
FENPROPATHRIN
First draft prepared by
E. Bosshard
Federal Office of Public Health
Schwerzenbach, Switzerland
EXPLANATION
Fenpropathrin is an ingestion and contact synthetic pyrethroid
insecticide and acaricide used against various pests in cotton,
grapes, ornamentals, fruits and vegetables and other field crops.
The compound was considered for the first time by the present
Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Biokinetics and biotransformation of fenpropathrin was studied
in rats, goats, cows and chickens. In all the species investigated
the compound was efficiently absorbed and excreted in urine and
faeces. The biotransformation pattern was similar in the various
species (Crawford, 1975; Crawford & Hutson, 1976, 1977; Kaneko et
al., 1981; Ku & Doran, 1990a,b).
Rats
After oral application of single doses of 2.4-26.8 mg/kg bw
14C-acid and 14C-alcohol-labelled fenpropathrin, 25%-60% of the
radiocarbon was eliminated in urine and 40%-70% in faeces (Crawford,
1975; Kaneko et al., 1987). Excretion was rapid and amounted to
95% of the dose within 48 hours. Residues in tissues were low.
After a single oral dose of 1.5 mg/kg bw of 14C-benzyl-
labelled fenpropathrin, 42-70% of the administered radioactivity was
excreted in urine and 25-63% in faeces. Highest residues of 0.6 ppm
were measured in fat tissue and liver one day after treatment.
Residues in all other tissues were less than 0.1 ppm (Crawford,
1975).
The lipophilic nature of the compound together with its very
rapid elimination indicated that metabolism to polar products must
be very effective.
Cows
Two lactating cows were fed twice daily for 21 days a diet
containing 0.2 ppm 14C-labelled fenpropathrin. Fenpropathrin was
rapidly absorbed and eliminated and an equilibrium established
within five days. Total urinary and faecal excretion amounted to
48% and 39%, respectively. Residue levels in milk and tissues were
below the limits of detection of 0.001-0.008 ppm (Crayford, 1975).
Hens
Hens fed with 14C-cyclo- or 14C-benzyl-fenpropathrin at doses
of 0.5 or 5 mg/kg bw for 10 days showed rapid excretion and
biotransformation. 14C-levels were low in eggs (< 0.5 ppm) and
meat (< 0.6 ppm). Metabolites were identical to those found in
rats (Ku & Doran, 1990b).
Biotransformation
Rats
On the basis of the identified metabolites, the major
biotransformation reactions of fenpropathrin in rats consisted of
oxidation at the methyl groups of the acid moiety and at the 2'-and
4'-positions of the alcohol moiety, cleavage of the ester linkage
and the conjugation of the resultant carboxylic acids, alcohols and
phenols with glucuronic acid, sulfuric acid and glycine. Most of
the urinary metabolites were ester-cleaved ones. The predominant
urinary metabolites derived from the acid moiety were identified as
TMPA1-glucuronide and TMPA-CH2OH (trans). Other metabolites
identified were TMPA-COOH (trans), TMPA-CH2OH-lactone in free form
or as glucuronide. The major urinary metabolites derived from the
alcohol moiety were PB2 acid in free form and as glycine
conjugate, 4'-OH-PB acid-sulfate and 2'-OH-PB acid-sulfate. The
urinary metabolites from the alcohol moiety were similar to those
from other pyrethroids (e.g., fenvalerate, deltamethrin,
cypermethrin). Almost all faecal metabolites retained the ester
linkage. The major faecal metabolite was identified as CH2OH
(trans)-fenpropathrin, followed by COOH (trans)-fenpropathrin, 4'-
OH-fenpropathrin and 4'-OH-, CH2OH(trans)-fenpropathrin. Depending
on the dose administered, 30-50% of the applied radioactivity was
excreted in faeces as parent compound. A maximum of 0.3% of the
applied radiocarbon was exhaled as 14CO2 after administration of
14C-acid or 14C-alcohol fenpropathrin. Fenpropathrin and TMPA were
the major components of 14C in tissues. No sex difference was
apparent (Ruzo et al., 1978; Ohkawa et al., 1979; Kaneko et al.,
1987).
An aryl-hydroxylated ester (alpha-cyano-3-(4-
hydroxyphenoxy)benzyl ester) was identified in bile. The ester was
eliminated in the bile presumably conjugated (Crawford & Hutson,
1977).
Goats
14C-benzyl- and 14C-cyclo-fenpropathrin were orally
administered to lactating goats at 50 ppm for five days. As in the
other species the majority of the radiolabel was excreted rapidly
and 14C levels were low in milk and tissues. The parent
1TMPA: 2,2,3,3-Tetramethylcyclopropane carboxylic acid.
2PB acid: 3-Phenoxybenzoic acid.
fenpropathrin was the major component of residues in milk, meat, fat
and heart. In kidney and liver, the major metabolites resulting
from hydrolysis and oxidation reactions of the benzyl ring moiety
were identified as PB acid and its glycine derivative as well as 4'-
OH-PB acid. Other metabolites found in milk and tissue samples were
TMPA and its oxidative derivatives including TMPA-CH2OH, TMPA-COOH
and TMPA-CH2OH-lactone (Ku & Doran, 1990a).
The proposed metabolic pathway for fenpropathrin in mammals is
given in Figure 1.
Toxicological studies
Acute toxicity studies
Signs of acute toxicity of fenpropathrin were typical of
pyrethroid intoxication and included decreased motor activity,
hyperexcitability, tremors, diarrhoea and salivation. Survivors
recovered from the clinical signs within a few days (Hiromori et
al., 1982a). The results of the studies on the acute toxicity of
fenpropathrin are summarized in Table 1. Fenpropathrin has been
classified as moderately hazardous by WHO (WHO, 1992).
The acute oral toxicity of the following impurities were tested
in mice: alpha-cyano-4-phenoxybenzyl 2,2,3,3-tetramethyl-1-
cyclopropane carboxylate and alpha-(3-phenoxybenzoyl)-3-
phenoxybenzyl 2,2,3,3-tetramethyl-1-cyclopropane carboxylate, both
showing an oral LD50 of > 5000 mg/kg bw in both sexes (Misaki &
Kohda, 1981a).
Another impurity tested was 2,2,3,3-tetramethylcyclopropane
carboxylic anhydride, showing an oral LD50 in mice of 1450 mg/kg bw
in males and 1880 mg/kg bw in females (Misaki & Kohda, 1981b).
Short-term toxicity studies
Mice
Groups of mice (CD-1; 8/sex/group) were fed diets containing 0,
100, 200 or 300 ppm fenpropathrin for 4 weeks. No adverse reactions
were observed in any of the treated animals (Colley et al.,
1981a). A second study was performed at dietary concentrations of
0, 500, 1000 or 1500 ppm for 4 weeks. At 1000 ppm reduced body-
weight gain and higher liver weights were observed. At 1500 ppm
mortalities occurred at the very beginning of the study (Colley et
al., 1981b).
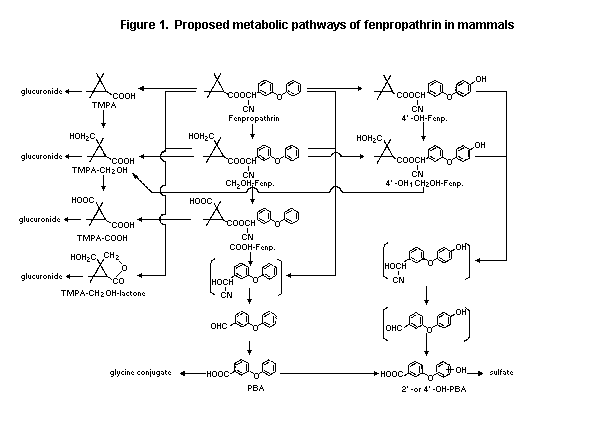 Table 1. Acute toxicity of fenpropathrin (technical material)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Rat M oral 54 97 Kohda, 1975
F oral 49 97 Kohda, 1975
M oral 71 91.8 Hiromori et al., 1983b
Misaki et al., 1983
F oral 67 91.8 Hiromori et al., 1983b
Misaki et al., 1983
M oral 77 97.3 Hiromori et al., 1983a
Misaki et al., 1983
F oral 67 97.3 Hiromori et al., 1983a
Misaki et al., 1983
M oral 164 97.3 Hiromori et al., 1983b
F oral 107 97.3 Hiromori et al., 1983b
M&F oral 60 93.8 Omodaka et al., 1986a
F oral 70 93.8 Omodaka et al., 1986a
M&F inhalative (vapour) > 0.009 µg/l* 94.5 Bruce et al., 1986
M&F inhalative (Xylene EC mist) > 96 µg/l* 97 Kohda et al., 1976
M dermal (sol. in corn oil) 1600 97 Kohda, 1976
F dermal (sol. in corn oil) 870 97 Kohda, 1976
M&F dermal (viscous liquid) > 5000 93.8 Omodaka et al., 1986b
M sc 1410 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 225 97 Kohda & Kadota, 1976b
F ip 180 97 Kohda & Kadota, 1976b
Mouse M oral 67 97 Kohda & Kadota, 1975
F oral 58 97 Kohda & Kadota, 1975
M oral 135 93.8 Suzuki et al., 1986
F oral 154 93.8 Suzuki et al., 1986
M inhalative (Xylene EC mist) 100 µg/l* 97 Kohda et al., 1976
F inhalative (Xylene EC mist) 43 µg/l* 97 Kohda et al., 1976
Table 1 (contd)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Mouse M dermal 740 97 Kohda & Kadota, 1976a
F dermal 920 97 Kohda & Kadota, 1976a
M sc 1350 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 230 97 Kohda & Kadota, 1976b
F ip 210 97 Kohda & Kadota, 1976b
M iv 4.5 ? Summitt & Albert, 1976
Rabbit M oral 675 96.2 Hara & Suzuki, 1980
F oral 510 96.2 Hara & Suzuki, 1980
M&F dermal > 2000 ? Marroquin et al., 1981
Dog M&F oral > 1000 96.2 Pence et al., 1978
* LC50
Rats
Fenpropathrin (purity 96%) was fed to groups of Carworth Farm E
rats (12/sex/dose; control 24/sex) at dietary concentrations of 0,
2, 10, 50 or 250 ppm over 13 weeks. The treatment did not influence
the general health and behaviour, haematology, clinical chemistry or
pathology of treated animals. Body-weight gain was slightly
increased at all dose levels. A slight increase in spleen weight in
male rats at 250 ppm was the only change concerning organ weights
and may present a borderline effect. The NOAEL was > 250 ppm,
equal to 18 mg/kg/day (males) and 21 mg/kg bw/day females) (Hend &
Butterworth, 1975).
In a similar study, rats (12/sex/group; control 24/sex) were
fed diets containing fenpropathrin (purity 97%) at concentrations of
0, 3, 30, 100, 300 or 600 ppm over 13 weeks. After 5 weeks of
exposure, most females and one male showed tremors and reduced body-
weight gain at 600 ppm. The tremors disappeared towards the end of
the study. Changes in a few haematological parameters were marginal
and were - though statistically significant - not considered
biologically significant. Slight increases in plasma ALP were
observed at 600 ppm. Organ weights were not influenced by treatment
and no abnormal histopathological findings were observed. The NOAEL
was 300 ppm, equal to 17 mg/kg bw/day (males) and 24 mg/kg bw/day
(females) based on the appearance of clinical signs and reduced
body-weight gain at 600 ppm (Hend & Butterworth, 1976a).
In a third study, groups of rats (SPF SD(Crj:CD); 12/sex/dose)
were fed concentrations of 0, 15, 50, 150, 450 or 600 ppm
fenpropathrin (purity 93.1%) over a period of 13 weeks. One female
died at 600 ppm. A dose-dependent depression of body-weight gain
was observed at 600 ppm and 450 ppm in female rats, and at 600 ppm
in male rats. Food consumption was reduced at the beginning of the
study at 600 ppm in males and at 450 ppm and above in females; food
efficiency was reduced in females treated with 600 ppm. An increase
in urinary protein and specific gravity was observed in males at 450
ppm and above. Haematological and ophthalmological parameters as
well as organ weights were not influenced by the treatment. A
slightly elevated alkaline phosphatase activity was noted in females
of the 600 ppm group. There was no evidence of treatment-related
histopathological changes in any organ. The NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain and
urinalysis at higher dose levels (Yoshida et al., 1986).
Rabbits
In a 21-day dermal toxicity study with fenpropathrin (purity
91.4%), groups of New Zeeland white rabbits (10/sex/dose) received
dermal applications on either intact or abraded skin at dose levels
of 0, 500, 1200 or 3000 mg/kg bw. The treatment did not influence
survival, body weight, food consumption, haematology, clinical
chemistry, organ weights, or macroscopic or microscopic findings.
Single instances of slight skin reactions were observed at the
application sites of animals treated with 500 and 1200 mg/kg bw and
in most animals at 3000 mg/kg bw. The NOAEL was > 3000 mg/kg bw
based on systemic toxicity (Riley et al., 1982).
Dogs
Fenpropathrin (purity 96.2%) was administered in the diet to
groups of beagle dogs (6/sex/dose) at concentrations of 0, 250, 500
or 1000/750 ppm over 13 weeks (1000 ppm for 3 weeks, 750 ppm for the
rest of the study because of the appearance of severe tremors and
ataxia). The doses were adjusted for purity. Treatment did not
affect survival, food consumption, ophthalmoscopy, clinical
chemistry, urinalysis, organ weights, gross or microscopic
pathology. Treatment-related clinical signs consisted of emesis,
tremors, ataxia, diarrhoea, lethargic appearance and salivation.
Some effects were observed in all treatment groups. The frequency
of the observed clinical signs in all of the compound-treated groups
decreased after week 5. One high-dose male dog was sacrificed
moribund. Reduced body-weight gain was observed at concentrations of
500 and 1000/750 ppm. Haematocrit and haemoglobin values and the
erythrocyte count in females at 1000/750 ppm were decreased. The
NOAEL was < 250 ppm, equal to 7 mg/kg bw/day in males and 10 mg/kg
bw/day in females, based on the occurrence of emesis at 250 ppm
(Pence et al., 1980a,b).
Fenpropathrin (purity 92.5%) was fed to groups of beagle dogs
(4/sex/dose) at dietary concentrations of 0, 100, 250 or 750 ppm
(adjusted to 100% active ingredient) over a period of 52 weeks. One
high-dose male died in the middle of the study after exhibiting
tremor and ataxia. Emesis occurred at an increased incidence in the
mid-dose and high-dose females but without a clear dose-response
relationship. The incidence of tremors however showed a clear dose-
related increase at 250 and 750 ppm (males: 0, 0, 49, 181; females:
0, 1, 42, 168 observations at 0, 100, 250 and 750 ppm,
respectively). Ataxia occurred at 750 ppm. Reduced body-weight
gain was observed at 750 ppm and at 250 ppm in females. The
treatment did not influence ophthalmoscopy, haematology, clinical
chemistry, urinalyses, gross or microscopic pathology. The only
significant difference concerning organ weight was an increase in
the relative kidney weight in females at 750 ppm. The NOAEL was 100
ppm, equal to 3 mg/kg bw/day, based on reduced body-weight gain and
occurrence of clinical signs at 250 ppm and higher (Pence et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
In a 104-week feeding study, groups of mice (Charles River (UK)
CD-1; 52/sex/group in main study; 40/sex/group in satellite groups
for interim sacrifice) were fed fenpropathrin (purity 91.4%-92.5%)
at concentrations of 0, 40, 150 or 600 ppm. These concentration
levels were based on two range-finding studies (Colley et al.,
1981a,b). Treatment did not affect the general behaviour, with the
exception of a marginal increase in the number of females at 600 ppm
showing hyperactivity at week 78. No effects on survival rate, body
weight, food consumption, food efficiency, urinalysis, macroscopic
pathology or organ weights were reported. Changes in haematological
parameters at 600 ppm included slight decreases in Hb and MCHC in
males and an increase in MCHC in females at week 25. Because these
changes were small, occurring only on some occasions and only in one
sex, they are not considered to be toxicologically significant.
Sporadic changes in some clinical chemical parameters were not
considered to be of toxicological significance (e.g., decrease in
urea nitrogen in males at 600 ppm, increase in GOT and GPT levels at
all dose levels without clear dose-response relationship). An
increased incidence in lung tumours (adenoma and adenocarcinoma) was
observed in males and females of treated groups. The incidences in
the satellite groups (spontaneous deaths and interim sacrifice after
26, 52 and 78 weeks) were 10, 12.5, 18 and 2.5% for males and 7.5,
12.5, 7.5 and 7.5% for females at 0, 40, 150 and 600 ppm,
respectively. The incidences in the main study were 12, 23, 35 and
31% for males and 10, 29, 19 and 13% females at 0, 40, 150 and 600
ppm, respectively. The incidences did not show a clear dose-
response relationship. The absence of any statistical significance
or significant trend supports the interpretation that the increased
incidences in the treatment groups were not due to a tumorigenic
activity of fenpropathrin. Moreover, data on the incidence of lung
tumours in historical controls showed that incidences of pulmonary
adenoma and adenocarcinoma in untreated animals may vary between 7
and 36%. There was no sex difference concerning lung tumours in
these controls. Therefore the results of this study gave no
evidence of fenpropathrin-induced carcinogenic potential. The NOAEL
was the highest dose tested, 600 ppm, equal to 56 mg/kg bw/day
(males) and 65 mg/kg bw/day (females) (Colley et al., 1985, 1987).
Rats
Groups of rats (COBS) were fed diets containing 0, 1, 5, 25,
125 or 500 ppm fenpropathrin (purity 97%) for two years. The group
sizes were 12/sex and 48/sex for the satellite and main study
controls, respectively; while the treatment groups included 6/sex
and 24/sex for the satellite and main study, respectively. The
treatment did not affect the survival, general health, behaviour of
the animals, clinical chemical or haematological parameters. The
depression in body-weight gain observed at 500 ppm was statistically
significant in females only. Food consumption of treated animals
was comparable with control animals at most observation times.
Absolute spleen weight increased at 500 ppm in females of the 6-
month satellite group and kidney weights decreased in females of all
treatment groups in the 12-month satellite groups without showing a
clear dose-response relationship. In the 2-year main study groups,
no treatment-related alterations in organ weights were observed.
Gross pathological examination showed a higher incidence of
white/grey foci or plaques in the lungs at 125 and 500 ppm in
females at 6 and 24 months. In the 2-year main groups, the number
of deaths attributable to renal failure in males at 500 ppm was
greater than in the other groups, but no increase was observed when
animals from satellite and main study groups were combined.
The histopathological neoplastic and non-neoplastic changes
found were consistent with the range and severity of changes usually
observed in this rat strain and did not give any evidence of
carcinogenicity. The NOAEL was 125 ppm, equal to 5 mg/kg bw/day
based on depression in body-weight gain at higher dose levels (Hend
& Gellatly, 1979; Okuno, 1981; Aitken & Rushton, 1981).
Single animals of a satellite group fed the above-mentioned
concentrations for 18 months were used for functional and
neurochemical investigations. For results see "Special Studies on
Neurotoxicity" (Hend & Gellatly, 1980).
Fenpropathrin (purity 91.4-92.5%) was fed to groups of rats
(Charles River CD; 50/sex/dose in main study and 15/sex/dose in
satellite groups for interim sacrifice) at dietary concentrations of
0, 50, 150, 450 or 600 ppm for 104 weeks. The dose levels were
selected based on the results of range-finding studies (Colley et
al., 1982a,b; Heywood, 1982). Clinical signs were restricted to
body tremors, most prevalent in females receiving 600 ppm, but were
also observed in males at the highest dose levels in the first few
weeks of the study and in females at 450 ppm.
Mortality increased in males and females receiving 600 ppm and
in females receiving 450 ppm during the first 26 weeks of treatment;
subsequently, mortality was less than that of control animals
resulting in highest survival rates in males at 600 ppm and in
females at 450 ppm. Exposure of main group females receiving 600
ppm was terminated after 52 weeks. A slightly higher food
consumption was reported for the high-dose females during the first
3 months of treatment, most probably resulting in the mortalities
observed. Body-weight gain was reduced in females at 600 ppm and
food utilization efficiency was marginally impaired.
Ophthalmological examination did not reveal treatment-related ocular
lesions. No abnormal haematological findings were reported.
Changes in clinical chemistry parameters at 450 and 600 ppm included
slightly reduced creatinine levels and reduced total protein levels
in males. The differences were minimal however and - though
statistically significant - most probably of no biological
significance. Urinalysis did not indicate any kidney lesions.
Macroscopic examination revealed no findings which were
treatment-related with the exception of an increased incidence of
alopecia in females at 600 ppm at 52 weeks. Changes in organ
weights were observed on some occasions but did not show a
treatment-related pattern and histopathological examination did not
reveal changes attributable to treatment. A few lymphoreticular
tumours were seen in treated groups of male rats, but none in
control group. The increases observed were not dose-related and
were within the incidence range of the historical controls for this
rat strain. There was no indication of a treatment-related increase
in tumour incidence or non-neoplastic organ changes. The electron
microscopic examination of tibial nerve did not reveal treatment-
related effects. The NOAEL was 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses (Warren
et al., 1986; Fish et al., 1986; Dean et al., 1987).
Reproduction studies
Rats
In a 3-generation reproduction study, fenpropathrin (purity
97%) was fed to rats (COBS; 30/sex/group) at dietary concentrations
of 0, 5, 25 or 250 ppm. Treatment did not influence the general
health condition or mortality, body-weight gain, food consumption,
pregnancy rates, litter size or litter weight. However, pup weights
in the F3a generation were reduced at 250 ppm. No macroscopic or
microscopic lesions were attributable to treatment. A very small
number of cases of locomotor incoordination occurred in pups fed 5
and 250 ppm. Most pups in the 5 ppm group showing these signs were
from one litter in the F1b generation. No similar lesions were
observed in the second litter of that pair nor in the following
generation and no macroscopic lesions were identified in these
animals. Histopathological examination (F2 parents, F3b pups) did
not reveal treatment-related tissue lesions. The NOAEL was 25 ppm,
equal to 1.6 mg/kg bw/day, based on decreased pup weights in the
F3a generation at 250 ppm (Fleming, 1979; Hend et al., 1979; Else
& Rushton, 1981).
In a second 3-generation study, groups of rats (CrL: COBS CD
(SD)BR; 11-28/sex/dose) were fed diets containing 0, 40, 120 or 360
ppm fenpropathrin (purity 92.5%). Animals of the last generation
(F2) were reared to maturity. At 360 ppm, most females that
littered showed body tremors associated with muscle twitches and
increased sensitivity. These signs were generally observed during
the lactation period. Increased mortality occurred at 360 ppm in
females only; 18/28 females at this dose died, most during the
lactation period of the F1b females. At 120 ppm, 2/24 F1b females
died during lactation. Mean body-weight gain of males in the F1
and F2 generations were reduced at 360 ppm. F1b females at 120
ppm showed also some indication of retarded weight gain. Treatment
had no influence on mating performance and no macroscopic or
microscopic abnormalities were observed at terminal autopsy that
were considered to be associated with treatment. At 360 ppm, F0
females showed a slight increase in liver weights. A few F2b pups
at 120 ppm showed body tremors prior to weaning and 2/3 of these
pups died subsequently. A slight reduction in litter size was
observed at 360 ppm in F0 animals (second mating) and in both
matings of F1b animals from days 4 to 21. At 360 ppm, pup weights
were lower in all generations. The NOAEL was 40 ppm, equal to 3
mg/kg bw/day, based on depression of body-weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above (Cozens et al., 1986).
Special studies on embryotoxicity/teratogenicity
Rats
Fenpropathrin (purity 96.2%) was fed by intubation to groups of
rats (Fischer 344 CDF; 27/28 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.4, 2 or 10 mg/kg bw/day. One mid-
dose and 9 high-dose females were found dead during the study.
Clinical signs noted at a somewhat greater incidence in the treated
animals included soft faeces, red or lacrimating eyes, alopecia or
hunched appearance. Post-dose tremors occurred on a few occasions
in some high-dose females. Body-weight gain was reduced in high-
dose females during the treatment period, and increased slightly
afterwards, resulting in an overall change comparable to the control
group. Food consumption was also reduced at the highest dose level.
Various gross lesions were found in animals that died during the
study. The findings included discolorations of organs, but no
specific target organ was identified. The pregnancy rate was lower
in the high-dose group compared to the other dose groups but other
reproduction parameters (e.g., number of corpora lutea, resorptions,
fetal viability) were not impaired by treatment. No visceral or
skeletal anomalies were noted in the pups. Visceral or skeletal
variants did not show dose-related increases. No teratogenic
effects were observed. The NOAEL for maternal toxicity was 2 mg/kg
bw/day. The NOAEL for embryotoxicity and teratogenicity was 10 mg/kg
bw/day (Pence et al., 1980c; Cox, 1986, 1987).
Groups of rats (Fischer-344; 30 females/dose group) were orally
treated with fenpropathrin (purity 91.9%) at dose levels of 0, 0.44,
1.6, 2.2, 3.3, 6.5 or 11 mg/kg bw/day (corresponding to 0, 0.4, 1.5,
2, 3, 6 or 10 mg/kg bw/day active ingredient) from day 6 to day 15
of gestation. Seven animals died in the highest dose group. Signs
of toxicity included ataxia, sensitivity to external stimuli,
tremors, prostration and convulsions. At 6 and 10 mg/kg bw/day,
mean maternal body-weight gains and food consumption were lower
during the dosing period. Pregnancy rate of high-dose females was
slightly lower (90%) compared to the other treatment groups (93%-
97%). Treatment had no influence on reproduction parameters such as
resorptions, fetal viability or fetal body weight. No visceral or
skeletal anomalies were observed that were considered treatment-
related. Skeletal variations were noted in all groups and included
variations in the stage of ossification and rib counts, but not in a
dose-dependent pattern. A higher incidence of incomplete
ossification of the sternebrae was observed in all treatment groups
but without a clear dose-response relationship. The highest
incidences with respect to some differences in ossification (e.g.,
5th/6th sternebrae incomplete ossification, asymmetric ossification
of sternebrae) were noted at 10 mg/kg bw. Statistical analysis
revealed no significant trend for the overall incidence of delayed
ossification of the 5th/6th sternebrae. The NOAEL for maternal
toxicity was 3 mg/kg bw/day. The NOAEL for embryotoxicity and
teratogenicity was 10 mg/kg bw/day (Morseth, 1990).
Rabbits
Groups of rabbits (banded Dutch; 20-30 females/group) were fed
fenpropathrin (purity 97%) at dose levels of 0, 1.5, 3 or 6 mg/kg
bw/day from day 6 to day 18 of gestation. No clinical signs of
intoxication were observed. A lower number of rabbits survived to
term at 3 mg/kg bw/day but this was unrelated to treatment.
Treatment had no effect on body weight of the dams. No adverse
effects were observed with respect to pre-implantation loss, number
of resorptions, fetal deaths, litter size, or pup weight. Increased
incidence in minor skeletal and visceral abnormalities (e.g.,
enlarged fontanelles, interparietal bones, fused sternebrae, extra
centres of ossification) were noted at 3 mg/kg bw but not at the
higher dose. Therefore these changes were not considered treatment-
related. The NOAEL for maternotoxicity, embryotoxicity and
teratogenicity was 6 mg/kg bw/day (van der Pauw et al., 1975; Dix,
1975).
Groups of rabbits (New Zeeland white; 17-19 females/dose) were
treated with oral doses of 0, 4, 12 or 36 mg/kg bw/day fenpropathrin
(purity 92.5%) from days 7 to 19 of gestation. A dose-related
increase in the incidence of grooming was observed in all dose
groups. At 12 and 36 mg/kg bw/day, flicking of forepaws was also
observed, with a few animals at 36 mg/kg bw/day showing shaky
movements and tremor. Slight reduction in body-weight gain and
reduced food consumption were noted at 36 mg/kg bw/day. One dam at
12 and two at 36 mg/kg bw failed to maintain their pregnancies. One
dam at each dosage (including control) was sacrificed in poor
condition. Treatment did not adversely affect litter parameters
(e.g., litter size, fetal weight). There was no dose-related
increase in the incidence of malformations. The NOAEL was 4 mg/kg
bw/day with respect to maternotoxicity and 36 mg/kg bw/day for
fetotoxicity and teratogenicity (Cozens et al., 1985).
Special studies on genotoxicity
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The results are
summarized in Table 2.
Special studies on neurotoxicity
Hens
Six laying hens were treated with daily oral doses of 1 g/kg
bw/day for five days. Dosing was repeated after three weeks and the
birds sacrificed three weeks later. A positive control group was
treated with tri-ortho-tolyl phosphate (TOTP). No signs of
intoxication were noted. Histological examination of nervous
tissues revealed no lesions (Milner & Butterworth, 1977).
Rats
Groups of rats (6/sex/group) were fed diets containing 0 or 900
ppm fenpropathrin (purity not specified) for up to 25 days. Tremors
and high mortality were noted. On histological examination of the
sciatic nerve, swelling and disintegration of nerve axons were
observed whereas no myelin lesions were noted (Hend & Butterworth,
1976b).
The neurotoxic effect of fenpropathrin in rats was assessed by
means of the slip angle test. Oral doses of 0, 10, 25, 50, 75 or
100 mg/kg bw were administered to male rats (10/dose) and the angle
at which the treated animals slipped from the board determined. The
mean slip angle (MSA) decreased dose-dependently at 50 mg/kg bw and
higher. Clinical signs observed in animals treated with doses of 25
mg/kg bw and above consisted of slight to severe tremor, ataxia and
limb paralysis appearing at 3-7 hours after administration. The
effects on MSA and toxic signs almost disappeared after 24 hours.
Dose-related mortalities were observed at 50 mg/kg bw and above
(Hiromori et al., 1986b).
Table 2. Mutagenicity of fenpropathrin
Test system Test object Concentration Purity % Results Reference
non-activated activated
In vitro
Ames test S. typhimurium 50-5000 µg/plate 92.5 negative negative Izumozaki et al., 1984
(various strains) Yoshitake et al., 1987
Ames test S. typhimurium 10-1000 µg/plate 97.0 negative1 negative Suzuki, 1977
(various strains)
Reverse mutation Saccharomyces 92.5 negative negative Hara et al., 1984
mitotic crossing cerevisiae D7
over and mitiotic
gene convn. assay
Mammalian cell Mouse lymphoma 50-400 µg/ml (-activ). 91.4 negative negative Richold et al., 1982b
mutation assay L5178Y cells 30-300 µg/ml (+activ).
Gene mutation V79 Chinese 50-500 µg/ml 92.4 negative negative Yoshitake et al., 1988
assay hamster cells
DNA repair assay HeLa S3 cells 0.16-2.5 µg/ml ? negative negative Richold et al., 1982a
Rec-assay Bacillus subtilis 10-5000 µg/disc 97.0 negative negative Kishida et al., 1980
M45 rec and H17
DNA repair assay Bacillus subtilis 100-10 000 µg/disc 92.5 negative negative Yoshitake et al., 1986
Chromosome damage Chinese hamster 10 or 20 mg/kg bw 97.0 negative negative Dean, 1975
bone marrow cells orally on two
succcessive days
Table 2 (contd)
Test system Test object Concentration Purity % Results Reference
non-activated activated
Chromosome Chinese hamster 50-500 µg/ml (-activ). 92.5 negative negative McSheehy & Nunziata,
aberration assay ovary cells (CHO) 500-5000 µg/ml (+activ). 1984
Chromosome CHO-K1 cells 10-30 µg/ml (-activ). 92.4 negative negative Yoshitake et al., 1989
aberration assay 250-1000 µg/ml (+activ).
Chromosome Chiense hamster 0.003-0.1 mmol/l 92.5 negative negative Yoshitake et al., 1989b
aberration assay ovarian cells
(CHO-K1)
Sister chromatid Chinese hamster 0.003-0.1 mmol/l 92.5 negative negative Hara & Suzuki, 1984b
exchange ovarian cells
(CHO-K1)
In vivo
Micronucleus test Mouse femur cells 50-200 mg/kg 92.5 negative negative Hara & Suzuki, 1984a,c
intraperitoneally
Host mediated S. cerevisiae JD1 10-20 mg/kg bw orally 97.0 negative negative Brooks et al., 1976
assay (in male mice)
1 +activation by hepatic S-9 fractions obtained from 6 different mouse strains
Functional testing was performed on 2 animals/sex at 0, 125 or
500 ppm (satellite group of long-term study in rats, Hend &
Gellatly, 1979) using the "inclined plane test". Preliminary
results revealed an impaired performance in animals fed 500 ppm.
Measurements of ß-glucuronidase activity (parameter indicative of
Wallerian degeneration in nerves) did not reveal a clear increase as
would be expected as a result of toxic neuropathy. Macroscopy and
histopathology of sciatic and tibial nerves did not show treatment-
related changes (Hend & Gellatly, 1980).
Special study on effects on enzymes
A preliminary investigation was performed to study the possible
effect of fenpropathrin on the induction of hepatic microsomal
enzymes. Four Charles River CD rats were fed dietary concentrations
of 1, 10, 100 or 1000 ppm over two weeks. No significant difference
was observed between the activities of control and test livers in
the in vitro O-dealkylation of 14C-chlorfenvinphos and no dose-
related increase in liver weights was induced. Therefore, the
results of this study do not provide any evidence for an inducing
effect of fenpropathrin on liver microsomal enzymes (Creedy &
Potter, 1976).
Special study on antidotes
The therapeutic potency of intraperitoneally administered
methocarbamol [3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate] was
examined against acute oral intoxication of rats after treatment
with lethal doses of fenpropathrin (100 mg/kg bw) and some other
pyrethroids. Methocarbamol was initially administered
intraperitoneally at a dose of 400 mg/kg bw followed by repeated
doses of 200 mg/kg bw. Treatment with methocarbamol reduced the
mortality from 60% to 0% and depressed tremors (Hiromori et al.,
1986a).
Special study on paresthesia
Rabbits
Facial paresthesia activities following exposure to
fenpropathrin and other pyrethroids were estimated using a rabbit
model. To determine the intensity of facial paresthesia changes in
behaviour after dermal application of the pyrethroids were recorded.
Doses applied ranged from 0.0001-10 mg/animal. The frequency of
licking and/or biting after application showed a clear dose-response
relationship with all pyrethroids tested. Post-treatment with
vaseline-based 5% benzocaine ointment (local anaesthetic) was
effective in reducing the intensity of animal response. Post-
treatment by application of undiluted vitamin E was also effective
for treatment of facial paresthesia (Hiromori & Takemura, 1983).
Special studies on irritation and sensitization
The test material (purity 90.2%) caused no skin irritation and
only very slight eye irritation in rabbits (Matsubara et al.,
1978).
Two skin sensitization studies were conducted with guinea-pigs.
No sensitization reactions occurred (Okuno et al., 1975; Suzuki &
Miyamoto, 1981).
Observations in humans
In a field study in Japan, six workers participated in two
tests using a 5% emulsifiable concentrate. The spray concentrations
were 25-50 ppm (a.i.), the spraying time 2 hours. A motor mounted
sprayer was used. The workers wore protective clothing. No effects
were reported (e.g., headache, nasal discharge, itching, burning,
pain in face or limbs) (Fujita, 1980).
Some workers exposed to pyrethroids in the laboratory during
formulation processes or in field trials in England reported
transient abnormal facial sensations. A clinical and
electrophysiological study using an electromyograph was carried out
on 23 workers. Electrophysiological studies were also carried out
on an equal number of age and sex-matched control subjects who had
no contact with pyrethroids. Nineteen had experienced at least one
episode of abnormal facial sensations and 13/23 had experienced
several such episodes. Permethrin, cypermethrin, fenpropathrin and
fenvalerate were most frequently used. Symptoms were limited to the
face. Characteristically, the symptoms appeared 30 minutes to eight
hours after exposure. Since exposure to the different pyrethroids
varied, it was not possible to determine which compounds were more
likely to produce symptoms. Electrophysiological investigation
showed statistically significant increase in maximal nerve
conduction velocity in exposed workers compared to controls, which
is contrary to what is expected in subclinical neuropathy. The
increase must therefore be fortuitous. This conclusion is supported
by the observation that no difference in nerve conduction velocity
was noted in workers more exposed than others (Le Quesne et al.,
1980; Matsumoto et al., no date).
In a survey of 18 operators using a 10% fenpropathrin product,
no adverse effects were reported, with the exception of slight nasal
irritation. All operators wore protective clothing (Shell Chemical
Ltd., 1978).
COMMENTS
After oral administration of fenpropathrin to rats, the
compound was almost completely absorbed and eliminated in urine and
in faeces. The major biotransformation reactions consisted of
oxidation at the methyl groups of the acid moiety and at the 2'-and
4'-positions of the alcohol moiety, cleavage of the ester linkage
followed by glucuronide, sulfate or glycine conjugation.
Fenpropathrin has been tested for acute toxicity and it has
been classified as moderately hazardous by WHO.
In a short-term feeding study in rats conducted at dietary
concentrations of 0, 3, 30, 100, 300 or 600 ppm over thirteen weeks,
the NOAEL was 300 ppm, equal to 17 mg/kg bw/day, based on reduced
body-weight gain and the appearance of clinical signs at higher dose
levels. In a second 13-week study in rats, the NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain at
higher dose levels.
A one-year study in dogs conducted at dose levels of 0, 100,
250 or 750 ppm revealed a NOAEL of 100 ppm equal to 3 mg/kg bw/day,
based upon reduced body-weight gain and clinical signs (emesis,
tremors) at 250 ppm.
A long-term toxicity/carcinogenicity study was performed in
mice over 2 years at 0, 40, 150 and 600 ppm. The NOAEL was the
highest dose tested, 600 ppm, equal to 56 mg/kg bw/day. There was
no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study in rats conducted
at dietary concentrations of 0, 1, 5, 25, 125 or 500 ppm over two
years, the NOAEL was 125 ppm, equal to 5 mg/kg bw/day, based on
depression in body-weight gain at 500 ppm. There was no evidence of
carcinogenicity.
A second long-term toxicity/carcinogenicity study in rats
performed at dietary concentrations of 0, 50, 150, 450 or 600 ppm
over two years revealed a NOAEL of 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses. There
was no evidence of carcinogenicity.
In a multigeneration reproduction study in rats, fenpropathrin
was administered at dietary levels of 0, 5, 25 or 250 ppm. The
NOAEL was 25 ppm, equal to 1.6 mg/kg bw/day, based on decreased pup
weights in the F3a generation at 250 ppm.
In a second multigeneration reproduction study conducted at
dose levels of 0, 40, 120 or 360 ppm, the NOAEL was 40 ppm, equal to
3 mg/kg bw/day, based on depression of body weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above.
Two oral teratogenicity studies in rats were performed at dose
levels of 0, 0.4, 2 or 10 mg/kg bw/day and 0, 0.4, 1.5, 2, 3, 6 or
10 mg/kg bw/day. The NOAELs were 2 and 3 mg/kg bw/day in the two
studies, respectively, with respect to maternotoxic effects and a
NOAEL of 10 mg/kg bw/day in both for embryotoxicity and
teratogenicity.
In an oral teratogenicity study in rabbits at dose levels of 0,
1.5, 3 or 6 mg/kg bw/day, the NOAEL was 6 mg/kg bw/day. In a second
study with oral doses of 0, 4, 12 or 36 mg/kg bw/day the NOAEL was 4
mg/kg bw/day with respect to maternotoxicity.
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The Meeting concluded
that fenpropathrin was not genotoxic.
Based upon studies in hens and rats, fenpropathrin exhibited no
potential for delayed neurotoxicity.
Data on observations in humans were not suitable for the
estimation of an acceptable daily intake.
An ADI of 0-0.03 mg/kg bw was established based on a NOAEL of 3
mg/kg bw/day in the multigeneration reproduction study in rats, the
teratogenicity studies in rats and the one-year feeding study in
dogs, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Mouse: 600 ppm, equal to 56 mg/kg bw/day (two-year study)
Rat: 150 ppm, equal to 7 mg/kg bw/day (two-year study)
40 ppm, equal to 3 mg/kg bw/day (reproduction study)
3 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 4 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Aitken, R. & Rushton, B. (1981) Histopathology report for compound
WL41706. 26, 53 and 104 week study in rats. Inveresk report project
415386. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0046.
Brooks, T.M., Dean, B.J. & Thorpe, E. (1976) Toxicity studies with
WL 41706 in the host mediated assay. Shell research report TLGR 0003
76. Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0007.
Bruce, E.D., Griffis, L.C. & Wong, Z.A. (1986) The acute vapor
inhalation toxicity of Danitol technical (SX-1713) in mice and rats.
Chevron Study CEHC 2545. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0170.
Colley, J., Gopinath, C. & Offer, J.M. (1987) S-3206: Two-year
feeding study in mice (Addendum to final report). Huntingdon report
SMO 149 861391 (Addendum to SMO 149 8640 (FT-51-0135)). Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-71-0180.
Colley, J., Heywood, R., Street, A.E., Gopinath, C., Offer, J.M.,
Gibson, W.A., & Almond, R.H. (1985) S-3206: Two year feeding study
in mice. Huntingdon report SMO 14984607. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-51-0135.
Colley, J., Welch, P.J., Heywood,R., Prentice, D.E. & Gibson, W.A.
(1981a) S-3206 Preliminary assessment of toxicity to mice by dietary
administration for four weeks. Huntingdon report SMO 121 81289.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0047.
Colley, J., Welch, P.J., Heywood, R., Prentice,D.E. & Gibson, W.A.
(1981b) S-3206 Second preliminary assessment of toxicity to mice by
dietary administration for four weeks. Huntingdon report SMO 139
81574. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0049.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Isaacs, K.R., Gibson, W.A. & Almond, R.H. (1982a) S-3206 Toxicity to
rats by dietary administration for four weeks. Huntingdon report SMO
146 8210. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0064.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Gibson, W.A. &
Almond, R.H. (1982b) S-3206 Toxicity to rats by dietary
administration for four weeks. Huntingdon report SMO 158 82335.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0074.
Cox, R.H. (1986) Teratology study in rats S-3206. Addendum I to
final report. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-61-0176.
Cox, R.H. (1987) S-3206: Amendment II to final report teratology
study in rats. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-71-0188.
Cozens, D.D., Barton, S.J., Offer, J.M., Gibson, W.A. & Anderson, A.
(1986) Effect of S-3206 on multiple generations of the rat.
Huntingdon report SMO 164 85707. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
61-0159.
Cozens, D.D., Hughes, E.W., Masters, R.E. & Anderson, A. (1985) The
effect of S-3206 on pregnancy of the New Zealand white rabbit.
Huntingdon report SMO 181 84667. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
51-0134.
Crawford, M.J. (1975) The metabolism of WL 41706 in mammals. The
fate of a single oral dose of (14C) WL 41706 in the rat. Shell
Research TLGR 0071 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FM-51-
0002.
Crawford, M.J. & Hutson, D.H. (1976) Metabolic fate of (14C) WL
41706 in rats. Shell Research TLGR. 0034 76. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FM-61-0001.
Crawford, M.J. & Hutson, D.H. (1977) The metabolism of the
pyrethroid insecticide (+)-alpha-cyano-3-phenoxybenzyl 2,2,3,3-
tetramethyl-cyclopropanecarboxylate, WL 41706, in the rat. Pestic.
Sci., 8: 579-599. Submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FM-71-0050.
Crayford, J.V. (1975) The excretion and residues of radioactivity in
cows fed (14C) WL 41706 in their diet. Shell Research TLGR 0096 75.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FR-51-0015.
Creedy, C.L. & Potter, D. (1976) The effect of feeding WL 41706 on
the microsomal mono-oxygenase system of rat liver. Shell Research
report TLGR 0043 76. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0009.
Dean, B.J. (1975) Toxicity studies with WL 41706 (S-3206).
Chromosome studies on bone marrow cells of Chinese Hamsters after
two daily oral doses of WL 41706 (S-3206). Shell Research report
TLGR 0104 75. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-51-0003.
Dean, G.A., Gopinath, C. & Fish, L.E. (1987) Potential tumorigenic
and toxic effects in prolonged dietary administration to rats
(Addendum to final report). Huntingdon report SMO 167 87185
(Addendum to SMO 167 851348). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0181.
Dix, K.M. (1975) Toxicity studies of WL 41706: Teratological studies
in rabbits given WL 41706 orally. Shell Research report TLGR 0103 75
(Addendum to FT-51-0006). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-51-
0133.
Else, R. & Rushton, B. (1981) Histopathology report for compound WL
41706 3-generation study in rats. Inveresk report project 415370.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0069.
Fish, C.J., Lewis, D.J. & Gopinath, C. (1986) Electron micrograph
Addendum to histopathology report No SMO/167 S-3206 potential
tumorigenic and toxic effects in prolonged dietary administration to
rats. Huntingdon report SMO 167 86602 (Addendum to SMO 167 851348
(FT-61-0161)). Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0162.
Fleming, D.J. (1979) Toxicity studies on the insecticide WL 41706: A
three generation reproduction study (minus histopathology) in rats -
Corrigendum to FT-91-0027. Shell Research report TLGR 79 0071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-01-0028.
Fujita, Y. (1980) Interview survey concerning complaints connected
with use of S3206 in the field. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0040.
Hara, S. & Suzuki, T. (1980) Acute oral toxicity of S-3206 in
rabbits. Unpublished Sumitomo study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-00-0039.
Hara, M. & Suzuki, T. (1984a) Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0106.
Hara, M. & Suzuki, T. (1984b) In vitro sister chromatid exchanges
test of S-3206 in CHO-K1 cells. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-40-0108.
Hara, M. & Suzuki, T. (1984c) Addendum: Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0114.
Hara, M., Yamada, F., Kogiso, S., Yoshitake, A., Matsuo, M. &
Miyamoto, J. (1984) Mutagenicity test of S-3206 in Saccharomyces
cerevisiae D7. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-40-
0121.
Hend, R.W. & Butterworth, S.T.G (1975) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0031 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0001.
Hend, R.W. & Butterworth, S.T.G (1976a) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0020 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0013.
Hend, R.W. & Butterworth, S.T.G. (1976b) Toxicity studies on the
insecticide WL 41706: A short-term feeding study in rats. Shell
Research report TLGR 0041 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0011.
Hend, R.W. & Gellatly, J.B.M. (1979) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival,
bodyweight, food intake, organ weights, clinical chemistry,
hematology and gross pathological observations of rats exposed to WL
41706 for up to two years. Shell Research report TLGR 79 062.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0026.
Hend, R.W. & Gellatly, J.B.M. (1980) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival, body
weight, food intake, organ weights, clinical chemistry, hematology
and gross pathological observations of rats exposed to WL 41706 for
up to two years, Results of studies after 18 months. Shell Research
report TLGR 79 062 (Addendum to FT-91-0026). Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0032.
Hend, R.W., Gellatly, J.B.M. & Fleming, D.J. (1979) Toxicity studies
on the insecticide WL 41706: A three generation reproduction study
(minus histopathology) in rats. Shell Research report TLGR 79 071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0027.
Heywood, R. (1982) An appraisal of the toxicological findings on
rats receiving a synthetic pyrethroid S-3206 (fenpropathrin) by
dietary administration for 4 weeks. Huntingdon report (unnumbered,
1982). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0075.
Hiromori, T. Hosokawa, S. & Miyamoto, J. 1982a: Fenpropathrin
toxicology overview - Acute toxicity and safety in use. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-20-0079.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1982b) Acute oral toxicity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-20-0076.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1983a) Acute oral toxicity of S-3206 (97.3%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0082.
Hiromori, T., Misaki, Y., Seki, T., Hosokawa, S. & Miyamoto, J.
(1983b) Acute oral toxicity of S-3206 (91.8%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0081.
Hiromori, T., Nakanishi, T., Kawaguchi, S., Sako, H., Suzuki, T. &
Miyamoto, J. (1986a) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci. 11: 9-14.
Hiromori, T., Nakanishi, T., Suzuki, T., Kato, T. & Miyamoto, J.
(1986b) The mean slip angle test of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-60-0153.
Hiromori, T. & Takemura, T. (1983) Short report on facial
paresthesia following exposure to fenpropathrin, cypermethrin,
fenvalerate or permethrin in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0087.
Izumozaki, H., Hara, M. & Suzuki, T. (1984) Gene mutation test of S-
3206 in bacterial system. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-40-0107.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism
of fenvalerate and the (2S, alphaS)-isomer in rats and mice. J.
Pestic. Sci. 6: 317-326.
Kaneko, H., Shiba, K., Yoshitake, A. & Miyamoto, J. (1987)
Metabolism of fenpropathrin (S-3206) in rats. J. Pestic. Sci. 12:
385-395.
Kishida, F., Suzuki, H., Miyamoto, J. (1980) Studies on DNA damaging
capacity of S-3206 with Bacillus subtilis. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-00-0038.
Kohda, H. (1975) Acute oral toxicity of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-50-0018.
Kohda, H. (1976) Acute dermal toxicity of S-3206 in rats.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0019.
Kohda, H. & Kadota, T. (1975) Acute oral toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-50-
0035.
Kohda, H. & Kadota, T. (1976a) Acute dermal toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0036.
Kohda, H. & Kadota, T. (1976b) Acute subcutaneous and
intraperitoneal toxicity of S-3206 technical in rats and mice.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0037.
Kohda, H., Kadota, T. & Miyamoto, J. (1976) Acute inhalation
toxicity of S-3206 and S-5602 in mice an rats. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number AT-50-0043.
Ku, H.S. & Doran, T.J. (1990a) A study to determine the nature of
the residue in milk, meat and tissue from lactating goats dosed with
14C fenpropathrin. Ricerca Inc report project 89 0109. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0041.
Ku, H.S. & Doran, T.J. (1990b) A study to determine the nature of
the residue in poultry end eggs from chickens dosed with 14C
fenpropathrin. Ricerca Inc., report project 89 0084. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0042.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
Marroquin, F., Richter, W.R., Myer, J.R. & Johnson, D.E. (1981) S-
3206 technical grade: Acute dermal toxicity (LD50) study in rabbits
(TSCA 7/79) (EPA 8/78) (OSHA). IRDC report project 491 002.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0051.
Matsubara, T., Hara, S. & Kadota, T. (1978) Primary eye and skin
irritation tests of S-3206 in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-80-0023.
Matsumoto,S., Suzuki, T., Kato, T. & Yamada, H. (no date) Comments
on the facial sensation-inducing potential of fenpropathrin.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-80-0195.
McSheehy, T. & Nunziata, A. (1984) Chromosome aberrations in Chinese
hamster ovary (CHO) cells in vitro. Test substance: S-3206 Final
report 113-005. Life Science Research Roma report project 113 005.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-41-0104.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 41706
to adult hens. Shell Research report TLGR 0068 77. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FW-81-0006.
Misaki, Y., Hiromori, T., Hosokawa, S. & Miyamoto, J. (1983)
Comments on acute oral toxicity of fenpropathrin technicals with
high purity and lower purity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0083.
Misaki, Y. & Kohda, H. (1981a) Acute oral toxicity of two impurities
of S-3206 (technical) in mice. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0044.
Misaki, Y. & Kohda, H. (1981b) Acute oral toxicity of 2,2,3,3-
tetramethylcyclopropane carboxylic anhydride in mice. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-90-0045.
Morseth, S.L. (1990) Rat teratology study with S-3206. Hazleton
report project 343 216. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-01-
0216.
Ohkawa, H., Kaneko, H., Tsuji, H. & Miyamoto, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
Okuno, Y. (1981) Comment on possible weight changes of kidney and no
effect levels in a 2-year chronic toxicity study on WL 41706
(fenpropathrin). Comments 0n FT-91-0026 and FT-11-9946. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-10-0048.
Okuno, Y., Kadota, T. & Miyamoto, J. (1975) Skin sensitization study
of S-3206 in guinea-pigs. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-50-0024.
Omodaka, H., Misaki, Y. & Okuno, Y. (1986a) Acute oral toxicity of
S-3206 in rats. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0138.
Omodaka, H., Suzuki, T. & Kato, T. (1986b) Acute dermal toxicity
study of S-3206 in rats. Unpublished Sumitomo study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
60-0139.
Pauw van der, C.L., Dix, K.M., Blanchard, P.M., McCarthy, W.V. &
Stevenson, D.E. 1975: Teratological studies in rabbits given WL
41706 orally. Shell Research report TLGR 0103 75. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-51-0006.
Pence, D.H., Alsaker, R.D., Hepner, K.E., Dawkins, B.G., Vargus,
K.J., Hagen, W.H. & Hitzelberg, D.A. (1984) Chronic study in dogs S-
3206 T.G. Final report. Hazleton report project 343 153. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-41-0122.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980a) Subchronic toxicity study in dogs S-3206.
Final report. Hazleton report project 343 125. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0034.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980b) Subchronic toxicity study in dogs S-3206.
Addendum to final report. Hazleton report project 343 125. Addendum
to FT-01-0034. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-01-0041.
Pence, D.H., Pence, L.A., Durloo, R. and Cameron, J. (1980c)
Teratology study in rats S-3206. Final report. Hazleton report
project 343 122. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-010031.
Pence, D.H., Weatherholtz, W.M. & Cameron, J.T. (1978) Oral dose
range finding study in dogs, S-3206. Final report. Hazleton report
project 343 123. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-91-0025.
Richold, M., Allen, J.A. & Proudlock, R.J. (1982a) Autoradiographic
assessment of DNA repair in mammalian cells after exposure to S-3206
(fenpropathrin). Huntingdon report SMO 143 81881. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-21-0068.
Richold, M., Grantham, C.J. & Basham, K.J. (1982b) An assessment of
the mutagenic potential of S-3206 using an in vitro mammalian cell
test system (with comment by J. Miyamoto). Huntingdon report SMO 144
8252. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference numberFT-21-0060.
Riley, J.H., Nair, K.P.C., Kopplin, J.R., Johnson, D.E., Ott, S.S.,
Meyer, J.R. & Spicer, E.J.F. (1982) S-3206 (technical grade) 21-day
dermal study in rabbits (EPA) (technical product). IRDC report
project 491 010. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-21-0058.
Ruzo, L.O., Unai, T. & Casida, J.E. 1978: Decamethrin metabolism in
rats. J. Agric. Food Chem. 26: 918-925.
Shell Chemical (UK) Limited (1978) A survey of operator experience
with fenpropathrin 10% EC - A pyrethroid insecticide/acaricide for
use in fruit and ornamental crops in 1986. Shell UK report 16th
January 1987. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-71-0179.
Summitt, L.M. & Albert, J.R. (1976) Intravenous toxicity of SD 41706
(1-24-0-0) in the mouse. Shell Development Co report TIR 74 110 76.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0010.
Suzuki, H. (1977) Studies on mutagenicity of some pyrethroids on
Salmonella strains in the presence of mouse hepathic S9 fractions.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number AT-70-0157.
Suzuki, T. & Miyamoto, J. (1981) Skin sensitization test of S-3206
in guinea-pigs. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-10-
0078.
Suzuki, T., Sako, H. & Okuno, Y. (1986) Acute oral toxicity study of
S-3206 in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0175.
Warren, S., Heywood, R., Street, A.E., Gopinath, C., Browne, J.G.,
Gibson, W.A., Reed, L.E. & Anderson, A. (1986) S-3206: Potential
tumorigenic and toxic effects in prolonged dietary administration to
rats (Final report. Huntingdon report SMO 167 851348. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-61-0161.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yoshida, A., Kosaka, T., Miyaoka, T., Maita, K. & Goto, S. (1986) S-
3206: 13-week oral subchronic toxicity study in rats. Inst. Environ.
Toxicol. Tokyo report 17th, July 1986. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-61-0166.
Yoshitake, A., Kogiso, S. & Hara, M. (1990) In vitro chromosomal
aberration test of S-3206 in Chinese Hamster ovary cells (CHO-K1).
Sumitomo study M83-54. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-00-
0215.
Yoshitake, A., Kogiso, S., Kato, H. & Hara, M. (1988) In vitro
gene mutation test of S-3206 in V79 Chinese Hamster cells in
culture. Sumitomo study 1174. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-80-
0209.
Yoshitake, A. Kogiso, S. & Yamada, F. (1986) Bacterial DNA repair
test of S-3206. Sumitomo study MUT86020. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-60-0165.
Yoshitake, A., Kogiso, S & Yamada, F. (1987) Reverse mutation test
of S-3206 in bacterial systems. Sumitomo study 704. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-70-0182.
Yoshitake, A., Kogiso, S., Yamamoto, K. & Hara, M. (1989) In vitro
chromosomal aberration test of S-3206 in Chinese Hamster ovary cells
(CHO-K1). Sumitomo study 1730. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-90-
0200.
Table 1. Acute toxicity of fenpropathrin (technical material)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Rat M oral 54 97 Kohda, 1975
F oral 49 97 Kohda, 1975
M oral 71 91.8 Hiromori et al., 1983b
Misaki et al., 1983
F oral 67 91.8 Hiromori et al., 1983b
Misaki et al., 1983
M oral 77 97.3 Hiromori et al., 1983a
Misaki et al., 1983
F oral 67 97.3 Hiromori et al., 1983a
Misaki et al., 1983
M oral 164 97.3 Hiromori et al., 1983b
F oral 107 97.3 Hiromori et al., 1983b
M&F oral 60 93.8 Omodaka et al., 1986a
F oral 70 93.8 Omodaka et al., 1986a
M&F inhalative (vapour) > 0.009 µg/l* 94.5 Bruce et al., 1986
M&F inhalative (Xylene EC mist) > 96 µg/l* 97 Kohda et al., 1976
M dermal (sol. in corn oil) 1600 97 Kohda, 1976
F dermal (sol. in corn oil) 870 97 Kohda, 1976
M&F dermal (viscous liquid) > 5000 93.8 Omodaka et al., 1986b
M sc 1410 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 225 97 Kohda & Kadota, 1976b
F ip 180 97 Kohda & Kadota, 1976b
Mouse M oral 67 97 Kohda & Kadota, 1975
F oral 58 97 Kohda & Kadota, 1975
M oral 135 93.8 Suzuki et al., 1986
F oral 154 93.8 Suzuki et al., 1986
M inhalative (Xylene EC mist) 100 µg/l* 97 Kohda et al., 1976
F inhalative (Xylene EC mist) 43 µg/l* 97 Kohda et al., 1976
Table 1 (contd)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Mouse M dermal 740 97 Kohda & Kadota, 1976a
F dermal 920 97 Kohda & Kadota, 1976a
M sc 1350 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 230 97 Kohda & Kadota, 1976b
F ip 210 97 Kohda & Kadota, 1976b
M iv 4.5 ? Summitt & Albert, 1976
Rabbit M oral 675 96.2 Hara & Suzuki, 1980
F oral 510 96.2 Hara & Suzuki, 1980
M&F dermal > 2000 ? Marroquin et al., 1981
Dog M&F oral > 1000 96.2 Pence et al., 1978
* LC50
Rats
Fenpropathrin (purity 96%) was fed to groups of Carworth Farm E
rats (12/sex/dose; control 24/sex) at dietary concentrations of 0,
2, 10, 50 or 250 ppm over 13 weeks. The treatment did not influence
the general health and behaviour, haematology, clinical chemistry or
pathology of treated animals. Body-weight gain was slightly
increased at all dose levels. A slight increase in spleen weight in
male rats at 250 ppm was the only change concerning organ weights
and may present a borderline effect. The NOAEL was > 250 ppm,
equal to 18 mg/kg/day (males) and 21 mg/kg bw/day females) (Hend &
Butterworth, 1975).
In a similar study, rats (12/sex/group; control 24/sex) were
fed diets containing fenpropathrin (purity 97%) at concentrations of
0, 3, 30, 100, 300 or 600 ppm over 13 weeks. After 5 weeks of
exposure, most females and one male showed tremors and reduced body-
weight gain at 600 ppm. The tremors disappeared towards the end of
the study. Changes in a few haematological parameters were marginal
and were - though statistically significant - not considered
biologically significant. Slight increases in plasma ALP were
observed at 600 ppm. Organ weights were not influenced by treatment
and no abnormal histopathological findings were observed. The NOAEL
was 300 ppm, equal to 17 mg/kg bw/day (males) and 24 mg/kg bw/day
(females) based on the appearance of clinical signs and reduced
body-weight gain at 600 ppm (Hend & Butterworth, 1976a).
In a third study, groups of rats (SPF SD(Crj:CD); 12/sex/dose)
were fed concentrations of 0, 15, 50, 150, 450 or 600 ppm
fenpropathrin (purity 93.1%) over a period of 13 weeks. One female
died at 600 ppm. A dose-dependent depression of body-weight gain
was observed at 600 ppm and 450 ppm in female rats, and at 600 ppm
in male rats. Food consumption was reduced at the beginning of the
study at 600 ppm in males and at 450 ppm and above in females; food
efficiency was reduced in females treated with 600 ppm. An increase
in urinary protein and specific gravity was observed in males at 450
ppm and above. Haematological and ophthalmological parameters as
well as organ weights were not influenced by the treatment. A
slightly elevated alkaline phosphatase activity was noted in females
of the 600 ppm group. There was no evidence of treatment-related
histopathological changes in any organ. The NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain and
urinalysis at higher dose levels (Yoshida et al., 1986).
Rabbits
In a 21-day dermal toxicity study with fenpropathrin (purity
91.4%), groups of New Zeeland white rabbits (10/sex/dose) received
dermal applications on either intact or abraded skin at dose levels
of 0, 500, 1200 or 3000 mg/kg bw. The treatment did not influence
survival, body weight, food consumption, haematology, clinical
chemistry, organ weights, or macroscopic or microscopic findings.
Single instances of slight skin reactions were observed at the
application sites of animals treated with 500 and 1200 mg/kg bw and
in most animals at 3000 mg/kg bw. The NOAEL was > 3000 mg/kg bw
based on systemic toxicity (Riley et al., 1982).
Dogs
Fenpropathrin (purity 96.2%) was administered in the diet to
groups of beagle dogs (6/sex/dose) at concentrations of 0, 250, 500
or 1000/750 ppm over 13 weeks (1000 ppm for 3 weeks, 750 ppm for the
rest of the study because of the appearance of severe tremors and
ataxia). The doses were adjusted for purity. Treatment did not
affect survival, food consumption, ophthalmoscopy, clinical
chemistry, urinalysis, organ weights, gross or microscopic
pathology. Treatment-related clinical signs consisted of emesis,
tremors, ataxia, diarrhoea, lethargic appearance and salivation.
Some effects were observed in all treatment groups. The frequency
of the observed clinical signs in all of the compound-treated groups
decreased after week 5. One high-dose male dog was sacrificed
moribund. Reduced body-weight gain was observed at concentrations of
500 and 1000/750 ppm. Haematocrit and haemoglobin values and the
erythrocyte count in females at 1000/750 ppm were decreased. The
NOAEL was < 250 ppm, equal to 7 mg/kg bw/day in males and 10 mg/kg
bw/day in females, based on the occurrence of emesis at 250 ppm
(Pence et al., 1980a,b).
Fenpropathrin (purity 92.5%) was fed to groups of beagle dogs
(4/sex/dose) at dietary concentrations of 0, 100, 250 or 750 ppm
(adjusted to 100% active ingredient) over a period of 52 weeks. One
high-dose male died in the middle of the study after exhibiting
tremor and ataxia. Emesis occurred at an increased incidence in the
mid-dose and high-dose females but without a clear dose-response
relationship. The incidence of tremors however showed a clear dose-
related increase at 250 and 750 ppm (males: 0, 0, 49, 181; females:
0, 1, 42, 168 observations at 0, 100, 250 and 750 ppm,
respectively). Ataxia occurred at 750 ppm. Reduced body-weight
gain was observed at 750 ppm and at 250 ppm in females. The
treatment did not influence ophthalmoscopy, haematology, clinical
chemistry, urinalyses, gross or microscopic pathology. The only
significant difference concerning organ weight was an increase in
the relative kidney weight in females at 750 ppm. The NOAEL was 100
ppm, equal to 3 mg/kg bw/day, based on reduced body-weight gain and
occurrence of clinical signs at 250 ppm and higher (Pence et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
In a 104-week feeding study, groups of mice (Charles River (UK)
CD-1; 52/sex/group in main study; 40/sex/group in satellite groups
for interim sacrifice) were fed fenpropathrin (purity 91.4%-92.5%)
at concentrations of 0, 40, 150 or 600 ppm. These concentration
levels were based on two range-finding studies (Colley et al.,
1981a,b). Treatment did not affect the general behaviour, with the
exception of a marginal increase in the number of females at 600 ppm
showing hyperactivity at week 78. No effects on survival rate, body
weight, food consumption, food efficiency, urinalysis, macroscopic
pathology or organ weights were reported. Changes in haematological
parameters at 600 ppm included slight decreases in Hb and MCHC in
males and an increase in MCHC in females at week 25. Because these
changes were small, occurring only on some occasions and only in one
sex, they are not considered to be toxicologically significant.
Sporadic changes in some clinical chemical parameters were not
considered to be of toxicological significance (e.g., decrease in
urea nitrogen in males at 600 ppm, increase in GOT and GPT levels at
all dose levels without clear dose-response relationship). An
increased incidence in lung tumours (adenoma and adenocarcinoma) was
observed in males and females of treated groups. The incidences in
the satellite groups (spontaneous deaths and interim sacrifice after
26, 52 and 78 weeks) were 10, 12.5, 18 and 2.5% for males and 7.5,
12.5, 7.5 and 7.5% for females at 0, 40, 150 and 600 ppm,
respectively. The incidences in the main study were 12, 23, 35 and
31% for males and 10, 29, 19 and 13% females at 0, 40, 150 and 600
ppm, respectively. The incidences did not show a clear dose-
response relationship. The absence of any statistical significance
or significant trend supports the interpretation that the increased
incidences in the treatment groups were not due to a tumorigenic
activity of fenpropathrin. Moreover, data on the incidence of lung
tumours in historical controls showed that incidences of pulmonary
adenoma and adenocarcinoma in untreated animals may vary between 7
and 36%. There was no sex difference concerning lung tumours in
these controls. Therefore the results of this study gave no
evidence of fenpropathrin-induced carcinogenic potential. The NOAEL
was the highest dose tested, 600 ppm, equal to 56 mg/kg bw/day
(males) and 65 mg/kg bw/day (females) (Colley et al., 1985, 1987).
Rats
Groups of rats (COBS) were fed diets containing 0, 1, 5, 25,
125 or 500 ppm fenpropathrin (purity 97%) for two years. The group
sizes were 12/sex and 48/sex for the satellite and main study
controls, respectively; while the treatment groups included 6/sex
and 24/sex for the satellite and main study, respectively. The
treatment did not affect the survival, general health, behaviour of
the animals, clinical chemical or haematological parameters. The
depression in body-weight gain observed at 500 ppm was statistically
significant in females only. Food consumption of treated animals
was comparable with control animals at most observation times.
Absolute spleen weight increased at 500 ppm in females of the 6-
month satellite group and kidney weights decreased in females of all
treatment groups in the 12-month satellite groups without showing a
clear dose-response relationship. In the 2-year main study groups,
no treatment-related alterations in organ weights were observed.
Gross pathological examination showed a higher incidence of
white/grey foci or plaques in the lungs at 125 and 500 ppm in
females at 6 and 24 months. In the 2-year main groups, the number
of deaths attributable to renal failure in males at 500 ppm was
greater than in the other groups, but no increase was observed when
animals from satellite and main study groups were combined.
The histopathological neoplastic and non-neoplastic changes
found were consistent with the range and severity of changes usually
observed in this rat strain and did not give any evidence of
carcinogenicity. The NOAEL was 125 ppm, equal to 5 mg/kg bw/day
based on depression in body-weight gain at higher dose levels (Hend
& Gellatly, 1979; Okuno, 1981; Aitken & Rushton, 1981).
Single animals of a satellite group fed the above-mentioned
concentrations for 18 months were used for functional and
neurochemical investigations. For results see "Special Studies on
Neurotoxicity" (Hend & Gellatly, 1980).
Fenpropathrin (purity 91.4-92.5%) was fed to groups of rats
(Charles River CD; 50/sex/dose in main study and 15/sex/dose in
satellite groups for interim sacrifice) at dietary concentrations of
0, 50, 150, 450 or 600 ppm for 104 weeks. The dose levels were
selected based on the results of range-finding studies (Colley et
al., 1982a,b; Heywood, 1982). Clinical signs were restricted to
body tremors, most prevalent in females receiving 600 ppm, but were
also observed in males at the highest dose levels in the first few
weeks of the study and in females at 450 ppm.
Mortality increased in males and females receiving 600 ppm and
in females receiving 450 ppm during the first 26 weeks of treatment;
subsequently, mortality was less than that of control animals
resulting in highest survival rates in males at 600 ppm and in
females at 450 ppm. Exposure of main group females receiving 600
ppm was terminated after 52 weeks. A slightly higher food
consumption was reported for the high-dose females during the first
3 months of treatment, most probably resulting in the mortalities
observed. Body-weight gain was reduced in females at 600 ppm and
food utilization efficiency was marginally impaired.
Ophthalmological examination did not reveal treatment-related ocular
lesions. No abnormal haematological findings were reported.
Changes in clinical chemistry parameters at 450 and 600 ppm included
slightly reduced creatinine levels and reduced total protein levels
in males. The differences were minimal however and - though
statistically significant - most probably of no biological
significance. Urinalysis did not indicate any kidney lesions.
Macroscopic examination revealed no findings which were
treatment-related with the exception of an increased incidence of
alopecia in females at 600 ppm at 52 weeks. Changes in organ
weights were observed on some occasions but did not show a
treatment-related pattern and histopathological examination did not
reveal changes attributable to treatment. A few lymphoreticular
tumours were seen in treated groups of male rats, but none in
control group. The increases observed were not dose-related and
were within the incidence range of the historical controls for this
rat strain. There was no indication of a treatment-related increase
in tumour incidence or non-neoplastic organ changes. The electron
microscopic examination of tibial nerve did not reveal treatment-
related effects. The NOAEL was 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses (Warren
et al., 1986; Fish et al., 1986; Dean et al., 1987).
Reproduction studies
Rats
In a 3-generation reproduction study, fenpropathrin (purity
97%) was fed to rats (COBS; 30/sex/group) at dietary concentrations
of 0, 5, 25 or 250 ppm. Treatment did not influence the general
health condition or mortality, body-weight gain, food consumption,
pregnancy rates, litter size or litter weight. However, pup weights
in the F3a generation were reduced at 250 ppm. No macroscopic or
microscopic lesions were attributable to treatment. A very small
number of cases of locomotor incoordination occurred in pups fed 5
and 250 ppm. Most pups in the 5 ppm group showing these signs were
from one litter in the F1b generation. No similar lesions were
observed in the second litter of that pair nor in the following
generation and no macroscopic lesions were identified in these
animals. Histopathological examination (F2 parents, F3b pups) did
not reveal treatment-related tissue lesions. The NOAEL was 25 ppm,
equal to 1.6 mg/kg bw/day, based on decreased pup weights in the
F3a generation at 250 ppm (Fleming, 1979; Hend et al., 1979; Else
& Rushton, 1981).
In a second 3-generation study, groups of rats (CrL: COBS CD
(SD)BR; 11-28/sex/dose) were fed diets containing 0, 40, 120 or 360
ppm fenpropathrin (purity 92.5%). Animals of the last generation
(F2) were reared to maturity. At 360 ppm, most females that
littered showed body tremors associated with muscle twitches and
increased sensitivity. These signs were generally observed during
the lactation period. Increased mortality occurred at 360 ppm in
females only; 18/28 females at this dose died, most during the
lactation period of the F1b females. At 120 ppm, 2/24 F1b females
died during lactation. Mean body-weight gain of males in the F1
and F2 generations were reduced at 360 ppm. F1b females at 120
ppm showed also some indication of retarded weight gain. Treatment
had no influence on mating performance and no macroscopic or
microscopic abnormalities were observed at terminal autopsy that
were considered to be associated with treatment. At 360 ppm, F0
females showed a slight increase in liver weights. A few F2b pups
at 120 ppm showed body tremors prior to weaning and 2/3 of these
pups died subsequently. A slight reduction in litter size was
observed at 360 ppm in F0 animals (second mating) and in both
matings of F1b animals from days 4 to 21. At 360 ppm, pup weights
were lower in all generations. The NOAEL was 40 ppm, equal to 3
mg/kg bw/day, based on depression of body-weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above (Cozens et al., 1986).
Special studies on embryotoxicity/teratogenicity
Rats
Fenpropathrin (purity 96.2%) was fed by intubation to groups of
rats (Fischer 344 CDF; 27/28 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.4, 2 or 10 mg/kg bw/day. One mid-
dose and 9 high-dose females were found dead during the study.
Clinical signs noted at a somewhat greater incidence in the treated
animals included soft faeces, red or lacrimating eyes, alopecia or
hunched appearance. Post-dose tremors occurred on a few occasions
in some high-dose females. Body-weight gain was reduced in high-
dose females during the treatment period, and increased slightly
afterwards, resulting in an overall change comparable to the control
group. Food consumption was also reduced at the highest dose level.
Various gross lesions were found in animals that died during the
study. The findings included discolorations of organs, but no
specific target organ was identified. The pregnancy rate was lower
in the high-dose group compared to the other dose groups but other
reproduction parameters (e.g., number of corpora lutea, resorptions,
fetal viability) were not impaired by treatment. No visceral or
skeletal anomalies were noted in the pups. Visceral or skeletal
variants did not show dose-related increases. No teratogenic
effects were observed. The NOAEL for maternal toxicity was 2 mg/kg
bw/day. The NOAEL for embryotoxicity and teratogenicity was 10 mg/kg
bw/day (Pence et al., 1980c; Cox, 1986, 1987).
Groups of rats (Fischer-344; 30 females/dose group) were orally
treated with fenpropathrin (purity 91.9%) at dose levels of 0, 0.44,
1.6, 2.2, 3.3, 6.5 or 11 mg/kg bw/day (corresponding to 0, 0.4, 1.5,
2, 3, 6 or 10 mg/kg bw/day active ingredient) from day 6 to day 15
of gestation. Seven animals died in the highest dose group. Signs
of toxicity included ataxia, sensitivity to external stimuli,
tremors, prostration and convulsions. At 6 and 10 mg/kg bw/day,
mean maternal body-weight gains and food consumption were lower
during the dosing period. Pregnancy rate of high-dose females was
slightly lower (90%) compared to the other treatment groups (93%-
97%). Treatment had no influence on reproduction parameters such as
resorptions, fetal viability or fetal body weight. No visceral or
skeletal anomalies were observed that were considered treatment-
related. Skeletal variations were noted in all groups and included
variations in the stage of ossification and rib counts, but not in a
dose-dependent pattern. A higher incidence of incomplete
ossification of the sternebrae was observed in all treatment groups
but without a clear dose-response relationship. The highest
incidences with respect to some differences in ossification (e.g.,
5th/6th sternebrae incomplete ossification, asymmetric ossification
of sternebrae) were noted at 10 mg/kg bw. Statistical analysis
revealed no significant trend for the overall incidence of delayed
ossification of the 5th/6th sternebrae. The NOAEL for maternal
toxicity was 3 mg/kg bw/day. The NOAEL for embryotoxicity and
teratogenicity was 10 mg/kg bw/day (Morseth, 1990).
Rabbits
Groups of rabbits (banded Dutch; 20-30 females/group) were fed
fenpropathrin (purity 97%) at dose levels of 0, 1.5, 3 or 6 mg/kg
bw/day from day 6 to day 18 of gestation. No clinical signs of
intoxication were observed. A lower number of rabbits survived to
term at 3 mg/kg bw/day but this was unrelated to treatment.
Treatment had no effect on body weight of the dams. No adverse
effects were observed with respect to pre-implantation loss, number
of resorptions, fetal deaths, litter size, or pup weight. Increased
incidence in minor skeletal and visceral abnormalities (e.g.,
enlarged fontanelles, interparietal bones, fused sternebrae, extra
centres of ossification) were noted at 3 mg/kg bw but not at the
higher dose. Therefore these changes were not considered treatment-
related. The NOAEL for maternotoxicity, embryotoxicity and
teratogenicity was 6 mg/kg bw/day (van der Pauw et al., 1975; Dix,
1975).
Groups of rabbits (New Zeeland white; 17-19 females/dose) were
treated with oral doses of 0, 4, 12 or 36 mg/kg bw/day fenpropathrin
(purity 92.5%) from days 7 to 19 of gestation. A dose-related
increase in the incidence of grooming was observed in all dose
groups. At 12 and 36 mg/kg bw/day, flicking of forepaws was also
observed, with a few animals at 36 mg/kg bw/day showing shaky
movements and tremor. Slight reduction in body-weight gain and
reduced food consumption were noted at 36 mg/kg bw/day. One dam at
12 and two at 36 mg/kg bw failed to maintain their pregnancies. One
dam at each dosage (including control) was sacrificed in poor
condition. Treatment did not adversely affect litter parameters
(e.g., litter size, fetal weight). There was no dose-related
increase in the incidence of malformations. The NOAEL was 4 mg/kg
bw/day with respect to maternotoxicity and 36 mg/kg bw/day for
fetotoxicity and teratogenicity (Cozens et al., 1985).
Special studies on genotoxicity
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The results are
summarized in Table 2.
Special studies on neurotoxicity
Hens
Six laying hens were treated with daily oral doses of 1 g/kg
bw/day for five days. Dosing was repeated after three weeks and the
birds sacrificed three weeks later. A positive control group was
treated with tri-ortho-tolyl phosphate (TOTP). No signs of
intoxication were noted. Histological examination of nervous
tissues revealed no lesions (Milner & Butterworth, 1977).
Rats
Groups of rats (6/sex/group) were fed diets containing 0 or 900
ppm fenpropathrin (purity not specified) for up to 25 days. Tremors
and high mortality were noted. On histological examination of the
sciatic nerve, swelling and disintegration of nerve axons were
observed whereas no myelin lesions were noted (Hend & Butterworth,
1976b).
The neurotoxic effect of fenpropathrin in rats was assessed by
means of the slip angle test. Oral doses of 0, 10, 25, 50, 75 or
100 mg/kg bw were administered to male rats (10/dose) and the angle
at which the treated animals slipped from the board determined. The
mean slip angle (MSA) decreased dose-dependently at 50 mg/kg bw and
higher. Clinical signs observed in animals treated with doses of 25
mg/kg bw and above consisted of slight to severe tremor, ataxia and
limb paralysis appearing at 3-7 hours after administration. The
effects on MSA and toxic signs almost disappeared after 24 hours.
Dose-related mortalities were observed at 50 mg/kg bw and above
(Hiromori et al., 1986b).
Table 2. Mutagenicity of fenpropathrin
Test system Test object Concentration Purity % Results Reference
non-activated activated
In vitro
Ames test S. typhimurium 50-5000 µg/plate 92.5 negative negative Izumozaki et al., 1984
(various strains) Yoshitake et al., 1987
Ames test S. typhimurium 10-1000 µg/plate 97.0 negative1 negative Suzuki, 1977
(various strains)
Reverse mutation Saccharomyces 92.5 negative negative Hara et al., 1984
mitotic crossing cerevisiae D7
over and mitiotic
gene convn. assay
Mammalian cell Mouse lymphoma 50-400 µg/ml (-activ). 91.4 negative negative Richold et al., 1982b
mutation assay L5178Y cells 30-300 µg/ml (+activ).
Gene mutation V79 Chinese 50-500 µg/ml 92.4 negative negative Yoshitake et al., 1988
assay hamster cells
DNA repair assay HeLa S3 cells 0.16-2.5 µg/ml ? negative negative Richold et al., 1982a
Rec-assay Bacillus subtilis 10-5000 µg/disc 97.0 negative negative Kishida et al., 1980
M45 rec and H17
DNA repair assay Bacillus subtilis 100-10 000 µg/disc 92.5 negative negative Yoshitake et al., 1986
Chromosome damage Chinese hamster 10 or 20 mg/kg bw 97.0 negative negative Dean, 1975
bone marrow cells orally on two
succcessive days
Table 2 (contd)
Test system Test object Concentration Purity % Results Reference
non-activated activated
Chromosome Chinese hamster 50-500 µg/ml (-activ). 92.5 negative negative McSheehy & Nunziata,
aberration assay ovary cells (CHO) 500-5000 µg/ml (+activ). 1984
Chromosome CHO-K1 cells 10-30 µg/ml (-activ). 92.4 negative negative Yoshitake et al., 1989
aberration assay 250-1000 µg/ml (+activ).
Chromosome Chiense hamster 0.003-0.1 mmol/l 92.5 negative negative Yoshitake et al., 1989b
aberration assay ovarian cells
(CHO-K1)
Sister chromatid Chinese hamster 0.003-0.1 mmol/l 92.5 negative negative Hara & Suzuki, 1984b
exchange ovarian cells
(CHO-K1)
In vivo
Micronucleus test Mouse femur cells 50-200 mg/kg 92.5 negative negative Hara & Suzuki, 1984a,c
intraperitoneally
Host mediated S. cerevisiae JD1 10-20 mg/kg bw orally 97.0 negative negative Brooks et al., 1976
assay (in male mice)
1 +activation by hepatic S-9 fractions obtained from 6 different mouse strains
Functional testing was performed on 2 animals/sex at 0, 125 or
500 ppm (satellite group of long-term study in rats, Hend &
Gellatly, 1979) using the "inclined plane test". Preliminary
results revealed an impaired performance in animals fed 500 ppm.
Measurements of ß-glucuronidase activity (parameter indicative of
Wallerian degeneration in nerves) did not reveal a clear increase as
would be expected as a result of toxic neuropathy. Macroscopy and
histopathology of sciatic and tibial nerves did not show treatment-
related changes (Hend & Gellatly, 1980).
Special study on effects on enzymes
A preliminary investigation was performed to study the possible
effect of fenpropathrin on the induction of hepatic microsomal
enzymes. Four Charles River CD rats were fed dietary concentrations
of 1, 10, 100 or 1000 ppm over two weeks. No significant difference
was observed between the activities of control and test livers in
the in vitro O-dealkylation of 14C-chlorfenvinphos and no dose-
related increase in liver weights was induced. Therefore, the
results of this study do not provide any evidence for an inducing
effect of fenpropathrin on liver microsomal enzymes (Creedy &
Potter, 1976).
Special study on antidotes
The therapeutic potency of intraperitoneally administered
methocarbamol [3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate] was
examined against acute oral intoxication of rats after treatment
with lethal doses of fenpropathrin (100 mg/kg bw) and some other
pyrethroids. Methocarbamol was initially administered
intraperitoneally at a dose of 400 mg/kg bw followed by repeated
doses of 200 mg/kg bw. Treatment with methocarbamol reduced the
mortality from 60% to 0% and depressed tremors (Hiromori et al.,
1986a).
Special study on paresthesia
Rabbits
Facial paresthesia activities following exposure to
fenpropathrin and other pyrethroids were estimated using a rabbit
model. To determine the intensity of facial paresthesia changes in
behaviour after dermal application of the pyrethroids were recorded.
Doses applied ranged from 0.0001-10 mg/animal. The frequency of
licking and/or biting after application showed a clear dose-response
relationship with all pyrethroids tested. Post-treatment with
vaseline-based 5% benzocaine ointment (local anaesthetic) was
effective in reducing the intensity of animal response. Post-
treatment by application of undiluted vitamin E was also effective
for treatment of facial paresthesia (Hiromori & Takemura, 1983).
Special studies on irritation and sensitization
The test material (purity 90.2%) caused no skin irritation and
only very slight eye irritation in rabbits (Matsubara et al.,
1978).
Two skin sensitization studies were conducted with guinea-pigs.
No sensitization reactions occurred (Okuno et al., 1975; Suzuki &
Miyamoto, 1981).
Observations in humans
In a field study in Japan, six workers participated in two
tests using a 5% emulsifiable concentrate. The spray concentrations
were 25-50 ppm (a.i.), the spraying time 2 hours. A motor mounted
sprayer was used. The workers wore protective clothing. No effects
were reported (e.g., headache, nasal discharge, itching, burning,
pain in face or limbs) (Fujita, 1980).
Some workers exposed to pyrethroids in the laboratory during
formulation processes or in field trials in England reported
transient abnormal facial sensations. A clinical and
electrophysiological study using an electromyograph was carried out
on 23 workers. Electrophysiological studies were also carried out
on an equal number of age and sex-matched control subjects who had
no contact with pyrethroids. Nineteen had experienced at least one
episode of abnormal facial sensations and 13/23 had experienced
several such episodes. Permethrin, cypermethrin, fenpropathrin and
fenvalerate were most frequently used. Symptoms were limited to the
face. Characteristically, the symptoms appeared 30 minutes to eight
hours after exposure. Since exposure to the different pyrethroids
varied, it was not possible to determine which compounds were more
likely to produce symptoms. Electrophysiological investigation
showed statistically significant increase in maximal nerve
conduction velocity in exposed workers compared to controls, which
is contrary to what is expected in subclinical neuropathy. The
increase must therefore be fortuitous. This conclusion is supported
by the observation that no difference in nerve conduction velocity
was noted in workers more exposed than others (Le Quesne et al.,
1980; Matsumoto et al., no date).
In a survey of 18 operators using a 10% fenpropathrin product,
no adverse effects were reported, with the exception of slight nasal
irritation. All operators wore protective clothing (Shell Chemical
Ltd., 1978).
COMMENTS
After oral administration of fenpropathrin to rats, the
compound was almost completely absorbed and eliminated in urine and
in faeces. The major biotransformation reactions consisted of
oxidation at the methyl groups of the acid moiety and at the 2'-and
4'-positions of the alcohol moiety, cleavage of the ester linkage
followed by glucuronide, sulfate or glycine conjugation.
Fenpropathrin has been tested for acute toxicity and it has
been classified as moderately hazardous by WHO.
In a short-term feeding study in rats conducted at dietary
concentrations of 0, 3, 30, 100, 300 or 600 ppm over thirteen weeks,
the NOAEL was 300 ppm, equal to 17 mg/kg bw/day, based on reduced
body-weight gain and the appearance of clinical signs at higher dose
levels. In a second 13-week study in rats, the NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain at
higher dose levels.
A one-year study in dogs conducted at dose levels of 0, 100,
250 or 750 ppm revealed a NOAEL of 100 ppm equal to 3 mg/kg bw/day,
based upon reduced body-weight gain and clinical signs (emesis,
tremors) at 250 ppm.
A long-term toxicity/carcinogenicity study was performed in
mice over 2 years at 0, 40, 150 and 600 ppm. The NOAEL was the
highest dose tested, 600 ppm, equal to 56 mg/kg bw/day. There was
no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study in rats conducted
at dietary concentrations of 0, 1, 5, 25, 125 or 500 ppm over two
years, the NOAEL was 125 ppm, equal to 5 mg/kg bw/day, based on
depression in body-weight gain at 500 ppm. There was no evidence of
carcinogenicity.
A second long-term toxicity/carcinogenicity study in rats
performed at dietary concentrations of 0, 50, 150, 450 or 600 ppm
over two years revealed a NOAEL of 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses. There
was no evidence of carcinogenicity.
In a multigeneration reproduction study in rats, fenpropathrin
was administered at dietary levels of 0, 5, 25 or 250 ppm. The
NOAEL was 25 ppm, equal to 1.6 mg/kg bw/day, based on decreased pup
weights in the F3a generation at 250 ppm.
In a second multigeneration reproduction study conducted at
dose levels of 0, 40, 120 or 360 ppm, the NOAEL was 40 ppm, equal to
3 mg/kg bw/day, based on depression of body weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above.
Two oral teratogenicity studies in rats were performed at dose
levels of 0, 0.4, 2 or 10 mg/kg bw/day and 0, 0.4, 1.5, 2, 3, 6 or
10 mg/kg bw/day. The NOAELs were 2 and 3 mg/kg bw/day in the two
studies, respectively, with respect to maternotoxic effects and a
NOAEL of 10 mg/kg bw/day in both for embryotoxicity and
teratogenicity.
In an oral teratogenicity study in rabbits at dose levels of 0,
1.5, 3 or 6 mg/kg bw/day, the NOAEL was 6 mg/kg bw/day. In a second
study with oral doses of 0, 4, 12 or 36 mg/kg bw/day the NOAEL was 4
mg/kg bw/day with respect to maternotoxicity.
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The Meeting concluded
that fenpropathrin was not genotoxic.
Based upon studies in hens and rats, fenpropathrin exhibited no
potential for delayed neurotoxicity.
Data on observations in humans were not suitable for the
estimation of an acceptable daily intake.
An ADI of 0-0.03 mg/kg bw was established based on a NOAEL of 3
mg/kg bw/day in the multigeneration reproduction study in rats, the
teratogenicity studies in rats and the one-year feeding study in
dogs, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Mouse: 600 ppm, equal to 56 mg/kg bw/day (two-year study)
Rat: 150 ppm, equal to 7 mg/kg bw/day (two-year study)
40 ppm, equal to 3 mg/kg bw/day (reproduction study)
3 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 4 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Aitken, R. & Rushton, B. (1981) Histopathology report for compound
WL41706. 26, 53 and 104 week study in rats. Inveresk report project
415386. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0046.
Brooks, T.M., Dean, B.J. & Thorpe, E. (1976) Toxicity studies with
WL 41706 in the host mediated assay. Shell research report TLGR 0003
76. Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0007.
Bruce, E.D., Griffis, L.C. & Wong, Z.A. (1986) The acute vapor
inhalation toxicity of Danitol technical (SX-1713) in mice and rats.
Chevron Study CEHC 2545. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0170.
Colley, J., Gopinath, C. & Offer, J.M. (1987) S-3206: Two-year
feeding study in mice (Addendum to final report). Huntingdon report
SMO 149 861391 (Addendum to SMO 149 8640 (FT-51-0135)). Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-71-0180.
Colley, J., Heywood, R., Street, A.E., Gopinath, C., Offer, J.M.,
Gibson, W.A., & Almond, R.H. (1985) S-3206: Two year feeding study
in mice. Huntingdon report SMO 14984607. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-51-0135.
Colley, J., Welch, P.J., Heywood,R., Prentice, D.E. & Gibson, W.A.
(1981a) S-3206 Preliminary assessment of toxicity to mice by dietary
administration for four weeks. Huntingdon report SMO 121 81289.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0047.
Colley, J., Welch, P.J., Heywood, R., Prentice,D.E. & Gibson, W.A.
(1981b) S-3206 Second preliminary assessment of toxicity to mice by
dietary administration for four weeks. Huntingdon report SMO 139
81574. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0049.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Isaacs, K.R., Gibson, W.A. & Almond, R.H. (1982a) S-3206 Toxicity to
rats by dietary administration for four weeks. Huntingdon report SMO
146 8210. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0064.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Gibson, W.A. &
Almond, R.H. (1982b) S-3206 Toxicity to rats by dietary
administration for four weeks. Huntingdon report SMO 158 82335.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0074.
Cox, R.H. (1986) Teratology study in rats S-3206. Addendum I to
final report. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-61-0176.
Cox, R.H. (1987) S-3206: Amendment II to final report teratology
study in rats. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-71-0188.
Cozens, D.D., Barton, S.J., Offer, J.M., Gibson, W.A. & Anderson, A.
(1986) Effect of S-3206 on multiple generations of the rat.
Huntingdon report SMO 164 85707. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
61-0159.
Cozens, D.D., Hughes, E.W., Masters, R.E. & Anderson, A. (1985) The
effect of S-3206 on pregnancy of the New Zealand white rabbit.
Huntingdon report SMO 181 84667. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
51-0134.
Crawford, M.J. (1975) The metabolism of WL 41706 in mammals. The
fate of a single oral dose of (14C) WL 41706 in the rat. Shell
Research TLGR 0071 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FM-51-
0002.
Crawford, M.J. & Hutson, D.H. (1976) Metabolic fate of (14C) WL
41706 in rats. Shell Research TLGR. 0034 76. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FM-61-0001.
Crawford, M.J. & Hutson, D.H. (1977) The metabolism of the
pyrethroid insecticide (+)-alpha-cyano-3-phenoxybenzyl 2,2,3,3-
tetramethyl-cyclopropanecarboxylate, WL 41706, in the rat. Pestic.
Sci., 8: 579-599. Submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FM-71-0050.
Crayford, J.V. (1975) The excretion and residues of radioactivity in
cows fed (14C) WL 41706 in their diet. Shell Research TLGR 0096 75.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FR-51-0015.
Creedy, C.L. & Potter, D. (1976) The effect of feeding WL 41706 on
the microsomal mono-oxygenase system of rat liver. Shell Research
report TLGR 0043 76. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0009.
Dean, B.J. (1975) Toxicity studies with WL 41706 (S-3206).
Chromosome studies on bone marrow cells of Chinese Hamsters after
two daily oral doses of WL 41706 (S-3206). Shell Research report
TLGR 0104 75. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-51-0003.
Dean, G.A., Gopinath, C. & Fish, L.E. (1987) Potential tumorigenic
and toxic effects in prolonged dietary administration to rats
(Addendum to final report). Huntingdon report SMO 167 87185
(Addendum to SMO 167 851348). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0181.
Dix, K.M. (1975) Toxicity studies of WL 41706: Teratological studies
in rabbits given WL 41706 orally. Shell Research report TLGR 0103 75
(Addendum to FT-51-0006). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-51-
0133.
Else, R. & Rushton, B. (1981) Histopathology report for compound WL
41706 3-generation study in rats. Inveresk report project 415370.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0069.
Fish, C.J., Lewis, D.J. & Gopinath, C. (1986) Electron micrograph
Addendum to histopathology report No SMO/167 S-3206 potential
tumorigenic and toxic effects in prolonged dietary administration to
rats. Huntingdon report SMO 167 86602 (Addendum to SMO 167 851348
(FT-61-0161)). Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0162.
Fleming, D.J. (1979) Toxicity studies on the insecticide WL 41706: A
three generation reproduction study (minus histopathology) in rats -
Corrigendum to FT-91-0027. Shell Research report TLGR 79 0071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-01-0028.
Fujita, Y. (1980) Interview survey concerning complaints connected
with use of S3206 in the field. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0040.
Hara, S. & Suzuki, T. (1980) Acute oral toxicity of S-3206 in
rabbits. Unpublished Sumitomo study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-00-0039.
Hara, M. & Suzuki, T. (1984a) Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0106.
Hara, M. & Suzuki, T. (1984b) In vitro sister chromatid exchanges
test of S-3206 in CHO-K1 cells. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-40-0108.
Hara, M. & Suzuki, T. (1984c) Addendum: Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0114.
Hara, M., Yamada, F., Kogiso, S., Yoshitake, A., Matsuo, M. &
Miyamoto, J. (1984) Mutagenicity test of S-3206 in Saccharomyces
cerevisiae D7. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-40-
0121.
Hend, R.W. & Butterworth, S.T.G (1975) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0031 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0001.
Hend, R.W. & Butterworth, S.T.G (1976a) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0020 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0013.
Hend, R.W. & Butterworth, S.T.G. (1976b) Toxicity studies on the
insecticide WL 41706: A short-term feeding study in rats. Shell
Research report TLGR 0041 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0011.
Hend, R.W. & Gellatly, J.B.M. (1979) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival,
bodyweight, food intake, organ weights, clinical chemistry,
hematology and gross pathological observations of rats exposed to WL
41706 for up to two years. Shell Research report TLGR 79 062.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0026.
Hend, R.W. & Gellatly, J.B.M. (1980) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival, body
weight, food intake, organ weights, clinical chemistry, hematology
and gross pathological observations of rats exposed to WL 41706 for
up to two years, Results of studies after 18 months. Shell Research
report TLGR 79 062 (Addendum to FT-91-0026). Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0032.
Hend, R.W., Gellatly, J.B.M. & Fleming, D.J. (1979) Toxicity studies
on the insecticide WL 41706: A three generation reproduction study
(minus histopathology) in rats. Shell Research report TLGR 79 071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0027.
Heywood, R. (1982) An appraisal of the toxicological findings on
rats receiving a synthetic pyrethroid S-3206 (fenpropathrin) by
dietary administration for 4 weeks. Huntingdon report (unnumbered,
1982). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0075.
Hiromori, T. Hosokawa, S. & Miyamoto, J. 1982a: Fenpropathrin
toxicology overview - Acute toxicity and safety in use. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-20-0079.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1982b) Acute oral toxicity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-20-0076.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1983a) Acute oral toxicity of S-3206 (97.3%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0082.
Hiromori, T., Misaki, Y., Seki, T., Hosokawa, S. & Miyamoto, J.
(1983b) Acute oral toxicity of S-3206 (91.8%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0081.
Hiromori, T., Nakanishi, T., Kawaguchi, S., Sako, H., Suzuki, T. &
Miyamoto, J. (1986a) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci. 11: 9-14.
Hiromori, T., Nakanishi, T., Suzuki, T., Kato, T. & Miyamoto, J.
(1986b) The mean slip angle test of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-60-0153.
Hiromori, T. & Takemura, T. (1983) Short report on facial
paresthesia following exposure to fenpropathrin, cypermethrin,
fenvalerate or permethrin in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0087.
Izumozaki, H., Hara, M. & Suzuki, T. (1984) Gene mutation test of S-
3206 in bacterial system. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-40-0107.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism
of fenvalerate and the (2S, alphaS)-isomer in rats and mice. J.
Pestic. Sci. 6: 317-326.
Kaneko, H., Shiba, K., Yoshitake, A. & Miyamoto, J. (1987)
Metabolism of fenpropathrin (S-3206) in rats. J. Pestic. Sci. 12:
385-395.
Kishida, F., Suzuki, H., Miyamoto, J. (1980) Studies on DNA damaging
capacity of S-3206 with Bacillus subtilis. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-00-0038.
Kohda, H. (1975) Acute oral toxicity of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-50-0018.
Kohda, H. (1976) Acute dermal toxicity of S-3206 in rats.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0019.
Kohda, H. & Kadota, T. (1975) Acute oral toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-50-
0035.
Kohda, H. & Kadota, T. (1976a) Acute dermal toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0036.
Kohda, H. & Kadota, T. (1976b) Acute subcutaneous and
intraperitoneal toxicity of S-3206 technical in rats and mice.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0037.
Kohda, H., Kadota, T. & Miyamoto, J. (1976) Acute inhalation
toxicity of S-3206 and S-5602 in mice an rats. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number AT-50-0043.
Ku, H.S. & Doran, T.J. (1990a) A study to determine the nature of
the residue in milk, meat and tissue from lactating goats dosed with
14C fenpropathrin. Ricerca Inc report project 89 0109. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0041.
Ku, H.S. & Doran, T.J. (1990b) A study to determine the nature of
the residue in poultry end eggs from chickens dosed with 14C
fenpropathrin. Ricerca Inc., report project 89 0084. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0042.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
Marroquin, F., Richter, W.R., Myer, J.R. & Johnson, D.E. (1981) S-
3206 technical grade: Acute dermal toxicity (LD50) study in rabbits
(TSCA 7/79) (EPA 8/78) (OSHA). IRDC report project 491 002.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0051.
Matsubara, T., Hara, S. & Kadota, T. (1978) Primary eye and skin
irritation tests of S-3206 in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-80-0023.
Matsumoto,S., Suzuki, T., Kato, T. & Yamada, H. (no date) Comments
on the facial sensation-inducing potential of fenpropathrin.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-80-0195.
McSheehy, T. & Nunziata, A. (1984) Chromosome aberrations in Chinese
hamster ovary (CHO) cells in vitro. Test substance: S-3206 Final
report 113-005. Life Science Research Roma report project 113 005.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-41-0104.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 41706
to adult hens. Shell Research report TLGR 0068 77. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FW-81-0006.
Misaki, Y., Hiromori, T., Hosokawa, S. & Miyamoto, J. (1983)
Comments on acute oral toxicity of fenpropathrin technicals with
high purity and lower purity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0083.
Misaki, Y. & Kohda, H. (1981a) Acute oral toxicity of two impurities
of S-3206 (technical) in mice. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0044.
Misaki, Y. & Kohda, H. (1981b) Acute oral toxicity of 2,2,3,3-
tetramethylcyclopropane carboxylic anhydride in mice. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-90-0045.
Morseth, S.L. (1990) Rat teratology study with S-3206. Hazleton
report project 343 216. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-01-
0216.
Ohkawa, H., Kaneko, H., Tsuji, H. & Miyamoto, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
Okuno, Y. (1981) Comment on possible weight changes of kidney and no
effect levels in a 2-year chronic toxicity study on WL 41706
(fenpropathrin). Comments 0n FT-91-0026 and FT-11-9946. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-10-0048.
Okuno, Y., Kadota, T. & Miyamoto, J. (1975) Skin sensitization study
of S-3206 in guinea-pigs. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-50-0024.
Omodaka, H., Misaki, Y. & Okuno, Y. (1986a) Acute oral toxicity of
S-3206 in rats. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0138.
Omodaka, H., Suzuki, T. & Kato, T. (1986b) Acute dermal toxicity
study of S-3206 in rats. Unpublished Sumitomo study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
60-0139.
Pauw van der, C.L., Dix, K.M., Blanchard, P.M., McCarthy, W.V. &
Stevenson, D.E. 1975: Teratological studies in rabbits given WL
41706 orally. Shell Research report TLGR 0103 75. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-51-0006.
Pence, D.H., Alsaker, R.D., Hepner, K.E., Dawkins, B.G., Vargus,
K.J., Hagen, W.H. & Hitzelberg, D.A. (1984) Chronic study in dogs S-
3206 T.G. Final report. Hazleton report project 343 153. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-41-0122.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980a) Subchronic toxicity study in dogs S-3206.
Final report. Hazleton report project 343 125. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0034.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980b) Subchronic toxicity study in dogs S-3206.
Addendum to final report. Hazleton report project 343 125. Addendum
to FT-01-0034. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-01-0041.
Pence, D.H., Pence, L.A., Durloo, R. and Cameron, J. (1980c)
Teratology study in rats S-3206. Final report. Hazleton report
project 343 122. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-010031.
Pence, D.H., Weatherholtz, W.M. & Cameron, J.T. (1978) Oral dose
range finding study in dogs, S-3206. Final report. Hazleton report
project 343 123. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-91-0025.
Richold, M., Allen, J.A. & Proudlock, R.J. (1982a) Autoradiographic
assessment of DNA repair in mammalian cells after exposure to S-3206
(fenpropathrin). Huntingdon report SMO 143 81881. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-21-0068.
Richold, M., Grantham, C.J. & Basham, K.J. (1982b) An assessment of
the mutagenic potential of S-3206 using an in vitro mammalian cell
test system (with comment by J. Miyamoto). Huntingdon report SMO 144
8252. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference numberFT-21-0060.
Riley, J.H., Nair, K.P.C., Kopplin, J.R., Johnson, D.E., Ott, S.S.,
Meyer, J.R. & Spicer, E.J.F. (1982) S-3206 (technical grade) 21-day
dermal study in rabbits (EPA) (technical product). IRDC report
project 491 010. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-21-0058.
Ruzo, L.O., Unai, T. & Casida, J.E. 1978: Decamethrin metabolism in
rats. J. Agric. Food Chem. 26: 918-925.
Shell Chemical (UK) Limited (1978) A survey of operator experience
with fenpropathrin 10% EC - A pyrethroid insecticide/acaricide for
use in fruit and ornamental crops in 1986. Shell UK report 16th
January 1987. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-71-0179.
Summitt, L.M. & Albert, J.R. (1976) Intravenous toxicity of SD 41706
(1-24-0-0) in the mouse. Shell Development Co report TIR 74 110 76.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0010.
Suzuki, H. (1977) Studies on mutagenicity of some pyrethroids on
Salmonella strains in the presence of mouse hepathic S9 fractions.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number AT-70-0157.
Suzuki, T. & Miyamoto, J. (1981) Skin sensitization test of S-3206
in guinea-pigs. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-10-
0078.
Suzuki, T., Sako, H. & Okuno, Y. (1986) Acute oral toxicity study of
S-3206 in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0175.
Warren, S., Heywood, R., Street, A.E., Gopinath, C., Browne, J.G.,
Gibson, W.A., Reed, L.E. & Anderson, A. (1986) S-3206: Potential
tumorigenic and toxic effects in prolonged dietary administration to
rats (Final report. Huntingdon report SMO 167 851348. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-61-0161.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yoshida, A., Kosaka, T., Miyaoka, T., Maita, K. & Goto, S. (1986) S-
3206: 13-week oral subchronic toxicity study in rats. Inst. Environ.
Toxicol. Tokyo report 17th, July 1986. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-61-0166.
Yoshitake, A., Kogiso, S. & Hara, M. (1990) In vitro chromosomal
aberration test of S-3206 in Chinese Hamster ovary cells (CHO-K1).
Sumitomo study M83-54. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-00-
0215.
Yoshitake, A., Kogiso, S., Kato, H. & Hara, M. (1988) In vitro
gene mutation test of S-3206 in V79 Chinese Hamster cells in
culture. Sumitomo study 1174. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-80-
0209.
Yoshitake, A. Kogiso, S. & Yamada, F. (1986) Bacterial DNA repair
test of S-3206. Sumitomo study MUT86020. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-60-0165.
Yoshitake, A., Kogiso, S & Yamada, F. (1987) Reverse mutation test
of S-3206 in bacterial systems. Sumitomo study 704. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-70-0182.
Yoshitake, A., Kogiso, S., Yamamoto, K. & Hara, M. (1989) In vitro
chromosomal aberration test of S-3206 in Chinese Hamster ovary cells
(CHO-K1). Sumitomo study 1730. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-90-
0200.
 Table 1. Acute toxicity of fenpropathrin (technical material)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Rat M oral 54 97 Kohda, 1975
F oral 49 97 Kohda, 1975
M oral 71 91.8 Hiromori et al., 1983b
Misaki et al., 1983
F oral 67 91.8 Hiromori et al., 1983b
Misaki et al., 1983
M oral 77 97.3 Hiromori et al., 1983a
Misaki et al., 1983
F oral 67 97.3 Hiromori et al., 1983a
Misaki et al., 1983
M oral 164 97.3 Hiromori et al., 1983b
F oral 107 97.3 Hiromori et al., 1983b
M&F oral 60 93.8 Omodaka et al., 1986a
F oral 70 93.8 Omodaka et al., 1986a
M&F inhalative (vapour) > 0.009 µg/l* 94.5 Bruce et al., 1986
M&F inhalative (Xylene EC mist) > 96 µg/l* 97 Kohda et al., 1976
M dermal (sol. in corn oil) 1600 97 Kohda, 1976
F dermal (sol. in corn oil) 870 97 Kohda, 1976
M&F dermal (viscous liquid) > 5000 93.8 Omodaka et al., 1986b
M sc 1410 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 225 97 Kohda & Kadota, 1976b
F ip 180 97 Kohda & Kadota, 1976b
Mouse M oral 67 97 Kohda & Kadota, 1975
F oral 58 97 Kohda & Kadota, 1975
M oral 135 93.8 Suzuki et al., 1986
F oral 154 93.8 Suzuki et al., 1986
M inhalative (Xylene EC mist) 100 µg/l* 97 Kohda et al., 1976
F inhalative (Xylene EC mist) 43 µg/l* 97 Kohda et al., 1976
Table 1 (contd)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Mouse M dermal 740 97 Kohda & Kadota, 1976a
F dermal 920 97 Kohda & Kadota, 1976a
M sc 1350 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 230 97 Kohda & Kadota, 1976b
F ip 210 97 Kohda & Kadota, 1976b
M iv 4.5 ? Summitt & Albert, 1976
Rabbit M oral 675 96.2 Hara & Suzuki, 1980
F oral 510 96.2 Hara & Suzuki, 1980
M&F dermal > 2000 ? Marroquin et al., 1981
Dog M&F oral > 1000 96.2 Pence et al., 1978
* LC50
Rats
Fenpropathrin (purity 96%) was fed to groups of Carworth Farm E
rats (12/sex/dose; control 24/sex) at dietary concentrations of 0,
2, 10, 50 or 250 ppm over 13 weeks. The treatment did not influence
the general health and behaviour, haematology, clinical chemistry or
pathology of treated animals. Body-weight gain was slightly
increased at all dose levels. A slight increase in spleen weight in
male rats at 250 ppm was the only change concerning organ weights
and may present a borderline effect. The NOAEL was > 250 ppm,
equal to 18 mg/kg/day (males) and 21 mg/kg bw/day females) (Hend &
Butterworth, 1975).
In a similar study, rats (12/sex/group; control 24/sex) were
fed diets containing fenpropathrin (purity 97%) at concentrations of
0, 3, 30, 100, 300 or 600 ppm over 13 weeks. After 5 weeks of
exposure, most females and one male showed tremors and reduced body-
weight gain at 600 ppm. The tremors disappeared towards the end of
the study. Changes in a few haematological parameters were marginal
and were - though statistically significant - not considered
biologically significant. Slight increases in plasma ALP were
observed at 600 ppm. Organ weights were not influenced by treatment
and no abnormal histopathological findings were observed. The NOAEL
was 300 ppm, equal to 17 mg/kg bw/day (males) and 24 mg/kg bw/day
(females) based on the appearance of clinical signs and reduced
body-weight gain at 600 ppm (Hend & Butterworth, 1976a).
In a third study, groups of rats (SPF SD(Crj:CD); 12/sex/dose)
were fed concentrations of 0, 15, 50, 150, 450 or 600 ppm
fenpropathrin (purity 93.1%) over a period of 13 weeks. One female
died at 600 ppm. A dose-dependent depression of body-weight gain
was observed at 600 ppm and 450 ppm in female rats, and at 600 ppm
in male rats. Food consumption was reduced at the beginning of the
study at 600 ppm in males and at 450 ppm and above in females; food
efficiency was reduced in females treated with 600 ppm. An increase
in urinary protein and specific gravity was observed in males at 450
ppm and above. Haematological and ophthalmological parameters as
well as organ weights were not influenced by the treatment. A
slightly elevated alkaline phosphatase activity was noted in females
of the 600 ppm group. There was no evidence of treatment-related
histopathological changes in any organ. The NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain and
urinalysis at higher dose levels (Yoshida et al., 1986).
Rabbits
In a 21-day dermal toxicity study with fenpropathrin (purity
91.4%), groups of New Zeeland white rabbits (10/sex/dose) received
dermal applications on either intact or abraded skin at dose levels
of 0, 500, 1200 or 3000 mg/kg bw. The treatment did not influence
survival, body weight, food consumption, haematology, clinical
chemistry, organ weights, or macroscopic or microscopic findings.
Single instances of slight skin reactions were observed at the
application sites of animals treated with 500 and 1200 mg/kg bw and
in most animals at 3000 mg/kg bw. The NOAEL was > 3000 mg/kg bw
based on systemic toxicity (Riley et al., 1982).
Dogs
Fenpropathrin (purity 96.2%) was administered in the diet to
groups of beagle dogs (6/sex/dose) at concentrations of 0, 250, 500
or 1000/750 ppm over 13 weeks (1000 ppm for 3 weeks, 750 ppm for the
rest of the study because of the appearance of severe tremors and
ataxia). The doses were adjusted for purity. Treatment did not
affect survival, food consumption, ophthalmoscopy, clinical
chemistry, urinalysis, organ weights, gross or microscopic
pathology. Treatment-related clinical signs consisted of emesis,
tremors, ataxia, diarrhoea, lethargic appearance and salivation.
Some effects were observed in all treatment groups. The frequency
of the observed clinical signs in all of the compound-treated groups
decreased after week 5. One high-dose male dog was sacrificed
moribund. Reduced body-weight gain was observed at concentrations of
500 and 1000/750 ppm. Haematocrit and haemoglobin values and the
erythrocyte count in females at 1000/750 ppm were decreased. The
NOAEL was < 250 ppm, equal to 7 mg/kg bw/day in males and 10 mg/kg
bw/day in females, based on the occurrence of emesis at 250 ppm
(Pence et al., 1980a,b).
Fenpropathrin (purity 92.5%) was fed to groups of beagle dogs
(4/sex/dose) at dietary concentrations of 0, 100, 250 or 750 ppm
(adjusted to 100% active ingredient) over a period of 52 weeks. One
high-dose male died in the middle of the study after exhibiting
tremor and ataxia. Emesis occurred at an increased incidence in the
mid-dose and high-dose females but without a clear dose-response
relationship. The incidence of tremors however showed a clear dose-
related increase at 250 and 750 ppm (males: 0, 0, 49, 181; females:
0, 1, 42, 168 observations at 0, 100, 250 and 750 ppm,
respectively). Ataxia occurred at 750 ppm. Reduced body-weight
gain was observed at 750 ppm and at 250 ppm in females. The
treatment did not influence ophthalmoscopy, haematology, clinical
chemistry, urinalyses, gross or microscopic pathology. The only
significant difference concerning organ weight was an increase in
the relative kidney weight in females at 750 ppm. The NOAEL was 100
ppm, equal to 3 mg/kg bw/day, based on reduced body-weight gain and
occurrence of clinical signs at 250 ppm and higher (Pence et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
In a 104-week feeding study, groups of mice (Charles River (UK)
CD-1; 52/sex/group in main study; 40/sex/group in satellite groups
for interim sacrifice) were fed fenpropathrin (purity 91.4%-92.5%)
at concentrations of 0, 40, 150 or 600 ppm. These concentration
levels were based on two range-finding studies (Colley et al.,
1981a,b). Treatment did not affect the general behaviour, with the
exception of a marginal increase in the number of females at 600 ppm
showing hyperactivity at week 78. No effects on survival rate, body
weight, food consumption, food efficiency, urinalysis, macroscopic
pathology or organ weights were reported. Changes in haematological
parameters at 600 ppm included slight decreases in Hb and MCHC in
males and an increase in MCHC in females at week 25. Because these
changes were small, occurring only on some occasions and only in one
sex, they are not considered to be toxicologically significant.
Sporadic changes in some clinical chemical parameters were not
considered to be of toxicological significance (e.g., decrease in
urea nitrogen in males at 600 ppm, increase in GOT and GPT levels at
all dose levels without clear dose-response relationship). An
increased incidence in lung tumours (adenoma and adenocarcinoma) was
observed in males and females of treated groups. The incidences in
the satellite groups (spontaneous deaths and interim sacrifice after
26, 52 and 78 weeks) were 10, 12.5, 18 and 2.5% for males and 7.5,
12.5, 7.5 and 7.5% for females at 0, 40, 150 and 600 ppm,
respectively. The incidences in the main study were 12, 23, 35 and
31% for males and 10, 29, 19 and 13% females at 0, 40, 150 and 600
ppm, respectively. The incidences did not show a clear dose-
response relationship. The absence of any statistical significance
or significant trend supports the interpretation that the increased
incidences in the treatment groups were not due to a tumorigenic
activity of fenpropathrin. Moreover, data on the incidence of lung
tumours in historical controls showed that incidences of pulmonary
adenoma and adenocarcinoma in untreated animals may vary between 7
and 36%. There was no sex difference concerning lung tumours in
these controls. Therefore the results of this study gave no
evidence of fenpropathrin-induced carcinogenic potential. The NOAEL
was the highest dose tested, 600 ppm, equal to 56 mg/kg bw/day
(males) and 65 mg/kg bw/day (females) (Colley et al., 1985, 1987).
Rats
Groups of rats (COBS) were fed diets containing 0, 1, 5, 25,
125 or 500 ppm fenpropathrin (purity 97%) for two years. The group
sizes were 12/sex and 48/sex for the satellite and main study
controls, respectively; while the treatment groups included 6/sex
and 24/sex for the satellite and main study, respectively. The
treatment did not affect the survival, general health, behaviour of
the animals, clinical chemical or haematological parameters. The
depression in body-weight gain observed at 500 ppm was statistically
significant in females only. Food consumption of treated animals
was comparable with control animals at most observation times.
Absolute spleen weight increased at 500 ppm in females of the 6-
month satellite group and kidney weights decreased in females of all
treatment groups in the 12-month satellite groups without showing a
clear dose-response relationship. In the 2-year main study groups,
no treatment-related alterations in organ weights were observed.
Gross pathological examination showed a higher incidence of
white/grey foci or plaques in the lungs at 125 and 500 ppm in
females at 6 and 24 months. In the 2-year main groups, the number
of deaths attributable to renal failure in males at 500 ppm was
greater than in the other groups, but no increase was observed when
animals from satellite and main study groups were combined.
The histopathological neoplastic and non-neoplastic changes
found were consistent with the range and severity of changes usually
observed in this rat strain and did not give any evidence of
carcinogenicity. The NOAEL was 125 ppm, equal to 5 mg/kg bw/day
based on depression in body-weight gain at higher dose levels (Hend
& Gellatly, 1979; Okuno, 1981; Aitken & Rushton, 1981).
Single animals of a satellite group fed the above-mentioned
concentrations for 18 months were used for functional and
neurochemical investigations. For results see "Special Studies on
Neurotoxicity" (Hend & Gellatly, 1980).
Fenpropathrin (purity 91.4-92.5%) was fed to groups of rats
(Charles River CD; 50/sex/dose in main study and 15/sex/dose in
satellite groups for interim sacrifice) at dietary concentrations of
0, 50, 150, 450 or 600 ppm for 104 weeks. The dose levels were
selected based on the results of range-finding studies (Colley et
al., 1982a,b; Heywood, 1982). Clinical signs were restricted to
body tremors, most prevalent in females receiving 600 ppm, but were
also observed in males at the highest dose levels in the first few
weeks of the study and in females at 450 ppm.
Mortality increased in males and females receiving 600 ppm and
in females receiving 450 ppm during the first 26 weeks of treatment;
subsequently, mortality was less than that of control animals
resulting in highest survival rates in males at 600 ppm and in
females at 450 ppm. Exposure of main group females receiving 600
ppm was terminated after 52 weeks. A slightly higher food
consumption was reported for the high-dose females during the first
3 months of treatment, most probably resulting in the mortalities
observed. Body-weight gain was reduced in females at 600 ppm and
food utilization efficiency was marginally impaired.
Ophthalmological examination did not reveal treatment-related ocular
lesions. No abnormal haematological findings were reported.
Changes in clinical chemistry parameters at 450 and 600 ppm included
slightly reduced creatinine levels and reduced total protein levels
in males. The differences were minimal however and - though
statistically significant - most probably of no biological
significance. Urinalysis did not indicate any kidney lesions.
Macroscopic examination revealed no findings which were
treatment-related with the exception of an increased incidence of
alopecia in females at 600 ppm at 52 weeks. Changes in organ
weights were observed on some occasions but did not show a
treatment-related pattern and histopathological examination did not
reveal changes attributable to treatment. A few lymphoreticular
tumours were seen in treated groups of male rats, but none in
control group. The increases observed were not dose-related and
were within the incidence range of the historical controls for this
rat strain. There was no indication of a treatment-related increase
in tumour incidence or non-neoplastic organ changes. The electron
microscopic examination of tibial nerve did not reveal treatment-
related effects. The NOAEL was 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses (Warren
et al., 1986; Fish et al., 1986; Dean et al., 1987).
Reproduction studies
Rats
In a 3-generation reproduction study, fenpropathrin (purity
97%) was fed to rats (COBS; 30/sex/group) at dietary concentrations
of 0, 5, 25 or 250 ppm. Treatment did not influence the general
health condition or mortality, body-weight gain, food consumption,
pregnancy rates, litter size or litter weight. However, pup weights
in the F3a generation were reduced at 250 ppm. No macroscopic or
microscopic lesions were attributable to treatment. A very small
number of cases of locomotor incoordination occurred in pups fed 5
and 250 ppm. Most pups in the 5 ppm group showing these signs were
from one litter in the F1b generation. No similar lesions were
observed in the second litter of that pair nor in the following
generation and no macroscopic lesions were identified in these
animals. Histopathological examination (F2 parents, F3b pups) did
not reveal treatment-related tissue lesions. The NOAEL was 25 ppm,
equal to 1.6 mg/kg bw/day, based on decreased pup weights in the
F3a generation at 250 ppm (Fleming, 1979; Hend et al., 1979; Else
& Rushton, 1981).
In a second 3-generation study, groups of rats (CrL: COBS CD
(SD)BR; 11-28/sex/dose) were fed diets containing 0, 40, 120 or 360
ppm fenpropathrin (purity 92.5%). Animals of the last generation
(F2) were reared to maturity. At 360 ppm, most females that
littered showed body tremors associated with muscle twitches and
increased sensitivity. These signs were generally observed during
the lactation period. Increased mortality occurred at 360 ppm in
females only; 18/28 females at this dose died, most during the
lactation period of the F1b females. At 120 ppm, 2/24 F1b females
died during lactation. Mean body-weight gain of males in the F1
and F2 generations were reduced at 360 ppm. F1b females at 120
ppm showed also some indication of retarded weight gain. Treatment
had no influence on mating performance and no macroscopic or
microscopic abnormalities were observed at terminal autopsy that
were considered to be associated with treatment. At 360 ppm, F0
females showed a slight increase in liver weights. A few F2b pups
at 120 ppm showed body tremors prior to weaning and 2/3 of these
pups died subsequently. A slight reduction in litter size was
observed at 360 ppm in F0 animals (second mating) and in both
matings of F1b animals from days 4 to 21. At 360 ppm, pup weights
were lower in all generations. The NOAEL was 40 ppm, equal to 3
mg/kg bw/day, based on depression of body-weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above (Cozens et al., 1986).
Special studies on embryotoxicity/teratogenicity
Rats
Fenpropathrin (purity 96.2%) was fed by intubation to groups of
rats (Fischer 344 CDF; 27/28 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.4, 2 or 10 mg/kg bw/day. One mid-
dose and 9 high-dose females were found dead during the study.
Clinical signs noted at a somewhat greater incidence in the treated
animals included soft faeces, red or lacrimating eyes, alopecia or
hunched appearance. Post-dose tremors occurred on a few occasions
in some high-dose females. Body-weight gain was reduced in high-
dose females during the treatment period, and increased slightly
afterwards, resulting in an overall change comparable to the control
group. Food consumption was also reduced at the highest dose level.
Various gross lesions were found in animals that died during the
study. The findings included discolorations of organs, but no
specific target organ was identified. The pregnancy rate was lower
in the high-dose group compared to the other dose groups but other
reproduction parameters (e.g., number of corpora lutea, resorptions,
fetal viability) were not impaired by treatment. No visceral or
skeletal anomalies were noted in the pups. Visceral or skeletal
variants did not show dose-related increases. No teratogenic
effects were observed. The NOAEL for maternal toxicity was 2 mg/kg
bw/day. The NOAEL for embryotoxicity and teratogenicity was 10 mg/kg
bw/day (Pence et al., 1980c; Cox, 1986, 1987).
Groups of rats (Fischer-344; 30 females/dose group) were orally
treated with fenpropathrin (purity 91.9%) at dose levels of 0, 0.44,
1.6, 2.2, 3.3, 6.5 or 11 mg/kg bw/day (corresponding to 0, 0.4, 1.5,
2, 3, 6 or 10 mg/kg bw/day active ingredient) from day 6 to day 15
of gestation. Seven animals died in the highest dose group. Signs
of toxicity included ataxia, sensitivity to external stimuli,
tremors, prostration and convulsions. At 6 and 10 mg/kg bw/day,
mean maternal body-weight gains and food consumption were lower
during the dosing period. Pregnancy rate of high-dose females was
slightly lower (90%) compared to the other treatment groups (93%-
97%). Treatment had no influence on reproduction parameters such as
resorptions, fetal viability or fetal body weight. No visceral or
skeletal anomalies were observed that were considered treatment-
related. Skeletal variations were noted in all groups and included
variations in the stage of ossification and rib counts, but not in a
dose-dependent pattern. A higher incidence of incomplete
ossification of the sternebrae was observed in all treatment groups
but without a clear dose-response relationship. The highest
incidences with respect to some differences in ossification (e.g.,
5th/6th sternebrae incomplete ossification, asymmetric ossification
of sternebrae) were noted at 10 mg/kg bw. Statistical analysis
revealed no significant trend for the overall incidence of delayed
ossification of the 5th/6th sternebrae. The NOAEL for maternal
toxicity was 3 mg/kg bw/day. The NOAEL for embryotoxicity and
teratogenicity was 10 mg/kg bw/day (Morseth, 1990).
Rabbits
Groups of rabbits (banded Dutch; 20-30 females/group) were fed
fenpropathrin (purity 97%) at dose levels of 0, 1.5, 3 or 6 mg/kg
bw/day from day 6 to day 18 of gestation. No clinical signs of
intoxication were observed. A lower number of rabbits survived to
term at 3 mg/kg bw/day but this was unrelated to treatment.
Treatment had no effect on body weight of the dams. No adverse
effects were observed with respect to pre-implantation loss, number
of resorptions, fetal deaths, litter size, or pup weight. Increased
incidence in minor skeletal and visceral abnormalities (e.g.,
enlarged fontanelles, interparietal bones, fused sternebrae, extra
centres of ossification) were noted at 3 mg/kg bw but not at the
higher dose. Therefore these changes were not considered treatment-
related. The NOAEL for maternotoxicity, embryotoxicity and
teratogenicity was 6 mg/kg bw/day (van der Pauw et al., 1975; Dix,
1975).
Groups of rabbits (New Zeeland white; 17-19 females/dose) were
treated with oral doses of 0, 4, 12 or 36 mg/kg bw/day fenpropathrin
(purity 92.5%) from days 7 to 19 of gestation. A dose-related
increase in the incidence of grooming was observed in all dose
groups. At 12 and 36 mg/kg bw/day, flicking of forepaws was also
observed, with a few animals at 36 mg/kg bw/day showing shaky
movements and tremor. Slight reduction in body-weight gain and
reduced food consumption were noted at 36 mg/kg bw/day. One dam at
12 and two at 36 mg/kg bw failed to maintain their pregnancies. One
dam at each dosage (including control) was sacrificed in poor
condition. Treatment did not adversely affect litter parameters
(e.g., litter size, fetal weight). There was no dose-related
increase in the incidence of malformations. The NOAEL was 4 mg/kg
bw/day with respect to maternotoxicity and 36 mg/kg bw/day for
fetotoxicity and teratogenicity (Cozens et al., 1985).
Special studies on genotoxicity
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The results are
summarized in Table 2.
Special studies on neurotoxicity
Hens
Six laying hens were treated with daily oral doses of 1 g/kg
bw/day for five days. Dosing was repeated after three weeks and the
birds sacrificed three weeks later. A positive control group was
treated with tri-ortho-tolyl phosphate (TOTP). No signs of
intoxication were noted. Histological examination of nervous
tissues revealed no lesions (Milner & Butterworth, 1977).
Rats
Groups of rats (6/sex/group) were fed diets containing 0 or 900
ppm fenpropathrin (purity not specified) for up to 25 days. Tremors
and high mortality were noted. On histological examination of the
sciatic nerve, swelling and disintegration of nerve axons were
observed whereas no myelin lesions were noted (Hend & Butterworth,
1976b).
The neurotoxic effect of fenpropathrin in rats was assessed by
means of the slip angle test. Oral doses of 0, 10, 25, 50, 75 or
100 mg/kg bw were administered to male rats (10/dose) and the angle
at which the treated animals slipped from the board determined. The
mean slip angle (MSA) decreased dose-dependently at 50 mg/kg bw and
higher. Clinical signs observed in animals treated with doses of 25
mg/kg bw and above consisted of slight to severe tremor, ataxia and
limb paralysis appearing at 3-7 hours after administration. The
effects on MSA and toxic signs almost disappeared after 24 hours.
Dose-related mortalities were observed at 50 mg/kg bw and above
(Hiromori et al., 1986b).
Table 2. Mutagenicity of fenpropathrin
Test system Test object Concentration Purity % Results Reference
non-activated activated
In vitro
Ames test S. typhimurium 50-5000 µg/plate 92.5 negative negative Izumozaki et al., 1984
(various strains) Yoshitake et al., 1987
Ames test S. typhimurium 10-1000 µg/plate 97.0 negative1 negative Suzuki, 1977
(various strains)
Reverse mutation Saccharomyces 92.5 negative negative Hara et al., 1984
mitotic crossing cerevisiae D7
over and mitiotic
gene convn. assay
Mammalian cell Mouse lymphoma 50-400 µg/ml (-activ). 91.4 negative negative Richold et al., 1982b
mutation assay L5178Y cells 30-300 µg/ml (+activ).
Gene mutation V79 Chinese 50-500 µg/ml 92.4 negative negative Yoshitake et al., 1988
assay hamster cells
DNA repair assay HeLa S3 cells 0.16-2.5 µg/ml ? negative negative Richold et al., 1982a
Rec-assay Bacillus subtilis 10-5000 µg/disc 97.0 negative negative Kishida et al., 1980
M45 rec and H17
DNA repair assay Bacillus subtilis 100-10 000 µg/disc 92.5 negative negative Yoshitake et al., 1986
Chromosome damage Chinese hamster 10 or 20 mg/kg bw 97.0 negative negative Dean, 1975
bone marrow cells orally on two
succcessive days
Table 2 (contd)
Test system Test object Concentration Purity % Results Reference
non-activated activated
Chromosome Chinese hamster 50-500 µg/ml (-activ). 92.5 negative negative McSheehy & Nunziata,
aberration assay ovary cells (CHO) 500-5000 µg/ml (+activ). 1984
Chromosome CHO-K1 cells 10-30 µg/ml (-activ). 92.4 negative negative Yoshitake et al., 1989
aberration assay 250-1000 µg/ml (+activ).
Chromosome Chiense hamster 0.003-0.1 mmol/l 92.5 negative negative Yoshitake et al., 1989b
aberration assay ovarian cells
(CHO-K1)
Sister chromatid Chinese hamster 0.003-0.1 mmol/l 92.5 negative negative Hara & Suzuki, 1984b
exchange ovarian cells
(CHO-K1)
In vivo
Micronucleus test Mouse femur cells 50-200 mg/kg 92.5 negative negative Hara & Suzuki, 1984a,c
intraperitoneally
Host mediated S. cerevisiae JD1 10-20 mg/kg bw orally 97.0 negative negative Brooks et al., 1976
assay (in male mice)
1 +activation by hepatic S-9 fractions obtained from 6 different mouse strains
Functional testing was performed on 2 animals/sex at 0, 125 or
500 ppm (satellite group of long-term study in rats, Hend &
Gellatly, 1979) using the "inclined plane test". Preliminary
results revealed an impaired performance in animals fed 500 ppm.
Measurements of ß-glucuronidase activity (parameter indicative of
Wallerian degeneration in nerves) did not reveal a clear increase as
would be expected as a result of toxic neuropathy. Macroscopy and
histopathology of sciatic and tibial nerves did not show treatment-
related changes (Hend & Gellatly, 1980).
Special study on effects on enzymes
A preliminary investigation was performed to study the possible
effect of fenpropathrin on the induction of hepatic microsomal
enzymes. Four Charles River CD rats were fed dietary concentrations
of 1, 10, 100 or 1000 ppm over two weeks. No significant difference
was observed between the activities of control and test livers in
the in vitro O-dealkylation of 14C-chlorfenvinphos and no dose-
related increase in liver weights was induced. Therefore, the
results of this study do not provide any evidence for an inducing
effect of fenpropathrin on liver microsomal enzymes (Creedy &
Potter, 1976).
Special study on antidotes
The therapeutic potency of intraperitoneally administered
methocarbamol [3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate] was
examined against acute oral intoxication of rats after treatment
with lethal doses of fenpropathrin (100 mg/kg bw) and some other
pyrethroids. Methocarbamol was initially administered
intraperitoneally at a dose of 400 mg/kg bw followed by repeated
doses of 200 mg/kg bw. Treatment with methocarbamol reduced the
mortality from 60% to 0% and depressed tremors (Hiromori et al.,
1986a).
Special study on paresthesia
Rabbits
Facial paresthesia activities following exposure to
fenpropathrin and other pyrethroids were estimated using a rabbit
model. To determine the intensity of facial paresthesia changes in
behaviour after dermal application of the pyrethroids were recorded.
Doses applied ranged from 0.0001-10 mg/animal. The frequency of
licking and/or biting after application showed a clear dose-response
relationship with all pyrethroids tested. Post-treatment with
vaseline-based 5% benzocaine ointment (local anaesthetic) was
effective in reducing the intensity of animal response. Post-
treatment by application of undiluted vitamin E was also effective
for treatment of facial paresthesia (Hiromori & Takemura, 1983).
Special studies on irritation and sensitization
The test material (purity 90.2%) caused no skin irritation and
only very slight eye irritation in rabbits (Matsubara et al.,
1978).
Two skin sensitization studies were conducted with guinea-pigs.
No sensitization reactions occurred (Okuno et al., 1975; Suzuki &
Miyamoto, 1981).
Observations in humans
In a field study in Japan, six workers participated in two
tests using a 5% emulsifiable concentrate. The spray concentrations
were 25-50 ppm (a.i.), the spraying time 2 hours. A motor mounted
sprayer was used. The workers wore protective clothing. No effects
were reported (e.g., headache, nasal discharge, itching, burning,
pain in face or limbs) (Fujita, 1980).
Some workers exposed to pyrethroids in the laboratory during
formulation processes or in field trials in England reported
transient abnormal facial sensations. A clinical and
electrophysiological study using an electromyograph was carried out
on 23 workers. Electrophysiological studies were also carried out
on an equal number of age and sex-matched control subjects who had
no contact with pyrethroids. Nineteen had experienced at least one
episode of abnormal facial sensations and 13/23 had experienced
several such episodes. Permethrin, cypermethrin, fenpropathrin and
fenvalerate were most frequently used. Symptoms were limited to the
face. Characteristically, the symptoms appeared 30 minutes to eight
hours after exposure. Since exposure to the different pyrethroids
varied, it was not possible to determine which compounds were more
likely to produce symptoms. Electrophysiological investigation
showed statistically significant increase in maximal nerve
conduction velocity in exposed workers compared to controls, which
is contrary to what is expected in subclinical neuropathy. The
increase must therefore be fortuitous. This conclusion is supported
by the observation that no difference in nerve conduction velocity
was noted in workers more exposed than others (Le Quesne et al.,
1980; Matsumoto et al., no date).
In a survey of 18 operators using a 10% fenpropathrin product,
no adverse effects were reported, with the exception of slight nasal
irritation. All operators wore protective clothing (Shell Chemical
Ltd., 1978).
COMMENTS
After oral administration of fenpropathrin to rats, the
compound was almost completely absorbed and eliminated in urine and
in faeces. The major biotransformation reactions consisted of
oxidation at the methyl groups of the acid moiety and at the 2'-and
4'-positions of the alcohol moiety, cleavage of the ester linkage
followed by glucuronide, sulfate or glycine conjugation.
Fenpropathrin has been tested for acute toxicity and it has
been classified as moderately hazardous by WHO.
In a short-term feeding study in rats conducted at dietary
concentrations of 0, 3, 30, 100, 300 or 600 ppm over thirteen weeks,
the NOAEL was 300 ppm, equal to 17 mg/kg bw/day, based on reduced
body-weight gain and the appearance of clinical signs at higher dose
levels. In a second 13-week study in rats, the NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain at
higher dose levels.
A one-year study in dogs conducted at dose levels of 0, 100,
250 or 750 ppm revealed a NOAEL of 100 ppm equal to 3 mg/kg bw/day,
based upon reduced body-weight gain and clinical signs (emesis,
tremors) at 250 ppm.
A long-term toxicity/carcinogenicity study was performed in
mice over 2 years at 0, 40, 150 and 600 ppm. The NOAEL was the
highest dose tested, 600 ppm, equal to 56 mg/kg bw/day. There was
no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study in rats conducted
at dietary concentrations of 0, 1, 5, 25, 125 or 500 ppm over two
years, the NOAEL was 125 ppm, equal to 5 mg/kg bw/day, based on
depression in body-weight gain at 500 ppm. There was no evidence of
carcinogenicity.
A second long-term toxicity/carcinogenicity study in rats
performed at dietary concentrations of 0, 50, 150, 450 or 600 ppm
over two years revealed a NOAEL of 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses. There
was no evidence of carcinogenicity.
In a multigeneration reproduction study in rats, fenpropathrin
was administered at dietary levels of 0, 5, 25 or 250 ppm. The
NOAEL was 25 ppm, equal to 1.6 mg/kg bw/day, based on decreased pup
weights in the F3a generation at 250 ppm.
In a second multigeneration reproduction study conducted at
dose levels of 0, 40, 120 or 360 ppm, the NOAEL was 40 ppm, equal to
3 mg/kg bw/day, based on depression of body weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above.
Two oral teratogenicity studies in rats were performed at dose
levels of 0, 0.4, 2 or 10 mg/kg bw/day and 0, 0.4, 1.5, 2, 3, 6 or
10 mg/kg bw/day. The NOAELs were 2 and 3 mg/kg bw/day in the two
studies, respectively, with respect to maternotoxic effects and a
NOAEL of 10 mg/kg bw/day in both for embryotoxicity and
teratogenicity.
In an oral teratogenicity study in rabbits at dose levels of 0,
1.5, 3 or 6 mg/kg bw/day, the NOAEL was 6 mg/kg bw/day. In a second
study with oral doses of 0, 4, 12 or 36 mg/kg bw/day the NOAEL was 4
mg/kg bw/day with respect to maternotoxicity.
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The Meeting concluded
that fenpropathrin was not genotoxic.
Based upon studies in hens and rats, fenpropathrin exhibited no
potential for delayed neurotoxicity.
Data on observations in humans were not suitable for the
estimation of an acceptable daily intake.
An ADI of 0-0.03 mg/kg bw was established based on a NOAEL of 3
mg/kg bw/day in the multigeneration reproduction study in rats, the
teratogenicity studies in rats and the one-year feeding study in
dogs, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Mouse: 600 ppm, equal to 56 mg/kg bw/day (two-year study)
Rat: 150 ppm, equal to 7 mg/kg bw/day (two-year study)
40 ppm, equal to 3 mg/kg bw/day (reproduction study)
3 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 4 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Aitken, R. & Rushton, B. (1981) Histopathology report for compound
WL41706. 26, 53 and 104 week study in rats. Inveresk report project
415386. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0046.
Brooks, T.M., Dean, B.J. & Thorpe, E. (1976) Toxicity studies with
WL 41706 in the host mediated assay. Shell research report TLGR 0003
76. Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0007.
Bruce, E.D., Griffis, L.C. & Wong, Z.A. (1986) The acute vapor
inhalation toxicity of Danitol technical (SX-1713) in mice and rats.
Chevron Study CEHC 2545. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0170.
Colley, J., Gopinath, C. & Offer, J.M. (1987) S-3206: Two-year
feeding study in mice (Addendum to final report). Huntingdon report
SMO 149 861391 (Addendum to SMO 149 8640 (FT-51-0135)). Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-71-0180.
Colley, J., Heywood, R., Street, A.E., Gopinath, C., Offer, J.M.,
Gibson, W.A., & Almond, R.H. (1985) S-3206: Two year feeding study
in mice. Huntingdon report SMO 14984607. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-51-0135.
Colley, J., Welch, P.J., Heywood,R., Prentice, D.E. & Gibson, W.A.
(1981a) S-3206 Preliminary assessment of toxicity to mice by dietary
administration for four weeks. Huntingdon report SMO 121 81289.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0047.
Colley, J., Welch, P.J., Heywood, R., Prentice,D.E. & Gibson, W.A.
(1981b) S-3206 Second preliminary assessment of toxicity to mice by
dietary administration for four weeks. Huntingdon report SMO 139
81574. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0049.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Isaacs, K.R., Gibson, W.A. & Almond, R.H. (1982a) S-3206 Toxicity to
rats by dietary administration for four weeks. Huntingdon report SMO
146 8210. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0064.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Gibson, W.A. &
Almond, R.H. (1982b) S-3206 Toxicity to rats by dietary
administration for four weeks. Huntingdon report SMO 158 82335.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0074.
Cox, R.H. (1986) Teratology study in rats S-3206. Addendum I to
final report. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-61-0176.
Cox, R.H. (1987) S-3206: Amendment II to final report teratology
study in rats. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-71-0188.
Cozens, D.D., Barton, S.J., Offer, J.M., Gibson, W.A. & Anderson, A.
(1986) Effect of S-3206 on multiple generations of the rat.
Huntingdon report SMO 164 85707. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
61-0159.
Cozens, D.D., Hughes, E.W., Masters, R.E. & Anderson, A. (1985) The
effect of S-3206 on pregnancy of the New Zealand white rabbit.
Huntingdon report SMO 181 84667. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
51-0134.
Crawford, M.J. (1975) The metabolism of WL 41706 in mammals. The
fate of a single oral dose of (14C) WL 41706 in the rat. Shell
Research TLGR 0071 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FM-51-
0002.
Crawford, M.J. & Hutson, D.H. (1976) Metabolic fate of (14C) WL
41706 in rats. Shell Research TLGR. 0034 76. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FM-61-0001.
Crawford, M.J. & Hutson, D.H. (1977) The metabolism of the
pyrethroid insecticide (+)-alpha-cyano-3-phenoxybenzyl 2,2,3,3-
tetramethyl-cyclopropanecarboxylate, WL 41706, in the rat. Pestic.
Sci., 8: 579-599. Submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FM-71-0050.
Crayford, J.V. (1975) The excretion and residues of radioactivity in
cows fed (14C) WL 41706 in their diet. Shell Research TLGR 0096 75.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FR-51-0015.
Creedy, C.L. & Potter, D. (1976) The effect of feeding WL 41706 on
the microsomal mono-oxygenase system of rat liver. Shell Research
report TLGR 0043 76. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0009.
Dean, B.J. (1975) Toxicity studies with WL 41706 (S-3206).
Chromosome studies on bone marrow cells of Chinese Hamsters after
two daily oral doses of WL 41706 (S-3206). Shell Research report
TLGR 0104 75. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-51-0003.
Dean, G.A., Gopinath, C. & Fish, L.E. (1987) Potential tumorigenic
and toxic effects in prolonged dietary administration to rats
(Addendum to final report). Huntingdon report SMO 167 87185
(Addendum to SMO 167 851348). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0181.
Dix, K.M. (1975) Toxicity studies of WL 41706: Teratological studies
in rabbits given WL 41706 orally. Shell Research report TLGR 0103 75
(Addendum to FT-51-0006). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-51-
0133.
Else, R. & Rushton, B. (1981) Histopathology report for compound WL
41706 3-generation study in rats. Inveresk report project 415370.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0069.
Fish, C.J., Lewis, D.J. & Gopinath, C. (1986) Electron micrograph
Addendum to histopathology report No SMO/167 S-3206 potential
tumorigenic and toxic effects in prolonged dietary administration to
rats. Huntingdon report SMO 167 86602 (Addendum to SMO 167 851348
(FT-61-0161)). Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0162.
Fleming, D.J. (1979) Toxicity studies on the insecticide WL 41706: A
three generation reproduction study (minus histopathology) in rats -
Corrigendum to FT-91-0027. Shell Research report TLGR 79 0071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-01-0028.
Fujita, Y. (1980) Interview survey concerning complaints connected
with use of S3206 in the field. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0040.
Hara, S. & Suzuki, T. (1980) Acute oral toxicity of S-3206 in
rabbits. Unpublished Sumitomo study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-00-0039.
Hara, M. & Suzuki, T. (1984a) Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0106.
Hara, M. & Suzuki, T. (1984b) In vitro sister chromatid exchanges
test of S-3206 in CHO-K1 cells. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-40-0108.
Hara, M. & Suzuki, T. (1984c) Addendum: Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0114.
Hara, M., Yamada, F., Kogiso, S., Yoshitake, A., Matsuo, M. &
Miyamoto, J. (1984) Mutagenicity test of S-3206 in Saccharomyces
cerevisiae D7. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-40-
0121.
Hend, R.W. & Butterworth, S.T.G (1975) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0031 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0001.
Hend, R.W. & Butterworth, S.T.G (1976a) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0020 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0013.
Hend, R.W. & Butterworth, S.T.G. (1976b) Toxicity studies on the
insecticide WL 41706: A short-term feeding study in rats. Shell
Research report TLGR 0041 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0011.
Hend, R.W. & Gellatly, J.B.M. (1979) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival,
bodyweight, food intake, organ weights, clinical chemistry,
hematology and gross pathological observations of rats exposed to WL
41706 for up to two years. Shell Research report TLGR 79 062.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0026.
Hend, R.W. & Gellatly, J.B.M. (1980) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival, body
weight, food intake, organ weights, clinical chemistry, hematology
and gross pathological observations of rats exposed to WL 41706 for
up to two years, Results of studies after 18 months. Shell Research
report TLGR 79 062 (Addendum to FT-91-0026). Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0032.
Hend, R.W., Gellatly, J.B.M. & Fleming, D.J. (1979) Toxicity studies
on the insecticide WL 41706: A three generation reproduction study
(minus histopathology) in rats. Shell Research report TLGR 79 071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0027.
Heywood, R. (1982) An appraisal of the toxicological findings on
rats receiving a synthetic pyrethroid S-3206 (fenpropathrin) by
dietary administration for 4 weeks. Huntingdon report (unnumbered,
1982). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0075.
Hiromori, T. Hosokawa, S. & Miyamoto, J. 1982a: Fenpropathrin
toxicology overview - Acute toxicity and safety in use. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-20-0079.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1982b) Acute oral toxicity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-20-0076.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1983a) Acute oral toxicity of S-3206 (97.3%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0082.
Hiromori, T., Misaki, Y., Seki, T., Hosokawa, S. & Miyamoto, J.
(1983b) Acute oral toxicity of S-3206 (91.8%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0081.
Hiromori, T., Nakanishi, T., Kawaguchi, S., Sako, H., Suzuki, T. &
Miyamoto, J. (1986a) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci. 11: 9-14.
Hiromori, T., Nakanishi, T., Suzuki, T., Kato, T. & Miyamoto, J.
(1986b) The mean slip angle test of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-60-0153.
Hiromori, T. & Takemura, T. (1983) Short report on facial
paresthesia following exposure to fenpropathrin, cypermethrin,
fenvalerate or permethrin in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0087.
Izumozaki, H., Hara, M. & Suzuki, T. (1984) Gene mutation test of S-
3206 in bacterial system. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-40-0107.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism
of fenvalerate and the (2S, alphaS)-isomer in rats and mice. J.
Pestic. Sci. 6: 317-326.
Kaneko, H., Shiba, K., Yoshitake, A. & Miyamoto, J. (1987)
Metabolism of fenpropathrin (S-3206) in rats. J. Pestic. Sci. 12:
385-395.
Kishida, F., Suzuki, H., Miyamoto, J. (1980) Studies on DNA damaging
capacity of S-3206 with Bacillus subtilis. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-00-0038.
Kohda, H. (1975) Acute oral toxicity of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-50-0018.
Kohda, H. (1976) Acute dermal toxicity of S-3206 in rats.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0019.
Kohda, H. & Kadota, T. (1975) Acute oral toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-50-
0035.
Kohda, H. & Kadota, T. (1976a) Acute dermal toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0036.
Kohda, H. & Kadota, T. (1976b) Acute subcutaneous and
intraperitoneal toxicity of S-3206 technical in rats and mice.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0037.
Kohda, H., Kadota, T. & Miyamoto, J. (1976) Acute inhalation
toxicity of S-3206 and S-5602 in mice an rats. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number AT-50-0043.
Ku, H.S. & Doran, T.J. (1990a) A study to determine the nature of
the residue in milk, meat and tissue from lactating goats dosed with
14C fenpropathrin. Ricerca Inc report project 89 0109. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0041.
Ku, H.S. & Doran, T.J. (1990b) A study to determine the nature of
the residue in poultry end eggs from chickens dosed with 14C
fenpropathrin. Ricerca Inc., report project 89 0084. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0042.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
Marroquin, F., Richter, W.R., Myer, J.R. & Johnson, D.E. (1981) S-
3206 technical grade: Acute dermal toxicity (LD50) study in rabbits
(TSCA 7/79) (EPA 8/78) (OSHA). IRDC report project 491 002.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0051.
Matsubara, T., Hara, S. & Kadota, T. (1978) Primary eye and skin
irritation tests of S-3206 in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-80-0023.
Matsumoto,S., Suzuki, T., Kato, T. & Yamada, H. (no date) Comments
on the facial sensation-inducing potential of fenpropathrin.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-80-0195.
McSheehy, T. & Nunziata, A. (1984) Chromosome aberrations in Chinese
hamster ovary (CHO) cells in vitro. Test substance: S-3206 Final
report 113-005. Life Science Research Roma report project 113 005.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-41-0104.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 41706
to adult hens. Shell Research report TLGR 0068 77. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FW-81-0006.
Misaki, Y., Hiromori, T., Hosokawa, S. & Miyamoto, J. (1983)
Comments on acute oral toxicity of fenpropathrin technicals with
high purity and lower purity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0083.
Misaki, Y. & Kohda, H. (1981a) Acute oral toxicity of two impurities
of S-3206 (technical) in mice. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0044.
Misaki, Y. & Kohda, H. (1981b) Acute oral toxicity of 2,2,3,3-
tetramethylcyclopropane carboxylic anhydride in mice. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-90-0045.
Morseth, S.L. (1990) Rat teratology study with S-3206. Hazleton
report project 343 216. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-01-
0216.
Ohkawa, H., Kaneko, H., Tsuji, H. & Miyamoto, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
Okuno, Y. (1981) Comment on possible weight changes of kidney and no
effect levels in a 2-year chronic toxicity study on WL 41706
(fenpropathrin). Comments 0n FT-91-0026 and FT-11-9946. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-10-0048.
Okuno, Y., Kadota, T. & Miyamoto, J. (1975) Skin sensitization study
of S-3206 in guinea-pigs. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-50-0024.
Omodaka, H., Misaki, Y. & Okuno, Y. (1986a) Acute oral toxicity of
S-3206 in rats. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0138.
Omodaka, H., Suzuki, T. & Kato, T. (1986b) Acute dermal toxicity
study of S-3206 in rats. Unpublished Sumitomo study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
60-0139.
Pauw van der, C.L., Dix, K.M., Blanchard, P.M., McCarthy, W.V. &
Stevenson, D.E. 1975: Teratological studies in rabbits given WL
41706 orally. Shell Research report TLGR 0103 75. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-51-0006.
Pence, D.H., Alsaker, R.D., Hepner, K.E., Dawkins, B.G., Vargus,
K.J., Hagen, W.H. & Hitzelberg, D.A. (1984) Chronic study in dogs S-
3206 T.G. Final report. Hazleton report project 343 153. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-41-0122.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980a) Subchronic toxicity study in dogs S-3206.
Final report. Hazleton report project 343 125. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0034.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980b) Subchronic toxicity study in dogs S-3206.
Addendum to final report. Hazleton report project 343 125. Addendum
to FT-01-0034. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-01-0041.
Pence, D.H., Pence, L.A., Durloo, R. and Cameron, J. (1980c)
Teratology study in rats S-3206. Final report. Hazleton report
project 343 122. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-010031.
Pence, D.H., Weatherholtz, W.M. & Cameron, J.T. (1978) Oral dose
range finding study in dogs, S-3206. Final report. Hazleton report
project 343 123. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-91-0025.
Richold, M., Allen, J.A. & Proudlock, R.J. (1982a) Autoradiographic
assessment of DNA repair in mammalian cells after exposure to S-3206
(fenpropathrin). Huntingdon report SMO 143 81881. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-21-0068.
Richold, M., Grantham, C.J. & Basham, K.J. (1982b) An assessment of
the mutagenic potential of S-3206 using an in vitro mammalian cell
test system (with comment by J. Miyamoto). Huntingdon report SMO 144
8252. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference numberFT-21-0060.
Riley, J.H., Nair, K.P.C., Kopplin, J.R., Johnson, D.E., Ott, S.S.,
Meyer, J.R. & Spicer, E.J.F. (1982) S-3206 (technical grade) 21-day
dermal study in rabbits (EPA) (technical product). IRDC report
project 491 010. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-21-0058.
Ruzo, L.O., Unai, T. & Casida, J.E. 1978: Decamethrin metabolism in
rats. J. Agric. Food Chem. 26: 918-925.
Shell Chemical (UK) Limited (1978) A survey of operator experience
with fenpropathrin 10% EC - A pyrethroid insecticide/acaricide for
use in fruit and ornamental crops in 1986. Shell UK report 16th
January 1987. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-71-0179.
Summitt, L.M. & Albert, J.R. (1976) Intravenous toxicity of SD 41706
(1-24-0-0) in the mouse. Shell Development Co report TIR 74 110 76.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0010.
Suzuki, H. (1977) Studies on mutagenicity of some pyrethroids on
Salmonella strains in the presence of mouse hepathic S9 fractions.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number AT-70-0157.
Suzuki, T. & Miyamoto, J. (1981) Skin sensitization test of S-3206
in guinea-pigs. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-10-
0078.
Suzuki, T., Sako, H. & Okuno, Y. (1986) Acute oral toxicity study of
S-3206 in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0175.
Warren, S., Heywood, R., Street, A.E., Gopinath, C., Browne, J.G.,
Gibson, W.A., Reed, L.E. & Anderson, A. (1986) S-3206: Potential
tumorigenic and toxic effects in prolonged dietary administration to
rats (Final report. Huntingdon report SMO 167 851348. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-61-0161.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yoshida, A., Kosaka, T., Miyaoka, T., Maita, K. & Goto, S. (1986) S-
3206: 13-week oral subchronic toxicity study in rats. Inst. Environ.
Toxicol. Tokyo report 17th, July 1986. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-61-0166.
Yoshitake, A., Kogiso, S. & Hara, M. (1990) In vitro chromosomal
aberration test of S-3206 in Chinese Hamster ovary cells (CHO-K1).
Sumitomo study M83-54. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-00-
0215.
Yoshitake, A., Kogiso, S., Kato, H. & Hara, M. (1988) In vitro
gene mutation test of S-3206 in V79 Chinese Hamster cells in
culture. Sumitomo study 1174. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-80-
0209.
Yoshitake, A. Kogiso, S. & Yamada, F. (1986) Bacterial DNA repair
test of S-3206. Sumitomo study MUT86020. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-60-0165.
Yoshitake, A., Kogiso, S & Yamada, F. (1987) Reverse mutation test
of S-3206 in bacterial systems. Sumitomo study 704. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-70-0182.
Yoshitake, A., Kogiso, S., Yamamoto, K. & Hara, M. (1989) In vitro
chromosomal aberration test of S-3206 in Chinese Hamster ovary cells
(CHO-K1). Sumitomo study 1730. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-90-
0200.
Table 1. Acute toxicity of fenpropathrin (technical material)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Rat M oral 54 97 Kohda, 1975
F oral 49 97 Kohda, 1975
M oral 71 91.8 Hiromori et al., 1983b
Misaki et al., 1983
F oral 67 91.8 Hiromori et al., 1983b
Misaki et al., 1983
M oral 77 97.3 Hiromori et al., 1983a
Misaki et al., 1983
F oral 67 97.3 Hiromori et al., 1983a
Misaki et al., 1983
M oral 164 97.3 Hiromori et al., 1983b
F oral 107 97.3 Hiromori et al., 1983b
M&F oral 60 93.8 Omodaka et al., 1986a
F oral 70 93.8 Omodaka et al., 1986a
M&F inhalative (vapour) > 0.009 µg/l* 94.5 Bruce et al., 1986
M&F inhalative (Xylene EC mist) > 96 µg/l* 97 Kohda et al., 1976
M dermal (sol. in corn oil) 1600 97 Kohda, 1976
F dermal (sol. in corn oil) 870 97 Kohda, 1976
M&F dermal (viscous liquid) > 5000 93.8 Omodaka et al., 1986b
M sc 1410 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 225 97 Kohda & Kadota, 1976b
F ip 180 97 Kohda & Kadota, 1976b
Mouse M oral 67 97 Kohda & Kadota, 1975
F oral 58 97 Kohda & Kadota, 1975
M oral 135 93.8 Suzuki et al., 1986
F oral 154 93.8 Suzuki et al., 1986
M inhalative (Xylene EC mist) 100 µg/l* 97 Kohda et al., 1976
F inhalative (Xylene EC mist) 43 µg/l* 97 Kohda et al., 1976
Table 1 (contd)
Species Sex Route LD50 Purity References
(mg/kg bw) (%)
Mouse M dermal 740 97 Kohda & Kadota, 1976a
F dermal 920 97 Kohda & Kadota, 1976a
M sc 1350 97 Kohda & Kadota, 1976b
F sc 900 97 Kohda & Kadota, 1976b
M ip 230 97 Kohda & Kadota, 1976b
F ip 210 97 Kohda & Kadota, 1976b
M iv 4.5 ? Summitt & Albert, 1976
Rabbit M oral 675 96.2 Hara & Suzuki, 1980
F oral 510 96.2 Hara & Suzuki, 1980
M&F dermal > 2000 ? Marroquin et al., 1981
Dog M&F oral > 1000 96.2 Pence et al., 1978
* LC50
Rats
Fenpropathrin (purity 96%) was fed to groups of Carworth Farm E
rats (12/sex/dose; control 24/sex) at dietary concentrations of 0,
2, 10, 50 or 250 ppm over 13 weeks. The treatment did not influence
the general health and behaviour, haematology, clinical chemistry or
pathology of treated animals. Body-weight gain was slightly
increased at all dose levels. A slight increase in spleen weight in
male rats at 250 ppm was the only change concerning organ weights
and may present a borderline effect. The NOAEL was > 250 ppm,
equal to 18 mg/kg/day (males) and 21 mg/kg bw/day females) (Hend &
Butterworth, 1975).
In a similar study, rats (12/sex/group; control 24/sex) were
fed diets containing fenpropathrin (purity 97%) at concentrations of
0, 3, 30, 100, 300 or 600 ppm over 13 weeks. After 5 weeks of
exposure, most females and one male showed tremors and reduced body-
weight gain at 600 ppm. The tremors disappeared towards the end of
the study. Changes in a few haematological parameters were marginal
and were - though statistically significant - not considered
biologically significant. Slight increases in plasma ALP were
observed at 600 ppm. Organ weights were not influenced by treatment
and no abnormal histopathological findings were observed. The NOAEL
was 300 ppm, equal to 17 mg/kg bw/day (males) and 24 mg/kg bw/day
(females) based on the appearance of clinical signs and reduced
body-weight gain at 600 ppm (Hend & Butterworth, 1976a).
In a third study, groups of rats (SPF SD(Crj:CD); 12/sex/dose)
were fed concentrations of 0, 15, 50, 150, 450 or 600 ppm
fenpropathrin (purity 93.1%) over a period of 13 weeks. One female
died at 600 ppm. A dose-dependent depression of body-weight gain
was observed at 600 ppm and 450 ppm in female rats, and at 600 ppm
in male rats. Food consumption was reduced at the beginning of the
study at 600 ppm in males and at 450 ppm and above in females; food
efficiency was reduced in females treated with 600 ppm. An increase
in urinary protein and specific gravity was observed in males at 450
ppm and above. Haematological and ophthalmological parameters as
well as organ weights were not influenced by the treatment. A
slightly elevated alkaline phosphatase activity was noted in females
of the 600 ppm group. There was no evidence of treatment-related
histopathological changes in any organ. The NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain and
urinalysis at higher dose levels (Yoshida et al., 1986).
Rabbits
In a 21-day dermal toxicity study with fenpropathrin (purity
91.4%), groups of New Zeeland white rabbits (10/sex/dose) received
dermal applications on either intact or abraded skin at dose levels
of 0, 500, 1200 or 3000 mg/kg bw. The treatment did not influence
survival, body weight, food consumption, haematology, clinical
chemistry, organ weights, or macroscopic or microscopic findings.
Single instances of slight skin reactions were observed at the
application sites of animals treated with 500 and 1200 mg/kg bw and
in most animals at 3000 mg/kg bw. The NOAEL was > 3000 mg/kg bw
based on systemic toxicity (Riley et al., 1982).
Dogs
Fenpropathrin (purity 96.2%) was administered in the diet to
groups of beagle dogs (6/sex/dose) at concentrations of 0, 250, 500
or 1000/750 ppm over 13 weeks (1000 ppm for 3 weeks, 750 ppm for the
rest of the study because of the appearance of severe tremors and
ataxia). The doses were adjusted for purity. Treatment did not
affect survival, food consumption, ophthalmoscopy, clinical
chemistry, urinalysis, organ weights, gross or microscopic
pathology. Treatment-related clinical signs consisted of emesis,
tremors, ataxia, diarrhoea, lethargic appearance and salivation.
Some effects were observed in all treatment groups. The frequency
of the observed clinical signs in all of the compound-treated groups
decreased after week 5. One high-dose male dog was sacrificed
moribund. Reduced body-weight gain was observed at concentrations of
500 and 1000/750 ppm. Haematocrit and haemoglobin values and the
erythrocyte count in females at 1000/750 ppm were decreased. The
NOAEL was < 250 ppm, equal to 7 mg/kg bw/day in males and 10 mg/kg
bw/day in females, based on the occurrence of emesis at 250 ppm
(Pence et al., 1980a,b).
Fenpropathrin (purity 92.5%) was fed to groups of beagle dogs
(4/sex/dose) at dietary concentrations of 0, 100, 250 or 750 ppm
(adjusted to 100% active ingredient) over a period of 52 weeks. One
high-dose male died in the middle of the study after exhibiting
tremor and ataxia. Emesis occurred at an increased incidence in the
mid-dose and high-dose females but without a clear dose-response
relationship. The incidence of tremors however showed a clear dose-
related increase at 250 and 750 ppm (males: 0, 0, 49, 181; females:
0, 1, 42, 168 observations at 0, 100, 250 and 750 ppm,
respectively). Ataxia occurred at 750 ppm. Reduced body-weight
gain was observed at 750 ppm and at 250 ppm in females. The
treatment did not influence ophthalmoscopy, haematology, clinical
chemistry, urinalyses, gross or microscopic pathology. The only
significant difference concerning organ weight was an increase in
the relative kidney weight in females at 750 ppm. The NOAEL was 100
ppm, equal to 3 mg/kg bw/day, based on reduced body-weight gain and
occurrence of clinical signs at 250 ppm and higher (Pence et al.,
1984).
Long-term toxicity/carcinogenicity studies
Mice
In a 104-week feeding study, groups of mice (Charles River (UK)
CD-1; 52/sex/group in main study; 40/sex/group in satellite groups
for interim sacrifice) were fed fenpropathrin (purity 91.4%-92.5%)
at concentrations of 0, 40, 150 or 600 ppm. These concentration
levels were based on two range-finding studies (Colley et al.,
1981a,b). Treatment did not affect the general behaviour, with the
exception of a marginal increase in the number of females at 600 ppm
showing hyperactivity at week 78. No effects on survival rate, body
weight, food consumption, food efficiency, urinalysis, macroscopic
pathology or organ weights were reported. Changes in haematological
parameters at 600 ppm included slight decreases in Hb and MCHC in
males and an increase in MCHC in females at week 25. Because these
changes were small, occurring only on some occasions and only in one
sex, they are not considered to be toxicologically significant.
Sporadic changes in some clinical chemical parameters were not
considered to be of toxicological significance (e.g., decrease in
urea nitrogen in males at 600 ppm, increase in GOT and GPT levels at
all dose levels without clear dose-response relationship). An
increased incidence in lung tumours (adenoma and adenocarcinoma) was
observed in males and females of treated groups. The incidences in
the satellite groups (spontaneous deaths and interim sacrifice after
26, 52 and 78 weeks) were 10, 12.5, 18 and 2.5% for males and 7.5,
12.5, 7.5 and 7.5% for females at 0, 40, 150 and 600 ppm,
respectively. The incidences in the main study were 12, 23, 35 and
31% for males and 10, 29, 19 and 13% females at 0, 40, 150 and 600
ppm, respectively. The incidences did not show a clear dose-
response relationship. The absence of any statistical significance
or significant trend supports the interpretation that the increased
incidences in the treatment groups were not due to a tumorigenic
activity of fenpropathrin. Moreover, data on the incidence of lung
tumours in historical controls showed that incidences of pulmonary
adenoma and adenocarcinoma in untreated animals may vary between 7
and 36%. There was no sex difference concerning lung tumours in
these controls. Therefore the results of this study gave no
evidence of fenpropathrin-induced carcinogenic potential. The NOAEL
was the highest dose tested, 600 ppm, equal to 56 mg/kg bw/day
(males) and 65 mg/kg bw/day (females) (Colley et al., 1985, 1987).
Rats
Groups of rats (COBS) were fed diets containing 0, 1, 5, 25,
125 or 500 ppm fenpropathrin (purity 97%) for two years. The group
sizes were 12/sex and 48/sex for the satellite and main study
controls, respectively; while the treatment groups included 6/sex
and 24/sex for the satellite and main study, respectively. The
treatment did not affect the survival, general health, behaviour of
the animals, clinical chemical or haematological parameters. The
depression in body-weight gain observed at 500 ppm was statistically
significant in females only. Food consumption of treated animals
was comparable with control animals at most observation times.
Absolute spleen weight increased at 500 ppm in females of the 6-
month satellite group and kidney weights decreased in females of all
treatment groups in the 12-month satellite groups without showing a
clear dose-response relationship. In the 2-year main study groups,
no treatment-related alterations in organ weights were observed.
Gross pathological examination showed a higher incidence of
white/grey foci or plaques in the lungs at 125 and 500 ppm in
females at 6 and 24 months. In the 2-year main groups, the number
of deaths attributable to renal failure in males at 500 ppm was
greater than in the other groups, but no increase was observed when
animals from satellite and main study groups were combined.
The histopathological neoplastic and non-neoplastic changes
found were consistent with the range and severity of changes usually
observed in this rat strain and did not give any evidence of
carcinogenicity. The NOAEL was 125 ppm, equal to 5 mg/kg bw/day
based on depression in body-weight gain at higher dose levels (Hend
& Gellatly, 1979; Okuno, 1981; Aitken & Rushton, 1981).
Single animals of a satellite group fed the above-mentioned
concentrations for 18 months were used for functional and
neurochemical investigations. For results see "Special Studies on
Neurotoxicity" (Hend & Gellatly, 1980).
Fenpropathrin (purity 91.4-92.5%) was fed to groups of rats
(Charles River CD; 50/sex/dose in main study and 15/sex/dose in
satellite groups for interim sacrifice) at dietary concentrations of
0, 50, 150, 450 or 600 ppm for 104 weeks. The dose levels were
selected based on the results of range-finding studies (Colley et
al., 1982a,b; Heywood, 1982). Clinical signs were restricted to
body tremors, most prevalent in females receiving 600 ppm, but were
also observed in males at the highest dose levels in the first few
weeks of the study and in females at 450 ppm.
Mortality increased in males and females receiving 600 ppm and
in females receiving 450 ppm during the first 26 weeks of treatment;
subsequently, mortality was less than that of control animals
resulting in highest survival rates in males at 600 ppm and in
females at 450 ppm. Exposure of main group females receiving 600
ppm was terminated after 52 weeks. A slightly higher food
consumption was reported for the high-dose females during the first
3 months of treatment, most probably resulting in the mortalities
observed. Body-weight gain was reduced in females at 600 ppm and
food utilization efficiency was marginally impaired.
Ophthalmological examination did not reveal treatment-related ocular
lesions. No abnormal haematological findings were reported.
Changes in clinical chemistry parameters at 450 and 600 ppm included
slightly reduced creatinine levels and reduced total protein levels
in males. The differences were minimal however and - though
statistically significant - most probably of no biological
significance. Urinalysis did not indicate any kidney lesions.
Macroscopic examination revealed no findings which were
treatment-related with the exception of an increased incidence of
alopecia in females at 600 ppm at 52 weeks. Changes in organ
weights were observed on some occasions but did not show a
treatment-related pattern and histopathological examination did not
reveal changes attributable to treatment. A few lymphoreticular
tumours were seen in treated groups of male rats, but none in
control group. The increases observed were not dose-related and
were within the incidence range of the historical controls for this
rat strain. There was no indication of a treatment-related increase
in tumour incidence or non-neoplastic organ changes. The electron
microscopic examination of tibial nerve did not reveal treatment-
related effects. The NOAEL was 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses (Warren
et al., 1986; Fish et al., 1986; Dean et al., 1987).
Reproduction studies
Rats
In a 3-generation reproduction study, fenpropathrin (purity
97%) was fed to rats (COBS; 30/sex/group) at dietary concentrations
of 0, 5, 25 or 250 ppm. Treatment did not influence the general
health condition or mortality, body-weight gain, food consumption,
pregnancy rates, litter size or litter weight. However, pup weights
in the F3a generation were reduced at 250 ppm. No macroscopic or
microscopic lesions were attributable to treatment. A very small
number of cases of locomotor incoordination occurred in pups fed 5
and 250 ppm. Most pups in the 5 ppm group showing these signs were
from one litter in the F1b generation. No similar lesions were
observed in the second litter of that pair nor in the following
generation and no macroscopic lesions were identified in these
animals. Histopathological examination (F2 parents, F3b pups) did
not reveal treatment-related tissue lesions. The NOAEL was 25 ppm,
equal to 1.6 mg/kg bw/day, based on decreased pup weights in the
F3a generation at 250 ppm (Fleming, 1979; Hend et al., 1979; Else
& Rushton, 1981).
In a second 3-generation study, groups of rats (CrL: COBS CD
(SD)BR; 11-28/sex/dose) were fed diets containing 0, 40, 120 or 360
ppm fenpropathrin (purity 92.5%). Animals of the last generation
(F2) were reared to maturity. At 360 ppm, most females that
littered showed body tremors associated with muscle twitches and
increased sensitivity. These signs were generally observed during
the lactation period. Increased mortality occurred at 360 ppm in
females only; 18/28 females at this dose died, most during the
lactation period of the F1b females. At 120 ppm, 2/24 F1b females
died during lactation. Mean body-weight gain of males in the F1
and F2 generations were reduced at 360 ppm. F1b females at 120
ppm showed also some indication of retarded weight gain. Treatment
had no influence on mating performance and no macroscopic or
microscopic abnormalities were observed at terminal autopsy that
were considered to be associated with treatment. At 360 ppm, F0
females showed a slight increase in liver weights. A few F2b pups
at 120 ppm showed body tremors prior to weaning and 2/3 of these
pups died subsequently. A slight reduction in litter size was
observed at 360 ppm in F0 animals (second mating) and in both
matings of F1b animals from days 4 to 21. At 360 ppm, pup weights
were lower in all generations. The NOAEL was 40 ppm, equal to 3
mg/kg bw/day, based on depression of body-weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above (Cozens et al., 1986).
Special studies on embryotoxicity/teratogenicity
Rats
Fenpropathrin (purity 96.2%) was fed by intubation to groups of
rats (Fischer 344 CDF; 27/28 females/dose) from days 6 through 15 of
gestation at dose levels of 0, 0.4, 2 or 10 mg/kg bw/day. One mid-
dose and 9 high-dose females were found dead during the study.
Clinical signs noted at a somewhat greater incidence in the treated
animals included soft faeces, red or lacrimating eyes, alopecia or
hunched appearance. Post-dose tremors occurred on a few occasions
in some high-dose females. Body-weight gain was reduced in high-
dose females during the treatment period, and increased slightly
afterwards, resulting in an overall change comparable to the control
group. Food consumption was also reduced at the highest dose level.
Various gross lesions were found in animals that died during the
study. The findings included discolorations of organs, but no
specific target organ was identified. The pregnancy rate was lower
in the high-dose group compared to the other dose groups but other
reproduction parameters (e.g., number of corpora lutea, resorptions,
fetal viability) were not impaired by treatment. No visceral or
skeletal anomalies were noted in the pups. Visceral or skeletal
variants did not show dose-related increases. No teratogenic
effects were observed. The NOAEL for maternal toxicity was 2 mg/kg
bw/day. The NOAEL for embryotoxicity and teratogenicity was 10 mg/kg
bw/day (Pence et al., 1980c; Cox, 1986, 1987).
Groups of rats (Fischer-344; 30 females/dose group) were orally
treated with fenpropathrin (purity 91.9%) at dose levels of 0, 0.44,
1.6, 2.2, 3.3, 6.5 or 11 mg/kg bw/day (corresponding to 0, 0.4, 1.5,
2, 3, 6 or 10 mg/kg bw/day active ingredient) from day 6 to day 15
of gestation. Seven animals died in the highest dose group. Signs
of toxicity included ataxia, sensitivity to external stimuli,
tremors, prostration and convulsions. At 6 and 10 mg/kg bw/day,
mean maternal body-weight gains and food consumption were lower
during the dosing period. Pregnancy rate of high-dose females was
slightly lower (90%) compared to the other treatment groups (93%-
97%). Treatment had no influence on reproduction parameters such as
resorptions, fetal viability or fetal body weight. No visceral or
skeletal anomalies were observed that were considered treatment-
related. Skeletal variations were noted in all groups and included
variations in the stage of ossification and rib counts, but not in a
dose-dependent pattern. A higher incidence of incomplete
ossification of the sternebrae was observed in all treatment groups
but without a clear dose-response relationship. The highest
incidences with respect to some differences in ossification (e.g.,
5th/6th sternebrae incomplete ossification, asymmetric ossification
of sternebrae) were noted at 10 mg/kg bw. Statistical analysis
revealed no significant trend for the overall incidence of delayed
ossification of the 5th/6th sternebrae. The NOAEL for maternal
toxicity was 3 mg/kg bw/day. The NOAEL for embryotoxicity and
teratogenicity was 10 mg/kg bw/day (Morseth, 1990).
Rabbits
Groups of rabbits (banded Dutch; 20-30 females/group) were fed
fenpropathrin (purity 97%) at dose levels of 0, 1.5, 3 or 6 mg/kg
bw/day from day 6 to day 18 of gestation. No clinical signs of
intoxication were observed. A lower number of rabbits survived to
term at 3 mg/kg bw/day but this was unrelated to treatment.
Treatment had no effect on body weight of the dams. No adverse
effects were observed with respect to pre-implantation loss, number
of resorptions, fetal deaths, litter size, or pup weight. Increased
incidence in minor skeletal and visceral abnormalities (e.g.,
enlarged fontanelles, interparietal bones, fused sternebrae, extra
centres of ossification) were noted at 3 mg/kg bw but not at the
higher dose. Therefore these changes were not considered treatment-
related. The NOAEL for maternotoxicity, embryotoxicity and
teratogenicity was 6 mg/kg bw/day (van der Pauw et al., 1975; Dix,
1975).
Groups of rabbits (New Zeeland white; 17-19 females/dose) were
treated with oral doses of 0, 4, 12 or 36 mg/kg bw/day fenpropathrin
(purity 92.5%) from days 7 to 19 of gestation. A dose-related
increase in the incidence of grooming was observed in all dose
groups. At 12 and 36 mg/kg bw/day, flicking of forepaws was also
observed, with a few animals at 36 mg/kg bw/day showing shaky
movements and tremor. Slight reduction in body-weight gain and
reduced food consumption were noted at 36 mg/kg bw/day. One dam at
12 and two at 36 mg/kg bw failed to maintain their pregnancies. One
dam at each dosage (including control) was sacrificed in poor
condition. Treatment did not adversely affect litter parameters
(e.g., litter size, fetal weight). There was no dose-related
increase in the incidence of malformations. The NOAEL was 4 mg/kg
bw/day with respect to maternotoxicity and 36 mg/kg bw/day for
fetotoxicity and teratogenicity (Cozens et al., 1985).
Special studies on genotoxicity
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The results are
summarized in Table 2.
Special studies on neurotoxicity
Hens
Six laying hens were treated with daily oral doses of 1 g/kg
bw/day for five days. Dosing was repeated after three weeks and the
birds sacrificed three weeks later. A positive control group was
treated with tri-ortho-tolyl phosphate (TOTP). No signs of
intoxication were noted. Histological examination of nervous
tissues revealed no lesions (Milner & Butterworth, 1977).
Rats
Groups of rats (6/sex/group) were fed diets containing 0 or 900
ppm fenpropathrin (purity not specified) for up to 25 days. Tremors
and high mortality were noted. On histological examination of the
sciatic nerve, swelling and disintegration of nerve axons were
observed whereas no myelin lesions were noted (Hend & Butterworth,
1976b).
The neurotoxic effect of fenpropathrin in rats was assessed by
means of the slip angle test. Oral doses of 0, 10, 25, 50, 75 or
100 mg/kg bw were administered to male rats (10/dose) and the angle
at which the treated animals slipped from the board determined. The
mean slip angle (MSA) decreased dose-dependently at 50 mg/kg bw and
higher. Clinical signs observed in animals treated with doses of 25
mg/kg bw and above consisted of slight to severe tremor, ataxia and
limb paralysis appearing at 3-7 hours after administration. The
effects on MSA and toxic signs almost disappeared after 24 hours.
Dose-related mortalities were observed at 50 mg/kg bw and above
(Hiromori et al., 1986b).
Table 2. Mutagenicity of fenpropathrin
Test system Test object Concentration Purity % Results Reference
non-activated activated
In vitro
Ames test S. typhimurium 50-5000 µg/plate 92.5 negative negative Izumozaki et al., 1984
(various strains) Yoshitake et al., 1987
Ames test S. typhimurium 10-1000 µg/plate 97.0 negative1 negative Suzuki, 1977
(various strains)
Reverse mutation Saccharomyces 92.5 negative negative Hara et al., 1984
mitotic crossing cerevisiae D7
over and mitiotic
gene convn. assay
Mammalian cell Mouse lymphoma 50-400 µg/ml (-activ). 91.4 negative negative Richold et al., 1982b
mutation assay L5178Y cells 30-300 µg/ml (+activ).
Gene mutation V79 Chinese 50-500 µg/ml 92.4 negative negative Yoshitake et al., 1988
assay hamster cells
DNA repair assay HeLa S3 cells 0.16-2.5 µg/ml ? negative negative Richold et al., 1982a
Rec-assay Bacillus subtilis 10-5000 µg/disc 97.0 negative negative Kishida et al., 1980
M45 rec and H17
DNA repair assay Bacillus subtilis 100-10 000 µg/disc 92.5 negative negative Yoshitake et al., 1986
Chromosome damage Chinese hamster 10 or 20 mg/kg bw 97.0 negative negative Dean, 1975
bone marrow cells orally on two
succcessive days
Table 2 (contd)
Test system Test object Concentration Purity % Results Reference
non-activated activated
Chromosome Chinese hamster 50-500 µg/ml (-activ). 92.5 negative negative McSheehy & Nunziata,
aberration assay ovary cells (CHO) 500-5000 µg/ml (+activ). 1984
Chromosome CHO-K1 cells 10-30 µg/ml (-activ). 92.4 negative negative Yoshitake et al., 1989
aberration assay 250-1000 µg/ml (+activ).
Chromosome Chiense hamster 0.003-0.1 mmol/l 92.5 negative negative Yoshitake et al., 1989b
aberration assay ovarian cells
(CHO-K1)
Sister chromatid Chinese hamster 0.003-0.1 mmol/l 92.5 negative negative Hara & Suzuki, 1984b
exchange ovarian cells
(CHO-K1)
In vivo
Micronucleus test Mouse femur cells 50-200 mg/kg 92.5 negative negative Hara & Suzuki, 1984a,c
intraperitoneally
Host mediated S. cerevisiae JD1 10-20 mg/kg bw orally 97.0 negative negative Brooks et al., 1976
assay (in male mice)
1 +activation by hepatic S-9 fractions obtained from 6 different mouse strains
Functional testing was performed on 2 animals/sex at 0, 125 or
500 ppm (satellite group of long-term study in rats, Hend &
Gellatly, 1979) using the "inclined plane test". Preliminary
results revealed an impaired performance in animals fed 500 ppm.
Measurements of ß-glucuronidase activity (parameter indicative of
Wallerian degeneration in nerves) did not reveal a clear increase as
would be expected as a result of toxic neuropathy. Macroscopy and
histopathology of sciatic and tibial nerves did not show treatment-
related changes (Hend & Gellatly, 1980).
Special study on effects on enzymes
A preliminary investigation was performed to study the possible
effect of fenpropathrin on the induction of hepatic microsomal
enzymes. Four Charles River CD rats were fed dietary concentrations
of 1, 10, 100 or 1000 ppm over two weeks. No significant difference
was observed between the activities of control and test livers in
the in vitro O-dealkylation of 14C-chlorfenvinphos and no dose-
related increase in liver weights was induced. Therefore, the
results of this study do not provide any evidence for an inducing
effect of fenpropathrin on liver microsomal enzymes (Creedy &
Potter, 1976).
Special study on antidotes
The therapeutic potency of intraperitoneally administered
methocarbamol [3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate] was
examined against acute oral intoxication of rats after treatment
with lethal doses of fenpropathrin (100 mg/kg bw) and some other
pyrethroids. Methocarbamol was initially administered
intraperitoneally at a dose of 400 mg/kg bw followed by repeated
doses of 200 mg/kg bw. Treatment with methocarbamol reduced the
mortality from 60% to 0% and depressed tremors (Hiromori et al.,
1986a).
Special study on paresthesia
Rabbits
Facial paresthesia activities following exposure to
fenpropathrin and other pyrethroids were estimated using a rabbit
model. To determine the intensity of facial paresthesia changes in
behaviour after dermal application of the pyrethroids were recorded.
Doses applied ranged from 0.0001-10 mg/animal. The frequency of
licking and/or biting after application showed a clear dose-response
relationship with all pyrethroids tested. Post-treatment with
vaseline-based 5% benzocaine ointment (local anaesthetic) was
effective in reducing the intensity of animal response. Post-
treatment by application of undiluted vitamin E was also effective
for treatment of facial paresthesia (Hiromori & Takemura, 1983).
Special studies on irritation and sensitization
The test material (purity 90.2%) caused no skin irritation and
only very slight eye irritation in rabbits (Matsubara et al.,
1978).
Two skin sensitization studies were conducted with guinea-pigs.
No sensitization reactions occurred (Okuno et al., 1975; Suzuki &
Miyamoto, 1981).
Observations in humans
In a field study in Japan, six workers participated in two
tests using a 5% emulsifiable concentrate. The spray concentrations
were 25-50 ppm (a.i.), the spraying time 2 hours. A motor mounted
sprayer was used. The workers wore protective clothing. No effects
were reported (e.g., headache, nasal discharge, itching, burning,
pain in face or limbs) (Fujita, 1980).
Some workers exposed to pyrethroids in the laboratory during
formulation processes or in field trials in England reported
transient abnormal facial sensations. A clinical and
electrophysiological study using an electromyograph was carried out
on 23 workers. Electrophysiological studies were also carried out
on an equal number of age and sex-matched control subjects who had
no contact with pyrethroids. Nineteen had experienced at least one
episode of abnormal facial sensations and 13/23 had experienced
several such episodes. Permethrin, cypermethrin, fenpropathrin and
fenvalerate were most frequently used. Symptoms were limited to the
face. Characteristically, the symptoms appeared 30 minutes to eight
hours after exposure. Since exposure to the different pyrethroids
varied, it was not possible to determine which compounds were more
likely to produce symptoms. Electrophysiological investigation
showed statistically significant increase in maximal nerve
conduction velocity in exposed workers compared to controls, which
is contrary to what is expected in subclinical neuropathy. The
increase must therefore be fortuitous. This conclusion is supported
by the observation that no difference in nerve conduction velocity
was noted in workers more exposed than others (Le Quesne et al.,
1980; Matsumoto et al., no date).
In a survey of 18 operators using a 10% fenpropathrin product,
no adverse effects were reported, with the exception of slight nasal
irritation. All operators wore protective clothing (Shell Chemical
Ltd., 1978).
COMMENTS
After oral administration of fenpropathrin to rats, the
compound was almost completely absorbed and eliminated in urine and
in faeces. The major biotransformation reactions consisted of
oxidation at the methyl groups of the acid moiety and at the 2'-and
4'-positions of the alcohol moiety, cleavage of the ester linkage
followed by glucuronide, sulfate or glycine conjugation.
Fenpropathrin has been tested for acute toxicity and it has
been classified as moderately hazardous by WHO.
In a short-term feeding study in rats conducted at dietary
concentrations of 0, 3, 30, 100, 300 or 600 ppm over thirteen weeks,
the NOAEL was 300 ppm, equal to 17 mg/kg bw/day, based on reduced
body-weight gain and the appearance of clinical signs at higher dose
levels. In a second 13-week study in rats, the NOAEL was 150 ppm,
equal to 8 mg/kg bw/day, based on depression of body-weight gain at
higher dose levels.
A one-year study in dogs conducted at dose levels of 0, 100,
250 or 750 ppm revealed a NOAEL of 100 ppm equal to 3 mg/kg bw/day,
based upon reduced body-weight gain and clinical signs (emesis,
tremors) at 250 ppm.
A long-term toxicity/carcinogenicity study was performed in
mice over 2 years at 0, 40, 150 and 600 ppm. The NOAEL was the
highest dose tested, 600 ppm, equal to 56 mg/kg bw/day. There was
no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study in rats conducted
at dietary concentrations of 0, 1, 5, 25, 125 or 500 ppm over two
years, the NOAEL was 125 ppm, equal to 5 mg/kg bw/day, based on
depression in body-weight gain at 500 ppm. There was no evidence of
carcinogenicity.
A second long-term toxicity/carcinogenicity study in rats
performed at dietary concentrations of 0, 50, 150, 450 or 600 ppm
over two years revealed a NOAEL of 150 ppm, equal to 7 mg/kg bw/day,
based on the appearance of clinical signs at higher doses. There
was no evidence of carcinogenicity.
In a multigeneration reproduction study in rats, fenpropathrin
was administered at dietary levels of 0, 5, 25 or 250 ppm. The
NOAEL was 25 ppm, equal to 1.6 mg/kg bw/day, based on decreased pup
weights in the F3a generation at 250 ppm.
In a second multigeneration reproduction study conducted at
dose levels of 0, 40, 120 or 360 ppm, the NOAEL was 40 ppm, equal to
3 mg/kg bw/day, based on depression of body weight gain, increased
mortality in females and the occurrence of tremors in pups at 120
ppm and above.
Two oral teratogenicity studies in rats were performed at dose
levels of 0, 0.4, 2 or 10 mg/kg bw/day and 0, 0.4, 1.5, 2, 3, 6 or
10 mg/kg bw/day. The NOAELs were 2 and 3 mg/kg bw/day in the two
studies, respectively, with respect to maternotoxic effects and a
NOAEL of 10 mg/kg bw/day in both for embryotoxicity and
teratogenicity.
In an oral teratogenicity study in rabbits at dose levels of 0,
1.5, 3 or 6 mg/kg bw/day, the NOAEL was 6 mg/kg bw/day. In a second
study with oral doses of 0, 4, 12 or 36 mg/kg bw/day the NOAEL was 4
mg/kg bw/day with respect to maternotoxicity.
Fenpropathrin has been adequately tested in a series of in
vitro and in vivo genotoxicity assays. The Meeting concluded
that fenpropathrin was not genotoxic.
Based upon studies in hens and rats, fenpropathrin exhibited no
potential for delayed neurotoxicity.
Data on observations in humans were not suitable for the
estimation of an acceptable daily intake.
An ADI of 0-0.03 mg/kg bw was established based on a NOAEL of 3
mg/kg bw/day in the multigeneration reproduction study in rats, the
teratogenicity studies in rats and the one-year feeding study in
dogs, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Mouse: 600 ppm, equal to 56 mg/kg bw/day (two-year study)
Rat: 150 ppm, equal to 7 mg/kg bw/day (two-year study)
40 ppm, equal to 3 mg/kg bw/day (reproduction study)
3 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 4 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 100 ppm, equal to 3 mg/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Aitken, R. & Rushton, B. (1981) Histopathology report for compound
WL41706. 26, 53 and 104 week study in rats. Inveresk report project
415386. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0046.
Brooks, T.M., Dean, B.J. & Thorpe, E. (1976) Toxicity studies with
WL 41706 in the host mediated assay. Shell research report TLGR 0003
76. Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0007.
Bruce, E.D., Griffis, L.C. & Wong, Z.A. (1986) The acute vapor
inhalation toxicity of Danitol technical (SX-1713) in mice and rats.
Chevron Study CEHC 2545. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0170.
Colley, J., Gopinath, C. & Offer, J.M. (1987) S-3206: Two-year
feeding study in mice (Addendum to final report). Huntingdon report
SMO 149 861391 (Addendum to SMO 149 8640 (FT-51-0135)). Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-71-0180.
Colley, J., Heywood, R., Street, A.E., Gopinath, C., Offer, J.M.,
Gibson, W.A., & Almond, R.H. (1985) S-3206: Two year feeding study
in mice. Huntingdon report SMO 14984607. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-51-0135.
Colley, J., Welch, P.J., Heywood,R., Prentice, D.E. & Gibson, W.A.
(1981a) S-3206 Preliminary assessment of toxicity to mice by dietary
administration for four weeks. Huntingdon report SMO 121 81289.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0047.
Colley, J., Welch, P.J., Heywood, R., Prentice,D.E. & Gibson, W.A.
(1981b) S-3206 Second preliminary assessment of toxicity to mice by
dietary administration for four weeks. Huntingdon report SMO 139
81574. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-11-0049.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Isaacs, K.R., Gibson, W.A. & Almond, R.H. (1982a) S-3206 Toxicity to
rats by dietary administration for four weeks. Huntingdon report SMO
146 8210. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0064.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Gibson, W.A. &
Almond, R.H. (1982b) S-3206 Toxicity to rats by dietary
administration for four weeks. Huntingdon report SMO 158 82335.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0074.
Cox, R.H. (1986) Teratology study in rats S-3206. Addendum I to
final report. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-61-0176.
Cox, R.H. (1987) S-3206: Amendment II to final report teratology
study in rats. Hazleton report project 343 122 (Addendum to FT-01-
0031). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-71-0188.
Cozens, D.D., Barton, S.J., Offer, J.M., Gibson, W.A. & Anderson, A.
(1986) Effect of S-3206 on multiple generations of the rat.
Huntingdon report SMO 164 85707. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
61-0159.
Cozens, D.D., Hughes, E.W., Masters, R.E. & Anderson, A. (1985) The
effect of S-3206 on pregnancy of the New Zealand white rabbit.
Huntingdon report SMO 181 84667. Unpublished study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
51-0134.
Crawford, M.J. (1975) The metabolism of WL 41706 in mammals. The
fate of a single oral dose of (14C) WL 41706 in the rat. Shell
Research TLGR 0071 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FM-51-
0002.
Crawford, M.J. & Hutson, D.H. (1976) Metabolic fate of (14C) WL
41706 in rats. Shell Research TLGR. 0034 76. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FM-61-0001.
Crawford, M.J. & Hutson, D.H. (1977) The metabolism of the
pyrethroid insecticide (+)-alpha-cyano-3-phenoxybenzyl 2,2,3,3-
tetramethyl-cyclopropanecarboxylate, WL 41706, in the rat. Pestic.
Sci., 8: 579-599. Submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FM-71-0050.
Crayford, J.V. (1975) The excretion and residues of radioactivity in
cows fed (14C) WL 41706 in their diet. Shell Research TLGR 0096 75.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FR-51-0015.
Creedy, C.L. & Potter, D. (1976) The effect of feeding WL 41706 on
the microsomal mono-oxygenase system of rat liver. Shell Research
report TLGR 0043 76. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0009.
Dean, B.J. (1975) Toxicity studies with WL 41706 (S-3206).
Chromosome studies on bone marrow cells of Chinese Hamsters after
two daily oral doses of WL 41706 (S-3206). Shell Research report
TLGR 0104 75. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-51-0003.
Dean, G.A., Gopinath, C. & Fish, L.E. (1987) Potential tumorigenic
and toxic effects in prolonged dietary administration to rats
(Addendum to final report). Huntingdon report SMO 167 87185
(Addendum to SMO 167 851348). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0181.
Dix, K.M. (1975) Toxicity studies of WL 41706: Teratological studies
in rabbits given WL 41706 orally. Shell Research report TLGR 0103 75
(Addendum to FT-51-0006). Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-51-
0133.
Else, R. & Rushton, B. (1981) Histopathology report for compound WL
41706 3-generation study in rats. Inveresk report project 415370.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-21-0069.
Fish, C.J., Lewis, D.J. & Gopinath, C. (1986) Electron micrograph
Addendum to histopathology report No SMO/167 S-3206 potential
tumorigenic and toxic effects in prolonged dietary administration to
rats. Huntingdon report SMO 167 86602 (Addendum to SMO 167 851348
(FT-61-0161)). Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-61-0162.
Fleming, D.J. (1979) Toxicity studies on the insecticide WL 41706: A
three generation reproduction study (minus histopathology) in rats -
Corrigendum to FT-91-0027. Shell Research report TLGR 79 0071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-01-0028.
Fujita, Y. (1980) Interview survey concerning complaints connected
with use of S3206 in the field. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0040.
Hara, S. & Suzuki, T. (1980) Acute oral toxicity of S-3206 in
rabbits. Unpublished Sumitomo study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-00-0039.
Hara, M. & Suzuki, T. (1984a) Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0106.
Hara, M. & Suzuki, T. (1984b) In vitro sister chromatid exchanges
test of S-3206 in CHO-K1 cells. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-40-0108.
Hara, M. & Suzuki, T. (1984c) Addendum: Micronucleus test of S-3206.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-40-0114.
Hara, M., Yamada, F., Kogiso, S., Yoshitake, A., Matsuo, M. &
Miyamoto, J. (1984) Mutagenicity test of S-3206 in Saccharomyces
cerevisiae D7. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-40-
0121.
Hend, R.W. & Butterworth, S.T.G (1975) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0031 75. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-71-
0001.
Hend, R.W. & Butterworth, S.T.G (1976a) Toxicity studies on the
insecticide WL 41706: A three month feeding study in rats. Shell
Research report TLGR 0020 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0013.
Hend, R.W. & Butterworth, S.T.G. (1976b) Toxicity studies on the
insecticide WL 41706: A short-term feeding study in rats. Shell
Research report TLGR 0041 76. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-61-
0011.
Hend, R.W. & Gellatly, J.B.M. (1979) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival,
bodyweight, food intake, organ weights, clinical chemistry,
hematology and gross pathological observations of rats exposed to WL
41706 for up to two years. Shell Research report TLGR 79 062.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0026.
Hend, R.W. & Gellatly, J.B.M. (1980) Toxicity studies on the
insecticide WL 41706: Results of physical appearance, survival, body
weight, food intake, organ weights, clinical chemistry, hematology
and gross pathological observations of rats exposed to WL 41706 for
up to two years, Results of studies after 18 months. Shell Research
report TLGR 79 062 (Addendum to FT-91-0026). Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0032.
Hend, R.W., Gellatly, J.B.M. & Fleming, D.J. (1979) Toxicity studies
on the insecticide WL 41706: A three generation reproduction study
(minus histopathology) in rats. Shell Research report TLGR 79 071.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-91-0027.
Heywood, R. (1982) An appraisal of the toxicological findings on
rats receiving a synthetic pyrethroid S-3206 (fenpropathrin) by
dietary administration for 4 weeks. Huntingdon report (unnumbered,
1982). Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-21-0075.
Hiromori, T. Hosokawa, S. & Miyamoto, J. 1982a: Fenpropathrin
toxicology overview - Acute toxicity and safety in use. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-20-0079.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1982b) Acute oral toxicity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-20-0076.
Hiromori, T., Misaki, Y., Ito, S., Hosokawa, S. & Miyamoto, J.
(1983a) Acute oral toxicity of S-3206 (97.3%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0082.
Hiromori, T., Misaki, Y., Seki, T., Hosokawa, S. & Miyamoto, J.
(1983b) Acute oral toxicity of S-3206 (91.8%) in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-30-0081.
Hiromori, T., Nakanishi, T., Kawaguchi, S., Sako, H., Suzuki, T. &
Miyamoto, J. (1986a) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci. 11: 9-14.
Hiromori, T., Nakanishi, T., Suzuki, T., Kato, T. & Miyamoto, J.
(1986b) The mean slip angle test of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-60-0153.
Hiromori, T. & Takemura, T. (1983) Short report on facial
paresthesia following exposure to fenpropathrin, cypermethrin,
fenvalerate or permethrin in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0087.
Izumozaki, H., Hara, M. & Suzuki, T. (1984) Gene mutation test of S-
3206 in bacterial system. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-40-0107.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism
of fenvalerate and the (2S, alphaS)-isomer in rats and mice. J.
Pestic. Sci. 6: 317-326.
Kaneko, H., Shiba, K., Yoshitake, A. & Miyamoto, J. (1987)
Metabolism of fenpropathrin (S-3206) in rats. J. Pestic. Sci. 12:
385-395.
Kishida, F., Suzuki, H., Miyamoto, J. (1980) Studies on DNA damaging
capacity of S-3206 with Bacillus subtilis. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-00-0038.
Kohda, H. (1975) Acute oral toxicity of S-3206 in rats. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-50-0018.
Kohda, H. (1976) Acute dermal toxicity of S-3206 in rats.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0019.
Kohda, H. & Kadota, T. (1975) Acute oral toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-50-
0035.
Kohda, H. & Kadota, T. (1976a) Acute dermal toxicity of S-3206
technical in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0036.
Kohda, H. & Kadota, T. (1976b) Acute subcutaneous and
intraperitoneal toxicity of S-3206 technical in rats and mice.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-60-0037.
Kohda, H., Kadota, T. & Miyamoto, J. (1976) Acute inhalation
toxicity of S-3206 and S-5602 in mice an rats. Unpublished Sumitomo
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number AT-50-0043.
Ku, H.S. & Doran, T.J. (1990a) A study to determine the nature of
the residue in milk, meat and tissue from lactating goats dosed with
14C fenpropathrin. Ricerca Inc report project 89 0109. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0041.
Ku, H.S. & Doran, T.J. (1990b) A study to determine the nature of
the residue in poultry end eggs from chickens dosed with 14C
fenpropathrin. Ricerca Inc., report project 89 0084. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FM-01-0042.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
Marroquin, F., Richter, W.R., Myer, J.R. & Johnson, D.E. (1981) S-
3206 technical grade: Acute dermal toxicity (LD50) study in rabbits
(TSCA 7/79) (EPA 8/78) (OSHA). IRDC report project 491 002.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-11-0051.
Matsubara, T., Hara, S. & Kadota, T. (1978) Primary eye and skin
irritation tests of S-3206 in rabbits. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-80-0023.
Matsumoto,S., Suzuki, T., Kato, T. & Yamada, H. (no date) Comments
on the facial sensation-inducing potential of fenpropathrin.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number FT-80-0195.
McSheehy, T. & Nunziata, A. (1984) Chromosome aberrations in Chinese
hamster ovary (CHO) cells in vitro. Test substance: S-3206 Final
report 113-005. Life Science Research Roma report project 113 005.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-41-0104.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 41706
to adult hens. Shell Research report TLGR 0068 77. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FW-81-0006.
Misaki, Y., Hiromori, T., Hosokawa, S. & Miyamoto, J. (1983)
Comments on acute oral toxicity of fenpropathrin technicals with
high purity and lower purity in rats. Unpublished Sumitomo study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-30-0083.
Misaki, Y. & Kohda, H. (1981a) Acute oral toxicity of two impurities
of S-3206 (technical) in mice. Unpublished Sumitomo study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-00-0044.
Misaki, Y. & Kohda, H. (1981b) Acute oral toxicity of 2,2,3,3-
tetramethylcyclopropane carboxylic anhydride in mice. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-90-0045.
Morseth, S.L. (1990) Rat teratology study with S-3206. Hazleton
report project 343 216. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-01-
0216.
Ohkawa, H., Kaneko, H., Tsuji, H. & Miyamoto, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
Okuno, Y. (1981) Comment on possible weight changes of kidney and no
effect levels in a 2-year chronic toxicity study on WL 41706
(fenpropathrin). Comments 0n FT-91-0026 and FT-11-9946. Unpublished
Sumitomo study submitted to WHO by Sumitomo Chemical Company Limited
under the reference number FT-10-0048.
Okuno, Y., Kadota, T. & Miyamoto, J. (1975) Skin sensitization study
of S-3206 in guinea-pigs. Unpublished Sumitomo study submitted to
WHO by Sumitomo Chemical Company Limited under the reference number
FT-50-0024.
Omodaka, H., Misaki, Y. & Okuno, Y. (1986a) Acute oral toxicity of
S-3206 in rats. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0138.
Omodaka, H., Suzuki, T. & Kato, T. (1986b) Acute dermal toxicity
study of S-3206 in rats. Unpublished Sumitomo study submitted to WHO
by Sumitomo Chemical Company Limited under the reference number FT-
60-0139.
Pauw van der, C.L., Dix, K.M., Blanchard, P.M., McCarthy, W.V. &
Stevenson, D.E. 1975: Teratological studies in rabbits given WL
41706 orally. Shell Research report TLGR 0103 75. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-51-0006.
Pence, D.H., Alsaker, R.D., Hepner, K.E., Dawkins, B.G., Vargus,
K.J., Hagen, W.H. & Hitzelberg, D.A. (1984) Chronic study in dogs S-
3206 T.G. Final report. Hazleton report project 343 153. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-41-0122.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980a) Subchronic toxicity study in dogs S-3206.
Final report. Hazleton report project 343 125. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-01-0034.
Pence, D.H., Dawkins, B.G., Alsaker, R.D., Weatherholtz, W.M. &
Marshall, P.M. (1980b) Subchronic toxicity study in dogs S-3206.
Addendum to final report. Hazleton report project 343 125. Addendum
to FT-01-0034. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-01-0041.
Pence, D.H., Pence, L.A., Durloo, R. and Cameron, J. (1980c)
Teratology study in rats S-3206. Final report. Hazleton report
project 343 122. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-010031.
Pence, D.H., Weatherholtz, W.M. & Cameron, J.T. (1978) Oral dose
range finding study in dogs, S-3206. Final report. Hazleton report
project 343 123. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-91-0025.
Richold, M., Allen, J.A. & Proudlock, R.J. (1982a) Autoradiographic
assessment of DNA repair in mammalian cells after exposure to S-3206
(fenpropathrin). Huntingdon report SMO 143 81881. Unpublished study
submitted to WHO by Sumitomo Chemical Company Limited under the
reference number FT-21-0068.
Richold, M., Grantham, C.J. & Basham, K.J. (1982b) An assessment of
the mutagenic potential of S-3206 using an in vitro mammalian cell
test system (with comment by J. Miyamoto). Huntingdon report SMO 144
8252. Unpublished study submitted to WHO by Sumitomo Chemical
Company Limited under the reference numberFT-21-0060.
Riley, J.H., Nair, K.P.C., Kopplin, J.R., Johnson, D.E., Ott, S.S.,
Meyer, J.R. & Spicer, E.J.F. (1982) S-3206 (technical grade) 21-day
dermal study in rabbits (EPA) (technical product). IRDC report
project 491 010. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-21-0058.
Ruzo, L.O., Unai, T. & Casida, J.E. 1978: Decamethrin metabolism in
rats. J. Agric. Food Chem. 26: 918-925.
Shell Chemical (UK) Limited (1978) A survey of operator experience
with fenpropathrin 10% EC - A pyrethroid insecticide/acaricide for
use in fruit and ornamental crops in 1986. Shell UK report 16th
January 1987. Unpublished study submitted to WHO by Sumitomo
Chemical Company Limited under the reference number FT-71-0179.
Summitt, L.M. & Albert, J.R. (1976) Intravenous toxicity of SD 41706
(1-24-0-0) in the mouse. Shell Development Co report TIR 74 110 76.
Unpublished study submitted to WHO by Sumitomo Chemical Company
Limited under the reference number FT-61-0010.
Suzuki, H. (1977) Studies on mutagenicity of some pyrethroids on
Salmonella strains in the presence of mouse hepathic S9 fractions.
Unpublished Sumitomo study submitted to WHO by Sumitomo Chemical
Company Limited under the reference number AT-70-0157.
Suzuki, T. & Miyamoto, J. (1981) Skin sensitization test of S-3206
in guinea-pigs. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-10-
0078.
Suzuki, T., Sako, H. & Okuno, Y. (1986) Acute oral toxicity study of
S-3206 in mice. Unpublished Sumitomo study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-60-
0175.
Warren, S., Heywood, R., Street, A.E., Gopinath, C., Browne, J.G.,
Gibson, W.A., Reed, L.E. & Anderson, A. (1986) S-3206: Potential
tumorigenic and toxic effects in prolonged dietary administration to
rats (Final report. Huntingdon report SMO 167 851348. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-61-0161.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yoshida, A., Kosaka, T., Miyaoka, T., Maita, K. & Goto, S. (1986) S-
3206: 13-week oral subchronic toxicity study in rats. Inst. Environ.
Toxicol. Tokyo report 17th, July 1986. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-61-0166.
Yoshitake, A., Kogiso, S. & Hara, M. (1990) In vitro chromosomal
aberration test of S-3206 in Chinese Hamster ovary cells (CHO-K1).
Sumitomo study M83-54. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-00-
0215.
Yoshitake, A., Kogiso, S., Kato, H. & Hara, M. (1988) In vitro
gene mutation test of S-3206 in V79 Chinese Hamster cells in
culture. Sumitomo study 1174. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-80-
0209.
Yoshitake, A. Kogiso, S. & Yamada, F. (1986) Bacterial DNA repair
test of S-3206. Sumitomo study MUT86020. Unpublished study submitted
to WHO by Sumitomo Chemical Company Limited under the reference
number FT-60-0165.
Yoshitake, A., Kogiso, S & Yamada, F. (1987) Reverse mutation test
of S-3206 in bacterial systems. Sumitomo study 704. Unpublished
study submitted to WHO by Sumitomo Chemical Company Limited under
the reference number FT-70-0182.
Yoshitake, A., Kogiso, S., Yamamoto, K. & Hara, M. (1989) In vitro
chromosomal aberration test of S-3206 in Chinese Hamster ovary cells
(CHO-K1). Sumitomo study 1730. Unpublished study submitted to WHO by
Sumitomo Chemical Company Limited under the reference number FT-90-
0200.
