
PENCONAZOLE
First draft prepared by
J.E.M. van Koten-Vermeulen and E.M. den Tonkelaar
National Institute of Public Health and Environmental Protection
Bilthoven, Netherlands
EXPLANATION
Penconazole is a systemic triazole fungicide with preventive
and curative properties for the control of powdery mildew. It stops
the development of fungi by interfering with the biosynthesis of
sterols in cell membranes. It is used on fruit, especially apples
and grapes, and vegetables. The compound was considered for the
first time by the present Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Mice
Groups of Cr:CD(ICR)BR mice (20/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm penconazole;
purity 98.7%) for 13 weeks. At the end of the study the mice were
given orally (10 mice/sex) or intravenously (10/mice/sex) a tracer
dose of triazole-14C-penconazole. Urine was collected 24 and 48 h
after dosing and faeces were collected at 48 h after dosing. Most of
the 14Cin urine was excreted in the first 24 h after dosing both
after oral and i.v. administration. Total recovery of 14C in urine
amounted to 47-66% for males and 63-78% for females irrespective of
the previously administered dose or the route of administration.
After i.v. or oral adminis-tration, male mice excreted more 14C in
the faeces than female mice, 19-31% and 9-17%, respectively (Hiles,
1987b).
Rats
Groups of two Tif:RAIf (SPF) rats/sex were given a single oral
dose of 0.5 or 25 mg/kg bw 14C-penconazole (triazole-labelled,
purity >98%) in ethanol/PEG 200/water (2/3/5, v/v) by gavage. One
rat/sex served as a control. Urine, faeces and expired CO2 were
collected at 24 h intervals. The rats were sacrificed after 6 days
and tissues were collected and analyzed for radioactivity. In both
dose groups most of the radioactivity was eliminated in the urine
(62-65% in males and 74-85% in females) and faeces (34-43% in males
and 13-32% in females). Most of the excretion occurred within the
first 24 h after administration. Especially at the high-dose level,
females excreted a higher amount in the urine than the males. Minor
amounts were detected in the expired air (ca 0.1%). At the 0.5 mg/kg
bw dose level, most tissue residues were at or below the detection
limit. Only in lungs (0.003-0.005 mg/kg), liver (0.003-0.004),
kidney (0.002 mg/kg) and carcass (0.003 mg/kg) were detectable
amounts of radioactivity found. The residues found at 25 mg/kg bw
were about 20 times higher than those found at the 0.5 mg/kg bw dose
level (Hamböck, 1980).
Groups of Crl:CD(SD)BR rats (5/sex/group) were given a single
dose of unlabelled penconazole (purity 98.7%) corresponding to 0,
10, 100, 300, 500, 1000 or 2400 ppm in the diet, immediately
followed by the administration by gavage of 0.1 mg 14C-triazole-
labelled penconazole. Urine was collected 24 and 48 hours after
dosing and faeces were collected 48 h after dosing. Rats were
sacrificed 48 h after dosing. Most of the radioactivity was
recovered in the first 24 h urine: 34-46% and 56-86% in males and
females, respectively. After 48 h, the urinary excretion ranged from
46-55% in males and from 69-90% in females. Recoveries in faeces
ranged from 19-27% in males and from 9-13% in females. Average total
recoveries were 80% and 93% for males and females, respectively. In
female rats, total recoveries were higher at all dose levels but
there was no apparent correlation between excretion of radioactivity
and the pretreatment dose level (LeVan, 1987).
Groups of Crl:CD(SD)BR rats (13/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm non-labelled
penconazole (purity 98.7%), for at least 13 weeks prior to a single
oral or i.v. administration of 0.1 mg of 14C-penconazole (triazole
ring-labelled) to 5 rats/sex/group; 3 rats/sex/group were available
for replacement. Rats were sacrificed 48 h after receiving the
radioactive dose. Urine was collected for 0-24 h and 24-48 h after
radioactive dosing and faeces for 48 h. The samples were analyzed
for radioactivity.
The administered radioactivity was recovered primarily in the
first 24 h urine sample after both oral and i.v. dosing, with
considerably higher amounts in female rats than in male rats. Total
recovery in urine ranged from 73-77% in female rats and from 49-53%
in male rats after an i.v. dose, and from 74-79% in female rats and
from 48-59% in male rats after an oral dose. In faeces, 22-29% was
recovered in males and 12-17% in females after i.v. adminis-tration.
After oral administration, 26-31% was recovered in male rats and 13-
16% in females rats. There was no relationship between pre-treatment
doses and excretion of radioactivity (Hiles, 1987a).
Five Wistar rats/sex received a single oral dose of 0.5 or 50
mg penconazole/kg bw (14C-phenyl-labelled; purity >99%). Urine
and faeces were collected up to 96 h. Independent of the
administered dose, male rats excreted equal amounts via the urine
(46.9% and 41.1%) and faeces (43.5%-47.0%), female rats excreted
69.0% and 72.2% via the urine and 21.1% and 18.1% in the faeces in
the low- and high-dose, respectively. No radioactivity (<0.01%) was
found in the expired air. In both sexes, detectable tissue residues
at 0.5 mg/kg bw were found only in liver, kidney, femur and
intestinal tract. In males at 50 mg/kg bw the highest residual
activity was found in the intestinal tract (4.08 ppm penconazole
equivalents) followed by liver, kidney, adrenals, skin, carcass,
blood and plasma (0.15 to 1.46 ppm). In females at 50 mg/kg bw the
residual activity was 2 to 9 times lower than in males (Van Dijk,
1987).
In a bile excretion study, 9 h after the oral administration of
0.5 mg 14C-penconazole/kg bw to males and females, 48.7% and 28.8%
of the administered dose was excreted via the bile, respectively.
After 48 h, biliary, urinary and faecal excretion represented 54.6%,
28.2% and 4.7% of the administered radioactivity for male rats and
40.2%, 47.9% and 2.0% for female rats. Fourteen daily treatments
with 0.5 mg penconazole/kg bw/day followed by a final treatment with
14C-labelled penconazole had no influence on the rate and route of
excretion (Van Dijk, 1987).
Hens
Groups of two laying hens received oral doses equivalent to 5
ppm in the feed of 14C-triazole-labelled penconazole or 14C-
phenyl ring-labelled penconazole for 16 consecutive days. Excreta
and eggs were collected daily. Regardless of the radiolabel, nearly
99% of the radioactivity was eliminated with the excreta. Total
radioactivity in the tissues amounted to 0.008% and 0.025-0.08% for
the triazole and phenyl labels, respectively. Individual tissue
residues for both labels ranged from <0.003 ppm to 0.025 ppm. The
radioactivity recovered in the eggs reached a plateau on day 10 with
0.022 ppm (yolks) and 0.010 ppm (whites) for the triazol label, and
on day 11 with 0.022 ppm (yolks) and 0.005 ppm (whites) for the
phenyl label (Murphy & Capps, 1988a).
Goats
Two lactating goats were given an oral dose equivalent to 5 ppm
in the feed of 14C-triazole-labelled penconazole or 14C-phenyl
ring-labelled penconazole for 10 consecutive days. Daily samples of
urine, faeces and milk as well as samples of blood, expired CO2,
and volatiles were collected. The goats were sacrificed 24 h after
the last dose. Excretion in the urine amounted to 92% and 77% for
the triazole and phenyl label, respectively; 8% of the radioactivity
was recovered in the faeces for both labels. About 0.2 and 0.1% of
the administered dose was recovered as total tissue residue for the
triazole- and phenyl-label, respectively. Individual 14C tissue
levels were lower than 0.017 ppm penconazole equivalents except for
kidney and liver with 14C levels of 0.061 and 0.103 ppm for the
triazole-label, and 0.036 and 0.075 ppm for the phenyl-label,
respectively. The radioactivity recovered in milk reached a plateau
around day 4 with 0.013 ppm for the triazole-label and 0.009 for the
phenyl-label. Total recovery for the phenyl-label was lower, but
this was due to a technical error (Murphy & Capps, 1988b).
Biotransformation
Rats
Twenty male Tif:RAIf (SPF) rats were given by gavage single
oral dosages of 22.8 mg 14C-triazole-labelled penconazole (> 98%
purity)/kg bw. Urine and faeces were collected 24 h and 48 h after
dosing and analyzed for radioactivity. The pooled 0-48 h urine and
faeces samples contained 62% and 33% of the dose, respectively.
Numerous metabolites, mostly conjugated, were identified in urine
and faeces (13 urinary metabolites were identified by mass and NMR
spectroscopy). Only 0.8% of the administered dose was excreted with
the faeces as unchanged penconazole. A major pathway of metabolism
is the stepwise oxidation and shortening of the alkyl side chain
(see Figure 1). Also free triazole (about 15%) was excreted in urine
and faeces, demonstrating another major independent pathway, namely
cleavage at the nitrogen-carbon bond between the triazole ring and
the pentyl moiety. Also oxidation of the triazole ring moiety,
producing the 3-(or 5-)-hydroxy derivative, is observed.
Conjugation, in particular glucuronidation, of the hydroxylated
metabolites was also found (Hamböck, 1982, 1984).
Quantitative differences of the metabolite pattern of male and
female urines were studied in groups of two male and female rats
following a single oral dose of 0.5 mg/kg bw or 25 mg/kg bw
triazole-labelled penconazole (Hamböck, 1980). Renal excretion was
60-63% for the males and 73-85% for the females. Males excreted more
via the faeces (35-37%) than females (14-31%). Although the pattern
of metabolites found in the 0-24 h urine samples was qualitatively
the same in males and females (irrespective of the dose), polar
metabolites, consisting of conjugated hydroxy derivatives, were much
more abundant in females, accounting for 45% of the administered
dose as compared to 3% in males. Males tend to excrete more free
triazole (7% of the dose) than females (1%). Carboxylic acid
metabolites are also more abundant in males. (Hamböck, 1985).
In a toxicokinetic study with 14C phenyl-labelled
penconazole, similar metabolite patterns were found in urine, bile
and extractable faeces of males and females given one oral dose of
0.5 or 50 mg/kg bw. A total of 5 metabolites were in common to both
sexes in urine, faeces and bile. Quantitatively more polar
conjugated metabolites were found in the metabolite patterns of the
females. Conjugated CGA 127841 was found in urine, faeces and bile.
Unconjugated CGA 127841 was found in bile, liver and kidney and CGA
189659 was found in faeces, liver and kidney (Van Dijk, 1987). These
metabolites were not found by Hamböck, but they are essential
intermediates in the metabolic pathway of penconazole (see Figure
1).
Hens
Characterization of the radioactivity in the excreta of laying
hens dosed with triazole or phenyl 14C-labelled penconazole for 16
consecutive days revealed only 0.8% and 3.7% unchanged parent
compound, respectively. After administration of both labels a
carboxylic acid (metabolite 1) was the major metabolite accounting
for 22% of the radioactive extractables. The metabolic pattern was
comparable to female rats (no free triazole being detected) (Murphy
& Capps, 1988a).
Goats
In goats fed a diet containing 5 ppm of 14C-labelled
penconazole (triazole as well as phenyl ring) no parent compound was
detected in the urine, while in faeces 17 and 21% parent compound
were detected following administration of the phenyl- and triazole-
label, respectively. The metabolic patterns in tissues, excreta and
milk were the same for both labels, with the major metabolite
identified as a carboxylic acid (metabolite 1). The metabolic
pattern was comparable to female rats (no free triazole being
detected) (Murphy & Capps, 1988b).
Effects on drug metabolizing liver enzymes
Groups of 6 male RAI albino rats or groups of 6 male Mag mice
were orally administered 14 daily doses of 0, 10, 80, 160 or 320 mg
penconazole/kg bw/day (purity 98,7%). The animals were fasted for 24
h after the last administration and then killed. The livers were
removed and biochemical determinations were performed in the
homogenates, microsomal and cytosolic fractions. Part of the livers
from mice and rats from the control and the high-dose group were
fixed for electron microscopy.
Relative liver weight was increased at doses > 80 mg/kg
bw/day in both rats and mice. At the highest dose, total liver DNA
content was increased to 120% in rats and to 125% in mice.
Microsomal protein, phospholopid contents and enzyme activities were
significantly increased in both mice and rats at 80 mg/kg bw/day and
above. Cytochrome P-450, ethoxycoumarin O-deethylase and epoxide
hydrolase were also significantly increased in rats at 10 mg/kg
bw/day. Electron microscopy revealed proliferation of smooth
endoplasmic reticulum membranes in both species (Waechter et al.,
1985)
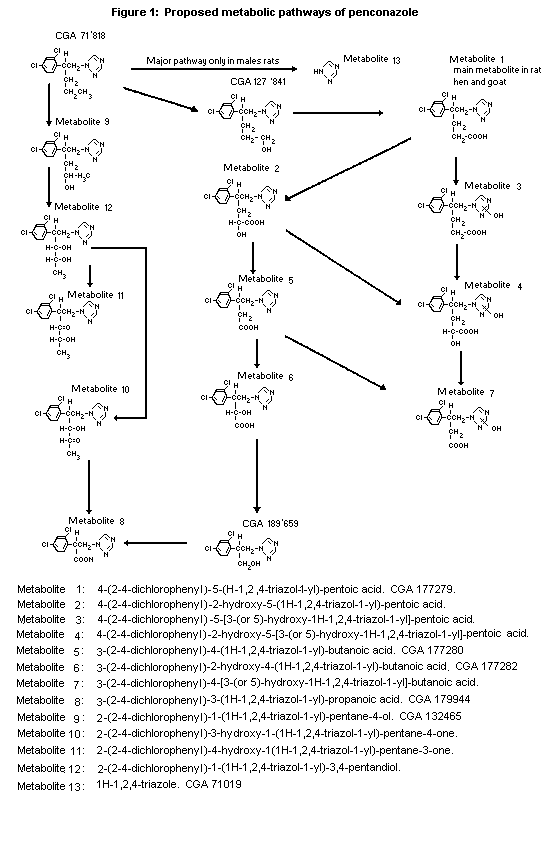 Metabolites 9, 10, 11 and 12 were mainly found as glucuronides
or other conjugates.
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of penconazole
Species Sex Route LD50 LC50 Purity Reference
mg/kg bw mg/m3
Mouse M&F oral 2444 88.4 Sarasin (1980)
Rat M&F oral 2125 88.4 Bathe & Gfeller (1980a)
M&F dermal >3000 88.4 Bathe & Gfeller (1980b)
M&F inhal >4046 96.1 Hartmann & Gfeller (1987)
Chinese hamster M&F oral >5000 88.4 Bathe & Gfeller (1980c)
Rabbit M&F oral 971 88.4 Kobel (1981)
Penconazole shows low acute oral toxicity to mice, rats and
hamsters, but is slightly more toxic to rabbits.
Short-term toxicity studies
Mice
Groups of Cr:CD(ICR)BR mice (15/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm penconazole
(purity 98.7%) equivalent to 0, 1.5, 15, 45, 75, 150 or 360 mg/kg
bw/day for 13 weeks. Histopathology was only reported for the liver.
No treatment-related effects were observed on clinical signs,
mortality, ophthalmoscopy, food consumption, food efficiency,
haematology or urinalysis. Body-weight was lower at 2400 ppm.
Cholesterol levels were decreased at 1000 and 2400 ppm, ALAT
increased in high-dose males and total protein and albumin were
decreased in females at 2400 ppm. Relative liver weight was
significantly increased at 500, 1000 and 2400 ppm. An increased
incidence of centrilobular hypertrophy of the liver was observed in
males at doses > 500 ppm and in females at the highest dose.
Hepatic degeneration and vacuolisation was also observed in high-
dose males. Coagulative necrosis in the liver was observed in males
at 1000 and 2400 ppm. The NOAEL in this study was 300 ppm in the
diet, equivalent to 45 mg/kg bw/day (Hiles 1987b).
Rats
Groups of Tif:RAIf(SPF) rats (10/sex/group) were orally
administered 0, 20, 100 or 500 mg penconazole (purity 91.7%)/kg
bw/day for 28 days. Since no marked symptoms of toxicity showed up
during the first week of treatment the doses were increased to 100,
500 or 1000 mg/kg bw/day on day 8 of treatment. After increasing the
doses, 3 male and 3 female high-dose rats showed marked apathia and
lateral body position at day 10 but the animals recovered on day 11.
No effects were observed on mortality, ophthalmic or hearing
examinations. In the mid- and high-dose groups male mean body weight
was decreased, food consumption and food conversion were decreased
in males and females, haemoglobin and haematocrit values were
decreased in females and ALP and ALAT as well as cholesterol and
total proteins were increased in males and females and total
globulin was increased and A/G ratio was decreased in females.
Phosphate and glucose were increased in high-dose females. Albumin
increased in high-dose males and females. Urine volume was increased
in males at the highest dose and in females at the mid- and high-
dose. Relative liver, kidney and adrenal weight was increased in
mid- and high-dose males and females. Relative brain, spleen and
thyroid weight were increased in high-dose females. Enlarged livers
with slight hypertrophy of the hepatocytes were observed in 8/10 and
3/10 males and females at the mid-dose and in all animals at the
highest dose. The lowest dose (20-100 mg/kg bw/day) was considered
as the NOAEL (Basler et al., 1984).
Groups of Tif:RAIf(SPF) rats (20/sex/group) were fed diets
containing 0, 30, 300 or 3000 ppm (equal to 2.1, 19.4 or 202.3 mg
penconazole/kg bw/day for males and 2.1, 20.7 or 208.6 mg
penconazole/kg bw/day for females, respectively) (purity 91.7%) for
3 months. No effects were observed on clinical signs, mortality,
food and water consumption, food conversion, hearing tests or eye
examinations. Body weight was decreased in males and females at the
highest dose. An increase in total plasma protein and albumin was
observed in male rats at all doses and a trend to an increase in
total plasma proteins was seen in females at 3000 ppm. GGT was
slightly increased in females at 300 and 3000 (significantly) ppm.
Cholesterol levels were increased in both sexes at 3000 and also in
females at 300 ppm. A significant dose-related increase in relative
liver weight was observed at all dose levels. Relative testes
weights were increased in males at 3000 ppm and brain, kidney and
thymus weight were increased in high dose females. At histopathology
minimal hypertrophy of the hepatocytes was seen in 20/20 male rats
and 9/20 female rats at 3000 ppm. In this study no NOAEL was
identified, although the liver weight increase at 30 ppm was
marginal (Basler et al., 1982).
In another 3 month feeding study groups of Tif:RAIf(SPF) rats
(20/sex/group) were fed diets containing 0, 10, 30 or 100 ppm
penconazole (purity 91.7%) (equal to 0.8, 2.1 or 7.1 mg
penconazole/kg bw/day for males and 0.8, 2.1 or 7.3 mg
penconazole/kg bw/day for females, respectively). No effects were
observed on clinical signs, mortality, body-weight, food and water
consumption, food conversion, eye examinations, hearing tests, organ
weight or histopathology. Total proteins were increased in male and
female rats at 30 and 100 ppm. Total globulin was increased in high
dose females. GGT was slightly increased in females at both 30 and
100 ppm. Liver weight was increased at 10 and 30 ppm in male rats
only, but not at 100 ppm. No liver histopathological effects were
observed. The NOAEL in this study was 10 ppm penconazole, equal to
0.8 mg/kg bw/day (Basler et al., 1983).
In an additional study groups of albino [Crl:CD(SD)BR] rats
(15/sex/group) were fed diets containing 0, 10, 100, 300, 500, 1000
or 2400 ppm penconazole (purity 98,7%) equivalent to 0.5, 5, 15, 25,
50 or 120 mg penconazole/kg bw/day for 90 days. In this study only
the liver as target organ was studied histopathologically.
Observations included clinical signs mortality, body-weight, food
consumption, food efficiency, ophthalmoscopy, haematology, clinical
chemistry (including GGT), urinalysis, organ weights, macroscopy and
histopathology. Female body weight gain was lower at 500, 1000 and
2400 ppm and food consumption was decreased at the highest dose.
Urea nitrogen was increased in males at all dose levels, but not
clearly dose-related. Relative liver weight was significantly
increased in rats at 500 (females only), 1000 and 2400 ppm. Relative
kidney weight was increased in rats at 2400 ppm. At histopathology
the incidence of vacuolization, degeneration and centrilobular
hyperthrophy of hepatocytes was increased at 500 ppm and above. The
NOAEL in this study was 300 ppm, equivalent to 15 mg/kg bw/day
(Hiles, 1987c).
Rabbits
Groups of New Zeeland white rabbits (5/sex/group) were given
1000, 1500 or 2000 mg penconazole/kg bw/day (purity 91.7%) by dermal
application once/day for 5 days/week for 21 days. Two satellite
groups received 0 or 2000 mg penconazole/kg bw/day for 21 days
followed by a recovery period of 14 days. Transient signs of
dyspnea, curved body position and ruffled fur were observed at all
dose levels including control rabbits, but symptoms disappeared
during the recovery period. No dose-related effects were observed on
mortality, body-weight, food consumption and food conversion,
haematology, clinical chemistry, macroscopy and histopathology. The
NOAEL in this study was > 2000 mg/kg bw/day (Seifert et al.,
1983).
Dogs
Groups of beagle dogs (10/sex/group) were fed diets containing
0, 100, 500, or 5000/2500 ppm penconazole (purity 91.7%), equal to
3.0, 16.9 or 133 mg penconazole/kg bw/day for males and 3.3, 16.7 or
139 mg penconazole/kg bw/day for females, respectively, for 12
months. Due to poor feed intake the highest dose was lowered to 2500
ppm in week 20. An interim kill on 4 dogs/sex/group was carried out
at week 14 and 2 dogs/sex/group were kept for a 4-week recovery
period. Observations included clinical signs, mortality, body-
weight, food consumption, food conversion, ophthalmoscopy, auditory
perception, haematology, blood chemistry, urinalysis, macroscopy,
organ weights and histopathology.
An increased incidence of vomiting was observed in high-dose
dogs during the first three months of treatment; thereafter
occasionally vomiting was observed only in females. The high-dose
dogs lost body-weight and had a reduced food consumption during the
first months. After lowering the dosage food consumption and male
bodyweights were comparable to the controls but the mean weight of
the females remained lower than the control value. ASAT, ALP, OCT,
GGT and ALAT activities were increased in dogs at the highest dose
during the whole administration period. Total globulin concentration
was increased in males at the highest dose during weeks 13, 26 and
53, consequently the total protein was slightly increased and the
A/G ratio was decreased. Terminal body-weight of high-dose male and
female dogs were decreased after 3 months. At this time relative
liver weights were increased at 500 and 5000 ppm. Relative kidney
weight was increased at the highest dose in both males and females.
Relative brain and adrenal weight were increased and relative heart
weight was decreased in high-dose females. All males at 5000 ppm
showed a reduction in testes weights and atrophy of the seminiferous
epithelium. At macroscopy emaciation was observed in dogs at the
highest dose after 3 months. At histopathology after 3 months,
minimal lesions characterized by monocellular hepatocyte necrosis
associated with inflammatory cell infiltration were noticed in
nearly all interim sacrificed dogs at the highest dose and in one
male dog at 500 ppm. After 12 months, testes weights were reduced in
males at 2500 ppm and liver weight was increased in females at 500
and 2500 ppm. At histopathology, bilateral tubular atrophy and
reduced spermatogenesis in the testes was observed in the high-dose
males and an increased incidence of inflammation with fibrosis in
the peripheral lobular region of the liver occurred in the mid- and
high-dose animals. The only abnormalities found after the recovery
period were decreased relative testes weights and tubular atrophy at
2500 ppm. The NOAEL in this study was 100 ppm, equal to 3.0 mg/kg
bw/day for males and 3.3 mg/kg bw/day for females (Gfeller et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Tif:MAGf(SPF) mice (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%),
equal to 0.75, 9.8, 19.3 or 40.8 mg/kg bw/day for males and 0.67,
8.8, 17.2 or 35.7 mg/kg bw/day for females, for 24 months. An
interim kill was carried out on 10 mice/sex/group at 52 weeks. No
effects were observed on mortality, clinical signs, body-weight,
food and water consumption, food conversion, ophthalmic and hearing
inspections, haematology, blood chemistry, urinalysis, macroscopy or
histopathology. Relative liver weight was increased in high-dose
males and females at the interim kill. At the end of the study
adrenal weight (not dose-related) and prostate weight were increased
in males at 75, 150 and 300 ppm. There was no treatment-related
increase in tumour incidence (Basler et al., 1985b). Since
weighing of prostate glands is not very reliable and the increased
weight was not found at 53 weeks, the Meeting concluded that this
effect was probably biologically insignificant. The NOAEL was
therefore 150 ppm, equal to 19.3 mg/kg bw/day for males and 17.2
mg/kg bw/day for females based on the increased liver weight.
Rats
Groups of Tif:RAIf(SPF) rats (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%) for
24 months. An interim kill was carried out on 10 rats/sex/group at
12 months. Twenty rats/sex/group were maintained for laboratory
investigations and sacrificed after 24 months. The remaining 50
rats/sex/group were sacrificed after 116/117 weeks. No effects were
observed on mortality, clinical signs, body-weight, food and water
consumption, food conversion, ophthalmic and hearing inspections,
haematology, blood chemistry, urinalysis, macroscopy or
histopathology. At the interim kill, liver weight was increased in
females at 150 and 300 ppm and pituitary weight was decreased in
high-dose males. A tendency to increased liver weight was still
observed in females at the highest dose at 104 weeks. Tumour
incidence was not enhanced. The NOAEL in this study was 75 ppm,
equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively (Basler et al., 1985b).
Reproduction studies
Rats
Penconazole (purity 91.7%) was administered in the diet to
groups of 20 Tif:RAIf(SPF) rats/sex at concentrations of 0, 80, 400
or 2000 ppm (continuously over a period of 110 days to the parental
generations (F0 and F1), including a 12-day mating period for
each generation at the age of 3 months. These dose levels were equal
to 5.5-6.5, 28.5-31 or 146-166 mg/kg bw/day for males and 7.5-8.5,
40-42.5 or 202-227 mg/kg bw/day for females during the whole period.
Parental rats were killed after weaning of the F1 and F2
generation, respectively. Observations included clinical signs,
body-weight, food consumption, fertility index, implantation rate,
litter size, live and dead fetuses, sex ratio, organ weight,
macroscopy and histopathology.
A slightly depressed body-weight gain and food consumption was
seen at the highest dose in F0 females. Parturation was delayed
and the average duration of pregnancy was 21.1, 21.2, 21.3 and 21.6
days for the 0, 80, 400 and 2000 ppm group. Four dams died, two (1
at 400 and 1 at 2000 ppm) at or a short time after parturation, one
(at 2000 ppm) after 4 days and one (at 2000 ppm) after 11 days.
Testes weights of F0 males were slightly increased at 2000 ppm
(liver weight was not determined).
Body-weight gain of F1 pups was slightly reduced at 2000 ppm.
The activity index (ELP, exploratory locomotion pattern) was
slightly but not significantly depressed at 400 and 2000 ppm; there
was however no effect on general development and behaviour. At 2000
ppm, relative liver weight was increased in male and female F1
pups and brain and ovary weight was increased in F1 female pups.
Body-weight gain and food consumption of F1 parents was slightly
reduced at 2000 ppm. One dam died at 400 ppm and 2 at 2000 ppm at or
some days after parturation. The average pregnancy duration was
increased at the highest dose (21.3, 21.4, 21.2 and 21.8 days at 0,
80, 400 and 2000 ppm, respectively). At 2000 ppm, relative liver
weight and relative ovary weight were increased in F1 parents,
while relative brain and testes weight were increased in F1 males.
A decreased implantation rate was observed at 2000 ppm.
The ELP was slightly but not significantly decreased in high-
dose F2 pups without any effect on general development and
behaviour. Relative liver weight was increased in high-dose F2
pups.
Histopathological examination of the livers of F1 adults
revealed slight hypertrophy of the hepatocytes mainly in the
centrilobular region in 17/20 males and in 16/16 females at 2000 ppm
and in 5/19 males and 14/16 females at 400 ppm. Additionally, small
recent necrosis in the liver was observed in 2/16 high-dose females.
No histopathological changes were seen in weanling rats from both
F1 and F2 generations. The NOAEL in this study was 80 ppm, equal
to 5.5-6.5 mg/kg bw/day and 7.5-8.5 mg/kg bw/day for males and
females, respectively (Fritz et al., 1983a; Fritz et al.,
1983b).
Groups of 30 Charles River COBS CD rats/sex were fed diets
containing 0, 25, 250 or 2500 ppm penconazole (purity not given).
After 63 days of treatment the rats were mated to start a two-
generation (1 litter/generation) study. The F1 parents (randomly
selected) were mated after 84 days of treatment. Only weights of
ovaries and testes were determined. At 2500 ppm body-weight and food
consumption were decreased in F0 females and body-weight was
decreased in F1 parents. Mating indices were slightly reduced in
the F0 and F1 generations at 2500 ppm, but fertility indices
were not affected. Reduced pup weight and an increase in the number
of stillborn pups or pups that died during the first days of
lactation was observed at the highest dose. Relative ovary weight
was significantly increased in females of the F0 and the F1
generation at 2500 ppm. The NOAEL in this study is 250 ppm,
equivalent to 12.5 mg/kg bw/day (Schardein, 1987)
Special studies on embryotoxicity and/or teratogenicity
Rats
Groups of 25 pregnant Tif:RAIf(SPF) female rats were
administered orally by intubation 0, 30, 100 or 300 mg penconazole
(purity 88.4%)/kg bw/day suspended in CMC from days 6 to 15 of
gestation. The dams were observed for clinical signs, body-weight
and food consumption and were killed at day 21 of gestation. The
number of total implantations, resorptions, live and dead fetuses
were recorded and the uterus was weighed. Fetuses were weighed,
sexed and examined for external, visceral and skeletal
malformations. Two dams at 300 mg/kg bw/day died shortly before
autopsy. Maternal bodyweight gain in the highest dose group was
slightly reduced. Food consumption was reduced in all experimental
groups. An increase in the numbers of unossified phalangeal nuclei
of the hind limb was observed at 100 and 300 mg/kg bw/day (not dose-
related). At the highest dose, acaudia (taillessness) was observed
in 1/182 fetuses, and the overall incidence of skeletal anomalies
including irregularly shaped sternebrae was increased at the highest
dose. The NOAEL for maternal and embryofetal toxicity was 30 mg/kg
bw/day.
In a supplementary study, 0 or 300 mg penconazole/kg bw/day was
administered by gavage to groups of 15 pregnant rats from days 6-15
of gestation and another group of 15 rats received 450 mg
penconazole/kg bw/day from days 10-14 of gestation. Body-weight gain
and food consumption were decreased at both dose levels. Four and 2
females died shortly before autopsy at 300 and 450 mg/kg bw/day,
respectively. The average weight of live fetuses was decreased at
300 and 450 mg/kg bw/day. The incidence of unossified phalangeal
nuclei of the forelimb was increased at 450 mg/kg bw/day and the
incidence of unossified phalangeal nuclei of the hind limbs and
calcaneus were increased at both 300 and 450 mg/kg bw/day. No
irregular ossification of sternebrae was observed at 300 mg/kg
bw/day. At 450 mg/kg bw/day an irregularly ossified sixth sternebrae
was recorded in one foetus (Fritz & Giese, 1981). The increased
incidence of these variants may indicate delayed fetal development,
but in the absence of any associated malformations should not be
considered as indicative of teratogenicity.
Groups of 25 pregnant Charles River rats were administered by
gavage 0, 5, 100, or 500 mg penconazole (purity 98.7%)/kg bw/day in
corn oil from days 6-15 of gestation. The females were observed for
clinical signs, body-weight and food consumption and sacrificed on
day 20 of gestation. The number of implantation sites, resorption
sites, corpora lutea were recorded. Fetuses were weighed, sexed and
examined for external, internal and visceral anomalies. Two high-
dose dams and 1 dam at 5 mg/kg bw/day died during the study. High-
dose dams exhibited damp fur, crusty eye, crusty muzzle, crusty
nose, and yellow/brown stained fur as well as staggered gait,
emaciated loose stools, weakness and/or lethargy (5/25) and clear
salivation (4/25). Body-weight and body-weight gain were
significantly decreased at the highest dose. Food consumption was
decreased from days 6-13 at 100 and 500 mg/kg bw/day. At the highest
dose, the number of early and late resorption sites was increased
and the number of viable fetuses as well as the fetal weight were
decreased. The number of runts and litters with runts as well as the
occurrence of cervical ribs was increased at 500 mg/kg bw/day. No
teratogenic effects were observed. The NOAEL for maternal and
embryofetal toxicity in this study was 100 mg/kg bw/day (Salamon,
1985).
Rabbits
Groups of 20 pregnant chinchilla rabbits received 0, 25, 75 or
150 mg penconazole (purity 91.7%)/kg bw/day orally by intubation
from day 6 to 18 of pregnancy. Observations included clinical signs,
body-weight gain, food consumption. Dams were killed on day 28 of
pregnancy and fetuses were removed by caesarean section. No effects
were observed on the number of corpora lutea, total implantations,
early and late resorptions, live and dead fetuses, sex ratio uterus
and fetal weight, external and visceral examination and skeletal
malformations.
At 150 mg/kg bw/day average food consumption was slightly
reduced and the number of early resorptions was slightly but not
signficantly increased. At the highest dose,one fetus with
microphthalmia and one fetus showing microphthalmia associated with
internal hydrocephaly were seen. The NOAEL for maternal toxicity and
for embryotoxicity was 75 mg/kg bw/day (Giese & Suter, 1982).
Groups of 20 pregnant New Zeeland white rabbits were orally
dosed by gavage with 0, 10, 50 or 200 mg penconazole/kg bw/day from
days 7 to 19 of gestation. Observation included clinical signs,
body-weight and food consumption. All dams were killed on gestation
day 29 and fetuses were delivered by caesarean section. During the
treatment period decreased defecation and urination were observed in
high-dose animals and body-weight and food consumption were
decreased. A significant increase in body-weight and mean food
consumption was observed from gestation day 20-29. The number of
early resorptions was slightly increased and the number of viable
fetuses was slightly lower at the highest dose. At 200 mg/kg bw/day,
a slightly increased incidence of the number of fetuses with
unossified hyoid body and/or arches (marginal at 50 mg/kg bw/day)
and of fetuses with reduced ossification of the skull was observed.
No teratogenic effects were observed. The NOAEL in this study for
maternal and/or embryotoxicity was 50 mg/kg bw/day (Nemec et al.,
1985).
Special studies on mutagenicity
A number of genotoxicity tests have been carried out with
penconazole. The results are summarized in Table 2.
Special studies on skin and eye irritation and skin sensitization
Penconazole (purity 88.4%) produced slight erythema in 3 male
and 3 female New Zeeland white rabbits when applied under occlusive
conditions to the shaven intact and abraded skin for 24 hours, which
disappeared within 48 h (Ullmann & Gfeller, 1980b).
Penconazole (0.1 mg) was inserted into the conjuctival sac of
the left eye of nine New Zeeland white rabbits. At 30 seconds after
treatment, the eyes of 3/9 rabbits were washed with physiological
saline. In unrinsed eyes slight damage of the cornea and slight
conjunctival redness and chemosis were observed up to 7 days after
treatment. Rinsed eyes were normal 4 days after exposure. Two
rabbits died during the experiment. Bloody exudate in the pleural
and abdominal cavities was observed at autopsy (Ullmann & Gfeller,
1980a).
In a similar study, nine New Zeeland white albino rabbits were
given doses of 100 mg of undiluted penconazole (purity not given)
into the conjunctival sac of the left eye. Three of nine eyes were
washed. Conjunctival redness, chemosis and discharge was observed in
all animals at one hour after application which lasted up to 7 days
after treatment. The iris showed swelling but these changes
disappeared within 24 h. Corneal opacity and positive fluorescein
staining occurred but did not occur in any animal at 48 h (washed
eyes) and on day 7 (non-washed eyes) (Kuhn, 1988).
Penconazole (purity 88.4%) was tested for skin sensitization in
10 male and 10 female Pirbright white guinea-pigs in an optimization
test. No sensitization was observed when the guinea-pigs received 10
intracutaneous injections (0.1 ml of a 0.1% suspension of
penconazole in propylene glycol) followed 14 days later by a single
challenge injection (0.1 ml of 0.1% suspension of penconazole in
propylene glycol) (Ulmann & Gfeller,1980c).
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vitro
Ames testa,b S. typhimurium 250-20250 µg/ml in 92.8 negative Arni & Müller (1980)
TA100, TA98 DMSO;a > 6750 µg/ml
TA1535, TA1537 toxic, and b 20250
µg/ml toxic
Ames testa,b S. typhimurium 100-25600 µg/ml in 91.7 negative c Deparade & Arni (1984)
TA98, TA100 acetone
TA1535, TA1537
Yeast testa,b S. cerevisiae 0.1-40 µg/ml in DMSO 91.7 negative Arni & Müller (1983)
(mitotic gene D7
conversion)
Transformation mouse 2.625, 5.25, 10.5, 91.7 negative c Beilstein & Müller (1984a)
assay embryofibroblasts 21.0 and 42.0 µg/ml
BALB/3T3 in DMSO
Mouse lymphoma L5178Y mouse (a):6.75-100 µg/ml, 91.7 negative c Beilstein & Müller (1984b)
assay a,b lymphoma cells 100 µg/ml toxic;
(TK+/-) (b):8.25-110 µg/ml,
> 110 µg/ml toxic;
both in DMSO
DNA repair human fibroblasts 0, 0,32, 1.6 and 91.7 negative c Puri & Müller (1983)
test 40 µg/ml.
DNA repair rat hepatocytes 0, 032, 1.6, 8.0, 91.7 negative c Puri & Müller (1984)
test 40 µg/ml
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vivo
Host mediated S. typhimurium 0, 350, 700 and 1400 91.7 negative Arni & Müller (1984)
assay (intra TA98, TA100 and mg/kg orally
sanguine) TA1535
in male NMRI mice
Host mediated L 5178Y-cells 0, 813 mg/kg bw 91.7 negative Strasser & Müller (1982)
assay in DBA/Bom/SPF orally
mice
Nucleus anomaly Chinese hamster 0, 417, 834 and 1668 92.8 negative c Hool & Langauer (1982)
test bone marrow cells mg/kg orally in PEG 400
per day on 2 consecutive
days
Sister chromatid Chinese hamster 0, 417, 834 and 1668 91.7 negative c Hool & Arni (1983a)
exchange assay bone marrow mg/kg orally in PEG 400
Chromosome NMRI mice 0, 91, 272 and 816 mg/kg 91.7 negative Hool & Arni (1983b)
aberration assay spermatogonia bw orally in PEG
400 per day on 5
consecutive days; at
816 mg/kg
all mice died
Dominant lethal Male NMRI mice 0, 272 and 816 mg/kg 91.7% negative Hool & Arni (1983c)
assay bw orally
a without metabolic activation
b with metabolic activation
c positive controls yielded positive result(s)
COMMENTS
Penconazole administered orally to mice and rats was rapidly
absorbed and excreted, predominantly in the urine. Female rats
eliminated more in the urine and less in the faeces than male rats.
Very low residues were found in organs and tissues in both males and
females.
Numerous metabolites were identified in urine and faeces. The
major metabolic pathways involved oxidation of the pentyl side chain
to alcohols and acids with sequential cleavage of the terminal
carbon. As the oxidation products were conjugated, the resulting
metabolic patterns were complex. More polar and conjugated products
were found in female rats. Cleavage of the alkyl bridge between the
two rings led to the formation of 1,2,4-triazole, which was a major
metabolic route in male rats.
Penconazole showed low acute oral toxicity to mice, rats and
hamsters, but was slightly more toxic to rabbits. The World Health
Organization has classified penconazole as unlikely to present acute
hazard in normal use (WHO, 1992).
Short-term studies with mice, rats and dogs indicated that the
liver was the primary target organ. In a special study with male
rats, induction of microsomal liver enzymes was demonstrated. In a
13-week study in mice, increased liver weight and liver hyperthrophy
were observed at 500, 1000 and 2400 ppm. The NOAEL was 300 ppm,
equivalent to 45 mg/kg bw/day.
In three 90-days studies performed with rats, liver weight and
liver histopathology were the main effects, except for the second
study. In the first study, increase in relative liver weight was
observed at all dietary levels (30, 300 or 3000 ppm), while in the
third study (dietary levels of 10, 100, 300, 500, 1000 or 2400 ppm)
the NOAEL was 300 ppm, equivalent to 15 mg/kg bw/day. In the second
study (dietary levels of 10, 30 or 100) some biochemical parameters
were affected at 30 and 100 ppm. The NOAEL was 10 ppm, equal to 0.8
mg/kg bw/day.
In a one-year study in dogs, increased liver weights and
histopathological liver effects were observed at dietary
concentrations of 500 and 2500/5000 ppm. At the highest dose level
reduced testis weight and athrophic changes were also observed. The
NOAEL was 100 ppm, equal to 3.0 and 3.3 mg/kg bw/day for males and
females, respectively.
In a two-year feeding study in mice (dietary concentrations of
0, 5, 75, 150 or 300 ppm) increased liver weight at interim kill was
seen at 300 ppm. The NOAEL in this study was 150 ppm, equal to 19.3
mg/kg bw/day for males and 17.2 mg/kg bw/day for females. In a long-
term study in rats (dietary concentrations of 0, 5, 75, 150 or 300
ppm) liver weight was increased at 150 and 300 ppm. The NOAEL was 75
ppm, equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively. Penconazole was not carcinogenic in mice or rats.
Two two-generation reproduction studies in rats were reviewed.
In the first study (dietary concentrations of 0, 80, 400 or 2000
ppm), mortality and delayed parturation were observed, as well as
decreased body-weight gain of parents and pups and increased
relative liver weights in parents and pups at 2000 ppm. Hyperthrophy
of liver cells was found at 400 and 2000 ppm. The NOAEL was 80 ppm,
equal to 5.5-6.5 mg/kg bw/day for males and 7.5-8.5 mg/kg bw/day for
females. In the second study (dietary concentrations of 0, 25, 250
or 2500 ppm), liver weights were not determined. The main effect at
2500 ppm was reduced body-weight gain of parents and pups and
mortality of pups during lactation. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
Embryotoxicity/fetotoxicity was observed in three
teratogenicity studies with rats. In two studies (gavage doses of 0,
30, 100 or 300 mg/kg bw/day in one study and 0, 300 or 450 mg/kg
bw/day in the other), maternal toxicity, an increased number of
resorptions, decreased pup weight and delayed ossification were
observed at the high doses. Maternal toxicity or embryotoxicity were
not observed at 30 mg/kg bw/day. In the third study (gavage doses of
0, 5, 100 or 500 mg/kg bw/day), the NOAEL was 100 mg/kg bw/day. In
two teratogenicity studies with rabbits (doses of 0, 25, 75 or 150
mg/kg bw/day in one study and 0, 10, 50 or 200 mg/kg bw/day in the
other), maternal body-weight and food consumption were reduced and
the number of early resorptions was increased at the highest doses.
Overall, the NOAEL was 75 mg/kg bw/day for both maternal toxicity
and embryotoxicity.
After reviewing the available in vitro and in vivo short-
term genotoxicity data, the Meeting concluded that penconazole was
not genotoxic.
An ADI was allocated on the basis of the NOAEL determined from
the one-year study in dogs, which was supported by the NOAEL from
the long-term study in rats. A safety factor of 100 was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 17 mg/kg bw/day (two-year study)
Rat: 75 ppm, equal to 3.8 mg/kg bw/day (two-year study)
80 ppm, equal to 5.5 mg/kg bw/day (two-generation
reproduction study
30 mg/kg bw/day (teratology study)
Rabbit: 75 mg/kg bw/day (teratology study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arni, P. & Müller, D. (1980) Salmonella/mammalian-microsome
mutagenicity test with CGA 71818 (test for mutagenic properties in
bacteria). Unpublished report experiment No. 800550 dated 29
September 1980 from Ciba-Geigy Protection of Health and Environment,
Toxicology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Arni, P. & Müller, D. (1983) Saccharomyces cerevisiae
D7/mammalian-microsome mutagenicity test in vitro with CGA 71818.
Unpublished report experiment No. 811560 dated 27 June 1983 from
Ciba Geigy, Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Müller, D. (1984) Intrasanguine host-mediated assay with
S. typhimurium. Unpublished report experiment No. 830749 dated 3
May 1984 from Ciba Geigy, Protection of Health and Environment, GU
2.3, Experimental Pathology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Basler, W., Gfeller, W., Zak, F., Zakova, N. & Grieve, A.P. (1982)
CGA 71818: 3 months toxicity study in rats. Unpublished final
report, GU project No. 801194 dated 24 February 1982 from Ciba-Geigy
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Komarek, J., Zak, F. & Beri, J. (1983) CGA 71818: 3
month toxicity study in rats. Unpublished final report GU project
No. 821054 dated 17 August 1983 from Ciba-Geigy Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd. Basle, Switzerland.
Basler, W., Komarek, J., Zak, F. & Skorpil, V. (1984) CGA 71818: 28-
day oral cumulative toxicity study in rats. Unpublished final
report, GU project No. 820822 dated 28 June 1984 from Ciba-Geigy,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Gretener, P., Zak, F. & Krinke, G. (1985a) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in mice.
Unpublished final report GU project No. 811414 dated 1 July 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Basler, W., Gretener, P., Zak, F. & Naylor, D.C. (1985b) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in rats.
Unpublished final draft GU project no.: 811415 d.d. 25 June 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980a) Acute oral LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800553 dated 28
May 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980b) Acute dermal LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800559 dated 19
March 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980c) Acute oral LD50 in the Chinese
hamster of technical CGA 71818. Unpublished report project No.
800555 dated 19 March 1980 from Ciba-Geigy, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Beilstein, P. & Müller, D. (1984a) BALB/3T3 cell transformation
assay with CGA 71818. Unpublished report test No. 830755 dated 6
September 1984 from Ciba-Geigy, Protection of Health and
Environment, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Beilstein, P. & Muller, D. (1984b) L5178Y/TK+- mouse lymphoma
mutagenicity test. CGA 71'818 tech. ( in vitro test for mutagenic
properties of chemical substances in mammalian cells). Proj. No.:
811524. Unpublished report dated 9 October 1984 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Deparade, E. & Arni, P. (1984) Salmonella/mammalian-microsome
mutagenicity test. Unpublished report project No. 839759 dated 20
February 1984 from Ciba-Geigy Protection of Health and Environment,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H. & Giese, K. (1981) Report on CGA 71818 tech. teratology
study (seg.II) in rats. Unpublished report test No. 800549 d.d. 25
August 1981 from Ciba-Geigy, Reproductive Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983a) Report on CGA
71818 tecnical. 2-generation toxicity study in rats. Unpublished
report test No. 811416 from Ciba-Geigy, Toxicology GU 2,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983b) Two-generation
reproduction study in rats with CGA 71818 technical. Additional data
submission. Unpublished report test No. 811416 from Ciba-Geigy,
Experimental Toxicology, Sisseln, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Gfeller, W., Zak, F. & Naylor, D.C. (1984) CGA 71818 12 month
toxicity study in dogs. Unpublished final report GU project No.
801187 dated February 1984 from Ciba-Geigy, GU2 Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Giese, K. & Suter, H.P. (1982) Report on CGA 71818 tech. Teratology
study in rabbits. Unpublished report test No. 811354 dated March
1982 from Ciba-Geigy Reproductive Toxicology GU 2.1, Sisseln,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hamböck, H. (1980) Distribution, degradation and excretion of CGA
71818 in the rat. Unpublished project report 41/80 dated 6 November
1982 from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1982) The major urinary metabolites of CGA 71818 in the
rat. Unpublished project (status) report 15/82 dated 17 May 1982
from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1984) The metabolic fate of CGA 71818 in the rat.
Unpublished project report 23/83 dated 10 May 1984 from Ciba-Geigy,
AG 2.52, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hamböck, H. (1985) Sex dependency of the metabolite pattern of CGA
71818 after oral administration to rats. Addendum to project report
41/80. Unpublished report 1/85 dated 11 January 1985 from Ciba-Geigy
AG 2.52 Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hartmann, H.R. & Gfeller, W. (1987) CGA 71818 tech. Acute aerosol
inhalation toxicity in the rat. Unpublished final report GU project
No. 871169 d.d. 6 August 1987 from Ciba-Geigy Ltd, GU 2 Toxicology,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987a) CGA 71818 technical. Kinetic study in albino
rats with CGA 71818 technical. Unpublished report HLA 6117-122 dated
24-2-1987 from Hazleton Laboratories America, Inc. Madison,
Wisconsim 53704. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987b) 90-Day subchronic dietary toxicity and kinetic
study in albino mice with CGA-71818 technical. Unpublished report
HLA 6117-121 dated 4-3-1987 from Hazleton Laboratories America, Inc.
Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hiles, R.A. (1987c) 90-Day subchronic dietary toxicity and kinetic
study in albino rats with CGA-71818 technical. Unpublished report
HLA 6117-120 dated 14-04-1987 from Hazleton Laboratories America,
Inc. Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Arni, P. (1983a) Sister chromatid exchange study CGA
71818 Chinese hamster. Unpublished report dated 2-06-1983 project
No. 811523 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983b) Chromosome studies in male germinal
epithelium CGA 71818 mouse. Unpublished report dated 17-06-1983
project No. 811520 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983c) Dominant lethal test, mouse 8 weeks CGA
71818. Unpublished report dated 2-12-1983 project No. 811519 from
Ciba-Geigy Ltd., Protection of Health and Environment, Experimental
Pathology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Langauer, M. (1982) Nucleus anomaly test in somatic
interphase nuclei. CGA 71818 Chinese hamster. Unpublished report
dated 20-1-1982 experiment No. 800551 from Ciba-Geigy Ltd,
Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Kobel, W. (1981) Acute oral LD50 in the rabbit of technical CGA
71818. Unpublished report dated 4-3-1981, project No. 800554 from
Ciba-Geigy Ltd., Exp. Toxicology Sisseln, Switzerland, Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1988) Primary eye irritation study in rabbits EPA
guidelines No. 81-4. Unpublished report dated 31-03-1988 project
no.: 5303-88 from Stillmeadow Inc. Houston, Texas, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
LeVan, L.W. (1987) Acute kinetic study with CGA-71818 technical in
albino rats. Unpublished report dated 2-03-1987 project No. HLA
6117-123 from Hazleton Laboratories America Inc. Madison, Wisconsin,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988a) CGA-71818 Metabolism in hens - a
summary. Unpublished report No. ABR-88009 dated 20-05-1988 from
Ciba-Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988b) CGA-71818 Metabolism in goats - a
summary. Unpublished report No. ABR-88008 d.d.20-05-1988 from Ciba-
Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Nemec, M.D. & Keets, S.A., Leist, P.L. & Mercieca, M.D. (1985) A
teratology study (segment II) in albino rabbits with CGA-71818
technical. Unpublished report No. WIL-82004 dated 31-07-1985 from
WIL Research Laboratories Inc., Ashland, Ohio, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Müller, D. (1983) Autoradigraphic DNA repair test on
human fibroblast CGA 71818 . Unpublished report dated 2-12-1983
project No. 811657 from Ciba-Geigy Ltd.,Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Muller, D. (1984) Autradiographic DNA repair test on rat
hepatocytes. CGA 71'818 ( in vitro test for DNA-damaging
properties). Proj. No.: 811522. Unpublished report dated 30 January
1984 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Salamon, C.M. (1985) CGA-71818 technical teratology study in rats.
Unpublished report No. 450-2087 dated 16-09-1985 from American
Biogenics Corp., Decatur, Illinois, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sarasin, G. (1980) Report on acute oral LD50 in the mouse of CGA
71818 technical. Unpublished report No. 800552 dated 20-10-1980 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Schardein, J.L. (1987) Two-generation reproduction study in albino
rats with CGA 71818. Unpublished report No. 382-119 dated 14-08-1987
from International Research and Development Corp., Mattawan,
Michigan, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Seiffert, G., Komarek, J., Zak, F., Malik, C. & Zakova, N. (1983)
CGA 71818 21 day repeated dose dermal toxicity study in rabbits.
Unpublished report dated September 1983, project No. 820206 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Strasser, F.F. & Müller, D. (1982) Point mutation assay with mouse
lymphoma cells. Host-mediated assay with CGA 71818. Unpublished
report project No. 811355 dated 5-01-1982 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980a) Report on eye irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800557 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland.Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980b) Report on skin irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800558 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980c) Report on skin sensitizing
(contact allergenic) efect in guinea-pigs of technical CGA 71818.
Unpublished report project No. 800560 dated 12-06-1980 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Van Dijk, A. (1987) (U-14C) phenyl CGA 71818: Absorption,
distribution, excretion and metabolism after single oral and
repeated oral administration to the rat. Unpublished report project
No. 075666 dated 11-06-1987 from RCC Umweltchemie AG, Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Stäubli, W. (1985) The effect of
penconazole on drug metabolizing enzymes in the livers of male rats
and mice. Unpublished report dated April 1985 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Metabolites 9, 10, 11 and 12 were mainly found as glucuronides
or other conjugates.
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of penconazole
Species Sex Route LD50 LC50 Purity Reference
mg/kg bw mg/m3
Mouse M&F oral 2444 88.4 Sarasin (1980)
Rat M&F oral 2125 88.4 Bathe & Gfeller (1980a)
M&F dermal >3000 88.4 Bathe & Gfeller (1980b)
M&F inhal >4046 96.1 Hartmann & Gfeller (1987)
Chinese hamster M&F oral >5000 88.4 Bathe & Gfeller (1980c)
Rabbit M&F oral 971 88.4 Kobel (1981)
Penconazole shows low acute oral toxicity to mice, rats and
hamsters, but is slightly more toxic to rabbits.
Short-term toxicity studies
Mice
Groups of Cr:CD(ICR)BR mice (15/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm penconazole
(purity 98.7%) equivalent to 0, 1.5, 15, 45, 75, 150 or 360 mg/kg
bw/day for 13 weeks. Histopathology was only reported for the liver.
No treatment-related effects were observed on clinical signs,
mortality, ophthalmoscopy, food consumption, food efficiency,
haematology or urinalysis. Body-weight was lower at 2400 ppm.
Cholesterol levels were decreased at 1000 and 2400 ppm, ALAT
increased in high-dose males and total protein and albumin were
decreased in females at 2400 ppm. Relative liver weight was
significantly increased at 500, 1000 and 2400 ppm. An increased
incidence of centrilobular hypertrophy of the liver was observed in
males at doses > 500 ppm and in females at the highest dose.
Hepatic degeneration and vacuolisation was also observed in high-
dose males. Coagulative necrosis in the liver was observed in males
at 1000 and 2400 ppm. The NOAEL in this study was 300 ppm in the
diet, equivalent to 45 mg/kg bw/day (Hiles 1987b).
Rats
Groups of Tif:RAIf(SPF) rats (10/sex/group) were orally
administered 0, 20, 100 or 500 mg penconazole (purity 91.7%)/kg
bw/day for 28 days. Since no marked symptoms of toxicity showed up
during the first week of treatment the doses were increased to 100,
500 or 1000 mg/kg bw/day on day 8 of treatment. After increasing the
doses, 3 male and 3 female high-dose rats showed marked apathia and
lateral body position at day 10 but the animals recovered on day 11.
No effects were observed on mortality, ophthalmic or hearing
examinations. In the mid- and high-dose groups male mean body weight
was decreased, food consumption and food conversion were decreased
in males and females, haemoglobin and haematocrit values were
decreased in females and ALP and ALAT as well as cholesterol and
total proteins were increased in males and females and total
globulin was increased and A/G ratio was decreased in females.
Phosphate and glucose were increased in high-dose females. Albumin
increased in high-dose males and females. Urine volume was increased
in males at the highest dose and in females at the mid- and high-
dose. Relative liver, kidney and adrenal weight was increased in
mid- and high-dose males and females. Relative brain, spleen and
thyroid weight were increased in high-dose females. Enlarged livers
with slight hypertrophy of the hepatocytes were observed in 8/10 and
3/10 males and females at the mid-dose and in all animals at the
highest dose. The lowest dose (20-100 mg/kg bw/day) was considered
as the NOAEL (Basler et al., 1984).
Groups of Tif:RAIf(SPF) rats (20/sex/group) were fed diets
containing 0, 30, 300 or 3000 ppm (equal to 2.1, 19.4 or 202.3 mg
penconazole/kg bw/day for males and 2.1, 20.7 or 208.6 mg
penconazole/kg bw/day for females, respectively) (purity 91.7%) for
3 months. No effects were observed on clinical signs, mortality,
food and water consumption, food conversion, hearing tests or eye
examinations. Body weight was decreased in males and females at the
highest dose. An increase in total plasma protein and albumin was
observed in male rats at all doses and a trend to an increase in
total plasma proteins was seen in females at 3000 ppm. GGT was
slightly increased in females at 300 and 3000 (significantly) ppm.
Cholesterol levels were increased in both sexes at 3000 and also in
females at 300 ppm. A significant dose-related increase in relative
liver weight was observed at all dose levels. Relative testes
weights were increased in males at 3000 ppm and brain, kidney and
thymus weight were increased in high dose females. At histopathology
minimal hypertrophy of the hepatocytes was seen in 20/20 male rats
and 9/20 female rats at 3000 ppm. In this study no NOAEL was
identified, although the liver weight increase at 30 ppm was
marginal (Basler et al., 1982).
In another 3 month feeding study groups of Tif:RAIf(SPF) rats
(20/sex/group) were fed diets containing 0, 10, 30 or 100 ppm
penconazole (purity 91.7%) (equal to 0.8, 2.1 or 7.1 mg
penconazole/kg bw/day for males and 0.8, 2.1 or 7.3 mg
penconazole/kg bw/day for females, respectively). No effects were
observed on clinical signs, mortality, body-weight, food and water
consumption, food conversion, eye examinations, hearing tests, organ
weight or histopathology. Total proteins were increased in male and
female rats at 30 and 100 ppm. Total globulin was increased in high
dose females. GGT was slightly increased in females at both 30 and
100 ppm. Liver weight was increased at 10 and 30 ppm in male rats
only, but not at 100 ppm. No liver histopathological effects were
observed. The NOAEL in this study was 10 ppm penconazole, equal to
0.8 mg/kg bw/day (Basler et al., 1983).
In an additional study groups of albino [Crl:CD(SD)BR] rats
(15/sex/group) were fed diets containing 0, 10, 100, 300, 500, 1000
or 2400 ppm penconazole (purity 98,7%) equivalent to 0.5, 5, 15, 25,
50 or 120 mg penconazole/kg bw/day for 90 days. In this study only
the liver as target organ was studied histopathologically.
Observations included clinical signs mortality, body-weight, food
consumption, food efficiency, ophthalmoscopy, haematology, clinical
chemistry (including GGT), urinalysis, organ weights, macroscopy and
histopathology. Female body weight gain was lower at 500, 1000 and
2400 ppm and food consumption was decreased at the highest dose.
Urea nitrogen was increased in males at all dose levels, but not
clearly dose-related. Relative liver weight was significantly
increased in rats at 500 (females only), 1000 and 2400 ppm. Relative
kidney weight was increased in rats at 2400 ppm. At histopathology
the incidence of vacuolization, degeneration and centrilobular
hyperthrophy of hepatocytes was increased at 500 ppm and above. The
NOAEL in this study was 300 ppm, equivalent to 15 mg/kg bw/day
(Hiles, 1987c).
Rabbits
Groups of New Zeeland white rabbits (5/sex/group) were given
1000, 1500 or 2000 mg penconazole/kg bw/day (purity 91.7%) by dermal
application once/day for 5 days/week for 21 days. Two satellite
groups received 0 or 2000 mg penconazole/kg bw/day for 21 days
followed by a recovery period of 14 days. Transient signs of
dyspnea, curved body position and ruffled fur were observed at all
dose levels including control rabbits, but symptoms disappeared
during the recovery period. No dose-related effects were observed on
mortality, body-weight, food consumption and food conversion,
haematology, clinical chemistry, macroscopy and histopathology. The
NOAEL in this study was > 2000 mg/kg bw/day (Seifert et al.,
1983).
Dogs
Groups of beagle dogs (10/sex/group) were fed diets containing
0, 100, 500, or 5000/2500 ppm penconazole (purity 91.7%), equal to
3.0, 16.9 or 133 mg penconazole/kg bw/day for males and 3.3, 16.7 or
139 mg penconazole/kg bw/day for females, respectively, for 12
months. Due to poor feed intake the highest dose was lowered to 2500
ppm in week 20. An interim kill on 4 dogs/sex/group was carried out
at week 14 and 2 dogs/sex/group were kept for a 4-week recovery
period. Observations included clinical signs, mortality, body-
weight, food consumption, food conversion, ophthalmoscopy, auditory
perception, haematology, blood chemistry, urinalysis, macroscopy,
organ weights and histopathology.
An increased incidence of vomiting was observed in high-dose
dogs during the first three months of treatment; thereafter
occasionally vomiting was observed only in females. The high-dose
dogs lost body-weight and had a reduced food consumption during the
first months. After lowering the dosage food consumption and male
bodyweights were comparable to the controls but the mean weight of
the females remained lower than the control value. ASAT, ALP, OCT,
GGT and ALAT activities were increased in dogs at the highest dose
during the whole administration period. Total globulin concentration
was increased in males at the highest dose during weeks 13, 26 and
53, consequently the total protein was slightly increased and the
A/G ratio was decreased. Terminal body-weight of high-dose male and
female dogs were decreased after 3 months. At this time relative
liver weights were increased at 500 and 5000 ppm. Relative kidney
weight was increased at the highest dose in both males and females.
Relative brain and adrenal weight were increased and relative heart
weight was decreased in high-dose females. All males at 5000 ppm
showed a reduction in testes weights and atrophy of the seminiferous
epithelium. At macroscopy emaciation was observed in dogs at the
highest dose after 3 months. At histopathology after 3 months,
minimal lesions characterized by monocellular hepatocyte necrosis
associated with inflammatory cell infiltration were noticed in
nearly all interim sacrificed dogs at the highest dose and in one
male dog at 500 ppm. After 12 months, testes weights were reduced in
males at 2500 ppm and liver weight was increased in females at 500
and 2500 ppm. At histopathology, bilateral tubular atrophy and
reduced spermatogenesis in the testes was observed in the high-dose
males and an increased incidence of inflammation with fibrosis in
the peripheral lobular region of the liver occurred in the mid- and
high-dose animals. The only abnormalities found after the recovery
period were decreased relative testes weights and tubular atrophy at
2500 ppm. The NOAEL in this study was 100 ppm, equal to 3.0 mg/kg
bw/day for males and 3.3 mg/kg bw/day for females (Gfeller et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Tif:MAGf(SPF) mice (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%),
equal to 0.75, 9.8, 19.3 or 40.8 mg/kg bw/day for males and 0.67,
8.8, 17.2 or 35.7 mg/kg bw/day for females, for 24 months. An
interim kill was carried out on 10 mice/sex/group at 52 weeks. No
effects were observed on mortality, clinical signs, body-weight,
food and water consumption, food conversion, ophthalmic and hearing
inspections, haematology, blood chemistry, urinalysis, macroscopy or
histopathology. Relative liver weight was increased in high-dose
males and females at the interim kill. At the end of the study
adrenal weight (not dose-related) and prostate weight were increased
in males at 75, 150 and 300 ppm. There was no treatment-related
increase in tumour incidence (Basler et al., 1985b). Since
weighing of prostate glands is not very reliable and the increased
weight was not found at 53 weeks, the Meeting concluded that this
effect was probably biologically insignificant. The NOAEL was
therefore 150 ppm, equal to 19.3 mg/kg bw/day for males and 17.2
mg/kg bw/day for females based on the increased liver weight.
Rats
Groups of Tif:RAIf(SPF) rats (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%) for
24 months. An interim kill was carried out on 10 rats/sex/group at
12 months. Twenty rats/sex/group were maintained for laboratory
investigations and sacrificed after 24 months. The remaining 50
rats/sex/group were sacrificed after 116/117 weeks. No effects were
observed on mortality, clinical signs, body-weight, food and water
consumption, food conversion, ophthalmic and hearing inspections,
haematology, blood chemistry, urinalysis, macroscopy or
histopathology. At the interim kill, liver weight was increased in
females at 150 and 300 ppm and pituitary weight was decreased in
high-dose males. A tendency to increased liver weight was still
observed in females at the highest dose at 104 weeks. Tumour
incidence was not enhanced. The NOAEL in this study was 75 ppm,
equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively (Basler et al., 1985b).
Reproduction studies
Rats
Penconazole (purity 91.7%) was administered in the diet to
groups of 20 Tif:RAIf(SPF) rats/sex at concentrations of 0, 80, 400
or 2000 ppm (continuously over a period of 110 days to the parental
generations (F0 and F1), including a 12-day mating period for
each generation at the age of 3 months. These dose levels were equal
to 5.5-6.5, 28.5-31 or 146-166 mg/kg bw/day for males and 7.5-8.5,
40-42.5 or 202-227 mg/kg bw/day for females during the whole period.
Parental rats were killed after weaning of the F1 and F2
generation, respectively. Observations included clinical signs,
body-weight, food consumption, fertility index, implantation rate,
litter size, live and dead fetuses, sex ratio, organ weight,
macroscopy and histopathology.
A slightly depressed body-weight gain and food consumption was
seen at the highest dose in F0 females. Parturation was delayed
and the average duration of pregnancy was 21.1, 21.2, 21.3 and 21.6
days for the 0, 80, 400 and 2000 ppm group. Four dams died, two (1
at 400 and 1 at 2000 ppm) at or a short time after parturation, one
(at 2000 ppm) after 4 days and one (at 2000 ppm) after 11 days.
Testes weights of F0 males were slightly increased at 2000 ppm
(liver weight was not determined).
Body-weight gain of F1 pups was slightly reduced at 2000 ppm.
The activity index (ELP, exploratory locomotion pattern) was
slightly but not significantly depressed at 400 and 2000 ppm; there
was however no effect on general development and behaviour. At 2000
ppm, relative liver weight was increased in male and female F1
pups and brain and ovary weight was increased in F1 female pups.
Body-weight gain and food consumption of F1 parents was slightly
reduced at 2000 ppm. One dam died at 400 ppm and 2 at 2000 ppm at or
some days after parturation. The average pregnancy duration was
increased at the highest dose (21.3, 21.4, 21.2 and 21.8 days at 0,
80, 400 and 2000 ppm, respectively). At 2000 ppm, relative liver
weight and relative ovary weight were increased in F1 parents,
while relative brain and testes weight were increased in F1 males.
A decreased implantation rate was observed at 2000 ppm.
The ELP was slightly but not significantly decreased in high-
dose F2 pups without any effect on general development and
behaviour. Relative liver weight was increased in high-dose F2
pups.
Histopathological examination of the livers of F1 adults
revealed slight hypertrophy of the hepatocytes mainly in the
centrilobular region in 17/20 males and in 16/16 females at 2000 ppm
and in 5/19 males and 14/16 females at 400 ppm. Additionally, small
recent necrosis in the liver was observed in 2/16 high-dose females.
No histopathological changes were seen in weanling rats from both
F1 and F2 generations. The NOAEL in this study was 80 ppm, equal
to 5.5-6.5 mg/kg bw/day and 7.5-8.5 mg/kg bw/day for males and
females, respectively (Fritz et al., 1983a; Fritz et al.,
1983b).
Groups of 30 Charles River COBS CD rats/sex were fed diets
containing 0, 25, 250 or 2500 ppm penconazole (purity not given).
After 63 days of treatment the rats were mated to start a two-
generation (1 litter/generation) study. The F1 parents (randomly
selected) were mated after 84 days of treatment. Only weights of
ovaries and testes were determined. At 2500 ppm body-weight and food
consumption were decreased in F0 females and body-weight was
decreased in F1 parents. Mating indices were slightly reduced in
the F0 and F1 generations at 2500 ppm, but fertility indices
were not affected. Reduced pup weight and an increase in the number
of stillborn pups or pups that died during the first days of
lactation was observed at the highest dose. Relative ovary weight
was significantly increased in females of the F0 and the F1
generation at 2500 ppm. The NOAEL in this study is 250 ppm,
equivalent to 12.5 mg/kg bw/day (Schardein, 1987)
Special studies on embryotoxicity and/or teratogenicity
Rats
Groups of 25 pregnant Tif:RAIf(SPF) female rats were
administered orally by intubation 0, 30, 100 or 300 mg penconazole
(purity 88.4%)/kg bw/day suspended in CMC from days 6 to 15 of
gestation. The dams were observed for clinical signs, body-weight
and food consumption and were killed at day 21 of gestation. The
number of total implantations, resorptions, live and dead fetuses
were recorded and the uterus was weighed. Fetuses were weighed,
sexed and examined for external, visceral and skeletal
malformations. Two dams at 300 mg/kg bw/day died shortly before
autopsy. Maternal bodyweight gain in the highest dose group was
slightly reduced. Food consumption was reduced in all experimental
groups. An increase in the numbers of unossified phalangeal nuclei
of the hind limb was observed at 100 and 300 mg/kg bw/day (not dose-
related). At the highest dose, acaudia (taillessness) was observed
in 1/182 fetuses, and the overall incidence of skeletal anomalies
including irregularly shaped sternebrae was increased at the highest
dose. The NOAEL for maternal and embryofetal toxicity was 30 mg/kg
bw/day.
In a supplementary study, 0 or 300 mg penconazole/kg bw/day was
administered by gavage to groups of 15 pregnant rats from days 6-15
of gestation and another group of 15 rats received 450 mg
penconazole/kg bw/day from days 10-14 of gestation. Body-weight gain
and food consumption were decreased at both dose levels. Four and 2
females died shortly before autopsy at 300 and 450 mg/kg bw/day,
respectively. The average weight of live fetuses was decreased at
300 and 450 mg/kg bw/day. The incidence of unossified phalangeal
nuclei of the forelimb was increased at 450 mg/kg bw/day and the
incidence of unossified phalangeal nuclei of the hind limbs and
calcaneus were increased at both 300 and 450 mg/kg bw/day. No
irregular ossification of sternebrae was observed at 300 mg/kg
bw/day. At 450 mg/kg bw/day an irregularly ossified sixth sternebrae
was recorded in one foetus (Fritz & Giese, 1981). The increased
incidence of these variants may indicate delayed fetal development,
but in the absence of any associated malformations should not be
considered as indicative of teratogenicity.
Groups of 25 pregnant Charles River rats were administered by
gavage 0, 5, 100, or 500 mg penconazole (purity 98.7%)/kg bw/day in
corn oil from days 6-15 of gestation. The females were observed for
clinical signs, body-weight and food consumption and sacrificed on
day 20 of gestation. The number of implantation sites, resorption
sites, corpora lutea were recorded. Fetuses were weighed, sexed and
examined for external, internal and visceral anomalies. Two high-
dose dams and 1 dam at 5 mg/kg bw/day died during the study. High-
dose dams exhibited damp fur, crusty eye, crusty muzzle, crusty
nose, and yellow/brown stained fur as well as staggered gait,
emaciated loose stools, weakness and/or lethargy (5/25) and clear
salivation (4/25). Body-weight and body-weight gain were
significantly decreased at the highest dose. Food consumption was
decreased from days 6-13 at 100 and 500 mg/kg bw/day. At the highest
dose, the number of early and late resorption sites was increased
and the number of viable fetuses as well as the fetal weight were
decreased. The number of runts and litters with runts as well as the
occurrence of cervical ribs was increased at 500 mg/kg bw/day. No
teratogenic effects were observed. The NOAEL for maternal and
embryofetal toxicity in this study was 100 mg/kg bw/day (Salamon,
1985).
Rabbits
Groups of 20 pregnant chinchilla rabbits received 0, 25, 75 or
150 mg penconazole (purity 91.7%)/kg bw/day orally by intubation
from day 6 to 18 of pregnancy. Observations included clinical signs,
body-weight gain, food consumption. Dams were killed on day 28 of
pregnancy and fetuses were removed by caesarean section. No effects
were observed on the number of corpora lutea, total implantations,
early and late resorptions, live and dead fetuses, sex ratio uterus
and fetal weight, external and visceral examination and skeletal
malformations.
At 150 mg/kg bw/day average food consumption was slightly
reduced and the number of early resorptions was slightly but not
signficantly increased. At the highest dose,one fetus with
microphthalmia and one fetus showing microphthalmia associated with
internal hydrocephaly were seen. The NOAEL for maternal toxicity and
for embryotoxicity was 75 mg/kg bw/day (Giese & Suter, 1982).
Groups of 20 pregnant New Zeeland white rabbits were orally
dosed by gavage with 0, 10, 50 or 200 mg penconazole/kg bw/day from
days 7 to 19 of gestation. Observation included clinical signs,
body-weight and food consumption. All dams were killed on gestation
day 29 and fetuses were delivered by caesarean section. During the
treatment period decreased defecation and urination were observed in
high-dose animals and body-weight and food consumption were
decreased. A significant increase in body-weight and mean food
consumption was observed from gestation day 20-29. The number of
early resorptions was slightly increased and the number of viable
fetuses was slightly lower at the highest dose. At 200 mg/kg bw/day,
a slightly increased incidence of the number of fetuses with
unossified hyoid body and/or arches (marginal at 50 mg/kg bw/day)
and of fetuses with reduced ossification of the skull was observed.
No teratogenic effects were observed. The NOAEL in this study for
maternal and/or embryotoxicity was 50 mg/kg bw/day (Nemec et al.,
1985).
Special studies on mutagenicity
A number of genotoxicity tests have been carried out with
penconazole. The results are summarized in Table 2.
Special studies on skin and eye irritation and skin sensitization
Penconazole (purity 88.4%) produced slight erythema in 3 male
and 3 female New Zeeland white rabbits when applied under occlusive
conditions to the shaven intact and abraded skin for 24 hours, which
disappeared within 48 h (Ullmann & Gfeller, 1980b).
Penconazole (0.1 mg) was inserted into the conjuctival sac of
the left eye of nine New Zeeland white rabbits. At 30 seconds after
treatment, the eyes of 3/9 rabbits were washed with physiological
saline. In unrinsed eyes slight damage of the cornea and slight
conjunctival redness and chemosis were observed up to 7 days after
treatment. Rinsed eyes were normal 4 days after exposure. Two
rabbits died during the experiment. Bloody exudate in the pleural
and abdominal cavities was observed at autopsy (Ullmann & Gfeller,
1980a).
In a similar study, nine New Zeeland white albino rabbits were
given doses of 100 mg of undiluted penconazole (purity not given)
into the conjunctival sac of the left eye. Three of nine eyes were
washed. Conjunctival redness, chemosis and discharge was observed in
all animals at one hour after application which lasted up to 7 days
after treatment. The iris showed swelling but these changes
disappeared within 24 h. Corneal opacity and positive fluorescein
staining occurred but did not occur in any animal at 48 h (washed
eyes) and on day 7 (non-washed eyes) (Kuhn, 1988).
Penconazole (purity 88.4%) was tested for skin sensitization in
10 male and 10 female Pirbright white guinea-pigs in an optimization
test. No sensitization was observed when the guinea-pigs received 10
intracutaneous injections (0.1 ml of a 0.1% suspension of
penconazole in propylene glycol) followed 14 days later by a single
challenge injection (0.1 ml of 0.1% suspension of penconazole in
propylene glycol) (Ulmann & Gfeller,1980c).
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vitro
Ames testa,b S. typhimurium 250-20250 µg/ml in 92.8 negative Arni & Müller (1980)
TA100, TA98 DMSO;a > 6750 µg/ml
TA1535, TA1537 toxic, and b 20250
µg/ml toxic
Ames testa,b S. typhimurium 100-25600 µg/ml in 91.7 negative c Deparade & Arni (1984)
TA98, TA100 acetone
TA1535, TA1537
Yeast testa,b S. cerevisiae 0.1-40 µg/ml in DMSO 91.7 negative Arni & Müller (1983)
(mitotic gene D7
conversion)
Transformation mouse 2.625, 5.25, 10.5, 91.7 negative c Beilstein & Müller (1984a)
assay embryofibroblasts 21.0 and 42.0 µg/ml
BALB/3T3 in DMSO
Mouse lymphoma L5178Y mouse (a):6.75-100 µg/ml, 91.7 negative c Beilstein & Müller (1984b)
assay a,b lymphoma cells 100 µg/ml toxic;
(TK+/-) (b):8.25-110 µg/ml,
> 110 µg/ml toxic;
both in DMSO
DNA repair human fibroblasts 0, 0,32, 1.6 and 91.7 negative c Puri & Müller (1983)
test 40 µg/ml.
DNA repair rat hepatocytes 0, 032, 1.6, 8.0, 91.7 negative c Puri & Müller (1984)
test 40 µg/ml
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vivo
Host mediated S. typhimurium 0, 350, 700 and 1400 91.7 negative Arni & Müller (1984)
assay (intra TA98, TA100 and mg/kg orally
sanguine) TA1535
in male NMRI mice
Host mediated L 5178Y-cells 0, 813 mg/kg bw 91.7 negative Strasser & Müller (1982)
assay in DBA/Bom/SPF orally
mice
Nucleus anomaly Chinese hamster 0, 417, 834 and 1668 92.8 negative c Hool & Langauer (1982)
test bone marrow cells mg/kg orally in PEG 400
per day on 2 consecutive
days
Sister chromatid Chinese hamster 0, 417, 834 and 1668 91.7 negative c Hool & Arni (1983a)
exchange assay bone marrow mg/kg orally in PEG 400
Chromosome NMRI mice 0, 91, 272 and 816 mg/kg 91.7 negative Hool & Arni (1983b)
aberration assay spermatogonia bw orally in PEG
400 per day on 5
consecutive days; at
816 mg/kg
all mice died
Dominant lethal Male NMRI mice 0, 272 and 816 mg/kg 91.7% negative Hool & Arni (1983c)
assay bw orally
a without metabolic activation
b with metabolic activation
c positive controls yielded positive result(s)
COMMENTS
Penconazole administered orally to mice and rats was rapidly
absorbed and excreted, predominantly in the urine. Female rats
eliminated more in the urine and less in the faeces than male rats.
Very low residues were found in organs and tissues in both males and
females.
Numerous metabolites were identified in urine and faeces. The
major metabolic pathways involved oxidation of the pentyl side chain
to alcohols and acids with sequential cleavage of the terminal
carbon. As the oxidation products were conjugated, the resulting
metabolic patterns were complex. More polar and conjugated products
were found in female rats. Cleavage of the alkyl bridge between the
two rings led to the formation of 1,2,4-triazole, which was a major
metabolic route in male rats.
Penconazole showed low acute oral toxicity to mice, rats and
hamsters, but was slightly more toxic to rabbits. The World Health
Organization has classified penconazole as unlikely to present acute
hazard in normal use (WHO, 1992).
Short-term studies with mice, rats and dogs indicated that the
liver was the primary target organ. In a special study with male
rats, induction of microsomal liver enzymes was demonstrated. In a
13-week study in mice, increased liver weight and liver hyperthrophy
were observed at 500, 1000 and 2400 ppm. The NOAEL was 300 ppm,
equivalent to 45 mg/kg bw/day.
In three 90-days studies performed with rats, liver weight and
liver histopathology were the main effects, except for the second
study. In the first study, increase in relative liver weight was
observed at all dietary levels (30, 300 or 3000 ppm), while in the
third study (dietary levels of 10, 100, 300, 500, 1000 or 2400 ppm)
the NOAEL was 300 ppm, equivalent to 15 mg/kg bw/day. In the second
study (dietary levels of 10, 30 or 100) some biochemical parameters
were affected at 30 and 100 ppm. The NOAEL was 10 ppm, equal to 0.8
mg/kg bw/day.
In a one-year study in dogs, increased liver weights and
histopathological liver effects were observed at dietary
concentrations of 500 and 2500/5000 ppm. At the highest dose level
reduced testis weight and athrophic changes were also observed. The
NOAEL was 100 ppm, equal to 3.0 and 3.3 mg/kg bw/day for males and
females, respectively.
In a two-year feeding study in mice (dietary concentrations of
0, 5, 75, 150 or 300 ppm) increased liver weight at interim kill was
seen at 300 ppm. The NOAEL in this study was 150 ppm, equal to 19.3
mg/kg bw/day for males and 17.2 mg/kg bw/day for females. In a long-
term study in rats (dietary concentrations of 0, 5, 75, 150 or 300
ppm) liver weight was increased at 150 and 300 ppm. The NOAEL was 75
ppm, equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively. Penconazole was not carcinogenic in mice or rats.
Two two-generation reproduction studies in rats were reviewed.
In the first study (dietary concentrations of 0, 80, 400 or 2000
ppm), mortality and delayed parturation were observed, as well as
decreased body-weight gain of parents and pups and increased
relative liver weights in parents and pups at 2000 ppm. Hyperthrophy
of liver cells was found at 400 and 2000 ppm. The NOAEL was 80 ppm,
equal to 5.5-6.5 mg/kg bw/day for males and 7.5-8.5 mg/kg bw/day for
females. In the second study (dietary concentrations of 0, 25, 250
or 2500 ppm), liver weights were not determined. The main effect at
2500 ppm was reduced body-weight gain of parents and pups and
mortality of pups during lactation. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
Embryotoxicity/fetotoxicity was observed in three
teratogenicity studies with rats. In two studies (gavage doses of 0,
30, 100 or 300 mg/kg bw/day in one study and 0, 300 or 450 mg/kg
bw/day in the other), maternal toxicity, an increased number of
resorptions, decreased pup weight and delayed ossification were
observed at the high doses. Maternal toxicity or embryotoxicity were
not observed at 30 mg/kg bw/day. In the third study (gavage doses of
0, 5, 100 or 500 mg/kg bw/day), the NOAEL was 100 mg/kg bw/day. In
two teratogenicity studies with rabbits (doses of 0, 25, 75 or 150
mg/kg bw/day in one study and 0, 10, 50 or 200 mg/kg bw/day in the
other), maternal body-weight and food consumption were reduced and
the number of early resorptions was increased at the highest doses.
Overall, the NOAEL was 75 mg/kg bw/day for both maternal toxicity
and embryotoxicity.
After reviewing the available in vitro and in vivo short-
term genotoxicity data, the Meeting concluded that penconazole was
not genotoxic.
An ADI was allocated on the basis of the NOAEL determined from
the one-year study in dogs, which was supported by the NOAEL from
the long-term study in rats. A safety factor of 100 was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 17 mg/kg bw/day (two-year study)
Rat: 75 ppm, equal to 3.8 mg/kg bw/day (two-year study)
80 ppm, equal to 5.5 mg/kg bw/day (two-generation
reproduction study
30 mg/kg bw/day (teratology study)
Rabbit: 75 mg/kg bw/day (teratology study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arni, P. & Müller, D. (1980) Salmonella/mammalian-microsome
mutagenicity test with CGA 71818 (test for mutagenic properties in
bacteria). Unpublished report experiment No. 800550 dated 29
September 1980 from Ciba-Geigy Protection of Health and Environment,
Toxicology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Arni, P. & Müller, D. (1983) Saccharomyces cerevisiae
D7/mammalian-microsome mutagenicity test in vitro with CGA 71818.
Unpublished report experiment No. 811560 dated 27 June 1983 from
Ciba Geigy, Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Müller, D. (1984) Intrasanguine host-mediated assay with
S. typhimurium. Unpublished report experiment No. 830749 dated 3
May 1984 from Ciba Geigy, Protection of Health and Environment, GU
2.3, Experimental Pathology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Basler, W., Gfeller, W., Zak, F., Zakova, N. & Grieve, A.P. (1982)
CGA 71818: 3 months toxicity study in rats. Unpublished final
report, GU project No. 801194 dated 24 February 1982 from Ciba-Geigy
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Komarek, J., Zak, F. & Beri, J. (1983) CGA 71818: 3
month toxicity study in rats. Unpublished final report GU project
No. 821054 dated 17 August 1983 from Ciba-Geigy Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd. Basle, Switzerland.
Basler, W., Komarek, J., Zak, F. & Skorpil, V. (1984) CGA 71818: 28-
day oral cumulative toxicity study in rats. Unpublished final
report, GU project No. 820822 dated 28 June 1984 from Ciba-Geigy,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Gretener, P., Zak, F. & Krinke, G. (1985a) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in mice.
Unpublished final report GU project No. 811414 dated 1 July 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Basler, W., Gretener, P., Zak, F. & Naylor, D.C. (1985b) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in rats.
Unpublished final draft GU project no.: 811415 d.d. 25 June 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980a) Acute oral LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800553 dated 28
May 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980b) Acute dermal LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800559 dated 19
March 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980c) Acute oral LD50 in the Chinese
hamster of technical CGA 71818. Unpublished report project No.
800555 dated 19 March 1980 from Ciba-Geigy, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Beilstein, P. & Müller, D. (1984a) BALB/3T3 cell transformation
assay with CGA 71818. Unpublished report test No. 830755 dated 6
September 1984 from Ciba-Geigy, Protection of Health and
Environment, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Beilstein, P. & Muller, D. (1984b) L5178Y/TK+- mouse lymphoma
mutagenicity test. CGA 71'818 tech. ( in vitro test for mutagenic
properties of chemical substances in mammalian cells). Proj. No.:
811524. Unpublished report dated 9 October 1984 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Deparade, E. & Arni, P. (1984) Salmonella/mammalian-microsome
mutagenicity test. Unpublished report project No. 839759 dated 20
February 1984 from Ciba-Geigy Protection of Health and Environment,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H. & Giese, K. (1981) Report on CGA 71818 tech. teratology
study (seg.II) in rats. Unpublished report test No. 800549 d.d. 25
August 1981 from Ciba-Geigy, Reproductive Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983a) Report on CGA
71818 tecnical. 2-generation toxicity study in rats. Unpublished
report test No. 811416 from Ciba-Geigy, Toxicology GU 2,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983b) Two-generation
reproduction study in rats with CGA 71818 technical. Additional data
submission. Unpublished report test No. 811416 from Ciba-Geigy,
Experimental Toxicology, Sisseln, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Gfeller, W., Zak, F. & Naylor, D.C. (1984) CGA 71818 12 month
toxicity study in dogs. Unpublished final report GU project No.
801187 dated February 1984 from Ciba-Geigy, GU2 Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Giese, K. & Suter, H.P. (1982) Report on CGA 71818 tech. Teratology
study in rabbits. Unpublished report test No. 811354 dated March
1982 from Ciba-Geigy Reproductive Toxicology GU 2.1, Sisseln,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hamböck, H. (1980) Distribution, degradation and excretion of CGA
71818 in the rat. Unpublished project report 41/80 dated 6 November
1982 from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1982) The major urinary metabolites of CGA 71818 in the
rat. Unpublished project (status) report 15/82 dated 17 May 1982
from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1984) The metabolic fate of CGA 71818 in the rat.
Unpublished project report 23/83 dated 10 May 1984 from Ciba-Geigy,
AG 2.52, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hamböck, H. (1985) Sex dependency of the metabolite pattern of CGA
71818 after oral administration to rats. Addendum to project report
41/80. Unpublished report 1/85 dated 11 January 1985 from Ciba-Geigy
AG 2.52 Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hartmann, H.R. & Gfeller, W. (1987) CGA 71818 tech. Acute aerosol
inhalation toxicity in the rat. Unpublished final report GU project
No. 871169 d.d. 6 August 1987 from Ciba-Geigy Ltd, GU 2 Toxicology,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987a) CGA 71818 technical. Kinetic study in albino
rats with CGA 71818 technical. Unpublished report HLA 6117-122 dated
24-2-1987 from Hazleton Laboratories America, Inc. Madison,
Wisconsim 53704. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987b) 90-Day subchronic dietary toxicity and kinetic
study in albino mice with CGA-71818 technical. Unpublished report
HLA 6117-121 dated 4-3-1987 from Hazleton Laboratories America, Inc.
Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hiles, R.A. (1987c) 90-Day subchronic dietary toxicity and kinetic
study in albino rats with CGA-71818 technical. Unpublished report
HLA 6117-120 dated 14-04-1987 from Hazleton Laboratories America,
Inc. Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Arni, P. (1983a) Sister chromatid exchange study CGA
71818 Chinese hamster. Unpublished report dated 2-06-1983 project
No. 811523 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983b) Chromosome studies in male germinal
epithelium CGA 71818 mouse. Unpublished report dated 17-06-1983
project No. 811520 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983c) Dominant lethal test, mouse 8 weeks CGA
71818. Unpublished report dated 2-12-1983 project No. 811519 from
Ciba-Geigy Ltd., Protection of Health and Environment, Experimental
Pathology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Langauer, M. (1982) Nucleus anomaly test in somatic
interphase nuclei. CGA 71818 Chinese hamster. Unpublished report
dated 20-1-1982 experiment No. 800551 from Ciba-Geigy Ltd,
Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Kobel, W. (1981) Acute oral LD50 in the rabbit of technical CGA
71818. Unpublished report dated 4-3-1981, project No. 800554 from
Ciba-Geigy Ltd., Exp. Toxicology Sisseln, Switzerland, Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1988) Primary eye irritation study in rabbits EPA
guidelines No. 81-4. Unpublished report dated 31-03-1988 project
no.: 5303-88 from Stillmeadow Inc. Houston, Texas, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
LeVan, L.W. (1987) Acute kinetic study with CGA-71818 technical in
albino rats. Unpublished report dated 2-03-1987 project No. HLA
6117-123 from Hazleton Laboratories America Inc. Madison, Wisconsin,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988a) CGA-71818 Metabolism in hens - a
summary. Unpublished report No. ABR-88009 dated 20-05-1988 from
Ciba-Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988b) CGA-71818 Metabolism in goats - a
summary. Unpublished report No. ABR-88008 d.d.20-05-1988 from Ciba-
Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Nemec, M.D. & Keets, S.A., Leist, P.L. & Mercieca, M.D. (1985) A
teratology study (segment II) in albino rabbits with CGA-71818
technical. Unpublished report No. WIL-82004 dated 31-07-1985 from
WIL Research Laboratories Inc., Ashland, Ohio, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Müller, D. (1983) Autoradigraphic DNA repair test on
human fibroblast CGA 71818 . Unpublished report dated 2-12-1983
project No. 811657 from Ciba-Geigy Ltd.,Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Muller, D. (1984) Autradiographic DNA repair test on rat
hepatocytes. CGA 71'818 ( in vitro test for DNA-damaging
properties). Proj. No.: 811522. Unpublished report dated 30 January
1984 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Salamon, C.M. (1985) CGA-71818 technical teratology study in rats.
Unpublished report No. 450-2087 dated 16-09-1985 from American
Biogenics Corp., Decatur, Illinois, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sarasin, G. (1980) Report on acute oral LD50 in the mouse of CGA
71818 technical. Unpublished report No. 800552 dated 20-10-1980 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Schardein, J.L. (1987) Two-generation reproduction study in albino
rats with CGA 71818. Unpublished report No. 382-119 dated 14-08-1987
from International Research and Development Corp., Mattawan,
Michigan, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Seiffert, G., Komarek, J., Zak, F., Malik, C. & Zakova, N. (1983)
CGA 71818 21 day repeated dose dermal toxicity study in rabbits.
Unpublished report dated September 1983, project No. 820206 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Strasser, F.F. & Müller, D. (1982) Point mutation assay with mouse
lymphoma cells. Host-mediated assay with CGA 71818. Unpublished
report project No. 811355 dated 5-01-1982 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980a) Report on eye irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800557 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland.Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980b) Report on skin irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800558 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980c) Report on skin sensitizing
(contact allergenic) efect in guinea-pigs of technical CGA 71818.
Unpublished report project No. 800560 dated 12-06-1980 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Van Dijk, A. (1987) (U-14C) phenyl CGA 71818: Absorption,
distribution, excretion and metabolism after single oral and
repeated oral administration to the rat. Unpublished report project
No. 075666 dated 11-06-1987 from RCC Umweltchemie AG, Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Stäubli, W. (1985) The effect of
penconazole on drug metabolizing enzymes in the livers of male rats
and mice. Unpublished report dated April 1985 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Metabolites 9, 10, 11 and 12 were mainly found as glucuronides
or other conjugates.
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of penconazole
Species Sex Route LD50 LC50 Purity Reference
mg/kg bw mg/m3
Mouse M&F oral 2444 88.4 Sarasin (1980)
Rat M&F oral 2125 88.4 Bathe & Gfeller (1980a)
M&F dermal >3000 88.4 Bathe & Gfeller (1980b)
M&F inhal >4046 96.1 Hartmann & Gfeller (1987)
Chinese hamster M&F oral >5000 88.4 Bathe & Gfeller (1980c)
Rabbit M&F oral 971 88.4 Kobel (1981)
Penconazole shows low acute oral toxicity to mice, rats and
hamsters, but is slightly more toxic to rabbits.
Short-term toxicity studies
Mice
Groups of Cr:CD(ICR)BR mice (15/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm penconazole
(purity 98.7%) equivalent to 0, 1.5, 15, 45, 75, 150 or 360 mg/kg
bw/day for 13 weeks. Histopathology was only reported for the liver.
No treatment-related effects were observed on clinical signs,
mortality, ophthalmoscopy, food consumption, food efficiency,
haematology or urinalysis. Body-weight was lower at 2400 ppm.
Cholesterol levels were decreased at 1000 and 2400 ppm, ALAT
increased in high-dose males and total protein and albumin were
decreased in females at 2400 ppm. Relative liver weight was
significantly increased at 500, 1000 and 2400 ppm. An increased
incidence of centrilobular hypertrophy of the liver was observed in
males at doses > 500 ppm and in females at the highest dose.
Hepatic degeneration and vacuolisation was also observed in high-
dose males. Coagulative necrosis in the liver was observed in males
at 1000 and 2400 ppm. The NOAEL in this study was 300 ppm in the
diet, equivalent to 45 mg/kg bw/day (Hiles 1987b).
Rats
Groups of Tif:RAIf(SPF) rats (10/sex/group) were orally
administered 0, 20, 100 or 500 mg penconazole (purity 91.7%)/kg
bw/day for 28 days. Since no marked symptoms of toxicity showed up
during the first week of treatment the doses were increased to 100,
500 or 1000 mg/kg bw/day on day 8 of treatment. After increasing the
doses, 3 male and 3 female high-dose rats showed marked apathia and
lateral body position at day 10 but the animals recovered on day 11.
No effects were observed on mortality, ophthalmic or hearing
examinations. In the mid- and high-dose groups male mean body weight
was decreased, food consumption and food conversion were decreased
in males and females, haemoglobin and haematocrit values were
decreased in females and ALP and ALAT as well as cholesterol and
total proteins were increased in males and females and total
globulin was increased and A/G ratio was decreased in females.
Phosphate and glucose were increased in high-dose females. Albumin
increased in high-dose males and females. Urine volume was increased
in males at the highest dose and in females at the mid- and high-
dose. Relative liver, kidney and adrenal weight was increased in
mid- and high-dose males and females. Relative brain, spleen and
thyroid weight were increased in high-dose females. Enlarged livers
with slight hypertrophy of the hepatocytes were observed in 8/10 and
3/10 males and females at the mid-dose and in all animals at the
highest dose. The lowest dose (20-100 mg/kg bw/day) was considered
as the NOAEL (Basler et al., 1984).
Groups of Tif:RAIf(SPF) rats (20/sex/group) were fed diets
containing 0, 30, 300 or 3000 ppm (equal to 2.1, 19.4 or 202.3 mg
penconazole/kg bw/day for males and 2.1, 20.7 or 208.6 mg
penconazole/kg bw/day for females, respectively) (purity 91.7%) for
3 months. No effects were observed on clinical signs, mortality,
food and water consumption, food conversion, hearing tests or eye
examinations. Body weight was decreased in males and females at the
highest dose. An increase in total plasma protein and albumin was
observed in male rats at all doses and a trend to an increase in
total plasma proteins was seen in females at 3000 ppm. GGT was
slightly increased in females at 300 and 3000 (significantly) ppm.
Cholesterol levels were increased in both sexes at 3000 and also in
females at 300 ppm. A significant dose-related increase in relative
liver weight was observed at all dose levels. Relative testes
weights were increased in males at 3000 ppm and brain, kidney and
thymus weight were increased in high dose females. At histopathology
minimal hypertrophy of the hepatocytes was seen in 20/20 male rats
and 9/20 female rats at 3000 ppm. In this study no NOAEL was
identified, although the liver weight increase at 30 ppm was
marginal (Basler et al., 1982).
In another 3 month feeding study groups of Tif:RAIf(SPF) rats
(20/sex/group) were fed diets containing 0, 10, 30 or 100 ppm
penconazole (purity 91.7%) (equal to 0.8, 2.1 or 7.1 mg
penconazole/kg bw/day for males and 0.8, 2.1 or 7.3 mg
penconazole/kg bw/day for females, respectively). No effects were
observed on clinical signs, mortality, body-weight, food and water
consumption, food conversion, eye examinations, hearing tests, organ
weight or histopathology. Total proteins were increased in male and
female rats at 30 and 100 ppm. Total globulin was increased in high
dose females. GGT was slightly increased in females at both 30 and
100 ppm. Liver weight was increased at 10 and 30 ppm in male rats
only, but not at 100 ppm. No liver histopathological effects were
observed. The NOAEL in this study was 10 ppm penconazole, equal to
0.8 mg/kg bw/day (Basler et al., 1983).
In an additional study groups of albino [Crl:CD(SD)BR] rats
(15/sex/group) were fed diets containing 0, 10, 100, 300, 500, 1000
or 2400 ppm penconazole (purity 98,7%) equivalent to 0.5, 5, 15, 25,
50 or 120 mg penconazole/kg bw/day for 90 days. In this study only
the liver as target organ was studied histopathologically.
Observations included clinical signs mortality, body-weight, food
consumption, food efficiency, ophthalmoscopy, haematology, clinical
chemistry (including GGT), urinalysis, organ weights, macroscopy and
histopathology. Female body weight gain was lower at 500, 1000 and
2400 ppm and food consumption was decreased at the highest dose.
Urea nitrogen was increased in males at all dose levels, but not
clearly dose-related. Relative liver weight was significantly
increased in rats at 500 (females only), 1000 and 2400 ppm. Relative
kidney weight was increased in rats at 2400 ppm. At histopathology
the incidence of vacuolization, degeneration and centrilobular
hyperthrophy of hepatocytes was increased at 500 ppm and above. The
NOAEL in this study was 300 ppm, equivalent to 15 mg/kg bw/day
(Hiles, 1987c).
Rabbits
Groups of New Zeeland white rabbits (5/sex/group) were given
1000, 1500 or 2000 mg penconazole/kg bw/day (purity 91.7%) by dermal
application once/day for 5 days/week for 21 days. Two satellite
groups received 0 or 2000 mg penconazole/kg bw/day for 21 days
followed by a recovery period of 14 days. Transient signs of
dyspnea, curved body position and ruffled fur were observed at all
dose levels including control rabbits, but symptoms disappeared
during the recovery period. No dose-related effects were observed on
mortality, body-weight, food consumption and food conversion,
haematology, clinical chemistry, macroscopy and histopathology. The
NOAEL in this study was > 2000 mg/kg bw/day (Seifert et al.,
1983).
Dogs
Groups of beagle dogs (10/sex/group) were fed diets containing
0, 100, 500, or 5000/2500 ppm penconazole (purity 91.7%), equal to
3.0, 16.9 or 133 mg penconazole/kg bw/day for males and 3.3, 16.7 or
139 mg penconazole/kg bw/day for females, respectively, for 12
months. Due to poor feed intake the highest dose was lowered to 2500
ppm in week 20. An interim kill on 4 dogs/sex/group was carried out
at week 14 and 2 dogs/sex/group were kept for a 4-week recovery
period. Observations included clinical signs, mortality, body-
weight, food consumption, food conversion, ophthalmoscopy, auditory
perception, haematology, blood chemistry, urinalysis, macroscopy,
organ weights and histopathology.
An increased incidence of vomiting was observed in high-dose
dogs during the first three months of treatment; thereafter
occasionally vomiting was observed only in females. The high-dose
dogs lost body-weight and had a reduced food consumption during the
first months. After lowering the dosage food consumption and male
bodyweights were comparable to the controls but the mean weight of
the females remained lower than the control value. ASAT, ALP, OCT,
GGT and ALAT activities were increased in dogs at the highest dose
during the whole administration period. Total globulin concentration
was increased in males at the highest dose during weeks 13, 26 and
53, consequently the total protein was slightly increased and the
A/G ratio was decreased. Terminal body-weight of high-dose male and
female dogs were decreased after 3 months. At this time relative
liver weights were increased at 500 and 5000 ppm. Relative kidney
weight was increased at the highest dose in both males and females.
Relative brain and adrenal weight were increased and relative heart
weight was decreased in high-dose females. All males at 5000 ppm
showed a reduction in testes weights and atrophy of the seminiferous
epithelium. At macroscopy emaciation was observed in dogs at the
highest dose after 3 months. At histopathology after 3 months,
minimal lesions characterized by monocellular hepatocyte necrosis
associated with inflammatory cell infiltration were noticed in
nearly all interim sacrificed dogs at the highest dose and in one
male dog at 500 ppm. After 12 months, testes weights were reduced in
males at 2500 ppm and liver weight was increased in females at 500
and 2500 ppm. At histopathology, bilateral tubular atrophy and
reduced spermatogenesis in the testes was observed in the high-dose
males and an increased incidence of inflammation with fibrosis in
the peripheral lobular region of the liver occurred in the mid- and
high-dose animals. The only abnormalities found after the recovery
period were decreased relative testes weights and tubular atrophy at
2500 ppm. The NOAEL in this study was 100 ppm, equal to 3.0 mg/kg
bw/day for males and 3.3 mg/kg bw/day for females (Gfeller et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Tif:MAGf(SPF) mice (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%),
equal to 0.75, 9.8, 19.3 or 40.8 mg/kg bw/day for males and 0.67,
8.8, 17.2 or 35.7 mg/kg bw/day for females, for 24 months. An
interim kill was carried out on 10 mice/sex/group at 52 weeks. No
effects were observed on mortality, clinical signs, body-weight,
food and water consumption, food conversion, ophthalmic and hearing
inspections, haematology, blood chemistry, urinalysis, macroscopy or
histopathology. Relative liver weight was increased in high-dose
males and females at the interim kill. At the end of the study
adrenal weight (not dose-related) and prostate weight were increased
in males at 75, 150 and 300 ppm. There was no treatment-related
increase in tumour incidence (Basler et al., 1985b). Since
weighing of prostate glands is not very reliable and the increased
weight was not found at 53 weeks, the Meeting concluded that this
effect was probably biologically insignificant. The NOAEL was
therefore 150 ppm, equal to 19.3 mg/kg bw/day for males and 17.2
mg/kg bw/day for females based on the increased liver weight.
Rats
Groups of Tif:RAIf(SPF) rats (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%) for
24 months. An interim kill was carried out on 10 rats/sex/group at
12 months. Twenty rats/sex/group were maintained for laboratory
investigations and sacrificed after 24 months. The remaining 50
rats/sex/group were sacrificed after 116/117 weeks. No effects were
observed on mortality, clinical signs, body-weight, food and water
consumption, food conversion, ophthalmic and hearing inspections,
haematology, blood chemistry, urinalysis, macroscopy or
histopathology. At the interim kill, liver weight was increased in
females at 150 and 300 ppm and pituitary weight was decreased in
high-dose males. A tendency to increased liver weight was still
observed in females at the highest dose at 104 weeks. Tumour
incidence was not enhanced. The NOAEL in this study was 75 ppm,
equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively (Basler et al., 1985b).
Reproduction studies
Rats
Penconazole (purity 91.7%) was administered in the diet to
groups of 20 Tif:RAIf(SPF) rats/sex at concentrations of 0, 80, 400
or 2000 ppm (continuously over a period of 110 days to the parental
generations (F0 and F1), including a 12-day mating period for
each generation at the age of 3 months. These dose levels were equal
to 5.5-6.5, 28.5-31 or 146-166 mg/kg bw/day for males and 7.5-8.5,
40-42.5 or 202-227 mg/kg bw/day for females during the whole period.
Parental rats were killed after weaning of the F1 and F2
generation, respectively. Observations included clinical signs,
body-weight, food consumption, fertility index, implantation rate,
litter size, live and dead fetuses, sex ratio, organ weight,
macroscopy and histopathology.
A slightly depressed body-weight gain and food consumption was
seen at the highest dose in F0 females. Parturation was delayed
and the average duration of pregnancy was 21.1, 21.2, 21.3 and 21.6
days for the 0, 80, 400 and 2000 ppm group. Four dams died, two (1
at 400 and 1 at 2000 ppm) at or a short time after parturation, one
(at 2000 ppm) after 4 days and one (at 2000 ppm) after 11 days.
Testes weights of F0 males were slightly increased at 2000 ppm
(liver weight was not determined).
Body-weight gain of F1 pups was slightly reduced at 2000 ppm.
The activity index (ELP, exploratory locomotion pattern) was
slightly but not significantly depressed at 400 and 2000 ppm; there
was however no effect on general development and behaviour. At 2000
ppm, relative liver weight was increased in male and female F1
pups and brain and ovary weight was increased in F1 female pups.
Body-weight gain and food consumption of F1 parents was slightly
reduced at 2000 ppm. One dam died at 400 ppm and 2 at 2000 ppm at or
some days after parturation. The average pregnancy duration was
increased at the highest dose (21.3, 21.4, 21.2 and 21.8 days at 0,
80, 400 and 2000 ppm, respectively). At 2000 ppm, relative liver
weight and relative ovary weight were increased in F1 parents,
while relative brain and testes weight were increased in F1 males.
A decreased implantation rate was observed at 2000 ppm.
The ELP was slightly but not significantly decreased in high-
dose F2 pups without any effect on general development and
behaviour. Relative liver weight was increased in high-dose F2
pups.
Histopathological examination of the livers of F1 adults
revealed slight hypertrophy of the hepatocytes mainly in the
centrilobular region in 17/20 males and in 16/16 females at 2000 ppm
and in 5/19 males and 14/16 females at 400 ppm. Additionally, small
recent necrosis in the liver was observed in 2/16 high-dose females.
No histopathological changes were seen in weanling rats from both
F1 and F2 generations. The NOAEL in this study was 80 ppm, equal
to 5.5-6.5 mg/kg bw/day and 7.5-8.5 mg/kg bw/day for males and
females, respectively (Fritz et al., 1983a; Fritz et al.,
1983b).
Groups of 30 Charles River COBS CD rats/sex were fed diets
containing 0, 25, 250 or 2500 ppm penconazole (purity not given).
After 63 days of treatment the rats were mated to start a two-
generation (1 litter/generation) study. The F1 parents (randomly
selected) were mated after 84 days of treatment. Only weights of
ovaries and testes were determined. At 2500 ppm body-weight and food
consumption were decreased in F0 females and body-weight was
decreased in F1 parents. Mating indices were slightly reduced in
the F0 and F1 generations at 2500 ppm, but fertility indices
were not affected. Reduced pup weight and an increase in the number
of stillborn pups or pups that died during the first days of
lactation was observed at the highest dose. Relative ovary weight
was significantly increased in females of the F0 and the F1
generation at 2500 ppm. The NOAEL in this study is 250 ppm,
equivalent to 12.5 mg/kg bw/day (Schardein, 1987)
Special studies on embryotoxicity and/or teratogenicity
Rats
Groups of 25 pregnant Tif:RAIf(SPF) female rats were
administered orally by intubation 0, 30, 100 or 300 mg penconazole
(purity 88.4%)/kg bw/day suspended in CMC from days 6 to 15 of
gestation. The dams were observed for clinical signs, body-weight
and food consumption and were killed at day 21 of gestation. The
number of total implantations, resorptions, live and dead fetuses
were recorded and the uterus was weighed. Fetuses were weighed,
sexed and examined for external, visceral and skeletal
malformations. Two dams at 300 mg/kg bw/day died shortly before
autopsy. Maternal bodyweight gain in the highest dose group was
slightly reduced. Food consumption was reduced in all experimental
groups. An increase in the numbers of unossified phalangeal nuclei
of the hind limb was observed at 100 and 300 mg/kg bw/day (not dose-
related). At the highest dose, acaudia (taillessness) was observed
in 1/182 fetuses, and the overall incidence of skeletal anomalies
including irregularly shaped sternebrae was increased at the highest
dose. The NOAEL for maternal and embryofetal toxicity was 30 mg/kg
bw/day.
In a supplementary study, 0 or 300 mg penconazole/kg bw/day was
administered by gavage to groups of 15 pregnant rats from days 6-15
of gestation and another group of 15 rats received 450 mg
penconazole/kg bw/day from days 10-14 of gestation. Body-weight gain
and food consumption were decreased at both dose levels. Four and 2
females died shortly before autopsy at 300 and 450 mg/kg bw/day,
respectively. The average weight of live fetuses was decreased at
300 and 450 mg/kg bw/day. The incidence of unossified phalangeal
nuclei of the forelimb was increased at 450 mg/kg bw/day and the
incidence of unossified phalangeal nuclei of the hind limbs and
calcaneus were increased at both 300 and 450 mg/kg bw/day. No
irregular ossification of sternebrae was observed at 300 mg/kg
bw/day. At 450 mg/kg bw/day an irregularly ossified sixth sternebrae
was recorded in one foetus (Fritz & Giese, 1981). The increased
incidence of these variants may indicate delayed fetal development,
but in the absence of any associated malformations should not be
considered as indicative of teratogenicity.
Groups of 25 pregnant Charles River rats were administered by
gavage 0, 5, 100, or 500 mg penconazole (purity 98.7%)/kg bw/day in
corn oil from days 6-15 of gestation. The females were observed for
clinical signs, body-weight and food consumption and sacrificed on
day 20 of gestation. The number of implantation sites, resorption
sites, corpora lutea were recorded. Fetuses were weighed, sexed and
examined for external, internal and visceral anomalies. Two high-
dose dams and 1 dam at 5 mg/kg bw/day died during the study. High-
dose dams exhibited damp fur, crusty eye, crusty muzzle, crusty
nose, and yellow/brown stained fur as well as staggered gait,
emaciated loose stools, weakness and/or lethargy (5/25) and clear
salivation (4/25). Body-weight and body-weight gain were
significantly decreased at the highest dose. Food consumption was
decreased from days 6-13 at 100 and 500 mg/kg bw/day. At the highest
dose, the number of early and late resorption sites was increased
and the number of viable fetuses as well as the fetal weight were
decreased. The number of runts and litters with runts as well as the
occurrence of cervical ribs was increased at 500 mg/kg bw/day. No
teratogenic effects were observed. The NOAEL for maternal and
embryofetal toxicity in this study was 100 mg/kg bw/day (Salamon,
1985).
Rabbits
Groups of 20 pregnant chinchilla rabbits received 0, 25, 75 or
150 mg penconazole (purity 91.7%)/kg bw/day orally by intubation
from day 6 to 18 of pregnancy. Observations included clinical signs,
body-weight gain, food consumption. Dams were killed on day 28 of
pregnancy and fetuses were removed by caesarean section. No effects
were observed on the number of corpora lutea, total implantations,
early and late resorptions, live and dead fetuses, sex ratio uterus
and fetal weight, external and visceral examination and skeletal
malformations.
At 150 mg/kg bw/day average food consumption was slightly
reduced and the number of early resorptions was slightly but not
signficantly increased. At the highest dose,one fetus with
microphthalmia and one fetus showing microphthalmia associated with
internal hydrocephaly were seen. The NOAEL for maternal toxicity and
for embryotoxicity was 75 mg/kg bw/day (Giese & Suter, 1982).
Groups of 20 pregnant New Zeeland white rabbits were orally
dosed by gavage with 0, 10, 50 or 200 mg penconazole/kg bw/day from
days 7 to 19 of gestation. Observation included clinical signs,
body-weight and food consumption. All dams were killed on gestation
day 29 and fetuses were delivered by caesarean section. During the
treatment period decreased defecation and urination were observed in
high-dose animals and body-weight and food consumption were
decreased. A significant increase in body-weight and mean food
consumption was observed from gestation day 20-29. The number of
early resorptions was slightly increased and the number of viable
fetuses was slightly lower at the highest dose. At 200 mg/kg bw/day,
a slightly increased incidence of the number of fetuses with
unossified hyoid body and/or arches (marginal at 50 mg/kg bw/day)
and of fetuses with reduced ossification of the skull was observed.
No teratogenic effects were observed. The NOAEL in this study for
maternal and/or embryotoxicity was 50 mg/kg bw/day (Nemec et al.,
1985).
Special studies on mutagenicity
A number of genotoxicity tests have been carried out with
penconazole. The results are summarized in Table 2.
Special studies on skin and eye irritation and skin sensitization
Penconazole (purity 88.4%) produced slight erythema in 3 male
and 3 female New Zeeland white rabbits when applied under occlusive
conditions to the shaven intact and abraded skin for 24 hours, which
disappeared within 48 h (Ullmann & Gfeller, 1980b).
Penconazole (0.1 mg) was inserted into the conjuctival sac of
the left eye of nine New Zeeland white rabbits. At 30 seconds after
treatment, the eyes of 3/9 rabbits were washed with physiological
saline. In unrinsed eyes slight damage of the cornea and slight
conjunctival redness and chemosis were observed up to 7 days after
treatment. Rinsed eyes were normal 4 days after exposure. Two
rabbits died during the experiment. Bloody exudate in the pleural
and abdominal cavities was observed at autopsy (Ullmann & Gfeller,
1980a).
In a similar study, nine New Zeeland white albino rabbits were
given doses of 100 mg of undiluted penconazole (purity not given)
into the conjunctival sac of the left eye. Three of nine eyes were
washed. Conjunctival redness, chemosis and discharge was observed in
all animals at one hour after application which lasted up to 7 days
after treatment. The iris showed swelling but these changes
disappeared within 24 h. Corneal opacity and positive fluorescein
staining occurred but did not occur in any animal at 48 h (washed
eyes) and on day 7 (non-washed eyes) (Kuhn, 1988).
Penconazole (purity 88.4%) was tested for skin sensitization in
10 male and 10 female Pirbright white guinea-pigs in an optimization
test. No sensitization was observed when the guinea-pigs received 10
intracutaneous injections (0.1 ml of a 0.1% suspension of
penconazole in propylene glycol) followed 14 days later by a single
challenge injection (0.1 ml of 0.1% suspension of penconazole in
propylene glycol) (Ulmann & Gfeller,1980c).
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vitro
Ames testa,b S. typhimurium 250-20250 µg/ml in 92.8 negative Arni & Müller (1980)
TA100, TA98 DMSO;a > 6750 µg/ml
TA1535, TA1537 toxic, and b 20250
µg/ml toxic
Ames testa,b S. typhimurium 100-25600 µg/ml in 91.7 negative c Deparade & Arni (1984)
TA98, TA100 acetone
TA1535, TA1537
Yeast testa,b S. cerevisiae 0.1-40 µg/ml in DMSO 91.7 negative Arni & Müller (1983)
(mitotic gene D7
conversion)
Transformation mouse 2.625, 5.25, 10.5, 91.7 negative c Beilstein & Müller (1984a)
assay embryofibroblasts 21.0 and 42.0 µg/ml
BALB/3T3 in DMSO
Mouse lymphoma L5178Y mouse (a):6.75-100 µg/ml, 91.7 negative c Beilstein & Müller (1984b)
assay a,b lymphoma cells 100 µg/ml toxic;
(TK+/-) (b):8.25-110 µg/ml,
> 110 µg/ml toxic;
both in DMSO
DNA repair human fibroblasts 0, 0,32, 1.6 and 91.7 negative c Puri & Müller (1983)
test 40 µg/ml.
DNA repair rat hepatocytes 0, 032, 1.6, 8.0, 91.7 negative c Puri & Müller (1984)
test 40 µg/ml
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vivo
Host mediated S. typhimurium 0, 350, 700 and 1400 91.7 negative Arni & Müller (1984)
assay (intra TA98, TA100 and mg/kg orally
sanguine) TA1535
in male NMRI mice
Host mediated L 5178Y-cells 0, 813 mg/kg bw 91.7 negative Strasser & Müller (1982)
assay in DBA/Bom/SPF orally
mice
Nucleus anomaly Chinese hamster 0, 417, 834 and 1668 92.8 negative c Hool & Langauer (1982)
test bone marrow cells mg/kg orally in PEG 400
per day on 2 consecutive
days
Sister chromatid Chinese hamster 0, 417, 834 and 1668 91.7 negative c Hool & Arni (1983a)
exchange assay bone marrow mg/kg orally in PEG 400
Chromosome NMRI mice 0, 91, 272 and 816 mg/kg 91.7 negative Hool & Arni (1983b)
aberration assay spermatogonia bw orally in PEG
400 per day on 5
consecutive days; at
816 mg/kg
all mice died
Dominant lethal Male NMRI mice 0, 272 and 816 mg/kg 91.7% negative Hool & Arni (1983c)
assay bw orally
a without metabolic activation
b with metabolic activation
c positive controls yielded positive result(s)
COMMENTS
Penconazole administered orally to mice and rats was rapidly
absorbed and excreted, predominantly in the urine. Female rats
eliminated more in the urine and less in the faeces than male rats.
Very low residues were found in organs and tissues in both males and
females.
Numerous metabolites were identified in urine and faeces. The
major metabolic pathways involved oxidation of the pentyl side chain
to alcohols and acids with sequential cleavage of the terminal
carbon. As the oxidation products were conjugated, the resulting
metabolic patterns were complex. More polar and conjugated products
were found in female rats. Cleavage of the alkyl bridge between the
two rings led to the formation of 1,2,4-triazole, which was a major
metabolic route in male rats.
Penconazole showed low acute oral toxicity to mice, rats and
hamsters, but was slightly more toxic to rabbits. The World Health
Organization has classified penconazole as unlikely to present acute
hazard in normal use (WHO, 1992).
Short-term studies with mice, rats and dogs indicated that the
liver was the primary target organ. In a special study with male
rats, induction of microsomal liver enzymes was demonstrated. In a
13-week study in mice, increased liver weight and liver hyperthrophy
were observed at 500, 1000 and 2400 ppm. The NOAEL was 300 ppm,
equivalent to 45 mg/kg bw/day.
In three 90-days studies performed with rats, liver weight and
liver histopathology were the main effects, except for the second
study. In the first study, increase in relative liver weight was
observed at all dietary levels (30, 300 or 3000 ppm), while in the
third study (dietary levels of 10, 100, 300, 500, 1000 or 2400 ppm)
the NOAEL was 300 ppm, equivalent to 15 mg/kg bw/day. In the second
study (dietary levels of 10, 30 or 100) some biochemical parameters
were affected at 30 and 100 ppm. The NOAEL was 10 ppm, equal to 0.8
mg/kg bw/day.
In a one-year study in dogs, increased liver weights and
histopathological liver effects were observed at dietary
concentrations of 500 and 2500/5000 ppm. At the highest dose level
reduced testis weight and athrophic changes were also observed. The
NOAEL was 100 ppm, equal to 3.0 and 3.3 mg/kg bw/day for males and
females, respectively.
In a two-year feeding study in mice (dietary concentrations of
0, 5, 75, 150 or 300 ppm) increased liver weight at interim kill was
seen at 300 ppm. The NOAEL in this study was 150 ppm, equal to 19.3
mg/kg bw/day for males and 17.2 mg/kg bw/day for females. In a long-
term study in rats (dietary concentrations of 0, 5, 75, 150 or 300
ppm) liver weight was increased at 150 and 300 ppm. The NOAEL was 75
ppm, equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively. Penconazole was not carcinogenic in mice or rats.
Two two-generation reproduction studies in rats were reviewed.
In the first study (dietary concentrations of 0, 80, 400 or 2000
ppm), mortality and delayed parturation were observed, as well as
decreased body-weight gain of parents and pups and increased
relative liver weights in parents and pups at 2000 ppm. Hyperthrophy
of liver cells was found at 400 and 2000 ppm. The NOAEL was 80 ppm,
equal to 5.5-6.5 mg/kg bw/day for males and 7.5-8.5 mg/kg bw/day for
females. In the second study (dietary concentrations of 0, 25, 250
or 2500 ppm), liver weights were not determined. The main effect at
2500 ppm was reduced body-weight gain of parents and pups and
mortality of pups during lactation. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
Embryotoxicity/fetotoxicity was observed in three
teratogenicity studies with rats. In two studies (gavage doses of 0,
30, 100 or 300 mg/kg bw/day in one study and 0, 300 or 450 mg/kg
bw/day in the other), maternal toxicity, an increased number of
resorptions, decreased pup weight and delayed ossification were
observed at the high doses. Maternal toxicity or embryotoxicity were
not observed at 30 mg/kg bw/day. In the third study (gavage doses of
0, 5, 100 or 500 mg/kg bw/day), the NOAEL was 100 mg/kg bw/day. In
two teratogenicity studies with rabbits (doses of 0, 25, 75 or 150
mg/kg bw/day in one study and 0, 10, 50 or 200 mg/kg bw/day in the
other), maternal body-weight and food consumption were reduced and
the number of early resorptions was increased at the highest doses.
Overall, the NOAEL was 75 mg/kg bw/day for both maternal toxicity
and embryotoxicity.
After reviewing the available in vitro and in vivo short-
term genotoxicity data, the Meeting concluded that penconazole was
not genotoxic.
An ADI was allocated on the basis of the NOAEL determined from
the one-year study in dogs, which was supported by the NOAEL from
the long-term study in rats. A safety factor of 100 was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 17 mg/kg bw/day (two-year study)
Rat: 75 ppm, equal to 3.8 mg/kg bw/day (two-year study)
80 ppm, equal to 5.5 mg/kg bw/day (two-generation
reproduction study
30 mg/kg bw/day (teratology study)
Rabbit: 75 mg/kg bw/day (teratology study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arni, P. & Müller, D. (1980) Salmonella/mammalian-microsome
mutagenicity test with CGA 71818 (test for mutagenic properties in
bacteria). Unpublished report experiment No. 800550 dated 29
September 1980 from Ciba-Geigy Protection of Health and Environment,
Toxicology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Arni, P. & Müller, D. (1983) Saccharomyces cerevisiae
D7/mammalian-microsome mutagenicity test in vitro with CGA 71818.
Unpublished report experiment No. 811560 dated 27 June 1983 from
Ciba Geigy, Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Müller, D. (1984) Intrasanguine host-mediated assay with
S. typhimurium. Unpublished report experiment No. 830749 dated 3
May 1984 from Ciba Geigy, Protection of Health and Environment, GU
2.3, Experimental Pathology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Basler, W., Gfeller, W., Zak, F., Zakova, N. & Grieve, A.P. (1982)
CGA 71818: 3 months toxicity study in rats. Unpublished final
report, GU project No. 801194 dated 24 February 1982 from Ciba-Geigy
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Komarek, J., Zak, F. & Beri, J. (1983) CGA 71818: 3
month toxicity study in rats. Unpublished final report GU project
No. 821054 dated 17 August 1983 from Ciba-Geigy Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd. Basle, Switzerland.
Basler, W., Komarek, J., Zak, F. & Skorpil, V. (1984) CGA 71818: 28-
day oral cumulative toxicity study in rats. Unpublished final
report, GU project No. 820822 dated 28 June 1984 from Ciba-Geigy,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Gretener, P., Zak, F. & Krinke, G. (1985a) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in mice.
Unpublished final report GU project No. 811414 dated 1 July 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Basler, W., Gretener, P., Zak, F. & Naylor, D.C. (1985b) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in rats.
Unpublished final draft GU project no.: 811415 d.d. 25 June 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980a) Acute oral LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800553 dated 28
May 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980b) Acute dermal LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800559 dated 19
March 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980c) Acute oral LD50 in the Chinese
hamster of technical CGA 71818. Unpublished report project No.
800555 dated 19 March 1980 from Ciba-Geigy, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Beilstein, P. & Müller, D. (1984a) BALB/3T3 cell transformation
assay with CGA 71818. Unpublished report test No. 830755 dated 6
September 1984 from Ciba-Geigy, Protection of Health and
Environment, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Beilstein, P. & Muller, D. (1984b) L5178Y/TK+- mouse lymphoma
mutagenicity test. CGA 71'818 tech. ( in vitro test for mutagenic
properties of chemical substances in mammalian cells). Proj. No.:
811524. Unpublished report dated 9 October 1984 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Deparade, E. & Arni, P. (1984) Salmonella/mammalian-microsome
mutagenicity test. Unpublished report project No. 839759 dated 20
February 1984 from Ciba-Geigy Protection of Health and Environment,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H. & Giese, K. (1981) Report on CGA 71818 tech. teratology
study (seg.II) in rats. Unpublished report test No. 800549 d.d. 25
August 1981 from Ciba-Geigy, Reproductive Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983a) Report on CGA
71818 tecnical. 2-generation toxicity study in rats. Unpublished
report test No. 811416 from Ciba-Geigy, Toxicology GU 2,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983b) Two-generation
reproduction study in rats with CGA 71818 technical. Additional data
submission. Unpublished report test No. 811416 from Ciba-Geigy,
Experimental Toxicology, Sisseln, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Gfeller, W., Zak, F. & Naylor, D.C. (1984) CGA 71818 12 month
toxicity study in dogs. Unpublished final report GU project No.
801187 dated February 1984 from Ciba-Geigy, GU2 Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Giese, K. & Suter, H.P. (1982) Report on CGA 71818 tech. Teratology
study in rabbits. Unpublished report test No. 811354 dated March
1982 from Ciba-Geigy Reproductive Toxicology GU 2.1, Sisseln,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hamböck, H. (1980) Distribution, degradation and excretion of CGA
71818 in the rat. Unpublished project report 41/80 dated 6 November
1982 from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1982) The major urinary metabolites of CGA 71818 in the
rat. Unpublished project (status) report 15/82 dated 17 May 1982
from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1984) The metabolic fate of CGA 71818 in the rat.
Unpublished project report 23/83 dated 10 May 1984 from Ciba-Geigy,
AG 2.52, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hamböck, H. (1985) Sex dependency of the metabolite pattern of CGA
71818 after oral administration to rats. Addendum to project report
41/80. Unpublished report 1/85 dated 11 January 1985 from Ciba-Geigy
AG 2.52 Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hartmann, H.R. & Gfeller, W. (1987) CGA 71818 tech. Acute aerosol
inhalation toxicity in the rat. Unpublished final report GU project
No. 871169 d.d. 6 August 1987 from Ciba-Geigy Ltd, GU 2 Toxicology,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987a) CGA 71818 technical. Kinetic study in albino
rats with CGA 71818 technical. Unpublished report HLA 6117-122 dated
24-2-1987 from Hazleton Laboratories America, Inc. Madison,
Wisconsim 53704. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987b) 90-Day subchronic dietary toxicity and kinetic
study in albino mice with CGA-71818 technical. Unpublished report
HLA 6117-121 dated 4-3-1987 from Hazleton Laboratories America, Inc.
Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hiles, R.A. (1987c) 90-Day subchronic dietary toxicity and kinetic
study in albino rats with CGA-71818 technical. Unpublished report
HLA 6117-120 dated 14-04-1987 from Hazleton Laboratories America,
Inc. Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Arni, P. (1983a) Sister chromatid exchange study CGA
71818 Chinese hamster. Unpublished report dated 2-06-1983 project
No. 811523 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983b) Chromosome studies in male germinal
epithelium CGA 71818 mouse. Unpublished report dated 17-06-1983
project No. 811520 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983c) Dominant lethal test, mouse 8 weeks CGA
71818. Unpublished report dated 2-12-1983 project No. 811519 from
Ciba-Geigy Ltd., Protection of Health and Environment, Experimental
Pathology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Langauer, M. (1982) Nucleus anomaly test in somatic
interphase nuclei. CGA 71818 Chinese hamster. Unpublished report
dated 20-1-1982 experiment No. 800551 from Ciba-Geigy Ltd,
Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Kobel, W. (1981) Acute oral LD50 in the rabbit of technical CGA
71818. Unpublished report dated 4-3-1981, project No. 800554 from
Ciba-Geigy Ltd., Exp. Toxicology Sisseln, Switzerland, Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1988) Primary eye irritation study in rabbits EPA
guidelines No. 81-4. Unpublished report dated 31-03-1988 project
no.: 5303-88 from Stillmeadow Inc. Houston, Texas, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
LeVan, L.W. (1987) Acute kinetic study with CGA-71818 technical in
albino rats. Unpublished report dated 2-03-1987 project No. HLA
6117-123 from Hazleton Laboratories America Inc. Madison, Wisconsin,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988a) CGA-71818 Metabolism in hens - a
summary. Unpublished report No. ABR-88009 dated 20-05-1988 from
Ciba-Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988b) CGA-71818 Metabolism in goats - a
summary. Unpublished report No. ABR-88008 d.d.20-05-1988 from Ciba-
Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Nemec, M.D. & Keets, S.A., Leist, P.L. & Mercieca, M.D. (1985) A
teratology study (segment II) in albino rabbits with CGA-71818
technical. Unpublished report No. WIL-82004 dated 31-07-1985 from
WIL Research Laboratories Inc., Ashland, Ohio, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Müller, D. (1983) Autoradigraphic DNA repair test on
human fibroblast CGA 71818 . Unpublished report dated 2-12-1983
project No. 811657 from Ciba-Geigy Ltd.,Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Muller, D. (1984) Autradiographic DNA repair test on rat
hepatocytes. CGA 71'818 ( in vitro test for DNA-damaging
properties). Proj. No.: 811522. Unpublished report dated 30 January
1984 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Salamon, C.M. (1985) CGA-71818 technical teratology study in rats.
Unpublished report No. 450-2087 dated 16-09-1985 from American
Biogenics Corp., Decatur, Illinois, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sarasin, G. (1980) Report on acute oral LD50 in the mouse of CGA
71818 technical. Unpublished report No. 800552 dated 20-10-1980 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Schardein, J.L. (1987) Two-generation reproduction study in albino
rats with CGA 71818. Unpublished report No. 382-119 dated 14-08-1987
from International Research and Development Corp., Mattawan,
Michigan, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Seiffert, G., Komarek, J., Zak, F., Malik, C. & Zakova, N. (1983)
CGA 71818 21 day repeated dose dermal toxicity study in rabbits.
Unpublished report dated September 1983, project No. 820206 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Strasser, F.F. & Müller, D. (1982) Point mutation assay with mouse
lymphoma cells. Host-mediated assay with CGA 71818. Unpublished
report project No. 811355 dated 5-01-1982 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980a) Report on eye irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800557 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland.Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980b) Report on skin irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800558 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980c) Report on skin sensitizing
(contact allergenic) efect in guinea-pigs of technical CGA 71818.
Unpublished report project No. 800560 dated 12-06-1980 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Van Dijk, A. (1987) (U-14C) phenyl CGA 71818: Absorption,
distribution, excretion and metabolism after single oral and
repeated oral administration to the rat. Unpublished report project
No. 075666 dated 11-06-1987 from RCC Umweltchemie AG, Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Stäubli, W. (1985) The effect of
penconazole on drug metabolizing enzymes in the livers of male rats
and mice. Unpublished report dated April 1985 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Metabolites 9, 10, 11 and 12 were mainly found as glucuronides
or other conjugates.
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of penconazole
Species Sex Route LD50 LC50 Purity Reference
mg/kg bw mg/m3
Mouse M&F oral 2444 88.4 Sarasin (1980)
Rat M&F oral 2125 88.4 Bathe & Gfeller (1980a)
M&F dermal >3000 88.4 Bathe & Gfeller (1980b)
M&F inhal >4046 96.1 Hartmann & Gfeller (1987)
Chinese hamster M&F oral >5000 88.4 Bathe & Gfeller (1980c)
Rabbit M&F oral 971 88.4 Kobel (1981)
Penconazole shows low acute oral toxicity to mice, rats and
hamsters, but is slightly more toxic to rabbits.
Short-term toxicity studies
Mice
Groups of Cr:CD(ICR)BR mice (15/sex/group) were fed diets
containing 0, 10, 100, 300, 500, 1000 or 2400 ppm penconazole
(purity 98.7%) equivalent to 0, 1.5, 15, 45, 75, 150 or 360 mg/kg
bw/day for 13 weeks. Histopathology was only reported for the liver.
No treatment-related effects were observed on clinical signs,
mortality, ophthalmoscopy, food consumption, food efficiency,
haematology or urinalysis. Body-weight was lower at 2400 ppm.
Cholesterol levels were decreased at 1000 and 2400 ppm, ALAT
increased in high-dose males and total protein and albumin were
decreased in females at 2400 ppm. Relative liver weight was
significantly increased at 500, 1000 and 2400 ppm. An increased
incidence of centrilobular hypertrophy of the liver was observed in
males at doses > 500 ppm and in females at the highest dose.
Hepatic degeneration and vacuolisation was also observed in high-
dose males. Coagulative necrosis in the liver was observed in males
at 1000 and 2400 ppm. The NOAEL in this study was 300 ppm in the
diet, equivalent to 45 mg/kg bw/day (Hiles 1987b).
Rats
Groups of Tif:RAIf(SPF) rats (10/sex/group) were orally
administered 0, 20, 100 or 500 mg penconazole (purity 91.7%)/kg
bw/day for 28 days. Since no marked symptoms of toxicity showed up
during the first week of treatment the doses were increased to 100,
500 or 1000 mg/kg bw/day on day 8 of treatment. After increasing the
doses, 3 male and 3 female high-dose rats showed marked apathia and
lateral body position at day 10 but the animals recovered on day 11.
No effects were observed on mortality, ophthalmic or hearing
examinations. In the mid- and high-dose groups male mean body weight
was decreased, food consumption and food conversion were decreased
in males and females, haemoglobin and haematocrit values were
decreased in females and ALP and ALAT as well as cholesterol and
total proteins were increased in males and females and total
globulin was increased and A/G ratio was decreased in females.
Phosphate and glucose were increased in high-dose females. Albumin
increased in high-dose males and females. Urine volume was increased
in males at the highest dose and in females at the mid- and high-
dose. Relative liver, kidney and adrenal weight was increased in
mid- and high-dose males and females. Relative brain, spleen and
thyroid weight were increased in high-dose females. Enlarged livers
with slight hypertrophy of the hepatocytes were observed in 8/10 and
3/10 males and females at the mid-dose and in all animals at the
highest dose. The lowest dose (20-100 mg/kg bw/day) was considered
as the NOAEL (Basler et al., 1984).
Groups of Tif:RAIf(SPF) rats (20/sex/group) were fed diets
containing 0, 30, 300 or 3000 ppm (equal to 2.1, 19.4 or 202.3 mg
penconazole/kg bw/day for males and 2.1, 20.7 or 208.6 mg
penconazole/kg bw/day for females, respectively) (purity 91.7%) for
3 months. No effects were observed on clinical signs, mortality,
food and water consumption, food conversion, hearing tests or eye
examinations. Body weight was decreased in males and females at the
highest dose. An increase in total plasma protein and albumin was
observed in male rats at all doses and a trend to an increase in
total plasma proteins was seen in females at 3000 ppm. GGT was
slightly increased in females at 300 and 3000 (significantly) ppm.
Cholesterol levels were increased in both sexes at 3000 and also in
females at 300 ppm. A significant dose-related increase in relative
liver weight was observed at all dose levels. Relative testes
weights were increased in males at 3000 ppm and brain, kidney and
thymus weight were increased in high dose females. At histopathology
minimal hypertrophy of the hepatocytes was seen in 20/20 male rats
and 9/20 female rats at 3000 ppm. In this study no NOAEL was
identified, although the liver weight increase at 30 ppm was
marginal (Basler et al., 1982).
In another 3 month feeding study groups of Tif:RAIf(SPF) rats
(20/sex/group) were fed diets containing 0, 10, 30 or 100 ppm
penconazole (purity 91.7%) (equal to 0.8, 2.1 or 7.1 mg
penconazole/kg bw/day for males and 0.8, 2.1 or 7.3 mg
penconazole/kg bw/day for females, respectively). No effects were
observed on clinical signs, mortality, body-weight, food and water
consumption, food conversion, eye examinations, hearing tests, organ
weight or histopathology. Total proteins were increased in male and
female rats at 30 and 100 ppm. Total globulin was increased in high
dose females. GGT was slightly increased in females at both 30 and
100 ppm. Liver weight was increased at 10 and 30 ppm in male rats
only, but not at 100 ppm. No liver histopathological effects were
observed. The NOAEL in this study was 10 ppm penconazole, equal to
0.8 mg/kg bw/day (Basler et al., 1983).
In an additional study groups of albino [Crl:CD(SD)BR] rats
(15/sex/group) were fed diets containing 0, 10, 100, 300, 500, 1000
or 2400 ppm penconazole (purity 98,7%) equivalent to 0.5, 5, 15, 25,
50 or 120 mg penconazole/kg bw/day for 90 days. In this study only
the liver as target organ was studied histopathologically.
Observations included clinical signs mortality, body-weight, food
consumption, food efficiency, ophthalmoscopy, haematology, clinical
chemistry (including GGT), urinalysis, organ weights, macroscopy and
histopathology. Female body weight gain was lower at 500, 1000 and
2400 ppm and food consumption was decreased at the highest dose.
Urea nitrogen was increased in males at all dose levels, but not
clearly dose-related. Relative liver weight was significantly
increased in rats at 500 (females only), 1000 and 2400 ppm. Relative
kidney weight was increased in rats at 2400 ppm. At histopathology
the incidence of vacuolization, degeneration and centrilobular
hyperthrophy of hepatocytes was increased at 500 ppm and above. The
NOAEL in this study was 300 ppm, equivalent to 15 mg/kg bw/day
(Hiles, 1987c).
Rabbits
Groups of New Zeeland white rabbits (5/sex/group) were given
1000, 1500 or 2000 mg penconazole/kg bw/day (purity 91.7%) by dermal
application once/day for 5 days/week for 21 days. Two satellite
groups received 0 or 2000 mg penconazole/kg bw/day for 21 days
followed by a recovery period of 14 days. Transient signs of
dyspnea, curved body position and ruffled fur were observed at all
dose levels including control rabbits, but symptoms disappeared
during the recovery period. No dose-related effects were observed on
mortality, body-weight, food consumption and food conversion,
haematology, clinical chemistry, macroscopy and histopathology. The
NOAEL in this study was > 2000 mg/kg bw/day (Seifert et al.,
1983).
Dogs
Groups of beagle dogs (10/sex/group) were fed diets containing
0, 100, 500, or 5000/2500 ppm penconazole (purity 91.7%), equal to
3.0, 16.9 or 133 mg penconazole/kg bw/day for males and 3.3, 16.7 or
139 mg penconazole/kg bw/day for females, respectively, for 12
months. Due to poor feed intake the highest dose was lowered to 2500
ppm in week 20. An interim kill on 4 dogs/sex/group was carried out
at week 14 and 2 dogs/sex/group were kept for a 4-week recovery
period. Observations included clinical signs, mortality, body-
weight, food consumption, food conversion, ophthalmoscopy, auditory
perception, haematology, blood chemistry, urinalysis, macroscopy,
organ weights and histopathology.
An increased incidence of vomiting was observed in high-dose
dogs during the first three months of treatment; thereafter
occasionally vomiting was observed only in females. The high-dose
dogs lost body-weight and had a reduced food consumption during the
first months. After lowering the dosage food consumption and male
bodyweights were comparable to the controls but the mean weight of
the females remained lower than the control value. ASAT, ALP, OCT,
GGT and ALAT activities were increased in dogs at the highest dose
during the whole administration period. Total globulin concentration
was increased in males at the highest dose during weeks 13, 26 and
53, consequently the total protein was slightly increased and the
A/G ratio was decreased. Terminal body-weight of high-dose male and
female dogs were decreased after 3 months. At this time relative
liver weights were increased at 500 and 5000 ppm. Relative kidney
weight was increased at the highest dose in both males and females.
Relative brain and adrenal weight were increased and relative heart
weight was decreased in high-dose females. All males at 5000 ppm
showed a reduction in testes weights and atrophy of the seminiferous
epithelium. At macroscopy emaciation was observed in dogs at the
highest dose after 3 months. At histopathology after 3 months,
minimal lesions characterized by monocellular hepatocyte necrosis
associated with inflammatory cell infiltration were noticed in
nearly all interim sacrificed dogs at the highest dose and in one
male dog at 500 ppm. After 12 months, testes weights were reduced in
males at 2500 ppm and liver weight was increased in females at 500
and 2500 ppm. At histopathology, bilateral tubular atrophy and
reduced spermatogenesis in the testes was observed in the high-dose
males and an increased incidence of inflammation with fibrosis in
the peripheral lobular region of the liver occurred in the mid- and
high-dose animals. The only abnormalities found after the recovery
period were decreased relative testes weights and tubular atrophy at
2500 ppm. The NOAEL in this study was 100 ppm, equal to 3.0 mg/kg
bw/day for males and 3.3 mg/kg bw/day for females (Gfeller et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Tif:MAGf(SPF) mice (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%),
equal to 0.75, 9.8, 19.3 or 40.8 mg/kg bw/day for males and 0.67,
8.8, 17.2 or 35.7 mg/kg bw/day for females, for 24 months. An
interim kill was carried out on 10 mice/sex/group at 52 weeks. No
effects were observed on mortality, clinical signs, body-weight,
food and water consumption, food conversion, ophthalmic and hearing
inspections, haematology, blood chemistry, urinalysis, macroscopy or
histopathology. Relative liver weight was increased in high-dose
males and females at the interim kill. At the end of the study
adrenal weight (not dose-related) and prostate weight were increased
in males at 75, 150 and 300 ppm. There was no treatment-related
increase in tumour incidence (Basler et al., 1985b). Since
weighing of prostate glands is not very reliable and the increased
weight was not found at 53 weeks, the Meeting concluded that this
effect was probably biologically insignificant. The NOAEL was
therefore 150 ppm, equal to 19.3 mg/kg bw/day for males and 17.2
mg/kg bw/day for females based on the increased liver weight.
Rats
Groups of Tif:RAIf(SPF) rats (80/sex/group) were fed diets
containing 0, 5, 75, 150 or 300 ppm penconazole (purity 91.7%) for
24 months. An interim kill was carried out on 10 rats/sex/group at
12 months. Twenty rats/sex/group were maintained for laboratory
investigations and sacrificed after 24 months. The remaining 50
rats/sex/group were sacrificed after 116/117 weeks. No effects were
observed on mortality, clinical signs, body-weight, food and water
consumption, food conversion, ophthalmic and hearing inspections,
haematology, blood chemistry, urinalysis, macroscopy or
histopathology. At the interim kill, liver weight was increased in
females at 150 and 300 ppm and pituitary weight was decreased in
high-dose males. A tendency to increased liver weight was still
observed in females at the highest dose at 104 weeks. Tumour
incidence was not enhanced. The NOAEL in this study was 75 ppm,
equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively (Basler et al., 1985b).
Reproduction studies
Rats
Penconazole (purity 91.7%) was administered in the diet to
groups of 20 Tif:RAIf(SPF) rats/sex at concentrations of 0, 80, 400
or 2000 ppm (continuously over a period of 110 days to the parental
generations (F0 and F1), including a 12-day mating period for
each generation at the age of 3 months. These dose levels were equal
to 5.5-6.5, 28.5-31 or 146-166 mg/kg bw/day for males and 7.5-8.5,
40-42.5 or 202-227 mg/kg bw/day for females during the whole period.
Parental rats were killed after weaning of the F1 and F2
generation, respectively. Observations included clinical signs,
body-weight, food consumption, fertility index, implantation rate,
litter size, live and dead fetuses, sex ratio, organ weight,
macroscopy and histopathology.
A slightly depressed body-weight gain and food consumption was
seen at the highest dose in F0 females. Parturation was delayed
and the average duration of pregnancy was 21.1, 21.2, 21.3 and 21.6
days for the 0, 80, 400 and 2000 ppm group. Four dams died, two (1
at 400 and 1 at 2000 ppm) at or a short time after parturation, one
(at 2000 ppm) after 4 days and one (at 2000 ppm) after 11 days.
Testes weights of F0 males were slightly increased at 2000 ppm
(liver weight was not determined).
Body-weight gain of F1 pups was slightly reduced at 2000 ppm.
The activity index (ELP, exploratory locomotion pattern) was
slightly but not significantly depressed at 400 and 2000 ppm; there
was however no effect on general development and behaviour. At 2000
ppm, relative liver weight was increased in male and female F1
pups and brain and ovary weight was increased in F1 female pups.
Body-weight gain and food consumption of F1 parents was slightly
reduced at 2000 ppm. One dam died at 400 ppm and 2 at 2000 ppm at or
some days after parturation. The average pregnancy duration was
increased at the highest dose (21.3, 21.4, 21.2 and 21.8 days at 0,
80, 400 and 2000 ppm, respectively). At 2000 ppm, relative liver
weight and relative ovary weight were increased in F1 parents,
while relative brain and testes weight were increased in F1 males.
A decreased implantation rate was observed at 2000 ppm.
The ELP was slightly but not significantly decreased in high-
dose F2 pups without any effect on general development and
behaviour. Relative liver weight was increased in high-dose F2
pups.
Histopathological examination of the livers of F1 adults
revealed slight hypertrophy of the hepatocytes mainly in the
centrilobular region in 17/20 males and in 16/16 females at 2000 ppm
and in 5/19 males and 14/16 females at 400 ppm. Additionally, small
recent necrosis in the liver was observed in 2/16 high-dose females.
No histopathological changes were seen in weanling rats from both
F1 and F2 generations. The NOAEL in this study was 80 ppm, equal
to 5.5-6.5 mg/kg bw/day and 7.5-8.5 mg/kg bw/day for males and
females, respectively (Fritz et al., 1983a; Fritz et al.,
1983b).
Groups of 30 Charles River COBS CD rats/sex were fed diets
containing 0, 25, 250 or 2500 ppm penconazole (purity not given).
After 63 days of treatment the rats were mated to start a two-
generation (1 litter/generation) study. The F1 parents (randomly
selected) were mated after 84 days of treatment. Only weights of
ovaries and testes were determined. At 2500 ppm body-weight and food
consumption were decreased in F0 females and body-weight was
decreased in F1 parents. Mating indices were slightly reduced in
the F0 and F1 generations at 2500 ppm, but fertility indices
were not affected. Reduced pup weight and an increase in the number
of stillborn pups or pups that died during the first days of
lactation was observed at the highest dose. Relative ovary weight
was significantly increased in females of the F0 and the F1
generation at 2500 ppm. The NOAEL in this study is 250 ppm,
equivalent to 12.5 mg/kg bw/day (Schardein, 1987)
Special studies on embryotoxicity and/or teratogenicity
Rats
Groups of 25 pregnant Tif:RAIf(SPF) female rats were
administered orally by intubation 0, 30, 100 or 300 mg penconazole
(purity 88.4%)/kg bw/day suspended in CMC from days 6 to 15 of
gestation. The dams were observed for clinical signs, body-weight
and food consumption and were killed at day 21 of gestation. The
number of total implantations, resorptions, live and dead fetuses
were recorded and the uterus was weighed. Fetuses were weighed,
sexed and examined for external, visceral and skeletal
malformations. Two dams at 300 mg/kg bw/day died shortly before
autopsy. Maternal bodyweight gain in the highest dose group was
slightly reduced. Food consumption was reduced in all experimental
groups. An increase in the numbers of unossified phalangeal nuclei
of the hind limb was observed at 100 and 300 mg/kg bw/day (not dose-
related). At the highest dose, acaudia (taillessness) was observed
in 1/182 fetuses, and the overall incidence of skeletal anomalies
including irregularly shaped sternebrae was increased at the highest
dose. The NOAEL for maternal and embryofetal toxicity was 30 mg/kg
bw/day.
In a supplementary study, 0 or 300 mg penconazole/kg bw/day was
administered by gavage to groups of 15 pregnant rats from days 6-15
of gestation and another group of 15 rats received 450 mg
penconazole/kg bw/day from days 10-14 of gestation. Body-weight gain
and food consumption were decreased at both dose levels. Four and 2
females died shortly before autopsy at 300 and 450 mg/kg bw/day,
respectively. The average weight of live fetuses was decreased at
300 and 450 mg/kg bw/day. The incidence of unossified phalangeal
nuclei of the forelimb was increased at 450 mg/kg bw/day and the
incidence of unossified phalangeal nuclei of the hind limbs and
calcaneus were increased at both 300 and 450 mg/kg bw/day. No
irregular ossification of sternebrae was observed at 300 mg/kg
bw/day. At 450 mg/kg bw/day an irregularly ossified sixth sternebrae
was recorded in one foetus (Fritz & Giese, 1981). The increased
incidence of these variants may indicate delayed fetal development,
but in the absence of any associated malformations should not be
considered as indicative of teratogenicity.
Groups of 25 pregnant Charles River rats were administered by
gavage 0, 5, 100, or 500 mg penconazole (purity 98.7%)/kg bw/day in
corn oil from days 6-15 of gestation. The females were observed for
clinical signs, body-weight and food consumption and sacrificed on
day 20 of gestation. The number of implantation sites, resorption
sites, corpora lutea were recorded. Fetuses were weighed, sexed and
examined for external, internal and visceral anomalies. Two high-
dose dams and 1 dam at 5 mg/kg bw/day died during the study. High-
dose dams exhibited damp fur, crusty eye, crusty muzzle, crusty
nose, and yellow/brown stained fur as well as staggered gait,
emaciated loose stools, weakness and/or lethargy (5/25) and clear
salivation (4/25). Body-weight and body-weight gain were
significantly decreased at the highest dose. Food consumption was
decreased from days 6-13 at 100 and 500 mg/kg bw/day. At the highest
dose, the number of early and late resorption sites was increased
and the number of viable fetuses as well as the fetal weight were
decreased. The number of runts and litters with runts as well as the
occurrence of cervical ribs was increased at 500 mg/kg bw/day. No
teratogenic effects were observed. The NOAEL for maternal and
embryofetal toxicity in this study was 100 mg/kg bw/day (Salamon,
1985).
Rabbits
Groups of 20 pregnant chinchilla rabbits received 0, 25, 75 or
150 mg penconazole (purity 91.7%)/kg bw/day orally by intubation
from day 6 to 18 of pregnancy. Observations included clinical signs,
body-weight gain, food consumption. Dams were killed on day 28 of
pregnancy and fetuses were removed by caesarean section. No effects
were observed on the number of corpora lutea, total implantations,
early and late resorptions, live and dead fetuses, sex ratio uterus
and fetal weight, external and visceral examination and skeletal
malformations.
At 150 mg/kg bw/day average food consumption was slightly
reduced and the number of early resorptions was slightly but not
signficantly increased. At the highest dose,one fetus with
microphthalmia and one fetus showing microphthalmia associated with
internal hydrocephaly were seen. The NOAEL for maternal toxicity and
for embryotoxicity was 75 mg/kg bw/day (Giese & Suter, 1982).
Groups of 20 pregnant New Zeeland white rabbits were orally
dosed by gavage with 0, 10, 50 or 200 mg penconazole/kg bw/day from
days 7 to 19 of gestation. Observation included clinical signs,
body-weight and food consumption. All dams were killed on gestation
day 29 and fetuses were delivered by caesarean section. During the
treatment period decreased defecation and urination were observed in
high-dose animals and body-weight and food consumption were
decreased. A significant increase in body-weight and mean food
consumption was observed from gestation day 20-29. The number of
early resorptions was slightly increased and the number of viable
fetuses was slightly lower at the highest dose. At 200 mg/kg bw/day,
a slightly increased incidence of the number of fetuses with
unossified hyoid body and/or arches (marginal at 50 mg/kg bw/day)
and of fetuses with reduced ossification of the skull was observed.
No teratogenic effects were observed. The NOAEL in this study for
maternal and/or embryotoxicity was 50 mg/kg bw/day (Nemec et al.,
1985).
Special studies on mutagenicity
A number of genotoxicity tests have been carried out with
penconazole. The results are summarized in Table 2.
Special studies on skin and eye irritation and skin sensitization
Penconazole (purity 88.4%) produced slight erythema in 3 male
and 3 female New Zeeland white rabbits when applied under occlusive
conditions to the shaven intact and abraded skin for 24 hours, which
disappeared within 48 h (Ullmann & Gfeller, 1980b).
Penconazole (0.1 mg) was inserted into the conjuctival sac of
the left eye of nine New Zeeland white rabbits. At 30 seconds after
treatment, the eyes of 3/9 rabbits were washed with physiological
saline. In unrinsed eyes slight damage of the cornea and slight
conjunctival redness and chemosis were observed up to 7 days after
treatment. Rinsed eyes were normal 4 days after exposure. Two
rabbits died during the experiment. Bloody exudate in the pleural
and abdominal cavities was observed at autopsy (Ullmann & Gfeller,
1980a).
In a similar study, nine New Zeeland white albino rabbits were
given doses of 100 mg of undiluted penconazole (purity not given)
into the conjunctival sac of the left eye. Three of nine eyes were
washed. Conjunctival redness, chemosis and discharge was observed in
all animals at one hour after application which lasted up to 7 days
after treatment. The iris showed swelling but these changes
disappeared within 24 h. Corneal opacity and positive fluorescein
staining occurred but did not occur in any animal at 48 h (washed
eyes) and on day 7 (non-washed eyes) (Kuhn, 1988).
Penconazole (purity 88.4%) was tested for skin sensitization in
10 male and 10 female Pirbright white guinea-pigs in an optimization
test. No sensitization was observed when the guinea-pigs received 10
intracutaneous injections (0.1 ml of a 0.1% suspension of
penconazole in propylene glycol) followed 14 days later by a single
challenge injection (0.1 ml of 0.1% suspension of penconazole in
propylene glycol) (Ulmann & Gfeller,1980c).
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vitro
Ames testa,b S. typhimurium 250-20250 µg/ml in 92.8 negative Arni & Müller (1980)
TA100, TA98 DMSO;a > 6750 µg/ml
TA1535, TA1537 toxic, and b 20250
µg/ml toxic
Ames testa,b S. typhimurium 100-25600 µg/ml in 91.7 negative c Deparade & Arni (1984)
TA98, TA100 acetone
TA1535, TA1537
Yeast testa,b S. cerevisiae 0.1-40 µg/ml in DMSO 91.7 negative Arni & Müller (1983)
(mitotic gene D7
conversion)
Transformation mouse 2.625, 5.25, 10.5, 91.7 negative c Beilstein & Müller (1984a)
assay embryofibroblasts 21.0 and 42.0 µg/ml
BALB/3T3 in DMSO
Mouse lymphoma L5178Y mouse (a):6.75-100 µg/ml, 91.7 negative c Beilstein & Müller (1984b)
assay a,b lymphoma cells 100 µg/ml toxic;
(TK+/-) (b):8.25-110 µg/ml,
> 110 µg/ml toxic;
both in DMSO
DNA repair human fibroblasts 0, 0,32, 1.6 and 91.7 negative c Puri & Müller (1983)
test 40 µg/ml.
DNA repair rat hepatocytes 0, 032, 1.6, 8.0, 91.7 negative c Puri & Müller (1984)
test 40 µg/ml
Table 2. Results of mutagenicity assays on penconazole
Test system Test object Concentration Purity Results References
of penconazole (%)
In vivo
Host mediated S. typhimurium 0, 350, 700 and 1400 91.7 negative Arni & Müller (1984)
assay (intra TA98, TA100 and mg/kg orally
sanguine) TA1535
in male NMRI mice
Host mediated L 5178Y-cells 0, 813 mg/kg bw 91.7 negative Strasser & Müller (1982)
assay in DBA/Bom/SPF orally
mice
Nucleus anomaly Chinese hamster 0, 417, 834 and 1668 92.8 negative c Hool & Langauer (1982)
test bone marrow cells mg/kg orally in PEG 400
per day on 2 consecutive
days
Sister chromatid Chinese hamster 0, 417, 834 and 1668 91.7 negative c Hool & Arni (1983a)
exchange assay bone marrow mg/kg orally in PEG 400
Chromosome NMRI mice 0, 91, 272 and 816 mg/kg 91.7 negative Hool & Arni (1983b)
aberration assay spermatogonia bw orally in PEG
400 per day on 5
consecutive days; at
816 mg/kg
all mice died
Dominant lethal Male NMRI mice 0, 272 and 816 mg/kg 91.7% negative Hool & Arni (1983c)
assay bw orally
a without metabolic activation
b with metabolic activation
c positive controls yielded positive result(s)
COMMENTS
Penconazole administered orally to mice and rats was rapidly
absorbed and excreted, predominantly in the urine. Female rats
eliminated more in the urine and less in the faeces than male rats.
Very low residues were found in organs and tissues in both males and
females.
Numerous metabolites were identified in urine and faeces. The
major metabolic pathways involved oxidation of the pentyl side chain
to alcohols and acids with sequential cleavage of the terminal
carbon. As the oxidation products were conjugated, the resulting
metabolic patterns were complex. More polar and conjugated products
were found in female rats. Cleavage of the alkyl bridge between the
two rings led to the formation of 1,2,4-triazole, which was a major
metabolic route in male rats.
Penconazole showed low acute oral toxicity to mice, rats and
hamsters, but was slightly more toxic to rabbits. The World Health
Organization has classified penconazole as unlikely to present acute
hazard in normal use (WHO, 1992).
Short-term studies with mice, rats and dogs indicated that the
liver was the primary target organ. In a special study with male
rats, induction of microsomal liver enzymes was demonstrated. In a
13-week study in mice, increased liver weight and liver hyperthrophy
were observed at 500, 1000 and 2400 ppm. The NOAEL was 300 ppm,
equivalent to 45 mg/kg bw/day.
In three 90-days studies performed with rats, liver weight and
liver histopathology were the main effects, except for the second
study. In the first study, increase in relative liver weight was
observed at all dietary levels (30, 300 or 3000 ppm), while in the
third study (dietary levels of 10, 100, 300, 500, 1000 or 2400 ppm)
the NOAEL was 300 ppm, equivalent to 15 mg/kg bw/day. In the second
study (dietary levels of 10, 30 or 100) some biochemical parameters
were affected at 30 and 100 ppm. The NOAEL was 10 ppm, equal to 0.8
mg/kg bw/day.
In a one-year study in dogs, increased liver weights and
histopathological liver effects were observed at dietary
concentrations of 500 and 2500/5000 ppm. At the highest dose level
reduced testis weight and athrophic changes were also observed. The
NOAEL was 100 ppm, equal to 3.0 and 3.3 mg/kg bw/day for males and
females, respectively.
In a two-year feeding study in mice (dietary concentrations of
0, 5, 75, 150 or 300 ppm) increased liver weight at interim kill was
seen at 300 ppm. The NOAEL in this study was 150 ppm, equal to 19.3
mg/kg bw/day for males and 17.2 mg/kg bw/day for females. In a long-
term study in rats (dietary concentrations of 0, 5, 75, 150 or 300
ppm) liver weight was increased at 150 and 300 ppm. The NOAEL was 75
ppm, equal to 3.8 and 4.0 mg/kg bw/day for males and females,
respectively. Penconazole was not carcinogenic in mice or rats.
Two two-generation reproduction studies in rats were reviewed.
In the first study (dietary concentrations of 0, 80, 400 or 2000
ppm), mortality and delayed parturation were observed, as well as
decreased body-weight gain of parents and pups and increased
relative liver weights in parents and pups at 2000 ppm. Hyperthrophy
of liver cells was found at 400 and 2000 ppm. The NOAEL was 80 ppm,
equal to 5.5-6.5 mg/kg bw/day for males and 7.5-8.5 mg/kg bw/day for
females. In the second study (dietary concentrations of 0, 25, 250
or 2500 ppm), liver weights were not determined. The main effect at
2500 ppm was reduced body-weight gain of parents and pups and
mortality of pups during lactation. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
Embryotoxicity/fetotoxicity was observed in three
teratogenicity studies with rats. In two studies (gavage doses of 0,
30, 100 or 300 mg/kg bw/day in one study and 0, 300 or 450 mg/kg
bw/day in the other), maternal toxicity, an increased number of
resorptions, decreased pup weight and delayed ossification were
observed at the high doses. Maternal toxicity or embryotoxicity were
not observed at 30 mg/kg bw/day. In the third study (gavage doses of
0, 5, 100 or 500 mg/kg bw/day), the NOAEL was 100 mg/kg bw/day. In
two teratogenicity studies with rabbits (doses of 0, 25, 75 or 150
mg/kg bw/day in one study and 0, 10, 50 or 200 mg/kg bw/day in the
other), maternal body-weight and food consumption were reduced and
the number of early resorptions was increased at the highest doses.
Overall, the NOAEL was 75 mg/kg bw/day for both maternal toxicity
and embryotoxicity.
After reviewing the available in vitro and in vivo short-
term genotoxicity data, the Meeting concluded that penconazole was
not genotoxic.
An ADI was allocated on the basis of the NOAEL determined from
the one-year study in dogs, which was supported by the NOAEL from
the long-term study in rats. A safety factor of 100 was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 17 mg/kg bw/day (two-year study)
Rat: 75 ppm, equal to 3.8 mg/kg bw/day (two-year study)
80 ppm, equal to 5.5 mg/kg bw/day (two-generation
reproduction study
30 mg/kg bw/day (teratology study)
Rabbit: 75 mg/kg bw/day (teratology study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arni, P. & Müller, D. (1980) Salmonella/mammalian-microsome
mutagenicity test with CGA 71818 (test for mutagenic properties in
bacteria). Unpublished report experiment No. 800550 dated 29
September 1980 from Ciba-Geigy Protection of Health and Environment,
Toxicology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Arni, P. & Müller, D. (1983) Saccharomyces cerevisiae
D7/mammalian-microsome mutagenicity test in vitro with CGA 71818.
Unpublished report experiment No. 811560 dated 27 June 1983 from
Ciba Geigy, Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Müller, D. (1984) Intrasanguine host-mediated assay with
S. typhimurium. Unpublished report experiment No. 830749 dated 3
May 1984 from Ciba Geigy, Protection of Health and Environment, GU
2.3, Experimental Pathology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Basler, W., Gfeller, W., Zak, F., Zakova, N. & Grieve, A.P. (1982)
CGA 71818: 3 months toxicity study in rats. Unpublished final
report, GU project No. 801194 dated 24 February 1982 from Ciba-Geigy
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Komarek, J., Zak, F. & Beri, J. (1983) CGA 71818: 3
month toxicity study in rats. Unpublished final report GU project
No. 821054 dated 17 August 1983 from Ciba-Geigy Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd. Basle, Switzerland.
Basler, W., Komarek, J., Zak, F. & Skorpil, V. (1984) CGA 71818: 28-
day oral cumulative toxicity study in rats. Unpublished final
report, GU project No. 820822 dated 28 June 1984 from Ciba-Geigy,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Basler, W., Gretener, P., Zak, F. & Krinke, G. (1985a) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in mice.
Unpublished final report GU project No. 811414 dated 1 July 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Basler, W., Gretener, P., Zak, F. & Naylor, D.C. (1985b) CGA 71818:
Lifetime carcinogenicity and chronic toxicity study in rats.
Unpublished final draft GU project no.: 811415 d.d. 25 June 1985
from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980a) Acute oral LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800553 dated 28
May 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980b) Acute dermal LD50 in the rat of
technical CGA 71818. Unpublished report project No. 800559 dated 19
March 1980 from Ciba-Geigy, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Gfeller, W. (1980c) Acute oral LD50 in the Chinese
hamster of technical CGA 71818. Unpublished report project No.
800555 dated 19 March 1980 from Ciba-Geigy, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Beilstein, P. & Müller, D. (1984a) BALB/3T3 cell transformation
assay with CGA 71818. Unpublished report test No. 830755 dated 6
September 1984 from Ciba-Geigy, Protection of Health and
Environment, Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Beilstein, P. & Muller, D. (1984b) L5178Y/TK+- mouse lymphoma
mutagenicity test. CGA 71'818 tech. ( in vitro test for mutagenic
properties of chemical substances in mammalian cells). Proj. No.:
811524. Unpublished report dated 9 October 1984 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Deparade, E. & Arni, P. (1984) Salmonella/mammalian-microsome
mutagenicity test. Unpublished report project No. 839759 dated 20
February 1984 from Ciba-Geigy Protection of Health and Environment,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H. & Giese, K. (1981) Report on CGA 71818 tech. teratology
study (seg.II) in rats. Unpublished report test No. 800549 d.d. 25
August 1981 from Ciba-Geigy, Reproductive Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983a) Report on CGA
71818 tecnical. 2-generation toxicity study in rats. Unpublished
report test No. 811416 from Ciba-Geigy, Toxicology GU 2,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Fritz, H., Suter, H.P., Zak, F. & Skorpil, V. (1983b) Two-generation
reproduction study in rats with CGA 71818 technical. Additional data
submission. Unpublished report test No. 811416 from Ciba-Geigy,
Experimental Toxicology, Sisseln, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Gfeller, W., Zak, F. & Naylor, D.C. (1984) CGA 71818 12 month
toxicity study in dogs. Unpublished final report GU project No.
801187 dated February 1984 from Ciba-Geigy, GU2 Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Giese, K. & Suter, H.P. (1982) Report on CGA 71818 tech. Teratology
study in rabbits. Unpublished report test No. 811354 dated March
1982 from Ciba-Geigy Reproductive Toxicology GU 2.1, Sisseln,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hamböck, H. (1980) Distribution, degradation and excretion of CGA
71818 in the rat. Unpublished project report 41/80 dated 6 November
1982 from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1982) The major urinary metabolites of CGA 71818 in the
rat. Unpublished project (status) report 15/82 dated 17 May 1982
from Ciba-Geigy AG 2.52, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hamböck, H. (1984) The metabolic fate of CGA 71818 in the rat.
Unpublished project report 23/83 dated 10 May 1984 from Ciba-Geigy,
AG 2.52, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hamböck, H. (1985) Sex dependency of the metabolite pattern of CGA
71818 after oral administration to rats. Addendum to project report
41/80. Unpublished report 1/85 dated 11 January 1985 from Ciba-Geigy
AG 2.52 Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hartmann, H.R. & Gfeller, W. (1987) CGA 71818 tech. Acute aerosol
inhalation toxicity in the rat. Unpublished final report GU project
No. 871169 d.d. 6 August 1987 from Ciba-Geigy Ltd, GU 2 Toxicology,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987a) CGA 71818 technical. Kinetic study in albino
rats with CGA 71818 technical. Unpublished report HLA 6117-122 dated
24-2-1987 from Hazleton Laboratories America, Inc. Madison,
Wisconsim 53704. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hiles, R.A. (1987b) 90-Day subchronic dietary toxicity and kinetic
study in albino mice with CGA-71818 technical. Unpublished report
HLA 6117-121 dated 4-3-1987 from Hazleton Laboratories America, Inc.
Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hiles, R.A. (1987c) 90-Day subchronic dietary toxicity and kinetic
study in albino rats with CGA-71818 technical. Unpublished report
HLA 6117-120 dated 14-04-1987 from Hazleton Laboratories America,
Inc. Madison, Wisconsin 53704. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Arni, P. (1983a) Sister chromatid exchange study CGA
71818 Chinese hamster. Unpublished report dated 2-06-1983 project
No. 811523 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983b) Chromosome studies in male germinal
epithelium CGA 71818 mouse. Unpublished report dated 17-06-1983
project No. 811520 from Ciba-Geigy Ltd, Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. & Arni, P. (1983c) Dominant lethal test, mouse 8 weeks CGA
71818. Unpublished report dated 2-12-1983 project No. 811519 from
Ciba-Geigy Ltd., Protection of Health and Environment, Experimental
Pathology, Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Hool, G. & Langauer, M. (1982) Nucleus anomaly test in somatic
interphase nuclei. CGA 71818 Chinese hamster. Unpublished report
dated 20-1-1982 experiment No. 800551 from Ciba-Geigy Ltd,
Protection of Health and Environment, Toxicology, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Kobel, W. (1981) Acute oral LD50 in the rabbit of technical CGA
71818. Unpublished report dated 4-3-1981, project No. 800554 from
Ciba-Geigy Ltd., Exp. Toxicology Sisseln, Switzerland, Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kuhn, J.O. (1988) Primary eye irritation study in rabbits EPA
guidelines No. 81-4. Unpublished report dated 31-03-1988 project
no.: 5303-88 from Stillmeadow Inc. Houston, Texas, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
LeVan, L.W. (1987) Acute kinetic study with CGA-71818 technical in
albino rats. Unpublished report dated 2-03-1987 project No. HLA
6117-123 from Hazleton Laboratories America Inc. Madison, Wisconsin,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988a) CGA-71818 Metabolism in hens - a
summary. Unpublished report No. ABR-88009 dated 20-05-1988 from
Ciba-Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Murphy, R.T. & Capps, T.M. (1988b) CGA-71818 Metabolism in goats - a
summary. Unpublished report No. ABR-88008 d.d.20-05-1988 from Ciba-
Geigy Corp. Greensboro, NC, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Nemec, M.D. & Keets, S.A., Leist, P.L. & Mercieca, M.D. (1985) A
teratology study (segment II) in albino rabbits with CGA-71818
technical. Unpublished report No. WIL-82004 dated 31-07-1985 from
WIL Research Laboratories Inc., Ashland, Ohio, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Müller, D. (1983) Autoradigraphic DNA repair test on
human fibroblast CGA 71818 . Unpublished report dated 2-12-1983
project No. 811657 from Ciba-Geigy Ltd.,Protection of Health and
Environment, Toxicology, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. & Muller, D. (1984) Autradiographic DNA repair test on rat
hepatocytes. CGA 71'818 ( in vitro test for DNA-damaging
properties). Proj. No.: 811522. Unpublished report dated 30 January
1984 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Salamon, C.M. (1985) CGA-71818 technical teratology study in rats.
Unpublished report No. 450-2087 dated 16-09-1985 from American
Biogenics Corp., Decatur, Illinois, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sarasin, G. (1980) Report on acute oral LD50 in the mouse of CGA
71818 technical. Unpublished report No. 800552 dated 20-10-1980 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Schardein, J.L. (1987) Two-generation reproduction study in albino
rats with CGA 71818. Unpublished report No. 382-119 dated 14-08-1987
from International Research and Development Corp., Mattawan,
Michigan, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Seiffert, G., Komarek, J., Zak, F., Malik, C. & Zakova, N. (1983)
CGA 71818 21 day repeated dose dermal toxicity study in rabbits.
Unpublished report dated September 1983, project No. 820206 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Strasser, F.F. & Müller, D. (1982) Point mutation assay with mouse
lymphoma cells. Host-mediated assay with CGA 71818. Unpublished
report project No. 811355 dated 5-01-1982 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980a) Report on eye irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800557 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland.Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980b) Report on skin irritation in the
rabbit after single application of technical CGA 71818. Unpublished
report project No. 800558 dated 28-05-1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L. & Gfeller, W. (1980c) Report on skin sensitizing
(contact allergenic) efect in guinea-pigs of technical CGA 71818.
Unpublished report project No. 800560 dated 12-06-1980 from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Van Dijk, A. (1987) (U-14C) phenyl CGA 71818: Absorption,
distribution, excretion and metabolism after single oral and
repeated oral administration to the rat. Unpublished report project
No. 075666 dated 11-06-1987 from RCC Umweltchemie AG, Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Waechter, F., Bentley, P. & Stäubli, W. (1985) The effect of
penconazole on drug metabolizing enzymes in the livers of male rats
and mice. Unpublished report dated April 1985 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
