
ABAMECTIN: Addendum
First draft prepared by
E. Bosshard,
Federal Office of Public Health, Division of Food Science,
Schwerzenbach, Switzerland
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Special studies
Sensitivity of CF-1 mice
Comments
Toxicological evaluation
References
Explanation
Abamectin comprises at least 80% avermectin B1a and not more
than 20% avermectin B1b. Abamectin was first evaluated by the
Joint Meeting in 1992, when it was allocated an ADI of 0-0.0001
mg/kg bw on the basis of the lowest NOAEL of 0.05 mg/kg bw per day
for maternal toxicity observed in a study of teratogenicity in mice
and a two-generation study of reproductive toxicity in rats. A
safety factor of 500 was applied because of concern about the
teratogenicity of the delta-8,9 isomer in CF-1 mice. The isomer is a
photolytic degradation product which forms a variable part of the
residue on crops. Abamectin was re-evaluated by the 1994 Joint
Meeting in order to consider new information.
This monograph addendum presents the data on abamectin
submitted since 1992, comprising a study of the photo-oxidative
stability of avermectin B1a, the main component of abamectin, and
a study of the sensitivity of CD-1 mice to the toxicity of
abamectin, and summarizes briefly the results of the studies
described in the full monograph.
Abamectin has a close structural relationship to ivermectin, a
widely used therapeutic agent against onchocerciasis in humans, and
the two compounds have similar toxic effects in various animal
species. In 1992, the Joint FAO/WHO Expert Committee on Food
Additives (JECFA) changed the ADI for ivermectin from 0-0.0002 to
0-0.001 mg/kg bw (WHO, 1990, 1993a). The present Meeting therefore
also considered additional data on ivermectin, comprising the
results of two studies in primates and a published report of a study
in humans, which were reviewed and summarized by JECFA (Dalgard et
al., 1986; Hendricks & Lankas, 1986 [summarized in WHO, 1991];
Pacqui et al., 1990 [summarized in WHO, 1993b]).
Evaluation for acceptable daily intake
1. Biochemical aspects
Under photolytic conditions in the laboratory and in the field,
abamectin undergoes isomerization around the 8,9-double bond to
produce small amounts of the delta-8,9 isomer. A study was conducted
to investigate the stability of 0.15 EC formulations of avermectin
B1a and the delta-8,9 isomer by exposing thin films of the
compounds to simulated solar radiation for 1-23 h. Slightly higher
degradation rates were seen for the delta-8,9 isomer than for
avermectin B1a (Demchak & MacConnell, 1986).
(a) Absorption, distribution and excretion
Rats were given oral doses of 0.14 or 1.4 mg/kg bw per day of
abamectin or 1.4 mg/kg bw per day of the delta-8,9 isomer. Over
seven days, the percentages excreted in urine were 0.3-1% of the
administered dose of abamectin and 0.4% of the dose of the isomer.
The animals eliminated 69-82% of the dose of abamectin and 94% of
the dose of isomer in faeces (Annex I, reference 67; Chiu & Lu,
1989).
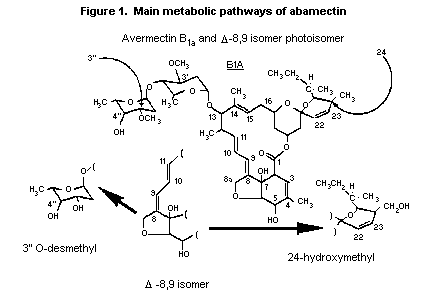
(b) Biotransformation
In rats, goats and cattle, unchanged parent compound accounted
for up to 50% of the total radioactive residues in tissues. The
24-hydroxymethyl derivative of abamectin was found in rats, goats
and cattle treated with the compound and in rats treated with the
delta-8,9 isomer, and the 3"-O-demethyl derivative was found in rats
and cattle administered abamectin and in rats administered the
isomer (Annex I, reference 67; Chiu & Lu, 1989). The metabolic
pathways of abamectin are shown in Figure 1.
Figure 1. Main metabolic pathways of abamectin
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of abamectin is summarized in Table 1.
In monkeys given a single oral dose of abamectin, the NOAEL was
1 mg/kg bw per day, with a plasma concentration of 38 ng/ml, and the
LOAEL was 2 mg/kg bw per day, with a plasma concentration of 76
ng/ml (Gordon et al., 1985). The same NOAEL and LOAEL were
observed with ivermectin in monkeys (WHO, 1991).
Table 1. Acute toxicity of abamacetin and its delta-8,9 isomer
Species Sex Route LD50 Component Purity
(mg/kg bw) (%)
Abamectin
Mouse F Oral 13.6-23.8 B1a NR
B1b 98.4
Pregnantb 11.8-19.0 B1a + B1b 94
Non-pregnantb 15.0-41.3 B1a + B1b 94
Rat M&F Oral approx. 11 B1a NR
M&F Oral 8.7-12.8 B1a + B1b 91
Dog M&F Oral approx. 8 B1a NR
B1a + B1b
Monkey M&F Oral > 24 B1a + B1b
Rat Dermal > 330 B1a +B1b 87±9.4
Rabbit Dermal > 1600 B1a + B1b 91.4
B1a + B1b 94
Delta-8,9 isomer
Mouse M&F Oral > 80
(CF-1)
NR, not reported
a From Annex I, reference 67
b Five-day mortality
(b) Short-term toxicity
As shown in Table 2, abamectin induced acute effects on the
central nervous system, manifested as tremors and mydriasis,
particularly in dogs. CD-1 mice were less sensitive than rats and
dogs.
(c) Long-term toxicity and carcinogenicity
Long-term studies of toxicity in mice and rats are summarized
in Table 3. The effects in the long-term study of abamectin in rats
showed a steep dose-response relationship.
(d) Reproductive toxicity
Studies of the reproductive toxicity of abamectin in rodents
(Annex I, reference 67) reveal that pregnant CF-1 mice are
particularly sensitive to effects on the central nervous system, as
tremors were observed at a dose as low as 0.16 mg/kg bw per day in a
study of maternal toxicity. No data on mice were available for the
delta-8,9 isomer. In rats, the NOAELs and LOELs for maternal
toxicity in most of the studies were higher than in CD-1 mice,
ranging from > 0.4 to 1 mg/kg bw per day. In one study, however,
an NOAEL of 0.12 mg/kg bw per day was found on the basis of reduced
mating performance at a higher dose level.
Studies of embryo- and fetotoxicity (Annex I, reference 67)
have been performed only in rats. The NOAELs in the different
studies were 0.1-0.3 mg/kg bw per day. The NOAELs and LOELs in most
studies were lower for embryo- and fetotoxicity than for maternal
toxicity, indicating that the developing organism is particularly
sensitive. Increased pup mortality and reduced weight gain were
consistent findings after postnatal exposure. The delta-8,9 isomer
had no adverse effects in rats at the doses tested.
(e) Embryotoxicity and teratogenicity
Studies on the embryotoxicity and teratogenicity of abamectin
and the delta-8,9 isomer in rodents are summarized in Table 5.
Mice
In pregnant CF-1 mice treated with abamectin, the NOAEL for
maternal toxicity, manifested as tremors, weight loss and death, was
0.05 mg/kg bw per day, and the NOAEL for embryotoxicity and
teratogenicity was 0.2 mg/kg bw per day. At higher doses (0.4 and
0.8 mg/kg bw per day), at which pronounced maternal toxicity occurs,
an increased incidence of cleft palates was seen in comparison with
concurrent control. The increase was not related to dose, and the
incidences are within the range of those of historical controls (up
to 3%) (Annex I, reference 67).
Table 2. Short-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 12 weeks, 0, 2, 5, 10, 20 ppm and 8 (40) 12 (60) Decreased body weight gain
(CD-1) 3 weeks: 40, 60 ppm (diet)
Rat 8 weeks: 0, 5, 10, 15, 20/25, 0.5 (10) 0.75 (15) Tremors, reduced body
40, 60 ppm (diet) weight gain
Dog 12 weeks: 0, 0.25, 0.5, 1, 0.5 1 Mydriasis
4/2 mg/kg bw per day (diet)
18 weeks: 0, 0.25, 0.5, 2, 0.25 0.5 Tremors, ataxia, mydriasis,
8 mg/kg bw per day (gavage) hepatocellular vacuolation
(avermectin B1a) and lipid accumulation,
single death
53 weeks: 0, 0.25, 0.5, 0.25 0.5 Single instances of mydriasis,
1 mg/kg bw per day (diet) marginal effect
From Annex 1, reference 67
Table 3. Long-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 94 weeks: 0, 2, 4, 8 mg/kg bw 4 8 Tremors, increased
(CD-I) per day (diet) mortality, reduced body
weight gain; no
tumorigenicity
Rat 104 weeks: 0, 0.75, 1.5, 2/2.5/2 1.5 2/2.5 Tremors (after increase in
mg/kg bw per day (diet) dose after 10 weeks),
moribund condition; no
tumorigenicity
From Annex 1, reference 67
In study 7 in Table 5, the delta-8,9 isomer was maternally
toxic in CF-1 mice, inducing tremors, decreased weight gain and
death, at doses above 0.1 mg/kg bw per day. The NOAEL for
embryotoxicity and teratogenicity was > 0.03 mg/kg bw per day.
The NOAEL for maternotoxicity was based on the finding of one
moribund animal among 25 treated animals at 0.5 mg/kg bw per day. A
marked increase in the incidence of cleft palates, which exceeded
the range seen in historical controls, was observed at this
maternally toxic dose. An increased incidence of cleft palates was
seen at the NOAEL for maternal toxicity, but the value was clearly
within the range of that of historical controls. An increased
incidence of exencephaly was found at 0.03 mg/kg bw per day, which
slightly exceeded the historical control range (up to 1.6%), so that
the increase can be regarded as a borderline effect (Annex I,
reference 67). The results of this study indicate that teratogenic
effects occur at maternally toxic doses.
In a second study of the delta-8,9 isomer in mice (study 8),
the NOAEL for maternal toxicity was 0.1 mg/kg bw per day and that
for embryotoxicity and teratogenicity was 0.05 mg/kg bw per day.
Increased incidences of cleft palate and exencephaly were observed
over those in concurrent controls at doses of 0.1 mg/kg bw per day
and higher. Most of the anomalies were found at 0.1 mg/kg bw per day
in one litter, but the range observed in historical controls was
often exceeded (Annex I, reference 67). It is remarkable, therefore,
that no dose-response relationship was seen with respect to these
malformations. Its absence renders interpretation of these results
difficult, and the conclusion that teratogenicity occurred in the
absence of maternotoxicity in this study seems to be questionable.
In a third study in mice (study 9), the NOAEL for maternal and
embrotoxicity and teratogenicity was > 0.06 mg/kg bw per day
(Annex I, reference 67). Although increased incidences of cleft
palate and exencephaly were seen in comparison with concurrent
controls, there was no dose-response relationship, and the
incidences were within the historical control range.
Assessment of the maternal toxicity of the delta-8,9 isomer in
these three studies was difficult because of the very steep
dose-response curve and the uncertainty of the end-points used
(decreased body weight and slight tremors).
Table 4. Reproductive toxicity of abamectin and its delta-8,9 isomer
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Abamectin
Mouse 0, 0.06, 0.16, 0.33 mg/kg bw per day 0.06 0.16 Maternal toxicity: tremors
(CF-1) on days 6-15 of gestation (diet)
Rat 14 weeks after exposure in utero: > 0.4 > 0.4 No effects
0, 0.1, 0.2, 0.4 mg/kg bw per day
(gavage) (avermectin B1a)
Two generations (F0, F1) 0, 0.5, 1, < 0.5 0.5 Embryo- and fetotoxicity:
2/1.5 mg/kg bw per day (oral) Reduced pup weight;
(avermectin B1a) increased pup mortality;
developmental
retardation (eye opening)
1 2/1.5 Maternal toxicity: tremors,
deaths, decreased body
weight gain, weight loss
Two generations (F0, F1): 0.1 0.2 Embryo- and fetotoxicity:
0, 0.1, 0.2, 0.4 mg/kg bw per day spastic movements, reduced
(oral) (avermectin B1a) weight gain, developmental
retardation (eye opening)
> 0.4 > 0.4 Maternal toxicity: no effects
Two generations (F0, F1): Embryo- and fetotoxicity in F1
0, 0.15, 0.5, 1.5, 5 mg/l 0.3 (1.5 mg/l) 0.7 (5 mg/l) pups: tremors, increased
(drinking-water) mortality, decreased weight
> 0.7 > 0.7 Maternal toxicity
Table 4 (contd)
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Two generations (F0, F1) 0.12 0.4 Embryo- and fetotoxicity:
0, 0.05, 0.12, 0.4 mg/kg bw increased mortality, reduced
per day (oral) weight
0.12 (?) 0.4 Maternal toxicity: reduced
body weight gain during
lactation in F0 and F1 not
related to dose
Delta-8,9 isomer
Rat Two generations (F0, F1) > 0.4 > 0.4 No treatment-related
0, 0.06, 0.12, 0.4 mg/kg bw effects
per day (gavage)
From Annex I, reference 67
Table 5. Embryotoxicity and teratogenicity of abamectin and its delta-8,9 isomer
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
Avermectin B1a
1 Mouse 20 0 Cleft palate (0.4)
(CF-1) 0.1 Tremors, death (1)
0.2
0.4 Tremors, death (3) Cleft palate (2.4)
0.8 Tremors, death (2) Cleft palate (2.5)
Avermectin B1b
2 Mouse 12 0
(CF-1) 0.025
0.05 Cleft palate, exencephaly (1)
0.075 Tremors, weight loss, death (2)
0.1
Abamectin
3 Rat 25 0 None Externala (0.3)
0.4 None
0.8 None Externala (0.7)
1.6 None Externala (0.6)
Abamectin
4 Rabbit 10 0
0.5
1
2
3 All animals: stupor, loss of body Not investigated
weight, reduced food and water
consumption; one moribund
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
5 Rabbit 18 0 Externala (2.1)
0.5 One death
1 One death
2 Reduced food and water Externala (7.4)
consumption, weight loss
Delta-8,9 isomer
6 Mouse 7-11 0
(CF-1) 1.5 Deaths Only group with litters; cleft
3 Deaths palate (29)
6.25 Deaths
12.5 Deaths
25 Deaths
50 Deaths
7 Mouse 12 0 Exencephaly (0.7)
(CF-1) 0.05 Decreased body weight gain,
fewer implantsb
0.1 ? Cleft palate (11), exencephaly (1.7)
0.5 Tremors, loss of body weight, Cleft palate (1), exencephaly (4)
single deaths
1 Tremors, decrease or loss of body Cleft palate (8), exencephaly (2)
weight; fewer implants, single
deaths
8 Mouse 25 0
(CF-1) 0.015 None Cleft palate (0.5)
0.03 None Exencephaly (1.3)
0.06 None Exencephaly (1.3)
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
9 Mouse 25 0 Exencephaly (0.4)
(CF-1) 0.015 Cleft palate (0.4), exencephaly
(0.4)
0.03 Cleft palate (0.4), exencephaly
(2)
0.1 Cleft palate (2)
0.5 Moribund (1) Cleft palate (10), exencephaly
(0.4)
10 Rat 25 0 No effect No effect
0.25 No effect No effect
0.5 No effect No effect
1 No effect No effect
From Annex I, reference 67. The historical control incidences of malformations in CF-1 mice (in % fetuses) were 0.3
(max. 3) for cleft palate and 0.3 (max. 1.6) for exencephaly. In Charles River rats, the incidences in historical
controls were 0.03 (max. 0.3) for cleft palate and 0.004 (max. 0.3) for gastroschisis.
a Cleft palate, exencephaly, gastroschisis, omphaloceles, clubbed forefeet
b Statistically nonsignificant and non-dose-related increases in the rates of resorptions and of dead fetuses in all
treated groups; fewer implants per female at 0.05 and 1 mg/kg bw per day
Rats
In rats treated with abamectin, the NOAEL for both maternal
toxicity, indicated by tremors and weight loss, and for
embryotoxicity and teratogenicity was > 1.6 mg/kg bw per day. At
0.8 and 1.6 mg/kg bw per day, the incidence of external
malformations was greater than that in concurrent controls (Annex I,
reference 67); the increase was not dose-related. The range of
incidences of each malformation in historical controls indicates
that the increases seen in animals treated with abamectin were not
related to treatment.
In rats treated with the delta-8,9 isomer, no maternal or
embryotoxicity or teratogenicity was seen at doses ranging from 0 to
1 mg/kg bw per day (Annex 1, reference 67).
(g) Special studies
Sensitivity of CF-1 mice
Multidrug resistance has been associated with over-expression
of permeability glycoprotein (P-glycoprotein), which is a component
of the plasma membrane in various cell types and species (Beck,
1997; Gottesman & Pastan, 1993). Cells that express high levels of
P-glycoprotein have decreased rates of drug uptake, decreased
steady-state levels of drugs and decreased drug retention (Beck et
al., 1983). Schinkel et al. (1994) used mice that had been
genetically engineered for disruption of the gene that encodes
P-glycoprotein and showed that ivermectin is a substrate for this
protein.
In a short-term study of oral toxicity, comparisons were made
of the sensitivity of CF-1 and CD-1 mice to abamectin and their
levels of P-glycoprotein, in order to determine whether low levels
of the protein could explain the particularly high sensitivity of
CD-1 mice to abamectin. Abamectin was administered orally in sesame
oil to groups of 49 male and 50 female CF-1 mice and five male and
five female CD-1 mice at 0.8 mg/kg bw per day for five days. A
control group received only the vehicle. The LD50 for abamectin
given orally is about 10 mg/kg bw for CF-1 mice and higher for CD-1
mice. Signs of severe neurotoxicity appeared in 12 female and five
male CF-1 mice 3-4 h after the first dose; no clinical signs of
toxicity were seen in any of the other CF-1 mice or in any of the
CD-1 or control mice. Immunohistochemical investigation of the
cerebellum, cerebral cortex and jejunum showed that the
abamectin-sensitive CF-1 mice had low levels of P-glycoprotein and
the abamectin-insensitive CF-1 mice had higher levels, which were
similar to those seen in CD-1 mice (Lankas et al., 1994).
These results indicate that about 17% of CF-1 mice are highly
sensitive to abamectin and that there is a strong correlation
between the P-glycoprotein level and sensitivity to abamectin.
Comments
In a study of photo-oxidative stability, the half-life of the
delta-8,9 isomer was 4.5 and that of avermectin B1a was 6.5 h.
Because of the close structural relationship between abamectin
and ivermectin, a widely used therapeutic agent against human
onchocerciasis and other parasitic diseases, and the very similar
toxic effects of the two compounds in various animal species,
additional data on ivermectin were considered, comprising the
results of two studies in primates and a published report of a study
in humans. A study in which expression of the P-glycoprotein (a
permeability protein associated with multiple drug resistance) was
correlated with sensitivity to avermectins in different mouse
strains was also considered.
A two-week study of the oral toxicity of ivermectin conducted
in immature rhesus monkeys at doses of 0, 0.3, 0.6 and 1.2 mg/kg bw
per day showed no adverse effects on body weight, clinical signs,
ophthalmoscopic end-points, haematological, or clinical chemical
parameters, or pathological manifestations. Thus, the NOAEL in this
study was > 1.2 mg/kg bw per day. In a second two-week study with
ivermectin, in which neonatal rhesus monkeys were administered doses
of 0, 0.04 or 0.1 mg/kg bw per day by nasogastric intubation, the
NOAEL was 0.1 mg/kg bw per day. The doses administered were 10-30
times the dose that would be received by the nursing infant of a
lactating mother who had been treated with ivermectin for
onchocerciasis.
New data were submitted which showed that the high sensitivity
of the CF-1 mouse to the toxicity of avermectins on the nervous
system is associated with a deficiency in the expression of
P-glycoprotein in both the epithelium of the small intestine and the
capillary endothelial cells of the blood-brain barrier. The
deficiency is associated with a marked increase in the concentration
of ivermectin in the brain and plasma after administration of the
compound. CD-1 mice and those of the CF-1 strain that have higher
levels of P-glycoprotein are less sensitive to the toxicity of
abamectin on the central nervous system than the approximately 17%
of CF-1 mice deficient in this protein. The oral LD50 for CF-1
P-glycoprotein-deficient mice was about one order of magnitude lower
than that of CD-1 mice and of CF-1 mice with higher levels of
P-glycoprotein. This heterogeneity in the CF-1 mouse strain may
explain the apparent absence of a dose-response relationship with
respect to maternal toxicity in the studies of teratogenicity. These
data cannot, however, be used to demonstrate a correlation between
P-glycoprotein deficiency and teratogenicity, although, given the
apparent absence of a dose-response relationship, such a correlation
might be inferred.
Extensive information available on the use of ivermectin in
animal and human health was reviewed at the fortieth meeting of the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1992.
That Committee concluded that 'despite the extremely wide use of
ivermectin, there is no evidence of significant incidences of
adverse effects on reproductive performance in treated animals and
the very limited data on reproductive toxicity in humans indicate
that ivermectin does not increase the incidence of birth defects.'
The Meeting confirmed that the lowest NOAEL was 0.05 mg/kg bw
per day for maternal toxicity in the studies of teratogenicity in
mice for abamectin and for the delta-8,9 isomer. The Joint Meeting
in 1992 considered that slight decreases in body-weight gain early
in the lactation period in one generation of rats in a study of
reproductive toxicity provided supporting evidence. The present
Meeting concluded that the NOAEL in the study on reproductive
toxicity in rats was 0.12 mg/kg bw per day on the basis of toxicity
to pups at the higher dose level.
The Meeting concluded that the CF-1 mouse strain is
heterogeneous with respect to sensitivity to abamectin and,
therefore, may be an inappropriate model for studying the toxicity
(including teratogenicity) of avermectins. The Meeting therefore
decided to base the ADI on the NOAEL of 0.12 mg/kg bw per day for
pup toxicity in the study of reproductive toxicity in rats. A safety
factor of 500 was applied because the concern about the
teratogenicity of the delta-8,9 isomer could not be assuaged by the
additional data.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 0.12 mg/kg bw per day (two-generation study of
reproductive toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Data on P-glycoprotein in other species, including humans
2. Establishment and validation of a more sensitive method to
assess neurotoxic effects of avermectins in rodents
3. Acute toxicity of the delta-8,9 isomer in CF-1 and CD-1 mice,
with measurements of P-glycoprotein and blood and brain levels
of the compound
4. Study of the teratogenicity in CD-1 and CF-1 mice of abamectin
and the delta-8,9 isomer with concurrent measurements of
P-glycoprotein, in order to correlate its presence or absence
with maternal toxicity and teratogenicity
References
Beck, W.T. (1987) The cell biology of multiple drug resistance.
Biochem. Pharmacol., 36, 2879-2887.
Beck, W.T., Cirtain, M.C. & Lefko, J.L. (1983) Energy-dependent
reduced drug binding as a mechanism of vinca alkaloid resistance in
human leukemic lymphoblasts. Mol. Pharmacol., 24, 485-492.
Chiu, S.-H. & Lu, A.Y.U. (1989) Metabolism and tissue residues. In:
Campbell, W.C., ed., Ivermectin and Abamectin, New York, Springer
Verlag, pp. 131-143.
Dalgard, D.W., Mistretta, L.H., Bromberg, N.M. & Vargas, K.J. (1986)
Sixteen day oral toxicity study of MK-0933 in immature rhesus
monkeys. Study no. TT-85-9033. Unpublished report prepared by Merck
Research Laboratories, Three Bridges, NJ, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Demchak, R.J. & MacConnell, J.G. (1986) Comparison of avermectin
B1a and its 8,9-Z isomer on petri dishes. Unpublished report dated
27 May 1986, prepared by Merck Research Laboratories, Three Bridges,
NJ, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J. & Rafferty, C.E. (1985) Oral toxicity and plasma level study in
monkeys with ivermectin (MK 0933) and abamectin (MK 0936). Study no.
T 85-013-0. Unpublished report prepared by Merck, Sharpe & Dohme
Research Laboratories, West Point, PA, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Gottesman, M.M. & Pastan, I. (1993) Biochemistry of multidrug
resistance mediated by the multidrug transporter. Ann. Rev.
Biochem., 62, 385-427.
Lankas, G.R., Cartwright, M.E. & Umbenhauer, D. (1994) Abamectin
(#177) Final report for TT #94-2775: Exploratory 5-day oral toxicity
study comparing abamectin sensitivity and P-glycoprotein levels in
CF-1 and CD-1 mice. Unpublished report dated 12 September 1994,
prepared by Merck, Sharpe & Dohme Research Laboratories, West Point,
PA, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Pacqué, M., Muñoz, B., Poetschke, G., Foose, J., Greene, B.M. &
Taylor, H.R. (1990) Pregnancy outcome after inadvertant ivermectin
treatment during community-based distribution. Lancet, 336,
1486-1489.
Schinkel, A.H., Smit, J.J.M., van Tellingen, O., Beijen, J.H.,
Wagenaar, E., van Deemter, L., Mol, C.A.A.M., van der Valk, M.A.,
Robanus-Maandag, E.C., te Riele, H.P.J., Berns, A.J.M. & Borst, P.
(1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to
a deficiency in the blood-brain barrier and to increased sensitivity
to drugs. Cell, 77, 491-502.
WHO (1990) Evaluation of Certain Veterinary Drug Residues in Food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 799), Geneva.
WHO (1991) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 27), Geneva.
WHO (1993a) Evaluation of Certain Veterinary Drug Residues in Food
(Fortieth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 832), Geneva.
WHO (1993b) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 31), Geneva.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of abamectin is summarized in Table 1.
In monkeys given a single oral dose of abamectin, the NOAEL was
1 mg/kg bw per day, with a plasma concentration of 38 ng/ml, and the
LOAEL was 2 mg/kg bw per day, with a plasma concentration of 76
ng/ml (Gordon et al., 1985). The same NOAEL and LOAEL were
observed with ivermectin in monkeys (WHO, 1991).
Table 1. Acute toxicity of abamacetin and its delta-8,9 isomer
Species Sex Route LD50 Component Purity
(mg/kg bw) (%)
Abamectin
Mouse F Oral 13.6-23.8 B1a NR
B1b 98.4
Pregnantb 11.8-19.0 B1a + B1b 94
Non-pregnantb 15.0-41.3 B1a + B1b 94
Rat M&F Oral approx. 11 B1a NR
M&F Oral 8.7-12.8 B1a + B1b 91
Dog M&F Oral approx. 8 B1a NR
B1a + B1b
Monkey M&F Oral > 24 B1a + B1b
Rat Dermal > 330 B1a +B1b 87±9.4
Rabbit Dermal > 1600 B1a + B1b 91.4
B1a + B1b 94
Delta-8,9 isomer
Mouse M&F Oral > 80
(CF-1)
NR, not reported
a From Annex I, reference 67
b Five-day mortality
(b) Short-term toxicity
As shown in Table 2, abamectin induced acute effects on the
central nervous system, manifested as tremors and mydriasis,
particularly in dogs. CD-1 mice were less sensitive than rats and
dogs.
(c) Long-term toxicity and carcinogenicity
Long-term studies of toxicity in mice and rats are summarized
in Table 3. The effects in the long-term study of abamectin in rats
showed a steep dose-response relationship.
(d) Reproductive toxicity
Studies of the reproductive toxicity of abamectin in rodents
(Annex I, reference 67) reveal that pregnant CF-1 mice are
particularly sensitive to effects on the central nervous system, as
tremors were observed at a dose as low as 0.16 mg/kg bw per day in a
study of maternal toxicity. No data on mice were available for the
delta-8,9 isomer. In rats, the NOAELs and LOELs for maternal
toxicity in most of the studies were higher than in CD-1 mice,
ranging from > 0.4 to 1 mg/kg bw per day. In one study, however,
an NOAEL of 0.12 mg/kg bw per day was found on the basis of reduced
mating performance at a higher dose level.
Studies of embryo- and fetotoxicity (Annex I, reference 67)
have been performed only in rats. The NOAELs in the different
studies were 0.1-0.3 mg/kg bw per day. The NOAELs and LOELs in most
studies were lower for embryo- and fetotoxicity than for maternal
toxicity, indicating that the developing organism is particularly
sensitive. Increased pup mortality and reduced weight gain were
consistent findings after postnatal exposure. The delta-8,9 isomer
had no adverse effects in rats at the doses tested.
(e) Embryotoxicity and teratogenicity
Studies on the embryotoxicity and teratogenicity of abamectin
and the delta-8,9 isomer in rodents are summarized in Table 5.
Mice
In pregnant CF-1 mice treated with abamectin, the NOAEL for
maternal toxicity, manifested as tremors, weight loss and death, was
0.05 mg/kg bw per day, and the NOAEL for embryotoxicity and
teratogenicity was 0.2 mg/kg bw per day. At higher doses (0.4 and
0.8 mg/kg bw per day), at which pronounced maternal toxicity occurs,
an increased incidence of cleft palates was seen in comparison with
concurrent control. The increase was not related to dose, and the
incidences are within the range of those of historical controls (up
to 3%) (Annex I, reference 67).
Table 2. Short-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 12 weeks, 0, 2, 5, 10, 20 ppm and 8 (40) 12 (60) Decreased body weight gain
(CD-1) 3 weeks: 40, 60 ppm (diet)
Rat 8 weeks: 0, 5, 10, 15, 20/25, 0.5 (10) 0.75 (15) Tremors, reduced body
40, 60 ppm (diet) weight gain
Dog 12 weeks: 0, 0.25, 0.5, 1, 0.5 1 Mydriasis
4/2 mg/kg bw per day (diet)
18 weeks: 0, 0.25, 0.5, 2, 0.25 0.5 Tremors, ataxia, mydriasis,
8 mg/kg bw per day (gavage) hepatocellular vacuolation
(avermectin B1a) and lipid accumulation,
single death
53 weeks: 0, 0.25, 0.5, 0.25 0.5 Single instances of mydriasis,
1 mg/kg bw per day (diet) marginal effect
From Annex 1, reference 67
Table 3. Long-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 94 weeks: 0, 2, 4, 8 mg/kg bw 4 8 Tremors, increased
(CD-I) per day (diet) mortality, reduced body
weight gain; no
tumorigenicity
Rat 104 weeks: 0, 0.75, 1.5, 2/2.5/2 1.5 2/2.5 Tremors (after increase in
mg/kg bw per day (diet) dose after 10 weeks),
moribund condition; no
tumorigenicity
From Annex 1, reference 67
In study 7 in Table 5, the delta-8,9 isomer was maternally
toxic in CF-1 mice, inducing tremors, decreased weight gain and
death, at doses above 0.1 mg/kg bw per day. The NOAEL for
embryotoxicity and teratogenicity was > 0.03 mg/kg bw per day.
The NOAEL for maternotoxicity was based on the finding of one
moribund animal among 25 treated animals at 0.5 mg/kg bw per day. A
marked increase in the incidence of cleft palates, which exceeded
the range seen in historical controls, was observed at this
maternally toxic dose. An increased incidence of cleft palates was
seen at the NOAEL for maternal toxicity, but the value was clearly
within the range of that of historical controls. An increased
incidence of exencephaly was found at 0.03 mg/kg bw per day, which
slightly exceeded the historical control range (up to 1.6%), so that
the increase can be regarded as a borderline effect (Annex I,
reference 67). The results of this study indicate that teratogenic
effects occur at maternally toxic doses.
In a second study of the delta-8,9 isomer in mice (study 8),
the NOAEL for maternal toxicity was 0.1 mg/kg bw per day and that
for embryotoxicity and teratogenicity was 0.05 mg/kg bw per day.
Increased incidences of cleft palate and exencephaly were observed
over those in concurrent controls at doses of 0.1 mg/kg bw per day
and higher. Most of the anomalies were found at 0.1 mg/kg bw per day
in one litter, but the range observed in historical controls was
often exceeded (Annex I, reference 67). It is remarkable, therefore,
that no dose-response relationship was seen with respect to these
malformations. Its absence renders interpretation of these results
difficult, and the conclusion that teratogenicity occurred in the
absence of maternotoxicity in this study seems to be questionable.
In a third study in mice (study 9), the NOAEL for maternal and
embrotoxicity and teratogenicity was > 0.06 mg/kg bw per day
(Annex I, reference 67). Although increased incidences of cleft
palate and exencephaly were seen in comparison with concurrent
controls, there was no dose-response relationship, and the
incidences were within the historical control range.
Assessment of the maternal toxicity of the delta-8,9 isomer in
these three studies was difficult because of the very steep
dose-response curve and the uncertainty of the end-points used
(decreased body weight and slight tremors).
Table 4. Reproductive toxicity of abamectin and its delta-8,9 isomer
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Abamectin
Mouse 0, 0.06, 0.16, 0.33 mg/kg bw per day 0.06 0.16 Maternal toxicity: tremors
(CF-1) on days 6-15 of gestation (diet)
Rat 14 weeks after exposure in utero: > 0.4 > 0.4 No effects
0, 0.1, 0.2, 0.4 mg/kg bw per day
(gavage) (avermectin B1a)
Two generations (F0, F1) 0, 0.5, 1, < 0.5 0.5 Embryo- and fetotoxicity:
2/1.5 mg/kg bw per day (oral) Reduced pup weight;
(avermectin B1a) increased pup mortality;
developmental
retardation (eye opening)
1 2/1.5 Maternal toxicity: tremors,
deaths, decreased body
weight gain, weight loss
Two generations (F0, F1): 0.1 0.2 Embryo- and fetotoxicity:
0, 0.1, 0.2, 0.4 mg/kg bw per day spastic movements, reduced
(oral) (avermectin B1a) weight gain, developmental
retardation (eye opening)
> 0.4 > 0.4 Maternal toxicity: no effects
Two generations (F0, F1): Embryo- and fetotoxicity in F1
0, 0.15, 0.5, 1.5, 5 mg/l 0.3 (1.5 mg/l) 0.7 (5 mg/l) pups: tremors, increased
(drinking-water) mortality, decreased weight
> 0.7 > 0.7 Maternal toxicity
Table 4 (contd)
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Two generations (F0, F1) 0.12 0.4 Embryo- and fetotoxicity:
0, 0.05, 0.12, 0.4 mg/kg bw increased mortality, reduced
per day (oral) weight
0.12 (?) 0.4 Maternal toxicity: reduced
body weight gain during
lactation in F0 and F1 not
related to dose
Delta-8,9 isomer
Rat Two generations (F0, F1) > 0.4 > 0.4 No treatment-related
0, 0.06, 0.12, 0.4 mg/kg bw effects
per day (gavage)
From Annex I, reference 67
Table 5. Embryotoxicity and teratogenicity of abamectin and its delta-8,9 isomer
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
Avermectin B1a
1 Mouse 20 0 Cleft palate (0.4)
(CF-1) 0.1 Tremors, death (1)
0.2
0.4 Tremors, death (3) Cleft palate (2.4)
0.8 Tremors, death (2) Cleft palate (2.5)
Avermectin B1b
2 Mouse 12 0
(CF-1) 0.025
0.05 Cleft palate, exencephaly (1)
0.075 Tremors, weight loss, death (2)
0.1
Abamectin
3 Rat 25 0 None Externala (0.3)
0.4 None
0.8 None Externala (0.7)
1.6 None Externala (0.6)
Abamectin
4 Rabbit 10 0
0.5
1
2
3 All animals: stupor, loss of body Not investigated
weight, reduced food and water
consumption; one moribund
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
5 Rabbit 18 0 Externala (2.1)
0.5 One death
1 One death
2 Reduced food and water Externala (7.4)
consumption, weight loss
Delta-8,9 isomer
6 Mouse 7-11 0
(CF-1) 1.5 Deaths Only group with litters; cleft
3 Deaths palate (29)
6.25 Deaths
12.5 Deaths
25 Deaths
50 Deaths
7 Mouse 12 0 Exencephaly (0.7)
(CF-1) 0.05 Decreased body weight gain,
fewer implantsb
0.1 ? Cleft palate (11), exencephaly (1.7)
0.5 Tremors, loss of body weight, Cleft palate (1), exencephaly (4)
single deaths
1 Tremors, decrease or loss of body Cleft palate (8), exencephaly (2)
weight; fewer implants, single
deaths
8 Mouse 25 0
(CF-1) 0.015 None Cleft palate (0.5)
0.03 None Exencephaly (1.3)
0.06 None Exencephaly (1.3)
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
9 Mouse 25 0 Exencephaly (0.4)
(CF-1) 0.015 Cleft palate (0.4), exencephaly
(0.4)
0.03 Cleft palate (0.4), exencephaly
(2)
0.1 Cleft palate (2)
0.5 Moribund (1) Cleft palate (10), exencephaly
(0.4)
10 Rat 25 0 No effect No effect
0.25 No effect No effect
0.5 No effect No effect
1 No effect No effect
From Annex I, reference 67. The historical control incidences of malformations in CF-1 mice (in % fetuses) were 0.3
(max. 3) for cleft palate and 0.3 (max. 1.6) for exencephaly. In Charles River rats, the incidences in historical
controls were 0.03 (max. 0.3) for cleft palate and 0.004 (max. 0.3) for gastroschisis.
a Cleft palate, exencephaly, gastroschisis, omphaloceles, clubbed forefeet
b Statistically nonsignificant and non-dose-related increases in the rates of resorptions and of dead fetuses in all
treated groups; fewer implants per female at 0.05 and 1 mg/kg bw per day
Rats
In rats treated with abamectin, the NOAEL for both maternal
toxicity, indicated by tremors and weight loss, and for
embryotoxicity and teratogenicity was > 1.6 mg/kg bw per day. At
0.8 and 1.6 mg/kg bw per day, the incidence of external
malformations was greater than that in concurrent controls (Annex I,
reference 67); the increase was not dose-related. The range of
incidences of each malformation in historical controls indicates
that the increases seen in animals treated with abamectin were not
related to treatment.
In rats treated with the delta-8,9 isomer, no maternal or
embryotoxicity or teratogenicity was seen at doses ranging from 0 to
1 mg/kg bw per day (Annex 1, reference 67).
(g) Special studies
Sensitivity of CF-1 mice
Multidrug resistance has been associated with over-expression
of permeability glycoprotein (P-glycoprotein), which is a component
of the plasma membrane in various cell types and species (Beck,
1997; Gottesman & Pastan, 1993). Cells that express high levels of
P-glycoprotein have decreased rates of drug uptake, decreased
steady-state levels of drugs and decreased drug retention (Beck et
al., 1983). Schinkel et al. (1994) used mice that had been
genetically engineered for disruption of the gene that encodes
P-glycoprotein and showed that ivermectin is a substrate for this
protein.
In a short-term study of oral toxicity, comparisons were made
of the sensitivity of CF-1 and CD-1 mice to abamectin and their
levels of P-glycoprotein, in order to determine whether low levels
of the protein could explain the particularly high sensitivity of
CD-1 mice to abamectin. Abamectin was administered orally in sesame
oil to groups of 49 male and 50 female CF-1 mice and five male and
five female CD-1 mice at 0.8 mg/kg bw per day for five days. A
control group received only the vehicle. The LD50 for abamectin
given orally is about 10 mg/kg bw for CF-1 mice and higher for CD-1
mice. Signs of severe neurotoxicity appeared in 12 female and five
male CF-1 mice 3-4 h after the first dose; no clinical signs of
toxicity were seen in any of the other CF-1 mice or in any of the
CD-1 or control mice. Immunohistochemical investigation of the
cerebellum, cerebral cortex and jejunum showed that the
abamectin-sensitive CF-1 mice had low levels of P-glycoprotein and
the abamectin-insensitive CF-1 mice had higher levels, which were
similar to those seen in CD-1 mice (Lankas et al., 1994).
These results indicate that about 17% of CF-1 mice are highly
sensitive to abamectin and that there is a strong correlation
between the P-glycoprotein level and sensitivity to abamectin.
Comments
In a study of photo-oxidative stability, the half-life of the
delta-8,9 isomer was 4.5 and that of avermectin B1a was 6.5 h.
Because of the close structural relationship between abamectin
and ivermectin, a widely used therapeutic agent against human
onchocerciasis and other parasitic diseases, and the very similar
toxic effects of the two compounds in various animal species,
additional data on ivermectin were considered, comprising the
results of two studies in primates and a published report of a study
in humans. A study in which expression of the P-glycoprotein (a
permeability protein associated with multiple drug resistance) was
correlated with sensitivity to avermectins in different mouse
strains was also considered.
A two-week study of the oral toxicity of ivermectin conducted
in immature rhesus monkeys at doses of 0, 0.3, 0.6 and 1.2 mg/kg bw
per day showed no adverse effects on body weight, clinical signs,
ophthalmoscopic end-points, haematological, or clinical chemical
parameters, or pathological manifestations. Thus, the NOAEL in this
study was > 1.2 mg/kg bw per day. In a second two-week study with
ivermectin, in which neonatal rhesus monkeys were administered doses
of 0, 0.04 or 0.1 mg/kg bw per day by nasogastric intubation, the
NOAEL was 0.1 mg/kg bw per day. The doses administered were 10-30
times the dose that would be received by the nursing infant of a
lactating mother who had been treated with ivermectin for
onchocerciasis.
New data were submitted which showed that the high sensitivity
of the CF-1 mouse to the toxicity of avermectins on the nervous
system is associated with a deficiency in the expression of
P-glycoprotein in both the epithelium of the small intestine and the
capillary endothelial cells of the blood-brain barrier. The
deficiency is associated with a marked increase in the concentration
of ivermectin in the brain and plasma after administration of the
compound. CD-1 mice and those of the CF-1 strain that have higher
levels of P-glycoprotein are less sensitive to the toxicity of
abamectin on the central nervous system than the approximately 17%
of CF-1 mice deficient in this protein. The oral LD50 for CF-1
P-glycoprotein-deficient mice was about one order of magnitude lower
than that of CD-1 mice and of CF-1 mice with higher levels of
P-glycoprotein. This heterogeneity in the CF-1 mouse strain may
explain the apparent absence of a dose-response relationship with
respect to maternal toxicity in the studies of teratogenicity. These
data cannot, however, be used to demonstrate a correlation between
P-glycoprotein deficiency and teratogenicity, although, given the
apparent absence of a dose-response relationship, such a correlation
might be inferred.
Extensive information available on the use of ivermectin in
animal and human health was reviewed at the fortieth meeting of the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1992.
That Committee concluded that 'despite the extremely wide use of
ivermectin, there is no evidence of significant incidences of
adverse effects on reproductive performance in treated animals and
the very limited data on reproductive toxicity in humans indicate
that ivermectin does not increase the incidence of birth defects.'
The Meeting confirmed that the lowest NOAEL was 0.05 mg/kg bw
per day for maternal toxicity in the studies of teratogenicity in
mice for abamectin and for the delta-8,9 isomer. The Joint Meeting
in 1992 considered that slight decreases in body-weight gain early
in the lactation period in one generation of rats in a study of
reproductive toxicity provided supporting evidence. The present
Meeting concluded that the NOAEL in the study on reproductive
toxicity in rats was 0.12 mg/kg bw per day on the basis of toxicity
to pups at the higher dose level.
The Meeting concluded that the CF-1 mouse strain is
heterogeneous with respect to sensitivity to abamectin and,
therefore, may be an inappropriate model for studying the toxicity
(including teratogenicity) of avermectins. The Meeting therefore
decided to base the ADI on the NOAEL of 0.12 mg/kg bw per day for
pup toxicity in the study of reproductive toxicity in rats. A safety
factor of 500 was applied because the concern about the
teratogenicity of the delta-8,9 isomer could not be assuaged by the
additional data.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 0.12 mg/kg bw per day (two-generation study of
reproductive toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Data on P-glycoprotein in other species, including humans
2. Establishment and validation of a more sensitive method to
assess neurotoxic effects of avermectins in rodents
3. Acute toxicity of the delta-8,9 isomer in CF-1 and CD-1 mice,
with measurements of P-glycoprotein and blood and brain levels
of the compound
4. Study of the teratogenicity in CD-1 and CF-1 mice of abamectin
and the delta-8,9 isomer with concurrent measurements of
P-glycoprotein, in order to correlate its presence or absence
with maternal toxicity and teratogenicity
References
Beck, W.T. (1987) The cell biology of multiple drug resistance.
Biochem. Pharmacol., 36, 2879-2887.
Beck, W.T., Cirtain, M.C. & Lefko, J.L. (1983) Energy-dependent
reduced drug binding as a mechanism of vinca alkaloid resistance in
human leukemic lymphoblasts. Mol. Pharmacol., 24, 485-492.
Chiu, S.-H. & Lu, A.Y.U. (1989) Metabolism and tissue residues. In:
Campbell, W.C., ed., Ivermectin and Abamectin, New York, Springer
Verlag, pp. 131-143.
Dalgard, D.W., Mistretta, L.H., Bromberg, N.M. & Vargas, K.J. (1986)
Sixteen day oral toxicity study of MK-0933 in immature rhesus
monkeys. Study no. TT-85-9033. Unpublished report prepared by Merck
Research Laboratories, Three Bridges, NJ, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Demchak, R.J. & MacConnell, J.G. (1986) Comparison of avermectin
B1a and its 8,9-Z isomer on petri dishes. Unpublished report dated
27 May 1986, prepared by Merck Research Laboratories, Three Bridges,
NJ, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J. & Rafferty, C.E. (1985) Oral toxicity and plasma level study in
monkeys with ivermectin (MK 0933) and abamectin (MK 0936). Study no.
T 85-013-0. Unpublished report prepared by Merck, Sharpe & Dohme
Research Laboratories, West Point, PA, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Gottesman, M.M. & Pastan, I. (1993) Biochemistry of multidrug
resistance mediated by the multidrug transporter. Ann. Rev.
Biochem., 62, 385-427.
Lankas, G.R., Cartwright, M.E. & Umbenhauer, D. (1994) Abamectin
(#177) Final report for TT #94-2775: Exploratory 5-day oral toxicity
study comparing abamectin sensitivity and P-glycoprotein levels in
CF-1 and CD-1 mice. Unpublished report dated 12 September 1994,
prepared by Merck, Sharpe & Dohme Research Laboratories, West Point,
PA, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Pacqué, M., Muñoz, B., Poetschke, G., Foose, J., Greene, B.M. &
Taylor, H.R. (1990) Pregnancy outcome after inadvertant ivermectin
treatment during community-based distribution. Lancet, 336,
1486-1489.
Schinkel, A.H., Smit, J.J.M., van Tellingen, O., Beijen, J.H.,
Wagenaar, E., van Deemter, L., Mol, C.A.A.M., van der Valk, M.A.,
Robanus-Maandag, E.C., te Riele, H.P.J., Berns, A.J.M. & Borst, P.
(1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to
a deficiency in the blood-brain barrier and to increased sensitivity
to drugs. Cell, 77, 491-502.
WHO (1990) Evaluation of Certain Veterinary Drug Residues in Food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 799), Geneva.
WHO (1991) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 27), Geneva.
WHO (1993a) Evaluation of Certain Veterinary Drug Residues in Food
(Fortieth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 832), Geneva.
WHO (1993b) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 31), Geneva.
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of abamectin is summarized in Table 1.
In monkeys given a single oral dose of abamectin, the NOAEL was
1 mg/kg bw per day, with a plasma concentration of 38 ng/ml, and the
LOAEL was 2 mg/kg bw per day, with a plasma concentration of 76
ng/ml (Gordon et al., 1985). The same NOAEL and LOAEL were
observed with ivermectin in monkeys (WHO, 1991).
Table 1. Acute toxicity of abamacetin and its delta-8,9 isomer
Species Sex Route LD50 Component Purity
(mg/kg bw) (%)
Abamectin
Mouse F Oral 13.6-23.8 B1a NR
B1b 98.4
Pregnantb 11.8-19.0 B1a + B1b 94
Non-pregnantb 15.0-41.3 B1a + B1b 94
Rat M&F Oral approx. 11 B1a NR
M&F Oral 8.7-12.8 B1a + B1b 91
Dog M&F Oral approx. 8 B1a NR
B1a + B1b
Monkey M&F Oral > 24 B1a + B1b
Rat Dermal > 330 B1a +B1b 87±9.4
Rabbit Dermal > 1600 B1a + B1b 91.4
B1a + B1b 94
Delta-8,9 isomer
Mouse M&F Oral > 80
(CF-1)
NR, not reported
a From Annex I, reference 67
b Five-day mortality
(b) Short-term toxicity
As shown in Table 2, abamectin induced acute effects on the
central nervous system, manifested as tremors and mydriasis,
particularly in dogs. CD-1 mice were less sensitive than rats and
dogs.
(c) Long-term toxicity and carcinogenicity
Long-term studies of toxicity in mice and rats are summarized
in Table 3. The effects in the long-term study of abamectin in rats
showed a steep dose-response relationship.
(d) Reproductive toxicity
Studies of the reproductive toxicity of abamectin in rodents
(Annex I, reference 67) reveal that pregnant CF-1 mice are
particularly sensitive to effects on the central nervous system, as
tremors were observed at a dose as low as 0.16 mg/kg bw per day in a
study of maternal toxicity. No data on mice were available for the
delta-8,9 isomer. In rats, the NOAELs and LOELs for maternal
toxicity in most of the studies were higher than in CD-1 mice,
ranging from > 0.4 to 1 mg/kg bw per day. In one study, however,
an NOAEL of 0.12 mg/kg bw per day was found on the basis of reduced
mating performance at a higher dose level.
Studies of embryo- and fetotoxicity (Annex I, reference 67)
have been performed only in rats. The NOAELs in the different
studies were 0.1-0.3 mg/kg bw per day. The NOAELs and LOELs in most
studies were lower for embryo- and fetotoxicity than for maternal
toxicity, indicating that the developing organism is particularly
sensitive. Increased pup mortality and reduced weight gain were
consistent findings after postnatal exposure. The delta-8,9 isomer
had no adverse effects in rats at the doses tested.
(e) Embryotoxicity and teratogenicity
Studies on the embryotoxicity and teratogenicity of abamectin
and the delta-8,9 isomer in rodents are summarized in Table 5.
Mice
In pregnant CF-1 mice treated with abamectin, the NOAEL for
maternal toxicity, manifested as tremors, weight loss and death, was
0.05 mg/kg bw per day, and the NOAEL for embryotoxicity and
teratogenicity was 0.2 mg/kg bw per day. At higher doses (0.4 and
0.8 mg/kg bw per day), at which pronounced maternal toxicity occurs,
an increased incidence of cleft palates was seen in comparison with
concurrent control. The increase was not related to dose, and the
incidences are within the range of those of historical controls (up
to 3%) (Annex I, reference 67).
Table 2. Short-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 12 weeks, 0, 2, 5, 10, 20 ppm and 8 (40) 12 (60) Decreased body weight gain
(CD-1) 3 weeks: 40, 60 ppm (diet)
Rat 8 weeks: 0, 5, 10, 15, 20/25, 0.5 (10) 0.75 (15) Tremors, reduced body
40, 60 ppm (diet) weight gain
Dog 12 weeks: 0, 0.25, 0.5, 1, 0.5 1 Mydriasis
4/2 mg/kg bw per day (diet)
18 weeks: 0, 0.25, 0.5, 2, 0.25 0.5 Tremors, ataxia, mydriasis,
8 mg/kg bw per day (gavage) hepatocellular vacuolation
(avermectin B1a) and lipid accumulation,
single death
53 weeks: 0, 0.25, 0.5, 0.25 0.5 Single instances of mydriasis,
1 mg/kg bw per day (diet) marginal effect
From Annex 1, reference 67
Table 3. Long-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 94 weeks: 0, 2, 4, 8 mg/kg bw 4 8 Tremors, increased
(CD-I) per day (diet) mortality, reduced body
weight gain; no
tumorigenicity
Rat 104 weeks: 0, 0.75, 1.5, 2/2.5/2 1.5 2/2.5 Tremors (after increase in
mg/kg bw per day (diet) dose after 10 weeks),
moribund condition; no
tumorigenicity
From Annex 1, reference 67
In study 7 in Table 5, the delta-8,9 isomer was maternally
toxic in CF-1 mice, inducing tremors, decreased weight gain and
death, at doses above 0.1 mg/kg bw per day. The NOAEL for
embryotoxicity and teratogenicity was > 0.03 mg/kg bw per day.
The NOAEL for maternotoxicity was based on the finding of one
moribund animal among 25 treated animals at 0.5 mg/kg bw per day. A
marked increase in the incidence of cleft palates, which exceeded
the range seen in historical controls, was observed at this
maternally toxic dose. An increased incidence of cleft palates was
seen at the NOAEL for maternal toxicity, but the value was clearly
within the range of that of historical controls. An increased
incidence of exencephaly was found at 0.03 mg/kg bw per day, which
slightly exceeded the historical control range (up to 1.6%), so that
the increase can be regarded as a borderline effect (Annex I,
reference 67). The results of this study indicate that teratogenic
effects occur at maternally toxic doses.
In a second study of the delta-8,9 isomer in mice (study 8),
the NOAEL for maternal toxicity was 0.1 mg/kg bw per day and that
for embryotoxicity and teratogenicity was 0.05 mg/kg bw per day.
Increased incidences of cleft palate and exencephaly were observed
over those in concurrent controls at doses of 0.1 mg/kg bw per day
and higher. Most of the anomalies were found at 0.1 mg/kg bw per day
in one litter, but the range observed in historical controls was
often exceeded (Annex I, reference 67). It is remarkable, therefore,
that no dose-response relationship was seen with respect to these
malformations. Its absence renders interpretation of these results
difficult, and the conclusion that teratogenicity occurred in the
absence of maternotoxicity in this study seems to be questionable.
In a third study in mice (study 9), the NOAEL for maternal and
embrotoxicity and teratogenicity was > 0.06 mg/kg bw per day
(Annex I, reference 67). Although increased incidences of cleft
palate and exencephaly were seen in comparison with concurrent
controls, there was no dose-response relationship, and the
incidences were within the historical control range.
Assessment of the maternal toxicity of the delta-8,9 isomer in
these three studies was difficult because of the very steep
dose-response curve and the uncertainty of the end-points used
(decreased body weight and slight tremors).
Table 4. Reproductive toxicity of abamectin and its delta-8,9 isomer
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Abamectin
Mouse 0, 0.06, 0.16, 0.33 mg/kg bw per day 0.06 0.16 Maternal toxicity: tremors
(CF-1) on days 6-15 of gestation (diet)
Rat 14 weeks after exposure in utero: > 0.4 > 0.4 No effects
0, 0.1, 0.2, 0.4 mg/kg bw per day
(gavage) (avermectin B1a)
Two generations (F0, F1) 0, 0.5, 1, < 0.5 0.5 Embryo- and fetotoxicity:
2/1.5 mg/kg bw per day (oral) Reduced pup weight;
(avermectin B1a) increased pup mortality;
developmental
retardation (eye opening)
1 2/1.5 Maternal toxicity: tremors,
deaths, decreased body
weight gain, weight loss
Two generations (F0, F1): 0.1 0.2 Embryo- and fetotoxicity:
0, 0.1, 0.2, 0.4 mg/kg bw per day spastic movements, reduced
(oral) (avermectin B1a) weight gain, developmental
retardation (eye opening)
> 0.4 > 0.4 Maternal toxicity: no effects
Two generations (F0, F1): Embryo- and fetotoxicity in F1
0, 0.15, 0.5, 1.5, 5 mg/l 0.3 (1.5 mg/l) 0.7 (5 mg/l) pups: tremors, increased
(drinking-water) mortality, decreased weight
> 0.7 > 0.7 Maternal toxicity
Table 4 (contd)
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Two generations (F0, F1) 0.12 0.4 Embryo- and fetotoxicity:
0, 0.05, 0.12, 0.4 mg/kg bw increased mortality, reduced
per day (oral) weight
0.12 (?) 0.4 Maternal toxicity: reduced
body weight gain during
lactation in F0 and F1 not
related to dose
Delta-8,9 isomer
Rat Two generations (F0, F1) > 0.4 > 0.4 No treatment-related
0, 0.06, 0.12, 0.4 mg/kg bw effects
per day (gavage)
From Annex I, reference 67
Table 5. Embryotoxicity and teratogenicity of abamectin and its delta-8,9 isomer
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
Avermectin B1a
1 Mouse 20 0 Cleft palate (0.4)
(CF-1) 0.1 Tremors, death (1)
0.2
0.4 Tremors, death (3) Cleft palate (2.4)
0.8 Tremors, death (2) Cleft palate (2.5)
Avermectin B1b
2 Mouse 12 0
(CF-1) 0.025
0.05 Cleft palate, exencephaly (1)
0.075 Tremors, weight loss, death (2)
0.1
Abamectin
3 Rat 25 0 None Externala (0.3)
0.4 None
0.8 None Externala (0.7)
1.6 None Externala (0.6)
Abamectin
4 Rabbit 10 0
0.5
1
2
3 All animals: stupor, loss of body Not investigated
weight, reduced food and water
consumption; one moribund
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
5 Rabbit 18 0 Externala (2.1)
0.5 One death
1 One death
2 Reduced food and water Externala (7.4)
consumption, weight loss
Delta-8,9 isomer
6 Mouse 7-11 0
(CF-1) 1.5 Deaths Only group with litters; cleft
3 Deaths palate (29)
6.25 Deaths
12.5 Deaths
25 Deaths
50 Deaths
7 Mouse 12 0 Exencephaly (0.7)
(CF-1) 0.05 Decreased body weight gain,
fewer implantsb
0.1 ? Cleft palate (11), exencephaly (1.7)
0.5 Tremors, loss of body weight, Cleft palate (1), exencephaly (4)
single deaths
1 Tremors, decrease or loss of body Cleft palate (8), exencephaly (2)
weight; fewer implants, single
deaths
8 Mouse 25 0
(CF-1) 0.015 None Cleft palate (0.5)
0.03 None Exencephaly (1.3)
0.06 None Exencephaly (1.3)
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
9 Mouse 25 0 Exencephaly (0.4)
(CF-1) 0.015 Cleft palate (0.4), exencephaly
(0.4)
0.03 Cleft palate (0.4), exencephaly
(2)
0.1 Cleft palate (2)
0.5 Moribund (1) Cleft palate (10), exencephaly
(0.4)
10 Rat 25 0 No effect No effect
0.25 No effect No effect
0.5 No effect No effect
1 No effect No effect
From Annex I, reference 67. The historical control incidences of malformations in CF-1 mice (in % fetuses) were 0.3
(max. 3) for cleft palate and 0.3 (max. 1.6) for exencephaly. In Charles River rats, the incidences in historical
controls were 0.03 (max. 0.3) for cleft palate and 0.004 (max. 0.3) for gastroschisis.
a Cleft palate, exencephaly, gastroschisis, omphaloceles, clubbed forefeet
b Statistically nonsignificant and non-dose-related increases in the rates of resorptions and of dead fetuses in all
treated groups; fewer implants per female at 0.05 and 1 mg/kg bw per day
Rats
In rats treated with abamectin, the NOAEL for both maternal
toxicity, indicated by tremors and weight loss, and for
embryotoxicity and teratogenicity was > 1.6 mg/kg bw per day. At
0.8 and 1.6 mg/kg bw per day, the incidence of external
malformations was greater than that in concurrent controls (Annex I,
reference 67); the increase was not dose-related. The range of
incidences of each malformation in historical controls indicates
that the increases seen in animals treated with abamectin were not
related to treatment.
In rats treated with the delta-8,9 isomer, no maternal or
embryotoxicity or teratogenicity was seen at doses ranging from 0 to
1 mg/kg bw per day (Annex 1, reference 67).
(g) Special studies
Sensitivity of CF-1 mice
Multidrug resistance has been associated with over-expression
of permeability glycoprotein (P-glycoprotein), which is a component
of the plasma membrane in various cell types and species (Beck,
1997; Gottesman & Pastan, 1993). Cells that express high levels of
P-glycoprotein have decreased rates of drug uptake, decreased
steady-state levels of drugs and decreased drug retention (Beck et
al., 1983). Schinkel et al. (1994) used mice that had been
genetically engineered for disruption of the gene that encodes
P-glycoprotein and showed that ivermectin is a substrate for this
protein.
In a short-term study of oral toxicity, comparisons were made
of the sensitivity of CF-1 and CD-1 mice to abamectin and their
levels of P-glycoprotein, in order to determine whether low levels
of the protein could explain the particularly high sensitivity of
CD-1 mice to abamectin. Abamectin was administered orally in sesame
oil to groups of 49 male and 50 female CF-1 mice and five male and
five female CD-1 mice at 0.8 mg/kg bw per day for five days. A
control group received only the vehicle. The LD50 for abamectin
given orally is about 10 mg/kg bw for CF-1 mice and higher for CD-1
mice. Signs of severe neurotoxicity appeared in 12 female and five
male CF-1 mice 3-4 h after the first dose; no clinical signs of
toxicity were seen in any of the other CF-1 mice or in any of the
CD-1 or control mice. Immunohistochemical investigation of the
cerebellum, cerebral cortex and jejunum showed that the
abamectin-sensitive CF-1 mice had low levels of P-glycoprotein and
the abamectin-insensitive CF-1 mice had higher levels, which were
similar to those seen in CD-1 mice (Lankas et al., 1994).
These results indicate that about 17% of CF-1 mice are highly
sensitive to abamectin and that there is a strong correlation
between the P-glycoprotein level and sensitivity to abamectin.
Comments
In a study of photo-oxidative stability, the half-life of the
delta-8,9 isomer was 4.5 and that of avermectin B1a was 6.5 h.
Because of the close structural relationship between abamectin
and ivermectin, a widely used therapeutic agent against human
onchocerciasis and other parasitic diseases, and the very similar
toxic effects of the two compounds in various animal species,
additional data on ivermectin were considered, comprising the
results of two studies in primates and a published report of a study
in humans. A study in which expression of the P-glycoprotein (a
permeability protein associated with multiple drug resistance) was
correlated with sensitivity to avermectins in different mouse
strains was also considered.
A two-week study of the oral toxicity of ivermectin conducted
in immature rhesus monkeys at doses of 0, 0.3, 0.6 and 1.2 mg/kg bw
per day showed no adverse effects on body weight, clinical signs,
ophthalmoscopic end-points, haematological, or clinical chemical
parameters, or pathological manifestations. Thus, the NOAEL in this
study was > 1.2 mg/kg bw per day. In a second two-week study with
ivermectin, in which neonatal rhesus monkeys were administered doses
of 0, 0.04 or 0.1 mg/kg bw per day by nasogastric intubation, the
NOAEL was 0.1 mg/kg bw per day. The doses administered were 10-30
times the dose that would be received by the nursing infant of a
lactating mother who had been treated with ivermectin for
onchocerciasis.
New data were submitted which showed that the high sensitivity
of the CF-1 mouse to the toxicity of avermectins on the nervous
system is associated with a deficiency in the expression of
P-glycoprotein in both the epithelium of the small intestine and the
capillary endothelial cells of the blood-brain barrier. The
deficiency is associated with a marked increase in the concentration
of ivermectin in the brain and plasma after administration of the
compound. CD-1 mice and those of the CF-1 strain that have higher
levels of P-glycoprotein are less sensitive to the toxicity of
abamectin on the central nervous system than the approximately 17%
of CF-1 mice deficient in this protein. The oral LD50 for CF-1
P-glycoprotein-deficient mice was about one order of magnitude lower
than that of CD-1 mice and of CF-1 mice with higher levels of
P-glycoprotein. This heterogeneity in the CF-1 mouse strain may
explain the apparent absence of a dose-response relationship with
respect to maternal toxicity in the studies of teratogenicity. These
data cannot, however, be used to demonstrate a correlation between
P-glycoprotein deficiency and teratogenicity, although, given the
apparent absence of a dose-response relationship, such a correlation
might be inferred.
Extensive information available on the use of ivermectin in
animal and human health was reviewed at the fortieth meeting of the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1992.
That Committee concluded that 'despite the extremely wide use of
ivermectin, there is no evidence of significant incidences of
adverse effects on reproductive performance in treated animals and
the very limited data on reproductive toxicity in humans indicate
that ivermectin does not increase the incidence of birth defects.'
The Meeting confirmed that the lowest NOAEL was 0.05 mg/kg bw
per day for maternal toxicity in the studies of teratogenicity in
mice for abamectin and for the delta-8,9 isomer. The Joint Meeting
in 1992 considered that slight decreases in body-weight gain early
in the lactation period in one generation of rats in a study of
reproductive toxicity provided supporting evidence. The present
Meeting concluded that the NOAEL in the study on reproductive
toxicity in rats was 0.12 mg/kg bw per day on the basis of toxicity
to pups at the higher dose level.
The Meeting concluded that the CF-1 mouse strain is
heterogeneous with respect to sensitivity to abamectin and,
therefore, may be an inappropriate model for studying the toxicity
(including teratogenicity) of avermectins. The Meeting therefore
decided to base the ADI on the NOAEL of 0.12 mg/kg bw per day for
pup toxicity in the study of reproductive toxicity in rats. A safety
factor of 500 was applied because the concern about the
teratogenicity of the delta-8,9 isomer could not be assuaged by the
additional data.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 0.12 mg/kg bw per day (two-generation study of
reproductive toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Data on P-glycoprotein in other species, including humans
2. Establishment and validation of a more sensitive method to
assess neurotoxic effects of avermectins in rodents
3. Acute toxicity of the delta-8,9 isomer in CF-1 and CD-1 mice,
with measurements of P-glycoprotein and blood and brain levels
of the compound
4. Study of the teratogenicity in CD-1 and CF-1 mice of abamectin
and the delta-8,9 isomer with concurrent measurements of
P-glycoprotein, in order to correlate its presence or absence
with maternal toxicity and teratogenicity
References
Beck, W.T. (1987) The cell biology of multiple drug resistance.
Biochem. Pharmacol., 36, 2879-2887.
Beck, W.T., Cirtain, M.C. & Lefko, J.L. (1983) Energy-dependent
reduced drug binding as a mechanism of vinca alkaloid resistance in
human leukemic lymphoblasts. Mol. Pharmacol., 24, 485-492.
Chiu, S.-H. & Lu, A.Y.U. (1989) Metabolism and tissue residues. In:
Campbell, W.C., ed., Ivermectin and Abamectin, New York, Springer
Verlag, pp. 131-143.
Dalgard, D.W., Mistretta, L.H., Bromberg, N.M. & Vargas, K.J. (1986)
Sixteen day oral toxicity study of MK-0933 in immature rhesus
monkeys. Study no. TT-85-9033. Unpublished report prepared by Merck
Research Laboratories, Three Bridges, NJ, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Demchak, R.J. & MacConnell, J.G. (1986) Comparison of avermectin
B1a and its 8,9-Z isomer on petri dishes. Unpublished report dated
27 May 1986, prepared by Merck Research Laboratories, Three Bridges,
NJ, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J. & Rafferty, C.E. (1985) Oral toxicity and plasma level study in
monkeys with ivermectin (MK 0933) and abamectin (MK 0936). Study no.
T 85-013-0. Unpublished report prepared by Merck, Sharpe & Dohme
Research Laboratories, West Point, PA, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Gottesman, M.M. & Pastan, I. (1993) Biochemistry of multidrug
resistance mediated by the multidrug transporter. Ann. Rev.
Biochem., 62, 385-427.
Lankas, G.R., Cartwright, M.E. & Umbenhauer, D. (1994) Abamectin
(#177) Final report for TT #94-2775: Exploratory 5-day oral toxicity
study comparing abamectin sensitivity and P-glycoprotein levels in
CF-1 and CD-1 mice. Unpublished report dated 12 September 1994,
prepared by Merck, Sharpe & Dohme Research Laboratories, West Point,
PA, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Pacqué, M., Muñoz, B., Poetschke, G., Foose, J., Greene, B.M. &
Taylor, H.R. (1990) Pregnancy outcome after inadvertant ivermectin
treatment during community-based distribution. Lancet, 336,
1486-1489.
Schinkel, A.H., Smit, J.J.M., van Tellingen, O., Beijen, J.H.,
Wagenaar, E., van Deemter, L., Mol, C.A.A.M., van der Valk, M.A.,
Robanus-Maandag, E.C., te Riele, H.P.J., Berns, A.J.M. & Borst, P.
(1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to
a deficiency in the blood-brain barrier and to increased sensitivity
to drugs. Cell, 77, 491-502.
WHO (1990) Evaluation of Certain Veterinary Drug Residues in Food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 799), Geneva.
WHO (1991) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 27), Geneva.
WHO (1993a) Evaluation of Certain Veterinary Drug Residues in Food
(Fortieth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 832), Geneva.
WHO (1993b) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 31), Geneva.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of abamectin is summarized in Table 1.
In monkeys given a single oral dose of abamectin, the NOAEL was
1 mg/kg bw per day, with a plasma concentration of 38 ng/ml, and the
LOAEL was 2 mg/kg bw per day, with a plasma concentration of 76
ng/ml (Gordon et al., 1985). The same NOAEL and LOAEL were
observed with ivermectin in monkeys (WHO, 1991).
Table 1. Acute toxicity of abamacetin and its delta-8,9 isomer
Species Sex Route LD50 Component Purity
(mg/kg bw) (%)
Abamectin
Mouse F Oral 13.6-23.8 B1a NR
B1b 98.4
Pregnantb 11.8-19.0 B1a + B1b 94
Non-pregnantb 15.0-41.3 B1a + B1b 94
Rat M&F Oral approx. 11 B1a NR
M&F Oral 8.7-12.8 B1a + B1b 91
Dog M&F Oral approx. 8 B1a NR
B1a + B1b
Monkey M&F Oral > 24 B1a + B1b
Rat Dermal > 330 B1a +B1b 87±9.4
Rabbit Dermal > 1600 B1a + B1b 91.4
B1a + B1b 94
Delta-8,9 isomer
Mouse M&F Oral > 80
(CF-1)
NR, not reported
a From Annex I, reference 67
b Five-day mortality
(b) Short-term toxicity
As shown in Table 2, abamectin induced acute effects on the
central nervous system, manifested as tremors and mydriasis,
particularly in dogs. CD-1 mice were less sensitive than rats and
dogs.
(c) Long-term toxicity and carcinogenicity
Long-term studies of toxicity in mice and rats are summarized
in Table 3. The effects in the long-term study of abamectin in rats
showed a steep dose-response relationship.
(d) Reproductive toxicity
Studies of the reproductive toxicity of abamectin in rodents
(Annex I, reference 67) reveal that pregnant CF-1 mice are
particularly sensitive to effects on the central nervous system, as
tremors were observed at a dose as low as 0.16 mg/kg bw per day in a
study of maternal toxicity. No data on mice were available for the
delta-8,9 isomer. In rats, the NOAELs and LOELs for maternal
toxicity in most of the studies were higher than in CD-1 mice,
ranging from > 0.4 to 1 mg/kg bw per day. In one study, however,
an NOAEL of 0.12 mg/kg bw per day was found on the basis of reduced
mating performance at a higher dose level.
Studies of embryo- and fetotoxicity (Annex I, reference 67)
have been performed only in rats. The NOAELs in the different
studies were 0.1-0.3 mg/kg bw per day. The NOAELs and LOELs in most
studies were lower for embryo- and fetotoxicity than for maternal
toxicity, indicating that the developing organism is particularly
sensitive. Increased pup mortality and reduced weight gain were
consistent findings after postnatal exposure. The delta-8,9 isomer
had no adverse effects in rats at the doses tested.
(e) Embryotoxicity and teratogenicity
Studies on the embryotoxicity and teratogenicity of abamectin
and the delta-8,9 isomer in rodents are summarized in Table 5.
Mice
In pregnant CF-1 mice treated with abamectin, the NOAEL for
maternal toxicity, manifested as tremors, weight loss and death, was
0.05 mg/kg bw per day, and the NOAEL for embryotoxicity and
teratogenicity was 0.2 mg/kg bw per day. At higher doses (0.4 and
0.8 mg/kg bw per day), at which pronounced maternal toxicity occurs,
an increased incidence of cleft palates was seen in comparison with
concurrent control. The increase was not related to dose, and the
incidences are within the range of those of historical controls (up
to 3%) (Annex I, reference 67).
Table 2. Short-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 12 weeks, 0, 2, 5, 10, 20 ppm and 8 (40) 12 (60) Decreased body weight gain
(CD-1) 3 weeks: 40, 60 ppm (diet)
Rat 8 weeks: 0, 5, 10, 15, 20/25, 0.5 (10) 0.75 (15) Tremors, reduced body
40, 60 ppm (diet) weight gain
Dog 12 weeks: 0, 0.25, 0.5, 1, 0.5 1 Mydriasis
4/2 mg/kg bw per day (diet)
18 weeks: 0, 0.25, 0.5, 2, 0.25 0.5 Tremors, ataxia, mydriasis,
8 mg/kg bw per day (gavage) hepatocellular vacuolation
(avermectin B1a) and lipid accumulation,
single death
53 weeks: 0, 0.25, 0.5, 0.25 0.5 Single instances of mydriasis,
1 mg/kg bw per day (diet) marginal effect
From Annex 1, reference 67
Table 3. Long-term toxicity of abamectin
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Mouse 94 weeks: 0, 2, 4, 8 mg/kg bw 4 8 Tremors, increased
(CD-I) per day (diet) mortality, reduced body
weight gain; no
tumorigenicity
Rat 104 weeks: 0, 0.75, 1.5, 2/2.5/2 1.5 2/2.5 Tremors (after increase in
mg/kg bw per day (diet) dose after 10 weeks),
moribund condition; no
tumorigenicity
From Annex 1, reference 67
In study 7 in Table 5, the delta-8,9 isomer was maternally
toxic in CF-1 mice, inducing tremors, decreased weight gain and
death, at doses above 0.1 mg/kg bw per day. The NOAEL for
embryotoxicity and teratogenicity was > 0.03 mg/kg bw per day.
The NOAEL for maternotoxicity was based on the finding of one
moribund animal among 25 treated animals at 0.5 mg/kg bw per day. A
marked increase in the incidence of cleft palates, which exceeded
the range seen in historical controls, was observed at this
maternally toxic dose. An increased incidence of cleft palates was
seen at the NOAEL for maternal toxicity, but the value was clearly
within the range of that of historical controls. An increased
incidence of exencephaly was found at 0.03 mg/kg bw per day, which
slightly exceeded the historical control range (up to 1.6%), so that
the increase can be regarded as a borderline effect (Annex I,
reference 67). The results of this study indicate that teratogenic
effects occur at maternally toxic doses.
In a second study of the delta-8,9 isomer in mice (study 8),
the NOAEL for maternal toxicity was 0.1 mg/kg bw per day and that
for embryotoxicity and teratogenicity was 0.05 mg/kg bw per day.
Increased incidences of cleft palate and exencephaly were observed
over those in concurrent controls at doses of 0.1 mg/kg bw per day
and higher. Most of the anomalies were found at 0.1 mg/kg bw per day
in one litter, but the range observed in historical controls was
often exceeded (Annex I, reference 67). It is remarkable, therefore,
that no dose-response relationship was seen with respect to these
malformations. Its absence renders interpretation of these results
difficult, and the conclusion that teratogenicity occurred in the
absence of maternotoxicity in this study seems to be questionable.
In a third study in mice (study 9), the NOAEL for maternal and
embrotoxicity and teratogenicity was > 0.06 mg/kg bw per day
(Annex I, reference 67). Although increased incidences of cleft
palate and exencephaly were seen in comparison with concurrent
controls, there was no dose-response relationship, and the
incidences were within the historical control range.
Assessment of the maternal toxicity of the delta-8,9 isomer in
these three studies was difficult because of the very steep
dose-response curve and the uncertainty of the end-points used
(decreased body weight and slight tremors).
Table 4. Reproductive toxicity of abamectin and its delta-8,9 isomer
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Abamectin
Mouse 0, 0.06, 0.16, 0.33 mg/kg bw per day 0.06 0.16 Maternal toxicity: tremors
(CF-1) on days 6-15 of gestation (diet)
Rat 14 weeks after exposure in utero: > 0.4 > 0.4 No effects
0, 0.1, 0.2, 0.4 mg/kg bw per day
(gavage) (avermectin B1a)
Two generations (F0, F1) 0, 0.5, 1, < 0.5 0.5 Embryo- and fetotoxicity:
2/1.5 mg/kg bw per day (oral) Reduced pup weight;
(avermectin B1a) increased pup mortality;
developmental
retardation (eye opening)
1 2/1.5 Maternal toxicity: tremors,
deaths, decreased body
weight gain, weight loss
Two generations (F0, F1): 0.1 0.2 Embryo- and fetotoxicity:
0, 0.1, 0.2, 0.4 mg/kg bw per day spastic movements, reduced
(oral) (avermectin B1a) weight gain, developmental
retardation (eye opening)
> 0.4 > 0.4 Maternal toxicity: no effects
Two generations (F0, F1): Embryo- and fetotoxicity in F1
0, 0.15, 0.5, 1.5, 5 mg/l 0.3 (1.5 mg/l) 0.7 (5 mg/l) pups: tremors, increased
(drinking-water) mortality, decreased weight
> 0.7 > 0.7 Maternal toxicity
Table 4 (contd)
Species Treatment Effect level Effects
(mg/kg bw per day)
NOAEL LOAEL
Two generations (F0, F1) 0.12 0.4 Embryo- and fetotoxicity:
0, 0.05, 0.12, 0.4 mg/kg bw increased mortality, reduced
per day (oral) weight
0.12 (?) 0.4 Maternal toxicity: reduced
body weight gain during
lactation in F0 and F1 not
related to dose
Delta-8,9 isomer
Rat Two generations (F0, F1) > 0.4 > 0.4 No treatment-related
0, 0.06, 0.12, 0.4 mg/kg bw effects
per day (gavage)
From Annex I, reference 67
Table 5. Embryotoxicity and teratogenicity of abamectin and its delta-8,9 isomer
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
Avermectin B1a
1 Mouse 20 0 Cleft palate (0.4)
(CF-1) 0.1 Tremors, death (1)
0.2
0.4 Tremors, death (3) Cleft palate (2.4)
0.8 Tremors, death (2) Cleft palate (2.5)
Avermectin B1b
2 Mouse 12 0
(CF-1) 0.025
0.05 Cleft palate, exencephaly (1)
0.075 Tremors, weight loss, death (2)
0.1
Abamectin
3 Rat 25 0 None Externala (0.3)
0.4 None
0.8 None Externala (0.7)
1.6 None Externala (0.6)
Abamectin
4 Rabbit 10 0
0.5
1
2
3 All animals: stupor, loss of body Not investigated
weight, reduced food and water
consumption; one moribund
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
Abamectin
5 Rabbit 18 0 Externala (2.1)
0.5 One death
1 One death
2 Reduced food and water Externala (7.4)
consumption, weight loss
Delta-8,9 isomer
6 Mouse 7-11 0
(CF-1) 1.5 Deaths Only group with litters; cleft
3 Deaths palate (29)
6.25 Deaths
12.5 Deaths
25 Deaths
50 Deaths
7 Mouse 12 0 Exencephaly (0.7)
(CF-1) 0.05 Decreased body weight gain,
fewer implantsb
0.1 ? Cleft palate (11), exencephaly (1.7)
0.5 Tremors, loss of body weight, Cleft palate (1), exencephaly (4)
single deaths
1 Tremors, decrease or loss of body Cleft palate (8), exencephaly (2)
weight; fewer implants, single
deaths
8 Mouse 25 0
(CF-1) 0.015 None Cleft palate (0.5)
0.03 None Exencephaly (1.3)
0.06 None Exencephaly (1.3)
Table 5 (contd)
Study Species No. Dose Maternal toxicity Malformations
no. (mg/kg bw (no. of animals) (% fetuses)
per day)
9 Mouse 25 0 Exencephaly (0.4)
(CF-1) 0.015 Cleft palate (0.4), exencephaly
(0.4)
0.03 Cleft palate (0.4), exencephaly
(2)
0.1 Cleft palate (2)
0.5 Moribund (1) Cleft palate (10), exencephaly
(0.4)
10 Rat 25 0 No effect No effect
0.25 No effect No effect
0.5 No effect No effect
1 No effect No effect
From Annex I, reference 67. The historical control incidences of malformations in CF-1 mice (in % fetuses) were 0.3
(max. 3) for cleft palate and 0.3 (max. 1.6) for exencephaly. In Charles River rats, the incidences in historical
controls were 0.03 (max. 0.3) for cleft palate and 0.004 (max. 0.3) for gastroschisis.
a Cleft palate, exencephaly, gastroschisis, omphaloceles, clubbed forefeet
b Statistically nonsignificant and non-dose-related increases in the rates of resorptions and of dead fetuses in all
treated groups; fewer implants per female at 0.05 and 1 mg/kg bw per day
Rats
In rats treated with abamectin, the NOAEL for both maternal
toxicity, indicated by tremors and weight loss, and for
embryotoxicity and teratogenicity was > 1.6 mg/kg bw per day. At
0.8 and 1.6 mg/kg bw per day, the incidence of external
malformations was greater than that in concurrent controls (Annex I,
reference 67); the increase was not dose-related. The range of
incidences of each malformation in historical controls indicates
that the increases seen in animals treated with abamectin were not
related to treatment.
In rats treated with the delta-8,9 isomer, no maternal or
embryotoxicity or teratogenicity was seen at doses ranging from 0 to
1 mg/kg bw per day (Annex 1, reference 67).
(g) Special studies
Sensitivity of CF-1 mice
Multidrug resistance has been associated with over-expression
of permeability glycoprotein (P-glycoprotein), which is a component
of the plasma membrane in various cell types and species (Beck,
1997; Gottesman & Pastan, 1993). Cells that express high levels of
P-glycoprotein have decreased rates of drug uptake, decreased
steady-state levels of drugs and decreased drug retention (Beck et
al., 1983). Schinkel et al. (1994) used mice that had been
genetically engineered for disruption of the gene that encodes
P-glycoprotein and showed that ivermectin is a substrate for this
protein.
In a short-term study of oral toxicity, comparisons were made
of the sensitivity of CF-1 and CD-1 mice to abamectin and their
levels of P-glycoprotein, in order to determine whether low levels
of the protein could explain the particularly high sensitivity of
CD-1 mice to abamectin. Abamectin was administered orally in sesame
oil to groups of 49 male and 50 female CF-1 mice and five male and
five female CD-1 mice at 0.8 mg/kg bw per day for five days. A
control group received only the vehicle. The LD50 for abamectin
given orally is about 10 mg/kg bw for CF-1 mice and higher for CD-1
mice. Signs of severe neurotoxicity appeared in 12 female and five
male CF-1 mice 3-4 h after the first dose; no clinical signs of
toxicity were seen in any of the other CF-1 mice or in any of the
CD-1 or control mice. Immunohistochemical investigation of the
cerebellum, cerebral cortex and jejunum showed that the
abamectin-sensitive CF-1 mice had low levels of P-glycoprotein and
the abamectin-insensitive CF-1 mice had higher levels, which were
similar to those seen in CD-1 mice (Lankas et al., 1994).
These results indicate that about 17% of CF-1 mice are highly
sensitive to abamectin and that there is a strong correlation
between the P-glycoprotein level and sensitivity to abamectin.
Comments
In a study of photo-oxidative stability, the half-life of the
delta-8,9 isomer was 4.5 and that of avermectin B1a was 6.5 h.
Because of the close structural relationship between abamectin
and ivermectin, a widely used therapeutic agent against human
onchocerciasis and other parasitic diseases, and the very similar
toxic effects of the two compounds in various animal species,
additional data on ivermectin were considered, comprising the
results of two studies in primates and a published report of a study
in humans. A study in which expression of the P-glycoprotein (a
permeability protein associated with multiple drug resistance) was
correlated with sensitivity to avermectins in different mouse
strains was also considered.
A two-week study of the oral toxicity of ivermectin conducted
in immature rhesus monkeys at doses of 0, 0.3, 0.6 and 1.2 mg/kg bw
per day showed no adverse effects on body weight, clinical signs,
ophthalmoscopic end-points, haematological, or clinical chemical
parameters, or pathological manifestations. Thus, the NOAEL in this
study was > 1.2 mg/kg bw per day. In a second two-week study with
ivermectin, in which neonatal rhesus monkeys were administered doses
of 0, 0.04 or 0.1 mg/kg bw per day by nasogastric intubation, the
NOAEL was 0.1 mg/kg bw per day. The doses administered were 10-30
times the dose that would be received by the nursing infant of a
lactating mother who had been treated with ivermectin for
onchocerciasis.
New data were submitted which showed that the high sensitivity
of the CF-1 mouse to the toxicity of avermectins on the nervous
system is associated with a deficiency in the expression of
P-glycoprotein in both the epithelium of the small intestine and the
capillary endothelial cells of the blood-brain barrier. The
deficiency is associated with a marked increase in the concentration
of ivermectin in the brain and plasma after administration of the
compound. CD-1 mice and those of the CF-1 strain that have higher
levels of P-glycoprotein are less sensitive to the toxicity of
abamectin on the central nervous system than the approximately 17%
of CF-1 mice deficient in this protein. The oral LD50 for CF-1
P-glycoprotein-deficient mice was about one order of magnitude lower
than that of CD-1 mice and of CF-1 mice with higher levels of
P-glycoprotein. This heterogeneity in the CF-1 mouse strain may
explain the apparent absence of a dose-response relationship with
respect to maternal toxicity in the studies of teratogenicity. These
data cannot, however, be used to demonstrate a correlation between
P-glycoprotein deficiency and teratogenicity, although, given the
apparent absence of a dose-response relationship, such a correlation
might be inferred.
Extensive information available on the use of ivermectin in
animal and human health was reviewed at the fortieth meeting of the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1992.
That Committee concluded that 'despite the extremely wide use of
ivermectin, there is no evidence of significant incidences of
adverse effects on reproductive performance in treated animals and
the very limited data on reproductive toxicity in humans indicate
that ivermectin does not increase the incidence of birth defects.'
The Meeting confirmed that the lowest NOAEL was 0.05 mg/kg bw
per day for maternal toxicity in the studies of teratogenicity in
mice for abamectin and for the delta-8,9 isomer. The Joint Meeting
in 1992 considered that slight decreases in body-weight gain early
in the lactation period in one generation of rats in a study of
reproductive toxicity provided supporting evidence. The present
Meeting concluded that the NOAEL in the study on reproductive
toxicity in rats was 0.12 mg/kg bw per day on the basis of toxicity
to pups at the higher dose level.
The Meeting concluded that the CF-1 mouse strain is
heterogeneous with respect to sensitivity to abamectin and,
therefore, may be an inappropriate model for studying the toxicity
(including teratogenicity) of avermectins. The Meeting therefore
decided to base the ADI on the NOAEL of 0.12 mg/kg bw per day for
pup toxicity in the study of reproductive toxicity in rats. A safety
factor of 500 was applied because the concern about the
teratogenicity of the delta-8,9 isomer could not be assuaged by the
additional data.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 0.12 mg/kg bw per day (two-generation study of
reproductive toxicity)
Estimate of acceptable daily intake for humans
0-0.0002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Data on P-glycoprotein in other species, including humans
2. Establishment and validation of a more sensitive method to
assess neurotoxic effects of avermectins in rodents
3. Acute toxicity of the delta-8,9 isomer in CF-1 and CD-1 mice,
with measurements of P-glycoprotein and blood and brain levels
of the compound
4. Study of the teratogenicity in CD-1 and CF-1 mice of abamectin
and the delta-8,9 isomer with concurrent measurements of
P-glycoprotein, in order to correlate its presence or absence
with maternal toxicity and teratogenicity
References
Beck, W.T. (1987) The cell biology of multiple drug resistance.
Biochem. Pharmacol., 36, 2879-2887.
Beck, W.T., Cirtain, M.C. & Lefko, J.L. (1983) Energy-dependent
reduced drug binding as a mechanism of vinca alkaloid resistance in
human leukemic lymphoblasts. Mol. Pharmacol., 24, 485-492.
Chiu, S.-H. & Lu, A.Y.U. (1989) Metabolism and tissue residues. In:
Campbell, W.C., ed., Ivermectin and Abamectin, New York, Springer
Verlag, pp. 131-143.
Dalgard, D.W., Mistretta, L.H., Bromberg, N.M. & Vargas, K.J. (1986)
Sixteen day oral toxicity study of MK-0933 in immature rhesus
monkeys. Study no. TT-85-9033. Unpublished report prepared by Merck
Research Laboratories, Three Bridges, NJ, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Demchak, R.J. & MacConnell, J.G. (1986) Comparison of avermectin
B1a and its 8,9-Z isomer on petri dishes. Unpublished report dated
27 May 1986, prepared by Merck Research Laboratories, Three Bridges,
NJ, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Gordon, L.R., Kornbrust, D.J., Douwning, G.V., Nickell, B.E., Buck,
J. & Rafferty, C.E. (1985) Oral toxicity and plasma level study in
monkeys with ivermectin (MK 0933) and abamectin (MK 0936). Study no.
T 85-013-0. Unpublished report prepared by Merck, Sharpe & Dohme
Research Laboratories, West Point, PA, USA. Submitted to WHO by
Merck Research Laboratories, Three Bridges, NJ, USA.
Gottesman, M.M. & Pastan, I. (1993) Biochemistry of multidrug
resistance mediated by the multidrug transporter. Ann. Rev.
Biochem., 62, 385-427.
Lankas, G.R., Cartwright, M.E. & Umbenhauer, D. (1994) Abamectin
(#177) Final report for TT #94-2775: Exploratory 5-day oral toxicity
study comparing abamectin sensitivity and P-glycoprotein levels in
CF-1 and CD-1 mice. Unpublished report dated 12 September 1994,
prepared by Merck, Sharpe & Dohme Research Laboratories, West Point,
PA, USA. Submitted to WHO by Merck Research Laboratories, Three
Bridges, NJ, USA.
Pacqué, M., Muñoz, B., Poetschke, G., Foose, J., Greene, B.M. &
Taylor, H.R. (1990) Pregnancy outcome after inadvertant ivermectin
treatment during community-based distribution. Lancet, 336,
1486-1489.
Schinkel, A.H., Smit, J.J.M., van Tellingen, O., Beijen, J.H.,
Wagenaar, E., van Deemter, L., Mol, C.A.A.M., van der Valk, M.A.,
Robanus-Maandag, E.C., te Riele, H.P.J., Berns, A.J.M. & Borst, P.
(1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to
a deficiency in the blood-brain barrier and to increased sensitivity
to drugs. Cell, 77, 491-502.
WHO (1990) Evaluation of Certain Veterinary Drug Residues in Food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 799), Geneva.
WHO (1991) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 27), Geneva.
WHO (1993a) Evaluation of Certain Veterinary Drug Residues in Food
(Fortieth report of the Joint FAO/WHO Expert Committee on Food
Additives) (WHO Technical Report Series, No. 832), Geneva.
WHO (1993b) Toxicological Evaluation of Certain Veterinary Drug
Residues in Food (WHO Food Additives Series, No. 31), Geneva.
