
METIRAM
First draft prepared by
M. Caris
Health Canada, Ottawa, Canada
EXPLANATION
Metiram is a non-systemic EBDC fungicide that contains zineb
and poly (ethylenethiuram disulfide). It was considered for the
first time at the present Joint Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Rats
Male and female Sprague-Dawley rats were administered a single
oral dose of 14C-labelled metiram at a dose of 5 or 50 mg/kg bw or
were pretreated with unlabelled test material, 5 mg/kg bw/day for 14
days followed by a single oral dose of 14C metiram of 5 mg/kg bw.
Over 98% of the administered 14C metiram dose was excreted within 7
days, with the majority of the dose excreted within the first 48
hours post-dosing. Mean recovered radioactivity in the faeces
accounted for 53.9-78.7% of the dose with a smaller percentage
excreted in the urine, 21.3-46.6%. Slightly lower proportions of
radioactivity were excreted in the urine at the high dose when
compared to the low dose, suggesting that absorption of metiram was
less at higher doses. Mean radioactivity excreted in the expired
air represented 0.4-1.1% of the dose. The patterns of excretion of
radioactivity were similar upon single or repeated exposure to
metiram. Comparatively, absorption was greater and excretion more
rapid in rats treated with 0.5 mg of 14C-ETU/kg bw, where 96% of
the dose was excreted in the urine and only 4.2% in the faeces
within 3 days post-dosing. Biliary excretion of 14C-labelled
metiram accounted for 14.3% and 7.1% of a single 5 mg/kg bw dose in
males and females, and 4.3% and 3.7% of a single 50 mg/kg bw dose in
either sex, respectively. Peak mean plasma concentrations of
radioactivity occurred after 4 hours in the 5 mg/kg bw dose and
after 6 hours following administration of a single oral dose of 50
mg/kg bw, respectively. After 7 daily oral doses of 14C metiram at
5 mg/kg bw/day, the highest concentrations of radioactivity were
found in the thyroid and kidneys with concentrations being slightly
higher in females than in males. Whole-body autoradiography of
these rats confirmed the results of the quantitative tissue
distributions. The comparisons between rats sacrificed at 24 hours
after a single dose or after multiple doses showed evidence of
accumulation of radioactivity in the body with multiple dosing
(Hawkins et al., 1985).
Absorption was studied in male CD rats treated dermally on
their shaved backs with 14C metiram at dose levels of 0.24 or 240
mg/kg bw equal to 0.004 or 4 mg/cm2, respectively. Groups of 4
animals were killed after 1, 2, 4 and 8 hours following application,
and two groups of 4 animals had the treated skin sites washed after
8 hours with subsequent sacrifice of the animals, 24 or 96 hours
post-treatment. Total radioactivity detected from urinary and
faecal excretion and tissue residue levels accounted for 0.9% and
0.2% of the radioactivity at the low and high dose, respectively.
The majority of the administered dose was recovered from the treated
skin of the rats. Concentrations of radioactivity in plasma and
tissues were generally below or at the limits of detection (Hawkins
et al., 1984).
Biotransformation
Rats
Thin layer chromatography (TLC) of the urine of rats treated
with 14C-metiram as single oral doses of 5 or 50 mg/kg bw, and a
single oral dose of 100 mg/kg bw of a 15N-metiram and 14C-metiram
suspension (1:1 ratio), revealed eight principal radioactive
components. Incubation with ß-glucuronidase/sulphatase did not
significantly alter the proportion of radioactive components. Polar
components accounted for the majority of the radioactivity (34-58%)
and were identified as ethylenediamine, N-acetyl-ethylenediamine and
possibly ethanolamine and oxalic acid. Another polar component was
an acidic compound with similar chromatographic properties to
glycine. Major less polar components were identified as
ethyleneurea (4-11%), ethylenethiourea (10-35%) and ethylene
bis(isothiocyanate) sulphide (1-3%). The proportion of radioactive
components in the bile of rats treated with a single oral dose of
14C metiram at 10 mg/kg bw as separated by TLC were similar to
those obtained in the urine. Metabolite patterns were determined
for kidney and liver extracts from rats treated with 14C metiram at
5 mg/kg bw for 7 days. Radioactive components in the kidney were
similar to those found in urine; however, of the liver extracts,
only the ß-glucuronidase/sulphatase treated sample provided a
satisfactory pattern which contained mainly polar material and only
traces of ethyleneurea and ethylenethiourea (Hawkins et al.,
1985).
The proposed metabolic pathway of metiram in animals is
depicted in Figure 1.
Toxicological studies
Acute toxicity studies
Acute toxicity studies with technical metiram have been
performed in the rat and mouse, the results of which are summarized
in Table 1. WHO has classified metiram as unlikely to present acute
hazard in normal use (WHO, 1992). Acute studies with the formulated
trade product, Polyram combi (consisting of 80% metiram complex
(technical active ingredient) and 20% formulating agents) were
similarly conducted in several animal species, the results of which
are given in Table 2. Both technical and formulated metiram, upon
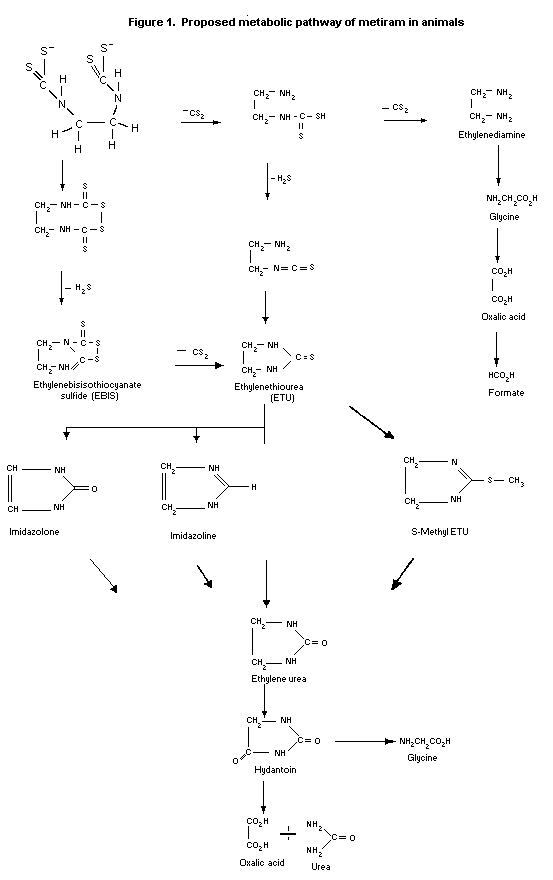 Table 1. Acute toxicity of technical metiram
Species Sex Route (vehicle) LD50 Test material Reference
(strain) (mg/kg bw)
Rat M+F oral > 6810 Technical grade Jackh, 1981
(SD) (distilled water) (purity unknown)
Rat M+F oral M: 9100 Technical grade Leuschner, 1979a
(SD) (HPMC) F: 8900 (purity unknown) + 2% ETU
M+F: 9000
Rat M+F oral 6500 Technical grade Hofmann, 1985a
(SD) (tragacanth) (purity unknown)
Rat M+F oral 10 000 Technical grade Hofmann, 1975
(SD) (CMC) (purity unknown)
Rat (SD) M+F dermal (water) > 2000 Technical grade Grundler, 1979
(77.5% purity) + 2% ETU
Mouse (NMRI) M+F intraperitoneal M: 208 Technical grade Leuschner, 1979b
(HPMC) F: 215 (purity unknown) + 2% ETU
M+F: 212
Mouse (NMRI) M+F intraperitoneal 80 Technical grade Hofmann, 1974a
(tragacanth) (purity unknown)
Rat (SD) M+F intraperitoneal M: 318 Technical grade Hofmann & Munk, 1975
(CMC) F: 317 (77.5% purity)
M+F: 318
Rat (SD) M+F inhalation 4 h > 5.7 (mg/l) Technical grade Klimisch & Zeller, 1980
(77.5% purity) + 2% ETU
Rat (SD) M+F inhalation 8 h (well Technical grade Hofmann, 1985b
tolerated) (purity unknown) + 2.2% ETU
Table 2. Acute toxicity of Polyram combi (formulated product)
Species Sex Route LD50 Test Reference
(strain) (vehicle) (mg/kg bw) material
Rat M+F oral > 10 000 Polyram combi Hofmann, 1985c
(SD) (tragacanth) (purity unknown)
Dog M+F oral * Polyram combi Chesterman et al., 1973
(beagle) (water) (purity unknown)
Mouse (NMRI) M+F intraperitoneal approx 80 Polyram combi Hofmann, 1974b
(tragacanth) (purity unknown)
Rat M+F dermal > 2000 Polyram combi Grundler, 1985
(SD) (77.5% purity)
Rat M+F inhalation, (well Polyram combi Hofmann, 1985d
(SD) 8 hr tolerated) (77.5% purity)
Guinea-pig inhalation, >0.8 (mg/L) Polyram combi Hofmann, 1985e
8 hr (purity unknown)
* Estimation of LD50 precluded due to high incidence of vomiting
oral and dermal administration, were practically non-toxic in the
rat with LD50 values of > 6500 mg/kg bw and > 2000 mg/kg bw,
respectively. Moderate acute toxicity was demonstrated upon
intraperitoneal administration of technical material in the mouse
(LD50 = 80-212 mg/kg bw) and rat (LD50 = 318 mg/kg bw). Technical
metiram when administered by the inhalation route resulted in an
LC50 value greater than 5.7 mg/l in the rat. Eight-hour inhalation
of the formulated material, Polyram combi was well tolerated in both
rats and guinea-pigs. Intentional spiking of the technical material
with ETU at levels of 2 to 2.2% did not markedly alter the acute
toxicity profile.
Short-term toxicity studies
Mice
In a preliminary assessment of the toxicity of metiram (96.8%
purity, containing 2.2% ETU), groups of 8 CFLP mice per sex were fed
dietary concentrations of 0, 100, 300, 1000 or 3000 ppm equal to 0,
16, 40, 120 or 399 mg/kg bw/day for males and 0, 15, 44, 142 or 433
mg/kg bw/day for females, respectively, for a period of 4 weeks.
Body-weight gain of males treated with metiram at 1000 and 3000 ppm
were significantly lower than that of the corresponding controls
during the first week of treatment with subsequent recovery of
weight gain in the ensuing weeks. Although no consistent effects on
body weight in treated females were observed on a weekly basis,
overall weight gain of females at the high dose of 3000 ppm was
marginally lower than that of the control animals. Significantly
increased liver weights were recorded in both sexes treated at
levels of 1000 and 3000 ppm. In the absence of routine
histopathological examination, no microscopic correlation could be
drawn from the increased liver weights. There were no effects of
treatment on survival, clinical signs, food consumption or gross
pathological changes. On the basis of the reported findings, a high
dietary level of 1000 ppm was selected for the long-term study in
mice (Hunter et al., 1976b).
A 3-month study was performed with groups of 10 B6C3F1
mice/sex treated with metiram (94.8% purity, ETU <0.2%) at
dietary levels of 0, 300, 1000, 3000 or 7500 ppm, equal to 0, 84,
302, 853 or 2367 mg/kg bw/day in males and 0, 133, 465, 1448 or 3565
mg/kg bw/day in females, respectively. Treatment-related effects
were observed with respect to serum T3 and T4 concentrations.
Serum T4 levels were significantly decreased in both sexes of mice
treated with metiram at dietary levels of 1000 ppm and higher,
whereas T3 levels were significantly increased in male mice treated
at the highest dietary level of 7500 ppm. Histopathological
findings attributable to treatment were denoted in the thyroid in
both sexes treated at dietary levels of 3000 ppm and higher as
minimal to slight hypertrophy and vacuolation of the follicular
epithelium. A slight increase in the severity of fatty degeneration
of the `X zone' in the adrenals was also observed in female mice
treated with metiram at 3000 and 7500 ppm. A dose-related increased
incidence in the presence of a refractile, granular material
presumed to be the test material or a metabolite, was noted in the
superficial layer of the urinary bladder urothelium in all groups of
treated mice. Since no morphological changes were observed in the
urothelium of the bladder to suggest disturbed epithelial function,
the presence of this granular material was considered to be of
doubtful toxicological consequence. Significantly increased organ
weights were recorded for the adrenals in females at dietary levels
of 3000 ppm and higher and for the liver in both sexes treated at
7500 ppm and in males at 3000 ppm. Statistically significant
increases in kidney and testicular weights were judged to be of no
biological consequence. No histopathological correlation could be
determined for the increased liver, kidney or testicular weights.
Group mean body weights and corresponding weight gains were
decreased in males treated at the highest dietary level of 7500 ppm.
Although significant decreases in body weights were recorded in
females treated at dietary levels of 1000 ppm and higher, there was
no dose-response relationship. There were no adverse treatment-
related effects on survival, food consumption or on blood
haematological parameters. The NOAEL for this study was 300 ppm,
equal to 84 mg/kg bw/day, based on decreased serum T4 levels in
both sexes at levels of 1000 ppm and above (Gelbke et al., 1992a).
Rats
Metiram (96.8% purity containing 2.2% ETU) was administered for
a period of 4 weeks to groups of 20 Sprague-Dawley CD rats/sex at
dietary levels of 0, 100, 300, 1000 or 3000 ppm, equal to 0, 10, 30,
100 or 296 mg/kg bw/day in males and 0, 11, 33, 114 or 292 mg/kg
bw/day in females, respectively. Following termination of treatment
with metiram, 10 rats per sex per group were retained for subsequent
2 and 4 week recovery periods with scheduled sacrifices of 5
rats/sex/group on each occasion. Clinical toxicity was manifest as
signs of hind limb paralysis in rats treated at the highest dietary
level of 3000 ppm, which due to severity, resulted in early
sacrifice of 3 females for humane reasons. Histopathological
examination confirmed gross morphological alterations in the muscles
of the hind limbs, characterized by atrophy of the fibres, often
with associated proliferation of sacrolemmal nuclei, and the
presence of groups of vacuoles within muscle fibres. After 4 weeks
of treatment, the microscopic changes were more marked and extensive
in females, occurring in all females and in 2 of 9 males treated at
3000 ppm. After a withdrawal period of 2 weeks, a varying degree of
atrophy of muscle fibres was still present in females previously
treated with 3000 ppm of metiram. Although atrophy was not evident
in animals after a 4-week recovery period, additional changes, not
apparent earlier, were the presence of vacuoles and/or areas of fat
cells within the skeletal fibres. Target organ effects of treatment
with metiram were also evident on the thyroid and kidney. Changes
in the thyroid were apparent as a dose-related increase in the
incidence and severity of thyroid hyperplasia in both sexes treated
at dietary levels of 300 ppm and higher. Microscopic examination of
the kidney revealed varying degrees of hydropic vacuolation of the
proximal tubular epithelial cells in 2 females from each of the 1000
and 3000 ppm groups in the absence of similar effects at any of the
other dose levels or corresponding controls. Examination of bone
marrow smears revealed a depressed number of myeloid cells in males
treated at the high dose after 4 weeks of treatment. Group mean
body-weight gains were significantly lower in males treated at 1000
ppm and higher, and in females treated at 300 ppm and higher when
compared to the controls. Decreased food intake was recorded in
both sexes treated at the highest dietary level of 3000 ppm. There
were no adverse effects of treatment noted upon ocular examination,
haematology, blood chemistry, urinalysis and estrus cycle as
assessed from vaginal smears. The NOAEL was 100 ppm, equal to 10
mg/kg bw/day (Hunter et al., 1976a).
Groups of 35 Sprague-Dawley CFY strain rats per sex were
treated with metiram (96.8% purity containing 2.2% ETU) at dietary
levels of 0, 50, 100, 300 or 900 ppm, equal to 0, 3, 6, 20 or
61 mg/kg bw/day for males and 0, 4, 8, 24 or 76 mg/kg bw/day for
females, respectively, for a period of 13 weeks followed by a 6-week
recovery period. The principal target organs were the thyroid and
skeletal muscle. Treatment-related effects on the thyroid were
manifest as increased thyroid weights of females treated at 300 and
900 ppm, decreased percentage uptake of 131I by the thyroid in males
in all treated groups and in females treated at 100 ppm and higher,
and decreased T4 levels in both sexes at 300 ppm and higher.
Microscopically, an increased incidence of males treated at 900 ppm
had slight to minimal hyperplasia of the thyroid. A minimally
higher incidence at 13 weeks of slight thyroid hyperplasia in
females at 900 ppm and in males at 100 and 300 ppm when compared to
the controls was considered to be of equivocal significance. There
were no significant changes in the thyroid observed after the 6-week
recovery period. Treatment-related morphological changes of the
skeletal muscle denoted by atrophy of the muscle fibres often
accompanied by fat cells and associated proliferation of sacrolemmal
nuclei, were evident in both sexes treated at dietary levels of 300
ppm and higher. Varying degrees of muscular atrophy were still
prevalent after a 6-week recovery period in rats previously treated
at 300 and 900 ppm. Other effects of treatment were exhibited at
the high-dose level of 900 ppm as clinical signs of hind limb
paralysis, decreased body weights and slight reduction in food
intake. There were no treatment-related effects observed with
respect to survival, water consumption, ophthalmoscopy, haematology,
urinalysis or examination of bone marrow smears. Although reduced
iodine uptake by the thyroid was observed at all dietary levels,
these changes were shown to be reversible following cessation of
treatment. At the lowest dietary levels of 50 and 100 ppm, the
effects on iodine uptake were not correlated with changes in thyroid
hormone levels or any overt morphological alterations of the thyroid
gland, thus rendering the toxicological significance of this finding
at 50 and 100 ppm as doubtful. The NOAEL for this study was
therefore 100 ppm, equal to 6 mg/kg bw/day, based on decreased serum
T4 levels and increased thyroid weights at dietary levels of 300
and 900 ppm (Hunter et al., 1977).
Metiram (94.8% purity, ETU <0.2%) was administered for a
period of 3 months to groups of 13 Wistar Chbb:THOM SPF rats per sex
at dietary levels of 0, 5, 80, 320 or 960 ppm, equal to 0, 0.4, 5.8,
23.5 or 73.9 mg/kg bw/day in males and 0, 0.4, 6.7, 27.3 or 88.8
mg/kg bw/day in females, respectively. Neurofunctional assessment
revealed general muscle weakness expressed as ataxia and
significantly reduced forelimb and hindlimb grip strength in females
treated at the highest dietary level of 960 ppm. Perfusion fixation
of tissues from selected rats from the control and treated groups
failed to reveal any associated neuropathological or morphological
alterations of the central or peripheral nervous systems. Thyroid
hormone levels revealed significantly decreased T4 levels in both
sexes treated at the high dose of 960 ppm. Significantly reduced
RBC values were recorded in both sexes at levels of 320 and 960 ppm.
In females, decreases in RBC values were accompanied by decreases in
mean haemoglobin and haematocrit levels. Several statistically
significant changes in blood biochemical parameters of uncertain
toxicological significance were observed at the highest dietary
level of 960 ppm. These changes were recorded as decreased ALAT and
ALP activity (females), decreased creatinine (both sexes), decreased
urea (males), and decreases in the electrolytes; potassium (both
sexes), sodium (females), calcium (both sexes) and magnesium (both
sexes). Decreased phosphorus levels were recorded in males at
dietary levels of 320 ppm and higher. Other effects of treatment
expressed in both sexes at the highest dietary level of 960 ppm were
decreased body weights and corresponding weight gains, as well as
significantly increased thyroid, liver, kidney and testes (males
only) weights. There were no treatment-related gross or
histopathological changes observed in any of the animals from the
treated groups. Treatment with metiram did not result in any
significant effects on survival, food consumption, ophthalmoscopy or
urinalysis. The NOAEL was 80 ppm, equal to 5.8 mg/kg bw/day, based
on changes in haematological and blood chemical parameters at 320
ppm (Gelbke et al., 1992b).
A 13-week inhalation study was conducted with groups of 28
Sprague-Dawley CD rats/sex exposed in nose-only chambers to metiram
(94% purity) 6 hours/day, 5 days/week at concentrations of 0, 2, 20
or 100 mg/m3. Concentrations of ETU in the exposure atmospheres
were calculated to be 0, 0.02, 0.33 or 1.8 mg/m3, respectively.
After termination of treatment, one-half of the rats were sacrificed
while the remaining animals were retained for a 13-week recovery
period. The NOAEL was 2 mg/m3. Pulmonary changes consisting of
"subacute alveolitis", characterized by accumulations of alveolar
macrophages within alveolar lumens, accompanied by some polymorpho-
nuclear leukocytes were observed in rats treated at 20 and 100
mg/m3. It was conjectured that the alveolitis observed was not
treatment-related, but rather, a non-specific dust reaction of the
polymeric active ingredient artificially ground to particle size
capable of reaching the alveoli. Intra-alveolar pigment deposition
was recorded in a single male and female from the high-dose treated
group. There was no evidence of damage to bronchial or bronchiolar
epithelium in any of the treated groups. Mean lung/trachea weights
were increased in both sexes treated at the high dose. Other
changes associated with treatment were depositions of golden brown
granular pigment, similar to that seen in the lungs, within the
renal cortices in the high dose animals and decreased overall body-
weight gain in both males and females treated at 20 (11% and 14%)
and 100 mg/m3 (26% and 33% less than corresponding controls,
respectively). After the 13-week recovery, effects of treatment
persisted in the lungs and kidneys of previously treated animals.
No treatment-related findings were noted on survival,
ophthalmoscopy, haematology or blood chemistry. Tissue residue
analysis performed on the urine, plasma, liver, lung and thyroid
revealed measurable levels of metiram and ETU in the urine and lung
in rats treated at the mid- and high-dose levels (Ulrich, 1986).
Rabbits
A dermal toxicity study was performed with groups of 5 New
Zeeland white KFM rabbits/sex treated with Polyram DF formulation of
metiram on the shaved skin of their backs under occlusive conditions
for 6 hours/day at concentrations of 0 (vehicle control, distilled
water), 25, 50 or 250 mg/kg bw/day for a minimum of 21 consecutive
daily applications. Dermal administration of metiram resulted in
minimal to moderate exfoliation and ulcerative dermatitis in the
skin of rabbits treated at the high-dose level. Similar effects
were not evident at lower dose levels, indicating a NOAEL for local
irritation to the skin of 50 mg/kg bw/day. In the absence of
treatment-related effects on survival, clinical signs, body weights,
food consumption, ophthalmoscopy, haematology, blood chemistry,
organ weights or histopathology of the liver and kidneys, the NOAEL
for systemic toxicity was greater than 250 mg/kg bw/day (Ullmann et
al., 1987).
Dogs
Groups of 4 beagle dogs/sex were treated with metiram (96.8%
purity containing 2.2% ETU) for a period of 4 weeks at dietary
levels of 0, 100, 300, 600 or 900 ppm, equal to 0, 5, 14, 28 or 41
mg/kg bw/day for males and 0, 5, 15, 27 or 43 mg/kg bw/day for
females, respectively. Effects observed were an increased frequency
of micro-follicles in the thyroids associated with minimal depletion
of colloid and minimal hyperplasia of the follicular epithelium in
2/4 males and 2/4 females treated at the high dose of 900 ppm when
compared to the controls. Other changes recorded were significantly
increased liver weights in dogs receiving 600 and 900 ppm, which
were not associated with any correlative histopathological findings.
Significantly increased water consumption was recorded in dogs
treated at 100, 600 and 900 ppm but not at 300 ppm. The significance
of increased fluid intake (calculated over 5-day periods) was
difficult to ascertain since there were no corresponding changes
noted in urinalysis parameters. Erythrocyte, haemoglobin and packed
cell volume values, although significantly decreased in dogs treated
at 900 ppm when compared to the controls, were found to be within
normal limits of variability and therefore of doubtful toxicological
consequence. The NOAEL, based on the aforementioned findings, was
300 ppm, equal to 14 mg/kg bw/day. There were no significant
effects of treatment on survival, clinical signs, body weights, food
consumption, ophthalmoscopy, blood chemistry or urinalysis
(Chesterman et al., 1978).
Metiram (93.6% purity, ETU < 0.2%) was given to groups of 5
beagle dogs per sex at dietary concentrations of 0, 30, 80, 1000 or
3000 ppm, equal to 0, 0.9, 2.5, 29.8 or 76.9 mg/kg bw/day in males
and 0, 1.1, 2.7, 29.9 or 92.7 mg/kg bw/day in females, respectively,
for a period of 52 weeks. The principal effects of treatment were
manifest on the thyroid. Microscopic examination revealed thyroid
follicular hyperplasia in dogs of both sexes treated at 1000 and
3000 ppm. This finding was associated with an increased size and
weight of this organ at 3000 ppm. Gross necropsy revealed thyroid
thickening in several animals from both the control and treated
groups. This observation was, however, only correlated with
histopathological change at dietary levels of 1000 ppm and higher,
rendering the significance of thyroid thickening at lower levels
uncertain. Serum T4 levels were decreased in both sexes at 3000 ppm
and in males at 1000 ppm. A dose-related increased incidence of dogs
with focal hepatic lipofuscin pigment deposition was noted in both
sexes at 1000 ppm and higher. Other effects of treatment with
metiram were described as an increased incidence of diarrhoea in
dogs treated at 80 (attributed to single male), 1000 and 3000 ppm;
slight evidence of anaemia (both sexes at 1000 and 3000 ppm)
involving an increased number of reticulocytes (both sexes at 3000
ppm); decreased body-weight gain at 3000 ppm in males and to a
lesser extent in females; and decreased food intake in both sexes at
3000 ppm and in females at 1000 ppm. Significant changes in blood
chemical parameters at dietary levels of 1000 ppm and higher were
evident as increased lipid, cholesterol, triglycerides, phospholipid
levels, ALP activity, and total protein, and decreased albumin and
A/G ratio as well as disturbances in the protein electrophoretic
pattern. There were no effects of treatment on survival,
neurological parameters, ophthalmoscopy or urinalysis. The NOAEL
for this study was 80 ppm, equal to 2.5 mg/kg bw/day (Corney et
al., 1991).
Monkeys
A preliminary toxicity assessment was performed with groups of
single male and female rhesus monkeys administered metiram (96.8%
purity containing 2.2% ETU) orally by gavage as a suspension in 0.5%
mucilage of tragacanth in water at dose levels of 50, 100 or 500
mg/kg bw/day for a period of 4 weeks. Treatment-related toxicity
was evident as salivation at the time of dosing or immediately
thereafter and occasional emesis in monkeys treated at 100 and 500
mg/kg bw/day, body-weight loss in the single female treated at 500
mg/kg bw/day and changes of the thyroid, representative of early
follicular hyperplasia in the single high-dose female. The low dose
of 50 mg/kg bw/day was well tolerated with no overt signs of
toxicity (Sortwell et al., 1977).
Metiram (96.8% purity containing 2.2% ETU) was administered
orally by gavage to groups of 4 rhesus monkeys/sex for a period of
26 weeks at dose levels of 0 (vehicle control, 0.5% tragacanth in
distilled water), 5, 15 or 75 mg/kg bw/day. After termination of
treatment, 1 male and 1 female were retained untreated for a period
of 15 weeks as recovery animals. In a separate, but concurrently
conducted study, a further 2 male and 2 female monkeys were treated
with metiram at doses of 0, 5 or 75 mg/kg bw/day for 26 weeks to
monitor thyroid function. The primary effects of treatment with
metiram were evident in the thyroid and were characterized at dose
levels of 15 and 75 mg/kg bw/day as minimal thyroid follicular
hyperplasia, significantly decreased T3 and T4 levels and
increased thyroid weights. After a 15-week recovery, morphological
changes of the thyroid were still apparent in monkeys previously
treated at the 15 and 75 mg/kg bw/day dose levels. Assessment of
thyroid function revealed an initial marked reduction in iodine
uptake at dose levels of 5 and 75 mg/kg bw/day, followed by a
significant increase in uptake during the latter part of the study.
In the absence of any correlation to thyroid hormone levels or
morphological alterations, no toxicological significance was
attributed to fluctuations in iodine uptake by the thyroid at the
lowest level of 5 mg/kg bw/day. Other effects resulting from
treatment were an increased incidence of salivation occurring at the
time of dosing at the high dose. Significantly increased liver
weights at the high-dose level were not associated with any
histopathological findings. Treatment with metiram did not
significantly affect survival, body-weight gains, food or water
consumption, ophthalmoscopy, haematology, urinalysis or examination
of bone marrow smears. The NOAEL was 5 mg/kg bw/day (Sortwell et
al., 1979).
Long-term toxicity/carcinogenicity studies
Mice
Dietary administration of metiram (96.8% purity containing 2.2%
ETU) to groups of 52 CFLP mice/sex at levels of 0, 100, 300 or 1000
ppm, equal to 0, 8, 24 or 79 mg/kg bw/day for males and 0, 9, 29 or
95 mg/kg bw/day for females, respectively, for a minimum period of
88 weeks resulted in a NOAEL for in-life parameters of 300 ppm,
equal to 24 mg/kg bw/day. Although there were no statistically
significant differences in mortality in the treated groups, a
marginally higher mortality incidence from week 40 onwards was
recorded in females treated at 1000 ppm, resulting in a minimum 25%
survival rate being reached in this group before the corresponding
controls. Group mean body weights were significantly decreased in
both sexes treated at the 1000 ppm dietary level. Food consumption
in males treated at 1000 ppm was only marginally (5%) lower than the
corresponding controls during the first year of treatment. Clinical
signs and examination of bone marrow smears were not adversely
affected by treatment with metiram. Under the conditions of the
present study, treatment with metiram at dietary levels up to and
including 1000 ppm failed to uncover any evidence of carcinogenic
potential (Hunter et al., 1979).
Rats
Groups of 50 Sprague-Dawley CD rats/sex were administered
metiram (96.8% purity containing 2.2% ETU) in the diet at levels of
0, 5, 20, 80 or 320 ppm, equal to 0.2, 0.8, 3.1 or 12.3 mg/kg bw/day
in males and 0, 0.2, 1.0, 3.8 or 15.5 mg/kg bw/day in females,
respectively, for 119 weeks for males and 111 weeks for females.
Satellite groups of 30 male and 30 female rats per group were used
for blood sampling and thyroid function tests and were then killed
after 102 weeks of treatment. Treatment with metiram resulted in a
NOAEL of 80 ppm (equal to 3.1 mg/kg bw/day), based on an increased
incidence of rats treated at the next higher dietary level of 320
ppm with muscular atrophy. There were no consistent treatment-
related effects with respect to survival, clinical signs, body
weights, food or water consumption, ophthalmoscopy, haematology,
blood chemistry, thyroid function, urinalysis or organ weights.
There was no evidence of metiram-induced carcinogenic potential
(Hunter et al., 1981).
Reproduction studies
Rats
A three-generation (two-litter per generation) reproduction
study was conducted with groups of 12 male and 24 female Crl:COBS
CD(SD)BR rats fed metiram (96.8% purity containing 2.2% ETU) at
dietary levels of 0, 5, 40 or 320 ppm, equal to 0, 0.2, 1.8 or 14.2
mg/kg bw/day in males and 0, 0.3, 2.3 or 19.8 mg/kg bw/day in
females, respectively. In the F0 generation, treatment with
metiram commenced at least 60 days prior to mating. In the F1 and
F2 generations, the F1b and F2b derived parental animals,
respectively were exposed to the test material for a minimum period
of 90 days. At day 21 post-partum, 10 F3a pups/sex from each group
were selected for detailed gross examination, recording of organ
weights and histopathological assessment. At the second pairing of
the F2b generation, all pregnant females from each of the dose
levels were sacrificed on day 20 of gestation for teratological
examination. Appearance of spermatozoa in the vaginal smear or the
presence of a copulation plug was considered to be day 0 of
gestation. Decreases in body-weight gain (4-7%) were noted in both
the F0 and F1 generation males and females treated at 320 ppm when
compared to the controls. Slightly depressed (7-8%) food intake was
observed in males fed metiram during the F0 and F1 generations.
Body weights and food consumption were not adversely affected by
treatment at lower dietary levels or in any of the treated groups
from the F2 generation when compared to the controls. There were
no treatment-related effects on parental mortality, clinical signs,
food conversion ratios, reproductive parameters or gross
pathological alterations. Statistically significant decreases in
the mean number of pups born were observed in all treated groups
during the second mating of both the F1 and F2 generations.
Although the differences were statistically significant, there was
no dose-response relationship and all values in the treated groups
generally fell within the reported laboratory standard values.
Total litter loss was significantly decreased at 320 ppm during the
second mating of the F1 generation. In the absence of similar
effects in the first mating of the F1 or in either of the matings
in the F0 and F2 generations, the toxicological significance of
this finding was dubious. Statistically significant decreases in
mean litter weights were observed in the treated groups during the
F1 and F2 generations. Since the mean pup weights in the treated
groups were comparable to the controls, the decreased mean litter
weights may, in part, be attributed to the correspondingly smaller
litter sizes observed in these groups. With regard to the
teratological component, there were no effects on pregnancy rate,
pre- or post-implantation loss, number of live fetuses, embryo/fetal
resorptions, fetal sex ratio or fetal/litter weights. Metiram was
not teratogenic as determined by gross, skeletal and visceral
examination of fetuses, following in utero exposure at dietary
levels as high as 320 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg
bw/day, based on decreased parental body weight and food consumption
recorded in the F0 and F1 generations treated at 320 ppm (Cozens
et al., 1981).
Special studies on teratogenicity
Rats
A preliminary range-finding study was performed with groups of
6 non-pregnant Crl:Cobs CD(SD)BR female rats administered metiram
(96.8% purity containing 2.2% ETU) orally by gavage at 0 (vehicle
control, sodium carboxymethyl cellulose), 150, 300, 600 or 1200
mg/kg bw/day for 10 consecutive days. Dose-related clinical effects
primarily affecting the nervous system were observed at all dose
levels. In the absence of microscopic examination,
histopathological correlation could not be ascertained. Other
effects of treatment were related to decreased body-weight gains at
all dose levels, depressed food intake at 300 mg/kg bw/day and
higher and decreased water consumption in rats treated at 600 and
1200 mg/kg bw/day (Palmer & Simmons, 1979a).
Groups of 20 time-mated female Crl:Cobs CD(SD)BR rats were
treated with metiram (96.8% purity containing 2.2% ETU) daily by
gavage during days 6 to 15 of gestation, inclusive, at dose levels
of 0 (vehicle control, sodium carboxymethyl cellulose), 40, 80 or
160 mg/kg bw/day. The day of mating, determined by the presence of
a vaginal plug or sperm in the vaginal smear, was designated as day
0 of gestation. Decreased body-weight gain (4-9%) was noted in the
high-dose animals. There were no significant effects with respect
to clinical signs, food or water consumption or gross organ/tissue
changes. A slight increase in the pre- and post-implantation loss
was recorded in the 160 mg/kg bw/day group, in conjunction with a
significant decrease in the mean number of live fetuses and
decreased litter weight in this group. It is unlikely that the
increased pre-implantation loss at the high dose was related to
treatment, since theoretically, treatment was not initiated until
after implantation occurred. Nevertheless, the combined effect of
pre- and post-implantation loss may have, to a certain degree,
influenced the decreased litter size and weight noted at the high
dose level. There were no effects of treatment on the incidence of
early or late resorptions, mean fetal weight or sex ratio. The
NOAEL for embryo/fetotoxicity was 80 mg/kg bw/day, based on slight
decreases in litter size and weights. Treatment with metiram failed
to uncover any evidence of teratogenic potential. The NOAEL for
maternal toxicity was 80 mg/kg bw/day, based on decreased body-
weight gain (Palmer & Simmons, 1979a).
Rabbits
In a range-finding study, groups of 3 pregnant Himalayan
rabbits were treated orally by gavage with metiram (97.9% purity,
ETU <0.2%) during days 7 through 19 of gestation at dose levels
of 0 (vehicle control, distilled water with 0.5% carboxymethyl
cellulose), 50, 100 or 200 mg/kg bw/day. Reduced body-weight gains
and food intake were recorded at the two highest dose levels. The
significance of the incidence of single abortion at each of the dose
levels, upon conduct of the definitive study, was confirmed to be
treatment-related. No further relevant details were provided
(Gelbke et al., 1988).
Metiram (97.9% purity, ETU < 0.2%) was administered orally
by gavage at 0 (vehicle control, distilled water with 0.5%
carboxymethyl cellulose), 10, 40 or 120 mg/kg bw/day to groups of 15
artificially inseminated Himalayan rabbits on days 7 through 19 of
gestation. The day of artificial insemination was designated as day
0 of gestation. Treatment with metiram elicited signs of maternal
toxicity at 40 and 120 mg/kg bw/day, denoted by abortion, decreased
body weight and food consumption, the latter associated with
reduction or absence of defecation. The high incidence of abortion
(8/15) recorded at 120 mg/kg bw/day, in conjunction with a single
death culminated in only 6/15 rabbits treated at this level with
litters available for examination. Although 2 of 15 animals aborted
at 40 mg/kg bw/day, there were, nevertheless, adequate numbers of
litters secured in the control, 10 and 40 mg/kg bw/day dose groups.
Since a dose level of 10 mg/kg bw/day was without significant
effects, this dose was considered the NOAEL for maternal toxicity.
The NOAEL for developmental toxicity was 40 mg/kg bw/day based on
the slight decrease in mean fetal weights recorded at 120 mg/kg
bw/day. A slight decrease in the number of live male fetuses in the
120 mg/kg bw/day group was considered to be of no toxicological
consequence, in light of the comparable number of female fetuses and
the total number of fetuses in the 120 mg/kg bw/day group relative
to the controls. There were no significant effects of treatment on
the mean number of live fetuses, early or late resorptions, post-
implantation loss, or placental weight. There was no evidence
suggestive of teratogenic potential with metiram at any of the dose
levels investigated (Gelbke et al., 1988).
Special studies on genotoxicity
Results of mutagenicity assays conducted with metiram are
presented in Table 3. The tests conducted to evaluate potential for
gene mutation in bacteria and induction of chromosomal aberration in
rat bone marrow were negative. Unscheduled DNA synthesis in rat
hepatocytes and a dominant lethal study in mice were also negative.
Although metiram demonstrated potential for inducing sister
chromatid exchange (SCE) in Chinese hamster ovary cells in the
absence and presence of mouse microsomal activation, there was
nevertheless, no significant induction of SCE observed with rat
microsomal activation. Moreover, an in vivo SCE assay in bone
marrow cells of the Chinese hamster was negative. Metiram exhibited
a weak promotion activity in mouse embryo fibroblasts in the absence
of a direct transformation response. The Meeting concluded that
metiram was not genotoxic.
Special studies on irritation and sensitization
The eye and skin irritation potential of technical metiram
(containing 2.2% ETU) were investigated in six Vienna white rabbits.
There were no signs of irritation when metiram was applied to the
external ear for 20 hours or to the backs for 1, 5 or 15 minutes.
When applied to the back for 20 hours, slight reddening occurred 24
hours after application with subsequent scaling after 8 days.
Metiram (50 mg) when introduced into the conjunctival sac of the eye
of these rabbits produced slight redness and edema after 1 hour,
with no signs of irritation after 8 days (Zeller, 1985a).
Polyram combi, a formulation of technical metiram, was
administered to eight Vienna white rabbits to determine skin and eye
irritation potential. A 50% aqueous suspension of the test material
when applied to the backs of the rabbits for a period of 20 hours,
resulted in slight erythema of the skin, which was reversible within
8 days. The instillation of 50 mg of Polyram combi to the
conjunctival sac of the rabbit eye caused redness and edema which
were reversible after 8 days. When 50 µl of a 20% aqueous
suspension was applied, slight reddening of the mucosa occurred,
whereas 50 µl of a 2% aqueous suspension was tolerated without any
irritation (Zeller, 1985b).
Metiram technical demonstrated severe sensitizing effects on
the skin of female Pirbright white, Dunkin Hartley guinea-pigs when
tested according to the methods of the Magnusson & Kligman
maximization test (Jackh, 1982).
Table 3. Genotoxicity of metiram
Test Test system Concentration Purity Results Reference
Reverse mutation S. typhimurium 0, 1, 3, 10, 31, Technical negative Oesch, 1977
(in vitro) TA 98, 100, 1537 100, 310, 1000, 1., 2. (rat)
2000 µg/plate
S. typhimurium 0, 1, 10, 50, 100, Technical + 2.2% ETU negative Gelbke & Engelhardt,
TA 98, 100, 1535, 1537 500, 2500 µg/plate 1., 2. 1985
(rat & mouse)
Reverse mutation/host S. typhimurium 500, 1670, Technical + 2.2% ETU negative Jagannath & Myhr, 1985
mediated (in vivo) TA 1530 5000 mg/kg bw
Male CD-1 mice (single oral doses)
(inoculated ip)
Point mutation HGPRT locus of Chinese 0, 0.681, 1.0, 4.64, Technical + 2.2% ETU negative, 1. Gelbke & Jackh, 1985
(in vitro) hamster ovary (CHO) cell 6.81, 10, 46.4, equivocal, 2.
line, K1 68.1, 100 µg/ml (rat)
HGPRT locus of Chinese 0.5, 1, 5, 10, 50, Premix 95% negative Hoffman & Jackh, 1990
hamster ovary (CHO) cell 100, 500 µg/ml 1., 2. (mouse)
line, K1
SCE (in vitro) Chinese hamster ovary 0, 40, 60, 80, 100, Technical + 2.2% ETU positive, 1. Ivett & Myhr, 1986a
CHO, WB1 cells 125, 150, 175, 200 positive, 2.
µg/ml (mouse)
negative, 2.
(rat)
SCE (in vivo) Chinese hamster, LMP 1000, 3330, 10 000 Premix 95% negative Miltenburger et al.,
stock (bone marrow) mg/kg bw 1990
Table 3 (contd)
Test Test system Concentration Purity Results Reference
Chromosome aberration Rat, male Fischer 344 0, 0.24, 1.2, 2.4 Technical + 2.2% ETU negative Ivett & Myhr, 1986b
(in vivo) (bone marrow) g/kg bw (acute) 0,
0.02, 0.1, 0.2 g/kg
bw (5 repeated oral
doses)
Transformation Mouse embryo fibroblasts 0, 0.1, 0.25, 0.5, Technical + 2.2% ETU transformation: Tu et al., 1985
promotion (in vitro) C3H-10T 1/2 (clone 8) 0.75, 1.0 µg/ml negative
cell system promotion
activity:
weakly
positive
Unscheduled DNA Rat (Fischer 344, male) 0.492, 1.23, 2.46, Technical + 2.2% ETU negative Cifone & Myhr, 1984
synthesis (in vitro) hepatocytes 4.92, 12.3, 24.6,
49.2, 160 µg/ml
Dominant lethal Mouse, CD-1 male 0, 600, 1200, 2400 Technical + 2.2% ETU negative Palmer & Simons, 1979b
(in vivo) mg/kg bw/day
Results:
1. = in the presence of metabolic activation derived from the indicated species
2. = in the absence of metabolic activation
COMMENTS
Metiram was incompletely absorbed when administered orally to
rats. Elimination was primarily via the faeces, with minimal
biliary excretion. Comparatively higher urinary excretion at low
doses suggested that metiram may be more poorly absorbed at higher
doses. Highest residual tissue levels were found in the thyroid and
kidney, with slightly higher concentrations present in females than
in males. Comparison of tissue residues after single or multiple
doses suggested slight accumulation in the body with multiple
dosing.
The metabolism of metiram has not been completely elucidated.
In the rat, the predominant urinary components were polar and were
identified as ethylenediamine, N-acetyl-ethylenediamine,
ethanolamine, oxalic acid and glycine. Major, less polar components
were ethyleneurea, ETU and EBIS.
Metiram was practically non-toxic upon acute oral, dermal and
inhalation administration to rats. WHO has classified metiram as
unlikely to present acute hazard in normal use.
The principal target organ upon repeated dietary exposure to
metiram was the thyroid.
Mice treated with metiram for three months at 0, 300, 1000,
3000 or 7500 ppm in the diet revealed minimal to slight hypertrophy
and vacuolation of the thyroid follicular epithelium in both sexes
at levels of 3000 ppm and higher. A NOAEL of 300 ppm (equal to 84
mg/kg bw/day) was based on decreased serum T4 levels in both sexes
at levels of 1000 ppm and above.
Thirteen-week dietary administration of metiram to SD CFY rats
at 0, 50, 100, 300 or 900 ppm revealed a NOAEL of 100 ppm (equal to
6 mg/kg bw/day) based on decreased serum T4 levels and increased
thyroid weights at dietary levels of 300 and 900 ppm. Slight to
minimal hyperplasia of the thyroid was observed at 900 ppm.
Although reduced iodine uptake by the thyroid was observed at all
dietary levels, these changes were shown to be reversible following
cessation of treatment. At the lowest dietary levels of 50 and 100
ppm, the effects on iodine uptake were not correlated with changes
in thyroid hormone levels or any overt morphological alterations of
the thyroid gland, thus rendering the toxicological significance of
this finding doubtful.
In a recently conducted three-month study with Wistar rats
receiving metiram at 0, 5, 80, 320 or 960 ppm in the diet, decreased
serum T4 levels and increased thyroid weights were observed at 960
ppm. Slight evidence of anaemia was observed at 320 ppm, indicating
a NOAEL of 80 ppm (equal to 5.8 mg/kg bw/day).
Other effects of treatment in the diet with metiram in the rat
were manifest as hind limb paralysis with corresponding atrophy of
muscle fibres. In the 13-week study with SD CFY rats, microscopic
changes in muscle fibres at levels of 300 ppm (equal to 20 mg/kg
bw/day) and higher were still prevalent in previously treated rats
after the 6-week recovery period. Muscular atrophy was observed in
a long-term study in SD CD rats treated at the highest level of 320
ppm (see below). General muscle weakness/ataxia and reduced grip
strength of the limbs with no histopathological consequences were
observed in the 3-month study with Wistar rats fed metiram at the
highest level of 960 ppm.
A 52-week study in dogs at dietary levels of 0, 30, 80, 1000 or
3000 ppm yielded a NOAEL of 80 ppm (equal to 2.5 mg/kg bw/day) based
on thyroid follicular hyperplasia with increased size, thickening
and weight of this organ, in conjunction with decreased serum T4
levels at dietary levels of 1000 ppm and above. Other effects
recorded at 1000 ppm and above were a dose-related increased
incidence of focal hepatic lipofuscin pigment deposition, slight
evidence of anaemia, diarrhoea and changes in blood biochemical
parameters. A preliminary 4-week study in dogs uncovered an
increased frequency of microfollicles in the thyroid, in association
with colloid depletion and minimal hyperplasia in both sexes treated
at the highest level of 900 ppm (equal to 41 mg/kg bw/day).
Metiram given by gavage to rhesus monkeys at dose levels of 0,
5, 15 or 75 mg/kg bw/day for a period of 26 weeks indicated a NOAEL
of 5 mg/kg bw/day based on significantly decreased serum T3 and T4
levels, increased thyroid weights and minimal thyroid follicular
hyperplasia at 15 and 75 mg/kg bw/day. Morphological changes of the
thyroid were still apparent after a 15-week recovery period. In the
absence of any correlation between thyroid hormone levels and
morphological alterations, no significance was attributed to
fluctuations in iodine uptake by the thyroid recorded at 5 mg/kg
bw/day.
Long-term dietary treatment of mice with metiram at 0, 100, 300
or 1000 ppm resulted in a NOAEL of 300 ppm (equal to 24 mg/kg
bw/day) based on decreased body weights recorded at 1000 ppm.
Chronic dietary administration of metiram to SD CD rats at 0,
5, 20, 80 or 320 ppm revealed muscular atrophy at 320 ppm (equal to
12 mg/kg bw/day), with a NOAEL of 80 ppm (equal to 3.1 mg/kg
bw/day).
Metiram was not carcinogenic when fed to mice or rats at
dietary levels of up to 1000 and 320 ppm, respectively.
A three-generation, two litter per generation reproduction
study in rats treated at 0, 5, 40 or 320 ppm in the diet failed to
reveal any adverse effects on reproductive parameters. The NOAEL
was 40 ppm (equal to 1.8 mg/kg bw/day), based on decreased parental
body weight and food consumption recorded in the F0 and F1
generations treated at 320 ppm.
Metiram when administered to pregnant rats at 0, 40, 80 or 160
mg/kg bw/day or rabbits at 0, 10, 40 or 120 mg/kg bw/day during
critical periods of organogenesis was not teratogenic at any dose.
The NOAEL for maternal toxicity in the rat was 80 mg/kg bw/day based
on decreased body-weight gain and in the rabbit the NOAEL was 10
mg/kg bw/day based on increased abortions, decreased body weights
and food consumption. The NOAELs for embryo/fetotoxicity were 80
mg/kg bw/day in the rat based on slight decreases in litter size and
weight and 40 mg/kg bw/day in the rabbit based on decreases in mean
fetal weights.
Metiram has been tested in a series of in vitro and in vivo
genotoxicity assays. The Meeting concluded that metiram is not
genotoxic.
The Meeting allocated an ADI of 0-0.03 mg/kg bw based on a
NOAEL of 2.5 mg/kg bw/day in the 52-week study in dogs, using a 100-
fold safety factor. This ADI is supported by the NOAEL of 3.1 mg/kg
bw/day observed in the long-term study in rats. This ADI served as
the basis for a group ADI that was established for metiram alone or
in combination with mancozeb, maneb, and zineb.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 300 ppm, equal to 24 mg/kg bw/day (88-week study)
Rat: 80 ppm, equal to 3.1 mg/kg bw/day (111-week study)
40 ppm, equal to 1.8 mg/kg bw/day
(reproduction study)
Rabbit: 10 mg/kg bw/day (teratogenicity study)
Dog: 80 ppm, equal to 2.5 mg/kg bw/day (52-week study)
Monkey: 5 mg/kg bw/day (26-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, maneb, and
zineb).
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Chesterman, H., Heywood, R. & Barker M. (1973) Polyram Combi acute
oral toxicity study in beagle dogs. Report BASF 73/0047, BASF
40/73703. Unpublished report dated October 5, 1973 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Chesterman, H., Heywood, R., Ball, S., Street, A. & Prentice, D.
(1978) Metiram (active ingredient) oral toxicity study in beagle
dogs (dietary intake for 4 weeks). Report BASF 78/0154, BSF
201/76422. Unpublished report dated April 26, 1978 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Cifone, M. & Myhr, B. (1984) Report on the evaluation of metiram
tech. in the rat primary hepatocyte unscheduled DNA synthesis assay.
Report RZ 84/209. Unpublished report dated July 5, 1984 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Corney, S.J., Allen, T.R., Janiak, T. & Springall, C. (1991) 52-week
oral toxicity (feeding) study with metiram premix 95% in the dog.
Report No. 91/10786. Unpublished report dated August 22, 1991 from
RCC, Research & Consulting Company Ltd., Itingen, Switzerland.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Cozens, D., Simons, R., Clark, R., Offer, J. & Gibson, W. (1981)
Effect of metiram technical on reproductive function of multiple
generations in the rat. Report RZ 81/132, BSF 200/80692. Unpublished
report dated March 18, 1981 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Gelbke, H. & Engelhardt, G. (1985) Report on the study of metiram
(techn.) in the Ames test. Report RZ 85/020. Unpublished report
dated February 7, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H. & Jackh, R. (1985) Report on a point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram. Report RZ 85/238. Unpublished report dated July 30, 1985
from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Gelbke, H., Hellwig, J. & Hildebrand, B. (1988) Report on the study
of the prenatal toxicity of metiram-premix 95% in rabbits after oral
administration (gavage). Report BASF 88/0154 and Supplement BASF
88/0262. Unpublished report dated May 26, 1988 from BASF, Department
of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. and Hildebrand, B. (1992a) Study of the oral
toxicity of metiram premix 95% in B6C3F1 mice. Administration in
the diet for 3 months. Report No. 92/11223. Unpublished report dated
October 16, 1992 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. & Hildebrand, B. (1992b) Study of the oral
toxicity of metiram premix 95% in Wistar rats. Administration in the
diet for 3 months including the examination of neurotoxicology
(neurofunctional observational battery). Report No. 92/11224.
Unpublished report dated October 16, 1992 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1979) Study of the acute dermal toxicity of "Metiram
tech. with 2% ETU" on the rat (Translation). Report RZ 79/032.
Unpublished report dated September 25, 1979 from BASF,
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1985) Report on the study of the acute dermal
toxicity of Polyram Combi (BAS 222 01 F) in the rat (Translation of
the original report dated September 25, 1979). Report RZ 85/026.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Girkin, R. & Jackson R. (1984) Report on the
study of dermal absorption of metiram in rats. Report RZ 85/158, HRC
BSF 411/84694. Unpublished report dated October 10, 1984 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Midgley, I., Biggs, S. & McCay, C. (1985)
The biokinetics and metabolism of 14C-metiram in the rat. Report
BASF 85/0470, HRC BSF 410/85720. Unpublished report dated October
28, 1985 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1974a) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF, Gewerbehygiene
und Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hofmann, H. (1974b) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF
Aktiengesellschaft, FRG.
Hofmann, H. & Munk, R. (1975) Acute intraperitoneal toxicity of the
technical active ingredient metiram to the rat. Report RZ 75/006.
Unpublished report dated June 4, 1975 from BASF, Gewerbehygiene und
Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1975) Acute oral toxicity of the technical active
ingredient metiram to the rat. Report RZ 75/005. Unpublished report
dated June 4, 1975 from BASF, Gewerbehygiene und Toxikologie, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1985a) Report on the study of the acute oral toxicity of
Polyram Combi, technical active ingredient, in the rat (Translation
of original report dated March 25, 1974). Report BASF 85/0485.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985b) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi, technical active
ingredient, in the rat (Translation of original report dated March
25, 1974). Report RZ 85/028. Unpublished report dated February 4,
1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985c) Report on the study of the acute oral toxicity
of Polyram Combi (BAS 222 01 F) to rats (Translation of original
report dated March 25, 1974). Report RZ 85/121. Unpublished report
dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985d) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi (BAS 222 01 F) in rats
(Translation of original report dated March 25, 1974). Report RZ
85/124. Unpublished report dated April 12, 1985 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985e) Report on the study of the acute inhalation
toxicity of the spray of a 5% aqueous suspension of Polyram Combi
(BAS 222 01 F) to guinea-pigs (Translation of original report dated
March 25, 1974). Report RZ 85/125. Unpublished report dated April
12, 1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein,
FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. & Jackh, R., 1990. Report on the point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram premix 95% with B6C3F1 mice microsomal fraction. Report
No. 90/0285. Unpublished report dated August 2, 1990 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A. & Prentice, D.
(1976a) Preliminary assessment of metiram toxicity to rats in
dietary administration for four weeks followed by a two and a four
week withdrawal period. Report RZ 76/024, BSF 172/76245. Unpublished
report dated October 20, 1976 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hunter, B., Bridges, J. & Prentice, D. (1976b) Preliminary
assessment of metiram toxicity to mice in dietary administration for
4 weeks. Report RZ 76/014, BSF 170/76108. Unpublished report dated
June 7, 1976 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A., Prentice, D. &
Offer, J. (1977) Metiram toxicity to rats in dietary administration
for 13 weeks followed by a 6 week withdrawal period (final report).
Report RZ 77/043, BSF/197/77612 and Addendum BASF 87/0205, BSF
197/8713. Unpublished report dated November 16, 1977 and addendum
dated May 29, 1987 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Prentice, D. & Gregson, R., 1979. Metiram
tumorigenicity to mice in long term dietary administration (final
report). Report RZ 79/033, BSF 198/78265. Unpublished report dated
June 5, 1979 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Street, A., Heywood, R., Prentice, D.,
Offer, J. & Gibson, W. (1981) Metiram toxicity and tumorigenicity in
prolonged dietary administration to the rat. Report RZ 81/280, BSF
199/80391, WNT No. 77/951 and Addendum BASF 89/0001. Unpublished
report dated May 7, 1981 and addendum date December 19, 1988 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986a) Report on the mutagenicity evaluation
of metiram in an in vitro sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells/amended final report. Report RZ
86/082. Unpublished report dated March 11, 1986 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986b) Report on the mutagenicity evaluation
of metiram technical K38/33A in the rat bone marrow cytogenetic
assay. Report RZ 86/265. Unpublished amended report dated August 4,
1986 from Litton Bionetics, Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jackh, R. (1981) Report on the study of the acute oral toxicity of
metiram technical grade in the rat (Translation). Report BASF
92/10669. Unpublished report dated July 9, 1981 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Jackh, R. (1982) Report on the study of the sensitizing effect of
metiram techn. in the guinea-pig -maximization test (Translation of
original report dated August 21, 1981). Report RZ 82/068.
Unpublished report dated February 16, 1982 from BASF, Department of
Toxicology, Ludwigshafen, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jagannath, D. & Myhr, B. (1985) Report on the mouse host-mediated
assay of metiram tech. K38/33A. Report RZ 85/210. Unpublished report
dated June 28, 1985, from Litton Bionetics Inc., Maryland, USA.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Klimisch, H.J. & Zeller, H. (1980) Report on the determination of
the acute inhalation toxicity LC50 metiram techn. with 2% ETU as a
dust aerosol after 4-hour exposure in Sprague-Dawley rats
(Translation of original report dated September 15, 1980). Report RZ
83/064. Unpublished report dated September 15, 1980 from
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Leuschner, F. (1979a) The acute oral toxicity of the preparation
metiram, techn. agent with 2% ETU in rats. Report BASF 79/0161, WNT-
Nr. 77/951. Unpublished report dated August 20, 1979 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Leuschner, F. (1979b) The acute intraperitoneal toxicity of the
preparation metiram, techn. agent with 2% ETU in mice. Report BASF
79/0162, WNT-Nr. 77/951. Unpublished report dated August 20, 1979
from Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Miltenburger, H., Volkner, W. & Heidemann, A. (1990) Sister
chromatid exchange assay in bone marrow cells of the Chinese hamster
with metiram premix 95%. Report No. 90/0569. Unpublished report
dated September 18, 1990 from CCR, Cytotest Cell Research Gmbh &
Co., Rossdorf, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Oesch, F. (1977) Ames test for metiram. Report RZ 77/027.
Unpublished report dated August 22, 1977 from the Institute of
Pharmacology, University of Mainz, Germany. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Palmer, A. & Simons, R. (1979a) Effect of metiram technical on
pregnancy of the rat. Report RZ 79/065, BSF 302/79616. Unpublished
report dated August 3, 1979 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Palmer, A. & Simons, R. (1979b) Dominant lethal assay of metiram
technical in the male mouse. Report RZ 79/069, BSF 304/79860.
Unpublished report dated November, 1979 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Heywood, R., Allen, D., Prentice, D. & Cherry, C.
(1977) Metiram preliminary oral toxicity study in rhesus monkeys.
Repeated dosage for 4 weeks. Report BASF 77/0149, BSF 265/77264.
Unpublished report dated April 15, 1977 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Allen, D., Heywood, R. & Street, A. (1979) Metiram
(containing 2.2% ethylenethiourea) oral toxicity study in rhesus
monkeys (repeated dosage for 26 weeks with recovery period). Final
report. Report BASF 79/0082, BSF 267/78263. Unpublished report dated
January 15, 1979 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Tu, A., Sivak, A., Breen, P. & Hatch, K. (1985) Report on the
evaluation of metiram in the C3H-10T 1/2 cell system for
transformation and promotion activities. Report RZ 85/209.
Unpublished report dated June 18, 1985 from Arthur D. Little, Inc.,
Maryland, USA. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ullmann, L., Sacher, R., Porricello, T., Luetkemeier, H., Vogel, W.,
Vogel, O., Wilson, J. & Terrier, H. (1987) Report on the subacute
21-day repeated-dose dermal toxicity study with polyram DF in
rabbits. Report BASF 87/0260, ZNT No. 86/314. Unpublished report
dated June 24, 1987 from RCC, Research and Consulting Company AG,
Switzerland. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ulrich, C. (1986) Report on the thirteen week subchronic inhalation
toxicity study on metiram in rats. Report RZ 86/407 and addendum
BASF 87/0414. Unpublished report dated December 31, 1986 and
addendum dated October 7, 1987 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. (1985a) Report on the study of the irritation of Polyram
Combi, technical active ingredient, to the skin and eye of rabbits
(Translation of original report dated March 25, 1974). Report RZ
85/027. Unpublished report dated February 4, 1985 from BASF,
Department of Toxicology, Ludwigshafen, FRG. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Zeller, H. (1985b) Report on the study of the irritation of Polyram
Combi (BAS 222 01 F) to the skin and eyes of rabbits (Translation of
original report dated March 25, 1974). Report RZ 85/122. Unpublished
report dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Table 1. Acute toxicity of technical metiram
Species Sex Route (vehicle) LD50 Test material Reference
(strain) (mg/kg bw)
Rat M+F oral > 6810 Technical grade Jackh, 1981
(SD) (distilled water) (purity unknown)
Rat M+F oral M: 9100 Technical grade Leuschner, 1979a
(SD) (HPMC) F: 8900 (purity unknown) + 2% ETU
M+F: 9000
Rat M+F oral 6500 Technical grade Hofmann, 1985a
(SD) (tragacanth) (purity unknown)
Rat M+F oral 10 000 Technical grade Hofmann, 1975
(SD) (CMC) (purity unknown)
Rat (SD) M+F dermal (water) > 2000 Technical grade Grundler, 1979
(77.5% purity) + 2% ETU
Mouse (NMRI) M+F intraperitoneal M: 208 Technical grade Leuschner, 1979b
(HPMC) F: 215 (purity unknown) + 2% ETU
M+F: 212
Mouse (NMRI) M+F intraperitoneal 80 Technical grade Hofmann, 1974a
(tragacanth) (purity unknown)
Rat (SD) M+F intraperitoneal M: 318 Technical grade Hofmann & Munk, 1975
(CMC) F: 317 (77.5% purity)
M+F: 318
Rat (SD) M+F inhalation 4 h > 5.7 (mg/l) Technical grade Klimisch & Zeller, 1980
(77.5% purity) + 2% ETU
Rat (SD) M+F inhalation 8 h (well Technical grade Hofmann, 1985b
tolerated) (purity unknown) + 2.2% ETU
Table 2. Acute toxicity of Polyram combi (formulated product)
Species Sex Route LD50 Test Reference
(strain) (vehicle) (mg/kg bw) material
Rat M+F oral > 10 000 Polyram combi Hofmann, 1985c
(SD) (tragacanth) (purity unknown)
Dog M+F oral * Polyram combi Chesterman et al., 1973
(beagle) (water) (purity unknown)
Mouse (NMRI) M+F intraperitoneal approx 80 Polyram combi Hofmann, 1974b
(tragacanth) (purity unknown)
Rat M+F dermal > 2000 Polyram combi Grundler, 1985
(SD) (77.5% purity)
Rat M+F inhalation, (well Polyram combi Hofmann, 1985d
(SD) 8 hr tolerated) (77.5% purity)
Guinea-pig inhalation, >0.8 (mg/L) Polyram combi Hofmann, 1985e
8 hr (purity unknown)
* Estimation of LD50 precluded due to high incidence of vomiting
oral and dermal administration, were practically non-toxic in the
rat with LD50 values of > 6500 mg/kg bw and > 2000 mg/kg bw,
respectively. Moderate acute toxicity was demonstrated upon
intraperitoneal administration of technical material in the mouse
(LD50 = 80-212 mg/kg bw) and rat (LD50 = 318 mg/kg bw). Technical
metiram when administered by the inhalation route resulted in an
LC50 value greater than 5.7 mg/l in the rat. Eight-hour inhalation
of the formulated material, Polyram combi was well tolerated in both
rats and guinea-pigs. Intentional spiking of the technical material
with ETU at levels of 2 to 2.2% did not markedly alter the acute
toxicity profile.
Short-term toxicity studies
Mice
In a preliminary assessment of the toxicity of metiram (96.8%
purity, containing 2.2% ETU), groups of 8 CFLP mice per sex were fed
dietary concentrations of 0, 100, 300, 1000 or 3000 ppm equal to 0,
16, 40, 120 or 399 mg/kg bw/day for males and 0, 15, 44, 142 or 433
mg/kg bw/day for females, respectively, for a period of 4 weeks.
Body-weight gain of males treated with metiram at 1000 and 3000 ppm
were significantly lower than that of the corresponding controls
during the first week of treatment with subsequent recovery of
weight gain in the ensuing weeks. Although no consistent effects on
body weight in treated females were observed on a weekly basis,
overall weight gain of females at the high dose of 3000 ppm was
marginally lower than that of the control animals. Significantly
increased liver weights were recorded in both sexes treated at
levels of 1000 and 3000 ppm. In the absence of routine
histopathological examination, no microscopic correlation could be
drawn from the increased liver weights. There were no effects of
treatment on survival, clinical signs, food consumption or gross
pathological changes. On the basis of the reported findings, a high
dietary level of 1000 ppm was selected for the long-term study in
mice (Hunter et al., 1976b).
A 3-month study was performed with groups of 10 B6C3F1
mice/sex treated with metiram (94.8% purity, ETU <0.2%) at
dietary levels of 0, 300, 1000, 3000 or 7500 ppm, equal to 0, 84,
302, 853 or 2367 mg/kg bw/day in males and 0, 133, 465, 1448 or 3565
mg/kg bw/day in females, respectively. Treatment-related effects
were observed with respect to serum T3 and T4 concentrations.
Serum T4 levels were significantly decreased in both sexes of mice
treated with metiram at dietary levels of 1000 ppm and higher,
whereas T3 levels were significantly increased in male mice treated
at the highest dietary level of 7500 ppm. Histopathological
findings attributable to treatment were denoted in the thyroid in
both sexes treated at dietary levels of 3000 ppm and higher as
minimal to slight hypertrophy and vacuolation of the follicular
epithelium. A slight increase in the severity of fatty degeneration
of the `X zone' in the adrenals was also observed in female mice
treated with metiram at 3000 and 7500 ppm. A dose-related increased
incidence in the presence of a refractile, granular material
presumed to be the test material or a metabolite, was noted in the
superficial layer of the urinary bladder urothelium in all groups of
treated mice. Since no morphological changes were observed in the
urothelium of the bladder to suggest disturbed epithelial function,
the presence of this granular material was considered to be of
doubtful toxicological consequence. Significantly increased organ
weights were recorded for the adrenals in females at dietary levels
of 3000 ppm and higher and for the liver in both sexes treated at
7500 ppm and in males at 3000 ppm. Statistically significant
increases in kidney and testicular weights were judged to be of no
biological consequence. No histopathological correlation could be
determined for the increased liver, kidney or testicular weights.
Group mean body weights and corresponding weight gains were
decreased in males treated at the highest dietary level of 7500 ppm.
Although significant decreases in body weights were recorded in
females treated at dietary levels of 1000 ppm and higher, there was
no dose-response relationship. There were no adverse treatment-
related effects on survival, food consumption or on blood
haematological parameters. The NOAEL for this study was 300 ppm,
equal to 84 mg/kg bw/day, based on decreased serum T4 levels in
both sexes at levels of 1000 ppm and above (Gelbke et al., 1992a).
Rats
Metiram (96.8% purity containing 2.2% ETU) was administered for
a period of 4 weeks to groups of 20 Sprague-Dawley CD rats/sex at
dietary levels of 0, 100, 300, 1000 or 3000 ppm, equal to 0, 10, 30,
100 or 296 mg/kg bw/day in males and 0, 11, 33, 114 or 292 mg/kg
bw/day in females, respectively. Following termination of treatment
with metiram, 10 rats per sex per group were retained for subsequent
2 and 4 week recovery periods with scheduled sacrifices of 5
rats/sex/group on each occasion. Clinical toxicity was manifest as
signs of hind limb paralysis in rats treated at the highest dietary
level of 3000 ppm, which due to severity, resulted in early
sacrifice of 3 females for humane reasons. Histopathological
examination confirmed gross morphological alterations in the muscles
of the hind limbs, characterized by atrophy of the fibres, often
with associated proliferation of sacrolemmal nuclei, and the
presence of groups of vacuoles within muscle fibres. After 4 weeks
of treatment, the microscopic changes were more marked and extensive
in females, occurring in all females and in 2 of 9 males treated at
3000 ppm. After a withdrawal period of 2 weeks, a varying degree of
atrophy of muscle fibres was still present in females previously
treated with 3000 ppm of metiram. Although atrophy was not evident
in animals after a 4-week recovery period, additional changes, not
apparent earlier, were the presence of vacuoles and/or areas of fat
cells within the skeletal fibres. Target organ effects of treatment
with metiram were also evident on the thyroid and kidney. Changes
in the thyroid were apparent as a dose-related increase in the
incidence and severity of thyroid hyperplasia in both sexes treated
at dietary levels of 300 ppm and higher. Microscopic examination of
the kidney revealed varying degrees of hydropic vacuolation of the
proximal tubular epithelial cells in 2 females from each of the 1000
and 3000 ppm groups in the absence of similar effects at any of the
other dose levels or corresponding controls. Examination of bone
marrow smears revealed a depressed number of myeloid cells in males
treated at the high dose after 4 weeks of treatment. Group mean
body-weight gains were significantly lower in males treated at 1000
ppm and higher, and in females treated at 300 ppm and higher when
compared to the controls. Decreased food intake was recorded in
both sexes treated at the highest dietary level of 3000 ppm. There
were no adverse effects of treatment noted upon ocular examination,
haematology, blood chemistry, urinalysis and estrus cycle as
assessed from vaginal smears. The NOAEL was 100 ppm, equal to 10
mg/kg bw/day (Hunter et al., 1976a).
Groups of 35 Sprague-Dawley CFY strain rats per sex were
treated with metiram (96.8% purity containing 2.2% ETU) at dietary
levels of 0, 50, 100, 300 or 900 ppm, equal to 0, 3, 6, 20 or
61 mg/kg bw/day for males and 0, 4, 8, 24 or 76 mg/kg bw/day for
females, respectively, for a period of 13 weeks followed by a 6-week
recovery period. The principal target organs were the thyroid and
skeletal muscle. Treatment-related effects on the thyroid were
manifest as increased thyroid weights of females treated at 300 and
900 ppm, decreased percentage uptake of 131I by the thyroid in males
in all treated groups and in females treated at 100 ppm and higher,
and decreased T4 levels in both sexes at 300 ppm and higher.
Microscopically, an increased incidence of males treated at 900 ppm
had slight to minimal hyperplasia of the thyroid. A minimally
higher incidence at 13 weeks of slight thyroid hyperplasia in
females at 900 ppm and in males at 100 and 300 ppm when compared to
the controls was considered to be of equivocal significance. There
were no significant changes in the thyroid observed after the 6-week
recovery period. Treatment-related morphological changes of the
skeletal muscle denoted by atrophy of the muscle fibres often
accompanied by fat cells and associated proliferation of sacrolemmal
nuclei, were evident in both sexes treated at dietary levels of 300
ppm and higher. Varying degrees of muscular atrophy were still
prevalent after a 6-week recovery period in rats previously treated
at 300 and 900 ppm. Other effects of treatment were exhibited at
the high-dose level of 900 ppm as clinical signs of hind limb
paralysis, decreased body weights and slight reduction in food
intake. There were no treatment-related effects observed with
respect to survival, water consumption, ophthalmoscopy, haematology,
urinalysis or examination of bone marrow smears. Although reduced
iodine uptake by the thyroid was observed at all dietary levels,
these changes were shown to be reversible following cessation of
treatment. At the lowest dietary levels of 50 and 100 ppm, the
effects on iodine uptake were not correlated with changes in thyroid
hormone levels or any overt morphological alterations of the thyroid
gland, thus rendering the toxicological significance of this finding
at 50 and 100 ppm as doubtful. The NOAEL for this study was
therefore 100 ppm, equal to 6 mg/kg bw/day, based on decreased serum
T4 levels and increased thyroid weights at dietary levels of 300
and 900 ppm (Hunter et al., 1977).
Metiram (94.8% purity, ETU <0.2%) was administered for a
period of 3 months to groups of 13 Wistar Chbb:THOM SPF rats per sex
at dietary levels of 0, 5, 80, 320 or 960 ppm, equal to 0, 0.4, 5.8,
23.5 or 73.9 mg/kg bw/day in males and 0, 0.4, 6.7, 27.3 or 88.8
mg/kg bw/day in females, respectively. Neurofunctional assessment
revealed general muscle weakness expressed as ataxia and
significantly reduced forelimb and hindlimb grip strength in females
treated at the highest dietary level of 960 ppm. Perfusion fixation
of tissues from selected rats from the control and treated groups
failed to reveal any associated neuropathological or morphological
alterations of the central or peripheral nervous systems. Thyroid
hormone levels revealed significantly decreased T4 levels in both
sexes treated at the high dose of 960 ppm. Significantly reduced
RBC values were recorded in both sexes at levels of 320 and 960 ppm.
In females, decreases in RBC values were accompanied by decreases in
mean haemoglobin and haematocrit levels. Several statistically
significant changes in blood biochemical parameters of uncertain
toxicological significance were observed at the highest dietary
level of 960 ppm. These changes were recorded as decreased ALAT and
ALP activity (females), decreased creatinine (both sexes), decreased
urea (males), and decreases in the electrolytes; potassium (both
sexes), sodium (females), calcium (both sexes) and magnesium (both
sexes). Decreased phosphorus levels were recorded in males at
dietary levels of 320 ppm and higher. Other effects of treatment
expressed in both sexes at the highest dietary level of 960 ppm were
decreased body weights and corresponding weight gains, as well as
significantly increased thyroid, liver, kidney and testes (males
only) weights. There were no treatment-related gross or
histopathological changes observed in any of the animals from the
treated groups. Treatment with metiram did not result in any
significant effects on survival, food consumption, ophthalmoscopy or
urinalysis. The NOAEL was 80 ppm, equal to 5.8 mg/kg bw/day, based
on changes in haematological and blood chemical parameters at 320
ppm (Gelbke et al., 1992b).
A 13-week inhalation study was conducted with groups of 28
Sprague-Dawley CD rats/sex exposed in nose-only chambers to metiram
(94% purity) 6 hours/day, 5 days/week at concentrations of 0, 2, 20
or 100 mg/m3. Concentrations of ETU in the exposure atmospheres
were calculated to be 0, 0.02, 0.33 or 1.8 mg/m3, respectively.
After termination of treatment, one-half of the rats were sacrificed
while the remaining animals were retained for a 13-week recovery
period. The NOAEL was 2 mg/m3. Pulmonary changes consisting of
"subacute alveolitis", characterized by accumulations of alveolar
macrophages within alveolar lumens, accompanied by some polymorpho-
nuclear leukocytes were observed in rats treated at 20 and 100
mg/m3. It was conjectured that the alveolitis observed was not
treatment-related, but rather, a non-specific dust reaction of the
polymeric active ingredient artificially ground to particle size
capable of reaching the alveoli. Intra-alveolar pigment deposition
was recorded in a single male and female from the high-dose treated
group. There was no evidence of damage to bronchial or bronchiolar
epithelium in any of the treated groups. Mean lung/trachea weights
were increased in both sexes treated at the high dose. Other
changes associated with treatment were depositions of golden brown
granular pigment, similar to that seen in the lungs, within the
renal cortices in the high dose animals and decreased overall body-
weight gain in both males and females treated at 20 (11% and 14%)
and 100 mg/m3 (26% and 33% less than corresponding controls,
respectively). After the 13-week recovery, effects of treatment
persisted in the lungs and kidneys of previously treated animals.
No treatment-related findings were noted on survival,
ophthalmoscopy, haematology or blood chemistry. Tissue residue
analysis performed on the urine, plasma, liver, lung and thyroid
revealed measurable levels of metiram and ETU in the urine and lung
in rats treated at the mid- and high-dose levels (Ulrich, 1986).
Rabbits
A dermal toxicity study was performed with groups of 5 New
Zeeland white KFM rabbits/sex treated with Polyram DF formulation of
metiram on the shaved skin of their backs under occlusive conditions
for 6 hours/day at concentrations of 0 (vehicle control, distilled
water), 25, 50 or 250 mg/kg bw/day for a minimum of 21 consecutive
daily applications. Dermal administration of metiram resulted in
minimal to moderate exfoliation and ulcerative dermatitis in the
skin of rabbits treated at the high-dose level. Similar effects
were not evident at lower dose levels, indicating a NOAEL for local
irritation to the skin of 50 mg/kg bw/day. In the absence of
treatment-related effects on survival, clinical signs, body weights,
food consumption, ophthalmoscopy, haematology, blood chemistry,
organ weights or histopathology of the liver and kidneys, the NOAEL
for systemic toxicity was greater than 250 mg/kg bw/day (Ullmann et
al., 1987).
Dogs
Groups of 4 beagle dogs/sex were treated with metiram (96.8%
purity containing 2.2% ETU) for a period of 4 weeks at dietary
levels of 0, 100, 300, 600 or 900 ppm, equal to 0, 5, 14, 28 or 41
mg/kg bw/day for males and 0, 5, 15, 27 or 43 mg/kg bw/day for
females, respectively. Effects observed were an increased frequency
of micro-follicles in the thyroids associated with minimal depletion
of colloid and minimal hyperplasia of the follicular epithelium in
2/4 males and 2/4 females treated at the high dose of 900 ppm when
compared to the controls. Other changes recorded were significantly
increased liver weights in dogs receiving 600 and 900 ppm, which
were not associated with any correlative histopathological findings.
Significantly increased water consumption was recorded in dogs
treated at 100, 600 and 900 ppm but not at 300 ppm. The significance
of increased fluid intake (calculated over 5-day periods) was
difficult to ascertain since there were no corresponding changes
noted in urinalysis parameters. Erythrocyte, haemoglobin and packed
cell volume values, although significantly decreased in dogs treated
at 900 ppm when compared to the controls, were found to be within
normal limits of variability and therefore of doubtful toxicological
consequence. The NOAEL, based on the aforementioned findings, was
300 ppm, equal to 14 mg/kg bw/day. There were no significant
effects of treatment on survival, clinical signs, body weights, food
consumption, ophthalmoscopy, blood chemistry or urinalysis
(Chesterman et al., 1978).
Metiram (93.6% purity, ETU < 0.2%) was given to groups of 5
beagle dogs per sex at dietary concentrations of 0, 30, 80, 1000 or
3000 ppm, equal to 0, 0.9, 2.5, 29.8 or 76.9 mg/kg bw/day in males
and 0, 1.1, 2.7, 29.9 or 92.7 mg/kg bw/day in females, respectively,
for a period of 52 weeks. The principal effects of treatment were
manifest on the thyroid. Microscopic examination revealed thyroid
follicular hyperplasia in dogs of both sexes treated at 1000 and
3000 ppm. This finding was associated with an increased size and
weight of this organ at 3000 ppm. Gross necropsy revealed thyroid
thickening in several animals from both the control and treated
groups. This observation was, however, only correlated with
histopathological change at dietary levels of 1000 ppm and higher,
rendering the significance of thyroid thickening at lower levels
uncertain. Serum T4 levels were decreased in both sexes at 3000 ppm
and in males at 1000 ppm. A dose-related increased incidence of dogs
with focal hepatic lipofuscin pigment deposition was noted in both
sexes at 1000 ppm and higher. Other effects of treatment with
metiram were described as an increased incidence of diarrhoea in
dogs treated at 80 (attributed to single male), 1000 and 3000 ppm;
slight evidence of anaemia (both sexes at 1000 and 3000 ppm)
involving an increased number of reticulocytes (both sexes at 3000
ppm); decreased body-weight gain at 3000 ppm in males and to a
lesser extent in females; and decreased food intake in both sexes at
3000 ppm and in females at 1000 ppm. Significant changes in blood
chemical parameters at dietary levels of 1000 ppm and higher were
evident as increased lipid, cholesterol, triglycerides, phospholipid
levels, ALP activity, and total protein, and decreased albumin and
A/G ratio as well as disturbances in the protein electrophoretic
pattern. There were no effects of treatment on survival,
neurological parameters, ophthalmoscopy or urinalysis. The NOAEL
for this study was 80 ppm, equal to 2.5 mg/kg bw/day (Corney et
al., 1991).
Monkeys
A preliminary toxicity assessment was performed with groups of
single male and female rhesus monkeys administered metiram (96.8%
purity containing 2.2% ETU) orally by gavage as a suspension in 0.5%
mucilage of tragacanth in water at dose levels of 50, 100 or 500
mg/kg bw/day for a period of 4 weeks. Treatment-related toxicity
was evident as salivation at the time of dosing or immediately
thereafter and occasional emesis in monkeys treated at 100 and 500
mg/kg bw/day, body-weight loss in the single female treated at 500
mg/kg bw/day and changes of the thyroid, representative of early
follicular hyperplasia in the single high-dose female. The low dose
of 50 mg/kg bw/day was well tolerated with no overt signs of
toxicity (Sortwell et al., 1977).
Metiram (96.8% purity containing 2.2% ETU) was administered
orally by gavage to groups of 4 rhesus monkeys/sex for a period of
26 weeks at dose levels of 0 (vehicle control, 0.5% tragacanth in
distilled water), 5, 15 or 75 mg/kg bw/day. After termination of
treatment, 1 male and 1 female were retained untreated for a period
of 15 weeks as recovery animals. In a separate, but concurrently
conducted study, a further 2 male and 2 female monkeys were treated
with metiram at doses of 0, 5 or 75 mg/kg bw/day for 26 weeks to
monitor thyroid function. The primary effects of treatment with
metiram were evident in the thyroid and were characterized at dose
levels of 15 and 75 mg/kg bw/day as minimal thyroid follicular
hyperplasia, significantly decreased T3 and T4 levels and
increased thyroid weights. After a 15-week recovery, morphological
changes of the thyroid were still apparent in monkeys previously
treated at the 15 and 75 mg/kg bw/day dose levels. Assessment of
thyroid function revealed an initial marked reduction in iodine
uptake at dose levels of 5 and 75 mg/kg bw/day, followed by a
significant increase in uptake during the latter part of the study.
In the absence of any correlation to thyroid hormone levels or
morphological alterations, no toxicological significance was
attributed to fluctuations in iodine uptake by the thyroid at the
lowest level of 5 mg/kg bw/day. Other effects resulting from
treatment were an increased incidence of salivation occurring at the
time of dosing at the high dose. Significantly increased liver
weights at the high-dose level were not associated with any
histopathological findings. Treatment with metiram did not
significantly affect survival, body-weight gains, food or water
consumption, ophthalmoscopy, haematology, urinalysis or examination
of bone marrow smears. The NOAEL was 5 mg/kg bw/day (Sortwell et
al., 1979).
Long-term toxicity/carcinogenicity studies
Mice
Dietary administration of metiram (96.8% purity containing 2.2%
ETU) to groups of 52 CFLP mice/sex at levels of 0, 100, 300 or 1000
ppm, equal to 0, 8, 24 or 79 mg/kg bw/day for males and 0, 9, 29 or
95 mg/kg bw/day for females, respectively, for a minimum period of
88 weeks resulted in a NOAEL for in-life parameters of 300 ppm,
equal to 24 mg/kg bw/day. Although there were no statistically
significant differences in mortality in the treated groups, a
marginally higher mortality incidence from week 40 onwards was
recorded in females treated at 1000 ppm, resulting in a minimum 25%
survival rate being reached in this group before the corresponding
controls. Group mean body weights were significantly decreased in
both sexes treated at the 1000 ppm dietary level. Food consumption
in males treated at 1000 ppm was only marginally (5%) lower than the
corresponding controls during the first year of treatment. Clinical
signs and examination of bone marrow smears were not adversely
affected by treatment with metiram. Under the conditions of the
present study, treatment with metiram at dietary levels up to and
including 1000 ppm failed to uncover any evidence of carcinogenic
potential (Hunter et al., 1979).
Rats
Groups of 50 Sprague-Dawley CD rats/sex were administered
metiram (96.8% purity containing 2.2% ETU) in the diet at levels of
0, 5, 20, 80 or 320 ppm, equal to 0.2, 0.8, 3.1 or 12.3 mg/kg bw/day
in males and 0, 0.2, 1.0, 3.8 or 15.5 mg/kg bw/day in females,
respectively, for 119 weeks for males and 111 weeks for females.
Satellite groups of 30 male and 30 female rats per group were used
for blood sampling and thyroid function tests and were then killed
after 102 weeks of treatment. Treatment with metiram resulted in a
NOAEL of 80 ppm (equal to 3.1 mg/kg bw/day), based on an increased
incidence of rats treated at the next higher dietary level of 320
ppm with muscular atrophy. There were no consistent treatment-
related effects with respect to survival, clinical signs, body
weights, food or water consumption, ophthalmoscopy, haematology,
blood chemistry, thyroid function, urinalysis or organ weights.
There was no evidence of metiram-induced carcinogenic potential
(Hunter et al., 1981).
Reproduction studies
Rats
A three-generation (two-litter per generation) reproduction
study was conducted with groups of 12 male and 24 female Crl:COBS
CD(SD)BR rats fed metiram (96.8% purity containing 2.2% ETU) at
dietary levels of 0, 5, 40 or 320 ppm, equal to 0, 0.2, 1.8 or 14.2
mg/kg bw/day in males and 0, 0.3, 2.3 or 19.8 mg/kg bw/day in
females, respectively. In the F0 generation, treatment with
metiram commenced at least 60 days prior to mating. In the F1 and
F2 generations, the F1b and F2b derived parental animals,
respectively were exposed to the test material for a minimum period
of 90 days. At day 21 post-partum, 10 F3a pups/sex from each group
were selected for detailed gross examination, recording of organ
weights and histopathological assessment. At the second pairing of
the F2b generation, all pregnant females from each of the dose
levels were sacrificed on day 20 of gestation for teratological
examination. Appearance of spermatozoa in the vaginal smear or the
presence of a copulation plug was considered to be day 0 of
gestation. Decreases in body-weight gain (4-7%) were noted in both
the F0 and F1 generation males and females treated at 320 ppm when
compared to the controls. Slightly depressed (7-8%) food intake was
observed in males fed metiram during the F0 and F1 generations.
Body weights and food consumption were not adversely affected by
treatment at lower dietary levels or in any of the treated groups
from the F2 generation when compared to the controls. There were
no treatment-related effects on parental mortality, clinical signs,
food conversion ratios, reproductive parameters or gross
pathological alterations. Statistically significant decreases in
the mean number of pups born were observed in all treated groups
during the second mating of both the F1 and F2 generations.
Although the differences were statistically significant, there was
no dose-response relationship and all values in the treated groups
generally fell within the reported laboratory standard values.
Total litter loss was significantly decreased at 320 ppm during the
second mating of the F1 generation. In the absence of similar
effects in the first mating of the F1 or in either of the matings
in the F0 and F2 generations, the toxicological significance of
this finding was dubious. Statistically significant decreases in
mean litter weights were observed in the treated groups during the
F1 and F2 generations. Since the mean pup weights in the treated
groups were comparable to the controls, the decreased mean litter
weights may, in part, be attributed to the correspondingly smaller
litter sizes observed in these groups. With regard to the
teratological component, there were no effects on pregnancy rate,
pre- or post-implantation loss, number of live fetuses, embryo/fetal
resorptions, fetal sex ratio or fetal/litter weights. Metiram was
not teratogenic as determined by gross, skeletal and visceral
examination of fetuses, following in utero exposure at dietary
levels as high as 320 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg
bw/day, based on decreased parental body weight and food consumption
recorded in the F0 and F1 generations treated at 320 ppm (Cozens
et al., 1981).
Special studies on teratogenicity
Rats
A preliminary range-finding study was performed with groups of
6 non-pregnant Crl:Cobs CD(SD)BR female rats administered metiram
(96.8% purity containing 2.2% ETU) orally by gavage at 0 (vehicle
control, sodium carboxymethyl cellulose), 150, 300, 600 or 1200
mg/kg bw/day for 10 consecutive days. Dose-related clinical effects
primarily affecting the nervous system were observed at all dose
levels. In the absence of microscopic examination,
histopathological correlation could not be ascertained. Other
effects of treatment were related to decreased body-weight gains at
all dose levels, depressed food intake at 300 mg/kg bw/day and
higher and decreased water consumption in rats treated at 600 and
1200 mg/kg bw/day (Palmer & Simmons, 1979a).
Groups of 20 time-mated female Crl:Cobs CD(SD)BR rats were
treated with metiram (96.8% purity containing 2.2% ETU) daily by
gavage during days 6 to 15 of gestation, inclusive, at dose levels
of 0 (vehicle control, sodium carboxymethyl cellulose), 40, 80 or
160 mg/kg bw/day. The day of mating, determined by the presence of
a vaginal plug or sperm in the vaginal smear, was designated as day
0 of gestation. Decreased body-weight gain (4-9%) was noted in the
high-dose animals. There were no significant effects with respect
to clinical signs, food or water consumption or gross organ/tissue
changes. A slight increase in the pre- and post-implantation loss
was recorded in the 160 mg/kg bw/day group, in conjunction with a
significant decrease in the mean number of live fetuses and
decreased litter weight in this group. It is unlikely that the
increased pre-implantation loss at the high dose was related to
treatment, since theoretically, treatment was not initiated until
after implantation occurred. Nevertheless, the combined effect of
pre- and post-implantation loss may have, to a certain degree,
influenced the decreased litter size and weight noted at the high
dose level. There were no effects of treatment on the incidence of
early or late resorptions, mean fetal weight or sex ratio. The
NOAEL for embryo/fetotoxicity was 80 mg/kg bw/day, based on slight
decreases in litter size and weights. Treatment with metiram failed
to uncover any evidence of teratogenic potential. The NOAEL for
maternal toxicity was 80 mg/kg bw/day, based on decreased body-
weight gain (Palmer & Simmons, 1979a).
Rabbits
In a range-finding study, groups of 3 pregnant Himalayan
rabbits were treated orally by gavage with metiram (97.9% purity,
ETU <0.2%) during days 7 through 19 of gestation at dose levels
of 0 (vehicle control, distilled water with 0.5% carboxymethyl
cellulose), 50, 100 or 200 mg/kg bw/day. Reduced body-weight gains
and food intake were recorded at the two highest dose levels. The
significance of the incidence of single abortion at each of the dose
levels, upon conduct of the definitive study, was confirmed to be
treatment-related. No further relevant details were provided
(Gelbke et al., 1988).
Metiram (97.9% purity, ETU < 0.2%) was administered orally
by gavage at 0 (vehicle control, distilled water with 0.5%
carboxymethyl cellulose), 10, 40 or 120 mg/kg bw/day to groups of 15
artificially inseminated Himalayan rabbits on days 7 through 19 of
gestation. The day of artificial insemination was designated as day
0 of gestation. Treatment with metiram elicited signs of maternal
toxicity at 40 and 120 mg/kg bw/day, denoted by abortion, decreased
body weight and food consumption, the latter associated with
reduction or absence of defecation. The high incidence of abortion
(8/15) recorded at 120 mg/kg bw/day, in conjunction with a single
death culminated in only 6/15 rabbits treated at this level with
litters available for examination. Although 2 of 15 animals aborted
at 40 mg/kg bw/day, there were, nevertheless, adequate numbers of
litters secured in the control, 10 and 40 mg/kg bw/day dose groups.
Since a dose level of 10 mg/kg bw/day was without significant
effects, this dose was considered the NOAEL for maternal toxicity.
The NOAEL for developmental toxicity was 40 mg/kg bw/day based on
the slight decrease in mean fetal weights recorded at 120 mg/kg
bw/day. A slight decrease in the number of live male fetuses in the
120 mg/kg bw/day group was considered to be of no toxicological
consequence, in light of the comparable number of female fetuses and
the total number of fetuses in the 120 mg/kg bw/day group relative
to the controls. There were no significant effects of treatment on
the mean number of live fetuses, early or late resorptions, post-
implantation loss, or placental weight. There was no evidence
suggestive of teratogenic potential with metiram at any of the dose
levels investigated (Gelbke et al., 1988).
Special studies on genotoxicity
Results of mutagenicity assays conducted with metiram are
presented in Table 3. The tests conducted to evaluate potential for
gene mutation in bacteria and induction of chromosomal aberration in
rat bone marrow were negative. Unscheduled DNA synthesis in rat
hepatocytes and a dominant lethal study in mice were also negative.
Although metiram demonstrated potential for inducing sister
chromatid exchange (SCE) in Chinese hamster ovary cells in the
absence and presence of mouse microsomal activation, there was
nevertheless, no significant induction of SCE observed with rat
microsomal activation. Moreover, an in vivo SCE assay in bone
marrow cells of the Chinese hamster was negative. Metiram exhibited
a weak promotion activity in mouse embryo fibroblasts in the absence
of a direct transformation response. The Meeting concluded that
metiram was not genotoxic.
Special studies on irritation and sensitization
The eye and skin irritation potential of technical metiram
(containing 2.2% ETU) were investigated in six Vienna white rabbits.
There were no signs of irritation when metiram was applied to the
external ear for 20 hours or to the backs for 1, 5 or 15 minutes.
When applied to the back for 20 hours, slight reddening occurred 24
hours after application with subsequent scaling after 8 days.
Metiram (50 mg) when introduced into the conjunctival sac of the eye
of these rabbits produced slight redness and edema after 1 hour,
with no signs of irritation after 8 days (Zeller, 1985a).
Polyram combi, a formulation of technical metiram, was
administered to eight Vienna white rabbits to determine skin and eye
irritation potential. A 50% aqueous suspension of the test material
when applied to the backs of the rabbits for a period of 20 hours,
resulted in slight erythema of the skin, which was reversible within
8 days. The instillation of 50 mg of Polyram combi to the
conjunctival sac of the rabbit eye caused redness and edema which
were reversible after 8 days. When 50 µl of a 20% aqueous
suspension was applied, slight reddening of the mucosa occurred,
whereas 50 µl of a 2% aqueous suspension was tolerated without any
irritation (Zeller, 1985b).
Metiram technical demonstrated severe sensitizing effects on
the skin of female Pirbright white, Dunkin Hartley guinea-pigs when
tested according to the methods of the Magnusson & Kligman
maximization test (Jackh, 1982).
Table 3. Genotoxicity of metiram
Test Test system Concentration Purity Results Reference
Reverse mutation S. typhimurium 0, 1, 3, 10, 31, Technical negative Oesch, 1977
(in vitro) TA 98, 100, 1537 100, 310, 1000, 1., 2. (rat)
2000 µg/plate
S. typhimurium 0, 1, 10, 50, 100, Technical + 2.2% ETU negative Gelbke & Engelhardt,
TA 98, 100, 1535, 1537 500, 2500 µg/plate 1., 2. 1985
(rat & mouse)
Reverse mutation/host S. typhimurium 500, 1670, Technical + 2.2% ETU negative Jagannath & Myhr, 1985
mediated (in vivo) TA 1530 5000 mg/kg bw
Male CD-1 mice (single oral doses)
(inoculated ip)
Point mutation HGPRT locus of Chinese 0, 0.681, 1.0, 4.64, Technical + 2.2% ETU negative, 1. Gelbke & Jackh, 1985
(in vitro) hamster ovary (CHO) cell 6.81, 10, 46.4, equivocal, 2.
line, K1 68.1, 100 µg/ml (rat)
HGPRT locus of Chinese 0.5, 1, 5, 10, 50, Premix 95% negative Hoffman & Jackh, 1990
hamster ovary (CHO) cell 100, 500 µg/ml 1., 2. (mouse)
line, K1
SCE (in vitro) Chinese hamster ovary 0, 40, 60, 80, 100, Technical + 2.2% ETU positive, 1. Ivett & Myhr, 1986a
CHO, WB1 cells 125, 150, 175, 200 positive, 2.
µg/ml (mouse)
negative, 2.
(rat)
SCE (in vivo) Chinese hamster, LMP 1000, 3330, 10 000 Premix 95% negative Miltenburger et al.,
stock (bone marrow) mg/kg bw 1990
Table 3 (contd)
Test Test system Concentration Purity Results Reference
Chromosome aberration Rat, male Fischer 344 0, 0.24, 1.2, 2.4 Technical + 2.2% ETU negative Ivett & Myhr, 1986b
(in vivo) (bone marrow) g/kg bw (acute) 0,
0.02, 0.1, 0.2 g/kg
bw (5 repeated oral
doses)
Transformation Mouse embryo fibroblasts 0, 0.1, 0.25, 0.5, Technical + 2.2% ETU transformation: Tu et al., 1985
promotion (in vitro) C3H-10T 1/2 (clone 8) 0.75, 1.0 µg/ml negative
cell system promotion
activity:
weakly
positive
Unscheduled DNA Rat (Fischer 344, male) 0.492, 1.23, 2.46, Technical + 2.2% ETU negative Cifone & Myhr, 1984
synthesis (in vitro) hepatocytes 4.92, 12.3, 24.6,
49.2, 160 µg/ml
Dominant lethal Mouse, CD-1 male 0, 600, 1200, 2400 Technical + 2.2% ETU negative Palmer & Simons, 1979b
(in vivo) mg/kg bw/day
Results:
1. = in the presence of metabolic activation derived from the indicated species
2. = in the absence of metabolic activation
COMMENTS
Metiram was incompletely absorbed when administered orally to
rats. Elimination was primarily via the faeces, with minimal
biliary excretion. Comparatively higher urinary excretion at low
doses suggested that metiram may be more poorly absorbed at higher
doses. Highest residual tissue levels were found in the thyroid and
kidney, with slightly higher concentrations present in females than
in males. Comparison of tissue residues after single or multiple
doses suggested slight accumulation in the body with multiple
dosing.
The metabolism of metiram has not been completely elucidated.
In the rat, the predominant urinary components were polar and were
identified as ethylenediamine, N-acetyl-ethylenediamine,
ethanolamine, oxalic acid and glycine. Major, less polar components
were ethyleneurea, ETU and EBIS.
Metiram was practically non-toxic upon acute oral, dermal and
inhalation administration to rats. WHO has classified metiram as
unlikely to present acute hazard in normal use.
The principal target organ upon repeated dietary exposure to
metiram was the thyroid.
Mice treated with metiram for three months at 0, 300, 1000,
3000 or 7500 ppm in the diet revealed minimal to slight hypertrophy
and vacuolation of the thyroid follicular epithelium in both sexes
at levels of 3000 ppm and higher. A NOAEL of 300 ppm (equal to 84
mg/kg bw/day) was based on decreased serum T4 levels in both sexes
at levels of 1000 ppm and above.
Thirteen-week dietary administration of metiram to SD CFY rats
at 0, 50, 100, 300 or 900 ppm revealed a NOAEL of 100 ppm (equal to
6 mg/kg bw/day) based on decreased serum T4 levels and increased
thyroid weights at dietary levels of 300 and 900 ppm. Slight to
minimal hyperplasia of the thyroid was observed at 900 ppm.
Although reduced iodine uptake by the thyroid was observed at all
dietary levels, these changes were shown to be reversible following
cessation of treatment. At the lowest dietary levels of 50 and 100
ppm, the effects on iodine uptake were not correlated with changes
in thyroid hormone levels or any overt morphological alterations of
the thyroid gland, thus rendering the toxicological significance of
this finding doubtful.
In a recently conducted three-month study with Wistar rats
receiving metiram at 0, 5, 80, 320 or 960 ppm in the diet, decreased
serum T4 levels and increased thyroid weights were observed at 960
ppm. Slight evidence of anaemia was observed at 320 ppm, indicating
a NOAEL of 80 ppm (equal to 5.8 mg/kg bw/day).
Other effects of treatment in the diet with metiram in the rat
were manifest as hind limb paralysis with corresponding atrophy of
muscle fibres. In the 13-week study with SD CFY rats, microscopic
changes in muscle fibres at levels of 300 ppm (equal to 20 mg/kg
bw/day) and higher were still prevalent in previously treated rats
after the 6-week recovery period. Muscular atrophy was observed in
a long-term study in SD CD rats treated at the highest level of 320
ppm (see below). General muscle weakness/ataxia and reduced grip
strength of the limbs with no histopathological consequences were
observed in the 3-month study with Wistar rats fed metiram at the
highest level of 960 ppm.
A 52-week study in dogs at dietary levels of 0, 30, 80, 1000 or
3000 ppm yielded a NOAEL of 80 ppm (equal to 2.5 mg/kg bw/day) based
on thyroid follicular hyperplasia with increased size, thickening
and weight of this organ, in conjunction with decreased serum T4
levels at dietary levels of 1000 ppm and above. Other effects
recorded at 1000 ppm and above were a dose-related increased
incidence of focal hepatic lipofuscin pigment deposition, slight
evidence of anaemia, diarrhoea and changes in blood biochemical
parameters. A preliminary 4-week study in dogs uncovered an
increased frequency of microfollicles in the thyroid, in association
with colloid depletion and minimal hyperplasia in both sexes treated
at the highest level of 900 ppm (equal to 41 mg/kg bw/day).
Metiram given by gavage to rhesus monkeys at dose levels of 0,
5, 15 or 75 mg/kg bw/day for a period of 26 weeks indicated a NOAEL
of 5 mg/kg bw/day based on significantly decreased serum T3 and T4
levels, increased thyroid weights and minimal thyroid follicular
hyperplasia at 15 and 75 mg/kg bw/day. Morphological changes of the
thyroid were still apparent after a 15-week recovery period. In the
absence of any correlation between thyroid hormone levels and
morphological alterations, no significance was attributed to
fluctuations in iodine uptake by the thyroid recorded at 5 mg/kg
bw/day.
Long-term dietary treatment of mice with metiram at 0, 100, 300
or 1000 ppm resulted in a NOAEL of 300 ppm (equal to 24 mg/kg
bw/day) based on decreased body weights recorded at 1000 ppm.
Chronic dietary administration of metiram to SD CD rats at 0,
5, 20, 80 or 320 ppm revealed muscular atrophy at 320 ppm (equal to
12 mg/kg bw/day), with a NOAEL of 80 ppm (equal to 3.1 mg/kg
bw/day).
Metiram was not carcinogenic when fed to mice or rats at
dietary levels of up to 1000 and 320 ppm, respectively.
A three-generation, two litter per generation reproduction
study in rats treated at 0, 5, 40 or 320 ppm in the diet failed to
reveal any adverse effects on reproductive parameters. The NOAEL
was 40 ppm (equal to 1.8 mg/kg bw/day), based on decreased parental
body weight and food consumption recorded in the F0 and F1
generations treated at 320 ppm.
Metiram when administered to pregnant rats at 0, 40, 80 or 160
mg/kg bw/day or rabbits at 0, 10, 40 or 120 mg/kg bw/day during
critical periods of organogenesis was not teratogenic at any dose.
The NOAEL for maternal toxicity in the rat was 80 mg/kg bw/day based
on decreased body-weight gain and in the rabbit the NOAEL was 10
mg/kg bw/day based on increased abortions, decreased body weights
and food consumption. The NOAELs for embryo/fetotoxicity were 80
mg/kg bw/day in the rat based on slight decreases in litter size and
weight and 40 mg/kg bw/day in the rabbit based on decreases in mean
fetal weights.
Metiram has been tested in a series of in vitro and in vivo
genotoxicity assays. The Meeting concluded that metiram is not
genotoxic.
The Meeting allocated an ADI of 0-0.03 mg/kg bw based on a
NOAEL of 2.5 mg/kg bw/day in the 52-week study in dogs, using a 100-
fold safety factor. This ADI is supported by the NOAEL of 3.1 mg/kg
bw/day observed in the long-term study in rats. This ADI served as
the basis for a group ADI that was established for metiram alone or
in combination with mancozeb, maneb, and zineb.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 300 ppm, equal to 24 mg/kg bw/day (88-week study)
Rat: 80 ppm, equal to 3.1 mg/kg bw/day (111-week study)
40 ppm, equal to 1.8 mg/kg bw/day
(reproduction study)
Rabbit: 10 mg/kg bw/day (teratogenicity study)
Dog: 80 ppm, equal to 2.5 mg/kg bw/day (52-week study)
Monkey: 5 mg/kg bw/day (26-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, maneb, and
zineb).
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Chesterman, H., Heywood, R. & Barker M. (1973) Polyram Combi acute
oral toxicity study in beagle dogs. Report BASF 73/0047, BASF
40/73703. Unpublished report dated October 5, 1973 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Chesterman, H., Heywood, R., Ball, S., Street, A. & Prentice, D.
(1978) Metiram (active ingredient) oral toxicity study in beagle
dogs (dietary intake for 4 weeks). Report BASF 78/0154, BSF
201/76422. Unpublished report dated April 26, 1978 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Cifone, M. & Myhr, B. (1984) Report on the evaluation of metiram
tech. in the rat primary hepatocyte unscheduled DNA synthesis assay.
Report RZ 84/209. Unpublished report dated July 5, 1984 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Corney, S.J., Allen, T.R., Janiak, T. & Springall, C. (1991) 52-week
oral toxicity (feeding) study with metiram premix 95% in the dog.
Report No. 91/10786. Unpublished report dated August 22, 1991 from
RCC, Research & Consulting Company Ltd., Itingen, Switzerland.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Cozens, D., Simons, R., Clark, R., Offer, J. & Gibson, W. (1981)
Effect of metiram technical on reproductive function of multiple
generations in the rat. Report RZ 81/132, BSF 200/80692. Unpublished
report dated March 18, 1981 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Gelbke, H. & Engelhardt, G. (1985) Report on the study of metiram
(techn.) in the Ames test. Report RZ 85/020. Unpublished report
dated February 7, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H. & Jackh, R. (1985) Report on a point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram. Report RZ 85/238. Unpublished report dated July 30, 1985
from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Gelbke, H., Hellwig, J. & Hildebrand, B. (1988) Report on the study
of the prenatal toxicity of metiram-premix 95% in rabbits after oral
administration (gavage). Report BASF 88/0154 and Supplement BASF
88/0262. Unpublished report dated May 26, 1988 from BASF, Department
of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. and Hildebrand, B. (1992a) Study of the oral
toxicity of metiram premix 95% in B6C3F1 mice. Administration in
the diet for 3 months. Report No. 92/11223. Unpublished report dated
October 16, 1992 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. & Hildebrand, B. (1992b) Study of the oral
toxicity of metiram premix 95% in Wistar rats. Administration in the
diet for 3 months including the examination of neurotoxicology
(neurofunctional observational battery). Report No. 92/11224.
Unpublished report dated October 16, 1992 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1979) Study of the acute dermal toxicity of "Metiram
tech. with 2% ETU" on the rat (Translation). Report RZ 79/032.
Unpublished report dated September 25, 1979 from BASF,
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1985) Report on the study of the acute dermal
toxicity of Polyram Combi (BAS 222 01 F) in the rat (Translation of
the original report dated September 25, 1979). Report RZ 85/026.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Girkin, R. & Jackson R. (1984) Report on the
study of dermal absorption of metiram in rats. Report RZ 85/158, HRC
BSF 411/84694. Unpublished report dated October 10, 1984 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Midgley, I., Biggs, S. & McCay, C. (1985)
The biokinetics and metabolism of 14C-metiram in the rat. Report
BASF 85/0470, HRC BSF 410/85720. Unpublished report dated October
28, 1985 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1974a) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF, Gewerbehygiene
und Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hofmann, H. (1974b) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF
Aktiengesellschaft, FRG.
Hofmann, H. & Munk, R. (1975) Acute intraperitoneal toxicity of the
technical active ingredient metiram to the rat. Report RZ 75/006.
Unpublished report dated June 4, 1975 from BASF, Gewerbehygiene und
Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1975) Acute oral toxicity of the technical active
ingredient metiram to the rat. Report RZ 75/005. Unpublished report
dated June 4, 1975 from BASF, Gewerbehygiene und Toxikologie, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1985a) Report on the study of the acute oral toxicity of
Polyram Combi, technical active ingredient, in the rat (Translation
of original report dated March 25, 1974). Report BASF 85/0485.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985b) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi, technical active
ingredient, in the rat (Translation of original report dated March
25, 1974). Report RZ 85/028. Unpublished report dated February 4,
1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985c) Report on the study of the acute oral toxicity
of Polyram Combi (BAS 222 01 F) to rats (Translation of original
report dated March 25, 1974). Report RZ 85/121. Unpublished report
dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985d) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi (BAS 222 01 F) in rats
(Translation of original report dated March 25, 1974). Report RZ
85/124. Unpublished report dated April 12, 1985 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985e) Report on the study of the acute inhalation
toxicity of the spray of a 5% aqueous suspension of Polyram Combi
(BAS 222 01 F) to guinea-pigs (Translation of original report dated
March 25, 1974). Report RZ 85/125. Unpublished report dated April
12, 1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein,
FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. & Jackh, R., 1990. Report on the point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram premix 95% with B6C3F1 mice microsomal fraction. Report
No. 90/0285. Unpublished report dated August 2, 1990 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A. & Prentice, D.
(1976a) Preliminary assessment of metiram toxicity to rats in
dietary administration for four weeks followed by a two and a four
week withdrawal period. Report RZ 76/024, BSF 172/76245. Unpublished
report dated October 20, 1976 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hunter, B., Bridges, J. & Prentice, D. (1976b) Preliminary
assessment of metiram toxicity to mice in dietary administration for
4 weeks. Report RZ 76/014, BSF 170/76108. Unpublished report dated
June 7, 1976 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A., Prentice, D. &
Offer, J. (1977) Metiram toxicity to rats in dietary administration
for 13 weeks followed by a 6 week withdrawal period (final report).
Report RZ 77/043, BSF/197/77612 and Addendum BASF 87/0205, BSF
197/8713. Unpublished report dated November 16, 1977 and addendum
dated May 29, 1987 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Prentice, D. & Gregson, R., 1979. Metiram
tumorigenicity to mice in long term dietary administration (final
report). Report RZ 79/033, BSF 198/78265. Unpublished report dated
June 5, 1979 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Street, A., Heywood, R., Prentice, D.,
Offer, J. & Gibson, W. (1981) Metiram toxicity and tumorigenicity in
prolonged dietary administration to the rat. Report RZ 81/280, BSF
199/80391, WNT No. 77/951 and Addendum BASF 89/0001. Unpublished
report dated May 7, 1981 and addendum date December 19, 1988 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986a) Report on the mutagenicity evaluation
of metiram in an in vitro sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells/amended final report. Report RZ
86/082. Unpublished report dated March 11, 1986 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986b) Report on the mutagenicity evaluation
of metiram technical K38/33A in the rat bone marrow cytogenetic
assay. Report RZ 86/265. Unpublished amended report dated August 4,
1986 from Litton Bionetics, Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jackh, R. (1981) Report on the study of the acute oral toxicity of
metiram technical grade in the rat (Translation). Report BASF
92/10669. Unpublished report dated July 9, 1981 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Jackh, R. (1982) Report on the study of the sensitizing effect of
metiram techn. in the guinea-pig -maximization test (Translation of
original report dated August 21, 1981). Report RZ 82/068.
Unpublished report dated February 16, 1982 from BASF, Department of
Toxicology, Ludwigshafen, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jagannath, D. & Myhr, B. (1985) Report on the mouse host-mediated
assay of metiram tech. K38/33A. Report RZ 85/210. Unpublished report
dated June 28, 1985, from Litton Bionetics Inc., Maryland, USA.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Klimisch, H.J. & Zeller, H. (1980) Report on the determination of
the acute inhalation toxicity LC50 metiram techn. with 2% ETU as a
dust aerosol after 4-hour exposure in Sprague-Dawley rats
(Translation of original report dated September 15, 1980). Report RZ
83/064. Unpublished report dated September 15, 1980 from
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Leuschner, F. (1979a) The acute oral toxicity of the preparation
metiram, techn. agent with 2% ETU in rats. Report BASF 79/0161, WNT-
Nr. 77/951. Unpublished report dated August 20, 1979 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Leuschner, F. (1979b) The acute intraperitoneal toxicity of the
preparation metiram, techn. agent with 2% ETU in mice. Report BASF
79/0162, WNT-Nr. 77/951. Unpublished report dated August 20, 1979
from Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Miltenburger, H., Volkner, W. & Heidemann, A. (1990) Sister
chromatid exchange assay in bone marrow cells of the Chinese hamster
with metiram premix 95%. Report No. 90/0569. Unpublished report
dated September 18, 1990 from CCR, Cytotest Cell Research Gmbh &
Co., Rossdorf, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Oesch, F. (1977) Ames test for metiram. Report RZ 77/027.
Unpublished report dated August 22, 1977 from the Institute of
Pharmacology, University of Mainz, Germany. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Palmer, A. & Simons, R. (1979a) Effect of metiram technical on
pregnancy of the rat. Report RZ 79/065, BSF 302/79616. Unpublished
report dated August 3, 1979 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Palmer, A. & Simons, R. (1979b) Dominant lethal assay of metiram
technical in the male mouse. Report RZ 79/069, BSF 304/79860.
Unpublished report dated November, 1979 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Heywood, R., Allen, D., Prentice, D. & Cherry, C.
(1977) Metiram preliminary oral toxicity study in rhesus monkeys.
Repeated dosage for 4 weeks. Report BASF 77/0149, BSF 265/77264.
Unpublished report dated April 15, 1977 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Allen, D., Heywood, R. & Street, A. (1979) Metiram
(containing 2.2% ethylenethiourea) oral toxicity study in rhesus
monkeys (repeated dosage for 26 weeks with recovery period). Final
report. Report BASF 79/0082, BSF 267/78263. Unpublished report dated
January 15, 1979 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Tu, A., Sivak, A., Breen, P. & Hatch, K. (1985) Report on the
evaluation of metiram in the C3H-10T 1/2 cell system for
transformation and promotion activities. Report RZ 85/209.
Unpublished report dated June 18, 1985 from Arthur D. Little, Inc.,
Maryland, USA. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ullmann, L., Sacher, R., Porricello, T., Luetkemeier, H., Vogel, W.,
Vogel, O., Wilson, J. & Terrier, H. (1987) Report on the subacute
21-day repeated-dose dermal toxicity study with polyram DF in
rabbits. Report BASF 87/0260, ZNT No. 86/314. Unpublished report
dated June 24, 1987 from RCC, Research and Consulting Company AG,
Switzerland. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ulrich, C. (1986) Report on the thirteen week subchronic inhalation
toxicity study on metiram in rats. Report RZ 86/407 and addendum
BASF 87/0414. Unpublished report dated December 31, 1986 and
addendum dated October 7, 1987 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. (1985a) Report on the study of the irritation of Polyram
Combi, technical active ingredient, to the skin and eye of rabbits
(Translation of original report dated March 25, 1974). Report RZ
85/027. Unpublished report dated February 4, 1985 from BASF,
Department of Toxicology, Ludwigshafen, FRG. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Zeller, H. (1985b) Report on the study of the irritation of Polyram
Combi (BAS 222 01 F) to the skin and eyes of rabbits (Translation of
original report dated March 25, 1974). Report RZ 85/122. Unpublished
report dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
 Table 1. Acute toxicity of technical metiram
Species Sex Route (vehicle) LD50 Test material Reference
(strain) (mg/kg bw)
Rat M+F oral > 6810 Technical grade Jackh, 1981
(SD) (distilled water) (purity unknown)
Rat M+F oral M: 9100 Technical grade Leuschner, 1979a
(SD) (HPMC) F: 8900 (purity unknown) + 2% ETU
M+F: 9000
Rat M+F oral 6500 Technical grade Hofmann, 1985a
(SD) (tragacanth) (purity unknown)
Rat M+F oral 10 000 Technical grade Hofmann, 1975
(SD) (CMC) (purity unknown)
Rat (SD) M+F dermal (water) > 2000 Technical grade Grundler, 1979
(77.5% purity) + 2% ETU
Mouse (NMRI) M+F intraperitoneal M: 208 Technical grade Leuschner, 1979b
(HPMC) F: 215 (purity unknown) + 2% ETU
M+F: 212
Mouse (NMRI) M+F intraperitoneal 80 Technical grade Hofmann, 1974a
(tragacanth) (purity unknown)
Rat (SD) M+F intraperitoneal M: 318 Technical grade Hofmann & Munk, 1975
(CMC) F: 317 (77.5% purity)
M+F: 318
Rat (SD) M+F inhalation 4 h > 5.7 (mg/l) Technical grade Klimisch & Zeller, 1980
(77.5% purity) + 2% ETU
Rat (SD) M+F inhalation 8 h (well Technical grade Hofmann, 1985b
tolerated) (purity unknown) + 2.2% ETU
Table 2. Acute toxicity of Polyram combi (formulated product)
Species Sex Route LD50 Test Reference
(strain) (vehicle) (mg/kg bw) material
Rat M+F oral > 10 000 Polyram combi Hofmann, 1985c
(SD) (tragacanth) (purity unknown)
Dog M+F oral * Polyram combi Chesterman et al., 1973
(beagle) (water) (purity unknown)
Mouse (NMRI) M+F intraperitoneal approx 80 Polyram combi Hofmann, 1974b
(tragacanth) (purity unknown)
Rat M+F dermal > 2000 Polyram combi Grundler, 1985
(SD) (77.5% purity)
Rat M+F inhalation, (well Polyram combi Hofmann, 1985d
(SD) 8 hr tolerated) (77.5% purity)
Guinea-pig inhalation, >0.8 (mg/L) Polyram combi Hofmann, 1985e
8 hr (purity unknown)
* Estimation of LD50 precluded due to high incidence of vomiting
oral and dermal administration, were practically non-toxic in the
rat with LD50 values of > 6500 mg/kg bw and > 2000 mg/kg bw,
respectively. Moderate acute toxicity was demonstrated upon
intraperitoneal administration of technical material in the mouse
(LD50 = 80-212 mg/kg bw) and rat (LD50 = 318 mg/kg bw). Technical
metiram when administered by the inhalation route resulted in an
LC50 value greater than 5.7 mg/l in the rat. Eight-hour inhalation
of the formulated material, Polyram combi was well tolerated in both
rats and guinea-pigs. Intentional spiking of the technical material
with ETU at levels of 2 to 2.2% did not markedly alter the acute
toxicity profile.
Short-term toxicity studies
Mice
In a preliminary assessment of the toxicity of metiram (96.8%
purity, containing 2.2% ETU), groups of 8 CFLP mice per sex were fed
dietary concentrations of 0, 100, 300, 1000 or 3000 ppm equal to 0,
16, 40, 120 or 399 mg/kg bw/day for males and 0, 15, 44, 142 or 433
mg/kg bw/day for females, respectively, for a period of 4 weeks.
Body-weight gain of males treated with metiram at 1000 and 3000 ppm
were significantly lower than that of the corresponding controls
during the first week of treatment with subsequent recovery of
weight gain in the ensuing weeks. Although no consistent effects on
body weight in treated females were observed on a weekly basis,
overall weight gain of females at the high dose of 3000 ppm was
marginally lower than that of the control animals. Significantly
increased liver weights were recorded in both sexes treated at
levels of 1000 and 3000 ppm. In the absence of routine
histopathological examination, no microscopic correlation could be
drawn from the increased liver weights. There were no effects of
treatment on survival, clinical signs, food consumption or gross
pathological changes. On the basis of the reported findings, a high
dietary level of 1000 ppm was selected for the long-term study in
mice (Hunter et al., 1976b).
A 3-month study was performed with groups of 10 B6C3F1
mice/sex treated with metiram (94.8% purity, ETU <0.2%) at
dietary levels of 0, 300, 1000, 3000 or 7500 ppm, equal to 0, 84,
302, 853 or 2367 mg/kg bw/day in males and 0, 133, 465, 1448 or 3565
mg/kg bw/day in females, respectively. Treatment-related effects
were observed with respect to serum T3 and T4 concentrations.
Serum T4 levels were significantly decreased in both sexes of mice
treated with metiram at dietary levels of 1000 ppm and higher,
whereas T3 levels were significantly increased in male mice treated
at the highest dietary level of 7500 ppm. Histopathological
findings attributable to treatment were denoted in the thyroid in
both sexes treated at dietary levels of 3000 ppm and higher as
minimal to slight hypertrophy and vacuolation of the follicular
epithelium. A slight increase in the severity of fatty degeneration
of the `X zone' in the adrenals was also observed in female mice
treated with metiram at 3000 and 7500 ppm. A dose-related increased
incidence in the presence of a refractile, granular material
presumed to be the test material or a metabolite, was noted in the
superficial layer of the urinary bladder urothelium in all groups of
treated mice. Since no morphological changes were observed in the
urothelium of the bladder to suggest disturbed epithelial function,
the presence of this granular material was considered to be of
doubtful toxicological consequence. Significantly increased organ
weights were recorded for the adrenals in females at dietary levels
of 3000 ppm and higher and for the liver in both sexes treated at
7500 ppm and in males at 3000 ppm. Statistically significant
increases in kidney and testicular weights were judged to be of no
biological consequence. No histopathological correlation could be
determined for the increased liver, kidney or testicular weights.
Group mean body weights and corresponding weight gains were
decreased in males treated at the highest dietary level of 7500 ppm.
Although significant decreases in body weights were recorded in
females treated at dietary levels of 1000 ppm and higher, there was
no dose-response relationship. There were no adverse treatment-
related effects on survival, food consumption or on blood
haematological parameters. The NOAEL for this study was 300 ppm,
equal to 84 mg/kg bw/day, based on decreased serum T4 levels in
both sexes at levels of 1000 ppm and above (Gelbke et al., 1992a).
Rats
Metiram (96.8% purity containing 2.2% ETU) was administered for
a period of 4 weeks to groups of 20 Sprague-Dawley CD rats/sex at
dietary levels of 0, 100, 300, 1000 or 3000 ppm, equal to 0, 10, 30,
100 or 296 mg/kg bw/day in males and 0, 11, 33, 114 or 292 mg/kg
bw/day in females, respectively. Following termination of treatment
with metiram, 10 rats per sex per group were retained for subsequent
2 and 4 week recovery periods with scheduled sacrifices of 5
rats/sex/group on each occasion. Clinical toxicity was manifest as
signs of hind limb paralysis in rats treated at the highest dietary
level of 3000 ppm, which due to severity, resulted in early
sacrifice of 3 females for humane reasons. Histopathological
examination confirmed gross morphological alterations in the muscles
of the hind limbs, characterized by atrophy of the fibres, often
with associated proliferation of sacrolemmal nuclei, and the
presence of groups of vacuoles within muscle fibres. After 4 weeks
of treatment, the microscopic changes were more marked and extensive
in females, occurring in all females and in 2 of 9 males treated at
3000 ppm. After a withdrawal period of 2 weeks, a varying degree of
atrophy of muscle fibres was still present in females previously
treated with 3000 ppm of metiram. Although atrophy was not evident
in animals after a 4-week recovery period, additional changes, not
apparent earlier, were the presence of vacuoles and/or areas of fat
cells within the skeletal fibres. Target organ effects of treatment
with metiram were also evident on the thyroid and kidney. Changes
in the thyroid were apparent as a dose-related increase in the
incidence and severity of thyroid hyperplasia in both sexes treated
at dietary levels of 300 ppm and higher. Microscopic examination of
the kidney revealed varying degrees of hydropic vacuolation of the
proximal tubular epithelial cells in 2 females from each of the 1000
and 3000 ppm groups in the absence of similar effects at any of the
other dose levels or corresponding controls. Examination of bone
marrow smears revealed a depressed number of myeloid cells in males
treated at the high dose after 4 weeks of treatment. Group mean
body-weight gains were significantly lower in males treated at 1000
ppm and higher, and in females treated at 300 ppm and higher when
compared to the controls. Decreased food intake was recorded in
both sexes treated at the highest dietary level of 3000 ppm. There
were no adverse effects of treatment noted upon ocular examination,
haematology, blood chemistry, urinalysis and estrus cycle as
assessed from vaginal smears. The NOAEL was 100 ppm, equal to 10
mg/kg bw/day (Hunter et al., 1976a).
Groups of 35 Sprague-Dawley CFY strain rats per sex were
treated with metiram (96.8% purity containing 2.2% ETU) at dietary
levels of 0, 50, 100, 300 or 900 ppm, equal to 0, 3, 6, 20 or
61 mg/kg bw/day for males and 0, 4, 8, 24 or 76 mg/kg bw/day for
females, respectively, for a period of 13 weeks followed by a 6-week
recovery period. The principal target organs were the thyroid and
skeletal muscle. Treatment-related effects on the thyroid were
manifest as increased thyroid weights of females treated at 300 and
900 ppm, decreased percentage uptake of 131I by the thyroid in males
in all treated groups and in females treated at 100 ppm and higher,
and decreased T4 levels in both sexes at 300 ppm and higher.
Microscopically, an increased incidence of males treated at 900 ppm
had slight to minimal hyperplasia of the thyroid. A minimally
higher incidence at 13 weeks of slight thyroid hyperplasia in
females at 900 ppm and in males at 100 and 300 ppm when compared to
the controls was considered to be of equivocal significance. There
were no significant changes in the thyroid observed after the 6-week
recovery period. Treatment-related morphological changes of the
skeletal muscle denoted by atrophy of the muscle fibres often
accompanied by fat cells and associated proliferation of sacrolemmal
nuclei, were evident in both sexes treated at dietary levels of 300
ppm and higher. Varying degrees of muscular atrophy were still
prevalent after a 6-week recovery period in rats previously treated
at 300 and 900 ppm. Other effects of treatment were exhibited at
the high-dose level of 900 ppm as clinical signs of hind limb
paralysis, decreased body weights and slight reduction in food
intake. There were no treatment-related effects observed with
respect to survival, water consumption, ophthalmoscopy, haematology,
urinalysis or examination of bone marrow smears. Although reduced
iodine uptake by the thyroid was observed at all dietary levels,
these changes were shown to be reversible following cessation of
treatment. At the lowest dietary levels of 50 and 100 ppm, the
effects on iodine uptake were not correlated with changes in thyroid
hormone levels or any overt morphological alterations of the thyroid
gland, thus rendering the toxicological significance of this finding
at 50 and 100 ppm as doubtful. The NOAEL for this study was
therefore 100 ppm, equal to 6 mg/kg bw/day, based on decreased serum
T4 levels and increased thyroid weights at dietary levels of 300
and 900 ppm (Hunter et al., 1977).
Metiram (94.8% purity, ETU <0.2%) was administered for a
period of 3 months to groups of 13 Wistar Chbb:THOM SPF rats per sex
at dietary levels of 0, 5, 80, 320 or 960 ppm, equal to 0, 0.4, 5.8,
23.5 or 73.9 mg/kg bw/day in males and 0, 0.4, 6.7, 27.3 or 88.8
mg/kg bw/day in females, respectively. Neurofunctional assessment
revealed general muscle weakness expressed as ataxia and
significantly reduced forelimb and hindlimb grip strength in females
treated at the highest dietary level of 960 ppm. Perfusion fixation
of tissues from selected rats from the control and treated groups
failed to reveal any associated neuropathological or morphological
alterations of the central or peripheral nervous systems. Thyroid
hormone levels revealed significantly decreased T4 levels in both
sexes treated at the high dose of 960 ppm. Significantly reduced
RBC values were recorded in both sexes at levels of 320 and 960 ppm.
In females, decreases in RBC values were accompanied by decreases in
mean haemoglobin and haematocrit levels. Several statistically
significant changes in blood biochemical parameters of uncertain
toxicological significance were observed at the highest dietary
level of 960 ppm. These changes were recorded as decreased ALAT and
ALP activity (females), decreased creatinine (both sexes), decreased
urea (males), and decreases in the electrolytes; potassium (both
sexes), sodium (females), calcium (both sexes) and magnesium (both
sexes). Decreased phosphorus levels were recorded in males at
dietary levels of 320 ppm and higher. Other effects of treatment
expressed in both sexes at the highest dietary level of 960 ppm were
decreased body weights and corresponding weight gains, as well as
significantly increased thyroid, liver, kidney and testes (males
only) weights. There were no treatment-related gross or
histopathological changes observed in any of the animals from the
treated groups. Treatment with metiram did not result in any
significant effects on survival, food consumption, ophthalmoscopy or
urinalysis. The NOAEL was 80 ppm, equal to 5.8 mg/kg bw/day, based
on changes in haematological and blood chemical parameters at 320
ppm (Gelbke et al., 1992b).
A 13-week inhalation study was conducted with groups of 28
Sprague-Dawley CD rats/sex exposed in nose-only chambers to metiram
(94% purity) 6 hours/day, 5 days/week at concentrations of 0, 2, 20
or 100 mg/m3. Concentrations of ETU in the exposure atmospheres
were calculated to be 0, 0.02, 0.33 or 1.8 mg/m3, respectively.
After termination of treatment, one-half of the rats were sacrificed
while the remaining animals were retained for a 13-week recovery
period. The NOAEL was 2 mg/m3. Pulmonary changes consisting of
"subacute alveolitis", characterized by accumulations of alveolar
macrophages within alveolar lumens, accompanied by some polymorpho-
nuclear leukocytes were observed in rats treated at 20 and 100
mg/m3. It was conjectured that the alveolitis observed was not
treatment-related, but rather, a non-specific dust reaction of the
polymeric active ingredient artificially ground to particle size
capable of reaching the alveoli. Intra-alveolar pigment deposition
was recorded in a single male and female from the high-dose treated
group. There was no evidence of damage to bronchial or bronchiolar
epithelium in any of the treated groups. Mean lung/trachea weights
were increased in both sexes treated at the high dose. Other
changes associated with treatment were depositions of golden brown
granular pigment, similar to that seen in the lungs, within the
renal cortices in the high dose animals and decreased overall body-
weight gain in both males and females treated at 20 (11% and 14%)
and 100 mg/m3 (26% and 33% less than corresponding controls,
respectively). After the 13-week recovery, effects of treatment
persisted in the lungs and kidneys of previously treated animals.
No treatment-related findings were noted on survival,
ophthalmoscopy, haematology or blood chemistry. Tissue residue
analysis performed on the urine, plasma, liver, lung and thyroid
revealed measurable levels of metiram and ETU in the urine and lung
in rats treated at the mid- and high-dose levels (Ulrich, 1986).
Rabbits
A dermal toxicity study was performed with groups of 5 New
Zeeland white KFM rabbits/sex treated with Polyram DF formulation of
metiram on the shaved skin of their backs under occlusive conditions
for 6 hours/day at concentrations of 0 (vehicle control, distilled
water), 25, 50 or 250 mg/kg bw/day for a minimum of 21 consecutive
daily applications. Dermal administration of metiram resulted in
minimal to moderate exfoliation and ulcerative dermatitis in the
skin of rabbits treated at the high-dose level. Similar effects
were not evident at lower dose levels, indicating a NOAEL for local
irritation to the skin of 50 mg/kg bw/day. In the absence of
treatment-related effects on survival, clinical signs, body weights,
food consumption, ophthalmoscopy, haematology, blood chemistry,
organ weights or histopathology of the liver and kidneys, the NOAEL
for systemic toxicity was greater than 250 mg/kg bw/day (Ullmann et
al., 1987).
Dogs
Groups of 4 beagle dogs/sex were treated with metiram (96.8%
purity containing 2.2% ETU) for a period of 4 weeks at dietary
levels of 0, 100, 300, 600 or 900 ppm, equal to 0, 5, 14, 28 or 41
mg/kg bw/day for males and 0, 5, 15, 27 or 43 mg/kg bw/day for
females, respectively. Effects observed were an increased frequency
of micro-follicles in the thyroids associated with minimal depletion
of colloid and minimal hyperplasia of the follicular epithelium in
2/4 males and 2/4 females treated at the high dose of 900 ppm when
compared to the controls. Other changes recorded were significantly
increased liver weights in dogs receiving 600 and 900 ppm, which
were not associated with any correlative histopathological findings.
Significantly increased water consumption was recorded in dogs
treated at 100, 600 and 900 ppm but not at 300 ppm. The significance
of increased fluid intake (calculated over 5-day periods) was
difficult to ascertain since there were no corresponding changes
noted in urinalysis parameters. Erythrocyte, haemoglobin and packed
cell volume values, although significantly decreased in dogs treated
at 900 ppm when compared to the controls, were found to be within
normal limits of variability and therefore of doubtful toxicological
consequence. The NOAEL, based on the aforementioned findings, was
300 ppm, equal to 14 mg/kg bw/day. There were no significant
effects of treatment on survival, clinical signs, body weights, food
consumption, ophthalmoscopy, blood chemistry or urinalysis
(Chesterman et al., 1978).
Metiram (93.6% purity, ETU < 0.2%) was given to groups of 5
beagle dogs per sex at dietary concentrations of 0, 30, 80, 1000 or
3000 ppm, equal to 0, 0.9, 2.5, 29.8 or 76.9 mg/kg bw/day in males
and 0, 1.1, 2.7, 29.9 or 92.7 mg/kg bw/day in females, respectively,
for a period of 52 weeks. The principal effects of treatment were
manifest on the thyroid. Microscopic examination revealed thyroid
follicular hyperplasia in dogs of both sexes treated at 1000 and
3000 ppm. This finding was associated with an increased size and
weight of this organ at 3000 ppm. Gross necropsy revealed thyroid
thickening in several animals from both the control and treated
groups. This observation was, however, only correlated with
histopathological change at dietary levels of 1000 ppm and higher,
rendering the significance of thyroid thickening at lower levels
uncertain. Serum T4 levels were decreased in both sexes at 3000 ppm
and in males at 1000 ppm. A dose-related increased incidence of dogs
with focal hepatic lipofuscin pigment deposition was noted in both
sexes at 1000 ppm and higher. Other effects of treatment with
metiram were described as an increased incidence of diarrhoea in
dogs treated at 80 (attributed to single male), 1000 and 3000 ppm;
slight evidence of anaemia (both sexes at 1000 and 3000 ppm)
involving an increased number of reticulocytes (both sexes at 3000
ppm); decreased body-weight gain at 3000 ppm in males and to a
lesser extent in females; and decreased food intake in both sexes at
3000 ppm and in females at 1000 ppm. Significant changes in blood
chemical parameters at dietary levels of 1000 ppm and higher were
evident as increased lipid, cholesterol, triglycerides, phospholipid
levels, ALP activity, and total protein, and decreased albumin and
A/G ratio as well as disturbances in the protein electrophoretic
pattern. There were no effects of treatment on survival,
neurological parameters, ophthalmoscopy or urinalysis. The NOAEL
for this study was 80 ppm, equal to 2.5 mg/kg bw/day (Corney et
al., 1991).
Monkeys
A preliminary toxicity assessment was performed with groups of
single male and female rhesus monkeys administered metiram (96.8%
purity containing 2.2% ETU) orally by gavage as a suspension in 0.5%
mucilage of tragacanth in water at dose levels of 50, 100 or 500
mg/kg bw/day for a period of 4 weeks. Treatment-related toxicity
was evident as salivation at the time of dosing or immediately
thereafter and occasional emesis in monkeys treated at 100 and 500
mg/kg bw/day, body-weight loss in the single female treated at 500
mg/kg bw/day and changes of the thyroid, representative of early
follicular hyperplasia in the single high-dose female. The low dose
of 50 mg/kg bw/day was well tolerated with no overt signs of
toxicity (Sortwell et al., 1977).
Metiram (96.8% purity containing 2.2% ETU) was administered
orally by gavage to groups of 4 rhesus monkeys/sex for a period of
26 weeks at dose levels of 0 (vehicle control, 0.5% tragacanth in
distilled water), 5, 15 or 75 mg/kg bw/day. After termination of
treatment, 1 male and 1 female were retained untreated for a period
of 15 weeks as recovery animals. In a separate, but concurrently
conducted study, a further 2 male and 2 female monkeys were treated
with metiram at doses of 0, 5 or 75 mg/kg bw/day for 26 weeks to
monitor thyroid function. The primary effects of treatment with
metiram were evident in the thyroid and were characterized at dose
levels of 15 and 75 mg/kg bw/day as minimal thyroid follicular
hyperplasia, significantly decreased T3 and T4 levels and
increased thyroid weights. After a 15-week recovery, morphological
changes of the thyroid were still apparent in monkeys previously
treated at the 15 and 75 mg/kg bw/day dose levels. Assessment of
thyroid function revealed an initial marked reduction in iodine
uptake at dose levels of 5 and 75 mg/kg bw/day, followed by a
significant increase in uptake during the latter part of the study.
In the absence of any correlation to thyroid hormone levels or
morphological alterations, no toxicological significance was
attributed to fluctuations in iodine uptake by the thyroid at the
lowest level of 5 mg/kg bw/day. Other effects resulting from
treatment were an increased incidence of salivation occurring at the
time of dosing at the high dose. Significantly increased liver
weights at the high-dose level were not associated with any
histopathological findings. Treatment with metiram did not
significantly affect survival, body-weight gains, food or water
consumption, ophthalmoscopy, haematology, urinalysis or examination
of bone marrow smears. The NOAEL was 5 mg/kg bw/day (Sortwell et
al., 1979).
Long-term toxicity/carcinogenicity studies
Mice
Dietary administration of metiram (96.8% purity containing 2.2%
ETU) to groups of 52 CFLP mice/sex at levels of 0, 100, 300 or 1000
ppm, equal to 0, 8, 24 or 79 mg/kg bw/day for males and 0, 9, 29 or
95 mg/kg bw/day for females, respectively, for a minimum period of
88 weeks resulted in a NOAEL for in-life parameters of 300 ppm,
equal to 24 mg/kg bw/day. Although there were no statistically
significant differences in mortality in the treated groups, a
marginally higher mortality incidence from week 40 onwards was
recorded in females treated at 1000 ppm, resulting in a minimum 25%
survival rate being reached in this group before the corresponding
controls. Group mean body weights were significantly decreased in
both sexes treated at the 1000 ppm dietary level. Food consumption
in males treated at 1000 ppm was only marginally (5%) lower than the
corresponding controls during the first year of treatment. Clinical
signs and examination of bone marrow smears were not adversely
affected by treatment with metiram. Under the conditions of the
present study, treatment with metiram at dietary levels up to and
including 1000 ppm failed to uncover any evidence of carcinogenic
potential (Hunter et al., 1979).
Rats
Groups of 50 Sprague-Dawley CD rats/sex were administered
metiram (96.8% purity containing 2.2% ETU) in the diet at levels of
0, 5, 20, 80 or 320 ppm, equal to 0.2, 0.8, 3.1 or 12.3 mg/kg bw/day
in males and 0, 0.2, 1.0, 3.8 or 15.5 mg/kg bw/day in females,
respectively, for 119 weeks for males and 111 weeks for females.
Satellite groups of 30 male and 30 female rats per group were used
for blood sampling and thyroid function tests and were then killed
after 102 weeks of treatment. Treatment with metiram resulted in a
NOAEL of 80 ppm (equal to 3.1 mg/kg bw/day), based on an increased
incidence of rats treated at the next higher dietary level of 320
ppm with muscular atrophy. There were no consistent treatment-
related effects with respect to survival, clinical signs, body
weights, food or water consumption, ophthalmoscopy, haematology,
blood chemistry, thyroid function, urinalysis or organ weights.
There was no evidence of metiram-induced carcinogenic potential
(Hunter et al., 1981).
Reproduction studies
Rats
A three-generation (two-litter per generation) reproduction
study was conducted with groups of 12 male and 24 female Crl:COBS
CD(SD)BR rats fed metiram (96.8% purity containing 2.2% ETU) at
dietary levels of 0, 5, 40 or 320 ppm, equal to 0, 0.2, 1.8 or 14.2
mg/kg bw/day in males and 0, 0.3, 2.3 or 19.8 mg/kg bw/day in
females, respectively. In the F0 generation, treatment with
metiram commenced at least 60 days prior to mating. In the F1 and
F2 generations, the F1b and F2b derived parental animals,
respectively were exposed to the test material for a minimum period
of 90 days. At day 21 post-partum, 10 F3a pups/sex from each group
were selected for detailed gross examination, recording of organ
weights and histopathological assessment. At the second pairing of
the F2b generation, all pregnant females from each of the dose
levels were sacrificed on day 20 of gestation for teratological
examination. Appearance of spermatozoa in the vaginal smear or the
presence of a copulation plug was considered to be day 0 of
gestation. Decreases in body-weight gain (4-7%) were noted in both
the F0 and F1 generation males and females treated at 320 ppm when
compared to the controls. Slightly depressed (7-8%) food intake was
observed in males fed metiram during the F0 and F1 generations.
Body weights and food consumption were not adversely affected by
treatment at lower dietary levels or in any of the treated groups
from the F2 generation when compared to the controls. There were
no treatment-related effects on parental mortality, clinical signs,
food conversion ratios, reproductive parameters or gross
pathological alterations. Statistically significant decreases in
the mean number of pups born were observed in all treated groups
during the second mating of both the F1 and F2 generations.
Although the differences were statistically significant, there was
no dose-response relationship and all values in the treated groups
generally fell within the reported laboratory standard values.
Total litter loss was significantly decreased at 320 ppm during the
second mating of the F1 generation. In the absence of similar
effects in the first mating of the F1 or in either of the matings
in the F0 and F2 generations, the toxicological significance of
this finding was dubious. Statistically significant decreases in
mean litter weights were observed in the treated groups during the
F1 and F2 generations. Since the mean pup weights in the treated
groups were comparable to the controls, the decreased mean litter
weights may, in part, be attributed to the correspondingly smaller
litter sizes observed in these groups. With regard to the
teratological component, there were no effects on pregnancy rate,
pre- or post-implantation loss, number of live fetuses, embryo/fetal
resorptions, fetal sex ratio or fetal/litter weights. Metiram was
not teratogenic as determined by gross, skeletal and visceral
examination of fetuses, following in utero exposure at dietary
levels as high as 320 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg
bw/day, based on decreased parental body weight and food consumption
recorded in the F0 and F1 generations treated at 320 ppm (Cozens
et al., 1981).
Special studies on teratogenicity
Rats
A preliminary range-finding study was performed with groups of
6 non-pregnant Crl:Cobs CD(SD)BR female rats administered metiram
(96.8% purity containing 2.2% ETU) orally by gavage at 0 (vehicle
control, sodium carboxymethyl cellulose), 150, 300, 600 or 1200
mg/kg bw/day for 10 consecutive days. Dose-related clinical effects
primarily affecting the nervous system were observed at all dose
levels. In the absence of microscopic examination,
histopathological correlation could not be ascertained. Other
effects of treatment were related to decreased body-weight gains at
all dose levels, depressed food intake at 300 mg/kg bw/day and
higher and decreased water consumption in rats treated at 600 and
1200 mg/kg bw/day (Palmer & Simmons, 1979a).
Groups of 20 time-mated female Crl:Cobs CD(SD)BR rats were
treated with metiram (96.8% purity containing 2.2% ETU) daily by
gavage during days 6 to 15 of gestation, inclusive, at dose levels
of 0 (vehicle control, sodium carboxymethyl cellulose), 40, 80 or
160 mg/kg bw/day. The day of mating, determined by the presence of
a vaginal plug or sperm in the vaginal smear, was designated as day
0 of gestation. Decreased body-weight gain (4-9%) was noted in the
high-dose animals. There were no significant effects with respect
to clinical signs, food or water consumption or gross organ/tissue
changes. A slight increase in the pre- and post-implantation loss
was recorded in the 160 mg/kg bw/day group, in conjunction with a
significant decrease in the mean number of live fetuses and
decreased litter weight in this group. It is unlikely that the
increased pre-implantation loss at the high dose was related to
treatment, since theoretically, treatment was not initiated until
after implantation occurred. Nevertheless, the combined effect of
pre- and post-implantation loss may have, to a certain degree,
influenced the decreased litter size and weight noted at the high
dose level. There were no effects of treatment on the incidence of
early or late resorptions, mean fetal weight or sex ratio. The
NOAEL for embryo/fetotoxicity was 80 mg/kg bw/day, based on slight
decreases in litter size and weights. Treatment with metiram failed
to uncover any evidence of teratogenic potential. The NOAEL for
maternal toxicity was 80 mg/kg bw/day, based on decreased body-
weight gain (Palmer & Simmons, 1979a).
Rabbits
In a range-finding study, groups of 3 pregnant Himalayan
rabbits were treated orally by gavage with metiram (97.9% purity,
ETU <0.2%) during days 7 through 19 of gestation at dose levels
of 0 (vehicle control, distilled water with 0.5% carboxymethyl
cellulose), 50, 100 or 200 mg/kg bw/day. Reduced body-weight gains
and food intake were recorded at the two highest dose levels. The
significance of the incidence of single abortion at each of the dose
levels, upon conduct of the definitive study, was confirmed to be
treatment-related. No further relevant details were provided
(Gelbke et al., 1988).
Metiram (97.9% purity, ETU < 0.2%) was administered orally
by gavage at 0 (vehicle control, distilled water with 0.5%
carboxymethyl cellulose), 10, 40 or 120 mg/kg bw/day to groups of 15
artificially inseminated Himalayan rabbits on days 7 through 19 of
gestation. The day of artificial insemination was designated as day
0 of gestation. Treatment with metiram elicited signs of maternal
toxicity at 40 and 120 mg/kg bw/day, denoted by abortion, decreased
body weight and food consumption, the latter associated with
reduction or absence of defecation. The high incidence of abortion
(8/15) recorded at 120 mg/kg bw/day, in conjunction with a single
death culminated in only 6/15 rabbits treated at this level with
litters available for examination. Although 2 of 15 animals aborted
at 40 mg/kg bw/day, there were, nevertheless, adequate numbers of
litters secured in the control, 10 and 40 mg/kg bw/day dose groups.
Since a dose level of 10 mg/kg bw/day was without significant
effects, this dose was considered the NOAEL for maternal toxicity.
The NOAEL for developmental toxicity was 40 mg/kg bw/day based on
the slight decrease in mean fetal weights recorded at 120 mg/kg
bw/day. A slight decrease in the number of live male fetuses in the
120 mg/kg bw/day group was considered to be of no toxicological
consequence, in light of the comparable number of female fetuses and
the total number of fetuses in the 120 mg/kg bw/day group relative
to the controls. There were no significant effects of treatment on
the mean number of live fetuses, early or late resorptions, post-
implantation loss, or placental weight. There was no evidence
suggestive of teratogenic potential with metiram at any of the dose
levels investigated (Gelbke et al., 1988).
Special studies on genotoxicity
Results of mutagenicity assays conducted with metiram are
presented in Table 3. The tests conducted to evaluate potential for
gene mutation in bacteria and induction of chromosomal aberration in
rat bone marrow were negative. Unscheduled DNA synthesis in rat
hepatocytes and a dominant lethal study in mice were also negative.
Although metiram demonstrated potential for inducing sister
chromatid exchange (SCE) in Chinese hamster ovary cells in the
absence and presence of mouse microsomal activation, there was
nevertheless, no significant induction of SCE observed with rat
microsomal activation. Moreover, an in vivo SCE assay in bone
marrow cells of the Chinese hamster was negative. Metiram exhibited
a weak promotion activity in mouse embryo fibroblasts in the absence
of a direct transformation response. The Meeting concluded that
metiram was not genotoxic.
Special studies on irritation and sensitization
The eye and skin irritation potential of technical metiram
(containing 2.2% ETU) were investigated in six Vienna white rabbits.
There were no signs of irritation when metiram was applied to the
external ear for 20 hours or to the backs for 1, 5 or 15 minutes.
When applied to the back for 20 hours, slight reddening occurred 24
hours after application with subsequent scaling after 8 days.
Metiram (50 mg) when introduced into the conjunctival sac of the eye
of these rabbits produced slight redness and edema after 1 hour,
with no signs of irritation after 8 days (Zeller, 1985a).
Polyram combi, a formulation of technical metiram, was
administered to eight Vienna white rabbits to determine skin and eye
irritation potential. A 50% aqueous suspension of the test material
when applied to the backs of the rabbits for a period of 20 hours,
resulted in slight erythema of the skin, which was reversible within
8 days. The instillation of 50 mg of Polyram combi to the
conjunctival sac of the rabbit eye caused redness and edema which
were reversible after 8 days. When 50 µl of a 20% aqueous
suspension was applied, slight reddening of the mucosa occurred,
whereas 50 µl of a 2% aqueous suspension was tolerated without any
irritation (Zeller, 1985b).
Metiram technical demonstrated severe sensitizing effects on
the skin of female Pirbright white, Dunkin Hartley guinea-pigs when
tested according to the methods of the Magnusson & Kligman
maximization test (Jackh, 1982).
Table 3. Genotoxicity of metiram
Test Test system Concentration Purity Results Reference
Reverse mutation S. typhimurium 0, 1, 3, 10, 31, Technical negative Oesch, 1977
(in vitro) TA 98, 100, 1537 100, 310, 1000, 1., 2. (rat)
2000 µg/plate
S. typhimurium 0, 1, 10, 50, 100, Technical + 2.2% ETU negative Gelbke & Engelhardt,
TA 98, 100, 1535, 1537 500, 2500 µg/plate 1., 2. 1985
(rat & mouse)
Reverse mutation/host S. typhimurium 500, 1670, Technical + 2.2% ETU negative Jagannath & Myhr, 1985
mediated (in vivo) TA 1530 5000 mg/kg bw
Male CD-1 mice (single oral doses)
(inoculated ip)
Point mutation HGPRT locus of Chinese 0, 0.681, 1.0, 4.64, Technical + 2.2% ETU negative, 1. Gelbke & Jackh, 1985
(in vitro) hamster ovary (CHO) cell 6.81, 10, 46.4, equivocal, 2.
line, K1 68.1, 100 µg/ml (rat)
HGPRT locus of Chinese 0.5, 1, 5, 10, 50, Premix 95% negative Hoffman & Jackh, 1990
hamster ovary (CHO) cell 100, 500 µg/ml 1., 2. (mouse)
line, K1
SCE (in vitro) Chinese hamster ovary 0, 40, 60, 80, 100, Technical + 2.2% ETU positive, 1. Ivett & Myhr, 1986a
CHO, WB1 cells 125, 150, 175, 200 positive, 2.
µg/ml (mouse)
negative, 2.
(rat)
SCE (in vivo) Chinese hamster, LMP 1000, 3330, 10 000 Premix 95% negative Miltenburger et al.,
stock (bone marrow) mg/kg bw 1990
Table 3 (contd)
Test Test system Concentration Purity Results Reference
Chromosome aberration Rat, male Fischer 344 0, 0.24, 1.2, 2.4 Technical + 2.2% ETU negative Ivett & Myhr, 1986b
(in vivo) (bone marrow) g/kg bw (acute) 0,
0.02, 0.1, 0.2 g/kg
bw (5 repeated oral
doses)
Transformation Mouse embryo fibroblasts 0, 0.1, 0.25, 0.5, Technical + 2.2% ETU transformation: Tu et al., 1985
promotion (in vitro) C3H-10T 1/2 (clone 8) 0.75, 1.0 µg/ml negative
cell system promotion
activity:
weakly
positive
Unscheduled DNA Rat (Fischer 344, male) 0.492, 1.23, 2.46, Technical + 2.2% ETU negative Cifone & Myhr, 1984
synthesis (in vitro) hepatocytes 4.92, 12.3, 24.6,
49.2, 160 µg/ml
Dominant lethal Mouse, CD-1 male 0, 600, 1200, 2400 Technical + 2.2% ETU negative Palmer & Simons, 1979b
(in vivo) mg/kg bw/day
Results:
1. = in the presence of metabolic activation derived from the indicated species
2. = in the absence of metabolic activation
COMMENTS
Metiram was incompletely absorbed when administered orally to
rats. Elimination was primarily via the faeces, with minimal
biliary excretion. Comparatively higher urinary excretion at low
doses suggested that metiram may be more poorly absorbed at higher
doses. Highest residual tissue levels were found in the thyroid and
kidney, with slightly higher concentrations present in females than
in males. Comparison of tissue residues after single or multiple
doses suggested slight accumulation in the body with multiple
dosing.
The metabolism of metiram has not been completely elucidated.
In the rat, the predominant urinary components were polar and were
identified as ethylenediamine, N-acetyl-ethylenediamine,
ethanolamine, oxalic acid and glycine. Major, less polar components
were ethyleneurea, ETU and EBIS.
Metiram was practically non-toxic upon acute oral, dermal and
inhalation administration to rats. WHO has classified metiram as
unlikely to present acute hazard in normal use.
The principal target organ upon repeated dietary exposure to
metiram was the thyroid.
Mice treated with metiram for three months at 0, 300, 1000,
3000 or 7500 ppm in the diet revealed minimal to slight hypertrophy
and vacuolation of the thyroid follicular epithelium in both sexes
at levels of 3000 ppm and higher. A NOAEL of 300 ppm (equal to 84
mg/kg bw/day) was based on decreased serum T4 levels in both sexes
at levels of 1000 ppm and above.
Thirteen-week dietary administration of metiram to SD CFY rats
at 0, 50, 100, 300 or 900 ppm revealed a NOAEL of 100 ppm (equal to
6 mg/kg bw/day) based on decreased serum T4 levels and increased
thyroid weights at dietary levels of 300 and 900 ppm. Slight to
minimal hyperplasia of the thyroid was observed at 900 ppm.
Although reduced iodine uptake by the thyroid was observed at all
dietary levels, these changes were shown to be reversible following
cessation of treatment. At the lowest dietary levels of 50 and 100
ppm, the effects on iodine uptake were not correlated with changes
in thyroid hormone levels or any overt morphological alterations of
the thyroid gland, thus rendering the toxicological significance of
this finding doubtful.
In a recently conducted three-month study with Wistar rats
receiving metiram at 0, 5, 80, 320 or 960 ppm in the diet, decreased
serum T4 levels and increased thyroid weights were observed at 960
ppm. Slight evidence of anaemia was observed at 320 ppm, indicating
a NOAEL of 80 ppm (equal to 5.8 mg/kg bw/day).
Other effects of treatment in the diet with metiram in the rat
were manifest as hind limb paralysis with corresponding atrophy of
muscle fibres. In the 13-week study with SD CFY rats, microscopic
changes in muscle fibres at levels of 300 ppm (equal to 20 mg/kg
bw/day) and higher were still prevalent in previously treated rats
after the 6-week recovery period. Muscular atrophy was observed in
a long-term study in SD CD rats treated at the highest level of 320
ppm (see below). General muscle weakness/ataxia and reduced grip
strength of the limbs with no histopathological consequences were
observed in the 3-month study with Wistar rats fed metiram at the
highest level of 960 ppm.
A 52-week study in dogs at dietary levels of 0, 30, 80, 1000 or
3000 ppm yielded a NOAEL of 80 ppm (equal to 2.5 mg/kg bw/day) based
on thyroid follicular hyperplasia with increased size, thickening
and weight of this organ, in conjunction with decreased serum T4
levels at dietary levels of 1000 ppm and above. Other effects
recorded at 1000 ppm and above were a dose-related increased
incidence of focal hepatic lipofuscin pigment deposition, slight
evidence of anaemia, diarrhoea and changes in blood biochemical
parameters. A preliminary 4-week study in dogs uncovered an
increased frequency of microfollicles in the thyroid, in association
with colloid depletion and minimal hyperplasia in both sexes treated
at the highest level of 900 ppm (equal to 41 mg/kg bw/day).
Metiram given by gavage to rhesus monkeys at dose levels of 0,
5, 15 or 75 mg/kg bw/day for a period of 26 weeks indicated a NOAEL
of 5 mg/kg bw/day based on significantly decreased serum T3 and T4
levels, increased thyroid weights and minimal thyroid follicular
hyperplasia at 15 and 75 mg/kg bw/day. Morphological changes of the
thyroid were still apparent after a 15-week recovery period. In the
absence of any correlation between thyroid hormone levels and
morphological alterations, no significance was attributed to
fluctuations in iodine uptake by the thyroid recorded at 5 mg/kg
bw/day.
Long-term dietary treatment of mice with metiram at 0, 100, 300
or 1000 ppm resulted in a NOAEL of 300 ppm (equal to 24 mg/kg
bw/day) based on decreased body weights recorded at 1000 ppm.
Chronic dietary administration of metiram to SD CD rats at 0,
5, 20, 80 or 320 ppm revealed muscular atrophy at 320 ppm (equal to
12 mg/kg bw/day), with a NOAEL of 80 ppm (equal to 3.1 mg/kg
bw/day).
Metiram was not carcinogenic when fed to mice or rats at
dietary levels of up to 1000 and 320 ppm, respectively.
A three-generation, two litter per generation reproduction
study in rats treated at 0, 5, 40 or 320 ppm in the diet failed to
reveal any adverse effects on reproductive parameters. The NOAEL
was 40 ppm (equal to 1.8 mg/kg bw/day), based on decreased parental
body weight and food consumption recorded in the F0 and F1
generations treated at 320 ppm.
Metiram when administered to pregnant rats at 0, 40, 80 or 160
mg/kg bw/day or rabbits at 0, 10, 40 or 120 mg/kg bw/day during
critical periods of organogenesis was not teratogenic at any dose.
The NOAEL for maternal toxicity in the rat was 80 mg/kg bw/day based
on decreased body-weight gain and in the rabbit the NOAEL was 10
mg/kg bw/day based on increased abortions, decreased body weights
and food consumption. The NOAELs for embryo/fetotoxicity were 80
mg/kg bw/day in the rat based on slight decreases in litter size and
weight and 40 mg/kg bw/day in the rabbit based on decreases in mean
fetal weights.
Metiram has been tested in a series of in vitro and in vivo
genotoxicity assays. The Meeting concluded that metiram is not
genotoxic.
The Meeting allocated an ADI of 0-0.03 mg/kg bw based on a
NOAEL of 2.5 mg/kg bw/day in the 52-week study in dogs, using a 100-
fold safety factor. This ADI is supported by the NOAEL of 3.1 mg/kg
bw/day observed in the long-term study in rats. This ADI served as
the basis for a group ADI that was established for metiram alone or
in combination with mancozeb, maneb, and zineb.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 300 ppm, equal to 24 mg/kg bw/day (88-week study)
Rat: 80 ppm, equal to 3.1 mg/kg bw/day (111-week study)
40 ppm, equal to 1.8 mg/kg bw/day
(reproduction study)
Rabbit: 10 mg/kg bw/day (teratogenicity study)
Dog: 80 ppm, equal to 2.5 mg/kg bw/day (52-week study)
Monkey: 5 mg/kg bw/day (26-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, maneb, and
zineb).
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Chesterman, H., Heywood, R. & Barker M. (1973) Polyram Combi acute
oral toxicity study in beagle dogs. Report BASF 73/0047, BASF
40/73703. Unpublished report dated October 5, 1973 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Chesterman, H., Heywood, R., Ball, S., Street, A. & Prentice, D.
(1978) Metiram (active ingredient) oral toxicity study in beagle
dogs (dietary intake for 4 weeks). Report BASF 78/0154, BSF
201/76422. Unpublished report dated April 26, 1978 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Cifone, M. & Myhr, B. (1984) Report on the evaluation of metiram
tech. in the rat primary hepatocyte unscheduled DNA synthesis assay.
Report RZ 84/209. Unpublished report dated July 5, 1984 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Corney, S.J., Allen, T.R., Janiak, T. & Springall, C. (1991) 52-week
oral toxicity (feeding) study with metiram premix 95% in the dog.
Report No. 91/10786. Unpublished report dated August 22, 1991 from
RCC, Research & Consulting Company Ltd., Itingen, Switzerland.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Cozens, D., Simons, R., Clark, R., Offer, J. & Gibson, W. (1981)
Effect of metiram technical on reproductive function of multiple
generations in the rat. Report RZ 81/132, BSF 200/80692. Unpublished
report dated March 18, 1981 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Gelbke, H. & Engelhardt, G. (1985) Report on the study of metiram
(techn.) in the Ames test. Report RZ 85/020. Unpublished report
dated February 7, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H. & Jackh, R. (1985) Report on a point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram. Report RZ 85/238. Unpublished report dated July 30, 1985
from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Gelbke, H., Hellwig, J. & Hildebrand, B. (1988) Report on the study
of the prenatal toxicity of metiram-premix 95% in rabbits after oral
administration (gavage). Report BASF 88/0154 and Supplement BASF
88/0262. Unpublished report dated May 26, 1988 from BASF, Department
of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. and Hildebrand, B. (1992a) Study of the oral
toxicity of metiram premix 95% in B6C3F1 mice. Administration in
the diet for 3 months. Report No. 92/11223. Unpublished report dated
October 16, 1992 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. & Hildebrand, B. (1992b) Study of the oral
toxicity of metiram premix 95% in Wistar rats. Administration in the
diet for 3 months including the examination of neurotoxicology
(neurofunctional observational battery). Report No. 92/11224.
Unpublished report dated October 16, 1992 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1979) Study of the acute dermal toxicity of "Metiram
tech. with 2% ETU" on the rat (Translation). Report RZ 79/032.
Unpublished report dated September 25, 1979 from BASF,
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1985) Report on the study of the acute dermal
toxicity of Polyram Combi (BAS 222 01 F) in the rat (Translation of
the original report dated September 25, 1979). Report RZ 85/026.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Girkin, R. & Jackson R. (1984) Report on the
study of dermal absorption of metiram in rats. Report RZ 85/158, HRC
BSF 411/84694. Unpublished report dated October 10, 1984 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Midgley, I., Biggs, S. & McCay, C. (1985)
The biokinetics and metabolism of 14C-metiram in the rat. Report
BASF 85/0470, HRC BSF 410/85720. Unpublished report dated October
28, 1985 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1974a) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF, Gewerbehygiene
und Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hofmann, H. (1974b) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF
Aktiengesellschaft, FRG.
Hofmann, H. & Munk, R. (1975) Acute intraperitoneal toxicity of the
technical active ingredient metiram to the rat. Report RZ 75/006.
Unpublished report dated June 4, 1975 from BASF, Gewerbehygiene und
Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1975) Acute oral toxicity of the technical active
ingredient metiram to the rat. Report RZ 75/005. Unpublished report
dated June 4, 1975 from BASF, Gewerbehygiene und Toxikologie, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1985a) Report on the study of the acute oral toxicity of
Polyram Combi, technical active ingredient, in the rat (Translation
of original report dated March 25, 1974). Report BASF 85/0485.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985b) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi, technical active
ingredient, in the rat (Translation of original report dated March
25, 1974). Report RZ 85/028. Unpublished report dated February 4,
1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985c) Report on the study of the acute oral toxicity
of Polyram Combi (BAS 222 01 F) to rats (Translation of original
report dated March 25, 1974). Report RZ 85/121. Unpublished report
dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985d) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi (BAS 222 01 F) in rats
(Translation of original report dated March 25, 1974). Report RZ
85/124. Unpublished report dated April 12, 1985 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985e) Report on the study of the acute inhalation
toxicity of the spray of a 5% aqueous suspension of Polyram Combi
(BAS 222 01 F) to guinea-pigs (Translation of original report dated
March 25, 1974). Report RZ 85/125. Unpublished report dated April
12, 1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein,
FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. & Jackh, R., 1990. Report on the point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram premix 95% with B6C3F1 mice microsomal fraction. Report
No. 90/0285. Unpublished report dated August 2, 1990 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A. & Prentice, D.
(1976a) Preliminary assessment of metiram toxicity to rats in
dietary administration for four weeks followed by a two and a four
week withdrawal period. Report RZ 76/024, BSF 172/76245. Unpublished
report dated October 20, 1976 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hunter, B., Bridges, J. & Prentice, D. (1976b) Preliminary
assessment of metiram toxicity to mice in dietary administration for
4 weeks. Report RZ 76/014, BSF 170/76108. Unpublished report dated
June 7, 1976 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A., Prentice, D. &
Offer, J. (1977) Metiram toxicity to rats in dietary administration
for 13 weeks followed by a 6 week withdrawal period (final report).
Report RZ 77/043, BSF/197/77612 and Addendum BASF 87/0205, BSF
197/8713. Unpublished report dated November 16, 1977 and addendum
dated May 29, 1987 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Prentice, D. & Gregson, R., 1979. Metiram
tumorigenicity to mice in long term dietary administration (final
report). Report RZ 79/033, BSF 198/78265. Unpublished report dated
June 5, 1979 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Street, A., Heywood, R., Prentice, D.,
Offer, J. & Gibson, W. (1981) Metiram toxicity and tumorigenicity in
prolonged dietary administration to the rat. Report RZ 81/280, BSF
199/80391, WNT No. 77/951 and Addendum BASF 89/0001. Unpublished
report dated May 7, 1981 and addendum date December 19, 1988 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986a) Report on the mutagenicity evaluation
of metiram in an in vitro sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells/amended final report. Report RZ
86/082. Unpublished report dated March 11, 1986 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986b) Report on the mutagenicity evaluation
of metiram technical K38/33A in the rat bone marrow cytogenetic
assay. Report RZ 86/265. Unpublished amended report dated August 4,
1986 from Litton Bionetics, Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jackh, R. (1981) Report on the study of the acute oral toxicity of
metiram technical grade in the rat (Translation). Report BASF
92/10669. Unpublished report dated July 9, 1981 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Jackh, R. (1982) Report on the study of the sensitizing effect of
metiram techn. in the guinea-pig -maximization test (Translation of
original report dated August 21, 1981). Report RZ 82/068.
Unpublished report dated February 16, 1982 from BASF, Department of
Toxicology, Ludwigshafen, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jagannath, D. & Myhr, B. (1985) Report on the mouse host-mediated
assay of metiram tech. K38/33A. Report RZ 85/210. Unpublished report
dated June 28, 1985, from Litton Bionetics Inc., Maryland, USA.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Klimisch, H.J. & Zeller, H. (1980) Report on the determination of
the acute inhalation toxicity LC50 metiram techn. with 2% ETU as a
dust aerosol after 4-hour exposure in Sprague-Dawley rats
(Translation of original report dated September 15, 1980). Report RZ
83/064. Unpublished report dated September 15, 1980 from
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Leuschner, F. (1979a) The acute oral toxicity of the preparation
metiram, techn. agent with 2% ETU in rats. Report BASF 79/0161, WNT-
Nr. 77/951. Unpublished report dated August 20, 1979 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Leuschner, F. (1979b) The acute intraperitoneal toxicity of the
preparation metiram, techn. agent with 2% ETU in mice. Report BASF
79/0162, WNT-Nr. 77/951. Unpublished report dated August 20, 1979
from Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Miltenburger, H., Volkner, W. & Heidemann, A. (1990) Sister
chromatid exchange assay in bone marrow cells of the Chinese hamster
with metiram premix 95%. Report No. 90/0569. Unpublished report
dated September 18, 1990 from CCR, Cytotest Cell Research Gmbh &
Co., Rossdorf, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Oesch, F. (1977) Ames test for metiram. Report RZ 77/027.
Unpublished report dated August 22, 1977 from the Institute of
Pharmacology, University of Mainz, Germany. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Palmer, A. & Simons, R. (1979a) Effect of metiram technical on
pregnancy of the rat. Report RZ 79/065, BSF 302/79616. Unpublished
report dated August 3, 1979 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Palmer, A. & Simons, R. (1979b) Dominant lethal assay of metiram
technical in the male mouse. Report RZ 79/069, BSF 304/79860.
Unpublished report dated November, 1979 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Heywood, R., Allen, D., Prentice, D. & Cherry, C.
(1977) Metiram preliminary oral toxicity study in rhesus monkeys.
Repeated dosage for 4 weeks. Report BASF 77/0149, BSF 265/77264.
Unpublished report dated April 15, 1977 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Allen, D., Heywood, R. & Street, A. (1979) Metiram
(containing 2.2% ethylenethiourea) oral toxicity study in rhesus
monkeys (repeated dosage for 26 weeks with recovery period). Final
report. Report BASF 79/0082, BSF 267/78263. Unpublished report dated
January 15, 1979 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Tu, A., Sivak, A., Breen, P. & Hatch, K. (1985) Report on the
evaluation of metiram in the C3H-10T 1/2 cell system for
transformation and promotion activities. Report RZ 85/209.
Unpublished report dated June 18, 1985 from Arthur D. Little, Inc.,
Maryland, USA. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ullmann, L., Sacher, R., Porricello, T., Luetkemeier, H., Vogel, W.,
Vogel, O., Wilson, J. & Terrier, H. (1987) Report on the subacute
21-day repeated-dose dermal toxicity study with polyram DF in
rabbits. Report BASF 87/0260, ZNT No. 86/314. Unpublished report
dated June 24, 1987 from RCC, Research and Consulting Company AG,
Switzerland. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ulrich, C. (1986) Report on the thirteen week subchronic inhalation
toxicity study on metiram in rats. Report RZ 86/407 and addendum
BASF 87/0414. Unpublished report dated December 31, 1986 and
addendum dated October 7, 1987 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. (1985a) Report on the study of the irritation of Polyram
Combi, technical active ingredient, to the skin and eye of rabbits
(Translation of original report dated March 25, 1974). Report RZ
85/027. Unpublished report dated February 4, 1985 from BASF,
Department of Toxicology, Ludwigshafen, FRG. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Zeller, H. (1985b) Report on the study of the irritation of Polyram
Combi (BAS 222 01 F) to the skin and eyes of rabbits (Translation of
original report dated March 25, 1974). Report RZ 85/122. Unpublished
report dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Table 1. Acute toxicity of technical metiram
Species Sex Route (vehicle) LD50 Test material Reference
(strain) (mg/kg bw)
Rat M+F oral > 6810 Technical grade Jackh, 1981
(SD) (distilled water) (purity unknown)
Rat M+F oral M: 9100 Technical grade Leuschner, 1979a
(SD) (HPMC) F: 8900 (purity unknown) + 2% ETU
M+F: 9000
Rat M+F oral 6500 Technical grade Hofmann, 1985a
(SD) (tragacanth) (purity unknown)
Rat M+F oral 10 000 Technical grade Hofmann, 1975
(SD) (CMC) (purity unknown)
Rat (SD) M+F dermal (water) > 2000 Technical grade Grundler, 1979
(77.5% purity) + 2% ETU
Mouse (NMRI) M+F intraperitoneal M: 208 Technical grade Leuschner, 1979b
(HPMC) F: 215 (purity unknown) + 2% ETU
M+F: 212
Mouse (NMRI) M+F intraperitoneal 80 Technical grade Hofmann, 1974a
(tragacanth) (purity unknown)
Rat (SD) M+F intraperitoneal M: 318 Technical grade Hofmann & Munk, 1975
(CMC) F: 317 (77.5% purity)
M+F: 318
Rat (SD) M+F inhalation 4 h > 5.7 (mg/l) Technical grade Klimisch & Zeller, 1980
(77.5% purity) + 2% ETU
Rat (SD) M+F inhalation 8 h (well Technical grade Hofmann, 1985b
tolerated) (purity unknown) + 2.2% ETU
Table 2. Acute toxicity of Polyram combi (formulated product)
Species Sex Route LD50 Test Reference
(strain) (vehicle) (mg/kg bw) material
Rat M+F oral > 10 000 Polyram combi Hofmann, 1985c
(SD) (tragacanth) (purity unknown)
Dog M+F oral * Polyram combi Chesterman et al., 1973
(beagle) (water) (purity unknown)
Mouse (NMRI) M+F intraperitoneal approx 80 Polyram combi Hofmann, 1974b
(tragacanth) (purity unknown)
Rat M+F dermal > 2000 Polyram combi Grundler, 1985
(SD) (77.5% purity)
Rat M+F inhalation, (well Polyram combi Hofmann, 1985d
(SD) 8 hr tolerated) (77.5% purity)
Guinea-pig inhalation, >0.8 (mg/L) Polyram combi Hofmann, 1985e
8 hr (purity unknown)
* Estimation of LD50 precluded due to high incidence of vomiting
oral and dermal administration, were practically non-toxic in the
rat with LD50 values of > 6500 mg/kg bw and > 2000 mg/kg bw,
respectively. Moderate acute toxicity was demonstrated upon
intraperitoneal administration of technical material in the mouse
(LD50 = 80-212 mg/kg bw) and rat (LD50 = 318 mg/kg bw). Technical
metiram when administered by the inhalation route resulted in an
LC50 value greater than 5.7 mg/l in the rat. Eight-hour inhalation
of the formulated material, Polyram combi was well tolerated in both
rats and guinea-pigs. Intentional spiking of the technical material
with ETU at levels of 2 to 2.2% did not markedly alter the acute
toxicity profile.
Short-term toxicity studies
Mice
In a preliminary assessment of the toxicity of metiram (96.8%
purity, containing 2.2% ETU), groups of 8 CFLP mice per sex were fed
dietary concentrations of 0, 100, 300, 1000 or 3000 ppm equal to 0,
16, 40, 120 or 399 mg/kg bw/day for males and 0, 15, 44, 142 or 433
mg/kg bw/day for females, respectively, for a period of 4 weeks.
Body-weight gain of males treated with metiram at 1000 and 3000 ppm
were significantly lower than that of the corresponding controls
during the first week of treatment with subsequent recovery of
weight gain in the ensuing weeks. Although no consistent effects on
body weight in treated females were observed on a weekly basis,
overall weight gain of females at the high dose of 3000 ppm was
marginally lower than that of the control animals. Significantly
increased liver weights were recorded in both sexes treated at
levels of 1000 and 3000 ppm. In the absence of routine
histopathological examination, no microscopic correlation could be
drawn from the increased liver weights. There were no effects of
treatment on survival, clinical signs, food consumption or gross
pathological changes. On the basis of the reported findings, a high
dietary level of 1000 ppm was selected for the long-term study in
mice (Hunter et al., 1976b).
A 3-month study was performed with groups of 10 B6C3F1
mice/sex treated with metiram (94.8% purity, ETU <0.2%) at
dietary levels of 0, 300, 1000, 3000 or 7500 ppm, equal to 0, 84,
302, 853 or 2367 mg/kg bw/day in males and 0, 133, 465, 1448 or 3565
mg/kg bw/day in females, respectively. Treatment-related effects
were observed with respect to serum T3 and T4 concentrations.
Serum T4 levels were significantly decreased in both sexes of mice
treated with metiram at dietary levels of 1000 ppm and higher,
whereas T3 levels were significantly increased in male mice treated
at the highest dietary level of 7500 ppm. Histopathological
findings attributable to treatment were denoted in the thyroid in
both sexes treated at dietary levels of 3000 ppm and higher as
minimal to slight hypertrophy and vacuolation of the follicular
epithelium. A slight increase in the severity of fatty degeneration
of the `X zone' in the adrenals was also observed in female mice
treated with metiram at 3000 and 7500 ppm. A dose-related increased
incidence in the presence of a refractile, granular material
presumed to be the test material or a metabolite, was noted in the
superficial layer of the urinary bladder urothelium in all groups of
treated mice. Since no morphological changes were observed in the
urothelium of the bladder to suggest disturbed epithelial function,
the presence of this granular material was considered to be of
doubtful toxicological consequence. Significantly increased organ
weights were recorded for the adrenals in females at dietary levels
of 3000 ppm and higher and for the liver in both sexes treated at
7500 ppm and in males at 3000 ppm. Statistically significant
increases in kidney and testicular weights were judged to be of no
biological consequence. No histopathological correlation could be
determined for the increased liver, kidney or testicular weights.
Group mean body weights and corresponding weight gains were
decreased in males treated at the highest dietary level of 7500 ppm.
Although significant decreases in body weights were recorded in
females treated at dietary levels of 1000 ppm and higher, there was
no dose-response relationship. There were no adverse treatment-
related effects on survival, food consumption or on blood
haematological parameters. The NOAEL for this study was 300 ppm,
equal to 84 mg/kg bw/day, based on decreased serum T4 levels in
both sexes at levels of 1000 ppm and above (Gelbke et al., 1992a).
Rats
Metiram (96.8% purity containing 2.2% ETU) was administered for
a period of 4 weeks to groups of 20 Sprague-Dawley CD rats/sex at
dietary levels of 0, 100, 300, 1000 or 3000 ppm, equal to 0, 10, 30,
100 or 296 mg/kg bw/day in males and 0, 11, 33, 114 or 292 mg/kg
bw/day in females, respectively. Following termination of treatment
with metiram, 10 rats per sex per group were retained for subsequent
2 and 4 week recovery periods with scheduled sacrifices of 5
rats/sex/group on each occasion. Clinical toxicity was manifest as
signs of hind limb paralysis in rats treated at the highest dietary
level of 3000 ppm, which due to severity, resulted in early
sacrifice of 3 females for humane reasons. Histopathological
examination confirmed gross morphological alterations in the muscles
of the hind limbs, characterized by atrophy of the fibres, often
with associated proliferation of sacrolemmal nuclei, and the
presence of groups of vacuoles within muscle fibres. After 4 weeks
of treatment, the microscopic changes were more marked and extensive
in females, occurring in all females and in 2 of 9 males treated at
3000 ppm. After a withdrawal period of 2 weeks, a varying degree of
atrophy of muscle fibres was still present in females previously
treated with 3000 ppm of metiram. Although atrophy was not evident
in animals after a 4-week recovery period, additional changes, not
apparent earlier, were the presence of vacuoles and/or areas of fat
cells within the skeletal fibres. Target organ effects of treatment
with metiram were also evident on the thyroid and kidney. Changes
in the thyroid were apparent as a dose-related increase in the
incidence and severity of thyroid hyperplasia in both sexes treated
at dietary levels of 300 ppm and higher. Microscopic examination of
the kidney revealed varying degrees of hydropic vacuolation of the
proximal tubular epithelial cells in 2 females from each of the 1000
and 3000 ppm groups in the absence of similar effects at any of the
other dose levels or corresponding controls. Examination of bone
marrow smears revealed a depressed number of myeloid cells in males
treated at the high dose after 4 weeks of treatment. Group mean
body-weight gains were significantly lower in males treated at 1000
ppm and higher, and in females treated at 300 ppm and higher when
compared to the controls. Decreased food intake was recorded in
both sexes treated at the highest dietary level of 3000 ppm. There
were no adverse effects of treatment noted upon ocular examination,
haematology, blood chemistry, urinalysis and estrus cycle as
assessed from vaginal smears. The NOAEL was 100 ppm, equal to 10
mg/kg bw/day (Hunter et al., 1976a).
Groups of 35 Sprague-Dawley CFY strain rats per sex were
treated with metiram (96.8% purity containing 2.2% ETU) at dietary
levels of 0, 50, 100, 300 or 900 ppm, equal to 0, 3, 6, 20 or
61 mg/kg bw/day for males and 0, 4, 8, 24 or 76 mg/kg bw/day for
females, respectively, for a period of 13 weeks followed by a 6-week
recovery period. The principal target organs were the thyroid and
skeletal muscle. Treatment-related effects on the thyroid were
manifest as increased thyroid weights of females treated at 300 and
900 ppm, decreased percentage uptake of 131I by the thyroid in males
in all treated groups and in females treated at 100 ppm and higher,
and decreased T4 levels in both sexes at 300 ppm and higher.
Microscopically, an increased incidence of males treated at 900 ppm
had slight to minimal hyperplasia of the thyroid. A minimally
higher incidence at 13 weeks of slight thyroid hyperplasia in
females at 900 ppm and in males at 100 and 300 ppm when compared to
the controls was considered to be of equivocal significance. There
were no significant changes in the thyroid observed after the 6-week
recovery period. Treatment-related morphological changes of the
skeletal muscle denoted by atrophy of the muscle fibres often
accompanied by fat cells and associated proliferation of sacrolemmal
nuclei, were evident in both sexes treated at dietary levels of 300
ppm and higher. Varying degrees of muscular atrophy were still
prevalent after a 6-week recovery period in rats previously treated
at 300 and 900 ppm. Other effects of treatment were exhibited at
the high-dose level of 900 ppm as clinical signs of hind limb
paralysis, decreased body weights and slight reduction in food
intake. There were no treatment-related effects observed with
respect to survival, water consumption, ophthalmoscopy, haematology,
urinalysis or examination of bone marrow smears. Although reduced
iodine uptake by the thyroid was observed at all dietary levels,
these changes were shown to be reversible following cessation of
treatment. At the lowest dietary levels of 50 and 100 ppm, the
effects on iodine uptake were not correlated with changes in thyroid
hormone levels or any overt morphological alterations of the thyroid
gland, thus rendering the toxicological significance of this finding
at 50 and 100 ppm as doubtful. The NOAEL for this study was
therefore 100 ppm, equal to 6 mg/kg bw/day, based on decreased serum
T4 levels and increased thyroid weights at dietary levels of 300
and 900 ppm (Hunter et al., 1977).
Metiram (94.8% purity, ETU <0.2%) was administered for a
period of 3 months to groups of 13 Wistar Chbb:THOM SPF rats per sex
at dietary levels of 0, 5, 80, 320 or 960 ppm, equal to 0, 0.4, 5.8,
23.5 or 73.9 mg/kg bw/day in males and 0, 0.4, 6.7, 27.3 or 88.8
mg/kg bw/day in females, respectively. Neurofunctional assessment
revealed general muscle weakness expressed as ataxia and
significantly reduced forelimb and hindlimb grip strength in females
treated at the highest dietary level of 960 ppm. Perfusion fixation
of tissues from selected rats from the control and treated groups
failed to reveal any associated neuropathological or morphological
alterations of the central or peripheral nervous systems. Thyroid
hormone levels revealed significantly decreased T4 levels in both
sexes treated at the high dose of 960 ppm. Significantly reduced
RBC values were recorded in both sexes at levels of 320 and 960 ppm.
In females, decreases in RBC values were accompanied by decreases in
mean haemoglobin and haematocrit levels. Several statistically
significant changes in blood biochemical parameters of uncertain
toxicological significance were observed at the highest dietary
level of 960 ppm. These changes were recorded as decreased ALAT and
ALP activity (females), decreased creatinine (both sexes), decreased
urea (males), and decreases in the electrolytes; potassium (both
sexes), sodium (females), calcium (both sexes) and magnesium (both
sexes). Decreased phosphorus levels were recorded in males at
dietary levels of 320 ppm and higher. Other effects of treatment
expressed in both sexes at the highest dietary level of 960 ppm were
decreased body weights and corresponding weight gains, as well as
significantly increased thyroid, liver, kidney and testes (males
only) weights. There were no treatment-related gross or
histopathological changes observed in any of the animals from the
treated groups. Treatment with metiram did not result in any
significant effects on survival, food consumption, ophthalmoscopy or
urinalysis. The NOAEL was 80 ppm, equal to 5.8 mg/kg bw/day, based
on changes in haematological and blood chemical parameters at 320
ppm (Gelbke et al., 1992b).
A 13-week inhalation study was conducted with groups of 28
Sprague-Dawley CD rats/sex exposed in nose-only chambers to metiram
(94% purity) 6 hours/day, 5 days/week at concentrations of 0, 2, 20
or 100 mg/m3. Concentrations of ETU in the exposure atmospheres
were calculated to be 0, 0.02, 0.33 or 1.8 mg/m3, respectively.
After termination of treatment, one-half of the rats were sacrificed
while the remaining animals were retained for a 13-week recovery
period. The NOAEL was 2 mg/m3. Pulmonary changes consisting of
"subacute alveolitis", characterized by accumulations of alveolar
macrophages within alveolar lumens, accompanied by some polymorpho-
nuclear leukocytes were observed in rats treated at 20 and 100
mg/m3. It was conjectured that the alveolitis observed was not
treatment-related, but rather, a non-specific dust reaction of the
polymeric active ingredient artificially ground to particle size
capable of reaching the alveoli. Intra-alveolar pigment deposition
was recorded in a single male and female from the high-dose treated
group. There was no evidence of damage to bronchial or bronchiolar
epithelium in any of the treated groups. Mean lung/trachea weights
were increased in both sexes treated at the high dose. Other
changes associated with treatment were depositions of golden brown
granular pigment, similar to that seen in the lungs, within the
renal cortices in the high dose animals and decreased overall body-
weight gain in both males and females treated at 20 (11% and 14%)
and 100 mg/m3 (26% and 33% less than corresponding controls,
respectively). After the 13-week recovery, effects of treatment
persisted in the lungs and kidneys of previously treated animals.
No treatment-related findings were noted on survival,
ophthalmoscopy, haematology or blood chemistry. Tissue residue
analysis performed on the urine, plasma, liver, lung and thyroid
revealed measurable levels of metiram and ETU in the urine and lung
in rats treated at the mid- and high-dose levels (Ulrich, 1986).
Rabbits
A dermal toxicity study was performed with groups of 5 New
Zeeland white KFM rabbits/sex treated with Polyram DF formulation of
metiram on the shaved skin of their backs under occlusive conditions
for 6 hours/day at concentrations of 0 (vehicle control, distilled
water), 25, 50 or 250 mg/kg bw/day for a minimum of 21 consecutive
daily applications. Dermal administration of metiram resulted in
minimal to moderate exfoliation and ulcerative dermatitis in the
skin of rabbits treated at the high-dose level. Similar effects
were not evident at lower dose levels, indicating a NOAEL for local
irritation to the skin of 50 mg/kg bw/day. In the absence of
treatment-related effects on survival, clinical signs, body weights,
food consumption, ophthalmoscopy, haematology, blood chemistry,
organ weights or histopathology of the liver and kidneys, the NOAEL
for systemic toxicity was greater than 250 mg/kg bw/day (Ullmann et
al., 1987).
Dogs
Groups of 4 beagle dogs/sex were treated with metiram (96.8%
purity containing 2.2% ETU) for a period of 4 weeks at dietary
levels of 0, 100, 300, 600 or 900 ppm, equal to 0, 5, 14, 28 or 41
mg/kg bw/day for males and 0, 5, 15, 27 or 43 mg/kg bw/day for
females, respectively. Effects observed were an increased frequency
of micro-follicles in the thyroids associated with minimal depletion
of colloid and minimal hyperplasia of the follicular epithelium in
2/4 males and 2/4 females treated at the high dose of 900 ppm when
compared to the controls. Other changes recorded were significantly
increased liver weights in dogs receiving 600 and 900 ppm, which
were not associated with any correlative histopathological findings.
Significantly increased water consumption was recorded in dogs
treated at 100, 600 and 900 ppm but not at 300 ppm. The significance
of increased fluid intake (calculated over 5-day periods) was
difficult to ascertain since there were no corresponding changes
noted in urinalysis parameters. Erythrocyte, haemoglobin and packed
cell volume values, although significantly decreased in dogs treated
at 900 ppm when compared to the controls, were found to be within
normal limits of variability and therefore of doubtful toxicological
consequence. The NOAEL, based on the aforementioned findings, was
300 ppm, equal to 14 mg/kg bw/day. There were no significant
effects of treatment on survival, clinical signs, body weights, food
consumption, ophthalmoscopy, blood chemistry or urinalysis
(Chesterman et al., 1978).
Metiram (93.6% purity, ETU < 0.2%) was given to groups of 5
beagle dogs per sex at dietary concentrations of 0, 30, 80, 1000 or
3000 ppm, equal to 0, 0.9, 2.5, 29.8 or 76.9 mg/kg bw/day in males
and 0, 1.1, 2.7, 29.9 or 92.7 mg/kg bw/day in females, respectively,
for a period of 52 weeks. The principal effects of treatment were
manifest on the thyroid. Microscopic examination revealed thyroid
follicular hyperplasia in dogs of both sexes treated at 1000 and
3000 ppm. This finding was associated with an increased size and
weight of this organ at 3000 ppm. Gross necropsy revealed thyroid
thickening in several animals from both the control and treated
groups. This observation was, however, only correlated with
histopathological change at dietary levels of 1000 ppm and higher,
rendering the significance of thyroid thickening at lower levels
uncertain. Serum T4 levels were decreased in both sexes at 3000 ppm
and in males at 1000 ppm. A dose-related increased incidence of dogs
with focal hepatic lipofuscin pigment deposition was noted in both
sexes at 1000 ppm and higher. Other effects of treatment with
metiram were described as an increased incidence of diarrhoea in
dogs treated at 80 (attributed to single male), 1000 and 3000 ppm;
slight evidence of anaemia (both sexes at 1000 and 3000 ppm)
involving an increased number of reticulocytes (both sexes at 3000
ppm); decreased body-weight gain at 3000 ppm in males and to a
lesser extent in females; and decreased food intake in both sexes at
3000 ppm and in females at 1000 ppm. Significant changes in blood
chemical parameters at dietary levels of 1000 ppm and higher were
evident as increased lipid, cholesterol, triglycerides, phospholipid
levels, ALP activity, and total protein, and decreased albumin and
A/G ratio as well as disturbances in the protein electrophoretic
pattern. There were no effects of treatment on survival,
neurological parameters, ophthalmoscopy or urinalysis. The NOAEL
for this study was 80 ppm, equal to 2.5 mg/kg bw/day (Corney et
al., 1991).
Monkeys
A preliminary toxicity assessment was performed with groups of
single male and female rhesus monkeys administered metiram (96.8%
purity containing 2.2% ETU) orally by gavage as a suspension in 0.5%
mucilage of tragacanth in water at dose levels of 50, 100 or 500
mg/kg bw/day for a period of 4 weeks. Treatment-related toxicity
was evident as salivation at the time of dosing or immediately
thereafter and occasional emesis in monkeys treated at 100 and 500
mg/kg bw/day, body-weight loss in the single female treated at 500
mg/kg bw/day and changes of the thyroid, representative of early
follicular hyperplasia in the single high-dose female. The low dose
of 50 mg/kg bw/day was well tolerated with no overt signs of
toxicity (Sortwell et al., 1977).
Metiram (96.8% purity containing 2.2% ETU) was administered
orally by gavage to groups of 4 rhesus monkeys/sex for a period of
26 weeks at dose levels of 0 (vehicle control, 0.5% tragacanth in
distilled water), 5, 15 or 75 mg/kg bw/day. After termination of
treatment, 1 male and 1 female were retained untreated for a period
of 15 weeks as recovery animals. In a separate, but concurrently
conducted study, a further 2 male and 2 female monkeys were treated
with metiram at doses of 0, 5 or 75 mg/kg bw/day for 26 weeks to
monitor thyroid function. The primary effects of treatment with
metiram were evident in the thyroid and were characterized at dose
levels of 15 and 75 mg/kg bw/day as minimal thyroid follicular
hyperplasia, significantly decreased T3 and T4 levels and
increased thyroid weights. After a 15-week recovery, morphological
changes of the thyroid were still apparent in monkeys previously
treated at the 15 and 75 mg/kg bw/day dose levels. Assessment of
thyroid function revealed an initial marked reduction in iodine
uptake at dose levels of 5 and 75 mg/kg bw/day, followed by a
significant increase in uptake during the latter part of the study.
In the absence of any correlation to thyroid hormone levels or
morphological alterations, no toxicological significance was
attributed to fluctuations in iodine uptake by the thyroid at the
lowest level of 5 mg/kg bw/day. Other effects resulting from
treatment were an increased incidence of salivation occurring at the
time of dosing at the high dose. Significantly increased liver
weights at the high-dose level were not associated with any
histopathological findings. Treatment with metiram did not
significantly affect survival, body-weight gains, food or water
consumption, ophthalmoscopy, haematology, urinalysis or examination
of bone marrow smears. The NOAEL was 5 mg/kg bw/day (Sortwell et
al., 1979).
Long-term toxicity/carcinogenicity studies
Mice
Dietary administration of metiram (96.8% purity containing 2.2%
ETU) to groups of 52 CFLP mice/sex at levels of 0, 100, 300 or 1000
ppm, equal to 0, 8, 24 or 79 mg/kg bw/day for males and 0, 9, 29 or
95 mg/kg bw/day for females, respectively, for a minimum period of
88 weeks resulted in a NOAEL for in-life parameters of 300 ppm,
equal to 24 mg/kg bw/day. Although there were no statistically
significant differences in mortality in the treated groups, a
marginally higher mortality incidence from week 40 onwards was
recorded in females treated at 1000 ppm, resulting in a minimum 25%
survival rate being reached in this group before the corresponding
controls. Group mean body weights were significantly decreased in
both sexes treated at the 1000 ppm dietary level. Food consumption
in males treated at 1000 ppm was only marginally (5%) lower than the
corresponding controls during the first year of treatment. Clinical
signs and examination of bone marrow smears were not adversely
affected by treatment with metiram. Under the conditions of the
present study, treatment with metiram at dietary levels up to and
including 1000 ppm failed to uncover any evidence of carcinogenic
potential (Hunter et al., 1979).
Rats
Groups of 50 Sprague-Dawley CD rats/sex were administered
metiram (96.8% purity containing 2.2% ETU) in the diet at levels of
0, 5, 20, 80 or 320 ppm, equal to 0.2, 0.8, 3.1 or 12.3 mg/kg bw/day
in males and 0, 0.2, 1.0, 3.8 or 15.5 mg/kg bw/day in females,
respectively, for 119 weeks for males and 111 weeks for females.
Satellite groups of 30 male and 30 female rats per group were used
for blood sampling and thyroid function tests and were then killed
after 102 weeks of treatment. Treatment with metiram resulted in a
NOAEL of 80 ppm (equal to 3.1 mg/kg bw/day), based on an increased
incidence of rats treated at the next higher dietary level of 320
ppm with muscular atrophy. There were no consistent treatment-
related effects with respect to survival, clinical signs, body
weights, food or water consumption, ophthalmoscopy, haematology,
blood chemistry, thyroid function, urinalysis or organ weights.
There was no evidence of metiram-induced carcinogenic potential
(Hunter et al., 1981).
Reproduction studies
Rats
A three-generation (two-litter per generation) reproduction
study was conducted with groups of 12 male and 24 female Crl:COBS
CD(SD)BR rats fed metiram (96.8% purity containing 2.2% ETU) at
dietary levels of 0, 5, 40 or 320 ppm, equal to 0, 0.2, 1.8 or 14.2
mg/kg bw/day in males and 0, 0.3, 2.3 or 19.8 mg/kg bw/day in
females, respectively. In the F0 generation, treatment with
metiram commenced at least 60 days prior to mating. In the F1 and
F2 generations, the F1b and F2b derived parental animals,
respectively were exposed to the test material for a minimum period
of 90 days. At day 21 post-partum, 10 F3a pups/sex from each group
were selected for detailed gross examination, recording of organ
weights and histopathological assessment. At the second pairing of
the F2b generation, all pregnant females from each of the dose
levels were sacrificed on day 20 of gestation for teratological
examination. Appearance of spermatozoa in the vaginal smear or the
presence of a copulation plug was considered to be day 0 of
gestation. Decreases in body-weight gain (4-7%) were noted in both
the F0 and F1 generation males and females treated at 320 ppm when
compared to the controls. Slightly depressed (7-8%) food intake was
observed in males fed metiram during the F0 and F1 generations.
Body weights and food consumption were not adversely affected by
treatment at lower dietary levels or in any of the treated groups
from the F2 generation when compared to the controls. There were
no treatment-related effects on parental mortality, clinical signs,
food conversion ratios, reproductive parameters or gross
pathological alterations. Statistically significant decreases in
the mean number of pups born were observed in all treated groups
during the second mating of both the F1 and F2 generations.
Although the differences were statistically significant, there was
no dose-response relationship and all values in the treated groups
generally fell within the reported laboratory standard values.
Total litter loss was significantly decreased at 320 ppm during the
second mating of the F1 generation. In the absence of similar
effects in the first mating of the F1 or in either of the matings
in the F0 and F2 generations, the toxicological significance of
this finding was dubious. Statistically significant decreases in
mean litter weights were observed in the treated groups during the
F1 and F2 generations. Since the mean pup weights in the treated
groups were comparable to the controls, the decreased mean litter
weights may, in part, be attributed to the correspondingly smaller
litter sizes observed in these groups. With regard to the
teratological component, there were no effects on pregnancy rate,
pre- or post-implantation loss, number of live fetuses, embryo/fetal
resorptions, fetal sex ratio or fetal/litter weights. Metiram was
not teratogenic as determined by gross, skeletal and visceral
examination of fetuses, following in utero exposure at dietary
levels as high as 320 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg
bw/day, based on decreased parental body weight and food consumption
recorded in the F0 and F1 generations treated at 320 ppm (Cozens
et al., 1981).
Special studies on teratogenicity
Rats
A preliminary range-finding study was performed with groups of
6 non-pregnant Crl:Cobs CD(SD)BR female rats administered metiram
(96.8% purity containing 2.2% ETU) orally by gavage at 0 (vehicle
control, sodium carboxymethyl cellulose), 150, 300, 600 or 1200
mg/kg bw/day for 10 consecutive days. Dose-related clinical effects
primarily affecting the nervous system were observed at all dose
levels. In the absence of microscopic examination,
histopathological correlation could not be ascertained. Other
effects of treatment were related to decreased body-weight gains at
all dose levels, depressed food intake at 300 mg/kg bw/day and
higher and decreased water consumption in rats treated at 600 and
1200 mg/kg bw/day (Palmer & Simmons, 1979a).
Groups of 20 time-mated female Crl:Cobs CD(SD)BR rats were
treated with metiram (96.8% purity containing 2.2% ETU) daily by
gavage during days 6 to 15 of gestation, inclusive, at dose levels
of 0 (vehicle control, sodium carboxymethyl cellulose), 40, 80 or
160 mg/kg bw/day. The day of mating, determined by the presence of
a vaginal plug or sperm in the vaginal smear, was designated as day
0 of gestation. Decreased body-weight gain (4-9%) was noted in the
high-dose animals. There were no significant effects with respect
to clinical signs, food or water consumption or gross organ/tissue
changes. A slight increase in the pre- and post-implantation loss
was recorded in the 160 mg/kg bw/day group, in conjunction with a
significant decrease in the mean number of live fetuses and
decreased litter weight in this group. It is unlikely that the
increased pre-implantation loss at the high dose was related to
treatment, since theoretically, treatment was not initiated until
after implantation occurred. Nevertheless, the combined effect of
pre- and post-implantation loss may have, to a certain degree,
influenced the decreased litter size and weight noted at the high
dose level. There were no effects of treatment on the incidence of
early or late resorptions, mean fetal weight or sex ratio. The
NOAEL for embryo/fetotoxicity was 80 mg/kg bw/day, based on slight
decreases in litter size and weights. Treatment with metiram failed
to uncover any evidence of teratogenic potential. The NOAEL for
maternal toxicity was 80 mg/kg bw/day, based on decreased body-
weight gain (Palmer & Simmons, 1979a).
Rabbits
In a range-finding study, groups of 3 pregnant Himalayan
rabbits were treated orally by gavage with metiram (97.9% purity,
ETU <0.2%) during days 7 through 19 of gestation at dose levels
of 0 (vehicle control, distilled water with 0.5% carboxymethyl
cellulose), 50, 100 or 200 mg/kg bw/day. Reduced body-weight gains
and food intake were recorded at the two highest dose levels. The
significance of the incidence of single abortion at each of the dose
levels, upon conduct of the definitive study, was confirmed to be
treatment-related. No further relevant details were provided
(Gelbke et al., 1988).
Metiram (97.9% purity, ETU < 0.2%) was administered orally
by gavage at 0 (vehicle control, distilled water with 0.5%
carboxymethyl cellulose), 10, 40 or 120 mg/kg bw/day to groups of 15
artificially inseminated Himalayan rabbits on days 7 through 19 of
gestation. The day of artificial insemination was designated as day
0 of gestation. Treatment with metiram elicited signs of maternal
toxicity at 40 and 120 mg/kg bw/day, denoted by abortion, decreased
body weight and food consumption, the latter associated with
reduction or absence of defecation. The high incidence of abortion
(8/15) recorded at 120 mg/kg bw/day, in conjunction with a single
death culminated in only 6/15 rabbits treated at this level with
litters available for examination. Although 2 of 15 animals aborted
at 40 mg/kg bw/day, there were, nevertheless, adequate numbers of
litters secured in the control, 10 and 40 mg/kg bw/day dose groups.
Since a dose level of 10 mg/kg bw/day was without significant
effects, this dose was considered the NOAEL for maternal toxicity.
The NOAEL for developmental toxicity was 40 mg/kg bw/day based on
the slight decrease in mean fetal weights recorded at 120 mg/kg
bw/day. A slight decrease in the number of live male fetuses in the
120 mg/kg bw/day group was considered to be of no toxicological
consequence, in light of the comparable number of female fetuses and
the total number of fetuses in the 120 mg/kg bw/day group relative
to the controls. There were no significant effects of treatment on
the mean number of live fetuses, early or late resorptions, post-
implantation loss, or placental weight. There was no evidence
suggestive of teratogenic potential with metiram at any of the dose
levels investigated (Gelbke et al., 1988).
Special studies on genotoxicity
Results of mutagenicity assays conducted with metiram are
presented in Table 3. The tests conducted to evaluate potential for
gene mutation in bacteria and induction of chromosomal aberration in
rat bone marrow were negative. Unscheduled DNA synthesis in rat
hepatocytes and a dominant lethal study in mice were also negative.
Although metiram demonstrated potential for inducing sister
chromatid exchange (SCE) in Chinese hamster ovary cells in the
absence and presence of mouse microsomal activation, there was
nevertheless, no significant induction of SCE observed with rat
microsomal activation. Moreover, an in vivo SCE assay in bone
marrow cells of the Chinese hamster was negative. Metiram exhibited
a weak promotion activity in mouse embryo fibroblasts in the absence
of a direct transformation response. The Meeting concluded that
metiram was not genotoxic.
Special studies on irritation and sensitization
The eye and skin irritation potential of technical metiram
(containing 2.2% ETU) were investigated in six Vienna white rabbits.
There were no signs of irritation when metiram was applied to the
external ear for 20 hours or to the backs for 1, 5 or 15 minutes.
When applied to the back for 20 hours, slight reddening occurred 24
hours after application with subsequent scaling after 8 days.
Metiram (50 mg) when introduced into the conjunctival sac of the eye
of these rabbits produced slight redness and edema after 1 hour,
with no signs of irritation after 8 days (Zeller, 1985a).
Polyram combi, a formulation of technical metiram, was
administered to eight Vienna white rabbits to determine skin and eye
irritation potential. A 50% aqueous suspension of the test material
when applied to the backs of the rabbits for a period of 20 hours,
resulted in slight erythema of the skin, which was reversible within
8 days. The instillation of 50 mg of Polyram combi to the
conjunctival sac of the rabbit eye caused redness and edema which
were reversible after 8 days. When 50 µl of a 20% aqueous
suspension was applied, slight reddening of the mucosa occurred,
whereas 50 µl of a 2% aqueous suspension was tolerated without any
irritation (Zeller, 1985b).
Metiram technical demonstrated severe sensitizing effects on
the skin of female Pirbright white, Dunkin Hartley guinea-pigs when
tested according to the methods of the Magnusson & Kligman
maximization test (Jackh, 1982).
Table 3. Genotoxicity of metiram
Test Test system Concentration Purity Results Reference
Reverse mutation S. typhimurium 0, 1, 3, 10, 31, Technical negative Oesch, 1977
(in vitro) TA 98, 100, 1537 100, 310, 1000, 1., 2. (rat)
2000 µg/plate
S. typhimurium 0, 1, 10, 50, 100, Technical + 2.2% ETU negative Gelbke & Engelhardt,
TA 98, 100, 1535, 1537 500, 2500 µg/plate 1., 2. 1985
(rat & mouse)
Reverse mutation/host S. typhimurium 500, 1670, Technical + 2.2% ETU negative Jagannath & Myhr, 1985
mediated (in vivo) TA 1530 5000 mg/kg bw
Male CD-1 mice (single oral doses)
(inoculated ip)
Point mutation HGPRT locus of Chinese 0, 0.681, 1.0, 4.64, Technical + 2.2% ETU negative, 1. Gelbke & Jackh, 1985
(in vitro) hamster ovary (CHO) cell 6.81, 10, 46.4, equivocal, 2.
line, K1 68.1, 100 µg/ml (rat)
HGPRT locus of Chinese 0.5, 1, 5, 10, 50, Premix 95% negative Hoffman & Jackh, 1990
hamster ovary (CHO) cell 100, 500 µg/ml 1., 2. (mouse)
line, K1
SCE (in vitro) Chinese hamster ovary 0, 40, 60, 80, 100, Technical + 2.2% ETU positive, 1. Ivett & Myhr, 1986a
CHO, WB1 cells 125, 150, 175, 200 positive, 2.
µg/ml (mouse)
negative, 2.
(rat)
SCE (in vivo) Chinese hamster, LMP 1000, 3330, 10 000 Premix 95% negative Miltenburger et al.,
stock (bone marrow) mg/kg bw 1990
Table 3 (contd)
Test Test system Concentration Purity Results Reference
Chromosome aberration Rat, male Fischer 344 0, 0.24, 1.2, 2.4 Technical + 2.2% ETU negative Ivett & Myhr, 1986b
(in vivo) (bone marrow) g/kg bw (acute) 0,
0.02, 0.1, 0.2 g/kg
bw (5 repeated oral
doses)
Transformation Mouse embryo fibroblasts 0, 0.1, 0.25, 0.5, Technical + 2.2% ETU transformation: Tu et al., 1985
promotion (in vitro) C3H-10T 1/2 (clone 8) 0.75, 1.0 µg/ml negative
cell system promotion
activity:
weakly
positive
Unscheduled DNA Rat (Fischer 344, male) 0.492, 1.23, 2.46, Technical + 2.2% ETU negative Cifone & Myhr, 1984
synthesis (in vitro) hepatocytes 4.92, 12.3, 24.6,
49.2, 160 µg/ml
Dominant lethal Mouse, CD-1 male 0, 600, 1200, 2400 Technical + 2.2% ETU negative Palmer & Simons, 1979b
(in vivo) mg/kg bw/day
Results:
1. = in the presence of metabolic activation derived from the indicated species
2. = in the absence of metabolic activation
COMMENTS
Metiram was incompletely absorbed when administered orally to
rats. Elimination was primarily via the faeces, with minimal
biliary excretion. Comparatively higher urinary excretion at low
doses suggested that metiram may be more poorly absorbed at higher
doses. Highest residual tissue levels were found in the thyroid and
kidney, with slightly higher concentrations present in females than
in males. Comparison of tissue residues after single or multiple
doses suggested slight accumulation in the body with multiple
dosing.
The metabolism of metiram has not been completely elucidated.
In the rat, the predominant urinary components were polar and were
identified as ethylenediamine, N-acetyl-ethylenediamine,
ethanolamine, oxalic acid and glycine. Major, less polar components
were ethyleneurea, ETU and EBIS.
Metiram was practically non-toxic upon acute oral, dermal and
inhalation administration to rats. WHO has classified metiram as
unlikely to present acute hazard in normal use.
The principal target organ upon repeated dietary exposure to
metiram was the thyroid.
Mice treated with metiram for three months at 0, 300, 1000,
3000 or 7500 ppm in the diet revealed minimal to slight hypertrophy
and vacuolation of the thyroid follicular epithelium in both sexes
at levels of 3000 ppm and higher. A NOAEL of 300 ppm (equal to 84
mg/kg bw/day) was based on decreased serum T4 levels in both sexes
at levels of 1000 ppm and above.
Thirteen-week dietary administration of metiram to SD CFY rats
at 0, 50, 100, 300 or 900 ppm revealed a NOAEL of 100 ppm (equal to
6 mg/kg bw/day) based on decreased serum T4 levels and increased
thyroid weights at dietary levels of 300 and 900 ppm. Slight to
minimal hyperplasia of the thyroid was observed at 900 ppm.
Although reduced iodine uptake by the thyroid was observed at all
dietary levels, these changes were shown to be reversible following
cessation of treatment. At the lowest dietary levels of 50 and 100
ppm, the effects on iodine uptake were not correlated with changes
in thyroid hormone levels or any overt morphological alterations of
the thyroid gland, thus rendering the toxicological significance of
this finding doubtful.
In a recently conducted three-month study with Wistar rats
receiving metiram at 0, 5, 80, 320 or 960 ppm in the diet, decreased
serum T4 levels and increased thyroid weights were observed at 960
ppm. Slight evidence of anaemia was observed at 320 ppm, indicating
a NOAEL of 80 ppm (equal to 5.8 mg/kg bw/day).
Other effects of treatment in the diet with metiram in the rat
were manifest as hind limb paralysis with corresponding atrophy of
muscle fibres. In the 13-week study with SD CFY rats, microscopic
changes in muscle fibres at levels of 300 ppm (equal to 20 mg/kg
bw/day) and higher were still prevalent in previously treated rats
after the 6-week recovery period. Muscular atrophy was observed in
a long-term study in SD CD rats treated at the highest level of 320
ppm (see below). General muscle weakness/ataxia and reduced grip
strength of the limbs with no histopathological consequences were
observed in the 3-month study with Wistar rats fed metiram at the
highest level of 960 ppm.
A 52-week study in dogs at dietary levels of 0, 30, 80, 1000 or
3000 ppm yielded a NOAEL of 80 ppm (equal to 2.5 mg/kg bw/day) based
on thyroid follicular hyperplasia with increased size, thickening
and weight of this organ, in conjunction with decreased serum T4
levels at dietary levels of 1000 ppm and above. Other effects
recorded at 1000 ppm and above were a dose-related increased
incidence of focal hepatic lipofuscin pigment deposition, slight
evidence of anaemia, diarrhoea and changes in blood biochemical
parameters. A preliminary 4-week study in dogs uncovered an
increased frequency of microfollicles in the thyroid, in association
with colloid depletion and minimal hyperplasia in both sexes treated
at the highest level of 900 ppm (equal to 41 mg/kg bw/day).
Metiram given by gavage to rhesus monkeys at dose levels of 0,
5, 15 or 75 mg/kg bw/day for a period of 26 weeks indicated a NOAEL
of 5 mg/kg bw/day based on significantly decreased serum T3 and T4
levels, increased thyroid weights and minimal thyroid follicular
hyperplasia at 15 and 75 mg/kg bw/day. Morphological changes of the
thyroid were still apparent after a 15-week recovery period. In the
absence of any correlation between thyroid hormone levels and
morphological alterations, no significance was attributed to
fluctuations in iodine uptake by the thyroid recorded at 5 mg/kg
bw/day.
Long-term dietary treatment of mice with metiram at 0, 100, 300
or 1000 ppm resulted in a NOAEL of 300 ppm (equal to 24 mg/kg
bw/day) based on decreased body weights recorded at 1000 ppm.
Chronic dietary administration of metiram to SD CD rats at 0,
5, 20, 80 or 320 ppm revealed muscular atrophy at 320 ppm (equal to
12 mg/kg bw/day), with a NOAEL of 80 ppm (equal to 3.1 mg/kg
bw/day).
Metiram was not carcinogenic when fed to mice or rats at
dietary levels of up to 1000 and 320 ppm, respectively.
A three-generation, two litter per generation reproduction
study in rats treated at 0, 5, 40 or 320 ppm in the diet failed to
reveal any adverse effects on reproductive parameters. The NOAEL
was 40 ppm (equal to 1.8 mg/kg bw/day), based on decreased parental
body weight and food consumption recorded in the F0 and F1
generations treated at 320 ppm.
Metiram when administered to pregnant rats at 0, 40, 80 or 160
mg/kg bw/day or rabbits at 0, 10, 40 or 120 mg/kg bw/day during
critical periods of organogenesis was not teratogenic at any dose.
The NOAEL for maternal toxicity in the rat was 80 mg/kg bw/day based
on decreased body-weight gain and in the rabbit the NOAEL was 10
mg/kg bw/day based on increased abortions, decreased body weights
and food consumption. The NOAELs for embryo/fetotoxicity were 80
mg/kg bw/day in the rat based on slight decreases in litter size and
weight and 40 mg/kg bw/day in the rabbit based on decreases in mean
fetal weights.
Metiram has been tested in a series of in vitro and in vivo
genotoxicity assays. The Meeting concluded that metiram is not
genotoxic.
The Meeting allocated an ADI of 0-0.03 mg/kg bw based on a
NOAEL of 2.5 mg/kg bw/day in the 52-week study in dogs, using a 100-
fold safety factor. This ADI is supported by the NOAEL of 3.1 mg/kg
bw/day observed in the long-term study in rats. This ADI served as
the basis for a group ADI that was established for metiram alone or
in combination with mancozeb, maneb, and zineb.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 300 ppm, equal to 24 mg/kg bw/day (88-week study)
Rat: 80 ppm, equal to 3.1 mg/kg bw/day (111-week study)
40 ppm, equal to 1.8 mg/kg bw/day
(reproduction study)
Rabbit: 10 mg/kg bw/day (teratogenicity study)
Dog: 80 ppm, equal to 2.5 mg/kg bw/day (52-week study)
Monkey: 5 mg/kg bw/day (26-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, maneb, and
zineb).
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Chesterman, H., Heywood, R. & Barker M. (1973) Polyram Combi acute
oral toxicity study in beagle dogs. Report BASF 73/0047, BASF
40/73703. Unpublished report dated October 5, 1973 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Chesterman, H., Heywood, R., Ball, S., Street, A. & Prentice, D.
(1978) Metiram (active ingredient) oral toxicity study in beagle
dogs (dietary intake for 4 weeks). Report BASF 78/0154, BSF
201/76422. Unpublished report dated April 26, 1978 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Cifone, M. & Myhr, B. (1984) Report on the evaluation of metiram
tech. in the rat primary hepatocyte unscheduled DNA synthesis assay.
Report RZ 84/209. Unpublished report dated July 5, 1984 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Corney, S.J., Allen, T.R., Janiak, T. & Springall, C. (1991) 52-week
oral toxicity (feeding) study with metiram premix 95% in the dog.
Report No. 91/10786. Unpublished report dated August 22, 1991 from
RCC, Research & Consulting Company Ltd., Itingen, Switzerland.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Cozens, D., Simons, R., Clark, R., Offer, J. & Gibson, W. (1981)
Effect of metiram technical on reproductive function of multiple
generations in the rat. Report RZ 81/132, BSF 200/80692. Unpublished
report dated March 18, 1981 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Gelbke, H. & Engelhardt, G. (1985) Report on the study of metiram
(techn.) in the Ames test. Report RZ 85/020. Unpublished report
dated February 7, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H. & Jackh, R. (1985) Report on a point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram. Report RZ 85/238. Unpublished report dated July 30, 1985
from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Gelbke, H., Hellwig, J. & Hildebrand, B. (1988) Report on the study
of the prenatal toxicity of metiram-premix 95% in rabbits after oral
administration (gavage). Report BASF 88/0154 and Supplement BASF
88/0262. Unpublished report dated May 26, 1988 from BASF, Department
of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. and Hildebrand, B. (1992a) Study of the oral
toxicity of metiram premix 95% in B6C3F1 mice. Administration in
the diet for 3 months. Report No. 92/11223. Unpublished report dated
October 16, 1992 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Gelbke, H., Mellert, W. & Hildebrand, B. (1992b) Study of the oral
toxicity of metiram premix 95% in Wistar rats. Administration in the
diet for 3 months including the examination of neurotoxicology
(neurofunctional observational battery). Report No. 92/11224.
Unpublished report dated October 16, 1992 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1979) Study of the acute dermal toxicity of "Metiram
tech. with 2% ETU" on the rat (Translation). Report RZ 79/032.
Unpublished report dated September 25, 1979 from BASF,
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Grundler, O.J. (1985) Report on the study of the acute dermal
toxicity of Polyram Combi (BAS 222 01 F) in the rat (Translation of
the original report dated September 25, 1979). Report RZ 85/026.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Girkin, R. & Jackson R. (1984) Report on the
study of dermal absorption of metiram in rats. Report RZ 85/158, HRC
BSF 411/84694. Unpublished report dated October 10, 1984 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Hawkins, D., Elsom, L., Midgley, I., Biggs, S. & McCay, C. (1985)
The biokinetics and metabolism of 14C-metiram in the rat. Report
BASF 85/0470, HRC BSF 410/85720. Unpublished report dated October
28, 1985 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1974a) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF, Gewerbehygiene
und Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hofmann, H. (1974b) Acute intraperitoneal toxicity of Polyram Combi
(technical active ingredient) to the mouse. Report RZ 74/011.
Unpublished report dated March 25, 1974 from BASF
Aktiengesellschaft, FRG.
Hofmann, H. & Munk, R. (1975) Acute intraperitoneal toxicity of the
technical active ingredient metiram to the rat. Report RZ 75/006.
Unpublished report dated June 4, 1975 from BASF, Gewerbehygiene und
Toxikologie, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1975) Acute oral toxicity of the technical active
ingredient metiram to the rat. Report RZ 75/005. Unpublished report
dated June 4, 1975 from BASF, Gewerbehygiene und Toxikologie, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann H. (1985a) Report on the study of the acute oral toxicity of
Polyram Combi, technical active ingredient, in the rat (Translation
of original report dated March 25, 1974). Report BASF 85/0485.
Unpublished report dated February 4, 1985 from BASF, Department of
Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985b) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi, technical active
ingredient, in the rat (Translation of original report dated March
25, 1974). Report RZ 85/028. Unpublished report dated February 4,
1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985c) Report on the study of the acute oral toxicity
of Polyram Combi (BAS 222 01 F) to rats (Translation of original
report dated March 25, 1974). Report RZ 85/121. Unpublished report
dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Hofmann, H. (1985d) Report on the study of the acute inhalation
toxicity (inhalation hazard) of Polyram Combi (BAS 222 01 F) in rats
(Translation of original report dated March 25, 1974). Report RZ
85/124. Unpublished report dated April 12, 1985 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hofmann, H. (1985e) Report on the study of the acute inhalation
toxicity of the spray of a 5% aqueous suspension of Polyram Combi
(BAS 222 01 F) to guinea-pigs (Translation of original report dated
March 25, 1974). Report RZ 85/125. Unpublished report dated April
12, 1985 from BASF, Department of Toxicology, Ludwigshafen/Rhein,
FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hofmann, H. & Jackh, R., 1990. Report on the point mutation test
carried out on CHO cells (HGPRT locus) with the test substance
metiram premix 95% with B6C3F1 mice microsomal fraction. Report
No. 90/0285. Unpublished report dated August 2, 1990 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A. & Prentice, D.
(1976a) Preliminary assessment of metiram toxicity to rats in
dietary administration for four weeks followed by a two and a four
week withdrawal period. Report RZ 76/024, BSF 172/76245. Unpublished
report dated October 20, 1976 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Hunter, B., Bridges, J. & Prentice, D. (1976b) Preliminary
assessment of metiram toxicity to mice in dietary administration for
4 weeks. Report RZ 76/014, BSF 170/76108. Unpublished report dated
June 7, 1976 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Heywood, R., Street, A., Prentice, D. &
Offer, J. (1977) Metiram toxicity to rats in dietary administration
for 13 weeks followed by a 6 week withdrawal period (final report).
Report RZ 77/043, BSF/197/77612 and Addendum BASF 87/0205, BSF
197/8713. Unpublished report dated November 16, 1977 and addendum
dated May 29, 1987 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Prentice, D. & Gregson, R., 1979. Metiram
tumorigenicity to mice in long term dietary administration (final
report). Report RZ 79/033, BSF 198/78265. Unpublished report dated
June 5, 1979 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Hunter, B., Barnard, A., Street, A., Heywood, R., Prentice, D.,
Offer, J. & Gibson, W. (1981) Metiram toxicity and tumorigenicity in
prolonged dietary administration to the rat. Report RZ 81/280, BSF
199/80391, WNT No. 77/951 and Addendum BASF 89/0001. Unpublished
report dated May 7, 1981 and addendum date December 19, 1988 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986a) Report on the mutagenicity evaluation
of metiram in an in vitro sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells/amended final report. Report RZ
86/082. Unpublished report dated March 11, 1986 from Litton
Bionetics Inc., Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Ivett, J. & Myhr, B. (1986b) Report on the mutagenicity evaluation
of metiram technical K38/33A in the rat bone marrow cytogenetic
assay. Report RZ 86/265. Unpublished amended report dated August 4,
1986 from Litton Bionetics, Maryland, USA. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jackh, R. (1981) Report on the study of the acute oral toxicity of
metiram technical grade in the rat (Translation). Report BASF
92/10669. Unpublished report dated July 9, 1981 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, FRG. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
Jackh, R. (1982) Report on the study of the sensitizing effect of
metiram techn. in the guinea-pig -maximization test (Translation of
original report dated August 21, 1981). Report RZ 82/068.
Unpublished report dated February 16, 1982 from BASF, Department of
Toxicology, Ludwigshafen, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Jagannath, D. & Myhr, B. (1985) Report on the mouse host-mediated
assay of metiram tech. K38/33A. Report RZ 85/210. Unpublished report
dated June 28, 1985, from Litton Bionetics Inc., Maryland, USA.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Klimisch, H.J. & Zeller, H. (1980) Report on the determination of
the acute inhalation toxicity LC50 metiram techn. with 2% ETU as a
dust aerosol after 4-hour exposure in Sprague-Dawley rats
(Translation of original report dated September 15, 1980). Report RZ
83/064. Unpublished report dated September 15, 1980 from
Gewerbehygiene und Toxikologie, FRG. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Leuschner, F. (1979a) The acute oral toxicity of the preparation
metiram, techn. agent with 2% ETU in rats. Report BASF 79/0161, WNT-
Nr. 77/951. Unpublished report dated August 20, 1979 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Leuschner, F. (1979b) The acute intraperitoneal toxicity of the
preparation metiram, techn. agent with 2% ETU in mice. Report BASF
79/0162, WNT-Nr. 77/951. Unpublished report dated August 20, 1979
from Laboratorium fur Pharmakologie und Toxikologie, Hamburg, FRG.
Submitted to WHO by BASF Aktiengesellschaft, FRG.
Miltenburger, H., Volkner, W. & Heidemann, A. (1990) Sister
chromatid exchange assay in bone marrow cells of the Chinese hamster
with metiram premix 95%. Report No. 90/0569. Unpublished report
dated September 18, 1990 from CCR, Cytotest Cell Research Gmbh &
Co., Rossdorf, FRG. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Oesch, F. (1977) Ames test for metiram. Report RZ 77/027.
Unpublished report dated August 22, 1977 from the Institute of
Pharmacology, University of Mainz, Germany. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Palmer, A. & Simons, R. (1979a) Effect of metiram technical on
pregnancy of the rat. Report RZ 79/065, BSF 302/79616. Unpublished
report dated August 3, 1979 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by BASF Aktiengesellschaft,
FRG.
Palmer, A. & Simons, R. (1979b) Dominant lethal assay of metiram
technical in the male mouse. Report RZ 79/069, BSF 304/79860.
Unpublished report dated November, 1979 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Heywood, R., Allen, D., Prentice, D. & Cherry, C.
(1977) Metiram preliminary oral toxicity study in rhesus monkeys.
Repeated dosage for 4 weeks. Report BASF 77/0149, BSF 265/77264.
Unpublished report dated April 15, 1977 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by BASF
Aktiengesellschaft, FRG.
Sortwell, R., Allen, D., Heywood, R. & Street, A. (1979) Metiram
(containing 2.2% ethylenethiourea) oral toxicity study in rhesus
monkeys (repeated dosage for 26 weeks with recovery period). Final
report. Report BASF 79/0082, BSF 267/78263. Unpublished report dated
January 15, 1979 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Tu, A., Sivak, A., Breen, P. & Hatch, K. (1985) Report on the
evaluation of metiram in the C3H-10T 1/2 cell system for
transformation and promotion activities. Report RZ 85/209.
Unpublished report dated June 18, 1985 from Arthur D. Little, Inc.,
Maryland, USA. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ullmann, L., Sacher, R., Porricello, T., Luetkemeier, H., Vogel, W.,
Vogel, O., Wilson, J. & Terrier, H. (1987) Report on the subacute
21-day repeated-dose dermal toxicity study with polyram DF in
rabbits. Report BASF 87/0260, ZNT No. 86/314. Unpublished report
dated June 24, 1987 from RCC, Research and Consulting Company AG,
Switzerland. Submitted to WHO by BASF Aktiengesellschaft, FRG.
Ulrich, C. (1986) Report on the thirteen week subchronic inhalation
toxicity study on metiram in rats. Report RZ 86/407 and addendum
BASF 87/0414. Unpublished report dated December 31, 1986 and
addendum dated October 7, 1987 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO
by BASF Aktiengesellschaft, FRG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. (1985a) Report on the study of the irritation of Polyram
Combi, technical active ingredient, to the skin and eye of rabbits
(Translation of original report dated March 25, 1974). Report RZ
85/027. Unpublished report dated February 4, 1985 from BASF,
Department of Toxicology, Ludwigshafen, FRG. Submitted to WHO by
BASF Aktiengesellschaft, FRG.
Zeller, H. (1985b) Report on the study of the irritation of Polyram
Combi (BAS 222 01 F) to the skin and eyes of rabbits (Translation of
original report dated March 25, 1974). Report RZ 85/122. Unpublished
report dated April 12, 1985 from BASF, Department of Toxicology,
Ludwigshafen, FRG. Submitted to WHO by BASF Aktiengesellschaft, FRG.
