
MYCLOBUTANIL
First draft prepared by M. Caris
Bureau of Chemical Safety
Health and Welfare Canada, Ottawa, Canada
EXPLANATION
Myclobutanil is a broad spectrum systemic fungicide of the
substituted triazole chemical class of compounds. The mode of action
of myclobutanil is by inhibition of sterol biosynthesis in fungi.
Myclobutanil was considered for the first time by the present
Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Mice
Male and female Crl:CD-1 mice were treated by gavage with a
single oral dose of 2, 20 or 200 mg 14C-myclobutanil/kg bw,
radiolabelled in the chlorophenyl ring, immediately following a 2-
week pretreatment period with unlabelled myclobutanil (81.1% purity)
at dietary levels of 10, 100 or 1000 ppm a.i. Excretion of
radioactivity after 96 h accounted for 81-107% of the administered
14C-radiolabel, with the majority of the radiolabel recovered
within 24-48 h post-dosing. Comparable amounts of radiolabel were
found in both the urine (with cage wash: 41-57%) and faeces (31-52%)
with no significant differences with respect to sex or dose level.
Orally administered 14C-myclobutanil was rapidly absorbed from the
gastrointestinal tract, with peak blood concentrations occurring
within 0.2-1 h post-dosing and with absorption half-lives of 0.04-
0.3 h. Elimination was generally biphasic with half-lives of 0.6-0.9
and 6.0-30.1 h, respectively. The single exception to the
elimination profile was the high-dose (200 mg/kg bw) treated male
group, where only one phase of elimination (half-life: 6.2 h) was
observed. Presence of 14C-radiolabel in groups of mice killed one-
hour after dosing revealed similar dose-related concentrations in
whole blood and plasma. Concentrations of radiolabel in the liver
were 4 to 11-fold higher than in the blood, although the ratio of
liver to blood 14C-concentration was shown to decrease with
increasing dose (Steigerwalt et al., 1986a).
Rats
Four Sprague Dawley rats/sex were administered a single oral
dose of 150 mg 14C-myclobutanil/kg bw, radiolabelled at the 3 and
5 carbons of the triazole ring and suspended in aqueous 0.5% methyl
cellulose. The major routes of elimination of the administered
radiolabel, as determined 7 days post-dosing, were via the urine
(48% males, 37% females) and faeces (51% males, 63% females). The
level of radioactivity in expired CO2 was minimal (0.01-0.02%).
Residual tissue levels were higher in males than in females,
representing 0.2-0.5% of the radiolabel, with highest concentrations
present in the intestine, liver and kidney (Streelman, 1984).
Myclobutanil 14C-labelled in the chlorophenyl ring was
administered by gavage to groups of male and female Crl:CD(SD)BR
rats as a single oral dose of 1 or 100 mg/kg bw or as a single high-
dose of 100 mg/kg bw preceded by a 14-day repeated exposure to
unlabelled myclobutanil (81.1% purity) at a dietary level of 1000
ppm a.i. An additional group of rats received a single intravenous
dose of 1 mg 14C-myclobutanil/kg bw. Total radioactive recovery
after 96 h, upon oral dosing ranged from 82-97% and following
intravenous administration was 77% in males and 82% in females. The
majority of the radiolabel was excreted via the urine (35-48%) and
faeces (32-46% of the radiolabel dose), regardless of route of
administration. 14C-myclobutanil was well absorbed (89-115%)
following oral administration as determined by the ratio of the
percentage dose excreted in the urine after oral and intravenous
dosing. Peak blood and tissue levels as studied at the high-dose of
100 mg/kg bw, occurred within 1 hour post-dosing. Elimination
kinetics of 14C-radiolabel was biphasic after a single oral dose
of 100 mg/kg bw alone (plasma half-lives of 5.3 and 25.7 h) or after
dietary pretreatment (plasma half-lives of 2.0 and 31.5 h). Residual
tissue levels in orally treated rats after 96 hours were generally
less than 1% of the dose, with highest concentrations present in the
liver, kidneys, adrenals, whole blood, thyroids and bone marrow
(Steigerwalt et al., 1986b).
Biotransformation
Mice
Metabolic profiles from the urine and faeces of mice treated
with 14C-myclobutanil (labelled in the chlorophenyl ring) at 2, 20
or 200 mg/kg bw following 2-week dietary pretreatment (Steigerwalt
et al., 1986a) revealed no significant sex or dose-related
differences in 14C-metabolite patterns. Myclobutanil was
extensively metabolized to more polar compounds. Unchanged parent
detected in the excreta accounted for 0.7 to 7.2% of the
administered dose. No further metabolic characterization was
performed.
Rats
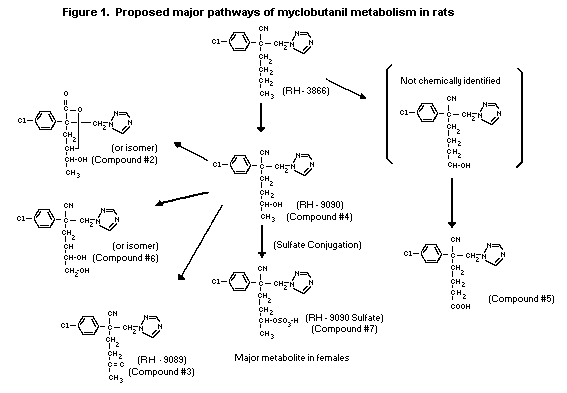
The proposed metabolic pathway in rats is depicted in Figure 1.
14C-Myclobutanil (labelled in the 3 and 5 carbons of the
triazole ring) was extensively metabolized when administered to SD
rats as a single oral dose of 150 mg/kg bw (Streelman, 1984).
Unchanged parent myclobutanil was estimated to represent only 2-3%
of the excreted dose. The predominant pathway of metabolism has been
suggested to be through a variety of oxygenation reactions of the
butyl group. The major polar metabolites identified including a
lactone, ketone, alcohol, carboxylate, dialcohol and sulfate
conjugate, were distributed uniformly in the urine and faeces of
both sexes. Quantitative differences in the metabolic profiles were
apparent most notably with the major metabolite, the sulfate
conjugate of RH-9090, which in females accounted for 75% and in
males for only 15% of the total.
 Myclobutanil, 14C-radiolabelled in the chlorophenyl ring was
extensively metabolized to more polar metabolites when administered
by gavage to groups of male and female rats as a single oral dose of
1 or 100 mg/kg bw (Steigerwalt et al., 1986b). 14C-Metabolites
of myclobutanil were qualitatively similar with respect to sex and
dose. Unchanged parent compound represented only 1-4% of the
excreted dose. In males, five fractions with more than 10% of the
excreted 14C were identified in the excreta compared with a single
major fraction in females, which accounted for 53-61% of the
radiolabel.
Effects on enzymes and other biochemical parameters
Mice
Sections of liver were taken from 4 randomly selected mice/sex
from the 6 highest dietary levels: 30, 100, 300, 1000, 3000 or 10
000 ppm a.i. of the 3-month dietary toxicity study with myclobutanil
in mice (Goldman et al., 1986) for determination of hepatic MFO
activity. Liver sections were analyzed for MFO activity using
aminopyrine and benzphetamine N-demethylation assays. MFO activity
per gram of liver as estimated by N-demethylation of benzphetamine
was significantly increased in males (2.1 to 3.3-fold) at 1000 ppm
and above, and in females (1.7 to 2.2-fold) at dietary levels of
3000 ppm and higher. Enzyme activity as measured by N-demethylation
of amino-pyrine revealed increased levels in males (2.2 to 2.3-fold)
at 3000 ppm and higher, and in females at 10 000 ppm (1.7-fold).
Hepatic microsomal protein was increased in both sexes at dietary
levels of 3000 ppm and higher.
Liver microsomal suspensions were prepared from 6 randomly
selected mice/sex treated with myclobutanil at 0, 20, 100 and 500
ppm a.i. as part of the long-term carcinogenicity study (Goldman &
Harris, 1986a). The microsomal suspensions were assayed for MFO
activity using aminopyrine N-demethylation after 3, 6 or 12 months
of treatment. Hepatic MFO activity per gram of liver was increased
after 3 months in females (1.3-fold) at 100 ppm, and in both sexes
(1.5 to 2-fold) at 500 ppm; after 6 months in both sexes at 100 ppm
(1.3 to 1.4-fold) and 500 ppm (2.8 to 2.9-fold); and after 12 months
in females (1.5-fold) at 100 ppm, and in both sexes (1.6 to 3.7-
fold) at 500 ppm. There were no treatment-related effects on hepatic
microsomal protein concentration after 3, 6 or 12 months.
Additional liver samples taken at 12 months were analyzed for
hepatic peroxisomal œ-oxidation activity by measuring the conversion
of acid insoluble 14C-palmitoyl-CoA (substrate) to the acid
soluble acetyl CoA by hepatic acyl-CoA oxidase. There were no
increases in peroxisomal 14C-palmitoyl-CoA oxidase activity
indicative of peroxisomal proliferation (Goldman & Harris, 1986a).
Rats
Mixed function oxidase activity was measured by aminopyrine and
benzphetamine N-demethylation assays on sections of liver taken from
3 randomly selected rats/sex from the control group and from dietary
level groups fed myclobutanil at 100 ppm and higher as part of a 3-
month study (O'Hara & DiDonato, 1984). Significant, dose-related
increases in MFO activity per gram of liver were recorded at dietary
levels of 300 ppm in males only (1.7 to 1.9-fold), and in both sexes
at 1000 ppm (1.8 to 2.3-fold), 3000 ppm (3 to 5.1-fold) and 10 000
ppm (4.1 to 8-fold higher). Microsomal protein was increased in both
sexes at 10 000 ppm and in males at 3000 ppm.
Livers from 6 randomly selected rats/sex/group were collected
from interim kills scheduled after 3, 6 or 12 months of treatment
with myclobutanil at dietary levels of 0, 50, 200 or 800 ppm as a
component of the long-term study in rats (Shellenberger et al.,
1986). Hepatic MFO analyzed by aminopyrine N-demethylation revealed
increased activity per gram of liver after 3 months at 200 ppm in
females (1.6-fold) and in both sexes at 800 ppm (1.5 to 1.8-fold),
and after 6 months in males at 800 ppm (1.5-fold). Increases in MFO
activity in females at 6 months and in both sexes at 12 months of
study were not significantly different from the controls. The
microsomal protein concentration in the treated groups was not
markedly different from the controls.
For determination of hepatic peroxisomal œ-oxidation activity,
additional sections of liver were obtained from 6 rats/sex/group at
the 12-month interim kill. There was no effect of myclobutanil
treatment on hepatic peroxisomal œ-oxidation activity at dietary
levels as high as 800 ppm (Shellenberger et al., 1986).
Toxicological studies
Acute toxicity studies
Acute toxicity studies with technical myclobutanil have been
performed in several animal species, the results of which are
summarized in Table 1. Myclobutanil, upon oral administration was
only slightly toxic to the mouse and rat with LD50 values of 1.36
to > 4.42 g/kg bw and 1.6 to 2.71 g/kg bw, respectively.
Myclobutanil was practically non-toxic by the inhalation and dermal
routes with a LC50 value greater than 5.1 mg/L in rats and LD50
values greater than 5 g/kg bw in the rabbit, respectively.
Table 1. Acute toxicity of technical myclobutanil
Species Sex Route Vehicle LD50 Puritya Reference
(strain) (g/kg bw)
Mouse M oral corn oil 3.23 81.1% Krzywicki & Krajewski (1983)
(CRCD-1)
Mouse M oral corn oil > 4.42 91.9% Morrison et al. (1984a)
(CRCD-1)
Mouse M+F oral corn oil M: 1.91 91.9% Morrison et al. (1986b)
(CRCD-1) F: 1.84
Mouse F oral corn oil 1.36 91.9% Romanello et al. (1986a)
(CRCD-1)
Mouse M+F oral corn oil M: 2.27 91.4% Shimizu (1987a)
(CRJ: CD-1 F: 2.44
(ICR))
Rat M+F oral corn oil M: 1.75 84.5% Krzywicki (1983)
(CR(CD)SD) F: 1.80
Rat M+F oral corn oil M: 1.6 91.9% Krzywicki & Morrison (1984a)
(CR(CD)SD) F: 2.29
Rat M+F oral corn oil M: 2.62 91.4% Shimizu (1987b)
(CRJ: CD-1 F: 2.71
(SD))
Rat M+F inhalation - LC50: 91.4% Fisher et al. (1987)
(Crl:CDBR) > 5.1 mg/L
air
Rabbit (NZW) M+F dermal - > 5.0 84.5% Krzywicki (1983)
Rabbit M+F dermal - > 5.0 91.9% Krzywicki & Bonin (1984)
(NZW)
a = active ingredient (a.i.)
Short-term toxicity studies
Mice
Myclobutanil (81.1% purity) was administered daily for a period
of 3 months to groups of 10 Crl:CD-1 (ICR) BR mice/sex at dietary
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm a.i.
(equal to 0, 0.4, 1.5, 4.8, 14.1, 42.1, 132, 542 or 2035 mg a.i./kg
bw/day in males and 0, 0.6, 2.1, 6.9, 22.9, 65.5, 232, 710 and 2027
mg a.i./kg bw/day in females, respectively). A NOAEL of 300 ppm,
equal to 42.1 mg/kg bw/day, was indicated based on treatment-related
hepatic alterations at dietary levels of 1000 ppm and higher,
manifested histomorphologically as hepatocytic inflammation,
centrilobular hypertrophy, vacuolation, and necrosis. Associated
hepatic changes were increases in liver weight, accentuated liver
lobular architecture, increased MFO activity, increased ALAT levels
and decreased cholesterol. Other microscopic treatment-related
changes pertained to increased cytoplasmic eosinophilia (> 1000
ppm males, > 3000 females) and hypertrophy of the zona
fasciculata cells of the adrenal gland (3000 ppm and higher in
both sexes). Treatment-related effects observed at dietary levels of
3000 ppm and above were decreased body-weights, increased ASAT,
decreased glucose, increased pigmentation of the liver Kupffer cells
and macrophages of the spleen, and a slight increase in lymphoid
necrosis of the spleen. Additional effects of treatment observed
only at the highest dietary level of 10 000 ppm were related to
scant faecal droppings and fluctuations in haematological (both
sexes: decrease HCT, decrease MCV, decrease MCH, increase MCHC;
males: decrease WBC, decrease lymphocytes, increase segmented
neutrophils; females: decrease haemoglobin and increase platelets)
and blood biochemical parameters (increased ALP, GGT and BUN).
Histopathological changes at 10 000 ppm were noted as bile duct
proliferation, slight increase in pigmentation of renal cortical
tubular cells, lymphoid necrosis of the thymus and mesenteric lymph
nodes, increased myeloid/erythroid ratio of bone marrow, immaturity
of the uterus and absence of corpora lutea in ovaries as well as
increased mononuclear cell infiltration of the skin (Goldman et
al., 1986).
Rats
Groups of 10 COBS-CD(SD) BR rats/sex were fed myclobutanil
(81.1% purity) for 3 months at dietary levels of 0, 10, 30, 100,
300, 1000, 3000, 10 000 or 30 000 ppm a.i. equal to 0, 0.5, 1.6,
5.2, 15.3, 51.5, 158, 585 or 1730 mg a.i./kg bw/day in males and 0,
0.7, 2.0, 6.9, 19.7, 65.8, 195, 665 or 1811 mg a.i./kg bw/day in
females, respectively. A NOAEL for this study was 100 ppm, equal to
5.2 mg/kg bw/day, as revealed by increased MFO activity in males at
300 ppm and in both sexes at higher dietary levels. Increased liver
weights and accentuated hepatic lobular architecture were observed
at 1000 ppm and above. Treatment-related changes introduced at
dietary levels of 3000 ppm were decreased body-weights, increased
cholesterol, hepatic centrilobular hypertrophy and necrosis,
increased kidney weights associated with renal congestion and
pigmentation of the convoluted tubular epithelium, and
histopathological alterations of the adrenal (increased cortical
vacuolization), ovary (congestion), thyroid (increase in small
follicles) and thymus (congestion). Additional effects of treatment
exhibited at 10 000 ppm were decreased food consumption, slight
haematological changes (decrease HCT, decrease Hb, decrease MCV and
increase RBC, increase platelets), increased GGT, pigmentation of
the liver Kupffer cells, hepatocytic vacuolation and coagulative
necrosis, increased pigmentation in the red pulp of the spleen, and
chronic pulmonary alveolitis. Treatment of rats with myclobutanil at
the highest dietary level of 30 000 ppm resulted in 100% mortality.
No effects of treatment were reported with respect to urinalysis and
ophthalmology (O'Hara & DiDonato, 1984).
A 13-week feeding study conducted with groups of 10 Crj:CD SD
rats/sex given myclobutanil (91.4% purity) in the diet at levels of
0, 100, 300 or 3000 ppm equal to 0, 6.2, 18.8 or 192 mg/kg bw/day in
males and 0, 6.9, 19.6 or 225 mg/kg bw/day in females respectively,
demonstrated a NOAEL of 300 ppm, equal to 18.8 mg/kg bw/day.
Treatment with myclobutanil at the highest dietary level of 3000 ppm
culminated in histomorphological alterations of the liver, kidney
and adrenal glands. Specific organ changes were characterized in the
liver as slight to moderate hepatocytic hypertrophy (10/10 males,
8/10 females) and in the kidney as slight vacuolar degeneration of
the renal tubular epithelium (7/10 males). Alterations of the
adrenal were described as vacuolization of the cortical cells (7/10
males), atrophy of the zona fasciculata (5/10 males) and fine
vacuolization of the zona glomerulosa (1/10 males). A single male
at 3000 ppm exhibited changes in the reproductive organs depicted in
the testes as moderate atrophy of the seminiferous tubule(s) and
giant cell-like changes with absence of sperm cells in the
epididymis. Other effects of treatment with myclobutanil were
decreased body-weight, blood chemistry changes (decreased bilirubin,
glucose and triglycerides), increased liver and kidney weights,
decreased adrenal weights and a slight increase in the number of
males with round cells in the urine. Decreased food intake was
observed in males during the first week of treatment. There were no
treatment-related effects on haematology or ophthalmoscopy (Shimizu,
1987c).
Dogs
A range-finding study was undertaken with 2 beagle dogs/sex per
group fed myclobutanil (84.5% purity) for a period of 4 weeks at
dietary levels of 0, 50, 250, 1000 or 4000 ppm a.i. equal to 0, 2.2,
10.5, 45.3 or 45 mg a.i./kg bw/day in males and 0, 2.0, 10.6, 39.3
or 47 mg a.i./kg bw/day in females respectively. Dogs treated at the
highest dietary level of 4000 ppm were sacrificed after 2 weeks of
treatment due to severely depressed food intake. The NOAEL for the
study was determined to be 250 ppm, equal to 10.5 mg/kg bw/day,
based on slightly decreased body-weight and food consumption
recorded during the first week of treatment in females at 1000 ppm.
There were no treatment-related consequences on clinical signs,
haematological and blood chemistry investigations (12 and 28 days),
or gross pathological examination (Goldman & Emmons, 1986).
Myclobutanil (81.1% purity) was administered to groups of 4
beagle dogs/sex for a period of 3 months at dietary levels of 0, 10,
200, 800 or 1600 ppm a.i. equal to 0, 0.3, 7.3, 29.1 or 56.8 mg
a.i./kg bw/day in males and 0, 0.4, 7.9, 32.4 or 58 mg a.i./kg
bw/day in females respectively. The NOAEL was determined to be 10
ppm, equal to 0.3 mg/kg bw/day, based on the dose-related incidence
of centrilobular or midzonal hepatocellular hypertrophy noted at 200
ppm (3/4 males) and in all animals of both sexes at 800 and 1600
ppm. Periportal hepatocytes were enlarged in a few of the more
severely affected livers. Liver weights were increased in males at
800 ppm and in both sexes at 1600 ppm. Treatment-related effects on
the kidney, evident as an increased incidence and severity of
unilateral chronic nephritis were observed in males at 800 ppm and
higher. Additional effects of treatment unveiled at the highest
level of 1600 ppm were related to decreased body-weights and food
consumption, due possibly to palatability of the diet. Slight
changes in haematological and blood chemistry values were within
range of normal variability and were not considered of toxicological
consequence. There were no effects of treatment on ophthalmology.
Increases in the ovarian weights at 800 and 1600 ppm were attributed
to estrus (McLaughlin & DiDonato, 1984).
Groups of 6 beagle dogs/sex were administered myclobutanil
(91.4% purity) daily for 12 months in the diet at levels of 0, 10,
100, 400 or 1600 ppm a.i. equal to 0, 0.3, 3.1, 14.3 or 54.2 mg
a.i./kg bw/day in males and 0, 0.4, 3.8, 15.7 or 58.2 mg a.i./kg
bw/day in females, respectively. The principal target organ was the
liver, demonstrating a NOAEL of 100 ppm, equal to 3.1 mg/kg bw/day,
based on hepatocellular hypertrophy, increased liver weights and
increased serum alkaline phosphatase levels at dietary
concentrations of 400 ppm and higher. At the highest level of 1600
ppm, livers displayed accentuated lobular architecture and in 4 of 6
females, the hepatocytes were expanded with large clear cytoplasmic
spaces. Other effects observed at 1600 ppm were evident as decreased
body-weight and food consumption, as well as changes in haematology
(decrease RBC, increase platelet count) and blood chemistry
(increase phosphorus, increase ALAT, increase GGT, decrease
albumin). Treatment with myclobutanil failed to elicit any adverse
effects on clinical signs, ophthalmological examination or
urinalysis (Goldman & Harris, 1986b).
Long-term toxicity/carcinogenicity studies
Mice
A 2-year study was conducted with Crl:CD-1 (ICR)BR mice fed
diets containing myclobutanil (90.4% purity) at levels of 0, 20, 100
or 500 ppm a.i. equal to 0, 2.7, 13.7 or 70.2 mg a.i./kg bw/day in
males and 0, 3.2, 16.5 or 85.2 mg a.i./kg bw/day in females,
respectively. A total of 110 males and 110 females per group were
assigned to the chronic phase of this bioassay. Interim sacrifices
were scheduled after 3 (10 mice/sex/group), 6 (10/sex/group) and 12
months (20/sex/group) of study. All surviving animals (70/sex/group,
maximum) were sacrificed after 24 months of continuous treatment.
Treatment with myclobutanil resulted in a NOAEL for in-life
parameters of 20 ppm, equal to 2.7 mg/kg bw/day, established on the
basis of increased MFO activity at 3, 6 and 12 months at dietary
levels of 100 and 500 ppm. At 500 ppm, target effects of treatment,
primarily on the liver were demonstrated by increased ALAT activity
(females, 3 months), increased liver weights (both sexes, 3 months),
and histopathological alterations comprising centrilobular
hypertrophy (males, 3/6/12 months), periportal vacuolation (males,
3/6/12 months; both sexes, 24 months), Kupffer cell pigmentation
(males, 6/12 months), hepatocellular necrosis (males, 12 months) and
hepatocellular alteration (tinctorial and dimensional properties:
both sexes, 24 months). There were no changes attributed to
treatment with myclobutanil with regard to survival, clinical signs,
body-weight, food consumption, ophthalmoscopy, haematology or
urinalysis. Myclobutanil was not oncogenic when administered to mice
for 2 years at dietary levels up to 500 ppm (Goldman & Harris,
1986a).
Rats
Groups of 110 Charles River Sprague Dawley rats/sex were
treated with myclobutanil (90.4% & 91.4% purity) for a period of up
to 24 months at dietary levels of 0, 50, 200 or 800 ppm a.i. equal
to 0, 2.5, 9.8 or 39.2 mg a.i./kg bw/day in males and 0, 3.2, 12.9
or 52.3 mg a.i./kg bw/day in females, respectively. Interim
sacrifices were performed after 3 and 6 months (10 rats/sex/group),
12 months (20/sex/group) and 17 months of study (18 males and 10
females/group). The survivors (52 male, 60 female/group, maximum)
were sacrificed after 24 months of treatment. Treatment with
myclobutanil indicated a NOAEL for in-life parameters of 50 ppm,
equal to 2.5 mg/kg bw/day. Effects of treatment at 200 ppm were
observed as decreased testes weights in association with slight
testicular atrophy at 24 months. At the highest dietary level of 800
ppm, testes weights were decreased at 12 and 24 months with slight
to moderate increases in the incidence of testicular atrophy. (The
seminiferous tubules were reported frequently found to be devoid of
spermatid formation and germinal epithelial cells; the tubules
appeared smaller than normal. In severe cases only Sertoli cells
remained). Increased ovary weights in females treated at 800 ppm and
sacrificed at 12 months were not correlated with any
histomorphological changes. Other treatment-related effects noted at
800 ppm were decreased body-weights (both sexes) and food
consumption (males), and increased liver weights (females).
Increased MFO activity was recorded at 200 ppm in females (3 months)
and at 800 ppm in both sexes (3 months) and in males (6 months).
There were no treatment-related effects on survival, clinical
signs, ophthalmoscopy, haematology, blood chemistry or urinalysis.
Treatment with myclobutanil at dietary levels up to 800 ppm failed
to uncover any evidence of carcinogenic potential (Shellenberger et
al., 1986).
Reproduction studies
Rats
A two-generation (two litter per generation) reproduction study
was conducted with groups of 25 CRI:CD(SD)BR rats/sex fed
myclobutanil (84.5% purity) at dietary levels of 0, 50, 200 or 1000
ppm a.i. equal to 0, 3.6, 14.7 or 73.6 mg a.i./kg bw/day in males
and 0, 4.3, 17.4 or 87 mg a.i./kg bw/day in females respectively. In
the F0 generation, treatment with myclobutanil commenced 8 weeks
before mating. In the F1 generation, the F1a litter-derived
parental animals were exposed to the test material throughout
weaning and for a minimum period of 8 weeks post-weaning. In both
generations, treatment continued throughout the reproductive phases.
Treatment-related effects on reproduction were denoted at the
highest dietary level of 1000 ppm by a decreased number of females
delivering litters (F0F1a and both matings from second
generation), decreased mean number of pups per litter (first mating
of second generation) and an increased number of stillborn pups (all
matings of both generations). An increase, albeit minimal in the
proportion of dead pups in both matings of the first generation was
similarly recorded at 200 ppm when compared to the controls. Effects
on the reproductive organs were evident in the second generation
F1 males treated at 1000 ppm as multifocal or diffuse atrophy of
the testes, decreased spermatozoa and/or necrotic spermatocytes of
the epididymides, as well as atrophy of the prostate. Systemic
toxicity was observed at 200 ppm as increased liver weights in males
of both parental generations in association with centrilobular
hepatocytic hypertrophy in the F1 generation males. At 1000 ppm,
increased liver weights and hepatocytic hypertrophy were observed in
males and females of both generations. Decreased body-weight in
males and depressed food intake in both sexes were recorded at 1000
ppm in both parental generations. Body-weights were similarly
decreased at 1000 ppm in both sexes of all filial generations. The
NOAEL for this study was determined to be 50 ppm, equal to 3.6 mg/kg
bw/day, for systemic and reproductive effects (Costlow & Harris,
1985).
Special studies on teratogenicity
Rats
A range-finding study was performed with groups of 8 mated
female Crl:CD(SD)BR rats treated orally by gavage with myclobutanil
(81.1% purity) at 0 (vehicle, corn oil), 32, 68, 100, 215, 464 or
700 mg a.i./kg bw/day on days 6 to 15 of gestation. Day 0 of
gestation was considered the day sperm were evident in the vaginal
smear. All surviving dams were killed on day 20 of gestation. The
NOAEL for maternal toxicity was 215 mg/kg bw/day. At dose levels of
464 and 700 mg/kg bw/day, treatment with myclobutanil resulted in
mortality (25% and 100%, respectively), decreased body-weights and
clinical signs of toxicity manifest as scant faeces,
chromodacryorrhea, red exudate around mouth, rough and urine-stained
hair coat, and salivation. Embryofetal toxicity was expressed at
levels of 68 mg/kg bw/day and higher as increased resorptions and
decreased viability indices (viable fetuses/implantation sites).
Decreased fetal weights were recorded at 464 mg/kg bw/day. In the
absence of detailed visceral and skeletal examinations of the
fetuses, the NOAEL for developmental toxicity was 32 mg/kg bw/day
(Costlow & Kane, 1984a).
Myclobutanil (84.5% purity) was administered orally by gavage
at 0 (vehicle, corn oil), 31, 94, 310 or 470 mg a.i./kg bw/day to
groups of 25 presumed pregnant Crl:CD(SD)BR rats from days 6 to 15
of gestation. The day on which sperm were found in the vaginal smear
was considered day 0 of gestation. All surviving dams were killed on
day 20 of gestation. A NOAEL for maternal toxicity was indicated at
94 mg/kg bw/day based on clinical signs of toxicity (rough hair
coat, desquamation and salivation) at doses of 310 mg/kg bw/day and
higher. At 470 mg/kg bw/day, red exudate around the mouth, scant
faeces and decreased body-weights were also observed. Decreased
viability indices (viable fetuses /implantation sites) and a slight
trend toward increasing resorption rate were recorded at 94 mg/kg
bw/day and higher resulting in a NOAEL for embryofetal toxicity of
31 mg/kg bw/day. An increased incidence of skeletal variations of
the ribs (7th cervical and 14th rudimentary ribs) was observed at
310 mg/kg bw/day and higher. Treatment with myclobutanil failed to
reveal any evidence of teratogenic potential (Costlow & Kane,
1984b).
Rabbits
A range-finding study was conducted with myclobutanil (84.5%
purity) administered orally by gavage at 0 (vehicle, 1% methyl
cellulose), 10, 31.6, 100, 215, 464 or 700 mg a.i./kg bw/day to
groups of 6 artificially inseminated New Zeeland white rabbits on
days 7 through 19 of gestation. The day of artificial insemination
was designated as day 0 of gestation. All surviving animals were
killed on day 29. Myclobutanil was lethal at doses of 464 mg/kg
bw/day and higher resulting in 100% mortality. Maternal toxicity
represented by clinical signs (irregular faeces and red-stained
urine) and decreased body-weights was noted at 215 mg/kg bw/day. An
increased incidence of resorptions and decreased litter size (viable
fetuses/litter) was observed at 215 mg/kg bw/day, resulting in an
overall NOAEL of 100 mg/kg bw/day. Viable fetuses from surviving
rabbits appeared normal upon gross examination (Costlow & Kane,
1984c).
Groups of 18 artificially inseminated female New Zeeland white
rabbits were administered myclobutanil (90.4% purity) at 0
(distilled water control), 0 (vehicle, 1% methyl cellulose), 20, 60
or 200 mg a.i./kg bw/day orally by gavage from day 7 through 19 of
gestation. The day of insemination was designated as day 0 of
gestation. All surviving rabbits were killed on day 29. The NOAEL
for maternal toxicity was 20 mg/kg bw/day, based on minimal,
transient body-weight loss at 60 mg/kg bw/day. Maternal animals at
the high-dose of 200 mg/kg bw/day experienced body-weight loss and
clinical signs reported as irregular faeces and blood-stained urine.
Embryofetal toxicity was manifest at 200 mg/kg bw/day as an
increased frequency of abortion and total litter resorption, an
increased incidence of litters with resorptions as well as reduced
litter size and fetal weight. Myclobutanil was not teratogenic when
administered to pregnant rabbits at dose levels of up to 200 mg/kg
bw/day (Costlow & Kane, 1984d).
Special studies on genotoxicity
Myclobutanil did not reveal any evidence of genotoxic potential
when investigated in a battery of assays specific for gene mutation
in microbial and mammalian cells or for detection of chromosomal
aberrations in cytogenetics studies. Myclobutanil did not induce
unscheduled DNA synthesis in isolated rat hepatocytes and was
negative in a DNA repair test with Bacillus subtilis. A dominant
lethal study in rats was also negative. The results of the
genotoxicity studies are presented in Table 2.
Special studies on irritation and sensitization
Eye irritation potential was studied in 9 male New Zeeland
white rabbits given technical myclobutanil with a purity of 78.4%
(Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984). Treatment of
the eyes with higher purity technical material produced both corneal
and conjunctival effects suggestive of moderate to severe irritating
potential. Myclobutanil with a purity of 78.4% was only slightly
irritating to the eyes of rabbits resulting in reversible
conjunctival effects.
A 0.5 ml aliquot of technical myclobutanil was applied dermally
to the shaved backs of 6 male New Zeeland rabbits under occluded
conditions for a 4-hour exposure period. Myclobutanil with a purity
of 78.4% (Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984) was
practically non-irritating to the skin of male rabbits.
Table 2. Genotoxicity of technical myclobutanil
Test Test system Concentration (vehicle) Puritya Results Reference
Reverse S. typhimurium 99% negative Byers & Lohse (1983)
Mutation TA 98, 100, 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(in vitro) TA 1535, 75, 250, 500, 1000, 5000 µg/plate
TA 1537 250, 750, 1500, 2500, 7500 µg/plate
(DMSO)
S. typhimurium 84.5% negative Byers & Chism (1983a)
TA 98, 100, 1535, 1537 75 - 7500 µg/plate (DMSO) 1., 2.
S. typhimurium 90.4% negative Byers & Chism (1983b)
TA 98, 100, 1535, 1537 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(DMSO)
S. typhimurium 91.4% negative Sutou (1987)
TA 98, 100, 1535, 1537 125, 250, 500, 1000, 2000 ug/plate 1., 2.
E. coli WP2 uvrA- (DMSO)
Point mutation Chinese hamster ovary, K1BH4 cell 1. 120-175 µg/ml 81.1% negative O'Neill et al. (1984)
(in vitro) line - HGPRT locus 2. 25-90 µg/ml 1., 2.
(DMSO)
Chromosome Chinese hamster ovary WB1 cells 1. 20, 30, 40, 50 µg/ml 91.9% negative Ivett (1985)
aberration 2. 25, 50, 75 µg/ml 1., 2.
(in vitro) (DMSO)
Chromosome Mouse (male CR CD-1) 0, 65, 260, 650 mg/kg bw 81.1% negative McLeod & McCarthy (1984)
aberration bone marrow (corn oil)
(in vivo)
Mouse 0, 117, 585, 1170 mg/kg bw 91.4% negative Sames & Frank (1987)
(male and female (corn oil)
Crl:CD-1(ICR)),
bone marrow
Table 2 (cont'd)
Test Test system Concentration (vehicle) Puritya Results Reference
DNA repair Bacillus subtilis 312.5, 625, 1250, 2500, 5000 ug/plate 91.4% negative Sutou (1987)
(in vitro) H17, M45 1., 2.
Unscheduled DNA Rat 0.1 - 1000 ug/ml 91.9% negative Muller (1986)
synthesis (CRCD, Crl:CDBR male) (DMSO)
(in vitro) hepatocytes
Dominant Rat, male 0, 10, 100, 735 mg/kg bw 91.4% negative Dearlove et al. (1986)
lethal Crl:COBS(SD)BR (corn oil)
(in vivo)
DMSO = dimethyl sulfoxide
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
a = active ingredient (a.i.)
The potential of technical myclobutanil to induce delayed
contact hypersensitivity in the Hartley guinea-pig using a modified
Buehler procedure, could not unequivocally be ascertained due to a
low incidence of animals with erythema response following challenge
doses with the test material (Bonin & Hazelton, 1987a). Technical
myclobutanil, when administered to guinea-pigs according to the
method of Magnusson and Kligman did not produce delayed contact
hypersensitivity (Kreuzmann, 1989).
Observations in humans
No information was available.
COMMENTS
Myclobutanil was rapidly absorbed when administered orally to
rats and mice. The principal routes of excretion were via the urine
and faeces, with no significant residual tissue accumulation. The
toxicokinetic model in the rodent did not differ markedly with
respect to species, sex, single versus repeated exposures or doses.
In both the rat and mouse, myclobutanil was extensively
metabolized to more polar compounds. The proposed metabolic pathway
of myclobutanil in the rat was through oxidation of the butyl group.
The major excretory metabolites were qualitatively similar with
respect to sex and dose.
Myclobutanil was only slightly toxic upon acute oral
administration to rats and mice. WHO has classified myclobutanil as
slightly hazardous (WHO, 1992).
The primary target organ upon repeated dietary exposure to
myclobutanil was the liver. Histomorphological changes,
characterized predominantly by centrilobular hepatocytic hypertrophy
in association with increased liver weights, were observed in all
species investigated. Microscopically, there was accentuated lobular
architecture, hepatocytic vacuolation, inflammation, necrosis, and
pigmentation of the Kupffer cells. Increased hepatic enzyme activity
in serum (ALAT, ASAT, GGT, ALP) were also observed. Hepatic
microsomal MFO activities in rats and mice were increased
correspondingly. There were no similar increases in hepatic
peroxisomal œ-oxidation activity that would have suggested
peroxisomal proliferation.
A three-month dietary study with myclobutanil in the mouse at
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm revealed
hepatic alterations at dietary levels of 1000 ppm and higher,
resulting in a NOAEL of 300 ppm, equal to 42.1 mg/kg bw/day.
Two 3-month studies in rats fed myclobutanil at levels of 0,
10, 30, 100, 300, 1000, 3000, 10 000, or 30 000 ppm and 0, 100, 300,
or 3000 ppm indicated a NOAEL of 100 ppm, equal to 5.2 mg/kg bw/day,
based on treatment-related hepatic effects.
The NOAEL for myclobutanil-related liver effects in dogs
treated for 3 months at 0, 10, 200, 800 or 1600 ppm was 10 ppm,
equal to 0.3 mg/kg bw/day. Treatment of dogs with myclobutanil for
12 months at dietary levels of 0, 10, 100, 400, or 1600 ppm resulted
in a NOAEL for hepatic effects of 100 ppm, equal to 3.1 mg/kg
bw/day. Myclobutanil administered to two dogs per sex at dietary
levels of up to 1000 ppm, equal to 39 mg/kg bw/day, did not produce
any hepatic changes after a period of 4 weeks.
Long-term dietary treatment of mice with myclobutanil for two
years at 0, 20, 100 or 500 ppm revealed a NOAEL of 20 ppm, equal to
2.7 mg/kg bw/day, based on increased MFO activity at 100 ppm as well
as more pronounced liver toxicity at 500 ppm and above. Myclobutanil
was not carcinogenic in mice.
A 24-month long-term toxicity/carcinogenicity study in rats at
dietary concentrations of 0, 50, 200 or 800 ppm revealed a NOAEL of
50 ppm, equal to 2.5 mg/kg bw/day, based on findings of testicular
atrophy and increased MFO activity at 200 ppm and above.
Myclobutanil was not carcinogenic in rats.
A two-generation reproduction study in rats at dietary
concentrations of 0, 50, 200 or 1000 ppm revealed a NOAEL of 50 ppm,
equal to 3.6 mg/kg bw/day, based on increased liver weights and an
increase in numbers of stillborn pups at 200 ppm and above. At 1000
ppm atrophy of the testes and prostate were observed.
An oral teratogenicity study in rats at gavage doses of 0, 31,
94, 310, or 470 mg/kg bw/day demonstrated clinical signs of toxicity
at 310 mg/kg bw/day and above, indicating a NOAEL of 94 mg/kg
bw/day. The NOAEL for embryofetal toxicity was 31 mg/kg bw/day.
There was no evidence of teratogenicity at doses up to 470 mg/kg
bw/day.
Myclobutanil was not teratogenic when administered to the
rabbit at gavage doses of 20, 60 or 200 mg/kg bw/day. A NOAEL for
maternal toxicity was 20 mg/kg bw/day, based on decreased body-
weight at 60 mg/kg bw/day and above. Embryofetal toxicity was
evident at 200 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that myclobutanil was not genotoxic.
An ADI was allocated on the basis of NOAELs in two-year feeding
studies in mice and rats, a reproduction study in rats and a one-
year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 20 ppm, equal to 2.7 mg/kg bw/day (two-year study)
Rat: 50 ppm, equal to 2.5 mg/kg bw/day (two-year study)
50 ppm, equal to 3.6 mg/kg bw/day (two-generation
reproduction study)
Dog: 100 ppm, equal to 3.1 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0 - 0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
1. Results of ongoing long-term studies in mice and rats
known to be in progress.
2. If the results of (1) show a carcinogenic response,
studies (a) to determine whether myclobutanil acts as a
tumour promoter in the two-stage rat liver bioassay and
(b) whether it causes inhibition of intercellular
communications.
3. Observations in humans.
REFERENCES
Bonin, R. & Hazelton, G.A. (1987a). RH-3866 technical: Delayed
contact hypersensitivity study in guinea-pigs. Report No. 87R-035.
Unpublished report dated June 25, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983a). Microbial mutagen assay. Report
No. 83R-162. Unpublished report dated October 19, 1983 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983b). RH-53,866: Microbial mutagen
assay. Report No. 83R-246. Unpublished report dated January 31, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Byers, M.J. & Lohse, K.L. (1983). RH-53,866: Microbial mutagen
assay. Report No. 82R-193. Unpublished report dated January 11, 1983
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Harris, J.C. (1985). RH-53,866: Two generation
reproduction study in rats. Report No. 84R-117. Unpublished report
dated August 21, 1985 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984a). Range-finding teratology study
with RH-53,866 in rats. Report No. 83R-023. Unpublished report dated
June 26, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984b). Teratology study with RH-53,866
in rats. Report No. 83R-024. Unpublished report dated June 22, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984c). Range-finding teratology study
with RH-53,866 in rabbits. Report No. 83R-216. Unpublished report
dated October 31, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984d). Teratology study with RH-53,866
in rabbits. Report No. 83R-217. Unpublished report dated November
15, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986). Dominant
lethal study of RH-53,866 administered orally via gavage to
Crl:COBS(SD)BR male rats. Report No. 018-011; R & H Report No. 86RC-
054. Unpublished report dated October 10, 1986 from Argus Research
Laboratories Inc., Horsham, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Fisher, J.R., Emmons H.F., & Hagan J.V. (1987). RH-3866 technical
acute inhalation toxicity study in rats. Report No. 87R-028.
Unpublished report dated August 31, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Goldman, P.R. & Emmons, H.F. (1986). RH-3866 (Experimental
Fungicide): Four week dietary range-finding study in dogs. Report
No. 83R-078. Unpublished report dated October 8, 1986 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Goldman, P.R. & Harris, J.C. (1986a). RH-3866: Dietary chronic and
oncogenicity study in mice. Report No. 84R-023. Unpublished report
dated October 17, 1986 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Goldman, P.R. & Harris; J.C. (1986b). RH-3866: One-year dietary
study in beagle dogs. Report No. 84R-078. Unpublished report dated
October 15, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Goldman, P.R., Harris, J.C. & Lampe, K.R. (1986). RH-3866: Three-
month dietary toxicity study in mice. Report No. 83R-136.
Unpublished report dated October 8, 1988 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of RH-53,866 technical
in an in vitro cytogenetic assay measuring chromosome aberration
frequencies in Chinese hamster ovary (CHO) cells. Report No. 20990;
R & H Report No. 85RC-011. Unpublished report dated April 26, 1985
from Litton Bionetics, Kensington, Maryland, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Kreuzmann, J.J. (1989). RH-53,866 technical: Guinea-pig maximization
test (Magnusson and Kligman method). Report 89-3655-21; R & H Report
No. 89RC-103. Unpublished report dated April 29, 1989 from Hill Top
Biolabs, Inc., Miamiville, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Krzywicki K.M. (1983). RH-53,866: Definitive oral LD50 in male and
female rats, dermal LD50 in male and female rabbits and skin and
eye irritation in male rabbits. Report No. 83R-125 A&B. Unpublished
report dated September 20, 1983 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Bonin, R. (1984). RH-53,866 technical: Definitive
dermal LD50 in male and female rabbits, and skin and eye
irritation in male rabbits. Report No. 84R-134 A&B. Unpublished
report dated August 3, 1984 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Krajewski, R.J. (1983). RH-53,866 technical:
Definitive oral LD50 in male mice. Report No. 83R-103. Unpublished
report dated July 6, 1983 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Krzywicki, K.M. & Morrison, R.D. (1984a). RH-53,866 technical:
Definitive oral LD50 in male and female rats. Report No. 84R-063
A&B. Unpublished report dated July 19, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLaughlin, J.E. & DiDonato, L.J. (1984). RH-3866 (Experimental
Fungicide): Three month dietary feeding study in dogs. Report No.
83R-204. Unpublished report dated August 7, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLeod, P.L. & McCarthy, K.L. (1984). RH-53,866 in vivo
cytogenetic study in mice. Report No. 84R-074. Unpublished report
dated July 23, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Morrison, R.D., Murphy, M.E., & Chan, P.K. (1984a). RH-53,866
technical: Acute oral LD50 in male mice. Report No. 84R-153.
Unpublished report dated August 3, 1984 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Morrison, R.D., Romanello, A.S., Krzywicki, K.M., & Hazelton, G.A.
(1986b). RH-3866 technical: Definitive oral LD50 in male and
female mice. Report No. 86R-088 A&B. Unpublished report dated August
14, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Muller, G. (1986). RH-53,866 technical in vitro unscheduled DNA
synthesis assay. Report No. 86R-084. Unpublished report dated July
22, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Hara, G.P. & DiDonato, L.J. (1984). RH-3866: Three month dietary
feeding study in rats. Report No. 83R-068. Unpublished report dated
August 7, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Neill, P.J., Foxall, S. & Byers, M.J. (1984). RH-53,866 technical:
CHO/HGPRT gene mutation assay. Report No. 84R-046. Unpublished
report dated May 29, 1984 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Quinn, J.A., Fujimoto, T.T., Egan, A.R. & Shaber, S.H. (1986).
Properties of RH-3866, a new triazole fungicide. Pesticide
Science, Vol. 17: 357-362. Submitted to WHO by Rohm & Haas
Company, Spring House, PA, USA.
Romanello, A.S., Krzywicki, K.M. & Hazelton, G.A. (1986a). RH-3866
technical definitive mouse oral LD50 in females. Report No. 85R-
247. Unpublished report dated May 22, 1986 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
Sames, J.L. & Frank, J.P. (1987). RH-53,866 technical: In vivo
cytogenetics study in mice. Report No. 87R-141. Unpublished report
dated October 30, 1987 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Shellenberger, T.G., Billups, L.H., Tegeris, A.S. & Green, D.S.
(1986). Chronic toxicity and oncogenicity study in rats with RH-
3866. Report No. 8342; R & H Report No. 85RC-061. Unpublished report
dated October 24, 1986 from Tegeris Laboratories, Inc., Laurel,
Maryland. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Shimizu Y. (1987a). Acute oral toxicity study with RH-3866 in mice.
Report No. NRILS 86-2052; R & H Report No. 87RC-036. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987b(. Acute oral toxicity study with RH-3866 in rats.
Report No. NRILS 86-2053; R & H Report No. 87RC-035. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987c). A thirteen week feeding subchronic study of RH-
3866 in rats. Report No. NRILS 86-2024;R & H Report No. 87RC-038.
Unpublished report dated September 1, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986a). RH-3866
kinetic and metabolism study in mice. Report No. 83R-175.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986b). RH-3866
kinetic and metabolism study in rats. Report No. 83R-144.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Streelman D.R. (1984). Material balance and metabolism study of
14C-RH-3866 in rats. Report No. TR 310-84-16. Unpublished report
dated June 22, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Sutou, S. (1987). Mutagenicity testing of RH-53,866 technical with
bacteria. Report No. NRILS 86-2112; R & H Report No. 87RC-037.
Unpublished report dated August 21, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Myclobutanil, 14C-radiolabelled in the chlorophenyl ring was
extensively metabolized to more polar metabolites when administered
by gavage to groups of male and female rats as a single oral dose of
1 or 100 mg/kg bw (Steigerwalt et al., 1986b). 14C-Metabolites
of myclobutanil were qualitatively similar with respect to sex and
dose. Unchanged parent compound represented only 1-4% of the
excreted dose. In males, five fractions with more than 10% of the
excreted 14C were identified in the excreta compared with a single
major fraction in females, which accounted for 53-61% of the
radiolabel.
Effects on enzymes and other biochemical parameters
Mice
Sections of liver were taken from 4 randomly selected mice/sex
from the 6 highest dietary levels: 30, 100, 300, 1000, 3000 or 10
000 ppm a.i. of the 3-month dietary toxicity study with myclobutanil
in mice (Goldman et al., 1986) for determination of hepatic MFO
activity. Liver sections were analyzed for MFO activity using
aminopyrine and benzphetamine N-demethylation assays. MFO activity
per gram of liver as estimated by N-demethylation of benzphetamine
was significantly increased in males (2.1 to 3.3-fold) at 1000 ppm
and above, and in females (1.7 to 2.2-fold) at dietary levels of
3000 ppm and higher. Enzyme activity as measured by N-demethylation
of amino-pyrine revealed increased levels in males (2.2 to 2.3-fold)
at 3000 ppm and higher, and in females at 10 000 ppm (1.7-fold).
Hepatic microsomal protein was increased in both sexes at dietary
levels of 3000 ppm and higher.
Liver microsomal suspensions were prepared from 6 randomly
selected mice/sex treated with myclobutanil at 0, 20, 100 and 500
ppm a.i. as part of the long-term carcinogenicity study (Goldman &
Harris, 1986a). The microsomal suspensions were assayed for MFO
activity using aminopyrine N-demethylation after 3, 6 or 12 months
of treatment. Hepatic MFO activity per gram of liver was increased
after 3 months in females (1.3-fold) at 100 ppm, and in both sexes
(1.5 to 2-fold) at 500 ppm; after 6 months in both sexes at 100 ppm
(1.3 to 1.4-fold) and 500 ppm (2.8 to 2.9-fold); and after 12 months
in females (1.5-fold) at 100 ppm, and in both sexes (1.6 to 3.7-
fold) at 500 ppm. There were no treatment-related effects on hepatic
microsomal protein concentration after 3, 6 or 12 months.
Additional liver samples taken at 12 months were analyzed for
hepatic peroxisomal œ-oxidation activity by measuring the conversion
of acid insoluble 14C-palmitoyl-CoA (substrate) to the acid
soluble acetyl CoA by hepatic acyl-CoA oxidase. There were no
increases in peroxisomal 14C-palmitoyl-CoA oxidase activity
indicative of peroxisomal proliferation (Goldman & Harris, 1986a).
Rats
Mixed function oxidase activity was measured by aminopyrine and
benzphetamine N-demethylation assays on sections of liver taken from
3 randomly selected rats/sex from the control group and from dietary
level groups fed myclobutanil at 100 ppm and higher as part of a 3-
month study (O'Hara & DiDonato, 1984). Significant, dose-related
increases in MFO activity per gram of liver were recorded at dietary
levels of 300 ppm in males only (1.7 to 1.9-fold), and in both sexes
at 1000 ppm (1.8 to 2.3-fold), 3000 ppm (3 to 5.1-fold) and 10 000
ppm (4.1 to 8-fold higher). Microsomal protein was increased in both
sexes at 10 000 ppm and in males at 3000 ppm.
Livers from 6 randomly selected rats/sex/group were collected
from interim kills scheduled after 3, 6 or 12 months of treatment
with myclobutanil at dietary levels of 0, 50, 200 or 800 ppm as a
component of the long-term study in rats (Shellenberger et al.,
1986). Hepatic MFO analyzed by aminopyrine N-demethylation revealed
increased activity per gram of liver after 3 months at 200 ppm in
females (1.6-fold) and in both sexes at 800 ppm (1.5 to 1.8-fold),
and after 6 months in males at 800 ppm (1.5-fold). Increases in MFO
activity in females at 6 months and in both sexes at 12 months of
study were not significantly different from the controls. The
microsomal protein concentration in the treated groups was not
markedly different from the controls.
For determination of hepatic peroxisomal œ-oxidation activity,
additional sections of liver were obtained from 6 rats/sex/group at
the 12-month interim kill. There was no effect of myclobutanil
treatment on hepatic peroxisomal œ-oxidation activity at dietary
levels as high as 800 ppm (Shellenberger et al., 1986).
Toxicological studies
Acute toxicity studies
Acute toxicity studies with technical myclobutanil have been
performed in several animal species, the results of which are
summarized in Table 1. Myclobutanil, upon oral administration was
only slightly toxic to the mouse and rat with LD50 values of 1.36
to > 4.42 g/kg bw and 1.6 to 2.71 g/kg bw, respectively.
Myclobutanil was practically non-toxic by the inhalation and dermal
routes with a LC50 value greater than 5.1 mg/L in rats and LD50
values greater than 5 g/kg bw in the rabbit, respectively.
Table 1. Acute toxicity of technical myclobutanil
Species Sex Route Vehicle LD50 Puritya Reference
(strain) (g/kg bw)
Mouse M oral corn oil 3.23 81.1% Krzywicki & Krajewski (1983)
(CRCD-1)
Mouse M oral corn oil > 4.42 91.9% Morrison et al. (1984a)
(CRCD-1)
Mouse M+F oral corn oil M: 1.91 91.9% Morrison et al. (1986b)
(CRCD-1) F: 1.84
Mouse F oral corn oil 1.36 91.9% Romanello et al. (1986a)
(CRCD-1)
Mouse M+F oral corn oil M: 2.27 91.4% Shimizu (1987a)
(CRJ: CD-1 F: 2.44
(ICR))
Rat M+F oral corn oil M: 1.75 84.5% Krzywicki (1983)
(CR(CD)SD) F: 1.80
Rat M+F oral corn oil M: 1.6 91.9% Krzywicki & Morrison (1984a)
(CR(CD)SD) F: 2.29
Rat M+F oral corn oil M: 2.62 91.4% Shimizu (1987b)
(CRJ: CD-1 F: 2.71
(SD))
Rat M+F inhalation - LC50: 91.4% Fisher et al. (1987)
(Crl:CDBR) > 5.1 mg/L
air
Rabbit (NZW) M+F dermal - > 5.0 84.5% Krzywicki (1983)
Rabbit M+F dermal - > 5.0 91.9% Krzywicki & Bonin (1984)
(NZW)
a = active ingredient (a.i.)
Short-term toxicity studies
Mice
Myclobutanil (81.1% purity) was administered daily for a period
of 3 months to groups of 10 Crl:CD-1 (ICR) BR mice/sex at dietary
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm a.i.
(equal to 0, 0.4, 1.5, 4.8, 14.1, 42.1, 132, 542 or 2035 mg a.i./kg
bw/day in males and 0, 0.6, 2.1, 6.9, 22.9, 65.5, 232, 710 and 2027
mg a.i./kg bw/day in females, respectively). A NOAEL of 300 ppm,
equal to 42.1 mg/kg bw/day, was indicated based on treatment-related
hepatic alterations at dietary levels of 1000 ppm and higher,
manifested histomorphologically as hepatocytic inflammation,
centrilobular hypertrophy, vacuolation, and necrosis. Associated
hepatic changes were increases in liver weight, accentuated liver
lobular architecture, increased MFO activity, increased ALAT levels
and decreased cholesterol. Other microscopic treatment-related
changes pertained to increased cytoplasmic eosinophilia (> 1000
ppm males, > 3000 females) and hypertrophy of the zona
fasciculata cells of the adrenal gland (3000 ppm and higher in
both sexes). Treatment-related effects observed at dietary levels of
3000 ppm and above were decreased body-weights, increased ASAT,
decreased glucose, increased pigmentation of the liver Kupffer cells
and macrophages of the spleen, and a slight increase in lymphoid
necrosis of the spleen. Additional effects of treatment observed
only at the highest dietary level of 10 000 ppm were related to
scant faecal droppings and fluctuations in haematological (both
sexes: decrease HCT, decrease MCV, decrease MCH, increase MCHC;
males: decrease WBC, decrease lymphocytes, increase segmented
neutrophils; females: decrease haemoglobin and increase platelets)
and blood biochemical parameters (increased ALP, GGT and BUN).
Histopathological changes at 10 000 ppm were noted as bile duct
proliferation, slight increase in pigmentation of renal cortical
tubular cells, lymphoid necrosis of the thymus and mesenteric lymph
nodes, increased myeloid/erythroid ratio of bone marrow, immaturity
of the uterus and absence of corpora lutea in ovaries as well as
increased mononuclear cell infiltration of the skin (Goldman et
al., 1986).
Rats
Groups of 10 COBS-CD(SD) BR rats/sex were fed myclobutanil
(81.1% purity) for 3 months at dietary levels of 0, 10, 30, 100,
300, 1000, 3000, 10 000 or 30 000 ppm a.i. equal to 0, 0.5, 1.6,
5.2, 15.3, 51.5, 158, 585 or 1730 mg a.i./kg bw/day in males and 0,
0.7, 2.0, 6.9, 19.7, 65.8, 195, 665 or 1811 mg a.i./kg bw/day in
females, respectively. A NOAEL for this study was 100 ppm, equal to
5.2 mg/kg bw/day, as revealed by increased MFO activity in males at
300 ppm and in both sexes at higher dietary levels. Increased liver
weights and accentuated hepatic lobular architecture were observed
at 1000 ppm and above. Treatment-related changes introduced at
dietary levels of 3000 ppm were decreased body-weights, increased
cholesterol, hepatic centrilobular hypertrophy and necrosis,
increased kidney weights associated with renal congestion and
pigmentation of the convoluted tubular epithelium, and
histopathological alterations of the adrenal (increased cortical
vacuolization), ovary (congestion), thyroid (increase in small
follicles) and thymus (congestion). Additional effects of treatment
exhibited at 10 000 ppm were decreased food consumption, slight
haematological changes (decrease HCT, decrease Hb, decrease MCV and
increase RBC, increase platelets), increased GGT, pigmentation of
the liver Kupffer cells, hepatocytic vacuolation and coagulative
necrosis, increased pigmentation in the red pulp of the spleen, and
chronic pulmonary alveolitis. Treatment of rats with myclobutanil at
the highest dietary level of 30 000 ppm resulted in 100% mortality.
No effects of treatment were reported with respect to urinalysis and
ophthalmology (O'Hara & DiDonato, 1984).
A 13-week feeding study conducted with groups of 10 Crj:CD SD
rats/sex given myclobutanil (91.4% purity) in the diet at levels of
0, 100, 300 or 3000 ppm equal to 0, 6.2, 18.8 or 192 mg/kg bw/day in
males and 0, 6.9, 19.6 or 225 mg/kg bw/day in females respectively,
demonstrated a NOAEL of 300 ppm, equal to 18.8 mg/kg bw/day.
Treatment with myclobutanil at the highest dietary level of 3000 ppm
culminated in histomorphological alterations of the liver, kidney
and adrenal glands. Specific organ changes were characterized in the
liver as slight to moderate hepatocytic hypertrophy (10/10 males,
8/10 females) and in the kidney as slight vacuolar degeneration of
the renal tubular epithelium (7/10 males). Alterations of the
adrenal were described as vacuolization of the cortical cells (7/10
males), atrophy of the zona fasciculata (5/10 males) and fine
vacuolization of the zona glomerulosa (1/10 males). A single male
at 3000 ppm exhibited changes in the reproductive organs depicted in
the testes as moderate atrophy of the seminiferous tubule(s) and
giant cell-like changes with absence of sperm cells in the
epididymis. Other effects of treatment with myclobutanil were
decreased body-weight, blood chemistry changes (decreased bilirubin,
glucose and triglycerides), increased liver and kidney weights,
decreased adrenal weights and a slight increase in the number of
males with round cells in the urine. Decreased food intake was
observed in males during the first week of treatment. There were no
treatment-related effects on haematology or ophthalmoscopy (Shimizu,
1987c).
Dogs
A range-finding study was undertaken with 2 beagle dogs/sex per
group fed myclobutanil (84.5% purity) for a period of 4 weeks at
dietary levels of 0, 50, 250, 1000 or 4000 ppm a.i. equal to 0, 2.2,
10.5, 45.3 or 45 mg a.i./kg bw/day in males and 0, 2.0, 10.6, 39.3
or 47 mg a.i./kg bw/day in females respectively. Dogs treated at the
highest dietary level of 4000 ppm were sacrificed after 2 weeks of
treatment due to severely depressed food intake. The NOAEL for the
study was determined to be 250 ppm, equal to 10.5 mg/kg bw/day,
based on slightly decreased body-weight and food consumption
recorded during the first week of treatment in females at 1000 ppm.
There were no treatment-related consequences on clinical signs,
haematological and blood chemistry investigations (12 and 28 days),
or gross pathological examination (Goldman & Emmons, 1986).
Myclobutanil (81.1% purity) was administered to groups of 4
beagle dogs/sex for a period of 3 months at dietary levels of 0, 10,
200, 800 or 1600 ppm a.i. equal to 0, 0.3, 7.3, 29.1 or 56.8 mg
a.i./kg bw/day in males and 0, 0.4, 7.9, 32.4 or 58 mg a.i./kg
bw/day in females respectively. The NOAEL was determined to be 10
ppm, equal to 0.3 mg/kg bw/day, based on the dose-related incidence
of centrilobular or midzonal hepatocellular hypertrophy noted at 200
ppm (3/4 males) and in all animals of both sexes at 800 and 1600
ppm. Periportal hepatocytes were enlarged in a few of the more
severely affected livers. Liver weights were increased in males at
800 ppm and in both sexes at 1600 ppm. Treatment-related effects on
the kidney, evident as an increased incidence and severity of
unilateral chronic nephritis were observed in males at 800 ppm and
higher. Additional effects of treatment unveiled at the highest
level of 1600 ppm were related to decreased body-weights and food
consumption, due possibly to palatability of the diet. Slight
changes in haematological and blood chemistry values were within
range of normal variability and were not considered of toxicological
consequence. There were no effects of treatment on ophthalmology.
Increases in the ovarian weights at 800 and 1600 ppm were attributed
to estrus (McLaughlin & DiDonato, 1984).
Groups of 6 beagle dogs/sex were administered myclobutanil
(91.4% purity) daily for 12 months in the diet at levels of 0, 10,
100, 400 or 1600 ppm a.i. equal to 0, 0.3, 3.1, 14.3 or 54.2 mg
a.i./kg bw/day in males and 0, 0.4, 3.8, 15.7 or 58.2 mg a.i./kg
bw/day in females, respectively. The principal target organ was the
liver, demonstrating a NOAEL of 100 ppm, equal to 3.1 mg/kg bw/day,
based on hepatocellular hypertrophy, increased liver weights and
increased serum alkaline phosphatase levels at dietary
concentrations of 400 ppm and higher. At the highest level of 1600
ppm, livers displayed accentuated lobular architecture and in 4 of 6
females, the hepatocytes were expanded with large clear cytoplasmic
spaces. Other effects observed at 1600 ppm were evident as decreased
body-weight and food consumption, as well as changes in haematology
(decrease RBC, increase platelet count) and blood chemistry
(increase phosphorus, increase ALAT, increase GGT, decrease
albumin). Treatment with myclobutanil failed to elicit any adverse
effects on clinical signs, ophthalmological examination or
urinalysis (Goldman & Harris, 1986b).
Long-term toxicity/carcinogenicity studies
Mice
A 2-year study was conducted with Crl:CD-1 (ICR)BR mice fed
diets containing myclobutanil (90.4% purity) at levels of 0, 20, 100
or 500 ppm a.i. equal to 0, 2.7, 13.7 or 70.2 mg a.i./kg bw/day in
males and 0, 3.2, 16.5 or 85.2 mg a.i./kg bw/day in females,
respectively. A total of 110 males and 110 females per group were
assigned to the chronic phase of this bioassay. Interim sacrifices
were scheduled after 3 (10 mice/sex/group), 6 (10/sex/group) and 12
months (20/sex/group) of study. All surviving animals (70/sex/group,
maximum) were sacrificed after 24 months of continuous treatment.
Treatment with myclobutanil resulted in a NOAEL for in-life
parameters of 20 ppm, equal to 2.7 mg/kg bw/day, established on the
basis of increased MFO activity at 3, 6 and 12 months at dietary
levels of 100 and 500 ppm. At 500 ppm, target effects of treatment,
primarily on the liver were demonstrated by increased ALAT activity
(females, 3 months), increased liver weights (both sexes, 3 months),
and histopathological alterations comprising centrilobular
hypertrophy (males, 3/6/12 months), periportal vacuolation (males,
3/6/12 months; both sexes, 24 months), Kupffer cell pigmentation
(males, 6/12 months), hepatocellular necrosis (males, 12 months) and
hepatocellular alteration (tinctorial and dimensional properties:
both sexes, 24 months). There were no changes attributed to
treatment with myclobutanil with regard to survival, clinical signs,
body-weight, food consumption, ophthalmoscopy, haematology or
urinalysis. Myclobutanil was not oncogenic when administered to mice
for 2 years at dietary levels up to 500 ppm (Goldman & Harris,
1986a).
Rats
Groups of 110 Charles River Sprague Dawley rats/sex were
treated with myclobutanil (90.4% & 91.4% purity) for a period of up
to 24 months at dietary levels of 0, 50, 200 or 800 ppm a.i. equal
to 0, 2.5, 9.8 or 39.2 mg a.i./kg bw/day in males and 0, 3.2, 12.9
or 52.3 mg a.i./kg bw/day in females, respectively. Interim
sacrifices were performed after 3 and 6 months (10 rats/sex/group),
12 months (20/sex/group) and 17 months of study (18 males and 10
females/group). The survivors (52 male, 60 female/group, maximum)
were sacrificed after 24 months of treatment. Treatment with
myclobutanil indicated a NOAEL for in-life parameters of 50 ppm,
equal to 2.5 mg/kg bw/day. Effects of treatment at 200 ppm were
observed as decreased testes weights in association with slight
testicular atrophy at 24 months. At the highest dietary level of 800
ppm, testes weights were decreased at 12 and 24 months with slight
to moderate increases in the incidence of testicular atrophy. (The
seminiferous tubules were reported frequently found to be devoid of
spermatid formation and germinal epithelial cells; the tubules
appeared smaller than normal. In severe cases only Sertoli cells
remained). Increased ovary weights in females treated at 800 ppm and
sacrificed at 12 months were not correlated with any
histomorphological changes. Other treatment-related effects noted at
800 ppm were decreased body-weights (both sexes) and food
consumption (males), and increased liver weights (females).
Increased MFO activity was recorded at 200 ppm in females (3 months)
and at 800 ppm in both sexes (3 months) and in males (6 months).
There were no treatment-related effects on survival, clinical
signs, ophthalmoscopy, haematology, blood chemistry or urinalysis.
Treatment with myclobutanil at dietary levels up to 800 ppm failed
to uncover any evidence of carcinogenic potential (Shellenberger et
al., 1986).
Reproduction studies
Rats
A two-generation (two litter per generation) reproduction study
was conducted with groups of 25 CRI:CD(SD)BR rats/sex fed
myclobutanil (84.5% purity) at dietary levels of 0, 50, 200 or 1000
ppm a.i. equal to 0, 3.6, 14.7 or 73.6 mg a.i./kg bw/day in males
and 0, 4.3, 17.4 or 87 mg a.i./kg bw/day in females respectively. In
the F0 generation, treatment with myclobutanil commenced 8 weeks
before mating. In the F1 generation, the F1a litter-derived
parental animals were exposed to the test material throughout
weaning and for a minimum period of 8 weeks post-weaning. In both
generations, treatment continued throughout the reproductive phases.
Treatment-related effects on reproduction were denoted at the
highest dietary level of 1000 ppm by a decreased number of females
delivering litters (F0F1a and both matings from second
generation), decreased mean number of pups per litter (first mating
of second generation) and an increased number of stillborn pups (all
matings of both generations). An increase, albeit minimal in the
proportion of dead pups in both matings of the first generation was
similarly recorded at 200 ppm when compared to the controls. Effects
on the reproductive organs were evident in the second generation
F1 males treated at 1000 ppm as multifocal or diffuse atrophy of
the testes, decreased spermatozoa and/or necrotic spermatocytes of
the epididymides, as well as atrophy of the prostate. Systemic
toxicity was observed at 200 ppm as increased liver weights in males
of both parental generations in association with centrilobular
hepatocytic hypertrophy in the F1 generation males. At 1000 ppm,
increased liver weights and hepatocytic hypertrophy were observed in
males and females of both generations. Decreased body-weight in
males and depressed food intake in both sexes were recorded at 1000
ppm in both parental generations. Body-weights were similarly
decreased at 1000 ppm in both sexes of all filial generations. The
NOAEL for this study was determined to be 50 ppm, equal to 3.6 mg/kg
bw/day, for systemic and reproductive effects (Costlow & Harris,
1985).
Special studies on teratogenicity
Rats
A range-finding study was performed with groups of 8 mated
female Crl:CD(SD)BR rats treated orally by gavage with myclobutanil
(81.1% purity) at 0 (vehicle, corn oil), 32, 68, 100, 215, 464 or
700 mg a.i./kg bw/day on days 6 to 15 of gestation. Day 0 of
gestation was considered the day sperm were evident in the vaginal
smear. All surviving dams were killed on day 20 of gestation. The
NOAEL for maternal toxicity was 215 mg/kg bw/day. At dose levels of
464 and 700 mg/kg bw/day, treatment with myclobutanil resulted in
mortality (25% and 100%, respectively), decreased body-weights and
clinical signs of toxicity manifest as scant faeces,
chromodacryorrhea, red exudate around mouth, rough and urine-stained
hair coat, and salivation. Embryofetal toxicity was expressed at
levels of 68 mg/kg bw/day and higher as increased resorptions and
decreased viability indices (viable fetuses/implantation sites).
Decreased fetal weights were recorded at 464 mg/kg bw/day. In the
absence of detailed visceral and skeletal examinations of the
fetuses, the NOAEL for developmental toxicity was 32 mg/kg bw/day
(Costlow & Kane, 1984a).
Myclobutanil (84.5% purity) was administered orally by gavage
at 0 (vehicle, corn oil), 31, 94, 310 or 470 mg a.i./kg bw/day to
groups of 25 presumed pregnant Crl:CD(SD)BR rats from days 6 to 15
of gestation. The day on which sperm were found in the vaginal smear
was considered day 0 of gestation. All surviving dams were killed on
day 20 of gestation. A NOAEL for maternal toxicity was indicated at
94 mg/kg bw/day based on clinical signs of toxicity (rough hair
coat, desquamation and salivation) at doses of 310 mg/kg bw/day and
higher. At 470 mg/kg bw/day, red exudate around the mouth, scant
faeces and decreased body-weights were also observed. Decreased
viability indices (viable fetuses /implantation sites) and a slight
trend toward increasing resorption rate were recorded at 94 mg/kg
bw/day and higher resulting in a NOAEL for embryofetal toxicity of
31 mg/kg bw/day. An increased incidence of skeletal variations of
the ribs (7th cervical and 14th rudimentary ribs) was observed at
310 mg/kg bw/day and higher. Treatment with myclobutanil failed to
reveal any evidence of teratogenic potential (Costlow & Kane,
1984b).
Rabbits
A range-finding study was conducted with myclobutanil (84.5%
purity) administered orally by gavage at 0 (vehicle, 1% methyl
cellulose), 10, 31.6, 100, 215, 464 or 700 mg a.i./kg bw/day to
groups of 6 artificially inseminated New Zeeland white rabbits on
days 7 through 19 of gestation. The day of artificial insemination
was designated as day 0 of gestation. All surviving animals were
killed on day 29. Myclobutanil was lethal at doses of 464 mg/kg
bw/day and higher resulting in 100% mortality. Maternal toxicity
represented by clinical signs (irregular faeces and red-stained
urine) and decreased body-weights was noted at 215 mg/kg bw/day. An
increased incidence of resorptions and decreased litter size (viable
fetuses/litter) was observed at 215 mg/kg bw/day, resulting in an
overall NOAEL of 100 mg/kg bw/day. Viable fetuses from surviving
rabbits appeared normal upon gross examination (Costlow & Kane,
1984c).
Groups of 18 artificially inseminated female New Zeeland white
rabbits were administered myclobutanil (90.4% purity) at 0
(distilled water control), 0 (vehicle, 1% methyl cellulose), 20, 60
or 200 mg a.i./kg bw/day orally by gavage from day 7 through 19 of
gestation. The day of insemination was designated as day 0 of
gestation. All surviving rabbits were killed on day 29. The NOAEL
for maternal toxicity was 20 mg/kg bw/day, based on minimal,
transient body-weight loss at 60 mg/kg bw/day. Maternal animals at
the high-dose of 200 mg/kg bw/day experienced body-weight loss and
clinical signs reported as irregular faeces and blood-stained urine.
Embryofetal toxicity was manifest at 200 mg/kg bw/day as an
increased frequency of abortion and total litter resorption, an
increased incidence of litters with resorptions as well as reduced
litter size and fetal weight. Myclobutanil was not teratogenic when
administered to pregnant rabbits at dose levels of up to 200 mg/kg
bw/day (Costlow & Kane, 1984d).
Special studies on genotoxicity
Myclobutanil did not reveal any evidence of genotoxic potential
when investigated in a battery of assays specific for gene mutation
in microbial and mammalian cells or for detection of chromosomal
aberrations in cytogenetics studies. Myclobutanil did not induce
unscheduled DNA synthesis in isolated rat hepatocytes and was
negative in a DNA repair test with Bacillus subtilis. A dominant
lethal study in rats was also negative. The results of the
genotoxicity studies are presented in Table 2.
Special studies on irritation and sensitization
Eye irritation potential was studied in 9 male New Zeeland
white rabbits given technical myclobutanil with a purity of 78.4%
(Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984). Treatment of
the eyes with higher purity technical material produced both corneal
and conjunctival effects suggestive of moderate to severe irritating
potential. Myclobutanil with a purity of 78.4% was only slightly
irritating to the eyes of rabbits resulting in reversible
conjunctival effects.
A 0.5 ml aliquot of technical myclobutanil was applied dermally
to the shaved backs of 6 male New Zeeland rabbits under occluded
conditions for a 4-hour exposure period. Myclobutanil with a purity
of 78.4% (Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984) was
practically non-irritating to the skin of male rabbits.
Table 2. Genotoxicity of technical myclobutanil
Test Test system Concentration (vehicle) Puritya Results Reference
Reverse S. typhimurium 99% negative Byers & Lohse (1983)
Mutation TA 98, 100, 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(in vitro) TA 1535, 75, 250, 500, 1000, 5000 µg/plate
TA 1537 250, 750, 1500, 2500, 7500 µg/plate
(DMSO)
S. typhimurium 84.5% negative Byers & Chism (1983a)
TA 98, 100, 1535, 1537 75 - 7500 µg/plate (DMSO) 1., 2.
S. typhimurium 90.4% negative Byers & Chism (1983b)
TA 98, 100, 1535, 1537 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(DMSO)
S. typhimurium 91.4% negative Sutou (1987)
TA 98, 100, 1535, 1537 125, 250, 500, 1000, 2000 ug/plate 1., 2.
E. coli WP2 uvrA- (DMSO)
Point mutation Chinese hamster ovary, K1BH4 cell 1. 120-175 µg/ml 81.1% negative O'Neill et al. (1984)
(in vitro) line - HGPRT locus 2. 25-90 µg/ml 1., 2.
(DMSO)
Chromosome Chinese hamster ovary WB1 cells 1. 20, 30, 40, 50 µg/ml 91.9% negative Ivett (1985)
aberration 2. 25, 50, 75 µg/ml 1., 2.
(in vitro) (DMSO)
Chromosome Mouse (male CR CD-1) 0, 65, 260, 650 mg/kg bw 81.1% negative McLeod & McCarthy (1984)
aberration bone marrow (corn oil)
(in vivo)
Mouse 0, 117, 585, 1170 mg/kg bw 91.4% negative Sames & Frank (1987)
(male and female (corn oil)
Crl:CD-1(ICR)),
bone marrow
Table 2 (cont'd)
Test Test system Concentration (vehicle) Puritya Results Reference
DNA repair Bacillus subtilis 312.5, 625, 1250, 2500, 5000 ug/plate 91.4% negative Sutou (1987)
(in vitro) H17, M45 1., 2.
Unscheduled DNA Rat 0.1 - 1000 ug/ml 91.9% negative Muller (1986)
synthesis (CRCD, Crl:CDBR male) (DMSO)
(in vitro) hepatocytes
Dominant Rat, male 0, 10, 100, 735 mg/kg bw 91.4% negative Dearlove et al. (1986)
lethal Crl:COBS(SD)BR (corn oil)
(in vivo)
DMSO = dimethyl sulfoxide
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
a = active ingredient (a.i.)
The potential of technical myclobutanil to induce delayed
contact hypersensitivity in the Hartley guinea-pig using a modified
Buehler procedure, could not unequivocally be ascertained due to a
low incidence of animals with erythema response following challenge
doses with the test material (Bonin & Hazelton, 1987a). Technical
myclobutanil, when administered to guinea-pigs according to the
method of Magnusson and Kligman did not produce delayed contact
hypersensitivity (Kreuzmann, 1989).
Observations in humans
No information was available.
COMMENTS
Myclobutanil was rapidly absorbed when administered orally to
rats and mice. The principal routes of excretion were via the urine
and faeces, with no significant residual tissue accumulation. The
toxicokinetic model in the rodent did not differ markedly with
respect to species, sex, single versus repeated exposures or doses.
In both the rat and mouse, myclobutanil was extensively
metabolized to more polar compounds. The proposed metabolic pathway
of myclobutanil in the rat was through oxidation of the butyl group.
The major excretory metabolites were qualitatively similar with
respect to sex and dose.
Myclobutanil was only slightly toxic upon acute oral
administration to rats and mice. WHO has classified myclobutanil as
slightly hazardous (WHO, 1992).
The primary target organ upon repeated dietary exposure to
myclobutanil was the liver. Histomorphological changes,
characterized predominantly by centrilobular hepatocytic hypertrophy
in association with increased liver weights, were observed in all
species investigated. Microscopically, there was accentuated lobular
architecture, hepatocytic vacuolation, inflammation, necrosis, and
pigmentation of the Kupffer cells. Increased hepatic enzyme activity
in serum (ALAT, ASAT, GGT, ALP) were also observed. Hepatic
microsomal MFO activities in rats and mice were increased
correspondingly. There were no similar increases in hepatic
peroxisomal œ-oxidation activity that would have suggested
peroxisomal proliferation.
A three-month dietary study with myclobutanil in the mouse at
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm revealed
hepatic alterations at dietary levels of 1000 ppm and higher,
resulting in a NOAEL of 300 ppm, equal to 42.1 mg/kg bw/day.
Two 3-month studies in rats fed myclobutanil at levels of 0,
10, 30, 100, 300, 1000, 3000, 10 000, or 30 000 ppm and 0, 100, 300,
or 3000 ppm indicated a NOAEL of 100 ppm, equal to 5.2 mg/kg bw/day,
based on treatment-related hepatic effects.
The NOAEL for myclobutanil-related liver effects in dogs
treated for 3 months at 0, 10, 200, 800 or 1600 ppm was 10 ppm,
equal to 0.3 mg/kg bw/day. Treatment of dogs with myclobutanil for
12 months at dietary levels of 0, 10, 100, 400, or 1600 ppm resulted
in a NOAEL for hepatic effects of 100 ppm, equal to 3.1 mg/kg
bw/day. Myclobutanil administered to two dogs per sex at dietary
levels of up to 1000 ppm, equal to 39 mg/kg bw/day, did not produce
any hepatic changes after a period of 4 weeks.
Long-term dietary treatment of mice with myclobutanil for two
years at 0, 20, 100 or 500 ppm revealed a NOAEL of 20 ppm, equal to
2.7 mg/kg bw/day, based on increased MFO activity at 100 ppm as well
as more pronounced liver toxicity at 500 ppm and above. Myclobutanil
was not carcinogenic in mice.
A 24-month long-term toxicity/carcinogenicity study in rats at
dietary concentrations of 0, 50, 200 or 800 ppm revealed a NOAEL of
50 ppm, equal to 2.5 mg/kg bw/day, based on findings of testicular
atrophy and increased MFO activity at 200 ppm and above.
Myclobutanil was not carcinogenic in rats.
A two-generation reproduction study in rats at dietary
concentrations of 0, 50, 200 or 1000 ppm revealed a NOAEL of 50 ppm,
equal to 3.6 mg/kg bw/day, based on increased liver weights and an
increase in numbers of stillborn pups at 200 ppm and above. At 1000
ppm atrophy of the testes and prostate were observed.
An oral teratogenicity study in rats at gavage doses of 0, 31,
94, 310, or 470 mg/kg bw/day demonstrated clinical signs of toxicity
at 310 mg/kg bw/day and above, indicating a NOAEL of 94 mg/kg
bw/day. The NOAEL for embryofetal toxicity was 31 mg/kg bw/day.
There was no evidence of teratogenicity at doses up to 470 mg/kg
bw/day.
Myclobutanil was not teratogenic when administered to the
rabbit at gavage doses of 20, 60 or 200 mg/kg bw/day. A NOAEL for
maternal toxicity was 20 mg/kg bw/day, based on decreased body-
weight at 60 mg/kg bw/day and above. Embryofetal toxicity was
evident at 200 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that myclobutanil was not genotoxic.
An ADI was allocated on the basis of NOAELs in two-year feeding
studies in mice and rats, a reproduction study in rats and a one-
year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 20 ppm, equal to 2.7 mg/kg bw/day (two-year study)
Rat: 50 ppm, equal to 2.5 mg/kg bw/day (two-year study)
50 ppm, equal to 3.6 mg/kg bw/day (two-generation
reproduction study)
Dog: 100 ppm, equal to 3.1 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0 - 0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
1. Results of ongoing long-term studies in mice and rats
known to be in progress.
2. If the results of (1) show a carcinogenic response,
studies (a) to determine whether myclobutanil acts as a
tumour promoter in the two-stage rat liver bioassay and
(b) whether it causes inhibition of intercellular
communications.
3. Observations in humans.
REFERENCES
Bonin, R. & Hazelton, G.A. (1987a). RH-3866 technical: Delayed
contact hypersensitivity study in guinea-pigs. Report No. 87R-035.
Unpublished report dated June 25, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983a). Microbial mutagen assay. Report
No. 83R-162. Unpublished report dated October 19, 1983 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983b). RH-53,866: Microbial mutagen
assay. Report No. 83R-246. Unpublished report dated January 31, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Byers, M.J. & Lohse, K.L. (1983). RH-53,866: Microbial mutagen
assay. Report No. 82R-193. Unpublished report dated January 11, 1983
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Harris, J.C. (1985). RH-53,866: Two generation
reproduction study in rats. Report No. 84R-117. Unpublished report
dated August 21, 1985 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984a). Range-finding teratology study
with RH-53,866 in rats. Report No. 83R-023. Unpublished report dated
June 26, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984b). Teratology study with RH-53,866
in rats. Report No. 83R-024. Unpublished report dated June 22, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984c). Range-finding teratology study
with RH-53,866 in rabbits. Report No. 83R-216. Unpublished report
dated October 31, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984d). Teratology study with RH-53,866
in rabbits. Report No. 83R-217. Unpublished report dated November
15, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986). Dominant
lethal study of RH-53,866 administered orally via gavage to
Crl:COBS(SD)BR male rats. Report No. 018-011; R & H Report No. 86RC-
054. Unpublished report dated October 10, 1986 from Argus Research
Laboratories Inc., Horsham, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Fisher, J.R., Emmons H.F., & Hagan J.V. (1987). RH-3866 technical
acute inhalation toxicity study in rats. Report No. 87R-028.
Unpublished report dated August 31, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Goldman, P.R. & Emmons, H.F. (1986). RH-3866 (Experimental
Fungicide): Four week dietary range-finding study in dogs. Report
No. 83R-078. Unpublished report dated October 8, 1986 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Goldman, P.R. & Harris, J.C. (1986a). RH-3866: Dietary chronic and
oncogenicity study in mice. Report No. 84R-023. Unpublished report
dated October 17, 1986 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Goldman, P.R. & Harris; J.C. (1986b). RH-3866: One-year dietary
study in beagle dogs. Report No. 84R-078. Unpublished report dated
October 15, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Goldman, P.R., Harris, J.C. & Lampe, K.R. (1986). RH-3866: Three-
month dietary toxicity study in mice. Report No. 83R-136.
Unpublished report dated October 8, 1988 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of RH-53,866 technical
in an in vitro cytogenetic assay measuring chromosome aberration
frequencies in Chinese hamster ovary (CHO) cells. Report No. 20990;
R & H Report No. 85RC-011. Unpublished report dated April 26, 1985
from Litton Bionetics, Kensington, Maryland, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Kreuzmann, J.J. (1989). RH-53,866 technical: Guinea-pig maximization
test (Magnusson and Kligman method). Report 89-3655-21; R & H Report
No. 89RC-103. Unpublished report dated April 29, 1989 from Hill Top
Biolabs, Inc., Miamiville, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Krzywicki K.M. (1983). RH-53,866: Definitive oral LD50 in male and
female rats, dermal LD50 in male and female rabbits and skin and
eye irritation in male rabbits. Report No. 83R-125 A&B. Unpublished
report dated September 20, 1983 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Bonin, R. (1984). RH-53,866 technical: Definitive
dermal LD50 in male and female rabbits, and skin and eye
irritation in male rabbits. Report No. 84R-134 A&B. Unpublished
report dated August 3, 1984 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Krajewski, R.J. (1983). RH-53,866 technical:
Definitive oral LD50 in male mice. Report No. 83R-103. Unpublished
report dated July 6, 1983 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Krzywicki, K.M. & Morrison, R.D. (1984a). RH-53,866 technical:
Definitive oral LD50 in male and female rats. Report No. 84R-063
A&B. Unpublished report dated July 19, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLaughlin, J.E. & DiDonato, L.J. (1984). RH-3866 (Experimental
Fungicide): Three month dietary feeding study in dogs. Report No.
83R-204. Unpublished report dated August 7, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLeod, P.L. & McCarthy, K.L. (1984). RH-53,866 in vivo
cytogenetic study in mice. Report No. 84R-074. Unpublished report
dated July 23, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Morrison, R.D., Murphy, M.E., & Chan, P.K. (1984a). RH-53,866
technical: Acute oral LD50 in male mice. Report No. 84R-153.
Unpublished report dated August 3, 1984 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Morrison, R.D., Romanello, A.S., Krzywicki, K.M., & Hazelton, G.A.
(1986b). RH-3866 technical: Definitive oral LD50 in male and
female mice. Report No. 86R-088 A&B. Unpublished report dated August
14, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Muller, G. (1986). RH-53,866 technical in vitro unscheduled DNA
synthesis assay. Report No. 86R-084. Unpublished report dated July
22, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Hara, G.P. & DiDonato, L.J. (1984). RH-3866: Three month dietary
feeding study in rats. Report No. 83R-068. Unpublished report dated
August 7, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Neill, P.J., Foxall, S. & Byers, M.J. (1984). RH-53,866 technical:
CHO/HGPRT gene mutation assay. Report No. 84R-046. Unpublished
report dated May 29, 1984 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Quinn, J.A., Fujimoto, T.T., Egan, A.R. & Shaber, S.H. (1986).
Properties of RH-3866, a new triazole fungicide. Pesticide
Science, Vol. 17: 357-362. Submitted to WHO by Rohm & Haas
Company, Spring House, PA, USA.
Romanello, A.S., Krzywicki, K.M. & Hazelton, G.A. (1986a). RH-3866
technical definitive mouse oral LD50 in females. Report No. 85R-
247. Unpublished report dated May 22, 1986 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
Sames, J.L. & Frank, J.P. (1987). RH-53,866 technical: In vivo
cytogenetics study in mice. Report No. 87R-141. Unpublished report
dated October 30, 1987 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Shellenberger, T.G., Billups, L.H., Tegeris, A.S. & Green, D.S.
(1986). Chronic toxicity and oncogenicity study in rats with RH-
3866. Report No. 8342; R & H Report No. 85RC-061. Unpublished report
dated October 24, 1986 from Tegeris Laboratories, Inc., Laurel,
Maryland. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Shimizu Y. (1987a). Acute oral toxicity study with RH-3866 in mice.
Report No. NRILS 86-2052; R & H Report No. 87RC-036. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987b(. Acute oral toxicity study with RH-3866 in rats.
Report No. NRILS 86-2053; R & H Report No. 87RC-035. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987c). A thirteen week feeding subchronic study of RH-
3866 in rats. Report No. NRILS 86-2024;R & H Report No. 87RC-038.
Unpublished report dated September 1, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986a). RH-3866
kinetic and metabolism study in mice. Report No. 83R-175.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986b). RH-3866
kinetic and metabolism study in rats. Report No. 83R-144.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Streelman D.R. (1984). Material balance and metabolism study of
14C-RH-3866 in rats. Report No. TR 310-84-16. Unpublished report
dated June 22, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Sutou, S. (1987). Mutagenicity testing of RH-53,866 technical with
bacteria. Report No. NRILS 86-2112; R & H Report No. 87RC-037.
Unpublished report dated August 21, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Myclobutanil, 14C-radiolabelled in the chlorophenyl ring was
extensively metabolized to more polar metabolites when administered
by gavage to groups of male and female rats as a single oral dose of
1 or 100 mg/kg bw (Steigerwalt et al., 1986b). 14C-Metabolites
of myclobutanil were qualitatively similar with respect to sex and
dose. Unchanged parent compound represented only 1-4% of the
excreted dose. In males, five fractions with more than 10% of the
excreted 14C were identified in the excreta compared with a single
major fraction in females, which accounted for 53-61% of the
radiolabel.
Effects on enzymes and other biochemical parameters
Mice
Sections of liver were taken from 4 randomly selected mice/sex
from the 6 highest dietary levels: 30, 100, 300, 1000, 3000 or 10
000 ppm a.i. of the 3-month dietary toxicity study with myclobutanil
in mice (Goldman et al., 1986) for determination of hepatic MFO
activity. Liver sections were analyzed for MFO activity using
aminopyrine and benzphetamine N-demethylation assays. MFO activity
per gram of liver as estimated by N-demethylation of benzphetamine
was significantly increased in males (2.1 to 3.3-fold) at 1000 ppm
and above, and in females (1.7 to 2.2-fold) at dietary levels of
3000 ppm and higher. Enzyme activity as measured by N-demethylation
of amino-pyrine revealed increased levels in males (2.2 to 2.3-fold)
at 3000 ppm and higher, and in females at 10 000 ppm (1.7-fold).
Hepatic microsomal protein was increased in both sexes at dietary
levels of 3000 ppm and higher.
Liver microsomal suspensions were prepared from 6 randomly
selected mice/sex treated with myclobutanil at 0, 20, 100 and 500
ppm a.i. as part of the long-term carcinogenicity study (Goldman &
Harris, 1986a). The microsomal suspensions were assayed for MFO
activity using aminopyrine N-demethylation after 3, 6 or 12 months
of treatment. Hepatic MFO activity per gram of liver was increased
after 3 months in females (1.3-fold) at 100 ppm, and in both sexes
(1.5 to 2-fold) at 500 ppm; after 6 months in both sexes at 100 ppm
(1.3 to 1.4-fold) and 500 ppm (2.8 to 2.9-fold); and after 12 months
in females (1.5-fold) at 100 ppm, and in both sexes (1.6 to 3.7-
fold) at 500 ppm. There were no treatment-related effects on hepatic
microsomal protein concentration after 3, 6 or 12 months.
Additional liver samples taken at 12 months were analyzed for
hepatic peroxisomal œ-oxidation activity by measuring the conversion
of acid insoluble 14C-palmitoyl-CoA (substrate) to the acid
soluble acetyl CoA by hepatic acyl-CoA oxidase. There were no
increases in peroxisomal 14C-palmitoyl-CoA oxidase activity
indicative of peroxisomal proliferation (Goldman & Harris, 1986a).
Rats
Mixed function oxidase activity was measured by aminopyrine and
benzphetamine N-demethylation assays on sections of liver taken from
3 randomly selected rats/sex from the control group and from dietary
level groups fed myclobutanil at 100 ppm and higher as part of a 3-
month study (O'Hara & DiDonato, 1984). Significant, dose-related
increases in MFO activity per gram of liver were recorded at dietary
levels of 300 ppm in males only (1.7 to 1.9-fold), and in both sexes
at 1000 ppm (1.8 to 2.3-fold), 3000 ppm (3 to 5.1-fold) and 10 000
ppm (4.1 to 8-fold higher). Microsomal protein was increased in both
sexes at 10 000 ppm and in males at 3000 ppm.
Livers from 6 randomly selected rats/sex/group were collected
from interim kills scheduled after 3, 6 or 12 months of treatment
with myclobutanil at dietary levels of 0, 50, 200 or 800 ppm as a
component of the long-term study in rats (Shellenberger et al.,
1986). Hepatic MFO analyzed by aminopyrine N-demethylation revealed
increased activity per gram of liver after 3 months at 200 ppm in
females (1.6-fold) and in both sexes at 800 ppm (1.5 to 1.8-fold),
and after 6 months in males at 800 ppm (1.5-fold). Increases in MFO
activity in females at 6 months and in both sexes at 12 months of
study were not significantly different from the controls. The
microsomal protein concentration in the treated groups was not
markedly different from the controls.
For determination of hepatic peroxisomal œ-oxidation activity,
additional sections of liver were obtained from 6 rats/sex/group at
the 12-month interim kill. There was no effect of myclobutanil
treatment on hepatic peroxisomal œ-oxidation activity at dietary
levels as high as 800 ppm (Shellenberger et al., 1986).
Toxicological studies
Acute toxicity studies
Acute toxicity studies with technical myclobutanil have been
performed in several animal species, the results of which are
summarized in Table 1. Myclobutanil, upon oral administration was
only slightly toxic to the mouse and rat with LD50 values of 1.36
to > 4.42 g/kg bw and 1.6 to 2.71 g/kg bw, respectively.
Myclobutanil was practically non-toxic by the inhalation and dermal
routes with a LC50 value greater than 5.1 mg/L in rats and LD50
values greater than 5 g/kg bw in the rabbit, respectively.
Table 1. Acute toxicity of technical myclobutanil
Species Sex Route Vehicle LD50 Puritya Reference
(strain) (g/kg bw)
Mouse M oral corn oil 3.23 81.1% Krzywicki & Krajewski (1983)
(CRCD-1)
Mouse M oral corn oil > 4.42 91.9% Morrison et al. (1984a)
(CRCD-1)
Mouse M+F oral corn oil M: 1.91 91.9% Morrison et al. (1986b)
(CRCD-1) F: 1.84
Mouse F oral corn oil 1.36 91.9% Romanello et al. (1986a)
(CRCD-1)
Mouse M+F oral corn oil M: 2.27 91.4% Shimizu (1987a)
(CRJ: CD-1 F: 2.44
(ICR))
Rat M+F oral corn oil M: 1.75 84.5% Krzywicki (1983)
(CR(CD)SD) F: 1.80
Rat M+F oral corn oil M: 1.6 91.9% Krzywicki & Morrison (1984a)
(CR(CD)SD) F: 2.29
Rat M+F oral corn oil M: 2.62 91.4% Shimizu (1987b)
(CRJ: CD-1 F: 2.71
(SD))
Rat M+F inhalation - LC50: 91.4% Fisher et al. (1987)
(Crl:CDBR) > 5.1 mg/L
air
Rabbit (NZW) M+F dermal - > 5.0 84.5% Krzywicki (1983)
Rabbit M+F dermal - > 5.0 91.9% Krzywicki & Bonin (1984)
(NZW)
a = active ingredient (a.i.)
Short-term toxicity studies
Mice
Myclobutanil (81.1% purity) was administered daily for a period
of 3 months to groups of 10 Crl:CD-1 (ICR) BR mice/sex at dietary
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm a.i.
(equal to 0, 0.4, 1.5, 4.8, 14.1, 42.1, 132, 542 or 2035 mg a.i./kg
bw/day in males and 0, 0.6, 2.1, 6.9, 22.9, 65.5, 232, 710 and 2027
mg a.i./kg bw/day in females, respectively). A NOAEL of 300 ppm,
equal to 42.1 mg/kg bw/day, was indicated based on treatment-related
hepatic alterations at dietary levels of 1000 ppm and higher,
manifested histomorphologically as hepatocytic inflammation,
centrilobular hypertrophy, vacuolation, and necrosis. Associated
hepatic changes were increases in liver weight, accentuated liver
lobular architecture, increased MFO activity, increased ALAT levels
and decreased cholesterol. Other microscopic treatment-related
changes pertained to increased cytoplasmic eosinophilia (> 1000
ppm males, > 3000 females) and hypertrophy of the zona
fasciculata cells of the adrenal gland (3000 ppm and higher in
both sexes). Treatment-related effects observed at dietary levels of
3000 ppm and above were decreased body-weights, increased ASAT,
decreased glucose, increased pigmentation of the liver Kupffer cells
and macrophages of the spleen, and a slight increase in lymphoid
necrosis of the spleen. Additional effects of treatment observed
only at the highest dietary level of 10 000 ppm were related to
scant faecal droppings and fluctuations in haematological (both
sexes: decrease HCT, decrease MCV, decrease MCH, increase MCHC;
males: decrease WBC, decrease lymphocytes, increase segmented
neutrophils; females: decrease haemoglobin and increase platelets)
and blood biochemical parameters (increased ALP, GGT and BUN).
Histopathological changes at 10 000 ppm were noted as bile duct
proliferation, slight increase in pigmentation of renal cortical
tubular cells, lymphoid necrosis of the thymus and mesenteric lymph
nodes, increased myeloid/erythroid ratio of bone marrow, immaturity
of the uterus and absence of corpora lutea in ovaries as well as
increased mononuclear cell infiltration of the skin (Goldman et
al., 1986).
Rats
Groups of 10 COBS-CD(SD) BR rats/sex were fed myclobutanil
(81.1% purity) for 3 months at dietary levels of 0, 10, 30, 100,
300, 1000, 3000, 10 000 or 30 000 ppm a.i. equal to 0, 0.5, 1.6,
5.2, 15.3, 51.5, 158, 585 or 1730 mg a.i./kg bw/day in males and 0,
0.7, 2.0, 6.9, 19.7, 65.8, 195, 665 or 1811 mg a.i./kg bw/day in
females, respectively. A NOAEL for this study was 100 ppm, equal to
5.2 mg/kg bw/day, as revealed by increased MFO activity in males at
300 ppm and in both sexes at higher dietary levels. Increased liver
weights and accentuated hepatic lobular architecture were observed
at 1000 ppm and above. Treatment-related changes introduced at
dietary levels of 3000 ppm were decreased body-weights, increased
cholesterol, hepatic centrilobular hypertrophy and necrosis,
increased kidney weights associated with renal congestion and
pigmentation of the convoluted tubular epithelium, and
histopathological alterations of the adrenal (increased cortical
vacuolization), ovary (congestion), thyroid (increase in small
follicles) and thymus (congestion). Additional effects of treatment
exhibited at 10 000 ppm were decreased food consumption, slight
haematological changes (decrease HCT, decrease Hb, decrease MCV and
increase RBC, increase platelets), increased GGT, pigmentation of
the liver Kupffer cells, hepatocytic vacuolation and coagulative
necrosis, increased pigmentation in the red pulp of the spleen, and
chronic pulmonary alveolitis. Treatment of rats with myclobutanil at
the highest dietary level of 30 000 ppm resulted in 100% mortality.
No effects of treatment were reported with respect to urinalysis and
ophthalmology (O'Hara & DiDonato, 1984).
A 13-week feeding study conducted with groups of 10 Crj:CD SD
rats/sex given myclobutanil (91.4% purity) in the diet at levels of
0, 100, 300 or 3000 ppm equal to 0, 6.2, 18.8 or 192 mg/kg bw/day in
males and 0, 6.9, 19.6 or 225 mg/kg bw/day in females respectively,
demonstrated a NOAEL of 300 ppm, equal to 18.8 mg/kg bw/day.
Treatment with myclobutanil at the highest dietary level of 3000 ppm
culminated in histomorphological alterations of the liver, kidney
and adrenal glands. Specific organ changes were characterized in the
liver as slight to moderate hepatocytic hypertrophy (10/10 males,
8/10 females) and in the kidney as slight vacuolar degeneration of
the renal tubular epithelium (7/10 males). Alterations of the
adrenal were described as vacuolization of the cortical cells (7/10
males), atrophy of the zona fasciculata (5/10 males) and fine
vacuolization of the zona glomerulosa (1/10 males). A single male
at 3000 ppm exhibited changes in the reproductive organs depicted in
the testes as moderate atrophy of the seminiferous tubule(s) and
giant cell-like changes with absence of sperm cells in the
epididymis. Other effects of treatment with myclobutanil were
decreased body-weight, blood chemistry changes (decreased bilirubin,
glucose and triglycerides), increased liver and kidney weights,
decreased adrenal weights and a slight increase in the number of
males with round cells in the urine. Decreased food intake was
observed in males during the first week of treatment. There were no
treatment-related effects on haematology or ophthalmoscopy (Shimizu,
1987c).
Dogs
A range-finding study was undertaken with 2 beagle dogs/sex per
group fed myclobutanil (84.5% purity) for a period of 4 weeks at
dietary levels of 0, 50, 250, 1000 or 4000 ppm a.i. equal to 0, 2.2,
10.5, 45.3 or 45 mg a.i./kg bw/day in males and 0, 2.0, 10.6, 39.3
or 47 mg a.i./kg bw/day in females respectively. Dogs treated at the
highest dietary level of 4000 ppm were sacrificed after 2 weeks of
treatment due to severely depressed food intake. The NOAEL for the
study was determined to be 250 ppm, equal to 10.5 mg/kg bw/day,
based on slightly decreased body-weight and food consumption
recorded during the first week of treatment in females at 1000 ppm.
There were no treatment-related consequences on clinical signs,
haematological and blood chemistry investigations (12 and 28 days),
or gross pathological examination (Goldman & Emmons, 1986).
Myclobutanil (81.1% purity) was administered to groups of 4
beagle dogs/sex for a period of 3 months at dietary levels of 0, 10,
200, 800 or 1600 ppm a.i. equal to 0, 0.3, 7.3, 29.1 or 56.8 mg
a.i./kg bw/day in males and 0, 0.4, 7.9, 32.4 or 58 mg a.i./kg
bw/day in females respectively. The NOAEL was determined to be 10
ppm, equal to 0.3 mg/kg bw/day, based on the dose-related incidence
of centrilobular or midzonal hepatocellular hypertrophy noted at 200
ppm (3/4 males) and in all animals of both sexes at 800 and 1600
ppm. Periportal hepatocytes were enlarged in a few of the more
severely affected livers. Liver weights were increased in males at
800 ppm and in both sexes at 1600 ppm. Treatment-related effects on
the kidney, evident as an increased incidence and severity of
unilateral chronic nephritis were observed in males at 800 ppm and
higher. Additional effects of treatment unveiled at the highest
level of 1600 ppm were related to decreased body-weights and food
consumption, due possibly to palatability of the diet. Slight
changes in haematological and blood chemistry values were within
range of normal variability and were not considered of toxicological
consequence. There were no effects of treatment on ophthalmology.
Increases in the ovarian weights at 800 and 1600 ppm were attributed
to estrus (McLaughlin & DiDonato, 1984).
Groups of 6 beagle dogs/sex were administered myclobutanil
(91.4% purity) daily for 12 months in the diet at levels of 0, 10,
100, 400 or 1600 ppm a.i. equal to 0, 0.3, 3.1, 14.3 or 54.2 mg
a.i./kg bw/day in males and 0, 0.4, 3.8, 15.7 or 58.2 mg a.i./kg
bw/day in females, respectively. The principal target organ was the
liver, demonstrating a NOAEL of 100 ppm, equal to 3.1 mg/kg bw/day,
based on hepatocellular hypertrophy, increased liver weights and
increased serum alkaline phosphatase levels at dietary
concentrations of 400 ppm and higher. At the highest level of 1600
ppm, livers displayed accentuated lobular architecture and in 4 of 6
females, the hepatocytes were expanded with large clear cytoplasmic
spaces. Other effects observed at 1600 ppm were evident as decreased
body-weight and food consumption, as well as changes in haematology
(decrease RBC, increase platelet count) and blood chemistry
(increase phosphorus, increase ALAT, increase GGT, decrease
albumin). Treatment with myclobutanil failed to elicit any adverse
effects on clinical signs, ophthalmological examination or
urinalysis (Goldman & Harris, 1986b).
Long-term toxicity/carcinogenicity studies
Mice
A 2-year study was conducted with Crl:CD-1 (ICR)BR mice fed
diets containing myclobutanil (90.4% purity) at levels of 0, 20, 100
or 500 ppm a.i. equal to 0, 2.7, 13.7 or 70.2 mg a.i./kg bw/day in
males and 0, 3.2, 16.5 or 85.2 mg a.i./kg bw/day in females,
respectively. A total of 110 males and 110 females per group were
assigned to the chronic phase of this bioassay. Interim sacrifices
were scheduled after 3 (10 mice/sex/group), 6 (10/sex/group) and 12
months (20/sex/group) of study. All surviving animals (70/sex/group,
maximum) were sacrificed after 24 months of continuous treatment.
Treatment with myclobutanil resulted in a NOAEL for in-life
parameters of 20 ppm, equal to 2.7 mg/kg bw/day, established on the
basis of increased MFO activity at 3, 6 and 12 months at dietary
levels of 100 and 500 ppm. At 500 ppm, target effects of treatment,
primarily on the liver were demonstrated by increased ALAT activity
(females, 3 months), increased liver weights (both sexes, 3 months),
and histopathological alterations comprising centrilobular
hypertrophy (males, 3/6/12 months), periportal vacuolation (males,
3/6/12 months; both sexes, 24 months), Kupffer cell pigmentation
(males, 6/12 months), hepatocellular necrosis (males, 12 months) and
hepatocellular alteration (tinctorial and dimensional properties:
both sexes, 24 months). There were no changes attributed to
treatment with myclobutanil with regard to survival, clinical signs,
body-weight, food consumption, ophthalmoscopy, haematology or
urinalysis. Myclobutanil was not oncogenic when administered to mice
for 2 years at dietary levels up to 500 ppm (Goldman & Harris,
1986a).
Rats
Groups of 110 Charles River Sprague Dawley rats/sex were
treated with myclobutanil (90.4% & 91.4% purity) for a period of up
to 24 months at dietary levels of 0, 50, 200 or 800 ppm a.i. equal
to 0, 2.5, 9.8 or 39.2 mg a.i./kg bw/day in males and 0, 3.2, 12.9
or 52.3 mg a.i./kg bw/day in females, respectively. Interim
sacrifices were performed after 3 and 6 months (10 rats/sex/group),
12 months (20/sex/group) and 17 months of study (18 males and 10
females/group). The survivors (52 male, 60 female/group, maximum)
were sacrificed after 24 months of treatment. Treatment with
myclobutanil indicated a NOAEL for in-life parameters of 50 ppm,
equal to 2.5 mg/kg bw/day. Effects of treatment at 200 ppm were
observed as decreased testes weights in association with slight
testicular atrophy at 24 months. At the highest dietary level of 800
ppm, testes weights were decreased at 12 and 24 months with slight
to moderate increases in the incidence of testicular atrophy. (The
seminiferous tubules were reported frequently found to be devoid of
spermatid formation and germinal epithelial cells; the tubules
appeared smaller than normal. In severe cases only Sertoli cells
remained). Increased ovary weights in females treated at 800 ppm and
sacrificed at 12 months were not correlated with any
histomorphological changes. Other treatment-related effects noted at
800 ppm were decreased body-weights (both sexes) and food
consumption (males), and increased liver weights (females).
Increased MFO activity was recorded at 200 ppm in females (3 months)
and at 800 ppm in both sexes (3 months) and in males (6 months).
There were no treatment-related effects on survival, clinical
signs, ophthalmoscopy, haematology, blood chemistry or urinalysis.
Treatment with myclobutanil at dietary levels up to 800 ppm failed
to uncover any evidence of carcinogenic potential (Shellenberger et
al., 1986).
Reproduction studies
Rats
A two-generation (two litter per generation) reproduction study
was conducted with groups of 25 CRI:CD(SD)BR rats/sex fed
myclobutanil (84.5% purity) at dietary levels of 0, 50, 200 or 1000
ppm a.i. equal to 0, 3.6, 14.7 or 73.6 mg a.i./kg bw/day in males
and 0, 4.3, 17.4 or 87 mg a.i./kg bw/day in females respectively. In
the F0 generation, treatment with myclobutanil commenced 8 weeks
before mating. In the F1 generation, the F1a litter-derived
parental animals were exposed to the test material throughout
weaning and for a minimum period of 8 weeks post-weaning. In both
generations, treatment continued throughout the reproductive phases.
Treatment-related effects on reproduction were denoted at the
highest dietary level of 1000 ppm by a decreased number of females
delivering litters (F0F1a and both matings from second
generation), decreased mean number of pups per litter (first mating
of second generation) and an increased number of stillborn pups (all
matings of both generations). An increase, albeit minimal in the
proportion of dead pups in both matings of the first generation was
similarly recorded at 200 ppm when compared to the controls. Effects
on the reproductive organs were evident in the second generation
F1 males treated at 1000 ppm as multifocal or diffuse atrophy of
the testes, decreased spermatozoa and/or necrotic spermatocytes of
the epididymides, as well as atrophy of the prostate. Systemic
toxicity was observed at 200 ppm as increased liver weights in males
of both parental generations in association with centrilobular
hepatocytic hypertrophy in the F1 generation males. At 1000 ppm,
increased liver weights and hepatocytic hypertrophy were observed in
males and females of both generations. Decreased body-weight in
males and depressed food intake in both sexes were recorded at 1000
ppm in both parental generations. Body-weights were similarly
decreased at 1000 ppm in both sexes of all filial generations. The
NOAEL for this study was determined to be 50 ppm, equal to 3.6 mg/kg
bw/day, for systemic and reproductive effects (Costlow & Harris,
1985).
Special studies on teratogenicity
Rats
A range-finding study was performed with groups of 8 mated
female Crl:CD(SD)BR rats treated orally by gavage with myclobutanil
(81.1% purity) at 0 (vehicle, corn oil), 32, 68, 100, 215, 464 or
700 mg a.i./kg bw/day on days 6 to 15 of gestation. Day 0 of
gestation was considered the day sperm were evident in the vaginal
smear. All surviving dams were killed on day 20 of gestation. The
NOAEL for maternal toxicity was 215 mg/kg bw/day. At dose levels of
464 and 700 mg/kg bw/day, treatment with myclobutanil resulted in
mortality (25% and 100%, respectively), decreased body-weights and
clinical signs of toxicity manifest as scant faeces,
chromodacryorrhea, red exudate around mouth, rough and urine-stained
hair coat, and salivation. Embryofetal toxicity was expressed at
levels of 68 mg/kg bw/day and higher as increased resorptions and
decreased viability indices (viable fetuses/implantation sites).
Decreased fetal weights were recorded at 464 mg/kg bw/day. In the
absence of detailed visceral and skeletal examinations of the
fetuses, the NOAEL for developmental toxicity was 32 mg/kg bw/day
(Costlow & Kane, 1984a).
Myclobutanil (84.5% purity) was administered orally by gavage
at 0 (vehicle, corn oil), 31, 94, 310 or 470 mg a.i./kg bw/day to
groups of 25 presumed pregnant Crl:CD(SD)BR rats from days 6 to 15
of gestation. The day on which sperm were found in the vaginal smear
was considered day 0 of gestation. All surviving dams were killed on
day 20 of gestation. A NOAEL for maternal toxicity was indicated at
94 mg/kg bw/day based on clinical signs of toxicity (rough hair
coat, desquamation and salivation) at doses of 310 mg/kg bw/day and
higher. At 470 mg/kg bw/day, red exudate around the mouth, scant
faeces and decreased body-weights were also observed. Decreased
viability indices (viable fetuses /implantation sites) and a slight
trend toward increasing resorption rate were recorded at 94 mg/kg
bw/day and higher resulting in a NOAEL for embryofetal toxicity of
31 mg/kg bw/day. An increased incidence of skeletal variations of
the ribs (7th cervical and 14th rudimentary ribs) was observed at
310 mg/kg bw/day and higher. Treatment with myclobutanil failed to
reveal any evidence of teratogenic potential (Costlow & Kane,
1984b).
Rabbits
A range-finding study was conducted with myclobutanil (84.5%
purity) administered orally by gavage at 0 (vehicle, 1% methyl
cellulose), 10, 31.6, 100, 215, 464 or 700 mg a.i./kg bw/day to
groups of 6 artificially inseminated New Zeeland white rabbits on
days 7 through 19 of gestation. The day of artificial insemination
was designated as day 0 of gestation. All surviving animals were
killed on day 29. Myclobutanil was lethal at doses of 464 mg/kg
bw/day and higher resulting in 100% mortality. Maternal toxicity
represented by clinical signs (irregular faeces and red-stained
urine) and decreased body-weights was noted at 215 mg/kg bw/day. An
increased incidence of resorptions and decreased litter size (viable
fetuses/litter) was observed at 215 mg/kg bw/day, resulting in an
overall NOAEL of 100 mg/kg bw/day. Viable fetuses from surviving
rabbits appeared normal upon gross examination (Costlow & Kane,
1984c).
Groups of 18 artificially inseminated female New Zeeland white
rabbits were administered myclobutanil (90.4% purity) at 0
(distilled water control), 0 (vehicle, 1% methyl cellulose), 20, 60
or 200 mg a.i./kg bw/day orally by gavage from day 7 through 19 of
gestation. The day of insemination was designated as day 0 of
gestation. All surviving rabbits were killed on day 29. The NOAEL
for maternal toxicity was 20 mg/kg bw/day, based on minimal,
transient body-weight loss at 60 mg/kg bw/day. Maternal animals at
the high-dose of 200 mg/kg bw/day experienced body-weight loss and
clinical signs reported as irregular faeces and blood-stained urine.
Embryofetal toxicity was manifest at 200 mg/kg bw/day as an
increased frequency of abortion and total litter resorption, an
increased incidence of litters with resorptions as well as reduced
litter size and fetal weight. Myclobutanil was not teratogenic when
administered to pregnant rabbits at dose levels of up to 200 mg/kg
bw/day (Costlow & Kane, 1984d).
Special studies on genotoxicity
Myclobutanil did not reveal any evidence of genotoxic potential
when investigated in a battery of assays specific for gene mutation
in microbial and mammalian cells or for detection of chromosomal
aberrations in cytogenetics studies. Myclobutanil did not induce
unscheduled DNA synthesis in isolated rat hepatocytes and was
negative in a DNA repair test with Bacillus subtilis. A dominant
lethal study in rats was also negative. The results of the
genotoxicity studies are presented in Table 2.
Special studies on irritation and sensitization
Eye irritation potential was studied in 9 male New Zeeland
white rabbits given technical myclobutanil with a purity of 78.4%
(Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984). Treatment of
the eyes with higher purity technical material produced both corneal
and conjunctival effects suggestive of moderate to severe irritating
potential. Myclobutanil with a purity of 78.4% was only slightly
irritating to the eyes of rabbits resulting in reversible
conjunctival effects.
A 0.5 ml aliquot of technical myclobutanil was applied dermally
to the shaved backs of 6 male New Zeeland rabbits under occluded
conditions for a 4-hour exposure period. Myclobutanil with a purity
of 78.4% (Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984) was
practically non-irritating to the skin of male rabbits.
Table 2. Genotoxicity of technical myclobutanil
Test Test system Concentration (vehicle) Puritya Results Reference
Reverse S. typhimurium 99% negative Byers & Lohse (1983)
Mutation TA 98, 100, 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(in vitro) TA 1535, 75, 250, 500, 1000, 5000 µg/plate
TA 1537 250, 750, 1500, 2500, 7500 µg/plate
(DMSO)
S. typhimurium 84.5% negative Byers & Chism (1983a)
TA 98, 100, 1535, 1537 75 - 7500 µg/plate (DMSO) 1., 2.
S. typhimurium 90.4% negative Byers & Chism (1983b)
TA 98, 100, 1535, 1537 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(DMSO)
S. typhimurium 91.4% negative Sutou (1987)
TA 98, 100, 1535, 1537 125, 250, 500, 1000, 2000 ug/plate 1., 2.
E. coli WP2 uvrA- (DMSO)
Point mutation Chinese hamster ovary, K1BH4 cell 1. 120-175 µg/ml 81.1% negative O'Neill et al. (1984)
(in vitro) line - HGPRT locus 2. 25-90 µg/ml 1., 2.
(DMSO)
Chromosome Chinese hamster ovary WB1 cells 1. 20, 30, 40, 50 µg/ml 91.9% negative Ivett (1985)
aberration 2. 25, 50, 75 µg/ml 1., 2.
(in vitro) (DMSO)
Chromosome Mouse (male CR CD-1) 0, 65, 260, 650 mg/kg bw 81.1% negative McLeod & McCarthy (1984)
aberration bone marrow (corn oil)
(in vivo)
Mouse 0, 117, 585, 1170 mg/kg bw 91.4% negative Sames & Frank (1987)
(male and female (corn oil)
Crl:CD-1(ICR)),
bone marrow
Table 2 (cont'd)
Test Test system Concentration (vehicle) Puritya Results Reference
DNA repair Bacillus subtilis 312.5, 625, 1250, 2500, 5000 ug/plate 91.4% negative Sutou (1987)
(in vitro) H17, M45 1., 2.
Unscheduled DNA Rat 0.1 - 1000 ug/ml 91.9% negative Muller (1986)
synthesis (CRCD, Crl:CDBR male) (DMSO)
(in vitro) hepatocytes
Dominant Rat, male 0, 10, 100, 735 mg/kg bw 91.4% negative Dearlove et al. (1986)
lethal Crl:COBS(SD)BR (corn oil)
(in vivo)
DMSO = dimethyl sulfoxide
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
a = active ingredient (a.i.)
The potential of technical myclobutanil to induce delayed
contact hypersensitivity in the Hartley guinea-pig using a modified
Buehler procedure, could not unequivocally be ascertained due to a
low incidence of animals with erythema response following challenge
doses with the test material (Bonin & Hazelton, 1987a). Technical
myclobutanil, when administered to guinea-pigs according to the
method of Magnusson and Kligman did not produce delayed contact
hypersensitivity (Kreuzmann, 1989).
Observations in humans
No information was available.
COMMENTS
Myclobutanil was rapidly absorbed when administered orally to
rats and mice. The principal routes of excretion were via the urine
and faeces, with no significant residual tissue accumulation. The
toxicokinetic model in the rodent did not differ markedly with
respect to species, sex, single versus repeated exposures or doses.
In both the rat and mouse, myclobutanil was extensively
metabolized to more polar compounds. The proposed metabolic pathway
of myclobutanil in the rat was through oxidation of the butyl group.
The major excretory metabolites were qualitatively similar with
respect to sex and dose.
Myclobutanil was only slightly toxic upon acute oral
administration to rats and mice. WHO has classified myclobutanil as
slightly hazardous (WHO, 1992).
The primary target organ upon repeated dietary exposure to
myclobutanil was the liver. Histomorphological changes,
characterized predominantly by centrilobular hepatocytic hypertrophy
in association with increased liver weights, were observed in all
species investigated. Microscopically, there was accentuated lobular
architecture, hepatocytic vacuolation, inflammation, necrosis, and
pigmentation of the Kupffer cells. Increased hepatic enzyme activity
in serum (ALAT, ASAT, GGT, ALP) were also observed. Hepatic
microsomal MFO activities in rats and mice were increased
correspondingly. There were no similar increases in hepatic
peroxisomal œ-oxidation activity that would have suggested
peroxisomal proliferation.
A three-month dietary study with myclobutanil in the mouse at
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm revealed
hepatic alterations at dietary levels of 1000 ppm and higher,
resulting in a NOAEL of 300 ppm, equal to 42.1 mg/kg bw/day.
Two 3-month studies in rats fed myclobutanil at levels of 0,
10, 30, 100, 300, 1000, 3000, 10 000, or 30 000 ppm and 0, 100, 300,
or 3000 ppm indicated a NOAEL of 100 ppm, equal to 5.2 mg/kg bw/day,
based on treatment-related hepatic effects.
The NOAEL for myclobutanil-related liver effects in dogs
treated for 3 months at 0, 10, 200, 800 or 1600 ppm was 10 ppm,
equal to 0.3 mg/kg bw/day. Treatment of dogs with myclobutanil for
12 months at dietary levels of 0, 10, 100, 400, or 1600 ppm resulted
in a NOAEL for hepatic effects of 100 ppm, equal to 3.1 mg/kg
bw/day. Myclobutanil administered to two dogs per sex at dietary
levels of up to 1000 ppm, equal to 39 mg/kg bw/day, did not produce
any hepatic changes after a period of 4 weeks.
Long-term dietary treatment of mice with myclobutanil for two
years at 0, 20, 100 or 500 ppm revealed a NOAEL of 20 ppm, equal to
2.7 mg/kg bw/day, based on increased MFO activity at 100 ppm as well
as more pronounced liver toxicity at 500 ppm and above. Myclobutanil
was not carcinogenic in mice.
A 24-month long-term toxicity/carcinogenicity study in rats at
dietary concentrations of 0, 50, 200 or 800 ppm revealed a NOAEL of
50 ppm, equal to 2.5 mg/kg bw/day, based on findings of testicular
atrophy and increased MFO activity at 200 ppm and above.
Myclobutanil was not carcinogenic in rats.
A two-generation reproduction study in rats at dietary
concentrations of 0, 50, 200 or 1000 ppm revealed a NOAEL of 50 ppm,
equal to 3.6 mg/kg bw/day, based on increased liver weights and an
increase in numbers of stillborn pups at 200 ppm and above. At 1000
ppm atrophy of the testes and prostate were observed.
An oral teratogenicity study in rats at gavage doses of 0, 31,
94, 310, or 470 mg/kg bw/day demonstrated clinical signs of toxicity
at 310 mg/kg bw/day and above, indicating a NOAEL of 94 mg/kg
bw/day. The NOAEL for embryofetal toxicity was 31 mg/kg bw/day.
There was no evidence of teratogenicity at doses up to 470 mg/kg
bw/day.
Myclobutanil was not teratogenic when administered to the
rabbit at gavage doses of 20, 60 or 200 mg/kg bw/day. A NOAEL for
maternal toxicity was 20 mg/kg bw/day, based on decreased body-
weight at 60 mg/kg bw/day and above. Embryofetal toxicity was
evident at 200 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that myclobutanil was not genotoxic.
An ADI was allocated on the basis of NOAELs in two-year feeding
studies in mice and rats, a reproduction study in rats and a one-
year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 20 ppm, equal to 2.7 mg/kg bw/day (two-year study)
Rat: 50 ppm, equal to 2.5 mg/kg bw/day (two-year study)
50 ppm, equal to 3.6 mg/kg bw/day (two-generation
reproduction study)
Dog: 100 ppm, equal to 3.1 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0 - 0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
1. Results of ongoing long-term studies in mice and rats
known to be in progress.
2. If the results of (1) show a carcinogenic response,
studies (a) to determine whether myclobutanil acts as a
tumour promoter in the two-stage rat liver bioassay and
(b) whether it causes inhibition of intercellular
communications.
3. Observations in humans.
REFERENCES
Bonin, R. & Hazelton, G.A. (1987a). RH-3866 technical: Delayed
contact hypersensitivity study in guinea-pigs. Report No. 87R-035.
Unpublished report dated June 25, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983a). Microbial mutagen assay. Report
No. 83R-162. Unpublished report dated October 19, 1983 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983b). RH-53,866: Microbial mutagen
assay. Report No. 83R-246. Unpublished report dated January 31, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Byers, M.J. & Lohse, K.L. (1983). RH-53,866: Microbial mutagen
assay. Report No. 82R-193. Unpublished report dated January 11, 1983
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Harris, J.C. (1985). RH-53,866: Two generation
reproduction study in rats. Report No. 84R-117. Unpublished report
dated August 21, 1985 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984a). Range-finding teratology study
with RH-53,866 in rats. Report No. 83R-023. Unpublished report dated
June 26, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984b). Teratology study with RH-53,866
in rats. Report No. 83R-024. Unpublished report dated June 22, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984c). Range-finding teratology study
with RH-53,866 in rabbits. Report No. 83R-216. Unpublished report
dated October 31, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984d). Teratology study with RH-53,866
in rabbits. Report No. 83R-217. Unpublished report dated November
15, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986). Dominant
lethal study of RH-53,866 administered orally via gavage to
Crl:COBS(SD)BR male rats. Report No. 018-011; R & H Report No. 86RC-
054. Unpublished report dated October 10, 1986 from Argus Research
Laboratories Inc., Horsham, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Fisher, J.R., Emmons H.F., & Hagan J.V. (1987). RH-3866 technical
acute inhalation toxicity study in rats. Report No. 87R-028.
Unpublished report dated August 31, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Goldman, P.R. & Emmons, H.F. (1986). RH-3866 (Experimental
Fungicide): Four week dietary range-finding study in dogs. Report
No. 83R-078. Unpublished report dated October 8, 1986 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Goldman, P.R. & Harris, J.C. (1986a). RH-3866: Dietary chronic and
oncogenicity study in mice. Report No. 84R-023. Unpublished report
dated October 17, 1986 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Goldman, P.R. & Harris; J.C. (1986b). RH-3866: One-year dietary
study in beagle dogs. Report No. 84R-078. Unpublished report dated
October 15, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Goldman, P.R., Harris, J.C. & Lampe, K.R. (1986). RH-3866: Three-
month dietary toxicity study in mice. Report No. 83R-136.
Unpublished report dated October 8, 1988 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of RH-53,866 technical
in an in vitro cytogenetic assay measuring chromosome aberration
frequencies in Chinese hamster ovary (CHO) cells. Report No. 20990;
R & H Report No. 85RC-011. Unpublished report dated April 26, 1985
from Litton Bionetics, Kensington, Maryland, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Kreuzmann, J.J. (1989). RH-53,866 technical: Guinea-pig maximization
test (Magnusson and Kligman method). Report 89-3655-21; R & H Report
No. 89RC-103. Unpublished report dated April 29, 1989 from Hill Top
Biolabs, Inc., Miamiville, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Krzywicki K.M. (1983). RH-53,866: Definitive oral LD50 in male and
female rats, dermal LD50 in male and female rabbits and skin and
eye irritation in male rabbits. Report No. 83R-125 A&B. Unpublished
report dated September 20, 1983 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Bonin, R. (1984). RH-53,866 technical: Definitive
dermal LD50 in male and female rabbits, and skin and eye
irritation in male rabbits. Report No. 84R-134 A&B. Unpublished
report dated August 3, 1984 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Krajewski, R.J. (1983). RH-53,866 technical:
Definitive oral LD50 in male mice. Report No. 83R-103. Unpublished
report dated July 6, 1983 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Krzywicki, K.M. & Morrison, R.D. (1984a). RH-53,866 technical:
Definitive oral LD50 in male and female rats. Report No. 84R-063
A&B. Unpublished report dated July 19, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLaughlin, J.E. & DiDonato, L.J. (1984). RH-3866 (Experimental
Fungicide): Three month dietary feeding study in dogs. Report No.
83R-204. Unpublished report dated August 7, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLeod, P.L. & McCarthy, K.L. (1984). RH-53,866 in vivo
cytogenetic study in mice. Report No. 84R-074. Unpublished report
dated July 23, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Morrison, R.D., Murphy, M.E., & Chan, P.K. (1984a). RH-53,866
technical: Acute oral LD50 in male mice. Report No. 84R-153.
Unpublished report dated August 3, 1984 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Morrison, R.D., Romanello, A.S., Krzywicki, K.M., & Hazelton, G.A.
(1986b). RH-3866 technical: Definitive oral LD50 in male and
female mice. Report No. 86R-088 A&B. Unpublished report dated August
14, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Muller, G. (1986). RH-53,866 technical in vitro unscheduled DNA
synthesis assay. Report No. 86R-084. Unpublished report dated July
22, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Hara, G.P. & DiDonato, L.J. (1984). RH-3866: Three month dietary
feeding study in rats. Report No. 83R-068. Unpublished report dated
August 7, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Neill, P.J., Foxall, S. & Byers, M.J. (1984). RH-53,866 technical:
CHO/HGPRT gene mutation assay. Report No. 84R-046. Unpublished
report dated May 29, 1984 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Quinn, J.A., Fujimoto, T.T., Egan, A.R. & Shaber, S.H. (1986).
Properties of RH-3866, a new triazole fungicide. Pesticide
Science, Vol. 17: 357-362. Submitted to WHO by Rohm & Haas
Company, Spring House, PA, USA.
Romanello, A.S., Krzywicki, K.M. & Hazelton, G.A. (1986a). RH-3866
technical definitive mouse oral LD50 in females. Report No. 85R-
247. Unpublished report dated May 22, 1986 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
Sames, J.L. & Frank, J.P. (1987). RH-53,866 technical: In vivo
cytogenetics study in mice. Report No. 87R-141. Unpublished report
dated October 30, 1987 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Shellenberger, T.G., Billups, L.H., Tegeris, A.S. & Green, D.S.
(1986). Chronic toxicity and oncogenicity study in rats with RH-
3866. Report No. 8342; R & H Report No. 85RC-061. Unpublished report
dated October 24, 1986 from Tegeris Laboratories, Inc., Laurel,
Maryland. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Shimizu Y. (1987a). Acute oral toxicity study with RH-3866 in mice.
Report No. NRILS 86-2052; R & H Report No. 87RC-036. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987b(. Acute oral toxicity study with RH-3866 in rats.
Report No. NRILS 86-2053; R & H Report No. 87RC-035. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987c). A thirteen week feeding subchronic study of RH-
3866 in rats. Report No. NRILS 86-2024;R & H Report No. 87RC-038.
Unpublished report dated September 1, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986a). RH-3866
kinetic and metabolism study in mice. Report No. 83R-175.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986b). RH-3866
kinetic and metabolism study in rats. Report No. 83R-144.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Streelman D.R. (1984). Material balance and metabolism study of
14C-RH-3866 in rats. Report No. TR 310-84-16. Unpublished report
dated June 22, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Sutou, S. (1987). Mutagenicity testing of RH-53,866 technical with
bacteria. Report No. NRILS 86-2112; R & H Report No. 87RC-037.
Unpublished report dated August 21, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Myclobutanil, 14C-radiolabelled in the chlorophenyl ring was
extensively metabolized to more polar metabolites when administered
by gavage to groups of male and female rats as a single oral dose of
1 or 100 mg/kg bw (Steigerwalt et al., 1986b). 14C-Metabolites
of myclobutanil were qualitatively similar with respect to sex and
dose. Unchanged parent compound represented only 1-4% of the
excreted dose. In males, five fractions with more than 10% of the
excreted 14C were identified in the excreta compared with a single
major fraction in females, which accounted for 53-61% of the
radiolabel.
Effects on enzymes and other biochemical parameters
Mice
Sections of liver were taken from 4 randomly selected mice/sex
from the 6 highest dietary levels: 30, 100, 300, 1000, 3000 or 10
000 ppm a.i. of the 3-month dietary toxicity study with myclobutanil
in mice (Goldman et al., 1986) for determination of hepatic MFO
activity. Liver sections were analyzed for MFO activity using
aminopyrine and benzphetamine N-demethylation assays. MFO activity
per gram of liver as estimated by N-demethylation of benzphetamine
was significantly increased in males (2.1 to 3.3-fold) at 1000 ppm
and above, and in females (1.7 to 2.2-fold) at dietary levels of
3000 ppm and higher. Enzyme activity as measured by N-demethylation
of amino-pyrine revealed increased levels in males (2.2 to 2.3-fold)
at 3000 ppm and higher, and in females at 10 000 ppm (1.7-fold).
Hepatic microsomal protein was increased in both sexes at dietary
levels of 3000 ppm and higher.
Liver microsomal suspensions were prepared from 6 randomly
selected mice/sex treated with myclobutanil at 0, 20, 100 and 500
ppm a.i. as part of the long-term carcinogenicity study (Goldman &
Harris, 1986a). The microsomal suspensions were assayed for MFO
activity using aminopyrine N-demethylation after 3, 6 or 12 months
of treatment. Hepatic MFO activity per gram of liver was increased
after 3 months in females (1.3-fold) at 100 ppm, and in both sexes
(1.5 to 2-fold) at 500 ppm; after 6 months in both sexes at 100 ppm
(1.3 to 1.4-fold) and 500 ppm (2.8 to 2.9-fold); and after 12 months
in females (1.5-fold) at 100 ppm, and in both sexes (1.6 to 3.7-
fold) at 500 ppm. There were no treatment-related effects on hepatic
microsomal protein concentration after 3, 6 or 12 months.
Additional liver samples taken at 12 months were analyzed for
hepatic peroxisomal œ-oxidation activity by measuring the conversion
of acid insoluble 14C-palmitoyl-CoA (substrate) to the acid
soluble acetyl CoA by hepatic acyl-CoA oxidase. There were no
increases in peroxisomal 14C-palmitoyl-CoA oxidase activity
indicative of peroxisomal proliferation (Goldman & Harris, 1986a).
Rats
Mixed function oxidase activity was measured by aminopyrine and
benzphetamine N-demethylation assays on sections of liver taken from
3 randomly selected rats/sex from the control group and from dietary
level groups fed myclobutanil at 100 ppm and higher as part of a 3-
month study (O'Hara & DiDonato, 1984). Significant, dose-related
increases in MFO activity per gram of liver were recorded at dietary
levels of 300 ppm in males only (1.7 to 1.9-fold), and in both sexes
at 1000 ppm (1.8 to 2.3-fold), 3000 ppm (3 to 5.1-fold) and 10 000
ppm (4.1 to 8-fold higher). Microsomal protein was increased in both
sexes at 10 000 ppm and in males at 3000 ppm.
Livers from 6 randomly selected rats/sex/group were collected
from interim kills scheduled after 3, 6 or 12 months of treatment
with myclobutanil at dietary levels of 0, 50, 200 or 800 ppm as a
component of the long-term study in rats (Shellenberger et al.,
1986). Hepatic MFO analyzed by aminopyrine N-demethylation revealed
increased activity per gram of liver after 3 months at 200 ppm in
females (1.6-fold) and in both sexes at 800 ppm (1.5 to 1.8-fold),
and after 6 months in males at 800 ppm (1.5-fold). Increases in MFO
activity in females at 6 months and in both sexes at 12 months of
study were not significantly different from the controls. The
microsomal protein concentration in the treated groups was not
markedly different from the controls.
For determination of hepatic peroxisomal œ-oxidation activity,
additional sections of liver were obtained from 6 rats/sex/group at
the 12-month interim kill. There was no effect of myclobutanil
treatment on hepatic peroxisomal œ-oxidation activity at dietary
levels as high as 800 ppm (Shellenberger et al., 1986).
Toxicological studies
Acute toxicity studies
Acute toxicity studies with technical myclobutanil have been
performed in several animal species, the results of which are
summarized in Table 1. Myclobutanil, upon oral administration was
only slightly toxic to the mouse and rat with LD50 values of 1.36
to > 4.42 g/kg bw and 1.6 to 2.71 g/kg bw, respectively.
Myclobutanil was practically non-toxic by the inhalation and dermal
routes with a LC50 value greater than 5.1 mg/L in rats and LD50
values greater than 5 g/kg bw in the rabbit, respectively.
Table 1. Acute toxicity of technical myclobutanil
Species Sex Route Vehicle LD50 Puritya Reference
(strain) (g/kg bw)
Mouse M oral corn oil 3.23 81.1% Krzywicki & Krajewski (1983)
(CRCD-1)
Mouse M oral corn oil > 4.42 91.9% Morrison et al. (1984a)
(CRCD-1)
Mouse M+F oral corn oil M: 1.91 91.9% Morrison et al. (1986b)
(CRCD-1) F: 1.84
Mouse F oral corn oil 1.36 91.9% Romanello et al. (1986a)
(CRCD-1)
Mouse M+F oral corn oil M: 2.27 91.4% Shimizu (1987a)
(CRJ: CD-1 F: 2.44
(ICR))
Rat M+F oral corn oil M: 1.75 84.5% Krzywicki (1983)
(CR(CD)SD) F: 1.80
Rat M+F oral corn oil M: 1.6 91.9% Krzywicki & Morrison (1984a)
(CR(CD)SD) F: 2.29
Rat M+F oral corn oil M: 2.62 91.4% Shimizu (1987b)
(CRJ: CD-1 F: 2.71
(SD))
Rat M+F inhalation - LC50: 91.4% Fisher et al. (1987)
(Crl:CDBR) > 5.1 mg/L
air
Rabbit (NZW) M+F dermal - > 5.0 84.5% Krzywicki (1983)
Rabbit M+F dermal - > 5.0 91.9% Krzywicki & Bonin (1984)
(NZW)
a = active ingredient (a.i.)
Short-term toxicity studies
Mice
Myclobutanil (81.1% purity) was administered daily for a period
of 3 months to groups of 10 Crl:CD-1 (ICR) BR mice/sex at dietary
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm a.i.
(equal to 0, 0.4, 1.5, 4.8, 14.1, 42.1, 132, 542 or 2035 mg a.i./kg
bw/day in males and 0, 0.6, 2.1, 6.9, 22.9, 65.5, 232, 710 and 2027
mg a.i./kg bw/day in females, respectively). A NOAEL of 300 ppm,
equal to 42.1 mg/kg bw/day, was indicated based on treatment-related
hepatic alterations at dietary levels of 1000 ppm and higher,
manifested histomorphologically as hepatocytic inflammation,
centrilobular hypertrophy, vacuolation, and necrosis. Associated
hepatic changes were increases in liver weight, accentuated liver
lobular architecture, increased MFO activity, increased ALAT levels
and decreased cholesterol. Other microscopic treatment-related
changes pertained to increased cytoplasmic eosinophilia (> 1000
ppm males, > 3000 females) and hypertrophy of the zona
fasciculata cells of the adrenal gland (3000 ppm and higher in
both sexes). Treatment-related effects observed at dietary levels of
3000 ppm and above were decreased body-weights, increased ASAT,
decreased glucose, increased pigmentation of the liver Kupffer cells
and macrophages of the spleen, and a slight increase in lymphoid
necrosis of the spleen. Additional effects of treatment observed
only at the highest dietary level of 10 000 ppm were related to
scant faecal droppings and fluctuations in haematological (both
sexes: decrease HCT, decrease MCV, decrease MCH, increase MCHC;
males: decrease WBC, decrease lymphocytes, increase segmented
neutrophils; females: decrease haemoglobin and increase platelets)
and blood biochemical parameters (increased ALP, GGT and BUN).
Histopathological changes at 10 000 ppm were noted as bile duct
proliferation, slight increase in pigmentation of renal cortical
tubular cells, lymphoid necrosis of the thymus and mesenteric lymph
nodes, increased myeloid/erythroid ratio of bone marrow, immaturity
of the uterus and absence of corpora lutea in ovaries as well as
increased mononuclear cell infiltration of the skin (Goldman et
al., 1986).
Rats
Groups of 10 COBS-CD(SD) BR rats/sex were fed myclobutanil
(81.1% purity) for 3 months at dietary levels of 0, 10, 30, 100,
300, 1000, 3000, 10 000 or 30 000 ppm a.i. equal to 0, 0.5, 1.6,
5.2, 15.3, 51.5, 158, 585 or 1730 mg a.i./kg bw/day in males and 0,
0.7, 2.0, 6.9, 19.7, 65.8, 195, 665 or 1811 mg a.i./kg bw/day in
females, respectively. A NOAEL for this study was 100 ppm, equal to
5.2 mg/kg bw/day, as revealed by increased MFO activity in males at
300 ppm and in both sexes at higher dietary levels. Increased liver
weights and accentuated hepatic lobular architecture were observed
at 1000 ppm and above. Treatment-related changes introduced at
dietary levels of 3000 ppm were decreased body-weights, increased
cholesterol, hepatic centrilobular hypertrophy and necrosis,
increased kidney weights associated with renal congestion and
pigmentation of the convoluted tubular epithelium, and
histopathological alterations of the adrenal (increased cortical
vacuolization), ovary (congestion), thyroid (increase in small
follicles) and thymus (congestion). Additional effects of treatment
exhibited at 10 000 ppm were decreased food consumption, slight
haematological changes (decrease HCT, decrease Hb, decrease MCV and
increase RBC, increase platelets), increased GGT, pigmentation of
the liver Kupffer cells, hepatocytic vacuolation and coagulative
necrosis, increased pigmentation in the red pulp of the spleen, and
chronic pulmonary alveolitis. Treatment of rats with myclobutanil at
the highest dietary level of 30 000 ppm resulted in 100% mortality.
No effects of treatment were reported with respect to urinalysis and
ophthalmology (O'Hara & DiDonato, 1984).
A 13-week feeding study conducted with groups of 10 Crj:CD SD
rats/sex given myclobutanil (91.4% purity) in the diet at levels of
0, 100, 300 or 3000 ppm equal to 0, 6.2, 18.8 or 192 mg/kg bw/day in
males and 0, 6.9, 19.6 or 225 mg/kg bw/day in females respectively,
demonstrated a NOAEL of 300 ppm, equal to 18.8 mg/kg bw/day.
Treatment with myclobutanil at the highest dietary level of 3000 ppm
culminated in histomorphological alterations of the liver, kidney
and adrenal glands. Specific organ changes were characterized in the
liver as slight to moderate hepatocytic hypertrophy (10/10 males,
8/10 females) and in the kidney as slight vacuolar degeneration of
the renal tubular epithelium (7/10 males). Alterations of the
adrenal were described as vacuolization of the cortical cells (7/10
males), atrophy of the zona fasciculata (5/10 males) and fine
vacuolization of the zona glomerulosa (1/10 males). A single male
at 3000 ppm exhibited changes in the reproductive organs depicted in
the testes as moderate atrophy of the seminiferous tubule(s) and
giant cell-like changes with absence of sperm cells in the
epididymis. Other effects of treatment with myclobutanil were
decreased body-weight, blood chemistry changes (decreased bilirubin,
glucose and triglycerides), increased liver and kidney weights,
decreased adrenal weights and a slight increase in the number of
males with round cells in the urine. Decreased food intake was
observed in males during the first week of treatment. There were no
treatment-related effects on haematology or ophthalmoscopy (Shimizu,
1987c).
Dogs
A range-finding study was undertaken with 2 beagle dogs/sex per
group fed myclobutanil (84.5% purity) for a period of 4 weeks at
dietary levels of 0, 50, 250, 1000 or 4000 ppm a.i. equal to 0, 2.2,
10.5, 45.3 or 45 mg a.i./kg bw/day in males and 0, 2.0, 10.6, 39.3
or 47 mg a.i./kg bw/day in females respectively. Dogs treated at the
highest dietary level of 4000 ppm were sacrificed after 2 weeks of
treatment due to severely depressed food intake. The NOAEL for the
study was determined to be 250 ppm, equal to 10.5 mg/kg bw/day,
based on slightly decreased body-weight and food consumption
recorded during the first week of treatment in females at 1000 ppm.
There were no treatment-related consequences on clinical signs,
haematological and blood chemistry investigations (12 and 28 days),
or gross pathological examination (Goldman & Emmons, 1986).
Myclobutanil (81.1% purity) was administered to groups of 4
beagle dogs/sex for a period of 3 months at dietary levels of 0, 10,
200, 800 or 1600 ppm a.i. equal to 0, 0.3, 7.3, 29.1 or 56.8 mg
a.i./kg bw/day in males and 0, 0.4, 7.9, 32.4 or 58 mg a.i./kg
bw/day in females respectively. The NOAEL was determined to be 10
ppm, equal to 0.3 mg/kg bw/day, based on the dose-related incidence
of centrilobular or midzonal hepatocellular hypertrophy noted at 200
ppm (3/4 males) and in all animals of both sexes at 800 and 1600
ppm. Periportal hepatocytes were enlarged in a few of the more
severely affected livers. Liver weights were increased in males at
800 ppm and in both sexes at 1600 ppm. Treatment-related effects on
the kidney, evident as an increased incidence and severity of
unilateral chronic nephritis were observed in males at 800 ppm and
higher. Additional effects of treatment unveiled at the highest
level of 1600 ppm were related to decreased body-weights and food
consumption, due possibly to palatability of the diet. Slight
changes in haematological and blood chemistry values were within
range of normal variability and were not considered of toxicological
consequence. There were no effects of treatment on ophthalmology.
Increases in the ovarian weights at 800 and 1600 ppm were attributed
to estrus (McLaughlin & DiDonato, 1984).
Groups of 6 beagle dogs/sex were administered myclobutanil
(91.4% purity) daily for 12 months in the diet at levels of 0, 10,
100, 400 or 1600 ppm a.i. equal to 0, 0.3, 3.1, 14.3 or 54.2 mg
a.i./kg bw/day in males and 0, 0.4, 3.8, 15.7 or 58.2 mg a.i./kg
bw/day in females, respectively. The principal target organ was the
liver, demonstrating a NOAEL of 100 ppm, equal to 3.1 mg/kg bw/day,
based on hepatocellular hypertrophy, increased liver weights and
increased serum alkaline phosphatase levels at dietary
concentrations of 400 ppm and higher. At the highest level of 1600
ppm, livers displayed accentuated lobular architecture and in 4 of 6
females, the hepatocytes were expanded with large clear cytoplasmic
spaces. Other effects observed at 1600 ppm were evident as decreased
body-weight and food consumption, as well as changes in haematology
(decrease RBC, increase platelet count) and blood chemistry
(increase phosphorus, increase ALAT, increase GGT, decrease
albumin). Treatment with myclobutanil failed to elicit any adverse
effects on clinical signs, ophthalmological examination or
urinalysis (Goldman & Harris, 1986b).
Long-term toxicity/carcinogenicity studies
Mice
A 2-year study was conducted with Crl:CD-1 (ICR)BR mice fed
diets containing myclobutanil (90.4% purity) at levels of 0, 20, 100
or 500 ppm a.i. equal to 0, 2.7, 13.7 or 70.2 mg a.i./kg bw/day in
males and 0, 3.2, 16.5 or 85.2 mg a.i./kg bw/day in females,
respectively. A total of 110 males and 110 females per group were
assigned to the chronic phase of this bioassay. Interim sacrifices
were scheduled after 3 (10 mice/sex/group), 6 (10/sex/group) and 12
months (20/sex/group) of study. All surviving animals (70/sex/group,
maximum) were sacrificed after 24 months of continuous treatment.
Treatment with myclobutanil resulted in a NOAEL for in-life
parameters of 20 ppm, equal to 2.7 mg/kg bw/day, established on the
basis of increased MFO activity at 3, 6 and 12 months at dietary
levels of 100 and 500 ppm. At 500 ppm, target effects of treatment,
primarily on the liver were demonstrated by increased ALAT activity
(females, 3 months), increased liver weights (both sexes, 3 months),
and histopathological alterations comprising centrilobular
hypertrophy (males, 3/6/12 months), periportal vacuolation (males,
3/6/12 months; both sexes, 24 months), Kupffer cell pigmentation
(males, 6/12 months), hepatocellular necrosis (males, 12 months) and
hepatocellular alteration (tinctorial and dimensional properties:
both sexes, 24 months). There were no changes attributed to
treatment with myclobutanil with regard to survival, clinical signs,
body-weight, food consumption, ophthalmoscopy, haematology or
urinalysis. Myclobutanil was not oncogenic when administered to mice
for 2 years at dietary levels up to 500 ppm (Goldman & Harris,
1986a).
Rats
Groups of 110 Charles River Sprague Dawley rats/sex were
treated with myclobutanil (90.4% & 91.4% purity) for a period of up
to 24 months at dietary levels of 0, 50, 200 or 800 ppm a.i. equal
to 0, 2.5, 9.8 or 39.2 mg a.i./kg bw/day in males and 0, 3.2, 12.9
or 52.3 mg a.i./kg bw/day in females, respectively. Interim
sacrifices were performed after 3 and 6 months (10 rats/sex/group),
12 months (20/sex/group) and 17 months of study (18 males and 10
females/group). The survivors (52 male, 60 female/group, maximum)
were sacrificed after 24 months of treatment. Treatment with
myclobutanil indicated a NOAEL for in-life parameters of 50 ppm,
equal to 2.5 mg/kg bw/day. Effects of treatment at 200 ppm were
observed as decreased testes weights in association with slight
testicular atrophy at 24 months. At the highest dietary level of 800
ppm, testes weights were decreased at 12 and 24 months with slight
to moderate increases in the incidence of testicular atrophy. (The
seminiferous tubules were reported frequently found to be devoid of
spermatid formation and germinal epithelial cells; the tubules
appeared smaller than normal. In severe cases only Sertoli cells
remained). Increased ovary weights in females treated at 800 ppm and
sacrificed at 12 months were not correlated with any
histomorphological changes. Other treatment-related effects noted at
800 ppm were decreased body-weights (both sexes) and food
consumption (males), and increased liver weights (females).
Increased MFO activity was recorded at 200 ppm in females (3 months)
and at 800 ppm in both sexes (3 months) and in males (6 months).
There were no treatment-related effects on survival, clinical
signs, ophthalmoscopy, haematology, blood chemistry or urinalysis.
Treatment with myclobutanil at dietary levels up to 800 ppm failed
to uncover any evidence of carcinogenic potential (Shellenberger et
al., 1986).
Reproduction studies
Rats
A two-generation (two litter per generation) reproduction study
was conducted with groups of 25 CRI:CD(SD)BR rats/sex fed
myclobutanil (84.5% purity) at dietary levels of 0, 50, 200 or 1000
ppm a.i. equal to 0, 3.6, 14.7 or 73.6 mg a.i./kg bw/day in males
and 0, 4.3, 17.4 or 87 mg a.i./kg bw/day in females respectively. In
the F0 generation, treatment with myclobutanil commenced 8 weeks
before mating. In the F1 generation, the F1a litter-derived
parental animals were exposed to the test material throughout
weaning and for a minimum period of 8 weeks post-weaning. In both
generations, treatment continued throughout the reproductive phases.
Treatment-related effects on reproduction were denoted at the
highest dietary level of 1000 ppm by a decreased number of females
delivering litters (F0F1a and both matings from second
generation), decreased mean number of pups per litter (first mating
of second generation) and an increased number of stillborn pups (all
matings of both generations). An increase, albeit minimal in the
proportion of dead pups in both matings of the first generation was
similarly recorded at 200 ppm when compared to the controls. Effects
on the reproductive organs were evident in the second generation
F1 males treated at 1000 ppm as multifocal or diffuse atrophy of
the testes, decreased spermatozoa and/or necrotic spermatocytes of
the epididymides, as well as atrophy of the prostate. Systemic
toxicity was observed at 200 ppm as increased liver weights in males
of both parental generations in association with centrilobular
hepatocytic hypertrophy in the F1 generation males. At 1000 ppm,
increased liver weights and hepatocytic hypertrophy were observed in
males and females of both generations. Decreased body-weight in
males and depressed food intake in both sexes were recorded at 1000
ppm in both parental generations. Body-weights were similarly
decreased at 1000 ppm in both sexes of all filial generations. The
NOAEL for this study was determined to be 50 ppm, equal to 3.6 mg/kg
bw/day, for systemic and reproductive effects (Costlow & Harris,
1985).
Special studies on teratogenicity
Rats
A range-finding study was performed with groups of 8 mated
female Crl:CD(SD)BR rats treated orally by gavage with myclobutanil
(81.1% purity) at 0 (vehicle, corn oil), 32, 68, 100, 215, 464 or
700 mg a.i./kg bw/day on days 6 to 15 of gestation. Day 0 of
gestation was considered the day sperm were evident in the vaginal
smear. All surviving dams were killed on day 20 of gestation. The
NOAEL for maternal toxicity was 215 mg/kg bw/day. At dose levels of
464 and 700 mg/kg bw/day, treatment with myclobutanil resulted in
mortality (25% and 100%, respectively), decreased body-weights and
clinical signs of toxicity manifest as scant faeces,
chromodacryorrhea, red exudate around mouth, rough and urine-stained
hair coat, and salivation. Embryofetal toxicity was expressed at
levels of 68 mg/kg bw/day and higher as increased resorptions and
decreased viability indices (viable fetuses/implantation sites).
Decreased fetal weights were recorded at 464 mg/kg bw/day. In the
absence of detailed visceral and skeletal examinations of the
fetuses, the NOAEL for developmental toxicity was 32 mg/kg bw/day
(Costlow & Kane, 1984a).
Myclobutanil (84.5% purity) was administered orally by gavage
at 0 (vehicle, corn oil), 31, 94, 310 or 470 mg a.i./kg bw/day to
groups of 25 presumed pregnant Crl:CD(SD)BR rats from days 6 to 15
of gestation. The day on which sperm were found in the vaginal smear
was considered day 0 of gestation. All surviving dams were killed on
day 20 of gestation. A NOAEL for maternal toxicity was indicated at
94 mg/kg bw/day based on clinical signs of toxicity (rough hair
coat, desquamation and salivation) at doses of 310 mg/kg bw/day and
higher. At 470 mg/kg bw/day, red exudate around the mouth, scant
faeces and decreased body-weights were also observed. Decreased
viability indices (viable fetuses /implantation sites) and a slight
trend toward increasing resorption rate were recorded at 94 mg/kg
bw/day and higher resulting in a NOAEL for embryofetal toxicity of
31 mg/kg bw/day. An increased incidence of skeletal variations of
the ribs (7th cervical and 14th rudimentary ribs) was observed at
310 mg/kg bw/day and higher. Treatment with myclobutanil failed to
reveal any evidence of teratogenic potential (Costlow & Kane,
1984b).
Rabbits
A range-finding study was conducted with myclobutanil (84.5%
purity) administered orally by gavage at 0 (vehicle, 1% methyl
cellulose), 10, 31.6, 100, 215, 464 or 700 mg a.i./kg bw/day to
groups of 6 artificially inseminated New Zeeland white rabbits on
days 7 through 19 of gestation. The day of artificial insemination
was designated as day 0 of gestation. All surviving animals were
killed on day 29. Myclobutanil was lethal at doses of 464 mg/kg
bw/day and higher resulting in 100% mortality. Maternal toxicity
represented by clinical signs (irregular faeces and red-stained
urine) and decreased body-weights was noted at 215 mg/kg bw/day. An
increased incidence of resorptions and decreased litter size (viable
fetuses/litter) was observed at 215 mg/kg bw/day, resulting in an
overall NOAEL of 100 mg/kg bw/day. Viable fetuses from surviving
rabbits appeared normal upon gross examination (Costlow & Kane,
1984c).
Groups of 18 artificially inseminated female New Zeeland white
rabbits were administered myclobutanil (90.4% purity) at 0
(distilled water control), 0 (vehicle, 1% methyl cellulose), 20, 60
or 200 mg a.i./kg bw/day orally by gavage from day 7 through 19 of
gestation. The day of insemination was designated as day 0 of
gestation. All surviving rabbits were killed on day 29. The NOAEL
for maternal toxicity was 20 mg/kg bw/day, based on minimal,
transient body-weight loss at 60 mg/kg bw/day. Maternal animals at
the high-dose of 200 mg/kg bw/day experienced body-weight loss and
clinical signs reported as irregular faeces and blood-stained urine.
Embryofetal toxicity was manifest at 200 mg/kg bw/day as an
increased frequency of abortion and total litter resorption, an
increased incidence of litters with resorptions as well as reduced
litter size and fetal weight. Myclobutanil was not teratogenic when
administered to pregnant rabbits at dose levels of up to 200 mg/kg
bw/day (Costlow & Kane, 1984d).
Special studies on genotoxicity
Myclobutanil did not reveal any evidence of genotoxic potential
when investigated in a battery of assays specific for gene mutation
in microbial and mammalian cells or for detection of chromosomal
aberrations in cytogenetics studies. Myclobutanil did not induce
unscheduled DNA synthesis in isolated rat hepatocytes and was
negative in a DNA repair test with Bacillus subtilis. A dominant
lethal study in rats was also negative. The results of the
genotoxicity studies are presented in Table 2.
Special studies on irritation and sensitization
Eye irritation potential was studied in 9 male New Zeeland
white rabbits given technical myclobutanil with a purity of 78.4%
(Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984). Treatment of
the eyes with higher purity technical material produced both corneal
and conjunctival effects suggestive of moderate to severe irritating
potential. Myclobutanil with a purity of 78.4% was only slightly
irritating to the eyes of rabbits resulting in reversible
conjunctival effects.
A 0.5 ml aliquot of technical myclobutanil was applied dermally
to the shaved backs of 6 male New Zeeland rabbits under occluded
conditions for a 4-hour exposure period. Myclobutanil with a purity
of 78.4% (Krzywicki, 1983) or 91.9% (Krzywicki & Bonin, 1984) was
practically non-irritating to the skin of male rabbits.
Table 2. Genotoxicity of technical myclobutanil
Test Test system Concentration (vehicle) Puritya Results Reference
Reverse S. typhimurium 99% negative Byers & Lohse (1983)
Mutation TA 98, 100, 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(in vitro) TA 1535, 75, 250, 500, 1000, 5000 µg/plate
TA 1537 250, 750, 1500, 2500, 7500 µg/plate
(DMSO)
S. typhimurium 84.5% negative Byers & Chism (1983a)
TA 98, 100, 1535, 1537 75 - 7500 µg/plate (DMSO) 1., 2.
S. typhimurium 90.4% negative Byers & Chism (1983b)
TA 98, 100, 1535, 1537 75, 250, 750, 2500, 7500 µg/plate 1., 2.
(DMSO)
S. typhimurium 91.4% negative Sutou (1987)
TA 98, 100, 1535, 1537 125, 250, 500, 1000, 2000 ug/plate 1., 2.
E. coli WP2 uvrA- (DMSO)
Point mutation Chinese hamster ovary, K1BH4 cell 1. 120-175 µg/ml 81.1% negative O'Neill et al. (1984)
(in vitro) line - HGPRT locus 2. 25-90 µg/ml 1., 2.
(DMSO)
Chromosome Chinese hamster ovary WB1 cells 1. 20, 30, 40, 50 µg/ml 91.9% negative Ivett (1985)
aberration 2. 25, 50, 75 µg/ml 1., 2.
(in vitro) (DMSO)
Chromosome Mouse (male CR CD-1) 0, 65, 260, 650 mg/kg bw 81.1% negative McLeod & McCarthy (1984)
aberration bone marrow (corn oil)
(in vivo)
Mouse 0, 117, 585, 1170 mg/kg bw 91.4% negative Sames & Frank (1987)
(male and female (corn oil)
Crl:CD-1(ICR)),
bone marrow
Table 2 (cont'd)
Test Test system Concentration (vehicle) Puritya Results Reference
DNA repair Bacillus subtilis 312.5, 625, 1250, 2500, 5000 ug/plate 91.4% negative Sutou (1987)
(in vitro) H17, M45 1., 2.
Unscheduled DNA Rat 0.1 - 1000 ug/ml 91.9% negative Muller (1986)
synthesis (CRCD, Crl:CDBR male) (DMSO)
(in vitro) hepatocytes
Dominant Rat, male 0, 10, 100, 735 mg/kg bw 91.4% negative Dearlove et al. (1986)
lethal Crl:COBS(SD)BR (corn oil)
(in vivo)
DMSO = dimethyl sulfoxide
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
a = active ingredient (a.i.)
The potential of technical myclobutanil to induce delayed
contact hypersensitivity in the Hartley guinea-pig using a modified
Buehler procedure, could not unequivocally be ascertained due to a
low incidence of animals with erythema response following challenge
doses with the test material (Bonin & Hazelton, 1987a). Technical
myclobutanil, when administered to guinea-pigs according to the
method of Magnusson and Kligman did not produce delayed contact
hypersensitivity (Kreuzmann, 1989).
Observations in humans
No information was available.
COMMENTS
Myclobutanil was rapidly absorbed when administered orally to
rats and mice. The principal routes of excretion were via the urine
and faeces, with no significant residual tissue accumulation. The
toxicokinetic model in the rodent did not differ markedly with
respect to species, sex, single versus repeated exposures or doses.
In both the rat and mouse, myclobutanil was extensively
metabolized to more polar compounds. The proposed metabolic pathway
of myclobutanil in the rat was through oxidation of the butyl group.
The major excretory metabolites were qualitatively similar with
respect to sex and dose.
Myclobutanil was only slightly toxic upon acute oral
administration to rats and mice. WHO has classified myclobutanil as
slightly hazardous (WHO, 1992).
The primary target organ upon repeated dietary exposure to
myclobutanil was the liver. Histomorphological changes,
characterized predominantly by centrilobular hepatocytic hypertrophy
in association with increased liver weights, were observed in all
species investigated. Microscopically, there was accentuated lobular
architecture, hepatocytic vacuolation, inflammation, necrosis, and
pigmentation of the Kupffer cells. Increased hepatic enzyme activity
in serum (ALAT, ASAT, GGT, ALP) were also observed. Hepatic
microsomal MFO activities in rats and mice were increased
correspondingly. There were no similar increases in hepatic
peroxisomal œ-oxidation activity that would have suggested
peroxisomal proliferation.
A three-month dietary study with myclobutanil in the mouse at
levels of 0, 3, 10, 30, 100, 300, 1000, 3000 or 10 000 ppm revealed
hepatic alterations at dietary levels of 1000 ppm and higher,
resulting in a NOAEL of 300 ppm, equal to 42.1 mg/kg bw/day.
Two 3-month studies in rats fed myclobutanil at levels of 0,
10, 30, 100, 300, 1000, 3000, 10 000, or 30 000 ppm and 0, 100, 300,
or 3000 ppm indicated a NOAEL of 100 ppm, equal to 5.2 mg/kg bw/day,
based on treatment-related hepatic effects.
The NOAEL for myclobutanil-related liver effects in dogs
treated for 3 months at 0, 10, 200, 800 or 1600 ppm was 10 ppm,
equal to 0.3 mg/kg bw/day. Treatment of dogs with myclobutanil for
12 months at dietary levels of 0, 10, 100, 400, or 1600 ppm resulted
in a NOAEL for hepatic effects of 100 ppm, equal to 3.1 mg/kg
bw/day. Myclobutanil administered to two dogs per sex at dietary
levels of up to 1000 ppm, equal to 39 mg/kg bw/day, did not produce
any hepatic changes after a period of 4 weeks.
Long-term dietary treatment of mice with myclobutanil for two
years at 0, 20, 100 or 500 ppm revealed a NOAEL of 20 ppm, equal to
2.7 mg/kg bw/day, based on increased MFO activity at 100 ppm as well
as more pronounced liver toxicity at 500 ppm and above. Myclobutanil
was not carcinogenic in mice.
A 24-month long-term toxicity/carcinogenicity study in rats at
dietary concentrations of 0, 50, 200 or 800 ppm revealed a NOAEL of
50 ppm, equal to 2.5 mg/kg bw/day, based on findings of testicular
atrophy and increased MFO activity at 200 ppm and above.
Myclobutanil was not carcinogenic in rats.
A two-generation reproduction study in rats at dietary
concentrations of 0, 50, 200 or 1000 ppm revealed a NOAEL of 50 ppm,
equal to 3.6 mg/kg bw/day, based on increased liver weights and an
increase in numbers of stillborn pups at 200 ppm and above. At 1000
ppm atrophy of the testes and prostate were observed.
An oral teratogenicity study in rats at gavage doses of 0, 31,
94, 310, or 470 mg/kg bw/day demonstrated clinical signs of toxicity
at 310 mg/kg bw/day and above, indicating a NOAEL of 94 mg/kg
bw/day. The NOAEL for embryofetal toxicity was 31 mg/kg bw/day.
There was no evidence of teratogenicity at doses up to 470 mg/kg
bw/day.
Myclobutanil was not teratogenic when administered to the
rabbit at gavage doses of 20, 60 or 200 mg/kg bw/day. A NOAEL for
maternal toxicity was 20 mg/kg bw/day, based on decreased body-
weight at 60 mg/kg bw/day and above. Embryofetal toxicity was
evident at 200 mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that myclobutanil was not genotoxic.
An ADI was allocated on the basis of NOAELs in two-year feeding
studies in mice and rats, a reproduction study in rats and a one-
year study in dogs, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 20 ppm, equal to 2.7 mg/kg bw/day (two-year study)
Rat: 50 ppm, equal to 2.5 mg/kg bw/day (two-year study)
50 ppm, equal to 3.6 mg/kg bw/day (two-generation
reproduction study)
Dog: 100 ppm, equal to 3.1 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0 - 0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
1. Results of ongoing long-term studies in mice and rats
known to be in progress.
2. If the results of (1) show a carcinogenic response,
studies (a) to determine whether myclobutanil acts as a
tumour promoter in the two-stage rat liver bioassay and
(b) whether it causes inhibition of intercellular
communications.
3. Observations in humans.
REFERENCES
Bonin, R. & Hazelton, G.A. (1987a). RH-3866 technical: Delayed
contact hypersensitivity study in guinea-pigs. Report No. 87R-035.
Unpublished report dated June 25, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983a). Microbial mutagen assay. Report
No. 83R-162. Unpublished report dated October 19, 1983 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Byers, M.J. & Chism, E.M. (1983b). RH-53,866: Microbial mutagen
assay. Report No. 83R-246. Unpublished report dated January 31, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Byers, M.J. & Lohse, K.L. (1983). RH-53,866: Microbial mutagen
assay. Report No. 82R-193. Unpublished report dated January 11, 1983
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Harris, J.C. (1985). RH-53,866: Two generation
reproduction study in rats. Report No. 84R-117. Unpublished report
dated August 21, 1985 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984a). Range-finding teratology study
with RH-53,866 in rats. Report No. 83R-023. Unpublished report dated
June 26, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984b). Teratology study with RH-53,866
in rats. Report No. 83R-024. Unpublished report dated June 22, 1984
from Rohm and Haas Company, Spring House, PA, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Costlow, R.D. & Kane, W.W. (1984c). Range-finding teratology study
with RH-53,866 in rabbits. Report No. 83R-216. Unpublished report
dated October 31, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Costlow, R.D. & Kane, W.W. (1984d). Teratology study with RH-53,866
in rabbits. Report No. 83R-217. Unpublished report dated November
15, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986). Dominant
lethal study of RH-53,866 administered orally via gavage to
Crl:COBS(SD)BR male rats. Report No. 018-011; R & H Report No. 86RC-
054. Unpublished report dated October 10, 1986 from Argus Research
Laboratories Inc., Horsham, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Fisher, J.R., Emmons H.F., & Hagan J.V. (1987). RH-3866 technical
acute inhalation toxicity study in rats. Report No. 87R-028.
Unpublished report dated August 31, 1987 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Goldman, P.R. & Emmons, H.F. (1986). RH-3866 (Experimental
Fungicide): Four week dietary range-finding study in dogs. Report
No. 83R-078. Unpublished report dated October 8, 1986 from Rohm and
Haas Company, Spring House, PA, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Goldman, P.R. & Harris, J.C. (1986a). RH-3866: Dietary chronic and
oncogenicity study in mice. Report No. 84R-023. Unpublished report
dated October 17, 1986 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Goldman, P.R. & Harris; J.C. (1986b). RH-3866: One-year dietary
study in beagle dogs. Report No. 84R-078. Unpublished report dated
October 15, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Goldman, P.R., Harris, J.C. & Lampe, K.R. (1986). RH-3866: Three-
month dietary toxicity study in mice. Report No. 83R-136.
Unpublished report dated October 8, 1988 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of RH-53,866 technical
in an in vitro cytogenetic assay measuring chromosome aberration
frequencies in Chinese hamster ovary (CHO) cells. Report No. 20990;
R & H Report No. 85RC-011. Unpublished report dated April 26, 1985
from Litton Bionetics, Kensington, Maryland, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, PA, USA.
Kreuzmann, J.J. (1989). RH-53,866 technical: Guinea-pig maximization
test (Magnusson and Kligman method). Report 89-3655-21; R & H Report
No. 89RC-103. Unpublished report dated April 29, 1989 from Hill Top
Biolabs, Inc., Miamiville, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, PA, USA.
Krzywicki K.M. (1983). RH-53,866: Definitive oral LD50 in male and
female rats, dermal LD50 in male and female rabbits and skin and
eye irritation in male rabbits. Report No. 83R-125 A&B. Unpublished
report dated September 20, 1983 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Bonin, R. (1984). RH-53,866 technical: Definitive
dermal LD50 in male and female rabbits, and skin and eye
irritation in male rabbits. Report No. 84R-134 A&B. Unpublished
report dated August 3, 1984 from Rohm and Haas Company, Spring
House, PA, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Krzywicki, K.M. & Krajewski, R.J. (1983). RH-53,866 technical:
Definitive oral LD50 in male mice. Report No. 83R-103. Unpublished
report dated July 6, 1983 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Krzywicki, K.M. & Morrison, R.D. (1984a). RH-53,866 technical:
Definitive oral LD50 in male and female rats. Report No. 84R-063
A&B. Unpublished report dated July 19, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLaughlin, J.E. & DiDonato, L.J. (1984). RH-3866 (Experimental
Fungicide): Three month dietary feeding study in dogs. Report No.
83R-204. Unpublished report dated August 7, 1984 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
McLeod, P.L. & McCarthy, K.L. (1984). RH-53,866 in vivo
cytogenetic study in mice. Report No. 84R-074. Unpublished report
dated July 23, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Morrison, R.D., Murphy, M.E., & Chan, P.K. (1984a). RH-53,866
technical: Acute oral LD50 in male mice. Report No. 84R-153.
Unpublished report dated August 3, 1984 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Morrison, R.D., Romanello, A.S., Krzywicki, K.M., & Hazelton, G.A.
(1986b). RH-3866 technical: Definitive oral LD50 in male and
female mice. Report No. 86R-088 A&B. Unpublished report dated August
14, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Muller, G. (1986). RH-53,866 technical in vitro unscheduled DNA
synthesis assay. Report No. 86R-084. Unpublished report dated July
22, 1986 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Hara, G.P. & DiDonato, L.J. (1984). RH-3866: Three month dietary
feeding study in rats. Report No. 83R-068. Unpublished report dated
August 7, 1984 from Rohm and Haas Company, Spring House, PA, USA.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
O'Neill, P.J., Foxall, S. & Byers, M.J. (1984). RH-53,866 technical:
CHO/HGPRT gene mutation assay. Report No. 84R-046. Unpublished
report dated May 29, 1984 from Rohm and Haas Company, Spring House,
PA, USA. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Quinn, J.A., Fujimoto, T.T., Egan, A.R. & Shaber, S.H. (1986).
Properties of RH-3866, a new triazole fungicide. Pesticide
Science, Vol. 17: 357-362. Submitted to WHO by Rohm & Haas
Company, Spring House, PA, USA.
Romanello, A.S., Krzywicki, K.M. & Hazelton, G.A. (1986a). RH-3866
technical definitive mouse oral LD50 in females. Report No. 85R-
247. Unpublished report dated May 22, 1986 from Rohm and Haas
Company, Spring House, PA, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, PA, USA.
Sames, J.L. & Frank, J.P. (1987). RH-53,866 technical: In vivo
cytogenetics study in mice. Report No. 87R-141. Unpublished report
dated October 30, 1987 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Shellenberger, T.G., Billups, L.H., Tegeris, A.S. & Green, D.S.
(1986). Chronic toxicity and oncogenicity study in rats with RH-
3866. Report No. 8342; R & H Report No. 85RC-061. Unpublished report
dated October 24, 1986 from Tegeris Laboratories, Inc., Laurel,
Maryland. Submitted to WHO by Rohm and Haas Company, Spring House,
PA, USA.
Shimizu Y. (1987a). Acute oral toxicity study with RH-3866 in mice.
Report No. NRILS 86-2052; R & H Report No. 87RC-036. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987b(. Acute oral toxicity study with RH-3866 in rats.
Report No. NRILS 86-2053; R & H Report No. 87RC-035. Unpublished
report dated July 9, 1987 from NRI Life Science, Kanagawa, Japan.
Submitted to WHO by Rohm and Haas Company, Spring House, PA, USA.
Shimizu Y. (1987c). A thirteen week feeding subchronic study of RH-
3866 in rats. Report No. NRILS 86-2024;R & H Report No. 87RC-038.
Unpublished report dated September 1, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986a). RH-3866
kinetic and metabolism study in mice. Report No. 83R-175.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Steigerwalt, R.B., Udinsky, J.R. & Longacre, S.L. (1986b). RH-3866
kinetic and metabolism study in rats. Report No. 83R-144.
Unpublished report dated August 29, 1986 from Rohm and Haas Company,
Spring House, PA, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, PA, USA.
Streelman D.R. (1984). Material balance and metabolism study of
14C-RH-3866 in rats. Report No. TR 310-84-16. Unpublished report
dated June 22, 1984 from Rohm and Haas Company, Spring House, PA,
USA. Submitted to WHO by Rohm and Haas Company, Spring House, PA,
USA.
Sutou, S. (1987). Mutagenicity testing of RH-53,866 technical with
bacteria. Report No. NRILS 86-2112; R & H Report No. 87RC-037.
Unpublished report dated August 21, 1987 from NRI Life Science,
Kanagawa, Japan. Submitted to WHO by Rohm and Haas Company, Spring
House, PA, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
