetate on pregnancy in the rats. Bull. Environm. Contam. Toxicol.
24: 936-939 (A19995). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1974a) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished Report 16454
(A16454) from Hoechst Pharma Forschung Toxicologie. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Hollander H. & Weigand W. (1974b) Triphenyltin acetate. Acute oral
toxicity (LD50) in guinea pigs. Unpublished Report (A15562) of
Hoechst Pharma Forschung Toxicologie. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974c) Triphenyltin acetate.
Subchronic (30-day) oral toxicity testing in rats and guinea pigs.
Unpublished Report (A37587) of Hoechst Pharma Research Toxicology.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1974d) Triphenyltin acetate dermal
toxicity in rats and rabbits. Unpublished Data (A15561) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1974e) Triphenyltin acetate skin and
eye irritation in rabbits. Unpublished Report (15564) of Hoechst
Pharma Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Hollander H. & Weigand W. (1980) Acute oral toxicity of fentin
acetate technical (Hoe 02782) in male rabbits. Unpublished Report
(A28616) of Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand, W. (1981) Dust inhalation of HOE 29664 -
active ingredient in the male and female SPF-Wistar rat. 4 h -LC50.
Unpublished Report 335/81 (A22293) from Hoechst Pharma Forschung
Toxicologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Hollander, H. & Weigand W. (1986) Fentin acetate - active
ingredient technical. Testing for acute dust inhalation toxicity in
the male and female SPF rat 4-hour LC50. Unpublished Report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ishaaya, I., Engel, J.L. & Casida, J.E. (1976) Dietary
triorganotins affect lymphatic tissues and blood composition of
mice. Pestic. Biochem. Physiol. 6: 270-279. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main. (A31628)
Jung, R. & Miltenburger, H.G. (1986) Fentin acetate - substance
technical (Code: Hoe 002782 OF ZD97 0002). Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with
V79 cells. Unpublished Report LMP 229 (A35212) of Hoechst
Laboratory for mutagenicity testing. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Jung, R. & Weigand, W. (1986) Fentin acetate - substance technical,
Code: Hoe 002782 OF ZD97 0002. Study of the mutagenic potential in
strains of Salmonella typhimurium (Ames test) and Escherichia
coli. Unpublished Report 86.0042 (A33021) of Hoechst Pharma
Research Toxicology. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kavlock, R.J. (1985) Triphenyltin hydroxide developmental
toxicology study. Unpublished Report of United States Environmental
Protection Agency (A31424). Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1986) Kinetics in the rats following
single and repeated administration of 2 mg/kg and single
administration of 10 mg/kg body weight. Unpublished Report
01-L42-0489-86 (A34186) of Hoechst Radiochemisches Laboratorium.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M. & Eckert, H.G. (1989) Addendum to report
01-L42-0565-89. Hoe 029664 (TPTH)-113Sn. Accumulation and depletion
study in rats after single and repeated oral administration of 2
mg/kg body weight. Unpublished Report 01-L42-582-90 (A43434) of
Pharma Forschung GB-L Radiochemisches Laboratorium Hoechst,
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Kellner, H.M., Eckert, H.G. & Bürkle, W.L. (1989) Hoe 029664
(TPTH)-113Sn. Absorption studies in rats with bile fistula after
a single oral dose of approx. 2 mg/kg body weight. Unpublished
Report 01-L42-0564-89 (A41409) of Hoechst Pharma Foreschung
Radiochemisches Laboratorium Productent-wicklung GB-C, Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of HOE 029664 - substance technical by its effects on
cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished Report A31614 from Microtest Research Limited, UK.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Lachmann, G. & Siegemund, B. (1987) Exhalation experiment with
triphenyltinhydroxide (Hoe 029664) in rats. Unpublished Report
(A34601) of Battelle-Institute. V., Frankfurt-am-Main. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Laveglia, J., Hagen, C.A. & Nemec, M.D. (1985) 21-Day dermal
toxicity study in rats with triphenyltin hydroxide (Code: Hoe
029664 OF ZD97 0001 technical substance). Unpublished Final Report
(A31616) project no WIL-39018 from American Hoechst Company, New
Jersey. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. (1988) Fentin acetate-substance technical (Code HOE
002782 OF ZD97 0002). Repeated dose dermal toxicity study (21
applications in 29 days) with a 14 day withdrawal period.
Unpublished Report (A39231) 3104 TSR of Pharma Forschung
Toxicologie und Pathologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981a) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 29664 O F AT201) to the male rat.
Unpublished Report (A22115) no 182/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981b) Acute oral toxicity of HOE 29664
- Active ingredient (Code: HOE 19664 O F AT201) to the female rat.
Unpublished Report (A22114) 183/81 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981c) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 O F AT201) to the
female rat. Unpublished Report (A22292) 184/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981d) Acute percutaneous toxicity of
HOE 29664 -active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished Report (A22112) 229/81 from Hoechst Pharma
Forschung Toxicologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981e) HOE 29664 - active ingredient
(Code: HOE 29664 O F AT201). Irritance to the rabbit skin and eye
mucosa. Unpublished Report (A22113) 104/81 from Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Leist, K.H. & Weigand, W. (1981f) Test for sensitizing properties
of Fentin hydroxide-technical (Code : HOE 29664 O F AT201) in
guinea-pig (according to Buehler). Unpublished Report (A22433)
724/81 from Hoechst Pharma Forschung Toxicologie. Submitted to WHO
by Hoechst AG., Frankfurt-am-Main.
Leist, K.H., Weigand, W. & Kramer, Dr. (1982) Range-finding test
von Hoe 29664-Wirkstoff (Fentinhydroxid) (Code: Hoe 29664 O F
AT201) im 30-Tage-Fuetterungsversuch and SPF-Wistar-Ratten.
Unpublished Report (A24003) 426/82 from Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Loquet, C.(1985) Fentin acetate (Code: Hoe 002782 OF ZD97 0001)
Micronucleus test in mice. Unpublished Report 1592 MAS (A32196) of
Centre International de Toxicologie at Misery. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990a) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH) substance
technical (Code HOE 029664 OFZD97 0004) in mice. Unpublished Report
L08252SN01 (A44053) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
MacCormick, D.L. & Thomas, P.T. (1990b) 28-Day oral diet
immunotoxicity study of triphenyltin hydroxide (TPTH), substance
technical (Code HOE 029664 OFZD97 0004) in rats. Unpublished Report
L08252SN02 (A44054) of Life Science Research. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Manzo, L. & Richelmi, P. (1981) Poisoning by triphenyltin acetate.
Report of two cases and determination of tin in blood and urine by
neutron activation analysis. Clinical Toxicology, 18(11)
1343-1353. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
(A44030)
Milone, M.F. & Hirsch, E. (1985a). Study of the capacity of the
test article HOE 029664 - Substance technical grade - (Code No. HOE
029664 OF ZD97 0004) to induce gene conversion in saccharomyces
cerevisiae. Unpublished Report (A32085) experiment no.M 890 from
RBM, Ivrea, Italy. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1985b) Study of the capacity of the test
article HOE 029664 - Substance technical grade (Code HOE 029664 OF
ZD97 0004) to induce gene mutation in Schizosaccharomyces pombe.
Unpublished Report (A31613) from RBM Italy. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986a) Study of the capacity of the
article fentin-acetate-substance technical grade (Code No. Hoe
002782 OF ZD97 0002) to induce gene conversion in Saccharomyces
cerevisiae. Unpublished Report M1023/1-2 (A34018) of Instituto di
Ricerche Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst
AG., Frankfurt-am-Main.
Milone, M.F. & Hirsch, E. (1986b) Fentin acetate substance
technical grade. Forward Mutation test in S. pombe P1.
Unpublished Report M898 (A32812) of Instituto di Ricerche
Biomedische 'Antoine Marxer'. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Mueller, W. (1987) Evaluation of HOE 029664 OF ZD97 0004 in the
in vivo cytogenetic test in bone marrow cells of the Chinese
hamster - chromosome analysis. Unpublished Report 87.0031 (A36080)
from Hoechst Pharma research Toxicology and Pathology. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Nagamatsu, K., Kido, Y., Urakubo, G., Aida, Y., Ikeda, Y. & Suzuki,
Y. (1978) Absorption, distribution and excretion of triphenyltin
acetate and stannic chloride in the guinea pig. Japanese Journal
of Hygiene, 33: 486-496.
NCI (1984) Mouse lymphoma with triphenyltin hydroxide. Unpublished
Report (A30130), National Cancer Institute. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS no. 76-87-9 (A199994). Technical report series
No.139. US Department of Health, Education, and Welfare.
Nunziata, A. & Consonni, R.F. (1988) Chromosome aberrations in
human lymphocytes cultured in vitro. Test substance: HOE 029664
Fentin hydroxide substance technical (Code: Hoe 029664 OF ZD97
0004). Unpublished Final Report 157024-M-04487 (A38456) from Life
Science Research Roma Toxicology Centre S.P.A. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Ravert, J. & Parke, G.S.E. (1976) Investigation of teratogenic and
toxic potential of technical triphenyltinhydroxide (lot #SWRAM-1K).
Unpublished Report GT85.0797 (31618) of Cannon Laboratories, Inc.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Ravert, J., Parke, G.S. & Charles, S.J. (1978) The dominant lethal
assay of technical TPTH (Triphenyltin hydroxide) in Sprague-Dawley
rats. Unpublished Report (A24394) from Cannon Laboratories Inc.,
Reading. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Resnis, P., Craine, E.M. & Nemec, M.D. (1986) A dermal absorption
study in rats with 14C-triphenyltin hydroxide. Unpublished Report
(WIL-39020) (32810) of WIL Research Laboratories. A subsidiary of
Great Lakes Chemical Corporation, Ashland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Richold, M., Jones, E. & Fleming, P.M. (1981) Ames metabolic
activation test to assess the potential mutagenic effect of HOE
29664 OF AT201. Unpublished Report 450/81A (A21735) from Huntingdon
Research Centre, GBR. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1985a) One generation teratology and reproductive
study in rats with triphenyltin hydroxide (Code: HOE 029664 OF ZD97
0001 Technical substance). Unpublished Final Report project
WIL-39013 (A31617) from Wil Research Laboratories Inc., Ashland
USA. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Rodwell, D.E. (1985b) A teratology study in rats with triphenyltin
hydroxide (Code: HOE 029664 OF ZD97 0001 Technical Substance).
Unpublished Report WIL-39011 (A31079) of WIL Research Laboratories
Inc, Ashland USA. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Rodwell, D.E. (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
Report WIL-39012 (A35220) of WIL Research Laboratories, Ashland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Sachsse, K., Frei, T., Luetkemeier, H., Vogel, W., Pappritz, G. &
Terrier, C. (1987) TPTH - Substance technical (Hoe 029664 OF ZD 97
0004) Chronic oral toxicity 52-week deeding study in beagle dogs.
Unpublished Report 047013/047024 (A35733) of RCC Research and
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Schollmeier, U. & Leist, K.H. (1989) Triphenyltin hydroxide -
active ingredient technical (Code: Hoe 029664 OF ZD97 0004).
Testing for sensitising properties in the Pirbright-White guinea
pig according to the technique of Buehler. Unpublished Report
89.1006 (A42667) of Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Scholz & Weigand, W., (1969b) Triphenyltin acetate Study No. 2116.
Unpublished Data A37396 from Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Seinen, W. & Penninks, A.H. (1987) Immunotoxicity of triphenyltin
compounds. Department pharmacology, pharmacy and toxicity. Faculty
of Veterinary Sciences, University of Utrecht, The Netherlands.
Document A35456. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Snow, R.L. & Hays, R.L. (1983) Phasic distribution of seminiferous
tubulus in rats treated with triphenyltin compounds. Bull.
Environ. Contam. Toxicol. 31: 658-665. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986a) 13-week oral toxicity (feeding) study
with TPTH-technical (code: HOE 029664 OF ZD97 0004 in the mouse.
Unpublished Report project 046991 (A32420) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Suter, P. & Horst, K. (1986b) 13-week oral toxicity (feeding) study
with TPTH-technical (Code : HOE 029664 OF ZD97 0004) in the rat.
Unpublished Report project 046978 (A32419) from RCC, Itingen,
Switzerland. Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Vogel, O.,
Armstrong, J., Ehlers, H.A., Müller, E. & Terrier, Ch. (1989a)
TPTH-technical (Code: HOE 029664 OF ZD97 0004) oncogenicity 80-week
feeding study in mice. Unpublished Report 047002 (A40467) of RCC,
Research and Consulting Company AG., Itingen, Switzerland.
Submitted to WHO by Hoechst AG., Frankfurt-am-Main.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Schlotke, B.,
Vogel, O., Ehlers, H.A., Mueller, E. & Terrier, Ch. (1989b)
TPTH-technical (Code: HOE 029664 OF ZD97 0007) chronic
toxicity/oncogenicity 104-week feeding study in rats. Unpublished
Report 046980 (A40468) of RCC, Research and Consulting Company AG.,
Itingen, Switzerland. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Terrel, Y & Reddy, A.K. (1978) Report: 18-month carcinogenicity
study of technical TPTH 96.3% lot no. SWRAM-1K in CD1 mice.
Unpublished Report project 6E-725 (A26239) from Cannon
Laboratories, Inc. Reading. Submitted to WHO by Hoechst AG.,
Frankfurt-am-Main.
Traul, K.A., Takayama, K., Kachevsky, V., Hink, R.J. & Wolff, J.S.
(1981) A rapid in vitro assay for carcinogenicity of chemical
substances in mammalian cells utilizing an attachment-independence
endpoint. J. Appl. Toxicol. 1(3), 190-195.
Ueda, K. & Iijima, K. (1961) Acute toxicity of Brestan Technical
(Triphenyltin Acetate) for mice. Unpublished Report A15046 from
Laboratory of Hygienics, Tokyo Dental College, Japan. Submitted to
WHO by Hoechst AG., Frankfurt-am-Main.
Wu, R,M., Chang, Y.C. & Chiu, H.C. (1990) Acute triphenyltin
intoxication: a case report. J. Neurol. Neurosurg. Psych., 53,
356-357.
Young, D.L. (1986) A dietary two-generation reproduction study in
rats with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final Report project WIL-39022 (A35378) from
WIL research laboratories, Inc. Ashland USA. Submitted to WHO by
Hoechst AG., Frankfurt-am-Main.
