
ABAMECTIN
First draft prepared by E. Bosshard
Federal Office of Public Health,
Schwerzenbach, Switzerland
EXPLANATION
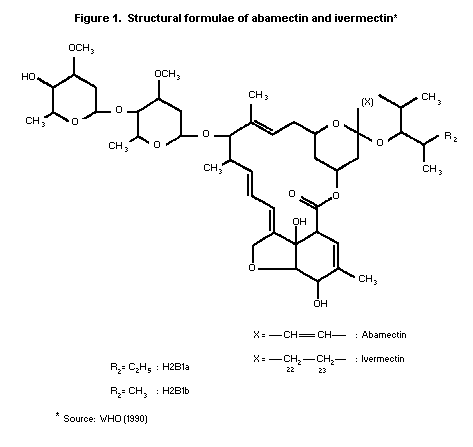
Abamectin is a macrocyclic lactone product derived from the
soil microorganism Streptomyces avermitilis. Abamectin contains at
least 80% avermectin B1a and not more than 20% avermectin B1b
(see Figure 1). It is used as an insecticide and acaricide. The
compound was considered for the first time by the present Meeting.
Because of the very similar biological and toxicological
properties of the individual B1a and B1b components, they can be
considered to be equivalent. Abamectin is degraded photolytically to
the delta-8,9-isomer which therefore forms a part of the residue.
In addition to data on abamectin, human data on ivermectin,
which is structurally similar, were considered.
 EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
A study was carried out to evaluate the tissue distribution and
elimination of 3H- and 14C-labelled avermectin B1a in a sesame
oil vehicle after oral administration to male and female rats.
Sixty-two CRCD strain rats/sex were divided into groups and dosed as
follows: a single high dose (1.4 mg/kg bw); a single low dose (0.14
mg/kg bw); fourteen daily low doses (0.14 mg/kg bw/day) of
unlabelled avermectin B1a, followed by a single low dose of
tritium-labelled avermectin B1a; a single high dose (1.4 mg/kg bw)
of a mixture of tritium - and 14C-labelled avermectin B1a; a
single dose of vehicle only to serve as controls for groups with
single dosing regimen or fifteen daily doses of vehicle only to
serve as controls for the multiple dosing experiment. The control
groups were sacrificed seven days after the last (or single) dose,
while rats from the treated groups were sacrificed on days 1, 2, 4
and 7 after dosing. Urine and faeces were collected each day after
dosing while the organs were collected from all rats at time of
sacrifice. All samples were assayed for total radioactivity.
Elimination in the urine was 0.3-0.6% of the applied dose in females
and 0.8-1.1% in males over the 7 days collection period. Elimination
in faeces ranged from 69-77% and 70-82% in females and males,
respectively. Residue levels in liver ranged from about 0.001-0.02
ppm, in kidney from 0.003-0.06 ppm, in muscle from 0.001-0.02 ppm
and in fat from 0.008-0.01 ppm in the different dosing groups, 7
days post-dose. The total residue level in the organs of the females
were generally higher than in males. The residue levels were dose
dependent, the residues in the high dose groups being roughly ten-
fold greater than in the low dose groups, whereas the depletion
rates were similar among the different tissues. The total
radioactive residues depleted from the tissues with half-lives of
approximately 1.2 days indicating that the residues did not persist.
Repeated dosing did not influence elimination rates or residue
levels. The tritiated avermectin B1a residue levels and depletion
rates were comparable to the 14C residue levels. Thus the tritium
label at the 5-position of avermectin B1a was not labile during
the course of the study. The stability of the label was also
demonstrated in an experiment for volatile/exchangeable tritium,
demonstrating that less than 2% of the tritiated avermectin B1a
sample was volatile (Alvaro et al., 1984).
Selected tissue samples (liver, kidney, muscle, fat) were
analyzed for unchanged avermectin B1a and metabolites. Two
metabolites that were also formed in vitro after rat liver
microsomal incubation, in addition to unchanged avermectin B1a,
accounted for most of the residues: The metabolites were identified
as 24-hydroxymethyl-avermectin B1a (24-OHMe-B1a) and 3"-desmethyl
avermectin B1a (3"-DM-B1a) (Maynard 1986b). A minor metabolite
was identified as ß-alpha-hydroxy-avermectin B1a (Gruber, 1988).
Toxicological studies
Acute toxicity studies
The main clinical signs were ataxia and tremors found in all
species investigated and irrespective of the route of
administration. The data are summarized in Tables 1 and 2.
Table 1: Acute toxicity of abamectin
Species Sex Route LD50 Component Reference
(mg/kg bw) (purity)
Mouse F oral 13.6-23.8 B1a Mandel (1977)
19.8 B1b (98.4%) Gordon et al. (1984a)
5-day mortality:
pregnant: 11.8-19.0 B1a+B1b (94%) Gordon et al. (1984b)
non pregnant: 15.0-41.3 B1a+B1b (94%) Gordon et al. (1985a)
Rat F,M oral ca. 11 B1a Mandel, 1977
F,M oral 8.7-12.8 B1a+B1b (91%) Robertson et al. (1981a)
Dog M,F oral ca. 8 B1a Robertson and Allen (1976)
B1a+B1b Gordon et al. (1984f)
Monkey M,F oral > 24 B1a+B1b Gordon et al. (1985b)
Rat dermal >330 B1a/B1b (87%/9.4%) Gordon & Mandel (1978)
Rabbit dermal >1600 B1a+B1b (91.4%) Robertson et al. (1981b)
B1a + B1b (94%) Gordon et al. (1983d)
Delta-8,9-isomer (photolytic degradation product)
Mouse M,F oral > 80 (Gordon et al. 1984c
& 1984e)
Table 2: Dermal and ocular irritation studies with abamectin,
technical material
Species Sex Target Findings Reference
Organ
Rabbit M,F eye very slight Robertson et al. (1981c)
irritation
Rabbit M,F skin non irritating on Robertson et al. (1981d)
intact or abraded
skin
Guinea- M,F skin: negative Gordon et al. (1983a)
pig sensitization
Short-term toxicity studies
Mice
In a twelve-week dietary range-finding study groups of mice
(CD-1 strain;15/sex/group) were fed dietary concentrations of 0,
2/40 (increase in week 9), 5, 10 or 20 ppm abamectin over 12 weeks
and 40 or 60 ppm over 3 weeks. There were no physical signs or
mortality. Decrease in body-weight gain at 60 ppm was the only
change observed. The NOAEL in this study was 40 ppm, equal to 8
mg/kg bw/day (Gordon et al., 1982f).
Rats
In an eight-week oral range-finding study in rats abamectin
(purity 94%) was administered to groups of rats (Charles River CD
albino rats, 10/sex/group) at dietary concentrations of 0, 5, 10,
15, 20/25 (increase in week 7), 40 or 60 ppm. Due to mortality and
the appearance of severe clinical signs of toxicity (tremors,
decreased activity) in the first study week, remaining animals at 40
and 60 ppm were sacrificed on days 15 and 5, respectively. These
groups were replaced by groups at 10 and 15 ppm in study week 5.
Decreases in mean body-weight gain were seen at 15, 20/25 (3 and 5%
decrease in males and females, respectively) and 40 ppm. Tremors
occurring at 15 and 20/25 ppm were no longer evident after study
week 1. One animal at 5 ppm showed slight tremors on day 2 only. No
gross or microscopic examination was performed. Based on clinical
signs and body-weight effects it appears that there is a steep dose-
response curve. Therefore the doses recommended for the
carcinogenicity study were 0.75, 1.5 and 2.0 mg/kg bw/day (Gordon
et al., 1982g).
Dogs
In a twelve-week oral range-finding study abamectin (purity
94%) was fed to groups of beagle dogs (2/sex/dose) at doses of 0,
0.25, 0.5, 1 or 4/2 mg/kg bw/day. Because of signs of toxicity
(tremors, weakness, incoordination, disorientation) and markedly
decreased food consumption at 4 mg/kg bw/day in one dog, this dose
was decreased to 2 mg/kg bw in study week 4. Inability to constrict
pupils after light stimulus was observed in some dogs at 1 mg/kg
bw/day or higher doses. Depression of body-weight gain or weight
loss occurred in dogs at 2 mg/kg bw/day and higher. No gross or
microscopic examination was performed. Based on the clinical signs
and body-weight effects recommended doses for the one-year oral dog
study were 0.25, 0.5 and 1 mg/kg/ bw/day. The NOAEL in this study
was 0.5 mg/kg bw/day (Gordon et al., 1982e).
Avermectin B1a (purity not specified) was orally administered
by gavage to beagle dogs (15/sex/dose) at dose levels of 0, 0.25,
0.5, 2 or 8 mg/kg bw/day over a period of 18 weeks. The treatment
did not affect parameters of urinalyses, ophthalmologic examination
or organ weights. Deaths occurred at incidences of 1/30, 3/30 and
3/30 at 0.5, 2 and 8 mg/kg bw/day, respectively. Signs of toxicity
at levels of > 0.5 mg/kg bw/day consisted of whole body muscular
tremors, ataxia, mydriasis and ptyalism (hypersalivation); in
addition, tonic convulsions and emesis occurred at 2 and 8 mg/kg
bw/day. Reduced body-weight gain was only observed at dose levels of
0.5 and 2 mg/kg bw/day, but not in surviving dogs at 8 mg/kg bw/day.
At 8 mg/kg bw/day changes in haematologic and serum biochemical
parameters included slightly increased values of haemoglobin,
haematocrit, erythrocytes, nonsegmented neutrophils and glucose
level. Electrocardiographic changes (elongation of the QT interval
and bradycardia) were noted at 8 mg/kg bw/day only at the beginning
of the study. Histopathologic changes were seen only in animals that
died or were killed moribund and consisted of diffuse hepato-
cellular vacuolation (without lipid accumulation) at dose levels of
0.5, 2 and 8 mg/kg bw/day and edema of the gallbladder at 2 and 8
mg/kg bw/day. The NOAEL in this study was 0.25 mg/kg bw/day
(Robertson & Allen 1976).
Groups of beagle dogs (6/sex/dose) were fed abamectin (purity
> 89%) for 53 weeks at concentrations resulting in doses of 0,
0.25, 0.5 or 1 mg/kg bw/day. The doses were selected on the basis of
the results of a previous range-finding study (Gordon et al.,
1982e). The compound administration had no effect on
ophthalmological examination, urinalyses or organ weights. Decreased
or even absence of constriction of pupils to light was evident at
0.5 mg/kg bw/day (occurrence rate 3%) and 1 mg/kg bw/day (15%) and
as single instances and in single animals also at 0.25 mg/kg bw/day.
At 1 mg/kg bw/day one dog was found dead (week 38), two others were
killed moribund (weeks 33 and 38). Reduced body-weight gain most
probably due to food unpalatability and therefore reduced food
consumption was observed at the highest dose level. A slight
decrease in serum urea nitrogen at 1 mg/kg bw/day was most probable
a consequence of decreased protein intake at this dose level. Slight
leukocytosis and increased packed cell volume was noted in one dog
killed in poor condition. There were no gross or microscopic changes
that could be attributed to treatment. The NOAEL in this study was
< 0.25 mg/kg bw/day, the single instance of mydriasis at this
dose being considered a borderline effect (Gordon et al., 1982i).
Long-term toxicity carcinogenicity studies
Mice
Abamectin (purity 90%) was administered in the diet to groups
of mice (Crl:CD-1(ICR)BR;74/sex/group; 24/sex/group for interim
sacrifice) at concen-trations resulting in doses of 0, 2, 4 or 8
mg/kg bw/day over a period of 94 weeks. In females, treatment-
related tremors were observed in all dose groups and deaths occurred
at 4 and 8 mg/kg bw/day. These effects were not observed in the 12-
week range-finding study at dosage levels up to 11 mg/kg bw/day (60
ppm). No explanation could be found for the sensitivity of these
female mice. Plasma samples taken from affected and unaffected
animals revealed no significant differences. A subsequent batch of
females did not show this unexpected sensitivity and following
restart of the study with a new group of female mice, tremors
occurred only in single animals at 8 mg/kg bw/day. In males, tremors
occurred in single moribund animals of the control and 8 mg/kg
bw/day group. In this group the mortality rate was increased:
towards the end of the study the mortality reached 60% compared to
45% in controls. Body-weight gain was reduced about 21% in females
and 7% in males at 8 mg/kg bw/day. There was a dose-related increase
in food consumption in treated females but a decrease in food
efficiency at 8 mg/kg bw/day. The treatment had no effect on
ophthalmic examinations, haematologic or biochemical parameters,
organ weights, gross pathology, histopathologic changes (with the
exception of a higher incidence of dermatitis at 2 and 8 mg/kg
bw/day in males). The tumour incidence was not increased by the
treatment. The NOAEL in this study was 4 mg/kg bw/day (Gordon et
al., 1983b).
Rats
Abamectin (purity 91%) was fed to groups of rats (Crl:CD(SD)Br;
65/sex/group) at dietary concentrations resulting in doses of 0,
0.75, 1.5 or 2/2.5/2 mg/kg bw/day over two years. Since no effects
attributable to abamectin were observed through study week 10, the
high dose level was increased to 2.5 mg/kg/ bw/day in week 11.
Because of the appearance of severe signs of CNS toxicity, the dose
was decreased again to 2 mg/kg bw/day in study week 13 for the
remainder of the study. No treatment-related changes with respect to
ophthalmoscopic, haematologic or serum biochemical parameters,
urinalyses and organ weights were observed. Increases in body-weight
gain were seen in all dosage groups during the first year on study.
By the end of the study comparable mean body-weight gains for all
groups of females were found, but an increased gain was noticed in
males. The increases in body-weight gain are considered treatment-
related, but the effect is not considered as an adverse effect.
Clinical signs consisting of tremors appeared in study week 12
correlated with the increase in dosage from 2 to 2.5 mg/kg bw/day in
week 11. The tremors in all the affected animals persisted
intermittently until sacrifice despite reduction in the high-dose
level back to 2 mg/kg bw/day in week 13. Several high-dose group
animals, which exhibited tremors, were sacrificed in a moribund
condition. The increase in mortality reflects the induction of
tremors during the early stage of the study and subsequent sacrifice
of the affected animals. Following the reduction in dose to 2
mg/kg/bw/day no new cases of tremors occurred and mortality in all
treated and control groups was comparable. No increase in tumour
incidence or treatment-related non-neoplastic histopathologic
changes were observed. The NOAEL in this study was 1.5 mg/kg bw/day
(Gordon et al., 1982h).
Reproduction studies
Mice
In a ten-day dietary maternotoxicity study abamectin (purity >
88%) was fed at dietary concentrations resulting in target dose
levels of 0, 0.1, 0.3 or 0.6 mg/kg bw/day (actual doses = 0.06,
0.16, or 0.33 mg/kg bw/day) to groups of pregnant mice (albino CF1;
20 females/group) on days 6 through 15 of gestation. Marked tremors
at 0.33 and at 0.16 mg/kg bw/day were observed. Reproductive
parameters (numbers of implants, resorptions and live and dead
fetuses) were not influenced. The NOAEL in this study was 0.06 mg/kg
bw/day (Gordon et al., 1983c).
Rats
One hundred-and-fifty F1 offspring, from five groups of F0
female rats (Charles River CD), that had been exposed in utero to
avermectin B1a at dosage levels of 0 (two groups), 0.1, 0.2 or 0.4
mg/kg bw/day were selected for a fourteen-week study of oral
toxicity. Groups of weanling rats (15/sex/group) received avermectin
B1a at doses of 0, 0.1, 0.2 or 0.4 mg/kg bw/day by gavage. The
treatment did not have any effects on mortality, ocular changes,
haematology, serum biochemistry, organ weights or gross and
microscopic tissue alterations. The increased body-weight gain of
male rats at 0.4 mg/kg bw/day was considered as treatment-related
but not as an adverse effect. The NOAEL in this study was 0.4 mg/kg
bw/day (Norbury and Wolf, 1977).
Avermectin (B1a) was administered orally to three groups of
female rats (12 females/dose group) at dose levels of 0, 0.5, 1, or
2/1.5 mg/kg bw/day from 14 days before mating throughout gestation
and lactation until day 21 postpartum. The high dose was reduced
after five doses to 1.5 mg/kg bw/day due to whole body muscular
tremors at 2 mg/kg bw/day. Two deaths occurred at the high dose, one
animal at this dose level became moribund. The significant decrease
in body-weight gain in the 1.5 mg/kg bw/day group was a result of
weight loss of the 3 moribund or dead animals. Weight gain increases
were observed during some periods at 1 and 1.5 mg/kg bw/day. A
treatment-related decrease in the number of live pups per litter on
day 1 postpartum at 1.5 mg/kg bw was observed. The pup weights were
decreased at this dose level on day 1. Pups at all dosage levels
showed decreases in average weight per litter throughout the study.
Dose-related increase in mortality among pups at all dose levels
were observed resulting in survival rates of 0, 14% and 76%, in the
1.5, 1 and 0.5 mg/kg bw/day groups respectively, compared to 98% in
the control group. There was a developmental retardation (eye
opening) at 0.5 and 1 mg/kg bw/day in surviving pups. The NOAELs in
this study were < 0.5 mg/kg bw/day for embryo-fetotoxicity and 1
mg/kg bw/day for maternotoxicity (MSDRL, 1977a).
Avermectin (B1a) was administered orally to three groups of
15 female rats at dose levels of 0, 0.1, 0.2 or 0.4 mg/kg bw/day
from 14 days before mating, throughout mating, gestation and
lactation until day 21 postpartum. No maternotoxic effects were
observed and the reproduction status was not adversely affected.
Among pups at 0.2 and 0.4 mg/kg bw/day a dose-related incidence of
spastic movements was noted increasing in severity with increasing
dose. At 0.2 and 0.4 mg/kg bw/day reduction in average pup weight
was observed (dose-related). Developmental retardation (eye opening,
ear opening, hear growth), occurred at 0.2 and 0.4 mg/kg bw/day
(dose-related). The NOAEL in this study was 0.1 mg/kg bw/day for
embryo-fetotoxicity (MSDRL, 1977b).
In an oral range-finding study (multigeneration) abamectin was
administered to groups of rats (Crl: CD (SD) BR Sprague-Dawley; 12
females/group) in the drinking water at concentrations of 0, 0.15,
0.5, 1.5 or 5 mg/l. Treatment was conducted from 14 days prior to
cohabitation with untreated males through day 21 postpartum and
subsequently through the F1 generation. Water consumption in
groups 0.15-1.5 mg/l increased during the lactation period and
decreased slightly in the 5 mg/l group. No accurate measurement of
water consumption could be performed prior to transfer to delivery
boxes on day 17 of gestation. Thereafter mean levels of water
consumption were comparable in all groups resulting in dose ranges
of 0.017-0.037, 0.066-0.127, 0.192-0.396, or 0.556-0.685 mg/kg
bw/day for the 0.15, 0.5, 1.5 mg/l or 5 mg/l group, respectively. In
the F0 generation the only effect observed was an increase in
body-weight gain in the 5 mg/l group in the first study week. In the
F1 generation an increase in postnatal mortality at 5 mg/l (53%
compared to 1% in control) was observed, physical signs observed in
pups at the highest dose level consisted of tremors; pup weights in
the 5 mg/l group were decreased. 3/116 Fetuses (2.6%) (from 2
litters) in the 5 mg/l group showed single malformations (1 cleft
palate, 1 sternebral malformation, 1 sternebral variation). One
fetus in the 0.5 mg/l group had lumbar ribs. Because of the single
incidences in the study groups and because of the occurrence of
these alterations in historical control groups it is questionable if
these effects are treatment-related (Gordon et al., 1981).
In a two-generation study, groups of rats
(Crl:COBSTMCDTM(SD) BR/30/sex/group) were orally treated
(gavage) with dosages of 0, 0.05, 0.12 or 0.4 mg abamectin/kg bw/day
. F0 and F1b rats were mated to produce F1a and F1b litters,
F2a and F2b litters. At 0.12 and 0.4 mg/kg bw/day both sexes of
the F0 rats during the first gestation period (F0-F1a) showed
increased body-weight gains, whereas during the subsequent lactation
period (F0-F1a) a reduction in body-weight gain compared to
control was observed at these two dose levels particularly on the
first days of lactation. Similar changes were also observed in the
next generation. During the second mating period of F0 rats (to
produce the F1b litters) mating performance was reduced (reduction
of incidence of mating) at 0.4 mg/kg bw/day as compared to control
animals. The reduction in mating performance was presumed to be
associated with irregular estrous cycles observed during the second
mating period of F0 female rats. This effect was not observed in
matings resulting in the F1a litters or the F2a and F2b
litters. During the lactation periods of both generations effects in
the 0.4 mg/kg bw/day group consisted of increased pup mortality,
reduced viability and lactation indices and lower average pup body-
weights. The incidence of pups which appeared thin and weak was
increased. These effects were less severe in the F2 litters than
in the F1 litters. No skeletal anomalies associated with the test
agent were revealed. Histopathological examination showed retinal
anomalies in 3/4 males and 1/5 females in the high-dose group
compared wich 1/10 in the control group (F1b weanlings). The
anomalies consisted of single or multiple retinal folds of many
layers that included pigment epithelium. The same lesion was seen in
one control female and in one male in the 0.05 and 0.12 mg/kg bw/day
groups of the F1b weanlings. In the F1b parental animals (both
sexes) only 1/35 showed this retinal anomaly. In the F2b weanlings
histopathology was performed on a greater number of animals. There
was a significant increase in retinal anomalies in the high-dose
group with the following incidences (M + F): 4.6% and 22% in the
control and 0.4 mg/kg bw/day, respectively. As will become evident
from the results of a reproduction study in rats conducted with
comparable doses of the delta-8,9-isomer of abamectin the control
incidence of retinal anomalies (4.6%) in the present study is much
smaller than in the other study, where a control incidence of 20%
was found. Therefore the difference observed may be incidental
following an unusually low incidence of this lesion in the
concurrent control group of the present study.
Among both the F1b and F2b weanlings the high-dose group
pups were the smallest. One hypothesis to explain the presence of
retinal folds in the high-dose group weanlings and the disappearance
with maturity proposed by the study authors is that the ocular globe
size is relatively smaller in the small pups (where there was
pronounced pup toxicity) relative to the size of the retina which
perhaps carried on at its normal growth rate. Therefore it may be
assumed that the retinal effects observed are a secondary effect.
Reversibility of the retinal anomalies is evidenced by its absence
in the high dose adults. The NOAELs in this study were 0.12 mg/kg
bw/day for pup toxicity and retinal anomalies and 0.05 mg/kg bw/day
for maternotoxicity (Gordon et al., 1982n).
Special studies on embryotoxicity/teratogenicity
Mice
Avermectin B1a was administered orally (gavage) to groups of
mice (albino CF1 strain/20 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.1, 0.2, 0.4 or 0.8 mg/kg bw/day.
The doses of the B1a component were calculated as the parent
compound.
Deaths occurred in the 0.1, 0.4 and 0.8 mg/kg bw/day groups (1,
3 and 2 animals respectively), in most cases preceded by tremors.
Body-weight gain was not influenced by the treatment; neither was
the reproductive status (number of implants, resorptions, live and
dead fetuses per litter). An increased incidence of cleft palate at
0.4 (4/165; 2.4%) and 0.8 mg/kg bw/day (5/199; 2.5%) was observed
compared to a control incidence of 0.4%. The NOAELs in this study
were < 0.1 mg/kg bw/day for maternotoxicity and 0.2 mg/kg bw/day
for embryo-fetotoxicity and teratogenicity (Robertson 1977a).
Groups of mice (albino CF1 strain/20 female/group) were
administered avermectin B1a by gavage from day 6 through 15 of
gestation at dose levels of 0, 0.025, 0.05, 0.075 or 0.1 mg/kg
bw/day. One animal died at 0.1 mg/kg bw/day and tremors were
observed in several additional mice at this dose. One animal at
0.075 mg/kg bw/day exhibited muscular tremors and became moribund.
Maternal body-weight gains were unaffected at any dosage level. The
NOAEL in this study was 0.05 mg/kg bw/day for maternotoxicity
(Robertson, 1977b).
In an oral maternotoxicity study the minor component of
abamectin, avermectin B1b was administered by gavage to groups of
mice (Crl:CF1 BR; 12 females/dose) at dose levels of 0, 0.025, 0.05,
0.075 or 0.1 mg/kg bw/day on days 6 through 15 of gestation. At the
0.075 mg/kg bw/day dose level two deaths occurred preceded by weight
loss and tremors. Single fetuses at all dose levels showed
malformations consisting of exencephaly and cleft palates resulting
in incidences of about 1% in all dose groups. The highest incidences
for historical control groups are 1.6% for exencephaly and 1.3 % for
cleft palates (overall incidence 0.3% for exencephaly and cleft
palate each). There was no evidence of fetotoxicity. The NOAEL in
this study was 0.05 mg/kg bw/day for maternotoxicity (Gordon et
al., 1985e).
Rats
In a range-finding study groups of mated female rats (Charles
River; 10/group) were administered abamectin by gavage at dose
levels of 0, 0.25, 0.5, 1 or 2 mg/kg bw/day on gestation days 6
through 17. One female at 2 mg/kg bw/day exhibited weight loss and
tremors and was sacrificed after receiving 12 doses. There was no
other evidence of maternal toxicity. At 0.25 and 2 mg/kg bw/day
there was a slightly increased body-weight gain that is considered
to be treatment-related but not an adverse effect. The NOAEL in this
study was 1 mg/kg bw/day (Gordon et al., 1982j).
In a rat teratology study groups of mated rats (Charles River;
25 females/group) were orally treated (gavage) with abamectin
(purity 94%) at dosage levels of 0, 0.4, 0.8 or 1.6 mg/kg bw/day on
days 6 through 19 of gestation. There was no evidence of maternal
toxicity. Slightly increased maternal body-weight gain was seen at
all dosage levels between days 6 and 14 of gestation. An increased
incidence of fetuses with external fetal malformations was observed
(exencephaly, cleft palate, gastroschisis) at 0.8 and 1.6 mg/kg
bw/day. The incidences were: 2/279 (0.7%) and 2/326 (0.6%),
respectively, compared to 1/319 (0.3%) in the control group.
Historical control incidence of cleft palate in this rat strain is
0.03% (overall) or 0.3% (single study with highest incidence).
Gastroschisis alone has an overall incidence of 0.004% or the
highest incidence in a single study of 0.3%. Based on the fact, that
in historical controls one single malformation (cleft palate) may
occur in up to 0.3% of control animals and no dose-response-
relationship is evident, it may be assumed that the increase
observed may either not be treatment-related or may be a borderline
effect. Visceral examination indicated a higher incidence of
distended ureters in the treated groups up to 3% compared to 0% in
controls), but without any dose-effect relationship. This effect is
observed at incidences of up to 7% in historical control groups.
Skeletal examination revealed a higher incidence of fetuses with
lumbar ribs and count variations at 1.6 mg/kg bw/day: at 1.6 mg/kg
bw/day an incidence of 72/326 (22%) was found, compared to 41/320
(13%) at 0.4, and 45/279 (16%) at 0.8 mg/kg bw/day compared to
44/319 (14%) in the control group. The increase at 1.6 mg/kg bw/day
may not be treatment-related because higher incidences are observed
in historical controls. For example, in historical controls lumbar
ribs occurred at an incidence of 14% (overall) or 27.8% (single
study with highest incidence). The NOAELs in this study were 1.6
mg/kg bw/day for maternotoxicity and < 1.6 mg/kg bw/day for
fetotoxicity and teratogenicity (Gordon et al., 1982k).
Rabbit
In a range-finding study groups of New Zeeland albino rabbits
(10 females/dose) were given abamectin at oral dosage levels of 0,
0.5, 1, 2 or 3 mg/kg bw/day on gestational days 6 through 18.
Maternotoxicity was observed at 3 mg/kg bw/day. One female at 3
mg/kg bw/day was sacrificed moribund on day 16 of gestation after 11
doses. All females at 3 mg/kg bw/day were in a stupor after the
fourth and subsequent doses and showed yellow or green discharge
from nose or mouth. Moreover animals at 3 mg/kg bw/day had a
decreased food and water consumption, and showed body-weight loss.
The NOAEL in this study was 2 mg/kg bw/day (Gordon et al., 1982).
In a teratogenicity study groups of female New Zeeland albino
rabbits (18/dose) were given abamectin (purity 94%) at oral dosage
levels of 0, 0.5, 1 or 2 mg/kg bw/day on day 6 through 27 of
gestation. There were single deaths in all the treatment groups. At
2 mg/kg bw/day the animals showed decreased food and water
consumption and weight loss. Fetuses of the 2 mg/kg bw/day showed
cleft palates, omphaloceles and clubbed forefeet at higher
incidences than in the concurrent control group: 7.4% versus 2.1% in
concurrent control. The incidence of clubbed fore foot alone in
historical control groups is 0.2% (overall) and 3.9%, respectively,
in a single study with highest incidence. No data are available
concerning historical control incidences of cleft palates and
omphaloceles in rabbits. The clearly higher incidence of these
malformations at 2 mg/kg bw/day is therefore considered to be
treatment-related. With respect to skeletal fetal examination higher
incidences of skeletal terata (vertebral malformations, branched and
fused ribs) were observed at 0.5, 1 and 2 mg/kg bw/day with
incidences of 4%, 2% and 4% of the fetuses showing these
malformations respectively, compared to 0% in the concurrent control
group. In historical controls single vertebral malformations may
occur at incidences of up to 0.5% (overall) (e.g., caudal vertebral
malformations) with the highest value of 9% in a single study (e.g.,
fused ribs). At 0.5 and 2 mg/kg bw/day there were increased
incidences of incompletely ossified sites particularly in sternebrae
and metacarpals. These effects were more pronounced in the highest
dose group. Because the incidences observed at 1 mg/kg bw/day were
comparable to the incidences in the control group the absence of a
dose-relationship in the lower dose levels of 0.5 and 1 mg/kg bw/day
indicates that the increased incidence at 0.5 mg/kg bw/day is
possibly not treatment-related. The NOAEL in this study was 1 mg/kg
bw/day for maternotoxicity, embryo-fetotoxicity, and teratogenicity
(Gordon et al., 1982m).
Special studies on genotoxicity
A number of genotoxicity studies have been conducted with
abamectin. The results are summarized in Table 3.
Special studies on the delta-8,9-isomer of avermectin B
Biochemical aspects
Rat
Male rats were orally (gavage) dosed with 1.4 mg3H-labelled
delta-8,9-isomer avermectin B1a/kg bw. The delta-8,9-isomer of
abamectin is a photolytic degradation product of abamectin. The
stability of the tritium label at the 5th carbon position was
previously demonstrated with avermectin B1a in rats. Within 7 days
after dosing 94% of the administered dose was eliminated with the
faeces, and 0.4% in the urine. The levels of residues in liver,
kidney, fat and muscle ranged from 0.28 ppm (muscle) to 1.4 ppm
(fat) on day 1 after dose and decreased to a range of 0.017 to 0.1,
respectively, on day 7 after dosing. The half-lives for the tissue
residues in muscle, kidney, liver and fat varied between 1.45 and
1.64 days. As major metabolite the 3"-desmethyl-delta-8,9 isomer was
isolated. The 3"-DM-delta-8,9 isomer was also isolated and
identified after rat liver microsomal incubation of the delta-8,9-
isomer. The minor metabolite 24-hydroxymethyl-Delta-8,9-isomer,
(also formed in vitro), was identified.
By comparison, the elimination by rats of both the delta-8,9-
isomer avermectin B1a in this study and the avermectin B1a in a
previous study was very similar. In both studies 94-96% of the
administered dose were recovered within 7 days after dosing in the
excreta. The tissue residue levels were also demonstrated to be
similar for both compounds. The average half-life for the residue in
the edible tissues was 1.1 and 1.5 days for avermectin B1a and
delta-8,9-isomer, respectively. The metabolites generated were very
similar with rats metabolizing both compounds to the 3"-desmethyl
and the 24-hydroxymethyl metabolites. Similarly, both avermectin
B1a and ivermectin B1 (22,23-dihydro-avermectin B1) have been
shown to be primarily metabolized to the 3"-desmethyl and/or 24-
hydroxymethyl metabolite(s) by many other animal species (Maynard
et al., 1986a).
Table 3. Results of genotoxicity assays on abamectin
Test System Test Object Concentration Results Reference
(purity)
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1982a)
typhimurium (precipitate at conc.
> 3000 µg/plate)
± activation
(94%)
Ames test Salmonella 1-2000 µg/plate * negative Skeggs (1976)
typhimurium
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1985f)
typhimurium without activation
(89%)
3-1000 µg/plate
± activation
(94%)
V-79 mammalian V-79 Chinese 0.03 - 0.05 mM negative Gordon et al. (1982b)
cell assay hamster lung cells (+ activation)
(HGPRT Locus) 0.003-0.006 mM
(- activation)
Alkaline elution Rat hepatocytes 0.01-0.6 mM positive in Gordon et al. (1982c)
assay in vitro concentrations
(DNA single (> 0.2 mM)
strand breaks)
Test System Test Object Concentration Results Reference
(purity)
Table 3 (contd)
Test System Test Object Concentration Results Reference
(purity)
In vitro Chinese hamster 0.005 - 0.025 mM negative Gordon et al. (1985g)
chromosomal ovary cells with activation
aberration
0.01 - 0.035 mM
without activation
Alkaline elution Rat oral up to 10.6 mg/kg bw negative Gordon et al. (1982d)
assay in vivo (approx. LD50)
(DNA single
strand breaks)
In vivo cytogenetic Mouse oral 1, 2, 4, negative Blazak et al. (1983)
assay 12 mg/kg bw
(mouse bone marrow) (94%)
Delta-8,9-isomer: Salmonella 10-3000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at conc.
> 1000 µg/plate)
(91,6%)
Polar degradates: Salmonella 100-10 000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at
10 000 µg/plate)
* avermectin B1a
Embryotoxicity/teratogenicity and reproduction
Mice
The delta-8,9-isomer of abamectin was given by gavage in sesame
oil to groups of 7-11 mated female mice (Crl:CF1 BR) at doses of 0,
1.5, 3, 6.25, 12.5, 25, or 50 mg/kg bw/day on day 6 through 15 of
gestation. Treatment-related deaths occurred at all dosage levels.
The groups at 3 mg/kg bw/day and higher doses were terminated on
days 6-8 of gestation. There was a slight decrease in body-weight
gain on single days at 1.5 mg/kg bw/day compared to control. Because
of early termination, no body-weights were measured after the
beginning of dosing in the other dose groups. Fetal effects became
apparent at 1.5 mg/kg bw/day (the only group with litters) as an
increased incidence of fetuses with cleft palate (24/83 (29%)
compared to 0% in the concurrent control. The NOAEL in this study
was <1.5 mg/kg bw/day for maternotoxicity and teratogenicity
(Gordon et al., 1984d).
A subsequent study with the delta-8,9-isomer was performed to
establish a no-effect level. Oral doses were administered to groups
of mice (Crl: CF1 BR; 12 mated females/dose) at levels of 0, 0.05,
0.1, 0.5 or 1 mg/kg bw/day on days 6 through 15 of gestation. Single
females at 0.5 and 1 mg/kg bw/day showed clinical signs including
tremors and loss of body-weight. Decreases in body-weight gain were
observed at 0.05 and 1 mg/kg bw/day, but not at 0.1 mg/kg bw/day. No
dose-related increase in the incidence of resorptions and dead
fetuses occurred in any treatment groups. In addition there were
fewer implants per female in the 0.05 (10.4 per female) and 1 mg/kg
bw/day (9.8 per female) groups compared to 12.5 per female in the
control group resulting in decreases in live fetuses per female at
0.05 (8.7) and 1 mg/kg bw/day (8.3) compared to control (11.3).
These differences from control and particularly those at the low
dose level were probably not treatment-related since the changes
were slight and were not observed at the intermediate dose levels.
An increased incidence of cleft palates were observed at dose
levels of 0.1 mg/kg bw/day and above with incidences of 13/115
(11%), 1/90 (1%) and 7/91 (8%) at dose levels of 0.1, 0.5, and 1
mg/kg bw/day, respectively, compared to 0% in the concurrent control
group. The highest historical control incidence of cleft palates was
reported to be 3% in one study (overall 0.3%). There were also
slightly increased incidences of exencephaly at dose levels of 0.1
mg/kg bw/day and above: of 1/115 (1.7%), 4/90 (4%), and 2/91 (2%) at
the dosage levels of 0.1, 0.5 and 1.0 mg/kg bw/day, respectively,
compared to 1/136 (0.7%) in the concurrent control. The highest
incidence of historical control incidence was reported to be 1.6% in
a single study (overall 0.3%). As with the incidence of cleft
palates no dose-response-relationship was evident but the observed
incidences were clearly higher than the range of historical controls
reported. The NOAELs in this study were 0.1 mg/kg bw/day for
maternotoxicity and 0.05 mg/kg bw/day for fetotoxicity and
teratogenicity (Gordon et al., 1984d).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR; 25 females/dose group) at dosage
levels of 0, 0.015, 0.03 or 0.06 mg/kg bw/day on days 6 through 15
of gestation (original intent was to have dosage levels of 0.025,
0.05 and 0.1 mg/kg bw/day; but an error in preparation resulted in
different dose levels). No signs of maternotoxicity were observed.
Increased incidences of exencephaly in the 0.03 and 0.06 mg/kg
bw/day groups were found, but without any dose-response
relationship. The incidence in both groups was about 1.3% compared
to 0% in the concurrent control and the 0.015 mg/kg bw/day group.
Compared to the incidences of historical controls the present
incidence of 1.3% is slightly smaller than the highest incidence of
1.6% of a single study in historical control animals. A single fetus
(1/210) in the 0.015 mg/kg bw/day group had a cleft palate resulting
in an incidence of about 0.5%. The NOAELs in this study were 0.06
mg/kg bw/day for maternotoxicity and < 0.06 mg/kg bw/day for
teratogenicity (Gordon et al., 1985c).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR;25 females/group) on days 6 through 15
of gestation at dosage levels of 0, 0.015, 0.03, 0.1 or 0.5 mg/kg
bw/day. One animal at 0.5 mg/kg bw/day was sacrificed in a moribund
condition. Incidence of cleft palate was 24/233 (10%), 6/279 (2%),
and single fetuses (0.4%) at 0.5, 0.1, and at both 0.03 and 0.015
mg/kg bw/day, respectively. The overall incidence of cleft palate in
historical controls is reported to be 0.3%. The incidences of
exencephaly of about 0.4% that occurred in single fetuses in the
control, 0.015 and 0.5 mg/kg bw/day groups and in 5/238 (2%) in the
0.03 mg/kg bw/day group did not show any dose-response-relationship.
This compares with, but is greater than, the overall incidence of
0.3% and is less than 1.6% (highest incidence) of historical control
animals except at 0.03 mg/kg bw/day. The NOAELs in this study were
0.1 mg/kg bw/day for maternotoxicity and 0.03 mg/kg bw/day for
embryo-fetotoxicity and terato-genicity (Gordon et al., 1985d).
Rats
In an oral study groups of rats (Sprague-Dawley Crl:CD(SD)BR;
25 females/group) were treated with dosage levels of 0, 0.25, 0.5 or
1.0 mg/kg bw/day of the delta 8,9-isomers of abamectin on days 6
through 17 of gestation. The body-weight gain in treatment groups
was slightly greater than in the control group. This effect on body-
weight gain was seen in previous teratology and reproductive studies
with the parent compound at comparable doses (Gordon et al.,
1982k,n). As in the previous studies, this increased body-weight
gain is considered treatment-related but not an adverse effect. No
evidence of embryo- or fetotoxicity or teratogenicity at any dose
level was given. The NOAEL in this study was > 1 mg/kg bw/day
(Gordon et al., 1987b)
In a single generation study groups of rats (Sprague-Dawley
[Crl: CD(SD)BR]; 20 females/dose) were orally treated (gavage) at
dosage levels of 0, 0.06, 0.12 or 0.4 mg of the delta-8,9-isomer of
abamectin/kg bw/day. The period of dosing was fifteen days prior to
cohabitation through day 20 of lactation. There were no treatment-
related effects on F0 female reproductive performance (mating
index, fertility, length of gestation). Histopathological
examination of the F1 weanlings revealed retinal anomalies in all
groups including controls, consisting particularly of intra-retinal
folds. The incidences were 20, 16, 16 and 22% in control, 0.06, 0.12
and 0.4 mg/kg bw/day groups, respectively. More severe retinal
anomalies were identified in animals of the low- and mid-dose groups
compared to control animals. These changes were compared with the
severity of retinal anomalies observed in control weanling rats of a
previous two-generation study conducted with abamectin (Gordon et
al., 1982n). According to the statement of the study authors the
retinal anomalies observed in the present study have comparable
severity as those in the previous control animals, but no original
data were presented. The study authors conclude that no ocular
lesions were produced as a result of the administration of the
compound. The fact that no clear dose-response with regard to the
incidence and severity of these retinal effects is observed supports
the suggestion that in fact they are not caused by the treatment
(Gordon et al., 1987d).
Polar degradates (unidentified mixture of residues generated
in vitro and in a field study on oranges).
In two oral developmental toxicity studies groups of female
mice (Crl:CF1 BR; 25/group) were orally treated (gavage) at dosage
levels of 0, 0.25, 0.5 and 1 mg/kg bw/day on days 6 through 15 of
gestation. The treatment did not have maternotoxic effects and did
not influence the reproductive performance. On fetal examination
there was no evidence of embryotoxic/fetotoxic or teratogenic
effects of the compounds (Gordon et al., 1987c, 1988).
Genotoxicity studies with metabolites
See Table 3.
Antidote study of abamectin intoxication in dogs
Ipecac given at a rate of 30 ml/dog by stomach tube 15 min
after a lethal oral dose of abamectin of 8 mg/kg bw induced vomiting
within 15-45 min after ipecac was given. This treatment was adequate
to prevent coma and death in dogs given a lethal dose of abamectin.
Ipecac treatment only after 30 min or longer after the dose of
abamectin did not prevent coma and death of the dogs (Gordon et
al., 1984f).
Observations in humans and non-human primates
The comparison of the toxicological data of the two members of
the avermectin family of compounds, abamectin and ivermectin,
reveals a lot of similarities. Ivermectin has been used extensively
in humans at an oral therapeutic dose of 0.2 mg/kg bw for the
treatment of onchocerciasis without serious drug-related adverse
effects (Greene et al., 1989). Therefore the human experience with
a closely related compound offers useful information to be
considered in the evaluation of abamectin.
Comparative studies with ivermectin in monkeys
Safety assessment studies with abamectin and ivermectin have
shown that the susceptibility of different animal species to these
compounds varies considerably. The pregnant mouse is the most
sensitive mammal examined so far. In view of the similarity in
structure and toxicity of abamectin and ivermectin and the diversity
of responses among the different species it was suggested that an
acute oral toxicity study in rhesus monkeys might serve as a
valuable source of information about the potential toxicity of the
compounds in primates. Such a study might provide a more rational
basis for predicting the toxicity of abamectin in humans.
The purpose of the study was to determine the minimum toxic
dose (mTD) of abamectin and ivermectin in monkeys and to determine
the plasma levels of drug at that dose.
Single oral doses of abamectin or ivermectin were given to four
rhesus monkeys (2/sex) each at intervals of 2-3 weeks, in the
following chronological dose levels: 0.2, 0.5, 1, 2, 4, 6, 8, 12 or
24 mg/kg bw. The most sensitive indicator of toxicity was emesis
occurring at dose levels of 2 mg/kg bw and higher. The incidence of
emesis was dose-related and the time after dosing when emesis
occurred tended to decrease with increasing dose. Marked mydriasis
(lack of pupillary constriction) was noted only at 24 mg
abamectin/kg bw. Less pronounced mydriasis was observed after doses
of 24 mg ivermectin/kg bw. At 24 mg/kg bw both compounds induced
slight to moderate sedation.
Since no emesis occurred with doses below 2 mg/kg bw and since
no other drug-related physical signs of toxicity were observed at
lower doses, it was concluded that emesis was the most appropriate
physical sign for characterizing minimum toxic doses of the
compounds in monkeys and that the mTD was 2 mg/ kg bw for both
compounds. This dose is tenfold higher than the human dose for
onchocerciasis therapy of 200 µg/kg bw.
Plasma concentrations of ivermectin were similar to the levels
of abamectin for up to 4 h following the 2 mg/kg bw doses, but
thereafter ivermectin levels were higher on the average than those
of abamectin. Peak levels occurred between 8-24 h post-dose with
maximum values of 110 ng/ml for ivermectin and 76 ng/ml for
abamectin after a 2 mg/kg bw dose. The average maximum plasma level
following the 24 mg/kg bw dose of ivermectin was 680 ng/ml, after
the same dose of abamectin it was 390 ng/ml. Despite roughly
proportional increases in plasma levels with increased doses, the
severity of clinical signs did not worsen appreciably and no
tremors, convulsions or deaths occurred even at a dose of 24 mg/kg
bw. The dose-response curve for acute toxicity of abamectin and
ivermectin in monkeys seems to be much flatter than for mice,
indicating that in primates, clinical evidence of intoxication
(emesis) may occur well in advance of serious or life-threatening
toxicity. Mean peak plasma level measured in humans given the 200
µg/kg bw therapeutic dose of ivermectin was 20 ng/ml (Gordon et
al., 1985b).
Observations in humans
Whereas for abamectin no observations in humans are reported,
information is available concerning the therapeutic use and the
safety of ivermectin, extensively tested in human onchcerciasis. In
a single yearly dose it suppresses microfilaria in the skin and eyes
and prevents disease progression in most infected persons. A single
oral dose of 30 µg/kg bw or 50 µg/kg bw exhibited microfilaricidal
activity. Transient pruritus was observed soon after treatment, but
no abnormal laboratory results were produced (Aziz et al., 1982).
In a 12-month follow-up study investigating the efficacy of
different dosages of the drug it was shown that doses of 100 µg/kg
bw did not produce different reactions from the placebo group,
whereas dose levels of 150 and 200 µg/kg bw only produced mild
reversible clinical reactions (Mazzotti-like reactions). It is
suggested therefore that 150 µg/kg bw is probably the optimal dose
in terms of its antiparasitic activity and side effects. A single
oral dose of 150 µg/kg bw annually induces a minimal reaction in 5-
15% of infected adults and a more significant reaction in about 1%.
Its use was associated with hypotension, usually occurring within 24
h of administration in 1 out of 1000 persons (Greene et al.,
1989).
COMMENTS
Following oral administration of abamectin to rats, 69-82% of
the administered dose was eliminated in the faeces and only 1% in
the urine. Biliary excretion was the major cause of the high level
of faecal excretion. Biotrans-formation proceeds mainly by
demethylation and hydroxylation.
Orally administered abamectin elicited dose-dependent CNS
effects, including tremors and ataxia.
In a one-year dietary study in dogs at doses of 0, 0.25, 0.5 or
1 mg/kg bw/day, a borderline NOAEL of 0.25 mg/kg bw/day was
determined, despite single instances of mydriasis at this lowest-
dose level.
In a two-year long-term/carcinogenicity study in mice,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 2, 4 or 8 mg/kg bw/day. The NOAEL was 4 mg/kg bw/day,
based on the occurrence of tremors, a higher mortality rate and
reduced body-weight gain at 8 mg/kg bw/day. Abamectin was not
carcinogenic in the mouse.
In a two-year long-term/carcinogenicity study in rats,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 0.75, 1.5 or 2 mg/kg bw/day. The NOAEL was 1.5 mg/kg
bw/day. Higher doses caused CNS toxicity. Abamectin was not
carcinogenic in rats.
In two one-generation reproduction studies in rats avermectin
B1a was administered in the diet at concentration resulting in
dosage levels ranging from 0.1 to 2 mg/kg bw/day in rats.
Maternotoxicity was observed at dose levels above 1 mg/kg bw/day.
Fetotoxicity, consisting of reduced pup survival rates, reduced pup
weight growth and retardation became evident at dose levels of 0.5
mg/kg bw/day and higher. The NOAEL for fetotoxicity was 0.1 mg/kg
bw/day.
In a two-generation reproduction study in rats at dose levels
of 0.05, 0.12 or 0.4 mg abamectin/kg bw/day, the NOAEL for
maternotoxicity was 0.05 mg/kg bw/day, based on reduced maternal
body-weight gain during lactation at 0.12 mg/kg bw/day and above.
The NOAEL for pup toxicity was 0.12 mg/kg bw/day, based on increased
mortality and lowered pup weights at 0.4 mg/kg bw/day.
The teratogenic potential of abamectin administered by gavage
was investigated in mice, rats and rabbits. Teratogenic effects,
including cleft palates, omphaloceles and clubbed fore feet, were
observed at maternotoxic doses in mice and rabbits. The NOAEL for
teratogenicity in the most sensitive species, the mouse (CF1 strain)
was 0.2 mg/kg bw/day, while for maternotoxicity the NOAEL was 0.05
mg/kg bw/day, based on the occurrence of tremors and deaths at
higher doses.
Various studies to investigate the teratogenic potential of the
delta-8,9-isomer have been conducted in mice and rats. Similar
teratogenic effects to those seen with abamectin were observed in
the most sensitive species, the mouse. The NOAELs for
maternotoxicity in the mouse were 0.1 mg/kg bw/day, and for
fetotoxicity/teratogenicity, 0.05 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that abamectin was not genotoxic.
Although no human data were available on abamectin, extensive
data on field and community-based trials with ivermectin in humans
infected with Onchocera spp. were available (WHO, 1993). The main
effects noted were those arising from the death of parasites, the
so-called Mazzotti reaction, which is characterized by arthralgia,
pruritus, fever, hypertension, tachycardia, headache, and ocular
changes. Very limited data in humans indicate that ivermectin does
not increase the incidence of birth defects, although it is
teratogenic in mice, rats and rabbits (WHO, 1990).
The available data provided adequate toxicological information
to permit the allocation of an ADI for abamectin and its delta-8,9-
isomer, based on the NOAELs for abamectin of 0.05 mg/kg bw/day in
the teratogenicity study in mice and in the two-generation
reproduction study in rats. The NOAEL for the delta-8,9-isomer was
0.05 mg/kg bw/day in the teratogenicity study in mice. A safety
factor of 500 was used because of concern over the teratogenicity of
the delta-8,9-isomer which forms part of the residue in food.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Abamectin (and components avermectin B1a and B1b):
Mouse: 4 mg/kg bw/day (2 year feeding study)
0.05 mg/kg bw/day (teratology study, maternotoxicity)
0.2 mg/kg bw/day (teratology study, teratogenicity)
Rat: 1.5 mg/kg bw/day (2-year study)
0.1 mg/kg bw/day (1-generation reproduction study)
0.05 mg/kg bw/day (2-generation reproduction study,
maternotoxicity)
0.12 (2-generation reproduction study, pup toxicity)
Dog 0.25 mg/kg (borderline) (1-year study)
Delta-8,9-isomer
Mouse 0.1 mg/kg bw/day (teratology study, maternotoxicity)
0.05 mg/kg bw/day (teratology study, teratogenicity)
Estimate of acceptable daily intake for humans (abamectin and
delta-8,9-isomer)
0-0.0001 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing studies on the mechanism of central nervous system
toxicity.
2. Observations in humans.
REFERENCES
Alvaro, R.F., Green, M.L., Halley, B.A., Maynard, M.S., &
Meriwether, H.T. (1984) Distribution and clearance of avermectin
B1a in rats. Unpublished report ARM-1 prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Aziz, M.A., Biallo, S., Diopp, I. and Lariviere, M. (1982) Efficacy
and tolerance of ivermectin in human onchocerciasis. Lancet, 24
July, p. 171-173. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Blazak, W.F., Mitchell, A.D., & Skinner, W.A. (1983) An assessment
of the mutagenic potential of abamectin (mk 0936) utilizing the in
vivo mouse bone marrow cytogenetics assay. Study Number TT 83-900-
6. Unpublished report prepared by SRI International, Menlo Park, CA,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. & Mandel, J. (1978) Acute dermal toxicity study in rats
with abamectin (avermectin B1; C-076). Study Number TT 78-3607.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Nickell, B.E., Collevechio, K., Mutchler, M., & Clark,
R.L. (1981) Oral-range-finding study (multigeneration) in rats with
abamectin (MK 0936). Study No. 82-707-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Cook, M.M., Berglund, R.M., & Prato, M.
(1982a) Microbial mutagen test on abamectin (MK 0936) with and
without rat liver enzyme activation. Study No. TT 82-8013.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., & Patterson, S.K. (1982b) V-79
mammalian cell mutagenesis assay with abamectin (MK 0936). Study No.
TT 82-8506, 82-8510, 82-8512, 82-8519. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982c) In vitro alkaline elution/rat hepatocyte
assay with abamectin (MK 0936). Study No. TT 82-8520, 82-8523, 82-
8525, 82-8526. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982d) In vivo alkaline elution/rat hepatocyte assay
with abamectin (MK 0936). Study No. TT 82-8302. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1982e) Twelve-week oral
range-finding study in dogs given abamectin (MK 0936). Study No. TT
82-073-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Peter, Ch.P., Nickell, B.E., Buck, J.F., & Schultz,
A.K. (1982f) Twelve-week oral dietary range-finding study in mice
given abamectin (MK 0936). Study No. TT82-082-0,-2,-2. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Lankas, G.R., Nickell, B.E., Buck, J.F., & Jackson, L.
(1982g) Eight-week dietary range-finding study in rats given
abamectin (MK 0936). Study No. TT 82-075-0, -1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Skolnick, E.M. (1982h) One-hundred-
five week dietary carcinogenicity and toxicity study of abamectin
(MK 0936) in rats with a 53-week interim necropsy. Study No. TT 82-
099-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. Allen, H.L., Nickell, B.E., Satiritz, S.M., Powzaniuk,
W., Roux, L., & McKeon, J. (1982i) Fifty-three week dietary study in
dogs given abamectin (MK 0936). Study No. TT 82-104-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Weller,
J.V. (1982j) Oral range-finding study in pregnant rats given
abamectin (MK 0936). Study Number TT 82-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982k) Oral teratology study in rats given abamectin
(MK 0936). Study No. TT 82-705-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982l) Oral range finding study in pregnant rabbits
given abamectin (MK 0936). Study No. TT 82-706-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Vetter,
C.M. (1982m) Oral teratology study in rabbits given abamectin (MK
0936). Study No. TT 82-706-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Mildred, S.C., & Hoberman, H.M. (1982n) Reproductive
effects of abamectin (MK 0936) administered orally by gavage to Crl:
COBS CD(SD)BR rats for two Generations. Study No. TT 82-9010.
Unpublished report prepared by Argus Research Laboratories, Horsham,
Pennsylvania, USA and by Merck Sharp & Dohme Research Laboratories,
West Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three
Bridges, NJ, USA.
Gordon, L.R., Mandel, J., McDonald, J.S., Nickell, B.E., Powzaniuk,
W., & Bielinski, T.C. (1983a) Guinea pig skin maximization test with
abamectin (MK 0936). Study No. TT 83-2506. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Lankas, G.R., Fabry, A., Nickell, B.E., Powzaniuk, W.,
Buck, J.F., Satiritz, S.M., & Schultz, A. (1983b) Ninety-four week
dietary carcinogenicity and toxicity study in mice given abamectin
(MK 0936). Study No. TT 83-002-0,-1,-2,-3. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon L.R., Minsker, D.H., Nickell, B.E., Collevechio, K., &
Battisti, G.A. (1983c) Ten-day dietary maternotoxicity study in mice
given abamectin (MK 0936). Study No. TT 83-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Nickell, B.E., Mandel, J., Powzaniuk,
W., & Stolz, W.W. (1983d) Acute dermal toxicity study in rabbits
given abamectin (MK 0936). Study No. TT 83-064-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Allen, H.L., Nickell, B.E., Mandel,
J., Powzaniuk, W., & McAfee, J.L. (1984a) Acute oral toxicity study
in mice given Avermectin B1b. Study No. TT 84-107-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1984b)
Five-day acute oral toxicity study in pregnant and non-pregnant cf1
mice with abamectin (MK 0936). Study No. TT 84-2842-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Mandel, J., Majka, J.A., & McAfee, J.L. (1984c) Acute
oral toxicity study in mice given the Delta-8,9-isomer of abamectin
(MK 0936). Study No. 84-112-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., & Landis, D.K. (1984d) Oral maternotoxicity study in mice with
the Delta-8,9 isomer of abamectin (avermectin B1). Study No. 84-
722-0,-1. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. (1984e) Acute oral toxicity study in mice with Delta-
8,9-isomer of abamectin (MK 0936). Study No. TT 84-2820. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1984f) Exploratory non-
specific antidote study of abamectin (MK 0936) intoxication in dogs.
Study No. 84-085-0. Unpublished report prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1985a)
Five-day acute oral toxicity study in pregnant and nonpregnant CF1
mice with abamectin (MK 0936). Study No. TT 85-2593. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J., & Rafferty, C.E. (1985b) Oral toxicity and plasma level study in
monkeys with Ivermectin (MK 0933) and abamectin (MK 0936). Study No.
T 85-013-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Landis,
D.K. (1985c) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. TT 85-710-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Geiger,
J.E. (1985d) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. 85-710-1. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., Powzaniuk, W., & Landis, D.K. (1985e) Oral maternotoxicity study
in mice with Avermectin B1b. Study No. 84-721-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Sina, J., Patterson, S.K., Berglind, R.M., Prato, M.,
& Quillin, F. (1985f) Microbial mutagenesis assays with abamectin
(avermectin B1; MK 0936). Study No. TT 85-8005 and 85-8051
Unpublished reports prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Galloway, S., Patterson, S.K., Armstrong, M., Bean,
Ch.L., & Deasy, D. (1985g) Abamectin (MK 0936) assay for chromosomal
aberrations in vitro, in chinese hamster ovary cells Study No. TT
85-8631, 85-8632, 85-8635. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Sina, J.F., Wright, S.P., Prato, M., & Quillin, F.
(1987a) Microbial mutagenesis assays of the Delta-8,9-isomer and
polar degradates of abamectin (Avermectin B1; MK 0936). Study Nos.
TT 87-8046, 87-8047, 87-8058. Unpublished reports prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Wise,L.D., Jensen, R.D., Nickell, B.E., Collevechio,
K., & Vetter, C.H. (1987b) Oral developmental toxicity study in rat
given the Delta-8,9-Isomer of abamectin (avermectin B1). Study No.
TT 87-715-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Minsker, D.H., Anderson, C.A., Nickell, B.E.,
Collevechio, K. & Deyerle-Brooks, K.A. (1987c) Oral developmental
toxicity study in mice given the polar degradates of abamectin (MK
0936). Study No. TT 87-717-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon L.R., Wise, L.D., Vonderfecht, St.L., Nickell, B.E.,
Collevechio, K., Powzaniuk, W., & McMahon, M.G. (1987d) Single
generation study in rats with the Delta-8,9-isomer of abamectin
(avermectin B1). Study No. 87-716-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Wise, L.E., Allen, M.L., Nickell, B.E.,
Collovechio,K., Powzaniuk, W., & Sina, J.L. (1988) Oral
developmental toxicity study in mice with L-930,463 (polar
degradate). Study No. TT 88-713-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Greene, B.M., Brown, K.R., & Taylor, H.R. (1989) Use of Ivermectin
in humans, In: Ivermectin and Abamectin, ed. by Campbell, W.C.,
Springerverlag, Chapter 21, p. 311-323. Submitted to WHO by MSDRL,
Three Bridges, NJ, USA.
Gruber, V.I. (1988) Identification of ß-alpha-hydroxy-avermectin
B1a as a metabolite of avermectin B1a in rats. Unpublished
report of Merck sharp and Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Mandel, J. (1977) Acute oral toxicity studies in mice and rats with
avermectin B1a (C-076(B1a)). Study Nos. TT 77-3248, 77-3250, 77-
3264, 77-3787, 77-3789, 77-3788, 77-3337 and 77-3346. Unpublished
reports prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986a) The metabolism
of Delta-8,9-Z-isomer avermectin B1a in rats. Unpublished report
ARM-2, prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986b) The metabolism
of avermectin B1a in rats. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
MSDRL (1977a) Oral reproduction study in rats with avermectin B1a
(C-076(B1a)). Study Number 77-706-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
MSDRL (1977b) Oral reproduction study in rats. Study Number 77-712-0
with avermectin B1a (C-076(B1a)). Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Norbury K.C. & Wolf, G.L. (1977) Fourteen-week oral toxicity study
in rats following in utero exposure to avermectin B1a (C-
076(B1a)). Study number TT 77-043-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. & Allen, H.L. (1976) Eighteen-week oral toxicity
study in dogs with avermectin B1a (C-076(B1a)). Study No. TT 76-
073-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. (1977a) Oral teratology study in mice with
avermectin B1a (C-076(B1a)). Study No. TT 77-705-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T. (1977b) Ten-day oral toxicity study in pregnant mice
with avermectin B1a (C-076(B1a)). Study No. TT 77-717-1.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel., J., Everett, M.A., Wolf,
G.L., & Powzaniuk, W., (1981a) Acute oral toxicity study in rats
with abamectin (avermectin B1). Study No. TT 81-2937. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Wolf, G.L., Stolz,
W.W., & Bielinski, T.C. (1981b) Acute dermal toxicity study in
rabbits with abamectin (avermectin B1). Study No. TT 81-3021.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981c) Acute ocular irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2940. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981d) Primary dermal irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2941, 81-2943, 81-
2945. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Skeggs, H. (1976) Microbial mutagenesis assay with avermectin B1a
(C-076(B1a)). Study No. TT 76-8052. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
WHO (1990) Evaluation of certain veterinary drug residues in food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 799, Geneva.
WHO (1993) Toxicological evaluation of certain veterinary drug
residues in food. WHO Food Additive Series No. 31, Geneva.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
A study was carried out to evaluate the tissue distribution and
elimination of 3H- and 14C-labelled avermectin B1a in a sesame
oil vehicle after oral administration to male and female rats.
Sixty-two CRCD strain rats/sex were divided into groups and dosed as
follows: a single high dose (1.4 mg/kg bw); a single low dose (0.14
mg/kg bw); fourteen daily low doses (0.14 mg/kg bw/day) of
unlabelled avermectin B1a, followed by a single low dose of
tritium-labelled avermectin B1a; a single high dose (1.4 mg/kg bw)
of a mixture of tritium - and 14C-labelled avermectin B1a; a
single dose of vehicle only to serve as controls for groups with
single dosing regimen or fifteen daily doses of vehicle only to
serve as controls for the multiple dosing experiment. The control
groups were sacrificed seven days after the last (or single) dose,
while rats from the treated groups were sacrificed on days 1, 2, 4
and 7 after dosing. Urine and faeces were collected each day after
dosing while the organs were collected from all rats at time of
sacrifice. All samples were assayed for total radioactivity.
Elimination in the urine was 0.3-0.6% of the applied dose in females
and 0.8-1.1% in males over the 7 days collection period. Elimination
in faeces ranged from 69-77% and 70-82% in females and males,
respectively. Residue levels in liver ranged from about 0.001-0.02
ppm, in kidney from 0.003-0.06 ppm, in muscle from 0.001-0.02 ppm
and in fat from 0.008-0.01 ppm in the different dosing groups, 7
days post-dose. The total residue level in the organs of the females
were generally higher than in males. The residue levels were dose
dependent, the residues in the high dose groups being roughly ten-
fold greater than in the low dose groups, whereas the depletion
rates were similar among the different tissues. The total
radioactive residues depleted from the tissues with half-lives of
approximately 1.2 days indicating that the residues did not persist.
Repeated dosing did not influence elimination rates or residue
levels. The tritiated avermectin B1a residue levels and depletion
rates were comparable to the 14C residue levels. Thus the tritium
label at the 5-position of avermectin B1a was not labile during
the course of the study. The stability of the label was also
demonstrated in an experiment for volatile/exchangeable tritium,
demonstrating that less than 2% of the tritiated avermectin B1a
sample was volatile (Alvaro et al., 1984).
Selected tissue samples (liver, kidney, muscle, fat) were
analyzed for unchanged avermectin B1a and metabolites. Two
metabolites that were also formed in vitro after rat liver
microsomal incubation, in addition to unchanged avermectin B1a,
accounted for most of the residues: The metabolites were identified
as 24-hydroxymethyl-avermectin B1a (24-OHMe-B1a) and 3"-desmethyl
avermectin B1a (3"-DM-B1a) (Maynard 1986b). A minor metabolite
was identified as ß-alpha-hydroxy-avermectin B1a (Gruber, 1988).
Toxicological studies
Acute toxicity studies
The main clinical signs were ataxia and tremors found in all
species investigated and irrespective of the route of
administration. The data are summarized in Tables 1 and 2.
Table 1: Acute toxicity of abamectin
Species Sex Route LD50 Component Reference
(mg/kg bw) (purity)
Mouse F oral 13.6-23.8 B1a Mandel (1977)
19.8 B1b (98.4%) Gordon et al. (1984a)
5-day mortality:
pregnant: 11.8-19.0 B1a+B1b (94%) Gordon et al. (1984b)
non pregnant: 15.0-41.3 B1a+B1b (94%) Gordon et al. (1985a)
Rat F,M oral ca. 11 B1a Mandel, 1977
F,M oral 8.7-12.8 B1a+B1b (91%) Robertson et al. (1981a)
Dog M,F oral ca. 8 B1a Robertson and Allen (1976)
B1a+B1b Gordon et al. (1984f)
Monkey M,F oral > 24 B1a+B1b Gordon et al. (1985b)
Rat dermal >330 B1a/B1b (87%/9.4%) Gordon & Mandel (1978)
Rabbit dermal >1600 B1a+B1b (91.4%) Robertson et al. (1981b)
B1a + B1b (94%) Gordon et al. (1983d)
Delta-8,9-isomer (photolytic degradation product)
Mouse M,F oral > 80 (Gordon et al. 1984c
& 1984e)
Table 2: Dermal and ocular irritation studies with abamectin,
technical material
Species Sex Target Findings Reference
Organ
Rabbit M,F eye very slight Robertson et al. (1981c)
irritation
Rabbit M,F skin non irritating on Robertson et al. (1981d)
intact or abraded
skin
Guinea- M,F skin: negative Gordon et al. (1983a)
pig sensitization
Short-term toxicity studies
Mice
In a twelve-week dietary range-finding study groups of mice
(CD-1 strain;15/sex/group) were fed dietary concentrations of 0,
2/40 (increase in week 9), 5, 10 or 20 ppm abamectin over 12 weeks
and 40 or 60 ppm over 3 weeks. There were no physical signs or
mortality. Decrease in body-weight gain at 60 ppm was the only
change observed. The NOAEL in this study was 40 ppm, equal to 8
mg/kg bw/day (Gordon et al., 1982f).
Rats
In an eight-week oral range-finding study in rats abamectin
(purity 94%) was administered to groups of rats (Charles River CD
albino rats, 10/sex/group) at dietary concentrations of 0, 5, 10,
15, 20/25 (increase in week 7), 40 or 60 ppm. Due to mortality and
the appearance of severe clinical signs of toxicity (tremors,
decreased activity) in the first study week, remaining animals at 40
and 60 ppm were sacrificed on days 15 and 5, respectively. These
groups were replaced by groups at 10 and 15 ppm in study week 5.
Decreases in mean body-weight gain were seen at 15, 20/25 (3 and 5%
decrease in males and females, respectively) and 40 ppm. Tremors
occurring at 15 and 20/25 ppm were no longer evident after study
week 1. One animal at 5 ppm showed slight tremors on day 2 only. No
gross or microscopic examination was performed. Based on clinical
signs and body-weight effects it appears that there is a steep dose-
response curve. Therefore the doses recommended for the
carcinogenicity study were 0.75, 1.5 and 2.0 mg/kg bw/day (Gordon
et al., 1982g).
Dogs
In a twelve-week oral range-finding study abamectin (purity
94%) was fed to groups of beagle dogs (2/sex/dose) at doses of 0,
0.25, 0.5, 1 or 4/2 mg/kg bw/day. Because of signs of toxicity
(tremors, weakness, incoordination, disorientation) and markedly
decreased food consumption at 4 mg/kg bw/day in one dog, this dose
was decreased to 2 mg/kg bw in study week 4. Inability to constrict
pupils after light stimulus was observed in some dogs at 1 mg/kg
bw/day or higher doses. Depression of body-weight gain or weight
loss occurred in dogs at 2 mg/kg bw/day and higher. No gross or
microscopic examination was performed. Based on the clinical signs
and body-weight effects recommended doses for the one-year oral dog
study were 0.25, 0.5 and 1 mg/kg/ bw/day. The NOAEL in this study
was 0.5 mg/kg bw/day (Gordon et al., 1982e).
Avermectin B1a (purity not specified) was orally administered
by gavage to beagle dogs (15/sex/dose) at dose levels of 0, 0.25,
0.5, 2 or 8 mg/kg bw/day over a period of 18 weeks. The treatment
did not affect parameters of urinalyses, ophthalmologic examination
or organ weights. Deaths occurred at incidences of 1/30, 3/30 and
3/30 at 0.5, 2 and 8 mg/kg bw/day, respectively. Signs of toxicity
at levels of > 0.5 mg/kg bw/day consisted of whole body muscular
tremors, ataxia, mydriasis and ptyalism (hypersalivation); in
addition, tonic convulsions and emesis occurred at 2 and 8 mg/kg
bw/day. Reduced body-weight gain was only observed at dose levels of
0.5 and 2 mg/kg bw/day, but not in surviving dogs at 8 mg/kg bw/day.
At 8 mg/kg bw/day changes in haematologic and serum biochemical
parameters included slightly increased values of haemoglobin,
haematocrit, erythrocytes, nonsegmented neutrophils and glucose
level. Electrocardiographic changes (elongation of the QT interval
and bradycardia) were noted at 8 mg/kg bw/day only at the beginning
of the study. Histopathologic changes were seen only in animals that
died or were killed moribund and consisted of diffuse hepato-
cellular vacuolation (without lipid accumulation) at dose levels of
0.5, 2 and 8 mg/kg bw/day and edema of the gallbladder at 2 and 8
mg/kg bw/day. The NOAEL in this study was 0.25 mg/kg bw/day
(Robertson & Allen 1976).
Groups of beagle dogs (6/sex/dose) were fed abamectin (purity
> 89%) for 53 weeks at concentrations resulting in doses of 0,
0.25, 0.5 or 1 mg/kg bw/day. The doses were selected on the basis of
the results of a previous range-finding study (Gordon et al.,
1982e). The compound administration had no effect on
ophthalmological examination, urinalyses or organ weights. Decreased
or even absence of constriction of pupils to light was evident at
0.5 mg/kg bw/day (occurrence rate 3%) and 1 mg/kg bw/day (15%) and
as single instances and in single animals also at 0.25 mg/kg bw/day.
At 1 mg/kg bw/day one dog was found dead (week 38), two others were
killed moribund (weeks 33 and 38). Reduced body-weight gain most
probably due to food unpalatability and therefore reduced food
consumption was observed at the highest dose level. A slight
decrease in serum urea nitrogen at 1 mg/kg bw/day was most probable
a consequence of decreased protein intake at this dose level. Slight
leukocytosis and increased packed cell volume was noted in one dog
killed in poor condition. There were no gross or microscopic changes
that could be attributed to treatment. The NOAEL in this study was
< 0.25 mg/kg bw/day, the single instance of mydriasis at this
dose being considered a borderline effect (Gordon et al., 1982i).
Long-term toxicity carcinogenicity studies
Mice
Abamectin (purity 90%) was administered in the diet to groups
of mice (Crl:CD-1(ICR)BR;74/sex/group; 24/sex/group for interim
sacrifice) at concen-trations resulting in doses of 0, 2, 4 or 8
mg/kg bw/day over a period of 94 weeks. In females, treatment-
related tremors were observed in all dose groups and deaths occurred
at 4 and 8 mg/kg bw/day. These effects were not observed in the 12-
week range-finding study at dosage levels up to 11 mg/kg bw/day (60
ppm). No explanation could be found for the sensitivity of these
female mice. Plasma samples taken from affected and unaffected
animals revealed no significant differences. A subsequent batch of
females did not show this unexpected sensitivity and following
restart of the study with a new group of female mice, tremors
occurred only in single animals at 8 mg/kg bw/day. In males, tremors
occurred in single moribund animals of the control and 8 mg/kg
bw/day group. In this group the mortality rate was increased:
towards the end of the study the mortality reached 60% compared to
45% in controls. Body-weight gain was reduced about 21% in females
and 7% in males at 8 mg/kg bw/day. There was a dose-related increase
in food consumption in treated females but a decrease in food
efficiency at 8 mg/kg bw/day. The treatment had no effect on
ophthalmic examinations, haematologic or biochemical parameters,
organ weights, gross pathology, histopathologic changes (with the
exception of a higher incidence of dermatitis at 2 and 8 mg/kg
bw/day in males). The tumour incidence was not increased by the
treatment. The NOAEL in this study was 4 mg/kg bw/day (Gordon et
al., 1983b).
Rats
Abamectin (purity 91%) was fed to groups of rats (Crl:CD(SD)Br;
65/sex/group) at dietary concentrations resulting in doses of 0,
0.75, 1.5 or 2/2.5/2 mg/kg bw/day over two years. Since no effects
attributable to abamectin were observed through study week 10, the
high dose level was increased to 2.5 mg/kg/ bw/day in week 11.
Because of the appearance of severe signs of CNS toxicity, the dose
was decreased again to 2 mg/kg bw/day in study week 13 for the
remainder of the study. No treatment-related changes with respect to
ophthalmoscopic, haematologic or serum biochemical parameters,
urinalyses and organ weights were observed. Increases in body-weight
gain were seen in all dosage groups during the first year on study.
By the end of the study comparable mean body-weight gains for all
groups of females were found, but an increased gain was noticed in
males. The increases in body-weight gain are considered treatment-
related, but the effect is not considered as an adverse effect.
Clinical signs consisting of tremors appeared in study week 12
correlated with the increase in dosage from 2 to 2.5 mg/kg bw/day in
week 11. The tremors in all the affected animals persisted
intermittently until sacrifice despite reduction in the high-dose
level back to 2 mg/kg bw/day in week 13. Several high-dose group
animals, which exhibited tremors, were sacrificed in a moribund
condition. The increase in mortality reflects the induction of
tremors during the early stage of the study and subsequent sacrifice
of the affected animals. Following the reduction in dose to 2
mg/kg/bw/day no new cases of tremors occurred and mortality in all
treated and control groups was comparable. No increase in tumour
incidence or treatment-related non-neoplastic histopathologic
changes were observed. The NOAEL in this study was 1.5 mg/kg bw/day
(Gordon et al., 1982h).
Reproduction studies
Mice
In a ten-day dietary maternotoxicity study abamectin (purity >
88%) was fed at dietary concentrations resulting in target dose
levels of 0, 0.1, 0.3 or 0.6 mg/kg bw/day (actual doses = 0.06,
0.16, or 0.33 mg/kg bw/day) to groups of pregnant mice (albino CF1;
20 females/group) on days 6 through 15 of gestation. Marked tremors
at 0.33 and at 0.16 mg/kg bw/day were observed. Reproductive
parameters (numbers of implants, resorptions and live and dead
fetuses) were not influenced. The NOAEL in this study was 0.06 mg/kg
bw/day (Gordon et al., 1983c).
Rats
One hundred-and-fifty F1 offspring, from five groups of F0
female rats (Charles River CD), that had been exposed in utero to
avermectin B1a at dosage levels of 0 (two groups), 0.1, 0.2 or 0.4
mg/kg bw/day were selected for a fourteen-week study of oral
toxicity. Groups of weanling rats (15/sex/group) received avermectin
B1a at doses of 0, 0.1, 0.2 or 0.4 mg/kg bw/day by gavage. The
treatment did not have any effects on mortality, ocular changes,
haematology, serum biochemistry, organ weights or gross and
microscopic tissue alterations. The increased body-weight gain of
male rats at 0.4 mg/kg bw/day was considered as treatment-related
but not as an adverse effect. The NOAEL in this study was 0.4 mg/kg
bw/day (Norbury and Wolf, 1977).
Avermectin (B1a) was administered orally to three groups of
female rats (12 females/dose group) at dose levels of 0, 0.5, 1, or
2/1.5 mg/kg bw/day from 14 days before mating throughout gestation
and lactation until day 21 postpartum. The high dose was reduced
after five doses to 1.5 mg/kg bw/day due to whole body muscular
tremors at 2 mg/kg bw/day. Two deaths occurred at the high dose, one
animal at this dose level became moribund. The significant decrease
in body-weight gain in the 1.5 mg/kg bw/day group was a result of
weight loss of the 3 moribund or dead animals. Weight gain increases
were observed during some periods at 1 and 1.5 mg/kg bw/day. A
treatment-related decrease in the number of live pups per litter on
day 1 postpartum at 1.5 mg/kg bw was observed. The pup weights were
decreased at this dose level on day 1. Pups at all dosage levels
showed decreases in average weight per litter throughout the study.
Dose-related increase in mortality among pups at all dose levels
were observed resulting in survival rates of 0, 14% and 76%, in the
1.5, 1 and 0.5 mg/kg bw/day groups respectively, compared to 98% in
the control group. There was a developmental retardation (eye
opening) at 0.5 and 1 mg/kg bw/day in surviving pups. The NOAELs in
this study were < 0.5 mg/kg bw/day for embryo-fetotoxicity and 1
mg/kg bw/day for maternotoxicity (MSDRL, 1977a).
Avermectin (B1a) was administered orally to three groups of
15 female rats at dose levels of 0, 0.1, 0.2 or 0.4 mg/kg bw/day
from 14 days before mating, throughout mating, gestation and
lactation until day 21 postpartum. No maternotoxic effects were
observed and the reproduction status was not adversely affected.
Among pups at 0.2 and 0.4 mg/kg bw/day a dose-related incidence of
spastic movements was noted increasing in severity with increasing
dose. At 0.2 and 0.4 mg/kg bw/day reduction in average pup weight
was observed (dose-related). Developmental retardation (eye opening,
ear opening, hear growth), occurred at 0.2 and 0.4 mg/kg bw/day
(dose-related). The NOAEL in this study was 0.1 mg/kg bw/day for
embryo-fetotoxicity (MSDRL, 1977b).
In an oral range-finding study (multigeneration) abamectin was
administered to groups of rats (Crl: CD (SD) BR Sprague-Dawley; 12
females/group) in the drinking water at concentrations of 0, 0.15,
0.5, 1.5 or 5 mg/l. Treatment was conducted from 14 days prior to
cohabitation with untreated males through day 21 postpartum and
subsequently through the F1 generation. Water consumption in
groups 0.15-1.5 mg/l increased during the lactation period and
decreased slightly in the 5 mg/l group. No accurate measurement of
water consumption could be performed prior to transfer to delivery
boxes on day 17 of gestation. Thereafter mean levels of water
consumption were comparable in all groups resulting in dose ranges
of 0.017-0.037, 0.066-0.127, 0.192-0.396, or 0.556-0.685 mg/kg
bw/day for the 0.15, 0.5, 1.5 mg/l or 5 mg/l group, respectively. In
the F0 generation the only effect observed was an increase in
body-weight gain in the 5 mg/l group in the first study week. In the
F1 generation an increase in postnatal mortality at 5 mg/l (53%
compared to 1% in control) was observed, physical signs observed in
pups at the highest dose level consisted of tremors; pup weights in
the 5 mg/l group were decreased. 3/116 Fetuses (2.6%) (from 2
litters) in the 5 mg/l group showed single malformations (1 cleft
palate, 1 sternebral malformation, 1 sternebral variation). One
fetus in the 0.5 mg/l group had lumbar ribs. Because of the single
incidences in the study groups and because of the occurrence of
these alterations in historical control groups it is questionable if
these effects are treatment-related (Gordon et al., 1981).
In a two-generation study, groups of rats
(Crl:COBSTMCDTM(SD) BR/30/sex/group) were orally treated
(gavage) with dosages of 0, 0.05, 0.12 or 0.4 mg abamectin/kg bw/day
. F0 and F1b rats were mated to produce F1a and F1b litters,
F2a and F2b litters. At 0.12 and 0.4 mg/kg bw/day both sexes of
the F0 rats during the first gestation period (F0-F1a) showed
increased body-weight gains, whereas during the subsequent lactation
period (F0-F1a) a reduction in body-weight gain compared to
control was observed at these two dose levels particularly on the
first days of lactation. Similar changes were also observed in the
next generation. During the second mating period of F0 rats (to
produce the F1b litters) mating performance was reduced (reduction
of incidence of mating) at 0.4 mg/kg bw/day as compared to control
animals. The reduction in mating performance was presumed to be
associated with irregular estrous cycles observed during the second
mating period of F0 female rats. This effect was not observed in
matings resulting in the F1a litters or the F2a and F2b
litters. During the lactation periods of both generations effects in
the 0.4 mg/kg bw/day group consisted of increased pup mortality,
reduced viability and lactation indices and lower average pup body-
weights. The incidence of pups which appeared thin and weak was
increased. These effects were less severe in the F2 litters than
in the F1 litters. No skeletal anomalies associated with the test
agent were revealed. Histopathological examination showed retinal
anomalies in 3/4 males and 1/5 females in the high-dose group
compared wich 1/10 in the control group (F1b weanlings). The
anomalies consisted of single or multiple retinal folds of many
layers that included pigment epithelium. The same lesion was seen in
one control female and in one male in the 0.05 and 0.12 mg/kg bw/day
groups of the F1b weanlings. In the F1b parental animals (both
sexes) only 1/35 showed this retinal anomaly. In the F2b weanlings
histopathology was performed on a greater number of animals. There
was a significant increase in retinal anomalies in the high-dose
group with the following incidences (M + F): 4.6% and 22% in the
control and 0.4 mg/kg bw/day, respectively. As will become evident
from the results of a reproduction study in rats conducted with
comparable doses of the delta-8,9-isomer of abamectin the control
incidence of retinal anomalies (4.6%) in the present study is much
smaller than in the other study, where a control incidence of 20%
was found. Therefore the difference observed may be incidental
following an unusually low incidence of this lesion in the
concurrent control group of the present study.
Among both the F1b and F2b weanlings the high-dose group
pups were the smallest. One hypothesis to explain the presence of
retinal folds in the high-dose group weanlings and the disappearance
with maturity proposed by the study authors is that the ocular globe
size is relatively smaller in the small pups (where there was
pronounced pup toxicity) relative to the size of the retina which
perhaps carried on at its normal growth rate. Therefore it may be
assumed that the retinal effects observed are a secondary effect.
Reversibility of the retinal anomalies is evidenced by its absence
in the high dose adults. The NOAELs in this study were 0.12 mg/kg
bw/day for pup toxicity and retinal anomalies and 0.05 mg/kg bw/day
for maternotoxicity (Gordon et al., 1982n).
Special studies on embryotoxicity/teratogenicity
Mice
Avermectin B1a was administered orally (gavage) to groups of
mice (albino CF1 strain/20 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.1, 0.2, 0.4 or 0.8 mg/kg bw/day.
The doses of the B1a component were calculated as the parent
compound.
Deaths occurred in the 0.1, 0.4 and 0.8 mg/kg bw/day groups (1,
3 and 2 animals respectively), in most cases preceded by tremors.
Body-weight gain was not influenced by the treatment; neither was
the reproductive status (number of implants, resorptions, live and
dead fetuses per litter). An increased incidence of cleft palate at
0.4 (4/165; 2.4%) and 0.8 mg/kg bw/day (5/199; 2.5%) was observed
compared to a control incidence of 0.4%. The NOAELs in this study
were < 0.1 mg/kg bw/day for maternotoxicity and 0.2 mg/kg bw/day
for embryo-fetotoxicity and teratogenicity (Robertson 1977a).
Groups of mice (albino CF1 strain/20 female/group) were
administered avermectin B1a by gavage from day 6 through 15 of
gestation at dose levels of 0, 0.025, 0.05, 0.075 or 0.1 mg/kg
bw/day. One animal died at 0.1 mg/kg bw/day and tremors were
observed in several additional mice at this dose. One animal at
0.075 mg/kg bw/day exhibited muscular tremors and became moribund.
Maternal body-weight gains were unaffected at any dosage level. The
NOAEL in this study was 0.05 mg/kg bw/day for maternotoxicity
(Robertson, 1977b).
In an oral maternotoxicity study the minor component of
abamectin, avermectin B1b was administered by gavage to groups of
mice (Crl:CF1 BR; 12 females/dose) at dose levels of 0, 0.025, 0.05,
0.075 or 0.1 mg/kg bw/day on days 6 through 15 of gestation. At the
0.075 mg/kg bw/day dose level two deaths occurred preceded by weight
loss and tremors. Single fetuses at all dose levels showed
malformations consisting of exencephaly and cleft palates resulting
in incidences of about 1% in all dose groups. The highest incidences
for historical control groups are 1.6% for exencephaly and 1.3 % for
cleft palates (overall incidence 0.3% for exencephaly and cleft
palate each). There was no evidence of fetotoxicity. The NOAEL in
this study was 0.05 mg/kg bw/day for maternotoxicity (Gordon et
al., 1985e).
Rats
In a range-finding study groups of mated female rats (Charles
River; 10/group) were administered abamectin by gavage at dose
levels of 0, 0.25, 0.5, 1 or 2 mg/kg bw/day on gestation days 6
through 17. One female at 2 mg/kg bw/day exhibited weight loss and
tremors and was sacrificed after receiving 12 doses. There was no
other evidence of maternal toxicity. At 0.25 and 2 mg/kg bw/day
there was a slightly increased body-weight gain that is considered
to be treatment-related but not an adverse effect. The NOAEL in this
study was 1 mg/kg bw/day (Gordon et al., 1982j).
In a rat teratology study groups of mated rats (Charles River;
25 females/group) were orally treated (gavage) with abamectin
(purity 94%) at dosage levels of 0, 0.4, 0.8 or 1.6 mg/kg bw/day on
days 6 through 19 of gestation. There was no evidence of maternal
toxicity. Slightly increased maternal body-weight gain was seen at
all dosage levels between days 6 and 14 of gestation. An increased
incidence of fetuses with external fetal malformations was observed
(exencephaly, cleft palate, gastroschisis) at 0.8 and 1.6 mg/kg
bw/day. The incidences were: 2/279 (0.7%) and 2/326 (0.6%),
respectively, compared to 1/319 (0.3%) in the control group.
Historical control incidence of cleft palate in this rat strain is
0.03% (overall) or 0.3% (single study with highest incidence).
Gastroschisis alone has an overall incidence of 0.004% or the
highest incidence in a single study of 0.3%. Based on the fact, that
in historical controls one single malformation (cleft palate) may
occur in up to 0.3% of control animals and no dose-response-
relationship is evident, it may be assumed that the increase
observed may either not be treatment-related or may be a borderline
effect. Visceral examination indicated a higher incidence of
distended ureters in the treated groups up to 3% compared to 0% in
controls), but without any dose-effect relationship. This effect is
observed at incidences of up to 7% in historical control groups.
Skeletal examination revealed a higher incidence of fetuses with
lumbar ribs and count variations at 1.6 mg/kg bw/day: at 1.6 mg/kg
bw/day an incidence of 72/326 (22%) was found, compared to 41/320
(13%) at 0.4, and 45/279 (16%) at 0.8 mg/kg bw/day compared to
44/319 (14%) in the control group. The increase at 1.6 mg/kg bw/day
may not be treatment-related because higher incidences are observed
in historical controls. For example, in historical controls lumbar
ribs occurred at an incidence of 14% (overall) or 27.8% (single
study with highest incidence). The NOAELs in this study were 1.6
mg/kg bw/day for maternotoxicity and < 1.6 mg/kg bw/day for
fetotoxicity and teratogenicity (Gordon et al., 1982k).
Rabbit
In a range-finding study groups of New Zeeland albino rabbits
(10 females/dose) were given abamectin at oral dosage levels of 0,
0.5, 1, 2 or 3 mg/kg bw/day on gestational days 6 through 18.
Maternotoxicity was observed at 3 mg/kg bw/day. One female at 3
mg/kg bw/day was sacrificed moribund on day 16 of gestation after 11
doses. All females at 3 mg/kg bw/day were in a stupor after the
fourth and subsequent doses and showed yellow or green discharge
from nose or mouth. Moreover animals at 3 mg/kg bw/day had a
decreased food and water consumption, and showed body-weight loss.
The NOAEL in this study was 2 mg/kg bw/day (Gordon et al., 1982).
In a teratogenicity study groups of female New Zeeland albino
rabbits (18/dose) were given abamectin (purity 94%) at oral dosage
levels of 0, 0.5, 1 or 2 mg/kg bw/day on day 6 through 27 of
gestation. There were single deaths in all the treatment groups. At
2 mg/kg bw/day the animals showed decreased food and water
consumption and weight loss. Fetuses of the 2 mg/kg bw/day showed
cleft palates, omphaloceles and clubbed forefeet at higher
incidences than in the concurrent control group: 7.4% versus 2.1% in
concurrent control. The incidence of clubbed fore foot alone in
historical control groups is 0.2% (overall) and 3.9%, respectively,
in a single study with highest incidence. No data are available
concerning historical control incidences of cleft palates and
omphaloceles in rabbits. The clearly higher incidence of these
malformations at 2 mg/kg bw/day is therefore considered to be
treatment-related. With respect to skeletal fetal examination higher
incidences of skeletal terata (vertebral malformations, branched and
fused ribs) were observed at 0.5, 1 and 2 mg/kg bw/day with
incidences of 4%, 2% and 4% of the fetuses showing these
malformations respectively, compared to 0% in the concurrent control
group. In historical controls single vertebral malformations may
occur at incidences of up to 0.5% (overall) (e.g., caudal vertebral
malformations) with the highest value of 9% in a single study (e.g.,
fused ribs). At 0.5 and 2 mg/kg bw/day there were increased
incidences of incompletely ossified sites particularly in sternebrae
and metacarpals. These effects were more pronounced in the highest
dose group. Because the incidences observed at 1 mg/kg bw/day were
comparable to the incidences in the control group the absence of a
dose-relationship in the lower dose levels of 0.5 and 1 mg/kg bw/day
indicates that the increased incidence at 0.5 mg/kg bw/day is
possibly not treatment-related. The NOAEL in this study was 1 mg/kg
bw/day for maternotoxicity, embryo-fetotoxicity, and teratogenicity
(Gordon et al., 1982m).
Special studies on genotoxicity
A number of genotoxicity studies have been conducted with
abamectin. The results are summarized in Table 3.
Special studies on the delta-8,9-isomer of avermectin B
Biochemical aspects
Rat
Male rats were orally (gavage) dosed with 1.4 mg3H-labelled
delta-8,9-isomer avermectin B1a/kg bw. The delta-8,9-isomer of
abamectin is a photolytic degradation product of abamectin. The
stability of the tritium label at the 5th carbon position was
previously demonstrated with avermectin B1a in rats. Within 7 days
after dosing 94% of the administered dose was eliminated with the
faeces, and 0.4% in the urine. The levels of residues in liver,
kidney, fat and muscle ranged from 0.28 ppm (muscle) to 1.4 ppm
(fat) on day 1 after dose and decreased to a range of 0.017 to 0.1,
respectively, on day 7 after dosing. The half-lives for the tissue
residues in muscle, kidney, liver and fat varied between 1.45 and
1.64 days. As major metabolite the 3"-desmethyl-delta-8,9 isomer was
isolated. The 3"-DM-delta-8,9 isomer was also isolated and
identified after rat liver microsomal incubation of the delta-8,9-
isomer. The minor metabolite 24-hydroxymethyl-Delta-8,9-isomer,
(also formed in vitro), was identified.
By comparison, the elimination by rats of both the delta-8,9-
isomer avermectin B1a in this study and the avermectin B1a in a
previous study was very similar. In both studies 94-96% of the
administered dose were recovered within 7 days after dosing in the
excreta. The tissue residue levels were also demonstrated to be
similar for both compounds. The average half-life for the residue in
the edible tissues was 1.1 and 1.5 days for avermectin B1a and
delta-8,9-isomer, respectively. The metabolites generated were very
similar with rats metabolizing both compounds to the 3"-desmethyl
and the 24-hydroxymethyl metabolites. Similarly, both avermectin
B1a and ivermectin B1 (22,23-dihydro-avermectin B1) have been
shown to be primarily metabolized to the 3"-desmethyl and/or 24-
hydroxymethyl metabolite(s) by many other animal species (Maynard
et al., 1986a).
Table 3. Results of genotoxicity assays on abamectin
Test System Test Object Concentration Results Reference
(purity)
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1982a)
typhimurium (precipitate at conc.
> 3000 µg/plate)
± activation
(94%)
Ames test Salmonella 1-2000 µg/plate * negative Skeggs (1976)
typhimurium
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1985f)
typhimurium without activation
(89%)
3-1000 µg/plate
± activation
(94%)
V-79 mammalian V-79 Chinese 0.03 - 0.05 mM negative Gordon et al. (1982b)
cell assay hamster lung cells (+ activation)
(HGPRT Locus) 0.003-0.006 mM
(- activation)
Alkaline elution Rat hepatocytes 0.01-0.6 mM positive in Gordon et al. (1982c)
assay in vitro concentrations
(DNA single (> 0.2 mM)
strand breaks)
Test System Test Object Concentration Results Reference
(purity)
Table 3 (contd)
Test System Test Object Concentration Results Reference
(purity)
In vitro Chinese hamster 0.005 - 0.025 mM negative Gordon et al. (1985g)
chromosomal ovary cells with activation
aberration
0.01 - 0.035 mM
without activation
Alkaline elution Rat oral up to 10.6 mg/kg bw negative Gordon et al. (1982d)
assay in vivo (approx. LD50)
(DNA single
strand breaks)
In vivo cytogenetic Mouse oral 1, 2, 4, negative Blazak et al. (1983)
assay 12 mg/kg bw
(mouse bone marrow) (94%)
Delta-8,9-isomer: Salmonella 10-3000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at conc.
> 1000 µg/plate)
(91,6%)
Polar degradates: Salmonella 100-10 000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at
10 000 µg/plate)
* avermectin B1a
Embryotoxicity/teratogenicity and reproduction
Mice
The delta-8,9-isomer of abamectin was given by gavage in sesame
oil to groups of 7-11 mated female mice (Crl:CF1 BR) at doses of 0,
1.5, 3, 6.25, 12.5, 25, or 50 mg/kg bw/day on day 6 through 15 of
gestation. Treatment-related deaths occurred at all dosage levels.
The groups at 3 mg/kg bw/day and higher doses were terminated on
days 6-8 of gestation. There was a slight decrease in body-weight
gain on single days at 1.5 mg/kg bw/day compared to control. Because
of early termination, no body-weights were measured after the
beginning of dosing in the other dose groups. Fetal effects became
apparent at 1.5 mg/kg bw/day (the only group with litters) as an
increased incidence of fetuses with cleft palate (24/83 (29%)
compared to 0% in the concurrent control. The NOAEL in this study
was <1.5 mg/kg bw/day for maternotoxicity and teratogenicity
(Gordon et al., 1984d).
A subsequent study with the delta-8,9-isomer was performed to
establish a no-effect level. Oral doses were administered to groups
of mice (Crl: CF1 BR; 12 mated females/dose) at levels of 0, 0.05,
0.1, 0.5 or 1 mg/kg bw/day on days 6 through 15 of gestation. Single
females at 0.5 and 1 mg/kg bw/day showed clinical signs including
tremors and loss of body-weight. Decreases in body-weight gain were
observed at 0.05 and 1 mg/kg bw/day, but not at 0.1 mg/kg bw/day. No
dose-related increase in the incidence of resorptions and dead
fetuses occurred in any treatment groups. In addition there were
fewer implants per female in the 0.05 (10.4 per female) and 1 mg/kg
bw/day (9.8 per female) groups compared to 12.5 per female in the
control group resulting in decreases in live fetuses per female at
0.05 (8.7) and 1 mg/kg bw/day (8.3) compared to control (11.3).
These differences from control and particularly those at the low
dose level were probably not treatment-related since the changes
were slight and were not observed at the intermediate dose levels.
An increased incidence of cleft palates were observed at dose
levels of 0.1 mg/kg bw/day and above with incidences of 13/115
(11%), 1/90 (1%) and 7/91 (8%) at dose levels of 0.1, 0.5, and 1
mg/kg bw/day, respectively, compared to 0% in the concurrent control
group. The highest historical control incidence of cleft palates was
reported to be 3% in one study (overall 0.3%). There were also
slightly increased incidences of exencephaly at dose levels of 0.1
mg/kg bw/day and above: of 1/115 (1.7%), 4/90 (4%), and 2/91 (2%) at
the dosage levels of 0.1, 0.5 and 1.0 mg/kg bw/day, respectively,
compared to 1/136 (0.7%) in the concurrent control. The highest
incidence of historical control incidence was reported to be 1.6% in
a single study (overall 0.3%). As with the incidence of cleft
palates no dose-response-relationship was evident but the observed
incidences were clearly higher than the range of historical controls
reported. The NOAELs in this study were 0.1 mg/kg bw/day for
maternotoxicity and 0.05 mg/kg bw/day for fetotoxicity and
teratogenicity (Gordon et al., 1984d).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR; 25 females/dose group) at dosage
levels of 0, 0.015, 0.03 or 0.06 mg/kg bw/day on days 6 through 15
of gestation (original intent was to have dosage levels of 0.025,
0.05 and 0.1 mg/kg bw/day; but an error in preparation resulted in
different dose levels). No signs of maternotoxicity were observed.
Increased incidences of exencephaly in the 0.03 and 0.06 mg/kg
bw/day groups were found, but without any dose-response
relationship. The incidence in both groups was about 1.3% compared
to 0% in the concurrent control and the 0.015 mg/kg bw/day group.
Compared to the incidences of historical controls the present
incidence of 1.3% is slightly smaller than the highest incidence of
1.6% of a single study in historical control animals. A single fetus
(1/210) in the 0.015 mg/kg bw/day group had a cleft palate resulting
in an incidence of about 0.5%. The NOAELs in this study were 0.06
mg/kg bw/day for maternotoxicity and < 0.06 mg/kg bw/day for
teratogenicity (Gordon et al., 1985c).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR;25 females/group) on days 6 through 15
of gestation at dosage levels of 0, 0.015, 0.03, 0.1 or 0.5 mg/kg
bw/day. One animal at 0.5 mg/kg bw/day was sacrificed in a moribund
condition. Incidence of cleft palate was 24/233 (10%), 6/279 (2%),
and single fetuses (0.4%) at 0.5, 0.1, and at both 0.03 and 0.015
mg/kg bw/day, respectively. The overall incidence of cleft palate in
historical controls is reported to be 0.3%. The incidences of
exencephaly of about 0.4% that occurred in single fetuses in the
control, 0.015 and 0.5 mg/kg bw/day groups and in 5/238 (2%) in the
0.03 mg/kg bw/day group did not show any dose-response-relationship.
This compares with, but is greater than, the overall incidence of
0.3% and is less than 1.6% (highest incidence) of historical control
animals except at 0.03 mg/kg bw/day. The NOAELs in this study were
0.1 mg/kg bw/day for maternotoxicity and 0.03 mg/kg bw/day for
embryo-fetotoxicity and terato-genicity (Gordon et al., 1985d).
Rats
In an oral study groups of rats (Sprague-Dawley Crl:CD(SD)BR;
25 females/group) were treated with dosage levels of 0, 0.25, 0.5 or
1.0 mg/kg bw/day of the delta 8,9-isomers of abamectin on days 6
through 17 of gestation. The body-weight gain in treatment groups
was slightly greater than in the control group. This effect on body-
weight gain was seen in previous teratology and reproductive studies
with the parent compound at comparable doses (Gordon et al.,
1982k,n). As in the previous studies, this increased body-weight
gain is considered treatment-related but not an adverse effect. No
evidence of embryo- or fetotoxicity or teratogenicity at any dose
level was given. The NOAEL in this study was > 1 mg/kg bw/day
(Gordon et al., 1987b)
In a single generation study groups of rats (Sprague-Dawley
[Crl: CD(SD)BR]; 20 females/dose) were orally treated (gavage) at
dosage levels of 0, 0.06, 0.12 or 0.4 mg of the delta-8,9-isomer of
abamectin/kg bw/day. The period of dosing was fifteen days prior to
cohabitation through day 20 of lactation. There were no treatment-
related effects on F0 female reproductive performance (mating
index, fertility, length of gestation). Histopathological
examination of the F1 weanlings revealed retinal anomalies in all
groups including controls, consisting particularly of intra-retinal
folds. The incidences were 20, 16, 16 and 22% in control, 0.06, 0.12
and 0.4 mg/kg bw/day groups, respectively. More severe retinal
anomalies were identified in animals of the low- and mid-dose groups
compared to control animals. These changes were compared with the
severity of retinal anomalies observed in control weanling rats of a
previous two-generation study conducted with abamectin (Gordon et
al., 1982n). According to the statement of the study authors the
retinal anomalies observed in the present study have comparable
severity as those in the previous control animals, but no original
data were presented. The study authors conclude that no ocular
lesions were produced as a result of the administration of the
compound. The fact that no clear dose-response with regard to the
incidence and severity of these retinal effects is observed supports
the suggestion that in fact they are not caused by the treatment
(Gordon et al., 1987d).
Polar degradates (unidentified mixture of residues generated
in vitro and in a field study on oranges).
In two oral developmental toxicity studies groups of female
mice (Crl:CF1 BR; 25/group) were orally treated (gavage) at dosage
levels of 0, 0.25, 0.5 and 1 mg/kg bw/day on days 6 through 15 of
gestation. The treatment did not have maternotoxic effects and did
not influence the reproductive performance. On fetal examination
there was no evidence of embryotoxic/fetotoxic or teratogenic
effects of the compounds (Gordon et al., 1987c, 1988).
Genotoxicity studies with metabolites
See Table 3.
Antidote study of abamectin intoxication in dogs
Ipecac given at a rate of 30 ml/dog by stomach tube 15 min
after a lethal oral dose of abamectin of 8 mg/kg bw induced vomiting
within 15-45 min after ipecac was given. This treatment was adequate
to prevent coma and death in dogs given a lethal dose of abamectin.
Ipecac treatment only after 30 min or longer after the dose of
abamectin did not prevent coma and death of the dogs (Gordon et
al., 1984f).
Observations in humans and non-human primates
The comparison of the toxicological data of the two members of
the avermectin family of compounds, abamectin and ivermectin,
reveals a lot of similarities. Ivermectin has been used extensively
in humans at an oral therapeutic dose of 0.2 mg/kg bw for the
treatment of onchocerciasis without serious drug-related adverse
effects (Greene et al., 1989). Therefore the human experience with
a closely related compound offers useful information to be
considered in the evaluation of abamectin.
Comparative studies with ivermectin in monkeys
Safety assessment studies with abamectin and ivermectin have
shown that the susceptibility of different animal species to these
compounds varies considerably. The pregnant mouse is the most
sensitive mammal examined so far. In view of the similarity in
structure and toxicity of abamectin and ivermectin and the diversity
of responses among the different species it was suggested that an
acute oral toxicity study in rhesus monkeys might serve as a
valuable source of information about the potential toxicity of the
compounds in primates. Such a study might provide a more rational
basis for predicting the toxicity of abamectin in humans.
The purpose of the study was to determine the minimum toxic
dose (mTD) of abamectin and ivermectin in monkeys and to determine
the plasma levels of drug at that dose.
Single oral doses of abamectin or ivermectin were given to four
rhesus monkeys (2/sex) each at intervals of 2-3 weeks, in the
following chronological dose levels: 0.2, 0.5, 1, 2, 4, 6, 8, 12 or
24 mg/kg bw. The most sensitive indicator of toxicity was emesis
occurring at dose levels of 2 mg/kg bw and higher. The incidence of
emesis was dose-related and the time after dosing when emesis
occurred tended to decrease with increasing dose. Marked mydriasis
(lack of pupillary constriction) was noted only at 24 mg
abamectin/kg bw. Less pronounced mydriasis was observed after doses
of 24 mg ivermectin/kg bw. At 24 mg/kg bw both compounds induced
slight to moderate sedation.
Since no emesis occurred with doses below 2 mg/kg bw and since
no other drug-related physical signs of toxicity were observed at
lower doses, it was concluded that emesis was the most appropriate
physical sign for characterizing minimum toxic doses of the
compounds in monkeys and that the mTD was 2 mg/ kg bw for both
compounds. This dose is tenfold higher than the human dose for
onchocerciasis therapy of 200 µg/kg bw.
Plasma concentrations of ivermectin were similar to the levels
of abamectin for up to 4 h following the 2 mg/kg bw doses, but
thereafter ivermectin levels were higher on the average than those
of abamectin. Peak levels occurred between 8-24 h post-dose with
maximum values of 110 ng/ml for ivermectin and 76 ng/ml for
abamectin after a 2 mg/kg bw dose. The average maximum plasma level
following the 24 mg/kg bw dose of ivermectin was 680 ng/ml, after
the same dose of abamectin it was 390 ng/ml. Despite roughly
proportional increases in plasma levels with increased doses, the
severity of clinical signs did not worsen appreciably and no
tremors, convulsions or deaths occurred even at a dose of 24 mg/kg
bw. The dose-response curve for acute toxicity of abamectin and
ivermectin in monkeys seems to be much flatter than for mice,
indicating that in primates, clinical evidence of intoxication
(emesis) may occur well in advance of serious or life-threatening
toxicity. Mean peak plasma level measured in humans given the 200
µg/kg bw therapeutic dose of ivermectin was 20 ng/ml (Gordon et
al., 1985b).
Observations in humans
Whereas for abamectin no observations in humans are reported,
information is available concerning the therapeutic use and the
safety of ivermectin, extensively tested in human onchcerciasis. In
a single yearly dose it suppresses microfilaria in the skin and eyes
and prevents disease progression in most infected persons. A single
oral dose of 30 µg/kg bw or 50 µg/kg bw exhibited microfilaricidal
activity. Transient pruritus was observed soon after treatment, but
no abnormal laboratory results were produced (Aziz et al., 1982).
In a 12-month follow-up study investigating the efficacy of
different dosages of the drug it was shown that doses of 100 µg/kg
bw did not produce different reactions from the placebo group,
whereas dose levels of 150 and 200 µg/kg bw only produced mild
reversible clinical reactions (Mazzotti-like reactions). It is
suggested therefore that 150 µg/kg bw is probably the optimal dose
in terms of its antiparasitic activity and side effects. A single
oral dose of 150 µg/kg bw annually induces a minimal reaction in 5-
15% of infected adults and a more significant reaction in about 1%.
Its use was associated with hypotension, usually occurring within 24
h of administration in 1 out of 1000 persons (Greene et al.,
1989).
COMMENTS
Following oral administration of abamectin to rats, 69-82% of
the administered dose was eliminated in the faeces and only 1% in
the urine. Biliary excretion was the major cause of the high level
of faecal excretion. Biotrans-formation proceeds mainly by
demethylation and hydroxylation.
Orally administered abamectin elicited dose-dependent CNS
effects, including tremors and ataxia.
In a one-year dietary study in dogs at doses of 0, 0.25, 0.5 or
1 mg/kg bw/day, a borderline NOAEL of 0.25 mg/kg bw/day was
determined, despite single instances of mydriasis at this lowest-
dose level.
In a two-year long-term/carcinogenicity study in mice,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 2, 4 or 8 mg/kg bw/day. The NOAEL was 4 mg/kg bw/day,
based on the occurrence of tremors, a higher mortality rate and
reduced body-weight gain at 8 mg/kg bw/day. Abamectin was not
carcinogenic in the mouse.
In a two-year long-term/carcinogenicity study in rats,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 0.75, 1.5 or 2 mg/kg bw/day. The NOAEL was 1.5 mg/kg
bw/day. Higher doses caused CNS toxicity. Abamectin was not
carcinogenic in rats.
In two one-generation reproduction studies in rats avermectin
B1a was administered in the diet at concentration resulting in
dosage levels ranging from 0.1 to 2 mg/kg bw/day in rats.
Maternotoxicity was observed at dose levels above 1 mg/kg bw/day.
Fetotoxicity, consisting of reduced pup survival rates, reduced pup
weight growth and retardation became evident at dose levels of 0.5
mg/kg bw/day and higher. The NOAEL for fetotoxicity was 0.1 mg/kg
bw/day.
In a two-generation reproduction study in rats at dose levels
of 0.05, 0.12 or 0.4 mg abamectin/kg bw/day, the NOAEL for
maternotoxicity was 0.05 mg/kg bw/day, based on reduced maternal
body-weight gain during lactation at 0.12 mg/kg bw/day and above.
The NOAEL for pup toxicity was 0.12 mg/kg bw/day, based on increased
mortality and lowered pup weights at 0.4 mg/kg bw/day.
The teratogenic potential of abamectin administered by gavage
was investigated in mice, rats and rabbits. Teratogenic effects,
including cleft palates, omphaloceles and clubbed fore feet, were
observed at maternotoxic doses in mice and rabbits. The NOAEL for
teratogenicity in the most sensitive species, the mouse (CF1 strain)
was 0.2 mg/kg bw/day, while for maternotoxicity the NOAEL was 0.05
mg/kg bw/day, based on the occurrence of tremors and deaths at
higher doses.
Various studies to investigate the teratogenic potential of the
delta-8,9-isomer have been conducted in mice and rats. Similar
teratogenic effects to those seen with abamectin were observed in
the most sensitive species, the mouse. The NOAELs for
maternotoxicity in the mouse were 0.1 mg/kg bw/day, and for
fetotoxicity/teratogenicity, 0.05 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that abamectin was not genotoxic.
Although no human data were available on abamectin, extensive
data on field and community-based trials with ivermectin in humans
infected with Onchocera spp. were available (WHO, 1993). The main
effects noted were those arising from the death of parasites, the
so-called Mazzotti reaction, which is characterized by arthralgia,
pruritus, fever, hypertension, tachycardia, headache, and ocular
changes. Very limited data in humans indicate that ivermectin does
not increase the incidence of birth defects, although it is
teratogenic in mice, rats and rabbits (WHO, 1990).
The available data provided adequate toxicological information
to permit the allocation of an ADI for abamectin and its delta-8,9-
isomer, based on the NOAELs for abamectin of 0.05 mg/kg bw/day in
the teratogenicity study in mice and in the two-generation
reproduction study in rats. The NOAEL for the delta-8,9-isomer was
0.05 mg/kg bw/day in the teratogenicity study in mice. A safety
factor of 500 was used because of concern over the teratogenicity of
the delta-8,9-isomer which forms part of the residue in food.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Abamectin (and components avermectin B1a and B1b):
Mouse: 4 mg/kg bw/day (2 year feeding study)
0.05 mg/kg bw/day (teratology study, maternotoxicity)
0.2 mg/kg bw/day (teratology study, teratogenicity)
Rat: 1.5 mg/kg bw/day (2-year study)
0.1 mg/kg bw/day (1-generation reproduction study)
0.05 mg/kg bw/day (2-generation reproduction study,
maternotoxicity)
0.12 (2-generation reproduction study, pup toxicity)
Dog 0.25 mg/kg (borderline) (1-year study)
Delta-8,9-isomer
Mouse 0.1 mg/kg bw/day (teratology study, maternotoxicity)
0.05 mg/kg bw/day (teratology study, teratogenicity)
Estimate of acceptable daily intake for humans (abamectin and
delta-8,9-isomer)
0-0.0001 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing studies on the mechanism of central nervous system
toxicity.
2. Observations in humans.
REFERENCES
Alvaro, R.F., Green, M.L., Halley, B.A., Maynard, M.S., &
Meriwether, H.T. (1984) Distribution and clearance of avermectin
B1a in rats. Unpublished report ARM-1 prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Aziz, M.A., Biallo, S., Diopp, I. and Lariviere, M. (1982) Efficacy
and tolerance of ivermectin in human onchocerciasis. Lancet, 24
July, p. 171-173. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Blazak, W.F., Mitchell, A.D., & Skinner, W.A. (1983) An assessment
of the mutagenic potential of abamectin (mk 0936) utilizing the in
vivo mouse bone marrow cytogenetics assay. Study Number TT 83-900-
6. Unpublished report prepared by SRI International, Menlo Park, CA,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. & Mandel, J. (1978) Acute dermal toxicity study in rats
with abamectin (avermectin B1; C-076). Study Number TT 78-3607.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Nickell, B.E., Collevechio, K., Mutchler, M., & Clark,
R.L. (1981) Oral-range-finding study (multigeneration) in rats with
abamectin (MK 0936). Study No. 82-707-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Cook, M.M., Berglund, R.M., & Prato, M.
(1982a) Microbial mutagen test on abamectin (MK 0936) with and
without rat liver enzyme activation. Study No. TT 82-8013.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., & Patterson, S.K. (1982b) V-79
mammalian cell mutagenesis assay with abamectin (MK 0936). Study No.
TT 82-8506, 82-8510, 82-8512, 82-8519. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982c) In vitro alkaline elution/rat hepatocyte
assay with abamectin (MK 0936). Study No. TT 82-8520, 82-8523, 82-
8525, 82-8526. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982d) In vivo alkaline elution/rat hepatocyte assay
with abamectin (MK 0936). Study No. TT 82-8302. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1982e) Twelve-week oral
range-finding study in dogs given abamectin (MK 0936). Study No. TT
82-073-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Peter, Ch.P., Nickell, B.E., Buck, J.F., & Schultz,
A.K. (1982f) Twelve-week oral dietary range-finding study in mice
given abamectin (MK 0936). Study No. TT82-082-0,-2,-2. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Lankas, G.R., Nickell, B.E., Buck, J.F., & Jackson, L.
(1982g) Eight-week dietary range-finding study in rats given
abamectin (MK 0936). Study No. TT 82-075-0, -1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Skolnick, E.M. (1982h) One-hundred-
five week dietary carcinogenicity and toxicity study of abamectin
(MK 0936) in rats with a 53-week interim necropsy. Study No. TT 82-
099-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. Allen, H.L., Nickell, B.E., Satiritz, S.M., Powzaniuk,
W., Roux, L., & McKeon, J. (1982i) Fifty-three week dietary study in
dogs given abamectin (MK 0936). Study No. TT 82-104-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Weller,
J.V. (1982j) Oral range-finding study in pregnant rats given
abamectin (MK 0936). Study Number TT 82-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982k) Oral teratology study in rats given abamectin
(MK 0936). Study No. TT 82-705-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982l) Oral range finding study in pregnant rabbits
given abamectin (MK 0936). Study No. TT 82-706-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Vetter,
C.M. (1982m) Oral teratology study in rabbits given abamectin (MK
0936). Study No. TT 82-706-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Mildred, S.C., & Hoberman, H.M. (1982n) Reproductive
effects of abamectin (MK 0936) administered orally by gavage to Crl:
COBS CD(SD)BR rats for two Generations. Study No. TT 82-9010.
Unpublished report prepared by Argus Research Laboratories, Horsham,
Pennsylvania, USA and by Merck Sharp & Dohme Research Laboratories,
West Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three
Bridges, NJ, USA.
Gordon, L.R., Mandel, J., McDonald, J.S., Nickell, B.E., Powzaniuk,
W., & Bielinski, T.C. (1983a) Guinea pig skin maximization test with
abamectin (MK 0936). Study No. TT 83-2506. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Lankas, G.R., Fabry, A., Nickell, B.E., Powzaniuk, W.,
Buck, J.F., Satiritz, S.M., & Schultz, A. (1983b) Ninety-four week
dietary carcinogenicity and toxicity study in mice given abamectin
(MK 0936). Study No. TT 83-002-0,-1,-2,-3. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon L.R., Minsker, D.H., Nickell, B.E., Collevechio, K., &
Battisti, G.A. (1983c) Ten-day dietary maternotoxicity study in mice
given abamectin (MK 0936). Study No. TT 83-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Nickell, B.E., Mandel, J., Powzaniuk,
W., & Stolz, W.W. (1983d) Acute dermal toxicity study in rabbits
given abamectin (MK 0936). Study No. TT 83-064-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Allen, H.L., Nickell, B.E., Mandel,
J., Powzaniuk, W., & McAfee, J.L. (1984a) Acute oral toxicity study
in mice given Avermectin B1b. Study No. TT 84-107-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1984b)
Five-day acute oral toxicity study in pregnant and non-pregnant cf1
mice with abamectin (MK 0936). Study No. TT 84-2842-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Mandel, J., Majka, J.A., & McAfee, J.L. (1984c) Acute
oral toxicity study in mice given the Delta-8,9-isomer of abamectin
(MK 0936). Study No. 84-112-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., & Landis, D.K. (1984d) Oral maternotoxicity study in mice with
the Delta-8,9 isomer of abamectin (avermectin B1). Study No. 84-
722-0,-1. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. (1984e) Acute oral toxicity study in mice with Delta-
8,9-isomer of abamectin (MK 0936). Study No. TT 84-2820. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1984f) Exploratory non-
specific antidote study of abamectin (MK 0936) intoxication in dogs.
Study No. 84-085-0. Unpublished report prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1985a)
Five-day acute oral toxicity study in pregnant and nonpregnant CF1
mice with abamectin (MK 0936). Study No. TT 85-2593. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J., & Rafferty, C.E. (1985b) Oral toxicity and plasma level study in
monkeys with Ivermectin (MK 0933) and abamectin (MK 0936). Study No.
T 85-013-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Landis,
D.K. (1985c) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. TT 85-710-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Geiger,
J.E. (1985d) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. 85-710-1. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., Powzaniuk, W., & Landis, D.K. (1985e) Oral maternotoxicity study
in mice with Avermectin B1b. Study No. 84-721-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Sina, J., Patterson, S.K., Berglind, R.M., Prato, M.,
& Quillin, F. (1985f) Microbial mutagenesis assays with abamectin
(avermectin B1; MK 0936). Study No. TT 85-8005 and 85-8051
Unpublished reports prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Galloway, S., Patterson, S.K., Armstrong, M., Bean,
Ch.L., & Deasy, D. (1985g) Abamectin (MK 0936) assay for chromosomal
aberrations in vitro, in chinese hamster ovary cells Study No. TT
85-8631, 85-8632, 85-8635. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Sina, J.F., Wright, S.P., Prato, M., & Quillin, F.
(1987a) Microbial mutagenesis assays of the Delta-8,9-isomer and
polar degradates of abamectin (Avermectin B1; MK 0936). Study Nos.
TT 87-8046, 87-8047, 87-8058. Unpublished reports prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Wise,L.D., Jensen, R.D., Nickell, B.E., Collevechio,
K., & Vetter, C.H. (1987b) Oral developmental toxicity study in rat
given the Delta-8,9-Isomer of abamectin (avermectin B1). Study No.
TT 87-715-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Minsker, D.H., Anderson, C.A., Nickell, B.E.,
Collevechio, K. & Deyerle-Brooks, K.A. (1987c) Oral developmental
toxicity study in mice given the polar degradates of abamectin (MK
0936). Study No. TT 87-717-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon L.R., Wise, L.D., Vonderfecht, St.L., Nickell, B.E.,
Collevechio, K., Powzaniuk, W., & McMahon, M.G. (1987d) Single
generation study in rats with the Delta-8,9-isomer of abamectin
(avermectin B1). Study No. 87-716-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Wise, L.E., Allen, M.L., Nickell, B.E.,
Collovechio,K., Powzaniuk, W., & Sina, J.L. (1988) Oral
developmental toxicity study in mice with L-930,463 (polar
degradate). Study No. TT 88-713-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Greene, B.M., Brown, K.R., & Taylor, H.R. (1989) Use of Ivermectin
in humans, In: Ivermectin and Abamectin, ed. by Campbell, W.C.,
Springerverlag, Chapter 21, p. 311-323. Submitted to WHO by MSDRL,
Three Bridges, NJ, USA.
Gruber, V.I. (1988) Identification of ß-alpha-hydroxy-avermectin
B1a as a metabolite of avermectin B1a in rats. Unpublished
report of Merck sharp and Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Mandel, J. (1977) Acute oral toxicity studies in mice and rats with
avermectin B1a (C-076(B1a)). Study Nos. TT 77-3248, 77-3250, 77-
3264, 77-3787, 77-3789, 77-3788, 77-3337 and 77-3346. Unpublished
reports prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986a) The metabolism
of Delta-8,9-Z-isomer avermectin B1a in rats. Unpublished report
ARM-2, prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986b) The metabolism
of avermectin B1a in rats. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
MSDRL (1977a) Oral reproduction study in rats with avermectin B1a
(C-076(B1a)). Study Number 77-706-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
MSDRL (1977b) Oral reproduction study in rats. Study Number 77-712-0
with avermectin B1a (C-076(B1a)). Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Norbury K.C. & Wolf, G.L. (1977) Fourteen-week oral toxicity study
in rats following in utero exposure to avermectin B1a (C-
076(B1a)). Study number TT 77-043-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. & Allen, H.L. (1976) Eighteen-week oral toxicity
study in dogs with avermectin B1a (C-076(B1a)). Study No. TT 76-
073-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. (1977a) Oral teratology study in mice with
avermectin B1a (C-076(B1a)). Study No. TT 77-705-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T. (1977b) Ten-day oral toxicity study in pregnant mice
with avermectin B1a (C-076(B1a)). Study No. TT 77-717-1.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel., J., Everett, M.A., Wolf,
G.L., & Powzaniuk, W., (1981a) Acute oral toxicity study in rats
with abamectin (avermectin B1). Study No. TT 81-2937. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Wolf, G.L., Stolz,
W.W., & Bielinski, T.C. (1981b) Acute dermal toxicity study in
rabbits with abamectin (avermectin B1). Study No. TT 81-3021.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981c) Acute ocular irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2940. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981d) Primary dermal irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2941, 81-2943, 81-
2945. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Skeggs, H. (1976) Microbial mutagenesis assay with avermectin B1a
(C-076(B1a)). Study No. TT 76-8052. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
WHO (1990) Evaluation of certain veterinary drug residues in food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 799, Geneva.
WHO (1993) Toxicological evaluation of certain veterinary drug
residues in food. WHO Food Additive Series No. 31, Geneva.
 EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
A study was carried out to evaluate the tissue distribution and
elimination of 3H- and 14C-labelled avermectin B1a in a sesame
oil vehicle after oral administration to male and female rats.
Sixty-two CRCD strain rats/sex were divided into groups and dosed as
follows: a single high dose (1.4 mg/kg bw); a single low dose (0.14
mg/kg bw); fourteen daily low doses (0.14 mg/kg bw/day) of
unlabelled avermectin B1a, followed by a single low dose of
tritium-labelled avermectin B1a; a single high dose (1.4 mg/kg bw)
of a mixture of tritium - and 14C-labelled avermectin B1a; a
single dose of vehicle only to serve as controls for groups with
single dosing regimen or fifteen daily doses of vehicle only to
serve as controls for the multiple dosing experiment. The control
groups were sacrificed seven days after the last (or single) dose,
while rats from the treated groups were sacrificed on days 1, 2, 4
and 7 after dosing. Urine and faeces were collected each day after
dosing while the organs were collected from all rats at time of
sacrifice. All samples were assayed for total radioactivity.
Elimination in the urine was 0.3-0.6% of the applied dose in females
and 0.8-1.1% in males over the 7 days collection period. Elimination
in faeces ranged from 69-77% and 70-82% in females and males,
respectively. Residue levels in liver ranged from about 0.001-0.02
ppm, in kidney from 0.003-0.06 ppm, in muscle from 0.001-0.02 ppm
and in fat from 0.008-0.01 ppm in the different dosing groups, 7
days post-dose. The total residue level in the organs of the females
were generally higher than in males. The residue levels were dose
dependent, the residues in the high dose groups being roughly ten-
fold greater than in the low dose groups, whereas the depletion
rates were similar among the different tissues. The total
radioactive residues depleted from the tissues with half-lives of
approximately 1.2 days indicating that the residues did not persist.
Repeated dosing did not influence elimination rates or residue
levels. The tritiated avermectin B1a residue levels and depletion
rates were comparable to the 14C residue levels. Thus the tritium
label at the 5-position of avermectin B1a was not labile during
the course of the study. The stability of the label was also
demonstrated in an experiment for volatile/exchangeable tritium,
demonstrating that less than 2% of the tritiated avermectin B1a
sample was volatile (Alvaro et al., 1984).
Selected tissue samples (liver, kidney, muscle, fat) were
analyzed for unchanged avermectin B1a and metabolites. Two
metabolites that were also formed in vitro after rat liver
microsomal incubation, in addition to unchanged avermectin B1a,
accounted for most of the residues: The metabolites were identified
as 24-hydroxymethyl-avermectin B1a (24-OHMe-B1a) and 3"-desmethyl
avermectin B1a (3"-DM-B1a) (Maynard 1986b). A minor metabolite
was identified as ß-alpha-hydroxy-avermectin B1a (Gruber, 1988).
Toxicological studies
Acute toxicity studies
The main clinical signs were ataxia and tremors found in all
species investigated and irrespective of the route of
administration. The data are summarized in Tables 1 and 2.
Table 1: Acute toxicity of abamectin
Species Sex Route LD50 Component Reference
(mg/kg bw) (purity)
Mouse F oral 13.6-23.8 B1a Mandel (1977)
19.8 B1b (98.4%) Gordon et al. (1984a)
5-day mortality:
pregnant: 11.8-19.0 B1a+B1b (94%) Gordon et al. (1984b)
non pregnant: 15.0-41.3 B1a+B1b (94%) Gordon et al. (1985a)
Rat F,M oral ca. 11 B1a Mandel, 1977
F,M oral 8.7-12.8 B1a+B1b (91%) Robertson et al. (1981a)
Dog M,F oral ca. 8 B1a Robertson and Allen (1976)
B1a+B1b Gordon et al. (1984f)
Monkey M,F oral > 24 B1a+B1b Gordon et al. (1985b)
Rat dermal >330 B1a/B1b (87%/9.4%) Gordon & Mandel (1978)
Rabbit dermal >1600 B1a+B1b (91.4%) Robertson et al. (1981b)
B1a + B1b (94%) Gordon et al. (1983d)
Delta-8,9-isomer (photolytic degradation product)
Mouse M,F oral > 80 (Gordon et al. 1984c
& 1984e)
Table 2: Dermal and ocular irritation studies with abamectin,
technical material
Species Sex Target Findings Reference
Organ
Rabbit M,F eye very slight Robertson et al. (1981c)
irritation
Rabbit M,F skin non irritating on Robertson et al. (1981d)
intact or abraded
skin
Guinea- M,F skin: negative Gordon et al. (1983a)
pig sensitization
Short-term toxicity studies
Mice
In a twelve-week dietary range-finding study groups of mice
(CD-1 strain;15/sex/group) were fed dietary concentrations of 0,
2/40 (increase in week 9), 5, 10 or 20 ppm abamectin over 12 weeks
and 40 or 60 ppm over 3 weeks. There were no physical signs or
mortality. Decrease in body-weight gain at 60 ppm was the only
change observed. The NOAEL in this study was 40 ppm, equal to 8
mg/kg bw/day (Gordon et al., 1982f).
Rats
In an eight-week oral range-finding study in rats abamectin
(purity 94%) was administered to groups of rats (Charles River CD
albino rats, 10/sex/group) at dietary concentrations of 0, 5, 10,
15, 20/25 (increase in week 7), 40 or 60 ppm. Due to mortality and
the appearance of severe clinical signs of toxicity (tremors,
decreased activity) in the first study week, remaining animals at 40
and 60 ppm were sacrificed on days 15 and 5, respectively. These
groups were replaced by groups at 10 and 15 ppm in study week 5.
Decreases in mean body-weight gain were seen at 15, 20/25 (3 and 5%
decrease in males and females, respectively) and 40 ppm. Tremors
occurring at 15 and 20/25 ppm were no longer evident after study
week 1. One animal at 5 ppm showed slight tremors on day 2 only. No
gross or microscopic examination was performed. Based on clinical
signs and body-weight effects it appears that there is a steep dose-
response curve. Therefore the doses recommended for the
carcinogenicity study were 0.75, 1.5 and 2.0 mg/kg bw/day (Gordon
et al., 1982g).
Dogs
In a twelve-week oral range-finding study abamectin (purity
94%) was fed to groups of beagle dogs (2/sex/dose) at doses of 0,
0.25, 0.5, 1 or 4/2 mg/kg bw/day. Because of signs of toxicity
(tremors, weakness, incoordination, disorientation) and markedly
decreased food consumption at 4 mg/kg bw/day in one dog, this dose
was decreased to 2 mg/kg bw in study week 4. Inability to constrict
pupils after light stimulus was observed in some dogs at 1 mg/kg
bw/day or higher doses. Depression of body-weight gain or weight
loss occurred in dogs at 2 mg/kg bw/day and higher. No gross or
microscopic examination was performed. Based on the clinical signs
and body-weight effects recommended doses for the one-year oral dog
study were 0.25, 0.5 and 1 mg/kg/ bw/day. The NOAEL in this study
was 0.5 mg/kg bw/day (Gordon et al., 1982e).
Avermectin B1a (purity not specified) was orally administered
by gavage to beagle dogs (15/sex/dose) at dose levels of 0, 0.25,
0.5, 2 or 8 mg/kg bw/day over a period of 18 weeks. The treatment
did not affect parameters of urinalyses, ophthalmologic examination
or organ weights. Deaths occurred at incidences of 1/30, 3/30 and
3/30 at 0.5, 2 and 8 mg/kg bw/day, respectively. Signs of toxicity
at levels of > 0.5 mg/kg bw/day consisted of whole body muscular
tremors, ataxia, mydriasis and ptyalism (hypersalivation); in
addition, tonic convulsions and emesis occurred at 2 and 8 mg/kg
bw/day. Reduced body-weight gain was only observed at dose levels of
0.5 and 2 mg/kg bw/day, but not in surviving dogs at 8 mg/kg bw/day.
At 8 mg/kg bw/day changes in haematologic and serum biochemical
parameters included slightly increased values of haemoglobin,
haematocrit, erythrocytes, nonsegmented neutrophils and glucose
level. Electrocardiographic changes (elongation of the QT interval
and bradycardia) were noted at 8 mg/kg bw/day only at the beginning
of the study. Histopathologic changes were seen only in animals that
died or were killed moribund and consisted of diffuse hepato-
cellular vacuolation (without lipid accumulation) at dose levels of
0.5, 2 and 8 mg/kg bw/day and edema of the gallbladder at 2 and 8
mg/kg bw/day. The NOAEL in this study was 0.25 mg/kg bw/day
(Robertson & Allen 1976).
Groups of beagle dogs (6/sex/dose) were fed abamectin (purity
> 89%) for 53 weeks at concentrations resulting in doses of 0,
0.25, 0.5 or 1 mg/kg bw/day. The doses were selected on the basis of
the results of a previous range-finding study (Gordon et al.,
1982e). The compound administration had no effect on
ophthalmological examination, urinalyses or organ weights. Decreased
or even absence of constriction of pupils to light was evident at
0.5 mg/kg bw/day (occurrence rate 3%) and 1 mg/kg bw/day (15%) and
as single instances and in single animals also at 0.25 mg/kg bw/day.
At 1 mg/kg bw/day one dog was found dead (week 38), two others were
killed moribund (weeks 33 and 38). Reduced body-weight gain most
probably due to food unpalatability and therefore reduced food
consumption was observed at the highest dose level. A slight
decrease in serum urea nitrogen at 1 mg/kg bw/day was most probable
a consequence of decreased protein intake at this dose level. Slight
leukocytosis and increased packed cell volume was noted in one dog
killed in poor condition. There were no gross or microscopic changes
that could be attributed to treatment. The NOAEL in this study was
< 0.25 mg/kg bw/day, the single instance of mydriasis at this
dose being considered a borderline effect (Gordon et al., 1982i).
Long-term toxicity carcinogenicity studies
Mice
Abamectin (purity 90%) was administered in the diet to groups
of mice (Crl:CD-1(ICR)BR;74/sex/group; 24/sex/group for interim
sacrifice) at concen-trations resulting in doses of 0, 2, 4 or 8
mg/kg bw/day over a period of 94 weeks. In females, treatment-
related tremors were observed in all dose groups and deaths occurred
at 4 and 8 mg/kg bw/day. These effects were not observed in the 12-
week range-finding study at dosage levels up to 11 mg/kg bw/day (60
ppm). No explanation could be found for the sensitivity of these
female mice. Plasma samples taken from affected and unaffected
animals revealed no significant differences. A subsequent batch of
females did not show this unexpected sensitivity and following
restart of the study with a new group of female mice, tremors
occurred only in single animals at 8 mg/kg bw/day. In males, tremors
occurred in single moribund animals of the control and 8 mg/kg
bw/day group. In this group the mortality rate was increased:
towards the end of the study the mortality reached 60% compared to
45% in controls. Body-weight gain was reduced about 21% in females
and 7% in males at 8 mg/kg bw/day. There was a dose-related increase
in food consumption in treated females but a decrease in food
efficiency at 8 mg/kg bw/day. The treatment had no effect on
ophthalmic examinations, haematologic or biochemical parameters,
organ weights, gross pathology, histopathologic changes (with the
exception of a higher incidence of dermatitis at 2 and 8 mg/kg
bw/day in males). The tumour incidence was not increased by the
treatment. The NOAEL in this study was 4 mg/kg bw/day (Gordon et
al., 1983b).
Rats
Abamectin (purity 91%) was fed to groups of rats (Crl:CD(SD)Br;
65/sex/group) at dietary concentrations resulting in doses of 0,
0.75, 1.5 or 2/2.5/2 mg/kg bw/day over two years. Since no effects
attributable to abamectin were observed through study week 10, the
high dose level was increased to 2.5 mg/kg/ bw/day in week 11.
Because of the appearance of severe signs of CNS toxicity, the dose
was decreased again to 2 mg/kg bw/day in study week 13 for the
remainder of the study. No treatment-related changes with respect to
ophthalmoscopic, haematologic or serum biochemical parameters,
urinalyses and organ weights were observed. Increases in body-weight
gain were seen in all dosage groups during the first year on study.
By the end of the study comparable mean body-weight gains for all
groups of females were found, but an increased gain was noticed in
males. The increases in body-weight gain are considered treatment-
related, but the effect is not considered as an adverse effect.
Clinical signs consisting of tremors appeared in study week 12
correlated with the increase in dosage from 2 to 2.5 mg/kg bw/day in
week 11. The tremors in all the affected animals persisted
intermittently until sacrifice despite reduction in the high-dose
level back to 2 mg/kg bw/day in week 13. Several high-dose group
animals, which exhibited tremors, were sacrificed in a moribund
condition. The increase in mortality reflects the induction of
tremors during the early stage of the study and subsequent sacrifice
of the affected animals. Following the reduction in dose to 2
mg/kg/bw/day no new cases of tremors occurred and mortality in all
treated and control groups was comparable. No increase in tumour
incidence or treatment-related non-neoplastic histopathologic
changes were observed. The NOAEL in this study was 1.5 mg/kg bw/day
(Gordon et al., 1982h).
Reproduction studies
Mice
In a ten-day dietary maternotoxicity study abamectin (purity >
88%) was fed at dietary concentrations resulting in target dose
levels of 0, 0.1, 0.3 or 0.6 mg/kg bw/day (actual doses = 0.06,
0.16, or 0.33 mg/kg bw/day) to groups of pregnant mice (albino CF1;
20 females/group) on days 6 through 15 of gestation. Marked tremors
at 0.33 and at 0.16 mg/kg bw/day were observed. Reproductive
parameters (numbers of implants, resorptions and live and dead
fetuses) were not influenced. The NOAEL in this study was 0.06 mg/kg
bw/day (Gordon et al., 1983c).
Rats
One hundred-and-fifty F1 offspring, from five groups of F0
female rats (Charles River CD), that had been exposed in utero to
avermectin B1a at dosage levels of 0 (two groups), 0.1, 0.2 or 0.4
mg/kg bw/day were selected for a fourteen-week study of oral
toxicity. Groups of weanling rats (15/sex/group) received avermectin
B1a at doses of 0, 0.1, 0.2 or 0.4 mg/kg bw/day by gavage. The
treatment did not have any effects on mortality, ocular changes,
haematology, serum biochemistry, organ weights or gross and
microscopic tissue alterations. The increased body-weight gain of
male rats at 0.4 mg/kg bw/day was considered as treatment-related
but not as an adverse effect. The NOAEL in this study was 0.4 mg/kg
bw/day (Norbury and Wolf, 1977).
Avermectin (B1a) was administered orally to three groups of
female rats (12 females/dose group) at dose levels of 0, 0.5, 1, or
2/1.5 mg/kg bw/day from 14 days before mating throughout gestation
and lactation until day 21 postpartum. The high dose was reduced
after five doses to 1.5 mg/kg bw/day due to whole body muscular
tremors at 2 mg/kg bw/day. Two deaths occurred at the high dose, one
animal at this dose level became moribund. The significant decrease
in body-weight gain in the 1.5 mg/kg bw/day group was a result of
weight loss of the 3 moribund or dead animals. Weight gain increases
were observed during some periods at 1 and 1.5 mg/kg bw/day. A
treatment-related decrease in the number of live pups per litter on
day 1 postpartum at 1.5 mg/kg bw was observed. The pup weights were
decreased at this dose level on day 1. Pups at all dosage levels
showed decreases in average weight per litter throughout the study.
Dose-related increase in mortality among pups at all dose levels
were observed resulting in survival rates of 0, 14% and 76%, in the
1.5, 1 and 0.5 mg/kg bw/day groups respectively, compared to 98% in
the control group. There was a developmental retardation (eye
opening) at 0.5 and 1 mg/kg bw/day in surviving pups. The NOAELs in
this study were < 0.5 mg/kg bw/day for embryo-fetotoxicity and 1
mg/kg bw/day for maternotoxicity (MSDRL, 1977a).
Avermectin (B1a) was administered orally to three groups of
15 female rats at dose levels of 0, 0.1, 0.2 or 0.4 mg/kg bw/day
from 14 days before mating, throughout mating, gestation and
lactation until day 21 postpartum. No maternotoxic effects were
observed and the reproduction status was not adversely affected.
Among pups at 0.2 and 0.4 mg/kg bw/day a dose-related incidence of
spastic movements was noted increasing in severity with increasing
dose. At 0.2 and 0.4 mg/kg bw/day reduction in average pup weight
was observed (dose-related). Developmental retardation (eye opening,
ear opening, hear growth), occurred at 0.2 and 0.4 mg/kg bw/day
(dose-related). The NOAEL in this study was 0.1 mg/kg bw/day for
embryo-fetotoxicity (MSDRL, 1977b).
In an oral range-finding study (multigeneration) abamectin was
administered to groups of rats (Crl: CD (SD) BR Sprague-Dawley; 12
females/group) in the drinking water at concentrations of 0, 0.15,
0.5, 1.5 or 5 mg/l. Treatment was conducted from 14 days prior to
cohabitation with untreated males through day 21 postpartum and
subsequently through the F1 generation. Water consumption in
groups 0.15-1.5 mg/l increased during the lactation period and
decreased slightly in the 5 mg/l group. No accurate measurement of
water consumption could be performed prior to transfer to delivery
boxes on day 17 of gestation. Thereafter mean levels of water
consumption were comparable in all groups resulting in dose ranges
of 0.017-0.037, 0.066-0.127, 0.192-0.396, or 0.556-0.685 mg/kg
bw/day for the 0.15, 0.5, 1.5 mg/l or 5 mg/l group, respectively. In
the F0 generation the only effect observed was an increase in
body-weight gain in the 5 mg/l group in the first study week. In the
F1 generation an increase in postnatal mortality at 5 mg/l (53%
compared to 1% in control) was observed, physical signs observed in
pups at the highest dose level consisted of tremors; pup weights in
the 5 mg/l group were decreased. 3/116 Fetuses (2.6%) (from 2
litters) in the 5 mg/l group showed single malformations (1 cleft
palate, 1 sternebral malformation, 1 sternebral variation). One
fetus in the 0.5 mg/l group had lumbar ribs. Because of the single
incidences in the study groups and because of the occurrence of
these alterations in historical control groups it is questionable if
these effects are treatment-related (Gordon et al., 1981).
In a two-generation study, groups of rats
(Crl:COBSTMCDTM(SD) BR/30/sex/group) were orally treated
(gavage) with dosages of 0, 0.05, 0.12 or 0.4 mg abamectin/kg bw/day
. F0 and F1b rats were mated to produce F1a and F1b litters,
F2a and F2b litters. At 0.12 and 0.4 mg/kg bw/day both sexes of
the F0 rats during the first gestation period (F0-F1a) showed
increased body-weight gains, whereas during the subsequent lactation
period (F0-F1a) a reduction in body-weight gain compared to
control was observed at these two dose levels particularly on the
first days of lactation. Similar changes were also observed in the
next generation. During the second mating period of F0 rats (to
produce the F1b litters) mating performance was reduced (reduction
of incidence of mating) at 0.4 mg/kg bw/day as compared to control
animals. The reduction in mating performance was presumed to be
associated with irregular estrous cycles observed during the second
mating period of F0 female rats. This effect was not observed in
matings resulting in the F1a litters or the F2a and F2b
litters. During the lactation periods of both generations effects in
the 0.4 mg/kg bw/day group consisted of increased pup mortality,
reduced viability and lactation indices and lower average pup body-
weights. The incidence of pups which appeared thin and weak was
increased. These effects were less severe in the F2 litters than
in the F1 litters. No skeletal anomalies associated with the test
agent were revealed. Histopathological examination showed retinal
anomalies in 3/4 males and 1/5 females in the high-dose group
compared wich 1/10 in the control group (F1b weanlings). The
anomalies consisted of single or multiple retinal folds of many
layers that included pigment epithelium. The same lesion was seen in
one control female and in one male in the 0.05 and 0.12 mg/kg bw/day
groups of the F1b weanlings. In the F1b parental animals (both
sexes) only 1/35 showed this retinal anomaly. In the F2b weanlings
histopathology was performed on a greater number of animals. There
was a significant increase in retinal anomalies in the high-dose
group with the following incidences (M + F): 4.6% and 22% in the
control and 0.4 mg/kg bw/day, respectively. As will become evident
from the results of a reproduction study in rats conducted with
comparable doses of the delta-8,9-isomer of abamectin the control
incidence of retinal anomalies (4.6%) in the present study is much
smaller than in the other study, where a control incidence of 20%
was found. Therefore the difference observed may be incidental
following an unusually low incidence of this lesion in the
concurrent control group of the present study.
Among both the F1b and F2b weanlings the high-dose group
pups were the smallest. One hypothesis to explain the presence of
retinal folds in the high-dose group weanlings and the disappearance
with maturity proposed by the study authors is that the ocular globe
size is relatively smaller in the small pups (where there was
pronounced pup toxicity) relative to the size of the retina which
perhaps carried on at its normal growth rate. Therefore it may be
assumed that the retinal effects observed are a secondary effect.
Reversibility of the retinal anomalies is evidenced by its absence
in the high dose adults. The NOAELs in this study were 0.12 mg/kg
bw/day for pup toxicity and retinal anomalies and 0.05 mg/kg bw/day
for maternotoxicity (Gordon et al., 1982n).
Special studies on embryotoxicity/teratogenicity
Mice
Avermectin B1a was administered orally (gavage) to groups of
mice (albino CF1 strain/20 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.1, 0.2, 0.4 or 0.8 mg/kg bw/day.
The doses of the B1a component were calculated as the parent
compound.
Deaths occurred in the 0.1, 0.4 and 0.8 mg/kg bw/day groups (1,
3 and 2 animals respectively), in most cases preceded by tremors.
Body-weight gain was not influenced by the treatment; neither was
the reproductive status (number of implants, resorptions, live and
dead fetuses per litter). An increased incidence of cleft palate at
0.4 (4/165; 2.4%) and 0.8 mg/kg bw/day (5/199; 2.5%) was observed
compared to a control incidence of 0.4%. The NOAELs in this study
were < 0.1 mg/kg bw/day for maternotoxicity and 0.2 mg/kg bw/day
for embryo-fetotoxicity and teratogenicity (Robertson 1977a).
Groups of mice (albino CF1 strain/20 female/group) were
administered avermectin B1a by gavage from day 6 through 15 of
gestation at dose levels of 0, 0.025, 0.05, 0.075 or 0.1 mg/kg
bw/day. One animal died at 0.1 mg/kg bw/day and tremors were
observed in several additional mice at this dose. One animal at
0.075 mg/kg bw/day exhibited muscular tremors and became moribund.
Maternal body-weight gains were unaffected at any dosage level. The
NOAEL in this study was 0.05 mg/kg bw/day for maternotoxicity
(Robertson, 1977b).
In an oral maternotoxicity study the minor component of
abamectin, avermectin B1b was administered by gavage to groups of
mice (Crl:CF1 BR; 12 females/dose) at dose levels of 0, 0.025, 0.05,
0.075 or 0.1 mg/kg bw/day on days 6 through 15 of gestation. At the
0.075 mg/kg bw/day dose level two deaths occurred preceded by weight
loss and tremors. Single fetuses at all dose levels showed
malformations consisting of exencephaly and cleft palates resulting
in incidences of about 1% in all dose groups. The highest incidences
for historical control groups are 1.6% for exencephaly and 1.3 % for
cleft palates (overall incidence 0.3% for exencephaly and cleft
palate each). There was no evidence of fetotoxicity. The NOAEL in
this study was 0.05 mg/kg bw/day for maternotoxicity (Gordon et
al., 1985e).
Rats
In a range-finding study groups of mated female rats (Charles
River; 10/group) were administered abamectin by gavage at dose
levels of 0, 0.25, 0.5, 1 or 2 mg/kg bw/day on gestation days 6
through 17. One female at 2 mg/kg bw/day exhibited weight loss and
tremors and was sacrificed after receiving 12 doses. There was no
other evidence of maternal toxicity. At 0.25 and 2 mg/kg bw/day
there was a slightly increased body-weight gain that is considered
to be treatment-related but not an adverse effect. The NOAEL in this
study was 1 mg/kg bw/day (Gordon et al., 1982j).
In a rat teratology study groups of mated rats (Charles River;
25 females/group) were orally treated (gavage) with abamectin
(purity 94%) at dosage levels of 0, 0.4, 0.8 or 1.6 mg/kg bw/day on
days 6 through 19 of gestation. There was no evidence of maternal
toxicity. Slightly increased maternal body-weight gain was seen at
all dosage levels between days 6 and 14 of gestation. An increased
incidence of fetuses with external fetal malformations was observed
(exencephaly, cleft palate, gastroschisis) at 0.8 and 1.6 mg/kg
bw/day. The incidences were: 2/279 (0.7%) and 2/326 (0.6%),
respectively, compared to 1/319 (0.3%) in the control group.
Historical control incidence of cleft palate in this rat strain is
0.03% (overall) or 0.3% (single study with highest incidence).
Gastroschisis alone has an overall incidence of 0.004% or the
highest incidence in a single study of 0.3%. Based on the fact, that
in historical controls one single malformation (cleft palate) may
occur in up to 0.3% of control animals and no dose-response-
relationship is evident, it may be assumed that the increase
observed may either not be treatment-related or may be a borderline
effect. Visceral examination indicated a higher incidence of
distended ureters in the treated groups up to 3% compared to 0% in
controls), but without any dose-effect relationship. This effect is
observed at incidences of up to 7% in historical control groups.
Skeletal examination revealed a higher incidence of fetuses with
lumbar ribs and count variations at 1.6 mg/kg bw/day: at 1.6 mg/kg
bw/day an incidence of 72/326 (22%) was found, compared to 41/320
(13%) at 0.4, and 45/279 (16%) at 0.8 mg/kg bw/day compared to
44/319 (14%) in the control group. The increase at 1.6 mg/kg bw/day
may not be treatment-related because higher incidences are observed
in historical controls. For example, in historical controls lumbar
ribs occurred at an incidence of 14% (overall) or 27.8% (single
study with highest incidence). The NOAELs in this study were 1.6
mg/kg bw/day for maternotoxicity and < 1.6 mg/kg bw/day for
fetotoxicity and teratogenicity (Gordon et al., 1982k).
Rabbit
In a range-finding study groups of New Zeeland albino rabbits
(10 females/dose) were given abamectin at oral dosage levels of 0,
0.5, 1, 2 or 3 mg/kg bw/day on gestational days 6 through 18.
Maternotoxicity was observed at 3 mg/kg bw/day. One female at 3
mg/kg bw/day was sacrificed moribund on day 16 of gestation after 11
doses. All females at 3 mg/kg bw/day were in a stupor after the
fourth and subsequent doses and showed yellow or green discharge
from nose or mouth. Moreover animals at 3 mg/kg bw/day had a
decreased food and water consumption, and showed body-weight loss.
The NOAEL in this study was 2 mg/kg bw/day (Gordon et al., 1982).
In a teratogenicity study groups of female New Zeeland albino
rabbits (18/dose) were given abamectin (purity 94%) at oral dosage
levels of 0, 0.5, 1 or 2 mg/kg bw/day on day 6 through 27 of
gestation. There were single deaths in all the treatment groups. At
2 mg/kg bw/day the animals showed decreased food and water
consumption and weight loss. Fetuses of the 2 mg/kg bw/day showed
cleft palates, omphaloceles and clubbed forefeet at higher
incidences than in the concurrent control group: 7.4% versus 2.1% in
concurrent control. The incidence of clubbed fore foot alone in
historical control groups is 0.2% (overall) and 3.9%, respectively,
in a single study with highest incidence. No data are available
concerning historical control incidences of cleft palates and
omphaloceles in rabbits. The clearly higher incidence of these
malformations at 2 mg/kg bw/day is therefore considered to be
treatment-related. With respect to skeletal fetal examination higher
incidences of skeletal terata (vertebral malformations, branched and
fused ribs) were observed at 0.5, 1 and 2 mg/kg bw/day with
incidences of 4%, 2% and 4% of the fetuses showing these
malformations respectively, compared to 0% in the concurrent control
group. In historical controls single vertebral malformations may
occur at incidences of up to 0.5% (overall) (e.g., caudal vertebral
malformations) with the highest value of 9% in a single study (e.g.,
fused ribs). At 0.5 and 2 mg/kg bw/day there were increased
incidences of incompletely ossified sites particularly in sternebrae
and metacarpals. These effects were more pronounced in the highest
dose group. Because the incidences observed at 1 mg/kg bw/day were
comparable to the incidences in the control group the absence of a
dose-relationship in the lower dose levels of 0.5 and 1 mg/kg bw/day
indicates that the increased incidence at 0.5 mg/kg bw/day is
possibly not treatment-related. The NOAEL in this study was 1 mg/kg
bw/day for maternotoxicity, embryo-fetotoxicity, and teratogenicity
(Gordon et al., 1982m).
Special studies on genotoxicity
A number of genotoxicity studies have been conducted with
abamectin. The results are summarized in Table 3.
Special studies on the delta-8,9-isomer of avermectin B
Biochemical aspects
Rat
Male rats were orally (gavage) dosed with 1.4 mg3H-labelled
delta-8,9-isomer avermectin B1a/kg bw. The delta-8,9-isomer of
abamectin is a photolytic degradation product of abamectin. The
stability of the tritium label at the 5th carbon position was
previously demonstrated with avermectin B1a in rats. Within 7 days
after dosing 94% of the administered dose was eliminated with the
faeces, and 0.4% in the urine. The levels of residues in liver,
kidney, fat and muscle ranged from 0.28 ppm (muscle) to 1.4 ppm
(fat) on day 1 after dose and decreased to a range of 0.017 to 0.1,
respectively, on day 7 after dosing. The half-lives for the tissue
residues in muscle, kidney, liver and fat varied between 1.45 and
1.64 days. As major metabolite the 3"-desmethyl-delta-8,9 isomer was
isolated. The 3"-DM-delta-8,9 isomer was also isolated and
identified after rat liver microsomal incubation of the delta-8,9-
isomer. The minor metabolite 24-hydroxymethyl-Delta-8,9-isomer,
(also formed in vitro), was identified.
By comparison, the elimination by rats of both the delta-8,9-
isomer avermectin B1a in this study and the avermectin B1a in a
previous study was very similar. In both studies 94-96% of the
administered dose were recovered within 7 days after dosing in the
excreta. The tissue residue levels were also demonstrated to be
similar for both compounds. The average half-life for the residue in
the edible tissues was 1.1 and 1.5 days for avermectin B1a and
delta-8,9-isomer, respectively. The metabolites generated were very
similar with rats metabolizing both compounds to the 3"-desmethyl
and the 24-hydroxymethyl metabolites. Similarly, both avermectin
B1a and ivermectin B1 (22,23-dihydro-avermectin B1) have been
shown to be primarily metabolized to the 3"-desmethyl and/or 24-
hydroxymethyl metabolite(s) by many other animal species (Maynard
et al., 1986a).
Table 3. Results of genotoxicity assays on abamectin
Test System Test Object Concentration Results Reference
(purity)
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1982a)
typhimurium (precipitate at conc.
> 3000 µg/plate)
± activation
(94%)
Ames test Salmonella 1-2000 µg/plate * negative Skeggs (1976)
typhimurium
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1985f)
typhimurium without activation
(89%)
3-1000 µg/plate
± activation
(94%)
V-79 mammalian V-79 Chinese 0.03 - 0.05 mM negative Gordon et al. (1982b)
cell assay hamster lung cells (+ activation)
(HGPRT Locus) 0.003-0.006 mM
(- activation)
Alkaline elution Rat hepatocytes 0.01-0.6 mM positive in Gordon et al. (1982c)
assay in vitro concentrations
(DNA single (> 0.2 mM)
strand breaks)
Test System Test Object Concentration Results Reference
(purity)
Table 3 (contd)
Test System Test Object Concentration Results Reference
(purity)
In vitro Chinese hamster 0.005 - 0.025 mM negative Gordon et al. (1985g)
chromosomal ovary cells with activation
aberration
0.01 - 0.035 mM
without activation
Alkaline elution Rat oral up to 10.6 mg/kg bw negative Gordon et al. (1982d)
assay in vivo (approx. LD50)
(DNA single
strand breaks)
In vivo cytogenetic Mouse oral 1, 2, 4, negative Blazak et al. (1983)
assay 12 mg/kg bw
(mouse bone marrow) (94%)
Delta-8,9-isomer: Salmonella 10-3000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at conc.
> 1000 µg/plate)
(91,6%)
Polar degradates: Salmonella 100-10 000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at
10 000 µg/plate)
* avermectin B1a
Embryotoxicity/teratogenicity and reproduction
Mice
The delta-8,9-isomer of abamectin was given by gavage in sesame
oil to groups of 7-11 mated female mice (Crl:CF1 BR) at doses of 0,
1.5, 3, 6.25, 12.5, 25, or 50 mg/kg bw/day on day 6 through 15 of
gestation. Treatment-related deaths occurred at all dosage levels.
The groups at 3 mg/kg bw/day and higher doses were terminated on
days 6-8 of gestation. There was a slight decrease in body-weight
gain on single days at 1.5 mg/kg bw/day compared to control. Because
of early termination, no body-weights were measured after the
beginning of dosing in the other dose groups. Fetal effects became
apparent at 1.5 mg/kg bw/day (the only group with litters) as an
increased incidence of fetuses with cleft palate (24/83 (29%)
compared to 0% in the concurrent control. The NOAEL in this study
was <1.5 mg/kg bw/day for maternotoxicity and teratogenicity
(Gordon et al., 1984d).
A subsequent study with the delta-8,9-isomer was performed to
establish a no-effect level. Oral doses were administered to groups
of mice (Crl: CF1 BR; 12 mated females/dose) at levels of 0, 0.05,
0.1, 0.5 or 1 mg/kg bw/day on days 6 through 15 of gestation. Single
females at 0.5 and 1 mg/kg bw/day showed clinical signs including
tremors and loss of body-weight. Decreases in body-weight gain were
observed at 0.05 and 1 mg/kg bw/day, but not at 0.1 mg/kg bw/day. No
dose-related increase in the incidence of resorptions and dead
fetuses occurred in any treatment groups. In addition there were
fewer implants per female in the 0.05 (10.4 per female) and 1 mg/kg
bw/day (9.8 per female) groups compared to 12.5 per female in the
control group resulting in decreases in live fetuses per female at
0.05 (8.7) and 1 mg/kg bw/day (8.3) compared to control (11.3).
These differences from control and particularly those at the low
dose level were probably not treatment-related since the changes
were slight and were not observed at the intermediate dose levels.
An increased incidence of cleft palates were observed at dose
levels of 0.1 mg/kg bw/day and above with incidences of 13/115
(11%), 1/90 (1%) and 7/91 (8%) at dose levels of 0.1, 0.5, and 1
mg/kg bw/day, respectively, compared to 0% in the concurrent control
group. The highest historical control incidence of cleft palates was
reported to be 3% in one study (overall 0.3%). There were also
slightly increased incidences of exencephaly at dose levels of 0.1
mg/kg bw/day and above: of 1/115 (1.7%), 4/90 (4%), and 2/91 (2%) at
the dosage levels of 0.1, 0.5 and 1.0 mg/kg bw/day, respectively,
compared to 1/136 (0.7%) in the concurrent control. The highest
incidence of historical control incidence was reported to be 1.6% in
a single study (overall 0.3%). As with the incidence of cleft
palates no dose-response-relationship was evident but the observed
incidences were clearly higher than the range of historical controls
reported. The NOAELs in this study were 0.1 mg/kg bw/day for
maternotoxicity and 0.05 mg/kg bw/day for fetotoxicity and
teratogenicity (Gordon et al., 1984d).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR; 25 females/dose group) at dosage
levels of 0, 0.015, 0.03 or 0.06 mg/kg bw/day on days 6 through 15
of gestation (original intent was to have dosage levels of 0.025,
0.05 and 0.1 mg/kg bw/day; but an error in preparation resulted in
different dose levels). No signs of maternotoxicity were observed.
Increased incidences of exencephaly in the 0.03 and 0.06 mg/kg
bw/day groups were found, but without any dose-response
relationship. The incidence in both groups was about 1.3% compared
to 0% in the concurrent control and the 0.015 mg/kg bw/day group.
Compared to the incidences of historical controls the present
incidence of 1.3% is slightly smaller than the highest incidence of
1.6% of a single study in historical control animals. A single fetus
(1/210) in the 0.015 mg/kg bw/day group had a cleft palate resulting
in an incidence of about 0.5%. The NOAELs in this study were 0.06
mg/kg bw/day for maternotoxicity and < 0.06 mg/kg bw/day for
teratogenicity (Gordon et al., 1985c).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR;25 females/group) on days 6 through 15
of gestation at dosage levels of 0, 0.015, 0.03, 0.1 or 0.5 mg/kg
bw/day. One animal at 0.5 mg/kg bw/day was sacrificed in a moribund
condition. Incidence of cleft palate was 24/233 (10%), 6/279 (2%),
and single fetuses (0.4%) at 0.5, 0.1, and at both 0.03 and 0.015
mg/kg bw/day, respectively. The overall incidence of cleft palate in
historical controls is reported to be 0.3%. The incidences of
exencephaly of about 0.4% that occurred in single fetuses in the
control, 0.015 and 0.5 mg/kg bw/day groups and in 5/238 (2%) in the
0.03 mg/kg bw/day group did not show any dose-response-relationship.
This compares with, but is greater than, the overall incidence of
0.3% and is less than 1.6% (highest incidence) of historical control
animals except at 0.03 mg/kg bw/day. The NOAELs in this study were
0.1 mg/kg bw/day for maternotoxicity and 0.03 mg/kg bw/day for
embryo-fetotoxicity and terato-genicity (Gordon et al., 1985d).
Rats
In an oral study groups of rats (Sprague-Dawley Crl:CD(SD)BR;
25 females/group) were treated with dosage levels of 0, 0.25, 0.5 or
1.0 mg/kg bw/day of the delta 8,9-isomers of abamectin on days 6
through 17 of gestation. The body-weight gain in treatment groups
was slightly greater than in the control group. This effect on body-
weight gain was seen in previous teratology and reproductive studies
with the parent compound at comparable doses (Gordon et al.,
1982k,n). As in the previous studies, this increased body-weight
gain is considered treatment-related but not an adverse effect. No
evidence of embryo- or fetotoxicity or teratogenicity at any dose
level was given. The NOAEL in this study was > 1 mg/kg bw/day
(Gordon et al., 1987b)
In a single generation study groups of rats (Sprague-Dawley
[Crl: CD(SD)BR]; 20 females/dose) were orally treated (gavage) at
dosage levels of 0, 0.06, 0.12 or 0.4 mg of the delta-8,9-isomer of
abamectin/kg bw/day. The period of dosing was fifteen days prior to
cohabitation through day 20 of lactation. There were no treatment-
related effects on F0 female reproductive performance (mating
index, fertility, length of gestation). Histopathological
examination of the F1 weanlings revealed retinal anomalies in all
groups including controls, consisting particularly of intra-retinal
folds. The incidences were 20, 16, 16 and 22% in control, 0.06, 0.12
and 0.4 mg/kg bw/day groups, respectively. More severe retinal
anomalies were identified in animals of the low- and mid-dose groups
compared to control animals. These changes were compared with the
severity of retinal anomalies observed in control weanling rats of a
previous two-generation study conducted with abamectin (Gordon et
al., 1982n). According to the statement of the study authors the
retinal anomalies observed in the present study have comparable
severity as those in the previous control animals, but no original
data were presented. The study authors conclude that no ocular
lesions were produced as a result of the administration of the
compound. The fact that no clear dose-response with regard to the
incidence and severity of these retinal effects is observed supports
the suggestion that in fact they are not caused by the treatment
(Gordon et al., 1987d).
Polar degradates (unidentified mixture of residues generated
in vitro and in a field study on oranges).
In two oral developmental toxicity studies groups of female
mice (Crl:CF1 BR; 25/group) were orally treated (gavage) at dosage
levels of 0, 0.25, 0.5 and 1 mg/kg bw/day on days 6 through 15 of
gestation. The treatment did not have maternotoxic effects and did
not influence the reproductive performance. On fetal examination
there was no evidence of embryotoxic/fetotoxic or teratogenic
effects of the compounds (Gordon et al., 1987c, 1988).
Genotoxicity studies with metabolites
See Table 3.
Antidote study of abamectin intoxication in dogs
Ipecac given at a rate of 30 ml/dog by stomach tube 15 min
after a lethal oral dose of abamectin of 8 mg/kg bw induced vomiting
within 15-45 min after ipecac was given. This treatment was adequate
to prevent coma and death in dogs given a lethal dose of abamectin.
Ipecac treatment only after 30 min or longer after the dose of
abamectin did not prevent coma and death of the dogs (Gordon et
al., 1984f).
Observations in humans and non-human primates
The comparison of the toxicological data of the two members of
the avermectin family of compounds, abamectin and ivermectin,
reveals a lot of similarities. Ivermectin has been used extensively
in humans at an oral therapeutic dose of 0.2 mg/kg bw for the
treatment of onchocerciasis without serious drug-related adverse
effects (Greene et al., 1989). Therefore the human experience with
a closely related compound offers useful information to be
considered in the evaluation of abamectin.
Comparative studies with ivermectin in monkeys
Safety assessment studies with abamectin and ivermectin have
shown that the susceptibility of different animal species to these
compounds varies considerably. The pregnant mouse is the most
sensitive mammal examined so far. In view of the similarity in
structure and toxicity of abamectin and ivermectin and the diversity
of responses among the different species it was suggested that an
acute oral toxicity study in rhesus monkeys might serve as a
valuable source of information about the potential toxicity of the
compounds in primates. Such a study might provide a more rational
basis for predicting the toxicity of abamectin in humans.
The purpose of the study was to determine the minimum toxic
dose (mTD) of abamectin and ivermectin in monkeys and to determine
the plasma levels of drug at that dose.
Single oral doses of abamectin or ivermectin were given to four
rhesus monkeys (2/sex) each at intervals of 2-3 weeks, in the
following chronological dose levels: 0.2, 0.5, 1, 2, 4, 6, 8, 12 or
24 mg/kg bw. The most sensitive indicator of toxicity was emesis
occurring at dose levels of 2 mg/kg bw and higher. The incidence of
emesis was dose-related and the time after dosing when emesis
occurred tended to decrease with increasing dose. Marked mydriasis
(lack of pupillary constriction) was noted only at 24 mg
abamectin/kg bw. Less pronounced mydriasis was observed after doses
of 24 mg ivermectin/kg bw. At 24 mg/kg bw both compounds induced
slight to moderate sedation.
Since no emesis occurred with doses below 2 mg/kg bw and since
no other drug-related physical signs of toxicity were observed at
lower doses, it was concluded that emesis was the most appropriate
physical sign for characterizing minimum toxic doses of the
compounds in monkeys and that the mTD was 2 mg/ kg bw for both
compounds. This dose is tenfold higher than the human dose for
onchocerciasis therapy of 200 µg/kg bw.
Plasma concentrations of ivermectin were similar to the levels
of abamectin for up to 4 h following the 2 mg/kg bw doses, but
thereafter ivermectin levels were higher on the average than those
of abamectin. Peak levels occurred between 8-24 h post-dose with
maximum values of 110 ng/ml for ivermectin and 76 ng/ml for
abamectin after a 2 mg/kg bw dose. The average maximum plasma level
following the 24 mg/kg bw dose of ivermectin was 680 ng/ml, after
the same dose of abamectin it was 390 ng/ml. Despite roughly
proportional increases in plasma levels with increased doses, the
severity of clinical signs did not worsen appreciably and no
tremors, convulsions or deaths occurred even at a dose of 24 mg/kg
bw. The dose-response curve for acute toxicity of abamectin and
ivermectin in monkeys seems to be much flatter than for mice,
indicating that in primates, clinical evidence of intoxication
(emesis) may occur well in advance of serious or life-threatening
toxicity. Mean peak plasma level measured in humans given the 200
µg/kg bw therapeutic dose of ivermectin was 20 ng/ml (Gordon et
al., 1985b).
Observations in humans
Whereas for abamectin no observations in humans are reported,
information is available concerning the therapeutic use and the
safety of ivermectin, extensively tested in human onchcerciasis. In
a single yearly dose it suppresses microfilaria in the skin and eyes
and prevents disease progression in most infected persons. A single
oral dose of 30 µg/kg bw or 50 µg/kg bw exhibited microfilaricidal
activity. Transient pruritus was observed soon after treatment, but
no abnormal laboratory results were produced (Aziz et al., 1982).
In a 12-month follow-up study investigating the efficacy of
different dosages of the drug it was shown that doses of 100 µg/kg
bw did not produce different reactions from the placebo group,
whereas dose levels of 150 and 200 µg/kg bw only produced mild
reversible clinical reactions (Mazzotti-like reactions). It is
suggested therefore that 150 µg/kg bw is probably the optimal dose
in terms of its antiparasitic activity and side effects. A single
oral dose of 150 µg/kg bw annually induces a minimal reaction in 5-
15% of infected adults and a more significant reaction in about 1%.
Its use was associated with hypotension, usually occurring within 24
h of administration in 1 out of 1000 persons (Greene et al.,
1989).
COMMENTS
Following oral administration of abamectin to rats, 69-82% of
the administered dose was eliminated in the faeces and only 1% in
the urine. Biliary excretion was the major cause of the high level
of faecal excretion. Biotrans-formation proceeds mainly by
demethylation and hydroxylation.
Orally administered abamectin elicited dose-dependent CNS
effects, including tremors and ataxia.
In a one-year dietary study in dogs at doses of 0, 0.25, 0.5 or
1 mg/kg bw/day, a borderline NOAEL of 0.25 mg/kg bw/day was
determined, despite single instances of mydriasis at this lowest-
dose level.
In a two-year long-term/carcinogenicity study in mice,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 2, 4 or 8 mg/kg bw/day. The NOAEL was 4 mg/kg bw/day,
based on the occurrence of tremors, a higher mortality rate and
reduced body-weight gain at 8 mg/kg bw/day. Abamectin was not
carcinogenic in the mouse.
In a two-year long-term/carcinogenicity study in rats,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 0.75, 1.5 or 2 mg/kg bw/day. The NOAEL was 1.5 mg/kg
bw/day. Higher doses caused CNS toxicity. Abamectin was not
carcinogenic in rats.
In two one-generation reproduction studies in rats avermectin
B1a was administered in the diet at concentration resulting in
dosage levels ranging from 0.1 to 2 mg/kg bw/day in rats.
Maternotoxicity was observed at dose levels above 1 mg/kg bw/day.
Fetotoxicity, consisting of reduced pup survival rates, reduced pup
weight growth and retardation became evident at dose levels of 0.5
mg/kg bw/day and higher. The NOAEL for fetotoxicity was 0.1 mg/kg
bw/day.
In a two-generation reproduction study in rats at dose levels
of 0.05, 0.12 or 0.4 mg abamectin/kg bw/day, the NOAEL for
maternotoxicity was 0.05 mg/kg bw/day, based on reduced maternal
body-weight gain during lactation at 0.12 mg/kg bw/day and above.
The NOAEL for pup toxicity was 0.12 mg/kg bw/day, based on increased
mortality and lowered pup weights at 0.4 mg/kg bw/day.
The teratogenic potential of abamectin administered by gavage
was investigated in mice, rats and rabbits. Teratogenic effects,
including cleft palates, omphaloceles and clubbed fore feet, were
observed at maternotoxic doses in mice and rabbits. The NOAEL for
teratogenicity in the most sensitive species, the mouse (CF1 strain)
was 0.2 mg/kg bw/day, while for maternotoxicity the NOAEL was 0.05
mg/kg bw/day, based on the occurrence of tremors and deaths at
higher doses.
Various studies to investigate the teratogenic potential of the
delta-8,9-isomer have been conducted in mice and rats. Similar
teratogenic effects to those seen with abamectin were observed in
the most sensitive species, the mouse. The NOAELs for
maternotoxicity in the mouse were 0.1 mg/kg bw/day, and for
fetotoxicity/teratogenicity, 0.05 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that abamectin was not genotoxic.
Although no human data were available on abamectin, extensive
data on field and community-based trials with ivermectin in humans
infected with Onchocera spp. were available (WHO, 1993). The main
effects noted were those arising from the death of parasites, the
so-called Mazzotti reaction, which is characterized by arthralgia,
pruritus, fever, hypertension, tachycardia, headache, and ocular
changes. Very limited data in humans indicate that ivermectin does
not increase the incidence of birth defects, although it is
teratogenic in mice, rats and rabbits (WHO, 1990).
The available data provided adequate toxicological information
to permit the allocation of an ADI for abamectin and its delta-8,9-
isomer, based on the NOAELs for abamectin of 0.05 mg/kg bw/day in
the teratogenicity study in mice and in the two-generation
reproduction study in rats. The NOAEL for the delta-8,9-isomer was
0.05 mg/kg bw/day in the teratogenicity study in mice. A safety
factor of 500 was used because of concern over the teratogenicity of
the delta-8,9-isomer which forms part of the residue in food.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Abamectin (and components avermectin B1a and B1b):
Mouse: 4 mg/kg bw/day (2 year feeding study)
0.05 mg/kg bw/day (teratology study, maternotoxicity)
0.2 mg/kg bw/day (teratology study, teratogenicity)
Rat: 1.5 mg/kg bw/day (2-year study)
0.1 mg/kg bw/day (1-generation reproduction study)
0.05 mg/kg bw/day (2-generation reproduction study,
maternotoxicity)
0.12 (2-generation reproduction study, pup toxicity)
Dog 0.25 mg/kg (borderline) (1-year study)
Delta-8,9-isomer
Mouse 0.1 mg/kg bw/day (teratology study, maternotoxicity)
0.05 mg/kg bw/day (teratology study, teratogenicity)
Estimate of acceptable daily intake for humans (abamectin and
delta-8,9-isomer)
0-0.0001 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing studies on the mechanism of central nervous system
toxicity.
2. Observations in humans.
REFERENCES
Alvaro, R.F., Green, M.L., Halley, B.A., Maynard, M.S., &
Meriwether, H.T. (1984) Distribution and clearance of avermectin
B1a in rats. Unpublished report ARM-1 prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Aziz, M.A., Biallo, S., Diopp, I. and Lariviere, M. (1982) Efficacy
and tolerance of ivermectin in human onchocerciasis. Lancet, 24
July, p. 171-173. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Blazak, W.F., Mitchell, A.D., & Skinner, W.A. (1983) An assessment
of the mutagenic potential of abamectin (mk 0936) utilizing the in
vivo mouse bone marrow cytogenetics assay. Study Number TT 83-900-
6. Unpublished report prepared by SRI International, Menlo Park, CA,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. & Mandel, J. (1978) Acute dermal toxicity study in rats
with abamectin (avermectin B1; C-076). Study Number TT 78-3607.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Nickell, B.E., Collevechio, K., Mutchler, M., & Clark,
R.L. (1981) Oral-range-finding study (multigeneration) in rats with
abamectin (MK 0936). Study No. 82-707-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Cook, M.M., Berglund, R.M., & Prato, M.
(1982a) Microbial mutagen test on abamectin (MK 0936) with and
without rat liver enzyme activation. Study No. TT 82-8013.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., & Patterson, S.K. (1982b) V-79
mammalian cell mutagenesis assay with abamectin (MK 0936). Study No.
TT 82-8506, 82-8510, 82-8512, 82-8519. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982c) In vitro alkaline elution/rat hepatocyte
assay with abamectin (MK 0936). Study No. TT 82-8520, 82-8523, 82-
8525, 82-8526. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982d) In vivo alkaline elution/rat hepatocyte assay
with abamectin (MK 0936). Study No. TT 82-8302. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1982e) Twelve-week oral
range-finding study in dogs given abamectin (MK 0936). Study No. TT
82-073-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Peter, Ch.P., Nickell, B.E., Buck, J.F., & Schultz,
A.K. (1982f) Twelve-week oral dietary range-finding study in mice
given abamectin (MK 0936). Study No. TT82-082-0,-2,-2. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Lankas, G.R., Nickell, B.E., Buck, J.F., & Jackson, L.
(1982g) Eight-week dietary range-finding study in rats given
abamectin (MK 0936). Study No. TT 82-075-0, -1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Skolnick, E.M. (1982h) One-hundred-
five week dietary carcinogenicity and toxicity study of abamectin
(MK 0936) in rats with a 53-week interim necropsy. Study No. TT 82-
099-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. Allen, H.L., Nickell, B.E., Satiritz, S.M., Powzaniuk,
W., Roux, L., & McKeon, J. (1982i) Fifty-three week dietary study in
dogs given abamectin (MK 0936). Study No. TT 82-104-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Weller,
J.V. (1982j) Oral range-finding study in pregnant rats given
abamectin (MK 0936). Study Number TT 82-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982k) Oral teratology study in rats given abamectin
(MK 0936). Study No. TT 82-705-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982l) Oral range finding study in pregnant rabbits
given abamectin (MK 0936). Study No. TT 82-706-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Vetter,
C.M. (1982m) Oral teratology study in rabbits given abamectin (MK
0936). Study No. TT 82-706-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Mildred, S.C., & Hoberman, H.M. (1982n) Reproductive
effects of abamectin (MK 0936) administered orally by gavage to Crl:
COBS CD(SD)BR rats for two Generations. Study No. TT 82-9010.
Unpublished report prepared by Argus Research Laboratories, Horsham,
Pennsylvania, USA and by Merck Sharp & Dohme Research Laboratories,
West Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three
Bridges, NJ, USA.
Gordon, L.R., Mandel, J., McDonald, J.S., Nickell, B.E., Powzaniuk,
W., & Bielinski, T.C. (1983a) Guinea pig skin maximization test with
abamectin (MK 0936). Study No. TT 83-2506. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Lankas, G.R., Fabry, A., Nickell, B.E., Powzaniuk, W.,
Buck, J.F., Satiritz, S.M., & Schultz, A. (1983b) Ninety-four week
dietary carcinogenicity and toxicity study in mice given abamectin
(MK 0936). Study No. TT 83-002-0,-1,-2,-3. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon L.R., Minsker, D.H., Nickell, B.E., Collevechio, K., &
Battisti, G.A. (1983c) Ten-day dietary maternotoxicity study in mice
given abamectin (MK 0936). Study No. TT 83-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Nickell, B.E., Mandel, J., Powzaniuk,
W., & Stolz, W.W. (1983d) Acute dermal toxicity study in rabbits
given abamectin (MK 0936). Study No. TT 83-064-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Allen, H.L., Nickell, B.E., Mandel,
J., Powzaniuk, W., & McAfee, J.L. (1984a) Acute oral toxicity study
in mice given Avermectin B1b. Study No. TT 84-107-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1984b)
Five-day acute oral toxicity study in pregnant and non-pregnant cf1
mice with abamectin (MK 0936). Study No. TT 84-2842-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Mandel, J., Majka, J.A., & McAfee, J.L. (1984c) Acute
oral toxicity study in mice given the Delta-8,9-isomer of abamectin
(MK 0936). Study No. 84-112-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., & Landis, D.K. (1984d) Oral maternotoxicity study in mice with
the Delta-8,9 isomer of abamectin (avermectin B1). Study No. 84-
722-0,-1. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. (1984e) Acute oral toxicity study in mice with Delta-
8,9-isomer of abamectin (MK 0936). Study No. TT 84-2820. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1984f) Exploratory non-
specific antidote study of abamectin (MK 0936) intoxication in dogs.
Study No. 84-085-0. Unpublished report prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1985a)
Five-day acute oral toxicity study in pregnant and nonpregnant CF1
mice with abamectin (MK 0936). Study No. TT 85-2593. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J., & Rafferty, C.E. (1985b) Oral toxicity and plasma level study in
monkeys with Ivermectin (MK 0933) and abamectin (MK 0936). Study No.
T 85-013-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Landis,
D.K. (1985c) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. TT 85-710-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Geiger,
J.E. (1985d) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. 85-710-1. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., Powzaniuk, W., & Landis, D.K. (1985e) Oral maternotoxicity study
in mice with Avermectin B1b. Study No. 84-721-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Sina, J., Patterson, S.K., Berglind, R.M., Prato, M.,
& Quillin, F. (1985f) Microbial mutagenesis assays with abamectin
(avermectin B1; MK 0936). Study No. TT 85-8005 and 85-8051
Unpublished reports prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Galloway, S., Patterson, S.K., Armstrong, M., Bean,
Ch.L., & Deasy, D. (1985g) Abamectin (MK 0936) assay for chromosomal
aberrations in vitro, in chinese hamster ovary cells Study No. TT
85-8631, 85-8632, 85-8635. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Sina, J.F., Wright, S.P., Prato, M., & Quillin, F.
(1987a) Microbial mutagenesis assays of the Delta-8,9-isomer and
polar degradates of abamectin (Avermectin B1; MK 0936). Study Nos.
TT 87-8046, 87-8047, 87-8058. Unpublished reports prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Wise,L.D., Jensen, R.D., Nickell, B.E., Collevechio,
K., & Vetter, C.H. (1987b) Oral developmental toxicity study in rat
given the Delta-8,9-Isomer of abamectin (avermectin B1). Study No.
TT 87-715-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Minsker, D.H., Anderson, C.A., Nickell, B.E.,
Collevechio, K. & Deyerle-Brooks, K.A. (1987c) Oral developmental
toxicity study in mice given the polar degradates of abamectin (MK
0936). Study No. TT 87-717-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon L.R., Wise, L.D., Vonderfecht, St.L., Nickell, B.E.,
Collevechio, K., Powzaniuk, W., & McMahon, M.G. (1987d) Single
generation study in rats with the Delta-8,9-isomer of abamectin
(avermectin B1). Study No. 87-716-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Wise, L.E., Allen, M.L., Nickell, B.E.,
Collovechio,K., Powzaniuk, W., & Sina, J.L. (1988) Oral
developmental toxicity study in mice with L-930,463 (polar
degradate). Study No. TT 88-713-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Greene, B.M., Brown, K.R., & Taylor, H.R. (1989) Use of Ivermectin
in humans, In: Ivermectin and Abamectin, ed. by Campbell, W.C.,
Springerverlag, Chapter 21, p. 311-323. Submitted to WHO by MSDRL,
Three Bridges, NJ, USA.
Gruber, V.I. (1988) Identification of ß-alpha-hydroxy-avermectin
B1a as a metabolite of avermectin B1a in rats. Unpublished
report of Merck sharp and Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Mandel, J. (1977) Acute oral toxicity studies in mice and rats with
avermectin B1a (C-076(B1a)). Study Nos. TT 77-3248, 77-3250, 77-
3264, 77-3787, 77-3789, 77-3788, 77-3337 and 77-3346. Unpublished
reports prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986a) The metabolism
of Delta-8,9-Z-isomer avermectin B1a in rats. Unpublished report
ARM-2, prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986b) The metabolism
of avermectin B1a in rats. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
MSDRL (1977a) Oral reproduction study in rats with avermectin B1a
(C-076(B1a)). Study Number 77-706-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
MSDRL (1977b) Oral reproduction study in rats. Study Number 77-712-0
with avermectin B1a (C-076(B1a)). Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Norbury K.C. & Wolf, G.L. (1977) Fourteen-week oral toxicity study
in rats following in utero exposure to avermectin B1a (C-
076(B1a)). Study number TT 77-043-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. & Allen, H.L. (1976) Eighteen-week oral toxicity
study in dogs with avermectin B1a (C-076(B1a)). Study No. TT 76-
073-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. (1977a) Oral teratology study in mice with
avermectin B1a (C-076(B1a)). Study No. TT 77-705-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T. (1977b) Ten-day oral toxicity study in pregnant mice
with avermectin B1a (C-076(B1a)). Study No. TT 77-717-1.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel., J., Everett, M.A., Wolf,
G.L., & Powzaniuk, W., (1981a) Acute oral toxicity study in rats
with abamectin (avermectin B1). Study No. TT 81-2937. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Wolf, G.L., Stolz,
W.W., & Bielinski, T.C. (1981b) Acute dermal toxicity study in
rabbits with abamectin (avermectin B1). Study No. TT 81-3021.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981c) Acute ocular irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2940. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981d) Primary dermal irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2941, 81-2943, 81-
2945. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Skeggs, H. (1976) Microbial mutagenesis assay with avermectin B1a
(C-076(B1a)). Study No. TT 76-8052. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
WHO (1990) Evaluation of certain veterinary drug residues in food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 799, Geneva.
WHO (1993) Toxicological evaluation of certain veterinary drug
residues in food. WHO Food Additive Series No. 31, Geneva.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
A study was carried out to evaluate the tissue distribution and
elimination of 3H- and 14C-labelled avermectin B1a in a sesame
oil vehicle after oral administration to male and female rats.
Sixty-two CRCD strain rats/sex were divided into groups and dosed as
follows: a single high dose (1.4 mg/kg bw); a single low dose (0.14
mg/kg bw); fourteen daily low doses (0.14 mg/kg bw/day) of
unlabelled avermectin B1a, followed by a single low dose of
tritium-labelled avermectin B1a; a single high dose (1.4 mg/kg bw)
of a mixture of tritium - and 14C-labelled avermectin B1a; a
single dose of vehicle only to serve as controls for groups with
single dosing regimen or fifteen daily doses of vehicle only to
serve as controls for the multiple dosing experiment. The control
groups were sacrificed seven days after the last (or single) dose,
while rats from the treated groups were sacrificed on days 1, 2, 4
and 7 after dosing. Urine and faeces were collected each day after
dosing while the organs were collected from all rats at time of
sacrifice. All samples were assayed for total radioactivity.
Elimination in the urine was 0.3-0.6% of the applied dose in females
and 0.8-1.1% in males over the 7 days collection period. Elimination
in faeces ranged from 69-77% and 70-82% in females and males,
respectively. Residue levels in liver ranged from about 0.001-0.02
ppm, in kidney from 0.003-0.06 ppm, in muscle from 0.001-0.02 ppm
and in fat from 0.008-0.01 ppm in the different dosing groups, 7
days post-dose. The total residue level in the organs of the females
were generally higher than in males. The residue levels were dose
dependent, the residues in the high dose groups being roughly ten-
fold greater than in the low dose groups, whereas the depletion
rates were similar among the different tissues. The total
radioactive residues depleted from the tissues with half-lives of
approximately 1.2 days indicating that the residues did not persist.
Repeated dosing did not influence elimination rates or residue
levels. The tritiated avermectin B1a residue levels and depletion
rates were comparable to the 14C residue levels. Thus the tritium
label at the 5-position of avermectin B1a was not labile during
the course of the study. The stability of the label was also
demonstrated in an experiment for volatile/exchangeable tritium,
demonstrating that less than 2% of the tritiated avermectin B1a
sample was volatile (Alvaro et al., 1984).
Selected tissue samples (liver, kidney, muscle, fat) were
analyzed for unchanged avermectin B1a and metabolites. Two
metabolites that were also formed in vitro after rat liver
microsomal incubation, in addition to unchanged avermectin B1a,
accounted for most of the residues: The metabolites were identified
as 24-hydroxymethyl-avermectin B1a (24-OHMe-B1a) and 3"-desmethyl
avermectin B1a (3"-DM-B1a) (Maynard 1986b). A minor metabolite
was identified as ß-alpha-hydroxy-avermectin B1a (Gruber, 1988).
Toxicological studies
Acute toxicity studies
The main clinical signs were ataxia and tremors found in all
species investigated and irrespective of the route of
administration. The data are summarized in Tables 1 and 2.
Table 1: Acute toxicity of abamectin
Species Sex Route LD50 Component Reference
(mg/kg bw) (purity)
Mouse F oral 13.6-23.8 B1a Mandel (1977)
19.8 B1b (98.4%) Gordon et al. (1984a)
5-day mortality:
pregnant: 11.8-19.0 B1a+B1b (94%) Gordon et al. (1984b)
non pregnant: 15.0-41.3 B1a+B1b (94%) Gordon et al. (1985a)
Rat F,M oral ca. 11 B1a Mandel, 1977
F,M oral 8.7-12.8 B1a+B1b (91%) Robertson et al. (1981a)
Dog M,F oral ca. 8 B1a Robertson and Allen (1976)
B1a+B1b Gordon et al. (1984f)
Monkey M,F oral > 24 B1a+B1b Gordon et al. (1985b)
Rat dermal >330 B1a/B1b (87%/9.4%) Gordon & Mandel (1978)
Rabbit dermal >1600 B1a+B1b (91.4%) Robertson et al. (1981b)
B1a + B1b (94%) Gordon et al. (1983d)
Delta-8,9-isomer (photolytic degradation product)
Mouse M,F oral > 80 (Gordon et al. 1984c
& 1984e)
Table 2: Dermal and ocular irritation studies with abamectin,
technical material
Species Sex Target Findings Reference
Organ
Rabbit M,F eye very slight Robertson et al. (1981c)
irritation
Rabbit M,F skin non irritating on Robertson et al. (1981d)
intact or abraded
skin
Guinea- M,F skin: negative Gordon et al. (1983a)
pig sensitization
Short-term toxicity studies
Mice
In a twelve-week dietary range-finding study groups of mice
(CD-1 strain;15/sex/group) were fed dietary concentrations of 0,
2/40 (increase in week 9), 5, 10 or 20 ppm abamectin over 12 weeks
and 40 or 60 ppm over 3 weeks. There were no physical signs or
mortality. Decrease in body-weight gain at 60 ppm was the only
change observed. The NOAEL in this study was 40 ppm, equal to 8
mg/kg bw/day (Gordon et al., 1982f).
Rats
In an eight-week oral range-finding study in rats abamectin
(purity 94%) was administered to groups of rats (Charles River CD
albino rats, 10/sex/group) at dietary concentrations of 0, 5, 10,
15, 20/25 (increase in week 7), 40 or 60 ppm. Due to mortality and
the appearance of severe clinical signs of toxicity (tremors,
decreased activity) in the first study week, remaining animals at 40
and 60 ppm were sacrificed on days 15 and 5, respectively. These
groups were replaced by groups at 10 and 15 ppm in study week 5.
Decreases in mean body-weight gain were seen at 15, 20/25 (3 and 5%
decrease in males and females, respectively) and 40 ppm. Tremors
occurring at 15 and 20/25 ppm were no longer evident after study
week 1. One animal at 5 ppm showed slight tremors on day 2 only. No
gross or microscopic examination was performed. Based on clinical
signs and body-weight effects it appears that there is a steep dose-
response curve. Therefore the doses recommended for the
carcinogenicity study were 0.75, 1.5 and 2.0 mg/kg bw/day (Gordon
et al., 1982g).
Dogs
In a twelve-week oral range-finding study abamectin (purity
94%) was fed to groups of beagle dogs (2/sex/dose) at doses of 0,
0.25, 0.5, 1 or 4/2 mg/kg bw/day. Because of signs of toxicity
(tremors, weakness, incoordination, disorientation) and markedly
decreased food consumption at 4 mg/kg bw/day in one dog, this dose
was decreased to 2 mg/kg bw in study week 4. Inability to constrict
pupils after light stimulus was observed in some dogs at 1 mg/kg
bw/day or higher doses. Depression of body-weight gain or weight
loss occurred in dogs at 2 mg/kg bw/day and higher. No gross or
microscopic examination was performed. Based on the clinical signs
and body-weight effects recommended doses for the one-year oral dog
study were 0.25, 0.5 and 1 mg/kg/ bw/day. The NOAEL in this study
was 0.5 mg/kg bw/day (Gordon et al., 1982e).
Avermectin B1a (purity not specified) was orally administered
by gavage to beagle dogs (15/sex/dose) at dose levels of 0, 0.25,
0.5, 2 or 8 mg/kg bw/day over a period of 18 weeks. The treatment
did not affect parameters of urinalyses, ophthalmologic examination
or organ weights. Deaths occurred at incidences of 1/30, 3/30 and
3/30 at 0.5, 2 and 8 mg/kg bw/day, respectively. Signs of toxicity
at levels of > 0.5 mg/kg bw/day consisted of whole body muscular
tremors, ataxia, mydriasis and ptyalism (hypersalivation); in
addition, tonic convulsions and emesis occurred at 2 and 8 mg/kg
bw/day. Reduced body-weight gain was only observed at dose levels of
0.5 and 2 mg/kg bw/day, but not in surviving dogs at 8 mg/kg bw/day.
At 8 mg/kg bw/day changes in haematologic and serum biochemical
parameters included slightly increased values of haemoglobin,
haematocrit, erythrocytes, nonsegmented neutrophils and glucose
level. Electrocardiographic changes (elongation of the QT interval
and bradycardia) were noted at 8 mg/kg bw/day only at the beginning
of the study. Histopathologic changes were seen only in animals that
died or were killed moribund and consisted of diffuse hepato-
cellular vacuolation (without lipid accumulation) at dose levels of
0.5, 2 and 8 mg/kg bw/day and edema of the gallbladder at 2 and 8
mg/kg bw/day. The NOAEL in this study was 0.25 mg/kg bw/day
(Robertson & Allen 1976).
Groups of beagle dogs (6/sex/dose) were fed abamectin (purity
> 89%) for 53 weeks at concentrations resulting in doses of 0,
0.25, 0.5 or 1 mg/kg bw/day. The doses were selected on the basis of
the results of a previous range-finding study (Gordon et al.,
1982e). The compound administration had no effect on
ophthalmological examination, urinalyses or organ weights. Decreased
or even absence of constriction of pupils to light was evident at
0.5 mg/kg bw/day (occurrence rate 3%) and 1 mg/kg bw/day (15%) and
as single instances and in single animals also at 0.25 mg/kg bw/day.
At 1 mg/kg bw/day one dog was found dead (week 38), two others were
killed moribund (weeks 33 and 38). Reduced body-weight gain most
probably due to food unpalatability and therefore reduced food
consumption was observed at the highest dose level. A slight
decrease in serum urea nitrogen at 1 mg/kg bw/day was most probable
a consequence of decreased protein intake at this dose level. Slight
leukocytosis and increased packed cell volume was noted in one dog
killed in poor condition. There were no gross or microscopic changes
that could be attributed to treatment. The NOAEL in this study was
< 0.25 mg/kg bw/day, the single instance of mydriasis at this
dose being considered a borderline effect (Gordon et al., 1982i).
Long-term toxicity carcinogenicity studies
Mice
Abamectin (purity 90%) was administered in the diet to groups
of mice (Crl:CD-1(ICR)BR;74/sex/group; 24/sex/group for interim
sacrifice) at concen-trations resulting in doses of 0, 2, 4 or 8
mg/kg bw/day over a period of 94 weeks. In females, treatment-
related tremors were observed in all dose groups and deaths occurred
at 4 and 8 mg/kg bw/day. These effects were not observed in the 12-
week range-finding study at dosage levels up to 11 mg/kg bw/day (60
ppm). No explanation could be found for the sensitivity of these
female mice. Plasma samples taken from affected and unaffected
animals revealed no significant differences. A subsequent batch of
females did not show this unexpected sensitivity and following
restart of the study with a new group of female mice, tremors
occurred only in single animals at 8 mg/kg bw/day. In males, tremors
occurred in single moribund animals of the control and 8 mg/kg
bw/day group. In this group the mortality rate was increased:
towards the end of the study the mortality reached 60% compared to
45% in controls. Body-weight gain was reduced about 21% in females
and 7% in males at 8 mg/kg bw/day. There was a dose-related increase
in food consumption in treated females but a decrease in food
efficiency at 8 mg/kg bw/day. The treatment had no effect on
ophthalmic examinations, haematologic or biochemical parameters,
organ weights, gross pathology, histopathologic changes (with the
exception of a higher incidence of dermatitis at 2 and 8 mg/kg
bw/day in males). The tumour incidence was not increased by the
treatment. The NOAEL in this study was 4 mg/kg bw/day (Gordon et
al., 1983b).
Rats
Abamectin (purity 91%) was fed to groups of rats (Crl:CD(SD)Br;
65/sex/group) at dietary concentrations resulting in doses of 0,
0.75, 1.5 or 2/2.5/2 mg/kg bw/day over two years. Since no effects
attributable to abamectin were observed through study week 10, the
high dose level was increased to 2.5 mg/kg/ bw/day in week 11.
Because of the appearance of severe signs of CNS toxicity, the dose
was decreased again to 2 mg/kg bw/day in study week 13 for the
remainder of the study. No treatment-related changes with respect to
ophthalmoscopic, haematologic or serum biochemical parameters,
urinalyses and organ weights were observed. Increases in body-weight
gain were seen in all dosage groups during the first year on study.
By the end of the study comparable mean body-weight gains for all
groups of females were found, but an increased gain was noticed in
males. The increases in body-weight gain are considered treatment-
related, but the effect is not considered as an adverse effect.
Clinical signs consisting of tremors appeared in study week 12
correlated with the increase in dosage from 2 to 2.5 mg/kg bw/day in
week 11. The tremors in all the affected animals persisted
intermittently until sacrifice despite reduction in the high-dose
level back to 2 mg/kg bw/day in week 13. Several high-dose group
animals, which exhibited tremors, were sacrificed in a moribund
condition. The increase in mortality reflects the induction of
tremors during the early stage of the study and subsequent sacrifice
of the affected animals. Following the reduction in dose to 2
mg/kg/bw/day no new cases of tremors occurred and mortality in all
treated and control groups was comparable. No increase in tumour
incidence or treatment-related non-neoplastic histopathologic
changes were observed. The NOAEL in this study was 1.5 mg/kg bw/day
(Gordon et al., 1982h).
Reproduction studies
Mice
In a ten-day dietary maternotoxicity study abamectin (purity >
88%) was fed at dietary concentrations resulting in target dose
levels of 0, 0.1, 0.3 or 0.6 mg/kg bw/day (actual doses = 0.06,
0.16, or 0.33 mg/kg bw/day) to groups of pregnant mice (albino CF1;
20 females/group) on days 6 through 15 of gestation. Marked tremors
at 0.33 and at 0.16 mg/kg bw/day were observed. Reproductive
parameters (numbers of implants, resorptions and live and dead
fetuses) were not influenced. The NOAEL in this study was 0.06 mg/kg
bw/day (Gordon et al., 1983c).
Rats
One hundred-and-fifty F1 offspring, from five groups of F0
female rats (Charles River CD), that had been exposed in utero to
avermectin B1a at dosage levels of 0 (two groups), 0.1, 0.2 or 0.4
mg/kg bw/day were selected for a fourteen-week study of oral
toxicity. Groups of weanling rats (15/sex/group) received avermectin
B1a at doses of 0, 0.1, 0.2 or 0.4 mg/kg bw/day by gavage. The
treatment did not have any effects on mortality, ocular changes,
haematology, serum biochemistry, organ weights or gross and
microscopic tissue alterations. The increased body-weight gain of
male rats at 0.4 mg/kg bw/day was considered as treatment-related
but not as an adverse effect. The NOAEL in this study was 0.4 mg/kg
bw/day (Norbury and Wolf, 1977).
Avermectin (B1a) was administered orally to three groups of
female rats (12 females/dose group) at dose levels of 0, 0.5, 1, or
2/1.5 mg/kg bw/day from 14 days before mating throughout gestation
and lactation until day 21 postpartum. The high dose was reduced
after five doses to 1.5 mg/kg bw/day due to whole body muscular
tremors at 2 mg/kg bw/day. Two deaths occurred at the high dose, one
animal at this dose level became moribund. The significant decrease
in body-weight gain in the 1.5 mg/kg bw/day group was a result of
weight loss of the 3 moribund or dead animals. Weight gain increases
were observed during some periods at 1 and 1.5 mg/kg bw/day. A
treatment-related decrease in the number of live pups per litter on
day 1 postpartum at 1.5 mg/kg bw was observed. The pup weights were
decreased at this dose level on day 1. Pups at all dosage levels
showed decreases in average weight per litter throughout the study.
Dose-related increase in mortality among pups at all dose levels
were observed resulting in survival rates of 0, 14% and 76%, in the
1.5, 1 and 0.5 mg/kg bw/day groups respectively, compared to 98% in
the control group. There was a developmental retardation (eye
opening) at 0.5 and 1 mg/kg bw/day in surviving pups. The NOAELs in
this study were < 0.5 mg/kg bw/day for embryo-fetotoxicity and 1
mg/kg bw/day for maternotoxicity (MSDRL, 1977a).
Avermectin (B1a) was administered orally to three groups of
15 female rats at dose levels of 0, 0.1, 0.2 or 0.4 mg/kg bw/day
from 14 days before mating, throughout mating, gestation and
lactation until day 21 postpartum. No maternotoxic effects were
observed and the reproduction status was not adversely affected.
Among pups at 0.2 and 0.4 mg/kg bw/day a dose-related incidence of
spastic movements was noted increasing in severity with increasing
dose. At 0.2 and 0.4 mg/kg bw/day reduction in average pup weight
was observed (dose-related). Developmental retardation (eye opening,
ear opening, hear growth), occurred at 0.2 and 0.4 mg/kg bw/day
(dose-related). The NOAEL in this study was 0.1 mg/kg bw/day for
embryo-fetotoxicity (MSDRL, 1977b).
In an oral range-finding study (multigeneration) abamectin was
administered to groups of rats (Crl: CD (SD) BR Sprague-Dawley; 12
females/group) in the drinking water at concentrations of 0, 0.15,
0.5, 1.5 or 5 mg/l. Treatment was conducted from 14 days prior to
cohabitation with untreated males through day 21 postpartum and
subsequently through the F1 generation. Water consumption in
groups 0.15-1.5 mg/l increased during the lactation period and
decreased slightly in the 5 mg/l group. No accurate measurement of
water consumption could be performed prior to transfer to delivery
boxes on day 17 of gestation. Thereafter mean levels of water
consumption were comparable in all groups resulting in dose ranges
of 0.017-0.037, 0.066-0.127, 0.192-0.396, or 0.556-0.685 mg/kg
bw/day for the 0.15, 0.5, 1.5 mg/l or 5 mg/l group, respectively. In
the F0 generation the only effect observed was an increase in
body-weight gain in the 5 mg/l group in the first study week. In the
F1 generation an increase in postnatal mortality at 5 mg/l (53%
compared to 1% in control) was observed, physical signs observed in
pups at the highest dose level consisted of tremors; pup weights in
the 5 mg/l group were decreased. 3/116 Fetuses (2.6%) (from 2
litters) in the 5 mg/l group showed single malformations (1 cleft
palate, 1 sternebral malformation, 1 sternebral variation). One
fetus in the 0.5 mg/l group had lumbar ribs. Because of the single
incidences in the study groups and because of the occurrence of
these alterations in historical control groups it is questionable if
these effects are treatment-related (Gordon et al., 1981).
In a two-generation study, groups of rats
(Crl:COBSTMCDTM(SD) BR/30/sex/group) were orally treated
(gavage) with dosages of 0, 0.05, 0.12 or 0.4 mg abamectin/kg bw/day
. F0 and F1b rats were mated to produce F1a and F1b litters,
F2a and F2b litters. At 0.12 and 0.4 mg/kg bw/day both sexes of
the F0 rats during the first gestation period (F0-F1a) showed
increased body-weight gains, whereas during the subsequent lactation
period (F0-F1a) a reduction in body-weight gain compared to
control was observed at these two dose levels particularly on the
first days of lactation. Similar changes were also observed in the
next generation. During the second mating period of F0 rats (to
produce the F1b litters) mating performance was reduced (reduction
of incidence of mating) at 0.4 mg/kg bw/day as compared to control
animals. The reduction in mating performance was presumed to be
associated with irregular estrous cycles observed during the second
mating period of F0 female rats. This effect was not observed in
matings resulting in the F1a litters or the F2a and F2b
litters. During the lactation periods of both generations effects in
the 0.4 mg/kg bw/day group consisted of increased pup mortality,
reduced viability and lactation indices and lower average pup body-
weights. The incidence of pups which appeared thin and weak was
increased. These effects were less severe in the F2 litters than
in the F1 litters. No skeletal anomalies associated with the test
agent were revealed. Histopathological examination showed retinal
anomalies in 3/4 males and 1/5 females in the high-dose group
compared wich 1/10 in the control group (F1b weanlings). The
anomalies consisted of single or multiple retinal folds of many
layers that included pigment epithelium. The same lesion was seen in
one control female and in one male in the 0.05 and 0.12 mg/kg bw/day
groups of the F1b weanlings. In the F1b parental animals (both
sexes) only 1/35 showed this retinal anomaly. In the F2b weanlings
histopathology was performed on a greater number of animals. There
was a significant increase in retinal anomalies in the high-dose
group with the following incidences (M + F): 4.6% and 22% in the
control and 0.4 mg/kg bw/day, respectively. As will become evident
from the results of a reproduction study in rats conducted with
comparable doses of the delta-8,9-isomer of abamectin the control
incidence of retinal anomalies (4.6%) in the present study is much
smaller than in the other study, where a control incidence of 20%
was found. Therefore the difference observed may be incidental
following an unusually low incidence of this lesion in the
concurrent control group of the present study.
Among both the F1b and F2b weanlings the high-dose group
pups were the smallest. One hypothesis to explain the presence of
retinal folds in the high-dose group weanlings and the disappearance
with maturity proposed by the study authors is that the ocular globe
size is relatively smaller in the small pups (where there was
pronounced pup toxicity) relative to the size of the retina which
perhaps carried on at its normal growth rate. Therefore it may be
assumed that the retinal effects observed are a secondary effect.
Reversibility of the retinal anomalies is evidenced by its absence
in the high dose adults. The NOAELs in this study were 0.12 mg/kg
bw/day for pup toxicity and retinal anomalies and 0.05 mg/kg bw/day
for maternotoxicity (Gordon et al., 1982n).
Special studies on embryotoxicity/teratogenicity
Mice
Avermectin B1a was administered orally (gavage) to groups of
mice (albino CF1 strain/20 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.1, 0.2, 0.4 or 0.8 mg/kg bw/day.
The doses of the B1a component were calculated as the parent
compound.
Deaths occurred in the 0.1, 0.4 and 0.8 mg/kg bw/day groups (1,
3 and 2 animals respectively), in most cases preceded by tremors.
Body-weight gain was not influenced by the treatment; neither was
the reproductive status (number of implants, resorptions, live and
dead fetuses per litter). An increased incidence of cleft palate at
0.4 (4/165; 2.4%) and 0.8 mg/kg bw/day (5/199; 2.5%) was observed
compared to a control incidence of 0.4%. The NOAELs in this study
were < 0.1 mg/kg bw/day for maternotoxicity and 0.2 mg/kg bw/day
for embryo-fetotoxicity and teratogenicity (Robertson 1977a).
Groups of mice (albino CF1 strain/20 female/group) were
administered avermectin B1a by gavage from day 6 through 15 of
gestation at dose levels of 0, 0.025, 0.05, 0.075 or 0.1 mg/kg
bw/day. One animal died at 0.1 mg/kg bw/day and tremors were
observed in several additional mice at this dose. One animal at
0.075 mg/kg bw/day exhibited muscular tremors and became moribund.
Maternal body-weight gains were unaffected at any dosage level. The
NOAEL in this study was 0.05 mg/kg bw/day for maternotoxicity
(Robertson, 1977b).
In an oral maternotoxicity study the minor component of
abamectin, avermectin B1b was administered by gavage to groups of
mice (Crl:CF1 BR; 12 females/dose) at dose levels of 0, 0.025, 0.05,
0.075 or 0.1 mg/kg bw/day on days 6 through 15 of gestation. At the
0.075 mg/kg bw/day dose level two deaths occurred preceded by weight
loss and tremors. Single fetuses at all dose levels showed
malformations consisting of exencephaly and cleft palates resulting
in incidences of about 1% in all dose groups. The highest incidences
for historical control groups are 1.6% for exencephaly and 1.3 % for
cleft palates (overall incidence 0.3% for exencephaly and cleft
palate each). There was no evidence of fetotoxicity. The NOAEL in
this study was 0.05 mg/kg bw/day for maternotoxicity (Gordon et
al., 1985e).
Rats
In a range-finding study groups of mated female rats (Charles
River; 10/group) were administered abamectin by gavage at dose
levels of 0, 0.25, 0.5, 1 or 2 mg/kg bw/day on gestation days 6
through 17. One female at 2 mg/kg bw/day exhibited weight loss and
tremors and was sacrificed after receiving 12 doses. There was no
other evidence of maternal toxicity. At 0.25 and 2 mg/kg bw/day
there was a slightly increased body-weight gain that is considered
to be treatment-related but not an adverse effect. The NOAEL in this
study was 1 mg/kg bw/day (Gordon et al., 1982j).
In a rat teratology study groups of mated rats (Charles River;
25 females/group) were orally treated (gavage) with abamectin
(purity 94%) at dosage levels of 0, 0.4, 0.8 or 1.6 mg/kg bw/day on
days 6 through 19 of gestation. There was no evidence of maternal
toxicity. Slightly increased maternal body-weight gain was seen at
all dosage levels between days 6 and 14 of gestation. An increased
incidence of fetuses with external fetal malformations was observed
(exencephaly, cleft palate, gastroschisis) at 0.8 and 1.6 mg/kg
bw/day. The incidences were: 2/279 (0.7%) and 2/326 (0.6%),
respectively, compared to 1/319 (0.3%) in the control group.
Historical control incidence of cleft palate in this rat strain is
0.03% (overall) or 0.3% (single study with highest incidence).
Gastroschisis alone has an overall incidence of 0.004% or the
highest incidence in a single study of 0.3%. Based on the fact, that
in historical controls one single malformation (cleft palate) may
occur in up to 0.3% of control animals and no dose-response-
relationship is evident, it may be assumed that the increase
observed may either not be treatment-related or may be a borderline
effect. Visceral examination indicated a higher incidence of
distended ureters in the treated groups up to 3% compared to 0% in
controls), but without any dose-effect relationship. This effect is
observed at incidences of up to 7% in historical control groups.
Skeletal examination revealed a higher incidence of fetuses with
lumbar ribs and count variations at 1.6 mg/kg bw/day: at 1.6 mg/kg
bw/day an incidence of 72/326 (22%) was found, compared to 41/320
(13%) at 0.4, and 45/279 (16%) at 0.8 mg/kg bw/day compared to
44/319 (14%) in the control group. The increase at 1.6 mg/kg bw/day
may not be treatment-related because higher incidences are observed
in historical controls. For example, in historical controls lumbar
ribs occurred at an incidence of 14% (overall) or 27.8% (single
study with highest incidence). The NOAELs in this study were 1.6
mg/kg bw/day for maternotoxicity and < 1.6 mg/kg bw/day for
fetotoxicity and teratogenicity (Gordon et al., 1982k).
Rabbit
In a range-finding study groups of New Zeeland albino rabbits
(10 females/dose) were given abamectin at oral dosage levels of 0,
0.5, 1, 2 or 3 mg/kg bw/day on gestational days 6 through 18.
Maternotoxicity was observed at 3 mg/kg bw/day. One female at 3
mg/kg bw/day was sacrificed moribund on day 16 of gestation after 11
doses. All females at 3 mg/kg bw/day were in a stupor after the
fourth and subsequent doses and showed yellow or green discharge
from nose or mouth. Moreover animals at 3 mg/kg bw/day had a
decreased food and water consumption, and showed body-weight loss.
The NOAEL in this study was 2 mg/kg bw/day (Gordon et al., 1982).
In a teratogenicity study groups of female New Zeeland albino
rabbits (18/dose) were given abamectin (purity 94%) at oral dosage
levels of 0, 0.5, 1 or 2 mg/kg bw/day on day 6 through 27 of
gestation. There were single deaths in all the treatment groups. At
2 mg/kg bw/day the animals showed decreased food and water
consumption and weight loss. Fetuses of the 2 mg/kg bw/day showed
cleft palates, omphaloceles and clubbed forefeet at higher
incidences than in the concurrent control group: 7.4% versus 2.1% in
concurrent control. The incidence of clubbed fore foot alone in
historical control groups is 0.2% (overall) and 3.9%, respectively,
in a single study with highest incidence. No data are available
concerning historical control incidences of cleft palates and
omphaloceles in rabbits. The clearly higher incidence of these
malformations at 2 mg/kg bw/day is therefore considered to be
treatment-related. With respect to skeletal fetal examination higher
incidences of skeletal terata (vertebral malformations, branched and
fused ribs) were observed at 0.5, 1 and 2 mg/kg bw/day with
incidences of 4%, 2% and 4% of the fetuses showing these
malformations respectively, compared to 0% in the concurrent control
group. In historical controls single vertebral malformations may
occur at incidences of up to 0.5% (overall) (e.g., caudal vertebral
malformations) with the highest value of 9% in a single study (e.g.,
fused ribs). At 0.5 and 2 mg/kg bw/day there were increased
incidences of incompletely ossified sites particularly in sternebrae
and metacarpals. These effects were more pronounced in the highest
dose group. Because the incidences observed at 1 mg/kg bw/day were
comparable to the incidences in the control group the absence of a
dose-relationship in the lower dose levels of 0.5 and 1 mg/kg bw/day
indicates that the increased incidence at 0.5 mg/kg bw/day is
possibly not treatment-related. The NOAEL in this study was 1 mg/kg
bw/day for maternotoxicity, embryo-fetotoxicity, and teratogenicity
(Gordon et al., 1982m).
Special studies on genotoxicity
A number of genotoxicity studies have been conducted with
abamectin. The results are summarized in Table 3.
Special studies on the delta-8,9-isomer of avermectin B
Biochemical aspects
Rat
Male rats were orally (gavage) dosed with 1.4 mg3H-labelled
delta-8,9-isomer avermectin B1a/kg bw. The delta-8,9-isomer of
abamectin is a photolytic degradation product of abamectin. The
stability of the tritium label at the 5th carbon position was
previously demonstrated with avermectin B1a in rats. Within 7 days
after dosing 94% of the administered dose was eliminated with the
faeces, and 0.4% in the urine. The levels of residues in liver,
kidney, fat and muscle ranged from 0.28 ppm (muscle) to 1.4 ppm
(fat) on day 1 after dose and decreased to a range of 0.017 to 0.1,
respectively, on day 7 after dosing. The half-lives for the tissue
residues in muscle, kidney, liver and fat varied between 1.45 and
1.64 days. As major metabolite the 3"-desmethyl-delta-8,9 isomer was
isolated. The 3"-DM-delta-8,9 isomer was also isolated and
identified after rat liver microsomal incubation of the delta-8,9-
isomer. The minor metabolite 24-hydroxymethyl-Delta-8,9-isomer,
(also formed in vitro), was identified.
By comparison, the elimination by rats of both the delta-8,9-
isomer avermectin B1a in this study and the avermectin B1a in a
previous study was very similar. In both studies 94-96% of the
administered dose were recovered within 7 days after dosing in the
excreta. The tissue residue levels were also demonstrated to be
similar for both compounds. The average half-life for the residue in
the edible tissues was 1.1 and 1.5 days for avermectin B1a and
delta-8,9-isomer, respectively. The metabolites generated were very
similar with rats metabolizing both compounds to the 3"-desmethyl
and the 24-hydroxymethyl metabolites. Similarly, both avermectin
B1a and ivermectin B1 (22,23-dihydro-avermectin B1) have been
shown to be primarily metabolized to the 3"-desmethyl and/or 24-
hydroxymethyl metabolite(s) by many other animal species (Maynard
et al., 1986a).
Table 3. Results of genotoxicity assays on abamectin
Test System Test Object Concentration Results Reference
(purity)
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1982a)
typhimurium (precipitate at conc.
> 3000 µg/plate)
± activation
(94%)
Ames test Salmonella 1-2000 µg/plate * negative Skeggs (1976)
typhimurium
Ames test Salmonella 100-10 000 µg/plate negative Gordon et al. (1985f)
typhimurium without activation
(89%)
3-1000 µg/plate
± activation
(94%)
V-79 mammalian V-79 Chinese 0.03 - 0.05 mM negative Gordon et al. (1982b)
cell assay hamster lung cells (+ activation)
(HGPRT Locus) 0.003-0.006 mM
(- activation)
Alkaline elution Rat hepatocytes 0.01-0.6 mM positive in Gordon et al. (1982c)
assay in vitro concentrations
(DNA single (> 0.2 mM)
strand breaks)
Test System Test Object Concentration Results Reference
(purity)
Table 3 (contd)
Test System Test Object Concentration Results Reference
(purity)
In vitro Chinese hamster 0.005 - 0.025 mM negative Gordon et al. (1985g)
chromosomal ovary cells with activation
aberration
0.01 - 0.035 mM
without activation
Alkaline elution Rat oral up to 10.6 mg/kg bw negative Gordon et al. (1982d)
assay in vivo (approx. LD50)
(DNA single
strand breaks)
In vivo cytogenetic Mouse oral 1, 2, 4, negative Blazak et al. (1983)
assay 12 mg/kg bw
(mouse bone marrow) (94%)
Delta-8,9-isomer: Salmonella 10-3000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at conc.
> 1000 µg/plate)
(91,6%)
Polar degradates: Salmonella 100-10 000 µg/plate negative Gordon et al. (1987a)
reverse mutation typhimurium ± activation
test and E. coli (precipitation at
10 000 µg/plate)
* avermectin B1a
Embryotoxicity/teratogenicity and reproduction
Mice
The delta-8,9-isomer of abamectin was given by gavage in sesame
oil to groups of 7-11 mated female mice (Crl:CF1 BR) at doses of 0,
1.5, 3, 6.25, 12.5, 25, or 50 mg/kg bw/day on day 6 through 15 of
gestation. Treatment-related deaths occurred at all dosage levels.
The groups at 3 mg/kg bw/day and higher doses were terminated on
days 6-8 of gestation. There was a slight decrease in body-weight
gain on single days at 1.5 mg/kg bw/day compared to control. Because
of early termination, no body-weights were measured after the
beginning of dosing in the other dose groups. Fetal effects became
apparent at 1.5 mg/kg bw/day (the only group with litters) as an
increased incidence of fetuses with cleft palate (24/83 (29%)
compared to 0% in the concurrent control. The NOAEL in this study
was <1.5 mg/kg bw/day for maternotoxicity and teratogenicity
(Gordon et al., 1984d).
A subsequent study with the delta-8,9-isomer was performed to
establish a no-effect level. Oral doses were administered to groups
of mice (Crl: CF1 BR; 12 mated females/dose) at levels of 0, 0.05,
0.1, 0.5 or 1 mg/kg bw/day on days 6 through 15 of gestation. Single
females at 0.5 and 1 mg/kg bw/day showed clinical signs including
tremors and loss of body-weight. Decreases in body-weight gain were
observed at 0.05 and 1 mg/kg bw/day, but not at 0.1 mg/kg bw/day. No
dose-related increase in the incidence of resorptions and dead
fetuses occurred in any treatment groups. In addition there were
fewer implants per female in the 0.05 (10.4 per female) and 1 mg/kg
bw/day (9.8 per female) groups compared to 12.5 per female in the
control group resulting in decreases in live fetuses per female at
0.05 (8.7) and 1 mg/kg bw/day (8.3) compared to control (11.3).
These differences from control and particularly those at the low
dose level were probably not treatment-related since the changes
were slight and were not observed at the intermediate dose levels.
An increased incidence of cleft palates were observed at dose
levels of 0.1 mg/kg bw/day and above with incidences of 13/115
(11%), 1/90 (1%) and 7/91 (8%) at dose levels of 0.1, 0.5, and 1
mg/kg bw/day, respectively, compared to 0% in the concurrent control
group. The highest historical control incidence of cleft palates was
reported to be 3% in one study (overall 0.3%). There were also
slightly increased incidences of exencephaly at dose levels of 0.1
mg/kg bw/day and above: of 1/115 (1.7%), 4/90 (4%), and 2/91 (2%) at
the dosage levels of 0.1, 0.5 and 1.0 mg/kg bw/day, respectively,
compared to 1/136 (0.7%) in the concurrent control. The highest
incidence of historical control incidence was reported to be 1.6% in
a single study (overall 0.3%). As with the incidence of cleft
palates no dose-response-relationship was evident but the observed
incidences were clearly higher than the range of historical controls
reported. The NOAELs in this study were 0.1 mg/kg bw/day for
maternotoxicity and 0.05 mg/kg bw/day for fetotoxicity and
teratogenicity (Gordon et al., 1984d).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR; 25 females/dose group) at dosage
levels of 0, 0.015, 0.03 or 0.06 mg/kg bw/day on days 6 through 15
of gestation (original intent was to have dosage levels of 0.025,
0.05 and 0.1 mg/kg bw/day; but an error in preparation resulted in
different dose levels). No signs of maternotoxicity were observed.
Increased incidences of exencephaly in the 0.03 and 0.06 mg/kg
bw/day groups were found, but without any dose-response
relationship. The incidence in both groups was about 1.3% compared
to 0% in the concurrent control and the 0.015 mg/kg bw/day group.
Compared to the incidences of historical controls the present
incidence of 1.3% is slightly smaller than the highest incidence of
1.6% of a single study in historical control animals. A single fetus
(1/210) in the 0.015 mg/kg bw/day group had a cleft palate resulting
in an incidence of about 0.5%. The NOAELs in this study were 0.06
mg/kg bw/day for maternotoxicity and < 0.06 mg/kg bw/day for
teratogenicity (Gordon et al., 1985c).
The delta-8,9-isomer of avermectin B1 was orally administered
to groups of mice (Crl:CF1 BR;25 females/group) on days 6 through 15
of gestation at dosage levels of 0, 0.015, 0.03, 0.1 or 0.5 mg/kg
bw/day. One animal at 0.5 mg/kg bw/day was sacrificed in a moribund
condition. Incidence of cleft palate was 24/233 (10%), 6/279 (2%),
and single fetuses (0.4%) at 0.5, 0.1, and at both 0.03 and 0.015
mg/kg bw/day, respectively. The overall incidence of cleft palate in
historical controls is reported to be 0.3%. The incidences of
exencephaly of about 0.4% that occurred in single fetuses in the
control, 0.015 and 0.5 mg/kg bw/day groups and in 5/238 (2%) in the
0.03 mg/kg bw/day group did not show any dose-response-relationship.
This compares with, but is greater than, the overall incidence of
0.3% and is less than 1.6% (highest incidence) of historical control
animals except at 0.03 mg/kg bw/day. The NOAELs in this study were
0.1 mg/kg bw/day for maternotoxicity and 0.03 mg/kg bw/day for
embryo-fetotoxicity and terato-genicity (Gordon et al., 1985d).
Rats
In an oral study groups of rats (Sprague-Dawley Crl:CD(SD)BR;
25 females/group) were treated with dosage levels of 0, 0.25, 0.5 or
1.0 mg/kg bw/day of the delta 8,9-isomers of abamectin on days 6
through 17 of gestation. The body-weight gain in treatment groups
was slightly greater than in the control group. This effect on body-
weight gain was seen in previous teratology and reproductive studies
with the parent compound at comparable doses (Gordon et al.,
1982k,n). As in the previous studies, this increased body-weight
gain is considered treatment-related but not an adverse effect. No
evidence of embryo- or fetotoxicity or teratogenicity at any dose
level was given. The NOAEL in this study was > 1 mg/kg bw/day
(Gordon et al., 1987b)
In a single generation study groups of rats (Sprague-Dawley
[Crl: CD(SD)BR]; 20 females/dose) were orally treated (gavage) at
dosage levels of 0, 0.06, 0.12 or 0.4 mg of the delta-8,9-isomer of
abamectin/kg bw/day. The period of dosing was fifteen days prior to
cohabitation through day 20 of lactation. There were no treatment-
related effects on F0 female reproductive performance (mating
index, fertility, length of gestation). Histopathological
examination of the F1 weanlings revealed retinal anomalies in all
groups including controls, consisting particularly of intra-retinal
folds. The incidences were 20, 16, 16 and 22% in control, 0.06, 0.12
and 0.4 mg/kg bw/day groups, respectively. More severe retinal
anomalies were identified in animals of the low- and mid-dose groups
compared to control animals. These changes were compared with the
severity of retinal anomalies observed in control weanling rats of a
previous two-generation study conducted with abamectin (Gordon et
al., 1982n). According to the statement of the study authors the
retinal anomalies observed in the present study have comparable
severity as those in the previous control animals, but no original
data were presented. The study authors conclude that no ocular
lesions were produced as a result of the administration of the
compound. The fact that no clear dose-response with regard to the
incidence and severity of these retinal effects is observed supports
the suggestion that in fact they are not caused by the treatment
(Gordon et al., 1987d).
Polar degradates (unidentified mixture of residues generated
in vitro and in a field study on oranges).
In two oral developmental toxicity studies groups of female
mice (Crl:CF1 BR; 25/group) were orally treated (gavage) at dosage
levels of 0, 0.25, 0.5 and 1 mg/kg bw/day on days 6 through 15 of
gestation. The treatment did not have maternotoxic effects and did
not influence the reproductive performance. On fetal examination
there was no evidence of embryotoxic/fetotoxic or teratogenic
effects of the compounds (Gordon et al., 1987c, 1988).
Genotoxicity studies with metabolites
See Table 3.
Antidote study of abamectin intoxication in dogs
Ipecac given at a rate of 30 ml/dog by stomach tube 15 min
after a lethal oral dose of abamectin of 8 mg/kg bw induced vomiting
within 15-45 min after ipecac was given. This treatment was adequate
to prevent coma and death in dogs given a lethal dose of abamectin.
Ipecac treatment only after 30 min or longer after the dose of
abamectin did not prevent coma and death of the dogs (Gordon et
al., 1984f).
Observations in humans and non-human primates
The comparison of the toxicological data of the two members of
the avermectin family of compounds, abamectin and ivermectin,
reveals a lot of similarities. Ivermectin has been used extensively
in humans at an oral therapeutic dose of 0.2 mg/kg bw for the
treatment of onchocerciasis without serious drug-related adverse
effects (Greene et al., 1989). Therefore the human experience with
a closely related compound offers useful information to be
considered in the evaluation of abamectin.
Comparative studies with ivermectin in monkeys
Safety assessment studies with abamectin and ivermectin have
shown that the susceptibility of different animal species to these
compounds varies considerably. The pregnant mouse is the most
sensitive mammal examined so far. In view of the similarity in
structure and toxicity of abamectin and ivermectin and the diversity
of responses among the different species it was suggested that an
acute oral toxicity study in rhesus monkeys might serve as a
valuable source of information about the potential toxicity of the
compounds in primates. Such a study might provide a more rational
basis for predicting the toxicity of abamectin in humans.
The purpose of the study was to determine the minimum toxic
dose (mTD) of abamectin and ivermectin in monkeys and to determine
the plasma levels of drug at that dose.
Single oral doses of abamectin or ivermectin were given to four
rhesus monkeys (2/sex) each at intervals of 2-3 weeks, in the
following chronological dose levels: 0.2, 0.5, 1, 2, 4, 6, 8, 12 or
24 mg/kg bw. The most sensitive indicator of toxicity was emesis
occurring at dose levels of 2 mg/kg bw and higher. The incidence of
emesis was dose-related and the time after dosing when emesis
occurred tended to decrease with increasing dose. Marked mydriasis
(lack of pupillary constriction) was noted only at 24 mg
abamectin/kg bw. Less pronounced mydriasis was observed after doses
of 24 mg ivermectin/kg bw. At 24 mg/kg bw both compounds induced
slight to moderate sedation.
Since no emesis occurred with doses below 2 mg/kg bw and since
no other drug-related physical signs of toxicity were observed at
lower doses, it was concluded that emesis was the most appropriate
physical sign for characterizing minimum toxic doses of the
compounds in monkeys and that the mTD was 2 mg/ kg bw for both
compounds. This dose is tenfold higher than the human dose for
onchocerciasis therapy of 200 µg/kg bw.
Plasma concentrations of ivermectin were similar to the levels
of abamectin for up to 4 h following the 2 mg/kg bw doses, but
thereafter ivermectin levels were higher on the average than those
of abamectin. Peak levels occurred between 8-24 h post-dose with
maximum values of 110 ng/ml for ivermectin and 76 ng/ml for
abamectin after a 2 mg/kg bw dose. The average maximum plasma level
following the 24 mg/kg bw dose of ivermectin was 680 ng/ml, after
the same dose of abamectin it was 390 ng/ml. Despite roughly
proportional increases in plasma levels with increased doses, the
severity of clinical signs did not worsen appreciably and no
tremors, convulsions or deaths occurred even at a dose of 24 mg/kg
bw. The dose-response curve for acute toxicity of abamectin and
ivermectin in monkeys seems to be much flatter than for mice,
indicating that in primates, clinical evidence of intoxication
(emesis) may occur well in advance of serious or life-threatening
toxicity. Mean peak plasma level measured in humans given the 200
µg/kg bw therapeutic dose of ivermectin was 20 ng/ml (Gordon et
al., 1985b).
Observations in humans
Whereas for abamectin no observations in humans are reported,
information is available concerning the therapeutic use and the
safety of ivermectin, extensively tested in human onchcerciasis. In
a single yearly dose it suppresses microfilaria in the skin and eyes
and prevents disease progression in most infected persons. A single
oral dose of 30 µg/kg bw or 50 µg/kg bw exhibited microfilaricidal
activity. Transient pruritus was observed soon after treatment, but
no abnormal laboratory results were produced (Aziz et al., 1982).
In a 12-month follow-up study investigating the efficacy of
different dosages of the drug it was shown that doses of 100 µg/kg
bw did not produce different reactions from the placebo group,
whereas dose levels of 150 and 200 µg/kg bw only produced mild
reversible clinical reactions (Mazzotti-like reactions). It is
suggested therefore that 150 µg/kg bw is probably the optimal dose
in terms of its antiparasitic activity and side effects. A single
oral dose of 150 µg/kg bw annually induces a minimal reaction in 5-
15% of infected adults and a more significant reaction in about 1%.
Its use was associated with hypotension, usually occurring within 24
h of administration in 1 out of 1000 persons (Greene et al.,
1989).
COMMENTS
Following oral administration of abamectin to rats, 69-82% of
the administered dose was eliminated in the faeces and only 1% in
the urine. Biliary excretion was the major cause of the high level
of faecal excretion. Biotrans-formation proceeds mainly by
demethylation and hydroxylation.
Orally administered abamectin elicited dose-dependent CNS
effects, including tremors and ataxia.
In a one-year dietary study in dogs at doses of 0, 0.25, 0.5 or
1 mg/kg bw/day, a borderline NOAEL of 0.25 mg/kg bw/day was
determined, despite single instances of mydriasis at this lowest-
dose level.
In a two-year long-term/carcinogenicity study in mice,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 2, 4 or 8 mg/kg bw/day. The NOAEL was 4 mg/kg bw/day,
based on the occurrence of tremors, a higher mortality rate and
reduced body-weight gain at 8 mg/kg bw/day. Abamectin was not
carcinogenic in the mouse.
In a two-year long-term/carcinogenicity study in rats,
abamectin was administered in the diet at concentrations resulting
in doses of 0, 0.75, 1.5 or 2 mg/kg bw/day. The NOAEL was 1.5 mg/kg
bw/day. Higher doses caused CNS toxicity. Abamectin was not
carcinogenic in rats.
In two one-generation reproduction studies in rats avermectin
B1a was administered in the diet at concentration resulting in
dosage levels ranging from 0.1 to 2 mg/kg bw/day in rats.
Maternotoxicity was observed at dose levels above 1 mg/kg bw/day.
Fetotoxicity, consisting of reduced pup survival rates, reduced pup
weight growth and retardation became evident at dose levels of 0.5
mg/kg bw/day and higher. The NOAEL for fetotoxicity was 0.1 mg/kg
bw/day.
In a two-generation reproduction study in rats at dose levels
of 0.05, 0.12 or 0.4 mg abamectin/kg bw/day, the NOAEL for
maternotoxicity was 0.05 mg/kg bw/day, based on reduced maternal
body-weight gain during lactation at 0.12 mg/kg bw/day and above.
The NOAEL for pup toxicity was 0.12 mg/kg bw/day, based on increased
mortality and lowered pup weights at 0.4 mg/kg bw/day.
The teratogenic potential of abamectin administered by gavage
was investigated in mice, rats and rabbits. Teratogenic effects,
including cleft palates, omphaloceles and clubbed fore feet, were
observed at maternotoxic doses in mice and rabbits. The NOAEL for
teratogenicity in the most sensitive species, the mouse (CF1 strain)
was 0.2 mg/kg bw/day, while for maternotoxicity the NOAEL was 0.05
mg/kg bw/day, based on the occurrence of tremors and deaths at
higher doses.
Various studies to investigate the teratogenic potential of the
delta-8,9-isomer have been conducted in mice and rats. Similar
teratogenic effects to those seen with abamectin were observed in
the most sensitive species, the mouse. The NOAELs for
maternotoxicity in the mouse were 0.1 mg/kg bw/day, and for
fetotoxicity/teratogenicity, 0.05 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that abamectin was not genotoxic.
Although no human data were available on abamectin, extensive
data on field and community-based trials with ivermectin in humans
infected with Onchocera spp. were available (WHO, 1993). The main
effects noted were those arising from the death of parasites, the
so-called Mazzotti reaction, which is characterized by arthralgia,
pruritus, fever, hypertension, tachycardia, headache, and ocular
changes. Very limited data in humans indicate that ivermectin does
not increase the incidence of birth defects, although it is
teratogenic in mice, rats and rabbits (WHO, 1990).
The available data provided adequate toxicological information
to permit the allocation of an ADI for abamectin and its delta-8,9-
isomer, based on the NOAELs for abamectin of 0.05 mg/kg bw/day in
the teratogenicity study in mice and in the two-generation
reproduction study in rats. The NOAEL for the delta-8,9-isomer was
0.05 mg/kg bw/day in the teratogenicity study in mice. A safety
factor of 500 was used because of concern over the teratogenicity of
the delta-8,9-isomer which forms part of the residue in food.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Abamectin (and components avermectin B1a and B1b):
Mouse: 4 mg/kg bw/day (2 year feeding study)
0.05 mg/kg bw/day (teratology study, maternotoxicity)
0.2 mg/kg bw/day (teratology study, teratogenicity)
Rat: 1.5 mg/kg bw/day (2-year study)
0.1 mg/kg bw/day (1-generation reproduction study)
0.05 mg/kg bw/day (2-generation reproduction study,
maternotoxicity)
0.12 (2-generation reproduction study, pup toxicity)
Dog 0.25 mg/kg (borderline) (1-year study)
Delta-8,9-isomer
Mouse 0.1 mg/kg bw/day (teratology study, maternotoxicity)
0.05 mg/kg bw/day (teratology study, teratogenicity)
Estimate of acceptable daily intake for humans (abamectin and
delta-8,9-isomer)
0-0.0001 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing studies on the mechanism of central nervous system
toxicity.
2. Observations in humans.
REFERENCES
Alvaro, R.F., Green, M.L., Halley, B.A., Maynard, M.S., &
Meriwether, H.T. (1984) Distribution and clearance of avermectin
B1a in rats. Unpublished report ARM-1 prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Aziz, M.A., Biallo, S., Diopp, I. and Lariviere, M. (1982) Efficacy
and tolerance of ivermectin in human onchocerciasis. Lancet, 24
July, p. 171-173. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Blazak, W.F., Mitchell, A.D., & Skinner, W.A. (1983) An assessment
of the mutagenic potential of abamectin (mk 0936) utilizing the in
vivo mouse bone marrow cytogenetics assay. Study Number TT 83-900-
6. Unpublished report prepared by SRI International, Menlo Park, CA,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. & Mandel, J. (1978) Acute dermal toxicity study in rats
with abamectin (avermectin B1; C-076). Study Number TT 78-3607.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Nickell, B.E., Collevechio, K., Mutchler, M., & Clark,
R.L. (1981) Oral-range-finding study (multigeneration) in rats with
abamectin (MK 0936). Study No. 82-707-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Cook, M.M., Berglund, R.M., & Prato, M.
(1982a) Microbial mutagen test on abamectin (MK 0936) with and
without rat liver enzyme activation. Study No. TT 82-8013.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., & Patterson, S.K. (1982b) V-79
mammalian cell mutagenesis assay with abamectin (MK 0936). Study No.
TT 82-8506, 82-8510, 82-8512, 82-8519. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982c) In vitro alkaline elution/rat hepatocyte
assay with abamectin (MK 0936). Study No. TT 82-8520, 82-8523, 82-
8525, 82-8526. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Bradley, M.O., Patterson, S.K., Taylor, V.E., &
Dysart, G.R. (1982d) In vivo alkaline elution/rat hepatocyte assay
with abamectin (MK 0936). Study No. TT 82-8302. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1982e) Twelve-week oral
range-finding study in dogs given abamectin (MK 0936). Study No. TT
82-073-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Peter, Ch.P., Nickell, B.E., Buck, J.F., & Schultz,
A.K. (1982f) Twelve-week oral dietary range-finding study in mice
given abamectin (MK 0936). Study No. TT82-082-0,-2,-2. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Lankas, G.R., Nickell, B.E., Buck, J.F., & Jackson, L.
(1982g) Eight-week dietary range-finding study in rats given
abamectin (MK 0936). Study No. TT 82-075-0, -1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Bokelman, D.L., & Skolnick, E.M. (1982h) One-hundred-
five week dietary carcinogenicity and toxicity study of abamectin
(MK 0936) in rats with a 53-week interim necropsy. Study No. TT 82-
099-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. Allen, H.L., Nickell, B.E., Satiritz, S.M., Powzaniuk,
W., Roux, L., & McKeon, J. (1982i) Fifty-three week dietary study in
dogs given abamectin (MK 0936). Study No. TT 82-104-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Weller,
J.V. (1982j) Oral range-finding study in pregnant rats given
abamectin (MK 0936). Study Number TT 82-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982k) Oral teratology study in rats given abamectin
(MK 0936). Study No. TT 82-705-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., &
Siriani, L.B. (1982l) Oral range finding study in pregnant rabbits
given abamectin (MK 0936). Study No. TT 82-706-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Vetter,
C.M. (1982m) Oral teratology study in rabbits given abamectin (MK
0936). Study No. TT 82-706-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Mildred, S.C., & Hoberman, H.M. (1982n) Reproductive
effects of abamectin (MK 0936) administered orally by gavage to Crl:
COBS CD(SD)BR rats for two Generations. Study No. TT 82-9010.
Unpublished report prepared by Argus Research Laboratories, Horsham,
Pennsylvania, USA and by Merck Sharp & Dohme Research Laboratories,
West Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three
Bridges, NJ, USA.
Gordon, L.R., Mandel, J., McDonald, J.S., Nickell, B.E., Powzaniuk,
W., & Bielinski, T.C. (1983a) Guinea pig skin maximization test with
abamectin (MK 0936). Study No. TT 83-2506. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Lankas, G.R., Fabry, A., Nickell, B.E., Powzaniuk, W.,
Buck, J.F., Satiritz, S.M., & Schultz, A. (1983b) Ninety-four week
dietary carcinogenicity and toxicity study in mice given abamectin
(MK 0936). Study No. TT 83-002-0,-1,-2,-3. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon L.R., Minsker, D.H., Nickell, B.E., Collevechio, K., &
Battisti, G.A. (1983c) Ten-day dietary maternotoxicity study in mice
given abamectin (MK 0936). Study No. TT 83-705-1. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Nickell, B.E., Mandel, J., Powzaniuk,
W., & Stolz, W.W. (1983d) Acute dermal toxicity study in rabbits
given abamectin (MK 0936). Study No. TT 83-064-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., McDonald, J.S., Allen, H.L., Nickell, B.E., Mandel,
J., Powzaniuk, W., & McAfee, J.L. (1984a) Acute oral toxicity study
in mice given Avermectin B1b. Study No. TT 84-107-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1984b)
Five-day acute oral toxicity study in pregnant and non-pregnant cf1
mice with abamectin (MK 0936). Study No. TT 84-2842-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Mandel, J., Majka, J.A., & McAfee, J.L. (1984c) Acute
oral toxicity study in mice given the Delta-8,9-isomer of abamectin
(MK 0936). Study No. 84-112-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., & Landis, D.K. (1984d) Oral maternotoxicity study in mice with
the Delta-8,9 isomer of abamectin (avermectin B1). Study No. 84-
722-0,-1. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R. (1984e) Acute oral toxicity study in mice with Delta-
8,9-isomer of abamectin (MK 0936). Study No. TT 84-2820. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Bokelman, D.L., & Stone, C.A. (1984f) Exploratory non-
specific antidote study of abamectin (MK 0936) intoxication in dogs.
Study No. 84-085-0. Unpublished report prepared by Merck Sharp &
Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., McDonald, J.S., Mandel, J., & McAfee, J.L. (1985a)
Five-day acute oral toxicity study in pregnant and nonpregnant CF1
mice with abamectin (MK 0936). Study No. TT 85-2593. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J., & Rafferty, C.E. (1985b) Oral toxicity and plasma level study in
monkeys with Ivermectin (MK 0933) and abamectin (MK 0936). Study No.
T 85-013-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Landis,
D.K. (1985c) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. TT 85-710-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Nickell, B.E., Collevechio, K., & Geiger,
J.E. (1985d) Oral teratology study in mice with the Delta-8,9-Isomer
of abamectin (avermectin B1). Study No. 85-710-1. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Gordon, L.R., Clark, R.L., Allen, H.L., Nickell, B.E., Collevechio,
K., Powzaniuk, W., & Landis, D.K. (1985e) Oral maternotoxicity study
in mice with Avermectin B1b. Study No. 84-721-0. Unpublished report
prepared by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Sina, J., Patterson, S.K., Berglind, R.M., Prato, M.,
& Quillin, F. (1985f) Microbial mutagenesis assays with abamectin
(avermectin B1; MK 0936). Study No. TT 85-8005 and 85-8051
Unpublished reports prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Galloway, S., Patterson, S.K., Armstrong, M., Bean,
Ch.L., & Deasy, D. (1985g) Abamectin (MK 0936) assay for chromosomal
aberrations in vitro, in chinese hamster ovary cells Study No. TT
85-8631, 85-8632, 85-8635. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Sina, J.F., Wright, S.P., Prato, M., & Quillin, F.
(1987a) Microbial mutagenesis assays of the Delta-8,9-isomer and
polar degradates of abamectin (Avermectin B1; MK 0936). Study Nos.
TT 87-8046, 87-8047, 87-8058. Unpublished reports prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Wise,L.D., Jensen, R.D., Nickell, B.E., Collevechio,
K., & Vetter, C.H. (1987b) Oral developmental toxicity study in rat
given the Delta-8,9-Isomer of abamectin (avermectin B1). Study No.
TT 87-715-0. Unpublished report prepared by Merck Sharp & Dohme
Research Laboratories, West Point, Pennsylvania, USA. Submitted to
WHO by MSDRL, Three Bridges, NJ, USA.
Gordon, L.R., Minsker, D.H., Anderson, C.A., Nickell, B.E.,
Collevechio, K. & Deyerle-Brooks, K.A. (1987c) Oral developmental
toxicity study in mice given the polar degradates of abamectin (MK
0936). Study No. TT 87-717-0. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Gordon L.R., Wise, L.D., Vonderfecht, St.L., Nickell, B.E.,
Collevechio, K., Powzaniuk, W., & McMahon, M.G. (1987d) Single
generation study in rats with the Delta-8,9-isomer of abamectin
(avermectin B1). Study No. 87-716-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Gordon, L.R., Wise, L.E., Allen, M.L., Nickell, B.E.,
Collovechio,K., Powzaniuk, W., & Sina, J.L. (1988) Oral
developmental toxicity study in mice with L-930,463 (polar
degradate). Study No. TT 88-713-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Greene, B.M., Brown, K.R., & Taylor, H.R. (1989) Use of Ivermectin
in humans, In: Ivermectin and Abamectin, ed. by Campbell, W.C.,
Springerverlag, Chapter 21, p. 311-323. Submitted to WHO by MSDRL,
Three Bridges, NJ, USA.
Gruber, V.I. (1988) Identification of ß-alpha-hydroxy-avermectin
B1a as a metabolite of avermectin B1a in rats. Unpublished
report of Merck sharp and Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
Mandel, J. (1977) Acute oral toxicity studies in mice and rats with
avermectin B1a (C-076(B1a)). Study Nos. TT 77-3248, 77-3250, 77-
3264, 77-3787, 77-3789, 77-3788, 77-3337 and 77-3346. Unpublished
reports prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986a) The metabolism
of Delta-8,9-Z-isomer avermectin B1a in rats. Unpublished report
ARM-2, prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Maynard, M.S., Wislocki, P.G., & Lu, A.Y.H. (1986b) The metabolism
of avermectin B1a in rats. Unpublished report prepared by Merck
Sharp & Dohme Research Laboratories, West Point, Pennsylvania, USA.
Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
MSDRL (1977a) Oral reproduction study in rats with avermectin B1a
(C-076(B1a)). Study Number 77-706-0. Unpublished report prepared
by Merck Sharp & Dohme Research Laboratories, West Point,
Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges, NJ,
USA.
MSDRL (1977b) Oral reproduction study in rats. Study Number 77-712-0
with avermectin B1a (C-076(B1a)). Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Norbury K.C. & Wolf, G.L. (1977) Fourteen-week oral toxicity study
in rats following in utero exposure to avermectin B1a (C-
076(B1a)). Study number TT 77-043-0. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. & Allen, H.L. (1976) Eighteen-week oral toxicity
study in dogs with avermectin B1a (C-076(B1a)). Study No. TT 76-
073-0. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T. (1977a) Oral teratology study in mice with
avermectin B1a (C-076(B1a)). Study No. TT 77-705-0. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T. (1977b) Ten-day oral toxicity study in pregnant mice
with avermectin B1a (C-076(B1a)). Study No. TT 77-717-1.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel., J., Everett, M.A., Wolf,
G.L., & Powzaniuk, W., (1981a) Acute oral toxicity study in rats
with abamectin (avermectin B1). Study No. TT 81-2937. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Wolf, G.L., Stolz,
W.W., & Bielinski, T.C. (1981b) Acute dermal toxicity study in
rabbits with abamectin (avermectin B1). Study No. TT 81-3021.
Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981c) Acute ocular irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2940. Unpublished
report prepared by Merck Sharp & Dohme Research Laboratories, West
Point, Pennsylvania, USA. Submitted to WHO by MSDRL, Three Bridges,
NJ, USA.
Robertson, R.T., McDonald, J.S., Mandel, J., Stolz, W.W., &
Bielinski, T.C. (1981d) Primary dermal irritation study in rabbits
with abamectin (avermectin B1). Study No. TT 81-2941, 81-2943, 81-
2945. Unpublished report prepared by Merck Sharp & Dohme Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
MSDRL, Three Bridges, NJ, USA.
Skeggs, H. (1976) Microbial mutagenesis assay with avermectin B1a
(C-076(B1a)). Study No. TT 76-8052. Unpublished report prepared by
Merck Sharp & Dohme Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by MSDRL, Three Bridges, NJ, USA.
WHO (1990) Evaluation of certain veterinary drug residues in food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 799, Geneva.
WHO (1993) Toxicological evaluation of certain veterinary drug
residues in food. WHO Food Additive Series No. 31, Geneva.
